Abstract
Background
Annual lung cancer screening (LCS) with low-dose chest computed tomography for high-risk individuals reduces lung cancer mortality, with greater reduction observed in Black participants in clinical trials. While racial disparities in lung cancer mortality exist, less is known about disparities in LCS participation. We conducted a systematic review to explore LCS participation in Black compared with White patients in the USA.
Methods
A systematic review was conducted through a search of published studies in MEDLINE, PubMed, EMBASE, Web of Science, and Cumulative Index to Nursing and Allied-Health Literature Database, from database inception through October 2020. We included studies that examined rates of LCS participation and compared rates by race. Studies were pooled using random-effects meta-analysis.
Results
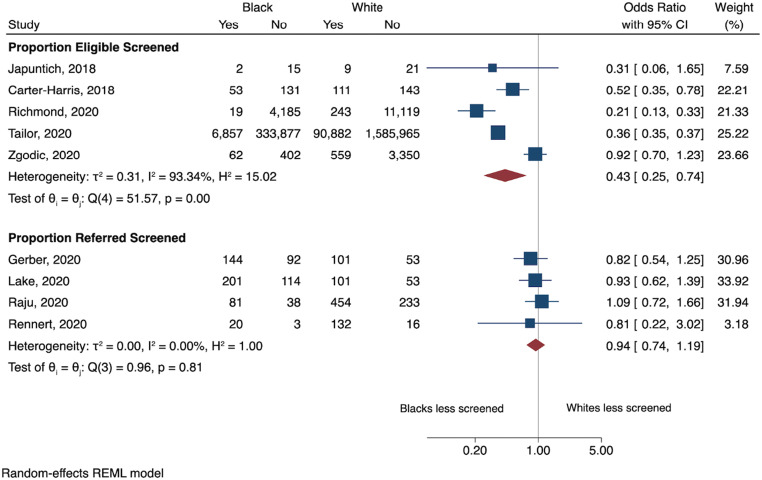
We screened 18,300 titles/abstracts; 229 studies were selected for full-text review, of which nine studies met inclusion criteria. Studies were categorized into 2 groups: studies that reported the screening rate among an LCS-eligible patient population, and studies that reported the screening rate among a patient population referred for LCS. Median LCS participation rates were 14.4% (range 1.7 to 62.6%) for eligible patient studies and 68.5% (range 62.6 to 88.8%) for referred patient studies. The meta-analyses showed screening rates were lower in the Black compared to White population among the LCS-eligible patient studies ([OR]=0.43, [95% CI: 0.25, 0.74]). However, screening rates were the same between Black and White patients in the referred patient studies (OR=0.94, [95% CI: 0.74, 1.19]).
Discussion
Black LCS-eligible patients are being screened at a lower rate than White patients but have similar rates of participation once referred. Differences in referrals by providers may contribute to the racial disparity in LCS participation. More studies are needed to identify barriers to LCS referral and develop interventions to increase provider awareness of the importance of LCS in Black patients.
Trial Registry
PROSPERO; No.: CRD42020214213; URL: http://www.crd.york.ac.uk/PROSPERO
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07613-2.
KEY WORDS: lung cancer screening, meta-analysis, race, systematic review
INTRODUCTION
The burden of lung cancer mortality in the United States (US) is unevenly distributed, with Black males having the highest rate of age-adjusted lung cancer incidence and increased mortality1. The Surveillance, Epidemiology, and End Results (SEER) program registry has shown higher standardized lung cancer case-fatality rates for Black compared to White patients with early-stage lung cancer2. This is thought to be due to multiple factors including disproportionate excess risk of lung cancer from tobacco use, tumor biology with tendency for more aggressive disease, and, importantly, inequities in healthcare access leading to substandard care in both screening and treatment of lung cancer3. Annual lung cancer screening (LCS) with low-dose chest computed tomography (LDCT) in high-risk patients reduces lung cancer mortality4, with greater mortality reduction observed in Black participants in the National Lung Screening Trial (NLST)5. This trial subsequently became the foundation for the original LCS eligibility criteria released in 2013 by the United States Preventive Service Task Force (USPSTF): adults ages 55 to 80 years with at least a 30-pack-year smoking history, who are actively smoking or quit within the last 15 years 6.
However, there was increasing evidence that the 2013 USPSTF criterion of “high-risk patients” did not align with lung cancer risk in the Black population and thus missed a significant cohort of at-risk patients who could benefit from LCS7,8. Black patients who developed lung cancer often smoked fewer than the minimum smoking threshold with an overall shorter smoking history compared to White patients9,10, and presented at a younger age7. The recent decision by the USPSTF to expand LCS eligibility by lowering the age minimum to 50 years and minimum pack-year smoking history to 20 pack-years reflects the ongoing efforts to address this variability in lung cancer risk by race11. Thus, while LCS with LDCT has the potential to bring equity to the lung cancer burden in the Black patient population, the expanded screening criteria will only yield a benefit in lung cancer mortality if LCS is adopted by a large and diverse patient population.
As a relatively new cancer screening practice in the US, the process of LCS with LDCT has not yet become as ubiquitous or streamlined as a preventive care measure compared to colonoscopy for colorectal cancer screening or mammography for breast cancer screening. Overall, there has been low uptake of LCS in the US, with nationwide screening rates of only 2.0 to 3.9% among eligible adults12. Low participation may in part be related to the complexity of cancer screening. All cancer screening is a multi-step process which requires (1) identification of eligible patients, (2) shared decision making about the screening process between the provider and patient, (3) referral for the screening test by a provider, (4) completion of the screening test by the patient, and (5) ongoing participation in subsequent follow-up studies. Each step presents with unique challenges driven by provider or patient factors, which may serve as potential targets for intervention.
Little is known about racial disparities in LCS participation and behaviors in clinical practice, in part due to low rates of racial and ethnic minorities in the large clinical trials. In the NLST, Black individuals constituted only 4.5% of the study cohort despite making up 14% of the US population13,14. While there is increasing recognition that Black patients have lower participation in LCS15, it is not yet clear where in the cancer screening process the disparities become manifest. Given the greater potential for LCS to reduce lung cancer mortality in Black patients, it is important to understand racial disparities in LCS participation, as well as where in the cancer screening process this occurs. We performed a systematic review to explore LCS participation among Black compared with White patients in the US.
METHODS
Protocol
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines16. The protocol was published (PROSPERO: CRD42020214213) prior to commencing the review.
Eligibility Criteria
The Patient-Intervention-Comparator-Outcome-Study Design (PICO) criteria were used to determine eligibility of the articles based on the type of study design, type of population, type of exposure, and outcome. All observational and randomized clinical trials were eligible. Articles not from a clinical study (editorials, narrative reviews) were excluded. We focused our review on studies which examined rates of LDCT acquisition for LCS and included race as a demographic factor. Given the limited availability of literature including race beyond White and Black or African American race, analysis was restricted to studies which included Black race identified as follows: Black, African American, Non-Hispanic Black, and Non-White/Caucasian (majority of the Non-White population were Black).
Data Sources and Searches
The literature search was conducted in collaboration with a clinical librarian (AB) using a combination of free text and index terms focusing on three concepts: lung cancer, screening with low-dose CT scan, and uptake (receipt of screening CT). We searched the following databases from their inception until October 13, 2020: MEDLINE (Ovid), PubMed, EMBASE (Ovid), Center Register of Controlled Trials (Cochrane CENTRAL), CINAHL (ebsco), and Web of Science-Core Collection. No restrictions on publication language were applied and search strategies were piloted prior to use. Search strategies for all databases are presented in Appendix Table 1. We also searched the reference lists of included or relevant articles to identify additional references.
Study Selection
The initial screening of titles and abstracts was conducted independently by two investigators (Y.K., L.B.). Abstracts included by either reviewer underwent full-text review. Full texts of selected studies were reviewed based on the selection criteria (Y.K., L.B., B. B, K.A.). Disagreements were resolved by consensus.
Data Extraction and Quality Assessment
To minimize error, two reviewers used a data collection form to extract information (patient and study characteristics, patient eligibility criteria for lung cancer screening, and meta-analysis outcomes (defined as LDCT performed)) from included studies. The restrictions on the study populations were based on eligibility for LCS which includes age and smoking history. For studies with missing or incomplete data on eligibility criteria, race, or LDCT referral or participation, we attempted to contact authors to retrieve those data. The Newcastle-Ottawa Scale (NOS) for cohort and case-control studies17 was used for quality assessment of the selected observational studies by two investigators (Y.K., L.B.) using a numerical score out of 9 points, with higher scores indicating higher quality of the study.
Data Synthesis and Meta-analysis
When at least two studies were available with comparable outcomes for the purposes of our meta-analysis (LDCT performed), we performed random-effects meta-analyses and estimated pooled odds ratios (ORs) with 95% CI using the restricted maximum likelihood method18. We evaluated heterogeneity visually and with the I2 statistic. I2 values of 25%, 50%, and 75% were considered low, medium, and high heterogeneity, respectively19. Meta-regression and tests for publication bias were not performed due to the limited number of included studies20. Heterogeneity was explored by sensitivity analysis by removing individual studies sequentially (Appendix Table 2). Statistical analysis was performed using Stata/IC, version 16.1 (StataCorp, College Station, Texas).
RESULTS
Study Selection
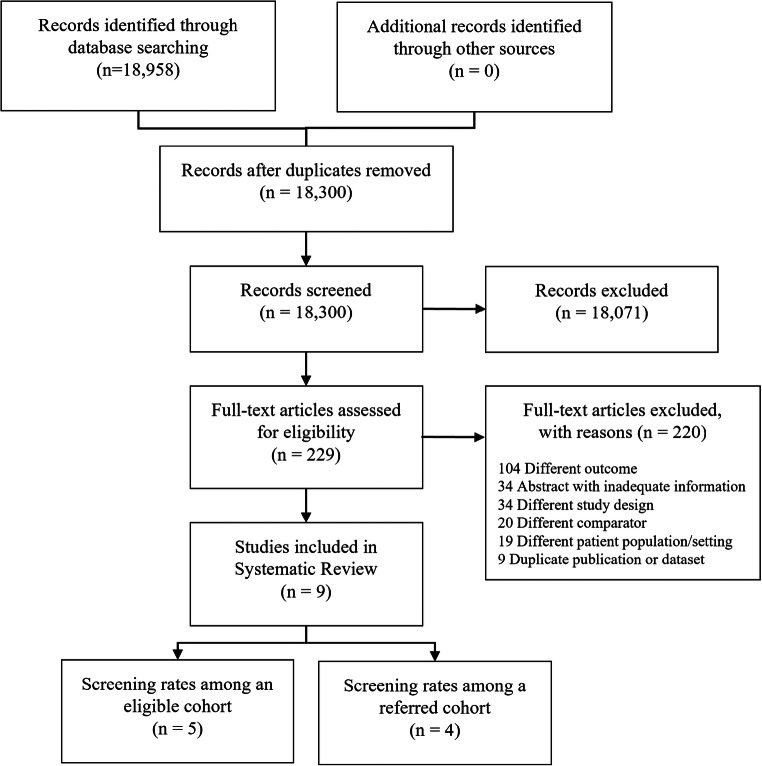
The medical database search yielded 18,300 studies, of which 229 studies were selected for full-text review. Nine studies21–29 were identified for inclusion in the systematic review (Fig. 1).
Figure 1.
Evidence search and selection.
Common reasons for exclusion after full-text review included different outcome of interest from the meta-analyses, missing race as a patient characteristic, abstracts with inadequate information, duplicate publications, and review articles without original data.
Study Characteristics
The included studies were composed of 1 prospective cohort study29, 3 retrospective cohort studies21,22,28, 1 retrospective case-control study24, and 4 cross-sectional studies23,25–27. All studies were conducted in the US at single healthcare centers22,24,25,28, regional healthcare networks21,26, community-based recruitment29, or utilized databases including Medicare27 and the Behavioral Risk Factor Surveillance System (BRFSS) data from 10 states23. No clinical trial met criteria for inclusion into the systematic review or meta-analysis.
Main characteristics and findings of the 9 included studies are summarized in Table 1.
Table 1.
Characteristics of Included Studies
| Author, year | Study type | Data source/setting | Study period | State | Eligibility criteria | Outcome assessment | N | % Black | Screening rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Studies of patient population eligible for screening | |||||||||
| Carter-Harris, 2018 | Prospective cohort | Community-based recruitment | Jan–Feb, 2017 | IN | USPSTF | Self-reported* | 438 | 42 | 62.2 |
| Japuntich, 2018 | Cross-sectional | Single center/health org. | 2016 | RI | USPSTF | Self-reported | 134 | 49 | 23.0 |
| Richmond, 2020 | Retrospective cohort | Community based cancer center | Jan–June, 2016 | NC | Current smokers >55y | EMR | 15,566‡ | 27 | 1.6 |
| Tailor, 2020 | Cross-sectional | Medicare | 2016 | N/A | USPSTF, 65–79y | Medicare claims data | 2,531,725‡ | 13.5 | 4.1 |
| Zgodic, 2020 | Cross-sectional | BRFSS | 2017 | Mult† | USPSTF | Self-reported | 4373 | 13.4§ | 14.4 |
| Studies of patient population referred for screening | |||||||||
| Gerber, 2020 | Case-control | Parkland Health and Hospital System | 2017–2019 | TX | USPSTF/NLST | EMR | 453 | 52.1 | 61.6 |
| Lake, 2020 | Retrospective cohort | Single academic center | 2015–2017 | PA | USPSTF | EMR | 675 | 46.7 | 70.7 |
| Raju, 2020 | Retrospective case-control | Single academic center | 2015–2016 | OH | USPSTF | EMR | 818 | 14.6 | 66.3 |
| Rennert, 2020 | Cross-sectional | Prisma Health System | 2016–2017 | SC | USPSTF | EMR | 171 | 13.5§ | 88.8 |
*Includes intent to screen in addition to screened
†10 states (including FL, GA, KS, ME, MD, MO, NV, OK, VT, WY)
‡Estimated
§Non-White
USPSTF criteria (2013): adults ages 55 to 80 years with at least a 30-pack-year smoking history, who are actively smoking or quit within the last 15 years
Abbreviations: USPSTF, United States Preventive Task Force; EMR, electronic medical record; Mult, multiple; NLST, National Lung Cancer Screening Trial; BRFSS, Behavioral Risk Factor Surveillance System
The studies were subdivided into those which examined rates of LCS in a baseline population of (a) LCS-eligible patients22,23,25,27,29 (5 eligible studies), or (b) patients referred for LCS by a provider21,24,26,28 (4 referred studies). The eligible versus referred studies were separated because they used a different denominator of a screening eligible population versus a population both eligible and referred for screening. Therefore, these rates of screening were not comparable. For the eligible studies, the method of determining an eligible patient cohort varied by study. Three studies23,25,29 applied the 2013 USPSTF LCS criteria to their study population cohort which included (a) recruited patients from the community in the state of Indiana (n=438 eligible patients, 62.2% screening rate)29, (b) a random patient sample from a healthcare organization electronic medical record (EMR; n=134, 23% screening rate)25, or (c) BRFSS reported data where smoking history was provided (n=4373, 14.4% screening rate)23. Two studies estimated the LCS-eligible population using a combination of census data, county or state level smoking data, and either 2013 USPSTF criteria (n=2,531,725, 13.5% screening rate)27 or an age threshold of 55 years or above with any smoking history (n=15,566, 1.6% screening rate)22. The eligible patients who completed LDCTs were identified through self-report23,25,29, EMR review22, or Medicare claims data27.
For the referred studies, all patients in the referred patient population met the 2013 USPSTF LCS criteria and had a referral placed by a provider in the EMR (total n=2117). The referred patients who completed LDCTs were identified through EMR review.
Among the eligible studies, the number of participants ranged from 134 to 4373; two studies22,27 used an estimated total cohort population. The median number of participants for the referred studies was 564 (range 171 to 818). Basic demographics were similar for eligible and referred studies with average age of 65.2 years and 64.0 years, respectively, and average proportion of male participants 50% and 52% respectively. When comparing the eligible versus referred studies, the median screening rates were 14.4% (range 1.7 to 62.6%) and 68.5% (range 62.6 to 88.8%), respectively.
Additional demographics, comorbidities, clinical variables, and socioeconomic variables reported in each study are summarized in Appendix Table 3. Six studies21,23,24,26,28,29 reported socioeconomic variables (insurance category, education level, neighborhood level) but did not adjust for these factors in LCS participation rates.
Quality Appraisal
Study quality was based on three main elements: selection, comparability, and outcome. Overall, the studies had a median NOS score of 5 (range 2–8) out of a possible 9 points (Table 2). Studies lost points primarily due to being single-center studies or lack of adjustment for a comprehensive set of potential confounders. Specifically for the eligible studies, use of self-reported data or use of estimates to calculate the number of eligible patients in the study cohort resulted in lower quality appraisals.
Table 2.
Study Quality Assessment Using Newcastle-Ottawa Quality Assessment Scale
| Author, year | Selection | Comparability | Outcome | NOS score | Limitations |
|---|---|---|---|---|---|
| Carter-Harris, 2018 | 0*00 | 00 | 0*0 | 2/9 | Community recruitment, self-reported outcome, no adjustment |
| Japuntich, 2018 | **00 | *0 | 0*0 | 4/9 | 1 site, self-reported outcome, adjustment for age only |
| Richmond, 2020 | 00** | 00 | **0 | 4/9 | Estimated eligible, no adjustment |
| Tailor, 2020 | 0*** | ** | *** | 8/9 | Estimated eligible |
| Zgodic, 2020 | **00 | ** | 0*0 | 5/9 | Self-reported outcome |
| Gerber, 2020 | 00** | 00 | *** | 5/9 | Part of a clinical trial, no adjustment |
| Lake, 2020 | 0*** | ** | *** | 8/9 | 1 site |
| Raju, 2020 | 0*** | 00 | *** | 6/9 | 1 site, no adjustment |
| Rennert, 2020 | 0*** | 00 | *** | 6/9 | 1 site, no adjustment |
NOS, Newcastle Ottawa Scale
*Asterisk indicates item achieves 1 point, 0 indicates 0 points in the NOS category
Meta-analysis of LCS Participation by Race
The meta-analyses were performed separately for the 5 eligible studies and the 4 referred studies (Fig. 2). For the 5 eligible studies, the meta-analysis showed lower LCS participation by Black compared to White patients eligible for LCS ([OR]=0.43, [95% CI: 0.25, 0.74]). However, there was no significant difference in LCS participation between Black and White patients among the 4 studies of patients referred for LCS (OR=0.94, [95% CI: 0.74, 1.19]). Heterogeneity was high for studies reporting eligible patients screened (I2=93%) and low for studies of referred patients screened (I2=0%). The high heterogeneity for studies of eligible patients screened was explored using sensitivity analysis but no substantial difference was seen on heterogeneity or pooled effect by sequentially removing individual studies (Appendix Table 2).
Figure 2.
Meta-analysis of lung cancer screening utilization by race.
DISCUSSION
Here, we present the first systematic review to focus on the relationship between race and LCS participation in the US. The meta-analyses revealed that Black LCS eligible patients are being screened at a lower rate than White patients [Summary OR=0.43 (0.25–0.74)]. However, once referred for LCS by a provider, the difference in LCS participation between Black and White patients was almost negligible [Summary OR=0.94 (0.74–1.19)]. This result demonstrates that racial disparity is present in LCS participation. Importantly, racial disparity in LCS participation is attenuated once patients are referred for screening by a provider. This indicates that racial disparities in LCS participation for the higher-risk Black population may be reduced by more equitable provider referral practices.
Currently, little is known about LCS referral practices in the US. The complexity of the LCS eligibility criteria poses a challenge for conducting large-scale research on LCS participation because screening eligibility cannot be easily extracted from an EMR. This was exhibited in the literature search for this systematic review, where only one abstract specifically examined rates of referral among an eligible population as a primary outcome. This abstract by Kats and colleagues reported that Black race and higher age were associated with lower rates of referral30. This is consistent with prior systematic reviews which have summarized how implicit bias towards racial and ethnic minorities by healthcare providers can negatively affect healthcare outcomes31,32.
The patient-provider relationship is crucial in cancer screening, including LCS, where shared-decision making is a required step. Establishing a strong patient-provider relationship may be particularly challenging for Black patients because of underlying discrimination or by the lack of a regular primary care provider (PCP). Nonetheless, the relationship between perceived racism or medical discrimination and cancer screening participation is not straightforward. Crawley and colleagues found lower rates of colorectal cancer screening among patients who had experienced medical discrimination compared to those who had not33. However, other studies observed weak or no correlation between perceived discrimination or racism and use of preventive health services, including cancer screening, after adjusting for socioeconomic status (SES)34,35. As many of these studies were limited to single institutions without a standardized method to evaluate racism or discrimination, it is difficult to draw conclusions.
In conjunction with the interpersonal racism that may manifest as provider racial bias in cancer screening referral practices, structural racism creates barriers to healthcare access and thus equitable LCS participation in the US. For patients to participate in LCS, they must have access to a PCP who can inform them of the LCS process and place a referral. Studies have shown that contact with a regular PCP is an important factor determining cancer screening referral and participation36. Residential racial segregation in the US has had long standing effects including perpetuation of inequities in wealth, education, and access to healthcare, which have also manifested through cancer disparities37. Greater residential segregation is associated with later stage of lung cancer diagnosis, lower likelihood of undergoing surgery for early stage lung cancer, and higher lung cancer–specific mortality38,39.
Thus, the relationship between residential segregation, SES, the social/political construct of race in the US, and cancer outcomes is complex. The literature used in this systematic review did not consistently include or adjust for socioeconomic variables which would have allowed us to better distinguish between interpersonal and structural racism that may be affecting LCS participation. Historically, studies on breast cancer screening or colorectal cancer screening have shown that even after adjusting for SES, racial disparities in rates of screening persist between Black and White patients40,41. Similarly, in our systematic review, Tailor and colleagues who used Medicare data (indicating equal insurance status) still demonstrated 64% lower odds of LCS participation among Black patients27. In addition, a recent systematic review by Sosa and colleagues provided examples of studies where SES variables such as household income or education were associated with lower LCS participation42. Thus, while there is evidence that racial disparities exist in cancer screening after accounting for SES, targeted work in clarifying the interaction between SES and race in LCS participation is needed.
The discordance in the meta-analysis results between the two study groups, eligible patients and referred patients, illustrates the important role of providers and their referral practices in creating disparities in LCS participation. Therefore, interventions should target providers, especially PCPs, in several domains to motivate conversations between PCPs and patients. First, increased education on LCS in general is necessary, as qualitative studies on physician perspectives on LCS have shown that only 47–58% of providers are well-informed on the 2013 USPSTF guidelines43,44. Second, we must raise awareness among PCPs of the greater benefits of LCS in Black patients and how the updated USPSTF LCS eligibility guidelines better align with lung cancer risk in the Black population. Third, continued emphasis is needed on the importance of trust and PCP engagement in the shared decision making process for cancer screening, and the potential provider biases contributing to racial disparities in referral patterns44,45.
In general, identifying who is eligible for LCS complicates the referral process because of the need to assess the patient’s smoking history46. Unlike other cancer screening criteria which are predominantly based on gender and age, LCS eligibility also relies on the following: total pack-year smoking history and, among former smokers, time since last use. As there is no standardized method of incorporating smoking histories into the EMR, this information cannot be easily or reliably located in a patient’s chart or developed into EMR advisories. The discordance rate between the smoking history within a patient’s EMR and the history obtained during a shared decision-making discussion has been reported to be as high as 90%47. On a more systemic level, considering ways to have more accurate and updated smoking history data or simplified LCS criteria to generate EMR reminders for providers may facilitate the referral process48.
Limitations
The present study has several limitations. First, publication bias likely affected inferences from this systematic review. Given that it has been less than a decade since the formal implementation of LCS by the USPSTF, many LCS programs are still in their nascency with inadequate data. Second, two studies in the eligible group relied on estimates of a LCS-eligible population and three studies used patient self-report of LCS completion. This, in addition to the significant heterogeneity between the studies, may impact the reliability of the meta-analysis estimates. One study by Richmond and colleagues used a different eligibility criterion of current smokers >55 years without a total pack-year cutoff. With a broader eligibility criterion, the percentage of screened patients may be lower than what would have been observed if USPSTF criteria had been used instead. Finally, several factors limited the potential to extrapolate the results to the general US population. Most studies represented only one medical center or hospital system, with only two23,27 using a cohort across multiple states. Academic centers may have a higher LCS participation rate due to their ability to establish centralized LCS referral programs. While geographic locations varied by study, not all US regions were represented equally and there is heterogeneity in prevalence of smokers in each state.
CONCLUSION
In summary, we demonstrate racial disparities exist in LCS participation in the US. Eligible Black patients are less likely to participate in LCS compared to eligible White patients despite the higher burden of lung cancer in the Black population. However, the racial disparity is attenuated among patients referred to LCS, suggesting the importance of the referral step in the LCS process as a target to reduce disparities. There is a need to promote LCS referral among providers with particular emphasis on the significant benefits of LCS in the Black patient population. More studies are needed to identify barriers to LCS referral and better understand both the provider and patient characteristics that may influence LCS participation.
Supplementary Information
(DOCX 275 kb)
(DOCX 16 kb)
Author Contribution:
Y.K. and L.B. screened articles, extracted data, and performed the analyses. B.B. and K.A. contributed to study review for selection and disagreements were resolved in consensus by Y.K., L.B., B.B., K.A., and A.B. conducted the literature search and C.G. conducted the statistical analysis for the meta-analyses. All authors have contributed substantially to the manuscript. Y.K. and L.B. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding:
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Health Services Research and Development #CIN 13-407 and Dr. Kunitomo’s efforts are sponsored by HSR&D post-doctoral fellowship VA Office of Academic Affairs.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Prior Presentations: not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–33. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 2.Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and Ethnic Disparities in Early-Stage Lung Cancer Survival. Chest. 2017;152(3):587–97. doi: 10.1016/j.chest.2017.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315–32. doi: 10.1038/s41416-020-01038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(10):971–87. doi: 10.1001/jama.2021.0377. [DOI] [PubMed] [Google Scholar]
- 5.Tanner NT, Gebregziabher M, Halbert CH, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the National Lung Screening Trial: Implications for widespread implementation. Am J Respir Crit Care Med. 2015;192(2):200–8. doi: 10.1164/rccm.201502-0259OC. [DOI] [PubMed] [Google Scholar]
- 6.Moyer VA. Screening for lung cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2014;160(5):330–8. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 7.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 8.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American Adult Smokers. JAMA Oncol. 2019;5(9):1318–24. doi: 10.1001/jamaoncol.2019.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CC, Matthews AK, Rywant MM, Hallgren E, Shah RC. Racial disparities in eligibility for low-dose computed tomography lung cancer screening among older adults with a history of smoking. Cancer Causes Control. 2019;30(3):235–40. doi: 10.1007/s10552-018-1092-2. [DOI] [PubMed] [Google Scholar]
- 10.Fiscella K, Winters P, Farah S, Sanders M, Mohile SG. Do Lung Cancer Eligibility Criteria Align with Risk among Blacks and Hispanics? PLoS One. 2015;10(11):e0143789. doi: 10.1371/journal.pone.0143789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krist AH, Davidson KW, Mangione CM, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962–70. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 12.Pham D, Bhandari S, Pinkston C, Oechsli M, Kloecker G. Lung Cancer Screening Registry Reveals Low-dose CT Screening Remains Heavily Underutilized. Clin Lung Cancer. 2020;21:e206–e211. doi: 10.1016/j.cllc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Duda C, Mahon I, Chen MH, et al. Impact and costs of targeted recruitment of minorities to the National Lung Screening Trial. Clin Trials. 2011;8(2):214–23. doi: 10.1177/1740774510396742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamir C, Budiman A, Noe-Bustamante L, Mora L. Facts About the U.S. Black Population. Pew Res. Cent. 2021;Available from: https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/ Accessed: June 20, 2021
- 15.Lam ACL, Aggarwal R, Cheung S, et al. Predictors of participant nonadherence in lung cancer screening programs: a systematic review and meta-analysis. Lung Cancer. 2020;146:134–44. doi: 10.1016/j.lungcan.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O’Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa, Ottawa Hosp. Res. Inst. 2000; Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed: May 9, 2021.
- 18.Raudenbush SW. Analyzing Effect Sizes: Random-Effects Models. In: Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. New York: Russel Sage Foundation; 2009. pp. 295–316. [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 6. Cochrane; 2020.
- 21.Gerber DE, Hamann HA, Chavez C, et al. Tracking the Nonenrolled: Lung Cancer Screening Patterns Among Individuals not Accrued to a Clinical Trial. Clin Lung Cancer. 2020;21(4):326–32. doi: 10.1016/j.cllc.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richmond J, Mbah OM, Dard SZ, et al. Evaluating Potential Racial Inequities in Low-dose Computed Tomography Screening for Lung Cancer. J Natl Med Assoc. 2020;112(2):209–14. doi: 10.1016/j.jnma.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zgodic A, Zahnd WE, Miller DP, Studts JL, Eberth JM. Predictors of Lung Cancer Screening Utilization in a Population-Based Survey. J Am Coll Radiol. 2020;17(12):1591–1601. doi: 10.1016/j.jacr.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Raju S, Khawaja A, Han X, Wang X, Mazzone PJ. Lung Cancer Screening: Characteristics of Nonparticipants and Potential Screening Barriers. Clin Lung Cancer. 2020;21(5):e329–e336. doi: 10.1016/j.cllc.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Japuntich SJ, Krieger NH, Salvas AL, Carey MP. Racial Disparities in Lung Cancer Screening: An Exploratory Investigation. J Natl Med Assoc. 2018;110(5):424–7. doi: 10.1016/j.jnma.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Rennert L, Zhang L, Lumsden B, et al. Factors influencing lung cancer screening completion following participation in shared decision-making: A retrospective study in a U.S. academic health system. Cancer Treat Res Commun. 2020;24:0–5. doi: 10.1016/j.ctarc.2020.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tailor TD, Tong BC, Gao J, Henderson LM, Choudhury KR, Rubin GD. Utilization of Lung Cancer Screening in the Medicare Fee-for-Service Population. Chest. 2020;158(5):2200–10. doi: 10.1016/j.chest.2020.05.592. [DOI] [PubMed] [Google Scholar]
- 28.Lake M, Shusted CS, Juon HS, et al. Black patients referred to a lung cancer screening program experience lower rates of screening and longer time to follow-up. BMC Cancer. 2020;20(1):561. doi: 10.1186/s12885-020-06923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter-Harris L, Slaven JE, Jr, Monahan PO, Shedd-Steele R, Hanna N, Rawl SM. Understanding lung cancer screening behavior: Racial, gender, and geographic differences among Indiana long-term smokers. Prev Med Rep. 2018;10:49–54. doi: 10.1016/j.pmedr.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kats DJ, Adie Y, Timat A, Tarabichi Y. Providers at an Academic Safety-Net Hospital System Tend to Refer Patients for Lung Cancer Screening Based on Patient Risk, but Demographic Disparities Persist. Am J Respir Crit Care Med. 2019;199:A1004. [Google Scholar]
- 31.Maina IW, Belton TD, Ginzberg S, Singh A, Johnson TJ. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc Sci Med. 2018;199:219–29. doi: 10.1016/j.socscimed.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Paradies Y, Truong M, Priest N. A systematic review of the extent and measurement of healthcare provider racism. J Gen Intern Med. 2014;29(2):364–87. doi: 10.1007/s11606-013-2583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawley LM, Ahn DK, Winkleby MA. Perceived medical discrimination and cancer screening behaviors of racial and ethnic minority adults. Cancer Epidemiol Biomarkers Prev a Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol. 2008;17(8):1937–44. doi: 10.1158/1055-9965.EPI-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shariff-Marco S, Klassen AC, Bowie JV. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010;100(2):364–74. doi: 10.2105/AJPH.2009.163899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trivedi AN, Ayanian JZ. Perceived discrimination and use of preventive health services. J Gen Intern Med. 2006;21(6):553–8. doi: 10.1111/j.1525-1497.2006.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson EB, Ostroff JS, DuHamel KN, et al. Impact of provider-patient communication on cancer screening adherence: A systematic review. Prev Med. 2016;93:96–105. doi: 10.1016/j.ypmed.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pleasant VA, Griggs JJ. Contemporary Residential Segregation and Cancer Disparities. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(25):2739–41. doi: 10.1200/JCO.21.01328. [DOI] [PubMed] [Google Scholar]
- 38.Annesi CA, Poulson MR, Mak KS, et al. The Impact of Residential Racial Segregation on Non-Small Cell Lung Cancer Treatment and Outcomes. Ann Thorac Surg. 2022;113(4):1291–8. doi: 10.1016/j.athoracsur.2021.04.096. [DOI] [PubMed] [Google Scholar]
- 39.Krieger N, Wright E, Chen JT, Waterman PD, Huntley ER, Arcaya M. Cancer Stage at Diagnosis, Historical Redlining, and Current Neighborhood Characteristics: Breast, Cervical, Lung, and Colorectal Cancers, Massachusetts, 2001-2015. Am J Epidemiol. 2020;189(10):1065–75. doi: 10.1093/aje/kwaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May FP, Bromley EG, Reid MW, et al. Low uptake of colorectal cancer screening among African Americans in an integrated Veterans Affairs health care network. Gastrointest Endosc. 2014;80(2):291–8. doi: 10.1016/j.gie.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones BA, Dailey A, Calvocoressi L, et al. Inadequate follow-up of abnormal screening mammograms: findings from the race differences in screening mammography process study (United States) Cancer Causes Control. 2005;16(7):809–21. doi: 10.1007/s10552-005-2905-7. [DOI] [PubMed] [Google Scholar]
- 42.Sosa E, D’Souza G, Akhtar A, et al. Racial and socioeconomic disparities in lung cancer screening in the United States: A systematic review. CA Cancer J Clin. 2021;71(4):299–314. doi: 10.3322/caac.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raz DJ, Wu GX, Consunji M, et al. Perceptions and utilization of lung cancer screening among primary care physicians. J Thorac Oncol. 2016;11(11):1856–62. doi: 10.1016/j.jtho.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triplette M, Kross EK, Mann BA, et al. An Assessment of Primary Care and Pulmonary Provider Perspectives on Lung Cancer Screening. Ann Am Thorac Soc. 2018;15(1):69–75. doi: 10.1513/AnnalsATS.201705-392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raz DJ, Wu GX, Consunji M, et al. The Effect of Primary Care Physician Knowledge of Lung Cancer Screening Guidelines on Perceptions and Utilization of Low-Dose Computed Tomography. Clin Lung Cancer. 2018;19(1):51–7. doi: 10.1016/j.cllc.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang GX, Baggett TP, Pandharipande PV, et al. Barriers to Lung Cancer Screening Engagement from the Patient and Provider Perspective. Radiology. 2019;290(2):278–287. doi: 10.1148/radiol.2018180212. [DOI] [PubMed] [Google Scholar]
- 47.Modin HE, Fathi JT, Gilbert CR, et al. Pack-Year Cigarette Smoking History for Determination of Lung Cancer Screening Eligibility. Comparison of the Electronic Medical Record versus a Shared Decision-making Conversation. Ann Am Thorac Soc. 2017;14(8):1320–5. doi: 10.1513/AnnalsATS.201612-984OC. [DOI] [PubMed] [Google Scholar]
- 48.Triplette M, Donovan LM, Crothers K, Madtes DK, Au DH. Prediction of Lung Cancer Screening Eligibility Using Simplified Criteria. Ann Am Thorac Soc. 2019;16(10):1280–5. doi: 10.1513/AnnalsATS.201903-239OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 275 kb)
(DOCX 16 kb)