Abstract
Morphological changes, especially cytoskeletal alterations, in lipopolysaccharide (LPS)-induced vascular endothelial cell injury were studied by using LPS-susceptible bovine aortic endothelial cells (BAEC). BAEC in cultures with LPS showed cell rounding, shrinking, and intercellular gap formation. In those cells, LPS caused the disorganization of actin, tubulin, and vimentin. LPS also induced a reduction in the F-actin pool and an elevation in the G-actin pool. Cytoskeletal disorganization affected transendothelial permeability across the endothelial monolayer. Pretreatment of BAEC with sodium arsenite (SA) prevented alterations in LPS-induced BAEC injury. However, posttreatment with SA had no protective effect on them. SA upregulated the expression of heat shock protein in the presence of LPS. The role of SA in prevention of LPS-induced BAEC injury is discussed.
Bacterial lipopolysaccharide (LPS), an outer membrane component of gram-negative bacteria, has been shown to directly induce systemic injury of vascular endothelial cells and cause systemic inflammatory response syndrome or multiorgan failure (3). In an in vitro culture system, LPS was reported to induce the injury of bovine aortic endothelial cells (BAEC) directly in the absence of nonendothelial-cell-derived host mediators (11, 13, 20, 23). The LPS-induced BAEC injury is accompanied by altered cell morphology, intercellular gap formation, and increased transendothelial permeability (12, 21, 24). It was possible that cytoskeletal alterations in LPS-induced BAEC injury were closely linked to intercellular gap formation and endothelial barrier dysfunction (12). However, there were few reports on detailed alterations of cytoskeleton in morphological changes of LPS-induced BAEC injury (12). Furthermore, knowledge regarding factors preventing the alterations in LPS-induced BAEC injury is very limited (15, 16, 26).
Sodium arsenite (SA) is known to be a standard inducer of the heat shock response in vitro and can lead to heat shock protein (HSP) expression in vascular endothelial cells (4, 5). Several reports suggest that SA prevented LPS-induced endothelial cell injury via enhanced heat shock response (6, 27, 32). Therefore, it was of particular interest to determine if and how SA affected morphological changes in LPS-induced BAEC injury. In the present study, we examined the detailed cytoskeletal alterations in LPS-induced vascular endothelial cell injury by using LPS-susceptible BAEC and, furthermore, observed the effect of SA on them. Here we discuss the role of SA in the prevention of LPS-induced BAEC injury.
MATERIALS AND METHODS
Materials.
LPS from Escherichia coli O55:B5 was obtained from Sigma Chemical Co., St. Louis, Mo. LPS was dissolved at a concentration of 1 mg/ml in distilled water and diluted in culture medium for experiments. SA (Wako Pure Chemicals, Osaka, Japan) was dissolved at a concentration of 1 mM and diluted to 100 μM in culture medium for experiments.
Cell culture.
BAEC were obtained from the Health Science Resource Bank (Tokyo, Japan) and maintained in Ham's F-12K medium (Sigma) containing 10% heat-inactivated horse serum (Gibco-BRL, Grand Island, N.Y.) at 37°C under 5% CO2. The cells were washed gently with Hank's balanced salt solution (Sigma) and detached with trypsin-EDTA solution (Gibco-BRL). The cells were counted and suspended in a 96-well plate or 12-well plate. In experiments with LPS treatment, culture medium was supplemented with noninactivated 1% horse serum because our preliminary experiments with 1% heat-inactivated serum caused attenuation of LPS action.
Pretreatment with SA.
For preparation of SA-pretreated BAEC, BAEC were cultured with 100 μM SA for 90 min at 37°C. The culture medium containing SA was removed and washed with the fresh culture medium. These cells were used as SA-pretreated BAEC for the experiments. In some experiments, BAEC were pretreated with various concentrations of SA.
Fluorescent staining of F-actin, tubulin, and vimentin.
BAEC were seeded on glass coverslips and incubated for 48 h. Untreated and SA-pretreated BAEC were cultured with various concentrations of LPS. The coverslips were incubated for 6 h, and then cells were fixed with 3.5% formaldehyde for 20 min and permeabilized with 0.1% Triton X-100 for 10 min. The cells were blocked with 2% bovine serum albumin (BSA) for 1 h. For F-actin analysis, cells were stained with fluorescein-phalloidin (Sigma) for 20 min. For vimentin and tubulin analyses, cells were incubated with a 1:10 dilution of antivimentin antibody (Progen, Heidelberg, Germany) or a 1:200 dilution of antitubulin antibody (Sigma) for 1 h followed by six washes with phosphate-buffered saline. Fluorescein isothiocyanate-conjugated antimouse immunoglobulin G (IgG) or antirabbit IgG antibody was added to the cells, which were then incubated for 30 min. After being washed, the cells were inspected for organization of F-actin, vimentin, and tubulin under a fluorescence microscope.
Assay of transendothelial permeability.
Transendothelial flux of 14C-BSA was assayed as described by Goldblum et al. (12) with some modifications. Briefly, BAEC (3 × 104 cells/0.5 ml) were seeded on mini cell culture inserts (0.4-μm pore size; Nunc, Roskilde, Denmark). The inserts were placed in 24-well plates with 0.5 ml of medium serving as the lower compartment. The cells on the upper compartment of the inserts were treated with various concentrations of LPS for 6 h for various exposure times. 14C-BSA was obtained from Amersham, Arlington Heights, Ill. The baseline barrier function of each confluent endothelial monolayer was determined by applying an equivalent amount of 14C-BSA (5,000 dpm/0.5 ml) to the upper compartment for 1 h at 37°C, after which 0.5 ml of medium from the lower compartment was removed. The medium removed was mixed with 4.5 ml of scintillation fluid in a glass vial and counted in a Beckman beta counter. An endothelial cell monolayer retaining more than 97% 14C-BSA was used for further experiments.
F-actin quantification by spectrofluorometry.
F-actin in BAEC was measured as described by Suttorp et al. (31). BAEC were seeded in 12-well plates (5 × 104 cells/well) in 1 ml of medium and cultured for 48 h. Untreated and SA-pretreated BAEC were cultured with LPS (100 ng/ml) for 6 h. The monolayers were washed in buffer A (KCl, 75 mM; MgSO4, 3 mM; EGTA, 1 mM; imidazole, 1 mM, dithiothreitol, 0.2 mM; aprotinin, 10 μg/ml; phenylmethylsulfonyl fluoride, 0.1 mM) and fixed with 3.7% formaldehyde for 15 min. Monolayers were permeabilized with buffer A containing 0.1% Triton X-100 for 5 min, stained with N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]phallicidin (0.3 μmol, 20 min), and extracted with methanol at −20°C overnight. Extracts were harvested into cuvettes, and fluorescence was measured at a 465-nm excitation and 535-nm emission and expressed in arbitrary fluorescence units per milligram of total cell protein.
G-actin quantification by DNase I inhibition assay.
Sets of experiments identical to those used to measure F-actin were used to measure the G-actin pool. The concentration of G-actin was measured by using a DNase I inhibition assay as described by Heacock and Bamburg (14). The cells were washed with 2 ml of Hanks buffered saline solution and extracted with 0.5 ml of extraction solution containing 0.1% Triton X-100, 100 mM CaCl2, 2 mM MgCl2, 1 μg of pepstatin per ml, 10 mM ATP, 10 μg of trypsin inhibitor per ml, 0.5 mM phenylmethylsulfonyl fluoride, 0.2 mM dithiothreitol, and 10 mM HEPES (pH 7.5) for 5 min. Extracts were centrifuged at 15,000 × g for 20 s for removal of cell debris. The supernatants were chilled on ice until assayed. Fifty microliters of extract was mixed with 25 μl of actin depolymerizing buffer containing 1.5 M guanidine hydrochloride (Wako), and the reaction mixture was incubated for 20 min on ice. DNase I (750 μl) at 6 pg/ml was added to the reaction mixture, and the reaction was initiated by addition of 50 μl of calf thymus DNA (Sigma) at an optical density at 260 nm of 30. The reaction mixture was incubated for 5 to 10 min, and 60% perchloric acid (200 μl) was added at 4°C. After 30 min, the reaction mixture was centrifuged at 15,000 × g for 2 min at 4°C. The optical density at 260 nm of the supernatant was measured. Pure bovine skeletal muscle actin (Sigma) was used as the standard. The G-actin pool was expressed as G-actin per total cell protein (micrograms per milligram).
DNA synthesis.
DNA synthesis in BAEC was assayed by incorporation of [3H]thymidine into the cells. Cells (3 × 104/100 μl) were plated in 96-well plates and incubated with various concentrations of LPS for 24 h. [3H]thymidine (0.5 μCi/well; Amersham) was added to the cultures. Eighteen hours later, cells were harvested on glass fiber filter discs. The discs were inserted into a glass vial with scintillation fluid (1 ml). Radioactivity was counted as counts per minute in a Beckman beta counter. The time course of [3H]thymidine incorporation in BAEC treated with 10 μg/ml was also monitored for 8 h.
Immunoblotting for HSP27 and -70.
Untreated and SA-pretreated BAEC were seeded in 35-mm-diameter culture dishes (4 × 105 cells/dish) and incubated with various concentrations of LPS for 6 h. Cells were lysed directly in lysis buffer and boiled for 10 min at 100°C. Aliquots of equal amounts of protein (20 μg/lane) were loaded onto a 4 to 20% gradient gel, run under reducing conditions, and transferred to the membrane. The membranes were treated with 5% BSA for 1 h, rinsed, and incubated with a 1:1,000 dilution of rabbit polyclonal anti-HSP27 (StressGen, Victoria, Canada) or rabbit polyclonal anti-HSP70 (Upstate Biotechnology) antibody for 1 h. The blots were further treated with a 1:3,000 dilution of horseradish peroxidase-conjugated protein G for 1 h. The immune complex on the blots was detected with the enhanced chemiluminescence (ECL) substrate (New England Nuclear, Boston, Mass.) and exposed to Kodak XAR X-ray film.
RESULTS
LPS-induced morphological changes of BAEC and prevention of them by SA pretreatment.
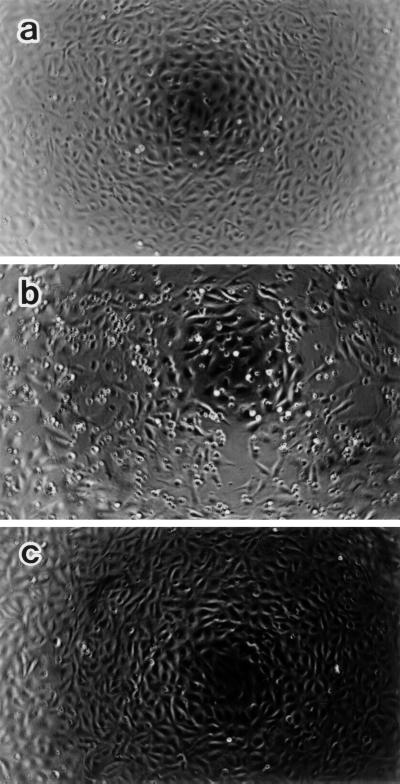
Morphological changes of BAEC in cultures with LPS at 0.01 or 10 μg/ml were inspected under a phase-contrast microscope. As shown in Fig. 1, BAEC treated with LPS at 10 μg/ml for 10 h became round and retracted, with formation of intercellular dilations and gaps. A part of the cells detached from the plastic dish bottom. On the other hand, SA-pretreated BAEC retained the original morphology in response to LPS (10 μg/ml), and their shapes were the same as that in the medium control monolayer (Fig. 1c). The number of BAEC in cultures with LPS was less than that of untreated BAEC, whereas the number of SA-pretreated BAEC did not alter with LPS exposure. The treatment with LPS at 0.01 μg/ml as well as at 10 μg/ml resulted in similar morphological changes of BAEC (data not shown).
FIG. 1.
Phase-contrast micrographs showing BAEC morphology. Untreated (a and b) and SA-pretreated (c) BAEC were cultured with medium alone (a) or with LPS (10 μg/ml) (b and c) for 10 h. Note that LPS induces cell shrinking, rounding, and intercellular gaps in BAEC (b), but not in SA-pretreated BAEC (c). Original magnification, ×25.
LPS-induced disorganization of F-actin, tubulin, and vimentin, and its prevention by SA pretreatment.
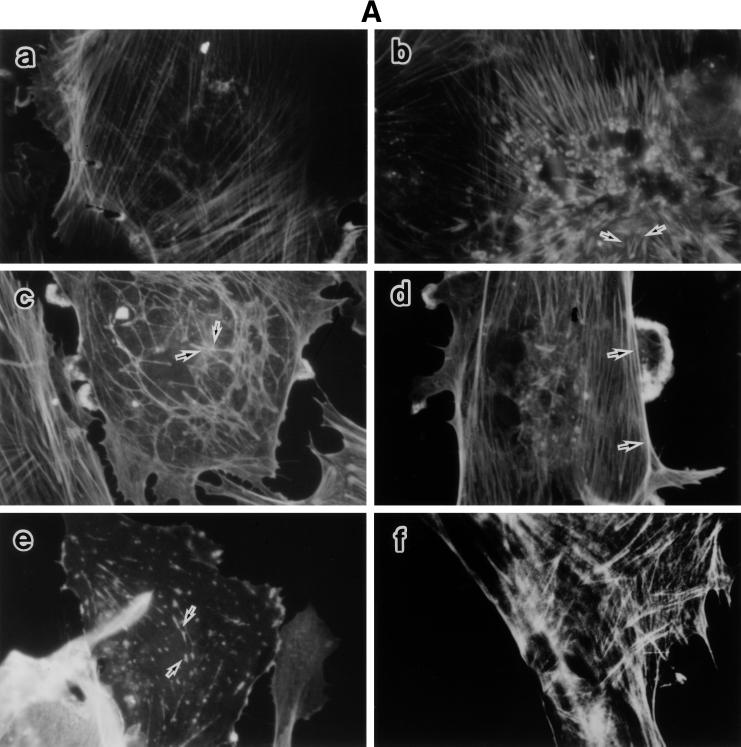
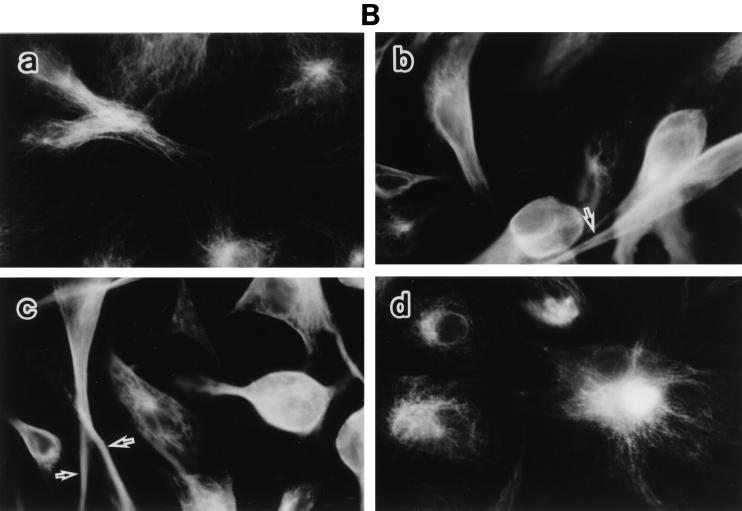
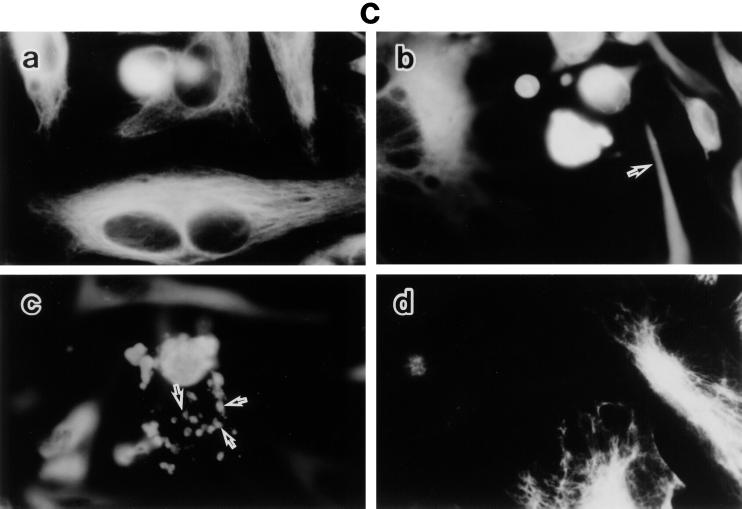
To characterize the detailed morphological changes of LPS-treated BAEC, we studied the organization of F-actin, tubulin, and vimentin in those cells, as well as the effect of SA pretreatment on it (Fig. 2). Untreated and SA-pretreated BAEC were cultured with various concentrations of LPS for 6 h. The monolayers were stained for the expression of F-actin, tubulin, and vimentin and inspected under a fluorescence microscope. Fluorescent microscopic analysis of actin demonstrated transcytoplasmic actin filament in a continuous manner in untreated BAEC (Fig. 2A, a). BAEC exposed to 0.01 μg of LPS per ml exhibited extensive formation of orthogonal stress fibers in the form of short spikes, mainly in the center of the cell (Fig. 2A, b). At 0.1 and 1 μg of LPS per ml, the actin filaments without stress fibers were polymerized marginally (Fig. 2A, c and d). At 10 μg of LPS per ml, actin was seen as a knotted staining throughout the cells with a streak of marginal actin accumulation (Fig. 2A, e). Pretreatment with SA completely inhibited LPS-induced actin disorganization and maintained normal continuous actin filament, like that of untreated BAEC (Fig. 2A, f). The staining patterns of tubulin in cultures with medium alone were observed as dense networks filling the entire cell to the end of cell processes (Fig. 2B, a). Treatment of BAEC with LPS (0.01 μg/ml) caused loss of the filamentous network and homogenous staining in the center of the cell, accompanied by bundling of the microtubules in the cell processes (Fig. 2B, b). The exposure of BAEC to a high dose of LPS (10 μg/ml) resulted in a similar staining pattern of tubulin (Fig. 2B, c). However, SA-pretreated BAEC restored the filamentous microtubule, like untreated cells (Fig. 2B, d). Vimentin filaments in untreated BAEC were located as a dense filamentous network from the nuclear lamina to the cell periphery (Fig. 2C, a). A homogenous staining pattern of vimentin was not observed in untreated control cells. In BAEC treated with 0.01 μg of LPS per ml, filamentous staining of vimentin was replaced by a homogenous staining pattern (Fig. 2C, b). Especially, the nuclear region was strongly stained, suggesting a concentrated vimentin distribution. In BAEC treated with a high dose (10 μg/ml) of LPS, fragmented staining of vimentin was scattered around densely stained round cells (Fig. 2C, c). Pretreatment of BAEC with SA inhibited the formation of blebs and retained normal filamentous vimentin networks (Fig. 2C, d).
FIG. 2.
LPS-induced disorganization of F-actin, tubulin, and vimentin and its prevention by SA pretreatment. Fluorescent micrographs show F-actin (A), tubulin (B), and vimentin (C) organization in response to LPS. Untreated and SA-pretreated BAEC were cultured with various concentrations of LPS for 6 h. (A) For estimation of F-actin organization, BAEC were cultured with medium alone (a) or 0.01 (b), 0.1 (c), 1 (d), or 10 (e) μg of LPS per ml. SA-pretreated BAEC (f) were cultured with 10 μg of LPS per ml. Arrows indicate the extensive spike formation of F-actin (b), its peripheral accumulation (c and d), and the knotted form of actin staining (e). (B) For estimation of tubulin organization, BAEC were cultured with medium alone (a) or 0.01 (b) or 10 (c) μg of LPS per ml. SA-pretreated BAEC (d) were cultured with 10 μg of LPS per ml. Arrows indicate loss of the filamentous network. (C) The same samples were used for the staining of vimentin organization. Arrows in panels b and c show homogenous vimentin staining and cell blebs and round bodies surrounding the cell, respectively. Original magnification, ×500.
LPS-induced alteration of the F- and G-actin pools and its prevention by SA pretreatment.
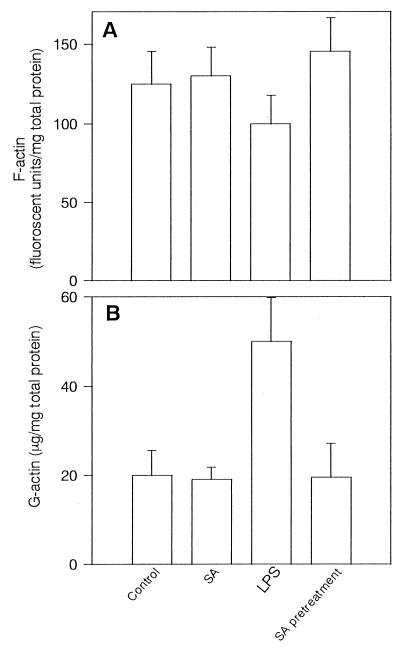
Because of marked actin disorganization in LPS-treated BAEC, we intended to examine alterations in the F- and G-actin pools. In preliminary experiments, LPS (100 ng/ml) significantly decreased the F-actin pool 2 h after its addition (an approximately 20% reduction), and its decrease continued up to 10 h (data not shown). Therefore, the effect of LPS on the F-actin pool was investigated 6 h after its addition (Fig. 3A). Alteration of the F-actin pool was not seen in untreated BAEC during 10 h, whereas LPS (100 ng/ml) decreased the F-actin pool significantly (P < 0.01). On the other hand, pretreatment with SA prevented the decrease in the F-actin pool. Treatment with SA alone did not exhibit any effect on the F-actin pool. From preliminary studies, the increase in the G-actin pool appeared 2 h after treatment of LPS (0.1 μg), and it gradually increased up to 6 h (data not shown). The exposure of BAEC to LPS increased the G-actin pool in a dose-dependent manner. Therefore, the G-actin pool was investigated 6 h after the addition of LPS (100 ng/ml) (Fig. 3B). The G-actin pool was markedly augmented with exposure of BAEC to LPS for 6 h. However, pretreatment of BAEC with SA abolished the increase in G-actin pool. Treatment with SA alone did not affect the G-actin pool.
FIG. 3.
LPS-induced alteration of the F- and G-actin pools and its prevention by SA pretreatment. F-actin (A) and G-actin (B) pools were determined in untreated and SA-pretreated BAEC 6 h after cultivation with LPS (100 ng/ml). The F-actin pool is expressed as the mean fluorescent units per milligram of total cell protein of triplicates ± standard deviation in three independent experiments. G-actin in the same samples was assayed by DNase I inhibition assay, and each bar represents the mean G-actin in micrograms per milligram of total cell protein of triplicates ± standard deviation in three independent experiments.
LPS-induced enhancement of transendothelial flux of 14C-BSA and its prevention by SA pretreatment.
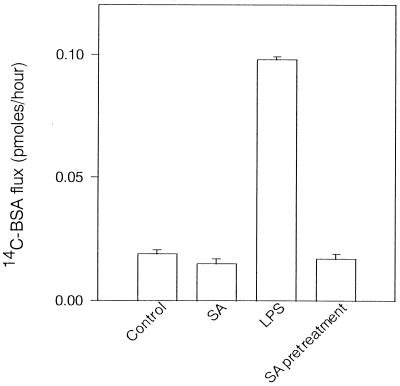
In the preceding paragraphs, we demonstrated that LPS caused morphological changes in BAEC, accompanied by cell detachment, gap formation between adjacent cells, and disorganization of cytoskeletal filaments. The relationship between those morphological changes and endothelial barrier function was investigated. Endothelial barrier function of BAEC in response to LPS was assessed by measuring the 14C-BSA flux across the endothelial monolayer. The mean 14C-BSA flux of medium control was 0.015 pmol/h, and treatment of BAEC with LPS dose dependently increased the 14C-BSA flux across the monolayer. The maximum increase in the 14C-BSA flux across the monolayer was 0.15 pmol/h in treatment with 10 μg of LPS per ml. The time course of the increase of transendothelial 14C-BSA was monitored after treatment with LPS (0.1 μg/ml). The 14C-BSA flux did not change within 30 min after the addition of LPS, and thereafter it gradually increased up to 24 h. Furthermore, we studied the effect of pretreatment with SA on LPS-induced transendothelial permeability (Fig. 4). Pretreatment of the monolayer with SA completely inhibited the increase in 14C-albumin flux across the endothelial monolayer, although treatment with LPS alone enhanced 14C-BSA flux across BAEC monolayers markedly.
FIG. 4.
Effect of pretreatment or posttreatment with SA on transendothelial 14C-BSA flux in cultures of BAEC with LPS. SA-pretreated BAEC were cultured with LPS (100 ng/ml) for 6 h. For posttreatment with LPS, BAEC were cultured with LPS for 6 h and further incubated with SA for 90 min, followed by washing. Transendothelial flux was determined by cultivation of BAEC with 14C-BSA for 1 h. Each bar represents the mean 14C-BSA flux (picomoles per hour) of triplicates ± standard deviation in three independent experiments.
LPS-induced reduction of [3H]thymidine incorporation in BAEC and its prevention by SA pretreatment.
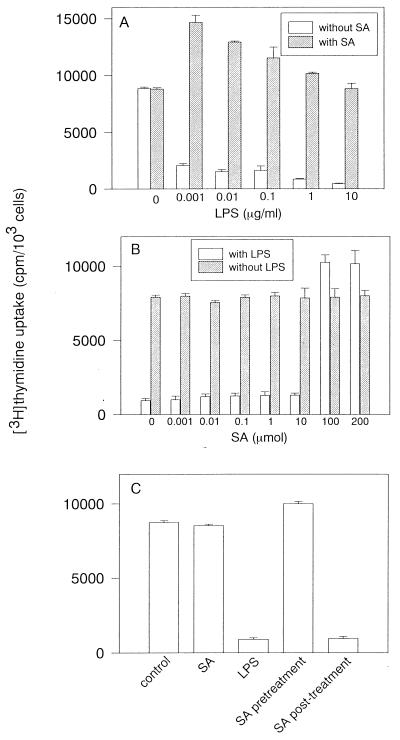
The [3H]thymidine incorporation was studied for the proliferative activity of LPS-damaged BAEC. Preliminary studies demonstrated that the decrease in DNA synthesis was found in BAEC cultured with LPS (10 μg/ml) for 4 h, followed by the [3H]thymidine incorporation assay for 18 h (data not shown). Untreated and SA-pretreated BAEC were cultured with various concentrations of LPS for 4 h, and subsequently the [3H]thymidine incorporation was determined 18 h after addition of [3H]thymidine (Fig. 5A). The [3H]thymidine incorporation was markedly reduced with the addition of 0.001 μg of LPS per ml. DNA synthesis of BAEC treated with LPS was approximately 15-fold less than that of untreated control cells. In contrast, pretreatment of BAEC with SA markedly enhanced [3H]thymidine incorporation in the presence of a lower concentration of LPS (0.001 to 0.1 μg/ml). Even at a higher concentration of LPS (10 μg/ml), SA-pretreated BAEC maintained almost the same [3H]thymidine incorporation as untreated BAEC. In pretreatment with various concentrations of SA, SA at higher than 100 μM enhanced [3H]thymidine incorporation in the presence of LPS (1 μg/ml), but SA at lower than 10 μM did not (Fig. 5B). Next, the effect of pretreatment or posttreatment with SA on LPS-induced DNA synthesis reduction was studied (Fig. 5C). [3H]thymidine incorporation in SA-pretreated BAEC was significantly enhanced in cultures with LPS (1 μg/ml). However, posttreatment with SA resulted in the reduction of [3H]thymidine incorporation in response to LPS, and the degree of its reduction was the same as that in BAEC treated with LPS alone. Treatment with SA alone did not affect [3H]thymidine incorporation of BAEC.
FIG. 5.
LPS-induced reduction of [3H]thymidine incorporation in BAEC and its prevention by SA. (A) Effect of SA on [3H]thymidine incorporation in cultures of BAEC with addition of various concentrations of LPS. SA-pretreated BAEC were cultured with various concentrations of LPS for 24 h and further incubated with [3H]thymidine for 18 h. (B) Effect of pretreatment with various concentrations of SA on [3H]thymidine incorporation in the presence or absence of LPS (1 μg/ml). (C) Effect of pretreatment or posttreatment with SA on [3H]thymidine incorporation in cultures of BAEC with LPS. SA-pretreated BAEC were cultured with LPS (10 μg/ml) for 8 h. Posttreatment with SA was performed after cultivation of BAEC with LPS for 8 h. BAEC were further incubated with [3H]thymidine for 18 h. [3H]thymidine incorporation for DNA synthesis is expressed as the mean counts per minute of triplicates ± standard deviation in five independent experiments.
Expression of HSP in SA-pretreated BAEC.
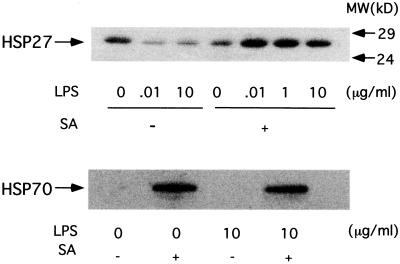
Since pretreatment with SA, an inducer of stress response, prevented LPS-induced BAEC injury, we further investigated SA-induced HSP expression in BAEC. The expression of constitutive HSP27 and inducible HSP70 was examined by immunoblotting of SA-pretreated BAEC cultured with or without LPS (Fig. 6). Exposure of BAEC to LPS (0.01 and 10 μg/ml) downregulated the expression of HSP27, although untreated BAEC showed the constitutive expression of HSP27. On the other hand, SA pretreatment profoundly enhanced HSP27 expression in the presence of LPS (0.01, 1, and 10 μg/ml). The immunoblotting analysis demonstrated no detectable band of inducible HSP70 in BAEC cultured with medium or LPS (10 μg/ml) alone. On the other hand, SA definitely induced the expression of HSP70 in BAEC in the presence or absence of LPS.
FIG. 6.
Immunoblotting analysis of HSP27 and HSP70 expression. Untreated and SA-pretreated BAEC were cultured with various concentrations of LPS for 6 h. Extracts from cells treated with 10 μg of LPS per ml or those treated with 0.01, 0.1, 1, and 10 μg of LPS per ml were analyzed by immunoblotting. MW, molecular mass.
DISCUSSION
In the present study, we demonstrated that LPS induced characteristic morphological changes in BAEC, accompanied by cytoskeletal disorganization, and that the alterations were prevented by SA pretreatment. The disorganization of F-actin, tubulin, and vimentin became clear in LPS-induced BAEC injury. Previously, Goldblum et al. (12) reported that the discrete disruptions of actin microfilament occurred exclusively at the cell-to-cell interface in LPS-induced BAEC injury. In the present study, however, LPS at the same concentration induced the formation of orthogonal stress fibers with short spikes in BAEC. A higher concentration of LPS caused the assembly and polymerization of actin filament and finally disruptions of them. This finding was also supported by analysis of the F- and G-actin pools. Moreover, we demonstrated the detailed disorganization of tubulin and vimentin. This is the first report on disorganization of tubulin and vimentin in LPS-induced endothelial injury. In LPS-treated BAEC, tubulin and vimentin lost their filamentous network and accumulated in the nuclear region with a homogenous staining pattern. Considering that LPS-induced BAEC injury is based on apoptotic cell death (8, 10, 32), the disorganization of actin, tubulin, and vimentin might reflect the apoptotic process of BAEC.
SA pretreatment prevented cytoskeletal disorganization in LPS-treated BAEC injury. How could SA prevent LPS-induced BAEC injury? It was of particular interest that SA upregulated HSP27 expression in BAEC in the presence of LPS. Recently, HSP27 was reported to affect microfilament extension (25). HSP27 homologs have been characterized as the F-actin modulating protein, inhibiting F-actin polymerization (2). Furthermore, Loktionova et al. (18) have reported that heat shock response prevents the F-actin disruption and aggregation. Furthermore, HSP27 was reported to prevent apoptotic cell death (19). It was likely that augmented expression of HSP27 might participate in the prevention of LPS-increased BAEC injury through stabilization of actin, tubulin, and vimentin. We also demonstrated that SA induced the expression of newly synthesized HSP70. Since there are a number of reports on the protective role of HSP70 in LPS-induced tissue injury (9, 17, 22, 28), it was suggested that the prevention of LPS-induced injury by SA might be related to SA-induced heat shock response.
LPS resulted in dilatation between adjacent cells, and the intercellular gap formation led to endothelial barrier dysfunction, as determined by enhanced transendothelial permeability. This finding was consistent with the previous report of Goldblum et al. (12). Several studies demonstrated that endothelial cell cytoskeletal filaments, especially actin, might be important determinants of endothelial permeability (7, 24, 29, 30). Furthermore, the present study demonstrated that monolayers exposed to LPS showed the disorganization of tubulin and vimentin. The disorganization of tubulin and vimentin as well as actin must be involved in intercellular gap formation in LPS-treated BAEC. The importance of actin filaments in increased endothelial permeability was supported by the finding that the stabilization of F-actin with phalloidin protects against LPS-enhanced endothelial permeability (1, 12). Therefore, stabilization of tubulin, vimentin, and actin might also be effective in prevention of endothelial barrier dysfunction. The LPS-induced enhanced transendothelial permeability might manifest as systemic tissue edema in endotoxic shock.
LPS exhibited differential action on DNA synthesis in the presence and absence of SA. LPS has been reported to reduce DNA synthesis in BAEC (8, 11). For the first time, it has been demonstrated that SA promoted DNA synthesis of BAEC in the presence of a low concentration of LPS. Although the exact mechanism underlying the phenomenon is unclear, vascular endothelial cells undergoing a stress response might tend toward cell proliferation after stimulation with noxious agents, like LPS. The stimulation of DNA synthesis in BAEC by the combination of SA and LPS may indicate not only rescue of the endothelial cells from LPS-induced injury, but also promotion of cell proliferation, thereby helping in the process of healing of LPS-induced injury.
ACKNOWLEDGMENTS
We are grateful to K. Takahashi and A. Morikawa for excellent technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Alexander J S, Hechtman H B, Shepro D. Phalloidin enhances endothelial barrier function and reduces inflammatory permeability in vitro. Microvasc Res. 1988;35:308–315. doi: 10.1016/0026-2862(88)90085-4. [DOI] [PubMed] [Google Scholar]
- 2.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerizing-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 3.Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun J N, Halversen S, Sorensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Brostrom C O, Brostrom M A. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- 5.Burdon R H. Temperature and animal cell protein synthesis. Symp Soc Exp Biol. 1987;41:113–133. [PubMed] [Google Scholar]
- 6.DeMeester S L, Buchman T G, Qiu Y, Jacob A K, Dunning K, Hotchkiss R S, Karl I, Cobb J P. Heat shock induces IkappaB-alpha and prevents stress-induced endothelial cell apoptosis. Arch Surg. 1997;132:1283–1287. doi: 10.1001/archsurg.1997.01430360029005. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–286. [PubMed] [Google Scholar]
- 8.Drab-Weiss E A, Hansra I K, Blazek E R, Rubin D B. Aminothiols protect endothelial cell proliferation against inhibition by lipopolysaccharide. Shock. 1998;10:423–429. doi: 10.1097/00024382-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Feinstein D L, Galea E, Aquino D A, Li G C, Xu H, Reis D J. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NF kappaB activation. J Biol Chem. 1996;271:17724–17732. doi: 10.1074/jbc.271.30.17724. [DOI] [PubMed] [Google Scholar]
- 10.Frey E A, Finlay B B. Lipopolysaccharide induces apoptosis in a bovine endothelial cell line via a soluble CD14 dependent pathway. Microb Pathog. 1998;24:101–109. doi: 10.1006/mpat.1997.0178. [DOI] [PubMed] [Google Scholar]
- 11.Gartner S L, Sieckmann D G, Kang Y H, Watson L P, Homer L D. Effects of lipopolysaccharide, lipid A, lipid X and phorbol ester on cultured bovine endothelial cells. Lab Investig. 1988;59:181–191. [PubMed] [Google Scholar]
- 12.Goldblum S E, Ding X, Brann T W, Campbell-Washington J. Bacterial lipopolysaccharide induces actin reorganization, intracellular gap formation and endothelial barrier dysfunction in pulmonary vascular endothelial cells: concurrent F-actin depolymerization and new actin synthesis. J Cell Physiol. 1993;157:13–23. doi: 10.1002/jcp.1041570103. [DOI] [PubMed] [Google Scholar]
- 13.Harlan J M, Harker L A, Reidy M A, Gajdusek C M, Schwartz S M, Striker G E. Lipopolysaccharide-mediated bovine endothelial injury in vitro. Lab Investig. 1983;48:269–274. [PubMed] [Google Scholar]
- 14.Heacock C S, Bamburg J R. The quantitation of G- and F-actin in cultured cells. Anal Biochem. 1983;135:22–36. doi: 10.1016/0003-2697(83)90725-x. [DOI] [PubMed] [Google Scholar]
- 15.Hoyt D G, Mannix R J, Rusnak J M, Pitt B R, Lazo J S. Collagen is a survival factor against LPS-induced apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Physiol. 1995;269:L171–L177. doi: 10.1152/ajplung.1995.269.2.L171. [DOI] [PubMed] [Google Scholar]
- 16.Hoyt D G, Mannix R J, Gerritsen M M E, Watkins S C, Lazo J S, Pitt B R. Integrins inhibit LPS-induced DNA strand breakage in cultured lung endothelial cells. Am J Physiol. 1996;270:L689–L694. doi: 10.1152/ajplung.1996.270.4.L689. [DOI] [PubMed] [Google Scholar]
- 17.Klosterhalfen B, Hauptmann S, Offner F A, Amo-Takyi B, Tons C, Winkeltau G, Affify M, Kupper W, Kirkpatrick C J, Mittermayer C. Induction of heat shock protein 70 by zinc-bis-(DL-hydrogenaspartate) reduces cytokine liberation, apoptosis, and mortality rate in a rat model of LD100 endotoxemia. Shock. 1997;7:254–262. doi: 10.1097/00024382-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Loktionova S A, Ilyinskaya O P, Kabakov A E. Early and delayed tolerance to simulated ischemia in heat-preconditioned endothelial cells: a role for HSP27. Am J Physiol. 1998;275:H2147–H2158. doi: 10.1152/ajpheart.1998.275.6.H2147. [DOI] [PubMed] [Google Scholar]
- 19.Mehlen P, Mehlen A, Godet J, Arrigo A P. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- 20.Meyrick B, Hoover R, Jones M R, Berry L C, Jr, Brigham K L. In vitro effects of endotoxin on bovine and sheep lung microvascular and pulmonary artery endothelial cells. J Cell Physiol. 1989;138:165–174. doi: 10.1002/jcp.1041380122. [DOI] [PubMed] [Google Scholar]
- 21.Meyrick B O, Ryan U S, Brigham K L. Direct effects of E. coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986;122:140–151. [PMC free article] [PubMed] [Google Scholar]
- 22.Morikawa A, Kato Y, Sugiyama T, Koide N, Kawai M, Fukada M, Yoshida T, Yokochi T. Altered expression of constitutive and inducible type heat shock proteins in response to lipopolysaccharide as an experimental endotoxic shock model. FEMS Immunol Med Microbiol. 1998;21:37–45. doi: 10.1111/j.1574-695X.1998.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen D B, Mosier D A, Clinkenbeard K D, Confer A W. Direct effects of Pasteurella haemolytica lipopolysaccharide on bovine pulmonary endothelial cells in vitro. Am J Vet Res. 1989;50:1633–1637. [PubMed] [Google Scholar]
- 24.Phillips P G, Tsan M F. Hyperoxia causes increased albumin permeability of cultured endothelial monolayers. J Appl Physiol. 1988;64:1196–1202. doi: 10.1152/jappl.1988.64.3.1196. [DOI] [PubMed] [Google Scholar]
- 25.Piotrowicz R S, Hickey E, Levin E G. Heat shock protein 27 kDa expression and phosphorylation regulates endothelial cell migration. FASEB J. 1998;12:1481–1490. doi: 10.1096/fasebj.12.14.1481. [DOI] [PubMed] [Google Scholar]
- 26.Podolski J L, Mooteri S N, Drab-Weiss E A, Onoda J M, Saclarides T J, Rubin D B. Aminothiol WR-1065 protects endothelial cell morphology against alterations induced by lipopolysaccharide. Shock. 1998;10:430–435. doi: 10.1097/00024382-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro S P, Villar J, Downey G P, Edelson J D, Slutsky A S. Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: evidence for TNF-alpha posttranslational regulation. Am J Respir Crit Care Med. 1996;154:1843–1850. doi: 10.1164/ajrccm.154.6.8970379. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder S, Bischoff J, Lehmann L E, Hering R, von Spiegel T, Putensen C, Hoeft A, Stuber F. Endotoxin inhibits heat shock protein 70 (HSP70) expression in peripheral blood mononuclear cells of patients with severe sepsis. Intensive Care Med. 1999;25:52–57. doi: 10.1007/s001340050786. [DOI] [PubMed] [Google Scholar]
- 29.Shasby D M, Shasby S S, Sullivan J M, Peach M J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982;51:657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- 30.Stasek J E, Jr, Patterson C E, Garcia J G. Protein kinase C phosphorylates caldesmon 77 and vimentin and enhances albumin permeability across cultured bovine pulmonary artery endothelial cell monolayers. J Cell Physiol. 1992;153:62–75. doi: 10.1002/jcp.1041530110. [DOI] [PubMed] [Google Scholar]
- 31.Suttorp N, Polley M, Seybold J, Schnittler H, Seeger W, Grimminger F, Aktories K. Adenosine diphosphate-ribosylation of G-actin by botulinum C2 toxin increases endothelial permeability in vitro. J Clin Investig. 1991;87:1575–1584. doi: 10.1172/JCI115171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong H R, Mannix R J, Rusnak J M, Boota A, Zar H, Watkins S C, Lazo J S, Pitt B R. The heat-shock protein attenuates lipopolysaccharide-mediated apoptosis in cultured sheep pulmonary aortic endothelial cells. Am J Respir Cell Mol Biol. 1996;15:745–751. doi: 10.1165/ajrcmb.15.6.8969269. [DOI] [PubMed] [Google Scholar]