Abstract
Objective:
To determine whether cognitive and psychological symptom profiles differentiate clinical diagnostic classifications (e.g., history of mild traumatic brain injury [mTBI], posttraumatic stress disorder [PTSD]) in military personnel.
Method:
U.S. Active-Duty Service Members (n=209, 89% male) with a history of mTBI (n=56), current PTSD (n=23), combined mTBI+PTSD (n=70), or orthopedic injury controls (n=60) completed a neuropsychological battery assessing cognitive and psychological functioning. Latent profile analysis was performed to determine how neuropsychological outcomes of individuals clustered together. Diagnostic classifications (i.e., mTBI, PTSD, mTBI+PTSD, orthopedic injury controls) within each symptom profile were examined.
Results:
A five-profile model had the best fit. The profiles differentiated subgroups with high (34.0%,) or normal (21.5%) cognitive and psychological functioning, cognitive symptoms (19.1%), psychological symptoms (15.3%), and combined cognitive and psychological symptoms (10.0%). The symptom profiles differentiated participants as would generally be expected. Participants with PTSD were mainly represented in the psychological symptom sub-group, while orthopedic injury controls were mainly represented in the high functioning subgroup. Further, ~79% of participants with comorbid mTBI and PTSD were represented in a symptomatic group (~24% = cognitive symptoms, ~29% = psychological symptoms, 26% = combined cognitive/psychological symptoms). Our results also showed that ~70% of military personnel with a history of mTBI were represented in the high and normal functioning groups.
Conclusions:
These results demonstrate both overlapping and heterogeneous symptom and performance profiles in military personnel with a history of mTBI, PTSD, and/or mTBI+PTSD. The overlapping profiles may underscore why these diagnoses are often difficult to diagnose and treat, but suggests that advanced statistical models may aid in identifying profiles representing symptom and cognitive performance impairments within patient groups and enable identification of more effective treatment targets.
Keywords: Combat, Concussion, PTSD, Cognition, Latent profile analysis, Military, TBI
Introduction
Traumatic brain injury (TBI) is a significant health concern among Service Members and Veterans of the recent military conflicts in Iraq and Afghanistan and follow-on conflicts. Approximately 10–20% of military personnel returning from combat report TBI or probable TBI [1–7], with more than 80% of these being mild TBI [mTBI; 8]. Many military personnel with TBI show cognitive symptoms including executive dysfunction and disinhibition, slower processing speed, impaired attention, and concentration [3, 9]. Other commonly reported symptoms across TBI severity include headaches, chronic pain, sleep disturbance, depression, anxiety, and irritability [10–12]. There is a critical need for additional research, as diagnostic and prognostic accuracy following TBI is complicated by several factors including the clinical and functional heterogeneity observed between individual patients and across research cohorts.
Further complicating clinical and functional outcomes are the number of comorbidities experienced by Service Members and Veterans following mTBI, especially TBIs that occur during combat deployment. Research among Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) Veterans clearly shows that posttraumatic stress disorder (PTSD) is a common comorbidity among Veterans with TBI and deployment history. An estimated 12–25% of military personnel experience PTSD and PTSD symptomatology, which seems to be affected by the nature and degree of combat exposure [3, 6, 13, 14]. Hoge and colleagues found that in ~44% of soldiers who had lost consciousness, ~27% reported PTSD symptoms [7]. Individuals with PTSD and no history of TBI also show impaired executive functioning, memory, and attention [3, 7, 15]. There may be a bidirectional relationship between PTSD and cognition, such that PTSD contributes to worse cognitive functioning, but cognition may also influence the risk of developing and maintaining PTSD [16]. Scott and colleagues conducted a meta-analysis of neurocognitive functioning in PTSD, and reported medium effect sizes (>.45) for impairment in various domains (verbal learning, speed of information processing, attention/working memory, and verbal memory) [17]. Compared to those with a history of mTBI only, PTSD and depression may be associated with more persistent cognitive difficulties [18]. Furthermore, PTSD is a potential risk factor for dementia later in life [19], whereas the findings are mixed regarding the association between TBI and dementia [20].
Thus, TBI and comorbid PTSD among Service Members is a significant health challenge as they have a complex set of symptoms and features with significant overlap (e.g., impaired attention, memory, executive functioning [3]; sleep disturbance and pain [21]; depression, anxiety, fatigue, irritability, anger and hyperarousal [22]). Many studies indicate that the neuropsychological profile of comorbid TBI and PTSD is worse than that of either one alone [3]. Although many military personnel who have mTBI and/or PTSD will recover to normal functioning, a large proportion still require medical or other intervention [13]. Even in isolation, the diagnosis and treatment of these conditions is complicated and depends heavily on subjective reporting of symptoms, but clinical care and diagnosis are additionally challenging and complex when TBI and PTSD co-occur [1, 23]. Thus, methods that can account for the role of both TBI and psychiatric comorbidities in functional outcomes may provide more effective diagnostic and treatment options.
Recently, latent profile analysis (LPA) has been used to identify latent or unobserved clusters in TBI and PTSD clinical data [24] and has demonstrated the utility of identifying performance and symptom profiles. Using latent class analysis (LCA), similar to LPA but for categorical indicators, Jaramillo and colleagues identified distinct symptom classes that were associated with patient groups (TBI, PTSD, Depression, or combination of diagnosis) [25]. Further, symptom classes differentiated patient subgroups; more TBI patients were represented in Moderate and High Distress Groups, while PTSD and depression were more likely in the Behavioral Health and High Distress symptom profiles. Additionally, the High Distress group also had the highest proportion of TBI with a combination of PTSD and depression. Furthermore, LCA has been used to show which postconcussion symptom profiles in individuals with mTBI may be associated with protracted recovery and long-term endorsement of postconcussion symptoms [26]. Another LPA study of former National Football League players found five distinct profiles of self-reported functioning, related to neurobehavioral, physical and psychosocial functioning [27]. Given the difficulty in determining management strategies and prognosis for individuals with a history of mTBI, current PTSD, and mTBI+PTSD, using advanced statistical techniques to identify profiles representing symptom and cognitive performance impairments, regardless of clinical diagnostic classification, may enable identification of more effective treatment targets in these patients.
In the current study, LPA was used to determine whether self-report symptoms on psychological measures and performance on neuropsychological tests clustered individuals into classes and whether these classes represented predefined diagnostic characteristics (i.e., mTBI, PTSD, mTBI+PTSD or orthopedic injury controls) in Active-Duty Service Members. We first predicted that the measures included in the current study would separate individuals into classes representing psychological symptoms, cognitive symptoms, and normal functioning. Second, we expected that military personnel with mTBI or mTBI and comorbid PTSD would be represented in both the cognitive and psychological symptom classes, PTSD would be more represented in the psychological symptoms class.
Methods
Participants
Participants (N = 209; 186 males, 23 females) were Active-Duty U.S. Service Members recruited at a large military treatment facility following an initial chart review to determine eligibility. The study consisted of three demographically-comparable groups including mTBI, PTSD, and orthopedic injury controls being treated in their respective clinics (Table 1). All participants exceeded the standard threshold for the Test of Memory Malingering (TOMM; >45 for Trial 1). As anticipated in this active-duty Service Member population, participants had Wide Range Achievement Test-version 4 (WRAT-4) Word Reading scores in the average to high-average range (mean = 98.4, SD = 10.5) and were presumed to be functionally within normal limits. This research was approved and monitored by the local hospital IRB and Human Research Protection Office (HRPO) at the U.S. Army Medical Department Medical Research and Materiel Command (USAMRMC). After explanation of the research (including aims, risks, and benefits), each participant provided written informed consent.
Table 1.
Demographic Characteristics
| All | OI Control | PTSD | mTBI | mTBI+PTSD | |
|---|---|---|---|---|---|
|
|
|||||
| N | 209 | 60 | 23 | 56 | 70 |
| Age | 33.8 (8.8) | 37.4 (7.3) | 37.9 (6.2) | 29.2 (7.3) | 33.0 (10.0) |
| Sex (Male/Female) | 186 / 23 | 48 / 12 | 21 / 2 | 53 / 3 | 64 / 6 |
| Race | |||||
| White | 148 [70.8%] | 38 [63.3%] | 13 [56.5%] | 46 [82.1%] | 51 [72.9%] |
| Black or African-American | 33 [15.8%] | 18 [30.0%] | 4 [17.4%] | 4 [7.1%] | 7 [10.0%] |
| American Indian or Alaska Native | 2 [1.0%] | 0 [0.0%] | 0 [0.0%] | 2 [3.6%] | 0 [0.0%] |
| Native Hawaiian or Other Pacific Islander | 4 [1.9%] | 1 [1.7%] | 0 [0.0%] | 0 [0.0%] | 3 [4.3%] |
| Asian | 5 [2.4%] | 1 [1.7%] | 3 [13.0%] | 0 [0.0%] | 1 [1.4%] |
| More than one race | 17 [8.1%] | 2 [3.3%] | 3 [13.0%] | 4 [7.1%] | 8 [11.4%] |
| Ethnicity | |||||
| Hispanic or Latino | 59 [28.2%] | 13 [21.7%] | 10 [43.5%] | 16 [28.6%] | 20 [28.6%] |
| Not Hispanic or Latino | 150 [71.8%] | 47 [78.3%] | 13 [56.5%] | 40 [71.4%] | 50 [71.4%] |
| Education | |||||
| High School Diploma | 98 [46.9%] | 15 [25.0%] | 8 [34.8%] | 41 [73.2%] | 34 [48.6%] |
| GED | 7 [3.3%] | 0 [0.0%] | 0 [0.0%] | 1 [1.8%] | 6 [8.6%] |
| Associates Degree | 43 [20.6%] | 13 [21.7%] | 5 [21.7%] | 12 [21.4%] | 13 [18.6%] |
| College Degree (BA/BS) | 34 [16.3%] | 16 [26.7%] | 6 [26.1%] | 2 [3.6%] | 10 [14.3%] |
| Post Graduate Degree | 27 [12.9%] | 16 [26.7%] | 4 [17.4%] | 0 [0.0%] | 7 [10.0%] |
| Years of Military Service | 11.9 (7.9) | 15.7 (6.7) | 15.0 (6.2) | 7.5 (6.5) | 11.2 (8.4) |
| Number of Deployments | 1.9 (1.0) | 2.0 (0.9) | 2.1 (1.3) | 1.8 (1.0) | 1.9 (1.0) |
| PTSD Checklist – Military Version | 43.8 (18.4) | 27.2 (12.4) | 60.9 (11.5) | 33.8 (7.1) | 60.5 (10.3) |
| Mechanism of Injury | |||||
| Blast | - | - | - | 42 [75.0%] | 38 [54.3%] |
| Vehicular | - | - | - | 1 [1.8%] | 8 [11.4%] |
| Fall | - | - | - | 6 [10.7%] | 6 [8.6%] |
| Other | - | - | - | 7 [12.5%] | 18 [25.7%] |
| Number of Prior TBI Reported | - | - | - | 2.0 (3.5) | 3.9 (6.2) |
| Time Since Injury (Months) | |||||
| High Functioning | - | - | - | 9.0 (5.4) | - |
| Normal Functioning | - | - | - | 12.5 (7.1) | 9.1 (4.5) |
| Psychological Symptoms | - | - | - | - | 9.9 (6.3) |
| Cognitive Symptoms | - | - | - | 9.7 (4.8) | 7.4 (4.7) |
| Combined Cognitive / Psychological Symptoms | - | - | - | - | 12.0 (5.4) |
| Loss of Consciousness | |||||
| High Functioning | - | - | - | 13 [54.2%]* | - |
| Normal Functioning | - | - | - | 4 [26.7%]* | 5 [33.3%]* |
| Psychological Symptoms | - | - | - | - | 10 [50.0%]* |
| Cognitive Symptoms | - | - | - | 7 [41.2%]* | 6 [35.3%]* |
| Combined Cognitive / Psychological Symptoms | - | - | - | - | 11 [61.1%]* |
| Post-Traumatic Amnesia | |||||
| High Functioning | - | - | - | 3 [12.5%]* | - |
| Normal Functioning | - | - | - | 2 [13.3%]* | 2 [13.3%]* |
| Psychological Symptoms | - | - | - | - | 0 [0.0%]* |
| Cognitive Symptoms | - | - | - | 0 [0.0%]* | 2 [11.8%]* |
| Combined Cognitive / Psychological Symptoms | - | - | - | - | 6 [33.3%]* |
Note: Mean with standard deviation in parentheses. Frequency with percentage in square brackets.
Percentage of diagnostic group within symptom class. One OI control was missing data for deployments. Three participants (2 mTBI and 1 mTBI+PTSD) had missing data for number of prior TBI and one mTBI participant reported multiple prior TBIs but did not quantify the number. One mTBI+PTSD had missing data for loss of consciousness. Two mTBI participants had missing data for post-traumatic amnesia. Time since injury did not differ between mTBI and mTBI+PTSD groups or between classes.
mTBI Participants
mTBI participants (n = 126) were Active-Duty Service Members who took part in a large cognitive rehabilitation clinical trial study (Study of Cognitive Rehabilitation Effectiveness (SCORE!) [28]. The diagnosis of mTBI was made using Veteran Affairs (VA)/Department of Defense Clinical Practice Guidelines [29] following a screening interview and medical record review by experienced TBI and Concussion Center medical staff. Furthermore, each participant was required to have persistent cognitive symptoms, defined as a Neurobehavioral Symptom Inventory (NSI) score of 3 or higher on any of the four cognitive symptoms [28]. Furthermore, participants were included if they were 18–55 years old, sustained a closed head injury during deployment (OEF/OIF/Operation New Dawn [OND]) activities 3–24 months prior to recruitment, and were fluent in English. Given that mTBI often coexists with PTSD, we further classified our mTBI group into mTBI with potential comorbid PTSD using the Posttraumatic Stress Disorder Checklist – Military Version (PCL-M) cut-off score of >45 [30–32]. This resulted in a mTBI-only group (n = 56; PCL-M: M = 33.8, SD = 7.1) and mTBI with PTSD group (n = 70; PCL-M: M = 60.5, SD = 10.3).
PTSD Participants
PTSD participants (n = 23) included Active-Duty Service Members recruited through the hospital Behavioral Health Clinic. Potential participants underwent a screening interview and medical record review and were excluded if they had a lifetime history of TBI. PTSD participants were required to have a deployment-related Clinician Administered PTSD Scale (CAPS; DSM-IV criteria) confirmed diagnosis of PTSD at the time of data collection. Given that TBI inclusion criteria required that the injury occurred during deployment, PTSD trauma exposure must also have occurred during this time. PTSD participants were selected using similar inclusion criteria including age range, deployment history, and English fluency.
Orthopedic Participants
Orthopedic injury controls (n = 60) were Active-Duty Service Members recruited through the hospital Orthopedic Clinic. Orthopedic injury was defined as a joint injury, typically in the extremity. Potential participants underwent a screening interview and medical record review and were excluded if they had a lifetime history of TBI and/or a PTSD diagnosis according to the standardized CAPS (DSM-IV criteria). Orthopedic injury control participants had the same inclusion criteria as above.
Across groups, participants were excluded if they had neurologic comorbidities (i.e., seizures, psychosis), history of moderate/severe TBI, spinal cord injury, were on scheduled narcotic pain medications, or unable to use their dominant hand. As participants also completed a neuroimaging battery that is not reported in the current study, participants with abnormal MRI findings were also excluded and referred for appropriate evaluation. Attempts were made to include patients in each group such that the groups would be similar in age, rank, and sex distribution.
Measures
Demographic, Clinical, and Cognitive Variables
Participants meeting inclusion criteria were surveyed for demographic information (e.g., age, sex, number of deployments, etc.) and injury history (e.g., time since injury, loss/alteration of consciousness, post-traumatic amnesia, etc.) if applicable (Table 1). Participants completed self-report psychological questionnaires including the Alcohol Use Disorder Identification Test (AUDIT), NSI, PCL-M, and the Depression and Anxiety subscales from the Symptom Checklist-90 (SCL-90). Technician-administered assessments were used to assess cognitive performance: memory: Total Recall, Short Delay Free Recall, Long Delay Free Recall and Recognition Hits from the California Verbal Learning Test-II (CVLT-II); verbal fluency: Delis-Kaplan Executive Function System (D-KEFS Category and Letter Fluency subtests); attention: Paced Auditory Serial Addition Test (PASAT); executive function: time to complete Trail Making Test Part B minus Part A (TMT B-A); working memory and processing speed: Working Memory Index and Processing Speed Indices from the Wechsler Adult Intelligence Scale-IV (WAIS-IV); and performance validity indicators: TOMM. Mean self-report scores or mean performance for each group are included in Supplemental Table 1. Reaction time (i.e., TMT B-A) and self-report measures were reflected at the model interpretation stage so that higher scores reflected better performance or fewer symptoms.
Statistical Approach
LPA using maximum likelihood parameter estimates with robust standard errors was completed in MPlus 7.3 using the 15 cognitive and psychological test scores as indicators. N=1000 random starts was used to replicate the best log-likelihood and ensure the global solutions. To identify the best fitting model, a series of LPA models with different numbers of profiles were performed until the best log-likelihood for the k+1 profile could not be replicated, or the model failed to converge. Models were then compared iteratively based on the following heuristics which were used to guide model selection (i.e., optimal symptom clusters): (1) lower information criterion values (Akaike Information Criterion [AIC], Bayesian Information Criterion [BIC] values, and sample-size adjusted BIC values [SABIC]) indicating a better model fit; (2) Lo-Mendell-Rubin Likelihood [LMR] [33] test value and Bootstrapped Likelihood Ratio Test [BLRT] [34] where a smaller p value (e.g., p < .05) for LMR and BLRT tests indicate a better model fit of the k profile model compared to the k-1 profile model [24, 35]; (3) higher entropy values close to 1 indicate an excellent classification of subjects into its corresponding latent classes with a cut-off of 0.80 or higher [36]; (4) conceptual meaning based on comparison of standardized means; and (5) size of the smallest derived profile (as profiles constituting less than 5% of the sample considered likely to over-fit the data). Although it is recommended to report various fit indices, BIC and BLRT are the best indices in determining the number of classes across various models, with lower BIC and a significant (p < .05) BLRT indicating a better fit of k profiles model than k-1 profiles model [37]. When deciding the final model, besides statistical fit indices, model parsimony, clinical meaningfulness, and profile size are also considered. For instance, if an additional profile in k profiles model adds no substantial new information from the prior k-1 profiles solution [38], the new profile would not be retained.
Post-estimation provided information on the proportion of mTBI, PTSD, mTBI+PTSD and orthopedic injury controls for each of the identified classes. One-way ANOVA models with class membership as the between-subjects variable were used to assess separation between classes. Significant ANOVAs were followed with Tukey’s HSD post-hoc tests to determine significant differences between classes (p < 0.05). After the best fit model was identified, two neuropsychologists (D.F.T. and E.A.W) who were blinded to participant representation (i.e., clinical diagnosis of mTBI, PTSD, or mTBI+PTSD) within each class, assigned semantic labels to each class profile (e.g., cognitive symptoms, psychological symptoms) based on test scores for each indicator within a class. For example, a class with normal cognitive function but high psychological symptoms was labelled “Psychological Symptoms” class. Thus, class labels were determined using a data driven analytical approach, not based on prior knowledge of clinical diagnostic classification.
Results
Class Determination
The cognitive and psychological indicator variables were fit into latent profile models with k=2 to 5 classes. A model with 5 classes fit the data best among all models. The 5-class model minimized the log-likelihood value, AIC, BIC and SABIC relative to the 2–4-class models (Table 2). The BLRT demonstrated that the 5-class model performed significantly better than the other models. While the LMR for the 5-class model was not significant, the entropy values were well above the .8 threshold and were relatively stable for all k-class models up to k=5. An examination of the model fit statistics and conceptual meaning indicated that a fifth class was well differentiated from the other classes (Table 2). One-way ANOVAs comparing class membership for the 5-class model demonstrated good separation between the classes on all indicators (p<0.05) except for the AUDIT (p=0.052).
Table 2.
Model Fit Statistics for Latent Profile Analysis Models
| Classes | Loglikelihood | AIC | BIC | SABIC | BLRT | pBLRT | LMR | pLMR | Entropy |
|---|---|---|---|---|---|---|---|---|---|
| 2 | −9995.52 | 20083.04 | 20236.79 | 20091.04 | 796.14 | <0.001 | 786.94 | 0.006 | 0.92 |
| 3 | −9853.76 | 19831.53 | 20038.75 | 19842.30 | 283.52 | <0.001 | 280.24 | 0.205 | 0.90 |
| 4 | −9744.99 | 19645.97 | 19906.68 | 19659.53 | 217.55 | <0.001 | 215.04 | 0.071 | 0.93 |
| 5 | −9650.68 | 19489.35 | 19803.53 | 19505.69 | 188.62 | <0.001 | 186.44 | 0.212 | 0.94 |
Note: Lower values of AIC, BIC, SABIC are evidence of a better fitting model.
pBLRT < .05 indicates that the k-class model is a better fit to the data than the k-1 class model.
pLMR small p-values indicate that the k-class model fits better to data than the k-1 class model.
Entropy value close to 1 indicates excellent classification of subjects into latent classes.
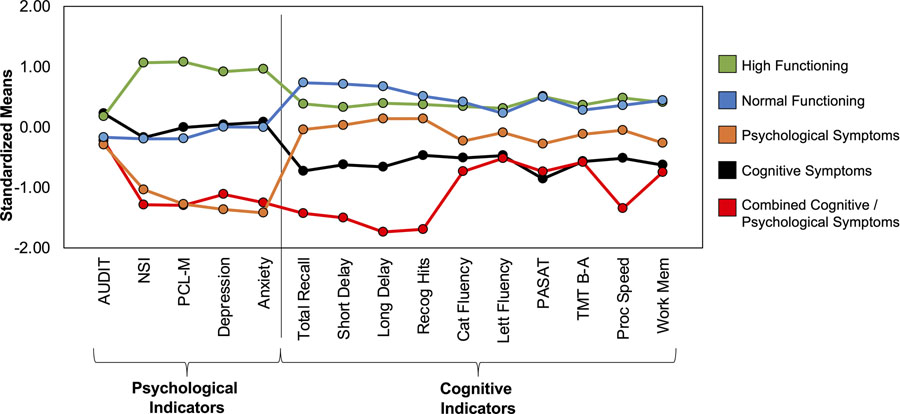
Standardized means for the psychological symptoms or neuropsychological performance were used to categorize the 5 distinct classes into: 1) High Functioning, 2) Normal Cognitive and Psychological Functioning, 3) Cognitive Symptoms, 4) Psychological Symptoms, and 5) Combined Cognitive and Psychological Symptoms (Figure 1).
Figure 1. Standardized means plotted for the individual psychological and cognitive indicators for each class.
Tests are presented such that higher standardized means represent fewer symptoms/better performance. Psychological Indicators: AUDIT = Alcohol Use Disorder Identification Test; NSI = Neurobehavioral Symptom Inventory; PCL-M = Posttraumatic stress disorder Checklist – Military Version; Depression = Symptom Checklist-90 (SCL-90) Depression subscale; Anxiety = SCL-90 Anxiety Subscale; Cognitive Indicators: Total Recall = California Verbal Learning Test-II (CVLT-II) total recall; Short Delay = CVLT-II short delay free recall; Long Delay = CVLT-II long delay free recall; Recog Hits = CVLT-II recognition hits; Cat Fluency = Delis-Kaplan Executive Function System (D-KEFS) category fluency; Lett Fluency = D-KEFS Letter Fluency; PASAT = Paced auditory serial addition test; TMT B-A = Trail Making Test Part B minus Part A; Proc speed = Wechsler Adult Intelligence Scale-IV (WAIS-IV) – Processing Speed Index; Work Mem = WAIS-IV Working Memory Index.
The standardized means for the psychological or cognitive indicators for each class are presented in Figure 2. As expected, the high functioning group performed well on most psychological and cognitive indicators (Figure 2). The normal cognitive and psychological functioning group also performed well on all psychological and cognitive indicators but reported more psychological symptoms than the high functioning group. The cognitive symptoms group showed primarily poor cognitive performance but relatively intact psychological functioning, while the psychological symptoms group showed worse psychological functioning but intact cognitive performance. The combined cognitive and psychological symptoms group demonstrated both worse psychological functioning and cognitive performance.
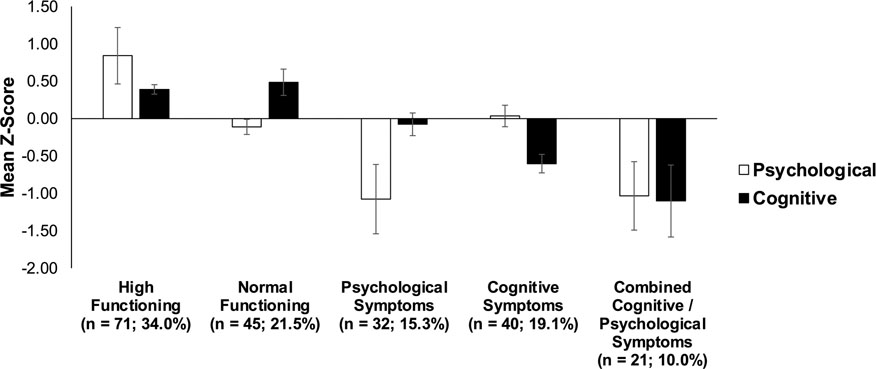
Figure 2. Standardized means collapsed across the psychological and cognitive indicators for each class.
The high functioning (34.0% of the overall sample; n = 71) and normal functioning (21.5%; n = 45) groups performed well across all psychological and cognitive indicators. The cognitive symptoms group (19.1%; n = 40) showed primarily poor cognitive performance but relatively intact psychological functioning, while the psychological symptoms group (15.3%; n = 32) showed worse psychological functioning but intact cognitive performance. The combined cognitive and psychological symptoms group (10.0%; n = 21) demonstrated both worse psychological functioning and cognitive performance.
Diagnostic Category Prediction
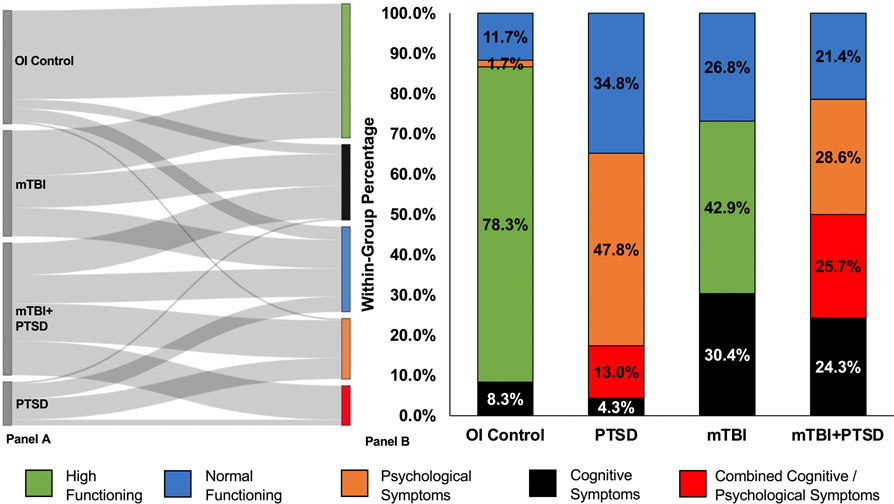
We next determined how the class assignments differed based on diagnostic classifications. Figure 3, panel A shows how participants from each diagnostic category were distributed to each class. Most of the orthopedic injury controls were classified in the high or normal functioning group, with ~10% classified in the cognitive symptoms and psychological symptoms groups (Figure 3 panel b). Participants with PTSD were represented in four classes, with the majority represented in the psychological symptoms group followed by normal functioning, combined cognitive and psychological symptoms, and cognitive symptoms groups. Military personnel with mTBI were classified in the high and normal functioning groups, with ~30% in the cognitive symptoms group. Those with comorbid mTBI+PTSD had high representation in all dysfunction groups with 24.3% in the cognitive symptoms group, 28.6% in the psychological symptoms group, and 25.7% in the combined cognitive and psychological symptoms group, with the remainder in the normal functioning group.
Figure 3. Class assignments for each diagnostic category.
Panel A. Distribution of participants from each diagnostic category to each class. The LPA sorted participants based on psychological symptom and cognitive performance indicators. Left side of panel shows the diagnostic group, right side of panel shows the class. The thickness of each line between diagnostic group and class represents number of participants (thicker line = more participants). Panel B. The percentage of class assignments for each diagnostic classification. While each diagnostic classification was primarily represented by one of the classes (e.g., Combined Cognitive/ Psychological Symptoms class for comorbid mild traumatic brain injury and post-traumatic stress disorder (mTBI+PTSD), High Functioning class for the orthopedic injury controls (OI Control)), the same diagnostic classification was represented by multiple classes, demonstrating heterogeneity of symptom presentation. mTBI = mild traumatic brain injury only; PTSD = post-traumatic stress disorder only.
Discussion
Given the heterogeneity of outcomes associated with mTBI, as well as overlapping impairments in cognitive and psychological functioning in patients diagnosed with either mTBI or PTSD, it is often difficult to develop sensitive diagnostic techniques and efficacious rehabilitation strategies. Further complicating this picture, many military personnel who experience mTBI in combat also develop comorbid PTSD, which requires more complex treatment strategies. However, diagnosis and TBI treatment strategies, particularly for mTBI and comorbid PTSD, are difficult to implement and frequently ineffective [1, 23, 39–41], likely due to the heterogeneity in mTBI outcomes, patient characteristics, symptom profiles, and cognitive symptoms or overlapping symptom profiles occurring across diagnostic groups [3, 21, 42, 43]. Thus, using advanced analytical techniques to identify specific psychological symptom and cognitive performance profiles may be more effective than relying on clinical diagnosis classification alone.
We sought to determine whether self-report psychological symptoms and cognitive performance indicators regardless of diagnostic classifiers (e.g., mTBI, PTSD, mTBI+PTSD) clustered in distinct psychological and cognitive profiles. We then examined whether the symptom and performance profiles were representative of each diagnostic group, or whether there were overlapping symptom and performance clusters across groups. We found that a 5-class model best represented the psychological symptom and cognitive performance data. The classes differentiated five specific profiles characterizing high and normal functioning, primary cognitive symptoms, primary psychological symptoms, and combined cognitive and psychological symptoms. The high and normal functioning group included the largest proportion of participants which was mainly represented by orthopedic injury controls. The cognitive symptoms group included the next highest proportion of the sample, and mainly included participants with mTBI and mTBI with comorbid PTSD. Both the combined cognitive and psychological symptoms and psychological symptoms groups included the smallest proportion of the sample, but mainly consisted of participants with PTSD and mTBI with comorbid PTSD. Although we had predicted that the symptom and performance indicators would result in three clusters, we did not expect a differentiation between high and normal functioning, or the mTBI with comorbid PTSD resulting in an additional cluster with lower cognitive and psychological functioning.
In general, the cognitive and psychological outcome profiles differentiated participants as would be expected based on their diagnostic classifications. Specifically, participants with PTSD had the highest proportion of representation in the psychological symptoms and combined psychological and cognitive symptoms classes, while the majority of mTBI participants showed normal to high function with ~30% showing cognitive symptoms. Complicating this picture, however, military personnel with mTBI and comorbid PTSD were dispersed across the symptom classes, with high representation in the combined cognitive and psychological symptoms group, but a similar proportion also represented in the normal functioning group. This supports the notion that although treatment can be developed and implemented based on diagnostic labels, we must be aware that even within a patient group, individuals show differences in degrees of cognitive symptoms and psychological symptoms. The history of a brain injury alone cannot reliably predict outcome, although it may be one of many factors that may contribute to a given psychological and cognitive presentation. Moreover, these results demonstrate why it is often more complex and difficult to develop effective treatment strategies for mTBI with comorbid PTSD, as they can show a range of functional impairment. However, it should be noted that military personnel with mTBI were classified in the cognitive symptoms group, but also represented ~70% of the normal and high functioning groups. Thus, these results suggest that the majority of mTBI patients are resilient to neurotrauma and may not manifest persistent symptoms, albeit the current findings reflect psychological and cognitive functioning within the chronic phase of recovery (3–24 months post-injury for the mTBI group), whereas this may not reflect findings in a more acute post-injury setting.
Potential clinical implications.
While many individuals who sustain mTBI appear to recover rapidly and well, a subset of individuals demonstrate a prolonged recovery course, which indicate the need for personalized intervention. As previously discussed, treatment of mTBI, PTSD, and mTBI+PTSD is complex, and unfortunately, many generalized treatment approaches for mTBI are often not successful [39–41]. The current findings highlight the importance of assessing factors that may complicate recovery and treatment, such as the comorbid presence of mTBI and PTSD and possibly other factors that were not the focus of this study. Patients may present for evaluation following a known history of mTBI, without recognition that the response to the traumatic nature of the injury may also contribute to recovery. Given that there is a high degree of overlap between PTSD and mTBI in terms of cognitive and psychological sequelae, co-occurrence of these conditions may serve to exacerbate and prolong the effects of either one alone [44].
As expected, the psychological symptoms group was mainly composed of those who were diagnosed with PTSD, either singly or in combination with mTBI. Thus, appropriate diagnosis of PTSD may serve to identify effective psychotherapeutic interventions and guide more accurate prognosis. Serial assessment of treatment response in this comorbid mTBI+PTSD group (and identification of which treatments were provided) would lead to improved understanding of effective interventions for these individuals. Also of interest, however, is that the diagnostic categories with participants with a history of mTBI had the highest proportion of cognitive symptoms (mTBI followed by mTBI+PTSD), which is expected given that mTBI is a neurological injury that affects cognitive functioning.
The findings also illustrate that absence of a history of mTBI or PTSD may not fully protect against cognitive impairment, as a small group of orthopedically injured participants also demonstrated cognitive deficits and psychological symptoms on assessment. A number of non-neurologic factors may contribute to cognitive dysfunction in this group, such as sleep quality [45], chronic pain [46–49], medication [50, 51] and/or substance use [52, 53], and potentially other comorbid medical conditions such as sleep apnea [54, 55]. Such individuals would benefit from a personalized assessment to identify individual factors that may contribute to cognitive dysfunction, with the aim of providing targeted interventions to ameliorate their effects.
Limitations.
Although our data provide valuable insight into the benefits of using symptoms and cognitive performance profiles and how these profiles are associated with participant diagnostic groups, there are some limitations that need to be considered. First, mTBI with comorbid PTSD was not diagnosed prior to study enrollment. Instead, cut-off scores on the PCL-M were used to define this group. As this is a self-report measure, the outcomes on the PCL-M may not match clinician-confirmed diagnoses. Second, there were unequal sample sizes within each of the diagnostic classification groups. Although we used proportions to represent groups within symptom and performance clusters, these results must be confirmed in studies with larger sample sizes and matched groups. Third, the mTBI participants enrolled in this study may not be representative of all individuals with a history of mTBI, as presence of cognitive complaints was a requirement for study participation. Finally, although our sample of female Veterans is consistent with the proportion of women deployed to combat, with such a small sample in the study we cannot account for the effect of sex in these results.
Conclusion
Findings from this study demonstrated that although mTBI, PTSD, and mTBI+PTSD diagnostic categories may be helpful in prediction of symptom presentation severity and cognitive and functional performance, there are areas of symptom overlap which can complicate treatment effectiveness. These overlapping symptoms and performance clusters also provide support for why it may be difficult to diagnose and treat mTBI, PTSD, and mTBI with comorbid PTSD. Furthermore, these results argue for the importance of patient-centered treatment strategies and multidisciplinary coordinated care, and that regardless of diagnostic classification, cognitive and psychological functional abilities must be considered when evaluating treatment outcomes.
Supplementary Material
Acknowledgements
The view(s) expressed herein are those of the author and do not reflect the official policy or position of the Defense and Veterans Brain Injury Center, Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense, or the U.S. Government.
Conflicts of Interest and Source of Funding
Carrie Esopenko has received presentation honoraria from New York University. Douglas Cooper is employed as a researcher by the Defense and Veterans Brain Injury Center and the Foundation for Advancing Veterans Health Research. Amy O. Bowles is currently receiving grants (W81XWH-18–2-0070, W81XWH-11–2-0222, W81XWH-15-PORP-ARA) through the Department of Defense Congressionally Directed Medical Research Programs (CDMRP). For the remaining authors none were declared.
This work is supported in part by the Defense and Veterans Brain Injury Centers, the U.S. Army Medical Research and Materiel Command (USAMRMC; W81XWH-13–2-0025), and also supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the Psychological Health/Traumatic Brain Injury Research Program LongTerm Impact of Military Relevant Brain Injury Consortium (LIMBIC) Award W81XWH18PH/TBIRPLIMBIC under Awards No. W81XWH1920067 and W81XWH1320095, and by the U.S. Department of Veterans Affairs Awards No. I01 CX002097, I01 CX002096, I01 HX003155, I01 RX003444, I01 RX003443, I01 RX003442, I01 CX001135, I01 CX001246, I01 RX001774, I01 RX 001135, I01 RX 002076, I01 RX 001880, I01 RX 002172, I01 RX 002173, I01 RX 002171, I01 RX 002174, and I01 RX 002170. The U.S. Army Medical Research Acquisition Activity, 839 Chandler Street, Fort Detrick MD 217025014 is the awarding and administering acquisition office. This work is also supported in part by R61NS120249 to FGH, ELD, DFT, and EAW. Financial support was provided to CE through the School of Health Professions at Rutgers Biomedical and Health Sciences.
Footnotes
List of Supplemental Digital Content
References
- 1.Burke HS, Degeneffe CE, and Olney MF, A New Disability for Rehabilitation Counselors: Iraq War Veterans with Traumatic Brain Injury and Post-Traumatic Stress Disorder. Journal of Rehabilitation, 2009. 75(3): p. 5–14. [Google Scholar]
- 2.Belanger H, Uomoto J, and Vanderploeg R, The Veterans Health Administration System of Care for Mild Traumatic Brain Injury: Costs, Benefits, and Controversies. J Head Trauma Rehabil, 2009. 24(1): p. 4–13. [DOI] [PubMed] [Google Scholar]
- 3.Dolan S, et al. , Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychol Rev, 2012. 22(1): p. 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneiderman AI, Braver ER, and Kang HK, Understanding Sequelae of Injury Mechanisms and Mild Traumatic Brain Injury Incurred during the Conflicts in Iraq and Afghanistan: Persistent Postconcussive Symptoms and Posttraumatic Stress Disorder. American journal of epidemiology, 2008. 167(12): p. 1446–1452. [DOI] [PubMed] [Google Scholar]
- 5.Terrio H, et al. , Traumatic Brain Injury Screening: Preliminary Findings in a US Army Brigade Combat Team. J Head Trauma Rehabil, 2009. 24(1): p. 14–23. [DOI] [PubMed] [Google Scholar]
- 6.Tanielian T and Jaycox LH, Invisible Wounds of War : Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. 2008, Santa Monica, UNITED STATES: RAND Corporation, The. [Google Scholar]
- 7.Hoge CW, et al. , Mild Traumatic Brain Injury in U.S. Soldiers Returning from Iraq. The New England journal of medicine, 2008. 358(5): p. 453–463. [DOI] [PubMed] [Google Scholar]
- 8.Health.mil. DoD TBI Worldwide Numbers. 2021 June 6, 2021]; Available from: https://health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Development/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers. [Google Scholar]
- 9.Rice VJ, et al. , The Effect of Traumatic Brain Injury (TBI) on Cognitive Performance in a Sample of Active Duty U.S. Military Service Members. Mil Med, 2020. 185(Suppl 1): p. 184–189. [DOI] [PubMed] [Google Scholar]
- 10.Lange RT, et al. , Long-term neurobehavioural symptom reporting following mild, moderate, severe, and penetrating traumatic brain injury in U.S. military service members. Neuropsychol Rehabil, 2020. 30(9): p. 1762–1785. [DOI] [PubMed] [Google Scholar]
- 11.Lew HL, et al. , Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev, 2009. 46(6): p. 697–702. [DOI] [PubMed] [Google Scholar]
- 12.Vasterling JJ, et al. , Long-term negative emotional outcomes of warzone TBI. Clin Neuropsychol, 2020. 34(6): p. 1088–1104. [DOI] [PubMed] [Google Scholar]
- 13.Golding H, et al. , Understanding recent estimates of PTSD and TBI from Operations Iraqi Freedom and Enduring Freedom. Journal of rehabilitation research and development, 2009. 46(5): p. vii–xiv. [DOI] [PubMed] [Google Scholar]
- 14.Hoge CW, et al. , The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry, 2014. 1(4): p. 269–77. [DOI] [PubMed] [Google Scholar]
- 15.Vasterling JJ, et al. , Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology, 2002. 16(1): p. 5–14. [DOI] [PubMed] [Google Scholar]
- 16.Jacob SN, Dodge CP, and Vasterling JJ, Posttraumatic stress disorder and neurocognition: A bidirectional relationship? Clin Psychol Rev, 2019. 72: p. 101747. [DOI] [PubMed] [Google Scholar]
- 17.Scott JC, et al. , A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull, 2015. 141(1): p. 105–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasterling JJ, et al. , Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br J Psychiatry, 2012. 201(3): p. 186–92. [DOI] [PubMed] [Google Scholar]
- 19.Burnes DP and Burnette D, Broadening the etiological discourse on Alzheimer’s disease to include trauma and posttraumatic stress disorder as psychosocial risk factors. J Aging Stud, 2013. 27(3): p. 218–24. [DOI] [PubMed] [Google Scholar]
- 20.LoBue C, et al. , Neurodegenerative Dementias After Traumatic Brain Injury. J Neuropsychiatry Clin Neurosci, 2018. 30(1): p. 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balba NM, et al. , Increased Sleep Disturbances and Pain in Veterans With Comorbid Traumatic Brain Injury and Posttraumatic Stress Disorder. J Clin Sleep Med, 2018. 14(11): p. 1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasterling JJ, Jacob SN, and Rasmusson A, Traumatic Brain Injury and Posttraumatic Stress Disorder: Conceptual, Diagnostic, and Therapeutic Considerations in the Context of Co-Occurrence. J Neuropsychiatry Clin Neurosci, 2018. 30(2): p. 91–100. [DOI] [PubMed] [Google Scholar]
- 23.McAllister TW, Psychopharmacological issues in the treatment of TBI and PTSD. Clin Neuropsychol, 2009. 23(8): p. 1338–67. [DOI] [PubMed] [Google Scholar]
- 24.Yeates KO, et al. , Derivation and Initial Validation of Clinical Phenotypes of Children Presenting with Concussion Acutely in the Emergency Department: Latent Class Analysis of a Multi-Center, Prospective Cohort, Observational Study. J Neurotrauma, 2019. 36(11): p. 1758–1767. [DOI] [PubMed] [Google Scholar]
- 25.Jaramillo CA, et al. , Subgroups of US IRAQ and Afghanistan veterans: associations with traumatic brain injury and mental health conditions. Brain imaging and behavior, 2015. 9(3): p. 445–455. [DOI] [PubMed] [Google Scholar]
- 26.Hsu H-H, et al. , Long-Term Presentation of Postconcussion Symptoms and Associated Factors: Analysis of Latent Class Modeling. Archives of clinical neuropsychology, 2021. 36(1): p. 62–73. [DOI] [PubMed] [Google Scholar]
- 27.Brett BL, et al. , Distinct latent profiles based on neurobehavioural, physical and psychosocial functioning of former National Football League (NFL) players: an NFL-LONG Study. J Neurol Neurosurg Psychiatry, 2021. 92(3): p. 282–290. [DOI] [PubMed] [Google Scholar]
- 28.Cooper DB, et al. , Cognitive Rehabilitation for Military Service Members With Mild Traumatic Brain Injury: A Randomized Clinical Trial. J Head Trauma Rehabil, 2017. 32(3): p. E1–e15. [DOI] [PubMed] [Google Scholar]
- 29.Department of Veterans Affairs and D.o. Defense, VA/DoD Clinical Practice Guideline for the Management of Concussion-Mild Traumatic Brain Injury. 2016.
- 30.Karstoft KI, et al. , Diagnostic accuracy of the posttraumatic stress disorder checklist-civilian version in a representative military sample. Psychol Assess, 2014. 26(1): p. 321–5. [DOI] [PubMed] [Google Scholar]
- 31.Bolzenius JD, et al. , Diffusion Imaging Findings in US Service Members With Mild Traumatic Brain Injury and Posttraumatic Stress Disorder. J Head Trauma Rehabil, 2018. 33(6): p. 393–402. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard EB, et al. , Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther, 1996. 34(8): p. 669–73. [DOI] [PubMed] [Google Scholar]
- 33.Lo Y, Mendell NR, and Rubin DB, Testing the number of components in a normal mixture. Biometrika, 2001. 88(3): p. 767–778. [Google Scholar]
- 34.McLachlan GJ, On Bootstrapping the Likelihood Ratio Test Statistic for the Number of Components in a Normal Mixture. Journal of the Royal Statistical Society: Series C (Applied Statistics), 1987. 36(3): p. 318–324. [Google Scholar]
- 35.Tein JY, Coxe S, and Cham H, Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct Equ Modeling, 2013. 20(4): p. 640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark S and Muthén B, Relating Latent Class Analysis Results to Variables not Included in the Analysis. 2009.
- 37.Nylund KL, Asparouhov T, and Muthén BO, Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. 2007. 14: p. 535–569. [Google Scholar]
- 38.Lubke G and Neale MC, Distinguishing Between Latent Classes and Continuous Factors: Resolution by Maximum Likelihood? Multivariate Behav Res, 2006. 41(4): p. 499–532. [DOI] [PubMed] [Google Scholar]
- 39.DeWitt DS, et al. , Pre-Clinical Testing of Therapies for Traumatic Brain Injury. J Neurotrauma, 2018. 35(23): p. 2737–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein FC, et al. , Very Early Administration of Progesterone Does Not Improve Neuropsychological Outcomes in Subjects with Moderate to Severe Traumatic Brain Injury. Journal of neurotrauma, 2017. 34(1): p. 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clifton GL, et al. , Lack of Effect of Induction of Hypothermia after Acute Brain Injury. New England Journal of Medicine, 2001. 344(8): p. 556–563. [DOI] [PubMed] [Google Scholar]
- 42.Stein MB and McAllister TW, Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry, 2009. 166(7): p. 768–76. [DOI] [PubMed] [Google Scholar]
- 43.Vasterling JJ, et al. , Neuropsychological Outcomes of Army Personnel Following Deployment to the Iraq War. JAMA : the journal of the American Medical Association, 2006. 296(5): p. 519–529. [DOI] [PubMed] [Google Scholar]
- 44.Brenner LA, Vanderploeg RD, and Terrio H, Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: burden of adversity hypothesis. Rehabil Psychol, 2009. 54(3): p. 239–246. [DOI] [PubMed] [Google Scholar]
- 45.Brownlow JA, Miller KE, and Gehrman PR, Insomnia and Cognitive Performance. Sleep Med Clin, 2020. 15(1): p. 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Distel DF, et al. , Cognitive Dysfunction in Persons with Chronic Spinal Cord Injuries. Phys Med Rehabil Clin N Am, 2020. 31(3): p. 345–368. [DOI] [PubMed] [Google Scholar]
- 47.Berryman C, et al. , Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev, 2014. 34(7): p. 563–79. [DOI] [PubMed] [Google Scholar]
- 48.Block C and Cianfrini L, Neuropsychological and neuroanatomical sequelae of chronic non-malignant pain and opioid analgesia. NeuroRehabilitation, 2013. 33(2): p. 343–66. [DOI] [PubMed] [Google Scholar]
- 49.Berryman C, et al. , Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain, 2013. 154(8): p. 1181–96. [DOI] [PubMed] [Google Scholar]
- 50.Sargent L, et al. , Anticholinergic Drug Induced Cognitive and Physical Impairment: Results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci, 2020. 75(5): p. 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neylan TC, et al. , Acute cognitive effects of the hypocretin receptor antagonist almorexant relative to zolpidem and placebo: a randomized clinical trial. Sleep, 2020. 43(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lappin JM and Sara GE, Psychostimulant use and the brain. Addiction, 2019. 114(11): p. 2065–2077. [DOI] [PubMed] [Google Scholar]
- 53.Toledo-Fernandez A, et al. , Exploring the prevalence of substance-induced neurocognitive disorder among polysubstance users, adding subjective and objective evidence of cognitive impairment. Psychiatry Res, 2020. 288: p. 112944. [DOI] [PubMed] [Google Scholar]
- 54.Li N, et al. , Correlation of sleep microstructure with daytime sleepiness and cognitive function in young and middle-aged adults with obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol, 2019. 276(12): p. 3525–3532. [DOI] [PubMed] [Google Scholar]
- 55.Grigg-Damberger M and Ralls F, Cognitive dysfunction and obstructive sleep apnea: from cradle to tomb. Curr Opin Pulm Med, 2012. 18(6): p. 580–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.