Abstract
Background
Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear.
Objective
To determine the effect of state-imposed regulation on gabapentin prescribing for Medicare Part D enrollees from 2013 to 2018.
Design
Population-based difference-in-difference(DID) analysis study utilizing the Medicare Part D Prescriber Public Use File.
Participants
All eligible Medicare Part D prescribers excluding those outside of the fifty states and the District of Columbia were included in our analysis. Prescriber data and key sociodemographic variables were organized by state and year. States with a gabapentin schedule change or PDMP regulation enacted before 2019 were included in the intervention group. For the Schedule V DID analysis, a control group of the ten highest opioid-prescribing states was used.
Interventions
States with gabapentin schedule changes or PDMP regulation before January 1, 2019, were included and compared to control states that did not implement these policies.
Main Measures
Total days’ supply of gabapentin per enrollee per year was the primary outcome variable.
Key Results
The mean total days’ supply of gabapentin per enrollee increased 41% from 19.71 to 27.81 total days’ supply per enrollee per year between 2013 and 2018. After adjustment, Schedule V gabapentin regulation resulted in a reduction of 8.37 total days of gabapentin prescribed per enrollee (95% confidence interval of − 10.34 to − 6.39). In contrast, PDMP regulation resulted in a reduction of 1.01 total days of gabapentin prescribed per enrollee (95% confidence interval of − 1.74 to − 0.29).
Conclusions
Classifying gabapentin as a Schedule V drug results in substantial reduction in total days prescribed whereas PDMP regulation results in modest reduction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-07314-2.
INTRODUCTION
Gabapentin was first approved for use in the USA in 1993 as an adjunct therapy for partial seizures in adults.1,2 It has since been approved by the US Food & Drug Administration (FDA) for partial seizure adjunct therapy in children, and postherpetic neuralgia. However, gabapentin is frequently prescribed off-label for all types of pain and co-administered with opioids in a variety of health-care settings.3–6 From 2013 to 2018, gabapentin prescriptions increased dramatically from 44 million prescriptions per year to 67 million prescriptions per year making gabapentin the 6th most commonly prescribed drug in the USA in 2018.7,8
Gabapentinoids are prescribed off-label for a wide variety of disorders such as neuropathic pain, fibromyalgia, alcohol withdrawal, insomnia, migraine, mania, and bipolar disorder.9,10 In addition, gabapentinoids are increasingly used in combination with or as an alternative to opioids for all pain types.11–13 As off-label use of gabapentinoids in the treatment of pain has expanded, the risks of gabapentin to patients are increasingly becoming apparent. In 2019, the US Food and Drug Administration (FDA) published a statement requiring new manufacturer warnings for the risk of respiratory depression and increased risk of opioid overdose and death associated with co-use of gabapentinoids and opioids.14–16 While serious harm and adverse events reported with gabapentin are often at doses far exceeding recommendations, gabapentin is not without risk at therapeutic doses. The risk of gabapentinoid and opioid co-use in the Medicare population was investigated, showing an increased risk of overdose for co-users, regardless of dosage, that equates with high-dose opioid consumption.17 Reports of gabapentin misuse describe effects of relaxation, euphoria, dissociation, and sedation, especially at higher doses.18,19 There have also been reports of withdrawal when high doses are abruptly halted and evidence of diversion due to the ease of obtaining gabapentin.3,6,10,20–22 Gabapentin is increasingly prescribed in the geriatric population where the risk of side effects is greater, and multiple comorbidities make it more difficult to recognize the adverse effects.23
In response to surging off-label use of gabapentin and increasing awareness of the medication risks, several states have taken the highly unusual step of passing state-specific regulations for gabapentin. In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death.24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in their states’ Prescription Drug Monitoring Program (PDMP). The impact of these state policies moving beyond FDA and Drug Enforcement Association (DEA) regulation is unclear. The objective of this study is to evaluate the effects of state-specific gabapentin legislation on gabapentin prescribing in the Medicare Part D enrollee population.
METHODS
Data Source
We used data from the Centers for Medicare & Medicaid Services (CMS) Part D Public Use File that included all gabapentin prescriptions written by providers and filled on behalf of Medicare Part D enrollees for the years 2013 through 2018.29 The dataset included all enrollees who received gabapentin from a prescriber in the 50 states and the District of Columbia. We excluded prescribers located in US territories. In 2018 Medicare Part D beneficiaries comprise 70% of Medicare enrollees, and 70% of these enrollees are age 65 or greater.30 Data from providers who wrote fewer than ten gabapentin prescriptions or fewer than fifty Medicare prescriptions in a given year of the study period were suppressed from the dataset for patient privacy purposes. Gabapentin prescriptions were sorted by state generating one record per state per year for total gabapentin claims and total gabapentin days’ supply. We weighted states by population size using US Census data. We excluded pregabalin in our study as pregabalin is already a federal Schedule V controlled substance, still patented, more expensive, and comprised only 12.6% of overall gabapentinoid prescriptions in this dataset.
Variables
Our primary outcome was the total days’ supply of gabapentin per enrollee per state. CMS defines total days’ supply as the “aggregate number of day’s supply for which this drug was dispensed.”31FDA-approved gabapentin dosing ranges from 300 to 3600 mg, with a wide range of doses depending on the indication. Off-label dosages may also be highly variable. Our secondary outcomes were total gabapentin claims per enrollee and total gabapentin days’ supply per claim.
We included state-level covariates from the US Census American Community Survey data from 2013 through 2018 including median age, sex, and race (percent non-Hispanic white, percent non-Hispanic black, percent Asian or Pacific Islanders, and percent race not classified).32 We used the National Survey on Drug Use and Health (NSDUH) data from 2013 through 2018 to obtain state-level rates of alcohol use in the past month (age 12 or older estimate), tobacco use in the past month (age 12 or older estimate), nonmedical use/misuse of pain relievers in the past year (age 12 or older estimate), and serious mental illness in the past year (age 18 or older estimate) for each year of our study period.33 NSDUH data for nonmedical use of pain relievers was not available for 2015 and was therefore not included in the analysis.
We compared two interventions including (1) class V scheduling of gabapentin and (2) gabapentin inclusion in the state PDMP using a control group of similar states. The Schedule V change for gabapentin was implemented in three states including Kentucky, Tennessee, and West Virginia (Table 1). These three states were all among the top quartile for opioids prescribed per 100 persons according to the 2018 National Institute on Drug Abuse; therefore, we used a control group of 10 states also in the top quartile for opioid prescribing (Alabama, Arkansas, Georgia, Indiana, Kansas, Louisiana, Missouri, Mississippi, Oklahoma, South Carolina).34 This control group was intended to assess prescribing outcomes in states with comparable environments, as the link between opioid abuse and gabapentinoids has been well described.3,21,35 The gabapentin PDMP regulation group included nine states that enacted legislation that took effect before December 31, 2018, including Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming (Table 1). We compared the gabapentin PDMP regulation group to a control group consisting of all other states with no PDMP or scheduling regulations for gabapentin. These intervention and control groups had similar rates of opioid prescriptions per enrollee.
Table 1.
Summary of Gabapentin Policy Changes 2013–2018
| State | Policy start date | Gabapentin policy type |
|---|---|---|
| Kansas | 7/25/2017 | PDMP Reporting for Gabapentin |
| Kentucky | 3/3/2017 | Schedule V Classification of Gabapentin |
| Massachusetts | 8/1/2017 | PDMP Reporting for Gabapentin |
| Minnesota | 8/1/2016 | PDMP Reporting for Gabapentin |
| Nebraska | 1/1/2018 | PDMP Reporting for Gabapentin |
| New Jersey | 5/7/2018 | PDMP Reporting for Gabapentin |
| North Dakota | 8/1/2017 | PDMP Reporting for Gabapentin |
| Ohio | 12/1/2016 | PDMP Reporting for Gabapentin |
| Tennessee | 7/1/2018 | Schedule V Classification of Gabapentin |
| Virginia | 2/23/2017 | PDMP Reporting for Gabapentin |
| West Virginia | 6/7/2018 | Schedule V Classification of Gabapentin |
| Wyoming | 7/1/2017 | PDMP Reporting for Gabapentin |
Statistical Analysis
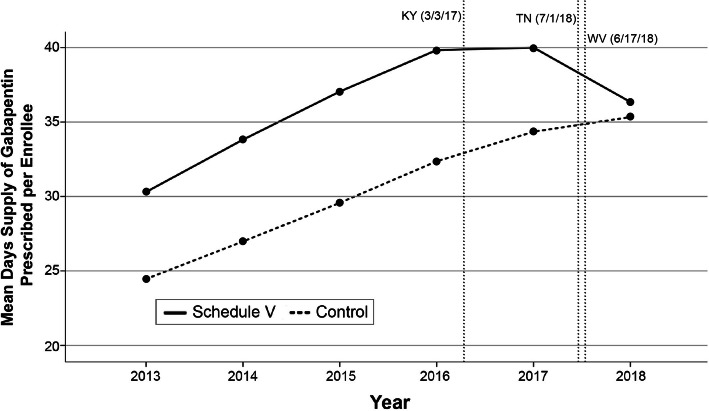
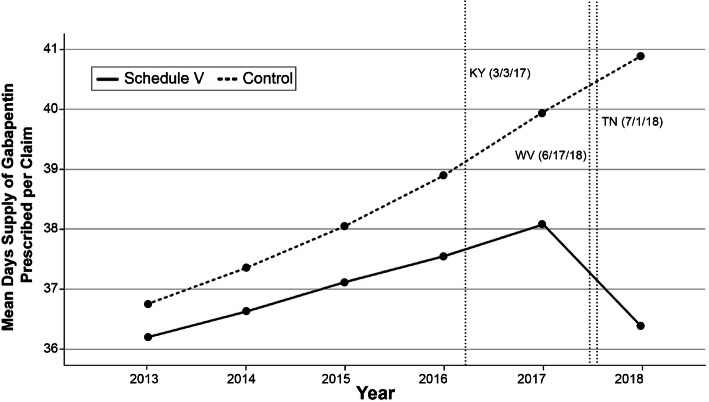
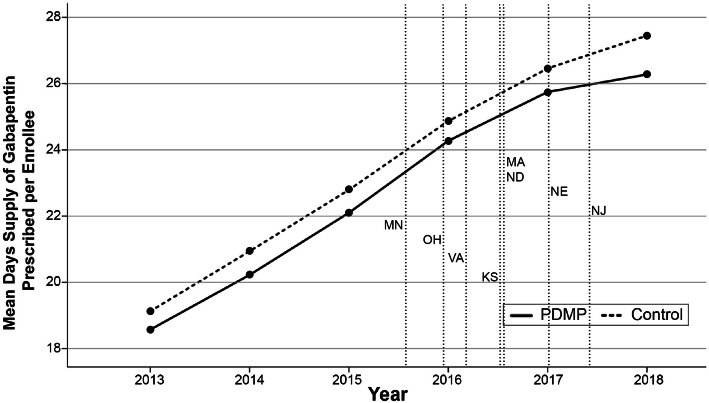
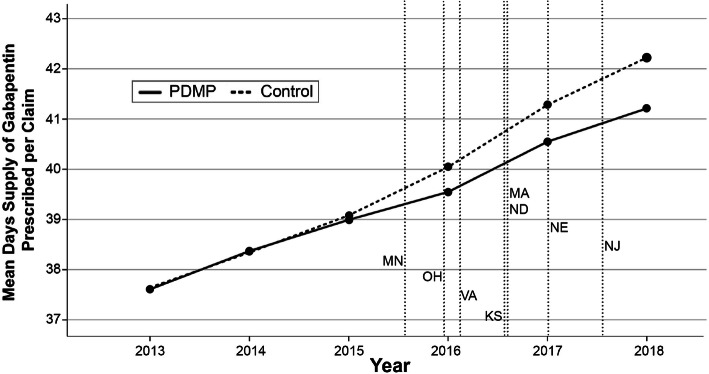
We developed DID models for each of intervention. To assess the suitability of a DID model, pre-trend analyses were graphed showing the unadjusted mean days’ supply of gabapentin per enrollee and claim for each cohort from 2013 to 2018 (Figs. 1, 2, 3, and 4). We conducted visual pre-trend analysis to show that this assumption is met by relatively similar slopes for pre-trend time periods (2017 for the Schedule V cohort and before 2016 for the PDMP cohort). The first model assessed the effect of Schedule V gabapentin regulation on prescribing rates by a DID regression comparing the total days’ supply of gabapentin, total gabapentin claims per Medicare Part D enrollee, and total days per claim. This model compared states with Schedule V regulation in place during the study period to a control group with no gabapentin regulation. The second model assessed the effect of gabapentin PDMP regulation on prescribing rates by a DID regression comparing the total days’ supply of gabapentin, total gabapentin claims per enrollee, and total days per claim in states with PDMP regulation to a control group with no gabapentin regulation. Both models adjusted for time of regulation, sex, race, ethnicity, year, age, and each state’s rate of serious mental illness, tobacco use, alcohol use, and pain medication misuse/abuse. We accounted for the timing of regulation by appointing states values between 0 and 1 for each year of the study period that either Schedule V or PDMP regulation was in effect.36 Where 0 represented no regulation, 1 represented regulation in place for an entire year, and a value between 0 and 1 represented the fraction of a year that each regulation was in place. For example, a state that passed regulation taking effect on July 1, 2017, was appointed a value of 0 for 2016, 0.504 for 2017, and a value of 1 for 2018.
Figure 1.
Schedule V regulation states vs. control states’ unadjusted mean days’ supply of gabapentin prescribed per Medicare Part D enrollee from 2013 to 2018
Figure 2.
Schedule V regulation states vs. control states unadjusted mean days’ supply of gabapentin prescribed per Medicare Part D claim from 2013 to 2018
Figure 3.
PDMP regulation states vs. control states unadjusted mean days’ supply of gabapentin prescribed per Medicare Part D enrollee from 2013 to 2018
Figure 4.
PDMP regulation states vs. control states unadjusted mean days’ supply of gabapentin prescribed per Medicare Part D claim from 2013 to 2018
We also analyzed gabapentin-prescribing patterns by prescriber specialty for the Schedule V and PDMP intervention cohorts. All gabapentin prescribers were organized into five specialty categories: primary care, anesthesia/pain, neurology, medical & surgical specialties, and other prescribers. the primary care group included general medicine, internal medicine, family medicine, geriatric medicine, and pediatric medicine prescribers. The anesthesia/pain group included anesthesia, pain management, and physical & rehabilitation medicine. The medical & surgical specialty group included all other physician specialties. The other prescribers group included trainees and mid-level prescribers. These categorizations were selected after considering the indications for gabapentin, the volume of prescribers in each specialty, and after conducting primary analyses to assess rates of prescribing for each CMS-designated specialty code during the study period.
Statistical analyses were performed using SPSS Version 27 (SPSS, Chicago, IL, USA) from April 2021 to June 2021. We used multivariate linear regression models for each function performed. The Wayne State University Institutional Review Board exempted the study from board approval and informed consent because this study was not considered human subject research. The authors adhered to the STROBE observational study guidelines.37
RESULTS
Our dataset included 531,780 gabapentin prescribers. Over the 6-year period, there were 5,761,798,472 total days’ supply of gabapentin prescribed to Medicare Part D enrollees via 143,737,549 claims submitted by prescribers with an average of 40.09 days prescribed per claim. The mean total days’ supply of gabapentin per Medicare Part D enrollee increased 41% (from 19.70 to 27.81 total days’ supply per enrollee per year) over the 6-year period of our study, and the mean total claims per enrollee increased 26% (from 0.53 to 0.67 claims per enrollee per year).
Schedule V Regulation
When we compared states with gabapentin Schedule V regulation to a control group in a DID regression analysis (Table 2), we estimated a decrease of 8.37 total days’ supply of gabapentin per enrollee per year (95% confidence interval (CI) of − 10.34 to − 6.39). When we compared the Schedule V regulation group to the control, we found a decrease of 0.10 claims per enrollee (CI − 0.15 to − 0.05) and a decrease of 4.85 total days per claim (CI − 5.95 to − 3.74).
Table 2.
Difference-in-Difference Results Showing Changes in Gabapentin Prescribing After Implementing Regulation for Schedule V and PDMP Cohorts. Difference Values Based on Raw Gabapentin Prescribing Data 2013–2018, and DID Estimates Comparing Control and Intervention Groups After Accounting for Covariates
| Schedule V regulation cohort | Control states (n = 10) |
States exposed to policy (n = 3) |
Difference-in-differences estimate | |||||
|---|---|---|---|---|---|---|---|---|
| 2013 | 2018 | Difference | 2013 | 2018 | Difference | Estimate | 95% CI | |
| Total days per enrollee | 24.23 | 34.74 | 10.51 | 30.30 | 36.34 | 6.04 | − 8.37 | − 10.34 to − 6.39 |
| Claims per enrollee | 0.66 | 0.85 | 0.19 | 0.84 | 1.00 | 0.16 | − 0.10 | − 0.15 to − 0.05 |
| Total days per claim | 36.75 | 40.90 | 4.15 | 36.19 | 36.36 | 0.17 | − 4.85 | − 5.95 to − 3.74 |
| PDMP regulation cohort |
Control states (n = 39) |
States exposed to policy (n = 9) |
Difference-in-differences estimate | |||||
| 2013 | 2018 | Difference | 2013 | 2018 | Difference | Estimate | 95% CI | |
| Total days per enrollee | 19.07 | 27.38 | 8.31 | 18.94 | 26.84 | 7.90 | − 1.01 | − 1.74 to − 0.29 |
| Claims per enrollee | 0.51 | 0.65 | 0.14 | 0.51 | 0.66 | 0.15 | − 0.014 | − 0.03 to 0.006 |
| Total days per claim | 37.73 | 42.43 | 4.70 | 37.22 | 40.56 | 3.34 | − 0.89 | − 1.57 to − 0.20 |
PDMP Regulation
States that added new gabapentin PDMP regulation during our study period from 2013 to 2018 were compared to a control group in a regression analysis model to generate DID estimates (Table 2). We estimated a decrease of 1.01 total days’ supply of gabapentin per enrollee per year (CI of − 1.74 to − 0.29) with gabapentin PDMP regulation. When we compared the PDMP intervention group to the control, we also found a decrease of 0.01 claims per enrollee (CI − 0.03 to 0.006) and a decrease of 0.89 total days per claim (CI − 1.57 to − 0.20).
Specialty Data
Prescribers were categorized into five designated groups: primary care, anesthesia/pain, neurology, medical & surgical specialties, and other prescribers. Our analysis included 217,729 primary care prescribers, 21,959 anesthesia/pain prescribers, 17,108 neurology prescribers, 115,673 medical & surgical specialty prescribers, and 159,311 other prescribers. These groups were used to assess DID estimates for total days prescribed and claims per enrollee for the Schedule V and PDMP regulation cohorts using the same control groups as our previous models (Table 3). We found that the largest changes in prescribing were in the primary care and other prescriber groups in the Schedule V cohort. The DID estimate for primary care in the Schedule V cohort was a decrease of 5.68 total days per enrollee (CI − 7.04 to − 4.31) and 0.070 claims per enrollee (CI − 0.11 to − 0.04). The DID estimate for other prescribers in the Schedule V cohort was a decrease of 2.20 total days per enrollee (CI − 3.41 to − 0.99) and 0.03 claims per enrollee (CI − 0.07 to 0.001).
Table 3.
Difference-in-Difference Results for Medical Specialties Sub-analysis of Schedule V and PDMP Cohorts. Difference Values Comparing Raw Gabapentin Days Supply for Each Prescriber Group in 2013–2018, and DID Estimates Comparing Control and Intervention Groups After Accounting for Covariates
| Schedule V regulation cohort | Control states (n = 10) |
States exposed to policy (n = 3) |
Difference-in-differences estimate | |||||
|---|---|---|---|---|---|---|---|---|
| 2013 | 2018 | Difference | 2013 | 2018 | Difference | Estimate | 95% CI | |
| Primary care total days per enrollee | 15.60 | 20.57 | 4.96 | 18.09 | 19.97 | 1.87 | − 5.68 | − 7.04 to − 4.31 |
| Anesthesia/pain total days per enrollee | 1.66 | 2.21 | 0.55 | 1.79 | 2.27 | 0.48 | 0.57 | 0.10 to 1.03 |
| Neurology total days per enrollee | 1.50 | 1.64 | 0.14 | 1.73 | 1.59 | − 0.14 | − 0.28 | − 0.44 to − 0.13 |
| Medical & surgical specialties total days per enrollee | 3.08 | 3.59 | 0.51 | 3.30 | 3.29 | − 0.01 | − 0.65 | − 0.95 to − 0.35 |
| Other prescribers total days per enrollee | 2.42 | 6.34 | 3.92 | 5.38 | 9.22 | 3.84 | − 2.20 | − 3.41 to − 0.99 |
| PDMP regulation cohort |
Control states (n = 39) |
States exposed to policy (n = 9) |
Difference-in-differences estimate | |||||
| 2013 | 2018 | Difference | 2013 | 2018 | Difference | Estimate | 95% CI | |
| Primary care total days per enrollee | 12.03 | 16.08 | 4.06 | 12.07 | 15.80 | 3.72 | − 0.73 | − 1.20 to − 0.26 |
| Anesthesia/pain total days per enrollee | 1.12 | 1.47 | 0.35 | 0.90 | 1.13 | 0.23 | − 0.10 | − 0.21 to − 0.012 |
| Neurology total days per enrollee | 1.29 | 1.42 | 0.12 | 1.31 | 1.51 | 0.20 | − 0.04 | − 0.11 to 0.022 |
| Medical & surgical specialties Total days per enrollee | 2.16 | 2.62 | 0.47 | 2.05 | 2.53 | 0.48 | − 0.07 | − 0.18 to 0.040 |
| Other prescriber total days per enrollee | 2.47 | 5.78 | 3.31 | 2.61 | 5.72 | 3.11 | − 0.07 | − 0.42 to 0.29 |
In the PDMP regulation DID analysis, there was an estimated decrease of 0.73 total days’ supply of gabapentin in the primary care cohort (CI − 1.20 to − 0.26).
DISCUSSION
We found widespread and escalating gabapentin prescribing in the Medicare Part D population. In 2018, gabapentin prescriptions accounted for an average of 27.81 days per enrollee per year, a 41% increase from 2013. Our findings indicate that Schedule V changes to gabapentin implemented in three states significantly reduced gabapentin prescribing behavior in Medicare Part D enrollee prescribers. In contrast, we found a modest decrease in gabapentin prescribing for states that implemented PDMP regulation.
It is unusual for states to take drug regulation into their own hands. While prescription drug monitoring has been a state issue for many years, drug scheduling is typically conducted at the federal level. Gabapentin is not currently classified as a controlled substance by any federal organization. Furthermore, much of the state-specific regulation regarding other scheduled drugs is on the topic of drug crimes whereas these laws for gabapentin are intended to curb distribution by prescribers as a state-specific response to the role gabapentinoids play in the opioid epidemic.35 Our data shows that states with the greatest rates of gabapentin prescribing are the same states that have high rates of opioid prescribing.5 Others have suggested that gabapentin abuse potential stems from the wide variety of off-label uses as well as a shift towards prescribing gabapentinoids as an opioid alternative.4,5,10
Whereas PDMP regulation alone provides a form of oversight to monitor gabapentin prescribing, Schedule V regulation for gabapentin has a greater impact on prescribers, pharmacies, and patients. Schedule V drugs are typically defined as those with a low potential for abuse and include drugs with low quantities of opioids. In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance.38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky prescribers are also required to use controlled substance security prescription pads or certified secured electronic prescriptions for gabapentin. In all three states with Schedule V gabapentin regulation, prescription, and dispensation data is added to each state’s PDMP. Pharmacies are required to monitor the gabapentin inventory more closely and follow controlled substance requirements for storage and disposal and must also follow federal limitations on refill and partial fill rules for gabapentin. Schedule V regulation also increases penalties for illegal possession and intent to manufacture or distribute gabapentin by unauthorized individuals.39
Federal law does not limit the quantity or number of refills of Schedule V controlled substances, but some states and health insurance companies place limits. In Kentucky, Tennessee, and West Virginia, Schedule V controlled substances cannot be prescribed with more than five refills and prescriptions cannot be filled more than 6 months after they were written.24,27,40 We observed that a substantial proportion of the overall decrease seen in Schedule V states occurred as a result of fewer days prescribed per prescription claim. These findings may be a consequence of prescribers writing shorter prescriptions or pharmacists limiting the quantity prescribed. In addition to each state’s controlled substance laws, the additional awareness and caution that state legislators, professional associations, and the media placed on gabapentin may have also contributed to the observed changes in prescribing behavior.24,35
Prescribers are at a crossroads as this recent gabapentin regulation is largely a reflexive response to the opioid epidemic. Many have suggested that the increased gabapentin prescribing rates are likely a response to a strong emphasis on the reduction and elimination of opioid prescriptions.6,23,41 When we examined gabapentin prescribing in specialty-specific cohorts, we observed that the greatest reduction in gabapentin prescribing with Schedule V legislation occurred in primary care; however, we observed increased prescribing by pain/anesthesia providers. Gabapentin was recommended as a component of multimodal anesthesia for the management of perioperative pain in the 2016 guidelines from a consortium of professional societies including the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists.13 These guidelines may help to explain our findings showing the anesthesia/pain group with an increased total days’ supply of gabapentin in our analysis. In addition, some patients receiving gabapentin from a primary care provider before these regulations were enacted may be seeing pain specialists for gabapentin prescriptions.
It is important to recognize that new regulation and prescribing restrictions may also impact patients who rely on gabapentin to manage chronic pain conditions such as trigeminal neuralgia, neurologic diseases, and seizure disorders, among other conditions.5,12 Patients in states with Schedule V regulation may require more frequent visits to their health-care providers and pharmacy. In addition, prescribers and pharmacists may be hesitant to prescribe and dispense higher quantities of gabapentin to these patients due to this regulation.
Since the period of our study, three additional states, North Dakota, Michigan, and Virginia, have implemented Schedule V regulation, and two states, Illinois and Utah, added state-mandated PDMP requirements for gabapentin. As states continue to implement their own specific gabapentin regulations, it is important to evaluate the effect of public health interventions, regulation, and policy on prescribing behavior. Our data support that this policy is effective at lowering rates of gabapentin prescribing in the Medicare Part D enrollee population. To support the national imperative to reduce gabapentin prescribing, the FDA may consider changing the federal gabapentin schedule classification to Schedule V.
Limitations
There are many limitations to consider before drawing conclusions from our findings. The population included all Medicare Part D prescribers who wrote at least ten gabapentin prescriptions for one or more of the years included in our study period. Data from prescribers writing fewer than ten prescriptions was suppressed. Location data is based on prescriber location; therefore, prescriptions written in one state may have been filled and consumed in another state. The data source limited our ability to identify doses and concentrations of gabapentin prescribed, and we did not calculate clustered standard errors based on the prescriber. Further investigation of gabapentin prescribing and its effects on patients would improve our understanding of this issue. Due to the nature of our data source, we were unable to track the frequency of primary care, specialty care, or emergency department visits for our patient cohorts, to assess changes that may have resulted from gabapentin regulation. While our data demonstrates changes in gabapentin prescribing in the Medicare Part D population, we cannot determine whether those changes influenced overall patient outcomes and further study is needed to determine the benefit/drawbacks of gabapentin regulation on this population. Finally, our data is limited to the Medicare Part D population.
CONCLUSIONS
Gabapentin is one of the most prescribed drugs in the USA and is commonly used off-label. However, the adverse effects of gabapentin are increasingly being recognized leading some states to target gabapentin for state-specific regulation. We demonstrate that implementation of Schedule V controlled substance regulation significantly reduces gabapentin prescribing and causes a reduction in total days’ supply per enrollee and days per claim. In contrast, we demonstrate minimal impact on gabapentin prescribing in states that added gabapentin PDMP regulation.
Supplementary Information
(DOCX 12 kb)
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.U.S. Food & Drug Administration. NEURONTIN (Gabapentin) Label. Accessed June 7, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
- 2.U.S. Food & Drug Administration. GRALISE (Gabapentin) Label. Accessed June 8, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022544s026lbl.pdf
- 3.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160–1174. doi: 10.1111/add.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman CW, Brett AS. Gabapentin and Pregabalin for Pain — Is Increased Prescribing a Cause for Concern? N Engl J Med. 2017;377(5):411–414. doi: 10.1056/NEJMp1704633. [DOI] [PubMed] [Google Scholar]
- 5.Pauly NJ, Delcher C, Slavova S, Lindahl E, Talbert J, Freeman PR. Trends in gabapentin prescribing in a commercially insured U.S. Adult population, 2009-2016. J Manag Care Spec Pharm. 2020;26(3):246–252. doi: 10.18553/jmcp.2020.26.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292–294. doi: 10.1001/jamainternmed.2017.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medicine Use and Spending in the U.S.; 2019. Accessed June 15, 2021. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/medicine-use-and-spending-in-the-us%2D%2D-a-review-of-2018-outlook-to-2023.pdf?_ = 1623766932941
- 8.Medicines Use and Spending in the U.S.; 2017. Accessed June 18, 2021. www.quintilesimsinstitute.org
- 9.McAnally H, Bonnet U, Kaye AD. Gabapentinoid Benefit and Risk Stratification: Mechanisms Over Myth. Pain Ther. 2020;9(2):441–452. doi: 10.1007/s40122-020-00189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: The case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy. 2018;11:109–116. doi: 10.2147/RMHP.S168504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021–1026. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 12.Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm. 2003;9(6):559–568. doi: 10.18553/jmcp.2003.9.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou R, Gordon DB, De Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive commi. J Pain. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food & Drug Administration. Neurontin, Gralise, Horizant (Gabapentin) and Lyrica, Lyrica CR (Pregabalin): Drug Safety Communication - Serious Breathing Problems.; 2019. Accessed June 14, 2021. https://www.fda.gov/safety/medical-product-safety-information/neurontin-gralise-horizant-gabapentin-and-lyrica-lyrica-cr-pregabalin-drug-safety-communication
- 15.Cavalcante AN, Sprung J, Schroeder DR, Weingarten TN. Multimodal analgesic therapy with gabapentin and its association with postoperative respiratory depression. Anesth Analg. 2017;125(1):141–146. doi: 10.1213/ANE.0000000000001719. [DOI] [PubMed] [Google Scholar]
- 16.Pergolizzi JV. Exploring the Combination of Gabapentinoids and Opioids for Postoperative Analgesia. JAMA Netw Open. 2020;3(12):e2032139. doi: 10.1001/jamanetworkopen.2020.32139. [DOI] [PubMed] [Google Scholar]
- 17.Z L, B S, K CK, et al. Dual-trajectories of opioid and gabapentinoid use and risk of subsequent drug overdose among Medicare beneficiaries in the United States: a retrospective cohort study. Addiction. 2021;116(4):819–830. doi: 10.1111/ADD.15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell LS, Coomer TN, Jacob GK, Lenz RJ. Gabapentin controlled substance status. J Am Pharm Assoc. Published online 2021. 10.1016/j.japh.2021.01.025 [DOI] [PubMed]
- 19.Smith BH, Higgins C, Baldacchino A, Kidd B, Bannister J. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):406–407. doi: 10.3399/bjgp12X653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487–488. doi: 10.1176/appi.ajp.2014.14101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580–1589. doi: 10.1111/add.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185–1215. doi: 10.1016/j.euroneuro.2017.08.430. [DOI] [PubMed] [Google Scholar]
- 23.Goodman CW, Brett AS. Gabapentinoids for Pain: Potential Unintended Consequences. Vol 100.; 2019. . www.aafp.org/afp. [PubMed]
- 24.Hawk DB. HOUSE BILL 1832 AN ACT to amend Tennessee Code Annotated, Title 39, Chapter 17, Part 4; Title 41, Chapter 21, Part 2; Title 53 and Title 63, relative to controlled or addictive substances. Published online 2018:1-27.
- 25.West Virginia pharmacy board: Gabapentin a “drug of concern.” Accessed June 8, 2021. https://www.whsv.com/content/news/West-Virginia-pharmacy-board-Gabapentin-a-drug-of-concern-462383043.html
- 26.WEST VIRGINIA CODE CHAPTER 60A. UNIFORM CONTROLLED SUBSTANCES ACT. A-2-212. Schedule V.; :2-3. https://code.wvlegislature.gov/60A-2-212/
- 27.Section 902 KAR 55:015 - Schedules of controlled substances, 902 Ky. Admin. Regs. 55:015 | Casetext Search + Citator. Accessed June 14, 2021. https://casetext.com/regulation/kentucky-administrative-regulations/title-902-cabinet-for-health-and-family-services-department-for-public-health/chapter-55-controlled-substances/section-902-kar-55015-schedules-of-controlled-substances
- 28.Faryar KA, Webb AN, Bhandari B, Price TG, Bosse GM. Trending gabapentin exposures in Kentucky after legislation requiring use of the state prescription drug monitoring program for all opioid prescriptions#. Clin Toxicol. 2019;57(6):398–403. doi: 10.1080/15563650.2018.1538518. [DOI] [PubMed] [Google Scholar]
- 29.Medicare Provider Utilization and Payment Data: Part D Prescriber | CMS. Accessed June 7, 2021. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Part-D-Prescriber
- 30.Juliette Cubanski, Anthony Damico, Tricia Neuman. Medicare Part D in 2018: The Latest on Enrollment, Premiums, and Cost Sharing | KFF. Published May 17, 2018. Accessed June 14, 2021. https://www.kff.org/medicare/issue-brief/medicare-part-d-in-2018-the-latest-on-enrollment-premiums-and-cost-sharing/
- 31.Medicare Fee-For Service Provider Utilization & Payment Data Part D Prescriber Public Use File: A Methodological Overview.; 2020. Accessed June 14, 2021. https://nppes.cms.hhs.gov/.
- 32.American Community Survey (ACS). Accessed June 14, 2021. https://www.census.gov/programs-surveys/acs
- 33.National Survey on Drug Use and Health | CBHSQ Data. Accessed June 14, 2021. https://www.samhsa.gov/data/data-we-collect/nsduh-national-survey-drug-use-and-health
- 34.Opioid Summaries by State | National Institute on Drug Abuse (NIDA). Accessed June 14, 2021. https://www.drugabuse.gov/drug-topics/opioids/opioid-summaries-by-state
- 35.Vestal C. Abuse of Opioid Alternative Gabapentin Is on the Rise | The Pew Charitable Trusts. Stateline. Published 2018. Accessed June 15, 2021. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/05/10/abuse-of-opioid-alternative-gabapentin-is-on-the-rise
- 36.Yarbrough C. Prescription Drug Monitoring Programs Produce a Limited Impact on Painkiller Prescribing in Medicare Part D. Health Serv Res. 2018;53(2):671–689. doi: 10.1111/1475-6773.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ Br Med J 2007;335(7624):806. 10.1136/BMJ.39335.541782.AD [DOI] [PMC free article] [PubMed]
- 38.Kentucky Cabinet for Health and Family Services. Important Notice: Gabapentin Becomes a Schedule 5 Controlled Substance in Kentucky.
- 39.New Statue Gabapentin 06-18. https://www.tn.gov/content/dam/tn/health/healthprofboards/New Statue Gabapentin 06-18.pdf
- 40.West Virginia Legislature’s Office of Reference & Information. West Virginia Code CHAPTER 60A. UNIFORM CONTROLLED SUBSTANCES ACT. Accessed June 28, 2021. https://www.wvlegislature.gov/wvcode/code.cfm?chap = 60A&art = 3#01
- 41.Throckmorton DC, Gottlieb S, Woodcock J. The FDA and the Next Wave of Drug Abuse — Proactive Pharmacovigilance. N Engl J Med. 2018;379(3):205–207. doi: 10.1056/nejmp1806486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb)