Abstract
Background
There is general consensus that hand hygiene is the most effective way to prevent healthcare-associated infections. However, low rates of compliance amongst healthcare workers have been reported globally. The coronavirus disease 2019 pandemic has further emphasized the need for global improvement in hand hygiene compliance by healthcare workers.
Aim
This comprehensive systematic review provides an up-to-date compilation of clinical trials, reported between 2014 and 2020, assessing hand hygiene interventions in order to inform healthcare leaders and practitioners regarding approaches to reduce healthcare-associated infections using hand hygiene.
Methods
CINAHL, Cochrane, EMbase, Medline, PubMed and Web of Science databases were searched for clinical trials published between March 2014 and December 2020 on the topic of hand hygiene compliance among healthcare workers. In total, 332 papers were identified from these searches, of which 57 studies met the inclusion criteria.
Findings
Forty-five of the 57 studies (79%) included in this review were conducted in Asia, Europe and the USA. The large majority of these clinical trials were conducted in acute care facilities, including hospital wards and intensive care facilities. Nurses represented the largest group of healthcare workers studied (44 studies, 77%), followed by physicians (41 studies, 72%). Thirty-six studies (63%) adopted the World Health Organization's multi-modal framework or a variation of this framework, and many of them recorded hand hygiene opportunities at each of the ‘Five Moments’. However, recording of hand hygiene technique was not common.
Conclusion
Both single intervention and multi-modal hand hygiene strategies can achieve modest-to-moderate improvements in hand hygiene compliance among healthcare workers.
Keywords: Systematic review, Hand hygiene, Hand hygiene opportunities, Compliance, Clinical trial, Healthcare worker, HAI, HCAI
Introduction
Healthcare-associated infections (HCAIs) are defined as infections that arise following use of a healthcare service, and are associated with increased patient morbidity and mortality [1,2]. HCAIs have been estimated to affect 7% of patients in developed countries and >25% of patients in developing countries [3]. In Europe, this equates to 3.2 million people being affected with HCAIs in acute care hospitals each year, contributing to approximately 37,000 deaths [4]. Economically, HCAIs have a negative impact on insurers and health systems. Across Europe, HCAIs have been shown to cause 25 million extra days of hospital stay and associated treatment, resulting in an economic burden of €13–24 billion per year [5]. With the current focus on viral disease, it is notable that nosocomial influenza alone has been reported to elevate care-related costs in the Netherlands by between €4934 and €10,665 per patient [6], while one outbreak in the USA involving 18 patients had a defined impact of US$112,131 [7].
Establishing effective monitoring and reporting of HCAIs and hospital-acquired infections (HAIs) is integral to the evaluation of control measures within healthcare systems and to enable implementation of appropriate changes. However, such surveillance can be resource intensive and, therefore, presents a significant challenge to healthcare systems worldwide, particularly in developing countries. The inaccuracy of microbiological data, poor access to imaging equipment, and lack of up-to-date medical record-keeping resources are just a few of the additional hurdles faced in the developing world [5]. As such, there are relatively few published data on HCAI surveillance in these regions. A search conducted by the World Health Organization (WHO) found that 300 papers were published between 1995 and 2008, of which only 80 (27%) demonstrated rigorous methodological efforts [5].
It is now well established that the most effective way to prevent HCAIs is to practice hand hygiene (HH) [8]. Chen et al. calculated that for every US dollar spent on HH promotion, there would be a savings of almost US$24 [9]. Pittet et al. further demonstrated that hygiene promotion would cost <1% of the projected costs associated with HCAIs [10]. More specifically, data from a mathematical model used by Cummings et al. showed that in a 200-bed hospital, the annual costs related to meticillin-resistant Staphylococcus aureus infection would total US$1,779,283, and 1% improvement in HH compliance could achieve savings of US$39,650 [11].
HH guidelines were first developed in the 1980s by the US Centers for Disease Control and Prevention in an effort to control the hospital environment and prevent specific nosocomial infections [12,13]. In 2000, Pittet et al. reported sustained improvement of HH compliance and reduction of nosocomial infections after initiation of a HH promotion programme involving educational posters and increased access to alcohol-based hand rub (ABHR) in University of Geneva Hospitals [8]. In 2005, WHO introduced the ‘Global Patient Safety Challenge’ campaign, which emphasized multi-modal intervention strategies incorporating health system structural changes, education and training, feedback, reminders and patient safety [14]. In 2008, WHO launched another global initiative – ‘Clean Care is Safer Care’ – to improve HH compliance among healthcare workers (HCWs) [15,16]. Along with these interventions, WHO developed ‘My Five Moments for Hand Hygiene’ which encouraged HCWs to perform HH before touching a patient, before clean/aseptic procedures, after body fluid exposure/risk, after touching a patient, and after touching a patient's surroundings [5]. In subsequent years, the USA, Canada, the UK and Ireland, amongst many other countries, have revised and updated national guidelines for HH based on these campaigns [1].
The adoption and effectiveness of multi-modal HH strategies has been the focus of many clinical studies in recent years [[17], [18], [19], [20], [21], [22]]. In addition to the strategies suggested by WHO, it has been suggested that improved adherence to HH guidelines could accrue from the engagement of HCWs in identifying barriers to HH compliance [12,18], involvement of patients to encourage good HH practice by physicians [23,24], use of positive role-modelling [25,26], use of reinforcing strategies (e.g. rewards) [27,28], and application of behavioural change theories [[29], [30], [31], [32]] as well as personality factors (i.e. theory of thinking styles) [33]. Indeed, the list of possible interventions is extensive, and different combinations of interventions can lead to different outcomes. It is, therefore, important to identify the specific intervention or combination of interventions that may most impact the target population.
In the context of the coronavirus disease 2019 (COVID-19) pandemic, the importance of HH compliance among HCWs has been brought into focus. This systematic review builds on the authors' previous systematic review of this topic published in this journal [1], which focused on the period 2010–2015, and is designed to inform healthcare leaders and practitioners regarding the effectiveness and character of HH promotion strategies internationally over the past 6 years using evidence from published clinical trials with sound methodological designs.
Methods
Scope
Searches involved literature published between 1st March 2014 and 31st December 2020 indexed in CINAHL, Cochrane, EMbase, Medline, PubMed or Web of Science on the topic of HH compliance among healthcare professionals.
Systematic approach to finding appropriate literature
Searches were performed in CINAHL, Cochrane, EMbase, Medline, PubMed and Web of Science in October 2020 and January 2021 for full articles published on the topic of HH compliance. More specifically, searches sought to identify clinical trials. Papers that were not published in English were excluded. Only full original research papers and reviews were included. Editorial opinions, letters to the editor, other ‘opinion’-based publications, poster presentations and conference proceedings that were not published as full articles were excluded.
Search methodology
Title and abstract fields were searched for publications containing the words: ‘hand hygiene’, ‘handwashing’ and ‘compliance’. Boolean operators were used to combine search components. For example, the PubMed search was: (hand hygiene) OR (hand washing) AND compliance (hand hygiene [Title/Abstract]) AND compliance [Title/Abstract]. The CINAHL search was: (hand hygiene) OR (hand washing) AND compliance. The EMbase search was: (hand hygiene) OR (hand washing) AND compliance. The Web of Science search was: (hand hygiene) OR (hand washing) AND compliance AND article, abstract of published item [document type]. The Web of Science search was processed further to exclude results that were not full papers or papers from unrelated disciplines. The Cochrane search was: (hand hygiene) OR (hand washing) AND (compliance). The Medline search was: (hand hygiene) OR (hand washing) AND compliance.
Critical appraisal and synthesis
Two reviewers (CC and TD) reviewed the search results, titles and abstracts independently. Consensus on eligibility for inclusion was agreed; where discrepancies arose, these were resolved by discussion with CPD. These potentially eligible articles were retrieved and read, resulting in the final selection of eligible studies. Those articles retrieved by the search but deemed ineligible for further analysis, as they did not report on HH compliance per se, are listed in Table S1 (see online supplementary material).
Studies that met the following criteria were included: empirical studies conducted in study settings that included acute or non-acute healthcare, long-term care of the elderly, long-term paediatric care and primary care facilities; samples from countries with developed or developing economies; compliance with HH measured either by observation or electronic counters; results of HH compliance rates published; and published in the English language. Studies set in domestic or school settings were excluded. Four studies where compliance was measured by self-reporting were excluded.
Of the 332 papers identified by the search, 57 studies (17%) were deemed eligible for inclusion in this review. Data were extracted by examining study characteristics using the following headings: country of origin; study objectives; study setting; target population; study design; interventions; HH recording method; and study outcomes. A lack of homogeneity of the studies selected was identified on extraction of study characteristics, so formal meta-analysis was not possible. However, analysis was achieved by collating data manually and compiling the results in tables. Studies were further analysed by region and income status according to World Bank classification [34].
Results
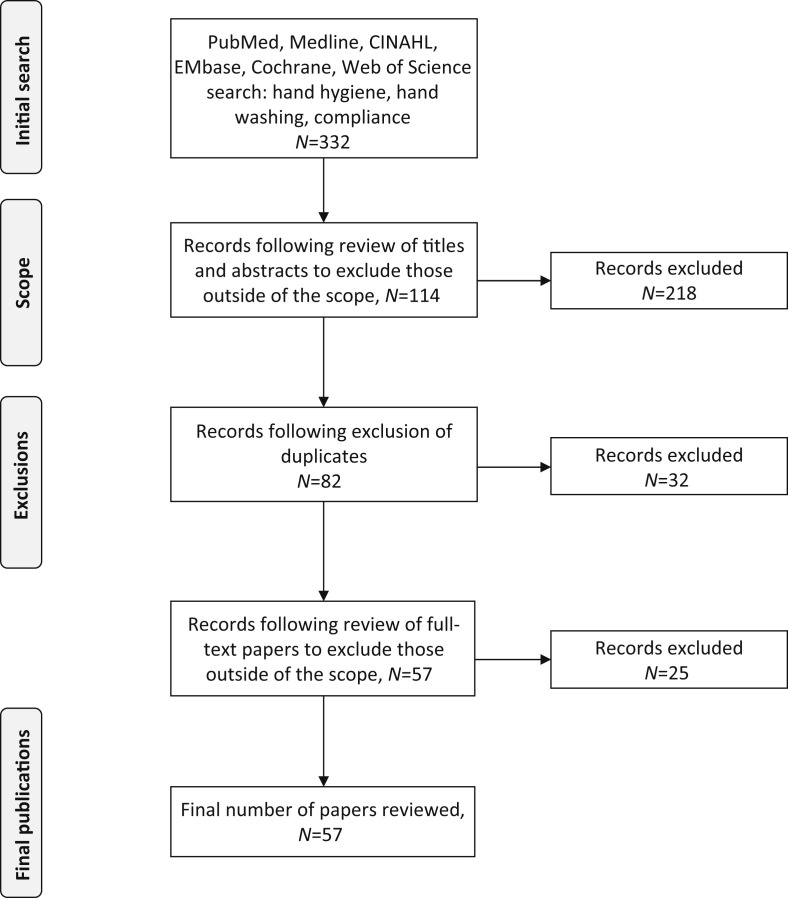
This search yielded 332 publications, of which 218 were excluded as they were outside the scope of this review. A further 32 records were excluded following screening for duplicates. On closer reading, a further 25 papers were found not to meet the criteria and were excluded. In total, 275 papers were excluded (Table S1, see online supplementary material), leaving 57 papers in this review (Figure 1 ). The most common reason for exclusion was that a study was not a full clinical trial (85 papers). Other common reasons for exclusion were: the study was not performed in a healthcare setting (70 papers); the study did not measure HH compliance specifically (55 papers); the data were inconclusive or results were not recorded (18 papers); the study population did not comprise HCWs (16 papers); the study was not published as a full study/involved abstract alone (11 papers); compliance was reported using self-reporting measures (nine papers); and the study was not published in the English language (three papers). Fifteen studies were excluded for more than one reason, and are accounted for in the categories listed above.
Figure 1.
PRISMA flow diagram.
Geographic location
The geographic breadth of the studies included in this review reflects recognition of the importance of HH globally. The analysis of countries involved incorporated definitions of global geographic regions and income levels according to World Bank classification [34]. The specific number of studies included per country and their income status classification can be found in Table I .
Table I.
Geographic location of included studies
| Region according to World Bank classification [34] | Country | Number of studies included | Income according to World Bank classification [34] | Corresponding studies |
|---|---|---|---|---|
| Africa (Sub-Saharan Africa) | Ghana | 1 | Middle (lower middle) | [40] |
| Tanzania | 1 | Middle (lower middle) | [38] | |
| Ethiopia | 1 | Low | [37] | |
| Zimbabwe | 1 | Low | [39] | |
| Asia (East Asia and the Pacific) | China | 4 | Middle (upper middle) | [56,70,82,86] |
| Indonesia | 2 | Middle (upper middle) | [77,84] | |
| Malaysia | 1 | Middle (upper middle) | [62] | |
| Thailand | 1 | Middle (upper middle) | [55] | |
| Japan | 2 | High | [50,72] | |
| Asia (South Asia) | India | 3 | Middle (lower middle) | [41,51,79] |
| Pakistan | 1 | Middle (lower middle) | [54] | |
| Asia (Central Asia) | Turkey | 1 | Middle (upper middle) | [52] |
| Europe | Multiple European countries | 2 | High | [35,36] |
| Denmark | 1 | High | [47] | |
| Finland | 1 | High | [53] | |
| France | 1 | High | [60] | |
| Germany | 5 | High | [43,45,46,65,74] | |
| Italy | 2 | High | [3,85] | |
| Netherlands | 1 | High | [90] | |
| Switzerland | 3 | High | [58,61,80] | |
| Middle East and North Africa | Egypt | 1 | Middle (lower middle) | [59] |
| Iran | 1 | Middle (upper middle) | [88] | |
| Saudi Arabia | 2 | High | [57,71] | |
| United Arab Emirates | 1 | High | [78] | |
| North America | Canada | 2 | High | [64,68] |
| Multiple US states | 1 | High | [42] | |
| Boston | 1 | High | [66] | |
| California | 1 | High | [81] | |
| Florida | 2 | High | [49,75] | |
| Iowa | 2 | High | [44,67] | |
| Massachusetts | 1 | High | [48] | |
| New York | 1 | High | [63] | |
| Ohio | 2 | High | [12,83] | |
| Utah | 1 | High | [69] | |
| South America | Argentina | 1 | Middle (upper middle) | [87] |
| Brazil | 2 | Middle (upper middle) | [73,89] |
Sixteen studies (28%) were performed in European countries, 15 (26%) in Asian countries and 14 (25%) in North American countries. There were five studies from Middle Eastern countries (9%), four from African countries (7%) and three from South American countries (5%). Two of the European studies involved research sites from multiple countries: Derde et al. primarily focused on Western European countries including the Netherlands, the UK, Belgium, Poland, France, Portugal, Latvia, Greece, Slovenia, Italy, Spain and Luxembourg [35]; and Lytsy et al. primarily focused on Eastern European countries including Latvia, Lithuania, Russia and Sweden [36]. Overall, 35 (61%) studies were conducted in high-income countries, 13 (23%) in upper-middle-income countries and 7 (12%) in lower-middle-income countries; only two (4%) studies were performed in low-income countries. Studies conducted in low-resource settings, as identified by the authors, included all four studies performed in African countries [[37], [38], [39], [40]] and one study performed in India [41].
Clinical setting
Among the studies reviewed, 801 clinical settings were identified (Table II ), including: medical/surgical wards (N=361); dispensaries (N=162); adult intensive care units (ICUs) (N=72); whole organizations (N=61); operating rooms (N=56); primary healthcare centres (N=38); nursing homes (N=18); clinical services (N=11); laboratories (N=8); rehabilitation centre units (N=5); haematopoietic stem cell transplant units (N=4); neonatal ICUs (N=2); paediatric ICUs (N=2); a paediatric long-term care facility (N=1); and a high-dependency unit (HDU) (N=1). Several studies involved implementation of interventions across more than one clinical setting. For instance, in the study conducted by Nyamadzawo et al., HH compliance was assessed in an adult ICU (N=1), a paediatric ICU (N=1), a neonatal ICU (N=1), an HDU (N=1), medical wards (N=4) and surgical wards (N=2) in Zimbabwe [39]. Other studies included data from clinical settings across multiple geographic locations. For instance, Staats assessed the use of sanitizer dispensers from 42 hospitals across the USA [42]. Similarly, Lytsy et al. included data on ABHR consumption from two hospitals in Latvia and Lithuania, six hospitals in Russia, and three hospitals in Sweden [36].
Table II.
Clinical setting
| Clinical setting (N) | No. of clinical settings | Study |
|---|---|---|
| Adult ICU (N=72) | 1 | King et al. [75] |
| 1 | Kuruno et al. [72] | |
| 1 | Saharman et al. [84] | |
| 1 | Yilmaz et al. [52] | |
| 3 | Chakravarthy et al. [41] | |
| 1 | Medeiros et al. [89] | |
| 5 | Su et al. [86] | |
| 1 | van der Kooi et al. [58] | |
| 6 | Apisarnthanarak et al. [55] | |
| 13 | Derde et al. [35] | |
| 1 | Fox et al. [81] | |
| 2 | Ellison et al. [48] | |
| 1 | Nyamadzawo et al. [39] | |
| 1 | Rodrigeuz et al. [87] | |
| 10 | von Lengerke et al. [46] | |
| 10 | von Lengerke et al. [45] | |
| 11 | Reisinger et al. [44] | |
| 1 | Renaudin et al. [60] | |
| 2 | Visnovsky et al. [69] | |
| Neonatal ICU (N=2) | 1 | Chhapola and Brar [79] |
| 1 | Nyamadzawo et al. [39] | |
| Paediatric ICU (N=1) | 1 | Nyamadzawo et al. [39] |
| Paediatric long-term care facility (N=1) | 1 | Larson et al. [63] |
| Nursing home (N=18 nursing homes, N=124 nursing home units) | 18 | Teesing et al. [90] |
| High-dependency unit (N=1) | 1 | Nyamadzawo et al. [39] |
| Primary healthcare centre (N=38) | 13 | Labi et al. [40] |
| 1 | Nour-Eldein and Ali Mohamed [59] | |
| 24 | Wiedenmayer et al. [38] | |
| Whole organization (N=61) | 2 | Labi et al. [40] |
| 1 | Xiong et al. [56] | |
| 2 | Lea et al. [70] | |
| 1 | Stewardson et al. [61] | |
| 1 | Harrabi et al. [57] | |
| 5 | Sopirala et al. [12] | |
| 42 | Staats [42] | |
| 1 | Mu et al. [82] | |
| 1 | Farhoudi et al. [88] | |
| 2 | Moller-Sorenson et al. [47] | |
| 1 | Stevenson et al. [83] | |
| 2 | Derksen et al. [74] | |
| Operating room (N=56) | 1 | Khan and Nausheen [54] |
| 14 | Wiedenmayer et al. [38] | |
| 11 | Laurikainen et al. [53] | |
| 30 | Nobile et al. [3] | |
| Medical/surgical ward (N=361) | 11 | Schmitz et al. [37] |
| 12 | Tschudin-Sutter et al. [80] | |
| 4 | Diefenbacher et al. [65] | |
| 1 | Kai et al. [50] | |
| 5 | Santoaningsih et al. [77] | |
| 207 | Wiedenmayer et al. [38] | |
| 1 | Fouad and Eltaher [71] | |
| 38 | Lytsy et al. [36] | |
| 2 | Lee et al. [62] | |
| 6 | Nyamadzawo et al. [39] | |
| 20 | Aghdassi et al. [43] | |
| 1 | Muller et al. [68] | |
| 4 | Ng et al. [78] | |
| 11 | Reisinger et al. [44] | |
| 1 | Vander Weg et al. [67] | |
| 6 | Visnovsky et al. [69] | |
| 2 | Benudis et al. [66] | |
| 1 | Beyfus et al. [49] | |
| 2 | Marra et al. [73] | |
| 19 | Nobile et al. [3] | |
| 1 | Mukherjee et al. [51] | |
| 6 | Donati et al. [85] | |
| Rehabilitation centre unit (N=5) | 5 | Pong et al. [64] |
| Dispensary (N=162) | 155 | Staats [42] |
| 7 | Wiedenmayer et al. [38] | |
| Haematopoietic stem cell transplant unit (N=4) | 2 | von Lengerke et al. [46] |
| 2 | von Lengerke et al. [45] | |
| Clinical service (N=11) | 11 | Nobile et al. [3] |
| Laboratory (N=8) | 8 | Wiedenmeyer et al. [38] |
| Total=801 |
ICU, intensive care unit.
Overall, the most studied clinical settings were medical and surgical wards (N=361). Collectively, these included 207 wards across seven hospitals in Tanzania [38], 20 wards from one hospital in Germany [43] and 19 wards from one orthopaedic hospital in Italy [3]. Dispensaries were the second most commonly assessed clinical setting (N=162). These included 155 dispensaries throughout the USA [42] and seven dispensaries in Tanzania [38]. Adult ICUs were the third most frequently studied clinical setting, involving 13 adult ICUs in Europe [35], 11 adult ICUs in the USA [44] and 10 adult ICUs in Germany [45,46].
Healthcare worker category
The broad categories of HCWs participating in the interventions were nurses, physicians, healthcare assistants and ‘other’ staff (technicians, radiology team members, physiotherapists, paramedical personnel, medical/nursing students) (Table III ). While the majority of the studies reported including one or a combination of these HCW types, they did not typically specify the number of participating HCWs in each category. However, von Lengerke et al. described what appears to be the largest single cohort of participants, specifically 572 nurses and 515 physicians [45,46]. It is notable that a number of studies failed to observe, or at least report, the types of HCWs assessed. These studies mainly involved use of electronic devices for behavioural reminders or ABHR/soap dispensers, where it may not have been possible to identify the professional role of the HCW specifically. For example, in the study by Moller Sorenson et al., an electronic device was used to provide a reminder to perform handwashing after restroom visits [47]. Although the restrooms were restricted to HCWs, the exact profession of the HCW accessing the facilities could not be identified. Furthermore, in the studies by Ellison et al. and Beyfus et al., electronic HH ABHR/soap dispensers were installed to estimate HH compliance from HH events [48] and volume dispensed [49], respectively. In these studies, overall HH compliance within the clinical setting was monitored; however, close assessment of individual HCW activity was not measured. The absence of such descriptive and quantitative data makes it difficult to draw any conclusions from these studies regarding particular HCW roles.
Table III.
Healthcare worker category
| Study | Nurse (N) | Physician (N) | Healthcare assistant (N) | Other (N) | Total sample size (N) |
|---|---|---|---|---|---|
| Aghdassi et al. [43] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Apisarnthanarak et al. [55] | Yes (75) | Yes (15) | Yes (25) | Yes (10) | Yes (125) |
| Benudis et al. [66] | Yes (no data) | Yes (no data) | Yes (no data) | Yes (no data) | Yes (91) |
| Beyfus et al. [49] | No data | No data | No data | No data | Yes (150) |
| Chakravarthy et al. [41] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Chhapola and Brar [79] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Diefenbacher et al. [65] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Derde et al. [35] | No data | No data | No data | No data | No data |
| Derksen et al. [74] | Yes (no data) | Yes (no data) | No data | Yes (no data) | Yes (140) |
| Donati et al. [85] | Yes (121) | No data | No data | No data | Yes (121) |
| Ellison et al. [48] | No data | No data | No data | No data | No data |
| Farhoudi et al. [88] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Fouad and Eltaher [71] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Fox et al. [81] | Yes (no data) | No data | No data | No data | No data |
| Harrabi et al. [57] | Yes (21) | Yes (15) | No data | No data | Yes (36) |
| Kai et al. [50] | No data | Yes (no data) | No data | No data | No data |
| Khan and Nausheen [54] | No data | Yes (59) | Yes (32) | Yes (59) | Yes (150) |
| King et al. [75] | Yes (no data) | Yes (no data) | No data | Yes (no data) | Yes (404) |
| Kuruno et al. [72] | Yes (no data) | No data | No data | No data | No data |
| Labi et al. [40] | Yes (397) | Yes (5) | No data | Yes (177) | Yes (574) |
| Larson et al. [63] | No data | Yes (no data) | No data | Yes (no data) | No data |
| Laurikainen et al. [53] | Yes (263) | Yes (367) | No data | Yes (55) | Yes (685) |
| Lea et al. [70] | Yes (no data) | Yes (no data) | No data | Yes (no data) | Yes (220) |
| Lee et al. [62] | Yes (10) | Yes (2) | No data | No data | Yes (12) |
| Lytsy et al. [36] | Yes (no data) | Yes (no data) | No data | No data | No data |
| Marra et al. [73] | No data | No data | No data | No data | No data |
| Medeiros et al. [89] | Yes (no data) | Yes (no data) | No data | No data | No data |
| Moller-Sorenson et al. [47] | No data | No data | No data | No data | No data |
| Mu et al. [82] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Mukherjee et al. [51] | No data | Yes (42) | No data | No data | Yes (42) |
| Muller et al. [68] | Yes (10) | No data | No data | No data | Yes (10) |
| Ng et al. [78] | Yes (118) | Yes (62) | No data | Yes (39) | Yes (219) |
| Nobile et al. [3] | Yes (no data) | Yes (no data) | No data | No data | No data |
| Nour-Eldein and Ali Mohamed [59] | Yes (no data) | Yes (no data) | No data | Yes (no data) | Yes (82) |
| Nyamadzawo et al. [39] | Yes (86) | No data | No data | No data | Yes (86) |
| Pong et al. [64] | Yes (no data) | Yes (no data) | No data | Yes (no data) | Yes (511) |
| Reisinger et al. [44] | No data | No data | No data | No data | No data |
| Renaudin et al. [60] | No data | No data | No data | No data | No data |
| Rodriguez et al. [87] | Yes (466) | Yes (183) | No data | Yes (56) | Yes (705) |
| Saharman et al. [84] | Yes (77) | Yes (14) | No data | Yes (6) | Yes (97) |
| Santoaningsih et al. [77] | Yes (no data) | Yes (no data) | No data | Yes (no data) | Yes (284) |
| Schmitz et al. [37] | No data | No data | No data | No data | No data |
| Sopirala et al. [12] | Yes (no data) | No data | No data | No data | No data |
| Staats [42] | Yes (3752) | Yes (no data) | No data | Yes (no data) | Yes (5247) |
| Stevenson et al. [83] | Yes (no data) | Yes (no data) | Yes (no data) | Yes (no data) | No data |
| Stewardson et al. [61] | Yes (no data) | Yes (no data) | Yes (no data) | Yes (no data) | No data |
| Su et al. [86] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Teesing et al. [90] | Yes (782) | No data | No data | No data | Yes (782) |
| Tschudin-Sutter et al. [80] | Yes (101) | Yes (no data) | No data | Yes (no data) | Yes (194) |
| van der Kooi et al. [58] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Vander Weg et al. [67] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| Visnovsky et al. [69] | Yes (no data) | Yes (no data) | No data | Yes (no data) | No data |
| von Lengerke et al. [46] | Yes (572) | Yes (515) | No data | No data | Yes (1087) |
| von Lengerke et al. [45] | Yes (572) | Yes (515) | No data | No data | Yes (1087) |
| Wiedenmayer et al. [38] | Yes (112) | Yes (no data) | Yes (no data) | Yes (no data) | Yes (236) |
| Xiong et al. [56] | No data | No data | No data | Yes (84) | Yes (84) |
| Yilmaz et al. [52] | Yes (15) | Yes (20) | No data | Yes (15) | Yes (50) |
Among the studies that quantified the number and category of HCWs, nurses were most commonly included. There were 44 studies (77%) involving nurses, and 17 of them quantified the number of nurses participating (total 6978 nurses). In fact, seven studies focused solely on nurses. For instance, Nyamadzawo et al. included a total of 86 HCWs, all of whom were nursing staff [39]. Physicians accounted for the second largest group of HCWs assessed. From 41 studies (72%) that reported some level of physician involvement, 11 referred to a total of 1257 physicians as study participants. Although not specified as such within the study objectives, the studies by Kai et al. and Mukherjee et al. recruited physicians alone [50,51]. In the study by Yilmaz et al., more physicians were studied than nurses (N=20 vs 15) [52]. Similarly, in the study by Laurikainen et al., 367 physicians and 263 nurses were included [53]. Furthermore, a total of six studies (11%) involved healthcare assistants. Amongst these six studies, Khan and Nausheen quantified 32 healthcare assistants [54] and Apisarnthanarak et al. listed 25 healthcare assistants [55]. Finally, 33 studies (58%) included HCWs that fell within the ‘other’ category. Of these 33 studies, 10 identified a total of 543 ‘other’ staff. For example, in the study by Xiong et al., 84 nursing students were observed during their clinical rotations and were considered as hospital staff [56].
Hand hygiene opportunities
HH opportunities (HHOs) are ubiquitous and can be defined as a point of time when any one or more of the ‘Five Moments’ outlined by WHO is present and observed, either directly or electronically [5]. Twenty-nine of the included studies (51%) quantified HHOs across multiple clinical settings (Table IV ). For example, Nyamadzawo et al. reported 659 HHOs across four medical wards, two surgical wards, one adult ICU, one paediatric ICU, one neonatal ICU and one HDU [39]. Furthermore, a large data set was reported by Staats, gathered over 3 years and involving 20 million HHOs within 42 hospitals and 155 dispensaries [42]. Of similar scale, Ellison et al. reported over 13.7 million HHOs over a period of 25 weeks within two adult ICUs [48]. Among the remaining 1,370,483 opportunities observed, a large proportion were observed from 30 adult ICUs (N=76,346) and 57 medical and surgical wards (N=813,000).
Table IV.
Hand hygiene opportunities (HHOs)
| Study | Clinical setting (N) | Observation method (number of HHOs) |
|---|---|---|
| van der Kooi et al. [58] | Medical/surgical ward (1) | Direct (59,122) |
| Vander Weg et al. [67] | Medical/surgical ward (1) | Direct (52,065) |
| Derde et al. [35] | Adult ICUs (13) | Direct (41,558) |
| Chhapola and Brar [79] | Neonatal ICU (1) | Direct (28,726) |
| Mu et al. [82] | Whole organization (1) | Direct (27,852) |
| Aghdassi et al. [43] | Medical/surgical wards (20) | Direct (21,424) |
| Reisinger et al. [44] | Medical/surgical wards + ICU (11) | Direct (13,195) |
| Stewardson et al. [61] | Whole organization (1) | Direct (12,579) |
| Rodriguez et al. [87] | Adult ICU (1) | Direct (10,429) |
| Saharman et al. [84] | Adult ICUs (2) | Direct (7187) |
| Kuruno et al. [72] | Adult ICU (1) | Direct (6050) |
| Lee et al. [62] | Medical/surgical wards (2) | Direct (4895) |
| Medeiros et al. [89] | Adult ICU (1) | Direct (4837) |
| Labi et al. [40] | Whole organization (2) | Direct (4296) |
| Primary healthcare centre (13) | ||
| Chakravarthy et al. [41] | Adult ICUs (3) | Direct (3612) |
| Tschudin-Sutter et al. [80] | Medical/surgical wards (12) | Direct (2923) |
| Santoaningsih et al. [77] | Medical/surgical wards (5) | Direct (2766) |
| Stevenson et al. [83] | Whole organization (1) | Direct (2654) |
| Su et al. [86] | Medical/surgical wards (5) | Direct (2079) |
| Schmitz et al. [37] | Medical/surgical wards (4) | Direct (2000) |
| Diefenbacher et al. [65] | Medical/surgical wards (4) | Direct (1894) |
| Apisarnthanarak et al. [55] | Adult ICUs (6) | Direct (1872) |
| Fouad and Eltaher [71] | Medical/surgical ward (1) | Direct (1374) |
| Teesing et al. [90] | Nursing homes (18) | Direct (1000) |
| Renaudin et al. [60] | Adult ICU (1) | Direct (801) |
| Nyamadzawo et al. [39] | Adult ICU (1) | Direct (659) |
| Neonatal ICU (1) | ||
| Paediatric ICU (1) | ||
| High-dependency unit (1) | ||
| Medical/surgical wards (6) | ||
| Donati et al. [85] | Medical/surgical wards (6) | Direct (448) |
| Derksen et al. [74] | Whole organization (2) | Direct (267) |
| Farhoudi et al. [88] | Whole organization (1) | Direct (255) |
| Staats [42] | Whole organization (42) | |
| Dispensaries (155) | Electronic (20 million) | |
| Ellison et al. [48] | Adult ICUs (2) | Electronic (>13.7 million) |
| Marra et al. [73] | Medical/surgical wards (2) | Electronic (648,815) |
| Pong et al. [64] | Rehabilitation centre units (5) | Electronic (402,849) |
| Kai et al. [50] | - | - |
| Khan and Nausheen [54] | - | - |
| King et al. [75] | - | - |
| Yilmaz et al. [52] | - | - |
| Nour-Eldein and Ali Mohamed [59] | - | - |
| Wiedenmayer et al. [38] | - | - |
| Xiong et al. [56] | - | - |
| Lea et al. [70] | - | - |
| Fox et al. [81] | - | - |
| Lytsy et al. [36] | - | - |
| Moller-Sorenson et al. [47] | - | - |
| von Lengerke et al. [46] | - | - |
| von Lengerke et al. [45] | - | - |
| Harrabi et al. [57] | - | - |
| Muller et al. [68] | - | - |
| Ng et al. [78] | - | - |
| Visnovsky et al. [69] | - | - |
| Benudis et al. [66] | - | - |
| Beyfus et al. [49] | - | - |
| Larson et al. [63] | - | - |
| Laurikainen et al. [53] | - | - |
| Nobile et al. [3] | - | - |
| Sopirala et al. [12] | - | - |
| Mukherjee et al. [51] | - | - |
| Total | Direct (318,819) Electronic (>34.8 million) |
|
| Range | Direct (255–59,122) Electronic (402,849–20 million) |
|
| Mean | Direct (10,994) Electronic (8.7 million) |
ICU, intensive care unit.
Hand hygiene compliance interventions
The majority of studies involved multi-modal approaches, although 17 (30%) studies used a single intervention (Table V ). Harrabi et al. did not describe the intervention specifically, but referred to their set of HH interventions as ‘diverse activities’ as part of an intervention to reduce HCAIs at a military hospital [57]. In 50 studies (88%), HH was the primary focus of the intervention, while seven studies (12%) [35,38,50,52,[58], [59], [60]] reported HH as part of an intervention focused on another measure. In the study by Kai et al., for example, HH was one element of a six-part intervention named ‘Stop the Contamination’ aimed at reducing infection at the site of venepuncture [50].
Table V.
Intervention types and compliance outcomes
| Study | Year | Type of intervention(s) | No. of interventions | Compliance at baseline | Compliance post intervention (effect increase/decrease) |
|---|---|---|---|---|---|
| Aghdassi et al. [43] | 2020 | Education and training, reminder, infrastructural improvement, feedback | 4 | 59% | 61% (+2%) P=0.03 |
| Apisarnthanarak et al. [55]c | 2015 | Education and training, infrastructural improvement | 2 | 66% | 84% (+18%) P=0.84, 0.02, 0.005 |
| Benudis et al. [66] | 2020 | Reminders | 1 | Clear percentage data could not be extrapolated from the paper which reports that HH compliance improved by 1.3 percentage points per month during the intervention period, P=0.0005 | |
| Beyfus et al. [49] | 2016 | Reminders | 1 | HHOs only (dispenser volume). Average volume dispensed in stations with eyes was 279 cc vs 246 cc in stations without eyes, P=0.009 | |
| Chakravarthy et al. [41] | 2015 | Education and training, reminders, infrastructural improvement, feedback, leadership | 5 | 37% | 82% (+45%) P=0.0001 |
| Chhapola and Brar [79] | 2015 | Education and training, reminders, feedback | 3 | 46% | 69% (+23%) P≤0.0001 |
| Diefenbacher et al. [65] | 2019 | Feedback, leadership, teamwork | 3 | HHOs alone. HH dispenser usage almost doubled during the intervention, and increases were sustained during the post-intervention phase, P=0.004 | |
| Derde et al. [35] | 2014 | Education and training | 1 | 52% | 77% (+25%) P-value not stated |
| Derksen et al. [74] | 2020 | Reminders, infrastructural improvement | 2 | 47% | 95% (+48%) P<0.001 |
| Donati et al. [85] | 2020 | Education and training, reminders, feedback, leadership | 4 | 63% | 77% (+14%) P=0.031 |
| Ellison et al. [48] | 2015 | Reminders | 1 | 26% | 37% (+11%) P<0.001 |
| Farhoudi et al. [88] | 2016 | Education and training, reminders, infrastructural improvement, feedback, leadership | 5 | 30% | 71% (+41%) P<0.001 |
| Fouad and Eltaher. [71] | 2020 | Education and training, feedback | 2 | 31% | 46% (+15%) P<0.01 |
| Fox et al. [81] | 2015 | Education and training, reminders, feedback | 3 | 48% | 75% (+27%) P-value not stated |
| Harrabi et al. [57] b | 2017 | Intervention not described in detail | Intervention not described in detail | 70% | 77% (+7%) P-value not stated |
| Kai et al. [50] | 2020 | Education and training | 1 | 6% | 56% (+50%) P<0.0001 |
| Khan and Nausheen [54] | 2017 | Feedback | 1 | 15% | 81% (+66%) P<0.0001 |
| King et al. [75] | 2016 | Reminders | 2 (sensory and visual cue are two separate reminders) | 15% | 47% (+32%) olfactory prime, P=0.0001; male eyes, P=0.038; female eyes, P=0.626 |
| Kuruno et al. [72] | 2017 | Education and training, feedback | 2 | 16% | 57% (+41%) P<0.05 |
| Labi et al. [40] | 2019 | Education and training, infrastructural improvement, leadershipa | 2 | 29% | 68% (+39%) P<0.0001 |
| Larson et al. [63] | 2018 | Education and training, feedback, leadership, teamwork | 4 | HHOs alone. Increase in number of HH events in one of three sites, P=0.0003. Results for Site 1 were not significant, P=0.59 | |
| Laurikainen et al. [53] | 2016 | Feedback | 1 | HHOs alone. Post intervention showed increases in ABHR dispenser usage in one of three sites, P=0.0003 | |
| Lea et al. [70] | 2016 | Leadership | 1 | 38% | 67% (+29%) P-value not stated |
| Lee et al. [62]b | 2020 | Education and training, leadership, teamwork | 3 | 49% | 66% (+17%) P-value not stated |
| Lytsy et al. [36]b | 2016 | Education and training, feedback | 2 | 78% | 79% (+1%) P-value not stated |
| Marra et al. [73] | 2014 | Reminders, feedback, leadershipa | 2 | HHOs alone. ABHR dispenser usage higher in intervention units. P-values varied by study site, all were significant | |
| Medeiros et al. [89] | 2015 | Education and training, reminders, infrastructural improvement, feedback, leadership | 5 | 27% | 58% (+31%) P=0.0001 |
| Moller-Sorenson et al. [47] | 2016 | Reminders | 1 | 66% | 91% (+25%) P<0.0001 |
| Mu et al. [82] | 2016 | Education and training, infrastructural improvement, feedback, leadership | 4 | 38% | 76% (+38%) P<0.0001 |
| Mukherjee et al. [51] | 2020 | Education and training, feedback, leadership | 3 | 34% | 100% (+66%) P-value not stated |
| Muller et al. [68] | 2014 | Feedback | 1 | 66% | 68% (+2%) P-value not stated |
| Ng et al. [78]b | 2019 | Education and training, infrastructural improvement, leadership | 3 | 64% | 82% (+16%) P=0.01 |
| Nobile et al. [3] | 2018 | Education and training, feedback, leadershipa | 2 | HHOs alone. Decrease in breaches of HH protocol during intervention period, P=0.000 | |
| Nour-Eldein and Ali Mohamed [59] | 2016 | Education and training | 1 | 20% | 50% (+30%) P<0.001 |
| Nyamadzawo et al. [39] | 2020 | Education and training, infrastructural improvement | 2 | 48% | 68% (+20%) P<0.001 |
| Pong et al. [64] | 2018 | Education and training, reminders, feedback, teamwork | 4 | 25% | 63% (+38%) P<0.0001 |
| Reisinger et al. [44]c | 2014 | Reminders | 1 | 34% on room entry, 52% on room exit | 39% on entry into room, P=0.79; 57% on exit from room, P=0.54 |
| Renaudin et al. [60]b | 2017 | Education and training | 1 | 88% | 77% (-11%) P-value not stated |
| Rodrigeuz et al. [87]b | 2015 | Education and training, reminders, infrastructural improvements, feedback, leadership | 5 | 62% | 76% (+14%) P<0.0001 |
| Saharman et al. [84] | 2019 | Education and training, reminders, feedback, leadership | 4 | 27% | 77% (+50%) P<0.001 |
| Santoaningsih et al. [77]b | 2017 | Education and training, leadership | 2 | 16% | 27% (+11%) P<0.001 |
| Schmitz et al. [37] | 2014 | Education and training, reminders, infrastructural improvement, feedback, leadership | 5 | 2% | 12% (+10%) P<0.001 |
| Sopirala et al. [12] | 2014 | Education and training, reminders, feedback, leadership | 4 | 30% | 93% (+63%) P=0.001 |
| Staats [42] | 2017 | Reminders | 1 | HHOs alone. Total dispenser use increased by 62.02%, P<0.0001 | |
| Stevenson et al. [83] | 2014 | Education and training, reminders, infrastructural improvement, feedback | 4 | HHOs alone. HHOs increased by 20.1% compared with 3.1% in control group, baseline not specified, P=0.001 | |
| Stewardson et al. [61] | 2016 | Education and training, reminders, infrastructural improvement, feedback, leadership, teamwork | 5 | 66% | 74% (+8%) P<0.0001 |
| Su et al. [86] | 2015 | Education and training, reminders, infrastructural improvement, feedback, leadership | 5 | 52% | 80% (+28%) P=0.004 |
| Teesing et al. [90] | 2020 | Education and training, reminders, feedback, leadership, teamwork | 5 | 12% | 36% (+14%) P-value not stated |
| Tschudin-Sutter et al. [80] | 2019 | Education and training, reminders, feedback | 3 | HH baseline not clearly stated | |
| van der Kooi et al. [58] | 2018 | Education and training | 1 | 36% | 58% (+22%) P<0.0001 |
| Vander Weg et al. [67]b | 2019 | Reminders | 1 | 56% | 57% (+1%) P=0.14, P=0.06 |
| Visnovsky et al. [69]c | 2019 | Infrastructural improvement (intervention involved reduction in contact precautions) | 1 | HH compliance net change could not be extrapolated. Data not significant for effect when personal protective equipment removed, P=0.07; or for change in intervention group, P=0.29 | |
| von Lengerke et al. [46] | 2017 | Education and training, feedback, leadershipa | 2 | 54% | 70% (+16%) P=0.005 |
| von Lengerke et al. [45] | 2019 | Education and training, feedback, leadershipa | 2 | 54% | 70% (+16%) P=0.005 |
| Wiedenmayer et al. [38] | 2020 | Education and training, reminders, infrastructural improvement, feedback, leadership | 5 | 30% | 56% (+26%) P<0.001 |
| Xiong et al. [56] | 2016 | Education and training | 1 | Records increase in intervention group performance of proper HH technique, P=0.00. Percentage compliance data for HHOs not recorded | |
| Yilmaz et al. [52]b | 2016 | Education and training, feedback | 2 | 28% | 73% (+45%) P<0.001 |
| Mean HH compliance | Mean baseline 41% | Mean post intervention 67% (mean net effect +26%) |
ABHR, alcohol-based hand rub; HH, hand hygiene; HHO, hand hygiene opportunity.
All percentage data have been rounded to the nearest integer. Studies that did not record compliance percentage data are marked as HHO alone. Where data could not be extrapolated from the studies, this is noted in the ‘Compliance post intervention’ column.
Leadership is alluded to in the study but not considered by this review to be part of the intervention. This is discussed further in the Results section under ‘Hand hygiene compliance interventions’.
Data were extrapolated from figures provided in the tables of these papers.
Study was excluded from mean calculations as the results failed to reach significance.
Of the interventions described, education and training were the most common, featuring in 40 studies (70%). Other interventions included performance feedback [featured in 32 studies (56%)], HH reminders [used in 27 studies (47%)], and provision of HH materials and/or infrastructure including ABHR [used in 17 studies (30%)]. Leadership was referred to in 26 studies (46%); however, the significance of leadership as a component of the intervention varied (and, while noted in the individual paragraphs below, is discussed in more detail later in this review). Teamwork was a specific focus of the intervention in five studies (9%) [[61], [62], [63], [64], [65]].
Seventeen studies (30%) used a single intervention. Five of these studies involved the use of a reminder, which was either electronic [47,66] or visual in the form of signage at HH stations [44,49,67]. Benudis et al. incorporated patients into their study by using an electronic bracelet that vibrated if the HCW had not cleaned his/her hands properly within 30 s of entering the patient's bed space, and alerted the patient via a green or red light on the HCW's bracelet that the HCW had or had not cleaned their hands [66]. Three studies (17%) used feedback as their sole intervention. This was provided by staff peers [53,54] or through an electronic device [68]. Two studies (11%) that used a single intervention involved the removal of elements of hygiene procedures to measure the effect of their absence on compliance [60,69]. These included introducing a personal-protective-equipment-free zone [69] or removal of extra patient contact procedures [60]. Education was the sole intervention used in four studies (22%) [35,50,56,59]. These four educational interventions included comparison of multi-media and textbooks for HH education [56], use of a Microsoft PowerPoint presentation with instructions [59], instructional videos [50], and HH training alongside training for ICU staff in chlorhexidine washing of patients [35]. Three studies (17%), each of which had one sole intervention, did not describe their HH intervention clearly [42,58,70]. In two of these studies, the intervention was referred to as part of a broader intervention but not in specific detail [58,70], while Staats noted the use of performance feedback but did not describe how that feedback was provided [42].
Twelve of the 17 studies that employed a single intervention provided percentage data on HH compliance both before and after the intervention. However, the results of two of these studies [44,69] did not reach significance. Of those studies recording percentage data, there were mixed results. Kai et al. [50] and Khan and Nausheen [54] recorded the most successful interventions, with 49% and 66% improvement, respectively. It should be noted, however, that both of these studies started from a low baseline (6% and 15%, respectively). Kai et al. educated junior and senior residents in the emergency department using an instructional video that explained the elements of a hygiene bundle, while Khan and Nausheen used feedback provided by staff peers on HH performance. Of the studies starting from a high baseline, Moller Sorenson et al. [47] achieved an increase in compliance from 66% to 91% using an electronic monitoring system that provided a reminder to perform handwashing after restroom visits, and Derde et al. [35] showed an increase in compliance from 52% to 77% after implementing a HH training programme for ICU staff alongside training in chlorhexidine washing of patients. The worst performing interventions were detailed by Renaudin et al. [60], whereby rates of compliance from 62% to 81% were recorded subsequent to removal of extra patient contact precautions, resulting in an average of 77% and an overall decrease of 11% from a very high baseline of 88%. Muller et al. [68] reported an increase of just 2% from a baseline of 66% using an electronic feedback system, citing lack of real-time feedback, staff distrust of feedback figures, and challenges in understanding the data as reasons for the low level of change.
Sixteen studies (28%) used a combination of two interventions. These included a variety of combinations with no discernible pattern. Education and training interventions included: production and dissemination of training materials [3]; practical HH training in HH skills [3,36,45,46]; education on ABHR usage [39,71]; interventional workshops [71]; and slide presentations [36,72]. In one study, the educational intervention was repeated every 3 months [55]. Fouad and Eltaher [71] provided the most extensive educational intervention within this group of studies. Their intervention was modelled on the WHO ‘Save Lives: Clean your Hands’ campaign and involved staff workshops, PowerPoint presentations and practical training regarding the use of ABHR. In the study by Marra et al., reminders were given through an electronic handwash recording system linked to HCW badges that enabled real-time feedback if handwashing was not performed [73]. Derksen et al. provided reminders using posters highlighting the importance of HH to prevent the spread of COVID-19 [74], while King et al. used two reminders – one olfactory prime using a citrus smell cue and one visual prime involving a photo of eyes placed above gel dispensers [75]. Forms of feedback included were facilitated through electronic systems [48,73] and staff training sessions [52], while the feedback method was not described in one study [40]. Additionally, von Lengerke et al. [45,46], who replicated the same HH data in their two papers included in this review, provided tailored feedback to staff during interviews with a team of clinical managers, medical psychologists and health economists which focused on the reasons for non-compliance. Five of the studies employing two interventions involved improvement of HH infrastructure. Four of these studies involved the provision of ABHR [39,48,55,74], and the remaining study [40] did not state directly what aspect of infrastructure was improved but was linked to the United Nations International Children's Emergency Fund (UNICEF) Water, Sanitation and Hygiene (WASH) campaign highlighting provision of basic sanitation facilities such as sinks and running water [76]. While eight of these studies [3,40,45,46,65,73,74,77] made reference to a leadership component of their intervention, only one study [77] considered it to be a meaningful part of the intervention. Teamwork was included as part of the intervention in one of these studies [65].
Thirteen of the studies that used two interventions provided percentage data on HH compliance, although this was not significant in one study [55]. These studies had a wide range of baseline compliance, ranging from 15% to 48%, and had mixed levels of improvement. The best performing intervention was detailed by Derksen et al. [74], who used posters to raise awareness of the need for HH to prevent COVID-19 and also increased the provision of ABHR, leading to an increase in compliance from a baseline of 47%–95% post intervention. This increase was not sustained, however, and decreased to 79% during the second period of the study. Of note, Nyamadwazo et al. recorded baseline compliance of 48% and emphasized that this figure was much higher than compliance rates typically reported from neighbouring peer African nations, which are often <5% [39]. Nyamadwazo et al. attributed this higher baseline compliance to the fact that their study was performed in a relatively well-resourced urban setting, whereas studies in neighbouring countries were performed in rural settings where access to basic HH infrastructure, such as running water, disinfectants and adequate sink facilities, is less common. They also noted that their baseline compliance figure may have been increased as they only recorded whether or not HH took place, rather than whether full HH was performed.
Six studies (12%) used three interventions [51,62,[78], [79], [80], [81]]. All of these studies used an educational intervention delivered by staff to staff. In the study by Lee et al., staff were identified as HH change agents [62], while Ng et al. [78] used an ultraviolet hand scanner glow germ solution to demonstrate the proper HH technique. In the study by Mukherjee et al., surgical internees attended repeated phases of an educational intervention if they did not use the perfect HH technique. The initial phase used video instruction, while later phases used in-person HH tutorials delivered by surgical consultants [51]. The most common interventions combined with education in this group of studies were reminders and feedback [64,[79], [80], [81]]. Meaningful leadership interventions were implemented in three studies [51,64,78], and teamwork was used as part of the intervention in two studies. Only one study [78] worked to improve the HH infrastructure.
Only five of the studies that used three interventions [51,62,78,79,81] recorded HH compliance data, while one study [80] did not record baseline compliance although a 10.9% improvement was noted post intervention. These studies all started from relatively high baselines, ranging from 24% to 64%. Most had moderate levels of improvement, although Mukherjee et al. eventually recorded 100% compliance with pre-operative HH technique amongst surgical internees after six phases of their intervention!
A further eight studies (14%) used a combination of four interventions [12,43,63,64,[82], [83], [84], [85]]. All of these studies used both education and performance feedback as part of their intervention. Where educational interventions were described in detail, they were provided through video [43], training in HH by the clinical microbiology team [12], interactive lessons [64,84,85] or dissemination of training materials focused on HH technique [63]. One study [83] used a multi-faceted educational intervention including games, videos, poster competitions, demonstrations, lectures, displays and dissemination of educational materials. Performance feedback was derived from staff during active work [43,84], at staff meetings [12], using a whiteboard visible in the staff area [82], or was not described in detail [63,85]. Of note, Stevenson et al. implemented a positive feedback programme through a recognition and awards programme for good HH compliance [83]. Reminders were provided by e-mail [43], delivered at monthly meetings and on bulletin boards [12], or using posters [82,84]. In the study by Pong et al. [64], reminders were delivered through posting information next to handwashing stations, as well as through vibration and light cues on staff ID badges to prompt handwashing and acknowledge when handwashing was recorded by the system. Supply of HH infrastructure, including ABHR, was used in two studies [82]. Five of the eight studies that used a combination of four interventions [12,43,82,84,85] used a meaningful leadership intervention, and two studies involved teamwork as part of their intervention [63,64]. Leadership and teamwork interventions are discussed in more detail at the end of this section. Monitoring [82,85] and interviews [84] were noted as elements of interventions; however, these were not described as discrete interventions.
Of the eight studies that used a combination of four interventions, one [83] did not record both baseline and post-intervention compliance data, and one [63] recorded HHOs alone. The remaining studies captured percentage data for HH compliance at baseline and post intervention. Of note was the study by Mu et al. [82], which reported an improvement in HH compliance from a baseline of 38% - 76% using posters, provision of HH infrastructure such as ABHR, education and training, and feedback using a whiteboard to net a difference of 38%.
Nine studies (16%) involved five interventions [37,38,41,61,[86], [87], [88], [89], [90]]. All of these studies used education and training, HH reminders, HH infrastructure improvement, performance feedback and either teamwork or leadership interventions, except for the study by Teesing et al. [90] which did not enhance the HH infrastructure. It was common amongst this group of studies to use a prescribed framework provided by an accredited external organization, such as the International Nosocomial Infection Control Consortium (INICC) [41,86,89], UNICEF [38] or WHO [61,88], or an adaption of the WHO multi-modal intervention [37,90]. In the studies that used the full WHO multi-modal intervention, reminders were provided using a pocketbook on HH technique provided to staff. Feedback could be given verbally or by a report card [61], and visual cues were provided by posters placed in offices, nurses' stations or HH stations. The level of administrative involvement varied from support via e-mail [61] to recruitment of staff HH champions [37]. Farhoudi et al. noted the creation of a ‘climate of safety’, but this was not described in detail [88]. The educational interventions also varied. For example, while Farhoudi et al. [88] used PowerPoint and HH education forms, other studies used more intensive workshops involving ‘train the trainer’ sessions for infection control personnel. One study [90] involved an e-learning module in combination with staff educational meetings, live tutorials, posters and a staff photo competition. Patient involvement was noted in one study [61], whereby patients were invited to ask HCWs to wash their hands before touching them, although the study did not measure the uptake or effect of this request. Studies that implemented the INICC framework used an identical combination of administrative involvement at infection control meetings, ABHR supply, poster reminders, staff education sessions and monthly feedback from the INICC headquarters staff based on HH compliance figures. Rodriguez et al., who designed their own intervention, were influenced by the WHO ‘Clean Care is Safer Care’ campaign, and differed only slightly from other studies using the WHO framework in that they used more visible involvement of leadership by combining e-mail involvement with a storyboard of the project in a visible area signed by hospital leaders, complemented by active participation of hospital leaders in HH-focused ward rounds [87]. Wiedenmayer et al. [38] implemented HH measures as part of the UNICEF WASH campaign [76], including HH infrastructure improvement, training and education, performance feedback, reminders in the work place and institutional leadership through ‘system change’.
All of the nine studies that used five interventions recorded baseline and post-intervention HH compliance data. While most studies had moderate baseline compliance ranging between 27% and 52%, some studies had higher baseline compliance at 62% [87] and 66% [61], and one study reported very low baseline compliance at 2% [37]. The greatest increases were seen in studies that used the INICC framework (average net effect 35%), whereas studies that employed the WHO frameworks had an average net effect of 18%. It should be noted that Schmitz et al., who used this framework, reported an increase of 11% despite starting with baseline compliance of 2% [37]. The study using the UNICEF intervention [38], derived from the WASH campaign framework, reported an increase of 26%, while Rodriguez et al. [87] reported a net effect of 14%, albeit from a relatively high baseline of 62%.
Leadership per se was discussed in 26 studies (46%). Many of these, however, only included passing references to leadership involvement. Leadership involvement ranged from the management being aware that a study was taking place to leadership demonstrating active participation in the design and implementation of the HH intervention. For this review, only the 18 studies (32%) in which leadership was a key component of the intervention or in which leaders were actively involved in the design or implementation of the intervention were considered, and they are appraised comprehensively below.
In the study by Lea et al., the intervention was designed and implemented by management [70]. Santoaningsih et al. used staff role models who were given active roles in training [77]. Role models received training about the HH intervention through presentations, discussion and practical training. The study by Ng et al. involved religious consultation from local religious leaders as part of a HH education programme that acknowledged the appropriateness of using ABHR for HCWs practising the Muslim faith [78]. Lee et al. appointed change agents to provide education and feedback on the use of proper HH techniques [62]. Leaders were encouraged to overtly correct, educate and congratulate staff on correct procedure. This was not dissimilar to the study by Sopirala et al., which created a programme in which ‘link nurses’ were trained by the clinical microbiology team [12]. These nurses monitored HH and reported hygiene issues to clinical epidemiology physicians, after which the reports were addressed at monthly staff meetings. The link nurses also distributed information to staff at meetings, prepared information bulletin boards, and provided on-the-spot feedback when compliance was breached. The study by Donati et al. also used link nurses who received separate HH training and were appointed as leaders responsible for promoting HH amongst their peer staff group [85]. The study by Larson et al. involved explicit leadership commitment, active staff participation, conducting workflow assessments, training focused on the WHO ‘Five Moments’, and electronic HH monitoring and feedback [63]. Su et al. included administrative feedback to staff as part of their intervention [86]. In the study by Mu et al., monitoring was performed by the department of HAI management who produced quarterly reports on HH [82]. Saharman et al. used role models to encourage staff HH compliance [84]. In the study by Stewardson et al., departmental HH targets were sent by e-mail and signed off by hospital directors, while the intervention also used interviews and focus groups to involve HCWs in designing the intervention [61]. Leaders also showed commitment by attending executive walk-arounds. The study by Rodriguez et al. involved explicit leadership commitment in the form of a signed letter that was circulated to staff, and participation of hospital directors and unit leads in ward rounds oriented towards HH [87]. Schmitz et al. used staff HH champions. HH champions were trained in accordance with the WHO HH observation method, and performed weekly observations of the wards, and provided feedback and encouragement to staff on HH performance [37]. Farhoudi et al. [88] and Weidenmeyer et al. [38] reported that their interventions created a climate of safety throughout the institution. Medeiros et al. included administrative feedback to staff as part of the intervention [89]. Teesing et al. included management meetings in their intervention, and management also provided non-financial incentives to well-performing staff groups [90]. In the study by Mukherjee et al., surgical consultants provided HH tutorials directly to surgical internees [51].
A number of studies lacked clarity regarding the manner in which stated leadership was part of the intervention, although leadership was referred to in the study. For example, although the study by Chavravarthy et al. followed the same INICC multi-modal strategies that were used in the study by Medeiros et al. (i.e. leadership is part of the intervention), Chavravarthy et al. did not expand on the role of administrative support beyond noting that it was included [41]. In the study by Marra et al., the wearing of badges that monitored HH compliance was controlled by nurse managers [73]. The research by von Lengerke et al. [45,46] involved the use of medical psychologists and health economists as part of tailored discussions with health staff to remediate non-compliance. Labi et al. noted the positive effect on HH resulting from a positive attitude to HH amongst hospital leaders [40].
Teamwork per se was discussed in six studies (11%) [[61], [62], [63], [64], [65],90]. Stewardson et al. conducted interviews and focus groups to involve HCWs in study design and intervention [61]. Nobile et al. involved staff as part of the design and implementation of the intervention to ensure active participation, while Teesing et al. included a HH photo competition amongst staff [3,90]. In the study by Diefenbacher et al., goal setting was completed with the involvement of staff at a moderated team session [65]. Pong et al. displayed the HH compliance performance of each nursing unit on a screen visible to participating staff members in order to encourage unit teamwork [64]. Larson et al. noted that staff teams were involved directly in developing an intervention in order to encourage ‘buy-in’ and to individualize feedback [63].
Hand hygiene compliance outcomes
HH compliance was measured either by direct observation or by electronic recording. The observation was based on whether or not the HCW complied with best practice relating to an HHO. Direct observation was either overt or covert, and was performed by varying categories of staff across projects including volunteers, students, clinical staff, administrative staff and infection control personnel. Methods of electronic recording included video camera monitoring [54]; radio frequency feedback linked to a HH station alone [48,63], or a HH station in combination with an employee badge [42,64,73]; a HH station in combination with wearable technology other than badges [66,68]; or an electronic medical record that provided HH reminders [81].
In total, 50 studies (88%) recorded data on compliance, while seven studies (12%) [3,42,49,53,63,65,73] recorded only the number of HHOs. Of the 50 studies that recorded compliance, 34 (68%) directly identified both baseline and post-intervention HH compliance in the intervention group. A further 12 studies (24%) [36,44,52,55,57,60,62,67,69,77,78,87] showed the results in tables, and the authors of this review calculated the appropriate percentage results using the tables provided. These include studies that provided results for individual groups involved in the study but did not give an overall figure. In those cases, the results of all intervention groups involved in the study were added together to obtain overall compliance figures. Three of these studies [44,55,69] did not achieve significance so were excluded from the calculated means discussed below. Four of the 50 studies (8%) that recorded compliance [56,66,80,83] did not record figures for one of either baseline or post-intervention compliance, or recorded compliance with technique alone rather than percentage of completed HHOs. For example, Stevenson et al. did not specify baseline compliance but reported a 20% improvement in compliance [83].
Twenty-three studies (46%) measured baseline compliance in an intervention group as well as in a control group that received either a different intervention or no intervention. Of these 23 studies, 19 compared an intervention group with no intervention. Four studies compared the intervention with another intervention [58,60,80,85]. van der Kooi et al., for example, assigned groups to either a HH intervention, a central venous catheter insertion technique intervention, or both interventions [58].
There was wide variation in baseline compliance, ranging from 2% to 88% (mean 41%). Mean HH compliance post intervention was 67%. The mean net effect of interventions on HH compliance was 26%. Individual figures for each study are shown in Table V.
Discussion
This review appraised clinical trials published between 1st March 2014 and 31st December 2020 that reported HH compliance in the context of reducing HCAIs.
Geographic location
As an economical, accessible and effective method of reducing HAIs and associated cost burdens, HH is of global importance. The geographic spread of the studies included in this review suggests, however, that HH clinical trials performed in the study period were primarily conducted in Asia, Europe and the USA which, together, accounted for 45 of the 57 studies included (79%). Data from full clinical trials focusing on HH are also concentrated on a relatively small minority of the global population. Asia constitutes 60% of the global population [91], yet in this review, studies performed in Asian countries accounted for 26% of studies (N=15), which is almost equal to the number of studies from European states (10% of global population) and only one more than the 14 studies from North America (5% of global population). Of note, these figures differ from a previous review in which studies performed in Asia accounted for a much smaller percentage of the total [1], perhaps highlighting increased awareness of, and clinical interest in, HH in Asian countries. It should be noted that the current review involved a more comprehensive database search (i.e. inclusion of additional sources) than the authors' previous review [1], and this may have influenced this variance.
These trends are more pronounced when other regions are examined. This review identified just three studies (5%) from South America and four studies (7%) from African countries that met the inclusion criteria, corroborating the finding by Schmitz et al. [37] who noted a paucity of clinical HH research in African countries. The reasons for the relative lack of clinical HH research include a lack of basic HH infrastructure, particularly in rural settings [39,40]. Further discussion of the difficulty of HCAI surveillance in developing countries due to the lack of quality laboratory data, standardized medical records and varying quality of facilities can be found in other studies [92].
This review also noted a concentration of HH clinical trials amongst wealthier countries. High- and upper-middle-income countries represent 53% of the global population [91], but studies from these regions accounted for 48 of the 57 (84%) papers included in this review. Baseline HH compliance in high- and upper-middle-income countries also tended to be higher, at 44%, compared with a figure of 29% for lower-middle and lower-income countries. This aligns with previous studies that have reported baseline compliance rates ranging from 5% to 89%, with an average rate of 39% [92]. Baseline compliance also differed by region. The 12 European studies in this review that provided a baseline HH compliance percentage showed average baseline compliance of 57%, which was slightly higher than the value (49%) from 11 European countries in the PROHIBIT study [58]. In comparison, Nyamadwazo et al. [39], whose study was based in Zimbabwe, noted that their baseline compliance of 48% was much higher than figures from studies in neighbouring peer African nations, which commonly record figures close to 5% [37]. Baseline compliance figures in South America were as high as 62% in one study [87] and as low as 27% in another [89].
Collectively, these data suggest a concentration of HH clinical research in wealthier countries. Given that rates of HCAIs are higher in developing countries [93], this geographic focus clearly represents a limitation of HH research to date, and limits the extent to which conclusions can be generalized to developing regions.
Healthcare facility
In total, 801 clinical settings were identified in which HH interventions were performed. While wards accounted for 361 of these facilities, the results may be skewed by a large single study which contributed 207 of these wards [38].
This study supports the view that HAIs are a major study interest in acute care facilities, including both hospital wards and ICUs. This interest is appropriate as data from the European Centre for Disease Control (ECDC) have indicated that the prevalence of patients with HCAIs is highest in intensive care facilities (previously reported as 8% [94] and 6% [4] in general acute care facilities in Europe) [93].
COVID-19 has increased focus on maintaining strong HH compliance, both inside and external to the acute care setting, as well as emphasizing consistent HH compliance throughout the whole organization [95,96]. The inclusion of data from primary care and long-term care facilities is therefore welcomed, as is the inclusion of study data encompassing entire facilities. Specifically, in this review, 38 primary care facilities were featured. Thirty-seven of these were accounted for across two studies [38,40] that originated from African countries as part of the UNICEF WASH campaign. Overall, primary care facilities only featured in three of the included studies (5%); a trend similar to previous reviews [1,97]. Given that HH can be improved at primary care level [98], a greater focus on these facilities is warranted in future research. Twelve studies included data on HH across a whole organization, including a total of 61 facilities.
A notable lack of data regarding long-term care facilities was identified in this review. Only two studies [63,90] were included (4%). Given that the majority of people in long-term care facilities are older adults [99], and that ECDC has reported prevalence of HCAIs of 3% in long-term care facilities [99], more research in this area focusing on elderly patients is warranted in the context of an ageing global population [100].
Healthcare worker category
Reliance on HH to reduce HCAIs requires involvement of all HCWs who interact with patients and patient environments [101]. Further, attitudes, perceptions and knowledge regarding HH, which may vary between different HCW groups [102] or amongst HCWs of differing skill levels [38], have been shown to affect HH compliance rates [52,56,59,84]. Nurses represent the largest group within the healthcare profession; they featured in 44 (77%) studies included in this review and represent the overwhelming majority of participants in those studies that recorded figures. One study showed higher HH compliance in doctors [41], while 10 studies showed higher HH compliance in nurses [45,46,53,61,83,84,[86], [87], [88], [89]] (note that two of these studies [45,46] included the same HH compliance data). Notably, significant data showed lower HH compliance in laboratory staff and pharmacy staff, as well as staff with lower levels of qualification such as medical attendants [38]. von Lengerke et al. [46] suggested that higher compliance in nurses may be due to having more HHOs associated with their duties, leading to enhanced habit formation. As such, it is reasonable to suggest that further research on the factors influencing compliance rates among different professional groups could consider the effectiveness of nurses as role models within professional groups and as drivers for improvement in HH practices [103].
Hand hygiene opportunities
WHO outlines five key moments when HH should be performed [92]. In that context, Labi et al. [40] noted that HH compliance was worst before Moments 1 and 2 (the two steps before touching a patient or beginning a procedure), and best after Moment 5 (after touching a patient's surroundings). The overwhelming majority of studies evaluating the WHO methods recorded HHOs at each of the five moments, except for one study [73] which only recorded whether HH took place during a patient interaction, and one study which adapted the guideline into four moments but covered the same bases [90]. INICC recommended that HH should be performed before patient contact as well as before and after aseptic procedures [104], although studies included in this review using the INICC framework recorded HHOs before patient contact as well as either before aseptic procedures [86,89] or after patient contact [41].
HHOs are the recommended unit for HH analysis. Direct observation by a trained observer [92] provides rich data about the HH event; however, it has limitations, including time and staffing demands, the ability to record only a minority of HH events, and the potential for inducing a Hawthorne effect [105]. For example, one study [41] that used direct observers cited a limited budget as the reason why more information about HHOs could not be recorded. Another method which has been shown to be effective, albeit based on limited data [105], is the use of electronic technology. Two studies in this review used electronic methods to capture 20 million [42] and 13.7 million [48] HHOs. Of the eight studies (14%) included in this review employing electronic technology as part of their intervention [42,48,63,64,66,68,73,81], three [64,66,81] reported moderate to high levels of net effect on HH compliance, one of which started with high baseline compliance [68], and three studies [48,68,73] reported only minor improvements.
Importantly, staff attitudes toward HH recording technology may influence study outcomes. Participants reported negative attitudes to the monitoring devices in two studies [63,66]. Specifically, Larson et al. [63] noted that staff distrusted the accuracy of the collated data, while in the USA [66], there were negative attitudes towards the ‘big brother’-like perception of electronic monitoring, leading to formal withdrawal from the study or sharp decreases in electronic bracelet usage among staff shortly after the study began.
Cost is also an important consideration for adoption of technology-based interventions. Marra et al. [73] discussed a cost of US$50,000 for the design and development of electronic HH monitoring technology for a 20-bed ICU. Larson et al. [63] did not define costs but referred to high costs.
HH technique per se has a large influence on whether HH is effective in preventing microbial transmission [106]. WHO recommends 11 steps for soap and water or eight steps for ABHR [92]. However, only six studies recorded whether the full HH technique was used [3,36,51,53,70,80]. Aside from those using electronic monitors linked to ABHR dispensers, a further five studies recorded whether HH had taken place and whether this had included the use of ABHR [35,59,78,82,90]. The complexity of the technique used may, in itself, have an influence on HH. Tschudin-Sutter et al. [80] reported HH compliance of 76% in a group with a modified three-step version of the WHO HH measures compared with 65% in a group observing a six-step technique.
It is evident that the influence of large multi-national organizations on HH is considerable. Thirty-five studies (61%) used the WHO design directly or a WHO-influenced design. Twenty-one studies (37%) did not use the WHO guidelines in their study design. Of note, only one of the studies that used electronic recording used the WHO guidelines [63].
Hand hygiene compliance interventions
Six broad types of interventions were used in varying formats across all studies. These included: education and training; provision of HH infrastructure; reminders; performance feedback; teamwork interventions; and leadership interventions/administrative support. The varying combinations of interventions, as well as the differing formats used, make it difficult to ascertain the relationship between type of intervention and improved outcomes. Unfortunately, this review is unable to improve understanding of whether HH improvements are enhanced with the number of interventions used [19,97,[107], [108], [109]]. However, this review supports previous evidence that multi-modal approaches to HH are favoured by clinical researchers.
Hand hygiene compliance outcomes
Of the 46 studies that provided both baseline and post-intervention data on HH compliance, three did not reach significance and were excluded from the mean overall calculations [44,55,69], leaving 43 studies. The mean baseline compliance from these studies was 41%. All except one study [60] showed an increase in HH compliance post intervention, ranging from 1% [67] to 66% [54], with the mean net effect being an increase of 26%. Calculation of an overall post-intervention compliance rate for the intervention group resulted in mean compliance of 67%. Crucially, the net effect tended to decrease as baseline compliance increased to a higher range. More specifically, the average net effect in the nine studies with baseline compliance <25% was an increase of 32%; for the 19 studies with baseline compliance between 25% and 49%, the average net effect increased by 35%; for the 13 studies with baseline compliance between 50% and 75%, the average net effect increased by 16%; and for the two studies with baseline compliance >75%, the average net effect increased by 1% [36] and decreased by 11% [60].
Not all of the studies included in this review defined the duration of the control and intervention periods. Of the 52 studies (91%) that did, 32 (56%) were conducted over a period ≤1 year. von Lengerke et al. [45,46] observed the longest baseline period (5 years). Some studies found that their improvements were sustained beyond the study period. Of the studies that provided follow-up data, Chakravarthy et al. [41] provided data for the longest period. They followed a 3-month baseline measurement period with a 4-month intervention period and then an average of 17.2 months of follow-up measurements, with a range of 4–52 months. Peak HH compliance was 90% at 2-year follow-up and decreased to 82% at 3-year follow-up. In light of this, it seems that targeted research on sustainable interventions is needed, and future studies may consider collecting data at intervals following completion of the intervention period to gauge its sustainable efficacy.
Highlighting the reasons for compliance helps to optimize interventions and explain non-adherence. Several studies commented on the reasons why staff did or did not observe the HH protocol. This was often ascertained from staff surveys or was commented on by the authors. For example, the tolerability of ABHR by staff and the aversion to skin irritation was noted in two studies [36,37]. Perceptions about HH also influenced compliance. For example, data from the included studies noted an association between low compliance and lower staff prioritization of HH [57], staff rejection of HH as an effective measure to prevent HH [84], or lack of knowledge about HH amongst HCWs [52,56,59]. In the study by Ng et al. [78], religious beliefs about ABHR and its appropriateness among a largely Muslim staff cohort influenced HH. Other factors contributing to HH were habituation to HH [45,46,63], heightened awareness of microbial transmission during a pandemic [74], staff work load [48], convenience of accessing HH material [39,80] and the quality of feedback [54]. In the study by Lee et al. [62], staff felt obliged to perform HH to avoid disappointing HH leaders, similar to the effect seen with role models in the study by Santosaningsih et al. [77]. Khan and Nausheen noted that meaningful feedback encouraged a sense of competition between departments and helped to achieve HH compliance [54]. Renaudin et al. included a discussion on the effect of personal protective equipment on HH [60], while the improvements in HH relating to punitive sanctions for non-compliance were discussed by Mu et al. [82] and Pong et al. [64].
In conclusion, of the papers reviewed, 39 (68%) were designed using the WHO framework or the INICC framework, attesting to the sizeable influence of multi-national organizations on the landscape of HH clinical research. While this review did not clearly identify trends in outcome associated with multi-modal approaches vs single interventions, all but one study achieved a positive outcome. This leads to the conclusion that both multi-modal and single intervention studies can achieve modest to moderate increases in HH compliance across a variety of healthcare environments. Particularly important areas for future research include the influence of HCWs' perceptions and attitudes regarding HH on compliance, HH behaviours amongst the broader healthcare team, the effectiveness of different intervention types among varying professional groups, and the effect of additional intervention components on outcomes in multi-modal intervention studies. Similarly, greater clarity on the effectiveness and limitations of electronic HH promotion methods is needed to fully harness the potential of technology to reduce HAIs, and expansion of international global HH guidelines to include electronic recording would be welcome. Additionally, more focus is needed on areas lacking in the literature, such as primary care and long-term care facilities, as well as greater geographic diversity. Future studies may also deepen analysis by recording whether HH technique was properly observed and whether improvements were sustained in follow-up assessments. The standardization of intervention methodology, research setting, and the recording of HHO and HH technique would greatly improve understanding of HH and its effect on HCAIs. Overall, HH remains a promising strategy to reduce HCAIs across all healthcare settings.
Conflict of interest statement
None declared.
Funding sources
None.
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committee on human experimentation with the Declaration of Helsinki 1975, as revised in 2008. The authors assert that ethical approval for publication was not required by their local ethics committee as this article used publicly accessible documents as evidence.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.03.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kingston L., O'Connell N.H., Dunne C.P. Hand hygiene-related clinical trials reported since 2010: a systematic review. J Hosp Infect. 2016;92:309–320. doi: 10.1016/j.jhin.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Healthcare Research and Quality . AHRQ; Rockville, MD: 2012. Patient safety primers: healthcare-associated infections. Secondary patient safety primers: healthcare-associated infections.https://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/hais/index.html Available at: [last accessed December 2020] [Google Scholar]
- 3.Nobile M., Conti C., Bastianelli A., Piscitelli A., Calori G.M., Navone P. Promotion of hand hygiene: the experience of the orthopaedic hospital Gaetano Pini-CTO, Milan, Italy. Ann Ig. 2018;30:229–236. doi: 10.7416/ai.2018.2214. [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2013. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals. [Google Scholar]
- 5.World Health Organization . WHO; Geneva: 2009. WHO guidelines for hand hygiene in health care. [Google Scholar]
- 6.Marbus S.D., Schweitzer V.A., Groeneveld G.H., Oosterheert J.J., Schneeberger P.M., van der Hoek W., et al. Incidence and costs of hospitalized adult influenza patients in the Netherlands: a retrospective observational study. Eur J Health Econ. 2020;21:775–785. doi: 10.1007/s10198-020-01172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sendi P., Dräger S., Batzer B., Walser S., Dangel M., Widmer A.F. The financial burden of an influenza outbreak in a small rehabilitation centre. Influenza Other Respir Viruses. 2020;14:72–76. doi: 10.1111/irv.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittet D., Hugonnet S., Harbarth S., Mourouga P., Sauvan V., Touveneau T., et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection control programme. Lancet. 2000;356:1307–1312. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.C., Sheng W.H., Wang J.T., Chang S.C., Lin H.C., Tien K.L., et al. Effectiveness and limitations of hand hygiene promotion on decreasing healthcare-associated infections. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittet D., Sax H., Hugonnet S., Harbarth S. Cost implications of successful hand hygiene promotion. Infect Control Hosp Epidemiol. 2004;25:264–266. doi: 10.1086/502389. [DOI] [PubMed] [Google Scholar]
- 11.Cummings K.L., Anderson D.J., Kaye K.S. Hand hygiene noncompliance and the cost of hospital-acquired methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31:357–364. doi: 10.1086/651096. [DOI] [PubMed] [Google Scholar]
- 12.Sopirala M., Yahle-Dunbar L., Smyer J., Wellington L., Dickman J., Zikri N., et al. Infection control link nurse program: an interdisciplinary approach in targeting health care-acquired infection. Am J Infect Control. 2014;42:353–359. doi: 10.1016/j.ajic.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons B.P. CDC guidelines for the prevention and control of nosocomial infections. Guideline for hospital environmental control. Am J Infect Control. 1983;11:97–120. doi: 10.1016/0196-6553(83)90122-0. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . WHO; Geneva: 2005. Global patient safety challenge: 2005–2006. [Google Scholar]
- 15.Pittet D., Allegranzi B., Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4–10. doi: 10.1016/j.jiph.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Secondary tools for institutional safety climate. WHO; Geneva: 2009. Tools for institutional safety climate.https://www.who.int/gpsc/5may/tools/safety_climate/en/ Available at: [last accessed December 2020] [Google Scholar]
- 17.Naikoba S., Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers – a systematic review. J Hosp Infect. 2001;47:173–180. doi: 10.1053/jhin.2000.0882. [DOI] [PubMed] [Google Scholar]
- 18.Ellingson K., Haas J.P., Aiello A.E., Kusek L., Maragakis L.L., Olmsted R.N., et al. Strategies to prevent healthcare-associated infections through hand hygiene. Infect Control Hosp Epidemiol. 2014;35(Suppl. 2):S155–S178. doi: 10.1017/s0899823x00193900. [DOI] [PubMed] [Google Scholar]
- 19.Schweizer M.L., Reisinger H.S., Ohl M., Formanek M.B., Blevins A., Ward M.A., et al. Searching for an optimal hand hygiene bundle: a meta-analysis. Clin Infect Dis. 2014;58:248–259. doi: 10.1093/cid/cit670. [DOI] [PubMed] [Google Scholar]
- 20.Gould D.J., Moralejo D., Drey N., Chudleigh J.H. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev. 2010;9:CD005186. doi: 10.1002/14651858.CD005186.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Pittet D. Improving adherence to hand hygiene practice: a multidisciplinary approach. Emerg Infect Dis. 2001;7:234–240. doi: 10.3201/eid0702.010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huis A., van Achterberg T., de Bruin M., Grol R., Schoonhoven L., Hulscher M. A systematic review of hand hygiene improvement strategies: a behavioural approach. Implement Sci. 2012;7:92. doi: 10.1186/1748-5908-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerr D.M., Allpress A.L., Heath J., Bornemann R., Bennett E. Decreasing hospital-associated rotavirus infection: a multidisciplinary hand hygiene campaign in a children's hospital. Pediatr Infect Dis J. 2005;24:397–403. doi: 10.1097/01.inf.0000160944.14878.2b. [DOI] [PubMed] [Google Scholar]
- 24.Doron S.I., Kifuji K., Hynes B.T., Dunlop D., Lemon T., Hansjosten K., et al. A multifaceted approach to education, observation, and feedback in a successful hand hygiene campaign. Jt Comm J Qual Patient Saf. 2011;37:3–10. doi: 10.1016/s1553-7250(11)37001-8. [DOI] [PubMed] [Google Scholar]
- 25.Snow M., White G.L., Alder S.C., Stanford J.B. Mentor's hand hygiene practices influence student's hand hygiene rates. Am J Infect Control. 2006;34:18–24. doi: 10.1016/j.ajic.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Erasmus V., Brouwer W., van Beeck E.F., Oenema A., Daha T.J., Richardus J.H., et al. A qualitative exploration of reasons for poor hand hygiene among hospital workers: lack of positive role models and of convincing evidence that hand hygiene prevents cross-infection. Infect Control Hosp Epidemiol. 2009;30:415–419. doi: 10.1086/596773. [DOI] [PubMed] [Google Scholar]
- 27.Aboumatar H., Ristaino P., Davis R.O., Thompson C.B., Maragakis L., Cosgrove S., et al. Infection prevention promotion program based on the PRECEDE model: improving hand hygiene behaviors among healthcare personnel. Infect Control Hosp Epidemiol. 2012;33:144–151. doi: 10.1086/663707. [DOI] [PubMed] [Google Scholar]
- 28.Talbot T.R., Johnson J.G., Fergus C., Domenico J.H., Schaffner W., Daniels T.L., et al. Sustained improvement in hand hygiene adherence: utilizing shared accountability and financial incentives. Infect Control Hosp Epidemiol. 2013;34:1129–1136. doi: 10.1086/673445. [DOI] [PubMed] [Google Scholar]
- 29.O'Boyle C.A., Henly S.J., Duckett L.J. Nurses' motivation to wash their hands: a standardized measurement approach. Appl Nurs Res. 2001;14:136–145. doi: 10.1053/apnr.2001.24412. [DOI] [PubMed] [Google Scholar]
- 30.Jenner E.A., Fletcher B.C., Watson P., Jones F.A., Miller L., Scott G.M. Discrepancy between self-reported and observed hand hygiene behaviour in healthcare professionals. J Hosp Infect. 2006;63:418–422. doi: 10.1016/j.jhin.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Sax H., Uçkay I., Richet H., Allegranzi B., Pittet D. Determinants of good adherence to hand hygiene among healthcare workers who have extensive exposure to hand hygiene campaigns. Infect Control Hosp Epidemiol. 2007;28:1267–1274. doi: 10.1086/521663. [DOI] [PubMed] [Google Scholar]
- 32.Maskerine C., Loeb M. Improving adherence to hand hygiene among health care workers. J Contin Educ Health Prof. 2006;26:244–251. doi: 10.1002/chp.77. [DOI] [PubMed] [Google Scholar]
- 33.Sladek R.M., Bond M.J., Phillips P.A. Why don't doctors wash their hands? A correlational study of thinking styles and hand hygiene. Am J Infect Control. 2008;36:399–406. doi: 10.1016/j.ajic.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 34.World Bank. World Bank country and lending groups. Washington, DC: World Bank; n.d. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [last accessed November 2020].
- 35.Derde L.P.G., Cooper B.S., Goossens H., Malhotra-Kumar S., Willems R.J.L., Gniadkowski M., et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lytsy B., Melbarde-Kelmere A., Hambraeus A., Liubimova A., Aspevall O. A joint, multilateral approach to improve compliance with hand hygiene in 4 countries within the Baltic region using the World Health Organization's Save Lives: Clean Your Hands model. Am J Infect Control. 2016;44:1208–1213. doi: 10.1016/j.ajic.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz K., Kempker R.R., Tenna A., Stenehjem E., Abebe E., Tadesse L., et al. Effectiveness of a multimodal hand hygiene campaign and obstacles to success in Addis Ababa, Ethiopia. Antimicrob Resist Infect Control. 2014;3:8. doi: 10.1186/2047-2994-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiedenmayer K., Msamba V.S., Chilunda F., Kiologwe J.C., Seni J. Impact of hand hygiene intervention: a comparative study in health care facilities in Dodoma region, Tanzania using WHO methodology. Antimicrob Resist Infect Control. 2020;9:80. doi: 10.1186/s13756-020-00743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyamadzawo A., Nishio J., Okada S., Nyamakura R. Effect of using portable alcohol-based handrub on nurses' hand hygiene compliance and nasal carriage of Staphylococcus aureus in a low-income health setting. Am J Infect Control. 2020;48:473–479. doi: 10.1016/j.ajic.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Labi A., Obeng-Nkrumah N., Nuertey B.D., Issahaku S., Ndiaye N.F., Baffoe P., et al. Hand hygiene practices and perceptions among healthcare workers in Ghana: a WASH intervention study. J Infect Dev Ctries. 2019;13:1076–1085. doi: 10.3855/jidc.11045. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarthy M., Myatra S.N., Rosenthal V.D., Udwadia F.E., Gokul B.N., Divatia J.V. The impact of the International Nosocomial Infection Control Consortium (INICC) multicenter, multidimensional hand hygiene approach in two cities of India. J Infect Public Health. 2015;8:177–186. doi: 10.1016/j.jiph.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Staats B.R. Motivating process compliance through individual electronic monitoring: an empirical examination of hand hygiene in healthcare. Manag Sci. 2016;63:1271–1656. [Google Scholar]
- 43.Aghdassi S.J.S., Schröder C., Lemke E., Behnke M., Fliss P.M., Plotzki C., Wenk J. A multimodal intervention to improve hand hygiene compliance in peripheral wards of a tertiary care university centre: a cluster randomised controlled trial. Antimicrob Resist Infect Control. 2020;9:113. doi: 10.1186/s13756-020-00776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reisinger H.S., Perencevich E.N., Morgan D.J., Forrest G.N., Shardell M., Schweizer M.L. Improving hand hygiene compliance with point-of-use reminder signs designed using theoretically grounded messages. Infect Control Hosp Epidemiol. 2014;35:593–594. doi: 10.1086/675827. [DOI] [PubMed] [Google Scholar]
- 45.von Lengerke T., Ebadi E., Schock B., et al. Impact of psychologically tailored hand hygiene interventions on nosocomial infections with multidrug-resistant organisms: results of the cluster-randomized controlled trial PSYGIENE. Antimicrob Resist Infect Control. 2019;8:56. doi: 10.1186/s13756-019-0507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Lengerke T., Ebadi E., Schock B., Krauth C., Lange K., Stahmeyer J.T. Promoting hand hygiene compliance. Dtsch Arztebl Int. 2017;114:29–36. doi: 10.3238/arztebl.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moller-Sorensen H., Korshin A., Mogensen T., Hoiby N. New technology markedly improves hand-hygiene performance among healthcare workers after restroom visits. J Hosp Infect. 2016;92:337–339. doi: 10.1016/j.jhin.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Ellison R.T., Barysauskas C.M., Rundensteiner E.A., Wang D., Barton B. A prospective controlled trial of an electronic hand hygiene reminder system. Open Forum Infect Dis. 2015;2 doi: 10.1093/ofid/ofv121. :ofv121-ofv21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beyfus T.A., Dawson N.L., Danner C.H., Rawal B., Gruber P.E., Petrou S.P. The use of passive visual stimuli to enhance compliance with handwashing in a perioperative setting. Am J Infect Control. 2016;44:496–499. doi: 10.1016/j.ajic.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 50.Kai M., Miyamoto K., Akamatsu K., Tsujita A., Nishio M. Effect of a bundle-approach intervention against contamination of blood culture in the emergency department. J Infect Chemother. 2020;26:785–789. doi: 10.1016/j.jiac.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee R., Roy P., Parik M. Achieving perfect hand washing: an audit cycle with surgical internees. Indian J Surg. 2020 doi: 10.1007/s12262-020-02619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yilmaz G., Aydin H., Aydin M., Saylan S., Ulusoy H., Koksal I. Staff education aimed at reducing ventilator-associated pneumonia. J Med Microbiol. 2016;65:1378–1384. doi: 10.1099/jmm.0.000368. [DOI] [PubMed] [Google Scholar]
- 53.Laurikainen E., Rintala E., Kaarto A.M., Routamaa M. Adherence to surgical hand rubbing directives in a hospital district of Southwest Finland. Infect Dis. 2016;48:116–121. doi: 10.3109/23744235.2015.1089591. [DOI] [PubMed] [Google Scholar]
- 54.Khan A., Nausheen S. Compliance of surgical hand washing before surgery: role of remote video surveillance. J Pak Med Assoc. 2017;67:92–96. [PubMed] [Google Scholar]
- 55.Apisarnthanarak A., Eiamsitrakoon T., Mundy L.M. Behavior-based interventions to improve hand hygiene adherence among intensive care unit healthcare workers in Thailand. Infect Control Hosp Epidemiol. 2015;36:517–521. doi: 10.1017/ice.2015.1. [DOI] [PubMed] [Google Scholar]
- 56.Xiong P., Zhang J., Wang X., Wu T.L., Hall B.J. Effects of a mixed media education intervention program on increasing knowledge, attitude, and compliance with standard precautions among nursing students: a randomized controlled trial. Am J Infect Control. 2017;45:389–395. doi: 10.1016/j.ajic.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrabi I., Al-Ghamdi S., Cubelo P. Effectiveness of an intervention program to improve compliance with hand hygiene among health staff in NAFH. J Health Allied Sci NU. 2017;7:16–20. [Google Scholar]
- 58.van der Kooi T., Sax H., Pittet D., van Dissel J., van Benthem B., Walder B., et al. Prevention of hospital infections by intervention and training (PROHIBIT): results of a pan-European cluster-randomized multicentre study to reduce central venous catheter-related bloodstream infections. Intensive Care Med. 2018;44:48–60. doi: 10.1007/s00134-017-5007-6. [DOI] [PubMed] [Google Scholar]
- 59.Nour-Eldein H., Ali Mohamed R. Effect of education intervention on prevention of bloodborne infections for health care workers in family medicine centers, Suez Canal University in Ismailia City, Egypt. Middle East. J Fam Med. 2016;7 [Google Scholar]
- 60.Renaudin L., Llorens M., Goetz C., Gette S., Citro V., Poulain S., et al. Impact of discontinuing contact precautions for MRSA and ESBLE in an intensive care unit: a prospective noninferiority before and after study. Infect Control Hosp Epidemiol. 2017;38:1342–1350. doi: 10.1017/ice.2017.196. [DOI] [PubMed] [Google Scholar]
- 61.Stewardson A.J., Sax H., Gayet-Ageron A., Touveneau S., Longtin Y., Zingg W., et al. Enhanced performance feedback and patient participation to improve hand hygiene compliance of health-care workers in the setting of established multimodal promotion: a single-centre, cluster randomised controlled trial. Lancet Infect Dis. 2016;16:1345–1355. doi: 10.1016/S1473-3099(16)30256-0. [DOI] [PubMed] [Google Scholar]
- 62.Lee Y.F., McLaws M.L., Ong L.M., Amir Husin S., Chua H.H., Wong S.Y., et al. Hand hygiene promotion delivered by change agents – two attitudes, similar outcome. Infect Control Hosp Epidemiol. 2020;41:273–279. doi: 10.1017/ice.2019.339. [DOI] [PubMed] [Google Scholar]
- 63.Larson E.L., Murray M.T., Cohen B., Simpser E., Pavia M., Jackson O., et al. Behavioral interventions to reduce infections in pediatric long-term care facilities: the Keep It Clean for Kids Trial. Behav Med. 2018;44:141–150. doi: 10.1080/08964289.2017.1288607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pong S., Holliday P., Fernie G. Effect of electronic real-time prompting on hand hygiene behaviors in health care workers. Am J Infect Control. 2018;46:768–774. doi: 10.1016/j.ajic.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 65.Diefenbacher S., Fliss P.M., Tatzel J., Wenk J., Keller J. A quasi-randomized controlled before–after study using performance feedback and goal setting as elements of hand hygiene promotion. J Hosp Infect. 2019;101:399–407. doi: 10.1016/j.jhin.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Benudis A., Stone S., Sait A.S., Mahoney I., Price L.L., Moreno-Koehler A., et al. Pitfalls and unexpected benefits of an electronic hand hygiene monitoring system. Am J Infect Control. 2019;47:1102–1106. doi: 10.1016/j.ajic.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Vander Weg M.W., Perencevich E.N., O'Shea A.M.J., Jones M.P., Vaughan Sarrazin M.S., Franciscus C.L., et al. Effect of frequency of changing point-of-use reminder signs on health care worker hand hygiene adherence: a cluster randomized clinical trial. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller M.P., Levchenko A.I., Ing S., Pong S.M., Fernie G.R. Electronic monitoring of individual healthcare workers' hand hygiene event rate. Infect Control Hosp Epidemiol. 2014;35:1189–1192. doi: 10.1086/677631. [DOI] [PubMed] [Google Scholar]
- 69.Visnovsky L.D., Zhang Y., Leecaster M.K., Safdar N., Barko L., Haroldsen C., et al. Effectiveness of a multisite personal protective equipment (PPE)-free zone intervention in acute care. Infect Control Hosp Epidemiol. 2019;40:761–766. doi: 10.1017/ice.2019.111. [DOI] [PubMed] [Google Scholar]
- 70.Lea Y. Application of PDCA cycle in the management of medical staff hand hygiene in community hospitals. Acta Med Mediterr. 2016;32 [Google Scholar]
- 71.Fouad M., Eltaher S. Hand hygiene initiative: comparative study of pre- and postintervention outcomes. East Mediterr Health J. 2020;26:198–205. doi: 10.26719/2020.26.2.198. [DOI] [PubMed] [Google Scholar]
- 72.Kuruno N., Kasahara K., Mikasa K. Hand hygiene compliance in a universal gloving setting. Am J Infect Control. 2017;45:830–834. doi: 10.1016/j.ajic.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 73.Marra A.R., Sampaio Camargo T.Z., Magnus T.P., Blaya R.P., Dos Santos G.B., Guastelli L.R., et al. The use of real-time feedback via wireless technology to improve hand hygiene compliance. Am J Infect Control. 2014;42:608–611. doi: 10.1016/j.ajic.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Derksen C., Keller F.M., Lippke S. Obstetric healthcare workers' adherence to hand hygiene recommendations during the COVID-19 pandemic: observations and social-cognitive determinants. Appl Psychol Health Well Being. 2020;12:1286–1305. doi: 10.1111/aphw.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.King D., Vlaev I., Everett-Thomas R., Fitzpatrick M., Darzi A., Birnbach D.J. ‘Priming’ hand hygiene compliance in clinical environments. Health Psychol. 2016;35:96–101. doi: 10.1037/hea0000239. [DOI] [PubMed] [Google Scholar]
- 76.United Nations International Children's Emergency Fund . UNICEF; New York: 2016. Strategy for water, sanitation and hygiene 2016–2030. [Google Scholar]
- 77.Santosaningsih D., Erikawati D., Santoso S., Noorhamdani N., Ratridewi I., Candradikusuma D., et al. Intervening with healthcare workers' hand hygiene compliance, knowledge, and perception in a limited-resource hospital in Indonesia: a randomized controlled trial study. Antimicrob Resist Infect Control. 2017;6:23. doi: 10.1186/s13756-017-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ng W.K., Shaban R.Z., van de Mortel T. The effect of a hand hygiene program featuring tailored religion-relevant interventions on healthcare workers' hand rubbing compliance and beliefs in the United Arab Emirates: a cohort study. Infect Dis Health. 2019;24:115–123. doi: 10.1016/j.idh.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Chhapola V., Brar R. Impact of an educational intervention on hand hygiene compliance and infection rate in a developing country neonatal intensive care unit. Int J Nurs Pract. 2015;21:486–492. doi: 10.1111/ijn.12283. [DOI] [PubMed] [Google Scholar]
- 80.Tschudin-Sutter S., Sepulcri D., Dangel M., Ulrich A., Frei R., Widmer A.F. Simplifying the World Health Organization protocol: 3 steps versus 6 steps for performance of hand hygiene in a cluster-randomized trial. Clin Infect Dis. 2019;69:614–620. doi: 10.1093/cid/ciy948. [DOI] [PubMed] [Google Scholar]
- 81.Fox C., Wavra T., Drake D.A., Mulligan D., Bennett Y.P., Nelson C., et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses' hand washing. Am J Crit Care. 2015;24:216–224. doi: 10.4037/ajcc2015898. [DOI] [PubMed] [Google Scholar]
- 82.Mu X., Xu Y., Yang T., Zhang J., Wang C., Liu W., et al. Improving hand hygiene compliance among healthcare workers: an intervention study in a hospital in Guizhou Province, China. Braz J Infect Dis. 2016;20:413–418. doi: 10.1016/j.bjid.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stevenson K.B., Searle K., Curry G., Boyce J.M., Harbarth S., Stoddard G.J., et al. Infection control interventions in small rural hospitals with limited resources: results of a cluster-randomized feasibility trial. Antimicrob Resist Infect Control. 2014;3:10. doi: 10.1186/2047-2994-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saharman Y.R., Aoulad Fares D., El-Atmani S., Sedono R., Aditianingsih D., Karuniawati A., et al. A multifaceted hand hygiene improvement program on the intensive care units of the National Referral Hospital of Indonesia in Jakarta. Antimicrob Resist Infect Control. 2019;8:93. doi: 10.1186/s13756-019-0540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donati D., Miccoli G.A., Cianfrocca C., Di Stasio E., De Marinis M.G., Tartaglini D. Effectiveness of implementing link nurses and audits and feedback to improve nurses' compliance with standard precautions: a cluster randomized controlled trial. Am J Infect Control. 2020;48:1204–1210. doi: 10.1016/j.ajic.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 86.Su D., Hu B., Rosenthal V.D., Li R., Hao C., Pan W., et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach in five intensive care units in three cities of China. Public Health. 2015;129:979–988. doi: 10.1016/j.puhe.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez V., Giuffre C., Villa S., Almada G., Prasopa-Plaizier N., Gogna M., et al. A multimodal intervention to improve hand hygiene in ICUs in Buenos Aires, Argentina: a stepped wedge trial. Int J Qual Health Care. 2015;27:405–411. doi: 10.1093/intqhc/mzv065. [DOI] [PubMed] [Google Scholar]
- 88.Farhoudi F., Sanaei Dashti A., Hoshangi Davani M., Ghalebi N., Sajadi G., Taghizadeh R. Impact of WHO Hand Hygiene Improvement Program implementation: a quasi-experimental trial. Biomed Res Int. 2016;2016:7026169. doi: 10.1155/2016/7026169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medeiros E.A., Grinberg G., Rosenthal V.D., Bicudo Angelieri D., Buchner Ferreira I., Bauer Cechinel R., et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach in 3 cities in Brazil. Am J Infect Control. 2015;43:10–15. doi: 10.1016/j.ajic.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Teesing G.R., Erasmus V., Nieboer D., Petrignani M., Koopmans M.P.G., Vos M.C., et al. Increased hand hygiene compliance in nursing homes after a multimodal intervention: a cluster randomized controlled trial (HANDSOME) Infect Control Hosp Epidemiol. 2020;41:1169–1177. doi: 10.1017/ice.2020.319. [DOI] [PubMed] [Google Scholar]
- 91.World Bank. Population, total. Washington, DC: World Bank: n.d. Available at: https://data.worldbank.org/indicator/SP.POP.TOTL [last accessed October 2020].
- 92.World Health Organization . Clean Care Is Safer Care. Comparison of national and sub-national guidelines for hand hygiene. WHO; Geneva: 2009. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge. [PubMed] [Google Scholar]
- 93.Rosenthal V.D. Health-care-associated infections in developing countries. Lancet. 2011;377:186–188. doi: 10.1016/S0140-6736(10)62005-3. [DOI] [PubMed] [Google Scholar]
- 94.European Centre for Disease Prevention and Control . ECDC annual epidemiological report for 2017. ECDC; Stockholm: 2019. Healthcare-associated infections acquired in intensive care units. [Google Scholar]
- 95.World Health Organization . WHO; Geneva: 2020. COVID-19 strategy update. [Google Scholar]
- 96.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2020. COVID-19 infection prevention and control measures for primary care, including general practitioner practices, dental clinics and pharmacy settings: first update. [Google Scholar]
- 97.Gould D.J., Moralejo D., Drey N., Chudleigh J.H., Taljaard M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev. 2017;9:CD005186. doi: 10.1002/14651858.CD005186.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martín-Madrazo C., Soto-Díaz S., Cañada-Dorado A., Salinero-Fort M.A., Medina-Fernández M., Carrillo de Santa Pau E., et al. Cluster randomized trial to evaluate the effect of a multimodal hand hygiene improvement strategy in primary care. Infect Control Hosp Epidemiol. 2012;33:681–688. doi: 10.1086/666343. [DOI] [PubMed] [Google Scholar]
- 99.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2014. Point prevalence survey of healthcare-associated infections and antimicrobial use in European long-term care facilities. [Google Scholar]
- 100.United Nations . United Nations; New York: 2017. World population ageing 2017 – highlights. ST/ESA/SER.A/397. [Google Scholar]
- 101.Mannion R., Davies H. Understanding organisational culture for healthcare quality improvement. BMJ. 2018;363 doi: 10.1136/bmj.k4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kingston L.M., O'Connell N.H., Dunne C.P. A comparative study of hand hygiene and alcohol-based hand rub use among Irish nursing and medical students. Nurse Educ Today. 2018;63:112–118. doi: 10.1016/j.nedt.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 103.Haessler S., Bhagavan A., Kleppel R., Hinchey K., Visintainer P. Getting doctors to clean their hands: lead the followers. BMJ Qual Saf. 2012;21:499–502. doi: 10.1136/bmjqs-2011-000396. [DOI] [PubMed] [Google Scholar]
- 104.Rosenthal V.D., Maki D.G., Graves N. The International Nosocomial Infection Control Consortium (INICC): goals and objectives, description of surveillance methods, and operational activities. Am J Infect Control. 2008;36:e1–12. doi: 10.1016/j.ajic.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Boyce J.M. Measuring healthcare worker hand hygiene activity: current practices and emerging technologies. Infect Control Hosp Epidemiol. 2011;32:1016–1028. doi: 10.1086/662015. [DOI] [PubMed] [Google Scholar]
- 106.Widmer A.E., Dangel M. Alcohol-based handrub: evaluation of technique and microbiological efficacy with international infection control professionals. Infect Control Hosp Epidemiol. 2004;25:207–209. doi: 10.1086/502379. [DOI] [PubMed] [Google Scholar]
- 107.World Health Organization . WHO; Geneva: 2018. WHO advocacy toolkit: ‘Save lives: clean your hands’. [Google Scholar]
- 108.Rosenthal V.D., Bijie H., Maki D.G., Mehta Y., Apisarnthanarak A., Medeiros E.A., et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am J Infect Control. 2012;40:396–407. doi: 10.1016/j.ajic.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 109.Rosenthal V.D. International Nosocomial Infection Control Consortium (INICC) resources: INICC multidimensional approach and INICC surveillance online system. Am J Infect Control. 2016;44:e81–90. doi: 10.1016/j.ajic.2016.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.