Acquired Bleeding Disorders
Coagulopathy of Major Bleeding (Trauma, PPH, Vascular/surgical, ECMO, GI bleeding, etc.)
OC 11.5
Correction of coagulopathy in trauma haemorrhage – Still room for improvement. A secondary analysis of the ITACTIC trial
C. Lindsay 1; K. Baksaas‐Aasen2; N. Juffermans3; N. Curry4; M. Maegele5; J. Stensballe6; S. Stanworth7; R. Davenport8; P. Næss2; C. Gaarder9; K. Brohi1
1 Queen Mary University of London, London, England, United Kingdom; 2 Oslo University Hospital & University of Oslo, Oslo, Oslo, Norway; 3 Amsterdam University Medical Centre, Amsterdam, Noord‐Holland, Netherlands; 4 University of Oxford, Oxford, England, United Kingdom; 5 Cologne‐Merheim Medical Centre, University of Witten/Herdecke, Cologne, Nordrhein‐Westfalen, Germany; 6 Copenhagen University Hospital, Copenhagen, Hovedstaden, Denmark; 7 Oxford University Hospitals/NHS Blood and Transplant, Oxford, England, United Kingdom; 8 Centre for Trauma Science, Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom; 9 Oslo University Hospital & University of Oslo, Amsterdam, Oslo, Norway
Background: Viscoelastic haemostatic assays (VHAs) have advantages over conventional coagulation tests in terms of speed, sensitivity and ability to direct interventions. However, in the ITACTIC trial, comparing major haemorrhage protocols augmented with VHA or CCT guided intervention, there was no overall outcome benefit observed in the VHA arm, despite patients receiving a greater number of targeted interventions more quickly.
Aims: We aimed to explore the effect of targeted transfusion therapies on coagulation parameters in all patients, and a coagulopathic subgroup in the ITACTIC cohort.
Methods: We analysed a subgroup of ITACTIC patients, co‐enrolled into the ACIT observational study of coagulation and inflammation after trauma (REC 16/LO/0004 & 07/Q0603/2). We compared coagulation values at baseline and after 4, 8 and 12 units in the whole cohort and in a subgroup who received at least 8 units of RBCs and were coagulopathic at any time point (defined as EXTEM CA5 < 40 mm).
Results: 139 patients met the inclusion criteria. Overall, coagulopathy worsened substantially during bleeding (EXTEM CA5 36 mm at baseline vs 28 mm at 12 units). VHA and CCT tests were closer to normal in survivors and in those who received targeted treatment but were not different between the two ITACTIC trial arms. In the subgroup of coagulopathic patients who received at least 8 RBCs, correction of coagulopathy was achieved in more survivors than non survivors (29% vs 13%). Coagulopathy was not corrected in any patient who received only empiric treatment, while targeted therapy corrected coagulation tests in only 25% of subjects. Correction of coagulopathy was greater in the VHA arm (33% vs 16%), and targeted transfusion therapy was administered earlier in this group (100 mins vs 121 mins).
Conclusion(s): Current treatments for trauma induced coagulopathy are not sufficient to correct coagulopathy in the majority of bleeding trauma patients.
OC 11.4
Dynamic changes in activated protein C during major haemorrhage protocol and associated fibrinolytic response
A. Thaventhiran 1; K. Brohi2; R. Davenport3
1 Barts and the London School of Medicine and Dentistry, Tunbridge Wells, England, United Kingdom; 2 Queen Mary University of London, London, England, United Kingdom; 3 Centre for Trauma Science, Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom
Background: Major trauma haemorrhage is associated with an early increase in activated Protein C (aPC). Consumption of PAI‐1 by aPC and loss of inhibitory control over tissue plasminogen activator (tPA) are key to the hyperfibrinolytic response in Acute Traumatic Coagulopathy (ATC). Resuscitation with balanced transfusion primarily supports coagulation rather than directly targeting the mediators of ATC. We wished to characterise the relationship between aPC, fibrinolytic response and clinical outcome during transfusion to understand the potential therapeutic value of aPC pathway modulation.
Aims: Determine the relative importance of dynamic aPC levels during major trauma haemorrhage on fibrinolytic markers and outcome.
Methods: Prospective cohort study of trauma patients admitted to a level 1 trauma centre who received 4+ red blood cells (RBC) units with elevated aPC (>3 ng/ml) on admission. Samples were collected after transfusion of 4, 8 and 12 RBC units for aPC and fibrinolytic markers assay. Patients were stratified into DECREASING aPC (decreasing during resuscitation) vs INCREASE/HIGH aPC (increasing or persistently elevated during resuscitation).
Results: Thirty‐seven patients were analysed and 58% had INCREASING/HIGH aPC during the bleeding period. This subgroup were more injured than DECREASING aPC patients (Injury Severity Score: 41 vs 25, p = 0.03) but had similar shock severity (base deficit 10.8 vs 7.85 mEq/L, p = 0.165). Mortality was significantly higher in INCREASING/HIGH aPC (67% vs 25%) despite comparable RBC:FFP transfusion ratios. Plasmin‐Antiplasmin levels mirrored aPC levels during bleeding (Pearson r = 0.773, p = <0.001) whilst PAI‐1 was inversely associated with aPC (F‐test = 4.478 p = 0.0032). Both groups had similar thrombin generation and an overall decline in fibrinogen during MHP resuscitation.
Conclusion(s): Persistently elevated aPC despite balanced MHP transfusion was associated with increased mortality. Direct modulation of aPC (to mitigate PAI‐1 neutralisation) may represent a targeted intervention to treat ATC for improving patient outcomes.
OC 61.3
Changes in fibrinolysis during extracorporeal membrane oxygenation in critically ill adult patients
A. Doyle 1; K. Parmar2; K. Breen3; N. Barrett2; A. Retter1; B. Hunt1
1 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 2 Guy's & St Thomas NHS Foundation Trust, London, England, United Kingdom; 3 Guy's & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Rates of major haemorrhage during the use of Extracorporeal Membrane Oxygenation (ECMO) are high. The fibrinolytic system plays a role in bleeding and survival following trauma and cardiopulmonary bypass but its role during ECMO is poorly described.
Aims: To assess the changes in markers of fibrinolysis and fibrinolytic enzymes during veno‐venous ECMO and their roles in haemostatic complications during ECMO.
Methods: Blood samples were taken from 17 patients >18 years old at a single, high‐volume ECMO centre. Samples were taken at pre‐ECMO, 1‐hour, 1‐day, 2‐days, 7‐days, pre‐decannulation, 1‐hour and 1‐day after, and during major bleeding. Samples were analysed by ELISA for fibrinogen, Plasmin‐Antiplasmin (PAP), D‐Dimer, tPA antigen (Ag), uPA Ag, PAI‐1 and TAFI according to manufacturer’s protocols. Ethical approval was gained for the study with nominated consultee consent.
Results: Fibrinogen, PAI‐1, D‐Dimer and PAP levels were increased before ECMO whereas other factors were normal. Fibrinogen fell 1‐hour after cannulation. D‐Dimers increased over the first 2‐days of ECMO and stabilised at 7‐days. PAP increased at 2‐ and 7‐days. D‐Dimers and PAP fell 1‐day after decannulation in comparison to pre‐decannulation. There was a positive correlation between D‐Dimer and PAP during ECMO (R = +0.61). Patient with intracranial haemorrhage (n = 4) at ECMO initiation had a higher increase in D‐Dimer than those without (median change +2421 vs ‐705 ng/mL FEU, p = 0.069). D‐Dimer levels were increased in those with PE (n = 4) to those without (n = 13) at ECMO initiation (median 9948 vs PE 6192 ng/mL FEU, p = 0.047). During non‐intracranial major haemorrhage (n = 3), TAFI was lower compared to those without (median 114.5 vs 154.5%, p = 0.004) whereas PAI‐1 levels were higher (median = 111 ng/mL vs 54.3 ng/mL, p = 0.003).
Conclusion(s): Fibrinolysis is activated during ECMO in critically unwell patients, with D‐Dimers reflective of fibrinolytic activity. This study suggests that fibrinolysis may have a role in major bleeding during ECMO.
OC 61.5
National Incidence of bleeding‐related hospitalisations and mortality by anatomical and ISTH site classification in England 2014‐2019
K. Creeper 1; A. Stafford2; S. Choudhuri3; N. Tumian4; K. Breen5; A. Cohen6
1 Guy's and St Thomas's NHS Foundation Trust, Kings College London, London, England, United Kingdom; 2 Curtin University, Perth, Western Australia, Australia; 3 Northern Care Alliance, Manchester, Manchester, England, United Kingdom; 4 Guy's and St Thomas's NHS Foundation Trust and Department of Hematology, Hospital Canselor Tuanku Muhriz University Kebangsaan Malaysia, CHERAS, Kuala Lumpur, Malaysia; 5 Guy's & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom; 6 Guy's and St Thomas's NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Acute bleeding is common and associated with increased morbidity and mortality. Epidemiological studies evaluating national data on burden, incidence and annual trends in hospitalisations and mortality associated with bleeding sites are valuable yet are lacking.
Aims: To report the burden and incidence of hospitalisation and mortality based on anatomical and ISTH critical site and non‐critical site classification, and to review national trends in England.
Methods: A population‐based review of people in England between 2014 and 2019 either admitted to an acute care ward, or who died was undertaken. Admitted patients were identified using the Hospital Episode Statistic database. Mortality data and population estimates were obtained from the Office of National Statistics. Bleeding events were selected based on the International Statistical Classification of Diseases version 10 codes. Patients with inherited or acquired coagulation or platelet disorders, and those who were not admitted (emergency department or outpatient clinic) were excluded. Analyses were performed based on anatomical site, critical site and non‐critical site ISTH classifications. Annual incidence rates of admissions were calculated as per 100,000 patient years, and deaths per 100, 000 people.
Results: 1,112,873 (34.4%) gastrointestinal, 725,213 (22.4%) genitourinary, 280,003 (8.6%) intracranial and 478,499 (14.7%) obstetric bleeding related hospitalisations were observed during the study period. Intracranial bleeding was associated with the highest mean annual mortality incidence of 15 per 100,000 people. (Figure 1). Hospitalisation for non‐critical site bleeding was 5.50 times more common than critical site bleeding (83.4% versus 15.2%, respectively). Mean annual mortality for critical site bleeding was higher than non‐critical site bleeding (OR 4.62, CI 4.42‐4.82, p < 0.001) (Figure 2).
Conclusion(s): Gastrointestinal bleeding accounted for the highest hospitalisation burden. Intracranial and critical site bleeding are associated with a high mean mortality rate compared to other anatomical sites. Further analysis of these anatomical bleeding classifications is required.
OC 11.2
Functional characterisation of coagulopathy in isolated traumatic brain injury
C. Lindsay 1; J. Ross2; H. Tucker2; A. Rossetto3; M. Carver2; J. Pott4; A. Almuwallad5; R. Stoner2; J. Wohlgemut2; R. Davenport2; K. Brohi1
1 Queen Mary University of London, London, England, United Kingdom; 2 Centre for Trauma Sciences, Queen Mary University of London, London, England, United Kingdom; 3 Centre for Trauma Science, Blizard Institute, Queen Mary University, London, United Kingdom, London, England, United Kingdom; 4 Centre for Trauma Sciences, Queen Mary University of London, London, England, United Kingdom; 5 Centre for Trauma Sciences, London, England, United Kingdom
Background: The existence and nature of a coagulopathy associated with isolated Traumatic Brain Injury (iTBI) remains unclear. However, recent randomised controlled trials suggest that iTBI may be more common and important than has previously been appreciated.
Aims: We aimed to describe the coagulation abnormalities present on admission in iTBI patients; iTBI characteristics associated with their development; and their relationship with outcomes in terms of intracranial pressure (ICP), 7‐day progression of intracranial haemorrhage (ICH), and mortality.
Methods: We analysed admission coagulation tests from a subgroup of iTBI patients (Head Abbreviated Injury Score of 3 or higher, and below 3 in all other body regions) enrolled in the ACIT prospective observational study of coagulopathy and inflammation in trauma (REC 07/Q0603/2). Evidence of raised ICP was defined as ICP > 25 mmHg or basal cistern compression on CT. We used modified Rotterdam Scoring to explore the combined effect of injury burden and signs of raised ICP on coagulation.
Results: 237 patients had with severe iTBI. At least one coagulation abnormality was present in 66% of individuals, EXTEM CA5 < 40 mm (45%), EXTEM ML < 5% (44%), fibrinogen < 2 g/L (38%) and EXTEM CT > 80 s (29%). Coagulation abnormalities were more common in those with the highest modified Rotterdam score than the lowest (86% vs 55%), head AIS 5 versus 3 (70% vs 62%) and with raised ICP (80% vs 60%). Coagulation abnormalities were present in a greater proportion of patients with adverse outcomes, across a range of tests (table 1). The presence of any coagulation abnormality was associated with evidence of increased ICP (31% vs 16.3%), 7‐day progression of ICH (67% vs 51%) and higher 7‐day mortality (21% vs 7.5%).
Conclusion(s): Admission iTBI coagulopathy is common and associated with severity and raised ICP on admission. ITBI coagulopathy was associated with substantially worse outcomes and represents a potential opportunity for therapeutic intervention. TABLE 1 Proportion of abnormal admission values by outcome
TABLE 1 Proportion of abnormal admission values by outcome
OC 24.1
Synergy of red blood cells and tranexamic acid in the inhibition of fibrinolysis
A. Raska 1; K. Kalman2; B. Egri3; I. Roberts4; K. Kolev5; N. Wohner1
1 Semmelweis University, Department of Biochemistry, Department of Hematology and Internal Medicine, HCEMM‐SU Thrombosis and Haemostasis Research Group, Budapest, Budapest, Hungary; 2 Semmelweis University, Department of Biochemistry, HCEMM‐SU Thrombosis and Haemostasis Research Group, Budapest, Budapest, Hungary; 3 Semmelweis University, Budapest, Budapest, Hungary; 4 Clinical Trials Unit, London School of Hygiene & Tropical Medicine, London, England, United Kingdom; 5 Semmelweis University, Department of Biochemistry, Budapest, Budapest, Hungary
Background: Postpartum haemorrhage (PPH) is the leading cause of maternal death world‐wide. Maternal anaemia strongly increases the risk of PPH. The Woman trial showed that the antifibrinolytic tranexamic acid (TXA) reduces PPH deaths. The Woman‐2 trial is now assessing whether TXA can prevent PPH in anaemic women. Low RBC‐counts promote fibrinolysis by altering fibrin structure and plasminogen activation. We explore the effect of low RBC counts on the potency of TXA.
Aims: To examine if the RBC content of fibrin clots affects the anti‐fibrinolytic potency of TXA.
Methods: We used ball sedimentation assay (BS) and elastic motion thromboelastography (ClotPro) to monitor the lysis of fibrin containing plasminogen and tissue‐type plasminogen activator (tPA) at various RBC‐counts and TXA concentrations. We examined the anti‐fibrinolytic potency of TXA with parallel line bioassay analysis of lysis times and with dose‐response curves of lysis inhibition, over a range of tPA and TXA concentrations.
Results: Compared to RBC‐free fibrin, the anti‐fibrinolytic potency of 4–64 μM TXA was increased in the presence of RBC at 10–40%(V/V). This effect was consistent at all TXA concentrations and RBC counts in both BS and ClotPro assays. The maximal increase in TXA potency was 2.7‐fold at 64 μM TXA and 20% (V/V) RBC (Fig. 1A). The prolongation of lysis time in response to varying TXA concentration indicated that RBC increased the number of target sites available for TXA binding (Ltmax in Fig. 1B) without any significant change in the TXA concentration needed to achieve a half‐maximal effect (Ks in Fig. 2B). These extra TXA binding sites are probably presented by the higher fraction of plasmin residing in unbound form in a fibrin meshwork of thinner fibers which is formed in the presence of RBCs.
Conclusion(s): RBCs increase the anti‐fibrinolytic potency of TXA. Higher doses of TXA may be required to inhibit fibrinolysis in severely anaemic women.
OC 24.5
Modest performance of available risk prediction models when used to predict hemorrhage in patients with chronic liver disease
A. Afzal 1; L. Suhong2; B. Gage3; K. Sanfilippo4
1 Washington University in St Louis, Saint Louis, Missouri, United States; 2 Washington University in St Louis, Saint Louis, Missouri, United States; 3 Washington University School of Medicine St. Louis, St Louis, Missouri, United States; 4 Washington University School of Medicine St. Louis, St. Louis, Missouri, United States
Background: Patients with chronic liver disease (CLD) have a unique hemostatic profile with simultaneous reduction in factors promoting and opposing thrombosis, and multiple abnormalities on conventional coagulation tests. Available risk prediction models for anticoagulant‐related major bleeding (MB) were not developed and validated in the CLD population, and hence, may not predict MB among these patients to assess the risk‐versus‐benefit of anticoagulation.
Aims: We aimed to evaluate the performance of existing (anticoagulant‐related) bleeding risk prediction models in a cohort of CLD patients.
Methods: Using the Veterans Health Administrative data, we identified patients with CLD (using previously validated methods) who were started on anticoagulant therapy between 2001 and 2018. We identified hemorrhage within 12 months of anticoagulant therapy through previously validated ICD‐9/10 codes present in primary or secondary position of inpatient diagnoses codes. We evaluated the predictive performance of three risk prediction models in the CLD cohort, and assigned points as recommended (Table 1). The association between the score assigned by each model and MB was measured using competing‐risk analysis by Fine and Grey. We evaluated each model’s discrimination using Harrell’s c‐statistic.
Results: Among 19,871 CLD patients, 761 experienced hemorrhage (variceal or non‐variceal) within twelve months of initiation of anticoagulation. The median time to hemorrhage from initiation of anticoagulation was 60 days. The increase in risk of MB per point increase in risk prediction score is presented in Table 2. The c‐statistic for HEMORR2HAGES and HAS‐BLED was 0.56, and it was 0.51 for VTE‐BLEED.
Conclusion(s): In this cohort of 19,871 veterans with CLD, available risk prediction models predicted anticoagulant‐related MB only slightly better than random chance. There is hence, a need to develop and validate a risk prediction model in patients with CLD to accurately identify those at a higher risk of anticoagulant‐related bleeding.
OC 61.2
Thrombosis and major bleeding in patients with severe COVID‐19 supported by extracorporeal membrane oxygenation (ECMO) – Multicentre observational study in UK
D. Arachchillage 1; I. Rajakaruna2; I. Scott3; M. Laffan4; S. Ledot5; R. Jooste6; A. Vuylsteke6; Y. Hakeem7
1 Department of Immunology and Inflammation, Imperial College London, London, England, United Kingdom; 2 University of East London, London, England, United Kingdom; 3 NHS Grampian, Aberdeen, Scotland, United Kingdom; 4 Centre for Haematology, Imperial College London, London, England, United Kingdom; 5 Royal Brompton Hospital, London, England, United Kingdom; 6 Royal Papworth Hospital NHS Foundation Trust, Cambridge, England, United Kingdom; 7 The University Hospitals of Leicester NHS Trust, Leicester, England, United Kingdom
Background: Support with extracorporeal membrane oxygenation (ECMO) can be life saving for patients with coronavirus [Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2)]‐induced severe respiratory failure. However, bleeding and thrombosis are major complications in patients supported with ECMO and thrombosis is itself a prominent feature of severe COVID‐19.
Aims: To assess the rate of thrombosis and major bleeding (MB) and their impact on mortality in patients supported with veno‐venous (VV)‐ECMO.
Methods: This was a multicentre observational study of 320 consecutive patients (≥18 years) with severe COVID‐19 supported by VV‐ECMO in four nationally commissioned ECMO centres in UK from 1st March 2020 to 31st December 2021.
Results: Median age (range) was 48 years (19–75) and 71.2% were male. Overall, the 180‐day mortality was 36.6%% (117/320). The rate of MB was 27.5% (88/320), of which intracranial bleeding (ICH) was the most common [35.3% (31/88) [ followed by pulmonary haemorrhage [26.1% (23/88). Gastrointestinal bleeding accounted for 15.9% (14/88) of MB and the remainder (22.7%) had bleeding at other sites. There were 136 thrombotic events (42.5%) consisting of 80.1% (109/136) venous thrombosis (VTE) and 19.9% (27/136) arterial thromboses. Of the 109 patients with VTE, 73.4% (80/109) had pulmonary embolism (PE), 17.4% (19/109) had deep vein thrombosis (DVT) and 9.2% (10/109) had combined DVT and PE. Ischaemic stroke accounted for >50% of the arterial thrombosis (51.6%, 14/27). HIT occurred in 9.7% (31/320) and 80.6% (25/31) developed thrombosis. MB and ICH were associated with 3.51‐fold (95% CI 2.56–8.41) and 6.71‐fold (CI) 2.56–16.54] increased risk of mortality and PE with a 2.23‐fold (95% CI1.29–3.89) risk of mortality.
Conclusion(s): MB and thrombosis including HIT are frequent complications in patients supported with VV‐ECMO which significantly increase the risk of mortality. This highlights the need for prospective studies assessing the optimal haemostasis management in patients on VV‐ECMO.
OC 61.1
Risk factors for bleeding after recent medical hospitalization: The Medical Inpatient Thrombosis and Hemostasis Study (MITH)
M. Gergi 1; K. Wilkinson2; A. Sparks1; H. Al‐Samkari3; N. Smith4; N. Roetker5; T. Plante6; M. Cushman7; A. Repp1; C. Holmes8; N. Zakai9
1 University of Vermont, Burlington, Vermont, United States; 2 Larner College of medicine at the University of vermont, Burlington, Vermont, United States; 3 Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, United States; 4 Department of Epidemiology, University of Washington; Department of Health Service, University of Washington; Seattle Epidemiologic Research and Information Center; Department of Veterans Affairs, Office of Research and Development, Seattle, WA 98105, USA, Seattle, Washington, United States; 5 Hennepin Healthcare Research Institute, Minneapolis, Minnesota, United States; 6 University of Vermont Medical Center, Burlington, Vermont, United States; 7 Larner College of Medicine at the University of Vermont, Colchester, Vermont, United States; 8 University of Vermont College of Medicine, Burlington, Vermont, United States; 9 University of Vermont, Colchester, Vermont, United States
Background: Assessing bleeding risk in recently discharged medical patients is essential in decisions regarding venous thrombosis prophylaxis. Studies assessing post‐discharge bleeding risk in this population of patients are lacking. To appropriately risk stratify people for post‐discharge venous thrombosis prophylaxis, bleeding risk needs to be better understood.
Aims: To quantify the risk of and risk factors for bleeding after medical hospital discharge.
Methods: We followed all primary care patients aged ≥18 years at the University of Vermont Medical Center’s primary care clinics from July 2010 to September 2019. We applied validated computable phenotypes to electronic health record data and identified post‐discharge (PD) bleeding events that required re‐hospitalization within 90 days of the index hospital discharge. We report age and sex‐adjusted odds ratios (OR) for putative bleeding risk factors.
Results: Between 2010‐2019, there were a total of 14,266 medical hospitalizations and 1216 PD‐bleeding events requiring re‐admission. The bleeding rate for patients who did not have any medical hospitalizations in the past 90 days was 2.9 per 1000‐person years compared to 98.9 per 1000 person‐years for PD‐bleeding up to 90 days after discharge. History of cancer, liver disease, renal failure, heart disease and bleeding disorder were associated with increased risk of PD‐bleeding. Patients with anemia and thrombocytopenia or elevated creatinine at the time of discharge were also at increased risk for bleeding. The highest OR (6.2) was observed in patients who presented with bleeding at the time of the index admission (Table 1).
Conclusion(s): Bleeding requiring re‐hospitalization is common after discharge from medical hospitalizations. There are easily identifiable, common, and strong risk factors for post‐discharge bleeding. Development of risk assessment models for post‐discharge bleeding may help guide patient care and is the focus of ongoing research.
OC 11.1
Cryoprecipitate transfusion in trauma patients attenuates hyperfibrinolysis and restores normal clot structure and strength; results from a sub‐study of the FEISTY trial
G. Morrow 1; T. Feller2; Z. McQuilten3; E. Wake4; R. Ariëns5; J. Winearls4; N. Mutch6; M. Laffan7; N. Curry8
1 University of Oxford, Aberdeen, Scotland, United Kingdom; 2 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds., Leeds, England, United Kingdom; 3 Monash University, Melbourne, Victoria, Australia; 4 Gold Coast University Hospital, Gold Coast, Queensland, Australia; 5 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom; 6 University of Aberdeen, Aberdeen, Scotland, United Kingdom; 7 Centre for Haematology, Imperial College London, London, England, United Kingdom; 8 University of Oxford, Oxford, England, United Kingdom
Background: Fibrinogen rapidly reaches critically low levels during traumatic haemorrhage. Hyperfibrinolysis is common and exacerbates hypofibrinogenaemia. The Fibrinogen Early in Severe Trauma studY (FEISTY;NCT02745041) is the first randomised controlled trial comparing the clinical effects of cryoprecipitate and fibrinogen concentrate (Fg‐C) during traumatic haemorrhage.
Aims: To compare the effect of Fg‐C or cryoprecipitate supplementation on clot structure, strength and fibrinolysis in severely injured patients enrolled to FEISTY.
Methods: Paired plasma samples pre‐ and post‐fibrinogen replacement were examined for PAI‐1 and FXIII antigen and activity levels and plasmin generation. Fibrin clot structure was analysed using confocal microscopy and mechanical properties of individual fibres investigated using atomic force microscopy (AFM). Healthy donor plasma was used as a control.
Results: Plasmin generation was significantly reduced in patients treated with cryoprecipitate, but did not change with Fg‐C. PAI‐1 activity and antigen levels were increased post‐cryoprecipitate treatment, but not Fg‐C. There was a significant increase in FXIII post‐cryoprecipitate, whereas a significant decrease was observed in the Fg‐C cohort. FXIII activity analysis revealed trauma patients had significantly lower levels than controls. Upon hospital admission trauma patients formed clots with significantly fewer fibrin fibres, that were shorter in length and reduced cross‐links compared to controls. Cryoprecipitate transfusion restored the fibrin network, with fibres comparable to those observed in normal plasma, whereas Fg‐C did not restore normal clot structure. AFM analysis confirmed that fibres formed after cryoprecipitate transfusion required more energy to rupture than their Fg‐C counterparts.
Conclusion(s): In severely injured, bleeding trauma patients, cryoprecipitate supplementation attenuated plasmin generation and increased PAI‐1 and FXIII levels. Patients given cryoprecipitate showed a homogeneous fibrin network with increased fibres than those with Fg‐C. Moreover, these fibres showed increased resistance to mechanical stress. Our data indicate that cryoprecipitate is a superior source of fibrinogen to manage bleeding in trauma coagulopathy by increasing stability against mechanical disruption and fibrinolysis.
Management/Treatments of Acquired Bleeding
OC 24.4
Factor XIII levels correlate with fibrinogen concentrations in patients with venous malformations and chronic disseminated intravascular coagulopathy
N. Fordham 1; J. Clark1; A. Taylor1; K. Sibson1; L. Solman1; M. Glover1; M. Mathias2
1 Great Ormond Street Hospital for Children, London, England, United Kingdom; 2 Great Ormond Street Hospital for Children, NHS Foundation Trust, London, England, United Kingdom
Background: Patients with slow flow venous malformations (VM) have a coagulopathy which can worsen with age. This is characterised by disseminated intravascular coagulopathy (DIC) and heralded by elevated D‐dimers, but severe cases can lead to depletion of fibrinogen and significant haemorrhage. Little data exists on extended coagulation parameters in this cohort of patients.
Aims: The identification of clinically important coagulation abnormalities, to guide management of surgery and haemorrhage in VM patients.
Methods: A single‐centre retrospective data review on all patients attending the VM service between June 2014 and June 2021 with factor XIII assays and basic coagulation screens assessed.
Results: 84 patients with VMs were identified. Where assessed, the majority (48/77) of patients had elevation of D‐dimers at initial review, but the prothrombin time (PT) and activated partial thromboplastin time (APTT) were abnormal in only 8 patients. Extended PT and APTT based factor assays revealed two patients had a mild reduction in factor V, common in DIC. Fibrinogen concentration was decreased in ~24% (20/84) of VM patients. Thrombocytopenia (<150 × 109/L) was rare (5/84 patients) and predicted for a worsening coagulopathy. Almost all patients (15/16) with a fibrinogen concentration of <1.5 g/L had a low factor XIII level, some profound (range 8‐62), whereas a fibrinogen > 1.5 g/L predicted for a normal factor XIII.
Conclusion(s): Our data suggests that acquired factor XIII deficiency is more common and severe in patients with VMs (15/84) than previously described, and clinicians should be aware of this. We suggest that all patients with VMs should have a basic coagulation screen assessed, including PT, APTT, D‐dimers and fibrinogen concentration. Where the coagulation profile is normal, little is gained from extended factor screening, however, a factor XIII level should be measured in all patients with a low fibrinogen.
OC 24.2
validation of a treatment decision algorithm for the use of andexanet alfa in acute life‐threatening bleeding from DIRCT factor Xa inhibitors
J. Beyer‐Westendorf 1; J. Koscielny2
1 University Hospital Carl Gustav Carus Dresden, Germany, Dresden, Sachsen, Germany; 2 Charité – Universitätsmedizin Berlin, Berlin, Berlin, Germany
Background: Andexanet alfa (AA) is approved for reversing direct factor Xa inhibitors (DXI) in life‐threatening bleeding. However, AA label only provides dosing recommendations but does not specify in whom AA reversal is indicated or not. Under‐treatment (continuous bleeding) and over‐treatment (thrombotic risk) are clinical concerns. Anti‐Xa activity tests are often not available to guide treatment decisions. A clinical decision pathway reducing the risk or over‐ and under‐treatment is urgently needed.
Aims: To validate an AA treatment decision algorithm derived from an interdisciplinary German expert consensus group (Figure 1).
Methods: The algorithm was retrospectively applied to 100 cases (acute life threatening DXI bleeding and documented anti‐Xa activity levels), identified in charts from Dresden University Hospital, Germany and Charitè Berlin, Germany. The algorithm performance was compared against a label‐conform decision to “reverse all”. Correctness of treatment indication was judged against three commonly used clinical AA treatment thresholds for anti‐Xa activities: <35 ng/ml; <50 ng/ml and <75 ng/ml.
Results: Of the 100 patients, 7% had aXa activities <35 ng/ml; 11% <50 ng/ml and 21% <75 ng/ml but all would normally have received AA reversal. Application of a cut‐off “last intake >18 hours” (as applied in AA trials and used in clinical routine) would have excluded 12% from AA therapy, of whom 4 (33%) had aXa activities >75 ng/ml. Table 1 indicates that, without an algorithm, the risk of over‐treatment increases with increasing treatment indication thresholds. Application of the algorithm resulted in “correct indications” in 67‐73% without delay from aXa activity test, which increased to 89‐95% if 22% of cases would receive testing. The algorithm reduced risk of over‐treatment considerably without increasing the risk of under‐treatment.
Conclusion(s): The proposed algorithm can improve AA treatment decisions and reduces the risk of over‐treatment. Further validation is currently ongoing.
OC 11.3
Improved survival in severely bleeding trauma patients treated with low‐titer group O whole blood compared to component therapy
E. Mihalko 1; S. Shea2; K. Thomas3; M. Huff4; D. Scheurer5; G. Bochicchio6; P. Spinella7
1 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States; 2 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 3 Department of Pediatrics, Washington University in St. Louis, St. Louis, Missouri, United States; 4 Department of Pediatrics Washington University, St. Louis, Missouri, United States; 5 Department of Surgery Washington University, Pittsburgh, Pennsylvania, United States; 6 Department of Surgery, Washington University in St. Louis, St. Louis, Missouri, United States; 7 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA; Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States
Background: Low‐titer group O whole blood (LTOWB) or component therapy (CT) may be used to resuscitate hemorrhaging trauma patients. LTOWB has biologic and logistical benefits and may improve survival.
Aims: Evaluate outcomes in trauma patients treated with LTOWB or CT.
Methods: In this prospective observational study, adult trauma patients with massive transfusion protocol (MTP) activations were enrolled (IRB#201909200). CT group was enrolled from 2017‐2019 and LTOWB group from 2019‐2021. LTOWB replaced CT in the MTP in 2019, allowing for comparison. Primary outcome was 24‐hour mortality. Secondary outcomes included 28‐day mortality and 72‐hour weight‐adjusted total blood product use. Data is presented as median (IQR) or proportions and analyzed via Wilcoxon rank‐sum test or Fisher’s exact test. Multivariable logistic regression (MVLR) and adjusted Cox regression were performed to determine independent associations.
Results: We enrolled 384 patients. Patient demographics were similar between LTOWB (N = 192) and CT (N = 192) cohorts (Table1) with injury severity scores (ISS) of 22(13‐33) and 25(17‐34) (p = 0.026), respectively. Unadjusted 24‐hour mortality was not statistically different between LTOWB vs. CT cohorts: 19% vs. 24%, respectively (p = 0.322). In stratified analyses, LTOWB was associated with survival for patients with hemostatic dysfunction (Maximum Clot Formation(MCF) < 60 mm) and shock (more negative base excess) (Figure1). In an MVLR model, adjusting for MCF and ISS, LTOWB improved the odds of 24‐hour survival by 12% (Odds Ratio = 0.89; 95% Confidence Interval = 0.81‐0.97; p = 0.011), and also by an adjusted Cox regression model with a Hazard Ratio = 0.51; (95%CI = 0.27‐0.94); p = 0.033). The use of LTOWB trended towards improved 28‐day survival with a Hazard Ratio = 0.66; (95%CI = 0.41‐1.08); p = 0.092), (Figure1E). Additionally, LTOWB patients received significantly less total blood products (80.9(41.6‐139.3)mL/kg vs. 48.9(25.9‐106.9)mL/kg; p < 0.001).
Conclusion(s): LTOWB treatment in hemorrhagic trauma patients was independently associated with improved 24‐hour survival with a 40% relative‐reduction in total blood product usage over 72‐hours, indicating support for LTOWB in severely bleeding trauma patients with MTP.
Novel Therapies in the Management of Acquired Bleeding
OC 24.3
VMX‐C001 is an effective factor Xa inhibitor reversal agent and displays a favorable pharmacodynamic profile in animal models
D. Verhoef 1; T. Gomes2; H. Spronk3; G. Short4; P. Reitsma1
1 VarmX, Leiden, The Netherlands. 2Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands, 2 VarmX, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands, 3 Maastricht University, Maastricht, The Netherlands., Maastricht, Zuid‐Holland, Netherlands, 4 VarmX, Leiden, The Netherlands., Cambridge, England, United Kingdom
Background: VMX‐C001, a modified form of human zymogen factor X, is being developed to stop or prevent bleeding in patients taking factor Xa inhibitors and is currently in human phase I studies.
Aims: To examine the pharmacodynamics of VMX‐C001 and factor Xa inhibitor reversal in rats and cynomolgus monkeys by means of thrombin generation (TG).
Methods: Subsequent to dose range finding studies, 2‐week toxicity studies were performed at doses of 20, 50 and 100 IU/kg/day VMX‐C001, followed by a two‐week recovery period.
Results: In rats, regardless of sex, there were no VMX‐C001 related changes compared to pre‐dose and placebo in endogenous thrombin potential (ETP), thrombin peak height and time to initiation of thrombin generation (ie. lag time). Similarly, administration of VMX‐C001 to monkeys was not associated with significant changes in TG parameters. There was no overshooting of TG parameters regardless of dose level, dose period, sex, or species. During dosing, reversal of anticoagulation was examined in two males and females receiving VMX‐C001 (20 IU/kg/day) by ex‐vivo spiking of plasma from the VMX‐C001 dosed animals with edoxaban (250 ng/ml). Five minutes after intravenous administration, VMX‐C001 corrected the effect of edoxaban on ETP and lag time and restored peak height to near normal levels (Figure 1). In another setup, five male monkeys received rivaroxaban by oral gavage (10 mg/kg) and VMX‐C001 was administered once 3 hours after the factor Xa inhibitor. Rivaroxaban (plasma level 407 ng/ml) was associated with markedly suppressed TG parameters. These changes were fully reversed after administration of 50 IU/kg VMX‐C001 (Figure 2).
Conclusion(s): VMX‐C001 displays a favorable pharmacodynamic profile where repeated administration of VMX‐C001, at levels of 20, 50 and 100 IU/kg in rats and monkeys is not associated with changes in TG parameters. In addition, VMX‐C001, at a dose of 20 IU/kg, restores thrombin generation in the presence of factor Xa inhibitors.
OC 61.4
Supercharged platelets as a novel therapy for reducing blood loss post‐cardiac surgery
A. Crosby 1; S. Mookerjee1; A. Waller2; H. Foster3; C. Ghevaert4; D. Wilcox5; L. Du
1 Cambridge University, Cambridge, England, United Kingdom; 2 University of Cambridge, Braintree, England, United Kingdom; 3 University of Cambridge, Cambridge, England, United Kingdom; 4 Cambridge Univeristy, Cambridge, England, United Kingdom; 5 Medical College of Wisconsin, MIlwaukee, Wisconsin, United States
Background: Cardiovascular surgery is one of the surgical disciplines that requires the most blood components for transfusion. In addition to surgical techniques, the transfusion of blood components and pharmacological interventions, measures to restore haemostasis in actively bleeding patients include the administration of concentrated coagulation factors including recombinant FVIIa (rFVIIa). However, this has huge cost implications given the very short half‐life of clotting factors and an inherent risk of adverse thrombotic events, resulting in increases in hospital, morbidity and mortality.
Aims: We sought to determine whether loading donor MK’s and subsequently MK’s generated in vitro from iPSC’s with FVIIa, would enhance the haemostatic ability of their platelet progeny, with a view to translating this to the clinic.
Methods: Immunofluorescence and flow cytometry were used to determine whether donor platelets endocytose extracellular rFVIIa. Using the bleeding time NRG mouse model, made profoundly thrombocytopenic through antibody depletion, we intravenously administered donor‐derived platelets loaded with rFVIIa. Using viral transduction we over‐expressed 3 specific transcription factors (FLI1, TAL1 and GATA1) (FoP) to generate MK’s from iPSC’s and virally transduced these MK’s with FVIIa.
Results: We have shown that donor platelets endocytose rFVIIa and target it to their alpha granules. We have also shown a reduction in early haemorrhage in the NRG mouse administered donor platelets loaded with FVIIa, compared with those administered donor platelets or PBS (Figure 1). We have also shown that we can generate MK’s from iPSC’s and that they express FVIIa (Figure 2).
Conclusion(s): We have demonstrated that donor platelets ‘supercharged’ with rFVIIa have an enhanced haemostatic ability and that we can ‘over‐express’ FVIIa in our FOP platelets. We are currently determining whether FoP platelets, which over‐express FVIIa, exhibit a greater haemostatic ability than FoP platelets, which will enable the clinic to have a more effective, safe, continuous and universal supply of platelets.
Arterial Thromboembolism
Acute Coronary Syndromes
OC 18.2
Plasma exosomes reflect myocardial injury detected by cardiac magnetic resonance in STEMI patients
M. Zarà 1; A. Baggiano2; C. Banfi3; J. Campodonico2; C. Tedesco4; P. Amadio3; S. Gili2; G. De Dona2; L. Sandrini3; G. Marenzi2; G. Pontone2; S. Barbieri3
1 Centro Cardiologico Monzino, Pavia, Lombardia, Italy; 2 Cantro Cardiologico Monzino IRCCS, Milano, Lombardia, Italy; 3 Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy; 4 Cantro Cardiologico Monzino IRCCS, milano, Lombardia, Italy
Background: Exosomes are a subgroup of extracellular vesicles released by cells and detectable in all body fluids. Their release and cargo are influenced by cellular microenvironment, thus mirroring cell/organ physio‐pathological condition. The concentration and cargo of plasma exosomes released during ST‐elevation myocardial infarction (STEMI) well reflect the clinical progression of the disease, suggesting their potential as biomarkers. Cardiac Magnetic Resonance (CMR) precisely detects STEMI‐induced myocardial injury, by several parameters including microvascular obstruction (MVO) and myocardial salvage index (MSI), which predict functional recovery and risk of further cardiovascular events. However, it is not always applicable due to cost and availability reasons.
Aims: To assess whether plasma exosomes, specifically platelet‐derived exosomes, reflect myocardial injury as detected by CMR after STEMI.
Methods: Fourty‐two patients with STEMI were enrolled, underwent CMR within 1 week and concomitantly, blood was collected. Plasma exosomes were isolated by commercial kits, their concentration and size distribution determined by Nanoparticle Tracking Analysis, and GPIIbIIIa expression assessed by ELISA kit.
Results: Patients with anterior STEMI and those with late revascularization ( > 3 h from symptoms onset) displayed a higher number of circulating plasma exosomes (p < 0.001 and p < 0.05, respectively). Exosome dimension was smaller in patients with MVO (p < 0.01) and MSI < 0.5 (p < 0.05). Similarly, the expression of platelet marker GPIIbIIIa was lower in patients with anterior STEMI (p < 0.01) and MVO (p < 0.05). Specifically, exosome GPIIbIIIa expression and dimension significantly discriminated between patients with and without MVO in ROC curve analysis, with areas under the curve ranging from 0.70 to 0.77.
Conclusion(s): The main finding of our study is that plasma exosome profile well reflects CMR‐assessed myocardial injury after STEMI. In particular, the exosome dimension and the expression of platelet marker GPIIbIIIa is independently associated with MVO. Future studies with larger populations are required to confirm the role of platelet‐exosomes in risk stratification after STEMI.
Atherosclerosis
OC 18.5
APAC treatment limits collar‐induced carotid atherosclerotic plaque development in ApoE‐/‐ mice
I. Bot1; A. Jouppila 2; L. Delfos1; E. Hemme1; P. Kovanen3; R. Lassila4
1 Division of BioTherapeutics, Leiden Academic Centre for Drug Research, Leiden University, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 2 Clinical Research Institute HUCH, Helsinki, Finland, Helsinki, Uusimaa, Finland; 3 Wihuri Research Institute, Helsinki, Finland, Helsinki, Uusimaa, Finland; 4 Helsinki University Hospital, and University of Helsinki, Finland, Helsinki, Uusimaa, Finland
Background: Mimics of mast cell‐derived heparin proteoglycans can be tailored to molecules carrying both antiplatelet (AP) and anticoagulant (AC) properties. These dual APAC constructs can also shield adhesion molecules, i.e., von Willebrand factor, P‐selectin and VCAM‐1 expressed by endothelial cells upon atherosclerosis development. We hypothesize that via vascular targeting, APAC prevents macrophage accumulation and lesion development.
Aims: Our aim was to determine the efficacy of APAC in inhibiting atherosclerosis in apoE‐/‐ mice.
Methods: Male western‐type diet fed apoE‐/‐ mice were equipped with perivascular carotid artery collars (diameter 0.5 mm and length 2 mm) to induce atherosclerosis. This collar triggers various effects on shear forces along the artery. In this model, mRNA expression of adhesion molecules, i.e., ICAM‐1, VCAM‐1, P‐Selectin and Platelet Factor 4 (PF4) are upregulated upon lesion development (all p < 0.05 at 2 weeks after collar placement versus control arteries). Mice were treated with 0.2 mg/kg APAC or vehicle control (i.v, 3x weekly, n = 12‐14 per group) for 2.5 weeks either at lesion initiation or 2.5 weeks afterwards. At 5 weeks after collar placement, mice were sacrificed. Data are mean ± SEM.
Results: APAC treatment did not affect body weight or plasma total cholesterol levels of the mice during the experiments. Interestingly, when APAC treatment was started from the lesion initiation carotid artery plaque size was reduced by over 50% (APAC: 50 ± 10*10^3 versus controls: 102 ± 13*10^3 μm2; p < 0.01). This observation was aligned with reduced plaque macrophage area (APAC: 20 ± 5*10^3 versus controls: 33 ± 5*10^3 μm2) and collagen content (APAC: 13 ± 4*10^3 versus controls: 28 ± 6*10^3 μm2; p < 0.05). When APAC treatment was started at 2.5 weeks after the lesion initiation, APAC decreased necrosis (p < 0.05) and propagation of atherosclerotic lesions.
Conclusion(s): We report that APAC effectively inhibits atherosclerotic lesion development when administered to apoE‐/‐ mice. APAC may have potential as therapeutic agent to prevent or attenuate atherosclerosis.
OC 18.1
Therapeutic potential of CD146 extracellular vesicles for the treatment of atherosclerosis
C. Dubrou1; M. Blin1; K. Fallague1; R. Bachelier2; C. Chareyre1; S. Robert3; R. Lacroix4; F. Dignat‐George5; N. Bardin6; M. Blot‐Chabaud1; A. Leroyer 1
1 INSERM/Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France; 2 Aix‐Marseille Univ, INSERM, INRAE, C2VN, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Aix Marseille University, INSERM 1263, INRAE, C2VN, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 4 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN, Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 5 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN ‐ Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France., Marseille, Provence‐Alpes‐Cote d'Azur, France; 6 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN ‐Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: Atherosclerosis remains the biggest cause of death worldwide despite the wide use of LDL‐lowering therapy, thus targeting inflammation during its pathogenesis appears to be an alternative to the actual therapies. We previously showed that the expression of the adhesion molecule CD146 on foamy macrophages allows to reduce CCL5 and subsequent inflammation within the atherosclerotic plaque.
Aims: As extracellular vesicles may serve as potential clinical delivery devices through specific interactions with target cells, we propose to delay the progression of atherosclerosis by delivering athero‐protective CD146 using extracellular vesicles in order to overexpress it within atheroma.
Methods: For this, we generated and purified extracellular vesicles from mouse endothelial cells which strongly express CD146 and from mouse endothelial cells deleted in CD146 as a control.
Results: Mouse bone‐marrow‐derived macrophages were stimulated with CD146 extracellular vesicles previously labeled with CMFDA and we demonstrated that vesicles were rapidly incorporated by macrophages within 15 minutes. In vivo, CD146 extracellular vesicles were able to reach the inflammatory atherosclerotic sites in both heart and aorta within 30 minutes after their injection. Macrophages in culture were further stimulated with extracellular vesicles and both qPCR, flow cytometry and ELISA analysis showed that CD146 extracellular vesicles induce macrophage polarization towards an anti‐inflammatory phenotype by decreasing TNF‐α, IL‐6, CCL‐5 and increasing IL‐10, TGF‐β. Then, ApoE ‐/‐ mice were intraveneously injected with extracellular vesicles once every 15 days during 6 weeks. We demonstrated that the delivery of CD146 using extracellular vesicles to ApoE ‐/‐ mice significantly delayed atherosclerosis (decrease of 53% plaque area), decreased plaque inflammation (decrease of 46% of neutrophils content and 87% of CCL5 content), and promoted the increase of CD206 expression, hallmark of anti‐inflammatory “M2” macrophages whereas the expression of CD16/32 “M1” marker was decreased.
Conclusion(s): Altogether, our study has demonstrated proof‐of‐concept that delivering CD146 using extracellular vesicles delays inflammation and atherosclerosis.
Atrial Fibrillation
OC 71.4
Effectiveness and safety of edoxaban therapy in daily‐care patients with atrial fibrillation. Results from the Dresden NOAC Registry
J. Beyer‐Westendorf 1; L. Tittl2; C. Köhler2
1 University Hospital Carl Gustav Carus Dresden, Germany, Dresden, Sachsen, Germany; 2 Thrombosis Research Unit, University Hospital Carl Gustav Carus, Dresden, Sachsen, Germany
Background: Edoxaban is a non‐vitamin K dependent oral anticoagulant (NOAC) licensed for stroke prevention in atrial fibrillation (SPAF).
Aims: Confirmation of effectiveness and safety of edoxaban for SPAF in unselected patients in routine clinical care.
Methods: In the prospective, non‐interventional DRESDEN NOAC REGISTRY a network of more than 230 physicians enrolled >5000 NOAC patients who received prospective central follow up by the registry office. All reported outcome events (stroke/transient ischemic attack/systemic embolism; ISTH bleeding; death) are centrally adjudicated using standard scientific definitions.
Results: During 1st January 2016 and 31st August 2021, 1258 patients receiving edoxaban for SPAF were enrolled (Table 1). The mean duration of follow‐up was 927.1 ± 562.2 days with a mean duration under edoxaban exposure of 790.3 ± 577.2 days. Edoxaban was discontinued by 274 patients (10.1/100 patient‐years; 95% CI 8.9‐11.3). The combined endpoint of stroke/TIA/systemic embolism occurred at a rate of 1.7/100 patients‐years (95% CI 1.3‐2.3) in the intention‐to‐treat analysis and at 1.3/100 patient‐years (95% CI 0.9‐1.9) in the on‐treatment analysis (censored 3 days after last edoxaban intake). There were no significant differences between the rates of ISTH major bleeding in patients receiving edoxaban 30 mg (3.6/100 patient‐years; 95% CI 2.2‐5.5) compared with the 60 mg dose (2.5/100 patient‐years; 95% CI 1.8‐3.2) in the on‐treatment analysis (Table 2). During follow‐up 151 patients (12.0%) died. Causes of death were non‐stroke cardiovascular events (n = 50), followed by infection/sepsis (n = 40) and terminal malignant disease (n = 31), age related deaths (n = 11), fatal bleedings (n = 8), stroke (n = 5) and other reasons (n = 6).
Conclusion(s): Our rates of effectiveness and safety outcomes bleedings are in line with latest real‐world data (such as ETNA‐AF registry) and the ENGAGE‐AF trial that led to approval of edoxaban. Non‐thrombotic cardiovascular and infectious diseases were the leading causes of death in our cohort, whereas fatal stroke and fatal bleeding were rare.
OC 71.5
Assessment of DOACs in GEriatrics (ADAGE) study: Rivaroxaban and apixaban plasma concentrations and thrombin generation profiles in very elderly patients with non valvular atrial fibrillation
G. Foulon‐Pinto1; C. Lafuente‐Lafuente2; G. Jourdi3; J. Le Guen4; F. Tall5; E. Puymirat4; M. Delrue6; L. Riviere7; F. Ketz8; I. Gouin9; F. Mullier10; P. Gaussem11; E. Pautas12; T. Lecompte13; E. Curis14, V. Siguret 3
1 AP‐HP.Nord University of Paris, Paris, Ile‐de‐France, France; 2 Hospital Groupe Pitié‐Salpêtrière‐Charles Foix/Sorbonne Paris University, Ivry‐sur‐Seine, Ile‐de‐France, France; 3 INSERM UMR‐S‐1140/University of Paris, Paris, Ile‐de‐France, France; 4 Hôpital Européen Georges Pompidou/University of Paris, Paris, Ile‐de‐France, France; 5 Rotschild Hospital/Sorbonne Paris University, Paris, Ile‐de‐France, France; 6 AP‐HP.Nord/University of Paris, 75010, Ile‐de‐France, France; 7 Hospital Group Pitié‐Salpêtrière‐Charles Foix/ Paris Sorbonne University, Ivry‐sur‐Seine, Ile‐de‐France, France; 8 Hospital Group Pitié‐Salpêtrière‐Charles‐Foix/Paris Sorbonne University, IIvry‐sur‐Seine, Ile‐de‐France, France; 9 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, Institut de recherche en santé, environnement et travail ‐ IRSET, Inserm UMR_S 1085, Univ Rennes, CHU Rennes, France, Rennes, Bretagne, France, 10 University Hospital of Namur, Namur, Namur, Belgium, 11 Department of Biological Hematology, European Hospital Georges Pompidou, AP‐HP, Innovative Therapies in Haemostasis, Paris University, INSERM U1140, Paris, France, Paris, Ile‐de‐France, France, 12 Hospital Group Pitié‐Salpêtrière‐Charles Foix ‐ Paris Sorbonne University, Ivry‐sur‐Seine, Ile‐de‐France, France, 13 University of Nancy, France, Nancy, Lorraine, France, 14 Faculty of Pharmacy/University of Paris, Paris, Ile‐de‐France, France
Background: A growing number of very elderly patients with non‐valvular atrial fibrillation (NVAF) receive direct oral anticoagulants (DOAC) to prevent ischemic stroke and embolic events. However, no study specifically investigated xaban pharmacokinetics (PK) and pharmacodynamics (PD) in these frail polymedicated patients at high hemorrhagic and thrombotic risks.
Aims: To investigate: i/ xaban concentration‐time profiles; ii/ thrombin generation (TG); and iii/ clinical outcomes 6‐months after inclusion in very elderly NVAF patients receiving rivaroxaban or apixaban.
Methods: ADAGE (NCT02464488) was a prospective exploratory academic multicenter study, enrolling NVAF patients aged ≥80‐years from geriatrics units, receiving xaban for at least 4 days. Each patient had 1‐5 samples at different time‐points after DOAC intake over a 20‐day period. TG was investigated using ST‐Genesia (Drugscreen, Thromboscreen with/without thrombomodulin). Clinical outcomes were collected at 6‐months.
Results: Two‐hundred‐and‐fifteen patients (women 71.1%, mean age 87 ± 4‐years) were included, 104‐rivaroxaban and 111‐apixaban, 79.5% receiving reduced‐dose regimen (i.e. 15 mg qd and 2.5 mg bid, respectively). We observed important inter‐individual variabilities (CV) of Cmax (47% ‐ 45%) and Cmin (38% ‐ 65%) in 15 mg‐rivaroxaban and 2.5 mg‐apixaban patients, respectively. Dose regimen was associated with plasma concentration and TG peak‐height at Tmax (p = 0.0058 and 0.0074) and Tmin (p = 0.0222 and 0.0516) in apixaban samples, respectively, but not in rivaroxaban samples (multivariate analysis). Moreover, substantial variability of TG peak‐height was noticed at a given plasma concentration for both xabans, suggesting the important impact on TG of the underlying coagulation status in very elderly patients. Major bleeding, thrombotic event and death rates were 6.0%, 2.3%, and 18.1%, respectively, without association with PK/PD data.
Conclusion(s): Our study provides original PK/PD data in very elderly frail patients receiving xabans in real‐life setting. The potential clinical impact of such data deserves to be extensively investigated in the context of an aging world.
OC 71.3
Bleeding outcomes for patients on anticoagulant and antiplatelet therapy
J. Schaefer 1; J. Errickson1; X. Kong1; M. Ali2; S. Edupuganti1; B. Haymart1; S. Kaatz3; D. DeCamillo1; E. Kline‐Rogers4; V. Shah3; S. Sood1; J. Froehlich4; G. Barnes1
1 University of Michigan, Ann Arbor, Michigan, United States; 2 Beaumont Hospital, Royal Oak, Michigan, United States; 3 Henry Ford Hospital, Detroit, Michigan, United States; 4 University of Michigan Health, Ann Arbor, Michigan, United States
Background: For patients anticoagulated with a direct oral anticoagulant (DOAC) or warfarin for the indications of non‐valvular atrial fibrillation and/or venous thromboembolism, adding concomitant aspirin (ASA) therapy can increase bleeding risk with uncertain antithrombotic benefit. It is unclear if outcomes with apixaban or rivaroxaban and ASA differ from warfarin and ASA.
Aims: To assess bleeding and thrombotic event rates for apixaban and rivaroxaban with aspirin as compared to warfarin and aspirin.
Methods: Within the six‐center Michigan Anticoagulation Quality Improvement Initiative (MAQI2), we analyzed registry data for warfarin or DOAC treated patients with atrial fibrillation and/or venous thromboembolism without a clear indication for concomitant ASA (e.g., recent myocardial infarction or history of heart valve replacement) who were taking ASA. Warfarin+ASA treated patients were propensity matched to DOAC+ASA treated patients who had at least 3 months of follow‐up data. The primary outcome was bleeding. Secondary outcomes included episodes of thrombosis, healthcare utilization, and death. Event rates were compared using Poisson regression. Residual differences between matched groups were included in the Poisson regression models as appropriate.
Results: 1,328 warfarin+ASA patients were matched to 872 on apixaban+ASA and 377 on rivaroxaban+ASA. Patient demographics, co‐morbidities, indication for anticoagulation, history of bleeding or clotting, medications, and duration of follow‐up were fairly similar after matching. Patients were followed for a mean (standard deviation) of 23.8 months (26.0 months) for warfarin versus apixaban and 24.5 months (29.4 months) for warfarin versus rivaroxaban. Bleeding and thrombotic outcomes between apixaban+ASA were similar to warfarin+ASA (Table 1), aside for more emergency room visits for bleeding with warfarin+ASA. We observed increased bleeding and thrombotic events with rivaroxaban+ASA compared to warfarin+ASA (Table 2).
Conclusion(s): Patients treated with rivaroxaban+ASA may experience worse clinical outcomes compared to warfarin+ASA while outcomes between apixaban+ASA and warfarin+ASA seem similar.
OC 71.1
Safety of off‐label dose reduction of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation
C. Van Den Dries1; R. Pajouheshnia2; R. van den Ham2; P. Souverein2; C. Moons3; A. Hoes4; G. Geersing4; S. van Doorn 5
1 Julius Center for Health Sciences and Primary Care, UMC Utrecht/Utrecht University, Utrecht, Utrecht, Netherlands; 2 Utrecht University, Utrecht, Utrecht, Netherlands; 3 UMC Utrecht/Utrecht University, Utrecht, Utrecht, Netherlands; 4 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 5 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: While non‐vitamin K antagonist oral anticoagulants (NOACs) are increasingly prescribed, concerns have emerged about patients receiving a lower dose against guideline recommendations, i.e. off‐label dose reduction. The clinical impact of off‐label dose reduction remains unclear.
Aims: To investigate the effects of off‐label NOAC dose reduction compared to on‐label standard dosing in atrial fibrillation (AF) patients in routine care.
Methods: Population‐based cohort study using data from the United Kingdom Clinical Practice Research Datalink, comparing adults with non‐valvular AF receiving an off‐label reduced NOAC dose to patients receiving an on‐label standard dose. Outcomes were ischaemic stroke, major bleeding, non‐major bleeding and mortality. Inverse probability of treatment weighting (IPTW) on the propensity score was applied to adjust for confounding.
Results: Off‐label dose reduction occurred in 2,466 patients (8.0%), compared to 18,108 (58.5%) on‐label standard dose users. Median age was 80 years (interquartile range (IQR) 73.0‐86.0) versus 72 years (IQR 66‐78), respectively. Incidence rates were higher in the off‐label dose reduction group compared to the on‐label standard dose group, for ischaemic stroke (0.94 vs. 0.70 per 100 person years), major bleeding (1.48 vs. 0.83), non‐major bleeding (6.78 vs. 6.16) and mortality (10.12 vs 3.72). IPTW resulted in an adjusted hazard ratio of 1.07 (95%CI 0.65‐1.74) for ischaemic stroke; 0.98 (95%CI 0.65‐1.48) for major bleeding; 0.89 (95%CI 0.74‐1.08) for non‐major bleeding; and 1.48 (95%CI 1.25‐1.76) for mortality.
Conclusion(s): In this large population‐based study, the risk of ischaemic stroke, non‐major bleeding and major bleeding was similar in AF patients receiving an off‐label reduced NOAC dose compared to on‐label standard dose users, while mortality risk appeared to be higher. Therefore, altogether, off‐label dose reduction seems unlikely to be a fruitful strategy when aiming to reduce bleeding risk in (high‐risk) AF patients. FIGURE 1 Forest plot showing the main results, comparing off‐label dose reduction to on‐label standard dosing. Event rates are incidence rates per 100 person years. ATT = average treatment effect among the treated
FIGURE 1 Forest plot showing the main results, comparing off‐label dose reduction to on‐label standard dosing. Event rates are incidence rates per 100 person years. ATT = average treatment effect among the treated
OC 71.2
PAUSE‐2 randomized pilot trial to compare two strategies (PAUSE vs. ASRA) for perioperative DOAC management
J. Douketis; J. Duncan; M. St John; P. Gross; S. Schulman
McMaster University, Hamilton, Ontario, Canada
Background: There is uncertainty about how to manage patients taking a direct oral anticoagulant (DOAC) who need a high‐bleed‐risk surgery/neuraxial anesthesia. There are two possible strategies: (i) PAUSE management, derived from the PAUSE study, requires DOAC interruption for 2 days before and after a high‐bleed‐risk surgery/neuraxial procedure, without heparin bridging or DOAC level testing; (ii) ASRA management, derived from the American Society of Regional Anesthesia guidelines, requires DOAC interruption for 3‐5 days, resumption within 24 hours post‐op and heparin bridging and DOAC levels in selected patients.
Aims: This pilot study aims to assess feasibility of a larger RCT and to identify possible safety signal concerns in either strategy.
Methods: PAUSE‐2 pilot is an open‐label, randomized controlled trial (RCT) that compares the PAUSE vs. ASRA strategy for perioperative DOAC management in patients with atrial fibrillation needing a high‐bleed‐risk surgery/neuraxial anesthesia. We hypothesize that PAUSE management will be as safe as ASRA for the outcomes of major bleeding (2.5% both arms, 2% non‐inferiority [NI] margin) and stroke/systemic embolism (0.5% both arms, 1% NI margin). A secondary outcome is residual DOAC levels, measured just before surgery. Patients are followed from randomization (3‐5 days pre‐surgery) until 30 days post‐surgery. PAUSE‐2 was approved by local research ethics boards; 80% of eligible patients consented to participate.
Results: As of January 28, 2022, 90 recruited patients from 3 clinical sites completed the 30‐day follow‐up. The patient/surgery characteristics are shown in Table 1. In a blinded analysis of all patients, there was no stroke/systemic embolism, major bleeding or deaths. One patient had a pulmonary embolism. Results of residual DOAC levels are ongoing, to be disclosed at the time of public presentation.
Conclusion(s): The PAUSE‐2 pilot RCT demonstrates feasibility to conduct a large, adequately‐powered trial. It successfully randomized patients to two perioperative DOAC management strategies, and does not appear to have safety signal concerns. TABLE 1 PAUSE‐2 RCT pilot patient/surgery characteristics
TABLE 1 PAUSE‐2 RCT pilot patient/surgery characteristics
Cardiovascular Risk Factors
OC 18.3
MiR‐146a seeds a pro‐inflammatory state in acute myocardial infarction of young patients promoting recurrence
A. de los Reyes‐García 1; J. Rivera Caravaca2; S. Aguila Martinez3; N. García‐Barberá4; L. Zapata‐Martínez4; M. Lozano5; F. Marín2; C. Martínez4; R. Gonzalez‐Conejero Hilla4
1 Instituto Murciano de Investigación Biomédica, Universidad de Murcia, Hospital Universitario Morales Meseguer, Murcia, Murcia, Spain; 2 Hospital Virgen de la Arrixaca, Murcia, Murcia, Spain; 3 Centro Regional de Hemodonación, Murcia, Murcia, Spain; 4 Centro Regional de Hemodonación. Hospital Morales Meseguer. Universidad de Murcia. Imib‐Arrixaca, Murcia, Murcia, Spain; 5 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain
Background: Inflammation and atherosclerosis have an important function in acute coronary syndrome (ACS). Neutrophils are responsible for one of the most important elements involved in thrombosis/inflammation crosstalk, neutrophil extracellular traps (NETs). miR‐146a‐5p has important anti‐inflammatory functions. Our group previously described that its levels are regulated by rs2431697 influencing NET formation. Here, we evaluated the potential association between NET markers and outcomes of ACS in young adults (<45 years).
Aims: In these subjects, differences in etiology and risk factors may lead the prognosis and treatments. Therefore, an early detection of subclinical disease might prevent coronary events or recurrences.
Methods: 300 ACS patients (44 ± 4 years) and 300 healthy blood donors (44 ± 4 years) were recruited. The informed consent was obtained for each subject and the study was approved by local ethics committee. NETs in plasma were evaluated by quantifying cell‐free DNA (cfDNA) using SYTOX green, and citrullinated histone‐3 (citH3)/DNA complexes by ELISA. Rs2431697 was genotyped using TaqMan probes. Patients were followed‐up up from January 2015 until March 2020 and all adverse events were recorded.
Results: We found a positive correlation between citH3‐DNA complexes and cfDNA levels with Killip‐Kimball score (p = 0.002 and p < 0.001), suggesting that patients with more severe ACS had higher levels of NETosis. We also found higher Killip‐Kimball score in those patients with cfDNA levels above Q4 (p = 0.003). Patients with citH3‐DNA levels above Q4 had more stroke recurrence (p = 0.026). Finally, when cfDNA and citH3‐DNA levels were combined with the T allele of rs2431697 as a risk factor, we found a higher risk of ischemic event recurrence (p = 0.024).
Conclusion(s): The present research shows an association between NETs’ markers and rs2431697 T allele increasing the risk of recurrence of cardiovascular events in young patients with ACS. These findings support the clinical relevance of circulating NETs as novel markers in ACS and as a potential therapeutic target.
OC 45.1
Circulatory microRNA‐411‐5p as a novel prognostic biomarker for major adverse cardiac events in patients with atrial fibrillation
S. Nopp 1; M. van der Bent2; O. Königsbrügge3; D. Kraemmer1; J. Wojta1; I. Pabinger4; C. Ay4; A. Nossent2
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 4 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Reliable risk prediction of cardiovascular (CV) events in patients with atrial fibrillation (AF) is essential for optimizing prevention strategies. However, current clinical and routine laboratory markers lack accuracy in predicting major adverse cardiovascular events (MACE).
Aims: In this study, we sought to investigate circulating microRNAs as prognostic biomarkers for MACE in patients with AF.
Methods: We conducted a 3‐stage nested case‐control study on 418 patients with AF, who were followed‐up for the occurrence of MACE (stroke, myocardial infarction, or CV death). First, total small‐RNA sequencing was performed in 13 patients with MACE and 13 controls matched by age, sex, and CHA2DS2‐VASc score. Eight candidate microRNAs showing differential expression in CV death were selected and measured using reverse‐transcription quantitative polymerase chain reaction (qPCR) in 42 patients with CV death and 55 matched controls. To validate our findings and investigate broader clinical applicability, we also measured these microRNAs in 37 patients with MACE and 65 controls. Results of the qPCR‐measurements for each candidate microRNA were handled according to a data‐handling pipeline and analyzed using Cox regression.
Results: In the screening cohort, we detected 184 well‐expressed microRNAs but did not find differentially expressed microRNAs below an FDR‐adjusted p‐value threshold of 0.05 for predicting MACE. However, significant results were obtained in the subgroup of patients with CV death. We, therefore, proceeded with a nested case‐control study on patients with CV death and selected 8 microRNAs (miR‐483‐3p, miR‐122‐3p, miR‐150‐5p, miR‐127‐3p, miR‐1908‐5p, miR‐15a‐5p, miR‐411‐5p, and miR‐625‐5p) for further qPCR‐analysis. In those, microRNA‐411‐5p was associated with CV death with a hazard ratio (HR) of 1.98 (95%CI 1.06‐3.72). Validation in patients who developed MACE held similar findings; microRNA‐411‐5p showed an HR of 2.12 (95%CI 1.16‐4.11) for the occurrence of MACE.
Conclusion(s): Circulating microRNA‐411‐5p could be a valuable prognostic biomarker for MACE in patients with AF.
Cerebrovascular Disorders
OC 45.5
Endothelial‐targeted recombinant CD39 as novel therapy for brain ischaemia
N. Lee1; C. Selan2; J. Chia2; R. Medcalf3; D. Wright4; X. Wang5; K. Peter5; S. Robson6; M. Sashindranath1; H. Nandurkar 7
1 Australian Centre for Blood Diseases and Monash University, Melbourne, Victoria, Australia; 2 Australian Centre for Blood Diseases, Monash University, Melbourne, Victoria, Australia; 3 Monash University, Melbourne, Victoria, Australia; 4 Department of Neurosciences, Monash University, Melbourne, Victoria, Australia; 5 Baker Institute, University of Melbourne, Melbourne, Victoria, Australia; 6 Harvard University, Boston, Massachusetts, United States; 7 Alfred Health and the Australian Centre for Blood Diseases and Monash University, Melbourne, Victoria, Australia
Background: Ischaemia‐reperfusion injury (IRI) contributes to acute ischaemic stroke (AIS) as well as to hypoxia induced brain injury (HIBI) that follows delayed resuscitation post cardiopulmonary arrest. IRI contains inflammation, thrombosis and activation of endothelium (EC). 'Anti‐VCAM‐CD39' is designed to target the antiinflammatory and antithrombotic properties of soluble CD39 (previously demonstrated by others and us) to activated EC by the incorporation of a scFV tag recognising the EC activation receptor VCAM‐1. CD39 activity hydrolyses ATP and ADP to AMP with subsequent conversion to adenosine.
Aims: To demonstrate that anti‐VCAM‐CD39 will improve stroke outcomes in murine models of AIS and HIBI when given as a single agent, and maximise the benefit of tPA in AIS. Doses on anti‐VCAM‐CD39 used here do not perturb haemostasis.
Methods: Models of transient middle cerebral artery occlusion (MCAo) and of global forebrain ischaemia by dual carotid artery ligation (DCAL) were utilised (models published by us: Scientific reports, 2020; 10, 1‐13). The test drugs (anti‐VCAM‐CD39 and various controls were given after 3 hours after ischaemia of 30 min. Assessments at 24 h included function (Bederson score and footslips), infarct volume and perfusion (MRI), albumin extravasation and serum vWF.
Results: In the HIBI (DCAL) model, anti‐VCAM‐CD39 (0.125 mg/kg) demonstrated small infarct volumes, lesser albumin extravasation and caspase activation and lower plasma vWF compared with saline and anti‐VCAM‐Inactive CD39 (controls) (data and statistics as shown in (Fig.1) and lower neurological deficit score (Saline: 4, n = 19; anti‐VCAM_CD39: 2, n = 14, p = 0.0001, One‐way ANOVA). In the MCAo model, anti‐VCAM‐CD39 (0.5 mg/kg), demonstrated lower infarct volumes and better perfusion (Fig 2), lower albumin extravasation and vWF level p < 0.5 for all parameters). Co‐administration with tPA was synergistic in improving function and infarct volumes (p < 0.05).
Conclusion(s): Endothelial‐targeted soluble CD39 (at a dose that dose that does not perturb haemostasis) significantly improved outcomes in focal stroke as well as global brain ischaemia.
OC 45.3
Non‐invasive in vivo thrombus imaging in patients with ischaemic stroke or transient ischaemic attack
B. Whittington 1; D. Newby1; E. Tzolos1; M. Williams1; J. Wardlaw2; E. Van Beek3; R. Bing1; T. Clark1; C. Lucatelli1; W. Whiteley1; P. Slomka4; M. Dweck1; A. Stephens5; N. Koglin5
1 University of Edinburgh, Edinburgh, Scotland, United Kingdom; 2 Univeristy of Edinburgh, Edinburgh, Scotland, United Kingdom; 3 Unversity of Edinburgh, Edinburgh, Scotland, United Kingdom; 4 Cedars‐Sinai Medical Center, Los Angeles, California, United States; 5 Life Molecular Imaging, Berlin, Berlin, Germany
Background: 18F‐GP1 is a novel positron‐emitting radiotracer that is highly specific for activated platelets and thrombus. It is a small molecule derivative of elarofiban and has a high and specific binding affinity for the activated glycoprotein IIb/IIIa receptor on activated platelets. Preliminary studies using 18F‐GP1 demonstrated excellent in vivo binding properties in a range of conditions including left ventricular thrombus following myocardial infarction, pulmonary thromboembolism, deep vein thrombosis and coronary thrombosis.
Aims: In a pilot proof‐of‐concept study, we aimed to determine its potential clinical application in establishing the role and origin of thrombus in stroke.
Methods: Eleven patients with recent ischaemic stroke (n = 9) or transient ischaemic attack (n = 2) underwent 18F‐GP1 positron emission tomography (PET) and computed tomography (CT) angiography at median of 11 [4‐17] days from symptom onset. 18F‐GP1 uptake maximum and mean target‐to‐background ratios (TBR) was assessed in the carotid arteries and brain
Results: 18F‐GP1 uptake was identified in 9/11 patients: 5 in the carotid arteries only, 2 in the brain only and 2 in both the brain and carotid arteries. In those with carotid uptake, 4 had >70% stenosis and 3 had non‐stenotic disease. One case had bilateral stenotic disease but only the culprit carotid artery showed 18F‐GP1 uptake. Overall, uptake was higher in the ipsilateral carotid artery (TBRmax 2.51 ± 0.95, TBR mean 1.75 ± 0.48) compared to the contralateral non‐culprit artery (TBRmax 1.66 ± 0.55, TBRmean 0.79 ± 0.20). Four cases showed visual uptake within the brain (TBRmax of 6.63 ± 2.27 and TBRmean 3.73 ± 2.67) corresponding to areas of CT‐defined infarction. There was no brain 18F‐GP1 uptake in the 7 cases without CT‐defined acute cerebral infarction.
Conclusion(s): 18F‐GP1 PET‐CT is a non‐invasive method of identifying in vivo cerebrovascular thrombosis which holds major promise in understanding the role and origin of thrombus in stroke. Acknowledgments British Heart Foundation funded this work (RG/16/10/32375, FS/CRTF/21/24129). Life Molecular Imaging provided reagents for 18F‐GP1. FIGURE 1 Bilateral severe carotid stenosis with focal 18F‐GP1 uptake (yellow/red) in culprit right common carotid bifurcation with ipsilateral brain uptake consistent with carotid artery thromboembolism and cerebral infarction. Severe soft plaque with severe stenosis of contralateral non culprit internal carotid artery with no 18F‐GP1 uptake
FIGURE 1 Bilateral severe carotid stenosis with focal 18F‐GP1 uptake (yellow/red) in culprit right common carotid bifurcation with ipsilateral brain uptake consistent with carotid artery thromboembolism and cerebral infarction. Severe soft plaque with severe stenosis of contralateral non culprit internal carotid artery with no 18F‐GP1 uptake
OC 45.4
Transcriptomic analysis of plasma and thrombus extracellular vesicles (Evs) for the molecular diagnosis of ischemic stroke etiology
F. Machado1; J. Marta‐Enguita1; C. Roncal1; J. Rodriguez1; J. Páramo2; R. Bermejo3; R. Muñoz4; J. Orbe 5
1 Cima‐Universidad de Navarra, Pamplona, Navarra, Spain; 2 Clínica Universidad de Navarra, Pamplona, Navarra, Spain; 3 Hospital Universitario de Navarra, Pamplona, Navarra, Spain; 4 Complejo Hospital de Navarra, Pamplona, Navarra, Spain; 5 Centro de Investigación Médica Aplicada (CIMA), Universidad de Navarra, Pamplona, Navarra, Spain
Background: Ischemic stroke (IS) results from an acute thrombus obstruction of a cerebral artery that causes high morbidity and mortality worldwide. Almost 45% of all IS are from unknown origin complicating the precise secondary treatment. Extracellular vesicles (EVs) are membrane nanoparticles that contain nucleic acids from the cell of origin. The study of their content might help to define a personalized molecular pattern to guide clinicians in the diagnosis and the therapeutic strategy for each patient.
Aims: To analyse the transcriptomic content of circulating and thrombus derived EVs according to IS etiology to assess their molecular signature.
Methods: We isolate EVs from IS patients with large vessels occlusion by high‐speed centrifugation (2 × 20.000 g, 70 min) from citrated platelet‐free plasma and Accutase‐digested thrombus. To characterize EVs size and cellular origin, nanoparticle tracking analysis (NTA) and flow cytometry were performed. Transcriptomic profiles of medium/large EVs were generated using the MARS‐Seq RNA‐Seq protocol (n = 5/group).
Results: EVs uptaking Carboxyfluorescein succinimidyl ester (CFSE) were mainly from platelet and erythrocyte origin. NTA shows a polydisperse heterogeneous vesicle distribution (133.5‐300 nm). By RNAseq we identified 95 differentially expressed genes (DEG) in plasma EVs (p < 0.01) and 21 in thrombus EVs (p < 0.01), which could discriminate IS patients by etiology. Among these DEG, plasma EVs from cardioembolic patients were enriched in circulatory system development, regulation of neuron death and T cell activation pathways, whereas, thrombi EVs presented differences in transcripts related to hematopoiesis and catabolic process. In atherothrombotic patients, lipid catabolic process, and regulation of MAPK were the enriched pathways in plasma while ion and transmembrane transport were in thrombi EVs.
Conclusion(s): Transcriptomic analysis of circulating and thrombus‐derived EVs in IS patients might be useful to identify molecular signatures of IS etiologies. Funding: ISCIII (PI19/00065"), co‐funded by ERDF, "A way to make Europe", RICORs‐ictus (RD21/0006/0008), CIBERCV (CB16/11/00371), and Virto Group (Navarra, Spain).
OC 45.2
Foudroyant cerebral venous (sinus) thrombosis triggered through CLEC‐2 and GPIIb/IIIa dependent platelet activation
D. Stegner1; V. Göb 2; V. Krenzlin3; S. Beck4; K. Hemmen5; M. Schuhmann6; B. Schörg7; C. Hackenbroch6; F. May8; P. Burkard9; J. Pinnecker5; A. Zernecke6; P. Rosenberger10; A. Greinacher11; B. Pichler7; K. Heinze12; G. Stoll6; B. Nieswandt13
1 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 2 University Hospital and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 3 University of Mainz, Mainz, Rheinland‐Pfalz, Germany, 4 University Hospital and Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 5 Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 6 University Hospital Würzburg, Würzburg, Bayern, Germany; 7 Eberhard Karls University Tübingen, Tübingen, Baden‐Wurttemberg, Germany; 8 CSL Behring, Marburg, Hessen, Germany; 9 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Germany, Würzburg, Bayern, Germany, 10 University Hospital Tübingen, Tübingen, Baden‐Wurttemberg, Germany, 11 University Medicine Greifswald, Greifswald, Mecklenburg‐Vorpommern, Germany, 12 Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany, 13 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: Cerebral venous (sinus) thrombosis (CVST, CVT) is an unusual manifestation of venous thrombosis causing severe neurological impairment and seizures. Molecular mechanisms underlying CVT, potentially involving pathological platelet activation, are currently unknown.
Aims: Recent studies suggested a role for platelet ITAM signaling in venous thrombosis. Therefore, we investigated a potential role of the hemITAM receptor CLEC‐2 in CVT.
Methods: Mice were monitored following the administration of the anti‐CLEC‐2 antibody, INU1, or its fab‐fragment. Platelet count and activation were assessed by flow cytometry, and thrombus formation was analyzed intravitally or in tissue sections using different imaging modalities.
Results: Administration of INU1 fab‐fragments, but not INU1‐IgG, triggered within minutes a CVT‐like thrombotic syndrome in mice, characterized by tonic/myoclonic seizures, platelet consumption and death. Brain autopsy showed thrombi mainly in the cortical venules. Transcranial intravital microscopy revealed rapidly progressing thrombosis in the superior sagittal sinus, a main site of CVT in humans. PET/MRI and light‐sheet fluorescence microscopy confirmed that INU1‐fab induced thrombosis is limited to cerebral veins. Defective CLEC‐2 signaling protected mice from INU1‐fab induced symptoms and lethality. Lack of dense granule secretion or treatment with Aspirin or Clopidogrel delayed the onset of CVT but did not prevent thrombocytopenia or lethality. In contrast, prophylactic GPIIb/IIIa blockade abolished platelet consumption, CVT and death. Unlike heparin, the current first line treatment of CVT, GPIIb/IIIa blockade was also highly beneficial when applied therapeutically after symptom onset.
Conclusion(s): These results point to aberrant platelet activation as a major trigger of CVT and suggests targeting GPIIb/IIIa or CLEC2‐ITAM signaling as a potential therapeutic option in foudroyant CVT. FIGURE 1 CLEC‐2 dependent platelet activation results in cerebral venous thrombosis. The bivalent anti‐CLEC‐2 antibody INU1 triggers platelet activation in vitro, while this is not the case for the monovalent INU1‐fab fragment. In vivo, however, INU1‐fab leads in the rapid formation of thrombi in the cerebral veins, here shown for the superior sagittal sinus. As a result, mice suffer from neurological symptoms like seizures (parts of the figure were created in BioRender)
FIGURE 1 CLEC‐2 dependent platelet activation results in cerebral venous thrombosis. The bivalent anti‐CLEC‐2 antibody INU1 triggers platelet activation in vitro, while this is not the case for the monovalent INU1‐fab fragment. In vivo, however, INU1‐fab leads in the rapid formation of thrombi in the cerebral veins, here shown for the superior sagittal sinus. As a result, mice suffer from neurological symptoms like seizures (parts of the figure were created in BioRender)
Peripheral Artery Disease
OC 18.4
The role of neutrophil extracellular traps (NETs) in lower extremity artery disease (LEAD)‐specific thrombosis
M. Thakur 1; N. Angliker2; M. Siegrist3; M. Kuijpers4; M. Schindewolf3; I. Baumgartner3; Y. Döring5
1 Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland. 2. Department for BioMedical Research, University of Bern, Switzerland., Olten, Solothurn, Switzerland, 21. Division of Angiology, Swiss Cardiovascular Center, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland. 2. Department for BioMedical Research, University of Bern, Switzerland, bern, Bern, Switzerland, 31. Division of Angiology, Swiss Cardiovascular Center, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland. 2. Department for BioMedical Research, University of Bern, Switzerland, Bern, Bern, Switzerland; 4 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands, 51. Division of Angiology, Swiss Cardiovascular Center, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland. 2. Department for BioMedical Research, University of Bern, Switzerland. 3. Institute for Cardiovascular Prevention (IPEK), Ludwig‐Maximilians‐University Munich (LMU), 80336 Munich, Germany. 4. DZHK (German Centre for Cardiovascular Research), Partner Site Munich Heart Alliance, 80336 Munich, Germany., Bern, Bern, Switzerland
Background: Lower‐extremity arterial disease (LEAD) is a significant health problem affecting 230 million people worldwide. Thrombotic complications are a hallmark of LEAD pathophysiology and recent genome wide associations studies have identified the risk variant Factor V Leiden specifically in LEAD emphasizing the importance of arterial thrombosis in LEAD. Arterial thrombi also consists of neutrophil extracellular traps (NETs), however, their prevalence and role in LEAD is very limited.
Aims: Explore the prevalence and role of NETs (negatively charged DNA‐scaffold) in the LEAD‐specific thrombus. Establishment of the ferric chloride induction thrombosis in the femoral artery of Apoe‐/‐ mice. Effect of therapeutic interference with NETs on the LEAD‐specific thrombi.
Methods: We measured the prevalence of NETs in LEAD versus carotid thromboendarterectomy patient samples. In our in‐vitro assays, we used flow chambers to perfuse the NETs with labelled fibrinogen to enable clot formation in absence or presence of NETs inhibitors like DNase1 or Oleylamine (neutralize negatively charged DNA scaffold). Furthermore, we performed thromboelastometry assays and functional assays using similar conditions. Apoe‐/‐ mice (WD 21 weeks) were used for FeCl3 induced thrombosis in the femoral arteries in absence and presence of NET modifying reagents. 2‐photon intravital microscopy and histology was used to evaluate the role of NETs and its inhibitors in thrombus formation. For statistical analyses we have used Mann‐Whitney, Kruskal‐Wallis with Dunn's multiple comparison.
Results: We saw strongly abolished fibrin clotting in presence of NET interfering agents. Correspondingly, thromboelastometry assays also revealed extended clotting time in presence of NET interference in‐vitro. FeCl3‐induced thrombosis in femoral artery showed significant number of NETs in the thrombi leading to arterial occlusion.
Conclusion(s): LEAD specific thrombosis differs from other arterial thrombi with significantly higher prevalence of NETs. NETs inhibition leads to depletion of thrombus formation both in‐vivo and in‐vitro. Therefore, preventing NETs release could represent a potential strategy to prevent LEAD‐specific thrombosis.
Coagulation and Natural Anticoagulants
Animal Models in Thrombosis and Hemostasis
OC 74.2
Generation of zebrafish f9like mutant by CRISPR/Cas9 technology
S. Dhinoja; A. De Maria; P. Jagadeeswaran
University of North Texas, Denton, Texas, United States
Background: Coagulation factor IX participates in the intrinsic pathway of coagulation. Previously we have shown that zebrafish have three factor IX genes: f9a, f9b, and f9like, and found that f9like is functionally similar to human coagulation factor X. At present, there are no knockout models for these genes to corroborate previous findings.
Aims: To create and characterize f9like knockout mutant zebrafish using CRISPR Cas 9 technology.
Methods: Three different gRNAs targeting exon 8 of the f9like gene were synthesized, and they were microinjected along with Cas9 protein into the yolk sac of the single‐cell wild‐type zebrafish embryos. The embryos were reared, and at 2.5 months post‐fertilization, DNA was isolated from tail clips and amplified by PCR. The PCR products were run on polyacrylamide gels and the gel‐purified PCR products were sequenced by the Sanger method.
Results: We injected approximately 300 zebrafish embryos and screened 60 adult fish for insertions or deletions in exon 8 using PAGE. Exon 8 was targeted as it encompassed the catalytic domain of the gene. Out of 60 fish, 9 fish showed two bands, one corresponding to the wild‐type and the other either had deletion or insertion. These fish individually were crossed with wild‐type fish, and only one of them transmitted the mutation to the next generation. The DNA bands from this homozygous mutant were sequenced, and the chromatograms revealed that there was a 14 bp deletion that generated a frameshift mutation causing premature termination and thus, yielded an f9like founder mutant line. We are currently characterizing this mutant with coagulation and bleeding assays.
Conclusion(s): We successfully created an f9like mutant that has 14bp deletion in exon 8 by CRISPR/Cas9 method. This f9like mutant should be useful in the creation of double mutants with the already known fx mutant to test whether it shows embryonic and developmental lethality.
OC 44.2
Genetic deletion of Prenylcysteine Oxidase 1 (PCYOX1) impairs arterial thrombosis in mice
P. Amadio; C. Banfi; M. Zarà; M. Brioschi; S. Ghilardi; L. Sandrini; S. Barbieri
Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy
Background: Prenylcysteine Oxidase 1 (PCYOX1) is an enzyme involved in the degradation of prenylated proteins. It is expressed in different tissue, including vascular and blood cells. We recently showed that the secretome from PCYOX1‐silenced cells reduced platelet adhesion to both fibrinogen and endothelial cells, suggesting a potential contribution of PCYOX1 into thrombus formation.
Aims: In this study, we used a knock‐out murine model to analyze the contribution of PCYOX1 in arterial thrombosis.
Methods: All procedures were performed in PCLOX1 knock‐out (Pcloxy1KO) mice and compared to littermate wild type (WT) mice. Ferric Chloride (FeCl3) arterial injury and pulmonary thromboembolism models were used to explore the impact of PCLOX1 on thrombosis. The phenotype and function of platelets were studied by flow cytometry and functional tests. The presence of Pcyox1 in washed platelets was assessed by mass spectrometry analysis.
Results: In vivo studies show that Pcloxy1KO mice are protected from collagen‐induced pulmonary thromboembolism and show delayed FeCl3 induced carotid thrombus. Pcloxy1KO mice displayed normal blood cells count, vascular procoagulant activity and plasma fibrinogen levels. Deletion of Pcloxy1 reduced the platelet/leukocyte aggregates in whole blood, as well as the platelet aggregation, the alpha granules release and the αIIbβ3 integrin activation in platelet‐rich plasma in response to ADP or TRAP. Pcyox1 was great expressed in washed platelets isolated from WT mice. Washed platelets from Pcloxy1KO and WT animals have similar phosphorylation pathway activation, adhesion ability and aggregation. The presence of Pcloxy1‐/‐ plasma impaired agonist‐induced WT platelet aggregation.
Conclusion(s): Our findings show that the absence of Pcloxy1 results in platelets hyporeactivity and impaired arterial thrombosis, suggesting that Pcloxy1 may represent a novel target for antithrombotic therapy.
OC 44.3
A new mouse model of impaired fibrin α‐chain crosslinking shows increased venous thrombembolism
C. Duval 1; T. Feller2; H. McPherson1, R. Ariëns3
1 University of Leeds, Leeds, England, United Kingdom; 2 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds., Leeds, England, United Kingdom; 3 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom
Background: Factor XIII (FXIII) crosslinks fibrin and incorporates fibrinolysis inhibitors into the clot, increasing resistance to mechanical strain and proteolytic degradation. We recently demonstrated that in‐vivo thromboembolisation is increased in a mouse strain with abolished fibrin α‐chain crosslinking (PNAS, 2021). However, the role of α‐chain crosslinking in clot formation and stability in‐ and ex‐vivo is not yet known.
Aims: The aims of this study were to generate a new genetically modified murine model of impaired fibrin α‐chain crosslinking and investigate its impact on venous thromboembolism.
Methods: Genetically modified FGA4X mice were generated by mutating the fibrin α‐chain glutamine residues involved in crosslinking by FXIII (αQ241N/Q243N/Q257N/Q518N). Mice phenotype was characterised, and clot properties were analysed by ROTEM and clot contraction assay. Pulmonary embolism was investigated by Xtreme live optical imaging, following FeCl3 injury to the inferior vena cava.
Results: Compared to WT, FGA4X mice showed no differences in haematological parameters. Fibrinogen levels were reduced (1.0 ± 0.1 vs 1.5 ± 0.1 mg/ml). Fibrin α‐α, but not γ‐γ, crosslinking was significantly reduced (35.6 ± 6.2 vs 83.7 ± 4.0 %). ROTEM analysis of whole blood showed that clotting time was increased (37.0 ± 4.0 vs 47.0 ± 6.0 sec) and maximum clot firmness was reduced (15.8 ± 2.9 vs 22.9 ± 3.0 mm, FIBTEM). Clot contraction experiments showed that FGA4X mice generated larger (46.7 ± 1.4 vs 30.8 ± 1.5 μl) and heavier (44.3 ± 0.7 vs 31.6 ± 1.8 mg) clots than WT, containing more red blood cells (+21 %). Pulmonary embolism was significantly increased (~1.8‐fold) in FGA4X mice compared to WT, at 0.5, 1, 2, 4, 24 hrs post‐injury of the inferior vena cava.
Conclusion(s): Our data show that α‐α crosslinking plays a significant role in clot stability during thrombosis, and protects against clot fragmentation and subsequent pulmonary embolism. In combination with our previous study, these data indicate that α‐ and γ‐chain crosslinking play important complementary roles in protection against venous thromboembolism.
Coagulation Factors and Inhibitors
OC 44.5
High α2‐macroglobulin levels are a risk factor for cardiovascular events
R. de Laat‐Kremers 1; S. Constanzo2; Q. Yan3; A. Di Castelnuovo4; A. De Curtis2; C. Cerletti2; M. Donati2; B. de Laat5; L. Iacoviello2
1 Synapse Research Institute, Maastricht, Limburg, Netherlands; 2 Department of Epidemiology and Prevention, IRCCS Neuromed, Pozzilli, Italy, Varese, Lombardia, Italy; 3 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands; Maastricht, Limburg, Netherlands; 4 Mediterranea Cardiocentro, Napoli, Italy, Varese, Lombardia, Italy; 5 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: α2‐macroglobulin (α2M) is a very versatile endopeptidase inhibitor that plays a role in growth, inflammation and coagulation. Hypercoagulability is a risk factor for thrombotic events. Therefore, the inactivation of thrombin, the key coagulation enzyme, is tightly regulated via antithrombin and α2M. Antithrombin deficiency has been related to thrombosis and cardiovascular events, while this is still unknown for α2M.
Aims: We analyzed the association of α2M levels with the occurrence of cardiovascular events in a large sample of general Italian population (n = 19,688).
Methods: We determined α2M levels in the baseline samples of the prospective Moli‐sani cohort and analyzed the association of α2M levels with cardiovascular events in the 4.3 years follow‐up period. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated by multivariable cox regression based on α2M quintiles and adjusted for age and sex, BMI, smoking and oral contraceptive use.
Results: 432 subjects suffered from a cardiovascular disease (CVD) event (2.2%); most events were non‐fatal (82%) and 18% were fatal. The percentage of CVD events was 2.2‐fold higher in subjects in the highest α2M quintile compared to the lowest quintile. The highest quintile of α2M (α2MQ5) was significantly associated with overall CVD events (HRQ1‐4vs5 = 1.93; CI = 1.57‐2.38; p < 0.001) in the crude model and after adjustment for age, sex, current smoking, BMI, and oral contraceptive use (HRQ1‐4vs5 = 1.30; CI = 1.04‐1.62; p = 0.019). α2MQ5 was especially associated with the risk of fatal CVD (HRQ1‐4vs5 = 1.84; CI = 1.13‐2.99; p = 0.015). From all CVD events, 368 were classified as coronary heart disease (CHD) events. α2MQ5 was significantly associated with fatal CHD events in the crude model (ORQ5vsQ1‐4 = 3.95 (2.31‐6.77); p < 0.001), and after adjustment for age, sex, BMI, smoking and oral contraceptive use (ORQ5vsQ1‐4 = 1.88; (1.07‐3.29); p = 0.027).
Conclusion(s): We showed for the first time in a prospective cross‐sectional cohort that α2M in the highest quintile of the normal range is a marker for CVD‐related death.
OC 65.2
Andexanet alfa is associated with a significant reduction in in‐hospital mortality compared to 4F‐PCC in a real‐world analysis
P. Dobesh 1; C. Coleman2; J. Ulloa3; B. Lovelace4; T. Dettling4; B. Koch4; G. Fermann5
1 University of Nebraska Medical Center College of Pharmacy, Omaha, Nebraska, United States; 2 School of Pharmacy, University of Connecticut, Hartford, Connecticut, United States; 3 Outcomes Insights, Westlake Village, California, United States; 4 Alexion, AstraZeneca Rare Disease, Boston, Massachusetts, United States; 5 University of Cincinnati, Cincinnati, Ohio, United States
Background: Anticoagulant‐related major bleeding is associated with increased morbidity and mortality. There are limited real‐world comparative‐effectiveness data on the reversal agents andexanet alfa (AA) and four‐factor prothrombin complex concentrate (4F‐PCC) for factor Xa (FXa) inhibitors.
Aims: We describe the utilization and outcomes associated with managing FXa inhibitor or enoxaparin‐related bleeding hospitalizations with either AA or 4F‐PCC in routine practice.
Methods: This retrospective study included patients who were ≥18 years of age, had an ICD‐10‐CM diagnosis code of D68.32 indicating bleeding due to extrinsic factors (ie, anticoagulant) as part of a hospital admission, and received treatment with AA or 4F‐PCC during the index hospitalization. Categorical variables were compared using the χ2 test. Continuous variables were compared using Student’s t‐test. The primary outcome was in‐hospital mortality, which was analyzed using a multivariate logistic regression.
Results: This study included data from 2,830 patients (AA, n = 1,366; 4F‐PCC, n = 1,464) across 184 US hospitals between 5/2018 and 9/2021. Unadjusted baseline characteristics, including time since last anticoagulant dose, were similar between groups (Table 1). Gastrointestinal (GI) was the most common type of bleed, and most bleeds were spontaneous (Table 1). In‐hospital mortality occurred in 6% (n = 78) and 8% (n = 121) of patients in the AA and 4F‐PCC groups, respectively (p = 0.008). In the adjusted analysis of in‐hospital mortality risk (Table 2), treatment with AA demonstrated a 31% lower likelihood of death compared to 4F‐PCC (OR, 0.69; 95% CI, 0.49‐0.98). Factors associated with higher odds of death included intracranial hemorrhage (versus GI), presence of CKD, impaired mental status, and a DNR order.
Conclusion(s): In this large retrospective study, management of FXa inhibitor or enoxaparin‐related bleeding hospitalizations with AA was associated with a lower in‐hospital mortality rate when compared with 4F‐PCC. This finding should be confirmed in a prospective, randomized controlled trial. Funding: Alexion, AstraZeneca Rare Disease.
OC 67.3
Regulatory function of the anticoagulant protein S in patients with Chuvash polycythemia
D. Melancon 1; V. Pereira2; B. Shah3; A. Sergueeva4; G. Miasnikova5; V. Gordeuk3, R. Majumder6
1 LSU Health Science Center, New Orleans, Louisiana, United States; 2 LSU Health Science center, New Orleans, Louisiana, United States; 3 University of Illinois Chicago, Chicago, Illinois, United States; 4 N. Ulianov Chuvash State University, Cheboksary, Chuvashia, Russia; 5 Chuvash Republic Clinical Hospital, Cheboksary, Chuvashia, Russia; 6 Department of Biochemistry, LSU Health Science Center, New Orleans, Louisiana, United States
Background: Chuvash erythrocytosis is an inherited disorder prevalent among people in the Chuvash Republic of the Russian Federation. The disorder is caused by a homozygous R200W substitution in the von Hippel Lindau (VHL) gene. The mutation impairs binding of pVHL to hypoxia‐inducible factor 1‐alpha (HIF‐1α) and HIF‐1β; resulting elevated HIF‐1 upregulates the hypoxic response. Upregulation of the hypoxic response produces increased concentrations of erythropoietin, the hormone that promotes production of red blood cells; elevated erythropoietin causes erythrocytosis. Affected individuals experience increases in stroke and other thrombotic events. Importantly, increased thrombosis is unrelated to the increased concentration of hemoglobin.
Aims: We found that hypoxia downregulates expression of the anticoagulant Protein S (PS). We seek to determine whether upregulation of HIF‐1 in patients with Chuvash erythrocytosis causes diminished amounts of PS, which may explain the increased risks of arterial and venous thromboembolic events.
Methods: Enzyme‐linked immunosorbent assays (ELISA) were performed to measure total PS concentration in Chuvash erythrocytosis and Chuvash control plasma. Immunoblotting was used to confirm whether that PS levels in Chuvash plasma correlated at the protein expression level. We are planning to obtain citrated plasma samples to perform thrombin generation assays.
Results: By ELISA, total PS as a percent of normal for Chuvash plasma had a mean value of 76.56% (n = 25) compared with a mean of 100.7% for control plasma (n = 22). This significant decrease in total PS was confirmed by immunoblotting.
Conclusion(s): Our results indicate that persons with Chuvash erythrocytosis have significantly lower total PS, a condition that may contribute to a hypercoagulable state. We will perform thrombin generation assays on Chuvash erythrocytosis plasma to determine whether coagulability returns to normal with addition of PS. If confirmed, the findings may suggest a path for PS‐centered treatment and intervention to combat thrombosis in hypercoagulable patients with Chuvash erythrocytosis.
OC 55.5
Prothrombin circulates in human plasma in multiple covalent forms with differing activities
M. Schreuder; D. Butera; J. Chiu; P. Hogg
Centenary Institute, University of Sydney, Sydney, New South Wales, Australia
Background: Disulfide bonds are covalent links between two cysteine residues and their formation was thought to be complete in the mature functioning protein. Recent studies demonstrated that circulating fibrinogen and human platelet αIIbβ3 integrin are constitutively produced in multiple disulfide‐bonded states where different disulfides are unformed in different molecules. Here, we examine the disulfide‐bonded state of the coagulation protein prothrombin which is proteolytically converted into the key protease thrombin.
Aims: To determine the redox state of the 12 disulfide bonds in circulating human prothrombin and to define the functions of the different prothrombin covalent states.
Methods: The redox state of disulfide bonds in prothrombin from healthy donors or cultured cells was assessed using differential cysteine labelling and mass spectrometry. Prothrombin variants comprising a single ablated disulfide bond by replacing both Cys with Ala were produced in HEK293 cells.
Results: Six disulfide bonds are unformed in ~1 in 10 to ~1 in 5 molecules of prothrombin from the plasmas of healthy donors. Recombinant prothrombin from HEK293 cells displayed an identical redox state. To assess the functional consequences of these unformed bonds, the six disulfide bond prothrombin mutants were produced. Ablation of one disulfide bond did not significantly alter the redox state of other disulfide bonds, indicating that disulfide formation in prothrombin is not interdependent. Functional assessment of prothrombin variants by the prothrombin time assay indicated that the clotting potential of four disulfide bond‐variants are reduced by ~2‐30‐fold compared to wild‐type. Furthermore, the efficiency of hydrolysis of chromogenic substrate for 2 out of 4 thrombin mutants are decreased by 2‐5‐fold.
Conclusion(s): These findings indicate that prothrombin circulates in multiple covalent forms in human plasma. Functional assays suggest that the covalent forms are less effectively converted to thrombin and exhibit differing proteolytic activities. Potentially, these covalent forms could have distinct functions towards different thrombin ligands.
OC 67.4
Congenital factor VII deficiency in a large cohort of Portuguese patients—molecular profiling, pathogenicity stratification and genotype‐phenotype correlation
C. Silva Pinto 1; I. Rocha2; C. Catarino3; R. Salvado4; M. Costa5; F. Rodrigues6; S. Nobre Fernandes7; C. Geraldes8; T. Fidalgo9
1 Functional Unit of Molecular Haematology ‐ Clinical Haematology Service, CHUC, Coimbra, Coimbra, Portugal; 2 Molecular Hematology Laboratory ‐ Clinical Hematology Service, CHUC, Coimbra, Coimbra, Portugal; 3 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisbon, Lisboa, Portugal; 4 Blood and Transfusion Medicine Department, Hemophilia Reference Centre, Centro Hospitalar e Universitário de Coimbra, Coimbra, Coimbra, Portugal; 5 Immunohemotherapy Service, Hospital de Viseu, Viseu, Viseu, Portugal; 6 Immunohemotherapy Service, Hospital Santa Maria, CHLN, Lisbon, Lisboa, Portugal; 7 Immunohemotherapy Service, Hospital de S. João, Porto, Porto, Portugal; 8 Clinical Hematology Department, Centro Hospitalar e Universitário de Coimbra, Coimbra, Coimbra, Portugal; 9 Unidade Funcional de Hematologia Molecular, Serviço de Hematologia Clínica, Coimbra, Coimbra, Portugal
Background: Congenital factor VII deficiency (FVIID), the most frequent autosomal recessive rare bleeding disorder, displays wide molecular heterogeneity and poor correlation between FVII plasma levels and bleeding phenotype. The need for establishing clinical significance for a variant requires its stratification according to ACMG recommendations, allowing a more accurate interpretation and classification.
Aims: To analyze and classify based on current criteria, F7 variants identified in a large cohort of patients diagnosed over 26 years with FVIID and establish genotype‐phenotype correlation.
Methods: A cohort of 404 patients of Portuguese origin [175F:229M; median age:27y(0d,89y)] diagnosed with FVIID (FVII:C 0‐58%; NR:60‐143%) were genotyped for variants and modulating polymorphisms [rs510317,rs510335, rs5742910, rs561241, rs6039, c.795_805+26[6/7](VNTR[6/7]) and rs6046] in the F7 by PCR/Direct Sanger sequencing/MLPA. Statistical analysis: one‐way ANOVA.
Results: In this cohort, 185/404 patients (46%) presented variants. In total, 33 different rare variants were identified: 11 had never been reported. ACMG guidelines allowed variant classification as distributed in table 1, including novel. The most frequent variants were: c.1109C > T(p.Cys370Phe) (30%) and c.430+1G > A (23%), both classified as pathogenic and in heterozygosity associated with FVII:C 31.6 ± 10.9% and 29.2 ± 7.6%, respectively.
Conclusion(s): These data showed that homozygous and compound heterozygous patients had lower FVII:C levels and were associated with severe clinical phenotypes(Table 1/Figure 1). The most severe hemorrhagic events occurred in young children (newborn,3‐month‐old) both homozygous for c.430+1G > A, that died following cerebral hemorrhage. In these cases, prenatal diagnosis was carried out. Thus, functional studies allowed the diagnosis of moderate/severe FVIID. Conversely, mild FVIID showed an overlap of FVII levels associated with heterozygous pathogenic variants and polymorphisms, and molecular studies were essential to distinguish between these two groups. In conclusion, variant classification was crucial to the utility of molecular diagnosis in clinical practice and was a powerful tool that ensured a better understanding of genotype‐phenotype correlations in FVII deficient Portuguese patients, allowing more assertive counseling and therapy.
OC 36.4
A tethered artificial protease reveals the location of key regulatory motifs in factor V
F. Ayombil 1; R. Toso1; R. Camire2
1 Division of Hematology and the Raymond G. Perelman Center for Cellular and Molecular Therapeutics, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 2 Division of Hematology and the Raymond G. Perelman Center for Cellular and Molecular Therapeutics, The Children’s Hospital of Philadelphia. Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, Philadelphia, Pennsylvania, United States
Background: Conserved acidic (AR2, 1493‐1537) and basic (BR, 963‐1008) regions within the FV B‐domain play a key role in regulating cofactor function. Elimination of BR through splicing (e.g., FV‐short) or proteolytic removal of BR and AR2 produce an active cofactor. Biochemical evidence suggests AR2, and BR form a tight interaction. The role of AR1 (656‐697) at the C‐terminus of the heavy chain (HC) is not defined.
Aims: To investigate the spatial relationships between AR1, AR2 and BR.
Methods: FV‐short or FV‐810 were incubated with BR fragments (FV‐BR or TFPIα‐BR) tethered to an artificial protease, FeBABE (Fe‐p‐bromoacetamidobenzyl‐EDTA). Once activated, FeBABE cuts proteins if near ( < 12 Å) the contact site of the target protein. FV cleavage products were identified by immunoblotting and N‐terminal sequencing.
Results: BR‐FeBABE bound FV‐short or FV‐810 with high affinity. When activated, BR‐FeBABE cut FV‐short and FV‐810 to yield distinct proteolytic fragments (~95 kDa and ~190 kDa) derived from the C‐terminus of the A2‐ and B‐domains. Using truncated HC derivatives of FVa, we found that cleavage within the A2 domain occurs near residues 675‐685 which is part of AR1. Additional blotting and N‐terminal sequencing revealed that cleavage yielding the ~190 kDa band is within AR2. These data suggest that BR engages both AR1 and AR2 and defines a key epitope for this functional unit. In control experiments, acetylated BR‐FeBABE, free hydroxy radicals, or titration with unlabeled BR fragments, revealed that cleavage of FV‐short and FV‐810 was specific. Further, in competitive studies, FXaS195A, APC‐DEGR, and TFPIα all inhibited cleavage of FV‐short and FV‐810 by BR‐FeBABE consistent with their high affinity binding to cofactor‐like species.
Conclusion(s): These studies document a physical interaction between BR and AR1/AR2, and help define how these key functional landmarks regulate the expression of cofactor activity when FV is converted to FVa or when FV‐short is bound to TFPIα.
OC 44.4
High plasma levels of C1 esterase inhibitor reduce intrinsic pathway‐initiated coagulation and are associated with reduced future risk of venous thromboembolism
S. Grover 1; O. Snir2; K. Hindberg2; T. Englebert1; S. Brækkan3; Z. Pólai4; V. Morelli5; H. Farkas4; S. Jensen2; A. Wolberg1; T. Mollnes6; T. Ueland6; N. Mackman1; J. Hansen3
1 University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States; 2 UiT – The Arctic University of Norway, Tromso, Troms, Norway; 3 Thrombosis Research Center, Department of Clinical Medicine, UiT ‐ The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway; 4 Semmelweis University, Budapest, Pest, Hungary; 5 UiT ‐ The Arctic University of Norway, Tromsø, Norway, Tromsø, Troms, Norway; 6 University of Oslo, Oslo, Oslo, Norway
Background: C1 esterase inhibitor (C1INH) is a broad acting serine protease inhibitor that, as a major endogenous inhibitor of factor (F) XIIa, FXIa and plasma kallikrein, may have important anticoagulant functions in the setting of venous thromboembolism (VTE).
Aims: In this study, we investigated the effect of exogenous and endogenous C1INH on plasma thrombin generation and the association between plasma C1INH levels and risk of future VTE in a large, nested case‐control cohort.
Methods: Thrombin generation was evaluated in normal human plasma supplemented with purified C1INH by calibrated automated thrombography. Thrombin generation was also assessed in plasma from patients with hereditary angioedema (HAE) caused by C1INH deficiency (n = 20) and compared to age and sex matched controls (n = 20). In the nested case‐control cohort, containing 405 VTE patients and 829 age and sex matched controls derived from the Tromsø 4 study, plasma C1INH levels were determined by immunoassay. Logistic regression analysis was used to determine the odds ratios (ORs) for deep vein thrombosis (DVT) and VTE across C1INH quartiles.
Results: Addition of exogenous C1INH dose‐dependently inhibited intrinsic pathway‐initiated thrombin generation (p < 0.0001) but not tissue factor‐initiated thrombin generation (p > 0.05). In contrast, plasma from patients with HAE, particularly those with C1INH activity < 25% of normal, supported increased intrinsic pathway‐initiated thrombin generation compared to controls (p < 0.05). In the nested case‐control study, high plasma C1INH levels were associated with lowered ORs for DVT and VTE. Subjects with plasma C1INH levels in the highest quartile had an OR of 0.68 (95% CI, 0.49‐0.96) for DVT and 0.58 (95% CI, 0.39‐0.89) for VTE when compared to individuals with C1INH in the lowest quartile.
Conclusion(s): Plasma C1INH demonstrates significant anticoagulant activity in intrinsic pathway‐initiated thrombin generation. Consistent with the anticoagulant activity of C1INH high plasma levels of C1INH were associated with reduced risk of VTE.
OC 36.2
Hydrophobic patch (PLVIVG 1481‐1486) in FV‐short crucial for its synergistic TFPI‐cofactor activity with protein S and the formation of a FXa‐inhibitory complex comprising FV‐short, protein S and TFPI
B. Dahlback; S. Tran
Lund University, Malmö, Skane Lan, Sweden
Background: The natural factor V (FV) splice isoform FV‐Short (FV756‐1458) functions in synergy with protein S as a TFPIα‐cofactor in inhibition of FXa. TFPIα binds to an exposed acid region (AR2; 1493‐1537) in the B domain and protein S binds to the FV‐Short/TFPIα‐complex. The preAR2 (1458‐1492) is crucial for the synergistic TFPIα‐cofactor activity between FV‐Short and protein S and for assembly of a trimolecular FXa‐inhibitory complex between FV‐Short, protein S and TFPIα.
Aims: To identify which part of preAR2 is required for the synergistic TFPIα‐cofactor activity between FV‐Short and protein S and the assembly of the FXa‐inhibitory complex.
Methods: New FV‐Short truncation variants FV709‐1476, FV712‐1478, FV712‐1481, FV712‐1484, FV712‐1487, and FV712‐1490 were created and tested in the FXa‐inhibition assay to identify the site required for the synergistic TFPIα cofactor activity between protein S and FV‐Short. A microtiter‐based assay analyzed the binding between FV‐Short variants, protein S and TFPIα.
Results: Three of the FV‐Short variants (FV709‐1476, FV712‐1478, FV712‐1481) were fully active as synergistic TFPIα cofactors with protein S. FV712‐1484 showed intermediate activity and FV712‐1487 and FV712‐1490 were inactive. Although TFPIα bound to all FV‐Short variants in the absence of protein S, strongest binding was observed with FV712‐1478 and FV712‐1481. In the absence of TFPIα no direct binding of protein S was observed to any of the FV‐Short variants. However, in presence of TFPIα, efficient cooperative binding was demonstrated between protein S, TFPIα and FV709‐1476, FV712‐1478, or FV712‐1481. In contrast, even in the presence of TFPIα, no binding of protein S was observed when FV712‐1484, FV712‐1487, or FV712‐1490 were tested.
Conclusion(s): A short hydrophobic patch in preAR2 (PLVIVG, 1481‐1486) in FV‐Short is crucial for the synergistic TFPIα‐cofactor activity between FV‐Short and protein S and for the cooperative assembly of a trimolecular FXa‐inhibitory complex between FV‐Short, protein S and TFPIα. FIGURE 1 (A) Cooperative interaction between FV‐Short, protein S and TFPI as basis for efficient FXa inhibition. (B) Hydrophobic patch PLVIVG (1481‐1486) in FV‐Short crucial for function
FIGURE 1 (A) Cooperative interaction between FV‐Short, protein S and TFPI as basis for efficient FXa inhibition. (B) Hydrophobic patch PLVIVG (1481‐1486) in FV‐Short crucial for function
OC 36.1
The CLEC4M locus participates to the regulation of FV plasma levels: Insights from a new genome‐wide association study conducted in 4,373 participants
D. Tregouet 1; A. Martinez‐Perez2; M. Kleber3; A. van Hylckama Vlieg4; M. Germain5; J. Deleuze6; J. Souto2; N. Smith7; M. Sabater‐Lleal8; G. Delgado9; F. Rosendaal10; P. Morange11
1 INSERM UMR1219 Bordeaux Population Health Research Center, Bordeaux, Aquitaine, France; 2 Genomics of Complex Diseases Group, Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau, Barcelona, Catalonia, Spain; 3 Department of Medicine, University Heidelberg, Mannheim Medical Faculty, Mannheim, Baden‐Wurttemberg, Germany; 4 Leiden University, Medical Center, Leiden, Zuid‐Holland, Netherlands; 5 University Bordeaux, INSERM UMR 1219, Bordeaux Population Health Research Center, Bordeaux, Aquitaine, France; 6 National Center of Human Genomics Research, Evry, Ile‐de‐France, France; 7 Department of Epidemiology, University of Washington; Department of Health Service, University of Washington; Seattle Epidemiologic Research and Information Center; Department of Veterans Affairs, Office of Research and Development, Seattle, WA 98105, USA, Seattle, Washington, United States; 8 Sant Pau Biomedical Research Institute (IIB Sant Pau), Barcelona, Spain; Cardiovascular Medicine Unit, Department of Medicine, Karolinska Institutet, Stockholm, Sweden, Barcelona, Catalonia, Spain; 9 V Medicine Clinic, University of Heidelberg, Mannheim, Baden‐Wurttemberg, Germany, 10 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands, 11 C2VN, INRAE, INSERM Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: Factor V (FV) is a key molecular player in the coagulation cascade. FV plasma levels have been associated with several human diseases, including thrombosis, bleeding and diabetic complications. Two genes have been robustly found to contribute in the inter‐individual variability of plasma FV levels: structural F5 gene and PLXDC2, a gene that has been recently identified in a genome wide association study (GWAS) conducted in 510 individuals.
Aims: To identify new genetic factors that influence the variability of FV plasma levels
Methods: A meta‐analysis of 5 independent GWAS totaling 4,373 participants of European ancestry was conducted using the inverse‐variance methodology
Results: Three loci reached the genome‐wide significance level of 5 x 10‐8 including the two already known loci: F5 with lead variant rs6027 (β = ‐0.151 ± 0.008; p = 1.97 10‐76 ) and PLXDC2 with lead variant rs927826 (β = ‐0.056 ± 0.005; p = 2.36 10‐31). One novel locus was detected, CLEC4M, with lead variant rs594793 (β = ‐0.025 ± 0.004; p = 2.22 10‐8) whose association with FV plasma levels replicated in an independent sample of 1,019 individuals (β = ‐0.022 ± 0.009; p = 0.022). Conditional analysis did not reveal any additional genome‐wide significant signal at the CLEC4M locus. Interestingly, the association of rs594793 with FV plasma levels was unaltered after adjusting for plasma levels of von Willebrand factor (VWF), which have also been found to be influenced by genetic variants at CLEC4M. Consistently, the two CLEC4M polymorphisms, rs571497 and rs2277998, which have been associated with VWF plasma levels were in modest linkage disequilibrium with the rs594793 (r2 = 0.14 and r2 = 0.49) and did not exhibit a strong association with FV plasma levels (p = 6.2 10‐4 and p = 1.9 10‐3)
Conclusion(s): This work identifies CLEC4M as a novel locus contributing to the regulation of FV plasma levels through VWF‐independent mechanisms, and this locus deserves to be more fully explored
OC 36.5
The TFPIα C‐terminal tail is essential for the synergistic enhancement of TFPIα mediated inhibition of FXa by protein S and FV‐short
M. Gierula 1; V. Noakes2; I. Salles‐Crawley3; J. Crawley2; J. Ahnström2
1 Imperial College, London, England, United Kingdom; 2 Imperial College London, London, England, United Kingdom; 3 St George's University of London, London, England, United Kingdom
Background: TFPIα is dependent on its synergistic cofactors, protein S (PS) and factor (F)V‐short, for efficient inhibition of FXa. Both cofactors interact with TFPIα, PS through Kunitz 3 residues including Arg199 and Glu226 and FV‐short with the TFPIα C‐terminus. While the TFPIα‐PS interaction is needed for enhancement by PS alone, it is not clear whether direct interactions between TFPIα and both its cofactors are needed for the synergistic enhancement.
Aims: Determine the importance of the TFPIα‐PS and TFPIα‐FV‐short interactions for synergistic enhancement of FXa inhibition.
Methods: FXa inhibition assays were used to study TFPIα enhancement in the presence and absence of PS and/or FV‐short. TFPIα variants unable to bind either PS (R199Q/E226Q) or FV‐short (ΔCT, aa 1–249), were used to investigate how abolished interactions with either cofactor affected their ability to enhance TFPIα, alone or in synergy.
Results: Both TFPIα R199Q/E226Q and TFPIα ΔCT inhibited FXa, with ΔCT showing moderately reduced activity (<2‐fold) compared to WT. In the absence of FV‐short, PS enhanced WT TFPIα with an EC50 of 42 ± 12 nM with only minimal enhancement of R199Q/E226Q. However, when PS was titrated in the presence of FV‐short (2 nM), FXa inhibition by TFPIα R199Q/E226Q was efficiently enhanced by PS (EC50: 2.9 ± 0.77 and 1.55 ± 0.29 for WT and R199Q/E226Q, respectively), suggesting that FV‐short rescues the synergistic enhancement when the TFPIα‐PS interaction is absent. The inhibition of FXa by TFPIα ΔCT was not enhanced by increasing concentration of FV‐short (0–4 nM). In contrast to TFPIα R199Q/E226Q, the addition of PS had little effect on the TFPIα ΔCT‐mediated inhibition of FXa.
Conclusion(s): The lack of enhancement of TFPIα by PS alone, observed in the absence of a TFPIα‐PS interaction, can be rescued by the presence of FV‐short. In contrast, an interaction between FV‐short and TFPIα is essential for the synergetic enhancement by PS and FV‐short and full anticoagulant function of TFPIα.
OC 55.1
Retrotransposon insertions: A challenging detection and characterization solved by long‐read nanopore sequencing. Relevance in antithrombin deficiency
B. de la Morena‐Barrio 1; J. Cuencia‐Guardiola2; P. Garrido‐Rodríguez3; A. Sanchis‐juan4; M. de la Morena‐Barrio3; R. Cifuentes‐Riquelme3; C. Bravo‐Pérez5; J. Padilla6; A. Miñano5; F. Vidal7; M. Lozano3; W. Ouwehand8; J. Fernández‐Breis2; J. Corral3
1 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain, Murcia, Murcia, Spain; 2 Departamento de Informática y Sistemas, Universidad de Murcia, CEIR Campus Mare Nostrum, IMIB‐Arrixaca, Facultad de Informática, Campus de Espinardo, 30100, Murcia, Spain., Murcia, Murcia, Spain; 3 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 4 Department of Haematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK., Cambridge, England, United Kingdom; 5 Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 6 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain., Murcia, Murcia, Spain; 7 Coagulopaties congènites. Banc de Sang i Teixits. Barcelona. Unitat de Diagnòstic i Teràpia Molecular. Vall d’Hebron Institut de Recerca, Universitat Autònoma de Barcelona (VHIR‐UAB). Barcelona., Barcelona, Catalonia, Spain; 8 Department of Haematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK. Department of Haematology, University College London Hospitals, London, NW1 2BU, UK, Cambridge, England, United Kingdom
Background: Retrotransposons are repetitive mobile sequences of DNA spread throughout the genome. Retrotransposon insertions (RI) are important for genome evolution, but they can also trigger diseases. As current molecular technologies hardly detect RI, their localization in the genome is largely unknown, and their role in genetic diseases could be underestimated. Antithrombin deficiency (ATD) is a dominant monogenic disorder caused by defects in SERPINC1, although causes are not yet known in up to 20% of cases.
Aims: To use long‐read whole genome nanopore sequencing (LR‐WGS) to identify RI in the human genome, particularly in SERPINC1, as cause of ATD.
Methods: LR‐WGS, done on the PromethION platform, was performed in 24 unrelated patients with ATD, 14 with gross SERPINC1 defects, and 10 with unknown defects. A bioinformatic pipeline was developed to detect, localize, and characterize RI in the genome. Validation of SERPINC1 RI was done by PCR and sequencing.
Results: An insertion of 2.4Kb in intron 6 of SERPINC1 not reported in GnomAD was identified in 2 ATD patients with unknown cause. De novo assembly of the inserted sequence revealed a new SVA retrotransposon, antisense oriented, with a tandem site duplication of 14bp. These data and phasing results supported a founder effect. Primers flanking the breakpoint did not amplify the insertion, which was only verified using SVA inner and SERPINC1 flanking primers. Pedigree studies confirmed the segregation of RI with ATD. Whole genome analysis of RI, revealed a median of 396 polymorphic or novel RI/patient, mainly SINE. A median of 230 RI/patient directly affected genes, 12 of them in coding regions.
Conclusion(s): LR‐WGS allowed the first detection and the complete characterization of a new SVA insertion in SERPINC1 causing ATD. A novel pipeline for localization, and characterization of RI in the genome has been developed which can potentially identify pathogenic elements. PI21/00174, PMP21/00052 (ISCIII&FEDER).
Contact Pathway
LB 01.5
Antiplatelet effects of inhibiting coagulation factor XI in a non‐human primate model of atherosclerosis
H. Vu1; T. Kohs1; J. Pang1; K. Jordan1; T. Zheng1; P. Kievit2; J. Johnson1; M. Wallisch3; M. Hinds1; C. Puy1; C. Lorentz3; E. Tucker4; D. Gailani5; J. Aslan1; J. Shatzel1; O. McCarty6; I. Parra‐Izquierdo 1
1 Oregon Health & Science University, Portland, Oregon, United States; 2 Oregon National Primate Research Center, Portland, Oregon, United States; 3 Aronora, Inc., Portland, Oregon, United States; 4 Aronora, Inc, Portland, Oregon, United States; 5 Vanderbilt University, Nashville, Tennessee, United States; 6 Oregon Health and Science University, Portland, Oregon, United States
Background: Inhibition of coagulation factor (F)XI represents a potentially safe and effective strategy to treat thromboinflammatory conditions in which platelets play a prominent role, including atherosclerosis. We recently generated evidence from a proof‐of‐concept phase 2 study showing that FXI inhibition may reduce inflammation in patients undergoing hemodialysis. Moreover, our previous studies demonstrated that FXI inhibition decreased inflammation in a non‐human primate (NHP) model of sepsis and mitigated atherosclerosis development in a low‐density lipoprotein receptor‐deficient mice model.
Aims: To determine the effects of inhibiting FXI on systemic platelet function in a diet‐induced NHP model of atherosclerosis.
Methods: NHPs were fed a standard chow diet (lean) or high fat diet (obese) for at least 24 months. Following establishment of early atherosclerosis, a function blocking FXI antibody was administered to obese NHPs. Blood was drawn at baseline, 1, and 7 days after treatment to assess platelet function in whole blood.
Results: Platelets from obese NHPs showed increased P‐selectin and phosphatidylserine exposure in response to platelet hemostatic agonists targeting glycoprotein VI (GPVI) and protease‐activated receptors (PAR‐1), compared to platelets from lean NHPs. GPVI and PAR‐1 agonists also promoted increased platelet‐leukocyte aggregates (PLA) formation in whole blood from obese NHPs. Following treatment with a FXI function‐blocking antibody, activated partial thromboplastin time increased in obese NHPs. Inhibition of FXI in obese NHPs decreased platelet sensitivity to GPVI and PAR‐1 agonists 1 and 7 days after treatment, compared to basal levels. Moreover, FXI inhibition reduced PLA formation following stimulation with agonists. Conversely, phosphatidylserine exposure following antibody treatment remained unchanged compared to basal activation levels.
Conclusion(s): Atherosclerotic NHPs showed an increase in systemic platelet sensitivity to hemostatic agonists compared to healthy NHPs. Inhibition of FXI reversed the increased platelet sensitivity and reduced PLA formation in obese NHPs, suggesting that FXI may modulate exacerbated platelet function characteristics of cardiovascular disease.
OC 03.5
An integrative biology approach to model the role of the feedback activation of FXI by thrombin in the tissue factor pathway
H. Lakshmanan1; A. Estonilo2; S. Reitsma3; A. Melrose3; T. Zheng3; J. Maddala4; D. Gailani5; P. Jurney2; C. Puy3; O. McCarty 6
1 Oregon Health & Science University, PORTLAND, Oregon, United States; 2 San Jose State University, San Jose, California, United States; 3 Oregon Health & Science University, Portland, Oregon, United States; 4 West Virginia University, Morgantown, West Virginia, United States; 5 Vanderbilt University, Nashville, Tennessee, United States; 6 Oregon Health and Science University, Portland, Oregon, United States
Background: Blood coagulation is a network of biochemical reactions regulated in part by the feedback activation of zymogens and inhibitors by the serine protease thrombin. While experiments using purified components enabled the discovery of each component in the reaction network, an integrative systems biology approach is required to understand the interplay between the components of the coagulation system.
Aims: Our goal is to delineate the contribution of the feedback activation of coagulation factor XI (FXI) by thrombin to the tissue factor (TF)‐mediated thrombin generation through an integrative biology approach of systems‐based modeling and experiments.
Methods: We improved the robustness of the existing ordinary differential equation‐based model of the TF pathway of thrombin generation, the Hockin‐Mann (HM) model, through integrating mathematical modeling and in vitro experiments. Thrombin generation measured in plasma using fluorogenic substrate‐based assay revealed that the contribution of FXI to thrombin generation depended on the concentration of TF or activated coagulation factor X (FXa) used to initiate coagulation. Using this experimental data, we modified the kinetic parameters of the HM model and extended it to include the reaction kinetics of feedback activation of FXI by thrombin to construct the extended (ext.) HM model.
Results: Simulations of thrombin generation using the ext.HM model revealed that the contribution of FXI to the TF pathway of thrombin generation can be eliminated by the selective removal of the inhibitory function of tissue factor pathway inhibitor (TFPI), a Kunitz‐type serine protease inhibitor of FXa and TF‐activated coagulation factor VII (FVIIa) complex. We validated the predictions made using the ext.HM model via measuring thrombin generation in plasmas depleted of FXI in the presence of function‐blocking antibodies against TFPI.
Conclusion(s): Together, our work is a demonstration of the utility of integrating computational systems biology and experiments in understanding complex biochemical reaction networks.
OC 03.4
Glycated albumin modulates the contact system with implications for the kallikrein‐kinin and intrinsic coagulation systems
L. Hardy 1; D. Bohinc2; K. Bane3; S. Heal4; E. Hethershaw5; M. Ali4; R. Foster4; C. Longstaff6; T. Renne7; E. Stavrou8; H. Philippou4
1 Leeds Institute of Cardiovascular & Metabolic Medicine (LICAMM), University of Leeds, Leeds, West Yorkshire, United Kingdom, Leeds, England, United Kingdom; 2 Case Western Reserve University, Cleveland, Ohio, United States; 3 CWRU School of Medicine, Cleveland, Ohio, United States; 4 University of Leeds, Leeds, England, United Kingdom; 5 Unversity of Leeds, Leeds, England, United Kingdom; 6 National Institute for Biological Standards and Control, Hertfordshire, England, United Kingdom; 7 Institute for Clinical Chemistry and Laboratory Medicine, Eppendorf, Hamburg, Germany; 8 Case Western Reserve University School of Medicine, Cleveland, Ohio, United States
Background: Factor(F) XII is a coagulation factor at the frontier of coagulation, inflammation, innate immunity, and fibrinolysis. Misfolded proteins are factor XII (FXII) activators that can trigger the proinflammatory kallikrein‐kinin system (KKS) and the intrinsic pathway of coagulation, via plasma kallikrein (PKa) and activated Factor XI (FXIa), respectively. The chronic hyperglycaemic conditions in diabetes mellitus (DM) patients induce a non‐enzymatic Maillard reaction that denatures plasma proteins to form advanced glycation end products (AGEs) which accumulate within the vasculature and tissues.
Aims: Human serum albumin (HSA) is the most abundant protein in plasma and is sensitive to glycation in vivo. We investigated the relationship between plasma glycation levels and activation of the contact pathway in diabetic patients and characterised the effects of HSA‐AGE on FXII, PK, FXI activation, and thrombus formation.
Methods: Plasma was obtained from 10 DM patients (2 T1DM, 8 T2DM), 10 age‐matched euglycemic volunteers and were subjected to immunoblotting for activated FXII(a) and cleaved high molecular weight kininogen (cHK). Plasma PKa activity was measured via chromogenic assay. Kinetic modulation of FXII, PK, FXI and their respective full active serine proteases by in vitro generated HSA‐AGE were explored using chromogenic assays, plasma clotting assays, and an in vitro flow model using whole blood.
Results: Plasma obtained from DM patients has increased plasma AGEs, FXIIa, cHK, along with increased plasma PKa enzymatic activity levels which positively correlated with HbA1C levels. In vitro generated HSA‐AGE triggered FXIIa‐dependent PKa activation but limits procoagulant intrinsic pathway activation by directly inhibiting FXIa, and separately, activated Factor IX (FIXa)‐dependent Factor X (FX) activation in plasma.
Conclusion(s): These data suggest a proinflammatory role of AGEs in the pathophysiology of DM via FXII and KKS activation. The expected procoagulant signal from FXII activation was lost through the inhibition of FXIa and (FIXa)‐dependent Factor X (FX) activation.
OC 74.3
Characterization and dissection of new structural variants in FXI deficiency by nanopore sequencing
B. de la Morena‐Barrio 1; Á. Palomo2; A. Rubio3; J. Padilla4; L. Martin5; R. Cifuentes‐Riquelme6; C. Bravo‐Pérez3; A. Miñano3; V. Vicente6; M. Lozano6; F. vidal7; J. Corral6; M. de la Morena‐Barrio6
1 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain, Murcia, Murcia, Spain; 2 Centro Materno‐Infantil del Hospital Regional Universitario Carlos de Haya, Málaga, Málaga, Andalucia, Spain; 3 Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 4 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain., Murcia, Murcia, Spain; 5 Unitat de Genòmica de Malalties Complexes. IIB‐Sant Pau. Barcelona., Barcelona, Catalonia, Spain; 6 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 7 Coagulopaties congènites. Banc de Sang i Teixits. Barcelona. Unitat de Diagnòstic i Teràpia Molecular. Vall d’Hebron Institut de Recerca, Universitat Autònoma de Barcelona (VHIR‐UAB). Barcelona., Barcelona, Catalonia, Spain
Background: Molecular diagnosis of FXI deficiency, usually restricted to F11 sequencing, has identified up to 280 variants, mostly SNVs or Indels. Only 3 causative structural variants (SV) have been reported: two whole gene deletions and one partial deletion.
Aims: Identification and characterization of new SVs causing FXI deficiency.
Methods: We studied 272 patients with FXI deficiency from 105 families identified by prolonged aPTT and characterized by functional and immunological methods. F11 was sequenced and SVs were identified by MLPA. The characterization of SVs was done by long‐read nanopore sequencing using real‐time enrichment methods in a MinION device. Validation involved PCR and NGS/sanger sequencing.
Results: Two cases with CRM‐ deficiency and no F11 defects detected by sequencing, carried heterozygous SVs identified by MLPA. P1 had a duplication of exons 8&9 located in intron 4, that segregated in 5 family members (FXI:C = 44.3%). P2, with severe deficiency (FXI:C = 5%), had a complete deletion of F11 in one allele, and a deleterious variant of maternal origin in the other allele (p.Phe295Cys). His father and sister had normal FXI levels and no F11 defects. STR analysis confirmed paternity. Deep NGS of F11 ( > 2000 reads) in 2 tissues of the patient discarded a mosaicism. Nanopore sequencing accurately defined the extension of the deletion (7 MB, 142 genes) and duplication (1648 bp); and characterized the breakpoints, identifying repetitive elements (RE) in both cases. (Figure 1 and Figure 2)
Conclusion(s): SVs affecting F11, probably formed by recombination of RE, are heterogeneous in type and extension and may be relatively frequent (2%) in FXI deficiency. We detected the first de novo deletion of F11, which probably occurred during the spermatogenesis. These data support the screening of SVs affecting F11 in FXI deficiency. MLPA is able to detect SVs, but nanopore sequencing is the best method to characterize at nucleotide level these SVs. PI21/00137 (ISCIII&FEDER)
OC 74.4
Identification of a site on Factor XII required for interactions with polyphosphate and other soluble polyanions
A. Shamanaev 1; M. Litvak2; S. Dickeson2; D. Gailani3
1 Vanderbilt University Medical Canter, Nashville, Tennessee, United States; 2 Vanderbilt University Medical Center, Nashville, Tennessee, United States; 3 Vanderbilt University, Nashville, Tennessee, United States
Background: During contact activation, the plasma protein FXII binds to polyanionic surfaces through its heavy chain, and undergoes autocatalytic conversion to the protease FXIIa. Previously we showed that the first epidermal growth factor (EGF1) domain of FXII is required for autoactivation on polyphosphate, kaolin and ellagic acid. EGF1 contains twelve basic amino acids including a patch of highly conserved lysines residues that could mediate polyanion‐binding.
Aims: Identify FXII amino acids involved in surface‐binding and autoactivation.
Methods: FXII with alanine replacements for Lys73, Lys74 and Lys76, alone (FXII‐Ala73, FXII‐Ala74, FXII‐Ala76) or in combination (FXII‐Ala73‐76) were expressed. FXII‐ with EGF1 replaced with EGF1 from the FXII‐homolog Pro‐HGFA (FXII‐EGF1) served as control. FXII autoactivation was assessed with negatively charged substances (polyphosphate, dextran sulfate, heparin, ellagic acid, kaolin, and silica). Binding was assessed by pull‐down experiments with heparin and kaolin.
Results: FXII autoactivated on all substances tested while FXII‐EGF1 did not. While FXII‐Ala73, FXII‐Ala74, FXII‐Ala76 autoactivated similarly to FXII on all surfaces, FXII‐Ala73‐76 failed to autoactivate with polyphosphate, dextran sulfate or heparin. FXII‐Ala73‐76 autoactivated with ellagic acid, kaolin, and silica. FXII‐Ala73, FXII‐Ala74, FXII‐Ala76 bound heparin more weakly than FXII, while FXII‐Ala73‐76 did not bind heparin. All proteins appeared to bind kaolin normally.
Conclusion(s): A cluster of basic amino acids at the N‐terminus of the FXII EGF1 domain mediates binding and supports FXII autoactivation with the soluble polyanions polyphosphate, heparin and dextran sulfate. However, these residues are not required for autoactivation on insoluble surfaces such as kaolin, silica or ellagic acid. As the chimera FXII‐EGF1 fails to autoactivate on soluble and insoluble surfaces, it appears that different parts of EGF1 support autoactivation, depending on the type of surface.
OC 74.5
Factor XI and plasma kallikrein apple domain structures reveal alternate kininogen bound complexes
J. Emsley 1; C. Li1; A. Bar Barroeta2; J. Meijers3
1 University of Nottingham, nottingham, England, United Kingdom; 2 Sanquin, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Molecular Hematology, Sanquin Research, Amsterdam, the Netherlands, Department of Experimental Vascular Medicine, Amsterdam UMC, University of Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: High molecular weight kininogen (HK) is a key cofactor for appropriate assembly and activation of the contact system. How it interacts with prekallikrein (PK) and Factor XI (FXI) is unknown. The PK‐HK interaction serves as a critical exosite to facilitate efficient HK cleavage and bradykinin generation.
Aims: To understand the structure of the complex formed between HK and PK, and to compare this with the FXI‐HK complex.
Methods: Key residues involved in the HK interaction with PK and FXI were analysed by protein crystallography using a 31 amino acid HK D6 domain peptide to determine the complex structure. Interactions of the full length FXI‐HK complex were studied in solution by Hydrogen Deuterium exchange Mass Spectrometry (HDX‐MS).
Results: We determined a 2.3Å PK‐HK fragment crystal structure revealing that the HK sequence WIPDIQ (W569‐Q574) binds to the A1 domain and FNPISDFPDT (F582‐T591) to the A2 domain. The intervening HK‐peptide residues 575‐581 are not observed in the electron density and are assumed to be flexible. A 3.2Å FXI‐HK fragment crystal structure reveals an identical interaction in the A2 domain but an alternate, straightened conformation of HK with no flexible linker that extends into the A3 domain. The FXI‐HK interaction at A3 was unexpected so we analysed the full length FXI‐HK complex in solution using HDX which detected FXI interactions with A2 and A3. Unlike the PK‐HK interaction no interactions were observed between HK and the FXI A1 domain.
Conclusion(s): Although PK and FXI both bind to HK, they do so via interactions with different apple domains. Apple 2 is involved in HK binding to both PK and FXI, but an important exosite of the HK F582‐T591 sequence shifts from the A1 domain in PK to the A3 domain in FXI.
OC 03.2
The prekallikrein and factor XII heavy chains in surface‐independent reactions
P. Srivastava 1; S. Kumar1; A. Shamanaev2; M. Sun3; D. Gailani4
1 Vanderbilt University Medical Center, Nashville, Tennessee, United States; 2 Vanderbilt University Medical Canter, Nashville, Tennessee, United States; 3 Vanderbilt University Medical Center, Nashville, Tennessee, United States; 4 Vanderbilt University, Nashville, Tennessee, United States
Background: The plasma zymogens prekallikrein and factor XII (FXII) are reciprocally converted to their protease forms, kallikrein and FXIIa, when blood is exposed to surfaces by a process called contact activation. During contact activation the non‐catalytic heavy chains of prekallikrein and FXII mediate surface‐binding. Prekallikrein and FXII also undergo reciprocal activation in a surface‐independent manner. The roles of the heavy chains of these proteins in this setting are not well understood.
Aims: Determine the roles of the heavy chains and catalytic light chains of PK and Factor XII in surface‐independent reciprocal activation, and in PKa and FXIIa‐mediated proteolytic reactions.
Methods: Cysteines that connect the heavy chains and catalytic domains (CD) of prekallikrein (Cys‐364 and Cys‐484) and FXII (Cys‐340 and Cys467) were changed to serine. The resulting zymogen proteins, PK‐Ser‐364,484 and FXII‐Ser‐340,467, were compared to wild‐type FXII and PK in reciprocal activation reactions using chromogenic substrates for detection. When PK‐Ser‐364,484 and FXII‐Ser‐340,467 are activated the heavy chains and catalytic domains dissociate. Isolated catalytic domains (PKa‐CD and FXIIa‐CD) were prepared from PK‐Ser‐364,484 and FXII‐Ser‐340,467.
Results: In prekallikrein‐FXII reciprocal activation assays, replacing PK with PK‐Ser‐364,484 markedly slowed reactions, while replacing FXII with FXII‐Ser‐340,467 accelerated reactions. Kallikrein and PKa‐CD activated FXII comparably. Similarly, FXIIa and FXIIa‐CD activated PK comparably. Cleavage of the cofactor high‐molecular‐weight kininogen (HK) by PKa‐CD was significantly reduced when compared to PKa.
Conclusion(s): Our data indicate that during surface‐independent reciprocal activation of PK and FXII, the PK heavy chain is required, probably for the proper interaction with FXIIa. The FXII heavy chain, in contrast, is not required for surface‐independent FXII activation, and may actually slow the reaction by obscuring the FXII activation cleavage site. The kallikrein heavy chain is required for proper cleavage of HK, consistent with an exosite on the kallikrein heavy chain required for HK binding.
OC 03.3
Bradykinin liberation from high molecular weight kininogen by plasma kallikrein on endothelial cell membranes is modulated by ambient concentration of C1 inhibitor and prolylcarboxypeptidase
A. Merkulova1; S. Abdalian2; G. Forbes3; P. Zeng3; A. Pinheiro3; A. Schmaier 4
1 Case Western Reserve University, Clevelan, Ohio, United States; 2 Kansas City School of Medicine, Kansas City, Missouri, United States; 3 Case Western Reserve University, Cleveland, Ohio, United States; 4 Case Western Reserve University/University Hospitals Cleveland Medical Center, Cleveland, Ohio, United States
Background: What regulates bradykinin (BK) formation in the intravascular compartment?
Aims: These studies examine how variable concentrations of C1 inhibitor (C1INH) and prolylcarboxypeptidase (PRCP) influence high molecular weight kininogen (HK) cleavage and BK liberation by plasma kallikrein (PKa) formed on endothelial cells (EC).
Methods: This investigation was performed with microvascular EC and purified reagents.
Results: On EC, PK, C1INH, PRCP, and HK are expressed on non‐permeabilized EC and PK and HK, PK and PRCP, PK and C1INH, and PRCP‐C1INH colocalize. EC PK activation with HK is modulated by C1INH. Twenty to 620 nM C1INH blocks PK activation from 23% to 73% inhibition, respectively. PKa inhibitor Mab M202‐H03 or rEPI‐KAL2 (Takeda) completely blocks its formation on EC (IC50 = 10 nM) or (IC50 = 25 nM), respectively. A PRCP inhibitor (Calbiochem) or chloroquine completely inhibits PKa formation (IC50 = 10 micromolar) or (IC50 = 1.5 micromolar), respectively. Forming PKa on EC cleaves HK completely to a 65 kDa H‐chain and 46 kDa L‐chain. Two micromolar C1INH blocks 50% of cHK formation. 0.5 to 2.5 micromolar C1INH decrease BK formation on EC from 2.5 nM to 1 nM. Factor XII (FXII) does not activate when incubated with EC alone for 1 h. If incubated with EC in the presence of HK and PK, FXII becomes alpha FXIIa. Using siRNA knockdowns of PRCP, the inhibitory effect of 0.25 to 1 micromolar C1INH on PKa is magnified from 49 to 82% to 76 to 93%, respectively. When EC are transfected with a PRCP plasmid increasing PK activation by 35%, the 0.25 to 1 micromolar C1INH inhibitory effect on PKa is reduced from 36 to 93% to 0 to 55%, respectively.
Conclusion(s): These studies indicate that BK formed on EC is modulated by C1INH, PK and PRCP. These pathways are independent of activated factor XII and maybe important for the pathogenesis of the hereditary angioedemas. (Figure 1). FIGURE 1 Model for BK Formation in Normals and C1INH Deficiency
FIGURE 1 Model for BK Formation in Normals and C1INH Deficiency
OC 44.1
Activation of the plasma contact system triggers the fat embolism syndrome
S. Konrath 1; S. Hemkemeyer1; R. Mailer1; K. Püschel1; B. Ondruschka1; T. Renné2
1 University Medical Center Hamburg‐Eppendorf, Hamburg, Hamburg, Germany; 2 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany
Background: Fat embolism syndrome (FES) is a rare but severe thrombo‐inflammatory complication associated with neurologic changes, petechial rash, and thrombosis including pulmonary embolism. It occurs after major trauma with fractures of long tubular bones and involves the release of bone marrow (BM) components into the circulation. The mechanism underlying FES is poorly understood, and targeted therapy remains to be developed.
Aims: We studied the mechanism of FES and focused on the role of the procoagulant and proinflammatory factor XII (FXII)‐driven plasma contact system.
Methods: We analyzed post mortem lung tissue of FES patients by immunohistochemistry. We used chromogenic assays, real time thrombin generation analysis and clotting assays in patient and mouse plasma to assess FXII activities and tested for FXII‐driven signaling in primary and cultured endothelial cells. A murine lethal pulmonary embolism model was used to study the prothrombogenic potential of BM‐derived lipids in vivo.
Results: Synthetic, bovine and human BM‐derived lipids activated FXII and initiated coagulation time‐ and dose‐dependently. Contact system inhibitors and inherited contact factor deficiencies abrogated lipid‐induced thrombin formation. BM‐derived lipids induced plasma kallikrein activity and triggered plasma clotting in a FXII‐dependent manner. Further, BM‐derived lipids induced the generation of the proinflammatory mediator bradykinin that increased intracellular calcium levels and nitric oxide production in endothelial cells. Immunostaining of FES patient lungs revealed lipid depositions in close proximity to activated FXII and fibrin. Wild‐type mice challenged by intravenously injected BM‐derived lipids developed lethal pulmonary emboli, whereas FXII‐deficient animals were protected and survived BM‐derived lipid challenge.
Conclusion(s): We show that the procoagulant and proinflammatory FXII‐driven plasma contact system mediates FES in vitro and in vivo. Targeting the contact system presents a novel therapeutic option for FES patients.
OC 03.1
Factor XII heavy chain structure
J. Emsley 1; M. saleem2; C. Li1; H. Philippou3
1 University of Nottingham, nottingham, England, United Kingdom; 2 University of the Punjab, Lahore, Pakistan; 3 University of Leeds, Leeds, England, United Kingdom
Background: Human Factor XII is a zymogen form of coagulation protease factor XIIa which plays an important role in the initiation of intrinsic pathway of coagulation as well as inflammation through the activation of prekallikrein.
Aims: To understand how FXII recognises ligands by determining the structure of the FXII N‐terminal domains (heavy chain).
Methods: We prepared a recombinant FXII heavy chain (FXIIHC), crystallised this and determine the X‐ray structure.
Results: The FXII heavy chain (FXIIHC) crystal structure that revealed a torc shape with a head to tail interaction between the fibronectin type II domain (FnII) latch loop and the kringle domain. Contrary to current understanding, we observe a dimer of two interlocking FXIIHC torc shapes, which buries a large surface area. A structure of the isolated FnII domain suggests a FXIIHC torc structure unlatching mechanism.
Conclusion(s): The FXIIHC structure provides a holistic framework to understand how multiple domains assemble and facilitate coordinated ligand interactions with a variety of ligands including negatively charged surfaces.
Critical Care and Perioperative
OC 65.4
Idarucizumab for reversal of dabigatran: Multicenter real‐world experience
P. Barzallo Burbano 1; C. Fernández Maqueda2; M. Meijón3; N. Revilla3; M. Blanco Bañares4; B. Fernández5; R. Rodríguez González6; M. Sola Aparicio7; B. Rosado Sierra7; S. Asenjo8; I. Gutiérrez Jomarrón9; P. Pilar Llamas Sillero10; P. Prieto Martínez2; R. De Santiago Álvarez2; J. Díez Martín11; G. Pérez Rus1; C. Pascual Izquierdo11
1 Hospital General Universitario Gregorio Marañón, Madrid, Spain, Madrid, Madrid, Spain; 2 Hospital Universitario Puerta de Hierro, Madrid, Spain, Madrid, Madrid, Spain; 3 Hospital Universitario Ramón y Cajal, Madrid, Spain, Madrid, Madrid, Spain; 4 Hospital Universitario La Paz, Madrid, Spain, Madrid, Madrid, Spain; 5 Hospital Universitario de Móstoles, Madrid, Spain, Madrid, Madrid, Spain; 6 Hospital Severo Ochoa, Madrid, Spain, Madrid, Madrid, Spain; 7 Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Madrid, Spain; 8 Hospital Clínico San Carlos, Madrid, Spain, Madrid, Madrid, Spain; 9 Hospital Universitario Príncipe de Asturias, Madrid, Madrid, Spain, 10 Fundación Jiménez Diaz, Madrid, Spain, Madrid, Madrid, Spain, 11 Deparment of Hematology, Hospital General Universitario Gregorio Marañón, Madrid, Spain., Madrid, Madrid, Spain
Background: Idarucizumab is a humanized monoclonal antibody that binds to dabigatran and reverses its anticoagulant activity. It´s indicated for imminent surgery and life‐threatening bleeding.
Aims: Describe the current experience with idarucizumab in different centers in Madrid.
Methods: Patients with prescription of idarucizumab between June 2016 and October 2021 were included. Qualitative data are presented as frequencies and percentages. Quantitative data are presented as mean or median. Cumulative survival was calculated in a 30‐day post‐infusion period.
Results: A total of 101 patients from 10 hospitals in Madrid were included. Ninety‐seven percent received dabigatran for prevention of embolism in nonvalvular atrial fibrillation and 3% received it for the treatment of thromboembolic disease. The mean age was 72.5 ± 14.6, and 58.4% were men. The main indication for idarucizumab was reversal of anticoagulation for surgery (46.5%), followed by persistent bleeding (44.5%). The most common type of procedure was cardiac surgery, heart transplantation was a common indication (20/28). Gastrointestinal bleeding was the most common type of bleeding, followed by intracranial bleeding. The median time between infusion of idarucizumab and cessation of bleeding or onset of surgery was 3 hours. No reports of excessive bleeding during surgery or after fibrinolysis were noted. One patient with dabigatran intoxication had melenas. A case of auricular thrombosis occurred in a patient with a heart transplant. Full 30‐day follow‐up was available for 98 patients, during this period 14 died. Cumulative survival after a follow‐up period of 30 days was 85% (Figure 1). Seventy‐one percent resumed anticoagulation after a median of 4 days, 58.3% were bridged with low molecular weight heparin and 63.4% resumed anticoagulation at discharge.
Conclusion(s): Idarucizumab is effective for the reversal of dabigatran anticoagulation. It was used safely in patients awaiting a heart transplant. No cases of bleeding after infusion or during surgery were reported, except for a case of auricular thrombosis.
OC 65.5
Factors influencing anti‐Xa assays in different groups of critically ill and non‐critically ill patients receiving unfractionated heparin: A multicenter prospective study
D. Lasne1; M. Toussaint‐Hacquard2; C. Delassasseigne3; A. Bauters4; C. Flaujac5; P. Savard6; C. Mouton3; E. De Maistre7; A. STEPANIAN8; J. Georges9; A. Gros9; A. Mansour10; G. Leroy11; R. Jouffroy1; M. Mattei2; A. PONTIS12; M. NEUWIRTH13; E. Curis14; V. Siguret15; I. Gouin 12
1 AP‐HP.Centre/University of Paris, Paris, Ile‐de‐France, France; 2 University Hospital of Nancy, Nancy, Lorraine, France; 3 University Hospital of Bordeaux, Pessac, Aquitaine, France; 4 University Hospital of Lille, Lille, Nord‐Pas‐de‐Calais, France, 5 laboratoire de biologie médicale‐ secteur hémostase ‐ André Mignot hospital, Versailles, Le Chesnay, Ile‐de‐France, France; 6 University Hospital of Dijon, Dijon, Franche‐Comte, France; 7 University Hospital of Dijon, Dijon, Bourgogne, France; 8 Lariboisière hospital/University of Paris, Paris, Ile‐de‐France, France; 9 André Mignot hospital, Versailles, Versailles, Ile‐de‐France, France, 10 University Hospital of Rennes, Rennes, Bretagne, France, 11 University Hospital of Lille, Versailles, Ile‐de‐France, France, 12 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, Institut de recherche en santé, environnement et travail ‐ IRSET, Inserm UMR_S 1085, Univ Rennes, CHU Rennes, France, Rennes, Bretagne, France, 13 AP‐HP.Nord/University of Paris, Paris, Ile‐de‐France, France, 14 University Paris Descartes, Paris, Ile‐de‐France, France, 15 AP‐HP.Nord/University of Paris, PARIS, Ile‐de‐France, France
Background: Anti‐factor Xa (anti‐FXa) assays used to monitor unfractionated heparin (UFH) may vary in their responsiveness to UFH. Since dextran sulfate (DS) dissociates the UFH/neutralizing protein complexes, its presence in reagents could contribute to this heterogeneity.
Aims: To evaluate in a prospective multicenter non‐interventional study the effect of different reagents, containing or not DS, and of blood collection tubes, on anti‐FXa levels in patients receiving UFH.
Methods: In‐patients from 8 centers receiving UFH were included: group (G)1, cardio‐pulmonary bypass (CPB) 5‐10 min after protamine neutralization (n = 39); G2, intensive care unit (ICU) 1‐5 days after CPB (n = 35); G3, medical ICU (n = 53); G4, medical patients (n = 38). For each patient, blood was collected into both citrated/citrate‐theophylline‐adenosine‐dipyridamole (CTAD) tubes. Anti‐FXa assays were centrally assessed, using five commercially available chromogenic assays, including two without DS, on both citrated/CTAD plasma samples, with different analyzers. We used a linear mixed‐effects model to evaluate the potential effect of DS and citrate/CTAD on anti‐FXa levels.
Results: Citrated and CTAD plasma samples from 165 patients were analyzed. Table 1 shows the anti‐FXa values according to reagents, analyzers and tubes (n = 2273). The model showed that anti‐FXa levels were 15.1% higher in CTAD than in citrated tubes (95% confidence interval [+8.7;+21.9]), whatever the group. Reagents containing DS led to higher anti‐Xa values than those without, especially in G1 (Table 2). Accordingly, in CPB patients after protamine neutralization (G1), on citrate tubes, 6% of the assays with DS versus 77% of the assays without DS had anti‐FXa level below the limit of quantification (0.1 IU/mL; Table 2).
Conclusion(s): The observed variability according to reagents and tubes could lead to different treatment decision‐making. In critically‐ill patients, anti‐FXa results should be interpreted with caution and knowledge of the performances of the reagents in the context of UFH neutralization or dose adjustment decision.
OC 67.5
The Von Clauss assay does not accurately measure total fibrinogen levels after traumatic injury. Have we been thinking about fibrinogen replacement all wrong?
M. DeBot 1; I. Lacroix2; A. D'Alessandro3; E. Moore4; C. Silliman2; A. Sauaia2; T. Schaid1; C. Erickson2; A. Cralley2; M. Cohen2; K. Hansen2
1 University of Colorado, Denver, Colorado, United States; 2 University of Colorado, Aurora, Colorado, United States; 3 Department of Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States; 4 Ernest E Moore Shock Trauma Center at Denver Health, Denver, Colorado, United States
Background: The Von Clauss assay (FbVC), which estimates fibrinogen concentration based on the rate of thrombin‐induced fibrin polymerization, is the standard clinical fibrinogen measurement. During acute physiological stress, oxidation alters the capacity of fibrinogen for fibrin polymerization and thus decreases the accuracy of FbVC. To date, there are no reports on the accuracy of FbVC after trauma.
Aims: Our aim was to evaluate the accuracy of FbVC versus total fibrinogen levels measured via mass spectrometry (FbMS). We hypothesized that severe injury reduces the accuracy of FbVC, and that fibrinogen repletion increases total fibrinogen levels without improving fibrin polymerization.
Methods: Blood was collected from injured patients at a level I trauma center in the ED, and at 6 and 24hr after hospital arrival. Fibrinogen levels were measured via targeted liquid chromatography coupled with mass spectrometry and analyzed with clinical data, treatments, outcomes, and FbVC.
Results: At 6hr after injury, patients who received cryoprecipitate showed a 13% increase in FbMS while patients who did not receive cryoprecipitate showed no change in FbMS (p = 0.03, Figure 1). No significant difference was seen in FbVC between patients who did and did not receive cryoprecipitate. Patients with minor trauma (NISS < 15, BE > ‐10 or INR < 1.4) showed correlations between FbMS and FbVC which were similar to healthy controls (Figure 2); whereas severely injured patients with shock or coagulopathy had poor correlations between FbMS and FbVC.
Conclusion(s): The Von Clauss Assay underestimates fibrinogen levels in hemorrhagic shock. Decreases in FbVC may reflect impaired fibrinogen polymerization rather than decreased availability and therefore FbVC may not respond to fibrinogen replacement. FbVC should be used with caution to guide fibrinogen replacement during resuscitation and correcting shock and the oxidative milieu of trauma may be more effective at improving fibrin polymerization than fibrinogen repletion alone.
OC 65.1
Prediction of apixaban or rivaroxaban concentrations based on low molecular weight heparin anti‐Xa activity using nomograms: A useful tool in emergency clinical situations like thrombolysis in stroke
C. Brakta1; A. Stepanian2; R. Elayeb3; M. Neuwirth4; E. Curis5; M. Delrue6, V. Siguret 1
1 INSERM UMR‐S‐1140/University of Paris, Paris, Ile‐de‐France, France; 2 Lariboisière hospital/University of Paris, Paris, Ile‐de‐France, France; 3 Lariboisière hospital/University of Paris, 75010, Ile‐de‐France, France; 4 AP‐HP.Nord/University of Paris, Paris, Ile‐de‐France, France; 5 Faculty of Pharmacy/University of Paris, 75006, Ile‐de‐France, France; 6 AP‐HP.Nord/University of Paris, 75010, Ile‐de‐France, France
Background: Direct oral anticoagulants (DOACs) are now widely used for the prevention and treatment of thrombo‐embolism. In emergency situations such as invasive procedures or intravenous thrombolysis in acute ischemic stroke, plasma DOAC concentration measurement may be useful for decision‐making: different thresholds (30, 50, 100 ng/mL) have been proposed according to procedures. However, specific DOAC assays with rapid turnaround time are not widely available in contrast to low molecular weight heparin (LMWH) anti‐Xa activity (IU/mL).
Aims: To develop nomograms to predict apixaban and rivaroxaban concentrations up to 100 ng/mL, based on LMWH anti‐Xa activity.
Methods: Patients on apixaban or rivaroxaban referred to our laboratory for DOAC determination were included and separated into derivation and validation cohorts. On the same plasma sample, we measured using STA®‐Liquid‐Anti‐Xa (Stago®): i/LWMH‐anti‐Xa (IU/mL; Multi‐HEP®‐Stago® calibrator); ii/DOAC concentrations (ng/mL) with the DOAC specific test set‐up and calibrators (apixaban or rivaroxaban). Different reagent and calibrator batches were used over the study period. We built regression models from patients of the derivation cohort using R software. Model performances including sensitivity, specificity and negative predictive values for different clinically relevant thresholds are being studied on the validation cohort.
Results: Models were built from the derivation cohorts comprising 85 apixaban and 68 rivaroxaban samples. The obtained nomogram predicting apixaban concentrations (ng/mL) according to LMWH anti‐Xa activity (IU/mL) is given on Figure 1. The model predicted that <30, <50 or <100 ng/mL apixaban thresholds corresponded to LMWH anti‐Xa values of 0.12, 0.92 and 1.63 IU/mL; it accurately predicted xaban concentrations in the validation cohort (n = 67, apixaban). The validation of the rivaroxaban model is on‐going.
Conclusion(s): These easy‐to‐use nomograms allow clinical pathologists accurately predicting apixaban and rivaroxaban concentrations in emergency situations when specific DOAC assessments are not rapidly available. FIGURE 1 Nomogram for the prediction of apixaban concentrations (ng/mL) based on LMW H anti‐Xa activity (IU/mL), with 95% confidence interval (CI)
FIGURE 1 Nomogram for the prediction of apixaban concentrations (ng/mL) based on LMW H anti‐Xa activity (IU/mL), with 95% confidence interval (CI)
OC 67.1
Antithrombin deficiency and altered heparin responsiveness during veno‐arterial extracorporeal life support: A prospective study
A. Mansour 1; M. Berahou2; J. Odot2; A. Pontis3; F. NEDELEC‐GAC4; A. Parasido5; F. Reizine6; Y. Launey2; A. Ousmen7; R. Garlantezec7; E. Flecher5; N. Nesseler8; I. Gouin9
1 Cardiothoracic Intensive Care Unit, Department of Anesthesia Cardiothoracic Intensive Care Unit, Department of Anesthesia and Critical Care, Pontchaillou, University Hospital of Rennes, Institut de recherche en santé, environnement et travail ‐ IRSET, Inserm UMR_S 1085, Univ Rennes, CHU Rennes, France 1085, Univ Rennes, CHU Rennes, France, Rennes, Bretagne, France; 2 Department of Anesthesia and Critical Care, Pontchaillou, University Hospital of Rennes, CHU Rennes, France, Rennes, Bretagne, France; 3 Department Biological Hematology, Pontchaillou, University Hospital of Rennes, CHU Rennes, France, Rennes, Bretagne, France; 4 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, France, Rennes, Bretagne, France; 5 Department of Cardiothoracic surgery, Pontchaillou, University Hospital of Rennes, CHU Rennes, France, Rennes, Bretagne, France; 6 Service des maladies infectieuses et r animation m dicale, Rennes University Hospital, Rennes, France, Rennes, Bretagne, France, 7 epidemiology biostatistics and occupational health, Pontchaillou, University Hospital of Rennes, CHU Rennes, France, Rennes, Bretagne, France; 8 Cardiothoracic Intensive Care Unit, Department of Anesthesia and Critical Care, Pontchaillou, University Hospital of Rennes, Univ Rennes, CHU Rennes, France, Rennes, Bretagne, France; 9 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, Institut de recherche en santé, environnement et travail ‐ IRSET, Inserm UMR_S 1085, Univ Rennes, CHU Rennes, France, Rennes, Bretagne, France
Background: During VA‐ECLS, unfractionated heparin (UFH) is administered to prevent hemocompatibility‐related adverse events. Antithrombotic properties of UFH rely on plasma antithrombin (AT) activity. Given the underlying critical illness and blood exposure to shear stress and non‐biological surfaces, coagulopathy including decreased AT levels and apparent altered heparin responsiveness (AHR) are frequently associated with thrombosis and bleeding. While there is currently no evidence of a beneficial effect on heparin responsiveness or adverse events, AT supplementation is routinely administered during ECLS.
Aims: 1) Determine dynamic changes of plasma AT levels during the first week of ECLS support 2) Evaluate the association between AT deficiency and AHR
Methods: Adults receiving VA‐ECLS were prospectively included (CHU Rennes, France). All patients received UFH using a standardized protocol (target anti‐FXa 0.3‐0.5IU.mL‐1). For each patient, arterial blood was withdrawn on citrated tubes, at 11 time‐points (H0 at ECMO initiation, H+2, H+6, H+12, H+24 and daily up to D7). Anti‐FXa activity and AT level were measured (STA Liquid anti‐Xa®, Stachrom AT®; Stago). Data regarding clinical management and heparin dosage were collected. A new index was created to evaluate heparin responsiveness (HRI‐Heparin Responsiveness Index) measured at each time‐points as follows: HRI = heparin dosage [IU.kg‐1.h‐1]/anti‐FXa activity [IU.mL−1].
Results: Fifty patients including 42% post‐cardiotomy ECMO were included between April 2020 and May 2021, with a total of 403 samples. Median ECMO duration was 7 days (IQR 4‐12). Median AT level was 48% (37‐60) at H0. AT levels significantly increased throughout the follow‐up, without any supplementation (Figure 1). Overall, 49(98%) patients had at least one AT value below 80%, 45(90%) below 70% and 35(70%) below 50%. AT level was not correlated to heparin responsiveness evaluated by anti‐FXa activity, heparin dosage or HRI (r = 0.04, p = 0.57; Figure 2).
Conclusion(s): Antithrombin deficiency is common during the first 72 hours of VA‐ECMO support and is not related to altered heparin responsiveness.
FVIII/IX
OC 74.1
Substitution of the factor IX activation sites for characterization of the factor IX activation pathway and partially activated intermediates
J. Vatandoost 1; S. Cheng2; K. Cheung3; N. Shifai4; M. Mayorga4; V. Strijbis5; M. Bos6
1 Leiden University Medical Center/Hakim Sabzevari University, Leiden, Zuid‐Holland, Netherlands; 2 Leiden University Medical Center, leiden, Zuid‐Holland, Netherlands; 3 Div. Thrombosis and Hemostasis, Einthoven Laboratory, for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 4 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 5 Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 6 Leiden University Medical Center
Background: Conversion of the zymogen factor (F)IX to its activated serine protease state FIXa‐beta proceeds through sequential cleavage at Arg145 and Arg180 by the extrinsic tissue factor‐FVIIa complex or by intrinsic FXIa. Proteolysis at Arg145 results in the activation intermediate FIX‐alpha that displays limited amidolytic activity, while single cleavage at Arg180 generates partially active FIXa‐alpha. As modifications in FIX aimed at improving its activity may affect the activation pathway and activity of intermediate species, detailed knowledge on these key processes is essential.
Aims: Generate FIX variants comprising substitutions at the Arg145 and Arg180 activation sites to establish a framework for detailed characterization of FIX activation species and variants.
Methods: Recombinant variants FIX‐R145Q, FIX‐R180Q, and FIX‐R145Q‐R180Q were generated and purified to homogeneity. The variants were functionally assessed for both extrinsic and intrinsic activation pathways.
Results: Under conditions in which wild‐type (WT‐) FIX was efficiently converted to FIXa‐beta by both the extrinsic and intrinsic activators, FIX‐R180Q was mostly proteolyzed to FIX‐alpha while FIX‐R145Q was only partially converted to FIXa‐alpha. In case of the latter, prolonged incubations with supraphysiological FXIa concentration led to additional protein products suggestive of aspecific proteolysis of the FIX heavy chain. These findings confirm that cleavage at Arg145 site is most efficient, which is required for Arg180 to become available for subsequent proteolysis. No proteolysis was observed when incubating FIX‐R145Q‐R180Q with the extrinsic or intrinsic activators. Extrinsically or intrinsically triggered thrombin generation assessments in which FIX‐deficient plasma was supplemented with FIX variant demonstrated 50% activity for FIX‐R145Q relative to WT‐FIX, while no thrombin generation was observed for the other variants, in line with earlier observations.
Conclusion(s): These FIX variants allow for analysis of the FIX activation pathways. We next aim to obtain detailed insight into the contribution of activation intermediates to FIX function, which is essential for the knowledge‐based design of therapeutic FIX variants.
OC 55.2
Characterization of the factor VIII and LRP1 interaction suggests a dynamic binding mode with switching of alternative multiple canonical bivalent and non‐canonical electrostatic contacts
H. Chun1; J. Kurasawa2; P. Olivares1; E. Marakasova1; S. Shestopal1; G. Hassink3; E. Karnaukhova1; M. Migliorini4; J. Obi5; A. Smith5; P. Wintrode5; P. Durai6; K. Park6; D. Deredge5; D. Strickland4; A. Sarafanov 1
1 Food and Drug Administration, Silver Spring, Maryland, United States; 2 AstraZeneca, Gaithersburg, Maryland, United States; 3 GSK‐Rockville Center for Vaccines Research, Rockville, Maryland, United States; 4 University of Maryland School of Medicine, Baltimore, Maryland, United States; 5 University of Maryland School of Pharmacy, Baltimore, Maryland, United States; 6 Korea Institute of Science and Technology, Gangneung, Seoul‐t'ukpyolsi, Republic of Korea
Background: Deficiency in blood coagulation factor VIII (FVIII) results in life‐threating bleeding (Hemophilia A), which is treated by infusions of therapeutic FVIII. Understanding FVIII plasma clearance mechanisms facilitates generation of prolonged plasma lifetime FVIII to improve the disease treatment. An important FVIII clearance receptor is the hepatic low‐density lipoprotein receptor‐related protein 1 (LRP1). Previous studies indicated multiplicity of FVIII binding sites for LRP1 and complexity of the molecules’ interaction, at the same time, defined to be bivalent. However, the exact mechanism of this interaction remains unknown.
Aims: To characterize the bivalent sites of LRP1 and build a model of its interaction with FVIII.
Methods: Recombinant ligand‐binding complement‐type repeat (CR) fragments of LRP1 and their mutant variants were generated using a baculovirus system and tested for interactions with FVIII using surface plasmon resonance, a tissue culture model of LRP1‐mediated internalization of FVIII, hydrogen‐deuterium exchange mass‐spectrometry, and in silico docking.
Results: We identified a series of adjacent LRP1 CR doublets providing minimal requisite sites for binding FVIII and showed an additive effect of other CR domains on FVIII‐LRP1 affinity. A CR doublet was found to provide the bivalent interaction following the canonical mode of ligand recognition by the receptor in addition to the non‐canonical electrostatic contributions of other CR domains. For selected CR doublets, we found numerous contact sites on FVIII.
Conclusion(s): We characterized LRP1 bivalent binding sites for FVIII, confirmed their relevance to the canonical mode, and described a real‐time LRP1 interactive site. We propose that LRP1 interacts with FVIII via a string of CR domains providing both canonical bivalent and non‐canonical electrostatic contacts switched over time in a dynamic mode (Fig. 1) that represents a novel mechanism of biomolecular interaction. Disclaimer: This is an informal communication, and it represents the authors’ own best judgment. These comments do not bind or obligate FDA. Figure 1: Chun et al. FVIII and LRP1 interact in a dynamic mode
Figure 1: Chun et al. FVIII and LRP1 interact in a dynamic mode
Protein C Pathway
OC 65.3
A phase 2 trial evaluating the safety and antithrombotic efficacy of AB002, a first‐in‐class protein C activator in end‐stage renal disease patients on heparin‐free hemodialysis
N. Verbout 1; C. Lorentz1; B. Markway1; M. Wallisch1; J. Shatzel2; E. Tucker1
1 Aronora, Inc., Portland, Oregon, United States; 2 Oregon Health & Science University, Portland, Oregon, United States
Background: AB002 is a protein C activator enzyme under clinical investigation to safely prevent and treat thrombosis. In animal models, AB002 has potent antithrombotic and anti‐inflammatory activity without measurable bleeding side‐effects. This phase 2, randomized, double‐blind, placebo‐controlled trial in end‐stage renal disease (ESRD) patients undergoing hemodialysis is the first to clinically assess the safety and efficacy of therapeutic protein C activation.
Aims: ESRD patients are at increased risk of both clotting and bleeding, thus we sought to evaluate the safety and efficacy of AB002 as a short‐acting antithrombotic to safely prevent clotting in the extracorporeal circuit during hemodialysis.
Methods: In this single‐site study, 36 ESRD patients undergoing heparin‐free hemodialysis received a single dose of placebo or AB002 (1.5 μg/kg or 3.0 μg/kg) (NCT03963895). Patients underwent five heparin‐free sessions over 10 days and were dosed on the fourth session. The primary endpoint was safety and tolerability and the secondary endpoints were clotting severity in the dialysis circuit and pharmacodynamics. The study was approved by the local ethics review board and all patients provided informed consent.
Results: AB002 demonstrated a favorable safety profile, with no treatment‐related adverse events. Clinically relevant bleeding did not occur in any patient, and AB002 did not affect time to hemostasis at the vascular access site. Drug exposure was confirmed by a transient, dose‐dependent elevation of APC‐PCI. Compared to placebo and pre‐treatment sessions, clotting severity in the dialysis circuit was significantly reduced in patients receiving AB002. The frequency of thrombo‐occlusive events requiring circuit changeout was 13.9% (21/155) on days in which AB002 was not administered, compared to 0% (0/12) and 8.3% (1/12) at the 1.5 μg/kg and 3.0 μg/kg dose levels, respectively. AB002 also reduced dialysis‐induced thrombin‐antithrombin generation by up to 75%.
Conclusion(s): Results from this trial support further investigation of AB002 to safely prevent and treat thrombosis in at‐risk populations. NIH R44HL147695.
OC 67.2
Personalized assessment of the protein C pathway using endothelial colony forming cells
N. Schwarz 1; J. Müller2; H. Yadegari3; J. Oldenburg4; B. Pötzsch5; H. Rühl5
1 Institute of Experimental Haematology and Transfusion Medicine, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany, 3 University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 4 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 5 Institute of Experimental Haematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: The endothelium provides an anticoagulatory and anti‐inflammatory system by promoting the formation of activated protein C (APC). Mutations such as factor V Leiden (FVL) or inflammatory signaling can affect the protein C (PC) pathway and modulate hemostasis and host defence.
Aims: Aim of this study was to establish an assay system using human plasma and endothelial colony forming cells (ECFCs), allowing personalized assessment of the PC pathway.
Methods: Citrated, defibrinated plasma was added to ECFCs, which were cultivated from peripheral blood mononuclear cells, in 24‐well microtiter plates and coagulation was initiated by the 1 pM tissue factor. Thrombin and subsequent APC generation was quantified over time using corresponding oligonucleotide‐based enzyme capture assays (OECAs) after quenching the reaction using the reversible thrombin inhibitor argatroban. By treating ECFCs with cytokines, inflammatory stimulation was assessed. FVL carriers and non‐carriers (n = 3 each) were studied using the established ex vivo system.
Results: Similar thrombin and APC formation rates in normal pooled plasma were measured in the presence of ECFCs and human umbilical vein endothelial cells (HUVECs). Under inflammatory stimulation by treating ECFCs with 1 ng/mL interleukin 1 beta, peak APC formation was decreased (1.12 ± 0.04 nmol/L versus 3.66 ± 0.13 nmol/L, p = 7·10‐9). As evidenced by a higher ratio between the area under curve (AUC) of APC generation and that of thrombin, thrombin‐dependent APC generation was significantly higher in FVL carriers compared to non‐carriers (0.085 ± 0.014 versus 0.03 ± 0.019, p = 6·10‐4).
Conclusion(s): The established ex vivo model was shown to be suitable for measuring plasma thrombin and APC formation on ECFCs and to detect inter‐individual differences. Therefore, the assay system allows for personalized assessment of the PC pathway. It might contribute to further explanation of mechanisms modulating the thrombogenicity in inflammatory states, or of FVL and other mutations affecting the PC pathway.
Regulation of Coagulation
OC 55.4
Protein arginine deiminase 4 inactivates tissue factor pathway inhibitor by post‐translational modification of functional arginine residues
S. Thomassen1; B. Bouwens1; K. Wichapong1; D. Suylen1; F. Bouwman2; T. Hackeng1; R. Koenen 3
1 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 2 Department of Human Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Center, Maastricht, Maastricht, Limburg, Netherlands; 3 Maastricht University, Maastricht, Limburg, Netherlands
Background: Tissue factor pathway inhibitor (TFPI) is an important regulator of coagulation and a link between inflammation and thrombosis. During thrombotic events, TFPI is proteolytically inactivated by neutrophil elastase while bound to neutrophil extracellular traps (NETs). Protein arginine deiminase 4 (PAD4) catalyzes conversion of arginine to citrulline (termed “citrullination”) and is crucial for NET formation.
Aims: The aim of this work was to investigate whether PAD4 can directly modify the activity of TFPI by citrullination of its functional arginines.
Methods: Citrullination of TFPI and of TFPI‐constructs by PAD4 was studied by western blotting and mass spectrometry. Binding of TFPI by PAD4 was investigated using a solid‐phase assay. Functional consequences were investigated by factor Xa inhibition and by thrombin generation assays.
Results: Nanomolar PAD4 amounts sufficed to abolish factor Xa inhibition by TFPI (image 1). A citrullinated mutant Kunitz 2 domain did not inhibit factor Xa. Citrullination of full‐length TFPI was found to be time‐ and concentration‐dependent. Negatively charged phospholipids inhibited citrullination and truncated variants K1K2 and TFPI 1‐161, and the isolated K2 domain were less efficiently citrullinated by PAD4. TFPI bound to PAD4 with nanomolar affinity and involved the basic C‐terminus (image 2). Thrombin generation in TFPI‐deficient plasma demonstrated reduced anticoagulant activity of citrullinated TFPI. Mass spectrometry demonstrated citrullination of surface‐exposed arginine residues in TFPI after incubation with PAD4.
Conclusion(s): Full‐length TFPI is sensitive to citrullination by PAD4 and this causes a loss of factor Xa inhibition. This process might play a role in the increased thrombosis risk associated with inflammation.
Tissue Factor Pathway
OC 36.3
Analysis of TFPI‐protein S anticoagulant function in vivo
A. Petri 1; P. Sasikumar1; P. Badia Folgado1; D. Jones2; J. Ahnström1; I. Salles‐Crawley3; J. Crawley1
1 Imperial College London, London, England, United Kingdom; 2 The Francis Crick Institute, London, England, United Kingdom; 3 St George's University of London, London, England, United Kingdom
Background: In human plasma, TFPIα is the most effective anticoagulant TFPI form. TFPIα plasma levels appear to be determined by plasma levels of FVshort, which functions as a carrier protein diminishing renal filtration. TFPIα anticoagulant function is appreciably augmented by its cofactor, protein S. In vivo characterisation of the TFPIα – protein S pathway in mice is confounded by the embryonic lethality of TFPI‐deficiency in mice, the apparent lack of both TFPIα and FVshort in murine plasma, and uncertainty as to whether murine TFPIα is augmented by protein S.
Aims: To model/interrogate the TFPIα–protein S anticoagulant pathway in vivo.
Methods: FXa inhibition/CAT assays were used to measure enhancement of human/murine TFPIα function by human/murine protein S. Mice were injected with recombinant human TFPIα ( ± an in‐house non‐inhibitory anti‐TFPIα mAb), and its clearance monitored by ELISA. The influence of human TFPIα on laser‐induced thrombus formation was measured.
Results: Unlike human TFPIα, murine TFPIα was not enhanced by murine/human protein S, due to amino acid differences in the murine TFPI K3 domain. Injected human TFPIα was cleared rapidly (minutes) from circulation in mice, likely due to the absence of FVshort. However, formation of a complex between TFPIα and a non‐inhibitory mouse anti‐human TFPI K3 mAb appreciably extended the plasma half‐life of human TFPIα enabling its persistence in circulation. In the laser‐induced thrombosis model, we measured reduced fibrin deposition in the presence of human TFPIα demonstrating its anticoagulant function in vivo.
Conclusion(s): Using human TFPIα in complex with a non‐inhibitory mAb enables interrogation of the TFPIα – protein S anticoagulant pathway in vivo (as murine TFPIα is not enhanced by protein S). This also provides a novel means of augmenting endogenous TFPIα levels through the use of non‐inhibitory anti‐TFPI mAbs to reduce renal filtration, which may represent a potential new anticoagulant strategy.
COVID and Coagulation
COVID and Coagulation, Basic Science
OC 57.5
Downregulation of thrombomodulin‐thrombin‐activated protein C pathway as a mechanism for SARS‐CoV‐2 induced endotheliopathy and microvascular thrombosis
S. Agarwal 1; P. Jacobi2; S. Sartain2
1 Texas Children's Hospital/ Baylor College of Medicine, Houston, Texas, United States; 2 Texas Children's Hospital/Baylor College of Medicine, Houston, Texas, United States
Background: There is emerging evidence of microvascular thrombosis and thrombotic microangiopathy (TMA) induced by COVID‐19, presumably from endothelial injury or “endotheliopathy”. Thrombomodulin (TM) is an endothelial glycoprotein that plays a crucial role as a natural anticoagulant, binding thrombin to activate protein C (PC). TM is shed from endothelial surface during injury. We hypothesize SARS‐CoV‐2 spike proteins cause direct microvascular endothelial injury, leading to TM shedding, decreased activation of PC, and consequently, microvascular thrombosis in COVID‐19.
Aims: To assess: 1) endothelial injury (by soluble TM [sTM] levels) in a cohort of critically‐ill COVID‐19 pediatric patients; 2) endothelial injury (TM shedding) in vitro by SARS‐CoV‐2 spike proteins and the subsequent functional consequence in activated PC (APC) levels.
Methods: sTM in plasma samples from SARS‐CoV‐2 positive patients admitted to Texas Children’s Hospital Pediatric Intensive Care Unit (n = 34) and healthy controls (n = 38) were measured by ELISA. IRB approval and waiver of informed consent were obtained. In vitro, confluent glomerular microvascular endothelial cells (GMVECs) were incubated for 24 hours in the presence or absence (control) of purified SARS‐CoV‐2 spike proteins, S1 and S2. In some experiments, cell lysates were collected, and TM was measured by ELISA; in others, GMVECs were further supplemented with PC and thrombin for 1 hour, followed by supernatant collection for APC measurement by ELISA.
Results: sTM levels were significantly higher in the COVID‐19 pediatric patients (p < 0.01) (Fig. 1). In vitro, surface bound TM (Fig 2a) and soluble APC (Fig 2b) were significantly lower in GMVECs after addition of spike proteins (p < 0.05).
Conclusion(s): We provide evidence of endothelial injury in COVID‐19 patients and demonstrate a potential pathway of SARS‐CoV‐2 induced thrombosis. Decreased surface‐bound TM results in lower amount of thrombin‐TM complex, hence lesser activation of PC, likely leading to a pro‐thrombotic state. These findings in GMVECs could explain the vulnerability of kidneys to COVID‐19‐induced TMA.
OC 33.4
Detection of platelet‐activating antibodies associated with VITT by flow cytometry: An Italian experience
S. Sorrentino 1; F. Cesari2; A. Gori2; R. De Cristofaro3; B. Giusti4; A. Rogolino2; E. Sticchi2; E. De Candia3; R. Marcucci2
1 Unità Malattie Emorragiche e Trombotiche, Fondazione Policlinico Universitario Agostino Gemelli IRCSS, Rome, Italy, Rome, Lazio, Italy; 2 Dept Experimental and Clinical Medicine, University of Florence, AOU Careggi, Florence, Italy, Florence, Toscana, Italy; 3 Catholic University of Sacred Heart, Translational Medicine and Surgery Department, Rome, Lazio, Italy; 4 University of Florence, Department of Clinical and Applied Medicine, Florence, Toscana, Italy
Background: Rare cases of thrombocytopenia and thrombotic complications after adenoviral vaccination (VITT) caused by platelet activating anti‐platelet factor 4 (PF4)/polyanions antibodies, have been reported. The laboratory diagnosis of VITT, similarly to the diagnosis of heparin induced thrombocytopenia (HIT), requires immunoassays for antibodies identification and platelet activating functional tests to confirm pathogenicity of anti‐PF4/polyanions antibodies.
Aims: We compared flow cytometry (FC) measurement of p‐selectin exposure and heparin‐induced platelet activation test (HIPA) as functional tests to confirm VITT diagnosis in two Italian centers.
Methods: Anti‐PF4/polyanions antibodies were assessed using immunoloassays (ELISA Lifecode PF4 IgG and Asserachrom HPIA‐IgG assays; HemosIL AcuStar HIT‐IgG (PF4‐H) assay) and functional assays (FC measurement of p‐selectin exposure in platelet rich plasma and HIPA in the presence of VITT samples + buffer/low/high heparin concentration.
Results: Thirteen VITT patients [6 M/7 F; median age 56 (33‐78)] (Table 1) were all positive to ELISA and negative to AcuStar assays. Functional assay by FC identified three different patterns: 1) a typical VITT p‐selectin exposure pattern in 7 patients showing platelet activation with buffer only, which was moderately reduced with low heparin and completely abolished with high heparin doses (Fig.1A); 2) 4 VITT patients investigated under IvIg therapy showed low or no platelet activation (Fig.1B); 3) a typical HIT pattern – platelet activation with low heparin dose abolished by high heparin dose‐ was found in 2 VITT patients (Fig.1C). The HIPA testing was positive in 11 VITT patients. Three patients were no more positive to both ELISA and FC analysis after 7, 17 and 24 days of therapy (Fig.1A).
Conclusion(s): FC measurement of P‐selectin exposure was a simple and reliable method 1) to detect platelet‐activating anti‐PF4/polyanions antibodies in VITT patients, 2) to discriminate VITT from HIT, thus helping for the choise of the anticoagulant treatment, 3) to follow the response to therapies.
OC 57.2
Inversed platelet/fibrin ratio in shear‐dependent thrombus formation between patients with COVID‐19 and sepsis identifies subtherapeutic GPIIb/IIIa blockade as a potential therapeutic target
L. Weiss 1; M. Drayss2; M. Zeitlhöfler3; K. Mott1; S. Frantz4; B. Nieswandt5; D. Weismann4; H. Schulze6
1 University Hospital Wuerzburg, Würzburg, Bayern, Germany; 2 Institute of Experimental Biomedicine, Chair I, University Hospital Würzburg; Department of Internal Medicine I, University Hospital Wuerzburg, Wuerzburg, Bayern, Germany; 3 Institute of Experimental Biomedicine, University Hospital Würzburg, Würzburg, Bayern, Germany; 4 Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Bayern, Germany; 5 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 6 Institute of Experimental Biomedicine, Chair I, University Hospital Wuerzburg, Centre of Inherited Blood Cell Disorders, University Hospital Wuerzburg, Würzburg, Bayern, Germany
Background: Thrombotic events are frequent and life‐threating complications of COVID‐19, but are also observed in patients with bacterial sepsis. Disseminated thrombosis may occur despite strict anticoagulation, suggesting that platelets play a direct, but yet undefined role. Several studies demonstrate altered platelet function in COVID‐19, but the impact of platelets in COVID‐19 and sepsis remains poorly understood.
Aims: Platelet phenotype and function were comprehensively assessed in over 100 patients with either COVID‐19 (non‐ECMO, all‐ICU, n = 23), bacterial infection without sepsis (SOFA‐score < 2; n = 29), or sepsis/septic shock (SOFA‐score ≥2; n = 49) at multiple time points during the disease.
Methods: Patients were recruited at the local University Hospital (Ethical vote 94/19). Platelet phenotype and function were studied using flow cytometry (lumino‐)aggregometry and whole‐mount transmission electron‐microscopy. Thrombus formation was investigated using a collagen‐ and tissue factor‐coated flow chamber model at arterial shear rate (1000s‐1). Thrombi were imaged by confocal microscopy.
Results: Upon stimulation with ADP or CRP‐XL platelets of infection patients without sepsis showed reduced PAC‐1 binding and CD62P exposition. In sepsis patients reactivity was even more impaired and highly associated with disease severity (mean normalized geo‐MFI PAC‐1 infection: 0.56 vs sepsis: 0.25, p < 0.01; ROC‐AUC: 0.76, p < 0.001). Intriguingly, platelets of COVID‐19 patients were more responsive towards stimulation compared to comparably‐ill ICU patients with sepsis. This relative hyper‐reactivity was reflected by increased clot‐formation in the flow chamber, compared to sepsis patients (mean surface coverage: 36% vs. 19%, p < 0.05). Thrombi of COVID‐19 patients were platelet‐rich with little fibrin, in contrast to healthy donors or sepsis patients showing increased amount of fibrin and less platelets. Subtherapeutic doses of GPIIb/IIIa blockers eptifibatide or tirofiban, which had minor effect in control blood, sufficiently prevented thrombus formation in COVID‐19 samples under arterial flow.
Conclusion(s): Our findings provide evidence that low dose GPIIb/IIIa blockade might act as a powerful therapeutic tool in COVID‐19 patients.
OC 57.3
Cell‐based high throughput screening to identify FDA/EMA approved drugs that inhibit the endothelial pro‐thrombotic switch during severe COVID19 infection
D. Jones 1; G. Saginc Giannoustas2; C. Peghaire3; D. Pirri2; L. Braga4; E. Schneider5; M. Appiah2; G. Birdsey2; T. McKinnon1; R. Thwaites2; M. Giacca5; A. Randi1
1 Imperial College London, London, England, United Kingdom; 2 Imperial College London, london, England, United Kingdom; 3 University of Bordeaux, Bordeaux, Aquitaine, France; 4 University of Trieste, Trieste, Friuli‐Venezia Giulia, Italy; 5 King's college London, london, England, United Kingdom
Background: A major complication of COVID19 is severe endothelial injury with micro‐ and macro‐thrombotic disease in the lung and other organs. Several studies have identified high levels of inflammatory cytokines (“cytokine storm”), powerful activators of the endothelium, in plasma of severe COVID19 patients; indeed, COVID19 plasma was shown to activate endothelial cells (EC) in vitro. A consequence of EC activation is loss of anti‐coagulant function, with release of pro‐thrombotic Von Willebrand Factor (VWF). High levels of plasma VWF in severe COVID19 patients indicate systemic endothelial activation and increased risk of thrombosis.
Aims: To identify drugs that decrease endothelial activation and VWF release, which may have a therapeutic impact in COVID19 patients.
Methods: We established an in vitro model of endothelial activation driven by 6 cytokines selected because of their high levels in COVID19 plasma. Cells were treated with the 6‐cytokine cocktail for 24 hr; endothelial activation was confirmed by a panel of markers including ICAM1, measured by RT‐qPCR and immunofluorescence (IF).
Results: The treatment induced release of VWF and increased VWF‐platelet string formation in a platelet flow‐based assay. To identify drugs that blocked cytokine‐induced VWF release, a high‐throughput screening was carried out in human umbilical vein EC (HUVEC); VWF and ICAM1 expression were detected by IF; DAPI was used as nuclear stain. High content imaging screen of 3049 drugs from FDA/EMA‐approved drug libraries identified drugs able to decrease VWF release following cytokine treatment. Top hits from several therapeutic classes including anti‐inflammatory, anti‐viral and hormones were taken forward for validation. Two hits were confirmed to inhibit cytokine‐induced VWF release and VWF‐platelet string formation. Selected findings were validated in lung microvascular EC.
Conclusion(s): This study identified candidate drugs that reduce the enhanced VWF release caused by the “cytokine storm” typical of severe COVID19; these may be beneficial in the treatment of the pro‐thrombotic risk in COVID19 patients.
OC 05.5
Endothelial cell activation, Weibel‐Palade body secretion and enhanced angiogenesis in severe COVID‐19
E. Karampini 1; S. Elliott2; H. Fogarty3; J. O'Sullivan4; C. Bergin5; C. Ni Cheallaigh6; M. Byrne7; I. Martin‐Loeches8; P. Mallon9; G. Curley1; S. Glavey1; R. Preston10; S. Ward11; J. O'Donnell11
1 Royal College of Surgeons in Ireland, RCSI Dublin, Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Carlow, Ireland; 3 Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 4 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland., Dublin, Dublin, Ireland; 5 St James's Hospital, Dublin, Dublin, Ireland; 6 Department of Infectious Diseases, St James’s Hospital, Dublin, Ireland., Dublin, Dublin, Ireland; 7 National Coagulation Centre, St James’s Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 8 Department of Intensive Care Medicine, St James’s Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 9 UCD, Dublin, Dublin, Ireland, 10 Royal College of Surgeons in Ireland, Dublin, Dublin, Ireland, 11 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland
Background: Severe COVID‐19 is associated with marked endothelial cell (EC) activation that plays a key role in immunothrombosis and pulmonary microvascular occlusion. However, the biological mechanisms through which SARS‐CoV‐2 causes EC activation and damage remain poorly defined.
Aims: We investigated EC activation in patients with acute COVID‐19, and in particular focused on how proteins stored within Weibel‐Palade bodies (WPBs) may impact key aspects of disease pathogenesis.
Methods: 39 patients with confirmed COVID‐19 were recruited. Weibel‐Palade body biomarkers [von Willebrand factor (VWF), angiopoietin‐2 (Ang‐2) and osteoprotegerin (OPG)] and soluble thrombomodulin (sTM) levels were determined. In addition, EC activation and angiogenesis were assessed in the presence or absence of COVID‐19 plasma incubation.
Results: Markedly elevated plasma VWF:Ag, Ang‐2, OPG and sTM levels were observed in acute COVID‐19 patients. The increased levels of both sTM and WPB components (VWF, OPG and Ang‐2) correlated with COVID‐19 severity. Incubation of COVID‐19 plasma with ECs triggered enhanced VWF secretion and increased Ang‐2 expression (Figure 1). In keeping with the autopsy reports of intussusceptive angiogenesis, treatment with COVID‐19 plasma also caused significantly increased EC angiogenesis (Figure 1).
Conclusion(s): We propose that as COVID‐19 develops, progressive loss of TM and increased sTM, as well as increased Ang‐2 expression result in loss of EC quiescence, WPB exocytosis, and a local pro‐angiogenic state. FIGURE 1 (A) Effects of COVID‐19 plasma incubation on EC biology ex vivo. VWF secretion (%) from HUVECs after treatment with either healthy control plasma, or acute COVID‐19 plasma (moderate, critical or fatal respectively) (n = 3, Kruskal‐Wallis test, ns ‐ not significant; *p < 0.05; **p < 0.01; ***p < 0.001). (B) Representative images of enhanced HUVEC tube formation after incubation with acute COVID‐19 plasma compared to control plasma. (C) Significantly increased nodes per field and total branching length (t‐test, **p < 0.01)
FIGURE 1 (A) Effects of COVID‐19 plasma incubation on EC biology ex vivo. VWF secretion (%) from HUVECs after treatment with either healthy control plasma, or acute COVID‐19 plasma (moderate, critical or fatal respectively) (n = 3, Kruskal‐Wallis test, ns ‐ not significant; *p < 0.05; **p < 0.01; ***p < 0.001). (B) Representative images of enhanced HUVEC tube formation after incubation with acute COVID‐19 plasma compared to control plasma. (C) Significantly increased nodes per field and total branching length (t‐test, **p < 0.01)
OC 33.1
Functional studies on the interaction between anti‐PF4 antibodies and anticoagulants in vaccine‐induced thrombotic thrombocytopenia
A. Singh 1; F. Toma2; G. Uzun3; T. Wagner4; L. Pelzl5; J. Zlamal6; K. Weich5; S. Nowak‐Harnau3; U. Rothbauer4; K. Althaus5; T. Backchoul6
1 Institute for Clinical and Experimental Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany, 2 Institute for Clinical and Experimental Transfusion Medicine, University Hospital Tübingen, Tübingen, Baden‐Wurttemberg, Germany; 3 Center for Clinical Transfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 4 NMI Natural and Medical Sciences Institute at the University of Tuebingen, Reutlingen, Reutlingen, Baden‐Wurttemberg, Germany; 5 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 6 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: With increasing number of vaccinations against SARS‐CoV‐2, rare but life threatening thrombotic events at unusual sites have been reported, and collectively this phenomenon is termed as vaccine‐induced immune thrombotic thrombocytopenia (VITT). Pathophysiology of VITT is similar to that of heparin‐induced thrombocytopenia (HIT), and associated with platelet‐activating antibodies against platelet factor 4 (PF4).
Aims: Current guidelines for anticoagulation in VITT patients are issued accordingly, with a focus on non‐heparin anticoagulants. In this study, we investigated the interactions of heparin, danaparoid, fondaparinux and argatroban with VITT‐Ab/PF4 complexes.
Methods: We utilized an in‐house enzyme immunoassays (EIA) to estimate antibody binding, inhibition and dissociation of preformed PF4‐VITT complexes. Using biolayer interferometry (BLI), we analyzed binding kinetics and dissociation of complexes in real time. In a flow‐based ex vivo model, we assessed the impact of anticoagulants on VITT‐mediated thrombus formation.
Results: We found that heparin and danaparoid not only inhibited VITT IgG binding to PF4 but were also able to effectively dissociate preformed PF4/IgG complexes in EIA. In BLI, binding of PF4 specific antibodies was observed for all VITT samples tested, and we found remarkable changes in their dissociation after addition of various anticoagulants. Furthermore, IgGs from VITT patients induce increased thrombus formation in comparison to the healthy controls (mean % SAC ± SEM: 11.59 ± 0.57 vs. 1.99 ± 0.34 respectively, p < 0.001), which can further be effectively inhibited with danaparoid and heparin (mean % SAC ± SEM 2.82 ± 0.50 and 1.85 ± 0.56. p < 0.001). Fondaparinux and argatroban inhibited thrombus formation; however, they did not affect antibody binding.
Conclusion(s): Taken together, our data shed a light on suitability of anticoagulants in VITT, and indicate that negatively charged anticoagulants can disrupt VITT‐Ab/PF4 interactions, which might serve as an approach to reduce antibody‐mediated complications in VITT. Our results should be confirmed, however, in a clinical setting before a recommendation regarding the selection of anticoagulation in VITT patients could be made.
OC 57.1
SARS‐CoV‐2 spike protein enhanced thrombosis is inhibited by tipiracil in mice
W. Li 1; R. Roytenberg1; A. DeHart2; K. Denning1; H. Yue1
1 Joan C. Edwards School of Medicine at Marshall University, Huntington, West Virginia, United States; 2 Marshall University, Huntington, West Virginia, United States
Background: COVID‐19 is accompanied by excessive systemic thrombotic events, but the mechanism is unknown. All major COVID‐19 vaccines were associated with thrombosis. Thymidine phosphorylase (TYMP) plays an important role in platelet activation, thrombosis, and inflammation. TYMP expression is significantly increased in COVID‐19 patients.
Aims: To test the hypothesis that TYMP mediates SARS‐CoV‐2 spike protein (SP)‐enhanced thrombosis.
Methods: Transfection of plasmid encoding SP or the receptor‐binding domain (RBD) with human ACE2 was conducted in COS‐7 cells. BEAS‐2B cells were treated with SP or RBD containing COS‐7 cell lysates, and TYMP expression and activation of NF‐κB were examined. K18‐hACE2 transgenic (ACE2‐TG) mice were intraperitoneally treated with SP or RBD containing COS‐7 cells lysates, and thrombosis was assessed three days later using the FeCl3 injury‐induced carotid artery thrombosis model.
Results: SP and RBD led to ACE2 shedding, significantly increased TYMP expression, and NF‐κB activation in BEAS‐2B cells. In comparison to wildtype mice, ACE2‐TG mice are anti‐thrombotic and had significantly prolonged thrombosis time. Treating ACE2‐TG mice with COS‐7 cells transfected with empty plasmid did not affect the thrombosis. However, treating the ACE2‐TG mice with SP‐ or RBD‐containing COS‐7 cell lysates dramatically enhanced thrombosis and significantly shortened time to occlusive thrombi formation. SP is more powerful than RBD in enhancing thrombosis. SP‐enhanced thrombosis was dramatically inhibited by simultaneously feeding the mice with 1 mg/kg of tipiracil. TYMP is expressed in human type II alveolar epithelial cells and bronchial epithelium. By using the MGH Emergency Department COVID‐19 Cohort with Olink Proteomics TYMP data and Receiver Operating Characteristic analysis, we found TYMP is a sensitive and specific marker in diagnosing COVID‐19 (AUC 0.8721, p < 0.0001).
Conclusion(s): SARS‐CoV‐2 SP and RBD are pro‐inflammatory and pro‐thrombotic. SP/RBD‐induced thrombosis is inhibited by tipiracil, a TYMP inhibitor. TYMP is a sensitive marker for COVID‐19 diagnosis. Targeting TYMP could be a novel effective treatment for COVID‐19. FIGURE 1 A & B. SARS‐CoV‐2 spike protein and its receptor‐binding domain enhanced thymidine phosphorylase expression in BEAS‐2B cells. C. SARS‐CoV‐2 spike protein and its receptor‐binding domain enhanced thrombosis in the K18‐hACE transgenic mice. SP‐enhanced thrombosis was inhibited by Tipiracil (TPI), a selective thymidine phosphorylase inhibitor
FIGURE 1 A & B. SARS‐CoV‐2 spike protein and its receptor‐binding domain enhanced thymidine phosphorylase expression in BEAS‐2B cells. C. SARS‐CoV‐2 spike protein and its receptor‐binding domain enhanced thrombosis in the K18‐hACE transgenic mice. SP‐enhanced thrombosis was inhibited by Tipiracil (TPI), a selective thymidine phosphorylase inhibitor
OC 05.2
Proteome and phosphoproteome analyses of SARS‐CoV‐2‐infected humanized K18‐ACE2 mice reveal hyperactive phenotype of circulating platelets
S. Subramaniam 1; R. Matthew Hekman2; A. Jayaraman3; A. Kateri O'Connell4; M. Paige Montanaro5; D. Kenney6; M. Ericsson7; S. Walachowski3; B. Blum2; K. Ravid8; N. A Crossland9; F. Douam9; A. Emili2; M. Bosmann10
1 Department of Medicine, Boston University, Boston, MA, USA, Boston, Massachusetts, United States; 2 Center for Network Systems Biology, Boston University, Boston, MA, USA, Boston, Massachusetts, United States; 3 Pulmonary Center, Department of Medicine, Boston University, Boston, MA, USA, Boston, Massachusetts, United States; 4 National Emerging Infectious Diseases Laboratories (NEIDL) and Department of Microbiology, Boston University, Boston, MA, USA, Boston, Massachusetts, United States; 5 National Emerging Infectious Diseases Laboratories (NEIDL) and Department of Pathology and Laboratory Medicine, Boston University, Boston, USA, Boston, Massachusetts, United States; 6 National Emerging Infectious Diseases Laboratories (NEIDL) and Department of Microbiology, Boston University, Boston, USA, Boston, Massachusetts, United States; 7 Electron Microscopy Facility at Harvard Medical School, Harvard University, Boston, Massachusetts, United States; 8 Whitaker Cardiovascular Institute and Department of Medicine, Boston University School of Medicine, Boston, MA, USA, Boston, Massachusetts, United States; 9 Boston University, National Emerging Infectious Diseases Laboratories (NEIDL), Boston MA, USA, Boston, Massachusetts, United States, 10 BU Pulmonary Center, Department of Medicine and National Emerging Infectious Diseases Laboratories (NEIDL), Boston University, Boston MA, USA; Center for Thrombosis and Hemostasis, University Medical Center of the Johannes Gutenberg‐ University, Mainz, Germany, Boston, Massachusetts, United States
Background: Platelets are effectors of hemostasis and play a major role in coordinating immune and inflammatory activities. Suitable animal models are needed to study COVID‐19‐associated coagulopathy and platelet effector functions in COVID‐19, which are currently poorly understood.
Aims: We aimed to characterize alterations of platelets isolated from K18‐hACE2 transgenic mice infected with SARS‐CoV‐2.
Methods: Heterozygous K18‐hACE2 (human ACE2) and C57BL/6J mice were used to study SARS‐CoV‐2 infectivity. Lung infection, infiltration, and platelet aggregation were characterized with histology and immunohistochemistry. Platelet response to SARS‐CoV‐2 infection was quantified by mass spectrometry analysis of proteomics and phosphoproteomics. Western blotting, ELISA, and multiplex plasma profiling were performed to validate the proteomics and phosphoproteomics data.
Results: SARS‐CoV‐2 inoculated (10E6PFU, i.n.) K18‐hACE2 mice started to lose weight at 4 days post‐infection (dpi) and showed 90% lethality at 7‐dpi in association with viral neuroinvasion. Histopathologic findings of infected K18‐hACE2 mice included progressive lymphohistiocytic interstitial pneumonia with absence of diffuse alveolar damage. Lungs of infected K18‐hACE2 mice (2‐/4‐dpi) showed mild increase in CD61+ aggregates compared to sham mice, but no overt tissue thrombosis. Gene ontology and pathway analyses of platelet proteomics and phosphoproteomics revealed that SARS‐CoV‐2 infection significantly upregulates the complement‐coagulation cascades (F2/12/13, Tfpi, C1ra, Cd55, C4bp) and platelet activation‐adhesion‐degranulation proteins (Vwf, Itgb3/5, Selp, Pecam1) and chemokine (Pf4, Cxcl5/12) signaling at 2‐dpi. However, interferon (Ddx58, Trim25, Mapk3) signaling was dominant at 4‐dpi. Activation of proteomics and phosphoproteomics protein markers were highly correlated with platelet activation and interferon signaling at 2‐/4‐dpi, respectively. Plasma chemokine (e.g., Ccl8 and Pf4) and cytokines (e.g., IL6) were significantly elevated at 2‐/4‐dpi. SARS‐CoV‐2 spike protein was abundant at 2‐/4‐dpi in the lungs but not in platelets and kidneys, which correlated with no infectious virus in the serum.
Conclusion(s): Platelet re‐programming towards activation‐degranulation‐aggregation is likely attributable to a pneumonia‐induced elevated circulatory factors (e.g., cytokines)‐driven response rather than direct platelet infection.
OC 05.3
Hypoxia promotes metabolic and functional platelet exhaustion in critically ill COVID‐19 patients
L. lombardi1; F. Maiorca2; R. Marrapodi1; N. Scafa3; S. Cesaroni4; B. Corica4; G. Romiti4; P. Pasculli4; M. Ciardi1; F. Ruberto5; F. Pugliese6; F. Pulcinelli1; C. Mastroianni1; S. Basili4; L. Stefanini 1
1 Sapienza University of Rome, Rome, Lazio, Italy; 2 Sapienza University of Rome, ROMA, Lazio, Italy; 3 Sapienza University of Rome, Roma, Lazio, Italy; 4 Sapienza University of Rome, roma, Lazio, Italy, 5 sapienza university of rome, roma, Lazio, Italy; 6 Sapienza university of Rome, Rome, Lazio, Italy
Background: Severe Coronavirus disease 2019 (COVID‐19) has been associated with a dysregulated cytokine production, lymphocyte and monocyte exhaustion, and immunothrombotic complications that reduce gas exchange in the lungs and contribute to multiorgan failure.
Aims: The objective of this study was to characterize the interplay between platelets and the dysregulated immune phenotype that drives disease severity.
Methods: To achieve this goal, we performed a high‐throughput flow cytometric profiling of the phenotype and interactions of platelets circulating in the blood of Sars‐COV2‐positive subjects upon hospitalization. Patients were stratified into non‐ICU (n = 35) and critically ill ICU (n = 25) patients and compared to sex‐ and age‐matched Sars‐COV2‐negative patients (n = 15) and healthy volunteers (n = 20). All participants gave written informed consent. The study was approved by the Ethics Committee of our institution.
Results: Platelets from ICU patients had dysfunctional mitochondria and a non‐adhesive phenotype. Displayed significantly less glycoprotein (GP)Ibalpha and GPVI on the surface and failed to present active integrin alphaIIbbeta3 and P‐selectin on the plasma membrane in response to exogenous stimuli. Platelet hypo‐responsiveness positively correlated with the Horowitz index (PaO2/FiO2 ratio), a measure of lung function, and with the D‐dimer concentration, a surrogate marker of ongoing thrombosis. Exposure of platelets from healthy volunteers to acute hypoxic conditions (1% O2) recapitulated this phenotype in vitro. Despite the low adhesiveness, platelets of ICU patients bound avidly to innate immune cells. Interactions with monocytes and NK cells increased with severity, even though these leukocytes subpopulations were reduced in the circulation of ICU patients. Platelet‐T cell aggregates were doubled in non‐ICU patients compared to controls but were not detectable among the ICU patients.
Conclusion(s): In summary, platelets from COVID‐19 patients who have reduced lung function present features of metabolic and functional exhaustion and bind primarily innate but not adaptive immune cells, thus promoting the dysregulated immune response that drives COVID19 severity.
OC 05.4
Biphasic thrombus formation and resolution during a coronavirus infection in the liver
M. Salzmann 1; J. Kral‐Pointner1; P. Gibler1; J. Wojta1; R. Plasenzotti1, P. Hohensinner2
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Center for Biomedical Research, Medical University of Vienna, Vienna, Wien, Austria
Background: The liver is the main producer of coagulation factors, which are activated in response to a variety of viruses, presumably to contain and limit virus spreading. Neutrophils and their extracellular traps (NETs) often build the molecular and structural basis for such thrombi that can cause adverse complications and organ damage in patients. Using the murine coronavirus m‐CoV, we analysed the consequences of a coronavirus infection in the liver.
Aims: Analysis of haemostatic alterations and immunothrombosis in a virus infected liver.
Methods: C57Bl/6 mice were nasally infected with m‐CoV (MHV‐A59). Livers were collected 2, 4 and 10 days after infection, and virus burden, tissue damage, thrombus formation, expression of coagulation factors and neutrophil activation were analysed via histology and qPCR.
Results: NET formation occurred rapidly and U‐shaped over the course of the infection: 1.55 thrombi/mm2 liver section were present 2 days after m‐CoV infection, which decreased to 0.50 thrombi/mm2 at day 4 and increased again to 1.04 thrombi/mm2 at day 10. Neutrophil counts significantly increased until day 4 and showed highest MPO activity on day 2. M‐CoV virus is mainly present 4 days after infection and coincided with highest percentage of damaged tissue (4.58%). Although mRNAs of factors II, V, VII, VIII, IX, and X were not significantly altered, we could detect a significant peak in plasminogen mRNA on day 2 and PAI 1 mRNA on day 4. Plasma concentrations of MCP‐1, IFN‐γ, IL‐6 and TNF α were also highest at day 4.
Conclusion(s): We describe a U‐shaped liver thrombosis development during a coronavirus infection. Liver NETs are rapidly formed after a viral infection and are resolved due to increased plasminogen expression. Peak virus burden of the liver and increased systemic inflammation markers induce expression of liver PAI‐1 and activation of recruited neutrophils, which favours NET formation anew.
OC 13.3
Temporal changes in coagulation, endothelial dysfunction and fibrinolysis biomarkers are associated with illness severity in patients with COVID‐19: Canadian COVID‐19 Prospective Cohort Study (CanCOV)
G. Juneja 1; M. Castelo2; C. Yeh1; V. Sabourin1; B. Hansen3; A. Cheung3; M. Herridge3; P. Kim1
1 McMaster University; Thrombosis & Atherosclerosis Research Institute (TaARI), Hamilton, Ontario, Canada; 2 University of Toronto, Toronto, Ontario, Canada; 3 University of Toronto; University Health Network, Toronto, Ontario, Canada
Background: The mechanisms by which COVID‐19 results in severe illness in some individuals remains poorly defined. Identification of biomarkers associated with disease severity could be useful in defining the mechanisms of COVID‐19 pathology and predicting disease course.
Aims: Identify trajectories of biomarkers of coagulation, endothelial dysfunction, and fibrinolysis that are associated with COVID‐19 severity.
Methods: Longitudinal plasma samples were collected from 99 patients in the Canadian COVID‐19 Prospective Cohort Study (CanCOV) (23 outpatients, 31 ward patients, and 45 intensive care unit (ICU) patients). Plasma was quantified using 1) ELISAs for plasminogen, soluble thrombomodulin (sTM), plasminogen activator inhibitor‐1 (PAI‐1), α2‐antiplasmin, D‐dimer, thrombin‐activatable fibrinolysis inhibitor (TAFI), and fibrinogen, and 2) in‐house functional assays for clot lysis times and activated TAFI (TAFIa) levels. Biomarker values were log‐transformed and linear mixed effects models were used to compare trajectories in ICU and ward patients compared to outpatients from date of symptom onset.
Results: Among the 45 ICU patients, 24 (53%) died. There were no deaths in the other patient groups. D‐dimer (Fig 1A) and sTM (Fig 1B) were significantly elevated for both hospitalized and ICU cohorts when compared with outpatients. PAI‐1 (Fig 1C) was significantly elevated only in the ICU group between days 1 and 40. Plasminogen (Fig 1D) significantly decreased only in the ICU group from day 25 onwards. TAFIa (Fig 1E) increased over time only in the ICU cohort, with the levels being significant from day 35. Fibrinogen (Fig 1F) displayed similar trends as plasminogen whereby only the ICU was significantly decreased from day 25. α2‐antiplasmin, TAFI, and clot lysis times were not significantly different compared to COVID‐19 outpatients.
Conclusion(s): D‐dimer and sTM showed the strongest associations with moderate and severe COVID‐19 compared to mild disease. PAI‐1, plasminogen, TAFIa, and fibrinogen may additionally be useful in identifying patients who become critically ill. FIGURE 1 Linear mixed effects models used to compare trajectories in ICU and ward patients with respect to outpatients from the date of symptom onset
FIGURE 1 Linear mixed effects models used to compare trajectories in ICU and ward patients with respect to outpatients from the date of symptom onset
OC 05.1
SARS‐CoV‐2 RBD and its variants can induce platelet activation and clearance: Implications for vaccinations and antibody therapy against COVID‐19
X. Ma1; J. Liang1; G. Zhu2; P. Bhoria 1; D. MacKeigan3; L. Lin1; Z. Chen4; D. Karakas4; Z. Liu1; C. Shen1; Y. Li4; J. Dou1; Z. Rousseau4; H. Shaykhalishahi1; J. Zhang1; T. Ni4; P. Chen1; H. Zhang4; O. Rotstein4; H. Ni2
1 St. Michael's Hospital, Toronto, Ontario, Canada; 2 St. Michael's Hospital, Keenan Research Centre, Toronto, Ontario, Canada; 3 University of Toronto, Toronto, Ontario, Canada; 4 St. Michael's Hospital/University of Toronto, Toronto, Ontario, Canada
Background: Severe COVID‐19 is associated with platelet activation, thrombosis, and thrombocytopenia, but the mechanisms remain unclear. Similarly, very rare cases of COVID‐19 vaccine‐induced‐thrombotic‐thrombocytopenia (VITT) are also poorly understood. Both infection and vaccination utilize the receptor‐binding domain (RBD) of the spike protein for virus‐host cell entry and to elicit an immune response, respectively. Interestingly, the RBD contains an “RGD” integrin‐binding motif that may facilitate platelet binding.
Aims: To determine whether the RBD binds platelets and causes platelet activation/clearance.
Methods: We intravenously injected different doses (0.25, 0.5, 1.0μg/g) of recombinant RBD into mice and measured platelet counts post‐injection using a Z2 Series Coulter. Flow cytometry detected RBD/RBD variants binding to platelets and associated platelet activation, apoptosis, and desialylation. Human gel‐filtered platelet aggregation was induced by ADP, Collagen and Thrombin. Six anti‐RBD monoclonal antibodies (mAbs) were generated and tested in a SARS‐CoV‐2 Vero cell infection model with the envelop gene quantified by RT‐qPCR to determine the virus replication.
Results: RBD injection caused platelet clearance in a dose‐dependent manner. The RBD could also bind to platelets, induce activation and potentiate platelet aggregation in vitro. Our preliminary data also showed the RBD Delta variant has greater potential in inducing platelet activation. Interestingly, the RBD bound β3‐/‐ platelets ~50% less relative than wildtype mice. Consistently, mutating the RGD motif to RGE, and preincubating platelets with the β3 inhibitor Eptifibatide also reduced RBD binding to platelets. Our novel anti‐RBD mAbs 4F2 and 4H12 inhibited RBD‐induced platelet activation and RBD‐potentiated platelet aggregation in vitro, and prevented RBD‐induced platelet clearance in vivo. Importantly, these mAbs also inhibited SARS‐CoV‐2 viral replication in a dose‐dependent manner.
Conclusion(s): Our data demonstrate that the RBD could directly bind to platelets partially via β3 integrin. RBD‐induced platelet activation and clearance may contribute to thrombosis and thrombocytopenia observed in clinical cases of COVID‐19 and VITT.
COVID and Coagulation, Clinical
LB 02.2
Hemorrhagic, coagulopathic, and thrombotic (HECTOR) complications among critically‐ill patients with COVID‐19: An International COVID‐19 critical care consortium study
J. Fanning 1; R. Fanning2; N. Weaver3; M. Griffee4; S. Cho1; M. Panigada5; A. Zaaqoq6; N. White7; N. Obonyo8; A. Labib9; Y. Chia10; B. Fan11; R. Arora12; D. Chiumello13; A. Torres14; J. Suen15; G. Li Bassi15; G. Peek16; J. Fraser15; H. Dalton17
1 Johns Hopkins Medicine, Baltimore, Maryland, United States; 2 University of Melbourne, Melbourne, Queensland, Australia; 3 University of Newcastle, Newcastle, New South Wales, Australia; 4 University of Utah, Salt Lake City, Utah, United States, 512. Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico di Milano, Milan, Lombardia, Italy; 6 MedStar Washington Hospital Center, Georgetown University, Washington, District of Columbia, United States; 7 Queensland University of Technology, Brisbane, Queensland, Australia; 8 Initiative to Develop African Research Leaders (IDeAL)/KEMRI‐Wellcome Trust Research Programme, Kilifi, Kenya; Wellcome Trust Centre for Global Health Research at Imperial College London, United Kingdom, Kilifi, North‐Eastern, Kenya; 9 Hamad General Hospital, Hamad, Al Wakrah, Qatar, 10 National University of Singapore, Singapore, Not Applicable, Singapore, 11 National University of Singapoore, Singapore, Not Applicable, Singapore, 12 University of Manitoba, Winnipeg, Manitoba, Canada, 13 San Paolo University Hospital, Milan, Lombardia, Italy, 14 Centre de Investigación Bioedica En Red ‐ Enfermedades Respiratorias, Barcelona, Catalonia, Spain, 15 University of Queensland, Brisbane, Queensland, Australia, 16 University of Florida, Gainesville, Florida, United States, 17 Inova Fairfax Hospital, Falls Church, Virginia, United States
Background: Hemorrhage, coagulopathy and thrombosis (HECTOR) are reported complications of coronavirus disease 2019 (COVID‐19); however, more information is needed on the prevalence of these complications and their associated outcomes in intensive care unit (ICU) settings.
Aims: To determine the prevalence and outcomes of HECTOR complications in ICU patients with COVID‐19.
Methods: Observational cohort study spanning 229 ICUs across 32 countries. Patients ≥16 years admitted for severe COVID‐19 from 1st January 2020, through 31st December 2021 were included. Patient characteristics and clinical data were collected. Survival analysis estimated the instantaneous impact of HECTOR complications on ICU‐mortality and discharge.
Results: HECTOR complications occurred in 1,735 (14%) of 11,972 study‐eligible patients. Acute thrombosis occurred in 1,249 (10%) patients, including 712 (57%) with pulmonary embolism, 413 (33%) with myocardial infarction, 93 (7.4%) with deep vein thrombosis, and 49 (3.9%) with ischemic stroke. Hemorrhagic complications were reported in 582 (4.9%) patients, including 276 (48%) with gastrointestinal hemorrhage, 83 (14%) with hemorrhagic stroke, and 77 (13%) with pulmonary hemorrhage. Disseminated intravascular coagulation occurred in 11 (0.09%) patients. Univariate analysis identified diabetes, hypertension, cardiac and kidney disease and ECMO as statistically‐significant risk factors for HECTOR complications. Patients with versus without HECTOR complications suffered higher ICU‐mortality at 28 days (25%vs.13%, p < 0.001), 90 days (32%vs.15%, p < 0.0001) and overall (44%vs.36%, p < 0.001). Among ICU survivors, the ICU stay was longer (median days 19vs.12, p < 0.001). ICU mortality was similar between patients with and without HECTOR complications (HR = 1.01, 95%CI 0.92‐1.12, p = 0.783) where an increased hazard of ICU mortality with hemorrhage (HR = 1.26, 1.09‐1.45, p = 0.002) was balanced by a reduced hazard of thrombosis (HR = 0.88, 0.79‐0.99, p = 0.03). Kaplan‐Meier curves are presented in the Figure.
Conclusion(s): HECTOR events are frequent complications of severe COVID‐19 in ICU patients. Hemorrhagic, but not thrombotic complications are associated with increased ICU‐mortality. FIGURE 1 Kaplan‐ever survival curves for patients with (red) versus without (blue) HECTOR complications
FIGURE 1 Kaplan‐ever survival curves for patients with (red) versus without (blue) HECTOR complications
OC 57.4
Decreased DNase activity in severe COVID‐19 correlates with increased circulating NET burden
C. Martens 1; S. Kraisin2; L. Velásquez Pereira3; R. Lavend'homme4; C. Jacobs5; L. Vanderbeke6; E. Wauters7; P. Verhamme3; T. Witsch8; M. Jacquemin9; J. Wauters10; K. Martinod1
1 KU Leuven, Leuven, Vlaams‐Brabant, Belgium; 2 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 3 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Vlaams‐Brabant, Belgium; 4 Center for Molecular and Vascular Biology, University of Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 5 Laboratory for Clinical Infectious and Inflammatory Disorders, UZ Leuven, Leuven, Vlaams‐Brabant, Belgium; 6 Laboratory of Clinical Microbiology, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Vlaams‐Brabant, Belgium; 7 Department of Pneumology, UZ Leuven; Laboratory of Respiratory Diseases and Thoracic Surgery (BREATHE), Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Vlaams‐Brabant, Belgium; 8 Invasive Cardiology, University Hospital Basel; Department of Cardiology and Angiology I, University Heart Center, Freiburg‐Bad Krozingen; Faculty of Medicine, University of Freiburg, Freiburg, Germany, Freiburg, Baden‐Wurttemberg, Germany; 9 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium; Department of Vascular Medicine and Hemostasis, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium, 10 Medical Intensive Care Unit, Department of Internal Medicine, UZ Leuven; Laboratory for Clinical Infectious and Inflammatory Disorders, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Vlaams‐Brabant, Belgium
Background: COVID‐19 represents a “perfect storm” for release of neutrophil extracellular traps (NETs) and resultant pathology from immunothrombosis. Levels of NETs in circulation are regulated by endogenous plasma DNases, which have been shown to be reduced in various diseases including myocardial infarction and sepsis. We previously reported elevated NET biomarkers in admission samples from our first wave study cohort.
Aims: To characterize DNase activity and biomarkers of released NETs in the context of COVID‐19 immunothrombosis.
Methods: With ethical permission and informed consent, we prospectively collected citrated platelet‐poor plasma samples from patients admitted to the COVID ward (55 patients) or intensive care unit (216 patients) from March 2020‐December 2021 as part of the COntAGIouS trial at UZ Leuven in Belgium (NCT04327750), with special attention paid to sample preparation and storage to preserve NET fragments and DNase activity. Consecutive samples were obtained within 48 hours of admission, between days 6‐8, and upon hospital or ICU discharge. Analysis was batch‐performed for MPO, MPO‐DNA, PF4, sP‐selectin, citrullinated histones, DNase activity, VWF:Ag, and FVIII:Ag levels.
Results: In ICU patients, MPO, VWF, sP‐selectin, and NET biomarkers were elevated throughout hospitalization, peaking at day 6‐8 after admission, whereas PF4 and FVIII remained highly elevated through the time of ICU discharge. DNase activity was decreased in admission samples, normalized at day 6‐8, and strongly increased at the time of discharge, indicating a potential compensatory mechanism. DNase activity was negatively correlated with MPO‐DNA values (r = ‐0.29, p = 0.0013). sPselectin and NET levels were significantly higher in admission samples for patients who experienced a thrombotic event in the period during hospitalization, including pulmonary embolism, DVT, myocardial infarction, and/or stroke.
Conclusion(s): Elevated NET levels and decreased DNase activity in plasma are correlated in severe COVID‐19, together with elevated markers of thrombotic risk. Approaches to restore DNase activity in plasma may be beneficial in COVID‐19‐associated immunothrombosis.
OC 13.5
A multicenter randomized clinical trial of modulation of host thromboinflammatory response in hospitalized patients with COVID‐19: The DAWn‐Antico study
M. Engelen 1; J. Wauters1; J. Gunst1; C. Wouters1; C. Vandenbriele1; S. Rex1; M. Thomeer2; T. Fivez2; D. Mesotten2; D. Ruttens2; L. Heytens3; P. Verhamme4; T. Vanassche5
1 University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 2 Ziekenhuis Oost‐Limburg (ZOL), Genk, Limburg, Belgium; 3 Ziekenhuizen GasthuisZusters Antwerpen (GZA), Antwerpen, Antwerpen, Belgium; 4 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 5 University Hospitals Leuven, KULeuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium
Background: Thromboinflammation in severe COVID‐19 is associated with disease severity and inferior outcome. Evidence suggests that the kallikrein pathway potentially plays a vital role in COVID‐19 associated thromboinflammation as it both activates downstream inflammatory pathways and contact‐mediated coagulation.
Aims: To investigate whether modulation of this pronounced thromboinflammatory response can improve outcomes in hospitalized patients with severe COVID‐19.
Methods: This multicenter randomized clinical trial was approved by the ethics committee and supported by the KU Leuven COVID‐19 fund and Research Foundation Flanders (FWO). After informed consent, eligible patients were 1:2 randomized to receive standard of care (SOC) or SOC plus study intervention (figure 1). The intervention consisted of off‐label ‐ kallikrein‐inhibiting ‐ aprotinin combined with low molecular weight heparin (LMWH). Additionally, patients with predefined hyperinflammation were treated with the interleukin‐1 receptor antagonist anakinra. The primary endpoint was time to sustain a 2‐point improvement in the WHO ordinal scale for clinical status.
Results: Three hospitals in Belgium included 102 patients (35 SOC vs. 67 intervention). Twenty‐five patients from the intervention group (37%) were treated with anakinra. Patients had elevated D‐dimers (mean 1012.4 μg/L; SD 991.9 μg/L) and C‐reactive protein (mean 81.4 mg/L; SD 59.6 mg/L) at admission confirming baseline activation of coagulation and inflammatory pathways. During hospitalization, 37% of patients were admitted to the ICU (29% SOC vs. 42% intervention), and 20% needed invasive ventilation (12% SOC vs. 25% intervention). The intervention did not affect the time to sustained clinical improvement or hospital discharge (figure 2), nor secondary clinical endpoints. Except for D‐dimers at day 3, there was no significant C‐reactive protein or D‐dimer reductions. There were no differences in treatment‐related adverse events.
Conclusion(s): In hospitalized COVID‐19 patients, additional modulation of thromboinflammation with high‐dose aprotinin and LMWH with or without anakinra was feasible and safe but did not improve clinical nor biochemical outcomes.
OC 33.2
Hyperinflammatory state and death in patients affected by COVID‐19: Role of vaccines
L. Marchini; M. Vedovati; C. Becattini
University of Perugia, Perugia, Umbria, Italy
Background: Systemic hyperinflammation in patients with COVID‐19 is associated with a higher probability of severe disease and poor prognosis. The COVID‐19 vaccines are expected to be associated with a lower risk of moderate‐severe disease. However, data on the effects of vaccination on the inflammatory state and adverse outcomes in patients affected by COVID‐19 are lacking.
Aims: To evaluate the association of hyperinflammation state and adverse outcome among vaccinated and unvaccinated patients for SARS‐CoV‐2 infection.
Methods: We performed an observational study on COVID‐19 patients admitted to a non‐ICU ward at Perugia Hospital from August to December 2021. The inclusion criteria were: age ≥ 18 years, hospitalization due to respiratory failure, and SARS‐CoV‐2 infection confirmed by nasopharyngeal RT‐PCR swab. A patient was defined as vaccinated after a full cycle. Study outcomes were all‐cause‐death or symptomatic venous thromboembolism (VTE) and hyperinflammation state (defined as at least four values among CRP, LDH, ferritin, CPK, D‐dimer above the threshold).
Results: Overall, 182 patients were included (mean age 68 years, range 18‐98). All‐cause death occurred in 16 patients (8.8%). Vaccinated patients were older (76 vs 61 years); they had a higher rate of comorbidities and a lower rate of NIV/HFNC requirement than unvaccinated patients. After age adjustment, the hyperinflammation state was significantly more frequent in unvaccinated compared to vaccinated patients (65 vs 37%, p = 0.004). Lack of vaccination was an independent predictor of in‐hospital all‐cause‐death (HR 2.71, 95% CI 1.05‐7.00, p = 0.040) and all‐cause‐death or symptomatic VTE (HR 3.10, 95% CI 1.31‐7.35, p = 0.010). The risk of symptomatic VTE was not significantly higher in unvaccinated compared to vaccinated patients (5 vs 1%, HR 5.66, 95% CI 0.63‐50.85).
Conclusion(s): Covid‐19 vaccination is associated with a lower hyperinflammation state and lower risk of death compared to lack of vaccination. These findings should be confirmed in a larger population. FIGURE 1 The cumulative risk of death or symptomatic VTE in vaccinated and unvaccinated patients
FIGURE 1 The cumulative risk of death or symptomatic VTE in vaccinated and unvaccinated patients
OC 33.3
Absence of hypercoagulability after nCoV‐19 vaccination: An observational longitudinal study
C. Simion 1; E. Campello1; C. Bulato2; L. Spiezia3; D. Tormene4; C. Radu2; S. Gavasso2; N. Perin2; M. Sartori3; P. Simioni5
1 University of Padova, Padova, Veneto, Italy; 2 Unviersity of Padova, Padova, Veneto, Italy; 3 Padova University Hospital, Padova, Italy, Padova, Veneto, Italy; 4 Padova university hospital, Padova, Veneto, Italy; 5 Padua University Hospital, Padua, Veneto, Italy
Background: A large number of daily requests to exclude possible prothrombotic risk factors for COVID‐19 vaccines were received.
Aims: Our aim was to longitudinally evaluate coagulation profiles in a series of healthy subjects who received COVID‐19 vaccination and assess hypercoagulability thereafter.
Methods: Volunteers awaiting a first or second dose of either the ChAdOx1 or BNT162b2 vaccine were enrolled. Venous samples were obtained at baseline (before the vaccine) and longitudinally 3 ± 2 days (T1) and 10 ± 2 days after the vaccine (T2). Global coagulation monitoring was assessed via platelet count, whole blood thromboelastometry and impedance aggregometry, plasma thrombin generation and anti‐PF4/heparin IgG antibodies. Informed consent was obtained and the study was approved by the local medical ethics committee.
Results: One hundred and twenty‐two subjects were enrolled (61 [50%] ChAdOx1 and 61 BNT162b2). The ChAdOx1 cohort showed a slight but transient increase in thrombin generation (mainly endogenous thrombin potential [ETP] with thrombomodulin and ETP ratio) at T1, which promptly decreased at T2. In addition, the second dose of either vaccine was associated with increased thrombin peak, ETP with thrombomodulin and ETP ratio. At baseline, 3.2% of the ChAdOx1 cohort and 1.6% BNT162b2 cohort were positive for PF4/heparin antibodies with a stable titre through T1 and T2 (Figure 1). No relevant differences were detected in platelet count and aggregation, or thromboelastometry parameters. No thrombotic or haemorrhagic events occurred.
Conclusion(s): We can confirm that no clinically meaningful hypercoagulability occurred after either vaccine, albeit keeping in mind that thrombin generation may increase in the first days after the second dose of either vaccine and after the first dose of the ChAdOx1 vaccine. FIGURE 1
FIGURE 1
OC 13.4
Cardio‐embolic vulnerability predicts hospitalisation in primary care clinically suspected and confirmed COVID‐19 patients: A model development and validation study
F. van Royen 1; L. Joosten2; M. van Smeden3; P. Slottje4; F. Rutten3; G. Geersing3; S. van Doorn2
1 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands, Utrecht, Utrecht, Netherlands; 2 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 3 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 4 Dept. General Practice, Academic network of general practice, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Cardio‐embolic conditions were shown to be predictive of clinical deterioration in hospitalised patients with coronavirus disease 2019 (COVID‐19). Whether this also holds for outpatients managed in primary care is yet unknown.
Aims: The aim of this study was to determine the incremental prognostic value of cardio‐embolic vulnerability in predicting the risk of hospital referral in primary care COVID‐19 outpatients.
Methods: Data were retrospectively collected from three large Dutch primary care registries. Consecutive adult patients seen in primary care for COVID‐19 symptoms in the ‘first wave’ of COVID‐19 infections (March 1 2020 to June 1 2020) and in the ‘second wave’ (June 1 2020 to April 15 2021) were included. A multivariable logistic regression model was fitted to predict hospital referral within 90 days after first COVID‐19 consultation in primary care. Data from the ‘first wave’ was used for derivation. Age, sex, the interaction between age and sex, and the number of cardiovascular and thrombo‐embolic conditions and/or diabetes (0, 1, or ≥2) were pre‐specified as candidate predictors. This full model was (i) compared to a simple model including only age and sex and its interaction, and (ii) externally validated in COVID‐19 patients during the ‘second wave’.
Results: There were 5,475 patients included for model development and 16,693 for external validation. The full model performed better than the simple model (likelihood ratio test p < 0.001). Older male patients with multiple cardio‐embolic conditions and/or diabetes had the highest predicted risk of hospital referral, reaching risks above 15‐20%, whereas on average this risk was 5.1%. The temporally validated c‐statistic was 0.747 (95%CI 0.729‐0.764) and the model showed good calibration upon validation.
Conclusion(s): For patients with COVID‐19 symptoms managed in primary care, the risk of hospital referral was on average 5.1%. Older, male and cardio‐embolic vulnerable COVID‐19 patients are more at risk for hospital referral. FIGURE 1 Plotted risk of hospital referral in (clinically suspected and confirmed) COVID‐19 primary care patients at a certain age and stratified by sex and by the number of cardiovascular diseases and thrombo‐embolic conditions and/or diabetes (i.e. 0, 1 or ≥2). Confidence intervals are shown in grey
FIGURE 1 Plotted risk of hospital referral in (clinically suspected and confirmed) COVID‐19 primary care patients at a certain age and stratified by sex and by the number of cardiovascular diseases and thrombo‐embolic conditions and/or diabetes (i.e. 0, 1 or ≥2). Confidence intervals are shown in grey
OC 13.1
Arterial thromboembolism risk assessment model in hospitalized COVID‐19 patients
P. Li 1; Y. Lee2; Q. Jehangir2; C. Lin3; G. Krishnamoorthy2; A. Sule2; D. Apala2; A. Halabi2; K. Patel2; D. Wang3; L. Poisson3; G. Nair4
1 Henry Ford Health System, DETROIT, Michigan, United States; 2 St. Joseph Mercy Oakland Hospital, Pontiac, Michigan, United States; 3 Henry Ford Health System, Detroit, Michigan, United States; 4 William Beaumont Hospital, Royal Oak, Michigan, United States
Background: Patients with SARS‐CoV‐2 infection are at an increased risk of cardiovascular and thrombotic complications portending an extremely poor prognosis. COVID‐19 infection is known to be an independent risk factor for acute ischemic stroke (AIS) and myocardial infarction (MI).
Aims: We propose to develop risk assessment model (RAM) that can risk stratify hospitalized COVID‐19 patients for arterial thromboembolism (ATE).
Methods: This multicenter, retrospective study included adult patients admitted with PCR proven SARS‐CoV‐2 infection between 3/1/2020 and 9/5/2021. The composite outcome was in‐hospital ATE events, including AIS, MI, and other ATE identified by ICD‐10 codes. 49 variables, including baseline demographics, past medical history, presenting vitals and laboratory values, were categorized with multiple imputation to impute missing values. Variables selected by LASSO regression were used to build the final RAM.
Results: Among 3531 patients from training cohort (admitted before 12/31/2020), 548 (15.5%) patients developed acute ATE, compared to 285 of 2508 (11.4%) in the validation cohort (admitted after 12/31/2020). The final score included 16 items: male gender (1); Non‐African American race (1); Age 40‐59 (2), Age 60+ (4); Systolic blood pressure > = 160mmHg (1); History of cerebrovascular accident (1), Coronary artery disease (1), Smoking (1); Leukocytes > 11 K/uL (1), B‐type natriuretic peptide > 100 pg/mL (1), Lactate dehydrogenase > 192 U/L (1), Creatinine > 1.4 mg/dL (1), Aspartate aminotransferase > 41 U/L (1), Troponin‐I > 0.03 ng/mL (1), Troponin‐I > 0.09 ng/mL (3), Interleukin‐6 > 5 pg/mL(1), Potassium < 3.5 mEq/L(1), Magnesium < 1.8 mg/mL (1). RAM had a good discrimination for ATE (training AUC 0.777, 95% CI 0.756–0.797; validation AUC 0.749, 95% CI 0.721–0.778). The validation cohort was stratified as low‐risk (score 0‐8), intermediate‐risk (score 9‐13) and high‐risk groups (score 14+), with the incidence of ATE 2.1%, 11.3%, and 31.1%, respectively.
Conclusion(s): Our prediction model based on 16 parameters commonly available at hospital admission showed moderate performance in identifying hospitalized COVID‐19 patients at low and high risk for ATE.
OC 13.2
Convalescent plasma therapy does not increase the risk of thromboembolism in the treatment of COVID‐19: A systematic review and meta‐analysis
P. Li 1; A. Li2; P. Yu3; M. Khalid4; M. Laureano5; M. Crowther6
1 University of Toronto, Toronto, Ontario, Canada; 2 University of Ottawa, Ottawa, Ontario, Canada; 3 McMaster University, Faculty of Health Sciences, Hamilton, Ontario, Canada; 4 McMaster University, Michael G. DeGroote School of Medicine, Hamilton, Ontario, Canada; 5 McMaster University, Michael D. DeGroote School of Medicine, Hamilton, Ontario, Canada; 6 McMaster University, Department of Medicine, Hamilton, Ontario, Canada
Background: Convalescent plasma therapy (CPT) has been issued emergency use authorization for the treatment of SARS‐CoV‐2 (COVID‐19) by the United States Food and Drug Administration. The presence of coagulation factors in CPT in combination with the pro‐thrombotic state COVID‐19 patients are in may potentiate their risk of thrombotic events.
Aims: To assess the risk of venous thromboembolisms (VTE) and arterial thromboembolisms (ATE) in COVID‐19 patients undergoing CPT.
Methods: MEDLINE, Embase, and Cochrane CENTRAL were searched for randomized controlled trials that investigated the safety and efficacy of CPT and standard of care (SOC) against placebo or SOC alone in adult COVID‐19 patients. Study selection and data extraction were done in duplicate. The primary outcome was the development of VTE and ATE. The secondary outcomes were 30‐day mortality, clinical improvement, length of hospitalization (LOH), sepsis/fever, and major adverse cardiovascular events (MACE). Meta‐analysis was conducted using the Mantel‐Haenszel random effects model. Binary endpoints and continuous endpoints were synthesized using odd ratios (OR) and mean differences respectively with 95% confidence intervals (CI).
Results: 17 randomized controlled trials including 18566 patients were included (Table 1). The risk of VTE and ATE did not differ between the CPT and the control group. There were also no significant differences in 30‐day mortality, clinical improvement, LOH, risk of sepsis/fever, and MACE. A summary of outcomes is illustrated in Table 2.
Conclusion(s): Treatment of COVID‐19 with CPT does not appear to be associated with an increased risk of VTE, ATE, or adverse events but also does not appear to provide mortality or clinical benefit.
Platelet Function and Interactions
OC 33.5
Anti‐PF4 IgG levels in patients with VITT remain high at 7 months following vaccination and can still activate platelets
S. Montague 1; C. Smith2; C. Lodwick3; C. Stoneley4; M. Roberts4; G. Lowe5; W. Lester4; S. Watson1; P. Nicolson6
1 University of Birmingham, Birmingham, UK, Birmingham, England, United Kingdom; 2 University of Birmingham, Birmingham, England, United Kingdom; 3 Worcester Acute Hospitals NHS Trust, Worcester, England, United Kingdom; 4 University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 5 University Hospitals Birmingham NHS Trust, Birmingham, England, United Kingdom; 6 University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK, Birmingham, England, United Kingdom
Background: Vaccine‐induced immune thrombocytopenia and thrombosis (VITT) is a new syndrome that occurs 4‐30 days following COVID‐19 vaccination with adenoviral vector vaccines. VITT is characterised by thrombocytopenia, thrombosis, highly elevated levels of D‐dimers and anti‐platelet factor 4 (PF4) antibodies. Anti‐PF4 antibodies activate platelets via FcγRIIA driving pathophysiology. However, the evolution of these antibodies and their ability to activate platelets after initial treatment remains unknown.
Aims: To determine how anti‐PF4 antibody levels in VITT patients change following recovery and the ability of patient serum to activate platelets.
Methods: We followed‐up seven discharged VITT patients from diagnosis up to 280 days (range: 199‐280) post‐vaccination and measured anti‐PF4 antibodies and other biomarkers, including PF4 levels, in patient serum. We tested the ability of patient serum to activate healthy and patient platelets using light transmission aggregometry with and without PF4 addition. We also assessed platelet function of patients’ platelets at the latest follow‐up timepoint.
Results: Anti‐PF4 IgG antibody levels remained high in 6 out of 7 patients up to 7 months post‐vaccination. The other patient received rituximab. Diagnostic patient serum strongly activated control (n = 3) and patient platelets, either alone or with PF4. Most follow‐up serum alone (5 out of 7 patients) was weaker at stimulating platelets, despite similar anti‐PF4 antibody levels. However, PF4‐enhanced serum‐mediated platelet activation was detectable in 3 out 7 patients beyond 150 days post‐vaccination. Patients’ PF4 serum levels were reduced at diagnosis compared to follow‐up (p < 0.0001, n = 7) but returned to healthy control levels during follow‐up. Patients’ platelet responses and FcγRIIA levels were similar to controls.
Conclusion(s): The reduction in serum‐mediated platelet activation during follow‐up, despite similar PF4 antibody levels remains unexplained. Further assessment is required to determine if levels of high affinity anti‐PF4 antibodies are reduced during follow‐up. Additional understanding is also required to assess duration of ongoing anticoagulation for VITT patients.
Diagnostics and OMICs
Biomarkers of Thrombosis and Hemostasis
LB 02.4
Platelets as versatile effectors of thromboinflammation in chronic myeloproliferative neoplasms
A. Krishnan 1; Z. Shen2; W. Du1; C. Perkins1; L. Fechter1; V. Natu1; H. Maecker1; J. Rowley3; J. Gotlib1; J. Zehnder1
1 Stanford University, Palo Alto, California, United States; 2 Harvard University, Stanford, California, United States; 3 University Of Utah, Salt Lake City, Utah, United States
Background: Patients with Philadelphia (Ph)‐negative myeloproliferative neoplasms (MPN), such as polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF), often elicit unique clinical features, such as a tendency toward both thrombosis and hemorrhage, splenomegaly (at times massive) and clinical manifestations of microcirculatory disturbances such as ocular migraine, Raynaud phenomenon, and erythromelalgia. Although an increase in one or more blood cell lineages contributes to these morbid sequelae, the qualitative abnormalities of myeloid cells that increase vascular risk or disease progression are not well understood.
Aims:
Considering the critical role of inflammation in the vascular risk; and considering emerging evidence for platelet functional interplay between thrombosis, inflammation, and cancer, we study the platelet transcriptome in a large clinical cohort of the three chronic progressive MPN phenotypes, ET, PV and MF.
Methods: MPN patient blood samples were obtained with informed consent and approval by the Stanford Institutional Review Board. Established high‐quality reproducible methods were followed for platelet isolation, purification, RNA isolation, high‐throughput sequencing, and bioinformatic analyses
Results: Using platelet RNA‐sequencing (RNA‐seq), we quantify how gene expression, alternative splicing and the associated molecular pathways are altered in each of ET (n = 24), PV (n = 33) and MF (n = 42) with respect to healthy controls (n = 11). Differential markers in each of ET, PV and MF highlight candidate genes as potential mediators of the pro‐thrombotic and pro‐fibrotic phenotypes in MPNs
Conclusion(s): In summary, we present comprehensive platelet RNA profiling of a large (100+) clinical cohort of hematologic disorders (across all three chronic progressive subtypes), that includes both expression and splicing changes as a function of disease. Our findings indicate that the platelet transcriptome offers a unique window into cross‐functional molecular mechanisms of thromboinflammation in myeloproliferative neoplasms, thus expanding our classical understanding of platelet function in hemostasis and thrombosis. MPN platelets reveal a chronic proinflammatory state that likely contributes to disease pathogenesis.
OC 17.1
Growth differentiation factor‐15 is related to adverse prognosis in patients with polyvascular disease receiving dual antithrombotic therapy
E. Krivosheeva 1; A. Komarov2; A. Dobrovolsky2; E. Titaeva2; O. Pogorelova3; T. Balakhonova3; E. Panchenko2
1 National Medical Research Center of Cardiology Ministry of Health of the Russian Federation, Moscow, Moskva, Russia; 2 National Medical Research Center of Cardiology Ministry of Health of the Russian Federation, Department of Clinical Problems of Atherothrombosis, Moscow, Moskva, Russia; 3 National Medical Research Center of Cardiology Ministry of Health of the Russian Federation, Ultrasound Division, Moscow, Moskva, Russia
Background: Polyvascular disease may require long‐term dual antithrombotic therapy (DAT) with aspirin and low‐dose rivaroxaban. This combination resulted in decrease of thrombotic events at the cost of some excess of major bleedings. We proposed that biochemical markers of vascular damage (growth differentiation factor‐15 (GDF‐15) and von Willebrand factor (VWF)) may improve risk stratification for better choice of proper treatment regimen.
Aims: To investigate the prognostic value of GDF‐15 and VWF levels in patients with chronic polyvascular disease receiving long‐term DAT (aspirin+ rivaroxaban 2.5 mg BID).
Methods: Data obtained from single center prospective Registry of Long‐term AnTithrombotic TherApy (REGATTA‐1 NCT04347200). In this analysis we include subgroup of patients with polyvascular disease (CAD+peripheral atherosclerosis) receiving DAT (n = 58; 72,4% males, median age 67 [IQR 62; 70] years). Median duration of follow up period was 10 months [IQR 8.0; 12.0]. Primary outcome was a composite of bleeding (BARC 2‐5) and MACE. Plasma samples for GDF‐15 and VWF were taken before DAT started and analyzed using ELISA.
Results: Frequency of primary outcome during follow up period was 15.5 % (there were 7 bleeding events and only 2 MACE). Median GDF‐15 level was 1147.7 pg/ml [IQR 882.6; 1435.9]. Median VWF level was 157.5% [116.0; 205.0]. According to ROC analysis GDF‐15 level > 1548 pg/ml (AUC = 0.710; p = 0.0211; CI 0.576–0.821) and VWF level > 157% (AUC = 0.701; p = 0.0182; CI 0.566–0.814) increase the probability of primary outcome. Event free curves for GDF‐15 and VWF levels are shown on the picture. Significant relationship was found between GFD‐15 and VWF (r = 0.32; p = 0.0153). Only GDF‐15 level ( > 1548 pg/ml) remained significant in multiple regression model: OR 11.0; CI 2.20‐55.9; р = 0.0035.
Conclusion(s): High GDF‐15 ( > 1548 pg/ml) is related to adverse outcomes (mostly, bleeding events) in patients with chronic polyvascular disease receiving long‐term DAT. FIGURE 1 Kaplan‐Meier curves
FIGURE 1 Kaplan‐Meier curves
Epigenetics, OMICs and Bioinformatics
OC 69.5
An integrative approach to diagnose heparin‐induced thrombocytopenia: development, validation, and implementation of a multivariable prediction model
H. Nilius1; A. Cuker2; S. Haug3; C. Nakas4; J. Studt5; D. Tsakiris6; A. Greinacher7; A. Mendez8; A. Schmidt9; W. Wuillemin10; B. Gerber11; J. Kremer Hovinga12; P. Vishnu13; L. Graf14; A. Kashev3; R. Sznitman3; T. Backchoul15; M. Nagler 16
1 Inselspital, Bern University Hospital, Bern, Bern, Switzerland; 2 University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, United States; 3 University of Bern, Bern, Bern, Switzerland; 4 University of Bern, University of Thessaly, Volos, Thessaloniki, Greece; 5 Zurich University Hospital, Zurich, Zurich, Switzerland; 6 Basel University Hospital, Basel, Basel‐Stadt, Switzerland; 7 University Medicine Greifswald, Greifswald, Mecklenburg‐Vorpommern, Germany; 8 Cantonal Hospital Aarau, Aarau, Aargau, Switzerland; 9 City Hospital Waid and Triemli, Zurich, Zurich, Switzerland, 10 Cantonal Hospital of Lucerne, Lucerne, Luzern, Switzerland, 11 Oncology Institute of Southern Switzerland, Bellinzona, Ticino, Switzerland, 12 Department of Hematology and Central Hematology Laboratory, Bern University Hospital, University of Bern, Bern, Bern, Switzerland, 13 CHI Franciscan Medical Group, Seattle, Washington, United States, 14 Centre for Laboratory Medicine, St. Gallen, Sankt Gallen, Switzerland, 15 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany, 16 Inselspital, Bern University Hospital, and University of Bern, Bern, Bern, Switzerland
Background: Interpreting clinical information and laboratory test results to diagnose heparin‐induced thrombocytopenia (HIT) is challenging, particularly for the inexperienced.
Aims: We aimed to develop, validate, and implement an easy‐to‐use multivariable diagnostic prediction model integrating clinical information and laboratory test results.
Methods: We conducted a prospective cohort study including 1’448 patients with suspected HIT from 11 study centers. Detailed clinical information was collected, and various immunoassays were conducted. The washed platelet heparin‐induced platelet activation assay (HIPA) served as the reference standard. Using 75% of the patients, five different machine‐learning models were trained per immunoassay and internally validated on the remaining 25%.
Results: Sufficient sample material was available in 1’393 individuals. HIPA was positive in 119 patients (prevalence 8.5%). The following variables were selected for the final models: (1) immunoassay test result (either ELISA, chemiluminescent immunoassay [CLIA], or particle‐gel immunoassay [PaGIA]), (2) platelet nadir, (3) unfractionated heparin use, (4) CRP, (5) timing of thrombocytopenia, and (6) the likelihood of other causes of thrombocytopenia. The c‐statistic of all models in the validation dataset was 0.99 (95%CI: 0.97, 1.00). Compared to the currently recommended diagnostic algorithm (4Ts score, immunoassay), the number of false‐negative patients was reduced by 64.3% (CLIA), 66.6% (PaGIA), and 45.5% (ELISA). False‐positive individuals were reduced by 29.2% (CLIA), 72.1% (PaGIA), and 53.1% (ELISA).
Conclusion(s): We developed, validated, and implemented an accurate and easy‐to‐use decision support tool for the diagnosis of HIT (http://www.toradi‐hit.org), which has the potential to reduce overtreatment and delayed diagnosis. FIGURE 1 Graphical abstract
FIGURE 1 Graphical abstract
OC 69.2
Deep learning of structural variations: Genomic and bioinformatic lessons from antithrombin deficiency
B. de la Morena‐Barrio 1; J. Cuencia‐Guardiola2; C. Orlando3; A. Sanchis‐juan4; J. García5; J. Padilla6; M. de la Morena‐Barrio7; M. Puruunen8; K. Stouffs3; R. Cifuentes‐Riquelme7; C. Bravo‐Pérez9; R. Benito10; V. Vicente7; F. vidal11; J. Hernández‐Rivas12; W. Ouwehand13; J. Fernández‐Breis2; K. Jochmans3; M. Lozano7; J. Corral7
1 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain, Murcia, Murcia, Spain; 2 Departamento de Informática y Sistemas, Universidad de Murcia, CEIR Campus Mare Nostrum, IMIB‐Arrixaca, Facultad de Informática, Campus de Espinardo, 30100, Murcia, Spain., Murcia, Murcia, Spain; 3 Department of Haematology, Vrije Universiteit Brussel (VUB), Universitair Ziekenhuis Brussel (UZ Brussel), Brussels, Belgium, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 4 Department of Haematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK., Cambridge, England, United Kingdom; 5 Cancer Research Center (IBMCC) CSIC‐University of Salamanca; Instituto de Investigación Biomédica (IBSAL); Department of Hematology, University Hospital of Salamanca; Department of Medicine, University of Salamanca, Salamanca, Spain, Salamanca, Castilla y Leon, Spain; 6 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain., Murcia, Murcia, Spain; 7 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 8 National Heart, Lung and Blood Institute's. The Framingham Heart Study, Framingham, MA, US., Framingham, Massachusetts, United States; 9 Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain, 10 IBSAL, CIC, IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain, Salamanca, Castilla y Leon, Spain, 11 Coagulopaties congènites. Banc de Sang i Teixits. Barcelona. Unitat de Diagnòstic i Teràpia Molecular. Vall d’Hebron Institut de Recerca, Universitat Autònoma de Barcelona (VHIR‐UAB). Barcelona., Barcelona, Catalonia, Spain, 12 IBSAL, CIC, IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain; Department of Hematology, Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain, Salamanca, Castilla y Leon, Spain, 13 Department of Haematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK. Department of Haematology, University College London Hospitals, London, NW1 2BU, UK, Cambridge, England, United Kingdom
Background: Structural Variants (SVs) cover a range of gross genetic alterations ( > 50bp) that account for about 1% of the differences among human genomes and play a role in phenotypic variation and disease susceptibility. However, they are scarcely studied, because of the limitations of current detection methods, particularly short‐read sequencing methods. Furthermore, read mapping‐based methods may detect SVs but have poor accuracy and do not fully characterize the SV at nucleotide‐level resolution. Whole genome nanopore sequencing (WGNS) has emerged as a promising technology that might fully characterize SVs.
Aims: To undertake a molecular dissection of SVs involved in antithrombin deficiency (ATD), and to improve the identification of SVs along the genome using WGNS and CGH data of these patients.
Methods: Thirty‐nine unrelated patients with ATD caused by SVs were evaluated by MLPA, CGH array, Long‐Range PCR and WGNS. We analyzed circumstances that affect SVs calling results using these methods, such as reference genome version, aligner and variant caller combinations.
Results: Most SVs affecting SERPINC1 were deletions (82.1%), but tandem duplications, deletion of introns, and retrotransposon insertions were also detected (Fig.1). Their size ranged from 193bp to 8Mb, 54% involved neighboring genes, and all but 2 had repetitive elements and/or microhomologies involved in their breakpoints. The bioinformatic comparison of results in 7 patients revealed that coverage analysis of WGNS improved whole genome SVs detection. Thus, we developed a new tool called disCoverage, which rescued 14 SVs detected by CGH (Fig.2) by considering coverage data.
Conclusion(s): A wide range of SVs of different types and size generated by a common mechanism may cause ATD. WGNS is an appropriate method to identify and characterize SVs at nucleotide level, independently of their size or type. The developed disCoverage tool may be used to improve SVs detection by WGNS. PI21/00174, PMP21/00052(ISCIII&FEDER).
Laboratory Diagnostics
OC 17.4
Effects of andexanet alfa on different thrombin generation and anti‐Xa‐methods in vitro
Y. Henskens 1; A. Gulpen2; M. Bruurs3; R. van Oerle4; K. Winckers5; R. Olie6; A. ten Cate‐Hoek7; H. Spronk8; H. ten Cate9
1 Central Diagnostic Laboratory Maastricht University Medical Centre +, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands., Maastricht, Limburg, Netherlands; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 3 Central Diagnostic Laboratory, Maastricht University Medical Centre +, Maastricht, Limburg, Netherlands; 4 Central Diagnostic Laboratory, MUMC+ and Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands;, Maastricht, Limburg, Netherlands; 5 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 6 Departments of Biochemistry nd vascular medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands;, Maastricht, Limburg, Netherlands; 7 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 8 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 9 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands
Background: Andexanet alfa is a recombinant human factor Xa, used to treat life threatening bleedings caused by oral aXa inhibitors. It has been suggested that andexanet interacts with TFPI and could increase the risk for thrombosis. Conventional anti‐Xa‐tests to measure the andexanet effect are not yet applicable due to plasma sample dilutions, causing dissociation of andexanet from the Xa‐inhibitor. Thrombin generation (TG) assays could offer more clinical relevant information on the andexanet effect.
Aims: The effects of direct Xa inhibitors, andexanet alfa and TFPI were studied in vitro using two different thrombin generation assays and a traditonal or a modified aXa assay.
Methods: Various concentrations of rivaroxaban/apixaban with or without andexanet/anti‐TFPI were added to normal pool or individual plasma’s. TG was measured using several reagents by manual calibrated automated thrombogram (CAT)‐1 method (Thrombinoscope) or automated ST Genesia. AXa levels were determined using a commercial (Hyphen) or modified aXa protocol.
Results: An andexanet concentration of 25 μg/mL and 100 μg/mL restored TG in samples containing rivaroxaban/apixaban concentrations of respectively 0‐200 ng/mL and > 500 ng/ml. An overshoot of baseline TG levels was noticed after adding andexanet, which was reversed by a TFPI‐antibody. Small differences were found between the CAT‐1 and ST Genesia measurement. The commercial anti‐FXa test still showed high DOAC levels after the addition of andexanet, which did not correlate with TG levels. The modified test resulted in more expected levels of Xa‐inhibitors but a complete inhibition by andexanet was not seen.
Conclusion(s): The addition of andexanet alfa to normal plasma samples spiked with rivaroxaban or apixaban reversed the effects of the DOACs in both thrombin generation assays resulting in more thrombin generation compared to baseline. The influence of TFPI in the increase in thrombin generation after andexanet‐alfa was proven by using anti‐TFPI. Traditional and modified aXa assays are not suitable for andexanet monitoring.
OC 17.5
Direct Oral Anti‐Coagulant (DOAC) external quality assessment (EQA) scheme assay results and interpretations from UKNEQAS BC DOAC Survey 19 2021
C. Reilly‐Stitt 1; A. Lowe1; I. Jennings2; S. Kitchen3; D. Kitchen4; I. Walker1
1 UK NEQAS Blood Coagulation, SHEFFIELD, England, United Kingdom; 2 UK NEQAS BC, Sheffield, England, United Kingdom, 3 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 4 UK NEQAS Blood Coagulation, Sheffield, England, United Kingdom
Background: DOAC EQA scheme requires participants to perform DOAC assays and results are performance assessed.
Aims: The assay interpretation is a recent addition to the NEQAS BC programme with clinical and laboratory staff being asked to provide interpretations.
Methods: Data were collected as part of the DOAC exercise survey 19. Participants were asked to provide interpretations from: below level of detection; anticoagulant present at sub‐therapeutic level; anticoagulant detected, unable to state if therapeutic; anticoagulant present at therapeutic level or anticoagulant present at supra‐therapeutic level. The clinical scenario associated with Survey 19 stated that the patient had taken dose of anticoagulant three hours before medical assessment.
Results: The interpretations for the DOAC assays were either from: anticoagulant nurses (2%); biomedical scientists (34%); clinicians (33%) or clinical scientists (9%). 22% of participants did not provide an interpretation. From the interpretative data returned on Survey 19, none of the participants interpreted the assays results as below the level of detection. The majority of interpretations returned for dabigatran, apixaban and edoxaban indicated that levels of the drug were at a therapeutic level. The returns for rivaroxaban interpretation included 45% of participants who had indicated that the drug was at a level above the therapeutic range. In clinical scenarios that state the level of drug is unknown, an interpretation of “anticoagulant detected, unable to state if therapeutic” might stimulate the requesting clinician to review the time and dosage administered to the patient.
Conclusion(s): DOAC monitoring is not required for the vast majority of patients receiving these anticoagulants. However measuring DOAC levels is indicated in some settings including but not exclusively: bleeding risk; thrombotic risk; compliance and or accurate interpretation of other laboratory assays. Our data indicate that there is currently substantial variability in how the same levels of DOACs is interpreted. TABLE 1 Results and interpretations of DOAC survey 19
TABLE 1 Results and interpretations of DOAC survey 19
OC 17.3
A collaborative study to harmonize thrombin generation assays for measurement of procoagulant activity in immune globulins
M. Shea 1; L. Parunov2; S. Williams3; J. Hogwood3; S. Surov1; Y. Liang1; E. Gray3; M. Ovanesov1
1 US Food & Drug Administration, Silver Spring, Maryland, United States; 2 US Food and Drug Administration, Silver Spring, Maryland, United States; 3 National Institute for Biological Standards and Control, Medicines and Healthcare products Regulatory Agency, Potters Bar, England, United Kingdom
Background: Thrombin generation (TG) assay is widely used to identify procoagulant samples of immune globulin (IG) product, yet there have been issues with reproducibility of results and lab‐to‐lab variability. In addition, there is a lack of a standardized TG assay protocol to measure procoagulant activity in IG products.
Aims: To address these issues, the Global Working Group for the Measurement of Procoagulant Activity of Immunoglobulins organized a study with 10 participants, including IG manufacturers, TG kit manufacturers and national regulatory bodies.
Methods: Performance of a total of 13 TG methods was evaluated. The methods included assays based on reagent kits from Technothrombin (Technoclone) and Calibrated Automated Thrombogram (Stago), as well as both automated and manual TG versions. Participants tested 7 IG samples of varying procoagulant activity and evaluated assay conditions (e.g., tissue factor and phospholipids, plasma, or diluent) that can deliver robust assay performance and a consistent response to a wide range of procoagulant samples. Potencies of samples were analyzed centrally using TG parameters reported by participants. The different TG parameters were also compared. All TG methods were evaluated by limit test using thrombin peak height value of a low activity IG sample as cut‐off.
Results: Most labs were able to discern between samples with low and high activity. Quantitative measurement of procoagulant activity was found sensitive to assay conditions, which were different between labs. Regardless of the source of reagents used, good agreement was observed between labs that had optimized their assays (e.g., used lower tissue factor levels, FXI‐deficient plasma) to be sensitive to a wide range of IG samples.
Conclusion(s): Further analysis of this study may help with standardization of TG assay techniques in quality control and clinical laboratory applications.
OC 17.2
The measurement of a next‐generation FVIII Mimetic, Mim8, using tissue factor triggered calibrated automated thrombography
A. Bowyer 1; S. Kitchen2; M. Ezban3
1 Sheffield Teaching Hospitals NHSFT, Sheffield, England, United Kingdom, 2 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 3 Novo Nordisk A/S, Måløv, Hovedstaden, Denmark
Background: Mim8 is a next‐generation bispecific antibody that mimics the action of factor VIIIa in patients with haemophilia A (HA). Mim8 complexes with factor X and factor IXa to accelerate activation of factor X. Previous studies have mostly assessed thrombin generation using activated factor XI or tissue factor as a trigger (1).
Aims: To assess the thrombin generation capacity of severe HA plasma spiked with Mim8 using low concentration of tissue factor as a trigger.
Methods: Severe HA plasma (HRF) was spiked with Mim8 0‐20 μg/mL (Novo Nordisk). Calibrated Automated Thrombography (CAT) was performed using Stago PPP‐reagent low (low concentration of tissue factor) on Fluoroskan Ascent. Pooled normal plasma (PNP, Precision Biologic) was used as a normal comparison. Samples were tested on 4 times on different days.
Results: The mean Endogenous thrombin potential (ETP) and mean peak thrombin of unspiked, severe HA plasma were 234.6nM/min and 10.8nM respectively. The addition of 1 μg/mL Mim8 increased the mean ETP to 713.3nM/min and peak thrombin to 39.1nM. At 2 μg/mL Mim8, all parameters, except time to peak (TTP), were within normal limits (data shown in table 1 and figure 1); the mean ETP was 1440.1nM/min and peak thrombin was 118nM. TTP was normal in at 5 μg/mL Mim8. Plateaux were observed at 5‐20 μg/mL Mim8 for all parameters (Kruskal‐Wallis p > 0.05).
Conclusion(s): The addition of 5μg/mL Mim8 to HA plasma normalised all CAT parameters however, higher concentrations of Mim8 did not further increase thrombin generation. Mim8 restored the thrombin generation of severe HA plasma to normal levels in a low tissue factor‐triggered thrombin generation assay.
Megakaryocytes and Thrombopoiesis
OC 69.4
Synthetic microRNA switch technology enables to detect the immune‐biased megakaryocytes from heterogenous iPSC‐derived megakaryocyte progenitor cell lines
S. Chen 1; A. Yuzuriha1; K. Fujio1; K. Hashimoto1; Y. Fujita1; S. Paul2; N. Takayama2; T. Yamamoto1; N. Sugimoto1; H. Saito1; K. Eto1
1 Kyoto University, Kyoto, Kyoto, Japan; 2 Chiba University, Chiba, Chiba, Japan
Background: Platelets play roles in not only hemostasis but also innate immunity. It has also been suggested that megakaryocytes (MKs) per se can be distinguished into hemostasis‐biased and immune‐biased populations. Meanwhile, although the expression levels of several microRNAs (miRNAs) are studied in thrombopoiesis and megakaryopoiesis, there is no study based on the activities of miRNAs. We developed a miRNA switch technology which reflects the endogenous activity of miRNA and exemplified to be useful for specific cell isolation (Miki, et al. Cell Stem Cell, 2015).
Aims: By using miRNA switch, we sought to examined whether iPSC‐derived immortalized MK progenitor cell lines (imMKCLs), useful for ex vivo manufacturing of platelets applicable to human patients (Ito, et al. Cell, 2018; Sugimoto, et al. ASH abstract, 2021), contain immune‐biased MKs.
Methods: We applied miRNA switch library screening to imMKCLs and focused on those displaying broadly separated populations. The subpopulations were subsequently subjected to RNA sequencing analysis.
Results: According to the screening, we identified two subpopulations with differential activity of let‐7a‐5p and let‐7g‐5p in the proliferation phase of imMKCLs. Unexpectedly, the bulk RNA‐seq analysis of two subpopulations at proliferation and maturation stages revealed that the let‐7 low‐responsive (let‐7 low) imMKCLs exhibited an immune‐skewed transcriptional signatures. TNF signaling was found to significantly enriched in let‐7 low imMKCLs in both stages. In the maturation stage, interferon responsive gene set was significantly enriched in let‐7 low imMKCLs, while platelet activation signaling was enriched in let‐7 high imMKCLs. In addition, the let‐7 low hESC‐derived CD34+ hematopoietic progenitor cells also exhibited a similar immune‐skewed signature. While lung MKs are reported to exhibit immune‐skewed gene expression signatures, our study revealed that the immune‐skewed subpopulations also exist in iPSC/ESC‐derived MKs.
Conclusion(s): We found that let‐7 miRNA switches enabled to identify a subpopulation with immune properties in imMKCLs derived from iPSCs.
Nanotechnology and Novel Biomolecules
OC 69.1
SERPIN‐inspired nanomedicine targeting to activated neutrophils provides safe and effective thromboprotection
D. Bohinc 1; M. Cruz1; E. Andraska2; J. Alvikas3; S. Raghunathan1; N. Masters1; K. Bane4; N. van Kleef5; M. Nieman1; C. Maas6; S. De Maat6; K. Neeves7; M. Neal8; A. Sen Gupta1; E. Stavrou9
1 Case Western Reserve University, Cleveland, Ohio, United States; 2 University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 3 UPMC Montefiore, Pittsburgh, Pennsylvania, United States; 4 CWRU School of Medicine, Cleveland, Ohio, United States; 5 University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 6 UMC Utrecht, Utrecht, Utrecht, Netherlands; 7 University of Colorado Denver, Aurora, Colorado, United States; 8 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States; 9 Case Western Reserve University School of Medicine, Cleveland, Ohio, United States
Background: Neutrophils are the first responders to tissue damage and play a critical role in host defense against infection. However, unrestricted recruitment and function of activated neutrophils can prolong inflammation and contribute to the development of conditions such as thrombosis, tumor progression, autoimmunity, and chronic wounds. Functional characterization of neutrophils from individuals with chronic inflammatory states identified a distinct pathogenic sub‐population that exhibits baseline activation, prolonged lifespan and susceptibility to form NETs.
Aims: We previously designed a nanoparticle (NP) system that simultaneously targets activated neutrophils and platelets. Here, we aimed to develop and characterize a stand‐alone therapeutic strategy that exclusively targets activated neutrophils without the ubiquitous inhibition of resting cells, and independently of activated platelets.
Methods: We created a nanomedicine system that uniquely utilizes an α₁‐antitrypsin (AAT)‐derived peptide to confer binding specificity to neutrophil elastase (HNE) on activated neutrophils.
Results: We employed enzyme‐sensitive probes and FRET to characterize the interaction of NEBP with free and membrane‐bound HNE. NEBP showed dose‐dependent binding to free HNE (Fig 1A) and importantly, a higher affinity for membrane‐bound HNE compared to AAT (Fig 1B‐C). Moreover, NEBP did not interact with Proteinase 3, Cathepsin G (Fig 1D‐F) or non‐neutrophil proteases, including tPA and plasmin (Fig 1G‐J). In vitro visualization of NEBP‐assembled NPs showed specific targeting to activated neutrophils only, with partial trafficking to lysosomes (Fig 2A‐C). In vivo efficacy studies with a model drug (hydroxychloroquine‐HCQ) showed that drug‐loaded NEBP‐NPs significantly reduced inferior vena cava thrombus weights using considerably lower HCQ concentrations (Fig 2E).
Conclusion(s): We describe for the first time a novel nanoplatform that exclusively binds to activated neutrophils and demonstrate that targeting primed neutrophils, alone can be highly efficacious and safer than systemic drug treatment. These studies establish a mechanistic roadmap for cell‐ and activation state‐specific targeting that can be applied to several neutrophil‐driven pathologies.
Platelet Function and Interactions
OC 69.3
Homology directed repair edited megakaryocytes for rapid screening of platelet gene variant functions
Y. Kosaka1; S. Jacob1; E. Montenont1; J. Rowley 2
1 University of Utah, Salt Lake City, Utah, United States; 2 University Of Utah, Salt Lake City, Utah, United States
Background: Functional studies are urgently needed for the mechanistic and clinical interpretation of the numerous variants found by association studies and genetic testing, but are largely missing for variants affecting platelets. The paucity of functional variant validation has been in part because of the inability to make genetic modifications in anucleate cells. We previously published a robust approach to delete and survey platelet gene functions in megakaryocytes called CRIMSON (CRISPR‐edited megakaryocytes for rapid screening of platelet gene functions). Here we describe CRIMSON HD (CRIMSON with Homology Directed Repair (HDR)) for the rapid generation and functional testing of precise point mutations, insertions, or deletions in primary megakaryocytes.
Aims: Generate and functionally test genetic variants in human CD34+ cell derived megakaryocytes using HDR.
Methods: Human cord blood CD34+ cells were transfected with CRISPR/CAS9 targeting exon 5 of ITGA2B, and a homologous DNA donor harboring a 6 nucleotide insertion. After differentiation into megakaryocytes, Sanger sequencing was used to validate insertion. Megakaryocyte αIIb surface expression and agonist induced ligand binding were measured by flow cytometry.
Results: We tested CRISPR/CAS9 directed HDR for CD34+ derived megakaryocytes using a homologous DNA donor harboring a two amino acid in frame insertion (Figure 1A‐B) described to cause loss of αIIb function without affecting surface expression. As shown in Figure 1C, DNA sequence analysis indicated almost complete (96%) inclusion of the insertion in the genome of HDR edited megakaryocytes. Consistent with this, whereas CRISPR/CAS9 cutting without HDR resulted > 90% loss of CD41 and CD61 surface expression, inclusion of R192T193 rescued their expression (Figure 2). Despite rescue of expression, MKs with the R192T193 insertion lost the ability to bind PAC‐1 or fibrinogen after agonist stimulation.
Conclusion(s): CRIMSON HD can be used to rapidly generate and functionally test platelet associated genetic variants in their native cellular and DNA context for both mechanistic insight and clinical interpretation.
Fibrinolysis and Proteolysis
Fibrinogen and Factor XIII
OC 76.5
Clot destabilisation by the inclusion of fibrin with truncations in the αC‐region
H. McPherson 1; C. Duval1; T. Feller2; R. Ajjan1; R. Ariëns3
1 University of Leeds, Leeds, England, United Kingdom; 2 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds., Leeds, England, United Kingdom; 3 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom
Background: The fibrinogen αC‐region is comprised of an αC‐domain and connector, and contains binding sites for FXIIIa, α2‐antiplasmin and plasminogen. Heterozygous dysfibrinogenaemia due to mutation within the αC‐region may increase bleeding risk. The effects of mixtures between WT and truncation variants in a plasma environment are unknown.
Aims: To investigate the impact of αC‐region truncations on fibrinolysis of clots made with mixed WT and truncated αC‐region fibrinogens.
Methods: Fibrinolysis of recombinant WT, α390 and α220 fibrinogens produced in CHO‐cells was studied by turbidimetric assay with and without α2‐antiplasmin, and analysis of D‐dimer elution from plasma‐fibrinogen deficient clots. Clot structure of WT fibrinogen spiked with increasing percentages of truncated fibrinogens was analysed by laser‐scanning‐confocal‐microscopy.
Results: In the presence of FXIIIa and α2‐antiplasmin, resistance to fibrinolysis was increased for WT and α390, but not for α220 clots, which lack the α2‐antiplasmin binding site. Clot porosity was reduced for α390 (2.75x10‐8 ± 2.97‐9Ks) compared to WT clots (3.65x10‐8 ± 2.54‐9Ks; p = 0.0167). Permeation experiments in the presence of plasminogen and tPA showed reduced time to clot collapse in α390 compared to WT (89 ± 6 and 106 ± 4mins; p = 0.0204). Due to the weak nature of α220 no data could be determined by permeation. WT clots with increasing percentages of α390 and α220 showed increasingly abnormal clot structure. Clots spiked with α390 fibrin became denser with shorter fibres and α220 spiked clots became more porous with short, stunted fibres.
Conclusion(s): Increased resistance to fibrinolysis was observed with the addition of α2‐antiplasmin for both WT and α390 but not α220 fibrin. Reduced porosity did not provide resistance to fibrinolysis for fibrinogen lacking the αC‐region. Inclusion of truncated fibrinogen altered clot structure in a dose‐dependent manner and could result in clot destabilisation in vivo. These data show important mechanisms by which the risk of bleeding may be increased in patients with heterozygous dysfibrinogenaemia. FIGURE 1 Altered clot architecture of WT fibrinogen spiked with αC‐region truncations
FIGURE 1 Altered clot architecture of WT fibrinogen spiked with αC‐region truncations
OC 29.5
Dysregulated fibrinogen γ‐chain cross‐linking in FibγΔ5 mice drives acute liver injury after acetaminophen overdose
L. Poole 1; D. Groeneveld1; H. Cline‐Fedewa1; M. Flick2; J. Luyendyk3
1 Michigan State University, East Lansing, Michigan, United States; 2 University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States; 3 Department of Pathobiology and Diagnostic Investigation, Michigan State University, East Lansing, Michigan, United States
Background: Hepatic fibrin(ogen) deposition and platelet accumulation are hallmarks of liver injury driven by acetaminophen (APAP) overdose. The mechanisms mediating initial platelet accumulation in the injured liver are not understood.
Aims: We tested the hypothesis that hepatic platelet accumulation in the APAP‐injured liver is mediated by fibrin(ogen) engagement of the platelet integrin αIIbβ3.
Methods: Male wild‐type mice and mice expressing normal levels of a mutant fibrinogen incapable of engaging integrin αIIbβ3 through the c‐terminal domain of the fibrinogen γ chain (FibγΔ5 mice) were challenged with APAP (300 mg/kg, i.p.), and liver tissues were collected 24 hours after challenge.
Results: Contrary to our hypothesis, hepatic platelet accumulation was modestly increased in FibγΔ5 mice compared to wild‐type mice after APAP challenge. Earlier reports indicate similar fibrin(ogen) cross‐linking occurs in wild‐type and FibγΔ5 mice. Surprisingly, fibrinogen γ‐chain cross‐linking, assessed by capillary western blotting, was unique in livers of APAP‐challenged FibγΔ5 mice compared to wild‐type, favoring accumulation of high molecular weight cross‐linked γ‐chain complexes (~220 and 350 kDa) and absence of γ‐γ dimer. Moreover, we discovered using chain‐selective antibodies that γ‐γ dimer does not form in vitro in FibγΔ5 plasma or purified fibrinogen with addition of thrombin. Additionally, scanning electron microscopy revealed that in vitro clots formed from purified FibγΔ5 fibrinogen appeared to be composed of a denser network of thinner fibers compared to wild‐type. Finally, APAP‐induced liver necrosis was increased in FibγΔ5 mice compared to wild‐type mice. This affect appeared to be independent of platelet integrin αIIbβ3‐fibrinogen interactions, as treatment of wildtype mice with an αIIbβ3 inhibitor had no effect on APAP‐induced hepatic necrosis.
Conclusion(s): These results indicate that FibγΔ5 mice have defective fibrinogen γ‐chain cross‐linking with a complete absence of γ‐γ dimer formation and increased high molecular weight γ‐chain cross‐linking. Furthermore, this aberrant γ‐chain cross‐linking may exacerbate liver injury following APAP overdose.
OC 76.1
Functional characterization and structural modeling of DNA‐aptamers targeting activated coagulation factor XIII
N. Shahidi Hamedani 1; A. Biswas2; O. Rudan3; C. Meyring2; G. Mayer4; J. Oldenburg5; J. Müller2; B. Pötzsch2
1 Institute of Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Germany, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Department of Integrated Oncology, CIO Bonn, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 4 Life and Medical Sciences Institute, University of Bonn, Bonn, Nordrhein‐Westfalen, Germany; 5 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Coagulation factor XIII (FXIII) is a protransglutaminase which plays an important role in clot stabilization and composition by cross‐linking the α‐ and γ‐chains of fibrin and increasing the resistance of the clot to mechanical and proteolytic challenges. FXIII inhibition might lead to higher susceptibility of formed clot to fibrinolysis.
Aims: To study the inhibitory effect of FXIIIa‐binding aptamers on clot formation and lysis.
Methods: In this study, we selected six DNA aptamers specific for activated FXIII (FXIIIa) using Capillary Electrophoresis‐Systematic Evolution of Ligands by EXponential enrichment (CE‐SELEX) and investigated the functional characterization of FXIIIa after aptamer binding in terms of fibrinogen binding of FXIIIa, alpha2‐antiplasmin (α2AP) incorporation to fibrinogen and fibrin clot formation using plasma‐based thromboelastometry (rotational thromboelastometry analysis, ROTEM).
Results: One of these aptamers, named FA12, efficiently captures FXIIIa even in the presence of zymogenic FXIII subunits. Furthermore, this aptamer inhibits the incorporation of FXIII and α2‐antiplasmin (α2AP) into fibrin(ogen) with IC50‐values of 38 nM and 17 nM, respectively. In addition to FA12, also another aptamer, FA2, demonstrated significant effects in ROTEM analysis where spiking of the aptamers into plasma decreased clot stiffness and elasticity (p < 0.0001). The structure–function correlations determined by combining modeling/docking strategies with quantitative in vitro assays revealed spatial overlap of the FA12 binding site with the binding sites of two FXIII substrates, fibrinogen and α2AP, while FA2 binding sites only overlap those of fibrinogen.
Conclusion(s): Two DNA aptamers specific for FXIIIa could inhibit incorporation of FXIIIa and α2AP to fibrin clot and therefore can be considered as an interesting candidate molecule for the development of FXIIIa‐targeting therapeutic strategies.
OC 76.2
A zebrafish model of dysfibrinogenemia caused by hotspot mutations in the human fibrinogen gamma chain
R. Fish 1; M. Richard2; M. Neerman‐Arbez3
1 Faculty of Medicine, University of Geneva, Switzerland., Geneva, Geneve, Switzerland; 2 University of Geneva, Geneva, Geneve, Switzerland; 3 Department of Genetic Medicine and Development, Faculty of Medicine, University of Geneva, Geneva, Switzerland, Geneva, Geneve, Switzerland
Background: Mutations affecting fibrinogen quality lead to unpredictable bleeding and thrombotic events in patients with dysfibrinogenemia. With dominant inheritance, dysfibrinogenemia is caused by heterozygous missense mutations in fibrinogen genes. Mutation of arginine 301 to cysteine or histidine in the fibrinogen gamma chain (γR301C, γR301H), affecting D:D interactions between adjacent fibrin monomers and consequently fibrin polymerization, are frequently detected dysfibrinogenemia‐associated mutations.
Aims: To generate a zebrafish model of dysfibrinogenemia caused by γR301 mutations. Once established, the model should allow us to study the effects of additional genetic variation on the dysfibrinogenemia phenotype.
Methods: A morpholino antisense oligonucleotide was used to reduce zebrafish fibrinogen γ chain expression. Fibrinogen γR295C and γR295H cDNA, orthologous to human γR301 mutations, were expressed using transgenesis. In a second approach, a large deletion in the zebrafish fgg gene was generated using CRISPR‐Cas9 genome editing. Laser‐induced injury was used to assess coagulation and venous thrombosis in zebrafish larvae.
Results: Transient morpholino (MO)‐induced knock‐down of fibrinogen γ mRNA prevented laser‐induced venous thrombosis measured as time to occlusion (TTO) in zebrafish larvae. This was rescued by expression of γ cDNA but not with γR295C or γR295H cDNA, where TTO was prolonged compared to control larvae (mean TTO in controls 29s, MO + γ cDNA 29s, MO + γR295C 56s and MO + γR295H 46s, n = 7 to 16). We also produced zebrafish with an 8.1kb deletion in the fgg gene. With laser injury, homozygous mutant larvae showed no blood coagulation or thrombosis, and will now serve as a stable knock‐out background to study the effects of γR295C and γR295H expression.
Conclusion(s): Using fibrinogen γ chain mRNA knock‐downs we demonstrate dysfunctional clotting in larval zebrafish expressing γR295C and γR295H after laser injury. Our stable γ chain mutant with γR295C or γR295H expression will allow us to study the phenotypic effects of further genetic variation in dysfibrinogenemia.
OC 29.4
Development of recombinant antibodies targeting the coagulation factor XIII‐B subunit for research and therapeutic use
J. Byrnes; C. Bracken; J. Wells
University of California, San Francisco, San Francisco, California, United States
Background: Plasma coagulation factor XIII (FXIII) is a heterotetramer composed to two A and two B subunits (FXIII‐A2B2). The FXIII‐A subunits, once activated, catalyze covalent crosslinking in the fibrin network, whereas the FXIII‐B subunits stabilize the FXIII‐A subunits in circulation. The FXIII‐B subunits also mediate the binding of FXIII‐A2B2 to fibrinogen. Interestingly, this binding interaction enhances FXIII activation rate, which in turn modulates the retention of red blood cells in venous thrombi, and consequently, thrombus size. Therefore, therapeutic disruption of the FXIII‐fibrinogen interaction represents a potential approach to reduce venous thrombus burden.
Aims: Develop and characterize recombinant anti‐FXIII‐B antibodies.
Methods: Recombinant FXIII‐B was expressed in mammalian cells and used as a capture antigen in phage display with a library of phage expressing fragment antigen binding domains (Fabs, Figure 1). Binding affinities were measured using bio‐layer interferometry.
Results: Recombinant FXIII‐B bound fibrinogen, as expected (KD = 46.9 nM). Fab‐Phage selection yielded seven clones that bound immobilized FXIII‐B with nanomolar affinity (KD = 2.7‐22.2 nM). Stronger affinities were observed with solution‐phase FXIII‐B (KD = 0.7‐11.3 nM), likely due to the avidity of dimeric FXIII‐B in solution. None of the Fabs were competitive for fibrinogen binding to FXIII‐B, but three of these clones bound plasma‐derived FXIII‐A2B2, although with reduced affinity relative to FXIII‐B alone. Conversion of these three Fabs to bivalent IgGs dramatically improved the binding affinity for recombinant FXIII‐B (KD < 100 pM). These three clones in IgG format were still non‐competitive for fibrinogen binding. Consistent with this observation, epitope binning revealed that these clones bound the same epitope on FXIII‐B.
Conclusion(s): Fab‐Phage display is an effective strategy to generate new recombinant anti‐FXIII‐B antibodies. These antibodies represent tools to support studies investigating FXIII‐B structure and function. Efforts using different selection approaches and phage display libraries to identify antibodies that block FXIII‐fibrinogen binding and modulate FXIII function in coagulation are ongoing. FIGURE 1 Phage display strategy to identify anti‐FXIII‐B antibodies
FIGURE 1 Phage display strategy to identify anti‐FXIII‐B antibodies
OC 46.2
Fibrin film formation is enhanced by red blood cells and reduces platelet spreading
G. Alkarithi 1; F. Macrae2; C. Duval2; R. Ariëns3
1 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, UK., Leeds, England, United Kingdom; 2 University of Leeds, Leeds, England, United Kingdom; 3 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom
Background: Thrombi are heterogeneous, consisting of fibrin, red blood cells (RBCs), and platelets. Recent studies show that fibrin films develop on the surface of clots, playing a protective role. Evidence is emerging that fibrin films are also present in intravascular thrombi; however, little is known about the role of these films and their interactions with blood cells and the vasculature.
Aims: Investigate the effect of haematocrit on fibrin films, the role of fibrin films in platelet spreading and the impact of inflamed endothelial cells on fibrin film formation.
Methods: Blood was obtained from healthy volunteers. Haematocrit was adjusted to 10, 30, 50, 70% by dilution with autologous plasma. Effects on clot film coverage was investigated by scanning electron microscopy. Platelets spreading on clot surfaces in the presence or absence of film was investigated by laser scanning confocal microscopy (LSCM). Human umbilical vein endothelial cells (HUVECs) were stimulated with lipopolysaccharide (LPS), interleukin‐1β (IL‐1β) or tumour necrosis factor‐α (TNFα). Inflammation markers, including monocyte chemoattractant protein‐1 (MCP‐1) and tissue factor, were analysed by enzyme‐linked immunoassay. Effects of HUVEC inflammation on fibrin film was analysed by LSCM.
Results: Blood clots throughout the haematocrit range developed fibrin film; however, clots with 10% haematocrit showed less film coverage than those with a higher haematocrit. Spreading of platelets on clots with film was reduced compared to clots without film or fibrin monomer layer. MCP‐1 level was high in all treated HUVECS whilst tissue factor level was high in TNFα treated cells. There were no significant differences in film thickness in clots made with purified fibrinogen on the HUVECS with or without inflammation.
Conclusion(s): Our data show that RBCs support fibrin film coverage on clots, and that fibrin films impede platelet spreading. These findings may have important implications for boundary formation in clots and thrombi. FIGURE 1 Platelets spreading on BSA, fibrin monomer, clots without and with film
FIGURE 1 Platelets spreading on BSA, fibrin monomer, clots without and with film
OC 46.3
Neutrophil cathepsin G cleaves fibrinogen affecting clot structure
S. Smith; J. Morrissey
University of Michigan, Ann Arbor, Michigan, United States
Background: There is growing evidence that neutrophils contribute to the pathophysiology of thrombosis. Neutrophils are recruited to a site of injury, where they leave the vasculature and degranulate, releasing enzymes such as cathepsin G (CatG) and elastase, the latter of which has been previously reported to cleave fibrinogen at multiple sites, including several within the alphaC‐domain. Because the alphaC‐domain is relatively unstructured, we would expect it to be susceptible to proteolysis. The alphaC‐domain plays important roles in fiber growth, mechanical stability, and susceptibility of the clot to fibrinolysis.
Aims: To evaluate the consequences of cleavage of fibrinogen by CatG for fibrin clot structure.
Methods: Purified human fibrinogen was incubated with buffer or purified neutrophil CatG. Aliquots were removed at various time points, boiled, then subjected to SDS‐PAGE. Bands were submitted for N terminal sequencing. Protein was clotted with thrombin, and polymerization was monitored by assessing turbidity in a microplate reader. Fibrinolysis of these clots was triggered with plasmin. Viscoelastic properties were evaluated using thromboelastometry. FXIIIa‐mediated crosslinking activity was followed with SDS‐PAGE.
Results: We have identified four CatG cleavage sites within fibrinogen Aalpha, one within the N terminal fibrinopeptide A (L9) and three within the alphaC‐domain (F394, F500, M517). When CatG‐cleaved fibrinogen is then clotted by thrombin, polymerization is faster than that observed for control fibrinogen, and the resulting clot is markedly more turbid. CatG‐cleaved fibrinogen also results in clots that are weaker (amplitude values 40% lower), and are more susceptible to plasmin‐mediated fibrinolysis (time to 50% lysis decreased approximately 2 fold). FXIIIa mediated cross‐linking is reduced as indicated by delayed appearance of gamma dimers and alpha polymers.
Conclusion(s): The modification of fibrinogen by CatG results in changes in clot structure and function. Release of CatG from activated neutrophils may affect clot formation with inflammation, with potential consequences for thrombosis.
OC 29.2
Surface exposure of cellular factor XIII on activated platelets and platelet microparticles is unrelated to the elevation of cytoplasmic Ca2+ concentration
L. Somodi 1; I. Beke Debreceni1; G. Kis1; M. Cozzolino1; J. Kappelmayer1; M. Antal1; G. Panyi1; H. Bárdos1; N. Mutch2; L. Muszbek3
1 University of Debrecen, Debrecen, Hajdu‐Bihar, Hungary; 2 University of Aberdeen, Aberdeen, Scotland, United Kingdom; 3 University of Debrecen, Medical Faculty, Department of Laboratory Medicine, Division of Clinical Laboratory Science,, Debrecen, Hajdu‐Bihar, Hungary
Background: Platelets contain a high amount of potentially active A subunit dimer of coagulation factor XIII (cellular FXIII; cFXIII). It is of cytoplasmic localization, not secreted, but becomes translocated to the surface of platelets activated by convulxin and thrombin (CVX+Thr) (Mitchell et al. Blood 2014;124:3982‐90).
Aims: To explore the difference in cFXIII translocation on platelets and platelet microparticles between receptor mediated and non‐receptor mediated platelet activation and how the translocation is related to cytoplasmic Ca2+ concentration. A further aim was to shed some light on the mechanism of cFXIII translocation.
Methods: Gel‐filtered platelets were activated by CVX+Thr or Ca2+‐ionophore (calcimycin). The translocation of cFXIII and phosphatidylserine (PS) to the surface of activated platelets and platelet‐derived microparticles was investigated by flow cytometry, immunofluorescence and immune electron microscopy. Fluo‐4‐AM fluorescence was used for the measurement of intracellular Ca2+ concentration. The role of RhoA and the activation of cFXIII were tested by studying the effect of their inhibitors.
Results: Receptor mediated activation by CVX+Thr exposed cFXIII and PS to the surface of over 60% and 30% of platelets, respectively. Electron microscopy revealed microparticles with preserved membrane structure and also microparticles devoid of labeling for membrane glycoprotein CD41a. cFXIII was observed on both types of microparticles but was more abundant in the absence of CD41a. cFXIII translocation was eliminated by Rhosin, a RhoA inhibitor, while only partial decrease could be achieved by T101, a transglutaminase inhibitor. Non‐receptor mediated activation of platelets by calcimycin highly elevated cytoplasmic Ca2+ concentration, it induced the translocation of PS to the surface of platelets and microparticles, but failed to expose cFXIII.
Conclusion(s): The elevation of intracellular Ca2+ concentration is sufficient for the translocation of PS from the internal layer of the membrane, while the translocation of cFXIII from the platelet cytoplasm requires additional receptor mediated mechanism(s).
OC 29.3
Fibrinogen αC‐connector is critical for fibrin fiber assembly and mechanical strength
A. Daraei1; T. Dement2; H. Belcher2; N. Hudson2; M. Guthold 3
1 UCLA, Winston‐Salem, North Carolina, United States; 2 East Carolina University, Greenville, North Carolina, United States; 3 Wake Forest University, Winston Salem, North Carolina, United States
Background: Fibrinogen has two large αC regions (alpha221–610) that consists of the unstructured, 60 nm long αC‐connector (α221–391) and the folded αC‐domain (392–610). The unfolded αC‐connector contains ten repeat segments, each consisting of 13 amino acids, running from α264‐391.
Aims: Investigate the role of the αC‐connector in fibrin fiber assembly and mechanical strength.
Methods: Using a transient fibrinogen expression system, we obtained variant Aα∆1‐10, in which all ten repeat units are deleted. We used SEM imaging and AFM‐based nanomanipulation methods, to investigate fibrin fiber morphology and mechanical properties of uncrosslinked fibers formed from variant Aα∆1‐10 fibers and wild‐type fibers.
Results: Fibers formed from the AαΔ1‐10 variant showed dramatically different mechanical and morphological properties. Compared to wild type fibers, extensibility was reduced by a factor of 1.7 (from 2.1 to 1.26) and the modulus (stiffness) decreased by a factor of 3 (from 3 MPa to 1 MPa). SEM images show that AαΔ1‐10 fibers often form a flat ribbon‐like fiber, failing to form the round, cylindrical fibers seen in wild type fibers.
Conclusion(s): The αC‐connector region of fibrinogen (specifically α264‐391) plays a key role in proper fibrin fiber assembly, and it is a major contributor to the mechanical strength of fibrin fibers, and thus to the strength of blood clots. FIGURE 1 Variant and wild‐type fibers
FIGURE 1 Variant and wild‐type fibers
OC 29.1
Both α‐ and γ‐chain crosslinks mediated by FXIIIa affect fibrin fibre resistance to rupture
T. Feller 1; C. Duval2; S. Connell3; R. Ariëns4
1 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds., Leeds, England, United Kingdom; 2 University of Leeds, Leeds, England, United Kingdom; 3 Molecular and Nanoscale Physics Group, School of Physics, University of Leeds., Leeds, England, United Kingdom; 4 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom
Background: Mechanical strength of individual fibrin fibres is essential to prevent clot breakdown and subsequent embolism. As the last step of clot formation, activated coagulation factor XIII (FXIIIa) crosslinks the α‐ and γ‐chains of fibrin, thus increasing clot stiffness. We recently showed that the absence of γ‐chain crosslinks leads to decreased rupture stress and toughness of fibrin fibres in murine plasma‐purified fibrin (FGG3X, γQ398N/Q399N/K406R), resulting in more frequent pulmonary embolism (PNAS, 2021).
Aims: To investigate how α‐crosslinks alter the mechanical behaviour of fibrin fibres, we generated a novel murine model (FGA4X, αQ241N/Q243N/Q257N/Q518N) with impaired α‐α crosslinking, and compared individual fibre biomechanical behaviour with that of wild type (WT) fibres.
Methods: Clots from FGA4X, FGG3X or WT murine plasma were made on a striated surface using 8‐times diluted plasma with the addition of 0.5 U/ml thrombin and 10 mM CaCl2. Individual fibrin fibres were stretched using lateral force sensing atomic force microscopy (AFM) combined with fluorescence microscopy, and the resulting stress‐strain behaviour of each fibre was analysed.
Results: Compared with WT fibrin fibres, FGA4X fibres ruptured at 1.8‐fold lower stress (p < 0.001) and had 1.3‐fold lower toughness (ns). Similarly, a 1.9‐fold decrease in rupture stress (p < 0.0001) was found for FGG3X fibres with a somewhat larger, 1.9‐fold decrease for toughness (p < 0.0005), when compared with their WT counterparts. Both WT and 4X fibres gained ~70% extra length after rupture compared with their original length, showing a degree of unrecoverable deformation. Such deformation indicates permanently broken bonds, which represent potential places for rupture within the fibre.
Conclusion(s): Lower rupture stress and fibre toughness indicate that deficiency in both α‐ and γ‐chain crosslinking results in fibres that are more prone to rupture. These data indicate that α‐ and γ‐chain crosslinking play complementary roles in generating key biomechanical properties of fibrin clots for the prevention of embolism.
Fibrinolytic Factors and Inhibitors
OC 76.4
CM‐352 reduces rivaroxaban‐associated ICH by mechanisms dependent on MMP‐10 and fibrinolysis inhibition
M. Navarro‐Oviedo1; J. Marta‐Enguita1; C. Roncal2; J. Rodriguez2; B. Zandio3; R. Lecumberri4; F. Machado1; J. Oyarzabal1; A. Pineda‐Lucena1; J. Páramo4; R. Muñoz3; J. Orbe 5
1 Cima‐Universidad de Navarra, Pamplona, Navarra, Spain; 2 Cima Universidad de Navarra, Pamplona, Navarra, Spain; 3 Complejo Hospital de Navarra, Pamplona, Navarra, Spain; 4 Clínica Universidad de Navarra, Pamplona, Navarra, Spain; 5 Centro de Investigación Médica Aplicada (CIMA), Universidad de Navarra, Pamplona, Navarra, Spain
Background: Intracranial haemorrhage (ICH) is one of the major devastating complications of anticoagulation. DOACs antiXa, aside from their anticoagulant effects, enhance fibrinolysis, thus patients treated with FXa inhibitors experiencing bleeding might benefit from antifibrinolytic compounds. Matrix metalloproteinases (MMPs) inhibition has been proposed as a novel pharmacological approach for ICH treatment.
Aims: We evaluated the effects of CM‐352 (MMPs‐fibrinolysis inhibitor) in an experimental ICH model associated with rivaroxaban as compared with clinically used prothrombin complex concentrate (PCC).
Methods: ICH was induced by collagenase injection into the striatum of WT (C57BL/6J) anticoagulated mice (rivaroxaban) and Mmp10 ‐/‐ mice. Hematoma volume and neurological deficits were measured 24h later by diaminobenzidine staining and different behavioural test. Circulating plasminogen activator inhibitor‐1 (PAI‐1) activity and interleukin‐6 (IL‐6) were measured in plasma samples and local inflammation was assessed by neutrophil infiltration. Finally, fibrinolytic effects of MMP‐10 and rivaroxaban were evaluated by thromboelastometry and thrombin‐activatable fibrinolysis inhibitor (TAFI) activation assays.
Results: PCC and CM‐352 treatments, diminished haemorrhage volume (46%, p < 0.01 and 64%, p < 0.001, respectively) and ameliorated functional outcome in rivaroxaban‐ICH. Interestingly, only CM‐352 decreased neutrophil infiltration in the haemorrhage area at 24h. The effect of CM‐352 could be related to MMP‐10 inhibition since Mmp10‐/‐ mice showed lower haemorrhage volume, IL‐6 levels and neutrophil infiltration, as well as better neurological score and increased PAI‐1 after experimental ICH. Finally, we found that CM‐352 reduced MMP‐10 and rivaroxaban‐related fibrinolytic effects in thromboelastometry and TAFI activation.
Conclusion(s): CM‐352 treatment, by diminishing fibrinolysis related to MMPs and rivaroxaban‐, might be a novel antihaemorrhagic strategy for rivaroxaban‐associated ICH. Funding: ISCIII (PI15/01807 and PI19/00065"), co‐funded by ERDF, "A way to make Europe", grants from the Spanish Society of Thrombosis and Haemostasis (SETH), Navarra Government (02/2015), and Virto Group (Navarra, Spain). Patent family (EP 12382285) was licensed to Hemostatics Pharmaceuticals S.L.
OC 76.3
Molecular basis for the conformational selectivity of thrombomodulin for TAFI or protein C
M. Boffa 1; J. Ackersviller2; H. Marier2; R. Moric2; H. Khan2
1 University of Western Ontario, London, Ontario, Canada; 2 Department of Biochemistry, University of Western Ontario, London, Ontario, Canada
Background: Thrombomodulin (TM) is an endothelial cell cofactor for the activation of protein C (PC) and thrombin‐activatable fibrinolysis inhibitor (TAFI). EGF5 and 6 of TM bind thrombin, and EGF4 binds to PC in the ternary complex with thrombin. Met388 in the linker between EGF4 and EGF5 clamps these two domains in a specific orientation required for PC cofactor activity. TAFI cofactor activity additionally requires EGF3, which presumably binds to TAFI. However, the nature of the TM‐thrombin‐TAFI catalytic complex and how TM might discriminate between PC and TAFI remains unclear.
Aims: Our goal was to use recombinant variants of TM to reveal key structural features that mediate selectivity for PC or TAFI.
Methods: We expressed a series of soluble TM variants encompassing EGF3‐6 in HEK293 cells. These included (i) systematic substitutions of Met388 for all other residues; and (ii) EGF3 and 4 domain swap variants consisting of EGF3‐3‐5‐6, EGF4‐3‐5‐6, and EGF4‐4‐5‐6. We measured thrombin‐mediated PC and TAFI activation in the presence of the TM variants.
Results: Most Met388 substitutions had minimal impact on TAFI cofactor activity, whereas PC cofactor activity was only retained when Met388 was conservatively substituted (Leu/Tyr/Phe/Ile). Oxidation of EGF3‐6 with chloramine T virtually eliminated PC cofactor activity while only mildly decreasing TAFI cofactor activity. Of the domain swap variants, only EGF3‐3‐5‐6 was able to support TAFI activation, and only EGF4‐4‐5‐6 was able to support PC activation.
Conclusion(s): The specific orientation of EGF4 and 5 mediated by Met388 is dispensable for TAFI cofactor activity. In the context of TAFI activation, EGF4 acts as a flexible spacer to position TAFI‐binding EGF3. These features provide a mechanism for TM to discriminate between PC and TAFI as substrates. Specifically, during inflammatory responses where Met388 might be oxidized by reactive oxygen species, TM would retain TAFI cofactor activity to maintain clot stability while losing its unwanted anticoagulant functions.
Plasminogen Activation in the CNS and Immunity
OC 46.1
Streptococcal surface enolase (SEN) is overshadowed by plasminogen‐binding group A Streptococcal M‐protein (PAM) for human plasminogen acquisition and activation
S. Tjia‐Fleck 1; Y. Ayinuola2; B. Readnour2; Z. Liang3; V. Ploplis3; F. Castellino3
1 University of Notre Dame, south bend, Indiana, United States; 2 University of Notre Dame, Notre Dame, Indiana, United States; 3 W.M. Keck Center for Transgene Research, Notre Dame, Indiana, United States
Background: Human plasmin (hPm) proteolytic activity is utilized by various pathogenic bacteria, such as Group A Streptococcus (GAS), to increase dissemination and pathogenicity. GAS is a human‐selective Gram‐positive bacterium that utilizes hPm through the expression of surface human plasminogen (hPg) receptors. Once hPg is recruited to the cell surface, it is converted into active hPm. There are at least three hPg receptors on the GAS cell surface with different binding affinities. Plasminogen‐binding Group A Streptococcal M‐protein (PAM) is typically found on skin‐tropic Pattern D GAS strains, such as AP53. PAM binds hPg with a very high affinity (Kd ~1 nM) and is able to stimulate hPg activation in the presence of GAS‐secreted streptokinase (SK) and host hPg activators such as urokinase. Unlike PAM, streptococcal surface enolase (SEN) is found on the surface of most strains and its importance in hPg acquisition by skin‐tropic GAS strains is unknown.
Aims: An investigation of SEN structure and the contribution of SEN in hPg binding and activation.
Methods: AUC was used to determine the protein size. SEN octameric structure was imaged using CryoEM. Binding affinity of SEN and hPg was determined by ELISA and SPR. Surface expression of SEN was determined using FACS and SEM.
Results: The data generated showed that the 47 kDa monomer unit of recombinant SEN oligomerizes to form highly stable octamer in solution. The octameric SEN forms a complex with hPg, characterized by a Kd of ~100 nM. Although SEN tightly binds hPg, it does not stimulate hPg activation by SK or urokinase. Whole GAS cell studies demonstrated that the surface expression of SEN is diminished upon deletion of the PAM gene. hPg binding to AP53 cells has a lower affinity for SEN compared to PAM.
Conclusion(s): This study strongly suggests that SEN plays a secondary role as a hPg receptor in skin‐tropic GAS strains.
Thrombolytic Therapy
OC 46.5
Carbamylation in acute ischemic stroke thrombi impairs t‐PA‐mediated thrombolysis and increases thrombus frailty
V. Ollivier 1; A. Freiherr Von Seckendorff2; M. Solo Nomenjanahary1; M. Bourrienne3; J. Desilles4; M. Mazighi5; B. Ho‐Tin‐Noé6
1 INSERM UNIT 1148, PARIS, Ile‐de‐France, France; 2 Rothschild Foundation Hospital, PARIS, Ile‐de‐France, France; 3 Hôpital Beaujon (APHP); INSERM LVTS U1148 Université de Paris, CLICHY, Ile‐de‐France, France; 4 Rothschild Foundation Hospital, paris, Ile‐de‐France, France; 5 Rothschild Foundation Hospital Paris, paris, Ile‐de‐France, France; 6 Inserm, Paris, Ile‐de‐France, France
Background: Carbamylation is a post‐translational modification that results from the interaction between isocyanic acid with free functional groups of proteins. It preferentially occurs on the ε‐NH2 of lysine residues, thus generating homocitrulline (HCit). Importantly, conversion of lysine residues of fibrin(ogen) to HCit was shown to impair t‐PA‐mediated fibrinolysis. Although non‐enzymatic, carbamylation can be promoted by neutrophils through their myeloperoxidase (MPO) activity that favors isocyanic acid formation. We and others have shown that neutrophils are important components of thrombi responsible for large vessel occlusion (LVO)‐related acute ischemic stroke (AIS). It remains unknown whether carbamylation occurs in such thrombi.
Aims: We aimed to determine whether carbamylation occurs in AIS thrombi and whether it affects recanalization therapies.
Methods: A total of 30 thrombi from AIS patients with LVO, all recovered by endovascular therapy, were analyzed for the presence of carbamylated products by anti‐HCit immunostaining and by quantitative measurement of fibrin(ogen)‐ and von Willebrand factor (vWF)‐associated HCit. Correlations between fibrin(ogen) carbamylation and thrombolysis resistance were investigated in ex vivo thrombolysis assays in which AIS thrombi were treated with a mixture of tPA/plasminogen, and lysis supernatants analyzed for quantification of carbamylated fibrin(ogen). The impact of carabamylation on mechanical thrombectomy was determined by comparing the response to thrombectomy of non‐carbamylated and carbamylated blood clots produced in vitro and injected in‐flow in a Biomodex Evias Plus simulation system.
Results: All AIS thrombi contained carbamylated fibrin(ogen) and vWF whose levels were positively correlated to thrombus MPO and DNA content. Resistance to ex vivo thrombolysis was significantly correlated with thrombus carbamylated fibrin(ogen), MPO, and DNA content. Carbamylation led to an increase in thrombus frailty and fragmentation during simulated thrombectomy procedure.
Conclusion(s): Carbamylation occurs in AIS thrombi and negatively interferes with both pharmacological thrombolysis and mechanical thrombectomy.
OC 46.4
von Willebrand Factor‐targeting thrombolysis to overcome tissue plasminogen activator resistance in vivo
M. van Moorsel; C. Maas; S. De Maat
UMC Utrecht, Utrecht, Utrecht, Netherlands
Background: Intravenous administration of recombinant human tissue Plasminogen Activator (rh‐tPA) is the standard‐of‐care for pharmacological treatment of Acute Ischemic Stroke (AIS), although it is only effective in < 33% of patients. rh‐tPA requires binding to fibrin to activate plasminogen and initiate thrombolysis, limiting its applicability to fibrin‐dependent thrombi.. In contrast, Microlyse is a thrombolytic agent that targets vWF rather than fibrin to initiate plasminogen activation. Microlyse effectively attenuates symptoms in a mouse model for thrombotic thrombocytopenic purpura, a VWF‐driven form of microvascular thrombosis.
Aims: To compare rh‐tPA with Microlyse in two AIS mouse models that are either fibrin‐ or vWF‐dependent.
Methods: AIS was induced in the middle cerebral artery (MCA) by either direct infusion of thrombin or by topical application of FeCl3 (Fig. 1). Full vascular occlusion (stable for > 20 minutes) was confirmed Laser Doppler flowmetry. Next, mice were intravenously administered vehicle, 0.8mg/kg Microlyse or 10mg/kg tPA by bolus‐injection in‐ (10% of total dose) and infusion for 40 minutes via (90% of total dose) in the tail vein. Brain tissue reperfusion was monitored with Laser Speckle imaging, starting 10 minutes before‐ until 10 minutes after treatment. After 24 hours, the extent of ischemic injury was investigated by T2w‐MRI.
Results: In the fibrin‐dependent model, both rh‐tPA (35.8 ± 17.1%; p = 0.001) and Microlyse (39.3 ± 13.1%; p < 0.0001) increased early reperfusion (T = 70min; vehicle = 15.6 ± 7.5%). Consequently, both rh‐tPA (18.9 ± 11.2 mm3; p = 0.033) and Microlyse (16.1 ± 13.9 mm3; p = 0.018) reduced lesion volumes, indicating lowered ischemic injury (vehicle = 26.6 ± 5.6 mm3). In the vWF‐dependent model, rh‐tPA (7.6 ± 8.8%; p = 0.216) nor Microlyse (16.3 ± 13.9%; p = 0.151) increased early reperfusion (vehicle = 10.1 ± 7.9%). However, only Microlyse (13.9 ± 11.4 mm3; p < 0.001) but not rh‐tPA (23.6 ± 11.1 mm3; p = 0.188) significantly reduced lesion volumes (vehicle = 30.3 ± 10.9 mm3).
Conclusion(s): Altogether, whereas rh‐tPA only reduced ischemic injury in a fibrin‐dependent AIS model, Microlyse was effective in both models (table 1), supporting applicability irrespective of the thrombus composition.
Hemophilia and Rare Bleeding Disorders
Acquired Hemorrhagic Coagulation Disorders
OC 63.5
Cytokine profile of patients with acquired hemophilia A (AHA) in a longitudinal study
J. Frade‐Guanaes 1; A. Racanelli1; L. Siqueira1; C. Costa‐Lima1; S. Medina1; N. Foschi1; L. De Lima1; A. Francisco1; M. Colella1; S. Montalvão2; G. Yamaguti‐Hayakawa1; M. Ozelo1
1 Hemocentro UNICAMP, Department of Internal Medicine, School of Medical Sciences, University of Campinas, Campinas, Sao Paulo, Brazil, 22Hemostasis and Thrombosis Laboratory, Hematology and Hemotherapy Center, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil
Background: Acquired Hemophilia A (AHA) is an autoimmune disease caused by the production of autoantibodies against factor VIII. There is limited data about the pathophysiology of the disease, including T and B cells response.
Aims: To determine the cytokine expression through T and B cells activation in a longitudinal evaluation of patients with AHA.
Methods: This study included 18 patients diagnosed with AHA from a single center. Peripheral blood mononuclear cells were isolated before immunosuppressive therapy (IST), after achieving complete remission, and at relapse/failure. Cells were cultivated with RPMI‐1640 medium, with 7.5x105 cells/well in a 48‐well plate. After 24h, cells were stimulated with full‐length recombinant FVIII concentrate (rFVIII). After incubation for 24 h, cells were analyzed by flow cytometry (FACS).
Results: IST consisted of prednisone with cyclophosphamide (72.2%), only cyclophosphamide (11.1%) and, rituximab as second‐line therapy (16.7%). 50% of patients (n = 9) achieved complete remission, and 27.8% (n = 5) were considered as failure (2) or relapse (3). Three patients are still under initial follow‐up, and one patient died and was censured for the longitudinal analysis (table 1). We observed a significant increase in the production of CD4 cytokine subsets in both responding and non‐responding (failure/relapse) patients at baseline when compared with healthy individuals. This difference was not observed when patients achieved remission. Also, we observed increased production of Th17 [IL‐17A (p = 0.0002), IL‐21 (p = 0.007), TGF‐b (p = 0.04)], Th2 [IL‐4 (p = 0.004)] and Th1 cytokines [TNF‐a (p = 0.006), IFN‐γ (p = 0.001)] between all AHA at baseline and controls. Interestingly, as we previously reported for BAFF (B‐cell activating factor), baseline levels of IFN‐γ were markedly increased in non‐responding patients when compared to patients with sustained complete remission (figure 1).
Conclusion(s): Our findings suggest that BAFF levels in B cells and IFN‐ γ in T cells at the diagnosis are potential biomarkers for AHA prognosis and treatment response.
OC 40.2
Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis in patients with acquired hemophilia A: Primary analysis results from a Phase III study (AGEHA)
M. Shima 1; K. Amano2; Y. Ogawa3; K. Yoneyama4; R. Ozaki4; R. Kobayashi4; E. Sakaida5; M. Saito6; T. Okamura7; T. Ito8; N. Hattori9; S. Higasa10; N. Suzuki11; Y. Seki12; K. Nogami1
1 Nara Medical University, Kashihara, Nara, Japan; 2 Tokyo Medical University Hospital, Tokyo, Tokyo, Japan; 3 Gunma University Graduate School of Medicine, Maebashi, Gumma, Japan; 4 Chugai Pharmaceutical Co., Ltd., Tokyo, Tokyo, Japan; 5 Chiba University Hospital, Chiba, Chiba, Japan; 6 Aiiku Hospital, Sapporo, Hokkaido, Japan; 7 St. Mary’s Hospital, Kurume, Fukuoka, Japan; 8 National Hospital Organization Sendai Medical Center, Sendai, Miyagi, Japan; 9 Showa University School of Medicine, Tokyo, Tokyo, Japan, 10 Hyogo College of Medicine Hospital, Nishinomiya, Hyogo, Japan, 11 Nagoya University Hospital, Nagoya, Aichi, Japan, 12 Niigata University Medical and Dental Hospital, Minami‐uonuma, Niigata, Japan
Background: Emicizumab is a bispecific antibody that mimics the cofactor function of activated factor (F) VIII and prevents bleeds in patients with congenital hemophilia A (PwCHA) regardless of inhibitor status. Given its mechanism of action, emicizumab would also be efficacious in patients with acquired hemophilia A (PwAHA). However, no prospective clinical studies have been performed to explore optimal dosing regimen or completion criteria for emicizumab in PwAHA.
Aims: To report the primary analysis results from a prospective, multicenter, open‐label phase III study evaluating the efficacy, safety, and pharmacokinetics of emicizumab in PwAHA (AGEHA; JapicCTI‐205151).
Methods: PwAHA aged ≥18 years received emicizumab subcutaneously at 6 mg/kg on Day 1 and 3 mg/kg on Day 2 followed by 1.5 mg/kg once weekly from Day 8 onwards. Dosing completion criteria comprised FVIII activity > 50 IU/dL and no coagulation factor product use for bleeds within 72 hours. AGEHA was designed as a descriptive study with no primary or secondary endpoints and conducted in accordance with relevant ethical standards.
Results: By the cutoff date (April 23, 2021), 12 patients on immunosuppressive therapy had been enrolled and 11 of them (91.7%) had completed emicizumab treatment. Mean trough plasma emicizumab concentration reached steady state rapidly (1 week) achieving the efficacious level that was established in PwCHA ( > 30 μg/mL). During the pre‐treatment period, 6 (50.0%) patients experienced 30 treated bleeds, 27 of which were major bleeds. During the on‐treatment period, 5 treated bleeds occurred in 2 (16.7%) patients, and no major bleeds occurred in any patient (Figure). Emicizumab was well tolerated with no treatment discontinuation. One asymptomatic, non‐serious deep vein thrombosis was reported during emicizumab treatment and resolved without any treatment. No patients developed anti‐emicizumab antibodies impacting pharmacokinetics (Table).
Conclusion(s): These results suggest emicizumab prophylaxis with the tested dosing regimen and completion criteria has a favorable benefit–risk profile in PwAHA.
Hemophilia – Basic
OC 07.4
Exploring the conformational landscape of Factor VIII B domain in order to generate an all atom full length structure of the coagulation Factor VIII protein
S. Ramaraje Urs 1; S. Singh2; H. Javed3; D. Fenel4; J. Teulon5; G. Schoehn5; J. Pellequer5; B. Ma6; D. Ugurlar6; D. Imhof7; J. Oldenburg8; A. Biswas1
1 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 4 Institut de Biologie Structurale, University Grenoble Alpes, Grenoble, Rhone‐Alpes, France; 5 Institut de Biologie Structurale, University Grenoble Alpes, Grenoble, Rhone‐Alpes, France; 6 Materials and Structural Analysis Division, Thermo Fisher Scientific, Achtseweg Noord, Eindhoven, Noord‐Brabant, Netherlands; 7 University of Bonn, Bonn, Nordrhein‐Westfalen, Germany; 8 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: The B domain of coagulation Factor VIII (FVIII) has a heavily glycosylated and highly disordered structure. Traditional structural characterization methods are unable to resolve the structure of the B domain and hence of full length FVIII (FL‐FVIII).
Aims: Our study aims to structurally characterize the FVIII B domain using integrated hybrid methodology [Figure 01]. Bioanalytical techniques such as cross‐linking mass spectrometry (XL‐MS) are being used on purified FL‐FVIII to identify interdomain (between B domain and other domains of FVIII) and intradomain (within B domain) interface residues. The interdomain and intradomain interface residues will be used as modeling restraints for modeling the structure of the B domain and of FL‐FVIII. Simultaneously biophysical techniques such as AFM and cryo‐EM are being carried out on the purified FL‐FVIII. The structural models will be validated by matching/fitting/docking them onto biophysical maps/images generated.
Methods: Plasma concentrates and recombinant FL‐FVIII products are further purified by various chromatography methods to remove all excipients. One mM of DSSO crosslinker is used to crosslink 10 uM of pure FL‐FVIII and the cross‐linked product is further purified by SEC (size exclusion chromatography). Glycosyl residues in FL‐FVIII were removed by de‐glycosylation reaction using 5000 units of PNGase‐F enzyme. Negative staining of the purified FL‐FVIII followed by 2D classification was done towards future cryo‐EM studies. Air and liquid AFM imaging was also performed on the highly pure FL‐FVIII. The FL‐FVIII model from the alpha‐fold database was modified in silico to incorporate all post‐translational modification and subjected to all‐atom MD simulation.
Results: Pure FL‐FVIII was successfully crosslinked with DSSO towards future XL‐MS data. Negative staining and AFM of FL‐FVIII showed structural heterogeneity in FL‐FVIII which is also reflected in the simulation trajectory of the alpha‐fold model of FL‐FVIII.
Conclusion(s): FL‐FVIII is structurally heterogeneous owing to the conformational variability of the B domain. FIGURE 1 Integrated Hybrid (IH) approaches towards solving the structure of the FVIII B domain and FL‐FVIII
FIGURE 1 Integrated Hybrid (IH) approaches towards solving the structure of the FVIII B domain and FL‐FVIII
OC 07.5
Mathematical modeling to identify clotting factor combinations that enhance thrombin generation in hemophilia
M. Stobb1; K. Leiderman2; S. Sindi3; A. Fogelson 4; K. Neeves5; D. Monroe6
1 Coe College, Cedar Rapids, Iowa, United States; 2 Colorado School of Mines, Golden, Colorado, United States; 3 University of California Merced, Merced, California, United States; 4 University of Utah, Salt Lake City, Utah, United States; 5 University of Colorado Denver, Aurora, Colorado, United States; 6 UNC Blood Research Center, Chapel Hill, North Carolina, United States
Background: Our model previously identified that low normal levels (50%‐75%) of factor V (FV) enhanced thrombin generation in hemophilia A (Link, JTH, 18, 2020). This enhancement was found through a computational exploration through normal level factor combinations.
Aims: To identify factor levels that enhance thrombin generation in hemophilia B, C, and normal type blood using the methods developed in our previous work.
Methods: Plasma levels for factors II, V, VII, VIII, IX, X, XI, tissue factor pathway inhibitor (TFPI), and antithrombin (AT) were fixed at one of three values: 50%, 100%, or 150% of normal. Our mathematical model individually simulated all possible combinations of these three factor levels (19,683 combinations) for normal, hemophilia A, B, and C type blood. For each combination of factor levels, tissue factor (TF) was varied between 1 and 15 fmol/cm^2, resulting in well over one million independent simulations. Combinations of factor levels were identified as thrombin enhancing when they resulted in at least a 10 fold increase in detectable thrombin levels.
Results: For all four simulated cases (normal, hemophilia A, B, and C) low normal (50%) levels of FV and high normal (150%) levels of factor II led to an increase in total thrombin. For normal and hemophilia C, high normal levels of factors VIII and IX increased thrombin generation, while high normal levels of factor VIII increased thrombin generation in hemophilia B. There was moderate enhancement of thrombin in hemophilia A with low normal levels of factors IX and FXI, and also AT, but only when FVIII was severely deficient (1%). When FVIII levels were raised to 10%, high normal levels of FIX resulted in increased thrombin.
Conclusion(s): Our mathematical model identified new and testable combinations of parameters that enhance thrombin generation in normal, hemophilia A, B, and C blood.
OC 70.5
Bleeding in non‐severe haemophilia A and B – data from the PedNet study group
M. de Kovel 1; C. Königs2; S. Ranta3; C. Escuriola Ettingshausen4; K. Fischer5
1 PedNet Haemophilia Research Foundation, Utrecht, Utrecht, Netherlands; 2 University Hospital Frankfurt, Goethe University, Frankfurt, Hessen, Germany; 3 Department of Women's and Children's Health, Karolinska Univeristy Hospital and Karolinska Institute, Stockholm, Stockholms Lan, Sweden; 4 Hämophilie Zentrum Rhein Main (HZRM), Mörfelden‐Walldorf, Germany, Moerfelden‐Walldorf, Hessen, Germany; 5 University Medical Center Utrecht, University Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands
Background: The clinical observation that bleeding phenotype is milder in haemophilia B has never been established in large datasets.
Aims: To compare bleeding patterns between children with non‐severe haemophilia A and B.
Methods: Haemophilia patients included in the PedNet Registry (Clin.gov.trial‐NCT02979119) by January 1st, 2020 with baseline factor FVIII/FIX activity between 1%‐25% (0.01 UI/mL‐0.25 UI/mL) with follow up information available, were included in this study. Patients were followed from diagnosis to: January 1st, 2020, 18 years of age, start of prophylaxis, or inhibitor diagnosis. ABRs and A(J)BRs were established by negative binomial modelling. Onset of bleeding was analysed using Kaplan‐Meier survival. Groups were compared using regression analysis and Log Rank tests, respectively.
Results: 825 patients were included: 237 moderate HA, 92 moderate HB, 404 mild HA and 92 mild HB patients. Total follow up was 5249 patient years for HA, 1354 patient years for HB. Median age at last FU was 9.7 years (IQR 6.0 – 13.8) for HA, 8.6 years (IQR 5.0 – 12.6) for HB. Onset of bleeding was comparable between haemophilia types. Median age at first bleed was 3.7 years (95%CI 3.1 – 4.3) for HA vs 4.8 years (95%CI 3.5 – 6.1) for HB (p‐value 0.432). Median age at first joint bleed was 10.8 years (95%CI 9.2 – 12.4) for HA vs > 12.5 years for HB (p‐value 0.167). Moderate HA showed a more severe bleeding type in both ABR (1.12 in HA vs 0.47 in HB, p < 0.001) and AJBR (0.29 vs 0.10, p = 0.001)(Figure 1). For mild haemophilia, bleeding rates were very low and appeared similar in HA and HB: ABR 0.21 vs 0.16 (p = 0.212), AJBR 0.05 vs 0.03 (p = 0.283).
Conclusion(s): Moderate HA patients have a significantly more severe phenotype than moderate HB. Bleeding tendency in mild haemophilia was very low and no differences between the two types were observed. FIGURE 1 Annualized (joint) bleeding rate (established by negative binomial modelling) according to haemophilia type and severity
FIGURE 1 Annualized (joint) bleeding rate (established by negative binomial modelling) according to haemophilia type and severity
OC 20.5
Haemophilia‐mediated bleeding induces red pulp macrophage expansion
H. Hawerkamp 1; A. Yeow1; C. Byrne1; A. Chevalier1; L. Matarazzo1; J. O'Sullivan2; V. Kelly3; B. Gangadharan4; B. Reipert4; J. O'Donnell5; P. Turecek6; P. Fallon7
1 School of Medicine, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin 2, Ireland, Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland., Dublin, Dublin, Ireland; 3 School of Biochemistry& Immunology, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin 2, Ireland., Dublin, Dublin, Ireland; 4 Baxalta Innovations GmbH, A Member of the Takeda Group of Companies, Vienna, Austria., Vienna, Wien, Austria; 5 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 6 Baxalta Innovations GmbH, a Takeda company, Vienna, Wien, Austria; 7 Trinity College Dublin, Dublin, Dublin, Ireland
Background: Haemophilia A is an X‐linked bleeding disorder caused by a deficiency in the blood clotting protein, factor VIII (F8). Patients with haemophilia suffer from recurrent bleeding episodes of varying severity. The initial inflammation in joints is caused by synovial macrophages that constantly remove the accumulating iron deposits from erythrocytes entering the joints in a bleeding episode. Another cell type important in the iron recycling are splenic red pulp macrophages (RPM). Due to their unique location their main task is to scavenge damaged or aged erythrocytes.
Aims: To investigate underlying immunological mechanisms in haemophilia A, we have generated a novel factor 8 deficient (F8em1) mouse with a deletion in exon 1 using CRISPR/Cas technology. Here we aim to investigate RPMs in haemophilia, and especially their changes and adaptations during bleeding episodes in spontaneous bleeding and a model of haemophilic arthropathy.
Methods: RPMs in spleens from F8em1 and wild‐type mice were analysed via flow cytometry using their characteristic low expression of the marker CD11b and high expression of macrophage marker F4/80. Changes in RPM were analysed in F8em1 mice with spontaneous bleeding and in modelled haemophilic arthropathy. We further investigated the receptor for iron uptake (transferrin receptor, CD71) during bleeding episodes via flow cytometry. Systemic iron concentrations were measured during modelled haemophilic arthropathy.
Results: While F8em1 mice without bleeds do not differ from WT animals in their RPM percentage, both spontaneous bleeding and modelled haemophilic arthropathy, significantly increased RPM. This percentage inversely correlated with hematocrit. The expansion of RPM is seen together with a strong upregulation of CD71. We further report a strong iron release before the onset of RPM expansion.
Conclusion(s): F8em1 mice respond to a bleeding with a strong expansion of RPMs, most profound at 7 days after injury. This expansion is probably facilitated by a rapid systemic iron overload.
OC 37.5
Liver sinusoidal endothelial cells as a novel target for tolerance induction to FVIII
M. Leoni 1; M. Miranda2; K. Fijnvandraat3; S. Lacroix‐Desmazes4; J. Voorberg2
1 Department of Molecular Hemostasis, Sanquin Research, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Pediatric Hematology, Amsterdam UMC, University of Amsterdam, Emma Children’s Hospital, Amsterdam, Noord‐Holland, Netherlands; 4 INSERM, Paris, Ile‐de‐France, France
Background: A major complication of current treatment of hemophilia A is the development of FVIII inhibitory antibodies. Currently, immune tolerance induction (ITI) is used to eradicate FVIII inhibitors. Since ITI fails in 30% of patients within the EDUC8 consortium we are exploring novel ways to induce tolerance to FVIII.
Aims: Explore the potential of targeting FVIII or FVIII derived peptides to liver sinusoidal endothelial cells (LSEC) since these cells have been shown to promote tolerance instead of immunity towards the antigens presented on their surface.
Methods: Human hepatic sinusoidal endothelial cells were cultured and characterized. We studied their phenotype by FACS analysis and confocal imaging using specific antibodies against FVIII, stabilin‐2 and other markers of LSEC and endothelial cells. In a parallel approach we also employed stabilin‐2 transfected HEK293 cells as a model system for stabilin‐2 dependent targeting of LSEC.
Results: Our results show that primary human hepatic endothelial cells express von Willebrand factor. Primary human liver endothelial cells also express VE‐Cadherin and evidence was obtained for expression of stabilin‐2 on the cell membrane employing confocal microscopy. FACS analysis confirmed these findings and provided evidence for the expression of CD32, previously shown to be expressed on LSEC. In parallel we established a transient system for expressing stabilin‐2 in HEK293 cells which will be used to study the interaction of FVIII and VWF with stabilin‐2 and explore whether the internalization is linked to activation of tolerogenic pathways in LSEC.
Conclusion(s): Our findings indicate that LSECs are a potential target for the induction of tolerance. The primary human hepatic endothelial cells used in this study express several LSEC specific markers. The presence of stabilin‐2 on the surface of these cells will allow us to target this receptor employing recombinant proteins containing FVIII immunogenic domains or peptides with the ultimate goal of inducing tolerance to FVIII.
OC 07.3
Whole genome expression analysis resolves the correlation between genetic knockout of single genes and the obtained phenotype in relation to FVIII secretion
R. Al Rifai 1; M. Jamil2; M. Ibrahim2; N. Nüsgen2; H. Singer2; J. Oldenburg3; O. El‐maarri2
1 Uniklinikum Bonn, bonn, Nordrhein‐Westfalen, Germany; 2 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: We have shown that GABARAPs proteins modulate FVIII secretion; however, GABARAP gene KO decreased FVIII secretion by 40%, whereas GABARAPL1 and GABARAPL2 gene KOs increased FVIII secretion by 70 and 100%, respectively.
Aims: To identify the underlying reason, we performed gene rescue experiments and 3’RNAseq to identify DEGs. Our overall aim is to identify the link between the individual gene knockouts and FVIII secretion.
Methods: We rescued each KO by transient transfection of the corresponding gene; FVIII activities in the medium were measured using chromogenic assay. 3’RNA sequencing data results of wild type HEK28 cells and the eight knockouts were analysed using bioinformatics tools to determine DEGs and the affected pathways.
Results: The reintroduction of CANX, CALR, LMAN1, MCFD2 and GABARAP returned the FVIII secretion to direction of wild type values; in contrast, GABARAPL1 and GABARAPL2 did not show a significant rescue. Pairwise comparison between knockouts and HEK28 at 5% FDR showed 199, 68 and 1087 DEGs in CALR, GABARAPL1 and GABARAPL2 knockout respectively; all other KOs showed low DEG counts. IPA analysis of the DEG showed significant enrichment for pathways related to energy metabolism, among which ATP production was predicted high and low for increased and decreased FVIII secretion knockout respectively.
Conclusion(s): The rescue process was successful for transporter molecules (CANX, LMAN1, MCFD2 and GABARAP) returning the FVIII secretion values to normal, while the others (CALR, GABARAPL1 and L2) did not reach normal values. The RNA‐seq analyses showed that the latter are involved in the metabolism of the cells requiring longer time and more suitable conditions for rescue. 3'RNA sequencing results underscore the need for high ATP for the release of FVIII from the ER, these knockouts are considerably showing the change in ATP production correlated with change in FVIII secretion.
OC 20.3
Osteoclast role in haemophilic bone disease
S. Lancellotti 1; G. Battafarano2; M. Sacco3; M. Tardugno3; L. Di Gennaro4; R. De Cristofaro3; A. Del Fattore2
1 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Rome, Lazio, Italy; 2 Bone Physiopathology Group, Multifactorial Disease and Complex Phenotype Research Area, Bambino Gesù Children’s Hospital, Rome, Lazio, Italy; 3 Catholic University of Sacred Heart, Translational Medicine and Surgery Department, Rome, Lazio, Italy; 4 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Center for Hemorrhagic and Thrombotic Diseases and Haemophilia Center, Rome, Rome, Lazio, Italy
Background: Bone disease is a significant complication of haemophilia A (HA), but its pathogenesis still remains unknown. The reduction of bone mineral density seems due to perturbations of the Receptor Activator of Nuclear factor‐B RANK Ligand (RANKL) and osteoprotegerin (OPG) pathways. In haemophilic alterations of bone remodeling, osteoclasts seem to play an important role. Osteoclasts are multinucleated cells which derived from the CD14+ monocyte/macrophage lineage. Until recently, the identity of osteoclast progenitors has not been well defined, but evidences report that CD16−CD14+ rather than CD16+CD14+ monocytes were prone to differentiate into osteoclasts [1].
Aims: This study aims to evaluate whether the osteoclastogenesis could be influenced by the FVIII, von Willebrand Factor (VWF) and thrombin and to assess the osteoclastogenic potential from two different HA patients.
Methods: Osteoclastogenesis of healthy donors‐derived PBMC (Peripheral Blood Mononuclear Cells) were assessed in the presence of plasma derived VWF/FVIII complex, rVWF, rFVIII and thrombin. FACS‐analysis, in vitro assays and gene expression analysis were performed to evaluate osteoclast precursors and osteoclastogenic potential of PBMC isolated from two haemophilic patients.
Results: VWF appears to play a major role to regulate osteoclast differentation from healthy donor‐derived PBMC. Indeed, it inhibits by itself ~45% the osteoclastogenesis comparable to OPG, and even more if is complexed with FVIII (53%inhibition). Thrombin reduces osteoclast differentiation with variable effects (30‐50% inhibition). PBMC from HA patients showed increased ability to form mature osteoclasts compared to those obtained from healthy controls. Osteoclast precursors (CD16−CD14+CD11b+) are significantly higher in HA patients than age and sex matched controls (~33%). Moreover, RNA expression analysis performed on patient’s osteoclasts revealed higher levels of RANK, TRAF6, CATHEPSIN‐K and TCIRG1 genes expression compared to matched controls.
Conclusion(s): All these data support that bone loss observed in haemophilic patients could be related to increased osteoclast formation and activity and that coagulation factors directly impact on bone cells.
OC 37.4
A humanized mouse model to assess the therapeutic potential of IdeS in hemophilia A with inhibitors
M. Bou Jaoudeh 1; V. Daventure2; S. Delignat2; J. astermark3; H. Lévesque4; V. Proulle5; S. Lacroix‐Desmazes6
1 INSERM UMRS1138 ‐ Sorbonne University, Paris, Ile‐de‐France, France; 2 Inserm, Paris, Ile‐de‐France, France; 3 Skåne University Hospital, skane, Skane Lan, Sweden; 4 Rouen, France; 5 INSERM UMRS1138, Paris, Ile‐de‐France, France; 6 INSERM, Paris, Ile‐de‐France, France
Background: The development of inhibitory anti‐factor VIII (FVIII) antibodies (inhibitors) is the major drawback of replacement therapy in patients with hemophilia A (HA). IdeS is a proteinase from Streptococcus pyogenes that specifically degrades human IgG. We previously showed that IdeS efficiently hydrolyzes polyclonal anti‐FVIII IgG in patients’ plasma as well as recombinant monoclonal anti‐FVIII IgG in vitro.
Aims: To develop a humanized inhibitor‐positive HA mouse model and test the clinical relevance of IdeS in eliminating human anti‐FVIII IgG.
Methods: FVIII‐deficient mice were passively immunized with a human monoclonal anti‐FVIII IgG1 (BO2C11, 1200 Bethesda units (BU)/kg). On day‐1 (d1), passively immunized mice were treated with IdeS (0.6 mg/kg) or with PBS. BO2C11 levels were monitored for 4 days. On d4, all mice were injected with 200 UI/kg of human recombinant FVIII. FVIII recovery and blood loss in a tail‐clip bleeding model were assessed two hours later.
Results: Passively immunized mice had steady BO2C11 levels around 10 BU/mL between d1 and d4. Injection of IdeS induced a drop in BO2C11 levels: 8.3 ± 1.4 versus 0.7 ± 0.4 BU/mL in PBS‐treated and IdeS‐treated mice. The recovery of injected FVIII was higher in IdeS‐treated mice than in PBS‐treated mice (0.47 ± 0.51 vs 0.02 ± 0.02 IU/mL, p = 0.0004). Likewise, following FVIII injection, IdeS‐treated mice demonstrated lower blood loss than PBS‐treated mice (16 ± 26 vs 74 ± 65 μl, p = 0.0289) in a tail‐clip model, that did not differ from naïve FVIII‐treated mice (24 ± 21 μl, p = 0.3).
Conclusion(s): We validated a reproducible humanized inhibitor‐positive mouse model of HA. Using this model, we present proof of concept for the removal of FVIII inhibitors by IdeS, opening a time window for the efficient use of FVIII replacement therapy. Aside from the use of by‐passing agents and/or emicizumab, IdeS‐mediated transient removal of FVIII inhibitors may be a new therapeutic option to improve the management of inhibitor‐positive patients.
OC 30.2
Efficacy of emicizumab prophylaxis in patients with severe hemophilia A in Germany: Real‐life‐data documented by eDiary smart medication
W. Mondorf1; E. Hermann2; C. Escuriola Ettingshausen 3; F. Ronald4; K. Holstein5; R. Klamroth6; U. Sachs7
1 Haemostas Frankfurt, Frankfurt, Hessen, Germany; 2 Universitätsklinikum des Saarlandes, Homburg/Saar, Saarland, Germany; 3 Hämophilie Zentrum Rhein Main (HZRM), Mörfelden‐Walldorf, Germany, Moerfelden‐Walldorf, Hessen, Germany; 4 SRH Kurpfalzkrankenhaus Heidelberg, Heidelberg, Baden‐Wurttemberg, Germany; 5 Universitätstklinikum Eppendorf, Hamburg, Hamburg, Germany; 6 Vivantes Klinikum, Friedrichshain, Berlin, Berlin, Germany; 7 Universitätsklinikum Gießen Marburg, Giessen, Hessen, Germany
Background: Real‐life data showing the efficacy of emicizumab treatment in patients with severe hemophilia A (PwSHA) in Germany are limited.
Aims: The aim of the study was to find out whether a change in ABR or AJBR could be detected in the real data of smart medication eDiary after switching patients from FVIII concentrate to emicizumab.
Methods: ABR, AJBR and proportion of bleed‐free patients were documented using the electronic diary platform smart medication. Data of PwSHA before and after switch of treatment with FVIII concentrates to emicizumab were evaluated retrospectively. Included were patients with > 24 weeks of electronic documentation after switch. Data up to and including December 2021 were analyzed.
Results: 39 PwSHA from 7 HTCs using smart medication could be included. The median age was 42 years (IQR 35.5); 81% were 18 years and older. 13 PwSHA started with electronic documentation together with the switch to emizicumab. Data from paper documentation prior to switch in these patients were not available. In 26 patients complete electronic documentation before and after switch could be evaluated. After switch to emicizumab, the mean AJBR was 0.45, the mean ABR 0.73 in all patients. In the subgroup of 26 PwSHA with a > 24 weeks documentation before and after switch, the mean AJBR was 2.28 before and 0.68 after switch, the mean ABR dropped from 5.89 to 1.09 after switch. The proportion of bleeding‐free patients increased from 54% before to 77% after switching to emicizumab. Despite of additional FVIII treatment in of 7 (27%) patients after the switch to emicizumab, only 2 needed additional FVIII due to joint bleeds.
Conclusion(s): Real‐life data documentation by using electronic diary show a significant decrease of bleeding episodes by switching PwSHA on prophylactic treatment from FVIII concentrates to emicizumab, a more effective prophylaxis, consistent with data from clinical trials, may be discussed.
OC 47.5
The impact of N‐linked glycosylation in C1 domain of FVIII on immunogenicity
M. Fan 1; S. Wang2; J. Zhang3; X. Cai1; T. Chao1; L. Li2; W. Xiao3; B. Konkle4; C. Miao1
1 Seattle Children's Research Institute, Seattle, Washington, United States; 2 Georgia State University, Atlanta, Georgia, United States; 3 Indiana University, Indianapolis, Indiana, United States; 4 Washington Center for Bleeding Disorders and the University of Washington, Seattle, Washington, United States
Background: We previously demonstrated that lower FVIII inhibitor titers were detected in hemophilia A (HemA) mice injected with FVIII plasmid containing Glutamine to Asparagine substitution in C1 domain (N2118Q) than the mice with BDD‐FVIII plasmid via hydrodynamic gene delivery. It suggested that the elimination of N‐glycosylation in C1 domain reduced the immunogenicity and a glycoepitope may exist in C1 domain.
Aims: To examine the impact of FVIII glycosylation following AAV‐mediated gene therapy and further characterize the important immunogenic glycoepitope region in C1 domain.
Methods: HemA mice were injected with AAV‐FVIII and AAV‐N2118Q, followed by challenge with repeated FVIII injections. FVIII activity and inhibitor titer were examined over time. To characterize the immunogenic region around 2118 site, three sets of 15‐amino‐acid peptides were designed with partly overlapping and with or without mannosylation (MP1/NGP1; MP2/NGP2; MP3/NGP3). Cell proliferation and cytokine levels in supernatant were evaluated from mouse splenocytes or human PBMCs cultured with different peptides. Immunization studies in HemA mice using these peptides and FVIII challenge are being carried out.
Results: FVIII expression was initially detected in all AAV treated mice for eight weeks, however, subsequently dropped to very low or undetectable at week 12 in BDD group, whereas was only slightly decreased in N2118Q group. Following FVIII challenge, N2118Q group showed higher resistance to inhibitor formation than BDD group. Higher proliferation rates or cytokine levels were detected in mouse cells and human PBMCs isolated from inhibitor subjects cultured with two mannosylated peptides (MP1 and MP2) but not with MP3 and all non‐mannosylated peptides. Cells from control non‐inhibitor subjects did not exhibit increased proliferation when stimulated with all peptides.
Conclusion(s): Elimination of N‐glycosylation in C1 domain reduced FVIII immunogenicity following both plasmid‐ and AAV‐mediated gene therapy. The glycoepitope surrounding N2118 in FVIII was identified and characterized in both human and mouse cells.
OC 50.4
Differences in bleeding profiles and venous clot structures between emicizumab and factor VIII‐treated mice
T. Sefiane 1; V. Muczynski2; X. Heiligenstein3; O. Christophe2; C. Denis2; C. Casari4; P. Lenting2
1 UMR_S 1176 INSERM, Le Kremlin‐Bicêtre, France, Le Kremlin Bicetre, Ile‐de‐France, France; 2 INSERM U1176, Le Kremlin Bicetre, Ile‐de‐France, France; 3 Cryocapcell, Le Kremlin Bicetre, Ile‐de‐France, France; 4 Inserm U1176 ‐ HITh, Le Kremlin Bicetre, Ile‐de‐France, France
Background: Emicizumab is a bispecific antibody used to treat hemophilia A with/without inhibitors. We previously reported that emicizumab partially corrects bleeding in factor VIII (FVIII)‐deficient mice following tail‐clip injury. Using a milder bleeding model of tail vein‐transection (TVT), we further analyzed emicizumab’s hemostatic efficacy in mice.
Aims: To analyze the bleeding profile and clot stability/structure following FVIII or emicizumab treatment.
Methods: FVIII‐deficient mice received low‐dose FVIII (5 IU/kg) or emicizumab (5 mg/kg) intravenously before TVT, and bleeding profiles were evaluated with/without removing the occlusive clot (wound challenge). Alternatively, tail sections were collected 10 minutes post‐injury and prepared for microscopic analysis by immunofluorescence (fibrin, platelets and red blood cells (RBCs)) or scanning electron microscopy (SEM).
Results: Without wound challenge, blood loss was similar in FVIII‐treated mice (28 ± 7 μL) or emicizumab‐treated mice (32 ± 15 μL; p = 0.99; Figure 1). In FVIII‐treated mice, each wound challenge was followed by rapid bleeding arrest and minor blood loss (135 ± 33 μL after 2 challenges). No differences were observed between mice treated with FVIII or FVIII‐Fc. In emicizumab‐treated mice, wound challenges were associated with prolonged times to arrest, spontaneous rebleeding thereafter and abundant additional blood loss (533 ± 165 μL; p < 0.0001 versus FVIII with challenges). Immunofluorescence analysis of occlusive clots revealed that FVIII‐derived clots contain larger areas of co‐localization between aggregated RBCs and platelets compared to emicizumab‐derived clots (21 ± 4% vs 4 ± 1% of RBC‐signal overlapping platelet‐signal; p = 0.0031). SEM‐analysis further confirmed differences in clot‐structure between both treatments (Figure 2). Compact RBC‐structures were observed in clots from FVIII‐treated mice, with individual RBCs being mostly indistinguishable. In contrast, the outer‐surface of clots from emicizumab‐treated mice consisted of a fractured structure harbouring individual RBCs and apparent ruptured fibrils.
Conclusion(s): Clots forming in the presence of FVIII or emicizumab show different structure and density, which correlate with wound challenge response and clot stability. Our data suggest mechanistic differences in clot formation.
OC 37.3
Mice possess a more limited natural anti‐FVIII antibody repertoire than humans that is generated primarily by marginal zone B cells
M. Cormier 1; E. Burnett2; C. Hough3; D. Lillicrap4
1 Queen's University, Kingston, Ontario, Canada; 2 Department of Pathology and Molecular Medicine, Queen's University, Kingston, Ontario, Canada; 3 Department of Pathology & Molecular Medicine, Queen's University, Kingston, Ontario, Canada; 4 Department of Pathology and Molecular Medicine, Queen’s University, Kingston, Ontario, Canada
Background: 30% of severe hemophilia A patients develop neutralizing anti‐FVIII antibodies. The significance of natural non‐neutralizing antibodies (NNAs) to FVIII remains unclear.
Aims: To characterize the natural anti‐FVIII antibody repertoire in mice and humans.
Methods: Natural anti‐FVIII NNAs were quantified in naïve wild‐type and FVIII‐/‐ C57Bl6 mice aged 6‐20 weeks and in healthy human donors. Murine B cells were harvested and cultured ex vivo in lipopolysaccharide to measure IgM production. Marginal zone B cells (MZBs) and follicular B cells (FOBs) were sorted based on CD21 and CD23 expression. MZBs were depleted via intraperitoneal injections of anti‐CD49 and anti‐CD11a antibodies. Anti‐FVIII antibodies were detected by ELISA and quantified against purified immunoglobulin standards. Inhibitory activity was measured via Bethesda assay.
Results: Non‐neutralizing anti‐FVIII IgM, but not IgG nor IgA, was detectable in naïve wild‐type and FVIII‐/‐ mice and increased with age (p < 0.001). Anti‐FVIII IgM was produced by splenic B cells isolated from naïve FVIII‐/‐ mice when stimulated with lipopolysaccharide ex vivo and increased with age (p < 0.05). Total IgM production remained consistent across ages. Anti‐FVIII IgM was shown to bind FVIII in vivo, as intravenous administration of FVIII reduced plasma anti‐FVIII IgM levels by 44% after 24 hours (p < 0.01). Following depletion of MZBs in naïve FVIII‐/‐ mice, anti‐FVIII IgM was significantly reduced compared to mice receiving isotype control antibodies (p < 0.001). When sorted and cultured with lipopolysaccharide, MZBs produced almost all anti‐FVIII IgM compared to FOBs (p < 0.01). In human donors, FVIII‐specific IgM was detected at the highest concentration, followed by IgG then IgA. No inhibitory antibodies were detected.
Conclusion(s): FVIII‐specific NNAs are present in both mice and humans. MZBs are the predominant source of natural anti‐FVIII IgM in mice. Anti‐FVIII IgM levels rise with age and fall following exogenous FVIII administration, suggesting MZB‐derived IgM may be involved in the immune surveillance of FVIII.
OC 47.3
Towards explaining the unique bleeding pattern of acquired hemophilia A: Impact of FVIII‐containing immune complexes on hemostasis
O. Oleshko 1; A. Klingberg2; U. Curth3; S. Werwitzke2; A. Tiede1
1 Hannover Medical School, Hannover, Niedersachsen, Germany; 2 Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Hannover Medical School, Hannover, Niedersachsen, Germany; 3 Institute for Biophysical Chemistry, Hannover Medical School, Hannover, Niedersachsen, Germany
Background: Acquired hemophilia A (AHA) is a bleeding disorder due to neutralizing autoantibodies against FVIII. Its clinical presentation significantly differs from that in congenital hemophilia A (CHA) with or without alloantibody inhibitors, but the underlying reason remains unknown. We have previously shown that anti‐FVIII antibodies form immune complexes (IC) when FVIII protein concentration exceeds the normal range, which is expected in AHA but not CHA. These complexes incorporate von Willebrand factor (VWF) and might therefore disturb primary hemostasis.
Aims: To explore the functional impact of VWF‐containing FVIII‐IC on hemostasis.
Methods: IC were detected and characterized by analytical ultracentrifugation (AUC). FVIII‐depleted normal plasma was supplemented with VWF, washed fluorescently‐labeled platelets from healthy volunteers, and a mix of 7 monoclonal anti‐FVIII IgG antibodies (Ab1‐7) with or without FVIII. A Bioflux flow chamber model under high shear rates and calibrated automated thrombography were used to assess the primary and secondary hemostasis, respectively.
Results: Formation of IC by Ab1‐7 was observed in the presence of FVIII, but not in its absence. VWF was incorporated into FVIII‐IC as shown by AUC. VWF‐dependent platelet adherence and aggregation under high shear stress was significantly impaired (by 20 to 100%), depending on platelet donor and experimental conditions, in the presence of FVIII‐containing IC as compared to Ab1‐7 alone. In contrast, thrombin generation capacity was not impaired by FVIII‐IC.
Conclusion(s): Our data demonstrate that FVIII‐IC contain vWF and disturb platelet function in an ex vivo model of primary hemostasis. We suggest that FVIII‐IC occurring in AHA disturb hemostasis more severely than just the absence of FVIII in CHA with or without inhibitors. The severe impairment of primary hemostasis, combined with the suppression of FVIII activity, could contribute to the unique bleeding pattern of AHA. The study was supported by “Early Career Research Grant 2021” from the Society of Thrombosis and Haemostasis Research (GTH e.V.).
OC 37.2
Follicular regulatory T cells promote anti‐FVIII inhibitor development in hemophilia A mice
W. Jing1; J. Chen1; Y. Cai1; J. Schroeder1; A. Dent2; Q. Shi 1
1 Medical College of Wisconsin, Blood Research Institute, Children's Research Institute, Milwaukee, Wisconsin, United States; 2 Dept. of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, Indiana, United States
Background: The development of anti‐FVIII inhibitory antibodies (inhibitors) is one major complication of FVIII protein replacement therapy in hemophilia A patients. Our previous study demonstrated that the induction of activated follicular helper T (Tfh) cells in FVIIInull mice is critical for FVIII inhibitor development. Follicular regulatory T (Tfr) cells are a specialized subset of T cells identified in the germinal center (GC) that can modulate Tfh cell activation of B cells and antibody development.
Aims: To investigate the role of Tfr cells in FVIII immune responses.
Methods: Tfr cells in the spleen of immunized FVIIInull mice were characterized by flow cytometry analysis. Tfr deficient Foxp3‐Cre Bcl6fl/fl (BCL6FC) mice were used to determine the role of Tfr cells in anti‐FVIII inhibitor generation.
Results: FVIII immunization increased the activated Tfr cells in the spleen of mice that produced anti‐FVIII inhibitors. The percentage of activated Tfr cells significantly increased in inhibitor‐producing mice compared to saline‐treated controls and non‐inhibitor‐producing mice (0.88 ± 0.2%, 0.33 ± 0.16%, and 0.36 ± 0.2%, respectively). The Tfh/Tfr ratio also significantly increased in inhibitor‐producing mice, suggesting a preferential expansion of Tfh cells relative to Tfr cells in inhibitor‐producing mice. Loss of Tfr cells led to significantly decreased neutralizing anti‐FVIII inhibitor levels, with titers in BCL6FC mice markedly lower than in wild‐type mice upon rhF8 immunization (22 ± 12 and 145 ± 82 BU/ml, respectively). However, the total anti‐FVIII IgG titers were similar between the BCL6FC and wild‐type groups, suggesting that Tfr cells help generate high affinity anti‐FVIII antibodies. FVIII immunization could still efficiently induce Tfh cell activation in BCL6FC mice. However, both GC Tfh cells and GC B cells were reduced in FVIII immunized BCL6FC mice, suggesting that Tfr cells promote FVIII‐specific GC responses.
Conclusion(s): we found that FVIII immunization induces Tfr cell activation/expansion and that Tfr cells have an unexpected helper role in promoting anti‐FVIII inhibitor generation.
OC 37.1
Transplacental delivery of recombinant Fc‐fused factor VIII (rFVIIIFc) in FVIII‐deficient mice
A. Reyes Ruiz 1; S. Lacroix‐Desmazes2; S. Delignat1; A. Mimoun3; V. Daventure1; J. Dimitrov1
1 Inserm, Paris, Ile‐de‐France, France; 2 INSERM, Paris, Ile‐de‐France, France; 3 Sorbonne Université, Paris, Ile‐de‐France, France
Background: Injection to pregnant FVIII‐KO mice of the Fc‐fused A2 and C2 domains of FVIII leads to their transplacental delivery and induction of partial FVIII‐immune tolerance in the offspring. Hence, the transplacental delivery of the entire FVIII should confer complete FVIII‐immune tolerance. However, recombinant Fc‐fused FVIII (rFVIIIFc) crosses the placenta in amounts insufficient to foster FVIII‐tolerance. Understanding the rFVIIIFc properties associated with its reduced transplacental delivery could optimize rFVIIIFc transcytosis to levels compatible with tolerance induction.
Aims: To decipher the molecular mechanisms that account for the poor transplacental delivery of rFVIIIFc in FVIII‐deficient mice.
Methods: Different Fc‐fused proteins were generated: rFVIIIFcN297A, Fc‐fused A2, A3C1, C1 and C2 domains of FVIII. Pregnant FVIII‐KO mice were injected at day 17.5 of gestation with 170‐330 picomoles of the molecules. Blood was collected after 5 min and 4 h from mothers and 4 h from fetuses. The concentration of the molecules was quantified by ELISA.
Results: rFVIIIFcN297A that cannot bind to Fcγ receptors (FcγRs), crossed the placenta to similar levels to that of rFVIIIFc (0.06 ± 0.08 and 0.03 ± 0.01 nM, respectively), suggesting that binding of rFVIIIFc to mothers’ FcRγs does not account for its poor transplacental delivery. Preliminary data show that the Fc‐fused light chain of FVIII is poorly transferred through the placenta (0.04 ± 0.005 nM), suggesting that moieties in the light chain might be implicated in the retention of rFVIIIFc in mother’s circulation or placenta. Interestingly, A2Fc, A3C1Fc, C1Fc and C2Fc were all transferred to levels > 1 nM in fetus’s blood.
Conclusion(s): The reduced transplacental delivery of rFVIIIFc is not due to its binding to FcγRs. As the C1 and C2 domains of FVIII are implicated in the interaction with different molecules of the coagulation cascade or catabolic receptors, we hypothesize that co‐engagement of the C1 and C2 domains of rFVIIIFc may explain, at least in part, the reduced transcytosis of rFVIIIFc through the placenta.
OC 07.2
Macrophage Galactose Lectin (MGL) is a clearance receptor determining Factor VIII half‐life in haemophilia A patients
S. Ward1; T. Guest*1; J. O'Sullivan1; A. Chion1; J. Fazavana1; P. Fallon2; R. Preston3; J. Johnsen4; S. Pipe5; P. Turecek6; J. O'Donnell 7
1 Royal College of Surgeons in Ireland, Dublin, Dublin, Ireland; 2 Trinity College Dublin, Dublin, Dublin, Ireland; 3 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 4 University of Washington, Seattle, Washington, United States; 5 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States; 6 Baxalta Innovations GmbH, a Takeda company, Vienna, Wien, Austria; 7 Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland
Background: Although most plasma FVIII circulates in complex with VWF, a minority (3‐5%) circulates as free‐FVIII which is rapidly cleared. Modelling suggests that 20% of total FVIII is cleared as free‐FVIII. Critically, the mechanisms of free‐FVIII clearance remain poorly understood. Recent studies have implicated the Macrophage Galactose Lectin (MGL) in modulating VWF clearance.
Aims: Since VWF and FVIII share similar glycosylation, we investigated the role of MGL in FVIII clearance
Methods: FVIII binding to MGL was assessed in immunosorbent and cell‐based assays. In vivo FVIII clearance was assessed in MGL‐deficient murine models. Finally, VWF‐/‐/FVIII‐/‐ mice were generated and used to specifically study free‐FVIII clearance in the presence or absence of clodronate‐induced macrophage depletion or MGL inhibition
Results: In vitro binding studies demonstrated dose‐dependent binding of human FVIII to MGL. Sequential exoglycosidase digestions defined a critical role for FVIII O‐glycans in regulating MGL interaction. Plasma FVIII levels were significantly higher in MGL1‐/‐ mice compared to wild‐type controls (FVIII:C 88.5 ± 10.6 versus 124.0 ± 15.7%; p < 0.05). Combined inhibition of both MGL1 and MGL2 receptors in mice was associated with a further increase in endogenous FVIII (FVIII:C 169.4 ± 15.3%, p < 0.05). To investigate whether MGL contributes to free‐FVIII clearance, PK studies were performed in VWF‐/‐/FVIII‐/‐ mice. Clodronate‐induced macrophage depletion inhibited free‐FVIII clearance in VWF‐/‐/FVIII‐/‐ mice (MRT 21.8 ± 2.5 versus 29.7 ± 3.1 min). Interestingly, specific anti‐MGL 1/2 blocking also attenuated free‐FVIII clearance (MRT 94.8 ± 4.6 mins versus 49.4 ± 3.6; p < 0.001).
Conclusion(s): Cumulatively, these findings demonstrate that MGL plays an important role in regulating macrophage‐mediated clearance of both VWF‐bound FVIII and free‐FVIII in vivo. We propose that this novel FVIII clearance pathway may be of particular clinical importance in patients with type 2N or type 3 VWD. Further studies will be required to determine whether MGL inhibition may afford a novel therapeutic target to extend FVIII half‐life
OC 20.1
Neutrophil extracellular traps promote joint injury in hemophilia
T. Kaminski; T. Brzoska; E. Tutuncouglu; M. Ragni; P. Sundd
University of Pittsburgh, Pittsburgh, Pennsylvania, United States
Background: Hemophilic arthropathy (HA) is major morbidity affecting hemophilia patients. Epidemiological evidence suggests that recurring episodes of joint‐bleeding contribute to the development of HA in 70‐85%of hemophilic patients. Despite major advances in the treatment to prevent joint bleeding, HA continues to be major morbidity affecting hemophilia patients and the etiological mechanism contributing to the progression of HA remains elusive.
Aims: Recent evidence suggests that the accumulation of blood in the joints may lead to the release of erythrocyte‐derived DAMPs (eDAMPs) such as heme and hemoglobin that can promote sterile inflammation by priming innate immune pathways in neutrophils. The aim of the study was to identify pathways contributing to the development of HA.
Methods: In the study, we used a model of puncture‐induced knee joint injury in FVIII‐total‐knockout (F8TKO) mice and blood samples from hemophilia patients diagnosed with HA. Intravital multi‐photon‐excitation fluorescence microscopy imaging of injured joint cavity in living F8TKO or control mice was used to evaluate NETs formation within the joint structure. Imaging‐flow‐cytometry and ELISA assays were used to estimate the number of circulating NETs in patients diagnosed with HA and mice after the knee‐injury procedure. Scoring of the bleeding severity, histology, IHC and confocal imaging of joints were conducted to classify the joint injury in mice.
Results: F8TKO but not control mice manifested knee‐joint injury and severity of bleeding 5‐days‐post knee‐injury. Progression of knee‐joint injury was associated with the increased neutrophil accumulation and NETs shedding within the synovium of F8TKO mice. Circulating NETs were significantly abundant in the plasma of hemophilia patients diagnosed with HA and F8TKO following knee‐injury but not plasma of control humans or mice.
Conclusion(s): These findings are the first to suggest that NETs contribute to pathogenesis of HA in hemophilia. Currently, experiments are underway to identify the innate immune pathways that promote NETs shedding, leading to joint‐damage in hemophilia.
OC 21.1
Rescue of an FVIII splicing variant with engineered U1snRNAs
L. Peretto 1; R. Tarantino2; A. Borchiellini3; F. Bernardi4; A. Follenzi5; M. Pinotti1; D. Balestra1
1 Department of Life Sciences and Biotechnology, University of Ferrara, Ferrara, Italy, Ferrara, Emilia‐Romagna, Italy; 2 University of Ferrara, Ferrara, Emilia‐Romagna, Italy; 3 A.O.U. Città della Salute e della Scienza di Torino, Torino, Piemonte, Italy; 4 Department of Life Sciences and Biotechnology, University of Ferrara, Italy., Ferrara, Emilia‐Romagna, Italy, 5 University of Piemonte Orientale, Novara, Italy, Novara, Piemonte, Italy
Background: Hemophilia A (HA) is an X‐linked recessive hemorrhagic disorder caused by coagulation factor VIII (FVIII) deficiency. Among all HA‐causing mutations, those affecting splicing are relatively frequent, particularly in severe forms. These mutation types, often leading to exon skipping, can be rescued by RNA therapeutics based on variants of the key U1snRNA spliceosomal component, as shown in several human disease models.
Aims: To dissect the molecular mechanisms of the F8 c.1752+5g>c variant associated with moderate HA (FVIII: C<5) in two brothers, and assess the U1snRNA‐mediated rescue.
Methods: Creation of expression vectors for the wild‐type (pIVS11wt) and mutant (pIVS11+5c) F8 minigenes and for the engineered U1 snRNAs designed to base pairs to the mutated 5’ss (compensatory U1snRNA) or to less‐conserved downstream intronic sequences (Exon Specific U1snRNA). Transient transfection of various human hepatoma cell lines and RT‐PCR to evaluate splicing patterns and their modulation by U1snRNA variants.
Results: Bioinformatic analysis did not predict an impact on splicing for the F8 c.1752+5g > c mutation. However, splicing pattern analysis of the hepatoma cells expressing the pIVS11+5c variant revealed that the change leads to exon 11 skipping, with low levels of correctly spliced transcripts (~10 %). Co‐transfection of U1snRNA variants significantly improves FVIII exon 11 definition and thus inclusion, with the compensatory U1snRNA associated with exon 11 inclusion up to 92%.
Conclusion(s): We provided experimental evidence that the c.1752+5g>c mutation impairing F8 exon 11 definition and lead to exon skipping with trace levels of correct transcripts, in accordance with the HA patients’ phenotype. Importantly, exon inclusion of the defective exon can be efficiently restored by the use of appropriately designed U1snRNA variants, which are currently under investigation through lentiviral‐mediated delivery in Blood‐Born Endothelial Cells (BOECs) isolated from HA patients.
OC 47.1
FVIII‐reactive CD4 T cells isolated from non‐hemophilic blood respond to multiple epitopes in the FVIII protein
K. Pratt1; P. Vir 2; A. Karim2; D. Gunasekera2; A. Stering3; K. Lieuw4
1 Uniformed Services University, Bethesda, Maryland, United States; 2 Uniformed Services University of the Health Sciences, Bethesda, Maryland, United States; 3 Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center, Bethesda, Maryland, United States; 4 Uniformed Services University of the Health Sciences and Madigan Army Medical Center, Bethesda, Maryland, United States
Background: Neutralizing anti‐factor VIII (FVIII) antibodies (“inhibitors”) afflict ~30% of severe hemophilia A (HA) patients, while antibodies against self‐FVIII can also occur in non‐hemophilic individuals. The latter can cause the rare but potentially life‐threatening autoimmune disorder “acquired HA”. CD4 T cells provide help for antibody generation, class switching and affinity maturation. Interestingly, a series of non‐hemophilic individuals were recently shown by the Maillère group to circulate FVIII‐specific CD4 T cells (PMID 29296830), suggesting thymic escape of FVIII‐reactive T cells contributes to the relatively high immunogenicity of FVIII compared to other self‐proteins.
Aims: To investigate mechanisms by testing the fine specificity and phenotypes of FVIII‐reactive CD4 T cells in non‐hemophilic blood donors using ELISPOT assays, HLA‐ClassII tetramer staining and flow cytometry.
Methods: Peripheral blood mononuclear cells (PBMCs) were isolated from blood donors and expanded in vitro via FVIII stimulation. Interferon‐gamma ELISPOT assays were then carried out stimulating the lines with pooled synthetic, overlapping FVIII peptides (spanning the FVIII A1, A2, A3, C1 and C2 domains). Positive responses to specific peptide pools were further decoded by ELISPOT assays using IEDB‐predicted individual peptides as stimulants. We attempted to identify immunodominant epitopes and isolate FVIII‐specific T‐cell clones via peptide‐loaded HLA‐ClassII tetramer staining.
Results: Immunodominant epitopes restricted to HLA‐DRB1* 01:01, 07:01 and 15:01 were identified, and a clone restricted to HLA‐DRB1*01:01 was isolated. Responses to multiple peptide epitopes clearly confirmed FVIII specificity and ruled out alternative explanations for IFN‐gamma secretion upon stimulation with FVIII protein, e.g. molecular mimicry or other off‐target antigen stimulation of the responding cells.
Conclusion(s): Acquired HA entails a breakdown of tolerance mechanisms that prevent expansion of FVIII‐reactive T cells in the periphery. Identification of HLA‐restricted T‐cell epitopes recognized by non‐hemophilic immune systems will enable us to study autoimmune and tolerance mechanisms and to ask if these are the same epitopes driving hemophilic inhibitor responses.
OC 07.1
Role of GABARAP protein in the intracellular distribution of FVIII
S. El‐Hazzouri 1; H. Singer1; M. Ibrahim1; N. Nüsgen1; J. Oldenburg2; O. El‐maarri1
1 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: We previously showed intracellular interaction between GABARAP (an ATG8 protein) and FVIII; this finding suggested the involvement of the autophagy machinery in FVIII intracellular trafficking. However, the precise role of GABARAP on the intracellular distribution and transportation of FVIII remain to be elucidated.
Aims: To investigate the role of GABARAP and its effect on intracellular distribution of FVIII.
Methods: HEK293 cell lines harboring single CRISPR/Cas9 KOs in CANX, CALR, LMAN1, MCFD2 and GABARAP were subjected to immunofluorescent staining. Co‐localization data and correlation analysis of FVIII with different cellular markers of different cell compartments (ER, ERGIC, Golgi, Autophagosome, Lysosome, Endosome and Proteasome) was clustered, evaluated and analyzed.
Results: We observed in the GABARAP‐KO, relatively to wild type, a decrease in FVIII colocalization in ER (CALX, p < 0.0001, CALR, p = 0.0086), Trans‐Golgi (TGN46, p = 0.0031), Autophagosomes (LC3, p < 0.0001), Lysosomes (LAMP1, p = 0.0603) and proteasomes (26S p = 0.0004); while a relative increase was observed at the ERGIC compartment (LMAN1, MCFD2), endosomal markers (RAB7, p < 0.0001, RAB11, p = 0.24, VAMP8 p = 0.0055), Furin (p = 0.0003) and GABARAPL1 (p < 0.0001). The KO of Calnexin and Calreticulin showed relatively similar FVIII distribution to HEK28 while LMAN1 and MCFD2 KOs where largely characterized by a drop of FVIII co‐localization with all markers except ER and GABARAPs markers.
Conclusion(s): Our detailed correlation analysis show a significant impact of GABARAP protein on the intracellular distribution of FVIII especially between autophagy/lysosomal vesicles and endosomal compartments
OC 50.1
Transplantation of fetal liver and adult bone marrow cells for phenotypic correction of hemophilia A
S. Akula 1; S. Merlin2; A. Cottonaro1; A. Follenzi3; M. Sanchez4; E. Borroni2; V. Kalandadze2; T. Garcia Leal4; L. Serrano Ramos5; A. Liras5, S. Buzzi1
1 Universita del piemonte orientale, Novara, Piemonte, Italy, 2 universita del piemonte orientale, novara, Piemonte, Italy, 3 University of Piemonte Orientale, Novara, Italy, Novara, Piemonte, Italy; 4 Centro Andaluz de Biología del Desarrollo (CABD), madrid, Madrid, Spain; 5 Complutense University of Madrid, madrid, Madrid, Spain
Background: We previously showed that adult‐derived FVIII‐producing cells, i.e. liver sinusoidal endothelial cells (LSECs) and hematopoietic stem cells (HSCs), can be used for the treatment of adult HA mice. However, after transplantation in busulfan‐conditioned newborn mice, adult LSEC/HSC cannot efficiently engraft compared to murine fetal liver (FL) hemato/vascular cells from day 11‐13 of gestation (E11‐E13) that strongly reconstitute the hematopoietic compartment and showed multi‐organ endothelial reconstitution potential and FVIII secretion ability.
Aims: To investigate the ability of FL cells in repopulating the hemato/vascular compartment following transplantation in newborn HA mice without preconditioning.
Methods: We transplanted adult BM or FLE11‐E13 cells from GFP mice into newborn and adult HA mice pre‐treated (+BU) or not (noBU) with busulfan. The engraftment level and FVIII activity was assessed starting from 4w after transplantation and followed‐up for 18 m. Bleeding phenotype correction was verified by hemarthrosis induction and bleeding assay.
Results: In all BU‐conditioned groups we observed long term (18m) stable GFP+ blood engraftment (>60%) with up to 16% FVIII activity following FL and BM cells transplantation. Interestingly, without pre‐conditioning we observed lower but stable engraftment (≤12%) and consequent FVIII activity (1‐4%) after FL cells transfer, while BM cells did not engraft in noBU mice. Tail bleed challenge and induced hemarthrosis experiments further confirmed the phenotypic correction in all mice receiving FL/BM+BU and in FLnoBU mice. Additionally, we observed an increased survival rate in corrected mice (80%) compared to HA controls (30%). None of the transplanted mice developed anti‐FVIII or anti‐GFP antibodies.
Conclusion(s): Transplantation of HSCs from adult BM and FL may provide a novel, more stable and highly promising preclinical model for pediatric HA treatment. Our results show FL cells having higher engraftment ability, thus paving the way for studies aimed at maximizing the engraftment and proliferation of donor FVIII‐corrective cells while minimizing/avoiding harming pre‐conditioning regimens.
Hemophilia – Clinical
LB 01.1
A Phase 3 study (ATLAS‐PPX) to evaluate efficacy and safety of fitusiran, an siRNA therapeutic, in people with haemophilia A or B who have switched from prior factor or bypassing agent prophylaxis
G. Kenet 1; B. Nolan2; B. Zulfikar3; B. Antmen4; P. Kampmann5; T. Matsushita6; C. You7; K. Vilchevska8; C. Bagot9; A. Sharif10; F. Peyvandi11; G. Young12; C. Negrier13; T. Quan14; S. Poloskey15; C. Sussebach16; F. Shammas17; S. Andersson18; B. Mei18; K. Kavakli19
1 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 2 Children's Health Ireland at Crumlin, Dublin, Dublin, Ireland; 3 Istanbul University Oncology Institute, Istanbul, Istanbul, Turkey; 4 Department of Pediatric Hematology/Oncology and Bone Marrow Transplantation Unit, Faculty of Medicine, Acibadem University, Adana Hospital, Adana, Adana, Turkey; 5 Department of Hematology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Hovedstaden, Denmark; 6 Department of Transfusion Medicine, Nagoya University Hospital, Nagoya, Aichi, Japan; 7 Department of Pediatrics, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, South Korea, Daejeon, Ch'ungch'ong‐namdo, Republic of Korea; 8 National Children’s Specialized Hospital OHMATDYT, Kyiv, Kyyiv, Ukraine; 9 Department of Haematology, Glasgow Royal Infirmary, Glasgow, England, United Kingdom, 10 Hospital Sultanah Aminah, Johor Bahru, Johor, Malaysia, 11 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy, 12 Hemostasis and Thrombosis Center, Cancer and Blood Diseases Institute, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, California, United States, 13 Louis Pradel University Hospital, Claude Bernard University Lyon 1, Lyon, Auvergne, France, 14 Sanofi, Chengdu, Sichuan, China (People's Republic), 15 Sanofi, Cambridge, Massachusetts, United States, 16 Sanofi, Frankfurt, Hessen, Germany, 17 Sanofi, Montreal, Quebec, Canada, 18 Sanofi, Cambridge, MA, USA, Cambridge, Massachusetts, United States, 19 Department of Hematology, Ege University Faculty of Medicine, Children's Hospital, Izmir, Turkey, Izmir, Izmir, Turkey
Background: Fitusiran, a subcutaneous (SC) investigational siRNA therapeutic, targets antithrombin to rebalance haemostasis in people with haemophilia A or B (PwHA/B), irrespective of inhibitor status. In previous Phase 3 trials, once‐monthly fitusiran prophylaxis significantly reduced annualised bleeding rate (ABR) in PwHA/B, with or without inhibitors versus episodic/on‐demand treatment.
Aims: To evaluate the efficacy and safety of fitusiran versus prior factor/bypassing agent (BPA) prophylaxis in PwHA/B, with or without inhibitors.
Methods: This Phase 3, multinational, open‐label study (NCT03549871) included males aged ≥12 years with hemophilia A or B, with or without inhibitors, who had prior factor/BPA prophylaxis. Participants continued factor/BPA prophylaxis (6 months) before switching to once‐monthly 80 mg SC fitusiran prophylaxis (7 months). Primary endpoint was ABR in the factor/BPA prophylaxis period (Day‐168 to Day‐1) and fitusiran efficacy period (Day 29 to Day 190). Secondary endpoints included spontaneous ABR (AsBR), joint ABR (AjBR), and health‐related quality of life (HRQoL). Safety and tolerability were assessed.
Results: Of 80 enrolled participants, 65 (inhibitor/non‐inhibitor, n = 19/46; haemophilia A/haemophilia B, n = 50/15) were eligible for ABR analyses. Median observed (IQR) ABRs were 4.4 (2.2; 10.9) with factor/BPA and 0.0 (0.0; 2.3) with fitusiran prophylaxis; 41 participants (63.1%) experienced zero treated bleeds with fitusiran. Fitusiran achieved statistically significant reductions in estimated ABR, AsBR and AjBR versus factor/BPA prophylaxis (Table 1). Fitusiran significantly improved HRQoL versus factor/BPA as measured by Haem‐A‐QOL total score (LS mean difference ‐4.6 [95% CI: ‐7.6; ‐1.5; p < 0.01]). Serious adverse events (SAEs) occurred in 5/65 participants (7.7%) with factor/BPA and 9/67 (13.4%) with fitusiran prophylaxis. Two participants (3.0%) experienced suspected or confirmed thromboembolic events with fitusiran (Table 2).
Conclusion(s): Once‐monthly fitusiran prophylaxis significantly reduced bleeding versus factor/BPA prophylaxis with a median ABR of zero in PwHA/B with and without inhibitors, resulting in a meaningful improvement in HRQoL. Reported AEs were generally consistent with previously identified risks of fitusiran. TABLE 1 Bleeding events in the ATLAS‐PPX study (fitusiran efficacy and factor/BPA prophylaxis period*)
TABLE 1 Bleeding events in the ATLAS‐PPX study (fitusiran efficacy and factor/BPA prophylaxis period*) TABLE 2 Safety results from the ATLAS‐PPX study
TABLE 2 Safety results from the ATLAS‐PPX study
LB 01.2
Concizumab prophylaxis in patients with haemophilia A or B with inhibitors: Efficacy and safety results from the primary analysis of the phase 3 explorer7 Trial
V. Jiménez Yuste 1; P. Angchaisuksiri2; G. Castaman3; K. Cepo4; J. Haaning4; S. Jacobsen4; J. Mahlangu5; T. Matsushita6; K. Nogami7; A. Shapiro8
1 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain; 2 Mahidol University, Bangkok, Krung Thep, Thailand; 3 Careggi University Hospital, Florence, Toscana, Italy; 4 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark; 5 University of the Witwatersrand, National Health Laboratory Service and Hemophilia Comprehensive Care Center, Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, Gauteng, South Africa; 6 Department of Transfusion Medicine, Nagoya University Hospital, Nagoya, Aichi, Japan; 7 Nara Medical University, Kashihara, Nara, Japan; 8 Indiana Hemophilia and Thrombosis Center, Indianapolis, Indiana, United States
Background: Concizumab is a subcutaneously administered anti‐tissue factor pathway inhibitor (TFPI) antibody in development as once‐daily prophylaxis for all haemophilia patients. explorer7 (NCT04083781) primary analysis results are presented.
Aims: Explorer7 assessed concizumab efficacy and safety in haemophilia A/B with inhibitor (HAwI/HBwI) patients.
Methods: Patients were randomised 1:2 to no prophylaxis (arm 1; ≥24 weeks) or concizumab prophylaxis (arm 2; ≥32 weeks), or assigned to concizumab prophylaxis (arms 3&4). After treatment restart following pause due to thromboembolic events, patients received a 1.0 mg/kg concizumab loading dose, followed by an initial 0.20 mg/kg daily dose, with potential adjustment to 0.15 or 0.25 mg/kg based on plasma concizumab concentration at week 4. The primary analysis compared number of treated spontaneous and traumatic bleeding episodes between arms 1 and 2 (using negative binomial regression). Safety, patient‐reported outcomes, and pharmacokinetics/pharmacodynamics were assessed. Informed consent/ethics committee approval were obtained.
Results: Of 133 enrolled patients, 33 were randomised to concizumab (arm 2) and 19 to no prophylaxis (arm 1) (28 and 14 completed ≥32/24 weeks of treatment at the primary analysis cut‐off, respectively); the remaining 81 were assigned to concizumab (arms 3&4). Estimated mean annualised bleeding rate (ABR) was 1.7 (95% CI, 1.0–2.9) for concizumab versus 11.8 (95% CI, 7.0–19.9) for no prophylaxis (ABR ratio, 0.14 [95% CI, 0.07–0.29]; p < 0.001). Median ABR on concizumab was 0 (Figure 1). Twenty‐one (63.6%) concizumab patients had zero treated bleeds at 24 weeks (including those who discontinued before 24 weeks) versus two (10.5%) on no prophylaxis. No thromboembolic events were reported after treatment restart (Table 1). Positive trends were observed across 36‐Item Short‐Form Health Survey (SF‐36v2) domains with concizumab. Concizumab exposure was stable over time.
Conclusion(s): Concizumab prophylaxis effectively reduced ABR versus no prophylaxis and was considered safe and well tolerated in HAwI/HBwI patients, the latter of which have the greatest unmet need.

LB 01.4
Efficacy, safety, and pharmacokinetics of once‐weekly efanesoctocog alfa (BIVV001) prophylaxis in previously treated patients with severe hemophilia A: Results from the phase 3 XTEND‐1 study
A. von Drygalski 1; P. Chowdary2; R. Kulkarni3; S. Susen4; B. Konkle5; J. Oldenburg6; D. Matino7; R. Klamroth8; A. Weyand9; V. Jimenez Yuste10; K. Nogami11; S. Poloskey12; B. Winding13; A. Willemze14; K. Knobe15
1 Division of Hematology/Oncology, Department of Medicine, University of California San Diego, San Diego, California, United States; 2 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom; 3 Michigan State University, East Lansing, Michigan, United States; 4 Centre Hospitalier Universitaire de Lille, Université de Lille, Lille, Nord‐Pas‐de‐Calais, France; 5 Washington Center for Bleeding Disorders and the University of Washington, Seattle, Washington, United States; 6 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 7 Division of Hematology & Thromboembolism, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 8 Vivantes Klinikum, Friedrichshain, Berlin, Berlin, Germany; 9 Division of Hematology/Oncology, Department of Pediatrics, University of Michigan, Ann Arbour, Michigan, United States, 10 Hospital Universitario de La Paz, Autónoma University, Madrid, Madrid, Spain, 11 Nara Medical University, Kashihara, Nara, Japan, 12 Sanofi, Cambridge, Massachusetts, United States, 13 Sobi, Stockholm, Stockholms Lan, Sweden, 14 Sanofi, Amsterdam, Noord‐Holland, Netherlands, 15 Sanofi, Chilly‐Mazarin, Ile‐de‐France, France
Background: Efanesoctocog alfa is a new class of factor VIII (FVIII) replacement designed to overcome the von Willebrand factor‐imposed half‐life ceiling.
Aims: To evaluate the efficacy, safety, and pharmacokinetics of efanesoctocog alfa in previously treated patients, ≥12 years, with severe hemophilia A.
Methods: After informed consent, patients on prior FVIII prophylaxis entered Arm A (52 weeks once‐weekly intravenous efanesoctocog alfa prophylaxis [50 IU/kg]) (NCT04161495). Patients receiving prior on‐demand therapy entered Arm B (26 weeks on‐demand efanesoctocog alfa [50 IU/kg], then 26 weeks once‐weekly prophylaxis [50 IU/kg]). A subset had enrolled in a 12‐month observational pre‐study. Primary endpoint was Arm A annualized bleed rate (ABR). Secondary endpoints included observational pre‐study versus on‐study intra‐patient ABR (key secondary), bleed treatment, physical health, pain, joint health, pharmacokinetics, and safety.
Results: One female and 132 males enrolled in Arm A; 26 males in Arm B. Arm A mean (SD) and median (IQR) ABR were 0.71 (1.43) and 0.00 (0.00–1.04), respectively. Intra‐patient ABR comparison demonstrated superior bleed protection with efanesoctocog alfa versus prior FVIII prophylaxis (p < 0.001; Figure). Most bleeds (96.7%) resolved with one efanesoctocog alfa injection, and 94.9% of responses were rated excellent/good. Once‐weekly efanesoctocog alfa provided high sustained factor activity (Figure) consistent with earlier studies. Efanesoctocog alfa prophylaxis was associated with significant improvements from baseline in physical health (p = 0.0001), pain (p = 0.0276), and joint health (p = 0.0101) at Week 52 (Table). Inhibitor development to FVIII was not detected. The most common treatment‐emergent adverse events ( > 5% of participants overall) were headache, arthralgia, fall, and back pain.
Conclusion(s): Once‐weekly efanesoctocog alfa prophylaxis was well‐tolerated, provided superior bleed protection to prior prophylaxis, and showed clinically meaningful improvements in physical health, pain, and joint health. Efanesoctocog alfa prophylaxis provided high sustained factor activity within normal to near‐normal levels ( > 40%) for most of the week and > 10% at Day 7.
Funding: Sanofi and Sobi. FIGURE 1 Intra‐patient ABR comparison (A); sequential PK subgroup analysis, Week 1 and Week 26 FVIII activity versus time profiles (B)
FIGURE 1 Intra‐patient ABR comparison (A); sequential PK subgroup analysis, Week 1 and Week 26 FVIII activity versus time profiles (B) TABLE 1 Secondary endpoints
TABLE 1 Secondary endpoints
OC 40.5
Crimson 1: A Phase 3 study to evaluate the efficacy and safety of subcutaneous marzeptocog alfa (activated) for on‐demand treatment of bleed events in subjects with hemophilia A or B, with inhibitors
S. Rangarajan 1; S. Desai2; S. Apte3; D. Vaghasiya4; J. Mahlangu5; V. Ramanan6; L. Makhaldiani7; B. Korczowski8; M. Timofeeva9; S. Volkova10; S. Pinnamaraju2; J. Yun2; L. Neuman2; T. Knudsen2; B. Kim2
1 University of Southampton, Southhampton, England, United Kingdom; 2 Catalyst Biosciences, South San Francisco, California, United States; 3 Sahyadri Speciality Hospital, Pune, India, Pune, Maharashtra, India; 4 Sahyadri Super Specialty Hospital, Pune, Maharashtra, India; 5 University of the Witwatersrand and NHLS, Johannesburg, South Africa; 6 Grant Medical Foundation, Ruby Hall Clinic, Pune, Maharashtra, India; 7 K.Eristavi National Centre of Experimental and Clinical Surgery, Tbilisi, Guatemala, Georgia; 8 Institute of Medical Sciences, Medical College, University of Rzeszów, Rzeszów, Podkarpackie, Poland; 9 Federal State Budgetary Scientific Institution,Kirov Research Institute of Hematology and Blood Transfusion of Federal Medical and Biological Agency, Kirov, Kirov, Russia, 10 MEDIS, LLC, Novgorod, Novgorod, Russia
Background: A significant number of persons with hemophilia A (PwHA) or PwHB develop inhibitors to FVIII or FIX, respectively, and become refractory to factor replacement treatment. Marzeptocog alfa (activated; MarzAA) is an engineered, activated FVII (FVIIa) with improved pharmacokinetics developed for subcutaneous (SC) administration in PwHA/PwHB with inhibitors.
Aims: To evaluate the efficacy and safety of MarzAA versus standard of care (SOC; i.e., NovoSeven®, recombinant FVIIa [rFVIIa], or activated prothrombin complex concentrate) for treatment of bleeds in PwHA/PwHB with inhibitors.
Methods: Enrollment of approximately 60 subjects was planned (consented per local Ethics Committee) to observe a total of 488 eligible, treated bleeds, 244 with each treatment type in a cross‐over study design, to demonstrate non‐inferiority (margin: ‐0.12) in effective treatment (investigator assessment of “Excellent” or “Good” hemostasis at 24 hours after initial dose) with MarzAA versus SOC.
Results: Due to changes in business strategy, the Sponsor terminated enrollment and dosing early on 15 November 2021. At that time, 18 subjects had been randomized and enrolled, with observation of 74 eligible bleeds (30 and 44 bleeds in 8 and 11 subjects treated with MarzAA and SOC, respectively). The proportion of effective treatment at 24 hours post‐initial dose was 86.2% versus 86.5% for subjects treated with MarzAA versus SOC, respectively. One subject treated with SOC reported a serious adverse event of ureterolithiasis unrelated to treatment. No thromboembolic events were reported. One subject treated with MarzAA was found to have low‐titer cross‐reactive anti‐drug antibodies (ADAs) with neutralizing antibody assessments pending.
Conclusion(s): While this study was terminated early by the Sponsor, the accumulated data suggest that SC MarzAA has the potential to treat bleeding episodes safely and effectively in PwHA/PwHB with inhibitors. Further investigation into ADAs in one subject will be reported at the Congress.
OC 27.5
Predictive performance of pharmacokinetic‐guided prophylactic dosing of factor concentrates in hemophilia A and B (OPTI‐CLOT TARGET study) – Preliminary results
T. Goedhart 1; L. Bukkems2; M. Coppens3; K. Fijnvandraat4; S. Schols5; R. Schutgens6; J. Eikenboom7; F. Heubel‐Moenen8; P. Ypma9; L. Nieuwenhuizen10; K. Meijer11; F. Leebeek12; R. Mathôt13; M. Cnossen14
1 Erasmus MC Sophia Children’s Hospital, University Medical Center Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Clinical Pharmacology ‐ Hospital Pharmacy, Amsterdam UMC, University of Amsterdam, Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 3 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 5 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands; 6 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 7 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 8 Department of Hematology, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 9 Department of Hematology, Haga Hospital, The Hague, Netherlands, The Hague, Zuid‐Holland, Netherlands, 10 Department of Hematology, Máxima Medical Center, Eindhoven, Netherlands; Radboud university medical center, Hemophilia Treatment Center, Nijmegen – Eindhoven – Maastricht, Netherlands, Eindhoven, Noord‐Brabant, Netherlands, 11 University Medical Center Groningen, Groningen, Groningen, Netherlands, 12 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 13 Department of Hospital Pharmacy, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands, 14 Department of Pediatric Hematology, Sophia Children’s Hospital, Erasmus University Medical Center, Erasmus University Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Pharmacokinetic(PK)‐guided dosing is used to individualize factor VIII (FVIII) and factor IX (FIX) therapy.
Aims: Investigate the predictive performance of PK‐guided prophylactic dosing of factor concentrates in hemophilia patients.
Methods: In this multicenter, prospective cohort study, hemophilia patients of all ages on prophylaxis with standard half‐life (SHL) and extended half‐life (EHL) factor concentrates received PK‐guided dosing. Treating physicians set individual target levels based on previous FVIII/FIX levels, physical activities and bleeding phenotype. During 9 months follow‐up, at least four measured FVIII/FIX levels per patient were compared to corresponding predictions obtained by Bayesian forecasting. Predictive performance was adequate when ≥80% of the measured FVIII/FIX levels were within ± 25% of the prediction. Bias and accuracy were calculated using mean error (ME) and mean absolute error (MAE), respectively. During post‐hoc analysis, predictive performance was assessed allowing maximal difference of 1 (trough), 5 (mid) and 15 (peak) IU/dL. Ethical approval and informed consent was obtained.
Results: Fifty patients were included (Table 1). Twenty‐seven patients completed the study (January, 2022). Median targeted FVIII/FIX trough level under PK‐guidance was 2 IU/dL [IQR 1‐4]. Predictive performance of 189 levels is shown in Figure 1. Sixty‐six percent of levels (54% trough, 77% mid, 72% peak) were within ± 25% of prediction. MAE was 0.7 (trough), 2.6 (mid) and 10.5 (peak) IU/dL. According to post‐hoc analysis, 77% (trough), 92% (mid) and 78% (peak) of levels were within set limits. Patients who completed the study had a median number of total and spontaneous bleeds of one (IQR 0‐3) and zero (IQR 0‐1), respectively.
Conclusion(s): The prespecified predictive performance target was not achieved, partly due to high measurement error especially in trough levels. In our opinion, the predictive performance of PK‐guidance in clinical practice is better represented by post‐hoc analysis, the ME and MAE. These low errors were regarded as clinically irrelevant in most cases.
OC 30.5
Emicizumab prophylaxis for the treatment of people with moderate or mild hemophilia A without factor VIII inhibitors: Results from the primary analysis of the HAVEN 6 Study
C. Hermans 1; C. Negrier2; M. Lehle3; P. Chowdary4; O. Catalani3; V. Jiménez Yuste5; B. Beckermann3; C. Schmitt3; G. Ventriglia3; J. Windyga6; A. Kiialainen3; R. d'Oiron7; P. Moorehead8; S. Koparkar9; V. Teodoro10; A. Shapiro11; J. Oldenburg12
1 Cliniques Universitaires Saint‐Luc, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 2 Louis Pradel University Hospital, Claude Bernard University Lyon 1, Lyon, Auvergne, France; 3 F. Hoffmann‐La Roche Ltd., Basel, Basel‐Stadt, Switzerland; 4 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom; 5 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain; 6 Institute of Hematology and Transfusion Medicine, Warsaw, Mazowieckie, Poland; 7 CRC‐MHC, Hôpital de Bicetre AP‐HP, Le Kremlin Bicetre, France, Le Kremlin Bicêtre, Ile‐de‐France, France; 8 Memorial University of Newfoundland, St John’s, Newfoundland and Labrador, Canada; 9 Genentech, Inc., San Francisco, California, United States, 10 F. Hoffmann‐La Roche Ltd., Basel, California, United States, 11 Indiana Hemophilia and Thrombosis Center, Indianapolis, Indiana, United States, 12 University Clinic Bonn, Institute of Experimental Haematology and Transfusion Medicine, Bonn, Germany, Bonn, Berlin, Germany
Background: Emicizumab is a bispecific monoclonal antibody that substitutes for missing activated factor (F)VIII in people with hemophilia A (HA).
Aims: This primary analysis of HAVEN 6 (NCT04158648) aims to assess safety and efficacy of emicizumab prophylaxis in people with non‐severe HA without FVIII inhibitors.
Methods: HAVEN 6 is a Phase III, open‐label study of emicizumab in people with moderate or mild HA without FVIII inhibitors who warrant prophylaxis as assessed by Investigator. Informed consent and ethics approval were obtained. Participants received subcutaneous emicizumab 3 mg/kg weekly for 4 weeks, then 1.5 mg/kg weekly, 3 mg/kg every 2 weeks, or 6 mg/kg every 4 weeks. Safety endpoints include adverse events (AEs), serious AEs (SAEs) and AEs of special interest, including thromboembolic events (TEs) and thrombotic microangiopathies (TMAs). Efficacy endpoints include negative binomial regression model estimates of annualized bleed rates (ABRs).
Results: As of 30‐Oct‐2021, 72 participants (70.8% [n = 51] moderate; 29.2% [n = 21] mild; 95.8% [n = 69] male; 4.2% [n = 3] female) received emicizumab. Median follow‐up was 55.6 weeks. At baseline, 37 participants (51.4%) were on FVIII prophylaxis; 24 (33.3%) had target joints. Within 24 weeks prior to study entry, participants had a median (range) of 2.0 (0–96) bleeds and a model‐based ABR (95% CI) of 10.1 (6.93–14.76). Sixty participants (83.3%) had ≥1 AE and 15 (20.8%) had ≥1 emicizumab‐related AE; no AEs led to treatment withdrawal/modification/interruption (Table 1). Ten SAEs were reported by eight participants (11.1%), none emicizumab‐related. There were no deaths or TMAs. One participant experienced a Grade 1 thrombosed hemorrhoid unrelated to emicizumab, classified as a TE. Model‐based ABRs (95% CI) were 0.9 (0.55–1.52) for treated bleeds, and 2.3 (1.67–3.12) for all bleeds (Table 2). Forty‐eight participants (66.7%) had zero treated bleeds.
Conclusion(s): These data show continued efficacy and a favorable safety profile of emicizumab in people with non‐severe HA without FVIII inhibitors who warrant prophylaxis.
OC 63.4
Little discrepancy of one‐stage and chromogenic assays in a large international cohort of patients with non‐severe hemophilia A and B (DYNAMO study)
A. Zwagemaker1; F. Kloosterman1; S. Gouw2; S. Boyce3; P. Brons4; M. Cnossen5; P. Collins6; J. Eikenboom7; C. Hay8; S. Jackson9; M. Kruip10; B. Laros‐van Gorkom11; C. Male12; L. Nieuwenhuizen13; S. Shapiro14; K. Fijnvandraat15; M. Coppens 16
1 Pediatric Hematology, Emma Children's Hospital, Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Haematology, University Hospital Southampton, Southampton, United Kingdom, Southampton, England, United Kingdom; 4 Department of Pediatric Hemato‐Oncology, Radboud University Medical Center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 5 Department of Pediatric Hematology, Sophia Children’s Hospital, Erasmus University Medical Center, Erasmus University Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 Medical School of Cardiff University, Cardiff, Wales, United Kingdom; 7 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 8 University Department of Haematology, Manchester Royal Infirmary, Manchester, England, United Kingdom; 9 Adult Bleeding Disorders Program of BC ‐ Adult Division St. Paul's Hospital, Vancouver, British Columbia, Canada, Vancouver, British Columbia, Canada, 10 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 11 Department of Hematology, Radboud university medical center, Nijmegen, The Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, The Netherlands, Nijmegen, Gelderland, Netherlands, 12 Medical University of Vienna, Austria, Vienna, Wien, Austria, 13 Department of Hematology, Máxima Medical Center, Eindhoven, Netherlands; Radboud university medical center, Hemophilia Treatment Center, Nijmegen – Eindhoven – Maastricht, Netherlands, Eindhoven, Noord‐Brabant, Netherlands, 14 Oxford University Hospitals National Health Service Foundation Trust, University of Oxford, and Oxford National Institute for Health Research Biomedical Research Centre, Oxford, England, United Kingdom, 15 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands, 16 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Measurements of coagulation factor activity are essential for hemophilia management and are generally performed with the one‐stage or chromogenic assay. Currently it is suggested that around one‐third of patients with non‐severe hemophilia A have assay discrepancy. These data are limited for non‐severe hemophilia B. Knowledge on the extent of assay discrepancy in perspective of previous literature may facilitate further interpretation of this phenomenon.
Aims: To investigate the extent of assay discrepancy in moderate and mild hemophilia A and B.
Methods: Patients with non‐severe hemophilia A and B were included from the multicenter international DYNAMO study. Central measurements of FVIII and FIX activity levels were performed by the one‐stage (Actin FS reagents) and chromogenic assay (FVIII Siemens, FIX Rossix). Assay discrepancy was defined as a ratio > 2.0 or < 0.5 between the assay results according to the SSC ISTH. Ethics approval and written consent was obtained (ClinicalTrials.gov: NCT03623295).
Results: A total of 221 patients were included of whom 4 patients (2%) showed assay discrepancy. This corresponded to 2/175 hemophilia A patients and 2/46 hemophilia B patients. Another 4 patients did not meet the criteria but exhibited a potential clinically relevant absolute difference > 10 IU/dL between the assay results. The median difference between the assays was generally low with 1.1 IU/dL (IQR 0.5‐2.1) for the total cohort, 1.0 IU/dL (IQR 0.4‐1.9) in case of higher one‐stage results and 1.3 IU/dL (IQR 0.7‐2.4) in case of higher chromogenic results (Figure 1). Thirteen patients in this cohort had a F8/F9 mutation associated with assay discrepancy in at least 3 patients in previous literature. Most of these patients (92%) had no discrepant results in our study.
Conclusion(s): Little assay discrepancy was observed, even in those persons with mutations previously associated with discrepancy. This suggests that the previously reported discrepancy could be more dependent on laboratory‐ than on patient‐related factors. FIGURE 1 Scatterplot of centrally measured factor activity with one‐stage vs. chromogenic assay. The colored dots represent patients with assay discrepancy (red dots) or patients that did not meet these criteria but had an absolute difference > 10 IU/dL between the assay results (yellow dots). The line reflects x = y
FIGURE 1 Scatterplot of centrally measured factor activity with one‐stage vs. chromogenic assay. The colored dots represent patients with assay discrepancy (red dots) or patients that did not meet these criteria but had an absolute difference > 10 IU/dL between the assay results (yellow dots). The line reflects x = y
OC 27.4
Recombinant factor VIII Fc showed better prophylactic effectiveness compared to standard half‐life factor VIII in haemophilia A – Results from A‐SURE, a 24‐month prospective, non‐interventional study
J. Oldenburg 1; C. Hay2; F. Peyvandi3; A. Tagliaferri4; P. Holme5; M. Alvarez‐Román6; C. Biron‐Andréani7; H. Malmström8; S. Lethagen8
1 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 University Department of Haematology, Manchester Royal Infirmary, Manchester, England, United Kingdom; 3 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy; 4 Regional Reference Center for Inherited Bleeding Disorders, University Hospital of Parma, Parma, Emilia‐Romagna, Italy, 5 Department of Haematology, Oslo University Hospital 2Research Institute of Internal Medicine, Oslo University Hospital 3Institute of Clinical Medicine, University of Oslo, Oslo, Norway, Oslo, Oslo, Norway; 6 Hospital Universitario La Paz, Madrid, Madrid, Spain; 7 Haemophilia Treatment Centre, Department of Biological Haematology, University Hospital Montpellier, Montpellier, Languedoc‐Roussillon, France; 8 Swedish Orphan Biovitrum AB, Stockholm, Stockholms Lan, Sweden
Background: Recombinant factor VIII Fc (rFVIIIFc) was the first approved extended half‐life (EHL) FVIII product for people with haemophilia A (PwHA). Efficacy and safety were established in phase 3 studies and their extension.
Aims: To report direct comparative real‐world effectiveness of rFVIIIFc prophylaxis vs. a matched treatment group on standard half‐life (SHL) FVIII.
Methods: A‐SURE (NCT02976753) was a 24‐month prospective, non‐interventional, European multicentre study in PwHA comparing rFVIIIFc with SHL FVIII prophylaxis (with ethics committee approvals and informed consents). Considering different aims for prophylactic regimens, three primary endpoints were defined, including annualised bleeding rate (ABR), injection frequency, and factor consumption. Each included patient on rFVIIIFc was matched by age and last prescribed weekly SHL FVIII dose prior to baseline at local site level to a patient on SHL FVIII. To reduce potential effects of confounding, propensity scores were estimated based on patient characteristics at baseline and adjusted for in the statistical analysis. All primary endpoints were analysed with Generalized Linear Mixed Models, including the ABR endpoint for which poor model fit ruled out the use of the predefined negative binomial regression model.
Results: 356 PwHA were enrolled and eligible, 186 in the rFVIIIFc group and 170 in the SHL FVIII group. All primary endpoints i.e. mean ABR, annualized injection frequency and annualized factor consumption, were significantly lower in the rFVIIIFc group compared to the SHL FVIII group during the 24‐month prospective period (Table 1). rFVIIIFc was well tolerated, consistent with the established safety profile. No inhibitors occurred, including in patients with a previous inhibitor history.
Conclusion(s): The 24‐month prospective non‐interventional A‐SURE study with a solid comparative design including a matched control group, demonstrated significantly lower ABR, injection frequency and factor consumption with rFVIIIFc compared to SHL FVIII. Thus, rFVIIIFc shows beneficial effects on disease and treatment burden compared to SHL FVIII in a real‐world setting. TABLE 1 Primary study endpoints
TABLE 1 Primary study endpoints
OC 30.4
Emicizumab real world experience using the entire‐vial based dosing regimen in for patients with haemophilia A: An update
K. van der Zwet 1; A. Donners2; R. Schutgens3; K. Fischer4
1 University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands, Netherlands, Utrecht, Netherlands; 2 Department of Clinical Pharmacy, University Medical Center Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands; 3 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 4 University Medical Center Utrecht, University Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands
Background: Emicizumab is a subcutaneous FVIII mimetic that effectively reduces bleeds in patients with haemophilia A. The registered maintenance dose is based on body‐weight: 6 mg/kg/4 weeks (with varying intervals). The dose per administration, given an individual weight difference, often does not match vial content leading to medication spillage. The long elimination half‐life of emicizumab enables use of entire vials by varying the dosing interval (7 to 28 days). Entire‐vial based dosing was introduced for patients receiving emicizumab.
Aims: To report real‐world experience of the entire‐vial based dosing regimen of emicizumab in patients with haemophilia A by investigating emicizumab concentrations.
Methods: Monocenter, observational cohort study. All haemophilia A patient receiving emicizumab as maintenance therapy for > 2 months with plasma emicizumab concentrations available, were included. Loading doses were administered according to label. Maintenance treatment was dosed 6 mg/kg/4 weeks using entire vial regimen by varying interval 7 to 28 days. Plasma emicizumab concentrations were measured using a fully validated mass spectrometry‐method.
Results: A total of 89 patients were included see table 1. Their median age at start of emicizumab was 18.5 years (P25‐P75 (IQR): 8.8 – 53.1), weight was 66.0 kg (IQR 31.5‐ 85.7). Patients received a median of 115 days (IQR 103‐370) emicizumab maintenance treatment. The median emicizumab dose of 5.9 mg/kg/4 weeks administered in 7 – 28 days intervals, with 31% of patients using intervals > 12 days. Median plasma concentration of emicizumab was 61.7 μg/ml (IQR 49.0 – 79.9) with no differences observed between adults and children, see figure 1. A total of 74 patients (83%) demonstrated emicizumab concentration ≥ 40 μg/ml during maintenance therapy using entire vials based dose regimen.
Conclusion(s): Dosing emicizumab using entire vials with varying intervals to achieve 6 mg/kg/4 weeks, resulted in effective concentrations of emicizumab in both children and adults. This dosing strategy can be applied to avoid medication spillage.
OC 63.2
Inter‐individual FVIII clearance heterogeneity in PWH in the iPATH study – biological determinants and clinical importance
E. Elsheikh 1; M. Lavin2; N. Larkin3; N. O'Connell4; K. Ryan5; M. Byrne6; E. Singleton5; P. Fallon7; J. Johnsen8; S. Pipe9; P. Turecek10; J. O'Donnell11
1 Royal College of Surgeons Ireland, St James Hospital, Dublin8, Dublin 8, Ireland, 2 St James Hospital, Dublin, Dublin, Dublin, Ireland; 3 St James's Hospital,Dublin8, Dublin, Dublin, Ireland; 4 St. James Hospital, Dublin, Ireland; 5 National Coagulation Centre,St James's Hospital,Dublin8, Dublin, Dublin, Ireland; 6 National Coagulation Centre, St James’s Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 7 Trinity College Dublin, Dublin, Dublin, Ireland; 8 University of Washington, Seattle, Washington, United States; 9 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States, 10 Baxalta Innovations GmbH, a Takeda company, Vienna, Wien, Austria, 11 Royal College of Surgeons Ireland, St James Hospital, Dublin8, Dublin, Dublin, Ireland
Background: Significant inter‐individual variation in FVIII clearance has been described in patients with severe haemophilia A (PWH). However the mechanisms underlying this heterogeneity and its clinical significance remain poorly defined.
Aims: We studied in vivo clearance of rFVIII (Advate) in a large population of Irish PWH using MyPKFit and investigated factors influencing half‐life and clinical outcomes.
Methods: Patients were recruited from the National Coagulation Centre in Dublin. All patients provided written informed consent. Clinical data were collected retrospectively from electronic medical records and patients diaries for the period from January 2013 through January 2018.
Results: 54 PWH were enrolled median age 36 years, range( 18‐75). Overall, median annualized bleed rate (ABR) was 3.7, median Haemophilia Joint Health Score (HJHS) was 26 (range 0‐51), median FVIII dose was 52.4 IU/kg/week (range 5.7‐128.6). Although the median rFVIII half‐life was 11.4 hours, marked inter‐patient variability was observed (range 7.7‐20.1 hours). FVIII half‐life correlated significantly with endogenous plasma VWF:Ag levels (r = 0.5378, p < 0.0001). In keeping with this finding, FVIII half‐life was significantly reduced in group O compared to non‐O PWH (p = 0.02). In addition, FVIII half‐life increased significantly with age (p < 0.0001). Interestingly, FVIII usage was reduced in older PWH, despite the fact HJHS and ABR both increased progressively with age. Finally, we observed that dose of FVIII prophylaxis used in PWH correlated inversely with FVIII half‐life despite the fact that PK‐driven FVIII dosing was not used in Ireland in the study period. We hypothesize that PWH with faster FVIII clearance have increased bleed rates that lead to higher intensity FVIII prophylaxis over time.
Conclusion(s): These data highlight that multiple factors contribute to marked inter‐individual variation in rFVIII clearance in PWH. Moreover, this clearance heterogeneity has direct translational relevance. Further studies will be required to define the biological mechanisms underpinning this variability, and to develop personalised treatment regimens for PWH.
OC 63.3
Safety and efficacy of recombinant factor IX fusion protein (rIX‐FP) in previously untreated patients with hemophilia B
R. Lemons 1; M. Wang2; J. Curtin3; A. Huth‐Kuehne4; L. Lepatan5; C. Male6; F. Peyvandi7; M. Von Depka Prondzinski8; W. McKeand9; W. Seifert10; J. Oldenburg11
1 University of Utah, Department of Pediatrics and Primary Children’s Hospital, Salt Lake City, Utah, United States, Salt Lake City, Utah, United States; 2 University of Colorado Hemophilia and Thrombosis Center, Aurora, Colorado, United States; 3 The Children’s Hospital at Westmead, New South Wales, Australia, Westmead, New South Wales, Australia; 4 Kurpfalzkrankenhaus Heidelberg GmbH, Heidelburg, Germany, Heidelberg, Baden‐Wurttemberg, Germany; 5 Perpetual Succour Hospital, Cebu, Philippines, Cebu City, Cebu, Philippines, 6 Medical University of Vienna, Austria, Vienna, Wien, Austria; 7 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy; 8 Werlhof Institute, Hannover, Germany, Hannover, Niedersachsen, Germany; 9 CSL Behring, King of Prussia, United States, King of Prussia, Pennsylvania, United States, 10 CSL Behring, Marburg, Germany, Marburg, Hessen, Germany, 11 University Clinic Bonn, Institute of Experimental Haematology and Transfusion Medicine, Bonn, Germany, Bonn, Berlin, Germany
Background: Recombinant fusion protein linking coagulation factor IX (FIX) with albumin (rIX‐FP) has been shown to be efficacious and well tolerated with prolonged dosing intervals of up to 21 days in previously treated patients.
Aims: This study evaluated the safety and efficacy of prophylaxis with rIX‐FP in previously untreated patients (PUPs).
Methods: PUPs with severe/moderately severe hemophilia B (FIX ≤2%) who had never been treated with FIX products received weekly rIX‐FP prophylaxis (25–50 IU/kg, up to a maximum of 75 IU/kg) over ≥ 50 exposure days (EDs). Primary outcomes were the safety of rIX‐FP, including the development of FIX inhibitors, and pharmacokinetic (PK) parameters. Secondary outcomes included total annualized and spontaneous bleeding rates (ABR and AsBR).
Results: Twelve PUPs with a mean (range) age of 1.3 (0‐11) years received routine prophylaxis. Mean (SD) exposure to rIX‐FP was 68.3 (38.0) EDs. One 11‐year‐old PUP (8.3%, 95% CI 1.5–35.4) developed an inhibitor against FIX after eight EDs which was recorded as a related serious adverse event (AE) and the patient was discontinued from the study. Most treatment‐emergent AEs were unrelated to rIX‐FP and mild or moderate in intensity (Table 1). PK parameters were evaluated in 8 PUPs after a single infusion of 50 IU/kg rIX‐FP (Table 2). The mean steady‐state FIX activity trough was > 10%. In the 12 PUPs on the routine prophylaxis regimen, total ABR ranged from 0 to 3.89. Nine PUPs had an AsBR of 0. Six PUPs reported a total of 23 joint bleeding episodes; 13 episodes were reported in the PUP that had developed an inhibitor to FIX. Across all treatments, 93.8% of spontaneous bleeding events were successfully controlled with 1 or 2 rIX‐FP infusions.
Conclusion(s): This study confirmed the safety and efficacy of rIX‐FP when used for routine prophylaxis and on‐demand treatment in pediatric PUPs.
OC 70.4
Real‐world management of delivery in a large cohort of haemophilia carriers: Focus on maternal and neonatal outcomes
B. Pollio 1; I. Ricca2; N. Spano3; C. Linari4; U. Ramenghi3; F. Quaglino2; R. Albiani2
1 Regina Margherita Children Hospital Azienda Ospedaliero‐Universitaria Città della Salute e della Scienza, Torino, Piemonte, Italy; 2 AOU Città della Salute e della Scienza di Torino, Torino, Piemonte, Italy; 3 Turin University of medicine, Torino, Piemonte, Italy; 4 Città della Salute e della Scienza di Torino, Torino, Piemonte, Italy
Background: The optimal modality of delivery in hemophilia carriers s still a matter of debate.
Aims: We evaluated the maternal and neonatal outcomes in a real‐world large cohort of haemophilia carriers.
Methods: Our work is a single‐institution retrospective observational study. Family history of haemorrhagic disease, prenatal diagnosis, planned and effective mode of delivery, maternal and neonatal haemorrhages were collected from clinical records and parental interviews. Statistical analyses were performed using STATA/SE 16.1.
Results: 59 children with mild, moderate, or severe haemophilia born between 1999 and 2020 and 53 carriers were included. Prenatal diagnosis was performed in 11 cases and in 7 cases the diagnosis was defined as “suspected” according to the family history. Sporadic cases were 34, familiar cases were 25. Vaginal delivery was performed in 29 cases of which 2 induced delivery and 1 instrumental delivery with vacuum. C‐section was performed in 29 cases, of which 9 emergency c‐sections. 2 women were treated with blood transfusion for postpartum haemorrhage following emergency C‐section delivery. Children with severe haemophilia were more likely to be born by c‐section (p = 0,022). 2 cephalohaematomas were diagnosed after vaginal delivery without instruments, while no child was affected by intracranial haemorrhage at the time of birth. 7 minor haemorrhages in sampling sites were found. There was no significant difference in frequency for cephalohaematomas between vaginal delivery and C‐section (p = 0,491). Treatment with an infusion of FVIII was requested in 3 cases.
Conclusion(s): Natural childbirth in our experience appears safe for both the mother and the fetus in sporadic cases. Although there are no evidence‐based recommendations, the elective cesarean section before labor was the preferred modality of delivery in familiar cases. Prenatal diagnosis of haemophilia is a maternal risk factor for cesarean delivery.
OC 40.4
Safety and efficacy of emicizumab in people with hemophilia A enrolled in the hemophilia natural history study (ATHN 7)
T. Buckner1; S. L. Carpenter2; C. Kempton3; L. Lee4; L. Malec5; T. McLean6; P. Morton4; J. Staber7; M. Wang8; M. Recht 9
1 Hemophilia and Thrombosis Center, University of Colorado, School of Medicine, Aurora, California, United States; 2 Kansas City Regional Hemophilia Center, Kansas City, Montana, United States; 3 Hemophilia of Georgia Center for Bleeding and Clotting Disorders of Emory, Atlanta, Georgia, United States; 4 Genentech, Inc., South San Francisco, California, United States; 5 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 6 Wake Forest University Health Sciences, Winston‐Salem, North Carolina, United States; 7 Iowa Hemophilia and Thrombosis Center – University of Iowa, Iowa City, Iowa, United States; 8 University of Colorado Hemophilia and Thrombosis Center, Aurora, Colorado, United States; 9 American Thrombosis and Hemostasis Network, Rochester, New York, United States
Background: ATHN 7, A Natural History Study of the Safety, Effectiveness, and Practice of Treatment for People with Hemophilia (NCT03619863) monitors the use of current hemophilia therapies, including emicizumab, a bispecific monoclonal antibody that bridges activated factor (F)IX and FX, substituting for the function of deficient activated FVIII in people with hemophilia A (PwHA).
Aims: To report the baseline demographic characteristics of participants and real‐world safety and efficacy of emicizumab in ATHN 7.
Methods: ATHN 7 is a longitudinal, prospective observational cohort study being conducted at 26 American Thrombosis and Hemostasis Network (ATHN)‐affiliated sites. Ethics approval was obtained and participants and/or parents/guardians provided consent. PwHA receiving care at participating sites are eligible for inclusion. Demographic and clinical information is collected through participant interview and medical record review. Adverse events (AEs) are documented. Here, annualized bleed rates (ABRs) were calculated for PwHA without FVIII inhibitors treated with emicizumab.
Results: As of August 31, 2021, demographic information was available for 249 emicizumab‐treated PwHA (Table 1). Baseline inhibitor status was available for 236 individuals (59 with inhibitor, 177 without inhibitor). For the entire cohort, median (range) duration of emicizumab exposure was 77.2 (0.14–141.00) weeks. Fifteen AEs were reported in 9 participants, including 6 injection‐site reactions in one participant. There was one death due to hemorrhagic shock, which was deemed unrelated to emicizumab. No thrombotic events or thrombotic microangiopathies were reported. Effectiveness in the PwHA without FVIII inhibitors is summarized in Table 2. Mean (standard deviation) ABRs were 1.30 (2.78) for treated bleeds and 0.73 (2.07) for treated joint bleeds, with similar values for the participants with moderate or severe HA.
Conclusion(s): This is the largest multi‐center, prospective observational experience of emicizumab‐treated PwHA in the US. No new safety signals were identified and ABRs for PwHA without FVIII inhibitors were consistent across the different severities of HA.
OC 70.2
Safety of intramuscular COVID‐19 vaccination in patients with hemophilia
A. Tiede 1; H. Leise1; S. Horneff2; J. Oldenburg3; S. Halimeh4; C. Heller5; C. Königs6; K. Holstein7; C. Pfrepper8
1 Hannover Medical School, Hannover, Niedersachsen, Germany; 2 Bonn University, Bonn, Nordrhein‐Westfalen, Germany; 3 University Clinic Bonn, Institute of Experimental Haematology and Transfusion Medicine, Bonn, Germany, Bonn, Berlin, Germany; 4 Gerinnungszentrum Rhein‐Ruhr, Duisburg, Nordrhein‐Westfalen, Germany; 5 Frankfurt University, Frankfurt, Hessen, Germany; 6 University Hospital Frankfurt, Goethe University, Frankfurt, Hessen, Germany; 7 Universitätstklinikum Eppendorf, Hamburg, Hamburg, Germany; 8 University Hospital Leipzig, Leipzig, Germany, Leipzig, Sachsen, Germany
Background: Guidelines recommend that patients with hemophilia should preferably receive vaccination subcutaneously rather than intramuscularly. COVID‐19 vaccines, however, are only licensed for intramuscular application.
Aims: To assess the safety of intramuscular COVID‐19 vaccination in patients with hemophilia.
Methods: This observational multicenter study consisted of two parts. Part A enrolled consecutive patients with hemophilia A (HA) and B (HB) of all ages and severities and assessed injection site bleeding and other complications within 30 days of vaccination. Part B enrolled patients providing informed consent for more detailed data collection including medication and prophylaxis around the time of vaccination.
Results: Four hundred and sixty‐one patients were enrolled by six institutions into part A (HA 389 [84%], HB 72 [16%]; severe 291 [63%], moderate 61 [13%], mild 109 [24%]). The primary endpoint injection site bleeding occurred in 7 patients (1.5%, 95% confidence interval 0.6‐3.1%), including 5 with severe HA, 1 moderate HA, and 1 mild HB. Analysis of 214 patients in part B revealed that 97% of patients with severe hemophilia, who were not on emicizumab, had received factor prophylaxis before vaccination, either as part of their regular regime (60%) or additionally (40%). Only one patient on emicizumab received additional factor. The bleeding patients with severe HA had received factor within 2‐17h before vaccination and had no clinical characteristics that could have explained the bleeding. Factor was also given to 26% and 60% of mild and moderate patients not on regular prophylaxis, respectively. The two bleeding patients in this group had not received concentrate before vaccination. Other side effects of vaccination were comparable with studies in the general population.
Conclusion(s): This is the first study reporting on the safety of intramuscular COVID‐19 vaccination in hemophilia. The rate of injection site bleeding was below 3%, comparable with the general population.
OC 70.3
Unmet medical needs in patient‐reported outcomes and sports activity in people with haemophilia A/B with or without inhibitors: Historical data from patients entering a non‐interventional study
J. Mahlangu 1; R. Brown Frandsen2; A. Liu2; C. Eskelund3
1 University of the Witwatersrand and NHLS, Johannesburg, South Africa; 2 Novo Nordisk A/S, Søborg, Denmark, Søborg, Hovedstaden, Denmark; 3 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark
Background: Preventing and treating bleeding episodes while reducing treatment burden, improving quality of life (QoL) and increasing physical activity is the aim of a holistic approach to haemophilia management.
Aims: To describe unmet medical needs of patients with HA/HB with or without inhibitors using their historical treatment burden, QoL and sports activity.
Methods: Historical data were collected from patients entering the non‐interventional study, explorer6 (NCT03741881). Male patients ≥12 years old with severe HA (FVIII activity < 1%), severe/moderate HB (FIX activity ≤2%) or HA/HB with inhibitors of any severity were included. Patients with previous/current concizumab or emicizumab treatment were excluded. Each patient was treated according to their country’s standard‐of‐care (SoC). Historical treatment burden (assessed by Haemophilia Treatment Experience Measure [Hemo‐TEM]), QoL (assessed by Short Form 36 Health Survey Version 2 [SF‐36v2]) and sports activity (assessed by National Hemophilia Association’s “Playing It Safe: Activity Ratings Chart” [stepsforliving.hemophilia.org]) were collected and described.
Results: Overall, 231 patients (147 without inhibitors and 84 with inhibitors) with a median age of 28 years (range, 12–78 years) were enrolled. Mean Hemo‐TEM total scores [standard deviations] tended to be higher in patients without inhibitors treated episodically (HA: 36.1 [30.2]; HB: 28.5 [14.2]) than patients treated with prophylaxis (PPX; HA: 20.9 [14.9]; HB: 16.2 [11.9]). SF‐36v2 summary scores were lowest in patients with inhibitors and/or treated episodically (Figure 1). In the month before enrolment, lower proportions of patients with inhibitors and/or treated episodically performed sports activities and rarely those considered high‐risk (Figure 2).
Conclusion(s): We describe treatment burden, QoL and sports activity in patients with HA/HB, with or without inhibitors, from > 30 countries. Patients treated episodically had a higher treatment burden and rarely participated in sports in high‐risk categories. Emerging treatments may help to address unmet needs and treatment burden and improve QoL for patients with haemophilia.
OC 27.3
Individualized target trough FVIII level and optimized prophylaxis in pediatric patients with hemophilia A
K. Huang 1; D. Ai2; Y. Zhen2; G. Li2; Z. Chen2; R. Wu3
1 Beijing Children's Hospital, Capital Medical University, Beijing, Beijing, China (People's Republic); 2 Beijing Children's Hospital, Beijing, Beijing, China (People's Republic); 3 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic)
Background: Prophylaxis is the standard treatment for hemophilia A(HA). Considering the inter‐individual variability of pharmacokinetics (PK), bleeding phenotype and joint vulnerability, an individualized prophylactic protocol is vital to optimizing the therapy of HA.
Aims: To investigate the clinical outcomes of the new proposed PK‐guided dosing strategy which combined the comprehensive evaluation system for escalation.
Methods: Patients with severe HA and without FVIII inhibitors were enrolled. After a 72 h washout period and a single‐dose infusion of 50IU/kg of their routine used FVIII concentrate, each one received a PK test with a five‐point design. The trough levels were calculated by WAPPS‐Hemo. The bleeding rates (ABR, annualized bleeding rate; AJBR, annualized joint bleeding rate) were estimated from six months before enrollment to the study exit. The ultrasound and HJHS were used to evaluate the patients’ joints (both sides of ankles, knees, and elbows) every 12 months. The escalation criteria depended on joint bleeds, US scores and HJHS scores. Their quality of life was assessed by CHOKLAT sheets.
Results: Fifty‐eight severe HA boys who had an observational period of over 2 years were analyzed. Their age and body weight was 5.3(2.8,6.9)years and 21.5(16,25)kg respectively. At baseline, 34 of them had a trough level of <1IU/dL and seven target joints were detected according to the previous definition. During the study period, 47 escalations were observed. Joint bleeds count the most proportion (48.3%,N = 28). Significantly reduced ABR [0(0,6)vs.4(0,8),p < 0.0001] and AJBR[0(0,0.25)vs.0(0,2),p < 0.0001] was observed at study exit as well as the trend of decreased bleeding rates as the study progressed. Also,85% (6/7) of the target joints vanished during the study. Statistical improvement of US scores (p = 0.04) and HJHS scores (p = 0.02) was also reported at the study exit.
Conclusion(s): This newly proposed PK‐guided dosing strategy could reduce bleeding rates, eliminate target joints and improve impaired joints, which could be an optimal individualized prophylactic protocol.
OC 30.3
Treatment experience in hemophilia patients with and without inhibitors on concizumab prophylaxis: Hemo‐TEM results from the phase 2 explorer4 & 5 studies
M. Mancuso 1; J. Skov Neergaard2; K. Cepo2; T. Porstmann3
1 IRCCS Humanitas Research Hospital, Milan, Lombardia, Italy; 2 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark; 3 Novo Nordisk Health Care AG, Zurich, Zurich, Switzerland
Background: Haemophilia is associated with significant treatment burden. Concizumab, an anti‐tissue factor pathway inhibitor antibody in clinical development as a subcutaneous, prophylactic treatment, aims to improve patient experience by reducing treatment burden. explorer4 and explorer5 (NCT03196284/NCT03196297) investigated daily concizumab prophylaxis in patients with haemophilia A/B with inhibitors (HAwI/HBwI), and severe HA, respectively. Haemophilia Treatment Experience Measure (Hemo‐TEM), a 26‐item questionnaire with five domains developed to measure treatment burden in haemophilia patients, was used to investigate the value of subcutaneous prophylaxis with concizumab.
Aims: Assess impact of concizumab subcutaneous prophylaxis on treatment burden.
Methods: explorer4 and 5 comprised main (≤24 weeks) and extension parts (≤94 and ≤102 weeks, respectively). In explorer4, HAwI/HBwI patients were randomised 2:1 to concizumab prophylaxis or bypassing agent on‐demand. On‐demand patients switched to concizumab prophylaxis in the extension. In explorer5, all patients received concizumab. Hemo‐TEM was completed at baseline, 24 and ≥76 weeks. Scores range from 0–100; lower scores indicate lower treatment burden. Individual domain and total scores are calculated. Hemo‐TEM development and validation are presented separately.
Results: Twenty‐five and 29 patients completed explorer4 and 5, respectively. In explorer4, mean baseline Hemo‐TEM total scores were similar between concizumab (25.0) and on‐demand arms (26.2). After 24 weeks of treatment, the concizumab arm demonstrated change from baseline in mean Hemo‐TEM total score of ‐15.3, indicating lower treatment burden versus baseline, with no change in the on‐demand arm (Table 1). Similarly, change in mean Hemo‐TEM total score from baseline (21.9) was ‐12.8 for patients in explorer5 after 24 weeks (Table 2). Improved scores were observed for all Hemo‐TEM domains, with sustained improvements at last observational visit in both trials (Table 1, 2).
Conclusion(s): Hemo‐TEM scores improved after ≥24 weeks of concizumab subcutaneous prophylaxis during explorer4 and explorer5, in patients with and without inhibitors, illustrating the potential value of concizumab in reducing treatment burden.
OC 40.3
Consumption of on‐demand factor concentrates and bypassing agents for management of breakthrough bleeds with fitusiran prophylaxis in people with haemophilia A or B: An analysis of two phase 3 studies
A. Srivastava 1; R. Wu2; C. You3; K. Kavakli4; J. Bartelt‐Hofer5; R. Rosim6; Z. Qiu7; V. Cano8; S. Andersson9; S. Pipe10
1 Christian Medical College, Vellore, India, Vellore, Tamil Nadu, India; 2 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic); 3 Department of Pediatrics, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, South Korea, Daejeon, Ch'ungch'ong‐namdo, Republic of Korea; 4 Department of Hematology, Ege University Faculty of Medicine, Children's Hospital, Izmir, Turkey, Izmir, Izmir, Turkey; 5 Sanofi Genzyme, Chilly‐Mazarin, France, Chilly‐Mazarin, Ile‐de‐France, France; 6 Sanofi Genzyme, São Paulo, Brazil, São Paulo, Sao Paulo, Brazil; 7 Sanofi, Bridgewater, NJ, USA, Bridgewater, New Jersey, United States; 8 Sanofi Genzyme, Cambridge, MA, USA, Cambridge, Massachusetts, United States; 9 Sanofi, Cambridge, MA, USA, Cambridge, Massachusetts, United States, 10 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States
Background: Fitusiran, an investigational siRNA prophylactic, targets antithrombin mRNA to rebalance haemostasis in people with haemophilia A or B (PwHA/B), irrespective of inhibitor status. For PwHA/B receiving fitusiran, reduced doses of on‐demand clotting factor concentrates (CFC) or bypassing agents (BPA) are recommended to treat breakthrough bleeds.
Aims: To explore BPA/CFC consumption with fitusiran prophylaxis vs on‐demand BPA/CFC in PwHA/B, with or without inhibitors.
Methods: Two randomised, open‐label, Phase 3 trials (NCT03417102 and NCT03417245) enrolled males ≥12 years with severe haemophilia A/B with inhibitors (ATLAS‐INH) and without (ATLAS‐A/B). Eligible participants were randomised 2:1 to once‐monthly 80 mg subcutaneous fitusiran or on‐demand BPA (ATLAS‐INH) or CFC (ATLAS‐A/B) for 9 months. Annualised weight‐adjusted BPA/CFC consumption, numbers of treated bleeds and infusions per bleed were assessed.
Results: Overall, 118 participants were randomised to fitusiran, 19 to on‐demand BPA and 40 to on‐demand CFC. Total consumption of aPCC and rFVIIa was 97.5% and 98.2% lower in the fitusiran arm vs on‐demand BPA arm; overall mean consumption of FVIII and FIX was lower in the fitusiran arm vs on‐demand CFC arm (95.9% and 94.7%, respectively) (Table 1). Total number of treated bleeds were lower in the fitusiran arm vs the on‐demand BPA and CFC arms by 82.0% and 79.2%, respectively. In ATLAS‐INH, participants who received fitusiran required fewer mean injections (1.2 vs 3.7) and lower mean BPA doses per bleed vs participants who received on‐demand BPA (Table 2). In ATLAS‐A/B, participants in both arms required a mean 1.2 injections per bleed; participants who received fitusiran required lower mean CFC doses vs participants who received on‐demand CFC (Table 2).
Conclusion(s): Fitusiran prophylaxis reduced total BPA/CFC consumption by reducing number of treated bleeds, number of injections and BPA/CFC doses required to treat breakthrough bleeds in PwHA/B with and without inhibitors, by ~95% or more thereby reducing treatment burden.
OC 70.1
Similar Sports injuries in Dutch people with haemophilia and the general population: results of a 12‐month prospective study
O. Versloot 1; E. Kemler2; J. Blokzijl3; M. Schuuring4; M. Timmer3; M. Bartels5; W. van Beers3; K. van Galen3; I. Kremer Hovinga3; R. Schutgens1; M. Suijker3; P. van der Valk3; J. van der Net4; K. Fischer6
1 UMC Utrecht, Utrecht, Utrecht, Netherlands; 2 VeiligheidNL, Amsterdam, Noord‐Holland, Netherlands, 3 van Creveldkliniek, UMC Utrecht, Utrecht, Utrecht, Netherlands; 4 Wilhelmina Children's Hospital, Utrecht, Utrecht, Netherlands, 5 van Creveldkliniek, UMC Utrecht, UTrecht, Utrecht, Netherlands; 6 University Medical Center Utrecht, University Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands
Background: Although sports participation in Dutch people with haemophilia (PWH) is similar to the general population (GP), sports injury data have not been compared to the GP.
Aims: To assess sports injuries in PWH according to severity and compare them to the general population.
Methods: Sports injury data and bleeds following sports injuries were prospectively collected for 12 months in PWH aged 6‐49 without inhibitors who played sports at least once weekly. National GP data was collected retrospectively with a 3 month recall period. Data on 12‐months’ Sports injuries in PWH were compared according to severity and 3‐months’ data were compared to the GP. Groups were compared using non‐parametric methods.
Results: Data from 125 participants aged 6‐49 (41 children, 90% haemophilia A; 54% severe, 91% severe on prophylaxis) were included. Eighty‐seven sports injuries were reported by 51 participants. Participants with severe haemophilia reported more sports injuries than those with non‐severe haemophilia (69% vs. 31%; p < 0.01), but a shorter time loss after injury (median 7 (IQR: 3‐19) vs. 14 (7‐25) days; p = 0.05). Injuries reported by PWH were evenly distributed across the annual seasons (summer, autumn, winter, spring; p = 0.25). PWH reported a similar number of sports injuries as the GP (18% (CI: 12‐25%) vs. 16% (14‐18%)) over 3‐month’ periods, but a higher injury rate (6.0 vs. 3.4 injuries/1000 hrs exposure). Both PWH and GP mostly reported lower extremity injuries (PWH: 66%; GP: 62%).
Conclusion(s): These results showed different sports injury patterns in severe and non‐severe haemophilia. This could indicate a tendency of participants with severe haemophilia to be more prone to report their sports injuries (reporting bias), or increased risk‐taking by participants with non‐severe haemophilia. Furthermore, injury risk was similar in PWH and GP, suggesting that sustaining injuries occurred independent of haemophilia.
OC 20.4
IL‐6 is a biomarker for joint bleeds in hemophilia
L. Knowles1; C. Wolter2; L. Beyer1; M. Menger3; M. Laschke3; U. Grün4; E. Hermann5; J. Pilch 6
1 Institute of Clinical Hemostaseology and Transfusion Medicine, Homburg, Saarland, Germany; 2 Saarland University, Homburg, Saarland, Germany; 3 Institute for Clinical and Experimental Surgery, Homburg, Saarland, Germany; 4 Department of Orthopedic Surgery, Homburg, Saarland, Germany; 5 Universitätsklinikum des Saarlandes, Homburg/Saar, Saarland, Germany; 6 Saarland University Medical Center, Homburg, Saarland, Germany
Background: Deregulated macrophage function in hemophilia correlates with impaired wound healing.
Aims: To identify the underlying inflammatory stimulus of macrophage activation in hemophilia.
Methods: To assess the acute‐phase response in hemophilia patients, we collected blood from 59 adult patients with mostly severe hemophilia A and B compared to 54 healthy controls. To analyze inflammation in the context of bleeding, we induced a needle puncture injury in the knee joint of transgenic hemophilia mice.
Results: Compared to age‐ and weight‐matched controls, blood levels of interleukin‐6 (IL‐6), C‐reactive protein (CRP) and LPS‐binding protein (LBP) were significantly elevated in the entire cohort of hemophilia patients but exhibited a particularly pronounced increase in obese hemophilia patients with a BMI > 30. Subgroup analysis of the remaining non‐obese hemophilia patients (BMI of 18‐29) revealed a significant spike of IL‐6 and CRP in connection with a de‐novo increase of soluble IL‐6 receptor α (sIL‐6Rα) in patients with a bleeding event within the last month. Hemophilia patients that did not experience recent bleeding, on the other hand, had IL‐6, CRP and sIL‐6Rα blood concentrations at levels similar to that seen in healthy controls. The pivotal role of IL‐6 as a marker of bleeding in hemophilia was confirmed in hemophilia patients with acute bleeding events as well as in transgenic hemophilia mice after needle puncture of the knee, which caused an extensive hematoma and a nearly 150‐fold increase of IL‐6 blood levels within 7 days of the injury compared to needle‐punctured control mice. Notably, the IL‐6 blood levels shrunk to only a 4‐fold elevation in hemophilia mice over controls after 28 days, when the hematoma was completely replaced by arthrofibrosis.
Conclusion(s): IL‐6 is a potential biomarker for monitoring the extent and duration of joint bleeds in hemophilia and, as such, may be a predictor of bleeding‐induced joint damage.
OC 27.2
Clinical outcomes following prophylaxis with octocog alfa in patients with moderate hemophilia A: 8‐Year interim read‐out of the real‐world AHEAD International study
M. Ozelo1; C. Hermans 2; K. Khair3; B. Guillet4; R. Guerra5; J. Gu5; L. Tang6; M. Carcao7
1 Hemocentro UNICAMP, Department of Internal Medicine, School of Medical Sciences, University of Campinas, Campinas, Sao Paulo, Brazil; 2 Saint‐Luc University Hospital, Université Catholique de Louvain, Louvain, Vlaams‐Brabant, Belgium; 3 Haemnet, London, England, United Kingdom; 4 CRC‐MHC, CHU de Rennes – Hôpital Pontchaillou, Rennes, France, Rennes, Bretagne, France; 5 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States; 6 Takeda Pharmaceuticals International AG, Zürich, Zurich, Switzerland; 7 Division of Hematology/Oncology, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada
Background: AHEAD (NCT02078427, started in 2011) is an ongoing, international study evaluating long‐term outcomes in patients with severe/moderate hemophilia treated with octocog alfa or rurioctocog alfa pegol in routine clinical practice.
Aims: Characterize clinical outcomes in patients with moderate HA (factor VIII 1–≤5%) receiving standard or pharmacokinetic (PK)‐guided prophylaxis or on‐demand treatment with octocog alfa.
Methods: This is an 8‐year interim read‐out (data cutoff: July 01, 2021) of the prospective, noninterventional, multicenter AHEAD study (104 study centers). The primary endpoint is overall Gilbert score (primary endpoint; assessed using pain, bleeding, and physical examination). Secondary endpoints include annualized bleeding rates (ABR) and joint bleeding rates (AJBRs), EuroQol‐5 dimensions (EQ‐5D), and safety. Ethics committee approval and informed consent were obtained.
Results: 868 patients were enrolled; 287 had moderate HA; 116 (40%) received standard, 33 (11%) PK‐guided, and 50 unspecified prophylaxis. 86 patients (30%) were treated on‐demand and 2 received immune tolerance induction. Average Gilbert scores (all joints) were low across treatment groups (Mean [SD], years 1–7: standard, 0.0–3.1 [0.0–4.2; n = 1–33], PK‐guided: 0.0–3.0 [0.0–4.2; n = 1–5], on‐demand: 1.1–2.5 [1.5–2.7; n = 0–15]). ABRs and AJBRs were consistently lower, and zero bleed rates were consistently higher, in patients receiving prophylaxis versus on‐demand therapy (Tables 1 and 2). EQ‐5D visual analog scale scores were similar between patients receiving standard (Mean, years 1–8: 60.0–99.0 [n = 1–53]) and PK‐guided (57.5–89.0 [n = 1–10]) prophylaxis. Adverse events (AEs) were experienced by 454 (52.3%) of 868 patients, with serious AEs reported in 159 (18.3%) patients; 16 AEs (1.8%) were related to octocog alfa, of which 11 were serious AEs.
Conclusion(s): These real‐world findings from the 8‐year AHEAD interim data read‐out suggest that patients with moderate HA may also benefit from a prophylaxis regimen with octocog alfa.
OC 63.1
Decreased thrombin generation profile is associated with severe bleeding phenotype in hemophilia: Data from the hemophilia in the Netherlands study
M. Verhagen 1; W. van Heerde2; J. van der Bom3; E. Beckers4; N. Blijlevens5; M. Coppens6; S. Gouw7; J. Jansen8; F. Leebeek9; L. van Vulpen10; D. Meijer8; S. Schols11
1 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, Nijmegen, the Netherlands; Laboratory of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 2 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, the Netherlands; Enzyre BV, Novio Tech Campus, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 3 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands; Center for Clinical Transfusion Research, Sanquin‐Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 4 Department of Hematology, Maastricht University Medical Center, Maastricht University, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 5 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 6 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 7 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 8 Department of Laboratory Medicine, Laboratory of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 9 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 10 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, University Utrecht, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands, 11 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands
Background: Heterogeneity in clinical bleeding phenotype is observed in hemophilia patients with similar factor VIII or factor IX activity levels. Thrombin generation (TG) as a global hemostasis assay may contribute to better predict which patients are at increased risk of bleeding.
Aims: To quantify the association between clinical bleeding phenotype and TG profile in hemophilia patients.
Methods: The Nijmegen Hemostasis Assay (NHA), which simultaneously measures TG and plasmin generation, was performed in 863 hemophilia patients (adults and children) originating from the sixth Hemophilia in the Netherlands study. Patients using prophylaxis underwent a washout period. Ethical approval and written informed consent were obtained. Severe clinical bleeding phenotype was defined as an annual bleeding rate (ABR) ≥5 or an annual joint bleeding rate (AJBR) ≥3. Correlations were determined using Spearman’s correlation test.
Results: TG parameters significantly differed between hemophilia patients and healthy controls (thrombin peak height (TPH) in severe 1.0nM [IQR 1.0‐1.0], moderate 25.5 nM [1.0‐46.5], mild hemophilia A 47.4 nM [27.9‐71.1], and controls 266.5 nM [252.8‐280.6]; thrombin potential in severe 1.0 nM·min [1.0‐1.0], moderate 607.6 nM·min [1.0‐1083.0], mild hemophilia A 1034.0 nM·min [668.4‐1398.0], and controls 2088.0 nM·min [1988.0‐2175.0]). Weak but significant correlations were found between clinical bleeding phenotype and TPH (ABR r = ‐0.2571; AJBR r = ‐0.2776) or thrombin potential (ABR r = ‐0.2752; AJBR r = ‐0.3084) (Figure 1). A severe clinical bleeding phenotype was observed in patients with a TPH < 35% and a thrombin potential < 70%, independent of hemophilia severity. Median TPH and thrombin potential were significantly lower in patients with a severe clinical bleeding phenotype versus a mild clinical bleeding phenotype (TPH: 0.4% vs 17%; thrombin potential 0.05% vs 46%) (Figure 2).
Conclusion(s): A decreased TG profile is associated with severe clinical bleeding phenotype in hemophilia patients. TG parameters in combination with clinical bleeding severity may be better tools to personalize prophylactic replacement therapy, irrespective of hemophilia severity.
OC 20.2
Artificial intelligence for the point‐of‐care ultrasound‐based detection of joint effusion in patients with hemophilia
R. Gualtierotti 1; S. Arcudi2; M. Colussi3; S. Mascetti3; G. Cafasso4; E. Biguzzi5; C. Bettini3; F. Peyvandi6
1 Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, MILAN, Lombardia, Italy; 3 Department of Computer Science, University of Milan, Milan, Lombardia, Italy; 4 Division of Hematology, University of Milan, Milan, Italy., Milan, Lombardia, Italy; 5 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 6 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Musculoskeletal ultrasound (MSK‐US) is a non‐invasive and easily accessible diagnostic tool for joint health assessment of patients with hemophilia. The early identification of hemarthrosis by a remote MSK‐US‐based system performed by general practitioners or the patients themselves sending images to the Comprehensive Care Center could lead to a better personalized management of patients with hemophilia. A computer‐aided diagnosis (CAD) system for the automatic detection of joint effusion could support the physicians in prioritizing interventions
Aims: Our aim was to assess the feasibility of the CAD system by developing a deep‐learning algorithm to automatically recognize joint capsule distension in MSK‐US images.
Methods: Images of the suprapatellar bursa longitudinal scan (SPB‐LS) of the knee at 30°‐flexion were collected and labeled by an expert MSK‐US operator. The learning algorithm is based on an object detection framework that is trained to detect the normal and the distended joint recesses
Results: We recruited two‐hundred consecutive adult patients with hemophilia referring to our Center from October 2020 to December 2021, aged 44.7 ± 18.6 years and 50 sex‐ and age‐matched healthy controls. A total of 8,634 MSK‐US images were collected (2,267 knee scans). Of these, 450 were considered valid (SPB‐LS) and used for the learning task. We used 330 images for training and 120 images for testing the classifier, leading to the detection of joint recess distensions with an 88% accuracy (80% sensitivity; 93% specificity; Figure 1).
Conclusion(s): Our AI‐based algorithm showed that the CAD system for the automatic detection of joint recess distension in patients with hemophilia is feasible. Performance may further improve with a larger training set. This is the first step for the development of a telemedicine system to early detect hemarthrosis in patients with hemophilia, thus prompting early personalized management with the most appropriate therapeutic intervention. FIGURE 1 Longitudinal scan of a normal suprapatellar bursa of the knee at 30°‐flexion. Orange square indicates the rotula, the light blue square identifies the suprapatellar bursa or joint recess and the green square corresponds to the femur
FIGURE 1 Longitudinal scan of a normal suprapatellar bursa of the knee at 30°‐flexion. Orange square indicates the rotula, the light blue square identifies the suprapatellar bursa or joint recess and the green square corresponds to the femur
OC 47.4
A post hoc analysis of individuals with severe hemophilia A and inhibitors from the PUPs A‐LONG study
M. Carcao 1; M. Schiavulli2; R. Kulkarni3; P. Rendo4; M. Foster5; E. Santagostino6; S. Casiano5; C. Königs7
1 Division of Hematology/Oncology, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada; 2 A.O.R.N. Santobono‐Pausilipon, Napes, Campania, Italy; 3 Michigan State University, East Lansing, Michigan, United States; 4 Sanofi, Waltham, Massachusetts, United States; 5 Sanofi, Cambridge, Massachusetts, United States; 6 Sobi, Stockholm, Stockholms Lan, Sweden; 7 University Hospital Frankfurt, Goethe University, Frankfurt, Hessen, Germany
Background: PUPs A‐LONG evaluated extended half‐life recombinant factor VIII Fc fusion protein (rFVIIIFc) in previously untreated patients (PUPs) with severe hemophilia A.
Aims: Provide a descriptive post hoc analysis of inhibitor subjects from PUPs A‐LONG.
Methods: PUPs A‐LONG was an open‐label, multicenter, Phase 3 study (NCT02234323) evaluating safety and efficacy of rFVIIIFc in male PUPs < 6 years old with severe hemophilia A. The primary endpoint was occurrence of inhibitor development, defined as 2 inhibitor tests of ≥0.6 BU/mL (low titer: ≥0.6 and < 5 BU/mL; high titer: ≥5 BU/mL) ≥2 weeks apart. Post hoc analyses included patient treatment regimen patterns and timing of inhibitor development, descriptive statistics of first inhibitor development, Kaplan–Meier analyses of time to first inhibitor‐positive test by treatment regimen and by titer, and consumption.
Results: Twenty‐eight of 103 subjects (27%) who received rFVIIIFc developed an inhibitor during PUPs A‐LONG (Table 1). Twenty‐four of these subjects were on prophylaxis as their final treatment regimen, of which 18 (75%) switched from on demand (Figure 1). Of 14 subjects with high‐titer inhibitors (HTI), half (n = 7) had prior low‐titer inhibitors (LTI). Inhibitor development followed central venous access device (CVAD) placement in 17% of subjects who underwent the procedure (n = 7/42) and in 20% of subjects who had intense factor exposure (IFE) (n = 9/44). Five of 9 subjects (56%) experienced IFE due to CVAD placement (Table 1). Matching inhibitor‐positive and ‐negative subjects with IFE by exposure days indicated no significant difference between groups in cumulative dose (IU/kg) administered until inhibitor development, nor was there correlation between groups (unmatched) for total consumption over time.
Conclusion(s): PUPs A‐LONG post hoc analyses show half of HTI subjects initially develop LTI. The frequency of inhibitor development was comparable following IFE or CVAD placement, although analyses are based on limited patient numbers. Identifying factors that predispose individuals to inhibitor development remains a priority.
OC 47.2
Prevalence and characteristics of non‐neutralizing FVIII‐specific antibodies in persons with hemophilia A – preliminary results
I. Oomen 1; M. Miranda2; P. Allacher3; E. Beckers4; M. Coppens5; M. Driessens6; J. Eikenboom7; K. Fijnvandraat1; S. Hassan8; L. Hooimeijer9; P. Kaijen2; F. Leebeek10; F. Rosendaal11; S. Schols12; H. Schweiger13; C. Smit14; L. van Vulpen15; J. van der Bom11; J. Voorberg2; S. Gouw16
1 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Institute Krems Bioanalytics, IMC University of Applied Sciences Krems, Krems, Niederosterreich, Austria; 4 Department of Hematology, Maastricht University Medical Center, Maastricht University, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 5 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 6 VSOP, Dutch organisation for rare and genetic disorders, Soest, Utrecht, Netherlands; 7 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 8 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 9 Pediatric Hematology, Beatrix Children’s Hospital, University Medical Center Groningen, Groningen, Groningen, Netherlands, 10 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 11 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands, 12 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands, 13 Institute Krems Bioanalytics, IMC University of Applied Sciences Krems, Krems, Austria, Krems, Niederosterreich, Austria, 14 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands, 15 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, University Utrecht, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands, 16 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Development of neutralizing antibodies (inhibitors) is a major complication of hemophilia A treatment, which renders replacement therapy ineffective and increases susceptibility to major bleeding. Non‐neutralizing FVIII‐specific antibodies (NNA) that appear to increase clearance of exogenous clotting factors and limit its hemostatic efficacy have been identified recently. We hypothesize that FVIII‐binding antibodies form a spectrum from low titer, low affinity NNA and high affinity antibodies with or without neutralizing potential, to inhibitors detectable with the widely used Bethesda assay, depicted in Figure 1. We further hypothesize that a subset of NNA are in fact low‐titer inhibitors with titers below the lowest detection limit of the Bethesda assay.
Aims: To assess the prevalence and characteristics of NNA among persons with hemophilia A (PwHA), and to investigate whether these NNA have neutralizing potential.
Methods: PwHA with an available plasma sample were included from the cross‐sectional unselected nationwide ‘Hemophilia in the Netherlands’ (HIN) cohort. The NNA prevalence and isotype characteristics (IgA, IgM and IgG subclasses) are assessed in the overall population using a highly sensitive and fully validated ELISA. Samples with confirmed specificity for FVIII will be assessed for apparent affinity with a competition‐based ELISA approach. The presence of inhibitors will be assessed using the Nijmegen Bethesda assay and the novel Nijmegen low‐titer inhibitor assay.
Results: In total, 801 PwHA were included. 71 patients (8.9%) had a positive Nijmegen low‐titer inhibitor assay, of whom 15 (21.1%) had also a positive Nijmegen Bethesda Assay (Figure 2). Results of NNA prevalence and characteristics will be available at the time of the ISTH 2022.
Conclusion(s): This study is the first to describe the full spectrum of anti‐FVIII antibodies in an unselected population of PwHA. Improved knowledge of the prevalence and characteristics of NNA will eventually contribute to understanding of differences in pharmacokinetics and potentially identify patients at risk for inhibitor development.
OC 30.1
Emicizumab prophylaxis in people with severe haemophilia A without inhibitors: Outcomes from the UK Haemophilia Centre Doctors' Organisation
C. Wall 1; H. Xiang2; B. Palmer2; P. Chowdary3; P. Collins4; G. Hall5; M. Mathias6; C. Percy7; P. Sartain2; S. Shapiro8; D. Stephensen9; K. Talks10; C. Hay1
1 Manchester Royal Infirmary, Manchester, England, United Kingdom; 2 National Haemophilia Database, Manchester, England, United Kingdom; 3 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom; 4 Medical School of Cardiff University, Cardiff, Wales, United Kingdom; 5 Oxford Haemophilia and Thrombosis Centre, Oxford, England, United Kingdom; 6 Great Ormond Street Hospital for Children, NHS Foundation Trust, London, England, United Kingdom; 7 University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 8 Oxford University Hospitals National Health Service Foundation Trust, University of Oxford, and Oxford National Institute for Health Research Biomedical Research Centre, Oxford, England, United Kingdom; 9 East Kent Hospitals University NHS Trust, Faculty of Health and Wellbeing, Canterbury Christ Church University, Canterbury, England, United Kingdom, 10 Newcastle Haemophilia Comprehensive Care Centre, Royal Victoria Infirmary, Newcastle upon Tyne, England, United Kingdom
Background: The uptake of emicizumab in persons with severe haemophilia A (pSHA) without inhibitors is heterogeneous. We present UK outcomes since its introduction in 2019.
Aims: To determine bleeding outcomes in pSHA treated with emicizumab in UK clinical practice
Methods: An observational study was conducted of 673 pSHA who switched from FVIII prophylaxis to emicizumab between 01/08/19 and 30/09/21. A within‐person comparison of annualised bleed (ABR) and joint‐bleed rate (AJBR) with prior FVIII prophylaxis was conducted in people with ≥6 months pre and post‐switch Haemtrack home‐therapy data using Wilcoxon signed rank test. Change in proportion reporting zero‐treated bleeds was analysed using the McNemar test.
Results: Emicizumab was prescribed to 673 non‐inhibitor pSHA, 36.9% of the UK‐registered cohort, including 85 (12.6%) with an inhibitor history. A within‐person comparison with previous FVIII prophylaxis was conducted in 401 pSHA broken down by age (table 1). A within‐person sub‐analysis of 144 people reporting bleeding (table 2) showed that 73% (105/144) had a reduced bleeding after switching (p < 0.001). Relatively few subjects had target joints using ISTH criteria; 35 switchers had 51 target joints and 45 non‐switchers had 69 target joints. After a median 21 months follow‐up, 74% of emicizumab switchers experienced fewer and 6% more target joints versus 42% fewer and 33% more target joints in non‐switchers (p = 0.004). Recurrent FVIII inhibitors were reported in 4/85 (4.7%) people at risk. One person (0.15%) developed a low level anti‐drug antibody (ADA).
Conclusion(s): Switching to emicizumab resulted in significantly improved bleed control in all age groups (p < 0.001) and in 65‐80% no treated bleeds were reported. In subjects who reported bleeding, clinically and statistically significant reductions in ABR were observed. Target joints resolved more frequently than in people who continued FVIII prophylaxis. ADAs were uncommon and recurrent FVIII inhibitors occurred in ~5% of those at risk.
OC 27.1
The type and frequency of bleeds in 870 children with severe hemophilia A and B on primary prophylaxis: Data from the PedNet study group
H. van den Berg 1; M. Alvarez Roman2; M. Carcao3; H. Chambost4; K. Fischer5; N. Grentenkort‐Andersson6; G. Kenet7; C. Königs8; R. Ljung9; C. Male10; J. Motwani11
1 PedNet, Baarn, Utrecht, Netherlands; 2 Unidad de Coagulopatías, Hopital Universitario La Paz, Madird, Castilla‐La Mancha, Spain; 3 Division of Hematology/Oncology, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada; 4 APHM, Department of Pediatric Hematology Oncology, La Timone Children’s Hospital & Aix Marseille Univ, INSERM, INRA, C2VN, Marseille, Provence‐Alpes‐Cote d'Azur, France; 5 University Medical Center Utrecht, University Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands; 6 Department of Clinical Sciences, Lund University, Lund; Department of Pediatrics and Malmö Centre for Thrombosis and Haemostasis, Skåne University Hospital, Malmo, Skane Lan, Sweden; 7 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 8 University Hospital Frankfurt, Goethe University, Frankfurt, Hessen, Germany; 9 Lund University, Department of Clinical Sciences, Malmo, Skane Lan, Sweden, 10 Medical University of Vienna, Austria, Vienna, Wien, Austria, 11 Birmingham Women's and Children's NHS Foundation Trust, Birmingham, England, United Kingdom
Background: Few data exist on long‐term follow‐up of type and frequency of bleedings in children 0‐18 years with severe hemophilia on primary prophylaxis with FVIII/FIX.
Aims: Comparison of reported bleeds in real‐world data between different age groups in children with severe hemophilia A (SHA) or B (SHB) on primary prophylaxis.
Methods: The PedNet registry is a multicenter prospective observational cohort study (Clin.gov.trial NCT02979119). For this study we included all children with SHA or SHB without history of inhibitors born between 2000‐2020. Data of all bleeds were collected from the start of prophylaxis until January 2021. Bleeds were defined according to the ISTH definition. Bleeding severity was defined as minor when characterized by mild pain, minimal swelling, minimal motion restriction and resolving within 24 hours of treatment, and as major when symptoms persisted > 24 hours. Annual bleeding rate (ABR) was based on reported bleeds, using a negative binominal model. Patients were divided into three age groups: 0‐5 years, 6‐11 years, and 12‐18 years.
Results: We included 737 children with SHA and 133 with SHB. The total follow‐up years on prophylaxis for SHA was 5838, median 7.5, (IQR 4.0‐11.6) and for SHB 1042 years, median 7.7 (IQR 3.7‐11.1). Total ABR was 2.8 for SHA and 2.0 for SHB, major joint bleeds were a median (IQR) of 0.36 (0.30‐0.43) for SHA and 0.28 (0.21‐0.37) for SHB over all three age groups. Minor joint bleeds were a median (IQR) 0.56 (0.51‐0.61) for SHA and 0.42(0.34‐0.52) for SHB. Minor other bleeds were reported as 1.9 (1.7‐2.1) for SHA 1.4(1.1‐1.7) for SHB and between 0‐5 years (Figure 1).
Conclusion(s): Primary prophylaxis was equally protective in all groups. Reported bleeds in SHB were lower in all groups. In younger patients, the higher reporting of minor bleeds might reflect inadequate prophylaxis or high parental anxiety. FIGURE 1 Comparison of type of bleeds (ABR) in severe hemophilia A and B between 0‐5, 6‐11, and 12‐18 years
FIGURE 1 Comparison of type of bleeds (ABR) in severe hemophilia A and B between 0‐5, 6‐11, and 12‐18 years
OC 50.2
Fitusiran, an investigational siRNA therapeutic targeting antithrombin: Analysis of antithrombin levels and thrombin generation from a phase 3 study in people with hemophilia A or B without inhibitors
S. Pipe 1; A. Srivastava2; R. Klamroth3; G. Kenet4; H. Tran5; L. Fetita6; Z. Qiu7; S. Andersson8; B. Mei8; S. Rangarajan9
1 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States; 2 Christian Medical College, Vellore, India, Vellore, Tamil Nadu, India; 3 Vivantes Klinikum, Friedrichshain, Berlin, Berlin, Germany; 4 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 5 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia; 6 Sanofi Genzyme, Paris, France, Paris, Ile‐de‐France, France; 7 Sanofi, Bridgewater, NJ, USA, Bridgewater, New Jersey, United States; 8 Sanofi, Cambridge, MA, USA, Cambridge, Massachusetts, United States; 9 University of Southampton, Southhampton, England, United Kingdom
Background: Hemophilia is characterized by deficiency of FVIII or FIX resulting in impaired thrombin generation (TG) and ineffective clot formation. Fitusiran is an investigational siRNA therapeutic which targets antithrombin to enhance TG and rebalance hemostasis in people with hemophilia (PwH) A or B, irrespective of inhibitor status.
Aims: Longitudinal assessment of changes in antithrombin levels and TG over time in PwH A or B without inhibitors with fitusiran prophylaxis.
Methods: This analysis of a Phase 3, multinational, randomised, open‐label study (NCT03417245) included males aged ≥12 years with severe hemophilia A or B without inhibitors, previously treated on‐demand. Participants were randomised 2:1 to receive once‐monthly 80 mg subcutaneous fitusiran prophylaxis (fitusiran arm) or on‐demand factor concentrates (OD arm) for 9 months. The primary endpoint was annualized bleeding rate (ABR). Exploratory endpoints included changes in antithrombin levels and mean peak height assessed by TG over time.
Results: Overall, 120 participants were randomised; 79 (98.8%) in the fitusiran arm and 37 (92.5%) in the OD arm completed the study. On day 15, there was a 71.6% mean reduction from baseline in antithrombin levels in the fitusiran arm, with a further reduction to 79.8% on day 29 and maintained at 85.3%–88.6% from day 43 onwards (figure 1). There was a mean increase in TG of 17.1 nM from baseline in the fitusiran arm on day 15, increasing to 24.4 nM on day 29 and maintained at 29.8.1–43.1 nM from day 43 onwards (figure 2). These results corresponded with an 89.9% reduction in estimated ABR with fitusiran vs OD factor concentrates.
Conclusion(s): Fitusiran reached target pharmacodynamic effect of antithrombin lowering and increased TG by day 29 and demonstrated a consistent effect throughout the study. These findings and a reduction in ABR suggest fitusiran has the potential to rebalance hemostasis in PwH A or B without inhibitors.
Hemophilia Gene Therapy
LB 01.3
Relationship between transgene‐produced FVIII and bleeding rates 2 years after gene transfer with valoctocogene roxaparvovec: Results from GENEr8‐1
J. Mahlangu 1; H. Chambost2; S. Chou3; A. Dunn4; A. von Drygalski5; R. Kaczmarek6; G. Kenet7; M. Laffan8; A. Leavitt9; B. Madan10; J. Mason11; J. Oldenburg12; M. Ozelo13; F. Peyvandi14; D. Quon15; M. Reding16; S. Shapiro17; H. Yu18; T. Robinson18; S. Pipe19
1 University of the Witwatersrand, National Health Laboratory Service and Hemophilia Comprehensive Care Center, Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, Gauteng, South Africa; 2 APHM, Department of Pediatric Hematology Oncology, La Timone Children’s Hospital & Aix Marseille Univ, INSERM, INRA, C2VN, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Keelung, Taiwan (Republic of China); 4 Nationwide Children's Hospital Division of Hematology, Oncology, and Bone Marrow Transplant and The Ohio State University College of Medicine, Columbus, Ohio, United States; 5 Division of Hematology/Oncology, Department of Medicine, University of California San Diego, San Diego, California, United States; 6 Indiana University School of Medicine, IUPUI‐Wells Center for Pediatric Research, and Hirszfeld Institute of Immunology and Experimental Therapy, Indianapolis, Indiana, United States; 7 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 8 Centre for Haematology, Imperial College London, London, England, United Kingdom; 9 University of California San Francisco, San Francisco, California, United States, 10 Guy's & St. Thomas' NHS Foundation Trust, London, England, United Kingdom, 11 Queensland Haemophilia Centre, Royal Brisbane and Women's Hospital, and University of Queensland, Brisbane, Queensland, Australia, 12 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany, 13 Hemocentro UNICAMP, Department of Internal Medicine, School of Medical Sciences, University of Campinas, Campinas, Sao Paulo, Brazil, 14 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy, 15 Orthopaedic Hemophilia Treatment Center, Los Angeles, California, United States, 16 Center for Bleeding and Clotting Disorders, University of Minnesota, Minneapolis, Minnesota, United States, 17 Oxford University Hospitals National Health Service Foundation Trust, University of Oxford, and Oxford National Institute for Health Research Biomedical Research Centre, Oxford, England, United Kingdom, 18 BioMarin Pharmaceutical, Inc., Novato, California, United States, 19 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States
Background: Valoctocogene roxaparvovec (AAV5‐hFVIII‐SQ) gene transfer reduced bleeding through endogenous factor VIII (FVIII) production over 2 years in people with severe hemophilia A. The relationship between transgene‐produced FVIII activity levels and bleed frequency is not well characterized.
Aims: To assess the relationship between endogenous, transgene‐derived FVIII activity and bleeding using phase 3 trial data.
Methods: The open‐label, multicenter phase 3 trial GENEr8‐1 (NCT03370913) evaluated efficacy and safety of 6 × 1013 vg/kg valoctocogene roxaparvovec in adult men with severe hemophilia A (FVIII ≤1 IU/dL) without inhibitors or anti‐AAV5 antibodies. Bleeds and FVIII treatment were self‐reported and counted from the end of regular prophylaxis (scheduled for week 4) to data cutoff. Follow‐up was divided into 4‐ or 6‐week intervals, where the number of treated joint bleeds was paired with median FVIII activity. Baseline comparisons used the rollover population (112 participants enrolling from a noninterventional study). A negative binomial regression model predicting annualized joint bleeding rate and 95% confidence interval (CI) for given FVIII activity was built using data from all 134 participants.
Results: Median follow‐up was 110.9 weeks (n = 134). Overall, 83 (61.9%) participants reported 450 bleeds; 39 (29.1%) participants reported 268 treated bleeds. Of treated bleeds, 56.0% were joint bleeds. In the rollover population, the proportion of participants with no bleeds, regardless of treatment, increased during each year (Table). Using data from 134 participants, the negative binomial model predicted increasing bleeds with decreasing FVIII; 1.4 (95% CI, 0.9–2.2) and 1.0 (95% CI, 0.7–1.5) treated joint bleeds/year were predicted with FVIII of 5 IU/dL per one‐stage and chromogenic assay, respectively (Figure).
Conclusion(s): Estimates of treated joint bleeding rates per FVIII activity, particularly using the one‐stage assay, align with those using epidemiological data in people with hemophilia A on standard therapy, suggesting transgene‐derived FVIII provides similar protection as native or exogenous FVIII.

OC 12.5
Liver sinusoidal endothelial cells targeted with ultrasound mediated gene delivery shows long term FVIII expression in hemophilia A mice
S. Lawton 1; M. Manson2; M. Fan3; T. Chao3; P. Kim3; C. Campbell3; C. Chen3; X. Cai4; A. Vander Kooi3; C. Miao3
1 Seattle Children's Research Institute, SEATTLE, Washington, United States; 2 Seattle Children's Research Institute ‐ Seattle, Washington, Seattle, Washington, United States; 3 Seattle Children's Research Institute, Seattle, Washington, United States; 4 Seattle Children's Research Institute, seattle, Washington, United States
Background: Ultrasound‐mediated gene delivery (UMGD) with microbubbles (MBs) is a potentially effective method of non‐viral gene delivery to treat hemophilia A (HA). Previously, we found liver sinusoidal endothelial cells (LSECs) could be targeted with UMGD at a lower power than hepatocytes. We hypothesized that gene expression with lower energy conditions would be comparable to higher energy conditions, however, with less damage to the liver or induction of anti‐FVIII inhibitors.
Aims: In this study, we explored US conditions that target LSECs with UMGD to induce sustained FVIII expression in HA mice.
Methods: Mouse liver was injected via the portal vein with a combination of plasmid DNA and MBs. Simultaneously, a pulsed therapeutic US transducer was applied to the liver surface for one minute at 1.1 MHz frequency and 14 Hz PRF. HA mice were separated into two different US condition groups, low energy (LE; 50 W/cm2, 150us PD) targeting predominantly endothelial cells, or high energy (HE; 110 W/cm2, 150 us PD) targeting predominantly hepatocytes. A high‐expressing, endothelial‐specific hFVIII plasmid, pUCOE‐ICAM2‐hF8/N6‐X10, was used. To study hFVIII activity over 84 days, APTT was run on plasma. Livers were sectioned, stained, and imaged with a Leica DM6000 fluorescent microscope.
Results: FVIII activity levels for both the LE and HE groups stabilized around 10% at 84 days post‐treatment (Figure 1). RNAscope©® Multiplex Fluorescent staining showed colocalization of hFVIII and Lyve‐1 (LSEC marker) at D7 and D120. Transaminase levels indicated the LE group had lower transient liver damage than the HE group in the first week with both groups returning to baseline by week two.
Conclusion(s): We show LSECs can be targeted for transfection using lower energy than a previously established hepatocyte targeting condition and produced FVIII activity over 84 days. The ability to target different cell types with UMGD can be applied to a multitude of genetic conditions. FIGURE 1 HA mice in both the LE and HE ultrasound treatment groups had hFVIII activity levels between 5‐25% with stabilization of around 10% at day 84
FIGURE 1 HA mice in both the LE and HE ultrasound treatment groups had hFVIII activity levels between 5‐25% with stabilization of around 10% at day 84 FIGURE 2 Livers from LE mice harvested at day 7 (A) and day 120 (B) showed expression of hFVIII mRNA shown in green, and Lyve‐1 mRNA, an endothelial‐specific marker, in red. Colocalization appears yellow
FIGURE 2 Livers from LE mice harvested at day 7 (A) and day 120 (B) showed expression of hFVIII mRNA shown in green, and Lyve‐1 mRNA, an endothelial‐specific marker, in red. Colocalization appears yellow
OC 01.5
Innate and adaptive immune responses to adeno‐associated viral gene therapy in the severe hemophilia A dog model
P. Batty 1; A. Mo2; M. Ashrafali3; B. Yates3; A. Gonzalez3; B. Handyside3; L. Harpell2; D. Hurlbut2; A. Menard4; A. Pender2; L. Razon3; C. Sihn3; A. Winterborn2; S. Fong3; D. Lillicrap5
1 University College London, London, United Kingdom; 2 Queen's University, Canada, Kingston, Ontario, Canada; 3 BioMarin Pharmaceutical, USA, Novato, California, United States; 4 Kingston Health Sciences Center, Canada, Kingston, Ontario, Canada; 5 Department of Pathology and Molecular Medicine, Queen’s University, Kingston, Ontario, Canada
Background: Adeno‐associated viral (AAV) gene therapy is under investigation for the treatment of severe hemophilia. The predominant adverse event in clinical trials has been elevation of alanine aminotransferase (ALT) levels. Although capsid directed cytotoxic T‐cell responses have been reported, the underlying mechanism(s) remain unclear.
Aims: To evaluate the early immune response to AAV5‐cFVIII in the severe hemophilia dog model.
Methods: Severe hemophilia A dogs were treated using one of three AAV5‐B‐domain deleted canine Factor VIII constructs with a hybrid liver promoter (AAV5‐HLP‐cFVIII, Table 1). Capsid immune responses were evaluated using total antibody and ELISPOT assays. Serum innate immune responses were evaluated using a cytokine array (n = 16) and complement function. Vector genome (VG) copies and cFVIII mRNA were quantified in liver biopsies at baseline and 3‐months post‐treatment using ddPCR.
Results: Ten male hemophilia dogs (median = 4.3 years, 0.8 – 9.0 years) received a single AAV5‐HLP‐cFVIII infusion (dose = 6.0e13 – 2.0e14 vg/kg). FVIII expression was seen for dogs treated with the codon‐optimized constructs (n = 7). Dose response was seen at 3 months post‐treatment, with higher one‐stage FVIII:C in the high‐dose (2e14 vg/kg, mean = 15.6 ± 0.3 IU/dL) compared to the low‐dose cohort (6‐8e13 vg/kg, mean = 6.2 ± 1.0 IU/dL). Liver biopsies at 3‐months demonstrated AAV5‐HLP‐cFVIII in all animals, with correlation between VG and cFVIII mRNA copies (r = 0.8, p = 0.01). Anti‐AAV5 capsid antibodies were detected starting day 7 post‐treatment. No new FVIII inhibitors were detected. Dogs displayed variable serum cytokine profiles, with no change in mean levels 24‐hours post‐dosing. Higher baseline and post‐dose levels of TNF‐alpha, IL2, IL6, IL7, IL15 and IL18 were observed in the dog with a FVIII inhibitor history (MEM2018). No capsid specific T‐cell responses or complement activation was detected.
Conclusion(s): FVIII expression was seen using co‐AAV5‐HLP‐cFVIII vectors in the hemophilia dog model. No blood biomarkers of innate immune activation were detected, with further liver studies ongoing. TABLE 1 Outcomes following AAV5‐HLP‐cFVIII in the severe hemophilia A dog model. HLP = Hybrid liver promoter. nco = non‐codon optimized. co = codon optimized. dg = diploid genome. OSA = One stage FVIII:C (canine normal pooled standard). Follow up as of January 17th 2021 or study end‐point. * History of FVIII inhibitor. WBCT = Whole blood clot time (normal: 4‐6 minutes). n/a = not available
TABLE 1 Outcomes following AAV5‐HLP‐cFVIII in the severe hemophilia A dog model. HLP = Hybrid liver promoter. nco = non‐codon optimized. co = codon optimized. dg = diploid genome. OSA = One stage FVIII:C (canine normal pooled standard). Follow up as of January 17th 2021 or study end‐point. * History of FVIII inhibitor. WBCT = Whole blood clot time (normal: 4‐6 minutes). n/a = not available
OC 01.3
Elucidating the mechanism behind the AAV‐derived factor VIII assay discrepancy
A. Sternberg; B. Samelson‐Jones; L. George
The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States
Background: Hemophilia A (HA) AAV‐mediated gene transfer clinical trials demonstrated transgene‐derived FVIII:C is ~2‐fold higher by one‐stage clotting assay (OSA) than chromogenic assay (CSA). Plasma FVIII antigen (FVIII:Ag) concentrations correlate with CSA‐determined FVIII:C. The underlying mechanism is unclear and curious, particularly because this is not observed with recombinant proteins of the same amino acid sequence. Possible hypotheses that may explain the OSA vs CSA‐determined FVIII:C differences include enhanced activation (e.g. altered vWF affinity or enhanced thrombin or FXa cleavage) or, alternatively, differences in the function of the transgene‐derived FVIIIa species. Understanding the mechanism(s) of the OSA/CSA discrepancy is necessary to determine which assay best represents in vivo AAV‐derived FVIII hemostatic efficacy and thereby patient management.
Aims: We sought to interrogate the mechanism of the OSA/CSA discrepancy of transgene‐derived FVIII.
Methods: Male HA/CD4KO or HA/vWF‐/‐ mice were infused with AAV8‐BDD‐hFVIII (1E11‐5E11 vg/mouse). FVIII:C was measured by OSA and CSA against a standard curve of recombinant BDD‐hFVIII (rFVIII) purified from BHK cells reconstituted in mouse plasma. Thrombin generation assays (TGA) and 2‐stage clotting assays (2SA) were also performed.
Results: Like humans, transgene‐derived FVIII:C in mice determined by OSA was ~2‐fold CSA‐determined FVIII:C, and FVIII:Ag closely correlated with CSA FVIII:C. Consistent with prior human studies, shortened lag time in a TGA was recapitulated in plasma from AAV‐treated HA mice. The OSA/CSA discrepancy was maintained in a vWF‐free system. FVIII:C determined by 2SA was higher for transgene‐derived FVIII compared to rFVIII.
Conclusion(s): These data demonstrate the OSA/CSA discrepancy is observed in humans and mice, and therefore not species specific. The discrepency was maintained in the absence of vWF, supporting that it is not related to interactions with vWF. Interestingly, FVIII:C by 2SA is higher for transgene‐derived versus recombinant FVIII, suggesting gene therapy derived FVIIIa has enhanced function relative to rFVIII.
OC 21.5
Comparative effectiveness of valoctocogene roxaparvovec and prophylactic factor VIII replacement estimated through propensity scoring
H. Liu1; C. Hawes2; C. Hsu1; P. You1; X. Yang1; V. Newman2; T. Robinson1; A. Hatswell 3; D. Hinds1
1 BioMarin Pharmaceutical, Inc., Novato, California, United States; 2 BioMarin Europe Ltd, London, England, United Kingdom; 3 Delta Hat, Nottingham, England, United Kingdom
Background: A prospective, noninterventional study (NIS) followed 294 adults with severe hemophilia A (SHA) receiving prophylactic factor VIII (FVIII). Of these participants, 112 rolled over into a single‐arm, multicenter, phase 3 trial (GENEr8‐1; NCT03370913), which evaluated the efficacy and safety of valoctocogene roxaparvovec—an adeno‐associated virus serotype 5 (AAV5) gene therapy that transfers a B‐domain deleted FVIII cDNA to hepatocytes—in adults with SHA without preexisting anti‐AAV5 antibodies or FVIII inhibitors.
Aims: To compare bleeding outcomes among adults with SHA treated with valoctocogene roxaparvovec vs prophylactic FVIII replacement using propensity scoring.
Methods: Post‐hoc analysis of the 112 participants who rolled over into GENEr8‐1 (intervention cohort) compared to 73 participants enrolled in the NIS who were negative for FVIII inhibitors, anti‐AAV5 antibodies, and HIV; had ≥6 months follow‐up; and did not enroll in GENEr8‐1 (control cohort). Comparable cohorts were generated based on propensity scores (PS) using standardized mortality ratio weighting (SMRW) to weight the control cohort to match baseline characteristics of the intervention cohort. Cohorts were compared regarding mean annualized bleeding rate (ABR, treated and all bleeds) and the proportion of participants with zero bleeds (treated and all bleeds). Additional PS adjustment methodologies were evaluated.
Results: Baseline characteristics between cohorts were more similar after PS adjustment with generally smaller standardized mean differences (SMD) post‐weighting (Table 1). Mean treated and all bleeds ABR were significantly lower (absolute differences of –3.6 (p < 0.001) and –3.6 (p < 0.001), respectively) in the intervention vs control cohorts (Table 2). Proportions of participants with zero treated (79.5% vs 32.9%; p < 0.001) and all bleeds (52.7% vs 28.5%; p = 0.003) were significantly higher in the intervention vs control cohorts (Table 2).
Conclusion(s): Results of PS analysis were consistent with GENEr8‐1 findings, with participants receiving valoctocogene roxaparvovec demonstrating lower ABRs and higher proportions of participants with zero bleeds than participants receiving prophylactic FVIII. Funded by BioMarin.
OC 01.1
Base and prime editing of DNA as a new therapeutic option for hemophilia A
E. Tonetto 1; S. Pignani2; G. Roman2; A. Follenzi3; F. Bernardi4; D. Liu5; M. Pinotti1; D. Balestra1
1 Department of Life Sciences and Biotechnology, University of Ferrara, Ferrara, Italy, Ferrara, Emilia‐Romagna, Italy; 2 Department of Health Sciences, University of Piemonte Orientale, Novara, Italy, Novara, Piemonte, Italy, 3 University of Piemonte Orientale, Novara, Italy, Novara, Piemonte, Italy; 4 Department of Life Sciences and Biotechnology, University of Ferrara, Italy., Ferrara, Emilia‐Romagna, Italy; 5 Howard Hughes Medical Institute, Harvard University, Cambridge, USA, Cambridge, Massachusetts, United States
Background: Replacement therapy as well as non‐replacement therapy, albeit significantly improved Hemophilia A (HA) treatment, do not provide a definitive cure and still have limitations. This led to exploitation of gene therapy and genome editing approaches based on homologous recombination (HSR), which are not optimized yet. Recently, DNA base (BE) and prime (PE) editing approaches non‐relying on HSR have been developed to cleanly install or revert point mutations which, in HA, are the most represented ones when excluded the IVS22 inversion.
Aims: To exploit BE and PE approaches and correct representative F8 missense and nonsense variants at DNA level and rescue FVIII expression.
Methods: Cellular models created by expression of recombinant FVIII variants; design and delivery of BE and PE components and evaluation of secreted FVIII levels (ELISA, coagulation/chromogenic assays).
Results: In transient and stable expression systems, the c.6046C>T(p.R2016W), c.6496C>T(p.R2166*), c.6545G>A(p.R2182H), c.6682C>T(p.R2228*), c.6683G>A(p.R2228Q) led to reduced secreted FVIII levels (p.R2166*>p.R2228Q>p.R2016W>p.R2182H) while the p.R2228* change produced appreciable FVIII protein levels attributable to a truncated isoform. Screening of a large sets of BE/PE variants and guide RNAs identified combinations that rescued FVIII secretion for the p.R2166*, p.R2182H and p.R2228Q mutations. Extensive studies on the p.R2166* and p.R2228Q changes demonstrated that the BE and PE‐mediated reversion of mutations, detectable at DNA levels, resulted in appreciable rescue of secreted FVIII protein and activity levels (up to 20% of FVIII‐WT).
Conclusion(s): For the first time, the BE and PE approach has been applied to Hemophilia A. Data on two relatively frequent missense and nonsense mutations causing severe HA provided the proof‐of‐principle of efficacy of base editing in cellular models, and studies in HA blood‐outgrowth endothelial cells (BOECs) are currently in progress. This lay the foundation for studies with BE/PE in animal models, which might open a new avenue toward an innovative and personalized HA cure.
OC 01.2
Improvements in health‐related quality of life in adults with severe or moderately severe hemophilia B after receiving etranacogene dezaparvovec gene therapy
R. Itzler1; J. Miller2; R. Robson2; P. Monahan3; S. Pipe 4
1 CSL Behring, King of Prussia, PA, USA, King of Prussia, Pennsylvania, United States; 2 Everest Clinical Research, Little Falls, NJ, USA, Little Falls, New Jersey, United States; 3 CSL Behring, King of Prussia, PA, USA, King Of Prussia, Pennsylvania, United States; 4 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States
Background: Despite current prophylactic therapy for hemophilia B, breakthrough bleeding still occurs negatively affecting health‐related quality of life (HRQoL). The pivotal Phase 3 HOPE‐B trial of the investigational AAV gene transfer product, etranacogene dezaparvovec, demonstrated sustained factor IX (FIX) activity and bleed protection superior to FIX concentrate prophylaxis.
Aims: Here we report the impact on HRQoL.
Methods: Fifty‐four adults with hemophilia B (FIX≤2%) received standard of care FIX concentrate prophylaxis in the 6‐month lead‐in period followed by a single infusion of etranacogene dezaparvovec. HRQoL was assessed with the hemophilia‐specific Hem‐A‐QoL during the lead‐in and at 6 and 12 months after etranacogene dezaparvovec. Repeated measures linear mixed models estimated the difference in scores before and after gene therapy. A one‐sided p‐value ≤0.025 for the post‐treatment, lead‐in period was considered statistically significant. The analyses were not adjusted for multiplicity.
Results: Significant model‐based mean differences in scores and the percentage improvement compared with the lead‐in period were as follows: Total Score (least square (LS) mean ‐5.50; p < 0.0001; 21.5%), domains ‘Treatment’ (LS mean ‐14.88; p < 0.0001; 59.0%), ‘Feelings’ (LS mean ‐9.42; p < 0.0001; 45.7%), ‘Future’ (LS mean ‐5.02; p = 0.0023; 16.2%) ‘Work/School’ (LS mean ‐4.99; p = 0.0036; 28.8%). The p‐value was slightly above the p < 0.025 threshold for the ‘Physical Health’ domain (LS mean ‐4.21; p = 0.0278; 13.5%). Results were not significant for the five remaining Hem‐A‐QoL domains. ‘Treatment’ reflects how burdened patients are by their hemophilia treatments. ‘Feelings’ reflects current emotions associated with having hemophilia. ‘Future’ reflects concerns about how hemophilia will affect their life plans. ‘Work/School’ reflects how well patients think they perform these responsibilities.
Conclusion(s): HRQoL improvements after gene therapy demonstrate that etranacogene dezaparvovec can reduce the burden associated with hemophilia and FIX prophylactic therapy. This may contribute to how hemophilia B patients view their work/school performance as well as providing a sense of optimism for the future.
OC 12.4
An enhanced hemostatic factor VIII variant for hemophilia A gene therapy: Prothrombotic and immunological risk assessment
A. Sternberg; R. Davidson; C. Martos‐Rus; A. Wilhelm; L. George
The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States
Background: Despite phenotypic improvement in clinical trials, universally sustained AAV‐mediated FVIII expression at levels necessary to eliminate bleeding has not been achieved. Like the success of FIX‐Padua for HB gene therapy, we hypothesized an enhanced hemostatic‐function FVIII‐variant transgene for HA gene transfer may achieve hemostatic efficacy at lower AAV vector doses thereby overcoming vector dose‐dependent efficacy limitations while eliminating bleeding. We described a FVIII variant (FVIII‐R336Q/R562Q; FVIII‐QQ) resistant to APC‐mediated inactivation with ~5‐fold enhanced hemostatic function in vivo (Wilhelm et al. Blood 2021).
Aims: We investigated the safety and efficacy of FVIII‐QQ for HA AAV‐mediated gene therapy.
Methods: AAV‐hFVIII‐WT and AAV‐hFVIII‐QQ vectors were administered to HA/CD4KO mice; FVIII:C was determined by COAMATIC chromogenic assay and AAV‐treated cohorts were followed for survival or analyzed by tail‐clip assay. WT mice zygotes underwent CRISPR/cas9 editing to generate FVIII‐QQ mice. Recombinant hFVIII‐WT or hFVIII‐QQ, both produced in our lab, was intravenously administered to immunocompetent HA mice 6 times weekly at 0.2 μg/mouse followed by 5 μg/mouse on week 7 and Bethesda titer was determined.
Results: The vector dose EC50 and EC80 of blood loss post tail‐clip for AAV‐hFVIII‐QQ was 4.7 and 10‐fold lower, respectively, than AAV‐hFVIII‐WT supporting a 5‐10‐fold benefit of the hFVIII‐QQ transgene. Ongoing studies of HA/CD4KO mice expressing hFVIII‐WT and hFVIII‐QQ in the mild and normal ranges of FVIII:C thus far demonstrate no significant survival differences for at least 3 months post‐vector (Figure1). Homozygous female/hemizygous male mice endogenously expressing FVIII‐QQ via CRISPR‐mediated gene editing are viable, fertile and the same weight as littermate controls. Immunocompetent HA mice treated with recombinant hFVIII‐WT or hFVIII‐QQ demonstrated expected inhibitor development that did not statistically differ (Figure2).
Conclusion(s): These data demonstrate a hemostatic advantage of using the FVIII‐QQ transgene for AAV‐mediated gene transfer. Preliminary murine safety studies did not demonstrate evidence of FVIII‐QQ enhanced prothrombotic or immunological risk relative to FVIII‐WT.
OC 12.3
Rescue of the endogenous FVIII expression in hemophilia A mice using CRISPR/Cas9 mRNA LNPs
C. Chen; X. Cai; C. Miao
Seattle Children's Research Institute, Seattle, Washington, United States
Background: Correction of hemophilia A (HA) patients’ nonsense mutation by gene editing can regain expression of missing protein permanently. Furthermore, lipid nanoparticle (LNP) technology can provide an efficient gene delivery system. We investigate if LNP technology combined with gene‐editing tools using CRISPR/Cas9 can achieve targeted gene correction in a specific HA mouse model.
Aims: To examine gene delivery efficiency of LNP in vitro and regain FVIII function in HA mice using CRISPR/Cas9 mRNA LNPs in vivo.
Methods: LNPs carrying luciferase mRNA were examined for transfection efficiency in HepG2 and HUVEC cells. The efficiency of two sgRNAs was examined in vitro using a T7E1 assay. In vivo transfection efficiency was investigated by immunofluorescent staining of the liver following intravenous injection of GFP mRNA LNPs into the mice. Immunodeficient HA mice (NSG HA) with indel mutation in FVIII exon 1 were used as a gene‐editing animal model. NSG HA Mice were hydrodynamically injected with FVIII exon 1 targeting sgRNA/Cas9 expressing plasmid. Subsequently, CRISPR/Cas9 mRNA LNPs were synthesized and intravenously injected into NSG HA mice. FVIII activity was examined by aPTT assay and gene editing was verified by DNA sequencing.
Results: The data suggested that mRNA LNPs can efficiently transfect both HepG2 and HUVEC cells, and can be delivered to hepatocytes and liver sinusoidal endothelial cells (LSECs) in vivo. NSG HA mice treated with a hydrodynamic injection of sgRNA/Cas9 plasmids regained FVIII expression.. Subsequently, CRISPR/Cas9 mRNA LNPs delivered into NSG HA mice successfully induced indel mutation in mutant FVIII gene and rescued endogenous FVIII activity.
Conclusion(s): Our LNPs can transfect both hepatocytes and LSECs in vitro and in vivo. LNP carrying CRISPR/Cas9 mRNA and mF8sgRNA can induce indel mutation in the target site, leading to therapeutic levels of FVIII expression. These technologies have the potential to repair FVIII mutations and recover FVIII gene expression in HA patients.
OC 12.2
AAV mediated CRISPR/Cas9 based therapeutic gene‐editing with a bypass coagulation factor in a murine model of hemophilia
P. Sarangi 1; S. Bammidi1; R. Sambasivan2; G. Jayandharan1
1 Indian Institute of Technology Kanpur, Kanpur, Uttar Pradesh, India; 2 IISER Tirupati, Tirupati, Andhra Pradesh, India
Background: It is imperative to develop a sustainable therapy that targets both hemophilia A/B including the inhibitor‐positive patients using a bypass coagulation agent. We reasoned that a gene‐editing based strategy to introduce coagulation Factor(F)VIIa into the host genome may alleviate the phenotype in hemophilia.
Aims: To develop precise gene editing vectors containing activated murine (m)FVIIa to augment hemostasis in a murine model of hemophilia B.
Methods: We designed a novel AAV vector containing mFVIIa to target the murine Rosa26 locus using CRISPR/Cas9 mediated gene editing. The novel triple vectors designed for expression of guide RNA(AAV8 gRNA), Cas9(AAV2 mutant Cas9) and mFVIIa(AAV8‐mFVIIa) flanked by homology arms were validated by T7 endonuclease assay and HDR‐genotyping. Groups of hemophilia B mice were administered with AAV carrying gRNA, Cas9 (1 × 10e11), and mFVIIa with homology arms (2 × 10e11) in addition to control groups. We then assessed the functional rescue by coagulation function tests such as the prothrombin time (PT) assay, FVIIa activity and a hemostatic challenge assay.
Results: Our T7 assay revealed a cleavage efficiency of 20‐42% and further sequencing demonstrated the targeted integration of mFVIIa into the Rosa26 locus. The PT assay performed on the samples collected after 8‐weeks showed a significant PT reduction in mice that received the gene‐editing vectors (22%) and a 13% decline in mice that received only the AAV‐FVIIa in comparison to the mock‐treated animals. A long‐term follow‐up at 15 weeks showed a similar finding. Furthermore, the coagulation FVIIa activity in mice that received triple gene‐editing vectors was ~4 fold higher (122.5% vs 28.8%) than the mock group. Further tail‐clip assay revealed a significant reduction of blood loss in hemophilia B mice injected with only FVIIa or gene editing vectors.
Conclusion(s): We have observed a long‐term expression of a bypass coagulation FVIIa and phenotypic rescue in hemophilia B models by a novel gene editing strategy. FIGURE 1
FIGURE 1
OC 12.1
Transgene expression patterns after AAV8 delivery using hepatocyte promoter elements reveal expression in both hepatocytes and liver sinusoidal endothelial cells (LSECs)
J. Lindgren 1; D. Sabatino1; G. Nguyen1; A. Follenzi2; A. Henriksen1; R. Fama1
1 Children's Hospital of Philadelphia Center for Cellular and Molecular Therapeutics, Philadelphia, Pennsylvania, United States, 2 University of Piemonte Orientale, Novara, Italy, Novara, Piemonte, Italy
Background: Adeno‐associated virus (AAV)‐mediated gene therapy for hemophilia A (HA) is actively in clinical development. Due to the packaging constraints of the AAV vector, minimized promoter elements derived from hepatocyte specific genes have been developed to direct factor VIII (FVIII) expression to the liver. The human alpha‐one antitrypsin/ApoE promoter‐enhancer (hAAT/ApoE) (Pasi 2020) and the transthyretin (TTR) promoter (George 2021) are used in HA clinical studies to achieve liver‐specific FVIII expression. While the primary endogenous site of FVIII synthesis is liver sinusoidal endothelial cells (LSECs), the cellular profile of transgene expression in the liver after AAV gene therapy utilizing these promoters is not known.
Aims: Determine the cellular specificity of the hAAT/ApoE and TTR promoters after systemic delivery of AAV8 vectors.
Methods: AAV8‐TTR‐GFP (n = 4) and AAV8‐hAAT/ApoE‐GFP (n = 3) were administered intravenously to HA‐CD4KO mice (1e11 vg/mouse). LSECs and hepatocytes were analyzed for GFP expression by flow cytometry (FACS). CD31 and CD146 were used to isolate and label LSECs for flow analysis, while albumin was used to label hepatocytes. Immunofluorescence (IF) staining was conducted in parallel to evaluate GFP expression in LSECs and hepatocytes.
Results: AAV8‐TTR‐GFP produced GFP expression in a similar proportion of hepatocytes (83.3% GFP+/albumin+ +/‐ 12.8%) and LSECs (71.8% GFP+/CD146+/CD31+ +/‐ 15.9%)(Figure 1). Delivery of AAV8‐hAAT/ApoE‐GFP also resulted in GFP expression in both hepatocytes (76.2% GFP+/albumin+ +/‐ 15.4%) and LSECs (87.9% GFP+/CD146+/CD31+ +/‐6.4%). Immunofluorescent staining of liver confirms FACS data, showing transgene expression in both hepatocytes and LSECs.
Conclusion(s): AAV8 constructs under control of minimized hAAT/ApoE and TTR promoter elements drive transgene expression in hepatocytes and LSECs. Further studies are required to understand the biological consequences of expressing FVIII in hepatocytes and LSECs after AAV gene therapy. FIGURE 1 Flow cytometry analysis of GFP expression across hepatocytes and LSECs after systemic delivery of AAV8‐GFP
FIGURE 1 Flow cytometry analysis of GFP expression across hepatocytes and LSECs after systemic delivery of AAV8‐GFP
OC 21.4
Effect of growth on AAV‐mediated expression of FIX‐R338L in juvenile hemophilia B dogs
T. Nichols1; S. Rakhe2; V. Arruda3; B. Samelson‐Jones4; M. Caughey5; E. Merricks6; J. Murphy2; D. Pittman 7
1 Department of Medicine and Pathology and Lab Medicine, UNC, Chapel Hill, North Carolina, United States; 2 Rare Disease Research, Pfizer Inc., Cambridge, Massachusetts, United States; 3 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 4 The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 5 UNC Biomedical Engineering, Chapel Hill, North Carolina, United States; 6 UNC Department of Pathology and Lab Medicine (FOBRL), Chapel Hill, North Carolina, United States; 7 Rare Disease Research, Pfizer Inc., Windham, New Hampshire, United States
Background: The human liver doubles in mass 8‐9 months from birth and again by 3‐4 years. Liver growth and expanding blood volume may reduce FIX levels in pediatric recipients of hemophilia B (HB) gene therapy. HB dogs exhibit a similar phenotype and have proven to be highly predictive of the human experience with gene therapy. HB dogs with severe spontaneous bleeding can be monitored post‐gene therapy to assess the effect of normal growth on FIX transgene levels and bleeding phenotype.
Aims: To determine the effect of growth on FIX levels in male juvenile HB dogs following treatment with an AAV‐delivered, high‐activity canine FIX‐R338L.
Methods: Twelve HB dogs received an AAV encoding FIX‐R338L variant under a liver specific promoter at age 3 or 6 months to model 2‐6‐year‐ or 6‐12‐year‐ old children, respectively. Six dogs per age group, were dosed at 5E+11 (2 dogs), 2.5E+12 (3 dogs) or 5E+12 (1 dog) vg/kg. Body weights, liver size (ultrasound), clinical chemistries and hemostatic activity were monitored by whole blood clotting (WBCT), thromboelastography (TEG) and activated partial thromboplastin time (aPTT).
Results: Dogs were followed for ≥12 months, during a period of liver growth (right lateral lobe: 35.7 ± 5.4 to 93.5 ± 10.6 mm, Figure 1) and blood volume expansion (estimated ~0.1 L to ~2 L). Treatment was well tolerated. To date, no spontaneous bleeds have occurred post‐treatment. Increasing vector dose was associated with progressive shortening of clotting times (Figure 2). Stable, durable reductions of the aPTT, WBCT and TEG R values were observed in all dogs, indicative of FIX activity.
Conclusion(s): AAV‐mediated gene therapy is associated with long‐term correction of the laboratory and clinical phenotype of HB. This durable efficacy was observed in growing juvenile HB dogs treated at an early age (3 or 6 months) and was sustained for ≥ 1 year (ongoing observation) despite liver growth and blood volume expansion.
OC 21.2
Hemostatic results for up to 6 years following treatment with valoctocogene roxaparvovec, an AAV5‐hFVIII‐SQ gene therapy for severe hemophilia A
M. Laffan 1; S. Rangarajan2; W. Lester3; E. Symington4; B. Madan5; D. Hart6; M. Li7; T. Robinson8; G. Pierce9; W. Wong7
1 Centre for Haematology, Imperial College London, London, England, United Kingdom; 2 University of Southampton, Southhampton, England, United Kingdom; 3 University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 4 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 5 Guy's & St. Thomas' NHS Foundation Trust, London, England, United Kingdom; 6 The Royal London Hospital Haemophilia Centre, Barts and The London School of Medicine and Dentistry, London, England, United Kingdom; 7 BioMarin Pharmaceutical Inc., Novato, California, United States; 8 BioMarin Pharmaceutical, Inc., Novato, California, United States; 9 Independent, La Jolla, California, United States
Background: Sustained clinical benefit was demonstrated up to 5 years following a single 6E13 vg/kg dose of valoctocogene roxaparvovec (AAV5‐hFVIII‐SQ), an investigational gene therapy for severe hemophilia A, in a Phase 1/2 trial (NCT02576795). All 7 participants showed sustained improvements in factor FVIII (FVIII) activity, annualized treated bleeding rate, and use of exogenous FVIII. Similar results were seen for up to 4 years in the 6 participants who received a 4E13 vg/kg dose. No participants chose to resume FVIII prophylaxis. Although these results indicate that valoctocogene roxaparvovec provides substantial hemostatic efficacy, longer follow‐up is needed to monitor safety and determine how long the transgene will produce FVIII and protect against bleeding.
Aims: To report safety and efficacy of valoctocogene roxaparvovec up to 6 years after administration in a Phase 1/2 trial.
Methods: Adult male participants with severe hemophilia A who had previously been treated with FVIII received a single intravenous dose of valoctocogene roxaparvovec at 6E13 vg/kg (n = 7) or 4E13 vg/kg (n = 6).
Results: Updated, detailed safety and efficacy assessments from 6‐year follow‐up data from the 6E13 vg/kg cohort and 5‐year follow‐up data from the 4E13 vg/kg cohort will be shared at the ISTH 2022 Congress. Presented endpoints will include summary and individual participant FVIII activity, annualized treated bleeding and FVIII utilization rates, details of bleeding events, and adverse events.
Conclusion(s): The 6‐year data from this Phase 1/2 study of valoctocogene roxaparvovec reported at ISTH 2022 will provide the most up‐to‐date, long‐term follow‐up data currently available for investigational use of AAV‐mediated therapy for hemophilia A. Funded by BioMarin Pharmaceutical Inc.
OC 21.3
Treatment of canine hemophilia A via intraosseous delivery of a platelet‐specific factor VIII‐lentiviral vector
C. Rementer 1; C. Li1; T. Nichols2; X. Cai1; J. Joo1; X. Wang1; E. Merricks3; H. Ochs1; D. Rawlings1; C. Miao1
1 Seattle Children's Research Institute, Seattle, Washington, United States; 2 Department of Medicine and Pathology and Lab Medicine, UNC, Chapel Hill, North Carolina, United States; 3 UNC Department of Pathology and Lab Medicine (FOBRL), Chapel Hill, North Carolina, United States
Background: Hemophilia A (HemA) is a genetic disease resulting from a factor VIII (FVIII) deficiency. Traditional protein infusion to treat HemA is costly and requires repeated dosing.
Aims: We demonstrated previously that intraosseous (IO) gene therapy via delivery of lentiviral vectors (LVs) into bone marrow targeting FVIII expression in platelets successfully treated HemA mice, including mice that had developed FVIII inhibitors. Here, we investigated the treatment of HemA dogs using this approach.
Methods: A lentiviral vector incorporating a platelet‐specific promoter Gp1bα and canine FVIII gene was injected into the tibia or iliac bones of 4 HemA dogs. Prior to injection, the dogs were treated with an immune modulation regimen to minimize the immune response. Following the procedure, blood samples were taken at various timepoints.
Results: All dogs recovered well from the procedure and had blood chemistry values within normal ranges. Expression of cFVIII was examined in platelets and plasma isolated from LV‐treated dogs by ELISA and aPTT assays. Canine FVIII can be detected in platelets with the highest expression at 5‐10 mU/108 platelets around 1‐2 months post‐procedure and expression persisted for the experimental duration in all treated dogs. The correction of HemA phenotype was evaluated by whole blood clotting time (WBCT) and thromboelastography. WBCT was shortened in multiple time points shortly after IO gene therapy, indicating improved hemostasis. Furthermore, the IO gene therapy was well tolerated and did not produce any toxicity as evaluated by CBC and blood chemistry analysis. Encouragingly, the dogs experienced fewer bleeding events per year after gene therapy treatment compared with the baseline prior treatment.
Conclusion(s): We have established an IO‐LV gene therapy protocol to treat HemA dogs successfully with persistent effects of treatment over 2‐4 years. Our study demonstrated a potential strategy for safe and effective application of gene therapy in vivo for treating HemA patients.
Novel Biotherapeutics in Hemophilia
OC 50.5
Mim8 is associated with improved thrombin generation vs. emicizumab in patients with haemophilia A, with and without inhibitors
J. Windyga 1; P. Chowdary2; F. Jaime3; C. Eskelund4; P. Jørgensen4; M. Kreilgaard4; J. Mahlangu5
1 Institute of Hematology and Transfusion Medicine, Warsaw, Mazowieckie, Poland; 2 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom; 3 Hospital Universitario Regional de Málaga, Málaga, Andalucia, Spain; 4 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark; 5 University of the Witwatersrand and NHLS, Johannesburg, South Africa
Background: Factor VIII (FVIII) replacement is the standard of care for patients with haemophilia A (HA). Mim8 is a bispecific antibody bridging factor IXa/X (FIXa/FX), with enhanced haemostatic properties in vitro and in HA mouse models, compared with emicizumab. FRONTIER1 (NCT04204408) is a phase 1/2 study investigating the safety, tolerability, pharmacokinetics, and pharmacodynamics of subcutaneous Mim8 in healthy participants and patients with severe HA, independent of FVIII inhibitor status.
Aims: We report on peak thrombin generation, and laboratory markers in response to Mim8 or emicizumab.
Methods: The phase 2 part of FRONTIER1 is open‐label, with Mim8 administered subcutaneously over 12 weeks, across four multiple ascending dose cohorts, targeting average plasma exposure of 1–9 μg/ml, through dosing weekly (cohorts 1–3) or every four weeks (cohort 4); cohorts 3/4 targeted the same plasma exposure. FRONTIER1 is ongoing with a fifth ascending dose cohort. An additional cohort of patients treated with emicizumab was included for comparison. Informed consent and ethics committee approval were acquired.
Results: 32 patients on Mim8 (cohorts 1 [n = 7], 2 [n = 9], 3 [n = 8], and 4 [n = 8]) and 10 on emicizumab were included. Peak thrombin levels increased with Mim8 dose, at lower plasma concentrations than with emicizumab, indicating higher potency for Mim8 (Figure 1). Mean peak thrombin levels were comparable between patients on emicizumab and Mim8 in cohort 2. No dose‐dependent changes in D‐dimer, fibrinogen, platelets, or FIXa/FX antigen levels were observed; most values remained within the normal range. A relative increase in prothrombin fragments 1 and 2, with stabilisation at steady state, was seen for both Mim8‐ and emicizumab‐treated patients, in correlation with the respective thrombin peak increase.
Conclusion(s): A dose‐dependent increase in thrombin generation was observed in Mim8‐treated patients, reaching higher peak thrombin levels than in emicizumab patients. Laboratory parameters showed no signs of exaggerated coagulation. FIGURE 1 Peak thrombin levels in patients treated with increasing doses of Mim8 and a clinically recommended dose of emicizumab
FIGURE 1 Peak thrombin levels in patients treated with increasing doses of Mim8 and a clinically recommended dose of emicizumab
OC 50.3
Improved procoagulant activity of hemophilia B causing dysfunctional factor IX variants with factor VIII mimetics
J. Chau1; A. Sternberg1; A. Pishko2; L. George1; B. Samelson‐Jones 1
1 The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 2 University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: While the FVIII‐mimetic emicizumab has been widely adapted for hemophilia A (with subcutaneous administration), prophylaxis for hemophilia B (HB) still requires intravenous FIX administration. Previous studies investigating the effect of emicizumab in HB suggested that emicizumab can enhance the FIX activity of wild‐type FIX about tenfold, but HB patients with dysfunctional FIX variants were non‐responders to emicizumab (Ogiwara et al. 2017). We recently demonstrated that the mechanism of the high‐specific activity of FIX‐Padua was due to an enhanced interaction between FIXa/FVIIIa and the increased activity of FIX‐Padua compared to FIX‐WT is ablated with emicizumab (Samelson‐Jones et al 2019).
Aims: We now test the converse hypothesis: HB‐causing FIX missense variants due to dysfunctional FIXa/FVIIIa interactions can be rescued with FVIII mimetics.
Methods: Procoagulant activity of recombinant FIX protein and patient samples were assayed by either aPTT assay, one‐stage FIX activity, TGA, and ROTEM.
Results: Recombinant protein of high‐prevalence HB‐causing FIX variants with dysfunctional FIXa/FVIIIa interactions (G93S, R338P, E387K, and I397T) and severe‐to‐mild HB phenotypes demonstrated increased FIX activity with the addition of therapeutic amounts (300 nM) of emicizumab (Figure 1A). The FIX activities of these recombinant FIX variants without emicizumab is comparable to the clinical data. Emicizumab also corrected the thrombin generation of these variants (Figure 1B). In contrast, HB‐causing FIX variants with missense mutations outside FVIIIa‐interacting motifs (R248Q and A390V) had no improvement in FIX activity or thrombin generation. In samples from patients with FIX‐I397T, emicizumab normalized the aPTT‐based clotting time in plasma and substantially improved the ROTEM clot time in whole blood (Figure 1C,D).
Conclusion(s): HB‐causing FIX missense variants with dysfunctional FIXa/FVIIIa interactions are highly‐susceptible to procoagulant improvement with FVIII‐mimetics like emicizumab. Given emicuzmab’s excellent safety record in non‐inhibitor HA patients with expanding use into mild HA, consideration of treatment of HB patients with these variants could be considered. FIGURE 1
FIGURE 1
OC 40.1
FRONTIER1: A phase 1/2 dose escalation study of a novel factor VIIIa mimetic bispecific antibody, Mim8, for evaluation of safety, pharmacokinetics, and efficacy
P. Chowdary 1; F. Jaime2; J. Mahlangu3; C. Eskelund4; R. Meldgaard4; P. Persson4; J. Windyga5
1 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom; 2 Hospital Universitario Regional de Málaga, Málaga, Andalucia, Spain; 3 University of the Witwatersrand and NHLS, Johannesburg, South Africa; 4 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark; 5 Institute of Hematology and Transfusion Medicine, Warsaw, Mazowieckie, Poland
Background: Mim8 is a subcutaneously administered novel factor IXa/X bispecific antibody in development for patients with haemophilia A (PwHA) with or without inhibitors, which has demonstrated an efficacious haemostatic effect in nonclinical studies.
Aims: FRONTIER1 (EudraCT:2019‐000465‐20; NCT04204408) aims to investigate safety, tolerability, pharmacokinetics, and pharmacodynamics of single ascending doses of Mim8 in healthy participants; and multiple ascending doses of Mim8 in PwHA, with or without inhibitors. Additionally, the study aims to provide data for dose‐setting in subsequent FRONTIER studies.
Methods: In the single ascending dose (SAD) phase, healthy subjects received single ascending doses of Mim8 (targeting plasma exposures of 0.05–3 μg/ml) or placebo. In the multiple ascending dose (MAD) phase, PwHA received multiple ascending doses of Mim8 targeting an average plasma exposure of 1 μg/ml (cohort 1, QW dosing), 3 μg/ml (cohort 2, QW dosing), or 9 μg/ml (cohort 3, QW dosing, and cohort 4, Q4W dosing). Informed consent and ethics committee approval were obtained.
Results: Mim8 was well tolerated following both single and multiple dosing, and no thromboembolic events or related serious adverse events were reported. No occurrences of anti‐Mim8 antibodies were reported. The increases in AUC and Cmax with increasing dose were consistent with dose‐proportionality (Figure 1). Data from the SAD section suggests that T1/2 was 30.4 days, and Tmax was 9.1 days. During the 12‐week observation period, 15 treated bleeds were reported in 8 patients, of which 13 bleeds (9 traumatic) were observed in 6 patients from the lowest dose cohort. The 2 bleeds in patients from cohorts 2 and 3 were traumatic, thus neither treated joint nor spontaneous bleeds were observed beyond cohort 1 (Table 1).
Conclusion(s): Pharmacokinetic properties were consistent with dose‐proportionality and support weekly and monthly dosing approaches. Mim8 was well tolerated and no occurrences of anti‐Mim8 antibodies were reported. FRONTIER1 provides encouraging data supporting further clinical development.
Rare Bleeding Disorders
OC 78.5
Exploring hidden bleeding phenotypes by screening FXIII and Fibrinogen genes
S. Singh 1; N. Mir‐Montazeri1; M. Jamil1; B. Pezeshkpoor2; A. Sharma3; J. Dodt4; P. Volkers4; E. Hethershaw5; H. Philippou6; V. Ivaskevicius7; J. Oldenburg8; A. Biswas9
1 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Medical Faculty, University of Bonn, Germany, Bonn, Nordrhein‐Westfalen, Germany; 3 All India Institute of Medical Sciences, New Delhi, Delhi, India; 4 Paul‐Ehrich institute, Langen, Hessen, Germany; 5 Unversity of Leeds, Leeds, England, United Kingdom; 6 University of Leeds, Leeds, England, United Kingdom; 7 Uniklinikum bonn, Bonn, Nordrhein‐Westfalen, Germany; 8 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 9 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: The collaborative functional status of products encoded by Factor XIII (FXIII) and Fibrinogen genes, define the clot architecture in an individual. Genetic variations that even mildly influence either of the FXIII or Fibrinogen integrity at levels ranging from expression to complex assemblies; affects the integrity and functionality of fibrin clot.
Aims: This study aims to address the milder form of inherited heterozygous FXIII deficiency (FXIII plasma levels 20‐60%) and to understand its genetic and clinical burden.
Methods: Sample Collection: Blood samples corresponding to apparently healthy controls [n = 752 (unbiased cohort)] were collected at the University Clinic Bonn, Germany (Figure 1). Additionally, another group of sample of patients with suspected mild FXIII deficiency referred to the outpatient department of our hemophilia center were also collected (biased‐control cohort). Genetic characterization: DNA extracted from whole blood was analysed by NGS using Illumina based custom kits. Extracted plasma was tested for FXIII/Fibrinogen functional status using photometric assay, alpha‐2‐anti‐plasmin incorporation assay, Pentylamine incorporation assay in‐house FXIII antigenic ELISA and Fibrinogen activity.
Results: FXIII activity levels show good correlation with FXIII antigen, Fibrinogen and amongst different FXIIII activity assays. NGS analysis showed multiple heterozygous missense variants in F13A1 (43), F13B (23), FGA (26), FGB (4) and FGG (11) genes. In silico evaluations of heterozygous missense variants suggest that some of them could disrupt dimeric or heteromeric assembly of the complex. The cysteines of F13B gene appear to be mutational hotspots for mild FXIII deficiency.
Conclusion(s): There is a large inter‐individual variability in circulating plasma FXIII levels (35‐150%), with 6 individuals out of 752 with mild FXIII deficiency (prevalence: 0.8%) which is much higher than the theoretical estimate of 0.1%. In silico analysis suggests that the heterozygous missense variants largely affects the complex assembly of FXIII in plasma. FIGURE 1 Experimental work‐flow for assessing the prevalence of mild FXIII deficiency among German Caucasian population
FIGURE 1 Experimental work‐flow for assessing the prevalence of mild FXIII deficiency among German Caucasian population
OC 78.4
Haemorrhagic sub‐phenotypes in hereditary haemorrhagic telangiectasia are associated with high impact DNA variants in platelet/coagulation genes responsible for general population bleeding disorders
K. Joyce1; E. Onabanjo2; S. Brownlow2; F. Nur2; K. Olupona2; K. Fakayode2; M. Sroya3; G. Thomas3; T. Ferguson2; J. Redhead2; C. Millar2; N. Cooper3; M. Layton3; F. Boardman‐Pretty4; M. Caulfield4; G. Research Consortium4; C. Shovlin 3
1 Imperial College School of Medicine, London, England, United Kingdom; 2 Imperial College Healthcare NHS Trust, London, England, United Kingdom; 3 Imperial College London, London, England, United Kingdom; 4 Genomics England, London, England, United Kingdom
Background: Hereditary haemorrhagic telangiectasia (HHT) is an endothelial vasculopathy inherited as an autosomal dominant trait, due to a heterozygous loss‐of‐function variant in ACVRL1, ENG or SMAD4. Abnormal HHT vascular structures often cause anaemia due to recurrent haemorrhage, with published evidence for anaemia out‐of‐proportion to haemorrhage in one‐third of severely anaemic patients. HHT‐causal genes do not predict the severity of haematological complications.
Aims: Our aims were to test whether rare, high‐impact variation in genes beyond the primary HHT‐causative gene could play a role in the variable degrees of haemorrhage and anaemia exhibited by HHT patients.
Methods: We tested for chance inheritance and clinical associations of rare deleterious variants where loss‐of‐function causes bleeding or haemolytic disorders in the general population. In double‐blinded analyses, all 104 HHT patients from a single reference centre recruited to the 100,000 Genomes Project were assigned to sub‐phenotype severity scales, and whole genome sequencing data tested for high impact variants in 75 HHT‐independent genes encoding coagulation factors, platelet, haemoglobin, erythrocyte enzyme and erythrocyte membrane constituents.
Results: Rare variants (all GnomAD allele frequencies < 0.003) were identified in 38/104 (36.5%) of the HHT patients, and in 56 (75%) of the 75 HHT‐unrelated genes. Likely deleteriousness assignments by Combined Annotation Dependent Depletion (CADD) scores > 15 were supported by gene‐level mutation significance cutoff (MSC) scores. CADD > 15 variants were found for 1 in 10 patients within platelet genes; 1 in 8 within coagulation genes; and 1 in 4 within erythrocyte haemolytic genes. In blinded analyses, patients with greater haemorrhagic severity that had been attributed solely to HHT vessels had more CADD‐deleterious variants in platelet (Spearman ρ = 0.25, p = 0.008) and coagulation (Spearman ρ = 0.21, p = 0.024) genes. However, the HHT cohort had 60% fewer deleterious variants in platelet and coagulation genes than expected (Mann Whitney p = 0.021).
Conclusion(s): HHT patients with deleterious variants in genes relevant to haemorrhage/anaemia display more haemorrhagic phenotypes.
OC 78.3
HMB‐001 – A novel bispecific antibody accumulating and targeting endogenous FVIIa to activated platelets for subcutaneous prophylaxis in multiple bleeding disorders including Glanzmann thrombasthenia
H. Ostergaard 1; M. Zivkovic2; P. Gandhi1; M. Kjelgaard‐Hansen3; M. Broberg3; T. Elm3; M. Stougaard Kyhn3; M. Løvgreen3; E. Olsen1; C. Rea4; B. Sorensen5; S. Bjorn1; R. Schutgens2; R. Urbanus6; J. Faber1
1 Hemab Therapeutics, Copenhagen, Hovedstaden, Denmark; 2 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 3 Novo Nordisk, Copenhagen, Hovedstaden, Denmark; 4 Hemab Therapeutics, London, England, United Kingdom; 5 Hemab Therapeutics, Cambridge, Massachusetts, United States; 6 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: HMB‐001 is a bispecific antibody that binds and accumulates endogenous FVIIa in circulation. Upon vessel injury, HMB‐001 promotes local FX activation and thrombin generation by placing FVIIa on the surface of activated platelets via binding to the TREM‐like transcript 1 (TLT‐1) receptor. The activity of HMB‐001 thus builds on the mechanism of action (MoA) of recombinant FVIIa (rFVIIa) and has the potential to prevent bleeds in multiple hemostatic disorders of which Glanzmann Thrombasthenia (GT) is the primary focus. GT is characterized by defective platelet aggregation and frequent bleeding episodes. With no available prophylactic treatment in GT, HMB‐001 could address a significant unmet need.
Aims: Investigate the therapeutic potential of HMB‐001 in GT and hemophilia A (HA).
Methods: A PK/PD study was performed in cynomolgus monkey with antibody and FVIIa quantification using ELISA and activity assays, respectively. The activity of HMB‐001 was studied by thromboelastography in human HA (FVIII‐depleted) whole blood and in a platelet aggregation assay with GT platelets. The effect rFVIIa +/‐ HMB‐001 on bleeding was determined in the tail vein transection (TVT) model in HA mice.
Results: Multiple‐dose subcutaneous administration of HMB‐001 in cynomolgus monkeys resulted in the accumulation of endogenous FVIIa to low nM levels. Upon supplementation of corresponding levels of rFVIIa to ex vivo models of GT and HA, HMB‐001 was shown to potentiate the activity of FVIIa by 6‐14 fold in a TLT‐1 dependent manner reaching activity levels in the therapeutic range by comparison to rFVIIa. A similar enhancement of the hemostatic activity of FVIIa by HMB‐001 was demonstrated in the mouse TVT injury model upon co‐administration of rFVIIa and HMB‐001.
Conclusion(s): Combining ex vivo and animal models, the present study supports that HMB‐001 can enhance the activity of endogenous FVIIa to levels that are considered therapeutically effective in GT and HA based on clinical experience with rFVIIa.
OC 78.2
Whole exome sequencing in patients with rare bleeding disorders: Data from the RBiN study
S. Willems 1; J. Saes2; A. Simons3; J. Weiss3; K. Meijer4; M. Cnossen5; R. Schutgens6; M. Peters7; L. Nieuwenhuizen8; P. Den Exter9; I. Kruis10; N. Blijlevens11; W. van Heerde12; S. Schols13
1 Radboud university medical center, Nijmegen, The Netherlands;, Nijmegen, Gelderland, Netherlands; 2 Department of Hematology, Radboud university medical center, Nijmegen, The Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, The Netherland, Nijmegen, Gelderland, Netherlands; 3 Department of Human Genetics, Radboud university medical center, Nijmegen, The Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, The Netherlands, Nijmegen, Gelderland, Netherlands; 4 University Medical Center Groningen, Groningen, Groningen, Netherlands; 5 Department of Pediatric Hematology, Sophia Children’s Hospital, Erasmus University Medical Center, Erasmus University Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 7 Department of Pediatric Hematology, University Medical Center Utrecht and University Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands; 8 Department of Hematology, Máxima Medical Center, Eindhoven, Netherlands; Radboud university medical center, Hemophilia Treatment Center, Nijmegen – Eindhoven – Maastricht, Netherlands, Eindhoven, Noord‐Brabant, Netherlands; 9 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands, 10 Netherlands Hemophilia Society, Nijkerk, Netherlands, Nijkerk, Gelderland, Netherlands, 11 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands, 12 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, the Netherlands; Enzyre BV, Novio Tech Campus, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands, 13 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands
Background: Rare bleeding disorders (RBD) encompass a heterogenous group of rare coagulation factor deficiencies and disorders of fibrinolysis. Little is known about the genetic background, especially in patients with mild bleeding disorders.
Aims: To improve understanding in genetic variants and investigate the role for whole exome sequencing (WES) in the diagnostics of RBD.
Methods: The Rare Bleeding Disorders in The Netherlands (RBiN) study is a nation‐wide cross‐sectional multicenter study, which was conducted between November 2017 and February 2019 among patients with a hereditary RBD from all six Dutch Hemophilia Treatment Centers. WES was conducted with genomic DNA of included patients after informed consent; a selected gene panel was used including 156 genes proven to be involved in thrombosis and hemostasis. Variants with allele frequencies above 1% were not included.
Results: WES results were available for 156 of the 263 included patients; 46 patients had severe factor deficiency. In 119 (76%) patients, a disease‐causing class 4/5 genetic variant was found. These cases were considered solved (Table). In 34 patients of the solved cases group, additional pathogenic variants (class 3, 4 or 5) were found in other genes. Homozygous or compound heterozygous variants were found in 21 (14%) patients, all with severe coagulation factor deficiency. Moreover, 26 patients (19 with severe factor deficiency) had two variants in one gene, although unknown whether in cis or trans configuration. An additional number of 14 (9%) patients had a genetic variant that was likely or possibly causative of their RBD. No disease‐causing pathogenic genetic variants were found in patients with PA1‐1 deficiency or hyperfibrinolysis.
Conclusion(s): The diagnostic yield of WES in patients with RBD is high, with the exception of patients with hyperfibrinolysis and PAI‐1 deficiency. In 24% of RBD patients, bleeding phenotype may be explained by the interplay of pathogenic variants in multiple hemostasis genes and needs further attention. TABLE 1 Overview of the number of patients in each RBD and yield of whole exome sequencing. A case was considered ‘solved’ if the genetic variant confirmed the diagnosis of the RBD in question. * Baseline coagulation factor level was used. If not available, the centrally measured coagulation factor level was used. ** Euglobulin clot lysis time ratio was used for patients with hyperfibrinolysis
TABLE 1 Overview of the number of patients in each RBD and yield of whole exome sequencing. A case was considered ‘solved’ if the genetic variant confirmed the diagnosis of the RBD in question. * Baseline coagulation factor level was used. If not available, the centrally measured coagulation factor level was used. ** Euglobulin clot lysis time ratio was used for patients with hyperfibrinolysis
OC 78.1
Study of missense changes involving cysteine residues in F11: Implications on folding, dimerization, activity and FXI deficiency
C. Bravo‐Pérez 1; A. Rubio1; Á. Palomo2; J. Esteban3; F. Bauduer4; A. Miñano1; R. Cifuentes‐Riquelme5; B. de la Morena‐Barrio6; P. Garrido‐Rodríguez5; J. Rojo1; V. Vicente5; M. Lozano5; J. Corral5; M. de la Morena‐Barrio5
1 Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 2 Centro Materno‐Infantil del Hospital Regional Universitario Carlos de Haya, Málaga, Málaga, Andalucia, Spain; 3 Hospital Virgen del Castillo de Yecla, Murcia, Yecla, Murcia, Spain; 4 Centre Hospitalier de la Côte Basque, Bayonne, Bayonne, Aquitaine, France; 5 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 6 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain, Murcia, Murcia, Spain
Background: Factor XI (FXI) has 17 intramolecular disulfide bonds, and circulates as a disulfide‐linked homodimer. F11 genetic variants affecting cysteines (Cys) have been identified in patients with FXI deficiency.
Aims: To study the impact of F11 variants affecting Cys on folding, dimerization and function of FXI.
Methods: Assessment of F11 missense variants removing/creating Cys, from our cohort of 105 unrelated FXI‐deficient patients, and from literature review. Recombinant expression of wild‐type FXI and selected variants was conducted in HEK293.
Results: Overall, 40 variants were identified involving Cys (Fig.1). Twenty‐five removed Cys. Only p.Cys599Tyr (a new variant from our cohort), on the catalytic domain, caused qualitative (CRM+) FXI deficiency. Considering cases with quantitative (CRM‐) deficiency, mean FXI:C was <50% (38.0 ± 10.8%) in heterozygotes, suggesting an underlying dominant‐negative effect. A CRM‐ case in our cohort eliminated two Cys in the same allele: p.Cys255Tyr and p.Cys339Phe, the latter implicated in FXI dimerization. Our recombinant model demonstrated that the rp.Cys339Phe precluded dimer formation, but not monomer secretion. Fifteen missense variants introduced new Cys. Three of them caused CRM+ deficiency. We highlight a new variant from our cohort, p.Phe295Cys, identified in a hemizygous case due to concurrent de novo deletion of the wild‐type allele. This variant allowed the secretion of two dimeric forms of FXI, one with an aberrant electrophoretic mobility. The recombinant model confirmed two alternative conformations by differential intramolecular disulfide architecture (Fig.2).
Conclusion(s): Our study confirms the importance of intramolecular disulfide bonds in FXI folding and function. Only one new variant, removing Cys from the catalytic domain, caused CRM+ deficiency. Missense change of p.Cys339, by preventing dimerization, provoked CRM‐ deficiency in vivo. Other mutations, such as p.Phe295Cys, create new Cys residues, that can result in aberrant dimer formation with impaired activity. Further research on the implications of FXI intramolecular disulfide bond exchanges is needed. Funding:ISCIII&FEDER:PI21/00137;CM20/00094.
Hemostatic System in Cancer, Inflammation and Immunity
Coagulation Proteins Beyond Hemostasis
OC 28.5
Coagulation FXI regulates endothelial cell barrier function by inducing VE‐cadherin proteolysis in an ADAM10‐dependent manner
C. Puy1; K. Jordan1; N. Nguyen2; C. Lorentz3; E. Tucker3; D. Gailani4; O. McCarty 5
1 Oregon Health & Science University, Portland, Oregon, United States; 2 Oregn Health & Science University, Portland, Oregon, United States; 3 Aronora, Inc., Portland, Oregon, United States; 4 Vanderbilt University, Nashville, Tennessee, United States; 5 Oregon Health and Science University, Portland, Oregon, United States
Background: Proteolysis of vascular endothelial (VE)‐cadherin is a biomarker for disruption of endothelial cell (EC) barrier function, with elevated levels of soluble VE‐cadherin associated with a therosclerosis and sepsis. Factor XI (FXI), known for its role in the coagulation cascade, also plays a regulatory role in inflammation. While a relationship between FXI activity, VE‐cadherin expression levels, and endothelial barrier function has been observed, the mechanisms by which this process is mediated are unknown.
Aims: To determine whether activated FXI (FXIa) regulates EC barrier function by inducing VE‐cadherin proteolysis.
Methods: Human umbilical vein ECs were stimulated with FXIa (30 and 5 nM) for six hours. VE‐cadherin proteolysis and expression levels were analyzed by Western blot and immunofluorescence. ECs permeability was quantified by measuring the flux of Evans blue‐albumin across the ECs. Immunoprecipitation and Western blot analysis were used to study FXIa‐PAI‐1 or ‐very low density lipoprotein receptor (VLDLR) interactions.
Results: FXIa (5 nM) generated a VE‐cadherin C‐terminal fragment and decreased VE‐cadherin expression on ECs; this process was reversed by the serine protease inhibitor, PPACK. Thrombin, kallikrein, or FXIIa did not induce VE‐cadherin proteolysis. FXIa increased ECs permeability, yet did not induce the expression of VCAM‐1. In contrast, while VCAM‐1 expression was upregulated by tumor necrosis factor‐alpha or vascular endothelial growth factor, neither of these induced VE‐cadherin proteolysis. An inhibitor of a disintegrin and metalloproteinase 10 (ADAM10) abrogated the effects of FXIa on ECs, including proteolysis of VE‐cadherin. Similarly, the low density lipoprotein antagonist, receptor‐associated protein (RAP), inhibited FXIa‐induced VE‐cadherin proteolysis by preventing FXIa complex formation with PAI‐1 and VLDLR on ECs.
Conclusion(s): These results suggest that FXIa disrupts VE‐cadherin‐mediated EC permeability by increasing ADAM10 activity through complex formation and internalization by PAI‐1 and VLDLR. It remains to be seen whether this pathway represents a druggable target to protect EC barrier function in inflammatory diseases by inhibiting FXIa.
OC 66.4
Increased endothelial secretion and reduced circulatory clearance contribute to elevated plasma von Willebrand factor levels in multiple myeloma
C. Comerford 1; S. Pal Singh Dhami2; P. Murphy3; S. Patmore2; S. Ward2; U. Budde4; J. O'Donnell2; S. Glavey3; J. Quinn3; J. O'Sullivan5
1 Royal College of Surgeons in Ireland, Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 3 Department of Haematology, Beaumont Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 4 Medilys Laborgesellschaft mbH, Department of Hämostaseology, Hamburg, Germany, Hamburg, Hamburg, Germany; 5 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland., Dublin, Dublin, Ireland
Background: Multiple myeloma (MM) is associated with one of the highest rates of cancer associated thrombosis, with both venous and arterial events reported. The biological basis for this remains poorly understood. Elevated plasma von Willebrand Factor antigen (VWF:Ag) levels are an independent risk factor for thrombosis and poorer survival in other malignancies.
Aims: To systematically characterise VWF:Ag and activity in MM patients and provide novel mechanistic insight into the biology of thrombotic risk in MM.
Methods: Plasma samples were collected from 105 patients with MM/precursor MM. 10 primary bone marrow (BM)MM samples were obtained. VWF:Ag and propeptide (VWFpp) were measured using ELISA. Endothelial cells (ECs) were co‐cultured with human myeloma cell line (HMCLs) or primary MM cell supernatant.
Results: Elevated plasma VWF:Ag levels were observed in newly diagnosed/relapsed MM compared with precursor MM (332.7IU/dL vs 133.1IU/dL; p < 0.0001). VWF:Ag correlated with markers of disease activity, including paraprotein level. Highest levels were observed with poorest response to MM therapy (402IU/dL vs 154.4IU/dL; p < 0.05). Ultralarge VWF multimers were present in relapsed MM, suggesting increased thrombotic risk associated with treatment refractory disease. Increased VWFpp levels were observed across the cohort, indicating acute endothelial activation. However, impaired circulatory clearance of VWF is also suspected, with reduced VWFpp/VWF:Ag ratios (0.69 vs 1.48; p < 0.0001) and increased VWF half‐life evident. We also observed VWF multimers locally within the BM niche. Supporting this, supernatant from both HMCL and primary MM cells promoted endothelial secretion of VWF, which was shown to directly interact with MM cells.
Conclusion(s): Our findings provide novel mechanistic insight into contributory roles of both increased secretion and reduced clearance of VWF underpinning elevated VWF levels in MM. We also demonstrate multimeric VWF within the BM niche, raising the possibility that VWF may not only contribute to the prothrombotic phenotype but could also be implicated in local disease progression.
OC 28.4
Protective role of plasminogen deficiency in non‐alcoholic fatty liver disease and glucose dysmetabolism
W. Hur; Y. Nguyen; K. King; Y. Patel; M. Flick
University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States
Background: Obesity affects over 40% of Americans and predisposes individuals to metabolic syndrome, which is associated with increased risk of cardiovascular events. A documented clinical manifestation of obesity is a shift in the hemostatic system to a procoagulant and anti‐fibrinolytic state with elevated fibrinogen, fibrin deposition in multiple tissues and suppressed plasmin(ogen) activation and plasmin activity. Whereas recent studies have documented a function contribution of fibrinogen to progression of obesity and metabolic syndrome, a potential role for plasminogen has remained largely undefined.
Aims: Determine the contribution of plasmin(ogen) itself to the development of obesity and associated disease sequelae.
Methods: The impact of plasminogen deficiency on high fat diet (HFD)‐driven weight gain, metabolic inflammation, and obesity‐associated pathologies was analyzed by challenging plasminogen‐deficient (Plg‐) and control (Plg+) mice with low‐fat diet (LFD) or HFD for 20 weeks.
Results: Over the course of 20 weeks, Plg‐ mice gained as much weight as Plg+ mice on HFD, although the epididymal white adipose tissue of HFD‐fed Plg‐ mice had a greater mass than that of HFD‐fed Plg+ mice. In contrast, livers of HFD‐challenged Plg+ mice were significantly larger than that of HFD‐fed Plg‐ mice. HFD‐fed Plg+ animals displayed a fatty liver disease phenotype characterized by histological evidence of steatosis, elevated triglyceride content, and hepatocellular injury (i.e., elevated plasma ALT). However, HFD‐fed Plg‐ mice were protected from developing each of these pathologies. HFD‐fed Plg+ mice also developed hypercholesterolemia, whereas cholesterol levels in Plg‐ mice were comparable to LFD‐fed mice. Notably, whereas HFD‐fed Plg+ mice showed evidence of diabetes as revealed by compromised glucose clearance, HFD‐fed Plg‐ mice were partially protected.
Conclusion(s): Collectively, our data suggest that plasmin(ogen) contributes to HFD‐induced fatty liver disease and glucose dysmetabolism, and that plasminogen deficiency imposes a state of metabolically healthy obesity.
OC 28.3
Coagulation factor XII induces persistent uPAR‐integrin β1 signaling promoting DNA damage and senescence in diabetic kidneys
A. Elwakiel 1; K. Shahzad1; I. Gadi1; R. Rana1; D. Gupta1; J. Manoharan1; S. Kohli1; T. Renné2; B. Isermann1
1 Institute of laboratory medicine, clinical chemistry and molecular diagnostics, Leipzig University, Leipzig, Sachsen, Germany; 2 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany
Background: Diabetic kidney disease (DKD) is the leading cause of renal failure worldwide. Diabetes is associated with impaired coagulation system proteins. Whether FXII signaling, which has been linked with a number of inflammatory and fibrotic diseases, plays a role in DKD is currently unknown.
Aims: To identify the role of coagulation FXII in DKD and to elucidate the involved signaling mechanisms.
Methods: Persistent type‐1 diabetes was induced and maintained for 24 weeks in wild type (wt) and FXII knockout mice (FXII‐/‐). Kidney bulk RNA sequencing was performed to identify possible involved pathways. Ex‐vivo analyses of human and mice samples and in vitro work were conducted to obtain mechanistic insights.
Results: Increased renal tubular expression of FXII was observed in human and mouse diabetic kidneys. Additionally, FXII was significantly increased in urine samples obtained from two independent DKD human cohorts. Diabetic FXII‐/‐ mice showed reduced albuminuria and renal histopathological changes compared to diabetic wt mice. RNA sequencing revealed differential expressed genes (DEGs) in diabetic FXII‐/‐ versus diabetic wt mice. Functional annotation of DEGs identified pathways related to cell cycle checkpoints associated with DNA damage, senescence, and integrin signaling. Mechanistically, FXII induced and stabilized renal tubular expression of urokinase‐type plasminogen activator receptor (uPAR), which has been linked to senescence and is known to signal through integrins. FXII induced prolonged tubular uPAR signaling via integrin β1. This signaling axis promoted ROS generation, persistent DNA damage, and senescence. Blocking uPAR or integrin β1 ameliorated FXII‐induced deleterious effects. Targeting FXII in mice with established DKD using morpholino oligos halted DKD progression and partially reversed the disease.
Conclusion(s): FXII‐uPAR‐integrin β1 signaling axis promotes renal tubular DNA damage and senescence in diabetic kidneys. Inhibition of FXII or the related signaling pathway could be a promising therapeutic approach to prevent senescence in DKD and to halt the progression of the disease.
OC 66.1
The factor XII‐uPAR axis drives ovarian cancer maintenance and progression
K. Bane1; A. Gandhi2; J. McAnulty2; D. Bohinc3; A. DiFeo2; E. Stavrou 4
1 CWRU School of Medicine, Cleveland, Ohio, United States; 2 University of Michigan, Ann Arbor, Michigan, United States; 3 Case Western Reserve University, Cleveland, Ohio, United States; 4 Case Western Reserve University School of Medicine, Cleveland, Ohio, United States
Background: Epithelial ovarian cancer (EOC) is the leading cause of cancer death in women. Most commonly, EOC forms loosely attached outgrowths that transit through the peritoneal fluid and attach to new sites. This unusual route of dissemination is associated with tumor heterogeneity, development of resistant disease, and abdominal organ obstruction. The urokinase receptor uPAR has long been recognized to assist the directional movement of migrating cells and elicits a plethora of cellular responses, several of which are mediated through its association with coagulation Factor XII (FXII).
Aims: Because FXII and uPAR can be produced by both ovarian tumor cells and the host, we investigated if the FXII‐uPAR axis synergistically influences tumor biology.
Methods: We accessed publicly available single‐cell RNA‐seq data from human ovarian tumors, performed ex vivo silencing studies, and used in vivo murine models of EOC.
Results: Single‐cell RNAseq data showed that FXII is robustly expressed in EOC tumor cells themselves, whereas uPAR is enriched in tumor stromal cells (Fig 1A). Based on these findings, we next investigated if absence of host FXII and uPAR is sufficient at reducing tumor growth & dissemination. In murine studies, ID8 EOC cells resulted in significantly higher tumor burden in WT mice compared to F12‐/‐ and Plaur‐/‐ mice (Fig 1B). Invariably, loss of host FXII or uPAR resulted in tumors with significantly reduced vascularity, increased apoptotic rates and reduced epithelial‐to‐mesenchymal transition (EMT, Fig 1C‐G). However, FXII deficiency was uniquely associated with increased expression of epithelial E‐cadherin, indicative of reduced tumor invasive potential. These findings were recapitulated after silencing of F12 expression with an siRNA strategy (Fig 1C‐G).
Conclusion(s): These data show that FXII‐uPAR are differentially expressed in the ovarian tumor microenvironment where they support tumor maintenance and progression. Targeting this interaction may prove to be an effective therapeutic strategy for persistent or recurrent EOC. FIGURE 1 FXII deficiency results in decreased EOC dissemination
FIGURE 1 FXII deficiency results in decreased EOC dissemination
OC 28.1
Factor X – Protease activated receptor‐2 signaling in the regulation of diet‐induced obesity
D. Kaur 1; M. Thati1; W. Ruf2
1 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Mainz, Rheinland‐Pfalz, Germany; 2 Center for Thrombosis and Hemostasis, Johannes Gutenberg University Medical Center, Mainz, Rheinland‐Pfalz, Germany
Background: Tissue factor (TF) and PAR2 signaling regulate insulin resistance (IR) and metabolic inflammation in mouse models of diet‐induced obesity (DIO). While TF‐PAR2 signaling in adipocytes regulates IR, TF‐PAR2 signaling in monocyte/macrophages regulates metabolic inflammation. However, the proteases that modulate TF‐PAR2 signaling remain unclear. TF together with coagulation factor FXa, FVIIa, and endothelial protein C receptor (EPCR) forms distinct ternary or binary protein complexes that modulate PAR2 signaling in a cell‐specific manner. Beyond adipose tissue and macrophages, the intestine also plays a critical role in the manifestation of DIO.
Aims: Given that both PAR2 and FXa are expressed in the intestinal epithelial cells, we aimed to investigate the role of the intestinal FXa‐PAR2 axis in the regulation of DIO.
Methods: For this study, we employed FXa/FVIIa signaling resistant PAR2 mutant (PAR2‐G37I) mice and a complementary mouse model with intestinal epithelial cell specific‐FX deletion (FXfl/fl Villincre). These mice were fed a high‐fat diet (HFD) for 16 weeks and features of DIO including weight gain, glucose tolerance, and insulin sensitivity were determined. Tissue samples were harvested for ex vivo analysis.
Results: Upon HFD feeding, when compared to wild‐type (WT) mice, PAR2‐G37I mutant mice showed reduced weight gain and improved glucose tolerance. These changes were associated with a specific reduction in the postprandial intestinal hormone; glucose‐dependent insulinotropic polypeptide (GIP). In contrast, no difference in glucagon‐like peptide (GLP‐1) levels was observed. Incongruent with PAR2‐G37I mutants, FXfl/fl Villincre mice showed reduced early‐onset obesity and a specific reduction of postprandial GIP. Despite reduced weight gain, FXfl/fl Villincre mice were not protected against obesity‐induced glucose intolerance when compared to HFD‐fed FXfl/fl control mice.
Conclusion(s): These results suggest that FXa‐PAR2 signaling in the intestinal epithelium plays an important role in the regulation of postprandial hormone GIP and early‐onset obesity. However, additional proteases or cell types may play a role in regulating glucose homeostasis.
Complement and Hemostatic System
OC 28.2
Pathway‐based rare variant burden analysis identifies a role for the complement system in purpura fulminans
P. Bendapudi 1; S. Nazeen2; J. Ryu1; O. Soylemez2; B. Rouaisnel3; M. Colling4; B. Pasko1; A. Robbins3; M. Bouzinier2; L. Tomczak1; L. Collier1; S. Ram5; A. Toth‐Petroczy2; E. Fieg2; W. Dzik4; J. Hudspeth6; O. Pozdnyakova7; R. Maas2; S. Sunyaev2; J. Losman3
1 Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States; 2 Brigham and Women's Hospital, Boston, Massachusetts, United States; 3 Dana Farber Cancer Institute, Boston, Massachusetts, United States; 4 Massachusetts General Hospital, Boston, Massachusetts, United States; 5 University of Massachusetts, Worcester, Massachusetts, United States; 6 Boston Medical Center, Boston, Guatemala, United States; 7 Brigham and Women's Hospital, Boston, Guatemala, United States
Background: Purpura fulminans (PF) is a rare subtype of sepsis‐associated coagulopathy that disproportionately affects younger patients and is characterized by uncontrolled systemic thrombosis frequently resulting in end organ damage and death. The complement system is responsible for the initial inflammatory response to infection and has long been considered a key driver of inflammation in severe sepsis and PF.
Aims: We sought to determine whether rare coding variants in the complement system predispose patients to severe sepsis with coagulopathy.
Methods: We performed whole exome sequencing on the Boston PF Cohort (N = 40). Using a novel rare variant trend test (RVTT), we pursued a pathway‐based approach comparing the burden of putatively deleterious rare coding variants in the complement system in PF patients vs. unselected patients with sepsis (N = 87). We subsequently cloned and functionally characterized several novel variants in the integrin complement receptors 3 and 4 (CR3/CR4).
Results: Using RVTT in an ancestry‐stratified analysis, we found a significantly increased burden of rare, putatively deleterious coding variants (ΔP variants) in PF patients compared to unselected patients with sepsis (p = 0.013) (Table). By contrast, no association was found when the RVTT was repeated with synonymous variants or predicted functionally neutral missense variants. Similarly, gene sets from neither the glycolysis pathway nor the coagulation system showed enrichment for ΔP variants in the PF cohort. We cloned and characterized the 11 unique ΔP variants identified in genes encoding subunits of CR3 and CR4 in the PF cohort. CR3 is typically associated with anti‐inflammatory signaling, while CR4 promotes a pro‐inflammatory state. We found that 7/7 (100%) of CR3 variants showed decreased activity, while 6/7 (85.7%) CR4 variants showed increased activity in a dual‐luciferase reporter assay for NF‐κB (Figure).
Conclusion(s): Our results suggest that rare inherited defects in the complement system predispose individuals to the maladaptive hyperinflammatory response of severe sepsis.
Infection and Hemostatic Factors
OC 72.2
Neutrophils protect against Staphylococcus aureus endocarditis but the impact of NET release is negated by coagulases
S. Meyers 1; M. Lox2; S. Kraisin2; C. Martens3; L. Liesenborghs2; L. Frederix2; D. Missiakas4; P. Baatsen5; T. Vanassche6; P. Verhamme2; K. Martinod3
1 Center for Molecular and Vascular Biology, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 2 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 3 KU Leuven, Leuven, Vlaams‐Brabant, Belgium; 4 Department of Microbiology, University of Chicago, Chicago, IL, USA, Chicago, Illinois, United States; 5 EM‐Platform of the VIB Bio Imaging Core and VIB Center for Brain and Disease Research, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 6 University Hospitals Leuven, KULeuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium
Background: Infective endocarditis (IE) is characterized by an infected thrombus. How bacteria bypass the immune system and cause these complex thrombi remains unclear. Neutrophils, via release of neutrophil extracellular traps (NETs), lie at this interface between host defense and thrombosis.
Aims: We aimed to determine the role of neutrophils/NETs in IE.
Methods: We injected mice with Staphylococcus aureus i.v. and stimulated the aortic endothelium locally with histamine, resulting in IE on inflamed valves. We identified neutrophils and NETs in IE by immunostaining, performed antibody‐mediated neutrophil depletion in WT animals, and determined the role of NETs by using neutrophil‐specific PAD4‐knockout mice. In addition, we used S. aureus deficient in nuclease (Δnuc) or both coagulase and von Willebrand factor‐binding protein (Δcoa/Δvwb) to induce IE.
Results: Neutrophils and neutrophils releasing NETs were present in thrombi and within large cellular infiltrates in the surrounding aortic wall in mice with IE. Neutrophil depletion significantly increased endocarditis incidence and led to persistent bacteremia. However, incidence of IE and bacteremia was similar between NETosis‐impaired mice and wild‐type controls. As S. aureus nucleases degrade NETs, we tested Δnuc S. aureus in our model. This mutant did not affect endocarditis incidence, bacteremia and infiltration size. To investigate if bacteria shielded off neutrophils via a coagulase‐induced fibrin layer, mice were infected with Δcoa/Δvwb S. aureus. These mice had improved survival, decreased bacteremia, smaller infiltrates, and decreased tissue destruction. Significantly more NETs were present inside infected thrombi induced by the mutant, which correlated to decreased bacteria and cell death in the surrounding vessel wall.
Conclusion(s): Neutrophils are protective against IE independent of NET release. S. aureus‐induced fibrin likely shields neutrophils from entering the thrombus. In absence of this protective fibrin layer, NETs may be able to constrain the infection and hamper tissue damage, a key hallmark of valve destruction in IE.
Platelets and Cancer
OC 66.2
Platelet‐mediated potentiation of the lung pre‐metastatic niche in breast cancer
H. Roweth 1; M. Malloy2; Q. Guo3; I. Becker4; S. McAllister3; E. Battinelli3
1 Harvard Medical School, Brigham and Women's Hospital, BOSTON, Massachusetts, United States; 2 Brigham and Women's Hospital, Boston, Massachusetts, United States; 3 Harvard Medical School, Brigham and Women's Hospital, Boston, Massachusetts, United States; 4 Boston Children's Hospital, Boston, Massachusetts, United States
Background: Tumor metastasis is the principal cause of death in breast cancer patients and the lungs are a disproportionately common site for metastasis. Primary tumors can orchestrate this organotropism by recruiting bone marrow‐derived cells (BMDCs) to pre‐metastatic lungs, partly through increased lung lysyl oxidase (Lox) expression. In turn, BMDCs create an immune‐suppressive microenvironment commonly referred to as the pre‐metastatic niche (PMN), which supports the seeding of circulating tumor cells. Although platelets recruit BMDCs during wound healing and promote several hallmarks of metastasis, their contributions to the PMN are unknown.
Aims: The aim of the study was to assess if platelets promote BMDC infiltration into pre‐metastatic lungs and to identify platelet cytokines that may recruit BMDCs to the lung PMN.
Methods: Mammary tumors (E0771) were generated in thrombopoietin‐deficient (Thpo‐/‐) mice and lungs extracted for flow cytometric analysis and immunofluorescent staining of cryopreserved tissue sections.
Results: Increased platelet sequestration was observed in the lungs of tumor‐bearing Thpo+/+ mice compared to tumor‐naïve controls. As expected, platelets were reduced (≈90%) in Thpo‐/‐ mice, with no changes in circulating immune cell populations or primary tumor growth. However, the lungs of Thpo‐/‐ tumor‐bearing mice had statistically significant reductions in B cells and inflammatory monocytes, correlating with a reduced Lox staining in lung tissue. Plasma CXCL5 and platelet‐derived growth factor were diminished in tumor‐bearing Thpo‐/‐ mice and platelets from tumor‐bearing mice had increased lipocalin‐2, osteopontin, and S100A8/A9, which can be secreted upon platelet activation.
Conclusion(s): The pre‐metastatic lungs of platelet‐deficient mice had reduced infiltration of metastasis‐promoting BMDCs, which could result from reduced Lox expression. Platelets sequestered in pre‐metastatic lungs may recruit BMDCs to the PMN by secreting CXCL5, osteopontin, and/or S100A8/9, since they were enriched in several of these niche‐promoting factors, suggesting platelet content can be altered by the primary tumor to potentiate the PMN. FIGURE 1 Lowering platelet counts affects the lung pre‐metastatic niche
FIGURE 1 Lowering platelet counts affects the lung pre‐metastatic niche
OC 66.3
The lung pro‐thrombotic niche drives cancer‐associated thromboembolism via exosomal ITGB2
S. Lucotti 1; Y. Ogitani2; C. Kenific1; L. Bojmar1; M. Cioffi1; P. Lauritzen1; H. Molina3; S. Heissel3; H. Lengel4; X. Jing5; H. Zhang1; I. Matei1; E. O'Reilly4; W. Jarnagin4; D. Jones4; J. Bussel1; D. Kelsen4; J. Bromberg4; D. Simeone5; D. Lyden1
1 Weill Cornell Medicine, New York, New York, United States; 2 Daiichi‐Sankyo Co., LTD, Tokyo, Tokyo, Japan; 3 The Rockefeller University, New York, New York, United States; 4 Memorial Sloan Kettering Cancer Center, New York, New York, United States; 5 New York University Langone Health, New York, New York, United States
Background: Thromboembolism (TE) is a common complication in cancer patients, especially at the metastatic stage, and the second leading cause of cancer‐related deaths. The prevention of TE still remains an unmet clinical need due to the lack of predictive biomarkers and the bleeding risk associated with routine anti‐coagulants.
Aims: Our aim is to dissect the role of exosomes as initiator of cancer‐associated TE, in order to develop exosome‐based markers for TE risk and targets for TE prevention.
Methods: Exosomes were isolated from tissues of mice with melanoma (B16F10), breast cancer (MMTV‐PyMT), lung cancer (Lkb1‐/‐ KrasG12D/WT), and PDAC (KPC), or cell lines. The pro‐thrombotic effect of exosomes was studied in vivo (platelet count, D‐Dimer, platelet/fibrin staining), and in vitro (LTA and flow cytometry).
Results: We found that exosomes from pre‐metastatic and metastasis‐bearing lungs of tumor‐bearing mice induce extensive pulmonary embolism in naïve mice and package high levels of integrin beta 2 (ITGB2). Instead, exosomes from normal lungs, cell lines, tumors, or other metastasis‐bearing organs did not show any pro‐thrombotic properties. Monocytes/macrophages infiltrating pre‐/post‐metastatic lungs were the main source of ITGB2+ exosomes. We found that ITGB2 interacts directly or through fibrin with different platelet ligands, and induce their activation and aggregation. Importantly, blockade of ITGB2 on lung exosomes or systemically in mice prevented exosome‐induced platelet aggregation and TE. Finally, we showed that exosomal ITGB2 levels are elevated in the plasma of Stage IV PDAC patients prior to TE events in comparison to patients with no history of TE.
Conclusion(s): We provide the first evidence of the establishment of a pro‐thrombotic lung niche in different cancer types. We have identified exosomal ITGB2 as a new target for the prevention and/or treatment of TE, as well as a potential “liquid biopsy” analyte for the stratification of patients at high risk of TE.
OC 72.3
JAK2V617F mutation exacerbates the thrombo‐inflammatory response to cerebral venous sinus thrombosis in mice
M. Bourrienne 1; M. Solo‐nomenjanahary2; D. Faille3; J. GAY4; S. Loyau5; J. Villeval6; V. Edmond6; I. Plo6; B. Ho‐Tin‐Noé7; M. Mazighi8; N. Ajzenberg9
1 Hôpital Beaujon (APHP); INSERM LVTS U1148 Université de Paris, CLICHY, Ile‐de‐France, France; 2 INSERM U1148, Laboratory for Vascular Translational Science, PARIS, Ile‐de‐France, France; 3 Université de Paris ‐ APHP, PARIS CEDEX 18, Ile‐de‐France, France; 4 Université de Paris ‐ APHP, PARIS, Ile‐de‐France, France; 5 INSERM U1148, Laboratory for Vascular Translational Science, Paris, Ile‐de‐France, France; 6 INSERM UMR1287, Université Paris Saclay, Gustave Roussy, Villejuif, Ile‐de‐France, France; 7 INSERM, PARIS, Ile‐de‐France, France; 8 Département de neurologie, Hôpital Lariboisière (APHP), FHU NeuroVasc, INSERM U1148, Laboratory for Vascular Translational Science, PARIS, Ile‐de‐France, France; 9 APHP, Paris, Ile‐de‐France, France
Background: Cerebral venous sinus thrombosis (CVST) is a rare cause of stroke. JAK2V617F mutation, the most frequent mutation in myeloproliferative neoplasms (MPNs), has been reported to be associated with CVST. Worse outcome has been observed in MPN‐related CVST patients with particularly higher thrombotic recurrence. However, the underlying related pathophysiological mechanisms are still poorly understood.
Aims: To investigate whether JAK2V617F mutation predisposes to specific pathophysiological processes and/or worse prognosis using a mouse model of CVST.
Methods: A new CVST model was achieved by autologous clot injection into the superior sagittal sinus. Clinical, biological and imaging outcomes, were analyzed in mice expressing JAK2V617F mutation only in hematopoietic cells (Jak2V617F) compared to Jak2WT mice. On day 1 (D1) after CVST, parenchymal injury was monitored (magnetic resonnance imaging, immuno‐histology, intravital microscopy). Platelet‐neutrophil agregates (PNAs) and neutrophil activation were determined by flow cytometry analysis of whole blood samples.
Results: At D1 after CVST, survival rate was decreased in Jak2V617F mice compared to Jak2WT mice (78.5% versus 100%, p = 0.04). Two hours after CVST, intravital microscopy demonstrated increased neutrophil and platelet adhesion in cortical cerebral veins in Jak2V617F mice compared to Jak2WT mice. At D1, higher level of circulating PNAs (69 ± 18% vs 23 ± 13%, p < 0.01) and increased expression of CD11b on neutrophils were also observed in Jak2V617F mice. At D1, occurrence of intracranial hemorrhage was higher in Jak2V617F compared to Jak2WT mice (69% vs 36% respectively, p = 0.03). Following sacrifice at D1, brain immunohistological analyses showed that neutrophils localized within cortical haemorrhagic area suggesting involvement of neutrophils in the bleeding process.
Conclusion(s): Our study shows that the JAK2V617F mutation leads to a specific CVST pattern with increased intracranial hemorrhage and mortality. Our results suggest that neutrophils could contribute to hemorrhagic complications as part of the thrombo‐inflammatory response. FIGURE 1 Representative intravital microscopy images showing cerebral vascular network after CVST in Jak2WT (A) and Jak2V617F (B) mice. In Jak2V617F mice, CVST is accompanied by increased adhesion of rhodamine 6G‐labelled platelets/leukocytes and neutrophils (Ly6G) in the cortical veins
FIGURE 1 Representative intravital microscopy images showing cerebral vascular network after CVST in Jak2WT (A) and Jak2V617F (B) mice. In Jak2V617F mice, CVST is accompanied by increased adhesion of rhodamine 6G‐labelled platelets/leukocytes and neutrophils (Ly6G) in the cortical veins
Platelets and Infection
OC 72.5
Toll‐like receptor 4‐dependent and Independent platelet‐dependent thrombosis in SARS‐CoV‐2 infection
V. Cammisotto 1; R. Carnevale2; S. Bartimoccia2; C. Nocella2; M. Bufano2; V. Castellani2; L. Loffredo2; S. Sciarretta2; A. Coluccia2; R. Silvestri2; G. Ceccarelli2; A. Oliva2; M. Venditti2; F. Pugliese2; C. Mastroianni1; O. Turriziani2; P. Pignatelli3; F. Violi4
1 Sapienza University of Rome, Rome, Lazio, Italy; 2 Sapienza university of Rome, Rome, Lazio, Italy; 3 Sapienza University, Rome, Lazio, Italy; 4 Thrombosis and Haemostasis journal, Berlin, Berlin, Germany
Background: Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is associated with an increased risk of venous and arterial thrombosis but the underlying mechanism if still unclear.
Aims: The study would identify a mechanism implicated in platelet activation and thrombus growth during SARS‐CoV‐2 infection.
Methods: We performed a cross‐sectional analysis of platelet function in 30 SARS‐CoV‐2 and 20 healthy subjects (HS) by measuring Nox2‐derived oxidative stress and thromboxane (Tx) B2 and investigated if administration of monoclonal antibodies against the Spike(S) protein of SARS‐CoV‐2 affects platelet activation. Furthermore, we investigated in vitro if the Spike(S) protein of SARS‐CoV‐2 or plasma from SARS‐CoV‐2 enhanced platelet activation.
Results: Ex vivo studies showed enhanced platelet Nox2‐derived oxidative stress and TxB2 biosynthesis and under laminar flow platelet‐dependent thrombus growth in SARS‐CoV‐2 compared to controls; both effects were lowered by Nox2 and Toll‐like receptor 4(TLR4) inhibitors. Two hours after administration of monoclonal antibodies a significant inhibition of platelet activation was observed in SARS‐CoV‐2 patients compared to untreated ones. In vitro study showed that S protein functionally interacts with platelet TLR4, and a docking simulation analysis suggested that TLR4 binds to S protein via three receptor‐binding domains; furthermore, in platelets from SARS‐CoV‐2 S protein co‐immunoprecipitated with TLR4. Plasma from SARS‐CoV‐2 patients incubated with normal platelets enhanced platelet activation and Nox2‐related oxidative stress, an effect blunted by TNF‐alpha inhibitor; this effect was recapitulated by an in vitro study documenting that TNF‐alpha alone promoted platelet activation and amplified the platelet response to S protein via p47phox up‐regulation.
Conclusion(s): The study identifies two TLR4‐dependent and independent pathways promoting platelet‐dependent thrombus growth and suggests inhibition of TLR4 or p47phox as a tool to counteract thrombosis in SARS‐CoV‐2.
Platelets and Inflammation
LB 02.3
Sirtuin 1 as an endogenous inhibitor of NETosis
L. Wang1; C. Yuen1; S. Wong 2
1 Lee Kong Chian School of Medicine, Nanyang Technological University Singapore, Singapore, Not Applicable, Singapore; 2 Nanyang Technological University Singapore, Singapore, Singapore
Background: Neutrophil extracellular traps (NETs) are implicated in thrombosis, stroke, and delayed wound healing, conditions that are prevalent in diabetes. While diabetes is known to predispose neutrophils to NETosis, a knowledge gap persists for years as to how diabetes upregulates NETosis.
Aims: The study sought to dissect the dysregulated pathways that exacerbates NETosis in diabetes.
Methods: NETosis propensity was examined in blood neutrophils freshly isolated from humans and mice, as well as differentiated HL‐60 cells (dHL‐60 cells), by immunofluorescence microscopy. The role of Sirtuin 1 (SIRT1) was examined by targeted knockdown via siRNA transfection in dHL‐60 cells.
Results: Screening of anti‐aging compounds showed that pharmacological inhibition of SIRT1 using Ex‐527 increased NETosis in neutrophils isolated from healthy humans and mice. Excessive NETosis was also observed in dHL‐60 cells transfected with siRNA targeting SIRT1, confirming SIRT1 as an intrinsic inhibitor of NETosis. Interestingly, Ex‐527 gave no further increase in NETosis in neutrophils isolated from diabetic mice, suggesting that SIRT1 is defective in diabetes, leading to a full‐blown NETosis level which is not exacerbated by exogenous inhibition using Ex‐527. We next examined the relationship between SIRT1 and peptidylarginine deiminase 4 (PAD4), an enzyme critical for NETosis. Ex‐527 increased the levels of H4Cithigh neutrophils isolated from healthy subjects and mice, as well as dHL‐60 cells cultured in basal glucose, when challenged. Notably, the effect was abolished in neutrophils isolated from diabetic mice and dHL‐60 cells that had been primed in high glucose, indicating that SIRT1 acts upstream of PAD4 and limits NETosis via PAD4 inhibition in healthy neutrophils and dHL‐60 cells, but not those under diabetic or hyperglycemic conditions.
Conclusion(s): SIRT1, the endogenous inhibitor of NETosis, is dysfunctional in diabetes, thus causing excessive NETosis. Strategies that can revitalize SIRT1 activity are promising in suppressing NETosis and hence curtail NET‐mediated diabetic complications.
OC 66.5
Mitochondrial dysfunction in platelets from patients with JAK2 V617F essential thrombocythemia and polycythemia vera underlies thrombo‐hemorrhagic complications
O. Esparza1; B. McMahon2; G. Hernandez3; D. Le3; M. Kelher4; T. Nemkov5; A. D'Alessandro5; J. Lopez6; P. Davizon‐Castillo 7
1 University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States; 2 Division of Hematology, University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States; 3 Department of Pediatrics Hematology/Oncology and Bone Marrow Transplantation, University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States; 4 Department of Surgery, University of Colorado Anschutz Medical Campus/Vitalant Research Institute, Aurora, Colorado, United States; 5 Department of Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States; 6 Division of Hematology, University of Washington/Bloodworks NW Research Institute, Seattle, Washington, United States; 7 Department of Pediatrics Hematology/Oncology and Bone Marrow Transplantation, Hemophilia and Thrombosis Center, University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States
Background: Myeloproliferative neoplasms are a group of pro‐inflammatory clonal hematopoietic disorders. Patients with JAK2 V617F essential thrombocythemia (ET) and polycythemia vera (PV) experience thrombo‐hemorrhagic events. We have previously identified that platelets from patients with ET/PV exhibit dysmorphic mitochondria. In light of these changes, we hypothesize that platelet metabolism and function are altered.
Aims: We sought to identify and characterize the metabolic and functional alterations of platelets from patients with JAK2 V617F ET/PV.
Methods: Platelets from sex and age‐matched healthy controls and individuals with ET/PV were studied. Platelet activation, calcium mobilization and mitochondrial polarization were determined by flow cytometry. Thrombus formation under flow was measured with the Total Thrombus‐formation Analysis System analyzer. Platelet bioenergetics were studied with the Seahorse extracellular flux analyzer. Semi‐quantitative metabolomics was performed using the Vanquish UHPLC system. Clot retraction analysis was calculated by weight of extruded serum.
Results: ET/PV platelets demonstrated elevated cytoplasmic calcium under resting conditions and after stimulation with thrombin (Figure 1A). ET/PV platelets displayed greater membrane depolarization when compared to healthy controls (p = 0.03). ET/PV platelets also exhibited decreased basal respiration and ATP‐linked respiration (Figure 1B) and reduced ADP and ATP pools (Figure 1C). Functionally, ET/PV platelets exposed more procoagulant phosphatidylserine on the outer membrane leaflet and, under arterial flow conditions, formed thrombi faster (Figure 2A‐B). Despite the accelerated clot formation, platelet contraction was significantly decreased in ET/PV platelets (p = 0.04).
Conclusion(s): We show that JAK2 V617F ET/PV platelets have dysfunctional mitochondria (i.e., perturbed calcium flux and loss of mitochondrial membrane potential), leading to defective energy production. Energy‐demanding processes such as maintaining plasma membrane phospholipid polarity and clot retraction may be disrupted in ET/PV platelets leading to a procoagulant phenotype, accelerated thrombus formation, and an unstable clot. Our findings may explain why patients with ET/PV are at an increased risk for thrombo‐hemorrhagic complications.
OC 72.4
Toxicity of neutrophil extracellular traps (NETs) and the effects of platelet factor 4 (PF4) on NET thrombogenicity: Therapeutic implications
A. Ngo 1; C. Field2; A. Sarkar1; I. Yarovoi1; G. Zhao1; L. Rauova1; M. Kowalska1; M. Poncz1; K. Gollomp1
1 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 2 University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: Sepsis is a life‐threatening response to infection. Neutrophils prevent sepsis by releasing NETs to entrap bacteria; yet most experimental NET‐directed therapies are designed to accelerate NET degradation. We hypothesize that NET degradation products induce greater harm than intact NETs, and propose the alternative strategy of NET‐stabilization with PF4.
Aims: We compared coagulopathy and endothelial dysfunction induced by intact or degraded NETs and cell‐free (cf)DNA, and effects of PF4 on those properties.
Methods: Human neutrophils were stimulated to release NETs and treated with mild DNase to liberate high‐molecular‐weight (hmw) NETs. High and low molecular weight (lmw) cfDNA and single‐stranded (ss) DNA were also studied. We assessed effects of PF4 on DNA/NET/ssDNA‐induced thrombin and fibrin generation in normal, factor (F) XII‐ and FXI‐depleted plasma. Effects of PF4 on DNA‐induced endothelial dysfunction were studied using human umbilical vein endothelial cells (HUVECs) exposed to DNA/ssDNA and stained for von Willebrand factor (vWF) release. HmwDNA was administered intravenously to wildtype (WT) and PF4‐/‐ mice. Cremaster venous thrombosis and plasma thrombin‐anti‐thrombin (TAT) were assessed.
Results: HmwNETs/DNA were less thrombogenic than lmwNETs/DNA or ssDNA. PF4 delayed thrombin and fibrin generation induced by NET/DNA fragments of any length. PF4 did not affect DNA‐induced fibrin generation in FXII‐ or FXI‐depleted plasma, but supplementation with FXII or FXI rescued PF4 anticoagulant effects. LmwDNA and ssDNA induced vWF release by HUVECs, which was prevented by addition of PF4. Infused cfDNA markedly enhanced fibrin accumulation and neutrophil recruitment into thrombi in PF4‐/‐ compared to WT mice, corresponding to higher TAT levels in PF4‐/‐ mice.
Conclusion(s): NET‐stabilization with PF4 decreases thrombosis by (1) inhibiting DNase digestion of intact NETs and subsequent liberation of prothrombotic cfDNA, and (2) preventing further digestion of circulating cfDNA into shorter and more prothrombotic fragments. These studies further support our hypothesis that NET‐stabilization with PF4 is protective in sepsis, meriting further translational studies.
OC 72.1
Platelet HMGB1 mediates pathological NET formation in ischemic stroke
F. Denorme 1; I. Portier1; Y. Kosaka1; J. Rustad1; M. Cody1; C. Yost1; M. Neal2; J. Majersik1; R. Campbell1
1 University of Utah, Salt Lake City, Utah, United States; 2 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States
Background: Ischemic stroke provokes a strong inflammatory response which is associated with worse outcomes in patients. Classic anti‐inflammatory strategies have been unsuccessful in clinical trials for ischemic stroke, implying other mechanisms contribute to injurious inflammation in ischemic stroke.
Aims: In this study, we investigated mechanistic regulators of neutrophil extracellular trap (NET) formation in stroke and if they contribute to ischemic stroke outcomes.
Methods: Brain tissue and plasma from ischemic stroke patients and healthy matched controls were analyzed for the presence of NETs (n = 28 per group). Flow cytometry was used to characterize platelet function. For murine stroke studies, mice were subjected to transient middle cerebral artery occlusion.
Results: NET forming neutrophils were found in brain tissue of all stroke patients examined while NETs were absent in healthy brain tissue. Specific markers of NET formation including citrullinated histone H3 (H3cit) and MPO‐DNA complexes were significantly elevated in plasma from stroke patients compared to controls. Interestingly, H3cit and MPO‐DNA complexes significantly correlated with worse long‐term stroke outcomes (r = 0.45, p = 0.024 and r = 0.507, p = 0.01, respectively). Next, we observed increased plasma and platelet‐surface expressed high mobility group box 1 (HMGB1) in ischemic stroke patients compared to matched controls, and HMGB1 correlated with plasma NETs (r = 0.433, p = 0.0019). Blocking HMGB1 in vitro prevented platelet‐induced NET formation. Mechanistically, depleting platelets in mice before stroke reduced plasma HMGB1 levels as well as NET formation and improved outcomes after ischemic stroke (p < 0.005). Treatment of thrombocytopenic mice with recombinant HMGB1 increased stroke‐induced NETs to similar levels of non‐platelet depleted mice and exacerbated stroke outcomes. In agreement with these results, platelet‐specific HMGB1 knockout mice had significantly reduced NETs after stroke and greatly improved stroke outcomes (p < 0.001).
Conclusion(s): Our results support a pathological role for platelet derived HMGB1 in mediating NET formation in ischemic stroke and warrant further investigation into targeting NETs to improve stroke outcomes.
Pediatrics
Bleeding in Neonates and Children
OC 39.4
Clinical characteristics and management of children with severe factor VII deficiency‐ analysis of a United Kingdom cohort
T. Biss 1; J. Motwani2; J. Grainger3; M. Mathias4; S. Stokley5; A. Kelly6; J. Payne7; J. Alamelu8; S. Green9; E. Chalmers10; N. Bhatnagar11
1 The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, England, United Kingdom; 2 Birmingham Women's and Children's NHS Foundation Trust, Birmingham, England, United Kingdom; 3 Royal Manchester Children's Hospital, Manchester, England, United Kingdom; 4 Great Ormond Street Hospital for Children NHS Foundation Trust, London, England, United Kingdom; 5 Nottingham University Hospitals NHS Trust, Nottingham, England, United Kingdom; 6 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 7 Sheffield Children's NHS Foundation Trust, Sheffield, England, United Kingdom; 8 Evelina London Children's Hospital, London, England, United Kingdom; 9 Hull University Teaching Hospitals NHS Trust, Hull, England, United Kingdom; 10 Royal Hospital for Children, Glasgow, England, United Kingdom; 11 Oxford University Hospitals NHS Foundation Trust, Oxford, England, United Kingdom
Background: Severe factor VII (FVII) deficiency is a rare bleeding disorder. Bleeding phenotype and management in children with severe FVII deficiency is poorly described.
Aims: This retrospective, observational study aimed to characterise bleeding phenotype and management in individuals aged <18 years with severe FVII deficiency.
Methods: The UK National Haemophilia Database was scrutinised for individuals aged <18 years with baseline FVII <0.05 IU/ml. Individual centres were contacted to provide additional data.
Results: 27 individuals were identified, 16 males and 11 females, from 9 centres. Additional data were available for 26 children, aged 3 months–15 years (median age: 10.5 years). FVII was 10 years, intracranial haemorrhage (ICH, 4), muscle haematoma (2), haemarthrosis (2), cephalohaematoma (1), dental extraction bleeding (1), gastrointestinal bleeding (1), umbilical cord bleeding (1). ICH occurred at birth, day 4 of life, 2 and 6 months of age and none required surgical management. Four individuals had no bleeding symptoms. Ten receive prophylaxis with FVII concentrate, 9 recombinant and 1 plasma‐derived. Prophylaxis was commenced for significant bleeding (4 ICH, 1 muscle haematomata, 1 mucosal bleeding) or for primary prophylaxis (4). Only one commenced prophylaxis after 2 months of age. Treatment‐related complications were reported in two individuals, FVII inhibitor and multiple central venous line‐related thromboses.
Conclusion(s): Serious bleeding in severe FVII deficiency, including ICH, occurs early in life. Frequent non‐severe bleeding symptoms are mouth/nose bleeding and menorrhagia. More than one third require prophylaxis and this is started early in life.
OC 39.2
Predictors of platelet function disorders in pediatric patients
B. Doshi 1; E. Thompson2; A. Obstfeld3; M. Lambert1
1 Division of Hematology, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 2 Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, Pennsylvania, United States; 3 Department of Pathology and Laboratory Medicine, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States
Background: In pediatric patients, data about the presentation, etiologies, and accuracy of diagnosis of qualitative platelet function disorders (PFD) are limited. Understanding the patterns of PFD and presenting signs and symptoms could inform the utility and efficacy of laboratory testing.
Aims: The objectives of this study were to (1) understand the presenting indications and severity of bleeding that best predict abnormal platelet function testing, (2) identify clinical and demographic factors associated with PFD, and (3) assess the accuracy of diagnosis.
Methods: This was a single center, retrospective cohort study of patients aged 0–21 years evaluated by pediatric hematology for a bleeding disorder which included platelet function testing. Charts were abstracted for demographics, test indication, and platelet function testing results. Retrospective bleeding severity scores (ISTH BAT) were completed for all patients.
Results: Of 446 pediatric patients who underwent platelet light transmission aggregometry (LTA) testing, 151 (33.8%) had abnormal results including 86 (57%) males and 65 (43%) females. The most common test indications were epistaxis (28%), bruising (23.3%), heavy menses (16.1%) family history of bleeding (15.2%), petechiae (11.7%), and surgical hemorrhage (11.2%). Most patients had defects in aggregation and secretion to ADP, epinephrine, or both. Age, BAT score and >1 indication for testing did not correlate with increased odds of abnormal LTA. Male sex was associated with abnormal LTA (OR 1.77, 95% CI 1.18–2.63). Of 85 patients with abnormal initial LTA, 22 (25.9%) had normal results on subsequent testing.
Conclusion(s): In this pediatric cohort, abnormal LTA correlated with male sex but not BAT score, age, or multiple indications for testing. Notably, 25% of patients with an abnormal initial LTA normalized on subsequent testing. These data highlight the need for systematic, prospective studies of pediatric PFD to inform need for laboratory testing and diagnostics.
OC 39.5
The immediate effect of COVID‐19 vaccination on anticoagulation control in adolescents and young adults using vitamin K antagonists
C. Visser 1; A. Yousefi2; M. Nierman3; M. Huisman4; A. Gulpen5; C. van Ommen6; M. Kruip7
1 Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Haematology, Erasmus MC, Erasmus University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 3 Department of Thrombosis and Anticoagulation, Atalmedial Medical Diagnostics Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 5 Department of Internal medicine, the Elkerliek Hospital, Helmond, the Netherlands, Elkerliek, Noord‐Brabant, Netherlands; 6 Erasmus Medical Center‐Sophia Children's, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 7 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: The European Medicine Agency has authorized COVID‐19 vaccination in young adults from 12 years onwards. COVID‐19 vaccination is associated with a negative effect on the quality of anticoagulation stability in adult vitamin K antagonists (VKA) users, due to an increased risk of supra‐ and subtherapeutic INRs after the first vaccination. It is unknown whether this effect is also observed in adolescents and young adults (AYA) using VKAs.
Aims: To investigate whether the COVID‐19 vaccine also affects anticoagulation stability in AYAs using VKA.
Methods: A case‐crossover study was performed in a cohort of AYAs (12–30 years) using VKA. INR results before vaccination, the reference period, were compared with the first INR after the first and, if applicable, second vaccination. Vaccination is deemed safe when the INR is <3.5. Anticoagulation clinics were encouraged to measure the INR within 2 weeks after vaccination.
Results: Ninety‐six AYAs were included, with a median age [IQR] of 25 [7] years, of whom 53.1% were female and 67.7% used acenocoumarol. The majority of AYAs (69.8%) received the BNT162b2 vaccine. [Table 1]. The percentage of INR results within range was significantly lower after the first vaccination (60/97 (62.5%) vs. 40/97 (41.7%), p = 0.004) due to an increase in supratherapeutic INRs (12/97 (12.5%) vs. 30/97 (31.3%), p = 0.005) [Figure 1]. The percentages of subtherapeutic INRs (24/97 (25.0%) vs. 26/97 (27.1%), p = 0.864) and INRs ≥5 (1/97 (1.0%) vs. 2/97 (2.1%), p = 1.000) before and after first vaccination were similar. No differences were observed after the second vaccination compared to before or after the first vaccination. Complications after vaccination occurred less often than before vaccination (3.0 vs. 20.0, p = 0.012) and were non‐severe.
Conclusion(s): COVID‐19 vaccination is also associated with a negative effect on anticoagulation stability in AYA VKA users, but not with an increase in complications. Still, it is advisable to monitor the INR shortly after vaccination.
OC 39.1
Phenotypic switch of platelet function from preterm to term neonates – Daily life data from the Platelets In Neonatal Infants Study (PLINIUS)
M. Drayss 1; L. Weiss2; K. Mott2; C. Haertel3; C. Speer4; H. Schulze5; O. Andres6
1 Institute of Experimental Biomedicine, Chair I, University Hospital Würzburg; Department of Internal Medicine I, University Hospital Wuerzburg, Wuerzburg, Bayern, Germany; 2 University Hospital Wuerzburg, Würzburg, Bayern, Germany; 3 Department of Pediatrics, University Hospital Wuerzburg, Wuerzbug, Bayern, Germany; 4 Department of Pediatrics, University Hospital Wuerzburg, Wuerzburg, Bayern, Germany; 5 Institute of Experimental Biomedicine, Chair I, University Hospital Wuerzburg, Centre of Inherited Blood Cell Disorders, University Hospital Wuerzburg, Würzburg, Bayern, Germany; 6 Department of Pediatrics, University Hospital Wuerzburg, Centre of Inherited Blood Cell Disorders, University Hospital Wuerzburg, Wuerzburg, Bayern, Germany
Background: Prematurity is a major risk factor for neonatal hemorrhage, sepsis, and thrombosis. Platelet receptor function in premature infants and its transformation from fetal to postnatal reactivity is yet incompletely understood.
Aims: Platelet receptor expression and reactivity to agonists were studied in premature and term infants and correlated with their respective gestational age (GA).
Methods: We recruited a cohort of 68 subjects comprising seven early preterm (27–31 weeks GA), 26 preterm (32–36 weeks GA) and 13 term neonates. Eight Infants (<2 years), five children (2–12 years) and nine adolescents/adults (>12 years) served as controls. After informed consent, blood was drawn at three different time points (days 0–2, 3–7, and 8–14 respectively) and analyzed by flow cytometry. The study was approved by the local ethics committee.
Results: Expression levels of the major surface receptors for fibrinogen (CD41/CD61), collagen (GPVI; CD29/CD49b), von‐Willebrand factor (CD42a/CD42b), and TLR‐4 were overall reduced, but showed a strong linear correlation with decreased platelet size (forward scatter), indicating an unaltered surface density. P‐selectin (CD62P) surface neo‐exposition (figure 1A) upon stimulation with ADP or GPVI‐activating antibody HY101 increased significantly with rising GA, whereas reactivity towards TRAP‐6 stimulation was unaltered (figure 1B). Unexpectedly, integrin activation (PAC‐1 binding, figure 2) upon ADP or TRAP‐6 was blunted in neonates and infants. In contrast, the proportion of platelet‐leukocyte (CD45+/CD41+) and platelet‐monocyte aggregates (CD14+/CD41+) were significantly increased in preterm and term neonates without clinical signs of infection (mean 41% vs. 20%, p < 0.001, and 7% vs. 5%, p < 0.05, respectively).
Conclusion(s): Our findings imply that platelet signaling maturation from fetal to neonatal platelet function is uncoupled and is not directly associated with receptor surface expression. Development of alpha granule release precedes GPIIb/IIIa integrin activation, an observation which might be exploited to better understand this phenotypic switch. Our data suggest an immune modulatory role of neonatal platelets beyond hemostasis.
OC 39.3
Mapping hemorrhagic stroke in the fetal and neonatal period as a consequence of thrombocytopenia
A. Farley; S. Lloyd; M. Dayton; C. Biben; O. Stonehouse; A. Terreaux; S. Taoudi
WEHI, Melbourne, Victoria, Australia
Background: Severe thrombocytopenia is a major clinical problem, it affects 1 in 2000 live births, 30 % of babies admitted to neonatal intensive care units, and 70% of babies with extremely low birth weight. Severe thrombocytopenia is most commonly caused by fetal and neonatal alloimmune thrombocytopenia (FNAIT). The main complication of FNAIT is intra‐cerebral hemorrhage (ICH) which carries significant risk of life‐long morbidities and death but a causative role of thrombocytopenia leading to ICH was unknown.
Aims: To demonstrate that platelets are required throughout development to maintain cerebral vasculature integrity and to prevent ICH. To understand the level of thrombocytopenia that would lead to ICH and to define the platelet count required to prevent/minimise ICH.
Methods: We have used two models of severe thrombocytopenia, the Nfe2 knock out mouse line which are deficient in platelets and secondly, a clinically relevant model of FNAIT. In both models we have determined the extent of thrombocytopenia by analysis of the peripheral blood and we used image analysis to determine brain bleeds and location.
Results: In the absence of platelets, brain bleeds occur as early at E11.5‐E12 and persist through development. That the first bleeds always occur in the ganglionic eminence region of the brain. We demonstate that bleeds occur in different regions of the brain depending on when thrombocytopenia occurs and by controlling the level of thrombocytopenia we can determine the platelet count required to reduce the risk of ICH.
Conclusion(s): We demonstrate that platelets are required throughout development for maintaining cerebral vasculature integrity and that ICH occurs in regions of the brain which in humans, can lead to neurologic damage. By tuning platelet counts in utero we have defined levels of thrombocytopenia that have differential risk of developing ICH and additionally, by the second week after birth the cerebral vasculature has developed a resilience to severe thrombocytopenia.
Thrombosis in Neonates and Children
OC 08.5
Clinical prediction tool to identify children at risk for pulmonary embolism
T. Tiratrakoonseree 1; S. Charoenpichitnun2; R. Natesirinilkul3; N. Songthawee4; P. Komvilaisak5; P. Pongpicha6; J. Vaewpanich6; N. Sirachainan7
1 Faculty of Medicine Ramathibodi Hospital, Mahidol University, Pakkret, Nonthaburi, Thailand; 2 Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangbon, Krung Thep, Thailand; 3 Faculty of Medicine Chiang Mai University, Chiang Mai, Thailand, Chiang Mai, Chiang Mai, Thailand; 4 Department of Pediatric, Faculty of Medicine Prince of Songkla University, Hat Yai District, Songkhla, Thailand; 5 Srinagarind hospital, Department of Pediatric, Faculty of Medicine Khon Kaen University, Mueang Khon Kaen District, Khon Kaen, Thailand; 6 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Ratchathewi, Krung Thep, Thailand; 7 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Background: The diagnosis of pediatric pulmonary embolism (PE) is usually delayed due to non‐specific signs and symptoms. The Wells score is highly effective among adult patients, but the application to the pediatric population has shown relatively lower sensitivity and specificity. The establishment of a new PE prediction tool for pediatric patients is warranted.
Aims: To establish a clinical prediction tool for pediatric PE.
Methods: A multi‐center retrospective study, approved by the Institutional Review Board, included children ≤18 years of age who underwent computed tomography pulmonary angiogram due to the suspicion of PE. The patients were diagnosed at four university hospitals from 2006 to 2022. The patients were divided into PE‐positive, and PE‐negative groups. The reported risk factors (Table 1) for venous thromboembolism (VTE) were compared between the two groups using a univariate logistic regression analysis. Those with a p‐value <0.10 were selected for further analysis by a multivariate model. A clinical prediction tool was created using the ROC curve obtained from the data of the significant risk factors (p < 0.05).
Results: A total of 89 patients had at least one clinical presentation of PE. Of those patients, 36 (40.4%) were grouped as PE‐positive and 53 (59.6%) as PE‐negative. Four risk factors, including congenital heart disease/pulmonary surgery, thrombophilia, history of previous VTE, and nephrotic syndrome, showed statistically significant differences between the two groups (Table 1). The ROC curve demonstrated a clinical prediction tool that yields an 83.3% sensitivity and a 64.2% specificity in the patients with suspected PE who had one of the four risk factors.
Conclusion(s): The study identified a clinical prediction tool for the suspicion of PE in this population. Pediatric patients who show clinical presentations of PE and have at least one of the significant risk factors should be further investigated for the diagnosis of PE.
OC 08.3
Venous thromboembolism in a large series of pediatric patients with acute lymphoblastic leukemia. Risk factors and use of prophylaxis
R. Berrueco 1; A. Ruiz‐Llobet2; S. Gassiot3; E. Sarrate4; J. Zubicaray5; J. Dapena6; A. Menárguez7; M. Panesso7; C. Montoya8; J. Vagace9; J. Molina10; M. García‐Morín11; M. García‐Abos12; M. Mendoza13; F. Lendinez14; P. Palomo15; M. Tallón16; B. González17; E. Urrutia18; J. Serna19
1 Hospital Sant Joan de Déu, Esplugues Llobregat, Catalonia, Spain; 2 Hospital Sant Joan de Déu, Esplugues de Llobregat, Catalonia, Spain; 3 Sant Joan de Déu Hospital, Esplugues de LLobregat, Catalonia, Spain; 4 Sant Joan de Déu Hospital, Esplugues de Llobregat, Ceuta, Spain; 5 Hospital Infantil Universitario Niño Jesús, Madrid, Madrid, Spain; 6 Hospital Sant Joan de Déu, Barcelona, Catalonia, Spain; 7 Hospital Universitario Vall d'Hebrón, Barcelona, Catalonia, Spain; 8 Hospital de Alicante, Alicante, Comunidad Valenciana, Spain; 9 Complejo Hospitalario Universitario de Badajoz., Badajoz, Extremadura, Spain; 10 Hospital Universitario Reina Sofía, Córdoba, Andalucia, Spain; 11 Hospital Gregorio Marañón, Madrid, Madrid, Spain; 12 Hospital Universitario Donostia, Donostia, Pais Vasco, Spain; 13 Hospital Universitario de Salamanca, Salamanca, Castilla y Leon, Spain; 14 Hospital Torrecárdenas, Almería, Andalucia, Spain; 15 Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain; 16 Hospital Álvaro Cunquerio Vigo, Vigo, Galicia, Spain; 17 Hospital Universitario La Paz, Madrid, Madrid, Spain; 18 Hospital Materno Infantil Virgen de las Nieves, Granada, Andalucia, Spain; 19 Hospital Sant Pau i la Santa Creu, Barcelona, Catalonia, Spain
Background: Symptomatic venous thromboembolism (VTE) is diagnosed in 3%–14% of patients during pediatric acute lymphoblastic leukemia (ALL) therapy. There are well‐known risk factors, but the role of others as inherited thrombophilia is still controversial. Prophylaxis with low molecular weight heparin (LMWH) has been described, but its use is not globally accepted.
Aims: To evaluate VTE rate and VTE risk factors in ALL patients treated following the same protocol, and safety and effectiveness of LMWH administration as thromboprophylaxis in children with inherited thrombophilia.
Methods: A retrospective multicentric study in ALL patients 1–18 years old following SEHOP‐PETHEMA‐2013 treatment guideline was performed to evaluate VTE rate, anticoagulant treatment, outcome, risk factors, and safety and usefulness of LMWH administration as primary thromboprophylaxis in children with inherited thrombophilia.
Results: A total of 652 patients were included in the study. VTE incidence was 8.7%. Most of the cases occurred during induction therapy, associated to central venous catheter. Univariant analysis showed that family history of thrombosis, presence of mediastinal mass, high‐risk treatment group and inherited thrombophilia were statistically significant risk factors. Inherited thrombophilia was also an independent risk VTE factor in multivariate analysis (figure 1). LMWH administration was related to a decrease of VTE in patients with inherited thrombophilia and those with T‐ALL phenotype.
Conclusion(s): Most of VTE cases occurred in patients without inherited thrombophilia, but when it is present, VTE risk is higher. LMWH administration was useful to decrease VTE in these patients. FIGURE 1 Thrombosis‐free cumulative survival
FIGURE 1 Thrombosis‐free cumulative survival
OC 08.4
Predictive risk factors for recurrent VTE in children
S. Laabar1; K. Fijnvandraat2; E. Rettenbacher3; S. Gouw4; I. Klaassen 5
1 University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Pediatric Hematology, Emma Children's Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Emma Children's Hospital/Amsterdam University Medical Centers, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Pediatric Hematology, Emma Children's Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 5 Emma Children's Hospital/Amsterdam University Medical Centers, Amsterdam, Noord‐Holland, Netherlands
Background: A serious long term complication following venous thromboembolism (VTE) is recurrent thrombosis. Prolongation of the initial anticoagulant therapy is effective in reducing recurrent VTE, but associated with bleeding complications. Therefore, identification of the patients at risk of recurrent VTE is crucial to target prolonged anticoagulation in this specific group.
Aims: The primary aim of this study was to determine these risk factors and the secondary aim was to determine the incidence of recurrent VTE and recurrent‐free survival.
Methods: For this retrospective cohort study all children (0–18 years) with a VTE between 2000 and 2021 treated at Emma Children's Hospital, Amsterdam UMC were eligible. The following determinants were studied: age, comorbidity, CVC, thrombusresolution, thrombophilic mutation, inflammatory status and thromboprophylaxis.
Results: During the observation period 703 patients were eligible and 637 (91%) are included in the current analysis. Recurrent VTE occurred in 134/637 patients (21%). Median age was comparable in the recurrence (4.5 ± 7.0 years) and non‐recurrence group (4 ± 7.2 years). Most frequent comorbidity in recurrent VTE was total parenteral nutrition (TPN) (22.4%) and active malignancy (20.9%) compared to infection (21.9%) and active malignancy (20.3%) in patients without recurrence. The median time to recurrence was 182 days after the initial VTE. Risk factors significantly associated with recurrent VTE were lack of thrombus resolution (p = 0.026, OR = 4.6), TPN dependency (p = 0.002, OR = 59.7), active inflammation at time of VTE (p = 0.011, OR = 5.0) and the presence of a thrombophilic mutation (p = 0.007, OR = 0.182).
Conclusion(s): This study demonstrated a high recurrence rate, that was associated with the following determinants: lack of thrombus resolution, TPN dependency, active inflammation at time of VTE and the presence of thrombophilic mutation. These findings may aid in targeting more personalized treatment strategies in the future.
OC 15.3
Real world use of apixaban for the prevention and treatment of thrombosis in children with cardiac and complex disease
C. Vanderpluym 1; P. esteso2; A. Helllinger2; C. Ventresco2; B. Hawkins2; S. O'Brien2; R. Kobayashi2; R. Williams3; M. Cetatoui2; J. Esch2
1 Boston Children's Hospital and Harvard School of Medicine, Boston, Massachusetts, United States; 2 Boston Children's Hospital, Boston, Massachusetts, United States; 3 Mattel Children's Hospital at UCLA, Los Angeles, California, United States
Background: Direct oral anticoagulants (DAOCS) are ubiquitous for thromboprophylaxis and treatment in adults.
Aims: We present our experience of apixaban use for prevention and treatment of thrombosis in children with congenital and acquired cardiac disease, using weight‐ and level‐based dosing.
Methods: Retrospective single‐center analysis of cardiac patients <19 years treated with apixaban (graduated/not enrolled/ineligible for any other DOAC trial). Patients were evaluated for safety (clinically relevant non‐major (CRNM) or major bleeding; thrombotic events) and efficacy (thrombus improvement). Peak apixaban levels were analyzed according to indication.
Results: Over three years (9/2018–9/2021) 220 children, median age 7 years (0.3–19), median weight 22.4 kg (4.8–160) received apixaban, totaling 51,013 patient‐days. 172 (78%) warranted thromboprophylaxis: 56 (25%) post cardiac surgery, 35 (16%) post catheterization, 22 (10%) failing Fontan, 15 (7%) heart failure, 17 (8%) Kawasaki/MIS‐C and 11 (5%) other (including 2 ventricular assist devices). 48 required thrombosis treatment: 11 (5%) arterial, 19 (9%) venous, 15 (7%) intracardiac, and 3 pulmonary (1%). Peak levels (apixaban chromogenic anti‐Xa assay, HE‐Stago2) were measured to be 158/220 ng/ml (72%), with median 166 ng/ml [23–474; n = 125] among thromboprophylaxis and 153 ng/ml [30–450; n = 33] among thrombosis treatment. Based on level and indication, dose adjustment resulted in lower repeat levels in thromboprophylaxis [median 123 ng/ml (23–450); n = 36] and higher level in treatment [202 ng/ml (55–450); n = 9]. There were 5 bleeding safety events (4 CRNM; 1 major, hemoptysis complicating empyema), all in the thromboprophylaxis group. Bleeding event rate was 3.6 per 100 patient‐years of apixaban. 16% of patients reported minor bleeding or minor adverse events (AEs), including 2 patients with leukopenia and 1 with transaminitis. Improvement in thrombosis was seen in 87% (n = 33/38); 1 patient had thrombosis progression (pulmonary embolism requiring surgical revision of pulmonary artery graft).
Conclusion(s): Apixaban use was feasible and safe utilizing commercially available tablets dosed to weight and adjusted based on peak apixaban levels.
OC 08.2
Thrombotic and bleeding complications in paediatric inflammatory multisystem syndrome temporarily associated with COVID‐19 (PIMS‐TS) in children: A systematic review
A. Al‐Huniti1; S. Renzi2; S. Ali3; K. Langenberg‐Ververgaert4; R. Laxer5; L. Brandão 6
1 Mayo Clinic, Rochester, Minnesota, United States; 2 CHUL Hospital, Quebec City, Quebec, Canada; 3 Leeds Children Hospital, Leeds, England, United Kingdom; 4 Princess Máxima Center for pediatric oncology, Utrecht, Utrecht, Netherlands; 5 The Hospital for Sick Children, Toronto, Ontario, Canada; 6 The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada, Toronto, Ontario, Canada
Background: Paediatric Inflammatory Multisystem Syndrome Temporarily Associated with COVID‐19 in Children (PIMS‐TS) is a newly defined inflammatory disease following COVID‐19 infection. Limited reports exist investigating thrombosis/bleeding in PIMS‐TS.
Aims: To describe the thrombotic and bleeding complications in this population.
Methods: A systematic literature review was conducted searching MEDLINE®, EMBASE and Cochrane Libraries from inception until May 29, 2021. Primary outcomes were prevalence of thrombotic and bleeding complications; secondary outcomes evaluated the anticoagulation regimens used.
Results: One hundred and thirty‐two studies met eligibility criteria (124 retrospective, 8 prospective; figure 1) describing 3240 patients (male 58%; mean age 8.3 years ± 2.2; table 1). PIMS‐TS subtypes (table 1) included shock (47%), fever and inflammation (21%) and Kawasaki disease (4%). Two‐thirds of patients (65%; 2118/3240) were critically ill, 40% (1295/3240) required inotropic use, and 2% (58/3240) required ECMO support. Half of patients received anticoagulation (51%; 1638/3240; therapeutic [120]; prophylactic [408]; risk‐stratified approach [14]; not specified [1096]) and/or antiplatelet therapy (51%; 1659/3240). Thrombotic events (TE) were reported in about 3% (62/2057) of patients, with DVT/PE (50%; 31/62) being the most common followed by ischemic strokes (IS, 21%; 13/62), and cardiac thrombosis (11%; 7/62). Clinically, TE patients required more mechanical ventilation (MV, 65% vs. 20%*) and ECMO support (35% vs. 1%*), presenting a higher mortality rate (11% vs. 2%*). Bleeding was reported in 2.6% (16/618), of which 60% (10/16) were major bleeding events (MBE). Patients with MBE were more likely to be on ECMO (50% vs. 2%*) and MV (70% vs. 17%*), and four patients died (40%, 4/10; 2 had concurrent TE).
Conclusion(s): Patients with PIMS‐TS are at risk of thrombosis despite widespread anticoagulation use, particularly in critically ill patients (i.e., requiring MV and/or ECMO). MBE are also prevalent in this same risk group and associated with mortality. A risk‐stratified anticoagulation approach requires further investigation. *p value <0.01.
OC 15.4
Menstrual bleeding in adolescents taking dabigatran for acute VTE treatment and secondary prevention
L. Brandão 1; I. Tartakovsky2; J. Van Ryn3; C. Miede4; M. Albisetti5
1 The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada, Toronto, Ontario, Canada; 2 Boehringer Ingelheim International GmbH, Ingelheim, Germany, Ingelheim, Rheinland‐Pfalz, Germany; 3 Boehringer Ingelheim International GmbH, Biberach, Germany, Biberach, Baden‐Wurttemberg, Germany; 4 mainanalytics GmbH, Sulzbach. Germany, Sulzbach, Saarland, Germany; 5 University Children's Hospital, Zürich, Switzerland, Zürich, Zurich, Switzerland
Background: Anticoagulation is associated with an increased risk of abnormal menstrual bleeding (AMB). AMB was less frequent with dabigatran etexilate (DE) than warfarin in adult women with venous thromboembolism (VTE), however AMB was not evaluated in adolescents anticoagulated with DE or standard of care (SOC) in pediatric studies.
Aims: Evaluate AMB events in DE‐ and SOC‐treated adolescent females aged 10 to <18 years for treatment and secondary VTE prophylaxis from the pediatric trial program.
Methods: AMBs were evaluated in adolescents in DIVERSITY (NCT01895777), where patients with confirmed VTE initially treated with heparins were randomized (2:1) to receive up to 3 months of DE or SOC and the secondary VTE prevention trial (NCT02197416), where patients with persistent VTE risk factors were anticoagulated with DE for up to 12 months (no SOC arm existed in this trial). AMB events and severity were reported by trial investigators and evaluated from adverse event data. All bleeding events (BE) from both trials were adjudicated. All events occurred on treatment and are reported descriptively.
Results: There were 93 and 72 adolescent females in DIVERSITY and the secondary VTE prevention trial, respectively. AMB occurred in 4/65 (6.2%) DE and 4/28 (14.3%) SOC patients in DIVERSITY (Table). Non‐menstrual BEs were reported in 13/65 (20.0%) DE and 11/28 (39.3%) SOC patients. No major but one Clinically Relevant Non‐Major (CRNM) BE was reported among AMB events in the DE. In the secondary prevention trial, AMB occurred in 9/72 (12.5%) patients treated with DE, with one CRNM BE, and non‐menstrual BEs in 20/72 (27.8%) patients with one major bleeding.
Conclusion(s): Despite small numbers, there were fewer AMBs associated with DE treatment than with SOC in DIVERSITY and an overall low AMB rate in the secondary VTE prevention trial across the adolescent female population from 10 to <18 years.
OC 08.1
High rate of recurrent thrombosis in children with unprovoked venous thromboembolism
H. Whitworth 1; R. Hubbard2; C. Leonard2; C. Witmer3; L. Raffini1
1 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 2 Department of Biostatistics, Epidemiology & Informatics Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 3 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States
Background: The high risk of recurrent venous thromboembolism (VTE) in adults with unprovoked VTE led to recommendations for prolonged anticoagulation. Little evidence guides anticoagulation duration for pediatric unprovoked VTE.
Aims: Estimate the incidence of recurrent VTE in children with unprovoked VTE and evaluate characteristics associated with recurrence.
Methods: We performed a single center, retrospective study including children aged 1 to <21 years with unprovoked index VTE between 1/1/2003 and 8/1/2021. Patients were followed from first VTE until recurrent VTE or censoring (death, age 21 years, or date of last contact). Analysis included descriptive statistics and estimated incidence of recurrent VTE with 95% confidence interval (CI). Time to recurrent VTE was modeled with Kaplan‐Meier estimator, and we compared the hazard of recurrence according to thrombophilia, age, and sex with univariate Cox proportional hazards regression. The Institutional Review Board exempted this study and the National Institutes of Health support HW.
Results: 85 children met inclusion criteria (Table 1) with 26 recurrent events in 250.26 person‐years (incidence rate = 104 [95% CI: 71–153] per 1000 person‐years). 61% (95% CI: 46%–73%) of children were free from recurrent VTE at 5 years (Figure 1). Univariate analysis demonstrated a higher hazard of recurrence associated with age at first VTE ≥12 years (HR 7.56, 95% CI: 1.60–35.83). There was no association with sex (HR 0.56, 95% CI: 0.25–1.27). When compared to no thrombophilia, low‐risk thrombophilia was not associated with recurrence (HR 0.89, 95% CI: 0.20–3.96), however high‐risk thrombophilia was significant (HR 3.27, 95% CI: 1.43–7.46). Outpatient anticoagulation is summarized in Table 1. One patient recurred during the index VTE admission and was excluded from anticoagulation analysis. 12 of 26 (46%) had recurrent VTE on anticoagulation.
Conclusion(s): We demonstrate a high rate of recurrent VTE in children with unprovoked VTE, especially those ≥12 years or with a high‐risk thrombophilia.
OC 15.2
The SAXOPHONE Study; A multi‐center, multi‐national randomized trial of apixaban versus standard of care anticoagulation for thromboprophylaxis in children with congenital or acquired heart disease
R. Payne 1; K. Burns2; A. Glatz3; C. Male4; A. Donti5; L. Brandão6; G. Balling7; C. VanderPluym8; F. Bu'Lock9; L. Kochilas10; B. Stiller11; J. Cnota12; A. Buendia13; O. Rahkonen14; O. Wheaton15; A. Reedy16; J. Dyme17; L. Carlson16; C. Crevar17; P. Monagle18
1 Indiana University School of Medicine, Indianapolis, Indiana, United States; 2 Heart Development and Structural Diseases Branch/Division of Cardiovascular Sciences, Bethesda, Maryland, United States; 3 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 4 Medical University of Vienna, Austria, Vienna, Wien, Austria; 5 Azienda Ospedaliera‐Universitaria‐ Ospedale di S. Orsola, Bologna, Italy, Bologna, Emilia‐Romagna, Italy; 6 The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada, Toronto, Ontario, Canada; 7 German Heart Center Munich, Technical University Munich, Munich, Bayern, Germany; 8 Children's Hospital, Harvard University, Boston, Massachusetts, United States; 9 East Midlands Congenital Heart Centre, University Hospitals of Leicester NHS Trust, Leicester, England, United Kingdom; 10 Children's Healthcare of Atlanta and Emory University, School of Medicine, Department of Pediatrics, Atlanta, Georgia, United States; 11 Department of Congenital Heart Defects and Pediatric Cardiology, University Heart Center Freiburg‐BadKrozingen, University of Freiburg, Freiburg‐BadKrozingen, Baden‐Wurttemberg, Germany; 12 Cincinnati Children's Hospital Medical Center, Cincinnati, OH, Cincinnati, Ohio, United States; 13 Departamento de Cardiología Pedíatrica, Instituto Nacional de Cardiología, Mexico City, Distrito Federal, Mexico; 14 New Children's Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Uusimaa, Finland; 15 HealthCore, Wilmington, Delaware, United States; 16 Bristol Myers Squibb, Inc, Lawrence Township, New Jersey, United States; 17 Bristol Myers Squibb, Inc., Lawrence Township, New Jersey, United States; 18 University of Melbourne, and Royal Children's Hospital, Melbourne, Victoria, Australia
Background: Vitamin K antagonists (VKA) or low molecular weight heparin (LMWH) are currently standard of care (SOC) for chronic anticoagulation in children with heart disease. Direct anticoagulants are the preferred SOC for thromboembolism prevention (TE) in adults due to their proven efficacy and safety without need for monitoring.
Aims: SAXOPHONE compared safety and tolerability of apixaban versus SOC for TE prevention in children with heart disease and assessed pharmacokinetics/pharmacodynamics (PK/PD).
Methods: Children 29 days to <18 years of age with heart disease requiring chronic thromboprophylaxis were randomized 2:1 apixaban:SOC for ≤12 months in this open‐label, phase 2, multi‐center trial. The primary safety endpoint was major or clinically relevant non‐major (CRNM) bleeding. Secondary endpoints were PK/PD and TE. Safety and efficacy events were reviewed by a blinded independent Event Adjudication Committee. Ethics committee review and informed consent were obtained. SAXOPHONE was funded by the Bristol Myers Squibb and Pfizer Alliance, with scientific leadership from NIH's Pediatric Heart Network.
Results: 198 participants were screened, 192 randomized, 188 treated (126 apixaban, 62 SOC) and included in the analysis across 33 sites in 12 countries over 4 years. Primary diagnosis was single ventricle in 72.9% of apixaban subjects and 76.2% of SOC. Overall 66.7% were post‐Fontan. One apixaban subject had 2 primary safety events (incidence rate [IR] 1.8/100 person‐years exposure [P‐Y]) versus 3 subjects in SOC with 4 events (IR 6.8/100 P‐Y). Serious adverse event rates were similar (apixaban 20.6% versus SOC 21.0%) but mild hematomas and epistaxis were reported more frequently with apixaban (6.3% versus 1.6%, and 15.9% versus 9.7%, respectively). There were no adjudicated TE events.
Conclusion(s): Thromboprophylaxis with apixaban was found to be safe and well tolerated in children with heart disease with numerically lower rate of major/CRNM bleeding versus SOC. PK/PD data will inform apixaban dosing for thromboprophylaxis in children.
OC 15.1
PREVAPIX‐ALL: Phase 3 study of the safety and efficacy of apixaban for thromboprophylaxis versus standard of care in newly diagnosed pediatric acute lymphoblastic leukemia or lymphoma (ALL/LL)
S. O'Brien 1; V. Rodriguez2; G. Lew3; J. Newburger4; C. Schultz5; E. Orgel6; K. Derr7; M. Ranalli8; A. Esbenshade9; J. Hochberg10; H. Kang11; Y. Dinikina12; C. Crevar13; M. Donovan13; J. Dyme13; N. Favatella13; L. Mitchell14
1 Nationwide Children's Hospital & The Ohio State University, Columbus, Ohio, United States; 2 Nationwide Children's Hospital & The Ohio State University, Columbus, Ohio, United States; 3 Bristol Myers Squibb, Inc, Summit, New Jersey, United States; 4 Department of Cardiology, Boston Children's Hospital & Department of Pediatrics, Harvard Medical School, Boston, Massachusetts, United States; 5 Nemours Center for Cancer and Blood Disorders, Nemours Children's Health, Wilmington, Delaware, United States; 6 Cancer and Blood Disease Institute, Children's Hospital Los Angeles, Los Angeles, California, United States; 7 Department of Pediatric Oncology, Geisinger, Danville, Pennsylvania, United States; 8 Division of Pediatric Hematology/Oncology, Nationwide Children's Hospital & The Ohio State University, Colulmbus, Ohio, United States; 9 Monroe Carell Jr. Children's Hospital at Vanderbilt, Vanderbilt University Medical Center, Nashville, Tennessee, United States; 10 Department of Pediatrics, New York Medical College, Valhalla, New York, United States; 11 Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Cancer Research Institute& Wide River Institute of Immunology, Seoul National University Children's Hospital, Seoul, Kyonggi‐do, Republic of Korea; 12 Department of Chemotherapy for Oncohematological Diseases and Bone Marrow Transplantation for Children, Almazov National Medical Research Centre, Saint Petersburg, Saint Petersburg City, Russia; 13 Bristol Myers Squibb, Inc., Lawrence Township, New Jersey, United States; 14 Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada
Background: Pediatric patients with ALL/LL are at increased risk of venous thromboembolism (VTE).
Aims: To assess efficacy and safety of prophylactic apixaban vs. standard‐of‐care (SOC) for VTE prevention during induction chemotherapy.
Methods: Following ethics committee approval and informed consent patients ≥1 to <18 years with (1) newly diagnosed ALL/LL, (2) central venous line, and (3) three or four drug induction chemotherapy with asparaginase, were randomized in an open label, randomized controlled trial conducted in collaboration with the Children's Oncology Group. Apixaban thromboprophylaxis was stopped at the end of induction (Figure). After induction, patients underwent VTE screening. The primary efficacy endpoint was a composite of symptomatic/asymptomatic VTE and VTE‐ related death. The primary safety endpoint was major bleeding.
Results: 512 patients (56.5% male, mean age 7.2 years) were randomized. VTE occurred in 12.1% (n = 31) of apixaban patients and 17.6% (n = 45) of SOC patients (Table). No differences were shown in the primary efficacy endpoint between study arms [RR: 0.69 (0.45–1.05), 1‐sided p‐value 0.04]. In obese patients (n = 82), 1 VTE occurred in the apixaban arm vs. 10 in the SOC arm [RR: 0.11 (0.02–0.74), treatment‐subgroup interaction p‐value 0.036]. There were 2 major bleeding events in each arm (including 1 event before treatment with apixaban). A numerically higher incidence of clinically relevant non‐major bleeding occurred in the apixaban arm (11 vs. 3 events) due to increased epistaxis.
Conclusion(s): PREVAPIX‐ALL is the first trial to assess primary prophylaxis using a direct oral anticoagulant in pediatric ALL/LL. Apixaban was not shown to be efficacious in the primary analysis but decreased VTE risk in obese patients. Major and clinically relevant non‐major bleeding was infrequent. No new safety signals were observed. Apixaban was found to be a safe pharmacologic prophylaxis agent in pediatric patients with ALL/LL receiving induction chemotherapy containing asparaginase.
OC 15.5
Validating direct oral anticoagulants (DOAC) for use in children by the Throm‐PED DOAC registry of the International Pediatric Thrombosis Network
S. Holzhauer 1; P. Monagle2; C. Male3; R. Bhat4; M. Bordbar5; R. Natesirinilkul6; S. Saini7; M. Simpson8; C. van Ommen9; L. Raffini10
1 Charité University Medicine, Charite, Berlin, Germany, Berlin, Berlin, Germany; 2 University of Melbourne, and Royal Children's Hospital, Melbourne, Victoria, Australia; 3 Medical University of Vienna, Austria, Vienna, Wien, Austria; 4 Northwestern Medicine, Chicago, Illinois, United States; 5 Shiraz University of Medical Sciences, shiraz, Fars, Iran; 6 Faculty of Medicine Chiang Mai University, Chiang Mai, Thailand, Chiang Mai, Chiang Mai, Thailand; 7 St. Louis Children's Hospital‐Washington Universit, St Luis, Missouri, United States; 8 Rush University Medical Center, Chicago, IL, USA, Chicago, Illinois, United States; 9 Erasmus Medical Center‐Sophia Children's, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 10 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States
Background: Dabigatran and rivaroxaban have recently been approved for treatment of venous thromboembolism in children. Based on the risk benefit profiles published so far, we expect DOACs to be widely used in children. The strict inclusion criteria for participation in the clinical trials limit their generalizability, particularly for those with serious medical conditions that account for a significant proportion of pediatric VTE patients in clinical practice.
Aims: To study efficacy and safety of DOACs in a large heterogeneous pediatric population. To expand our knowledge on treatment strategies and outcomes in children across different risk profiles and comorbidities including cancer and renal disease.
Methods: An international, multicentre, prospective observational cohort study of patients <18 years with thromboembolic disease treated with DOACs. Data collection includes thrombosis location, risk factors, medical conditions and concurrent medications. Outcomes (thrombus progression/recurrence and bleeding) are assessed every 3 months for 12 months. Additional variables include adherence, drug levels, renal function and dose adjustments.
Results: A total of 102 patients from 9 centers have been included in the registry to date, of which 81% of patients were >12 years old (Figure 1) and most 79% received rivaroxaban. Pulmonary embolism and thrombosis of the lower extremities were more common (30%) each, followed by cerebral thrombosis (16%). Risk factors included cancer (13%) and renal disease (5%). Of the 39 patients with follow‐up at 3 months, bleeding was reported in 3 (8%) patients and thrombosis progression in 2 (6%) (Table 1).
Conclusion(s): Real world data collection is essential to assess the benefit risk profile of DOACs in children, to identify specific patient groups at risk of worse outcome and to understand drug interactions and the need for dose adjustments. Further data on younger age groups and children with comorbidities are required.
Platelet Disorders, von Willebrand Disease and Thrombotic Microangiopathies
Acquired Thrombocytopenias
OC 73.2
Phascolarctobacterium was increased in ITP and negatively correlated with platelet counts
H. Wang; H. Bi; Z. Zeping
The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China (People's Republic)
Background: There are few studies on the intestinal flora of primary immune thrombocytopenia (ITP), and different studies present different and inconsistent characteristic flora of ITP. Specific differential flora cannot be determined at present.
Aims: To explore the difference of intestinal flora between ITP and healthy people, and to search for specific differential flora and possible mechanisms.
Methods: Feces from ITP patients and healthy controls (patients' families) were studied using 16S rRNA and metagenomic techniques. KEGG and GO functions were annotated. The relationship between intestinal flora and disease stage, severity and refractory cases was analyzed. Plasma IL‐2 and IL‐4 levels were detected to investigate the role of intestinal flora in ITP.
Results: (1) The α‐diversity and β‐diversity of intestinal flora in ITP were significantly altered, indicating that the composition and distribution of intestinal flora in ITP patients and healthy people were significantly different. The functional changes of intestinal flora in ITP were mainly manifested in cell movement, membrane transport, signal transduction and metabolism. (2) The numbers of Phascolarctobacterium were significantly increased in ITP patients (p < 0.005) at the genus level. Correlation analysis showed that increased Phascolarctobacterium was negatively correlated with platelet counts, but had no significant correlation with bleeding score, course of disease, PT, APTT and INR. (3) The intestinal flora of newly diagnosed ITP, persistent ITP and chronic ITP also differed at multiple classification levels. (4) Plasma IL‐2 concentration in ITP patients was significantly higher than that in healthy controls (9.871 ± 2.866 pg/ml vs. 8.527 ± 3.003 pg/ml, p < 0.05). Phascolarctobacterium were positively correlated with IL‐2.
Conclusion(s): Phascolarctobacterium were significantly increased in ITP patients, and were negatively correlated with platelet counts and positively correlated with IL‐2. Phascolarctobacterium can be used as a specific differential bacterium of ITP, and its specific mechanism may involve immune changes of ITP, which needs further study.
OC 73.3
Thrombopoietin receptor agonists (TPO‐RAs) in older patients with primary immune thrombocytopenia (ITP): Effective and safe? Real World Data from the UK adult ITP registry
P. Raheja 1; H. Miah2; A. Miah1; L. Taylor1; T. Biss3; G. Evans4; M. Scully5; J. Hermans6; S. Marshall7; M. Kilner8; R. Rayment9; L. Robinson10; S. Johns11; C. Rinaldi12; H. Jackson13; S. Ackroyd14; A. Newland1; D. Provan1; N. Cooper15; V. McDonald16
1 Royal London Hospital, London, England, United Kingdom; 2 Queen Mary University London, London, England, United Kingdom; 3 The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, England, United Kingdom; 4 East Kent Hospitals University NHS Foundation Trust, Kent, England, United Kingdom; 5 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 6 Nottingham University Hospitals NHS Trust, Nottingham, England, United Kingdom; 7 Sunderland Royal Hospital, Sunderland, England, United Kingdom; 8 Northumbria Healthcare NHS Foundation Trust, Northumbria, England, United Kingdom; 9 Cardiff and Vale, University Hospital of Wales, Cardiff, England, United Kingdom; 10 County Hospital, Wye Valley NHS Trust (Hereford), Wye, England, United Kingdom; 11 Royal Cornwall Hospital, Cornwall, England, United Kingdom; 12 Pilgrim Hospital, Pilgrim, England, United Kingdom; 13 Aneurin Bevan University Health Board, Royal Gwent, Gwent, England, United Kingdom; 14 Bradford Royal Infirmary, Bradford, England, United Kingdom; 15 Hammersmith Hospital, London, England, United Kingdom; 16 Barts Health NHS Trust, The Royal London Hospital, London, England, United Kingdom
Background: Adult primary immune thrombocytopenia (ITP) is a rare acquired immune‐mediated disorder. It may have a more aggressive course in older patients with the potential for more complex management due to co‐morbidities and possible drug interactions. Thrombopoietin receptor agonists (TPO‐RAs) have reported overall response rates of 50%–90% in ITP.
Aims: To assess outcomes after TPO‐RA in patients >60 years.
Methods: Data analysed from the UK adult primary ITP registry (https://www.qmul.ac.uk/itpregistry/) for response and events of special interest.
Results: There were 4590 participants as of 4th January 2021. Of these 485 patients (259M; 226F), median age 73 years (IQR 67.9–79.2) >60 years had received TPO‐RA therapy (253 eltrombopag and 232 romiplostim) (Table 1). The mean time from diagnosis to first TPO‐RA treatment was 1.8 years, with median 3 lines of treatment prior to TPO‐RA. The median platelet count up to 2 weeks prior to starting TPO‐RA was 88.7 × 109/L, from 2 to 4 weeks after TPO‐RA was 99 × 109/L and at 3–4 months was 97 × 109/L. The overall response (OR) rates at 3–4 months were 72.5%; at 6–7 months were 72% and at 1–2 years were 70%. Bleeding events were reduced amongst all groups following TPO‐RA treatment. In the year prior to starting TPO‐RA there were 316 muco‐cutaneous bleed events and 82 organ bleeds, in the year after there were 193 and 40, respectively. Cardiovascular (CV) risk factors before ITP diagnoses are shown in table 2. Over 50% had at least one risk factor for CV disease. Before/after TPO‐RA there were 60 and 17 arterial thrombotic events (ATE); 18 and 17 venous thrombotic events (VTE), respectively.
Conclusion(s): This data confirms efficacy of TPO‐RA in this group with comparable response rates to the literature based on platelet count response. There does not appear to be an increase in reported thrombotic events after therapy in this group, despite a large number of co‐morbidities.
OC 73.1
Updated main study period and long‐term extension (LTE) results with oral Bruton tyrosine kinase inhibitor rilzabrutinib in immune thrombocytopenia (ITP)
D. Kuter 1; M. Efraim2; Z. Kaplan3; J. Mayer4; P. Choi5; A. Jansen6; V. McDonald7; R. Baker8; R. Bird9; M. Garg10; J. Gumulec11; M. Kostal12; T. Gernsheimer13; W. Ghanima14; M. Yao15; N. Cooper16; A. Daak17
1 Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, United States; 2 Multiprofile Hospital for Active Treatment Sveta Marina EAD, Varna, Varna, Bulgaria; 3 Monash Medical Centre, Clayton, Victoria, Australia; 4 Masaryk University Hospital, Brno, Moravskoslezsky kraj, Czech Republic; 5 The Canberra Hospital, Garran, Australian Capital Territory, Australia; 6 Erasmus MC, University Medical Center, Rotterdam, Zuid‐Holland, Netherlands; 7 Barts Health NHS Trust, The Royal London Hospital, London, England, United Kingdom; 8 WA Centre of Thrombosis and Haemostasis (WACTH) Centre for Computational and Systems Medicine, Murdoch University; Perth Blood Institute., Perth, Western Australia, Australia; 9 Princess Alexandra Hospital, Woolloongabba, Queensland, Australia; 10 Leicester Royal Infirmary, Leicester, England, United Kingdom; 11 University Hospital Ostrava and Faculty of Medicine University of Ostrava, Ostrava, Moravskoslezsky kraj, Czech Republic; 12 University Hospital of Hradec Kralove, Hradec Kralove, Kralovehradecky kraj, Czech Republic; 13 University of Washington Medical Center, Seattle, Washington, United States; 14 Østfold Hospital Foundation, Sarpsborg, Ostfold, Norway; 15 Sanofi US Services Inc., Bridgewater, New Jersey, United States; 16 Hammersmith Hospital, London, England, United Kingdom, 17 Sanofi Genzyme, Cambridge, Massachusetts, United States
Background: Rilzabrutinib led to rapid and durable platelet responses and was well tolerated in a global phase I/II trial in adult patients with ITP (NCT03395210). We present additional findings observed during the main study period in patients who initiated 400 mg BID as well as patients who are continuing in the LTE.
Aims: Assess efficacy and safety of rilzabrutinib 400 mg BID.
Methods: Patients with 2 baseline platelet counts <30 × 109/L were required to have responded to ≥1 prior ITP therapy, but at baseline were unable to maintain an adequate response to prior/concomitant therapies. Primary endpoints were safety and efficacy: ≥2 consecutive platelet counts ≥50 × 109/L and increased ≥20 × 109/L from baseline without requiring rescue medication. All patients provided informed consent.
Results: As of 04May2021, 60 patients received rilzabrutinib, 45 initiated a 400 mg BID dose, 16 proceeded to the LTE. At enrollment, patients were heavily pretreated with a median of 4 unique prior therapies. Median ITP duration was 6.1 years and median platelet count was 15 × 109/L. With a median treatment duration of 168 days during the main period, 18 patients (40%) achieved the primary endpoint (Table). In primary responders, platelet counts ≥50 × 109/L were maintained for a median of 72% of the weeks and the median time to first platelet count ≥50 × 109/L was 12.5 days. Figure shows subgroup analysis. Overall, LTE patients received rilzabrutinib for a median of 478 days and 13/14 patients (93%) maintained platelet counts ≥50 × 109/L for ≥50% of their monthly visits in the LTE (Table). Five patients (11%) received rescue medication during the main period + LTE. All treatment‐related adverse events were grade 1/2 and transient without related thrombotic events or deaths.
Conclusion(s): Over prolonged treatment, rilzabrutinib 400 mg BID demonstrated a rapid and durable clinical activity and continued to be well‐tolerated in patients with ITP.
ADAMTS13 and TTP
OC 04.5
Immune medicated thrombotic thrombocytopenic purpura relapse is associated with HLA‐DRB1*15:01
M. Stubbs 1; A. Doyle2; C. Cheshire3; A. Levine3; M. Thomas4; J. Westwood1; R. Kleta3; D. Gale3; H. Stanescu3; M. Scully1
1 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom; 2 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 3 University College London, London, England, United Kingdom; 4 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom
Background: Despite significant advances in immune Thrombotic Thrombocytopenic Purpura (iTTP) care relapses occur in 30%–50% of patients. This causes significant morbidity and mortality, and the aetiology remains poorly understood.
Aims: We undertook HLA and genome‐wide analysis to identify genetic associations for iTTP relapse.
Methods: 155 iTTP patients (44 relapsed, 111 non‐relapsed) of European genetic ancestry were genotyped at 3,649,347 Single Nucleotide Polymorphisms genome‐wide. HLA imputation was performed using SNP2HLA (V1.0.3, European reference panel). Relapse data from the UK TTP registry (defined as ADAMTS13 relapse or clinical relapse) were used to identify iTTP relapse associations.
Results: Among 25 Class‐II HLA types tested, HLA‐DRB1*15:01 was identified as being associated with iTTP disease relapse (p = 0.0008, see Table 1). HLA‐DQB1*06:02 and HLA‐DQA1*01:02, which are in linkage disequilibrium with HLA‐DRB1*15:01, also showed evidence of association. HLA‐DRB1*11:01 and HLA‐DQA1*03:01 (associated with risk of iTTP) were not associated with disease relapse (p = 0.97 and 0.94 respectively). Genome‐wide association analysis revealed that no genetic markers outside HLA region were significantly associated with iTTP relapse.
Conclusion(s): Here we demonstrate HLA‐DRB1*15:01 being associated with iTTP relapse for the first time, but not HLA‐DRB1*11:01. HLA‐DRB1*11:01 is well established in iTTP risk, with functional studies demonstrating that this allele is associated with ADAMTS13 presentation in addition to autoreactive CD4+ Th‐cells. HLA‐DRB1*15:01 is associated with several other autoimmune conditions (notably multiple sclerosis and SLE). Subsequent functional studies in MS implicate HLA‐DRB1*15:01 in autoreactive T‐cell activity and antigen presentation, and it is possible that similar mechanisms are also important in iTTP relapse. This work has identified HLA‐DRB1*15:01 as a means to potentially individualise monitoring to reduce relapse risk, in addition to increasing understanding of the pathophysiology in iTTP relapse. TABLE 1 HLA Analysis in iTTP patients, comparing relapsing versus non‐relapsing individuals. HLA types reaching statistical significance with a Bonferroni correction for 25 alleles tested (p < 0.002) are shown in bold
TABLE 1 HLA Analysis in iTTP patients, comparing relapsing versus non‐relapsing individuals. HLA types reaching statistical significance with a Bonferroni correction for 25 alleles tested (p < 0.002) are shown in bold
OC 04.4
Disease relapse in immune thrombotic thrombocytopenic purpura and treatment with anti‐CD20 monoclonal antibodies – 10‐years' experience from the UK TTP Registry
A. Doyle 1; M. Stubbs2; T. Dutt3; W. Lester4; W. Thomas5; J. Vanveen6; J. Hermans7; T. Cranfield8; Q. Hill9; B. Hunt1; A. Clark10; T. Nokes11; S. Benjamin12; C. Bagot13; N. Cooper14; S. Austin15; M. Thomas2; J. Westwood2; M. Scully2
1 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 2 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 3 Liverpool University Hospitals NHS Foundation Trust, Liverpool, England, United Kingdom; 4 University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 5 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 6 Sheffield Teaching Hospitals NHS Trust, Sheffield, England, United Kingdom; 7 Nottingham University Hospitals NHS Trust, Nottingham, England, United Kingdom; 8 Portsmouth Hospitals University NHS Trust, Portsmouth, England, United Kingdom; 9 The Leeds Teaching Hospitals NHS Trust, Leeds, England, United Kingdom; 10 University Hospitals Bristol NHS Foundation Trust, Bristol, England, United Kingdom; 11 University Hospital Plymouth NHS Trust, Plymouth, England, United Kingdom; 12 Oxford University Hospital NHS Foundation Trust, Oxford, England, United Kingdom; 13 Department of Haematology, Glasgow Royal Infirmary, Glasgow, England, United Kingdom; 14 Hammersmith Hospital, London, England, United Kingdom; 15 St George's University Hospital NHS Foundation Trust, London, England, United Kingdom
Background: Relapse in immune Thrombotic Thrombocytopenic Purpura (iTTP) is well‐characterised with subclinical ADAMTS13 relapses prior to clinical relapses. Rituximab is effective in preventing relapses in patients with iTTP.
Aims: We describe patterns of disease relapse over a 10‐year period with a minimum 4‐year review and response to anti‐CD20 treatments.
Methods: 443 patients were identified between 2008 and 2018 from 30 sites. Relapse was defined as either clinical relapse, presenting with thrombocytopenia and/or ADAMTS13 relapse, with activity levels <15%/IU/dl. Clinical details, treatments and responses were reviewed.
Results: 312/443 patients (70.4%) had no relapse following presentation. 131/443 (29.6%) had relapse episodes (45 had clinical relapses only (10%), 56 had ADAMTS13 relapses only (13%) and 30 has both (7%)). Black Caribbeans ethnicity was more common in relapsing patients (16.8% vs. 7.1%, p = 0.017). 28/443 patients (6.3%) had ‘frequent’ relapses (>0.5 episodes/year) with the remaining (n = 103, 23.3%) having ‘infrequent’ relapses (≤0.5 episodes/year). 197 pre‐emptive treatment episodes were reported in 89 patients – 181 rituximab (91.9%), 1 obinutuzimab (0.5%), 15 ofatumumab (7.6%). Treatment response (ADAMTS13 activity ≥30%/IU/dl) occurred in 155/167 (92.8%) episodes (91% patients) with complete response (ADAMTS13 activity ≥60%/IU/dl) occurring in 132/167 treatment episodes (79.0%). 8/98 patients (8.2%) were considered minimally responsive to anti‐CD20 treatment (ADAMTS13 activity <30% despite treatment). No patients who initially responded to anti‐CD20 therapy subsequently lost response with retreatment. In patients with ‘frequent’ and ‘infrequent’ relapses, there was no difference in ∆ADAMTS13 activity following treatment (86.5% vs. 73.2%, p = 0.07) nor end‐of‐treatment absolute CD19‐positive lymphocytes (0 vs. 0.001 × 109/L, p = 0.62).
Conclusion(s): Approximately 30% have a relapsing disease pattern with 6% having frequent relapses requiring treatment ≤2‐yearly. Anti‐CD20 treatment was an efficacious option with a normalisation of ADAMTS13 activity in the majority with persisting responses with further relapses. However, a minority (8%) failed to achieve a significant increase in ADAMTS13 activity following anti‐CD20 treatment requiring alternative treatment modalities.
OC 04.2
Factors influencing time from initial presentation to start of plasma exchange in patients with acute thrombotic thrombocytopenic purpura in the United Kingdom
T. Bull1; R. McCulloch2; A. Doyle 3; P. Nicolson4; R. Shaw5; Z. Sayar6; A. Langridge7; M. Pettitt8; R. Perry8; D. Tucker9; M. Scully10
1 West Suffolk NHS Foundation Trust, UK, Bury St Edmunds, England, United Kingdom; 2 Gloucestershire Hospitals NHS Foundation Trust, Gloucester, UK, Gloucester, England, United Kingdom; 3 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 4 University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK, Birmingham, England, United Kingdom; 5 University of Liverpool, Liverpool, UK, Liverpool, England, United Kingdom; 6 University College London Hospitals NHS Foundation Trust, London, UK; Whittington Hospital, London, UK, London, England, United Kingdom; 7 Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle‐upon‐Tyne, UK, Newcastle‐Upon‐Tyne, England, United Kingdom; 8 Birmingham Centre for Observational and Prospective Studies, Birmingham Surgical Trials Consortium, Birmingham, UK, Birmingham, England, United Kingdom; 9 Royal Cornwall Hospitals NHS Trust, Truro, UK, Truro, England, United Kingdom; 10 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom
Background: Diagnosis of acute thrombotic thrombocytopenic (TTP) can be challenging and delays initiating plasma exchange (PEX) may increase morbidity and mortality.
Aims: To assess adherence to national guidelines and understand the causes delaying early PEX.
Methods: Adults ≥18 years presenting to UK hospitals between 2014 and 2019 with first episode acute TTP and ADAMTS13 level <10% were included. Time to treatment with PEX was defined as time of the first blood count sample in the laboratory to time of plasma release for PEX from blood bank.
Results: 148 patients treated at 80 hospitals were analysed with clinical outcomes described in Table 1. The median time to treatment was 15 h (95% CI 11.3–18.7) with 25% receiving PEX within 8‐h and 61% within 24‐h. Age <60 years, haemoglobin <100 g/L, presence of fragments and on‐site PEX were predictors for PEX initiation within 8 h (p = 0.004, <0.001, 0.001, 0.002 respectively). Travel distance between hospital transfers did not correlate with time to PEX (R 2 = 0.058, p = 0.61). Case review of 50 patients (35%) that received PEX >24 h showed that uncertain/delayed diagnosis to be the most common cause.
Conclusion(s): This is the first national multi‐centre audit of time to treatment with PEX for acute TTP. Results show that early treatment rates with PEX are low. Further analysis suggests that difficulties in the diagnosis of TTP in this cohort is a significant component to treatment delays as opposed to transfer and technical aspects of PEX delivery. That 22% of patients initiated PEX over 48 h from admission indicates the issue is relatively common. With several deaths occurring in this group, we suggest initiatives to increase early diagnosis should be prioritised. TABLE 1 Numbers of patients meeting key performance indicators for plasma exchange for acute thrombotic thrombocytopenic purpura
TABLE 1 Numbers of patients meeting key performance indicators for plasma exchange for acute thrombotic thrombocytopenic purpura
OC 04.3
N‐glycan‐mediated shielding of ADAMTS13 effectively prevents binding of pathogenic autoantibodies in high‐titer patients with immune‐mediated thrombotic thrombocytopenic purpura
T. Postmus 1; N. Graca2; B. Ercig2; B. Luken3; P. Coppo4; A. Veyradier5; K. Vanhoorelbeke6; J. Voorberg7
1 Sanquin Research, Amsterdam, The Netherlands, Duivendrecht, Noord‐Holland, Netherlands; 2 Department of Molecular Hematology, Sanquin Research, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Sanquinnovate, Amsterdam, Noord‐Holland, Netherlands; 4 Service d'Hématologie, Hôpital Saint‐Antoine, APHP.6‐SU, INSERM UMRS 1138, Centre de Recherche des Cordeliers, Paris, France, Paris, Ile‐de‐France, France; 5 Université Paris Diderot UFR Médecine, Paris, Ile‐de‐France, France; 6 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 7 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Thrombotic Thrombocytopenic Purpura (TTP) is a rare thrombotic disorder, with 1.5–6.0 new cases per million per year. The majority of TTP patients develop inhibitory antibodies targeting predominantly the spacer domain of ADAMTS13. ADAMTS13 is responsible for cleaving VWF multimers, thereby regulating platelet adhesion at sites of vascular perturbation. Inhibition of ADAMTS13 by pathogenic autoantibodies results in accumulation of VWF multimers which promote the formation of platelet‐rich microthrombi. We have previously shown that introduction of an N‐glycan in the spacer domain of ADAMTS13 prevents the binding of pathogenic autoantibodies that develop in patients with immune TTP.
Aims: Evaluate whether N‐glycan mediated shielding of ADAMTS13 effectively prevents binding of pathogenic autoantibodies and restores ADAMTS13 activity in high‐titer patients with immune‐mediated TTP.
Methods: We screened the N‐Glycan mutants against immune TTP (iTTP) patient antibody binding and for their activity in the presence and absence of samples derived from 28 patients with iTTP. In parallel we introduced additional modifications in the spacer domain and characterized the potential of these variants to escape the binding of pathogenic antibodies present in plasma of patients with iTTP.
Results: Newly tested patient samples reinforced our previous findings that N‐glycans can shield ADAMTS13 from antibody binding. The N‐glycan was both protective against inhibitory anti‐ADAMTS13 antibodies binding as well as able to restore ADAMTS13 activity especially in samples of patients with high titer inhibitors. Dose‐response experiments revealed that the N‐glycan modified ADAMTS13 was up to ten‐fold more effective when compared to wild type ADAMTS13 upon addition of patient plasma containing high titer inhibitors. Preliminary findings indicated that introduction of an additional N‐glycan in the spacer domain further prevented patient antibodies to bind and inhibit ADAMTS13.
Conclusion(s): Collectively our findings show that N‐glycan mediated shielding of ADAMTS13 effectively prevents the binding of pathogenic autoantibodies in high titer patients with immune TTP.
OC 04.1
Management of thrombotic thrombocytopenic purpura (TTP) without plasma exchange: An update on the Austrian experience
P. Knöbl 1; K. Eller2; V. Buxhofer‐Ausch3; J. Thaler4; K. Gleixner5; W. Sperr6
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Med. Univ. Graz, Dept. Nephrology, Graz, Steiermark, Austria; 3 Department of Internal Medicine I with Hematology, Stem Cell Transplantation, Hemostaseology and Medical Oncology, Ordensklinikum Linz Elisabethinnen, Linz, Austria, Linz, Oberosterreich, Austria; 4 Clinical Division of Haematology and Haemostaseology, Department of Medicine I, v, Vienna, Wien, Austria; 5 Med. Univ. Vienna, Dept. Med. 1, Vienna, Wien, Austria; 6 Med. Univ. Vienna, Dept. Med.1, Vienna, Wien, Austria
Background: Therapeutic plasma exchange (TPE) is still considered important for TTP, a severe disease caused by autoantibodies to ADAMTS13. However, exacerbations, refractory courses, and 10% mortality from organ failure are well known. Caplacizumab reduced the need for TPE and improved platelet recovery and survival. Recently, the successful treatment of patients with TTP only with caplacizumab and immunosuppression, but without TPE, has been reported.
Aims: Thus, we used a modified TTP management strategy, aiming to avoid TPE if platelet counts increased after a first dose of 10 mg caplacizumab, early addition of immunosuppression with steroids and rituximab, and continuing daily caplacizumab until ADAMTS13 increased >20%.
Methods: With this strategy 14 Austrian patients with TTP (3 male, 11 female, median age 51 years; range 29–75 years) and signs of severe organ dysfunction (high troponin, stroke, confusion, elevated creatinine) were treated. All patients had severe ADAMTS13 deficiency and detectable inhibitors.
Results: After the first iv dose of caplacizumab, platelet counts increased within 6 h in 11 patients, and organ dysfunction improved. In these patients, platelet counts remained in the normal range during caplacizumab, no exacerbations or relapses occurred. Organ dysfunction resolved completely within a few days. In 3 patients, platelet counts did not improve after the first dose of caplacizumab, and additional TPE was started. One patient had acute cytomegalovirus viremia, one patient had both human immunodeficiency virus and hepatitis B viremia, and one patient had immune thrombocytopenia. Up to 3 TPE sessions were necessary until platelet counts normalized.
Conclusion(s): In conclusion, the management of TTP is feasible without an absolute need for TPE. With caplacizumab and immunosuppression, complete clinical and ADAMTS13 remissions can be achieved. In patients without immediate response, additional conditions may be responsible. We suggest the initiation of a clinical trial to clarify whether TPE is still needed for TTP in the era of caplacizumab.
Blood Cells and Vessel Wall
OC 02.5
In vivo murine studies demonstrate that neutrophil activation by anti‐NAP2 antibodies contributes to vaccine‐induced immune thrombocytopenia and thrombosis (VITT)
C. Field 1; H. Kim2; M. Kowalska2; M. Weitzman2; G. Arepally3; D. Cines4; L. Rauova2; M. Poncz2
1 University of Pennsylvania and Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 2 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 3 Division of Hematology Duke University Medical Center, Durham, North Carolina, United States; 4 Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: VITT involves thrombocytopenia and thrombosis post‐initial anti‐SARS‐CoV‐2 adenoviral vaccination. Most patients are found to have platelet‐activating antibodies to the chemokine, platelet factor 4 (PF4) in the absence of heparin. VITT antibodies differ from those in heparin‐induced thrombocytopenia (HIT), in which PF4 is bound to heparin. Distinct epitope sites on PF4 for VITT and HIT antibodies were defined (PMID34233346). We noted that the VITT antigenic site is conserved in mouse (m) PF4, and in the platelet‐specific chemokine neutrophil‐activating peptide 2 (NAP2), both human and mouse. We observed that VITT antibodies bind strongly to NAP2. In an active patient with VITT, we found that VITT antibodies circulate as immune complexes containing either PF4 or NAP2. Importantly, VITT antibodies plus NAP2 activates platelets.
Aims: We tested in a passive‐immunization murine model with VITT antibodies the ability to induce neutrophil‐endothelial activation as an indicator of a prothrombotic state and identify the chemokines involved.
Methods: We studied two systems: a femoral vein and a cremaster venule model, using confocal intravital imaging and labeled neutrophils. VITT antibodies were infused into mice transgenic for FcγRIIA and lacking PF4 (FcγRIIA+/mPF4‐/‐).
Results: This led to an immediately reduced neutrophil rolling by ~80% (14–3 m/sec) (Figure 1A,B). Subsequent infusion of PF4 slowed neutrophil rolling by another ~80% (3–0.6 m/sec). In contrast, VITT antibodies did not slow neutrophil speed in FcγRIIA+/mPF4‐/‐/mNAP2‐/‐ mice (Figure 1C).
Conclusion(s): These data suggest that both NAP2 and PF4 contribute to thrombosis in VITT and may explain the pathogenesis of VITT in patients with no detectable anti‐PF4 antibodies. VITT may be prothrombotic because it involves co‐activation of neutrophils via NAP2 by way of CXCR2 and FcγRIIA. Targeting NAP2 pathobiology may enhance understanding of the pathogenesis of VITT and lead to new therapeutics. FIGURE 1 Passive immunization VITT studies. Transgenic mice for human FcγRIIA and were mPF4 knockouts (mPF4‐/‐). (A) Representative stills from studies of neutrophil rolling along cremaster venules. Shown are pre‐ and post‐ VITT IgG IV infusions (50 μg/g mouse) followed 5 min later by hPF4 (2 μg/g mouse). (B) Mean ± 1 SD of neutrophils velocity analyzed using Image J. (C) Transgenic mice for human FcγRIIA and double knockouts for mPF4 and mNAP2 were infused with VITT IgG (50 μg/g mouse) and were analyzed as mean ± 1 SD of neutrophils. Scale bar is shown as are yellow arrows indicating flow. Neutrophils labeled with anti‐Ly6G F(ab')2 (0.2 μg/g, clone 1A8, BD Biosciences). Number of independent studies shown in the bars. p value was analyzed by student T‐test.
FIGURE 1 Passive immunization VITT studies. Transgenic mice for human FcγRIIA and were mPF4 knockouts (mPF4‐/‐). (A) Representative stills from studies of neutrophil rolling along cremaster venules. Shown are pre‐ and post‐ VITT IgG IV infusions (50 μg/g mouse) followed 5 min later by hPF4 (2 μg/g mouse). (B) Mean ± 1 SD of neutrophils velocity analyzed using Image J. (C) Transgenic mice for human FcγRIIA and double knockouts for mPF4 and mNAP2 were infused with VITT IgG (50 μg/g mouse) and were analyzed as mean ± 1 SD of neutrophils. Scale bar is shown as are yellow arrows indicating flow. Neutrophils labeled with anti‐Ly6G F(ab')2 (0.2 μg/g, clone 1A8, BD Biosciences). Number of independent studies shown in the bars. p value was analyzed by student T‐test.
Endothelial Cell Signaling
OC 53.5
Loss of Rab27A targeting to Weibel‐Palade bodies in endothelial colony forming cells of patients with biallelic mutations in the guanine nucleotide exchange factor MAP‐kinase activating death domain
P. Burgisser1; M. Kat2; M. Swinkels3; S. Hordijk 4; G. Korenke5; T. Hónzik6; R. Bierings7
1 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam UMC, University of Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 4 Erasmus University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 5 Klinik für Neuropädiatrie und angeborene Stoffwechselerkrankungen, Klinikum Oldenburg, Oldenburg, Niedersachsen, Germany; 6 Department of Pediatrics and Adolescent Medicine, First Faculty of Medicine, Charles University and General University Hospital, Prague, Hlavni mesto Praha, Czech Republic; 7 Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Endothelial cells secrete hemostatic proteins such as Von Willebrand factor (VWF) from specific secretory organelles called Weibel‐Palade bodies (WPBs). WPBs acquire secretion competence through recruitment of Rab GTPases such as Rab27A and Rab3 and their effectors. We have recently shown that activation and WPB recruitment of Rab27A and Rab3 depend on the guanine nucleotide exchange factor MAP‐kinase activating death domain (MADD), which acts as a master regulator of WPB Rab recruitment and WPB exocytosis. Biallelic mutations in MADD have recently been linked to a rare, multisystem disorder that can also include bleeding abnormalities.
Aims: To determine the impact of disease‐causing mutations in MADD on the recruitment of its Rab GTPases to WPBs using patient‐derived endothelial cells.
Methods: Endothelial colony forming cells (ECFCs) and platelets were isolated from peripheral blood from 2 pediatric patients with biallelic mutations in MADD (patient 1:c.914G>T, c.(1862+1_1863−1)_(3759+1_3760−1)del: p.Gly305Val, p.?; patient 2:c.979C>T, c.4398delG: p.Arg327*, p.Leu1467Cysfs*20), unaffected heterozygous family members and matching controls. Intracellular localization of Rab27A in endothelial cells was determined using immunolocalization and counterstaining with VWF and VE‐cadherin. Imaging was performed using a Leica SP8 confocal microscope. This study was performed according to the declaration of Helsinki with written informed consent from all participants and family members.
Results: ECFCs of patients with biallelic MADD mutations contained WPBs with typical, elongated morphology that were indistinguishable from those in ECFCs of healthy controls and unaffected heterozygous family members. Rab27A localized to WPBs in ECFCs of healthy controls and family members, but was lost from WPBs in ECFCs from the two patients with distinct biallelic MADD mutations.
Conclusion(s): These studies further emphasize the role of MADD in the recruitment of Rab27A to WPBs in endothelial cells. Future studies are needed to determine the impact of disease‐causing mutations in MADD on the hemostatic response of the endothelium.
HIT
OC 02.3
“Off‐the‐shelf” cryopreserved platelets for the detection of HIT and VITT antibodies
A. Kanack 1; C. Jones2; N. Splinter3; R. Leger3; N. Heikal3; D. Chen3; R. Pruthi3; G. George4; Y. Abou‐Ismail5; G. Wool6; K. Gundabolu7; A. Padmanabhan3
1 Mayo cliinc, Rochester, Minnesota, United States; 2 Retham Technologies, Wauwatosa, Wisconsin, United States; 3 Mayo Clinic, Rochester, Minnesota, United States; 4 Univ of Colorado, Denver, Colorado, United States; 5 University of Utah, Salt Lake City, Utah, United States; 6 University of Chicago, Chicago, Illinois, United States; 7 University of Nebraska, Omaha, Nebraska, United States
Background: Heparin‐induced thrombocytopenia (HIT) and vaccine‐induced immune thrombotic thrombocytopenia (VITT) are life‐threatening disorders characterized by anti‐Platelet Factor 4 (PF4) antibodies. Tests such as the PF4‐polyanion enzyme‐linked immunosorbent assay (ELISA) lack specificity, and accurate functional tests such as the serotonin release assay (SRA) and PF4‐dependent P‐selectin expression assay (PEA) are technically complex and require fresh platelets; causing delays in diagnosis and overuse of alternative anticoagulants.
Aims: Develop a rapid, near‐patient assay for diagnosing HIT and VITT using readily available “off‐the‐shelf” platelets.
Methods: Normal donor platelets were cryopreserved in a trehalose‐based buffer. Thawed platelets were washed, treated with PF4 or heparin, and incubated with HIT or VITT samples before quantifying platelet thrombospondin‐1 (TSP1) release in the TSP1‐release assay (TRA). Results were expressed relative to TSP1 released by healthy donor serum‐treated cryopreserved platelets.
Results: Platelet‐activating (PEA‐positive) samples stimulated a 2.52‐fold mean increase in TSP1 release in the PF4‐TRA (Fig 1A), and an average 2.79‐fold increase in the heparin‐TRA (Fig 1B), both significantly higher than TSP1 release induced by PEA‐negative HIT‐suspected patients (including some with false‐positive ELISA results; red squares). Two non‐activating samples induced TSP1 release in the heparin‐TRA but were not inhibited by high‐dose heparin (100 U/ml; data not shown), ruling out HIT. Cryopreserved platelets stored for >1 year supported detection of HIT antibodies (data not shown). Lastly, four VITT patient samples induced elevated TSP‐1 release in the PF4‐TRA (Fig 1A) but did not in the Heparin‐TRA (Fig 1B).
Conclusion(s): The PF4‐ and Heparin‐TRA were highly accurate for diagnosing HIT/VITT and HIT, respectively. VITT samples did not activate heparin‐treated platelets, likely due to competition for the heparin‐binding site on PF4. The use of “off‐the‐shelf” cryopreserved platelets has the potential to transform the diagnostic testing paradigm by making near‐patient functional testing available for the rapid and accurate diagnosis of HIT and VITT.
OC 02.4
Persistence of Ad26.COV2.S‐associated VITT and specific detection of VITT antibodies
A. Padmanabhan 1; A. Kanack2; B. Singh1; G. George3; K. Gundabolu4; S. Koepsell4; Y. Abou‐Ismail5; K. Moser5; K. Smock5; D. Green6; A. Major7; C. Chan7; G. Wool7; M. Reding8; A. Ashrani1; A. Bayas9; D. Grill1
1 Mayo Clinic, Rochester, Minnesota, United States; 2 Mayo cliinc, Rochester, Minnesota, United States; 3 Univ of Colorado, Denver, Colorado, United States; 4 University of Nebraska, Omaha, Nebraska, United States; 5 University of Utah, Salt Lake City, Utah, United States; 6 New York University Langone Health, New York, New York, United States; 7 University of Chicago, Chicago, Illinois, United States; 8 Center for Bleeding and Clotting Disorders, University of Minnesota, Minneapolis, Minnesota, United States; 9 University of Augsburg, Augsburg, Bayern, Germany
Background: Some COVID‐19 vaccinated individuals develop anti‐platelet factor 4 (PF4) antibodies that cause thrombocytopenia and thrombosis; a rare syndrome referred to as vaccine‐induced immune thrombotic thrombocytopenia (VITT). Currently, information on the characteristics and persistence of anti‐PF4 antibodies that cause VITT after Ad26.COV2.S vaccination is limited, and available PF4‐polyanion enzyme‐linked immunosorbent assays (ELISAs) and functional diagnostic assays fail to differentiate Ad26.COV2.S and ChAdOx1 nCoV‐19‐associated VITT from similar clinical disorders, namely heparin‐induced thrombocytopenia (HIT) and spontaneous HIT.
Aims: Evaluate the persistence of anti‐PF4 antibodies in Ad26.COV2.S‐associated VITT and correlate findings with clinical and laboratory variables such as thrombosis and platelet counts. Develop/investigate laboratory tools that differentiate VITT antibodies from HIT and spontaneous HIT.
Methods: Blood samples from VITT and HIT patient cohorts were tested in antigen‐based and functional assays and correlated with clinical and laboratory features.
Results: While Ad26.COV2.S‐associated VITT patients were strongly positive in PF4‐polyanion ELISAs; they were frequently negative in the serotonin release assay (4 of 8 tested patients were negative). In contrast, the PF4‐dependent p‐selectin expression assay (PEA) that uses PF4‐treated platelets consistently diagnosed Ad26.COV2.S‐associated VITT. Most Ad26.COV2.S‐associated VITT antibodies persisted for >5 months in PF4‐polyanion ELISAs, while the PEA became negative earlier. Two patients had otherwise unexplained mild persistent thrombocytopenia (140–150,000/μl) six months after acute presentation. No recurrence of thrombosis was noted. Additionally, a novel un‐complexed PF4 ELISA specifically differentiated VITT secondary to Ad26.COV2.S and ChAdOx1 nCoV‐19 vaccination, from spontaneous HIT and HIT (Fig 1A‐ PF4/polyanion ELISA; Fig 1B‐ Un‐complexed PF4 ELISA; closed black circles‐ Ad26.COV2.S‐associated VITT; closed red circle‐ ChAdOx1 nCoV‐19‐associated VITT; ***p < 0.001; ****p < 0.0001). Its specificity was further confirmed by testing commonly‐encountered HIT‐suspected patient samples that are PF4/polyanion ELISA‐positive but negative in functional assays (1A–1B).
Conclusion(s): Ad26.COV2.S‐associated VITT antibodies are persistent, and the un‐complexed PF4 ELISA appears to be both sensitive and specific for VITT diagnosis.
OC 02.2
Cooperation between PF4‐specific antibodies in heparin‐induced thrombocytopenia: A new mechanism potentially contributing to hypercoagulability and thrombosis
S. Billy 1; C. Vayne2; N. Charuel3; L. Coënon3; M. Atsouawe3; C. Pouplard4; Y. Gruel2; J. Rollin2
1 Université de Tours, TOURS, Centre, France; 2 University Hospital of Tours, Tours, Centre, France; 3 Université de Tours, Tours, Centre, France, 4 University Hospital of Tours, Toiurs, Centre, France
Background: Heparin‐induced thrombocytopenia (HIT) is a severe complication of heparin therapy frequently associated with thrombosis. IgG antibodies against heparin‐modified platelet factor 4 (anti‐PF4/H) play a central role in HIT by activating platelets and leukocytes via FcγRIIA. However, some HIT patients concomitantly develop IgG antibodies to native PF4 (anti‐PF4). Molecular studies have suggested that anti‐PF4 IgG could promote the binding of anti‐PF4/H IgG to PF4 without heparin, but their effect on cell activation remains unknown.
Aims: To assess the combined effects of anti‐PF4/H and anti‐PF4 IgG antibodies on platelet activation and thrombus formation.
Methods: Monoclonal IgG antibodies with a human Fc and directed against PF4/H complexes (5B9) or native PF4 (1E12) were used. Cooperative effects of 5B9 and 1E12 on platelet activation were studied by serotonin release assay (SRA) and whole blood (WB) impedance aggregometry. Thrombosis formation induced in WB by 5B9 and/or 1E12 under shear stress (500s‐1) was assessed in microfluidic channels.
Results: While 5B9 20 μg/ml activated platelets only with low heparin concentrations, its co‐incubation with a non‐activating concentration of 1E12 (0.5 μg/ml) induced in SRA significant platelet activation without heparin. This cooperative effect was confirmed in WB by platelet aggregometry, and is FcγRIIA‐dependent since fully inhibited by IV.3, a blocking antibody. In WB under flow conditions, 5B9 alone didn't activate platelets at 100 μg/ml. But when co‐incubated with a non‐activating low concentration of 1E12 (2 μg/ml), the formation of fibrin‐containing platelet aggregates similar to those observed with 5B9 and heparin (1 IU/ml), was demonstrated.
Conclusion(s): For the first time, we have demonstrated the existence of functional cooperation between HIT antibodies exhibiting different specificities. Although the molecular mechanisms underlying this synergy are still unknown, these results suggest that IgG antibodies specific to PF4 alone may contribute at low concentrations to the risk of thrombosis in HIT.
OC 02.1
Complement receptors mediate binding of heparin‐induced thrombocytopenia immune complexes to Fc receptor bearing cells
H. Harris 1; S. Khandelwal2; L. Rauova3; D. Cines4; G. Arepally2
1 Duke University, Durham, North Carolina, United States; 2 Division of Hematology Duke University Medical Center, Durham, North Carolina, United States; 3 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 4 Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: Heparin‐induced thrombocytopenia (HIT) is a thrombotic disorder caused by antibodies to platelet factor 4 (PF4)/heparin complexes that activate white blood cells (WBCs) via Fcγ receptors (FcγRs). Complement activation by HIT antibodies plays an important role in HIT immune complex (IC) binding to WBCs. However, the effect of complement and complement receptors (CRs) on IC binding to WBC FcγRs is not known.
Aims: Examine the contributions of CR1 (CD35), CR3 (CD18/CD11b), CR4 (CD18/CD11c), C1qR (CD93), FcγRI (CD64), and FcγRIIA (CD32) in HIT IC binding to WBCs.
Methods: THP‐1 cells, a monocytic cell line expressing FcγRI/IIA but lacking CRs, were incubated with PF4, heparin, and IgG monoclonal HIT‐like antibody KKO (KKO ICs) with or without FcγRIIA blocking antibody (IV.3) in plasma or media. Whole blood was pre‐incubated with blocking antibodies to CR1/2/3/4 or FcγRI/II, followed by incubation with KKO ICs. Cell surface binding of C3c and KKO was measured by flow cytometry.
Results: THP‐1 cells incubated with KKO ICs in plasma caused substantial complement deposition that was unaccompanied by IgG binding (Figure 1A). In media without a source of complement, KKO ICs showed robust binding in a FcγRII dependent manner (70% reduction in binding by IV.3; Figure 1B). In whole blood, IC binding to neutrophils (Figure 2A) and monocytes (Figure 2B) was significantly diminished by anti‐CR1 (70%–85%), anti‐CR3 (20%–70%), or combined CR1/CR3 blockade (85%–95%), but unaffected by Fc γR blockade.
Conclusion(s): These studies suggest that in plasma and whole blood: (1) complement masks Fc regions on HIT ICs and thereby limits their binding to FcγRIIA, and (2) binding of KKO ICs to monocytes and neutrophils is initiated primarily through CR1 and CR3, not directly to FcγRs. These data demonstrate that CR inhibition could serve as a potential therapeutic strategy to prevent thrombosis in HIT. Funding: NIH Grant HL151730 (GMA/DBC/LR), ASH Medical Student Physician‐Scientist Award (HMH).
Inherited Thrombocytopenias
OC 64.4
Modeling gray platelet syndrome: longitudinal mouse studies and in vitro human iPSC‐derived megakaryocytes to investigate clinical and cellular features
J. Collins1; L. Mayer1; L. Kollipara2; A. Sickmann3; H. Zhou4; G. Hoffman5; W. Erber5; C. Ghevaert1; W. Ouwehand6; J. Guerrero 1
1 University of Cambridge, Cambridge, England, United Kingdom; 2 Leibniz‐Institut f»r Analytische Wissenschaften _ ISAS _ e.V., Dortmund, Nordrhein‐Westfalen, Germany; 3 Leibniz‐Institut für Analytische Wissenschaften – ISAS – e.V. Dortmund, Germany, Dortmund, Nordrhein‐Westfalen, Germany; 4 Zhejiang Hisun Pharmaceutical, Taizhou Zhejiang, Jiangsu, China (People's Republic); 5 the university of western australia, Perth, Western Australia, Australia; 6 Department of Haematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK. Department of Haematology, University College London Hospitals, London, NW1 2BU, UK, Cambridge, England, United Kingdom
Background: Our understanding of the gray platelet syndrome (GPS) has recently expanded with the presence of dysfunctional neutrophils, a pro‐inflammatory plasma proteome, autoantibodies and autoimmune disorders. However, the onset and evolution of classical phenotypes such as splenomegaly, emperipolesis and bone marrow fibrosis (BMF), as well as the role of Nbeal2 are still lacking.
Aims: Our aim was to investigate the phenotypes mentioned above.
Methods: Mice aged 3–22 months and genetically‐engineered cells (K562, CHRF, iPSC‐derived megakaryocytes) using mass‐spectrometry (MS) and electron microscopy (EM). For comparison, control and GPS human/murine platelets were used.
Results: Across all ages, spleen size and bone marrow emperipolesis were increased in Nbeal2‐/‐ mice. Mean spleen length was 2.9 ± 0.9 cm in Nbeal2‐/‐ mice compared to 1.8 ± 0.4 cm in controls (p‐value = 1.2 × 10−6). The percentage of megakaryocytes containing neutrophils were 68.2 ± 14.9 and 1.3 ± 1.3 (p‐value = 4.8 × 10−13) with 2.24 ± 0.85 and 0.77 ± 0.73 neutrophils/megakaryocyte (p‐value = 2.237 × 10−5) in Nbeal2‐/‐ and control mice, respectively. Pathological BMF (≥grade 2) was observed in 40% of Nbeal2‐/‐ mice older than 10 months. In vitro, absence of Nbeal2 was confirmed in all GPS cell models. The number of granules and vacuoles in GPS platelets was statistically different to that of control platelets. However, this level of significance was not reached with the GPS cell models (Fig. 1A). A proportion of α‐granule proteins identified by MS were lower in GPS platelets and in the in vitro‐generated Nbeal2‐/‐ cells when compared to their corresponding controls, e.g. PSEL and PF4, with only a fraction of these proteins reaching statistical significance, e.g. PDGF, THBS1, TIMP1 (Fig. 1B,C).
Conclusion(s): While splenomegaly and emperipolesis develop in Nbeal2‐/‐ mice at the earliest age studied, pathological BMF occurs from 10 months. The absence of Nbeal2 from GPS cell models has a smaller effect on the loss of α‐granules and their content when compared to platelets suggesting that α‐granule loss in GPS might predominantly occur after proplatelet formation or platelet release. FIGURE 1 α‐granules and their content in human and murine platelets and different GPS cell models.
FIGURE 1 α‐granules and their content in human and murine platelets and different GPS cell models.
OC 64.3
Novel GNE gene variants associated with severe congenital thrombocytopenia and platelet sialylation defect – follow up data after hematopoietic stem cell transplantation
B. Zieger 1; D. Boeckelmann2; W. Anani3; H. Falet4; J. Zhu3; H. Glonnegger1; H. Full5; F. Andresen1; M. Erlacher1; E. Lausch6; S. Fels1; B. Strahm1; P. Lang7; K. Hoffmeister8
1 Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology and Oncology, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 2 Division of Pediatric Hematology and Oncology, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 3 Translational Glycomics Center, Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 4 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 5 Clinic for Pediatric and Adolescent Medicine, SLK‐Kliniken Heilbronn, Heilbronn, Baden‐Wurttemberg, Germany; 6 Pediatric Genetics Section, Department of Pediatrics, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 7 Department of Pediatrics, Children's University Hospital, University of Tübingen, Tübingen, Baden‐Wurttemberg, Germany; 8 Versiti Translational Glycomics Center, Milwaukee, Wisconsin, United States
Background: The GNE gene encodes an enzyme that initiates and regulates the biosynthesis of N‐acetylneuraminic acid, a precursor of sialic acids. GNE mutations are classically associated with Nonaka myopathy and sialuria, following an autosomal recessive and autosomal dominant inheritance pattern. Single patients have been described with GNE variants, which only cause isolated thrombocytopenia.
Aims: To characterize the platelet defect in a young girl (6 month old) with life‐threatening intracranial bleeding and severe congenital thrombocytopenia (lowest platelet count 5 G/L), who needed weekly platelet transfusions.
Methods: Bone marrow analysis and comprehensive analyses for inherited thrombocytopenia: blood smear, platelet aggregometry (LTA), platelet flow cytometry (FC) and lectin array analysis of platelet glycoproteins. Molecular analysis was performed by HTS panel and direct sequencing. Protein modelling using PyMOL.
Results: Bone marrow analysis indicated slightly increased megakaryopoiesis with mature megakaryocytes of normal morphology and hypolobulated, immature cells. LTA showed severe impaired response to Col, ADP, and Epi. FC showed moderately impaired VWF‐binding after stimulation with ristocetin and severely decreased agonist‐induced alpha and dense granules secretion. HTS identified two novel compound heterozygous variants in GNE (NM_005476.6:c.1250C>T and c.1259G>A.). Both alterations (p.Thr417Met and p.Arg420Gln) are located in the nucleotide‐binding site of the N‐acetylmannosamin kinase domain of GNE. Crystal structure of GNE kinase domain in complex with ADP shows the hydrogen‐bonding interactions of T417 and R420 with ADP and in silico mutations of T417M and R420Q suggest disruption of ADP binding. Lectin array showed decreased α‐2,3 sialylation on platelets, consistent with loss of sialic acid synthesis and indicative of rapid platelet clearance.
Conclusion(s): The findings led to hematopoietic stem cell transplantation (HSCT) which normalized platelet counts (follow up 15 months after HSCT: 423 G/L, no signs of myopathy). The child developed normally. To our knowledge, this was the first time that HSCT was successfully performed in a patient with GNE defect.
OC 64.2
Platelet RNA sequencing and generation of an imMKCL‐based cell model for SLFN14 K219N deficiency show evidence of increased cellular stress
F. Ver Donck 1; K. Ramaekers1; C. Thys1; K. Peerlinck2; C. Van Geet3; K. Eto4; V. Labarque3; K. Freson1
1 Center for Molecular and Vascular Biology, University of Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 2 University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 3 Paediatric Hemato‐Oncology, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 4 Kyoto University, Kyoto, Kyoto, Japan
Background: Single nucleotide variants in SLFN14, which encodes an RNA endoribonuclease protein, are known to cause inherited thrombocytopenia. Patients with a SLNF14 variant also present with reduced platelet aggregation and ATP secretion. Despite mild laboratory defects, these patients display an obvious bleeding phenotype. The function of SLFN14 in megakaryocyte and platelet biology is not studied.
Aims: This study aims to characterize the platelet transcriptome in patients with a SLFN14 K219N variant using total RNA sequencing and to model the disease using the immortalized MegaKaryocyte Cell Line imMKCL (Nakamura et al. Cell Stem Cell 2014).
Methods: Patients' platelet RNA was extracted and used for total RNA sequencing. Bioinformatics analysis was performed in R using DESeq2 and WGCNA packages. The K219N variant was introduced in imMKCL using CRISPR/Cas9.
Results: The SLFN14 K219N variant was detected by WES in thrombocytopenia patients from a large pedigree. Platelet RNA was sequenced for two patients and 12 healthy controls. Differential gene expression analysis yielded 2831 and 2860 significantly (|log2FC|>1, FDR <0.05) upregulated and downregulated genes, respectively (Figure 1). Top 500 up‐ and downregulated genes were used for Reactome pathway analysis. Upregulated genes were enriched for mRNA splicing, rRNA processing and regulation of HSF1‐mediated heat shock response (all indicative of increased cellular stress). Downregulated genes were enriched for platelet function pathways (mainly integrin αIIbβ3). Gene co‐expression network analysis confirmed these pathways. Heterozygous SLFN14 K219N imMKCL showed a pronounced defect in megakaryocyte differentiation and proplatelet‐formation compared to the wild type condition. Heterozygous SLFN14 defective megakaryocytes contain numerous multilamellar bodies that occur after increased cellular stress. An almost complete block in megakaryopoiesis was detected for the homozygous SLFN14 K219N imMKCL.
Conclusion(s): Our results indicate dysregulation of gene transcription and translation as potential disease mechanism underlying SLFN14‐related thrombocytopenia. We are currently characterizing the endoribonuclease properties of mutant SLFN14. FIGURE 1 Volcano plot illustrating differentially expressed (blue down‐regulated, red up‐regulated) genes in SLNF14 K219N defective platelets.
FIGURE 1 Volcano plot illustrating differentially expressed (blue down‐regulated, red up‐regulated) genes in SLNF14 K219N defective platelets.
OC 64.1
The VarioPath project: Determining effect sizes of pathogenic variants in platelet disorder genes, using clinical data from 0.5M whole exome sequenced UK Biobank participants
J. Collins 1; L. Stefanucci2; M. Sims3; K. Freson4; E. Bruford2; K. Megy2; D. Vuckovic5
1 University of Cambridge, Loughton, England, United Kingdom; 2 University of Cambridge, Cambridge, England, United Kingdom; 3 Department of Haematology, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK, Cambridge, England, United Kingdom; 4 Center for Molecular and Vascular Biology, University of Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 5 Imperial college London, London, England, United Kingdom
Background: Accurate determination of the effect sizes of DNA variants causal of platelet disorders is essential for reliable reporting to clinicians and patients. However, this has only recently become feasible for rare variants, with the release of the whole‐exome‐sequencing (WES) and associated clinical data from 0.5 million UK Biobank (UKB) participants.
Aims: To calculate the effect sizes of rare pathogenic/likely pathogenic variants (P/LPVs) causal of autosomal dominant (AD)‐platelet disorders and determine the clinical sequelae of carrying a single P/LPV causal of recessive (AR)‐platelet disorders.
Methods: We identified 484 P/LPVs in platelet disorder genes, present in UKB WES data with a minor‐allele‐frequency <0.001. Single‐variant linear regression analysis calculated effect sizes of P/LPVs present in at least five out of 131,022 unrelated European UKB‐participants, after adjusting for relevant covariates and genomic principle components (significance at p‐value < 0.05 after Benjamini‐Hochberg multiple‐testing correction).
Results: Analysis of 19 P/LPVs for AD‐thrombocytopenia revealed four that significantly reduce platelet count and increase mean platelet volume (MPV); the largest effect sizes were −1.4 SD for platelet count and 1.7 SD for MPV, for GP1BA:p.Gln196Ter, present in 19 UKB‐participants. Three TUBB1‐variants, together observed in 96 participants, lower platelet count and increase MPV; two additional P/LPVs increase MPV only (Fig. 1). Analysis of 102 P/LPVs for AR‐platelet disorders, showed that GP9:p.Asn61Ser (causal of Bernard‐Soulier‐Syndrome in homozygosity/compound heterozygosity), significantly reduces platelet count in carriers (−0.6 SD, p = 3 × 10−14, n = 169). Paradoxically, platelet counts of carriers of one of three MPL variants (causal of Congenital‐Amegakaryocytic‐Thrombocytopenia (CAMT)) are significantly increased (Fig. 2).
Conclusion(s): For the first time ever, we have accurately calculated effect sizes of P/LPVs deemed causal of platelet disorders; we observe platelet count/volume effect sizes have generally been overestimated. Our results demonstrate the clinical penetrance of P/LPVs causal of AR‐platelet disorders. Unexpectedly, carrying a loss‐of‐function MPL variant, which in homozygosity/compound heterozygosity cause CAMT, increases platelet count.
Non‐coding RNAs
OC 22.5
Allele‐selective inhibition of murine von Willebrand factor in vitro and in vivo
Y. Jongejan 1; R. Dirven2; K. Paunovska3; N. Linthorst1; E. Schrader3; K. van der Gouw2; A. de Jong2; J. Dahlman3; B. van Vlijmen2; J. Eikenboom2
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 3 Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, Georgia, United States, Atlanta, Georgia, United States
Background: High von Willebrand factor (VWF) plasma levels are associated with (arterial) thrombosis. Antiplatelet therapy increases bleeding risk and often fails to prevent arterial thrombosis. Lowering of VWF through allele‐selective silencing of VWF with small interfering RNAs (siRNAs) would be a personalized approach which averts complete VWF knockdown and thus minimizes bleeding risk.
Aims: To investigate the feasibility of strain‐selective siRNA‐mediated VWF inhibition in vivo in mice.
Methods: 15 siRNAs targeting murine Vwf were studied on activity and allele‐selectivity in vitro in transient co‐transfected HEK293 cells expressing C57BL/6J (B6) and 129S1/SvImJ (129S) Vwf. Of those siRNAs three lead candidates were chosen. These candidates, a non‐selective siVwf, and two strain‐selective siRNAs (siVwf.B6 and siVwf.129S), together with corresponding scrambled controls were encapsulated in 7C1 polymeric nanoparticles for endothelial targeting in vivo. Male and female B6 and 129S mice were intravenously injected with the siRNA‐encapsulated nanoparticles. 72 h post‐injection, citrated blood and lung tissue were collected for measuring VWF plasma protein and Vwf mRNA expression, respectively.
Results: For both male and female mice, all three lead candidate siRNAs dose‐dependently inhibited Vwf expression on mRNA and plasma protein levels in the corresponding mouse strains (Figure 1). The highest inhibitory effect was shown at the dose of 1.5 mg siRNA/kg body weight. The median inhibition was 70% [63–95] on lung mRNA and 75% [66–82] on plasma levels for siVwf‐treated mice, 87% [85–90] and 83% [80–86] for siVwf.B6‐treated mice, and 70% [54–79] and 62% [51–72] for siVwf.129S‐treated mice.
Conclusion(s): We have shown efficient in vivo endothelial targeting of strain‐selective siRNAs with up to 90% inhibition of Vwf in corresponding mouse strains. Testing of these siRNAs in F1 hybrids of B6x129S mice for allele‐selectivity is currently ongoing. This study was financially supported by the Dutch Thrombosis Foundation (grant #2018‐1). FIGURE 1 Murine Vwf lung mRNA and plasma protein levels 72 h post‐injection with different siRNA doses. (A) Vwf mRNA expression in C57BL/6J (B6) and 129S1/SvImJ (129S) mice after treatment with 0.5–1–1.5 mg siVwf/kg body weight. (B) Vwf mRNA expression in B6 mice after treatment with 0.5–1–1.5 mg siVwf.B6/kg body weight. (C) Vwf mRNA expression in 129S mice after treatment with 0.5–1–1.5 mg siVwf.129S/kg body weight. (D) Plasma VWF levels in B6 and 129S mice after treatment with 0.5–1–1.5 mg siVwf/kg body weight. (E) Plasma VWF levels in B6 mice after treatment with 0.5–1–1.5 mg siVwf.B6/kg body weight. (F) Plasma VWF levels in 129S mice after treatment with 0.5–1–1.5 mg siVwf.129S/kg body weight. Data presented as individual datapoints (N = 4–6 animals) with median percentage of scrambled control siRNA per treatment group. Statistical analysis was performed using a two‐tailed Mann‐Whitney test with *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
FIGURE 1 Murine Vwf lung mRNA and plasma protein levels 72 h post‐injection with different siRNA doses. (A) Vwf mRNA expression in C57BL/6J (B6) and 129S1/SvImJ (129S) mice after treatment with 0.5–1–1.5 mg siVwf/kg body weight. (B) Vwf mRNA expression in B6 mice after treatment with 0.5–1–1.5 mg siVwf.B6/kg body weight. (C) Vwf mRNA expression in 129S mice after treatment with 0.5–1–1.5 mg siVwf.129S/kg body weight. (D) Plasma VWF levels in B6 and 129S mice after treatment with 0.5–1–1.5 mg siVwf/kg body weight. (E) Plasma VWF levels in B6 mice after treatment with 0.5–1–1.5 mg siVwf.B6/kg body weight. (F) Plasma VWF levels in 129S mice after treatment with 0.5–1–1.5 mg siVwf.129S/kg body weight. Data presented as individual datapoints (N = 4–6 animals) with median percentage of scrambled control siRNA per treatment group. Statistical analysis was performed using a two‐tailed Mann‐Whitney test with *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Platelet Antagonists and Novel Therapeutics
OC 73.4
Characterization of a novel anti‐GPVI antibody in a humanized GP6 (hGP6KI) mouse model
S. Navarro 1; A. Starke2; H. Brown2; S. Beck3; V. Orth4; J. Heemskerk5; M. Kuijpers6; D. Stegner7; B. Nieswandt8
1 University Hospital Würzburg, Würzburg, Bayern, Germany; 2 University hospital Würzburg, Würzburg, Bayern, Germany; 3 University Hospital and Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 4 Emfret Analytics, Eibelstadt, Bayern, Germany; 5 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 6 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 7 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 8 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: Glycoprotein VI (GPVI) is the major collagen receptor on platelets, it has been proposed as a promising anti‐platelet target as its blockade prevents experimental thrombosis without impairing hemostasis.
Aims: This study aimed to use a humanized GP6 mouse line (hGP6KI) to investigate the effect of a novel function blocking anti‐GPVI antibody (EMF‐1) in thrombus formation and hemostasis.
Methods: Flow‐cytometry, aggregometry and flow‐adhesion assay were used to investigate the in vitro and ex vivo effect of EMF‐1 on platelet function. Tail bleeding assay was performed to assess the effect on hemostasis and arterial thrombosis was studied in a model of mechanical aorta injury.
Results: A panel of anti‐human GPVI (huGPVI) monoclonal antibodies (mAbs) was generated and characterized. Several mAbs displayed GPVI inhibitory activity, among them EMF‐1 which completely inhibited collagen‐related peptide (CRP) and collagen‐induced platelet activation, aggregation and thrombus formation. Treatment of hGP6KI mice with EMF‐1 (5 μg/g body weight) induced transient thrombocytopenia and long‐lasting GPVI depletion from the platelet surface. In contrast, the Fab fragment of EMF‐1 did neither affect platelet counts nor GPVI surface expression but reduced GPVI‐dependent activation up to 72 h after injection. GPVI‐depleted hGP6KI mice did not show any significant increase in tail‐bleeding times, but were protected from thrombosis after mechanical injury of abdominal aorta. Finally, EMF‐1 Fab treatment only slightly increased tail‐bleeding time, but prevented stable arterial thrombus formation after injury.
Conclusion(s): Our study presents a new antibody‐based candidate compound to target human GPVI in vivo to prevent or limit arterial thrombosis without significantly altering hemostasis.
Platelet Function and Interactions
OC 73.5
Investigations to assess Impact of Syk‐inhibition on antibody‐mediated desialylation: Novel implications on therapy for immune thrombocytopenia
A. Singh 1; F. Toma2; K. Althaus3; T. Backchoul4
1 Institute for Clinical and Experimental Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany; 2 Institute for Clinical and Experimental Transfusion Medicine, University Hospital Tübingen, Tübingen, Baden‐Wurttemberg, Germany; 3 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 4 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Immune thrombocytopenia (ITP) is an autoantibody‐mediated bleeding disorder with increased clearance of platelets and megakaryocyte dysfunction. Previously, we showed that antibody‐mediated desialylation plays a pivotal role in pathophysiology of ITP, leading to impaired platelet adhesion and megakaryocyte differentiation via FcγRIIA receptor.
Aims: In current study, we aim to investigate whether spleen tyrosine kinase (Syk) inhibition can alter platelet dysfunction and desialylation in ITP.
Methods: Using in vitro cell culture and flow cytometry methods, we analyzed the impact of Syk inhibitors R406 and PRT318. Role of FcγRIIA was studied by crosslinking anti CD32 clone AT‐10 with the secondary Fab2 antibody to stimulate the receptor. Platelet function was determined as CD62P and PAC1 expression, and apoptosis as mitochondrial‐transmembrane potential (Δψ) depolarization and phosphatidylserine (PS) externalization. Human ITP AAb‐induced platelet desialylation was analyzed using a lectin binding assay (LBA) after incubation of platelets with ITP or healthy donor sera and flow cytometry.
Results: Our data shows that Syk inhibition can effectively alter antibody‐mediated biological effects in ITP. Activation of FcyRIIA via crosslinking resulted in significant increase in apoptosis and desialylation markers. R406 and PRT318 significantly inhibited apoptosis (mean %PS externalization ± SEM: 27.9 ± 2.1 vs. 9.1 ± 2.3 and 2.1 ± 90.50 respectively, p < 0.001) and desialylation (mean FI ± SEM 2.15 ± 0.17 vs. 1.25 ± 0.10 and 0.93 ± 0.16 respectively, p < 0.01). Sera from ITP patients induced desialylation in healthy washed platelets, which could be inhibited with both R406 and PRT318 (mean FI ± SEM 1.62 ± 0.10 vs. 1.13 ± 0.04 and 0.93 ± 0.05 respectively, p < 0.01). In ongoing studies, we explore the opportunity to restore impaired proplatelet formation by megakaryocytes with Syk‐inhibition, and investigate the possibility of reverting desialylation in an established ex vivo model of thrombopoiesis.
Conclusion(s): Our data indicates that Syk‐inhibition might be a promising approach to prevent antibody‐mediated platelet desialylation in ITP and might serve as a potential therapeutic approach to prevent patient complications during pathogenesis of ITP.
Platelet Function Disorders, Hereditary
OC 64.5
Effect of the DIAPH1 R1213* GOF variant in actin‐dependent cytoskeleton organization in megakaryocytes
V. Palma‐Barqueros1; L. Bury2; A. Zamora‐Canovas 3; H. Kuchi Bhotla4; A. Sánchez‐Fuentes1; N. Revilla5; A. Rodríguez‐Alen6; A. Torrecillas1; N. Bohdan1; V. Vicente7; M. Lozano7; J. Bastida8; P. Gresele4; J. Rivera9
1 Centro Regional de Hemodonación‐IMIB, Murcia, Murcia, Spain; 2 University of Perugia, Perugia, Umbria, Italy; 3 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain, Murcia, Murcia, Spain; 4 University of Perugia, Department of Medicine and Surgery, Section of Internal and Cardiovascular Medicine, Perugia, Umbria, Italy; 5 Hospital Universitario Ramón y Cajal, Madrid, Spain, Madrid, Madrid, Spain; 6 Hospital Virgen de la Salud, Toledo, Spain, Toledo, Castilla‐La Mancha, Spain; 7 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 8 Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Salamanca, Castilla y Leon, Spain; 9 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Murcia, Murcia, Spain
Background: To date 5 families carrying the gain‐of‐function (GOF) R1213* variant in DIAPH1 displaying macrothrombocytopenia (MTP), neutropenia (NT) and sensorineural hearing loss (HL), have been reported. The underlying molecular mechanisms by which this variant causes MTP are unknown.
Aims: To assess the impact of the R1213* variant on megakaryocytes (Mks)‐platelets from 7 patients of 2 unrelated families (Fig. 1A) (Spanish Project of Inherited Platelet Disorders).
Methods: Patients were in clinical follow‐up due to HL and MTP (79–125 × 109/L; MPV: 13.4–14.3 fL) and NT (0.82–2.2 × 109/L), with no history of bleeding or relevant infections (Fig. 1A). We assessed blood cell count/film; platelet aggregation; platelet glycoproteins expression and platelet activation by flow cytometry (FC); immunofluorescence (IF) and electron microscopy (EM); immunoblotting and RNA expression of cytoskeletal proteins in ultrapure purified platelets. We assessed by IF Mks cultures differentiated from CD34+ patient peripheral blood cells.
Results: Patients showed normal platelet glycoproteins expression and normal aggregation, fibrinogen binding or granule secretion with agonists. mRNA and protein levels of actin and DIAPH1 were altered, but not those of β1‐tubulin, filamin and α1‐actinin. Platelet β1‐tubulin was disorganized in (Fig. 1B), while other cytoskeletal proteins seemed unaltered. Patient platelets normally spread. EM showed rounded and largr platelets with normal organelle content (Fig. 1C). Both families displayed a severe defect in proplatelet formation (Mks forming proplatelets: <1% vs.30% in controls) (Fig. 1D,E). Remarkably, most Mks were forming clusters. The most striking feature was an exacerbation in F‐actin polymerization, which forms aberrant structures protruding from the cell and colocalizing with DIAPH1 (Fig. 1F). Actin could not solely account for cell clustering, since cytochalasin B (actin depolymerizing agent) did not impair it. Noteworthy, actin and αIIbβ3 were found at the cell union interface.
Conclusion(s): The GOF variant R1213* in DIAPH1 associates with cytoskeleton alterations, enhanced actin polymerization which could lead, along with other elements, to Mks clustering and subsequent proplatelets defect and thrombocytopenia. FIGURE 1
FIGURE 1
von Willebrand Factor Biology
OC 53.3
Syntaxin‐2 deficiency results in reduced von Willebrand factor expression in vitro and in vivo
M. Bowman 1; M. Hinds2; P. Lima2; E. Burnett3; C. Ng4; D. Lillicrap5; P. James2
1 Queen's University, Kingston, Canada, Kingston, Ontario, Canada; 2 Department of Medicine, Queen's University, Kingston, Canada, Kingston, Ontario, Canada; 3 Department of Pathology and Molecular Medicine, Queen's University, Kingston, Ontario, Canada; 4 Department of Pediatrics, University of Colorado – Anschutz Medical Campus, Aurora, USA, Aurora, Colorado, United States; 5 Department of Pathology and Molecular Medicine, Queen's University, Kingston, Ontario, Canada
Background: The genetic association data derived from the original CHARGE analysis identified syntaxin‐2 (STX2) as a candidate gene for influencing von Willebrand factor (VWF) plasma levels. STX2 is known to be involved in vesicular transport and protein trafficking. In endothelial cells (ECs) it may play important roles in mediating Weibel‐Palade body (WPB) exocytosis.
Aims: To investigate the in vitro and in vivo effect of STX2 deficiency on VWF synthesis and secretion.
Methods: STX2 expression in ECs was assessed via qRT‐PCR, western blot and confocal immunofluorescence microscopy. STX2 was depleted in human umbilical vein endothelial cells (HUVECs) using siRNA. Cell supernatants and lysates were collected 72 h later and VWF:Ag measured via ELISA. VWF mRNA expression was assessed in cell lysates using qRT‐PCR. Epimorphin/Stx2 knock out (Stx2‐/‐) mice were used to evaluate the in vivo effect of STX2 deficiency on VWF. Blood was collected from male and female 8–12 week old wild‐type (WT), heterozygous (Stx2+/‐) and Stx2‐/‐ mice for the assessment of plasma VWF levels. Tissues were harvested (kidney, lung, liver) for qRT‐PCR analysis of VWF in WT, Stx2+/‐ and Stx2‐/‐ mice.
Results: STX2 was observed in ECs but did not associate with VWF in WPBs (Figure 1A). Depletion of STX2 in HUVECs resulted in significantly lower VWF in the media compared to siCTRL HUVECs (Figure 1B). VWF mRNA levels were lower in siSTX2 HUVEC compared to siCTRL (Figure 1C). In vivo, Stx2‐/‐ mice had lower plasma VWF levels compared to Stx2+/‐ and WT littermates (Figure 2A). VWF mRNA expression was lower in kidney and lung tissues of Stx2‐/‐ mice compared to WT (Figure 2B).
Conclusion(s): STX2 deficiency leads to a decrease in VWF expression both in vitro and in vivo. The mechanism underlying this remains to be determined, as well as the role of STX2 in von Willebrand disease.
OC 53.4
O‐linked glycosylation of VWF modulates Weibel‐Palade body formation and VWF secretion
E. Karampini 1; S. Elliott2; S. Ward3; J. O'Sullivan4; I. Schoen5; J. O'Donnell3
1 Royal College of Surgeons in Ireland, RCSI Dublin, Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Carlow, Ireland; 3 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 4 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 5 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland
Background: Von Willebrand factor (VWF) is a key hemostatic glycoprotein that is predominantly synthesized and secreted by endothelial cells. During biosynthesis, VWF undergoes extensive N‐ and O‐linked glycosylation, before finally being stored in Weibel‐Palade bodies (WPBs). WPB morphology influences VWF string formation upon activation. Although glycan alterations have been implicated in von Willebrand disease pathophysiology, the role of glycosylation in regulating VWF intracellular trafficking remains poorly understood.
Aims: In this study, we investigated the role of O‐glycosylation on intracellular VWF trafficking, WPB formation and VWF secretory pathways.
Methods: HUVECs were treated with the O‐glycosylation inhibitor BG (Benzyl‐α‐GalNAc). Site‐directed mutagenesis was used to generate VWF mutants lacking specific O‐glycan chains. WPB morphology was studied using confocal microscopy. VWF secretion was assessed by ELISA.
Results: BG‐induced inhibition of O‐glycosylation in HUVECs dramatically affected WPB formation, without altering the morphology of other intracellular compartments like the trans‐Golgi network. WPBs were significantly shorter and more circular in BG‐treated cells compared to controls (p < 0.0001). BG treatment also impacted VWF and angiopoietin‐2 (Ang‐2) secretion in HUVECs. In particular, histamine‐stimulated VWF secretion was significantly reduced (p < 0.002) in BG‐treated cells whereas unstimulated VWF secretion was increased (p < 0.001) (Figure 1). In addition, BG‐treatment also resulted in significantly decreased length of VWF strings released from HUVECs in response to histamine‐induced activation (p = 0.045). Interestingly and in keeping with the BG data, site‐directed mutagenesis of the two O‐linked glycan clusters which flank the A1 domain, led to a decrease in pseudo‐WPB length (p < 0.0001) with WPB morphology mimicking that observed in BG‐treated HUVECs.
Conclusion(s): Our novel data suggest that specific O‐linked glycan determinants on VWF critically regulate intracellular trafficking and thereby influence WPB morphology and secretion of VWF strings. FIGURE 1 (A) VWF immunostaining in control and BG‐treated HUVECs (blue: DAPI, magneta: VWF, bottom: VWF single channel, scale bar: 10 μm). (B) Automated high‐throughput analysis of WPB length (n = 4, t‐test, ****p < 0.0001). (C) Stimulated (histamine‐induced) VWF secretion (n = 4, ratio paired t‐test, **p = 0.0017). (D) Unstimulated VWF release over 24 h (n = 4, ratio paired t‐test, ***p = 0.0010).
FIGURE 1 (A) VWF immunostaining in control and BG‐treated HUVECs (blue: DAPI, magneta: VWF, bottom: VWF single channel, scale bar: 10 μm). (B) Automated high‐throughput analysis of WPB length (n = 4, t‐test, ****p < 0.0001). (C) Stimulated (histamine‐induced) VWF secretion (n = 4, ratio paired t‐test, **p = 0.0017). (D) Unstimulated VWF release over 24 h (n = 4, ratio paired t‐test, ***p = 0.0010).
OC 53.1
Studying VWF secretion under flow using microfluidics and patient‐derived endothelial cells
I. van Moort 1; Y. Sakurai2; E. Hardy2; P. Burgisser3; E. Vital2; Y. Qiu2; F. Leebeek3; W. Lam2; R. Bierings4
1 Erasmus MC, University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 2 Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, USA & Department of Pediatrics, Division of Pediatric Hematology/Oncology, Aflac Cancer Center and Blood Disorders Center of Children's Healthcare of Atlanta, Emory University School of Medicine, Atlanta, USA, Atlanta, Georgia, United States; 3 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Von Willebrand factor (VWF) is secreted from endothelial cells into the vessel lumen to form platelet adhesive strings at sites of vascular damage. Quantitative and qualitative defects in VWF lead to the bleeding disorder von Willebrand disease (VWD). Large inter‐individual differences in bleeding phenotype exist in these patients that are currently not well understood but may be the result of variability in endothelial secretory responses. To study how endothelial secretion may affect the interplay with other blood components we need an ex vivo model to model the patient's hemostatic response using patient endothelium under flow.
Aims: To model VWF secretion in patients with VWD using an ex vivo blood vessel consisting of a microfluidic channel endothelialized with patient‐derived endothelial colony forming cells (ECFCs).
Methods: ECFCs were isolated from healthy volunteers and patients with VWD enrolled in the 2020‐BOEK‐MK study with informed consent. VWD patients were selected from the Willebrand in the Netherlands (WiN) study. PDMS microfluidic devices containing vascular channels (150 × 50 μm) were created using soft lithography. ECFCs were seeded in collagen‐coated channels and cultured for 48 h under flow (750s‐1). To stimulate regulated secretion, ECFCs were stimulated under flow with 100 μM histamine. VWF secretion was monitored using confocal microscopy.
Results: PDMS channels seeded with ECFCs formed 3D vessels consisting of confluent endothelial monolayers encapsulating a vessel lumen (figure 1). Immunostaining of VWF in healthy donor vessels revealed large numbers of Weibel‐Palade bodies per cell. VWD 2A ECFCs (heterozygous p.C1190R) also formed 3D vessels, however significant amounts of VWF were retained in the endoplasmic reticulum. Histamine induced formation of numerous VWF strings covering the apical side of the endothelium facing the vessel lumen.
Conclusion(s): Microfluidic bleeding devices containing patient‐derived ECFCs are promising models to study the cause of (variations in) bleeding abnormalities. This study is funded by NWO‐NWA.1160.18.038 and an ISTH‐EHA Training Fellowship. FIGURE 1 Vessel created from healthy ECFCs. (A)VE‐Cadherin; (B) VWF; (C) DAPI; (D) merge with VE‐Cadherin in Magenta, VWF in green, DAPI in blue.
FIGURE 1 Vessel created from healthy ECFCs. (A)VE‐Cadherin; (B) VWF; (C) DAPI; (D) merge with VE‐Cadherin in Magenta, VWF in green, DAPI in blue.
OC 53.2
Deep mutational scan of the VWF C domains to define mutations associated with VWD
T. Sparring 1; H. Madarati2; K. Singh3; C. Kretz2; D. Fowler; N. Popp
1 Thrombosis and Atherosclerosis Research Institute (TaARI), McMaster University, Toronto, Ontario, Canada; 2 McMaster University, Hamilton, Ontario, Canada; 3 Thrombosis and Atherosclerosis Research Institute (TaARI), McMaster University, Hamiton, Ontario, Canada
Background: Von Willebrand Factor (VWF) is a multimeric glycoprotein that recruits platelets to sites of blood vessel injury. Mutations in the VWF C domains can lead to intracellular retention and lower circulating VWF levels associated with the bleeding disorder von Willebrand disease (VWD). It's currently not well understood why some mutations lead to decreased VWF secretion, while others do not.
Aims: Apply deep mutational scanning to identify every possible missense mutation in VWF that either increase or decrease its secretion from cells.
Methods: We developed a VWF cell secretion assay in HEK293T cells using Fluorescence Activated Cell Sorting (FACS).
Results: HEK293T cells expressing VWF‐eGFP variants with known secretion defects have increased mean fluorescence intensity (MFI) (V1822G = 157 ± 5, C2693Y = 184 ± 2), compared to WT VWF‐eGFP (69 ± 2), thus validating the screen. The VWF mutagenesis library contains every amino acid substitution at each position of the C1‐CTCK domains in full length VWF. HEK293T cells expressing the VWF‐eGFP mutagenesis library exhibited a broad distribution of MFI compared to cells expressing WT VWF‐eGFP, confirming the presence of variants that affect secretion. Cells were sorted into upper and lower eGFP intensity bins and prepared for high‐throughput sequencing to identify VWF mutations associated with impaired secretion. Data analysis of this screen is on‐going. VWF mutations can lead to ER retention, which may cause secondary effects on cell viability. Cells expressing variants with the highest 10% eGFP intensity had upregulation of GRP78, CHOP, and ERP57 expression compared to cells expressing WT VWF. Therefore, missense mutations that cause impaired VWF secretion are associated with increased expression of markers associated with the adaptive and apoptotic unfolded‐protein response.
Conclusion(s): Deep mutational scanning using FACS provides a platform to identify all possible coding variants in VWF that impact its secretion. These data will provide a detailed structure/function analysis of VWF in mammalian cells and identify variants associated with VWD.
OC 22.4
Unique humanized mouse models of von Willebrand disease to implement the need for innovative therapeutic strategies
C. Casari 1; M. Heestermans2; G. Mc Cluskey2; I. Peyron2; C. Reperant2; O. Christophe3; C. Denis4; P. Lenting3
1 Inserm U1176 – HITh, Le Kremlin Bicetre, Ile‐de‐France, France; 2 Inserm U1176 – HITh, Le Kremlin‐Bicetre, Ile‐de‐France, France; 3 INSERM U1176, Le Kremlin Bicetre, Ile‐de‐France, France; 4 Inserm, Le Kremlin‐Bicêtre, Ile‐de‐France, France
Background: Patients suffering from von Willebrand disease (VWD) have reduced quality‐of‐life despite current treatment options. Moreover, innovation in VWD therapeutic strategies has essentially stalled and available treatments have remained unchanged for decades. Therefore, there is an unmet need to develop new therapeutic strategies for VWD‐patients.
Aims: To develop original mouse models to expedite pre‐clinical studies of innovative therapeutic approaches for VWD.
Methods: Mice expressing human von Willebrand factor (hVWF), either wild‐type or carrying the type 2A (p.R1597W) variant, and human GPIbalpha, but not the corresponding murine proteins, have been generated on an 129Sv genetic background (hVWF+/+/hGP1BA+/+ and hVWF(p.R1597W)+/+/hGP1BA+/+). VWF antigen (VWF:Ag), propeptide, multimer pattern and factor VIII (FVIII) activity were analyzed. Tail clip and tail vein transection (TVT) models were applied to assess bleeding tendency.
Results: hVWF+/+/hGP1BA+/+‐mice expressed 15 ± 4% VWF:Ag, 44 ± 8% FVIII activity and normal VWF multimers. hVWF(p.R1597W)+/+/hGP1BA+/+‐mice expressed 3 ± 1% VWF:Ag and 7 ± 1% FVIII activity combined with an abnormal multimer pattern, with only low multimers and few degradation bands visible. VWF propeptide/antigen ratio was higher in these mice, suggesting an accelerated VWF clearance. Antigen levels increased upon histamine‐treatment, evidencing the presence of a releasable VWF pool in both models. Despite the relatively low VWF:Ag levels, hVWF+/+/hGP1BA+/+ ‐mice displayed normal haemostatic responses in both the severe‐ (tail‐clip) and milder‐ (TVT) bleeding assays. In contrast, hVWF(p.R1597W)+/+/hGP1BA+/+‐mice had a severe bleeding phenotype. Interestingly, in the TVT model, although the amount of blood shed was consistent with severe bleeding, 57% of type 2A mice were capable of forming an occlusive, although unstable clot within 15 min of the injury, differing from the bleeding profile of VWF‐deficient mice.
Conclusion(s): We have developed unique humanized mouse models for VWD‐type 1 and VWD‐type 2A, which will be useful to test innovative therapeutic strategies for VWD.
OC 43.4
Plasmin‐cleaved VWF: A biomarker for microthrombosis
H. El Otmani 1; S. Smits1; N. van Kleef1; R. Frunt1; S. De Maat2; C. Maas2
1 University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 2 UMC Utrecht, Utrecht, Utrecht, Netherlands
Background: Von Willebrand factor (VWF) is an essential contributor to hemostasis, but also to arterial and microvascular thrombosis. VWF is cleaved by ADAMTS13 to limit its prothrombotic properties. Failure to do so can lead to microthrombosis as is seen in thrombotic thrombocytopenic purpura (TTP). In this setting, plasmin becomes active to cleave VWF, as we previously demonstrated during acute TTP attacks in human patients. Furthermore, induction of plasmin activity attenuates TTP pathogenesis in preclinical models in vivo. These combined findings suggest that plasmin is an endogenous regulator of VWF thrombogenicity. We therefore hypothesize that assessment of plasmin‐cleaved VWF (cVWF) reflects microvascular thrombosis (analogous to D‐dimer for VTE). However, no assays are available to investigate cVWF in pathophysiology.
Aims: To develop a bioassay to track plasmin‐mediated VWF cleavage in plasma.
Methods: Plasma‐purified VWF was cleaved with plasmin and characterized by Western blotting. Nanobodies (llama‐derived antibody fragments) were developed to discriminate between cVWF and intact VWF by phage display.
Results: We developed an ELISA‐based bioassay that recognizes cVWF, generated after plasmin cleavage. The product that is captured was characterized by Western blotting and consists of three characteristic disulfide‐linked main cleavage fragments (140, 110 and 70 kDa; under reducing conditions). The same products are also formed upon plasmin‐mediated destruction of platelet‐VWF microthrombi in vitro. In spiked citrated plasma, cVWF was detected with high sensitivity at concentrations (lower detection limit 100 ng/ml) that cannot be detected by Western blotting. Validations in TTP patient samples are currently ongoing.
Conclusion(s): Our studies demonstrate that cVWF can be discriminated from intact VWF in an ELISA‐based setup. Detection of cVWF may serve as a biomarker for pathologies involving microvascular thrombosis such as TTP, and possibly other diseases.
OC 43.5
Domain‐specific nanobodies distinguish intact and proteolyzed forms of von Willebrand factor in congenital von Willebrand disease
C. Kizlik‐Masson 1; I. Peyron2; S. Gangnard3; M. Clavel3; V. Regnault4; E. Jeanpierre5; A. Dupont6; O. Christophe3; C. Casari7; C. Denis3; S. Susen8; P. Lenting3
1 Inserm U1176, Le Kremlin Bicetre, Ile‐de‐France, France; 2 Inserm U1176 – HITh, Le Kremlin‐Bicetre, Ile‐de‐France, France; 3 INSERM U1176, Le Kremlin Bicetre, Ile‐de‐France, France; 4 Inserm 1116, Nancy, Lorraine, France; 5 Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 6 Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐ EGID, F‐59000 Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 7 Inserm U1176 – HITh, Le Kremlin Bicetre, Ile‐de‐France, France; 8 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France
Background: Proteolysis of von Willebrand factor (VWF) in its A2 domain by ADAMTS13 results in smaller‐sized multimers with lower hemostatic potential. Both increased shear stress and specific genetic variants in VWF may alter the susceptibility of ADAMTS13‐mediated proteolysis.
Aims: To develop nanobodies that are selective for cleaved and uncleaved forms of VWF.
Methods: Two nanobodies were generated that recognized intact VWF (designated KB‐VWF‐D3.1) and ADAMTS13‐cleaved VWF (KB‐VWF‐F1.1), respectively. Epitopes of both nanobodies were identified using purified VWF and derivatives thereof. Plasma from congenital VWD‐patients were analyzed for their interaction with both nanobodies.
Results: Nanobody KB‐VWF‐F1.1 (interacting with A2‐domain residues Met1606‐Arg1668) specifically bound to unfolded and ADAMTS13‐proteolyzed VWF, but not to globular VWF. KB‐VWF‐D3.1 bound to uncleaved VWF and lost its epitope upon ADAMTS13‐cleavage. Unexpectedly, its epitope was located in the VWF‐A3 domain, overlapping the collagen‐binding site. Both nanobodies were used to follow ADAMTS13‐mediated VWF degradation over time, resulting in increased recognition by KB‐VWF‐F1.1 paralleling the decreased recognition by KB‐VWF‐D3.1 (Fig. 1). Nanobodies were then applied to the analysis of VWD‐patient samples. VWF binding to KB‐VWF‐D3.1 was significantly reduced relative to controls (defined as 1.0) for all types: VWD‐type 1: 0.7 ± 0.3 (n = 20, p = 0.0002); VWD‐type 2A: 0.5 ± 0.2 (n = 46, p < 0.0001); VWD‐type 2B: 0.6 ± 0.2 (n = 24, p = <0.0001) and VWD‐type 2M: 0.7 ± 0.2 (n = 13, p = 0.001). Interestingly, binding was lower in VWD‐type 2A(IIA) (0.4 ± 0.2; n = 31) compared to VWD‐type 2A(IIE) (0.6 ± 0.2; n = 15; p = 0.026). As for KB‐VWF‐F1.1, binding was increased 8 ± 4‐fold for VWD‐type 2A(IIA) samples compared to controls (p < 0.0001), whereas no statistical significant increase in KB‐VWF‐F1.1 binding was detected for other VWD‐types.
Conclusion(s): We identified two novel nanobodies that recognize different configurations of VWF, dependent on the degree of ADAMTS13‐dependent proteolysis. Nanobody KB‐VWF‐D3.1 revealed the existence of ADAMTS13‐induced conformational changes outside the A2‐domain. Understanding how specific variants affect such changes could be insightful to better understand the corresponding phenotype of the patients. FIGURE 1 Binding of KB‐VWF‐F1.1 and KB‐VWF‐D3.1 to VWF in response to ADAMTS13‐mediated proteolysis. VWF was exposed to increased shear stress in the presence of ADAMTS13 for up to 3 h. Residual binding to KB‐VWF‐F1.1 and KB‐VWF‐D3.1 was then measured. Presented is relative binding (as a fraction of total VWF antigen) versus incubation time
FIGURE 1 Binding of KB‐VWF‐F1.1 and KB‐VWF‐D3.1 to VWF in response to ADAMTS13‐mediated proteolysis. VWF was exposed to increased shear stress in the presence of ADAMTS13 for up to 3 h. Residual binding to KB‐VWF‐F1.1 and KB‐VWF‐D3.1 was then measured. Presented is relative binding (as a fraction of total VWF antigen) versus incubation time
OC 22.2
Inhibition of von Willebrand factor through stabilization of the ristocetin‐binding site
N. Arce; R. Li
Aflac Cancer and Blood Disorders Center, Children's Healthcare of Atlanta, Department of Pediatrics, Emory University School of Medicine, Atlanta, USA, Atlanta, Georgia, United States
Background: Capture of platelets by von Willebrand factor (VWF) is an essential process carefully controlled by mechanosensitive elements within VWF. We recently demonstrated that, during activation, VWF undergoes a local conformational change in the autoinhibitory module (AIM) to expose the A1 domain for platelet binding. We further demonstrated that caplacizumab binds to the N‐terminal AIM (NAIM), inhibits AIM unfolding, and prevents platelet binding to VWF. The C‐terminal AIM (CAIM) is used by ristocetin to activate VWF.
Aims: We sought to discover nanobody binders to the CAIM and test if they could modulate VWF activity.
Methods: Using an immunized nanobody library displayed on yeast, we used flow sorting to selectively enrich nanobodies that would bind specifically to the CAIM and deplete those that would bind to either the NAIM or A1. A parallel plate flow chamber was utilized to evaluate VWF‐dependent platelet adhesion under shear.
Results: Two monoclonal nanobodies, Nd4 and Nd6, were identified to bind human AIM‐A1 protein, although with different affinities. Neither showed binding towards a VWF fragment lacking the CAIM, and neither could immunoblot VWF, thus localizing their epitope to a conformationally sensitive region that includes residues 1459–1472. Interestingly, Nd4 and Nd6 incompletely inhibited platelet adhesion in whole blood on the collagen surface at high shear rates, preserving VWF function at higher shear rates to a better degree than m(monomeric)Caplacizumab. In comparison, ARC1172, a well‐known inhibitor of VWF, completely inhibited adhesion at all shear rates.
Conclusion(s): Inhibition of VWF activity can be achieved through binding to residues in the CAIM, including those that ristocetin uses to activate VWF. Inhibition of certain parts of either the AIM or A1 can lead to differential effects on VWF activation and platelet capture under force. FIGURE 1 Figure depicting yeast display and isolation of nanobodies Nd4 and Nd6, their affinity towards VWF constructs, and inhibition of platelet adhesion. Inhibition of von Willebrand factor through stabilization of the ristocetin‐binding site
FIGURE 1 Figure depicting yeast display and isolation of nanobodies Nd4 and Nd6, their affinity towards VWF constructs, and inhibition of platelet adhesion. Inhibition of von Willebrand factor through stabilization of the ristocetin‐binding site
OC 43.1
Idntification of a von Willebrand factor homozygous deep intronic variant by next‐generation analysis, and subsequent validation by mRNA analysis and study of the patient‐derived endothelial cells
H. Yadegari 1; M. Jamil2; J. Oldenburg3
1 University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: A type 3 von Willebrand disease (VWD) index patient (IP) remains mutation‐negative after completion of the conventional diagnostic analysis, including multiplex ligation‐dependent probe amplification and sequencing of the promoter, exons, and flanking intronic regions of VWF gene (VWF).
Aims: In this study, we intended to elucidate causative mutation through DNA analysis of the entire VWF gene, VWF transcript analysis, and study of the patient‐derived endothelial colony‐forming cells (ECFCs).
Methods: Whole‐genome NGS analysis was executed on an Illumina platform. ECFCs were obtained from the peripheral blood of the IP and healthy donors. Total RNA from ECFCs were isolated, and VWF mRNA was quantified using the TaqMan assay. Additionally, Whole‐transcriptome RNA‐sequencing was completed on Illumina HiSeq 2500 V4 platform. Amount of VWF antigen (VWF:Ag) secreted into the medium and present in lysates of ECFCs were measured. Immune‐staining of the ECFCs was done by using anti‐human VWF and Aniopoeitin 2 antibodies. Subsequently, image acquisition was carried out using a Carl Zeiss Apotome.2 microscope.
Results: The NGS revealed a variant in the intronic region of VWF (997+118 T>G in intron 8), for the first time. The bioinformatics assessments (e.g. Illumina artificial intelligence (AI) splicing prediction software, SpliceAl) predicted this variant creates a new donor splice site (ss), which could outcompete the consensus 5′ donor ss at exon/intron 8. This would lead to an aberrant mRNA that contains a premature stop codon, targeting it to nonsense‐mediated mRNA decay. The subsequent quantitative real‐time PCR confirmed the virtual absence of VWF mRNA in IP ECFCs. Additionally, the IP ECFCs demonstrated a considerable reduction in VWF secretion (~6% of healthy donors), and they were devoid of endothelial‐specific secretory organelles, Weible‐palade bodies.
Conclusion(s): Our findings underline the potential of NGS in conjunction with RNA analysis, and patient‐derived cells studies for genetic diagnosis of mutation‐negative type 3 VWD patients.
OC 22.1
Angiopoietin‐2 binds to von Willebrand factor within endothelial cells and after release from Weibel Palade bodies and mediates von Willebrand factor dependent angiogenesis
T. McKinnon 1; R. Starke2; K. Smith2; G. Mobayen2; K. Paschalaki3; A. Randi1; M. Laffan4
1 Imperial College London, London, England, United Kingdom; 2 Imperial College, London, England, United Kingdom; 3 National Heart and Lung Institute, Imperial College London, London, England, United Kingdom; 4 Centre for Haematology, Imperial College London, London, England, United Kingdom
Background: Von Willebrand Factor (VWF) is a multimeric plasma glycoprotein essential for haemostasis, inflammation and angiogenesis. The majority of VWF is synthesised by endothelial cells (EC) and stored in Weibel‐Palade bodies (WPB) with other proteins including angiopoietin‐2 (Angpt‐2), a pro‐inflammatory and pro‐angiogenic mediator, which interacts with the endothelial receptor tyrosine kinase Tie‐2. We have previously shown that VWF regulates angiogenesis, raising the hypothesis that the angiogenic activity of VWF may be mediated by Angpt‐2.
Aims: In the present study we characterise the biochemical parameters defining the interaction between VWF and Angpt‐2 and investigate the role of Angpt‐2 in VWF‐dependent platelet capture and angiogenesis.
Methods: We use static binding assays, VWF‐platelet capture assay and in vitro fibrin bead angiogenesis assay.
Results: We demonstrate that Angpt‐2 binds to VWF with high affinity (KD,app ~3 nM) in a pH and calcium dependent manner. The interaction was predominantly localised to the VWF A1 domain; co‐immunoprecipitation experiments demonstrate that the complex persisted following stimulated secretion from EC and was present in plasma. Angpt‐2 was visible on VWF strings on stimulated EC. The VWF‐Angpt‐2 complex did not inhibit binding of Angpt‐2 to Tie‐2 and did not interfere with VWF platelet capture. However, Angpt‐2 is involved in VWF‐mediated control of angiogenesis, since two inhibitors of Angpt‐2 activity, namely a recombinant Tie2‐Fc chimeric protein and the anti‐Angpt‐2 function blocking antibody (AZ 3.19.3), were able to normalise sprouting angiogenesis in VWF‐deficient HUVEC. The AZ 3.19.3 Ab was able to inhibit the increased angiogenesis of endothelial colony forming cells (ECFC) from a type 3 VWD patient.
Conclusion(s): These data demonstrate a direct binding interaction between Angpt‐2 and VWF that persists after secretion. These results also identify Angpt‐2 as a mediator of VWF‐dependent angiogenesis, suggesting a mechanism through which VWF regulates angiogenesis in patients with VWD.
VWF and von Willebrand Factor Disorders – Clinical Conditions
OC 22.3
Effects of platelet‐inspired haemostatic nanoparticles on bleeding in von Willebrand disease murine models
S. Roullet 1; N. Luc2; J. Rayes3; F. Adam1; A. Ditto4; E. Gahagan4; C. Pawlowski4; M. Bruckman4; P. Lenting5; A. Sen Gupta2; C. Denis6
1 Inserm U1176 – HITh, Le Kremlin‐Bicêtre, Ile‐de‐France, France; 2 Case Western Reserve University, Cleveland, Ohio, United States; 3 University of Birmingham, Birmingham, England, United Kingdom; 4 Haima Therapeutics, Cleveland, Ohio, United States; 5 INSERM U1176, Le Kremlin Bicetre, Ile‐de‐France, France; 6 Inserm, Le Kremlin‐Bicêtre, Ile‐de‐France, France
Background: Platelet‐inspired nanoparticles that emulate platelet's injury site‐specific adhesion and aggregation functions via surface‐decoration with three biofunctional peptides (von Willebrand factor (VWF)/phospholipid‐binding peptides (VBP), collagen‐binding peptides (CBP) and fibrinogen‐mimetic peptides (FMP)), have shown haemostatic efficiency in pre‐clinical models of thrombocytopenia and trauma. Therefore, it was investigated whether such nanoparticles could be beneficial in the treatment of von Willebrand disease (VWD).
Aims: To assess haemostatic efficacy of platelet‐inspired nanoparticles in VWF‐KO and VWD‐type 2B mice following tail‐clip injury.
Methods: VWF‐KO and VWD‐type 2B mice received 2 mg/kg of different nanoparticle formulations: undecorated (control), single, double or triple peptide‐decorated (VBP and/or CBP and/or FMP). Ten minutes post‐injection, 3 mm of the tail tip was amputated and blood was collected over 20 min for VWF‐KO mice and 30 min for VWD‐type 2B mice. Quantitative data (mean ± SD) were compared with ANOVA and Tukey's multiple comparisons test.
Results: In VWF‐KO mice, triple peptide‐decorated particles significantly reduced bleeding compared to control particles (183 ± 174 μl vs. 539 ± 203 μl; p < 0.0001) and not statistically different than wild‐type mice (19 ± 18 μl; p = 0.2043). Here, single or double peptide‐decorated particles did not significantly reduce bleeding. In VWD‐type 2B mice, bleeding was significantly reduced with triple peptide‐decorated particles compared to control particles (631 ± 206 μl vs. 966 ± 199 μl, p = 0.0001) and with two combinations of double peptide‐decorated particles compared to control particles (587 ± 271 μl with CBP + VBP, p = 0.0003; 568 ± 286 μl with CBP+FMP, p < 0.0001). Double peptide‐decorated particles with VBP+FMP or single peptide‐decorated particles did not affect bleeding.
Conclusion(s): Platelet‐inspired biosynthetic nanoparticles significantly reduced bleeding in VWD murine models. In VWD‐type 2B mice, the importance of the CBP‐decoration highlights the pivotal role of collagen in particle anchoring and action. In VWF KO mice, all 3 peptides were required to achieve haemostatic efficiency. These results warrant further investigation of platelet‐inspired nanoparticles as a novel strategy for the haemostatic management of VWD.
OC 43.2
Long‐term reduction in blood transfusion incidence after correction of the shear‐induced von Willebrand factor defect by TAVR‐procedure
A. Rauch 1; F. Vincent2; T. Denimal2; H. Spillemaeker2; T. Pamart2; E. Jeanpierre3; M. Desvages1; M. Daniel1; A. Dupont1; E. Van Belle2; S. Susen4
1 University of Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐EGID, France, Lille, Nord‐Pas‐de‐Calais, France; 2 CHU Lille, Institut Coeur Poumon, Cardiology, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 3 Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 4 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France
Background: Aortic stenosis (AS) has been associated with an increased incidence of gastrointestinal (GI‐) bleeding from angiodysplasia. The acquired Von Willebrand factor (VWF) high‐molecular‐weight (HMW) defect in AS‐patients may account for this association. Whether trans‐catheter aortic valve replacement (TAVR), allowing an immediate correction of the shear‐induced VWF‐defect, translates into a long‐term reduction of blood transfusion is unknown.
Aims: To compare the long‐term incidence of blood transfusion before and after TAVR‐procedure.
Methods: We included 415 patients with severe‐AS scheduled for TAVR in Lille University Hospital from 2010 to 2016 (WITAVI trial). All the transfusion events registered by the regional blood bank (Etablissement Français du Sang), in a period spanning the 5‐years before and after TAVR‐procedure, were first collected. After retrieving from medical records the events associated with bleeding, a Poisson regression model was used to compare blood transfusion incidence before and after TAVR‐procedure, taking death as competing risk into account. VWF‐HMW ratio and PFA‐CADP were also measured before and at the end of TAVR‐procedure.
Results: Mean age at inclusion was 81.9 ± 7 and 200 patients died after TAVR (median follow‐up = 3.4 years). Before TAVR, transfusion incidence progressively increased annually (from 0.03 in year‐5 to 0.14 per‐patient‐year in year‐1, p < 0.0001). At the time of TAVR, pre‐procedural VWF HMW‐ratio and PFA‐CADP levels were significantly lower (both p < 0.0001) in patients with at least one pre‐procedural transfusion. After TAVR allowing a within‐day post‐procedural correction of VWF‐defect, a significant reduction in transfusion incidence was observed (RR = 0.53 [95% CI 0.38–0.72] compared to pre‐procedural period), regardless of vital status at the end of follow‐up (Table 1). After TAVR, the proportion of transfusion related to GI‐bleeding also significantly decreased (p = 0.006) and when the latter were explored endoscopically a ten‐fold reduction in GI‐angiodysplasia was observed (p < 0.0001).
Conclusion(s): TAVR is associated with a reduced incidence of blood transfusion and GI‐bleeding from angiodysplasia. TABLE 1 Reduction of blood transfusion incidence after TAVR regardless of vital status at the end of follow‐up
TABLE 1 Reduction of blood transfusion incidence after TAVR regardless of vital status at the end of follow‐up
OC 43.3
Clinical and phenotypic presentation of patients with low VWF in the Milan Center
O. Seidizadeh 1; A. Ciavarella2; L. Baronciani3; M. Pagliari4; G. Cozzi4; P. Colpani3; S. Siboni5; E. Biguzzi6; F. Peyvandi7
1 1. Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy 2. Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, Milan, Lombardia, Italy; 2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 4 Fondazione IRCCS Ca'Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milan, Lombardia, Italy; 5 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 6 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 7 Fondazione IRCCS Ca' Granda – Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Low von Willebrand factor (VWF) refers to patients with VWF levels of 30–50 IU/dl. Managing patients with low‐VWF levels is a clinical challenge.
Aims: To determine the clinical and phenotypic features of patients with low‐VWF.
Methods: We included 235 well‐characterized patients with low‐VWF levels (VWF antigen and/or VWF activity 30–50 IU/dl with a VWF activity/VWF antigen ratio >0.6). The ISTH bleeding assessment tool was used to assess the severity and frequency of clinical symptoms. The VWF propeptide (VWFpp) assay was performed to determine VWF clearance, with results compared to 120 healthy controls. The Mann‐Whitney U test was used to compare medians between two independent groups. The abnormal bleeding score (BS) was defined as ≥4 in adult males, ≥6 in adult females, and ≥3 in children.
Results: The median (range) of VWF antigen and activity was 52 IU/dl (30–72) and 40 IU/dl (30–55) respectively, with a VWF activity/VWF antigen ratio of 0.77. The median BS was 4 (n = 160, range 0–17) and 33% of patients had bleeding that required treatment. Abnormal bleeding was seen in 33% of children, 43% of females and 51% of males. Epistaxis, bruising, surgery, menorrhagia and bleeding from minor wounds were the most common clinical manifestations (Figure 1). Nevertheless, severe bleeding symptoms as GI bleeding and haemarthrosis were also observed. Patients VWFpp/VWF antigen ratio was 1.4 (n = 130, range 0.5–2.4), significantly higher than that of controls (median 0.98, range 0.6–1.6, p < 0.002). An increased clearance was observed in 35% of cases (n = 46) evaluated for VWFpp. No difference was found for the BS between cases with and without enhanced clearance.
Conclusion(s): Low‐VWF levels can be associated with significant bleeding and >42% of patients had an abnormal BS. Therefore, these patients should be considered to have a mild bleeding disorder rather than a risk factor for bleeding. We further demonstrated the important role of increased VWF clearance as a pathogenic mechanism. FIGURE 1 The reported frequency of bleeding manifestations with an International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH‐BAT) subscore ≥1 in patients with low von Willebrand factor. CNS, Central nervous system.
FIGURE 1 The reported frequency of bleeding manifestations with an International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH‐BAT) subscore ≥1 in patients with low von Willebrand factor. CNS, Central nervous system.
Platelets and Megakaryocytes
Megakaryocytes and Thrombopoiesis
OC 10.3
A novel model of RUNX1‐haploinsufficient (RUNX1Lo): Human megakaryocytes infused into mice further characterizes this defect and identifies potential therapeutics
K. Lee; H. Ahn; M. Poncz
Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States
Background: RUNX1 is a key transcriptional regulator in hematopoiesis and megakaryopoiesis. Heterozygous defects in RUNX1 underlie familial platelet disorder with associated myeloid malignancy (FPDMM). FPDMM has symptoms of mild‐to‐moderate thrombocytopenia and platelet defects. Also, RUNX1Lo increases the risk of myelodysplasia and acute myeloblastic leukemia.
Aims: Since RUNX1Lo animal models do not mimic FPDMM disease, rarely developing bleeding disorder or leukemia, we tried to establish a model system to understand better the underlying mechanisms of the observed phenotypes and to identify therapeutic interventions.
Methods: We have developed an ex‐vivo megakaryopoiesis system beginning with healthy human CD34+‐hematopoietic stem and progenitor cells and recapitulating FPDMM by knockdown of RUNX1 expression using lentiviral short‐hairpin RNA interference. We then xenotransfused these human CD34+‐derived megakaryocytes into immunocompromised mice for in‐vivo platelet studies.
Results: In‐vitro RUNX1Lo‐megakaryocyte yield was reduced, and the megakaryocytes showed a marked reduction in agonist response. In‐vivo murine studies of the released platelets from infused RUNX1Lo‐megakaryocytes were then characterized and therapeutic intervention tested. Infused RUNX1Lo‐megakaryocytes into immunocompromised NSG mice released fewer platelets/megakaryocytes (Figure 1). RUNX1Lo‐platelets poorly responded to agonists. Unlike studies of infused control megakaryocytes into NSG mice – which are also homozygous for an R1326H mutation in von Willebrand factor, switching species‐specific binding from mouse to human platelets – infused RUNX1Lo‐megakaryocytes are defective in thrombus formation in a Rose‐Bengal‐photochemical carotid injury model (Figure 2). The small‐molecule inhibitor RepSox that blocks the transforming‐growth factor‐beta pathway rescued RUNX1Lo‐megakaryopoiesis in vitro, platelets/RUNX1Lo‐megakaryocyte yield in vivo (Figure 1), and thrombus formation in the photochemical injury model (Figure 2).
Conclusion(s): This model recapitulates the defect in FPDMM megakaryocytes and platelets, identifies a previously unrecognized defect in thrombopoiesis, and demonstrates reversal of these defects in vivo by a drug. We believe that such studies of human RUNX1Lo‐megakaryocytes may be a useful approach for the preclinical assessment of potential therapeutics.
OC 09.4
Spatial transcriptomics reveals megakaryocyte heterogeneity in mouse bone marrow
J. Tilburg 1; A. Stone1; J. Billingsley2; D. Scoville3; J. Italiano1; K. Machlus4
1 Harvard Medical School, Boston, Massachusetts, United States; 2 Harvard, Boston, Massachusetts, United States; 3 Nanostring, Boston, Massachusetts, United States; 4 Vascular Biology Program, Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts, United States
Background: Megakaryocytes (MKs) are bone marrow resident cells with the pivotal role of platelet production. Recent endeavors using single‐cell RNA sequencing on murine MKs revealed that functions of megakaryocytes go beyond platelet production with evidence for immunoregulatory and stem cell niche supporting subpopulations. However, the relationship between the spatial organization of MKs and transcriptional heterogeneity is unclear and thus remains to be elucidated whether localization of MKs near sinusoids or at the proximal versus the distal side of bones might influence cell function.
Aims: The aim of this study was to systematically map the molecular, cellular, and spatial composition of MKs in the murine femur by combining transcriptomics with in situ spatial orientation.
Methods: Serial sections were obtained from a 3‐month‐old C57BL/6J mouse femur. Using the Nanostring GeoMx digital spatial profiling platform and the mouse whole transcriptome array we profiled 44 individual MKs divided over different regions throughout the bone marrow.
Results: Principle component analysis revealed clustering based on the proximal versus distal axes of the bone (p = 0.000175), while an association with adjacency to the vasculature was not observed (p = 0.1667). Pearson correlation on the distance to the proximal side of the bone showed 5 genes of interest (Fold Chance of >2 and p‐value of <0.05) with two and three genes overexpressed in the proximal and distal side, respectively (Figure 1). Of note, these genes included pro‐platelet basic protein (Pbpp) and platelet factor 4 (Pf4) with a higher expression in proximal MKs.
Conclusion(s): Transcriptional heterogeneity was observed in compartments previously thought to be homogeneous, with evidence for Pbpp and Pf4 being expressed more prominently in the proximal side of the femur. Moreover, we present a novel methodology to measure the whole transcriptome of individual MKs in situ and reveal that spatial organization should be considered while assing the MK transcriptome.
OC 26.5
Thyroid hormones and analogues promote the acute release of platelets from megakaryocytes: from blood donor biology to the production of platelets in vitro
H. Foster 1; N. Herbert2; A. Waller1; C. Di Buduo3; J. Fang4; A. Schmidt5; D. Howard1; M. Taimoor1; T. Moreau6; A. Meuller1; A. Evans1; R. Biswas7; E. Turro8; R. Fischer9; D. Wilcox10; K. Hoffmeister11; A. Balduini12; C. Ghevaert13
1 Wellcome‐MRC Cambridge Stem Cell Institute, University of Cambridge, Cambridge, England, United Kingdom; 2 NHS Blood and Transplant/University of Cambridge, Cambridge, England, United Kingdom; 3 Department of Molecular Medicine, University of Pavia, Pavia, Italy, Pavia, Lombardia, Italy; 4 Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 5 Versiti Blood Research Insitiute/Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 6 bit.bio, Cambridge, England, United Kingdom, 7 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 8 Icahn School of Medicine at Mount Sinai, New York, New York, United States; 9 Nuffield Department of Medicine/Univeristy of Oxford, Oxford, England, United Kingdom; 10 Medical College of Wisconsin, MIlwaukee, Wisconsin, United States; 11 Versiti Translational Glycomics Center, Milwaukee, Wisconsin, United States; 12 University of Pavia, Pavia, Italy, Pavía, Lombardia, Italy; 13 University of Cambridge, Cambridge, England, United Kingdom
Background: Many improvements have been made over the past decade in the safety and quality of platelet units intended for transfusion but significant problems still remain. Often there is no alternative for severely ill patients but to be given allogenic platelets which have very limited efficacy and uncertain immunomodulatory responses and so there is a critical need for alternative treatments. Unfortunately, our current knowledge of thrombopoiesis is limited and the mediators that are directly involved remain elusive.
Aims: To elucidate soluble factors in the blood that promote platelet formation and can be used as therapeutic treatments or to upscale in vitro platelet production for transfucion purposes.
Methods: 19 plateletpheresis donors who regularly donate we recruited to analysis the dynamics of platelet recovery post acute loss of platelets. Full blood counts were performed on various timepoints before and after donation to identify the most relevant timepoints to analyse further. Metabolomic, proteomic and cytokine/chemokine/growth factor analyses were performed on the plasma/serum of these donors to identify differentially expressed analytes in these relevant timepoints compared to baseline levels. These analystes were screened in platelet production assays to identify novel targets.
Results: Using metabolomics and proteomics screening methods, thyroid hormones have been identified as potent mediators of platelet production. Triiodothyronine (T3) as well as thyroid hormone analogues, GC‐1 (Sobetirome), KB2115 (Eprotirome) and MGL‐3196 showed a significant effect on platelet production, as well as proplatelet formation in vitro in both primary derived‐ and iPSC derived‐megakaryocytes. These platelets that are produced under the influence of thyroid hormones are functionally active, showing degranulation (P‐selectin exposure) and incorporation into thrombi.
Conclusion(s): We have identified for the first time that thyroid hormones directly promote platelet production which offers very interesting therapeutic opportunities.
OC 59.4
Novel variants in GALE caused syndromic macrothrombocytopenia disrupting thrombopoiesis and glycosylation
A. Marin‐Quilez 1; C. Di Buduo2; L. Díaz‐Ajenjo1; P. Soprano3; E. Vuelta1; I. Serramito‐Gómez1; S. Santos‐Mínguez1; C. Miguel‐García1; S. Tocino‐Antonio4; A. Zamora‐Canovas5; P. Ruiz‐Sala6; M. Peñarrubia7; E. Pardal8; J. Hernández‐Rivas9; J. González‐Porras10; R. Benito1; J. Rivera11; I. García‐Tuñón1; A. Balduini12; J. Bastida13
1 IBSAL, CIC, IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain, Salamanca, Castilla y Leon, Spain; 2 Department of Molecular Medicine, University of Pavia, Pavia, Italy, Pavia, Lombardia, Italy; 3 Department of Molecular Medicine, University of Pavia, Pavia, Italy, Pavía, Lombardia, Italy; 4 IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain, Salamanca, Castilla y Leon, Spain; 5 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain, Murcia, Murcia, Spain; 6 Centro de Diagnóstico de Enfermedades Moleculares, Universidad Autónoma de Madrid, CIBERER, IdIPAZ, Madrid, Spain, Madrid, Madrid, Spain; 7 Department of Hematology, Hospital Clínico Universitario de Valladolid, Spain, Valladolid, Castilla y Leon, Spain; 8 Department of Hematology, Hospital Virgen del Puerto, Plasencia, Spain, Plasencia, Extremadura, Spain; 9 IBSAL, CIC, IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain; Department of Hematology, Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain, Salamanca, Castilla y Leon, Spain; 10 Department of Hematology, Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain, Salamanca, Castilla y Leon, Spain; 11 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Murcia, Murcia, Spain; 12 University of Pavia, Pavia, Italy, Pavía, Lombardia, Italy; 13 Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Salamanca, Castilla y Leon, Spain
Background: Glycosylation is recognized as a key process for proper megakaryopoiesis and platelet formation. The enzyme UDP‐galactose‐4‐epimerase, encoded by GALE, is involved in protein glycosylation. There is currently scarce information about the performance of megakaryopoiesis in patients with thrombocytopenia associated with GALE variants.
Aims: Assessing the clinical and platelet phenotype in three patients with syndromic macrothrombocytopenia associated to GALE variants, and to investigate the role of GALE in glycosylation and thrombopoiesis.
Methods: Three patients from two unrelated families with lifelong severe thrombocytopenia, bleeding diathesis, mental retardation, mitral valve prolapse, and jaundice were enrolled in the Spanish Project of Inherited Platelet Disorders. Biallelic variants in GALE were identified by whole‐exome sequencing (WES) (Figure 1). Platelet phenotyping included full blood cells count, blood film, light transmission aggregometry, flow cytometry analysis of major surface platetet glycoproteins, and agonist‐induced granule secretion. UDP‐galactose‐4‐epimerase enzymatic activity was measured by HPLC/MS/MS. GALE and N‐acetyl‐galactosamine (LacNac) levels were evaluated by immunoblotting. Human megakaryocyte (Mk) culture from peripheral blood samples was assessed in one proband.
Results: WES revealed four variants affecting GALE, three of them previously unreported (Figure 1). Patients showed giant and grey platelets, impaired platelet aggregation with several agonists and severely reduced alpha (P‐selectin) and dense granule (CD63) secretion. UDP‐galactose‐4‐epimerase enzymatic activity was severely decreased in these patients. Immunoblotting showed reduced GALE in platelets and significant reduction of LacNAc, suggesting a hypoglycosylation pattern. In proband B.II.2, CD34+ derived Mks exhibited normal ploidy, and maturation, but impaired proplatelet formation. These Mks showed, similarly to patient platelets, normal surface levels of β3 integrin, but remarkable reduction in the externalization of GPIbα.
Conclusion(s): This study expands our knowledge on the GALE related thrombocytopenic disorder, emphasizing its critical role in platelet glycosylation, and providing further evidence that thrombocytopenia derives from altered proplatelet formation developing from GPIbα impairment. Funding: PI20/00926, GRS2135/A/2020, GRS2314/A/2021, FMM AP172142019. FIGURE 1 Family pedigrees and clinical characteristics of the three patients carrying GALE biallelic variants. y/o, years old; P, platelet count; MPV, mean platelet volume; BS, bleeding score (ISTH‐BAT).
FIGURE 1 Family pedigrees and clinical characteristics of the three patients carrying GALE biallelic variants. y/o, years old; P, platelet count; MPV, mean platelet volume; BS, bleeding score (ISTH‐BAT).
OC 75.2
GP1b‐FilaminA interaction regulates megakaryocyte localization and budding during platelet biogenesis
M. Ellis 1; A. Terreaux2; R. Smythe3; A. Eckly4; S. Cranmer5; J. Maclean3; I. Alwis3; J. Perdomo6; F. Passam1; S. Taoudi2; F. Lanza7; Y. Yuan8; J. Shaun1
1 Heart Research Institute, University of Sydney, Sydney, New South Wales, Australia; 2 WEHI, Melbourne, Victoria, Australia; 3 Heart Research Institute, The University of Sydney, Sydney, New South Wales, Australia; 4 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strassbourg, Alsace, France; 5 Monash University, Melbourne, New South Wales, Australia; 6 University of NSW, Kogarah, New South Wales, Australia; 7 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 8 Heart Research Institute, University of Sydney, Melbourne, Victoria, Australia
Background: Glycoprotein Ib(alpha) is a critical receptor on platelets and megakaryocytes and is anchored to the membrane skeleton by Filamin A. While GPIb and FlnA have well defined roles in platelet biogenesis, the critical nature of the interaction of these proteins in megakaryocyte biology has not been evaluated in vivo. We sought to determine the influence of disruption of the GPIba/FlnA interaction on in vivo platelet biogenesis, particularly focusing on the recently described phenomenon of megakaryocyte membrane budding.
Aims: Investigate the role of the GPIba/FlnA interaction and functional consequence of disruption of this interaction in a transgenic human GPIba mouse model.
Methods: We generated mice with either a wild‐type (WT) or FlnA‐binding mutant (FW) human GPIba transgene within a GPIba‐null mouse line. Platelet counts, platelet clearance, GPIba and GPIbb expression and proplatelet formation were assessed. We examined ultrastructure of megakaryocytes using BM TEM. Megakaryocyte distribution and budding were assessed using confocal and STED microscopy from bone marrow cryosections.
Results: Mice expressing mutant FW GPIba transgene exhibited a macrothrombocytopenia with platelet counts ranging from 150 to 200 × 106/ml with increased platelet volume. GPIb surface expression was preserved, albeit at lower levels than transgenic controls. Platelet clearance was normal and differentiation of FW megakaryocytes to proplatelets in vitro was preserved. Pulmonary proplatelets and bone marrow megakaryocytes numbers were normal in FW mice. The most striking defect in FW mice was the presence of dysregulated megakaryocyte budding, resulting in enlarged megakaryocyte buds that were ectopically released into the bone marrow interstitium. Expression of the cytoplasmic tail of GPIba in megakaryocytes was sufficient to restore normal megakaryocyte localization and budding and correct the macrothrombocytopenia.
Conclusion(s): These studies define a new mechanism of macrothrombocytopenia resulting from dysregulated megakaryocyte budding. The GPIba/FlnA interaction is dispensable for megakaryocyte budding, however it plays a major role in regulating megakaryocyte localization and budding morphogenesis.
OC 75.3
Platelet generation from circulating megakaryocytes is triggered in the lung vasculature
X. Zhao1; D. Alibhai1; T. Walsh1; N. Tarassova1; S. Birol1; C. Williams1; C. Neal1; E. Aitken1; A. Waller2; J. Ballester‐Beltran3; P. Gunning4; E. Hardeman5; E. Agbani6; I. Hers1; C. Ghevaert3; A. Poole 1
1 University of Bristol, Bristol, England, United Kingdom; 2 University of Cambridge, Braintree, England, United Kingdom; 3 University of Cambridge, Cambridge, England, United Kingdom; 4 University of New South Wales, Syndey, New South Wales, Australia; 5 University of New South Wales, Sydney, New South Wales, Australia; 6 University of Calgary, CALGARY, Alberta, Canada
Background: Platelets are derived from megakaryocytes (MKs), although the generation process is unclear. Only small numbers of platelets have been produced in systems outside the body, where bone marrow and lung are proposed as sites of platelet generation. The release of platelets requires cytoskeletal re‐organisation involving actin. Tropomyosin TPM4 has a role in platelet formation.
Aims: To investigate the mechanisms of platelet generation from MKs in the lung using a novel ex vivo mouse model.
Methods: We established an ex vivo mouse heart‐lung model (Fig. 1A) for perfusion of murine MKs. MKs and their derivates were imaged by confocal microscopy. The numbers of generated platelets (GPs) in the perfusate or retained in the lung were counted by FACS and two‐photon microscopy, respectively. Morphological and functional features of GPs were determined by FACS, transmission electron microscopy and in vitro thrombosis assays. A microfluidic chamber mimicking the lung vasculature system was designed to explore the mechanism of platelet generation in the lung (Fig. 1B). MKs from TPM4‐/‐ mice were introduced to study whether TPM4 is required for platelet generation in the lung.
Results: MKs can pass multiple times through the lung vasculature, leading to the generation of physiological levels of functional platelets (approximately 1,000–4,000 per MK), through a process involving nuclear marginalization and enucleation, prior to TPM4‐dependent fragmentation. GPs demonstrated morphological and functional features comparable to control platelets. Air ventilation and healthy pulmonary endothelial cells play critical roles in platelet generation.
Conclusion(s): The ex vivo mouse heart‐lung model allows the detailed study of platelet generation, and has enabled us to show that MKs can repeatedly passage through the lung vasculature under air ventilation, leading to enucleation and TPM4‐dependent fragmentation to generate platelets. The findings add to our understanding of why the lung is a primary site for platelet generation. FIGURE 1 Mouse platelets are generated in physiological quantities from megakaryocytes passaged multiple times through mouse pulmonary vasculature ex vivo.
FIGURE 1 Mouse platelets are generated in physiological quantities from megakaryocytes passaged multiple times through mouse pulmonary vasculature ex vivo.
OC 59.5
Glucocorticoids stimulate thrombopoiesis in murine megakaryocytes
M. Grodzielski; J. Cidlowski
National Institute of Environmental Health Sciences, NIH, Research Triangle Park, North Carolina, United States
Background: Cushing's syndrome is characterized by elevated levels of endogenous glucocorticoids. Patients commonly present high platelet counts and increased thromboembolism, but the underlying mechanism remains unclear. Healthy men treated with dexamethasone evidenced a significant rise in the number of platelets. Platelet levels follow a circadian pattern like that of circulating glucocorticoids.
Aims: Determine a direct effect of glucocorticoids on platelet production by megakaryocytes.
Methods: Megakaryocytes were differentiated from C57BL/6 bone marrow and fetal livers. Expression and functionality of glucocorticoid receptors were addressed by immunofluorescence, western blot, and RT‐qPCR. Megakaryocyte morphology, spreading and proplatelet formation, as well as in vitro platelet production, were analyzed by immunofluorescence, microscopic assays, and flow cytometry. In vivo effect was assessed by injecting dexamethasone to wild type C57BL/6 mice. Collected blood was examined using a hematology analyzer and a flow cytometer. Tail bleeding time was performed to explore any hemostatic outcome.
Results: Glucocorticoid receptor mRNA and protein were present in megakaryocytes. Glucocorticoid treatment induced nuclear translocation of the receptor and expression of Gilz and Fkbp5, two well‐known glucocorticoid‐responsive genes. A whole‐genome expression microarray revealed 1325 genes differentially transcribed by dexamethasone (70% activated and 30% repressed). These genes were related to numerous cellular functions, particularly to the activation of cytoplasm and cytoskeleton organization and microtubule dynamics. Megakaryocytes incubated with dexamethasone on fibronectin displayed increased spreading and shape change associated, with augmented podosome formation and microtubule polymerization. Glucocorticoids increased the percentage of proplatelet‐forming megakaryocytes and of platelet‐like particles. Single dose of dexamethasone resulted in higher mature and young platelet counts and induced a more rapid hemostatic response.
Conclusion(s): We demonstrated that megakaryocytes express functional glucocorticoid receptors conferring glucocorticoids the ability to modulate megakaryocyte transcriptome and thus its functions. Furthermore, we present evidence that glucocorticoids stimulate thrombopoiesis in vitro and in vivo, providing a novel mechanism of action of glucocorticoids in health and disease.
OC 59.1
Thrombopoiesis has a unique lipidomic profile enriched in polyunsaturated fatty acids that facilitates megakaryocyte maturation and platelet production
M. Barrachina 1; G. Pernes2; I. Becker3; D. Freire4; D. Groeneveld5; J. Luyendyk6; P. Meikle2; J. Italiano7; G. Lancaster2; A. Murphy2; K. Machlus8
1 Harvard Medical School, Boston Children's Hospital, Boston, Massachusetts, United States; 2 Baker Heart and Diabetes Institute, Melbourne, Victoria, Australia; 3 Boston Children's Hospital, Boston, Massachusetts, United States; 4 Boston Children's Hospital; Harvard Medical School, Boston, Massachusetts, United States; 5 Michigan State University, East Lansing, Michigan, United States; 6 Department of Pathobiology and Diagnostic Investigation, Michigan State University, East Lansing, Michigan, United States; 7 Harvard Medical School, Boston, Massachusetts, United States; 8 Vascular Biology Program, Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts, United States
Background: Lipids represent an extremely vast class of biomolecules, with essential roles in energy homeostasis, membrane structure, and signaling. Recent studies have uncovered that, lipids are key drivers of cell fate decisions during hematopoiesis. However, the contribution of lipids to megakaryopoiesis is unknown.
Aims: Define the trajectory and role of key lipids essential for megakaryocyte differentiation and platelet production.
Methods: We used targeted mass spectrometry to obtain a lipidomic profile of murine bone marrow hematopoietic stem and progenitor cells (HSPC), primary megakaryocyte progenitors, mature megakaryocytes, and platelets. Then, we targeted the incorporation and biosynthesis of fatty acids in vitro. Finally, we administered a high‐fat‐diet to mice to investigate how changes in dietary fatty acids affect megakaryocyte differentiation and platelet formation.
Results: Analysis of HSPC lipidomes revealed that cell populations clustered into distinct populations, with enrichment in polyunsaturated fatty acids (PUFAs) distinguishing mature from immature megakaryocytes. In primary murine HSPCs, inhibition of acyl‐coA synthetase, an enzyme essential for fatty acid metabolism, profoundly decreased megakaryocyte differentiation up to 70%. Similarly, MK maturation was significantly attenuated up to 80% after inhibition of de novo lipogenesis. To examine fatty acid incorporation, we supplemented HSPCs with saturated (palmitic, 16:0) and unsaturated (linoleic, 18:2) fatty acids, which significantly increased megakaryocyte area. Extending this in vivo, mice fed a high‐fat‐diet with increased palmitic acid and reduced PUFAs had significantly larger megakaryocytes, but reduced platelet counts compared with mice fed a standard chow diet.
Conclusion(s): Our data reveal that fatty acid metabolism and synthesis are critical for megakaryocyte differentiation. Increasing dietary saturated fatty acids and reducing PUFAs significantly altered both megakaryocyte maturation and platelet production, suggesting that thrombopoiesis can be modified in vivo by diet alone. Further explorations will clarify whether lipid uptake and biosynthesis pathways can be targeted therapeutically to modulate platelet levels.
OC 59.2
Ribosomal biogenesis inhibition facilitates megakaryocyte/platelet biased haematopoiesis
V. Bhoopalan 1; A. Kaur2; N. Hein2; K. Maclachlan3; R. Ferreira2; S. Man2; R. Hannan2; K. Hannan2; E. Gardiner4
1 John curtin school of medical research, ANU, Canberra city, Australian Capital Territory, Australia; 2 John Curtin School of Medical Research, ANU, Canberra, Australian Capital Territory, Australia; 3 Memorial Sloan Kettering Cancer Center, New York City, New York, United States; 4 John Curtin School of Medical Research, The Australian National University, Canberra, Australian Capital Territory, Australia
Background: Recently reported Megakaryocyte (MK)‐biased haematopoietic pathways can be detected in murine models of inflammation. Thrombopoietin (TPO) regulates normal thrombopoiesis by enhancing MK maturation. What triggers and controls the MK‐biased pathway remains vague. Ribosomal biogenesis inhibitor CX‐5461 is a candidate anti‐cancer therapeutic that has completed phase I/II clinical trials and is well‐tolerated. Treatment of mice and humans with CX‐5461 results in an increase in circulating platelets.
Aims: To explore mechanisms underpinning CX‐5461‐mediated thrombopoiesis.
Methods: C57BL/6 wildtype or TPO‐receptor‐deficient mice received three doses/week 35 mg/kg CX‐5461 or vehicle. Blood was collected for up to 42 days and enumerated by an automated blood analyser. Flow cytometry was used to assess platelet lifespan, receptor levels and function. Bone marrow was isolated, and MK number/ploidy and haematopoietic cell compartment were quantified. Plasma cytokine and TPO levels were assessed by ELISA, and liver TPO mRNA by RT‐qPCR.
Results: CX‐5461‐treated mice showed rapid, reversible ~1.7‐fold increases in platelets at day 7, while all other blood cells remained steady (lymphocytes, neutrophils) or decreased (red cells). Glycoprotein (GP)Ibα, αIIb and GPVI showed normal levels and function. A 2.3‐fold increase in reticulated platelets but normal platelet lifespan was measured. Inflammatory cytokines (IL‐6, TNFα, IL‐1β) were within normal ranges. A >2‐fold increase in MK numbers and hyperploidy was detected together with increased Sca1+MK (**p < 0.01), MK progenitors (**p < 0.01) and HSC (****p > 0.0005). CX‐5461‐induced platelet production was independent of TPO, as plasma TPO and liver TPO mRNA levels did not change with treatment, and CX‐5461 treatment of TPO receptor‐/‐ mice resulted in increased platelets (5‐fold, ***p < 0.0005, day 28).
Conclusion(s): CX‐5461 treatment triggered a TPO‐ and inflammation‐independent, platelet/MK‐biased haematopoietic pathway. This is a new and potentially advantageous feature of an anti‐cancer therapeutic which may ameliorate often observed chemotherapy‐induced thrombocytopenia. The application of ribosomal biogenesis inhibitors can enhance our understanding of the mechanisms governing thrombopoiesis.
OC 35.1
Light‐induced Ca2+ signaling triggers megakaryocyte polarization
Y. Zhang 1; S. Gao2; J. Yu‐Strzelczyk2; H. Kurz3; G. Nagel4; M. Bender5
1 University Hospital Wuerzburg and Rudolf Virchow Center, University of Würzburg, wuerzburg, Bayern, Germany; 2 Department of Neurophysiology, Institute of Physiology, Biocenter, University of Würzburg, Wuerzburg, Bayern, Germany; 3 Institute of Experimental Biomedicine – Chair I, University Hospital Wuerzburg and Rudolf Virchow Center, University of Würzburg, Wuerzburg, Bayern, Germany; 4 Department of Neurophysiology, Institute of Physiology, Biocenter, University of Würzburg, Wuerzbrug, Bayern, Germany; 5 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: Bone marrow megakaryocytes (MKs) extend proplatelets into sinusoidal blood vessels where these proplatelets undergo fission to release platelets. The molecular mechanisms that regulate megakaryocyte differentiation, polarization and proplatelet formation are only poorly understood. We expressed an optogenetic construct in primary MKs, which allows us to study these complex processes by spatiotemporally controlling cellular activity using light. A widely used optogenetic construct is Channelrhodopsin2 (ChR2), which is a blue light‐activated cation channel from the green alga Chlamydomonas reinhardtii. We modified ChR2 by molecular engineering to obtain higher Ca2+ conductance (ChR2‐XXM2.0), which allows us to investigate the role of Ca2+ in MK function.
Aims: We expressed ChR2‐XXM2.0 in MKs to manipulate Ca2+ signaling by light in a high spatiotemporal manner to better understand the influence of intracellular Ca2+ changes on MK function.
Methods: ChR2‐XXM2.0 was expressed in bone marrow‐derived MKs after viral transduction. Whole cell patch‐clamp was used to test the functionality of the channel. MKs were globally or locally illuminated and subsequent MK behavior was analyzed with confocal microscopy.
Results: ChR2‐XXM2.0 localized in the plasma membrane and the demarcation membrane system (DMS) in MKs. Blue light induced a significant photocurrent in ChR2‐XXM2.0 expressing MKs, which indicates cation influx. Global illumination of ChR2‐XXM2.0 expressing MKs spread on fibrinogen resulted in intracellular Ca2+ increase, stress fiber formation and more pronounced MK spreading. Local illumination triggered MK polarization and motility towards the direction of light. Using inhibitors and genetically‐modified mouse models, we show that MK polarization was dependent on Ca2+ influx, Cdc42 expression and myosin IIA activity. Impaired light‐induced polarization of Cdc42‐deficient MKs was rescued upon co‐expression of the Cdc42/ChR2‐XXM2.0.
Conclusion(s): We established optogenetics in bone marrow‐derived MKs. Light‐induced local Ca2+ influx triggers Cdc42‐ and myosin IIA‐dependent MK polarization. These findings also demonstrate that optogenetics allows for the precise manipulation of MK biology.
OC 26.2
A dose‐escalation phase 1 clinical trial of autologous iPSC‐derived platelets (iPLAT1)
N. Sugimoto 1; J. kanda2; S. Nakamura3; T. Kitano2; S. Shimizu1; A. Shigemasa1; H. Hirai2; M. Minami2; H. Tada2; D. Momose4; K. Koh4; M. Nogawa5; N. Watanabe6; M. Handa6; A. Sawaguchi7; M. Tanaka8; T. Hayashi8; Y. Tani8; A. Takaori‐Kondo9; K. Eto3
1 Center for iPS Cell Research and Application, Kyoto University, Kyoto, Kyoto, Japan; 2 Kyoto University Hospital, Kyoto, Kyoto, Japan; 3 Kyoto University, Kyoto, Kyoto, Japan; 4 Osaka General Hospital of West Japan Railway Company, Osaka, Osaka, Japan; 5 Japanese Red Cross Society, Tokyo, Tokyo, Japan; 6 Keio University School of Medicine, Tokyo, Tokyo, Japan; 7 University of Miyazaki, Miyazaki, Miyazaki, Japan; 8 Japanese Red Cross Society, Osaka, Osaka, Japan; 9 Department of Hematology, Kyoto University Hospital, Kyoto, Kyoto, Japan
Background: Platelet products are provided from blood banks which collect blood from healthy donors. However, approximately 5% of platelet transfusion patients are complicated with alloimmune platelet transfusion refractoriness (allo‐PTR) due to alloantibodies against class I human leukocyte antigens (HLA‐I) or human platelet antigens (HPA). In these cases, platelets from compatible donors are transfused, but for patients with rare HLA‐I or HPA, donors are difficult to find. Aiming to ultimately solve this issue, we had developed ex vivo production system of iPSC‐derived platelet products (iPSC‐PLTs).
Aims: We aimed to assess the safety and efficacy of autologous iPSC‐PLTs in an aplastic anemia patient with allo‐PTR due to rare HPA‐1 mismatch in Japan.
Methods: The iPSC‐PLTs were produced from megakaryocyte cell lines (imMKCLs) established from patient iPSCs using turbulent flow bioreactors and new drugs. In preclinical studies, iPSC‐PLTs were confirmed for quality, safety, and efficacy, including hemostasis in animal models. We then performed the first‐in‐human clinical trial of iPSC‐PLTs as a phase I study of autologous transfusion. Three escalating doses of 0.1 × 1011, 0.3 × 1011 and 1 × 1011 iPSC‐PLTs were sequentially administered.
Results: The primary endpoint was safety, and no significant adverse event was observed over one year. The secondary endpoint of corrected count increment showed no significant change in all dose cohorts. Circulation of larger size possibly iPSC‐PLTs was observed, but there was also a slight increase in d‐dimer.
Conclusion(s): This first‐in‐human clinical trial provides extensive resource of successful GMP‐based production of iPSC‐PLTs with no significant adverse event profile, as well as issues that needs to be overcome, thereby marking a first benchmark in realizing iPSC‐PLTs as a clinical measure. (Funded by the Japan Agency for Medical Research and Development; this trial was registered at Japan Registry of Clinical Trials as jRCTa050190117).
OC 75.1
A 3D microvasculature assay reveals novel sub‐cellular dynamics of megakaryocyte endothelial barrier interactions, proplatelet formation and release
N. Asquith 1; D. Freire1; S. Shelton2; K. Machlus3; R. Kamm2; J. Italiano4
1 Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts, United States; 2 Massachusetts Institute of Technology, Cambridge, Massachusetts, United States; 3 Vascular Biology Program, Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts, United States; 4 Harvard Medical School, Boston, Massachusetts, United States
Background: Megakaryocytes (MKs) generate platelets through podosome and proplatelet intermediates. The mechanisms by which MKs interact with and extravasate through the endothelial vessel wall, or how subpopulations of MKs multi‐task to also generate niche supporting cytokines or extracellular vesicles (EVs) remain unclear.
Aims: To investigate MK‐endothelial interactions, we have developed a 3D microvascular system, with which we aim to (1) identify how MKs penetrate the vascular space, (2) determine what cytoskeletal mechanisms power the transition from podosome to proplatelet formation, and (3) define how individual platelets and EVs are released.
Methods: Human umbilical vascular endothelial cells, human lung fibroblasts, and mouse hematopoietic stem and progenitor cells (HSPCs) were seeded into a fibrin hydrogel. The co‐cultures self‐assembled into open lumens and were labelled for live‐cell super resolution microscopy of proplatelet formation and MK‐endothelial interactions. Mature 3D networks were fixed and cross sectioned for scanning electron microscopy.
Results: We successfully created a 3D self‐forming co‐culture that supports HSPC differentiation into MKs. MK‐podosome structures were observed extending at the endothelial barrier by electron microscopy. MKs have been observed extending proplatelets within the vessel lumens and releasing platelet like particles (Figure 1). In our alternate microfluidic system, we observed that individual mouse megakaryocytes can simultaneously form proplatelets and release vesicles (Figure 2).
Conclusion(s): Conclusion: Our results suggest that our co‐culture systems are capable of recapitulating thrombopoiesis and the study of subcellular MK‐endothelial cell interactions. Simultaneous formation of MK‐EVs and platelets suggests the mechanism and the timing of the release of these structures could be linked. We are going to continue to increase the complexity of our model with the addition of flow, retroviral expression systems for cytoskeletal markers and by including CRISPR‐Cas9 edited MKs.
OC 75.5
Platelet release device: From bench‐scale to large culture platelet production
A. Pongerard 1; L. Mallo1; V. Do Sacramento1; O. Boiron2; F. Lanza1; C. Gachet1; Y. Knapp3; C. Strassel1
1 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 2 CNRS, Université Aix‐Marseille, Ecole Centrale Marseille, IRPHE UMR7342, F‐13000 Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Université Avignon, LAPEC UPR4278, F‐84000 Avignon, France, Avignon, Provence‐Alpes‐Cote d'Azur, France
Background: Platelet production is now feasible on a bench, but the remaining challenge is to move towards industrial production to enable clinical use. One way to achieve this goal is through the development of platelet release devices capable of producing bonafide platelets, at a high rate and in compliance with Good Manufacturing Practice.
Aims: Our study focused on the development of a platelet release device compatible with industrial requirements, allowing the production of important amounts of high quality cultured platelets.
Methods: From an analysis of flow generated during an established method of platelet release based on successive standard pipette up and down cycles, we designed a large‐scale platelet release device compatible with industrial constraints, examined its main parameters and studied the quality of the released platelets.
Results: The device is composed of a succession of 5 spheres, each containing two calibrated cones placed in a staggered pattern matching the dimensions used during manual pipetting. Computational flow dynamics analysis showed the generation of periodic unsteady flows. Placed in negative pressure, this device allows the continuous flow of large volumes of culture (100 ml/min) and elevated platelet yields (100 platelets per megakaryocyte). Analysis of platelet morphology by electron microscopy showed that these platelets exhibit similar features than native ones despite their larger size (5.60 μm ± 0.52 diameter culture platelets vs. 2.46 μm ± 0.55 diameter native platelets). They are discoid and express the key glycoproteins to ensure platelet functions. Functionally, cultured platelets are able to activate their GPIIb‐IIIa in response to thrombin 1 U/ml. Finally, we observed that released platelets recirculate at a very high efficiency following transfusion into immunodeficient mice with a half‐life 4.33 fold higher than native platelets.
Conclusion(s): We have developed a platelet release device that meets the requirements for the large‐scale production of cultured platelets suited for transfusion.
OC 59.3
Megakaryocyte engraftment and altered platelet function in a murine model of hematopoietic stem cell transplantation
K. Mott 1; C. Keß2; A. Frank2; S. Hölle3; Z. Nagy2; N. Schlegel4; M. Eyrich3; H. Schulze5
1 University Hospital Wuerzburg, Würzburg, Bayern, Germany; 2 Institute of Experimental Biomedicine, Chair I, University Hospital Wuerzburg, Wuerzburg, Germany, Würzburg, Bayern, Germany; 3 Department of Pediatric Hematology, Oncology and Stem Cell Transplantation, University Children's Hospital, University Medical Center, University of Wuerzburg, Wuerzburg, Germany, Würzburg, Bayern, Germany; 4 Department of General, Visceral, Transplant, Vascular and Pediatric Surgery, University Hospital Wuerzburg, Wuerzburg, Germany, Würzburg, Bayern, Germany; 5 Institute of Experimental Biomedicine, Chair I, University Hospital Wuerzburg, Centre of Inherited Blood Cell Disorders, University Hospital Wuerzburg, Würzburg, Bayern, Germany
Background: Total body irradiation (TBI) preceding hematopoietic stem cell transplantation (HSCT) remains a treatment option for hemato‐oncological diseases. Complications include bleeding due to poor megakaryocyte (MK) engraftment and prolonged thrombocytopenia. Underlying mechanisms for delayed platelet production are yet ill‐defined.
Aims: To analyze how TBI clinically impacts platelet engraftment and how TBI‐induced bone marrow (BM) remodeling influences MK engraftment and platelet function using a reporter‐based murine HSCT model.
Methods: C57B6/J wildtype mice were subjected to sublethal or lethal TBI followed by HSCT with BM from dsRedβ‐actin mice. Platelet production and function was assessed by flow cytometry and immunoblotting. MK number and location were determined in respect to BM sinusoids and matrix proteins in femur sections by confocal microscopy. MMP activity was assessed by immunoblot and zymography using MMP9‐/‐ mice as controls. Plasma chemokine levels were measured by ProcartaPlex luminex. Platelet engraftment kinetics was assessed in a retrospective single‐center cohort of 350 pediatric HSCT recipients.
Results: Our human HSCT analysis revealed that TBI‐conditioning is associated with prolonged thrombocytopenia and increased platelet transfusion demand compared to chemo‐based conditioning regimens. In TBI‐conditioned mice after HSCT, platelets were hyporeactive towards thrombin and CRP‐XL (Fig. 1A–C,E). While dsRed‐ (recipient‐derived) platelets were hyporeactive, dsRed+ (donor‐derived) platelets displayed over‐activation towards CRP‐XL (Fig. 1D), implying that two functionally distinct platelet populations are present in circulation. Syk phosphorylation was markedly reduced in response to CRP‐XL (Fig. 1F). Donor cell engraftment occurred in large clusters, containing Ly6G+ cells and MKs, while Ly6C+, B220+ and Ter119+ cells were homogeneously distributed (Fig. 2A), implying a specific MK/granulocytes engraftment pattern. Massive vasodilation occurred already 6 h after irradiation (Fig. 2B,C), including sinusoid‐specific MMP9‐mediated collagen IV degradation (Fig. 2D–F). CXCL1, CXCL9 and CCL7 were selectively upregulated (Fig. 2G–I), suggesting a role for HSC engraftment.
Conclusion(s): A specific MK engraftment pattern in response to TBI‐induced BM remodeling is associated with a mixed platelet chimerism comprising markedly different reactivity.
OC 75.4
Investigating the health and function of murine platelets generated using a new ex‐vivo lung system
N. Tarassova; X. Zhao; A. Poole
University of Bristol, Bristol, England, United Kingdom
Background: Producing fully functional platelets outside of the body has been challenging, with notably low platelet yields per megakaryocyte. We have recently designed a new murine lung‐based ex vivo vascular system that generates physiological levels of platelets or up to 3000 per megakaryocyte (https://www.biorxiv.org/content/10.1101/2021.11.01.466743v1).
Aims: This study builds on the functional analysis of these generated platelets conducted in the original report by looking specifically at their procoagulant features and mitochondrial health and function.
Methods: Cultured mouse megakaryocytes were passaged through the vasculature of the new mouse lung ex vivo system until platelets were generated. Using flow cytometry, tetramethylrhodamine methyl ester (TMRM) fluorescence was measured in resting and collagen‐related peptide + thrombin stimulated platelets (n = 8) to assess their mitochondrial depolarisation compared to control platelets isolated directly from mouse whole blood. Surface phosphatidylserine (PS) was assessed by annexin V binding to determine their procoagulant activity (n = 5).
Results: Surface activation of αIIbβ3 integrin and P‐Selectin exposure upon single agonist stimulation was normal. In their resting state, the generated platelets showed normal TMRM mean fluorescent intensity but significantly more of them (12% of the whole sample) exposed PS compared to control platelets (1.5%) (p < 0.005, Student's t‐test). Upon dual agonist stimulation, significantly fewer generated platelets start presenting procoagulant features compared to controls (p < 0.05). Notably, 19% have depolarised mitochondria and 20% expose PS compared to 38% and 35% of controls respectively.
Conclusion(s): Under basal conditions, the platelets generated in the mouse lung vasculature have healthy mitochondria but increased surface PS likely due to some degree of pre‐activation by exposure to tissue injury while in the ex vivo system. Once activated, they have reduced procoagulant activity compared to control platelets, possibly a mechanism that ensures young platelets remain quiescent until they are established in the blood system. Their functionality should now be assessed over time as they age in vitro.
Platelet Function and Interactions
OC 26.3
Novel flow cytometric diagnostic assay for primary immune thrombocytopenia
P. Woolley 1; A. Constantinescu‐Bercu2; M. Stubbs3; R. de Groot2; J. Westwood3; Y. Guo4; M. Scully5
1 University College London Hospitals NHS Foundation Trust, London, United Kingdom, London, England, United Kingdom; 2 University College London, London, England, United Kingdom; 3 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 4 Univsersity College London, London, England, United Kingdom; 5 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom
Background: Primary Immune Thrombocytopenia (ITP) remains a diagnosis of exclusion and the lack of diagnostic tests in this field makes conclusive diagnosis difficult.
Aims: A whole blood flow cytometry assay was developed to confirm the diagnosis of ITP and differentiate between immune versus non‐immune thrombocytopenia.
Methods: Samples of 50 patients with ITP, 50 normal controls and 30 thrombocytopenic controls were analysed; written consent was obtained. Whole blood was incubated with 3 platelet markers (CD41, CD42b and CD61) and 3 immunoglobulin markers (IgA/IgG and IgM). Sample collection was standardised to 10000 platelet events. Gating for platelet events established CD41/CD42b/CD61 positivity and with‐in these gates, IgA and IgG positive events were captured and formed the basis for a multinomial regression model.
Results: The 3 groups were of comparable ages and gender. Patients with ITP had a platelet count range of 3 × 109/L–213 × 109/L and patients with active disease, patients on treatment and patients in remission were included. Thrombocytopenic controls platelet count ranged from 4 × 109/L–96 × 109/L and normal controls platelet count ranged from 154 × 109/L–474 × 109/L. Receive operator curve (ROC) curve analysis confirmed a sensitivity of 87% and specificity of 84% (p = 0.0006) and this equated to a positive predictive value of 90% in confirming a diagnosis of ITP (Figure 1). This assay also confirmed a >99% probability of ITP at an IgG level >10 and a 90% probability at an IgA level of >18 (Figure 2). In contrast, 97.5% of thrombocytopenic and normal controls had IgG levels <3.7 and IgA levels of <11.5 for thrombocytopenic controls and <3.0 for normal controls.
Conclusion(s): This assay demonstrates the first clinically useful technique for confirming the diagnosis of ITP by whole blood flow cytometry. This assay enables the differentiation of patients with ITP from both normal controls and patients with thrombocytopenia not related to ITP.
OC 26.4
Thrombin and ADP pathways of platelet aggregation are dysregulated in participants with diabetes in the Framingham Heart Study
M. Chan 1; D. Masood1; M. Chen1; F. Thibord2; B. Nkambule3; A. Lachapelle1; J. Cunha4; R. Pashek5; A. Johnson1
1 NHLBI, Framingham, Massachusetts, United States; 2 Population Sciences Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, Boston, Massachusetts, United States; 3 NHLBI, Boston, Massachusetts, United States; 4 NHLBI and Boston University's Framingham Heart Study, Framingham, Massachusetts, United States; 5 NHLBI, Allston, Massachusetts, United States
Background: Cardiovascular disease is the leading cause of death in patients with diabetes. Though it is well known that platelets are critical in the development of thrombosis, there is limited evidence to show that diabetes modulates platelet function.
Aims: We investigated whether markers of diabetes modulated reactivity in five platelet assays.
Methods: Fasting blood was drawn from Framingham Heart Study participants. Sodium citrate anti‐coagulated blood was centrifuged to obtain platelet‐rich plasma, whilst hirudin anti‐coagulated blood was used for whole blood assays. Five platelet assays were conducted using a range of agonists: light transmission aggregometry (LTA), Optimul aggregometry, flow cytometry, Total Thrombus formation Analysis System and Multiplate aggregometry (MP). Linear mixed‐effects models were used with platelet reactivity traits corrected for age, sex, and aspirin‐use to observe the differences between participants with and without diabetes, and associations between platelet reactivity traits and blood glucose, HbA1c levels and anti‐diabetic medications.
Results: The FHS cohort consisted of a total of 3,429 participants. Participants with diabetes (N = 351) were significantly older (60.1 ± 8.5 vs. 53.8 ± 9.2 years, p = 1.39E‐15) and had greater aspirin use (38.7% vs. 19.5%). As expected, fasting blood glucose (p = 2.38E‐38) and HbA1c (p = 1.98E‐53) were significantly greater in those diagnosed with diabetes. In participants with diabetes, platelet reactivity in response to TRAP‐6 amide (4.48 μM) using MP was enhanced (β = 0.255, p = 5.29E‐06) whilst disaggregation to ADP (1.82 μM) in LTA also increased (β = 0.249, p = 1.31E‐05) corresponding with an overall blunted ADP response. Insulin and metformin use were similarly associated with increases in TRAP‐6 amide‐induced MP response and ADP disaggregation.
Conclusion(s): This study highlights the importance of thrombin receptors, ADP and glucose‐lowering drugs in platelet reactivity in patients with diabetes. Our results support utilization of vorapaxar, a thrombin receptor antagonist, in diabetics which has been shown to reduce cardiovascular events in diabetes in the TRA 2P‐TIMI 50 study (Cavender et al., 2014).
OC 49.4
Platelet‐released mitochondria interact with neutrophils, altering their phenotype and merging with the existing mitochondrial network
H. Allan 1; P. Ferreira2; M. Crescente3; P. Armstrong4; T. Warner4
1 Blizard Institute, Queen Mary University of London, London, England, United Kingdom; 2 Imperial College London, London, England, United Kingdom; 3 Manchester Metropolitan University, Manchester, England, United Kingdom; 4 Queen Mary, University of London, London, England, United Kingdom
Background: Activated platelets produce a heterogenous population of extracellular vesicles packaged with pro‐inflammatory and pro‐thrombotic molecules. A small subset of these vesicles contains functional mitochondria that actively respire and consume oxygen. Interactions between platelet vesicles and cells within the circulation have been described, however to date, mitochondria containing vesicles have not been investigated as a separate population. Notably, mitochondria are damaged associated molecular patterns which raises the question whether mitochondria vesicles will interact with and affect immune cells.
Aims: To investigate if platelet vesicles containing mitochondria interact with neutrophils thereby changing their phenotype.
Methods: Washed platelets (3 × 108/ml) stained with MitoTracker Orange (20 nm) were incubated with TRAP‐6 (20 μM; 2 h, 37°C) to stimulate the production of vesicles. Cell sorting was used to separate mitochondria negative (PMV) and positive (mitoPMV) vesicles. Isolated neutrophils were incubated with sorted vesicles (45 min, 37°C), prepared for immunofluorescence or analysed for the expression of CD66b, CD11b and CXCR2 by flow cytometry. Data were analysed using Image J, FlowJo v.10 and GraphPad Prism.
Results: MitoPMVs accounted for 19 ± 1% of the platelet vesicle population, had high levels of P‐selectin expression (96 ± 1%, n = 8) and interacted with and were internalised by neutrophils. Microscopy revealed mitoPMVs merged with the neutrophil mitochondria network. Furthermore, neutrophils incubated with mitoPMVs, but not those incubated with PMVs, showed significant increases in expression of CD66b (1.5 ± 0.2 fold; n = 6, p < 0.05) and CD11b (1.3 ± 0.1; n = 6, p < 0.05) with a concurrent reduction in CXCR2 expression (0.7 ± 0.07 fold, n = 6, p < 0.05).
Conclusion(s): Mitochondrial transfer has been demonstrated in numerous cells highlighting a mechanism in which mitochondrial function may be augmented. Here we show that mitochondria encapsulated within platelet vesicles are internalised by neutrophils and can merge with existing neutrophils networks. Further work is required to determine the contribution to neutrophil metabolic capacity but may be associated with neutrophils adopting an activated and phagocytic phenotype.
OC 77.1
Inhibition of the Class II PI 3‐kinase, PI3KC2a, prevents thrombosis in hyperlipidaemic mice
N. Setiabakti 1; V. Tarlac2; J. Hamilton2
1 Monash University, Melbourne, Victoria, Australia; 2 Australian Centre for Blood Diseases, Monash University, Melbourne, Victoria, Australia
Background: Inhibition of platelet activation is the basis of standard‐of‐care therapy for the prevention of arterial thrombosis. A major limitation of current anti‐platelet agents is inadequate efficacy in high‐risk populations, such as those with hyperlipidaemia. The class II PI3‐kinase, PI3KC2α, is an intracellular signaling enzyme that has been shown to be important for platelet function. Targeting PI3KC2α in mice results in striking anti‐thrombotic effects with preserved haemostatic function, making PI3KC2α a promising candidate to pursue improved anti‐thrombotic therapy. However, whether these effects are preserved in the setting of hyperlipidaemia remains unknown.
Aims: To examine whether genetic deficiency or pharmacological inhibition of PI3KC2α provides anti‐thrombotic effects in blood taken from hyperlipidaemic mice.
Methods: Thrombosis was evaluated using an ex vivo microfluidic whole‐blood continuous perfusion assay. Whole blood taken from hyperlipidaemic ApoE‐/‐ mice was flowed at arterial shear rates over type I collagen fibers and thrombus volume measured in real‐time via confocal microscopy for five minutes. The effect of genetic deficiency of PI3KC2α was examined by using blood from mice with combined deficiency of ApoE‐/‐ and PI3KC2α, while a recently‐developed PI3KC2α inhibitor, MIPS‐21335 (10 μM), pretreated in whole blood from ApoE‐/‐ mice to evaluate the effect of acute pharmacological inhibition of PI3KC2α.
Results: Hyperlipidaemia had the anticipated pro‐thrombotic effect, with thrombus volume 1.5‐fold greater in blood from ApoE‐/‐ mice than in blood from wild‐type mice (p = 0.009). This increased thrombosis in blood from ApoE‐/‐ mice was entirely prevented in blood from mice with a combined deficiency of ApoE and PI3KC2α (p = 0.007). Acute inhibition of PI3KC2α with MIPS‐21335 also reduced thrombosis in blood from ApoE‐/‐ mice, albeit to a lesser extent (p = 0.066).
Conclusion(s): These findings demonstrate that the anti‐thrombotic effect of PI3KC2α‐deficiency or ‐inhibition observed in normolipidaemia is largely retained in the face of hyperlipidaemia and suggests PI3KC2α is a promising target for anti‐thrombotic therapy in this high‐risk population.
OC 77.2
Na+/K+ ATPase alpha 1 subunit participates in platelet signaling and is a potential anti‐thrombotic target
O. Li 1; R. Aguilar2; R. Roytenberg3; H. Yue4; E. Thompson2; S. Pierre5; J. Liu3; W. Li4
1 Joan C. Edwards School of Medicine at Marshall University, Huntington, West Virginia, United States; 2 Department of Medicine, Joan C. Edwards School of Medicine, Huntington, West Virginia, United States; 3 Marshall University Joan C. Edwards School of Medicine, Huntington, West Virginia, United States; 4 Joan C. Edwards School of Medicine at Marshall University, Huntington, West Virginia, United States; 5 Marshall Institute for Interdisciplinary Research, Huntington, West Virginia, United States
Background: Thrombosis is a major cause of myocardial infarction and ischemic stroke. Sodium/potassium ATPase (NKA) is composited by alpha and beta subunits and plays an important role in maintaining the sodium and potassium gradient across the cell membrane. NKA has signaling functions, and its α1 subunit (ATP1A1) binds to and inhibits the activity of Src, a tyrosine kinase, which plays a critical role during platelet activation.
Aims: To test the hypothesis that ATP1A1 participates in platelet signaling activation and is an anti‐platelet and anti‐thrombotic target.
Methods: Wildtype and ATP1A1 heterozygous mice aged 10–14 weeks were used. A FeCl3‐induced carotid artery injury thrombosis model in combination with intravital microscopy was used for in vivo thrombosis study. Platelet aggregation and Cellix flow chamber assays were used to evaluate in vitro platelet function. Western blot, co‐IP, and blue native PAGE assays were used for the characterization of protein expressions and protein complex formations.
Results: ATP1A1 heterozygosity dramatically reduced its expression on platelets and inhibited in vivo thrombosis in male but not female mice. ATP1A1 heterozygosity did not affect initial platelet adhesion/aggregation on injured vessel walls and collagen‐coated surfaces. However, it significantly delayed second wave platelet activation in vivo and inhibited ADP‐induced platelet aggregation in vitro. ATP1A1 heterozygosity did not affect platelet intracellular sodium concentration, suggesting that the observed anti‐thrombotic phenotype is not due to the altered NKA function. Intraperitoneal injection of the NKA inhibitor Ouabain (100 ng/g of body weight) for 24 h significantly inhibited thrombosis in mice. ATP1A1 heterozygosity showed reduced ADP‐induced AKT activation in platelets. ATP1A1 forms a complex with the ADP receptor P2Y12, and pretreatment of human platelets with Ouabain inhibited ADP‐stimulated platelet aggregation in a dose‐dependent manner.
Conclusion(s): ATP1A1 participates in platelet ADP signaling, which is essential for ADP‐induced platelet activation. Targeting ATP1A1 could be a novel strategy for antiplatelet and antithrombotic therapy.
OC 77.3
Platelet factor XIII‐A regulates platelet function and promotes clot retraction and stability
J. Mitchell 1; G. Little2; A. Bye3; A. Unsworth4; R. Gaspar5; N. Kriek2; T. Sage2; A. Stainer5; D. Sangowawa5; N. Curry6; S. Shapiro7; M. Desborough6; C. Whyte8; J. Gibbins9; N. Mutch8; C. Jones9
1 University of Birmingham, Birmingham, England, United Kingdom; 2 University of Reading, READING, England, United Kingdom; 3 St Georges University London, London, England, United Kingdom; 4 Manchester Metropolitan University, Manchester, England, United Kingdom; 5 University of Reading, Reading, England, United Kingdom; 6 Oxford University Hospitals NHS Foundation Trust, Oxford, England, United Kingdom; 7 Oxford University Hospitals National Health Service Foundation Trust, University of Oxford, and Oxford National Institute for Health Research Biomedical Research Centre, Oxford, England, United Kingdom; 8 University of Aberdeen, Aberdeen, Scotland, United Kingdom; 9 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: Platelet factor XIII‐A (FXIII‐A) is externalised upon platelet activation and is functional in cross‐linking extracellular fibrin to stabilise thrombi against fibrinolysis. FXIII‐A is abundant inside platelets and has many intracellular substrates, suggesting it may be involved in platelet function.
Aims: Determine the role platelet FXIII‐A plays in platelet function.
Methods: Platelets were isolated from FXIII‐deficient patients and normal donors and assessed using confocal microscopy and flow cytometry. Clots or thrombi under flow were formed from FXIII‐depleted plasma with normal healthy or FXIII‐deficient platelets. FXIII was inhibited using a cell‐permeable transglutaminase inhibitor (TGI).
Results: FXIII‐deficient platelets contained no detectable FXIII‐A and were not functional in stabilising FXIII‐depleted thrombus lysis, compared to the stabilising effect achieved by healthy platelets confirming platelet FXIII‐A protects thrombi against fibrinolysis. Retraction of FXIII‐depleted plasma clots was reduced in the presence of FXIII‐deficient platelets and platelets with TGI, compared to untreated healthy platelets (p < 0.05) indicating platelet FXIII‐A plays roles in driving clot retraction. Fibrinogen binding to FXIII‐deficient platelets and platelets treated with TGI (p < 0.0001) was reduced when compared to untreated normal platelets, and FXIII‐deficient platelets had reduced sensitivity to CRP‐XL and TRAP6 stimulation, suggesting FXIII‐A may be involved in the signalling processes that regulate platelet activation. Furthermore, FXIII‐A activity co‐localized with the platelet cytoskeleton, suggesting FXIII‐A may participate cross‐link actin. Platelet spreading on collagen (p < 0.01) and fibrinogen (p < 0.05) was attenuated in TGI treated and FXIII‐deficient platelets compared to normal platelets. In line with this, FXIII‐deficient platelets and TGI‐treated normal platelets had reduced adherence to fibrinogen and formed smaller thrombi on fibrinogen under flow.
Conclusion(s): Fibrinogen binding, spreading, platelet sensitivity to agonists and clot retraction are attenuated in TGI treated and FXIII‐deficient platelets. These data suggest platelet FXIII‐A is actively involved in these processes, possibly by mediating intracellular cytoskeletal rearrangement.
OC 10.1
CD36‐dependent metabolic rewiring and mitochondrial dysfunction in platelets in response to hyperlipidaemia
L. Cheah; M. Hindle; K. Naseem
University of Leeds, Leeds, England, United Kingdom
Background: Atherogenic hyperlipidaemia leads to plasma oxidised low‐density lipoprotein (oxLDL) accumulation, associated with increased arterial thrombosis. Scavenger receptor CD36 translates oxidised lipid stress to platelet hyperactivity via oxLDL binding. Platelet hyperactivation requires energy, however, the effects of oxLDL on metabolic rewiring remains elusive.
Aims: Investigate the effects of oxLDL on metabolic rewiring and mitochondrial dysfunction.
Methods: Mice with platelet specific deletion of CD36 (CD36fl/fl/PF4cre) were used in comparison to CD36fl/fl littermates. Hyperlipidaemic mice were produced by high fat diet (HFD) feeding (20 weeks). To mimic these effects in vitro, oxLDL or native‐LDL (nLDL) was used.
Results: CD36fl/fl/PF4cre mice were fertile with no differences found in platelet counts and key haemostatic receptors expression. The metabolically disturbed environment of HFD led to whole body hyperlipidaemia and insulin resistance. Under these conditions, CD36fl/fl platelets were shown to increase glucose uptake, which was lost in CD36fl/fl/PF4cre. Increased glucose uptake was associated with elevated basal glycolysis which was CD36 dependent. Upon thrombin stimulation, glycolysis and glucose uptake were further enhanced in CD36fl/fl platelets. Interestingly, glycolysis but not glucose uptake was attenuated in CD36fl/fl/PF4cre. When CD36fl/fl platelets were oxLDL‐treated there was a significantly higher glucose uptake than nLDL, and this was lost in CD36fl/fl/PF4cre mice. The accumulation of TMRE dye (mitochondrial polarity marker) was higher in HFD platelets however it was reduced in CD36fl/fl/PF4cre compared to the CD36fl/fl platelets. This effect was phenocopied in oxLDL‐treated human platelets. Mitochondrial superoxide production were also increased in hyperlipidaemia and oxLDL treatment. Importantly the effects of oxLDL on glucose uptake and mitochondrial function were reduced by glycolytic inhibitors.
Conclusion(s): We have previously shown that activated platelets are highly glycolytic. However, under conditions of hyperlipidaemia that glucose metabolism is elevated and results in mitochondrial function alterations. These effects are CD36 dependent and likely to occur via interaction with oxLDL. This work was funded by BHF (RG/16/5/32250).
OC 09.3
Differential fate of von Willebrand factor and von Willebrand factor propeptide following release from platelet alpha‐granules
M. Swinkels 1; T. Lemmens2; P. Burgisser3; J. Slotman4; A. Houtsmuller4; J. Cosemans5; F. Leebeek3; J. Voorberg6; A. Jansen7; R. Bierings8
1 Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 3 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Optical Imaging Center, Department of Pathology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 5 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 6 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 7 Erasmus MC, University Medical Center, Rotterdam, Zuid‐Holland, Netherlands; 8 Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Platelet alpha‐granules contain Von Willebrand factor (VWF), which is stored in eccentric alpha‐granule nanodomains, and VWF propeptide (VWFpp). Differential release of VWF and VWFpp has been reported from endothelial cells. It is unclear if this also occurs during platelet alpha‐granule exocytosis and what happens post‐release. We have recently developed a quantitative 3D super‐resolution imaging workflow for platelet alpha‐granule content based on Structured Illumination Microscopy (SIM). With this we can study alpha‐granule cargo release following platelet activation in hundreds of platelets simultaneously.
Aims: To study release of VWF and VWFpp from alpha‐granules using quantitative super‐resolution microscopy.
Methods: Platelets were activated with PAR‐1 activating peptide (PAR‐1‐ap) or collagen‐related peptide (CRP‐XL). Alpha‐tubulin, VWF, VWFpp, SPARC and fibrinogen were imaged using 3D‐SIM, followed by semi‐automated analysis in ImageJ. Uptake of anti‐VWF nanobody during degranulation was used to identify alpha‐granules that partially released content. In vitro thrombi were generated by perfusion of plasma‐depleted blood, reconstituted with thrombin and fibrinogen, over collagen surfaces at 1000 s‐1 shear rate.
Results: VWF+ and VWFpp+ structures overlapped nearly completely (~90%) in resting platelets, implying they are stored in similar eccentric alpha‐granule nanodomains. A subset of VWF+/VWFpp+‐structures was released completely at 0.6 μM PAR‐1‐ap, but at higher concentration (20μM) significantly more VWFpp (85.3 ± 1.6%) was released than VWF (37.6 ± 1.4%). Release of other cargo was intermediate at 20 μM (SPARC: 62.2 ± 1.4%; fibrinogen: 51.9 ± 2.9%), providing further evidence for differential cargo release. Similar results were obtained using CRP‐XL. Anti‐VWF nanobody was incorporated in VWF+/VWFpp‐‐structures and increased with stimulus strength, demonstrating these were post‐exocytotic structures. In thrombi formed under flow, VWFpp was primarily localized in the core while VWF was more uniformly present in the thrombus shell.
Conclusion(s): VWF and VWFpp are differentially released from alpha‐granules and associate with distinct regions within the thrombus post‐release. This may affect how platelet‐derived VWF and VWFpp contribute to formation and stabilization of hemostatic clots.
OC 09.5
Role of disturbed blood flow in the intrinsic thrombogenicity of human carotid stents
A. Kern 1; Y. Kreinin2; R. Pop3; N. Korin2; P. Mangin4
1 University of Strasbourg, INSERM UMR_S1255, EFS‐Grand Est, STRASBOURG, Alsace, France; 2 Department of Biomedical Engineering Technion, Israel Institute of Technology, Haifa, Hefa, Israel; 3 Interventional Neuroradiology Department, Strasbourg University Hospitals, STRASBOURG, Alsace, France; 4 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France
Background: In around 20% of emergency endovascular procedures for acute anterior circulation stroke, there is a tight stenosis or occlusion of the cervical carotid artery in addition to intracranial arterial occlusion. Emergent stenting of the carotid leads to better recanalization rates and improved clinical outcomes, however, dual‐antiplatelet therapy is often avoided due to increased risk of haemorrhagic transformation. Early stent thrombosis (ST) occurs frequently in these patients (up to 21%) and has been associated with worse clinical outcomes. A better understanding of the mechanism of ST could improve the clinical management of these patients and open new avenues for the development of less thrombogenic stents.
Aims: To evaluate the role of rheology in carotid ST.
Methods: We developed an on‐scale macrofluidic flow chamber mimicking a human carotid artery to investigate the thrombogenicity of clinically‐used stents. Hemodynamic profile was characterized using computational fluid dynamics (CFD). Platelet aggregation was studied using fluorescence and scanning electron microscopy (SEM).
Results: Real‐time video‐macroscopy showed that anticoagulated whole blood perfused at physiological shear rates found in the human carotid, resulted in an accumulation of platelet thrombi on the stent struts within the first hour. SEM indicated that platelets adherent to the stents were activated as indicated by their shape change and filopodia extension. Interestingly, regions of platelet aggregation were not randomly distributed, and hot spots of thrombus formation were identified around the crossing of the stent mesh, suggesting a pivotal role of local rheology rather than the stent material. CFD allowed to precisely characterize the flow occurring at these hot spots and identify thrombogenic flow profiles. The use of anti‐platelet drugs and pharmacological agents identified integrin alphaIIbbeta3 as a trigger of thrombus formation on the stent struts.
Conclusion(s): Our results highlight the pro‐thrombogenic potential of local stent‐mediated blood flow disturbances and open ways to better prevent their occurrence.
OC 49.5
Mitochondrial ATP generation in stimulated platelets is essential for granule secretion but dispensable for aggregation and procoagulant activity
P. Kulkarni 1; M. Ekhlak2; V. Sonkar3; D. Dash2
1 Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India; 2 Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India; 3 Department of Molecular and Human Genetics, Institute of Science, Banaras Hindu University, Varanasi, Uttar Pradesh, India
Background: Platelets exhibit considerable plasticity in energy metabolism. Yet, we recently demonstrated that diverting the metabolic flux from aerobic glycolysis towards OXPHOS prevents platelet activation. In addition, several studies have established that inhibitors of OXPHOS alone do not compromise thrombin‐induced platelet aggregation.
Aims: We aim to determine if mitochondrial respiration is redundant for platelet bioenergetics and function in response to agonist‐stimulation.
Methods: We studied the effect of antimycin (complex III inhibitor), oligomycin (FoF1 ATP synthase inhibitor) and CCCP (uncoupler), on platelet responses to agonist‐stimulation by lumi‐aggregometry, flow cytometry, and shear‐based thrombosis studies. The underlying mechanism mediating the observed effects was explored by flow cytometry and biochemical assays. Significance in difference of means was determined by repeated measures ANOVA and Dunnett's multiple comparison test.
Results: Thrombin‐induced platelet αIIbβ3 integrin activation, aggregation and procoagulant activity were spared in presence of either oligomycin or antimycin, but were significantly retarded by CCCP. Cellular ATP level, however, remained unchanged under above conditions through a compensatory rise in glycolysis. CCCP, but neither oligomycin nor antimycin, profoundly abrogated thrombin‐induced mitochondrial ROS generation and calcium transients that are essential for platelet aggregation and procoagulant activity, respectively. Strikingly, both oligomycin and CCCP restrained platelet granule secretion, as evidenced from compromised ATP release and P‐selectin exposure, which was associated with diminished platelet‐neutrophil interactions and platelet thrombus formation on immobilized collagen under arterial shear. The effect of oligomycin and CCCP on thrombus growth was abolished in the presence of ticagrelor, an ADP receptor antagonist.
Conclusion(s): Mitochondrial ATP generation is dispensable for platelet aggregation and procoagulant activity, which are fueled by glycolytic ATP. However, maintenance of proton gradient across inner mitochondrial membrane plays a vital role in these processes by supporting ROS generation and mitochondrial calcium influx, respectively. Mitochondrial ATP is critical for platelet granule secretion, platelet‐neutrophil interaction and thrombus growth, especially when inadequately compensated by glycolysis. FIGURE 1 Scheme depicting the effects of mitochondrial uncoupler (CCCP) and inhibitors (antimycin and oligomycin) on platelet bioenergetics and agonist‐induced responses.
FIGURE 1 Scheme depicting the effects of mitochondrial uncoupler (CCCP) and inhibitors (antimycin and oligomycin) on platelet bioenergetics and agonist‐induced responses.
OC 10.4
The role of α‐synuclein and Cysteine String Protein‐α in platelet function
A. Smith 1; L. Omali2; S. Joshi2; S. Whiteheart3
1 University of Kentucky College of Medicine, Lexington, Kentucky, United States; 2 Molecular and Cellular Biochemistry Department, University of Kentucky College of Medicine, Lexington, KY USA, Lexington, Kentucky, United States; 3 Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, Lexington, KY, USA, Lexington, Kentucky, United States
Background: Platelets use SNARE‐mediated exocytosis to maintain hemostasis and thrombosis via secretion from three types of platelet granules: dense, α, and lysosomal. To understand how SNAREs are regulated, we determined if α‐synuclein, a potential v/R‐SNARE chaperone, and its binding partner, Cysteine String Protein‐α (CSPα), affect platelet secretion and thus hemostasis. These abundant proteins are the only detectible members of their respective families, present in platelets.
Aims: To address the role(s) of α‐synuclein and Cysteine String Protein‐α in platelets.
Methods: We examined the hemostatic phenotypes of α‐synuclein‐/‐ and CSPα‐/‐ mice. Secretion by platelets from these mice was measured using kinetic assays. Platelet aggregation and ADP release were examined with Lumi‐aggregometry. Platelet activation was also examined using cytometry. Hemostasis was evaluated in different injury contexts using tail‐bleeding, FeCl3 carotid injury, and jugular puncture models. The levels of the platelet secretory machinery were determined by western blotting.
Results: α‐Synuclein‐/‐ platelets were defective for dense granule release and less so from lysosomal granule release; however, α granule release was similar to wild‐type platelets. Tail‐bleeding times for α‐synuclein‐/‐ mice were slightly increased compared to wild‐type mice, but bleeding from CSPα‐/‐ mice was greatly increased. Occlusion times in the FeCl3 carotid injury model and cessation of bleeding in the jugular puncture model were similar between α‐synuclein‐/‐ and wild‐type mice. The dominant v/R‐SNARE, VAMP‐8, was reduced in α‐synuclein‐/‐ platelets, while the other v/R‐SNARE and t/Q‐SNARE levels remained unchanged. Further experiments are underway to determine how α‐synuclein and CSPα interact with the secretory machinery to affect hemostasis.
Conclusion(s): These experiments demonstrate a role for α‐synuclein and CSPα in platelet exocytosis and hemostasis and will fill gaps in our knowledge of α‐synuclein's physiological function and our understanding of how platelet exocytosis is regulated. This work is supported by the NIH/NHLBI (HL56652, HL138179, HL150818), VA, and an NSF KY‐WV LSAMP BD Fellowship (NSF HRD 2004710).
OC 41.4
PACSIN2 regulates platelet integrin β1 hemostatic function
R. Biswas 1; N. Eaton1; M. Schulte1; M. Plomann2; K. Hoffmeister3; H. Falet1
1 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 2 University of Cologne, Cologne, Nordrhein‐Westfalen, Germany; 3 Versiti Translational Glycomics Center, Milwaukee, Wisconsin, United States
Background: Upon vascular injury, platelets adhere to exposed matrix constituents through specific membrane receptors, including the von Willebrand factor receptor GPIbα and integrins β1 and β3, thereby initiating thrombus formation. The F‐BAR protein PACSIN2 colocalizes with GPIbα in platelets, where it associates with the cytoskeletal and scaffolding protein filamin A (FlnA).
Aims: We investigated the role of platelet PACSIN2 in regulating thrombus formation in vivo.
Methods: We determined platelet parameters in mice lacking PACSIN2 and platelet integrin β1.
Results: Pacsin2‐/‐ mice displayed delayed thrombus formation in a ferric chloride‐mediated carotid artery injury model. The phenotype was intrinsic to platelets, as it was conserved in chimeric mice lacking PACSIN2 in blood cells only and normalized by injection of control platelets. Pacsin2‐/‐ platelets repeatedly formed unstable thrombi that embolized abruptly in a laser‐induced cremaster muscle injury model. Following stimulation with thrombin and the GPVI‐specific collagen‐related peptide, Pacsin2‐/‐ platelets displayed increased activation of the integrin β1, as evidenced by 9EG7 antibody binding, and showed increased spreading to surfaces coated with the integrin β1‐specific peptide GFOGER. By contrast, Pacsin2‐/‐ platelets activated the integrin αIIbβ3 and expressed P‐selectin similar to controls. Pacsin2‐/‐ mice were crossed with Itgb1fl/fl Pf4‐iCre (Itgb1Plt‐/‐) mice specifically lacking platelet integrin β1 to determine whether hyperactive platelet integrin β1 contributed to the thrombus formation defects. Integrin β1 deletion normalized thrombus formation in the ferric chloride‐mediated carotid artery and laser‐induced cremaster muscle injury models. Importantly, Itgb1Plt‐/‐ Pacsin2‐/‐ platelets formed stable thrombi that did not embolize.
Conclusion(s): We conclude that Pacsin2‐/‐ mice displayed thrombus formation defects due to hyperactivation of platelet integrin β1. We hypothesize that PACSIN2 binding to FlnA negatively regulates platelet integrin β1 hemostatic function and are currently exploring how plasma fibronectin binding to platelet integrin α5β1 contributes to the thrombus formation defects of Pacsin2‐/‐ mice.
OC 26.1
Procoagulant platelet – Neutrophil interactions exacerbate ischemic stroke
F. Denorme 1; I. Portier1; Y. Kosaka1; A. Ajanel1; M. Cody1; B. Kile2; M. Rondina1; J. Majersik1; R. Campbell1
1 University of Utah, Salt Lake City, Utah, United States; 2 The University of Adelaide, Adelaide, South Australia, Australia
Background: While anti‐platelet drugs are commonly used to prevent ischemic stroke, they are incompletely effective. Preclinical studies identified platelet‐neutrophil interactions as important mediators of stroke. One subpopulation of platelets that preferentially interacts with neutrophils are procoagulant platelets.
Aims: To study the role of procoagulant platelets in ischemic stroke pathophysiology.
Methods: Stroke patient brain tissue and blood was analyzed for the presence of platelet‐neutrophil aggregates (PNAs) and procoagulant platelets. RNAseq was performed on platelets isolated from stroke patients and healthy donors. Mechanistic studies were performed in mice subjected to transient middle cerebral artery occlusion.
Results: Occlusive platelet‐neutrophil microthrombi were observed in stroke patient brain tissue. Stroke patients had increased PNAs and procoagulant platelets in the circulation compared to healthy donors. A strong correlation was observed between circulating procoagulant platelets and PNAs. RNAseq from platelets isolated from stroke patients uncovered several genes (ANO6, CAPN2 and MCUR1) associated with procoagulant platelet formation that were significantly increased compared to matched controls. Two distinct pathways regulate procoagulant platelet formation: platelet apoptosis, mediated by BAK and BAX; and platelet necrosis mediated, in part, by mitochondrial calcium uniporter (MCU). To analyze which pathway contributes to stroke outcomes, we subjected mice whose platelets were unable to undergo apoptosis (BAK KO/BAXfl/fl‐PF4‐cre) or necrosis (MCUfl/fl‐PF4‐cre) to stroke. After stroke, BAK KO/BAXfl/fl‐PF4‐cre mice had similar levels of procoagulant platelets and PNAs, and stroke outcomes were comparable to littermate controls. In contrast, MCUfl/fl‐PF4‐cre mice had reduced levels of procoagulant platelets and PNAs circulating after stroke, and were protected from ischemic stroke brain injury. This protection was attributed to decreased PNAs in the cerebral microvasculature, resulting in improved blood flow and reduced neuronal apoptosis.
Conclusion(s): Ischemic stroke patients have an increased potential to form procoagulant platelets, leading to detrimental platelet‐neutrophil interactions. Prevention of procoagulant platelet formation through inhibition of platelet MCU conferred protection from stroke in mice.
OC 41.3
Histidine‐rich glycoprotein binds GPIIb/IIIa and attenuates platelet aggregation
R. Malik 1; M. Neves2; J. Zhou1; R. Hussain1; J. Fredenburgh3; H. Ni4; J. Weitz1
1 McMaster University, Hamilton, ON, Canada | Thrombosis and Atherosclerosis Research Institute (TaARI), Hamilton, ON, Canada, Hamilton, Ontario, Canada; 2 Department of Laboratory Medicine and Pathobiology, University of Toronto | Keenan Research Center for Biomedical Science, St. Michael's Hospital, Toronto, ON, Canada | Canadian Blood Services Centre for Innovation, Toronto, ON, Canada, Toronto, Ontario, Canada; 3 McMaster University, Hamilton, ON, Canada | Thrombosis and Atherosclerosis Research Institute (TaARI), Hamilton, ON, Canada, Kitchener, Ontario, Canada; 4 St. Michael's Hospital, Keenan Research Centre, Toronto, Ontario, Canada
Background: Histidine‐rich glycoprotein (HRG) circulates in plasma at a concentration of 1–3 μM and is released from activated platelets. HRG downregulates the contact system by inhibiting (F) XIIa. In murine models, HRG attenuates FeCl3‐ and polyphosphate‐induced thrombosis and colocalizes with platelets and fibrin in thrombi. Therefore, we hypothesized that HRG binds to platelets and modulates their function.
Aims: Determine whether HRG binds to platelets and if so, identify the mechanism and consequences of this interaction.
Methods: The binding of HRG to glycoprotein (GP) Ibα, thrombin‐binding GPIbα peptide, and active GPIIb/IIIa was examined using surface plasmon resonance (SPR), ELISA, and receptor‐coated silica beads. HRG binding to washed human and murine platelets was assessed by flow cytometry. The effect of HRG on platelet aggregation in whole blood and platelet‐rich plasma was examined using multiple electrode and light transmission aggregometry, respectively.
Results: Using SPR, HRG binds to immobilized GPIbα and thrombin‐binding GPIbα peptide with Kd values of 1.0 nM and 0.2 nM, respectively. Using an ELISA, HRG binds immobilized GPIIb/IIIa with a Kd value of 247 nM. HRG binds to GPIIb/IIIa‐coated beads with a 4‐fold higher affinity than to GPIbα‐coated beads. HRG binding increases 3‐fold when resting platelets are activated with thrombin. HRG does not bind to resting platelets from GPIbα‐deficient mice or activated platelets from Beta3‐integrin‐deficient mice, suggesting that binding to resting and activated platelets is mediated by GPIbα and GPIIb/IIIa, respectively. HRG competes with fibrinogen for binding to GPIIb/IIIa on thrombin‐activated human or mouse platelets and inhibits aggregation in mouse whole blood or human platelet‐rich plasma. Finally, whole blood platelet aggregation is enhanced in HRG‐deficient mice compared with wild‐type mice.
Conclusion(s): HRG binds to GPIbα on resting platelets and GPIIb/IIIa on activated platelets where it competes with fibrinogen, thereby attenuating platelet aggregation. Therefore, HRG may modulate platelet aggregation as well as coagulation.
OC 49.3
A microfluidic model to study the initiation of venous thrombosis
L. Mereweather 1; J. van Batenburg‐Sherwood1; J. Ahnström1; I. Salles‐Crawley2; J. Crawley1
1 Imperial College London, London, England, United Kingdom; 2 St George’s University of London, London, England, United Kingdom
Background: Venous thromboembolism is the third highest cause of cardiovascular mortality. Low/aberrant flow in the venous valves is a major contributing risk factor in the development of thrombosis. Although both platelets and neutrophils are important in the initiation of venous thrombi in valve pockets, underlying mechanisms remain to be elucidated.
Aims: To develop an in vitro model to investigate the initiating events leading to thrombus formation in venous valves.
Methods: Primary HUVECs were cultured in the presence or absence of shear, either in straight channels, or in microchannels with a shape approximating pathogenic venous valves. Expression levels of haemostatic markers were quantified using qPCR, Western blot or immunostaining. Live imaging flow assays in endothelialised valve channels were used to quantify the recruitment of platelets and neutrophils.
Results: ECs cultured under static conditions (i.e. in venous pockets) showed significantly higher expression of VWF, EPCR and TFPI compared with ECs cultured under venous shear, while TM was downregulated at both mRNA and protein level. Within valve channels, ECs cultured in the linear section showed alignment in the direction of flow, whereas those in the venous pocket retained a cobblestone morphology, highlighting the different shear stresses present in each region. Flow assays using plasma‐free blood revealed the ability of neutrophils to bind to platelets/ECs specifically in the valve pocket, but not in the linear section. Furthermore, neutrophils captured in the pocket region formed NETs within 60 min.
Conclusion(s): Our data demonstrate the influence of flow on EC phenotype and subsequent capture and activation of platelets and neutrophils. Further experiments are under way to elucidate key receptors and signalling pathways involved in this process. Our fluidic venous valve model will be invaluable to investigate key initiating events leading to neutrophil capture on platelets and/or ECs and may highlight novel targets for therapeutic intervention.
OC 41.2
The GPIbα intracellular tail is important for VWF‐mediated platelet signaling events that promote platelet/neutrophil interactions under flow
A. Constantinescu‐Bercu1; J. Lee1; A. Petri1; P. Mangin2; K. Vanhoorelbeke3; J. Crawley1; I. Salles‐Crawley 4
1 Imperial College London, London, England, United Kingdom; 2 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 3 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 4 St George's University of London, London, England, United Kingdom
Background: The VWF‐GPIbα axis is not only important for platelet capture at sites of vessel injury but also in thromboinflammatory conditions, such as venous thrombosis. We recently demonstrated that human VWF‐bound platelets are ‘primed’ under flow, recruiting neutrophils and inducing NETosis via activated αIIbβ3. We also identified SLC44A2 as the neutrophil counter‐receptor for activated αIIbβ3. The SLC44A2 locus has been linked to venous thromboembolism risk in several genome‐wide association studies. To analyse the role of VWF‐priming in vivo, we generated a novel GpIbαδsig/δsig mouse with a 24 amino acid deletion of the GPIbα intracellular tail and showed that these mice exhibited normal haemostasis but impaired VWF‐GPIbα signalling.
Aims: To evaluate the (patho)physiological importance of VWF/flow‐dependent signalling through GPIbα.
Methods: GpIbαδsig/δsig mice have been previously generated. VWF‐mediated platelet priming was assessed on VWF surfaces under static and flow conditions in a microfluidic platform.
Results: As previously shown, although VWF‐mediated platelet capture under flow was unchanged in GpIbαδsig/δsig platelets compared to GpIbα+/+ platelets, they exhibited a significant decrease (50%) in αIIbβ3 activation. Defect in VWF‐GPIbα‐mediated signalling in GpIbαδsig/δsig platelets was further characterized by diminished calcium spike responses when stimulated with botrocetin. Consistent with a diminished expression of activated αIIbβ3, GpIbαδsig/δsig platelets exhibited a significantly reduced ability to recruit neutrophils compared to GpIbα+/+ platelets. Similar to VWF‐primed human platelets, GpIbα+/+ primed platelets recruited neutrophils under low shear and these interactions could be inhibited by αIIbβ3 or SLC44A2 blockade, but not by β2‐integrin inhibition. Neutrophil recruitment to VWF‐primed platelets was also significantly increased in the absence of plasma.
Conclusion(s): Our data suggest an important role for the GPIbα intracellular tail in transducing signals downstream of the VWF A1‐ GPIbα interaction and to mediate subsequent platelet‐neutrophil interactions. Additional thrombosis models and platelet signalling assays are underway to fully appreciate the pathophysiological role of the GPIbα A1 signalling.
OC 49.2
Spatio‐temporal control of fibrin formation by platelet glycoprotein V
S. Beck 1; P. Öftering2; R. Li3; K. Hemmen4; M. Nagy5; Y. Wang3; A. Zarpellon6; M. Schuhmann7; G. Stoll7; Z. Ruggeri6; K. Heinze8; J. Heemskerk9; W. Ruf10; D. Stegner11; B. Nieswandt12
1 University Hospital and Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 2 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Germany, Würzburg, Bayern, Germany; 3 Aflac Cancer and Blood Disorders Center, Children's Healthcare of Atlanta, Department of Pediatrics, Emory University School of Medicine, Atlanta, USA, Atlanta, Georgia, United States; 4 Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 5 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 6 Department of Molecular Medicine, Scripps Research, La Jolla, CA, USA, La Jolla, California, United States; 7 University Hospital Würzburg, Würzburg, Bayern, Germany; 8 Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 9 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 10 Center for Thrombosis and Hemostasis, Johannes Gutenberg University Medical Center, Mainz, Rheinland‐Pfalz, Germany; 11 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 12 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: Platelet activation and coagulation at sites of vascular injury are crucial for hemostasis but can promote thrombosis in vascular pathologies. During platelet activation, the abundant platelet glycoprotein (GP)V is cleaved by thrombin, however the function of membrane‐bound as well as cleaved GPV remains unknown.
Aims: To elucidate the role of platelet GPV cleavage and its functional relevance at sites of vascular injury.
Methods: We used genetic and pharmacological approaches (Gp5‐/‐ and Gp5dThr mice – thrombin‐insensitive GPV, new anti‐GPV antibodies, recombinant GPV ectodomain) to assess thrombus formation, thrombin generation and fibrin formation in vitro and in vivo.
Results: Platelets of Gp5‐/‐ mice were hyperreactive to low thrombin concentrations under conditions where GPIbα was required for platelet responsiveness to thrombin. In contrast to Gp5‐/‐ platelets, Gp5dThr platelets or blockade of thrombin‐mediated GPV cleavage were not hyperreactive, indicating a key regulatory role of membrane‐bound GPV for thrombin‐dependent platelet activation. Surprisingly, both Gp5‐/‐ and Gp5dThr mice showed accelerated thrombus formation following vascular injury in vivo, suggesting that cleaved GPV controls thrombus formation by a mechanism unrelated to regulation of platelet activation. We uncovered that soluble (s)GPV regulates thrombin‐dependent fibrin formation under flow. Specifically, we demonstrated (I) direct interaction of sGPV with thrombin in a pulldown assay, (II) accumulation of sGPV with fibrin in platelet‐free areas of thrombi by super‐resolution microscopy, (III) increased availability of free thrombin and fibrin formation in Gp5‐/‐ and Gp5dThr mice and after blockade of GPV cleavage, and (IV) inhibition of thrombus formation by sGPV. Thus, sGPV localized to growing thrombi, limited thrombin‐dependent fibrin formation and protected from arterial thrombosis without interfering with initial hemostatic platelet adhesion. Accordingly, genetic or pharmacologic defects in hemostatic platelet function were rescued by blockade of GPV cleavage.
Conclusion(s): GPV spatio‐temporally controls fibrin formation and thereby provides a platelet‐based mechanism that locally limits excessive fibrin formation and thrombus growth.
OC 09.1
The rich inner life of a thrombus – “next generation” analysis of platelet activity during thrombus formation in vivo
P. Larsson1; V. Tarlac2; A. McGovern3; J. Nunez‐Iglesias4; L. Faxälv5; S. Cody6; J. Hamilton2; N. Boknäs 7
1 Monash University, Melbourne, Victoria, Australia; 2 Australian Centre for Blood Diseases, Monash University, Melbourne, Victoria, Australia; 3 Department of Anatomy and Developmental Biology, Monash University, Melbourne, Victoria, Australia; 4 Anatomy and Developmental Biology, Monash University, Melbourne, Victoria, Australia; 5 University of Linkoping, Linkoping, Ostergotlands Lan, Sweden; 6 Monash Universiy, Melbourne, Victoria, Australia; 7 Region Östergötland, Linkoping, Ostergotlands Lan, Sweden
Background: During the last two decades, application of fluorescence microscopy to the study of thrombosis in vivo has provided valuable insights into the structural composition of a developing thrombus. However, the level of detail afforded by current experimental and analytical models is insufficient for a detailed characterization of the complex and highly dynamic processes that shape thrombus architecture.
Aims: To develop experimental and analytical protocols that provide an automatized, quantitative analysis of the movements and activation states of large numbers of individual platelets during thrombus formation in vivo.
Methods: Isolated platelets labelled with a platelet marker and a calcium indicator were injected into vehicle‐ or inhibitor‐treated recipient mice used for intravital microscopy. Laser injuries with precisely defined sizes and positions were generated in mesenteric veins and 3D time‐lapse data was acquired using a NikonA1R confocal platform. Image data was processed using a neural network trained for platelet segmentation. Pattern recognition algorithms based on gaussian kernel density functions and support vector regression were applied to identify spatiotemporal clusters of platelet activities during thrombus development.
Results: The application of our model enabled accurate spatial and temporal mapping of platelet recruitment and shedding, platelet packing density, platelet‐fibrin interactions and platelet intracellular calcium mobilization during thrombus formation. Further, 3D tracking of millions of observations of individual platelets combined with pattern recognition algorithms enabled a systematic and unbiased analysis of how coordinated platelet movements are generated by the propagation of multiple stimulatory waves of platelet signalling within a thrombus. Our results identify discrete clusters of non‐canonical platelet activities which shape thrombus architecture.
Conclusion(s): Large‐scale tracking of individual platelets during thrombus formation in vivo provides an unprecedented level of detail about the complex processes shaping thrombus formation. The analytical tools developed will help to refine our conceptual understanding of the hemostatic response to injury.
Platelet Proteomics and Genomics
OC 35.5
Quantitative phosphoproteomics and causal analysis reveal distinct and combinatorial signaling mechanisms in protease‐activated receptor PAR1 and PAR4 platelet activation programs
S. Reitsma 1; I. Parra‐Izquierdo1; J. Pang1; A. Melrose1; T. Kohs1; A. Reddy1; P. Wilmarth2; L. David1; O. McCarty3; J. Aslan1
1 Oregon Health & Science University, Portland, Oregon, United States; 2 Oregon health & Science University, Portland, Oregon, United States; 3 Oregon Health and Science University, Portland, Oregon, United States
Background: Intracellular signaling pathways downstream of platelet protease‐activated receptors (PARs) mediate hemostasis and, also contribute to thrombosis in vascular diseases through mechanisms that remain unspecified.
Aims: Here, we assess the hypothesis that platelet PAR1 and PAR4 each activate specific, as well as overlapping signaling systems to drive platelet responses underlying hemostasis and thrombosis. Our systems biology approach incorporates state‐of‐the‐art mass spectrometry, computational and cell physiological tools to measure and map phosphorylation events in platelet PAR responses.
Methods: Following platelet isolation from n = 4 healthy human donors (in accordance with Helsinki guidelines), washed platelets were stimulated with PAR1 agonist (TRAP6), PAR4 agonist (AYPGFK), thrombin, or vehicle, prior to lysis, digestion, phosphopeptide enrichment and 16plex tandem‐mass‐tag (TMT) labeling.
Results: Relative to resting platelets, we measured >1,000 significant phosphorylation events in response to PAR agonists (fold‐change >1.5; false discovery rate <0.01), including >600 phosphorylation events common to TRAP6, AYPGFK and thrombin stimulation. These included phosphorylation of well‐established mediators (GSK3α, PAK2) and more novel and emerging effectors in platelet activation pathways (BIN2, NKX3‐2). Specific PAR1 and PAR4 agonist responses of mechanistic and translational interest were also noted, including phosphorylation of PAR1 T410 or PAR4 S369; thrombin uniquely activated tyrosine kinase Fer Y714 phosphorylation, in a manner that may integrate PAR1 and PAR4 signaling. Functional flow cytometry assays reveal a role of Fer Y714 in platelet function when inhibited with E260 Fer inhibitor. Furthermore, CausalPath analysis identified >100 signaling relations among site‐specific phosphorylation changes downstream of PARs, around MAPK, PI3K/Akt, mTOR/S6K and other pathways.
Conclusion(s): In conclusion, we provide a quantitative omics study and causal analysis of platelet PAR signaling, including specific PAR1 and PAR4 agonist responses. Ultimately, this work will help to specify essential effectors, as well as biomarkers and therapeutic targets in platelet dysregulation, hyperactivity and thrombotic diseases.
OC 49.1
Procoagulant platelets have a distinct transcriptome that identifies the large GTPase dynamin as a driver in their formation
I. Tohidi‐Esfahani 1; S. Whittaker2; C. Lee2; H. Campbell2; K. Lai3; H. Liang2; M. Kockx2; M. Larance4; V. CHEN5
1 University College London Hospital, London, England, United Kingdom; 2 ANZAC Research Institute, University of Sydney, Sydney, New South Wales, Australia; 3 University of Sydney, Sydney, New South Wales, Australia; 4 Charles Perkins Centre, School of Life and Environmental Sciences, Faculty of Science; University of Sydney, New South Wales, Australia, Sydney, New South Wales, Australia; 5 ANZAC Research Institute, University of Sydney; Department of Haematology, Concord Repatriation General Hospital, Sydney, New South Wales, Australia
Background: The procoagulant platelet subpopulation is involved more in pathological thrombosis than haemostasis.
Aims: This study aimed to identify unique pathways through transcriptomics that predispose platelets to become procoagulant after activation, to facilitate targeting this subpopulation.
Methods: Washed platelets from healthy donors (n = 6) were sorted into procoagulant (GSAO+/P‐selectin+) and activated non‐procoagulant (GSAO‐/P‐selectin+) subpopulations (Hua, Blood 2015), after thrombin 2 U/ml plus collagen‐related peptide 4 μg/ml stimulation, using flow cytometry cell sorting. RNA was extracted, sequenced (single‐end, 75 base‐pair reads), with differentially expressed (DE, fold‐change >/=2, false discovery rate/FDR <0.05) genes identified using DESeq2 and edgeR tools. DE genes were input into Ingenuity Pathway Analysis (IPA). Functional validation was undertaken for candidate gene, Dynamin 1. Procoagulant platelet proportions were assessed by flow cytometry and confocal microscopy with inhibitors MiTMAB and dynasore.
Results: DESeq2 analysis yielded 1024 DE genes with a higher proportion upregulated in procoagulant platelets. Subpopulations segregated after unsupervised hierarchical clustering (Figure 1A,B). qPCR of a subset of genes (n = 30) from different donors (n = 10) confirmed differential expression within subpopulations. IPA identified integrin signalling, endocytosis and actin cytoskeleton‐related pathways as highly enriched in procoagulant platelets (Figure 1C). Dynamin 1, involved in both endocytosis and actin rearrangement, was 4.9‐fold increased (FDR <0.05, both DE tools), confirmed by qPCR (3.4‐fold increased, p < 0.01, Figure 1D). Dynamin inhibition by MiTMAB led to a dose‐dependent, selective reduction in agonist‐induced procoagulant platelet formation by flow cytometry (Figure 1E), and platelet “balloon” morphology by confocal microscopy (vehicle 21.6 ± 0.7%, MiTMAB 1.7 ± 0.3%, p = 0.0003), while >99% of platelets remained activated (P‐selectin+). Alternative dynamin inhibitor, dynasore, confirmed dose‐dependent reduction in procoagulant platelet formation (n = 6, p < 0.0001). Together, these suggest functional validation of transcriptomic differences.
Conclusion(s): The procoagulant subpopulation is transcriptionally distinct compared to activated non‐procoagulant platelets. Functional validation of RNA‐sequencing results by dynamin inhibition strongly suggests transcriptional differences are involved in determination of functionally distinct platelet subpopulations.
Platelet Receptors
OC 77.4
Pharmacological targeting of GPV in a novel humanized mouse model to modulate thrombus formation in vivo
P. Öftering 1; S. Beck2; D. Stegner3; B. Nieswandt4
1 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Germany, Würzburg, Bayern, Germany; 2 University Hospital and Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 3 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 4 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: Platelet glycoprotein (GP) V is part of the GPIb‐V‐IX complex, which mediates the initial platelet recruitment to sites of vascular injury. Despite its abundant expression, the physiological function of GPV has remained elusive. We have recently shown that GPV spatio‐temporally controls fibrin formation and thereby provides a platelet‐based mechanism that locally limits excessive fibrin formation and thrombus growth (Beck et al., ID: 1201886). Thus, pharmacological modulation of GPV might provide a therapeutical strategy to treat both thrombotic and hemorrhagic disease states.
Aims: To generate a model system to pharmacologically modulate human GPV in vitro and in vivo.
Methods: The extracellular domain of mouse GPV was replaced by the human sequence and the resulting hGP5KI mice, as well as their platelets, were characterized. Monoclonal antibodies (mAbs) against human GPV (LUM/B, LUM/C, LUM3‐5) were generated and characterized.
Results: hGP5KI mice displayed normal expression levels of hGPV and all other tested platelet membrane glycoproteins. Also, their activation and aggregation responses to different agonists were unaltered compared to controls. Thrombin‐mediated cleavage of hGPV and protease‐activated receptor (PAR)‐dependent activation was indistinguishable from control platelets, and no hyperreactivity to thrombin, as seen with Gp5‐/‐ platelets, was observed. Screening of newly generated anti‐hGPV mAbs identified LUM/C to block the collagen‐binding site on hGPV and LUM/B to interfere with its thrombin‐induced cleavage, while all other mAbs had no effect on platelet function. In vivo, none of the LUM antibodies affected platelet counts in hGP5KI mice. However, LUM/B significantly accelerated fibrin formation and thrombosis in vivo, confirming the proposed role of GPV in these processes.
Conclusion(s): Together, these results demonstrate that hGP5KI mice are a valuable model system for studying the impact of GPV on thrombus formation in a translational approach. Furthermore, the LUM/B antibody very consistently modulates fibrin and thrombus formation by interfering with thrombin‐dependent cleavage of hGPV.
OC 77.5
Comprehensive analysis of human glycoprotein Ibα O‐glycosylation
M. Hollenhorst 1; K. Tiemeyer1; K. Mahoney2; K. Aoki3; M. Ishihara3; V. Rangel‐Angarita2; C. Bertozzi4; S. Malaker2
1 Stanford University, Palo Alto, California, United States; 2 Yale University, New Haven, Connecticut, United States; 3 University of Georgia, 4 Stanford University, Stanford, California, United States
Background: Platelet glycoprotein (GP) Ibα is the major ligand‐binding subunit of the platelet GPIb‐IX‐V complex that binds von Willebrand Factor (VWF). Human GPIbα is heavily glycosylated, with 58 predicted O‐glycosites (amino acid sites of O‐glycosylation). GPIbα O‐glycans have been hypothesized to play key roles in platelet biology, possibly modulating VWF binding and regulating platelet clearance. The O‐glycosylation profile of GPIbα has previously been largely uncharacterized.
Aims: The aim of this study was to comprehensively analyze GpIbα O‐glycosylation in a site‐specific manner.
Methods: Two different preparations of the human GpIbα ectodomain were analyzed: (1) commercially available recombinant protein, and (2) endogenous protein purified from platelets. GpIbα ectodomain was digested using a combination of commercially available proteases and bacterial mucinase enzymes. Glycopeptides were analyzed by higher‐energy collisional dissociation (HCD) triggered electron‐transfer/higher‐energy collision dissociation (EThcD) mass spectrometry.
Results: We identified a total of 65 unique O‐glycosites in recombinant GpIbα (Figure 1). 12 O‐glycosites are located within the mucin domain and 11 within the VWF ligand binding domain (LBD). We identified a total of 30 O‐glycosites in endogenous GpIbα, including 11 within the mucin domain and one within the VWF LBD. In the analysis of the endogenous protein, intact O‐glycan masses were observed that were consistent with both sialylated and non‐sialylated core 1, core 2, Tn antigen, and ABO blood group antigens.
Conclusion(s): This is the first study to report a comprehensive analysis of GpIbα O‐glycosylation. This information lays the foundation for further studies to determine the functional and structural implications of GpIbα O‐glycosylation. FIGURE 1 Platelet glycoprotein (GP) Ibα O‐glycosites.
FIGURE 1 Platelet glycoprotein (GP) Ibα O‐glycosites.
OC 41.5
A novel and unique mechanism of αIIbβ3 regulation in platelets
C. Kusch1; D. Stegner2; D. Johnson 3; M. Meub4; P. Nurden5; C. Gross6; D. Semeniak6; J. Schenk7; M. Bender8; T. Vögtle9; K. Heinze7; M. Sauer10; A. Nurden5; B. Nieswandt8
1 Institute of Experimental Biomedicine I, University Hospital and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 2 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 3 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 4 Department of Biotechnology and Biophysics, Biocenter, University of Würzburg, Würzburg, Bayern, Germany; 5 Institut Hospitalo‐Universitaire LIRYC, Hôpital Xavier Arnozan, Pessac, Pessac, Aquitaine, France; 6 Institute of Experimental Biomedicine I, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 7 Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 8 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 9 Institute of Experimental Biomedicine I, University Hospital Würzburg, Würzburg, Bayern, Germany; 10 Department of Biotechnology and Biophysics, Biocenter, University of Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany
Background: Integrins are heterodimeric adhesion molecules which mediate cell‐cell and cell‐extracellular matrix interactions. As the dominant platelet integrin, αIIbβ3 plays a central role in in haemostasis, thrombosis, and inflammation.
Aims: In this study, we report a previously unrecognized pathway of αIIbβ3 regulation in platelets that is triggered by monoclonal antibodies (mAbs) against the integrin.
Methods: In vitro and in vivo studies were performed with anti‐αIIbβ3 mAbs (MWReg30, JON mAbs). MWReg30 (2 μg/g body weight) was administered intravenously to Fcgr3‐/‐, Fcgr3‐/‐:FcgR2b‐/‐ or Fcgr3‐/‐:Fcgr2bYF/YF mice and the fate of platelets was monitored ex vivo and in vivo.
Results: Binding of anti‐αIIbβ3 mAbs almost instantaneously induced clustering and capping of the integrin in platelets and the opsonized platelets were rapidly recruited to the liver in vivo. To avoid their clearance by Fcγ receptor (FcγR)‐bearing immune cells, further studies were performed in mice lacking FcγRIII (Fcgr3‐/‐). Ex vivo analysis of platelets following MWReg30 infusion demonstrated a virtually complete loss of αIIbβ3 and a ~50% reduction in CD9. As a result, the animals displayed a Glanzmann thrombasthenia (GT)‐like phenotype. To study this paradoxical loss of αIIbβ3/CD9 in situ, we performed intravital confocal laser‐scanning microscopy (IV‐LSM) of the liver. Almost immediately after injection of MWReg30, the opsonized platelets attached via the antibody Fc‐part to the inhibitory FcγRIIB on liver sinusoidal endothelial cells (LSEC). The targeted integrins clustered at the platelet ‘rear edge’ and finally segregated within a long protrusion or tether. The αIIbβ3‐devoid platelet body detached and returned into the circulation (Fig. 1). The flow‐dependent release of αIIbβ3/CD9‐enriched tethers (termed Platelet‐derived Integrin and Tetraspanin‐enriched Tethers, PITTs) was reproduced in vitro by employing surface‐adsorbed antibodies.
Conclusion(s): This data reveals a novel insight for integrin regulation by mechanosensing in platelets and presumably other cells. This work was supported by TR240 grant with project number 374031971 and BE5084/5‐1.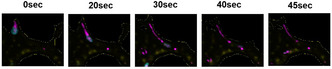 FIGURE 1 Intravital confocal laser‐scanning microscopy of the liver reveals the formation and deposition of PITTs on liver sinusoidal endothelial cells (LSECs) in mice and release of αIIbβ3‐depleted platelets which return to the circulation. WT platelets were labeled in vitro with MWReg30AF488 and anti‐GPIXAF546 and transfused into FcgR3‐/‐ mice. The vasculature was stained with anti‐CD105AF647 antibody.
FIGURE 1 Intravital confocal laser‐scanning microscopy of the liver reveals the formation and deposition of PITTs on liver sinusoidal endothelial cells (LSECs) in mice and release of αIIbβ3‐depleted platelets which return to the circulation. WT platelets were labeled in vitro with MWReg30AF488 and anti‐GPIXAF546 and transfused into FcgR3‐/‐ mice. The vasculature was stained with anti‐CD105AF647 antibody.
OC 10.2
Antibody‐mediated depletion of human CLEC‐2 in a novel humanized mouse model
H. Brown1; S. Beck 2; S. Navarro1; S. Watson3; B. Nieswandt4; D. Stegner5
1 University Hospital Würzburg, Würzburg, Bayern, Germany; 2 University Hospital and Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 3 University of Birmingham, Birmingham, UK, Birmingham, England, United Kingdom; 4Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 5 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany
Background: Platelet C‐type lectin‐like receptor 2 (CLEC‐2) is a unique platelet activation receptor signaling through a hemITAM motif. Due to its roles in thrombosis, tumor metastasis and inflammation CLEC‐2 has been suggested as therapeutic target. However, there are currently no methods to test potential human therapeutics targeting CLEC‐2, such as antibodies, in vivo.
Aims: We have therefore generated and characterized a humanized CLEC‐2 mouse (hCLEC‐2KI) and developed a novel monoclonal anti‐human CLEC‐2 antibody, HEL1, for in vivo testing.
Methods: Murine CLEC‐2 was replaced with the human variant and the mice, as well as their platelets characterized in vitro and in vivo. HEL1 was generated by hybridoma technology following immunization of rats with immunoprecipitated human CLEC‐2. HEL1 was characterized using human and hCLEC‐2KI platelets. CLEC‐2 regulation was assessed using the anti‐hCLEC‐2 monoclonal antibodies AYP1 and HEL1.
Results: hCLEC‐2KI mice were viable, fertile and born at Mendelian ratio, without evidence of blood‐lymphatic mixing. hCLEC‐2KI platelets displayed glycoprotein receptor expression, activation and aggregation comparable to wildtype controls. Likewise, tail bleeding times of hCLEC‐2KI mice were like those of WT mice. The novel antibody HEL1 activates platelets upon binding to CLEC‐2. However, HEL1‐fab fragments neither activate platelets nor affect rhodocytin‐induced platelet activation. Challenging hCLEC‐2KI mice with HEL1‐ or AYP1‐IgG, which bind different epitopes, resulted in a transient thrombocytopenia as well as CLEC‐2 depletion for more than 2 weeks, but did not affect hemostasis.
Conclusion(s): Here, we provide proof of principle that the hCLEC‐2KI mouse can be used to test anti‐hCLEC‐2 agents in vivo. In addition, we demonstrated that hCLEC‐2 can be immunodepleted. FIGURE 1 Antibody‐mediated depletion of human CLEC‐2 in a novel humanized mouse model. The novel anti‐human CLEC‐2 antibody HEL1 binds a different epitope than established AYP1 antibody, however, both antibodies activate platelets. In vivo, injection of either anti‐CLEC‐2 antibody in hCLEC‐2KI mice, that express human instead of murine CLEC‐2, results in CLEC‐2 depletion from the platelet surface for more than 2 weeks (parts of the figure were created in BioRender).
FIGURE 1 Antibody‐mediated depletion of human CLEC‐2 in a novel humanized mouse model. The novel anti‐human CLEC‐2 antibody HEL1 binds a different epitope than established AYP1 antibody, however, both antibodies activate platelets. In vivo, injection of either anti‐CLEC‐2 antibody in hCLEC‐2KI mice, that express human instead of murine CLEC‐2, results in CLEC‐2 depletion from the platelet surface for more than 2 weeks (parts of the figure were created in BioRender).
OC 41.1
Platelet αIIbβ3 integrin is produced in different covalent forms that have different functions
A. Pijning 1; M. Blyth2; M. Coote2; F. Passam3; J. Chiu4; P. Hogg4
1 Centenary Institute, Sydney, New South Wales, Australia; 2 Australian National University, Canberra, Australian Capital Territory, Australia; 3 Heart Research Institute, University of Sydney, Sydney, New South Wales, Australia; 4 Centenary Institute, University of Sydney, Sydney, New South Wales, Australia
Background: The αIIbβ3 integrin receptor coordinates platelet adhesion, activation, and mechano‐sensing in thrombosis and haemostasis. We identified covalent forms of αIIbβ3 integrin on healthy human platelets that differ from each other by the absence of specific disulfide bonds. A covalent form of the αIIb subunit missing the disulfide bond linking cysteines 490–545 comprises ~1 in 3 molecules on resting and activated platelets, whereas a covalent form of the β3 subunit missing the bond linking cysteines 177–184 comprises ~1 in 2 molecules. These covalent forms of αIIbβ3 are pre‐determined, as they are also produced by megakaryoblasts and transfected cells.
Aims: To determine the function and significance of alternate covalent forms of αIIbβ3 integrin.
Methods: Distribution of the alternate αIIbβ3 forms on platelets was measured by co‐immunoprecipitation and differential cysteine alkylation and mass spectrometry. The functions of the covalent forms were evaluated in cells expressing a mutant integrin with an ablated bond and by molecular dynamics (MD) simulations.
Results: The covalent form missing the αIIb Cys490‐Cys545 bond functions in focal adhesions where it has extended residency time due to decreased clathrin‐mediated internalisation. MD simulations revealed that absence of this bond propagates mechanical forces into the knee joint, resulting in a higher energy barrier for cycling between integrin conformations. The covalent form missing the β3 Cys177‐Cys184 disulfide bond does not bind the integrin’s physiological ligands. MD simulations indicate that absence of the bond has allosteric effects on the βI‐domain metal‐binding sites.
Conclusion(s): Megakaryocytes produce several covalent forms of αIIbβ3 integrin that have different functions. The disulfide bonds described here are conserved in all 18 integrin α subunits and 7 β subunits, suggesting that covalent regulation is conserved across integrins. The prevalence of different covalent forms of αIIbβ3 integrin varies considerably amongst individuals, which could contribute to the variability in thrombotic propensity in humans.
Platelet Signaling
OC 10.5
Aging activates platelet mTOR to induce platelet hyperreactivity
I. Portier; B. Manne; Y. Kosaka; F. Denorme; R. Campbell
University of Utah, Salt Lake City, Utah, United States
Background: Aging is an independent risk factor for the development of cardiovascular disease. We hypothesized that aging promotes platelet hyperreactivity and increases thrombotic risk.
Aims: In this study, we examined the mammalian target of rapamycin (mTOR) as a mechanistic regulator of platelet hyperreactivity in aging.
Methods: Platelets from young (<45 years) and aged (>70 years) humans were isolated to examine platelet aggregation and mTOR signaling. Young (8–12 weeks) and aged (>18 months) wild‐type (WT; mTORfl/fl) and platelet‐specific mTOR knockout mice (KO; mTORfl/fl‐Pf4‐Cre) were used to examine platelet activation. A microvascular thrombosis model using collagen and epinephrine was employed.
Results: In aged humans and mice, we observed significant (p < 0.01) basal activation of the mTOR pathway, including phosphorylation of AKT, 70S6K and 4E‐BP1. Aged platelets from humans and mice had significantly greater integrin activation and P‐selectin expression when activated. Additionally, aged human and murine platelets had significant greater aggregation compared to younger controls. Inhibition of mTOR either with torin‐1 in aged humans or genetic deletion in aged mice reversed this platelet hyperreactivity. In a collagen‐epinephrine thrombosis model, aged WT mice succumbed significantly (p < 0.001) faster compared to young WT mice, while aged KO mice had similar times to death as young WT mice. Mechanistically, we observed a significant increase in RAC‐GTP (p < 0.05) and phospho‐p47phox (p < 0.01) downstream of mTOR activation in aged WT mice. Additionally, we observed increased reactive oxygen species (ROS) in resting platelets and megakaryocytes in aged WT mice compared to young WT and young or aged KO mice. Increased ROS led to significantly greater phospho‐p38 and thromboxane generation in aged WT mice compared to young controls (p < 0.05). Phospho‐p38 and thromboxane generation upon activation were unchanged when comparing aged and young KO mice.
Conclusion(s): Aging induces platelet mTOR activation, leading to increased platelet hyperreactivity mediated through increased activation of p38 and thromboxane generation.
OC 35.3
Protein tyrosine phosphatase PTPN22 modulates platelet function and thrombosis
J. Qiao; X. Wang; G. Wei; L. Zeng; K. Xu
Xuzhou Medical University, Xuzhou, Jiangsu, China (People's Republic)
Background: Protein tyrosine phosphatase non‐receptor type 22 (PTPN22) is a protein tyrosine phosphatase and negatively regulates T cell signaling. However, whether it is expressed and functions in platelets remains unknown.
Aims: To investigate the expression and role of PTPN22 in platelet function.
Methods: Platelets were isolated from WT or PTPN22‐deficient mice to measure platelet aggregation, granule secretion, spreading and clot retraction along with the measurement of tail bleeding time, arterial and venous thrombus formation. In addition, quantitative phosphoproteomic analysis was conducted to indentify the potential target of PTPN22. Moreover, human platelets were treated with PTPN22 inhibitor LTV‐1 to measure platelet function.
Results: PTPN22 expression in both human and mouse platelets. Using PTPN22‐/‐ mice, we demonstrated that PTPN22 deficiency significantly shortened tail‐bleeding time and accelerated arterial thrombus formation without affecting venous thrombosis and the coagulation factor VIII and IX. Consistently, PTPN22‐deficient platelets exhibited enhanced platelet aggregation, spreading and clot retraction. Quantitative phosphoproteomic analysis revealed the significant difference of phosphodiesterase 5A (PDE5A) phosphorylation in PTPN22‐deficient platelets compared to wild‐type platelets after collagen‐related peptide (CRP) stimulation, which was confirmed by increased PDE5A phosphorylation (Ser92) in CRP‐treated PTPN22‐deficient platelets, concomitant with reduced cGMP level and VASP phosphorylation (Ser157/239). In addition, PTPN22 interacted with phosphorylated PDE5A (Ser92) and dephosphorylated it in activated platelets. Moreover, purified PTPN22 but not mutant form (C227S) possesses intrinsic serine phosphatase activity. Furthermore, inhibition of PTPN22 enhanced human platelet aggregation, spreading, clot retraction and increased PDE5A phosphorylation (Ser92). Finally, PTPN22 expression was significantly reduced in platelets of coronary artery disease patients.
Conclusion(s): In conclusion, our study demonstrates a novel role of PTPN22 in platelet function and arterial thrombosis, identifying new potential targets for future prevention of thrombotic or cardiovascular diseases.
OC 35.4
Critical role for RapGEF2 in PKC‐Rap1‐integrin signaling in platelets
D. Paul 1; E. Clark2; A. Beale2; W. Bergmeier2
1 University of North Carolina at Chapel Hill, UNC Blood Research Center, Chapel Hill, North Carolina, United States; 2 University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States
Background: The small GTPase Rap1 is a crucial regulator of platelet integrin activation, a critical event during the formation of both hemostatic and thrombotic plugs. Rap1 activation in response to cellular stimulation is controlled by guanine nucleotide exchange factors (GEFs). In platelets, CalDAG‐GEFI (RasGRP2) is the most highly expressed and functionally dominant GEF. However, genome‐wide association studies (GWAS) also suggest a significant role for RapGEF2 (PDZ‐GEFI) in platelet integrin inside‐out activation and coronary artery disease (CAD).
Aims: To examine the role of RapGEF2 in Rap1‐integrin signaling in platelets.
Methods: In this study we used mice deficient in RapGEF2 (Rapgef2‐mKO), CalDAG‐GEFI (Caldaggef1‐/‐), or both (DKO) to characterize the contribution of both proteins to platelet function. In vitro assays of integrin activation, granule secretion, platelet aggregation and Rap1 activation were used to assess platelet activation, while in vivo platelet function was assessed by saphenous vein laser injury and ferric chloride carotid injury.
Results: Rapgef2‐mKO platelets activated with various agonists showed minor defects in platelet integrin inside‐out activation. In contrast, integrin‐mediated aggregation in Caldaggef1‐/‐ platelets occurred with a delay and was dependent on protein kinase C (PKC) signaling and RapGEF2. PKC/RapGEF2 signaling was found to be critical for sustained Rap1 signaling. Consistent with these in vitro findings, thrombosis and hemostatic plug formation were markedly impaired in Caldaggef1‐/‐ but not Rapgef2‐mKO mice. However, hemostatic plugs in DKO mice were more unstable and bleeding times were significantly longer when compared to Caldaggef1‐/‐ mice.
Conclusion(s): In summary, here we provide genetic evidence that RapGEF2 operates downstream of PKC as an important regulator of sustained Rap1 signaling and integrin activation in platelets. Our findings are supportive of GWAS data suggesting a key role for RapGEF2 in platelet function and may have important implications for risk assessment and prevention strategies in CAD.
OC 35.2
The role of adenylyl cyclase 6 in platelet cyclic‐AMP signalling
B. Webb; M. Hindle; L. Cheah; N. Turner; K. Naseem
University of Leeds, Leeds, England, United Kingdom
Background: Platelet activation and haemostasis are constrained by endothelial‐derived prostacyclin (PGI2) acting through a cyclic adenosine‐5’‐monophosphate (cAMP) signalling pathway. Cyclic‐AMP signalling in platelets involves a complex network of multiple isoforms of both adenylyl cyclase (AC) and protein kinase A (PKA).
Aims: In the present study, we characterised the role of AC6, the predominant isoform in both human and murine platelets, on platelet function.
Methods: A platelet specific AC6 deficient mouse (AC6‐KO) was created using a Cre‐recombinase approach. Light transmission aggregometry, flow cytometry and intravital microscopy were used to assess platelet function. Western Blotting and EIA techniques were also used to assess downstream cAMP signalling and cAMP production, respectively.
Results: No difference in basal cAMP concentrations was observed between AC6‐KO and littermate controls, suggesting that AC6 does not control basal cAMP production. In contrast, AC6‐KO showed significantly reduced responses to PGI2 induced cAMP generation, although this was not completely ablated. Consistent with this data, the phosphorylation of key PKA substrates phosphoVASPSer239, phosphoVASPSer157 and glycogen synthase kinase beta were impaired in AC6‐KO platelets in response to both PGI2 and forskolin compared to controls. Having found that cAMP signalling was compromised in the absence of AC6, we examined its functional importance. In vitro studies showed that the absence of AC6 compromised the ability of PGI2 control thrombin, but not collagen activated platelets. The ability of PGI2 to inhibit thrombin‐induced aggregation, fibrinogen binding and P‐selectin expression was also impaired in AC6‐KO platelets. In vivo studies indicated an accelerated rate of thrombosis in response to ferric chloride injury.
Conclusion(s): These data confirm a key role of AC6 in controlling cAMP‐mediated platelet regulation in vitro and in vivo. However, given that the effects of PGI2 and cAMP signalling are not ablated, suggests that the regulation of haemostasis and thrombosis is linked to multiple AC isoforms.
OC 09.2
An extensional strain‐sensing mechanosome drives rapid adhesion‐independent platelet activation at supraphysiological hemodynamic gradients
N. Zainal Abidin 1; E. Poon2; C. Szydzik3; M. Timofeeva4; F. Akbaridoust4; R. Brazilek3; F. Tovar Lopez5; X. Ma6; C. Lav4; I. Marusic4; P. Thompson6; A. Mitchell5; A. Ooi4; J. Hamilton3; W. Nesbitt3
1 Monash University, Melbourne, Victoria, Australia; 2 St Vincent’s Hospital, University of Melbourne, Fitzroy, Victoria, Australia; 3 Australian Centre for Blood Diseases, Monash University, Melbourne, Victoria, Australia; 4 Dept of Mechanical Engineering, University of Melbourne, Melbourne, Victoria, Australia; 5 School of Engineering, RMIT University, Melbourne, Victoria, Australia; 6 Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia
Background: Severe stenosis or mechanical circulatory support devices impose supraphysiological strain on blood, leading to platelet thromboembolism. Current anti‐platelet therapies have limited efficacy, highlighting the need for more effective targeted treatments. Localised shear stress is known to drive thrombus formation mediated by adhesion receptors GPIb/V/IX and integrin αIIbβ3, but the exact mechanics of how platelets respond to haemodynamic gradients in free‐flow, independent of adhesion receptors is unknown.
Aims: We investigate the impact of supraphysiological hemodynamics on platelet activation and function and the signalling pathways involved.
Methods: We utilised axisymmetric step and hyperbolic microfluidic geometries that expose platelets to flow accelerations and defined extensional strain, respectively. We assessed real‐time calcium signalling by perfusing CAL520‐loaded platelets reconstituted +/− red blood cells through these platforms. Confocal imaging at defined regions of interest were normalised against +ve and −ve controls to obtain [Ca2+]c in nM. Platelet aggregation was measured by perfusing DiOC6‐labelled whole blood through stepped microchannels selectively coated with VWF, with aggregate dynamics over 180s quantified. Using a pharmacologic approach, we investigated the mechanotransduction mechanism underpinning platelet activation.
Results: Acute supraphysiological shear gradients trigger transient platelet [Ca2+]c flux that is attuned to extensional strain (ε) at flow acceleration, directly affecting immediate downstream platelet aggregation. Pharmacological screening indicates the [Ca2+]c flux occurs independently of adhesion receptor engagement and canonical pathways but is mediated by Piezo1 mechano‐sensing coupled to P2X1‐dependent signal amplification. We demonstrate a critical role for type II PI3KC2α in coupling the mechanosome to applied ε.
Conclusion(s): ε at flow acceleration plays a critical role in triggering adhesion‐independent platelet activation. We describe a novel ‘ε sensing mechanosome’ driven by mechanical activation of Piezo1 coupled to subsequent [Ca2+]c influx through P2X1, which primes platelets for immediate downstream aggregation. The class II PI3KC2α isoform plays a critical role in coupling the mechanosome to externally applied ε.
Vascular Biology
Blood Cells and Vessel Wall
OC 68.4
CD39‐bearing extracellular vesicles constrain platelet purinergic signaling‐dependent pulmonary thrombosis in sickle cell disease
T. Brzoska; E. Menchikova; T. Kaminski; S. Tofovic; E. Jackson; M. Gladwin; P. Sundd
University of Pittsburgh, Pittsburgh, Pennsylvania, United States
Background: Acute chest syndrome (ACS) is a type of acute lung injury and the leading cause of mortality among sickle cell disease (SCD) patients. ACS is often preceded by thrombocytopenia and involves massive thrombosis across pulmonary artery branches. Although, released during hemolysis, adenosine diphosphate (ADP) is known to activate platelets by stimulating their P2Y1 and P2Y12 purinergic receptors, antagonists of P2Y12 have not shown any benefit in ACS therapy. CD39 maintains ADP homeostasis by degrading excessive ADP. Though CD39 inhibits ADP‐dependent platelet activation, its role in the pathophysiology of SCD is still unidentified.
Aims: To assess CD‐39‐dependent platelet purinergic signaling in SCD.
Methods: To evaluate SCD platelet response to ADP in vivo we used a state‐of‐the‐art intravital lung microscopy and a novel in vivo model of ADP‐triggered thrombocytopenia in transgenic humanized SCD mice. Additionally, both mouse and human SCD platelet ADP‐dependent aggregation was examined using in vitro turbidimetric aggregation assay. Human Lung Microvascular Endothelial Cells (HMVEC‐L) were treated with hemin. Extracellular vesicles (EVs) were obtained from plasma and cell culture samples using size exclusion chromatography and subjected to nanoparticle tracking analysis. CD39 levels and activity were determined using ELISA, western blot and malachite green phosphate assays, respectively.
Results: Intravital lung microscopy and in vivo thrombocytopenia studies revealed that intravascular administration of ADP triggered acute pulmonary thrombosis in control but not in SCD mice. In vitro aggregation study demonstrated impaired SCD mouse and human platelet response to ADP, which was significantly augmented by a CD39 inhibitor. Hemin triggered shedding of CD39‐bearing EVs by HMVEC‐L. Endothelial cell‐derived SCD EVs, from mouse and human plasma, expressed higher CD39 levels and activity in comparison to control EVs.
Conclusion(s): Our findings suggest that CD39‐bearing EVs prevent ADP‐mediated platelet aggregation and pulmonary thrombosis in SCD. Current study explains why P2Y12 blockers are not effective in SCD therapy.
OC 38.4
Preventing CD62P‐mediated leukocyte infiltration and activation enhances thrombus resolution in mice
J. Kral‐Pointner 1; P. Haider2; P. Szabo3; C. Kaun2; M. Brekalo2; M. Salzmann1; K. Schneider3; S. Bleichart4; A. Kiss3; C. Hengstenberg5; C. Brostjan4; H. Bergmeister3; A. Assinger1; B. Podesser3; J. Wojta1; P. Hohensinner3
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Department of Internal Medicine II, Division of Cardiology/Medical University of Vienna, Vienna, Wien, Austria; 3 Center for Biomedical Research, Medical University of Vienna, Vienna, Wien, Austria; 4 Department of Surgery, Medical University of Vienna, Vienna, Wien, Austria; 5 Department of Internal Medicine II/Medical University of Vienna, Vienna, Wien, Austria
Background: Deep vein thrombosis and its complication pulmonary embolism and is a major health problem with an average annual incidence rate of 104–183 per 100 000 person‐years. After thrombus formation its resolution is essential to re‐establish blood flow.
Aims: In this funded study (Austrian Science Fund (FWF)), we aim to analyse the effect of CD62P‐mediated cell migration and activation on thrombus resolution post thrombus formation.
Methods: Thrombus formation was induced by inferior vena cava ligation and mice were treated after 1 day with a CD62P‐blocking antibody or isotype. The thrombus and the surrounding vessel were extracted for immunohistochemistry or flow cytometry. Data were analysed by unpaired Student’s t‐test or ANOVA.
Results: Localising neutrophils and macrophages in the thrombotic lesion revealed that they enter the thrombus and vessel wall from the caudal site. Neutrophils were predominantly present one day and monocytes/macrophages three days after vessel ligation. As leukocyte extravasation is promoted by endothelial and platelet CD62P, we blocked CD62P at day 1 after thrombus formation. This reduced aggregates between platelets and neutrophils or Ly6Chigh monocytes compared to isotype‐treated controls, leading to diminished neutrophils and Ly6Chigh monocytes in the cranial thrombus part. Continuous observation of thrombus volume by ultrasound revealed an accelerated thrombus breakdown after blocking CD62P, confirmed by decreased thrombus weight and length. To identify CD62P‐mediated effects on thrombus structure, we applied scanning electron microscopy and observed reduced fibrin density in thrombi of anti‐CD62P‐antibody‐treated mice. Corresponding, we found reduced tissue factor expression associated with macrophages and reduced neutrophil activation after CD62P inhibition.
Conclusion(s): We propose a CD62P‐mediated cross talk of vessel wall, platelets, monocytes and neutrophils resulting in activation of innate immune cells and increased tissue factor expression. This initial activation of immune cells strengthens the thrombus and delays subsequent resolution processes.
OC 38.5
Oxidized protein disulfide isomerase links oxidative stress to thrombus formation
M. Yang 1; S. Khan1; M. Chinnaraj2; C. Scartelli1; S. Patel1; K. Carroll3; N. Pozzi2; B. Smith4; R. Flaumenhaft1
1 Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States; 2 Saint Louis University, St. Louis, Missouri, United States; 3 Scripps Research Institute, Jupiter, Florida, United States; 4 Medical College of Wisconsin, Milwaukee, Wisconsin, United States
Background: Oxidative stress contributes to thrombosis in atherosclerosis, inflammation, infection and malignancy. How oxidants induce thrombus formation, however, is poorly understood. Protein disulfide isomerase (PDI) is a prothrombotic redox‐responsive enzyme. Yet the redox form of PDI responsible for thrombus formation is not known.
Aims: We hypothesized that oxidized PDI (oxPDI) transduces oxidative stress into a prothrombotic response.
Methods: Biochemical protein and enzymatic assays, platelet aggregometry, and in vivo murine thrombosis studies were used.
Results: Exposure of PDI to oxidants such as H2O2 and oxLDL resulted in loss of free thiols in PDI. Oxidants promoted generation of sulfenylated PDI, which spontaneously converted to disulfided PDI capable of transferring disulfides to substrate proteins. Evaluation of mutant PDIs and PDI fragments showed that sulfenylation required both PDI catalytic and substrate binding domains. While PDI alone failed to stimulate platelet aggregation, it augmented platelet aggregation induced by oxLDL, but not other agonists. The evaluation of oxPDI in vivo is complicated by the fact that infused oxPDI is quickly modified by the plasma redox environment. To circumvent this issue, we used LOC14, a compound that selectively binds PDI and forces it into an oxidized state. Evaluation by smFRET confirmed the oxidized conformation of PDI following LOC14 incubation and differential alkylation demonstrated the disulfided state. LOC14 enhanced platelet aggregation and this augmentation was inhibited by anti‐PDI antibodies. When infused into mice, LOC14 stimulated both platelet accumulation (4.6‐fold) and fibrin formation (2.4‐fold) following laser injury. OxLDL also markedly promoted both platelet accumulation and fibrin formation in this model and inhibition of PDI blocked oxLDL‐mediated thrombosis.
Conclusion(s): Structural elements within different PDI domains enable efficient conversion of oxygen free radicals to disulfides and transfer of these disulfides to substrate proteins. oxPDI is the prothrombotic form of the enzyme and serves as a link between oxidant stress and thrombus formation.
OC 38.3
Different contribution of monocyte‐ and platelet‐derived microvesicles to endothelial behavior
M. Brambilla 1; M. Talmon2; P. Canzano1; L. Fresu2; M. Conti3; A. Becchetti1; E. Tremoli4; M. Camera5
1 Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy; 2 University of Piemonte Orientale, Novara, Piemonte, Italy; 3 centro cardiologico monzino, milan, Lombardia, Italy; 4 Maria Cecilia Hospital, Cotignola, Toscana, Italy; 5 Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Circulating microvesicles (MVs) are recognized as both biomarkers and mediators of inflammation and endothelial dysfunction in many pathological conditions, including cardiovascular diseases. The two main MV‐subsets mediating these effects are those derived from monocytes and platelets (PLT), although the specific contribution of each of them is not well defined.
Aims: To assess how MVs released from PLT and monocytes influence processes involved in the vessel damage ‐i.e. oxidative stress, inflammation, leukocyte‐endothelial adhesion.
Methods: PLT and monocytes were isolated from healthy subjects (HS, n = 10) and acute myocardial infarction patients (AMI; n = 10). MVs, spontaneously released from AMI cells and those from stimulated HS‐platelet (TRAP‐6 20 μM, 30 min, 37°C) and ‐monocytes (LPS 10μg/ml, 16h, 37°C) were isolated, characterized by flow cytometry, and added to the culture medium of human vascular endothelial cells (hECV). Superoxide anion production, inflammatory markers (IL6, TNFalpha, NF‐κB mRNA expression), and hECV adhesiveness after MVs challenge were evaluated.
Results: Incubation of hECV with MVs released by activated PLT and monocytes triggered an oxidative burst (3‐fold increase) in a MV concentration‐dependent manner. Monocyte‐MVs doubled IL6, TNFalpha, and NF‐κB mRNA expression and monocyte‐endothelial adhesion which were only slightly influenced by PLT‐MVs. Interestingly, only AMI PLT‐MVs were able to affect both the redox state and the inflammatory phenotype (two‐fold increase). Conversely, AMI‐monocyte‐MVs upregulated hECV ICAM gene expression and twice the number of adhering monocytes. These functional effects were paralleled by an antigenic signature which reflected the overall activation status of parental cells, which results in an increased release of procoagulant and PLT‐Psel+ and monocyte‐CD16+‐derived MVs compared to HS spontaneously‐released MVs.
Conclusion(s): These data provide evidence that MVs derived from activated PLT and monocytes differently affect endothelial behavior. These functional effects mirror those induced by microvesicles spontaneously released by platelets and monocytes from AMI patients and support the antiplatelet treatment in this clinical setting.
OC 68.5
Neutrophil extracellular traps as a mediator of cerebral ischemia and reperfusion injury in ischemic stroke
M. De Wilde 1; S. Staessens1; L. Desender1; C. Tersteeg2; K. Vanhoorelbeke2; S. De Meyer2
1 Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, Kortrijk, West‐Vlaanderen, Belgium; 2 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium
Background: Thromboinflammatory processes are involved in cerebral ischemia/reperfusion injury in ischemic stroke, likely with neutrophil extracellular traps (NETs) as a mediator. However, the role of NETs in cerebral ischemia/reperfusion injury is yet to be clarified. In addition, the link between von Willebrand factor (VWF) and neutrophil recruitment in the ischemic brain might play a role in thromboinflammation, possibly by the formation of NETs.
Aims: Therefore, our aim was to investigate the involvement of NETs in cerebral ischemia/reperfusion injury and to explore the potential regulation of NETosis by VWF and the VWF‐cleaving protease ADAMTS13 in cerebral ischemia/reperfusion injury.
Methods: The filament‐induced transient middle cerebral artery occlusion model was used to induce 60 minutes focal cerebral ischemia in wild‐type (WT) and littermate Vwf‐knockout (KO) and Adamts13‐KO mice after which reperfusion was allowed. At different timepoints post‐ischemia, NETs (Ly6G, H3Cit, DNA) were identified in the mouse brains using quantitative immunofluorescence microscopy.
Results: NETs could be identified in the ipsilateral brain hemisphere. Interestingly, quantification of NETs at different timepoints, revealed that NETs can already be detected at 6 h, peak around 24 h and started to disappear again 48 h post‐ischemia. Remarkably, NETs were predominantly localized within the brain vasculature at different timepoints (12, 24, 48 h) post‐ischemia and to a lesser extent in the brain parenchyma, suggesting that NETs might play a role in secondary microthrombosis. Strikingly, NET formation was significantly decreased in Vwf‐KO mice and significantly increased in Adamts13‐KO mice compared to littermate WT mice 24 h post‐ischemia.
Conclusion(s): Our results put forward NETs as a thromboinflammatory mediator and indicate a role for VWF in promoting NETosis in the ischemic brain. Taken together, better understanding of thromboinflammatory processes leading to cerebral ischemia/reperfusion injury in ischemic stroke could serve as a basis for the development of novel treatment strategies.
OC 38.1
Protein tyrosine phosphatase 1B deficiency in vascular smooth muscle cells promotes perivascular fibrosis following arterial injury
R. Gogiraju 1; S. Gachkar2; D. Velmeden2; M. Bochenek3; K. Zifkos4; A. Hubert2; T. Münzel5; S. Offermanns6; K. Schäfer4
1 University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 2 Department of Cardiology, University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 3 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 5 Center for Cardiology – Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 6 Max‐Planck‐Institute for Heart and Lung Research, Bad Nauheim, Germany, Bad Nauheim, Hessen, Germany
Background: Smooth muscle cell (SMC) phenotype switching plays a central role during vascular remodeling. Growth factor receptors are negatively regulated by protein tyrosine phosphatases (PTP), including its prototype PTP1B.
Aims: To examine how reduction of PTP1B in SMCs affects the vascular remodeling response to injury.
Methods: Mice with inducible PTP1B deletion in SMCs (SMC.PTP1B‐KO) were generated by crossing mice expressing Cre.ERT2 recombinase under the Myh11 promoter with PTP1Bflox/flox mice and subjected to FeCl3 carotid artery injury.
Results: Genetic deletion of PTP1B in SMCs resulted in adventitia enlargement, perivascular α‐SMA+ and PDGFRβ+ myofibroblast expansion and collagen accumulation following vascular injury. Lineage tracing confirmed the appearance of Myh11‐Cre reporter cells in the remodeling adventitia, and SCA1+ CD45− vascular progenitor cell numbers increased. Elevated mRNA expression of TGFβ signaling components or enzymes involved in extracellular matrix remodeling and TGFβ liberation was seen in injured SMC.PTP1B‐KO mouse carotid arteries, and reduced mRNA transcript levels of contractile SMC marker genes were present already at baseline. Mechanistically, Cre recombinase (mice) or siRNA (cells) mediated downregulation of PTP1B or inhibition of ERK1/2 signaling in SMCs resulted in nuclear accumulation of KLF4, a central transcriptional repressor of SMC differentiation, whereas phosphorylation and nuclear translocation of SMAD2 was reduced. SMAD2 siRNA transfection increased protein levels of PDGFRβ and MYH10 while reducing ERK1/2 phosphorylation, thus phenocopying genetic PTP1B deletion.
Conclusion(s): Chronically reduced PTP1B levels in SMCs promote dedifferentiation, perivascular fibrosis and adverse remodeling following vascular injury by mechanisms involving a ERK1/2 phosphorylation‐driven shift from SMAD2 to KLF4 regulated gene transcription.
OC 38.2
Neutrophils and platelets collaborate to induce an increase NET formation during JAK2V617F positive myeloproliferative neoplasms
A. Guy 1; V. Gourdou‐Latyszenok2; L. Wolff‐Thrombini2; S. Favre2; S. Labrouche‐Colomer3; M. Renault2; C. James2
1 University Hospital Center of Bordeaux, Pessac, Aquitaine, France; 2 Univ. Bordeaux, INSERM, UMR 1034, Biology of Cardiovascular Diseases, Pessac, Aquitaine, France; 3 Univ. Bordeaux, INSERM, UMR 1034, Biology of Cardiovascular Diseases, Bordeaux, Aquitaine, France
Background: Thrombosis is a frequent complication during JAK2V617F myeloproliferative neoplasms (MPN) and neutrophils, especially through emission of neutrophil extracellular traps (NETs) were shown to promote thrombosis in MPN mouse models.
Aims: To assess whether JAK2V617F neutrophils alone are prone to NET formation, or whether they need to be activated to promote thrombosis.
Methods: We used two MPN mouse models with JAK2V617F expression in neutrophils: PF4‐iCre;JAK2V617/WT mice with JAK2V617F expression in all blood cells compartments and MRP8‐iCre;JAK2V617/WT mice with JAK2V617F expression only in neutrophils and monocytes. Thrombosis was analyzed in the lungs. Ex vivo NETosis was studied by immunofluorescence using histone 3 staining. In vivo NETosis was studied measuring plasmatic DNA and citrullinated histone 3 levels.
Results: We observed increased ex vivo NETosis in both models, but in vivo NETosis was only increased in PF4‐iCre; JAK2V617/WT mice. Besides, only PF4‐iCre; JAK2V617/WT mice had increased thrombosis formation, and NET inhibition reduced it. We hypothesized that increased inflammation or increased platelet activation could explain the differences in NET formation and thrombosis between PF4‐iCre; JAK2V617/WT and MRP8‐iCre; JAK2V617/WT mice. We could not find any evidence for the role of inflammation as (1) plasmatic TNF‐alpha concentration was similar, (2) addition of plasma from PF4‐iCre; JAK2V617/WT mice on JAK2V617F neutrophils did not increase ex vivo NETosis, (3) TNF‐alpha or LPS treatment of MRP8‐iCre; JAK2V617/WT mice did not increase thrombosis. We then focused on platelets and observed increased ex vivo NETosis when JAK2V617F platelets were coincubated with JAK2V617F neutrophils. Finally, treatment of PF4‐iCre; JAK2V617/WT mice with aspirin reduced in vivo NETosis and thrombosis formation.
Conclusion(s): We show here that the presence of JAK2V617F neutrophils together with JAK2V617F platelets is necessary to trigger increased NETosis and thrombosis in a mouse model of JAK2V617F MPN. Interestingly, platelet inhibition can inhibit NETosis and subsequent increased thrombosis formation.
Endothelial Cell Signaling
LB 02.5
Roles of FVIII in endothelial cell biology more than a coagulation factor
C. Olgasi1; A. Cucci2; S. Assanelli2; C. Sgromo2; C. Borsotti2; G. Walker2; I. Molineris3; F. Anselmi3; S. Oliviero3; A. Follenzi 4
1 Università del Piemonte Orientale, Novara, Piemonte, Italy; 2 Department of Health Sciences, Università del Piemonte Orientale, Novara, Piemonte, Italy; 3 Italian Institute for Genomic Medicine (IIGM), Università degli studi di Torino, Torino, Piemonte, Italy; 4 University of Piemonte Orientale, Novara, Italy, Novara, Piemonte, Italy
Background: Hemophilia A (HA) is a rare bleeding disorder caused by the absence or dysfunction of Factor FVIII (FVIII). Clinical manifestations are spontaneous bleedings that primarily consist of hemarthroses and intracranial hemorrhages. Standard therapies are ineffective in preventing the bleeding episodes and they can occur without any clear cause. To date, the impairment of vessel stability in HA patients and a correlation between FVIII and endothelial functionality has never been explained.
Aims: To elucidate the potential role of FVIII in endothelial stability and investigate significant differences in HA and healthy endothelial cells (ECs).
Methods: iPSCs‐derived ECs and Blood Outgrowth ECs (BOECs), both from HA patients and healthy donors, were used as ECs models. HA‐ECs were transduced with a lentiviral vector (LV) carrying the B‐deleted form of FVIII under the control of an endothelial specific‐promoter (LV‐VEC.FVIII). The transcriptomic profile of healthy, HA, and LV‐VEC.FVIII‐transduced HA ECs were evaluated by RNA Seq analysis and the differences in EC functionality were analyzed both in vitro and in vivo in a HA mouse model.
Results: Transcriptomic analysis revealed different gene expression in HA vs. healthy ECs in both ECs models. The impaired phenotype was partially attenuated in LV‐VEC.FVIII transduced HA ECs. Several genes were down regulated in HA ECs compared to healthy ECs, suggesting an impairment in HA ECs stability reproducible in both ECs systems. These data were validated in vitro, showing an impaired vessel‐formation capability, migration potential, and permeability for HA ECs. Finally, in a mouse model of severe HA, it was demonstrated an altered permeability and tubulogenesis potential of HA vessels when compared to wild‐type mice.
Conclusion(s): These results as confirmed in our EC models provide new insights into unexplored roles for FVIII besides coagulation, offering new therapeutic gene and cell therapy strategies in the management of HA patients.
OC 58.5
Epigenetic regulation of endothelial dysfunction in thromboembolic venous disease
M. Pilard 1; S. Robin2; F. Couturaud3; C. Lemarie4
1 INSERM 1304, GETBO, BREST, Bretagne, France; 2 INSERM, UMR 1304, BREST, Bretagne, France; 3 Inserm, Univ Brest, CHRU Brest, UMR 1304, GETBO, Departement de Medecine Interne et Pneumologie, Brest, France, Brest, Bretagne, France, 4 INSERM, UMR 1304, Brest, Bretagne, France
Background: Venous thromboembolism (VTE), which encompasses pulmonary embolism and deep vein thrombosis, is a frequent disease that is associated with vein wall fibrosis. Endothelial cells undergo phenotypical changes named endothelial‐to‐mesenchymal transition (EndMT), characterized by the loss of endothelial markers and the acquisition of mesenchymal markers involved in matrix deposition and fibrosis. Transforming growth factor (TGFβ) is the most potent inducer of EndMT. In chronic thromboembolic pulmonary hypertension, TGFβ induces EndMT and impairs thrombus resolution. However, the molecular mechanisms implicated in TGFβ signaling in the context of VTE are unknown. We hypothesized that epigenetic processes regulate the TGFβ signaling pathway in endothelial cells promoting EndMT and recurrent venous thromboembolism.
Aims: The aim of this study was to test if EndMT is regulated by epigenetic mechanisms in venous thromboembolism.
Methods: To study the role of histone deacetylase 6 (HDAC6) in EndMT, endothelial cells were transfected with a siRNA against HDAC6 or treated with a pharmacological inhibitor (TSC 20b). Endothelial cells were also treated with TGFβ and thrombin during 3–5 days. Real time PCR were performed to analyze endothelial and mesenchymal marker expression.
Results: Expression of the mesenchymal markers, calponin and α‐smooth muscle actin (SMA), is increased by TGFβ and thrombin. Interestingly these changes are inhibited in presence of siRNA or TSC 20b. Inhibition of HDAC6 also decrease the expression of TGFβ and Alk1 suggesting a role of HDAC6 in the regulation of the TGFβ pathway.
Conclusion(s): We found that treatment of endothelial cells with TGFβ and thrombin is associated with EndMT. Our preliminary data suggest that HDAC6 contribute to EndMT. Deciphering the mechanisms by which epigenetic pathways are regulating EndMT might lead to the discovery of novel biomarkers or new therapeutic targets would be instrumental in guiding decisions of treatment for patients with a high risk of recurrent VTE.
OC 58.4
Ibrutinib inhibits BMX‐dependent endothelial VCAM‐1 expression in vitro and pro‐atherosclerotic endothelial activation and platelet adhesion in vivo
T. Kohs 1; S. Olson1; K. Jordan1; T. Zheng1; A. Xie1; J. Hodovan1; M. Muller1; C. McArthur1; J. Johnson1; B. Sousa2; M. Wallisch3; P. Kievit4; J. Aslan1; J. Seixas2; G. Bernardes5; M. Hinds1; J. Lindner1; O. McCarty6; C. Puy1; J. Shatzel1
1 Oregon Health & Science University, Portland, Oregon, United States; 2 Instituto de Medicina Molecular João Lobo Antunes, Lisboa, Lisboa, Portugal; 3 Aronora, Inc., Portland, Oregon, United States; 4 Oregon National Primate Research Center, Portland, Oregon, United States; 5 Instituto de Medicina Molecular João Lobo Antunes, Lisboa, Lisboa, Portugal; 6 Oregon Health and Science University, Portland, Oregon, United States
Background: Inflammatory activation of the vascular endothelium leads to the overexpression of adhesion molecules, including vascular cell adhesion molecule‐1 (VCAM‐1), contributing to the pro‐thrombotic state underpinning atherogenesis. Although TEC family kinases (TFKs) mediate inflammatory cell and platelet activation, their contribution to vascular endothelial activation remain unclear.
Aims: Examine the role of TFK member, BMX, in endothelial cell (EC) activation in vitro and in atherosclerosis‐prone carotid arteries of obese nonhuman primates (NHPs) in vivo.
Methods: Human aortic ECs (HAECs) were stimulated with vascular endothelial growth factors (VEGF)‐A for 6 h. BMX phosphorylation and VCAM‐1 expression were measured by Western blot. To advance toward in vivo testing, two obese NHPs on a high‐fat diet were administered the TFK inhibitor, ibrutinib orally daily for 7 days at 10 mg·kg−1·day−1 and studied at baseline, and days 1 and 7 after drug administration. We measured platelet activation and aggregation in primate samples in response to glycoprotein VI‐agonist, cross‐linked collagen‐related peptide (CRP‐XL). Contrast‐enhanced ultrasound molecular imaging was used to measure platelet GPIbα and endothelial VCAM‐1 expression at the carotid bifurcation in obese NHPs during treatment.
Results: VEGF‐A increased VCAM‐1 expression and induced phosphorylation of BMX in HAECs. Ibrutinib or BMX inhibitors eliminated the ability of VEGF‐A to stimulate VCAM‐1 expression in HAECs. In a NHP model of early atherosclerosis, platelet aggregation and activation in response to CRP‐XL was abrogated following treatment with ibrutinib. Ibrutinib decreased the signal for both endothelial VCAM‐1 and platelet GPIbα compare to baseline, in vivo.
Conclusion(s): Our studies demonstrate that VEGF‐A signals through BMX to induce VCAM‐1 expression and that VCAM‐1 expression in HAECs is sensitive to ibrutinib. Treatment with ibrutinib decreased the markers of platelet deposition and endothelial cell activation in vivo. These findings suggest that TFKs may contribute to the pathogenesis of atherosclerosis and could represent a novel therapeutic target.
OC 58.2
Absence of protein tyrosine phosphatase‐1 in endothelial cells promotes venous thrombosis by mechanisms involving extracellular vesicles
K. Zifkos 1; M. Bochenek2; D. Pedrosa2; R. Gogiraju3; S. Robert4; L. Panicot‐Dubois4; W. Ruf5; P. Poncelet6; C. Dubois4; K. Schäfer1
1 University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 2 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 3 University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 Aix Marseille University, INSERM 1263, INRAE, C2VN, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 5 Center for Thrombosis and Hemostasis, Johannes Gutenberg University Medical Center, Mainz, Rheinland‐Pfalz, Germany; 6 Biocytex, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: PTP1B, a prototype protein tyrosine phosphatase, negatively regulates tyrosine kinase receptor signaling, and its absence has been associated with premature endothelial senescence. Cellular senescence and aging processes increase the risk of thrombosis, particularly in the venous system. Cellular senescence may also enhance the release of procoagulant extracellular vesicles (EVs).
Aims: To determine the contribution of endothelial PTP1B for venous thrombosis and the role of EVs therein.
Methods: Mice with tamoxifen‐inducible, Tie2.ERT2‐Cre mediated deletion of PTP1B (End.PTP1B‐KO) underwent inferior Vena cava (IVC) ligation. Coagulation assays were performed with murine whole blood or EVs isolated from it.
Results: Serial ultrasound measurements at different time points following IVC ligation revealed that endothelial PTP1B deficiency results in significantly larger thrombi at day 2, whereas thrombus resolution over 4 weeks was not altered. Histological analysis of IVC segments supported the ultrasound findings. Activation of the coagulation cascade, both in the intrinsic and extrinsic pathway, was significantly induced by End.PTP1B‐KO compared to End.PTP1B‐WT control plasma. Moreover, increased factor X activation and thrombin generation were seen both using plasma and EVs isolated from End.PTP1B‐KO mice. Intravital microscopy showed that PTP1B‐KO EVs enhanced the recruitment of endogenous leucocytes to the site of injury to a significantly greater extent than PTP1B‐WT control EVs, and this effect was less pronounced in End.PTP1B‐KO mice injected with PTP1B‐WT EVs. RT2 PCR profiler array analysis of primary endothelial cells revealed increased mRNA expression of markers of endothelial activation and activation‐induced cell death, including annexin V and Fas receptor, in End.PTP1B‐KO mice. Importantly, the procoagulant activity of PTP1B‐KO EVs was diminished using antibodies neutralizing tissue factor or P‐selectin glycoprotein ligand‐1, whereas antibodies targeting Fas ligand had no effect.
Conclusion(s): The results of this ongoing study suggest that endothelial PTP1B deficiency promotes venous thrombosis by endothelial activation and procoagulant EV formation.
OC 58.1
The amyloid peptide β increases endothelial permeability in a NADPH oxidase 1‐dependent manner: A link between Alzheimer’s disease and neurovascular inflammation
A. Tarafdar 1; N. Wolska2; C. Krisp3; H. Schlüter2; G. Pula4
1 CRUK‐TDL, Cambridge, England, United Kingdom; 2 University Medical Center Hamburg Eppendorf, Hamburg, Hamburg, Germany; 3 University Clinic Eppendorf, Hamburg, Hamburg, Germany; 4 University Medical Center Eppendorf Hamburg (UKE), Hamburg, Hamburg, Germany
Background: Alzheimer’s disease is the most common form of dementia and is associated with the accumulation of amyloid peptide β in the brain parenchyma. Vascular damage and microvascular thrombosis contribute to the neuronal degeneration and the loss of brain function typical of this disease.
Aims: In this study, we aimed to investigate the effects of amyloid peptide β on the neurovasculature and the blood‐brain barrier function.
Methods: We utilised 3xTG‐AD mice as a model of Alzheimer’s. A variety of techniques were utilised, ranging from brain histology studies to proteomics. The effect of amyloid peptide β on human primary endothelial cells was investigated using various immunochemistry techniques, electrical current impedance system (ECIS) experiments, and transwell cell permeability assays.
Results: Mouse brain proteomics highlighted pro‐inflammatory and pro‐oxidative changes concomitant with amyloid peptide β deposition in 3xTG‐AD mice at age 6 and 12 months. Upon detection of the phosphorylation of the endothelial cell‐cell interaction receptor VE‐cadherin in the hippocampus of 3xTG‐AD mice, we focused our attention on endothelial cells and utilised two types of primary endothelial cells cultured in vitro: (1) human umbilical vein endothelial cells (HUVECs) and (2) human brain microvascular endothelial cells (hBMECs). Using an electrical current impedance system (ECIS), we discovered that the treatment of human endothelial cells with amyloid peptide β causes a loss in their barrier function, which is oxidative stress‐dependent and similarly to our observation in mouse brain associates with VE‐cadherin phosphorylation. The activation of the superoxide anion‐generating enzyme NADPH oxidase 1 is responsible for the oxidative stress that leads to the disruption of barrier function in human endothelial cells in vitro.
Conclusion(s): In summary, we have identified a novel molecular mechanism that can explain how the accumulation of amyloid peptide β in the brain parenchyma may induce the loss of neurovascular function in Alzheimer’s patients.
Epigenetics, OMICs and Bioinformatics
OC 68.1
Identification of non‐coding genomic regions involved in the aetiology of bleeding, platelet, and thrombotic disorders
L. Stefanucci 1; M. Sims2; J. Lambourne3; F. Burden4; L. Grassi4; D. Seyres5; J. Stephens5; R. Tomaz5; F. Lugtu5; D. Greene5; N. Owens6; K. Megy5; S. Sivapalaratnam7; K. Freson8; L. Vallier5; E. Turro9; W. Ouwehand10; M. Frontini6
1 Wellcome Sanger Institute, Cambridge, England, United Kingdom; 2 Department of Haematology, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK., Cambridge, England, United Kingdom; 3 Department of Haematology, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK, Cambridge, England, United Kingdom; 4 Department of Haematology, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK, Cambridge, England, United Kingdom; 5 University of Cambridge, Cambridge, England, United Kingdom; 6 University of Exeter, Exeter, England, United Kingdom; 7 Barts NHS Trust, London, England, United Kingdom; 8 Center for Molecular and Vascular Biology, University of Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 9 Icahn School of Medicine at Mount Sinai, New York, New York, United States; 10 Department of Haematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK. Department of Haematology, University College London Hospitals, London, UK, Cambridge, England, United Kingdom
Background: There is an increasing understanding of the relationship between rare coding pathogenic variants and their clinical sequelae. However, for bleeding, platelet, and thrombotic disorders (BPD) a large portion of heritable cases remain without a molecular diagnosis. We have shown that some of these unexplained cases are caused by non‐coding variants in the gene‐regulatory space (Turro et al., 2020).
Aims: This study aims to identify the regulatory regions of 93 diagnostic‐grade BPD genes (Megy et al. 2019) in relevant cell types to increase the heritable‐BPD diagnostic yield.
Methods: We differentiated human induced pluripotent stem cells into megakaryocytes, endothelial cells and hepatocytes. A capture Hi‐C approach was used to identify regulatory regions of the 93 BPD genes (BPD‐regulome hereafter) in these cells. The BPD‐regulome was further constrained using statistical and experimental methods. We overlaid epigenetic features, RedPop and applied the BeviMed statistical approach (Greene et al., 2016; Turro et al., 2020). We searched the whole‐genome‐sequencing (WGS) data of 838 unexplained heritable BPD cases for putative pathogenic variants in the BPD‐regulome, and measured their effects on transcription in reporter assays.
Results: We produced the highest resolution interaction maps to date for the 93 BPD genes, identifying 62,027 interactions to putative regulatory elements. This framework identified 31 possible associations between rare variants in the BPD‐regulome of unexplained cases at a posterior probability >0.7. Moreover, a visual review of structural variants overlapping the BPD‐regulome identified three possibly explanatory aberrations, including the recently described pathogenic deletion in the HDAC6‐GATA1 locus (Turro et al. 2020). Finally, we showed that seven of these variants in the BPD‐regulome alter gene expression in reporter assays.
Conclusion(s): We generated a high‐resolution map of the BPD‐regulome in the relevant cell types. The regulatory regions defined in this study lay the foundation to explore WGS‐data from unexplained BPD cases for causal variants. FIGURE 1 A BPD regulatory region identified with the capture Hi‐C approach, differential use of the regulatory space in the three cell types, and effect of variants in BPD‐regulome. (A) RUNX1 chromatin structure in the three cell types relevant for human haemostasis (i.e. megakaryocytes, endothelial cells and hepatocytes, respectively orange, red and brown). Each cell type has the chromatin interactions identified with the capture Hi‐C (top track) and H3K27Ac to show regulatory regions (bottom track). (B) All the interactions identified per gene, in the different cell types, have been used for the dimensionality reduction (i.e. PCA) and show the different patterns of promoter interactions for some of the diagnostic‐grade genes. (C) Regulatory capacity of the regions identified with the capture Hi‐C approach and the effect of rare regulatory variants identified in the NIHR Rare‐Diseases cohort. The regulatory capacity of each region has been tested using the wild type sequence of the regulatory regions and the sequence harbouring the variant identified in the NIHR Rare‐diseases cohort. The horizontal black line is the Luciferase/Renilla ratio for the empty vector.
FIGURE 1 A BPD regulatory region identified with the capture Hi‐C approach, differential use of the regulatory space in the three cell types, and effect of variants in BPD‐regulome. (A) RUNX1 chromatin structure in the three cell types relevant for human haemostasis (i.e. megakaryocytes, endothelial cells and hepatocytes, respectively orange, red and brown). Each cell type has the chromatin interactions identified with the capture Hi‐C (top track) and H3K27Ac to show regulatory regions (bottom track). (B) All the interactions identified per gene, in the different cell types, have been used for the dimensionality reduction (i.e. PCA) and show the different patterns of promoter interactions for some of the diagnostic‐grade genes. (C) Regulatory capacity of the regions identified with the capture Hi‐C approach and the effect of rare regulatory variants identified in the NIHR Rare‐Diseases cohort. The regulatory capacity of each region has been tested using the wild type sequence of the regulatory regions and the sequence harbouring the variant identified in the NIHR Rare‐diseases cohort. The horizontal black line is the Luciferase/Renilla ratio for the empty vector.
Inflammation and Sepsis
OC 19.5
Protease‐Nexin‐1,a serpin participating in neutrophil recruitment through CD11a integrin regulation
C. Madjene 1; L. Venisse2; K. Aymonier3; Y. Boulaftali3; V. Arocas3; M. Bouton3
1 INSERM U1148 – Laboratory for vascular and translational Science ‐, Paris, Ile‐de‐France, France; 2 Inserm U1148, PARIS, Ile‐de‐France, France; 3 Inserm U1148, Paris, Ile‐de‐France, France
Background: Inflammation and coagulation are two closely linked processes essential in the defense of the body.Thrombin,a key serine protease in the coagulation‐cascade,is also involved in the inflammatory reaction.The activity of thrombin is mainly regulated by proteins belonging to the serpin superfamily. Among them, Protease‐Nexin‐1 (PN‐1)is the most effective tissue thrombin inhibitor.
Aims: Our aim is to understand how PN1 affects neutrophil functions.
Methods: PN1expression was analysed by immunocytochemistry and western blot on resting orLPS‐activated neutrophils.We analyzed in vivo by intravital‐microscopy the intravascular recruitment of neutrophils after inducing inflammation by topical deposition of LTB4 on mesenteric‐veins.We also analyzed the inflammation in vivo using models of peritonitis induced by LPS/thioglycolate.We compared by flow‐cytometry the production of ROS and the expression of the receptors expressed by WT/PN‐1deficient neutrophils.Neutrophil adhesion on ICAM‐1 matrix was studied.
Results: We showed that PN‐1 is secreted by neutrophils after stimulation with LPS.We have observed a significantly reduced ROS‐generation in PN‐1‐deficient neutrophils.In vivo models of peritonitis showed a decreased recruitment of PN‐1‐deficient neutrophils in the intraperitoneal lavages by 60%.We have demonstrated that the recruitment of neutrophils on inflamed mesenteric veins is less important in PN‐1‐KO mice.Since PN‐1 is also expressed by platelets,we compared neutrophil recruitment in PF4‐CRE + PN‐1Flox/Flox mice(Platelet PN1deficient mice)with the one observed in LY6G CRE + PN‐1F/Fmice(Neutrophil PN‐1deficient mice).No significant modification of neutrophil vascular recruitment was observed in mice exhibiting PN‐1deficient platelets and WT neutrophils,whereas a significant decreased neutrophil recruitment was observed in mice exhibiting PN‐1deficient neutrophils.We have also shown the significant decrease of PN‐1deficient neutrophils adhesion on ICAM‐1.We demonstrated by flow cytometry,a 2‐fold lower Mean Fluorescence Intensity(MFI) for the integrin CD11a on neutrophils from PN‐1‐deficient mice compared with neutrophils from WT mice.Such a deficit may explain the reduced neutrophil recruitment observed in PN1KOmice.
Conclusion(s): Our results demonstrated that PN‐1promotes neutrophil recruitment and activity and could play an important role in the inflammatory reaction.
OC 19.3
The endothelial inflammatory repertoire: A multi‐omics delineation of cytokine‐induced endothelial inflammation states
S. Groten 1; E. Smit2; E. Janssen2; B. van den Eshof2; F. van Alphen2; C. van der Zwaan2; A. meijer3; A. Hoogendijk2; M. van den Biggelaar2
1 Sanquin Research – Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Sanquin Research, Amsterdam, Noord‐Holland, Netherlands; 3 Sanquin Research/Utrecht University, Amsterdam, Noord‐Holland, Netherlands
Background: Hemostasis and inflammation are tightly intertwined processes in which vascular endothelial cells (ECs) form a dynamic interface between blood and tissue mediating critical steps in maintaining both anti‐ and pro‐ coagulation and inflammatory states. Detailed insight on the mechanistic processes that trigger endothelial inflammation could contribute to the design of intervention strategies at the crossroads between vascular inflammation and hemostasis.
Aims: Understanding the diversity of endothelial inflammatory responses.
Methods: To generate an overview of ECs responses, we evaluated changes in the proteome of blood derived ECs stimulated to a panel of 92 cytokines separately and in combination. We then performed a time‐resolved multi‐omics analysis on ECs exposed to TNFα and IFNγ, integrating transcriptome, whole (phospho‐) proteome, and secretome (Fig. 1).
Results: Inflammatory cytokines TNFα and IFNγ induced the highest number of significant proteomic events and exhibited distinct proteomic profiles. Our multi‐omics integration allowed for in‐depth mapping of endothelial inflammatory responses and highlighted distinct signatures as well as a drastically increased response through TNFα and IFNγ co‐stimulation on all omics levels. Regulatory hubs correlated to TNFα activation of the NFKB pathway and IFNγ signaling through the JAK/STAT pathway (Fig. 2). IFNγ also strongly upregulated MHCI and MHCII histocompatibility complexes and complement factors. Interestingly, several mRNAs, such as RelA, JAK3 and PECAM1 were synergistically regulated through combined TNFα and IFNγ stimulation. Moreover, ECs secreted a select subset of cytokines per inflammatory stimuli, which were synergistically increased through co‐stimulation, including CCL5, CCL8, CXCL9 and IL6.
Conclusion(s): In conclusion, our integrated analysis reveals (1) an in‐depth molecular mapping of endothelial inflammation on multiple omics levels, (2) the presence of distinct endothelial inflammatory states and (3) a synergistic endothelial response through combined TNFα and IFNγ stimulation. This study therefore supports the emerging role of ECs as active players in the progression of inflammation.
OC 19.2
Metabolic reprogramming is essential for myeloid cell‐dependent haemostatic activity
A. Rehill 1; G. Leon2; S. McCluskey2; I. Schoen2; J. O'Donnell3; A. Curtis4; R. Preston2
1 Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 3 Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 4 School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland
Background: Myeloid cell activation leading to enhanced tissue factor (TF) expression, decryption and procoagulant activity represents a key event in the initiation of thrombo‐inflammatory disease. Macrophage polarisation promotes rewiring of cellular metabolism to favour enhanced aerobic glycolysis, which allows for rapid generation of the energy required for phagocytosis and cytokine production.
Aims: To investigate whether metabolic reprogramming in response to inflammatory stimuli impacts myeloid cell procoagulant and antifibrinolytic activity.
Methods: Mouse bone marrow‐derived macrophages or human monocytes were skewed towards pro‐inflammatory (M1) or anti‐inflammatory (M2) polarisation states. Myeloid cell gene and protein expression was analysed using ELISA, RT‐PCR, flow cytometry and confocal microscopy. Additionally, we developed new myeloid cell‐based thrombin and plasmin generation assays to assess myeloid cell procoagulant function.
Results: We demonstrate that inhibition of inflammation‐associated metabolic reprogramming of myeloid cells ablates activated TF‐dependent thrombin generation. Treatment of macrophages or monocytes with 2‐Deoxy‐D‐glucose (2‐DG; hexokinase inhibitor) reduced F3 gene expression and significantly extended TF‐dependent lag‐time in a novel assay of myeloid cell‐dependent thrombin generation. We next assessed how inhibition of glycolysis restricts TF‐dependent procoagulant activity by measuring the impact of 2‐DG on cellular determinants of cell surface TF decryption. Notably, flow cytometric assessment of lactadherin binding to M1‐skewed macrophages revealed significantly reduced phosphatidylserine externalisation upon 2‐DG treatment. Furthermore, membrane localisation of acid sphingomyelinase, which degrades membrane sphingomyelin to promote TF procoagulant decryption, was significantly impaired by 2‐DG, suggesting a new role for activated macrophage metabolic reprogramming in facilitating TF decryption. Finally, we examined the role of glycolysis in controlling macrophage‐dependent plasmin generation. Enhanced M1‐skewed macrophage antifibrinolytic activity via increased plasminogen activator inhibitor‐1 gene expression and secretion was significantly attenuated by inhibition of glycolysis, leading to restoration of plasmin generation in 2‐DG treated, M1‐skewed macrophages.
Conclusion(s): The discovery of immunometabolic regulation of myeloid cell hypercoagulability opens new therapeutic possibilities for the mitigation of thrombo‐inflammatory disease.
OC 19.1
Rabeprazole is an agonist of HIF‐1 and promotes vascular repair and resolution of inflammatory lung injury
C. Evans 1; Y. Peng1; Z. Dai2; X. Zhang1; Y. Zhao1
1 Northwestern University, Chicago, Illinois, United States; 2 University of Arizona, Phoenix, Arizona, United States
Background: There are no effective treatments for inflammatory lung injury, including acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Severe COVID‐19 patients commonly suffer from ARDS. The repositioning of existing drugs is one possible strategy for treatment of ALI/ARDS. We previously showed that vascular repair and resolution of inflammatory lung injury is dependent upon endothelial hypoxia‐inducible factor 1 alpha (HIF1a) and forkhead box M1 (FoxM1).
Aims: To identify a candidate agonist of HIF1a/FoxM1 signaling that could effectively treat ALI/ARDS.
Methods: FDA‐approved drugs were screened using reporter assays, toxicity assays, and gene expression analyses in vitro. Inflammatory lung injury was assessed in a mouse model of lipopolysaccharide‐induced endotoxemia.
Results: We used high throughput screening of a library 1200 FDA‐approved drugs, along with hypoxia‐response element‐driven luciferase reporter assays, cell toxicity assays, and molecular analyses of human lung microvascular endothelial cells, to identify candidate drugs that enhance HIF1 signaling in vitro. One of these drugs, Rabeprazole (Aciphex), caused dose‐dependent increases in hypoxia‐response element activity without increasing cell death. By treating wild type mice orally with Rabeprazole on 2 consecutive days after sepsis challenge, we were able to identify a dose of Rabeprazole that is well tolerated and enhances vascular repair and resolution of inflammatory lung injury. In timeline studies, we found that Rabeprazole treatment reduces lung vascular leakage, edema, and inflammatory cytokine expression during the repair phrase. We next used conditional knockout mice to show that Rabeprazole increases vascular repair and resolution of inflammatory lung injury through endothelial HIF1a and FoxM1. Rabeprazole‐dependent decreases in lung vascular leakage, edema, and inflammatory cytokine expression were completely absent in the conditional HIF1a and FoxM1 knockout mice.
Conclusion(s): Rabeprazole improves murine vascular repair and resolution of sepsis‐induced inflammatory lung injury via endothelial HIF1a/FoxM1. This drug represents a promising candidate for repurposing to effectively treat ALI/ARDS.
Innate and Adaptive Immunity
OC 68.2
Endothelial toll‐like receptor 4 regulates arterial thrombus growth
K. Kiouptsi 1; A. Grill2; K. Mammadova2; J. Winterstein2; S. Jäckel2; K. Jurk3; C. Reinhardt4
1 University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Rheinland‐Pfalz, Germany; 2 Center for Thrombosis and Hemostasis/University Medical Center Mainz, Mainz, Rheinland‐Pfalz, Germany; 3 University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 Johannes Gutenberg University Mainz, Mainz, Rheinland‐Pfalz, Germany
Background: Pattern recognition receptors affect arterial thrombosis but the cell type‐specific function of these receptors remains elusive. While the microbiota promotes arterial thrombus growth via Toll‐like receptor (TLR)‐2, the role of other innate immune receptors remains poorly defined.
Aims: Therefore, we studied the role of TLR4 in arterial thrombus growth.
Methods: Using intravital epifluorescence microscopy, we analyzed platelet deposition in a mouse carotid artery ligation model. Thus, we studied the impact of TLR4 on arterial platelet deposition in global Tlr4‐deficient and endothelial‐specific Tlr4‐deficient mice generated by Cre‐Lox technology. Washed platelets isolated from either global Tlr4‐deficient or WT mice were stimulated by thrombin and the P‐selectin exposure and αIIbβ3 activation were analysed by flow cytometry. Ex vivo, thromboelastometry and VWF ELISA on plasma samples was performed.
Results: Applying the carotid artery ligation injury model to Tlr4 global knockout mice, we observed reduced platelet deposition to the ligation injury site relative to wild‐type (WT) controls. By thrombin ex vivo stimulation of washed WT and Tlr4‐deficient platelets, no differences were observed in platelet activation. Therefore, platelet TLR4 is not a main effector of arterial thrombus growth. To pinpoint the role of the endothelium, we studied a conditional knockout model with endothelial‐specific Tlr4‐deficiency. Platelet deposition to the arterial injury site was strongly impaired in Cre+ mice compared with Cre‐ controls, indicating that TLR4 expressed by endothelial cells is involved in arterial thrombus formation. Ex vivo clotting parameters did not differ between Tlr4 global knockout mice and controls. Consistent with reduced arterial platelet deposition in endothelial Tlr4‐deficiency, we found reduced von Willebrand factor (VWF) plasma levels in endothelial‐specific Tlr4‐deficient mice, which is likely responsible for the observed reduction in arterial thrombus growth.
Conclusion(s): Endothelial TLR4 plays a major role in platelet deposition to the ligation injury site at the common carotid artery in vivo.
OC 19.4
Induction of myeloid cell hypercoagulability is a maladaptive consequence of trained immunity
A. Rehill 1; S. McCluskey2; G. Leon2; A. Curtis3; C. McMahon4; H. Charles‐Messance5; F. Sheedy6; R. Preston2
1 Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 3 School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 4 Department of Haematology, Children’s Health Ireland (CHI) at Crumlin, Dublin, Ireland, Dublin, Dublin, Ireland; 5 School of Biochemistry and Immunology, Trinity College Dublin, Dublin, Dublin, Ireland; 6 School of Biochemistry and Immunology, Trinity College Dublin, Dublin, Ireland., Dublin, Dublin, Ireland
Background: Individuals with chronic inflammatory disease or haematological malignancies have an increased risk of venous thrombosis, although the molecular basis for this phenomenon remains poorly understood. We hypothesised that disease‐associated ‘trained immunity’ in myeloid cells, which describes long‐term epigenetic and metabolic modifications that occur in response to specific inflammatory stimuli that increases responsiveness to subsequent non‐specific inflammatory events, may contribute to hypercoagulability associated with chronic inflammatory disease.
Aims: To assess whether trained immunity enhanced myeloid cell procoagulant and antifibrinolytic activity.
Methods: Murine bone marrow‐derived macrophages were trained with β‐glucan or heme, washed, then left for 7 days before lipopolysaccharide (LPS) re‐stimulation. Macrophage gene expression and function were analysed by ELISA, RNA‐seq and cell‐based calibrated automated thrombinography. In vivo cell training was achieved by i.p. β‐glucan administration.
Results: Surprisingly, re‐stimulated β‐glucan‐ or heme‐trained macrophages exhibited enhanced procoagulant and antifibrinolytic gene expression compared to macrophages stimulated with LPS alone. Moreover, trained macrophage‐dependent thrombin generation was associated with significantly shortened lag‐time compared to LPS‐stimulated macrophages, which was dependent upon increased tissue factor activity and classical training‐associated metabolic and epigenetic modifications in trained macrophages. To assess whether haematopoietic progenitor cell training contributed to enhanced myeloid cell hypercoagulability in vivo, we performed transcriptomic analysis of splenic monocytes isolated from mice trained with β‐glucan 3 weeks prior to sacrifice, which identified up‐regulation of genes associated with both trained immunity and hypercoagulability. Furthermore, splenic monocytes isolated from β‐glucan‐trained mice exhibited enhanced procoagulant activity compared to control mice monocytes. Remarkably, monocyte procoagulant activity increased in parallel with the time period since β‐glucan training, consistent with the induction of a training‐dependent hypercoagulable state.
Conclusion(s): This study demonstrates that a lowered threshold for myeloid cell‐dependent hypercoagulability is a maladaptive consequence of innate immune cell memory. Furthermore, these data suggest that epigenetic and metabolic perturbations associated with trained immunity represent novel therapeutic vulnerabilities in immunothrombotic disease.
Stem Cells and Vascular Cell Growth
OC 68.3
Understanding the role of the renin‐angiotensin system in endothelial senescence in chronic obstructive pulmonary disease
K. Paschalaki 1; D. Gresham1; C. Pericleous1; M. MacLeod1; G. Donaldson1; J. Wedzicha1; J. Mason1; P. Barnes1; A. Randi2
1 National Heart and Lung Institute, Imperial College London, London, England, United Kingdom; 2 Imperial College London, London, England, United Kingdom
Background: Cardiovascular disease (CVD) is a major cause of morbidity and mortality in patients with chronic obstructive pulmonary disease (COPD). Endothelial senescence promotes vascular ageing and atherosclerosis. Endothelial‐colony‐forming‐cells (ECFC), provide non‐invasive access to endothelial cells in patients. The renin‐angiotensin system (RAS) and particularly Angiotensin (Ang)‐II are implicated in COPD pathogenesis and in CVD.
Aims: To investigate the role of AngII in endothelial senescence and vascular ageing in COPD, and test possible pharmacological interventions.
Methods: ECFC were isolated and expanded from peripheral blood samples of healthy non‐smokers, healthy smokers and COPD patients. Senescence was measured by senescence‐associated‐β‐galactosidase (SA‐β‐Gal) activity. Additional markers of senescence (p16, p21), DNA damage (γ‐H2AX, 53BP1) and selective senescence associated secretory phenotype (SASP) mediators were measured by immunofluorescence confocal microscopy. We use a high‐throughput ‘organ‐on‐a‐chip’ microfluidic platform (OrganoPlate, MIMETAS Netherlands) that allows the long‐term culture of endothelial cells and formation of microvessels for functional and immunofluorescent analysis.
Results: We have previously demonstrated in ECFC from COPD patients increased DNA damage response (DDR), ataxia‐telangiectasia‐mutated (ATM) activation and endothelial senescence due to epigenetic dysfunction involving the histone deacetylase sirtuin(SIRT)‐1, supporting the concept of accelerated endothelial ageing as a contributor to CVD in COPD. We now show that ECFC senescence is accompanied by a proinflammatory phenotype (increased IFN‐γ‐inducible‐protein‐10) in a subgroup of COPD patients not receiving treatment with inhaled‐corticosteroids. We have optimised in vitro 2D and 3D models of premature endothelial senescence caused by AngII. Pharmacological treatment with SIRT1 activators, ATM inhibitors, and AngII receptor blockers could inhibit the increased senescence in ECFC from COPD patients.
Conclusion(s): We demonstrate that ECFC from patients with COPD exhibit increased senescence possibly due to aberrant activation of the RAS system and could be amenable to treatment. These defects may contribute to endothelial dysfunction and CVD in COPD and could potentially constitute therapeutic targets for intervention.
Venous Thromboembolism
Antiphospholipid Syndrome
OC 06.5
Prevalence and severity of bleeding events in thrombotic antiphospholipid syndrome – Should they be included in the damage index for antiphospholipid syndrome?
P. Gaspar 1; F. Farinha2; Z. Sayar3; M. Efthymiou4; H. Cohen5; D. Isenberg6
1 North Lisbon University Hospital Centre, Lisbon, Portugal; Instituto de Medicina Molecular (IMM), Cellular Immunology Lab, Faculty of Medicine, University of Lisbon, Lisbon, Portugal, Póvoa De Santa Iria, Lisboa, Portugal; 2 Centre for Rheumatology, Division of Medicine, University College London, London, UK, London, England, United Kingdom; 3 University College London Hospitals NHS Foundation Trust, London, UK; Whittington Hospital, London, UK, London, England, United Kingdom; 4 Haemostasis Research Unit, Department of Haematology, University College London, London, UK, London, England, United Kingdom; 5 Department of Haematology, University College London Hospitals NHS Foundation Trust, London, UK; Haemostasis Research Unit, Department of Haematology, University College London, London, UK, London, England, United Kingdom; 6 Department of Rheumatology, University College London Hospitals NHS Foundation Trust, London, UK, London, England, United Kingdom
Background: Thrombotic antiphospholipid syndrome (APS) patients are treated with life‐long anticoagulation, associated with increased bleeding risk. In lupus, treatment‐related events (e.g.: diabetes, cataracts) are considered to be damage. In contrast, the Damage Index for Antiphospholipid Syndrome (DIAPS) does not include bleeding events (BE) in damage accrual.
Aims: To characterize the cumulative prevalence and severity of BE and its related damage in thrombotic APS patients.
Methods: Single‐centre retrospective analysis of thrombotic APS patients (2006 Sydney criteria). BE were classified according to ISTH definitions for minor‐BE (mi‐BE), clinically relevant non‐major‐BE (CRNM‐BE), and major‐BE in non‐surgical (Mns‐BE) and surgical (Ms‐BE) patients. Patients were then classified in two groups: (1) major‐BE: Mns‐BE and Ms‐BE; and (2) non‐major‐BE: mi‐BE and CRNM‐BE. Damage events were recorded as: (a) BE with persistent neurologic deficits; (b) BE complicated by total/partial organ resection; (c) BE resulting in decreased organ function.
Results: We identified 197 APS patients (female, 71.1%; primary APS, 65.9%; Caucasian, 72.4%; median age at APS onset 40 years; median follow‐up time, 10 years), all on anticoagulant and/or antiplatelet agents. 169 BE occurred in 80 patients (40.6%), of whom 50 (62.5%) had non‐major BE (126/169, 74.6% of events), and 30 (37.5%) had major‐BE (43/169, 25.4% of events) (Table 1). Mucocutaneous BE were the most frequent (32.5%) and none led to damage. Central nervous system (CNS) was the organ/system most affected by major‐BE (55.8%), followed by gastrointestinal (n = 7, 16.3%) and genitourinary (GU) (n = 4, 9.3%). Damage occurred in 11.8% (n = 20) of BE and affected 7.6% (n = 15) of patients (50.0% with major BE). Most of the damage was associated with CNS (8.3% of all BE) and GU events (2.4% of all BE).
Conclusion(s): Approximately 40% of patients experienced at least one BE and almost 20% of those were left with permanent disability. We suggest that BE should be incorporated into DIAPS. TABLE 1 Distribution of bleeding events and associated damage
TABLE 1 Distribution of bleeding events and associated damage
OC 06.4
Thrombocytopenia in primary antiphospholipid syndrome: Association with prognosis and clinical implications
Y. Shi 1; J. Zhao2; X. Zeng2
1 Chinese Academy of Medical Sciences, Peking Union Medical College Hospital, Beijing, Beijing, China (People's Republic); 2 Chinese Academy of Medical Sciences, Peking Union Medical Collge, Beijing, Beijing, China (People's Republic)
Background: Thrombocytopenia, a frequent clinical manifestation in patients with antiphospholipid syndrome (APS), could be an independent predictor of recurrent thrombotic, obstetrical and severe extra‐criteria events.
Aims: The aims of this study is to determine whether thrombocytopenia could be an independent predictor of recurrent thrombotic, obstetrical and severe extra‐criteria events.
Methods: This single‐center prospective study enrolled 218 consecutive patients diagnosed with primary APS between 2010 and 2021. Thrombocytopenia was defined as a platelet count <100 × 109/L.
Results: Our cohort included 74 (33.94%) patients with thrombocytopenia and 144 patients with a continuous normal platelet count. Comparison of baseline characteristics indicated that patients with thrombocytopenia had more visceral venous thromboses [10 (13.51%) vs. 5 (3.47%), p = 0.009] and extra‐criteria manifestations [mainly hemolytic anemia; 20 (27.03%) vs. 17 (11.81%), p = 0.007] than those with a continuous normal platelet count. Hypocomplementemia was more likely among patients with thrombocytopenia [19 (25.68%) vs. 16 (11.11%), p = 0.01]. The presence of aCL‐IgG/IgM, anti‐β2‐glycoprotein I and lupus anticoagulant were more frequently detected in patients with thrombocytopenia. In survival analysis, thrombotic, obstetrical, and severe extra‐criteria survival rates were significantly worse in patients with thrombocytopenia (Figure 1). In multivariate Cox regression (Table 1), thrombocytopenia was an independent risk factor for all endpoint events, including thrombotic events [hazard ratio (HR) 2.93, 95% confidence interval (CI) 1.31, 6.56, p = 0.009], pregnancy morbidity (HR 8.00, 95% CI 2.43, 26.37, p = 0.0006) and severe‐extra criteria events (HR 15.27, 95% CI 1.85, 125.98, p = 0.01). Twelve (16.22%) patients with thrombocytopenia appeared to have no response (NR) to treatment, and their minimum platelet count was significantly lower than that in the non‐NR group [22.5 (14.25, 36.25) vs. 68.5 (26.25, 99), p = 0.01]. Complement level and antibody profiles were not significantly different between NR and non‐NR groups.
Conclusion(s): Thrombocytopenia could identify primary APS patients at high risk of developing thrombotic events, pregnancy morbidity and severe extra‐criteria events.
OC 32.4
Gut microbiome composition and intestinal immunity in antiphospholipid syndrome patients versus healthy controls
V. Jansen 1; M. Davids1; D. van Mourik1; J. Levels1; S. Middeldorp2; M. Nieuwdorp1; T. van Mens1
1 Amsterdam UMC, Amsterdam, Noord‐Holland, Netherlands; 2 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands
Background: An increasing body of research indicates a role of the gut microbiome in the etiology of auto‐immunity and antiphospholipid syndrome (APS). A specific gut commensal, Roseburia intestinalis, expresses peptides that mimic the main epitopes in APS auto‐antigen beta‐2‐glycoprotein‐1 and is able to elicit the syndrome in APS‐prone mice. As evidenced by antibiotics experiments, the phenotype in APS mouse models is gut microbiome dependent.
Aims: To compare gut microbiota composition of APS patients to healthy controls.
Methods: Samples were selected from our biobank of patients with APS, the majority with an obstetric phenotype, and healthy controls were recruited alongside the APS patients from the same geographic region. For the current study we selected patients with beta‐2‐glycoprotein‐1 antibodies and excluded subjects with recent use of antibiotics or proton pump inhibitors. To determine differences in the gut microbiome we performed fecal shotgun metagenomics. Microbiome composition was assessed using Shannon Index and Bray‐curtis dissimilarity and general taxonomic abundance, all corrected for multiple testing. We measured fecal short chain fatty acids (SCFA) by high‐performance liquid chromatography because of their role in intestinal immunity, and calprotectin levels as a marker for intestinal inflammation.
Results: We included 15 patients and 16 controls, all female. Both alpha and beta gut microbiota diversity did not differ between APS patients and healthy controls [Figure 1]. R. intestinalis was not more abundant in APS patients, nor did we find other differentially abundant taxa. There were also no differences in fecal calprotectin levels between APS patients and controls, nor in SCFA [Table 1].
Conclusion(s): We found no differences in gut microbiota composition, intestinal inflammation and SCFA levels in APS patients compared to healthy controls. The reported effects of the gut microbiome on APS are likely mediated by other mechanisms than overabundance, SCFA production or local inflammation.
OC 06.2
Recurrence risk of a first systemic lupus erythematosus‐associated venous thromboembolism: a retrospective cohort study
S. Bhoelan 1; J. Borjas Howard1; V. Tichelaar1; P. van Daele2; L. Hak3; A. Voskuyl4; M. Limper5; R. goekoop6; O. Teng7; J. Vosters8; M. Bijl9; E. Zirkzee10; A. Schilder11; H. Bernelot Moens12; K. de Leeuw1; K. Meijer13
1 University Medical Centre Groningen, Groningen, Groningen, Netherlands; 2 Erasmus Medical Centre, Rotterdam, Zuid‐Holland, Netherlands; 3 Amsterdam University Medical Center – Location AMC, Amsterdam, Noord‐Holland, Netherlands; 4 Amsterdam University Medical Center – location VU Medical Centre, Amsterdam, Noord‐Holland, Netherlands; 5 University Medical Centre Utrecht, Utrecht, Utrecht, Netherlands; 6 Hagaziekenhuis, Den Haag, Zuid‐Holland, Netherlands; 7 Leiden University Medical Centre, Leiden, Zuid‐Holland, Netherlands; 8 Meander Medisch Centrum, Amersfoort, Utrecht, Netherlands; 9 Martini Hospital, Groningen, Groningen, Netherlands; 10 Maasstadziekenhuis, Rotterdam, Zuid‐Holland, Netherlands; 11 Medisch Centrum Leeuwarden, Leeuwarden, Friesland, Netherlands; 12 Ziekenhuisgroep Twente, Almelo, Overijssel, Netherlands, 13 University Medical Center Groningen, Groningen, Groningen, Netherlands
Background: The recurrence risk of systemic lupus erythematosus (SLE)‐associated venous thromboemblism (VTE) is currently not well known.
Aims: To determine the recurrence risk of SLE‐associated VTE and to explore the influence of provoking factors and SLE‐flare at time of index‐VTE.
Methods: A multi‐centre, retrospective follow‐up of a cohort of patients with a first SLE‐associated VTE and who discontinued anticoagulation therapy. As formal SLE‐diagnosis might not have been established at index‐VTE, all VTE occurring since one year before diagnosis of SLE was considered SLE‐associated. SLE‐flares were defined as SLEDAI‐index >4. The primary outcome was recurrent VTE, defined as any deep vein thrombosis. Incidence rates and cumulative incidences were calculated stratified by presence of a provoking factor and anti‐phospholipid syndrome/antibodies (APS) at index‐VTE. The hazard ratio (HR) for recurrence risk after SLE‐flare associated index‐VTE was estimated in a Cox‐regression, adjusted for provoking factor and APS.
Results: Eighty patients were included. Follow‐up was 8 (3–16) years (median, interquartile range); mean (±SD) age was 39 ± 16 years, 66 (83%) were female. Twenty‐one recurrent VTE occurred. In stratum ‘provoked index‐VTE’, recurrence rate in patients without APS was 1.1/100 person‐years (95% confidence interval [CI] 0.1–3.1) and in presence of APS 3.5/100 person‐years (95% CI 0.9–8.9), yielding cumulative incidences of 7.5% (95% CI 1.2–21.7%) and 31.4% (95% CI 6.3–61.6%) respectively. In stratum ‘unprovoked index‐VTE’, recurrence rate in patients without APS was 3.8/100 person‐years (95% CI 1.2–9.0) and in presence of APS 16.7/100 person‐years (95% CI 4.5–42.7), yielding cumulative incidences of 33.7% (95% CI 10.7%–58.9%) and 54.2% (95% CI 10.7%–84.5%) respectively. Fourty‐six index‐VTE were flare‐associated and the adjusted HR for recurrent VTE was 0.4 (95% CI 0.1–1.0).
Conclusion(s): APS is the main determinant for recurrence risk of SLE‐associated VTE irrespective of the presence of a provoking factor. SLE‐flare at index‐VTE might be associated with a lower recurrence risk.
OC 06.3
Combined oral contraceptive‐associated venous thromboembolism revealing an antiphospholipid syndrome: An international retrospective study of outcomes
J. GRIS 1; C. Bourguignon2; S. Bouvier3; E. Nouvellon4; J. Laurent5; A. Perez‐Martin5; E. Mousty6; M. Nikolaeva7; J. Khizroeva8; V. Bitsadze8; A. makatsariya8
1 University of Montpellier and University Hospital of Nîmes, Nîmes, Languedoc‐Roussillon, France; 2 Department of Haematology, University Hospital, Nîmes, Nîmes, Languedoc‐Roussillon, France; 3 Department of Haematology, University Hospital, Nîmes, NÎmes, Languedoc‐Roussillon, France; 4 Department of Haematology, Uinversity Hospital, Nimes, Languedoc‐Roussillon, France; 5 Department of Vascular Medicine, University Hospital, Nîmes, Languedoc‐Roussillon, France; 6 Department of Gynaecology and Obstetrics, University Hospital, Nîmes, Languedoc‐Roussillon, France; 7 Department of Obstetrics and Gynaecology, Altai State Medical University, Barnaul, Russian Federation, Barnaul, Altaisky krai, Russia; 8 Department of Obstetrics and Gynaecology, I.M. Sechenov First Moscow State Medical University, Moscow, Russian Federation, Moscow, Moskva, Russia
Background: Significant limitations in the data used to define the optimal thromboprophylaxis in patients with antiphospholipid antibodies (aPLAbs) and thrombosis include uncertainties in patients with an initial provoked venous thromboembolic event (VTE).
Aims: To describe thrombotic outcomes in women with a first combined oral contraceptives (COC)‐associated first VTE and positive aPLAbs, and a low risk of recurrence.
Methods: International multicentric retrospective study on patients referred for thrombophilia screening after a first COC‐associated VTE between January 1st 2010 and January 1st 2021, with low risk by HERDOO2 score, taking a low‐dose aspirin‐mediated secondary thromboprophylaxis, followed each year until 2021. Primary outcome: thrombosis recurrence rate. Secondary outcomes: recurrence rates by the initial VTE subtype (distal deep vein thrombosis DVT, proximal DVT, pulmonary embolism PE); major bleedings.
Results: Data from 264 patients (distal DVT: 62.9%, proximal DVT: 20.1%, PE: 17%), cumulating 1,327.7 patient.years of observation, were collected. Twenty‐two thrombosis occurred: 16 distal DVTs, 3 proximal DVTs, 1 PE and 2 transient ischaemic attacks, the recurrence rate of thrombosis being 1.66 per 100 patient.years (p.y; 95% CI: 1 – 2.3). The recurrence rate was 1.06, 2.88 and 2.57 per 100 p.y in women with distal DVT, proximal DVT and PE, respectively (p = 0.0336). No major bleeding occurred. Risk factors affecting thrombosis‐recurrence free survival of patients were the time between first COC intake and VTE (p < 0.0001), initial proximal DVT (p = 0.0035), active smoking (p = 0.0008), high positivity for anti‐b2GP1 IgG (p = 0.0181) and for anti‐b2GP1 IgM (p = 0.0321).
Conclusion(s): In women with thrombotic antiphospholipid syndrome diagnosed after a first COC‐induced VTE, with a low HERDOO2 score, under low‐dose aspirin secondary prophylaxis, those with distal DVT had a low risk of recurrence. Recurrence risks were higher in others and was modulated by simple cofactors. These observational data may give some clues for categorising the patients in future randomised controlled trials.
OC 32.3
Anti‐β2‐glycoprotein I antibodies cause activated protein C resistance by interfering with Factor V cleavage at Arginine 506
T. Noordermeer 1; S. Chemlal1; J. Molhoek2; R. Schutgens3; M. Limper4; P. de Groot5; J. Meijers6; R. Urbanus1
1 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 2 Central Diagnostic Laboratory, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 3 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 4 Department of Rheumatology and Clinical Immunology, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 5 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 6 Department of Molecular Hematology, Sanquin Research, Amsterdam, the Netherlands, Department of Experimental Vascular Medicine, Amsterdam UMC, University of Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: The acquired thrombotic risk factor known as lupus anticoagulant (LA) is detected as a phospholipid‐dependent prolongation of the clotting time and can be caused by autoantibodies against β2‐glycoprotein I (β2GPI). LA is associated with activated protein C (APC) resistance, which might contribute to thrombotic risk in patients with LA. How anti‐β2GPI antibodies cause APC resistance is currently unclear. We have previously shown that anti‐β2GPI antibodies cause LA by attenuating factor (F)V activation by Factor (F)Xa through a direct interaction with FV. As FV is central to the anticoagulant properties of APC, we hypothesized that the interaction between β2GPI‐antibody complexes and FV also causes APC resistance.
Aims: To investigate how anti‐β2GPI antibodies induce APC resistance.
Methods: The effects of model monoclonal anti‐β2GPI antibodies on APC resistance were studied in plasma and with purified coagulation factors.
Results: Anti‐β2GPI antibodies with LA activity caused APC resistance in LA‐sensitive clotting assays with the snake venom protein C activator protac. APC resistance was only observed at limiting phospholipid concentrations, underlining the association between APC resistance and LA. Anti‐β2GPI antibodies only interfered with APC‐mediated cleavage of FV, not FVa: When FV is activated with Russell's viper venom FV activator, the inhibitory effect of anti‐β2GPI antibodies on APC activity in plasma is lost. Also, anti‐β2GPI antibodies had no effect on APC‐mediated FVa inactivation in a purified system. Analysis of FV cleavage patterns after incubation with APC indicated that anti‐β2GPI antibodies attenuated APC‐mediated cleavage of R506 and R306 in FV. APC‐mediated cleavage at R506 is required for FV cofactor activity during inactivation of FVIIIa. Assays with purified coagulation factors confirmed that anti‐β2GPI antibodies interfered with the cofactor function of FV during inactivation of FVIIIa.
Conclusion(s): Anti‐β2GPI antibodies with LA activity contribute to a procoagulant state by causing APC resistance via interference with the cofactor function of FV during FVIIIa inactivation.
OC 48.1
Choice of anticoagulation in patients with low risk antiphospholipid syndrome
B. Bakow 1; Q. Phung2; D. Rabinovich3; J. Reagan1
1 The Warren Alpert Medical School of Brown University/Lifespan Cancer Institute, Providence, Rhode Island, United States; 2 The Warren Alpert Medical School of Brown University, Providence, Rhode Island, United States; 3 Kent Hospital, Providence, Rhode Island, United States
Background: Antiphospholipid syndrome (APS) is an acquired hypercoagulable state characterized by arterial or venous thrombosis and/or pregnancy‐related adverse outcome plus laboratory evidence of antiphospholipid antibodies. Current recommendations for secondary prevention of thrombosis in patients with APS favor warfarin over other forms of anticoagulation. These guidelines are based on data in high‐risk, triple positive patients, but the efficacy of non‐Vitamin K antagonists for secondary thrombosis prevention in low‐risk, single and double positive APS remains uncertain.
Aims: To assess incidence of recurrent thrombosis and major bleeding for patients with low‐risk APS treated with anticoagulants and antiplatelet agents.
Methods: Retrospective cohort study of patients who met revised criteria for APS between January 1, 2001 to April 1, 2021 and received care at the Lifespan Health System. Primary outcomes included recurrent arterial and venous thrombosis and WHO Grades 3 and 4 major bleeding.
Results: 188 patients were followed over a median duration of 110 months (Table 1). Of the total population, 160 patients were initiated on anticoagulation including warfarin (n = 105), low molecular weight heparin (LMWH) (n = 12), anti‐Xa inhibitors (n = 41), and direct thrombin inhibitors (n = 2). Patients on direct thrombin inhibitors were excluded from primary outcome analysis due to small sample size. Patients on warfarin, LMWH, and anti‐Xa inhibitors had no difference in incidence of recurrent thrombosis (Table 2). Major bleeding was seen in 10 patients taking warfarin but not in patients on other forms of anticoagulation. Only 1 patient on warfarin experienced a Grade 4 bleed due to intracranial hemorrhage necessitating intubation.
Conclusion(s): There were similar rates of recurrent thrombosis despite choice of anticoagulation in patients with low‐risk APS. Significantly, only patients on warfarin experienced major bleeding. There were no major bleeding episodes in patients on LMWH or anti‐Xa inhibitors. Limitations of our study include a retrospective study design and small population of patients on non‐Vitamin K antagonists.
OC 06.1
The effect of rituximab on antiphospholipid antibody titers in patients with antiphospholipid syndrome
K. Youkhana 1; H. Heiling1; A. Deal2; S. Moll1
1 University of North Carolina, Chapel Hill, Chapel Hill, North Carolina, United States; 2 University of North Carolina, Lineberger Comprehensive Cancer Center, Chapel Hill, North Carolina, United States
Background: Data on the effect of rituximab on antiphospholipid antibody (APLA) titers in patients with antiphospholipid syndrome (APS) are limited.
Aims: Determine the effect of rituximab on APLA titers in APS patients within 12 months of treatment.
Methods: Patients with APS, age ≥ 18, within the University of North Carolina Health Care Systems identified via electronic medical record review from 1994 to 2020 using: keyword rituximab, ICD codes for arterial/venous thromboses, APS/CAPS, and CPT codes for lupus anticoagulant (LA), anticardiolipin (aCL) IgM/IgG, and anti‐β2‐glycoprotein I (aβ2GPI) IgM/IgG. Definitions used: LA positive if DRVTT ratio ≥ 1.20 and/or LA‐aPTT HPN difference > 8 seconds, aCL/aβ2GPI: low <40, medium 40–100, and high >100. ‘Time zero’ includes the start and stop dates of the first treatment course with rituximab, with negative and positive times corresponding to the before the start date or after the stop date, respectively. Lab observations 12 months before and after time zero were collected but censored after the start of the second treatment, if applicable. Titer changes are described.
Results: 9/37 patients (1 catastrophic APS) were eligible for analysis (figure 1). Changes in aCL/aβ2GPI titers for each patient are presented in figure 2. LA: 6/9 were positive before rituximab; all remained positive after treatment. aCL IgG (6/9): 5/6 medium, 1/6 high. None became negative: 3/6 remained unchanged; 1/6 medium to high, 1/6 medium to low, and 1/6 high to medium. aCL IgM (3/9): all medium titers: 2/3 became negative, 1/3 unchanged. aβ2GPI IgG (6/9): 3/6 low, 1/6 medium, 2/6 high. After treatment 3/6 unchanged, 2/6 low to medium, 1/6 medium to high, and 1/6 negative to medium. aβ2GPI IgM (2/9): remained unchanged. 1/9 had a recurrent thrombotic event within 1 year of treatment.
Conclusion(s): Rituximab did not have a meaningful overall effect on the APLA titers.
Cancer Associated Thrombosis
OC 25.5
The incidence and risk factors of venous and arterial thrombosis in ovarian cancer patients‐ results from a population database
A. Lukey1; S. Penfound2; J. Hodgson2; W. Hopman2; G. Hanley1; M. Othman 2
1 University of British Columbia, Vancouver, British Columbia, Canada; 2 Queen's University, Kingston, Ontario, Canada
Background: Ovarian cancer (OC) patients are at high risk of thromboembolism due to the hypercoagulable state of malignancy. While this association has been well studied regarding venous thromboembolism (VTE), there is a lack of evidence regarding the risk of arterial thromboembolism (ATE). It is also unknown if other risk factors for thrombosis are involved. Appropriate identification of patients at high risk enables more targeted, effective and safe thromboprophylaxis.
Aims: To determine the incidence of thromboembolism in patients with OC from a Canadian population and evaluate the role of risk factors other than cancer.
Methods: We analysed comorbidities and incidence of thromboembolic events in a retrospective cohort study of all patients diagnosed with epithelial OC between January 1996, and December 2017, in British Columbia, Canada. The study was conducted using medical records obtained from PopDataBC, a de‐identified health record database. VTE, ATE and comorbidities in medical records were identified using ICD‐9‐CM and ICD‐10‐CM codes. The presence of comorbidities was compared between cancer patients who developed VTE or ATE and those who did not. Statistical analysis was performed using Chi‐squared test or Fishers exact test in cases where cell counts were <5 patients. A p‐value <0.05 was considered significant.
Results: Of 4,491 patients with epithelial OC (mean age of 61.4 years), 1.36% experienced ATE and 6.75% experienced VTE. Mean follow‐up time was 5.76 years. Sepsis was significantly associated with both VTE and ATE (table 1). Top four risk factors for ATE were: sepsis, peripheral vascular disease, open wound and intracranial injury. The occurrence of ATE and VTE was significantly associated with overall mortality (table 2).
Conclusion(s): Risk factors predicting thromboembolic events in OC patients are not consistent between both ATE and VTE. Thrombosis risk assessment is needed to reduce thrombosis occurrence and improve quality of care in this cohort.
OC 32.5
CD146‐positive tumors are associated with venous thromboembolism
A. Joshkon 1; J. Stalin1; W. Traboulsi1; L. Vivancos ‐Stalin1; M. Nollet1; R. Lacroix2; R. Bachelier1; F. Dignat‐George3; A. Bertaud1; A. leroyer1; N. Bardin4; M. Blot‐Chabaud1
1 INSERM/Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France; 2 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN, Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN – Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France., Marseille, Provence‐Alpes‐Cote d'Azur, France; 4 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN ‐Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France., Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: CD146 is a membrane glycoprotein physiologically present on all the vascular tree where it is involved in angiogenesis. Pathologically, CD146 is expressed by various tumors with a positive correlation between its expression and advanced cancer stages. First identified on melanoma cells, CD146 was thereafter found to be also expressed by many other cancers. In these tumors, CD146 and its secreted soluble form (sCD146), are involved in tumor growth, dissemination and in angiogenesis.
Aims: The aim of the study was to examine a potential role of CD146/sCD146 in cancer‐associated thromboembolism and to test the effect of antibodies targeting the molecule.
Methods: Two highly metastatic CD146‐positive cell lines, the ovarian HEY and melanoma A375, were used for in vitro and in vivo experiments. In vivo, preclinical models of nude mice xenografted with both cell lines were generated and the effect of the anti‐sCD146 antibody was tested by iv injection in the animals. RNA profiling was also performed to analyse differences between cancer cells treated with anti‐sCD146 mAb and cells treated with control IgG.
Results: We show a direct effect of sCD146 on TF expression in cancer cells in vitro. sCD146 upregulated surface TF expression, increased soluble TF in the cells’ supernatant along with an increase in factor Xa and increased the generation of microparticles. In vivo, sCD146 induced venous thromboembolism. An anti‐sCD146 antibody potently neutralized the adverse effect of sCD146 by decreasing the expression of genes related to thromboembolism and by reducing both the number of cancer microparticles and venous thromboembolism in mice.
Conclusion(s): We show a link between CD146 expression on the tumor cells and thrombotic events. Inhibiting the secreted form of CD146 with anti‐sCD146 mAb reduced both tumor dissemination and venous thromboembolism, suggesting that the use of this antibody could be of therapeutic interest in the treatment of CD146‐positive tumors. No conflict of interest.
OC 16.5
Available risk scores modestly predict hemorrhage in patients with cancer‐associated thrombosis (CAT)
K. Sanfilippo 1; S. Luo2; B. Gage3
1 Washington University School of Medicine St. Louis, St. Louis, Missouri, United States; 2 Washington University School of Medicine St. Louis, Saint Louis, Missouri, United States; 3 Washington University School of Medicine St. Louis, St Louis, Missouri, United States
Background: In patients with CAT, the risk of anticoagulant‐related major bleeding (MB) is more than double that of non‐cancer patients. Models that quantify MB risk in patients taking anticoagulants have been developed and validated in non‐cancer populations (i.e. atrial fibrillation, venous thromboembolism (VTE)). However, no validated risk model to quantify MB in cancer patients on anticoagulant therapy is available.
Aims: We aimed to evaluate the performance of existing bleeding risk models, derived in non‐cancer populations, in a cohort of patients with CAT on anticoagulant therapy.
Methods: Using a nationwide cohort of US Veterans, we identified 7489 patients with active cancer and newly diagnosed VTE prescribed anticoagulant therapy between 2012 and 2018. We identified MB within 6 months of anticoagulant start by the presence of ICD‐9/10 codes as previously validated. We evaluated the predictive performance of five models, assigning points as recommended. The association between each point increase and MB was measured using Fine and Gray analysis to account for the competing risk of cancer‐related death. We evaluated model discrimination using Harrell’s c‐statistic.
Results: The distribute of cancer type is shown in Figure 1. Mean age was 66.9 years and median overall survival 17.1 months. Of the cohort, anticoagulant therapy was as follows: 2314 warfarin, 4145 LMWH, 113 fondaparinux, and 1319 DOAC. 702 patients had a MB within 6 months of anticoagulant start. The mean time from anticoagulant start to MB was 55 days. The hazard per each point increase and MB is in Table 2. For model discrimination, the c‐statistic for each score is also listed in Table 2.
Conclusion(s): In this cohort of 7489 patients with active cancer and VTE on anticoagulant therapy, available risk models predicted MB modestly. The data support the need to use risk models derived in patients with cancer for accurate prediction of anticoagulant‐related MB.
OC 25.4
Thrombo‐inflammatory biomarkers in pulmonary embolism as stratified in cancer and non‐cancer patient groups
F. Siddiqui 1; A. Darki1; D. Hoppensteadt1; I. Darwish2; B. Kantarcioglu1; A. Tafur3; J. Fareed1; M. Monreal4
1 Loyola University Chicago, Maywood, Illinois, United States; 2 Loyola University Chicago, Stritch School of Medicine, Maywood, Illinois, United States; 3 Northshore University Hospital, Evanston, Illinois, United States; 4 Department of Internal Medicine, Hospital Universitari Germans Trias i Pujol. Badalona, Barcelona, Spain Chair for the Study of Thromboembolic Disease, Faculty of Health Sciences, UCAM – Universidad Católica San Antonio de Murcia, Spain, Barcelona, Andalucia, Spain
Background: The multifactorial and complex pathophysiology of pulmonary embolism (PE) involves dysregulation of hemostatic system including fibrinolytic imbalance, endothelial compromise, and generation of thrombotic mediators, along with cellular defects and hemodynamic derangement. Cancer is prevalent in PE patients and is considered an added risk factor for the adverse outcomes. Thrombo‐inflammatory biomarkers profiling provide means to risk stratify PE patients.
Aims: This study aimed to profile several of these biomarkers in normal and PE patients and to further demonstrate any potential difference between cancer and non‐cancer patients.
Methods: Four hundred patients of 18 years or older age, were included through enrolment in conjunction with IRB approved project by the pulmonary embolism response team (PERT) registry. Diagnosis of PE was confirmed by computed tomography (CT), angiography or ventilation perfusion imaging. Blood samples were drawn with in 24 h of confirmed diagnosis of acute PE. Control plasma samples were obtained from 50 healthy individuals. All plasma samples were analyzed for D‐Dimer, PAI‐1 antigen, tPA, TAFI‐a, vWF, CRP and IL‐6 were measured by ELISA methods. Microparticles were measured using a chromogenic functional method. Individual biomarkers were compared to normal plasma and further stratified into cancer and non‐cancer.
Results: All the individual biomarkers exhibited varying levels of elevation in PE patients compare to normal’s (Table 1). D‐Dimer and CRP showed the most pronounced increase. When the PE group stratified into cancer and non‐cancer patients, most of the biomarkers except D‐Dimer and microparticles showed varying degree of modest elevation in the cancer group (Table 2). vWF, IL6 and microparticles levels were found to be significantly different in the cancer group.
Conclusion(s): These studies demonstrate that thrombo‐inflammatory biomarkers are upregulated in PE patients. Furthermore, PE patients with cancer exhibit amplified levels of some of the biomarkers suggesting the severity of pathogenesis involving thrombo‐inflammatory mechanisms.
OC 16.4
COMPASS versus Khorana risk assessment model for predicting venous thromboembolic events in patients with non‐small cell lung cancer on active treatment with chemotherapy and immunotherapy
H. Abdel‐Razeq 1; B. Sharaf1; R. AlMasri2; R. Bater1; M. Abualsha'r1; O. Salama1; F. Tamimi1; K. Ashouri1; D. Abu Laban1; T. Salameh1; R. Al zughul1; Y. Alhalaseh1
1 King Hussein Cancer Center, Amman, 'Amman, Jordan; 2 Istishari Hospital, Amman, 'Amman, Jordan
Background: Non‐Small Cell Lung Cancer (NSCLC) is the most common lung cancer subtype. Cancer is known to increase the risk of VTE; majority of which are diagnosed in ambulatory settings. Several RAMs were developed to predict the occurrence of VTE in patients undergoing anti‐cancer therapy. Khorana RAM uses five clinical and laboratory variables to classify patients into 3 levels; low (0), intermediate (1–2), and high (≥3). COMPASS‐CAT RAM, however, uses criteria that include the use of hormonal therapy or anthracyclines, time since diagnosis, central venous catheter (CVC), disease stage, cardiovascular risk factors, recent hospitalization for acute illness, past history of VTE, and platelet count ≥350 × 109/L, and classify patients into Low/Intermediate (0–6) and high risk (≥7) levels.
Aims: We aim to study the predictors of VTE among NSCLC patients treated with chemotherapy and immunotherapy, and compare those two RAMs in predicting VTE.
Methods: Patients with confirmed diagnoses of NSCLC were retrospectively reviewed. Risk of VTE was assessed using both Khorana and COMPASS‐CAT RAMs.
Results: Between 2014 and 2020, a total of 508 patients (mean age ± SD, 58.4 ± 12.2 years) were enrolled. Most (n = 357) patients had adenocarcinoma, and 333 patients had metastatic disease (M1). VTE was confirmed in 76 (15.0%) patients; 51.3% were DVT while 34.2% were PE. Rates were higher among patients with M1‐disease, and those with adenocarcinoma, table. We applied the Khorana RAM on all enrolled patients; VTE rates were 21.2%, 14.1%, and 13.9% among the high, intermediate, and low‐risk, respectively. Nevertheless, by the COMPASS‐CAT RAM 37.4% were classified as high risk; 27.4% had VTE compared to 7.5% of Low/Intermediate risk patients, p < 0.001, table.
Conclusion(s): Patients with NSCLC are at high risk for VTE, especially those with adenocarcinoma or metastatic disease. Compared to Khorana RAM, COMPASS‐CAT RAM was better in identifying more high‐risk patients with higher VTE rate.
OC 25.2
Cabozantinib safety with different anticoagulants in patients with renal cell carcinoma (RCC)
A. Mesleh Shayeb 1; H. Dzimitrowicz2; D. Urman1; N. Dizman3; L. Meza3; A. Sivakumar4; C. Gan5; T. Zhang2; P. Barata6; M. Bilen4; X. Gao7; D. Heng5; S. Pal3; M. Kaymakcalan8; B. McGregor8; T. Choueiri8; R. Mckay1
1 University of California San Diego, San Diego, California, United States; 2 Duke University, Durham, North Carolina, United States; 3 City of Hope, Duarte, California, United States; 4 Emory University, Atlanta, Georgia, United States; 5 Tom Baker Cancer Center, Calgary, Alberta, Canada; 6 Tulane University, New Orleans, Louisiana, United States, 7 Massachusetts General Hospital, Boston, Massachusetts, United States, 8 Dana Farber Cancer Institute, Boston, Massachusetts, United States
Background: Anti‐Xa direct oral anticoagulants (DOACs) are widely used for venous thromboembolism (VTE) treatment in patients with cancer. But, in patients with RCC on cabozantinib, VTE management remains a challenge due to poor understanding of bleeding complications with concurrent use DOACs. Thus far, cabozantinib clinical trials excluded patients on DOACs while allowing concurrent use of low molecular weight heparin (LMWH).
Aims: Investigate cabozantinib safety profile with different anticoagulants in patients with RCC.
Methods: In this updated retrospective multicenter study (8 sites), patients with advanced RCC were allocated into four groups: cabozantinib with concomitant use (at least 1 week) of (1) no anticoagulant, (2) DOACs, (3) LMWH, or (4) warfarin. Primary safety endpoint was major bleeding proportions (defined per ISTH criteria) among the four groups. Secondary efficacy endpoint was new/recurrent VTE proportions while anticoagulated. Overall comparison between groups analysis was by Fisher exact test. Median overall survival between individuals with and without VTE was estimated by Kaplan‐Meier method and survival distribution by Logrank test.
Results: Between 2016 and 2020, 298 patients with RCC received cabozantinib (No anticoagulant 178, LMWH 41, DOAC 64, and warfarin 15). Median age was 62 years [IQR 53–69]. Most were White males, underwent nephrectomy, with intermediate/poor IMDC risk stratification stage 4 clear cell RCC, and had pulmonary metastases. No overall difference of major bleeding events was found between the four‐group comparison (p = 0.088, Table 1). No difference of new/recurrent VTE proportions was found among the different anticoagulant groups. Patients with a VTE had a statistically significant worse survival than patients without a VTE [HR 1.48 (CI 95% 1.05–2.08, p value 0.02), figure 1].
Conclusion(s): This cohort provides first real‐world experience on bleeding and thrombosis complications in patients with RCC. DOACs are safe and effective as an anticoagulant therapy in patients with RCC on cabozantinib. Importantly, VTE is associated with worse mortality in RCC.
OC 25.3
Venous thromboembolism and cancer. Incidence and sex difference
K. Glise Sandblad 1; J. Sörbo2; P. Karlsson3; A. Rosengren4; P. Hansson5
1 Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Department of Medicine, Geriatrics and Emergency Medicine, Östra, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden, Gothenburg, Vastra Gotaland, Sweden; 2 Department of Clinical Physiology, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden, Gothenburg, Vastra Gotaland, Sweden; 3 Department of Oncology, Inst of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Gothenburg, Vastra Gotaland, Sweden; 4 Department of Medicine, Geriatrics and Emergency Medicine, Östra, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden. Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, Gothenburg, Vastra Gotaland, Sweden; 5 Department of Medicine, Geriatrics and Emergency Medicine, Östra, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, Gothenburg, Vastra Gotaland, Sweden
Background: Knowledge of sex differences in venous thromboembolism (VTE) among cancer patients is limited.
Aims: To determine age‐ and sex‐specific incidence of first‐time VTE in patients with a diagnosis of cancer, and to study differences in prevalence of different cancers between men and women with a first time‐VTE.
Methods: Nationwide Swedish registry‐based case‐control study including all patients with first‐time venous thromboembolism (VTE) 1987–2018 and controls free of VTE, matched for age, sex, and county of residence. Incidence was determined by dividing number of patients with VTE and cancer, by the Swedish population in the same age group for each year. Odds ratios (OR) for a diagnosis of cancer (within 7 years of VTE diagnosis) was compared to matched controls, using conditional logistic regression, adjusting for multiple comorbidities.
Results: A total of 63,477 first time VTE patients had a prior or concomitant diagnosis of cancer. On a population level, incidence of VTE in patients with cancer was 22.0 per 100 000 for men and 23.0 per 100 000 for women. The incidence was higher in men compared to women after 64 years of age, Figure 1. When compared with matched controls, women with VTE had slightly higher odds ratio for malignancy than men, multivariable adjusted OR: 2.85 (99% CI 2.78–2.91) vs. 2.56 (2.50–2.62). Cancers with the highest OR were pancreatic cancer, liver, gallbladder and bile duct cancer and lung cancer, with no significant sex difference see Table 1.
Conclusion(s): Incidence rate of VTE in patients with a diagnosis of cancer was higher in men compared to women on a population level. The odds ratio of a diagnosis of cancer was slightly higher in women compared to men in patients with VTE compared to matched controls without VTE.
OC 16.3
Increased rate and risk factors of venous thromboembolism (VTE) in older patients (pts) with melanoma receiving chemotherapy and/or immune checkpoint inhibitors (ICI): A SEER‐Medicare analysis
T. Sussman 1; L. Vu2; S. Markt2; S. Koroukian2; A. Khorana3
1 Dana‐Faber Cancer Institute, Harvard Medical School, Boston, Massachusetts, United States; 2 Case Western Reserve University, Cleveland, Ohio, United States; 3 Cleveland Clinic, Taussig Cancer Institute and Case Comprehensive Cancer Center, Cleaveland, Ohio, United States
Background: Risk of VTE is 4–7 fold higher in cancer pts. Little is known about the risk of VTE in patients with melanoma on ICI compared to chemotherapy.
Aims: We assessed the incidence and risk factors of VTE in melanoma pts on ICI and/or chemotherapy.
Methods: We conducted a cohort study using SEER‐Medicare database to evaluate VTE in pts treated from 2008 to 2019 with ICI (ipilimumab, nivolumab, pembrolizumab) and/or chemotherapy within 2 years of treatment initiation. VTE of deep venous thrombosis and pulmonary embolism were identified by at least two outpatient claims or one inpatient claim. Clinical risk factors of VTE were evaluated by time‐varying Cox analysis.
Results: The cohort comprised 14,456 pts with median age 75 (24–101) years and 68.3% male. Of these, 8.0% received ICI, 69.5% chemotherapy, and 22.5% chemotherapy+ICI. Most had hypertension (72%), 36.4% had prior cerebrovascular disease, and 18% had atrial fibrillation. 11.1% were on anticoagulation. Incidence rates of VTE after treatment start are shown (Figure 1). VTE was highest at 3 months after starting therapy with 25 events per 100 person‐years in those receiving chemotherapy+ICI, 22 events with ICI alone and 19 events with chemotherapy alone. In multivariate analysis, prior history of VTE and hypertension were associated with increased risk of VTE (HR 2.88 [95% CI, 2.54–3.26] and HR 1.29 [95% CI, 1.14–1.46], respectively). Treatment with chemotherapy+ICI was associated with increased risk of VTE (HR 1.42 [95% CI, 1.26–1.59]) over chemotherapy alone (Figure 2). Treatment with ICI alone was associated with similar risk of VTE as chemotherapy alone (HR 1.08 [95% CI, 0.91–1.29]).
Conclusion(s): Highest rates of VTE were observed in pts receiving both ICI and chemotherapy and in the first 3–12 months after starting therapy. Risk factors include history of VTE, hypertension, and treatment with both chemotherapy + ICI. Further studies are needed to identify benefit of thromboprophylaxis.
OC 16.2
Hemorrhage risk in direct oral anticoagulant users receiving tamoxifen for breast cancer versus aromatase inhibitors: The STOP‐Bleed Cancer study
T. Wang 1; A. Clarke2; A. Awan1; M. Carrier1; M. Sood1
1 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 2 Ottawa Hospital Research Institute and ICES, Ottawa, Ontario, Canada
Background: Tamoxifen is commonly used as adjuvant therapy in breast cancer and is postulated to interfere with CYP3A4 and P‐gp pathways. This may pose a higher hemorrhage risk with concurrent direct oral anticoagulants (DOACs) use.
Aims: To assess the risk of hemorrhage in breast cancer patients co‐prescribed a DOAC and tamoxifen compared to aromatase inhibitors (AI).
Methods: This population‐based, retrospective cohort study was conducted among adults of age ≥ 66 who were prescribed tamoxifen compared to AI while on a DOAC in Ontario, Canada from June 2009 to November 2020. The primary outcome is major hemorrhage requiring emergency room visit or hospitalization after prescription until censored or the end of follow‐up on December 31, 2020. Overlap weighted Cox proportional hazard models, accounting for multiple covariates, were used to assess the association between hemorrhage and tamoxifen or AI use with a DOAC.
Results: Among a total of 4753 patients (98.4% women, mean age 77.4 years), 1179 (24.8%) were on tamoxifen and 3574 (75.2%) on AI. Rivaroxaban (53.2%) and apixaban (35.0%) were the most used DOACs. Patients taking AIs were younger, with higher Charlson comorbidity index score and more advanced stage (Table 1). Tamoxifen was not associated with a higher risk of major bleeding events compared to AI when combined with a DOAC [median follow‐up 166 days; Tamoxifen: 29/1179 (2.5%) vs. AI: 119/3574 (3.3%), absolute risk difference 0.8%; weighted hazard ratio 0.68, 95% confidence interval 0.44–1.05]. These results were similar in additional analyses using a more liberal definition of hemorrhage, accounting for kidney function, limiting follow‐up to 90 days, stratifying by incident/prevalent DOAC users, and accounting for cancer duration and the competing risk of death (Table 2).
Conclusion(s): This study suggests that in DOAC users, the concurrent use of tamoxifen poses no higher risk of hemorrhage compared to concurrent use of AI.
OC 16.1
External validation of the Khorana and Vienna‐CATS nomogram scores in cancer patients of the HYPERCAN cohort
M. Marchetti 1; C. Verzeroli2; C. Giaccherini2; L. Russo2; S. Gamba2; C. Tartari2; S. Bolognini2; C. Ticozzi2; A. Santoro3; F. De Braud4; G. Gasparini5; F. Petrelli6; F. Giuliani7; A. D'Alessio8; M. Minelli9; R. Labianca10; C. Morlotti11; P. Malighetti11; D. Spinelli12; A. Falanga13
1 ASST Papa Giovanni XXIII Bergamo, Italy, Dalmine, Lombardia, Italy; 2 ASST Papa Giovanni XXIII Bergamo, Italy, Bergamo, Lombardia, Italy; 3 IRCCS Humanitas Institute, Rozzano, Italy, MIlano, Lombardia, Italy; 4 IRCCS National Cancer Institute, Milan, Italy, Milano, Lombardia, Italy; 5 Hospital San Filippo Neri, Rome, Italy, Roma, Lazio, Italy; 6 Hospital Treviglio‐Caravaggio, Treviglio, Italy, Treviglio, Lombardia, Italy; 7 IRCCS Cancer Institute Giovanni Paolo II, Bari, Italy, Bari, Puglia, Italy; 8 Policlinico San Marco, Zingonia, Italy, Zingonia, Lombardia, Italy; 9 Hospital San Giovanni Addolorata, Rome, Italy, Roma, Lazio, Italy; 10 DIPO, ASST Papa Giovanni XXIII, Bergamo, Italy, Bergamo, Lombardia, Italy; 11 University of Bergamo, Bergamo, Italy, Bergamo, Lombardia, Italy; 12 University of Milan‐Bicocca, Milan, Italy, Bergamo, Lombardia, Italy; 13 ASST Papa Giovanni XXIII Bergamo and University of Milan‐Bicocca, Italy, Bergamo, Lombardia, Italy
Background: Identifying cancer outpatients at high risk of venous thromboembolism (VTE) is an unmet clinical need. The use of validated risk assessment models (RAMs) can be a relevant approach to hit this goal. Among several proposed RAMs, the Khorana risk score (KRS) and the Vienna‐CATS nomogram score have been developed and externally validated in newly diagnosed ambulatory cancer patients.
Aims: In a large prospective cohort of 1,286 outpatients with metastatic non‐small cell lung, colorectal, gastric, or breast cancers undergoing chemotherapy [the HYPERCAN study], we aimed to evaluate the performance of KRS and Vienna‐CATS nomogram score for VTE prediction.
Methods: KRS and Vienna‐CATS nomogram score were applied to the HYPERCAN cohort using pre‐chemotherapy patient variables (leucocyte and platelet counts, hemoglobin level, and BMI for KRS and D‐dimer level (HemosIL D‐dimer HS, Werfen) for Vienna‐CATS nomogram). All objectively confirmed VTE events within 6 months were considered for analysis.
Results: One hundred‐twenty VTE (10.3%) were recorded, including isolated deep vein thrombosis (DVT, 45.8%), pulmonary embolism (PE, 42.5%), and PE+DVT (11.7%). By ROC analysis, KRS provided a non‐relevant AUC of 0.39 for 6‐months VTE. Cumulative VTE incidence was 6.0 % (95% CI 3.8–9.7) for KRS <2 and 12.3% (95% CI 9.9–15.3) for KRS ≥2, p = ns. AUC for 6‐months VTE was 0.63 with Vienna‐CATS nomogram score. Cumulative VTE incidence was 7.2% (95% CI 5.6–9.2) in patients with a predicted VTE risk ≤5% (low risk) and 17% (95% CI 13.5–21.3) in those with a predicted VTE risk >5% (high risk), SHR = 2.4, p < 0.001.
Conclusion(s): In our cohort, the KRS showed a low performance, while the Vienna‐CATS nomogram score significantly categorized patients at low and high VTE risk. The addition of D‐dimer, a circulating coagulation biomarker, seems to be a promising approach towards reliable and clinically applicable RAMs for VTE prediction in these widespread cancers.
Genetic Risk Factors of Thrombosis
OC 42.5
Association of polygenic risk scores for severe mental illness with risk of venous thromboembolism
R. Strawbridge 1; J. Ward1; B. Cullen1; N. Graham1; J. Anderson1; J. Pell1; D. Lyall1; L. Lyall1; D. Smith2; M. Sabater‐Lleal3
1 University of Glasgow, Glasgow, Scotland, United Kingdom; 2 University of Edinburgh, Edinburgh, Scotland, United Kingdom; 3 Sant Pau Biomedical Research Institute (IIB Sant Pau), Barcelona, Spain; Cardiovascular Medicine Unit, Department of Medicine, Karolinska Institutet, Stockholm, Sweden, Barcelona, Catalonia, Spain
Background: Individuals with severe mental illness (SMI, including schizophrenia (SCZ), bipolar disorder (BD) and major depressive disorder (MDD)) have an increased risk of metabolic and cardiovascular diseases, including venous thromboembolism (VTE). There is growing evidence from genetic studies that SMI and cardiometabolic diseases share some pathological mechanisms.
Aims: The aim of this study was to determine whether genetic risk for SMI was associated with risk of VTE.
Methods: Using the large population‐based UK Biobank, we calculated genome‐wide polygenic risk scores (PRS) for MDD, BD and SCZ based on the largest meta‐analyses results from the Psychiatric Genomics consortium. These were assessed for impact on VTE risk (self‐reported deep vein thrombosis and/or pulmonary embolism, case n = 10786, control n = 285124), adjusting for age, sex, eight genetic principal components and genotyping chip. Secondary analyses included further adjustment for BMI, smoking, blood group, hormonal therapy and anti‐psychotic medication, exclusion of individuals with self‐reported SMI and sex‐stratified analyses.
Results: In self‐reported unrelated white British ancestry participants, PRS of MDD or BD‐increasing genetic variants were associated with increased risk of VTE (OR per SD of PRS = 1.08 (CIs 1.06–1.10), p < 0.001 and 1.03 (1.01–1.06) p = 0.002, respectively). The association was independent of age, sex, population structure, blood group, BMI, smoking, anti‐psychotic medication and exogenous hormones (women only). No association was observed between a PRS of SCZ‐increasing genetic variants and risk of VTE. Substituting family history of MDD for the PRS showed similar results.
Conclusion(s): Genetic loading for MDD and BD, but not SCZ was associated with an increased risk of VTE. If replicated, these results suggest that family history of SMI (in the absence of genetic data) should be considered in risk assessments of VTE.
OC 56.5
Insight into genetic predisposition to high plasma levels of Factor VIII (FVIII) and von Willebrand Factor (vWF) in a family with venous thrombosis
S. Spena 1; A. Cairo1; F. Gianniello1; E. Pappalardo2; M. Mortarino1; I. Garagiola3; I. Martinelli1; F. Peyvandi4
1 Angelo Bianchi Bonomi Haemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milano, Lombardia, Italy; 2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, Milan, Lombardia, Italy; 3 Angelo Bianchi Bonomi Haemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milan, Lombardia, Italy; 4 Fondazione IRCCS Ca’ Granda – Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: High levels of FVIII and vWF have been showed as independent risk factors for venous thromboembolism. However, little is known about the genetic factors responsible for their increase. In a woman referred to our Thrombosis unit following a venous thrombotic event, very high levels of FVIII and vWF were found at the acute episode and in subsequent measurements. High levels of FVIII and vWF were also detected in other family members, suggesting a possible genetic cause (Figure 1).
Aims: To identify variants/genes associated with high levels of FVIII and vWF.
Methods: Whole‐exome sequencing was performed in 12 family members (SureSelect‐Agilent). Data were analyzed according to the guidelines of the Broad Institute (https://software.broadinstitute.org/gatk/best‐practices/). Variants were annotated (Annovar) and filtered (KGGseq) considering an autosomal dominant inheritance. RNA levels were evaluated in PBMCs by qPCR (Thermo Fisher).
Results: 16 variants were identified in 11 genes, spread over a 8300‐Kb region on chromosome 5, including the low frequency rs13158382 located in the promoter of miR‐143/145 (Table 1). Since variants in the promoter of miR‐143/145 have been reported to affect their expression and reduced levels of miR‐145 have been associated with high vWF, the potential correlation between rs13158382 and vWF levels was evaluated. Lower mi‐R143/145 levels and higher vWF mRNA levels were measured in 3 family members carrying the rs13158382 variant compared to 3 family members without the variant. Analysis of repository data from the GEUVADIS project confirmed the observed genotype‐phenotype correlation. miRVaS tool predicted a structural change of pre‐miR‐143 with the rs13158382 which might affect Drosha recognition and hence the miRNA processing.
Conclusion(s): In this family‐based study, a genetic variant located in the miRNA‐143/145 promoter, potentially related to thrombotic event by affecting microRNA expression and levels of vWF and complexed FVIII, was identified. Further investigations are necessary to confirm the mechanisms underlying this genetic predisposition.
OC 56.2
Platelet RalA/B play pivotal roles in regulation of venous thrombosis by co‐ordinating P‐selectin‐HMGB1 interaction
Y. Li; C. Williams; B. Amulic; A. Poole
Uinversity of Bristol, Bristol, England, United Kingdom
Background: RalA and RalB are small GTPases which have important functions in cell proliferation and tumour metastasis but their exact roles in thrombosis are less clear. We reported previously that platelet restricted deletion of RalA and RalB (RalAB‐DKO) in mice resulted in near complete defect in P‐selectin externalisation upon activation but no other significant secretion defects were detected. We also showed that RalAB‐DKO resulted in near abolishment of thrombus formation.
Aims: We caught to investigate the underlying mechanism how deletion of RalAB resulted in near complete inhibition of venous thrombosis in mice.
Methods: In vitro NETosis was initiated in cultured mouse neutrophils by adding platelets and their releasates. NET+ cells were detected with confocal microscopy using anti‐CitH3. Thrombus sections were immunohistochemically stained and imaged as detailed in the figure legends.
Results: Confocal microscopy showed that in the WT mice P‐selectin colocalised with HMGB1 in the thrombi to form a multi‐layer scaffolding structure to stabilise the thrombi while P‐selectin and HMGB1 were completely separated in RalAB‐DKO (Fig. 1), indicating that platelet RalAB are required for both NETosis and interaction of P‐selectin‐HMGB1 during venous thrombosis. In an in vitro NETosis assay WT platelet releasates initiated NETosis in 27.83% neutrophils compared to only 1.99% neutrophils in RalAB‐DKO platelets. But 17.38% neutrophils were NET positive when RalAB‐DKO platelet releasates were substituted with WT platelet releasates, partially restoring the NETosis of RalAB‐DKO platelets (Fig. 2).
Conclusion(s): Our data suggest that activated platelets secrete signalling factors to initiate NETosis. It has been reported that deletion of platelet HMGB1 resulted in decreased venous thrombosis and NETosis in mice. We hypothesize that HMGB1 and P‐selectin could be the signalling factors platelets secrete during flow restriction in venous thrombosis. This work was funded by The British Heart Foundation.
OC 56.3
Endothelial overexpression of transforming growth factor beta induced in chronic thromboembolic pulmonary hypertension
M. Bochenek 1; K. Saar2; M. Nazari‐Jahantigh3; C. Wiedenroth4; M. Lankeit1; T. Münzel5; E. Mayer4; S. Guth4; A. Schober3; N. Hübner2; S. Konstantinides1; K. Schäfer6
1 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 2 Max‐Delbrück‐Center for Molecular Medicine, Berlin, Germany, Berlin, Berlin, Germany; 3 Institute for Prophylaxis and Epidemiology of Cardiovascular Diseases, Clinic of the University of Munich, Germany, München, Bayern, Germany; 4 Kerckhoff Heart and Thorax Center, Department of Thoracic Surgery, Bad Nauheim, Germany, Bad Nauheim, Hessen, Germany; 5 Center for Cardiology – Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 6 University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: Angiogenesis is a critical event during venous thrombus remodeling, and endothelial cell‐poor, fibrotic thrombi are obstructing the pulmonary artery lumen in patients with chronic thromboembolic pulmonary hypertension (CTEPH).
Aims: To examine the genetic footprint of endothelial cells from pulmonary thrombofibrotic material to better understand the non‐resolution of thrombus and fibrosis.
Methods: Cells outgrown from pulmonary endarterectomy specimens expressing endothelial cell markers (CTEPH‐ECs) were processed for whole gene microarray analysis. Spatial laser microdissection followed by nCounter gene expression analysis and immunohistochemistry of tissue microarrays were used to confirm expression of candidate proteins in CTEPH tissue. Plasma levels of candidate genes (TGFBI, TAGLN, FSTL3 and STC2) were examined in CTEPH patients. Finally, lentiviral overexpression of TGFBI was used to assess a role of TGFBI in the process of angiogenesis.
Results: Out of 26,808 genes examined, 527 were differentially regulated in CTEPH‐ECs compared to human pulmonary arterial endothelial cells (HPAECs). Biological pathway analysis and RT2 PCR profiler analysis confirmed that factors downstream of transforming growth factor beta (TGFβ) such as TGFβ‐induced (TGFBI) or transgelin (TAGLN), or involved in TGFβ signaling such as follistatin‐like 3 (FSTL3), were expressed at significantly higher levels in CTEPH‐ECs. Spatial laser microdissection followed by nCounter gene expression analysis and immunohistochemistry of tissue microarrays localized potential disease candidates to vessel‐rich regions. Whereas circulating levels of TAGLN and FSTL3 were also increased in patients with pulmonary arterial hypertension, only TGFBI plasma levels were specifically elevated in CTEPH patients and found to decrease after pulmonary endarterectomy. Lentiviral overexpression of TGFBI in HPAECs induced the expression of TAGLN, STC2 and the transcriptional repressor SNAI2, thus phenocopying gene expression patterns in CTEPH‐ECs.
Conclusion(s): Our findings strengthen the importance of significant endothelial alterations in the pathophysiology of CTEPH and suggest that overexpression of TGFBI in endothelial cells is causally involved in the thrombofibrosis.
OC 56.1
A cross‐ancestry meta‐analysis of venous thromboembolism genetic associations
F. Thibord 1; D. Klarin2; D. Chasman3; M. Teder‐Laving4; E. Goode5; K. Hveem6; A. Martinez‐Perez7; J. Emmerich8; X. Jouven9; K. Ito10; G. Cuellar Partida11; J. Haessler12; J. Hansen13; A. van Hylckama Vlieg14; P. Morange15; C. Kabrhel16; D. Tregouet17; S. Damrauer18; A. Johnson19; N. Smith20
1 Population Sciences Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, Boston, Massachusetts, United States; 2 Division of Vascular Surgery, Stanford University School of Medicine, Palo Alto, California, United States; 3 Division of Preventive Medicine, Brigham and Women's Hospital, Boston, Massachusetts, United Statesl 4 Institute of Genomics, University of Tartu, Tartu, Tartumaa, Estonia; 5 Department of Quantitative Health Sciences, Mayo Clinic, Rochester, Minnesota, United States; 6 HUNT Research Center, Department of Public Health and Nursing, Norwegian University of Science and Technology, Levanger, Sor‐Trondelag, Norway; 7 Genomics of Complex Diseases Group, Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau, Barcelona, Catalonia, Spain; 8 Department of vascular medicine, Paris Saint‐Joseph Hospital Group, University of Paris, Paris, Ile‐de‐France, France; 9 Department of Epidemiology, Université Paris Descartes, Sorbonne Paris Cité, Paris, Ile‐de‐France, France; 10 Laboratory for Cardiovascular Genomics and Informatics, RIKEN Center for Integrative Medical Sciences, Yokohama, Kanagawa, Japan; 11 23andMe, Inc., Sunnyvale, California, United States; 12 Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington, United States; 13 Thrombosis Research Center, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway; 14 Leiden University, Medical Center, Leiden, Zuid‐Holland, Netherlands; 15 C2VN, INRAE, INSERM Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France; 16 Emergency Medicine, Massachusetts General Hospital, Boston, Massachusetts, United States; 17 INSERM UMR1219 Bordeaux Population Health Research Center, Bordeaux, Aquitaine, France; 18 Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania, United States; 19 NHLBI, Framingham, Massachusetts, United States; 20 Department of Epidemiology, University of Washington; Department of Health Service, University of Washington; Seattle Epidemiologic Research and Information Center; Department of Veterans Affairs, Office of Research and Development, Seattle, WA 98105, USA, Seattle, Washington, United States
Background: Venous thromboembolism (VTE) is a cardiovascular event that manifests as deep vein thrombosis or pulmonary embolism. Over the last decade, genetic association studies uncovered up to 40 genetic loci associated with VTE risk.
Aims: To discover novel gene loci influencing VTE risk and extend our understanding of the genetic architecture of VTE, we conducted an expanded meta‐analysis of VTE genome wide association studies (GWAS).
Methods: We meta‐analyzed GWAS results from 14 studies, which included 81,669 VTE cases and over a million controls from European, African, Hispanic, East and South‐Asian ancestries. We first conducted a cross‐ancestry discovery meta‐analysis using a subset of 4 studies (n = 55,330 cases), while the remaining studies were reserved to replicate discovery loci that exceeded the significance threshold (P <5 × 10−8). The discovery and replication studies were then meta‐analyzed to create a combined, cross‐ancestry dataset along with Europeans and Africans ancestry‐specific meta‐analyses. Subsequent analyses included fine‐mapping, transcriptome and proteome‐wide association analyses.
Results: The discovery meta‐analysis revealed 85 genome‐wide significant loci, including 48 mapping to gene loci that were not previously reported for VTE. In the replication study, 46 out of the 48 novel loci had a concordant effect direction with the discovery analysis, of which 34 passed the Bonferroni‐corrected significance threshold, including 3 involving variants with low frequency and large effect sizes: ST3GAL4 (frequency = 0.021, OR = 1.19), ZMIZ1 (frequency = 0.029, OR = 1.12) and MAP1A (frequency = 0.027, OR = 0.85). The combined and ancestry‐stratified meta‐analyses established an additional 46 novel signals reaching genome‐wide significance. Conditional analyses revealed secondary signals at 20 loci, while proteome and transcriptome analyses identified putative genes at novel loci (e.g. TIMP3, TIMP4 and NFE2). VTE variants discovered with these analyses were mostly associated with blood traits, and platelet traits in particular.
Conclusion(s): This work substantially increases the known VTE genetic loci, unveiling new insights on the etiology of the disease and potential opportunities for new targeted treatments.
OC 32.1
Identifying novel stabilin‐2 plasma ligands using proximity labeling
M. Underwood; K. Golden; K. Desch
University of Michigan, Ann Arbor, Michigan, United States
Background: Damaging genetic variants in STAB2 increase the risk for venous thromboembolic disease and are associated with elevated levels of von Willebrand factor (VWF). STAB2 encodes stabilin‐2, a clearance receptor for glycosaminoglycans expressed by sinusoidal endothelial cells. We hypothesized that variant stabilin‐2 receptor contributes to venous thromboembolic disease through the reduced clearance of multiple unidentified procoagulant proteins in addition to VWF.
Aims: To better understand the role of stabilin‐2 in venous thromboembolic disease, we aimed to characterize the range of plasma protein ligands that bind to stabilin‐2 using proximity biotinylation.
Methods: In the presence of biotin and ATP, TurboID, a variant biotin ligase, converts biotin into short‐lived biotinoyl‐5′‐AMP, which reacts with nearby lysine residues. TurboID was fused to the extracellular domains of stabilin‐2 (STAB2‐TurboID) and low‐density lipoprotein receptor (LDLR‐TurboID) and stably expressed on the surface of 293 cells. Cells were incubated with pooled fibrinogen deficient plasma, followed by biotin and ATP to initiate labeling. Biotinylated proteins were purified from cell lysates, trypsin digested and identified using tandem mass spectrometry.
Results: Flow cytometry confirmed stabilin‐2 surface expression and binding to fluorescently labeled hyaluronic acid and acetylated LDL. Therefore, addition of TurboID did not interfere with receptor localization or function. From ~7x107 cells expressing STAB2‐TurboID, 146 proteins were identified; of these 20 were plasma proteins. For a comparable number of cells, LDLR‐TurboID labeled 262 proteins, 3 of these were plasma proteins (apolipoprotein‐B100, Apolipoprotein(a) and Apolipoprotein E). Besides self‐labeling, the most abundant plasma proteins labeled by STAB2‐TurboID but not LDLR‐TurboID were Gelsolin and Heparin cofactor II. Among the other unique proteins labeled we identified complement C3, prothrombin, complement C9, plasminogen and VWF.
Conclusion(s): We used a STAB2‐TurboID fusion protein to identify stabilin‐2 plasma protein ligands in vitro. These results suggest damaging variants in STAB2 increase the risk for venous thromboembolic disease through the interaction with multiple procoagulants/proinflammatory proteins.
Thrombophilia
OC 14.5
The severe thrombophilic state associated to inferior vena cava agenesis: Data from a multicentre cohort of 178 patients from the IVC Agenesis Study Group Registry
C. Bravo‐Pérez 1; A. Blanco1; N. Revilla2; S. Asenjo3; S. Bonanad4; N. Castro5; S. Marcellini6; P. López‐Sala7; D. Palomino8; M. Infante9; J. Corral10; J. Rodriguez‐Sevilla11; A. Rodríguez‐Alen12; T. Sevivas13; D. Morello14; V. Vicente15; M. Lozano15; M. de la Morena‐Barrio15; J. García‐Santos1
1 Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 2 Hospital Universitario Ramón y Cajal, Madrid, Spain, Madrid, Madrid, Spain; 3 Hospital Clínico San Carlos, Madrid, Spain, Madrid, Madrid, Spain; 4 Hospital Universitario y Politécnico La Fe, Valencia, Spain, Valencia, Comunidad Valenciana, Spain; 5 Hospital Universitario 12 de Octubre, Madrid, Spain, Madrid, Madrid, Spain; 6 Hospital General de Segovia, Segovia, Spain, Segovia, Castilla y Leon, Spain; 7 Hospital Universitario Donostia, San Sebastián, Spain, San Sebastián, Pais Vasco, Spain; 8 Complejo Asistencial Universitario de Salamanca, IBSAL, Salamanca, Spain, Salamanca, Castilla y Leon, Spain; 9 Hospital Universitario Infanta Leonor, Madrid, Spain, Madrid, Madrid, Spain; 10 University of Murcia, IMIB CIBERER, Murcia, Murcia, Spain; 11 Hospital del Mar, Barcelona, Spain, Barcelona, Catalonia, Spain; 12 Hospital Virgen de la Salud, Toledo, Spain, Toledo, Castilla‐La Mancha, Spain; 13 Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal, Coimbra, Coimbra, Portugal; 14 Hospital Clinico Universitario de Valencia. INCLIVA. Biomedial Research Institute., Valencia, Comunidad Valenciana, Spain; 15 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain
Background: Agenesis of inferior vena cava (AIVC) is a rare anomaly which increases the risk of thrombosis. Although the cause of AIVC is not known, it might be associated to intrauterine/perinatal thrombosis. We recently described a high penetrance of AIVC (70.8%) in severe thrombophilia secondary to homozygous antithrombin Budapest3.
Aims: To build a multicentre registry of patients with AIVC (the IVC Agenesis Study), aimed at improving knowledge on the associated thrombotic risk and underlying thrombophilias.
Methods: Systematic search of cases with radiologic evidence of AIVC was conducted in 15 hospitals from Spain and Portugal, using automated data‐extraction software in free‐text image reports from 2010 to 2021. Radiologic and clinical variables were collected.
Results: We recruited 178 AIVC patients (Table1). One hundred (56.5%) developed thrombosis, with early (median: 33.7 y.o.) and recurrent (33.0%) events. Only 48 (27.0%) had been referred for thrombophilia screening, and 19 (39.6%) carried a thrombophilic defect. Forty‐eight (27.0%) subjects had isolated hepatic AIVC, while 130 (73.0%) had infrarenal involvement. Demographics were similar among groups, but cases with infrarenal AIVC showed higher thrombotic rate (66.9% vs. 27.7%, p < 0.0001) and more severe clinical phenotype (Table1). Thrombosis associated to infrarenal AIVC tended to be located at lower extremities, as well as to have earlier onset and higher recurrence than those secondary to hepatic AIVC. Moreover, the profile of associated congenital malformations significantly differed between groups (Table1).
Conclusion(s): Our results demonstrate the existence of two forms of AIVC, with distinct thrombotic phenotypes. Different molecular and thrombophilic defects might be explored in these groups. But more remarkably, our findings show that, despite having high thrombotic risk, these patients remain out‐of‐the‐scope of hematologists. Our work supports the use of thromboprophylaxis in high‐risk situations, and of long‐term anticoagulation in symptomatic cases. Multidisciplinary management and future studies assessing the clinical utility of thrombophilia screening in AIVC should be guaranteed. Funding:ISCIII&FEDER:PI21/00174,CM20/00094.SETH:Premio López‐Borrasca/2021. TABLE 1 Clinical data and thrombotic history of the overall cohort of AIVC patients (n = 178) and comparison of cases with hepatic vs. infrarenal AIVC
TABLE 1 Clinical data and thrombotic history of the overall cohort of AIVC patients (n = 178) and comparison of cases with hepatic vs. infrarenal AIVC
OC 56.4
Targeted panel sequencing for identifying new rare genetic variants associated with venous thromboembolism (VTE): Application to 128 patients with unprovoked VTE and negative thrombophilia screening
P. Suchon 1; N. Saut2; M. Alessi3; D. Tregouet4; P. Morange5
1 Aix Marseille Univ, INSERM, INRAE, C2VN, Marseille, France; Laboratory of Haematology, La Timone Hospital, Marseille, France, marseille, Provence‐Alpes‐Cote d'Azur, France; 2 Laboratory of Haematology, La Timone Hospital, Marseille, France, marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Aix Marseille Université, Marseille, Provence‐Alpes‐Cote d'Azur, France; 4 INSERM UMR1219 Bordeaux Population Health Research Center, Bordeaux, Aquitaine, France; 5 C2VN, INRAE, INSERM Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: The genetic heritability of venous thromboembolism (VTE) is high (35%–60%). Rare variants have been proposed to cause severe rare inherited VTE whose identification is facilitated by the development of next generation sequencing technologies.
Aims: The aim of the present study was to identify candidate rare variants that could explain unprovoked VTE in 128 patients aged below 60yrs and presenting with negative thrombophilia screening, most of which with a family history of VTE.
Methods: Patients were sequenced using a targeted DNA panel of 20 coagulation genes . Identified rare variants (Minor Allele Frequencies MAF ≤0.1% in the general population) were then classified according to American College of Medical Genetics (ACMG) guidelines.
Results: The molecular screening of the regulatory and coding regions of the selected 20 genes (Table 1) identified 15 potentially pathogenic variants (ACMG Class 4&5) and 21 Class 3 variants of uncertain significance (VUS), most of them (31/36) being non synonymous ones. Among class 4–5 variants, 5 were located in inhibitors genes (SERPINC1, PROC, PROS1) and were identified in patients in whom thrombophilia screening had been performed under anticoagulant treatment preventing one from measuring inhibitors in their plasma samples. In addition, 10 class 4–5 variants were identified in F2, F5, F7, F10, FGA, TFPI among which 7 have never been reported in public database. Finally, 4 VUS were identified in SERPINC1 and 7 in PROS1, all in patient samples without deficiency of the corresponding protein. The PROS1 Heerlen S501P variant was present in 3 patients (MAF = 0.012) at a much higher frequency than that reported in the gnomAD database (MAF = 0.003).
Conclusion(s): This study allowed the discovery of potentially strongly VTE‐associated variants despite a negative thrombophilia screening and suggests that target panel sequencing could help identifying molecular defects in natural coagulation inhibitors in patients under anticoagulant treatment. Functional validation of newly identified variants remained to be performed.
OC 60.2
Impact of thrombophilia testing on treatment decision and outcome of thromboembolism and pregnancy morbidity: A single center retrospective cohort study
K. Vrotniakaite‐Bajerciene 1; T. Tritschler2; K. Jalowiec3; H. Broughton3; J. Brodard3; A. Haynes4; A. Rovo3; J. Kremer Hovinga5; D. Aujesky6; A. Angelillo‐Scherrer3
1 Bern University Hospital, Bern, Bern, Switzerland; 2 Inselspital, Bern University Hospital, Bern, Bern, Switzerland; 3 Bern University Hospital, Department of Hematology and Central Hematology Laboratory, Bern, Bern, Switzerland; 4 Bern University, CTU Bern, Bern, Bern, Switzerland; 5 Department of Hematology and Central Hematology Laboratory, Bern University Hospital, University of Bern, Bern, Bern, Switzerland; 6 Bern University Hospital, Department of General Internal Medicine, Bern, Bern, Switzerland
Background: Clinical utility of thrombophilia testing remains a topic of controversy since its introduction in clinical practice, because data showing its clinical usefulness and benefits for clinical decision‐making are limited.
Aims: To investigate the impact of thrombophilia work‐up on treatment decisions and recurrence of venous and arterial thromboembolic event and pregnancy morbidity in an observational study of unselected patients.
Methods: We conducted a single‐center retrospective cohort study of 3686 patients referred for thrombophilia consultation at the Bern University Hospital (Switzerland) from January 2010 to October 2020. Results of thrombophilia work‐up, and clinical and laboratory data were recorded. We studied the impact of thrombophilia testing results on treatment decisions according to guidelines and documented thromboembolic and pregnancy morbidity event after thrombophilia testing up to March 2021.
Results: In 3550 patients (94%), a partial or full thrombophilia testing was performed and 1258 patients (28.9%) displayed at least one thrombophilia (Figure 1A). Most patients were tested because of venous thromboembolism (2407, 65%), followed by patients with arterial thromboembolism (591, 16%) and with pregnancy morbidity (121, 3.3%). 341 asymptomatic subjects (30%), mainly patient family members, were also included. Only 211 (5.7%) work‐ups provided further guidance to extend, initiate or stop anticoagulation (Table 1). 2565 patients (70%) were followed‐up more than 30 days with a median follow‐up of 48 months (1 – 183 months). Patients with high‐risk thrombophilia had significantly more new venous thromboembolic events and new pregnancy morbidity compared to those without any thrombophilia or with low‐risk thrombophilia (Figure 1B).
Conclusion(s): Our study demonstrates the limited usefulness of thrombophilia work‐up on clinical decision‐making on all types of index thromboembolic events, pregnancy morbidity and asymptomatic family members. Selection criteria to identify high‐risk thrombophilia must be improved.
VTE Diagnosis
OC 31.4
Diagnostic accuracy of V/Q and Q SPECT/CT in patients with suspected pulmonary embolism: a systematic review and meta‐analysis
A. Venturini1; A. Squizzato 2; V. Pelitti1; B. Bellini1; M. Bernasconi3; A. Corso4; N. Riva5; T. Depalo4
1 Internal Medicine Residency Program, School of Medicine, University of Insubria, Varese and Como, Italy, Como, Lombardia, Italy; 2 University of Insubria, Varese and Como, Italy, Como, Lombardia, Italy; 3 Internal Medicine Unit, ASST Settelaghi, Cittiglio, Italy, Varese, Lombardia, Italy; 4 Nuclear Medicine Unit, ‘Sant’Anna’ Hospital, ASST Lariana, Como, Italy, Como, Lombardia, Italy; 5 Department of Pathology, Faculty of Medicine and Surgery, University of Malta, Msida, Malta, Msida, Not Applicable, Malta
Background: Computed tomography (CT) pulmonary angiography has simplified the diagnostic approach to patients with clinically suspected pulmonary embolism (PE), but alternative imaging tests are still advocated.
Aims: We aimed to systematically assess the diagnostic accuracy of ventilation/perfusion (V/Q) and Q single‐photon emission CT combined with low‐dose CT (SPECT/CT) for PE diagnosis.
Methods: Studies evaluating the diagnostic accuracy of SPECT/CT for the diagnosis of PE were systematically searched in MEDLINE and EMBASE databases (up to July 2021). QUADAS‐2 tool was used for risk of bias assessment of the primary studies. A bivariate random‐effects regression approach was used for summary estimates of both sensitivity and specificity. PROSPERO registration number is CRD42021276538.
Results: Six studies, for a total of 974 patients, were included. Weighted mean prevalence of PE was 24.9% at random‐effect model. Weighted mean inconclusive SPECT/CT results were 2.5% (95% CI, 0.2%–6.9%) at random‐effect model. After exclusion of technical inadequate results, SPECT/CT bivariate weighted mean sensitivity was 96% (95% confidence interval [CI], 93%–98%), with a bivariate weighted mean specificity of 96% (95% CI, 94%–97%). At subgroup analysis, for V/Q SPECT/CT bivariate weighted mean sensitivity and specificity were 97% (95% CI, 92%–99%) and 97% (95% CI, 95%–98%), while for Q SPECT/CT they were 95% (95% CI, 90%–98%) and 84% (95% CI, 61%–95%), respectively.
Conclusion(s): V/Q SPECT/CT has high sensitivity and specificity for the diagnosis of PE, meanwhile Q SPECT/CT has high sensitivity but limited specificity for the diagnosis of PE. Management studies will conclusively ascertain the actual role of SPECT/CT in the diagnostic workup of patients with suspected PE.
OC 31.5
Identifying which emergency department patients should be tested for pulmonary embolism
D. Christopher1; K. Grewal2; S. Ghazalbash3; F. Mowbray3; M. Zargoush3; K. de Wit 4
1 CanVECTOR, Burlington, Ontario, Canada; 2 Schwartz/Reisman Emergency Medicine Institute, Toronto, Ontario, Canada; 3 McMaster University, Hamilton, Ontario, Canada; 4 Queen's University, Kingston, Ontario, Canada
Background: Pulmonary embolism (PE) can be misdiagnosed wen emergency physicians do not consider PE as a potential diagnosis. Little research has focused on who should be tested for PE.
Aims: To develop a predictive model which identifies underlying PE diagnosis in emergency department patients.
Methods: Using linked population‐level administrative data from Ontario (Canada), we analyzed the first patient emergency department visit in each calendar year between 2015 and 2019. Patients were classified as presenting with PE if they were diagnosed with PE on that visit, within the following 30 days if there was no hospitalization, within seven days of hospitalization without surgery, or else prior to surgery within seven days. We collected patient data on age, sex, cancer, prior DVT and PE, pregnancy, coronary artery disease, COPD, diabetes, stroke, anticoagulant use, atrial fibrillation, recent hospitalization, recent surgery, presenting complaint and triage score. Logistic regression and 10‐fold cross‐validation were used to derive a model which predicts the diagnosis of PE for the year 2015, and subsequently validated the model performance on each calendar year 2016–2019.
Results: We analyzed 7,024,111 emergency department visits with 18751 cases of PE. Mean age 48.6, 52% female, 3% history of cancer, 1.7% stroke, 1% prior PE, 1.6% prior DVT, 1.9% recent admission and 4.4% recent surgery. Our final model predicting a diagnosis of PE contained age, prior PE, prior DVT, presenting complaint and triage category. The area under the curve for the predicting PE was 82.8 in 2015, and 84.5, 81.3, 78.4 and 80.6 for each subsequent year. The optimal cut‐off gave sensitivity estimates between 69.4% and 74.4% and specificity estimates 73.7% to 76.0% for each of the 5 years.
Conclusion(s): We derived a model which accurately predicts the diagnosis of PE in emergency department patients, with the potential to trigger PE testing in the emergency department. FIGURE 1 Model area under the curve for year 2015.
FIGURE 1 Model area under the curve for year 2015.
OC 60.4
Prognostic model for predicting the risk of symptomatic venous thromboembolism in children and adolescents with lymphoma
D. Evstratov 1; N. Myakova1; P. Zharkov2
1 Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 2 Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia
Background: Pediatric patients with lymphoma are in the higher risk of symptomatic venous thromboembolism (sVTE) compared with patients with other malignancies except acute lymphoblastic leukemia.
Aims: To evaluate pediatric lymphoma patients with high risk of sVTE.
Methods: Our study is a monocentric retrospective analysis of 262 patients aged <18 years with lymphoma that were treated in our Center since 2013–2019 year. Chi‐square test has been used for comparison of qualitative variables, Mann‐Whitney test – for comparison of quantitative variables. Risk factors were analyzed by univariate and multivariate analysis with logistic regression. ROC‐analysis was used for the determination of optimal cutoff. p‐Value <0.05 was considered as significant.
Results: In an univariate analysis such factors as age (12.1 ± 4.2 years vs. 10.3 ± 4.7 years; p = 0.009) with optimal cut‐off 12 years, non‐O blood group (10.3%, 95% CI: 5.8%–14.8% vs. 3.4%, 95% CI: 0%–7.2%; p = 0.084), the volume of mediastinal mass (OR = 1.075; 95% CI: 1.01–1.143, р = 0.02 for every 100 ml increment) with the optimal cutoff 250 ml, mean body mass index (BMI, 20.7 ± 5.1 vs. 17.9 ± 3.8 kg/m2, p = 0.005) with optimal cutoff 18 kg/m2 and ICU admittance during the first 30 days of hospitalization (ICU+) due to life‐threatening condition (OR = 3.96; 95% CI: 1.396–11.252, р = 0.01) were statistically significant associated with sVTE risk and subsequently included in the multivariate model (table 1). The optimal cutoff for “high risk group of sVTE” was ≥11 points. The cumulative incidence of sVTE in “high risk” and “standard risk” groups are presented in the figure 1.
Conclusion(s): The model developed in our Center allows to stratify pediatric patients with lymphoma in “high risk group of sVTE”.
OC 60.5
Artificial intelligence in the prediction of venous thromboembolism: A systematic review and pooled analysis
T. Chiasakul 1; B. Lam2; W. Robertson3; L. Lake3; R. Rosovsky4; I. Vlachos2; J. Zwicker2; R. Patell2
1 King Chulalongkorn Memorial Hospital, Arlington, Virginia, United States; 2 Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States; 3 National Blood Clot Alliance, Philadelphia, Pennsylvania, United States; 4 Massachusetts General Hospital, Boston, Massachusetts, United States
Background: Accurate diagnostic and prognostic predictions of venous thromboembolism (VTE) are crucial for VTE management. Artificial intelligence (AI) enables autonomous identification of the most predictive patterns from large complex data. Evidence regarding its performance in VTE prediction are emerging. Systematic reviews of current evidence are lacking.
Aims: To systematically review the performance of AI in the diagnosis and prediction of VTE and compare it to clinical risk assessment models (RAMs) or logistic regression models.
Methods: A systematic literature search was performed using PubMed, MEDLINE, EMBASE, and Web of Science from inception to April 20, 2021. Search terms included ‘artificial intelligence’ and ‘venous thromboembolism’. Eligible criteria were original studies evaluating AI in the prediction of VTE in adults and reporting one of the following outcomes: sensitivity, specificity, positive predictive value, negative predictive value, area under receiver operating curve (AUC), or c‐statistic. Studies assessing Natural Language Processing (NLP) accuracy to detect VTE from radiological reports or medical records were excluded. Risks of bias were assessed using the PROBAST tool. Unpaired t‐test was performed to compare the mean AUC from AI versus conventional methods (RAMs or logistic regression models).
Results: A total of 20 studies were included (Fig. 1). Numbers of participants ranged from 31 to 111,888. The AI methods included machine learning (11 studies), artificial neural network (4 studies), NLP (2 studies), support vector machines (1 study), Bayesian methods (1 study), and genetic programming (1 study). Twelve studies (60%) had both training and testing cohorts. Most studies had high risk of bias due to missing data handling and outcome determination. Among 14 studies where AUCs were reported, the mean AUC for AI vs. conventional methods were 0.79 (95% CI 0.74–0.85) vs. 0.61 (95% CI 0.54–0.68), respectively (P <.001) (Fig. 2).
Conclusion(s): The use of AI appears to improve the accuracy of diagnostic and prognostic prediction of VTE.
OC 31.3
Canadian prospective external validation of YEARS and age‐adjusted pulmonary embolism testing
K. de Wit 1; F. Al‐Haimus2; Y. Hu3; R. Ikesaka4; N. Chan4; Q. Ibrahim4; J. Klyn4; N. Clayton4; F. Germini5
1 Queen's University, Kingston, Ontario, Canada; 2 University of Toronto, Toronto, Ontario, Canada; 3 McMaster University, Ottawa, Ontario, Canada; 4 McMaster University, Hamilton, Ontario, Canada; 5 McMaster University Medical Centre, Hamilton, Ontario, Canada
Background: The frequency of emergency department pulmonary embolism (PE) testing is increasing, and CT pulmonary angiogram studies are commonplace. Neither YEARS nor age‐adjusted PE testing have been prospectively evaluated in North America.
Aims: To assess the diagnostic accuracy of YEARS and age‐adjusted algorithms in Canadian emergency departments.
Methods: This prospective diagnostic accuracy study was conducted in two tertiary care Canadian emergency departments, November 2019 to June 2021. Patients were included when they were tested for PE in the emergency department. We excluded repeat visits and cases without YEARS item documentation. Research ethics approved waiver of patient consent. The study was funded by PSI and SIDM. Physicians used a standardized order set for PE testing whereby all patients had D‐dimer testing (STA Liatest). If the D‐dimer was <500 ng/ml, PE was excluded. CT pulmonary angiography was performed when the D‐dimer was ≥ 500 ng/ml. Patients were followed for 30 days by medical record review in every hospital in the city. PE was independently adjudicated. Physicians systematically recorded the presence or absence of YEARS items (PE most likely, hemoptysis, signs of deep vein thrombosis) prior to D‐dimer or CT testing. We analyzed the diagnostic accuracy of YEARS and age‐adjusted D‐dimer (age‐adjustment (age x 10) was applied in patients where PE was not the most likely diagnosis and 500 ng/ml threshold when PE was most likely).
Results: 1704 patients were included, median age 62 (50, 74), 58% female, PE prevalence 8.0%. See Figure 1 for YEARS and Figure 2 for age‐adjusted testing results. YEARS sensitivity was 92.6% (87.0, 96.0), specificity 45.0% (42.5, 47.5), NPV 98.6% (97.4, 99.2), PPV 12.6% (10.7, 14.9). Age‐adjusted D‐dimer sensitivity was 100.0% (97.2, 100.0), specificity 32.4% (30.1, 34.8), NPV 100.0% (99.2, 100.0), PPV 11.4% (9.7, 13.3).
Conclusion(s): YEARS was less sensitive and more specific than age‐adjusted D‐dimer for PE diagnosis.
OC 60.3
Cost‐effectiveness of performing reference ultrasonography in patients with deep vein thrombosis
C. De Jong 1; W. van den Hout2; C. van Dijk3; L. van Dam4; G. Gautam5; W. Ghanima6; J. Gleditsch7; A. von Heijne5; H. Hofstee8; M. Hovens9; M. Huisman10; A. Mairuhu11; M. Nijkeuter12; M. van de Ree13; C. van Rooden14; R. Westerbeek15; J. Westerink16; E. Westerlund5; L. Kroft17; F. Klok1
1 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 2 Department of Biomedical Data Sciences – Medical Decision Making, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 National Health Care Institute, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 5 Department of Clinical Sciences, Karolinska Institutet, Danderyd Hospital, Stockholm, Stockholms Lan, Sweden; 6 Østfold Hospital Foundation, Sarpsborg, Ostfold, Norway; 7 Department of Radiology, Hospital of Ostfold, Kalnes, Sarpsborg, Ostfold, Norway; 8 Department of Internal Medicine, Haaglanden Medical Center, The Hague, Zuid‐Holland, Netherlands; 9 Department of Vascular Medicine, Rijnstate Hospital, Arnhem, Gelderland, Netherlands; 10 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 11 Department of Internal Medicine, Haga Teaching Hospital, Den Haag, Zuid‐Holland, Netherlands; 12 Department of Internal Medicine, University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 13 Department of Vascular Medicine, Diakonessen Hospital, Utrecht, Utrecht, Netherlands; 14 Department of Radiology, Haga Teaching Hospital, The Hague, Zuid‐Holland, Netherlands; 15 Department of Radiology, Deventer Hospital, Deventer, Overijssel, Netherlands; 16 Department of Vascular Medicine, University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 17 Department of Radiology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: The accurate diagnosis of recurrent ipsilateral deep vein thrombosis (DVT) with compression ultrasonography (CUS) is often hindered by chronic residual abnormalities after a previous DVT. Reference CUS, an additional ultrasound performed at the time of discontinuation of anticoagulant treatment, may improve the diagnostic work‐up of suspected recurrent ipsilateral DVT by providing baseline images for future comparison. Currently, it is unknown whether the application of reference CUS is cost‐effective.
Aims: To evaluate the cost‐effectiveness of applying reference CUS in patients with a previous DVT.
Methods: Patient‐level data of the Theia study (NCT02262052) and claims data were used in a decision analytic model to compare 12 scenarios for diagnostic management of suspected recurrent ipsilateral DVT. The scenarios included a clinical decision rule (CDR) and D‐dimer, CUS and/or magnetic resonance direct thrombus imaging (MRDTI), with and without reference CUS (Figure 1). Estimated total costs related to the associated mortality due to misdiagnosis, recurrent venous thromboembolism and bleeding during first year of treatment and follow‐up were compared.
Results: All six scenarios including reference CUS had higher estimated one‐year costs (€1763 to €1913; Figure 2), compared to the six scenarios without reference CUS (€1192 to €1474). Costs were higher since reference CUS results remained unused in patients without suspected recurrent DVT, which was determined to be 80% based on claims data. Estimated mortality was comparable in scenarios with reference CUS (14.8–17.9 per 10,000 patients) and without reference CUS (14.0–18.5 per 10,000 patients). None of the four potentially optimal scenarios (the ‘efficient frontier’) included reference CUS.
Conclusion(s): In this model, the one‐year healthcare costs of diagnostic strategies for suspected recurrent ipsilateral DVT including reference CUS are higher compared to strategies without reference CUS, without mortality benefit. These results inform policy‐making regarding use of healthcare resources during follow‐up after DVT and discourage the use of reference CUS in terms of cost‐effectiveness.
OC 31.2
External validation of the PEGeD diagnostic algorithm for suspected pulmonary embolism in an independent cohort
H. Robert‐Ebadi1; P. Roy2; O. Sanchez3; F. Verschuren4; G. Le Gal5; M. Righini 6
1 Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland, Geneva, Geneve, Switzerland; 2 University Hostpial of Angers, Angers, Pays de la Loire, France; 3 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 4 Saint‐Luc University Hospital, Bruxelles, Brussels Hoofdstedelijk Gewest, Belgium; 5 University of Ottawa, Ottawa, Ontario, Canada, 6 Division of Angiology and Hemostasis, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland, Geneva, Geneve, Switzerland
Background: Validated diagnostic algorithms are used to manage patients with clinically suspected pulmonary embolism (PE). The recently published PEGeD study proposed a new diagnostic strategy which appears to safely reduce the use of computed tomography pulmonary angiography (CTPA).
Aims: We aimed to externally validate this diagnostic strategy in an independent cohort.
Methods: We analyzed data from three prospective cohort studies of outpatients with suspected PE. As per the PEGeD algorithm using the Wells score with adapted thresholds, patients were classified as having a low, moderate or high clinical pre‐test probability (C‐PTP). PE was excluded with a D‐dimer <1000 ng/ml in case of low C‐PTP and <500 ng/ml in case of moderate C‐PTP. We assessed the yield and safety of this approach compared to previously validated algorithms.
Results: Among 3302 patients evaluated in our cohort, 1621 (49.0%) patients could have had PE excluded according to the PEGeD diagnostic algorithm, without the need for imaging. Of these patients, 38 (2.3%; 95% CI 1.7 – 3.2) were diagnosed with a symptomatic PE at initial testing or during the 3 month follow‐up period. On further analysis, 36 patients out of these 38 patients had a positive age‐adjusted D‐dimer. The risk of VTE among the 414 patients with a D‐dimer below 1000 ng/ml but above the age‐adjusted D‐dimer cutoff was 36/414 (8.7%; 95 % CI 6.4–11.8%).
Conclusion(s): We provide external validation of the PEGeD diagnostic algorithm in an independent cohort. Compared to standard algorithms, the PEGeD diagnostic strategy decreased the number of CTPA examinations required at presentation. However, in our high PE prevalence clinical setting (22%, versus 7% in the PEGeD study), the risk of false negatives in patients with a negative PEGeD algorithm but with a positive age‐adjusted D‐dimer deserves caution and further validation studies prior to implementation. FIGURE 1 Study flow chart
FIGURE 1 Study flow chart
OC 60.1
Performance of clinical decision rules for venous thromboembolism in patients with a history of thrombosis
V. Mai 1; F. Klok2; M. Righini3; S. Schulman4; V. Thiruganasambandamoorthy5; S. Kahn6; V. Bates7; A. Pecarskie7; M. Kovacs8; S. Visser9; S. Shivakumar10; M. Tan11; E. Martens12; M. Rodger13; D. Scarvelis7; A. Delluc7; P. Girard14; M. Huisman15; P. Wells7; G. Le Gal7
1 Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada; 2 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 3 Division of Angiology and Hemostasis, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland, Geneva, Geneve, Switzerland; 4 McMaster University, Hamilton, Ontario, Canada; 5 University of Ottawa, Department of Emergency Medicine, Ottawa, Ontario, Canada, Ottawa, Ontario, Canada; 6 Division of Internal Medicine, Department of Medicine, McGill University, Montreal, Québec, Canada, Montreal, Quebec, Canada; 7 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada; 8 Division of Hematology, Department of Medicine, University of Western Ontario, London, Ontario, Canada, London, Ontario, Canada; 9 Department of Emergency Medicine, Hôpital Monfort, Ottawa, Ontario, Canada, Ottawa, Ontario, Canada; 10 Department of Medicine, Dalhousie University, Nova Scotia Health, Halifax, Nova Scotia, Canada, Halifax, Nova Scotia, Canada; 11 Amsterdam University Medical Center, Amsterdam, Netherlands, Amsterdam, Noord‐Holland, Netherlands; 12 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 13 Department of Medicine, McGill University, McGill University Health Center, Montreal, Quebec, Canada, Montreal, Quebec, Canada; 14 Département de Pneumologie, Institut Mutualiste Montsouris, Paris, France; F‐CRIN INNOVTE Network, Saint‐Etienne, France, Saint‐Étienne, Rhone‐Alpes, France; 15 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Amongst patients with clinically suspected venous thromboembolism (VTE), about 20% are suspected recurrences. Diagnosing recurrent VTE may be challenging. Interpretation of diagnostic tests usually rely on clinical pre‐test probability based on clinical decision rules (CDR). However, current CDR have not been validated in patients with a history of VTE.
Aims: To assess and refine the diagnostic algorithm in patients with suspected recurrent VTE. In the present analysis, we reported the accuracy and usefulness of Wells deep vein thrombosis (DVT), Wells pulmonary embolism (PE) and revised Geneva CDR in patients with a clinically suspected recurrent VTE.
Methods: We conducted an international prospective multicenter observational cohort study (PREDICTORS study) of outpatients with suspected VTE recurrence seen in the Emergency Departments or Thrombosis Clinics. Diagnostic management was performed based on usual clinical practices. Standardized case report forms of predictors and diagnostic tests were completed. Wells DVT score was computed in patients with suspected DVT. Wells PE and revised Geneva score were computed in patients with suspected PE. All patients were followed for 3 months and all suspected recurrent episodes during follow‐up were independently adjudicated.
Results: 723 patients were included. Amongst patients with suspected DVT, 18/144 (12.5%) and 115/306 (37.6%) patients had a VTE recurrence at baseline and during follow‐up in the Wells DVT unlikely and likely groups, respectively. Amongst patients with suspected PE, 14/90 (15.6%), 64/183 (35.0%) and 25/44 (56.8%) patients had a VTE recurrence in the Wells PE low, moderate and high‐risk groups, respectively. Based on the revised Geneva score, VTE recurrence occurred in 4/50 (8.0%), 67/209 (32.1%) and 32/58 (55.2%) in the low, intermediate, and high‐risk groups, respectively.
Conclusion(s): Wells DVT, Wells PE and revised Geneva CDR are able to risk stratify patients with suspected VTE recurrence. Further studies are needed to improve the yield of non‐imaging diagnostic modalities. TABLE 1 Baseline characteristics of the patients
TABLE 1 Baseline characteristics of the patients
OC 31.1
Performance of the 4‐level pulmonary embolism clinical probability score (4PEPS) in the diagnostic management of pulmonary embolism
M. Stals 1; J. Knijp2; L. Beenen3; M. Coppens4; L. Faber5; H. Hofstee6; M. Hovens7; M. Huisman8; T. van der Hulle9; K. Kaasjager10; M. Kruip11; A. Mairuhu12; S. Middeldorp13; M. Ten Wolde14; F. Klok1; N. van Es15
1 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 2 Amsterdam UMC, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Radiology, Amsterdam University Medical Centres, Amsterdam, Noord‐Holland, Netherlands; 4 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 5 Department of Hematology, Red Cross Hospital, Beverwijk, the Netherlands, Beverwijk, Noord‐Holland, Netherlands; 6 Department of Internal Medicine, Haaglanden Medical Center, The Hague, Zuid‐Holland, Netherlands; 7 Department of Vascular Medicine, Rijnstate Hospital, Arnhem, Gelderland, Netherlands; 8 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 9 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 10 Department of Internal Medicine, University Medical Center Utrecht, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 11 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 12 Department of Internal Medicine, Haga Teaching Hospital, Den Haag, Zuid‐Holland, Netherlands; 13 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands; 14 Department of Internal Medicine, Flevo Hospital, Almere, the Netherlands., Almere, Flevoland, Netherlands; 15 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands
Background: Recommended diagnostic strategies for ruling out pulmonary embolism (PE) consist of clinical pre‐test probability (CPTP) assessment and D‐dimer testing. The recently published 4‐level Pulmonary Embolism Clinical Probability Score (4PEPS) integrates different aspects from currently available strategies to further reduce imaging testing.
Aims: To externally validate the diagnostic performance of 4PEPS in an independent cohort.
Methods: In this post‐hoc analysis of the YEARS study primary outcome measures were discrimination, calibration, efficiency (number of imaging tests potentially avoided), and failure rate (defined as venous thromboembolism (VTE) diagnosis at baseline or follow‐up in patients with negative 4PEPS algorithm). Patients were classified based on the twelve 4PEPS items based on prospectively collected data. Multiple imputation was used for missing 4PEPS items. Based on 4PEPS, PE was considered ruled out in patients with a very low CPTP without D‐dimer testing, in patients with a low CPTP and D‐dimer <1000 μg/L, and in patients with a moderate CPTP and D‐dimer below the age‐adjusted threshold. Efficiency and failure rate were compared to using the YEARS algorithm.
Results: Of the 3,465 patients, 474 (14%) were diagnosed with VTE at baseline or during 3‐month follow‐up (Table 1). Discriminatory performance of the 4PEPS items was good (area under ROC‐curve, 0.82; 95% CI, 0.80–0.84) as was calibration (Figure 2). Based on 4PEPS, PE was considered ruled out without imaging in 58% (95% CI 57–60) of patients (efficiency). Among these patients, 1.3% (95% CI 0.86–1.9) was diagnosed with VTE at baseline or during follow‐up (failure rate). Compared to the YEARS algorithm, efficiency was higher (58% vs. 48%), as was the failure rate (1.3% vs. 0.42%).
Conclusion(s): In this retrospective external validation, 4PEPS appeared to safely rule out PE, with a higher efficiency than the originally used YEARS algorithm but at the cost of a 3‐fold higher diagnostic failure rate.
VTE Epidemiology
OC 23.5
Identifying phenotypes of deep vein thrombosis and their relation to clinical outcomes beyond recurrence
A. Iding 1; A. Pallares Robles2; V. ten Cate3; H. ten Cate4; P. Wild3; A. ten Cate‐Hoek5
1 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 2 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Mainz, Rheinland‐Pfalz, Germany; 3 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 5 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: Deep vein thrombosis (DVT) is a multifactorial disease with several clinical outcomes, but current classifications are based on few factors and solely stratify recurrence risk.
Aims: We aimed to identify DVT phenotypes and assess their relation to recurrent venous thromboembolism (VTE), post‐thrombotic syndrome (PTS), arterial events and cancer.
Methods: Hierarchical clustering was performed on a single‐center cohort of patients with proximal DVT using 23 prospectively recorded characteristics (i.e., demographics, provoking factors, cardiovascular risk factors and comorbidities). Phenotypes were summarized by their discriminative characteristics. Cox regression was used for time‐to‐event analyses. Follow‐up duration was up to five years. The study was carried out in accordance with the Declaration of Helsinki and the medical ethics committee approved data collection.
Results: A total of 825 patients were clustered into four distinct phenotypes: (1, n = 112) younger females with estrogen therapy; (2, n = 268) older patients with a cardiovascular risk profile; (3, n = 128) patients with previous VTE and known thrombophilia; and (4, n = 317) patients without discriminative characteristics. Overall, risks of recurrence and other clinical outcomes were lowest in phenotype 1 and highest in phenotype 2, except for PTS and cancer for which phenotype 3 had the highest risk. Phenotype 4 had overall intermediate risks (Figure 1). Notably, phenotype 1 largely overlapped with current classifications of provoked DVT, while phenotypes 2, 3 and 4 harbored different proportions of unprovoked DVT.
Conclusion(s): Hierarchical clustering identified four distinct risk profiles among DVT patients that are not only associated with increasing risk for recurrence but also with outcomes beyond recurrence. Our results thereby highlight the limitations of current risk stratifications that dichotomize risks based on VTE provoking factors only. Moreover, patients with a cardiovascular risk profile were identified as being at the highest risk of arterial events and recurrence. FIGURE 1 Stacked bar chart visualizing hazard ratios of clinical outcomes per phenotype, relative to phenotype 1. Phenotypes were ordered by ascending recurrence risk, which was adjusted for the duration of anticoagulant therapy. Pie charts below each phenotype show their distribution according to a conventional classification. Patients were grouped as provoked or unprovoked based on the presence or absence of any transient provoking factor. Patients were designated as high‐risk if they had persistent provoking factors or were unprovoked with previous venous thromboembolism.
FIGURE 1 Stacked bar chart visualizing hazard ratios of clinical outcomes per phenotype, relative to phenotype 1. Phenotypes were ordered by ascending recurrence risk, which was adjusted for the duration of anticoagulant therapy. Pie charts below each phenotype show their distribution according to a conventional classification. Patients were grouped as provoked or unprovoked based on the presence or absence of any transient provoking factor. Patients were designated as high‐risk if they had persistent provoking factors or were unprovoked with previous venous thromboembolism.
OC 34.5
Identification of risk factors for major bleeding during anticoagulation and recurrent venous thromboembolism after its discontinuation in patients with a first unprovoked venous thromboembolism
C. Gabara 1; Y. Yamashita2; J. Aibar1; C. Zamora3; J. Moisés1; C. FONT1; R. Valle4; A. Bura‐Riviere5; L. Mazzolai6; A. Lorenzo7; A. Ballaz8; O. Madridano9; J. Suriñach10; J. Fernández‐Reyes11; A. Villalobos12; P. Verhamme13; J. López‐Sáez14; A. Gil‐Díaz15; J. Vela16; M. Monreal17
1 Hospital Clínic de Barcelona, Barcelona, Catalonia, Spain; 2 Kyoto University, Kyoto, Kyoto, Japan; 3 Hospital Clínc de Barcelona, Barcelona, Catalonia, Spain; 4 Hospital Sierrallana, Santander, Cantabria, Spain; 5 Hôpital de Rangueil, Toulouse, Languedoc‐Roussillon, France; 6 Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Vaud, Switzerland; 7 Hospital Universitario La Paz, Madrid, Madrid, Spain; 8 Hospital de Galdakao, Vizcaya, Pais Vasco, Spain; 9 Hospital Universitario Infanta Sofía, Madrid, Madrid, Spain; 10 Hospital Universitario Vall d'Hebron, Barcelona, Catalonia, Spain; 11 Complejo Hospitalario de Jaén, Jaén, Andalucia, Spain; 12 Hospital Regional Universitario de Málaga, Málaga, Andalucia, Spain; 13 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 14 Hospital Universitario de Puerto Real, Cádiz, Andalucia, Spain; 15 Hospital Universitario de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria, Canarias, Spain; 16 Hospital Universitario Miguel Servet, Zaragoza, Aragon, Spain; 17 Department of Internal Medicine, Hospital Universitari Germans Trias i Pujol. Badalona, Barcelona, Spain Chair for the Study of Thromboembolic Disease, Faculty of Health Sciences, UCAM – Universidad Católica San Antonio de Murcia, Spain, Barcelona, Andalucia, Spain
Background: After 3 months of anticoagulation for a first unprovoked venous thromboembolism (VTE), the decision to prolong therapy must consider the risk of both, bleeding and recurrences.
Aims: To identify independent predictors for major bleeding during anticoagulation and recurrent VTE after its discontinuation.
Methods: Consecutive patients with a first episode of unprovoked VTE from RIETE were included. We analysed their clinical characteristics and treatment to find predictors of major bleeding during anticoagulation, and VTE recurrences after its discontinuation. A multivariate analysis using a Cox proportional hazard regression analysis was performed.
Results: Among 33,262 patients included in the analysis, 685 (2.1%) suffered major bleeding during anticoagulation. Independent predictors were: age >80 years (hazard ratio [HR], 2.63; 95% CI, 1.84–3.76), chronic lung disease (HR, 1.29; 95% CI, 1.05–1.59), liver cirrhosis (HR, 4.07; 95% CI, 1.98–8.37); recent major bleeding (HR, 4.02; 95% CI, 2.61–6.20), anaemia (HR, 1.88; 95% CI, 1.60–2.21); CrCl levels 30–60ml/min (HR, 1.47; 95% CI, 1.20–1.80) or <30ml/min (HR, 1.96; 95% CI, 1.46–2.64) and concomitant use of corticosteroids (HR, 1.52; 95% CI, 1.18–1.94) or thrombolytic drugs (HR, 3.43; 95% CI, 2.42–4.85) (Table 1). Among 11,764 patients followed‐up after discontinuing anticoagulation, 1,403 (12%) developed VTE recurrences. Age >80 years (HR, 1.32; 95% CI, 1.08–1.61) and use of direct oral anticoagulants (HR, 1.25; 95% CI, 1.01–1.55) predicted the risk (Table 1).
Conclusion(s): Among patients with unprovoked VTE, a number of variables easily obtained at baseline may reliably predict the risk for major bleeeding during anticoagulation. To identify predictors of VTE recurrences after discontinuation was more elusive. TABLE 1 Multivariable analyses for the risk factors of major bleeding on‐anticoagulation therapy and recurrent VTE during off‐anticoagulation therapy
TABLE 1 Multivariable analyses for the risk factors of major bleeding on‐anticoagulation therapy and recurrent VTE during off‐anticoagulation therapy
OC 42.4
Venous thromboembolism associated with oestrogen‐containing contraceptives versus any hormonal risk factor: A comparison of recurrence rates
J. Knijp 1; H. Wiegers2; N. van Es2; M. Coppens3; S. Moll4; F. Klok5; S. Middeldorp6
1 Amsterdam UMC, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 2 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 3 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 University of North Carolina School of Medicine, Chapel Hill, North Carolina, United States; 5 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 6 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands
Background: In many studies reporting on recurrent venous thromboembolism (VTE), oestrogen‐containing contraceptives (OCC) as provoking factor is grouped together with other hormonal risk factors, such as hormonal replacement therapy or pregnancy and postpartum state. However, recurrence rates may differ across these groups, which questions whether this approach is justified.
Aims: To compare the pooled recurrence rate of subgroups including young women with OCC‐associated VTE exclusively versus women with OCC‐associated VTE together with other hormonal risk factors.
Methods: A systematic review and meta‐analysis was performed on the risk of recurrence after a first OCC‐associated VTE. For the present post‐hoc analysis, eligible studies were divided into two groups. The first group included studies that provided data separately for women with OCC‐associated VTE with a mean age <50 years. The second group included studies that provided data for women with OCC‐associated VTE, but either within a subgroup of mean age >50 years (i.e. mostly women with hormonal replacement therapy) or combined with pregnancy or postpartum state. Recurrence rates were pooled using Knapp‐Hartung random‐effects meta‐analysis. Differences between groups were tested for significance with the χ2 test.
Results: 26 studies were included [Table 1]. The pooled rate of recurrence was 1.57 (95% CI 1.10–2.23, I 2 = 82%) per 100 patient‐years in 14 studies that reported on young women with OCC‐associated VTE exclusively compared with 2.46 (95% CI 1.39–4.36, I 2 = 74%) per 100 patient‐years in 12 studies that reported on women with OCC‐associated VTE, either >50 years or combined with other hormonal factors (p = 0.14) [Figure1].
Conclusion(s): The recurrence rate was not significantly lower in studies that reported data for women with OCC‐associated VTE than in those grouping OCC with other hormonal risk factors. Since the numerical difference between these groups was nonetheless substantial, interpretation of existing studies and design of future studies on this topic should be cautious in grouping all hormonal causes.
OC 54.4
Development and validation of a clinical prediction model for 90‐day venous thromboembolism risk following total hip and total knee arthroplasty
B. Nemeth 1; M. Smeets1; A. Pedersen2; E. Kristiansen2; M. Whyte3; L. Roberts4; S. de Lusignan5; S. le Cessie6; S. Cannegieter1; R. Arya7
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Clinical Epidemiology and Department of Clinical Medicine, Aarhus University, Aarhus, Midtjylland, Denmark; 3 University of Surrey, Surrey, England, United Kingdom; 4 King's College Hospital, London, England, United Kingdom; 5 University of oxford, Oxford, England, United Kingdom; 6 Department of Clinical Epidemiology and Department of Medical Statistics and Bioinformatics, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 7 Kings College Hospital, London, England, United Kingdom
Background: Patients undergoing total hip (THA) or total knee arthroplasty (TKA) are at increased risk for postoperative venous thromboembolism (VTE). About 1.0%‐1.5% of patients still develop VTE despite thromboprophylaxis.
Aims: To develop and validate a clinical prediction model for 90‐day VTE risk following THA/TKA.
Methods: For model development, data on all patients undergoing THA/TKA between 2010 and 2018 were used from the Royal College of General Practitioners Research and Surveillance Centre (data from >290 practices in the United Kingdom). Data were linked to the NHS Hospital Episode Statistics and Office of National Statistics. Patients with missing linkage between datasets or those on permanent therapeutic anticoagulants were excluded. For model validation, data were used from the Danish Hip and Knee Arthroplasty Registry from 2017 to 2019, where the same exclusion criteria applied. 40 candidate predictors were selected to develop a penalized logistic regression model. Discrimination and calibration analyses were performed to assess model performance.
Results: 64,770 patients were included in the development data, of whom 1.4% developed VTE within 90‐days. The final prediction model consisted of age, sex, body mass index, history of coronary artery disease, history of lung disease, cystitis in the year before surgery, history of phlebitis, presence of varicose veins, history of VTE and type of procedure (THA/TKA). The Area Under Curve was 0.65 (95%Confidence Interval (CI) 0.63–0.67). 40,646 patients underwent THA/TKA in the validation cohort, 1.0% developed VTE. Here, the AUC was 0.63 (95% CI 0.61–0.66). The model showed good agreement between observed and predicted risks following recalibration of the model intercept (Figure 1 and Table 1).
Conclusion(s): This clinical prediction model for 90‐day VTE risk following THA/TKA performed well in both development and validation data. This model may be used to identify patients at either low or high risk for VTE following THA/TKA. Funding: The Netherlands Thrombosis Foundation and Sanofi.
OC 54.5
The risk of venous thromboembolism after minor surgical procedures: A population‐based case‐control study
M. Smeets 1; C. Touw2; F. Rosendaal3; B. Nemeth1; S. Cannegieter1
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Minor surgical procedures are classified, according to the Dutch national guidelines, as low risk procedures for venous thromboembolism (VTE) and hence, it is not advised to provide thromboprophylaxis. However, they include a wide variation of surgical procedures both in terms of procedure length and tissue damage.
Aims: To determine the VTE risk for several minor surgical procedures compared against one control group.
Methods: Data were used from the MEGA‐study (population‐based case‐control study into causes of VTE) and linked to the Dutch Hospital Registry data. Surgical procedures were assessed individually or grouped together in case of low numbers. Logistic regression was used to estimate odds ratios for the 90‐day relative risk for VTE following these procedures, adjusted for important confounders. The odds ratios at 1‐year after the procedures were also analyzed.
Results: 3,563 cases and 5,029 controls were assessed whose characteristics are described in table 1. A total of 104 participants, 74 cases and 30 controls, had undergone a minor surgical procedure within 90‐days of the index date, resulting in an approximately 3.5‐fold (OR 3.5, 95% CI 2.3–5.3) overall increased VTE risk (table 2). Venous stripping (OR 7.2, 95% CI 2.4–21.2), open hernia repair (OR 3.7, 95% CI 1.2–11.6) and laparoscopic cholecystectomy (OR 3.2, 95% CI 1.0–10.6) were associated with the highest postoperative risk. The other five procedures were weakly or not associated with an increased risk of VTE.
Conclusion(s): Although the procedures we studied are classified as having a low risk of VTE, we found that venous stripping, open hernia repair and laparoscopic cholecystectomy were associated with a clearly increased risk of VTE.
OC 14.3
Risk of long‐term mortality in elderly patients with venous thrombosis
H. Wang 1; F. Rosendaal2; A. van Hylckama Vlieg3
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 3 Leiden University, Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: Venous thrombosis (VT) is associated with a high risk of mortality, particularly shortly after the event. However, little is known about the long‐term mortality risk in patients aged 70 years and older with VT event.
Aims: To determine mortality in elderly patients with VT and controls subjects.
Methods: Analyses were performed in the AT‐AGE case‐control study. Between 2008 and 2011, we included 338 patients with a first objectively diagnosed deep vein thrombosis (DVT) or pulmonary embolism (PE) and 302 controls without VT, aged 70 years and older. All participants were followed up for a maximum of 12 years. The study (funded by the Netherlands Heart Foundation) was approved by the local Medical Ethical Committee and written informed consent was obtained from all participants. Information on dates and causes of death were retrieved from Statistics Netherland (CBS). 5 and 12‐year Cumulative incidences and incidence rates of mortality were calculated for all VT patients, controls, and for subgroups of VT (provoked/unprovoked and DVT/PE). As measures of relative risk, hazard ratios with 95% confidence interval (CI) were calculated.
Results: 374 Participants (216 patients and 158 controls) died over a follow‐up of 4922 person‐years (median duration of follow‐up: 9.4 years). The mortality rate was 90.4 per 1000 person‐years (95% CI: 78.7–103.3) for patients and 62.4 per 1000 person‐years (95% CI: 53.0–72.9) for controls. The table shows the 5 and 12‐year cumulative incidences and incidence rates of mortality. Incidences were higher in patients than controls. Overall, patients had a 1.4‐fold (95% CI 1.2–1.8) increased mortality rate than controls. The risk remained increased up to 5 years after the thrombotic event (Figure).
Conclusion(s): Elderly VT patients have an increased mortality up to 5 years after the event. More detailed analysis on causes of death as well as the role of comorbidities will be available at the ISTH conference.
OC 23.4
Association of inflammation and coagulation biomarkers with cerebral venous thrombosis clinical and imaging characteristics: Results from FPCCVT
P. Billoir1; V. Siguret2; L. Drouet3; E. Masson Fron4; A. Bagan Triquenot5; I. Crassard6; V. Le Cam Duchez 7
1 Normandie Univ., UNIROUEN, INSERM U1096, Vascular Hemostasis Unit, Rouen University Hospital, France, Rouen, Haute‐Normandie, France; 2 INSERM UMR‐S‐1140/University of Paris, Paris, Ile‐de‐France, France; 3 Cardiology, Lariboisière, APHP, Paris, Ile‐de‐France, France; 4 Biochemistry, Lariboisière, APHP, Paris, Ile‐de‐France, France; 5 Neurology department, Rouen University Hospital, Rouen, Haute‐Normandie, France; 6 Neurology, Lariboisière, APHP, Paris, Ile‐de‐France, France; 7 INSERM U1096 EnVI, Vascular Hemostasis Unit, Rouen University Hospital, Rouen, Haute‐Normandie, France
Background: Cerebral venous thrombosis (CVT) is a rare disease with highly variable clinical presentation and outcomes. Clinical studies suggest a role of inflammation and coagulation in CVT outcomes.
Aims: The aim of this study was to investigate the association of inflammation and hypercoagulability biomarkers with CVT clinical manifestations and prognosis.
Methods: This prospective multicentre study was conducted from July‐2011 to September‐2016. Consecutive patients referred to 21 French stroke units and who had a diagnosis of symptomatic CVT were included. High‐sensitivity C‐reactive‐protein (hs‐CRP), neutrophil‐to‐lymphocyte ratio (NLR), D‐dimer, and thrombin generation using calibrated‐automated‐thrombogram system were measured at different time‐points, until one month following anticoagulant therapy discontinuation.
Results: Two‐hundred‐and‐thirty‐one patients were included. Day 0 hs‐CRP levels, NLR and D‐dimer were increased in patients with initial consciousness disturbance versus those without (hs‐CRP: 10.2 mg/L [3.6–25.5] vs. 23.7 mg/L [4.8–60.0]; NLR: 3.51 [2.15–5.88] vs. 4.78 [3.10–9.59]; D‐dimer: 950 μg/L [520–2075] vs. 1220 μg/L [950–2445] respectively). Regarding parenchymal lesions, a D0 endogenous‐thrombin‐potential >1672 nM•min was associated with ischemic lesions (AUC: 0.69, Se: 76.9%, Sp: 60.5%). Using ROC curves, hs‐CRP and D‐dimer measured on D0 were good predictors of early death (with thresholds of 37.2 mg/L ‐AUC: 84.0%; Se: 75.0% Sp: 84.5%‐ and 1985 μg/L ‐AUC: 83.0%; Se: 100% Sp: 73.9%‐, respectively).
Conclusion(s): We reported associations between inflammation and hypercoagulability biomarkers with clinical outcomes in CVT. Moreover, thrombin generation was associated with ischemic parenchymal brain lesions. hs‐CRP and D‐dimer could predict clinical outcomes in CVT. The proposed D‐dimer and hs‐CRP cut‐offs need to be validated in an external cohort.
OC 42.3
Joint effect of ischemic stroke and obesity on the risk of incident venous thromboembolism
B. Tøndel 1; J. Sejrup2; V. Morelli3; E. Mathiesen4; I. Njølstad5; T. Wilsgaard5; J. Hansen6; S. Brækkan6
1 UiT – The Arctic University of Norway, Tromsø, Troms, Norway; 2 Thrombosis Research Center, Department of Clinical Medicine, UiT – the Arctic University of Norway, Tromsø, Troms, Norway; 3 UiT – The Arctic University of Norway, Tromsø, Norway, Tromsø, Troms, Norway; 4 Brain and Circulation Research Group, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Troms, Norway; 5 Epidemiology of Chronic Diseases Research Group, Department of Community Medicine, UiT – The Arctic University of Norway, Tromsø, Troms, Norway; 6 Thrombosis Research Center, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway
Background: Patients with ischemic stroke (IS) are at increased risk of venous thromboembolism (VTE). Obesity is prevalent in IS patients and a well‐established risk factor for VTE. However, whether obesity further increase the risk of VTE in patients with IS remains unclear.
Aims: To investigate the joint effect of IS and obesity on the risk of first‐time VTE in a population‐based cohort study.
Methods: Participants (n = 29 964) were recruited from 4 to 6th surveys of the Tromsø Study (conducted in 1994–95, 2001–02 and 2007–08) and followed through 2014. All incident events of IS and VTE during follow‐up were recorded. Obesity was defined as body mass index ≥30 kg/m2. Cox‐regression models with age as timescale and IS as a time‐dependent variable were used to estimate sex‐adjusted hazard ratios (HRs) of VTE according to combined categories of IS and obesity with exposure to neither risk factors as the reference group.
Results: During a median follow‐up time of 15.2 years, 1392 participants were diagnosed with a first‐time IS and 808 participants developed a first‐time VTE. Among those with IS, 51 developed a VTE, yielding an overall incidence rate of VTE of 7.5 per 1000 person‐years (95% CI: 5.7–9.8). In subjects without IS, obesity was associated with a 1.7‐fold higher risk of VTE (HR 1.70, 95% CI: 4.43–2.03) (Table 1). In non‐obese subjects, IS was associated with a 1.8‐fold higher risk of VTE (HR 1.75, 95% CI: 1.25–2.43). Obese subjects with IS had a 2.2‐fold increased risk (HR 2.18, 95% CI: 1.22–3.88). Thus, the combination of the two risk factors did not yield an excess risk of VTE. Similar results were obtained in subgroup analyses with pulmonary embolism and deep vein thrombosis as outcomes.
Conclusion(s): Obesity did not result in a more than additive risk of VTE in patients with IS. TABLE 1 Incidence rates (IRs) and hazard ratios (HRs) of venous thromboembolism (VTE), deep vein thrombosis (DVT) and pulmonary embolism (PE) according to ischemic stroke (IS) and obesity exposure: The Tromsø Study 1994–2014
TABLE 1 Incidence rates (IRs) and hazard ratios (HRs) of venous thromboembolism (VTE), deep vein thrombosis (DVT) and pulmonary embolism (PE) according to ischemic stroke (IS) and obesity exposure: The Tromsø Study 1994–2014
OC 25.1
Venous and arterial thromboembolism after incident colorectal cancer in the Netherlands: Incidence, predictors, and prognosis
R. Anijs 1; Q. Chen2; W. Lijfering2; S. Cannegieter2
1 Leiden University Medical Center, Amsterdam, Noord‐Holland, Netherlands, 2 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: Cancer patients have an increased risk of venous and arterial thromboembolism (VTE/ATE). Colorectal cancer (CRC) is the third most prevalent cancer type, but the size of the risks and predictors of TE after CRC and subsequent mortality are not well known in detail at nationwide level.
Aims: To determine incidence, predictors and prognosis of TE occurring within 1 year after incident CRC.
Methods: Using data from Statistics Netherlands (CBS) and the Netherlands Comprehensive Cancer Organization (IKNL), incident CRC patients were identified between January 2013 and December 2018. CRC patients were matched on a 1:2 ratio to cancer‐free controls on age, sex and cancer diagnosis date. Incidence rates and cumulative incidences were estimated for developing TE starting from date of diagnosis in 1 year follow‐up. Predictor variables for TE in were explored by univariable Cox regression analyses. All‐cause mortality in CRC was evaluated by multivariable Cox regression analyses, using TE as time‐dependent exposure.
Results: 68238 incident CRC patients were matched to 136476 control subjects. The 1 year cumulative incidence of VTE was 1.93% (95% CI 1.83–2.04) in cancer patients versus 0.24% (0.21–0.27) in control subjects (HR 8.85; 7.83–9.99). For ATE, this was 2.74% (2.62–2.87) in cancer patients versus 1.88% (1.81–1.95) in control subjects (HR 1.57; 1.47–1.66). Significant predictors for VTE were cancer stage, surgery, chemotherapy, asthma, prior VTE and smoking, and for ATE; age, prior ATE, Parkinson’s disease, peripheral artery disease, depression and physical health. TE in CRC patients led to an increased risk of all‐cause mortality (VTE HR; 3.68 (95% CI 3.30–4.10) and ATE HR; 3.05 (95% CI 2.75–3.39)), after adjusting for age, sex, comorbidities and tumor characteristics.
Conclusion(s): This Dutch nationwide study provides detailed information on size of VTE and ATE risks, their predictors and prognosis in patients with a CRC diagnosis. This information is important for targeted prevention of both types of TE in CRC.
OC 32.2
Pleiotropic influence of rosuvastatin on apolipoproteins and their association with coagulation factor levels: Results from the START trial
E. Camilleri 1; N. van Rein1; B. van Vlijmen2; J. Biedermann3; M. Kruip4; F. Leebeek5; F. van der Meer6; C. Cobbaert7; S. Cannegieter1; W. Lijfering1
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 3 Department of Haematology, Erasmus MC, Erasmus University Medical Center, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 5 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 7 Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Rosuvastatin decreases coagulation factors (F), among which FVIII, in venous thrombosis patients, but the mechanism is unknown. Apolipoproteins, which are responsible for the lipoprotein metabolism, may mediate this decrease through shared mechanisms of synthesis or regulatory pathways, as they have been associated with coagulation factors levels.
Aims: To investigate whether apolipoproteins are associated with statin‐related decrease of coagulation factors.
Methods: We measured levels of serum apolipoproteins (apo)A‐I, A‐II, A‐IV, (a), B‐100, C‐I, C‐II, C‐III and E and plasma coagulation factors (FVII, FVIII, FIX, FXI, von Willebrand factor[vWF]) in 126 patients randomized to 28 days of rosuvastatin use from the STAtin Reduces Thrombophilia trial. We studied the association between apolipoproteins and coagulation factor at baseline and the mean difference of apolipoprotein levels between baseline and day 28. We also determined the association between coagulation factor changes and the changes between apolipoprotein levels caused by rosuvastatin use (day 28 minus baseline) and adjusted for potential confounders (age, sex, body mass index). We estimated mean differences and 95% confidence intervals (CIs) using linear regression.
Results: At baseline, levels of all apolipoproteins, except apo(a), were positively associated with FVII, FIX and FXI. Apolipoprotein levels, except for apoA‐I, (a) and A‐IV, had decreased after rosuvastatin use (Figure 1). ApoB‐100 showed the biggest decrease of −0.43g/L (95% CI −0.46 to −0.40). Decreasing apoA‐IV, C‐I and C‐III levels were associated with decreasing FVII levels, whereas decreasing apoA‐II and B‐100 was associated with FXI decrease (Table 1). In contrast, apolipoprotein decrease was not associated with a FVIII and vWF decrease.
Conclusion(s): Rosuvastatin decreased the level of several apolipoproteins. This decrease was associated only with the decrease of liver‐derived coagulation factors FVII and FXI and not with FVIII/vWF, while the latter two showed the biggest decrease during rosuvastatin treatment. This suggests that rosuvastatin might decrease coagulation factors through other mechanisms than via apolipoproteins.
OC 54.3
Validation of the IMPROVE hospital‐acquired venous thrombosis risk assessment model in the medical inpatients thrombosis and hemostasis study (MITH) population
A. Sparks 1; K. Wilkinson1; A. Repp1; A. Li2; R. Thomas1; N. Roetker3; N. Zakai4
1 University of Vermont, Burlington, Vermont, United States; 2 Baylor College of Medicine, Houston, Texas, United States; 3 Hennepin Healthcare Research Institute, Minneapolis, Minnesota, United States; 4 University of Vermont, COLCHESTER, Vermont, United States
Background: Governmental and professional societies recommend assessing risk of hospital‐associated (HA) venous thrombosis (HA‐VTE) in medical patients, defined as a VTE occurring during hospitalization or up to 3 months after discharge. The IMPROVE risk assessment model (RAM) predicts HA‐VTE risk, and we sought to validate this RAM in an independent population.
Aims: To assess the performance of the IMPROVE RAM & IMPROVE score in an independent, external population of hospitalized medical patients.
Methods: The IMPROVE RAM was calculated for all medical admissions between 2010 and 2019 at the University of Vermont Medical Center (UVM) as part of the MITH study. Cox regression was used to estimate the hazard ratios (HR) for HA‐VTE risk factors and to examine model performance. This research is Institutional Review Board approved and funded by the National Institutes of Health, USA.
Results: Over 23,911 admissions there were 314 HA‐VTE events: 94 (30%) in‐hospital and 220 (70%) after discharge. Table 1 compares the results from the original IMPROVE‐VTE development cohort to the present UVM validation cohort, including the number of admissions and HA‐VTE events, as well as the prevalence of IMPROVE risk factors and their associations with HA‐VTE. Multivariable HR estimates were in general lower in the UVM population compared with the original IMPROVE study; one exception was active cancer, which showed a similar association in both studies. Assessing RAM performance, the AUC of the IMPROVE model in the UVM cohort was 0.64. HA‐VTE risks were overestimated at lower scores and underestimated at higher scores in the UVM cohort relative to the original IMPROVE cohort (Table 2).
Conclusion(s): The IMPROVE score, while associated with HA‐VTE risk, demonstrated reduced accuracy in the UVM validation cohort when compared to the IMPROVE development cohort. HA‐VTE RAMs with better external validity are needed that potentially assess in‐hospital and post‐discharge events as separate outcomes.
OC 14.1
Time trends in prognosis of pulmonary embolism in The Netherlands (2013–2018)
Q. Chen; N. van Rein; S. Cannegieter
Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: An update at nationwide level is warranted as inconsistent time trends in prognosis of pulmonary embolism (PE) have been reported in the Western world, which is relevant for both patients and physicians.
Aims: To assess time trends in prognosis of PE in The Netherlands (2013–2018).
Methods: With nationwide data from Statistics Netherlands, incident (hospitalized) PE patients (ICD‐10 code: I26) diagnosed between 1/1/2013 and 31/12/2018 were identified and categorized in six cohorts (i.e., Cohort 2013‐Cohort 2018) according to the calendar year of the diagnosis. All patients were followed until 31/12/2019 (or death) to estimate cumulative incidences of all‐cause mortality and major bleeding. Cox regression was employed to evaluate differences in outcomes between cohorts with adjustment for confounding. Age‐and‐sex‐standardized annual PE‐related mortality rates were also calculated, defined as numbers of PE‐related deaths (on the basis of death certificates) divided by age‐and‐sex‐standardized number of Dutch inhabitants in each calendar year.
Results: 61,322 incident PE patients were identified, of whom 12% also had deep vein thrombosis. The sizes and baseline characteristics were similar between cohorts (i.e., mean age 65 ± 16 years; male≈49%; three most prevalent comorbidities: cancer (≈28%), hypertension (≈22%), and diabetes (≈13%)). Overall, the 30‐day, 90‐day, 180‐day, 1‐year, and 5‐year cumulative incidences of all‐cause mortality were 10%, 16%, 20%, 24%, and 39%, respectively; the 30‐day and 180‐day cumulative incidences of major bleeding were 5% and 6%, respectively. Between cohorts, the more recent cohorts saw the higher cumulative incidences of all outcomes, but hazard ratios were not statistically significant after adjustment (Table). No significant difference was observed in standardized annual PE‐related mortality rates (2013–2018). The crude annual PE‐related mortality rates increased with age but were similar between sex (Figure).
Conclusion(s): The prognosis of PE, evaluated by short‐ or long‐term case‐fatality‐rate, major bleeding, and standardized PE‐related mortality rates, remained constant in The Netherlands between 2013 and 2018.
OC 42.1
The risk of incident venous thromboembolism attributed to overweight and obesity: The Tromsø Study
T. Frischmuth1; B. Tøndel2; S. Brækkan3; J. Hansen3; V. Morelli 4
1 Thrombosis Research Center, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Norway, Tromsø, Troms, Norway; 2 UiT – The Arctic University of Norway, Tromsø, Troms, Norway; 3 Thrombosis Research Center, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway; 4 UiT – The Arctic University of Norway, Tromsø, Norway, Tromsø, Troms, Norway
Background: Obesity is a well‐established risk factor for venous thromboembolism (VTE). Further, genetically elevated body mass index (BMI) is associated with a higher risk of VTE, supporting a causal association. However, the proportion of VTE events that can be attributed to obesity in the general population has been scarcely investigated.
Aims: To estimate the population attributable fraction (PAF) of VTE due to overweight and obesity in a population‐based cohort with repeated measurements of BMI.
Methods: Participants (n = 36,341) attending one or more surveys of the Tromsø Study 4–7 (enrolment: 1994–2016) were followed until 2020, and all incident VTEs were recorded. Overweight and obesity were defined by a baseline BMI of 25–30 kg/m2 and ≥30 kg/m2, respectively. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using time‐varying Cox regression models and adjusted for age and sex. The PAF was estimated applying a formula that takes into account the adjusted HR for overweight and obesity and their prevalence in VTE cases (pc) (PAF = pc[1 −1/HR]). Ethical approval and informed consent were obtained.
Results: During a median follow‐up of 13.9 years, 1,051 incident VTEs occurred. Compared with BMI <25 kg/m2, HRs for overweight and obesity were 1.40 (95% CI 1.21–1.61) and 1.86 (95% CI 1.58–2.20), respectively. The proportion of VTE events attributable to overweight and obesity was 24.6% (12.9% was due to overweight and 11.7% to obesity). Similar results were obtained for VTE subgroups (Table 1), and in analyses restricted to men, women, and the elderly (≥70 years).
Conclusion(s): Our findings suggest that almost 25% of all incident VTEs in the general population can be attributed to overweight and obesity. Public health interventions aimed to reduce the prevalence of overweight and obesity may substantially contribute to decrease the incidence of VTE. TABLE 1 Population attributable fraction (PAF) of overall venous thromboembolism (VTE) and VTE subgroups for overweight and obesity
TABLE 1 Population attributable fraction (PAF) of overall venous thromboembolism (VTE) and VTE subgroups for overweight and obesity
OC 54.2
Validation of a hospital‐acquired venous thrombosis risk assessment model: The medical inpatient thrombosis and hemostasis (MITH) study
N. Zakai 1; K. Wilkinson2; A. Sparks2; I. Koh3; N. Roetker4; A. Repp2; R. Thomas2; J. Schaefer5; N. Smith6; C. Holmes7; C. Hooper8; M. Cushman9
1 University of Vermont, COLCHESTER, Vermont, United States; 2 University of Vermont, Burlington, Vermont, United States; 3 SyllogisTeks, Burlington, Vermont, United States, 4 Hennepin Healthcare Research Institute, Minneapolis, Minnesota, United States; 5 University of Michigan, Ann Arbor, Michigan, United States; 6 Department of Epidemiology, University of Washington; Department of Health Service, University of Washington; Seattle Epidemiologic Research and Information Center; Department of Veterans Affairs, Office of Research and Development, Seattle, WA 98105, USA, Seattle, Washington, United States; 7 University of Vermont College of Medicine, Burlington, Vermont, United States; 8 Centers for Disease Control and Prevention, Atlanta, Georgia, United States; 9 Larner College of Medicine at the University of Vermont, Colchester, Vermont, United States
Background: To reduce the risk of hospital‐acquired venous thrombosis (HA‐VTE) in medical patients, guidelines recommend assessing HA‐VTE risk and providing prophylaxis for those at high risk. Risk assessment models (RAMS) including objective risk factors available at admission remain an unmet clinical need.
Aims: To develop and validate a RAM for HA‐VTE in medical inpatients using data available to providers within 24‐h of hospital admission.
Methods: We developed a HA‐VTE RAM at the University of Vermont Medical Center (Burlington, Vermont, USA, Table 1) and validated this RAM at Michigan Medicine (Ann Arbor, Michigan, USA, Table 2). HA‐VTE and the risk factors were identified using previously validated computable phenotypes. The RAM was developed using a Bayesian LASSO approach with model performance assessed using area under the receiver operating curves (AUC) and the slope of observed versus expected plot. People admitted with VTE were excluded. The research was approved by the Institutional Review Board funded by the National Institutes of Health and the Centers for Disease Control and Prevention, USA.
Results: Table 1 presents the risk factors, odds ratios (OR) and 95% credible intervals (CI) for the HA‐VTE RAM, which included 11 risk factors. For the development cohort, based on 219 events among 62,468 admissions, the AUC of the model was 0.75 and the observed versus expected slope was 1.11 (Table 2). In the validation cohort there were 48,265 admissions and 363 HA‐VTE events with a younger population and a higher incidence of HA‐VTE. The AUC and the observed versus expected slope were 0.69 and 0.89 (Table 2).
Conclusion(s): We developed and validated a HA‐VTE RAM in populations. The model fit and calibration are promising especially given these are two geographically diverse institutions. Further validation is in progress at additional hospitals as well as in people hospitalized with COVID‐19.
VTE Prophylaxis
LB 02.1
Intermediate versus low‐dose low‐molecular‐weight heparin in pregnant and postpartum women with a history of venous thromboembolism (Highlow Study)
I. Bistervels1; A. Buchmuller2; H. Wiegers3; F. Ní Áinle4; B. Tardy5; J. Donnelly6; P. Verhamme7; A. Jacobsen8; A. Hansen9; M. Rodger10; M. DeSancho11; R. Shmakov12; N. van Es3; M. Prins13; C. Chauleur14; S. Middeldorp 15
1 1) Amsterdam UMC location University of Amsterdam, Department of Vascular Medicine, Amsterdam, the Netherlands 2) Amsterdam Cardiovascular Sciences, Pulmonary Hypertension & Thrombosis, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Service de Médecine Vasculaire et Thérapeutique, CHU Saint Etienne, Saint‐Étienne, Rhone‐Alpes, France; 3 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 4 Department for Haematology, Mater Misericordiae University Hospital, Ireland, Dublin, Dublin, Ireland; 5 INSERM, CIC‐1408, Centre Hospitalier Universitaire de Saint‐Etienne, Saint‐Etienne, France, St Etienne, Rhone‐Alpes, France; 6 1) Department of Obstetrics and Gynaecology, Rotunda Hospital and Mater Misericordiae University Hospital, Dublin, Ireland 2) Royal College of Surgeons in Ireland, Dublin, Ireland 3) School of Medicine, University College Dublin (UCD), Dublin, Ireland, Dublin, Dublin, Ireland; 7 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 8 1) Department of Obstetrics and Gynaecology, Oslo University Hospital, Oslo, Norway 2) Faculty of Medicine, University of Oslo, Hospital, Oslo, Norway, Oslo, Oslo, Norway; 9 1) Department of Clinical Biochemistry, Aalborg University Hospital, Aalborg, Denmark 2) Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark, Aarhus, Midtjylland, Denmark; 10 Department of Medicine, McGill University, McGill University Health Center, Montreal, Quebec, Canada, Montreal, Quebec, Canada; 11 Weill Cornell Medicine, New York, New York, United States; 12 Institute of Obstetrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after V.I. Kulakov, Ministry of Healthcare of the Russian Federation, Moscow, Russia, Moscow, Moskva, Russia; 13 Department of Epidemiology and Technology Assessment, University of Maastricht, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 14 1) Service de Gynécologie Obstétrique, Centre Hospitalier Universitaire de St‐Etienne, Saint‐Etienne, France 2) INSERM, UMR1059, Equipe Dysfonction Vasculaire et Hémostase, Université Jean‐Monnet, F‐42055, Saint‐Etienne, France, St‐Etienne, Rhone‐Alpes, France; 15 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands
Background: Pregnancy‐related venous thromboembolism (VTE) is a leading cause of maternal morbidity and mortality. Thromboprophylaxis is indicated in pregnant women with a history of VTE. The optimal dose of low‐molecular‐weight heparin (LMWH) for antepartum and postpartum thromboprophylaxis is uncertain, as no randomized trials have been performed in this population.
Aims: To compare the efficacy and safety of intermediate versus low‐dose LMWH in pregnant women with a history of VTE.
Methods: In this international, open‐label randomized trial, pregnant women with a history of VTE were randomized before 14 weeks of gestational age to weight‐adjusted intermediate‐dose or fixed low‐dose LMWH until 6 weeks postpartum. The primary efficacy outcome was objectively confirmed VTE. The primary safety outcome was major bleeding. Outcomes were centrally adjudicated.
Results: A total of 1,110 pregnant women were randomized and included in the intention‐to‐treat population. VTE occurred in 11 of 555 (2.0%) women assigned to weight‐adjusted intermediate‐dose LMWH and in 16 of 555 (2.9%) assigned to fixed low‐dose LMWH (relative risk [RR], 0.69; 95% confidence interval [CI], 0.32–1.47; p = 0.33). VTE occurred antepartum in five (0.9%) and five (0.9%) women, and postpartum in six (1.1%) and 11 women (2.0%) in the intermediate‐dose and low‐dose groups, respectively. On‐treatment VTE in the per‐protocol population (n = 972) occurred in 1.0% and 2.4% (RR, 0.43; 95% CI, 0.15–1.20). On‐treatment major bleeding in the safety population (n = 1,045) occurred in 4.4% and in 3.8% receiving intermediate‐dose or low‐dose LMWH, respectively (RR, 1.16; 95% CI, 0.65–2.09).
Conclusion(s): In pregnant women with a history of VTE, weight‐adjusted intermediate‐dose LMWH during the combined antepartum and postpartum periods was not associated with a lower risk of recurrence than fixed low‐dose LMWH. The suggestion of greater efficacy of intermediate‐dose low‐molecular‐weight heparin vs. low‐dose low‐molecular weight heparin during the postpartum period should be regarded as hypothesis generating and needs confirmation in a future randomized controlled trial (ClinicalTrials.gov number, NCT01828697). TABLE 1 Patient characteristics
TABLE 1 Patient characteristics TABLE 2 Efficacy and safety outcomes
TABLE 2 Efficacy and safety outcomes
OC 48.4
Comparative effectiveness of alternative bridging therapies for subtherapeutic INR in ambulatory patients with left ventricular assist devices
G. Chung 1; E. Salem2; E. Sippola3; S. Shore3; L. Baumann Kreuziger2; G. Barnes3
1 University of Michigan, ANN ARBOR, Michigan, United States; 2 Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 3 University of Michigan, Ann Arbor, Michigan, United States
Background: Patients with left ventricular assist devices (LVAD) use warfarin to prevent life‐threatening thromboembolic complications. Warfarin’s anticoagulant effects are notoriously variable, requiring the use of temporary “bridging” anticoagulation when subtherapeutic. Comparative effectiveness data between hospitalization for unfractionated heparin (UFH) and outpatient management with low‐molecular‐weight heparin (LMWH) are lacking.
Aims: To compare 30‐day rates of bleeding and thrombotic events between patients bridged with LMWH vs. UFH for subtherapeutic INR occurring in the outpatient setting.
Methods: We conducted a retrospective cohort study of patients aged 18 years and older with LVAD implantation between January 1, 2014 and December 31, 2018 from two academic medical centers. Data were collected for each unintended subtherapeutic international normalized ratio (INR) episode occurring in the ambulatory setting for which either UFH or LMWH was used. Patients were followed for 30 days after UFH or LMWH was discontinued, assessing for bleeding and/or thromboembolic events. Given multiple bridging episodes per patient, multivariable logistic regression analysis adjusted for site‐ and patient‐level clustering along with LVAD type and HAS‐BLED score. The composite outcome was major bleeding or thromboembolism.
Results: Data were collected from 269 patients and 1438 bridging episodes. Compared to HeartMate 3, having a HeartMate II LVAD (OR: 0.34; 95% CI: 0.15–0.81; p = 0.015) or Heartware HVAD (OR: 0.24; 95% CI: 0.14–0.44; p < 0.001) was associated with lower odds of LMWH use. The 30‐day rate of major bleeding or thromboembolism was lower for patients receiving LMWH as compared to UFH (11/1169 [0.9%] vs. 8/195 [4.1%], adjusted OR: 0.31; 95% CI: 0.11–0.87; p = 0.026).
Conclusion(s): Outpatient LMWH bridging was associated with a lower risk of major adverse events for LVAD patients with subtherapeutic INR, compared to intravenous UFH therapy. If confirmed in prospective analyses, use of LMWH bridging as compared to hospital admission for UFH bridging is a potentially safer and lower‐cost management strategy for this high‐risk population.
OC 42.2
Deep vein thrombosis and pulmonary embolism rates in total hip and total knee arthroplasty patients: A claims database study
S. Simon 1; R. Patell2; B. Hollenbeck1
1 New England Baptist Hospital, Boston, Massachusetts, United States, 2 Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States
Background: Deep vein thrombosis (DVT) and pulmonary embolism (PE) are common post‐surgical complications after hip and knee arthroplasty. Venous thromboembolism (VTE) prophylaxis is used to reduce postoperative thrombosis but is associated with bleeding and strategies for different patient populations need to be optimized.
Aims: We investigated postoperative thrombosis and bleeding complications in a contemporary cohort, comparing effectiveness and bleeding by prophylactic agent and underlying risk factors.
Methods: All hip and knee arthroplasty patients from the 2017–2019 IBM MarketScan database who had continuous insurance enrollment three months prior to and following arthroplasty were included. We collected data on demographics, comorbidities, and thromboprophylactic medication. Ninety‐day cumulative incidence of postoperative VTE and bleeding were primary outcomes. Univariate analysis was performed to identify risk factors for DVT/PE. A multivariable analysis compared risk of DVT/PE and bleeding, by thromboprophylaxis agent used, adjusted for comorbidities. Patients on multiple prescription anticoagulants and those on chronic anticoagulation were excluded from the multivariable analysis.
Results: A total of 132,625 patients were included in this study. The average age was 61 years, 56.5% were female; 39.5% were hip arthroplasty and 60.5% knee. The 90‐day cumulative incidence of VTE was 2.84% (95% CI 2.75–2.93), and 90‐day surgical bleeding and all bleeding was 0.73% (95% CI 0.68–0.78) and 4.61% (95% CI 4.49–4.73), respectively. In the univariate analysis, those with a history of DVT/PE or hereditary hypercoagulable diagnosis had a highest VTE risk while outpatient surgery, younger age, and short length of stay had a low risk (Figure 1). Multivariable analysis adjusted for risk factors demonstrated a favorable VTE and safety profile for aspirin overall (Figure 2).
Conclusion(s): DVT/PE rates are driven more by the underlying risk factors than by thromboprophylaxis agent. Aspirin was associated with low DVT/PE and bleeding risk in lower risk populations, but should not be considered in high‐risk patients.
OC 23.3
Recurrent venous‐thromboembolism: association with thrombin generation and D‐Dimer
E. Biguzzi 1; K. Winckers2; T. Hackeng2; F. Rosendaal3; A. van Hylckama Vlieg4
1 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 3 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 4 Leiden University, Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: In patients with a first event of venous thrombo‐embolism (VT) clinicians must decide when to stop anticoagulation, evaluating the risk of recurrent thrombosis.
Aims: The aim of this study was to evaluate the association between recurrent VT and D‐Dimer and thrombin generation.
Methods: Analyses were performed in 1895 patients enrolled in the MEGA study (539 with unprovoked first VT) and followed up for recurrent VT (252 events). All patients gave informed consent and the study was approved by the Medical Ethics Committee of the Leiden University Medical Center. Thrombin generation was measured with low and high tissue factor concentration. Results obtained with high tissue factor (normalized ratio with and without activated protein C) are shown. The cumulative incidence of recurrent VT and incidence rates with 95% CI of recurrent VT were calculated. Hazard ratios and 95% CI were calculated by Cox proportional hazard regression models, to evaluate relative risks of VT associated with different levels of D‐Dimer and thrombin generation. For the combined analysis of D‐Dimer and thrombin generation, we chose the cut‐off of 215 ng/ml for D‐Dimer and the highest decile for thrombin generation.
Results: In patients with a first unprovoked VT, three levels of risk of recurrent VT were identified in the combined analysis: incidence rates per 100 patient‐years in low risk in patients with low D‐Dimer and low thrombin generation (1.36, 95% CI 0.42–2.30); intermediate risk in patients with high D‐Dimer (3.89, 95% CI 3.07–4.70) and high risk in patients with high D‐Dimer and thrombin generation (9.80, 95% CI 5.28–14.33).
Conclusion(s): The use of both tests allowed to identify patients with a high risk of recurrent VT, who could benefit from long‐term anticoagulation, in the absence of a severe bleeding risk.
OC 54.1
Rivaroxaban or placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer
C. Becattini 1; U. Pace2; F. Pirozzi3; A. Donini4; G. Avruscio5; F. Rondelli6; M. Boncompagni7; D. Chiari8; M. De Prizio9; A. Visonà10; R. De Luca11; F. Guerra12; A. Muratore13; G. Portale14; M. Righini15; S. Frasson16; F. Dentali17; P. Del Rio2; G. Gussoni16; G. Agnelli18
1 University of Perugia, Perugia, Umbria, Italy; 2 National Cancer Institute, "G. Pascale" Foundation, Napoli, Napoli, Campania, Italy; 3 Department of General Surgery, Santa Maria delle Grazie Hospital, Pozzuoli, Pozzuoli, Campania, Italy; 4 Oncology Surgery, University of Perugia, Perugia, Perugia, Umbria, Italy; 5 University Hospital of Padua, Padua, Padova, Veneto, Italy; 6 S. Giovanni Battista Hospital, Foligno, Foligno, Umbria, Italy; 7 General Surgery, S. Maria della Misericordia Hospital, Perugia, Perugia, Umbria, Italy; 8 Istituto Clinico Humanitas Mater Domini, Castellanza, Varese, Castellanza, Lombardia, Italy; 9 General Surgery, S. Donato Hospital, Arezzo, Arezzo, Toscana, Italy; 10 Vascular Medicine, S.Giacomo Apostolo Hospital, Catelfranco Veneto, Treviso, Treviso, Veneto, Italy; 11 Surgical Oncology IRCCS Istituto Tumori " Giovanni Paolo II, Bari, Bari, Puglia, Italy; 12 General Surgery, Ospedali Riuniti Marche Nord – Pesaro, Pesaro, Marche, Italy; 13 General Surgery, E. Agnelli Hospital – Pinerolo, Pinerolo, Piemonte, Italy; 14 General Surgery, Cittadella Hospital, AULSS 6 Euganea, Cittadella, Veneto, Italy; 15 Division of Angiology and Hemostasis, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland, Geneva, Geneve, Switzerland; 16 Research Department, FADOI Foundation, Milan, Milano, Lombardia, Italy; 17 ASST “Sette Laghi”, Insubria University, Varese, Varese, Lombardia, Italy; 18 University of Perugia, Italy, Perugia, Umbria, Italy
Background: The clinical benefit of extended prophylaxis for venous thromboembolism after laparoscopic surgery for cancer is unclear. The efficacy and safety of direct oral anticoagulants for this indication are unexplored.
Aims: To assess the efficacy and safety of extended prophylaxis with rivaroxaban in comparison to placebo after laparoscopic surgery for colorectal cancer.
Methods: PROLAPS‐II was a randomized, double‐blind, placebo‐controlled, investigator‐initiated, superiority study. Consecutive patients who had laparoscopic surgery for colorectal cancer. All patients received antithrombotic prophylaxis with low‐molecular‐weight heparin from surgery to randomization. Patients were randomized to receive rivaroxaban (10mg once daily) or placebo to be started at 7 ± 2 days after surgery and given for the subsequent 3 weeks. The primary study outcome was the composite of symptomatic objectively confirmed venous thromboembolism, asymptomatic ultrasonography‐detected deep vein thrombosis or venous thromboembolism‐related death at 28 ± 2 days after surgery. The primary safety outcome was major bleeding.
Results: Patient recruitment was prematurely closed due to study drug expiry after the inclusion of 582 of the 646 planned patients. A primary study outcome event occurred in 11 of 282 patients in the placebo group compared with three of 287 in the rivaroxaban group (3.9 vs. 1.0%; risk difference −0.029, 95% CI −0.054 to −0.003; p = 0.028). Major bleeding occurred in none of the patients in the placebo group and in two patients in the rivaroxaban group (0.7%, 95% CI 0–1.0). In addition, four patients withdrawn study treatment because of adverse effects (2 rivaroxaban and 2 placebo patients). The number needed to treat to achieve the prevention of one primary study outcome event was 34. The number needed to harm to have a major bleeding complication was 143.
Conclusion(s): Oral rivaroxaban was more effective than placebo for extended prevention of venous thromboembolism after laparoscopic surgery for colorectal cancer without an increase in major bleeding. (ClinicalTrials.gov. number NCT03055026).
VTE Treatment
OC 48.5
Brain cancers and the risk of intracranial hemorrhage with direct oral anticoagulants (DOACs) versus low molecular weight heparin (LMWH): A meta‐analysis of comparative studies
P. Haddad; D. Hammoud; K. Gallagher
OBVAMC/LSUHSC‐S, Shreveport, Louisiana, United States
Background: Patients with brain tumors, primary or metastatic, are at high risk for thromboembolic events. LMWH has been the standard treatment for many years. Recently, DOACs are more frequently used in cancer patients. However, there continues to be concerns regarding their safety in patients with primary and metastatic brain cancers especially with respect to intracranial hemorrhages (ICH). There are no trials in brain cancers comparing the safety of DOACs to LMWH in a head‐to‐head manner.
Aims: This meta‐analysis was conducted to compare the relative risk (RR) of ICH of DOACs to that of LMWH in patients with brain cancers.
Methods: A review of the medical literature was conducted using online databases. Inclusion criteria consisted of English language, diagnosis of primary brain cancers (PBC) or metastatic brain cancers (MBC), comparative studies using anticoagulation with LMWH versus DOACs, and studies that reported the incidence of ICH. A meta‐analysis using the fixed effects and random effects models was conducted.
Results: Three retrospective comparative studies with a total of 498 patients were included. Two studies reported on ICH incidence of DOACs versus LMWH in PBC and MBC separately and one reported on ICH incidence in MBC only. DOACs were found to have significantly lower RR of all types of ICH in patients with PBC (RR = 0.21, 95% CI 0.05–0.88) and a borderline significant lower RR in MBC (RR = 0.60, 95% CI 0.34–1.07) than LMWH. However, there was no significant difference between DOACs and LMWH with respect to major ICH in patients with MBC.
Conclusion(s): This is the first meta‐analysis to show that DOACs is associated with lower relative risk of all ICH compared to LMWH in patients with primary and metastatic brain cancers. In the absence of randomized clinical trials, it represents the most compelling data supporting the use of DOACs to treat thromboembolic events in these patient populations.
OC 62.5
Direct oral anticoagulant prescribing in patients with a very low bodyweight – Experience from King’s College Hospital
R. Clapham; V. Speed; R. Byrne; R. Patel; R. Arya; J. Patel
King's College Hospital, London, England, United Kingdom
Background: There is little published experience of prescribing direct oral anticoagulants (DOAC) in patients with low body weight (≤50kg). This cohort is increasing due to an aging population as well as more patients receiving DOACs for the treatment of cancer‐ associated venous thromboembolism (VTE). Our standard practice in this group has been to perform a DOAC plasma concentration to ensure they are not over‐exposed to anticoagulation.
Aims: To describe our experience of prescribing DOACs for patients with a body weight ≤50kg.
Methods: Patients prescribed a DOAC for atrial fibrillation and VTE, weighing ≤50kg who had a DOAC plasma concentration performed between 01/01/2018–31/12/2019, were identified from anticoagulation clinic records. Each case was reviewed and along with reporting the DOAC plasma concentration, 90 day outcomes (thrombosis and bleeding) from commencing the DOAC were identified from the medical records. Descriptive statistics was used to analyse the data.
Results: In total 95 patients were prescribed a DOAC during this period (Table 1 for detailed demographics). Patients were anticoagulated with apixaban 5 mg bd (2.1%), apixaban 2.5 mg bd (55.8%), edoxaban 30mg od (31.6%), and rivaroxaban 15mg od (10.5%). Nineteen patients had an active cancer diagnosis and 17 had documented poor dietary intake. No thrombotic complications occurred during 90 day follow‐up. There were 2 episodes of major bleeding and 3 episodes of clinically relevant non‐major bleeding. DOAC plasma concentrations are illustrated in Figure 1. Seventeen patients had true trough concentrations performed and all were within the expected reference range.
Conclusion(s): Low bodyweight patients are growing in number; often elderly, with significant renal impairment or a background of malignant disease. In our experience to date, exposure to the DOACs were within the respective reference ranges. Further data from a larger population is required to demonstrate safety from a bleeding perspective.
OC 62.4
FVIII and D‐dimer values at trough and peak concentrations of direct oral anticoagulants: important considerations for using these tests in assissting clinical decision for risk stratification scheme
S. Margetić 1; I. Ćelap2; S. Šupraha Goreta3
1 Department of Clinical Chemistry, Sestre milosrdnice University Hospital Center, Vinogradska 29, 10 000 Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 2 University Hospital Center Sestre milosrdnice, Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 3 Faculty of Pharmacy and Biochemistry, University of Zagreb, Zagreb, Grad Zagreb, Croatia
Background: FVIII and D‐dimer are included in different prediction models to risk stratification for thrombotic recurrence or anticoagulation cessation. However, the optimal timing of their measurement related to the last drug dose is not sufficiently examined.
Aims: To determine FVIII and D‐dimer levels at peak and trough plasma concentrations of DOACs in order to define optimal timing of blood drawing.
Methods: Concentrations of rivaroxaban (n = 32), apixaban (n = 24) and dabigatran (n = 28), D‐dimer levels and FVIII activities were measured at trough (before the next drug dose) and peak (2 h after drug intake) DOAC levels in circulation of outpatients during their regular control clinical examination. Rivaroxaban and apixaban were determined using specific chromogenic anti‐FXa assay, dabigatran with Innovance DTI assay, FVIII with APTT‐based coagulometric method (Actin FS/FVIII deficient plasma) and D‐dimer by quantitative immunoturbidimetric assay using monoclonal antibody (Innovance D‐dimer), all from Siemens Healthineers, Germany on BCSXP analyzer. Statistical analysis was done with Wilcoxon and Friedman tests. The study was funded by the Croatian Science Foundation as part of the research project IP‐2016‐06‐8208.
Results: In contrast to D‐dimer, FVIII values were significantly higher at trough in comparison with peak dabigatran and rivaroxaban concentrations (P = 0.013 and 0.024), whereas for apixaban FVIII also showed a trend of higher values at trough, but without significant difference (p = 0.850) (Table 1). Significantly higher values of both D‐dimer and FVIII were measured at trough and peak drug levels of apixaban compared to dabigatran and rivaroxaban (Table 1, P*).
Conclusion(s): Plasma concentration of DOACs significantly affects FVIII values, unlike D‐dimer. These findings are important in use of these tests in assissting clinical decisions related to risk stratification schemes for recurrent thrombotic event or anticoagulation cessation. For FVIII measurement blood drawing should be performed at trough DOAC levels exclusively whereas D‐dimer may be measured at both trough or peak drug levels in circulation. TABLE 1 Results of factor FVIII (FVIII) and D‐dimer levels at peak and trough concentrations of DOACs
TABLE 1 Results of factor FVIII (FVIII) and D‐dimer levels at peak and trough concentrations of DOACs
OC 62.2
Pharmacokinetics of direct oral anticoagulants after bariatric surgery: A retrospective cohort study
B. Gunka 1; D. Mackenzie2; A. Doumouras3; S. Mithoowani4
1 Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 2 Department of Surgery, McMaster University, Hamilton, Ontario, Canada; 3 Department of Surgery, McMaster University; Division of General Surgery, St Joseph's Healthcare, Hamilton, Ontario, Canada; 4 Department of Medicine, McMaster University, Hamilton, ON, Canada, Hamilton, Ontario, Canada
Background: Despite how commonly they are prescribed, there are few data specifically evaluating the pharmacokinetics of direct oral anticoagulants (DOACs) after bariatric surgery (BS).
Aims: To evaluate peak DOAC drug levels in patients after BS.
Methods: We performed a retrospective cohort study of patients who had BS in Hamilton, Canada between 2015 and 2021, received a DOAC post‐operatively and had at least one DOAC drug level measured using a drug‐specific anti‐Xa assay. Eligible patients were identified using the Ontario Bariatric Network database and Thrombosis Service records. Demographics, pharmacokinetic data and 30‐day clinical outcomes were summarized descriptively.
Results: Forty‐five patients had seventy‐two DOAC drug levels drawn: apixaban (n = 22), rivaroxaban (n = 39), dabigatran (n = 5) and edoxaban (n = 6). The most common procedures were sleeve gastrectomy (n = 35, 78%) and Roux‐en‐Y gastric bypass (n = 9, 20%). Mean body mass index (BMI) before BS was 51.8 kg/m2 (SD = 8). Drug levels were measured a median of 12.5 days post‐operatively. Thirty‐six patients (80%) had at least one peak drug level within the expected range seen in phase II‐III DOAC clinical trials. All four patients on dabigatran had low drug levels and were switched to apixaban (n = 3) or rivaroxaban (n = 1). Two patients on rivaroxaban with low drug levels were switched to apixaban. Ten patients had peak drug levels repeated a median of 6.1 months post‐operatively and all levels were in the expected range. Mean weight loss at follow‐up was 28 kg. One patient had a splenic infarct, and one had a pulmonary embolism within 30‐days of surgery.
Conclusion(s): Our study suggests that apixaban, rivaroxaban, and edoxaban are absorbed adequately after BS. Dabigatran should be used with caution. Limitations include a small sample size, brief follow‐up period, and use of peak drug levels as a surrogate for total anticoagulant exposure.
OC 62.3
Apixaban for venous thromboembolism treatment in obese patients with weight >120 kg or BMI >40 kg/m2
T. Groves; M. Rees; R. Alikhan
Cardiff and Vale UHB, Cardiff, Wales, United Kingdom
Background: The 2016 ISTH SSC guidelines recommended against DOAC use in patients with a BMI of >40 kg/m2 or weight >120kg and if used drug specific levels be measured. The 2021 ISTH update for treatment of VTE, suggest that standard doses of rivaroxaban & apixaban are options regardless of high BMI and weight. Fewer supportive data exist for apixaban than rivaroxaban.
Aims: To assess apixaban levels in patients > 120kg or BMI > 40 kg/m2 and correlate these with VTE recurrence.
Methods: Retrospective case series of patients prescribed apixaban for treatment of VTE with apixaban levels measured between 01/02/2016 and 30/12/20. All patients were followed up for 12 months to determine VTE recurrence.
Results: A total of 240 VTE patient requests and 424 apixaban levels, 242 peak/trough levels on 134 patients on apixaban 5 mg BD and 139 levels on 2.5 mg BD. Apixaban 5 mg bd 36 patients weight >120kg and 39 patients BMI >40kgm2. Figure 1 peak and trough levels are in keeping with those reported in the apixaban SPC www.medicines.org.uk/emc/product/2878/smpc. No patients on a dose of 5 mg BD and BMI >40kgm2 suffered a recurrence of VTE during either the initial treatment phase or for the duration of the follow up period. One patient weight 140 kg suffered a recurrent DVT on apixaban 5 mg bd 14 days after peak and trough levels (138 ng/ml and 54 ng/). The patient was non‐adherent to treatment with no dose of apixaban in the five days preceding recurrent DVT.
Conclusion(s): In line with ISTH 2016 recommendations apixaban levels were in keeping with those predicted with no evidence of clinical recurrence of VTE supporting the new ISTH 2021 recommendations for apixaban treatment of obese patients with VTE. FIGURE 1 Apixaban 5 mg bd peak and trough levels.
FIGURE 1 Apixaban 5 mg bd peak and trough levels.
OC 14.4
Evaluation of patients’ experience and related qualitative outcomes in venous thromboembolism: A scoping review
L. Genge 1; A. Krala2; T. Tritschler3; G. Le Gal4; N. Langlois5; C. West6; S. Dubois6; L. Duffett7; L. Skeith2
1 University of Calgary, Toronto, Ontario, Canada; 2 University of Calgary, Calgary, Alberta, Canada; 3 Inselspital, Bern University Hospital, Bern, Bern, Switzerland; 4 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada; 5 Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 6 CanVECTOR, Ottawa, Ontario, Canada, 7 Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario, Canada
Background: Venous thromboembolism (VTE) is a prevalent disease with high morbidity and mortality. VTE has well‐documented physical sequelae, however the psychological and emotional experience of patients is seldom evaluated in randomized controlled trials.
Aims: We conducted a scoping review of published qualitative studies aiming to understand the physical, psychological, and emotional impact of VTE as reflected from patients’ perspectives. This scoping review is part of a larger initiative to develop a core outcome set for VTE treatment studies. This research is funded by the Canadian Institutes of Health Research and CanVECTOR.
Methods: A systematic literature search was conducted to identify qualitative studies assessing patient experience of VTE. Two authors independently screened titles and abstracts using Covidence systematic review software. Full text reviews were conducted independently by two study team members. QSR International NVivo 12 software was used to perform systematic line‐by‐line coding of the Results and Discussion from all included articles. A modified method of “thematic synthesis” was used to collate themes upon reading and re‐reading of the publications.
Results: Our search strategy returned a total of 4944 citations; 28 were ultimately included in the analysis. The studies were conducted across 13 countries and representative of 436 participants including a spectrum of VTE sub‐populations. There were seven major themes identified; Acute Impacts: An Unforeseen Blow, Sustained Psychological Distress, Loss of Self: Life is Changed, Challenges of Thrombosis Management, Balancing Coping and Control, Negative Experience with the Medical System, and VTE in the Context of Other Conditions. Each major theme was comprised of additional subthemes (Figure 1).
Conclusion(s): The physical, psychological, and emotional impacts of VTE extend beyond the quantitative outcomes typically evaluated in clinical trials. An improved understanding of the outcomes most important to patients will improve patient‐centered research and care in VTE. FIGURE 1 Major themes and associated subthemes
FIGURE 1 Major themes and associated subthemes
OC 48.2
A ten‐year review of the impact of transition from warfarin to direct oral anticoagulant– has VTE treatment become safer?
B. Lui 1; B. Wee1; A. Kwok2; J. Lai2; Z. Khattak3; C. Donarelli3; P. Ho4; H. Lim1
1 Northern Pathology Victoria, Department of Haematology, Northern Health, Epping, VIC, Epping, Victoria, Australia; 2 University of Melbourne, Melbourne, Victoria, Australia; 3 Northern Health, Melbourne, Victoria, Australia; 4 Northern Health, Victoria, Australia, Epping, Victoria, Australia
Background: The introduction of direct oral anticoagulants (DOAC) has resulted in a paradigm shift in the management of venous thromboembolism (VTE) with DOAC the current agent of choice.
Aims: To evaluate the shift to DOAC in VTE management over the last decade on clinical outcomes, particularly in first unprovoked major VTEs.
Methods: A retrospective analysis of all VTE admissions in non‐cancer patients from January 2010 to December 2020 at Northern Health, Victoria, Australia. “Warfarin era” included events that occurred between January 2011 and December 2014 and “DOAC era” from January 2015.
Results: There were 2687 cases involving 2508 patients (45.9% males; median age 63 years). 98% were symptomatic and 1261 events (47%) were unprovoked (Table 1). 1003 events occurred during the warfarin era (79% managed with warfarin and 6% DOAC) and 1684 during the DOAC era (22% warfarin 66% DOAC). While recurrent thrombosis within 12 months from index event was comparable, there were fewer events beyond 12 months in the DOAC era compared to warfarin era (HR 0.694, 95% CI 0.511–0.943, p = 0.020) (Figure 1A). Clinically significant bleeding events were lower in the DOAC era (HR 0.633, 95% CI 0.410–0.978, p = 0.039) (Figure 1B), despite having more patients on prolonged anticoagulation (29.3% vs. 39.7%, p < 0.001). A subanalysis of first unprovoked major VTE events (n = 602) revealed longer therapeutic anticoagulation during the warfarin era (9 vs. 6 months, p < 0.001) although more patients were continued on low dose thromboprophylaxis in the DOAC era (19% vs. 1%, p < 0.001). There was a significant reduction in recurrent VTE beyond 12 months from the index event in the DOAC era (HR 0.315, 95% CI 0.132–0.750) with no difference in clinically significantly bleeding rates between the eras.
Conclusion(s): Treatment outcomes for VTE have improved over time with reduced rate of thrombotic and clinically significant bleeding complications in the DOAC era.
OC 48.3
Direct oral anticoagulants for the treatment of splanchnic vein thrombosis: A systematic review and meta‐analysis
A. Li 1; P. Li2; M. Zhang3; A. Eshaghpour4; M. Carrier5; M. Crowther6
1 University of Ottawa, Ottawa, Ontario, Canada; 2 University of Toronto, Toronto, Ontario, Canada; 3 Michael G. DeGroote School of Medicine, Hamilton, Ontario, Canada; 4 Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 5 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 6 McMaster University, Department of Medicine, Hamilton, Ontario, Canada
Background: Splanchnic vein thrombosis (SVT) is a form of atypical venous thrombosis (VTE) that occurs in the hepatic portal veins, mesenteric veins, splenic veins, and hepatic veins (Budd‐Chiari Syndrome, BCS). Current guidelines suggest the use of direct oral anticoagulants (DOACs) for the management of SVT; however, this indication remains off‐label in many countries due to the scarce evidence.
Aims: The goal of this study is to assess the efficacy and safety of DOACs for the treatment of SVT.
Methods: MEDLINE and Embase were searched for cohort studies and randomized controlled trials that assessed the use of DOACs against vitamin‐k antagonists (VKA), low‐molecular weight heparins (LMWH), or no anticoagulation for the treatment of SVT. Article screening and data collection was done in duplicate. The primary efficacy outcome was complete recanalization, and the primary safety outcome was major bleeding. Secondary outcomes included recurrent thrombosis, mortality, and partial recanalization. Meta‐analysis was done using DerSimonian and Laird random effects model and reported as odds ratios (ORs) with 95% confidence intervals (CI).
Results: A total of nine non‐randomized (n = 1180 patients) and one randomized study (n = 80 patients) were included for analysis (table 1). Meta‐analysis of odds ratios is found in table 2. The use of DOACs was associated with an increased rate of complete recanalization in randomized and non‐randomized studies when compared to VKAs or no anticoagulation and an increased rate of partial recanalization when compared to VKA, LMWH orVKAs, or no anticoagulation. Both bleeding, death, and recurrent thrombosis appeared to be reduced compared with VKAs in the single randomized study and occurred at a similar rate as the comparator therapy in the non‐randomized studies.
Conclusion(s): The use of DOACs for the treatment of SVT appears safe and effective. Ideally this intervention would be tested in a large, randomized study, but the low frequency of SVT makes conducting randomized studies difficult.
OC 34.3
Major gastrointestinal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: A score to identify high risk patients
J. Catella 1; L. Bertoletti2; F. Moustapha3; J. Nieto4; R. Valle5; j. Pedrajas6; A. Villalobos7; I. Quere8; G. Sarlon9; M. Monreal10
1 Hospices Civils de Lyon, LYON, Rhone‐Alpes, France; 2 CHU Saint Etienne, Saint‐Etienne, Rhone‐Alpes, France; 3 CHU Clermont Ferrand, Clermont Ferrand, Auvergne, France; 4 Hospital General Virgen de la Luz, Cuenca., Andalucia, Spain; 5 Hospital Sierrallana, Santander, Andalucia, Spain; 6 Hospital Clínico San Carlos, Madrid, Andalucia, Spain; 7 Hospital Regional Universitario de Málaga, Málaga, Andalucia, Spain; 8 Hopital St Eloi, montpellier, Provence‐Alpes‐Cote d'Azur, France; 9 Hopital la Timone, Marseille, Provence‐Alpes‐Cote d'Azur, France; 10 Department of Internal Medicine, Hospital Universitari Germans Trias i Pujol. Badalona, Barcelona, Spain Chair for the Study of Thromboembolic Disease, Faculty of Health Sciences, UCAM – Universidad Católica San Antonio de Murcia, Spain, Barcelona, Andalucia, Spain
Background: The gastrointestinal (GI) tract is a frequent site of bleeding in patients receiving anticoagulant therapy for venous thromboembolism (VTE).
Aims: We tried to identify at‐risk patients.
Methods: We used the RIETE registry to assess the clinical characteristics of patients developing major GI bleeding during the course of anticoagulation. Then, we built a predictive score based on multivariable analysis, aiming to identify patients at increased risk for major GI bleeding.
Results: We included 87,431 patients with acute VTE. During the course of anticoagulation, 778 (0.89%) suffered major GI bleeding, 815 (0.93%) non‐major GI bleeding and 1,462 (1.67%) had major bleeding outside the GI tract. During the first 30 days after major GI bleeding, 7.6% of patients re‐bled, 3.9% had VTE recurrences and 33% died. On multivariable analysis, male sex, age ≥70 years, initial VTE presentation as pulmonary embolism, active cancer, prior VTE, recent major bleeding in the GI tract, esophageal varicosities, anemia, abnormal prothrombin time, renal insufficiency and use of corticosteroids were associated to an increased risk for major GI bleeding. Using the predictive score, 39,591 patients (45%) were at low risk; 36,602 (42%) at intermediate‐risk; 9,315 (11%) at high‐risk; and 1,923 (2.2%) at very high risk. Their rates of major GI bleeding were: 0.21%, 0.96%, 2.41% and 6.08%, respectively. The c‐statistics was 0.771 (95% CI. 0.755–0.786).
Conclusion(s): Major GI bleeding is a severe complication in patients receiving anticoagulant therapy for VTE. At risk patients may be reliably identified using a number of variables easily available at baseline. FIGURE 1 Cumulative incidence of major GI bleeding during the first 365 days of anticoagulation, according to the prognostic score.
FIGURE 1 Cumulative incidence of major GI bleeding during the first 365 days of anticoagulation, according to the prognostic score.
OC 34.4
Long‐term risk of clinically relevant non‐major bleeding during extended anticoagulation for unprovoked venous thromboembolism: A systematic review and meta‐analysis
F. Khan 1; T. Tritschler2; P. Wells3; G. Le Gal3; M. Rodger4; D. Fergusson5
1 University of Ottawa, Ottawa, Ontario, Canada; 2 Inselspital, Bern University Hospital, Bern, Bern, Switzerland; 3 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada; 4 Department of Medicine, McGill University, McGill University Health Center, Montreal, Quebec, Canada, Montreal, Quebec, Canada; 5 Ottawa Hospital Research Institute, Ottawa, Ontario, Canada
Background: Clinically relevant non‐major bleeding (CRNMB) is an important safety outcome in clinical studies of venous thromboembolism (VTE) as it incurs time consuming management, a decreased perception of quality of life, and important healthcare costs. However, reliable estimates for the long‐term risk of CRNMB among patients with unprovoked VTE that are receiving extended anticoagulation are lacking.
Aims: To determine the incidence of CRNMB during extended anticoagulation of up to 5 years among patients with a first unprovoked VTE.
Methods: MEDLINE, Embase, and the the Cochrane Central Register of Controlled Trials were systematically searched for randomized controlled trials (RCTs) and prospective cohort studies reporting CRNMB events (as defined by the Internatinal Society on Thrombosis and Haemostasis or by the individual studies) among patients with a first unprovoked VTE who were to receive oral anticoagulation for a minimum of 6 additional months after completing at least 3 months of initial anticoagulant treatment. Unpublished data required for analyses were obtained from authors of included studies.
Results: Among the 8 RCTs and 7 cohort studies included in the analysis, 8443 patients received a vitamin K antagonist (VKA) and 6838 received a direct oral anticoagulant (DOAC). The incidence of CRNMB per 100 person‐years was 6.48 events (95% CI, 4.69–8.55 events) with VKAs and 5.01 events (95% CI, 3.71–6.49 events) with DOACs. The 5‐year cumulative incidence of CRNMB with VKAs was 21.7% (95% CI, 8.9%–40.0%) [Table 1]. Data were insufficient to estimate incidence of CRNMB beyond 2 years of extended anticoagulation with DOACs.
Conclusion(s): In patients receiving extended oral anticoagulation for unprovoked VTE, the long‐term risk of CRNMB is substantial. This risk may be important to consider in determining treatment duration for patients in whom the risk‐benefit ratio of major bleeding and recurrent VTE is closely balanced. TABLE 1 Incidence of clinically relevant non‐major bleeding
TABLE 1 Incidence of clinically relevant non‐major bleeding
OC 23.2
Serial D‐dimers after anticoagulant cessation in unprovoked venous thromboembolism: data from the REVERSE cohort study
Y. Xu 1; F. Khan1; M. Kovacs2; E. Sabri3; M. Righini4; M. Carrier5; S. Kahn6; P. Wells7; D. Anderson8; I. Chagnon9; M. Crowther10; R. White11; M. Rodger12; G. Le Gal7
1 University of Ottawa, Ottawa, Ontario, Canada; 2 Division of Hematology, Department of Medicine, University of Western Ontario, London, Ontario, Canada, London, Ontario, Canada; 3 University of Geneva, Ottawa, Ontario, Canada; 4 Division of Angiology and Hemostasis, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland, Geneva, Geneve, Switzerland; 5 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 6 Division of Internal Medicine, Department of Medicine, McGill University, Montreal, Québec, Canada, Montreal, Quebec, Canada; 7 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada; 8 Dalhousie University, Halifax, Nova Scotia, Canada; 9 University of Montreal, Montreal, Quebec, Canada; 10 McMaster University, Department of Medicine, Hamilton, Ontario, Canada; 11 University of California, Davis, Davis, California, United States; 12 Department of Medicine, McGill University, McGill University Health Center, Montreal, Quebec, Canada, Montreal, Quebec, Canada
Background: While several risk stratification tools have been developed to predict the risk of recurrence in patients with an unprovoked venous thromboembolism (VTE), only 1 in 4 patients are categorized as low‐risk. Rather than a one‐time measure, serial D‐dimer assessment holds promise to enhance the prediction of VTE recurrence after oral anticoagulant (OAC) cessation.
Aims: To assess the effectiveness and safety of serial D‐dimer measurement following OAC cessation, and to assess prediction of VTE recurrence using D‐dimer levels on and off OAC.
Methods: Using data from the REVERSE cohort, we compared VTE recurrence among patients with normal D‐dimer levels (defined as VIDAS D‐dimer <500 ng/ml in fibrinogen‐equivalent units) at OAC cessation and 1‐month follow‐up, to those with an elevated D‐dimer level at either time point. We also evaluated VTE recurrence based on absolute increase in D‐dimer levels between the two timepoints (e.g., ΔD‐dimer) according to quartiles.
Results: Among 214 patients with serial D‐dimer levels measured at OAC cessation and 1‐month follow‐up, an elevated D‐dimer level at either timepoint was associated with a numerically higher risk of recurrent VTE than patients with normal D‐dimer levels (6.9% vs. 4.2% per year, hazards ratio 1.6, 95% CI 0.9–2.7). Among women with <2 HERDOO2 risk factors, a normal D‐dimer level predicted very low risk of recurrence during follow‐up (0.8% per year, 95% CI 0.1–2.8). Similarly, recurrent VTE risk was 3% per year (95% CI 1.4–5.6) among patients in the lowest ΔD‐dimer quartile, despite 1 in 4 in the group having D‐dimer above cutoff values (Table).
Conclusion(s): Serial normal D‐dimer levels identifies patients at a lower risk of VTE recurrence. In addition, ΔD‐dimer, irrespective of its elevation above cutoff threshold, might predict VTE recurrence. Prospective validation of ΔD‐dimer on VTE recurrence is needed to identify subgroups who may safely discontinue OACs within populations considered to be at high‐risk. TABLE 1 Risk of VTE recurrence based on change in D‐dimer from baseline
TABLE 1 Risk of VTE recurrence based on change in D‐dimer from baseline
OC 23.1
Management of patients with unprovoked venous thromboembolism and a low risk of recurrence with the Vienna prediction model: A prospective cohort study
S. Eichinger 1; L. Eischer2; H. Sinkovec2; P. Gressenberger3; T. Gary4; M. Brodmann4; G. Heinze2; P. Kyrle5
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Medical University of Vienna, Wien, Wien, Austria; 3 Medical University of Graz, Graz, Steiermark, Austria; 4 Medical University Graz, Graz, Steiermark, Austria; 5 Department of Medicine I, Clinical Division of Hematology and Hemostasis, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: Patients with unprovoked venous thromboembolism (VTE) have a high recurrence risk and should receive extended oral anticoagulation (OAC). OAC may cause bleeding, and patients at low recurrence risk could benefit from limited OAC duration.
Aims: In a prospective management study, we evaluated the recurrence risk of VTE patients with a low recurrence risk predicted by the Vienna Prediction Model (VPM).
Methods: 520 patients with a first unprovoked VTE and a predicted one‐year recurrence risk of <4.4% by the VPM were included after OAC discontinuation. Exclusion criteria were previous VTE, VTE provoked by a temporary risk factor, by female hormone intake, cancer, known major thrombophilia, OAC >7 months or OAC for reasons other than VTE. Follow‐up was 2 years. The main outcome was recurrent VTE. One‐year and two‐years recurrence risks of 8% and 13% were pre‐specified as safety margins. Cumulative recurrence risk was estimated using the Kaplan‐Meier method. The VPM was recalibrated by adjusting the cumulative baseline risk to the observed values.
Results: Of 520 patients [median age 52; IQR 42–65 years, 289 (56%) men, 226 (43%) pulmonary embolism, 206 (40%) proximal and 88 (17%) distal deep vein thrombosis], 52 patients (30 men) had non‐fatal recurrent VTE (5.8/100 patient‐years, 95% CI 4.4–7.7). After one year the cumulative recurrence risk (5.2%, 95% CI 3.2–7.2) was significantly lower than the pre‐specified safety margin of 8% (p = 0.003). After 2 years the cumulative incidence (11.2%, 95% CI 8.3–14) was not significantly lower than the safety margin of 13% (p = 0.11). Because of the underestimated risk during the 2 year, we recalibrated the VPM. The VPM now allows stratification of low‐risk patients into different categories (Figure 1).
Conclusion(s): The recalibrated VPM identifies patients with unprovoked VTE at low risk of recurrence. Applying the VPM refines risk stratification, which facilitates treatment decisions on the duration of OAC. FIGURE 1 Predicted recurrence risk at one year and two years among men and women according to location of index VTE and categories of D‐Dimer levels.
FIGURE 1 Predicted recurrence risk at one year and two years among men and women according to location of index VTE and categories of D‐Dimer levels.
OC 14.2
Long‐term outcomes of superficial vein thrombosis in daily practice: 12‐month data from the INSIGHTS‐SVT study
E. Rabe1; U. Hoffmann2; A. Schimke3; A. Heinken4; F. Langer5; T. Noppeney6; D. Pittrow7; J. Klotsche8; H. Gerlach9; R. Bauersachs 10
1 University of Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Ludwig‐Maximilians‐University, Munich, Bayern, Germany; 3 Mylan Pharmaceuticals GmbH, Bad Homburg, Hessen, Germany; 4 Aspen GmbH, Munich, Bayern, Germany; 5 II. Medizinische Klinik und Poliklinik, Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 6 Martha‐Maria Hospital Nuremberg, Nuremberg, Bayern, Germany; 7 Institute for Clinical Pharmacology, Dresden, Bayern, Germany; 8 German Rheumatism Research Center Berlin (DRFZ), Berlin, Berlin, Germany; 9 Office for Vascular Diseases, Mannheim, Hessen, Germany; 10 Standort AGAPLESION Bethanien Krankenhaus, Frankfurt, Hessen, Germany
Background: Information about the long‐term course of superficial vein thrombosis (SVT) in daily practice is limited.
Aims: The INvestigating SIGnificant Health TrendS in the management of SVT study investigated venous thromboembolic events (VTE) and bleeding events over 12 months.
Methods: Prospective observational study, with objectively confirmed acute isolated SVT. Outcomes of interest were deep venous thrombosis (DVT), pulmonary embolism (PE), extension or recurrence of SVT, and, as safety outcome linked to antithrombotic treatment, bleeding events. NCT02699151.
Results: 872 patients with acute SVT as index event with 12‐month follow‐up were 60.6 ± 14.5 years old, 42.4% were women. The majority had varicose veins (76.4%) and often chronic venous insufficiency/ulceration (49.8%), or history of venous thrombosis (41.9%). 62.2% were treated with fondaparinux, 25.0% with low molecular weight heparin, 6.2% with other anticoagulation and 6.6% had no anticoagulation. At 12 months of follow‐up, the primary efficacy outcome (symptomatic VTE consisting of DVT, PE, recurrent or extending SVT) had occurred in 108 (14.3%) of patients. The most frequent VTE event was recurrent or extending SVT in 11.0%, followed by DVT in 2.7% and PE in 2.4%. Twelve patients (1.1%) died, and eight (1.8%) were hospitalized due to VTE. Bleeding events occurred in 2.2% of patients, however only 3 cases (0.3%) were major. The rate of primary efficacy outcome was highest in the LMWH group (22.4%) and lowest in the fondaparinux group (10.4%). In a multivariate model, factors associated with “late events”, i.e. those between 3 months and 12 months were: BMI, previous VTE, SVT in the saphenous veins, major surgery, severe systemic infection at entry.
Conclusion(s): Isolated SVT is a common but underrated condition. While the majority of events occurred within 3 months, the risk of symptomatic VTE remained elevated up to 12 months. Thus, in patients with risk factors, prolonged rather than short‐term anticoagulation therapy should be considered.
OC 34.2
Selective serotonin reuptake inhibitor use is associated with major bleeding during treatment with vitamin K antagonists: Results of a cohort study
L. Burggraaf 1; S. Bakker2; M. Kruip3; F. van der Meer4; W. Lijfering1; N. van Rein1
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Clinical Pharmacy and Toxicology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: Selective serotonin reuptake inhibitors (SSRIs) may increase the risk of major bleeding during vitamin K antagonist (VKA) (and other anticoagulant) treatment by decreasing platelet activation, increasing gastric acid secretion and decreasing VKA metabolism through cytochrome P450 2C9 (CYP2C9) inhibition.
Aims: To determine whether SSRIs cause major bleeding during VKA treatment and investigate the mechanisms behind this interaction.
Methods: Information on SSRI use and bleeding complications was obtained from patient records at the Anticoagulation Clinics of Leiden and Rotterdam of VKA initiators between 2006 and 2018. Conditional logistic regression and time‐dependent Cox regression were used to estimate the effect of SSRIs on a high INR (≥5) within 2 months after SSRI initiation and on major bleeding during the entire period of SSRI use, respectively. SSRI use was stratified for (non)‐CYP2C9 inhibitors. Participant consent was waived because the analysis used pre‐existing, coded data.
Results: 58,918 patients were included, of whom 1504 were SSRI users. SSRI initiation versus non‐use was associated with a 2.41‐fold (95% confidence interval [CI] 2.01–2.89) increased risk for a high INR, which was 3.14‐fold (95% CI 1.33–7.43) among CYP2C9 inhibiting SSRIs (table 1). SSRI use versus non‐use was associated with a 1.22‐fold (95% CI 0.99–1.50) increased risk for major bleeding in all SSRI users, which was 1.31‐fold (95% CI 0.62–2.72) in CYP2C9 inhibiting SSRIs compared to non‐users (table 2).
Conclusion(s): SSRIs are associated with an increased risk of high INR (≥5) and major bleeding. These risks were slightly more elevated for CYP2C9 inhibiting SSRI users, suggesting that this was due to a pharmacokinetic interaction (by CYP2C9 inhibition) as well as the effect of SSRIs on platelet activation. Therefore, we would suggest to prefer non‐CYP2C9 inhibiting SSRIs for patients already using a VKA, and intensify monitoring of INR shortly after initiation of SSRI.
OC 34.1
Bleeding in patients with genitourinary cancer compared to non‐genitourinary cancer treated with apixaban, rivaroxaban, or enoxaparin for acute venous thromboembolism
T. Lang 1; D. Vlazny1; D. Houghton1; A. Casanegra1; R. Meverden1; D. Froehling1; D. Hodge2; L. Peterson1; R. McBane1; W. Wysokinski1
1 Mayo Clinic, Rochester, Minnesota, United States; 2 Mayo Clinic, Jacksonville, Florida, United States
Background: Current guidelines either recommend low molecular weight heparin or advise caution when using direct oral anticoagulants for patients with cancer‐associated venous thromboembolism (Ca‐VTE) of genitourinary (GU) system.
Aims: Compare major bleeding (MB) and clinically relevant non‐major bleeding (CRNMB) rates in patients with GU to non‐GU cancers treated with apixaban, rivaroxaban or enoxaparin.
Methods: Consecutive patients (n = 3,758) with acute VTE between 03/01/2013 and 04/30/2021 were followed prospectively through the Mayo Clinic VTE registry (ClinicalTrials.gov: NCT03504007).
Results: Among 1,702 Ca‐VTE patients, 420 (mean age 64.8 years, 52.9% female) had GU cancers (163 urinary bladder/ureteral, 109 ovarian, 75 renal, 73 prostate) treated with apixaban (n = 174), rivaroxaban (n = 72), or enoxaparin (n = 132), and 1,282 (mean age 62.0 years, 43.0% female) with non‐GU cancers receiving apixaban (n = 520), rivaroxaban (n = 263), or enoxaparin (n = 424). The rates (all 100 person‐years) of MB [9.12 vs. 6.01, hazard ratio (HR) 1.49, 95% confidence limit (95% Cl) 0.97, 2.28] and CRNMB (8.72 vs. 6.04, HR 1.43, 95% Cl 0.93, 2.20) were similar in GU compared to non‐GU cancers treated with any anticoagulant. Rivaroxaban had higher MB rate in GU versus non‐GU cancers (10.43 vs. 3.26, HR 2.90, 95%Cl 1.09, 7.72) (Table 1). Patients with GU cancers receiving apixaban had lower MB rate (3.05) compared to rivaroxaban (10.43, HR 0.25, 95% CI 0.07, 1.84) and enoxaparin (10.82, HR 0.27, 95%Cl 0.08, 0.83) (Table 2). All differences remain significant after adjusting for age and renal function. CRNMB rates were similar throughout all comparisons. Separate analysis of patients with cancer involving only urinary bladder/ureteral showed similar MB and CRNMB rates compared to non‐GU cancers and amongst anticoagulants (data not shown).
Conclusion(s): Treatment with rivaroxaban is associated with higher MB rate in GU compared to non‐GU cancers. In GU group, apixaban has a lower rate of MB compared to rivaroxaban or enoxaparin.
OC 62.1
Effectiveness and safety of direct oral anticoagulants among octogenarians with venous thromboembolism: an international multi‐database cohort study
A. Douros 1; F. Basedow2; Y. Cui3; J. Dimakos1; J. Walker2; D. Enders2; V. Tagalakis1
1 McGill University, Montreal, Quebec, Canada, 2 Institute for Applied Health Research Berlin, Berlin, Berlin, Germany, 3 Lady Davis Institute, Montreal, Quebec, Canada
Background: Advanced age is an important risk factor for venous thromboembolism (VTE), recurrent VTE and related mortality. Moreover, older adults have a higher risk of anticoagulant related bleeding. However, the effects of direct oral anticoagulants (DOACs) among octogenarian patients with VTE remain poorly understood, complicating the benefit‐risk assessment of VTE treatment in this age group.
Aims: To assess the effectiveness and safety of DOACs compared to vitamin K antagonists (VKAs) among octogenarians with VTE.
Methods: We conducted an international cohort study using administrative healthcare databases from the Canadian province of Québec and Germany. We assembled two population‐based cohorts of octogenarians with incident VTE initiating treatment with DOACs or VKAs within 15 days of the VTE. Date of cohort entry was day 15 after the incident VTE. Study period spanned from 01/2012 to the most recent date of data availability (Québec: 12/2016; Germany: 12/2019). Using an as‐treated exposure definition, we compared DOACs to VKAs, thereby applying inverse probability of treatment weighting based on high‐dimensional propensity scores to balance exposure groups. Cox models estimated site‐specific hazard ratios (HRs) and 95% confidence intervals (CIs) of recurrent VTE, major bleeding, and all‐cause mortality. The results were meta‐analyzed using random‐effects models. Sensitivity analyses addressed the potential for different sources of bias.
Results: Our cohort included 6,737 octogenarians with VTE (Québec: n = 2,556; Germany: n = 4,181) who initiated use of DOACs (n = 3,778) or VKAs (n = 2,959). Compared to VKAs, DOACs were associated with similar risks of recurrent VTE (weighted HR, 0.80; 95% CI, 0.43–1.46; I 2 = 0.00), major bleeding (weighted HR, 0.96; 95% CI, 0.57–1.63; I 2 = 0.59), and all‐cause mortality (weighted HR, 1.04; 95% CI, 0.81–1.34; I 2 = 0.00). Sensitivity analyses yielded findings consistent with those of the primary analysis.
Conclusion(s): Among octogenarians with VTE, DOACs showed a comparable effectiveness and safety compared with VKAs. Our results support the use of DOACs in this high‐risk group. TABLE 1 Crude and adjusted hazard ratios of the study outcomes associated with use of DOACs compared to use of VKAs among octogenarians with VTE
TABLE 1 Crude and adjusted hazard ratios of the study outcomes associated with use of DOACs compared to use of VKAs among octogenarians with VTE
Women's Health
Coagulation Proteins Beyond Hemostasis
OC 51.1
Direct oral anticoagulants cause placental vascular abnormalities and epigenetic reprogramming in placenta and the offspring
K. Singh1; A. Gupta1; R. Younis1; H. Mai1; K. Shahzad2; B. Isermann2; S. Kohli 2
1 Institute for Laboratory Medicine, Leipzig University, Leipzig, Sachsen, Germany; 2 Institute of laboratory medicine, clinical chemistry and molecular diagnostics, Leipzig University, Leipzig, Sachsen, Germany
Background: Direct oral anticoagulants (DOACs) are increasingly used as antithrombotic agents in non‐pregnant individuals. Their use is discouraged in pregnancy due to their potential teratogenic effects. Several reports ask for better monitoring and reporting in cases of inadvertent DOAC use during pregnancy questioning whether DOACs may be safe to use during pregnancy.
Aims: We aim to study whether DOAC exposure during pregnancy impairs placental function and may have long‐lasting effects in the offspring.
Methods: To study the effect of DOACs during pregnancy, mice received factor IIa inhibitor (fIIai, dabigatran) alone or in combination with procoagulant extracellular vesicles (EVs, to incude placental thrombo‐inflammation). Placenta was evaluated for morphological alterations (H&E) and expression of trophoblast differentiation marker Gcm‐1. In vitro, trophoblast cells were treated with fIIai to study trophoblast differentiation and epigenetic regulation. Similarly, placenta, neonatal brain and kidney were studied for global epigenetic marks (DNMT1, HDAC3, H3K9me3, H3K9ac) using immunoblotting.
Results: EV‐induced pregnancy loss was prevented by fIIai treatment. However, the embryos showed growth restriction suggesting embryopathy. fIIai treatment on days 9.5 and 11.5 post‐coitus resulted in altered placental morphology at day 13.5 post‐coitus reflecting persistent impaired placental vascularization. fIIai reduced Gcm‐1 expression and altered epigenetic marks in vitro and in vivo. Remarkably, even neonatal brains and kidney showed dysregulated epigenetic marks suggesting that fIIai, which can cross the placenta, can epigenetically re‐program the offspring.
Conclusion(s): These results suggest that fIIai, while preventing thrombo‐inflammatory effects during pregnancy, has severe consequences on placental and embryonic development. These effects may be partially epigenetically programmed and can persist in the offspring. This may affect the offspring health. Further mechanistic studies are required to evaluate whether these effects are thrombin dependent, the mechanism underlying the altered epigenetic marks and their relevance. In line with current recommendations these results warrant caution regarding the use of fIIai in pregnancy.
Estrogens and Progestinics
OC 51.5
Effects of gender and hormonal status on thrombin generation in a large population‐based cohort of twins and their family members
S. Zwaveling 1; S. Bloemen2; E. Castoldi3; E. de Geus4; J. Hottenga4; T. Hackeng5; D. Boomsma4; C. Hemker6, G. Willemsen4
1 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Breukelen, Utrecht, Netherlands; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 3 CARIM, Maastricht University, Maastricht, Not Applicable, Netherlands; 4 Department of Biological Psychology, Amsterdam Public Health Research Institute, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 5 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 6 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Risk factors for venous thrombosis (VT) include age, gender, genetics and life‐style factors, e.g. oral contraceptive (OC) use. Thrombin generation (TG) correlates with a risk of VT.
Aims: We examined the association of TG with demographics, hormonal status and OC use in a large and well‐characterised cohort of twins and family members. Using a cotwin‐control design, the effect of OCs on TG within pairs discordant for OC use was determined.
Methods: TG data was available for 7,869 consenting participants (mean age: 45.1, range 18–98, 64.3% female) in the Netherlands Twin Register biobank. Data on demographics, hormonal status (incl. OC use) and medication use were obtained. TG was determined by calibrated automated thrombinography (CAT) in citrated plasma using 5 pM tissue factor (TF) and 4 μM phospholipids in the presence and absence of 20 nM thrombomodulin (TM). After excluding users of anticoagulants (n = 101), TG was compared in men, women with a natural menstrual cycle, women using estrogen‐containing OCs and post‐menopausal women. TG differences were also examined in female monozygotic twins concordant (N = 306) and discordant (N = 152) for OC use.
Results: TG of women showed shorter lag‐times, higher peak heights and higher endogenous thrombin potentials (ETPs) compared to men. Women using OCs had the most procoagulant TG profiles: higher ETP and peak, and shorter lag‐time. All groups differed significantly, with post‐menopausal women closer in value to men than women with a natural cycle. Similar patterns were seen in the presence of TM. The discordant cotwin‐control analyses confirmed the OC effect: even after controlling for familial factors. OC use was associated with a less favorable profile (Table 1).
Conclusion(s): This study shows that factors known to increase VT risk (notably OC use) act by increasing TG. Our findings in discordant female monozygotic twin pairs provide strong evidence for a prothrombotic profile caused by OC use. TABLE 1 TG parameters in MZF twin pairs concordant and discordant for OC use. In case of discordant twins, the non‐using twin is twin 1, the OC using twin is twin 2. In bold p < 0.000, Italic p < 0.05.
TABLE 1 TG parameters in MZF twin pairs concordant and discordant for OC use. In case of discordant twins, the non‐using twin is twin 1, the OC using twin is twin 2. In bold p < 0.000, Italic p < 0.05.
OC 52.2
Risk and impact of anticoagulation‐associated abnormal menstrual bleeding in women of reproductive age with venous thromboembolism – The TEAM‐VTE study
C. De Jong 1; M. Blondon2; C. Ay3; A. Buchmuller4; J. Beyer Westendorf5; J. Biechele6; L. Bertoletti7; G. Colombo8; M. Donadini9; S. Hendriks10; L. Jara‐Palomares11; S. Nopp12; P. Ruiz‐Artacho13; P. Stephan14; C. Tromeur14; T. Vanassche15; P. Westerweel16; F. Klok1
1 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 2 Division of Angiology and Hemostasis, Geneva University Hospitals and Faculty of Medicine, Geneva, Geneve, Switzerland; 3 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 4 Service de Médecine Vasculaire et Thérapeutique, CHU Saint Etienne, Saint‐Étienne, Rhone‐Alpes, France; 5 Department of Medicine, Division Hematology, Dresden University Hospital, Dresden, Sachsen, Germany; 6 Department of Oncology and Internal Medicine, Klinik Arlesheim, Arlesheim, Basel‐Landschaft, Switzerland; 7 CHU Saint Etienne, Saint‐Etienne, Rhone‐Alpes, France; 8 University of Insubria, Varese, Lombardia, Italy; 9 Department of Medicine and Surgery, University of Insubria and ASST Sette Laghi, Varese, Italy, Varese, Lombardia, Italy; 10 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 11 Medical Surgical Unit of Respiratory Diseases. Hospital Universitario Virgen del Rocio, Sevilla, Spain. Instituto de Biomedicina de Sevilla (IbiS), Sevilla, Spain. Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Sevilla, Andalucia, Spain; 12 Medical University of Vienna, Vienna, Wien, Austria; 13 Department of Internal Medicine, Clínica Universidad de Navarra, Madrid, Madrid, Spain; 14 Department of Internal Medicine and Chest Diseases, CHU de Brest, Brest, Bretagne, France; 15 University Hospitals Leuven, KULeuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 16 Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, Zuid‐Holland, Netherlands
Background: Women of reproductive age treated with anticoagulants have an increased risk of abnormal uterine bleeding (AUB). However, solid studies to quantify the prevalence of AUB are lacking.
Aims: To assess the incidence of (new‐onset) AUB and impact on quality of life (QoL).
Methods: The TEAM‐VTE study was an international, multicenter, prospective cohort study. Women aged 18–50 years treated with anticoagulants for venous thromboembolism (VTE) were included within one month after VTE diagnosis, before the next menstrual cycle, in 12 hospitals between August 2018 and September 2021. The study was terminated early because of slow recruitment due to the pandemic. Menstrual blood loss was measured by pictorial blood loss assessment charts (PBAC) at baseline for the last menstrual cycle before VTE diagnosis, and prospectively for each cycle during 3–6 month follow‐up. AUB was defined according to three definitions: PBAC score >100, PBAC score >150, or self‐reported increased menstrual volume. AUB‐related QoL was assessed at baseline and end of follow‐up using the Menstrual Bleeding Questionnaire (MBQ; higher scores indicate worse outcome).
Results: Of the 98 women (mean age: 34 years), 66% (65/98) met at least one of the three definitions of AUB during follow‐up (95% confidence interval (CI) 57%–75%; Table 1). AUB occurred in 60% (36/60) of women without AUB before VTE diagnosis (95% CI 47%–71%). Overall, QoL decreased significantly over time with a mean increase in MBQ score of 5.1 points (95% CI 2.2–7.9). When stratifying by AUB, this decrease in quality of life was only observed among women who had new‐onset AUB during the follow‐up (Figure 1).
Conclusion(s): Two out of three women who start anticoagulation for acute VTE suffer from AUB, with a considerable negative impact on QoL. These findings should be a call to action to increase awareness for this issue and to provide evidence‐based strategies for preventing and treating AUB in this setting.
Pregnancy and Pregnancy Complications
OC 52.5
Superficial venous thrombosis of the lower extremities during pregnancy and post partum: A Danish population‐based cohort study
H. Wiegers 1; D. Körmendiné Farkas2; E. Horváth‐Puhó3; S. Middeldorp4; N. van Es1; H. Toft Sørensen3
1 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Clinical Epidemiology, Aarhus University Hospital, Denmark, Midtjylland, Denmark; 3 Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Midtjylland, Denmark; 4 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands
Background: Data on the risk of lower extremity superficial venous thrombosis (SVT) during pregnancy and the postpartum period are lacking.
Aims: To examine: (1) the incidence rate of lower extremity SVT during pregnancy and in the postpartum period and (2) the risk of subsequent venous thromboembolism (VTE) within the same pregnancy.
Methods: This nationwide population‐based cohort study of all deliveries between 1997 and 2017 in Denmark was based on two nationwide health registries: the Danish National Patient Registry and the Medical Birth Registry covering all Danish hospitals and deliveries. Incidence rates per 1000 person‐years were calculated overall and separately per trimester and the postpartum period. Furthermore, the risk of VTE within the same pregnancy and postpartum period after a pregnancy‐related SVT was calculated compared to an age‐ and parity matched pregnant comparison cohort without previous SVT. Sensitivity analyses were performed on first pregnancies and on women without a history of VTE.
Results: A total of 1,276,046 deliveries in 733,951 women were included in this study. 710 SVT events occurred from conception up to 12 weeks post partum, yielding an overall incidence rate of 0.58 (95% CI, 0.54–0.63) per 1000 person years. The incidence rates of SVT per 1000 person‐years during the first, second, and third trimester were 0.13 (95% CI, 0.09–0.18), 0.24 (95% CI, 0.19–0.29), and 0.54 (95% CI, 0.46–0.62), respectively; the incidence rate was 1.69 (95% CI, 1.54–1.84) per 1000 person‐years during the postpartum period. In 10.4% of pregnancies with SVT, a VTE occurred during the antepartum or postpartum period, compared with 0.13% of pregnancies without SVT. Sensitivity analyses attenuated the results.
Conclusion(s): We found a low incidence rate of lower extremity SVT during pregnancy and post partum. However, if SVT during pregnancy occurs the risk to develop VTE within the same pregnancy is high. These results may inform decisions about anticoagulant management after pregnancy‐related SVT.
OC 52.3
Post‐partum hemorrhage in sub‐Saharan Africa – First results from a prospective study in Mozambique
M. Glenzer1; R. Barnes2; M. Correia3; E. Luis3; I. Boaventura3; V. Nhantumbo3; N. Manguele3; P. Silva3; A. von Drygalski 4
1 University of California, San Diego, San Diego, California, United States; 2 University of California San Diego, Dept. of Medicine, San Diego, USA, San Diego, California, United States; 3 Universidade Eduardo Mondlane, Depts. of Gynecology/Obstetrics and Hematology, Maputo, Maputo, Mozambique; 4 Division of Hematology/Oncology, Department of Medicine, University of California San Diego, San Diego, California, United States
Background: Maternal mortality in sub‐Saharan Africa is ~500–1,000 per 100,000 live births (vs. ~5–20 in developed countries). Postpartum hemorrhage (PPH) is responsible for 30%–50% of the deaths.
Aims: To study PPH, risk factors, and mortality in metropolitan Mozambique to inform future studies and intervention strategies.
Methods: Prospective data collection of deliveries at Maputo Central Hospital between February 2019 and January 2021. Data included age, HIV status, parity, delivery mode, delivery notes, vital signs, laboratory values, and fetal data. PPH was determined by charted diagnosis, blood loss >500 ml, transfusion, and/or notes indicating significant bleeding.
Results: 8,799 deliveries were analyzed. Median age and parity were 28 (Inter‐quartile range [IQR] 24–32) and 1 (IQR 0–2). Prevalence of HIV and anemia was 10% and 57% respectively. Incidence of PPH and maternal mortality was 14.5% and 1.3% respectively. Maternal mortality was strongly associated with PPH (adjusted odds ratio [AOR] 4.27; 95% confidence interval [CI] 2.56–7.14), fewer gestational weeks (AOR .75; CI .71‐.79), and rural living (AOR 1.84; CI 1.09–3.11). Uterine atony (UA; 1% prevalence) was associated with a strikingly high incidence of PPH (96.1%) and maternal mortality (8.8%). Parity of 5+ was associated with sharp increases in risk of PPH, maternal and infant mortality, and UA. Available labs from 1,202 distressed mothers revealed that lower hemoglobin level was strongly associated with PPH (AOR .75; CI 0.68–0.81), especially evident with shorter gestation. PPH was also associated with eclampsia (AOR 3.87; CI 2.94–5.12). Blood product transfusions were available for only ~1/3 of PPH cases. Antifibrinolytics were unavailable.
Conclusion(s): PPH remains a serious problem with high mortality even in metropolitan areas of sub‐Saharan Africa. Anemia is an important and modifiable risk factor (iron supplementation, prophylactic antifibrinolytics). It is critical to raise awareness and improve region‐specific prevention protocols.
OC 52.4
A case series examining the safety and efficacy of anticoagulation management in pregnant women with metallic heart valves
K. White 1; L. Millar1; K. Vonklemperer1; L. Bowles1; M. Hogg1; O. Glesa1; R. Howard2; R. Khan1; A. Deaner1
1 Barts Health NHS Trust, London, England, United Kingdom; 2 Queen's Hospital Romford, London, England, United Kingdom
Background: Metallic Heart Valves (MHVs) pose a high risk of thrombotic complications in pregnancy. Recent UKOSS data report 5% mortality. Anticoagulation management remains controversial, lacking clear guidance. At Barts NHS trust most women opt for Low Molecular Weight Heparin (LMWH) with intensive anti‐Xa monitoring with dose adjustment aiming for peak anti‐Xa of 0.8–1.2 IU/ml (valve dependant), trough >0.6 IU/ml.
Aims: To evaluate the safety and efficacy of Barts’ anticoagulation regimes in pregnant women with MHVs.
Methods: A single centre retrospective audit of anticoagulation management of MHV during pregnancy 2010–2022.
Results: We reviewed 19 pregnancies in 13 women. 15/19 (78.9%) anticoagulated with LMWH and aspirin; 2/19 (10.5%) LMWH and warfarin, 2/19 (10.5%) warfarin throughout pregnancy. The 2 pregnancies on warfarin throughout resulted in miscarriage at 12/40 and stillbirth at 24/40. 1/15 (6.67%) on LMWH experienced a miscarriage and there was one termination. 2/19 developed valve thrombus; one woman (incorrectly started on daily tinzaparin and aspirin without anti‐Xa monitoring) presented with an embolic coronary event at 9/40 and opted for termination. The other woman on therapeutic LMWH developed valve thrombus at 33/40 provoking a switch to warfarin – the thrombus resolved and she was delivered safely at 37/40. On average there was a 24 mg BD (0–35 mg) increase in LMWH dose, anti‐Xa levels were done every 11.6 days and 15.5% anti‐Xa results were out of range during pregnancy. 5 women had scheduled caesarean sections, 5 had normal deliveries at mean gestation at 36/40 (30–41), mean birth weight 2456g (1795–3180 g). Mean blood loss at delivery was 581mls with mean haemoglobin drop 22g/l. 3/19 (15.8%) patients required transfusion (1 placenta praevia, 1 pelvic subcutaneous haematoma).
Conclusion(s): An individualised MDT approach and tightly controlled LMWH dosing can result in good outcomes for women with MHV in pregnancy, but they remain high risk and require intensive medical surveillance and delivery planning.
OC 51.4
Spleen tyrosine kinase activation drives platelet procoagulation in antiphospholipid syndrome during pregnancy
E. Agbani 1; S. Chaturvedi2; A. Lee3; P. Skeith4; M. Barber4; L. Yamaura4; K. Main4; Y. Eslambolchi4; A. Garven4; E. Mahe5; A. Dufour4; A. Clarke4; M. Choi4; M. Poon4; A. Poole6; L. Skeith4
1 University of Calgary, Calgary, Alberta, Canada; 2 Johns Hopkins University, Baltimore, Maryland, United States; 3 University of Victoria, Victoria, British Columbia, Canada; 4 University of Calgary, Calgary, Alberta, Canada; 5 University of Calgary, University of Calgary, Alberta, Canada; 6 University of Bristol, Bristol, England, United Kingdom
Background: Pregnant patients with antiphospholipid syndrome (APS) develop thrombotic and obstetric complications despite therapy with low‐molecular‐weight heparin (LMWH) and aspirin. Platelets are known to play a role in thrombosis, but platelet characteristics in pregnant patients with APS are poorly understood. Elucidating platelet procoagulant mechanisms may allow us to identify novel drug targets to improve thrombotic or obstetric outcomes.
Aims: To elucidate platelet characteristics and procoagulant mechanisms in pregnant patients with APS.
Methods: Two pregnant patients with systemic lupus erythematosus and high‐titre triple positive antiphospholipid syndrome (APS/SLE) on LMWH/aspirin, and two pregnant control (PC) participants were followed at matched time points during pregnancy (REB19‐1447). We utilized a high‐resolution fluorescence imaging suite to visualise platelets in plasma, followed by a systematic analysis of procoagulant membrane dynamics and biochemical study.
Results: Circulating ballooned platelets typified all three trimesters of pregnancy in APS/SLE participants. Platelet activation and P‐selectin expression increased as pregnancy progressed and peaked during gestational week 24–28. Expectedly, phosphatidylserine (PS) externalisation (indicated by platelet membrane annexin‐V binding) and thrombin generation followed a similar temporal pattern. Additionally, PC platelets treated with plasma from APS/SLE participants (PC+) phenocopied the ballooning and procoagulation characteristics of APS/SLE platelets. Compared to PC platelets, APS/SLE platelets during the early 3rd trimester showed a four‐ to six‐fold increase in Spleen tyrosine kinase (Syk) phosphorylation (Tyr‐525/Tyr‐526) and a three‐fold increase in αIIbβ3 integrin activation. PC+ platelets also showed increased Syk phosphorylation but not integrin αIIbβ3 activation. Treatment of APS/SLE or PC+ platelets with the Syk inhibitor PRT‐060318 (10 μM) significantly attenuated Syk phosphorylation, P‐selectin expression, and PS exposure. Notably, PRT‐060318 reduced αIIbβ3 integrin activation in APS/SLE platelets only, and inhibited membrane ballooning in PC+ but not APS/SLE platelets.
Conclusion(s): We provide evidence that Syk‐driven platelet activation and procoagulation characterise pregnancy in APS/SLE patients. We are currently evaluating the mechanisms of antiphospholipid‐driven platelet activation and procoagulation.
OC 51.2
Changes in VWF, ADAMTS13, and complement in preeclampsia, HELLP syndrome and other obstetric complications: A single centre observational study
L. Neave 1; M. Thomas2; R. de Groot3; A. Doyle4; A. David5; K. Maksym6; M. Whitten7; M. Scully8
1 UCLH Hospitals NHS Trust/Haemostasis Research Unit, University College London, London, England, United Kingdom; 2 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 3 University College London, London, England, United Kingdom; 4 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 5 Elizabeth Garrett Anderson Institute for Women's Health, University College London, London, England, United Kingdom; 6 Fetal Medicine Unit, UCLH Hospitals NHS Trust/Institute for Women's Health, University College London, London, England, United Kingdom; 7 Elizabeth Garrett Anderson Institute for Women's Health, London, England, United Kingdom; 8 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom
Background: There is evidence, some conflicting, of changes in the VWF/ADAMTS13 axis, and also in the complement system, in preeclampsia and HELLP syndrome. Other obstetric complications are less studied in this regard.
Aims: To assess changes in VWF, ADAMTS13 and complement in preeclampsia, HELLP syndrome, fetal growth restriction (unexplained or uteroplacental), and new onset thrombocytopenia (<75 × 109/L), compared to normal pregnancies.
Methods: Pregnant women with a current or previous history of the obstetric complications of interest were recruited at a regional centre, in addition to gestational age‐matched normal pregnant controls. ADAMTS13 activity, VWF antigen and activity, CH50, and sC5b‐9 were measured in blood samples taken at recruitment. Clinical data was collected. Informed consent and regional ethics committee approval (18/NW/0552) were obtained. Data is summarised as median (range). Mann‐Whitney was used to compare groups (with significance level p < 0.05).
Results: 128 cases were analyzed. Comparing preeclampsia/HELLP (N = 42) with normal pregnancy (N = 48) (Table 1), ADAMTS13 activity was significantly lower in preeclampsia, VWF antigen and activity were significantly higher, and the VWF antigen:ADAMTS13 ratio was even higher (3.7 (1.9–6.5) vs. 2.1 (0.9–4.7), p < 0.0001). sC5b‐9 was higher in preeclampsia/HELLP than normal pregnancy (268 (162–628) vs. 189 (110–290), p < 0.0001), but there was no change in CH50. Abnormalities were greater in cases with features of HELLP (N = 9). Similar changes were not observed in fetal growth restriction (N = 13), thrombocytopenia (N = 10), or in pregnant women with a history of preeclampsia (N = 15) (Figure 1). The odds of VWF Ag:ADAMTS13 ratio >2 was 31.9 times higher in preeclampsia than normal pregnancy (95% CI 4.8–338.4), while the odds of sC5b‐9 >180 ng/ml was 11.0 times higher (2.6–49.7).
Conclusion(s): There is marked elevation in VWF levels, VWF antigen:ADAMTS13 ratio, and sC5b‐9 in preeclampsia/HELLP compared to normal pregnancies and certain other obstetric complications. This may result from endothelial activation, and may have potential therapeutically and diagnostically.
OC 51.3
Leptin and IL‐6 are linked to the mechanism underlying the prothrombotic state in obese pregnant women
S. McNeill 1; F. Jordan2; V. Gibson3; D. Freeman2; C. Bagot4
1 NHS Greater Glasgow and Clyde, GLASGOW, Scotland, United Kingdom; 2 University of Glasgow, Glasgow, Scotland, United Kingdom; 3 NHS Greater Glasgow and Clyde, Glasgow, Scotland, United Kingdom; 4 Department of Haematology, Glasgow Royal Infirmary, Glasgow, England, United Kingdom
Background: Venous thromboembolism (VTE) is the leading direct cause of maternal death, with obese women over‐represented in the mortality data. Obesity is associated with hyperleptinaemia and low‐grade chronic inflammation, and both are associated with a prothrombotic state. A similar pathophysiology may underly the prothrombotic state in obese pregnant women and understanding this mechanism may assist in the development of more effective VTE prevention strategies.
Aims: To investigate the role of adipokines in the pathophysiology underlying the prothrombotic state in obese pregnant women.
Methods: 262 women, grouped by BMI class, were recruited to a British Heart Foundation funded prospective longitudinal study, approved by the West of Scotland Research Ethics Committee. Following written informed consent, blood samples were obtained at the antenatal booking appointment and repeated at 28 weeks’ gestation. Thrombin generation was performed using the Calibrated Automated Thrombogram method. Protein S (PS) and fibrinogen were assayed by the ACL TOP analyser. Leptin, IL‐6, Tissue factor pathway inhibitor (TFPI), and plasminogen activator inhibitor‐1 (PAI‐1) were quantified by ELISA.
Results: Leptin (Fig. 1) and IL‐6 (Fig. 2) were significantly higher in obese compared to lean women at booking and 28 weeks’ gestation (all p < 0.001). At booking, multiple regression analysis, adjusted for BMI, age and parity, demonstrated leptin to be independently associated with fibrinogen (adjusted R 2 = 20.16%, p < 0.0001) and ETP (adjusted R 2 = 15.08%, p < 0.0001), and IL‐6 to be independently associated with fibrinogen (adjusted R 2 = 26.86%, p < 0.0001). At 28 weeks’, fibrinogen remained independently associated with leptin (adjusted R 2 = 15.33%, p < 0.0001) and IL‐6 (adjusted R 2 = 22.37%, p < 0.0001).
Conclusion(s): We have previously demonstrated obese women to have higher thrombin generation and fibrinogen in early pregnancy. We are the first to demonstrate, in pregnancy, that leptin and IL‐6 correlate with thrombin generation and fibrinogen, suggesting that the pathophysiology underlying the prothrombotic state in obese pregnant women is linked to leptin and IL‐6.
OC 52.1
Mode of delivery and intracranial hemorrhage in newborns with hemophilia: A systematic review and meta‐analysis
N. Radadia 1; D. Vyas2; E. Trinari3; A. Chan4; J. Petropoulos4; M. Bhatt5
1 McMaster University, Cambridge, Ontario, Canada; 2 University of North Carolina Greensboro, Greensboro, North Carolina, United States; 3 University of McMaster, Hamilton, Ontario, Canada; 4 McMaster University, Hamilton, Ontario, Canada, 5 McMaster Children's Hospital, Hamilton, Ontario, Canada
Background: Newborns with hemophilia have 44–60 times higher risk of neonatal intracranial hemorrhage (ICH). Mode of delivery has been explored as a prevention strategy. While initial small studies and meta‐analysis of 3 studies by Davies & Kadir (2015) suggested beneficial effect of caesarian section (CS) compared to spontaneous vaginal delivery (SVD), subsequent literature has reported no difference.
Aims: To perform a systematic review and meta‐analysis to compare odds of neonatal ICH in newborns with hemophilia born via CS and assisted VD (AVD) to SVD.
Methods: Systematic search of literature in MEDLINE, EMBASE, CINAHL, and Web of Science from inception to 2021. Abstracts and full texts were screened by two independent reviewers based on pre‐set inclusion/exclusion criteria. Risk of bias was evaluated using Newcastle Ottawa Scale (NOS). Heterogeneity was assessed using Cochran’s Q test and I 2 statistic and studies were assigned appropriate weight based on a random‐effects model. Meta‐analysis was conducted using SPSS software© and effect sizes were compared using odds ratios with p‐value <0.5 considered significant.
Results: Search yielded 1756 articles; 11 were included in final meta‐analysis (Figure 1). Of 2842 patients among 11 studies, 1864 (66%) were born via SVD, 784 (28%) CS, and 194 (6%) AVD; 68 (2.4%) suffered neonatal ICH. Based on NOS, 10 studies were deemed ‘good’ quality and 1 ‘fair’ quality. Heterogeneity was small with Cochran’s Q of 11.29 and I 2 statistic of 0.20. The odds of neonatal ICH with CS were similar to SVD (pooled OR: 1.27, 95% CI: 0.66–2.46) (Figure 2A). The odds of neonatal ICH with AVD were much higher compared to SVD (pooled OR: 12.88, 95% CI: 6.14–27.00) (Figure 2B).
Conclusion(s): Unlike previous reports, this larger meta‐analysis shows that CS is not associated with reduced risk of neonatal ICH when compared to SVD. As known, higher risk of neonatal ICH with AVD compared to SVD was confirmed.


