Abstract
Aim
Continuous intravenous (CIV) infusion of epinephrine for the treatment of anaphylaxis may be required if symptoms do not improve after intramuscular (IM) injection. As CIV infusion permits precise dose adjustment, we compared treatment course and adverse events following CIV infusion and IM injection of epinephrine for the management of anaphylaxis.
Methods
Medical records of patients, who were treated for anaphylaxis with epinephrine, were 18 years or older, and were admitted to our department from April 2005 to March 2016, were retrospectively reviewed. The cases were categorized as CIV infusion or IM injection, and treatment course and outcomes were compared between the two groups.
Results
Of the 142 eligible cases, there were 78 in the CIV infusion group and 64 in the IM injection group. The CIV infusion group had lower systolic blood pressure, more respiratory symptoms, and higher Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, but required a lower total dose of epinephrine, had fewer adverse events after epinephrine administration, and showed lower incidence of biphasic reactions. In addition, compared with the IM injection group, time to administration of epinephrine was significantly longer (P < 0.001), but time to resolution of symptoms, both from contact and epinephrine administration, was significantly shorter (P < 0.01 and P = 0.03, respectively).
Conclusion
Continuous intravenous infusion of epinephrine for the treatment of anaphylaxis may be safe, has fewer adverse events, improves symptoms, and is relatively easy to administer under ready conditions. CIV infusion of epinephrine may also reduce the incidence of biphasic reactions.
Keywords: adverse event, anaphylaxis, biphasic reaction, epinephrine, intravenous infusion
Our analysis shows that compared to intramuscular injection epinephrine, continuous intravenous (CIV) infusion of epinephrine for the treatment of anaphylaxis has fewer adverse events, improves symptoms, and is relatively easy to administer under ready conditions. CIV infusion of epinephrine may also reduce the incidence of biphasic reactions.
INTRODUCTION
Anaphylaxis is a serious and potentially life‐threatening allergic reaction that develops rapidly in response to allergens. The primary choice for the treatment of anaphylaxis is epinephrine, 1 which is typically administered as an intramuscular (IM) injection, as this route is both quick and safe. However, the effect is often inadequate, and repeated doses may be necessary. 2 In addition, once an IM injection is administered, amount of the drug cannot be adjusted, regardless of whether it is sufficient.
By contrast, continuous intravenous (CIV) infusion using a syringe pump and an intravenous line is rapid acting not only because of the intravascular route but also because epinephrine dose can be optimally adjusted with CIV; thus, it is used when symptoms do not improve after IM epinephrine. 3 A previous study in a canine model of anaphylactic shock has suggested that low‐dose CIV epinephrine significantly improved hemodynamics compared with subcutaneous, IM, or intravenous bolus administration. 4 Moreover, an initial CIV infusion of epinephrine was effective in a prospective study that used an initial CIV infusion of epinephrine and for resuscitation during anaphylaxis after immunotherapy. 5 Hence, we hypothesized that, compared with IM injection, CIV epinephrine as first‐line treatment would enable more effective management of anaphylaxis as the dose can be precisely controlled. However, CIV infusion requires a syringe pump, which may prolong the time to treatment initiation. Thus, we compared the treatment course and effects of epinephrine for the treatment of anaphylaxis, provided as CIV infusion or IM injection, in terms of time to symptom relief and number of adverse events.
METHODS
Study design and setting
This retrospective analysis reviewed the medical records of patients admitted to a single emergency medical center located in Gunma, Japan. Cases of patients with anaphylaxis, who were 18 years or older, were admitted to our department from April 2005 to March 2016, and were administered epinephrine, were eligible for inclusion. The emergency room has always been equipped with syringe pumps. At the time of diagnosis of anaphylaxis, preparation for epinephrine was started promptly. Method of epinephrine administration used was at the discretion of the treating physician. Cases were divided into two groups: CIV infusion and IM injection. This study was approved by the ethics committee of Gunma University Graduate School of Medicine (160122).
Exclusion criteria
We excluded patients with cardiac arrest, transferred to another hospital, and multiple routes of epinephrine administration (intravenous bolus infusion–CIV infusions and IM injection–CIV infusions).
Definition
Anaphylaxis was defined based on the three diagnostic criteria described in Table 1. 6 Biphasic anaphylaxis was defined as a uniphasic response characterized by typical symptoms, followed by an asymptomatic period of 1 h or longer, and a subsequent return of symptoms despite no further exposure to an antigen. IM injection (0.3–0.5 mg) was administered into the lateral aspect of the thigh. CIV infusions were provided using an infusion pump and were started at a rate of 0.1 μg/kg/min. Epinephrine dose was titrated to maintain systolic blood pressure at 90–140 mmHg, which was continuously and noninvasively monitored by the treating physician. When the initial symptoms improved, the administration of epinephrine was terminated. Saline solution was used for infusion, and the rate and volume were adjusted at the discretion of the physician.
Table 1.
Diagnostic criteria for anaphylaxis
| Anaphylaxis is highly likely when any one of the following three criteria is fulfilled: |
|---|
|
BP, blood pressure.
Data collection
Data were retrospectively collected and included information on age, sex, medications (beta‐blocker, antipsychotics), time of onset (morning, noon, evening, midnight), causative allergy, vital signs, symptoms, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, time from onset to administration of epinephrine, date of treatment, prognosis, initial volume of administration, total epinephrine dose, adverse events, biphasic reaction, and length of hospitalization.
The time interval from patient contact to resolution of symptoms was investigated as follows: A, time from contact to administration of epinephrine; B, time from administration of epinephrine to resolution of symptoms; and C, time from contact to resolution of symptoms (Fig. 1).
Fig. 1.
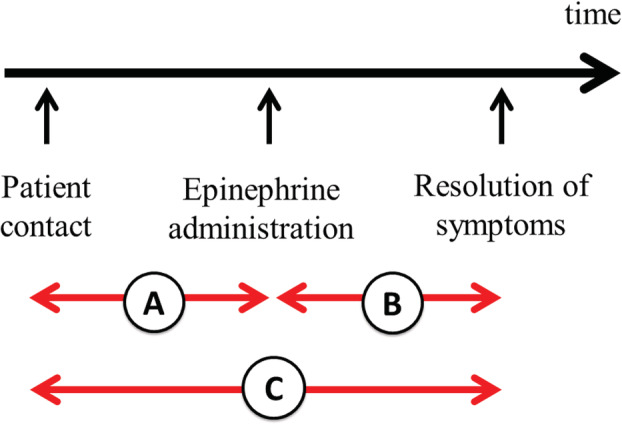
Time interval from patient contact to resolution of symptoms. (A) Time from contact to administration of epinephrine. (B) Time from administration of epinephrine to resolution of symptoms. (C) Time from contact to resolution of symptoms.
Statistical analyses
The Mann–Whitney U test was used for continuous data and the Pearson χ2 and Fisher exact tests were used for ordinal data. All statistical analyses were carried out on MedCalc 15.8 (MedCalc Software, Ostend, Belgium). P‐values <0.05 were considered statistically significant.
RESULTS
Of the 156 patients who were diagnosed with anaphylaxis and treated with epinephrine, 14 were excluded due to multiple methods of epinephrine administration. Thus, 142 patients were included in this study, with 78 in the CIV infusion group and 64 in the IM injection group (Fig. 2). All patients were monitored and administered steroids, and H1 and H2 blockers, as needed.
Fig. 2.
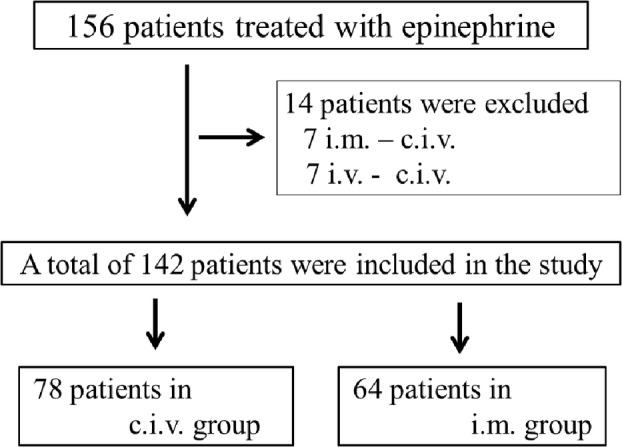
Flow diagram showing case selection. c.i.v., continuous intravenous infusion; i.m., intramuscular injection; i.v., intravenous injection.
Patient characteristics of the two groups are summarized in Table 2, and there were no significant differences in age, sex, medications, time of onset, causative allergen, SOFA score, and time from symptom onset to administration of epinephrine. APACHE II score was significantly higher in the CIV infusion group than in the IM group (median [interquartile range], 9 [6.3–12.0] versus 8 [6.0–10.3]; P = 0.02). There were no significant differences between the two groups in heart rate, respiratory rate, oxygen saturation, body temperature, and Glasgow Coma Scale; however, systolic pressure was significantly lower in the CIV infusion group (80 [70.0–104.8] mmHg versus 102.5 [90.0–121.3] mmHg; P < 0.001). There were no significant differences between the two groups in the prevalence of syncope, gastrointestinal symptoms (abdominal pain, vomiting, and diarrhea), and dermatological symptoms, but respiratory symptoms (stridor, wheezing, and dyspnea) were seen in significantly more cases in the CIV infusion group (48 [61.5%] versus 4 [6.3%]; P < 0.001).
Table 2.
Comparison of baseline characteristics
| Variables | Continuous intravenous infusion group (N = 78) | Intramuscular injection group (N = 64) | P‐value |
|---|---|---|---|
| Age (years) | 48 (32.3–63.5) | 51.5 (36.8–62.0) | 0.70 |
| Sex (female) | 36 (46.2) | 33 (51.6) | 0.61 |
| Medicine | |||
| Beta‐blocker | 2 (2.6) | 2 (3.1) | >0.99 |
| Antipsychotics | 0 | 1 (1.6) | 0.45 |
| Time of day | |||
| Morning | 3 (3.9) | 5 (7.8) | 0.47 |
| Noon | 33 (42.3) | 24 (37.5) | 0.61 |
| Evening | 22 (28.2) | 17 (26.6) | 0.85 |
| Midnight | 20 (25.6) | 18 (28.1) | 0.85 |
| Causative allergen | |||
| Foods | 37 (47.4) | 31 (48.4) | >0.99 |
| Medications | 19 (24.4) | 17 (26.6) | 0.85 |
| Insects | 18 (23.1) | 14 (21.9) | >0.99 |
| Unknown | 4 (5.1) | 2 (3.1) | 0.69 |
| Vital signs | |||
| Heart rate (per minute) | 96 (81.0–111.5) | 94 (81.8–103.8) | 0.72 |
| Systolic blood pressure (mmHg) | 80 (70.0–104.8) | 102.5 (90.0–121.3) | < 0.001 |
| Respiratory rate (per minute) | 24 (21.3–27.5) | 24 (22.0–26.0) | 0.92 |
| Oxygen saturation (%) | 92 (89.3–96.0) | 93.5 (92.0–96.3) | 0.07 |
| Body temperature (°C) | 36.6 (36.2–36.8) | 36.5 (36.2–36.9) | 0.7 |
| Glasgow Coma Scale | 14 (14–15) | 14 (14–15) | 0.68 |
| Symptoms | |||
| Stridor/wheezing/dyspnea | 48 (61.5) | 4 (6.3) | < 0.001 |
| Syncope | 4 (5.1) | 4 (6.3) | >0.99 |
| Abdominal pain/vomiting/diarrhea | 23 (29.5) | 17 (26.6) | 0.71 |
| Dermatological symptoms | 70 (90.0) | 61 (95.3) | 0.35 |
| APACHEII score | 9 (6.3–12.0) | 8 (6.0–10.3) | 0.02 |
| SOFA score | 3 (2–3) | 2 (2–3) | 0.19 |
| Time from onset to administration of epinephrine (min) | 29 (24.0–36.8) | 25 (20.8–35.0) | 0.07 |
Data are expressed as the group median (interquartile range) or n (%).
APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment.
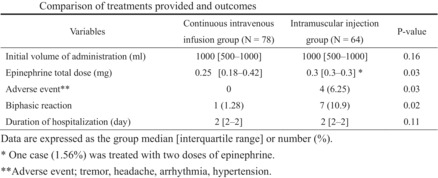
A comparison of treatment and outcomes is shown in Table 3. There were no significant differences between the two groups in the initial volume of saline administered and duration of hospitalization, but total epinephrine dose (median, 0.25 [0.18–0.42] mg versus 0.3 [0.3–0.3] mg; P = 0.03), number of adverse events (0 versus 4 [6.25%]; P = 0.03), and incidence of biphasic reactions (1 [1.28%] versus 7 [10.9%]; P = 0.02) were significantly lower in the CIV group. In addition, adverse events such as tremor, headache, arrhythmia (premature ventricular contraction), and hypertension occurred only in the IM group, and one patient (1.56%) required epinephrine two times in this group.
Table 3.
Comparison of treatments provided and outcomes
| Variables | Continuous intravenous infusion group (N = 78) | Intramuscular injection group (N = 64) | P‐value |
|---|---|---|---|
| Initial volume of administration (mL) | 1,000 (500–1,000) | 1,000 (500–1,000) | 0.16 |
| Epinephrine total dose (mg) | 0.25 (0.18–0.42) | 0.3 (0.3–0.3) † | 0.03 |
| Adverse event ‡ | 0 | 4 (6.25) | 0.03 |
| Biphasic reaction | 1 (1.28) | 7 (10.9) | 0.02 |
| Duration of hospitalization (days) | 2 (2–2) | 2 (2–2) | 0.11 |
Data are expressed as the group median (interquartile range) or n (%).
One case (1.56%) was treated with two doses of epinephrine.
Adverse event: tremor, headache, arrhythmia, hypertension.
We compared timelines between the two groups to determine whether time from contact to resolution of symptoms was shorter in the CIV group than in the IM group (Table 4). Time from contact to epinephrine administration was significantly longer in the CIV group (median, 7 [5–9] minutes versus 4 [3–5] minutes, P < 0.001; Table 4A); by contrast, time from epinephrine administration to symptom resolution was significantly shorter in the CIV group (median, 5 [4–5.75] minutes versus 9 [8–10] minutes, P < 0.01; Table 4B). Taken together, data presented in Table 4C indicate that time elapsed from contact to resolution of symptoms was significantly shorter in the CIV group than in the IM group (median, 12 [9, 10, 11, 12, 13, 14, 15] minutes versus 13 [11–15.25] minutes, respectively, P = 0.03).
Table 4.
Comparison of time elapsed from patient contact to resolution of symptoms
| Variables | Continuous intravenous infusion group (n = 78) | Intramuscular injection group (n = 64) | P‐value |
|---|---|---|---|
| A: Time from contact to administration of epinephrine (min) | 7 (5–9) | 4 (3–5) | <0.001 |
| B: Time from administration of epinephrine to resolution of symptoms (min) | 5 (4–5.75) | 9 (8–10) | <0.01 |
| C: Time from contact to resolution of symptoms (min) | 12 (9–15) | 13 (11–15.25) | 0.03 |
Data are expressed as the group median (interquartile range).
DISCUSSION
Epinephrine is essential for the management of anaphylaxis as its pharmacologic actions address the underlying pathophysiologic changes. 7 Our analysis shows that CIV infusion of epinephrine for anaphylaxis not only led to fewer adverse events and biphasic reactions but also shortened the time to resolution of symptoms. Epinephrine has multiple effects, namely, alpha‐1‐epinephrine agonist effects (greater vasoconstriction and peripheral vascular resistance but lower mucosal edema), beta‐1‐epinephrine agonist effects (higher inotropy and chronotropy), and beta‐2‐epinephrine agonist effects (increased bronchodilation). It also decreases mediator release from mast cells and basophils. 8
Adverse events and administration methods
Epinephrine in therapeutic doses, regardless of the method of administration, often causes mild transient pharmacologic effects, such as headache, tremor, dizziness, palpitations, and pallor. It may also rarely lead to serious adverse effects such as ventricular arrhythmias, angina, and abnormal hypertension. Intravenous bolus injection is typically used for the management of severe circulatory failure, but as adverse events can often occur, it is recommended that this method be used by experts alone. 9 Therefore, IM injection is the recommended route of epinephrine administration for anaphylaxis because it is associated with fewer adverse events. Campbell et al. 9 have reported that an IM injection of epinephrine for anaphylaxis had an adverse reaction in 1.3% of the patients, while we report that adverse events, which ranged from minor signs to arrhythmias, occurred in 6.25% of the cases. By contrast, although patients who were provided CIV infusion tended to have hypotension and a high APACHE II score, no adverse events were observed in this group, which is significantly lower than that observed in the IM injection group. This may be because epinephrine doses provided via IM injection and intravenous bolus infusion cannot be reversed after administration, whereas epinephrine dose provided through a CIV infusion can be adjusted using a syringe pump, which would have led to fewer adverse events. Thus, our analysis indicates that CIV infusion may be safer in the management of anaphylaxis.
Infusion methods and treatment timelines
We show that the time interval from patient contact to administration of epinephrine was significantly shorter in the IM group, probably because it is easy to administer an IM infusion. By contrast, time elapsed from contact to epinephrine administration takes longer as CIV infusion requires a secure intravenous route and priming of a syringe pump. Nevertheless, the effects of CIV epinephrine appear promptly and lead to symptom improvement, indicating faster symptom resolution after CIV compared with IM injection. Further, as the total time elapsed from patient contact to epinephrine administration and resolution of symptoms was significantly shorter in the CIV infusion group, it may be useful to maintain a primed syringe pump that is always available and ready for emergency use.
IM injection may occasionally require repeated dosing if there is a reduction in blood volume in the muscle during severe circulatory failure or in the presence of thick subcutaneous fat. 10 By contrast, because epinephrine is administered intravenously in the CIV methods, the effect of epinephrine appears stably. In this study, although many patients with low blood pressure were found in the CIV group, the total epinephrine dose was lower. CIV will produce significant hemodynamic improvement 4 and is considered to be better for anaphylaxis management, especially in cases with circulatory failure.
Biphasic reaction and CIV infusion
Biphasic reactions are characterized by the recurrence of anaphylactic symptoms without repeated exposure to an antigen. 11 Therefore, it is necessary to closely follow patients after initial treatment for anaphylaxis, and we show that biphasic reactions were significantly fewer in the CIV infusion group. The reported incidence of biphasic reactions ranges from 0.4% to 23.3%, 12 , 13 with a previous study from Japan reporting a rate of 10.8%. 14 Risk factors for biphasic reactions include prolonged time to epinephrine administration, 15 severity of the first phase of symptoms and multiorgan involvement, 16 need for more than once dose of epinephrine, and severe initial symptoms. 17 In this study, although time elapsed from onset to epinephrine administration tended to be longer in the CIV infusion group than in the IM injection group, this difference was not significant. The pathogenesis of biphasic reactions is not known, but several theories have been proposed, and it is possible that CIV epinephrine suppresses the occurrence of a biphasic reaction. One such theory posits that the biphasic response is due to a secondary rise in the mediator molecule following first response 18 ; hence, CIV infusion, rather than a single dose of epinephrine, may suppress secondary mediator elevation. To the best of our knowledge, no studies have compared the risk of biphasic reactions based on route of epinephrine administration, which is a novel aspect of this study.
Limitations
This was a single‐center retrospective study and small‐sample research with univariate analysis. There are some interpretation problems of the single‐center retrospective study, small numbers of cases and lack of adjustment for bias and confounding factors. In addition, a single‐center study may show a large effect than a multicenter study because it is targeted at carefully selected patients and treatment is performed by experts. 19 This study also needs to be careful with the interpretation. The route of epinephrine administration depended on the attending physician, and there may have been differences in the skill levels among the physicians, especially for CIV infusion. Besides, the physician may have preferentially selected the CIV infusion for patients with hemodynamically unstable symptoms. Moreover, our emergency room has always been equipped with resuscitation products and a syringe pump. Therefore, the time taken to initiate treatment may differ from that reported by others.
CONCLUSION
Compared with IM injections, epinephrine provided via CIV infusion for the treatment of anaphylaxis may be associated with fewer adverse events and results in quicker resolution of symptoms under ready‐to‐administer conditions, attesting to its safety and ease of use. In addition, epinephrine administered through CIV may reduce the incidence of a biphasic reaction.
DISCLOSURE
Approval of the research protocol with approval no. and committee name: This study was approved by the ethics committee of Gunma University Hospital (160122).
Informed Consent: Opt‐out mode.
Registry and Registration no. of the study/trial: N/A.
Animal Studies: N/A.
Conflict of Interest: None declared.
ACKNOWLEDGMENTS
The authors thank Enago (www.enago.jp) for the English language review.
Funding information
No funding information provided.
REFERENCES
- 1. Simons FER. Anaphylaxis. J. Aller. Clin. Immunol. 2010; 125: S161–81. [DOI] [PubMed] [Google Scholar]
- 2. Nandinee P, Kok WC, Alexander YGY et al. Use of multiple epinephrine doses in anaphylaxis: a systematic review and meta‐analysis. J. Aller. Clin. Immunol. 2021; 148: 1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soar J, Pumphrey R, Cant A et al. Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation 2008; 77: 157–69. [DOI] [PubMed] [Google Scholar]
- 4. Mink SN, Simons FE, Simons KJ, Becker AB, Duke K. Constant infusion of epinephrine, but not bolus treatment, improves haemodynamic recovery in anaphylactic shock in dogs. Clin. Exp. Allergy 2004; 34: 1776–83. [DOI] [PubMed] [Google Scholar]
- 5. Brown SG, Blackman KE, Stenlake V, Heddle RJ. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg. Med. J. 2004; 21: 149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sampson HA, Muñoz‐Furlong A, Campbell RL et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006; 117: 391–7. [DOI] [PubMed] [Google Scholar]
- 7. Simons FER. Anaphylaxis: evidence‐based long‐term risk reduction in the community. Immunol. Allergy Clin. North Am. 2007; 27: 231–48. [DOI] [PubMed] [Google Scholar]
- 8. Vadas P, Perelman B. Effect of epinephrine on platelet‐activating factor–stimulated human vascular smooth muscle cells. J. Allergy Clin. Immunol. 2012; 129: 1329–33. [DOI] [PubMed] [Google Scholar]
- 9. Campbell RL, Bellolio MF, Knutson BD et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J. Allergy Clin. Immunol. Pract. 2015; 3: 76–80. [DOI] [PubMed] [Google Scholar]
- 10. Song TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann. Allergy Asthma Immunol. 2005; 94: 539–42. [DOI] [PubMed] [Google Scholar]
- 11. Pourmand A, Robinson C, Syed W, Mazer‐Amirshahi M. Biphasic anaphylaxis: a review of the literature and implications for emergency management. Am. J. Emerg. Med. 2018; 36: 1480–5. [DOI] [PubMed] [Google Scholar]
- 12. Grunau BE, Li J, Yi TW et al. Incidence of clinically important biphasic reactions in emergency department patients with allergic reactions or anaphylaxis. Ann. Emerg. Med. 2014; 63: 736–44. [DOI] [PubMed] [Google Scholar]
- 13. Scranton SE, Gonzalez EG, Waibel KH. Incidence and characteristics of biphasic reactions after allergen immunotherapy. J. Allergy Clin. Immunol. 2009; 123: 493–8. [DOI] [PubMed] [Google Scholar]
- 14. Nomura T, Sekii H, Sugita M, Nakahara S. Association between biphasic reactions and the systems of symptoms and treatment in patients with anaphylaxis hospitalized from the emergency department. Acute Med. Surg. 2020; 7: e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu X, Lee S, Lohse CM, Hardy CT, Campbell RL. Biphasic reactions in emergency department anaphylaxis patients: a prospective cohort study. J .Allergy Clin. Immunol. Pract 2020; 8: 1230–8. [DOI] [PubMed] [Google Scholar]
- 16. Kraft M, Hofmeier KS, Ruëff F et al. Risk factors and characteristics of biphasic anaphylaxis. J. Allergy Clin. Immunol. 2020; 8: 3388–95. [DOI] [PubMed] [Google Scholar]
- 17. Shaker MS, Wallace DV, Golden DB et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J. Allergy Clin. Immunol. 2020; 145: 1082–123. [DOI] [PubMed] [Google Scholar]
- 18. Tole JW, Lieberman P. Biphasic anaphylaxis: review of incidence, clinical predictors, and observation recommendations. Immunol. Allergy Clin. North Am. 2007; 27: 309–26. [DOI] [PubMed] [Google Scholar]
- 19. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single‐center trials show larger treatment effects than multicenter trials: evidence from a meta‐epidemiologic study. Ann. Intern. Med. 2011; 155: 39–51. [DOI] [PubMed] [Google Scholar]


