Abstract
Introduction
Ankylosing spondylitis (AS) is a common form of chronic inflammatory rheumatic disease. Metallothionein-1 (MT-1) has been known to play an immunosuppressive role in various noninfectious inflammatory diseases, especially osteoarthritis and rheumatoid arthritis, thus inhibiting inflammation and pathogenesis in various diseases. However, whether MT-1 is related to AS is unclear. Here, we examined the levels of MT-1 in patients with AS and its correlation with the disease activity, complication, clinical indexes, and inflammatory cytokines and attempted to explain the effect of MT-1 on inflammation in AS.
Methods
The messenger RNA (mRNA) and protein expression of MT-1 in patients with AS were detected through real-time polymerase chain reaction and enzyme-linked immunosorbent assay. The associations between serum MT-1 protein level and clinical indexes or proinflammatory cytokines in AS were analyzed using the Spearman correlation test.
Results
The mRNAs and serum protein levels of MT-1 were significantly higher in patients with AS, especially in patients with active AS and patients with osteoporosis (OP) than in healthy controls (HCs), and no difference was observed between patients with inactive AS and HCs. Serum MT-1 levels positively correlated with disease activity, proinflammatory cytokines, and clinical indexes Ankylosing Spondylitis Disease Activity Score with C-Reactive Protein, C-reactive protein level, and erythrocyte sedimentation rate in patients with AS.
Conclusion
MT-1 expression was upregulated in patients with active AS but not in those with inactive AS and positively correlated with clinical indexes, especially in OP, as well as with proinflammatory cytokines tumor necrosis factor–alpha, interleukin (IL)-1β, and IL-6 in patients with AS.
Keywords: metallothionein-1, ankylosing spondylitis, noninfectious inflammatory diseases, inflammation cytokines, regulatory cytokines
Introduction
Ankylosing spondylitis (AS), a type of spondyloarthritis, is chronic inflammatory arthritis characterized by peri-fibrocartilaginous osteitis in the sacroiliac joint and enthesis bone, which manifests as back pain, limited spinal mobility, enthesitis, and peripheral articular and extra-articular manifestation.1,2 The disease typically begins in early adulthood (20–40 years),3 with a prevalence ranging from 0.1% to 0.8% in the adult population,4 and is more common in men than in women.3,5 Studies have shown that >90% of the patients with AS have a specific human leukocyte antigen known as HLA-B27.6 Although the cause of AS is unknown, accumulating evidence highlights that its underlying mechanism is autoimmune inflammation.7 Proinflammatory cytokines (tumor necrosis factor–alpha [TNF-α], interleukin [IL]-6, IL-17, and IL-23) were considerably elevated in the peripheral blood of patients with AS.8–11 The organs commonly affected by AS include the heart, lungs, eyes, colon, kidneys, and Achilles tendinitis. Osteoporosis (OP) is a complication of AS, and the prevalence of OP in patients with AS is between 13% (lumbar spine) and 16% (femoral neck).12 The patients with AS having OP implicates increased disease severity and a high risk of fractures.12–14
Metallothionein (MT) is a family of cysteine-rich, low molecular weight proteins and is divided into four subfamilies in humans and designated as MT-1, MT-2, MT-3, and MT-4.15 The main function of MTs is to scavenge free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), and combine both physiological (such as zinc, copper, and selenium) and xenobiotic (such as cadmium, mercury, silver, and arsenic) heavy metals through their thiol group of cysteine residues (represent nearly 30% of its constituent amino acid residue) to reduce oxidative stress and maintain the stability of intracellular heavy metal concentrations16,17 through which it participates in the regulation of metabolism and immunity.
Our previous study found that synovial inflammation and pathological symptoms in rheumatic mice were drastically suppressed by the intracellular expression of MT-1 and that in cell cultures favorable for T helper 17 (Th17) cell differentiation, MT-1 inhibits rheumatoid arthritis (RA) development by shifting the differentiation of CD4+ T cells toward regulatory T (Treg) cells and reducing the frequency of Th17 cells.18 A study showed that isolated peripheral blood mononuclear cells (PBMCs) and synovial cells from erosive inflammatory osteoarthritis (OA) treated with human recombinant MT-1 significantly reduced the expression of proinflammatory cytokines TNF-α, IL-6, and IL-17 in the cells.19 The studies indicate that MT-1 has an immunoregulatory function in inflammatory and autoimmune diseases.
The relationship between MT-1 and AS is unclear. In the present study, we investigated the expression of MT-1 messenger RNA (mRNA) in PBMCs and MT-1 protein in the serum of patients with AS and the correlation of serum MT-1 protein levels with the disease activity, complication, clinical indexes, as well as proinflammatory cytokines to elucidate the possible effects of MT-1 on patients with AS.
Materials and Methods
Patient Recruitment
In total, 67 adult patients with an AS diagnosis based on modified New York Criteria20 and 38 age- and sex-matched healthy volunteers were recruited from Shenzhen Futian Hospital for Rheumatic Diseases, China. Informed consent was obtained from the recruited participants. Patients with malignant tumors, infections, and other rheumatic diseases were excluded from this study. The demographic and clinical data of all the participants are listed in Table 1. All clinical manifestations and laboratory findings were recorded on the day of blood withdrawal. The AS disease activity was identified based on the Ankylosing Spondylitis Disease Activity Score with C-Reactive Protein (ASDAS-CRP).21,22 Active AS was defined as ASDAS-CRP ≥1.3. Kidney dysfunction was defined as urinary albumin excretion >18.6 µg/min for >3 months and glomerular filtration rate <60 mL/min/1.73 m2 for >3 months. Hepatic dysfunction was defined as total serum protein <64 g/L, serum albumin <35 g/L, and glutamic pyruvic transaminase >40 U/L. Hyperlipidemia was defined as low-density lipoprotein cholesterol >3.4 mmol/L, total cholesterol >5.2 mmol/L, and triglyceride >1.7 mmol/L. Polycythemia was defined as adult male red blood cell count >6.0 × 1012/L and hemoglobin >170 g/L and adult female red blood cell count >5.5 × 1012/L and hemoglobin>160 g/L.
Table 1.
The Demographic and Clinical Characteristics of Gout Patients, Hyperuricemia and HCs
| Characteristics | Active AS (n = 33) | Inactive AS (n = 34) | AS (n = 67) | HC (n = 38) |
|---|---|---|---|---|
| Age (years) | 40.45±9.98 | 36.91±9.92 | 38.66±10.1 | 42.19±10.24 |
| Sex, no. male /no. female) | 22/11 | 24/10 | 46/21 | 27/11 |
| Disease duration, (years) | 7.23±6.81 | 4.35±3.94 | 5.77±5.73 | – |
| HLA-B27 positive n (%) | 30 (91) | 13 (34) | 17 (67) | – |
| Anti-Inflammatory And Analgesic drugs (%) | 23 (70) | 20 (59) | 43 (64) | – |
| Bioinhibitor drugs | 13 (39) | 16 (47) | 29 (43) | – |
| Glucocorticoids | 4 (12) | 4 (12) | 8 (12) | – |
| Osteoporosis n (%) | 19 (58) | 12 (35) | 31 (46) | – |
| Kidney dysfunction n (%) | 9 (27) | 11 (32) | 20 (30) | – |
| Liver dysfunction n (%) | 13 (39) | 12 (35) | 25 (37) | – |
| Hypertension n (%) | 11 (33) | 10 (29) | 21 (31) | – |
| Hyperlipidemia n (%) | 6 (18) | 2 (6) | 8 (12) | – |
| Leukocytosis n (%) | 3 (9) | 4 (12) | 7 (10) | – |
| Polycythemia n (%) | 6 (18) | 4 (12) | 10 (15) | – |
| ALT | 27.06±17.68 | 24.74±27.61 | 24.4±16.2 | – |
| PLT | 252.76±64.58 | 263.82±79.87 | 258±73 | – |
| ESR (mm/h) | 27.47±16.9 | 18.72±11.39 | 23.03±15.02 | – |
| CRP (mg/L) | 10.7±7.99 | 2.95±1.29 | 6.77±6.88 | – |
| ASDAS-CRP | 2.05±0.56 | 0.76±0.19 | 1.59±0.62 | – |
Notes: Values are expressed as number (%), mean ± standard deviation (SD) unless otherwise indicated. There was no significant difference in age and sex between AS patients and healthy controls.
Abbreviations: HLA-B27, Human leukocyte antigens-B27; ALT, alanine aminotransferase; PLT, Platelet; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ASDAS-CRP, ankylosing spondylitis disease activity-CRP.
Blood Sample Collection and PBMC Isolation
Blood samples were collected in the morning. PBMCs were isolated using standard Ficoll-Paque Plus (TBD science, China) following the manufacturer’s instructions. The collected cells were used for RNA extractions. Serum samples were stored at −80 °C until the cytokines were determined.
RNA Extraction and Real-Time Polymerase Chain Reaction
Total RNAs were extracted with TRIzol reagent (Takara, Dalian, China) according to the manufacturer’s instructions. The RNA purity and quality were validated by absorbance on a microvolume spectrometer (NanoPhotometer N50, Implen, Germany) at 260 and 280 nm. Only samples with ratios from 1.8 to 2.0 were accepted for the next reverse transcription reaction. Complementary DNAs (cDNAs) were prepared with the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, USA). The primers were synthesized by Sangon Biotech (Shanghai, China): β-actin forward primer 5′-TCCTCTCCCAAGTCCACACAGG-3′, reverse primer 5′-GGGCACGAAGGCTCATCATTC-3′; MT-1 forward primer 5′-AGAGTGCAAATGCACCTCCTGC-3′, reverse primer 5′-CGGACATCAGGCACAGCAGCT-3′. Real-time polymerase chain reaction (RT-PCR) amplification reactions were prepared with the SYBR Green PCR kit (Transgen Biotech, China) and performed using the CFX96 RT-PCR system (Bio-Rad, USA). PCR products were verified using the melting curve analysis. The relative mRNA level of MT-1 was calculated with normalization to control the housekeeping gene β-actin and was reported using the 2−ΔΔct method.
Enzyme-Linked Immunosorbent Assay
Serum levels of MT-1 (EIAab Science Inc, Wuhan), TNF-α, IL-1β, and IL-6 were quantified using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, USA) according to the manufacturer’s instructions.
Statistical Analysis
All data were expressed as the mean ±standard error of the mean and were analyzed using GraphPad Prism software. Differences between the two groups were determined using a two-tailed Student’s t-test. The Spearman correlation test was used to investigate the correlations between serum MT-1 levels and laboratory values, as well as serum cytokine levels. A one-way analysis of variance was used for multiple comparisons. Here, p < 0.05 was considered statistically significant.
Results
Increased MT-1 mRNA and Serum Protein Levels in Patients with AS, Especially Those with Active AS
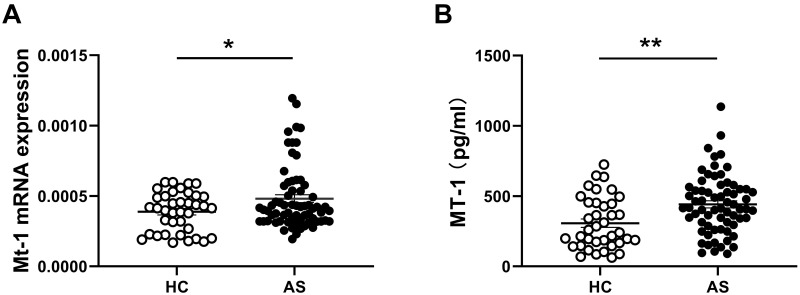
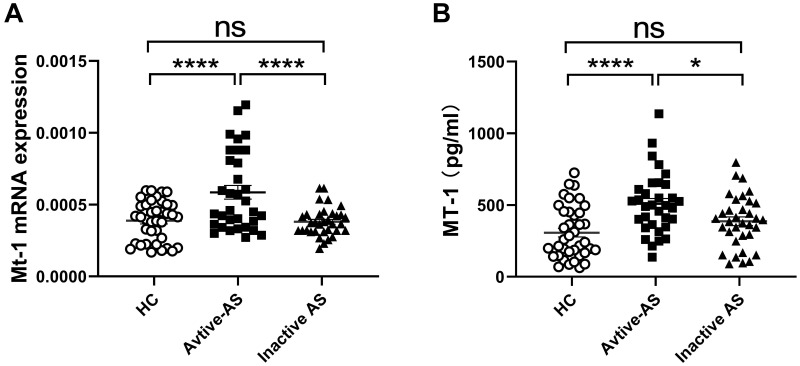
The level of MT-1 is increased in patients with OA and inflammatory bowel disease (IBD), both of which are complications of AS, and positively correlated with the disease activity, and MT-1 alleviates the symptoms and inflammation of diseases.19,23,24 To investigate the association between MT-1 and AS, the expressions of MT-1 mRNA in PBMCs and serum MT-1 protein levels from 67 patients with AS and 38 healthy controls (HCs) were detected using RT-PCR and ELISA, respectively. As shown in Figure 1A and B, the mRNA and protein levels of MT-1 were significantly higher in patients with AS than in HCs. Furthermore, we found that MT-1 expression was increased remarkably in patients with active AS compared with those with inactive AS and HCs, whereas the level of MT-1 was not different between patients with inactive AS and HCs (Figure 2A and B). Thus, we speculated that MT-1 is closely related to the AS disease activity and may be involved in AS pathogenesis.
Figure 1.
Comparison of MT1 mRNAs and protein levels between AS and HC. (A) Expressions of MT1 in PBMCs from AS patients (n = 67) and healthy controls (HC, n=38) were measured by RT-PCR, results are showed as mean± SEM. (B) Serum MT1 protein levels in AS patients (n= 67) and HC (n= 38) were measured by ELISA. Each symbol represents an individual patient and HC. Horizontal lines indicate median values. Differences between two groups were performed with unpaired Student’s t-test. *P<0.05; **P<0.01.
Figure 2.
Comparison of MT1 mRNAs and protein levels among AS patients with active disease and inactive disease as well as HC with tophi. (A) The mRNA expressions of MT1 in PBMCs from active AS patients (n=33) and inactive AS patients (n = 34) and healthy control (HC) (n = 38) were measured by RT-PCR; (B) Serum MT-1 protein levels in active AS patients (n= 33) and inactive AS patients (n = 34) and HC (n = 38) were measured by ELISA; Differences between two groups were performed with one-way ANOVA multiple comparisons. *P<0.05; ****P<0.0001.
Abbreviation: NS, no significant.
Correlation Between Serum MT-1 Levels in Patients with AS and ASDAS-CRP as Well as Other AS Laboratory Indexes
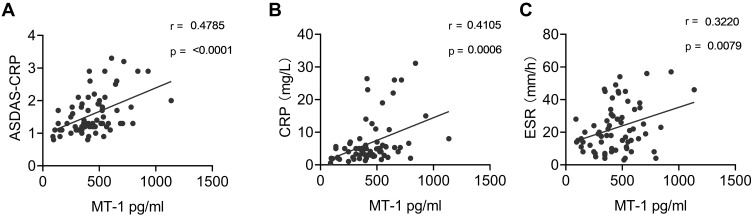
To further study the relevancy of the association between AS and MT-1, the correlation between the level of serum MT-1 protein and the values of ASDAS-CRP, C-reactive protein (CRP) level, and erythrocyte sedimentation rate (ESR) were examined. The results showed that serum MT-1 protein levels were positively correlated with ASDAS-CRP (r = 0.4785, p = 0.0001), CRP levels (r = 0.4105, p = 0.0006), and ESRs (r = 0.3220, p = 0.0079; Figure 3A–C), confirming that the level of MT-1 is positively correlated with AS activity and inflammation.
Figure 3.
Correlation of serum MT1 levels and AS clinical indexes. Serum MT1 levels in AS patients (n = 67) were positively correlated with ASDAS (A), CRP (B) and ESR (C). ASDAS, Ankylosing Spondylitis Disease Activity Score; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate. Each symbol represents an individual Gout patient. Spearman correlation analysis was used to calculate significance. P < 0.05 represents a significant difference.
Correlations Between Serum MT-1 Levels and as Clinical Features
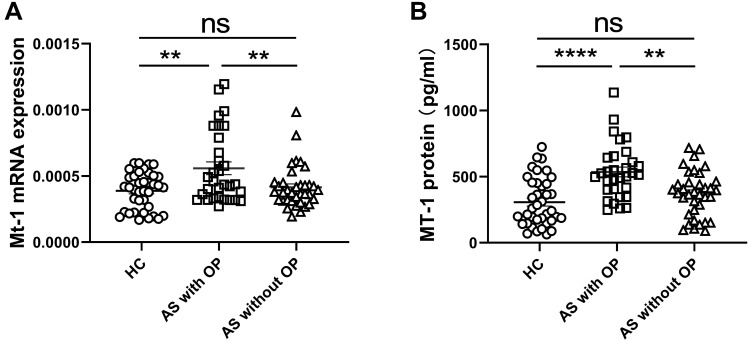
To assess the relationships between serum MT-1 protein levels and the clinical features of AS, the levels of MT-1 proteins in patients with and without AS definite clinical features were analyzed. The results showed that the serum MT-1 protein level was not significantly different among patients with hypertension, hyperlipidemia, liver dysfunction, kidney dysfunction, leukocytosis, and polycythemia (Table 2). Interestingly, the expressions of MT-1 were significantly higher in patients with osteoporosis (OP) than in patients without OP and HCs (Figure 4A and B). However, the levels of MT-1 in patients with AS without OP were not significantly higher than those in HCs (Figure 4A and B). Patients with AS were recruited in our study, and among 31 patients with OP, 22 (70.9%) had active AS, whereas among 36 patients without OP, 11 (30.6%) had active AS. These are consistent with the finding that the level of MT-1 is high in patients with active AS (Figure 2A and B) and positively correlated with the AS activity (Figure 3A–C), explaining why the expression of MT-1 in patients with AS without OP was not significantly higher than that in HCs. These results indicate that the expressions of MT-1 are closely related to AS with OP, AS activity, and AS severity.
Table 2.
Serum MT-1 Protein Levels in the Presence or Absence of as Clinical Characteristics
| Clinical Characteristics | n | Present Median (Interquartile Range) | n | Absent Median (Interquartile Range) | P- value |
|---|---|---|---|---|---|
| Osteoporosis | 31 | 514.35(400.12 to 643.39) | 36 | 374.26(214.15 to 456.21) | <0.0001 |
| Hypertension | 21 | 441.16(287.12 to 525.37) | 46 | 413.78(314.29 to 564.13) | ns |
| Hyperlipidemia | 8 | 454.81(248.19 to 500.99) | 59 | 417.36(314.29 to 549.25) | ns |
| Liver dysfunction | 25 | 415.19(345.13 to 528.36) | 42 | 440.42(287.12 to 549.25) | ns |
| Kidney dysfunction | 20 | 428.18(345.49 to 549.14) | 47 | 417.36(264.13 to 538.64) | ns |
| Leukocytosis | 7 | 467.46(168.55 to 528.36) | 60 | 416.27(314.29 to 549.14) | ns |
| Polycythemia | 10 | 448.69(394.26 to 527.46) | 57 | 415.19(295.72 to 549.14) | ns |
Notes: Differences between two groups were performed with unpaired Student’s t-test for nonparametric data. P< 0.05 represents a significant difference.
Abbreviation: NS, not significant.
Figure 4.
MT-1 mRNA and protein levels in AS patients with or without osteoporosis. The expression of MT1 mRNA (A) and MT1 protein levels in serum (B) were measured in AS patients with OP (n= 31) and without OP (osteoporosis, n= 36) as well as HC (n= 38). Results are showed as mean± SEM. Each symbol represents an individual patient and HC. Horizontal lines indicate median values. Differences between two groups were performed with one-way ANOVA multiple comparisons. **P<0.01; ****P<0.0001.
Abbreviation: NS, no significant.
Elevated Serum Levels of Proinflammatory Cytokines in Patients with AS and They Correlated with the Serum MT-1 Level
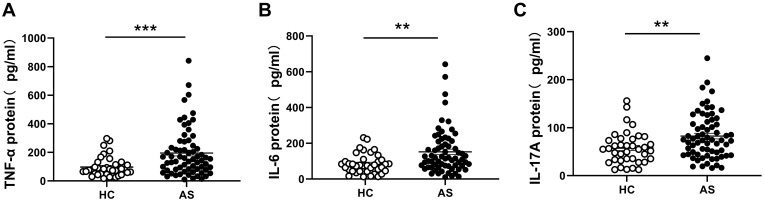
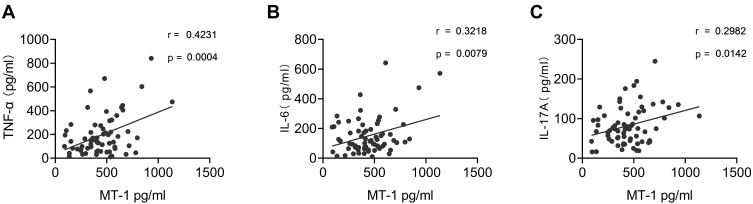
Studies have reported that proinflammatory cytokines play an important role in AS pathogenesis. The serum levels of TNF-α, IL-6, and IL-17 in patients with AS and HCs were detected using ELISA. Consistent with previous reports11,25 the results showed that TNF-α, IL-6, and IL-17 were remarkably higher in patients with AS than in HCs (Figure 5A–C). To investigate the correlation between MT-1 and inflammation cytokines in AS, we performed a correlation analysis of MT-1 with TNF-α, IL-6, and IL-17 by using the Spearman correlation test. MT-1 levels were positively correlated with the expression of TNF-α, IL-6, and IL-17 (Figure 6).
Figure 5.
Serum cytokines TNF-α, IL-6 and IL-17 levels in AS patients and HCs. The protein levels of TNF-α (A), IL-1β (B) and IL-6 (C) in serums from AS patients (n= 67) and HC (n= 38) were measured by ELISA. Results are showed as mean± SEM. Each symbol represents an individual gout patient and HC. Horizontal lines indicate median values. Differences between two groups were performed with unpaired Student’s t-test. **P<0.01; ***P<0.001.
Figure 6.
Correlation of serum MT-1 and pro-inflammatory cytokines in AS patients. Serum MT-1 levels were positively associated with TNF-α (A), IL-1β (B) and IL-6 (C). Each symbol represents an individual gout patient. Spearman correlation analysis was used to calculate significance. P < 0.05 represents a significant difference.
Discussion
The immunoregulatory activity of MT-1 has been elucidated by accumulated evidence. MT-1 producing dendritic cells preferentially drive naïve CD4+ T cell differentiating into Treg cells through inhibiting the phosphorylation of signal transducer and activator of transcription 1 (STAT)1/3.26 Our previous study showed that MT-1 can negatively regulate Th17 cell differentiation under Th17-skewing cell culture conditions by abolishing STAT3 phosphorylation.18 MT-1 has been proven to suppress the Wnt/β-catenin pathway by reducing the nuclear translocation of β-catenin and to inhibit phosphatidylinositol 3-kinase/protein kinase B (Akt) signaling by limiting Akt phosphorylation. The immunoregulatory function of MT-1 in the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway is contradictory, and the overexpression of the MT-1 gene in MT-knockout cells reduced TNF-α-induced NF-κB-dependent gene production through dampening IkBα degradation;27 on the other hand, MT-1 increases the migration and invasion of glioma cells by enhancing the activation of matrix metallopeptidase 9 through raising the p50 activity of NF-κB.28
The level of MT-1 has shown to increase the number of noninfectious inflammatory diseases, including multiple sclerosis,29,30 Parkinson disease,31 Alzheimer disease,32 amyotrophic lateral sclerosis,33 atopic dermatitis,34 IBD,24 OA,19 and RA.18 Furthermore, studies have demonstrated that MT-1 acts as an immune mediator to suppress inflammatory responses in these diseases by downregulating disease-related proinflammatory cytokines.18,19,24,29–34 Here, our results indicated that the levels of MT-1 mRNA in PBMCs and proteins in serum were significantly elevated in patients with AS (Figure 1), especially in patients with active AS (Figure 2) and in patients with OP (Figure 4) compared with HCs. Furthermore, results showed that the level of serum MT-1 was positively correlated with the disease activity and clinical indexes ASDAS-CRP, CRP level, and ESR (Figure 3). On the basis of the fact that MT-1 is upregulated in active inflammatory diseases and that they attenuate the activity of noninfectious inflammatory diseases18,19,24,29–34 it is reasonable to infer that MT-1 may play an immunoregulatory role in AS.
The mechanism by which MT-1 is upregulated in patients with active AS is still unclear. Studies have shown that MT-1 can be induced by proinflammatory cytokines TNF-α and IL-6, which have been demonstrated by intracellular overexpression of the TNF-α or IL-6 gene and injection of exogenous recombinant TNF-α or IL-6 proteins. These results were further confirmed by knockout TNF-α type 1 receptor and IL-6 with diminished levels of MT-1 in mice.35–40 Our published data showed that intracellular overexpression of MT-1 suppressed TNF-α, IL-6, and IL-17 in collagen-induced arthritis, and under Th17 cells–skewing culture conditions, recombinant MT-1 restrained the differentiation of naïve CD4+ T cells into Th17 cells but increased Treg-cell production.18 The present study indicated that the serum levels of TNF-α, IL-6, and IL-17 were significantly upregulated in patients with AS compared with HCs (Figure 5). Importantly, the results also showed that the serum level of MT-1 was positively correlated with the levels of proinflammatory cytokines TNF-α, IL-6, and IL-17 in patients with AS (Figure 6). Taken together, MT-1 can be induced by proinflammatory cytokines TNF-α and IL-6, whereas AS-related proinflammatory cytokines TNF-α, IL-6, and IL-17 are inhibited by MT-1 in immunoinflammatory diseases. Therefore, the elevated MT-1 in patients with active AS may influence the regulation of inflammation in AS.
During the active stage of AS, inflammation provokes a respiratory outburst to generate a large amount of superoxide; the oxidative stress exacerbates the inflammatory reaction and releases Zn2+ from the pool of MT-bound zinc in the cells. The levels of MT-1 can be drastically increased by hydrogen peroxide (H2O2) direct binding to an antioxidant-responsive element of the MT-1 gene promoter, whereas Zn2+ upregulates MT-1 production through the activation of a metal-responsive transcription factor, a transcriptional factor for metal-responsive elements in the promoter of the MT-1 gene.41–43 Thus, the high level of MT-1 is the result of orchestrating the intricate interplay among proinflammatory cytokines, ROS, and zinc ions, in which the inflammation, ROS, and Zn2+ form a positive feedforward and feedback loop to efficiently upregulate the level of MT-1. MT-1 has been shown to possess a strong antioxidant ability by scavenging a wide range of ROS and RNS, including H2O2, and serving as heavy metal acceptor and donor to maintain the stability of heavy metals in cells.44 The elevated MT-1 induced by ROS and heavy metals, in turn, suppresses the inflammation and restores the immune homeostasis, thereby avoiding tissue damaged by an excessive immune response and attenuating apoptosis by inhibiting caspase cascade activity in noninfectious inflammatory diseases41,44–48 Therefore, the expression of MT-1 was upregulated in patients with active AS (Figure 2) and positively correlated with the AS disease activity (Figure 3), which demonstrates the potential of MT-1 to contribute to the host defense system and act as an immune suppressor to mediate AS inflammation and pathogenic development through antioxidative stress.
Conclusions
Our study showed that the MT-1 level is significantly increased in patients with AS, and it is positively associated with proinflammatory cytokines, disease activity, complication, and clinical indexes. Furthermore, studies are needed to directly demonstrate the immunoregulatory function of MT-1 in AS and elucidate the immunoregulatory mechanism of MT-1 in AS.
Acknowledgments
This study was supported by grants from Shenzhen Science and Technology Basic Research Project JCYJ2019073015124040376, JCYJ20180504170414637, JCYJ20180302145033769 and JCYJ2019080915120563. Guangdong Provincial Key Laboratory of tissue and organ regional immunity and disease 2019B030301009. Shenzhen Futian Public Welfare Scientific Research Project FTWS2021006 and Sanming Project of Medicine in Shenzhen SZSM201602087.
Abbreviations
AS, Ankylosing Spondylitis; MT-1, Metallothionein-1; ASDAS, Ankylosing spondylitis disease activity; CRP, C-Reactive Protein; ESR, Erythrocyte Sedimentation Rate.
Ethics Statement
The study was approved by the Ethics Committee of Health Science Center of Shenzhen University, China. All study protocols complied with the Declaration of Helsinki. All participating patients and healthy volunteers have been signed the consent for publication.
Author Contributions
Yanmei Ma performed the experiments and wrote the manuscript. Jing Du designed, Zhihua yin, Hanying Dai, and Yazhi Wei contributed to data collection and analysis. Yuhao Xia and Lingyun Li prepared the figures. Zhizhong Ye and Zhong Huang edited the manuscript and supervised the study. All authors made significant contributions to the study conception and design, acquisition of data, or data analysis; participate in drafting or revising the article; agreed to submit to the current journal; gave final approval to the version to be published, and agree to take responsibility for all aspects of the work.
Disclosure
The authors declare no commercial or financial conflicts of interest in this work.
References
- 1.Garcia-Montoya L, Gul H, Emery P, Recent advances in ankylosing spondylitis: understanding the disease and management. F1000Res, 2018. 7. 1512 doi: 10.12688/f1000research.14956.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranganathan V, Gracey E, Brown MA, et al. Pathogenesis of ankylosing spondylitis - recent advances and future directions. Nat Rev Rheumatol. 2017;13(6):359–367. doi: 10.1038/nrrheum.2017.56 [DOI] [PubMed] [Google Scholar]
- 3.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84. doi: 10.1016/S0140-6736(16)31591-4 [DOI] [PubMed] [Google Scholar]
- 4.Robinson PC, van der Linden S, Khan MA, et al. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol. 2021;17(2):109–118. doi: 10.1038/s41584-020-00552-4 [DOI] [PubMed] [Google Scholar]
- 5.Stolwijk C, van Onna M, Boonen A, et al. Global prevalence of spondyloarthritis: a systematic review and meta-regression analysis. Arthritis Care Res. 2016;68(9):1320–1331. doi: 10.1002/acr.22831 [DOI] [PubMed] [Google Scholar]
- 6.Sheehan NJ. The ramifications of HLA-B27. J R Soc Med. 2004;97(1):10–14. doi: 10.1177/014107680409700102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JA. Update on ankylosing spondylitis: current concepts in pathogenesis. Curr Allergy Asthma Rep. 2015;15(1):489. doi: 10.1007/s11882-014-0489-6 [DOI] [PubMed] [Google Scholar]
- 8.Tan AL, Marzo-Ortega H, O’Connor P, et al. Efficacy of anakinra in active ankylosing spondylitis: a clinical and magnetic resonance imaging study. Ann Rheum Dis. 2004;63(9):1041–1045. doi: 10.1136/ard.2004.020800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkenbach A, Herold M, Wigand R. Interleukin-6 serum concentration in ankylosing spondylitis: a reliable predictor of disease progression in the subsequent year? Rheumatol Int. 2000;19(4):149–151. doi: 10.1007/s002960050119 [DOI] [PubMed] [Google Scholar]
- 10.Gratacós J, Collado A, Filella X, et al. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol. 1994;33(10):927–931. doi: 10.1093/rheumatology/33.10.927 [DOI] [PubMed] [Google Scholar]
- 11.Mei Y, Pan F, Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30(2):269–273. doi: 10.1007/s10067-010-1647-4 [DOI] [PubMed] [Google Scholar]
- 12.van der Weijden MA, Claushuis TAM, Nazari T, et al. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol. 2012;31(11):1529–1535. doi: 10.1007/s10067-012-2018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briot K, Roux C. Inflammation, bone loss and fracture risk in spondyloarthritis. RMD Open. 2015;1(1):e000052. doi: 10.1136/rmdopen-2015-000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geusens P, Vosse D, van der Linden S. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2007;19(4):335–339. doi: 10.1097/BOR.0b013e328133f5b3 [DOI] [PubMed] [Google Scholar]
- 15.Dai H, Wang L, Li L, et al. Metallothionein 1: a new spotlight on inflammatory diseases. Front Immunol. 2021;12:739918. doi: 10.3389/fimmu.2021.739918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlos Rodrigo MA, Jimenez Jimemez AM, Haddad Y, et al. Metallothionein isoforms as double agents - Their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist Updat. 2020;52:100691. doi: 10.1016/j.drup.2020.100691 [DOI] [PubMed] [Google Scholar]
- 17.Si M, Lang J. The roles of metallothioneins in carcinogenesis. J Hematol Oncol. 2018;11(1):107. doi: 10.1186/s13045-018-0645-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Li L, Li L, et al. Metallothionein-1 suppresses rheumatoid arthritis pathogenesis by shifting the Th17/Treg balance. Eur J Immunol. 2018;48(9):1550–1562. doi: 10.1002/eji.201747151 [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Gong Z, Hu S, et al. Metallothionein-1 is associated with osteoarthritis disease activity and suppresses proinflammatory cytokines production in synovial cells. Int Immunopharmacol. 2019;75:105815. doi: 10.1016/j.intimp.2019.105815 [DOI] [PubMed] [Google Scholar]
- 20.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 21.Lukas C, Landewé R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(1):18–24. doi: 10.1136/ard.2008.094870 [DOI] [PubMed] [Google Scholar]
- 22.van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(12):1811–1818. doi: 10.1136/ard.2008.100826 [DOI] [PubMed] [Google Scholar]
- 23.Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2006;20(3):451–471. doi: 10.1016/j.berh.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 24.Tsuji T, Naito Y, Takagi T, et al. Role of metallothionein in murine experimental colitis. Int J Mol Med. 2013;31(5):1037–1046. doi: 10.3892/ijmm.2013.1294 [DOI] [PubMed] [Google Scholar]
- 25.Bal A, Unlu E, Bahar G, et al. Comparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol. 2007;26(2):211–215. doi: 10.1007/s10067-006-0283-5 [DOI] [PubMed] [Google Scholar]
- 26.Spiering R, Wagenaar-Hilbers J, Huijgen V, et al. Membrane-bound metallothionein 1 of murine dendritic cells promotes the expansion of regulatory T cells in vitro. Toxicol Sci. 2014;138(1):69–75. doi: 10.1093/toxsci/kft268 [DOI] [PubMed] [Google Scholar]
- 27.Sakurai A, Hara S, Okano N, et al. Regulatory role of metallothionein in NF-kappaB activation. FEBS Lett. 1999;455(1–2):55–58. doi: 10.1016/S0014-5793(99)00839-X [DOI] [PubMed] [Google Scholar]
- 28.Ryu HH, Jung S, Jung TY, et al. Role of metallothionein 1E in the migration and invasion of human glioma cell lines. Int J Oncol. 2012;41(4):1305–1313. doi: 10.3892/ijo.2012.1570 [DOI] [PubMed] [Google Scholar]
- 29.Haase S, Linker RA. Inflammation in multiple sclerosis. Ther Adv Neurol Disord. 2021;14:17562864211007687. doi: 10.1177/17562864211007687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pegoretti V, Swanson KA, Bethea JR, et al. Inflammation and oxidative stress in multiple sclerosis: consequences for therapy development. Oxid Med Cell Longev. 2020;2020:7191080. doi: 10.1155/2020/7191080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pajares M, I. Rojo A, Manda G, et al. Inflammation in parkinson’s disease: mechanisms and therapeutic implications. Cells. 2020;9(7):1687. doi: 10.3390/cells9071687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forloni G, Balducci C. Alzheimer’s disease, oligomers, and inflammation. J Alzheimers Dis. 2018;62(3):1261–1276. doi: 10.3233/JAD-170819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon MS, Wosiski-Kuhn M, Gillespie R, et al. Inflammation, Immunity, and amyotrophic lateral sclerosis: I. Etiology and pathology. Muscle Nerve. 2019;59(1):10–22. doi: 10.1002/mus.26289 [DOI] [PubMed] [Google Scholar]
- 34.Guo JZ, Wang W-H, Li L-F, et al. The role of metallothionein in a dinitrofluorobenzene-induced atopic dermatitis-like murine model. Sci Rep. 2018;8(1):11129. doi: 10.1038/s41598-018-29410-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrasco J, Hernandez J, Bluethmann H, et al. Interleukin-6 and tumor necrosis factor-alpha type 1 receptor deficient mice reveal a role of IL-6 and TNF-alpha on brain metallothionein-I and -III regulation. Brain Res Mol Brain Res. 1998;57(2):221–234. doi: 10.1016/S0169-328X(98)00087-4 [DOI] [PubMed] [Google Scholar]
- 36.Schroeder JJ, Cousins RJ. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1990;87(8):3137–3141. doi: 10.1073/pnas.87.8.3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato M, Sasaki M, Hojo H. Tissue specific induction of metallothionein synthesis by tumor necrosis factor-alpha. Res Commun Chem Pathol Pharmacol. 1992;75(2):159–172. [PubMed] [Google Scholar]
- 38.Liu J, Liu YP, Sendelbach LE, Klaassen CD. Endotoxin induction of hepatic metallothionein is mediated through cytokines. Toxicol Appl Pharmacol. 1991;109(2):235–240. doi: 10.1016/0041-008X(91)90171-A [DOI] [PubMed] [Google Scholar]
- 39.Hernández J, Molinero A, Campbell IL, et al. Transgenic expression of interleukin 6 in the central nervous system regulates brain metallothionein-I and -III expression in mice. Brain Res Mol Brain Res. 1997;48(1):125–131. doi: 10.1016/S0169-328X(97)00087-9 [DOI] [PubMed] [Google Scholar]
- 40.Sato M, Sasaki M, Hojo H. Differential induction of metallothionein synthesis by interleukin-6 and tumor necrosis factor-alpha in rat tissues. Int J Immunopharmacol. 1994;16(2):187–195. doi: 10.1016/0192-0561(94)90075-2 [DOI] [PubMed] [Google Scholar]
- 41.Ruttkay-Nedecky B, Nejdl L, Gumulec J, et al. The role of metallothionein in oxidative stress. Int J Mol Sci. 2013;14(3):6044–6066. doi: 10.3390/ijms14036044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59(1):95–104. doi: 10.1016/S0006-2952(99)00301-9 [DOI] [PubMed] [Google Scholar]
- 43.Chen L, Ma L, Bai Q, et al. Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. J Biol Chem. 2014;289(32):22413–22426. doi: 10.1074/jbc.M114.548677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue K, Takano H, Shimada A, et al. Metallothionein as an anti-inflammatory mediator. Mediators Inflamm. 2009;2009:101659. doi: 10.1155/2009/101659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 46.Jarosz M, Olbert M, Wyszogrodzka G, et al. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25(1):11–24. doi: 10.1007/s10787-017-0309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry DK, Smyth MJ, Stennicke HR, et al. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997;272(30):18530–18533. doi: 10.1074/jbc.272.30.18530 [DOI] [PubMed] [Google Scholar]
- 48.Kang M, Zhao L, Ren M, et al. Reduced metallothionein expression induced by Zinc deficiency results in apoptosis in hepatic stellate cell line LX-2. Int J Clin Exp Med. 2015;8(11):20603–20609. [PMC free article] [PubMed] [Google Scholar]