SUMMARY:
Objective:
Cystic adrenal mass is a rare imaging presentation of pheochromocytoma. We aimed to describe the clinical, biochemical, and imaging characteristics of patients with cystic pheochromocytoma.
Design:
Single-center, retrospective study, 2000–2020.
Patients:
Consecutive patients with cystic pheochromocytoma identified from our institutional pathology and adrenal tumor database.
Results:
Of the 638 patients with pheochromocytomas, 21 (3.2%) had cystic pheochromocytomas (median age 57 years, 57% women). Most pheochromocytomas were discovered incidentally (57%) or due to symptoms of catecholamine excess (24%). The median tumor size was 6.4 cm. On imaging, cystic pheochromocytomas were round or oval (90%), heterogeneous lesions (86%) with a thick solid rim (median rim thickness 13.9 mm, unenhanced CT attenuation 40 HU, venous-phase CT attenuation 83 HU), and a median cystic component of 40% (unenhanced CT attenuation 17.6 HU, venous-phase CT attenuation 20.4 HU), and rarely with calcifications (15%). All 20 patients with biochemical testing had functioning tumors (adrenergic in 80%, noradrenergic in 20%). Total urinary metanephrine excretion correlated with the volume of the solid component (R2=0.75, P<0.0001) but not the cystic component (R2=0.04, P=0.4386). All patients underwent adrenalectomy (48% laparoscopic, 52% open), and the median duration of hospital stay was 4 days.
Conclusion:
Cystic pheochromocytomas are rare, large tumors with a phenotypic appearance that can masquerade as other adrenal cystic lesions. The degree of biochemical abnormality in cystic pheochromocytomas is associated with the volume of the solid component. All patients with adrenal cysts that have a solid component or an unenhanced attenuation >10 HU should undergo biochemical testing for pheochromocytoma.
Keywords: adrenalectomy, metanephrine, Hounsfield unit, solid component, cystic component
INTRODUCTION
Pheochromocytomas are rare catecholamine-secreting neuroendocrine tumors arising from the chromaffin cells in the adrenal medulla. In a population-based study of 1287 patients with adrenal tumors, pheochromocytomas represented 1.1%1. Most pheochromocytomas are discovered incidentally on imaging performed for reasons other than adrenal mass2 and are typically solid tumors with unenhanced attenuation on computed tomography (CT) of > 20 Hounsfield units (HU)3,4. While presence of focal cystic changes in pheochromocytomas are common5, presentation with a macroscopic cystic component is rare. These cases may pose a diagnostic challenge risking inadequate preparation for surgery due to misdiagnosis as an adrenal cyst. Due to its rarity, evidence regarding cystic pheochromocytomas is limited to case reports or small pathology-based series of up to six patients 6–10.
Accurate preoperative diagnosis of pheochromocytoma is important to assure the best perioperative outcomes. Our objectives were to (1) characterize the clinical, biochemical, imaging characteristics and outcomes of patients with cystic pheochromocytoma and (2) determine the association of the solid and cystic component volumes of pheochromocytoma with the degree of catecholamine excess.
METHODS
Patient selection
The study protocol was approved by our Institutional Review Board. Patients were included if 1) they were evaluated at our institution between January 1st, 2000 and December 31st, 2020; 2) histopathology was available and reported a cystic element; and 3) imaging (CT or magnetic resonance imaging [MRI]) demonstrated that ≥ 10% of pheochromocytoma volume was cystic. We first interrogated our longitudinal adrenal database on July 1st, 2021 to identify all patients with pheochromocytoma during the study period (n=638). We also interrogated the institutional pathology database of all pathology reports resulting from adrenalectomy using the terms “cyst” or “cystic” combined with term “pheochromocytoma” (n=87). Final cohort of patients meeting eligibility criteria (n=21) was reached after combining the search results from the two databases, and a detailed review of imaging and pathology reports.
Data collection
Information on clinical presentation, genetic testing, biochemical parameters, imaging phenotype, and management was collected. Biochemical parameters included plasma fractionated metanephrine (normal <0.5 nmol/L) and normetanephrine (normal <0.9 nmol/L), 24-hour urinary fractionated metanephrine (normal <2028 nmol/day) and normetanephrine (normal <4913 nmol/day), and 24-hour urinary fractionated catecholamines (epinephrine [ normal <115 nmol/day], norepinephrine [normal <473 nmol/day], dopamine [normal <2611 nmol/day]). Pheochromocytoma was classified as ‘adrenergic’ when both metanephrine and normetanephrine levels were elevated and ‘noradrenergic’ when only normetanephrine levels were elevated.
All imaging was reviewed by two investigators in duplicate (DP and PN). An abdominal radiologist with 3 years post fellowship experience (PN), calculated the total tumor volume, the volume of the solid and cystic components, unenhanced and contrast enhanced CT attenuation of the solid and cystic components, and rim thickness. At the time of imaging review and measurements, PN was blinded to patients’ clinical presentation and outcomes. All images were reviewed on a clinical workstation (EIZO RX360, EIZO inc. Cypress, California, USA) with all segmentation and measurements performed using imaging specific software (Visage Client 7.1.16, Visage imaging, Victoria, Australia)
Statistical analysis
Categorical data were presented as counts and percentages and continuous data were presented as median and interquartile ranges. Simple bivariate analysis was used to assess association between continuous variables. Statistical analysis was performed using JMP software, version 14.
RESULTS
Of 7724 patients with adrenal tumors evaluated at our institution between January 1, 2000, to December 31, 2020, 638 (8.3%) were pheochromocytomas. Cystic pheochromocytomas were diagnosed in 21 patients, representing 3.2% of all patients with pheochromocytomas, and 0.27% of all adrenal tumors.
The median age at presentation in the 21 patients with cystic pheochromocytomas was 57 years (IQR 51–67), and 12 (57%) were women. Mode of discovery was incidental in 12 (57%) patients, symptoms of catecholamine excess in 5 (24%), symptoms of mass effect in 2 (10%), genetic case detection testing in 1 (5%) patient, and 1 patient was not recognized to have pheochromocytoma until after adrenalectomy. Regardless of the mode of discovery, 13 (62%) patients endorsed symptoms and signs of catecholamine excess, with spells, palpitations, and paroxysmal or sustained hypertension being most common (Table 1). Genetic testing was pursued only in 6 (29%) patients and 3 patients had positive results: multiple endocrine neoplasia type 2A (n=1), neurofibromatosis type 1 (n=1), and von-Hippel Lindau disease (n=1). Seven (33%) patients had an active or history of extra-adrenal malignancy, including medullary thyroid cancer in the patient with multiple endocrine neoplasia type 2A and gastric cancer in the patient with neurofibromatosis type 1 (Table 1).
Table 1.
Presentation and management of patients with cystic pheochromocytoma
| N=21 | |
|---|---|
| Clinical presentation | |
|
| |
| Age at diagnosis, years, median (IQR) | 57 (51–67) |
|
| |
| Women, n (%) | 12 (57) |
|
| |
| Race, n (%) | |
| White | 18 (86) |
| Native American | 1 (5) |
| Unknown | 2 (10) |
|
| |
| Mode of discovery, n (%) | |
| Incidental | 13 (57) |
| Hormone excess symptoms | 5 (24) |
| Mass effect symptoms | 2 (10) |
| Genetic surveillance | 1 (5) |
| Post-adrenalectomy | 1 (5) |
|
| |
| Symptoms and signs of catecholamine excess, n (%) | 13 (62) |
|
| |
| Symptoms and signs, n (%) | |
| Palpitations | 8 (62) |
| Spells | 8 (62) |
| Paroxysmal hypertension | 7 (54) |
| Sustained hypertension | 2 (15) |
|
| |
| Genetic testing, n (%) | |
| Not tested | 15 (72) |
| Tested, negative | 3 (14) |
| Tested, positive | 3 (14) |
|
| |
| Type of pathogenic variant, n (%) | |
| VHL | 1 (33) |
| NF1 | 1 (33) |
| MEN 2A | 1 (33) |
|
| |
| Extra-adrenal malignancy, n (%) | 7 (33) |
|
| |
| Imaging phenotype | |
|
| |
| Site, n (%) | |
| Right | 15 (71) |
| Left | 6 (29) |
|
| |
| Tumor size, cm, median (IQR) | 6.4 (5–11.1) |
|
| |
| Volume, cm3, median (IQR) | |
| Total | 115 (47.4–654.1) |
| Cystic component | 48.7 (15.6–236.3) |
| Solid component | 61.2 (27.3–186.2) |
|
| |
| Composition, %, median (IQR) | |
| Cystic component | 40 (24–60) |
| Solid component | 60 (40–76) |
|
| |
| Unenhanced CT attenuation, HU, median (IQR), available for n=16 | |
| Cystic component | 17.6 (14.6–20) |
| Solid component | 40 (32.1–44.3) |
|
| |
| Enhanced CT attenuation#, HU, median (IQR), available for n=14 | |
| Cystic component | 20.4 (14.9–26.8) |
| Solid component | 83 (83.3–121) |
|
| |
| Other imaging characteristics | |
| Round/oval shape, n (%) | 19 (90) |
| Well defined borders, n (%) | 21 (100) |
| Heterogeneous, n (%) | 18 (86) |
| Rim thickness, mm, median (IQR) | 14 (8–22) |
| Calcification, n (%) available for n=20 | 3 (15) |
|
| |
| Biochemical evaluation | |
|
| |
| Preoperative evaluation for catecholamine excess, n (%) | 20 (95) |
|
| |
| Plasma fractionated metanephrines, nmol/L, median (IQR), available for n=18 | |
| Metanephrine (normal <0.5 nmol/L) | 2.7 (0.9–8.7) |
| Normetanephrine (normal <0.9 nmol/L) | 13.2 (3.2–23.6) |
|
| |
| Urinary fractionated metanephrine, nmol/day, median (IQR), available for n=16 | |
| Metanephrine (normal < 2028 nmol/day) | 15828 (5663–33731) |
| Normetanephrine (normal < 4913 nmol/day) | 22957 (9206–84472) |
|
| |
| Urinary fractionated catecholamines, nmol/day, median (IQR), available for n=15 | |
| Epinephrine (normal < 115 nmol/day) | 470 (104–1715) |
| Norepinephrine (normal <473 nmol/day) | 1093 (609–6461) |
| Dopamine (normal <2611 nmol/day) | 1222 (975–2126) |
|
| |
| Tumor type, n (%) | |
| Adrenergic | 16 (80) |
| Noradrenergic | 4 (20) |
|
| |
| Perioperative details | |
|
| |
| Preoperative medical therapy, n (%) | 20 (95) |
|
| |
| Alpha-adrenergic blockade | |
| Number of patients treated, n (%) | 20 (100) |
| Duration, days, median (IQR) | 15.5 (10.3–20) |
|
| |
| Metyrosine | |
| Number of patients treated, n (%) | 4 (20) |
| Duration, days, median (IQR) | 5 (2.8–8) |
|
| |
| Beta-adrenergic blockade, n (%) | 17 (85) |
|
| |
| Adrenalectomy, n (%) | |
| Laparoscopic | 10 (48) |
| Open | 11 (52) |
|
| |
| Duration of hospital stay, days, median (IQR) | 4 (2–6.5) |
|
| |
| Postoperative complications, n (%) | |
| Hypotension requiring vasopressors | 3 (14%) |
| Pulmonary edema | 1 (5%) |
| Retroperitoneal hemorrhage | 1 (5%) |
| Postoperative ileus | 1 (5%) |
Abbreviations – CT: Computed Tomography, HU: Hounsfield Unit, IQR: Interquartile range, MEN 2A: Multiple endocrine neoplasia type 2A, NF1: Neurofibromatosis type 1, VHL: Von Hippel Lindau
Measured during portal venous phase
Most patients presented with unilateral pheochromocytoma (15, 71% right, and 4, 19% left). In two patients with bilateral pheochromocytomas, only one was cystic. Cystic pheochromocytomas had a round or oval shape in 19 (90%) patients, and lobulated shape in the 2 (10%) patients. In 18 (86%) patients, cystic pheochromocytomas had heterogeneous appearance with septae present in 15 patients. On preoperative imaging, the median tumor size was 6.4 cm (IQR 5–11.1), and the median tumor volume was 115 cm3 (IQR 47.4–654.1). The cystic component of the pheochromocytomas varied widely and represented a median of 40% (IQR 24–60) corresponding to a median volume of 48.7 cm3 (IQR 15.6–236.3) (Table 2). The solid component of the pheochromocytomas represented a median of 60% (40–76%) corresponding to a volume of 61.2 cm3 (IQR 27.3–186.2). In 16 patients with unenhanced CT imaging available, the median attenuation was 17.6 HU (IQR 14.6–20) within the cystic component and 40 HU (IQR 32.1–44.3) in the solid component of pheochromocytoma. In the 14 patients with contrast-enhanced CT images available, the median attenuation of the cystic component was 20.35 HU (IQR 14.9–26.8), while in the solid component of pheochromocytoma, it was 93 HU (IQR 83.3–121). Only 3 (15%) patients demonstrated calcifications on CT. All pheochromocytomas were well-circumscribed and had a surrounding rim of variable thickness (median rim thickness of 14 mm [IQR 8–22]) that demonstrated significant rim enhancement on contrast-enhanced CT (Tables 1 and 2) (Figures 1 and 2).
Table 2.
Individual data of patients with cystic pheochromocytoma
| Patient indicator | Demographics and presentation | Catecholamine excess | Imaging presentation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Symptoms | Fold increment > upper normal range | Maximum tumor size, cm | Cystic volume, % | Solid volume, % | Unenhanced HU cystic component | Unenhanced HU solid component | |
| A | 42 | F | Yes | >10 | 11.2 | 75 | 25 | 30 | 48 |
| B | 50 | F | No | >10 | 9.1 | 77 | 23 | 30 | 50 |
| C | 56 | M | Yes | >10 | 11.7 | 68 | 32 | 14 | 39 |
| D | 59 | F | Yes | 5–10 | 4.1 | 26 | 74 | N/A | N/A |
| E | 55 | M | Yes | >10 | 8.2 | 50 | 50 | 20 | 46 |
| F | 63 | M | No | <5 | 2.9 | 15 | 85 | 19 | 29 |
| G | 66 | M | No | 5–10 | 3.8 | 24 | 76 | 13 | 45 |
| H | 57 | M | Yes | N/A | 18.9 | 40 | 60 | 18 | 35 |
| I | 75 | M | No | 5–10 | 6.4 | 53 | 47 | 14 | 34 |
| J | 76 | F | Yes | >10 | 5 | 49 | 51 | 17 | 42 |
| K | 69 | F | No | <5 | 12 | 64 | 36 | N/A | N/A |
| L | 55 | F | Yes | >10 | 8.2 | 28 | 72 | 17 | 43 |
| M | 86 | M | No | <5 | 5.5 | 44 | 56 | 18 | 28 |
| N | 52 | F | Yes | >10 | 6.5 | 29 | 71 | N/A | N/A |
| O | 65 | F | No | 5–10 | 4.4 | 24 | 76 | 17 | 32 |
| P | 4 | M | Yes | >10 | 5 | 24 | 76 | 27 | 41 |
| Q | 49 | M | Yes | >10 | 13.5 | 23 | 77 | 17 | 34 |
| R | 68 | F | Yes | 5–10 | 6.3 | 25 | 75 | N/A | N/A |
| S | 65 | F | No | >10 | 14.9 | 22 | 78 | 20 | 44 |
| T | 54 | F | Yes | <5 | 5.7 | 56 | 44 | 14 | 22 |
| U | 41 | F | Yes | >10 | 6.3 | 70 | 30 | N/A | N/A |
Abbreviations – F: Female, HU: Hounsfield Unit, M: Male, N/A: Not available
Figure 1. Computed tomography (CT) and magnetic resonance imaging (MRI) imaging of 21 patients with cystic pheochromocytomas.
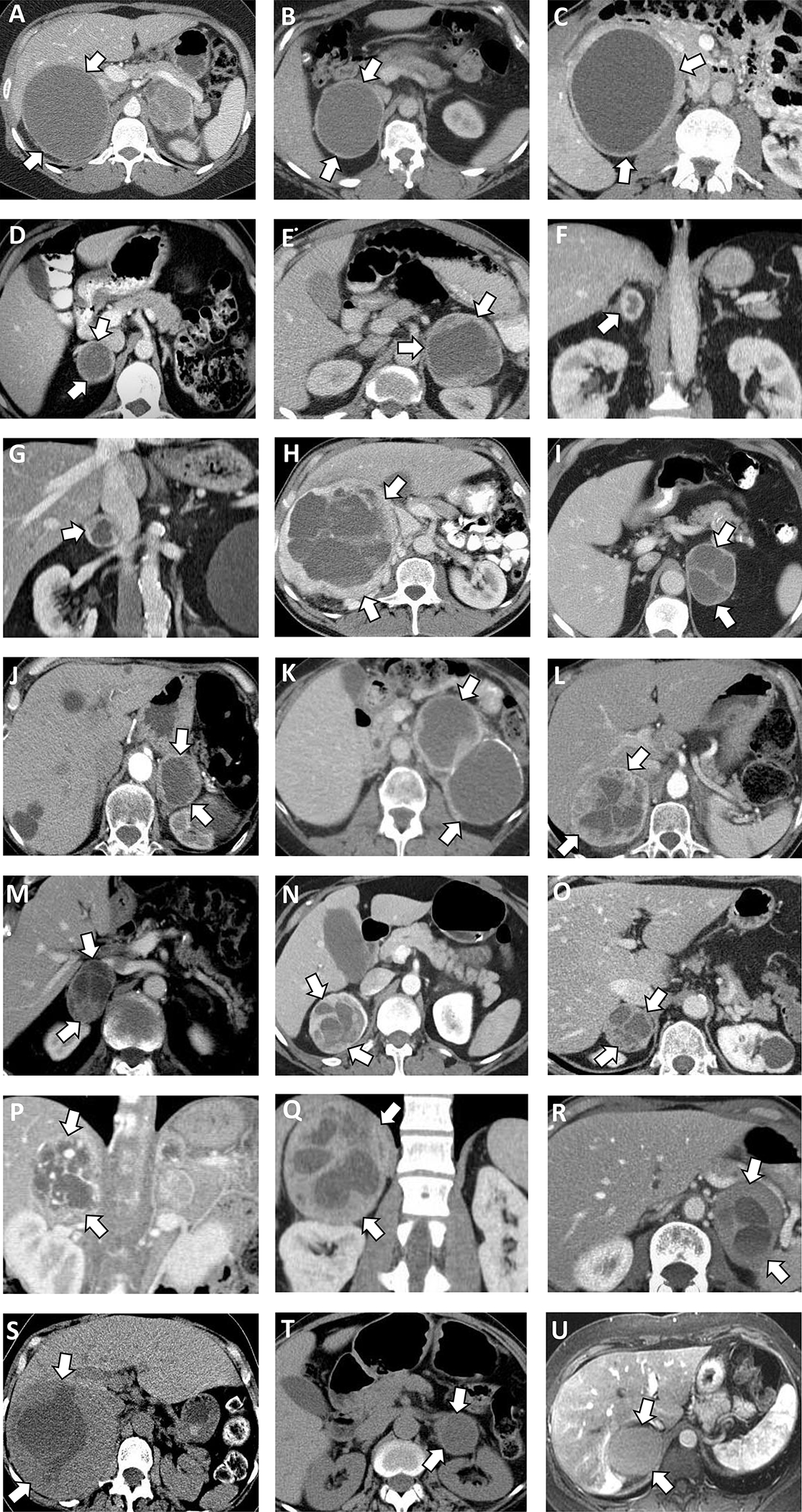
(A) Right 11.2 cm pheochromocytoma, cystic component 75%, on contrast enhanced CT.
(B) Right 9.1 cm pheochromocytoma, cystic component 77% on contrast enhanced CT.
(C) Right 11.7 cm pheochromocytoma, cystic component 68% on contrast enhanced CT.
(D) Right 4.1 cm pheochromocytoma, cystic component 26% on contrast enhanced CT.
(E) Left 8.2 cm pheochromocytoma, cystic component 50% on contrast enhanced CT.
(F) Left 2.9 cm pheochromocytoma, cystic component 15% on contrast enhanced CT.
(G) Right 3.8 cm pheochromocytoma, cystic component 24% on contrast enhanced CT.
(H) Right 18.9 cm pheochromocytoma, cystic component 40% on contrast enhanced CT.
(I) Left 6.4 cm pheochromocytoma, cystic component 53% on contrast enhanced CT.
(J) Left, 5.0 cm pheochromocytoma, cystic component 50% on contrast enhanced CT.
(K) Left 12.0 cm pheochromocytoma cystic component 64%, peripheral calcification on contrast enhanced CT.
(L) Right 8.2 cm pheochromocytoma, cystic component 28% on contrast enhanced CT.
(M) Right 5.5 cm pheochromocytoma, cystic component 44% on contrast enhanced CT.
(N) Right 6.5 cm pheochromocytoma, cystic component 29% on contrast enhanced CT.
(O) Right 4.4 cm pheochromocytoma, cystic component 24% on contrast enhanced CT.
(P) Right 5.0 cm pheochromocytoma, cystic component 24% on contrast enhanced CT.
(Q) Right 13.5 cm pheochromocytoma, cystic component 23% on contrast enhanced CT.
(R) Left 6.3 cm pheochromocytoma, cystic component 25% on contrast enhanced CT.
(S) Right 14.9 cm pheochromocytoma, cystic component 22% on unenhanced CT.
(T) Left 5.7 cm pheochromocytoma, cystic component 56% on unenhanced CT.
(U) Right 6.3 cm pheochromocytoma, cystic component 70% on contrast enhanced MRI.
Figure 2: Illustrating cystic pheochromocytoma appearance on unenhanced and contrast enhanced computed tomography (CT).
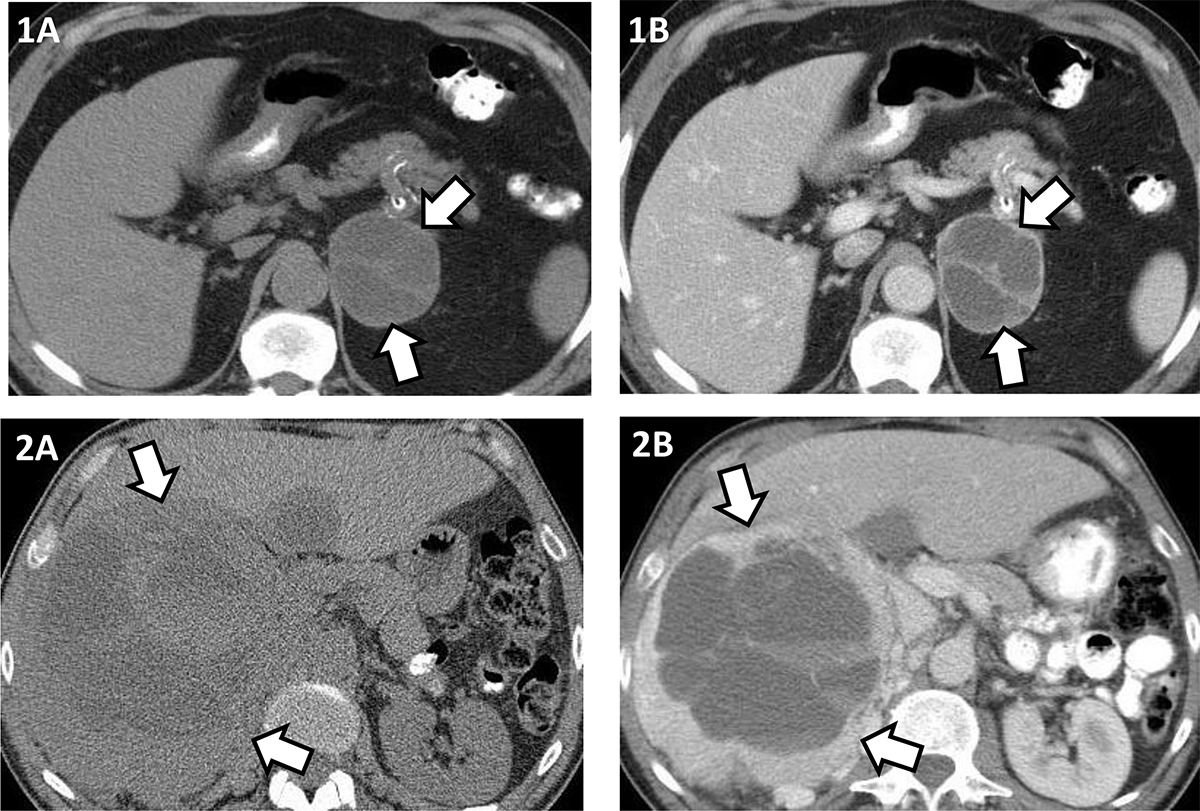
1A and 1B – Left 6.4 cm pheochromocytoma, cystic component 14 HU, solid component 34 HU on unenhanced CT (1A); cystic component 20 HU, solid component 90 HU, rim and septal enhancement on contrast enhanced CT (1B).
2A and 2B –Right 18.9 cm pheochromocytoma, cystic component 18 HU, solid component 35 HU on unenhanced CT (2A); cystic component 21 HU, solid component 132 HU, rim enhancement on contrast enhanced CT (2B).
For the 20 patients who had a preoperative evaluation by an endocrinologist, the primary reason for referral was a concern for pheochromocytoma in 12 (60%) and work-up for an adrenal mass in 8 (40%), with all ultimately recognized as pheochromocytoma preoperatively. The preoperative diagnosis in the 1 patient who did not have work-up for catecholamine excess was thought to be an adrenal metastasis from renal cell carcinoma. Plasma-free fractionated metanephrines were abnormal in all 18 patients who were tested with a median value of 2.7 nmol/L (IQR 0.9–8.7) for metanephrine and 13.2 nmol/L (IQR 3.2–23.6) for normetanephrine. Similarly, 24-hr urine testing was abnormal in all 16 patients who were tested with a median metanephrine excretion of 15828 nmol/day (IQR 5663–33731) and median normetanephrine excretion of 22957 nmol/day (IQR 9206–84472) (Table 1). Most patients (11, 55%) had plasma or urine fractionated metanephrines at least 10 times the upper limit range of normal or higher (Table 2). All 20 patients with preoperative work-up for catecholamine excess had functioning pheochromocytomas: 16 (80%) with adrenergic profile and 4 (20%) with noradrenergic profile (Table 1). Concentrations of the total urinary metanephrines were associated with the largest tumor diameter (R2=0.38, P=0.009), total tumor volume (R2=0.5, P=0.0021), and solid component volume (R2=0.75, P<0.0001), but not the cystic component volume of pheochromocytoma (R2=0.04, P=0.4386).
In 20 patients in whom pheochromocytoma was diagnosed before surgery, preoperative alpha-adrenergic blockade (phenoxybenzamine in 16, doxazosin in 4) for a median duration of 15.5 days (IQR 10.3–20) was administered. In addition, 17 (85%) patients were treated with beta-adrenergic blockade. Finally, 4 (20%) patients received metyrosine for a median duration of 5 days (IQR 2.8–8) prior to surgery, per our institutional protocol11. All 21 patients underwent adrenalectomy, 10 (47.6%, median tumor size 5.7 cm [IQR 4.3–6.4]) via laparoscopic approach and 11 (52.4%, median tumor size 10.1 cm [IQR 6.8–11.9]) via open laparotomy. The median duration of hospitalization was 4 days (IQR 2–6.5). Other than nausea and pain, the 30-day postoperative complications included hypotension and need for vasopressors in 3 patients, acute hypoxia due to pulmonary edema in 1, postoperative ileus in 1 patient and retroperitoneal hemorrhage in the 1 patient identified post-adrenalectomy. Four patients died between 29 to 91 months after surgery, 3 patients due to complications related to extra-adrenal malignancy, and one because of metastatic pheochromocytoma. (Table 1)
DISCUSSION
In this largest to date study of patients with cystic pheochromocytomas, we describe the prevalence, clinical presentation, biochemical parameters, imaging characteristics, and approaches to management.
We found that cystic pheochromocytomas are extremely rare and accounted for 3.2% of pheochromocytomas and 0.27% of adrenal tumors evaluated at our center over two decades. Prevalence of cystic pheochromocytoma in our study was similar to the prior study on cystic adrenal neoplasms from our institutional pathology database (1973–1998) 7. Most cystic pheochromocytomas in our study were found incidentally, while the symptoms of hormone excess led to the discovery of only a quarter of our patients.
Biochemical evaluation with plasma or urinary fractionated metanephrines is a mainstay of preoperative diagnosis of pheochromocytoma. However, biochemically silent pheochromocytomas occur in <5% of cases2. In a smaller study that included 6 patients with cystic pheochromocytoma, only 3 patients demonstrated catecholamine excess, suggesting a lower secretory capacity of cystic pheochromocytomas10. Our findings are discrepant from this study as all cystic pheochromocytomas in our series were biochemically active, and the majority of patients did report symptoms and signs of catecholamine excess, even when diagnosed incidentally based on imaging. We found the excretion of total urinary metanephrines was associated with the volume of the solid component but not the cystic component. Thus, a near-complete cystic pheochromocytoma could be less biochemically active, potentially explaining the differences to the previous smaller study. The cystic component is likely the result of hemorrhagic degeneration due to the large size and rich vascular supply of pheochromocytomas predisposing these tumors to a high frequency of spontaneous hemorrhage12.
In our cohort of patients with cystic pheochromocytoma, the peak incidence was during the 6th decade of life with a slight female predominance. We found cystic pheochromocytomas to be round or oval-shaped tumors with well-defined borders and rarely with calcifications. For unclear reasons, these tumors showed a predilection for the right side. The median unenhanced CT attenuation of the cystic component in cystic pheochromocytomas was similar to benign adrenal cysts, and that of the solid component was in the range of non-cystic pheochromocytoma. Unlike benign adrenal cysts, cystic pheochromocytomas were more likely to present as heterogeneous lesions and have a thick rim exhibiting significant enhancement following contrast administration (Figure 2). This thick rim comprises solid hypersecreting chromaffin tissue.
An open surgical approach may be favored over laparoscopic surgery in some patients with cystic pheochromocytoma due to the risk of intraoperative tumor capsule rupture, especially when the pheochromocytoma is large-sized or with a significant eccentric cystic component. Rupture of a pheochromocytoma intraoperatively is a potentially fatal complication due to hemorrhage and subsequent peritoneal deposits with pheochromocytoma tissue 13. In our series, nearly half of the patients, with markedly larger than average tumour size, underwent adrenalectomy via an open surgical approach. Most patients had an uneventful postoperative recovery. Vasoplegia following resection requiring ICU care and blood pressure support occurred in 14% of patients.
In comparison to patients with non-cystic pheochromocytomas, patients with cystic pheochromocytomas present with a larger size, and are more likely to undergo open adrenalectomy, however otherwise present with similar clinical symptoms2 (Table 3).
Table 3:
Comparison between cystic and noncystic pheochromocytoma
| Cystic pheochromocytoma | Non-cystic Pheochromocytoma* | |
|---|---|---|
| Median age, years (range) | 57 (51–67) | 52 (5–84) |
|
| ||
| Women, (%) | 57% | 52% |
|
| ||
| Mode of discovery, % | ||
| Incidental | 60% | 62% |
| Abdominal mass effect | 25% | - |
| Symptoms of hormone excess | 10% | 26% |
| Genetic surveillance | 5% | 12% |
|
| ||
| Symptoms of catecholamine excess regardless of the mode of discovery, % | 62% | 68% |
|
| ||
| Type of clinical manifestations, % | ||
| Palpitations | 62% | 56% |
| Spells | 62% | 37% |
| Paroxysmal hypertension | 54% | 40% |
| Sustained hypertension | 15% | - |
| Headaches | - | 43% |
|
| ||
| Tumor laterality, % | Predominantly unilateral | Predominantly unilateral, bilateral in 8% |
|
| ||
| Median tumor size, cm (range) | 6.4 (3–19) | 4.0 (8–26) |
|
| ||
| Median unenhanced CT attenuation, HU (range) | Cystic component: 18 (13–30) | 35 (18–58) |
| Solid component: 40 (22–50) | ||
|
| ||
| Median contrast enhancement, HU (range) | Cystic component: 20 (15–33) | 83 (22–187) |
| Solid component: 93 (67–132) | ||
|
| ||
| Biochemical profile, % | ||
| Adrenergic | 80% | 66% |
| Noradrenergic | 20% | 30% |
| Nonfunctioning | 0% | 4% |
|
| ||
| Median total plasma metanephrines, nmol/L (range) | 14.3 (3–223) | Median of 2.2–12.4 (0.4–397)# |
|
| ||
| Median total urinary metanephrines, nmol/L | 43000 (10030–494681) | Median of 6849–23176 (221–674816)# |
|
| ||
| Adrenalectomy, % | ||
| Laparoscopic | 48% | 76% |
| Open | 52% | 24% |
|
| ||
| Median hospital-stay following surgery, days (range) | 4 (1–17) | 3 (1–32) |
Abbreviations – CT: Computed Tomography, HU: Hounsfield Unit
Data from Gruber LM, Hartman RP, Thompson GB, et al. Pheochromocytoma Characteristics and Behavior Differ Depending on Method of Discovery. J Clin Endocrinol Metab. 2019;104(5):1386–1393.
Previously reported stratified based on mode of discovery, lowest in patients discovered based on genetic case detection, and highest in patients presenting with symptoms of catecholamine excess. Presented as range of medians.
One of the main strengths of our study is a relatively larger sample size compared to prior studies, enabling an analytical review of clinical, biochemical, and imaging characteristics of cystic pheochromocytomas. We acknowledge several limitations in our study, primarily due to the retrospective design, making biases related to information, imaging, and selection particularly likely. Additionally, the small sample size and this being an experience of a single center may limit the generalizability of our observations and results. However, considering the rarity of cystic pheochromocytoma, a prospective design with an adequate sample size may not be feasible.
In conclusion, cystic pheochromocytoma is a rare phenotypic variant, presenting various imaging appearances that could masquerade as other adrenal lesions. Though certain imaging characteristics such as a thick enhancing rim and heterogeneous parenchyma raise suspicion for a cystic pheochromocytoma, abnormal biochemical testing (plasma or urinary fractionated metanephrines) provides better preoperative diagnostic accuracy. Therefore, all patients with cystic lesions of the adrenal gland, except in those with an unenhanced CT attenuation of < 10 HU or with the typical appearance of a benign adrenal cyst (thin rim, homogenous appearance, no solid component or septae, and lack of intracystic contrast enhancement), should undergo evaluation for catecholamine excess to help with management decisions.
FUNDING STATEMENT:
This research was partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) USA under award K23DK121888 (to I.B). The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health USA.
Footnotes
CONFLICT OF INTEREST STATEMENT: IB reports advisory board participation with Corcept Therapeutics, HRA Pharma, Sparrow Pharmaceutics, Spruce, Recordati Rare Diseases, Adrenas, Lantheus outside the submitted work. Other authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
DATA AVIALABILITY STATEMENT:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Ebbehoj A, Li D, Kaur RJ, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(11):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruber LM, Hartman RP, Thompson GB, et al. Pheochromocytoma Characteristics and Behavior Differ Depending on Method of Discovery. J Clin Endocrinol Metab. 2019;104(5):1386–1393. [DOI] [PubMed] [Google Scholar]
- 3.Gruber LM, Strajina V, Bancos I, et al. Not all adrenal incidentalomas require biochemical testing to exclude pheochromocytoma: Mayo clinic experience and a meta-analysis. Gland Surg. 2020;9(2):362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canu L, Van Hemert JAW, Kerstens MN, et al. CT Characteristics of Pheochromocytoma: Relevance for the Evaluation of Adrenal Incidentaloma. J Clin Endocrinol Metab. 2019;104(2):312–318. [DOI] [PubMed] [Google Scholar]
- 5.Corwin MT, Mitchell AS, Wilson M, Campbell MJ, Fananapazir G, Loehfelm TW. Accuracy of focal cystic appearance within adrenal nodules on contrast-enhanced CT to distinguish pheochromocytoma and malignant adrenal tumors from adenomas. Abdom Radiol (NY). 2021;46(6):2683–2689. [DOI] [PubMed] [Google Scholar]
- 6.Rozenblit A, Morehouse HT, Amis ES Jr. Cystic adrenal lesions: CT features. Radiology. 1996;201(2):541–548. [DOI] [PubMed] [Google Scholar]
- 7.Erickson LA, Lloyd RV, Hartman R, Thompson G. Cystic adrenal neoplasms. Cancer. 2004;101(7):1537–1544. [DOI] [PubMed] [Google Scholar]
- 8.Chien HP, Chang YS, Hsu PS, et al. Adrenal cystic lesions: a clinicopathological analysis of 25 cases with proposed histogenesis and review of the literature. Endocr Pathol. 2008;19(4):274–281. [DOI] [PubMed] [Google Scholar]
- 9.Major P, Pedziwiatr M, Matlok M, et al. Cystic adrenal lesions - analysis of indications and results of treatment. Pol Przegl Chir. 2012;84(4):184–189. [DOI] [PubMed] [Google Scholar]
- 10.Andreoni C, Krebs RK, Bruna PC, et al. Cystic phaeochromocytoma is a distinctive subgroup with special clinical, imaging and histological features that might mislead the diagnosis. BJU Int. 2008;101(3):345–350. [DOI] [PubMed] [Google Scholar]
- 11.Gruber LM, Jasim S, Ducharme-Smith A, Weingarten T, Young WF, Bancos I. The Role for Metyrosine in the Treatment of Patients With Pheochromocytoma and Paraganglioma. J Clin Endocrinol Metab. 2021;106(6):e2393–e2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75–82. [DOI] [PubMed] [Google Scholar]
- 13.Rafat C, Zinzindohoue F, Hernigou A, et al. Peritoneal implantation of pheochromocytoma following tumor capsule rupture during surgery. J Clin Endocrinol Metab. 2014;99(12):E2681–2685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


