Abstract
Background
The Centers for Medicare & Medicaid Services requires decision aid use for lung cancer screening (LCS) shared decision-making. However, it does not require information about incidental findings, a potential harm of screening.
Objective
To assess the effect of incidental findings information in an LCS decision aid on screening intent as well as knowledge and valuing of screening benefits and harms.
Design
Randomized controlled trial conducted online between July 16, 2020, and August 22, 2020.
Participants
Adults 55–80 years, eligible for LCS.
Intervention
LCS video decision aid including information on incidental findings or a control video decision aid.
Main Measures
Intent to undergo LCS; knowledge regarding the benefit and harms of LCS using six knowledge questions; and valuing of six benefits and harms using rating (1–5 scale, 5 most important) and ranking (ranked 1–6) exercises.
Key Results
Of 427 eligible individuals approached, 348 (83.1%) completed the study (173 intervention, 175 control). Mean age was 64.5 years, 48.6% were male, 73.0% white, 76.3% with less than a college degree, and 64.1% with income < $50,000. There was no difference between the intervention and controls in percentage intending to pursue screening (70/173, 40.5% vs 73/175, 41.7%, diff 1.2%, 95% CI − 9.1 to 11.5%, p = 0.81). Intervention participants had a higher percentage of correct answers for the incidental findings knowledge than controls (164/173, 94.8% vs 129/175, 73.7%, 95% CI − 28.4 to − 13.8%, p < 0.01). Incidental findings had the fifth highest mean importance rating (4.0 ± 1.1) and the third highest mean ranking (3.6 ± 1.5). There was no difference in mean rating or ranking of incidental findings between intervention and control groups (rating 4.0 vs 3.9, diff 0.1, 95% CI − 0.2, 0.3, p = 0.51; ranking 3.6 vs 3.6, diff 0.02, 95% CI − 0.3, 0.3, p = 0.89).
Conclusions
Incidental findings information in a LCS decision aid did not affect LCS intent, but it resulted in more informed individuals regarding these findings. In formulating screening preferences, incidental findings were less important than other benefits and harms.
Trial Registration
ClinicalTrials.gov identifier: NCT04432753
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07409-4.
KEY WORDS: Primary care, Cancer screening, Lung cancer, Shared decision-making, Decision aids
INTRODUCTION
The mortality reduction observed in the National Lung Screening Trial (NLST) led the US Preventive Service Task Force (USPSTF) in 2013 to recommend offering annual screening with low-dose CT to high-risk individuals, 55–80-year-old current and former smokers who quit within the last 15 years with at least 30 pack years of smoking.1,2 Other national organizations similarly recommend screening.3–5 However due to potential harms (e.g., false positives leading to biopsies and complications, overdiagnosis), these organizations call for shared decision-making with persons eligible for screening. The Centers for Medicaid & Medicare Services (CMS) mandates a shared decision-making encounter to qualify for reimbursement of screening its beneficiaries for lung cancer.6
Within the CMS mandate for shared decision-making is a requirement for the use of a decision aid. Decision aids are tools created to improve the quality of health care decision-making.7 They provide information regarding options, such as potential benefits and harms of screening or not screening, and help elicit personal values and preferences. Evidence suggests that decision aids increase knowledge, accuracy of risk perceptions, and consistency between informed values and care choices.8 Prior research has demonstrated increases in knowledge of benefits and harms of lung cancer screening, such as mortality benefit, false positives, and overdiagnosis.9–11 In their decision memo to require use of a decision aid for lung cancer screening, CMS cites lung cancer screening as a “complex topic” with a balance of potential benefits and harms.6 It requires decision aids to include information on specific harms including false positives and follow-up diagnostic testing. However, incidental findings are not explicitly included as a required component, despite being a common potential harm.
Incidental findings can lead to harms through the downstream cascade of testing and referrals, separate from that of further evaluation for lung cancer, from findings on LDCT not related to lung cancer.12 Individuals affected by incidental findings and their providers report that these findings lead to psychological and physical harm as well as financial burden and lost time.13–15 Though incidental findings can potentially lead to benefit such as an early finding and treatment of cancer that would have otherwise caused morbidity and/or mortality in organs such as the thyroid, pancreas, and liver, it is estimated that they cause overall harm through the cascade of follow-up testing.16 There are no consensus recommendations for cancer screening for these organs in the general population due to lack of evidence for benefit. In a large subset of the NLST sample, investigators found extrapulmonary (cardiovascular, renal, hepatobiliary, adrenal, and thyroid) abnormalities in 19.6% of individuals thought to be in need of further investigation.17 A Veterans Affairs lung cancer screening study across eight centers found 40.7% of individuals had abnormalities requiring follow-up.18 Due to these findings and others with similar observations, some have called for this potential harm to be included in shared decision-making.19 However, there appears to be an absence of such information in both patient-provider discussions and in medical center information on screening.20,21 Given the high rate of incidental findings and apparent lack of disclosure to individuals of this potential harm, we sought to examine if the inclusion of information about incidental findings in a lung cancer screening video decision aid affects intent to undergo screening.
METHODS
Study Design
We conducted an online, parallel, double-blinded, randomized controlled trial between July 16 and August 22, 2020, with individuals recruited from a national survey company who were found to be eligible for screening according to the 2013 USPSTF recommendations. We randomized individuals to one of two groups, intervention or control. The intervention group viewed a lung cancer screening video decision aid that included information about incidental findings.22 The control group viewed the same decision aid without incidental findings information.23 Both groups reported their intent to pursue lung cancer screening, and screening intention was compared between groups. The Institutional Review Board of the University of North Carolina at Chapel Hill evaluated the proposal for this study and deemed it exempt from further review. The study protocol is available in Appendix 2. The trial was registered with ClinicalTrials.gov (NCT04432753) and is reported according to the 2010 CONSORT statement.24
Study Population
We recruited individuals in the USA through online panels using a national survey company.25 Individuals in these panels are recruited by the company and provide demographic data when signing up to receive surveys. This data was used to target these individuals for relevant surveys, and respondents receive a small monetary incentive to complete the survey. We sent email invitations to individuals 55–80 years old with a smoking history. We narrowed the sample using the 2013 USPSTF recommendations (55–80-year-old current and former smokers who quit within the last 15 years; 30 pack year minimum) for lung cancer screening through self-reported questions on age and smoking history (Table S1). Those meeting criteria could continue with the survey. We additionally collected demographic information for income, health insurance, and relationship status (Table S1). We ensured through quotas that our sample included at least 25% of respondents that were Black/African-American or Hispanic/Latino to better represent the lung cancer screening population in the USA because smoking and screening eligibility rates are thought to be higher proportionally among these races than the general population.26 We also ensured least 25% had less than college education as the online survey setting was likely to overrepresent white, highly educated respondents. Following eligibility verification, individuals were randomized by the survey software 1:1 to either the intervention or the control group. We sought a sample of at least 346 (approximately 173 in each group) to detect a 15% difference in screening intent between groups with an alpha of 0.05 and beta of 0.2. We chose a 15% difference as a reasonable threshold for a clinically relevant difference given the lack of a standard precedent for this comparison.
Intervention and Control
The intervention group viewed a four-and-a-half-minute video decision aid, covering the benefit and harms of screening, including information on incidental findings (Narrative S1).22 The decision aid was adapted from a previously developed and tested decision aid.9 The only addition of content to the video was a segment on incidental findings (for the intervention group only), which was 31 s in length. We developed this segment by adapting wording from health information on incidental findings and incidental findings reported from a study of a subset of NLST participants.17,27 We met with current and former smokers aged 50–80 years old and performed structured interviews to refine this segment through multiple iterations. The control group viewed the same video decision aid as the intervention group, but without the segment in incidental findings.23 Survey items for each group were the same. Respondents were unaware that there was a separate arm with different information about incidental findings. This was done to avoid influencing the perceived importance of this attribute (priming effect). Also, incidental findings are not currently a required component of screening discussions per CMS.6 For analysis, investigators were blinded to the respondent study arm.
Measurements and Outcomes
Our primary outcome was screening intent. Following viewing of the decision aid in their respective groups, both intervention and control groups indicated their agreement with a statement on their intent to pursue lung cancer screening, a previously tested survey item with a 4-point Likert scale (strongly agree, agree, disagree, strongly disagree) (Table S2).9 Before conducting the study, we used cognitive interviews to iteratively test and refine the wording of this item as well as the following secondary knowledge and attribute items below in (n = 6) 50–80-year-old former and current smokers.
Secondary outcomes included pre-post change in knowledge of screening-related benefit and harms, measured using 6 knowledge questions on the benefit (mortality benefit) and harms (false positives, procedures for false positives, overdiagnosis, out-of-pocket costs, and incidental findings) of lung cancer screening answered both before and after viewing the decision aid (Table S2). The questions on overdiagnosis and mortality benefit were adapted from previous studies.9,28 We created 4 new knowledge questions regarding incidental findings, out-of-pocket costs, false positives, and biopsies for false positives based on our formative work, prior research, and a review of the literature.9,11,29
Other secondary outcomes included the values individuals placed on screening-related benefit and harms (attributes), assessed using a rating and ranking exercise for six attributes corresponding to key screening benefits and harms (Table S3). Screening attributes were developed based on formative work, our own prior research, and a review of the literature.9,11,29 Attributes were positively framed. The benefit attribute was represented as avoiding death from lung cancer (mortality benefit). Harms attributes were represented as avoiding finding a harmless slow-growing “cancer” leading to unnecessary treatment that is not needed (overdiagnosis); avoiding false alarms (false positives); avoiding a lung biopsy for a nodule or “spot” that turns out not to be lung cancer (procedures of false positives); avoiding out-of-pocket costs for follow-up visits, tests, and procedures (out-of-pocket costs); and avoiding finding things outside of the lungs that will not likely cause a problem, but lead to more visits, tests, and procedures (incidental findings). Rating was conducted on a 1–5 scale with 5 being the most important. Respondents ranked each of the six attributes in order of importance (1–6) (Table S2).
As an exploratory outcome, we assessed respondent preferences for next steps in decision-making, an important ongoing area of research interest, by asking if they had a primary care provider (PCP) and whether they preferred further discussion with their PCP or a provider at a lung cancer screening center, or had no preference.21
Statistical Analysis
For the primary outcome, we compared screening intent between intervention and control groups with Pearson’s chi-squared testing. For our primary analysis, we included only those who completed the full survey. Analysis of secondary outcomes included comparison of mean pre- and post-scores for each knowledge question with McNemar’s chi-squared test and overall mean pre- and post-scores with a paired t test. We compared mean pre-, post-, and change in scores between intervention and control groups with unpaired t tests. Change in the incidental findings knowledge question between groups was compared with Pearson’s chi-squared testing. For rating and ranking, we compared mean ratings for attributes between the intervention and control groups with t tests. For ranking, we compared the proportion of first ranks for each attribute between intervention and control groups with chi-squared tests. We also compared mean rank for each attribute using unpaired t tests. Statistical analysis was carried out with STATA (StataCorp. 2017, Stata Statistical Software: Release 15, College Station, TX: StataCorp LLC).
RESULTS
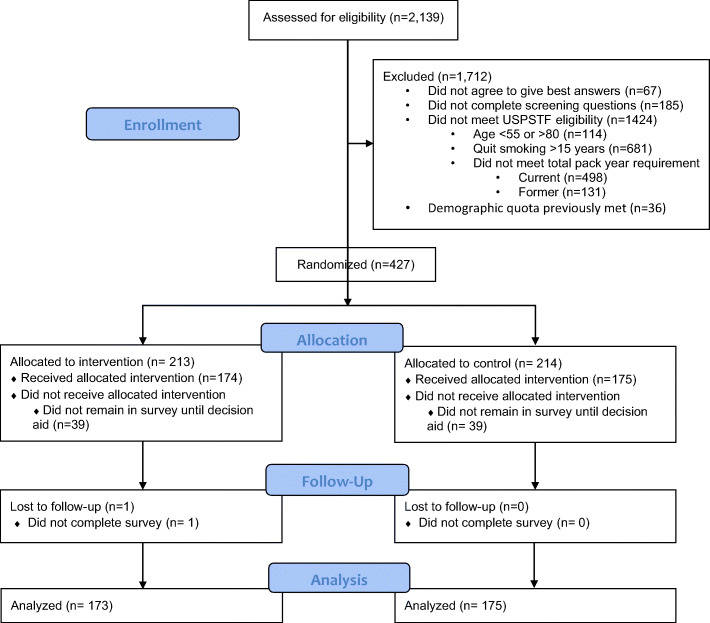
We sent the survey invitation to 2139 individuals. Of these, 1954 (91.4%) completed the initial eligibility screening questions regarding smoking history (Fig. 1). Of these, 427 were eligible and advanced to begin the full survey and were randomized. Of those randomized, 348 (83.1%) completed the survey (173 in the intervention group and 175 in the control group). Among completers, mean age was 64.5 years, 48.6% were male, 73.0% were white, 76.3% had less than a college degree, and 64.1% had income < $50,000 (Table 1).
Fig. 1.
CONSORT flow diagram.
Table 1.
Demographic Characteristics of Online Survey Respondents Eligible for Lung Cancer Screening (n = 348)
| Intervention group (n = 173) | Control group (n = 175) | Total | |
|---|---|---|---|
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | |
| Sex (male) | 87 (50.3) | 82 (46.9) | 169 (48.6) |
| Age | 64.5 (6.5) | 64.4 (6.1) | 64.5 (6.3) |
| Race | |||
| White | 120 (69.4) | 134 (76.6) | 254 (73.0) |
| Black or African-American | 35 (20.2) | 26 (14.9) | 61 (17.5) |
| Hispanic or Latino | 13 (7.5) | 14 (8.0) | 27 (7.8) |
| Other | 5 (2.9) | 1 (0.6) | 6 (1.7) |
| Smoking status (current) | 125 (72.3) | 120 (68.6) | 245 (70.4) |
| Pack years of smoking | |||
| Current | 52.3 (20.5) | 50.9 (27.6) | 51.6 (24.2) |
| Former | 61.4 (33.3) | 63.8 (41.6) | 61.4 (33.3) |
| Highest education level | |||
| Completed college | 42 (24.3) | 41 (23.4) | 83 (23.9) |
| Some college or technical school | 48 (27.8) | 57 (32.6) | 105 (30.2) |
| Completed high school or GED | 76 (43.9) | 70 (40.0) | 146 (42.0) |
| Some high school | 5 (2.9) | 7 (4.0) | 12 (3.5) |
| Some elementary/middle school | 2 (1.2) | 0 (0.0) | 2 (0.6) |
| Annual income | |||
| Less than $10k | 16 (9.3) | 10 (5.7) | 26 (7.5) |
| Less than $25k, ≥ $10k | 50 (28.9) | 44 (25.1) | 94 (27.0) |
| Less than $50k, ≥ $25k | 47 (27.2) | 56 (32.0) | 103 (29.6) |
| Less than $75k, ≥ $50k | 26 (15.0) | 36 (20.6) | 62 (17.8) |
| Less than $125k, ≥ $75k | 22 (12.7) | 17 (9.7) | 39 (11.2) |
| Greater than $125k | 9 (5.2) | 8 (4.6) | 17 (4.9) |
| Prefer not to answer | 3 (1.7) | 4 (2.3) | 7 (2.0) |
| Health insurance | |||
| Private | 43 (26.5) | 45 (27.4) | 88 (27.0) |
| Medicare | 91 (56.2) | 75 (45.7) | 166 (50.9) |
| Medicaid | 24 (14.8) | 25 (15.2) | 49 (15.0) |
| Military | 4 (2.5) | 11 (6.7) | 15 (4.6) |
| Other | 0 (0.0) | 7 (4.3) | 7 (2.2) |
| No coverage/do not know | 11 (6.4) | 12 (6.9) | 23 (6.6) |
Screening Intent
Overall, screening intent was balanced with 19.8% (n = 69) responding strongly agree, 39.1% (n = 136) agree, 29.0% (n = 101) disagree, and 12.1% (n = 42) strongly disagree with the statement that they planned to pursue lung cancer screening (Table 2). There was no difference in the percentage of respondents planning to pursue lung cancer screening between the intervention and control groups (70/173, 40.5% vs 73/175, 41.7%, diff 1.2%, 95% CI − 9.1 to 11.5%, p = 0.81).
Table 2.
Intent to Screen for Lung Cancer, “I Plan to Pursue Screening for Lung Cancer with an Annual Low-Dose CT Scan” (n = 348)
| Intervention group (n = 173) n (%) |
Control group (n = 175) n (%) |
Total n (%) |
|
|---|---|---|---|
| Strongly agree | 21 (12.1) | 21 (12.0) | 42 (12.1) |
| Agree | 49 (28.3) | 52 (29.7) | 101 (29.0) |
| Disagree | 65 (37.6) | 71 (40.6) | 136 (39.1) |
| Strongly disagree | 38 (22.0) | 31 (17.7) | 69 (19.8) |
Screening Knowledge
Overall, mean correct screening knowledge scores increased across groups before and after viewing the decision aid (pre 2.6 vs post 5.2 out of 6, diff + 2.7, 95% CI 2.5, 2.8, p < 0.01) (Table S4). Between groups, post-viewing mean correct scores were higher in the intervention group compared to those in the control group (5.3 vs 5.1, diff + 0.2, 95% CI − 0.01, 0.5, p = 0.07). Change in mean score from pre to post were similar (control 2.6 vs intervention 2.8, diff + 0.2, 95% CI − 0.1, 0.5, p = 0.20). For the question on incidental findings, there was no significant difference in percentage of correct answers between groups prior to viewing the decision aids (intervention 61.9% vs control 52.0%, p = 0.06). However, after viewing the decision aids, the intervention group had a higher percentage of individuals choosing the correct answer compared with the control group (164/173, 94.8% vs 129/175, 73.7%, 95% CI − 28.4 to − 13.8%, p < 0.01). The change in percentage of those with correct answers from pre to post was also significantly higher in the intervention group compared with the control (32.9% vs 21.7%, p = 0.02).
Rating and Ranking
Attribute Rating
Overall, respondents rated the attribute of incidental findings lowest in importance among the five harms attributes (mean rating 4.0 ± 1.1) (Table 3). Mean rating of this attribute did not differ between groups (intervention 4.0 vs control 3.9, diff + 0.1, 95% CI − 0.2, 0.3, p = 0.51). Avoiding false positives and overdiagnosis rated highest among screening attributes (mean rating: false alarms 4.2 ± 1.0, overdiagnosis 4.2 ± 1.1). These two harms were also rated highest within each arm. Those in the intervention group additionally rated procedures for false positives as a mean of 4.2 ± 1.0, and the control group also rated avoiding out-of-pocket costs for follow-up visits, tests, and procedures as a mean of 4.2 ± 1.2.
Table 3.
Rating of Lung Cancer Screening Benefit and Harms (n = 348)
| Intervention group (n = 173) | Control group (n = 175) | Total | |
|---|---|---|---|
| Rating mean (SD) | Rating mean (SD) | Rating mean (SD) | |
| Avoiding death from lung cancer | 3.7 (1.3) | 3.7 (1.3) | 3.7 (1.3) |
| Avoiding false alarms | 4.2 ( 1.0) | 4.2 (1.1) | 4.2 (1.0) |
| Avoiding a lung biopsy for a nodule or “spot” that turns out not to be lung cancer | 4.2 (1.0) | 4.1 (1.1) | 4.1 (1.1) |
| Avoiding finding a harmless cancer leading to treatment that is not needed (overdetection) | 4.2 (1.0) | 4.2 (1.2) | 4.2 (1.1) |
| Avoiding finding things outside of the lungs that will likely not cause a problem, but lead to more visits, tests, and procedures | 4.0 (1.1) | 3.9 (1.2) | 4.0 (1.1) |
| Avoiding out-of-pocket costs for follow-up visits, tests, and procedures | 4.1 (1.1) | 4.2 (1.2) | 4.1 (1.2) |
Ranking
Overall, respondents ranked the attribute for incidental findings fourth overall and third among harms (total number of 1st ranks 9.2%, n = 32; mean ranking 3.6 + 1.5) (Table 4). There was no significant difference between groups for this attribute for percentage of 1st ranks (intervention 9.5%, n = 16 vs control 10.9%, n = 19, p = 0.62) or mean ranking (control 3.6 vs intervention 3.6, diff 0.02, 95% CI − 0.3,0.3, p = 0.89). Avoiding death from lung cancer was rated highest most often (54.3%, n = 189) and had the highest (1–6 rankings with 1 being the highest) mean ranking of 2.5 + 1.9. The potential harm ranked highest most often was avoiding false positives (11.2%, n = 39), but the highest mean ranking among harms was avoiding a lung biopsy for a nodule or “spot’ that turns out not to be lung cancer (3.2 + 1.5).
Table 4.
Ranking of Lung Cancer Screening Benefit and Harms (n = 348)
| Intervention group (n = 173) | Control group (n = 175) | All participants (n = 348) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total 1st ranks n (%) | Total 6th ranks n (%) | Mean rank | Total 1st ranks n (%) | Total 6th ranks n (%) | Mean rank | Total 1st ranks | Total 6th ranks n (%) |
Mean rank | |
| Avoiding death from lung cancer | 90 (52.0) | 25 (14.5) | 2.5 (1.9) | 99 (56.6) | 19 (10.9) | 2.4 (1.9) | 189 (54.3) | 44 (12.6) | 2.5 (1.9) |
| Avoiding false alarms | 23 (13.3) | 37 (21.4) | 3.7 (1.7) | 16 (9.1) | 36 (20.6) | 3.7 (1.8) | 39 (11.2) | 73 (21.0) | 3.7 (1.7) |
| Avoiding a lung biopsy for a nodule or “spot” that turns out not to be lung cancer | 19 (11.0) | 19 (11.0) | 3.2 (1.5) | 19 (10.9) | 9 (5.1) | 3.2 (1.4) | 38 (10.9) | 28 (8.1) | 3.2 (1.5) |
| Avoiding finding a harmless cancer leading to treatment that is not needed (overdetection) | 10 (5.8) | 17 (9.8) | 3.7 (1.3) | 11 (6.3) | 21 (12.0) | 3.7 (1.4) | 21 (6.0) | 38 (10.9) | 3.7 (1.4) |
| Avoiding finding things outside of the lungs that will likely not cause a problem, but lead to more visits, tests, and procedures | 16 (9.2) | 19 (10.9) | 3.6 (1.5) | 16 (9.1) | 19 (10.9) | 3.6 (1.5) | 32 (9.2) | 38 (10.9) | 3.6 (1.5) |
| Avoiding out-of-pocket costs for follow-up visits, tests, and procedures | 15 (8.7) | 56 (32.4) | 4.2 (1.7) | 14 (8.0) | 71 (40.6) | 4.4 (1.7) | 29 (8.3) | 127 (36.5) | 4.3 (1.7) |
Preferences for Provider Discussion
Most respondents, 74.4% (n = 259), preferred to discuss lung cancer screening with a health care provider as a next step prior to making a screening decision (Table S5). Of those that wished to discuss further with a provider and had a PCP, 74.1% (n = 172) preferred to discuss with their PCP, 11.6% (n = 27) with a provider at a lung cancer screening center, and 14.2% (n = 33) had no preference (Table S6).
DISCUSSION
We found that including incidental findings information in a lung cancer screening decision aid did not affect screening intent. The lack of difference between groups occurred despite higher knowledge of incidental findings among those who received information on this potential harm of screening. The absence of effect on screening intent did, however, align with the relatively low importance (value) placed on avoiding incidental findings compared to other benefits and harms in rating and ranking exercises.
These findings suggest that incidental findings were not viewed by most participants as a substantial enough potential harm to move them away from screening. Such individuals may not have fully grasped the potential downsides (including costs) of further evaluation for findings that would likely never have caused health problems or have been identified apart from lung cancer screening. It is also possible that some individuals may have perceived incidental findings as a potential benefit of screening. Further study is necessary to fully characterize how individuals understand and value incidental findings within the context of lung cancer screening.
This study adds to the growing literature that decision aids enhance decision-relevant knowledge of lung cancer screening benefits and harms.9,11,30 Within the entire sample, knowledge improvement was significant with the mean post-decision aid viewing score greater than 80%. A majority of individuals in both groups answered the incidental findings knowledge question correctly prior to viewing the decision, indicating that this is not a novel concept for many individuals. Nevertheless, after watching the decision aid, the intervention group scored higher on this knowledge item than controls, and our findings are consistent with prior research suggesting that baseline knowledge of mortality benefit or the possibility of overdiagnosis is low.9–11,31
Our finding of a relatively high baseline knowledge of incidental findings contrasts with low baseline knowledge of the absolute likelihood of mortality benefit and possibility of overdiagnosis across groups. Only 8.6% of participants correctly estimated the approximate likelihood of benefitting from screening, with most greatly overestimating the probability. Similarly, only 13.2% of participants correctly answered the knowledge item about overdiagnosis. Post-decision aid scores showed increases of over 60% for these two concepts, which echoes previous findings regarding likelihood of benefit and overdiagnosis, and emphasizes the effectiveness of decision aids in helping to inform screen-eligible individuals about these critical aspects of screening.9,27
This study found that a majority of participants ranked the attribute of avoiding death from lung cancer first (most important). This is not surprising given it is the major benefit of screening, and screen-eligible individuals are undoubtedly aware that they are at increased risk for lung cancer. Nevertheless, we found that a substantial minority (45%) of participants ranked one of the avoiding harms attributes as most important, even above mortality benefit. We also found that the benefit attribute (avoiding death from lung cancer) had a lower mean importance rating than each of the harms attributes. Additionally, we found that screening intent was heterogeneous overall, as seen in previous studies.9,11,32 Taken together, these findings suggest that when informed about the tradeoffs, individuals vary in their preferences, and that a substantial minority of screen-eligible individuals appear to value avoiding the potential harms associated with screening over the potential benefits.
Our ranking data identified variation in which screening attribute was most important to decision-making, with a relatively even distribution in the percentage of first rankings. Although avoiding incidentals had a mean ranking that was similar to other harms attributes, it was highly valued (ranked most important) by a few individuals. Overall, these findings support the use of decision aids to inform individuals along with values assessment to identify what is most important to individual patients. Further study in the valuation of benefits and harms is an important consideration for future work, especially as lung cancer screening uptake is low across the USA and it is unknown if this is patient or provider driven.33–35
Limitations
Our study has limitations. Our sample was likely more white and more educated than the average individual eligible for lung cancer screening, though it was more diverse than the NLST study population.36
Second, the updated 2021 USPSTF guidelines for lung cancer screening broaden the eligible screening population by lowering the eligibility age (from 55 to 50) and the smoking exposure requirements—to include current or former smokers (who quit within the last 15 years) with at least 20 pack years of smoking. This study does not include the expanded population because this recommendation was not published at the time this study was conducted.37 Third, our study was conducted online, and the degree to which findings apply to the general screen-eligible population is uncertain. Further, this survey context limits our knowledge of respondent effort. However, the increase in knowledge scores after decision aid viewing indicates at least some effort and results were similar to those of a previous clinic-based study.9 Fourth, we did not assess knowledge or values for all potential benefits and harms. Instead, we focused on those highlighted by CMS and recommendation statements. Finally, we did not follow screening behavior beyond intent. However, self-reported screening intent can be predictive of screening behavior and is commonly used as a proxy for behavior.38
Conclusions
We found that including incidental findings information in a lung cancer screening video decision aid increased the degree to which eligible individuals were informed regarding this aspect of screening. However, in formulating screening preferences, this increased knowledge did not translate to greater value for avoiding incidental findings relative to other benefits and harms, and including incidental findings information did not affect intent to be screened for lung cancer overall. Nevertheless, because some screen-eligible individuals value avoiding incidental findings highly, it appears reasonable for this potential harm to be included in information about lung cancer screening.
Supplementary information
(DOCX 27 kb)
(DOCX 46 kb)
Funding
Dr. Clark’s time was supported by the Health Resources & Services Administration–funded primary care research fellowship at the University of North Carolina at Chapel Hill (T32-HP14001).
Declarations
Conflict of Interest
Dr. Clark was funded through the Health Resources & Services Administration T32-HP14001.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced Lung Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed]
- 2.Moyer VA. Screening for lung cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2014. 10.7326/m13-2771 [DOI] [PubMed]
- 3.Wender RC, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018. 10.3322/caac.21446 [DOI] [PubMed]
- 4.Mazzone PJ, Silvestri GA, Patel S, et al. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report. Chest. 2018. 10.1016/j.chest.2018.01.016 [DOI] [PubMed]
- 5.Kumar R, Reddy C, Kazerooni EA, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2018. 10.6004/jnccn.2018.0020 [DOI] [PMC free article] [PubMed]
- 6.Centers for Medicare & Medicaid Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId = 274. Published 2015. Accessed March 14, 2019.
- 7.International Patient Decision Aid Standards (IPDAS) Collaboration. What are patient decision aids? http://ipdas.ohri.ca/what.html. Published 2017. Accessed March 18, 2021.
- 8.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017. 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed]
- 9.Reuland DS, Cubillos L, Brenner AT, Harris RP, Minish B, Pignone MP. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med Inform Decis Mak. 2018. 10.1186/s12911-018-0582-1 [DOI] [PMC free article] [PubMed]
- 10.Volk RJ, Linder SK, Leal VB, et al. Feasibility of a patient decision aid about lung cancer screening with low-dose computed tomography. Prev Med. 2014;62:60–63. doi: 10.1016/j.ypmed.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark SD, Reuland DS, Brenner AT, Pignone MP. What is the effect of a decision aid on knowledge, values and preferences for lung cancer screening? An online pre–post study. BMJ Open. 2021;11(7):e045160. doi: 10.1136/bmjopen-2020-045160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RP, Sheridan SL, Lewis CL, et al. The harms of screening : A proposed taxonomy and application to lung cancer screening. JAMA Intern Med. 2014;174(2). 10.1001/jamainternmed.2013.12745 [DOI] [PubMed]
- 13.Casarella WJ. A patient’s viewpoint on a current controversy [1]. Radiology. 2002;224(3). 10.1148/radiol.2243020024 [DOI] [PubMed]
- 14.Rothberg MB. The $50 000 Physical. JAMA. 2014;311(21). 10.1001/jama.2014.3415 [DOI] [PubMed]
- 15.Ganguli I, Simpkin AL, Lupo C, et al. Cascades of Care After Incidental Findings in a US National Survey of Physicians. JAMA Netw Open. 2019;2(10). 10.1001/jamanetworkopen.2019.13325 [DOI] [PMC free article] [PubMed]
- 16.Wender R, Fontham ETH, Barrera E, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013. 10.3322/caac.21172 [DOI] [PMC free article] [PubMed]
- 17.Nguyen X V., Davies L, Eastwood JD, Hoang JK. Extrapulmonary Findings and Malignancies in Participants Screened With Chest CT in the National Lung Screening Trial. J Am Coll Radiol. 2017. 10.1016/j.jacr.2016.09.044 [DOI] [PubMed]
- 18.Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017. 10.1001/jamainternmed.2016.9022 [DOI] [PubMed]
- 19.Morgan L, Choi H, Reid M, Khawaja A, Mazzone PJ. Frequency of incidental findings and subsequent evaluation in low-dose computed tomographic scans for lung cancer screening. Ann Am Thorac Soc. 2017;14(9). 10.1513/AnnalsATS.201612-1023OC [DOI] [PubMed]
- 20.Brenner AT, Malo TL, Margolis M, et al. Evaluating Shared Decision Making for Lung Cancer Screening. JAMA Intern Med. 2018. 10.1001/jamainternmed.2018.3054 [DOI] [PMC free article] [PubMed]
- 21.Clark SD, Reuland DS, Enyioha C, Jonas DE. Assessment of Lung Cancer Screening Program Websites. In: JAMA Internal Medicine. Vol 180. ; 2020. 10.1001/jamainternmed.2020.0111 [DOI] [PMC free article] [PubMed]
- 22.Clark S, Reuland D, Brenner A, Jonas D. Benefit and Harms of Lung Cancer Screening. https://www.youtube.com/watch?v = T0nfBcoFpek. Published 2020. Accessed March 18, 2021.
- 23.Clark SD, Reuland DS, Brenner AT, Jonas DE. Lung cancer screening benefits and harms. https://www.youtube.com/watch?v = uAuFyKr-JtM. Published 2020. Accessed March 18, 2021.
- 24.The CONSORT statement. http://www.consort-statement.org/consort-2010. Accessed February 19, 2020.
- 25.Qualtrics. https://www.qualtrics.com/. Published 2020.
- 26.Pinsky PF, Lau YK, Doubeni CA. Potential Disparities by Sex and Race or Ethnicity in Lung Cancer Screening Eligibility Rates. Chest. 2021;160(1):341–350. doi: 10.1016/j.chest.2021.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agency for Healthcare Research & Quality. Is lung cancer screening right for me? https://effectivehealthcare.ahrq.gov/decision-aids/lung-cancer-screening/static/lung-cancer-screening-patient-encounter.pdf. Published 2016.
- 28.Hersch J, Barratt A, Jansen J, et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: A randomised controlled trial. Lancet 2015. 10.1016/S0140-6736(15)60123-4 [DOI] [PubMed]
- 29.Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(10):971–987. doi: 10.1001/jama.2021.0377. [DOI] [PubMed] [Google Scholar]
- 30.Volk RJ, Linder SK, Leal VB, et al. Feasibility of a patient decision aid about lung cancer screening with low-dose computed tomography. Prev Med (Baltim) 2014. 10.1016/j.ypmed.2014.02.006 [DOI] [PMC free article] [PubMed]
- 31.Housten AJ, Lowenstein LM, Leal VB, Volk RJ. Responsiveness of a Brief Measure of Lung Cancer Screening Knowledge. J Cancer Educ. 2018;33(4):842–846. doi: 10.1007/s13187-016-1153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dharod A, Bellinger C, Foley K, Case LD, Miller D. The Reach and Feasibility of an Interactive Lung Cancer Screening Decision Aid Delivered by Patient Portal. Appl Clin Inform. 2019. 10.1055/s-0038-1676807 [DOI] [PMC free article] [PubMed]
- 33.Seigel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA A Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 34.Huo J, Shen C, Volk RJ, Shih YCT. Use of CT and chest radiography for lung cancer screening before and after publication of screening guidelines: Intended and unintended uptake. JAMA Intern Med. 2017. 10.1001/jamainternmed.2016.9016 [DOI] [PMC free article] [PubMed]
- 35.Pham D, Bhandari S, Oechsli M, Pinkston CM, Kloecker GH. Lung cancer screening rates: Data from the lung cancer screening registry. J Clin Oncol. 2018;36(15_suppl). 10.1200/jco.2018.36.15_suppl.6504
- 36.Aberle DR, Adams AM, Berg CD, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010. 10.1093/jnci/djq434 [DOI] [PMC free article] [PubMed]
- 37.Force USPST. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 38.Sheeran P. Intention—Behavior Relations: A Conceptual and Empirical Review. Eur Rev Soc Psychol. 2002;12(1):1–36. doi: 10.1080/14792772143000003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 27 kb)
(DOCX 46 kb)