Abstract
Background
Newer glucose-lowering drugs, including sodium glucose co-transporter 2 inhibitors (SGLT2i) and GLP-1 agonists, have a key role in the pharmacologic management of type 2 diabetes. No studies have measured primary nonadherence for these two drug classes, defined as when a medication is prescribed for a patient but ultimately not dispensed to them.
Objective
To describe the incidence and predictors of primary nonadherence to SGLT2i (canagliflozin, empagliflozin) or GLP-1 agonists (dulaglutide, liraglutide, semaglutide) using a dataset that links electronic prescribing with health insurance claims.
Design and Participants
A retrospective cohort design using data of adult patients from a large health system who had at least one prescription order for a SGLT2i or GLP-1 agonist between 2012 and 2019. We used mixed-effects multivariable logistic regression to determine associations between sociodemographic, clinical, and provider variables and primary nonadherence.
Main Measures
Primary medication nonadherence, defined as no dispensed claim within 30 days of an electronic prescription order for any drug within each medication class.
Key Results
The cohort included 5146 patients newly prescribed a SGLT2i or GLP-1 agonist. The overall incidence of 30-day primary medication nonadherence was 31.8% (1637/5146). This incidence rate was 29.8% (n = 726) and 33.6% (n = 911) among those initiating a GLP-1 agonist and SGLT2i, respectively. Age ≥ 65 (aOR 1.37 (95% CI 1.09 to 1.72)), Black race vs White (aOR 1.29 (95% CI 1.02 to 1.62)), diabetic nephropathy (aOR 1.31 (95% CI 1.02 to 1.68)), and hyperlipidemia (aOR 1.18 (95% CI 1.01 to 1.39)) were associated with a higher odds of primary nonadherence. Female sex (aOR 0.86 (95% CI 0.75 to 0.99)), peripheral artery disease (aOR 0.73 (95% CI 0.56 to 0.94)), and having the index prescription ordered by an endocrinologist vs a primary care provider (aOR 0.76 (95% CI 0.61 to 0.95)) were associated with lower odds of primary nonadherence.
Conclusions
One third of patients prescribed SGLT2i or GLP-1 agonists in this sample did not fill their prescription within 30 days. Black race, male sex, older age, having greater baseline comorbidities, and having a primary care provider vs endocrinologist prescribe the index drug were associated with higher odds of primary nonadherence. Interventions targeting medication adherence for these newer drugs must consider primary nonadherence as a barrier to optimal clinical care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-07331-1.
The pharmacologic management of type 2 diabetes is undergoing a dramatic transformation. This is due in part to requirements of the FDA to assess the cardiovascular (CV) safety of newly approved glucose-lowering drugs, which resulted in publication of a substantial number of large CV outcome trials (CVOT) over the past 10 to 15 years.1–10 While the first class of new oral drugs (DPP-4 inhibitors) simply demonstrated noninferiority to placebo in terms of CV safety,11,12 several drugs in the newest classes (i.e., SGLT2i and GLP-1 agonists, approved between 2005 and 2017) were shown to actually reduce the risk of major adverse CV events (~ 12% reduction in HR), heart failure exacerbation, and stroke.13 These agents were also shown to reduce the risk of adverse renal outcomes including the progression of chronic kidney disease, albuminuria, or development of end-stage renal disease among patients (45% reduction) at higher baseline risk.14–17
As a result of these CVOTs, practice guidelines from the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) now recommend select agents within these classes first for patients with atherosclerotic cardiovascular disease (or risk factors), heart failure, or chronic kidney disease (CKD) who have not achieved glycemic goals despite maximally tolerated metformin and lifestyle interventions.18 Unfortunately, despite the rationale to preferentially select these drug classes for patients with strong clinical indications, recent data from large administrative claims studies suggest that underutilization is widespread.19,20 In one study, older patients and those with additional comorbidities such as CKD were less likely to be dispensed SGLT2 inhibitors, despite the fact that they are more likely to benefit from the cardiovascular and renal protective effects of this drug class.21
It is unclear whether the potential underutilization of these newer drug classes is related to lack of prescribing or lack of filling prescriptions (or both). A large number of studies have shown that higher out-of-pocket costs and other types of restrictions results in secondary medication nonadherence, generally defined as patients not refilling a prescription after the first dispensed claim.22–24 Fewer studies, however, have focused on the phenomenon of primary nonadherence, when a patient is prescribed a medication but does not fill it at the pharmacy. To our knowledge, no studies to date have described primary medication nonadherence to SGLT2i or GLP-1 agonists, despite their increasingly central role in the care of patients with diabetes. The objective of this study was to describe the incidence and predictors of primary nonadherence to these two classes of medications using a contemporary dataset that links electronic health records with health insurance claims within a large integrated health system.
METHODS
Data Sources and Study Population
We used a dataset linking electronic health record data from patients receiving care at a large health system including more than 20 hospitals and 800 clinics based in Pennsylvania with claims data from the integrated health care delivery system’s affiliated health plan.
We obtained these data from the PaTH Clinical Research Network, a Partner Network in PCORnet®, the National Patient-Centered Clinical Research Network. PCORnet® was developed with funding from the Patient-Centered Outcomes Research Institute® (PCORI®) to support the use of electronic healthcare data for patient-oriented comparative effectiveness research. Raw data was available in the PCORnet common data model format. Electronic health record and health plan claims data from patients were linked using a HIPAA-compliant unique identifier. This study was determined exempt by the Human Research Protection Office of the University of Pittsburgh.
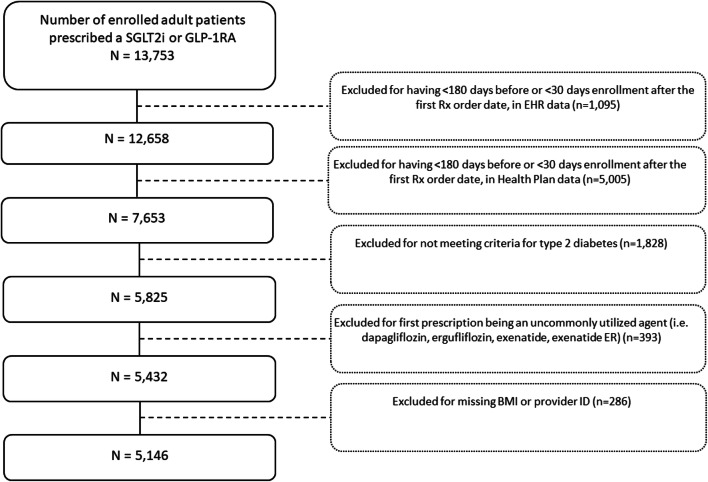
Our cohort included all patients aged 18 years or older who received at least one prescription order for any SGLT2i (canaglflozin, dapagliflozin, empagliflozin, ertugliflozin) or GLP-1 agonist (dulaglutide, exenatide, exenatide ER, liraglutide, semaglutide) between 2012 and 2019 (see Fig. 1 flow diagram). Patients were excluded if they did not have evidence of continuous enrollment in the health system or the health insurance plan for at least 180 days before and at least 30 days after their first prescription order date for one of the target drugs. We additionally excluded patients if they did not meet ICD-9-CM or ICD-10-CM diagnostic code criteria for type 2 diabetes (code list available upon request); if their first study medication was one of the least commonly dispensed SGLT2i or GLP-1 agonists in the health system (e.g., dapagliflozin, ertugliflozin, exenatide, or exenatide ER); and—for complete covariate adjustment—if they were missing a BMI value, or a provider ID for the prescription of the study drug during the baseline covariate assessment period.
Fig. 1.
Flow diagram for determining the final study cohort.
Outcomes
The primary outcome was the incidence of primary nonadherence to a SGLT2i or GLP-1 agonist, defined a priori as no dispensed claim within 30 days of the first electronic prescription order for any drug within each respective medication class.25 The 30-day time window is consistent with a quality metric established by the Pharmacy Quality Alliance. In this study, if a patient was prescribed one GLP-1 agonist and was able to fill their prescription within 30 days, we considered them adherent. However, if the patient had no dispensed claim for any GLP-1 agonist within 30 days, we considered them to have experienced primary nonadherence. We obtained order dates from EHR data and dispensing dates from health insurance claims.
Secondary outcomes included the incidence of 30-day primary nonadherence to each individual drug (i.e., dulaglutide, liraglutide, semaglutide, canagliflozin, or empagliflozin), where primary nonadherence is defined as no dispensed claim for that specific drug (i.e., if a patient is prescribed one GLP-1 agonist and does not have a dispensed claim for the specific medication within 30 days, we considered them nonadherent).
Predictors
We selected 35 candidate predictor variables a priori on the patient and provider levels that may affect the initiation of, or adherence to, a second-line glucose-lowering medication. These included demographic and clinical variables measured in the baseline period of 180 days before the index prescription such as age, gender, race, presence of coronary artery disease, heart failure, chronic kidney disease, and presence of diabetes complications such as diabetic nephropathy, neuropathy, and retinopathy. We used validated ICD-9-CM or ICD-10-CM diagnosis codes to define clinical comorbidities and National Drug Codes (NDCs) to define co-dispensed medications. We also included measures of engagement with the healthcare system such as the presence of laboratory tests (e.g., HbA1c, creatinine, total cholesterol based on Logical Observation Identifiers Names and Codes (LOINC)) during the assessment period and total number of hospitalizations. We included other common medications (e.g., ACE inhibitors, insulin, metformin, statins, sulfonylureas, TZDs), prescriber specialty (for the index drug), and index medication type (i.e., SGLT2i vs GLP-1 agonist), measured during the baseline period.
Statistical Analysis
We used means and standard deviations to summarize continuous measures and frequencies and proportions to summarize categorical variables. We reported the proportion of patients experiencing primary medication nonadherence within 30 (primary outcome), 7, and 14 days of follow-up. These proportions were reported both within each drug class individually and overall. We fit a mixed-effects multivariable logistic regression model to determine the associations between candidate predictors and the primary outcome. In the mixed model, we included a random intercept for the prescriber to account for the hierarchical nature of the data (i.e., multiple patients clustered within an individual prescriber).
Model selection and predictor variable operationalization was performed under the following considerations. We started with the inclusion of all investigator pre-specified variables and removed 4 variables with evidence of high collinearity with other variables (e.g., body weight was removed because it is highly collinear with BMI). Continuous variables in the regression model (i.e., BMI, total # of hospitalizations) were standardized to make estimated effect sizes more interpretable in relation to one another. The variables included in our final model included age; sex; race; Hispanic ethnicity; hypertension; coronary artery disease; heart failure; stroke; chronic kidney disease; end-stage renal disease; retinopathy; neuropathy; nephropathy; serious hyperglycemic events; serious hypoglycemic events; hyperlipidemia; food ulcers; peripheral artery disease; obesity; smoking status; standardized BMI; presence of the following laboratory tests: HbA1c, creatinine, and total cholesterol; provider specialty; standardized total # of hospitalizations; co-dispensed medications; index prescription year; and drug type (GLP-1 agonists or SGLT2i). We did not impute missing data because the percentage was very small (< 3%) and we found no evidence that the data were missing not at random. We assumed a type I error rate of 0.05, and no adjustments were made for multiplicity. All analyses were performed using R version 3.6.3, and logistic mixed-effects models were produced using the lmerTest package.26
We conducted two sensitivity analyses: (1) we repeated the multivariable model after excluding co-dispensed medications due to potentially high collinearity with the primary outcome (i.e., nonadherence to other classes of diabetes medications is related to nonadherence to SGLT2i and GLP-1 agonists), and (2) we varied the time window used to define primary nonadherence to be 7 or 14 days.
RESULTS
We linked EHR data from 13,753 patients who had received at least 1 prescription order for a GLP-1 agonist or SGLT2i to their claims data. Six thousand four hundred (44%) were excluded for not having sufficient enrollment in the health system and health plan. After exclusions, the final cohort size was 5146 (Fig. 1).
Baseline characteristics comparing the overall study population as well as those who experienced and did not experience primary medication nonadherence are shown in Table 1. Briefly, 91% of the overall cohort was under the age of 65, 47% were female, and 88% were White, 9% Black, and < 3% of other race. Seven out of 10 patients had hypertension at baseline, with 19% having a previous history of coronary artery disease. Approximately 10% had chronic kidney disease at baseline. The mean BMI was 35.3. Fifty-six percent of index prescriptions were written by a primary care provider while 24% were written by an endocrinologist. The index prescription year ranged from 2012 to 2019, with more patients initiating a target medication during 2017–2019 (> 75% of the overall cohort).
Table 1.
Baseline Characteristics of the Study Cohort, Overall and Comparing Those Who Were Adherent Vs Those with Primary Nonadherence
| Baseline characteristic* | Overall, n (%) (total n = 5146) |
Adherent, n (%) (n = 3509) |
Nonadherent, n (%) (n = 1637) |
|---|---|---|---|
| Age | |||
| < 65 | 4682 (91) | 3247 (92.5) | 1435 (87.7) |
| ≥ 65 | 464 (9) | 262 (7.5) | 202 (12.3) |
| Female | 2410 (46.8) | 1664 (47.4) | 746 (45.6) |
| Race | |||
| White | 4548 (88.4) | 3111 (88.7) | 1437 (87.8) |
| Black | 458 (8.9) | 299 (8.5) | 159 (9.7) |
| Other | 140 (2.7) | 99 (2.8) | 41 (2.5) |
| Hispanic | 28 (0.5) | 19 (0.5) | 9 (0.5) |
| Comorbidity | |||
| Hypertension | 3649 (70.9) | 2496 (71.1) | 1153 (70.4) |
| Hyperlipidemia | 3196 (62.1) | 2219 (63.2) | 977 (59.7) |
| Obesity | 1650 (32.1) | 1162 (33.1) | 488 (29.8) |
| Neuropathy | 1117 (21.7) | 766 (21.8) | 351 (21.4) |
| Coronary artery disease | 998 (19.4) | 645 (18.4) | 353 (21.6) |
| Smoker | 811 (15.8) | 533 (15.2) | 278 (17) |
| Nephropathy | 628 (12.2) | 403 (11.5) | 225 (13.7) |
| Chronic kidney disease | 488 (9.5) | 305 (8.7) | 183 (11.2) |
| Peripheral artery disease | 421 (8.2) | 304 (8.7) | 117 (7.1) |
| Retinopathy | 335 (6.5) | 228 (6.5) | 107 (6.5) |
| Congestive heart failure | 287 (5.6) | 181 (5.2) | 106 (6.5) |
| Foot ulcers | 166 (3.2) | 103 (2.9) | 63 (3.8) |
| Stroke | 118 (2.3) | 71 (2) | 47 (2.9) |
| Serious hyperglycemic event | 58 (1.1) | 38 (1.1) | 20 (1.2) |
| Serious hypoglycemic event | 24 (0.5) | 15 (0.4) | 9 (0.5) |
| End-stage renal disease | 13 (0.3) | 7 (0.2) | 6 (0.4) |
| BMI, mean [SD] | 35.3 [7.3] | 35.5 [7.3] | 35.0 [7.4] |
| HbA1c test | 3738 (72.6) | 2640 (75.2) | 1098 (67.1) |
| HbA1c (%), mean [SD] | 8.3 (1.7) | 8.3 (1.7) | 8.3 (1.7) |
| Creatinine test | 2857 (55.5) | 1988 (56.7) | 869 (53.1) |
| Creatinine (mg/dL), mean [SD] | 1 (0.3) | 1 (0.3) | 1 (0.4) |
| Total cholesterol test | 2679 (52.1) | 1866 (53.2) | 813 (49.7) |
| Total cholesterol (mg/dL), mean [SD] | 166.8 (46) | 166.7 (45.3) | 166.9 (47.7) |
| Prescriber specialty | |||
| Primary care, internal medicine | 2892 (56.2) | 1998 (56.9) | 894 (54.6) |
| Endocrinology | 1215 (23.6) | 923 (26.3) | 292 (17.8) |
| Cardiology | 203 (3.9) | 108 (3.1) | 95 (5.8) |
| Other | 836 (16.2) | 480 (13.7) | 356 (21.7) |
| Total # of hospitalizations, mean[SD] | 0.09 [0.4] | 0.08 [0.3] | 0.13 [0.5] |
| Total # of hospitalizations | |||
| 0 | 4756 (92.4) | 3282 (93.5) | 1474 (90) |
| 1 or more | 390 (7.6) | 227 (6.5) | 163 (10) |
| Co-dispensed medications | |||
| Metformin | 3295 (64) | 2489 (70.9) | 806 (49.2) |
| Sulfonylurea | 1267 (24.6) | 953 (27.2) | 314 (19.2) |
| TZD | 112 (2.2) | 76 (2.2) | 36 (2.2) |
| DPP4i | 1066 (20.7) | 817 (23.3) | 249 (15.2) |
| Insulin | 1647 (32) | 1238 (35.3) | 409 (25) |
| ACE inhibitor | 1387 (27) | 1052 (30) | 335 (20.5) |
| Angiotensin receptor blocker | 628 (12.2) | 462 (13.2) | 166 (10.1) |
| Statin | 3191 (62) | 2443 (69.6) | 748 (45.7) |
| Index prescription year | |||
| 2012–2014 | 145 (2.8) | 83 (2.4) | 62 (3.8) |
| 2015 | 397 (7.7) | 248 (7.1) | 149 (9.1) |
| 2016 | 673 (13.1) | 459 (13.1) | 214 (13.1) |
| 2017 | 994 (19.3) | 672 (19.2) | 322 (19.7) |
| 2018 | 1443 (28) | 1027 (29.3) | 416 (25.4) |
| 2019 | 1494 (29) | 1020 (29.1) | 474 (29) |
| Drug type | |||
| GLP-1 agonist | 2435 (47.3) | 1709 (48.7) | 726 (44.3) |
| SGLT2 inhibitor | 2711 (52.7) | 1800 (51.3) | 911 (55.7) |
*Baseline characteristics were measured during the 180-day baseline period (prior to the first prescription order)
The overall incidence of 30-day primary medication nonadherence was 31.8% (1637/5146). This incidence rate was 29.8% (n = 726) and 33.6% (n = 911) among those initiating a GLP-1 agonist and SGLT2i, respectively (Table 2). Using a shorter time window to define primary nonadherence resulted in higher incidence rates: 40.8% and 45.5% for the overall sample using 14 days and 7 days, respectively. The incidence of primary nonadherence by individual drug is reported in Appendix Table A.
Table 2.
Incidence of Primary Nonadherence in the Study Sample
| Outcome measure | Overall, n = 5146 | GLP-1 agonist, n = 2435 | SGLT2 inhibitor, n = 2711 |
|---|---|---|---|
| 30 days | 1637 (31.8%) | 726 (29.8%) | 911 (33.6%) |
| 14 days | 2099 (40.8%) | 950 (39%) | 1149 (42.4%) |
| 7 days | 2343 (45.5%) | 1067 (43.8%) | 1276 (47.1%) |
Several baseline clinical, demographic, and provider characteristics were associated with significantly higher or lower adjusted odds of experiencing primary nonadherence to GLP-1 agonists or SGLT2is (Table 3). The following characteristics were associated with higher odds of primary nonadherence: age ≥ 65 (aOR 1.37 (95% CI 1.09 to 1.72)), Black race vs White (aOR 1.29 (95% CI 1.02 to 1.62)), diabetic nephropathy (aOR 1.31 (95% CI 1.02 to 1.68)), hyperlipidemia (aOR 1.18 (95% CI 1.01 to 1.39)), and having the index prescription ordered by a cardiologist (aOR 1.88 (95% CI 1.32 to 2.65)) or other provider type (aOR 1.64 (95% CI 1.35 to 1.98)) vs a primary care provider/internist. An increase of one standard deviation from the mean number of hospitalizations at baseline was associated with a 14% higher adjusted odds (95% CI 1.06 to 1.22) of primary nonadherence.
Table 3.
Factors Associated with 30-Day Primary Medication Nonadherence to GLP-1 Agonists and SGLT2 Inhibitors
| Baseline characteristic | Primary nonadherence, n (%) | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|---|
| Age | |||||
| < 65 | 1435 (30.6%) | Ref | Ref | ||
| ≥ 65 | 202 (43.5%) | 1.67 | (1.36, 2.06) | 1.37 | (1.09, 1.72) |
| Male | 891 (32.6%) | Ref | Ref | ||
| Female | 746 (31%) | 0.94 | (0.83, 1.07) | 0.86 | (0.75, 0.99) |
| Race | |||||
| White | 1437 (31.6%) | Ref | Ref | ||
| Black | 159 (34.7%) | 1.25 | (1, 1.55) | 1.29 | (1.02, 1.62) |
| Other | 41 (29.3%) | 0.96 | (0.65, 1.41) | 1.08 | (0.72, 1.63) |
| Hispanic | 9 (32.1%) | 0.99 | (0.43, 2.28) | 1.37 | (0.57, 3.27) |
| Hypertension | 1153 (31.6%) | 0.94 | (0.82, 1.08) | 1.1 | (0.94, 1.28) |
| Coronary artery disease | 353 (35.4%) | 1.2 | (1.03, 1.4) | 1.05 | (0.87, 1.26) |
| Congestive heart failure | 106 (36.9%) | 1.24 | (0.95, 1.61) | 0.93 | (0.69, 1.26) |
| Stroke | 47 (39.8%) | 1.34 | (0.9, 1.99) | 1.25 | (0.82, 1.91) |
| Chronic kidney disease | 183 (37.5%) | 1.3 | (1.06, 1.6) | 1.05 | (0.8, 1.39) |
| End-stage renal disease | 6 (46.2%) | 1.81 | (0.57, 5.73) | 1.32 | (0.4, 4.43) |
| Retinopathy | 107 (31.9%) | 1.07 | (0.83, 1.37) | 1.25 | (0.95, 1.64) |
| Neuropathy | 351 (31.4%) | 0.97 | (0.83, 1.13) | 0.97 | (0.82, 1.15) |
| Nephropathy | 225 (35.8%) | 1.26 | (1.05, 1.52) | 1.31 | (1.02, 1.68) |
| Serious hyperglycemic event | 20 (34.5%) | 1.14 | (0.64, 2.04) | 1.13 | (0.61, 2.11) |
| Serious hypoglycemic event | 9 (37.5%) | 1.22 | (0.51, 2.91) | 1.42 | (0.56, 3.61) |
| Hyperlipidemia | 977 (30.6%) | 0.86 | (0.76, 0.98) | 1.18 | (1.01, 1.39) |
| Foot ulcers | 63 (38%) | 1.29 | (0.92, 1.8) | 1.14 | (0.79, 1.66) |
| Peripheral artery disease | 117 (27.8%) | 0.8 | (0.63, 1.02) | 0.73 | (0.56, 0.94) |
| Obesity | 488 (29.6%) | 0.85 | (0.75, 0.98) | 0.86 | (0.73, 1.01) |
| Smoker | 278 (34.3%) | 1.14 | (0.97, 1.35) | 1.05 | (0.87, 1.26) |
| BMI* | 0.94 | (0.88, 1) | 0.98 | (0.91, 1.06) | |
| HbA1c test | 1098 (29.4%) | 0.69 | (0.6, 0.79) | 0.7 | (0.59, 0.84) |
| Creatinine test | 869 (30.4%) | 0.88 | (0.78, 1) | 0.95 | (0.8, 1.13) |
| Total cholesterol test | 813 (30.3%) | 0.89 | (0.79, 1.01) | 1.08 | (0.92, 1.27) |
| Provider specialty | |||||
| Primary care, internal medicine | 894 (30.9%) | Ref | Ref | ||
| Endocrinology | 292 (24%) | 0.69 | (0.56, 0.86) | 0.76 | (0.61, 0.95) |
| Cardiology | 95 (46.8%) | 2.02 | (1.47, 2.77) | 1.88 | (1.33, 2.65) |
| Other | 356 (42.6%) | 1.72 | (1.44, 2.05) | 1.64 | (1.35, 1.98) |
| Total # of hospitalizations* | 1.15 | (1.08, 1.22) | 1.14 | (1.06, 1.22) | |
| Co-dispensed medications | |||||
| Metformin | 806 (24.5%) | 0.38 | (0.34, 0.44) | 0.51 | (0.45, 0.59) |
| Sulfonylurea | 314 (24.8%) | 0.62 | (0.53, 0.72) | 0.79 | (0.67, 0.93) |
| TZD | 36 (32.1%) | 0.97 | (0.63, 1.48) | 1.21 | (0.77, 1.88) |
| DPP4i | 249 (23.4%) | 0.56 | (0.47, 0.66) | 0.66 | (0.55, 0.78) |
| Insulin | 409 (24.8%) | 0.62 | (0.54, 0.71) | 0.62 | (0.53, 0.73) |
| ACE inhibitor | 335 (24.2%) | 0.58 | (0.5, 0.67) | 0.78 | (0.66, 0.92) |
| Angiotensin receptor blocker | 166 (26.4%) | 0.74 | (0.61, 0.9) | 0.81 | (0.65, 1) |
| Statin | 748 (23.4%) | 0.35 | (0.31, 0.4) | 0.44 | (0.38, 0.5) |
| Index prescription year | |||||
| 2012–2014 | 62 (42.8%) | ||||
| 2015 | 149 (37.5%) | 0.79 | (0.53, 1.19) | 0.76 | (0.49, 1.16) |
| 2016 | 214 (31.8%) | 0.64 | (0.43, 0.94) | 0.71 | (0.47, 1.09) |
| 2017 | 322 (32.4%) | 0.64 | (0.44, 0.94) | 0.74 | (0.48, 1.12) |
| 2018 | 416 (28.8%) | 0.54 | (0.37, 0.78) | 0.62 | (0.41, 0.93) |
| 2019 | 474 (31.7%) | 0.61 | (0.43, 0.89) | 0.76 | (0.5, 1.15) |
| Drug type | |||||
| GLP-1 agonist | 726 (29.8%) | Ref | Ref | ||
| SGLT2 inhibitor | 911 (33.6%) | 1.18 | (1.04, 1.34) | 1.14 | (0.99, 1.32) |
In Table 3, the bolded data (i.e. rows) are for predictors found to be statistically signifigant (95% Confidence Interval does not cross 1).
*Standardized
The following factors were associated with lower odds of primary nonadherence: female sex (aOR 0.86 (95% CI 0.75 to 0.99)), peripheral artery disease (aOR 0.73 (95% CI 0.56 to 0.94)), having an HbA1c test at baseline (aOR 0.70 (95% CI 0.59 to 0.84)), and having the index prescription ordered by an endocrinologist vs a primary care provider (aOR 0.76 (95% CI 0.61 to 0.95)). Being co-dispensed other diabetes medications was almost uniformly associated with lower odds of primary nonadherence (Table 3).
Results from sensitivity analyses excluding co-dispensed medications or using 14 days to define primary nonadherence were similar to the results from the primary analysis. In Appendix Table B, we list the variables for which statistical significance changed, with a comparison across models; in all cases, the direction of the effects remained similar.
DISCUSSION
In this study of patients receiving care at a large integrated healthcare system, 1 in 3 patients newly prescribed an SGLT2i or GLP-1 agonist did not fill their prescription within 30 days. Approximately 20% of patients in our cohort had evidence of coronary artery disease at baseline. Older age, Black race, male sex, having greater baseline comorbidities, and having a non-endocrinologist prescribe the index drug were associated with higher odds of primary nonadherence. These findings are clinically relevant given that many SGLT2i and GLP-1 agonists are medications with demonstrated cardiovascular and renal benefits.
The rate of primary nonadherence we report is similar to, or higher than, those reported in several other studies of primary nonadherence to diabetes medications.27–35 The higher rate of primary nonadherence reported in our study may be due to differences in the classes of medications being examined. For example, a prior study using pharmacy data alone reported a 12.9% rate of 30-day primary nonadherence to antidiabetic agents,29 a category that included biguanides, sulfonylureas, DPP-4 inhibitors, and thiazolidinediones. Since the vast majority of prescribed biguanides and sulfonylureas are available as low-cost generic drugs (and thus less likely to be subject to cost-related barriers), excluding those classes in the current analysis likely resulted in a higher rate of primary nonadherence. Changes in formulary practices over time may also partly explain higher rates of primary nonadherence for these two drug classes. For example, despite the commercial availability of 6 brand-name GLP-1 agonists and 4 SGLT2 inhibitors, clinical trial data from head-to-head comparisons are still limited or entirely unavailable at this time. The lack of superiority data allows insurers to employ utilization management (e.g., excluding non-preferred agents,36 placing some agents on very high cost-sharing tiers,37 or requiring failure of other glucose-lowering agents prior to allowing reimbursement38) to manage prescription drug spending.39,40 There may also be other patient-related reasons for high rates of primary nonadherence, because of cost concerns, or concerns about side effects, or other barriers to filling medications.
The relatively high rates of primary nonadherence to SGLT2i and GLP-1 agonists reported here may have important clinical implications. A large body of drug utilization literature suggests that newer agents have historically been underutilized relative to their potential benefits among the population of people living with type 2 diabetes.19–21,41–43 Some authors have reported a “treatment paradox,” where patients at the highest baseline risk of experiencing major adverse cardiovascular events or hospitalizations from heart failure (i.e., those with pre-existing disease or advanced age) were least likely to be dispensed SGLT2i or GLP-1 agonists.21,44 Although our primary analysis did not show a statistically significant association between baseline CVD/CKD and primary nonadherence, we did find that older age, and a history of nephropathy or hyperlipidemia, were associated with a higher likelihood of experiencing this outcome.
In terms of health equity concerns, we found that Black patients experienced higher odds of primary nonadherence to SGLT2i and GLP-1 agonists when compared to White patients. The race effect reported here is similar to other reports in secondary medication nonadherence, both from studies of SGLT2 inhibitors, and also novel anticoagulants, using commercial claims data.44–46 Additional research is needed to understand why these differences persist, both across primary and secondary nonadherence for higher-cost prescription drugs. Unfortunately, we did not have sufficient patient numbers to specifically examine primary nonadherence among other population subgroups such as Asians or Hispanic ethnicity.
In this study, patients receiving their index prescription from an endocrinologist experienced lower odds of primary medication nonadherence when compared to non-endocrine prescribers (including primary care providers, cardiologists, or others). The exact reasons for these differences deserve further study, although there are potential explanations to consider. Clinical endocrinologists might have a better sense of which brand-name alternative is more likely to be covered by insurance when compared to other specialties, as well as more time than other prescriber specialties to discuss therapeutic options and potential side effects of newer glucose-lowering medications. Alternatively, they may have ancillary staff better equipped to secure prior authorization for these classes of medications. It could also be that patients who see endocrinologists are more invested or engaged in their diabetes care and therefore more likely to overcome administrative or financial barriers to ensure that their prescribed medications are dispensed.
One interesting finding from our study was that being co-dispensed other glucose-lowering drugs for type 2 diabetes was associated with lower odds of primary nonadherence to SGLT2i and GLP-1 agonists. This finding is likely due to a healthy adherer effect, where patients more likely to be adherent to chronic diabetes medications are more likely to have their prescription for a newer glucose-lowering drug dispensed.
Our study has several limitations. First, our estimates of primary nonadherence may be subject to misclassification, such as when a patient chooses to fill a prescription for one of the study drugs without insurance (i.e., cash).47 However, we believe this is unlikely because of the extremely high list prices for these medications.37 Second, we could not accurately measure payor type (e.g., Medicare/Medicaid vs commercial) or patient socioeconomic status or level of education/familial support using the available data, which may confound the relationship between measured predictors and primary nonadherence. Third, our results may be limited by unmeasured confounding. For example, we did not have detailed formulary data to help determine which specific medications were covered by specific plans. In addition, patients who participate less in the healthcare system (e.g., by having fewer outpatient visits, fewer laboratory tests, and fewer co-dispensed medications) may also be less likely to fill a SGLT2i or GLP-1 agonist when prescribed. In part due to unmeasured confounding, the inferences we report here are not meant to be causal. Fourth, our results could have limited generalizability since they use data from only a single healthcare system. However, because the insurer in this study includes all types of insurance products (commercial, Medicaid, Medicare), it may be more generalizable than other payer sources of data.48
CONCLUSIONS
Approximately one third of patients first prescribed a SGLT2i or a GLP-1 agonist in this sample did not fill their medication within 30 days. Black race, male sex, older age, having greater baseline comorbidities, and having a primary care provider prescribe the index drug were associated with higher odds of primary nonadherence. These findings highlight a key deficiency in the utilization of these newer diabetes drugs and an opportunity for improving the pharmacotherapy of patients with diabetes.
Supplementary Information
(DOCX 25 kb)
Funding
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001856 and the National Institute of Diabetes and Digestive and Kidney Diseases (Luo) under Award Number K23DK120956. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Department of Veterans Affairs, or United States Government. The research reported in this article was conducted using PCORnet®, the National Patient-Centered Clinical Research Network. PCORnet® has been developed with funding from the Patient-Centered Outcomes Research Institute® (PCORI®). This work is partially supported through the Patient-Centered Outcomes Research Institute Program Award RI-CRN-2020-006. The views presented in this work are solely the responsibility of the authors and do not necessarily represent the views of organizations participating in, collaborating with, or funding PCORnet® or of the Patient-Centered Outcomes Research Institute® (PCORI®).
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 3.Regier EE, Venkat MV, Close KL. More than 7 years of hindsight: revisiting the FDA’s 2008 guidance on cardiovascular outcomes trials for Type 2 diabetes medications. Clinical Diabetes. 2016;34(4):173–180. doi: 10.2337/cd16-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors’ expert forum. Diabetes Care. 2018;41(1):14–31. doi: 10.2337/dci17-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. The Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 6.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 9.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: systematic review and meta-analysis of cardiovascular outcomes trials. Circulation. 2019;139(17):2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 10.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 11.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. Jama. 2007;298(2):194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 12.Scheen AJ. Cardiovascular effects of gliptins. Nature Reviews Cardiology. 2013;10(2):73. doi: 10.1038/nrcardio.2012.183. [DOI] [PubMed] [Google Scholar]
- 13.Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The Lancet Diabetes & Endocrinology. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 14.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 15.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. New England Journal of Medicine. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 16.Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. New England Journal of Medicine. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. The Lancet. 2019;394(10193):131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 18.Association AD. 9 Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 19.Dave CV, Schneeweiss S, Wexler DJ, Brill G, Patorno E. Trends in clinical characteristics and prescribing preferences for SGLT2 inhibitors and GLP-1 receptor agonists, 2013–2018. Diabetes Care. 2020;43(4):921–924. doi: 10.2337/dc19-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schernthaner G, Shehadeh N, Ametov AS, et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovascular Diabetology. 2020;19(1):1–17. doi: 10.1186/s12933-020-01154-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the US—the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technology & Therapeutics. 2019;21(12):702–712. doi: 10.1089/dia.2019.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau DT, Nau DP. Oral Antihyperglycemic Medication Nonadherence and Subsequent Hospitalization Among Individuals With Type 2 Diabetes. Diabetes Care. 2004;27(9):2149–2153. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- 23.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of Medication Nonadherence on Hospitalization and Mortality Among Patients With Diabetes Mellitus. Archives of internal medicine. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 24.Kang H, Lobo JM, Kim S, Sohn M-W. Cost-related medication non-adherence among US adults with diabetes. Diabetes Research and Clinical Practice. 2018;143:24–33. doi: 10.1016/j.diabres.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams AJ, Stolpe SF. Defining and measuring primary medication nonadherence: development of a quality measure. Journal of Managed Care & Specialty Pharmacy. 2016;22(5):516–523. doi: 10.18553/jmcp.2016.22.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software. 2017;82(13):1–26. [Google Scholar]
- 27.Tamblyn R, Eguale T, Huang A, Winslade N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Annals of Internal Medicine. 2014;160(7):441–450. doi: 10.7326/M13-1705. [DOI] [PubMed] [Google Scholar]
- 28.Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J. Prevalence of and factors associated with primary medication non-adherence in chronic disease: A systematic review and meta-analysis. International Journal of Clinical Practice. 2019;73(6):e13350. doi: 10.1111/ijcp.13350. [DOI] [PubMed] [Google Scholar]
- 29.Jackson TH, Bentley JP, McCaffrey I, David J, Pace P, Holmes E, West-Strum D. Store and prescription characteristics associated with primary medication nonadherence. Journal of Managed Care Pharmacy. 2014;20(8):824–832. doi: 10.18553/jmcp.2014.20.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Services Research. 2009;44(5p1):1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández A, Quan J, Moffet H, Parker MM, Schillinger D, Karter AJ. Adherence to newly prescribed diabetes medications among insured Latino and white patients with diabetes. JAMA Internal Medicine. 2017;177(3):371–379. doi: 10.1001/jamainternmed.2016.8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karter AJ, Parker MM, Solomon MD, et al. Effect of out-of-pocket cost on medication initiation, adherence, and persistence among patients with type 2 diabetes: the diabetes study of Northern California (DISTANCE) Health Services Research. 2018;53(2):1227–1247. doi: 10.1111/1475-6773.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. Journal of General Internal Medicine. 2010;25(4):284–290. doi: 10.1007/s11606-010-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raebel MA, Ellis JL, Carroll NM, et al. Characteristics of patients with primary non-adherence to medications for hypertension, diabetes, and lipid disorders. Journal of General Internal Medicine. 2012;27(1):57–64. doi: 10.1007/s11606-011-1829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer MA, Choudhry NK, Brill G, et al. Trouble getting started: predictors of primary medication nonadherence. The American Journal of Medicine. 2011;124(11):1081. e1089-1081. e1022. [DOI] [PubMed]
- 36.Huang X, Liu Z, Shankar RR, Rajpathak S. Description of anti-diabetic drug utilization pre-and post-formulary restriction of sitagliptin: findings from a national health plan. Current Medical Research and Opinion. 2015;31(8):1495–1500. doi: 10.1185/03007995.2015.1060211. [DOI] [PubMed] [Google Scholar]
- 37.Luo J, Feldman R, Rothenberger SD, Hernandez I, Gellad WF. Coverage, formulary restrictions, and out-of-pocket costs for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in the Medicare part D program. JAMA Network Open. 2020;3(10):e2020969–e2020969. doi: 10.1001/jamanetworkopen.2020.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung A, Mullins CD, Slejko JF, Haines ST, Shaya F, Lugo A. Using a budget impact model framework to evaluate antidiabetic formulary changes and utilization management tools. Journal of managed care & specialty pharmacy. 2019;25(3):342–349. doi: 10.18553/jmcp.2019.25.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Resneck JS. Refocusing medication prior authorization on its intended purpose. Jama. 2020;323(8):703–704. doi: 10.1001/jama.2019.21428. [DOI] [PubMed] [Google Scholar]
- 40.Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41(6):1299–1311. doi: 10.2337/dci18-0019. [DOI] [PubMed] [Google Scholar]
- 41.McCoy RG, Van Houten HK, Deng Y, et al. Comparison of diabetes medications used by adults with commercial insurance vs medicare advantage, 2016 to 2019. JAMA Network Open. 2021;4(2):e2035792–e2035792. doi: 10.1001/jamanetworkopen.2020.35792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen DH, Rungby J, Thomsen RW. Nationwide trends in glucose-lowering drug use, Denmark, 1999–2014. Clinical Epidemiology. 2016;8:381. doi: 10.2147/CLEP.S113211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao H, Laxy M, Benoit SR, Cheng YJ, Gregg EW, Zhang P. Trends in Total and Out-of-pocket Payments for Noninsulin Glucose-Lowering Drugs Among US Adults With Large-Employer Private Health Insurance From 2005 to 2018. Diabetes Care. 2021;44(4):925–934. doi: 10.2337/dc20-2871. [DOI] [PubMed] [Google Scholar]
- 44.Eberly LA, Yang L, Eneanya ND, et al. Association of Race/Ethnicity, Gender, and Socioeconomic Status With Sodium-Glucose Cotransporter 2 Inhibitor Use Among Patients With Diabetes in the US. JAMA Network Open. 2021;4(4):e216139–e216139. doi: 10.1001/jamanetworkopen.2021.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Essien UR, Magnani JW, Chen N, Gellad WF, Fine MJ, Hernandez I. Race/ethnicity and sex-related differences in direct oral anticoagulant initiation in newly diagnosed atrial fibrillation: a retrospective study of medicare data. Journal of the National Medical Association. 2020;112(1):103–108. doi: 10.1016/j.jnma.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Essien UR, Holmes DN, Jackson LR, et al. Association of Race/Ethnicity with oral anticoagulant use in patients with atrial fibrillation: findings from the outcomes Registry for better informed treatment of atrial fibrillation II. JAMA Cardiology. 2018;3(12):1174–1182. doi: 10.1001/jamacardio.2018.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karter AJ, Parker MM, Adams AS, et al. Primary non-adherence to prescribed medications. Journal of General Internal Medicine. 2010;25(8):763–763. doi: 10.1007/s11606-010-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernandez I, Good CB, Cutler DM, Gellad WF, Parekh N, Shrank WH. The contribution of new product entry versus existing product inflation in the rising costs of drugs. Health Affairs. 2019;38(1):76–83. doi: 10.1377/hlthaff.2018.05147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 25 kb)