Abstract
Objective
The first-line treatment for Cushing’s disease is transsphenoidal surgery, after which the rates of remission are 60 to 80%, with long-term recurrence of 20 to 30%, even in those with real initial remission. Drug therapies are indicated for patients without initial remission or with surgical contraindications or recurrence, and ketoconazole is one of the main available therapies. The objective of this study was to evaluate the safety profile of and the treatment response to ketoconazole in Cushing’s disease patients followed up at the endocrinology outpatient clinic of a Brazilian university hospital.
Patients and methods
This was a retrospective cohort of Cushing’s disease patients with active hypercortisolism who used ketoconazole at any stage of follow-up. Patients who were followed up for less than 7 days, who did not adhere to treatment, or who were lost to follow-up were excluded.
Results
Of the 172 Cushing’s disease patients who were followed up between 2004 and 2020, 38 received ketoconazole. However, complete data was only available for 33 of these patients. Of these, 26 (78%) underwent transsphenoidal surgery prior to using ketoconazole, five of whom (15%) had also undergone radiotherapy; seven used ketoconazole as a primary treatment. Ketoconazole use ranged from 14 days to 14.5 years. A total of 22 patients had a complete response (66%), three patients had a partial response (9%), and eight patients had no response to treatment (24%), including those who underwent radiotherapy while using ketoconazole. Patients whose hypercortisolism was controlled or partially controlled with ketoconazole had lower baseline 24-h urinary free cortisol levels than the uncontrolled group [times above the upper limit of normal: 0.62 (SD, 0.41) vs. 5.3 (SD, 8.21); p < 0.005, respectively] in addition to more frequent previous transsphenoidal surgery (p < 0.04). The prevalence of uncontrolled patients remained stable over time (approximately 30%) despite ketoconazole dose adjustments or association with other drugs, which had no significant effect. One patient received adjuvant cabergoline from the beginning of the follow-up, and it was prescribed to nine others due to clinical non-response to ketoconazole alone. Ten patients (30%) reported mild adverse effects, such as nausea, vomiting, dizziness, and loss of appetite. Only four patients had serious adverse effects that warranted discontinuation. There were 20 confirmed episodes of hypokalemia among 10/33 patients (30%).
Conclusion
Ketoconazole effectively controlled hypercortisolism in 66% of Cushing’s disease patients, being a relatively safe drug for those without remission after transsphenoidal surgery or whose symptoms must be controlled until a new definitive therapy is carried out. Hypokalemia is a frequent metabolic effect not yet described in other series, which should be monitored during treatment.
Keywords: Cushing’s disease, Cushing’s syndrome, hypercortisolism, treatment, ketoconazole
Introduction
Cushing’s disease (CD) results from a pituitary tumor that secretes adrenocorticotropic hormone (ACTH), which leads to chronic hypercortisolism. It is a potentially fatal disease with high morbidity and a mortality rate of up to 3.7 times than that of the general population (1–4) associated to several clinical–metabolic disorders caused by excess cortisol and/or loss of circadian rhythm (5). In general, its management is a challenge even in reference centers (6, 7).
Transsphenoidal surgery (TSS), the treatment of choice for CD, results in short-term remission in 60 to 80% of patients (8). However, recurrence rates of 20 to 30% are found in long-term follow-up, even in those with clear initial remission (9). Drug therapies can help control excess cortisol in patients without initial remission, in cases of recurrence, and in those with contraindications or high initial surgical risk (10).
Nevertheless, specific drugs that act on the pituitary adenoma, which could directly treat excess ACTH, have a limited effect, and only pasireotide is approved for this purpose in Brazil (11, 12). In this scenario, adrenal steroidogenesis blockers are important. One such off-label medication is the antifungal drug ketoconazole, a synthetic imidazole derivative that inhibits the enzymes CYP11A1, CYP17, CYP11B2, and CYP11B1. Because of its hepatotoxicity and the availability of other drugs, it has been withdrawn from the market in several countries (13). In Europe, it is still approved for use in CD, although in the United States, it is recommended for off-label use almost in CD (14–16). Due to the potential benefits for hypercortisolism, ketoconazole has been replaced by levoketoconazole, which the European Union has recently approved for CD with a lower expected hepatotoxicity (17).
Thus, when adrenal inhibitors are used as an alternative treatment for CD, information about the outcomes of drugs such as ketoconazole are important. Clinical studies on these effects in CD are scarce, mostly retrospective, multicenter, or from developed countries (14, 18). A recent meta-analysis on the therapeutic modalities for CD included only four studies (246 patients) that evaluated urinary cortisol response as a treatment outcome and eight studies (366 patients) describing the prevalence of some side effects: change in transaminase activity, digestive symptoms, skin rash, and adrenal insufficiency. Hypokalemia was not mentioned in this meta-analysis (19).
The objective of this study was to evaluate the safety profile of and treatment response to ketoconazole in CD patients followed during a long term in the endocrinology outpatient clinic of a Brazilian university hospital.
Patients and methods
Patients
We retrospectively evaluated 38 patients (27 women) diagnosed with CD. These patients, whose treatment included ketoconazole at any time between 2004 and 2020, are part of a prospective cohort series from the Hospital de Clínicas de Porto Alegre neuroendocrinology outpatient clinic.
The diagnostic criteria for hypercortisolism were based on high 24-h urinary free cortisol levels (24-h UFC) in at least two samples, non-suppression of serum cortisol after low-dose dexamethasone testing (>1.8 µg/dl), and/or loss of cortisol rhythm (midnight serum cortisol >7.5 µg/dl or midnight salivary cortisol >0.208 nmol/L). CD was diagnosed by normal or elevated ACTH levels, evidence of pituitary adenoma >0.6 cm on magnetic resonance image (MRI), and ACTH central/periphery gradient on inferior petrosal sinus catheterization when MRI was normal or showed an adenoma <0.6 cm.
CD was considered to be in remission after the improvement of hypercortisolism symptoms or clinical signs of adrenal insufficiency, associated with serum cortisol within reference values, normalization of 24-h UFC and/or serum cortisol <1.8 μg/dl at 8 am after 1 mg dexamethasone overnight, and/or normalization of midnight serum or salivary cortisol. In patients with active disease, to evaluate the ketoconazole treatment response, 24-h UFC was used as a laboratory parameter, as recommended in similar publications (14, 16, 20, 21), but in some cases, we considered elevated late night salivary cortisol and/or 1 mg dexamethasone overnight cortisol (even with normal 24-h UFC), given the greater assessment sensitivity seen through these two methods in the detection of early recurrence when compared with 24-h UFC (22).
Inclusion criteria
We included patients with CD and active hypercortisolism who used ketoconazole either as primary treatment, after TSS without hypercortisolism remission, or after a recurrence.
Exclusion criteria
We excluded patients with CD and active hypercortisolism who used ketoconazole but had <7 days of follow-up, irregular outpatient follow-up, treatment non-adherence, and incomplete medical records or those who were lost to follow-up.
Evaluated parameters
Prior to ketoconazole treatment, all patients underwent an assessment of pituitary function and hypercortisolism, including serum cortisol, ACTH, 24-hour UFC, cortisol suppression after 1 mg dexamethasone overnight, midnight serum cortisol, and/or midnight salivary cortisol. The evaluated parameters were sex, age at diagnosis, weight, height, prevalence and severity of hypertension and DM, pituitary tumor characteristics, prior treatment (surgery, radiotherapy, or other medications), symptoms at disease onset, biochemical tests (renal function, hepatic function, and lipid profile), number of medications used to treat associated comorbidities, data on medication tolerance, and reasons for discontinuation, when necessary.
The clinical parameters observed during treatment were control of blood pressure and hyperglycemia, anthropometric measurements (weight, height, and body mass index), jaundice, and any other symptoms or adverse effects reported by patients.
The biochemical evaluation included fasting glucose, glycated hemoglobin, lipid profile (total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglycerides), markers of liver damage (transaminases, bilirubin, gamma-glutamyl transferase, and alkaline phosphatase), electrolytes (sodium and potassium), and renal function (creatinine and urea). Hypecortisolism was accessed preferentially by 24-h UFC, however, late-night salivary cortisol and cortisol after 1 mg overnight dexamethasone could also be used.
Study design
This retrospective cohort study included patients with CD who were followed up at the Hospital de Clínicas de Porto Alegre Endocrinology Division, with their medical records from the first outpatient visit and throughout clinical follow-up collected. This study was approved by the Hospital de Clínicas de Porto Alegre Research Ethics Committee (number 74555617.0.0000.5327).
Outcomes
Hypercortisolism was considered controlled when the 24-h UFC and/or late-night salivary cortisol (LNSC) and/or overnight 1 mg dexamethasone suppression test (DST) levels were normalized in at least two consecutive assessments. Hypercortisolism was considered partially controlled when there was a 50% over-reduction in 24-h UFC and/or LNSC and/or DST levels but still above normal. A reduction lower than 50% in these parameters was considered as non-response.
We also assessed the ketoconazole doses that resulted in 24-h UFC normalization, maximum dose, medication tolerance, adverse effects, and changes in liver, kidney, and biochemical function. Due to the characteristics of this study, these outcomes were periodically evaluated in all patient consultations, which occurred usually every 2 to 4 months.
Data collection
This retrospective cohort evaluated outpatient medical records and any tests indicated by the attending physician as a pragmatic study. Ketoconazole use followed the department’s care protocol, which is based on national and international guidelines (4), and all patients received a similar care routine: the recommended initial prescription was generally taken in two to six doses at 100 to 300 mg/day. It was then increased by 200 mg every 2 to 4 months until hypercortisolism was controlled or side effects developed, especially those related to liver function. The maximum prescription was 1,200 mg/day. Clinical follow-up of these patients was performed 30 days after starting the medication and every 2–4 months thereafter (23).
Clinical, anthropometric, laboratory, and other exam data were collected through a review of the hospital’s electronic medical records for the entire follow-up period. Data from the first and last consultation were considered in the final analysis of all parameters.
Statistical analysis
Baseline population characteristics were described as mean and standard deviation (SD) or median with interquartile ranges (25–75) for continuous variables. The chi-square test was used to compare qualitative variables, and Student’s t-test or ANOVA was used to compare the quantitative variables. The Mann–Whitney U-test was used for unpaired data. P-values <0.05 were considered significant. Statistical analysis was performed in SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and R package geepack 1.3-1.
Results
Treatment with ketoconazole was indicated for 41 of the 172 CD patients. In 3/41 patients, ketoconazole was unallowed due to concomitant liver disease, and 38 received ketoconazole during CD treatment between 2004 and 2020. Of these, five were excluded due to insufficient data to determine the response to ketoconazole (short treatment time, irregular follow-up, incomplete medical records, or lost to follow-up). The baseline characteristics of every sample are shown in Table 1 . Thus, 33/41 patients were included in the final analysis. The patients were predominantly women (84.2%) and white (89.5%); 11 had microadenoma, 15 had macroadenoma, and 11 had no adenoma visualized. In 12/33 patients, pituitary imaging was not performed immediately before starting ketoconazole. Hypertension was observed in 26 patients (78%) and DM in 12 patients (36%). The mean age at CD diagnosis was 31.7 years.
Table 1.
Baseline clinical data of Cushing’s disease patients treated with ketoconazole.
| Variable | Total, n = 38 (%) |
|---|---|
| Sex (F/M) | 32/6 (84.2/15.8%) |
| Age (years) | 31.7 (11–51) |
| Ethnicity (White/non-White) | 34/4 (89.5/10.5%) |
| Facial plethora | 22 (57.9%) |
| Diabetes mellitus | 12 (36%) |
| Hypertension | 26 (78%) |
| Purpuric striae | 21 (55.3%) |
| Acne | 11 (28.9%) |
| Proximal weakness | 19 (50%) |
| Supraclavicular fat | 17 (44.7%) |
| “Buffalo hump” | 22 (57.9%) |
| Psychiatric illness | 12 (31.6%) |
| Microadenoma Macroadenoma Normal MRI Unavailable |
11 (28.94%) 15 (39.47%) 11 (28.94%) 1 (2.63%) |
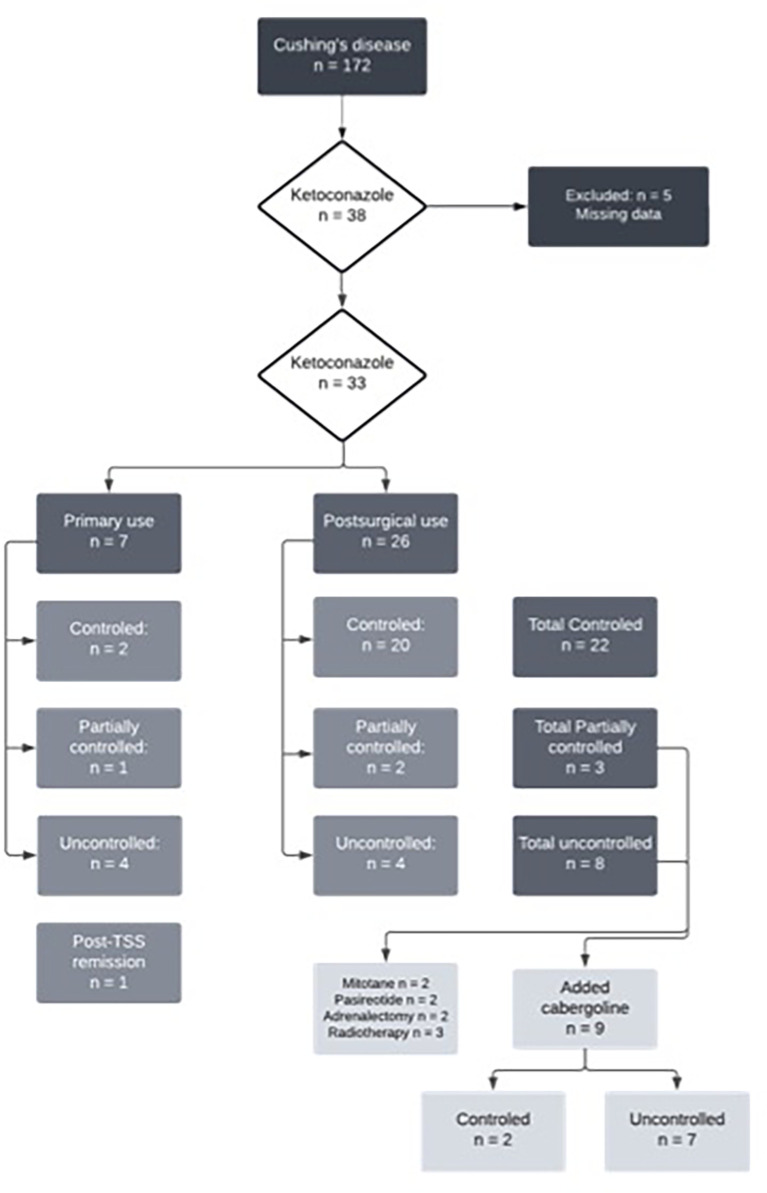
Of the 33 patients with complete data, 26 (78%) underwent TSS prior to starting ketoconazole, five of whom (15%) had also undergone radiotherapy. Thus, seven patients used ketoconazole as primary treatment since performing a surgical procedure was impossible at that time. Of these, four had no response to ketoconazole, one had a partial response, and two had a complete response. At follow-up, four of these patients underwent their first TSS, and three continued the ketoconazole therapy, achieving full UFC control. Among those who used ketoconazole after TSS (n = 26), 20 had a complete response, two had a partial response, and four had no response. Figure 1 shows the study flow chart and patient distribution throughout the treatment.
Figure 1.
Flowchart of ketoconazole treatment in Cushing's disease patients.
Individual patient data are described in Table 2 . The duration of ketoconazole use ranged from 14 days (in one patient who used it pre-TSS) to 14.5 years. The total follow-up time of the 22 patients with controlled CD ranged from 3 months to 14.5 years, with a mean of 5.33 years and a median of 4.8 years.
Table 2.
Individual data.
| ID | Sex | Time(months) | Prior TSS | Prior RDT | Dose variation (mg) a | Daily doses | Baseline ACTH | Baseline X > ULNUFC | Final X > ULNUFC | Final therapeutic response | Comorbidities | Altered liver function | Regular use | Intolerance/adverse effects | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 42 | Yes | No | 400–600 | 3 | 41 | 0.13 | 0.4b | Complete | SAH and DM | No | Yes | No | Continued use | |
| 2 | F | 7 | Yes | Yes | 100–200 | 2 | 88 | 1 | 0.79b | Complete | SAH | No | Yes | Yes, nausea | Interrupted due to intolerance | |
| 3 | F | 5 | No, pre-TSS use | No | 200–400 | 2 | 5.4 | 0.39 | Partial | SAH | Yes—drug hepatitis | Yes | Yes, hepatitis | Interrupted due to drug hepatitis | ||
| 4 | F | 120 | No | No | 200–800 | 4 | 76 | 2.92 | 0.8b | Complete | – | No | Yes | Yes | Pseudotumor cerebri resolved with acetazolamide | |
| 5 | F | 126 | Yes | No | 150–1,000 | 4 to 5 | 129 | 0.05 | -0.36 | Complete | Dyslipidemia | No | Yes | No | Continued use | |
| 6 | F | 90 | Yes | No | 300–1,000 | 4 to 5 | 26 | 3.9 | -0.7 | Complete | SAH and DM | No | Yes | No | Underwent radiotherapy | |
| 7 | F | 63 | Yes | No | 400–1,000 | 3 | 148 | 5.8 | -0.46 | Complete | SAH | No | Yes | No | Underwent radiotherapy | |
| 8 | F | 26 | No, pre-TSS use | No | 600–800 | 4 | 106 | 2.56 | 0.39b | No response | SAH and DM | No | Yes | No | Underwent TSS | |
| 9 | F | 81 | Yes | No | 600–1,200 | 5 | 24 | 22.2 | 5.68b | No response | SAH | No | Yes | Yes | Underwent bilateral adrenalectomy | |
| 10 | F | 86 | Yes | No | 400–1,200 | 4 to 5 | 65 | 0.72 | -0.47 | Complete | – | Important and isolated elevation of GGT | Yes | No | Underwent new TSS | |
| 11 | F | 51 | Yes | No | 400–800 | 2 | 48 | -0.68 b | -0.8 | Complete | SAH and dyslipidemia | Discrete and isolated elevation of GGT | Yes | No | Continued use | |
| 12 | F | 14 days | No | No | 600 | 3 | 121 | 4.3 | 3.5 | No response | SAH and DM | No | Yes | No | Underwent TSS | |
| 13 | F | 15 | Yes | No | 600–800 | 4 | 50 | 0.1 | -0.06 | Complete | SAH, DM and dyslipidemia | Discrete and isolated elevation of GGT | No | No | Continued use | |
| 14 | F | 85 | Yes | No | 200–800 | 4 | 37 | 2.11 | -0.38b | No response | SAH | Elevation of TGO and GPT, discontinued medication | Yes | Yes | Underwent radiotherapy | |
| 15 | F | 119 | Yes | No | 600–1,200 | 4 to 5 | 40 | 2.31 | 1.3 | Partial | SAH, DM and dyslipidemia | Discrete and isolated elevation of GGT | Yes | No | Underwent radiotherapy | |
| 16 | F | 14 | Yes | Yes | 600 | 3 | 300 | 0.27 | -0.06b | Partial | SAH and dyslipidemia | No | Yes | No | Underwent bilateral adrenalectomy | |
| 17 | M | 29 | Yes | No | 400–600 | 3 | 54 | 1.62 | -0.6 | Complete | – | No | Yes | Yes, loss of appetite | Ketoconazole suspended due to loss of appetite | |
| 18 | F | 5 | Yes | No | 400 | 2 | 65 | - 0.08 b | -0.8 | Complete | SAH and DM | Discrete and isolated GGT uptake | Yes | No | Underwent radiosurgery | |
| 19 | M | 42 | No, pre-TSS use | No | 400–1,200 | 2 to 6 | 51 | 3.67 | 2.8 | No response | SAH | Discrete elevation of TGO and GGT | No | No | Underwent TSS | |
| 20 | F | 6 | Yes | No | 400 | 2 | 115 | 3.9 | 2b | No response | – | No | Yes | No | Lost to follow-up | |
| 21 | F | 125 | Yes | Yes | 400–1,000 | 3 to 5 | 84 | 1.34 | -0.02 | Complete | SAH | No | Yes | Yes, diarrhea | Underwent bilateral adrenalectomy | |
| 22 | M | 34 | Yes | Yes | 100 | 2 | 0.3 | -0.8 | Complete | SAH, DM and dyslipidemia | No | Yes | No | Lost to follow-up | ||
| 23 | F | 53 | Yes | No | 200–600 | 2 to 4 | 61 | 1.19 | -0.6 | Complete | SAH and DM | No | Yes | Yes, dizziness | Continued use | |
| 24 | M | 3 | Yes | No | 600 | 3 | 1,696 | 34 | 0.8b | Complete | SAH | No | Yes | No | Underwent bilateral adrenalectomy | |
| 25 | F | 133 | Yes | No | 400 | 2 | 89 | 0.4 | -0.8 | Complete | SAH | No | Yes | No | Continued use | |
| 26 | F | 23 | Yes | No | 200–800 | 2 to 4 | 57 | 0.53 | -0.08 | No response | – | No | Yes | No | Underwent radiosurgery | |
| 27 | F | 38 | No, pre-TSS use | No | 400–600 | 2 to 3 | 102 | 4.1 | -0.3 | Complete | SAH and DM | No | Yes | Yes, dizziness and loss of appetite | Continued use | |
| 28 | M | 111 | Yes | No | 400—1,200 | 3 to 4 | 75 | 3.9 | -0.8 | Complete | SAH | Discrete elevation of TGO, GPT, and GGT | Yes | Yes, nausea | Underwent radiotherapy | |
| 29 | F | 8 | No, pre-TSS use | No | 300 | 3 | 92 | 2.2 | 0.8b | No response | SAH | No | Yes | No | Underwent TSS | |
| 30 | F | 132 | Yes | No | 200–400 | 2 to 3 | 20 | -0.2 b | -0.5 | Complete | SAH, DM and dyslipidemia | No | Yes | No | Continued use | |
| 31 | F | 40 | Yes | No | 400–800 | 2 to 3 | 21 | 0.7 | -0.99 | Complete | SAH and DM | No | Yes | No | Continued use | |
| 32 | F | 64 | Yes | No | 400–800 | 2 to 4 | 41 | -0.7 b | -0.6 | Complete | – | Discrete and isolated elevation of GGT | Yes | No | Underwent radiotherapy | |
| 33 | F | 173 | Yes | Yes | 400–800 | 2 to 4 | 40 | 0.05 | -0.5 | Complete | SAH | No | No | No | Pregnancy continued using ketoconazole without complications | |
RDT, radiotherapy; DM, diabetes mellitus; GGT, gamma glutamyl transferase; SAH, systemic arterial hypertension; TGO, oxaloacetic transaminase; GPT, glutamate–pyruvate transaminase; TSS, transsphenoidal surgery; ULN, upper limit of normal.
The highest dose used was not necessarily the final dose used.
In these patients, the criterion used to consider active hypercortisolism was late night salivary cortisol and/or elevated 1 mg dexamethasone overnight.
Therapeutic response
Relative therapeutic response data are described in Table 3 . Patients whose hypercortisolism was controlled or partially controlled with ketoconazole had lower baseline 24-h UFC than the uncontrolled group [times above the upper limit of normal: 0.62 (SD, 0.41) vs. 5.3 (SD, 8.21); p < 0.005, respectively], in addition to more frequent prior TSS (p < 0.04). In some patients (4/33), 24-h UFC was in the normal range at the beginning of ketoconazole therapy, but they were prescribed with the medication due to the clinical recurrence of CD associated to cortisol non-suppression after 1 mg dexamethasone overnight and/or abnormal midnight salivary or serum cortisol.
Table 3.
Baseline characteristics of Cushing’s disease patients according to therapeutic response to ketoconazole.
| Controlled/partially controlled | Uncontrolled | p | ||
|---|---|---|---|---|
| Sex (F/M) | 17/8 (68/32%) | 7/1 (87.5/12.5%) | 0.2281 | |
| Initial UFC (times > ULN) |
0.62 ± 0.41 (-0.71–0.73) | 5.3 ± 8.21 (-0.14–34) | < 0.005 | |
| Final UFC (times > ULN) |
-0.48 ± 0.55 (-0.99–1.3) | 5.13 ± 6.58 (-0.09–19.9) | < 0.005 | |
| ACTH | 83.6 ± 81.8 (20–300) | 172.3 ± 408.2 (1–1696) | 0.635 | |
| Basal cortisol | 18.8 ± 9.5 (8.1–43) | 26.5 ± 12.39 (5.8–47) | 0.092 | |
| BMI | 34.5 ± 7.6 (25.8–48) | 31.3 ± 6.5 (23.6–50.4) | 0.268 | |
| Final dose | 468 ± 314 (0–1,000) | 562 ± 184 (300–800) | 0.576 | |
| Fasting blood glucose | 111.5 ± 39.9 (69–177) | 120.7 ± 46.4 (77–219) | 0.881 | |
| Prior TSS (Y/N) | 22/3 (88/12%) | 4/4 (50/50%) | 0.043 | |
| RDT (Y/N) | 9/16 (36/64%) | 2/6 (25/75%) | 0.56 | |
| Regular use (Y/N) | 23/2 (92/8%) | 6/2 (75/25%) | 0.2 | |
ACTH, adrenocorticotropic hormone; TSS, transsphenoidal surgery; RDT, radiotherapy; UFC, urinary free cortisol; ULN, upper limit of normal.
Figure 2 shows that the prevalence of uncontrolled patients remained stable over time (approximately 30%) despite dose adjustments or association with other drugs, which led to no differences. When analyzing only the results of the last follow-up visit (eliminating fluctuations during follow-up), 22 patients had a complete response (66%), three patients had a partial response (9%), and eight patients had no response to ketoconazole treatment (24%), which includes patients who underwent radiotherapy during ketoconazole treatment.
Figure 2.
Prevalence of controlled hypercortisolism during follow-up of Cushing's disease patients treatesd with ketoconazole.
During follow-up, no significant differences were found in blood pressure control or in dehydroepiandrosterone sulfate, cortisol, ACTH, or glucose levels. Worsening of hypertension control was observed in association with hypokalemia in some cases, as described in side effects. The ketoconazole doses ranged from 100 to 1,200 mg per day, and there were no significant dose or response differences between the groups ( Table 4 ). Figure 3 shows the patients, their dosages, and 24-h UFC control at the first and last consultation, showing a trend toward hypercortisolism reduction in approximately 70% of the cohort (25 of 33). Only four patients used doses lower than 300 mg at the end of follow-up. One of them used before TSS and suspended its use after surgery. One patient, who has already undergone radiotherapy, discontinued ketoconazole due to intolerance, despite adequate control of hypercortisolism. Another one, who had also undergone radiotherapy, was lost to follow-up when it was controlled using 100 mg daily, and one remained controlled using 200 mg, without previous radiotherapy.
Table 4.
Final dose of ketoconazole used in patients with Cushing’s disease.
| Final dose | Controlled/partially controlled | Uncontrolled |
|---|---|---|
| ≤100 mg | 1 (4%) | 0 |
| 200 mg | 2 (8%) | 0 |
| 300 mg | 0 | 1 (12.5%) |
| 400 mg | 6 (24%) | 2 (25%) |
| 600 mg | 9 (36%) | 3 (37.5%) |
| 800 mg | 6 (24%) | 2 (25%) |
| 1,000 mg | 1 (4%) | 0 |
Figure 3.
First and last consultation 24çhour UFC results vs. ketoconazole dosage in Cushing's disease patients.
Side effects
Regarding adverse effects ( Table 5 ), there was no significant difference between the controlled/partially controlled group and the uncontrolled group regarding liver enzyme changes or drug intolerance. Mild adverse effects, including nausea, vomiting, dizziness, and loss of appetite, occurred in 10 patients (30%). Only four patients had serious adverse effects that warranted discontinuing the medication. In two cases, ketoconazole was discontinued due to a significantly acute increase in liver enzymes (drug-induced hepatitis) during the use of 400 and 800 mg of ketoconazole. Non-significant elevation of transaminases (up to three times the normal value) was observed in three cases. A slight increase in gamma-glutamyltransferase occurred in six patients. In these nine patients with elevated liver markers, the daily dose ranged from 400 to 1,200 mg. None of those with mild increases in liver markers needed to discontinue ketoconazole.
Table 5.
Adverse effects of ketoconazole in Cushing’s disease patients treated with ketoconazole.
| Controlled/partially controlled | Uncontrolled | p | |
|---|---|---|---|
| AST | 70 ± 230 (12–1,128) | 59 ± 90 (15–244) | 0.608 |
| ALT | 97 ± 341 (16–1,665) | 112 ± 214 (17–550) | 0.871 |
| Intolerance (Y/N) | 3/22 (12/88%) | 1/7% (12.5–87.5%) | 0.97 |
AST, aspartate transaminase; ALT, alanine aminotransferase.
One female patient developed pseudotumor cerebri syndrome, which was treated with acetazolamide. She did not need to discontinue ketoconazole, having used it for more than 10 years without new side effects and achieving complete control of hypercortisolism (24). Another patient became pregnant during follow-up while using the medication, but no maternal or fetal complications occurred (25).
Hypokalemia was also observed during follow-up. Twenty episodes of reduced potassium levels occurred in 10 patients over the course of treatment. Of these episodes, six occurred in controlled patients, three in partially controlled patients, and 11 in uncontrolled patients ( Table 6 ). The hypokalemia was managed with spironolactone (25 to 100 mg) and oral potassium supplementation.
Table 6.
Characteristics of Cushing’s disease patients who developed hypokalemia during ketoconazole treatment.
| ID | Dose (mg) | HK episodes | K value | Disease control | BP control | Previous medications in use |
|---|---|---|---|---|---|---|
| 9 | 1,200 | 7 | 3.3 3.3 3 2.8 2.8 2.8 3 |
No No No No Partial No No |
High High Normal Normal Normal High Normal |
HCTZ (suspension) enalapril, spironolactone, PPL, amlodipine, cabergoline, mitotane |
| 15 | 600 | 1 | 3.2 | Yes | Normal | HCTZ |
| 16 | 1,000 | 1 | 2.8 | Yes | High | Spironolactone, furosemide, enalapril, chlorthalidone, simvastatin |
| 8 | 600 | 1 | 3.4 | Yes | Normal | HCTZ |
| 10 | 800 | 1 | 3.1 | No | High | HCTZ |
| 14 | 800 | 1 | 3.3 | No | Normal | HCTZ and captopril |
| 19 | 1,200 | 1 | 2.9 | No | Unavailable | Amlodipine, atenolol, losartan, hydralazine, spironolactone |
| 25 | 400 | 1 | 3.2 | Partial | High | HCTZ |
| 28 | 1,200 | 3 | 2.9 2.6 3.3 |
Partial Partial No |
High High High |
Losartan, chlorthalidone, spironolactone, hydralazine |
| 27 | 400 400 600 |
3 | 3.1 3.4 3.4 |
Yes No Yes |
Normal Normal Normal |
Enalapril and HCTZ |
HK, hypokalemia; HCTZ, hydrochlorothiazide; PPL, propranolol.
Ketoconazole and associations
Of the patients who used an association of cabergoline and ketoconazole, one did so since the beginning of follow-up, while another nine were prescribed cabergoline during follow-up due to non-response to ketoconazole alone. Of these 10 patients, two did not start the medication due to problems in obtaining the drug. Thus, in two of the nine patients on the maximum tolerated dose of ketoconazole or who could not tolerate a higher dose due to hepatic enzymatic changes, 1.5–4.5 mg of cabergoline per week was associated. In patients not controlled with ketoconazole plus cabergoline, mitotane (two patients) or pasireotide (two patients) was added. Only two of nine patients responded to the combination of cabergoline and ketoconazole. Data on these associations are shown in Table 7 .
Table 7.
Effects of associating cabergoline with ketoconazole in Cushing’s disease patients.
| ID | Sex | Adenoma | Maximum daily (mg) dose of ketoconazole | Maximum weekly dose of cabergoline | Control | Other medications | Side effects | Outcome |
|---|---|---|---|---|---|---|---|---|
| 7 | F | Macro | 1,000 | 3.5 mg | No | Temozolomide and pasireotide | Gastrointestinal effects | RDT |
| 9 | F | Normal | 1,000 | 3.5 mg | No | Mitotane | AI symptoms | Adrenalectomy |
| 10 | F | Macro | 1,200 | 3 mg | Yes | – | – | Medications maintained |
| 14 | F | Macro | 800 | 1.5 mg | No | – | Elevation of transaminases | RDT |
| 15 | F | Micro | 1,200 | 3 mg | No | Mitotane | – | RDT |
| 21 | F | Normal | 600 | 2 mg | Yes | – | Gastrointestinal symptoms | Adrenalectomy |
| 26 | F | Macro | 400 | 2 mg | No | – | – | RDT |
| 28 | M | Macro | 1,200 | 4.5 mg | No | Pasireotide | Hyperglycemia, hypogonadism, and hypokalemia | RDT |
AI, adrenal insufficiency; ND, no data; RDT, radiotherapy.
Considering that one of the indications for the treatment of hypercortisolism may be complementary to radiotherapy, we analyzed the eight patients who underwent radiotherapy after transsphenoidal surgery. In these patients, doses of ketoconazole from 200 to 1,200 mg were used, and in six patients there was a normalization of the UFC in 1 to 60 months of treatment. Thus, the association of ketoconazole with radiotherapy was effective in normalizing the 24-h UFC in 75% of cases.
Clinical follow-up
New therapeutic approaches were attempted in some patients during follow-up: radiotherapy (eight patients), new TSS (five patients), and bilateral adrenalectomy (four patients). At the end of this analysis, 11 patients remained on ketoconazole, all with controlled hypercortisolism. Among the 11 patients who were not fully controlled by the last visit, five were using ketoconazole as pre-TSS therapy and underwent TSS as soon as possible, while three others underwent radiotherapy and two underwent bilateral adrenalectomy. One patient was lost to follow-up.
Discussion
According to the current consensus about CD, drug treatment should be reserved for patients without remission after TSS, those who cannot undergo surgical treatment, or those awaiting the effects of radiotherapy (4, 16). Drugs available in this context may act as adrenal steroidogenesis blockers (ketoconazole, osilodrostat, metyrapone, mitotane, levoketoconazole, and etomidate), in pituitary adenoma (somatostatinergic receptor ligands—pasireotide), dopamine receptor agonists (cabergoline), or glucocorticoid receptor blockers (mifepristone) (16, 26). Among these alternatives, the drug of choice still cannot be determined. Thus, the best option must be established individually, considering aspects such as remission potential, safety profile, availability, cost, etc. (16, 27, 28).
For over 30 years, ketoconazole has been prescribed off-label for CD patients with varied rates of remission of hypercortisolism, and it can be used in monotherapy or associated with other drugs (29, 30). The Brazilian public health system does not provide drugs for the treatment of CD, and among medications with a better profile for controlling hypercortisolism, such as osilodrostat, levoketoconazole, and pasireotide, only pasireotide has been approved by the national regulatory authority (ANVISA). Due to such pragmatic considerations, ketoconazole is among the most commonly used drugs in our health system, whether recently associated or not with cabergoline (7).
In this cohort, the most prevalent response type was complete (66%). Since 75% of the CD patients who used ketoconazole had a complete or partial response, there was a clear trend towards improvement in hypercortisolism. When only those who used ketoconazole post-TSS were evaluated, the rate of control increased to 76%. We found that patients with a higher initial 24-h UFC tended to have less control of excess cortisol, a difference that was not observed when analyzing ketoconazole dose or follow-up time.
In our series and at the prescribed doses, the combination of cabergoline and ketoconazole was not effective in the management of hypercortisolism since only two of nine patients (22%) had their 24-hour UFC normalized. However, it should be observed that this association was used in patients who had more severe CD and, consequently, were less likely to have a favorable response. The effects of cabergoline in CD patients remain controversial, although some studies have shown promising responses (31, 32).
Previous reviews found that the efficacy of ketoconazole for hypercortisolism control was quite heterogeneous, ranging from 14 to 100% in 99 patients (33, 34). Our cohort’s response rate was lower than that of Sonino et al. (89%) (20) but higher than that of a multicenter cohort by Castinetti et al. (approximately 50%) (14). Regarding other smaller series (35–37) our results reinforce some findings that demonstrate a percentage of control greater than 50% of the cases.
Our analyses showed a trend toward a response that continued, with some oscillations, over time. The rate of uncontrolled patients remained stable over time (approximately 30%), regardless of association with other drugs (cabergoline, mitotane, or pasireotide) or dose adjustments. Speculatively, it would appear that patients who respond to ketoconazole treatment would show some type of response as soon as therapy begins.
Our cohort has the longest follow-up time of any study on ketoconazole use in CD, nearly 15 years. Our results demonstrate that patients who benefit from ketoconazole (i.e., control of hypercortisolism and associated comorbidities) can safely use it for a long term since those who did not experience liver enzyme changes at the beginning of treatment also had no long-term changes.
Another relevant information for clinical practice is the result of treatment with ketoconazole associated with radiotherapy, which demonstrated normalizing the 24-h UFC in 75% of cases, a finding that reinforces the use of this therapeutic combination, especially in cases that are more resistant to different treatment modalities.
As described in the literature, adverse effects, such as nausea, vomiting, dizziness, headache, loss of appetite, and elevated transaminases, are relatively frequent (38). In our cohort, 10 patients (30%) had mild adverse effects, and four (12%) had more serious adverse effects requiring discontinuation. In other studies, up to 20% of patients required discontinuation due to side effects (14). We documented 20 episodes of hypokalemia during ketoconazole treatment, some with worsening blood pressure control. In most cases, hypokalemia has occurred in association with the use of diuretic drugs, which may have potentiated potassium spoliation, reinforcing the need of stringent surveillance in hypertensive Cushing’s disease patients using this combination. It can also result from the enzymatic blockade that could lead to the elevation of adrenal mineralocorticoid precursors (pex. deoxycorticosterone), with consequent sodium retention and worsening hypertension. Although it has not been analyzed in other series with ketoconazole, this side effect has been observed in patients who received other adrenal-blocking drugs, such as osilodrostat and metyrapone (16). This alteration seems to be transient in some patients; in our series, it was managed by suspending drugs that could worsen hypokalemia and introducing spironolactone and/or potassium supplementation. Hypokalemia may also result from continuing intense adrenal stimulation by ACTH and changes in the activity of the 11-beta-hydroxysteroid dehydrogenase enzyme, which increase the mineralocorticoid activity of cortisol, as observed in patients with severe hypercortisolism in uncontrolled CD (39). Hypogonadism occurred in one male patient. In two adolescent patients (one female and one male), hypercortisolism was effectively controlled without altering the progression of puberty. As described in other cohorts, this effect was expected due to the high doses, which block adrenal and testicular androgen production (20).
Thus, our findings confirm previous reports in the literature and add important information about the side effects and safety of long-term ketoconazole use in CD treatment. Our data reinforce the current recommendations about ketoconazole for recurrent cases or those refractory to surgery, including proper follow-up by an experienced team specializing in evaluating clinical and biochemical responses and potential adverse effects (7, 18, 40). Despite the severity of many of our CD patients, no ketoconazole-related death occurred during follow-up, including long-term observation. On the other hand, no patient progressed to definitive remission of hypercortisolism, even after many years of treatment with ketoconazole.
Conclusions
In our cohort of patients, ketoconazole proved to be an effective and safe alternative for CD treatment, although it can produce side effects that require proper identification and management, allowing effective long-term treatment. We found side effects that have been rarely described in the literature, including hypokalemia and worsening hypertension, which require specific care and management. Thus, ketoconazole is an effective alternative for CD patients who cannot undergo surgery, who do not achieve remission after pituitary surgery, or who have recurrent hypercortisolism.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Hospital de Clínicas de Porto Alegre Research Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CV and MAC created the research format. CV, RBM, and MCBC realized the search on medical records. CV performed the statistical analysis. MAC, ACVM, and TCR participated in the final data review and discussion. ACVM participated in the final data review and discussion as volunteer collaborator. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES), Ministry of Health - Brazil, through a PhD scholarship; and the Research Incentive Fund (FIPE) of Hospital de Clínicas de Porto Alegre.
Acknowledgments
The authors would like to thank the HCPA Research and Graduate Studies Group (GPPG) for the statistical technical support provided by Rogério Borges. We also thank the Research Incentive Fund of Hospital de Clínicas de Porto Alegre and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), by funds applied. We also thank the Graduate Program in Endocrinology and Metabolism (PPGEndo UFRGS) for all the support in the preparation of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Fleseriu M, Castinetti F. Updates on the role of adrenal steroidogenesis inhibitors in cushing’s syndrome: a focus on novel therapies. Pituitary (2016) 19(6):643–53. doi: 10.1007/s11102-016-0742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of cushing’s disease. Endocr Rev (2015) 36(4):385–486. doi: 10.1210/er.2013-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nieman LK, Biller BMK, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of cushing’s syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2008) 93(5):1526–40. doi: 10.1210/jc.2008-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieman LK, Biller BMK, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of cushing’s syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2015) 100(8):2807–31. doi: 10.1210/jc.2015-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pivonello R, De Martino M, De Leo M, Lombardi G, Colao A. Cushing’s syndrome. Endocrinol Metab Clin North (2008) 37(1):135–49. doi: 10.1016/j.ecl.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 6. Alexandraki KI, Grossman AB. Therapeutic strategies for the treatment of severe cushing’s syndrome. Drugs (2016) 76(4):447–8. doi: 10.1007/s40265-016-0539-6 [DOI] [PubMed] [Google Scholar]
- 7. Machado MC, Fragoso MCBV, Moreira AC, Boguszewski CL, Neto LV, Naves LA, et al. A review of cushing’s disease treatment by the department of neuroendocrinology of the Brazilian society of endocrinology and metabolism. Arch Endocrinol Metab (2018) 62(1):87–105. doi: 10.20945/2359-3997000000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rollin G, Ferreira NP, Czepielewski MA. Prospective evaluation of transsphenoidal pituitary surgery in 108 patients with Cushing's disease. Arq Bras Endocrinol Metabol. (2007) 51(8):1355–61. doi: 10.1590/s0004-27302007000800022 [DOI] [PubMed] [Google Scholar]
- 9. Patil CG, Prevedello DM, Lad SP, Lee Vance M, Thorner MO, Katznelson L, et al. Late recurrences of cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab (2008) 93(2):358–62. doi: 10.1210/jc.2007-2013 [DOI] [PubMed] [Google Scholar]
- 10. Rubinstein G, Osswald A, Zopp S, Ritzel K, Theodoropoulou M, Beuschlein F, et al. Therapeutic options after surgical failure in cushing’s disease: A critical review. Best Pract Res Clin Endocrinol Metab (2019) 33(2):101270. doi: 10.1016/j.beem.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 11. Zhao N, Yang X, Li C, Yin X. Efficacy and safety of pasireotide for Cushing's disease: A protocol for systematic review and meta-analysis. Medicine (Baltimore). (2020) 99(51):e23824. doi: 10.1097/MD.0000000000023824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, et al. Efficacy and safety of osilodrostat in patients with cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol (2020) 8(9):748–61. doi: 10.1016/S2213-8587(20)30240-0 [DOI] [PubMed] [Google Scholar]
- 13. Yan JY, Nie XL, Tao QM, Zhan SY, De Zhang Y. Ketoconazole associated hepatotoxicity: A systematic review and meta-analysis. Biomed Environ Sci (2013) 26(7):605–10. doi: 10.3967/0895-3988.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 14. Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, et al. Ketoconazole in cushing’s disease: Is it worth a try. J Clin Endocrinol Metab (2014) 99(5):1623–30. doi: 10.1210/jc.2013-3628 [DOI] [PubMed] [Google Scholar]
- 15. Castinetti F, Nieman LK, Reincke M, Newell-Price J. Approach to the patient treated with steroidogenesis inhibitors. J Clin Endocrinol Metab (2021) 106(7):2114–23. doi: 10.1210/clinem/dgab122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleseriu M, Auchus R, Bancos I, Bem-Shlomo A, Bertherat J, Biermasz NR, et al. Consensus on diagnosis and management of cushing’s disease: a guideline update. Lancet Diabetes Endocrinol (2021) 9(12):847–75. doi: 10.1016/s2213-8587(21)00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleseriu M, Pivonello R, Elenkova A, Salvatori R, Auchus RJ, Feelders RA, et al. Efficacy and safety of levoketoconazole in the treatment of endogenous cushing’s syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol (2019) 7(11):855–65. doi: 10.1016/S2213-8587(19)30313-4 [DOI] [PubMed] [Google Scholar]
- 18. Tritos NA. Adrenally directed medical therapies for cushing syndrome. J Clin Endocrinol Metab (2021) 106(1):16–25. doi: 10.1210/clinem/dgaa778 [DOI] [PubMed] [Google Scholar]
- 19. Simões Corrêa Galendi J, Correa Neto ANS, Demetres M, Boguszewski CL, dos S V. N. nogueira, “Effectiveness of medical treatment of cushing’s disease: A systematic review and meta-analysis,”. Front Endocrinol (Lausanne) (2021) 12:732240(September). doi: 10.3389/fendo.2021.732240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonino N, Boscaro M, Paoletta A, Mantero F, Zillotto D. Ketoconazole treatment in cushing’s syndrome: experience in 34 patients. Clin Endocrinol (Oxf) (1991) 35(4):347–52. doi: 10.1111/j.1365-2265.1991.tb03547.x [DOI] [PubMed] [Google Scholar]
- 21. Costenaro F, Rodrigues TC, Rollin GAF, Czepielewski MA. Avaliação do eixo hipotálamohipófise adrenal no diagnóstico e na remissão da doença de cushing. Arquivos Brasileiros Endocrinologia e Metabologia (2012). doi: 10.1590/S0004-27302012000300002 [DOI] [PubMed] [Google Scholar]
- 22. Amlashi FG, Swearinger B, Faje AT, Nachtigall LB, Miller KK, Klibanski A, et al. Accuracy of late-night salivary cortisol in evaluating postoperative remission and recurrence in cushing’s disease. J Clin Endocrinol Metab (2015) 100(10):3770–7. doi: 10.1210/jc.2015-2107 [DOI] [PubMed] [Google Scholar]
- 23. Silveiro SP, Satler F. Rotinas em endocrinologia. (Porto Alegre: Artmed; ) (2015). [Google Scholar]
- 24. Costenaro F, Rodrigues TC, Ferreira NP, da Costa TG, Schuch T, Boschi V, et al. Pseudotumor cerebri during cushing’s disease treatment with ketoconazole. Arq. Bras Endocrinol Metabol (2011). doi: 10.1590/s0004-27302011000400008 [DOI] [PubMed] [Google Scholar]
- 25. Costenaro F, Rodrigues TC, De Lima PB, Ruszczyk J, Rollin G, Czepielewski MA. A successful case of cushing’s disease pregnancy treated with ketoconazole. Gynecol Endocrinol (2015) 31(3):176–8. doi: 10.3109/09513590.2014.995615 [DOI] [PubMed] [Google Scholar]
- 26. Gadelha MR, Neto LV. Efficacy of medical treatment in cushing’s disease: A systematic review. Clin Endocrinol (Oxf) (2014) 80(1):1–12. doi: 10.1111/cen.12345 [DOI] [PubMed] [Google Scholar]
- 27. Fleseriu M, Petersenn S. New avenues in the medical treatment of cushing’s disease: Corticotroph tumor targeted therapy. J Neurooncol (2013) 114(1):1–11. doi: 10.1007/s11060-013-1151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fleseriu M, Petersenn S. Medical management of cushing’s disease: What is the future? Pituitary (2012) 15(3):330–41. doi: 10.1007/s11102-012-0397-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feelders RA, De Bruin C, Pereira AM, Romijn JÁ, Netea-Maier RT, Hermus AR, et al. Pasireotide alone or with cabergoline and ketoconazole in cushing’s disease. N Engl J Med (2010) 362(19):1846–8. doi: 10.1056/NEJMc1000094 [DOI] [PubMed] [Google Scholar]
- 30. Barbot M, Albiger N, Ceccato F, Zilio M, Frigo AC, Denaro Lc, et al. Combination therapy for cushing’s disease: Effectiveness of two schedules of treatment. should we start with cabergoline or ketoconazole? Pituitary (2014) 17(2):109–17. doi: 10.1007/s11102-013-0475-3 [DOI] [PubMed] [Google Scholar]
- 31. Vilar L, Naves LA, Azevedo MF, Arruda MJ, Arahata CM, Silva LM, et al. Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of cushing’s disease. Pituitary (2010) 13(2):123–9. doi: 10.1007/s11102-009-0209-8 [DOI] [PubMed] [Google Scholar]
- 32. Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, et al. The medical treatment of cushing’s disease: Effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab (2009) 94(1):223–30. doi: 10.1210/jc.2008-1533 [DOI] [PubMed] [Google Scholar]
- 33. Castinetti F, Morange I, Jaquet P, Conte-Devolx B, Brue T. Ketoconazole revisited: A preoperative or postoperative treatment in cushing’s disease. Eur J Endocrinol (2008). doi: 10.1530/EJE-07-0514 [DOI] [PubMed] [Google Scholar]
- 34. Loli P, Berselli ME, Tagliaferri M. Use of ketoconazole in the treatment of cushing’s syndrome. J Clin Endocrinol Metab (1986) 63(6):1365–71. doi: 10.1210/jcem-63-6-1365 [DOI] [PubMed] [Google Scholar]
- 35. Kakade HR, Kasaliwal R, Khadilkar KS, Jadhav S, Bukan A, Khare Sc, et al. Clinical, biochemical and imaging characteristics of cushing’s macroadenomas and their long-term treatment outcome. Clin Endocrinol (Oxf) (2014) 81(3):336–42. doi: 10.1111/cen.12442 [DOI] [PubMed] [Google Scholar]
- 36. Luisetto G, Zangari M, Camozzi V, Boscaro M, Sonino N, Fallo F. Recovery of bone mineral density after surgical cure, but not by ketoconazole treatment, in cushing’s syndrome. Osteoporos Int (2001) 12(11):956–60. doi: 10.1007/s001980170025 [DOI] [PubMed] [Google Scholar]
- 37. Huguet I, Aguirre M, Vicente A, Alramadan M, Quiroga I, Silva J, et al. Assessment of the outcomes of the treatment of cushing’s disease in the hospitals of castilla-la mancha. Endocrinol y Nutr (2015) 62(5):217–23. doi: 10.1016/j.endonu.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 38. Tritos NA, Biller BMK. Advances in the medical treatment of cushing disease. Endocrinol Metab Clin North Am (2020) 49(3):401–12. doi: 10.1016/j.ecl.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 39. Torpy D, Mullen N, Ilias I, Nieman L. Association of hypertension and hypokalemia with cushing’s syndrome caused by ectopic ACTH secretion. Ann N Y Acad Sci (2002) 970:134–44. doi: 10.1111/j.1749-6632.2002.tb04419.x [DOI] [PubMed] [Google Scholar]
- 40. Varlamov EV, Han AJ, Fleseriu M. “Updates in adrenal steroidogenesis inhibitors for cushing’s syndrome – a practical guide,”. Best Pract Res Clin Endocrinol Metab (2021) 35(1):101490. doi: 10.1016/j.beem.2021.101490 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.