Acquired Bleeding Disorders
Coagulopathy of Major Bleeding (Trauma, PPH, Vascular/surgical, ECMO, GI bleeding, etc.)
PB0475
Acquired von Willebrand disease in a patient undergoing extracorporeal membrane oxygenation
D. Cibele 1; C. Monteiro2; S. Silva3; M. Lopes4; L. Gonçalves5; S. Teixeira6; I. Machado1; M. Carvalho5; R. Roncon‐Albuquerque7; C. Koch5
1 Centro Hospitalar e Universitário São João, Porto, Porto, Portugal; 2 Center of Thrombosis and Haemostasis, Congenital Coagulopathies Reference Centre, Department of Immunohemotherapy, Centro Hospitalar Universitário São João, Porto, Porto, Portugal; 3 Department of Emergency and Intensive Care Medicine, Centro Hospitalar Universitário São João, Porto, Porto, Portugal; 4 Serviço de Imunohemoterapia, Hospital São João, Porto, Porto, Portugal; 5 Center of Thombosis and Haemostasis, Department of Immunohemotherapy,Centro Hospitalar e Universitário São João, Porto, Porto, Portugal; 6 Centro Hospitalar e Universitário São João, Maia, Porto, Portugal; 7 Department of Emergency and Intensive Care Medicine, Centro Hospitalar Universitário São João, Porto, Porto, Portugal
Background: Venoarterial Extracorporeal Membrane Oxygenation (VA‐ECMO) ensures a stable hemodynamic state in patients with life‐threatening cardiac failure. Although it requires anticoagulation therapy for prevention of thrombotic events, several ECMO intrinsic mechanisms result in the development of Acquired von Willebrand disease (AvWD) and platelet dysfunction, with an increased bleeding tendency.
Aims: To report the case of a patient under VA‐ECMO with AvWD associated bleeding.
Methods: A 54‐year‐old female, with several cardiovascular comorbidities, received VA‐ECMO support after an ST‐elevation myocardial infarction, Killip Class IV. While on a heart transplant waiting list, unfractionated heparin (UFH) was administered with minimal bleeding. During transplant surgery she required massive transfusion and, 5 days later, UFH was interrupted and a re‐sternotomy was performed due to a large hemothorax. After stabilization, UFH was briefly reintroduced, but consequent bleeding through thoracic drains required transfusion and hemostatic treatment. von Willebrand Factor (vWF) antigen (Ag) and vWF ristocetin cofactor (RCo) were determined (respectively, 2.81 and 1.61UI/ml; vWF:RCo/Ag ratio 0.57), while an angio‐computed tomography indicated that, besides several thoracic hematomas, an acute pulmonary embolism (PE) occurred. Nonetheless, clinical evolution was favorable and, on day 57, ECMO was removed. vWF:RCo and vWF:Ag were determined once more (2.06 and 1.96UI/ml; ratio 0.95).
Results: Forty four days after ECMO implementation, the patient presented with serious bleeding and AvWD was investigated ‐ despite the increased vWF:RCo and vWF:Ag, the low vWF:RCo/Ag ratio (< 0.7), enforced the diagnosis of AvWD. Desmopressin was used as a therapeutic strategy, but the simultaneous diagnosis of PE excluded treatment with vWF concentrate due to its thrombotic risk. As expected, AvWD was resolved immediately after ECMO was discontinued.
Conclusion(s): The presence of a delicate balance between thrombotic and bleeding risks is challenging in patients undergoing. There are no current consensuses on bleeding management due to AvWD.
PB0487
FXIII concentrate use in uncontralable bleeding after cardiac surgery– What is the role? – A case report
S. Teixeira 1; M. Carvalho2; I. Machado3; D. Cibele3; L. Gonçalves2; C. Koch2
1 Centro Hospitalar e Universitário São João, Maia, Porto, Portugal; 2 Center of Thombosis and Haemostasis, Department of Immunohemotherapy,Centro Hospitalar e Universitário São João, Porto, Porto, Portugal; 3 Centro Hospitalar e Universitário São João, Porto, Porto, Portugal
Background: Bleeding disorders in extracorporeal circulation remains a challenge. FXIII plays a critical role in clot stabilization which can be affected in patients submitted to cardiac surgery.
Aims: To present a case report of FXIII concentrate use in a bleeding diastasis of difficult control after cardiac surgery.
Methods: A 30‐years‐old woman recipient of a orthotopic heart transplant at 20 years of age due to familial dilated cardiomyopathy entered to the emergency department after several syncope episodes. On transthoracic echocardiogram she presented moderately impaired left ventricular systolic function and akinesia of the middle and basal segments of the inferior and posterior wall. Heart catheterization showed anterior descending artery with 70% stenosis, circumflex artery with 90% stenosis and right coronary artery with 50% stenosis. After team discussion coronary artery bypass graft (CABG) was performed.
Results: CABG was likely preceded by a perioperative acute myocardial infarction with need to rebuild vascular bridges and consequent prolongation of the cardiopulmonary bypass time. Hemorrhagic diathesis of difficult control ensued despite massive transfusion – 11 erythrocyte concentrate (EC), 12 Fresh Frozen Plasma (FFP), 3 platelet concentrates (PC) and 4 gr of fibrinogen‐ ECMO team was then mobilized. The patient remained in cardiogenic/hypovolemic shock and was reintervened needing 3 EC, cell‐saver, 4 FFP, 2 gr of fibrinogen, 1 PC, 1000 IU of prothrombin complex concentrate and desmopressin. Due to deterioration of clinical state she underwent surgery again, bleeding tendency persisted, ROTEM results as on Table 2 and FXIII of 57%, 6 EC, 1 PC, 4 FFP, 4 gr of fibrinogen and 2000 IU of FXIII (40 UI/kg) were administered. After all the transfusion products and surgical haemostasis the bleeding was controlled.
Conclusion(s): Cardiac surgery is a challenge in what concerns haemostasis and detailed consideration of all moments of coagulation is necessary trying to optimize care of these patients.


PB0480
Periarticular hemangioma as a cause of chronic arthropathy due to hemarthrosis
Z. Karakas 1; A. Unuvar2; Y. Yilmaz3; D. Tugcu4; G. Tanyildiz4; S. Karaman1
1 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, Istanbul, Istanbul, Turkey; 2 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, ISTANBUL, Istanbul, Turkey; 3 Istanbul University Istanbul Medical Faculty Department of Pediatric Hematology and Oncology, Istanbul, Istanbul, Turkey; 4 Istanbul University, Istanbul, Istanbul, Turkey
Background: Hemangioma is a benign vascular tumor that is commonly seen in pediatric age group. It is often localized at head/neck region. The rare localizations such as periarticular regions can result in misdiagnosis.
Aims: In this abstract, we aimed to present a patient with complaint of ankle pain and swelling for three years and has been diagnosed with periarticular hemangioma after further evaluations.
Methods: Patient was followed up by pediatric rheumatology and orthopedic outpatient clinics with complaint of right ankle pain and swelling for three years. He was diagnosed as juvenile idiopathic arthritis and received oral methotrexate. When the undulant complaints persisted on, the magnetic resonance imaging was run. The MRI scan revealed periarticular hemangioma and patient was referred to pediatric hematology and oncology clinic.
Results: There was swelling and decreased range of motion on right ankle and atrophy of calf muscle on first admission to our clinic. Other physical examinations were done and were within normal limit. Past medical history and family history was not specific. The laboratory results containing coagulation parameters were within normal limit. The MRI scan of right ankle showed hemarthrosis and one cm diameter nodular lesion at periarticular region. The radiology report and another center’s biopsy pathology report indicated hemangioma. Patient was evaluated for hemarthrosis and clinical and laboratory data guided us to hemangioma and was started oral propranolol. After three months, her complaints were regressed.
Conclusion(s): Periarticular hemangioma can cause to intra‐articular hemorrhage, hemarthrosis and by chronically mechanical irritation to arthropathy. The differential diagnosis can include hemophilia and rheumatologic disease. The MRI scan has priority for diagnosis. Pathological diagnosis might be invasive for childhood age group. The treatment is oral propranolol and/or arthroscopic surgical resection. Children with chronic arthropathy can be evaluated by periarticular hemangioma. Differential diagnosis such as hemophilic arthropathy should be taken into consideration.
PB0484
A comparison study of ROTEM® Sigma and ROTEM® Delta in a major tertiary hospital
N. Modica1; L. Kaminskis1; M. Anderson1; S. P'ng2; D. Pepperell 2
1 Haematology Department. PathWest Fiona Stanley Hospital, Perth, Western Australia, Australia; 2 Fiona Stanley Hospital, Perth, Western Australia, Australia
Background: The ROTEM Sigma is a cartridge based visco‐elastic instrument providing global assessment of the coagulation process. It supersedes the ROTEM Delta as a blood management tool to guide targeted component therapy.
Aims: Implementation of a new blood management device in the Transfusion Medicine Unit of a tertiary hospital requires careful assessment of potential differences to ensure continuity of service and consistent patient management. This study aimed to rule out potential measurement bias resulting from replacement of ROTEM Delta with ROTEM Sigma within the Fiona Stanley Network, and to verify manufacturer reported reference ranges. Study outcomes to be used to address suitability of current transfusion algorithms.
Methods: Blood from 51 healthy donors and 22 control samples manipulated with heparin to mimic a significant cohort of our patient population were run on the Delta and Sigma devices in tandem. Correlation across clinically relevant parameters derived from our ROTEM algorithm for critical bleeding were evaluated. (EXTEM CT +A5, INTEM CT, FIBTEM A5 and HEPTEM CT)
Results: EXTEM, INTEM and HEPTEM CT values displayed modest correlation (0.5, 0.7 and 0.45). Clot firmness at 5 minutes showed good correlation between devices (EXTEM A5 0.89 FIBTEM A5 0.98). A negative bias of 10.0% was recorded on the Sigma FIBTEM A5 (p < 0.00001). Normal ranges derived from this study were in correlation with those reported by Werfen group.
Conclusion(s): Parameters produced by both ROTEM devices used in the Algorithm for Critical Bleeding showed some statistical variation with no clinical significance. Slight bias in FIBTEM A5 unlikely to be reflected in clinical intervention as decision for administration of cryoprecipitate would be made with clinical circumstance and not algorithm alone. The comparison of these two systems can serve as an aid to other hospitals looking to implement this device.
PB0489
Audit of the use of Fibclot (Fibrinogen concentrate) in patients without congenital afibrinogenaemia
M. Ul‐haq 1; G. Baidya2; G. Gidley2; E. Horn2; A. Kanny2; J. Tarrant3
1 Leeds Teaching Hospitals, Blackburn, England, United Kingdom; 2 Leeds Teaching Hospitals, Leeds, England, United Kingdom; 3 Leeds Teaching hospitals, Leeds, England, United Kingdom
Background: Fibclot is licensed for use in patients with congenital hypo or afibrinogenaemia with a bleeding tendency. The recommended initial dose in an emergency situation is 0.05 g per kg of body weight aiming for a level of 1 g/l in non surgical bleeding.
Aims: To assess the indications and increments achieved in patients who had received Fiblclot.
Methods: This was an audit that collected information for the period March to December 2021; the point at which Fibclot had been introduced to the trust, using commissioning data and patient case notes.
Results: Information from 30 patients was analysed; 63% were women (n = 19) with a mean age of 41.47 years (range 0‐87). The clinical indications were: disseminated intravascular coagulopathy (DIC)‐10%, post‐partum haemorrhage (PPH)‐46.7%, acquired bleeding disorders 10%, liver disease 16.7%, cardiac surgery 10% and neonatal coagulopathy 6.7%. Mean plasma fibrinogen levels in all clinical indications pre and post Fibclot was 2.67 (range: 0.3‐4.5, median: 1.6) and 2.9 (range: 0.7‐5.1, median: 2.5) (n = 18). Excluding patients with PPH, mean plasma fibrinogen pre and post Fibclot was 1.48 (range: 0.3‐3.9) and 2.12 (range: 0.7‐5.1). In patients with DIC (n = 2), mean plasma fibrinogen levels pre and post Fibclot was 0.85 and 1.4. The mean and median Fibclot dosage was 0.04 g/kg (range of 0.02 g/kg‐0.08 g/kg). One patient with DIC experienced expressive dysphasia during the infusion of Fibclot. The infusion was stopped and a subsequent CT head was normal. Her symptoms resolved within 24 hours and she received further doses of Fibclot without neurological symptoms. There were no other documented side effects. Cessation and prevention of haemorrhage was achieved in 26/30 patients who survived until hospital discharge.
Conclusion(s): Fibclot has been used in patients with a range of clinical indications in order to raise fibrinogen levels to a sufficient degree to achieve haemastasis.
PB0483
Can thrombin generation assay predict bleeding risk in patients with severe liver failure?
A. Navaei 1; K. Bruzdoski2; V. Kostousov1; L. Hensch3; S. Hui1; J. Teruya1
1 Texas Children's Hospitals / Baylor College of Medicine, Houston, Texas, United States; 2 Texas Childrens Hosptials, Houston, Texas, United States; 3 Texas Children's Hospital / Baylor College of Medicine, Houston, Texas, United States
Background: Coagulopathy in liver failure may maintain a balanced state. Disruption of this balance will cause increased bleeding or thrombotic risks. Assessment of bleeding risk is essential to prevent undesired bleeding episodes or unnecessary therapy. Routine coagulation tests show poor correlation with bleeding risk in liver failure. Thrombin generation assay (TGA) provides unique perspective of coagulation and may be of benefit in this population.
Aims: Comparing standard coagulation assays with TGA and viscoelastometry to assess their correlation with bleeding.
Methods: After IRB approval, citrated plasma samples from 10 liver patients at a tertiary pediatric hospital were included in this descriptive study. Data were collected from routine hemostasis tests including international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, D‐dimer, and platelet count. TGA was performed using Genesia (Diagnostica Stago, USA) and viscoelastometry was performed via ROTEM (Werfen, USA). TGA values were reported as ratio or percentage of the reference plasma. Clinical bleeding episodes were obtained from electronic medical records. Descriptive statistics and ANOVA performed. Results were reported in mean+/‐SD.
Results: Median age was 4.5 (IQR 0.8‐15) years and female 60%. Thirty nine percent of samples were associated with bleeding episodes, ranging from mucocutaneous to intracranial hemorrhage. Samples were divided into two groups based on association with bleeding episodes. Results are presented in Table 1. D‐dimer, TGA lag time and EXTEM clotting time (CT) showed an association with bleeding episodes. Other parameters were not associated with bleeding episodes.
Conclusion(s): TGA lag time, EXTEM CT and D‐dimer can potentially be utilized as a predictive tool to assess bleeding risk in coagulopathy secondary to liver failure. Notably, INR did not show correlation with bleeding episodes. Further studies are warranted to assess utility of TGA and ROTEM in liver failure.

VPB0492
LEX‐211 (FARES‐II): a phase 3, prospective, active‐control randomised study of four‐factor prothrombin complex concentrate versus frozen plasma in bleeding adult cardiac surgery patients
K. Karkouti1; J. Callum 2; C. Solomon3; S. Knaub4
1 University of Toronto, Toronto, Ontario, Canada; 2 Queen's University, Kingston, Ontario, Canada; 3 Octapharma AG, Lachen, Zurich, Switzerland; 4 Octapharma AG, Lachen, Schwyz, Switzerland
Background: Cardiac surgery is often complicated by coagulopathic bleeding, leading to transfusion and poor outcomes. Prothrombin complex concentrate (PCC) and frozen plasma (FP) are used for coagulation factor replacement during surgery.
Aims: To demonstrate that four‐factor PCC (4F‐PCC, Octaplex®, Octapharma) is clinically non‐inferior to FP in terms of haemostatic effectiveness, as measured by the need for post‐therapy haemostatic interventions.
Methods: LEX‐211 (sponsor: Octapharma) will include patients (≥18 years) undergoing cardiac surgery with cardiopulmonary bypass (CPB) who require coagulation factor replacement due to post‐CPB bleeding and known/suspected coagulation factor deficiency. Exclusion criteria include heart transplant, insertion/removal of ventricular assist devices, high probability of death within 24 h, severe right heart failure, heparin contraindications, thromboembolic event (TEE) within 3 months and IgA deficiency. Approximately 500 patients will be randomised to PCC (20–25 IU/kg) or FP (10–15 ml/kg) (Figure 1). The primary endpoint is haemostatic response to PCC vs. FP, rated ‘effective’ if no further haemostatic intervention (systemic haemostatic agents, including second dose of study drug, or surgical re‐opening for bleeding) is required 60 min–24 h after initiation of first dose. Secondary endpoints include global haemostatic response (60 min–24 h), bleeding (24 h), blood product/coagulation factor usage (24 h, 7 d), surgical re‐exploration (24 h) and coagulation parameters (~1 h post‐treatment). Safety endpoints include serious treatment‐emergent adverse events (e.g., TEE, major adverse cardiac events), mechanical ventilation, ICU stay, hospitalisation and mortality (30 d).
Results: LEX‐211 is planned to start in Q2 2022. An unblinded interim analysis (100 evaluable patients/group) will test sample size assumptions and re‐estimate if necessary. Completion is expected Q1 2024.
Conclusion(s): The results of this study will inform clinical practice for bleeding cardiac surgery patients requiring coagulation factor replacement, potentially reducing blood product usage, and improving outcomes.

PB0488
Targeted Factor V replacement during major trauma haemorrhage
A. Thaventhiran 1; J. Lopez‐Tremoleda2; K. Brohi2; C. Thiemermann3; R. Davenport4
1 Barts and the London School of Medicine and Dentistry, Tunbridge Wells, England, United Kingdom; 2 Queen Mary University of London, London, England, United Kingdom; 3 The William Harvey Research Institute, Barts and The London School of Medicine & Dentistry, Queen Mary University of London, London, England, United Kingdom; 4 Centre for Trauma Science, Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom
Background: Factor Va (FVa) critical to thrombin generation, in a massive haemorrhage protocol (MHP) can only be supplemented as Fresh Frozen Plasma. It is unknown whether FFP can sufficiently maintain FV during MHP and ongoing bleeding in trauma.
Aims: Characterise the temporal changes of FV in trauma patients Determine the efficacy of Factor Va replacement in a murine model of Acute Traumatic Coagulopathy (ATC).
Methods: Trauma patients at a trauma centre were included with blood samples collected on admission and after transfusion of 4, 8 and 12 RBC units for FV assay. In a preclinical model of ATC three groups (n = 10) were infused with vehicle, rhFVa (resistant to aPC), or hFVa 30 minutes after haemorrhage. Animals were euthanased at 60 minutes to collect terminal blood samples for biomarkers of coagulation, fibrinolysis and FVa degradation.
Results: 207 trauma patients were included (Table 1). Admission FV was 41% lower in MHP patients compared to those without major injury and RBC transfusion < 4 units (60 u/dl vs 102 u/dl, p < 0.0001). Despite MHP, FV levels decreased to 36 u/dl (8U RBC) and 32 u/dl (12U RBC). Patients who died early (<12 h) had significantly lower FV at baseline than survivors (27 u/dl vs 60 u/dl). Compared to vehicle, mice infused with rhFVa or hFVa had significantly higher median survival rates at 60 minutes (44% vs 80% vs 88%). There was at least a three‐fold increase in plasmin‐antiplasmin levels in the vehicle group compared to rhFVa/hFVa (208 ng/ml vs 70 ng/ml vs 31 ng/ml, p < 0.0001) but with no difference in ROTEM clot strength. Plasmin degradation of hFVa was observed in both intervention groups but only aPC mediated degradation was seen in rhFVa animals.
Conclusion(s): FV in bleeding trauma patients is low on admission and not corrected by current MHP therapy. FVa replacement in addition to balanced resuscitation may represent a novel therapy for trauma haemorrhage.

PB0474
Acquired von‐Willebrand‐disease in ECMO or LVAD patients: a comparative cohort study
T. Bajorat 1; J. Hildebrandt1; B. Pannholzer2; D. Kowalski1; D. Juhl3; J. Kalbhenn4; A. Kowalski2; B. Zieger5; A. Haneya2; U. Nowak‐Göttl1
1 University Hospital Schleswig‐Holstein, Department for Hemostasis and Thrombosis, Institute for Clinical Chemistry, Kiel, Schleswig‐Holstein, Germany; 2 University Hospital Schleswig‐Holstein, Department of Cardiac and Vascular Surgery, Kiel, Schleswig‐Holstein, Germany; 3 University Hospital Schleswig‐Holstein, Department of Transfusion medicine, Lübeck, Schleswig‐Holstein, Germany; 4 University of Freiburg, Department of Anesthesiology and Critical Care, Freiburg, Baden‐Wurttemberg, Germany; 5 Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology and Oncology, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany
Background: Approximately 100% of patients necessitating ECMO or LVAD conduits develop post interventional acquired von‐Willebrand‐disease (aVWD), with only a small proportion presented with clinically relevant bleeding.
Aims: The aim of the present comparative cohort study of consecutively enrolled patients admitted to the cardiac surgery department (CSD) was to collect demographic, medical and laboratory data possibly associated with i) development of clinically relevant bleeding and/or ii) death during a three‐months follow‐up.
Methods: 507 white patients (LVAD n = 169) aged 18‐89 years (median: 61; male 80.3%) were enrolled. 78 of 507 patients (19.7%) presented with clinically relevant bleeding. Logistic regression adjusted for age, gender, BMI and liver function was performed.
Results: The analysis showed that i) the presence of blood group 0 versus non‐0 (OR/95%CI: 2.2/1.3‐3.6) and worse outcome within 30 days following intervention (OR/95%CI: 2.63/1.4‐5.0) was associated with symptomatic aVWD, and, vice versa, that patients with LVAD compared with ECMO insertion significantly less common develop clinically relevant aVWD (OR/95%CI: 0.55/0.33‐0.98). The overall death rate within 30 days post intervention was 71.2%. Furthermore we found that ii) ECMO versus LVAD implantation (OR/95%CI: 1.9/1.57‐2.38), symptomatic aVWD (OR/95%CI: 2.8/1.5‐5.4), the presence of blood group non‐0 versus 0 (OR/95%CI:1.7/1.06‐2.7), and increasing age per year (OR/95%CI: 1.01/1.002‐1.03) was independenly associated with worse outcome in the patients enrolled.
Conclusion(s): In the present comparative cohort study we found a clinical relevant bleeding rate of 19.7% in subjects with aVWD, with a significant higher proportion in patients receiving ECMO compared with LVAD devices. The development of symptomatic aVWD was associtated with the presence of blood group 0 and worse outcome.
PB0478
Changes in circulating H3 Histone levels during Extracorporeal Membrane Oxygenation in patients with severe respiratory failure with correlation to haemostatic complications
A. Doyle 1; K. Parmar2; A. Aswani2; K. Breen3; N. Barrett2; A. Retter1; B. Hunt1
1 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 2 Guy's & St Thomas NHS Foundation Trust, London, England, United Kingdom; 3 Guy's & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Circulating histones are part of damage associate molecular pattern of lung injury. They are also recognised as contributing to a prothrombotic tendency and thrombocytopenia. However, their role during extracorporeal membrane oxygenation (ECMO) has not been described.
Aims: 1) To assess changes in circulating H3.1 and citrullinated H3 (H3R8) levels, a marker of NETosis, in patients requiring veno‐venous ECMO 2) To correlate H3 with haemostatic events and thrombocytopenia
Methods: Blood samples were taken from 17 patients >18 years old at a single, high‐volume ECMO centre. Samples were taken at pre‐ECMO, 1‐hour, 1‐day, 2‐days, pre‐decannulation and 1‐day after. Samples were analysed by ELISA for H3.1 and H3R8 according to manufacturer’s protocols (Volition, Belgium). Ethical approval was gained for the study with nominated consultee consent for patient samples.
Results: H3.1 (reference range 0‐48 ng/ml) was significantly elevated prior to cannulation and continued to increase during the first 2 days (median 1271 to 1924 ng/ml, p = 0.031). There was a similar increase in H3R8 (reference range 0‐4.7 ng/ml) following the first 2 days (49.6 to 123.9 ng/ml, p = 0.012) however H3.1/H3R8 ratio remained stable (19.7 to 12.5, p = 0.26). There was a decrease from preceding to decannulation to 1‐day after in H3.1 (1321 to 953 ng/ml, p = 0.030) and H3R8 (58.2 to 36.0 ng/ml, p = 0.01). There was no significant difference in H3 levels in patients with pulmonary embolism, requiring circuit change, intracranial haemorrhage, or deep vein thrombosis compared to those without. There was no difference in H3.1 levels 24‐ and 48‐hours preceding the development of thrombocytopenia after 2‐days of ECMO.
Conclusion(s): These results suggest a combination of both lung injury and extracellular damage by the ECMO circuit contributing to high levels of circulating H3 histones. There was a lack of correlation between H3 histones to haemostatic events and thrombocytopenia, although given their highly elevated levels, it does not exclude their pathological role.
PB0476
Total thrombus formation analysis system (T‐TAS) detects poor clot formation in a swine model of polytrauma and hemorrhagic shock
A. Cralley 1; E. Moore2; M. DeBot3; T. Schaid3; C. Fox4; A. Ghasabyan3; S. Mitra5; P. Hom3; K. Hansen1; M. Cohen1; C. Silliman1; A. Sauaia1
1 University of Colorado, Aurora, Colorado, United States; 2 Ernest E Moore Shock Trauma Center atDenver Health, Denver, Colorado, United States; 3 University of Colorado, Denver, Colorado, United States; 4 University of Maryland, Baltimore, Maryland, United States; 5 University of Colorado, Department of Surgery, Aurora, Colorado, United States
Background: T‐TAS has gained popularity as a diagnostic tool to monitor antiplatelet therapy and predict bleeding risks. Its applicability to monitor trauma induced coagulopathy has not yet been assessed. The T‐TAS AR chip evaluates fibrin‐rich thrombus progression during arterial shear stress using platelet and coagulation system interactions. We hypothesized that the T‐TAS AR Chip measurements (T10, OT, and AUC30) would correlate with prothrombin time (PT) and thrombelastography (TEG) measurements in detecting coagulopathy.
Aims: Using PT and TEG as gold standards, we evaluated the diagnostic accuracy of T‐TAS.
Methods: 16 Yorkshire swine were randomized to undergo either instrumentation only (SHAM), or an injury model (INJURY: blast brain injury, femur fractures, hemorrhagic shock) followed by resuscitation. Native TEG, PT, and T‐TAS measurements were performed in tandem on citrated blood samples collected at baseline, and at 60 and 240 minutes post‐injury. Coagulopathy was defined as PT>14 sec ( >2 SD above baseline mean). Correlation of T‐TAS values with TEG and PT findings were conducted using Pearson’s correlation test.
Results: 4 Swine were assigned to the SHAM group and 12 to the INJURY group. The INJURY group developed coagulopathy at 60 min (PT = 15.4 ± 3.8 sec) and 240 min (PT = 14.91 ± 2.2 sec), while the SHAM group did not develop any coagulopathy. At baseline, the T‐TAS outcome T10 correlated strongly with TEG’s R‐Time (R = 0.7, p = 0.002) and Angle (R=‐0.67, p = 0.005), and correlated weakly with PT (R = 0.35. p = 0.18). R‐time remained highly correlated with T10 throughout the experiment for both groups. PT and Angle showed correlations with T‐TAS measurements at maximum coagulopathy in the INJURY group at 60 min. MA was not significantly correlated to any T‐TAS measurements.
Conclusion(s): T‐TAS T10 appears to be a sensitive marker for detecting hypocoagulability postinjury, with strong correlations to the TEG R‐Time. Other T‐TAS measurements only correlated with PT and Angle during PT‐detected hypocoagulability.


PB0485
Assessment of trauma‐induced coagulopathy using a microfluidic model of injury at venous and arterial shear regimes
S. Shea 1; R. Rassam1; R. Fonseca2; M. Canas2; K. Thomas3; K. Bochicchio2; G. Bochicchio2; P. Spinella4
1 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 2 Department of Surgery, Washington University in St. Louis, St. Louis, Missouri, United States; 3 Department of Pediatrics, Washington University in St. Louis, St. Louis, Missouri, United States; 4 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA; Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States
Background: Trauma is the leading cause of death in young individuals. 25% of trauma patients present with trauma‐induced‐coagulopathy (TIC), which increases mortality 400%. The underlying mechanisms of TIC are not fully understood.
Aims: We aimed to characterize flow‐dependent hemostatic function of trauma patient samples in a microfluidic model of injury.
Methods: Whole blood collected from Level I trauma patients at admission (N = 19) was perfused at venous and arterial shear through a microfluidic model of injury comprising an injury‐site (IS) and a collagen/tissue‐factor‐coated extravascular space. Hemostasis at the IS generates a clot seal, yielding endpoints of bleeding time (BT) and IS closure frequency (CF). A random subset of samples (N = 7) were stained with a fluorescent CD41 antibody to label platelets and mean fluorescence intensity (MFI) measured throughout perfusion. Trauma samples were compared to healthy controls (N = 2) via t‐test. Data are reported as mean (standard error of the mean(SEM)).
Results: Patients were 78% male, majority blunt injury (61%), and ISS was median 14 (interquartile range 5‐27). BT and CF for all groups are shown in Fig1. At low shear, control BT was 222 s(48 s), while trauma BT was 803 s(83 s) (p = 0.04). At high shear, control BT was 642 s(33 s), while trauma BT was 1011 s(67 s) (p = 0.09). There was a bimodal distribution in the trauma population, suggesting a possible division between those with hemostatic function and those without (Fig1). The CF was 100% in both controls, 63% for trauma in low shear, and 33% for trauma in high shear. Trauma samples demonstrated decreased platelet incorporation at low shear, yet increased platelet incorporation at high shear (Fig2).
Conclusion(s): We found delayed bleeding times in trauma samples at venous shear supported by a concomitant lack of platelet deposition. Bleeding times were delayed at high shear, despite substantial platelet deposition. Further characterization of TIC can optimize treatment approaches and identify novel therapeutics.


PB0479
A comparison of thrombin generation ex vivo circuit and in critically ill patients requiring Extracorporeal Membrane Oxygenation
A. Doyle 1; K. Parmar2; N. Gooby2; K. Breen3; N. Barrett2; A. Retter1; B. Hunt1
1 Guy’s & St Thomas’ NHS Foundation Trust, London, England, United Kingdom; 2 Guy’s & St Thomas NHS Foundation Trust, London, England, United Kingdom; 3 Guy’s & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Thrombin generation (TG) is increased with the use of extracorporeal circuits such as cardiopulmonary bypass. TG is also increased in patients with critical illness. The combination of these factors in patients receiving ECMO may therefore be significantly elevated but at present is poorly described.
Aims: To assess changes in TG markers in an ex vivo circuit of ECMO and in patients receiving veno‐venous ECMO for severe respiratory failure.
Methods: 6 ex vivo ECMO circuits with sodium citrate using healthy donor whole blood were run for 24‐hours at a high‐flow rate (4L/min). Blood samples were taken from 17 patients requiring ECMO prior to and 1‐day after starting, and prior to decannulation and 1‐day after. Samples were analysed by ELISA for Prothrombin Fragments (PF1+2, reference range 200‐1200 pmol/L), Thrombin‐Antithrombin (TAT, 0.8‐3.8μg/L) and D‐Dimers (< 400 ng/ml FEU) according to manufacturer’s protocols. Ethical approval was gained for the study with nominated consultee consent for patient samples.
Results: In the ex vivo circuit, there was no significant increase prior to circuit initiation to 24‐hours after in PF1+2 (median 97 to 101 pmol/L, p = 0.5), TAT (4.2 to 2.6 μg/L, p = 0.3) and D‐Dimers (462 to 506 ng/ml, p = 0.3). No circuit thrombosis was seen in any circuit. In critically ill patients, there was a significant increase prior to cannulation to 1‐day after in PF1+2 (median 729 to 1305 pmol/L, p = 0.03) and non‐significant increases in TAT (19.5 to 36.9μg/L, p = 0.7) and D‐Dimers (7398 to 9903 ng/ml, p = 0.3). There was a significant decrease from prior to decannulation to 1‐day after in PF1+2 (median 1453 to 658 pmol/L, p < 0.001), TAT (40.1 to 11.7μg/L, p < 0.001) and D‐Dimer (15450 to 11200 ng/ml, p = 0.05).
Conclusion(s): ECMO circuits lead to reversible increases TG in critically patients but not shown in an ex vivo model. Given elevated preceding TG markers in patients, this suggests that patient‐attributable factors may increase TG during ECMO.
VPB0491
Hyperfibrinolysis drives instabilities in trauma induced coagulopathy
A. Gosselin 1; V. Tutwiler2
1 Rutgers University, Central Square, New York, United States; 2 Rutgers University, New Brunswick, New Jersey, United States
Background: Trauma induced coagulopathy (TIC) leads to excessive bleeding following severe injury, by preventing the formation of stable blood clots, increasing transfusion requirements and mortality. TIC has several phenotypes, with increased clot degradation (hyperfibrinolysis) being among the most lethal. However, the mechanisms causing each phenotype are poorly defined.
Aims: To determine the mechanisms and factors present during TIC and their impact on structure and stability of blood clots after trauma.
Methods: Platelet poor plasma (PPP) was supplemented with tissue plasminogen activator (tPA), tissue factor (TF) and saline dilution to model the hyperfibrinolysis, hyperactivation and hemodilution consistent with clinical TIC. We examined fibrin formation in this model of TIC (STIC) following clotting initiation with CaCl2 and thrombin. Samples were tested with confocal microscopy, optical turbidity, and viscoelastic testing to determine the turbidity, storage modulus, and structural properties.
Results: Across testing modalities STIC samples showed significantly higher storage modulus and optical density compared to controls at 600 seconds (p < 0.05, Fig. 1A‐B). This was due in part to the polymerization rate being 9‐16 times faster in STIC samples vs control samples (p < 0.001, Fig 1C‐D). STIC samples had complete fibrin disintegration after 1800 seconds observed in confocal microscopy which corresponded with a complete loss of both structure and mechanical stability (Fig. 1A‐B). To determine which component was the main cause of mechanical instability, we varied the individual factors described above and determined that hyperfibrinolysis most accurately generated the loss of mechanical and structural integrity (Fig. 1A‐B). Addition of tranexamic acid, a fibrinolysis inhibitor, to the STIC samples caused a restoration of final mechanical properties compared to controls (Fig. 1A‐B).
Conclusion(s): This in vitro plasma model demonstrated that each individual factor leads to unique alterations in the structure and stability of plasma clots, however hyperfibrinolysis is the main mechanistic driver of clot instability seen our simulated TIC clots.

VPB0490
Audit on the use of fresh frozen plasma in a Tertiary Care Hospital
G. Ene 1; M. Raya Hinojosa2; L. Edo Caballero2; A. Carpi Medina2; A. Villaubi Serra2; N. Palo Mauriz2; E. Membrilla Fernandez2; J. Alvarez Garcia2
1 Banc de Sang i Teixits Hospital del Mar, Barcelona, Catalonia, Spain; 2 Hospital del Mar, Barcelona, Catalonia, Spain
Background: The administration of fresh frozen plasma (FFP) has been proved to be inappropriate in many hospital settings although many guidelines recommend its use in specific situations.
Aims: To audit the usage FFP in our hospital and to establish the need for a different approach on the use of blood components in managing bleeding and acquired coagulopathies.
Methods: Medical records of patients who received FFP in our hospital during 2021 were retrospectively studied.The recommendations of the British Committee for Standards in Hematology were used to determine the correct use of plasma.
Results: A total of 291 units were issued in 100 cases (89 patients, 68 male, mean age 66 years, range 21‐95 years).Bleeding related to surgery in the setting of altered coagulation, gastrointestinal bleeding and trauma represented the most common appropriate use of FFP. Seven units of FFP were used to revert DOACs in 5 patients (3 on Apixaban, 1 on Edoxaban, 1 on Rivaroxaban) and 19 units were administered to 11 patients on VKA (5 patients with no bleeding and 6 with major bleeding that required urgent reversal). In 12 patients 34 units of FFP were used to correct the coagulation of patients on LMWH. After a thorough review only in 49 cases (197 units) the use of FFP was according to guidelines (average 4 units/patient). In 51 cases (94 units) the use was not based on guidelines recommendations (average 1.8 units/patient).
Conclusion(s): In our study we have observed that there is great variability in recommendations and amount of FFP administered. Its use requires a proper understanding of its indications since in some situations may even be deleterious, delaying the use of more effective products. With an important decrease of blood donations, a critical eye should be placed on the use of FFP in managing bleeding as well as its use in non‐bleeding patients.
PB0473
Viscoelastic testing to guide transfusion management in patients with end stage liver disease: A systematic review and meta‐analysis
M. Al Moosawi 1; T. Hussaini2
1 The University of British Columbia, Vancouver, British Columbia, Canada; 2 University of British Columbia, Vancouver, British Columbia, Canada
Background: Standard coagulation tests (SCTs) such as INR/PT, PTT, and fibrinogen have limited utility in assessing coagulopathy in patients with end‐stage liver disease (ESLD) due to “re‐balanced” hemostatic activity. Observational data and small clinical trials have shown clinical utility for viscoelastic testing (VET) in this setting.
Aims: To compare transfusion requirements and clinical outcomes in patients with ESLD who are undergoing invasive procedures using VET‐guided vs SCT‐guided transfusions.
Methods: This was a systematic review and meta‐analysis. We searched MEDLINE Ovid, Embase Ovid, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and The WHO International Clinical Trial Registry Platform databases. Only parallel randomized controlled studies were included. The primary outcome was the number of patients transfused, and the average number of blood components transfused. Post‐operative bleeding, reoperation, and overall mortality were also captured.
Results: The preliminary analysis included data generated using MEDLINE Ovid search. 663 records were screened, of which 25 full texts were evaluated for eligibility, and 5 trials were included in the meta‐analysis (Figure 1). More patients in SCT group received blood transfusions versus the VET group (83.2% vs 46.7%; RR = 0.50, p = 0.12, 95% CI 0.21‐1.19). VET‐based transfusion led to a statistically significantly lower number of plasma units transfused (mean difference ‐1.34, 95% CI ‐1.78‐0.89, p < 0.05). There was no statistically significant difference in the mean number of pRBC or platelets transfused. Although there was a trend toward lower post‐operative bleeding in the VET group, this difference did not reach statistical significance (9.4% in VET and 16.8% in SCT, RR = 0.51, p = 0.12, 95%CI 0.33‐1.13). No difference in mortality was observed (26.9% in VET and 27.7% in SCT, RR = 1.02, p = 0.94, 95%CI 0.54‐ 1.93). Figure 2 shows the forest plots for each outcome.
Conclusion(s): VET‐guided transfusions in patients with ESLD resulted in a reduced proportion of patients transfused as well as lower mean plasma transfusion requirements.


PB0481
Are levels of direct anticoagulant at the onset of hip fracture surgery associated with post‐surgery bleeding?
A. Lubetsky 1; A. Levi2; R. keshet3
1 Sheba Medical Center, Tel‐Hashomer, Not Applicable, Israel; 2 Sheba Medical center, Tel‐Hashomer, HaMerkaz, Israel; 3 Sackler School of Medicine, Tel‐Aviv University, Tel‐Hashomer, HaMerkaz, Israel
Background: Hip fractures are a major cause of morbidity and mortality in elderly population, with 1‐year mortality rates up to 20%. Early surgery within 48 hours is associated with better morbidity and mortality post‐surgery. In patients treated with direct oral anticoagulants (DOACs), awaiting for the anticoagulant effect to subside in order to avoid excess surgical bleeding, may lead to delayed hip fracture surgery (HFS). There is limited data regarding the effect of DOAC levels at surgery on post‐surgical bleeding
Aims: To study the association between levels of DOACs at the start of HFS and post‐surgical bleeding in elderly patients
Methods: A retrospective cohort study of patients admitted to orthopedic or geriatric wards at Sheba Medical Center, with hip fractures between January 2016 to October 2019. Out of 1749 patients (age>65) with HFS 137 patients treated with DOACs and assessed for DOAC blood levels were studied. Half‐life and predicted level at surgery start were computed from sequential pre‐surgery blood levels. Outcome was post‐surgery bleeding divided into three bleeding classifications: Major bleed (MB), clinically relevant non major bleed (CRNMB) and No bleed
Results: Of the 137 patients, 64 (47%) had MB, 47 (34%) had CRNMB, 26 (19%) did not bleed. The predicted median level at surgery was 14.59 ng/ml for MB patients, 11.20 ng/ml for CRNMB patients and 14.41 ng/ml for patients with no bleeding with no significant difference among the three groups was found (p = 0.86). In all groups most patients (69%‐70%) had a level ≤ 30 ng/ml at surgery start (p = 0.98) (Figure 1).
Conclusion(s): Levels of DOAC at surgery start were not associated with post‐surgical bleeding in elderly DOAC treated patients who underwent HFS. Higher DOAC level pre‐surgery should not be the cause to delay surgery and consequently more patients could be operated upon in the desired 48 hours time frame

PB0482
Dysfunctional hemostasis in patients under extracorporeal life support. A rapid diagnostic approach with therapeutical guidance intentions
A. Moreno‐Castaño 1; E. Sandoval2; M. Pino2; S. Samanbar3; L. Bonastre2; P. Molina2; S. Fernández2; H. Ventosa4; G. Escolar4; P. Castro2; M. Diaz‐Ricart5
1 Hematopathology, Pathology Department, Centre de Diagnòstic Biomèdic. Hospital Clinic Barcelona, Barcelona, Catalonia, Spain; 2 Hospital Clinic Barcelona, Barcelona, Catalonia, Spain; 3 Hematopathology, Pathology Department, CDB, Hospital Clinic, IDIBAPS, University of Barcelona, Spain, Barcelona, Catalonia, Spain; 4 Hematopathology, Department of Pathology, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 5 Hospital Clinic, IDIBAPS, University of Barcelona, Spain, Barcelona, Catalonia, Spain
Background: Short‐term cardiopulmonary extracorporeal life supports (ECLS) are invasive devices whose use has increased exponentially during the COVID‐19 pandemic. Major bleeding is a main cause of morbi‐mortality in ECLS patients and acquired von Willebrand disease (aVWD) could justify this complication.
Aims: We aim at investigating the primary hemostasis alterations profile in ECLS patients, and to propose a potential treatment if bleeding.
Methods: Patients in ECLS at our center since June 2021 were included (n = 25). Primary hemostasis was evaluated by: von Willebrand Factor antigen (VWF:Ag) and activity (VWF:GPIbM) measurement (immunoturbidimetry), VWF multimeric analysis (agarose‐gels and immunoblotting), platelet function analysis (PFA‐200), and platelet activation (CD62P and CD63 expression by flow cytometry). Studies were performed 24 h after implant, each 7 days, and in the first week after ECLS extraction. T‐TAS® was used for hemostasis analysis in samples from bleeding patients, before and after in vitro addition of purified VWF. This study was approved by the Hospital Clinic’s Ethics Committee (HCB/2021/0200).
Results: After 24 h of ECLS implant, increased VWF:Ag levels and prolonged PFA occlusion times. In 60% of patients, altered VWF:GPIbM/VWF:Ag ratio ( < 0.7) and loss of VWF high molecular weight multimers (HMWM) were observed. CD62P expression was slightly higher in ECLS patients platelets than in controls (MFI±SD of 4.34 ± 2.2 vs. 3.27 ± 0.6, respectively; p = 0.3). Early after ECLS extraction, there was normalization of the VWF multimeric profile and PFA values. Interestingly, in samples from bleeding patients, addition of purified VWF reduced significantly the T‐TAS occlusion times (776 s±207 s vs. 1161 s±251 s, Mean±SD, post vs. pre, respectively; p = 0.033).
Conclusion(s): ECLS caused primary hemostasis alterations, leading to aVWD and platelet activation, solved early after support removal. Hemostatic efficiency in ECLS bleeding patients, with lack of HMWM, was corrected in vitro by providing functional purified VWF.


PB0477
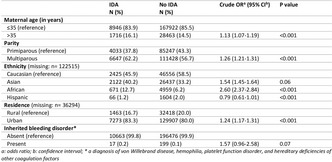
Age and sex distribution of the prevalence and incidence of hospitalisations and mortality due to bleeding in England 2014‐2019
K. Creeper 1; A. Stafford2; S. Choudhuri3; N. Tumian4; K. Breen5; A. Cohen6
1 Guy’s and St Thomas’s NHS Foundation Trust, Kings College London, London, England, United Kingdom; 2 Curtin University, Perth, Western Australia, Australia; 3 Northern Care Alliance, Manchester, Manchester, England, United Kingdom; 4 Guy’s and St Thomas’s NHS Foundation Trust and Department of Hematology, Hospital Canselor Tuanku Muhriz University Kebangsaan Malaysia, CHERAS, Kuala Lumpur, Malaysia; 5 Guy’s & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom; 6 Guy’s and St Thomas’s NHS Foundation Trust, Kings College London, LONDON, England, United Kingdom
Background: The relationship between age, sex and acute bleeding is described but not well defined in a national cohort. Studies evaluating demographics with respect to burden, incidence and trends in bleeding hospitalisations and mortality are infrequent.
Aims: To report the age and sex distribution of bleeding‐related hospitalisations and mortality.
Methods: A population‐based review of people in England between 2014 and 2019 either admitted to an acute care ward, or who died was undertaken. Admitted patients were identified using the Hospital Episode Statistic database. Mortality data and population estimates were obtained from the Office of National Statistics. Bleeding events were selected based on the International Statistical Classification of Diseases version 10 codes. Patients with inherited or acquired coagulation or platelet disorders, and those who were not admitted (emergency department or outpatient clinic) were excluded. Analyses were stratified by year, sex and age. Annual incidence of admissions was calculated per 100,000 patient years, and deaths per 100,000 people.
Results: There was a total of 3,238,427 hospitalisations, with a mean of 539,738 ± 6033 per year and 81,264 deaths with a mean of 13,544 ± 331 per year attributable to bleeding. The mean annual incident rate for bleeding related hospitalisations was 975 per 100,000 patient years and for mortality was 24.45. 58.9% (n = 1,908,153) of all hospitalisations occurred in females. Males had a higher mean annual mortality (OR 1.06, p < 0.001). Over the study period there was a trend of fewer deaths (χ2 test for trend 91.4, p < 0.001) (Figure 1). 68.6% (n = 55,786) of all deaths occurred in patients ≥ 75 years (Figure 2).
Conclusion(s): Females had a higher mean annual incidence of hospitalisation. Males had a higher annual incident mortality. The was a direct relationship between increasing age and incidence of bleeding related hospitalisation and mortality. The reasons for this require further investigation.


PB0486
Decompensated cirrhosis with bacterial infections shows a peculiar derangement of the hemostatic balance
E. Campello 1; A. Zanetto2; C. Bulato2; S. Gavasso2; G. Saggiorato2; N. Perin2; S. Shalaby2; P. Burra2; M. Senzolo3; P. Simioni4
1 University of Padova, Padova, Veneto, Italy; 2 Unviersity of Padova, Padova, Veneto, Italy; 3 Multivisceral Transplant Unit, University Hospital of Padua, Padua, Italy, Padova, Veneto, Italy; 4 Padua University Hospital, Padua, Veneto, Italy
Background: Decompensated cirrhosis with bacterial infections shows a peculiar derangement of the hemostatic balance.
Aims: To assess the factors responsible for bleeding tendency in BIs, we conducted a prospective study comparing all components of hemostasis (platelets, coagulation, and fibrinolysis) in hospitalized patients with decompensated cirrhosis with vs. without BIs.
Methods: Primary hemostasis assessment included whole blood platelet aggregation and von Willebrand factor (VWF). Coagulation assessment included procoagulant factors (fibrinogen, factor II, V, VII, VIII, IX, X, XI, XII, XIII), natural anticoagulants (protein C, protein S, antithrombin) and thrombomodulin‐modified thrombin generation test. Fibrinolysis assessment included fibrinolytic factors (plasminogen, t‐PA, PAI‐1, α2‐AP, TAFIa/ai) and plasmin‐antiplasmin complex (PAP).
Results: Eighty patients with decompensated cirrhosis were included (40 with and 40 without BIs). Severity of cirrhosis and platelet count were comparable between groups. At baseline, patients with cirrhosis and BIs had a significantly lower whole blood platelet aggregation, consistent with impaired platelet function, without significant differences in VWF. Regarding coagulation, BIs were associated with reduced procoagulant factors VII and XII, and a marked reduction of all natural anticoagulants. Thrombomodulin‐modified thrombin generation, however, was comparable between groups. Finally, although mixed potentially hypo‐fibrinolytic (low plasminogen) and hyper‐fibrinolytic (high t‐PA) changes were present in BIs, comparable levels of PAP were detected in both groups (Table 1). Upon resolution of infection (n = 29/40), platelet aggregation further deteriorated whereas coagulation and fibrinolysis factors returned to levels observed in patients without BIs (Figure 1).
Conclusion(s): In hospitalized patients with decompensated cirrhosis, BIs are associated with reduced whole blood platelet aggregation and a marked decrease of all natural anticoagulants, which may unbalance hemostasis and potentially increase the risk of bleeding and thrombosis.


Management/Treatments of Acquired Bleeding
PB0006
Delayed diagnosis of acquired hemophilia A results in worse clinical outcome
S. Stankovikj 1; N. Ridova2; S. Stojanovska2; T. Ristevska2; M. Cvetanoski2
1 University clinic of hematology, Skopje, Skopje, Macedonia; 2 University Clinic of Hematology, Skopje, Skopje, Macedonia
Background: Acquired hemophilia is a rare but potentially life‐threatening disease, involving development of autoantibodies directed against plasma coagulation factors. It is crucial to establish an early diagnosis and to initiate an appropriate treatment.
Aims: To present a case of patient with delayed diagnosis of acquired hemophilia A, his treatment and outcome.
Methods: A 66‐year‐old male was admitted to the hospital with a clinical presentation of a huge hematoma in his left leg and right chest. Both his medical and family history were negative for clotting disorder at any point before. The hematoma in his leg occurred one month earlier and it was treated as a muscle rupture.The blood tests revealed hemoglobin level of 76 g/L, WBC 12 x10(9)/L, and platelet count 693 x10(9)/L Screening hemostasis tests revealed prothrombin and thrombin time in normal range, but significantly prolonged activated partial thromboplastine time to 59 s (normal range 26‐34 s). Factor VIII level was 0.7%. The Bethesda assay confirmed inhibitor titre of 10.2 BU.
Results: The bleeding was treated with a by‐passing agent rVIIa (NovoSeven). Immunosuppressive treatment was started with Cyclophosphamide 100 mg and Prednison 100 mg daily. On the 8‐th day of hospitalization NovoSeven was stopped as factor VIII level increased to 6.2% and the inhibitors dropped to 1.1BU. However, the next day a new hemorrhage appeared in both legs and in left arm with a drop in hemoglobin level and development of a hemorrhagic shock which was promptly treated with red blood cell transfusions and other simptomathyc therapy. A new treatment with NovoSeven was initiated again and immunosuppressive treatment continued. The patient was discharged from the hospital on the 25‐th day of hospitalization with an ongoing treatment with Cyclophosphamide and Prednison.
Conclusion(s): This case report illustrates a condition with acquired hemophilia A with delayed diagnosis and a life‐threatening hemorrhage where the treatment was prolonged and the consummation of factor greatly increased.
PB0005
Acquired haemophilia presenting in a 14‐year‐old girl
J. Roganovic
Clinical Hospital Centre Rijeka, Rijeka, Primorsko‐Goranska, Croatia
Background: Acquired haemophilia A is a rare but potentially life‐threatening haemorrhagic disorder caused by autoantibodies directed mostly against coagulation factor VIII. Age distribution is bimodal, with a first peak occurring among young women in the postpartum period, and a second major peak of incidence among elderly patients. The disorder is rare in children less than 16 years old, and the incidence is 0.45/million/year.
Aims: The aim of this work is to present an extremely rare case of a 14‐year‐old girl with acquired haemophilia.
Methods: Case presentation
Results: A 14‐year‐old girl was admitted to the Clinical Hospital Centre Rijeka with a 2‐year history of bleeding tendency into the skin, muscles and soft tissues. Past medical and family histories were negative. Prior to admission the girl was seen by several paediatricians, rheumatologists and orthopaedists. An isolated prolongation of the activated partial thromboplastin was found, with FVIII levels 5% and FVIII inhibitor titre 3.5 BU. The fifth day she developed spontaneous extensive muscle bleed of the right thigh. The treatment was started with recombinant activated factor VII (rFVIIa), and progression of bleeding ceased. Because of further unavailability of bypassing therapy, anti‐haemorrhagic treatment was continued with recombinant FVIII concentrates. Along with the control of acute bleeding, the patient underwent immunosuppressive therapy with corticosteroids in combination with cyclophosphamide to eradicate autoantibodies. Factor VIII inhibitor levels gradually decreased. The girl was discharged on day 15, and remained on oral prednisolone and cyclophosphamide over the next 6 weeks. At 5‐year follow‐up, she has complete sustained clinical and laboratory response.
Conclusion(s): Although very uncommon, the diagnosis of acquired haemophilia should be considered in any child who presents with unexplained bleeding and a prolonged activated partial thromboplastin time. Acquired autoantibodies directed against coagulation factors may result in serious, life‐threatening bleeding. The management of acute bleeding and the inhibitor eradication are the mainstay of treatment.
PB0980
Intravenous ferric gluconate use in anemic heart failure patients at a large academic medical center
S. Coriolan; C. Merchan; A. Katz; T. Ahuja
NYU Langone Health, New York, New York, United States
Background: Iron deficiency is a comorbidity associated with heart failure as a result of increased circulating inflammatory cytokines. The 2017 ACC/AHA heart failure (HF) guidelines gives a Class IIB recommendation for use of intravenous (IV) iron in HF patients with NYHA functional class II or III symptoms and iron deficiency. Currently at our institution, there is no protocol to guide the dosing of ferric gluconate. We generally replete patients with IV iron for a total dose of 1 gram.
Aims: To evaluate the prescribing patterns and safety of IV ferric gluconate in iron deficient patients admitted with HF exacerbations.
Methods: This is a retrospective cohort study of all patients > 18 years of age with a HF diagnosis that received ferric gluconate between Jan 2019 and July 2021 at NYU Langone Health. The primary outcome was assessment of ferric gluconate based on anemia characteristics of HF patients. Secondary outcomes included tolerability, safety, blood transfusions, and hospital readmission within 30 days.
Results: Eighty‐one patients received IV ferric gluconate, with the majority of patients with HF with a LVEF < 30% (40%), NYHA functional class III (58%), and a history of anemia (54%). Most patients were on concomitant anticoagulants. Adverse events were rare. Hospital readmissions for HF exacerbations within 30 days were infrequent at 8%. Blood transfusions within 2 weeks of IV iron administration occurred in 24% of patients.
Conclusion(s): Overall, IV iron repletion can be considered a safe and tolerable method for managing anemia in HF. Patients on anticoagulant therapy may benefit from IV iron repletion. Based on tolerability, and early patient disposition, updating local practices to 250 mg IV ferric gluconate twice daily for 2 days may replenish iron stores sooner than 125 mg IV ferric gluconate daily. However, this may lead to increased drug cost, without an impact on hospital readmissions, and should be evaluated further.
VPB0998
Risk factors for intracranial hematomas during anticoagulant therapy
N. Vorobyeva 1; A. Shapkov2
1 Federal State Budgetary Institution “National Medical Research Center for Hematology” (Northern branch) Ministry of Health of Ru, Arckhangelsk, Arkhangelsk, Russia; 2 Federal State Budgetary Institution Northern State Medical University Ministry of Health of Russia, Arkhangelsk, Arckhangelsk, Arkhangelsk, Russia
Background: Anticoagulant therapy with vitamin K antagonists (VKAs) – warfarin and direct oral anticoagulants (DOACs), used to prevent thromboembolic complications, requires an assessment of all risk factors for bleeding, including comorbidity and its pharmacotherapy.
Aims: Analysis of intracranial hematomas against the background of prolonged anticoagulant therapy
Methods: A retrospective analysis of the case histories of 50 patients (23 women and 27 men) aged 46 to 83 years (Me = 67.4) admitted to the hospital in the period 2014‐2021 was performed. with a diagnosis of “intracerebral/subarachnoid hemorrhage”, confirmed clinically and using CT scan of the brain. We studied the causes, outcomes of complications, their frequency, comorbidity and pharmacotherapy, the level of INR (international normalized ratio) and blood pressure (blood pressure) during hospitalization.
Results: Lethal outcome occurred in 44% (n = 22) of patients. 36 patients (72%) took Warfarin, 14 patients (28%) – DOACs, of which 6 patients – Rivaroxaban, 7 – Apixaban, 1 – Dabigatran etexilate.Analysis of concomitant therapy showed that 12 patients were taking omeprazole, 5 patients were taking Digoxin, and 23 patients were taking atorvastatin/rosuvastatin. At the time of hospitalization, 15 patients had BP in the range: 160/100 – 179/109 mm Hg. and 28 patients – BP 180/110 mm Hg. and higher. 74% (n = 37) of patients had impaired renal function, 28% (n = 14) – liver function, 6% (n = 3) – thyroid gland, 6% (n = 3) – traumatic brain injury. INR value >3 at the time of hospitalization – in 91.67% (n = 33) of patients taking VKA – excessive level of hypocoagulation.
Conclusion(s): According to our analysis, BP, impaired renal and hepatic function, decompensated comorbidity, unsafe background pharmacotherapy, and polypharmacy may increase the likelihood of hemorrhagic complications. To avoid this, it is advisable to correct comorbidity,as well as patient adherence to treatment.
PB0992
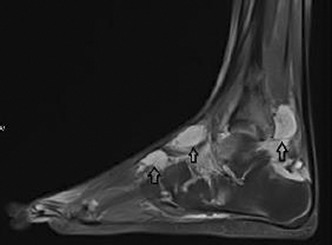
Rare cases of acquired bleeding disorders in adolescents and young adults
Y. Park
Kyung Hee University Hospital at Gangdong, Seoul, Seoul‐t’ukpyolsi, Republic of Korea
Background: Acquired bleeding disorder is very rare in adolescents and young adults (AYAs) but potentially life‐threatening clinical syndrome characterized by the sudden onset of bleeding in patients with a negative family and personal history.
Aims: We report two AYA patients with unusual clinical manifestations, who were diagnosed with acquired bleeding disorders.
Methods: We reviewed two patients who were diagnosed and treated in our clinics retrospectively.
Results: The first case was 19‐year‐old women with right lower leg painful swelling. She had no past and family history of bleeding disorder. Initial laboratory findings showed prolonged activated partial thromboplastin time (aPTT) and uncorrected mixing test. Factor VIII (FVIII) activity was below 1% and FVIII antibody was 22.4 Bethesda unit. Her diagnosis was acquired hemophilia A. Initial hemostatic treatments started with recombinant activated factor VII and oral steroid as immunosuppression therapy started. After 9 months, FVIII antibody was negative and FVIII activity was normalized. The second case was 17‐year‐old women with acquired von Willebrand syndrome (AVWS). She also had no past and family history of bleeding disorder. Initially, she was diagnosed with congenital von Willebrand disease. But when she visited the hospital again due to swelling and pain in both hands and wrists, the mixing aPTT was prolonged and low von Willebrand factor antigen (VWF:Ag) and VWF ristocetin cofactor activity (VWF:RCo) were low even after administration of coagulation factors. Her additional laboratory findings were hypoalbuminemia, proteinuria, and low C3 level. She was diagnosed with lupus nephritis and AVWS. She is being monitored with immunosuppressions, but aPTT is still prolonged and low VWF:Ag and VWF:RCo are low.
Conclusion(s): Acquired bleeding disorders are very rare in AYAs, but require a high index of suspicion and close collaboration with laboratories for specialized coagulation testing. An early diagnosis of acquired bleeding disorders is mandatory for starting the appropriate treatment.
PB0982
MGUS‐IgA with anti‐thrombin activity in a patient with a severe acquired haemorrhagic syndrome
A. Blanco1; M. Alberto2; M. Romero1; M. Martinuzzo3; S. Grosso1; M. Ingratti1; M. Arias 4; A. Sanchez‐Luceros5
1 National Academy of Medicine, Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 National Academy of Medicine, Ciudad Autonoma de buenos aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hematology and hemostasis division, Central Laboratory,Hospital Italiano de Buenos Aires, Argentina., Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Hospital Dr. César Milstein, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 5 National Academy of Medicine, CABA, Ciudad Autonoma de Buenos Aires, Argentina
Background: Acquired coagulation disorders are difficult to identify especially in presence of a lupus anticoagulant (LA).
Aims: We describe a 30‐year‐old man with a severe acquired haemorrhagic syndrome that could not be justified by laboratory findings.
Methods: Initial laboratory tests showed a strong LA, APTT 83 s not corrected by NPP (ICA30), not enhanced by incubation at 37°C, positive PNP, positive screen/confirm DRVVT, FVIII 3.5 IU/dl and FIX 4.5 IU/dl. Solid phase antiphospholipid antibodies (IgG, IgM, IgA: ACA, aBGPI, aPT, aPS), urea solubility and euglobulin lysis time were normal. VWD, thrombocytopathies and acquired haemophilia (FVIII 91%‐chromogenic assay) were excluded. During outpatient follow‐up for several years the positive LA remained.
Results: The patient was admitted to treat several major bleeding episodes (ISTH criteria) with aFVIIr. As clinical manifestations could not be justified by laboratory the presence of a heparinoid was considered and discarded. Finally, a MGUS‐IgA lambda (0.3 g/dl) was diagnosed. Complementary studies have shown decreased thrombin‐activated FXIII activity (13%) not corrected by NPP, but normal FXIIIA (95%) and FXIIIB (137%). Direct FXIII inhibitory effect was discarded (IgA‐FXIII immune‐complexes test), so we speculated that an anti‐thrombin effect might interfere with the activation of FVIII, FIX, FXIII. Immunocapture of IgA using a polyclonal anti‐human IgA was performed, followed by incubation with bovine thrombin (10IU/ml) and addition of thrombin chromogenic substrate. Colour development (405 nm) showed catalytic activity only in the presence of patient’s IgA (Figure 1). Patient’s IgA anti‐thrombin activity was confirmed by ELISA (Figure 2).
Conclusion(s): These results suggest that a MGUS‐IgA fraction interaction with thrombin might be interfering with its binding to natural substrates, explaining the haemorrhagic manifestations. Bypass agents in this case allowed the successful treatment of major bleeding episodes even before the defect was identified. We think that in patients with recurrent severe bleeding, extensive evaluation of haemostasis should be carried out in order to achieve correct diagnosis.


PB0989
Acquired factor V inhibitor in a patient with metastatic cancer
D. Dhami 1; P. Changizzadeh2; C. Solowiej Singh3; S. Dhami4; S. Muttana5
1 American University of Antigua College of Medicine, Glastonbury, Connecticut, United States; 2 Eastern Connecticut Hematology & Oncology, Norwich, CT, Norwich, Connecticut, United States; 3 American Universtiy of Antigua College of Medicine, Toronto, Ontario, Canada; 4 American Universtiy of Antigua College of Medicine, Glastonbury, Connecticut, United States; 5 American University of Antigua College of Medicine, Eastampton Township, New Jersey, United States
Background: Factor V is synthesized in the liver and is a cofactor to the prothrombinase complex that converts prothrombin to thrombin. Inhibitor formation against factor V is rare and has been associated with infections, antibiotics, surgery, malignancy, and autoimmune disorders. Factor V inhibitor has a wide range of clinical manifestations: from being asymptomatic to major bleeding.
Aims: To describe a case of an acquired factor V inhibitor associated with metastatic cancer.
Methods: Retrospective case review.
Results: This case involves an 85‐year‐old gentleman with distant history of right ileocolic stage II colon cancer, h/o of atrial fibrillation on apixaban, and TURBT for a high‐grade noninvasive papillary urothelial carcinoma followed by intravesical BCG. He underwent a TAVR complicated by incision site infection treated with cephalosporins. The pre‐procedure lab work revealed normal PT and aPTT. Following the TAVR, the patient had gradually increasing pelvic and pulmonary nodules with rising CEA concerning for metastatic disease. A biopsy was planned and pre‐biopsy laboratory testing revealed a remarkably elevated PT. Clinically, the patient exhibited signs of minor bleeding (epistaxis and bruising). Apixaban was discontinued once lab work revealed elevated PT and mixing studies showed lack of correction consistent with immediate acting inhibitor. Other labs include Factor V assay < 1% and Factor V Inhibitor of 4.1 Bethesda units. Due to the risk of bleeding and the patient’s preference to not consider any systemic chemotherapy, the planned biopsy was abandoned. The factor V inhibitor was likely related to metastatic disease. The patient was treated with Rituximab and subsequently IVIG resulting in a stepwise increase of factor V levels and simultaneous drop in factor V inhibitor.
Conclusion(s): Acquired factor V inhibitor can be associated with metastatic cancer and in the absence of being able to treat the underlying cause such as cancer, it can be treated with rituximab and IVIG.

PB0990
Acquired FXI deficiency (aFXIdef) in a HIV + patient with SLE. Alternative evolution to lupus Anticoagulant (LA) during immunosuppressive therapy
C. Seehaus1; M. Lopez 2; L. Barrera3; P. Quiñones Maffassanti4; L. Ferreyra Garrott5; V. Privitera6; F. Chuliber7; D. Penchasky8; E. Viñuales8; J. Arbelbide8; M. Martinuzzo9
1 Department of Hematology, Hospital Italiano de Buenos Aires, Argentina, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Hematology and hemostasis division, Central Laboratory,Hospital Italiano de Buenos Aires, Argentina., Ciudad Autonoma de buenos aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hematology and hemostasis division, Central Laboratory,Hospital Italiano de Buenos Aires, Argentina., Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Central Laboratory,Hospital Italiano de Buenos Aires, Argentina., Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Department of Rheumatology, Hospital Italiano de Buenos Aires, Argentina, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 6 Hospital Italiano de Buenos Aires, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 7 Hospital ItaIiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 8 Hospital Italiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 9 Hematology and hemostasis division, Central Laboratory,Hospital Italiano de Buenos Aires, Argentina., Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: Few cases with non‐neutralizing inhibitors causing aFXIdef, but frequently LAs are described in Systemic Lupus Erythematosus (SLE).
Aims: To describe a clinical case of an HIV+ patient with SLE who presented an aFXIdef, including FXI and LA evolution during treatment.
Methods: Not applicable, clinical case report.
Results: A 42 y.o. HIV+ female patient with a recent diagnosis of SLE was referred to Haematology Service in 2017 because of prolonged APTT and eventual renal biopsy requirement (Glomerular microhematuria and non‐nephrotic proteinuria). Prolonged APTT, low FXI,3U/dl and normal FVIII, IX and XII were detected. APTT, Silica Clotting time (SCT) and FXI presented negative mixing studies, SCT confirm was negative and Diluted Russell Viper Venom Time (DRVVT) normal, indicating LA not present. Anticardiolipin and anti β2glycoprotein I presented low positive results as well as ANA, Anti dsDNA and Anti‐Ro (SSA). Normal APTT until 2015 and 65 sec with negative mixing study in 2016, confirmed aFXIdef. Three fresh frozen plasma units’ infusion resulted in a moderate, but transient FXI increased up to 22 IU/dl at 2 hours, demonstrating abnormally fast half live falling to 5 IU/dl at 12 hours. Meprednisone and hydroxychloroquine treatment produced any improvement on FXI and renal manifestations. Mycophenolate was started, with several treatment changes according to clinical evolution. Renal biopsy was suspended. Clinical, treatment and laboratory evolution are shown in Figures 1 and 2. At the present time, aFXIdef disappear and LA is present (Figure 2). During aFXIdef periods without exposition to bleeding challenges, not bleeding was observed. Recently, while LA + and normal FXI levels, an inguinal hernioplasty and a annexhysterectomy for cervical intraepithelial neoplasia were performed without bleeding complications.
Conclusion(s): Proper coagulation laboratory diagnosis during evolution of SLE patients, when the coagulation disorders change alternatively according to immunosuppressive treatment, is important to take clinical decisions as exposition to bleeding challenges.


PB0993
Measurement of procoagulant platelets in platelet‐rich plasma by flow cytometer for the diagnosis of heparin‐induced thrombocytopenia
L. Pelzl 1; A. Karakuyu2; J. Zlamal3; I. Marini4; A. Singh5; H. Jaffal6; S. Hammer7; K. Althaus1; T. Backchoul3
1 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 2 Experimentelle und Klinische Transfusionsmedizin Tübingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 3 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 4 Transfusion Medicine, Medical Faculty of Tübingen, University Hospital Tübingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 5 Experimentelle und Klinische Transfusionsmedizin Tübingen, Tuebingen, Baden‐Wurttemberg, Germany; 6 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, tuebingen, Baden‐Wurttemberg, Germany; 7 Center for Clinical Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany
Background: Heparin‐induced thrombocytopenia (HIT) is caused by anti‐ PF4/heparinIgG antibodies, which activates platelets and leads to thrombocytopenia and thrombosis. The diagnosis of HIT can only be confirmed using functional assays such as the Heparin‐Induced Platelet Activation assay (HIPA assay). However, functional assays are technically demanding and routinely available only in specialized laboratories.
Aims: The aim of the current study was to establish a flow cytometer‐based method to detect procoagulant platelets in platelet‐rich plasma (PRP) for the diagnosis of HIT.
Methods: Sera samples from patients with HIT (HIT group) were incubated with PRP from healthy donors for different durations with (30, 60, 90 minutes). Procoagulant platelets were determined by double expression of P‐selectin (CD62p) and phosphatidylserine (PS) externalization by flow cytometry. CD32a‐mediated cross‐link and platelet stimulation with ionomycin were used as positive controls.
Results: Sera from HIT‐diagnosed patients but not from the control‐group induced a significant increase in the procoagulant platelet subpopulation in the presence of 0.2 U/ml heparin (% double positive CD62/PS: 1.2 ± 1.1 vs 18.5 ± 8.1, p = 0.0021). The optimal incubation time was 60 minutes. A donor dependency of the flow cytometric method was not observed (control vs HIPA+: 0.7 ± 0.59 vs19.0 ± 3.2, p = 0.0129). In addition, the use of washed platelets and PRP with HIT‐sera led to the same results in the flow cytometric method (34.2 ± 6.3 vs 30.1 ± 2.2, ns).
Conclusion(s): Our data suggest that PRP‐based protocol can be used to detect the ability of HIT antibodies to induce procoagulant platelets by flow cytometry. Ongoing study is currently investigating the clinical implementation of this protocol in the diagnostic work up for HIT.
VPB0997
Analytical performance of different laboratory methods for measuring susoctog‐alpha
C. Novembrino 1; I. Quaglia2; M. Boscolo‐Anzoletti3; E. Galbiati3; E. Dosio4; A. Valpreda4
1 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Lombardia, Italy; 2 Center for Thrombosis and Hemorrhagic Diseases, IRCCS Humanitas Researc Hospital, Rozzano, Milan, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Milan, Lombardia, Italy; 4 Center for Hemorrhagic and Thrombotic Diseases ‐ Clinical Biochemistry Laboratory ‐ A.O.U. City of Health and Science, Turin, Turin, Piemonte, Italy
Background: Acquired haemophilia A (AHA) is a severe bleeding disorder caused by the development of antibodies (inhibitors) to factor VIII (FVIII). Recombinant porcine factor VIII (rpFVIII, susoctocog alfa, Obizur®, Baxalta US Inc.‐Takeda company‐ Bannockburn, IL, USA) is indicated for the treatment of acute bleeding episodes in AHA, but there are scanty data regarding the laboratory methods for adequately monitoring the treatment.
Aims: This study involving 3 different laboratories aimed to evaluate the analytical performance of different assays for measuring rpFVIII.
Methods: Five spiked samples at different rpFVIII concentrations (from 0.05 U/ml to 1.5 U/ml) were analysed on three distinct days, in triplicate every day, with nine different combinations of reagents (SynthasIL and SynthaFax ‐Werfen‐ for one‐stage assay, Chromogenix Coamatic FVIII for chromogenic assay), FVIII depleted plasmas (with or without von Willebrand factor –vWF‐) and calibrators (HemosIL human calibrator plasma, porcine calibrator diluted in FVIII deficient plasma with or without vWF). All the assays were performed on ACL Top analysers (Werfen, Bedford, MA). Intra‐ and inter assay and inter‐laboratory Coefficient of Variation (CV%) were calculate together with percentage of recovery (% recovery) on the expected value.
Results: The results of the three laboratories are reported in table as total inter‐laboratory CV% (mean of CV% obtained for all the measures of the 5 samples in the three laboratories) and % recovery (mean of % recovery obtained for all the measures of the 5 samples in the three laboratories).
Conclusion(s): The present study highlights the impact of the calibrator and of the deficient plasma used in the assay on the accuracy of the results. The use of porcine standard (when available) and FVIII deficient plasma with vWF is recommended.
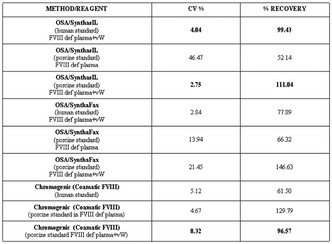
PB0001
Management of acquired haemophilia A in a Portuguese Center: 16 years and 16 cases
C. Catarino 1; F. Rodrigues2; A. Pereira2; J. Pestana3; C. Peixoto4; S. Campaniço4; L. Parusnikova4; C. Correia4; C. Pereira4; E. Parcelas5; J. Lucas6; D. Passos7; S. Croca7; P. Afonso4; P. Morgado7; C. Calisto7; S. Cruz7; M. Carriço7
1 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisbon, Lisboa, Portugal; 2 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisboa, Lisboa, Portugal; 3 Hospital do Espirito Santo ‐ Évora, Évora, Evora, Portugal; 4 Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisboa, Lisboa, Portugal; 5 Centro Hospitalar Lisboa Ocidental, Hospital de São Francisco Xavier, Lisboa, Lisboa, Lisboa, Portugal; 6 Hospital Nélio Mendonça, Funchal, Madeira, Portugal; 7 Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisboa, Lisboa, Portugal
Background: Management of acquired haemophilia A (AHA), a rare and severe autoimmune disease, is based in the control of bleeding, eradication of inhibitors and diagnosis of the underlying cause.
Aims: We, retrospectively, analyzed demographics, time for diagnosis and for eradication of inhibitors, symptoms, haemostatic and immunosuppressive therapy, prognosis, and underlying cause.
Methods: Between 2006 and 2021, 16 patients (9 males; 7 females), median age 76 years (62‐90) were diagnosed at our Center.
Results: Time from onset of symptoms to diagnosis was less than 1 week in 7 patients, between 1 and 4 weeks in 5 and more than 4 weeks in 3. Median FVIII was 3.5% (< 1‐21) and median higher inhibitor titer was 193 BU (3 ‐1250). Symptoms observed were muscular (11); subcutaneous (3); oropharyngeal (2); urinary (3); intracranial (1) and gastrointestinal (1). In 3 patients bleeding was related to surgical procedures. Hemostatic therapy was needed in 13 patients. Recombinant activated factor VII (rFVIIa) alone was effective in 9 /12 patients. In 1 patient, with a life‐threatening hemorrhage, association with activated prothrombin complexes (aPCC) was needed. aPCC alone was effective in another patient. No adverse events related to haemostatic treatment were observed. Eight patients (50%) received other blood components. In 10/11 patients who fully recovered, immunosuppression was done with corticoids only. In one patient, due to the severity of bleeding, cyclophosphamide and rituximab were associated with good results. Median time to remission was 26 days and no relapse was observed. AHA was classified as idiopathic in 11/16 (62.5%) patients. In the other cases, AHA was related to Epstein‐ Barr virus (1); malignancy (2) and autoimmune disease (2). Mortality was observed in 5/ 16 patients, being bleeding the cause of death in 3 patients.
Conclusion(s): Diagnosis, treatment and prognosis of AHA is influenced by the timing of diagnosis and adequate and effective treatment.

VPB0996
FiiRST‐2; a prospective, randomized study of clotting factor concentrates versus standard massive hemorrhage protocol in severely bleeding trauma patients
L. Da Luz1; J. Callum 2; A. Beckett3; H. Peng4; P. Engels5; N. Parry6; H. Tien1; A. Nathens1; J. Carroll7; D. Grewal7; K. Karkouti8
1 Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada; 2 Queen's University, Kingston, Ontario, Canada; 3 Saint Michael's Hospital, Toronto, Ontario, Canada; 4 Toronto Research Centre, Toronto, Ontario, Canada; 5 Hamilton General Hospital, Hamilton, Ontario, Canada; 6 London Health Sciences Centre, London, Ontario, Canada; 7 University Health Network, Sinai Health System and Women’s College Hospital, Toronto, Ontario, Canada; 8 University of Toronto, Toronto, Ontario, Canada
Background: Acquired fibrinogen deficiency and impaired thrombin generation are major drivers of acute traumatic coagulopathy (ATC), which coupled with bleeding is a leading cause of in‐hospital mortality in trauma patients. Early administration of clotting factor concentrates (fibrinogen concentrate [FC], prothrombin complex concentrate [PCC]) may be superior to the current standard of care (SOC); ratio‐based plasma resuscitation via a massive hemorrhage protocol (MHP).
Aims: To examine whether early administration of FC+PCC is superior to the current SOC in bleeding trauma patients.
Methods: FiiRST‐2 is a randomized, parallel‐control, superiority trial with a two‐armed, two stage design and adaptive interim analysis performed across 11 Canadian Level I trauma centers (funded by Octapharma). Bleeding trauma patients >16 years old (N = 350) will receive FC+PCC (intervention) or 4 units of red blood cells (RBC):plasma (control), plus platelets, until the second MHP pack has been given, MHP is terminated once bleeding has stopped, or 24 h has elapsed from admission (Figure 1). Exclusion criteria include receipt of >2 units RBCs before randomization, >3 h elapsed from injury, catastrophic brain injury, or known bleeding disorders. The primary endpoint is superiority in the number of composite allogeneic blood product units transfused within 24 h after administration. Secondary efficacy endpoints include RBC units transfused within the first 24 h following admission, ventilator‐free days, and 28 day all‐cause mortality. All adverse and serious adverse events will be assessed through 28 days.
Results: FiiRST‐2 has enrolled 60 patients at 4 sites to date. An interim analysis will be performed once 120 patients have completed the study. Completion is expected in Q1 2023.
Conclusion(s): This study will determine whether early use of coagulation factor concentrates (FC+PCC) is superior to the current SOC in bleeding trauma patients, potentially having an impact on clinical practice by improving management and outcomes for this high‐risk patient population.
PB0985
Clinical experience in factor Xa inhibitors´ reversal with prothrombin complex concentrate in emergency settings
J. Cerezo Martín 1; G. Mendez2; I. Romon2; B. González‐Mesones2; M. gonzalez2; M. Pérez2; S. Fernández‐Luis2; E. Ocio2
1 Sistema Cantabro de Salud, santander, Cantabria, Spain; 2 University Hospital Marqués de Valdecilla, Santander, Cantabria, Spain
Background: Studies evaluating clinical outcomes of direct oral anticoagulants (DOACs) related emergencies are scarce and heterogeneous and the optimal 4F‐PCC dose for reversal is still not well defined.
Aims: To describe the characteristics, management and outcomes of patients anticoagulated with an oral FXa inhibitor (FXa‐I) who were treated with 4F‐PCC. The secondary aim is to evaluate adverse‐related effects.
Methods: This is a retrospective and observational review of cases treated with 4F‐PCC between 2016 and 2020 for the reversal of FXa‐I because of major bleeding or an urgent surgery. Management of the DOAC‐related emergency was at the discretion of the treating physician. The effectiveness of the reversal was measured with the need for red blood cell (RBC) or fresh frozen plasma (FFP) transfusion within two weeks after reversal. We documented the occurrence of thrombotic events within four weeks of PCC administration and other related adverse‐effects.
Results: Forthy patients were included, all of them were anticoagulated because of atrial fibrillation. Twenty‐six (65%) patients were treated with apixaban and 17 (42.5%) patients were reversed because of an urgent surgery. The most common site of bleeding was intracranial (15%) (Table 1). Median dose of 4F‐PCC was 1000 UI. Only in 20 patients was the weight available in the medical history, being the median 4F‐PCC dose 14.3 UI/kg. Sixteen (40%) patients required a RBC or FFP transfusion. Patients reversed with a 4F‐PCC dose of 20U/Kg or greater needed less transfusions than patients reversed with inferior doses, OR 0.05 (CI95% 0.04‐0.597) (Table 2). No thromboembolic event was diagnosed. However, thromboprophylaxis was early administered in 25 patients (62.5%). A serious anaphylactic reaction was recorded in a patient.
Conclusion(s): The use of 4F‐PCC at low or intermediate doses and with posterior thromboprophylaxis may be a good treatment option in patients requiring DOAC reversal when an specific reversal agent is not available.

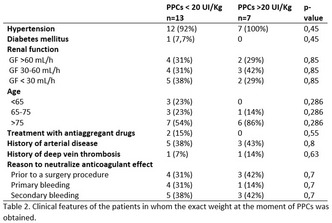
PB0987
Impact of amotosalen/UVA process for pathogen reduction in platelet concentrates on transfusion efficiency in cardiac surgery
B. Belkacem1; H. Hamzeh‐Cognasse2; A. Duchez3; M. Eyraud4; C. Arthaud4; A. Prier5; E. Audoux6; T. Ebermeyer7; P. Chavarin4; A. Kazra1; J. Morel8; J. Palao8; F. Cognasse 6
1 Department of Cardiovascular, University Hospital of Saint‐Etienne, France, Saint‐Etienne, Rhone‐Alpes, France; 2 SAINBIOSE Laboratory, INSERM, U1059, University of Lyon, Saint‐Étienne, Saint‐Etienne, Rhone‐Alpes, France; 3 Etablissement Français du Sang Auvergne‐Rhône‐Alpes & INSERM U1059, Decines‐Charpieu, Rhone‐Alpes, France; 4 Etablissement Français du Sang Auvergne‐Rhône‐Alpes, Decines‐Charpieu, Rhone‐Alpes, France; 5 Etablissement Français du Sang Auvergne‐Rhône‐Alpes, Decines‐Chapieu, Rhone‐Alpes, France; 6 Etablissement Français du Sang Auvergne‐Rhône‐Alpes & INSERM 1059, Decines‐Charpieu, Rhone‐Alpes, France; 7 SAINBIOSE Laboratory, INSERM, U1059, University of Lyon, Saint‐Étienne, France, Saint‐Étienne, Rhone‐Alpes, France; 8 Department of Anesthesiology and Intensive Care, Etienne University Hospital, Jean‐Monnet University, Saint‐Etienne, France, Saint‐Etienne, Rhone‐Alpes, France
Background: One of the most recent advances to improve blood safety and lower the risk of transfusion‐transmitted diseases is the pathogen reduction treatment (PRT) of platelet concentrates (PCs). The characteristics of PR‐treated PCs slightly differ from those of untreated PCs, and may affect transfusion outcome.
Aims: The aim of this research project is to see how effective amotosalen/UVA (INTERCEPT TM Blood System) PCs are when transfused to a group of cardiac surgery patients.
Methods: This study examined the influence of the PRT using amotosalen‐HCl and UVA light in a population of cardiac surgery patients.We analyzed the bleeding and platelet drop postoperatively in cardiac surgery with extracorporeal circulation. We selected 73 PCs after taking into account the medical exclusion criteria: 46 PCs without pathogen reduction treatment/Intercept® versus 27 PCs with pathogen reduction treatment/Intercept®.
Results: The decrease of patient platelet count between preoperative and H0 (start of surgery), preoperative and H6 (6 hours after) did not differ significantly with or without PRT. The volume of postoperative bleeding from cardiac surgery under extracorporeal circulation did not differ significantly regardless on whether the patient was transfused with PRT‐PC or untreated PC. No difference was found in the number of postoperative pneumopathies between the groups. Moreover, independently of the use of PRT for platelets we observed an inverse correlation between preoperative fibrinogen and bleeding at 24 hours (post‐surgery). A 1 mg/L increase in fibrinogen (preoperative) is associated with a 159 ml decrease in bleeding 24 hours after the start of the surgery.
Conclusion(s): In postoperative cardiac surgery, the use of platelets treated with amotosalen/UVA (INTERCEPT TM Blood System) for pathogen reduction does not appear to affect transfusion effectiveness and postoperative bleeding.
PB0988
A TEG‐guided treatment algorithm for major surgical bleeding
S. Dawson 1; A. Cain2
1 Northern School Anaesthesia and Intensive Care Medicine, Newcastle upon Tyne, England, United Kingdom; 2 Freeman Hospital, Newcastle Upon Tyne, England, United Kingdom
Background: Visco‐elastic assays such as thromboelastography (TEG) can reduce transfusion requirements and improve mortality across of range of settings including trauma, liver transplant, and bleeding post‐cardio‐pulmonary bypass. The role of TEG in the diagnosis and treatment of coagulopathy in major surgical bleeding is less well defined.
Aims: To evaluate the utility of a TEG‐guided algorithm in identification and treatment of coagulopathy associated with major surgical haemorrhage at a single tertiary centre.
Methods: A TEG‐guided treatment algorithm was adapted from the Copenhagen protocol. The electronic records of all patients who had a TEG sample run during a three‐month trial period from September to December 2021 were evaluated. Liver transplant patients were excluded. Data was collected on TEG results, blood loss, blood products administered in 24 hours pre‐ and post‐TEG specimen, and in‐hospital mortality. An unpaired T‐test was performed to compare outcomes between patients adhering to the treatment algorithm vs those whose treatment deviated from the suggested protocol.
Results: During three months, 25 TEGs were performed on 22 patients. Eight patients died. The most common TEG abnormalities were prolonged R‐time (5/25) and low MA (5/25), followed by low fibMA (4/25). The treatment algorithm was adhered to in 11/22 patients. Patients whose treatment adhered to the protocol post‐TEG showed a trend towards lower mean blood loss (2024 vs 2989 ml, p = 0.27), reduced mean RBC transfusion (1.45 vs 2.0 units, p = 0.27) and reduced FFP transfusion (0.91 vs 1.83, p = 0.15), however, none of the results reached statistical significance.
Conclusion(s): TEG was utilised in a high‐risk group of patients who experienced significant blood loss. A normal TEG result reduced unnecessary blood product administration. When the treatment algorithm was followed there was a trend towards reduced blood loss and transfusion requirements.


VPB0995
The clinical use of idarucizumab for dabigatran reversal in Aragón (Spain), new data
S. Angós Vázquez 1; A. Ortiz Lopez2; N. Gemperle Ortiz3; I. Rivas Estabén4; M. Moles Guerrero1; V. Murillo Cortés1; O. Gavín Sebastian1; M. Dobón Rebollo3; J. Calvo Viñas5; I. Cuesta Gallardo6; P. Paul Vidaller7; M. Paricio Moreno8; L. Palomera Bernal1
1 Hospital Lozano Blesa, Zaragoza, Aragon, Spain; 2 Hematology Resident, Zaragoza, Aragon, Spain; 3 Hospital Lozano Blesa, Zaragoza, Aragon, Spain; 4 Hospital Lozano Blesa, Andorra (Teruel), Aragon, Spain; 5 Hospital Miguel Servet, Zaragoza, Aragon, Spain; 6 Hospital Obispo Polanco, Teruel, Aragon, Spain; 7 Hospital Comarcal de Barbastro, Barbastro, Aragon, Spain; 8 Hospital de Alcañiz, Alcañiz, Aragon, Spain
Background: Idarucizumab is a specific reversal agent of dabigatran anticoagulant effect in major bleeding and emergency surgeries. Real‐world evidence for idarucizumab in Spain is limited.
Aims: Analyze idarucizumab management and results in current clinical practice in Aragón hospitals (Spain).
Methods: 46 patients that received idarucizumab (January 2016 – December 2021) were registered in this observational, multicenter, retrospective study. We analyzed clinical variables and laboratory tests; efficacy, safety and thrombogenic capacity of idarucizumab, and related mortality with its use.
Results: 46 patients needed idarucizumab in Aragon. Most patients were male (58.6%), ≥75 years (63%) and used dabigatran 110 mg (65.21%). The indications of idarucizumab were emergency surgeries in 63.04%; major bleeding in 30.43%, 1 off‐label use, 1 lumbar puncture and 1 suspected stroke. Surgeries requiring reversal anticoagulation were 31.03% abdominal surgery, 34.18% vascular, 13.79% traumas, 10.34% cardiothoracic, 6.89% urological and 3.44% neurological surgery. The use of the drug in major bleeding was in 50% with cerebral hemorrhage, 7.14% with retroperitoneal hemorrhage and 42.86% gastrointestinal bleeding. Adverse events were shown in three operated patients (10.34%); femoral artery bleeding, retrocardiac hematoma and cerebral hemorrhage. Another had kidney failure and none had thrombotic events after idarucizumab administration. Activated partial thromboplastin time (aPTT) or prothrombin times (PT) were prolonged in 86.95% patients prior to idarucizumab infusion. Complete reversal (aPTT ≤40 seconds) was shown in 76% of the patients. Six patients (13.04%) died in relation with intraventricular hemorrhage, heart transplant complications, septic shock, hypovolemic shock, bleeding from ruptured aneurysm and multi‐organic failure respectively.
Conclusion(s): Our series includes 46 patients treated with idarucizumab to reverse the anticoagulation effect of dabigatran in hospitals of Aragon. The clinical use of idarucizumab was safe and conducted according to the authorized label, although it would be recommendable the development of standardized hospital protocols to guarantee an optimal drug use.


PB0984
Direct comparison of Andexanet alfa and prothrombin complex concentrates in the reversal of direct factor Xa inhibitors – An in vitro study
H. Brinkman 1; M. Zuurveld2; J. Meijers3
1 Sanquin Research, Amsterdam, Noord‐Holland, Netherlands; 2 Sanquin Research, department of Molecular Hematology, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Molecular Hematology, Sanquin Research, Amsterdam, the Netherlands, Department of Experimental Vascular Medicine, Amsterdam UMC, University of Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Both Andexanet alfa and 4‐factor prothrombin complex concentrate (4F‐PCC) are clinically applied reversal agents for direct factor Xa inhibitors (FXaI) in emergency situations. Andexanet alfa is a recombinant modified human factor Xa. 4F‐PCC is a human plasma‐derived mixture of partially purified vitamin K‐dependent coagulation factors and may contain added heparin. Controversy exists whether Andexanet alfa is preferred over 4F‐PCC in correcting FXaI anticoagulation.
Aims: This in vitro study was designed to directly compare Andexanet alfa with two different 4F‐PCCs, one with and the other without added heparin, in their ability to correct FXaI anticoagulation.
Methods: Normal plasma was spiked with Apixaban or Rivaroxaban. Reversal of anticoagulation by the reversal agents was assessed using a thrombin generation assay and a fibrin generation‐clot lysis test.
Results: Andexanet alfa, applied at clinically recommended doses, was effective in restoring thrombin generation as evidenced by correction of thrombin generation lag time, peak thrombin and endogenous thrombin potential (ETP). Clotting time and clot resistance to fibrinolytic breakdown was also corrected over the full range of applied FXaI (0‐800 ng/ml). 4F‐PCC in increasing dose (0.625, 1.25 and 2 IU/ml; ~25, 50 and 80 IU/kg) only partially restored thrombin generation lag time and clotting time. Partial correction to over‐normalization of peak thrombin and ETP was observed, depending on FXaI concentration and PCC dose. Clot resistance to fibrinolytic breakdown was dose‐dependently improved to above normal. 4F‐PCC with added heparin was consistently less effective than 4F‐PCC without added heparin.
Conclusion(s): Both Andexanet alfa and 4F‐PCC improved coagulation in the presence of FXaI. While Andexanet alfa corrected all thrombin generation parameters; 4F‐PCC predominantly increased peak thrombin and ETP. Especially 4F‐PCC without added also improved clot stability against fibrinolytic breakdown. Whether heparin supplement to 4F‐PCC counteracts its prohemostatic effectivity in bleeding patients on FXaI remains to be investigated.
PB0983
Use of prothrombin complex concentrates in severe liver disease: in vitro dose effects on haemostasis parameters: COPIH Study
I. Ben Salah 1; M. Rousseaux1; M. Bourrienne2; L. Boudaoud1; C. Trichet1; P. Rautou3; F. Durand3; O. Roux3; E. de Raucourt4
1 Service Hématologie Biologique, Hôpital Beaujon (APHP), Clichy, Ile‐de‐France, France; 2 Hôpital Beaujon (APHP) ; INSERM LVTS U1148 Université de Paris, CLICHY, Ile‐de‐France, France; 3 Service d’Hépatologie et de Réanimation Hépato‐digestive, Hopital Beaujon (APHP), Clichy, Ile‐de‐France, France; 4 Centre de ressources et compétences des Maladies Hémorragiques Constitutionnelles rares, centre hospitalier de Versailles (André Mignot), Le chesnay, Ile‐de‐France, France
Background: Changes in pro‐ and anti‐coagulant pathways are common features in patients with severe liver disease leading to an increased risk of both bleeding and thrombotic complications. Common strategies to manage hemorrhage include fresh frozen plasma, platelets transfusion and prothrombin complex concentrates infusion (PCCs). PCCs offer a good alternative in patients with liver diseases by avoiding large‐volume infusion. However, conflicting results have been reported on optimal dosage of PCCs regarding thrombotic risk.
Aims: Hemostasis parameters were evaluated at baseline and, after in vitro addition in whole blood of two doses of PCCs in patients with severe liver diseases (acute liver failure or chronic liver disease).
Methods: PCC (Confidex®, CSL Behring) was added to reach 50% (low) or 100 % (high) of FII level at final concentration, which correspond respectively in vivo to 13 [9‐14] U/kg and 31 [29‐34] U/kg in patients. Then, rotational thromboelastometry (EXTEM) and thrombin generation (TG) assay (+/‐ thrombomodulin) were performed respectively, in whole blood and plasma. Results were compared with healthy controls and expressed as median [interquartile range].
Results: At baseline, patients had a coagulopathy characterized by significant prolonged INR, prolonged EXTEM clotting time and decreased thrombin generation without thrombomodulin. After PCC addition, INR was halved from baseline but did not improve significantly with increasing dose. TG parameters increased supraphysiologically in patients compared to baseline and controls. High‐dose of PCC was associated with higher thrombin peak and endogenous thrombin potential (ETP) than low dose (p < 0.05) (Table 1). In addition, PCC increased thrombomodulin resistance, and significant higher resistance was observed with the high‐dose (Table 1).
Conclusion(s): Our results support that, compared to high doses, low doses of PCC induce less hypercoagulability in patients with severe liver disease. Clinical studies are required to determine whether low doses of PCC may be used in these patients without increasing thrombotic risk.

PB0986
Efficacy and safety of prothrombin complex concentrate for major bleeding in patients treated with direct Factor‐Xa inhibitors
R. Clapham 1; V. Speed2; K. Nwankiti2; R. Byrne1; J. Patel2; J. Czuprynska1; R. Patel2; R. Arya2; L. Roberts1
1 King's College Hospital, London, England, United Kingdom; 2 Kings College Hospital, London, England, United Kingdom
Background: Treatment options for the reversal of direct Factor‐Xa inhibitors have been limited due to the lack of antidote. Our current practice for the management of a major bleed is to use prothrombin complex concentrate (PCC).
Aims: To determine the efficacy and safety of PCC in patients treated with direct Factor‐Xa inhibitors who present with major bleeding.
Methods: Patients issued with PCC between January 2019 and May 2021 were identified from blood bank records. The electronic patient record (EPR, Allscripts) was reviewed to identify use in patients on direct Factor‐Xa inhibitors with major bleeding. Primary outcomes were 30‐day mortality, re‐bleeding and thrombosis.
Results: PCC was issued to 105 patients, of which 48 (45.7%) received PCC for major bleeding associated with direct factor Xa inhibitor use. Intracranial haemorrhage (ICH) was the most common indication for PCC reversal (see Table 1 for patient characteristics). Prior to admission, 7 patients were on concomitant antiplatelet or non‐steroidal anti‐inflammatory medications. 17/48 (35.4%) patients had a Xa‐inhibitor drug level taken prior to receiving PCC. Nearly half of the patients received weight‐based PCC with a median dose of 2000 units i.e. 23.4 units/kg (range 1000 ‐2500 units). 5/45 (11.1%) patients died, however major bleeding was listed as the cause of death for just three patients (ICH, GI bleed and large abdominal wall haematoma). There was one episode of re‐bleeding where the patient developed an acute‐on chronic subdural haematoma at 30 days. There were no thromboembolic events within 30‐days of receiving PCC. 57.8% (26/45) of patients restarted anticoagulation after a median 14 days (range 1‐301 days) and 10/26 (38.5%) of these were following ICH.
Conclusion(s): Our rates of mortality, re‐bleeding and thrombosis were lower compared to current literature and suggest PCC to be a safe and effective option in the management of major bleeding in patients treated with direct Factor Xa inhibitors.

PB0991
Assessment of interference by emicizumab in the measurement of susoctocog alfa factor VIII activity using a chromogenic assay
L. Pan1; A. Mokdad 2; P. Turecek3; M. Robinson4; N. Jain1
1 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States; 2 Takeda, Sudbury, Massachusetts, United States; 3 Baxalta Innovations GmbH, a Takeda company, Vienna, Wien, Austria; 4 Laboratory Corporation of America Holdings, Englewood, Colorado, United States
Background: Recombinant porcine factor VIII (rpFVIII, susoctocog alfa) is indicated for the treatment of bleeding episodes (BEs) in adults with acquired hemophilia A (AHA). A key benefit of rpFVIII is the ability to measure factor VIII (FVIII) activity in plasma samples using the activated partial thromboplastin time (aPTT) clotting assay. Emicizumab, an anti‐FIXa/FX bispecific antibody, is being studied in patients with AHA to prevent or reduce the frequency of BEs, but it interferes with aPTT‐based clotting assays.
Aims: To evaluate whether the FVIII activity of rpFVIII can be measured in the presence of emicizumab using a chromogenic assay.
Methods: The FVIII activity of rpFVIII was measured using the Coatest SP4 FVIII chromogenic assay (Chromogenix) on the BCS XP analyzer (Siemens). Samples (rpFVIII 0, 0.05, or 1.20 U/ml; emicizumab 0, 30, or 100 μg/ml) were made by mixing intermediate solutions prepared in pooled congenital FVIII‐deficient plasma. High (1.500–0.150 IU/ml) and low (0.200–0.0075 IU/ml) calibration curves were prepared with normal reference plasma containing a known concentration of human FVIII (calibrated vs WHO 6th International Plasma Standard for FVIII). Samples were tested in duplicate at 3 dilutions either on the high curve (1:12, 1:24, and 1:48), prepared in SP4 assay buffer containing 1% bovine serum albumin, or on the low curve (1:5, 1:10, and 1:20), prepared in congenital FVIII‐deficient plasma.
Results: FVIII activity in unspiked plasma (zero rpFVIII) was below the lower limit of quantitation ( < 0.030 IU/ml) at all tested emicizumab concentrations. The FVIII activity of samples containing rpFVIII (0.05 or 1.20 U/ml) and emicizumab (30 or 100 μg/ml) fell within ±20% of the FVIII activity in corresponding samples containing rpFVIII only. Emicizumab concentrations ≤100 μg/ml did not interfere with the assay.
Conclusion(s): The FVIII activity of rpFVIII can be measured accurately in the presence of emicizumab (≤100 μg/ml) using the chromogenic Coatest SP4 FVIII assay.
PB0981
Nationwide analysis of pre‐hospital tranexamic acid in trauma patients at risk of bleeding
A. Almuwallad 1; A. Rossetto2; E. Cole2; R. Davenport3
1 Centre for Trauma Science, Blizard Institute, Queen Mary University of London, London, United Kingdom / Emeregency Medical Services Department, Faculty of Applied Medical Seciences, Jazan University, Kingdom of Saudi Arabia, London, England, United Kingdom; 2 Centre for Trauma Science, Blizard Institute, Queen Mary University, London, United Kingdom, London, England, United Kingdom; 3 Centre for Trauma Science, Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom
Background: Early tranexamic acid (TXA)improves survival in trauma haemorrhage with time to treatment a key determinant of efficacy. UK guidelines are for pre‐hospital (PH) medical services to administer TXA within one hour of arrival. Guideline compliance and characteristics of those patients who fail to receive early TXA are unknown.
Aims: Characterise pre‐hospital TXA use in trauma to identify undertreated patient subgroups.
Methods: A retrospective analysis of data submitted to the UK Trauma & Audit Research Network by Major Trauma Centres in England & Wales post‐regionalization of trauma care (2013‐2019). Patients at risk of bleeding and eligible for TXA (best practice tariff guidelines) were identified. Time to administration, injury characteristics and clinical outcomes were analysed. Shock was defined as heart rate ≥110/min and/or systolic blood pressure ≤90 mmHg.
Results: 51,310 patients were included. Overall PH TXA use significantly increased from 8% (2013‐15) to 27% (2017‐19). In most recent time period, older patients were less likely to receive PH TXA (16‐65 yrs: 32% vs >65 yrs 13%, p < 0.05). Mechanism of injury was associated with significant variation in rates of PH TXA use (gun/knife: 45% vs road traffic collision: 40% vs falls < 2 m: 3%). In shocked patients, 54% received PH TXA but significant variation administration persisted across age (16‐65 yrs: 60% vs >65 yrs 34%) and mechanism of injury (penetrating trauma: 65% vs road traffic collision: 70% vs falls < 2 m: 11%) subgroups. Average time to TXA for shocked patients was 51 (34‐89) mins with 60% receiving TXA < 1 h, and for older shocked patients was 80 (48‐154), with only 38% received TXA < 1 h from injury.
Conclusion(s): Early TXA administration in PH care has increased with regionalization of trauma care. Older patients and those with low energy mechanisms of injury are under‐treated subgroups who are failing to benefit from early TXA.
VPB0004
Evaluation of the utility of BAT (ISTH‐SSC bleeding assessment tool) questionnaire in the diagnostic of acquired von Willebrand syndrome
M. Costa‐Pinto1; M. Fernández2; N. Rodríguez2; A. Pérez‐Rodríguez2; M. Gómez‐del‐Castillo 2; T. Lado2; M. Lopez‐Fernandez3
1 Servicio de Hematología y Hemoterapia. Unidad de Trombosis y Hemostasia. Complejo Hospitalario Universitario A Coruña., La Coruña, Galicia, Spain; 2 Servicio de Hematología y Hemoterapia. Complejo Hospitalario Universitario A Coruña, La Coruña, Galicia, Spain; 3 Servicio de Hematologia y Hemoterapia. Complejo Hospitalario Universitario A Coruia, La Coruna, Galicia, Spain
Background: Acquired von Willebrand syndrome (AVWS) is a probably under‐diagnosed hemorrhagic condition, clinically similar to congenital von Willebrand disease (VWD), normally associated with other underlying conditions. Diagnostic of AVWS includes clinical symptomatology and a complex battery of laboratory tests. The utility of BAT (ISTH‐SCC bleeding assessment tool) questionnaire in the diagnostic of AVWS was not evaluated. In VWD BAT ≥ 3 is considered abnormal.
Aims: Describe path to diagnosis of AVWS and evaluate the utility of BAT questionnaire in AVWS.
Methods: We evaluated BAT questionnaire, and VWF laboratory test in patients with suspicion of AVWS (presence of underlying disease related with AVWS and recent hemorrhagic diathesis or high risk of hemorrhage). Laboratory study included: platelet function assay (PFA), ristocetin induced platelet agglutination (RIPA), VWF antigen (VWF:Ag), VWF ristocetin cofactor (VWF:RCo), VWF collagen binding (VWF:CB) and ratios, and VWF multimeric distribution (SDS‐agarose gel). Patients with 2‐3 laboratory criteria were included in AVWS group, and with 1‐0 criteria, in Control group (Table 1).
Results: A total of 82 patients were enrolled: 68 with CVD (cardiovascular disease), 6 with MG (monoclonal gammopathy), 5 with ET (essential thrombocytemia), 2 with cancer, and 1 with lupus. AVWS was confirmed in 50 (60.9%) patients and Control group 32 (39%). Probability of BAT ≥ 3 being related with AVWS diagnostic was 92% (OR = 12.7, CI 95% 3.9 – 40.9). There was significant difference in BAT values comparing AVWS group with Control group (Table 2). AVWS patients that were bleeders in moment of diagnosis had BAT > 3 (mean) in all underlying diseases except for ET. In Control group BAT was ≤ 3 despite presence of bleed.
Conclusion(s): Results suggest that BAT questionnaire is useful for the diagnostic of AVWS. Larger studies are necessary to compare BAT results in different underlying diseases and evaluate utility in hemorrhagic risk evaluation.


VPB0003
Acquired von Willebrand syndrome in patients with monoclonal gammopathy: a single centre experience
M. Costa‐Pinto1; M. Fernández2; N. Rodríguez2; A. Pérez‐Rodríguez2; M. Gómez‐del‐Castillo 2; T. Lado2; M. Lopez‐Fernandez3
1 Servicio de Hematología y Hemoterapia. Unidad de Trombosis y Hemostasia. Complejo Hospitalario Universitario A Coruña., La Coruña, Galicia, Spain; 2 Servicio de Hematología y Hemoterapia. Complejo Hospitalario Universitario A Coruña, La Coruña, Galicia, Spain; 3 Servicio de Hematologia y Hemoterapia. Complejo Hospitalario Universitario A Coruia, La Coruna, Galicia, Spain
Background: Acquired von Willebrand syndrome (AVWS) related with lymphoproliferative disorders (LD) is more frequent in patients with monoclonal gammopathy of undetermined significance (MGUS). The pathophysiological mechanisms can be autoantibodies against von Willebrand factor (VWF), or inmuno‐absorption by plasmatic cells. Diagnostic is complex and bleeding can be severe. The answer to different treatments is not fully established.
Aims: Describe the path to diagnosis of AVWS in patients with monoclonal gammopathy (MG) and the therapeutic approaches.
Methods: We have enrolled five patients with monoclonal component IgG or IgA without previous or family history of bleeding, who presented recent significant hemorrhage.We assessed BAT (ISTH‐SCC bleeding assessment tool) questionnaire and evaluated the hemorrhagic diathesis. Laboratory tests included primary and secondary hemostasis tests, and complete VWF study including multimeric analysis of VWF, and inhibitor to VWF. We evaluated the response to hemostatic treatments, and treatment of underlying disease.
Results: BAT results were > 3 (normal value < 3) in 4 of 5 patients (median 5.2); 3 patients needed hospitalization, blood products and FVIII/FVW concentrates administration, due to hemorrhagic episodes. VWF levels were very low. All patients had loss of VWF multimers. VWF inhibitor tests were negative. The response to IVIg (intravenous inmunoglobulins) was variable and transitory within 2 to 3 weeks. The answer to desmopressin and FVIII/VWF concentrates was poor or partial, due to the accelerated clearance of VWF. Response to the treatment of underlying disease was null, including: bortezomib, dexamethasone and cyclophosphamide; lenalidomide, and allogeneic bone marrow transplant.
Conclusion(s): Results suggest accelerated clearance of VWF multimers probably by absorption by malignant cells. Bleeding is frequent, can be severe, and sometimes blood products are needed. IVIg is the best option, but transitory. Response to treatment of underlying disease is null.


PB0994
Validation of the IMPROVE hospital‐acquired bleeding risk assessment model in the medical inpatients thrombosis and hemostasis study (MITH) population
K. Wilkinson 1; A. Sparks1; M. Gergi1; A. Repp1; H. Al‐Samkari2; N. Roetker3; N. Zakai4
1 University of Vermont, Burlington, Vermont, United States; 2 Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, United States; 3 Chronic Disease Research Group, Minneapolis, Minnesota, United States; 4 University of Vermont, Colchester, Vermont, United States
Background: Hospital‐acquired (HA) bleeding often receives less attention than HA‐venous thrombosis but may be more common. The International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) risk assessment model (RAM) is the only externally validated RAM for predicting HA‐bleeding in medical patients, but the validation was performed in a population designed to mimic the IMPROVE population.
Aims: To externally validate the IMPROVE bleeding RAM in unselected medical patients and by bleeding site.
Methods: Electronic health record data for medical admissions between 2010‐2019 at the University of Vermont Medical Center (UVMMC) were assessed. Major and clinically‐relevant non‐major bleeding and IMPROVE risk factors were defined using validated computable phenotypes. Logistic regression RAMs including the IMPROVE risk factors and anticoagulation intensity at admission were fit for overall and site‐specific bleeding. AUCs were calculated to assess model performance. The research received Institutional Review Board approval and was funded by the National Institutes of Health, USA.
Results: Table 1 presents the number of HA‐bleeding events and the prevalence of the IMPROVE RAM risk factors in the IMPROVE and the UVMMC populations. At UVMMC there were 57,165 admissions among 29,110 people and 1,992 HA‐bleeding events. Multivariable odds ratios tended to be smaller in the UVMMC population. The overall bleeding model had an AUC of 0.66 (Table 2). GI had the highest AUC (0.78) and Other Sites had the lowest AUC (0.63). When applying the points‐based system proposed by IMPROVE, the observed bleeding risk for a score < 7 versus >= 7 was 3.2% and 5.5%.
Conclusion(s): The IMPROVE HA‐bleeding score and the risk factors in the RAM are associated with bleeding. However, the risk score cut‐off poorly discriminated risk of bleeding clinically, a potential limitation to the clinical applicability of this RAM. The different performance by bleeding site suggests further refinement of RAMs by bleeding site is warranted.


PB0002
Emicizumab for the treatment of acquired hemophilia A: An update of the Vienna experience
P. Knöbl 1; J. Thaler2; P. Jilma3; P. Quehenberger4; K. Gleixner5; W. Sperr6
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Clinical Division of Haematology and Haemostaseology, Department of Medicine I, v, Vienna, Wien, Austria; 3 Department of Laboratory Medicine, Medical University of Vienna, Vienna, Wien, Austria; 4 Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 5 Med.Univ.Vienna, Dept.Med. 1, Vienna, Wien, Austria; 6 Med.Univ.Vienna, Dept.Med.1, Vienna, Wien, Austria
Background: Acquired hemophilia A (AHA) is a severe bleeding disorder caused by inhibiting autoantibodies to FVIII. For hemostatic treatment bypassing agents, human or porcine FVIII are currently standard of care. Recently, we reported on the advantages of emicizumab, a bispecific, FVIII‐mimetic therapeutic antibody, in patients with AHA. Good hemostatic activity, weekly (or longer) subcutaneous injections, early discharge, and reduced need for immunosuppression improve patients management.
Aims: Meanwhile, emicizumab has become standard of care for AHA in our center. This is an update on our experience.
Methods: Emicizumab was given at 3 mg/kg sc. weekly for 4 weeks, followed by 1.5 mg/kg in 2‐4 weeks intervals. For FVIII monitoring chromogenic assays with human and bovine reagents were used.
Results: Up to now, we have treated 20 patients with AHA according to this strategy, 11 male, 9 female, median age 79 yrs (range 51‐87). The initial FVIII activity was < 1%, the max. inhibitor 69 BU/ml (3‐2300). Most patients had severe bleeding, requiring RBC transfusions and therapy with rhFVIIa prior to emicizumab. APTT normalized 1‐3 days after the first dose, hemostatic efficacy was obtained and bypassing therapy was stopped after 4 (0‐9) days. FVIII exceeded 50%, indicating complete remission, after 115 days (median). All patients received immunosuppression with steroids and rituximab. 18 patients were discharged 2 weeks after the first emicizumab injection, and followed and treated every 3 weeks until complete remission. No breakthrough bleeding was observed. In 2 patients, all treatment was terminated within a few days because of advanced comorbidities, both patients expired.
Conclusion(s): In conclusion, our data confirm the advantageous properties of emicizumab for the management of AHA. Special attention is needed on the use of appropriate lab assays, the recognition of remission, and to avoid the concomitant use of APCC. A clinical trial on emicizumab for AHA is currently ongoing.
Novel Therapies in the Management of Acquired Bleeding
PB0999
Update in the management tools of thrombotic thrombocytopenic purpura (TTP) as one of the rare hematological disorders with a highlight on the cost value of such diseases
F. Alayoubi 1; G. Elgohary2
1 KSUMC, Riyadh, Ar Riyad, Saudi Arabia; 2 King Saud Univesrty, ryidh, Ar Riyad, Saudi Arabia
Background: TTP is a thrombotic microangiopathy (TMA) characterized by microangiopathic hemolytic anemia and thrombocytopenia developed due to deficiency of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13). T the absence of ADAMTS13 activity causes the formation of very large (ultra‐large) vWF multimers, which have an increased tendency to attach to the endothelium.
Aims: Primary objective: To correlate the diagnosis of TTP with the validated TTP scores (PLASMIC, French and Bentley scores) Secondary objective: To provide an estimated incidence of TTP at Our Center at King Khalid University Hospital. To evaluate the accuracy and quality of diagnostic work up done for patients with TTP To evaluate the therapeutic options provided for patients with TTP and assess their clinical outcomes.
Methods: Retrospective observation study was conducted in our King Khalid University Hospital. All patients who received PLEX at KKUH for definite or presumptive diagnosis of TTP between 2013‐2021.Hospital’s computer with eSiHi access and Blood bank patients’ record, Target Population / Sample Size (with sample 29) patients.
Results: The clinical judgment for diagnosing Thrombotic thrombocytopenic purpura (TTP) is in concordance with the validated TTP scores at King Khalid University Hospital (KKUH). The extensive review of patients presented to KKUH with TTP diagnosis is likely to provide us with the largest local sample size of eligible patients to compare the clinical outcomes of plasma exchange (PLEX) with the other additional forms of management.
Conclusion(s): In conclusion, a major challenge in diagnosing and managing patients with TTP where a rapid identification and management initiation has been proven to save patients’ lives. PLEX is the main curative option for these patients but many challenges face the initiation of PLEX. Here we seek to assess the local experience and gain more insight around the approach to similar cases in the future
VPB1006
Mini‐invasive surgical treatment of haemorrhoiadal bleeding in patients with cardiovascular disease under antithrombotic therapy
A. Tsitskarava
Pavlov First Saint Petersburg State Medical University, Saint Petersburg, Saint Petersburg City, Russia
Background: A large amount of comorbidity patients have a frequent complication such as gastrointestinal bleeding due to long‐term antithrombotic therapy. For instance, haemorrhoids and, consequently, haemorrhoidal bleeding significant decreases the quality of life for patients with cardiovascular disease, who have the high risk of thrombotic or ischemic events. Surgery for these patients requires mini‐invasive technic, radical result and minimize to risk of postoperative complications under antiplatelet or anticoagulant therapy.
Aims: The goal of research was to find surgical management of recurrent haemorrhoidal bleeding in cardiovascular patients with no influence to systemic haemostasis.
Methods: We studied 86 patients with cardiovascular disease and hemorrhoids. 45 patients from them had recurrent haemorrhoidal bleeding, causing to chronic anemia, need to constantly antiplatelet (ASA, Clopidogrel) or anticoagulant (VKA, NOACs) therapy and had not positive effect by basic treatment (phlebotonics and topical). In Group 1, contained in 23 patients, were underwent mini‐invasive doppler‐guided haemorrhoidal artery ligation with recto‐anal repair without interrupting antithrombotic therapy in the preoperative period. Group 2 was represented 22 patients in which were used haemorhhoidectomy by Milligan‐Morgan with 3‐7 days period of rejecting antithrombotic therapy.
Results: Despite to continued antithrombotic therapy in Group 1 was established the lower grade of intraoperative bleeding and postoperative pain compared to Group 2. In Group 1 the bridge‐therapy with LMWH was used in 6 patients with warfarin. No bleeding or thrombotic complications were observed postoperatively in this Group. All of these patients have been denying recurrent hemorrhoidal bleeding as well. In the early postoperative period hemorrhagic complications, requiring rectal revision and haemostasis, have been noted in 5 patients of Group 2.
Conclusion(s): Doppler‐guided haemorrhoidal artery ligation is the effective and secure method for treatment of haemorrhoids which did not require interrupting antithrombotic therapy and have not affected systemic haemostasis in patients with cardiovascular disease.
PB0007
Post‐partum acquired haemophilia A in the COVID era – Building the case for Emicizumab?
C. Crossette‐Thambiah 1; D. Arachchillage2; M. Laffan3
1 Imperial College London, London, England, United Kingdom; 2 Department of Immunology and Inflammation, Imperial College London, London, England, United Kingdom; 3 Centre for Haematology, Imperial College London, London, England, United Kingdom
Background: Both de‐novo and relapse of Acquired Haemophilia A (AHA) have been reported following SARS‐CoV‐2 infection and vaccination suggesting virus‐induced immune dysregulation as a potential mechanism. The current standard of care in the potentially fatal AHA, requires the use of bypassing agents (BPA) or porcine FVIII to achieve haemostasis and immunosuppression to suppress autoantibody production. The bispecific antibody emicizumab now offers the possibility to achieve the former and reduced need for the latter but is only approved solely for use in congenital haemophilia A.
Aims: We present a cluster of three AHA cases presenting between April to June 2021 at a single tertiary centre. Notably each patient received recent BNT162b2(Pfizer) vaccination.
Methods: Bypassing therapy and steroids were commenced with response in two cases. One case remained refractory to BPA, porcine FVIII, steroids and Azathioprine. Approval was sought for a subcutaneous biweekly injection of Emicizumab in order to avoid rituximab during the pandemic.
Results: In 10 months since initiation of emicizumab, no further bleeding and no thrombotic events have been reported. Factor VIII (chromogenic) is now detectable in all patients.
Conclusion(s): The close interaction between SARS‐Cov‐2 and the haemostatic system has been evident in the COVID era. The unusual clustering of cases presented here suggests that antibody responses to SARS‐Cov‐2 infection or vaccine may cross‐react with coagulation factors resulting in the immunohematological phenomenon of AHA. Furthermore, to our knowledge this is the first use of Emicizumab in acquired haemophilia in the post‐partum period. As well as providing excellent haemostatic efficacy and potential cost‐effectiveness, emicizumab provides several advantages for a new mother during the COVID‐19 pandemic. These include a return to home, contact with family and minimal attendance at hospital as well as avoiding immunosuppression conferring increased risk of infection and loss of protection from vaccinations. We note the recent application for use of emicizumab in AHA.
PB1002
Daratumumab as an adjunctive therapy for acquired hemophilia A with poor prognostic markers
C. Moonla 1; C. Polprasert1; S. Krittikarux2; S. Krajibthong3; T. Chajuwan4; A. Sukperm1; B. Akkawat1; P. Rojnuckarin1; N. Uaprasert1
1 Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Krung Thep, Thailand; 2 Department of Medicine, Sakaeo Crown Prince Hospital, Sa Kaeo, Sa Kaeo, Thailand; 3 Department of Medicine, Hua Hin Hospital, Hua Hin, Prachuap Khiri Khan, Thailand; 4 Department of Medicine, Sunpasitthiprasong Hospital, Ubon Ratchathani, Ubon Ratchathani, Thailand
Background: Acquired hemophilia A (AHA) patients with poor prognostic markers, factor VIII (FVIII) coagulant activity (FVIII:C) < 1 IU/dl or FVIII inhibitor titer >20 Bethesda units (BU)/ml, may fail to achieve remission after combined immunosuppressive therapy (IST). Anti‐CD38 monoclonal antibody daratumumab has a potential for inhibitor eradication by depleting anti‐FVIII autoantibody‐producing plasma cells in those patients.
Aims: We describe a case of AHA with major bleeding secondary to high‐titer FVIII inhibitors treated by daratumumab.
Methods: A 33‐year‐old previously healthy Thai man spontaneously developed large intramuscular hematomas at the right thigh and left buttock. Undetectable FVIII:C and high‐titer FVIII inhibitors by Nijmegen‐Bethesda assays confirmed AHA diagnosis. While bypassing agents (BAs), recombinant activated factor VII and activated prothrombin complex concentrate, were administered for hemostatic treatment, prednisolone 1 mg/kg/day plus 4‐week oral cyclophosphamide 1.5 mg/kg/day were prescribed. Four weekly doses of intravenous daratumumab 16 mg/kg were added after an uncontrolled bleeding and inadequate inhibitor suppression by IST for 1 week. Complete remission (CR) was defined as FVIII:C normalization, no active bleeding after 24‐hour discontinuation of hemostatic agents, and negative inhibitor (< 0.4 BU/ml) when using prednisolone < 15 mg/day.
Results: An initial inhibitor titer of 179.2 BU/ml was decreased to 141.6‐148.8 BU/ml, while FVIII:C of <1 IU/dl modestly elevated to 2.9–4.6 IU/dl and the hematomas expanded during the first week of treatment. The adjunctive daratumumab therapy could rapidly suppress FVIII inhibitors to the first negative titer and induce CR within 33 and 63 days, respectively. (Figure 1). All BAs were discontinued within 23 days. After prednisolone tapering‐off, FVIII inhibitor remained undetectable until 13 months of follow‐up. No infectious complications occurred despite transient hypogammaglobulinemia and CD19+ B‐cell depletion post‐daratumumab (Table 1).
Conclusion(s): Short‐course daratumumab combined with standard IST yields a dramatic decline of anti‐FVIII autoantibody titers and safe CR achievement in an AHA patient with poor prognostic markers.


PB1000
The loss of high molecular weight Von Willebrand factor multimers under shear conditions in sheep is not solely ADAMTS13 dependent
S. Deconinck 1; A. Schelpe2; I. Pareyn1; A. Vandenbulcke1; C. Tersteeg3; S. De Meyer3; K. Vanhoorelbeke3
1 Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 2 Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 3 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium
Background: Acquired von Willebrand syndrome has been linked to bleedings observed in mechanical circulatory support (MCS) device patients. We previously showed that blocking ADAMTS13, using the inhibitory anti‐ADAMTS13 monoclonal antibody (mAb) 17C7, prevented von Willebrand factor (VWF) degradation in in vitro MCS device systems with human and bovine blood. Surprisingly, blocking ovine ADAMTS13 did not prevent the loss of high molecular weight (HMW) VWF multimers in an in vitro MCS system.
Aims: To determine if other mechanisms (besides ADAMTS13) linked to the presence of blood cells could also contribute to the shear‐induced VWF degradation in sheep.
Methods: Ovine blood or plasma was supplemented with PBS (control) or mAb 17C7 (30 μg/ml) and vortexed for 10 minutes at 2500 rotations per minute using a vortex device (n = 4). The vortex assay allowed us to use smaller blood or plasma volumes compared to in vitro MCS device experiments. Next, VWF multimers were analyzed and the percentage of HMW VWF multimers was calculated relative to baseline (no shear set at 100% HMW VWF multimers).
Results: In line with our previous MCS device experiments with ovine blood, mAb 17C7 was not able to significantly prevent HMW VWF multimer loss in ovine blood after vortexing (26 ± 14% HMW VWF multimers in control versus 57 ± 15% in mAb 17C7‐treated ovine blood, P = 0.06). In contrast, vortexing ovine plasma in the presence of mAb 17C7 resulted in a significant inhibition of VWF degradation compared to control plasma (33 ± 14% HMW VWF multimers in control versus 81%±14% in mAb 17C7‐treated plasma, P = 0.03) and compared to mAb 17C7‐treated ovine blood (57 ± 15%, P = 0.04).
Conclusion(s): In sheep, shear‐induced VWF degradation is most likely a result of other blood cell proteases or the formation of VWF‐platelet complexes whereas it is mainly ADAMTS13‐dependent in human and bovine blood. Hence, these important differences between species should be taken into account when performing preclinical research.
PB0008
Neutralizing anti‐emicizumab antibodies in a patient with acquired hemophilia A
B. Pezeshkpoor 1; N. Sereda1; A. Berkemeier1; J. Müller2; S. Singh1; S. Ramaraje Urs2; C. Klein1; S. Horneff1; G. Goldmann1; N. Marquardt1; J. Oldenburg3
1 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Medical Faculty, University of Bonn, Germany, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Emicizumab is a bispecific monoclonal antibody with a therapeutic FVIII‐mimetic nature for prophylactic treatment of hemophilia A patients. Thus it seems to be an effective hemostatic therapy for Acquired Hemophilia A (AHA).
Aims: In the frame of this study, we diagnosed and characterized neutralizing anti‐emicizumab antibodies associated with the loss of treatment efficacy in an AHA patient.
Methods: For detection and characterization of ADAs, an in‐house multiplex microsphere‐based immunoassay based on Luminex was established. The established immunoassay allows simultaneous screening for anti‐FVIII and anti‐emicizumab antibodies. Furthuremore, emicizumab F(ab)2 Fragment was produced to allow the identification of the ADAs against the FIX‐F(ab) and FX‐F(ab) arms of Emicizumab. The screening for ADAs was performed (IgG1‐4 and IgA anti‐human antibodies). The specificity of the ADAs was confirmed by competitive immunoassays with 100μg/ml Emicizumab.
Results: A 83 year old male AHA patient was treated with pdFVIII and 1.5 mg/Kg emicizumab. In the beginning of his treatment he had expected levels of Emicizumab concentration in his plasma (~45μg/ml). 45 days after the start of the treatment a progressive decrease of the plasma concentration of emicizumab was observed. Five months later the plasma concentration of emicizumab dropped to < 10 μg/ml due to the supposed ADAs. Combined prednisolone and intravenous immunoglobulin treatment restored the plasma concentration of emicizumab. We were able to detect anti‐emicizumab antibodies in the course of treatment of IgG isotype. The IgG subtype analysis revealed the presence of IgG1 and IgG2 antibodies. Competitive assays confirmed the respective specificity of the ADAs against emicizumab.
Conclusion(s): Although the incidence of neutralizing anti‐emicizumab antibody is expected to be rare, with the increasing use of emicizumab in the therapy of AHA this number might rise. This study highlights the importance of a close monitoring and the need of laboratory assays to detect these antibodies.
VPB1007
Overview of hemostatic effects of exogenous fibrin monomer with systemic use in the experiment
V. Vdovin 1; I. Shakhmatov1; A. Momot2
1 FSBEI of Higher Education “Altai State Medical University” of the Ministry of Healthcare of the Russian Federation, Barnaul, Altaisky krai, Russia; 2 Altai Branch of FSBI «National Research Center for Hematology» Russian Ministry of Healthcare, Barnaul, Altaisky krai, Russia
Background: Trauma and surgery are often associated with large blood loss. Particular life threat circumstances are those that occur against the background of taking antithrombotic drugs. Clinical practice has a range of hemostatic drugs that are widely used to limit blood loss. However, there is a risk of thrombosis with their use. The search for effective and safe drugs with a systemic hemostatic effect remains relevant.
Aims: To present data on the presence of systemic hemostatic activity of exogenous fibrin monomer of human origin in dosed trauma in animals in the experiment against the background of antithrombotic and fibrinolytic therapy.
Methods: Studies were performed in accordance with Directive 86/609/EEC and the Declaration of Helsinki. Suppression of the hemostasis system activity was carried out by heparin, warfarin, dabigatran, clopidogrel in combination with aspirin and streptokinase. We analyzed the reduction of local blood loss against the background of intravenous administration of exogenous fibrin monomer (FM 0.25 mg/kg) after a dosed liver injury in comparison with the use of protamine sulfate (with heparin therapy), prothrombin complex concentrate (with warfarin therapy), Eptacog alfa (with dabigatran etexilate), tranexamic acid (dual antiplatelet therapy). The wound surface in the area of liver injury was examined histologically, including staining for fibrin. The data were evaluated using the MedCalc Version 17.9.7 program.
Results: The ability of the applied dose of FM to decrease wound bleeding (by 2.9–11.0 times) was shown depending on the type of pharmacological effect. The systemic administration of FM did not affect the analyzed parameters of the hemostasis system, in contrast to the prothrombogenic effects of known drugs with systemic hemostatic effects. The selective formation of fibrin in the area of injury was demonstrated when using exogenous FM.
Conclusion(s): The hemostatic properties of FM can be considered as an alternative in the development of new drugs with a systemic hemostatic effect.
PB1005
Preclinical safety and toxicokinetics of VMX‐C001 – An intravenous bypassing agent for factor Xa inhibitors
D. Verhoef 1; T. Gomes2; G. Short3; P. Reitsma1
1 VarmX, Leiden, The Netherlands; 2 Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 3 VarmX, Leiden, The Netherlands, Cambridge, England, United Kingdom
Background: VMX‐C001, a modified form of human zymogen factor X, is being developed to stop or prevent bleeding in patients taking factor Xa inhibitors and is currently in human phase I studies.
Aims: To examine the safety pharmacology of VMX‐C001 in rats and cynomolgus monkeys.
Methods: Subsequent to dose range finding studies, 2‐week toxicity studies were performed at doses of 20, 50 and 100 IU/kg/day VMX‐C001, followed by a two‐week recovery period.
Results: In either species there were no unscheduled deaths, no clinical signs, no effects on body weight or food consumption nor on ECGs. There were also no hematology, coagulation (including TAT and d‐dimer), clinical chemistry and urinalysis changes during the dosing and recovery periods. In rats, no positive ADAs were observed at the end of dosing. At the end of recovery, one female rat was positive for both anti‐VMX‐C001 and anti‐factor X antibodies. One other female rat tested positive for anti‐factor X antibodies. In monkeys, several animals tested positive for antibodies against factor X or VMX‐C001. Only one animal screened positive against both factor X and VMX‐C001. None of the animals developed a coagulation abnormality indicating the antibodies had no functional significance. Analysis of VMX‐C001 levels in plasma revealed linear and dose proportional toxicokinetics without an effect of sex or period of administration. Accumulation of VMX‐C001 was not observed after repeated dosing. The distribution volume was 80‐100 ml/kg and was consistent with a single compartment model limited to the blood circulation. The half‐life of VMX‐C001 was 4‐5 hours in rats and 6‐8 hours in monkeys.
Conclusion(s): Administration of VMX‐C001 for 2 weeks was clinically well tolerated in rats and cynomolgus monkeys at levels of 20, 50 and 100 IU/kg/. The No‐Observed‐Adverse‐Effect Level (NOAEL) of VMX‐C001was considered to be the highest dose administered in both species.
PB1003
CT‐001 is a rapid clearing factor VIIa with enhanced hemostatic activity and safety in mouse models of acute bleeding
D. Sim; C. Mallari; J. Teare; M. Bauzon; T. Hermiston
Coagulant Therapeutics, Berkeley, California, United States
Background: Acute bleeding leads to significant morbidity and mortality. Recombinant wildtype Factor VIIa (WT FVIIa) had been reported to have some therapeutic effects in some clinical trials. However, its use was associated with thromboembolic events.
Aims: We sought to develop a novel FVIIa molecule (CT‐001) with enhanced activity and lower thrombogenicity.
Methods: CT‐001 has 4 N‐glycans (T106N/N145/V253N/N322) with terminal sialic acid removed to promote active clearance via the asialoglycoprotein receptor, and P10Q/K32E substitutions introduced to its Gla‐domain for enhanced phospholipid affinity and activity.
Results: In mice, CT‐001 had half‐lives of 0.048 h and 0.087 h and clearance of 963 ml/h/kg and 467 ml/h/kg at 1 and 3 mg/kg respectively, which were significantly faster than WT FVIIa (at 3 mg/kg, t1/2 = 1.8 h, Cl = 39 ml/h/kg). Interestingly, CT‐001 was efficacious in reducing blood loss even with its rapid clearance. In a severe hemorrhage mouse model with tail amputated 5 cm from the tip, 1 mg/kg CT‐001 treatment provided comparable efficacy as 3 mg/kg WT FVIIa (Fig 1). The fast clearance of CT‐001 resulted in significantly reduced thrombogenicity in a FeCl3‐induced carotid artery thrombosis mouse model (Fig 2). The low thrombogenicity of CT‐001 was further confirmed in a soluble tissue factor‐induced thrombosis model. Mice treated with 1 and 3 mg/kg of WT FVIIa had significantly lower survival rates of 38.9% (P < 0.005, log rank test) and 35.0% (P < 0.0015) respectively, in comparison to the survival rate of 87% for the saline‐treated mice. The survival rates with 1 and 3 mg/kg of CT‐001 were 94.4% and 83.3% respectively.
Conclusion(s): The data on CT‐001 demonstrate that a short duration of highly active FVIIa procoagulant activity can be an optimal treatment paradigm for acute bleeding.


PB1004
Waste not, want not: Evaluating the hemostatic properties of novel plasma supernatants produced from late‐storage low‐titer type O whole blood
A. Srinivasan* 1; E. Mihalko*2; K. Rahn2; J. Seheult3; P. Spinella4; A. Cap5; D. Triulzi6; M. Yazer6; M. Neal2; S. Shea7
1 University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 2 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States; 3 Department of Pathology, Mayo Clinic, Rochester, MN, Rochester, Minnesota, United States; 4 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA; Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States; 5 United States Army Institute of Surgical Research, JBSA‐Fort Sam Houston, Texas, San Antonio, Texas, United States; 6 Department of Pathology, University of Pittsburgh Medical Center, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States; 7 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States
Background: Low‐titer type O whole blood (LTOWB) may be superior to component therapy in the hemorrhaging trauma patient. Current US regulations limit LTOWB storage in citrate‐phosphate‐dextrose to 21 days. If unused before day 21, LTOWB units can be manufactured into red cell units, and the supernatant discarded. However, this plasma supernatant may have value as a novel transfusion product.
Aims: We evaluated hemostatic properties of the plasma supernatant produced from LTOWB at storage day 15 in comparison to liquid plasma of the same storage age. We hypothesized that LTOWB supernatant would have higher hemostatic potential due to higher platelet and microparticle content.
Methods: LTOWB supernatants (N = 12) were prepared from LTOWB units by manually expressing the supernatant from LTOWB units, and units were sampled aseptically on days 15, 21, and 26. Fresh, never‐frozen liquid plasma (N = 12) were sampled on days 3, 15, 21, and 26. Complete blood counts, prothrombin time (PT), activated partial thromboplastin time (PTT), fibrinogen (FGN), rotational thromboelastometry (ROTEM, EXTEM and INTEM reagents), thrombin generation (TG, platelet‐rich plasma reagent), and enumeration of microparticles using nanoparticle tracking analysis were performed at all timepoints. Groups were compared using a mixed effects model (alpha = 0.05).
Results: Across all timepoints, LTOWB supernatants had significantly higher platelet counts than liquid plasma (p = 0.0016) and had comparable PTT and FGN results despite an elevated PT (p = 0.002, Figure 1A). LTOWB supernatants had higher endogenous thrombin potential (p < 0.0001, Figure 2A) and peak thrombin generation (p < 0.0001), as well as increased ROTEM‐INTEM maximum clot firmness (p = 0.0004) and decreased clot formation time (p = 0.0483) (Figure 2B). Microparticle sizes and concentrations were increased in LTOWB supernatants throughout storage duration (p = 0.0007, Figure 1C).
Conclusion(s): Plasma supernatants separated from LTOWB after 15 days have superior in vitro hemostatic properties than liquid plasma of equivalent age and merit further study as a novel transfusion product. (*Authors contributed equally)


PB1001
A novel human factor VIIa chimera with increased tissue factor‐independent hemostatic and anti‐inflammatory activities
A. Fager 1; M. Hoffman2; D. Monroe3
1 Durham VA Medical Center, Duke University Medical Center, Durham, North Carolina, United States; 2 Durham VA Medical Center, Durham, North Carolina, United States; 3 University of North Carolina Blood Research Center, Chapel Hill, NC, USA., Chapel Hill, North Carolina, United States
Background: Despite advances in critical care, the treatment of intracranial hemorrhage (ICH) is primarily restricted to supportive measures. This is largely due to diffuse thrombin‐mediated inflammation and microvascular dysfunction that leads to neurotoxicity via mechanisms not amenable to surgical or hemostatic intervention. At pharmacologic doses, recombinant Factor VIIa (rFVIIa) binds to activated platelets and activates Factor X in a tissue factor (TF)‐independent manner. rFVIIa also binds endothelial protein C receptor (EPCR) to initiate signaling that promotes cytoprotective and anti‐inflammatory effects via cleavage of protease‐activated receptor (PAR)‐1. However, the efficacy of rFVIIa in ICH is limited by a significant risk of TF‐dependent thrombosis. We have designed a novel FVIIa chimera (PC‐FVIIa) to retain the hemostatic and anti‐inflammatory properties of rFVIIa while minimizing its ability to promote TF‐dependent thrombosis.
Aims: The purpose of this study was to characterize the in vitro activity of PC‐FVIIa.
Methods: Antithrombin active‐site titrations determined the proteolytic activity of PC‐FVIIa. The hemostatic activity of PC‐FVIIa was evaluating using standard FXa and thrombin generation assays. A cell‐surface ELISA evaluated the ability of PC‐FVIIa to cleave endothelial PAR‐1. The ability of PC‐FVIIa to protect against thrombin‐induced endothelial barrier disruption was determined in a well‐described transwell assay.
Results: PC‐FVIIa retains the proteolytic activity of rFVIIa. PC‐FVIIa had no measureable TF binding, but exhibited increased TF‐independent hemostatic activity compared to rFVIIa on phospholipids and activated platelets (Figure 1). PC‐FVIIa cleaved PAR‐1 more efficiently than rFVIIa or Activated Protein C (APC) and provided better protection against endothelial barrier disruption (Figure 2).
Conclusion(s): PC‐FVIIa has the potential for increased hemostatic and anti‐inflammatory activity compared to rFVIIa while decreasing the risk for thrombotic complications and providing distinct advantages over existing therapies for emergency hemostasis. This work was supported by the US Department of Veteran’s Affairs, Office of Research and Development. Additional support included an investigator‐initiated grant from Otello Medical, Inc.


Arterial Thromboembolism
Acute Coronary Syndromes
PB0012
Restrictive versus liberal transfusion strategy for anemic patients with acute myocardial infarction: A systematic review and meta‐analysis
R. Kou 1; J. Park1; A. Li2; M. Laureano3; M. Crowther4
1 McMaster University, Hamilton, Ontario, Canada; 2 University of Ottawa, Ottawa, Ontario, Canada; 3 McMaster University, Michael D. DeGroote School of Medicine, Hamilton, Ontario, Canada; 4 McMaster University, Department of Medicine, Hamilton, Ontario, Canada
Background: The optimal blood transfusion threshold for patients with acute myocardial infarction (MI) and anemia is unclear.
Aims: This meta‐analysis aimed to assess the safety of restrictive transfusion thresholds to liberal thresholds.
Methods: We searched MEDLINE, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials for randomized controlled trials comparing different transfusion strategies in patients with acute MI. Studies were excluded if they were: non‐randomized, animal studies, comparisons of other interventions, evaluating a different target population, or were in progress. Screening, data extraction, evaluation of methodological quality, and quality assessment of the body of evidence were conducted by two reviewers, with disagreement resolved through consensus and input of a third reviewer. Primary outcomes were all measured at 30‐days post‐randomization and included: All‐cause mortality, heart failure, recurrent MI, and stroke. A random‐effects model was used.
Results: Our search yielded a total of 7631 results. Three studies were eligible and were included in the analysis (n = 820 patients). In all studies, the restrictive and liberal thresholds were 8 g/dl and 10 g/dl hemoglobin, respectively. There was no significant difference in All‐cause mortality (RR = 0.63; 95% CI 0.15‐2.66; P = 0.53; I2 = 59%), Heart failure (RR = 1.17; 95% CI 0.31‐4.34; P = 0.82; I2 = 70%), Recurrence of MI (RR = 1.61; 95% CI 0.65‐4.02; P = 0.30; I2 = 0%), or Stroke (RR = 1.41; 95% CI 0.27‐7.43; P = 0.69; I2 = 0%).
Conclusion(s): Our meta‐analysis found no significant difference in the primary outcomes between liberal and restrictive transfusion strategies for patients with acute MI. We advise clinicians to interpret our results with caution given the degree of heterogeneity, the limited sample size, and high imprecision in the results from each outcome.


PB0011
Von Willebrand factor levels at 3 months after endothelial injury in Acute Coronary syndrome: Single center experience
C. García Herce 1; B. vegas2; S. Feijóo2; A. Alonso2; B. López2; P. roquero2; N. Acedo2
1 Hospital Universitario de La Princesa, SPAIN, Madrid, Spain; 2 Hospital Universitario de La Princesa, Madrid, Madrid, Spain
Background: Von Willebrand factor (vWf) is a plasma protein that is synthesized in endothelial cells. In the acute moment of ischemic cardiovascular disease, high vWf levels are reported in the literature. There is less evidence of vWf elevation after 3 months.
Aims: To analyze the vWf levels in patients with Acute Coronary Syndrome (ACS) after 3 months of the event in our center.
Methods: We carried out a single‐center prospective study, including consecutively 63 patients < 60 years admitted to Cardiology Unit with ACS between the years 2020 and 2021, in which we quantified vWf levels. The patients were divided into 2 groups according to vWf parameters: normal VWF levels (50%‐150%) or elevated (>150%). All patients gave informed consent.
Results: Table I shows the demographical and clinical characteristics of patients. Median time from the event to the blood test was 3.6 (3.3‐4.4) months. vWf median plasma levels were normal in 26 out of 63 (41.3%) and increased in 37 patients (58.7%). 78.3% of patients with high levels of vWf had previous medical history of arterial ischemia. 51.4% of patients with high levels of vWf had ST‐elevation myocardial infarction. Multiple artery coronary affection was observed in 14 patients with normal vWf (53.9%) and in 24 patients with increased vWf levels (64.9%). Multiple coronary stenosis >50% was observed in 15 patients with normal vWf (57.7%) and in 24 patients with increased vWf levels (64.9%).
Conclusion(s): Our results show that patients with increased plasma VWF levels after 3 months of a cardiovascular event, have more previous medical history of arterial ischemia and higher endothelial injury than patients with normal levels. Further studies are needed to confirm these results.

PB0009
Could pharmacoinvasive strategy using streptokinase be an alternative to primary percutaneous coronary intervention in ST elevation myocardial infarction in resource poor settings?
A. Alex 1; M. Gowri2; O. George3
1 Professor ‐ Department of Cardiology, Vellore, Tamil Nadu, India; 2 CMC‐ Vellore, Vellore, Tamil Nadu, India; 3 Christain Medical College, Vellore, Vellore, Tamil Nadu, India
Background: Primary Percutaneous Coronary Intervention (PCI) is the gold standard in the management of ST Elevation Myocardial Infarction (STEMI). Timely PCI remains a challenge especially in developing countries where the number of centers performing PCI are few and timely transfer to such centers are constrained by the dearth of emergency ambulance services and poor road infrastructure. Pharmacoinvasive strategy refers to routine angiography with a view to revascularize the infarct related vessel 3‐24 hours after fibrinolysis. Due to cost constraints, streptokinase is the widely used fibrinolytic agent in our setting as against western countries where tenecteplase is the preferred agent.
Aims: To study whether the incidence of composite end points (mortality, cardiogenic shock and re‐myocardial infarction) in Pharmacoinvasive strategy was non inferior to Primary PCI in patients with STEMI.
Methods: Patients admitted with a diagnosis of STEMI within a window period of 24 hours over a period of 9 months, who underwent either primary PCI or pharmacoinvasive therapy were included in the study. Primary end points (death within 30 days, re‐MI within 30 days, and cardiogenic shock) and secondary endpoints (arrhythmias, bleeding manifestations, ischemic stroke, ejection fraction, mechanical complications, duration of hospital stay) were investigated. Mortality data of these patients at 5 years were analyzed.
Results: 135 patients were analyzed out of which 95 were in the primary PCI and 43 were in the pharmacoinvasive arm. Though the hypothesis was non‐inferiority, analysis of composite of primary endpoint and mortality outcome suggested equivalence as shown in Table and Figure. There was no significant difference between the secondary outcomes between the two groups. The 5 year mortality was 13.4% and 14.2% in primary PCI and pharmacoinvasive arms respectively.
Conclusion(s): Though this is a small study, it showed that pharmacoinvasive therapy is as effective as primary PCI in the setting of STEMI.


PB0013
Elevated activated partial thromboplastin time‐based clot waveform analysis parameters are associated with acute myocardial infarction and its adverse outcomes
C. Ng 1; F. Uy2; M. Cheong3; W. Wong3; H. Ng3; K. Yeo2; C. Tan3
1 Nanyang Technological University, Singapore, Singapore; 2 Department of Cardiology, National Heart Centre Singapore, Singapore, Not Applicable, Singapore; 3 Department of Haematology, Singapore General Hospital, Singapore, Not Applicable, Singapore
Background: Activated partial thromboplastin time (aPTT)‐based clot waveform analysis (CWA) is a plasma‐based global haemostatic assay that has potential utility in various thrombotic and bleeding conditions. Elevated CWA parameters have previously been shown to be associated with hypercoagulability in venous thromboembolism, but its role in arterial thrombosis has not been studied.
Aims: We aimed to explore the relationship between aPTT‐based CWA and arterial thrombotic disease, by focusing on acute myocardial infarction (AMI). We hypothesised that CWA parameters will be positively associated with AMI.
Methods: In this retrospective case‐control study, CWA data was retrieved from pre‐procedure aPTT tests of AMI patients who underwent emergency cardiac catheterisation from April to December 2018, and control patients who underwent elective orthopaedic or urological procedures. Patients who were on anticoagulant or fibrinolytic treatment, had fever on presentation, active cancer, or ongoing chemotherapy or hormonal therapy were excluded. aPTT and its CWA parameters min1, min2 and max2 (representing the maximum velocity, maximum acceleration and maximum deceleration of clot formation kinetics respectively) were analysed for their association with the occurrence of AMI and adverse outcomes in AMI patients.
Results: 214 AMI patients and 109 control patients were included in this study (Table 1). Shortened aPTT below the reference range, and raised CWA parameters (min1, min2 and max2) above their respective reference ranges, was each significantly associated with the occurrence of AMI (Table 2). However, only elevated min1 and min2 demonstrated significant association with the presence of at least one adverse outcome (Table 2).
Conclusion(s): Elevated aPTT‐based CWA parameters are significantly associated with the occurrence of AMI and its adverse outcomes. Raised CWA may be a potential risk factor and prognostic marker for AMI. Future work is warranted to validate our findings.


PB0010
Overexpression of FXII as a risk of thrombosis in patients with SAMTER syndrome, angioneurotic edema, and its association with smoking rate and hematological parameters
B. Ascencio; M. Castillejos Lopez; A. Lira Mendoza; M. Jaime Capetillo; F. Vidal Martínez
Instituto Nacional de Enfermedades Respiratorias, Mexico, Distrito Federal, Mexico
Background: SAMTER syndrome (SSx), with its triad bronchial asthma, nasal polyps and aspirin allergy; It is an entity that must be recognized since many patients with asthma may have other risk factors for thrombotic events, complicating the use of antiplatelet therapy. In the world we only find 5 clinics that study SSx, in addition, a strong association between angioneurotic edema, SSx and an over‐expression of coagulation FXII has been found.
Aims: To study in our SAMTER clinic the expression of FXII in patients who have developed a picture of angioedema and assess their risk of thrombosis.
Methods: A cohort of 20 patients with SxS was studied, with manifestations of angioneurotic edema secondary to aspirin and non‐steroidal anti‐inflammatory drugs (NSAIDs), whose clinical history, search for thrombosis factors, samples were taken for the measurement of blood count (baseline and follow‐up) and FXII factor. Patients were classified as hyperactive and with normal levels of FXII. Descriptive statistics were performed on quantitative variables and frequency measurements on qualitative variables. Clinical history and hematological parameters were compared between the two study groups.
Results: A total of 21 patients were studied, 6 men and 15 women, 38% (8) were classified as hyperactive to FXII, while 71% (15) were smokers. A strongly significant association was found between smoking rate and Hyperactivity to FXII, with respect to hematological variables: Erythrocytes hemoglobin, hematocrit and lymphocytes showing an increase in their average in hyperactives compared with those not hyperactive to FXII.
Conclusion(s): There is a strong association between smoking rate, hematocrit, hemoglobin and erythrocytes and factor XII hyperactivity in a small cohort of patients with SAMTER syndrome and angioneurotic edema.


PB0015
Left atrium size is associated with fibrin clot ultrastructure in patients with acute myocardial infarction
A. Siniarski 1; S. Baker2; K. Stępień3; R. Ariëns4; A. Gackowski3; G. Gajos5; J. Nessler5
1 Jagiellonian University Medical College, Department of Coronary Artery Disease and Heart Failure, Krakow, Malopolskie, Poland; 2 Wake Forest University, Winston Salem, North Carolina, United States; 3 Jagiellonian University Medical College, Krakow, Malopolskie, Poland; 4 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom; 5 Jagiellonian University Medical College, Kraków, Malopolskie, Poland
Background: Patients with coronary artery disease (CAD) form denser clots with increased stiffness, and higher resistance to fibrinolysis, even when accounting for fibrinogen levels. CAD is associated with disturbances in echocardiographic measurements including impaired ejection fraction, wall motion abnormalities or dilation of heart chambers. Measurements of the heart chambers such as the left atrium could be important to predict thromboembolic risk. We hypothesize that echocardiographic parameters are associated with fibrin clot properties.
Aims: The aim of this study was to assess whether fibrin clot ultrastructure correlates with echocardiographic indicators in patients with AMI.
Methods: We analysed 21 patients hospitalized within 12 hours from the onset of first clinical symptoms of AMI. Clot formation was assessed using standard turbidity measurements. Clot structure was analysed using laser scanning confocal microscopy, scanning electron microscopy (SEM), and permeation experiments, while individual fiber size and internal structure were compared using turbidimetric analysis. Clot breakdown was determined using turbidity. Plasminogen was measured by, and fibrinogen by the Clauss method. Transthoracic echocardiography was used to determine heart chambers size including left atrial size and left atrial volume index.
Results: In simple regression analysis, LA diameter assessed in PLAX (Figure 1, panel A and C) and LVEF were significantly associated with fibrin clot density (Figure 1, panel B and D). Importantly, LVEF and LA diameter were not associated with each other (P = 0.4). Moreover, LAV and LAVI were significantly, negatively correlated with Lag time (r=‐0.42; p = 0.026 for both). Other fibrin clot and lysis characteristics failed to demonstrate association with measurements of the LA.
Conclusion(s): Our study indicates that LA size could serve as an important clinical factor associated with clot density and thus prothrombotic risk.

PB0014
Extreme long‐term stability of aspirin tablets and their ability to inhibit platelet aggregation
I. Sabnis1; N. Shaji1; K. O'Connor2; H. Judge1; R. Storey1; W. Parker 1
1 University of Sheffield, Sheffield, England, United Kingdom; 2 Sheffield Hallam University, Sheffield, England, United Kingdom
Background: Now 125 years old, aspirin (acetylsalicylic acid) is most‐commonly prescribed for its antiplatelet effect in the treatment/prevention of atherothrombotic events, including acute coronary syndromes. Aspirin is broken down by hydrolysis to salicylic acid (SA). Little information is available on the extreme long‐term stability of aspirin formulations and their ongoing ability to inhibit platelet aggregation.
Aims: We characterised the extreme long‐term biochemical stability and antiplatelet effect of aspirin tablets up to approximately 100 years old.
Methods: Plain aspirin tablets approximately 80 and 100 years old (Figure 1), as well as new stocks of aspirin and SA, were obtained and dissolved in saline. After assay optimisation, ultraviolet (UV) spectrophotometry against standard curves was used to quantify the current amounts of aspirin and SA in each formulation. To assess effects on platelet aggregation (PA), healthy volunteer blood was exposed to each preparation and light transmittance aggregometry performed using collagen (0.5 & 2 mcg/ml) and arachidonic acid (AA, 1 mmol/L) as agonists. Experiments were performed in quadruplicate.
Results: UV spectrophotometry performed on mixed solutions of new stocks demonstrated that concentrations (10‐100 micromol/L) of aspirin and SA could be accurately and specifically measured against a standard curve using absorbance at 290 nm minus 230 nm (aspirin), or 230 nm alone (SA). Compared to new stock, tablets 80 and 100 years old had significantly lost aspirin content, with a corresponding increase in SA, but still contained 60.6+/‐7.5% (mean+/‐SD) and 40.2+/‐3.5% of the original aspirin respectively (Figure 2). Formulations of both ages were still able to significantly inhibit collagen‐ and AA‐induced PA when diluted equivalent to 10 micromol/L‐stock, though were weaker than new aspirin in inhibiting collagen‐induced responses.
Conclusion(s): UV spectrophotometry is a convenient and accurate method to assess aspirin and SA content of tablets. In the extreme long‐term, aspirin tablets retain significant antiplatelet activity. The half‐life of active ingredient appears to be c.80‐100 years.


VPB0016
Outcomes of ST‐elevation myocardial infarction in patients with Antiphospholipid syndrome
P. Aung 1; K. Park2; H. Nge3
1 Memorial Healthcare System, Pembroke Pines, Florida, United States; 2 Memorial Healthcare System, Miramar, Florida, United States; 3 University of Medicine 1, Yangon, Yangon, Yangon, Myanmar (Burma)
Background: Antiphospholipid syndrome (APS) is an autoimmune disorder associated with arterial and venous thrombosis. It is also known to increase the risk of coronary artery disease and myocardial infarction by twofold. The impact of APS on ST‐elevation myocardial infarction (STEMI) remains to be elucidated.
Aims: To identify the outcomes of STEMI in patients with anti phospholipid syndrome
Methods: We conducted a retrospective analysis of the 2016 to 2018 Nationwide Inpatient Sample. Adult patients with APS (age ≥18) undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) were selected using the ICD‐10 diagnosis and procedure codes. Discharge‐level weight analysis was used to produce a national estimate. Propensity score matching (1:1) with age, female and comorbidity burden was performed and outcomes were compared between matched cohorts.
Results: During the study period, a total of 645,935 STEMI patients were identified of which 390 patients (0.06%) also had APS. In those who developed STEMI, 129,160 APS and non‐APS patients were propensity‐matched. In‐hospital mortality (12.82% vs 10.9%, p = 0.5), PCI rate (61.5% vs 68.2%. p = 0.7), medical treatment rate (37.2% vs 28.0%, p = 0.5), major adverse cardiac events (MACE) (15.4% vs 11.0%, p = 0.5) and cardiogenic shock (11.5% vs 13.2%, p =1.0) were similar in APS and non‐APS population. However, APS was associated with lower rate of coronary artery bypass graft surgery (CABG) (2.6% vs 5.1%, p = 0.006) and longer length of stay (8.1 ± 1.4 vs 4.7 ± 0.02, p = 0.014).
Conclusion(s): The outcomes of STEMI including in‐hospital mortality, major cardiovascular events, rate of PCI and medical treatment were similar in patients with APS and without APS. The lower rate of CABG was identified in patients with APS who developed STEMI but required a longer length of stay when compared to patients without APS.
Atherosclerosis
VPB0021
Correlations between oxidative stress, endothelial injury and coagulation activation in hypertensive patients with chronic kidney disease
O. Korzh; S. Krasnokutskiy; Y. Fylenko
Kharkiv Medical Academy of Postgraduate Education, Kharkiv, Kharkivs'ka Oblast', Ukraine
Background: Cardiovascular disease is a major cause of death in patients with chronic kidney disease (CKD). Both conditions are rising in incidence as well as prevalence, creating poor outcomes for patients and high healthcare costs.
Aims: The aim of the present study was to establish whether enhanced oxidative stress (OS), involving endothelial injury, activation of coagulation, and inflammatory reaction, could be implicated in hypertensive patients with CKD.
Methods: 122 patients with hypertension and CKD and 63 age‐ and sex‐matched healthy controls were included. Markers of OS, endothelial injury, coagulation, and cytokines, were measured in the plasma of hypertensive patients with CKD and of healthy controls by ELISA methods. Remodeling of the carotid arteries was assessed by measuring the intima‐media thickness (IMT) as a surrogate of atherosclerotic disease in all groups.
Results: Markers of OS, endothelial injury, and extrinsic coagulation pathway activation and IMT values were significantly elevated in hypertensive patients with CKD. The von Willebrand factor antigen (vWF:Ag) levels were more increased in the hypertensive patients with CKD than in control group. Furthermore, the plasma levels of tumour necrosis factor alpha, monocyte chemo‐attractant protein 1, and macrophage inflammatory protein 1 beta were significantly higher in hypertensive patients with CKD when compared with the controls. Both IMT and Cu/Zn superoxide dismutase were positively correlated with age, thrombomodulin, vWF:Ag, tissue factor, tissue factor pathway inhibitor, macrophage inflammatory protein 1 beta, and tumour necrosis factor alpha levels. Multivariate analysis identified vWF:Ag as the only independent variable significantly associated with an increased IMT.
Conclusion(s): The present study suggests that enhanced OS, involved pro‐atherogenic cytokine and chemokines levels, endothelial injury, and coagulation activation may constitute cardiovascular complications in hypertensive patients with CKD. The significant, independent association between IMT and vWF:Ag should be assessed in future studies to determine whether vWF:Ag elevation is causative or a by‐product of the increased IMT.
VPB0022
Emodin‐ mediated pulsed focused ultrasound sonodynamic therapy of early stage atherosclerosis in the rabbit common carotid artery
H. Mehrad
Islamic Azad University, Tabriz Branch‐ Faculty of Basic Sciences, Tabriz, Azarbayjan‐e Sharqi, Iran
Background: Atherosclerosis poses a severe threat to human health. Most acute cardiovascular events result from the rupture of an atherosclerotic plaque, and macrophages play a crucial role in the progression.
Aims: In this study, we developed an experimental pulsed focused ultrasound device, and investigated its effectiveness on foam cells reduction in the early stage atherosclerosis.
Methods: Briefly, New Zealand white rabbits underwent intravascular balloon injury at the right common carotid artery, before being fed a 1.5% cholesterol‐rich diet. After two weeks, the histopathology results showed macrophages foam cells‐rich early stage atherosclerosis formation. Then treatment group underwent sonodynamic therapy with pulsed focused ultrasound (F = 1.1 MHz, I = 12 w/cm2, Pulsed Duration = 23 ms) accompanied by emodin (50 mg/kg) administration.
Results: B‐mode ultrasound and histopathology results showed a significant reduction in the mean value for macrophage foam cells content and wall mean thickness in the treatment group compared with the other groups (P < 0.05).
Conclusion(s): Enhanced apoptotic effect of emodin, induced by sonodynamic therapy accompanied by cytotoxic effect of inertial cavitation, induced by collapsed bubbles, can cause to reduce the macrophages foam cells in the early stage atherosclerotic lesion and significantly reduce the intima‐ media thickness. Emodin‐mediated pulsed focused sonodynamic therapy may be a potential treatment to attenuate early stage atherosclerosis.
VPB0023
Inhibition of restenosis after orbital atherectomy of severely calcified artery using combined electrohydraulic low level shock wave therapy and 125I‐ mediated gamma‐intravascular brachytherapy
H. Mehrad
Islamic Azad University, Tabriz Branch‐ Faculty of Basic Sciences, Tabriz, Azarbayjan‐e Sharqi, Iran
Background: Three mechanisms are responsible for the development of restenosis: elastic recoil, intimal hyperplasia and late vascular constriction, all grouped under the catch phrase “negative remodeling”. Neointimal hyperplasia is usually defined in an artery as thickening of the intimal layer after an injury such as angioplasty, mechanical atherectomy or surgical repair. The orbital atherectomy method that are currently in use, cause to inflammation and subsequent restenosis.
Aims: The aim of this study was to evaluate the effect of combined electrohydraulic shock wave therapy and 125I‐ mediated gamma‐intravascular brachytherapy on inflammation and restenosis reduction after mechanical atherectomy of the animal model of severely calcified common carotid artery, wherein diagnostic ultrasound is adjuncted with atherectomy and combination therapy system, with a goal of increased safety.
Methods: Briefly, New Zealand white rabbits were submitted to common carotid artery severely calcified atherosclerotic stenosis. Then treatment group underwent B‐ mode ultrasound‐ guided orbital atherectomy followed by combined shock wave (V = 20 Kv, F = 0.5 Hz, Impulses = 120) therapy and gamma‐intravascular brachytherapy (125I‐, 23 Gy).
Results: Results from histopathology and ultrasonography showed a significant reduction in the mean value for macrophages and smooth muscle hyperplasia cells density after orbital atherectomy in the treated group compared with the other groups (p < 0.05).
Conclusion(s): Anti‐ inflammatory effect of shock waves accompanied by apoptotic effect of gamma‐ mediated brachytherapy, can cause to reduce the inflammation and smooth muscle hyperplasia cells in the intimal layer. These findings provide the basis for developing of combined low level electrohydraulic focused shock wave therapy and 125I‐ mediated gamma‐intravascular brachytherapy for a successful clinical application in the treatment of restenosis after orbital atherectomy.
PB0020
The effects of hyperglycemia on endothelial activation and the initiation of atherosclerosis
L. Mastrogiacomo1; R. Ballagh2; D. Venegas‐Pino2; G. Werstuck 3
1 Thrombosis and Atherosclerosis Research Institute, McMaster University, Hamilton, Ontario, Canada; 2 Thrombosis And Atherosclerosis Research Institute, McMaster University, Hamilton, Ontario, Canada; 3 Thrombosis and Atherosclerosis Research Institute, McMaster Universtiy, Hamilton, Ontario, Canada
Background: It is well established that patients with diabetes have an increased risk of developing atherosclerotic cardiovascular disease. The earliest detectable sign of atherosclerosis initiation is endothelial cell activation. Activated endothelial cells express adhesion proteins P‐selectin, E‐selectin, vascular cell adhesion molecule (VCAM)‐1, and intercellular adhesion molecule (ICAM)‐1 that function to recruit monocytes to the subendothelial layer.
Aims: This study examines the effect of hyperglycemia on endothelial cell activation and the initiation and progression of atherosclerosis.
Methods: Human aortic endothelial cells (HAECs) were exposed to control (5 mM) or elevated (30 mM) concentrations of glucose for 24 hours and the expression of adhesive proteins P‐selectin, E‐ selectin, VCAM‐1 and ICAM‐1 were quantified by RT‐PCR. Atherogenesis and adhesion protein expression was quantified by immunostaining cross sections of the ascending aorta of atherosclerosis‐prone normoglycemic (ApoE‐/‐) and hyperglycemic (ApoE‐/‐Ins2+/Akita) mice.
Results: In vitro studies revealed that exposure of HAECs to 30 mM glucose significantly increased the expression of P‐selectin, E‐ selectin and VCAM‐1, but not ICAM‐1. In vivo studies showed that, prior to atherosclerotic lesion development, 5‐week‐old hyperglycemic ApoE‐/‐ Ins2+/akita mice had significantly increased expression of P‐selectin, E‐ selectin and VCAM‐1 in the aortic sinus and increased macrophage infiltration, compared to normoglycemic ApoE‐/‐ controls. At 25 weeks of age, ApoE‐/‐ Ins2+/akita mice had significantly larger atherosclerotic lesions than ApoE‐/‐ controls. Similar endothelial activation was observed in heterozygous ApoE+/‐ Ins2+/akita mice, however detectable atherosclerotic lesions did not develop in the absence of dyslipidemia. Lowering blood glucose levels with an SGLT2 inhibitor did significantly reduce endothelial activation.
Conclusion(s): Together these findings support a causative role of hyperglycemia in the early initiation of atherogenesis and highlight the importance of blood glucose regulation as a strategy to prevent, and perhaps reverse, atherosclerotic CVD.
VPB0024
Some hemostasis parameters concentrations in coronary artery disease patients depending on arrhythmias
Y. Tyravska 1; V. Lizogub1; O. Savchuk2
1 Bogomolets National Medical University, Kyiv, Kyyiv, Ukraine; 2 Taras Shevchenko National University of Kyiv, Kyiv, Kyyiv, Ukraine
Background: Relationships between coronary artery disease (CAD) course and hemostasis indicators are well‐established. However, the influence of arrhythmia on hemostasis in CAD patients is still under question.
Aims: To investigate concentrations of soluble fibrin monomer complex (SFMC), prothrombin pool (PP), serotonin and factor von Willebrand (vWF) in CAD patients depending on arrhythmias.
Methods: In our cross‐sectional study, we recruited 102 CAD patients: 23 with stable angina (SA) (the median age 62.0 (58.5‐69.0) years, 13 males (56.5%)) and 79 with progressive unstable angina (UA) (the median age 64.0 (55.5‐70.0) years, 51 males (64.5%)). Arrhythmias (the quantity and quality of premature ventricular complexes, paroxysms of atrial fibrillation, supraventricular tachycardia) were analyzed. All patients passed over 24‐hour ECG monitoring during the first day of hospitalization. Blood plasma samples were drawn before treatment onset. We determined vWF and PP concentrations by ELISA. For assessment of SFMC concentration, we used the colorimetric ortho‐phenanthroline method. We analyzed the blood plasma serotonin content by the ion‐exchange chromatography with further measurement of serotonin on a fluorescence spectrophotometer. We used SPSS, v.22.0, particularly the Chi‐square test, Fisher’s exact test, Mann‐Whitney test, Spearman test for comparison of parameters among the groups and establish the relationships between them.
Results: Arrhythmias were registered in 57 patients (72.2%) with UA and 11 patients (47.8%) with SA (F = 0.043). Concentrations of observed hemostasis parameters are demonstrated in Table. Significant differences were registered in SFMC and vWF concentrations in UA patients depending on arrhythmias presence (p = 0.002, p = 0.009, respectively), but not in PP (p = 0.123) and serotonin concentrations (p = 0.264) as well as observed parameters concentrations in SA patients with or without arrhythmias. Correlations were noticed between presence of arrhythmia in UA patients and SFMC concentration (r = 0.454, p = 0.001) as well as vWF (r=‐0.376, p = 0.008).
Conclusion(s): Arrhythmias in UA patients unlike SA patients are suggested to enhance the trends of thrombophilia. Further investigations are warranted.
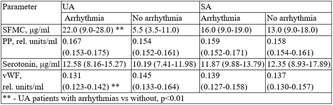
PB0017
High prevalence and wide variety of clonal haematopoiesis‐driver mutations in patients with severe aortic valve stenosis undergoing transcatheter aortic valve replacement
F. Lassalle1; N. Duployez2; F. Vincent1; A. Rauch1; M. Rosa1; B. Staels1; C. Preudhomme2; S. Susen3; E. Van Belle1; A. Dupont 1
1 Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐ EGID, F‐59000 Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 2 Department of Hematology, CHU Lille, F‐59000 Lille, Lille, Nord‐Pas‐de‐Calais, France; 3 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France
Background: Aortic valve stenosis (AVS), the most common acquired heart valve disease, is now considered as an active process of valve calcification mediating by chronic inflammation and similar to atherosclerosis. Clonal hematopoiesis of indeterminate potential (CHIP), defined by the presence of somatic mutations in hematopoietic stem cells without other hematologic abnormalities, was detected in 10 to 20% of general population aged>70 years and has been associated with atherosclerosis and inflammation. One recent study has reported the presence of mutations in CHIP‐genes, DNMT3A and TET2 in a third of patients with severe AVS and an association with mortality following transcatheter aortic valve replacement (TAVR), a procedure that elicits a thromboinflammatory state.
Aims: To assess the full profile of CHIP‐driver genes in AVS patients undergoing TAVR and determine whether CHIP is associated with thromboinflammatory state biological features (blood cell count, systemic inflammation/thrombosis markers).
Methods: High‐throughput sequencing of a 67‐genes panel was performed on blood‐DNA from 270 AVS patients, free of hematological malignancy and undergoing TAVR (age: 82 ± 7 years, 42% male).
Results: A total of 444 mutations (with a variant allele frequency > 1%) in 35 genes were detected in 202 of 270 patients (CHIP prevalence: 50%, 71% and 78% of patients aged < 70 years, 70‐80 years and >80 years). Despite a high prevalence of mutations in the most frequently mutated genes DNMT3A (129 patients, 48%) and TET2 (117 patients, 43%), mutations in PPM1D, ASXL1, SF3B1, TP53, CBL and SRSF2 were also identified in at least 5% of patients. However, the presence of CHIP was not associated with thromboinflammatory state biological features.
Conclusion(s): This study shows that CHIP prevalence is very high in AVS patients undergoing TAVR. These new data support the inflammatory and atherosclerotic background of AVS. Future studies clarifying the relationships between CHIP, AVS development and TAVR complications are needed.
PB0018
Activated protein C reduces maladaptive unfolded protein response (UPR) to ameliorate diabetes‐accelerated atherosclerosis
S. Fatima 1; I. Gadi2; S. Ambreen1; K. Singh3; A. Gupta3; A. Elwakiel2; S. Krishnan1; A. Methew4; H. Khawaja5; S. Kohli2; B. Isermann2; K. Shahzad2
1 University of Leipzig, Leipzig, Sachsen, Germany; 2 Institute of laboratory medicine, clinical chemistry and molecular diagnostics, Leipzig University, Leipzig, Sachsen, Germany; 3 Institute for Laboratory Medicine, Leipzig University, Leipzig, Sachsen, Germany; 4 Institute of laboratory medicine and molecular diagnostics University Hospital Leipzig Germany., Leipzig, Sachsen‐Anhalt, Germany; 5 Institute of laboratory medicine and molecular diagnostics University Hospital Leipzig Germany., Leipzig, Sachsen, Germany
Background: The mechanisms underlying the different atherosclerosis course in diabetic and non‐diabetic patients remain unknown. Hyperglycemia causes endothelial cells dysfunction, a key disease driver. Atherosclerotic plaques display markers of senescence and unfolded protein response (UPR). Plasma levels of coagulation protease, activated protein C decline in diabetes and atherosclerosis.
Aims: Our aim is to study the role activated protein C which is well known for its cytoprotective and anti‐inflamamtory functions. We hypothesized that activated protein C prevents diabetes induced accelerated atherosclerosis by reducing maladaptive UPR induced endothelial cell senescence.
Methods: To gain insights into pathomechanisms of diabetes induced atherosclerotic plaque development we cultured human coronary artery endothelial cells (HCAECs) under hyperglycemic (HG) or hyperlipidaemic (HL) conditions. ApoE‐/‐ mice (age 8 weeks) was made either diabetic by streptozotocin injections (A mouse model type 1 diabetes) or fed them HFD to induce hyperlipidemia. Mice were analyzed after 20 weeks of treatments.
Results: HG conditions induced strong barrier disruption as compared to HL (TEER, FITC dextran leakage) and protein expression of the senescence markers (p21, p16, p53) and UPR markers (XBP1, IRE1α and ATF6). Activated protein C restored barrier integrity, reduced glucose induced expression of senescence and UPR markers in vitro. Targeting IRE1α RNAase activity prevented HG induced cellular senescence. Ex vivo, diabetic ApoE‐/‐ mice revealed increased expression of senescence, inflammation and UPR markers within atherosclerotic lesion as compared with non‐diabetic ApoE‐/‐ mice.
Conclusion(s): Senescence associated inflammation and UPR are associated with glucose‐dependent endothelial cells dysfunction and loss of endothelial barrier integrity. These results demonstrate that diabetes‐induced atherosclerosis is associated with cellular senescence and UPR. Targeting cellular senescence and UPR (with aPC or IRE1α inhibitor) may be a useful therapy of atherosclerosis in diabetic patients.
PB0019
Bambi‐deficiency enhances adipose tissue browning, ameliorates dyslipidemia and confers protection against atherosclerosis
C. Lee1; O. Merhi El Hassan El Abdallah1; J. Monkman1; K. Woollard1; J. Crawley1; I. Salles‐Crawley 2
1 Imperial College London, London, England, United Kingdom; 2 St George's University of London, London, England, United Kingdom
Background: BAMBI is a TGFβ superfamily transmembrane protein that is highly expressed in platelets and endothelial cells. We have previously shown that endothelial Bambi plays a key role in thrombus formation and stability via modulating the endothelium anticoagulant properties. Several members of the TGFβ superfamily play a role in atherosclerosis and/or adipose tissue function however the role of BAMBI is not fully understood.
Aims: To evaluate the role of BAMBI in atherosclerosis.
Methods: Bambi‐/‐Ldlr‐/‐ and Bambi+/+Ldlr‐/‐ mice were fed a high fat diet (HFD) and aortic plaques analysed by oil red‐O staining or immunohistochemistry. Plasma cholesterol, triglyceride, thrombin‐antithrombin levels were determined. Adipose tissues from chow‐fed Bambi‐/‐ and Bambi+/+ mice were analysed by histology, qPCR and western‐blotting.
Results: Bambi deficiency significantly reduced the formation of atherosclerotic lesions in the descending aortas but not in aortic arches. Analysis of the plaque composition in aortic arches revealed no differences in the macrophage content or anticoagulant phenotype of the endothelium but an increase in collagen content in Bambi‐/‐Ldlr‐/‐ plaques. Plasma cholesterol and triglyceride levels were also significantly lower in Bambi‐/‐Ldlr‐/‐ mice. A significant reduction of body weight was observed in Bambi‐/‐ mice compared to Bambi+/+ mice fed a normal chow diet, and in Bambi‐/‐Ldlr‐/‐ males fed a HFD. No difference in adipose tissue weight was observed in Bambi‐/‐ mice compared to Bambi+/+ mice but adipocyte size was significantly reduced in white adipose tissues (WAT) with increased expression of uncoupling protein 1.
Conclusion(s): So far, our results suggest Bambi deficiency protects against atherosclerosis via modulation of lipid metabolism and favoring a stable plaque but not its influence on coagulation. We also highlight a role for BAMBI in adipose tissue although additional studies under HFD are warranted to confirm the browning phenotype of the subcutaneous WAT.
Atrial Fibrillation
PB0499
Bleeding risk assessment in end‐stage kidney disease: Validation of existing risk scores and evaluation of a machine learning‐based approach
S. Nopp 1; C. Spielvogel1; S. Schmaldienst2; R. Klauser‐Braun3; M. Lorenz4; B. Bauer5; I. Pabinger5; M. Säemann6; O. Königsbrügge7; C. Ay5
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Clinic Favoriten, Vienna, Wien, Austria; 3 Clinic Donaustadt, Vienna, Wien, Austria; 4 Vienna Dialysis Centre, Vienna, Austria, Vienna, Wien, Austria; 5 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 6 Clinic Ottakring, Vienna, Wien, Austria; 7 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: Patients with end‐stage kidney disease (ESKD) on hemodialysis (HD) are at increased risk for bleeding. However, despite relevant clinical implications regarding dialysis modalities or anticoagulation, no bleeding risk assessment strategy has been established in this challenging population.
Aims: This study sought to evaluate existing bleeding risk scores and develop a machine learning‐based prediction model for major bleeding in patients on HD.
Methods: Analyses on bleeding risk assessment models were performed in the population‐based Vienna InVestigation of Atrial fibrillation and thromboemboLism in patients on hemoDialysIs (VIVALDI) study including 625 patients. In this cohort study, patients were prospectively followed for a median observation period of 3.5 years for the occurrence of major bleeding. First, performances of existing bleeding risk scores (i.e., HAS‐BLED, HEMORR2HAGES, ATRIA, and four others) were evaluated in terms of discrimination and calibration. Second, four machine learning‐based prediction models that included clinical, dialysis‐specific, and laboratory parameters were developed and tested using Monte‐Carlo cross‐validation.
Results: Of 625 patients (median age: 66 years, 38% women), 89 (14.2%) developed major bleeding, with a 1‐year, 2‐year, and 3‐year cumulative incidence of 6.1% (95%CI 4.2‐8.0), 10.3% (95%CI 8.0‐12.8), and 13.5% (95%CI 10.8‐16.2), respectively. C‐statistics of seven contemporary bleeding risk scores ranged between 0.54 and 0.59 indicating poor discriminatory performance (Table 1). The HAS‐BLED score showed the highest C‐statistics of 0.59 (95% 0.53‐0.56). Similarly, all four machine learning‐based predictions models performed poorly in internal validation (C‐statistics ranging from 0.49‐0.55, Figure 1).
Conclusion(s): Existing bleeding risk scores and a machine learning approach including common clinical parameters fail to assist in bleeding risk prediction of patients on HD. Therefore, new approaches, including novel biomarkers, to improve bleeding risk prediction in patients on HD are needed.


PB0496
Management of non‐vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation in Spain. RE‐BELD study
O. Gavín Sebastian 1; V. Roldan Schilling2; V. Barrios3; J. Cosín‐Sales4; P. Díez‐Villanueva5; D. Riba6
1 Hospital Lozano Blesa, zaragoza, Aragon, Spain; 2 Hospital Universitario Morales Meseguer, Murcia, Murcia, Spain; 3 Hospital Universitario Ramón y Cajal, Madrid, Madrid, Spain; 4 Hospital Arnau de Vilanova, Valencia, Comunidad Valenciana, Spain; 5 Hospital Universitario La Princesa, Madrid, Madrid, Spain; 6 Boehringer‐Ingelheim Spain, Sant Cugat del Vallés (Barcelona), Catalonia, Spain
Background: Older age is an important risk factor for patients with atrial fibrillation (AF) and is associated with an increased risk of stroke and systemic embolism, as well as an increased risk of severe bleeding with oral anticoagulant therapy. The use of non‐vitamin K antagonist oral anticoagulants (NOACs) in the elderly population in routine clinical practice shows deficiencies and can be improved, with incorrect dosing being common in this population.
Aims: The main objective of the study was to describe the pattern of NOAC use in elderly patients with AF.
Methods: A national, non‐interventional, cross‐sectional and multicenter study was designed. Elderly (≥75 years) patients, with a AF diagnosis and undergoing NOAC treatment according to the SmPC at least 3 months before the study visit were recruited
Results: 503 patients were recruited (500 eligible, 3 excluded) in 36 Hematology, Cardiology and Geriatrics centers. The mean (±SD) age was 81.5 (±4.7) years, 50% women, mean time of AF diagnosis 5.5 (±5.3) years, 47.0% permanent AF, mean creatinine clearance of 57.3 (±18.9) ml/min, CHA2DS2‐VASc =4.33 (±1.36) and HASBLED =1.96 (±0.87). The prevalence of frailty, calculated using the clinical frailty scale (CFS > 4), was observed in 23.6% of the patients. Regarding oral anticoagulant (OAC) treatment, 57.4% of the patients were previously treated with VKAs. Of the 500 evaluable patients, 38.4% were treated with dabigatran, 15.2% with rivaroxaban, 33.2% with apixaban, and 13.2% with edoxaban. 8.6% of the patients have a NOAC switch and 3.6% receive antiplatelets concomitantly. Figure 1 shows the NOAC dosage patterns. A 48.4% and 88.6% of thromboembolic and bleeding events occurred after the start of anticoagulant treatment. Table 1 shows the thromboembolic and bleeding events associated to ACO treatment.
Conclusion(s): It is essential to develop effective strategies to improve the correct dosage of OAC treatment in elderly patients at usual clinical practice.


PB0493
Reach, adoption, and maintenance of a population health dashboard for safe DOAC prescribing: insights from the United States Veterans Health Affairs
G. Barnes 1; C. Chen2; P. Spoutz3; A. Allen4; J. Seagull1; J. Sussman1; M. Dorsch1
1 University of Michigan, Ann Arbor, Michigan, United States; 2 Ann Arbor VA Center for Clinical Management Research, Ann Arbor, Michigan, United States; 3 Veterans Affairs, Boise, Idaho, United States; 4 VA Salt Lake City, Salt Lake City, Utah, United States
Background: Direct oral anticoagulants (DOACs) are first line treatment for venous thrombosis and atrial fibrillation, but inappropriately prescribed for up to 1 in 7 patients. In 2016, the United States Veterans Health Affairs (VHA) implemented a population health dashboard to facilitate pharmacist monitoring of outpatient DOAC use.
Aims: To comprehensively analyze the nationwide VHA implementation of this novel care delivery model that aims to promote safe, evidence‐based anticoagulant prescribing.
Methods: This retrospective analysis was conducted using dashboard log‐in data across all 164 VHA sites between August 2016 and June 2020. Using the RE‐AIM implementation science evaluation framework, we calculated the reach (number of DOAC‐treated patients at sites with any dashboard use), adoption (number of VHA sites and total staff members with regular dashboard use), and maintenance (dashboard use 2+ days per month for at least 6 months) at the site‐level. Post‐hoc, we defined four phases of adoption (early, growth, full use, steady state) based on the pattern of dashboard use.
Results: The dashboard’s reach increased from an average of 413 to 1875 patients per site, achieving a maximum of 258,230 patients across 138 sites by June 2020. Early adoption was slow but then rapidly grew to 67 sites & 224 staff by December 2017 and to 130 sites & 627 staff during the full use phase (December 2018). Dashboard use was maintained (6+ months of consecutive use) by 18/36 (50%) sites by December 2017, 69/128 (54%) of sites by December 2018, and 126/150 (84%) sites by June 2020.
Conclusion(s): Nation‐wide use of a dashboard for safe DOAC prescribing followed a typically diffusion of innovation curve for reach and adoption. There was high site‐level sustained use between January 2019 and June 2020. How this novel electronic health tool and model of care impacted quality and outcomes remains to be assessed.
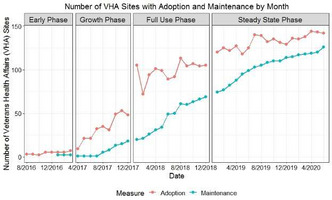

PB0495
The safety and efficacy of direct oral anticoagulants for atrial fibrillation in patients affected by hematologic malignancies: A single‐center experience
A. Serrao1; M. Chavez Orellana2; G. Assanto3; R. Mormile3; E. Baldacci3; C. Santoro4; A. Chistolini 2
1 Hematology, Sapienza University, Rome, Italy, Rome, Lazio, Italy; 2 Hematology, Sapienza University of Rome, Rome, Lazio, Italy; 3 Sapienza University of Rome, Rome, Lazio, Italy; 4 Hematology, University Hospital Policlinico Umberto I, Rome, Rome, Lazio, Italy
Background: Preexistent atrial fibrillation (AF) is not uncommon in cancer patients receiving therapy, and the risk of AF increases during the period adjacent to diagnosis and during active therapy. Managing anticoagulation in hematological malignancy patients with AF is a clinical challenge considering the risk of bleeding and thrombosis with limited data mainly concerning low molecular weight heparin.
Aims: The aim of our study was to evaluate the safety and efficacy of direct oral anticoagulants (DOACs) in patients affected by hematologic malignancies with AF.
Methods: A single‐center retrospective cohort study was performed by reviewing medical records of patients with a confirmed diagnosis of hematologic malignancy who presented a pre‐existing AF or developed AF during the clinical course and treated with DOACs. Oral anticoagulation was continued until a platelet count ≥50 x109/l.
Results: The characteristics of the 96 included patients are reported in Table 1. DOACs therapy was performed for a median time of 19 months (range 11‐107 months). During the entire follow‐up no thrombotic adverse event were reported. Thirty‐two patients (30.7%) required a suspension of DOACs: 14 (14.6%) for bleedings, 9 (9.3%) for toxicity or elevated creatinine levels and 7 (7.2%) for platelets < 50 x109/L; 11 of them discontinued permanently: 7 for a low platelet count and 4 for renal failure. The hemorrhagic adverse events were all clinical relevant non major but did not require definitive discontinuation of DOAC therapy. Univariate analysis did not show significant association of bleeding events with any of the baseline characteristics reported.
Conclusion(s): Although the limitations of this study due to the retrospective nature and the relative low number of patients, DOACs appeared to be effective and safe for the treatment of AF in patients with hematologic malignancies since the hemorrhagic adverse events did not represent a cause of DOACs definitive discontinuation.

PB0494
Apixaban plasma levels at time of elective surgery: Preliminary data from the DALI study
E. Camilleri 1; P. Shahbabai2; M. Rad3; E. van Dorp4; L. Visser2; S. Cannegieter1; N. van Rein1
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Hospital Pharmacy, Haga Teaching Hospital, The Hague, The Netherlands, Den Haag, Zuid‐Holland, Netherlands; 3 Anaesthesiology, Haga Hospital Juliana Children's Hospital, Den Haag, The Netherlands, Den Haag, Zuid‐Holland, Netherlands; 4 Anaesthesiology, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Before elective surgery, direct oral anticoagulants (DOAC) are discontinued following a standardized protocol based on the average DOAC half‐life and the patient’s kidney function. However, it is unclear whether this strategy is actually successful, since the average DOAC half‐life may not be applicable to the individual patient, which could result in high DOAC levels and increased bleeding risk.
Aims: To investigate DOAC levels prior to elective surgery and the associated blood loss.
Methods: The DALI study is an ongoing cohort in which 300 adult patients (100 each for apixaban, rivaroxaban and dabigatran) who have to cease DOAC treatment due to an elective procedure are planned to be included. We report here the results of the complete apixaban cohort. Blood was drawn just before surgery to determine apixaban levels (by liquid chromatography‐mass spectrometry), estimated glomerular filtration rate(eGFR) and activated partial thromboplastin and prothrombin time(aPTT, PT). Patients were split according to apixaban levels > or ≤30 ng/ml as well as by bleeding risk of surgery and eGFR(as both influence ceasing period). Blood loss during surgery and bleeding events in the 30 days after were retrieved from patient files.
Results: Table 1 describes the characteristics of the 101 included apixaban patients. Apixaban levels were >30 ng/ml in 13(13%) patients (Table 2), of whom two had eGFR < 30 ml/min. Median aPTT was 29 s (IQR 27‐31) and median PT was 14 s (IQR 12‐15). aPTT and PT were above‐range in 16(17%) and 30(33%) patients respectively, of whom 2(2%) and 4(4%) had apixaban levels >30 ng/ml. Median blood loss during surgery was 0 ml (IQR 0‐100). During the 30 days after, 3 patients (all with apixaban levels ≤30 ng/ml) received a blood transfusion and 10 had a minor bleeding (1 with apixaban levels >30 ng/ml).
Conclusion(s): With the current protocol,87% of the apixaban‐treated patients achieved levels ≤30 ng/ml and only one minor bleeding occurred in patients with levels>30 ng/ml.


PB0498
Atrial fibrillation: Trends in prevalence and antithrombotic prescriptions in the community
L. Joosten 1; A. de Boer2; E. van Eerde2; S. van Doorn1; A. Hoes2; M. Bots2; F. Rutten2; G. Geersing2
1 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 2 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: In the past decade, there have been many changes in the atrial fibrillation (AF) landscape, including changing treatment modalities. This raises the question how AF prevalence as well as choices in the prescription of oral anticoagulants have developed over time.
Aims: The aim of this study was to describe trends in AF prevalence and patterns of antithrombotic therapy prescriptions in the community.
Methods: Routine care data from the Dutch Julius General Practitioners’ Network (JGPN) were used to calculate the yearly prevalence of AF and to quantify the percentage of all patients who were prescribed a platelet inhibitor, vitamin K antagonist (VKA), non‐VKA oral anticoagulant (NOAC) or no antithrombotic medication. To explore whether certain patient characteristics are associated with selective prescription of oral anticoagulants (OAC), we applied logistic regression analyses.
Results: Results From 2008 through 2017, the JGPN database included 7459 unique AF patients. During this period, the prevalence of AF increased from 0.4% to 1.4% (Figure 1). The percentage of patients prescribed a VKA declined from 47% to 41%, whereas the percentage of patients prescribed a NOAC rose from 0% to 20% (Figure 2). In patients with new‐onset AF, older age, heart failure, diabetes mellitus, vascular disease and dementia were independently associated with a higher likelihood of VKA rather than NOAC prescription. In 2017, 25% of all the patients with AF and a CHA2DS2‐VASc score ≥ 2 were not prescribed OAC therapy (i.e. 8% with platelet inhibitor monotherapy and 17% without any antithrombotic therapy).
Conclusion(s): Between 2008 and 2017, AF prevalence in the community more than tripled. Prescription patterns showed possible ‘channelling’ of VKAs over NOACs in frailer, elderly patients, whereas still about one in every four AF patients with a CHA2DS2‐VASc score ≥ 2 was not prescribed any prophylactic OAC therapy.


VPB0504
Elevated 8‐isoprostane concentration is associated with thromboembolic events in patients with atrial fibrillation
P. Mołek 1; M. Ząbczyk2; J. Natorska2; A. Undas1
1 Jagiellonian University Medical College, Kraków, Malopolskie, Poland; 2 Jagiellonian University, Institute of Cardiology, John Paul II Hospital, Krakow, Malopolskie, Poland
Background: There is evidence that atrial fibrillation (AF) is associated with increased oxidative stress, however, its role in prediction of thromboembolic events is unknown.
Aims: To evaluate whether 8‐isoprostaglandin F2 (8‐isoprostane) levels may predict clinical outcomes in AF patients.
Methods: In this prospective study 243 AF patients (median age 69 years) were assessed. Serum levels of 8‐isoprostane, a marker of oxidative stress, along with plasma fibrin clot permeability (Ks), clot lysis time (CLT), endogenous thrombin potential (ETP), von Willebrand factor (VWF) were measured at baseline. Stroke or documented transient ischemic attacks (TIA), major bleeding, and death were recorded during a median follow‐up of 53 months while on anticoagulation, including 70.4% on direct oral anticoagulants. The study was approved by the Ethical Committee and conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Results: Increased 8‐isoprostane levels were observed in women, in patients with arterial hypertension, and those with paroxysmal or persistent AF. Patients with 8‐isoprostane levels ≥559 pg/ml (top quartile) compared with those with 8‐isoprostane < 250 pg/ml (bottom quartile) had 20% higher fibrinogen, 8% lower VWF concentrations, along with 10% lower Ks with no difference in CHA2DS2‐VASc score, CLT or ETP. Patients, who experienced thromboembolic events (n = 20, 1.9% per year) had 49% higher 8‐isoprostane concentrations compared to those free of such events (P < 0.01). Levels of 8‐isoprostane >459 pg/ml based on the optimal cut‐off value were associated with the thromboembolic events during follow‐up (hazard ratio 2.87, 95% confidence interval 1.17‐7.03, P = 0.02). There were no associations between 8‐isoprostane levels and major bleeding (2.0%/year) or all‐cause mortality (1.9%/year).
Conclusion(s): Increased 8‐isoprostane levels in AF patients are predictive of thromboembolic events despite anticoagulant therapy. We showed that enhanced oxidative stress in AF is associated with prothrombotic features which might explain its association with ischemic cerebrovascular events on anticoagulation.
VPB0503
Real‐ world effectiveness and safety of rivaroxaban in patients with non‐valvular atrial fibrillation and venous thromboembolism in Saudi Arabia
F. Alqahtani 1; O. Alosaimi2; S. Alqahani2; B. Balkhi2; m. alqahtani2; F. Alzamel2; A. Alhossan3; Y. Asiri3; A. AlHarbi2; H. Albaker2
1 King Saud Univesrity, Dammam‐Alkhobar, Ar Riyad, Saudi Arabia; 2 King Saud Univesrty, ryidh, Ar Riyad, Saudi Arabia; 3 King Saud Univesrty, ryide, Ar Riyad, Saudi Arabia
Background: Real‐world data on the safety and effectiveness of factor Xa inhibitor (rivaroxaban) among patients with non‐valvular atrial fibrillation and venous thromboembolism is scares.
Aims: Examine the safety and effectiveness of rivaroxaban in the largest academic center in the Kingdom of Saudi Arabia.
Methods: A retrospective observational study designed to examine the prescribing pattern, safety, and real world effectiveness of rivaroxaban in patients with NVAF/ and VTE Data on rivaroxaban prescriptions were collected analyzed. Bleeding outcomes were defined as per the ISTH definition
Results: A total of 2316 patients. The mean age is 61 years (±17.8) with 55% above the age of 60. .58% were females. The most common indication was VTE followed by NVAF. 23% of the patients were discharged on a 15 mg total daily dose. The incidence rate of recurrent thrombosis and recurrent stroke was 0.2%. The incidence rate of major bleeding was 1.1%. More than 50% of the patients who had major bleeding were on a 20 mg daily dose of rivaroxaban. 48% of the patients who had major bleeding have a high risk for bleeding according to HAS‐BLED Score (>2 scores).
Conclusion(s): In this real‐life cohort study, rivaroxaban prescribing is not consistent with the prescrining label or phase 3 randomized clinical trial data. However, it appears that the efficacy of the 20 mg dose is better than pivotal trial data for patients with VTE and NVAF. Moreover, in real world setting, rivaroxaban was associated with a lower risk of safety events such as recurrent thrombosis, recurrent stroke, MI, major bleeding, and non‐major bleeding in patients with VTE and NVAF. Key words: real‐world, rivaroxaban, VTE, stroke, major bleeding, non‐major bleeding
PB0501
Risk of anticoagulant‐related nephropathy early after initiating treatment
G. Tratar 1; T. Vižintin Cuderman2; A. Škoberne3; A. Koželj4; V. Lapuh4; L. Rozman4
1 University Medical Centre Ljubljana, Ljubljana, Ljubljana, Slovenia; 2 University Medical Centre Ljubljana, Department of Vascular Diseases, Ljubljana, Ljubljana, Slovenia; 3 University Medical Centre Ljubljana, Department of Nephrology, Ljubljana, Ljubljana, Slovenia; 4 University of Ljubljana, Faculty of Medicine, Ljubljana, Ljubljana, Slovenia
Background: Anticoagulant‐related nephropathy (ARN) is a potentially serious complication in patients receiving anticoagulant treatment. Retrospective studies indicate that ARN occurs in up to 20 % of patients exposed to supratherapeutic INR when treated with vitamin K antagonists but little is known about ARN in direct oral anticoagulants.
Aims: We wanted to determine the incidence of ARN in patients initiating anticoagulant treatment
Methods: We included consecutive anticoagulant‐naive patients referred to our anticoagulation clinic. The trial was approved by the national ethical committee and informed consent was obtained prior to inclusion. The choice of anticoagulant was left to the treating physician. Before initiation of therapy and after 4‐6 weeks of treatment, urine and blood samples were collected. We defined ARN as a rise in serum creatinine by at least 27 μmol/L or by at least 50 % after initiation of anticoagulant therapy. We also followed concentration of cystatin C and presence of proteinuria and erythrocyturia as markers of renal damage.
Results: We included 198 consecutive patients. All patients required treatment for atrial fibrillation. Patients received apixaban (57%), rivaroxaban (24%), dabigatran (15%) or warfarin (4%). Results are shown in Table 1. None of the patients developed ARN. The only significant difference was a slight rise in concentration of cystatin C but the absolute difference was negligible.
Conclusion(s): ARN appears to be rare in the first weeks after initiating anticoagulant treatment.

PB0497
Educational intervention effectiveness evaluated with time in therapeutic range in patients with atrial fibrillation and low educational level and social vulnerability in Corrientes Argentina
C. Giumelli; F. Francisco; J. Parras; M. Medina
Instituto de Cardiología de Corrientes, Corrientes, Corrientes, Argentina
Background: Atrial fibrillation (AF) is the most prevalent arrhythmia in adults and is related with an increased risk of stroke. Oral anticoagulation treatment, with vitamin k antagonists (VKA) significantly reduced the risk of stroke, but require adequate percentage of time in therapeutic range (TTR), greater than 65%. Limited knowledge of their condition and therapy may contribute to not reach this objective. Social and educational vulnerability defined as illiterated or less than 7 years school education and/or very low incomes (USD 250) are predictors for low TTR.
Aims: Evaluate the impact of educative videos to improve the TTR in patients with social and educational vulnerabilities
Methods: We compared the effect of an educational intervention with usual care in patients with nonvalvular atrial fibrillation (NVAF) treated with VKA. Prospective pre‐post study, comparing ”usual care management” care versus ”patient education”, using 4 educational videos containing information on the AF, symptoms, treatment, importance of adhering to recommendations and therapy followed by interviews with a doctor emphasizing the contents. They were carried out individually, and on two occasions the accompaniment of a cohabitant was requested. The primary endpoint was TTR and the secondary endpoint included knowledge.
Results: From 131 patients,110 were analysed. (see flowchart). The mean age was 71.3 ± 9.5 years, 56.5% male, the average CHA2DS2‐VASc 3.7 and 48.7% only had complete primary education with 10.4% illiterate. Characteristics Table 1. The mean pre‐intervention TTR was 61% and the post‐intervention was 77% (I95% CI 3‐28%) with P = 0.0053 that was maintained for a mean time of 300 days. The Hendriks questionnaire was carried out to evaluate knowledge that showed good performance in questions related to anticoagulant therapy and limiting questions related to symptoms and therapy directed to arrhythmia.
Conclusion(s): The use of videos as an educational intervention was effective in improving the TTR iin a population with socio‐educational vulnerability.


PB0500
Frequency, reasons and duration of hospitalization in patients with end‐stage kidney disease on hemodialysis: Prospective results of VIVALDI
D. Steiner 1; C. Ay2; A. Beyer2; S. Schmaldienst3; R. Klauser‐Braun4; M. Lorenz5; I. Pabinger2; M. Säemann6; O. Königsbrügge1
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 3 Clinic Favoriten, Vienna, Austria, Viennna, Wien, Austria; 4 Clinic Donaustadt, Vienna, Austria, Vienna, Wien, Austria; 5 Vienna Dialysis Centre, Vienna, Austria, Vienna, Wien, Austria; 6 Clinic Ottakring, Vienna, Austria, Vienna, Wien, Austria
Background: Patients with end‐stage kidney disease on hemodialysis (HD) suffer from frequent complications requiring hospitalization, causing substantial burden for patients and healthcare systems.
Aims: We aimed to assess frequency, reasons, and duration of hospitalization in HD patients and their association with atrial fibrillation (AF) and use of anticoagulation.
Methods: HD patients were recruited into a prospective cohort study (VIVALDI) after providing informed consent. The association of AF, anticoagulation, and time‐in‐therapeutic range (TTR) of phenprocoumon treatment with hospitalizations was analyzed using negative binomial regression.
Results: The analysis includes 625 patients with a median (25th‐75th percentile) observation time of 3.4 (2.9‐3.5) years. 238 (38.1%) patients had AF. Median number of hospitalizations per patient was 3 (1‐5) and median duration per hospitalization was 7.9 days (4.8‐12.9). The incidence rate of hospitalization was 1.7 per patient year in all patients and 1.9 in AF patients. Most frequent reasons for hospitalization were vascular access complication/intervention, infection/fever, and peripheral artery disease/amputation, while bleeding events and arrhythmia comprised 6.0% and 3.3% of all hospitalizations, respectively (Table 1). Patients with AF had 27% higher risk of hospitalization than non‐AF patients (incidence rate ratio [IRR] 1.27, 95% confidence interval [CI] 1.10‐1.47, p = 0.001). In AF patients, anticoagulation (phenprocoumon or enoxaparin, 41.6% of AF patients) was associated with an increased risk of all‐cause (IRR 1.38, 95%CI 1.14‐1.69, p = 0.001) and bleeding‐related hospitalization (IRR 1.96, 95%CI 1.06‐3.63, p = 0.031). There was no association between anticoagulation and stroke‐related hospitalization. In AF patients on phenprocoumon, increasing TTR was associated with significantly decreased risk of all‐cause (IRR 0.35, 95%CI 0.14‐0.87, p = 0.025), but not bleeding‐related hospitalization (IRR 0.13, 95%CI 0.01‐1.38, p = 0.09).
Conclusion(s): In HD patients, presence of AF and anticoagulation in AF patients were associated with a higher risk of all‐cause hospitalization, including bleeding related hospitalization in the latter. Increasing TTR in phenprocoumon patients lowered the risk of all‐cause, but not bleeding‐related hospitalization.

PB0502
Prediction of bleeding risk in patients with cancer receiving anticoagulants
E. Trinks‐Roerdink 1; G. Geersing1; F. Rutten1; S. van Doorn2
1 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 2 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: Cancer patients are frequently treated with anticoagulants given their increased risk of thrombosis. Nevertheless, when anticoagulated, they are at an increased risk of bleeding due to cancer‐related factors as well.
Aims: The aim was to externally validate existing bleeding risk models in patients with cancer receiving anticoagulants.
Methods: Based on clinical applicability and availability of predictors in our dataset, the following bleeding risk models were selected for external validation: VTE‐BLEED, HAS‐BLED, ORBIT, and ATRIA. The validation cohort was derived from routine healthcare data from general practices in the Netherlands. Patients with a new cancer episode during anticoagulant treatment – either a vitamin K antagonist, a direct oral anticoagulant, low molecular weight heparin or heparin indicated for either venous thromboembolism (VTE) or atrial fibrillation (AF) – were included. The outcome of this study was the composite of clinically‐relevant non‐major (CRNM) bleeding or major bleeding.
Results: The validation cohort included 1106 cancer patients. The mean age was 73.6 ± 11.2 years and 532 (48.1%) patients were males. In total, 310 patients (28%) had VTE, 753 patients (68%) AF and 43 patients (3.9%) both an AF and VTE. During a maximum of 3 years of follow‐up, 123 patients (11.1%) developed a CRNM or major bleeding (incidence rate of 6.3 per 100 person‐years (95% CI 5.2 ; 7.5)). No model allowed for calculation of calibration. The c‐statistics of the models varied between 0.59 and 0.61.
Conclusion(s): Bleeding is common in patients with cancer receiving anticoagulants. The predictive performance of existing bleeding risk models was poor to moderate. Updated or newly derived models are needed to accurately predict bleeding risk in this vulnerable population.
Cardiovascular Risk Factors
PB0511
Model of P2RY12 ‐T‐744C, (GPIbα) ‐ C482T, NOS3 ‐ T‐786C, MTHFR ‐ C667T gene interactions in patients with high cardiovascular risk
A. Esmaeil; E. Shorikov; D. Shorikova; O. Khukhlina; P. Shorikov
Bukovinian State Medical University, Chernivtsy, Chernivets'ka Oblast', Ukraine
Background: Multifactorial vascular damage is reflected by the presence of a significant number of biochemical markers, including a variability of platelet reactivity. The association of the haplotype of H2 presence with peripheral arterial damage due to gene interactions is undetermined.
Aims: to present the model of P2RY12 ‐T‐744C, (GPIbα) ‐ C482T, NOS3 ‐ T‐786C, MTHFR ‐ C667T gene interactions in patients with high cardiovascular risk.
Methods: The study included 100 patients with hypertension and diabetes mellitus type 2 (main group) with defined macroangiopathies and 50 healthy people (control group). The distribution of allelic polymorphisms was investigated by isolation DNA from leukocytes and polymerase chain reaction (PCR). For statistical analysis of the obtained results we used softwares MDR 3.0.2 (USA) and GMDR 0.7 (USA).
Results: To evaluate epistatic or complementary interaction of the genes we modeled the interaction of nucleotide polymorphisms by the method of multifactorial dimensional reduction (MDR). According to the analysis of this method in the sample of sick and healthy people, the optimal models of combinations of gene interactions were determined, with the establishment of their accuracy and reproducibility. As a result, we selected 2 models with the highest degree of reproducibility (10/10 ‐ 100%) ‐ for three loci of polymorphism and for four loci of polymorphism, Table I. The MDR method, when constructing a model of interaction, additionally defines classification rules (if‐then rules) for combinations of genotypes for assigning carriers of established combinations to the main or control group, which will allow to predict the probability of vascular complication, table II.
Conclusion(s): The risk of macrovascular complications in patients with hypertension and diabetes mellitus type 2 associated with allelic polymorphism depends not only on the type of monolocus polymorphism, but has a more complex nature of dependence due to intergenic multidirectional interactions.


PB0512
Associations of platelete P2y12 receptor polymorphism with macroangiopathies in patients with high cardiovascular risk
D. Shorikova; E. Shorikov; P. Shorikov; A. Esmaeil; O. Khukhlina
Bukovinian State Medical University, Chernivtsy, Chernivets'ka Oblast', Ukraine
Background: According to Aga Q.A.A. et al., the contribution of genetic factors to the variability of platelet reactivity is about 30%. Functional evaluation of the effect of polymorphic variants of the GRIbα gene on the rate of thrombosis was performed only in some small studies. Unndifferentiated the role of P2Y12 receptor polymorphism with macroangiopathies.
Aims: to establish the role of allelic polymorphisms P2RY12‐‐744C, (GPIbα)‐C482T in the development of vascular lesions in patients with arterial hypertension and diabetes mellitus type 2.
Methods: The study included 100 patients with hypertension and diabetes mellitus type 2 (main group) and 50 healthy people (control group). Patients underwent echocardiography, color duplex scanning of extracranial, brachiocephalic and femoral vessels. The distribution of allelic polymorphisms was investigated by isolation DNA from leukocytes and polymerase chain reaction (PCR).
Results: A monolocus analysis showed the presence of changes between the main and control groups (p < 0.05). The frequency distribution of alleles of the platelet glycoprotein receptor gene (C482T, GP1bα) was more uniform than the alleles of the P2RY12 receptor. Thus, the 482C allele was found in 90% of patients (its frequency in the group – 47.0 ± 1.8%), and the 482T allele ‐ in 96% of patients (total frequency in the group – 5.0 ± 1.8%). However, the analysis of associations of the C482T polymorphic locus of the GPIbα gene revealed a probable excess of the T allele frequency in patients with macroangiopathies (OR = 3.29 in the trend test, increasing the degree of heterozygosity for the allele " risk ", increase in the degree of allelic positivity (p < 0.001)).
Conclusion(s): Thus, based on data analysis, it can be assumed that allelic polymorphisms of GPIbα gene can be considered as genetic markers of markers associated with vascular damage in the general cohort of patients with combined hypertension and type 2 diabetes.

VPB0517
Relationship of decreased levels of omentin‐1 with prothrombotic state in coronary artery disease
O. Korzh; Y. Fylenko; S. Krasnokutskiy
Kharkiv Medical Academy of Postgraduate Education, Kharkiv, Kharkivs'ka Oblast', Ukraine
Background: Recent data suggest that various adipocytokines are dysregulated in coronary artery disease. Omentin‐1 has been linked to obesity, type 2 diabetes mellitus, inflammation and atherosclerosis.
Aims: The purpose of this study was to examine the relationship between omentin‐1 and prothrombotic factors in patients with coronary artery disease.
Methods: We consecutively evaluated 195 patients, who presented with coronary artery disease. We measured anthropometric and biochemical variables including renal function, plasma lipid profile, fasting glucose, insulin and C‐peptide, PAI‐1, prothrombin fragment 1+2 (F1+2), and D‐dimer as markers of a prothrombotic state, and C‐reactive protein (CRP) as a marker of proinflammatory state. The Homeostatic Model Assessment (HOMA) index was calculated as an index of sensitivity to insulin.
Results: Coronary artery disease patients had significantly lower levels of omentin‐1, D‐dimer, PAI‐1, F1+2 compared with healthy men. On univariate regression analysis, omentin‐1 was negatively related with PAI‐1 (r = ‐0.173, P < 0.05) and duration of diabetes (r = ‐0.141, P < 0.05), BMI (r = ‐0.214, P < 0.01), HOMA index (r = ‐0.347, P < 0.001), CRP (r = ‐0.375, P < 0.001), triglycerides (r = ‐0.193, P < 0.01) and LDL‐cholesterol (r = 0.190, P < 0.01), and directly related with HDL‐cholesterol (r = 0.179, P < 0.01). A multivariate analysis indicated that omentin‐1 levels are independently related with D‐dimer (β=0.203, P < 0.01), PAI‐1 and duration of diabetes (respectively: β=0.195 and β=0.157, both P < 0.05), LDL cholesterol (β = 0.164, P < 0.05), HOMA index (β = 0.236, P < 0.001), and CRP (β=0.278, P < 0.001).
Conclusion(s): These results indicate that a prothrombotic state is associated with reduced omentin‐1 level and duration of disease, insulin resistance and a proinflammatory status in coronary artery disease. These results suggest that decreased omentin‐1 levels in coronary artery disease may be novel biochemical risk factors for atherothrombotic complications, promoting to procoaculant reactions.
PB0509
Dual antiplatelet therapy is associated with high α‐tubulin acetylation in platelets from coronary artery disease patients
V. Robaux 1; S. Kautbally2; A. Ginion1; M. Dechamp3; S. Lejeune2; L. Bertrand1; S. Horman4; C. Beauloye5
1 UCLouvain, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 2 Cliniques Universitaires Saint‐Luc, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 3 Cliniques Universitaires Saint‐Luc and UCLouvain, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 4 Université catholique de louvain, Louvain‐La‐Neuve, Brabant Wallon, Belgium; 5 UCLouvain and Cliniques Universitaires Saint‐Luc, Brussels, Brussels Hoofdstedelijk Gewest, Belgium
Background: Platelet inhibition is the main treatment strategy to prevent atherothrombosis. Despite dual antiplatelet therapy (DAPT) combining aspirin and a P2Y12 receptor inhibitor, high platelet reactivity persists in some patients due to poor response to treatment and is associated with ischemic risk. It remains unknown if circulating platelets in high‐risk patients have different morphological characteristics which could participate in their pro‐thrombotic potential.
Aims: We postulated that α‐tubulin acetylation, a cytoskeletal modification known to regulate platelet shape change, could reflect circulating platelet reactivity and impact their morphology.
Methods: We collected arterial blood samples from 187 patients admitted for coronary angiography. Platelet reactivity was assessed in whole blood using multiplate analysis. Platelets were then isolated to evaluate α‐tubulin acetylation level by western blotting. 141 patients were taking aspirin among which 32 were treated with an additional P2Y12 inhibitor. DAPT didn’t achieve sufficient platelet inhibition in 8 out of 32 patients. Participants provided written informed consent and the study was approved by the institutional ethics committee.
Results: Platelet α‐tubulin acetylation was significantly increased in DAPT‐treated patients (p < 0.001). A minority of non‐treated patients (11.4%) exhibited high α‐tubulin acetylation (third acetyl α‐tubulin tertile) whereas a high level was observed in most patients on efficient DAPT (83.3%). Interestingly, the proportion of patients with high acetyl α‐tubulin (37.5%) level was drastically decreased in clopidogrel non‐responders. Multivariate logistic regression further supported that efficient DAPT is independently associated with a 27‐fold increase in the likelihood of being in the highest acetyl α‐tubulin tertile (p < 0.001).
Conclusion(s): We revealed high platelet α‐tubulin acetylation as a potential marker of efficient platelet inhibition by dual antiplatelet therapy. Since α‐tubulin acetylation is a hallmark of stable microtubules, its increase could contribute to maintaining resting morphology of circulating platelets.
VPB0519
Extracellular vesicles number and cell subtype may be influenced by diabetes mellitus and metformin in patients at high cardiovascular risk
P. Simeone 1; R. Liani2; G. Bologna2; R. Tripaldi2; A. Di Castelnuovo3; D. D'Ardes2; S. Miscia2; F. Cipollone2; M. Marchisio2; A. Consoli4; P. Lanuti2; F. Santilli4
1 Department of Medicine and Aging, and Center for Advanced Studies and Technology (CAST), Via Luigi Polacchi, Chieti, Italy, Pescara, Abruzzi, Italy; 2 Department of Medicine and Aging, Center for Advanced Studies and Technology (CAST), Chieti, Italy;, Chieti, Abruzzi, Italy; 3 Mediterranea Cardiocentro, Napoli, Italy, Napoli, Campania, Italy; 4 Department of Medicine and Aging, Center for Advanced Studies and Technology (CAST), Chieti, Italy;, Pescara, Abruzzi, Italy
Background: Extracellular vesicles (EV) represent a population of small vesicles deriving from all types of cells, generated by the extroflection of the plasma membrane, and released into the circulation. EV can have both pro‐ and anti‐atherothrombotic effects depending on the clinical setting, origin cell, stimuli, and different treatments might affect their levels.
Aims: The primary endpoint of our study was to compare the amount of circulating EV and specific EV subtypes derived from platelets, endothelial cells, and leucocytes in subject at high CV risk, with and without T2DM, and if any ongoing anti‐diabetic drugs could affect the levels of EV.
Methods: Fifty‐nine type 2 diabetes mellitus (T2DM) patients and forty patients without T2DM with or without prior vascular disease, were enrolled, this was a cross‐sectional study, extracellular vesicles were evaluated by flow cytometry analysis.
Results: After propensity score matching the levels of total EV (Cohen d = 0.55, p = 0.064), total Annexin+ EV (d = 0.49, p = 0.043), EV CD41a+ platelet derived EV (d = 0.51, p = 0.014), CD41a+/AnnexinV+ platelet derived EV (d = 0.50, p = 0.019) and CD45 leukocyte derived EV (d = 0.19, p = 0.012) were lower in diabetic patients vs. those without T2DM. Interestingly, platelet‐derived EV positive for AnnexinV were lower in patients on treatment with metformin (d = 0.72, p = 0.012).
Conclusion(s): Our study showed that, among high CV risk patients, treated with the state‐of‐the‐art preventive strategies, T2DM is associated with lower levels of total, platelet‐and leucocyte‐derived EV, and that this difference may be accounted for, at least in part, by ongoing treatment with metformin, the most widely prescribed oral antidiabetic agent.

PB0507
Insulin resistance links to procoagulant clot properties in young adult with type 1 diabetes: a cross‐sectional observation study
N. Kietsiriroje 1; S. Pearson2; R. Sagar2; R. Ariëns3; R. Ajjan2
1 University of Leeds, UK; Prince of Songkla University, Thailand, Headingley, Leeds, England, United Kingdom; 2 University of Leeds, Leeds, England, United Kingdom; 3 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom
Background: Individuals with Type 1 diabetes (T1D) and concomitant insulin resistance (IR) have a higher risk of vascular complications. Previous work has shown that fibrin clot properties can determine vascular risk.
Aims: We investigated the fibrin network in individuals with T1D and different degrees of IR, measured as estimated glucose disposal rate (eGDR).
Methods: A validated turbidimetric assay was employed to study plasma‐derived fibrin clot properties in 47 individuals with T1D. HbA1c, waist circumference, and presence of hypertension were used to calculate eGDR using an established formula. Patients were categorised into eGDR tertiles and plasma clot maximum turbidity and time to 50% lysis were assessed in each group. In addition to plasma clots, we investigated the effects of IR on clots made from purified fibrinogen to understand whether IR induces qualitative changes in fibrinogen that results in altered clot characteristics.
Results: Plasma clot maximum absorbance, a measure of clot density, was highest in the low eGDR tertile compared to the middle (T2) and high tertiles (T3) (0.22+/‐0.07, 0.19+/‐0.04 and 0.17+/‐0.04 AU, respectively; p = 0.004, T1 vs T3). Moreover, clot lysis time, an indicator of fibrinolysis potential, was longest in low eGDR tertile compared with middle and high (588+/‐163, 523+/‐104 and 470 ± 87 sec, respectively; p = 0.02, T1 vs T3). Clots made from fibrinogen (1 mg/ml) purified from pooled plasma of individuals with eGDR < 7 were denser than those with eGDR 7‐7.9 or eGDR>8 (23.3+/‐1.9, 22.4+/‐1.1 and 22.1+/‐1.0 fibers/100μm; p = 0.02, eGDR < 7 vs eGDR>8), analysed using confocal microscopy.
Conclusion(s): IR is associated with procoagulant clot properties in individuals with T1D due, at least in part, to qualitative changes in fibrinogen. This may represent one mechanism for increased vascular risk in T1D individuals with concomitant IR.

VPB0516
Lp(a) increase calcium phosphate aortic valve mineralization in patients with a previous myocardial infarction
A. de la Peña1; M. Flores‐Garcia 2; G. Cardoso‐Saldaña3; V. Herrera3; C. Linares‐Lopez4; J. Villegas‐Hernandez5; R. Ledezma6; M. Peña‐Duque5; M. Martinez‐ Rios5; E. Angles‐Cano7
1 Universidad Nacional Autonoma de Mexico/Instituto Nacional de Cardiologia, Mexico City, Distrito Federal, Mexico; 2 Instituto Nacional de Cardiología Ignacio Chávez, CDMX, Distrito Federal, Mexico; 3 Instituto Nacional de Cardiologia, CDMX, Distrito Federal, Mexico; 4 Universidad Nacional Autónoma de México, CDMX, Distrito Federal, Mexico; 5 Instituto Nacional de Cardiología, CDMX, Distrito Federal, Mexico; 6 Universidad Whesthill, CDMX, Distrito Federal, Mexico; 7 INSERM, Paris, Ile‐de‐France, France
Background: Lp(a) is composed by apolipoprotein(a), attached to the apolipoprotein B segment of an LDL‐like particle. Apo(a) has sequence homology with plasminogen, inhibiting fibrinolysis and causing atherothrombosis, infarction and ischaemic stroke..Apo(a) increases calcific valvular aortic stenosis through endothelial dysfunction and pro‐inflammatory responses, and pro osteogenic effects promoting calcification. X‐ray diffraction electron microscopy allowed the direct study of all the minerals found in the aortic valve. In addition to calcium, other minerals, whose chemical composition is associated with the mineralisation process, were present in the aortic valve stenosis. This approach may shed light to the Lp(a) promoted mineralization.
Aims: To identify the correlations between the plasma concentration of Lp(a) and the concentration of minerals deposited in the aortic valve in patients with aortic stenosis.
Methods: We conducted a cross‐sectional analytical study that included 68 patients, aged 19‐81 years old, both sexes, diagnosed with aortic stenosis, who underwent aortic valve replacement surgery at the Ignacio Chávez National Institute of Cardiology. Plasma concentration of Lp(a) was obtained by a nephelometric method. Aortic valves were preserved in 10% formaldehyde and conveniently prepared for X‐ray diffraction electron microscopy study. This study was approved by the INC ethics committee, followed all the ethical principles of the Declaration of Helsinki, and the participants provided their written informed consent. Statistical tests were used for parametric and non‐parametric variables, as appropriate, and Spearman's Rho.
Results: Aortic valve calcium as well as serum Lp(a), were 1.22, and 4 times higher in people with a previous myocardial infarction. Also only in those with a previous myocardial infarction a negative association with chlorus is observed.
Conclusion(s): High Lp(a) levels are associated with calcium deposition in the aortic valve.


PB0508
Differential quantitative proteomics reveals potential molecular mechanisms responsible for platelet hyperactivity and increased thrombotic risk in diabetes mellitus patients
R. Haghiri Limoudehi1; N. Wolska2; M. Moritz3; H. Voss3; H. Schlüter4; G. Pula 5
1 University Medical Center Hamburg‐Eppendorf (UKE), Hamburg, Hamburg, Germany; 2 University Medical Center Eppendorf (UKE), Hamburg (Germany), hamburg, Hamburg, Germany; 3 University Medical Center Eppendorf (UKE), Hamburg (Germany), Hamburg, Hamburg, Germany; 4 University Medical Center Hamburg Eppendorf, Hamburg, Hamburg, Germany; 5 University Medical Center Eppendorf Hamburg (UKE), Hamburg, Hamburg, Germany
Background: Diabetes mellitus (DM) is a common metabolic disease associated to increased cardiovascular risk. An increase in the responsiveness of platelets plays a key role in the onset and progression of cardiovascular diseases in DM patients, but the underlying molecular mechanisms remain to be elucidated.
Aims: Using differential quantitative bottom‐up proteomics and traditional biochemistry techniques, we aimed to identify the molecular events increasing the responsiveness of platelets in diabetes patients.
Methods: Peripheral blood samples from diabetes patients were obtained from the University Medical Center Eppendorf (Hamburg) and were selected based on glycated haemoglobin (HbA1c): 12 DM patients were selected for HbA1c values > 7.0% (hyperglycaemia), while 12 matching healthy controls were chosen with HbA1c < 6.0% (euglycaemia). Washed platelets were isolated by centrifugation and lysed by ultrasonication. After reduction and alkylation of cysteine residues, proteins were tryptic digested to peptides. Obtained raw data from the LC‐MS/MS measurement of peptides were processed with MaxQuant. Further statistical analysis was performed with Perseus Software.
Results: 1,552 platelet proteins were identified, of which 60 displayed levels statistically different in DM patients compared to healthy controls (44 upregulated, 16 downregulated). A subgroup of 21 upregulated proteins showed a level increase of 50% or more compared to healthy controls. Amongst proteins significantly upregulated in DM patients, the gene ontology analysis highlighted protein involved in intracellular trafficking, protein turnover, lipid metabolism and cell signalling.
Conclusion(s): Our proteomics analysis highlights some important differences in the biochemical make‐up of platelets, which are likely to have important functional consequences. Amongst the proteins expressed at significantly different level in DM patients, we aim to identify the causes of platelets hyperactivity. These proteins have the potential to become pharmacological targets for the development of novel antiplatelet drugs specifically designed for DM patients.

PB0513
Platelet P2Y12 receptor polymorphism and gene‐gene interactions in patients with high cardiovascular risk
P. Shorikov; D. Shorikova; E. Shorikov; A. Esmaeil; O. Khukhlina
Bukovinian State Medical University, Chernivtsy, Chernivets'ka Oblast', Ukraine
Background: Phenotypic manifestations of platelet aggregation may be modified by allelic polymorphism of genes associated with platelet functions and increases the risk of vascular complications. In the studies have shown that the occurrence of H2‐haplotype is associated with increased platelet ADP dependent aggregation with different concentrations of inducers, which may be due to increased platelet ADP receptor expression.
Aims: to establish gene‐gene interactions allelic between polymorphisms P2RY12‐‐744C, (GPIbα)‐C482T, NOS3 ‐ T‐786C, MTHFR ‐ C667T, P2RY12 ‐T‐744C, (GPIbα) ‐ C482T in patients with hypertension and diabetes mellitus type 2.
Methods: The study included 100 patients with hypertension and diabetes mellitus type 2 (main group) with defined macroangiopathies and 50 healthy people (control group). The distribution of allelic polymorphisms was investigated by PCR. For statistical analysis of the obtained results we used DeFinetti online programs (https://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
Results: According to this model, in the main and control group for the GPIbα gene the frequency of "risk" alleles was 53% and 38%, for the P2RY12 gene ‐ 36% and 28%, for the NOS3 gene ‐ 51% and 34%, for the MTHFR gene ‐ 46% and 33%, with a total median frequency of 48.5% for the main group and 33.5% for the control group. On average, there were 1.33 alleles per person in the control group, and 1.86 alleles in the main group, which was in 1.42 folds greater (t = 3.07; p = 0.022; p = 0.04 by median Mood test). Thus, in contrast to the single‐locus model of allelic polymorphism, carriers of "risky" allelic polymorphisms simultaneously for all 4 genes significantly (p < 0.05) increases the likelihood of macrovascular complications (Table).
Conclusion(s): The risk of macrovascular complications in patients with hypertension and diabetes mellitus 2 is a measure of the effect of a combination of polymorphisms of the four so‐called "risky" alleles.

VPB0514
LMWH safety and efficacy in pregnancy with rheumatic heart disease patients with heart valves replacement
F. Alqahtani 1; N. Alghamdi2; L. allehyani2; H. Alosaimi2; W. Almutairi2
1 King Saud Univesrity, Dammam‐Alkhobar, Ar Riyad, Saudi Arabia; 2 King Saud Univesrty, ryidh, Ar Riyad, Saudi Arabia
Background: The risk of thromboembolic complications is higher in pregnancy than at other times especially in Rheumatic heart disease patients who underwent heart valve replacement. Thus, maintenance of therapeutic anticoagulation during pregnancy is needed to diminish the chance of thromboembolic complications.
Aims: Investigated the efficacy and safety of LMWH in the management of RHD‐related valvular disease management in pregnant women.
Methods: A retrospective study was conducted at King Saud Medical City between January 2011‐ February 2020. All pregnant women with rheumatic heart disease and who had heart valve replacements were reviewed in this study. The reviewed patients were on one of the following regimens: LMWH, LMWH plus warfarin, or Unfarctinated heparin plus warfarin. Primary endpoints were thromboembolism, hemorrhagic complications, and fetal outcomes.
Results: A total of 744 pregnancies in 149 women were identified. The mean age ± SD of the women was 43.8 ± 12 years. Eighty‐six women (58%) were on LMWH regimen, thirty‐five women (23%) were on LMWH and warfarin regimen, and twenty‐eight women (19%) were Unfarctinated heparin and warfarin regimen. Overall, thromboembolic compilations developed in five (0.7 %) pregnancies of these, two were in the LMWH group, two were in the LMWH and warfarin group, and one in the heparin and warfarin group. In addition, significant hemorrhage occurred in five pregnancies. Of these, two occurred in the LMWH group, two in the LMWH and warfarin group, and one in the heparin and warfarin group.
Conclusion(s): This study presents the largest retrospective study of LMWH use in pregnant women with RHD and had a valve replacement. This study has assured that LMWH is safe and effective in preventing thrombosis in pregnancy. Further studies including randomized controlled trials investigating the use of LMWH for these indications are encouraged.
VPB0515
Assessing the cardiovascular risk in patients with Systemic Lupus Erythematosus: QRISK and GAPSS scores head to head
A. Barinotti; M. Radin; I. Cecchi; S. Foddai; E. Rubini; D. Roccatello; E. Menegatti; S. Sciascia
University of Turin, Turin, Piemonte, Italy
Background: Assessing the cardiovascular risk in SLE patients is fundamental in the clinical practice. Recently, the QRISK3 score has attempted to encompass for SLE augmented risk by adding items (e.g. corticosteroid use) that are missing in traditional CVD risk‐scores.
Aims: To apply/compare QRISK3 and adjusted Global AntiPhospholipid Syndrome Score (aGAPSS), a validated score to assess CVD risk in aPL‐positive patients, in a cohort of SLE patients.
Methods: 25‐85 years old patients with a confirmed diagnosis of SLE and/or of SAPS were recruited during a period of 6 months (Sep2019–Feb2020). QRISK3 was calculated using the official online calculator (https://qrisk.org/) and aGAPSS was calculated using the validated point values: aCL = 5, aβ2GPI = 4, LA = 4, aPS/PT = 3, hyperlipidemia = 3, hypertension = 1.
Results: The analysis included 142 SLE patients: 34 SAPS (23.9%) and 108 SLE patients without APS(76.1%) [mean age = 48 ± 12.9 (SAPS = 51.6 ± 12.8/SLE without APS = 46.9 ± 12.8)]. Table 1 summarizes patients characteristics. When focusing on cerebrovascular/coronary events, we found a statistical significance for aGAPSS (with event = 10.1 ± 6.2 vs. without event = 5.8 ± 6.1; p = 0.007), but not QRISK3. A significant association was observed between the occurrence of these events and high‐risk aGAPSS: p = 0.03 for aGAPSS≥8, p = 0.01 for aGAPSS≥9, p = 0.008 for aGAPSS≥10. aGAPSS but not QRISK3 strongly correlated with any thrombotic event occurrence, both at the uni‐ and multivariate analysis (univariate: with event = 8.17 ± 7.1 vs. without event = 5.41 ± 5.6; p = 0.012 /multivariate: p = 0.009). Male gender correlated with any thrombotic event occurrence both at uni‐ and multivariate(p = 0.017 and p = 0.03). When focusing on aPL‐profile, regardless the diagnosis, we found a statistical significance only with respect to aGAPSS(aPL+ =9.6 ± 6.3 vs. aPL‐ =4.1 ± 5.1; p < 0.001).
Conclusion(s): Our study showed that aGAPSS is the most valuable tool for assessing CVD risk in SLE patients. Adding the aPL‐profile as an item to the QRISK3 could be a useful strategy to improve it when considering patients with autoimmune diseases.

PB0505
Relation among Brain Derived Neurotrophic Factor, depression, and extracellular vesicles‐derived miRNA – Results from an Italian cohort
S. Barbieri 1; P. Amadio1; C. Macchi2; M. Zarà1; C. Favero2; G. Solazzo2; L. Vigna3; M. Greco2; M. Buoli2; C. Siroti2; A. Ieraci2; M. Ruscica2; V. Bollati2
1 Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy; 2 Università degli Studi di Milano, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Lombardia, Italy
Background: Obesity and depression are intertwined diseases often associated with increased risk of cardiovascular (CAD) complications. Despite modifications in the Brain‐Derived Neurotrophic Factor (BDNF) levels in the brain have been observed in subjects with depression and obesity, the relationships among peripheral BDNF, depression and obesity are not well‐defined, yet. Changes in extracellular vesicle (EV)‐derived miRNAs in blood have been related to obesity, depression and CAD.
Aims: In this study, we investigated the association among BDNF, depression and EV‐derived miRNAs related to atherothrombosis.
Methods: 743 overweight/obese individuals (BMI>25 kg/m2) of the SPHERE cohort were analyzed. A standard biochemical evaluation was assessed. BDNF plasma levels were measured by ELISA, EV‐miRNA isolated from plasma and analysed by TaqMan™ OpenArray™. Depression state was evaluated by the Beck Depression Inventory‐II (BDI‐II).
Results: In multivariate analysis, a negative association between BDNF and depression was found (p = 0.0004), and this relationship was modified by the high levels of inflammatory marker IF1γ, suggesting that a potential protective activity of BDNF is dependent on the high inflammatory levels. From the analysis of EV‐derived miRNAs emerged a clear that BDNF associated with about 80 upregulated and 59 downregulated miRNAs known to be related to cardiovascular diseases. miRNA target analysis showed that 18 of these genes fall in the atherothrombotic dataset. Interestingly, a network between genes involved in coagulation and inflammation specifically PTGS2, MTHFR, TNF‐a, IL6, IL1b, SERPIN1, PLAT, TFPI, TF and BDNF was found.
Conclusion(s): Overall these data provide a robust association among depression score, obesity, indicating and BDNF plasma levels, and support the hypothesis of a critical link between circulating BDNF levels and atherothrombotic risk.
VPB0518
Disentangle the relationship between depression and arterial thrombosis associated with BDNFVal66Met polymorphism: the role of α 2‐adrenergic receptor pathway
L. Sandrini 1; P. Amadio1; A. Ieraci2; M. Alessandro3; J. Werba4; P. Soprano3; A. Balduini5; M. Zarà1; A. Bonomi6; F. Veglia6; G. Colombo7; M. Popoli8; F. Lee9; E. Tremoli10; S. Barbieri1
1 Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy; 2 Università degli Studi di Milano, Milan, Lombardia, Italy; 3 Department of Molecular Medicine, University of Pavia and IRCCS San Matteo Foundation, Pavia, Lombardia, Italy; 4 Atherosclerosis research and prevention Unit, Centro Cardiologico Monzino, IRCCS, Milano, Lombardia, Italy; 5 University of Pavia, Pavia, Italy, Pavía, Lombardia, Italy; 6 Biostatistics Unit, Centro Cardiologico Monzino, IRCCS, Milano, Lombardia, Italy; 7 Immunology and Functional Genomic Unit, Centro Cardiologico Monzino, IRCCS, Milano, Lombardia, Italy; 8 Department of Pharmaceutical Sciences, Università degli Studi di Milano, Milano, Lombardia, Italy; 9 Department of Psychiatry, Weill Cornell Medical College, New York, New York, United States, 10 Maria Cecilia Hospital, Cotignola, Toscana, Italy
Background: Depression represents an independent risk factor for cardiovascular diseases, but the beneficial effects of antidepressants in patients with concomitant depression and cardiovascular events are still under debate. Brain‐Derived Neurotrophic Factor (BDNF)Val66Met polymorphism, related to depression, is associated with arterial thrombosis in mice, and with an increased risk of acute myocardial infarction in humans. Interestingly, BDNFMet/Met mice, as well as CAD patients, have hyper‐reactive platelets overexpressing α2A‐adrenergic receptor (α2A‐ADR), providing that this mouse model is a good tool to study novel strategies for these pathologies.
Aims: To investigate the implication of α2‐ADR pathway in depression and arterial thrombosis associated with BDNFVal66Met polymorphism.
Methods: Rauwolscine or desipramine were administered to wild‐type and BDNFMet/Met mice. Behaviour and carotid artery thrombosis were assessed by novelty‐suppressed feeding and FeCl3 model, respectively. Mouse platelets activation profile was characterized by flow cytometry. To examine the effect of mutation per see, in vitro experiments were performed in Hela and Ea.hy926 cells, and megakaryocytes, differentiated from peripheral blood CD34+ and CD45+ cells of healthy subjects, treated with ProBDNFVal and ProBDNFMet cleavable peptide; and in Hela cells transfected with BDNFVal (HelaVal) and BDNFMet (HelaMet) plasmids.
Results: HelaMet cells as well as both cell types and megakaryocytes treated with proBDNFMet‐peptide displayed a higher susceptibility to thrombosis and norepinephrine was crucial to up‐regulate procoagulant activity and enhance platelet generation. Rauwolscine, a α2‐ADR antagonist, decreased in vitro all the effects related to Met mutation, and in vivo abolished platelet hyper‐reactivity, reduced vascular procoagulant activity, normalized leukocyte, platelet and bone marrow megakaryocyte number, restored physiological norepinephrine levels and prevented arterial thrombosis in BDNFMet/Met mice. Finally, a norepinephrine reuptake‐inhibitor desipramine rescued the prothrombotic phenotype and the behavioural impairments in BDNFMet/Met mice.
Conclusion(s): The α2‐ADR pathway might represent a potential pharmacological target to concomitantly treat depression and reduce platelet hyperactivation and thrombosis in the carriers of the BDNFVal66Met polymorphism.
VPB0520
Assessment of thrombogenesis activity after endovascular treatment of coronary arteries
I. Vasilenko 1; D. Kassina2; G. Shekhyan2; M. Popov2; Z. Kardashova2
1 M.F. Vladimirsky Moscow Regional Clinical and Research Institute (MONIKI), A.N. Kosygin Russian State University, Moscow, Moskva, Russia; 2 M.F. Vladimirsky Moscow Regional Clinical and Research Institute (MONIKI), Moscow, Moskva, Russia
Background: Vascular stenosis is usually accompanied by impaired rheological properties of blood, leading to thrombotic complications. The detection of early stenting can improve the outcomes of stenting.
Aims: The study included 50 patients (23 men, 27 women) aged 49‐75, who were admitted for endovascular treatment. The investigations were performed dynamically: before stenting, 24 hours and 30 days after stenting. The parameters of global thrombodynamic test, platelet count, routine hemostasis indicatorswere assessed.
Methods: The study included 50 patients (23 men, 27 women) aged 49‐75, who were admitted for endovascular treatment. The investigations were performed dynamically: before stenting, 24 hours and 30 days after stenting. The parameters of global thrombodynamic test, platelet count, routine hemostasis indicatorswere assessed.
Results: It was found that compared to the control group, the rate of fibrin clot growth was increased by 32% in the middle‐aged group and by 38% in the elderly group (p < 0.05) in patients with coronary artery disease. The size of the clot after 30 minutes of follow‐up was 19.2% higher than that of the control group in the middle‐aged patients and 23% higher in the elderly patients (p < 0.05). The presence of spontaneous clots was detected in 10% of middle‐aged patients and in 17.5% of elderly patients. 24 hours after the operation a hypercoagulable state was registered in 16% of patients. On the 30th day after the operation a hypercoagulable state was registered in 24% of patients
Conclusion(s): The obtained results allowed us to identify some patients at risk of thrombosis and restenosis. The use of new approaches in clinical and laboratory practice can improve the outcomes of stenting by early diagnosis of hemostasis disorders.
PB0510
Multiple myeloma (MM) is associated with an increased risk of arterial thromboembolic events (ATE)
K. Sanfilippo 1; S. Luo2; A. Leader3; B. Gage4
1 Washington University School of Medicine St. Louis, St. Louis, Missouri, United States; 2 Washington University School of Medicine St. Louis, Saint Louis, Missouri, United States; 3 Rabin Medical Center, Petah Tikva, HaMerkaz, Israel; 4 Washington University School of Medicine St. Louis, St Louis, Missouri, United States
Background: Patients with newly diagnosed cancer have a 12‐month cumulative incidence of ATE of 1‐2% (Mulder et al., Leader et al.). Risk of venous thromboembolism in patients with cancer varies by cancer type. Whether risk of ATE also varies by cancer type in unknown.
Aims: We aimed to evaluate the incidence of ATE in patients with newly diagnosed MM starting treatment and determine if MM patients with ATE had a higher risk of mortality.
Methods: Using a nationwide cohort of US Veterans, we identified patients with newly diagnosed MM between 2006 and 2014 starting MM‐treatment. ATE was defined as incident ischemic stroke and/or myocardial infarction within the first 12 months of MM‐treatment using ICD codes as previously described. We analyzed the association between ATE and survival using a Cox proportional hazards model while adjusting for known confounders.
Results: Of the 2837 patients, mean age was 68.4 years. The mean number drugs included in first‐line therapy was two, and 22% of patients received three or more drugs (dexamethasone counted as one drug). Consistent with the US veteran population, 97.7% of patients were male and 30.4% Black. The 6‐month cumulative incidence of ATE for the cohort was 3.24% (95% CI 2.64‐3.94) and the 12‐month cumulative incidence was 4.83% (95% CI 4.08‐5.66). ATE was associated with a 3‐fold increased risk in mortality at 12 months after adjusting for known prognostic variables (Table 1).
Conclusion(s): In this cohort of 2837 patients with MM, the incidence of ATE was higher than reported in prior studies that included multiple cancer types. ATE in MM is associated with decreased survival after adjusting for potential confounders. Future studies should focus on identifying risk factors associated with ATE in MM as well as strategies to reduce this risk.

PB0506
Patient characteristics associated with arterial thromboembolism in incident hospitalized heart failure patients without other known indications for antithrombotic treatment: A nationwide cohort study
Q. Chen 1; N. van Rein1; M. Toorop2; W. Lijfering3; L. Tops1; S. Cannegieter1
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Leiden University Medical Center, Amsterdam, Noord‐Holland, Netherlands; 3 The Knowledge Institute of the Federation of Medical Specialists, Utrecht, Utrecht, Netherlands
Background: It is controversial whether heart failure (HF) patients in sinus rhythm without prior thromboembolism or known cardioembolic source would benefit from anticoagulation.
Aims: To explore patient characteristics associated with developing arterial thromboembolism in incident hospitalized HF patients without other known indications for antithrombotic treatment.
Methods: With nationwide data from Statistics Netherlands, we identified all adult patients admitted to hospitals with newly developed HF (ICD‐10 code: I50) in 2018, but only included those who were without atrial fibrillation, rheumatic mitral stenosis/mechanical heart valves, venous thromboembolism, myocardial infarction, ischemic stroke/transient ischemic attack/systemic arterial thromboembolism (ATE), and peripheral artery disease within the prior 5 years, and who did not receive antithrombotic agents within the prior 6 months. All included patients were followed for 1 year (or until death), and ATE was the study outcome. Cumulative incidences and hazard ratios of the outcome were estimated by the cumulative incidence competing risk method and Cox regression respectively among patients with different baseline characteristics.
Results: Among ≈41,000 incident HF patients in 2018 in The Netherlands, 6,199 (14.8%) were included (mean age 71.47 ± 15.40 years; 42.9% male). The five most prevalent comorbidities were hypertension (36.0%), other valvular heart disease (22.2%), diabetes (21.1%), chronic obstructive pulmonary disease (19.7%), and abnormal renal function (19.1%). 140 patients developed ATE within 1 year with a cumulative incidence of 2.26% (95% confidence interval 1.91‐2.65%). Old age, male sex, low income, and comorbidities including hypertension, aortic plaque, coagulopathy, and diabetes appeared to be risk factors of developing ATE (see the Figures).
Conclusion(s): The absolute risk of ATE appeared to be relatively low in HF patients who were without other indication for antithrombotic treatment when HF was diagnosed, among whom old age, male sex, low income and several comorbidities appeared to be risk factors.


Cerebrovascular Disorders
VPB0033
Prevention of stroke and other thromboembolic events in primary care: Alarming data from a Brazilian medium‐size city
M. Marcolino1; J. Oliveira1; D. Rios2; T. Pedroso 3; L. Sá4; M. Parreiras Martins5; T. Sales2; A. Ribeiro1
1 UFMG, Belo Horizonte, Minas Gerais, Brazil; 2 UFSJ, Divinópolis, Minas Gerais, Brazil; 3 UFMG, BELO HORIZONTE, Minas Gerais, Brazil; 4 FCMMG, Belo Horizonte, Minas Gerais, Brazil; 5 Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Background: Despite the development and increasingly common use of direct oral anticoagulants, warfarin is still widely used in clinical practice, mostly in low and middle income countries. Health literacy, patient knowledge regarding oral anticoagulation treatment, and medication adherence are essential to achieve the desired therapeutic effect of warfarin, despite their scarce information in clinical practice.
Aims: Evaluate the profile of patients using warfarin, followed up in primary care.
Methods: Consecutive adult (>=18 years‐old) on oral anticoagulation with warfarin being monitored at 27 primary care units in Divinópolis (MG) were recruited between November/2018 and February/2020. Clinical information was collected from medical records, and patients were invited to perform the following tests: Oral Anticoagulant Knowledge test; adaptation of the Treatment Adherence Measure (MAT) to oral anticoagulation; Short Assessment of Health Literacy for Portuguese‐speaking Adults (SAHLPA‐18) and Pharmacotherapy Complexity Index.
Results: Throughout, 162 patients were included (mean age 65 ± 12 years‐old, 55.6% women). The main indications for anticoagulation were non‐valvular atrial fibrillation/flutter (26.5%) and venous thromboembolism (24.1%). In 13.6% of patients, the reason for anticoagulation treatment was not recorded. The MAT‐adapted indicated that 83.3% of the patients were adherent to the treatment. The OAK showed only 11.7% of patients with an adequate level of knowledge about anticoagulant therapy. According to the SAHLPA‐18, 67.9% of patients have inadequate health literacy. In the review of medical records, there was lack of essential clinical information, such as INR results and conduct.
Conclusion(s): This study reveals an alarming scenario in the primary care setting, considering the short therapeutic window of warfarin, its pharmacological complexity, and its food and drugs interactions. Additionally, it was noticed a failure in the public health system, regarding lack of essential information about anticoagulant therapy. Thefore, these results highlighted the insufficient knowledge about warfarin therapy and the poor health literacy of the population assisted by primary care.
PB0030
A population cohort study to evaluate the risk of ischemic stroke among individuals with a new diagnosis of cancer compared to matched cancer‐free controls: impact of prior stroke history
D. Siegal 1; J. Cerasuolo2; M. Carrier3; P. Gross4; M. Kapral5; R. Lun6; M. Shamy6; R. Sutradhar5
1 University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 2 ICES, Hamilton, Ontario, Canada; 3 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 4 McMaster University, Hamilton, Ontario, Canada; 5 University of Toronto/ICES, Toronto, Ontario, Canada; 6 University of Ottawa, Ottawa, Ontario, Canada
Background: The association between cancer and ischemic stroke is not well understood.
Aims: To measure the risk of ischemic stroke in individuals with newly diagnosed cancer compared to cancer‐free controls in two matched cohorts based on prior history of ischemic stroke.
Methods: Using linked health databases in Ontario, Canada (2010 to 2019), we conducted a population‐based cohort study of adults with newly diagnosed cancer matched to controls (1:1) by age and sex. The primary outcome was the incidence of ischemic stroke. Subdistribution adjusted hazard ratios (aHR) were calculated. aHRs were estimated before and after 1.5‐years to account for interactions between time and exposure.
Results: Among 1,241,294 individuals without prior stroke, the rate of ischemic stroke was 4.6/1000 person‐years (95%CI 4.5‐4.7) in cancer patients and 3.5/1000 person‐years (95%CI 3.4‐3.6) in controls (p < 0.0001). At 1.5 years, the risk of ischemic stroke was higher in cancer patients (CIF estimate 0.71% vs. 0.49%; aHR 1.40, 95%CI 1.34‐1.47) (Table 1). At 5 years, the risk of ischemic stroke was lower in cancer patients (CIF estimate 1.60% vs. 1.68%; aHR 0.72, 95%CI 0.69‐0.74). Among 27,848 individuals with prior stroke, the rate of ischemic stroke was 26.9/1000 person‐years (95%CI 25.1‐28.9) in cancer patients and 22.0/1000 person‐years (95%CI 20.7‐23.4) in controls (p < 0.0001). The risk of ischemic stroke was similar at 1.5 years (CIF estimate 3.42% vs. 3.43; aHR 1.00, 95%CI 0.88‐1.14). At 5 years, the risk of ischemic stroke was lower in cancer patients (6.13% vs. 8.40%; aHR 0.53, 95%CI 0.46‐0.62). Figure 1 shows cumulative incidence function (CIF) curves for the overall cohort and cancer subtypes.
Conclusion(s): In individuals without prior stroke, those with newly diagnosed cancer had a 1.4‐fold higher risk of ischemic stroke at 1.5 years versus controls but the risk varied by cancer type. Future analyses will evaluate stroke risk factors, treatments, and outcomes.


PB0026
Decreased levels of factor VIII during acute ischemic stroke predict excellent outcome thirty days after
M. El Horri 1; F. Bemmoussat1; R. Messaoudi2; M. Moueden2; F. El Horri3; D. Benlaldj4; I. Khachaa1; G. Khitri5; A. Chikh Khelifa6; M. Smahi1; K. Benbouhadi1; K. Kebir1; S. Zatir1; L. Benmahdi1; H. Brouk7
1 Regional Military University Hospital of Oran, Oran, Oran, Algeria; 2 University Hospital Center of Oran, Oran, Oran, Algeria; 3 Mixed Hospital of Ras elma, Sidi Bel Abbes, Sidi Bel Abbes, Algeria; 4 University Hospital Center of Oran, Mostaghanem, Mostaganem, Algeria; 5 Regional Military University Hospital of Oran, Tlemcen, Tlemcen, Algeria; 6 Regional Military University Hospital of Oran, Saida, Saida, Algeria; 7 Faculty of medicine, University of Badji Mokhtar of Annaba, Annaba, Annaba, Algeria
Background: Increased levels of factor VIII (FVIII) have been associated with acute ischemic stroke(AIS), but its prognostic value remain controversial until now. That’s why FVIII testing during AIS, seems to be interested and also, can be useful for assessing the chances of recovery which is critical to optimize poststroke care.
Aims: To assess the prognostic value of factor VIII in the prediction excellent outcome.
Methods: The study was carried out on 46 adult patients with acute ischemic stroke. Blood samples were taken on admission, to measure FVIII activity. Neurological deficit of patients was determined according to modified Rankin scale (mRS). The end point of this study was excellent outcome at 30 days defined as mRS: (0 –1), during the follow‐up period.
Results: On the admission, elevated levels of FVIII, were associated with severity of AIS according to mRS: mean and IQR values: mRS < 5: 127% (73 – 148%); mRS ≥ 5: 193% (125 – 245%), p = 0.042. Moreover, a positive moderate correlation was found between FVIII levels and mRS at 30 days of follow‐up, with coefficient of Pearson (r = 0.600) and p < 0.0001. FVIII was significantly decreased in patients with excellent outcome (mRS: 0 – 1) comparing to the rest of patients, mean and IQR values: mRS ≤1: 98% (73 – 113%); mRS > 1: 171% (108 – 235%), p < 0.0001. Patients with factor VIII levels below 124% are more likely to have an excellent evolution30 days after AIS, with an Odds Ratio = 20 [CI: 3.72 – 107.65]; p < 0.0001; χ² = 15.97.
Conclusion(s): In patients with ischemic stroke, the decrease in factor VIII activity levels shows good prognosis value concording with excellent functional results at 30 days. These results may be useful both in clinical practice and in research but require validation by further studies.


PB0029
Safe timely resumption of pharmacologic thromboembolism prevention in hospitalized patients with a recent major bleed
H. Sharma; A. Burnett; O. Petrechko; B. Jackson; N. Khan; M. Mason; R. Garcia; C. Bartlett; J. Zimmerberg‐Helms
University of New Mexico Health Sciences Center, Albuquerque, New Mexico, United States
Background: Currently, there are no standardized guidelines for the safe resumption of chronic oral anticoagulation (OAC), or consideration of alternative means to thromboembolism prevention, following spontaneous or traumatic intracerebral hemorrhage (ICH). This is exacerbated by differing or nonexistent resumption protocols between hospitals and can lead to potential adverse events.
Aims: Our needs assessment, utilizing the Plan‐Do‐Study‐Act (PDSA) cycle, will identify deficits in post‐ICH management and opportunities to facilitate anticoagulation stewardship and improve patient management across the continuum of care.
Methods: A secured database was used to collect, de‐identify, and store retrospective data on 103 patients admitted for ICH over a six‐month period. 32 patients were taking OAC at the time of admission, and their charts were reviewed to evaluate common clinical characteristics and management practices such as time to initiation of venous thromboembolism prophylaxis and documentation of plan for OAC resumption, including timing and choice of OAC (Table 1).
Results: 12 patients (37.5%) did not survive hospital admission. Among these fatal cases, ICH was attributed as the primary cause of death in 9 individuals, and 3 deaths were secondary to acquired infection after hemorrhagic stabilization. Of the 20 surviving patients (62.5%), only 9 (45%) had a documented resumption plan in their discharge summary (Figure 1). Five resumed anticoagulation during admission, 3 were referred for left atrial appendage occlusion (LAAO), 2 had no resumption plan or alternative prevention documented at discharge, and 1 had OAC discontinued due to inappropriate prescription. Six (30%) patients did not have follow up scheduled with a neurologist or neurosurgeon.
Conclusion(s): Given the variability in practice seen at our institution, we believe the development of a standardized multidisciplinary post‐ICH protocol is needed. Electronic medical record (EMR)‐based workflow checkpoints and stepwise guidelines will equip clinicians with additional tools to decrease the likelihood of adverse events.


PB0032
Myeloperoxidase‐DNA serum titers and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage
V. Spalart1; P. Hendrix2; J. Oertel2; S. Hemmer2; J. Geisel3; H. Schneider4; K. Martinod1; J. Witsch 5
1 Centre for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 2 Department of Neurosurgery, Saarland University Medical Center, Homburg/Saar, Germany, Homburg, Saarland, Germany; 3 Department of Clinical Chemistry and Laboratory Medicine, Saarland University Medical Center, Homburg/Saar, Germany, Homburg, Saarland, Germany; 4 Universitätsklinikum Augsburg, Augsburg, Bayern, Germany; 5 Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: Myeloperoxidase (MPO)‐DNA complexes have been associated with arterial and venous thrombosis. A potential role in patients with aneurysmal subarachnoid hemorrhage (aSAH) is unknown.
Aims: Here, we sought to explore whether serum MPO‐DNA‐complexes are present in patients with aSAH, and whether levels of serum MPO‐DNA complexes are associated with delayed cerebral ischemia (DCI).
Methods: We performed a post‐hoc analysis of a prospective, blinded, observational single‐center biomarker study that enrolled consecutive patients with spontaneous SAH between July 2018 and September 2020. Serum samples obtained on admission and on hospital day 4 were analyzed for concentrations of MPO‐DNA complexes. The primary outcome was the occurrence of DCI defined as new infarction on brain CT‐scan. The secondary outcome was clinical vasospasm, a composite of clinical and transcranial doppler parameters. We used Wilcoxon’s signed‐rank‐test to assess for differences between paired measures.
Results: Among 100 patients with spontaneous SAH, mean age 59 (SD+13) years, 55% women, 78 patients had an aneurysmal SAH and complete DCI information. Among these, 29 (37%) developed DCI. MPO‐DNA complexes were detected in all serum samples. The median MPO‐DNA level was 33 ng/ml (IQR, 18‐43 ng/ml) on admission, and 22 ng/ml (IQR, 11‐31 ng/ml) on day 4 (Mann‐Whitney test: p = 0.015). In the primary outcome analysis, we found a significant reduction in MPO‐DNA levels from admission to day 4 in patients with DCI (paired test, p = 0.036) but not in those without DCI (p = 0.171). The secondary analysis showed a similar reduction in MPO‐DNA levels between admission and day 4 in patients with (p = 0.006), but not in those without clinical vasospasm (p = 0.473).
Conclusion(s): MPO‐DNA‐complexes are detectable in peripheral serum samples of patients with aSAH. A pronounced reduction in MPO‐DNA levels over the first days after aSAH might herald DCI. The potential of MPO‐DNA levels to serve as a biomarker of DCI requires further exploration.


PB0025
Primary hemostasis, coagulation and fibrinolysis markers in patients with intracranial large vessel occlusion stroke, on admission to hospital
A. Barakzie 1; A. Bruggeman2; G. Dirks3; D. Dippel4; M. Charles2; M. de Maat5
1 Department of Hematology, Erasmus University Medical Center, Rotterdam, The Netherlands, Papendrecht, Zuid‐Holland, Netherlands; 2 Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Hematology, Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Department of Neurology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and Department of Public Health, Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 5 Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Acute Ischemic Stroke (AIS) is most often caused by thromboembolic occlusion of the cerebral blood vessels. Changes in the primary hemostasis, coagulation, and fibrinolysis systems are important causal factors in thrombus formation but information on the levels at the acute moment of stroke are limited. Better understanding of hemostatic and fibrinolytic factors will help us to predict the risk of developing AIS and effects of treatments.
Aims: To study the levels and activities of primary hemostasis, coagulation, and fibrinolysis markers in patients with AIS.
Methods: We used data from patients with AIS of included in MR CLEAN‐NOIV trial. We measured hemostatic factors von Willebrand Factor Antigen (VWF:Ag), ADAMTS13, FVIII, fibrinogen, D‐dimer, and tissue‐Plasminogen Activator (tPA) in citrated plasma of AIS patients collected on admission to hospital. Hemostatic factors levels and activity were presented as either mean and standard deviation (SD) or median and inter‐quartile range (IQR) in case of skewedness.
Results: We included 131 AIS patients. ADAMTS13 activity (mean 65 ± SD 23%, (Ref. 41‐129%)) was normal in AIS patients, whereas the levels of VWF:Ag (mean 2.26 ± SD 1.10 U/ml, (Ref. 0.60‐1.40 U/ml)), FBG (mean 2.58 ± SD 1.44 g/l (Ref. 1.5‐3.6 g/l)), D‐dimer (median 1.07 mg/L [IQR 1.47], (Ref. < 0.50 mg/L)), FVIII (median 1.47 U/ml [IQR 1.41], (Ref. 0.60‐1.40 U/ml)), and tPA:Ag (median 10.69 ng/ml [IQR 10.97], (Ref. < 10 ng/ml)) were extremely increased in these patients compared to references (Ref). tPA:Ag in 49 patients was extremely high (>511 ng/ml).
Conclusion(s): In patients with AIS, we found increased levels of VWF:Ag, FBG, FVIII, D‐dimer and tPA:Ag on admission to hospital. These findings indicate that the primary hemostasis, coagulation, and fibrinolysis systems are disturbed in AIS patients. Increased levels of hemostatic and fibrinolytic markers might be involved in pathogenesis of AIS and clinical failure of AIS thrombolysis treatment.
PB0028
Thrombin generation predicts outcomes in patients with non‐traumatic intracerebral hemorrhage
L. Lóczi 1; R. Orbán‐Kálmándi2; T. Árokszállási3; M. Héja3; I. Fekete4; K. Fekete4; J. Tóth5; L. Csiba6; Z. Bagoly7
1 University of Debrecen; Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary; 2 University of Debrecen; Faculty of Medicine, Department of Laboratory Medicine, Division of Clinical Laboratory Sciences, Debrecen, Hajdu‐Bihar, Hungary; 3 University of Debrecen; Faculty of Medicine, Department of Neurology, Debrecen, Hajdu‐Bihar, Hungary; 4 University of Debrecen, Faculty of Medicine, Department of Neurology, Debrecen, Hajdu‐Bihar, Hungary; 5 University of Debrecen, Faculty of Medicine, Department of Radiology, Debrecen, Hajdu‐Bihar, Hungary; 6 University of Debrecen; Faculty of Medicine, Department of Neurology and ELKH‐DE Neurodegenerative and Cerebrovascular Research Group, Debrecen, Hajdu‐Bihar, Hungary; 7 University of Debrecen, Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary
Background: Non‐traumatic intracerebral hemorrhage (ICH) accounts for 10‐15% of all strokes and leads to a higher rate of mortality as compared to ischemic strokes.
Aims: Here we aimed to find out whether the thrombin generation assay (TGA) could predict outcomes in ICH patients.
Methods: In this prospective, observational study 87 consecutive ICH patients (age: 67 ± 12, male/female: 55/32) and 164 healthy controls were included. Exclusion criteria included aneurysm rupture, cancer, liver‐ or kidney failure or hemorrhagic diathesis. Detailed clinical and laboratory investigations were performed on admission. ICH volume was estimated based on CT performed on admission, day 14 and 90. TGA was performed on stored platelet poor plasma obtained on admission. Lag time, endogen thrombin potential (ETP), peak thrombin, time‐to‐peak were calculated. Short‐ and long‐term outcomes of ICH were defined at 14 days and 3 months post‐event according to the NIHSS and the modified Rankin Scale (mRS), respectively.
Results: Peak thrombin was significantly higher in patients as compared to controls (397.2 ± 93.9 vs. 306.3 ± 85.3 nM, p < 0.0001). Time to peak parameter was significantly longer in patients. Peak thrombin and ETP parameters significantly correlated with estimated ICH volume at day 90 (peak thrombin: r = 0.5383; 95%CI:0.2103‐0.7574, p = 0.0021, ETP: r = 0.3838, 95%CI:0.0161‐0.6600, p = 0.0363), ETP correlated with CRP on admission. Peak thrombin was significantly higher in patients with poor (mRS 2‐6) long‐term outcome (median [IQR] of mRS 0‐1: 402.5 [344.8‐473.8] vs. mRS 2‐6: 326.4 [294.2‐416.1] nM, p = 0.0096). Backward logistic regression analysis revealed that an elevated peak thrombin parameter (>339.1 nM) confers a significant risk for poor long‐term outcome (OR:7.27, 95%CI:2.18‐24.28, p = 0.001).
Conclusion(s): In patients with ICH, the extent of thrombin generation was increased as compared to healthy controls, which might be explained by the presence of higher inflammatory parameters in patients. Peak thrombin measured on admission might be useful to predict outcomes in ICH patients.
PB0027
Glenzocimab does not impact bleeding severity in two different mouse models of intracranial hemorrhage
H. Lebas1; S. Dupont2; E. Pascal3; M. Jandrot‐Perrus4; B. Ho‐Tin‐Noé 5
1 INSERM, PARIS, Ile‐de‐France, France; 2 INSERM UNIT 1148, PARIS, Ile‐de‐France, France; 3 INSERM Unit 1148, PARIS, Ile‐de‐France, France; 4 LVTS, UMR_S1148 INSERM, Université de Paris, France, PARIS, Ile‐de‐France, France; 5 INSERM, PARIS, Ile‐de‐France, France
Background: Because of the potential risk of worsening bleeding in case of hemorrhagic stroke, administration of antiplatelet drugs at the acute phase of ischemic stroke cannot be envisioned until the diagnosis of stroke and its ischemic nature have been confirmed by imaging. Reducing the delay between acute ischemic stroke (AIS) onset and treatment initiation is however critical for treatment efficacy. Glenzocimab, a novel antiplatelet drug targeting GPVI currently in phase 2 evaluation for AIS, has been shown to have no impact on hemostasis following traumatic injury or at sites of inflammation. Whether Glenzocimab treatment remains safe in the context of intracranial hemorrhage remains however unknown.
Aims: Here, we investigated and compared the safety of GPVI or GPIIbIIIa targeting in 2 mouse models of intracranial hemorrhage.
Methods: The severity of intracranial bleeding was compared between wild‐type, GPVI‐/‐, integrin beta3‐/‐, and mice humanized for GPVI (hGPVI) treated or not with glenzocimab or eptifibatide, in a model of hemorrhagic stroke caused by unilateral striatal injection of collagenase type VII, as well as in a model of hyperglycemia‐induced hemorrhagic transformation of cerebral ischemia‐reperfusion injury.
Results: GPVI deficiency or pharmacological inhibition with glenzocimab had no impact on bleeding severity in either model of intracranial hemorrhage. Conversely, GPIIbIIIa deficiency and eptifibatide treatment caused a significant increase in intracranial bleeding in both models and were associated with increased mortality in the model of hyperglycemia‐induced hemorrhagic transformation of cerebral ischemia‐reperfusion injury.
Conclusion(s): In contrast to eptifibatide, glenzocimab does not increase bleeding severity in case of intracranial hemorrhage. The safety of glenzocimab in the context of intracranial hemorrhage suggests that it could be administered in patients with suspicion of stroke, before assessment of its ischemic nature, thus allowing a drastic reduction in treatment initiation.
PB0031
Association between thromboembolus composition and first pass recanalisation after thrombectomy in ischemic stroke
S. Staessens1; S. Vandelanotte 2; O. François3; E. Boulleaux4; M. Bretzner4; B. Casolla5; D. Corseaux4; L. Puy6; L. Desender1; T. Dewaele3; C. Tersteeg7; K. Vanhoorelbeke7; P. Vanacker8; S. Susen9; C. Cordonnier6; T. Andersson10; S. De Meyer7
1 Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, Kortrijk, West‐Vlaanderen, Belgium; 2 Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium, Bruges, West‐Vlaanderen, Belgium; 3 Department of Medical Imaging, AZ Groeninge, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 4 University of Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐ EGID, F‐59000 Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 5 University of Lille, Inserm, CHU Lille, U1172 – LilNCog – Lille Neuroscience & Cognition / Stroke Unit, UR2CA‐URRIS Neurology, CHU Pasteur 2, Nice Cote d’Azur University, Nice, France., Lille, Nord‐Pas‐de‐Calais, France; 6 University of Lille, Inserm, CHU Lille, U1172 – LilNCog – Lille Neuroscience & Cognition, F‐59000 Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 7 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 8 Department of Neurology, AZ Groeninge, Kortrijk, Belgium / Department of Neurology, University Hospitals Antwerp, Antwerp, Belgium : Department of Translational Neuroscience, University of Antwerp, Antwerp, Belgium, Antwerpen, Antwerpen, Belgium; 9 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France, 10 Department of Medical Imaging, AZ Groeninge, Kortrijk, Belgium / Departments of Neuroradiology, Karolinska University Hospital, and Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden, Kortrijk, West‐Vlaanderen, Belgium
Background: When performing thrombectomy on ischemic stroke patients, achieving good recanalisation in the first pass (first pass effect, FPE) is associated with a good clinical outcome. Achieving FPE has therefore become an important objective of the procedure. The reasons why multiple attempts are sometimes needed are not fully understood, but the composition of the thromboembolus is considered to be an important factor.
Aims: The aim of this study was to investigate the link between thromboembolus composition and FPE.
Methods: In total, 497 stroke thromboemboli from the AZ Groeninge Hospital (Kortrijk, Belgium) and CHU Lille (Lille, France) were analysed histologically for 7 components: red blood cells (RBC), fibrin, von Willebrand factor, platelets, leukocytes, citrullinated histone 3 (marker for neutrophil extracellular traps) and DNA (intra‐ and extracellular). FPE was defined as obtaining a modified Thrombolysis in Cerebral Ischemia (mTICI) score of 2c/3 after the first pass. Thromboembolus histology was compared between cases in which a FPE was achieved and cases in which a mTICI score of 2c/3 was achieved after multiple passes.
Results: Good recanalisation (mTICI 2c/3) was obtained in 348 patients: 208 with an FPE and 140 needing multiple passes. Patients in which no good recanalisation was obtained (n = 149) were excluded for this analysis. Interestingly, thromboemboli from FPE cases contained significantly more RBC (median (IQR): 44.74% (29.53%‐58.13%) versus 39.85% (24.99%‐52.88%), p = 0.02) compared with patients in which multiple passes were needed. In addition, achieving an FPE was associated with better clinical outcome indicated by a lower modified ranking score at 90 days (median (IQR): 2 (1‐3) vs 3 (1‐5), p = 0.0003).
Conclusion(s): Achieving an FPE is more likely in patients with RBC‐richer thromboemboli and achieving an FPE results in better clinical outcome. Future research and technical improvements are needed to improve FPE‐rates in RBC‐poor thromboemboli.
Peripheral Artery Disease
VPB0523
Acute arterial limb ischemia after intake of amphetamine derivatives, report of two cases
A. Restrepo 1; A. Arango2; R. Herrera3; J. Arrieta3
1 Universidad de Antioquia, Envigado, Antioquia, Colombia; 2 Universidad Pontificia Bolivariana, Medellin, Antioquia, Colombia; 3 Universidad de Antioquia, Medellin, Antioquia, Colombia
Background: Illegal consumption of amphetamine derivatives from the phenylethylamine group is increasing worldwide, among the adverse effects reported in the literature are psychotic symptoms, rhabdomyolysis, lactic acidosis, renal failure, tachyarrhythmias, and encephalopathy secondary to vasculitis. However, given their mechanism of action as activators of dopamine, serotonin and catecholamine precursors, they have been associated with cardiovascular complications.
Aims: To describe two clinical cases with acute arterial ischemia secondary to vasospastic syndromes due to amphetamine derivatives
Methods: Descriptive, retrospective study
Results: A 38‐year‐old male with a history of MDMA, ecstasy, and 2C‐B use, was admitted with cyanosis of the third finger of the right hand, as well as the lower limbs, with sequential obstructive involvement of the arteries of the arms and bilateral distal infrapopliteal arteries suggestive of a vasospastic disorder. IV nitroglycerin and calcium channel blockers were started with subsequent resolution of the condition. A 27‐year‐old male with a history of consumption of 2C‐B, ketamine, and MDMA, was admitted due to cyanosis in the distal region of the lower limbs, with evidence of permeability of the arterial circulation and possible vasospastic effect. He was treated with calcium channel blockers and IV nitroglycerin with favorable evolution.
Conclusion(s): Acute limb ischemia secondary to vaso‐spastic syndromes has been associated with the consumption of substances such as cocaine and ergotamine. However, given the growing use of illicit substances derived from amphetamines, we were able to discover the effects of stimulation of peripheral alpha‐adrenergic, dopaminergic and serotonergic receptors, with the consequent effect on vascular smooth muscle. Since it is a rare complication, this phenomenon constitutes a clinical challenge that is difficult to identify. Knowledge of this entity and its management with vasodilators such as nitrates and the use of calcium channel blockers are of vital importance to impact adverse outcomes in the extremities.
VPB0524
Procoagulatory changes in patients with chronic lower limb ischemia
K. Komissarov1; V. Soldatenkov 2; L. Papayan3; S. Bessmeltsev1; N. Silina1; S. Kapustin2; V. Burakov1; N. Saltykova1; O. Kuzakbirdieva1; O. Matvienko4; O. Soldatenkova1
1 Russian Scientific Research Institute of Hematology and Transfusiology, Saint‐Petersburg, Saint Petersburg City, Russia; 2 Russian Research Institute of Hematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 3 Russian Research Institute of Haematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 4 Russian Research Institute of Hematology and Transfusiology, Saint‐Petersburg, Saint Petersburg City, Russia
Background: The risk factors for unfavorable peripheral artery disease (PAD) course and treatment failure in young patients are still unrevealed. One of the most promising research areas is the assessment of procoagulatory changes in these patients.
Aims: To determine the prognostic value of procoagulatory markers in patients with chronic lower limb ischemia.
Methods: We examined 17 patients under the age of 55 with chronic arterial insufficiency (stages IIa‐III according to Fontaine classification) and popliteal and tibial artery thrombosis and/or popliteal‐to‐distal bypass graft thrombosis. The following assays were performed: aPTT, PTI, thrombin time; antithrombin III, protein C, protein S, factor VIII (FVIII), factor IX (FIX) and von Willebrand factor (VWF) activity levels assessment; determination of homocysteine and fibrinogen plasma levels; testing for antiphospholipid antibodies; molecular genetic testing for inherited thrombophilia (FII, FV, FI, PAI‐1, GpIb, GpIIIa, MTHFR).
Results: The most unfavorable course of PAD accompanied by migratory thromboses of distal arterial segments, arterial bypass grafts thromboses, tissue loss was observed in 6 (35.3%) patients with the FI gene mutation 455G/A, which led to the persistent increase in fibrinogen plasma level (the average level was 4.57 g/l). In a single case, the combination of prothrombin gene mutation G20210A and FI gene mutation 455G/A determined the failure of reconstructive surgery and increased number of early post‐operative arterial and bypass grafts thromboses. In 9 (53%) subjects, the increased levels of FVIII and VWF activity were found.
Conclusion(s): The procoagulatory changes (FI gene mutation 455G/A with hyperfibrinogenemia, prothrombin gene mutation G20210A, elevation of FVIII and VWF activity levels) in patients with chronic lower limb ischemia may be predictors of unfavorable PAD course and failure of reconstructive interventions, and may require early, intensive and prolonged antithrombotic therapy.
PB0521
Use of antithrombotic agents for the prevention of coronary and peripheral arterial events in patients with end‐stage kidney disease on hemodialysis: Prospective results of the VIVALDI study
O. Königsbrügge 1; M. Lorenz2; S. Schmaldienst3; R. Klauser‐Braun4; I. Pabinger5; M. Säemann6; C. Ay5
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Vienna Dialysis Centre, Vienna, Austria, Vienna, Wien, Austria; 3 Clinic Favoriten, Vienna, Austria, Viennna, Wien, Austria; 4 Clinic Donaustadt, Vienna, Austria, Vienna, Wien, Austria; 5 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 6 Clinic Ottakring, Vienna, Austria, Vienna, Wien, Austria
Background: Antithrombotic agents are frequently used medications in patients with end‐stage kidney disease (ESKD) on hemodialysis (HD), because indications including atrial fibrillation, coronary heart disease and peripheral artery disease are highly prevalent in ESKD, although evidence on efficacy and safety is scarce. Vitamin‐K‐antagonists (VKA) are, however, suspected of accelerating vascular calcification and increasing the risk for arterial cardiovascular outcomes.
Aims: We aimed to investigate the incidence of arterial outcomes in HD patients and the association with antiplatelet agents and VKA treatment.
Methods: A cohort of 625 patients with ESKD on HD was prospectively observed for a median observation time of 3.4 years (25th to 75th percentile 2.9‐3.5 years) and occurrence of arterial outcomes were recorded and independently adjudicated. Participation in the study was confirmed with informed consent and with the approval of the local ethics committee. The occurrence of death was considered in competing risk regression.
Results: Absolute frequency of coronary and peripheral artery outcomes and incidence rates are presented in Table 1. Use of antiplatelet agents in secondary cardiovascular disease (CVD) prevention (N = 276 out of 367 patients with prior cardiovascular disease) was not statistically significantly associated with occurrence of arterial outcomes (table 2). Use of VKA in AF patients (N = 99 out of 238 patients with AF) was associated with increased risk of the composite outcome of peripheral artery revascularizations, acute limb ischemia, and any amputations (HR 1.81, 95% confidence interval 1.04‐3.16, p = 0.035) after adjustment for prior ischemic stroke, coronary artery disease, and peripheral artery disease (table 2).
Conclusion(s): Coronary and peripheral artery events are common cardiovascular events in HD patients. Neither VKA in AF patients nor antiplatelet agents in secondary CVD prevention was associated with reduced risk of outcomes, but use of VKA may be associated with increased risk of peripheral artery outcomes, albeit a potential confounding by indication remains.


PB0522
The combination of lipocalin‐2 and calprotectin improves risk assessment in advanced and early peripheral arterial disease
G. Saenz‐Pipaon1; S. Ravassa1; K. Lawaetz‐Larsen2; E. Martínez‐Aguilar3; J. Orbe 4; J. Rodriguez5; L. Fernandez‐Alonso3; A. Gonzalez5; J. Martin‐Ventura6; J. Páramo7; J. Lindholt2; C. Roncal5
1 CIma universidad de Navarra, Pamplona, Navarra, Spain; 2 Elite Centre of Medical Treatment of Arterial Diseases, Odense, Syddanmark, Denmark; 3 Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; 4 Centro de Investigación Médica Aplicada (CIMA), Universidad de Navarra, Pamplona, Navarra, Spain; 5 Cima Universidad de Navarra, Pamplona, Navarra, Spain; 6 IIS‐Fundación Jiménez Díaz, Madrid, Madrid, Spain; 7 Clínica Universidad de Navarra, Pamplona, Navarra, Spain
Background: It is important to find reliable markers assessing cardiovascular (CV) risk in peripheral artery disease (PAD). Previous transcriptomic analyses in plasma extracellular vesicles (EVs) revealed increased expression of calprotectin (S100A8/A9) and lipocalin 2 (LCN2) in PAD patients.
Aims: The aim of this study was to determine their prognostic value for CV risk assessment in PAD.
Methods: EVs were isolated from plasma and femoral plaques to determine LCN2 and calprotectin by qPCR and western blot. Their expression was also determined in human femoral plaques by immunohistochemistry. LCN2 and calprotectin were measured by ELISA in advanced PAD (CHN cohort, n = 331, serum), and early PAD (VIVA trial, n = 413, plasma). Patients were followed up for a mean of four years, recording as combined outcome CV death or amputation (CHN cohort) and CV death or major lower limb events (MALE, VIVA population).
Results: LCN2 and S100A9 were detected in plasma and femoral plaque EVs, and in femoral atherosclerotic tissues in regions rich in inflammatory cells. Increased levels of both proteins were associated with a higher risk of CV death or amputation (5.6‐fold after covariate adjustment, p<.001), and CV death or MALE (3‐fold, multivariate analysis, p<.001) in the CHN cohort and the VIVA trial respectively. Moreover, the addition of the combined variable to basal models, considering clinically relevant risk factors, improved reclassification for the studied outcome in both cohorts (p≤.024).
Conclusion(s): The transcriptomic analysis of circulating EVs (liquid biopsy) partially reflects both systemic and local changes (molecular signature), and enabled the identification of new prognostic biomarkers in PAD. Moreover, the combined assessment of LCN2 and calprotectin might be useful for risk stratification in advanced and early PAD. Funding: Ministry of Science and Innovation, Institute of Health Carlos III, co‐funded by the European Fund for Economic and Regional Development (FEDER) [PI18/01195 and CB16/11/00371]. Virto Group (Spain).
Coagulation and Natural Anticoagulants
Animal Models in Thrombosis and Hemostasis
VPB0527
Experimental model of rodent disseminated intravascular coagulation
F. Marchant 1; Y. Prado2; V. Alejandro3; F. Simon3; C. Otero
1 Millennium Institute in Immunology and Immunotherapy, santiago, Region Metropolitana, Chile; 2 Millennium Institute in Immunology and Immunotherapy, Santiago, Region Metropolitana, Chile; 3 Universidad Andrés Bello, Santiago, Region Metropolitana, Chile
Background: Sepsis is a syndrome characterized by a deregulated systemic inflammatory response of the organism during infectious processes. The systemic inflammation promotes a transition from an antithrombotic and anticoagulant endothelial phenotype that limits platelet adhesion and maintains hemostasis, to a highly adhesive phenotype characterized by increased expression of pro adhesive and procoagulant proteins. During its progression to severe sepsis, coagulation, and fibrinolysis, giving rise to a syndrome characterized by intravascular activation of coagulation without specific localization called disseminated intravascular coagulation (DIC). The processes described above are associated with microcirculation dysfunction, generating hypoperfusion, reducing the supply of oxygen and nutrients to the different tissues, causing organ failure, and finally death. Currently, DIC has become a very difficult to treat syndrome faced by patients and the medical team. This is directly related to the fact that DIC mortality has remained at very high levels over the last few years.
Aims: To generate a highly representative and rapid model of disseminated intravascular coagulation.
Methods: Male Sprague‐Dawley rats aged 8 weeks were anesthetized with isoflurane and by intravenous infusion of LPS (30 mg/kg/h serotype O55:B5) (n = 8) or the same volume of saline (n = 3). At 3 hours post‐infusion blood were collected, it was determined (I) prothrombin time (PT), (II) partial thromboplastin time (APTT), (III) fibrinogen levels (Fib), (IV) platelet count, (V) D‐dimer and (VI) percentage of perfused microvasculature by Sidestream Dark Field (SDF). The results are presented as ±SD. Significant differences were assessed by the t‐student test (Mann‐Whitney).
Results: LPS treated rats exhibited a decrease in platelet count, plasma fibrinogen and percentage of perfused vessels. On the other hand, we observed an increase in prothrombin time (PT), partial thromboplastin (APTT) and D‐Dimer in plasma.
Conclusion(s): This methodology will allow studying in an easy and fast way the alterations of hemostasis produced by systemic inflammation.
PB0525
Icodextrin as anti‐ adherence molecule affects cancer cells attachment to the fibrin deposits on the peritoneum surface
I. Adybiat 1; S. Mirshahi2; J. Soria1; M. Pocard1; M. Mirshahi3
1 CAP‐Paris Tech., INSERM U1275, Hôpital Lariboisière, Paris France, Paris, Ile‐de‐France, France; 2 Diagnostica Stago, Gennevilliers, Ile‐de‐France, France; 3 CAP‐Paris Tech., INSERM U1275, Université de Paris, Hôpital Lariboisière, Paris France, Paris, Ile‐de‐France, France
Background: In patients affected by cancer, tissue traction or peritoneal injury may increase the risk of cancer propagation.
Aims: the role of Icodextrin on cancer cell implantation at injured peritoneum
Methods: Thirty Balb/C mice were used. The mice were divided into three groups. All three groups had a tumor graft by105 CT26 murine cells intraperitoneally injection. The second also had peeling on the peritoneal wall and the third had injury induction and anti‐adhesion injection (icodextrin 4%). The animals were sacrificed after three weeks and the peritoneal membranes were fixed using 4% formaldehyde for 24 hours or by critical point drying (CPD). After simple preparation, they were sputter‐coated with gold or platinum, dried using the EMSCD500 apparatus from Leica and observed using an SEM FEG ZEISS ultra 55 SEM equipped with a LaB6 filament operating at 15 kV. Images were captured using “Orion” NCH Software.
Results: In the scarred area, induced by incision during surgery or by ell peeling, the fibrin network is a major element identified by electron microscopy. We demonstrated that i) a fiber network was deposited on the peritoneal wall. ii) cancer cells were adhering to fibrin deposits on the cicatrice zone. iii) the tumor nodule can be seen on the peritoneal surface surrounded by fibrin deposition as well as inside the nodules. iv) fibrin is an element of nodule matrix. v) Icodextrin decreased the fibrin ‐ cancer cell interaction and adhesion to the peritoneum.
Conclusion(s): These results suggest that the scarred areas are associated with fibrin deposits where cancer cells adhere and proliferate. In parallel, the fibrin deposits on the peritoneal surface are not degraded and Icodextrin can be used for cancer cells inhibition to be deposited on fibrin.
PB0528
Experimental investigation of the antiplatelet effect of Zingiber officinale (Ginger) ex vivo and in vitro
F. Marhoume 1; O. Ezmoury2; K. Lafhal2; N. Fdil2
1 Center of Regenerative Medicine, Laboratory of Cellular Therapy, Mohammed VI University Hospital, Marrakesh, Morocco., Marrakech, Marrakech‐Tensift‐Al Haouz, Morocco; 2 Metabolics Platform, Biochemistry Laboratory, Faculty of Medicine, Cadi Ayad University, Marrakech, Morocco, marrakech, Marrakech‐Tensift‐Al Haouz, Morocco
Background: It’s well recognized that blood platelets play crucial role in the pathologies linked to cardiovascular diseases (CVD). In this context, there is a growing interest in new and safer antiplatelets agent in human diet.
Aims: The aim for this study is to determine the anti‐aggregant effect of Zingiber officinale Ethanolic extract (E‐ZO) on collagen induced platelets aggregation in vitro, and by measuring the bleeding time and platelets count on rat ex vivo.
Methods: Phytochemical analysis was assessed by measuring DPPH free radical‐scavenging assay and identification & quantification of polyphenols compounds were determinate by HPLC analysis.
Results: Our finding demonstrated that E‐ZO extract showed a significant (P < 0.001) dose‐dependent inhibitory action which correlate well with data of bleeding time and platelets count that also highlight the in vivo anti‐thrombotic effect of the E‐ZO extract in platelets function. Data of Phytochemical analysis revealed a high amount of total polyphenols and flavonoids (0.7± 0.055 mg CAE/100 g DM), and the main polyphenols compounds identified and quantified by HPLC was vanillin (22.61%) in high
Conclusion(s): E‐ZO extract exhibited a very potent antioxidant activity. The present investigation has shown that Zingiber officinale extract has an anti‐aggregant and antioxidant effects in which flavonoids could be involved in particular vanillin.
VPB0531
Thrombotic state of readiness with increase of physical activity
A. Blazhko
Altai State Medical University, Barnaul, Russia, Barnaul, Altaisky krai, Russia
Background: Under the action of excessive stressors of different nature, hemostasis can respond by forming a state of thrombotic readiness.
Aims: The paper aims to assess the effect of a single intensive physical exercise of varying duration on the hemostasis system, microvasculature, and endothelium of rats.
Methods: The hemostasis system and microvasculature were investigated in 60 mature male Wistar white rats after a 4‐hour and 8‐hour physical exercise on a rat treadmill.
Results: It was found that a 4‐hour exercise resulted in an increase in the aggregation function of platelets with a subsequent increase in their number and hypercoagulation against the decrease in plasma anticoagulant activity. An 8‐hour exercise increased the negative changes identified before. Thus, there was an increase in soluble fibrin monomer complexes, a decrease in fibrinogen and platelets in the blood, a decrease in plasma anticoagulant and fibrinolytic activity, the development of endothelial dysfunction and congestive phenomena in the microcirculation area.
Conclusion(s): The increase in the duration of stress exposure training from 4 to 8 hours leads to an increase in the severity of signs of the thrombotic state of readiness.
PB0529
Investigations of the impact of different bleeding disorders using a humanized in vivo mouse model
L. Pelzl 1; A. Singh2; I. Marini3; K. Althaus1; T. Backchoul4
1 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 2 Experimentelle und Klinische Transfusionsmedizin Tübingen, Tuebingen, Baden‐Wurttemberg, Germany; 3 Transfusion Medicine, Medical Faculty of Tübingen, University Hospital Tübingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 4 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Immune thrombocytopenia (ITP) is a bleeding disorder due to multiple alterations. The immune system leads to increased platelet (PLT) clearance and bleeding in patients. Currently, no in vivo model system exists to predict the impact of autoantibodies on human platelets function in ITP.
Aims: To establish a humanized mouse model to investigate antibody–mediated destruction of human PLTs in vivo and to assess the impact on bleeding.
Methods: A monoclonal antibody against CD42b was injected in a NOD/SCID mouse model in order to deplete the murine platelets. Next, human PLTs were administrated into the tail vain and their survival was determined 1 and 5 hours post injection by flow cytometry (FC). The presence of human PLT was investigated by FC and calculated as % of circulating human PLTs. Finally, the functionality of human PLT in vivo was assessed by measuring the bleeding time.
Results: The CD42b antibody induced a drastic reduction of murine PLTs in the mouse bloodstream (< 5 %) without affecting the survival of the animals. Subsequently, human PLTs were injected into the mouse bloodstream via the tail vein, and 30 minutes post injection approximately 90% of the circulating PLTs were human. Next, IgGs from ITP patients were injected into the mouse circulation in order to investigate PLT clearance and function. Interestingly. A rapid decrease in PLT survival was observed compared to the control IgG (% survival of human PLT after 5 h, mean±SEM: ITP vs. control IgG: 54.75 ± 6.7 vs. 27.51 ± 5.14, n = 5 p = 0.0133). Bleeding time in the humanised mouse model was established to assess platelet function. A significant reduction in bleeding time was observed following injection of human PLTs (360.8 ± 20.5 vs 126 ± 10.73 seconds, n = 4, p = 0.0001).
Conclusion(s): Our results suggest that the NGS mouse model may be a promising tool to study human platelet function in different bleeding disorders.
PB0530
Characterization of disseminated intravascular coagulation (DIC) in a murine model of fecal‐induced peritonitis
N. Sharma 1; D. Dwivedi2; E. Cani3; M. Lalu4; A. Mendelson5; B. McDonald6; P. Liaw7
1 McMaster University ‐ The Thrombosis & Atherosclerosis Research Institute; 2 McMaster University ‐ The Thrombosis & Atherosclerosis Research Institute, Milton, Ontario, Canada; 3 McMaster University ‐ The Thrombosis & Atherosclerosis Research Institute, Vaughan, Ontario, Canada; 4 The Ottawa Hospital, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario, Canada; 5 Winnipeg Regional Health Authority, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada; 6 Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 7 McMaster University ‐ The Thrombosis & Atherosclerosis Research Institute, Hamilton, Ontario, Canada
Background: DIC is an acquired condition characterized by widespread intravascular coagulation that complicates many conditions including sepsis. The presence of DIC is an independent and strong predictor of organ dysfunction and mortality. For DIC diagnosis, the ISTH DIC scoring system uses platelet counts, the prothrombin time, D‐dimer, and fibrinogen. However, mouse models of DIC have not been developed in a standardized way which limits comparison between models and reduces translatability. One model of murine sepsis is the fecal‐induced peritonitis (FIP) model which does not require surgery and is easier to reproduce in a multi‐centre setting.
Aims: To characterize the time course of DIC in a murine model of sepsis and to investigate the correlation between DIC onset and immunothrombosis.
Methods: Male and female C57Bl/6 mice (10‐12 weeks old) received i.p. injections of 0.75 mg/g rat fecal slurry or 5% dextrose (controls). The mice were monitored every 4 h for temperature and the Murine Sepsis Score. The mice were culled at 4 h, 8 h, 12 h, and 24 h to assess changes in the DIC score. Blood and organs were harvested and plasma markers of immunothrombosis were quantified.
Results: Injection of 0.75 mg/g of fecal slurry resulted in 60% mortality at 72 hours, a finding we previously determined as members of the National Preclinical Sepsis Platform. In the current study, measurements of the components of the DIC score revealed that the onset of DIC occurs around 8 hours post‐FIP induction. The onset of DIC also correlates with elevations in markers of immunothrombosis including IL‐6, thrombin‐antithrombin complexes, myeloperoxidase, and extracellular DNA.
Conclusion(s): The onset of DIC occurs around 8 hours post‐FIP and correlates with elevations in markers of immunothrombosis. A standardized model for sepsis‐induced DIC will be useful to investigate the pathophysiology of DIC as well as to guide the optimal timing of novel therapies.
PB0526
Evaluation of intracerebral haematoma expansion of the new oral anticoagulants in rats.
R. Fonseca 1; J. Ferreira2; G. Carvalho3; F. Paiva4; P. Mourão5
1 Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil. Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil; 2 Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil.Instituto de Bioquímica Médica Leopoldo de Meis, Universidade Federal do Rio de Janeiro, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil; 3 Instituto de Ciências Biomédicas‐ ICB‐ UFRJ, Rio de Janeiro, Rio de Janeiro, Brazil; 4 Instituto de Física de São Carlos/ Universidade de São Paulo, São Carlos, Sao Paulo, Brazil; 5 Instituto de Bioquímica Médica Leopoldo de Meis/ Universidade Federal do Rio de Janeiro, Rio de Janeiro, Rio de Janeiro, Brazil
Background: Little is known about haematoma expansion that occurs after treatment with new oral anticoagulants (NOACs). Collagenase‐induced intracerebral hemorrhage (ICH) model has been used to investigate this event in a translational approach.
Aims: In this work, we evaluated the effects of warfarin and NOACs (dabigatran, rivaroxaban and apixaban) on haematoma expansion in rats using an ICH model.
Methods: Oral anticoagulants were given by gavage at different doses and, at the peak of their anticoagulant and antithrombotic activities, ICH was induced by injecting collagenase VII‐S (0.4U) into the left striatum. Haematomal lesion volume was determined 24 h later using MRI, Evans Blue extravasation and H&E‐stained brain sections. Neurological deficits were evaluated using Body Elevated Swing Test. Differences between groups were analyzed using analysis of variance followed by ANOVA test with Bioestat 5.0 software.
Results: At the highest dose (1.5 mg/kg), warfarin significantly increased hematoma volume ~3 fold, whereas no difference was observed with NOACs using T1‐weighted analysis. Quantification of Evans Blue extravasation in the brains of animals treated with oral anticoagulants showed that warfarin at 1 and 1.5 mg/kg and dabigatran (9 mg/kg) increased intracerebral bleeding ~3 fold. We observed a clear correlation between the images obtained by magnetic resonance and by histological staining, but no differences were observed in neurological tests.
Conclusion(s): The evaluation of ICH by in vivo models should be performed using different methodologies to better evaluate the effect of different anticoagulants on the expansion of intracerebral bleeding.

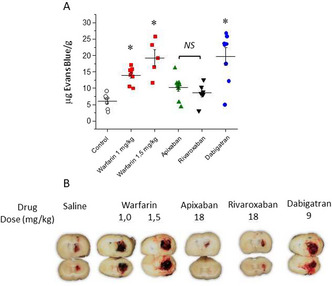
Coagulation Factors and Inhibitors
PB0542
Bio‐equivalence of potency adjusted approved heparin solutions compared to a newly developed heparin solution
E. Krupa 1; D. Hoppensteadt2; W. Jeske1; A. Farooqui1; A. Kouta1; O. Iqbal1; B. Kantarcioglu3; R. Krämer4; J. Fareed2
1 Loyola University Medical Center, Maywood, Illinois, United States; 2 Loyola University Chicago, Maywood, Illinois, United States; 3 Loyola University Chicago, Oak Park, Illinois, United States; 4 Heidelberg University, Heidelberg, Baden‐Wurttemberg, Germany
Background: Porcine mucosal heparin solutions with a defined potency adjusted concentrations are widely used for the anticoagulation in medical, surgical and interventional indications. Recently, Sintetica has developed a 20.000 IU (48 ml, 416 IU/ml) solution.
Aims: The purpose of this investigation is to compare the Sintetica heparin solution with other commercially available potency adjusted heparin solutions such as Fresenius, Roche, Hospira and Medefil heparins to demonstrate their bio‐equivalence.
Methods: Commercially available heparin solutions were obtained from Sintetica, Roche, Fresenius Kabi, Hospira and Medefil. USP reference standard was obtained from US Pharmacopeia. USP compliant anti‐Xa and anti‐IIa method based on chromogenic substrate assays. Thrombin generation studies were carried out using a fluorokinetic method (Diagnostico France). The quantification of glycosaminoglycan (GAG) content was made by using a fluorescence probe (Red Probe, Munster, Germany). The clot based assays and amidolytic assays were carried out on ACL Elite analyzer. HIT mediated platelet aggregation studies were carried out using HIT antibodies from clinically diagnosed patient serum. For the potency measurements calibration curves were prepared with the USP standard for cross‐referencing.
Results: In the USP compliant anti‐Xa and anti‐IIa assays all standardized solutions provided comparable results. Similarly in the clot‐based assays such as the aPTT, thrombin time, prothrombinase‐induced clotting time. All solutions produced comparable anticoagulant effects. Similarly in the thrombin generation assays all products produced comparable inhibitory responses. The HIT antibody mediated platelet aggregation was similar with all solutions. Total GAG content as measured by Red Probe was also comparable.
Conclusion(s): These result demonstrate that commercially available potency adjusted heparin solutions and the newly developed Sintetica heparin solution exhibit comparable anticoagulant and anti‐protease effects. Based on these results the Sintetica product is similar to the commercially available clinically approved potency adjusted heparin solutions. Furthermore these results suggest that these solutions are interchangeable for clinical use.
VPB0566
Quantification of FVIII inhibitors in patients on emicizumab prophylaxis ‐ which laboratory methods and reagents are appropriate?
M. Milos 1; D. Coen Herak2; S. Zupancic‐Salek3; E. Bilic4; R. Zadro5
1 University Hospital Centre Zagreb; Faculty of Pharmacy University of Mostar, Zagreb, Grad Zagreb, Croatia; 2 Department of Laboratory Diagnostics, University Hospital Centre Zagreb, Zagreb, Grad Zagreb, Croatia; 3 University of Zagreb, Haematology Dept, Zagreb, Grad Zagreb, Croatia; 4 Department of Pediatrics, University Hospital Centre Zagreb, Zagreb, Grad Zagreb, Croatia; 5 St. Catherine Specialty Hospital, Zagreb, Grad Zagreb, Croatia
Background: Laboratory monitoring of hemophilia A patients with inhibitors who received emicizumab prophylaxis is challenging, especially the assessment of FVIII inhibitors.
Aims: The aim of this study was to examine different laboratory methods for FVIII inhibitor quantification and to compare them with the recommended chromogenic method using bovine reagents.
Methods: The study included five hemophilia A patients and two samples from patients receiving emicizumab distributed from UK NEQAS for Blood Coagulation. In all samples aPTT (Actin FS), FVIII activity and inhibitor titers were determined using three different FVIII activity assays. One‐stage FVIII clotting assay (Actin FS as aPTT reagent) and chromogenic FVIII activity assay with bovine reagents (Factor VIII Chromogenic assay) were performed on Atellica COAG360 (Siemens Healthcare Diagnostics, Marburg, Germany) while chromogenic FVIII activity assay with human reagents (BIOPHEN FVIII:C, HYPHEN BioMed, France) was performed on Sysmex CS‐2000i (Sysmex, Kobe, Japan).
Results: As presented in Table, one‐stage clotting assay, as an extremely sensitive method for emicizumab, yielded false‐negative inhibitor results for all patients with known positive inhibitors and significant emicizumab activity (>6.0 IU/dl). Slightly lower FVIII inhibitor titers were obtained by using chromogenic method with human reagents as compared to the recommended method due to emicizumab interference, while false‐negative results were obtained in two samples with low inhibitor titers (patient 5 and Neqas sample 1).
Conclusion(s): Emicizumab interference with FVIII inhibitor quantification using the chromogenic method with human reagents is dependent on emicizumab activity and FVIII inhibitor titer, with more pronounced influence in patients with higher emicizumab activity and lower inhibitor titer.
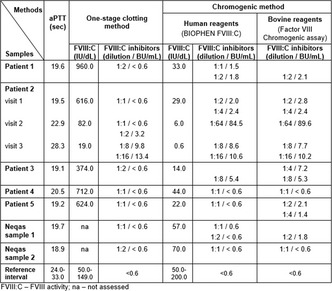
VPB0568
Implementation and scale‐uping of text messages for primary care patients using Warfarin: a real life study
V. Chagas 1; M. Rego Souza‐Silva2; M. Paiva Domingues3; D. Pereira2; L. Sá4; M. Almeida2; M. Raposo5; J. Oliveira6; T. Soares2; A. Baldoni7; M. Marcolino8; M. Parreiras Martins2
1 Universidade Federal de Viçosa ‐ UFV, Belo Horizonte, Minas Gerais, Brazil; 2 Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 3 Universidade Federal de São João Del‐Rei., Belo Horizonte, Minas Gerais, Brazil; 4 FCMMG, Belo Horizonte, Minas Gerais, Brazil; 5 Universidade Federal de São João Del‐Rei, Divinópolis, Minas Gerais, Brazil; 6 UFMG, Belo Horizonte, Minas Gerais, Brazil; 7 Universidade Federal de Minas Gerais., Belo Horizonte, Minas Gerais, Brazil; 8 Associate Professor and Internal Medicine Physician. Department of Internal Medicine, Medical School; and Telehealth Center, University Hospital, Universidade Federal de Minas Gerais., Belo Horizonte, Minas Gerais, Brazil
Background: Warfarin remains the most available oral anticoagulant, however therapy depends on adhesion. The use of text‐messages is a strategy that can be used to educate patients, but there are no studies evaluating this intervention in patients taking warfarin.
Aims: Our aim was to report the implementation of a pilot text‐messaging intervention to primary care patients taking warfarin in a middle‐sized Brazilian city.
Methods: A bank of 79 messages was drafted and validated. During six months, three times a week, 37 patients received semi‐personalized messages about anticoagulation with warfarin. At baseline and after three months, we assessed their knowledge and adherence with validated questionnaires. At the end of the follow‐up, participants answered a satisfaction questionnaire. Subsequently, a scale‐up stage was conducted, with an additional round of intervention including 82 patients. Seven months after the end of the second round, we interviewed the patients for their insights about the long‐term effects of this program. All patients signed informed consent. The study was approved by the Research and Ethics committee of the Universidade Federal de Minas Gerais.
Results: In the first round, 33 (89.2%) patients completed the follow‐up. Adherence measured by the MAT test was high in the pre‐intervention assessment and remained high after three months (96.7% vs. 93.3%). There was a trend to improve knowledge of warfarin therapy measured by the OAK test after three months (6.5% to 25.6% of correct answers, p = 0.0703). In the scale‐up phase, 23 patients were interviewed in the long‐term assessment. The main long‐term knowledge they reported was dietary information. Nine patients reported to have received the messages, but did not remember their content.
Conclusion(s): The intervention was well‐accepted by the patients, and had a positive impact on their knowledge about oral anticoagulation therapy. The scale‐up assessment showed the need to constantly monitor digital interventions.
PB0549
Comparative studies on clotting and antiprotease profile of heparins of ovine, bovine and porcine origin
E. Krupa 1; J. Lewis2; A. Farooqui1; O. Iqbal1; W. Jeske1; J. Walenga1; Y. Yao3; J. Fareed4
1 Loyola University Medical Center, Maywood, Illinois, United States; 2 Loyola University Chicago, Northfield, Illinois, United States; 3 Ronnsi Pharmaceutical, suzhou, Jiangsu, China (People's Republic); 4 Loyola University Chicago, Maywood, Illinois, United States
Background: Currently used unfractionated heparin is mainly derived from porcine mucosal tissue. Bovine and ovine mucosa is also used for the extraction of heparin. The availability of unfractionated heparin from non‐porcine origin will provide alternate options for anticoagulation.
Aims: This study is aimed to compare porcine heparin with newly developed ovine and bovine heparins.
Methods: Ten individual batches of ovine heparins were obtained from Ronnsi Pharmaceutical (Suzhou, China), 10 batches of porcine mucosal heparin were obtained from Medefil Laboratories (Glendale Heights, USA), 10 batches of ovine heparin were obtained from Kin Master (Passo Fundo, Brazil), and 13 additional batches of bovine heparin were obtained from Adeste (Sao Paulo, Brazil). All heparins were re‐constituted using saline to obtain a stock solution of 10 mg/ml. Normal human plasma was supplemented to obtain a concentration of 2.5 μg/ml. APTT and thrombin time measurements were measured using clot‐based assays, while anti‐Xa and anti‐IIa activities were measured using amidolytic assays. Activated clotting time measurements were measured in the whole blood clotting assays.
Results: Results were compiled for each of the heparins as a group means + 1 SD (Table 1). In both thrombin time and aPTT assays, ovine and porcine heparins produced comparable, much stronger anticoagulant effects in comparison to the bovine heparins. Similarly, in the ACT assays the ovine and porcine heparins produce stronger effects in comparison to two bovine groups of heparins. Batch variations were also noted.
Conclusion(s): These results show that the ovine and porcine heparins exhibit comparable anticoagulant and antiprotease effects. The bovine heparins from two different manufacturers show comparable results in all of the assays, however these effects were weaker when compared to porcine and ovine heparins. These results are suggestive of potency adjustment for bovine heparins to produce comparable anticoagulant and antiprotease actions using USP assays.

PB0532
Deciphering the role of small sulfated molecule PTS on AT3 structure and in vitro clot formation
I. Ahmad 1; M. Abid2; S. Deep3; M. Jairajpuri4
1 Indian Institute of Technology Delhi, New Delhi, Delhi, India; 2 Jamia Millia Islamia (A Central University), Delhi, Delhi, India; 3 Indian Institute of Technology Delhi, Delhi, Delhi, India; 4 Jamia Millia University (a central University), New Delhi, Delhi, India
Background: The human population suffers due to the disease burden caused by thromboembolic disorders. Thrombosis is a major life‐threatening condition with a high prevalence of cardio‐cerebrovascular complications. Antithrombin (AT3) is a serpin that inhibits thrombin, factor Xa. Anticoagulants are inhibitors (direct or indirect) of enzymes involved in the coagulation pathway. They enhance the proteinase inhibitory activity of AT3. The search is going on for new anticoagulants with significant activity and lesser side effects.
Aims: Our aim is here to design a new molecule pyrogallol trisulfate (PTS) and check its role in AT3 structure and clot formation.
Methods: The identification of PTS binding with AT3 was performed by molecular docking. Synthesis of PTS was achieved using triethylamine‐sulfur trioxide adduct and purified by RP‐HPLC. Structural modifications were characterized by FTIR, NMR, and Mass spectrometry. The cytotoxicity of pyrogallol and PTS was checked by MTT assay using a human embryonic kidney cell line (HEK‐293). The in vitro efficacy of PTS for its anticoagulant potential was performed by clotting assays APTT, PT, and TT. The impact of PTS interaction with AT3 on its secondary and tertiary structure was validated using Far‐UV CD and fluorescence spectrometry.
Results: The molecular docking results suggested PTS binds to AT3. PTS does not show cellular cytotoxicity even at the higher dosage tested. The results hinted that addition of sulfate moieties contributed to reduced cytotoxicity. In vitro anticoagulant assay of PTS with human plasma for clot formation, demonstrated a potentially prolonged effect on APTT, a moderate effect on PT and TT. This indicates the delay in clot formation upon plasma treatment with PTS. Far‐UV CD spectral analysis showed a marginal effect on the secondary structure while PTS treated AT3 showed changes in tertiary structure conformation.
Conclusion(s): The study suggests a potential anticoagulant effect of PTS in clot reduction with promising applications.
PB0541
Freeze‐dried plasma development and assessment of biochemical quality
A. Heger 1; M. Karlovits1; A. Hinz2; G. Bengtsson2; R. Moezzifard2; G. Gruber1
1 Octapharma PPGmbH, Vienna, Wien, Austria; 2 Octapharma AB, Stockholm, Stockholms Lan, Sweden
Background: OctaplasLG® is a frozen solvent/detergent‐treated, coagulation active plasma product for treating complex coagulation factor deficiencies and can be used as substitution therapy in situations where specific factor concentrates are not available, or in emergency situations where precise laboratory diagnosis is not possible. The recently developed freeze‐dried pharmaceutical form, OctaplasLG® Lyo, a more convenient lyophilized form, offers faster reconstitution and more flexibility in storage conditions (refrigerated/room temperature), thus increasing ease of logistics and utilization.
Aims: To compare the biochemical quality of OctaplasLG® Lyo with OctaplasLG® and single‐donor fresh frozen plasma (FFP) units.
Methods: Three OctaplasLG® Lyo batches for process performance qualification were manufactured at Octapharma AB (Stockholm, Sweden), freeze‐dried, and reconstituted with sterilized water. Twelve batches of OctaplasLG® and FFP units were used for comparison. All plasma samples were assessed for global coagulation parameters, coagulation factors and protease inhibitors, activation markers of coagulation and fibrinolysis, as well as important plasma proteins.
Results: Frozen OctaplasLG® and freeze‐dried OctaplasLG® Lyo demonstrate identical quality profiles upon thawing and reconstitution, respectively. All coagulation factor and protease inhibitor activity parameters were in line with levels mandated by the European Pharmacopoeia (Figure 1). FFP units show comparable coagulation factor activities, with higher protein S and plasmin inhibitor levels compared with the OctaplasLG® products. OctaplasLG® and OctaplasLG® Lyo parameters show lower bag‐to‐bag variation compared with FFP.
Conclusion(s): OctaplasLG® frozen and freeze‐dried products have equally high biochemical quality. The key features of the new freeze‐dried OctaplasLG® Lyo are the fast reconstitution time and flexibility of storage conditions (refrigerated/room temperature), which has advantages in emergency situations and in prehospital settings. In addition, the standardized content of plasma proteins in both OctaplasLG® products facilitates appropriate planning of high efficacy treatment.
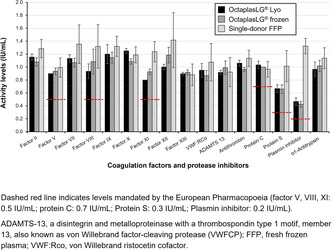
PB0559
LEX‐210: A phase 3, randomized, double‐blinded study of four‐factor prothrombin complex concentrate in patients with acute major bleeding on direct oral anticoagulant therapy with factor Xa inhibitors
R. Sarode 1; S. Maack2; C. Solomon3; S. Knaub2
1 University of Texas Southwestern Medical Center, Dallas, TX, Dallas, Texas, United States; 2 Octapharma AG, Lachen, Schwyz, Switzerland; 3 Octapharma AG, Lachen, Zurich, Switzerland
Background: Major bleeding associated with direct oral anticoagulant therapy with Factor Xa Inhibitors (FXaI) may be controlled using hemostatic agents such as prothrombin complex concentrates (PCCs). The efficacy/safety of PCCs for use to manage FXaI‐related bleeding requires further investigation.
Aims: To demonstrate the hemostatic efficacy/safety of four‐factor PCC (4F‐PCC; Octaplex®, Octapharma) in adults with FXaI‐related major bleeding.
Methods: LEX‐210 (NCT04867837) is a Phase 3, multicenter, prospective, randomized, double‐blinded, group‐sequential, parallel‐group, adaptive design study (sponsor: Octapharma). Patients (≥18 years) with acute major bleeding and FXaI activity equivalent to ≥100 ng/ml are eligible. Exclusion criteria include bleeding that is immediately life‐threatening and acute trauma for which FXaI reversal alone would not be expected to control bleeding. Approval from independent ethics committees will be sought prior to study start. Prior written informed consent will be obtained from each patient or the patient’s legally authorized representative. Approximately 200 patients will be enrolled and randomized 1:1 to receive 50 IU/kg or 15 IU/kg 4F‐PCC, to demonstrate superior hemostatic efficacy of the higher dose for emergency reversal of FXaI related major bleeding. The primary endpoint is the proportion of patients with effective (excellent/good) or non‐effective (poor/none) hemostasis within 24 h of 4F‐PCC, as determined by an independent adjudication committee consistent with predefined criteria based on Sarode et al. (Table 1) [1]. Secondary endpoints include change in endogenous thrombin potential (baseline to 1 h after PCC), 30‐day rates of thromboembolic events and all cause mortality, adverse events, vital signs, and laboratory parameters.
Results: LEX‐210 launched in Q4 2021 and will be performed across ~60 sites in North America and Europe. Completion is anticipated Q1 2024.
Conclusion(s): Results could confirm the hemostatic efficacy/safety of 4F‐PCC in the management of FXaI‐related major bleeding, potentially offering an alternative treatment for these patients. References: 1. Sarode R et al. Circulation 2013;128:1234‐43.

PB0565
Performance evaluation of the INNOVANCE anti‐Xa assay for the quantitative determination of apixaban on the Sysmex CN‐3000/CN‐6000 systems
D. Pahren; M. Wilkens; U. Groß; M. Merz; D. Bußfeld
Siemens Healthineers, Marburg, Hessen, Germany
Background: Apixaban (Eliquis) is a direct factor Xa inhibitor used in the prevention of thrombotic diseases. Due to its predictable pharmacokinetics and wide therapeutic window, no therapeutic range in which the clinical outcome is optimized has been established in conjunction with apixaban market approval. However, because hemostasis is a balance between the tendency to clot and the risk of bleeding, anticoagulants have an inherent risk of causing bleeding, which indeed is the most common side effect of apixaban. Although the manufacturer of apixaban has stated that regular monitoring is not needed, quantitative assays should be available to aid in the assessment of a patient’s anticoagulant status.
Aims: The INNOVANCE Anti‐Xa assay for the quantitative determination of apixaban is a chromogenic assay for which a new application for the Sysmex® CN‐3000/CN‐6000 Systems was developed. The aim of the studies was to assess the performance of this application.
Methods: The following studies were conducted using the Sysmex CN‐6000 System: limit of quantitation (LoQ), linearity, precision, dilution recovery, and method comparison versus the Sysmex CS‐5100 System. Performance characteristics were confirmed for the Sysmex CN‐3000 System by method comparison versus the Sysmex CN‐6000 System.
Results: Based on the results from the LoQ, linearity, and precision studies, the analytical measuring interval for the application was defined to be 20–350 ng/ml. An extended measuring interval (1:2 sample dilution) of 350–700 ng/ml was demonstrated by dilution‐recovery studies. Good comparability of the INNOVANCE Anti‐Xa assay on the Sysmex CN‐6000 System versus the INNOVANCE Anti‐Xa assay on the Sysmex CS‐5100 System could also be shown.
Conclusion(s): The INNOVANCE Anti‐Xa assay demonstrates good performance on the Sysmex CN‐3000/CN‐6000 System and can be used for the quantitative determination of apixaban in human citrated plasma.
VPB0571
In vitro effects of colloidal silica on clotting of plasma with or without coagulation factor deficiencies, heparization, lupus anticoagulant positivity, or high fibrinogen concentration
A. Kadowaki 1; M. Wakui2; Y. Fujimori3; H. Katagiri4; M. Murata2
1 LSI Medience Corporation, Takomachi, Katorigun, Chiba, Japan; 2 Department of Laboratory Medicine, Keio University School of Medicine, Shinjuku, Tokyo, Japan; 3 Office of Clinical Laboratory Technology, Keio University Hospital, Shinjuku, Tokyo, Japan; 4 Clinical Laboratory, Keio University Hospital, Shinjuku, Tokyo, Japan
Background: Activated partial thromboplastin time (APTT) reagents consist of phospholipids and activators such as ellagic acid and colloidal silica. However, anticoagulant effects of silica have also been reported (Margolis. Aust J Exp 1961).
Aims: To assess in vitro effects of colloidal silica on clotting of plasma with or without various coagulation abnormalities.
Methods: APTT reagents were prepared with three concentrations (low: 0.025 %; intermediate: 0.05 %; high: 0.1 %) of colloidal silica. Plasma with or without various coagulation factor deficiencies, heparization with unfractionated heparin (0.3IU/ml), lupus anticoagulant (LA) positivity, or high fibrinogen concentration (800 mg/dl) were subjected to APTT measurements using the automated analyzer STACIA (LSI Medience).
Results: In normal plasma, APTT at the low and intermediate concentrations of colloidal silica was similar while being prolonged at the high concentration, unexpectedly revealing anticoagulant effects. Similar observations were obtained from plasma with Factor II deficiency, heparization, LA positivity, or high fibrinogen concentration. Especially, Factor II deficiency and heparization enhanced APTT prolongation. When comparing APTT at the low and intermediate concentrations, APTT was shortened at the intermediate concentration in each coagulation factor deficiency except Factor II deficiency. Comparison of APTT measurements at the intermediate and high concentrations revealed differences in colloidal silica effects among various coagulation factor deficiencies. In the deficiency of Factor VII, VIII, or IX, APTT was prolonged at the high concentration, exhibiting anticoagulant effects. In the deficiency of Factor V, XI, or XII, APTT at the high concentration was roughly equivalent to that at the intermediate concentration, exhibiting neither procoagulant nor anticoagulant effects. In the deficiency of Factor X, APTT was shortened at the high concentration, exhibiting procoagulant effects.
Conclusion(s): It remains to be elucidated how coagulation factor deficiencies and heparization are relevant to colloidal silica effects. Basic aspects of colloidal silica impacting the intrinsic coagulation pathway should be addressed.

PB0533
Investigation of the epidemiology of inherited deficiency of coagulation factors in patients attending the Royal Hospital, Muscat, Oman
R. Al Ghaithi; S. Al Hashami; N. Al Amri; S. AL Shiyadi; S. Al Lamki; M. Al Yahyai; M. Al Riyami; M. Al Musalhi; I. Al Salmi
The Royal Hospital, Muscat, Masqat, Oman
Background: Inherited deficiencies of coagulation factors (IDF) are rare bleeding disorders with a variable bleeding tendency ranging from mild to severe. However, severity of bleeding does not necessarily correlate with the degree of factor (Fa) deficiency. High incidences of IDF occur in regions where consanguineous marriages are common [1].
Aims: We aimed to investigate the epidemiology of IDF among 416 patients (220 male and 196 female, median age 24 ± 22) referred to the Royal Hospital between 2018 and 2020.
Methods: Data files of 416 patients were analysed for the following parameters: Coagulation screen, Mixing study, Extrinsic factors, Intrinsic factors, Factor XIII level and Bethesda assay.
Results: Overall, 92/416(22%) patients were diagnosed with IDF. All patients had prolonged PT and/or APTT, which were corrected by normal plasma. A single factor deficiency in the extrinsic pathway was detected in 36 patients (FaV = 5, FaVII = 30 and FaX = 1) and in the intrinsic pathway in 49 patients (FaVIII = 24, FaIX = 2, FaXI = 7 and FaXII = 16). A combined deficiency of FaVIII & FaV, and FaIX & FaXI was detected in 3 and 1 patients respectively. Other deficiencies are afibrinogenemia (n = 1), High‐molecular‐weight kininogen (n = 1) and Prekallikrein (n = 1). Out of 10 patients diagnosed with severe hemophilia A (HA), one developed antibodies to FaVIII. In addition, FaXIII was analysed in 35 patients, of which 4(11.4%) patients had reduced FaXIII level. On the other hand 263(63%) patients had factors deficiency due to acquired causes including acquired HA, Vitamin K deficiency and liver dysfunction. The remaining 61(15%) patients had their factors level within normal range.
Conclusion(s): Our current study revealed that deficiency of FaVII is the most common among Omanis, followed by HA and deficiency of FaXII. Individuals with FaXII deficiency are asymptomatic and tend to be detected incidentally during routine laboratory screening. As such the true incident of FaXII deficiency among Omanis could be higher than reported here.
PB0537
Short‐term biological variation study of hemophilia and thrombophilia parameters in a population of Caucasian adults
A. Brochier; A. Mairesse; P. Saussoy; D. Gruson; M. van Dievoet
Catholic University of Louvain, Brussels, Brussels Hoofdstedelijk Gewest, Belgium
Background: Biological variation data obtained in a correct and standardized way is valuable to evaluate the analytical requirements and to assess the utility of a reference interval. No data were found in the literature on short‐term biological variation of thrombophilia and hemophilia parameters.
Aims: Our study aimed to determine analytical performance specifications for thrombophilia and hemophilia parameters. The studied parameters were protein C, protein S, activated protein C resistance (APCR), coagulation factors V, VIII, IX, XI and XII. Our second objective was to evaluate if our assays fulfill these criteria. Finally, we aimed to provide a robust tool for comparison of serial measurements of factor V, VIII, IX and XI.
Methods: A blood draw was performed weekly in 19 healthy Caucasian adults for 5 weeks. Hemophilia and thrombophilia parameters were measured in duplicate with Werfen reagents and analyzer. Biological variation components were calculated with nested analysis of variance after exclusion of outliers. This study was carried out at the clinical hemostasis laboratory of Saint‐ Luc University Hospital, Brussels, Belgium. Informed consent was obtained from each participant, and the study protocol was approved by the local Ethics Committee.
Results: The analytical coefficient of variation (CV) varied from 1.5% to 4.6%, the within‐subject CV from 1.6% to 8.9% and the between‐subject CV from 3.8% to 24.0%. All parameters showed a high individuality. For all parameters except APCR, the desirable analytical goal was met with our assays. A reference change value (RCV) of 21.8%, 28%, 20% and 17% was obtained for factor V, VIII, IX and XI respectively.
Conclusion(s): For most parameters, the analytical goals were met. A RCV was obtained for factors V, VIII, IX and XI, facilitating the comparison with previously obtained values in the follow‐up of patients with for example hepatic failure or mild hemophilia.
PB0550
Differential neutralization of unfractionated heparin and enoxaparin by andexanet alfa
J. Lewis 1; O. Iqbal2; W. Jeske2; D. Hoppensteadt3; F. Siddiqui2; B. Lewis2; J. Fareed3
1 Loyola University Chicago, Northfield, Illinois, United States; 2 Loyola University Medical Center, Maywood, Illinois, United States; 3 Loyola University Chicago, Maywood, Illinois, United States
Background: Andexanet alfa is a clinically used antidote for the neutralization of the bleeding effects of Direct Oral Anticoagulant (Direct Xa) agents such as Rivaroxaban and Apixaban. It is a molecularly modified factor Xa decoy protein with high specificity for factor Xa inhibitors. Unfractionated heparin (UFH) and low molecular weight heparins (LMWHs) exhibit both anti‐Xa and anti‐IIa activities.
Aims: The purpose of this study is to investigate the relative neutralization of the anticoagulant effects of UFH and enoxaparin by andexanet alfa in whole blood assays such as activated clotting time (ACT) and thrombelastography (TEG).
Methods: The neutralization profiles of UFH and enoxaparin were studied by andexanet alfa at various concentrations. The final concentration of UFH used for activated clotting time (ACT) was 10 μg/ml, and for enoxaparin was 25 μg/ml. Andexanet alfa was used in a concentration range of 6.25‐200 μg/ml. For the thromboelastography (TEG) analysis the concentration of all drugs were proportionately reduced by a factor of ten. The results were analyzed using R open source statistical software.
Results: Andexanet alfa at 200 μg/ml moderately neutralized UFH at the concentrations tested in ACT. The ANOVA test yielded a significant result when comparing the means of UFH treated at various andexanet alfa concentrations. The Tukey Post‐Hoc test revealed significant differences between the UFH and UFH treated with andexanet alfa at all concentrations tested except at 6.25 μg/ml. Andexanet alfa did not effectively neutralize enoxaparin. There was no concentration dependent change in the neutralization profiles of UFH and enoxaparin at a concentration range of 12.5 μg/ml ‐ 200 μg/ml. In the TEG assays, andexanet alfa at 10 μg/ml partially neutralized the anticoagulant effects of UFH at 1 μg/ml.
Conclusion(s): These studies indicate that andexanet alfa may be useful in neutralization of UFH and warrants in‐vivo validation.
PB0557
Effectiveness and safety of Direct Oral Anticoagulants (DOACs) in extremely obese compared to non‐extremely obese patients in an observational prospective single‐centre unselected patient registry
C. Moret Puig 1; S. Mojal2; M. Plaza Seijas3; R. Acosta‐Isaac4; J. Souto5
1 Hospital de la Santa Creu i Sant Pau, Barcelona, BARCELONA, Catalonia, Spain; 2 Hospital de la Santa Creu i Sant Pau, Barcelona, Catalonia, Spain; 3 Research Institute, Hospital de la Santa Creu i Sant Pau, Barcelona., Barcelona, Catalonia, Spain; 4 Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau., Barcelona, Catalonia, Spain; 5 Genomics of Complex Diseases Group, Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau, Barcelona, Catalonia, Spain
Background: Extreme obesity (BMI≥40 kg/m2 or weight≥120 kg) can affect DOACs’ pharmacokinetics, increasing the volume of distribution and the drug clearance. A systematic review and meta‐analysis of the impact of weight on DOAC in clinical trials showed no significant difference. To date, guidelines advise being cautious with prescribing DOACs to obese, especially with BMI≥40 kg/m2.
Aims: To compare effectiveness and safety between extremely obese and non‐extremely obese patients in a real‐world registry with Edoxaban prescription predominance (68.3%).
Methods: The patients, who have provided written informed consent, were assessed for demographic and clinical characteristics, validated laboratory tests, rigorous education and follow‐up inside the MACACOD registry (Clinical Application Model of Direct Oral Anticoagulants, NCT04042155) from July 2019 to January 2022. Ethical approval was obtained. Major thrombotic complications (MTC) comprised ischemic stroke and systemic embolism. Major hemorrhagic complications (MHC), events with a Bleeding Academic Research Consortium (BARC) scale above 2 points. Secondary outcomes are Clinically Relevant Non‐Major Thrombosis (CRNMT) or Bleedings (CRNMB), including peripheral thrombosis, myocardial infarction, transient ischemic attack and bleeding with a BARC scale of 2 points or less.
Results: Our registry includes 1030 patients with at least one follow‐up, 1000 anticoagulated for Atrial Fibrillation and 30 for venous thromboembolism. A total of 25 present extreme obesity (2.43%) and 1005 present non‐extreme obesity. The extremely obese cohort was significantly younger, with a higher Charlson Comorbidity Index (CCI) and better Creatinine Clearance (ClCr) (Table 1). However, there was a higher incidence of MHC and mortality in extremely obese than in non‐extremely obese. There’s no difference in MTC or CRNMT+B (Table 2).
Conclusion(s): In our registry, despite extremely obese patients being younger and with better ClCr, they are more comorbid and have more hemorrhagic complications and more mortality than non‐extremely obese patients, with no difference in the incidence of thrombotic complications. Acknowledgments to DAIICHI‐SANKYO Spain for the support.

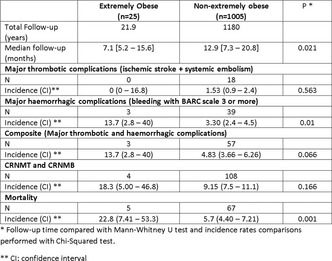
VPB0572
A study of the stability of fibrinogen and FXIII levels and microbial integrity of pooled cyroprecipitate stored at 1‐6°C and 20‐24°C for up to 120 hours
H. Kaur 1; A. Ang2; S. Chua2; P. Shu2; C. Tan3; W. Wong3
1 Health Sciences Authority, Singapore, Singapore; 2 Blood Services Group, Health Sciences Authority, Singapore, Not Applicable, Singapore; 3 Department of Haematology, Singapore General Hospital, Singapore, Not Applicable, Singapore
Background: Cryoprecipitate is an important source of fibrinogen and FXIII, both of which are depleted in trauma induced coagulopathy. Current standards stipulate its post‐thaw storage at 20‐24°C and transfusion within 4‐6 hours post‐thaw, however this is a potential limiting factor in its timely availability during major bleeding. Though recent studies demonstrate stability of fibrinogen levels in post‐thaw cryoprecipitate stored at 20‐24°C for up to 5 days, the risk of bacterial growth remains a concern.
Aims: To investigate the ex‐vivo haemostatic capacity and risk of bacterial contamination of thawed cryoprecipitate stored at 1‐6°C and room temperature (20‐24°C) over 120 hours
Methods: 10 units of pre‐pooled cryoprecipitate were thawed at 37°C and split into 2 arms (1‐6°C and 20‐24°C). Fibrinogen levels were assessed at 0, 24, 48, 72, 96 and 120 hours, while FXIII levels was assessed at 0 and 120 hour for each arm. For sterility testing, 10 units of pre‐pooled were thawed at 37°C and split into 2 arms (1‐6°C and 20‐24°C). Aerobic and anaerobic cultures were performed at 0, 24, 72 and 120 hour for each arm. Incubation period was 5 days at each time point.
Results: Decline in fibrinogen concentration was noted from Day 0 to 5 for the cryoprecipitate stored at 1‐6°C (median decline 3.5 g/L). Conversely, fibrinogen levels in cryoprecipitate stored at room temperature remained more constant (median decline 0.6 g/L). Nevertheless, all cryoprecipitate samples met HSA’s QC requirement of > 150 mg of fibrinogen/unit at Day 5. Likewise, there was a decline in FXIII levels from Day 0 to 5 at both 1‐6°C (median decline 27.0 IU/dl) and at 20‐24°C (median decline 12.5 IU/dl). The percentage decline in FXIII levels for both arms was however small. Sterility testing was negative for all units.
Conclusion(s): This study demonstrates potential feasibility in storing cryoprecipitate at 1‐6°C for 5 days while maintaining fibrinogen and FXIII levels.
VPB0576
Is plasma‐derived coagulation factor Xa safe for reversal of direct factor Xa inhibitor?
S. Surov; W. Jankowski; Z. Sauna; M. Ovanesov
US Food & Drug Administration, Silver Spring, Maryland, United States
Background: Several genetically modified variants of activated coagulation factor Xa (FXa) were proposed to achieve reversal of direct FXa inhibitor action during bleeding or perioperative management. The properties of FXa variants are often compared to unmodified, plasma‐derived (pd) FXa, but little is known about the efficacy and safety of pd‐FXa for this indication as examined in animal models of bleeding.
Aims: The aim of the study was to test the ability of pd‐FXa to safely reverse the action of a direct FXa inhibitor (apixaban) in vitro and in vivo.
Methods: Escalation of pd‐FXa dose was studied by mouse tail bleeding and thrombin generation in human plasma. CD1 mice were injected intravenously with apixaban or vehicle, followed by pd‐FXa 5 minutes later. After another 5 minutes, 3 mm of tail were cut, and bleeding was observed for 30 minutes.
Results: In vitro, reversal of apixaban effect on thrombin peak height in normal plasma was dependent on apixaban dose, e.g., 0.5 μM of apixaban was reversed with 33 μg/ml of pd‐FXa, and 2 μM of apixaban with 200 μg/ml. Progressive reduction and normalization of blood loss at 4 mg/kg of apixaban was observed at 0.25 to 8 mg/kg of pd‐FXa. pd‐FXa failed to reduce bleeding time. Without apixaban, pd‐FXa induced mortality within 30 minutes of injection at doses as low as 0.05 mg/kg.
Conclusion(s): High doses of human pd‐ FXa reverse some aspects of apixaban‐induced anticoagulation in vitro and in vivo. However, in agreement with its known thrombogenic effect, pd‐FXa shows substantial toxicity at sub‐pharmacological doses. Future development of FXa variants should focus on achieving reversal effects at doses that are sufficiently safe for patients with unknown or low level of direct FXa inhibitor. Disclosure: This paper is an informal communication and represents the authors’ best judgment. These comments do not bind or obligate FDA.
PB0534
Interference of PLX038 (pegylated topoisomerase inhibitor SN‐38) with coagulation assays
P. Alcedo Andrade 1; P. Desai2; S. Nichols2; K. Nghiem3; N. Patel3; A. Dulau‐Florea3; C. Pleyer4; A. Thomas2; C. Bolan4; R. Pruthi5; S. Kalsi6
1 Division of Intramural Research, National Heart, Lung, and Blood Institute. National Institutes of Health., Bethesda, Maryland, United States; 2 Developmental Therapeutics Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, United States; 3 Hematology Section, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, Maryland, United States; 4 Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, United States; 5 Mayo Clinic, Rochester, Minnesota, United States; 6 National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, United States
Background: A patient with metastatic breast cancer receiving PLX038, a novel pegylated form of the topoisomerase inhibitor SN‐38 (irinotecan active metabolite), showed abnormal pre‐procedural coagulation parameters, concerning for an acquired factor inhibitor.
Aims: To describe the interference of PLX038 with coagulation assays.
Methods: After informed consent, citrated blood samples were analyzed for PT, aPTT, aPTT long incubation, aPTT mixing studies, lupus anticoagulant (LAC), dilute Russell viper venom time (DRVVT), factor (F) activities, fibrinogen, thrombin time (TT), vWF:Ag, and vWF:RCo, using testing platforms and reagents shown in Table 1. The aPTT on patients who previously received the drug was retrospectively evaluated.
Results: See Table 1. On the Stago STA‐R Evolution analyzer using STA PTT Automate 5, prolonged aPTT did not correct with 50:50 mix, with FVIII, FIX, and FXI activities < 5% and non‐parallelism on serial dilution studies. In contrast, on the ACL Advance analyzer all aPTT and factor activity assays, including chromogenic FVIII and FIX, levels were normal. Normal studies on the Stago analyzer included PT, LAC, Fibrinogen, TT, vWF:Ag, vWF:RCo. DRVVT was normal on both analyzers. All 5 previously treated patients with aPTT evaluations performed on the Stago STA‐R Evolution analyzer both before and after PLX038 administration developed newly prolonged aPTT after receiving PLX038.
Conclusion(s): This first description of PLX038 interference with coagulation assays demonstrates a pattern of interference using the STA‐PTT assay (micronized silica activator), but not with the HemosIL SynthASil assay (colloidal silica activator), and no abnormalities on several other assays. Other pegylated agents have been shown to have a similar pattern of reagent‐ and assay‐specific interference (PMID: 31809565; 29984531; 19015207). Increasing use of pegylated drugs warrants greater awareness of this effect on routine laboratory parameters to avoid unnecessary testing, prolonged hospitalization, procedure delays, and inappropriate high‐risk treatments for acquired factor inhibitors.

PB0545
Efficacy and safety of Haemocer, a local hemostatic agent, in patients with congenital bleeding disorders: A pilot study
M. Karimi 1; S. Haghpanah2; N. Javanmardi2; H. Tavoosi3; S. Kamalian Fard2; N. Momtahan2
1 Hematology Research Center Shiraz University of Medical Sciences, Shiraz, Iran, Shiraz, Fars, Iran; 2 Shiraz University of Medical Sciences, Shiraz, Fars, Iran; 3 Shiraz University of Medical Sciences, Shiraz, Fars, Iran
Background: The safety and efficacy of Hemocer has been approved in different surgical procedures as a local hemostatic agent
Aims: To evaluate the efficacy and safety of Hemocer on the management of bleeding symptoms in patients with congenital bleeding disorders.
Methods: In this case series, 11 patients with hereditary bleeding disorders (6 Hemophila A, 3 Glanzman thromboastenia, one Vonwillbrand disease, and one Factor X deficiency) were investigated between April and September 2021. All patients were registered at Hemophilia center affiliated with Shiraz University of Medical Sciences, Shiraz, southern Iran. Outcome of all patients with bleeding symptoms were assessed after administration of HaemoCer TM PLUS. HaemoCer is a plant‐based hemostatic powder (absorbable polysaccharide) that augments the natural clotting cascade.
Results: Median age of patients was 13 (range: 5‐66) years, eight males and three females. Type of bleeding disorder was hemophilia A in about half of the patients. The most common presenting symptoms were bleeding at the time of circumcision (36.4%) followed by gingival bleeding (18.2%). The most prevalent bleeding symptoms were epistaxis (90.9%) and joint bleeding (63.6%). Table 1 demonstrates the details of clinical characteristics and outcome of each patient after using Hemocer. Overall, three patients (27.2%) (two epistaxis and one gingival bleeding) responded to Hemocer without requirement to any additional treatment. The rest of the patients needed further treatment for managing bleeding symptoms. The patients were followed for three months without occurring any adverse events. Bleeding symptoms occurred in 10 patients during the follow‐up period, only four patients agreed to use Hemocer again, which two of them responded and needed no further treatment.
Conclusion(s): It seems that the efficacy of Hemocer is not acceptable enough to use alone for managing the local bleeding symptoms in patients with congenital bleeding disorders. However, it appears safe based on this pilot study.

PB0552
A probable therapeutic for a child with inherited protein S deficiency
V. Pereira1; L. Thangada2; M. khosla3; R. Majumder 4
1 LSU Health Science center, New Orleans, Louisiana, United States; 2 LSU Health Science Center, New Orleans, Louisiana, United States; 3 LSU Health Science Center, new Orleans, Louisiana, United States; 4 Department of Biochemistry, LSU Health Science Center, New Orleans, Louisiana, United States
Background: Protein S (PS) is a vitamin K‐dependent anticoagulant that acts as a cofactor for APC and TFPI to degrade Factors Va/VIIIa and inhibit Factor Xa, respectively. Recently, we established a third function for PS, namely, inhibiting FIXa. Inherited deficiencies of PS are associated with increased risks of venous thrombosis. Oftentimes, patients are misdiagnosed as having PS deficiency, and, even after major thrombotic events when patients are diagnosed correctly, symptomatic control with anticoagulants is the only recourse. Thus, additional research concerning PS abnormalities is necessary to provide PS‐deficient individuals with treatments designed to target their condition for a better quality of life and better clinical outcomes
Aims: Our goal is to develop therapeutics for a girl with inherited PS deficiency.
Methods: Plasma was collected from a nine‐year‐old PS‐deficient child who had a history of neonatal stroke. Additional plasma was collected from her parents because her paternal grandfather was also PS‐deficient. We performed aPTT and thrombin generation assays with varying concentrations of PS added to the plasma samples. Immunoblotting was performed to assess expression of PS in all the aforesaid persons.
Results: The high thrombin generation in the child’s plasma was greatly responsive to exogenous PS, unlike her parents’ plasma. Immunoblotting revealed lower PS expression in the child, with the pattern being child< father< mother. Currently, genomic DNA from these individuals is being sequenced.
Conclusion(s): The low aPTT clotting times and high thrombin generation of the child’s plasma are indicative of the PS deficiency. Further genetic testing is needed to identify the specific molecular alteration responsible for the deficiency. In unpublished work, we found that residues E435 and E437 in the LGI domain of PS exclusively bind FIXa. Identifying the mutation in PS of the affected child will point the way to developing a therapeutic.
PB0556
Performance evaluation of the INNOVANCE anti‐Xa assay for the quantitative determination of rivaroxaban on the Sysmex CN‐3000/CN‐6000 Systems
D. Pahren; M. Wilkens; U. Groß; M. Merz; D. Bußfeld
Siemens Healthineers, Marburg, Hessen, Germany
Background: Rivaroxaban (Xarelto) is a direct factor Xa inhibitor used in the prevention of thrombotic diseases. Due to its predictable pharmacokinetics and wide therapeutic window, no therapeutic range in which the clinical outcome is optimized has been established in conjunction with rivaroxaban market approval. However, because hemostasis is a balance between the tendency to clot and the risk of bleeding, anticoagulants have an inherent risk of causing bleeding, which indeed is the most common side effect of rivaroxaban. Although the manufacturer of rivaroxaban has stated that regular monitoring is not needed, quantitative assays should be available to aid in the assessment of a patient’s anticoagulant status.
Aims: The INNOVANCE Anti‐Xa assay for the quantitative determination of rivaroxaban is a chromogenic assay for which a new application for the Sysmex® CN‐3000/CN‐6000 Systems was developed. The aim of the studies was to assess the performance of this application.
Methods: The following studies were conducted using the Sysmex CN‐6000 System: limit of quantitation (LoQ), linearity, precision, dilution recovery, and method comparison versus the Sysmex CS‐5100 System. Performance characteristics were confirmed for the Sysmex CN‐3000 System by method comparison versus the Sysmex CN‐6000 System.
Results: Based on the results from the LoQ, linearity, and precision studies, the analytical measuring interval for the application was defined to be 20–350 ng/ml. An extended measuring interval (1:2 sample dilution) of 350–700 ng/ml was demonstrated by dilution‐recovery studies. Good comparability of the INNOVANCE Anti‐Xa assay on the Sysmex CN‐6000 System versus the INNOVANCE Anti‐Xa assay on the Sysmex CS‐5100 System could also be shown.
Conclusion(s): The INNOVANCE Anti‐Xa assay demonstrates good performance on the Sysmex CN‐3000/CN‐6000 System and can be used for the quantitative determination of rivaroxaban in human citrated plasma.
PB0560
Use of heparin‐calibrated assays to estimate anti‐factor Xa activity of factor Xa inhibitors (FXaI): a literature correlation analysis
R. Sarode 1; P. Bassett2; J. Glossop2; S. Maack3; C. Solomon4
1 University of Texas Southwestern Medical Center, Dallas, TX, Dallas, Texas, United States; 2 Portland Medical Communications Ltd, Manchester, England, United Kingdom; 3 Octapharma AG, Lachen, Schwyz, Switzerland; 4 Octapharma AG, Lachen, Zurich, Switzerland
Background: Measurement of drug activity in patients on direct oral anticoagulant (DOAC) therapy with FXaI (rivaroxaban, apixaban, edoxaban) may guide the management of acute complications, such as major bleeding. As specific calibrated FXaI assays are not readily available, studies have evaluated correlation between heparin‐calibrated and specific FXaI assays, suggesting that heparin‐calibrated assays may be suitable to assess FXaI plasma activity. LEX‐210 (NCT04867837, Octapharma) will evaluate four‐factor PCC (Octaplex®) in patients with acute major bleeding on FXaI and aims to include patients with baseline anti‐Xa activity equivalent to ≥100 ng/ml according to the locally available test.
Aims: To enable LEX‐210 investigators to convert heparin‐calibrated anti‐factor Xa assay results in international units (IU)/ml to equivalent DOAC concentration in ng/ml, based on correlation analysis of published data, to recruit patients.
Methods: Literature was searched (PubMed, June 2021) for original data on correlations between heparin‐calibrated and FXaI‐specific assays. Data from animals or spiked (not patient‐derived) samples, or correlating against FXaI concentrations determined using liquid‐chromatography‐tandem mass spectrometry, were excluded. Fitted regression equations were extracted/calculated from included papers. Where >1 report was available for a device/reagent/FXaI combination, data were meta‐analysed.
Results: 8/57 screened articles contained relevant data. Correlation curves against FXaI‐calibrated anti‐Xa assays were obtained for heparin‐calibrated assays, for different combinations of device/reagent/calibrator. The correlation curves and corresponding conversion table for a commonly used device (STA‐R coagulation analyzer) and FXaI (rivaroxaban) are shown (Figure 1/Table 1). Fewer data were available for edoxaban. Enrolment in LEX‐210 is ongoing, with investigators consulting conversion tables where only heparin‐calibrated assays are available.
Conclusion(s): Conversion tables based on correlation data enable clinicians to estimate FXaI plasma activity using anti‐Xa assays calibrated for heparin, if FXaI‐calibrated assays are unavailable. Until FXaI calibrations become more widespread, this approach may be valuable for managing patients with reversal/hemostatic agents when presenting with FXaI‐related major bleeding.


VPB0570
Assessment of impact of metabolites of edoxaban and dabigatran on fibrin and thrombin generation assays
J. Evrard 1; R. Siriez1; V. Maloteau1; J. Dogné2; J. Douxfils3
1 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), Namur, Namur, Belgium; 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), 5000, Namur, Belgium, 31 Qualiblood s.a., 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS)., 5000, Namur, Belgium
Background: Edoxaban and dabigatran are factor Xa and direct thrombin inhibitors, respectively and are metabolized in pharmacologically active moiety. These anticoagulants are known to impact coagulation assays, and it is assumed that metabolites could also affect the thrombin and fibrin generation.
Aims: This study aims to compare the pharmacodynamics of DOACs and their metabolites in plasma on thrombin and fibrin generation.
Methods: Edoxaban and its metabolite (M4) were separately spiked in plasma for assessment of their pharmacodynamic properties. Edoxaban and M4 were also spiked together in plasma. The same experiment was performed with dabigatran and its metabolite (glucoronide‐dabigatran). Final concentrations in plasma were 30, 50 and 100 ng/ml. The control used is plasma spiked with a PBS solution. The thrombin generation assay (TGA) was assessed by the calibrated automated thrombogram (CAT) and the fibrin formation was assessed with the FibWave on ACL Top analyzer. A mixture of silica plus phospholipids (PLs) (intrinsic pathway) and tissue factor (±5 pM) plus PLs (±4μM) (extrinsic pathway) were used as intermediate reagent. The coagulation process was triggered by the addition of FluCa (TGA) or CaCl2 solution (FibWave).
Results: Edoxaban and M4 impacted more the extrinsic than the intrinsic pathway. Interesting, the M4‐metabolite more prolonged clotting time and more reduced the thrombin and fibrin generation than edoxaban. Dabigatran and glucuronide‐dabgiatran impacted acceleration in fibrin assay and time parameters in both assays. Contrary to edoxaban, glucuronide‐dabigatran did not seem to have a different pharmadynamic profile than dabigatran.
Conclusion(s): Contrary to edoxaban and M4‐metabolite, dabigatran and glucuronide‐dabigatran seemed to have similar influence the thrombin and fibrin generation. In the case of an increase in the M4/edoxaban ratio due to hepatic or renal impairments or in case of drug interactions, the impact of M4‐metabolite on coagulation could be more important at usual concentrations.
VPB0573
Real‐life behavior of direct oral anticoagulants in the elderly population with atrial fibrillation
B. Navarro Almenzar 1; F. García‐Candel2; J. Cerezo‐Manchado3
1 Hospital Virgen de la Arrixaca, Murcia, Murcia, Murcia, Spain; 2 Hospital Clínica Universitario Virgen de la Arrixaca, Murcia, Murcia, Spain; 3 Hospital Virgen del Castillo, Murcia, Murcia, Spain
Background: The prevalence of atrial fibrillation (AF) increases with age. In the population over 80 years, this prevalence reaches 17%. However, despite being the majority population, the evidence about the use of direct oral anticoagulants (DOAC) in this subgroup is not clear, especially regarding to the risk of bleeding.
Aims: To analyze the safety and effectiveness of DOAC in the elderly population with AF (≥ 80 years).
Methods: Retrospective multicenter study that included patients with AF on treatment with DOAC (rivaroxaban, apixaban, dabigatran or edoxaban) between January 1, 2013 and December 31, 2016, in Spanish hospitals (Hospital Clínico Universitario Virgen de la Arrixaca, Hospital Comarcal del Noroeste and Hospital Vega Baja). Mortality, bleeding and thromboembolic rates were analyzed. Mean follow‐up was 1.7 years. Statistical analysis was performed using the SPSS® program.
Results: A total of 2,492 patients with AF were included, of which 1,032 were ≥ 80 years. Table 1 shows the main characteristics of this population. When comparing with the general population, a higher mortality rate in the elderly population (10.4 vs 3.8/100 patient‐years, p < 0.001), and ischemic stroke (2.2 vs 1.4/100 patient‐years, p = 0.045) was observed, as well as a trend towards an increase in the rate of major bleeding (3.5 vs 2.6/100 patient‐years, p = 0.080) (Table 2). Most frequent major bleeding was digestive (57%). After dividing the group into age ranges (65‐74, 75‐84 and ≥ 85 years), the mortality and ischemic stroke rates gradually increased. However, this dynamic was not exactly fulfilled for major bleeding. In multivariate analysis, heart failure, chronic obstructive pulmonary disease and neoplasia were significantly associated with increased mortality, while valvulopathy and neoplasia were predictors of major bleeding.
Conclusion(s): The elderly population is especially susceptible, with a higher mortality and ischemic stroke rates, which progressively increase with age. The rate of major bleeding also tends to increase with age.


VPB0575
USP standardized mixtures of bovine, ovine and porcine heparin demonstrate comparable biologic effects to referenced single sourced heparins and may be interchangeable
G. Olson 1; W. Jeske2; O. Iqbal2; A. Farooqui2; F. Siddiqui2; D. Hoppensteadt1; A. Kouta2; J. Fareed1
1 Loyola University Chicago, Maywood, Illinois, United States; 2 Loyola University Medical Center, Maywood, Illinois, United States
Background: Unfractionated heparin (UFH) is the first line anticoagulant for the management of medical indications. Bovine and ovine mucosal sources represent alternate material to the commonly used porcine mucosa for production of UFH. Standardized heparins from various sources can be blended and their potency can be adjusted to exhibit comparable effects as the single sourced UFH.
Aims: The purpose of this study is to evaluate the pharmacologic profile of the blended heparin and compare these activities to that of the single sourced porcine, ovine and bovine heparins.
Methods: Clot based assays were used to study the anticoagulant effects of the heparins. The amidolytic anti‐Xa assay was used to assess the inhibitory effects of these heparins on these proteases. Protamine sulfate (PS) neutralization studies were performed to evaluate the reversal of anticoagulant effects in the heparins.
Results: The aPTT assay showed that at final concentrations of 5 μg/ml and 2.5 μg/ml porcine heparin significantly prolonged the aPTT compared to ovine, bovine, and blended heparins. The potency adjusted heparins demonstrated comparable aPTT values at all concentrations (U/ml) (Figure 1). The anti‐Xa assay showed that at all final concentrations between 10 μg/ml and 0.625 μg/ml porcine, ovine, and blended heparins produced significantly stronger Xa inhibition than bovine heparin. The potency adjusted heparins demonstrated comparable anti‐Xa inhibition at all concentrations (U/ml) (Figure 2). The protamine sulfate neutralization studies demonstrated complete neutralization at all concentrations for all the potency adjusted heparins in the aPTT and anti‐Xa assays.
Conclusion(s): These studies support the hypothesis that a blended heparin product from bovine, ovine, and porcine tissue, when standardized in USP unit‐equivalent proportions, exhibits a comparable anticoagulant profile to the single species heparins. These findings suggest that there is a potential for development of an alternate heparin to stabilize supply chain of this important anticoagulant and warrant clinical validation.


PB0538
Enzymatic activity of bound thrombin within fibrin fibers
J. Crossen 1; S. Diamond2
1 University of Pennsylvania School of Engineering and Applied Sciences, PHILADELPHIA, Pennsylvania, United States; 2 University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: It has been shown previously that thrombin becomes trapped within fibrin fibers during coagulation [Zhu, Chen, and Diamond, ATVB 2018], but not much is known about thrombin activity while bound, nor have there been direct measurements of clot‐bound thrombin by its enzymatic activity.
Aims: In this study, we investigated the activity and stability of thrombin bound to whole blood and plasma clots formed under flow.
Methods: A fluorogenic thrombin sensitive substrate BOC‐VPR‐AMC (628 Da, Km = 21 μM, kcat = 109 s‐1) was perfused over whole blood and plasma clots formed in microfluidic devices and washed with buffer to remove free thrombin. The flow was repeatedly stopped and started to allow for cleavage of the substrate by bound thrombin during stopped flow cycles and washed away during start flow cycles.
Results: It was observed that bound thrombin maintains its enzymatic activity over long periods of time under flow over repeated stop‐start cycles. Three stop/start cycles were averaged to approximate the substrate conversion V0 = 0.78 ± 0.03 μM/s and apparent active clot bound thrombin (IIa) concentration within the reaction volume of 9.24 ± 0.35 nM. The signal from the substrate cleavage comes from a 60 x 250 x 250 μm volume in which the clot forms upon a collagen/tissue factor patterned surface. Clot bound thrombin is confined to a thin fibrin layer ~10‐20 μM in height, therefore it is expected that the actual concentration of active clot bound thrombin is up to 10‐fold higher. This was further expanded to investigate the effect of clot bound thrombin of heparinized plasma perfused with substrate over fully formed clots.
Conclusion(s): Overall, it is important to fully understand the extent that fibrin acts as an anticoagulant via thrombin retention. Thrombin appears to remain stably bound and active within fibrin fibers of clots for long periods of time.

VPB0567
Comparative studies on the interaction of heparins from various origins with heparin cofactor II and antithrombin
N. Baig 1; A. Kouta1; M. Jaradeh2; D. Hoppensteadt3; W. Jeske1; J. Fareed3
1 Loyola University Medical Center, Maywood, Illinois, United States; 2 Loyola University Medical Center, Brookfield, Illinois, United States; 3 Loyola University Chicago, Maywood, Illinois, United States
Background: Unfractionated heparins (UFH) of mucosal origin are currently obtained from bovine, ovine, and porcine sources (BMH, OMH, and PMH respectively). Besides molecular and structural differences, these heparins have differential interactions with serpins such as antithrombin (AT) and heparin cofactor II (HCII).
Aims: The purpose of this study is to compare the inhibitory profiles of various heparins in systems enriched with AT and HCII.
Methods: All heparins (UFH’s and LMWH’s) were supplemented to AT and HCII enriched buffer systems at various gravimetric and potency equated concentrations. The relative inhibition of thrombin (IIa) was measured in terms of percent inhibition, which was calculated for each concentration in both systems for each of the three heparins. Inhibitory concentrations required to inhibit 50% of thrombin (IC‐50) was calculated for each UFH and LMWH.
Results: In the AT supplemented system using gravimetric concentrations of various UFH’s, PMH and OMH showed comparable values, whereas BMH exhibited much higher IC‐50’s. However, the LMWH’s of various origins showed comparable IC‐50 values. The potency adjusted heparins exhibited comparable results for all UFH’s (Figure 1). In the HCII supplemented system, all UFH’s exhibited similar IC‐50 values in both gravimetric and USP potency adjusted studies. Similarly, all LMWH’s demonstrated similar IC‐50 values (Figure 2). The relative IC‐50 values of the LMWH’s in both the AT and HCII enriched systems were higher than the UFH’s.
Conclusion(s): These results show that potency adjusted UFH’s exhibit comparable inhibition of IIa in AT and HCII enriched systems. The differing BMH results observed may partly be due to differences in charge density and AT‐binding consensus sequences. The nearly identical results of all LMWH’s may be due to the chemical modifications that occur during the manufacturing of these agents. Future studies may be warranted to further investigate these cofactors and their impacts on the function of various heparins.


VPB0569
Evaluation of the FibWave in edoxaban and healthy samples, a comparison with the APTT‐ and PT‐based clot waveform analysis
J. Evrard 1; R. Siriez1; J. Dogné2; J. Douxfils3
1 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), Namur, Namur, Belgium; 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), 5000, Namur, Belgium, 31 Qualiblood s.a., 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS)., 5000, Namur, Belgium
Background: Edoxaban is used for several thromboembolic diseases, known to impact coagulation assays and enhance FX‐dependent fibrinolysis and plasmin generation. The activated partial thromboplastin time (APTT) and prothrombin time (PT) are routinely available, but the sensitivity depends on reagent. Beside them, the CWA and FibWave are coagulation assays based on the analysis of clot formation kinetics.
Aims: This study compares the FibWave to the APTT‐ and PT‐based CWA and assesses the fibrinolytic activity in edoxaban and healthy samples.
Methods: APTT, PT and FibWave analysis were performed on 68 healthy individuals (23 men, 22 women not using combined hormonal contraception (no CHC) and 23 women using hormonal contraception (CHC) and 71 edoxaban samples (plasma samples were collected at Cthrough and/or Cmax. The APTT and PT were performed with SynthasIL and ReadiPlasTin. For the FibWave, the intrinsic and extrinsic pathways were assessed with diluted STA‐PTT‐Automate “FibIn” and with phospholipids (±4μM) plus tissue factor (±5 pM) “FibEx”, respectively. The fibrinolysis activity was assessed with tissue plasminogen activator “FibtPA”. For edoxaban samples, concentration was measured using a calibrated chromogenic anti‐Xa assay, Biophen® Direct Anti‐Xa Inhibitor.
Results: Significant differences were only observed in clotting time between healthy individuals with FibEx. Interestingly, with FibEx and PT‐based CWA, the women with hormonal contraception had a higher velocity, acceleration and deceleration of coagulation compared to the other groups (p<.05). Similarly, the fibrinolysis time and velocity were higher in women on CHC compared to men (p<.05). Pearson’s correlation test showed significant correlations between clotting times from APTT/PT/FibEx /FibIn assays and chromogenic measurements. The clotting time from FibEx demonstrated a better correlation (r = 0.89) than PT (r = 0.79), aPTT (r = 0.65) and FibIn (r = 0.61). The fibrinolysis time was prolonged in Cmax compared to Cthrough (p<.05).
Conclusion(s): The FibEx methodology has better potential to reflect the global coagulation activity compared to APTT‐ and PT‐based CWA in healthy and edoxaban samples.
PB0536
Ex vivo study of the concomitant use of plasma‐derived Factor VIII/Von Willebrand Factor and emicizumab in patients with severe Hemophilia A
M. Bravo 1; A. Raventós1; A. Pérez1; E. Arias Salgado2; M. Alvarez‐Román3; N. Butta2; V. Jiménez Yuste4; M. Costa1; T. Willis5
1 Grifols, Parets del Vallès, Catalonia, Spain; 2 Hospital Universitario La Paz‐IdiPaz, Madrid, Madrid, Spain; 3 Hospital Universitario La Paz, Madrid, Madrid, Spain; 4 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain; 5 Grifols, Research Triangle Park, North Carolina, United States
Background: Hemophilia A (HA) patients under emicizumab prophylaxis treatment may require the concomitant use of procoagulant factors for breakthrough bleedings or immune tolerance induction. Previous studies combined emicizumab and plasma‐derived Factor VIII/Von Willebrand Factor (pdFVIII/VWF) in vitro showing a non‐additive effect on thrombin generation (TG).
Aims: To evaluate the procoagulant effect of ex vivo combination of samples from HA patients treated with emicizumab with a pdFVIII/VWF concentrate (Fanhdi®, Grifols).
Methods: Blood samples from 14 adult patients with severe HA without inhibitors on prophylaxis with emicizumab and 12 healthy controls (HCs) were drawn in citrate plus corn trypsin inhibitor. Samples were spiked with increasing concentrations of pdFVIII/VWF (10–400 IU/dl), rFVIIa (0.9 μg/ml) or aPCC (0.5 IU/ml) and analyzed by two global coagulation assays after activation with low tissue factor levels. Whole blood was analyzed by ROTEM coagulation assay measuring clotting time (CT) and time to maximum clot formation velocity (MAXV‐t). Platelet poor plasma was analyzed by TG assay, measuring thrombin peak (TP) and endogenous thrombin potential (ETP) by calibrated automated thrombogram (Thrombinoscope™ software).
Results: Emicizumab treatment alone did not restore clotting to normal levels. The addition of pdFVIII/VWF at 50 IU/dl (≡25 IU/kg) restored these parameters within HCs normal range, similar to that observed after the addition of rFVIIa. Even higher doses of pdFVIII/VWF, up to 200IU/kg, did not result in excessive procoagulant profile. However, subtherapeutic dose of aPCC resulted in a synergistic procoagulant profile with coagulation values outside the normal range (Table 1). Moreover, coagulation parameters of both methods correlated at baseline and with increasing concentrations of pdFVIII/VWF.
Conclusion(s): The concomitant use of pdFVIII/VWF in HA patients with prophylaxis with emicizumab did not trigger a multiplying procoagulant effect. These results were aligned with previous in vitro data suggesting the low risk of overdose and thrombotic events of concomitant treatment with emicizumab and the pdFVIII/VWF concentrate.

PB0540
Investigation of GGCX interaction with VKD proteins
F. Forin 1; S. Ghosh1; J. Müller2; J. Oldenburg3; A. Biswas2; K. Czogalla1
1 Universitätsklinikum Bonn Institut für Experimentelle Hämatologie und Transfusionsmedizin, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: γ‐glutamyl carboxylase (GGCX) γ‐carboxylates vitamin K dependent (VKD) proteins including the procoagulatory factors FII, FVII, FIX, FX and anti‐coagulatory proteins Protein C (PC). This process is essential to maintain blood homeostasis. Interestingly, GGCX has different affinities for the propeptides of the VKD coagulatory proteins.
Aims: The aim of this study is to evaluate the mechanism of differential interaction of GGCX with VKD coagulatory proteins using in silico tools, which will be verified by in vitro assays.
Methods: In silico models of GGCX and FII, FVII, FIX, FX, and PC were downloaded from ITASSER and AlphaFold, where the propeptide and the Gla domain were further considered. These VKD coagulatory proteins were docked on both GGCX model in H‐dock server. The models with highest docking score were aligned and protein‐protein interaction between GGCX and VKD coagulatory proteins were analyzed in Protein Interaction Calculator server.
Results: The alignment of VKD coagulatory protein‐GGCX complex in ITASSER showed that these proteins are binding to GGCX in different clusters.FIX and PC clustered together, whereas FX binds nearby in a different orientation, and FVII and FII formed a third cluster. Interestingly, protein‐protein interaction analysis showed different interacting residues between ITASSER and AlphaFold models. Therefore, these substrate specific interacting residues in GGCX were mutated to alanine in silico. GGCX mutations f.e. R498A, E518A (ITASSER model) and E373A, Y379A (AlphaFold model) showed highest change in binding energy that will be further analyzed in vitro to obtain the closest model for the interaction analysis.
Conclusion(s): Our data indicates that VKD coagulatory proteins orient differentially once the propeptide of the VKD protein has bound to the same propeptide binding site in GGCX (495‐513). This leads to differential interaction between residues in GGCX and VKD coagulatory proteins. However, there are differences in the models of ITASSER and AlphaFold that will be further validated.
PB0544
Impact of fasting on INR in Ramadan on Muslim population taking warfarin.
A. Jahangir; S. khan; M. Quraishi; A. Taqi
Tabba Heart Institute, Karachi, Sindh, Pakistan
Background: Warfarin is one of the most extensively used anticoagulant globally. The mechanism of anticoagulation of warfarin is influenced by many factors like diet and underlying illness but fasting has variable results.
Aims: To assess the impact of fasting on INR of the patients who have been taking warfarin, in the month of Ramadan.
Methods: We conducted a retrospective observational study on 30 patients who were on warfarin for atrial fibrillation and prosthetic valve replacement. INR readings were recorded before, during and after Ramazan. INR samples were compared using McNemar Bowker test.
Results: Statistically significant differences observed among INR levels for the comparison group before vs during Ramadan (p = 0.024) and after vs during Ramadan (p = 0.027) but no significant difference observed for before vs after Ramadan. INR Supratherapeutic levels inclined from 26.7% to 70% from before Ramadan to during Ramadan and declined from 70% to 30% from during Ramadan to after Ramadan. TTR (time in therapeutic range) remained same before and after Ramadan i.e. 56.7% but declined to 23.3% during Ramadan. Whereas there is significant decline in sub therapeutic levels of INR in the month of Ramadan by 6.7% as compared to pre Ramadan 16.7% and post Ramadan 13.3% respectively. Out of 30 only 3 patients bleed resulting from supratherapeutic levels during Ramadan whereas no thrombotic events were reported.
Conclusion(s): Fasting has a significant impact on patients who have achieved TTR before Ramadan as fasting markedly increased INR in the month of Ramadan.


PB0553
The antithrombotic protein C activator thrombin mutant W215A/E217A (AB002, E‐WE) is slowly inhibited by the endogenous thrombin inhibitors antithrombin, protein C inhibitor, and heparin cofactor II
B. Markway 1; M. Carris2; N. Verbout1; M. Wallisch1; C. Lorentz1; A. Gruber1; E. Tucker1
1 Aronora, Inc., Portland, Oregon, United States; 2 Aronora, Portland, Oregon, United States
Background: AB002 is a recombinant thrombin analog (W215A/E217A, E‐WE thrombin) in phase 2 clinical development. Compared to thrombin, AB002 exhibits diminished activity toward prothrombotic substrates but can activate protein C when bound to thrombomodulin. These properties, in addition to a reduced rate of inhibition by antithrombin, presumably combine to produce a potent antithrombotic effect. However, the relative contributions of plasma serpins to AB002 inhibition is unknown.
Aims: To investigate the ability of antithrombin, protein C inhibitor (PCI), and heparin cofactor II (HCII) to inhibit AB002 in the absence or presence of cofactors, and to evaluate circulating serpin‐enzyme complexes following intravenous AB002 administration.
Methods: The time course of inhibitor complex formation following AB002 addition to prothrombin‐depleted plasma or buffer containing purified antithrombin, PCI, or HCII +/‐ heparin or thrombomodulin was evaluated by Western blot and SDS‐PAGE, respectively. Human plasma samples (n = 3) from an AB002 phase 1 clinical trial (NCT03453060) were evaluated for serpin‐enzyme complexes by ELISAs.
Results: AB002‐inhibitor complexes were not detected within 90 min of AB002 addition to plasma without addition of heparin or thrombomodulin (Figure 1). In buffer, the addition of either antithrombin or HCII to AB002 did not produce observable complex formation within 20 min, compared to PCI, which formed complexes within minutes. Thrombin rapidly complexed with all inhibitors. Heparin and thrombomodulin had the greatest effect on AB002‐HCII and AB002‐PCI, respectively. In AB002‐dosed subjects (4 mcg/kg), there was a temporal increase in PCI, HCII, and antithrombin complexes (Figure 2). HCII complexes were the most prevalent and peaked at 15 min post‐dose, returning to baseline by ~2 h post‐dose.
Conclusion(s): Our in vitro data suggest that, compared to thrombin, AB002 is relatively slowly inhibited by serpins including antithrombin, PCI, and HCII. All three inhibitors may play a role in regulating AB002’s effects in vivo, with relative contributions likely dependent on local availability of cofactors.


PB0555
Hypoplasminogenemia: an International retroSpecTive and prOspective cohoRt studY (HISTORY): the international registry of patients with plasminogen deficiency
M. Menegatti 1; J. Bowen2; M. Boscarino1; C. Nakar2; R. Palla3; S. Siboni4; M. Torres5; M. Batur6; R. Natesirinilkul7; C. Roberson2; H. McDaniel8; S. Al9; N. Rodriguez10; T. Celkan11; J. Ceresetto12; N. Thukral2; B. Hardesty2; F. Peyvandi13; A. Shapiro14
1 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 2 Indiana Hemophilia & Thrombosis Center, Indianapolis, IN, United States, Indianapolis, Indiana, United States; 3 Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 4 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 5 Cook Children’s Medical Center, Fort Worth, Texas, United States; 6 Van Yuzuncu Yil University, Ophthalmology Department, Van, Turkey, Van, Van, Turkey; 7 Faculty of Medicine Chiang Mai University, Chiang Mai, Thailand, Chiang Mai, Chiang Mai, Thailand; 8 Vanderbilt University Medical Center, Division of Pediatric Hematology/Oncology, Nashville, TN, United States, Nashville, Tennessee, United States; 9 Dokuz Eylül University Research Clinical Immunology, Izmir, Turkey, Izmir, Izmir, Turkey, 10 The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, TX, United States, Houston, Texas, United States, 11 İstinye University, Istanbul, Turkey, Istanbul, Istanbul, Turkey, 12 Servicio de Hematología y Hemoterapia. Hospital Británico de Buenos Aires, CABA, Ciudad Autonoma de Buenos Aires, Argentina, 13 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy, 14 Indiana Hemophilia and Thrombosis Center, Indianapolis, Indiana, United States
Background: Plasminogen (PLG) deficiency (PLGD), a rare inherited chronic disorder, affects ~1.6 per million population. Uncontrolled growth of fibrin‐rich pseudomembranes on mucous membranes of eyes, oral cavity, middle ears, respiratory, GU, GI, renal tracts, CNS, and skin is associated with morbidity and mortality. Ligneous conjunctivitis is the most common manifestation (~80%) and may lead to sight loss. Data regarding management of patients with PLG deficiency is scarce and based on case reports/series and small clinical trials. In 2021, the USFDA approved the first IV PLG concentrate; another specific therapy, PLG ophthalmologic concentrate, is not commercially available.
Aims: HISTORY is the first comprehensive retrospective/prospective international registry created to investigate PLGD natural history, and ultimately develop severity categories and treatment guidelines.
Methods: Up to 100 probands with their 1st degree non‐affected family members will be enrolled in the 4 ‐year study. Clinical, genetic, and laboratory data are collected at baseline, and PLGD‐related symptoms at study visits; unscheduled visits are included (new lesions, pregnancy). Specimens are collected for PLG activity (PLG:C), antigen (PLG:Ag), and molecular characterization.
Results: To date, 11 centers (6 countries) are actively enrolling with 115 subjects entered. Analysis was performed on 27 subjects (21 probands, 6 siblings) with complete data available; Table 1 includes demographic data and most frequently affected areas. Mean PLG:C and PLG:Ag were 15.3% (nr 80 – 132%) and 15.8 μg/ml (nr 70 – 215), respectively. Eyes and ear lesions were reported early in life, 1 and 3.5 years (median), respectively (Figure).
Conclusion(s): HISTORY is the first comprehensive retrospective/prospective international registry to document and evaluate PLGD current knowledge gaps. This first analysis demonstrates a significant decrease in PLG:C and PLG:Ag levels in affected patients, and that severe symptoms including eye and ear lesions occur early in life. Interestingly, the study has not yet recorded a subject of African descent.


VPB0577
Impacts of antithrombotic agents on cellular physiology in the pancreatic tumoral environment
H. Tran1; P. Van Dreden2; E. Mbemba3; I. Elalamy4; G. Gerotziafas 5
1 Clinical Research Department, Diagnostica Stago, Gennevilliers, France.; Sorbonne Université, INSERM UMR_S938, Research Group « Cancer‐Hemostasis‐Angiogenesis », Centre de Recherche Saint‐Antoine, Institut Universitaire de Cancérologie, Paris, France., Gennevilliers, Ile‐de‐France, France; 2 Diagnostica Stago, Gennevilliers, France., Gennevilliers, Ile‐de‐France, France; 3 Sorbonne Université, INSERM UMR_S938, Research Group « Cancer‐Hemostasis‐Angiogenesis », Centre de Recherche Saint‐Antoine, Institut Universitaire de Cancérologie, Paris, France., Paris, Ile‐de‐France, France; 4 Thrombosis and Haemostasis Centre, Biological Hematology Department, Hôpital Tenon, Assistance Publique‐Hôpitaux de Paris (AP‐HP), Sorbonne Université, Paris, France.; Department of Obstetrics and Gynecology, I M Sechenov First Moscow State Medical University, Moscow, Russia, Paris, Ile‐de‐France, France; 5 Sorbonne Université, INSERM UMR_S938, Research Group « Cancer‐Hemostasis‐Angiogenesis », Centre de Recherche Saint‐Antoine, Institut Universitaire de Cancérologie, Paris, France ; Thrombosis and Haemostasis Centre, Biological Hematology Department, Hôpital Tenon, Assistance Pu‐blique‐Hôpitaux de Paris (AP‐HP), Sorbonne Université, Paris, France., Paris, Ile‐de‐France, France
Background: Antithrombotic agents (AG) potentially exert an inhibition of the procoagulant properties of tumoral microenvironment and interfere with cancer cell biology, but this has been inadequately studied so far.
Aims: To investigate the role of LMWHs and the specific factor Xa inhibitors apixaban and fondaparinux on the procoagulant potential and physiology of pancreatic cancer cell line BXPC3 and their microenvironment.
Methods: BXPC3 cells were exposed to either enoxaparin, tinzaparin (2 anti‐Xa UI/ml), fondaparinux (2 μg/ml), or apixaban (2 μg/ml) for 48 h. The procoagulant properties of BXPC3 and their conditioned media (CM) were then assessed with the Calibrated Automated Thrombogram®. The viability of BXPC3 was tested with the MTT assay. mRNA expression of Tissue Factor (TF), Vascular Endothelial Growth Factor (VEGF), and Thrombospondin 1 (THSB1) was evaluated by RT‐qPCR. Residual concentrations of the AG in the conditioned media was measured with specific anti‐Xa amidolytic assays.
Results: Exposure to apixaban resulted in significant decrease of TF mRNA expression and a significant increase of that of THBS1. Fondaparinux and enoxaparin resulted in significant decrease of mRNA expression of VEGF. Cell viability was reduced by enoxaparin (14%), tinzaparin (11%), fondaparinux (12%), and apixaban (30%). AG did not significantly modify the impact of BXPC3 cells on thrombin generation but significantly impaired that capacity of the CM. Exposure to BXPC3 induced a significant decrease of the concentration of fondaparinux (27%), enoxaparin (48%) and tinzaparin (26%) in the CM, but did not significantly impact that of apixaban.
Conclusion(s): AG interfere with tumoral microenvironment not only by impairing the pro‐coagulant properties of cancer cells’ secretome, but also by altering cell viability, and gene expression of pro‐ and antiangiogenic factors VEGF and THBS1. Nonetheless, a degradation of LMWH and fondaparinux was observed after two days of exposure to cancer cells. Apixaban escaped of any deteriorating effect of cancer cells and preserved its antithrombotic potency.
PB0535
Structural basis of activated factor X binding to antithrombin not activated by heparinoids, insights from enhanced sampling molecular dynamics simulations
G. Balogh 1; Z. Bereczky2
1 Department of Laboratory Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hajdu‐Bihar, Hungary; 2 Faculty of Medicine, University of Debrecen, Debrecen, Hajdu‐Bihar, Hungary
Background: The activity of antithrombin, a critical regulator of the coagulation process, is significantly enhanced by high affinity heparin pentasaccharide against factor Xa and IXa. An X‐ray diffraction structure has been published for pentasaccharide‐activated antithrombin complexed with an inactivated factor Xa mutant. However, the currently available information about the binding of the non‐activated serpin to FXa is limited to mutagenesis data.
Aims: Our aim was to build model systems using protein docking and enhanced sampling molecular dynamics simulations that can describe the binding of factor Xa to antithrombin in its non‐activated state.
Methods: We have performed molecular docking using the available structures of the two proteins using HADDOCK (the GLA domain was removed from factor Xa). The highest‐ranked structure was then used in Gaussian Accelerated Molecular Dynamics simulations for further refinement of the structure and to evaluate its stability. Additionally, we started simulations based on the available X‐ray structure using the same method, both for systems containing and not containing the pentasaccharide.
Results: Based on the molecular docking and enhanced sampling molecular dynamics simulations, we propose a model structure in which factor Xa can interact with non‐activated antithrombin in a conformation with a “closed” hinge region and no heparin pentasaccharide bound. The simulations show differences in the interaction with the “exosite” of antithrombin compared to the available X‐ray structure for the complex with the activated form. The proposed complex conformation is in good agreement with the available mutagenesis data. From the simulations based on the X‐ray structure with the pentasaccharide deleted, we have investigated the changes in conformation compared to the ligand‐bound form. These changes provide information on how the pentasaccharide affects the interaction with FXa allosterically.
Conclusion(s): Our results provide a deeper insight into the mechanisms how pentasaccharide ligands activate antithrombin against its target factors.

PB0543
Extracellular vesicles from human body fluids trigger coagulation directly by exposing functional tissue factor/factor VIIa complexes
Y. Hu 1; C. Hau2; C. Ay3; A. Repa4; I. Pabinger3; R. Nieuwland2; J. Thaler5
1 Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 2 Laboratory of Experimental Clinical Chemistry, Vesicle Observation Center, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 4 Clinical Division of Neonatology, Paediatric Intensive Care & Neuropaediatrics, Department of Paediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 5 Clinical Division of Haematology and Haemostaseology, Department of Medicine I, v, Vienna, Wien, Austria
Background: Tissue factor (TF) is the transmembrane receptor of (activated) coagulation factor (F)VII(a). Together membrane‐bound TF and FVIIa form the so‐called “extrinsic tenase” complex, which directly triggers coagulation by activating FX to FXa. Surprisingly, TF occurs not only in the vessel wall, but is also present on extracellular vesicles (EVs) in body fluids such as amniotic fluid, milk, saliva, and urine. In amniotic fluid also FVII(a) has been encountered.
Aims: To investigate whether TF‐exposing EVs and FVII(a) form functional extrinsic tenase complexes in physiological body fluids.
Methods: Milk, saliva, and urine were collected from healthy breastfeeding women (n = 6), and amniotic fluid was collected from healthy women undergoing routine amniocentesis (n = 7). EVs were isolated by size exclusion chromatography (SEC) and coagulation (clotting) experiments were performed in the presence and absence of antibodies against TF and FVIIa in normal plasma and in FVII‐deficient plasma.
Results: All body fluids (5% vol/vol) triggered clotting of normal plasma (mean clotting time after addition of milk: 89 ± 27 s, amniotic fluid 375 ± 148 s, saliva 514 ± 110 s, urine 1672 ± 1081 s, all p‐values [compared to buffer] < 0.01) and of FVII‐deficient plasma (milk: 123 ± 74 s, followed by amniotic fluid 530 ± 139 s, saliva 902 ± 602 s, urine 2678 ± 642 s, all p‐values < 0.05), which was almost completely inhibited by anti‐FVII and to a lesser extend by anti‐TF. Fractionation of body fluids by SEC showed that only the fractions containing EVs triggered clotting and generated FXa in normal plasma and FVII‐deficient plasma, which again was almost completely inhibited by anti‐FVII and partially by anti‐TF.
Conclusion(s): We demonstrate that normal human body fluids contain functional extrinsic tenase complexes in the form of TF/FVIIa‐complex exposing EVs. These EVs activate FX directly, thereby bypassing a major part of the coagulation cascade and may provide direct hemostatic protection.

PB0554
Molecular, laboratory and clinical characteristics of inherited antithrombin deficiency: a large, single centre cohort study from the United Kingdom.
K. Mayger 1; A. Doyle2; M. Mitchell3; R. Wheeler4; B. Hunt2
1 Guys and St Thomas' NHS Trust, London, England, United Kingdom; 2 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 3 Viapath Analytics LLP, Guy's & St. Thomas' NHSFT, LONDON, England, United Kingdom; 4 Viapath Analytics LLP, LONDON, England, United Kingdom
Background: Inherited antithrombin (AT) deficiency is an autosomal dominant thrombophilia resulting from variants in SERPINC1. It is associated with a high risk of venous thromboembolism (VTE).
Aims: To describe a large patient cohort with AT deficiency.
Methods: A single centre registry of 109 patients (40 male: 69 female). Patient consent was obtained to perform PCR‐mediated Sanger sequencing for all SERPINC1 exons. Multiplex ligation‐dependent probe amplification was used for copy number analysis to detect large deletions or duplications. AT activity and antigen levels were measured using established assays (Hyphen Biophen, France).
Results: We identified 43 causative variants in SERPINC1 gene including 14 novel. The most frequent variant (n = 11) was AT Northwick Park (p.Arg425Cys). The variant types were: missense (66%), deletions (19% ‐one whole gene deletion), splice site (6%), duplications (2%), nonsense (1%), insertions (1%), unidentifiable (1%). Amongst all patients, 42% had Type 1 AT defects, 37% Type 2 and 21% were unclassified. Among Type 2 defects, 18% were heparin binding site (HBS), 15% reactive site (RS), and 3% pleiotropic (T2PE). Three unrelated patients had homozygous HBS defects for AT Budapest 3 (p.Leu131Phe). Two compound heterozygous variants (p.Asn450His and p.Arg457Thr) were identified in one family; consistent with Type 1 defect. FVL mutation was detected in 6 /77 (8%), of which five (83%) had VTE; PT20210 was detected in one case (1%). Table 1 describes the clinical characteristics of this cohort. The median age of VTE in all AT defects was < 50 years. There was a higher rate of VTE recurrence in those with Type 1 defects in comparison to Type 2 (57% vs 25%, d.f = 1, χ2=5.2, p = 0.02)
Conclusion(s): We demonstrate a high heterogeneity of SERPINC1 defects in the UK population with a predominance of Type 1. Approximately half of this cohort had no clear family history of VTE and/or AT deficiency.
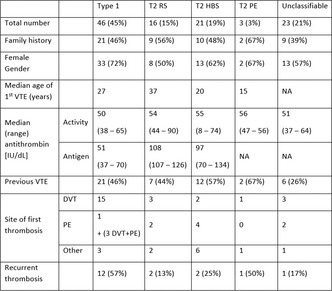
PB0548
Abelacimab, a factor XI/XIa antibody inhibits clotting in hemodialysis circuits ex vivo
K. Kovacevic 1; C. Schörgenhofer2; G. Langer2; Y. Khder3; B. Jilma2; J. Grafeneder4
1 Medical University of Vienna, Wien, Wien, Austria; 2 Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Wien, Austria; 3 MYRA LSS, Rosenau, Alsace, France; 4 Department of Emergency Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: Effective anticoagulants are needed to avoid blood clotting during extracorporeal circulation. Alternative to conventionally used heparins could be drugs targeting factor XI (FXI)/activated FXI (FXIa) such as the novel monoclonal antibody abelacimab.
Aims: We compared the effects of abelacimab used in combination with enoxaparin to enoxaparin alone.
Methods: Whole blood was collected from 10 healthy volunteers in two bags containing either 1.2 mg enoxaparin alone (control group) or 1.2 mg enoxaparin plus 5 mg abelacimab (treatment group). Each bag was attached to a hemodialysis device, and blood was circulated for a maximum of 3 hours or until the device stopped due to clotting, as assessed by a sudden rise in transmembrane pressure. We measured coagulation parameters, performed whole blood aggregation and thromboelastometry at baseline, 15 min, 30 min, 1 h, 2 h and 3 h after circuit initiation. The primary endpoint was time to filter clotting.
Results: 8 male and 2 female (mean age: 37 ± 7 years) volunteers were included. Abelacimab in combination with enoxaparin significantly prolonged the time to circuit clotting from a median of 120 min (IQR:97‐147) to 180 min (IQR:180‐180) (p < 0.001) and decreased the transmembrane pressure from 66 mmHg to 13 mmHg at the end of the circuit flow (p = 0.042). Furthermore, it preserved median fibrinogen levels (207 mg/dl vs. 178 mg/dl) (p = 0.038) and median platelets counts (135 x109/L vs 106 x109/L) (p = 0.002), improved ristocetin (22U vs. 2U) (p = 0.015); thrombin receptor activating peptide (29U vs. 19U) (p = 0.015) and arachidonic acid (23U vs. 17U) (p = 0.001) induced platelet aggregation; and prolonged clotting times in thromboelastometry (INTEM (p < 0.001) and HEPTEM (p = 0.003) at the end of the experiments.
Conclusion(s): In an ex vivo model of hemodialysis that is aggressive due to the frequent re‐circulation of blood and lack of endothelial cells, inhibition of FXI/FXIa by abelacimab prolonged time to circuit clotting, providing support for testing abelacimab in patients on hemodialysis.

PB0558
Thigh‐tourniquet application during knee arthroscopy does not induce a hypercoagulable state ‐ A randomized trial into the effect of tourniquet use on the coagulation system
B. Nemeth 1; C. Touw2; S. le Cessie3; R. Onstenk4; V. Wennemers4; P. Haen4; R. van den Wijngaard4; R. van Adrichem5; R. Nelissen6; T. Lisman7; S. Cannegieter1
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 Department of Clinical Epidemiology and Department of Medical Statistics and Bioinformatics, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 4 Department of Orthopaedic Surgery, Groene Hart Hospital, Gouda, Zuid‐Holland, Netherlands; 5 Department of orthopaedics, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 6 Department of orthopaedics Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 7 University Medical Center Groningen, Groningen, The Netherlands, Groningen, Not Applicable, Netherlands
Background: Knee arthroscopy is associated with an increased risk of venous thromboembolism (VTE), in which the role of thigh‐tourniquet application is unknown.
Aims: To study the effect of a thigh‐tourniquet application on coagulation, fibrinolysis, endothelial activation, inflammation and hypoxia.
Methods: A single center, non‐blinded, randomized controlled trial was conducted. Patients >18 years scheduled for knee arthroscopy were randomized to tourniquet or no tourniquet application. Most important exclusion criteria were: any coagulation disorder, pregnancy, hormonal contraceptive use or a history of VTE. Blood was drawn from the cubital vein preoperatively (T0), at the end of surgery (T1) and one hour after surgery (T2). At T1 and T2, blood was also drawn from the great saphenous vein of the operated leg (Figure 1). Lactate, white blood cell count (WBCC), von Willebrand Factor (VWF) antigen, E‐Selectin, thrombomodulin, factor (F)VIII activity, D‐dimer, thrombin generation potential and clot lysis time were measured. For each person, mean changes between T0 and T1 or T2 were obtained by a paired t‐tests. Differences in change between groups were obtained by linear mixed models.
Results: 286 patients were assessed for inclusion, 28 and 27 patients were randomized to tourniquet (Group I) and no tourniquet (Group II) application, respectively. Systemic and local measurements yielded similar results; therefore, only systemic results are shown. Postoperative changes of hypoxia (lactate) and inflammation (WBCC) markers did not differ between groups. This was also true for most markers of endothelial activation, coagulation and fibrinolysis. Only VWF and FVIII levels were different between groups. Whereas FVIII and VWF levels declined at T2 in the tourniquet group, these levels increased in the no‐tourniquet group resulting in a significant difference between groups (Table 1).
Conclusion(s): Tourniquet application during knee arthroscopy did not affect coagulation, fibrinolysis, endothelial activation, inflammation or hypoxia and therefore does not appear to explain the increased VTE risk. ClinicalTrials.gov:NCT02567903 Funding: Foundation Anna Fonds|NOREF
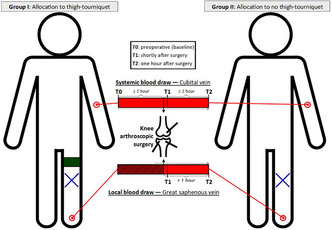

PB0561
Ca2+ and Zn2+ binding to human Protein Z (PZ) induces structural changes that augments its lipid binding
T. Sengupta 1; S. Gayathri2; S. Gupta3
1 Sri Sivasubramaniya Nadar College of Engineering, Chennai, Tamil Nadu, India; 2 Sosei Heptares, Cambridge, Cambridge, England, United Kingdom; 3 Indian Association for the Cultivation of Science, Kolkata, West Bengal, India
Background: Calcium ion plays a pivotal role in blood coagulation. Primarily, it helps in proper folding of the Gla domain through which coagulation proteins bind to the membrane surface. Zinc, the second most abundant cation in plasma and another key regulator of coagulation, has also been shown to bind in the Gla domains of a few coagulation proteins (fVIIa, PC, fXII). However, whether it can exert similar structural effect like Ca2+ is not clear.
Aims: The current study is therefore aimed to find if Zn2+ induces any conformational change in the Gla domain of the anticoagulant PZ, and help in its membrane binding. A comparison on the effects of Zn2+ and Ca2+ binding on the structure of PZ revealed structural changes that may have potential implications on the dynamics of the PZ‐ZPI complex and membrane binding
Methods: Full length apo‐PZ structure was modelled using homology modeling and geometry minimization. Ca2+ and Zn2+ bound holo structures of PZ and PZ‐ZPI complex were then subjected to MD simulation using AMBER.
Results: Ca2+ induces a conformational change in apo‐PZ where the Gla domain moves towards SP like domain. The folded Gla domain of the holo‐protein appears to create a pocket that may stabilize the PZ‐ZPI interaction. Replacing Ca2+ ions by Zn2+ in the holo structure showed no appreciable change in the overall conformation of PZ (Figure 1). However, de novo Zn2+ binding cannot cause the apo protein to fold as much as it does in presence of Ca2+. One Zn2+ was also found to be present in the EGF domain of PZ, inducing important structural changes that may influence membrane binding.
Conclusion(s): Zn2+ induces similar structural changes in the Gla domain of PZ as exerted by Ca2+ and therefore can regulate the activity of PZ‐ZPI system by augmenting the lipid binding of PZ.

PB0563
The ultra‐sensitive Nijmegen Inhibitor Assay detects very low‐titer factor VIII inhibitors
L. Valke 1; M. Verhagen2; B. Mulders3; R. Polenewen3; S. Schols4; W. van Heerde5; D. Meijer6
1 Radboud University Medical Center, Nijmegen, the Netherlands; Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands; 2 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, Nijmegen, the Netherlands; Laboratory of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 3 Department of Laboratory Medicine, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 4 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands; 5 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, the Netherlands; Enzyre BV, Novio Tech Campus, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 6 Department of Laboratory Medicine, Laboratory of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands
Background: Treatment of hemophilia A (HA) with factor VIII (FVIII) replacement therapy can be complicated by inhibitor formation against exogenous infused FVIII, rendering therapy ineffective. Inhibitors can also be formed against endogenous FVIII, causing acquired HA (AHA). In both cases inhibitor formation is related with an increased bleeding risk. The ISTH recommends the Nijmegen Bethesda Assay (NBA) for detecting and quantifying inhibitors, but the assay is only able to detect inhibitor levels above the cut‐off level of 0.6 NBU/ml.
Aims: Introduction of the ultra‐sensitive Nijmegen Inhibitor Assay (usNIA) for detecting very low‐titer FVIII inhibitors.
Methods: The usNIA is an adaptation of the NBA, in which the ratio of patient sample to normal pooled plasma is changed from 1:1 (NBA) to 9:1 (usNIA). Residual FVIII activity was determined with a chromogenic assay (FVIII:C, Biophen Biomed) using a low FVIII:C calibration line (0‐15%) and inhibitor titers were extrapolated. A recombinant FVIII inhibitor with a known titer was diluted (0.05‐0.80 NBU/ml) to generate a calibration curve and assess the usNIA lower limit of quantification (LLOQ, Figure 1). Furthermore, corresponding usNIA, NBA and FVIII activity levels were determined in sequential plasma samples from three patients with AHA to analyze the interplay between these parameters. Patients gave consent for research usage of left‐over diagnostic material
Results: Healthy controls (n = 49) had no detectable inhibitors (mean residual FVIII 109%, range 102‐116%). The usNIA LLOQ is determined as 0.1 NBU/ml, with an associated coefficient of variation of 12%. In AHA patients the usNIA detected FVIII inhibitory antibodies, even when the NBA indicated an undetectable titer (< 0.6 NBU/ml, table).
Conclusion(s): The usNIA is able to detect low‐titer FVIII inhibitor titers up to 0.1 NBU/ml. The usNIA will be further validated in clinical practice to prove its value in early inhibitor detection and therapy adjustments in patients with congenital and acquired HA.


VPB0574
Real‐life behavior of direct oral anticoagulants in the population with chronic kidney disease and atrial fibrillation
B. Navarro Almenzar 1; F. García‐Candel2; J. Cerezo‐Manchado3
1 Hospital Virgen de la Arrixaca, Murcia, Murcia, Murcia, Spain; 2 Hospital Clínica Universitario Virgen de la Arrixaca, Murcia, Murcia, Spain; 3 Hospital Virgen del Castillo, Murcia, Murcia, Spain
Background: Atrial fibrillation (AF) is the most prevalent arrhythmia in the world and the main cause of anticoagulation. Currently, many patients are being treated with direct oral anticoagulants (DOAC) for stroke prevention in this context. Among these patients, there is a considerable percentage with renal failure, in whom the clearance of the drug is decreased, with the potential bleeding effects that may result from accumulation of doses.
Aims: To analyze the safety and efficacy of DOAC in the population with atrial fibrillation and renal failure.
Methods: Retrospective study that included patients with AF and renal failure (clearance < 50 ml/min), who started treatment with DOAC (apixaban, rivaroxaban, dabigatran or edoxaban) between January 2013, and December 2016, in three Spanish hospitals (Hospital Clínico Universitario Virgen de la Arrixaca, Hospital Comarcal del Noroeste and Hospital Vega Baja). Rates of death, ischemic stroke and hemorrhagic events were analyzed. The mean follow‐up was 1.7 years. Statistical analysis was performed with SPSS® program.
Results: Of the 2,492 patients in the registry, 522 had kidney failure. Table 1 shows the main characteristics of this group. The most frequent origin of major bleeding was the digestive one (58%). When comparing with the group without renal failure, it was observed that this group had higher mortality (10.1 vs 4.6, p < 0.001), ischemic stroke (2.7 vs 1.5, p = 0.023) and major bleeding (4.1 vs 2.8, p = 0.043). When we divided groups according to creatinine clearance (15‐29, 30‐49 and ≥ 50 ml/min), we observed that the mortality (p < 0.001) and ischemic stroke (p = 0.072) rates increased with the grade of renal failure. We did not observed this effect for bleeding rate. In multivariate analysis, valvulopathy was an independent factor for death.
Conclusion(s): Patients with kidney failure have a higher risk of death, ischemic strokes and major bleeding, therefore a close monitoring of these patients is essential.


PB0546
Measurement of β‐antithrombin activity in healthy individuals and in patients with different types of antithrombin deficiency
É. Katona 1; R. Gindele2; É. Molnár2; E. Uj2; Z. Bereczky3
1 University of Debrecen, Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary; 2 Division of Clinical Laboratory Science, Department of Laboratory Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hajdu‐Bihar, Hungary; 3 Faculty of Medicine, University of Debrecen, Debrecen, Hajdu‐Bihar, Hungary
Background: The β glycoform of antithrombin (AT) has higher heparin affinity than α‐form and accounts for 5‐10% of total AT in normal plasma. β‐AT is thought to play a greater role in the prevention of thrombosis during vessel‐wall injury. It may have an impact on the thrombotic risk in AT deficiency. Since measurement of this isoform is not performed routinely, the level of β‐AT in these patients is unknown.
Aims: To determine the activity of total and β‐AT in healthy controls and in AT deficient patients.
Methods: Anti‐FXa chromogenic method in the presence of heparin and its modified version in the presence of high (1.1M) NaCl concentration were used in 50 controls and 79 patients.
Results: In the controls, the median (IQR) total AT activity was 105 (98‐116)% and the β‐AT activity was ‐ as expected – 8.9 (8.2‐9.2)%. The calculated β‐AT percentage of total AT activity (β‐AT%) was 8.3 (7.9‐8.8)%. In type II heparin binding site (IIHBS) deficient AT Budapest 3 heterozygotes (n = 50) the total AT activity, the β‐AT activity and the β‐AT% were 58 (54‐61)%, 8.4 (8.2‐8.9)% and 14.5 (13.9‐15.6)%, respectively. These results were in concordance with those measured in AT type IIHBS Basel and Padua (n = 2) patients. In AT Budapest 3 homozygotes (n = 9) total AT activity, β‐AT activity and β‐AT% were 17 (15‐26)%, 7.4 (6.8‐10.2)% and 41.6 (38.6‐48.0)%, respectively. On the contrary, in patients having type I AT defect – although total AT activity was comparable with type IIHBS heterozygotes, 52 (46‐53)% ‐ the β‐AT activity was significantly lower, 6.8 (6.5‐7.2)%.
Conclusion(s): Our results show that – while in type I quantitative AT deficiency the total and β‐AT activity are proportionally decreased ‐ the activity of β‐AT is less affected by type IIHBS AT mutations. These results support the clinical observations of less severe thrombotic phenotype in type IIHBS deficiency.
PB0562
Modifications in the thrombin active site S4 subpocket induce resistance to direct thrombin inhibitors
V. Strijbis 1; K. Cheung2; T. Rutten2; B. De Bruin2; P. Reitsma3; D. Verhoef3; M. Bos4
1 Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 2 Div. Thrombosis and Hemostasis, Einthoven Laboratory, for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands, 31VarmX, Leiden, The Netherlands. 2Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 4 Leiden University Medical Center
Background: Direct anticoagulants inhibit coagulation serine proteases by reversibly engaging their active site with high affinity. To expand the repertoire of specific reversal agents we have previously successfully modified the S4 active site subpocket to prevent specific ligand binding and preserve catalytic activity in factor Xa [Verhoef 2017 Nature Commun.]. Whether an analogous strategy can be applied to introduce resistance to direct inhibitors in thrombin is unclear.
Aims: Assess if specific substitutions or sequences in or around the S4 subsite derived from naturally occurring serine proteases introduce inhibitor resistance in (pro)thrombin.
Methods: Recombinant prothrombin variants were generated with substitution of the S4 subsite residue I174 or exchange of the 91‐99 sequence, directly adjacent to the S4 subsite, with that of human kallikrein‐3. Following purification using the CaptureSelect matrix selecting for fully gamma‐carboxylated prothrombin, the variants were biochemically assessed for their sensitivity towards direct thrombin inhibitors.
Results: The specific prothrombin clotting activity of the variants was 6‐fold (intrinsic clotting) to 10‐fold (extrinsic clotting) reduced relative to wild‐type prothrombin. Turbidity analyses of purified fibrinogen revealed that modification of the S4 subsite hampers the conversion of fibrinogen by thrombin. Consistent with this, the thrombin variants displayed a reduced catalytic efficiency towards the peptidyl substrate used in thrombin generation assessments. In case of the latter, substantial thrombin generation was attained when supplementing prothrombin‐deficient plasma with 180 μg/ml prothrombin variant. In this set‐up, the variants displayed a ~2‐fold reduced sensitivity for dabigatran relative to wild‐type prothrombin, while argatroban inhibition was unaffected. Analyses using a purified component system revealed an up to 24‐fold and 4‐fold reduced IC50 for inhibition of thrombin by dabigatran and argatroban, respectively (Figure 1).
Conclusion(s): Collectively, prothrombin variants with S4 subpocket modifications are able to generate thrombin in plasma inhibited by dabigatran and as such could have the potential to bypass anticoagulant therapy.

PB0564
Combined molecular dynamics and docking studies on direct oral anticoagulant binding to factor Xa variants
D. Veizaj 1; D. Poole III2; M. Schreuder3; P. Reitsma4; D. Verhoef4; D. Geerke5; M. Bos6
1 Div. Thrombosis and Hemostasis, Einthoven Laboratory, for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 AIMMS Division of Molecular and Computational Toxicology, Department of Chemistry and Pharmaceutical Sciences, Vrije Universiteit, Amsterdam, The Netherlands;, Amsterdam, Noord‐Holland, Netherlands; 3 Centenary Institute, University of Sydney, Sydney, New South Wales, Australia, 41VarmX, Leiden, The Netherlands. 2Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 5 AIMMS Division of Molecular and Computational Toxicology, Department of Chemistry and Pharmaceutical Sciences, Vrije Universiteit, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 6 Leiden University Medical Center
Background: For prevention and/or treatment of bleeding complications related to factor (F)Xa‐inhibiting direct oral anticoagulants (DOACs) specific reversal agents are required. Previously, we generated F174‐substituted human FX variants that demonstrated decreased sensitivity towards FXa‐DOACs. Our strategy for developing these bypassing FX variants relied on disruption of the FXa‐inhibitor binding site. While the latter was demonstrated employing Molecular Dynamics (MD) simulations, these studies did not quantitatively resolve to what extent the FXa‐inhibitor binding affinity was affected by the F174‐substitution.
Aims: Develop an in silico approach to assess differences in FXa‐inhibitor binding affinity between wild‐type and substituted FXa using combined molecular docking and MD simulations.
Methods: Starting from a crystal structure of wild‐type FXa and using PDB‐tools, the hydrogen prediction tool Protoss, and the AMBER biomolecular simulation package, conformations of wild‐type and substituted FXa variants were efficiently generated by employing an accelerated MD protocol on ns time scales. These conformations were used as template for subsequent FXa‐apixaban docking and scoring.
Results: Using our combined MD/docking approach, the effect of F174‐substitutions on apixaban binding was evaluated. No substantial differences in atom‐positional root‐mean‐square deviations (RMSDs) were observed during simulations of either wild‐type or F174‐substituted FXa, indicating that FXa stability was preserved following F174‐substitution. In contrast, the number of conformational clusters generated from accelerated MD simulation differed among the simulations, suggesting reduced conformational flexibility of the variants. Preliminary docking to initial conformations of FXa indicated loss in binding affinity of apixaban for F174A‐FXa and F174S‐FXa.
Conclusion(s): Our approach was used to identify possibly reduced FXa binding for apixaban in silico that is consistent with earlier in vitro observations for FXa variants with decreased sensitivity towards apixaban. Overall, our combined MD and molecular docking simulation strategy can be used to explore the binding of FXa‐DOACs to modified FXa variants.
PB0539
Low antithrombin levels are associated with low risk of cardiovascular death but are a risk factor for cancer mortality
L. Iacoviello1; R. de Laat‐Kremers 2; S. Constanzo1; Q. Yan3; A. Di Castelnuovo4; L. Van der Vorm5; A. De Curtis1; M. Ninivaggi6; C. Cerletti1; M. Donati1; B. de Laat5
1 Department of Epidemiology and Prevention, IRCCS Neuromed, Pozzilli, Italy, Varese, Lombardia, Italy; 2 Synapse Research Institute, Maastricht, Limburg, Netherlands; 3 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 4 Mediterranea Cardiocentro, Napoli, Italy, Varese, Lombardia, Italy; 5 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 6 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: Thrombosis is a common consequence of cardiovascular diseases (CVD) and cancer. Hypercoagulation plays a pivotal role in the pathophysiology of thrombosis. Therefore, the inactivation of key coagulation enzyme thrombin is tightly regulated by antithrombin (AT). Heterozygous deficiency of AT has been associated with thrombosis and cardiovascular death.
Aims: We aimed to study the relationship between AT levels and mortality in the general population, in particularly cardiovascular death and cancer‐related mortality.
Methods: We studied the association of AT levels and mortality in a prospective cohort sampled from the general Italian population (n = 19,676). AT levels were measured in the baseline samples, and mortality was recorded during a median follow‐up period of 8.2 years. The association of all‐cause, CVD‐related and cancer‐related mortality with variations in AT levels was assessed by Cox regression.
Results: In total, 989 subjects died during follow‐up, of which 373 deaths were CVD‐related and 353 were cancer‐related. AT levels were split into quartiles with ATQ1>94%, 94%≤ATQ2< 101%, 101%≤ATQ3< 105%, 105%≤ATQ4< 111%, ATQ5≥111%. Cox analysis revealed that the risk of cardiovascular death was reduced in subjects with low AT levels compared to subjects with higher AT levels, after adjustment for age, sex, smoking, BMI, diabetes, hypertension, hypercholesterolemia, history of CVD, history of cancer, vitamin K antagonists, antiplatelet medication, heparin and oral contraceptives (HRQ1vsQ2‐5: 0.72; 95% CI:0.57‐0.91; p = 0.006). On the contrary, low AT levels were associated with increased cancer mortality in a fully adjusted model (HRQ1vsQ2‐5: 1.41; 95% CI:1.22‐1.78; p = 0.003). All‐cause mortality was not associated with AT levels in the fully adjusted model (HRQ1vsQ2‐5: 0.99; 95% CI:0.86‐1.14; n.s.).
Conclusion(s): Low AT levels are associated with a lower risk of cardiovascular death, regardless of age, sex and medication. On the contrary, low AT levels, even before the development or diagnosis of cancer, are associated with a higher risk of cancer‐related death.
PB0551
Prevention of tumor cell‐induced coagulation activation by the small‐molecule FXIa inhibitor, BMS‐262084, is dependent on tissue factor (TF) expression levels
J. Mäder 1; M. Voigtländer2; C. Rolling2; A. Schulenkorf2; C. Lehr2; C. Bokemeyer2; L. Beckmann3; F. Langer2
1 UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 2 II. Medizinische Klinik und Poliklinik, Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 3 Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany
Background: Aberrant TF expression by tumor cells plays a critical role in the pathogenesis of cancer‐associated thrombosis (CAT). While targeting FXI is highly efficacious in preventing postoperative venous thromboembolism, its role in CAT is less clear.
Aims: To investigate FXI inhibition in the context of tumor cell‐induced coagulation activation.
Methods: A fibrin clot formation assay was used to measure the procoagulant activity (PCA) of recombinant human TF (rhTF) or tumor cells in the presence or absence of the small‐molecule FXIa inhibitor, BMS‐262084. Peak or trough concentrations of tinzaparin (0.85 and 0.2 IU/ml) and rivaroxaban (270 and 26 ng/ml) served as controls. Further, tumor cell‐induced thrombin generation and platelet aggregation were analyzed. FXIa amidolytic activity was assessed by a chromogenic assay.
Results: BMS‐262084 completely inhibited FXIa amidolytic activity at > 0.5 μM. In contrast to rivaroxaban and tinzaparin, 10 μM BMS‐262084 mitigated the PCA of rhTF only at low rhTF concentrations. Under these conditions, the effect of BMS‐262084 was comparable to that of peak tinzaparin and rivaroxaban levels. Similarly, the ability of BMS‐262084 to inhibit tumor cell‐induced fibrin formation, thrombin generation and platelet aggregation was inversely correlated with the magnitude of cellular TF expression. While in strongly TF‐expressing pancreatic BxPC‐3 cells FXI inhibition had negligible effects, it potently prevented tumor cell‐induced fibrin formation, thrombin generation and platelet aggregation in leukemic HL‐60 cells showing hardly any TF expression. Overall, the most prominent effects of BMS‐262084 were observed on peak and total thrombin generation, suggesting that FXI inhibition predominantly interfered with the propagation phase of coagulation. Consistently, BMS‐262084 already markedly reduced tumor cell‐induced thrombin generation at moderately TF‐expressing leukemic THP‐1 and breast MCF‐7 cells.
Conclusion(s): The efficacy of FXI inhibitors in preventing tumor cell‐induced coagulation activation may depend on TF expression levels. These findings have potential clinical implications for CAT prophylaxis and treatment.
PB0547
Antithrombin deficiency is associated with more compact plasma fibrin clot structure and impaired susceptibility to fibrinolysis
A. Klajmon1; M. Kopytek 2; J. Natorska3; A. Undas1; M. Ząbczyk3
1 Jagiellonian University Medical College, Kraków, Malopolskie, Poland; 2 Jagiellonian University Medical College, John Paul II Hospital, krakow, Malopolskie, Poland; 3 Jagiellonian University, Institute of Cardiology, John Paul II Hospital, Krakow, Malopolskie, Poland
Background: Inherited antithrombin deficiency is associated with increased risk of venous thromboembolism (VTE), largely due to insufficient thrombin inhibition. We hypothesized that antithrombin deficiency affects fibrin clot structure and function.
Aims: To evaluate plasma fibrin clot phenotype and factors affecting it in antithrombin‐deficient patients.
Methods: We assessed 37 VTE patients with genetically confirmed antithrombin deficiency (aged 40 ± 15 years) and 37 age‐ and sex‐matched VTE patients with normal antithrombin activity. Clot permeability in a thrombin‐based assay (Ks), clot lysis time (CLT), clot microstructure on scanning electron microscopy, along with thrombin generation, fibrinogen were assessed after >3 months since the VTE episode un blood samples obtained 24 hours since the last dose of direct oral anticoagulants (DOACs). The study was approved by the Ethical Committee and conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Results: Patients with antithrombin deficiency (median factor FIIa‐based antithrombin activity: 63%, range 55‐69%) had 21% reduced Ks (p < 0.001) in association with 30% thinner fibrin fibers and 27% prolonged CLT (p < 0.001) compared to controls. As expected, patients with antithrombin deficiency had 57% higher peak thrombin concentrations and 44% higher endogenous thrombin potential (ETP), while fibrinogen was similar. Type I (n = 22, 59.5%) compared to type II antithrombin‐deficient patients were characterized by lower Ks and longer CLT, along with higher thrombin generation (all p < 0.05). Ks (r = 0.38, p = 0.0083), CLT (r=‐0.44, p = 0.016), and ETP (r=‐0.37, p = 0.017) correlated with antithrombin activity in antithrombin‐deficient patients. ETP was not associated with fibrin clot properties. Addition of human antithrombin to deficient plasma to reach 100% resulted in thrombin generation inhibition, while Ks increased by 8% and CLT by 14%, but they were still lower than in controls.
Conclusion(s): Apart from increased thrombin generation, denser fibrin clot structure and poorly lysable clots might contribute to increased risk of VTE observed in antithrombin‐deficient patients.
Contact Pathway
PB0580
Microvesicles from stored platelets promote high molecular weight kininogen cleavage leading to bradykinin generation
D. Noubouossie1; M. Henderson1; A. Ilich2; P. Ellsworth1; M. Karafin1; R. Pawlinski1; M. Hoffman3; S. Strickland4; D. Monroe1; N. Key 1
1 University of North Carolina Blood Research Center, Chapel Hill, NC, USA., Chapel Hill, North Carolina, United States; 2 University of North Carolina, Chapel Hill, NC, USA., Chapel Hill, North Carolina, United States; 3 Veteran Affairs Medical Center, Durham, NC, USA., Durham, North Carolina, United States; 4 Patricia and John Rosenwald Laboratory of Neurobiology and Genetics, The Rockefeller University, New York, NY, USA., New York City, New York, United States
Background: The contact system (CS) links coagulation and inflammation. We previously demonstrated that microvesicles isolated from stored platelet apheresis units (sPLT‐MVs) activate the CS leading to thrombin generation.
Aims: To investigate if sPLT‐MVs also promote high molecular weight kininogen (HK) cleavage and bradykinin generation in a purified system.
Methods: sPLT‐MVs were isolated from expired apheresis platelet units by centrifugation, washed twice, and re‐suspended in Hepes‐buffered saline in the presence of physiologic plasma concentrations of HK and/or prekallikrein (PK). HK cleavage and bradykinin generation were measured after 0.5, 1‐ or 2‐hours incubation at 37⁰C. Cleavage of HK was assessed by a sandwich ELISA measuring residual intact HK (HKi). Bradykinin generation was quantified using a commercial ELISA. In some experiments, lisinopril was added to inhibit angiotensin converting enzyme, while avoralstat was added to block kallikrein (PKa).
Results: Following incubation of sPLT‐MVs with HK, the HKi concentration gradually decreased, whereas bradykinin levels increased, suggesting that sPLT‐MVs generate bradykinin from HK cleavage, independently of PKa, since these effects were not abolished by avoralstat (Fig 1A&B). However, the addition of PK further increased HK cleavage and bradykinin generation, both of which were inhibited by avoralstat (Fig 1C&D), suggesting the contribution of PKa formed by the activation of PK by sPLT‐MVs. Separately, the concentration of exogenously added bradykinin decreased following incubation with sPLT‐MVs, which was inhibited by the presence of lisinopril (Fig 1E), indicating the presence of angiotensin‐converting enzyme activity associated with sPLT‐MVs.
Conclusion(s): sPLT‐MVs cleave HK to generate bradykinin, although they also degrade bradykinin at a slower rate. sPLT‐MV‐mediated bradykinin formation may result from a] direct HK cleavage by sPLT‐MVs; and b] an indirect mechanism in which sPLT‐MVs activate PK to PKa that subsequently cleaves HK to generate bradykinin. The relevance of these mechanisms in plasma and in vivo following platelet transfusion warrants further studies.

PB0582
Elevated contact pathway activity in patients with acute venous thromboembolism
M. Nagy 1; A. Pallares Robles2; M. Visser3; V. ten Cate4; T. Koeck5; A. ten Cate‐Hoek6; H. ten Cate7; P. Wild4; H. Spronk8
1 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 2 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Mainz, Rheinland‐Pfalz, Germany; 3 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 4 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 5 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 6 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 7 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 8 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: Venous thromboembolism (VTE) is associated with excessive coagulation activity, which in part can be attributed to activation of the contact system. However, there is limited knowledge on the extent of contact activation in the setting of acute VTE.
Aims: To investigate the impact of contact activation in acute VTE.
Methods: Plasma samples were collected from patients with confirmed acute VTE (n = 321;VTEval study) and from population controls without history of VTE (n = 300;PREVENT‐it pilot study). Both studies were approved by the local ethics committee and Informed consent was obtained for all subjects. Patients were followed for 2 years for the primary endpoint (death or recurrent VTE). FXI levels, FXIa and plasma kallikrein (PKa) activity were determined in plasma samples with an aPTT‐ or thrombin generation‐based assay (FXI:c and CAT:FXIa, respectively) and with ELISA for enzyme‐inhibitor complexes (FXIa:alpha‐1 antitrypsin(a1AT), FXIa:antithrombin(AT), FXIa:C1‐inhibitor(C1‐inh), PKa:C1‐inh).
Results: VTE patients were slightly older, more often had a history of VTE and antithrombotic agent use compared to controls (Table 1). In VTE patients, higher FXI:c levels (124 ± 37% vs. 114 ± 28%), but lower CAT:FXIa levels were seen. This was accompanied by increased FXIa:a1AT, FXIa:AT and PKa:C1‐inh levels in patients compared with controls (312pM [238‐424] vs. 203 pM [144‐288]; 29 pM [23‐38] vs. 23 pM [20‐30]; 1.9 nM [1.2‐4.7] vs 1.4 nM [0.7‐3.5], respectively). In contrast, FXIa:C1inh levels were similar in both cohorts (84 pM [84‐151] vs 84 pM [84‐103]). Logistic regression models showed good discriminatory value for FXI:c and FXIa:a1AT (AUC = 0.64 [0.6/0.69] and AUC = 0.73 [0.69/0.77], respectively). After a 2‐year follow‐up, 81 recurrent VTE events or deaths occurred in the patient cohort, which were predicted by baseline levels of FXIa:a1AT and FXIa:C1Inh (Figure 1).
Conclusion(s): Elevated FXIa levels and activity are associated with acute and recurrent VTE suggesting an important risk contribution of FXI activation to VTE. These findings support studies with novel FXI(a) inhibitors for prevention of recurrent VTE.


PB0579
The Influence of plasma prekallikrein oligonucleotide antisense therapy on coagulation and fibrinolysis assays
L. Fijen 1; J. Meijers2; L. Bordone3; R. Petersen4; M. Levi4; D. Cohn4
1 Amsterdam UMC, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Experimental Vascular Medicine, Amsterdam University Medical Centers, Amsterdam, The Netherlands and Department of Molecular Hematology, Sanquin Research, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Ionis Pharmaceuticals, Carlsbad, California, United States; 4 Amsterdam UMC, University of Amsterdam, Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam, Noord‐Holland, Netherlands
Background: Congenital prekallikrein deficiency has been associated with increased risk of thrombosis in a few uncontrolled studies. Prekallikrein levels were reduced by 60‐65% in a recent phase 2 study with prekallikrein oligonucleotide antisense therapy (donidalorsen) for hereditary angioedema (HAE).
Aims: To estimate the effects of plasma prekallikrein reduction on coagulation and fibrinolysis.
Methods: Plasma samples were obtained from 16 HAE patients treated with donidalorsen and six patients with placebo. Informed consent was obtained from all participants and the study was approved by a recognized medical ethics committee. Calibrated automated thrombogram (CAT), clot lysis time (CLT), high‐molecular‐weight kininogen activity, factor XI activity, prothrombin fragment 1+2 (F1+2), thrombin‐antithrombin complexes, D‐dimer, plasminogen activity, plasmin‐α2‐antiplasmin complexes, and α2‐antiplasmin activity were measured before and after four months of treatment.
Results: No significant changes following donidalorsen treatment were observed between baseline and follow‐up: CAT lag time 3.0 min and 3.0 min, CAT peak thrombin concentration 109% and 129%, CAT endogenous thrombin potential 98% and 108%, factor XI activity 110% and 115%, F1+2 levels 251 pMol/L and 161 pMol/L, thrombin‐antithrombin complexes 2.6 μg/L and 1.9 μg/L, high‐molecular‐weight kininogen activity 87% and 93%, CLT with carboxypeptidase inhibitor 92% and 99%, CLT without carboxypeptidase inhibitor 87% and 93%, D‐dimer levels 0.65 μg/L and 0.36 μg/L, plasminogen activity 116% and 125%, plasmin‐α2‐antiplasmin complexes 533 ng/ml and 328 ng/ml, and α2‐antiplasmin activity 122% and 127%, respectively. There were also no differences between donidalorsen and placebo treatment.
Conclusion(s): Reduction of plasma prekallikrein by oligonucleotide antisense therapy in HAE patients does neither affect thrombin formation nor fibrinolytic activity. Our data show that that partial plasma prekallikrein reduction does not cause a procoagulant state and thereby suggest that it does not influence thrombotic risk. (Funded by Ionis Pharmaceuticals, Inc.; ISIS 721744‐CS2, ClinicalTrials.gov number: NCT04030598.)
PB0581
In vivo activation of the contact pathway by thrombin in sterile models of DIC in baboons
R. Keashari1; R. Silasi1; G. Regmi1; C. Lupu1; D. Gailani2; O. McCarty3; F. Lupu 1
1 Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, United States; 2 Vanderbilt University, Nashville, Tennessee, United States; 3 Oregon Health and Science University, Portland, Oregon, United States
Background: While data from in vitro and ex vivo systems support the conclusion that thrombin activates components of the contact system, particularly factor (F) XI, there is limited evidence for this process occurring in vivo.
Aims: We used baboon models of sterile disseminated intravascular coagulation (DIC) to test the hypothesis that thrombin promotes activation of contact phase proteases in vivo.
Methods: We used two models of thrombin‐mediated DIC: (i) intravenous bolus of a mixture of 36.6 pmol/kg FXa and 56.3 nmol/kg phosphatidylcholine/phosphatidylserine (PC/PS), followed by blood collection at select intervals; (ii) continuous intravenous infusion of thrombin (27 pmole/kg/min) for 60 min with periodic blood collection over 6 hours post‐challenge. Plasma levels of antithrombin (AT) complexes with FXIIa, FXIa, FIXa, thrombi and kallikrein as well as total and cleaved kininogen were measured by sandwich ELISAs.
Results: FXa:PC/PS infusion induced a rapid burst of thrombin generation that peaked 10 min post‐challenge, as shown by a rise in thrombin‐AT (TAT) complexes. This was paralleled by rapid generation of AT complexes with FXIIa, FXIa, FIXa and kallikrein, all reaching maximums within 10‐30 min post‐challenge, followed by a decrease at 60 min. Thrombin infusion led to a gradual increase in AT complexes, reaching maximums at 60 min. Total AT levels transiently decreased by 10‐20% after both challenges, then rapidly normalized. Changes in cleaved kininogen followed the pattern of kallikrein generation, with rapid increase after FXa‐PC/PS bolus and steady increase during thrombin infusion. Unlike AT, there was a slight transient increase in total kininogen, suggesting rapid release from tissue stores post‐thrombin generation.
Conclusion(s): Traditional models of coagulation are based on contact activation initiating thrombin production. Our data support the hypothesis that serine proteases including FXa and thrombin also contributes to activation of contact pathway proteases, indicating a more complex relationship between coagulation and contact activation.
PB0578
Generation and characterization of novel nanobodies inhibiting factor XI function
A. Bar Barroeta 1; J. Marquart2; K. Bakhtiari2; R. Urbanus3; J. Meijers4
1 Sanquin, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Sanquin, Dept. Molecular Hematology, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 4 Department of Molecular Hematology, Sanquin Research, Amsterdam, the Netherlands, Department of Experimental Vascular Medicine, Amsterdam UMC, University of Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Factor XI (FXI) is a promising target for novel anticoagulants considering that it is strongly involved in thromboembolic diseases, while fulfilling a mostly supportive role in haemostasis. Anticoagulants targeting FXI could therefore reduce the risk for thrombosis, without increasing the chance of bleeding side effects.
Aims: The generation and characterisation of nanobodies that can interfere with FXIa‐mediated activation of FIX.
Methods: After production of a library of FXI‐binding nanobodies, nanobodies were selected with a binding epitope located on the apple 3 domain, where the interaction between FXIa and FIX occurs. A system with purified proteins was used to test whether these nanobodies exhibited an inhibitory effect on FIX activation by FXIa. The effect of the nanobodies on coagulation was examined in plasma‐based coagulation assays. Additionally, the binding epitope of selected nanobodies was further elucidated by hydrogen deuterium exchange mass spectrometry.
Results: We identified five nanobodies that inhibit FIX activation by FXI. These nanobodies share a binding epitope with FIX on the apple 3 domain, and thereby prevent binding of FIX to FXIa. A sixth nanobody interferes with the FXI‐HK interaction by targeting a different region in the apple 3 domain.
Conclusion(s): We have produced six nanobodies against the apple 3 domain of FXI that could serve as candidates for anticoagulation therapy. Five of these inhibit coagulation by interfering with the FXIa‐FIX interaction. Interestingly, we have also produced a nanobody targeting the FXI apple 3 domain that hinders the FXI‐HK interaction, further elucidating the binding orientation of HK on FXI.
VPB0583
Procoagulant properties of titanium and titanium nitride
M. Litvak 1; A. Shamanaev2; A. Matafonov1; A. Kobrin3; E. Tucker4; M. Wallisch4; O. McCarty5; D. Gailani3
1 Vanderbilt University Medical Center, Nashville, Tennessee, United States; 2 Vanderbilt University Medical Canter, Nashville, Tennessee, United States; 3 Vanderbilt University, Nashville, Tennessee, United States; 4 Aronora, Inc., Portland, Oregon, United States; 5 Oregon Health and Science University, Portland, Oregon, United States
Background: Titanium (Ti), its alloys, and ceramics (titanium nitride or TiN) are widely used in medical devices/implants because of their strength, resistance to corrosion and presumed inertness. Titanium compounds are considered hemocompatible because they do not induce hemolysis, have low albumin and fibrinogen adsorption, and have low platelet retention. However, cardiovascular implants made of titanium, such as ventricular assist devices, require anticoagulation to prevent thrombosis.
Aims: To compare the interaction of the plasma contact system with titanium surfaces to the well‐known contact activator kaolin, and to study effects of contact activation inhibitors on titanium‐initiated contact activation.
Methods: Clotting assays in normal plasma and plasmas deficient in factor XII, (fXII), prekallikrein, or high‐molecular‐weight kininogen (HK), chromogenic substrate assays and pull‐down experiments were performed, using kaolin and nanoparticles made of silica (100 nm, 26.8 m2/g); Ti (70 nm, 20 m2/g), TiN (80 nm, 30‐50 m2/g) and aluminum (70 nm, 15‐30 m2/g).
Results: Using equivalent surface amounts, Ti and TiN nanopowders were comparable to kaolin for inducing plasma coagulation, factor XII (fXII) autoactivation, prekallikrein activation and HK cleavage. While Ti and TiN particles carry a negative charge like many contact inducers, their effects were not neutralized by the polycation Polybrene or changes in salt concentration. Aluminum nanospheres, which carry a positive charge, did not induce contact activation. Ti and TiN, but not aluminum, rapidly bind FXII, prekallikrein and HK in plasma. Antibodies against fXIIa, kallikrein or factor XI abrogated Ti‐ and TiN‐induced clotting. Anti‐fXII antibodies inhibited fXII binding to Ti, and reduced kallikrein generation and HK cleavage.
Conclusion(s): Titanium compounds are potent contact pathway activators, although the mechanism underlying their effect differs in some respects from contact activation on other negatively charged surfaces. Inhibitors of the contact system may be useful for mitigating prothrombotic and proinflammatory consequences of contact activation in patients with vascular devices made of titanium.
Critical Care and Perioperative
PB1015
Assessment of quantra analyzer on life‐threating bleeding under oral anticoagulants agents: A pilot study
D. Teissandier 1; C. Lucchini2; J. Bouillon‐Minois3; J. Schmidt4; T. Sinegre5; c. Morand6; A. Lebreton7; F. Moustafa4
1 CHU Clermont Ferrand, Clermont‐Ferrand, Auvergne, France; 2 Emergency Unit, CHU Clermont Ferrand, clermont‐ Ferrand, Auvergne, France; 3 Emergency Unit, CHU Clermont ferrand, CLERMONT FERRAND, Auvergne, France; 4 Emergency Unit, CHU Clermont Ferrand, Clermont‐Ferrand, Auvergne, France; 5 Haematology, CHU Clermont‐Ferrand, clermont‐Ferrand, Auvergne, France; 6 INRAE, THEIX, CLERMONT FERRAND, Auvergne, France; 7 Service d’hématologie biologique, CHU Clermont‐Ferrand, Clermont‐Ferrand, France, Clermont‐Ferrand, Rhone‐Alpes, France
Background: Bleeding is the most feared complication during oral anticoagulant therapy but for direct oral anticoagulant, biological assessment of reversion is still unclear.
Aims: The aim of this study is to assess, during major bleeding under oral anticoagulants, the effect of reversal therapy on hemostasis parameters using the Quantra® system.
Methods: We conducted a pilot study on anticoagulated patients (VKA, or direct oral anticoagulants (DOACs): dabigatran or apixaban) admitted to an emergency department for a life‐threating bleeding. Routine hemostasis parameters and those assessed by the Quantra are realized before and 30 min after the end of the reversal therapy.
Results: 5 patients were included: 3 patients on Apixaban therapy, 1 patient on dabigatran therapy and 1 patient on VKA therapy. Apixaban was reversed by 48.13+/‐3.23 UI/Kg of 4F‐PCC, dabigatran by idarucizumab (5 g) and VKA therapy by 25 UI/kg of 4F‐PCC. Regarding Quantra parameters, an increase value of Coagulation Time (CT) and Stiffness of the clot (CS) was found after reversal therapy for patients on apixaban therapy whereas for patients on dabigatran or VKA therapy we found a decrease of CT. Regarding routine hemostasis parameters, as expected, we observed an increase rate of factor II, V and X after reversal therapy by 4F‐PCC, a decrease of quick time (QT) regarding all anticoagulants agent and an increase of ACT for apixaban but a decrease of dabigatran or VKA.
Conclusion(s): If the Quantra analyzer could be of help for the management of major bleeding under oral anticoagulant agent in an emergency room, a larger multicenter study is needed to better understand the effect of reversal therapy on anticoagulant agent and more specially on DOACs.

VPB1018
Experience with the administration of Idarucizumab as a specific antagonist of Dabigatran in the emergency medicine
G. Ramazanov; E. Klychnikova; A. Vyshlova; E. Tazina; L. Akhmatkhanova; D. Cheboksarov; S. Petrikov
N.V. Sklifosovsky Research Institute for Emergency Medicine, Public Healthcare Institution of Moscow Healthcare Department, Moscow, Moskva, Russia
Background: The patients who are taking Direct oral anticoagulants (DOACs) may develop surgical or trauma emergencies that require prompt and safe neutralization of anticoagulant’s action. A modern anticoagulant should have a specific antagonist which is capable to neutralize the anticoagulant effect in the shortest possible time.
Aims: To investigate the administration of Idarucizumab as a specific antagonist of Dabigatran
Methods: The study included 6 patients with non‐valvular atrial fibrillation who received Dabigatran to prevent ischemic stroke (IS) and/or systemic embolism. There was performed a clinical and laboratory safety analysis of Idarucizumab for prothrombotic activity
Results: IS was developed in 3 patients on the background of Dabigatran’s administration that required systemic thrombolytic therapy. Acute surgical pathology – strangulated inguinal hernia – was developed in 1 patient. Another patient suffered a fracture of the left humerus as a result of a fall. And in 1 patient an intracerebral hemorrhage was developed while taking Dabigatran. The median values of hemostasis parameters before the administration of Idarucizumab were the following: Platelet count – 191*109/L (158; 241), APTT–26.2 (21.9; 35.0) sec, Thrombin time (TT) – 27.8 (25.0; 37.7) sec, INR–1.23 (1.12;1.29) and Fibrinogen’s level–2.79 (2.52; 3.69) g/L. Only TT was significantly lower – 16.8 (16.6; 18.1) sec (p < 0.05) – after the administration of Idarucizumab. Other parameters after the administration of Idarucizumab did not differ. None of the patients during the entire period of hospitalization developed clinically significant arterial and venous thrombotic complications. Of the three patients with IS who underwent systemic thrombolytic therapy after the administration of Idarucizumab, 2 patients developed asymptomatic hemorrhagic transformation of the area of cerebral ischemia in the form of type 1 petechial impregnation.
Conclusion(s): It was found that despite the fact that Idarucizumab quickly neutralizes the anticoagulant effect of Dabigatran its administration is not associated with clinical and laboratory manifestations of thrombotic complications.
PB1012
Review of a peri‐operative mobile application to conform anticoagulation management and review of safety and clinician satisfaction
J. Ruell; M. Thorpe; D. Fathoala; S. Allford
Musgrove Park Hospital, Taunton, England, United Kingdom
Background: A proposal for the Musgrove Park Hospital Haematology service to manage peri‐procedure anticoagulation bridging was made after several cancellations and key incidents were caused by inconsistent management. With limited resource the ‘Canadian Thrombosis’ application to guide anticoagulation in this setting was piloted within a revised clinical guideline.
Aims: To provide consistent, safe anticoagulation perioperative management according to an evidence base that the application has drawn from. Formal documentation of a clearly defined plan for perioperative care within our electronic patient record system was shared with hospital clinicians, general practitioners and patients. Education and support was provided. The aim was to simplify the process, minimize variation and develop a single overarching, practical guideline to improve perioperative anticoagulation management in a medium sized acute hospital setting undertaking common surgical procedures
Methods: The guideline was introduced in October 2020. The following prospective data was collected: 30‐day major bleeding events, thrombotic events and related mortality. The individualized patient plan provides clinicians with a guide to bleeding risk. As part of the evaluation of this service development, we reviewed patient and clinician satisfaction and compared this to operator satisfaction before the introduction of this new guideline. Data from the first 25 patients are presented here.
Results: Compliance with the guideline was excellent: all 25 patients had an anticoagulation perioperative plan documented in the electronic patient record. Clinical outcome was good: there have been no major bleeding or thrombotic events in the 21 patients who have progressed to surgery. All 15 clinicians involved reported increased user satisfaction relating to the change in application, clarity of plan, and patient outcome.
Conclusion(s): Use of perioperative anticoagulation guidelines based on the Canadian thrombosis application has resulted in improved patient care, high clinical engagement and user satisfaction with minimal resource requirements in an acute General Hospital setting
PB1016
Argatroban monitoring in critically ill patients and correlation with thrombin generation
G. Scheriau1; C. Schkömmer1; L. Wagner 2; N. Binder2; B. Steinlechner3
1 Department of Anesthesia, Intensive Care Medicine and Pain Medicine. Division of Cardiothoracic and Vascular Anesthesia Medical University Vienna, Austria, Vienna, Wien, Austria; 2 Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH, Vienna, Wien, Austria; 3 Department of Anesthesia, Intensive Care Medicine and Pain Medicine Division of Cardiothoracic and Vascular Anesthesia Medical University Vienna, Vienna, Wien, Austria
Background: Argatroban is a parenteral direct thrombin inhibitor used for anticoagulation in patients suffering from heparin induced thrombocytopenia (HIT). There is increasing evidence suggesting that inter‐individual differences are poorly reflected by argatroban plasma concentration in critically ill patients.
Aims: The aim of the study was to investigate the correlation of argatroban plasma concentration with blood coagulation activity when using standardized thrombin generation assay (TGA) measurement in individual patients.
Methods: Four patients who developed a HIT and were treated with argatroban at individual doses, samples were taken on five consecutive days. The argatroban concentration was determined with Technoclot DTI assay (Technoclone). Fully automated thrombin generation was determined with Ceveron RC High kit. All measurements were made on a Ceveron s100 analyzer (Technoclone). Calibration curves were made at the beginning of the study using the standard analyzer settings for the argatroban assay, and for the TGA.
Results: Patients 1, 2 and 3 were diagnosed with thrombosis on day 1. Patient 1 was treated with a constant dose of argatroban, his initially high TGA Peak of 461 nM thrombin normalized over time. Patients 2 and 3 had increasing doses of argatroban, but the peak thrombin level of patient 2 continued to show hypercoagulability, whereas the peak thrombin level of patient 3 was normalized. Patient 4 with an initially borderline peak thrombin value and increasing argatroban dose, showed increasing peak thrombin values and developed a thrombosis on day 4. The results are shown in Figure 1.
Conclusion(s): These data show that patient care may be individualized when coagulation activity, as determined by thrombin generation, is used as additional parameter in adjusting argatroban dose for prevention of a thrombosis. Thrombin generation measurement complements estimation of argatroban concentration, a static marker, because it additionally reflects the fluctuating hemostatic landscape in critically ill patients.

PB1010
Impact of fresh frozen plasma dosing in patients with a left ventricular assist device undergoing heart transplant
P. Dobesh 1; H. Brink2; M. Thorson1; T. Ruter1; M. Edwards1
1 University of Nebraska Medical Center College of Pharmacy, Omaha, Nebraska, United States; 2 Nebraska Medicine, Omaha, Nebraska, United States
Background: Warfarin is used in left ventricular assist device (LVAD) patients for the prevention of pump thrombosis. In the setting of bridge‐to‐transplant, warfarin reversal must be accomplished quickly when a heart becomes available. Although fresh frozen plasma (FFP) is commonly dosed at 10 ml/kg, dosing in this setting is not well defined.
Aims: Evaluate dosing of FFP [< 10 ml/kg=low‐dose (LD) vs. ≥10 ml/kg=high‐dose (HD)] on time to surgery, blood product use, and clinical outcomes post‐surgery.
Methods: In this retrospective study we identified LVAD patients undergoing heart transplant at our institution. Only patients receiving FFP prior to surgery were included. Data collection included baseline characters, surgical timing and blood product use, post‐surgical management, and 30‐day outcomes. Categorical data was evaluated using chi‐square analysis and continuous data was evaluated using student’s t‐test.
Results: We identified 153 eligible patients (n = 73 received LD and n = 80 received HD) from 9/2012 to 4/2021. All patients also received vitamin K 10 mg. Mean dose of FFP was significantly different between the groups (6.6 ± 2.0 ml/kg vs. 14.7 ± 4.0; p < 0.001). No differences were noted in baseline characteristics (Table). Although baseline INR was similar, HD‐FFP provided a significant reduction in INR prior to surgery (1.6 ± 0.3 vs. 1.4 ± 0.2; p < 0.001). There was also 25% absolute reduction in an INR >1.4 at the time of surgery with HD‐FFP (Table) Patients receiving HD‐FFP went to surgery about 2 hours quicker than those receiving LD‐FFP. There were also significant reductions in blood loss and red cell transfusion during surgery with HD‐FFP. Chest tube drainage was significantly less with HD‐FFP on the day of surgery and total over 5‐days. Clinical outcomes were similar between groups (Table).
Conclusion(s): HD‐FFP provides several benefits in LVAD patients undergoing heart transplant compared to LD‐FFP, although clinical outcomes are not altered. Larger, multicenter studies will help confirm that these patients should receive at least 10 ml/kg of FFP.

PB1011
Experience with the use of 4‐factor prothrombin complex concentrates for warfarin reversal in patients with a left ventricular assist device undergoing heart transplant
P. Dobesh 1; H. Brink2; M. Edwards1; T. Ruter1; M. Thorson1; C. Lemmons1
1 University of Nebraska Medical Center College of Pharmacy, Omaha, Nebraska, United States; 2 Nebraska Medicine, Omaha, Nebraska, United States
Background: Patients with a left ventricular assist device (LVAD) typically receive warfarin for the prevention of pump thrombosis. In the setting of bridge‐to‐transplant, warfarin reversal must be accomplished quickly when a heart becomes available. Although plasma is commonly used, limited data exist for use of four‐factor prothrombin complex concentrate (4FPCC) in this setting.
Aims: Describe our experience in using 4FPCC on time to surgery, blood product use, and clinical outcomes post‐surgery.
Methods: In this retrospective, observational study, we identified LVAD patients undergoing heart transplant at our institution who received 4FPCC for reversal. Data collection included baseline characteristics, surgical timing and blood product use, post‐surgical management, and 30‐day outcomes. Descriptive data are presented as mean ± standard deviation or percent incidence. Although a control group of patients not receiving 4FPCC is presented, no statistical comparisons were made due to the limited number of 4FPCC patients.
Results: We identified seven 4FPCC patients and 153 control patients from 9/2012 to 4/2021. Baseline characteristics are in the table. Patients receiving 4FPCC went to surgery about 6 hours faster than control patients. Although baseline INR was similar; 4FPCC provided a lower INR prior to surgery (1.5 ± 0.2 vs. 1.3 ± 0.2), with only one patient (14%) having an INR >1.4 at the time of surgery compared to 53% of control patients. Blood loss, plasma, and red blood cell use during surgery was numerically less in patients receiving 4FPCC. 4FPCC patients had less chest‐tube drainage post‐surgery (Table). 4FPCC patients had no major bleeds but a 43% rate of thrombosis, while 18% of control patients had major bleeding with 15% thrombosis.
Conclusion(s): 4FPCC may provide a faster time to surgery with less blood loss, blood product use, and chest‐tube drainage. Although there was no major bleeding, the number of thrombotic events is concerning. Larger studies are needed to confirm these observations.

PB1013
Educational resources on periprocedural anticoagulation management: A systematic review
J. Britto1; A. Alshenaiber1; N. Cantor1; A. Chen1; P. Grzela1; S. Sadeghian1; D. Singh1; C. Zhang1; J. Douketis1; T. Chan1; S. Mithoowani 2
1 McMaster University, Hamilton, Ontario, Canada; 2 Department of Medicine, McMaster University, Hamilton, ON, Canada, Hamilton, Ontario, Canada
Background: Periprocedural anticoagulation management is a clinical problem encountered by physicians across all specialties however knowledge translation is incomplete, leading to avoidable patient harm. The breadth and quality of teaching resources in this domain have never been formally assessed.
Aims: We conducted a systematic review of peer‐reviewed educational resources on periprocedural anticoagulation management to assess their quality and classify them according to an instructional design framework.
Methods: We searched OVID/MEDLINE, Embase and Web of Science for educational and review articles published between 2015 and 2021 on periprocedural management of patients on anticoagulants for any indication. Two reviewers independently screened studies for eligibility, extracted data (publication year, accompanying media, target audience) and assessed the educational quality using the revised METRIQ (rMETRIQ) score. Resources were classified as learning tasks, procedural information, supportive information, or part‐task practice according to the Four‐Component Instructional Design (4C/ID) model. This review was registered with PROSPERO.
Results: Of the 252 included articles, 97% were targeted to physicians, medical students, nurse practitioners or dentists. The target audience was made explicit in 135 articles (53.6%). 74% were targeted to Internal Medicine specialists and 53% to surgeons. Less than 5% of articles were targeted to Family Medicine or Emergency Medicine specialists. Common subtopics included direct oral anticoagulants, warfarin, atrial fibrillation, venous thromboembolism, bridging and anticoagulant reversal. 226 articles (89%) were of high‐quality per the rMETRIQ score; 248 articles were classified as supportive information and 4 as procedural information.
Conclusion(s): Most peer‐reviewed educational articles on periprocedural anticoagulation management were of high quality, however the great majority were supportive information. Very few resources were aimed at Family Medicine and Allied Health practitioners. Future educational publications should target a broader group of clinicians and take the form of just‐in‐time information, learning tasks (case studies), or part‐task practice (practice items) to better situate them within a whole‐task instructional design framework.


PB1009
Synergistic effect of the factor XIa inhibitor milvexian with heparin but not bivalirudin in activated clotting time assays in human whole blood in vitro
M. Bunce1; M. Chintala 2
1 Janssen Research & Development, Ardmore, Pennsylvania, United States; 2 Janssen Research & Development, Raritan, New Jersey, United States
Background: Milvexian is a small molecule FXIa inhibitor currently in Phase II clinical trials with the potential for reduced bleeding risk compared to currently approved direct oral anticoagulants (DOACs). Patients taking milvexian may require anticoagulation with heparin (UFH, LMWH) or bivalirudin for medical procedures such as percutaneous coronary intervention (PCI) or cardiopulmonary bypass (CPB) surgery. However, it is currently unknown what effect milvexian will have when combined with heparin or bivalirudin. Activated clotting time (ACT) is the standard whole‐blood coagulation assay used perioperatively to monitor anticoagulation levels for procedures such as PCI or CPB.
Aims: Assess impact of milvexian when added to UFH, enoxaparin (LMWH), or bivalirudin in ACT assay.
Methods: Milvexian (0.1 – 6 μM) was added to freshly drawn citrated whole blood obtained from healthy human donors either alone or combined with UFH (0.5 or 1 U/ml), enoxaparin (2 or 4 U/ml), or bivalirudin (12.5 μg/ml). ACT clotting times were measured on ACT Plus automated coagulation timers using High‐Range ACT (HR‐ACT) cartridges (Medtronic USA).
Results: 0.5 and 1 U/ml of UFH prolonged ACT by 1.3‐ and 1.6‐fold, 2 and 4 U/ml enoxaparin prolonged ACT by 1.3 and 1.5‐fold, and 12.5 μg/ml bivalirudin prolonged ACT by 2.45‐fold. Milvexian alone demonstrated a modest effect on HR‐ACT clotting times, with a maximal prolongation of 1.45‐fold at 6 μM. Combining milvexian with either UFH (1 U/ml) or enoxaparin (4 U/ml) potentiated the maximum ACT prolongation (4.0‐ and 4.28‐fold, respectively), whereas combining milvexian with bivalirudin had no effect compared to milvexian alone (1.55‐fold vs. 1.45‐fold, respectively).
Conclusion(s): Milvexian demonstrates a synergistic effect with UFH and enoxaparin, but not bivalirudin, in vitro in ACT assays. A clinical study may be required to confirm whether this synergy occurs in subjects taking milvexian and the potential implications if any in patients is unknown at this time.

PB1008
The impact of hematocrit on postoperative bleeding risk in patients undergoing robotic radical prostatectomy
S. Arcudi 1; A. Artoni2; E. De Lorenzis3; C. Silvani4; F. Spanu5; S. Villa6; M. Capecchi7; E. Montanari3; F. Peyvandi8
1 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, MILAN, Lombardia, Italy; 2 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, Milano, Lombardia, Italy; 3 Urology Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan; Dept. of Clinical Sciences and Community Health, University of Milan, Milan, Lombardia, Italy; 4 Urology Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Milan, Lombardia, Italy; 5 Clinical Laboratory, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy, Milan, Lombardia, Italy; 6 Foundation IRCCS Ca' Granda Ospedale Maggiore Policlinico, Department of Transfusion Medicine and Hematology, Milan, Lombardia, Italy; 7 Department of Biomedical Sciences for Health, Università degli Studi di Milano, Milan, Italy, Milan, Lombardia, Italy; 8 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Robot‐assisted radical prostatectomy (RARP) represents the first‐choice surgical procedure for the treatment of localized prostate cancer. The effects of blood parameters and blood groups on the bleeding risk had never been investigated.
Aims: To investigate whether blood parameters and/or blood groups may influence the bleeding risk in patients undergoing RARP.
Methods: We conducted a prospective cohort study enrolling consecutive patients undergoing RARP in our hospital, from January 2017 to December 2020. Patients were evaluated with full blood count and blood groups characterization before and after surgery. Demographic and clinical data were collected with particular attention to other known risk‐factors for RARP‐associated bleeding (prostate volume, BMI, smoking status, nerve sparing technique). Bleeding was assessed both as direct quantification of blood loss during surgery and the difference in hemoglobin values pre‐ and post‐surgery. Linear regression analyses were performed, corrected for confounders and known risk factors for RARP‐associated bleeding, and risk estimates were evaluated following Woolf's method.
Results: In the study period 191 consecutive patients were enrolled; mean age was 67.8 years. Mean blood loss was 366.2 ml [Table 1]. When analyzing the basal hematocrit with linear regression, a strong negative correlation was seen with the difference in hemoglobin pre‐ and post‐surgery (p < 0.001). The relationship remained strong after correction for age, smoke, BMI, prostate volume and nerve sparing (p < 0.001). Pre‐surgery hemoglobin levels were less associated with an increased bleeding risk (p = 0.07), while PT, aPTT and platelet count did not influence surgical losses. Individuals carrying the blood group O were not at increased risk for higher blood loss (OR = 0.76; 95%CI 0.4‐1.4; p = 0.4).
Conclusion(s): A lower basal hematocrit is associated with an increased bleeding during RARP. Lower basal platelet count and O blood group were not associated with greater bleeding.

PB1014
Thromboelastography characteristics in pediatric patients on extracorporeal membrane oxygenation (ECMO)
K. Regling 1; K. Cashen2; W. Hollon3; J. Callaghan4; P. Hines5; M. Chitlur1
1 Children's Hospital of Michigan, Central Michigan University, Detroit, Michigan, United States; 2 Duke Children's Hospital, Duke University, Durham, North Carolina, United States; 3 Children's Hospital of Michigan, Detroit, Michigan, United States; 4 Oakland University, Rochester, Michigan, United States; 5 Functional Fluidics, Detroit, Michigan, United States
Background: Extracorporeal membrane oxygenation (ECMO) can be lifesaving for critically ill patients of all ages. Balancing the risk of bleeding and thrombosis is challenging in pediatric patients and limited by our current anticoagulation and monitoring strategies. Novel monitoring of thrombin generation may be a key marker for these hemostatic complications and may be influenced by inflammation.
Aims: The aims of this study were to evaluate whether novel laboratory tests are better able to predict patients at risk for hemostatic complications compared to routine laboratory tests. We hypothesized that ECMO related thrombosis would be associated with increased thrombin generation and ECMO related bleeding would be associated with decreased thrombin generation.
Methods: An IRB approved prospective pilot study of patients requiring ECMO was conducted at Children’s Hospital of Michigan. Patients were enrolled within 24 hours of ECMO cannulation after informed consent. Patients aged 0‐17 years placed on venoarterial (VA) or venovenous (VV) ECMO were included. Included is the preliminary data of the first 32 patients enrolled.
Results: Table 1 shows demographic data for the cohort. The 32 patients had a total of 243 ECMO days. Subgroup analyses were performed for patients based on days of bleeding/thrombotic complications. Categories included “no event” (N = 174), “bleeding event” (N = 26) and “clotting event” (N = 43). Bleeding events had significantly decreased hemoglobin and fibrinogen levels and prolonged prothrombin time compared to no event and clotting events. Laboratory data for thromboelastography (TEG®) is shown in Table 2.
Conclusion(s): Bleeding events on ECMO are associated with increased mortality. Our findings suggest that lower fibrinogen levels, prolonged PT and decreased thrombin generation (as assessed by the TEG) are associated with an increased risk for major bleeding. Thrombin generation assay statistical analysis is pending. The results illustrate the challenges in monitoring ECMO anticoagulation and additional study of novel tests are needed to identify and avoid clinical complications.


VPB1020
Dielectric blood coagulometry for evaluation of coagulation activity of blood samples spiked with direct oral anticoagulant
A. Takemoto 1; N. Kondo2; H. Yamamoto2; Y. Yamamoto3; S. Toyama4; K. Higashino5; T. Uchida4
1 Tokyo Medical and Dental University, Graduate School of Medical and Dental Sciences, Bunkyo‐ku, Tokyo, Japan; 2 Department of Anesthesiology, Tokyo Medical and Dental University, Graduate School of Medical and Dental Sciences, Bunkyo‐ku, Tokyo, Japan; 3 Department of Anesthesiology, Tokyo Medical and Dental University, Graduate School of Medical and Dental Sciences, Bunko‐ku, Tokyo, Japan; 4 Department of Anesthesiology, Tokyo Medical and Dental University, Graduate School of Medical and Dental Sciences, Bunky‐ku, Tokyo, Japan; 5 Arkray Marketing Inc, Bunky‐ku, Tokyo, Japan
Background: Dielectric blood coagulometry (DBCM) is a coagulation test based on the measurement of dielectric permittivity change, We recently developed a new cartridge containing Russell’s viper venom to evaluate anticoagulation activity of anti Xa inhibitor.
Aims: (1)To evaluate correlation between coagulation time measured by this system (DBCM CT) and plasma concentration of DOACs, and (2) To evaluate correlation between DBCM CT and peak thrombin generation measured by calibrated automated thrombogram, using spiked blood samples
Methods: This study was approved by the institutional Ethical Committee(M2020‐373). After obtaining informed consent, whole blood samples are collected from 10 volunteers. Apixaban and rivaroxaban were added to make spiked blood samples with concentration of 25, 50, 100, 200 and 400 ng/ml. Our newly developed cartridges were used to measure DBCM CT. Plasma was isolated from same samples, and they are used in evaluation of thrombin generation using calibrated automated thrombogram (CAT), and in vitro quantitative determination of direct Factor Xa inhibitors (DiXaI test). Kruskal‐Wallis tests were performed for multiple comparison, and the difference between the samples with or without DOACs were analyzed by Steel tests as post‐hoc analyses. Spearman’s correlation test was performed to analyze correlation between DBCM CT and plasma concentration of DOACs measured by DiXaI test or peak thrombin generation. Statistical significance was defined as P < 0.05.
Results: Apixaban and rivaroxaban prolonged DBCM CT (Fig 1A, B), and DBCM CT correlated with plasma concentration measured by DiXaI test (Rs = 0.97, P < 0.001 for apixaban; Rs = 0.95, P < 0.001 for rivaroxaban). Peak thrombin levels showed significant correlation with DBCM CT Rs = 0.94, P < 0.001 for apixaban; Rs = 0.97, P < 0.001 for rivaroxaban, Fig 2).
Conclusion(s): These results suggest potential for clinical usefulness of DBCM as a point of care test to evaluate anticoagulation induced by DOACs. This study was supported by AMED 20im0210224 in Japan


PB1017
Effectivity of andexanet alfa for the reversal of factor Xa inhibitors under hemodilution
J. Wienhold 1; R. Rossaint2; H. Sieben2; O. Grottke3
1 RWTH Aachen University Hospital, Department of Anaesthesiology, Aachen, Nordrhein‐Westfalen, Germany; 2 RWTH Aachen University Hospital, Aachen, Nordrhein‐Westfalen, Germany; 3 Uniklinik RWTH Aachen, Aachen, Nordrhein‐Westfalen, Germany
Background: Andexanet alfa is a specific antidot for factor Xa (FXa) inhibitors and is licensed to treat patients under FXa inhibitor therapy and life‐threatening bleeding. Volume expanders are used in massive bleeding to compensate blood loss and maintain circulation. It is unknown if volume expanders influence the effectivity of andexanet to bind FXa inhibitors.
Aims: This study investigated the effectivity of andexanet for the reversal of FXa inhibitors under hemodilution using four different volume expanders.
Methods: After ethical approval, blood samples were drawn from five healthy volunteers and spiked with rivaroxaban (target concentration: 300 ng/ml). Samples were diluted with Ringer’s solution, 4% gelatin, 5% and 20% human albumin (HA) at two different dilution levels (20%, 50%), subsequently andexanet was added. Blood samples were analyzed using the russell ´s viper venom (RVV) test on a Clot Pro® device and a anti‐FXa activity chromogenic assay.
Results: Median rivaroxaban concentration was 273 ng/ml (254‐353) prior to hemodilution (Figure 1). Andexanet spiking of non‐anticoagulated blood samples prolonged RVV test’s clotting time (CT) (Figure 2). Hemodilution prolonged clotting parameters further and reduced mean clot firmness (MCF). After administration of andexanet, kinetic coagulation parameters in anticoagulated samples were reversed close to baseline in all groups. In three groups, there was a significant difference in RVV’s clotting time between non‐anticoagulated samples and anticoagulated samples with andexanet (4% gelatin 20%, 5% HA 20% and 20% HA 20% dilution). No significant differences between non‐anticoagulated samples with andexanet and anticoagulated samples with andexanet were observed. Andexanet administration decreased rivaroxaban concentrations in all groups to a median of < 15 ng/ml.
Conclusion(s): The results show that treatment with different volume expanders does not substantially affect the efficacy of andexanet to bind FXa inhibitors. Andexanet in non‐anticoagulated samples prolonged clotting time which could be explained by a competitive antagonism with human factor Xa, as described previously.1


VPB1019
Assessing the reversal efficacy of prothrombin complex concentrate (PCC) and andexanet alfa in rivaroxaban‐anticoagulated blood: Ex vivo study
F. Rayatdoost 1; K. Deventer1; H. Schöchl2; O. Grottke1
1 Uniklinik RWTH Aachen, Aachen, Nordrhein‐Westfalen, Germany; 2 AUVA Trauma Centre Salzburg, Salzburg, Salzburg, Austria
Background: In patients with factor‐Xa inhibitors associated bleeding, hemostatic restoration can be achieved by andexanet alfa (andexanet) or off‐label use of prothrombin complex concentrate (PCC). Although thrombin generation (TG) is a golden method to monitor the effects of reversal agents, conflicting results have been reported. Only limited data is available on head‐to‐head comparison.
Aims: Assessing the reversal potential of andexanet and PCC in rivaroxaban‐anticoagulated blood by TG and ex vivo flow chamber assays.
Methods: After ethical approval, blood samples of 6 donors were collected and spiked ex vivo with rivaroxaban (150 and 300 ng/ml). PCC (Cofact®; 25 and 50 U/kg) and andexanet (800 mg) were added at clinically recommended doses. TG was assessed with 5 pM tissue factor (TF) and 4 μM phospholipids. flow chamber experiments were performed to measure the impact on platelet aggregation and thrombus formation under high shear rate (1000 s‐1) over TF and collagen (Quantification by ImageJ).
Results: Rivaroxaban prolonged the lag time and decreased endogenous thrombin potential and peak height dose‐dependently. Independent of rivaroxaban concentration, andexanet normalized or overcompensated all TG parameters. PCC showed no significant improvement on TG. In flow chamber experiment, rivaroxaban (150 and 300 ng/ml) reduced platelet adhesion to 26% and 13%, and thrombus formation to 2.5% and 0.95% of baseline, respectively. Andexanet independent of anti‐FXa level, restored 90% and 100% of fibrin formation after 5 and 6 min running blood flow. However, after 6 min blood flow, this efficacy for PCC (25 U/kg) was 36% and 19%, and for PCC (50 U/kg) was 72% and 61% in 150 and 300 ng/ml rivaroxaban, respectively.
Conclusion(s): Results of TG assay do not consistently predict the effectiveness of hemostatic agents in vitro. Flow chamber experiment by simulating the physiological conditions shows the efficacies of andexanet and PCC in normalization of hemostasis alterations caused by rivaroxaban.
Hemostasis and Organ Dysfunction
PB1022
Monitoring hemostasis with Rotational Thromboelastometry (ROTEM) in patients undergoing aortic valve replacement surgery
A. García1; S. Sánchez1; D. Iyu 2; N. Marín2; J. García‐Estañ2; J. Moraleda1; F. García1
1 Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Murcia, Spain; 2 University of Murcia, Murcia, Murcia, Spain
Background: Hemostatic alterations are one of the most common postoperative complications in patients who undergo aortic valve replacement surgery with cardiopulmonary bypass (CPB). Excessive bleeding or thrombosis can be associated with a prolonged intensive care unit stay and an increase in morbidity and mortality. In this setting Rotational Thromboelastometry (ROTEM) is a point‐of‐care equipment that evaluates the viscoelastic properties of whole blood samples and permits an accurate analysis of the whole process of coagulation, which may allow the early detection and treatment of any hemostatic problem.
Aims: To evaluate the global hemostatic status, using ROTEM: clot initiation, formation, consolidation and lysis, of patients undergoing aortic valve replacement before, during and after the cardiopulmonary bypass procedure.
Methods: The citrated blood samples of 33 patients were analysed by ROTEM Delta in three different time points: Before (M1): in the absence of heparin; During (M2): in the presence of heparin, and After(M3): in the presence of protamine; the CPB procedure. The ROTEM analysis included the following tests: INTEM; EXTEM; FIBTEM and HEPTEM, in which Clotting Time (CT); Maximun Clot Firmness (MCF); Alpha Angle (α) and Maximun Lysis (ML) were measured, along with standard coagulation parameters such as: PT, aPTT, platelet count and fibrinogen.
Results: Complete ROTEM results were available within 15 minutes of test initiation. In INTEM, EXTEM and FIBTEM only M1 and M3 samples were analyzed (Table 1) whereas in HEPTEM all the time points were evaluated (Table 2), resulting, in all the cases, in a statistically significant difference in the different parameters, although all the values were within a normal physiological range. No hyperfibrinolysis was found in any of the patients.
Conclusion(s): According to our experimental conditions, the surgical procedure used appear not to have promoted significant clinical changes in the hemostatic status of patients undergoing aortic valve replacement surgery.


PB1021
Renal failure‐induced coagulation factor increase is similarly worsened by Haemodialysis and Haemodiafiltration
J. Caruana 1; K. Vella2; M. Cini Masini2; A. Vella2; M. Borg2; E. Farrugia3; L. Camilleri4; A. Gatt5
1 University of Malta, Zabbar, Malta; 2 Mater Dei Hospital, Department of Pathology, Msida, Malta, Msida, Not Applicable, Malta; 3 Mater Dei Hospital, Department of Nephrology, Msida, Malta, Msida, Not Applicable, Malta; 4 Department of Statistics & Operations Research, University of Malta, Msida, Malta, Msida, Not Applicable, Malta; 5 Department of Pathology, Faculty of Medicine and Surgery, University of Malta, Msida, Not Applicable, Malta
Background: End stage renal disease (ESRD) is associated with a paradoxical risk of bleeding and thrombotic events. Haemodialysis (HD) and haemodiafiltration (HDF) are different renal replacement therapies with an unclear effect on haemostasis.
Aims: To compare and contrast the effect of HD and HDF on haemostasis through analysis of clotting factors and procoagulant soluble P‐Selectin measurements.
Methods: Blood samples were collected from a total of 41 ESRD patients before and after HD(21) and HDF(20) respectively. Factor XIII (FXIII), Von Willebrand Factor (VWF) antigen, VWF activity, Fibrinogen antigen, Fibrinogen activity and soluble P‐Selectin (sP‐Selectin) were measured. Results from ESRD patients were compared to 20 healthy controls, compared in parallel (before vs after dialysis) and compared between the two therapies (HD vs HDF).
Results: Pre‐dialysis, FXIII and VWF activity in ESRD male patients were similar to healthy controls. FXIII increased significantly post‐dialysis. Fibrinogen assays, VWF antigen and VWF activity in the female cohort were significantly greater than those of the normal cohort, and these became even more elevated post‐dialysis (Table 1). sP‐Selectin levels were lower in renal patients (p = 0.004) and after treatment, results were similar to those of healthy controls (p = 0.333). Comparing the pre‐ vs post‐HD results, factor elevation was seen in all groups. With the exception of Fibrinogen, factor elevation was attributed to a physiological response other than dialysis‐related water loss. Comparing the pre‐ vs post‐HDF results, all analytes with the exception of VWF activity were significantly elevated. Elevation in Fibrinogen activity, FXIII and VWF antigen in the female cohort was also not solely caused by water loss (Table 2). Both HD and HDF resulted in coagulation factor increase. When comparing the factor levels in the HD and HDF groups, no significant difference emerged between the two interventions.
Conclusion(s): Factor elevation in ESRD patients was further increased post‐dialysis. HD and HDF equally contribute to coagulation factor changes.


PB1027
Efficacy of haemodialysis in the long‐lasting elimination of dabigatran
L. Vieira 1; S. Lopes2; R. Pombal3; R. Neto3; A. Magalhães3; D. Ferreira3; M. Figueiredo2
1 Centro Hospitalar Vila Nova de Gaia/Espinho, Porto, Portugal; 2 Centro Hospitalar Centro Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Porto, Portugal; 3 Centro Hospitalar Centro Vila Nova de Gaia/Espinho, Porto, Porto, Portugal
Background: Direct oral anticoagulants have a more predictable dose‐response, with less need for laboratory monitoring. However, clinical intercurrences may alter their metabolization. For dabigatran, renal function is particularly important, considering the preponderant role in its clearance. Besides idarucizumab, haemodialysis is also a tool in the elimination of its anticoagulant effect.
Aims: Description of a case of dabigatran intoxication in the setting of acute renal failure.
Methods: Collection of clinical data in SClínico® application.
Results: A 67‐year‐old German man was admitted to the emergency department of a Portuguese hospital, after being found fallen. Language‐barrier hindered communication, but it was later assessed that prostration and anorexia were progressively presented over the previous two weeks. Antecedents of diabetes, dyslipidaemia, arterial hypertension, chronic kidney disease and atrial fibrillation were inferred through is usual medication (which included dabigatran 150 mg twice daily). Analysis revealed a severe acute renal failure (Creatinine 8.22 mg/dl, Urea 281 mgd/L), with hyperkalaemia (K+ 9.5 mmol/L). Electrocardiogram presented sinus bradycardia with pronounced widening of the QRS‐complex. Dabigatran was in supratherapeutic levels (>460 ng/ml; trough range: 28‐155 ng/ml), without evidence of haemorrhage. Urgent haemodialysis was initiated. A central venous catheter was placed without complications. Idarucizumab was effective, but there was a rebound effect hours later. Four haemodialysis sessions were performed during the hospitalization and dabigatran concentration pre‐ and post‐haemodialysis was measured (Table 1). There was a mean decrease of 49% (42.9‐55.2%) in plasma dabigatran levels per session. Trough range was reached on the eleventh day. Renal failure, attributed to ischemic acute tubular necrosis associated with persistent hypotension, was stabilized and the patient returned to Germany.
Conclusion(s): Although haemodialysis was initiated due to hyperkalaemia and electrocardiographic findings, its high‐efficacy in long‐lasting elimination of dabigatran was demonstrated. The observed rebound‐effect and the average elimination rate per session emphasize that intra/extravascular balance of dabigatran, especially in intoxication cases, should be considered.

PB1024
Investigation of thrombin generation assay to assess vaso‐occlusive crisis in sickle cell disease
G. Feugray1; F. Kasonga2; M. Grall3; Y. Benhamou4; V. Le Cam Duchez 5; A. Lahary6; P. Billoir7
1 Normandie Univ, UNIROUEN, INSERM U1096, CHU Rouen, Vascular Hemostasis Unit, F‐76000 Rouen, France, Rouen, Haute‐Normandie, France; 2 CHU Rouen, Vascular Hemostasis Unit, F‐76000 Rouen, France, Rouen, Haute‐Normandie, France; 3 CHU Rouen, Department of Internal Medecine, F‐76000 Rouen, France, Rouen, Haute‐Normandie, France; 4 CHU Charles‐Nicolle, Rouen, Nord‐Pas‐de‐Calais, France; 5 INSERM U1096 EnVI, Vascular Hemostasis Unit, Rouen University Hospital, Rouen, Haute‐Normandie, France; 6 CHU Rouen, Hematology laboratory, F‐76000 Rouen, France, Rouen, Haute‐Normandie, France; 7 Normandie Univ, UNIROUEN, INSERM U1096, Vascular Hemostasis Unit, Rouen University Hospital, France, Rouen, Haute‐Normandie, France
Background: Sickle cell disease (SCD) is an inherited haemoglobinopathy disorder. The main consequence is synthesis of haemoglobin S leading to chronic haemolysis associated with morbidity.
Aims: The aim of this study was to investigate Thrombin Generation Assay (TGA) to assess hypercoagulability in SCD and TGA parameters as biomarkers of vaso‐occlusive crisis (VOC).
Methods: We performed TGA in platelet poor plasma with 1 pM of tissue factor and 4μM of phospholipid‐standardized concentration. We measured soluble thrombomodulin, soluble endothelial Protein C Receptor and Tissue Factor Pathway Inhibitor. Experiments were done in duplicate for patients and controls.
Results: A total of 113 patients with SCD, 83 at steady state and 30 during VOC, and 25 controls were included. Among the 83 patients at steady state, 37 were S/S‐1 S/β0, 20 were S/Sα3.7 and 19 were S/C‐7 S/β+. Among the 83 patients at steady state, 28 developed a VOC in the following year (median: 4 months [2.25‐6]). We observed an increase of peak and velocity associated with a reduction of lagtime and time to peak and no difference of endogenous thrombin potential (ETP) in patients compared to controls. Decreases of physiological anticoagulants were also observed. TGA confirmed prothrombotic state in all SCD genotypes or clinical status. The association of an ETP>1207 nM.min and a peak>228.5 nM presented a sensitivity of 73.5% and a specificity of 93.9% to predict VOC development in the following year.
Conclusion(s): We have demonstrated a hypercoagulable state in SCD induced by chronic haemolysis. ETP and peak could be used to predict VOC development and to initiate new therapeutics in SCD.


PB1025
Treatment with peptide KRRKPGP and warfarin changes anticoagulant effects and fibrinolysis in metabolic syndrome
T. Obergan; T. Shubina; L. Lyapina; M. Grigorjeva
Lomonosov Moscow State University, Moscow, Moskva, Russia
Background: Lipid and carbohydrate metabolism disorders, known as metabolic syndrome (MS), are also accompanied by the hemostasis system dysfunction, including hypercoagulation and hypofibrinolysis, which can lead to thrombosis. It has been shown that the treatment with glyproline family peptides (KKRRPGP, RKKRPGP) of MS animals had a positive effect on metabolic processes and hemostatic balance.
Aims: To carry out a comparative analysis of coagulation and fibrinolysis changes undergoing the glyproline peptide Lys‐Arg‐Arg‐Lys‐Pro‐Gly‐Pro (KRRKPGP) and the indirect anticoagulant warfarin treatment of experimental MS.
Methods: Briefly, adult male Wistar rats were fed high‐calorie food (3980 kcal/kg, 135% of standard feed) to simulate MS. After 6 weeks, MS‐rats were treated with either peptide KRRKPGP or warfarin (intragastric way, 100 μg/kg, once daily, 7 days); MS untreated rats (control) and health rats received 0.85% saline. Than hemostatic parameters of blood plasma were evaluated using standard methods: anticoagulant activity by APTT test and prothrombin time (PT); fibrinolysis was estimated by tests of total fibrinolytic activity (TFA), fibrindepolymerization (FDPA) and enzymatic fibrinolytic activities (EFA).
Results: Feeding animals with high‐calorie food for 6 weeks led to increased clotting properties (APTT and PT decreased to 73 and 56%) and inhibition of blood fibrinolysis (TFA, FDPA and EFA amounted to 60, 59 and 50% vs. health rats, respectively). Further peptide KRRKPGP or warfarin treatment resulted in an increase in the anticoagulant activity in both cases (APTT ‐ by 43 and 62% vs. MS‐control, respectively), although PT prolongation was observed only after the warfarin application (by 40% vs. MS‐control). At the same time, only KRRKPGP‐treatment significantly restored of impaired fibrinolysis: the changes of TFA, FDPA and EFA were 193, 176 and 285% vs. MS‐control.
Conclusion(s): Thus, warfarin had only anticlotting effects whereas the peptide KRRKPGP contributed to an increase both anticoagulant and fibrinolytic properties of blood when used in the MS conditions.
PB1023
Hemostasis in Yellow fever: Results from the 2018 outbreak in Brazil
L. Jardim 1; M. Brandão Franco1; B. Carvalho2; F. Basques2; D. RIbeiro3; L. Gallo4; S. Rezende5
1 Faculty of Medicine, Universidade Federal de Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil; 2 Hemocentro de Belo Horizonte, Fundação HEMOMINAS, Belo Horizonte, Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 3 University Hospital, Universidade Federal de Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil; 4 Hospital Eduardo de Menezes, Belo Horizonte, Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil; 5 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil
Background: Between 2017‐2018, Brazil faced the most expressive yellow fever(YF) outbreak, with 1,266 cases confirmed in humans and 415 deaths. YF is a viral fever which can evolve with a severe bleeding diathesis. To date, very few studies have investigated the hemostasis in these patients.
Aims: To perform a full evaluation of bleeding manifestations, death and hemostasis profile.
Methods: We evaluated clinical and laboratory characteristics of patients with YF admitted to a local hospital between December/2017‐April/2018.
Results: We included 46 patients, 34 with severe (SYF) and 12 with moderate (MYF) disease. Most were male (89%), median age 46 years (IQR,38‐53). A total of 12 (26%) died, all had SYF. At admission, median AST was 1,025 (IQR,266‐4219) and creatinine 1.4 (IQR,0.8‐2.5). A total of 59% versus 8% of patients with SYF versus MYF had bleeding, respectively (p = 0.003). Patients with SYF presented lower platelet counts (p = 0.001), more prolonged aPTT (p = 0.03), TT(p = 0.005), higher D‐dimer (p < 0.0001) and lower plasma levels of factors II (p = 0.0003), IX (p = 0.01) and X (p = 0.04). When compared with patients discharged alive, those who died presented more major bleeding(p = 0.03), hepatic encephalopathy (p = 0.002) and renal failure (p = 0.001). The INR(p = 0.003) and the aPTT (p = 0.002) were higher in SYF who died when compared with SYF who were discharged. SYF who died presented lower plasma levels of factors II (p = 0.02), V(p = 0.001), FVII (p = 0.005) and IX(p = 0.01). There was no difference in the levels of factor VIII, X or fibrinogen between patients who died and the ones discharged alive. Most patients (91%) had fibrinogen at normal levels and all patients presented increased TT.
Conclusion(s): Patients with YF develop a coagulopathy associated with deficiency of procoagulant factors of hepatic synthesis. Enlarged TT and aPTT and reduced factors II, V, VII, IX were associated with death in a univariate analysis. This is the first evaluation of a complete profile of hemostasis in YF.
PB1026
Whole blood better supports platelet measures of haemostasis compared to red cells and plasma: an ex vivo spiking study
A. Rossetto 1; P. Vulliamy2; L. Green3; R. Davenport4
1 Queen Mary Univeristy of London, UK, London, England, United Kingdom; 2 Queen Mary University of London, UK, London, England, United Kingdom; 3 Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom; 4 Centre for Trauma Science, Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom
Background: ¬Current prehospital haemostatic resuscitation is largely based on platelet‐poor blood components, e.g. red cells and plasma (RCP). Clinical trials have demonstrated improved outcomes compared to crystalloid resuscitation but the effect of these components and whole blood (WB) on trauma‐induced coagulopathy (TIC) have not been fully characterised.
Aims: Compare the ability of WB versus RCP to correct ex vivo platelet‐related haemostatic parameters in patients with TIC.
Methods: Samples were collected from trauma patients who activated the major haemorrhage protocol prehospital and were enrolled into a prospective cohort study (ISRCTN12962642) at a UK major trauma center in 2021/2022. Each sample was aliquoted and spiked ex vivo with a volume of RCP or WB equivalent to four units. ROTEM EXTEM and FIBTEM were performed on non‐spiked and spiked samples. Platelet count was measured using flow cytometry, while aggregation and agglutination with multiple electrode aggregometry. The original components and healthy‐donor samples were assayed as controls (REC 07/Q702/24). RCP and WB were stored at 4°C up to two weeks.
Results: Samples from 19 adult trauma patients and 5 healthy donors were analysed. Patients’ median age was 31 years, 89% were male, TIC was present in 68% (EXTEM A5 <=40 mm), 74% had platelet dysfunction (EXTEM‐FIBTEM A5 <=30 mm) and 39% died. Samples spiked with WB compared to RCP were characterised by better preserved and higher overall clot strength (EXTEM A5 37 mm [35‐42] vs 35 mm [30‐39], p = 0.001) and platelet‐related clot strength (EXTEM‐FIBTEM A5 30 mm [26‐33] vs 27 mm [24‐30], p = 0.001) (Figure 1A‐C). WB‐spiking reduced overall incidence of TIC and corrected platelet dysfunction in two samples (Figure 1B‐D). While both RCP and WB similarly corrected EXTEM CT (p = 0.501), other measures of clotting and fibrinolysis kinetics, platelet count, aggregation and agglutination were better preserved or ameliorated by WB (Figure 2).
Conclusion(s): When compared to RCP ex vivo, WB preserves or improves most platelet‐related haemostatic parameters.


Microparticles
PB1028
Extracellular vesicles of activated platelets possess shifted lipid‐rich biomolecular profile and display an increased procoagulant activity
E. Guerreiro 1; S. Kruglik2; J. Guigner3; A. Kuzmin4; S. Swamy1; N. Latysheva1; L. Wilsgård1; B. Østerud5; F. Sureau2; S. Bonneau2; J. Hansen6; O. Hellesø7; O. Snir8
1 Thrombosis research center ‐ University of Tromsø, Tromsø, Troms, Norway; 2 Institut de Biologie Paris‐Seine, Sorbonne Université, Paris, Ile‐de‐France, France; 3 L'Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie, Sorbonne Université, Paris, Paris, Ile‐de‐France, France; 4 Institute for Lasers, Photonics and Biophotonics, University at Buffalo, State University of New York, Buffalo, New York, United States; 5 University of Tromsø, Tromsø, Troms, Norway; 6 Thrombosis Research Center, Department of Clinical Medicine, UiT ‐ The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway; 7 Univeristy of Tromsø, Tromsø, Troms, Norway; 8 UiT – The Arctic University of Norway, Tromso, Troms, Norway
Background: Platelet activation and elevated proportions of platelet‐derived extracellular vesicles (PDEVs) are associated with an increased risk of venous thromboembolism (VTE), suggesting a role of PDEVs in the pathogenesis of the disease. The mechanisms, however, by which PDEVs may trigger and/or drive VTE are unclear, neither the functional output of EVs derived from resting or activated platelets.
Aims: In this study we aimed to perform a biomolecular characterization of EVs derived from resting and activated platelets at a single‐vesicle level and to study their respective procoagulant activity.
Methods: EVs of resting and in vitro activated platelets (TRAP6 and A23187) were isolated using a standard 20,000 g centrifugation protocol. PDEVs were characterized by nanoparticle tracking analysis, cryogenic transmission electron microscopy, and Raman tweezers microspectroscopy (RTM). Procoagulant phospholipids (PPL)‐dependent assay and a modified tissue factor (TF) activity assay were used to monitor the procoagulant activity of PDEVs.
Results: Biomolecular Component Analysis of the recorded Raman spectra of single PDEVs showed that EVs of resting platelets have a dominant protein‐rich profile, with high variation in biomolecular distribution. In contrast, EVs of activated platelets possess a lipid‐rich profile and a more homogenous biomolecular distribution (Figure 1). Moreover, EVs of activated platelets had an increased procoagulant activity, shown by a reduced plasma clotting time (9.2% +/‐ 5.8%) measured in a PPL‐dependent clotting assay. Specific blocking of phosphatidylserine (PS) and phosphatidylethanolamine (PE) blocked the procoagulant activity of such PDEVs in a dose dependent manner. Furthermore, PDEVs from activated platelets facilitate the activity of the TF: factor(F)VIIa complex and the downstream activation of prothrombin by 3.5‐fold.
Conclusion(s): EVs of activated platelets have a lipid‐rich biomolecular profile that is associated with an increased procoagulant activity. These findings may partly explain the observed association between platelet activation, elevated levels of PDEVs and VTE risk.

Protein C Pathway
PB1029
Identification and functional characterisation of alternatively spliced novel isoforms of human protein C (PROC) gene
S. Abul Kalam 1; F. Naaz2
1 Department of Biochemistry, School of Chemical and Life Sciences, Jamia Hamdard, NEW DELHI, Delhi, India; 2 Department of Biochemistry, School of Chemical and Life Sciences, Jamia Hamdard, New Delhi, Delhi, India
Background: Protein C regulates blood coagulation and is composed of several domains like gamma‐carboxyglutamic acid‐rich (GLA) domain, epidermal growth factor (EGF) like domains, a short activation peptide, and serine protease domain (SP). Several mutations spreading throughout the PROC gene have been identified that may contribute to protein C deficiency. Alternative splicing of the human PROC gene may also generate multiple variants. Different exons encode the domains present in the protein C and alternative splicing may lead to the addition or removal of some of these crucial domains, thus altering the functioning of the protein.
Aims: Identification and characterization of alternatively spliced novel isoforms of human protein C (PROC) gene
Methods: Gene and exon finding tools were used to predict novel exons of the human PROC gene, and alternative splicing patterns were elucidated. RT‐PCR and DNA sequencing were used to confirm predicted transcripts in the human liver, and their expression level was reported using real‐time PCR. MD simulation studies were performed using GROMACS.
Results: Six novel exons were predicted in the PROC gene using bioinformatics and the expression of two novel isoforms was reported in the human liver. These isoforms were found to have exons with different promoter regions and splicing patterns. Some critical domains were reported to be missing in these novel isoforms suggesting an alteration in the activity of the encoded protein. The MD simulation study revealed the structural integrity of novel isoforms.
Conclusion(s): The novel protein C isoforms identified in our study may have physiological significance and contribute to protein C's anticoagulant, anti‐inflammatory, and anti‐apoptotic properties.
PB1031
Real‐world treatment of severe congenital protein C deficiency with protein C concentrate including prophylaxis: A physician survey in Europe and the US
M. Wang 1; C. Siffel2; P. Turecek3; H. Gazda2; S. Fung4; E. Swallow5; A. Greatsinger5; E. Billmyer5; H. Hertfelder6
1 University of Colorado Hemophilia and Thrombosis Center, Aurora, Colorado, United States; 2 Takeda Pharmaceutical Company Limited, Lexington, Massachusetts, United States; 3 Baxalta Innovations GmbH, a Takeda company, Vienna, Wien, Austria; 4 Fung Consulting, Eching, Bayern, Germany; 5 Analysis Group, Inc., Boston, Massachusetts, United States; 6 Center for Blood Coagulation and Transfusion Medicine, University of Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Severe congenital protein C deficiency (SCPCD) is a rare, autosomal recessive disorder associated with thromboembolic events. Protein C concentrate (Baxalta US Inc., a Takeda company, Lexington, MA, USA) administered intravenously is approved for long‐term prophylaxis (LTP) in the US and short‐term prophylaxis (STP) in Europe for patients with SCPCD. Data on the real‐world use of protein C concentrate for LTP are limited.
Aims: To describe the real‐world use of protein C concentrate for LTP and STP administered either intravenously or subcutaneously in patients with SCPCD in Europe and the US.
Methods: Physicians with experience using protein C concentrate to treat patients with SCPCD completed an online survey. Information collected included physicians’ clinical practice, experience treating SCPCD (based on their best recollection), treatment strategies, and opinions on the subcutaneous use of protein C concentrate.
Results: This analysis included responses from 19 physicians (US, n = 7; Europe, n = 12) who reported using protein C concentrate to treat 32 patients (US, n = 8; Europe, n = 24), of whom 23 (71.9%) received LTP (Table 1). Among those receiving LTP, 16 patients (69.6%) received intravenous LTP and 12 (52.2%) received subcutaneous LTP. Five patients (21.7%) received both intravenous and subcutaneous LTP. The administration of subcutaneous LTP was reported only by physicians in Europe. Overall, 18 physicians indicated that they would like subcutaneous administration to be approved for protein C concentrate. Reasons for this included its convenience relative to intravenous administration, and the difficulty associated with intravenous administration when venous access is limited.
Conclusion(s): LTP with intravenous protein C concentrate is used in clinical practice in both Europe and the US for the treatment of patients with SCPCD. Subcutaneous administration of LTP is also practiced by some physicians in Europe. Physicians in both Europe and the US indicated their interest in having subcutaneous administration as an approved route of administration for protein C concentrate.

PB1030
Erythrocytes impair the anticoagulant function of the protein C system in whole blood thrombin generation
J. Wan1; J. Konings2; B. de Laat3; D. Huskens4; M. Roest 5
1 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 2 Synapse Research Institute, Maastricht, the Netherlands, MAASTRICHT, Limburg, Netherlands; 3 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands, 4 Synapse Research Institute, Maastricht, Limburg, Netherlands; 5 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: Erythrocyte disorders, such as polycythemia vera, sickle cell disease and malaria are often associated with an increased risk of venous thrombosis
Aims: To investigate the hypothesis that high erythrocyte numbers diminished protein C function and thereby increase the risk of thrombosis through impaired regulation of coagulation
Methods: The effects of activated protein C (APC) and thrombomodulin (TM) on calibrated thrombin generation (TG) were tested in whole blood (WB), platelet rich plasma (PRP), and platelet poor plasma (PPP), supplemented with various concentrations of erythrocytes. The effects of synthetic phospholipids supplementation on the activity of APC and TM in WB‐TG were determined. Furthermore, the correlation between erythrocyte count and TM modulated WB‐TG was tested in 119 healthy donors.
Results: APC and TM dose‐dependently prolonged the lag time and reduced the endogenous thrombin potential until the Peak (ETPp) and thrombin Peak in both PRP‐TG and WB‐TG (Fig. 1A & 1B). Interestingly, the anticoagulant effects of APC and TM on ETPp and thrombin Peak were 3 times less effective in WB than in PRP. Washed erythrocytes added to PPP dose‐dependently suppressed the inhibitory effect of APC on ETPp and thrombin Peak (Fig. 2A). The addition of synthetic phospholipids into WB dose‐dependently enhanced the inhibitory effect of APC (Fig. 2B). Erythrocyte counts were inversely correlated with the inhibitory effect of TM on the ETPp (r = ‐0.41, p < 0.001) measured in WB of 119 healthy donors, while platelet or leukocyte counts were not associated with APC activity.
Conclusion(s): We have shown that high erythrocyte numbers are inversely associated with the anticoagulant function of both TM and APC. This dose‐dependent inhibition of the protein C pathway may be an explanation for the high thrombosis risk in diseases, such as polycythemia vera, sickle cell disease and malaria.


Regulation of Coagulation
VPB1046
Changes in prothrombin time, activated partial thromboplastin time and thrombin time tests of patients administered with different types of anticoagulant in calabar metropolis, Nigeria
D. Okpokam 1; A. Josephine1; M. Kooffreh‐Ada2; E. Iyamah3; P. Zara‐Kokpa4; A. Emeribe1
1 University of Calabar, Calabar, Cross River, Nigeria; 2 University of Calabar Teaching Hospital, Calabar, Cross River, Nigeria; 3 University of Benin Teaching Hospital, Benin, Edo, Nigeria; 4 University of Port Harcourt, Port Harcourt, Rivers, Nigeria
Background: Anticoagulants are chemical substances that prevent or reduce coagulation of blood, and used in therapy for thrombotic disorder as a class of medication
Aims: The aim of the study is to evaluate some coagulation profiles in patients on anticoagulants in the University of Calabar Teaching Hospital, Calabar.
Methods: One hundred and sixty (160) subjects were enrolled, consisting of 80 test and 80 apparently healthy subjects, adult male and female, age range 18 to 70 years. Ethical clearance with the Reg. number (UCTH/HREC/33/528) was gotten. 4.5 ml of blood was aseptically collected with minimum stasis into a sample bottle containing 3.13% trisodium citrate for PT, APTT and TT. Estimation of PT, APTT and TT was assayed using Quick‐One Stage method.
Results: Over 50 percent of patient on anticoagulant were male and dabigatran (40%) comprised highest percentage, followed by heparin (19%), warfarin (15%), apixaban (13.8%) and clexane (11.3%). Deep vein thrombosis (31.3%) and ischemic stroke (23.8%) showed the highest percentage of diagnosis, while atrial fibrillation, cardiopulmonary bypass and heart failure were smallest (1.3%). There was a significant rise in the PT, APTT and TT level (19.6, 44.0 and 34.3) of patients on anticoagulants when compared to the control (11.3, 30.1 and 17.5) subjects respectively, also 45% of patients were in the middle of 28‐37 years. There was also significant statistical difference based on the type of drug use and the duration of taking the anticoagulant. A strong significant correlation between duration of use versus parameters assayed (p < 0.01) was found in patients on anticoagulant.
Conclusion(s): Therefore, assessing the sensitive PT, APTT and TT test for different anticoagulants used as a criterion to monitor patients should be considered and also edify clinicians about the test request to monitor their patients on anticoagulants.

Changes of some coagulation profile in patients administered anticoagulant therapy

PB1038
The participation of plant heparinoids in the regulation of blood clotting
E. Maystrenko; M. Lyapina; M. Kalugina; L. Lyapina
Lomonosov Moscow State University, Moscow, Moskva, Russia
Background: One of the urgent problems of modern medicine is the study of natural anticoagulants, the use of which does not cause adverse side effects on the organism. Many plants include heparin‐like components (heparinoids), which are effective in the development of processes that prevent thrombotic complications.
Aims: The purpose of our work was to identify anticoagulant effects in different types of heparinoids isolated from the roots of peonies and to establish their regulatory effect on blood clotting processes during the development of thrombosis.
Methods: Heparinoids were isolated from the roots of herbaceous peonies (Officinalis, Ivan Goroshankin). The mechanism of ah anticoagulant action was studied and their effect on the processes of fibrin and thrombosis was investigated. Laboratory animals – male Wistar rats. Anticoagulant activity was evaluated by tests of thrombin time (TV), prothrombin time (PV), APTT and total fibrinolytic activity (TFA). Heparinoids from peonies were administered orally to rats repeatedly for 5 days every 24 hours.
Results: 20 hours after the last administration of heparinoids in the blood of animals, an increase in TV was found by 13‐15%, PV ‐ by 9‐10%. APTT ‐ by 21‐24%, TFA ‐ by 25‐31%, possible mechanisms of activating action of heparinoids on anticoagulant activity of blood caused by blockade of coagulation factors. Prevention of fibrin formation processes and fibrin polymerization by heparinoids due to thrombin inhibition by plant heparinoids has been established. Thus, with hypercoagulation caused by the introduction of small doses of tissue thromboplastin, the use of heparinoids contributed to the restoration of normal blood clotting. The greatest effect was provided by the heparinoid from the roots of the Ivan Goroshankin peony. This heparinoid corresponded in molecular weight to low molecular weight heparin of animal origin
Conclusion(s): Vegetable heparinoids having an anticoagulant effect, are able to regulate blood clotting processes, especially with hypercoagulation shifts in the bloodstream
VPB1048
Evaluation of thrombin burst using thrombin time of clot waveform analysis
H. Wada; K. Shiraki; H. Shimpo
Mie Prefectural General Medical Center, Yokkaichi, Mie, Japan
Background: Thrombin burst has attracted many attentions as a physiological coagulation mechanism. However, the clinical evidence for it is scarce in routine methods.
Aims: The mechanism of thrombin burst was evaluated by a clot waveform analysis (CWA) to assess the thrombin time (TT).
Methods: The TT with a low concentration of thrombin (0.01~5.0 IU/ml) was evaluated using a CWA on ACL‐TOP system. We evaluated the CWA‐TT of plasma deficient for various clotting factors, calibration plasma, platelet‐poor plasma (PPP) and platelet‐rich plasma (PRP) obtained from healthy volunteers, patients with thrombocytopenia and patients with malignant disease.
Results: Although the TT‐CWA of calibration plasma was able to be evaluated with 0.01 IU/ml of thrombin, that of FVIII‐deficient plasma could not be evaluated. The peak time of CWA‐TT was significantly longer and the peak height significantly lower in various deficient plasma, especially FVIII‐deficient plasma than in calibration plasma. The second peak of the first derivative (1st DP‐2) was detected in PPP from healthy volunteers and was shorter and higher in PRP than in PPP. The 1st DP‐2 was not detected in PPP from patients with thrombocytopenia, and the 1st DP‐2 in PRP was significantly lower in patients with thrombocytopenia and significantly higher in patients with malignant disease than in healthy volunteers.
Conclusion(s): The CWA‐TT became abnormal in plasma deficient for various clotting factors and was significantly affected by platelet number, suggesting that the CWA‐TT may be a useful test for thrombin burst or hemostatic abnormalities.
PB1040
Histone‐3 plasma levels as a potential pre‐thrombotic marker up regulated in patients with digestive carcinomatosis
S. Mirshahi 1; I. aldybiat2; M. Ullah2; G. Contant1; J. Soria2; M. Pocard2; M. Mirshahi3
1 Diagnostica Stago, Gennevilliers, Ile‐de‐France, France; 2 CAP‐Paris Tech., INSERM U1275, Hôpital Lariboisière, Paris France, Paris, Ile‐de‐France, France; 3 CAP‐Paris Tech., INSERM U1275, Université de Paris, Hôpital Lariboisière, Paris France, Paris, Ile‐de‐France, France
Background: Histone H3 is one of the 5 major histone proteins that are involved in the chromatin structure in eukaryotic cells.
Aims: Previously, we reported the influence of DNA on Fibrin clot structure in peritoneal carcinomatosis [Ullah, ISTH 2021). Here, the presence of histone‐3 protein (H3) extracted from polynuclear (PN) cells in carcinomatosis patients, as well as its impact on plasma fibrinolysis are reported.
Methods: 112 carcinomatosis patients (colon = 35, ovary = 25, pseudomyxoma = 23, gastric =13, mesothelioma = 9, appendicle = 7) and 15 controls were analysed. Firstly, the polynuclear cells were purified through ficoll gradient protocol, the presence of H3 was demonstrated after PN activation by LPS. The H3 amount was quantified using two Elisa kits (R&D system). Plasmatic H4 and DNA were measured. Plasmas from 21 patients and controls were treated by thrombin (0.04U/ml) and the clots were exposed to t‐PA (10 ng/ml) for 25 minutes. The fibrin degradation products were quantified using STA Compact (Diagnostica Stago).
Results: i) The quantification of H3, H4 and DNA using LPS model in 112 carcinomatosis patients showed that pseudomyxoma, gastric and colon cancers have higher mean amounts of H3: 9.63 ng/ml, 4.9 ng/ml and 4.1 ng/ml respectively compared to control (0.55 ng/ml); ii) These results were confirmed with another test for H3 detection; iii) A high amount of H4 was observed in gastric cancer only; iv) The H3, H4, and DNA nuclear materials impacted the fibrinolysis. Clot degradation by t‐PA was defective in plasma with high H3 amounts: the D‐Dimer generation was decreased by 58%, 63%, 78% for ovarian (n = 5), pseudomyxoma (n = 9), and colic (n = 7) carcinomatosis respectively, compared with normal plasma. These results are consistent with the highest incidence of venous thrombosis in patients with this type of cancers, their metastatic and advancing stage [Ay. Thromb Haemost 2017].
Conclusion(s): These results indicate that plasma histone‐3 could be a potential biomarker for thromboembolism in peritoneal carcinomatosis.
PB1044
Reference interval of antithrombin, protein C, and protein S activities in healthy adults in Iran, the effect of age, sex, oral contraceptive intake, and menopause
S. Tabibian 1; M. Safa2; M. Khoshmirsafa3; M. Shekarabi4
1 Iranian comprehensive hemophilia care center, Tehran, Tehran, Iran; 2 Iran university of medical science, Tehran, Tehran, Iran; 3 Iran University of medical science, Tehran, Tehran, Iran; 4 Iran university of medical science, tehran, Tehran, Iran
Background: Antithrombin (AT), protein C (PC), and protein S (PS) are natural anticoagulant proteins that deficiency in each of them is associated with an increased risk of venous thromboembolism. The overlapping of plasma levels of AT, PC, and PS between healthy individuals and heterozygote carriers pose significant challenges in precise diagnosis.
Aims: This study aimed to evaluate the effect of most influencing variables on plasma levels of these proteins and propose specific reference intervals to improve the interpretation of the laboratory results.
Methods: This study was conducted on 1464 individuals who were referred to Massoud medical laboratory, Tehran, Iran, from 2019 to 2020. AT and PC were measured through chromogenic assay and PS plasma level with the clot‐based assay. A multivariable linear regression model was performed to evaluate the effect of sex, age, oral contraceptive (OCP) intake, and menopause state. Normal deviate z value was used for different subgroups to justify the need for a separate reference interval.
Results: 1200 verified healthy individuals (434 males, 766 females), aged between 18 to 69 years old were included in the study. The mean±SD age of the participants was 39.78 ± 11.79 years old. The age‐related effects for AT were found in men. In females, increasing age was associated with a rise in AT, PC, and PS plasma levels. No sex difference was found in AT plasma level. OCP taking is associated with a decrease in AT and an increase in PC plasma levels.
Conclusion(s): This is the largest study ever conducted on healthy individuals in the Iranian population. Using specific reference interval results in accurate diagnosis of true AT, PC, and PS deficiency.

PB1039
Plant heparinoids and their involvement in the regulation of blood coagulation
E. Maystrenko 1; M. Lyapina1; M. Kalugina1; S. Sorokoletov2
1 Lomonosov Moscow State University, Moscow, Moskva, Russia; 2 City Clinical Hospital named after S.P. Botkin, Moscow, Moskva, Russia
Background: One of the urgent problems of modern medicine is the study of natural anticoagulants, the use of which does not cause adverse side effects on the organism. Many plants include heparin‐like components (heparinoids), which are effective in the development of processes that prevent thrombotic complications.
Aims: The purpose of our work was to identify anticoagulant effects in different types of heparinoids isolated from the roots of peonies and to establish their regulatory effect on blood clotting processes during the development of thrombosis.
Methods: Heparinoids were isolated from the roots of herbaceous peonies (Officinalis, Ivan Goroshankin). The mechanism of ah anticoagulant action was studied and their effect on the processes of fibrin and thrombosis was investigated. Laboratory animals – male Wistar rats. Anticoagulant activity was evaluated by tests of thrombin time (TV), prothrombin time (PV), APTT and total fibrinolytic activity (TFA). Heparinoids from peonies were administered orally to rats repeatedly for 5 days every 24 hours.
Results: 20 hours after the last administration of heparinoids in the blood of animals, an increase in TV was found by 13‐15%, PV ‐ by 9‐10%. APTT ‐ by 21‐24%, TFA ‐ by 25‐31%, possible mechanisms of activating action of heparinoids on anticoagulant activity of blood caused by blockade of coagulation factors. Prevention of fibrin formation processes and fibrin polymerization by heparinoids due to thrombin inhibition by plant heparinoids has been established. Thus, with hypercoagulation caused by the introduction of small doses of tissue thromboplastin, the use of heparinoids contributed to the restoration of normal blood clotting. The greatest effect was provided by the heparinoid from the roots of the Ivan Goroshankin peony. This heparinoid corresponded in molecular weight to low molecular weight heparin of animal origin.
Conclusion(s): Vegetable heparinoids having an anticoagulant effect, are able to regulate blood clotting processes, especially with hypercoagulation shifts in the bloodstream.
PB1043
Complementary roles of red blood cells and platelets in high‐throughput whole‐blood thrombin generation
S. Sun 1; J. Zou2; J. Konings3; D. Huskens4; J. Wan5; C. Reutelingsperger2; H. ten Cate6; P. de Groot7; B. de Laat8; J. Heemskerk9; M. Roest10
1 Maastricht University, Maastricht, Limburg, Netherlands; 2 Department of biochemistry, Maastricht university, Maastricht, Limburg, Netherlands; 3 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands, 4 Synapse Research Institute, Maastricht, Limburg, Netherlands; 5 Blood Research Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA, Chapel Hill, North Carolina, United States; 6 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 7 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 8 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 9 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands, 10 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: A whole blood (WB) thrombin generation (TG) method was developed, allowing continuous and high‐throughput TG measurements. This method approaches physiology and promises new insights in the interactions of various blood cells and the coagulation process.
Aims: To investigate the contribution of both platelets and red blood cells (RBC) during different phases of clot formation applying WB‐TG.
Methods: Application of the automated 96‐well plate‐based tissue factor (TF) induced WB‐TG under conditions blocking or promoting platelet activation and/or RBC eryptosis. Platelet collagen receptors (GPVI) or thrombin receptors (PARs) were antagonized, and procoagulant phosphatidylserine (PS) was blocked with annexin A5. Parallel TG measurements were performed with platelet‐rich plasma (PRP).
Results: In TF‐triggered WB and PRP, TG parameters were optimal at 6 mM CaCl2 plus 3 mM MgCl2 for WB‐TG, and 11 mM CaCl2 plus 5.5 mM MgCl2 for PRP‐TG. At ≤1 pM TF, GPVI‐induced platelet activation caused a smaller increase in TG peak in WB than in PRP. Higher concentrations of dabigatran or rivaroxaban were needed to inhibit TG in WB than in PRP. Similarly, combined inhibition of PAR1/4 slightly reduced WB‐TG, and more potently reduced PRP‐TG. In a reconstituted system, prior treatment of RBCs with wildtype annexin A5 (but not mutated annexin A5) downregulated the WB‐TG in the initiation phase. Markedly, in WB, dose‐dependent addition of annexin A5, first enhanced the GPVI‐induced TG and then annulled all TG. Furthermore, annexin A5 delayed the onset of platelet ATP release in WB.
Conclusion(s): In WB, the role of agonist‐induced platelet activation on TG is complemented by thrombin‐generating RBC, in a manner downregulated by annexin A5. These findings are particularly relevant in RBC‐rich clotting in venous thromboembolism.
PB1036
The increase in plasma cell‐free DNA in patients with venous thromboembolism is independent of DNaseI activity
R. Herranz 1; J. Oto1; E. Plana2; F. Cana3; A. Fernández‐Pardo3; F. Ferrando4; A. Cid4; S. Bonanad5; P. Medina3
1 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 2 Angiology and Vascular Surgery Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 3 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 4 Haemostasis and Thrombosis Unit, Valencia, Comunidad Valenciana, Spain; 5 Hematology Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain
Background: Plasma cell‐free DNA (cfDNA), derived from cellular death or neutrophil extracellular traps (NETs) is increased in patients with venous thromboembolism (VTE) and is associated with an increased risk of VTE. Other thrombotic disorders, like thrombotic microangiopathies, display increased plasma cfDNA through an impaired plasma DNaseI activity.
Aims: To ascertain whether increased plasma cfDNA in VTE patients may be mediated by lower DNaseI activity, measured with two functional assays.
Methods: We obtained plasma samples from 126 VTE patients and 79 healthy volunteers (controls). In patients, plasma samples were obtained between 6 and 24 months after the thrombotic event. cfDNA was quantified with PicoGreen (Life Technologies) and DNaseI activity was measured with two different assays: DNaseI assay Kit (Abcam) after optimization and the single radial enzyme‐diffusion (SRED). Statistical analysis was performed with GraphPad (v.8.0.1).
Results: cfDNA levels were significantly increased in VTE patients (median 1799 ng/ml, 1st‐3rd quartiles 1661‐2088) than in controls (1632, 1185‐1753) (P < 0.0001). However, DNaseI activity was also increased in VTE patients (5.814 μU/ml, 5.139‐6.870) compared to controls (5.188, 4.794‐6.217) (P = 0.0041) when measured with the DNaseI assay Kit (Abcam); while, the increase was more discrete with the SRED method (0.49 cm2, 0.39‐0.61 and 0.45, 0.36‐0.52, respectively) (P = 0.0512). cfDNA and DNaseI activity, measured by any method, did not inversely correlate, suggesting an independent mechanism for cfDNA increase.
Conclusion(s): Patients with VTE have long‐lasting increased plasma levels of cfDNA and DNaseI activity. However, the increase in plasma cfDNA in VTE patients seems DNaseI‐independent, evidenced by two different functional assays. We speculate that the increase in plasma DNaseI might be a protective mechanism against the presence of high cfDNA levels. ISCIII‐FEDER (PI17/00495, PI20/00075, FI21/00171), GVA (ACIF/2017/138) and SETH.
PB1037
Rewarming from accidental hypothermia enhances whole blood coagulation in a mouse model
K. Horioka 1; H. Tanaka2
1 Karolinska Institutet, Stockholm, Blekinge Lan, Sweden; 2 Asahikawa Medical University, Asahikawa, Hokkaido, Japan
Background: Hypothermia triggers coagulation disorders, which can lead to the development of life‐threatening diseases in various organs. We previously reported that hypothermia induced platelet activation in the spleen, which resulted in microthrombosis after rewarming. However, the changes in whole blood clotting properties that occur in diseased states remain unclear.
Aims: Using thromboelastography, we investigated blood clotting activity and the effects of rewarming in a hypothermia‐induced mouse model.
Methods: C57Bl/6 mice were placed at ‐20°C under general anesthesia until their rectal temperature decreased to 15°C. One group of mice was kept at 4°C for 2 hours and then euthanized. Another group was rewarmed, kept in normal conditions for 24 hours, and then euthanized. Tissue and citrated whole blood samples were obtained from the mice for histopathological analysis, flow cytometry, and thromboelastography.
Results: Hypothermia induced the activation of platelets in the spleen; however, rewarming significantly reduced the number of activated platelets. Rewarming significantly increased the number of activated platelets in the peripheral blood, whereas no increase was observed in the hypothermia model. Thromboelastography analysis showed that whole blood samples from the rewarmed mice displayed an enhanced clotting strength.
Conclusion(s): Rewarming from hypothermia enhances whole blood coagulation activity due to an increase in the number of active platelets in peripheral blood. This phenomenon may lead to thrombotic disorders.
VPB1047
The FV‐A2086D mutation results in impaired anticoagulant function
N. Shimonishi 1; K. Ogiwara2; K. Nogami1
1 Nara Medical University, Kashihara, Nara, Japan; 2 Department of Pediatrics, Nara Medical University, Kashihara, Nara, Japan
Background: Coagulation factor V (FV) has procoagulant and anticoagulant functions. We reported the FV‐W1920R mutation in a patient with deep vein thrombosis and which had resistance to activated protein C (APC) (Nogami et al. Blood 2014). FV‐W1920R was located on the C1 domain in light chain. Recently FV‐A2086D mutation (FV Besançon), which located on the C2 domain in light chain, with deep vein thrombosis was reported (Castoldi et al. JTH 2021). However the mechanism of thrombosis associated with FV‐A2086D mutation was not fully evaluated.
Aims: To elucidate the mechanism(s) of anticoagulant functions in FV‐A2086D.
Methods: We expressed the full‐length FV using HEK293T cell. APC‐catalyzed inactivation of FVa‐A2086D was examined using the PT‐based clotting assay and western blotting. A property of FVa‐A2086D as the APC cofactor was examined in the FVIIIa degradation assay. Since the LCh region of FV is guessed to inhibit tissue factor TF‐induced procoagulant function, thrombin generation and diluted PT were measured using normal plasma added exogenous FVa.
Results: FVa‐WT:C was decreased by APC to ~10% of the initial level at 10 min. However, inactivation of FV‐A2086D a:C was delayed and remained at ~60% of the initial level, irrespective of the presence of protein S. FVIIIa was inactivated by APC with FV‐WT to ~25% of initial at 20 min. However, FVIIIa:C remained to ~50% in the presence of FV‐A2086D. Compared to normal plasma, the decreased peak thrombin and prolonged PT was shown in normal plasma added FVa‐WT. However, in normal plasma added FVa‐A2086D, all the results were comparable to normal plasma, suggestive of no inhibitory effect of FVa‐A2086D.
Conclusion(s): APC resistance of FV‐A2086D resulted from significant loss of FVa susceptibility to APC and APC cofactor activity. Moreover, FV‐A2086D lost anticoagulant function that inhibits TF‐induced procoagulant function. These impaired anticoagulant functions might have led to the thrombosis.
VPB1045
Transcriptome‐wide association studies reveal novel genes regulating plasma levels of hemostatic factors
N. Le 1; J. Huffman2; A. Morrison3; N. Smith4; P. De Vries3; M. Sabater‐Lleal5
1 Sant Pau Biomedical Research Institute (IIB Sant Pau), Barcelona, Spain, Barcelona, Catalonia, Spain; 2 Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC), VA Boston Healthcare System, Boston, MA, USA, Boston, Massachusetts, United States; 3 Human Genetics Center, Department of Epidemiology, Human Genetics, and Environmental Sciences, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX, USA, Houston, Texas, United States; 4 Department of Epidemiology, University of Washington; Department of Health Service, University of Washington; Seattle Epidemiologic Research and Information Center; Department of Veterans Affairs, Office of Research and Development, Seattle, WA 98105, USA, Seattle, Washington, United States; 5 Sant Pau Biomedical Research Institute (IIB Sant Pau), Barcelona, Spain; Cardiovascular Medicine Unit, Department of Medicine, Karolinska Institutet, Stockholm, Sweden, Barcelona, Catalonia, Spain
Background: Factor VIII (FVIII), von Willebrand factor (VWF), fibrinogen, antithrombin (AT), and protein C (PC) plasma levels are risk factors for venous thromboembolism (VTE). Genome‐wide association studies (GWAS) have identified genetic variants associated with plasma levels of these factors. By integrating GWAS and transcript expression, transcriptome‐wide association studies (TWAS) perform tests on biologically informed aggregates of variants, therefore reducing the multiple‐testing penalty and increasing power to find candidate causal associated genes.
Aims: To explore associations between the genetic component of gene expression and plasma levels of FVIII, VWF, fibrinogen, AT, and PC.
Methods: We leveraged summary data for FVIII (n = 42,125), VWF (n = 44,808), fibrinogen (n = 120,247), AT (n = 25,243), and PC (n = 19,285) from ongoing meta‐analyses of GWAS within the CHARGE Hemostasis Working Group and performed TWAS analyses via SPrediXcan in mechanistically related tissues to identify gene expression regulating plasma levels of these proteins. We then applied fine‐mapping and colocalization pipelines, using FOCUS and fastENLOC. Finally, TWAS‐based gene set enrichment was analyzed via GIGSEA to discover the regulatory network underlying plasma levels of studied factors.
Results: Overall, we detected differentially expressed genes in 15 loci associated with FVIII levels, 13 loci with VWF levels, 50 loci with fibrinogen levels, 4 loci with AT levels, and 10 loci associated with PC levels. We also found colocalization evidence between GWAS signals and gene expression of TWAS‐identified genes associated with FVIII (n = 6), VWF (n = 7), fibrinogen (n = 45), AT (n = 2), and PC (n = 2). Of these loci, RARS2, PSMB5, and SEPT9 (FVIII), RASIP1 (VWF), PCNX3, IRF8, and PTPN2 (fibrinogen), FCGRT (AT), and RP11‐13A1.1, and RP13‐942N8.1 (PC) were novel associations not detected by the corresponding GWAS input.
Conclusion(s): TWAS‐based approaches identified 10 novel significant loci associated with plasma levels of hemostatic factors, underlining the increased power to detect novel associations by integrating expression and genomic data.
PB1033
Tissue factor (TF) in peripheral blood mononuclear cells (PBMCs) is regulated by PACMA‐31 through transcriptional and posttranscriptional mechanisms
L. Beckmann 1; J. Mäder2; M. Voigtländer3; F. Klingler3; A. Schulenkorf3; C. Lehr3; C. Bokemeyer3; W. Ruf4; C. Rolling3; F. Langer3
1 Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 2 UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 3 II. Medizinische Klinik und Poliklinik, Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 4 Center for Thrombosis and Hemostasis, Johannes Gutenberg University Medical Center, Mainz, Rheinland‐Pfalz, Germany
Background: Thiol isomerase inhibitors, bacitracin and rutin, inhibit monocyte TF production under inflammatory conditions. Whether PACMA‐31, a more specific antagonist of protein disulfide isomerase with distinct pharmacologic properties, has the same antithrombotic effects, is unclear.
Aims: To investigate the effect of PACMA‐31 on the monocyte thrombo‐inflammatory response.
Methods: Isolated PBMCs or citrate‐anticoagulated whole blood were stimulated with lipopolysaccharide (LPS) and PACMA‐31 or DMSO vehicle. In some experiments, stimulation was carried out in the presence of the protease‐activated receptor 2 (PAR2) inhibitor ENMD547 or TF antibody 10H10. Cells were analyzed for TF antigen (flow cytometry, ELISA), procoagulant activity (PCA) (single‐stage clotting or Xa generation assay), and mRNA (RT‐PCR). Release of IL‐6 and TNFα was measured by ELISA.
Results: PACMA‐31 (25 μM) alone had no effect, but co‐incubation with LPS amplified monocyte TF production in whole blood. The effect was at least partially regulated on the mRNA level and could not be explained by increased phosphatidylserine membrane exposure. In contrast, equal PACMA‐31 concentrations were cytotoxic in purified PBMCs, and thus prevented LPS‐induced TF production. Titration of PACMA‐31 to 1 μM, however, recapitulated the potentiating effect with increased monocyte TF production and IL‐6 and TNFα release from LPS‐stimulated PBMCs. Western blot analysis revealed enhanced activation of the IκB‐NFκB‐signaling pathway. The stimulating effect of PACMA‐31 was prevented by co‐incubation with ENMD547, but not by TF antibody 10H10. In sharp contrast, short‐term incubation of LPS‐stimulated PBMCs with high PACMA‐31 concentrations (25 μM) was non‐cytotoxic and had no effect on TF antigen levels, but significantly reduced surface‐located TF PCA.
Conclusion(s): Regulation of monocyte TF by PACMA‐31 is time‐ and concentration‐dependent, involving transcriptional and posttranscriptional mechanisms. While short‐term incubation with high PACMA‐31 concentrations converts preformed monocyte TF into its cryptic, non‐coagulant form, long‐term incubation with low PACMA‐31 concentrations amplifies monocyte TF production by enhancing LPS‐dependent PAR2 signaling.
PB1035
Mathematical modeling suggests the importance of TFPI‐mediated inhibition of platelet‐surface bound proteins during coagulation under flow
A. Fogelson 1; K. Miyamara2; K. Leiderman2
1 University of Utah, Salt Lake City, Utah, United States; 2 Colorado School of Mines, Golden, Colorado, United States
Background: Our previously‐validated mathematical model of platelet deposition and coagulation under flow was extended to include TFPI’s action on platelet‐surface‐bound proteins.
Aims: To characterize the effectiveness of TPFI‐mediated inhibition of platelet‐bound FXa, partially‐activated FVa, and early prothrombinase on the timing and strength of thrombin production.
Methods: Differential equations for the concentrations of new species (e.g., partially‐activated factor V) were added to the model and existing equations were modified to reflect new reactions. Simulations were carried out for ranges of tissue factor exposure, flow shear rate, and values of the new kinetic rates and plasma concentrations of new species.
Results: The new model is sensitive to TFPI levels; thrombin production can be largely prevented by sufficiently high TFPI concentrations. The sensitivity is due entirely to reactions on the platelet surface and not to TPFI’s inhibition of TF:VIIa or fluid‐phase FXa. The new TPFI reactions increase the threshold level of TF needed to elicit a strong thrombin response under flow, and they delayed the onset of strong thrombin production, but had little effect on the eventual thrombin concentration reached with a strong response. The inhibitory effect of TPFI binding to platelet‐bound FXa was greater than that of its binding to platelet‐bound partially‐activated FV, and the two inhibitory effects were additive. The model includes platelet release of a mixture of FV and partially‐activated FV. Increasing the fraction of partially‐activated FV released accelerated thrombin production.
Conclusion(s): The model suggests that for coagulation under flow, the dominant effects of TFPI come from its inhibition of platelet‐bound coagulation factors, and the effects are a change in TF sensitivity and a delay in thrombin production.
PB1034
Calcium‐dependent monoclonal antibody against Gla‐domain of protein S efficiently inhibiting both protein C and TFPI anticoagulant pathways
B. Dahlback
Lund University, Malmö, Skane Lan, Sweden
Background: Vitamin K‐dependent protein S is an anticoagulant protein functioning as a cofactor in both protein C and TFPI pathways. The Gla‐domain of protein S mediates the interaction with negatively charged phospholipid, where it fulfills its anticoagulant activity. Inhibition of anticoagulant pathways is a means to rebalance the haemostatic system in bleeding disorders, offering therapeutic possibilities.
Aims: To investigate whether protein C and TFPI anticoagulant systems in plasma can be inhibited by an in‐house monoclonal antibody (mab47) against the Gla‐domain of protein S.
Methods: A calcium‐dependent mab47 against the Gla‐domain of protein S was added to human plasma and the anticoagulant activity of activated protein C (APC) or TFPI tested in a thrombin generation assay (TGA). The effects of the antibody on protein S anticoagulant activity were also tested in purified APC and TFPI systems.
Results: The mab47 was found to efficiently inhibit the anticoagulant activity of both APC and TFPI when included in the TGA. In TGA with added TFPI, the antibody dose‐dependently inhibited the anticoagulant effect of TFPI, approximately an equimolar concentration of antibody to protein S was required to neutralise the effect of TFPI. In a FXa‐inhibition assay using TFPI, protein S, FV‐Short and negatively charged phospholipid, addition of mab47 neutralized the synergistic TFPI‐cofactor activity of protein S and FV‐Short. In the TGA with added APC (0‐20 nM), addition of the mab47 inhibited the anticoagulant effect of APC.
Conclusion(s): We have identified a mab which efficiently inhibits the anticoagulant effect of protein S in both protein C and TFPI anticoagulant pathways. The antibody (mab47) is calcium‐dependent and directed against the Gla‐domain of protein S. Its mechanism of action is that it inhibits binding of protein S to the negatively charged phospholipid. The antibody has the potential to rebalance the haemostatic system in cases with bleeding disorders.
PB1041
Hypoxia increases coagulation which is more pronounced in women but is counterbalanced by a decreased platelet reactivity
M. Ninivaggi 1; H. Middelveld2; V. Schmalschläger3; M. Roest1; R. de Laat‐Kremers4; B. de Laat3
1 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 2 Department of Platelet pathophysiology, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 3 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 4 Synapse Research Institute, Maastricht, Limburg, Netherlands
Background: Hypoxia plays an important role in many pathologies, as e.g. chronic obstructive pulmonary disease and obstructive sleep apnea syndrome. These conditions are associated with an increased thrombosis risk and oxygen desaturation seems to be one of the major mediators of hypercoagulability.
Aims: The objective was to investigate the effect of exercise on thrombin generation (TG) and platelet activation under hypoxic conditions at high altitude.
Methods: Ten healthy volunteers were recruited for this study (50% male, mean age of 33 years). The study was conducted in two phases in which the subjects went up to an altitude of 3883 m firstly by cable car (passively), and secondly by walking (actively).
Results: As expected for both the passive and active ascend, the oxygen saturation decreased, while the heart rate increased significantly at high altitude (p < 0.005 and p < 0.05, respectively). Acute mountain sickness symptoms were observed independently of the ascend method. After the active ascend, platelet, white blood cell and granulocyte count were increased (p = 0.0051, p < 0.0007, and p = 0.0001, respectively), and lymphocyte was decreased (p < 0.0001). FVIII and von Willebrand factor were significantly increased after the active ascent (VWF, p = 0.0001 and p = 0.0016, respectively). TG analysis showed a prothrombotic trend at high altitude, especially after the active ascend. Interestingly, women had a more prothrombotic phenotype compared to men (Figure 1), indicated by a significantly higher peak height and ETP, and shorter lagtime, time‐to‐peak and velocity index. ETP inhibition by thrombomodulin was lower in women after the active ascend, albeit not significantly. Interestingly, platelet activation was reduced and delayed (p = 0.0073 and p = 0.0146, respectively) after the active ascend.
Conclusion(s): Hypoxia increased TG, as well as FVIII and VWF due to exercise. Women had a more prothrombotic phenotype compared to men, which was more defined at high altitude. We theorized that the observed hypercoagulability is counterbalanced by decreasing platelet activation.

PB1042
Coagulation activation in newly diagnosed AML is regulated by the heme enzyme myeloperoxidase
C. Rolling1; H. Quick 2; A. Beitzen‐Heineke1; S. Janjetovic3; J. Mäder1; C. Lehr1; C. Bokemeyer1; M. Haddad4; T. Renné4; W. Fiedler1; L. Beckmann5; F. Klingler1; F. Langer1
1 II. Medizinische Klinik und Poliklinik, Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 2 UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 3 Helios Klinikum Berlin‐Buch, Berlin, Germany, Berlin, Berlin, Germany; 4 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 5 Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany
Background: Patients with acute myeloid leukemia (AML) are at increased risk of thrombohemorrhagic complications. Overexpressed tissue factor (TF) on circulating AML blasts and shedding of TF‐bearing microvesicles (MV) contribute to systemic coagulation activation. We have recently shown that the heme enzyme myeloperoxidase (MPO) negatively regulates TF procoagulant activity (PCA) on myelomonocytic cells in vitro.
Aims: Subgroups of AML are characterized by MPO‐positive blasts. We therefore wanted to investigate the functional interaction of MPO and TF in AML in vivo.
Methods: We prospectively recruited 67 patients with newly diagnosed AML. The study was approved by the local ethics committee (PV5365). All patients and healthy controls (HC) provided written informed consent. TF PCA of isolated AML blasts was assessed by single‐stage clotting assay in the presence or absence of inhibitors against MPO catalytic activity (ABAH) or against surface MPO‐binding integrins (anti‐CD18). MV‐associated TF PCA was measured by chromogenic Xa generation assay. Plasma and intracellular MPO, prothrombin fragment F1+2 were analyzed by commercial ELISAs and D‐dimers by an immunoturbidimetric assay.
Results: AML patients had significantly higher MPO plasma levels compared to HC. Plasma MPO correlated with MPO‐positive circulating leukemic blasts and with intracellular MPO. Ex vivo incubation of isolated AML blasts with ABAH or anti‐CD18 either increased or decreased TF PCA, suggesting a divergent regulatory effect of MPO on TF activity. Importantly, modulation of TF by MPO catalytic activity required integrin‐dependent surface binding of the heme enzyme. Cellular TF PCA and F1+2 plasma levels strongly correlated, indicating that circulating leukemic blasts contributed to systemic coagulation activation in AML. In support of a regulatory role for MPO on TF, this effect was mitigated in patients with MPO plasma levels above the median.
Conclusion(s): Our study suggests that catalytically active MPO released by circulating myeloblasts regulates TF‐dependent coagulation activation in patients with newly diagnosed AML in a CD18‐dependent manner.
Tissue Factor Pathway
PB1051
Quantitative tissue factor pathway inhibitor antigen release and functionality after intravenous administration of heparins sourced from various species in non‐human primates
M. Jaradeh 1; N. Baig2; A. Kouta2; R. Duff2; L. Cera2; W. Jeske2; D. Hoppensteadt3; J. Fareed3
1 Loyola University Medical Center, Brookfield, Illinois, United States; 2 Loyola University Medical Center, Maywood, Illinois, United States; 3 Loyola University Chicago, Maywood, Illinois, United States
Background: Tissue factor pathway inhibitor (TFPI) is a multi‐domain Kunitz‐type inhibitor that plays an important role in regulating TFP through its release upon heparin administration. Currently, heparin is primarily porcine‐derived, however, recently it has been re‐sourced from bovine and ovine tissues.
Aims: In this context, we compared these heparins’ abilities to release endogenous TFPI following intravenous gravimetric and USP potency‐adjusted heparin dosages.
Methods: 10 mg/ml solutions of porcine, bovine, and ovine heparins were prepared. Individual groups of non‐human primates (n = 6) were intravenously injected with 0.5 mg/kg and 100U/kg dosages of each heparin type. Blood samples were drawn at 0‐, 15‐, 30‐, 60‐, and 120‐minutes post‐administration. Citrated blood samples were centrifuged to obtain platelet‐poor plasma and stored at ‐80C until analysis. TFPI antigen release and functionality were quantified via sandwich ELISA and a chromogenic, substrate‐based method, respectively. All results were calculated in terms of group means ±SD.
Results: Gravimetric porcine and ovine heparin dosages produced comparable TFPI release in both the functional and immunologic assays while bovine heparin demonstrated slightly lower peak TFPI antigen release and slightly higher peak function, as shown in Figure 1a and 1c, respectively. When compared following administration of USP‐adjusted 100U/kg dosages, all three heparins consistently demonstrated essentially identical TFPI antigen release and functional profiles, as shown in Figure 1b and 1d, respectively. Overall, any potential differences in the gravimetric dosing regimens were adequately corrected following USP potency adjustment in both TFPI antigen release and functionality, as seen in Figure 1. Furthermore, all three heparins demonstrated comparable pharmacokinetic profiles in both TFPI antigen release and function levels, as shown in Table 1.
Conclusion(s): These results demonstrate heparins from various sources are capable of comparatively releasing TFPI antigen. While the gravimetrically‐dosed studies demonstrated less consistent trajectories, potency‐based dosages allowed for improved pharmacokinetic homogeneity. These studies underscore the viability of alternative heparin sources as pharmacokinetic equivalents.


PB1049
Characterisation of anti‐TFPI Kunitz domain 3 antibodies that selectively inhibit protein S cofactor function
A. Petri1; P. Badia Folgado 1; D. Jones2; Y. Xu1; I. Salles‐Crawley3; J. Ahnström1; J. Crawley1
1 Imperial College London, London, England, United Kingdom; 2 The Francis Crick Institute, London, England, United Kingdom; 3 St George's University of London, London, England, United Kingdom
Background: The tissue factor pathway inhibitor (TFPI) anticoagulant pathway inhibits the initiation of coagulation. Only full‐length TFPIα functions as an effective anticoagulant in plasma and its function is greatly enhanced by its cofactor, protein S. TFPI Kunitz domain 3 (K3) plays a key role in the binding, and cofactor function, of protein S. How protein S augments TFPI function at a molecular level, and the physiological importance of this enhancement remains uncertain.
Aims: To generate and characterise anti‐human TFPI K3 monoclonal antibodies (mAbs) that inhibit/do not inhibit TFPI‐protein S cofactor function.
Methods: Mice were immunised with recombinant human TFPI K3 expressed in insect cells. Hybridoma clones were screened and selected based on mAb affinity, specificity, and inhibitory/non‐inhibitory function on the protein S enhancement of TFPI‐mediated FXa inhibition.
Results: Following selection of clones with immunoreactivity against human TFPI K3, two antibodies were selected for full characterisation (6H7 & 4D7). Analysis of the binding of these antibodies to either human TFPI K3 or full‐length TFPIα revealed high affinity binding (6H7: Kd = 0.3 nM (K3), Kd = 0.7 nM (TFPI)), (4D7: Kd = 0.6 nM (K3), Kd = 1.3 nM (TFPI)), and no cross‐reactivity with murine TFPI. Using FXa inhibition assays containing TFPI ±protein S; 6H7 completely blocked protein S enhancement of FXa inhibition by TFPI, whereas 4D7 had no effect on protein S cofactor function.
Conclusion(s): These data confirm the importance of the TFPI K3 domain in protein S‐dependent enhancement of TFPI function and reveal that distinct epitopes on the K3 are important for the interaction with protein S. These antibodies will pave the way for interrogation of protein S‐TFPI cofactor function in human plasma.
PB1050
Blockade of K‐ras signalling prevents coagulatory phenotype of alternatively activated macrophages
P. Haider1; J. Kral‐Pointner2; M. Salzmann2; B. Podesser3; J. Wojta2; P. Hohensinner 3
1 Department of Internal Medicine II, Division of Cardiology / Medical University of Vienna, Vienna, Wien, Austria; 2 Medical University of Vienna, Vienna, Wien, Austria; 3 Center for Biomedical Research, Medical University of Vienna, Vienna, Wien, Austria
Background: Alternatively acitvated macrophages are characterized by a procoagulatory phenotype including increased expression of tissue factor (TF) and reduced activation of urokinase‐type plasminogen activator (uPA) due to increased expression of plasminogen activator inhibitor 1 (PAI‐1).
Aims: To identify signaling pathways required for a procoagulatory phenotype of alternatively activated macrophages.
Methods: We studied the role of K‐ras in human macrophages as well as in a lung fibrosis model in mice, by inhibiting K‐ras signalling pharmacologically using fendiline (key experiments confirmed with a second K‐RAS inhibitor BAY‐293). Lung fibrosis was induced in mice by inhalative bleomycin application
Results: Alternative activation of macrophages (AAM) by IL‐4 and IL‐13 led to a significant phosphorylation of ERK1/2 in vitro and in vivo. K‐RAS inhibition significantly reduced the phosphorylation of ERK1/2 in macrophages leading to a significant reduction in AAM markers including TF and PAI‐1. K‐ras treated AAM showed significantly prolonged coagulation times as indicated by ROTEM measurements. Tissue Factor, uPA, PAI‐1 as well as collagen‐6 was reduced in K‐RAS inhibited AAM. In bleomycin‐induced lung fibrosis, mice treated with fendiline (K‐RAS inhibitor) showed significantly less pulmonary fibrosis. After initial weight loss associated with pulmonary inflammation, treated mice gained weight from day 9 on, whereas untreated mice lingered at their reduced weight.
Conclusion(s): Blocking intracellular K‐ras signalling in mice and human macrophages, reduced the fibrotic and coagulatory phenotype associated with pulmonary fibrosis. Mechanistically, fendiline led to a reduction of phosphorylated ERK1/2 and a downregulation of the coagulatory proteins TF, uPA, PAI‐1 and collagen‐ 6. These cellular alterations led to a prolonged coagulation potential. Treatment of mice with fendiline reduced bleomycin‐induced lung fibrosis.
PB1052
The higher activity of baboon versus human tissue factor implicates an extended exosite region
V. Zeng; F. Birkle; J. Morrissey
University of Michigan, Ann Arbor, Michigan, United States
Background: The “exosite” region of tissue factor (TF) facilitates macromolecular substrate binding to the TF/FVIIa complex, although the exact portion of TF which interacts with factor X (FX) remains unclear. We now report that baboon TF supports greater FX activation rates than does human TF. TF sequence alignments revealed a highly conserved basic residue at position 197 in most mammals (other than humans and some other higher primates), and an inserted arginine at this position is one of a few differences between human and baboon TF. We hypothesize that this arginine insertion is responsible for the increased TF activity, suggesting the possibility of an extended exosite region in TF.
Aims: Investigate the mechanism behind increased baboon TF activity.
Methods: Truncated human and baboon TF (soluble TF, or sTF), together with several sTF mutants, were characterized using FX and FIX activation assays in the presence of human FVIIa. We also tested the plasma clotting activity of baboon versus human membrane‐bound TF (memTF).
Results: Activity assays with baboon sTF and human FVIIa resulted in approximately twofold higher FX activation rates compared to human sTF/FVIIa. In plasma clotting assays, relipidated baboon memTF had faster clot times than did human memTF. Kinetic analyses of FX activation by sTF/FVIIa on membranes revealed similar Km but 1.4‐fold greater kcat for baboon sTF. No differences were found for FIX activation rates between human and baboon sTF. Inserting an arginine between residues 196 and 197 in human sTF supported FX activation rates comparable to those with baboon sTF.
Conclusion(s): A TF arginine or lysine residue conserved in most species except for humans and a few other higher primates is responsible for increased enzymatic activity of baboon versus human TF. Consequently, the TF exosite region responsible for FX binding may be more extensive than previously thought.
COVID and Coagulation
COVID and Coagulation, Basic Science
PB0602
The impact of Covid‐19 pandemic on the health and lifestyle hemophilia patients in a low resourced country and its effect on their activated partial thromboplastin time (aPTT) results
D. Ofosu 1; C. Obirikorang2; R. Ngala3; A. Asamoah4; R. Tuekpe Mawuli4; E. Owusu5; S. Quarshie4; A. Owusu Banahene4; M. Titus4; G. Asare Amponsah4
1 Deparment of Medical Laboratory Science (Basic and Applied Biology), University of Energy and Natural Resource, Sunyani, Brong‐Ahafo, Ghana; 2 Department of Molecular medicine, Kwame Nkurmah University of Science and Technology, kumasi, Ashanti, Ghana, 3 godwinfungamtama@gmail.com, kumasi, Ashanti, Ghana; 4 Basic and Applied Biology Department, University of Energy and Natural Resources, Sunyani, Brong‐Ahafo, Ghana; 5 Garden City University College, kumasi, Ashanti, Ghana
Background: Haemophilia is a genetic bleeding disorder caused by the deficiency of clotting factor‐VIII or factor‐IX. The challenge to have access to factor VIII and IX concentrate could have been compounded by the COVID‐19 pandemic and affected the health and lifestyle of haemophilia patients in an already low resourced lower‐middle‐income country like Ghana.
Aims: This study was aimed at determining the effect of COVID‐19 on prolonged Activated Partial Thromboplastin Time (aPTT) among haemophilia ‘A’ patients in the middle belt of Ghana.
Methods: We retrospectively evaluated, for 12 months before the COVID ‐19 pandemic and followed up on 45 haemophilia patients during the pandemic for this study. Questionnaires were administered to the study participants. Blood samples were collected before the COVID‐19 pandemic. Further samples were collected in the pandemic period into citrate tubes and tested for aPTT. SPSS v21 was used for the analysis of the data and P‐value < 0.05 was considered statistically significant.
Results: We found that aPTT test result of study participants increased significantly from the pre‐pandemic to the pandemic period (68.04 ± 15.97 s to 160.80 ± 87.90 s, p < 0.001) Also, there was a statistically significant increase between aPTT results of participants that could not visit the clinic during the pandemic to participants who visited (194.6 ± 86.5 s to 128.4 ± 77.9 s, p = 0.009). Finally, the comparison of aPTT of participants who were impacted by COVID‐19 restrictions to that of those who did not suffer restrictions was statistically different (168.3 ± 87.6 s to 124.3 ± 81.2 s, p = 0.042), with those who suffered restrictions having prolonged aPTT results.
Conclusion(s): We observed a very high increase in the aPTT of in the pandemic period compared to the pre‐pandemic period. Patients who suffered government restrictions and could not travel to assess health centres and patients who could not visit the hospital during the pandemic had a prolonged aPTT making them susceptible to prolonged bleeding episodes.
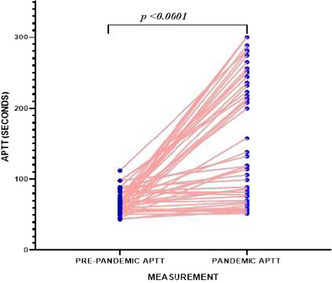

PB0590
Inflammatory convalescent plasma to treat COVID‐19: Impact of amotosalen/UVA pathogen reduction technology
F. Cognasse 1; H. Hamzeh‐Cognasse2; A. Duchez3; M. Eyraud4; C. Arthaud4; A. Prier5; E. Audoux1; O. Hequet4; B. Bonneaudeau6; S. Rochette‐Eribon4; F. Teyssier4; V. Barlet‐Excoffier4; P. Chavarin4; D. Legrand4; P. Richard6; P. Morel6; P. Tiberghien6
1 Etablissement Français du Sang Auvergne‐Rhône‐Alpes & INSERM 1059, Decines‐Charpieu, Rhone‐Alpes, France; 2 SAINBIOSE Laboratory, INSERM, U1059, University of Lyon, Saint‐Étienne, Saint‐Etienne, Rhone‐Alpes, France; 3 Etablissement Français du Sang Auvergne‐Rhône‐Alpes & INSERM U1059, Decines‐Charpieu, Rhone‐Alpes, France; 4 Etablissement Français du Sang Auvergne‐Rhône‐Alpes, Decines‐Charpieu, Rhone‐Alpes, France; 5 Etablissement Français du Sang Auvergne‐Rhône‐Alpes, Decines‐Chapieu, Rhone‐Alpes, France; 6 Etablissement Français du Sang, La Plaine, St Denis, France, Saint‐Denis, Ile‐de‐France, France
Background: Blood product in therapeutic transfusion are now commonly acknowledged to present biologically active constituents during processes of preparation. In the midst of worldwide COVI‐19 pandemic, preliminary evidence, suggest that convalescent plasma may lessen the severity of COVID‐19, particularly concerning patients with profound B‐cell lymphopenia and prolonged COVID‐19 symptoms.
Aims: This study examined the influence of photochemical pathogen reduction treatment (PRT) using amotosalen‐HCl and UVA light vs untreated control convalescent plasma (n = 72 – paired samples) ‐ cFFP.
Methods: This study investigated the soluble inflammatory factors: sCD40L, IFN‐alpha, IFN‐beta, IFN‐gamma, IL‐1 beta, IL‐6, IL‐8, IL‐10, IL‐18, TNF‐alpha and ex‐vivo inflammatory bioactivity on endothelial cells.
Results: We observed that IL‐8 concentrations were significantly decreased in cFFP w PRT, whereas IL‐18 concentration was increased. We observed after activation with cFFP w PRT and w/o PRT no significant modulation of IL‐6 released by endothelial cells. CD54 and CD31 expression in the presence of cFFP (w or w/o PRT) is close to negative controls, even if CD54 and CD31 were significant decreased in presence of cFFP w vs w/o PRT.
Conclusion(s): It appears valuable to carry on investigations, of IL‐18 and IL‐8, on both the physiopathology of PRT convalescent plasma treated and post marketing clinical trials. Further research, including a careful clinical evaluation of CCP‐treated patients, will be required to further define the clinical relevance of these findings.
VPB0617
Increased CD62P platelet expression in deceased Covid‐19 patients
U. Sukorini 1; A. Arjana2; T. Triyono3; I. Trisnawati; A. Yun Jufan; N. Rahmi Ananda
1 Faculty of Medicine Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Yogyakarta, Indonesia; 2 Faculty of Medicine Public Health and Nursing, Universitas Gadjah Mada, Sleman, Yogyakarta, Indonesia; 3 Faculty of Medicine, Nursing and Public Health, Universitas Gadjah Mada, Yogyakarta, Indonesia, Sleman, Yogyakarta, Indonesia
Background: There is an association between Coronavirus Disease 2019 (COVID‐19) and coagulation abnormalities. Platelet monitoring is important for COVID‐19 because abnormalities can occur in terms of quantity and quality. Impaired function of platelets can occur at the activation or aggregation stages. An increase in CD62P is associated with a 1.7‐fold increased risk of venous thrombosis. In the event of thrombosis, platelet activation causes interactions between fibrinogen and GP IIb/IIIa molecules which will form intracellular bonds between platelets, causing platelet aggregation. This suggests the role of CD62P as a major marker of platelet activation and may mediate cancer cell adhesion, inflammation, and thrombosis
Aims: This study aims to determine the description of platelet function as reflected in CD62P platelet expression in COVID‐19 patients
Methods: This study is a prospective study that take place from November 2020 to September 2021 at RSUP Dr. Sardjito, Yogyakarta, Indonesia. The subjects involved were adult patients aged over 18 years, men and women with confirmed COVID‐19 through PCR swab results. Patients with leukemia, history of coagulation disease, and immunodeficiency were excluded from this study. Flowcytometry analysis using FACS Canto was used to measure CD62P expression on platelets. Antibody used was anti human monoclonal CD41 PE and CD62P FITC antibody. The CD62P examination was carried out on the first day of treatment. Patients were grouped according to the severity of COVID‐19 as severe and non‐severe. Mann Whitney test was used to compare CD62P platelet expression percentage between groups.
Results: The CD62P platelet expression on day 1 of the deceased subjects were higher compared to the survived subjects (46.77% vs 43.38%; p = 0.04). On day 1, the severe subjects have a higher mean CD62P platelet expression compared to non‐severe subjects (47.88 % vs 39.75%).
Conclusion(s): CD62P platelet expression in deceased COVID‐19 subjects is higher compared to survived subjects.

PB0587
Platelet reactivity to SARS‐CoV‐2 Spike protein and domains
A. Cano‐Méndez; M. Damían‐Vázquez; N. García‐Larragoiti; S. López‐Castañeda; A. Ochoa‐Zarzosa; M. Viveros‐Sandoval
UMSNH, Morelia, Michoacan de Ocampo, Mexico
Background: COVID‐19 has affected millions of people worldwide over the past two years. SARS‐CoV‐ 2 uses the Spike (S) protein to infect target cells. An active immunothrombotic state has been described in severe stages of infection. Platelets are cells implicated in the pathophysiology of CoVID‐19, presumably by contributing to the release of inflammatory cytokines and exhibiting a procoagulant phenotype, although the platelet response in the disease has been studied, information on the cellular response to S protein domains is scarce.
Aims: To study platelet reactivity response to SARS‐CoV‐2 virus Spike protein and RBD domain.
Methods: Blood samples were obtained from healthy volunteers by venipuncture after signing an informed consent form, using 3.2% sodium citrate as anticoagulant. Platelet‐rich plasma (PRP) was obtained by slow centrifugation (100 g x 10 minutes). PRP was separated and resuspended in Tyrodes buffer. Platelet stimulation kinetics (1X107 cells/ml) was performed with S full protein and protein S receptor binding domain (RBD) [2 μg/ml], 37°C. PRP was also incubated with plasma from COVID‐19 patients [20 μl] for different times. Platelet activity was assessed by flow cytometry: CD41‐PECy7, CD62‐PE and PAC1‐FITC. We used ADP 20μM, collagen 0.19 mg/ml and epinephrine 100μM as platelet activation controls.
Results: We observed platelet reactivity after stimulation with protein S, highest activation was observed at 90 min with full protein and at 120 min with RBD domain when compared to the basal expression of selected markers and is similar to the observed with the positive control agonists. Stimulation of PRP with plasma from COVID‐19 patients show the presence of activation markers at 60 min. However, activation is lower than that observed with known activation agonists.
Conclusion(s): There is platelet reactivity to Spike protein, the RBD domain and with plasma from COVID‐19 affected subjects.
PB0597
Investigation of thrombin generation in COVID‐19 patients by a care setting design
G. Tiscia1; A. De Laurenzo1; F. Cappucci1; L. Fischetti1; G. Favuzzi1; D. Colaizzo1; E. Chinni2; E. Grandone 3
1 Fondazione IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Puglia, Italy; 2 Fondazione IRCCS "Casa Sollievo della Sofferenza", San Giovanni Rotondo, Puglia, Italy, 3 University of Foggia, Foggia, Puglia, Italy
Background: The coagulation system showed significant variations in COVID‐19 patients. These variations may parallel the disease stage of COVID‐19 toward either a hyper‐activation or coagulopathy syndrome. Classical clotting tests assist in exploring coagulation disorders, but they are unsuitable to examine prothrombotic conditions. In this regard, thrombin generation assay helps for a global assessment of the coagulation process, being appropriate for investigating hypercoagulable states and bleeding tendency.
Aims: It was investigated whether thrombin generation assay reveals coagulation variations in COVID‐19 patients by a care setting design.
Methods: From October to December 2020, it have been enrolled 27 and 40 patients with a confirmed COVID‐19 diagnosis who were hospitalised in an Intensive Care Unit (ICU) and a Medical Ward (MW), respectively. Also, 34 healthy subjects were included in this study. Thrombin generation parameters were evaluated using a Calibrated Automated Thrombogram system. Informed consent and approval by the local medical Ethics Committee were obtained.
Results: Lag‐Time and time‐to‐peak found in ICU and MW patients were significantly higher than those found in healthy subjects (Kruskal‐Wallis test: p < 0.0001). Endogenous‐Thrombin‐Potential and thrombin‐peak observed in ICU and MW patients were significantly lower than those observed in healthy subjects (Kruskal‐Wallis test: p < 0.0001). No statistically significant differences in all the parameters measured were observed between ICU and MW patients.
Conclusion(s): Thrombin generation assay performed in this study evidenced an acquired coagulopathy in COVID‐19 patients that, however, seems to be unrelated to the care setting and, in turn, to the clinical disease severity.
PB0603
The assessment of ROTEM test in patients with critical form of COVID‐19
V. Popov 1; L. Iliescu2; A. Badea1; C. Pirvu2; A. Grigorie1; O. Constantin1; M. Badoiu Niculae1; A. Calen1; A. Nichita2; A. Rus1; M. Andreescu1
1 Colentina Hospital Bucharest, Bucharest, Bucuresti, Romania; 2 Colentina Clinical Hospital, Bucharest, Bucuresti, Romania
Background: Patients with comorbidities could have severe COVID‐19 outcomes. Thrombosis or hemorrhages were frequently reported during COVID‐19 evolution
Aims: The study evaluated the role of ROTEM in the identification of patients with a high risk of vascular complications
Methods: We analyzed clinical, ROTEM, and laboratory data of 80 patients admitted to the intensive care unit (ICU) Department of our hospital, during 2020‐2021.
Results: Thrombosis was diagnosed in 26 cases (27.96%) and was more frequent in patients with severe pneumonia and ARDS, Chi‐squared 3.55, p = 0.05. Hemorrhages were identified in 21 cases (22.58%) and were less frequent in patients with severe ARDS, Chi‐squared = 4.67, p = 0.03. ROTEM analyses were focused on ExTEM, InTEM, and FibTEM clotting time (CT), clot amplitudes after five and ten minutes (A5/A10), maximum clot firmness (MCF), and maximum lysis (ML). In the ExTEM median CT (eCT) value was 90.5 (50‐207) s in patients with severe ARDS vs 75(58‐571)s in patients with mild/medium ARDS, p = 0.02; A5 44 (16‐68) in patient with thrombosis vs. 53 (12‐69) in patient without thrombosis; ML 6 (0‐104) in patients with sepsis vs 8 (0‐15) in patient without sepsis, p = 0.04.The median FibTEM CT (fCT) was 91 (53‐7913) s in patients with severe ARDS vs 76 (52‐8203)s in patients with mild/medium ARDS, p = 0.009;there were a significant correlation with ferritin level, rho = 0.22, p = 0.05. An increasing value of fCT during COVID‐19 evolution was observed especially in the critical form of COVID‐19. Lymphopenia was significant in patient with severe ARDS 0.48 x 1000/uL (0.02‐8.03) vs 0.67 x 1000/uL (0.14‐9.09) identified in patients with mild/medium ARDS, p = 0.05; it was not obtained significant correlations between lab test and ROTEM results.
Conclusion(s): The ROTEM test suggests showing hypercoagulability probably due to severe inflammation. Patients with a critical form of COVID‐19 developed thrombotic complications more frequently than hemorrhages. We have to check these results in large cohorts of patients
VPB0616
Interleukins that are mostly affected in severe coronavirus disease 2019 (COVID‐19)
M. Meiring 1; V. Nkuna1; L. Botes2; F. Smit1
1 University of the Free State, Bloemfontein, Free State, South Africa; 2 Central University of Technology, Bloemfontein, Free State, South Africa
Background: One of the major reasons people are severely affected by COVID‐19 is because of the combination of inflammation and thrombosis that they are subjected to. COVID‐19 is a disorder where thrombosis and inflammation play an essential role in the pathogenesis. Several predictive interleukin markers for COVID‐19 have been identified since its discovery These inflammatory markers include ( IL‐1‐alpha and ‐beta, IL‐6, IL‐8, IL‐10 and TNF‐alpha). The aim of this study was to determine the interleukin/s that are mostly affected by COVID‐19 in order to make recommendation on cost‐effective interleukin testing of COVID‐19 patients.
Aims: The aim of this study was to determine the interleukin/s that are mostly affected by COVID‐19 in order to make recommendation on cost‐effective interleukin testing of COVID‐19 patients.
Methods: We recruited 73 patients admitted with PCR confirmed severe COVID‐19 in the COVID‐19 wards at the COVID Unit of Universitas Hospitals in Bloemfontein, South Africa. The survival rate of these patients were 36%. Before testing, frozen citrated plasma samples were thawed at 37°C and assayed within 4 hours. The cytokine profile (IL‐1‐alpha and ‐beta, IL‐6, IL‐8, IL‐10 and TNF–alpha), were measured with enzyme assay kits from Biolegend (USA).
Results: IL‐10 levels were increased in all severe COVID‐19 patients. Only 40% of patients present with increased IL‐6 and IL‐1 beta levels. IL‐8 levels were increase in only 14% of patients, while only 1% of patients presented with increased IL‐1‐alpha and 4% with increased TNF‐alpha levels. There were no correlation between increased cytokine levels and survival in the patients.
Conclusion(s): We recommend the use of only IL‐10 as a marker for severe COVID‐19.
PB0600
ADAMTS13 activity and non‐parallel vWF Ristocetin CoFactor activity are associated with severity of COVID‐19 infection
S. MacDonald 1; D. White1; S. Cox‐Morton1; M. Sharp1; E. Duff1; C. Bridgeman1; T. Edwards1; C. Moore1; E. Vilar2; M. Besser1; E. Symington1; M. Robinson1; W. Thomas1
1 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 2 Univeristy of Hertfordshire, Hatfield, England, United Kingdom
Background: Increased von Willebrand factor (VWF) is common in COVID‐19 infection. vWF levels are reported at levels where pre‐dilution of samples is required. The assumption is that such dilutions respond linearly across the measurement range, and that this response is consistent across patient subgroups. ADAMTS13 levels have also been reported as reduced in those most severely affected. The interaction of these biomarkers has potential consequences to understanding pathophysiology of COVID‐19.
Aims: To investigate the linearity of dilution of high VWF levels in patients with COVID‐19 To compare the response in those mildly affected to those requiring more intensive therapy
Methods: This is a laboratory bases study investigating a convenience sample of fifty age and gender matched patients hospitalised during the first wave of the COVID‐19 pandemic (March to June 2020). Patients had been hospitalised for >24 hours before enrolment. Platelet RiCof activity was using the VW Select assay (BioData Corps, Horsham, USA). Dilutions were made using assay buffer provided in the kit. Data was collected in Microsoft Excel, before being analysed in both Microsoft Excel and the statistical programming environment R (R Core Team (2021)).
Results: The findings of Mancini et al were replicated here in that locale of patient admission was associated with a statistically significant reduction of ADAMTS13 activity (ITU mean 60.21 v non‐ITU mean 92.23). The activity of ADAMTS13 remained within what would be a reference range for diagnosis of TTP. VWF activity by Platelet RiCof was markedly raised. Serial dilution of samples demonstrated non‐parallelism, the response being most marked in severely affected patients.
Conclusion(s): Very high VWF levels disrupt the balance with mildly reduced ADAMTS13. In severe COVID‐19 infection, the effect manifests as non‐parallelism in platelet RiCof parameters. This calculated parameter classifies patients both by presence and severity of disease across all parameters in the platelet Ristocetin CoFactor assay.


VPB0608
Circulating levels of podoplanin and CLEC‐2 in COVID‐19: An exploratory study
I. Borba 1; F. Lima2; C. Peachazepi de Moraes3; M. Silva Barbosa2; D. Oliveira2; L. Velloso4; E. Mansur4; J. Annichino‐Bizzacchi5; F. Costa6; B. Bombassaro2; A. Palma2; E. de Paula4
1 University of Campinas, CAMPINAS, Sao Paulo, Brazil; 2 University of Campinas, Campinas, Sao Paulo, Brazil; 3 University of Campinas, Campinas, South Carolina, United States; 4 School of Medical Sciences of the University of Campinas, Campinas, Sao Paulo, Brazil; 5 Hemostasis and Thrombosis Laboratory, Hematology and Hemotherapy Center, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil; 6 Biology Institute of the University of Campinas, Campinas, Sao Paulo, Brazil
Background: Podoplanin (PDPN) and CLEC‐2 have been involved in the pathogenesis of thrombosis in inflammatory and neoplastic diseases. In addition, prior studies suggested that PDPN can be protective animal models of sepsis, as well as in models of acute lung injury.
Aims: To measure circulating levels of PDPN and CLEC‐2 in COVID‐19 and explore their relationship with clinical and laboratory markers of disease severity.
Methods: Thirty consecutive patients with COVID‐19 admitted due to hypoxemia and 30 age and sex‐matched controls were enrolled. PDPN and CLEC‐2 levels were measured by commercial ELISA kits on admission, and at day+4 (PDPN). Biomarkers of hemostasis were measured using commercial kits and clinical data were obtained from medical records.
Results: CLEC‐2 levels were similar between patients and controls, while lower PDPN levels were observed in patients when compared to healthy volunteers and these results were confirmed using two different ELISA kits. In addition, patients requiring intensive care presented lower PDPN levels compared to ward patients. PDPN levels were inversely correlated with biomarkers of coagulation and fibrinolysis activation.
Conclusion(s): Circulating levels of PDPN were reduced in patients with COVID‐19, and negatively correlated with markers of coagulation and fibrinolysis activation, as well as with and ICU need. Co‐expression of ACE2 and PDPN in alveolar epithelial cells could underlie these findings. Additional studies are warranted to confirm these observations in independent populations and to explore their potential mechanistic implications.


VPB0614
Coagulopathy associated with COVID‐19 (CAC)
H. Hadjer 1; B. Adel2; G. Faiza3; T. Amina4; A. Samia3
1 Hospital, Algers, Alger, Algeria, 2 Hospital, Blida, Blida, Algeria; 3 Hospital, Blida, Blida, Algeria; 4 Hospital, Alger, Alger, Algeria
Background: The Corona Virus Disease 19 (COVID‐19) characterized by pneumonia and acute respiratory distress syndrome. These patients with severe pneumonitis are at high risk of thrombotic complications, in relation to SARS‐CoV‐2‐induced coagulopathy
Aims: The purpose of this study : 1) evaluate the haemostasis parameters during Covid‐19 infection in cases admitted to intensive care; 2) Define CAC and its relationship with mortality; 3) determine differences between CAC and disseminated intravascular coagulation (DIC).
Methods: Study population: It was a monocentric prospective cohort, carried out at the Frantz Fanon University Hospital, Blida (Algeria) for a period of 3 months for patient with confirmed status or probable case according to the Algerian Note and admitted in ICU. Score system: For CAC diagnosis suggested by T. Iba et al. We consider a CAC if the patient has at least two of the following parameters: 1) platelets counts ; 2) prothrombin time ; 3) D‐dimer ; 4) presence of Macro and/or micro‐thrombosis. For DIC diagnosis we used ISTH criteria.
Results: We studied haemostasis parameters in 98 patients (75 deaths and 23 survivors). Respectively 12%, 50%, 26.5% and 39.5% of patients having platelet count < 100G/L, PT < 70%, aPTT ratio >1.2 and AT < 80% with p>0.5 between the deceased and survivors. The incidence of fibrinogen > 4 g/l and FVIII Ag >150% were respectively 41.2% P = 0.02, sHR = 1.3 and 59.5% p = 0.04. 15.1% have developed DIC (p = 0.710). 52.2% with CAC P = 0.035, sHR = 1.77. Logistic regression assessing COVID‐19 shows that CAC is an independent risk factor for mortality p = 0.045. The development of CAC exposes 6.75 higher risk of developing DIC.
Conclusion(s): Coagulopathy associated with COVID‐19 is correlated to high mortality risks. The biological and clinical results suggest that this Coagulopathy is distinct from DIC. We have proven that fibrinogen is a key element in distinguishing CAC from DIC.
PB0589
Inflammatory markers and auto‐Abs to type I IFNs in COVID‐19 Convalescent Plasma
F. Cognasse 1; H. Hamzeh‐Cognasse2; B. Bonneaudeau3; C. Pierre3; J. Huet3; C. Arthaud4; M. Eyraud4; A. Prier5; O. Hequet4; A. Fillet3; P. Chavarin4; D. Legrand4; P. Richard3; F. Pirenne3; J. Casanova6; S. Susen7; P. Morel3; K. Lacombe8; P. Bastard6; P. Tiberghien3
1 Etablissement Français du Sang Auvergne‐Rhône‐Alpes & INSERM 1059, Decines‐Charpieu, Rhone‐Alpes, France; 2 SAINBIOSE Laboratory, INSERM, U1059, University of Lyon, Saint‐Étienne, Saint‐Etienne, Rhone‐Alpes, France; 3 Etablissement Français du Sang, La Plaine, St Denis, France, Saint‐Denis, Ile‐de‐France, France; 4 Etablissement Français du Sang Auvergne‐Rhône‐Alpes, Decines‐Charpieu, Rhone‐Alpes, France; 5 Etablissement Français du Sang Auvergne‐Rhône‐Alpes, Decines‐Chapieu, Rhone‐Alpes, France; 6 University of Paris, Imagine Institute, Paris, France, Paris, Ile‐de‐France, France; 7 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 8 Sorbonne Université, Inserm IPLESP, Infectious Diseases Department, Saint‐Antoine Hospital, APHP, Paris, Rhone‐Alpes, France
Background: COVID‐19 convalescent plasma (CCP) contains neutralizing anti‐SARS‐CoV‐2 antibodies that may be useful as COVID‐19 passive immunotherapy in patients at risk of developing severe disease.
Aims: Such plasma from convalescent patients may also have additional immune‐modulatory properties when transfused to COVID‐19 patients.
Methods: CCP (n = 766) were compared to control non‐convalescent plasma (n = 166) for soluble inflammatory markers, ex‐vivo inflammatory bioactivity on endothelial cells, neutralizing auto‐Ab to type I IFNs, and reported adverse events in the recipients.
Results: CCP exhibited significantly higher IL‐6 and TNF‐alpha (0.531+/‐0.04 vs 0.271+/‐ 0.04; p = 0.0061 and 0.900+/‐0.07 vs 0.283+/‐ 0.07 pg/ml; p < 0.0001), respectively) and lower IL‐10 (0.731+/‐ 0.07 vs 1.22+/‐0.19 pg/ ml, p = 0.0034) levels than control plasma. Other inflammatory markers as well as ex‐vivo bioactivity did not differ significantly between CCP and control plasma. Neutralizing auto‐Abs against type I IFNs were detected in 14/766 (1.8 %) CCP. They were not associated with reported adverse events when transfused (n = 14). Inflammatory markers and bioactivity in CCP with or without auto‐Ab, or in CCP associated or not with adverse events in transfused patients, did not differ significantly. Overall, CCP exhibited moderately increased inflammatory markers compared to control plasma with no discernable differences in ex‐vivo bioactivity. Auto‐Ab to type I IFNs, detected in a small fraction of CCP, were not associated with reported adverse events or differences in inflammatory markers.
Conclusion(s): Further defining the clinical relevance of these findings will require further studies including careful clinical evaluation of patients treated with CCP.
PB0598
Demonstration of prothrombotic status in patients with COVID‐19 using thromboelastography and a novel T‐TAS assay
P. kundan1; K. Bliden1; U. Tantry2; s. Duhan3; s. arvind1; A. Singh1; P. Gurbel 4
1 SInai Center for Thrombosis Research and Drug Development, Baltimore, Maryland, United States; 2 Platelet and Thrombosis Research, Baltimore, Maryland, United States; 3 SInai Center for Thrombosis Research and Drug Developm, Baltimore, Maryland, United States; 4 Sinai Center for Thrombosis Research and Drug Development, Baltimore, MD, Maryland, United States
Background: COVID‐19 is associated with cytokine storm and hypercoagulability, indicating elevated risk for thrombotic events. Thromboelastography (TEG6s) assay has been used to demonstrate hypercoagulability in patients with COVID‐19. Total Thrombus formation Analysis System (T‐TAS) is a novel microfluidic assay that can be used to assess thrombogenicity in the presence of high shear as present in coronary arteries (PL chip) and 600/s shear stress indicating shear at large arteries (AR chip).
Aims: To demonstrate the prothrombotic state in COVID‐19 patients using a novel T‐TAS assay and TEG6s.
Methods: Blood samples were collected from 111 patients with COVID‐19 during hospitalization for TEG6s and T‐TAS analysis, and from 155 healthy volunteers who were not on any antithrombotic agents for TEG6s analysis. Previously published normal ranges for T‐TAS assays were used in the analysis.
Results: 57% of COVID‐19 patients were male and 65% were African American. All COVID‐19 patients were on prophylaxis or full dose heparin during hospitalization. With the T‐TAS AR chip, COVID‐19 patients exhibited significantly shorter values in initial occlusion time (T10) and occlusion time and higher area under the curve (AUC) (p < 0.001 for all), indicating a prothrombotic state. There were no differences in PL chip parameters. In COVID‐19 patients, TEG6s assay demonstrated reaction time within the normal range despite anticoagulant therapy, higher platelet‐fibrin clot strength, fibrinogen clot strength and fibrinogen levels compared to healthy subjects (p < 0.001 for all). There were no differences in total platelet function parameters (PL chip) between COVID‐19 patients and healthy subjects.
Conclusion(s): Our data indicate that COVID‐19 patients exhibit a prothrombotic state that can be assessed by a novel T‐TAS assay and TEG6s. This study also indicated that prothrombotic state in COVID‐19 patients may be driven by significantly higher fibrinogen levels in the presence of normal platelet function.
PB0584
Involvement of the contact pathway in COVID‐19 coagulopathy
M. Capecchi1; C. Novembrino2; M. Abbattista 3; M. Boscolo3; S. Griffini3; E. Grovetti3; A. Artoni4; L. Valenti5; D. Prati6; G. Grasselli7; F. Blasi8; M. Cugno3; F. Peyvandi9
1 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milano, Lombardia, Italy; 2 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milano, Lombardia, Italy; 4 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, Milano, Lombardia, Italy; 5 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Transfusion Medicine and Hematology and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milano, Lombardia, Italy; 6 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Transfusion Medicine and Hematology, Milano, Lombardia, Italy; 7 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Department of Anesthesia Intensive Care and Emergency and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milano, Lombardia, Italy; 8 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Respiratory Unit and Cystic Fibrosis Adult Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milano, Lombardia, Italy; 9 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: A novel acquired coagulopathy characterized by a severe procoagulant imbalance is common in COVID‐19 patients and is associated with the clinical severity of the disease.
Aims: Our study aims to elucidate the underlying mechanisms of coagulation activation in COVID‐19 patients.
Methods: Symptomatic COVID‐19 patients during Milan first wave were consecutively enrolled and stratified into 3 groups based on the intensity of care: low, requiring only high‐flow oxygen by nasal cannula; intermediate, requiring continuous positive airway pressure; high, requiring mechanical ventilation. Blood samples were tested for markers of activation of the intrinsic pathway (FXIa, FXIIa) together with its physiologic inhibitor (C1‐inhibitor), of the extrinsic pathway (FVIIa), of global activation of the coagulation cascade (D‐dimer, FDP, FM) and of fibrinolysis (plasminogen, t‐PA, α2‐antiplasmin, PAI‐1).
Results: 111 patients were included: 26 at low, 42 intermediate and 43 high care‐intensity. Median age was 59 ± 12 (34 patients >65 years); 32 patients (29%) developed a venous thrombosis and 12 (11%) died (Table). Median D‐dimer, FDP and FM plasma levels were higher in COVID‐19 patients compared to controls, with a gradient of increase across the three care intensities, while all the fibrinolytic pathway parameters were in the normal range. Median plasma levels of FVIIa were lower in COVID‐19 patients (27.5 mU/ml) than in controls (40.1 mU/ml) while median plasma levels of FXIIa and FXIa were higher in COVID‐19 patients (11.2 and 11.3 mU/ml) than in controls (7.2 and 5.5 mU/ml), with a gradient of increase across the three care intensities. C1‐inhibitor plasma levels were above the normal range in all the 3 COVID‐19 patients’ groups (Figure).
Conclusion(s): Our study showed a prevalent activation of the contact pathway over the extrinsic pathway of the coagulation cascade in COVID‐19 patients, which is proportional to the clinical severity of the infection, opening the possibility for targeted anticoagulant therapies.


PB0588
Persistent morphological alterations of endothelial colony‐forming cells after 9 months in patients recovered from COVID‐19
D. Chavarria‐Hernandez 1; A. Figueroa‐Torres2; V. Manuel‐Dominguez2; R. Arreola‐Diaz2; A. Chave‐Gonzalez2; A. Majluf Cruz3; J. Alvarado‐Moreno2
1 Instituto Politecnico Nacional (IPN), Instituto Mexicano del Seguro Social (IMSS), Mexico City, Distrito Federal, Mexico; 2 IMSS, Mexico City, Distrito Federal, Mexico; 3 Unidad de Investigación Medica de Trombosis, Hemostasia y Aterogénesis. Instituto Mexicano del Seguro Social, Mexico City, Distrito Federal, Mexico
Background: Patients who have had the disease Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) COVID‐19, mild, moderate or severe, has persisting symptoms weeks to moths after recovery of infection, with the risk of developing long‐terms effects. Some alterations are related to damage of the vascular endothelium, generating thrombosis, a direct relation of the endothelial dysfunction. There are not data related on frequency of endothelial colony‐forming cells (ECFCs) at 9 months of patients recovered from mild‐moderate COVID‐19 disease.
Aims: We analyzed the frequency and morphology of ECFCs from recovered COVID‐19 patients at 9 months post‐infection.
Methods: Human mononuclear cells (MNCs) were obtained from peripheral blood from 15 recovered COVID‐19 patients 9 months after disease, 10 healthy human volunteers with schedule of 2 vaccines (controls 1) and 10 healthy human volunteers without vaccines (controls 2), matched by age. All were men (25−50 years) without a history of major comorbidities. We assayed the frequency, morphological characteristics and proliferation of the ECFCs. The study was approved by the Scientific and Ethical Committee of the IMSS (number IMSS‐R‐2020‐785‐167). Informed written consent was obtained from all subjects before enrollment.
Results: ECFCs were detected in average on day 18 of culture in recovered COVID‐19 patients, on day 14 in controls 1 and day 21 in controls 2, respectively. In recovered COVID‐19 patients, ECFCs show abnormalities in size with elongations, large cytoplasm with prominent nuclei, similar to senescent state with no proliferative capacity when they were sub‐cultured. These findings were different from those observed in controls 1 and 2 respectively.
Conclusion(s): We demonstrate for the first time that ECFCs from patients recovered from COVID‐19 at 9 months has characteristics of dysfunctional endothelial similar to those observed in patients recovered after 30 days after infection. This finding may support our understanding of the physiology of dysfunctional endothelium from COVID‐19 disease at long term.
PB0604
Abridging molecular system network of COVID‐19 and thrombosis
M. Hussain1; A. Amanullah1; N. Jabeen2; B. Kantarcioglu3; F. Siddiqui 3; J. Fareed3
1 Dow University of Health Sciences, Karachi, Sindh, Pakistan; 2 University of Karachi, Karachi, Sindh, Pakistan; 3 Loyola University Chicago, Maywood, Illinois, United States
Background: The advance pathology of SARS‐CoV‐2 infection entails engagement of blood related ailment including thrombosis as secondary clinical manifestation. SARS‐CoV‐2‐Human protein‐protein interactome has been explored. Dysregulation of the several proteins and mutations in the genes have been linked with the incidence and progression of thrombosis.
Aims: Aim of the investigation is to develop and functionally analyze a combine molecular network of SARS‐CoV‐2‐Human and Thrombosis to delineate candidate molecule that could later be used for the prognosis and therapeutic intervention.
Methods: Briefly, two separate system networks were developed, one for over 500 humans protein that have shown to interact with the viral genome and 26 different proteins encoded by SARS‐CoV‐2 genome. The second network is based on the genes tagged for being aberrated genetically and/or in terms of expression in thrombosis. Both networks were combined as a singular entity after removing the redundant repetition and orphans’ nodes and edges by selective enrichment. The network then be dissected in different modules primarily based on the promiscuity of the nodes. Complete network and each module were assessed for in betweenness and shortest path length of edges.
Results: The data shown over 700 genes could be coalesced as a single network providing a molecular interplay that may underpin SARS‐CoV‐2 associated thrombosis. Over 16 modules were observed in the network with important candidate genes of thrombosis have been identified as hub due to the inter modular abridging potential. Identification of hub genes was further substantiated with the pathlength distance, lack of orphan edges and partner protein promiscuity. Biological functions and KEGG analysis of the holistic network and modular compartment further strengthen the predicted candidate gene status as central to the disease biology.
Conclusion(s): Candidate genes identified in the study could later be used as markers for prognosis of the pathology of COVID‐19 for thrombosis and/or developing therapeutic intervention.
PB0585
Prognostic value of troponin elevation at admission and during monitoring in COVID‐19 patients
A. Beauvais 1; N. Gendron2; A. Philippe3; B. Vedie1; M. Loriot1; P. Juvin1; O. Sanchez4; J. Diehl4; D. Smadja5; R. Chocron6
1 Hôpital Européen Georges Pompidou, Paris, Paris, Ile‐de‐France, France; 2 Hôpital européen Georges Pompidou, Inserm UMRS_1140, Paris, Ile‐de‐France, France; 3 Université de Paris, Innovative Therapies in Haemostasis, INSERM, F‐75006, Paris, France, Paris, Ile‐de‐France, France; 4 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 5 HEGP, Inserm UMRS_1140, Paris, Ile‐de‐France, France, 64Department of Emergency, AP‐HP, Georges Pompidou European Hospital, Paris, France, Paris, Ile‐de‐France, France
Background: Troponin seems to be a biological marker of interest in the risk‐stratification of COVID 19 at admission, but few studies focus on exploring its prognostic abilities during hospitalization.
Aims: To assess the ability of troponin levels at admission and during follow‐up to predict in‐hospital mortality in COVID‐19 patients.
Methods: Troponin was measured at admission and throughout hospitalization amongst COVID‐19 patients. We explored the prognostic ability of baseline troponin and kinetics on COVID‐19 patients outcomes using logistic regression and Cox model.
Results: Amongst 399 patients enrolled with confirmed COVID‐19, 247 had at least 2 troponin measurements during hospitalization and 319 (80%) survived, while 80 (20%) died during hospitalization. Elevated troponin upon arrival was significantly associated with mortality (Odds Ratio (OR) 5.65 (95% CI 3.24 – 9.88); p < 0.01). Patients with an elevated troponin level and underlying cardiovascular diseases were more likely to experience death than those without elevated troponin nor cardiovascular history (Hazard Ratio 4.62 (1.96‐10.91); p < 0.001). There seems to be a level‐dependent association between troponin level and in‐hospital mortality (p < 0.01). To assess the ability of troponin monitoring during the first 10 days of hospitalization to predict in‐hospital mortality we analyzed the ratio of troponin (ROT) between the highest level of troponin and the baseline troponin measurement. An increase over 75% of the ROT was not significantly associated with in hospital mortality (OR 1.02 (95% CI 1.01‐1.04); p = 0.12). During follow‐up, there was no significant differences of in‐mortality between patients with constant elevated troponin throughout hospitalization and those who went from a normal to an elevated troponin (p = 0.15).
Conclusion(s): For COVID‐19 patients, troponin seems to be a relevant factor for in‐hospital mortality and risk‐stratification at admission but its monitoring during follow‐up does not appear to be valuable in predicting disease progression.
PB0596
Anti‐SARS‐CoV‐2 vaccines do not induce a hypercoagulable state in healthy individuals
L. Garabet 1; A. Eriksson2; E. Tjønnfjord3; M. Olsen3; H. Jacobsen2; C. Tøvik Jørgensen2; Å. Mathisen2; M. Mowinckel4; M. Ahlen5; I. Sørvoll5; P. Sandset6; W. Ghanima7
1 Østfold Hospital, Grrålum, Ostfold, Norway; 2 Østfold Hospital, Grålum, Ostfold, Norway; 3 Østfold Hospital, Sarpsborg, Ostfold, Norway; 4 Oslo University Hospital, Oslo, Oslo, Norway; 5 University Hospital of North Norway, Tromsø, Troms, Norway; 6 University of Oslo, Oslo, Oslo, Norway; 7 Østfold Hospital Foundation, Sarpsborg, Ostfold, Norway
Background: Anti‐SARS‐CoV‐2 adenoviral‐vectored‐DNA vaccines have been linked to a rare but serious thrombotic post‐vaccine complication called the vaccine‐induced immune thrombotic thrombocytopenia (VITT). VITT has raised concerns regarding the possibilities of increased thrombotic risk and/thrombocytopenia after anti‐COVID‐19 vaccines.
Aims: To investigate whether anti‐SARS‐CoV‐2 vaccines can cause subclinical coagulation activation and increased thrombin generation leading to a hypercoagulable state.
Methods: The study included 567 healthcare personnel from two hospitals in Norway. Of these, 521 were recruited 11‐57 days post‐vaccination with the first dose of ChAdOx1‐S (Vaxzevria®, AstraZeneca, UK) vaccine, and 46 were recruited prospectively prior vaccination with an mRNA vaccine, either elasomeran (Spikevax, Moderna, n = 38) or tozinameran (Comirnaty, Pfize‐BioNTech, n = 8). In the latter group, samples were acquired before and 1‐2 weeks after vaccination. In addition to pre‐vaccination samples, 56 unvaccinated blood donors were recruited as controls (total n = 102). Thrombin generation, D‐dimer and free tissue factor pathway inhibitor antigen (free TFPI) were analyzed.
Results: None of the participants developed thrombosis/VITT or thrombocytopenia (platelet count < 100·109/L) after vaccination. There were no significant differences in D‐dimer, free TFPI or the parameters of thrombin generation between the two vaccine groups and the controls (Table 1). No differences in thrombin generation or free TFPI between the ChAdOx1‐S group and the mRNA group, the median D‐dimer level was slightly higher in the ChAdOx1‐S group, but both were within the normal range (Table 2). Thrombin generation, D‐dimer and free TFPI showed no changes after mRNA vaccination compared with baseline.
Conclusion(s): Anti‐COVID‐19 vaccines, both ChAdOx1‐S and mRNA, were not associated with significant increase in thrombin generation, D‐dimer or free TFPI compared with controls. Neither significant differences were observed between ChAdOx1‐S and mRNA vaccines nor changes after mRNA vaccines compared with baseline levels. Our results are reassuring in the sense that no subclinical activation in the coagulation system was observed with these vaccines.


PB0599
Association of heme/hemopexin/heme‐oxygenase 1 pathway with clinical and laboratory markers of severity in Covid‐19
F. Lima 1; C. Peachazepi de Moraes2; M. Silva Barbosa1; B. Bombassaro1; A. Palma1; E. Mansur3; L. Velloso1; E. de Paula3
1 University of Campinas, Campinas, Sao Paulo, Brazil; 2 University of Campinas, Campinas, South Carolina, United States; 3 School of Medical Sciences of the University of Campinas, Campinas, Sao Paulo, Brazil
Background: Heme‐oxygenase 1 (HO‐1) is an intracellular enzyme that is part of a broad anti‐inflammatory and antioxidative pathway, in which it metabolizes heme delivered to cells by hemopexin. Although initial suggestions of a direct action of SARS‐CoV‐2 on hemoglobin or heme have already been refuted, HO‐1 activation is recognized as part of the host response and as a potential therapeutic target in several diseases involving thrombosis and inflammation.
Aims: To evaluate circulating levels of hemopexin, heme and HO‐1 in COVID‐19, and their association with clinical and laboratory markers of disease severity.
Methods: Thirty consecutive patients with confirmed COVID‐19 admitted due to hypoxemia were enrolled, along with 30 age and sex‐matched healthy volunteers. HO‐1 and hemopexin were measured by ELISA, and heme was measured by a colorimetric method. Samples were obtained on admission. Coagulation and inflammatory biomarkers were measured using commercional kits.
Results: HO‐1 levels were higher in patients compared to healthy volunteers, and a trend towards higher hemopexin levels was also observed. In contrast heme levels were similar in patients and controls. A significant decrease in HO‐1 levels was observed at the 4th day of hospital stay, and the magnitude of this decrease (ΔHO‐1) was correlated with the number of days in intensive care. Moreover, admission HO‐1 levels were correlated with several biomarkers of hemostasis and fibrinolysis activation.
Conclusion(s): Upregulation of HO‐1 is observed in COVID‐19. HO‐1 levels on admission were associated with markers of coagulation and fibrinolysis activation. Persistance of high HO‐1 levels during admssion was associated with longer ICU stay.


PB0593
Increased percentage of extended Aα chain fibrinogen (αE) in COVID‐19 patients
J. de Vries 1; C. Visser1; M. van Ommen2; C. Rokx3; E. van Nood3; E. van Gorp4; M. Goeijenbier5; J. van den Akker6; H. Endeman6; D. Rijken7; M. Kruip7; M. Weggeman2; J. Koopman2; M. de Maat8
1 Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 Fibriant B.V., Leiden, Zuid‐Holland, Netherlands; 3 Department of Medical Microbiology and Infectious Diseases, Erasmus MC, Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Department of Internal Medicine & Department of Viroscience, Erasmus MC, Erasmus Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 5 Department of Viroscience & Department of Adult Intensive Care, Erasmus MC, Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 Department of Adult Intensive Care, Erasmus Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 7 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 8 Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Variation in fibrinogen caused by degradation or extension of the Aα chain affects the fibrin network structure and interaction of fibrin(ogen) with cells. The plasma level of these fibrinogen variants might be altered during COVID‐19, potentially affecting disease severity or the risk of thrombosis.
Aims: To investigate the level of fibrinogen Aα chain variants in plasma of COVID‐19 patients.
Methods: Using the Clauss assay, we measured plasma levels of functional fibrinogen. ELISAs were used to measure antigen levels of total, intact (non‐degraded Aα chain), and extended Aα chain fibrinogen (αE) in intensive care unit (ICU) patients with COVID‐19 (with and without thrombosis, at two time points), COVID‐19 ward patients without thrombosis, ICU patients with pneumococcal infection, and healthy controls. Ethical approval was obtained and written informed consent was obtained or an opt‐out procedure was in place.
Results: Higher levels of functional and intact fibrinogen were observed in COVID‐19 ICU patients (before diagnosis of thrombosis) with (n = 18) and without thrombosis (n = 19) and ICU patients with pneumococcal infection (n = 6) than in COVID‐19 ward patients (n = 10) and healthy controls (n = 7) (Figure 1). Total fibrinogen levels were higher in all ICU patients compared to healthy controls. Interestingly, the percentage of αE fibrinogen was significantly higher in COVID‐19 ICU patients who do and do not develop thrombosis than in healthy controls. COVID‐19 ICU patients who develop thrombosis also showed significantly higher percentages of αE fibrinogen than COVID‐19 ward patients. After diagnosis of thrombosis, functional fibrinogen levels were significantly higher in COVID‐19 ICU patients with thrombosis than in those without, while no differences were observed in intact, total, or αE fibrinogen (Figure 2).
Conclusion(s): Our results show that severe COVID‐19 is associated with increased percentages of αE fibrinogen, which may be the cause or consequence of severe disease, but does not explain the development of thrombosis.


PB0586
Repurposing of montelukast in COVID‐19 patients in order to modulate platelet activation
M. Brambilla 1; P. Canzano1; A. Becchetti1; M. Conti2; G. Rovati3; M. Camera3
1 Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy, 2 centro cardiologico monzino, milan, Lombardia, Italy; 3 Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Sustained platelet activation, thrombosis, vascular damage, fibrotic response as well as inflammatory overload are typical features of COVID‐19 pathology. Common denominator in these processes is leukotrienes (LTs). Elevated levels of LTE4 have been detected in bronchoalveolar lavage of COVID‐19 patients so the use of LT receptor antagonists as a potential therapeutic for COVID‐19 patient treatment has been hypothesized. A first phase III randomized double‐blind clinical trial testing montelukast in COVID‐19 patients has been indeed proposed.
Aims: To investigate whether montelukast affects the expression of the major markers of platelet activation such as tissue factor (TF), P‐selectin, as well as the formation of platelet‐leukocyte aggregates and microvesicle (MV) release observed in COVID‐19 syndrome.
Methods: Blood from healthy subjects (HS; n = 4‐6) was plasma‐depleted and reconstituted with plasma pools (n = 3) from COVID‐19 patients (4 patients/pool) or from the same HS blood donors. To assess the effect of montelukast on cell activation, blood from HS was preincubated for 30 minutes with the drug. Circulating cell‐associated TF expression, platelet activation markers, and MV release were analyzed by flow cytometry.
Results: Plasma from COVID‐19 patients significantly increased (4‐fold) the number of TF+‐ and P‐selectin+‐platelets of HS recapitulating the platelet activation status of COVID patients. Montelukast prevented platelet activation induced by plasma from COVID‐19 patients and it reduced the formation of circulating monocyte‐ and granulocyte‐platelet aggregates, decreasing the number of those TF+ by 4‐times. Finally, it completely inhibited the release of TF+ circulating MVs, reducing by more than 2‐times those derived from platelets.
Conclusion(s): Our data indicate that leukotrienes contribute to sustain platelet activation occurring in the COVID‐19 patient, which can however be prevented by treatment with montelukast. Until results from ongoing trials will be available, our data provide the molecular basis by which the drug may be effective in the treatment of COVID‐19.
VPB0612
Angiostatin and plasminogen levels as risk factors for mortality in hospitalized Covid‐19 patients
A. Franczak 1; S. Rathwell2; W. Alemayehu2; P. Jurasz3
1 University of Alberta, Edmonton, AB, Canada, Edmonton, Alberta, Canada; 2 Canadian VIGOUR Centre, University of Alberta, Edmonton, AB, Canada, Edmonton, Alberta, Canada; 3 Department of Pharmacology, Faculty of Medicine and Dentistry / Cardiovascular Research Centre / Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Alberta, Canada
Background: Covid‐19‐associated thrombotic complications are in part due to loss of plasminogen‐associated fibrinolytic activity. Angiostatin is a break‐down product of plasmin(ogen) with pro‐apoptotic properties under hypoxia and acidosis, conditions associated with Covid‐19. Hence, it may contribute to fatal outcome in Covid‐19.
Aims: To assess the relationship between plasminogen/angiostatin levels and mortality in hospitalized Covid‐19 patients.
Methods: Age‐ and sex‐matched plasma samples (n = 120) from Covid‐19 survivors and non‐survivors were obtained from Alberta Precision Laboratories (Canada). Angiostatin and plasminogen were quantified by immunoblot. Cox's proportional hazards regression model was used to determine the association between angiostatin/plasminogen levels and risk of death. Hazard ratios [HR] and 95% confidence intervals [CI] were estimated adjusting for time of sample collection (days from hospitalization to collection) and comorbidities (Charlson comorbidity score). The interaction between plasminogen/angiostatin levels and time of sample collection (before or after 7 days from hospital admission) was assessed and stratified HRs were estimated.
Results: Overall, compared to survivors, non‐survivors had a lower plasminogen level (mean±SD: 6.6 ± 2.1 and 5.6 ± 2.2 arbitrary units). Increase in plasminogen level was significantly associated with reduction in risk of death, independent of time of sample collection and comorbidities (Table 1). When stratified by time of sample collection, the association remained significant only for samples drawn within 7 days of admission (Table 2). Conversely, an overall difference was not observed in angiostatin concentrations (mean±SD: 136.0 ± 42.5 and 143.0 ± 48.9 μg/ml), and it was not associated with survival outcome (Table 1). However, when stratified by time of collection and adjusted for comorbidity burden, angiostatin was significantly associated with an increased risk of death for samples collected after 7 days from admission (Table 2).
Conclusion(s): Both angiostatin and plasminogen were associated with fatal outcome in Covid‐19, independent of comorbidities. These associations depended on the time from hospital admission to sample collection and likely reflect dynamic changes in the plasminogen‐angiostatin generation system associated with Covid‐19 disease progression.


PB0591
Coagulation parameters predict COVID‐19‐related thrombosis in a neural network with a positive predictive value of 98%
R. de Laat‐Kremers 1; R. de Jongh2; M. Ninivaggi3; A. Fiolet4; R. Fijnheer4; J. Remijn5; B. de Laat6
1 Synapse Research Institute, Maastricht, Limburg, Netherlands; 2 Department of Anesthesiology, Ziekenhuis Oost Limburg, Genk, Belgium, Genk, Limburg, Belgium; 3 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 4 Department of Internal Medicine, Meander Medical Center, Amersfoort, the Netherlands, Amersfoort, Utrecht, Netherlands; 5 Department of Clinical Chemistry, Meander Medical Center, Amersfoort, the Netherlands, Amersfoort, Utrecht, Netherlands; 6 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: Thrombosis is a major complication of a SARS‐CoV‐2 infection. COVID‐19 patients show changes in coagulation factor levels and functional coagulation tests that indicate an important role for the coagulation system in the pathogenesis of COVID‐19. However, the multifactorial nature of thrombosis complicates the prediction of thrombotic events based on a single hemostatic variable.
Aims: We used a neural network to predict future COVID‐19‐related thrombosis.
Methods: We developed neural networks for the prediction of thrombosis in COVID‐19 patients based on several dedicated coagulation parameters and general laboratory variables measured in plasma samples of 133 COVID‐19 patients collected at the time of hospital admission (cohort 1). The neural network was validated in a second cohort of 16 COVID‐19 patients admitted to the intensive care unit (cohort 2). In cohort 1 and 2, 19 and 7 patients respectively suffered from thrombosis during their hospital stay.
Results: The neural network predicts COVID‐19‐related thrombosis based on C‐reactive protein (relative importance 14%), sex (10%), thrombin generation (TG) time‐to‐tail (10%), α2‐macroglobulin (9%), TG curve width (9%), thrombin‐α2‐macroglobulin complexes (9%), plasmin generation lag time (8%), anti‐SARS‐CoV‐2 serum IgM (8%), TG lag time (7%), TG time‐to‐peak (7%), thrombin‐antithrombin complexes (5%), and age (5%). In developmental cohort 1, the neural network identified future thrombosis in COVID‐19 patients with a positive predictive value of respectively 98%. The neural network accurately ruled out thrombosis in COVID‐19 patients as the negative predictive value of the neural network was 86%. In validation cohort 2, the positive predictive value of the neural network was 100%, and a negative predictive value of 66%.
Conclusion(s): We developed a neural network that can accurately predict the occurrence of COVID‐19‐related thrombosis and is a promising algorithm to apply to other COVID‐19 patient cohorts. The prediction of COVID‐19 related thrombosis potentially can give clinicians the opportunity to increase anticoagulant therapy in high risk patients.
VPB0611
Alteration of circulating ACE2 regulating microRNAs profiles in patients with COVID‐19
Z. Wicik1; C. Eyileten 1; J. Jarosz‐Popek1; D. Jakubik1; A. Nowak1; M. Wolska2; A. Shahzadi3; D. Martins‐Jr4; S. Simões5; J. Siller‐Matula6; M. Postula1
1 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 2 Medical University of Warsaw, warsaw, Mazowieckie, Poland; 3 Istanbul Cerrahpasa University, Istanbul, Istanbul, Turkey; 4 Federal University of ABC, Sao Paulo, Sao Paulo, Brazil; 5 Federal Institute of Espírito Santo, Sao Paulo, Sao Paulo, Brazil, 6 Medical University of Vienna, Vienna, Wien, Austria
Background: SARS‐CoV‐2 tropism for the ACE2 receptor, along with the multifaceted inflammatory reaction, is likely to drive the generalized hypercoagulable state seen in patients with COVID‐19.
Aims: According to our previously published bioinformatics analysis, we aimed to analyze the diagnostic and predictive utility of miRNAs regulating ACE2 network (miR‐26b‐5p, miR‐10b‐5p, miR‐302c‐5p, hsa‐miR‐200b‐3p, hsa‐miR‐124‐3p) in patients with COVID‐19
Methods: We determined the expressions of ACE2‐related‐miRNAs in 79 hospitalized COVID‐19 patients and 32 healthy volunteers by PCR and monitored miRNAs patterns during the acute phase of COVID‐19, as well as the prognostic potential of these miRNAs as biomarkers.
Results: The expression levels of miR‐26b‐5p in COVID‐19 patients were found lower at the baseline, 7 and 21‐days after admission. compared to the healthy controls (p < 0.0001 for all time points). Similarly, miR‐10b‐5p expression levels were lower at the baseline and 21‐days post admission in COVID‐19 patients when compared to the healthy individuals (p = 0.001 in both time points). Moreover, expression levels of this miRNA were higher 7‐days post‐admission when compared to the baseline (p = 0.003). According to the ROC curve analysis, low miR‐200b‐3p expression presents predictive utility in assessment of the hospital length of stay and/or death (AUC:0.730, p = 0.002). According to the multivariable logistic regression model, low delta miR‐200p expression, together with diabetes mellitus (DM), are independent predictors of increased hospital length of stay and/or death (OR: 5.775; 95% CI, 1.572‐21.214; p = 0.008 and OR: 4.888; 95% CI, 1.001‐23.858; p = 0.050, respectively).
Conclusion(s): This study enabled us to better characterize changes in gene expression and signaling pathways related to COVID‐19 thrombosis. In this study we identified, characterized and validated miRNAs which could serve as novel, thrombosis‐related biomarkers of the COVID‐19, can be used for early stratification of patients and prediction of severity of infection development in an individual.


VPB0615
Therapeutic dose of enoxaparin associated with a better outcome for COVID‐19 hospitalized patients
F. Leite 1; S. Ferreira2; À. Leite3
1 Centro Hospitalar Universitário do Porto/UMIB/ICBAS ‐ Unit for Multidisciplinary Investigation in Biomedicine‐ Instituto de Ciências Biomédicas Abel Salazar, University of Porto, Porto, Portugal, PORTO, Porto, Portugal; 2 Department of Clinical Haematology, Centro Hospitalar Universitário do Porto, Porto, Portugal., PORTO, Porto, Portugal; 3 Centro de Estudos Filosóficos e Humanísticos, Universidade Católica Portuguesa, Braga, Portugal, PORTO, Porto, Portugal
Background: Coronavirus disease (COVID‐19) is associated with thromboinflammation that correlates with disease severity and mortality.
Aims: To identify biomarkers of coagulation and inflammation or therapeutic issues that are associated with the clinical outcome of COVID‐19 patients.
Methods: A single‐center retrospective study was performed of adult COVID‐19 positive admitted to Centro Hospitalar Universitário do Porto (CHUPorto) due to COVID 19 infection or acquired the infection during hospitalization from October to November 2020 (SARS COV‐2 infection second wave). Two dependent variables were defined: hospitalization outcome (dead or alive) and oxygen supplementation (needed or not). Data were collected by consulting patients' clinical files.
Results: Of 492 adults with SARS‐COV‐2 infection, 53.9% were male. The sample had a mean age of 70.44 years (SD = 15.36). The majority (87%) was symptomatic and 76.2% required oxygen supplementation. Regarding treatment, 89.2% received enoxaparin, mostly (68.5%) prophylactically. By the end of November, 12.8% of the sample (n = 63) had died; this number increased to 20.1% (n = 99) at the end of March 2021. Those who survived Covid 19 had lower plasmatic levels of CRP (C‐reactive protein) and D‐dimer (Table 1). Fibrinogen and CRP plasmatic levels were higher in patients who required oxygen supplementation in comparison to those who did not. The outcome dead or alive correlates with D‐dimer, oxygen supplementation, and enoxaparin regime; oxygen supplementation correlates with CRP, ferritin, fibrinogen, D‐dimer, hospitalization time, enoxaparin, enoxaparin regime. Symptomatic patients have higher values of CRP and fibrinogen in comparison to asymptomatic patients. Concerning therapeutic with enoxaparin [χ2(1) = 7.079; p = 0.008; Φ = ‐0.120], those who died mostly received enoxaparin (81.8%) and in prophylactic regime (58%) [χ2(1) = 19.559; p < 0.001; Φ = ‐0.211].
Conclusion(s): Inflammation and thrombosis are associated with a poorer clinical outcome and therapeutic dose of enoxaparin could be protective in COVID‐19 hospitalized patients.


PB0606
Stimulation of the ssRNA virus immune receptor, Toll‐like receptor 7, on megakaryocytes increases thrombopoesis
A. Waller 1; W. Lau2; H. Foster2; A. Evans2; J. Tregoning3; A. Poole4; A. Davidson4; C. Ghevaert2; J. Patterson5; M. Lawrence6; A. Mueller7
1 University of Cambridge, Braintree, England, United Kingdom; 2 University of Cambridge, Cambridge, England, United Kingdom; 3 Imperial College London, London, England, United Kingdom; 4 University of Bristol, Bristol, England, United Kingdom; 5 Xap Therapeutics, Cambridge, England, United Kingdom; 6 Kyoto University, Kyoto, Kyoto, Japan; 7 University of Cambridge, Berlin, Berlin, Germany
Background: TLR7/8 are immune receptors expressed in megakaryocytes which detects single‐stranded RNA viruses such as SARS‐CoV‐2. There is increasing evidence that in addition to raised platelet counts, severe infection with SARS‐CoV‐2 increases the risk of venous, arterial and microvascular coagulation.
Aims: To determine if ssRNA viruses are capable of increasing thrombopoeisis through direct interaction with megakaryocytes.
Methods: Cells were incubated with and without Gardiquimod (GDQ), a specific agonist of TLR7/8 in cord blood derived (CBMKs) and mouse bone marrow derived megakaryocytes (mMKs). TLR7/8‐/‐ iPSC derived megakaryocytes (iPSC‐MKs) were produced using CRISPR Cas9 editing of iPSCs and forward programming using an doxycyclin inducible cassette. GFP labelled SARS‐CoV‐2 virus was incubated with the TLR7/8‐/‐ iPSC‐MKs and wild‐type iPSC‐MKs.
Results: Incubation with GDQ increased platelet production in CBMKs and mMKs, and increased platelet function. Increased platelet counts were seen in mice treated with GDQ, and mice infected with influenza. Incubation with GDQ induced increased expression of IL1β in the parental iPSC‐MKs, however in the TLR7‐/‐ and TLR7/8‐/‐ MKs, no increased expression was observed. There was a significant increase in platelet production from the parental iPSC‐MKs in response to incubation with GDQ, which was not seen in the TLR7‐/‐ and TLR7/8‐/‐ MKs. Incubation of the GFP‐labelled SARS‐CoV‐2 virus with wild‐type MKs did not lead to a significant increase in fluorescence. Only very low level viral sequences were found in the cells post‐incubation demonstrating that penetration within the MKs is unlikely to be of significance. Studies are ongoing to ascertain whether SARS‐CoV‐2 induces outside in signalling leading to changes in transcription within the MKs (such as elevation of IL1β).
Conclusion(s): TLR7/8 agonists, including ssRNA genome viruses, increase platelet production and functionality from megakaryocytes. SARS‐CoV‐2 does not appear to penetrate and significantly replicate within MKs, however incubation with megakaryocytes did show elevated expression of IL1β.


PB0595
Factor XII activation by NET accumulation aggravates COVID‐19‐associated pulmonary thrombo‐inflammation
H. Englert 1; C. Rangaswamy1; C. Deppermann2; J. Sperhake1; C. Krisp1; D. Schreier1; E. Gordon3; S. Konrath4; M. Haddad5; G. Pula6; R. Mailer4; H. Schlüter1; S. Kluge1; F. Langer7; K. Püschel1; K. Panousis8; E. Stavrou9; C. Maas10; T. Renné5; M. Frye1
1 University Medical Center Hamburg Eppendorf, Hamburg, Hamburg, Germany; 2 University Medical Centre Mainz, Mainz, Rheinland‐Pfalz, Germany; 3 University of Queensland, Brisbane, Queensland, Australia; 4 University Medical Center Hamburg‐Eppendorf, Hamburg, Hamburg, Germany; 5 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 6 University Medical Center Eppendorf Hamburg (UKE), Hamburg, Hamburg, Germany; 7 II. Medizinische Klinik und Poliklinik, Zentrum für Onkologie, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 8 CSL Behring, Melbourne, Victoria, Australia; 9 Case Western Reserve University, Cleveland, Ohio, United States, 10 UMC Utrecht, Utrecht, Utrecht, Netherlands
Background: Coagulopathy and inflammation are hallmarks of COVID‐19 and are associated with increased mortality. Clinical and experimental data have revealed a role for neutrophil extracellular traps (NETs) in COVID‐19. Mechanisms that drive thrombo‐inflammation in COVID‐19 are poorly understood.
Aims: In this study, we aimed to investigate a possible role of NETs‐driven coagulation factor XII (FXII) activation in COVID‐19‐related thrombo‐inflammation.
Methods: We performed comprehensive proteomics and immunostaining of postmortem lung tissues from COVID‐19 patients and patients with other lung pathologies. We compared FXII and DNase1 activities in plasma samples from COVID‐19 patients and healthy control donors and determined NET‐induced FXII activation using a chromogenic substrate assay.
Results: FXII expression and activity were increased in the lung parenchyma, within the pulmonary vasculature and in fibrin‐rich alveolar spaces of postmortem lung tissues from COVID‐19 patients over controls. Active FXII (FXIIa) was increased in plasma of COVID‐19 patients. Furthermore, FXIIa colocalized with NETs in COVID‐19 lung tissue indicating that NETs accumulation leads to FXII activation in COVID‐19. Accumulation of NETs in COVID‐19 was at least in parts due to impaired DNA clearance by extracellular DNases. In plasma from COVID‐19 patients, DNase1 substitution improved NET dissolution and reduced FXII activation in vitro.
Conclusion(s): Collectively, our study shows that the NETs/FXIIa axis contributes to procoagulant and proinflammatory reactions in COVID‐19. Targeting NETs and FXIIa may offer a potential therapeutic strategy for interfering with the COVID‐19 lung pathology.
VPB0607
A comparison of eNOS‐based platelet subpopulation ratios and platelet VEGF content within COVID‐19
A. Asgari 1; B. Ritchie2; P. Jurasz3
1 Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB, Canada, Edmonton, Alberta, Canada; 2 Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada, edmonton, Alberta, Canada; 3 Department of Pharmacology, Faculty of Medicine and Dentistry / Cardiovascular Research Centre / Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Alberta, Canada
Background: Nitric oxide (NO) regulates both thrombosis and vascular leakage, two of the major complications of severe Covid19. Platelet‐derived NO inhibits adhesion/aggregation and within microvascular endothelial cells NO‐signalling mediates vascular endothelial growth factor (VEGF/VPF)‐induced permeability. Recently, we identified novel platelet subpopulations with a differential ability to produce NO and the presence/absence of endothelial nitric oxide synthase (eNOS). We showed that eNOS‐negative (eNOSneg) platelets initiate adhesion and aggregation, while eNOS‐positive (eNOSpos) platelets limit aggregate growth. As inflammatory cytokines are known to counter‐regulate eNOS and VEGF expression, we hypothesized that the ratio of eNOS‐negative to –positive platelets may be increased in COVID‐19 patients along with their VEGF content.
Aims: To determine the eNOS‐positive to –negative platelet ratio and platelet VEGF levels within the blood of hospitalized COVID‐19 patients.
Methods: Platelets were isolated from age‐ and sex‐matched COVID‐19 patients (ICU and non‐ICU) and COVID‐negative controls. Platelets were immunostained for eNOS, VEGF, and surface CD62P then analyzed using flow cytometry. A multiplex assay was used to measure plasma cytokines.
Results: The percentage of eNOSneg platelets within the blood of COVID‐19 patients (ICU and non‐ICU) was higher than that within healthy controls and their levels correlated with disease severity (81.2 ± 2.8% ICU vs. 66.0 ±3.1% non‐ICU vs. 6.1 ± 1.3% controls, P‐value < 0.001; Fig. 1). Accordingly, the percentage of eNOSpos platelets in COVID‐19 patients was lower compared to controls, while the platelet VEGF content and surface CD62P of COVID‐19 patients in ICU was higher than that of healthy controls (Fig. 2). Further, COVID‐19 patients demonstrated higher TNFα, IL‐6, and IL‐1β plasma concentrations than controls.
Conclusion(s): Preliminary data suggests that eNOS‐negative to ‐positive platelet ratios and VEGF content increase with COVID‐19 severity, potentially predisposing patients to thrombosis and enhanced vascular permeability upon platelet activation. These platelet changes may be due to the actions of inflammatory cytokines on megakaryocytes.


VPB0613
Unraveling the pathophysiological role of angiostatin in COVID‐19
A. Franczak 1; M. Joyce2; B. Ritchie3; L. Tyrrel2; P. Jurasz4
1 University of Alberta, Edmonton, AB, Canada, Edmonton, Alberta, Canada; 2 Department of Medical Microbiology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada, Edmonton, Alberta, Canada; 3 Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada, Edmonton, Alberta, Canada; 4 Department of Pharmacology, Faculty of Medicine and Dentistry / Cardiovascular Research Centre / Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Alberta, Canada
Background: Angiostatin is a break‐down product of plasmin(ogen). Physiologically, angiostatin is generated by platelets in an urokinase (uPA)‐dependent manner. During normoxia angiostatin has anti‐angiogenic/anti‐inflammatory effects and protects against lung injury, however during hypoxia/acidosis it is pro‐apoptotic. In SARS‐CoV infected mice, the uPA‐plasminogen pathway was shown to be the most transcriptionally enriched regulating sublethal vs. lethal infection. Similarly, uPA has been shown to be transcriptionally upregulated in SARS‐CoV‐2; however, the role of angiostatin has not been investigated.
Aims: To assess role of angiostatin in COVID‐19.
Methods: Plasma samples from COVID‐negative controls and from hospitalized COVID‐19 patients (n = 30) were collected (day 1, 7, 14, 28, 70) via the COVID‐19 Surveillance Collaboration study. WHO clinical progression scale was used to assess COVID‐19 severity. Angiostatin and plasminogen were quantified by immunoblot. VeroE6 cells were infected with SARS‐CoV‐2 and treated with angiostatin (140 μg/ml) for 24h at pH 7.5 or 6.9. Cell death was quantified by both TUNEL and the percentage of detached cells. Immunofluorescent staining against the spike protein was used to confirm cellular infection.
Results: Plasma angiostatin level was elevated in COVID‐19 patients compared to COVID‐negative controls at baseline. Both angiostatin and plasminogen increased with time of hospitalization in patients with severe COVID‐19, but not with mild‐to‐moderate disease (p = 0.05; Fig.1). In preliminary cell culture experiments, at pH = 7.5 angiostatin decreased the percentage of detached (26 ± 8% vs 67 ± 5%; p = 0.0004) and TUNEL‐positive VeroE6 (6 ± 6 vs 11 ± 7%; p = 0.07) following infection. Conversely, at pH = 6.9 angiostatin increased the percentage of detached cells following infection. Interestingly, angiostatin lowered the percentage of spike protein‐positive Vero E6 at both pH (Fig.2).
Conclusion(s): Angiostatin concentrations increase with disease progression in severe COVID‐19. This likely reflects angiostatin’s complex role in COVID‐19 pathophysiology. Angiostatin promotes cell death in acidotic microenvironments (associated with severe Covid‐19). Conversely, at physiological pH, angiostatin may have protective effects possibly by reducing viral entry and/or replication.


VPB0618
Thrombosis‐related circulating miR‐16‐5p is associated with disease severity in patients hospitalised for COVID‐19
C. Eyileten1; Z. Wicik 1; S. Simões2; D. Martins‐Jr3; D. Keshwani1; A. Shahzadi4; J. Jarosz‐Popek1; M. Wolska5; K. Klos6; W. Wlodarczyk6; D. Soldacki7; A. Chcialowski6; J. Siller‐Matula8; M. Postula1
1 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 2 Federal Institute of Espírito Santo, Sao Paulo, Sao Paulo, Brazil; 3 Federal University of ABC, Sao Paulo, Sao Paulo, Brazil; 4 Istanbul Cerrahpasa University, Istanbul, Istanbul, Turkey; 5 Medical University of Warsaw, warsaw, Mazowieckie, Poland; 6 Military Institute of Medicine, warsaw, Mazowieckie, Poland, 7 medical university of warsaw, warsaw, Mazowieckie, Poland, 8 medical university of vienna, vienna, Wien, Austria
Background: SARS‐CoV‐2 tropism for the ACE2 receptor, along with the multifaceted inflammatory reaction, is likely to drive the generalized hypercoagulable state seen in patients with COVID‐19.
Aims: We aimed identify microRNAs (miRNAs) play role in ACE2‐related thrombosis in coronavirus infection and validated the expressions of those miRNAs in 79 hospitalized COVID‐19 patients and 32 healthy volunteers and monitored miRNAs patterns during the acute phase of COVID‐19, as well as the prognostic potential biomarker.
Methods: Using the original bioinformatic workflow and network medicine approaches we reanalyzed four coronavirus‐related expression datasets and performed co‐expression analysis focused on thrombosis and ACE2 related genes. Blood RNA; quality RNA was assessed: fluorometric assay; RT‐PCR for miRNAs expression measurement.
Results: We identified EGFR, HSP90AA1, APP, TP53, PTEN, UBC, FN1, ELAVL1 and CALM1 as regulatory genes which could play a pivotal role in COVID‐19 related thrombosis. We also found miR‐16‐5p, miR‐27a‐3p, Let‐7b‐5p and miR‐155‐5p as regulators in coagulation and thrombosis process. We observed in separate cohort of COVID‐19 patients and healthy controls that (i) expression of miR‐16‐5p, miR‐27a‐3p and miR‐155‐5p increased during observation, compared to the baseline measurement; (ii) a low baseline miR‐16‐5p expression presents predictive utility in assessment of the hospital length of stay or death in follow‐up as a composite endpoint (AUC:0.810, 95% CI, 0.71‐0.91, p < 0.0001); (iii) low baseline expression of miR‐16‐5p and T2DM are independent predictors of increased length of stay or death according to a multivariate analysis (OR: 9.417; 95% CI, 2.647‐33.506; p = 0.0005 and OR: 6.257; 95% CI, 1.049‐37.316; p = 0.044).
Conclusion(s): This study enabled us to better characterize changes in gene expression and signaling pathways related to COVID‐19 thrombosis. In this study we identified, characterized and validated miRNAs which could serve as novel, thrombosis‐related biomarkers of the COVID‐19, can be used for early stratification of patients and prediction of severity of infection development in an individual.


VPB0610
IL‐2 (T330G) gene promoter polymorphism and its effect on lymphocyte‐platelet adhesion in patients with coronavirus infection SARS‐COV‐2 (COVID‐19)
A. Emelyanov; A. Emelyanova; E. Zaytseva; Y. Vitkovsky
Chita State Medical Academy, Chita, Zabaykalsky, Russia
Background: Cytokines are signaling molecules and involve lymphocytes and macrophages in the immune response. Lymphocyte‐platelet adhesion is one of the mechanisms that ensure the cooperation of various subpopulations of cells and allow them to migrate through the vascular wall into the tissue.
Aims: To study the function of lymphocyte‐platelet adhesion depending on SNP of IL‐2 (T330G) gene in healthy subjects and patients with COVID‐19.
Methods: The research involved 168 patients with COVID‐19 and 100 healthy people of the same age and gender. The participants of the study were Caucasian race and lived in the Trans‐Baikal Territory. The examine of LPA was carried out by the method of Yuri Vitkovsky et al. (1999). The SNP of IL‐2 (T330G) gene was determined by PCR. Such methods as Equilibrium Hardy‐Weinberg, χ2‐test, odds ratio descriptive statistics and the mean values (M) and standard deviation (SD) were used. Statistical significance was measured at the value p < 0.05.
Results: We found all the studied IL‐2 mutations in accordance with the Hardy‐Weinberg law (p>0.05). The heterozygous T/G of IL‐2 gene was registered significantly more frequently in the group of patients in comparison with the control group. Based on the obtained data on frequency distribution, the chance of developing SARS‐COV‐2 increased in carriers of the allele T and the T/T genotype of IL‐2 gene (p < 0.001). It was found that patients with SARS‐COV‐2 had an increase in the LPA index compared to the group of healthy individuals (15.2 ± 1.1%). The maximum amount of LPA was detected among patients with T/T genotype ‐ 39.7 ± 2.1%, the minimum amount – in the owners of G/G (31.4 ± 1.9%) (p< \0.001).
Conclusion(s): 1) SARS‐COV‐2 is accompanied by an increase in LPA function indicators, which depend on SNP of IL‐2 (T330G) gene. 2) Carriers of allele T and T/T genotype of IL‐2 gene (T330G) predispose to the development of COVID‐19.
PB0592
Altered fibrin network structure and fibrinolysis in intensive care unit patients with COVID‐19, not entirely explaining the increased risk of thrombosis
J. de Vries 1; C. Visser1; L. Geers2; J. Slotman3; N. van Kleef4; C. Maas5; H. Bax6; J. Miedema7; E. van Gorp8; M. Goeijenbier9; J. van den Akker10; H. Endeman10; D. Rijken2; M. Kruip2; M. de Maat11
1 Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 3 Optical Imaging Center, Department of Pathology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 4 University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 5 UMC Utrecht, Utrecht, Utrecht, Netherlands; 6 Department of Medical Microbiology and Infectious Diseases & Department of Internal Medicine, Erasmus MC, Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 7 Department of Pulmonary Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 8 Department of Internal Medicine & Department of Viroscience, Erasmus MC, Erasmus Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 9 Department of Viroscience & Department of Adult Intensive Care, Erasmus MC, Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 10 Department of Adult Intensive Care, Erasmus Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 11 Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: SARS‐CoV‐2 infection is associated with an increased incidence of thrombosis.
Aims: By studying the fibrin network structure of COVID‐19 patients, we aimed to unravel pathophysiological mechanisms that contribute to this increased risk of thrombosis. This may contribute to optimal prevention and treatment of COVID‐19 related thrombosis.
Methods: In this case‐control study, we collected plasma samples from intensive care unit (ICU) patients with COVID‐19, with and without confirmed thrombosis, between April and December 2020. Additionally, we collected plasma from COVID‐19 patients admitted to general wards without thrombosis, from ICU patients with pneumococcal infection, and from healthy controls. Fibrin fiber diameters and fibrin network density were quantified in plasma clots imaged with stimulated emission depletion (STED) microscopy and confocal microscopy. Finally, we determined the sensitivity to fibrinolysis. Ethical approval was obtained and written informed consent was obtained or an opt‐out procedure was in place.
Results: COVID‐19 ICU patients (n = 37) and ICU patients with pneumococcal disease (n = 7) showed significantly higher fibrin network densities and longer plasma clot lysis times than healthy controls (n = 7) (Figure 1). No differences were observed between COVID‐19 ICU patients with and without thrombosis, or ICU patients with pneumococcal infection. At a second time point, after thrombosis or at a similar time point in patients without thrombosis, we observed thicker fibers and longer lysis times in COVID‐19 ICU patients with thrombosis (n = 19) than in COVID‐19 ICU patients without thrombosis (n = 18).
Conclusion(s): Our results suggest that severe COVID‐19 is associated with a changed fibrin network structure and decreased susceptibility to fibrinolysis. Since these changes were not exclusive to COVID‐19 patients, they may not explain the increased thrombosis risk.

PB0601
Differential expression of platelet microRNAs in hospitalized patients with severe COVID‐19
M. Pócsi1; Z. Fejes2; H. Ghansah1; R. Sütő3; Z. Szabó3; S. Póliska4; Á. Gyetvai4; Z. Szentkereszty3; J. Kappelmayer4; B. Nagy 4
1 Department of Laboratory Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary, Debrecen, Hajdu‐Bihar, Hungary; 2 Faculty of Medicine, University of Debrecen, Debrecen, Hungary, Debrecen, Hajdu‐Bihar, Hungary; 3 Gyula Kenézy Campus, Intensive Care Unit, Faculty of Medicine, University of Debrecen, Debrecen, Hungary, Debrecen, Hajdu‐Bihar, Hungary; 4 University of Debrecen, Debrecen, Hajdu‐Bihar, Hungary
Background: Coronavirus disease 2019 (COVID‐19) is often associated with enhanced platelet activation and thrombotic complications which may be reflected by altered microRNA (miRNA) levels in blood cells.
Aims: We here investigated the expression of miRNAs in the background of platelet reactivity in severe COVID‐19 and evaluated them for the prediction of disease mortality.
Methods: Platelet miRNAs were isolated from leukocyte‐depleted platelets and profiled in COVID‐19 survivors and non‐survivors vs healthy controls using TaqMan Open Array. Candidate miRNAs showing at least 2‐fold alteration between two groups were validated by RT‐qPCR in 10 individuals from each group, and these data were correlated with clinical outcome in COVID‐19. Differentially regulated miRNAs were bioinformatically analyzed to identify their mRNA targets and Cytoscape ClueGO was used for functional enrichment analysis. Platelet reactivity was evaluated via quantification of P‐selectin expression, platelet‐leukocyte aggregates and platelet‐derived microparticles (PMPs) by flow cytometry.
Results: Increased platelet activation was detected via elevated level of P‐selectin positivity (6.5 vs 1.1%, P < 0.0001), platelet‐monocyte aggregates (40 vs 17 %, P = 0.0315) and PMPs (22 vs 18 PMPs/10^5 platelets, P = 0.3789) in severe COVID‐19 vs controls. Out of 340 detected platelet miRNAs, there were 127 attenuated (e.g. miR‐221) and 8 upregulated (e.g. miR‐320b) miRNAs in abnormal COVID‐19 platelets compared to normal samples. Furthermore, non‐survivors showing 28‐day mortality demonstrated a different profile of miRNAs vs survivors including 66 downregulated (e.g. miR‐126) and 4 upregulated (e.g. miR‐27b) miRNAs. Network analysis revealed the association of altered miRNAs with 241 genes involved in several mechanisms, such as regulation of cell activation, response to cytokines, and cell surface receptor signalling pathway involved in cell‐cell signalling. Among downregulated miRNAs, CD40LG was regulated by miR‐146a, miR‐30b targeted SERPINE1, while miR‐29b modulated the expression of CDC42.
Conclusion(s): Altered platelet miRNAs regulate the development of platelet reactivity in severe COVID‐19 and may serve as possible biomarkers to predict disease mortality.
PB0605
COVID‐19 disease is associated with a hypofibrinolytic state driven by elevated plasminogen activator inhibitor‐1 and its cofactor vitronectin
M. Simpson 1; C. Whyte1; G. Morrow2; C. Wallace1; A. Mentzer3; J. Knight3; S. Shapiro4; N. Curry3; C. Bagot5; H. Watson1; J. Cooper6; N. Mutch1
1 University of Aberdeen, Aberdeen, Scotland, United Kingdom; 2 University of Oxford, Aberdeen, Scotland, United Kingdom; 3 University of Oxford, Oxford, England, United Kingdom; 4 Oxford University Hospitals National Health Service Foundation Trust, University of Oxford, and Oxford National Institute for Health Research Biomedical Research Centre, Oxford, England, United Kingdom; 5 Department of Haematology, Glasgow Royal Infirmary, Glasgow, England, United Kingdom; 6 NHS Grampian, Aberdeen, Scotland, United Kingdom
Background: COVID‐19 disease arises from infection with severe acute respiratory cornonavirus‐2 (SARS‐CoV‐2). Severe disease is associated with a coagulopathy characterised by elevated D‐dimer levels, fibrin deposition in the lung, and a thrombotic incidence of approximately 30%, indicating catastrophic derailment of the haemostatic system.
Aims: To investigate whether SARS‐CoV‐2‐induced coagulopathy arises due to an imbalance in the fibrinolysis.
Methods: Citrated plasma was collected from 139 patients presenting with symptomatic COVID‐19, 24 patients with non‐COVID‐19 respiratory infection and 30 healthy controls in a dual‐centre study. Fibrinolytic biomarkers were evaluated including plasminogen activator inhibitor 1 (PAI‐1), tissue plasminogen activator (tPA), plasminogen, vitronectin and thrombin activatable fibrinolysis inhibitor (TAFI). Furthermore, diagnostic biomarkers including, fibrinogen, C‐reactive protein (CRP), D‐dimer and inflammatory cytokines were quantified. Clot lysis was evaluated using turbidity assays, plasma clot structure visualised by confocal microscopy and plasmin generation quantified by chromogenic substrate.
Results: PAI‐1 antigen, activity, and the cofactor for this serpin, vitronectin, were significantly elevated in patients with COVID‐19 compared to healthy controls and non‐COVID‐19 respiratory infection. Patients with COVID‐19 exhibit attenuated plasmin generation compared to healthy volunteers despite significant elevation in tPA. PAI‐1 correlated with inflammatory cytokines (IL‐1β, IL‐8 and TNF‐α). In line with this acute phase proteins, fibrinogen and CRP were high in patients with COVID‐19 but only CRP was increased compared to non‐COVID‐19 respiratory infections. Levels of PAI‐1 and vitronectin were associated with escalating oxygen support and a corresponding decrease in plasminogen. Importantly, patients with COVID‐19 disease exhibit resistance to fibrinolytic degradation by Actilyse®, however, this could be overcome by the PAI‐1 resistant form of tPA, Metalyse®.
Conclusion(s): We reveal that COVID‐19 disease promotes a hypofibrinolytic state due to elevated PAI‐1 and its stabilizing cofactor vitronectin. PAI‐1 correlates with inflammatory cytokines and disease severity thereby highlighting its potential prognostic power in the development of severe COVID‐19 disease.
PB0594
Hypercoagulability in COVID‐19 and its putative association with the downregulation of protein C signalling
B. dos Santos Silva 1; C. Jara2; D. Sidarta‐Oliveira3; L. Velloso3; W. Velander2; E. P Araujo3
1 University of Campinas ‐ UNICAMP, Osvaldo Cruz, Sao Paulo, Brazil; 2 University of Nebraska, Lincoln, Nebraska, United States; 3 University of Campinas ‐ UNICAMP, campinas, Sao Paulo, Brazil
Background: Hematological complications associated with prothrombotic events with extrapulmonary manifestations have been demonstrated in autopsies of patients affected by coronavirus disease 2019 (COVID‐19). Based on the close relationship of coagulation and immune response, we hypothesized that hypercoagulability in COVID‐19 could result from the activation of tissue factor (F3) and subsequent alterations in Activated Protein C (APC) signaling (Figure 1).
Aims: We aimed to identify changes in the expression of APC signaling network in liver, peripheral blood and nasal epithelium of COVID‐19 patients that may contribute to local and systemic disarrangement of hemostasis.
Methods: For the expression of PROC and receptor genes public single‐cell‐RNA‐sequencing datasets were analyzed from COVID‐19 patients and healthy individuals, using the toolkit Scanpy 1.7.2 in Phyton.
Results: The key compounds of Protein C (PC) activation and signaling; PROCR, F2R, THBD, S1PR1 and PROC were downregulated in COVID‐19 patients; a greater expression of F3 in all COVID‐19 tissues analyzed and upregulation of AGTR1, NFKB1, PTPN1, THBS1, PTGS2, PLAU, SERPINE1 and F5 pro‐inflammatory and procoagulant genes in the liver of COVID‐19 patients compared to control (Figure 2B, E and G). The hepatocyte PROC expression was changed in COVID‐19 patients from hepatocyte 4 ADH1B+ PCK1+ in healthy liver (Figure 2F) to hepatocyte 3 CYP2A6+ in the liver of COVID‐19 patients (Figure 2A). The ACE2 expression was increased in all COVID‐19 tissues (Figure 2B, E and G) overlapping the PROC expression in the epithelium (Figure 2D) and liver tissues (Figure 2A). There was a co‐expression of ACE2, PROC, PROS1, RHOA, and RAC1 in ciliated cells of COVID‐19 patients (Figure 2C‐D).
Conclusion(s): The results provide evidence indicating a deficient synthesis and activation of PC and its receptors in COVID‐19 patients that might contribute to a pronounced hypercoagulable state in response to endothelial COVID‐19‐ related injury. Financial support: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).


VPB0609
Characterization of TF positive small extracellular vesicles in SARS‐CoV‐2 infection
P. Canzano 1; M. Brambilla1; R. Frigerio2; M. Cretich2; A. Becchetti3; M. Conti3; M. Camera4
1 Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy; 2 National Research Council of Italy (SCITEC‐CNR), milan, Lombardia, Italy, 3 centro cardiologico monzino, milan, Lombardia, Italy, 4 centro cardiologico monzino, Università degli Studi di Milano, milan, Lombardia, Italy
Background: Coronavirus‐2 (SARS‐CoV‐2) infection causes a sustained prothrombotic state driven by a massive Tissue Factor (TF) expression in circulating platelets, leukocytes and microvesicles (MVs). Whether also circulating small extracellular vesicles (sEVs), in addition to large MVs (MVs), can contribute to this hypercoagulable scenario through TF expression is not yet known, mainly due to methodological issues in detecting and sizing the smallest vesicles.
Aims: To characterize TF expression and activity in circulating sEVs, compared to that of MVs, of COVID‐19 patients during acute phase infection and after symptom remission.
Methods: MVs and sEVs were isolated by plasma differential centrifugation from 10 COVID‐19 patients enrolled at acute phase infection (T0) and at six‐month‐follow‐up (T1). Ten healthy subjects (HS) were analyzed as controls. sEVs were counted by Nano Tracking Assay. In sEVs TF expression was analyzed within CD9/CD63/CD81pos events by imaging flow cytometry (IFC) and ExoView™ microarray, while TF activity by FXa generation assay. TF expression and activity in MVs were evaluated for comparison.
Results: By IFC analysis COVID‐19 patients at T0 have about 1.5‐ and 4‐fold higher number of TFpos‐sEVs and ‐MVs, respectively, compared to HS, with a trend toward reduction to physiological levels at T1. By microarray analysis sEVs behaved similarly (36 ± 12 and 25 ± 10 TFpos‐spots at T0 and T1, respectively; p = 0.0281). sEVs‐associated TF is functionally active thus able to partially support FXa formation as sEVs preincubation with TF‐neutralizing antibody reduced FXa generation by ~30%. However, although sEVs number is significantly higher compared to that measured for MVs (~600‐fold in HS), functional activity of sEV is one‐third lower compared to that of MVs.
Conclusion(s): These data suggest that, in COVID‐19 patients, the altered procoagulant phenotype could also be supported by TF carried by sEVs, although their functional activity is significantly lower than that of MVs.
COVID and Coagulation, Clinical
VPB0139
Predictive value of D‐dimer in Covid‐19
N. Vorobyeva 1; A. Vorobyeva2
1 Federal State Budgetary Institution "National Medical Research Center for Hematology" (Northern branch) Ministry of Health of Ru, Arckhangelsk, Arkhangelsk, Russia; 2 Federal State Budgetary Institution Northern State Medical University Ministry of Health of Russia, Arkhangelsk, Архангельск, Arkhangelsk, Russia
Background: The problem of determining the significance of individual laboratory markers that allows in the early stages of community‐acquired pneumonia, in including during SARS‐CoV‐2 infection, to predict both the nature of the course of the disease and possible poor prognosis.
Aims: To assess the predictive value of an increased D‐dimer in a new coronavirus infection caused by SARS‐CoV‐2.
Methods: The diagnostic significance of the D‐dimer level in predicting the severity of COVID19 infection was assessed based on the accumulated literature and real clinical practice. The methods of bibliographic and informational search in Scopus, CORE, eLIBRARY databases were used. The keywords for the search were: D‐dimer, DIC‐syndrome, SARS‐CoV‐2, COVID‐19. A total of 52 literary sources were found, of which 21 articles were selected for further analysis. To study real clinical practice, the level of D‐dimer was analyzed in 109 patients diagnosed with a confirmed new coronavirus infection caused by SARS‐CoV‐2, moderate pneumonia, hospitalized in the «covid» department of the Volosevich First City Clinical Hospital in Arkhangelsk.
Results: The D‐dimer is the most significant marker of the severity of the disease and predicting the risk of death in SARS‐CoV‐2 infection, both according to the literature and in real clinical practice. There is a relationship between the severity of the inflammatory process and the state of thrombinemia in community‐acquired pneumonia of both bacterial and viral genesis, including influenza and SARS‐CoV‐2
Conclusion(s): The main significance of elevated D‐dimer levels in patients with COVID‐19 is associated with the activation of coagulation against the background of systemic inflammation, ultimately leading to the disseminated intravascular coagulation syndrome, which must be taken into account in the treatment of the new coronavirus infection.
PB0093
Thromboembolism and its management in hospitalized COVID‐19 patients using modified caprini risk score: A single center experience in Ethiopia
T. Tadesse 1; A. Teklu2; A. Abiye2
1 Tikur Anbessa Specialized Hospital, College of Health Sciences, Addis Ababa University, Addis Ababa, Adis Abeba, Ethiopia; 2 School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Adis Abeba, Ethiopia
Background: Reports indicated association of COVID‐19 with coagulation dysfunction and venous thromboembolism (VTE) prevalence was in the range of 20–40%. All patients with COVID‐19 who are hospitalized should receive pharmacologic prophylaxis unless they have contraindications.
Aims: To assess VTE risk, incidence and its management in patients with COVID‐19 admitted to Tikur Anbessa Specialized Hospital (TASH).
Methods: A retrospective study was conducted among 146 COVID‐19 patients admitted to TASH. A pre‐tested data abstraction format was used to collect patients’ clinical information and VTE risk by using the modified Caprini Risk Score in COVID‐19. We used Statistical Package for the Social Sciences (SPSS) version 26 for data analysis. Descriptive statistics was used to summarize the findings and binary logistic regression analysis to assess association between the variables of interest.
Results: Result: Out of 146 patients, 57.53% were males and mean age was 45.56 ± 18.17 years. All patients were at risk of developing VTE. The most often observed VTE risk factors were being COVID‐19 symptomatic (88.40 %), serious lung diseases (56.2%) and age > 40 years (52.10 %). The incidence of VTE was 23 (15.75 %) and majorly (91.3%) occurred in highest VTE risk (≥ 5 score) patients, >40 years patients and in patients with severe COVID‐19 symptoms (100%). However, parenteral thromboprophylaxis was prescribed only for 98 (67.12 %) patients. Out of 23 patients developed VTE, 15 didn’t receive prophylaxis and the remaining 8 received the thromboprophylaxis. Unfractionated heparin (UFH) was the most widely used prophylaxis. For patients who developed VTE, majority of them 20 (86.96%) were given therapeutic dose of UFH.
Conclusion(s): All patients with COVID‐19 were at risk of developing VTE. Only one third received thromboprophylaxis. The incidence of VTE was high and majorly occurred in patients that didn’t receive prophylaxis.
PB0099
Arterial and venous thrombosis induced by vaccination with ChAdOx1 nCov‐19 (Astrazeneca) against Coronavirus 19 (COVID19)
A. Vilaseca 1; E. Valgolio2; M. Marchena3; N. Kujta4; B. Franco5; C. Capmany4; S. Pastoriza6
1 Clinica San Camilo, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 CLINICASANCAMILO, BUENOS AIRES, Buenos Aires, Argentina; 3 Clinica San Camilo, Ciudad, Ciudad Autonoma de Buenos Aires, Argentina; 4 CLINICA SAN CAMILO, CIUDAD AUTONOMA DE BUENOS AIRES, Ciudad Autonoma de Buenos Aires, Argentina; 5 CLINICASANCAMILO, CIUDAD AUTONOMA DE BUENOS AIRES, Ciudad Autonoma de Buenos Aires, Argentina; 6 CLINICAS SAN CAMILO, CIUDAD AUTONOMA DE BUENOS AIRES, Ciudad Autonoma de Buenos Aires, Argentina
Background: The biggest pharmaceutical break‐through during the Coronavirus 19 (COVID 19) pandemic was the development of vaccines. Vaccine‐induced thrombocytopenic thrombosis (VITT) is a rare but severe and/or fatal complication associated with adenoviral vector‐based vaccines. It is a prothrombotic disorder confirmed by the finding of antibodies against Platelet Factor 4 (PF4). To date, approximately 40 cases of VITT have been published, sixty‐seven percent (27/40) of patients were females, median age was 40.5 years. We describe a case occurs in a previously healthy young women receiving the first dose of ChAdOx1 nCov‐19 (Astrazeneca), 1 or 2 weeks after developing symptoms.
Aims: Describe clinical manifestations and management of a patient with arterial and venous thrombosis with thrombocytopenia following vaccination with ChAdOx1 nCov‐19.
Methods: Clinical history, laboratory data, diagnostic imaging (computed tomography, arterial and venous Doppler ultrasound and transcranial Doppler, cerebral arteriography), surgical intervention and electroencephalogram.
Results: A 27‐year‐old woman, without pathological history, arrived to the Emergency Room with expression aphasia and right hemiparesis, 10 days after vaccination for COVID 19 with ChAdOx1 nCov‐19. (Astrazeneca) CT scan: extensive left intraparenchymal hematoma. Arteriography: left sylvian thrombosis. Intercurred with bilateral radial arterial thrombosis, venous thrombosis and pulmonary thromboembolism. Thrombocytopenia, hypofibrinogenemia, elevated D‐dimer, and Positive PF4 Antibodies (Asserachrom HPIA Anti PF4/ Heparin IgG) confirmed the diagnosis of VITT. Treatment: evacuation of intraparenchymal hematoma, decompressive craniectomy and high doses of immunoglobulin, corticosteroids, and anticoagulation with Apixaban. She evolved with digital necrosis of the left thumb with partial amputation of said finger. Prolonged mechanical ventilation GCS (Glasgow Coma Scale) 10/15 and right hemiplegia.
Conclusion(s): Suspicion and early recognition of VITT is associated with better outcomes. Our patient presents the diagnostic criteria for VITT: thrombocytopenia, hypofibrinogenemia, elevated D‐dimer and positive PF4 Antibodies. The basis of treatment was, immunoglobulin, anticoagulation (avoiding heparin) and corticosteroids. Neurological sequelae were inherent to the initial bleeding event.


PB0100
Thromboelastography (TEG) as an outcome predictor marker of Covid‐19 patients in Malang Indonesia
S. Wardhani 1; D. Hermanto1; S. Fatonah2; J. Fajar1
1 Universitas Brawijaya, Malang, Jawa Timur, Indonesia; 2 Universitas Brawijaya/ dr Saiful Anwar General Hospital, Kota Malang, Jawa Timur, Indonesia
Background: Evidence suggested that severe COVID‐19, a condition associated with a high mortality rate, is aggravated by significant coagulopathy which manifests in the form of microthrombosis and venous thromboembolism(VTE). Therefore,easy and accurate markers are required to assess coagulopathic changes in Covid‐19 patients as the basis for providing prophylactic anticoagulant therapy to prevent thrombosis.
Aims: Our study aimed to assess the potential predictor of Thromboelastography(TEG) to estimate the outcome of COVID‐19 patients
Methods: A cross‐sectional study approved by local the ethical committee, was conducted in Dr.Saiful Anwar Hospital. The potential interest data, retrieved from medical records, were extracted, ie:TEG, thrombocytes levels, the severity of COVID‐19 patients, and themortality rate of COVID‐19 patients. The diagnosis of COVID‐19 was confirmed by reverse‐transcription polymerase chain reaction(RT‐PCR). The comparison between TEG and the risk of mortality among patients with COVID‐19 was analyzed using multiple logistic regression. We applied Graphad Prism version 9 (Graphad, Sandiego,CA) to analyze the data
Results: A total of 63 patients, employed from medical records, were included in this study, in which the survival rate was 65% and 22 patients died. Our study population between groups was age and sex‐matched(Tab1). Our current findings revealed that hypercoagulation TEG was associated with 12.67‐fold increase risk of mortality compared to those normocoagulation or hypocoagulation TEG(Fig1). Moreover, we also found that, among all TEG parameters, Lys30 was the only parameter having the greatest pivotal impact to govern the mortality of COVID‐19 patients. We found that higher levels of Lys30 (MD95%CI: 26.90[13.34‐40.47]) was associated with increase risk of mortality among patients with COVID‐19(Fig1). On the other hand, our study also confirmed that the levels of CI and MA were observed higher in mortality group than in survival group
Conclusion(s): Our current study indicated that hypercoagulation TEG, excess fibrinolysis (Lys30), CI and MA increased the risk of mortality among patients with COVID‐19


VPB0135
Vaccine‐induced thrombotic thrombocytopenia (VITT) in COVID‐19 vaccination: Demystified
A. Singh 1; C. Amrutha2; S. Vidyasagar2; N. Fatima3; S. Chauhan4
1 Department of Medicine, Kasturba Medical College & Hospital, Manipal Academy of Higher Education, Manipal, India, Manipal, Karnataka, India; 2 Kasturba Medical College & Hospital, Manipal Academy of Higher Education, Manipal, India, Manipal, Karnataka, India; 3 St. Pauls College of Pharmacy, Turkayamjal, Hayatnagar (md) R.R District Hyderabad, India – 501510, Hyderabad, Telangana, India; 4 Melaka Manipal Medical College, Manipal, Manipal, Karnataka, India
Background: The beacon of hope in the face of the COVID ‐19 pandemic has been the development of vaccinations. However, once these vaccines were administered to the masses, there were some rare complications reported. The most concerning among them was the development of a condition that presented with thrombosis, commonly in the cerebral and splanchnic veins in the presence of thrombocytopenia. This development led to a ban on some specific covid‐19 vaccines in certain countries.
Aims: To examine the evidence available regarding the vaccine‐associated complications and justify using these vaccines despite the risk.
Methods: Search methods The data were searched through popular electronic databases, including Google Scholar, Springer publications, World Health Organization guidelines, PubMed and Cochrane. All articles were arranged as per the information they have provided and arranged in a systematic way. Population Data were searched for the population of > 18 years of age who received any COVID‐19 vaccination irrespective of mortality status. Outcomes Occurrence of thrombotic events following COVID‐19 vaccination
Results: The data has been extracted and presented to help clinicians better understand and identify this post‐vaccination phenomenon. This study of recent data revealed a two‐fold benefit, diagnostic and therapeutic. While evaluating a patient with thrombocytopenia and thrombosis, it is prudent to consider vaccine‐induced thrombotic thrombocytopenia (VITT) as a differential as it helps avoid a battery of investigations to prove the disease's etiology. Moreover, the duration of anticoagulation can be restricted to 3 months in VITT, which otherwise would need to be continued life‐long in unexplained thrombotic events.
Conclusion(s): This study concluded that VITT has become an important differential diagnosis to consider in light of the current pandemic, especially to avoid a battery of unnecessary investigations to prove the etiology.


PB0047
Covid‐19 vaccination & Acquired Hemophilia A: the case of a middle aged woman
M. Daniel 1; C. Paris1; A. Hochart1; N. Trillot1; A. Rauch2; B. Wibaut1; E. Jeanpierre3; M. Desvages1; C. Zawadzki1; A. Bauters4; F. Lassalle1; A. Tournoys1; A. Dupont2; L. Terriou1; S. Susen5
1 Lille University Hospital, Lille, Nord‐Pas‐de‐Calais, France; 2 University of Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐EGID, France, Lille, Nord‐Pas‐de‐Calais, France; 3 Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 4 University Hospital of Lille, Lille, Nord‐Pas‐de‐Calais, France; 5 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France
Background: Acquired hemophilia A (AHA) is a rare bleeding condition defined by the development of autoantibodies directed against clotting factor VIII (FVIII). It occurs primarily in elderly patients. AHA is associated with comorbidities such as cancer, autoimmune disease and infections. There is growing literature on AHA during or following SARS‐CoV‐2 infection or vaccination. Six previous cases of AHA post Covid‐19 vaccination have been described; all patients were older than 65 years old (yo).
Aims: We report on a 45 yo woman presenting with AHA after COVID‐19 vaccination. She had no personal or family history of bleeding disorders. Eighteen days after her 2nd dose of Pfizer‐BioNTech COVID‐19 vaccine, she presented with recurring hematomas. Bleeding events were both spontaneous and provoked by minor traumas but with a disproportionate size. The aim of this report is to bring to light the association between AHA and COVID‐19 vaccine in a younger patient with no ongoing or recent pregnancy.
Methods: Case report
Results: Biological explorations showed a prolonged activated partial thromboplastin time (aPTT) at 75 seconds (ratio 2.42). FVIII was undetectable and von Willebrand factor explorations were normal. Anti‐FVIII antibodies were positive at 373 Bethesda units (BU/ml) confirming the diagnosis of AHA. There was no evidence of pregnancy, cancer, auto‐immune disorder or infectious disease. Immunosuppression was initiated with prednisone (1mg/kg), then intensified with rituximab. Bleeding was controlled with repeated infusions of activated prothrombin complex concentrate (aPCC, Feiba®) at a dose of 60 IU/kg/injection. A 3rd dose of Covid‐19 mRNA vaccine was contraindicated.
Conclusion(s): To our knowledge this is the first case of AHA post Covid‐19 vaccination in a patient under 50 yo. The exact mechanisms responsible for autoimmune disorders following vaccination are still unclear. However this report might suggest that AHA diagnosis in this context may not be a simple statistical coincidence. Physicians should remain wary of this possible complication.


PB0089
Clinical assessment of coagulation hemostasis in liver cirrhosis associated with COVID‐19
M. Salokhiddinov 1; O. Muminov2; Z. Qurbanova3
1 Zangiota‐2 COVID Specialized Centre, Tashkent, Toshkent, Uzbekistan; 2 Zangiota 2 COVID Specialized Hospital, Tashkent, Qashqadaryo, Uzbekistan; 3 Zangiota 2 COVID Specialized Hospital, Tashkent, Toshkent, Uzbekistan
Background: Since the beginning of the pandemic, our understanding of the hepatic repercussions of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and the resulting coronavirus disease 2019 has significantly progressed.
Aims: The primary objective of this study was to characterize coagulation hemostasis in patients with COVID‐19‐associated liver cirrhosis.
Methods: Our study involved 200 patients (confirmed COVID‐19 ) with liver cirrhosis, decompensation stage, Child‐Pugh class V as the objects of study. I group consisted of 50 patients with liver cirrhosis of HBV etiology, II group 30 patients with HBV + HDV liver cirrhosis, III groups 50 patients with cirrhosis of unknown etiology. The control group consisted of 20 healthy individuals. All patients were tested for coagulogram.
Results: In group I, activated partial thromboplastin time was 18.4 ± 2.3 c, prothrombin time was 9.5 ± 0.6 c, prothrombin index was 126 ± 6.4, and MNO was 0.79 ± 0.03. In group II, activated partial thromboplastin time was 15.2 ± 1.1 c, prothrombin time was 8.2 ± 0.4 c, prothrombin index was 146 ± 8.2, and the international normalized ratio was 0.68 ± 0.02. In group III, was 12.2 ± 0.8 c, prothrombin time was 7.9 ± 0.4 c, prothrombin index was 152 ± 10.4, and the international normalized ratio was 0.66 ± 0.02. In the control group, activated partial thromboplastin time was 32 ± 1.8 c, prothrombin time was 12.1 ± 0.2 c, prothrombin index was 99 ± 3.7, and the international normalized ratio was 1.01 ± 0.06.
Conclusion(s): To summarize, our findings demonstrate that severe COVID‐19 infection is associated with coagulopathy, which is associated with a bad prognosis. COVID‐19 coagulopathy, on the other hand, does not appear to be a form of disseminated intravascular coagulation. Additional research is required to elucidate additional probable mechanisms of this coagulopathy, including a thorough examination of the fibrinolytic system.
PB0090
Investigating levels of inflammatory biomarkers between SARS‐CoV‐2 vaccinated and unvaccinated subjects
N. Jabeen1; A. Amanullah2; M. Hussain2; B. Kantarcioglu3; F. Siddiqui 3; J. Fareed3
1 University of Karachi, Karachi, Sindh, Pakistan; 2 Dow University of Health Sciences, Karachi, Sindh, Pakistan; 3 Loyola University Chicago, Maywood, Illinois, United States
Background: To curb the threat of COVID‐19, vaccines of different forms and shape have been developed and assessed for their efficacy in the last one and a half year. Amongst those Inactivated viral vaccines developed in China, Sinopharm and Sinovac are the most frequently employed vaccines in Pakistan. It has been established that natural infection and certain forms of SARS‐CoV‐2 vaccine alters the clinical picture of blood.
Aims: In this study we have compared the levels of three inflammatory biomarkers namely PAI‐1, D‐Dimer and HAI‐IgG in the sera collected from SARS‐CoV‐2 Vaccinated and unvaccinated Subjects.
Methods: Briefly, 80 individuals, each as a cohort of SARS‐CoV‐2 vaccinated and unvaccinated were recruited with written consent after ethical approval for the study. From each subject 2 ml blood was drawn and plasma was separated to assess inflammatory biomarkers like PAI‐1, D‐Dimer and HIA IgG by ELISA. Additionally, platelets count were also monitored using automated counter.
Results: Our data show difference in the level of PAI‐1, D‐Dimer and HIA‐IgG between SARS‐CoV‐2 Vaccinated and unvaccinated subjects. However, the difference was found statistically in significant. Nevertheless, segregating the data based on the nature of vaccination, age and gender of the subjects shows interesting pattern that could be insightful in relation to the clinical outcome of the vaccine efficacy.
Conclusion(s): The findings in this regard could well be of clinical value, especially when the data is stratified with reference to different variables. Therefore, large scale studies are warranted with same, and few additional biomarkers would be of more resolving in relation to the host response against SARS‐CoV‐2 vaccination.
VPB0140
Genetic triggers prothrombogenic status in Covid‐19
N. Vorobyeva 1; A. Barteneva2
1 Federal State Budgetary Institution "National Medical Research Center for Hematology" (Northern branch) Ministry of Health of Ru, Arckhangelsk, Arkhangelsk, Russia; 2 Federal State Budgetary Institution Northern State Medical University Ministry of Health of Russia, Arkhangelsk, Arckhangelsk, Arkhangelsk, Russia
Background: Currently, the prothrombogenic status in Covid‐19 infection and the possible influence of genetic polymorphism of the hemostasis system are widely discussed.
Aims: The aim of the study was to evaluate the molecular and genetic features of the hemostasis system in patients with Covid‐19
Methods: The study was performed on the basis of the regional center for antithrombotic therapy Arkhangelsk.A molecular genetic analysis was performed for the presence of prothrombogenic mutations in patients (n = 100) with Covid‐19 in a covid hospital.
Results: Polymorphism in the F I G/A‐455 (fibrinogen) gene was found in 43% of patients (homozygote ‐ 6%, heterozygote ‐ 37%), polymorphism in the ITG B3 1565 T>C gene ‐ in 38% (26% ‐ heterozygous carrier and 12% ‐ homozygous). In our study, a mutation in the prothrombin F II gene G20210‐A (heterozygous allelic variant ‐ 2%) and a "true" prothrombogenic mutation of factor V (Leid) were found with the lowest (6% ‐ heterozygous state). Next, we analyzed the dependence of genetic polymorphism of hemostasis system factors F II 20210 G>A, F V 1691 G>A, PAI‐1 675 5G>4G and thrombinemia levels in patients with COVID‐19. The level of fibrinogen (>6.0 g/l; p < 0.05) was statistically significantly higher in the groups of patients with prothrombogenic polymorphism in the genes of coagulation factor V (F V), coagulation factor II (F II), PAI‐1 for the entire period of hospitalization. Upon admission, the levels of D‐dimer and fibrinogen were statistically significantly higher in patients with a mutation in the F II gene.
Conclusion(s): When treating Covid‐19, it is advisable to take into account the presence of molecular genetic markers of prothrombogenic status in a patient.
VPB0122
Correlation of Clot Waveform Analysis (CWA) Parameters with the Days of Hospitalization of CoVID‐19 Patients
E. Vagdatli; A. Chalvantzis; M. Issa; G. Variti; V. Katsikari; K. Bani
Hippokratio General Hospital, Thessaloniki, Greece
Background: The activation of coagulation is one of the most severe complications of CoVID‐19. The automatic optical end‐point coagulation analyzers have the ability to present the clot reaction curve of the PT, APTT and Fibrinogen(FIB) which is referred as a clot waveform analysis (CWA).
Aims: To investigate the ability of CWA parameters for the prognosis of the length of hospital stay of CoVID‐19 patients.
Methods: Coagulation tests and CWA on BCSXP System (SIEMENS) were performed in 268 CoVID‐19 patients upon hospital admission and prior to the initiation of anticoagulation therapy. The measured CWA parameters of PT, aPTT and FIB were the change in Absorbance(dmA) and the time difference from the starting time of the reaction (mixing of reagent and sample) until a specific absorbance change (absorbance threshold). The patients were divided into 3 groups depending on the duration of hospitalization: A: < 5 days (n = 42), B:6‐10 days (n = 81), C:>10days (n = 145). Variables were tested with Student’s t‐test or Mann–WhitneyU test for differences in distributions of dmA and dsec among three groups. Pearson's correlation coefficient was used for comparison among the above parameters of 268 patients and their days of hospitalization. P values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS27.
Results: A gradual increase in the values of dmA was detected from A to C group. Statistical significance was present in aPTT and PT among all groups but in FIB only between B and C. From the comparison of dsec, statistical significant decreased values were found only in FIB between A and C (p = 0.027) and B and C (p = 0.002). The comparison between CoVID‐19 patients and duration of hospitalization revealed statistical significant correlation of dmA in PT, aPTT (p < 0.001) and in FIB (p = 0.01).
Conclusion(s): CWA variables upon admission in COVID‐19 patients, may be utilized for the prognosis of the duration of hospitalization.
PB0044
Increased blood viscosity and red blood cell aggregation in patients with COVID‐19
C. Nougier1; E. Nader2; C. Boisson3; S. Poutrel4; J. Catella 5; F. Martin2; J. Charvet2; S. Girard6; S. Havard‐Guibert2; M. Martin2; H. Rezigue7; H. Desmurs‐Clavel8; C. Renoux9; P. Joly9; N. Guillot2; Y. Bertrand10; A. Hot11; Y. Dargaud12; P. Connes4
1 Laboratoire d'Hématologie, Groupement Hospitalier Est, Hospices Civils de Lyon, Lyon,France, lyon, Rhone‐Alpes, France; 2 Laboratoire Interuniversitaire de Biologie de la Motricité (LIBM) EA7424, Team VascularBiology and Red Blood Cell, Université ClaudeBernard Lyon 1, Villeurbanne, France; Laboratoire d'Excellence du Globule Rouge(Labex GR‐Ex), PRES Sorbonne, Paris, France, Lyon, Rhone‐Alpes, France; 3 Laboratoire Interuniversitaire de Biologie de la Motricité (LIBM) EA7424, Team VascularBiology and Red Blood Cell, Université ClaudeBernard Lyon 1, Villeurbanne, France; Laboratoire d'Excellence du Globule Rouge(Labex GR‐Ex), PRES Sorbonne, Paris, France, lyon, Rhone‐Alpes, France; 4 Laboratoire Interuniversitaire de Biologie de la Motricité (LIBM) EA7424, Team VascularBiology and Red Blood Cell, Université ClaudeBernard Lyon 1, Villeurbanne, France; Laboratoire d'Excellence du Globule Rouge (Labex GR‐Ex), PRES Sorbonne, Paris, France, Lyon, Rhone‐Alpes, France; 5 Laboratoire Interuniversitaire de Biologie de la Motricité (LIBM) EA7424, Université ClaudeBernard Lyon 1, Villeurbanne, France; Laboratoire d'Excellence du Globule Rouge(Labex GR‐Ex), PRES Sorbonne, Paris, France, lyon, Rhone‐Alpes, France; 6 Laboratoire d'Hématologie, Groupement Hospitalier Est, Hospices Civils de Lyon, Lyon,France, Lyon, Rhone‐Alpes, France; 7 Laboratoire d'Hématologie, Groupement Hospitalier Est, Hospices Civils de Lyon, Lyon,France, LYON, Rhone‐Alpes, France; 8 Service de Médecine Interne, Hôpital Edouard Herriot, Hospices Civils de Lyon, Lyon, France; GEMMAT, Groupe d'Etude Multidisciplinaire en Maladies Thrombotiques, Lyon, France, Lyon, Rhone‐Alpes, France; 9 Laboratoire Interuniversitaire de Biologie de la Motricité (LIBM) EA7424, Team VascularBiology and Red Blood Cell, Université Claude Bernard Lyon 1, Villeurbanne, France ; Laboratoire d'Excellence du Globule Rouge (Labex GR‐Ex), PRES Sorbonne, Paris, France ; Service de Biochimie et Biologie Moléculaire, Centre de Biologie et de Pathologie Est, Hospices Civils de Lyon, Lyon, France, Lyon, Rhone‐Alpes, France, 10 Institut d'Hématologique et d'Oncologique Pédiatrique, Hospices Civils de Lyon, Lyon, France, Lyon, Rhone‐Alpes, France, 11 Service de Médecine Interne, Hôpital Edouard Herriot, Hospices Civils de Lyon, Lyon, France, Lyon, Rhone‐Alpes, France, 12 GEMMAT, Groupe d'Etude Multidisciplinaire en Maladies Thrombotiques, Lyon, France4 Service de Médecine Intensive Réanimation, Hôpital Edouard Herriot, Lyon, France ; Unité d'Hémostase Clinique Hôpital Cardiologique Louis Pradel, Lyon, France ; UR4609 Hémostase & Thrombose, Université Claude Bernard Lyon 1, Lyon, LYON, Rhone‐Alpes, France
Background: Endothelial injury, hypercoagulability, and decreased blood flow are suspected to play a key role in thrombosis,multiorgan failure, and death of patients with COVID‐19.The link between increased blood viscosity and COVID‐19‐related complications and severity is not formerly established and can only be suspected.
Aims: The aim of this study was to (1) analyze blood viscosity, red blood cell (RBC) deformability, and aggregation in hospitalized patients with Coronavirus disease 19 (COVID‐19); (2) test the associations between impaired blood rheology and blood coagulation; and (3) test the associations between impaired blood rheology and several indicators of clinical severity.
Methods: A total of 172 patients with COVID‐19, hospitalized in COVID‐unit of the Internal Medicine Department (Lyon, France) participated in this study between January and May 2021. Clinical parameters were collected for each patient. Routine hematological/biochemical parameters, blood viscosity, RBC deformability and aggregation, and RBC senescence markers were measured on the first day of hospitalization. A control group of 38 healthy individuals was constituted to compare the blood rheological and RBC profile. Rotational thromboelastography was performed in 76 patients to study clot formation dynamics.
Results: Our study demonstrated that patients with COVID‐19 had increased blood viscosity despite lower hematocrit than healthy individuals, as well as increased RBC aggregation. In‐vitro experiments demonstrated a strong contribution of plasma fibrinogen in this RBC hyper‐aggregation. RBC aggregation correlated positively with clot firmness, negatively with clot formation time, and positively with the length of hospitalization. Patients with oxygen supplementation had higher RBC aggregation and blood viscosity than those without, and patients with pulmonary lesions had higher RBC aggregation and enhanced coagulation than those without.
Conclusion(s): This study is the first to demonstrate blood hyper‐viscosity and RBC hyper‐aggregation in a large cohort of patients with COVID‐19 and describe associations with enhanced coagulation and clinical outcomes.
PB0052
Assessing endothelial activation and risk of bleeding in patients receiving VV‐ECMO supportive therapy for COVID‐19 using non‐standard laboratory assays
T. Edwards 1; D. White1; S. Cox‐Morton1; E. Duff1; M. Sharp1; C. Bridgeman1; M. Kirk1; C. Moore1; M. Besser1; E. Symington1; M. Robinson1; W. Thomas1; S. MacDonald2
1 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 2 Cambridge University Hospitals NHS Foundation trusT, Cambridge, England, United Kingdom
Background: Critically ill patients infected with the SARS‐CoV‐2 virus are known to have a coagulopathy with a risk of thrombosis due to endothelial activation and systemic inflammation. Veno‐venous extracorporeal membrane oxygenation (VV ECMO) is recommended by the World Health Organisation (WHO) as a supportive therapy for patients with severe COVID‐19 infection when conventional ICU methods have proven ineffective. VV ECMO comes with haematological complications, including loss of high molecular weight von Willebrand factor multimers, consumption of clotting factors and premature activation of platelets. Laboratory methods to characterise haemostasis in these patients are required and may be clinically useful in predicting clinical outcome.
Aims: Can non‐standard methods provide clinically meaningful results in COVID‐19 positive patients supported by VV ECMO?
Methods: Tissue plasminogen activator and von Willebrand factor were quantified via Abcam SimpleStep ELISA in VV ECMO supported Covid‐19 patients and normal controls. Fibrinogen antigen concentration was quantified via Liaphen Fibrinogen Antigen assay in VV ECMO supported Covid‐19 patients and normal controls. VW Select ristocetin cofactor assay was used to assess von Willebrand Factor activity in VV ECMO supported COVID‐19 patients.
Results: Tissue plasminogen activator and von Willebrand factor concentrations are significantly increased in COVID‐19 patients supported by VV ECMO compared to healthy controls, which reflects endothelial damage displayed in critically unwell COVID patients. Fibrinogen levels were not significantly different between the two patient groups. The VW Select ristocetin cofactor assay detected patients with low vWF activity that would have otherwise been overlooked by standard methods.
Conclusion(s): Non‐conventional laboratory methods can be used to represent the extent of endothelial damage and risk of bleeding in patients who are COVID‐19 positive and anticoagulated on VV ECMO support. The assays help to characterise pathology of COVID‐19 used in conjunction with standard tests by providing clinically relevant results.


VPB0113
Evaluation of intravascular coagulation in patients with COVID‐19 pneumonia
B. Driss 1; M. Elhorri2; M. Moueden3
1 Faculty of Medicin University Oran 1 Algeria, Mostaganem, Mostaganem, Algeria; 2 HMRUO, Oran, Oran, Algeria; 3 University Hospital Center of Oran, Oran, Oran, Algeria
Background: Several studies reported the frequent occurrence of Disseminated Intravascular Coagulation (DIC) in COVID‐19 patients with acute respiratory distress syndrome (ARDS). The diagnosis of DIC by the validated ISTH DIC score, might be useful as readily assessable prognostic parameter.
Aims: The aim of our study was to explore DIC occurs and analyze the coagulation parameters of patients with COVID‐19 pneumonia admitted to our hospital.
Methods: A retrospective analysis of hospital‐based records of 62 COVID‐19 patients with ARDS. Data including D‐dimer, routine investigations (platelet count, prolonged prothrombin time, D‐dimer, fibrinogen and NLR ratio). DIC scorings within three days of admission were collected. The study was conducted in a tertiary care center catering to population of west Algeria.
Results: A total of 62 hospitalized patients with COVID‐19 ARDS were recruited in our study. Mean age of the subjects were 60.46 (29‐ 91) years, and 69.5% of the total patients were male. The coagulation parameters assessment demonstrated abnormal mean of PT (sec) 14.3 (11.1‐41.6), normal mean platelettes count 256 G/L (34‐768). However, mean D‐dimer level 1185 (150‐11000), fibrinogen level 5.02 g/l (2.1‐8.56) and NLR 7.3 (0.5‐19.9) were particularly elevated. Mean ISTH DIC Score were 1.68 (0‐5), One patients 1.6% withe DIC score 5 and confirmed DIC. 16 patients 25.8% withe DIC score 0. Our studies showed a significant positif correlation betwen NLR, ISTH score DIC and fibrinogen level respectively P < 0. 0001 and p < 0.001.
Conclusion(s): the DIC might not only be a concomitant finding, but even a pathophysiological process contributing to circulatory and organ failure, in COVID‐19 ARDS patients.
VPB0116
Prediction of severe covid, proposition of a severe index
I. Ghachem 1; M. Zouaouy2; M. Kaabar3; A. Bachali4
1 Hematology Laboratory, Taher Maamouri Hospital, Nabeul, Nabeul, Tunisia; 2 Hematology Laboratory Taher Maamouri Hospital, Nabeul, Nabeul, Tunisia; 3 Hematology Laboratory, Tahar Maamouri Hospital, Nabeul, Nabeul, Tunisia; 4 Laboratory Tahar Maamouri Hospital, Nabeul, Nabeul, Tunisia
Background: In a pandemic context with limited intensive care beds, it’s important to prioritize which patients will need to be hospitalized in intensive care.
Aims: We aimed to develop and evaluate a simple but accurate biological based index which is associated with intensive care hospitalization.
Methods: It’s a retrospective study including sex, age, CRP, Natriemia, D‐Dimers, Leukocytes, Polymorphonuclear Neutrophils (PMN), Lymphocytes (Ly), Platelets (Plt) from June to September 2021, in the covid unit and in the intensive care unit in Taher Maamouri Hospital, in Tunisia. Index was calculated as below: (Plt*PMN)/(Ly*age). ROC curves were obtained using SPSS software (IBM®, v22.0).
Results: Four hundreds and four patients were included in our study. Sex ratio was 1.38 and median age was 62 (from 3 to 98). Covid severity was significantly correlated with age, high leukocytes, PMN, Plt counts. Single parameters didn’t show a good area under the curve (AUC). However, for a 56.0 value, our index, which combined the better single parameters, was associated with an AUC of 0.71 (CI95: [0.66‐0.76], OR:4.7, p < 0.001) sensitivity of 65.5% and a specificity of 72.8%. The negative predictive value was 74.1% and the positive predictive value was 64.0%. Youden index was 0.38. Yule’s Q was 0.67 which shows a strong link between severity and our index.
Conclusion(s): Simple parameters may lack of signification, but combining them may improve their performance and may constitute a decision tool to help clinicians identify severe cases based on biological datas.
VPB0120
D‐Dimer levels at admission for predicting in‐hospital mortality in COVID‐19 patients
V. Irawan 1; L. Rotty2; P. Lasut2; C. Hendratta2; H. Haroen2
1 Faculty of Medicine, Sam Ratulangi University / Prof. Dr. R. D. Kandou General Hospital, Indonesia, Manado, Sulawesi Utara, Indonesia; 2 Division of Hematology and Medical Oncology, Department of Internal Medicine, Faculty of Medicine, Sam Ratulangi University / Prof. Dr. R. D. Kandou General Hospital, Indonesia, Manado, Sulawesi Utara, Indonesia
Background: Coronavirus disease 2019 (COVID‐19) has been associated with coagulation abnormalities. In achieving optimal patient management, early and effective markers are needed to predict the patient's clinical outcome, especially in areas with high incidence rates.
Aims: We aimed to determine an optimal cutoff level of NLR for predicting in‐hospital mortality in COVID‐19 patients in Indonesia.
Methods: We conducted a retrospective cohort study. We enrolled adult patients with confirmed RT‐PCR COVID‐19 who were hospitalized in the Prof. dr. R. D. Kandou General Hospital from October 2020 to June 2021. D‐dimer levels at admission and death event were collected and analyzed using ROC curves to determine the optimal cutoff level of D‐dimer to predict in‐hospital mortality.
Results: D‐dimer elevation (≥ 0.50 μg/ml) was obtained in 83.1% among 451 patients hospitalized with COVID‐19. The optimum cutoff D‐dimer level to predict in‐hospital mortality was 3.15 μg /ml with a sensitivity 67.39% and a specificity of 73.54%. There were 157 patients (34.81%) with D‐dimer ≥ 3.15 μg/ml, and 294 patients (65.19%) with D‐dimer < 3.15 μg/ml on admission. Median of D‐dimer level among survivors was 1.5 μg/ml and among non‐survivors was 6.52 μg/ml. Patients with D‐dimer levels ≥ 3.15 μg/ml had a higher incidence of mortality when comparing with those who with D‐dimer levels < 3.15 μg/ml (OR = 5.72, 95% CI (3.488 – 9.386), p = 0.0001).
Conclusion(s): D‐dimer level ≥ 3.15 μg/ml at admission could effectively predict in‐hospital mortality COVID‐19 patients in Indonesia. D‐dimer level could be the early and effective marker for COVID‐19 patients.
VPB0129
Thromboelastography Six: redefining heparin resistance in post COVID syndrome
J. Payne 1; V. Coba2; A. Marrocco2; T. Long2; N. Gupta3; A. Hodari‐Gupta2; I. Lopez‐Plaza2
1 Henry Ford Hospital, Royal Oak, Michigan, United States; 2 Henry Ford Hospital, Detroit, Michigan, United States; 3 Henry Ford, Detroit, Michigan, United States
Background: In the literature there has been minimal discussion of heparin resistance with regards to the post‐COVID syndrome. Since the COVID pandemic began, patients with COVID were suffering complications from VTE that suggested the presence of a hypercoagulable state. Generally this hypercoagulable state is described through the use of thromboelastogram six (TEG6) with a short R time, wide alpha angle and a very large amplitude.
Aims: In this case we were using the TEG6 as a guide for heparin therapy by following the CK‐R time compared to the CKH‐R time (with heparinase). We observed no bleeding or clotting events. While the patient was on ECMO, there were no changes in the delta‐P and the circuit was not replced during his clinical course. Additionally, he required no blood transfusions during this time.
Methods: An observational analysis of TEG6 in COVID‐19 ECMO patient while using an anti‐Xa heparin based protocol for therapeutic heparin therapy.
Results: On the initial TEG6, there was a significantly prolonged R time that was partially corrected with heparinase – with heparin dosing of 24 IU/kg/h (65,000 IU/day), see figure one. With the prolonged R time, heparin was descalated to 16 IU/kg/h and the coagulation profile was resent, see figure two. Additionally, the TEG6 MA remained elevated – consistent with the hypercoagulable post‐COVID syndrome. This occurred in the setting of a normal anti‐thrombin three level and platelet counts.
Conclusion(s): Through the use of TEG6, we were able to better characterize his coagulation profile and we were able to deescalate his dosing of heparin using the CK and CKH‐R time comparison. We propose using TEG6 to redefine heparin resistance in the post‐COVID syndrome of hypercoagulopathy, as older modalities may not be accurately reflecting this modern pathology.


VPB0131
Exogenous estrogen use for contraception and hormone replacement therapy and the risk of thrombosis in women with COVID‐19
J. Sabile 1; C. LaVasseur2; H. McMurry1; T. Kartika2; J. Shatzel3
1 Oregon Health and Science University, Portland, Oregon, United States; 2 Oregon Health & Sciences University, Portland, Oregon, United States; 3 Oregon Health & Science University, Portland, Oregon, United States
Background: The thrombotic risk associated with SARS‐CoV‐2 (COVID‐19) is well documented, although there is limited information on how additional prothrombotic risk factors impact persons who contract COVID‐19. Certain estrogen containing contraceptives and hormone replacement therapies are also well known to increase the risk of thrombosis. It is unknown if the risks of these agents and concurrent COVID‐19 infection are additive.
Aims: To investigate the relationship between COVID‐19 infection and concurrent exogenous estrogen on the rate of thrombotic events.
Methods: A retrospective analysis including all adult female patients diagnosed with COVID‐19 between January 2020‐2022 at our center also treated with hormone therapy. Female patients on hormone replacement therapy (HRT) for post‐menopausal symptoms were included in this analysis. HRT was defined as any treatment consisting of an estrogen analog with or without a progesterone. Thrombotic events were defined as any venous thromboembolism (VTE), cerebrovascular accident (CVA), myocardia infarction (MI), or any arterial clot/embolic event. Events were included if they were recorded as current SNOMED/ICD codes in the same encounter as the diagnosis of COVID‐19. Inpatients and outpatients were included in the analysis.
Results: 92 patients were identified who were diagnosed with COVID‐19 while on hormone therapy at the time of diagnosis. 18 of the patients were hospitalized. Mean age was 42 years and 59 years for all patients and hospitalized patients, respectively. The most common estrogen therapies were norethindrone‐estradiol (38%) and conjugated estrogen (14.1%). There were 3 (3.2%) total thrombotic events which all occurred in hospitalized patients. 2 patients experienced VTE while 1 patient experienced a cerebrovascular event with arterial clot.
Conclusion(s): This retrospective study demonstrates a low rate of thrombosis in patients treated with exogenous estrogen and with COVID‐19. The rate of thrombosis in the outpatient setting was low while thrombotic events occurred in hospitalized older patients. Further studies are warranted to explore this association.
VPB0124
Thrombocytopenia in COVID‐19 patients on ECMO
C. Jones 1; K. Chen1; V. Narendran2
1 University of Miami / Palm Beach Regional GME Consortium, West palm beach, Florida, United States; 2 JFK Medical Center, Atlantis, Florida, United States
Background: Thrombocytopenia is a common complication of COVID‐19 (coronavirus disease 2019). The possible mechanisms include decreased platelet production, increased platelet destruction, and consumption. Thrombocytopenia indicates a poor prognosis in COVID‐19 patients. Thrombocytopenia is often complicated in ECMO (extracorporeal membrane oxygenation) due to shearing force in the circuit and heparin‐induced thrombocytopenia (HIT). COVID‐19 patients on ECMO are at high risk of developing thrombocytopenia and bleeding.
Aims: The balance between bleeding prevention secondary to thrombocytopenia and thromboembolic prophylaxis is challenging in managing COVID‐19 patients. ECMO increases the risk of thrombocytopenia. Our study reports the incidences of thrombocytopenia and bleeding in COVID‐19 patients on ECMO.
Methods: We retrospectively reviewed the data of twenty‐three COVID‐19 patients on ECMO. Thrombocytopenia is defined by platelet levels lower than 150,000/uL. Incidences of thrombocytopenia and complications were recorded and analyzed.
Results: Twenty‐three COVID‐19 patients received ECMO. The mean age was 45‐year‐old. Eleven patients had at least one of the following pre‐ECMO comorbidity: ten patients had hypertension, eleven patients had diabetes and four patients had hyperlipidemia. None of the patients were active smokers or had chronic lung disease. Anticoagulation was initiated on the day of admission. Twenty‐one patients developed thrombocytopenia and HIT was excluded. Eighteen patients had hemorrhage requiring transfusion, with the gastrointestinal tract being the most common site. Thromboembolic prophylaxis was held for active bleeding or platelet count below 30,000/uL. The overall mortality rate was 69.6%.
Conclusion(s): In our study of ECMO‐managed COVID‐19 patients, 91.3% of patients developed thrombocytopenia and 78.3% of patients developed hemorrhage requiring transfusion. Anticoagulation is recommended to all hospitalized COVID‐19 patients unless there are contraindications due to the high risk of thromboembolism. However, anticoagulation further increases the risk of bleeding, which may lead to sudden deterioration and death. Further investigation into the mechanisms, implications, and management of thrombocytopenia will lead to significantly improved outcomes for COVID‐19 patients on ECMO.


PB0034
Heparin induced thrombocytopenia before and during COVID‐19 pandemia in Iran
M. Ahmadinejad1; M. Shahbazi1; A. Teimourpour1; M. Mojtabavi Naeini2; B. Asadi1; A. Rajabi3; M. Javan1; P. Eshghi
1 Blood Transfusion Research Center, High Institute For Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 2 Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 3 Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran
Background: The patients with COVID‐19 disease are at high risk for thrombosis. More aggressive thromboprophylaxis anticoagulation are considered in these patients. Several reports indicates a higher HIT incidence in the patients with COVID‐19 than other medical patients,
Aims: We conducted this study to evaluate the frequency of HIT among patients with COVID‐19 in comparison with non‐COVID‐19 patients.
Methods: The study population included patients who referred to the Iranian blood transfusion organization (IBTO) reference coagulation laboratory for anti‐PF4/H Ab testing (LFIA method, STic EXPERT, STAGO) during a 30 months period before and after the COVID‐19 pandemic in Iran. On 19 February 2020, Iran reported its first confirmed cases of infections in Qom. The patients have been divided into three groups: group1, Sep 2019‐Feb 2020 (before COVID‐19 pandemic in Iran), group 2: March 2020‐Aug 2020 (the first and second peaks), group 3: Sep2020‐ Feb 2021 (the third and fourth peaks). The demographic data; 4Ts score, Anti PF4‐Ab (LIFA), and HIPA test results were evaluated. The HIPA test was checked in cases with the positive result of LFIA test.
Results: A total number of 110 patients have been referred for anti‐PF4 Ab testing to our laboratory center during a 30 months period with the detail in table 1and 2. The rate of positive LFIA was 10 % and 33% in the non‐Covid‐19 and Covid‐19 patients respectively (OR = 4.1%, 95% CI, P‐value: 0.022). HIPA test was assessed in 4 cases of Covid‐19 and all were positive (definitive HIT).
Conclusion(s): Among 110 referral cases in 30 months period who were referred for Anti PF4/H Ab testing to our lab, 94 ( 85%) of cases were referred during the first year of the Covid‐19 pandemic in Iran. Regarding the rate of definite HIT (≥16%) among Covid‐19 patients, our data confirm the high rate of HIT in this group of patients.


PB0062
Rotational thromboelastometry in heparin monitoring in critical COVID‐19 disease
L. Schulting1; A. Hulshof 2; M. Mulder3; J. Sels3; H. ten Cate4; I. van der Horst5; B. van Bussel6; Y. Henskens7
1 ICU, Maastricht University Medical Centre +, Maastricht, Limburg, Netherlands; 2 Maastricht University Medical Centre +, The Nerherlands, Maastricht, Limburg, Netherlands; 3 ICU, Maastricht University Medical Centre +, The Netherlands, Maastricht, Limburg, Netherlands; 4 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 5 ICU, Maastricht University Medical Centre +,Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands., Maastricht, Limburg, Netherlands; 6 ICU and Care and Public Health Research Institute, Maastricht University Medical Centre+, Maastricht, the Netherlands., Maastricht, Limburg, Netherlands; 7 Central Diagnostic Laboratory Maastricht University Medical Centre +, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands., Maastricht, Limburg, Netherlands
Background: Critically‐ill COVID‐19 patients on UFH present with heparin resistance (defined as >35.000 IU/day) to reach target aPTT or aXa. APTT is the conventional method as aXa is time consuming and not widely available. APTT or aXa relate differently to doses of UFH, which questions their validity in COVID‐19 disease. Whole‐blood rotational thromboelastometry (ROTEM) INTEM/HEPTEM clotting time (CT) ratio could be a more functional alternative.
Aims: Observational study comparing ROTEM INTEM/HEPTEM CT ratio, aPTT and anti‐Xa in mechanically ventilated COVID‐19 patients on UFH/LMWH.
Methods: We studied a subcohort (n = 106/232) of mechanically ventilated COVID‐19 patients of the MaastrICCht cohort. We measured ROTEM, aPTT and aXa on UFH (n = 18) or therapeutic LMWH ( n = 88). Patients received UFH (ECMO/renal replacement therapy) or therapeutic LMWH (venous thromboembolism/cardiac arrhythmia). ROC and Linear regression analyses were assessed for diagnostic characteristics and associations.
Results: INTEM/HEPTEM CT ratio was significantly higher in UFH‐patients (1.4 [1.3‐1.4]) compared to LMWH‐patients (1.0 [1.0‐1.1], p < 0.001) (Table 1). Furthermore, ROC analysis showed an AUC of 0.935 for INTEM/HEPTEM CT ratio, 0.775 for aPTT and 0.632 for aXa, suggesting that INTEM/HEPTEM CT ratio detects presence of UFH more accurately than aPTT and aXa. At an INTEM/HEPTEM CT cut‐off of 1.1, sensitivity was 87.5% and specificity was 86.3% for the detection of UFH. Both APTT and aXa were positively associated with INTEM/HEPTEM CT ratio (β(95%CI) 0.006(0.004‐0.008) and 0.153 (0.071‐0.234) respectively, Table 2).Only, the association between aXa and INTEM/HEPTEM CT ratio was significantly stronger (p = 0.006) in UFH‐patients (β(95%CI) 0.310 (0.001‐0.619), compared to LMWH‐patients (β(95%CI) 0.094 (0.053‐0.135).
Conclusion(s): ROTEM INTEM/HEPTEM CT ratio can detect the presence of UFH opposed to the presence of therapeutic LMWH in mechanically ventilated COVID‐19 patients. INTEM/HEPTEM CT ratio was dependent on aPTT, irrespective of UFH or LMWH use. The association between aXa and INTEM/HEPTEM CT ratio was significantly stronger in patients on UFH than LMWH.
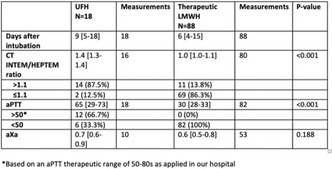

PB0092
Impact of COVID‐19 pandemic on pulmonary embolism incidence and characteristics: a 24 months perspective
P. Sterpone 1; M. Meroni2; W. Ageno3; M. Donadini4
1 Internal Medicine Residency Program, School of Medicine, University of Insubria, Varese, Magenta, Lombardia, Italy; 2 School of Medicine, University of Insubria, Varese, VARESE, Lombardia, Italy; 3 Department of Medicine and Surgery, Research Center on Thromboembolic Disorders and Antithrombotic Therapies, University of Insubria, Varese, Italy., VARESE, Lombardia, Italy; 4 Research Center on Thromboembolic Disorders and Antithrombotic Therapies, University of Insubria, Varese, VARESE, Lombardia, Italy
Background: Pulmonary embolism (PE) is the third most common cause of cardiovascular death with a case fatality rate of 10% at 30 days. COVID‐19 is associated with systemic inflammation and abnormal coagulation profile leading to an increased thromboembolic risk. Reported incidence of PE among COVID‐19 patients is highly variable and mainly short‐term studies are present in literature.
Aims: Evaluatethe impact of COVID‐19 on incidence and clinical, laboratoristic and radiological characteristics of PE over a 24‐month period
Methods: We conducted an observational cross‐sectional study on all patients diagnosed with PE in one of the seven hospitals of the Azienda Socio Sanitaria Territoriale Sette Laghi, during a 24 month period, going from 12 months before (pre‐COVID period) up to 12 months after (COVID period) February 21st 2020, day of first diagnosed italian case of COVID‐19. Data were collected on clinical, laboratoristic and radiological characteristics and Non‐COVID‐19 patients were compared to COVID‐19 patients.
Results: 489 patients with acute PE were identified: 217 in the pre‐COVID period and 272 in the COVID period of whom 50 patients had COVID‐19. The prevalence of PE in hospitalized patients with COVID‐19 was 1.1% (50/4568). In patients with PE and COVID‐19, male sex was more common (72% vs 42%;p‐value < 0.01), D‐Dimer values were significantly higher (> 9000 mcg/L in 50% of them vs 28% among non‐COVID patients; p‐value: 0.002), median Troponin T level was lower (32 ng/ml vs 14.5 ng/ml,; p‐value: 0.02), distal obstruction was more common (68.4% vs 48% p‐value: 0.03).
Conclusion(s): Incidence of PE has significantly increased after the onset of pandemic (55 more cases; 20%). Male sex appears to be a risk factor for PE in COVID‐19 patients. Despite the high procoagulant profile of COVID‐19 patients, our study found a predominantly distal distribution of PE and events appear to have lower severity indicators as compared to non‐COVID‐19 patients.

VPB0112
Incidence of thrombotic events in a population affected by Covid‐19 in a general hospital in Veracruz, Mexico
L. Del Carpio‐Orantes 1; S. García‐Méndez2; J. Sánchez‐Díaz3; A. Rosas‐Lozano3
1 Instituto Mexicano del Seguro Social, Veracruz, Veracruz‐Llave, Mexico; 2 Hospital Regional de Alta Especialidad de Oaxaca, veracruz, Veracruz‐Llave, Mexico; 3 Instituto Mexicano del Seguro Social, veracruz, Veracruz‐Llave, Mexico
Background: One of the main complications of Covid‐19 are the thrombotic events reported with different incidences in the different case series
Aims: To identify the incidence of thrombotic events in patients affected by Covid‐19 in a general hospital in Veracruz, Mexico during April to October 2020.
Methods: Descriptive and retrospective study in which the thrombotic events presented by patients who suffered from Covid‐19 during the period from April to October 2020 are analyzed.
Results: Data from 1,212 patients diagnosed with Covid‐19 are included, 641 (53%) men and 571 (47%) women, with a mean age of 49 years. 663 (54.7%) patients suffered from some chronic disease, systemic arterial hypertension was the most common comorbidity (29.2%), followed by diabetes (29.1%), obesity (13.9%), cardiovascular disease (5.4%), asthma (4.5 %), chronic kidney disease (3.8%) and COPD (2.1%). Six cases with various thrombotic events (50% venous and 50% arterial) were identified, predominantly in women (66%) and with mild Covid‐19 (66%), of which 1 had a poor prognosis (severe Covid). The incidence of thrombotic events was 0.5% in this case series.
Conclusion(s): Thrombotic events, although of low incidence, should always be taken into account during the follow‐up of patients with both mild and severe Covid‐19, since they add greater morbidity and mortality

VPB0115
Use of direct oral anticoagulants (DOACs) in prevention or treatment of Pulmonary Embolism (PE) associated with COVID‐19 pneumonia in ambulatory patients: experience of an Argentine center
S. Molnar; F. Gazzoni; M. Ferrero
Clinica Universitaria Reina Fabiola, Cordoba, Cordoba, Argentina
Background: Thrombotic events are frequent in COVID‐19 patients. The use of DOACs to treatment or extended prophylaxis is not clear in these population.
Aims: To know the prevalence of PE associated with COVID‐19 pneumonia in our population. To describe the use of DOACs for ambulatory prophylaxis after COVID‐19 pneumonia patients. To describe the use of DOACs for ambulatory treatment in PE COVID‐19 patients.
Methods: Retrospective registry of patients admitted for COVID‐19 pneumonia from May 2020 to December 2021. Internists evaluated thrombosis risk after discharge according to IMPROVE score and body mass index (BMI) ≥30 following a local guideline. Exclusion criteria: treatment with other anticoagulants, no follow‐up, death. Statistics: quantitative data through parametric analysis of variance and categorical data according to contingency tables
Results: 345 patients were admitted; 279 were evaluable (Table 1). 37.5% patients received extended prophylaxis (87/232: 54 Apixaban, 33 Rivaroxaban). Mean prophylaxis time 14.27 days (7‐28). There was no consensus among internists regarding prescribing ambulatory prophylaxis. 16.8% of COVID‐19 pneumonias had PE (47/279). 70% were incidental (imaging studies due to underlying disease) and 30% were symptomatic. 38 were on Apixaban, 9 on Rivaroxaban. The mean days on anticoagulation treatment was 92 days (20‐ 80). We had 4 thrombosis after discharge (Table 2). We have not recurrent thrombosis in the PE group. Bleeding events occurred in 3 cases on therapeutic doses. Only one was a major bleeding (hematuria).
Conclusion(s): Our prevalence of PE in COVID‐19 pneumonia was 16.8%. With respect to DOACs treatment we have not bleeding at prophylaxis doses and one mayor bleeding at therapeutic doses. We have four thrombosis after discharge. No recurrent thrombosis was seen in PE patients. The use of DOACs in extended prophylaxis or treatment after COVID‐19 pneumonia seems to be safe, with no differences with the non‐COVID‐19 population.


VPB0126
Assessing research collaboration networks and social media use among venous thromboembolism researchers during the COVID‐19 pandemic
D. Karsanji 1; J. King2; J. Godley2; D. Siegal3; T. Chan4; G. Le Gal5; M. Carrier6; S. Kahn7; M. Rodger8; N. Langlois9; C. MacGillivray10; A. Garven2; L. Skeith2
1 University of Manitoba, Calgary, Alberta, Canada; 2 University of Calgary, Calgary, Alberta, Canada; 3 University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 4 McMaster University, Hamilton, Ontario, Canada; 5 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada; 6 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 7 Division of Internal Medicine, Department of Medicine, McGill University, Montreal, Québec, Canada, Montreal, Quebec, Canada; 8 Department of Medicine, McGill University, McGill University Health Center, Montreal, Quebec, Canada, Montreal, Quebec, Canada; 9 Ottawa Hospital Research Institute, Ottawa, Ontario, Canada, 10 CanVECTOR Network, Ottawa, Ontario, Canada
Background: The ways in which research collaborations are formed and strengthened have evolved during the COVID‐19 pandemic due to restrictions limiting in‐person meetings. Given the need to rapidly adapt to online communication, and to accelerate COVID‐19 venous thromboembolism (VTE) research, social media has played an important role in all aspects of these interactions.
Aims: (1) Assess the size and geographic breadth of VTE researchers’ project collaborations before and during the early stages of the COVID‐19 pandemic; (2) Characterize how social media platforms are used by VTE researchers.
Methods: An online survey about research collaborations and social media use was distributed in June 2020 to VTE researchers via Twitter, CanVECTOR (n = 59) and INVENT (n = 389) research network websites and email lists. Research collaboration data were analyzed using ego‐centred social network analytic techniques to assess the size and composition of researchers’ VTE‐ and COVID‐related collaboration networks.
Results: Over half of respondents (23/45, 51%) reported leading at least one collaborative VTE research project in the past 2 years, with 16 (36%) currently leading COVID‐related VTE research. Eighteen (78%) respondents who led VTE research projects also contributed as a collaborator to VTE research projects over the past 2 years, with 17 (74%) contributing to COVID‐related VTE research. Research in the VTE field is inter‐institutional and international, but early COVID‐related collaborations tended to be more local (Table 1). Social media platforms were used primarily by VTE researchers to collect and disseminate COVID‐19 VTE research information.
Conclusion(s): Research in the VTE field is inter‐institutional and international, but early COVID‐related VTE research collaborations tended to be more local. Social media platforms may be useful in strengthening international collaborations between VTE researchers with similar interests.

VPB0130
Alterations in the coagulation profile and mortality due to covid‐19 during the first and second wave in peruvians: A retrospective cohort in a national reference center
R. Pichardo‐Rodriguez 1; W. Peña‐Oscuvilca2; L. cordova‐Cueva3; J. de la Cruz‐Vargas4; O. Ruiz‐Franco2; F. Garmendia‐Lorena2
1 Ricardo Palma University, Lima‐Peru., Lima, Lima, Peru; 2 Clinical Research Institute “Fausto Garmendia Lorena". National University "Mayor de San Marcos", Lima‐Peru., Lima, Lima, Peru; 3 Nephrology Service. National Hospital "Dos de Mayo", Lima‐Peru, Lima, Lima, Peru; 4 Research Institute in Biomedical Sciences (INICIB). Ricardo Palma University, Lima‐Peru., Lima, Lima, Peru
Background: Changes in the coagulation profile have been associated with adverse clinical outcomes in patients with COVID‐19. It is relevant to evaluate the changes in the coagulation profile and its impact on mortality from COVID‐19 during the first and second waves in Peru.
Aims: To evaluate the alterations and impact of the coagulation profile on mortality from COVID‐19 during the first and second waves in Peruvians.
Methods: Retrospective cohort carried out at the "Dos de Mayo" National Hospital, Lima‐Peru during the years 2020 and 2021. Peruvians over 18 years of age were included, excluding those with congenital hemostasis disorders, pneumonia caused by other agents and chronic users of anticoagulants. SARS‐COV2 pneumonia was defined as infiltrate in lung TEM + positive RT‐PCR. Alterations in coagulation parameters were determined by prolonged prothrombin time (PT>14s) and/or prolonged activated thromboplastin time (APTT>35s) and/or fibrinogen < 200 mg/dl and/or fibrinogen >400 mg/dl. dl. The sample was consecutive, reaching a sample of 300 patients from the first and 300 from the second wave. Data was collected and entered into a database and sent for analysis after quality control. Were presented summary measures for the qualitative and quantitative variables. A logistic regression equation was modeled to estimate the adjusted OR of the different coagulation parameters altered. The data were processed RStudio version 1.3.1093.
Results: A total of 600 patients were included. Male was the most frequent sex during the two periods. Figure 1 shows the baseline characteristics. Fibrinogen < 200 mg/dl during the first wave was associated with a lower risk of death (OR: 0.07; 95%‐CI: 0.01, 0.90). Table 2 shows the results of the multivariate analysis.
Conclusion(s): Apparently the changes in the coagulation profile caused by COVID‐19 in Peruvians during the first and second waves were not associated with higher mortality. Low fibrinogen seemed to offer protection during the first wave.


VPB0134
Blood coagulation parameters in hospitalized patients with COVID‐19 – data from Regional Center for transfusion medicine Shtip
M. Shorova 1; R. Grubovik‐Rastvorcev2; E. Petkovic3; D. Stambolieva4; N. Jovanova5
1 Institut for transfusion medicine of Republic of North Macedonia, Stip, Stip, Macedonia; 2 Institut for transfusion medicine Skopje, Skopje, Skopje, Macedonia; 3 Institute for transfusion medicine, Skopje, Skopje, Skopje, Macedonia; 4 Institute of transfusion medicine Skopje, Department Strumica, Strumica, Strumica, Macedonia; 5 Regioanl Center for transfusion medicine, stip, Stip, Macedonia
Background: Infection with COVID‐19 results in systemic inflammatory response and imbalance between homeostatic mechanisms of procoagulant and anticoagulant. Around 40% of hospitalized patients with COVID‐19 are at a high risk of development of venous thromboembolism. The coagulation tests can actually give us an indication of the severity of disease in any particular patient. Monitoring them is important as it can help shape care and determine the direction the patient is going.
Aims: The aim of our study was to analyze the characteristic of coagulation function of patients with COVID‐19, as important predictors of mortality.
Methods: We studied 726 hospitalized patients with confirmed COVID‐19 admitted to the Clinical Hospital Shtip, from January ‐ December 2021. Blood coagulation parameters included the measure of the PT,aPTT, INR, platelet count, fibrinogen and D‐dimer.Coagulation profile was done on CA‐600 (Sysmex), and the total platelet count was measured with Medonic‐M51.
Results: There were 510 abnormalities (70.2%) of coagulation parameters in 726 patients admitted to hospital. 48.9% were men, and 19.8% were healthcare workers. The median age was 62 years. 3.4% (17 patients), had a prolonged prothrombin time, >16 sec. 10.3% had a platelet count < 100 ×109/L and 3 patients (0.07%) had a platelet count < 50 ×109/L during their hospital course.Fibrinogen is generally normal or elevated(> 5g/l) in critical patients. D‐dimer levels were 69.4% of patients above the normal range (0–500 ng/ ml), average 1900 ng/ ml.
Conclusion(s): Coagulation abnormalities (including increased D‐dimer, elevated fibrinogen, and increasing prothrombin time) are typical findings in patients with COVID‐19 and are associated with poorer prognoses and survival. We found this analysis helpful not only to monitor for severity of disease, but also to potentially screen patients for VTE.We suggests that testing coagulation parameters may allow physicians to identify and treat patients with COVID‐19 more effectively to prevent complications.
VPB0141
A near real‐time surveillance algorithm to identify in‐hospital VITT cases
S. Williams; S. Sivapalaratnam; S. Vohra; P. Johnstone; A. Pentony; C. Gutteridge
Barts Health NHS Trust, London, England, United Kingdom
Background: In spring 2021, various groups described Vaccine‐induced immune thrombotic thrombocytopenia (VITT) a rare thrombotic syndrome associated with COVID‐19 vaccine ChAdOx1‐S (incidence 1:100,000 exposures). In the UK, reporting at a national level depended on specialists reporting cases through a variety of mechanisms, as national datafeeds were insufficiently granular to identify the VITT phenotype. Real‐world data analytics of EHRs to identify vaccine complications could strengthen national reporting.
Aims: We developed an algorithm to define VITT using a regional hospital and primary care data system. Secondary aims included a reporting dashboard for specialists and generalisable methods for identifying rare events in admitted inpatients.
Methods: A linked primary‐secondary care, near real‐time datafeed of patients admitted to Barts Health NHS Trust covering a 2.2 M population. An algorithm was developed to identify clinical features of VITT and categorise patients by likelihood of VITT, using SNOMED‐coded admission, vaccine and laboratory data (Table 1). Clinical validation of the algorithm’s output was completed.
Results: The algorithm identified 698 cases in routine data within Barts Health [definite (n = 1) probable (n = 10) and possible (n = 687)]. 91 patients were validated. The algorithm had low precision for defining separate VITT categories (9.1% ‘definite and probable’ and 32% ‘definite, probable and possible’) but identified the one definite case of VITT. Inter‐validator agreement was moderate (Randolph’s kappa 0.45). All cases identified clinically and included in the full dataset were also found by the algorithm (high recall). Dashboard design for care teams to flag future VITT cases for hospital in‐patients will be presented.
Conclusion(s): We have successfully demonstrated identification of thrombotic complications from COVID‐19 vaccines in EHR data. Alternative diagnosis trends could improve precision of the algorithm. Barriers to progress are lack of pathology data standards and complex approvals for data sharing. This study was supported by HDR‐UK.

VPB0106
Platelet parameters in patients with COVID‐19 and ST‐elevation myocardial infarction
N. Izmozherova; A. Popov; I. Antropova; A. Tsvetkov; L. Kadnikov; V. Ispavsky
Urals State Medical University, Ekaterinburg, Sverdlovsk, Russia
Background: COVID‐19 can affect the platelets function. A vital feature of the impact is the tendency to excessive thrombosis against the background of platelet hyperactivation.
Aims: The aim was determine the effect of COVID‐19 on platelet parameters in ST‐elevation myocardial infarction (STEMI).
Methods: This retrospective case‐control study included 66 patients (33 pairs) matched in terms of gender, age, and STEMI (Table 1). In each pair one of these patients was hospitalized with COVID‐19, and the other was hospitalized without COVID‐19, according to routing to different hospitals. The Mindray BC‐5150 automatic hematology analyzer was used to assess blood parameters. Erythrocyte sedimentation rate (ESR) analysis was performed by the Panchenkov method. «Statistica» data analysis package was used for statistical processing. Signed Informed Voluntary Consent form was obtained from all patients. The study was approved by the Local Ethics Committee of the Ural State Medical University. “Statistica” data analysis package was used for statistical processing.
Results: By the time of admission (see Table 2), white blood cells (WBC) count was lower in the group with STEMI and COVID‐19 compared with group without COVID‐19; mean platelet volume (MPV), platelet crit (PCT), ESR were higher in patients with COVID‐19. One week after, differences in MPV between the groups still persisted. In patients with STEMI and COVID‐19, there was a positive correlation between platelet count and WBC count (Spearman's ρ=0.42; p < 0.05) and a positive correlation between platelet count and ESR (Spearman's ρ=0.41; p < 0.05) at the time of admission. In patients without COVID‐19, these correlations were not found.
Conclusion(s): Patients with STEMI and COVID‐19 have higher MPV than patients without COVID‐19 at hospital admission and one week after. In patients with STEMI and COVID‐19, platelet count is positively correlated with WBC count and ESR; it may indicate an association between platelet count and inflammation in patients with COVID‐19 and STEMI.


PB0064
Lupus anticoagulants as an independent predictor for disease severity in COVID‐19 patients
H. Kim1; D. Chu2; S. Jang 2
1 Seoul National University Bundang Hospital, SeoulSeongnam, Kyonggi‐do, Republic of Korea; 2 Asan medical center, Seoul, Seoul‐t'ukpyolsi, Republic of Korea
Background: Lupus anticoagulants (LA) that appear in antiphospholipid syndrome, known as causes of thrombophilia, are commonly detected during various viral infection. As previously reported, LA is frequently detected in COVID‐19 patients who often exhibited thrombosis. However, clinical significance of LA remains unclear, and there are no accurate reports of LA detection patterns.
Aims: In this study, we performed to analyze clinical significance and detection pattern of LA in COVID‐19 patients.
Methods: We performed retrospective chart analysis of COVID‐19 patients who underwent LA test at Asan Medical Center from March 2020 to November 2021. We compared laboratory data and disease severity parameters, such as oxygen treatment, between LA‐negative and LA‐positive groups who detected LA at least once after infection. For LA‐positive patients who underwent multiple LA tests, subgroup analysis was performed to determine detection pattern of LA.
Results: A total of 219 COVID‐19 patients were enrolled, 148(67.6%) were LA‐positive. LA‐positive group more received high flow nasal cannula (p = 0.024). The LA‐positive group showed prolonged aPTT, high levels of fibrinogen and CRP (all p’s < 0.05). In a subgroup analysis, 127(86.5%) detected LA within 10days of infection and 87(58.8%) were detected LA within 5 days of infection. Among LA‐positive patients, 100 were confirmed negative by follow‐up test. All 87 patients showed LA‐negative conversion within 12 weeks, with exception of 13 patients who underwent follow‐up after 12 weeks. The median time to negative conversion was 10 days.
Conclusion(s): LA was found in high proportion of COVID‐19 patients. LA‐positive patients showed higher oxygen demand and higher level of inflammatory parameters. LA is expected to be a predictive indicator of disease severity in COVID‐19 patients. LA appears in early stages of infection and disappears rapidly within 10 days in half of cases. Therefore, it is recommended to perform LA test when confirmed COVID‐19 infection to predict the course of the disease.


PB0091
ADAMTS13 in the diagnosis of the severity of COVID‐19 mortality
L. Slavík 1; J. Ulehlova1; P. Bradacova2; M. Jancova3; J. Prochazkova1; A. Hlusi1
1 Department of Hematology‐Oncology, Faculty of Medicine and Dentistry, Palacký University Olomouc; University Hospital Olomouc, Olomouc, Olomoucky kraj, Czech Republic; 2 Department of Clinical Hematology, Masaryk Hospital Ústí nad Labem, Usti nad Labem, Ustecky kraj, Czech Republic; 3 Hematology‐Transfusion Department, Institut for Clinical and Experimental Medicine, Praha, Hlavni mesto Praha, Czech Republic
Background: Patients with severe respiratory failure come to the hospital emergency room, where they are triaged to support lung ventilation. Coagulation markers are one of the important methods for triage. The main method used so far is DDIM. Their elevation is due to the activation of coagulation with prothrombogenic potential, which manifests itself mainly in the microcirculation. These processes lead to the excessive release of Von Willebrand factor (VWF) from the Weibel Palade bodies and its cleavage by ADAMTS13, which is thus consumed.
Aims: The aim of our study was to assess the effect of ADAMTS13 levels on the prognosis of patients with acute respiratory failure due to COVID‐19 infection.
Methods: 46 patients coming to the emergency department were examined and admitted to the intensive care unit to support lung function based on clinical symptoms and elevated D‐Dimers. These patients were retrospectively examined for ADAMTS13.
Results: The levels of ADAMTS13 showed a statistically significant difference (P = 0.02) that corresponded to the clinical course of severe respiratory failure due to COVID‐19 infection. Ninghteen patients with a relatively mild course had an ADAMTS13 level of 0.74 IU/ml (0.28 ‐1.14 IU/ml), in contrast to a skip of 27 patients with a severe course, which had an ADAMTS13 level of 0.54 IU/ml (0.25 – 1.01 IU/ml).
Conclusion(s): The levels of D‐Dimer as a commonly used marker in triage of the COVID‐19 severity infection is mainly due to the activation of coagulation and its manifestations, especially in the microcirculation. The level of ADAMTS13 is a key marker in the early prognosis of the severity of COVID‐19 due to pulmonary insufficiency, whereas D‐DIm is only a consequence of this process. Supported by grant LF‐2022‐001 and MH CZ ‐ DRO (FNOl, 00098892)
PB0102
Prevalence of cerebrovascular MRI markers in hospitalized patients with COVID‐19
N. Wijers 1; T. van Lith2; W. Sluis3; F. Meijer4; K. Kamphuis‐van Ulzen5; J. de Bresser6; J. Dankbaar7; F. Klok8; A. Tuladhar9; S. Cannegieter10; M. Wermer11; B. van der Worp3; M. Huisman12; F. de Leeuw9
1 Leiden University Medical Centre department of Neurology, Leiden; The Netherlands, Leiden, Zuid‐Holland, Netherlands; 2 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Centre, Nijmegen, The Netherlands, Nijmegen, Gelderland, Netherlands; 3 Department of Neurology and Neurosurgery, Brain Center, University Medical Center Utrecht, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 4 Department of Medical Imaging, Radboud University Medical Center, Nijmegen, The Netherlands, Utrecht, Utrecht, Netherlands; 5 Department of Medical Imaging, Radboud University Medical Center, Nijmegen, The Netherlands, Nijmegen, Gelderland, Netherlands; 6 Department of Radiology, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 7 Department of Radiology and Nuclear Medicine, University Medical Center, Utrecht University, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands; 8 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 9 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands, 10 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands, 11 Department of Neurology, Leiden University Medical Center Leiden; The Netherlands, Leiden, Zuid‐Holland, Netherlands, 12 Department of Medicine ‐ Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: COVID‐19 is complicated by symptomatic ischemic stroke in 1% to 3% in small selected studies of hospitalized patients.
Aims: We aimed to assess the prevalence of cerebrovascular MRI markers and its causes in large samples of unselected patients with COVID‐19, compared to healthy controls, which is unknown.
Methods: CORONIS is an ongoing observational cohort study in adult hospitalized patients with COVID‐19. Patients without clinically overt stroke undergo standardized questionnaires, cognitive assessment, blood sampling, cardiac echo, telemetry and brain MRI within 90 days after COVID‐19 infection. Controls undergo standardized questionnaires and brain MRI. MRI markers include (acute) ischemic lesions, microbleeds, white matter hyperintensities, and intracranial vessel wall abnormalities and contrast enhancement. Brain MRI will be repeated after 3 months in a subset of patients. Cognitive function and functional outcome are assessed after 3 and 12 months after baseline measurements.
Results: This is an ongoing study with preliminary results. Between April and December 2021, we recruited 88 patients with a median age of 59 years (range 22‐82), of whom 59 (67%) are men and 25 (28%) had been admitted to the ICU. DWI lesions were found in 1 patient (1%), microbleeds in 22 patients (23%) and white matter hyperintensities in 66 (75%). Intracranial vessel wall enhancement was seen in 16%. Control results will follow.
Conclusion(s): The CORONIS study will provide insight into the frequency and risk factors of MRI markers of cerebrovascular lesions and its relation with long‐term functional outcome in hospitalized adult patients with COVID‐19.

VPB0114
BNT162b2 mRNA SARS‐CoV‐2 vaccination does not cause upregulation of endothelial activation markers or hypercoagulability: A prospective, single‐arm, longitudinal study
X. Lim1; B. Leung2; C. Sum3; G. Lim4; C. Chua1; T. Tu5; K. Ramanathan6; M. Huang2; H. Howe1; B. Fan 7
1 Department of Rheumatology, Allergy and Immunology, Tan Tock Seng Hospital, Singapore, Singapore, Not Applicable, Singapore; 2 Health and Social Sciences, Singapore Institute of Technology, Singapore, Singapore, Not Applicable, Singapore; 3 Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore, Singapore, Not Applicable, Singapore; 4 Clinical Research and Innovation Office, Tan Tock Seng Hospital, Singapore, Singapore, Not Applicable, Singapore; 5 Department of Neurology, National Neuroscience Institute, Singapore, Singapore, Not Applicable, Singapore; 6 Department of Cardiac, Thoracic and Vascular Surgery, National University Heart Centre, Singapore, Singapore, Not Applicable, Singapore; 7 Department of Haematology, Tan Tock Seng Hospital, Singapore, Singapore, Singapore
Background: Reports of thrombosis post COVID‐19 mRNA vaccination have sparked concerns about safety.
Aims: We prospectively evaluated blood samples of 18 participants who had received 2 doses of the BNT162b2 mRNA vaccine to determine if vaccination results in endothelial activation or hypercoagulability.
Methods: 18 participants who received the BNT162b2 vaccine were enrolled. Participants completed a questionnaire on their cardiovascular and thrombotic risk factors. Blood samples were collected at three time points: pre‐vaccination (day of vaccination), a median of 17 (IQR 16‐18) days after the first dose and a median of 9 (IQR 7.5–14.5) days after the second dose of BNT162b2 vaccine. Endothelial markers included ICAM‐1, VCAM‐1 and P‐selectin. Coagulation tests included PT and aPTT with clot waveform analysis, von Willebrand factor levels, Factor VIII and D‐dimer levels. Statistical tests of association between endothelial and coagulation parameters were performed with repeated measures ANOVA and Mauchly’s test of sphericity.
Results: The median age of the participants was 35 years (IQR 31 – 44), and 14 (78%) were female. 15 did not have any cardiovascular risk factors. There was a statistically significant increase in median ICAM levels post first (66.1ng/ml) and second dose of vaccination (69.5ng/ml)(p = 0.04), although this remained within the normal limit of ICAM levels. A statistically significant decrease in median PT (p = 0.005) and aPTT (p = 0.03) was observed post vaccination, with a corresponding statistically significant increase in aPTT clot waveform analysis (CWA) for maximum acceleration (max2)(p = 0.03) and maximum deceleration (max2)(p = 0.04) post first and second dose of vaccination. However, all evaluated endothelial and coagulation parameters remain within the reference ranges (Table 1).
Conclusion(s): Our findings provide reassuring preliminary data that BNT162b2 vaccination does not result in endothelial activation or hypercoagulability. Mild variations in endothelial markers and coagulation parameters, though statistically significant, remain within the reference ranges and may be related to an inflammatory immune response to vaccination.

VPB0118
Proper algorithm in donor‐recipient selection, the key to success in convalescent plasma treatment of Covid 19 patients
A. Grdinic 1; D. Vojvodic2
1 Sykehuset Østfold Kalnes, Sarpsborg, Oslo, Norway; 2 Institute for medical research Military medical academy, Belgrade, Vojvodina, Serbia
Background: Convalescent plasma (CP) obtained from patients following recovery from COVID‐19 is an option for treatment, since antibodies may have antiviral and anticoagulation effect.
Aims: The goal of this review is to present the latest evidence in the use of CP for COVID‐19, raise questions regarding donor selection, collection, testing of CP, timing and volume of CP, and offer recommendations for future research.
Methods: ‐Systematic review of all published available literature assessing the use of CP for COVID‐19. ‐Simple scoring was structured (0 min‐10max). Minimal score (O) gives maximal possibility of successfully treatment with CP in COVID‐19 patients and the opposite. This scoring system was applied into chosen 54 published studies.
Results: We analyzed data in 18 published Case reports, 31 Case series, 11 Observational studies, and 5 RTc. According to our score min.score 0 was not obtained in any type of study, either score 1 or 2. Only one observational study has score 3. In this group score vary from 3 to 9.5, but most studies had a high score (6 ‐9). In the group of case reports score was from 4 to 8 (the largest number had a score of 7). In case report studies the score vary from 4 (just one case) to 8. Higher scores were registered in the largest number of case reports also. In the case series that were the most numerous (31) available reports concerning research in CP treatment of COVID 9 patients, the minimum score was 5 (only one series), the max score was 10, and most series had a score of 6 to 9. The number of randomized studies was the lowest, only 5. In this group, the score ranged from 6.5 ‐9.
Conclusion(s): Despite a number of performed studies there is not god enough pretreatment estimation for success of CP treatment in COVID.
VPB0121
Evaluation of clot waveform patterns in Covid‐19 patients
E. Vagdatli; A. Chalvantzis; G. Variti; K. Bani; M. Issa; V. Katsikari
Hippokratio General Hospital, Thessaloniki, Thessaloniki, Greece
Background: Recent evidence has suggested the presence of unique coagulation abnormalities in patients with COVID‐19. Clot waveform analysis (CWA) has not been adequately described as a tool for the evaluation of coagulation.
Aims: To comparatively assess the changes in clot waveform analysis (CWA) parameters between COVID‐19 patients upon hospital admission and healthy individuals.
Methods: In this retrospective observational study, we evaluated 227 CoVID‐19 patients upon their hospital admission, prior to the initiation of anticoagulation therapy relative to 84 healthy individuals. Coagulation tests and CWA were performed on BCS® XP System (SIEMENS). The CWA parameters of PT, aPTT and Fifrinogen (FIB) were the change in Absorbance (dmA) and the time difference between the starting time of the reaction (mixing of reagent and sample) until a specific absorbance change (absorbance threshold). All statistical analyses were performed using SPSS27. Variables were tested with Student’s t tests or Mann–Whitney U tests for differences in distributions of dmA and dsec of PT, aPTT and FIB between two groups. P values < 0.05 were considered statistically significant.
Results: A statistically significant increase in the dmA values of PT, aPTT and FIB was detected in CoVID‐19 patients compared with the healthy individuals (p < 0.001) (fig.1). A statistically significant decrease in CoVID‐19 patients was found only for the dsec values of FIB (p < 0.001) (fig.2).
Conclusion(s): CWA variables upon admission in COVID‐19 patients may be used for the evaluation of their inflammatory response or/and hypercoagulopathy. Our results may help to identify patients at a high risk of thromboembolism.


VPB0123
Clot waveform analysis (CWA) parameters as indicator of Covid‐19 outcome
E. Vagdatli; G. Variti; A. Chalvantzis; V. Katsikari; K. Bani; M. Issa
Hippokratio General Hospital, Thessaloniki, Thessaloniki, Greece
Background: COVID‐19‐related systemic cytokine response induces the production of procoagulant factors, which predisposes patients to a prothrombotic state. CRP, the main acute inflammatory protein, has been related to the disease outcome. CWA is the global hemostatic assessment that evaluates clot formation kinetics during routine clotting tests.
Aims: To investigate the ability of CWA parameters to predict the severity of CoVID‐19.
Methods: In this retrospective observational study, we evaluated 227 CoVID‐19 patients upon hospital admission, prior to the initiation of anticoagulation therapy. CWA were performed on BCSXP System(SIEMENS). The measured CWA parameters of PT, aPTT and Fifrinogen(FIB) were the change in Absorbance(dmA) and the time difference from the starting time of the reaction (mixing of reagent and sample) until a specific absorbance change (absorbance threshold). The patients were divided into 5 groups based on the CRP values:A: < 6mg/l(n = 17), B:6‐25mg/l(n = 51), C:25‐50mg/l(n = 53), D:50‐100mg/dl(n = 43) and E:>100mg/l (n = 58). Variables were tested with Student’s t‐test or Mann–WhitneyU test for differences in distributions of dmA and dsec among five groups. Pearson's correlation coefficient was used for comparison of the above parameters of 227 patients and their CPR values. P values < 0.05 were considered statistically significant.
Results: A gradual increase in the values of dmA was detected from Α to Ε group. Statistical significance was present in aPTT among all groups, in PT in groups compared with D and E and finally in FIB in groups compared only with E. In the comparison of dsec, statistical significant decrease was observed in ΡΤ among Α and C, D, E and between Β and Ε, while in FIB among all groups. The comparison between CoVID‐9 patients and duration of hospitalization revealed statistical significant correlation in aPTT (both dsec and dmA) and in dmA of PT(p < 0.001).
Conclusion(s): CWA variables upon admission in COVID‐19 patients may be used for the prognosis of the patient outcome.
VPB0125
Thromboembolism in COVID‐19 patients on ECMO
C. Jones 1; K. Chen1; G. Cheng2; V. Narendran3
1 University of Miami / Palm Beach Regional GME Consortium, West palm beach, Florida, United States; 2 University of California, Los Angeles, Los Angeles, California, United States; 3 JFK Medical Center, Atlantis, Florida, United States
Background: Venous thromboembolism (VTE) is a common complication of COVID‐19 (coronavirus disease 2019), which often leads to sudden deterioration and death. Patients on extracorporeal membrane oxygenation (ECMO) are at risk of developing thromboembolism. Thrombus formation within the extracorporeal circuit is the main reason for systemic thromboembolism.
Aims: Thromboembolic prophylaxis is critical in managing COVID‐19 patients on ECMO. Anticoagulation is recommended to all hospitalized COVID‐19 patients. The prevalence of VTE in COVID‐19 patients on ECMO is unclear. We aim to investigate the VTE incidence and contribute to anticoagulation strategy and management in this specific population.
Methods: We retrospectively reviewed the data of 23 patients who were diagnosed with COVID‐19 and managed with ECMO. All patients received thromboembolic prophylaxis since admission. We report our findings of the incidences of thromboembolism.
Results: Twenty‐three adult patients who were diagnosed with COVID‐19 received ECMO support. The mean age of patients was 44.8‐year‐old. None of the patients were active smokers or had chronic lung disease. All patients received heparin for thromboembolic prophylaxis on admission. The overall VTE rate was 34.7%. Six patients developed deep vein thrombosis (DVT) (26%) with lower extremities induration. Two patients were found to have pulmonary embolism (PE) (8.7%). Four patients had clotted circuit that requiring exchange. No stroke or myocardial infarction (MI) was diagnosed in these patients. Heparin‐induced thrombocytopenia (HIT) was excluded in all cases.
Conclusion(s): According to Jenner’s systemic review, 34% of 2928 ICU‐managed COVID‐19 patients developed VTE, with 12.6% of PE and 16.1% of DVT. 529 patients (18.0%) received ECMO. When compared to our study, there were no statistically significant differences in the incidences of VTE, DVT, or PE between these two studies, although all our patients were on ECMO. Further investigation into the prevalence and management of thromboembolism in COVID‐19 patients on ECMO will lead to significantly improved outcomes for this specific patient population.


VPB0137
The difference of coagulation indicators between COVID‐19 and dengue haemorrhagic fever in children: A single‐centre study
S. Tatura 1; S. Gunawan2; R. Sugoro3
1 Medical Faculty of Sam Ratulangi University / Kandou General Hospital, Manado, Indonesia, Manado, Sulawesi Utara, Indonesia; 2 Department of Paediatrics, Medical Faculty of Sam Ratulangi University / Kandou General Hospital, Manado, Indonesia, manado, Sulawesi Utara, Indonesia; 3 Department of Paediatrics, Medical Faculty of Sam Ratulangi University / Kandou General Hospital, Manado, Indonesia, Manado, Sulawesi Utara, Indonesia
Background: COVID‐19 pandemic has become a serious issue worldwide, as a novel disease with various manifestations and symptoms, many of which are still unknown till date. Coagulopathy is a well‐known manifestation in COVID‐19, which also commonly presents in patients with Dengue Haemorrhagic Fever (DHF).
Aims: This study aimed to analyse the differences in prothrombin time (PT), activated partial thromboplastin time (aPTT), and D‐dimer level between COVID‐19 and DHF children.
Methods: This is a single‐centre prospective cohort study, conducted from April 2020 to September 2021 in R D Kandou General Hospital, Manado, Indonesia. All children aged 1 – < 18 years old diagnosed with Confirmed COVID‐19 or DHF (according to WHO 2011 criteria) were checked for PT, aPTT, and D‐dimer on the day of admission; after obtained informed consent from their parents/guardians. Subjects were then grouped into Confirmed COVID‐19 group and DHF group, in regards to their respective diagnosis. Data of coagulation markers between groups were analysed with t‐test, power 0.80, α 0.05, significant P < 0.05 using SPSS version 25.0.
Results: This study gathered 107 subjects, consisting of 46 COVID‐19 and 61 DHF subjects. Out of three laboratory markers, patients in COVID‐19 group had significant PT prolongation compared to DHF group (16.56 ± 7.1” vs. 13.0 ± 1.75”, respectively, p < 0.001). On the other hand, DHF group showed increasing trend of D‐dimer value and aPTT compared with COVID‐19 group, although no statistically significant.
Conclusion(s): This study finds significant PT prolongation in children with COVID‐19 compared to those of DHF. However, there were no significant difference of aPTT and D‐dimer between COVID‐19 and DHF children.
PB0042
Antithrombotics and antihistamine use correlated with increased COVID‐19 severity. Antithrombotics and antihistamine use correlated with increased COVID‐19 severity.
M. Khan1; V. Chan2; C. Maynard2; I. Asekomhe2; M. Brennan 1
1 Royal College of Surgeons in Ireland (RCSI), Dublin, Dublin, Ireland; 2 RCSI University of Medicine and Health Sciences, Dublin, Dublin, Ireland
Background: Many medications have been investigated for use in COVID‐19 with anticoagulants being recommended as thromboprophylaxis in hospitals.
Aims: To investigate the effect of prophylactic and prescribed medication on COVID‐19 severity.
Methods: An online survey was used to collect patient data relating to medication use prior to COVID‐19 diagnosis in recovered patients. Statistics were performed using one‐way ANOVA and t‐test. This was an international retrospective cohort study approved by the Royal College of Surgeons in Ireland Human Research Ethics Committee.
Results: 685 participants representing 32 countries responded (age range 18‐78 yrs). Antiplatelet and antithrombotic medication was associated with more severe disease, (28% severe vs. 8% mild). Aspirin and ibuprofen use after diagnosis was associated with increased length of disease; (aspirin 54.5 ± 3.1 days; control 34.8 ± 2.7 days, (P < 0.05); ibuprofen 54.7 ± 6.6 days; control 31.8 ± 2.8 days, P < 0.05). There was an increase in disease severity for patients taking antihistamines both before and after diagnosis (severe 28%, mild 7%; severe 33% mild 10%, respectively). Antihistamine use was associated with longer disease presentation in both groups (before diagnosis: antihistamine 47.5 ± 7.9 days, control 35.4 ± 2.7 days, P < 0.01; after diagnosis 51.9 ± 5.9 days, control 30.8 ± 2.6 days P < 0.05).
Conclusion(s): We anticipated a prophylactic effect of antithrombotic use prior to infection, however, these data do not support this. The association of ibuprofen and aspirin with severe presentation is likely due to their use for patients with more severe COVID‐19 or an underlying condition. Antihistamines inhibit the mast cell response which is important for fighting both the initial infection and the subsequent response. These results indicate that antithrombotics should only be used where there is an indicated thrombotic risk and antihistamines should be used with caution. Further work is required in a larger clinical study to confirm these findings.
PB0040
The predictive role of plasminogen activator inhibitor‐1 (PAI‐1) in the COVID‐19‐associated pneumonia
K. Bielosludtseva 1; T. Pertseva2
1 Dnipropetrovsk State Medical Academy, Dnipro, Dnipropetrovs'ka Oblast', Ukraine; 2 Dnipropetrovsk Medical Academy, Dnipro, Dnipropetrovs'ka Oblast', Ukraine
Background: PAI‐1 plays a key role in a wide range of physiological and pathological processes, including coagulation, fibrinolysis, inflammation.
Aims: The aim was to estimate the prognostic role of PAI‐1 on admission in hospitalized patients with confirmed COVID‐19 pneumonia.
Methods: We observed 2 groups: Main – 85 patients (age – 59 (52; 65) years, men – 45 (52.9%)), hospitalized with pneumonia on the background of laboratory‐confirmed (PCR) COVID‐19; Control – 25 healthy volunteers (age – 50.0 (35; 65) years, men – 13 (52.0%)). General analysis, determination of PAI‐1 (Human PAI‐1 ELISA Kit, Elabscience) performed at admission before starting of anticoagulant treatment, autopsy data, statistical analysis.
Results: At admission the level of PAI‐1 in Main group was in 60 times higher than in Control group (6.1 [0.15; 18] ng/ml versus 0.1 [0.09; 0.11] ng/ml, р=0.000). Despite the adequate treatment 12 patients died from COOVID‐19 pneumonia. Maximal levels of PAI‐1 were in died patients. ROC analysis shows the powerful reliable connection between increased level of PAI‐1 higher than 20.6 ng/ml at admission and COVID‐19 mortality (Fig.1) (OR = 219; CI = 95% (7.7056 to 6224.1404); p = 0.0016). Autopsy shown a lot of fibrin clots in lung vessels, which proves the theory that problem in fibrinolysis plays the crucial role in thrombogenesis in COVID‐19. Fig.2 demonstrates histological section of the artery of medium caliber, branching of the pulmonary artery, in horizontal projection, a fragment of vascular endothelial integrity violation was isolated, the arrow indicates a pale pink non‐nuclear mass in the form of threads – fibrin clot.
Conclusion(s): 1) problem in fibrinolysis plays the crucial role in thrombogenesis in COVID‐19; 2) increased level of PAI‐1 at admission more than 20 ng/l is associated with 200‐times higher risk of mortality.


PB0059
Influence of complement system genomic variations on the course of COVID‐19
J. Gumulec 1; J. Máca2; J. Sagan3; J. Škarda4; M. Kutěj5; N. Chobolova6; D. Kaspřák7
1 University Hospital Ostrava and Faculty of Medicine University of Ostrava, Ostrava, Moravskoslezsky kraj, Czech Republic; 2 Department of Anesthesiology and Intensive Care Medicine, University Hospital Ostrava, Czech Republic, Department of Intensive Care and Forensic Studies, Faculty Of Medicine, University of Ostrava, Czech Republic, Institute of Physiology and Pathophysiology, Faculty Of Medicine, University of Ostrava, Czech Republic, Ostrava, Moravskoslezsky kraj, Czech Republic; 3 Department of Infectious Medicine, University Hospital Ostrava, and Faculty of Medicine, University of Ostrava, Czech Republic, Ostrava, Moravskoslezsky kraj, Czech Republic; 4 Institute of Clinical and Molecular Pathology and Medical Genetics, University Hospital Ostrava and Faculty of Medicine, University of Ostrava, Czech Republic, Ostrava, Moravskoslezsky kraj, Czech Republic; 5 Department of Anesthesiology and Intensive Care Medicine, University Hospital Ostrava, Czech Republic, Department of Intensive Care and Forensic Studies, Faculty Of Medicine, University of Ostrava, Czech Republic, Department of Intensive Care and Forensic Studies, Faculty Of Medicine, University of Ostrava, Czech Republic, Faculty Of Medicine, Masaryk University in Brno, Czech Republic, Ostrava, Moravskoslezsky kraj, Czech Republic; 6 Department of Clinical Biochemistry, Institute of Laboratory Medicine, University Hospital Ostrava, University of Ostrava, Czech Republic, Ostrava, Moravskoslezsky kraj, Czech Republic; 7 Laboratory of Molecular Biology, Spadia LAB JSC, Czech Republic, Nový Jičín, Moravskoslezsky kraj, Czech Republic
Background: The pandemic of SARS‐CoV‐2 is a severe worldwide problem increasing morbidity and mortality.1, 2 Severe COVID‐19 presents as multiple organ failure caused by systemic inflammation, thrombin generation, and hypofibrinolysis. Diffuse microvascular thrombi and inter‐alveolar deposits of complement fragments are observed. The enhanced immunothrombosis is mediated by direct overactivation of complement by virus surface components or damaged cells.3‐5
Aims: The study aimed to find whether genetic changes responsible for complement dysregulation known in atypical hemolytic‐uremic syndrome (aHUS) can be found in severe COVID‐19 patients.
Methods: The study included adult COVID‐19 subjects undergoing extracorporeal membrane oxygenation support for severe acute respiratory distress syndrome. Two independent physicians signed informed consent, and the study was approved by a local ethics committee (No. 109/2021) and supported by the University Hospital fund. Next‐Generation Sequencing Panel of C3 component, membrane cofactor protein (CD46), complement factor B (CFB), complement factor H (CFH), complement factor H related genes 1‐5 (CFHR 1‐5), diacylglycerol Kinase Epsilon, thrombomodulin (THBD) and mannose‐binding lectin (MBL) genes were performed, with confirmations of positive results by Sanger sequencing.
Results: Twenty‐two patients (13 were male) aged 33 to 65 years were included. No pathogenic gene variants in the C3, CD46, CFB, CFH genes, CFHR 5, CFI, THBD were detected. However, we have shown the presence of modifiers (CFH‐H3 haplotype, MCP‐GGAAC haplotype, and CFH/CFHR1), which may, together with triggers (infection), increase the severity of the disease (aHUS).6‐8 Moreover, we have identified single nucleotide polymorphisms in exon 1 at codon 52 (c.154C>T) and 54 (c.161G>A) of the MBL2 gene promoter associated with low serum levels or dysfunctional MBL and higher incidence of infections.
Conclusion(s): We did not detect any complement‐related pathogenic gene variants known in aHUS. Thus, It is unlikely that complement dysregulation is the main factor influencing immunothrombosis in a cohort of the most severe COVID‐19 patients.
PB0066
Thromboprophylaxis outcome in childhood SARS‐CoV‐2 infection: A single‐center experience
M. Karimi 1; A. Sanaei Dashti2; S. Haghpanah2; Y. Mansoori3; T. Zarei3; A. Amanati2; M. Bordbar3
1 Hematology Research Center Shiraz University of Medical Sciences, Shiraz, Iran, Shiraz, Fars, Iran; 2 Shiraz University of Medical Sciences, Shiraz, Fars, Iran; 3 Shiraz University of Medical Sciences, shiraz, Fars, Iran
Background: Background The SARS‐CoV‐2 infection has been associated with a potentially severe inflammatory reaction, endothelial damage, and coagulation cascade activation that cause thrombosis. There is limited information on the thrombosis and anticoagulant therapy in children with COVID‐19 and no design pediatric‐specific recommendations for thromboprophylaxis in COVID‐19 are available.
Aims: This study aims to evaluate the outcome of thromboprophylaxis in children less than 18‐year‐old with COVID‐19 infection.
Methods: A retrospective study was conducted on 184 pediatric patients with confirmed COVID‐19 infection in southern Iran. A designed questionnaire was made to collect all demographic, clinical, and laboratory data. According to World Health Organization, the patients were classified as asymptomatic/mild, moderate, severe, and critically ill.
Results: The mean age of the patients was 7.04 ± 5.9 (1 week to < 18 years), 96 boys and 88 girls. Overall, 33 patients received anticoagulant therapy. The median of D‐dimer was insignificantly higher in patients taking anticoagulant therapy compared to another group. (P = 0.133). All variables were comparable between the two groups. The mortality rate was non‐significantly higher in patients who were not treated with anticoagulants (14%) compared to the thromboprophylaxis group (9%) (P = 0.567). In the critical group, two patients were complicated with disseminated intravascular coagulation (2.56%), one patient (1.28%) with deep vein thrombosis despite taking thromboprophylaxis, and one (1.28%) with pulmonary thromboembolism while the patient did not take anticoagulant during hospital admission Table 1).
Conclusion(s): Our data showed a lower rate of thrombosis (1.4% in moderate to severe/critically ill patients) than adult patients with COVID‐19 infection. Moreover, higher mortality rate was observed in patients without anticoagulant therapy, though statistically not significant. It may underline the role of anticoagulants in moderate to severe/critically ill children with COVID‐19 infection. Expert opinion and personal experience are necessary, while we have a significant knowledge gap in understanding COVID‐19‐associated coagulopathy and thrombotic risk in children.

PB0084
Use of telemedicine for outpatients' follow‐up during the COVID‐19 pandemic: The experience of an Italian pediatric center of hemostasis and thrombosis
I. Ricca; B. Pollio; S. Raso; F. Quaglino; R. Albiani
AOU Città della Salute e della Scienza di Torino, Torino, Piemonte, Italy
Background: During the SARS‐CoV‐2 emergency, we had the need to maintain a high‐quality follow‐up for outpatients in respect of social distancing. Telemedicine may contribute to assure health care at a distance using telecommunications technology.
Aims: We developed a tele visit protocol integrated into our local healthcare system with the aim to avoid lack of follow‐up in the chronic outpatients affected by haemostasis disorders.
Methods: We prospectively offered the tele visit option to all those cases in which physical examination, blood exams or immaging were not mandatory. An e‐mail address, a video call device (i.e. smartphone, tablet, computer) and a good internet connection were required. Written informed consent was signed prior to the visit. According with the Italian Guidelines for telemedicine and privacy rules, the tele visit was held on a protected and isolate online platform (Cisco Webex Inc.) and it could not be recorded. Final medical report was sent by e‐mail in encrypted form. At the end of the call, patient/caregivers were invited to fill in a brief feedback survey.
Results: From May to December 2021, 91 tele visits were performed (20% of our outpatient burden). Patients' characteristics are reported in the Table. 95% of patients welcome the tele visit option. Only 5 patients refused it: 2 because of lack of devices while 3 felt not confident with the internet context. No connection problems occurred; no call had to be converted into face‐to‐face visit. The feedback was generally good. In particular, 92% of patients expressed their propensity to use telemedicine also after pandemic. More details on the single statements are reported in Figure.
Conclusion(s): This preliminary experience in telemedicine proved to be feasible and efficient in a pandemic scenario. Based on these results and on patients' satisfaction, we are now considering its potential to become a valid alternative solution for the future healthcare service.

PB0097
Serial thrombin generation, while on LMWH prophylaxis in critically ill COVID‐19 patients from the prospective MaastrICCht cohort
T. van de Berg 1; M. Mulder2; T. Alnima3; H. Spronk4; R. van Oerle5; E. Beckers6; T. Hackeng7; A. Hulshof8; J. Sels2; Y. Henskens9; I. van der Horst10; H. ten Cate11; B. van Bussel12
1 Department of Biochemistry ‐ Cardiovascular Research Institute Maastricht (CARIM), Maastricht, Limburg, Netherlands; 2 ICU, Maastricht University Medical Centre +, The Netherlands, Maastricht, Limburg, Netherlands; 3 Radboud hospital Nijmegen, Nijmegen, Gelderland, Netherlands; 4 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 5 Central Diagnostic Laboratory, MUMC+ and Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands;, Maastricht, Limburg, Netherlands; 6 Department of Hematology, Maastricht University Medical Center, Maastricht University, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 7 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 8 Maastricht University Medical Centre +, The Nerherlands, Maastricht, Limburg, Netherlands; 9 Central Diagnostic Laboratory Maastricht University Medical Centre +, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands, Maastricht, Limburg, Netherlands; 10 ICU, Maastricht University Medical Centre +, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands, Maastricht, Limburg, Netherlands; 11 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Maastricht, Limburg, Netherlands; 12 MUMC+, Maastricht, Limburg, Netherlands
Background: COVID‐19 associated coagulopathy (CAC) has been associated with an increase in venous thromboembolic events. Most guidelines advise heparins in the management of this coagulopathy. However, the relative effectiveness of this strategy on the coagulation potential of COVID‐19 patients remains unclear.
Aims: We aim to describe the development of COIVID‐19 associated coagulopathy in patients treated with either low molecular weight heparins (LMWH) or unfractionated heparin (UFH) during the course of disease in an intensive care unit population. Additionally we evaluate the anticoagulant treatment in these patients in‐vitro.
Methods: We included 33 patients with confirmed COVID‐19 admitted at the ICU. Calibrated automated thrombinography (CAT) was measured at least twice over the course of 6 weeks after admission. Thrombin generation results were subsequently correlated with an extensive database of clinical and laboratory measurements.
Results: Anti‐Xa levels of all patients remained within their therapeutic range throughout the follow‐up. The mean (SE) endogenous thrombin potential (ETP) was 1727 (170) nM min and 1620 (460) nM min for ellagic acid (EA) and tissue factor (TF) respectively at inclusion. In line with this we found a mean (SE) PH of 352.9 (44.9) nM and 263.5 (95.7) nM for EA and TF. Despite adequate treatment thrombin generation parameters remained highly elevated. In vitro assessment alternative anticoagulants showed promising aspects of direct thrombin inhibition.
Conclusion(s): We showed that, in a cohort of critically ill COVID‐19 patients on low molecular weight heparins, despite apparent adequate anti‐coagulation doses evaluated by antiXa levels, thrombin generation potential remains high. Thrombin generation potential persists over the course of ICU admission and is independent of age, sex, body mass index, APACHE II score, cardiovascular disease and smoking status. The observations could, for a small part at the most, be explained by (anti)coagulation and thrombosis, inflammation and multi‐organ failure. Direct thrombin inhibition might be a promising alternative anticoagulant therapy in COVID‐19.


VPB0132
The impact of sex on D‐Dimer levels and disease outcomes in hospitalized COVID‐19 patients
O. Saville 1; Y. Tera2; Y. Deng3; M. Elbatarny4; M. Othman2
1 Queen's University, Belleville, Ontario, Canada; 2 Queen's University, Kingston, Ontario, Canada; 3 Queen’s University, Kingston, Ontario, Canada; 4 University of Toronto, Toronto, Ontario, Canada
Background: Males and females are similarly susceptible to COVID‐19 infection. Multiple studies report male mortality rate to be nearly double that of females. Hypercoagulability is common in severe COVID‐19 patients. D‐dimer was reported as a significant marker for disease severity and mortality risk. It is unclear whether D‐dimer levels differ between males and females. The effect of D‐dimers on disease outcomes remains under investigation.
Aims: To evaluate the sex difference of D‐dimer level in hospitalized COVID‐19 patients and to determine the effect of sex on disease outcomes.
Methods: We searched EMBASE for articles published prior to October 1, 2021, evaluating D‐dimer in adult males and females, hospitalized for COVID‐19 and reporting on mortality, ICU admission, hospital stay and thrombotic complications. 3225 articles were retrieved. Comparative, observational prospective or retrospective, or case control studies were included. Studies including pregnancy, children, or a secondary disease focus were excluded. We meta‐analysed data from 10 included studies using Cochrane RevMan 5 software.
Results: Of 11,827 hospitalized COVID‐19+ adults, 6519 (55%) were male and 5308 (45%) were female. Critical illness was experienced by 1681 (26%) males and 1228 (23%) females. Mortality occurred in 877 (13%) males and 548 (10%) females. In unadjusted analysis males had higher odds of experiencing critical illness and mortality. The Odds Ratios were 1.53 [95% CI: 1.36–1.72, I2 = 77%, p =< 0.00001] and 1.40 [95% CI: 1.24–1.57, I2 = 0%, p =< 0.00001], respectively. The mean difference between male and female D‐dimer level was 0.18 [95% CI: 0.13‐0.23, I2 = 83%, p =< 0.00001]. The reporting of D‐dimer assay calibration was inconsistent and D‐dimer unit magnitude varied greatly between studies.
Conclusion(s): Males have higher mean D‐dimer levels and are at higher risk of experiencing poor COVID‐19 outcomes than females. The diversity in D‐dimer reporting among different studies can impact data interpretation.


PB0080
Are drug‐drug interactions clinically relevant? Use of edoxaban concomitant to ritonavir in COVID‐19 hospitalized patients with atrial fibrillation
P. Olivera 1; D. Campoy1; C. Velásquez‐Escandón2; T. Canals3; K. Flores4; E. Johansson4; C. Hernandez5; O. Benitez5
1 Thrombosis and Hemostasis Unit, Department of Hematology, Hospital Universitari Vall d'Hebron, Barcelona, Catalonia, Spain; 2 Department of Hematology, Fundación Sanitària Mollet, Barcelona, Catalonia, Spain; 36. Department of Hematology, University Hospital Sant Joan de Reus, Tarragona, Tarragona, Catalonia, Spain; 45. Department of Hematology, General University Hospital of Catalonia, Barcelona, Barcelona, Catalonia, Spain; 5 Thrombosis and Hemostasis Unit Department of Hematology, Hospital Universitari Vall d'Hebron, Barcelona, Catalonia, Spain
Background: During the first wave of the SARS‐CoV‐2 pandemic, management of anticoagulation therapy in hospitalized patients with atrial fibrillation (AF) was simplified to low‐molecular‐weight heparin (LMWH), mainly due to the risk of drug‐drug interactions. However, not all potential drug‐drug interactions are clinically relevant. The metabolism of edoxaban by CYP3A4 is less than 4%, the risk of drug‐drug interactions with edoxaban is low. There are few data on the interaction between edoxaban and ritonavir.
Aims: To determine whether the effectiveness and safety of edoxaban or LMWH differed between patients with AF who had been hospitalized for COVID‐19 infection and received empirical treatment with ritonavir. In addition, we analyzed length of stay, the proportion of patients requiring admission to the intensive care unit, and mortality.
Methods: Observational, retrospective, and multicenter study that consecutively included hospitalized patients with non‐valvular AF who received anticoagulant treatment with LMWH or edoxaban concomitantly with empirical therapy for COVID‐19 infection.
Results: From March 5th to April 27th, 2020, 464 patients were included (80.3±7.7 years, 50.0% men, CHA2DS2‐VASc 4.1 ± 1.4; HAS‐BLED 2.6 ± 1.0). Regarding COVID‐19 therapy during hospitalization, patients were taking azithromycin (98.7%), hydroxychloroquine (89.7%), and ritonavir/lopinavir (81.5%). The mean length of hospital stay was 14.6 ± 7.2 days and mean total follow‐up (from admission to the last visit) was 31.6 ± 13.4 days. Furthermore, 12.9% of patients required admission to the intensive care unit, 18.5% of patients died, and 9.9% had a bleeding complication (34.8% major bleeding). Except for length of hospital stay, which was longer in patients taking LMWH (16.0 ± 7.7 vs. 13.3 ± 6.5 days; p = 0.005), data for the remaining outcomes were similar in patients treated with edoxaban and those treated with LMWH.
Conclusion(s): No significant differences were found between patients treated with edoxaban and patients treated with LMWH in terms of the percentage admitted to the intensive care unit, mortality rates, arterial and venous thromboembolic complications, and bleeds.


PB0037
Association of the FVIII/DD and FvW/DD indices as an indicator of severity and mortality in patients with COVID‐19
B. Ascencio; M. Jaime Capetillo; F. Vidal Martínez; M. Castillejos Lopez; P. Martel Palomo; A. Castorena Maldonado; l. Figueroa Hernández; M. Robledo Díaz; J. Martínez de Rafael; N. Ruíz Gómez
INSTITUTO NACIONAL DE ENFERMEDADES RESPIRATORIAS, Mexico, Distrito Federal, Mexico
Background: There are different studies on severity and mortality indices, such as the neutrophil/lymphocyte ratio (NLI), which has been relevant as a biomarker for mortality. However, indices where hemostatic factors are present, such as the FVIII/DD and FvW/DD ratio, have not been established for mortality in the Mexican population.
Aims: To determine whether the factor VIII and D‐dimer (FVIII/DD) and the von Willebrand factor (VWF/DD) indices can be useful as markers of poor prognosis and mortality and to identify patients with a poor prognosis in cases of COVID‐19 hospitalized from the National Institute of Respiratory Diseases.
Methods: A prospective, longitudinal study was carried out in which patients were admitted in the first 24 hours of hospitalization with a diagnosis of SARS‐CoV‐2 infection with a test confirmed by PCR; In a period from March to November 2020, laboratory data were recorded to generate the FVIII/DD and FvW/DD indices, including clinical, epidemiological data and discharge status.
Results: Data from 249 hospitalized patients with severe COVID‐19 were analyzed. The clinical and laboratory characteristics are described in Table 1. The FVIIIDD24H and FVWDD24H indices were constructed by dividing each FVIII data by its corresponding DD data. and in the same way it was done with the VWF. The medians of these indices were then compared between the presence or absence of cyanosis, ventilatory status, and mortality. A value >115 was established as the cut‐off point for the DD/FVIII and DD/FvW index to predict mortality, which are shown in Figure 1.
Conclusion(s): Low FVIIIDD24H and FVWDD24H levels on admission were associated with cianosis, severe COVID‐19 and mortality. Likewise, it was shown that the cut‐off point < or equal to 115 is a prognostic factor for mortality in this cohort of patients hospitalized for covid‐19.


PB0058
Contribution of biology to the diagnosis and follow‐up of VITT: A French multicentre experience
C. Pouplard1; C. Vayne2; E. Guery2; N. Ajzenberg3; P. Cauchie4; C. Cordonnier5; E. De Maistre6; M. Donnard7; N. Drillaud8; H. Galinat9; S. Goffard10; I. Gouin11; R. Marlu12; G. Mourey13; F. Mullier14; V. SIGURET15; S. Susen16; M. Tuffigo17; J. Rollin2; Y. Gruel 2
1 University Hospital of Tours, TOURS, Centre, France; 2 University Hospital of Tours, Tours, Centre, France; 3 APHP, Paris, Ile‐de‐France, France; 4 University Hopsital of Charleroi, Charleroi, Namur, Belgium; 5 University Hospital of Lille, Lille, Nord‐Pas‐de‐Calais, France; 6 University Hospital of Dijon, Dijon, Bourgogne, France; 7 University Hospital of Limoges, Limoges, Limousin, France; 8 University Hospital of Nantes, Nantes, Pays de la Loire, France; 9 University Hospital of Brest, Brest, Bretagne, France, 10 Hospital of Brive, Brive, Aquitaine, France; 11 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, Institut de recherche en santé, environnement et travail ‐ IRSET, Inserm UMR_S 1085, Univ Rennes, CHU Rennes, France, Rennes, Bretagne, France; 12 University Hospital of Grenoble, Grenoble, Auvergne, France; 13 EFS, Besançon, Bourgogne, France, 14 University Hospital of Namur, Namur, Namur, Belgium; 15 INSERM UMR‐S‐1140/University of Paris, Paris, Ile‐de‐France, France; 16 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 17 University Hospital of Angers, Angers, Pays de la Loire, France
Background: Vaccine‐induced immune thrombocytopenia (VITT) is a rare but serious complication of SARS‐COV‐2 vaccination and its diagnosis is based on both clinical and biological criteria.
Aims: To evaluate the performance of these criteria in a prospective cohort of patients with suspected VITT.
Methods: Among 105 consecutive patients (Table 1), 79 had received one (n = 71) or two (n = 8) injections of adenoviral vector vaccine, and 26 one (n = 18) or two (n = 8) doses of an mRNA vaccine. Most patients presented thrombosis either isolated (n = 40), or associated with thrombocytopenia (T+T = 45). Other patients had isolated thrombocytopenia (n = 15), or variable clinical manifestations. ELISA (PVS/PF4 HAT45G®) detected anti‐PF4 antibodies, and when present VITT diagnosis was confirmed using PF4 serotonin release assay.
Results: VITT was confirmed in 26 patients (both PVS/PF4 ELISA and PF4/SRA were positive) and probable in one case (PVS/PF4 ELISA OD = 2.4 but PF4/SRA negative). VITT was excluded in all patients who had received an mRNA vaccine, or in those with isolated thrombocytopenia or thrombosis or with other symptoms. In contrast, the presence of both thrombocytopenia and thrombosis 5 to 30 days after injection was highly suggestive of VITT with a specificity and PPV of 85% and 69%, respectively, these values increasing to 93.6% and 85% if patients had received adenoviral vector vaccine. Importantly, the PPV reached in these cases 100% when OD > 1 in ELISA. (Table)
Conclusion(s): The occurrence of thrombocytopenia and thromboses, 5 to 30 days after vaccination with an adenoviral vector is highly predictive of VITT. PF4/PVS ELISA allows to rule out VITT when OD is < 0.4 (NPV 100%) and to confirm the diagnosis when OD is > 1 without necessitating a platelet activation test. Moreover, the follow‐up of patients with ELISA is mandatory since anti‐PF4 antibodies may remain several weeks after VITT.

PB0071
Associations between hemostatic markers and mortality in COVID‐19—Compounding effects of amplified coagulation and decreased fibrinolytic activity
N. Boknäs1; C. Laine2; A. Hillarp3; A. Macwan4; K. Gustafsson1; M. Holmström2; T. Lindahl 5
1 Region Östergötland, Linkoping, Ostergotlands Lan, Sweden; 2 Linköping Univerisity, Linköping, Ostergotlands Lan, Sweden; 3 Oslo University, Oslo, Oslo, Norway; 4 Linköpings university, Linköping, Ostergotlands Lan, Sweden; 5 Linköping University, Linköping, Sweden, Linköping, Ostergotlands Lan, Sweden
Background: Several studies have reported on associations between laboratory parameters of hemostasis and the clinical outcomes of hospitalized patients with COVID‐19.
Aims: We applied an extensive panel of laboratory markers on a cohort of patients that were hospitalized for symptoms consistent with COVID‐19 to find predictive markers and increase understanding of the disease.
Methods: 217 patients were included in the study after being admitted to our hospital with a clinical suspicion of COVID‐19 during the spring 2020. 96 patients were diagnosed with COVID‐19. The clinical outcomes were monitored until September 2020.
Results: In the 96 patients that tested positive for SARS‐CoV‐2 (COVID‐19+), the cumulative incidences of death and venous thromboembolism were 24.0 % and 19.8 % as compared to 12.4 % (p = 0.031) and 11.6 % (p = 0.13) in the 121 patients that tested negative (COVID‐19‐). In COVID‐19+ patients, we found pronounced increases in plasma levels of von Willebrand factor (vWF) and fibrinogen. Among COVID‐19+ patients, excess mortality was observed in patients with laboratory signs of either coagulopathy or low fibrinolytic activity, with Odds Ratios (OR) for death of 4.7 (95% confidence interval (CI95) 1.7–12.9; p = 0.003) for D‐dimer >0.5 mg/L, 5.9 (CI 95 1.8–19.7; p = 0.004) for antithrombin (AT) ˂0.85 kIU/L and 4.9 (CI 95 1.3–18.3; p = 0.019) for plasmin‐antiplasmin complex (PAP) <1000 μg/L. Compounding increases in mortality was observed in COVID‐19+ patients with combined defects in both fibrinolysis and coagulation, with ORs for the death of 15.7 (CI95 4.3–57; p < 0.001) for patients with PAP <1000 μg/L and D‐dimer >0.5 mg/L and an OR of 15.5 (CI95 2.8–87, p = 0.002) for patients with PAP <1000 μg/L and AT ˂0.85 kIU/L.
Conclusion(s): In COVID‐19+ patients with high D‐dimer, low PAP was correlated to high mortality and associated with low levels of small fibrin fragments, indicating impaired fibrinolytic breakdown of fibrin.

PB0103
Evaluation of changes in antiphospholipid antibody titers following vaccination with SARS‐CoV‐2 mRNA in patients with autoimmune diseases
M. Yasuda; Y. Fujieda; K. Sakiyama; M. Tada; N. Tsuchida; K. Nishino; H. Moriya; M. Kono; M. Kato; O. Amengual; T. Atsumi
Department of Rheumatology, Endocrinology and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Hokkaido, Japan
Background: Severe coronavirus disease 2019 (COVID‐19) is characterized by a hypercoagulable state and antiphospholipid antibodies (aPLs) are detected in some cases. Since elevation of aPLs could be the risk of thrombosis.
Aims: We aim to evaluate the change of aPLs’ titers and the potential risk of thrombosis after vaccination against of SARS‐CoV‐2 in patients with antiphospholipid syndrome (APS) and systemic lupus erythematosus (SLE).
Methods: This study comprised patients with primary APS (PAPS), APS associated with systemic lupus erythematosus (SLE/APS), SLE aPL carriers (SLE/aPL+), and SLE without aPLs (SLE/aPL‐) who received the first and second dose of COVID‐19 mRNA vaccine. Serum anti‐cardiolipin antibody (aCLIgG, IgM), anti‐β2GPI antibody (aβ2GPI IgG, IgM) detected by chemiluminescent immunoassay (CLIA), and anti‐phosphatidylserine/prothrombin complex antibody (aPS/PT IgG, IgM) tested an in‐house enzyme‐linked immunosorbent assay (ELISA) were evaluated before and 4 weeks after vaccination. The cut‐off values were >20.0 U/ml for aCL (IgG, IgM)/aβ2GPI (IgG, IgM), >1.2 U/ml for aPS/PT IgG and >5.2 U/ml for aPS/PT IgM. A titer elevation of more than 10% after vaccination was defined as a significant elevation.
Results: A total of 32 patients were enrolled; 4 PAPS, 9 SLE‐APS, 3 SLE‐aPL+, and 16 SLE‐aPL‐. Among aPL+ patients (n = 16), there was no significant elevation of aPLs titers after vaccination (Table). Four weeks after vaccination, aPLs positivity was detected in three patients in the SLE/aPL‐ group (aCL IgG, 2 patients, aβ2GPI IgG 1 patients). In the SLE/aPL+ group, two SLE/APS patients had positive aβ2GPI IgG. No acute thrombotic events were observed during the observation period.
Conclusion(s): There was no significant change in aPL titers after vaccination in patients with APS and/or SLE. None of the patients developed a thrombotic event after vaccination.

VPB0133
Evaluation of D‐dimer concentrations measured with three methods in patients with COVID‐19
M. Capecchi1; E. Scalambrino 2; M. Clerici3; S. Testa4; C. Dellanoce4; F. Peyvandi5; A. Tripodi6
1 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milano, Lombardia, Italy; 2 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico di Milano, Italy, Milano, Lombardia, Italy; 3 Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 4 Azienda Ospedaliera Istituti Ospitalieri di Cremona, Centro Emostasi e Trombosi, Cremona, Lombardia, Italy; 5 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy; 6 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy
Background: COVID‐19 is associated with an increased risk of venous thrombosis, even when patients are on standard‐dose antithrombotic prophylaxis. Hence, the identification of biomarkers of thrombosis helps tailoring dosage of antithrombotic prophylaxis. D‐dimer has been extensively employed as a biomarker and cut‐off values at hospital admission have been proposed to stratify the risk of thrombosis and make decision on prophylaxis. However, D‐dimer measurement is not standardized, and it is unknown if the cut‐off values used for decision making can be used interchangeably between methods.
Aims: To assess for concordance of results obtained with different commercially available laboratory methods measuring D‐dimer.
Methods: Plasma samples collected from COVID‐19 patients at the Hospital of Cremona were evaluated for D‐dimer with three widely used immunoturbidimetric methods (Liatest D‐di, Stago, Asnieres, France; D‐dimer HS 500, Werfen, Orangeburg, NY; Innovance D‐dimer, Siemens, Marburg, Germany).
Results: A total of 87 COVID‐19 patients [54 male and 33 female, median age of 73 years (range 28–98)] were enrolled in the study. No significant differences were found between mean D‐dimer concentrations obtained with the three methods even when stratifying D‐dimer levels in 4 groups (<1000, 1000–2000, 2000–5000, >5000 ng/mL) (Figure 1). The three methods showed substantial result agreement [Stago‐vs‐Werfen and Siemens‐vs‐Stago (Cohen’s kappa coefficient of 0.760 and 0.699, respectively)] to an almost perfect agreement [Siemens‐vs‐Werfen (Cohen’s kappa coefficient of 0.811)], with a p‐value < 0.001. Results from the three methods showed a good linear correlation (Rho = 0.94) (Figure 2).
Conclusion(s): The relatively good concordance of D‐dimer results among the three investigated methods indicates that D‐dimer cut‐off values could be used interchangeably regardless of the method used for testing. The results pave the way to clinical trials aimed to assess the value of D‐dimer as a biomarker to make decision on the intensity of antithrombotic prophylaxis in COVID‐19 patients.


PB0039
The key role of plasminogen activator inhibitor‐1 (PAI‐1) in the thrombogenesis difference in COVID‐19‐associated pneumonia compared to bacterial pneumonia
K. Bielosludtseva 1; T. Pertseva2
1 Dnipropetrovsk State Medical Academy, Dnipro, Dnipropetrovs'ka Oblast', Ukraine; 2 Dnipropetrovsk Medical Academy, Dnipro, Dnipropetrovs'ka Oblast', Ukraine
Background: PAI‐1 plays a key role in a wide range of physiological and pathological processes, including coagulation, fibrinolysis, inflammation.
Aims: The aim was to estimate the diagnostic role of PAI‐1 on admission in hospitalized patients with COVID‐19 pneumonia, comparing with bacterial pneumonia.
Methods: We observed 3 groups: Main (1) – 85 patients (mean age – 59 (52; 65) years, men – 45 (52.9%)), hospitalized with pneumonia on the background of laboratory‐confirmed (PCR) COVID‐19, divided into 3 subgroups: subgroup 1 – 40 patients with moderate COVID‐19, subgroup 2 – 25 patients with severe COVID‐19, subgroup 3 – 20 patients with critical COVID‐19; Comparative (2) – 55 patients (mean age – 48.9 (34; 62) years, men – 30 (54.5%)), hospitalized with community‐acquired pneumonia of bacterial etiology (CABP) without COVID‐19; Control (3) – 25 healthy volunteers (average age – 50.0 (35; 65) years, men – 13 (52.0%)). General analysis, plasma level of PAI‐1 (Human PAI‐1 ELISA Kit, Elabscience) performed at admission before starting of anticoagulant treatment, statistical analysis.
Results: At admission the highest level of PAI‐1 (6.1 [0.15; 18] ng/ml) was in COVID‐19 patients (Main group) and exceeds the Control group (0.1 [0.09; 0.11] ng/ml), р1‐3 = 0.000) in more than 60 times. Whereas the level of PAI‐1 in patients with CABP didn’t differ from Control group (0.1 [0.09; 0.11] ng/ml), р2‐3 = 0.29) (Figure 1).Among COVID‐19 group the levels of PAI‐1 correlated with the disease severity (Figure 2).
Conclusion(s): 1) significantly increase of plasma levels of PAI‐1 in COVID‐19 pneumonia demonstrates the cornerstone in thrombogenesis of this disease – problems in fibrinolysis system, which is the main difference between CABP; 2) in hospitalized patients with COVID‐19 pneumonia the level of PAI‐1 is associated with the disease severity and could be the crucial marker for patients’ distribution.

PB0041
Lung ultrasound (LUS) for thrombosis in COVID‐19 patients
K. Bielosludtseva; K. Fuhol; M. Krykhtina
Dnipropetrovsk State Medical Academy, Dnipro, Dnipropetrovs'ka Oblast', Ukraine
Background: Lung thrombosis in hospitalized patients with COVID‐19 pneumonia is a great problem. Angiopulmography is difficult to do because of epidemiologic circumstances and patients’ oxygen dependency. Moreover, small focuses of thrombosis, which are common during COVID‐19 may not visualized during CT scan. LUS is staying popular for diagnosing and dynamic observation of COVID‐19 patients, but it is still unknown what parameters can be the really prediction of lung thrombosis.
Aims: The aim was to estimate the diagnostic role of LUS during verification of COVID‐19‐associated lung thrombosis and during its dynamic observations.
Methods: We observed 40 patients (age – 54.3 (48; 65), men – 6 (46.1%)) with severe COVID‐19 pneumonia. General clinical analysis, LUS during hospitalization and in dynamic, statistical analysis applied.
Results: 23 patients had signs of lung thrombosis on LUS: subpleural triangular hypoechoic lesion (consolidations) with absent lung sliding and vascularization in Color regime (Figure 1). After strengthening of anticoagulation treatment 20 patients had positive dynamic on LUS: decreasing of consolidation sizes and revascularization of lung tissue (Figure 2).
Conclusion(s): 1) combination of LUS signs: subpleural triangular hypoechoic lesion (consolidations) with absent lung sliding and vascularization in Color regime is quick, cheap, noninvasive test for verification of COVID‐19‐associated lung thrombosis 2) decreasing of consolidation sizes and revascularization of lung tissue during LUS could be the objective sign of adequate anticoagulant treatment.


VPB0104
Assessment of COVID ‐19 associated coagulopathy and multiple hemostatic markers in patients of upper Egypt
A. Abdelaal 1; A. Abu‐Elfatth1; L. Bakkar2; H. Abd El‐Azeem1; H. Hetta1; E. Badawy1
1 Assiut University Medical School, Assiut, Asyut, Egypt; 2 Assiut University Hospital, Assiut, Asyut, Egypt
Background: Coagulopathy is still a serious pattern of Coronavirus‐19 disease. Its form and severity could determine the path of the patient and his outcome.
Aims: ‐Evaluate COVID‐19 associated coagulopathy, thrombosis and disseminated intravascular coagulopathy among Egyptian patients. ‐Assess coagulation acute phase reactants; Von Willebrand factor (VWF), (FVIII), and acquired thrombophilia in the same patients and correlate them to severity and outcome of the patients.
Methods: Observational cross‐sectional study included 106 COVID‐19 patients, 51 controls, in accordance with Declaration of Helsinki and approved by ethics committees of Assiut University IBR 17300413. Informed consent was taken. Patients were divided according to severity and presence of thrombosis. Continuous variables compared with Student t test. Categorical data compared by Chi2 test. Correlation assessed by Pearson correlation. Diagnostic performance of VWF, lupus anticoagulant (LA) and antithrombin III (ATIII) were determined by ROC curve. Level of confidence was kept at 95%; p value was significant if <0.05
Results: Results showed significantly higher aPTT, VWF, D‐dimer, and LA1(screening) and LA2(confirmation) in patients than control group. Significantly higher INR, aPPT, D‐dimer FVIII, VWF, LA1,2 and lower ATIII were detected in severe group (Table 1). ATIII had high diagnostic accuracy in severity prediction. We found significantly higher INR and VWF among patients with thrombotic events. For prediction of thrombosis; VWF at cutoff >257.7 has 83.3% sensitivity and 83.3% specificity (Figure 1).
Conclusion(s): Our results are confirming the relationship between COVID‐19 associated coagulopathy and severity, poor outcome of the patients; INR, aPPT and D‐dimer are the bad prognostic markers. D‐dimer is a chief tool in diagnosis, severity evaluation but not thrombosis prediction. FVIII, VWF and ATIII are markers of severity. VWF could be used as assistant/alternative of D‐dimer in diagnosis, severity assessment and superior in thrombosis risk prediction. Sequent LA1, LA2 and their ratios to specify presence of LA didn't show difference between controls and patients.


PB0067
Safety of Sinopharm Covid‐19 vaccine in Iranian patients with congenital bleeding disorders: A preliminary report
M. Karimi 1; A. Shahsavani2; S. Haghpanah2; T. Zarei2; A. Bazrafshan2
1 Hematology Research Center Shiraz University of Medical Sciences, Shiraz, Iran, Shiraz, Fars, Iran; 2 Shiraz University of Medical Sciences, Shiraz, Fars, Iran
Background: There are limited data regarding safety of Sinopharm vaccine in patients with congenital bleeding disorders (CBD). The guidance published by World federation of hemophilia mainly recommends prophylaxis therapy in hemophilia patients with factor activity below 10% before getting vaccinated.
Aims: To evaluate side effects of Sinopharm vaccine in patients with CBD.
Methods: A total of 93 patients including 75 males and 18 females who filled the consent form, participated in this historical cohort study. A questionnaire was designed to report any experienced side effects. In case of continuing feel of discomfort at the injection site, it was recommended to administration of coagulation factor concentrate.
Results: The mean age of the patients was 37 ± 12.8 (18–78) years. The type of bleeding disorders is shown on table 1. The disease severity was mild in 41.9%, moderate in 8.9%, and severe in 41.9% of the patients. About 65% of patients had coagulation factor activity less than 10%. Comorbidities were seen in 7 patients. The most common reported side effects were headache (6.6%), feeling weakness (5.5%) and body pain (4.4%). Two patients (2.1%) experienced hematoma following vaccination in injection site, one severe hemophilia A patient who recovered without any treatment. Another one was a severe hemophilia B who received Factor concentrate resulted in disappearing hematoma.
Conclusion(s): No safety concerns were detected in patients who received two doses of Sinopharm vaccine. Moreover, our results showed that it seems vaccination is safe in patients with factor activity less than 10% without giving prophylaxis. This issue is important due to the limited access to coagulation factors in developing countries.

PB0068
Comparative profiles of routine and specialized biomarkers of coagulopathy and endotheliopathy in moderate and severe COVID‐19 disease: A prospective observational study
G. Bousquet1; R. Lacroix 2; L. Pahus3; S. Valera3; L. Arnaud3; E. Abdili4; F. Dignat‐Georges3; N. Hezard3; L. Papazian5; S. Hraiech6; J. Forel7; P. Morange8; C. Guervilly6; P. Chanez3
1 Assistance Publique des Hôpitaux de Marseille, Marseille, Provence‐Alpes‐Cote d'Azur, France; 2 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN, Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 APHM, Marseille, Provence‐Alpes‐Cote d'Azur, France; 4 APHM, Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 5 Aix‐Marseille Univ, APHM, Medical Intensive Care Unit, North Hospital‐Center for Studies and Research on Health Services and Quality of Life, EA3279 Research Unit, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 6 Aix‐Marseille Univ, APHM, Medical Intensive Care Unit, North Hospital ‐ Center for Studies and Research on Health Services and Quality of Life, EA3279 Research Unit, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 7 Aix‐Marseille Univ, APHM, Medical Intensive Care Unit, North Hospital ‐Center for Studies and Research on Health Services and Quality of Life, EA3279 Research Unit, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 8 C2VN, INRAE, INSERM Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: Endothelial injury and coagulation activation are prominent axis in coronavirus disease 19 (COVID‐19) pathogenesis. However, only few studies have compared profiles of biomarkers of coagulopathy and endotheliopathy with regard to the initial severity of COVID‐19 and the time course of the disease.
Aims: The main objective of our study is to point profiles of biomarkers of coagulopathy and endotheliopathy associated with initial severity of COVID‐19 and the time course of the disease.
Methods: In a prospective longitudinal study, we explored coagulation and endothelial function biomarkers at admission, at day 3 and at day 7 in two cohorts of severe and moderate COVID‐19 patients and according to outcome.
Results: We found that D‐dimers, free tissue factor pathway inhibition (TFPI) and extracellular vesicles – tissue factor (EV‐TF) at admission were associated with disease severity. Concerning outcome, TFPI, EV‐TF and von Willebrand factor antigen (VWF)/ADAMTS13 ratio at admission, at day 3 and at day 7 were associated with not favorable outcome defined as the onset of death or need of invasive mechanical ventilation. TFPI with a cut‐off value of 37.5 ng/ml had 87% sensitivity and 94% and specificity and EV‐TF with a cut‐off value of 13 fM had 87% sensitivity and 83% specificity. ROC curves analysis for severe outcome was significant with AUC = 0.90 for TFPI and AUC = 0.88 for EV‐TF (p < 0.001 for both).
Conclusion(s): We found a specific profile of specialized biomarkers of coagulopathy and endotheliopathy associated with severe form of COVID‐19 and not favorable outcome. We identified free TFPI and EV‐TF of potential interest to stratify patient’s risk or to design specific treatment targeting coagulation pathways. Additional studies are warranted to confirm our results and study the interaction between these biomarkers’ values and anticoagulation treatment.


PB0082
Biomarkers in COVID‐19: Association with disease severity and outcomes
M. Peralta 1; V. Muczynski2; P. Lane3; L. Pagala3; A. Riddell3; S. Bhagani4; B. Agarwal5; K. Gomez6; A. Drebes3; J. McVey7; T. Kotecha8; D. Martin8; A. Davenport8; A. Laurence9; A. Brachat10; T. Sood8; L. Lanning3; T. Gandhi3; P. Chowdary11
1 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free London NHS Foundation Trust, London, UK, LONDON, England, United Kingdom; 2 Research Department of Haematology, University College London (UCL) – Cancer Institute, London, United‐Kingdom, London, England, United Kingdom; 3 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free London NHS Foundation Trust, London, UK, London, England, United Kingdom; 4 Department of Infectious Diseases, Royal Free London NHS Foundation Trust, London, UK, London, England, United Kingdom; 5 Department of Intensive Care and Anaesthesia, Royal Free London NHS Foundation Trust, London, UK, London, England, United Kingdom; 6 Cancer Institute, University College London, London, UK, London, England, United Kingdom; 7 University of Surrey, Guilford, England, United Kingdom; 8 Royal Free London NHS Foundation Trust, London, UK, London, England, United Kingdom; 9 University College of London, London, UK, London, England, United Kingdom, 10 Biozentrum, University of Basel, Basel, Basel‐Landschaft, Switzerland, 11 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom
Background: In coronavirus disease 2019 (COVID‐19) the need for intervention increases with disease severity and a risk prediction model that incorporates biomarkers would be beneficial for identifying patients for treatment escalation.
Aims: To investigate biomarkers changes associated with disease severity and outcomes (mortality, thrombosis).
Methods: COVID‐19 patients were sampled between April 15 and May 31 2020. Disease severity was assessed by World Health Organization (WHO) ordinal scale. 132 systemic biomarkers were investigated by routine and multiplex assays and statistical analysis performed to characterise the biomarker profile of COVID‐19 patients associated with disease severity, duration, survival and thrombosis.
Results: The study enrolled 150 COVID‐19 positive adults and 16 healthy volunteers. The average age was 64 years, 59% were male, 85% had co‐morbidities, 33% had a thrombotic event, and 13% died. A cross comparative analysis of biomarkers identified 13 biomarkers common to severity, mortality and thrombosis with significant correlation; including endothelial dysfunction (VWF, tPA, TFPI), hypercatabolism (low albumin, Hb, FXIII) and inflammatory response (IL‐8, Osteopontin). Similarly, 14 biomarkers associated with severity and mortality included pro‐inflammatory cytokines and their receptors (sTNFRII, STNFRI, sIL2a, IL6, MIP1a), neutrophils (elevated WBC, Neutrophils, TIMP1) and tissue remodelling (SCGF, EG3A). Nine biomarkers common across severity and thrombosis were angiogenesis (VEGF, LYVE1, Follistatin), acute phase response (SAP, AGP) and clot formation (Fibrinogen and PAPs).
Conclusion(s): The biomarker profile associated with poorer outcomes indicates an inflammatory response, endothelial cell disruption, hypercoagulability and hypercatabolism. This study has identified several biomarkers that may be useful indicators of disease severity and progression. Further work is needed to determine how these may be used to direct clinical management.

VPB0105
COVID‐19 coagulopathies—Highlights On 2020‐2021 Reported Data
S. Anil Kumar 1; A. Pradhan1; A. Elsebaie1; K. Fainchtein1; A. Noureldin1; Y. Tera1; S. Kazi2; M. Othman1
1 Queen's University, Kingston, Ontario, Canada; 2 Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, England, United Kingdom
Background: COVID‐19 pandemic has evolved dramatically over the past 2 years and literature on COVID coagulopathy has been overwhelming. Understanding literature and assessing the quality of data available is a challenge which is furthermore complicated by the difficulty in defining the ‘waves of infection’ across the globe.
Aims: To provide highlights on literature regarding coagulation impairments, thrombotic complications, and anticoagulation use in severe COVID‐19 patients, over the past 2 years of the pandemic.
Methods: Performed a systematic search on MEDLINE, EMBASE and EPUB Ahead of Print AND Other Non‐Indexed Citations (from inception to 18th July 2021). Studies were eligible for inclusion if written in English, reporting severe COVID‐19/hospitalized patients and reporting coagulopathy data and thrombotic complications. Articles had to be published in journals with impact factor 3 or above. Data abstracted on country, total number of patients, age and sex, coagulation parameters, thrombotic complications, and anticoagulation data.
Results: Identified 62 studies (PRISMA in Figure 1). A total of 18,581 patients reported from 16 different countries (Figure 2) published between March 2020 to July 2021 were included in this review. Coagulation lab parameters were reported in most studies with considerable heterogeneity on data reported. A key finding is a pro‐coagulant profile with hypercoagulability more pronounced in ICU patients. Controversy existed around thrombocytopenia in association with severe or late disease. Elevated fibrinogen was reported in 37/41 (90%) studies. Elevated D‐dimer was consistently reported and was predictive of thrombosis and poor outcome. 46 (74%) studies reported VTE which occurred despite guideline‐recommended thromboprophylaxis. Anticoagulation was reported in all studies, but practices were diverse.
Conclusion(s): Evident heterogeneity of clinical and laboratory findings of reported studies with inconsistent reporting on coagulation parameters, units of measurement and relationship to disease outcomes. This study can help investigators carefully design future studies related to coagulopathy in COVID‐19.


VPB0107
Hemostasis markers determining unfavorable outcome in covid‐induced coagulopathy
I. Berger 1; A. Makhmudova2; M. Shamsutdinova3; A. Achilova2; O. Madasheva2
1 Head of the Scientific Laboratory of Hemostasis Pathology, Tashkent, Toshkent, Uzbekistan; 2 Republican Scientific and Practical Medical Center of Hematology (RSPMTSG) of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Toshkent, Uzbekistan; 3 Specialized multidisciplinary infectious diseases clinic for the treatment of coronavirus infection Zangiota 1, Tashkent, Toshkent, Uzbekistan
Background: We analyzed the laboratory parameters of 150 hospitalized patients with COVID‐19 associated pneumonia with severe damage to the lung fields (more than 55%). All patients were admitted to the intensive care unit on admission due to the severity of their condition. Analysis of laboratory data was carried out on the first day after admission. Of the 150 patients with severe course, 26 deaths (17.3%) and 124 (82.6%) recovery were recorded.
Aims: The aim was to identify the most significant marker of hemostasis that determines the outcome of the disease
Methods: The study of hemostasis parameters was carried out on an ACL‐TOP 350 coagulometer manufactured by Internetional Laboratory (USA), including indicators of D‐dimer, antithrombin III, protein C, free protein S, and other HemosIL reagents (USA).
Results: In patients with a lethal outcome, compared with surviving patients, there were observed: more severe lymphopenia, higher levels of leukocyte and neutrophil counts, higher values of D‐dimer concentration, PTI below 70%, and higher concentration values were recorded in biochemical studies blood urea and creatinine. 75% of patients had high levels of C reactive protein and D‐dimer in almost 100% of the cases among the deceased. Disease severity was associated with more pronounced changes in laboratory parameters. One of the parameters of the cytokine storm was taken IL ‐ 6, which in 35% of cases was more than 10 pg/ml in recovered patients and in 64.5% of cases with mortality.
Conclusion(s): Thus, it can be argued that the combined determination of the level of D‐dimer and IL‐6 has the highest predictive value in relation to the development of a severe form of COVID‐19.

VPB0117
Efficacy and safety of two heparin regimens for prevention of venous thromboembolism in hospitalized patients with COVID‐19: A meta‐analysis
M. Graziani 1; M. Vedovati2
1 Internal, Vascular, and Emergency Medicine Department at the University of Perugia, Italy, Perugia, Umbria, Italy; 2 University of Perugia, Perugia, Umbria, Italy
Background: Venous thromboembolism (VTE) is common in patients with coronavirus disease‐2019 (COVID‐19). The optimal heparin regimen remains unknown and should balance thromboembolic and bleeding risks.
Aims: The aim of this study is to evaluate the efficacy and safety of standard or higher heparin regimens for the prevention of VTE in patients hospitalized due to COVID‐19.
Methods: We performed a systematic literature search; studies reporting on hospitalized patients with COVID‐19 who received standard heparin prophylaxis vs. high (intermediate or therapeutic) heparin regimens were included if outcome events were reported by treatment group and more than 10 patients were included. Primary study outcome was in‐hospital VTE. Secondary study outcomes were major bleeding (MB), all‐cause death, fatal bleeding and fatal pulmonary embolism.
Results: Overall, 30 studies (10,637 patients) were included. Venous thromboembolic events occurred in 5.5% and in 8.8% of patients who received heparin prophylaxis with at high‐dose or standard‐dose, respectively (RR 0.70, 95% CI 0.54–0.90, I2 58.9%). MB was significantly higher in patients who received high‐ compared to the standard‐dose (3.8% vs. 2.1%, RR 1.89, 95% CI 1.41–2.53, I2 18.1%). Sub‐analyses showed a slight benefit associated with high‐dose heparin in patients admitted to non‐intensive care unit (ICU) but not in those to ICU. No significant differences were observed for mortality outcomes.
Conclusion(s): Heparin prophylaxis at high‐dose reduces the risk of VTE, but increased the risk of MB compared to the standard‐dose. No clinical benefit for heparin high‐dose was observed for ICU setting, but its role in the non‐ICU deserves further evaluation.
VPB0128
Characteristics of critically Ill COVID‐19 patients with positive heparin‐induced thrombocytopenia antibody
T. Nguyen; T. Tran; A. Vo
Cho Ray Hospital, Ho Chi Minh City, Ho Chi Minh, Vietnam
Background: COVID‐19 triggers a potentially fatal hypercoagulable state. Multiple mechanisms may contribute to this disorder, among which the production of abnormal antibodies, especially Heparin‐induced thrombocytopenia (HIT) antibodies. There have been a few single case reports about HIT in COVID‐19 patients. Nevertheless, risk factors for HIT in COVID‐19 patients are still under investigation.
Aims: We present a case series of critically ill COVID‐19 patients with positive HIT antibodies treated at Cho Ray Hospital from July 2021 to December 2021.
Methods: This is a retrospective case series report of critically ill COVID‐19 patients who were tested positive for HIT antibodies using an Anti‐PF4/Heparin antibody chemiluminescent assay with a cut‐off value of 1.0 U/ml. Demographic information, clinical and laboratory characteristics were described.
Results: 17 patients (9 female, 8 male) were included in the case series. Mean age was 59.24 ± 12.95. Mean heparin exposure duration was 13.94 ± 8.26 days for all types and 10.88 ± 7.15 days for unfractionated heparin. Mean HIT antibody titer was 2.45 ± 1.91 U/ml. 2/17 patients were also tested positive for antiphospholipid antibodies. Baseline platelet count was 235.82 ± 76.42 × 10e9/L. Nadir platelet count was 63.35 ± 51.58 × 10e9/L. Baseline D‐dimer was 6813.47 ± 15028.00 ng/ml. Peak D‐dimer was 14839.71 ± 16,436.64 ng/ml. Only 2/17 patients had proven thrombosis. 10/17 patients had secondary infections, most notably with Acinetobacter baumanii (6/17) and Klebsiella pneumoniae (5/17.) 16/17 patients perished in‐hospital.
Conclusion(s): From our case series, we suspect that beside long, continuous exposure to heparin, secondary bacterial infections can be an important risk factor for HIT in COVID‐19 patients. Furthermore, co‐positivity with antiphospholipid antibodies may occur and complicate treatment options. Lastly, critically ill COVID‐19 patients with positive HIT antibody seemed to have a very poor survival rate. However, whether this phenomenon is related to HIT remains to be investigated in future studies.

PB0043
Optimal anticoagulation intensity for patients with COVID‐19 treated with Tocilizumab
S. Bristogiannis 1; S. Paketci2; J. McCay2; A. Danga2; R. Haji2; K. Patel2; T. Sugai2; R. Kaczmarski2; J. Thachil3
1 Evaggelismos General Hospital, Athens, Greece; 2 The Hillingdon Hospitals NHS Foundation Trusts, London, England, United Kingdom; 3 NHS Manchester University Hospital, Manchester, England, United Kingdom
Background: Tocilizumab reduces the need and the duration of organ support and provides a survival benefit for patients at the early stages of COVID‐19 (Coronavirus Disease 2019) that have increasing oxygen needs and a significant inflammatory response. Contrary to expectations that this treatment would, also, break the vicious cycle of immunothrombosis of COVID‐19, it has been debated whether it is associated with a conversely increased venous thromboembolism (VTE) incidence. In the interim, society guidelines have updated their recommendations advising a prophylactic over that of an intermediate or treatment dose of anticoagulation but there is a lack of evidence based for this group of patients.
Aims: The purpose of this study was to compare the incidence of VTEs in patients with COVID‐19 treated with Tocilizumab in relationship to the thromboprophylaxis dose determined by NICE guidelines.
Methods: A retrospective, cohort study was performed including all patients with COVID‐19 admitted at NHS Hillingdon Hospital (UK) who required Tocilizumab between December 2020 and September 2021.
Results: Sixty‐three patients (20 females; 43 males) with a median age of 63 y.o. (17‐83) were analysed (Table 1). A Spearman’s rank correlation was run to determine the relationship between the anticoagulation strategy and the thromboembolic risk in this context. A moderate negative correlation was found between the anticoagulation intensity and the risk of a VTE, r (61) = −0.470, p = 0.000. Binary logistic regression was then used to determine the relationship between anticoagulation intensity and VTEs and Multinomial Logistic Regression for the opposite relationship. Treatment dose thromboprophylaxis is related with more VTEs however this is likely a reflection that patients with VTEs receive appropriate antithrombotic therapy (Table 2).
Conclusion(s): The present study suggests that patients with COVID‐19 that receive Tocilizumab are not at increased thromboembolic risk and thus standard thromboprophylaxis should suffice. Findings should be confirmed in randomized controlled trials.


PB0045
A pilot, open‐label, phase II clinical trial of nebulised recombinant tissue‐plasminogen activator in patients with COVID‐19 acute respiratory distress syndrome: The PACA trial
P. Chowdary 1; B. Agarwal2; S. Bhagani3; M. Lipman4; K. Gomez5
1 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom; 2 Department of Intensive Care and Anaesthesia, Royal Free London NHS Foundation Trust, London, UK, London, England, United Kingdom; 3 Department of Infectious Diseases, Royal Free London NHS Foundation Trust, London, UK, London, England, United Kingdom; 4 Respiratory Medicine, Royal Free London NHS Foundation Trust, London, UK and UCL Respiratory, University College London, London, UK, London, England, United Kingdom; 5 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, UK and Cancer Institute, University College London, London, UK, London, England, United Kingdom
Background: Alveolar fibrin deposition and pulmonary microthrombi are pathophysiological features of COVID‐19‐induced respiratory failure. Nebulised thrombolysis offers locally targeted therapy with potentially lower bleed risk than systemic administration.
Aims: To investigate the safety and potential for clinical efficacy of nebulised recombinant tissue plasminogen activator (rt‐PA) for improving oxygenation in patients hospitalised with COVID‐19 complicated by mild to severe acute respiratory distress syndrome (ARDS).
Methods: Patients hospitalised with severe COVID‐19 and a PaO2/FiO2 (P/F) ratio of <300 (units), requiring invasive mechanical ventilation (IMV) or non‐invasive respiratory support (NIRS) received 40–60 mg per day of rt‐PA, dosed for ≤14 days in a phase II, open‐label, single‐centre pilot study. Efficacy was assessed via improved oxygenation. Safety was assessed by treatment‐related serious adverse event bleeding and reduction of fibrinogen to <1.0–1.5 g/L.
Results: Nine (Cohort 1 [C1]; 6/9 IMV, 3/9 NIRS) and 26 adults (Cohort 2 [C2]; 12/26 IMV, 14/26 NIRS) received nebulised rt‐PA alongside standard of care. Matched historical controls (HC) (n = 18) were used for comparison in C1. Mean P/F ratio increased in both C1 groups from baseline (BL) to end of study (EOS) (rt‐PA; 154.4 to 298.8 vs. HC; 154.1 to 211.6); a linear mixed effect model confirmed higher P/F ratios in the rt‐PA group. Among C2 groups, greater improvements in mean P/F ratio from BL to EOS were seen in the NIRS group (NIRS; 125.5 to 239.4 vs. IMV; 120.3 to 188.2). Four potentially treatment‐related bleeds were reported; no clinically significant fibrinogen reductions were observed. Lower mortality was observed in the C1 rt‐PA group (11.1%) vs. the HC group (55.6%) and in the C2 NIRS group (21.4%) vs. the IMV group (41.7%).
Conclusion(s): Nebulised rt‐PA is well‐tolerated, improves oxygenation in patients with COVID‐19‐related ARDS, and merits a randomised controlled trial to confirm efficacy and potentially identify a subgroup of interest.
PB0048
Genetic variants and biological markers of coagulation and inflammation to predict the survival outcome upon SARS‐CoV‐2 infection in a cohort of patients from the first wave of COVID‐19 in Argentina
F. Aranda1; S. Perés1; E. Cunto2; V. Chediack2; M. Peralta2; A. Bocassi2; J. Valero2; M. Svencionis3; J. Chamorro2; T. Rubilar4; A. Crespi Abril4; G. de Larrañaga 5
1 Hospital of Infectious Diseases "Dr. Francisco J. Muñiz", Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Hospital Of Infectious Diseases "Dr. Francisco J. Muñiz", Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital Prof. Dr. Luis Güemes de Haedo, Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Centro para el Estudio de Sistemas Marinos (CONICET) e Universidad Nacional de la Patagonia San Juan Bosco, Puerto Madryn, Chubut, Argentina; 5 Hospital Of Infectious Diseases "Dr. Francisco J. Muñiz", Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: The severe clinical conditions and fatal outcomes resulting from SARS‐CoV‐2 infection have been associated with increased inflammatory and hypercoagulability processes, where an interplay between different comorbidities and genetic factors would be directly involved in the poor prognosis.
Aims: To study the association of different genetic variants, clinical variables and biological markers involved in inflammation and coagulation with the survival outcome of COVID‐19.
Methods: 204 unvaccinated COVID‐19 confirmed patients were grouped and compared according to their survival outcome. Inflammatory and hemostatic variables measured upon hospital admission, different clinical variables and the genotypic distributions of the genetic variants Factor II20210A (II20210A), Factor V Leiden (FVL), Fibrinogen Gamma 10034C>T (FGG10034C/T), Factor XI7872C>T (FXI7872C/T) and rs11385942 were compared between patients that either died (n = 63) or survived (n = 141). Differences between continuous variables were analyzed by either a t‐test or a U‐Mann Whitney test and differences in genotypic distributions were studied by a Chi‐square test. Cut off values were set for continuous variables significantly associated with a fatal outcome and their Odds Ratios (OR) of association were calculated through univariate logistic regression.
Results: The group of patients who died were significantly older, had a higher BMI index and presented with a lower platelet count, lower lymphocytes levels, higher levels of leukocytes and neutrophils, a higher neutrophils/lymphocytes ratio and higher levels of D‐dimer, Ferritin and LDH than survivors. No differences were observed in the genotypic distributions of the genetic variants studied between both groups.
Conclusion(s): In agreement with previous studies, age, obesity, and the levels of different hematological and plasmatic markers upon hospital admission would be useful predictors of a fatal outcome in COVID‐19 patients. Despite the typical exacerbation of inflammation/coagulation in severe COVID‐19, no association was found between the carriage of any of the proinflammatory/prothrombotic genetic variants studied and a higher risk of dying.

PB0078
Lupus anticoagulant as predictor for thrombosis in critically ill COVID‐19 patients: A retrospective cohort study
T. Noordermeer 1; R. Schutgens2; C. Visser3; E. Rademaker4; M. de Maat5; A. Jansen6; M. Limper7; O. Cremer8; M. Kruip9; H. Endeman10; C. Maas11; B. de Laat12; R. Urbanus1
1 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 2 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 3 Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 5 Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 Erasmus MC, University Medical Center, Rotterdam, Zuid‐Holland, Netherlands; 7 Department of Rheumatology and Clinical Immunology, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 8 Department of Intensive Care Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Utrecht, Utrecht, Netherlands; 9 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 10 Department of Adult Intensive Care, Erasmus Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 11 UMC Utrecht, Utrecht, Utrecht, Netherlands; 12 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: Thrombosis is a frequent and severe complication in COVID‐19 patients admitted to the intensive care unit (ICU). Lupus anticoagulant (LA) is a strong acquired risk factor for thrombosis in various diseases and is frequently observed in COVID‐19 patients. Whether LA is associated with thrombosis in patients with severe COVID‐19 is currently unclear.
Aims: To investigate if LA is associated with thrombosis in critically ill COVID‐19 patients.
Methods: The presence of LA and other antiphospholipid antibodies was assessed in COVID‐19 patients admitted to the ICU. Informed consent was obtained by an opt‐out approach and the study was approved by the local medical ethical committee. LA was determined with dilute Russell’s Viper Venom Time (dRVVT) and LA‐sensitive Activated Partial Thromboplastin Time (aPTT) reagents. Statistical analysis to study the association of LA and other antiphospholipid antibodies with thrombosis occurrence was performed using logistic regression.
Results: Out of 169 COVID‐19 patients, 116 (69%) tested positive for at least one antiphospholipid antibody upon admission to the ICU. Forty (24%) patients tested positive for LA; of whom 29 (17%) tested positive with a dRVVT, 19 (11%) tested positive with an LA‐sensitive aPTT and eight (5%) tested positive on both tests. Fifty‐eight (34%) patients developed thrombosis after ICU admission. The odds ratio (OR) for thrombosis in patients with LA based on a dRVVT was 2.4 (95%‐CI: 1.1–5.4), which increased to 5.1 (95%‐CI: 1.7–15.4) in patients on or below the median age of this study population (64 years). LA‐positivity based on a dRVVT or LA‐sensitive aPTT was only associated with thrombosis in patients younger than 65 years (OR: 4.2, 95%‐CI: 1.5–11.7).
Conclusion(s): LA on admission is strongly associated with thrombosis in critically ill COVID‐19 patients, especially in patients <65 years of age.
PB0088
Telehealth and health outreach mode of healthcare delivery in patients with bleeding disorders: Experience from a Tertiary Care Centre during COVID‐19 pandemic
M. Saleh; V. Mohammed; A. AlJefri; A. Alkofide; B. Abdo; B. Safi
King Faisal Specialist Hospital & Research Centre, Riyadh, Ar Riyad, Saudi Arabia
Background: Telehealth and health outreach facilities evolved as an integral component of healthcare delivery during COVID‐19 pandemic; digital healthcare tools such as Virtual Clinics (VCs) enterprise along with outreach facilities adapted for uninterrupted healthcare delivery. Pediatric Non‐Malignant Blood Disorders (NMBDs) section at King Faisal Specialist Hospital and Research Centre, Riyadh is a tertiary referral center for patients with bleeding disorders.
Aims: To maintain access to care facilitating comprehensive care through physician consults, laboratory investigations and blood products supply
Methods: Ambulatory care visits amongst children (≤14 years) collected and dashboard created utilizing REDCap for tracking visits (in‐person and virtual) during the pandemic (April’2020 to April’2022).
Results: Total of 7610 visits on 4610 patients identified with 72% (5520) in‐person and 27% (2100) virtual visits; increase in virtual visits by 328% (490 visits) observed while comparing 12 month period prior to the COVID‐19 pandemic ; in‐person visits decreased by 15% (6493 visits) with an overall increase of 10% in ambulatory care visits, indicative of substitutive care delivery mode. Majority of virtual visits observed amongst Hemoglobinopathies, 45%, Bone Marrow Failures, 36% and bleeding disorders, 19%; with an average of 2.0 virtual visits per patient observed. Amongst bleeding disorders majority of the cases were Hemophilia, 40% (87); Von Wille Brand, 30% (65); Rare bleeding disorders, 21% (46) and Glanzmann thrombasthenia 9% (21).Combination of telehealth and health outreach resulted in timely and uninterrupted laboratory result reviews, medication refills and dental referrals and factor‐replacement products were delivered in collaboration with outreach – satellite pharmacies.
Conclusion(s): In our experience, integrating telehealth in alliance with regional outreach facilities found effective in maintaining continuity of care, demonstrating an unexpected prospect in the face of adversity. We plan to continue virtual care to resolve patient access, clinician shortage and geographic disparities while supporting the National Healthcare Transformation Program driven by the Saudi Vision 2030.
VPB0127
Dynamics of hemostasis system’s parameters in patients with severe course of Covid‐19 undergoing extracorporeal membrane oxygenation
E. Klychnikova; I. Ivanov; E. Tazina; S. Zhuravel; K. Popugaev; S. Petrikov
N.V. Sklifosovsky Research Institute for Emergency Medicine, Public Healthcare Institution of Moscow Healthcare Department, Moscow, Moskva, Russia
Background: Extracorporeal membrane oxygenation’s (ECMO) support causes various hemostatic disorders that are poorly understood, especially in COVID‐19 patients with severe acute respiratory distress syndrome (ARDS)
Aims: To assess changes in hemostasis parameters during and without ECMO support in COVID‐19 patients with severe ARDS
Methods: A prospective study of 122 patients with a severe course of COVID‐19 was carried out. The average age was 59 ± 12 years. The patients’ condition, assessed by the NEWS and SOFA severity score, was 4.5 ± 1.4 and 6.1 ± 1.3 points, respectively. Retro‐prospective study of 130 patients with a severe course of COVID‐19 requiring the use of ECMO was carried out. The average age of patients was 55 ± 6 years. The patients’ condition, assessed by the RESP and SOFA severity score, was 1 ± 2 and 10 ± 2 points respectively. Median ECMO duration was 8 [IQR 4–14] days and median time from intubation to ECMO start was 1 [IQR 1–3] day. All patients were examined for PT, FG, Pr C, AT III, D‐dimer and platelet count. The research was performed on day 1 and 7 after ECMO application. Statistical analysis was carried out using Statistica 10.0 software.
Results: On day 1 patients with ECMO showed a significant decrease in the level of FG, PT, platelet count and the activity of AT III (p < 0.05) and an increase in the level of D‐dimer (p < 0.05). On day 7 there was also a decrease in the level of FG and PT, the activity of AT III, Pr C and an increase in the level of D‐dimer.
Conclusion(s): ECMO triggers early activation of coagulation, which leads to the consumption of FG, PT and platelets, followed by hypofibrinogenemia and thrombocytopenia that may possibly contribute to the high rate of hemorrhagic complications. On the other hand, a decrease in the activity of AT III and Pr C may contribute to thrombotic complications.

PB0038
Sources of heterogeneity in studies reporting the venous thrombotic risk in patients admitted with COVID‐19: A meta‐analysis and explorative meta‐regression
S. Bhoelan; C. Codreanu; V. Tichelaar; J. Borjas Howard; K. Meijer
1 University Medical Centre Groningen, Groningen, Groningen, Netherlands
Background: Meta‐analyses on venous thromboembolism (VTE) risk in admitted patients with COVID‐19 have shown substantial heterogeneity among included studies.
Aims: To explore sources of heterogeneity among studies estimating VTE risk in COVID‐19 patients.
Methods: A systematic review using the databases PubMed and Embase was performed searching for studies reporting VTE risk in patients admitted to the ward or intensive care unit (ICU). Analysis was performed with studies from which a incidence proportion could be retrieved. The pooled incidence of VTE and heterogeneity (I2) were estimated in a random‐effects model stratified by ICU or ward. Incidence estimates were logit‐transformed. The effect of 12 pre‐selected clinical and methodological variables were explored in a univariable linear meta‐regression model. Because of limited degrees of freedom, a multivariable model was constructed in a stepwise fashion. The outcome measure was regression coefficient with a 95% confidence interval (CI).
Results: Fifty‐three and 26 studies reported on incidence of VTE at the ICU and ward respectively. The pooled incidence at the ICU was 20.3% (95% CI 16.4‐24.8%, I2 95.4%) and at the ward was 4.7% (95% CI 3.0‐7.3%, I2 96.2%). In studies concerning ICU patients, asian region, VTE‐screening, standard error and date of publication were significantly associated with incidence of VTE in the univariable model. Two multivariable models were fitted (Table 1). In the multivariable model only standard error remained significantly associated. In studies concerning ward patients, average age, VTE‐screening, standard error were significantly associated in the univariable model. Two multivariable models were fitted (Table 2). Again, only standard error remained significantly associated.
Conclusion(s): Studies with a high standard error yielded a higher VTE incidence in COVID‐19, strongly suggesting publication bias. Remaining heterogeneity was not completely explained, but may be due to differences in setting/outcome definition and clinical practice.


PB0046
Association between thrombin generation and clinical characteristics in COVID‐19 patients
O. Cohen 1; N. Landau1; E. Avishai1; T. Barazani1; I. Budnik2; T. Livnat1; K. Asraf1; R. Doolman3; S. Levy‐Mendelovich1; E. Orly1; U. Manor1; E. Meltzer1; G. Segal1; G. Rahav1; G. Kenet4
1 Sheba Medical Center, Tel Aviv University, Ramat Gan, HaMerkaz, Israel; 2 Sechenov First Moscow State Medical University, Moscow, Moskva, Russia; 3 Bar Ilan University, Ramat Gan, HaMerkaz, Israel; 4 Sheba Medical Center & Tel Aviv University, Tel Aviv, Tel Aviv, Israel
Background: COVID‐19 disease is associated with coagulopathy and an increased risk of thrombosis. A high thrombin generation (TG) capacity has been reported among patients, however, association between TG, disease severity and outcomes has not been well described.
Aims: To assess the correlation of TG with the sequential organ failure assessment (SOFA) and sepsis‐induced coagulopathy (SIC) scores and clinical outcomes in COVID‐19 patients.
Methods: Plasma samples of hospitalized COVID‐19 patients were sequentially collected and analyzed for TG. Demographic and clinical data was retrieved from medical records. SOFA and SIC scores were calculated for all patients. Statistical analysis was performed using GraphPad Prism (version 7.0), USA.
Results: Thirty‐two patients (69% male), whose median age was 69 years were assessed. Among patients’ comorbidities hypertension, cardiovascular illness, dyslipidemia and malignancy prevailed. Only 3 patients did not receive anticoagulant therapy. The median (IQR; range) SOFA and SIC scores of our patients were 3 (2,6; 0–10) and 3 (2,4; 0–6), respectively. D‐dimers were uniformly increased. During hospitalization 2 patients had a thrombosis, 3 suffered a bleeding and 12 died. Initial Lag time, endogenous thrombin potential (ETP) and peak height (PH) of our patients did not significantly differ from the values obtained from non‐anticoagulated healthy controls. Nevertheless, patients who received higher than prophylactic doses of anticoagulant therapy had increased lag time (p = 0.003), lower ETP (p = 0.037) and a reduced PH (p = 0.006). ETP and PH were slightly increased among patients with severe and critical COVID‐19 as compared with mild and moderate patients (Figure 1). ETP correlated with the SIC score (p = 0.038), however there was no correlation between any of the TG parameters and the SOFA score (Figure 2) or mortality.
Conclusion(s): Thrombin generation could not predict disease severity among patients hospitalized with COVID‐19, however a correlation between ETP and the SIC score was noted and deserves attention.

PB0054
A role for Von Willebrand Factor‐ADAMTS13 axis imbalance and abnormal angiogenesis in long COVID syndrome
H. Fogarty 1; S. Ward2; L. Townsend3; E. Karampini2; S. Elliott4; M. Byrne5; C. Bergin3; J. O'Sullivan6; I. Martin‐Loeches7; P. Nadarajan8; C. Bannon3; R. Preston9; A. Rehill2; C. Ni Cheallaigh3; J. O'Donnell2
1 Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 3 Department of Infectious Diseases, St James’s Hospital, Dublin, Ireland., Dublin, Dublin, Ireland; 4 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Carlow, Ireland; 5 National Coagulation Centre, St James’s Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 6 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland., Dublin, Dublin, Ireland; 7 Department of Intensive Care Medicine, St James’s Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 8 Department of Respiratory Medicine, St James’s Hospital, Dublin, Ireland., Dublin, Dublin, Ireland; 9 Royal College of Surgeons in Ireland, Dublin, Dublin, Ireland
Background: Acute COVID‐19 is associated with marked endotheliopathy, VWF‐ADAMTS13 axis imbalance and abnormal pulmonary angiogenesis. Persistent endotheliopathy and elevated VWF levels have also been reported in convalescent COVID‐19 patients.
Aims: We investigated the hypothesis that altered pulmonary microvascular architecture may persist in COVID‐19 convalescence, resulting in ongoing endothelial cell (EC) activation and VWF‐ADAMTS13 axis imbalance, possibly contributing to Long COVID pathogenesis.
Methods: 50 patients (median age 50 years, 60% male, median 68 days post acute COVID‐19) were reviewed. Six‐minute‐walk tests (6MWT) were performed (median 6MWT distance 430m) and plasma samples collected. Plasma VWF:Ag and ADAMTS13 levels were measured by ELISA, and angiogenesis markers assessed by membrane‐based antibody array.
Results: Plasma VWF:Ag levels were significantly elevated in convalescent COVID‐19 patients compared to controls (1.1 vs. 0.84 IU/ml; p = 0.004), with 30% (15/50) having VWF:Ag levels above the upper limit of normal. In contrast, plasma ADAMTS13 was significantly reduced in convalescent COVID‐19 (median 467 ng/ml vs. 636 ng/ml p < 0.001). ADAMTS13 levels were significantly lower in those who required hospitalization for acute COVID‐19 compared with those managed as outpatients (median 454 ng/ml vs. 513 ng/ml, p = 0.04). Overall, the VWF/ADAMTS13 ratio was significantly elevated in convalescent COVID‐19 compared with controls (2.1 vs. 1.1 p = 0.0002) and interestingly was elevated in patients with reduced 6MWT distance (distance ≥430 m or <430 m: 1.8 vs. 2.4, p = 0.02). In total, 15 angiogenesis markers were elevated in convalescent COVID‐19 compared to controls. An additional 17 angiogenesis markers were unique to convalescent COVID‐19 and were not found in control plasma (Table 1).
Conclusion(s): Collectively, these novel findings demonstrate that endotheliopathy is sustained for months following acute COVID‐19 in some patients. As a result, plasma VWF levels are significantly increased; ADAMTS13 levels reduced, and there is ongoing dysregulation of angiogenesis. Further studies will be required to define whether these alterations play a role in Long COVID pathogenesis.

PB0055
Comparison of five different PF4 dependant immunoassays in the diagnosis of vaccine‐induced thrombotic thrombocytopenia (VITT)
T. Geevar 1; R. Dave1; D. M2; P. Elizabeth3; D. Daniel4; S. Nair1
1 Christian Medical College and Hospital, Vellore, Vellore, Tamil Nadu, India; 2 Christian Medical College, Vellore, Vellore, Tamil Nadu, India; 3 Christian Medical College, Vellore, Tamil Nadu, India; 4 Department of Transfusion Medicine and Immunohematology, Christian Medical College, Vellore, Vellore, Tamil Nadu, India
Background: Vaccination with ChAdOx1 Cov‐19 can rarely cause a condition called Vaccine‐induced Thrombotic Thrombocytopenia (VITT), characterised by acute thrombosis and thrombocytopenia, mediated by platelet‐activating antibodies against Platelet factor 4 (PF4), which clinically mimics heparin‐induced thrombocytopenia (HIT). VITT occurs between 4‐42 days after vaccination unrelated to heparin exposure and is associated with elevated D‐Dimer and high titre immunoglobulin‐G (IgG) class anti‐PF4 antibodies. The use of PF4/heparin immunoassays has been proposed as part of the diagnostic approach.
Aims: To compare and analyse five different types of immunoassays in suspected cases of VITT.
Methods: Blood samples from 21 patients suspected of having VITT were analysed using five different assays available for anti‐PF4 testing. These assays were divided into three groups: (1) IgG‐specific ELISA (Hyphen Biomed Zymutest HIA IgG); (2) polyspecific ELISA [IgG, IgA and IgM (IgGAM) Hyphen Biomed Zymutest HIA]; and (3) Rapid assays: (a) polyspecific Diamed PaGIA gel, (b) IgG‐specific HemosIL AcuStar HIT‐IgG and (c) Latex Immuno assay (LIA) ACL‐TOP. Results were interpreted as positive or negative using the manufacturers’ cut‐offs.
Results: In our study of 21 suspected VITT cases, 6 were positive for IgG and IgGAM ELISA, suggesting the possibility of vaccine induced anti‐PF4 antibodies. Five of the 6 cases were negative for all other rapid assays, while one was positive for PaGIA also. Two cases were positive only on LIA in ACL‐TOP. Both cases had recent history of vaccination and thrombocytopenia but presented with bleeding manifestations rather than thrombosis. One patient was positive only for IgGAM ELISA. He was a renal transplant recipient with thrombocytopenia, but no evidence of thrombosis. The interval between vaccination and presentation was more than 3 months.
Conclusion(s): Anti‐PF4 antibodies detected only on ELISAs and not on other platforms, suggest the possibility of VITT. In the appropriate clinical context, this could be viewed as laboratory “confirmation” of VITT.

PB0056
Bleeding risk of intramuscular injection of COVID‐19 vaccines in adult patients with therapeutic anticoagulation
N. Gendron 1; L. Khider2; C. Le Beller2; B. Espinasse3; C. Auditeau2; W. Amara2; G. Perrin2; D. Lebeaux2; A. Gaiffe4; S. Combret5; B. Bertin6; T. Mirault2; D. Smadja7; O. Sanchez8; C. Tromeur9; B. Planquette10; F. Couturaud11
1 Hôpital européen Georges Pompidou, Inserm UMRS_1140, Paris, Ile‐de‐France, France; 2 HEGP, Paris, Ile‐de‐France, France; 3 CHU Brest, Brest, Bretagne, France; 4 CHU Besançon, Besançon, Franche‐Comte, France; 5 CHU Dijon, Dijon, Bourgogne, France; 6 Hospices civils de Lyon, Lyon, Rhone‐Alpes, France; 7 HEGP, Inserm UMRS_1140, Paris, Ile‐de‐France, France; 8 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 9 Department of Internal Medicine and Chest Diseases, CHU de Brest, Brest, Bretagne, France, 10 APHP, Paris, Ile‐de‐France, France, 11 Inserm, Univ Brest, CHRU Brest, UMR 1304, GETBO, Departement de Medecine Interne et Pneumologie, Brest, France, Brest, Bretagne, France
Background: Concerns emerged for the management of intramuscular (IM) injections for COVID‐19 vaccines in patients with therapeutic anticoagulation.
Aims: The aim of the study was to evaluate the risk of bleeding events following IM vaccination in patients under therapeutic anticoagulation
Methods: We first performed a French multicentre prospective study including patient treated by anticoagulant therapy for venous thromboembolism between May 2021 and September 2021. Consecutive patients were asked to report bleeding events at the site of COVID‐19 vaccine injection during follow‐up. We next performed a request in the French national pharmacovigilance database to identify cases of bleeding events at the site of injections following COVID‐19 vaccine in patients under therapeutic anticoagulation between December, 27th, 2020 and June, 30th, 2021.
Results: Between May and September 2021, a total of 348 patients with anticoagulant therapy received 561 IM injections of COVD‐19 vaccines. Median age of patients was 68.4 years and 65.2% were males. Almost all patients were treated with direct oral anticoagulant (DOAC 96.6%), 11 (3.2%) patients with vitamin K antagonist and one (0.2%) with tinzaparin. Among them, 17.9% had pressure at the injection site after the injection and 4.2% had anticoagulant dose skipping before vaccination. After IM injections, a total of 3 (0.6%) bleeding events were observed, 2 (0.4%) minor and one (0.2%) clinically relevant non‐major bleeding. We next observed in the French national pharmacovigilance database a total of 13 bleeding events (all minor bleeding) at the site of injection in patients on therapeutic anticoagulation between December, 27th, 2020 and June, 30th, 2021. In France, 69,089,410 doses of COVID‐19 vaccine were administered during this period. These bleeding events correspond to a spontaneous notification rate of 0.19 cases (95% CI 0.09–0.29) reported per million of doses administered.
Conclusion(s): IM vaccination appears safe in patients under therapeutic anticoagulation in particular with DOAC, and may not require skipping doses.
PB0061
Standard dosing of enoxaparin versus unfractionated heparin in critically ill COVID19 patients: A multicenter cohort study
A. Hafiz 1; K. Al Sulaiman2; G. Korayem3; A. Altebainawi4; O. Aljuhani5
1 King Abdulaziz University, Jeddah, Makkah, Saudi Arabia; 2 College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Ar Riyad, Saudi Arabia; 3 Department of Pharmacy Practice, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Ar Riyad, Saudi Arabia; 4 Pharmaceutical Care Services, King Khalid Hospital, Hail Health Cluster, Hail, Ha'il, Saudi Arabia; 5 King Abdulaziz University, Jeddah, Makkah, Saudi Arabia
Background: Venous thromboembolism remains a major complication associated with a high incidence of mortality in COVID‐19 patients. Prophylactic anticoagulation utilizing either unfractionated heparin (UFH) or low molecular weight heparin (LMWH) remains an area of further investigation.
Aims: To evaluate the incidence of thrombotic, clinical outcomes of interest and safety of LMWH versus UFH in critically ill COVID‐19.
Methods: This is a multicenter, retrospective cohort study including adult critically ill patients with confirmed COVID‐19 and admitted to the ICU. Included patients were grouped based on the anticoagulation agent. The primary outcome was conformed arterial or venous thrombosis. Secondary outcomes included bleeding (major or minor), 30‐day mortality, hospital length of stay (LOS), ICU LOS, ventilator‐free days (VFDs) at 30 days, and ICU‐acquired complications.We adjusted comparisons for potential confounders using regression analysis. A p‐value < 0.05 will indicate statistical significance and all analyses will be performed using STATA version 9.4.
Results: 305 patients were included, 142 received UFH and 164 received LMWH. A statistical significant decrease in hospital LOS with the use of LMWH compared to UFH was observed (hazard ratio [HR] −0.28, p = 0.001).The incidence of thrombosis (odd ratio [OR] 0.24, p = 0.03), new atrial fibrillation (odd ratio [OR] −0.17, p = 0.04), acute kidney injury (odd ratio [OR] 0.25, p = 0.0005) and the need for transfusion (odd ratio [OR] 0.26, p = 0.02) was statistically significant as well favoring the LMWH group recipients. Major bleeding and minor bleeding were similar between groups (p = 0.90, p = 0.47) respectively.
Conclusion(s): LMWH might be linked with favorable outcomes in thrombosis and ICU complications reduction in critically ill patients with COVID‐19.
PB0079
Inhibitory potential of anticoagulant and antiplatelet agents on COVID‐19‐induced NETs release
J. Oliveira 1; L. Silva1; G. Damiani1; A. Santos1; B. Jacintho1; C. Vaz1; G. Mesquita1; J. Annichino‐Bizzacchi2; B. Mazetto1; E. de Paula3; F. de Andrade Orsi3
1 University of Campinas, Campinas, Sao Paulo, Brazil; 2 Hemostasis and Thrombosis Laboratory, Hematology and Hemotherapy Center, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil; 3 School of Medical Sciences of the University of Campinas, Campinas, Sao Paulo, Brazil
Background: Neutrophil extracellular traps (NETs) release is the one of the main mechanisms behind hypercoagulability and disease severity in severe acute respiratory syndromes. The identification of drugs capable of inhibiting this pathological mechanism is mandatory.
Aims: Neutrophil extracellular traps (NETs) release is the one of the main mechanisms behind hypercoagulability and disease severity in severe acute respiratory syndromes. The identification of drugs capable of inhibiting this pathological mechanism is mandatory.
Methods: Healthy neutrophils (20 × 103/well) were stimulated with phorbol myristate acetate (PMA) or sera from severe COVID‐19 patients (n = 16) in the presence or absence of dipyridamole (10 μM), aspirin (1 mM) and heparin (50 μg/mL). Neutrophils nuclei were stained with nuclear red and incubated with a medium containing the non‐permeable cell membrane marker Sytox Green. Cell images were obtained using IncuCyte ZOOM and the number of cells that suffered netosis was monitored over time. NETs release was determined after 1 h of incubation and the percentage of NETs was calculated dividing the number of green cells by the total number of cells per well.
Results: COVID‐19 induced NETs was lower in neutrophils pretreated with heparin (median 2.6%, IQR 2.6–2.9) than in non‐treated neutrophils (median 3.6%, IQR 3.2–4.0, p < 0.0001). Pretreatment with dipyridamole and aspirin did not change the effect of COVID‐19 sera in inducing NETs. A similar pattern of inhibition was observed with PMA stimulation, in which heparin decreased NETs by 3 times (NETs after PMA 43.2% and NETs after PMA and heparin 14.8%) while dipyridamole and aspirin did not significantly affect the release of PMA‐induced NETs (Figure 1). Figure 2 illustrates the identification of NETs.
Conclusion(s): Heparin was capable of inhibiting “in vitro” NETs release induced by COVID‐19, while dipyridamole and aspirin had no significant effect on this process. Such findings are in line with evidence that heparin use can improve COVID‐19 prognosis.


PB0085
Impaired exercise capacity in post‐COVID syndrome: The role of VWF‐ADAMTS13 axis
N. Prasannan 1; M. Heightman2; T. Hillman2; E. Wall2; R. Bell2; A. Kessler2; L. Neave3; A. Doyle4; A. Devaraj2; D. Singh5; H. Dehbi6; M. Scully1
1 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 2 UCLH, London, England, United Kingdom; 3 UCLH Hospitals NHS Trust/Haemostasis Research Unit, University College London, London, England, United Kingdom; 4 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 5 Health Services Laboratories, London, England, United Kingdom; 6 UCL, London, England, United Kingdom
Background: Post‐COVID syndrome (PCS) is an increasingly recognised complication of acute SARS‐CoV‐2 infection, characterised by persistent fatigue, reduced exercise tolerance, chest pain, shortness of breath and cognitive slowing. Acute COVID‐19 is strongly linked with increased risk of thrombosis; a prothrombotic state. Elevated Von Willebrand Factor (VWF) Antigen (Ag):ADAMTS13 ratio is associated with severity of acute COVID‐19 infection.
Aims: We hypothesised that the pro‐thrombotic state is implicated in the pathogenesis of PCS. We investigated specialist coagulation parameters associated with reduced exercise capacity in patients with PCS to identify the utility of these parameters to determine ongoing disease activity. We also investigated if an association exists between elevated VWF(Ag):ADAMTS13 ratio and impaired exercise capacity in patients with PCS.
Methods: Retrospective analysis of VWF(Ag):ADAMTS13 ratio in patients with PCS at a dedicated post‐COVID clinic. VWF(Ag):ADAMTS13 ratio was correlated with symptoms including exercise capacity as assessed by 1 minute sit‐to‐stand (STS) test and/or 6 minute walk test (6MWT). Peripheral oxygen desaturation ≥3% for 6MWT and STS test, and increase in lactate>1 from baseline during 6MWT were taken as markers of impaired exercise capacity.
Results: Elevated VWF(Ag):ADAMTS13 ratio (≥1.5) was found to be four times (OR 4.3) more likely in patients with impaired exercise capacity. 20% (56/276) had impaired exercise capacity, of which 55% (31/56) had a raised VWF(Ag):ADAMTS13 ratio ≥1.5 (p < 0.0001). A higher median VWF(Ag):ADAMTS13 ratio of 1.5 (IQR 1.2–1.7) in patients with abnormal exercise testing compared to 1.1 (IQR 0.9–1.4) in patients with normal exercise testing was found (p < 0.0001). FVIII and VWF(Ag) were elevated in 26% and 18% respectively and support a hypercoagulable state in patients with PCS.
Conclusion(s): These findings suggest possible ongoing microvascular/endothelial dysfunction in the pathogenesis of PCS and highlight the potential role for prophylactic anticoagulation in the management of these patients.


PB0094
Expression of extracellular vesicles in plasma and cerebrospinal fluid is associated with the procoagulant state in patients with neurological complications of COVID‐19
A. Taxiarchis 1; E. Rostami2; J. Virhammar3; E. Kumlien3; C. Karakoyun4; J. Antovic5; A. Antovic6
1 Karolinska Institutet, Solna, Stockholms Lan, Sweden; 2 Karolinska Institutet, Department of Neuroscience, Solna, Karolinska University Hospital, Stockholm, Sweden, Solna, Stockholms Lan, Sweden; 3 Department of Neuroscience, Neurology, Uppsala University, 752 37 Uppsala, Sweden, Uppsala, Uppsala Lan, Sweden; 4 Department of Neuroscience, Neurosurgery, Uppsala University, 752 37 Uppsala, Sweden, Uppsala, Uppsala Lan, Sweden; 5 Karolinska Institutet, Department of Molecular Medicine and Surgery, Solna, Karolinska University Hospital, Stockholm, Sweden., Solna, Stockholms Lan, Sweden; 6 Karolinska Institutet, Department of Medicine, Division of Rheumatology, Solna and Unit of Rheumatology, Karolinska University Hospital, Stockholm, Sweden, Stockholm, Stockholms Lan, Sweden
Background: Extracellular vesicles (EVs) have been described to be associated with hemostatic disturbances in different clinical settings.
Aims: In this study we have investigated EVs in plasma from patients with COVID‐19 in relation to the activation of coagulation. Moreover, we assessed the presence of EVs in the cerebrospinal fluid (CSF) of patients suffering neurological symptoms during COVID‐19.
Methods: Eighteen patients with COVID‐19 and neurological symptom admitted to the Uppsala University Hospital, Sweden were included. Median age of the patients was 64 (39–85) years, 39% were women. Twenty‐one aged matched healthy individuals were included as controls. Informed consent was obtained. EVs derived from platelets (CD61+), neutrophils (MPO+) and endothelial cells (CD51/61+); together with EVs‐expressing phosphatidylserine (PS+), tissue factor (CD142+), complement components C5b‐9 (TCC+), C3a and C4d were determined by flow cytometry. Overall hemostasis potential (OHP), including overall coagulation potential (OCP) and overall fibrinolytic potential (OFP) were measured and scanning electron microscopy of fibrin clots was performed.
Results: Significantly higher OCP (p < 0.01) and OHP (p < 0.001) and lower OFP (p < 0.05) were observed in Covid‐19 patients (p < 0.05), compared to controls. Denser fibrin structure was found in COVID‐19 patients (Figure 1). Increased concentrations of PS+, MPO+, CD61+ and TCC+ EVs were found in plasma from Covid‐19 patients compared to healthy controls, and the concentrations of PS+, CD61+ and TCC+ EVs were positively correlated with OCP and OHP in Covid‐19 patients. The presence of CD61+, CD51/61+, MPO+ EVs and EVs exposing PS and TCC was identified in the CSF obtained from 17 patients (Figure 2).
Conclusion(s): Procoagulant state together with elevated levels of circulating EVs of different cell origin was found in patients with Covid‐19. The unique finding of this study is the presence of EVs in CSF of Covid‐19 patients with neurologic manifestations. EVs may represent potentially novel biomarkers of blood‐brain barrier damage during SARS‐COV‐2 infection.


PB0096
Sera from donors of COVID‐19 convalescent plasma do not induce procoagulant phenotype in platelets
G. Uzun 1; A. Singh2; W. Abou‐Khalel3; L. Pelzl4; K. Weich4; S. Nowak‐Harnau1; K. Althaus4; P. Bugert5; H. Klüter5; T. Backchoul6
1 Center for Clinical Transfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 2 Institute for Clinical and Experimental Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany; 3 Institute for Clinical and Experimental Tranfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 4 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 5 Institute of Transfusion Medicine and Immunology, German Red Cross Blood Service Baden‐Württemberg – Hessen, Mannheim, Baden‐Wurttemberg, Germany; 6 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: COVID‐19 convalescent plasma (CCP) has been suggested to be beneficial to prevent disease progression in COVID‐19. However, concerns have been expressed whether plasma components in CCP can shift the already imbalanced coagulation system to a more hypercoagulable state.
Aims: To investigate the effect of CCP on platelet phenotype and activation.
Methods: We investigated platelets from CCP donors who had a history of mild COVID‐19 infection. Donors who did not have COVID‐19 were used as controls (non‐CCP donors). We analyzed phosphatidylserine (PS) externalization, CD62p expression, and GPVI shedding in healthy platelets after incubation with sera from CCP and non‐CCP donors using flow cytometry. The study protocol was approved by the ethics committee of the University Hospital of Tübingen.
Results: Forty‐seven CCP donors [22 Male, 25 Female; and mean age (±SD) 41.4 ± 13.7 years] with a history of mild COVID‐19 infection were included. Median duration after acute COVID‐19 infection was 97 days (range, 34–401). Compared to sera from non‐CCP donors, sera from CCP donors did not induce higher PS externalization (Fold increase [FI] of PS positive platelets: 1.16% ± 0.66 vs. 1.51% ± 0.74, respectively, p = 0.11) or increased the rate of CD62p/PS double positive procoagulant phenotype (FI in CD62p/PS positive platelets: 1.86 ± 0.87 vs. 1.37 ± 0.63, respectively, p = 0.10) in platelets from healthy persons. Of note, CD62p expression in healthy platelets after incubation with sera from CCP plasma donors was significantly lower compared to sera from non‐CCP donors (FI in CD62p: 2.09 ± 1.36 vs. 1.16 ± 0.45, p< 0.01). Sera‐mediated GPVI shedding was similar between non‐CCP and CCP donors (1.07 ± 0.16 vs. 1.27 ± 0.91, p = 0.52).
Conclusion(s): Our findings support data from clinical studies, which indicate that transfusion of CCP to treat or prevent severe COVID‐19 is not associated with increased risk of exacerbation of the coagulopathy in COVID‐19.
VPB0110
Long‐COVID‐19 syndrome: From the clinical evidences to a pathophysiological mechanism
P. Canzano 1; M. Brambilla1; A. Becchetti2; M. Conti2; P. Agostoni2; S. Rovai2; M. Pengo3; E. Tortorici3; M. Mancini2; D. Andreini2; A. Bonomi2; F. Veglia2; G. Parati3; M. Camera4
1 Centro Cardiologico Monzino IRCCS, Milan, Lombardia, Italy; 2 centro cardiologico monzino, milan, Lombardia, Italy; 3 istituto auxologico italiano, milan, Lombardia, Italy; 4 centro cardiologico monzino, Università degli Studi di Milano, milan, Lombardia, Italy
Background: Long‐COVID‐19 syndrome (LCS) is defined as symptoms persisting beyond initial phase of infection. Among them, pulmonary fibrotic damage remains in 25–30% of COVID‐19 patients at 3–6 month‐follow‐up. We documented that acute COVID‐19 patients have massive platelet activation characterized by the formation of platelet‐leukocyte aggregates (PLA), that may be involved in the pulmonary microthrombi found in autoptic specimens, and by a prothrombotic phenotype. No data are currently available on contribution of platelet activation to residual pulmonary impairment and procoagulant potential in LCS patients.
Aims: To characterize platelet activation, microvesicle (MV) profile, platelet thrombin generation capacity (pTGC) in LCS patients at 6‐month‐follow‐up (6mo‐FU) compared to acute COVID‐19 infection patients.
Methods: Twentyfour 6mo‐FU COVID‐19 patients with established LCS defined according to their residual pulmonary impairment assessed by Cardiopulmonary Exercise Testing (CPET) and 64‐rows‐CT scan evaluation were enrolled. Platelet activation (P‐selectin, Tissue Factor [TF] and PLA) and MV profile were evaluated by flow cytometry; pTGC by calibrated automated thrombogram. Fortysix patients enrolled during acute COVID‐19 infection and 46 healthy subjects (HS) were used for comparison.
Results: Dispnea in LCS patients was confirmed by CPET showing compromised alveolus‐capillary membrane diffusion and residual pulmonary impairment. TF+‐platelet and ‐MV levels were 3‐ and 2‐fold lower at 6mo‐FU compared to acute phase, being comparable to HS, as well as pTGC. At 6mo‐FU, the MV profile (total number and derived from different cells) returned to physiological levels. Conversely, although lower than that measured in acute phase, a 2.5‐fold higher platelet P‐selectin expression and PLA formation was observed at 6mo‐FU compared to HS. Interestingly, a significant correlation between PLA formation and residual pulmonary impairment was observed.
Conclusion(s): These data strengthen the hypothesis that the presence of PLA in the bloodstream, and thus also in the pulmonary microcirculation, may contribute to support pulmonary dysfunction still observed in LCS patients.
PB0049
Predictive value of Wells and Geneva score in suspected pulmonary thromboembolism in critically ill patients with COVID 19: A retrospective study
N. Diaconu; T. Cuzor
Institut of Cardiology, Chisinau, Chisinau, Moldova
Background: Critically ill patients with COVID 19 are at high risk for pulmonary embolism (PE). Specific PE prediction rules have not been validated in this population yet.
Aims: The present sub study of national project (20.80009.8007.28) aimed to assessed the Wells and revised Geneva scoring systems as predictors of PE in critically ill patients with coronavirus SARSCOV2.
Methods: Study included patients with PE. A sub analyses was performed on patients with COVID positive test. Pulmonary CT angiograms (CTAs) performed for suspected PE in critically ill adult patients were retrospectively identified. Wells and revised Geneva scores were calculated based on information from medical records. Patients were dichotomized into low and intermediate/high probability groups. The reliability of both scores as predictors of PE was determined using receiver operating characteristic (ROC) curve analysis.
Results: Of 108 patients, 42 (38.8%) were positive for PE based on pulmonary CTA. The mean group value of the Wells score was 3.8 points, with 46.2% of patients having a score of ≥3, which qualifies the patient with probable PE. The average value of the Geneva score represents 7.78 points, and 60.99% of patients qualify in the group with probable PE. According to the Wells and revised Geneva scores, (52.2%) patients and (39.1%) patients, respectively, were considered as low probability for PE. Of those considered as low risk by the Wells score, 25.8% had filling defects on CTA, including 2 patients with main pulmonary artery embolism. The area under the ROC curve was 0.64 for the Wells score and 0.56 for the revised Geneva score. Wells score >4 had a sensitivity of 38%, specificity of 77%, positive predictive value of 49%, and negative predictive value of 73% to predict risk of PE.
Conclusion(s): In this population of critically ill patients with covid 19, Wells and revised Geneva scores were not reliable predictors of PE.
PB0051
A multicenter cohort study of COVID‐19 associated venous thromboembolism in admitted patients followed longitudinally 3‐months after discharge
C. Drury 1; C. Lai2; G. Misselbrook3; A. Al Zaki2; G. Bhangu4; M. Conroy5; L. Eadie4; D. Kumar6; C. Li5; D. Manhas5; N. Singh4; J. Yuan4; A. Lee2; T. Wan7
1 University of British Columbia, Vancouver, British Columbia, Canada; 2 Division of Hematology, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; 3 Division of Critical Care Medicine, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; 4 Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; 5 Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; 6 Division of Respiratory Medicine, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada; 7 Division of General Internal Medicine, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada
Background: The incidence of venous thromboembolism (VTE) in patients with COVID‐19 during hospitalization and in the post‐discharge setting has been reported with wide variability. Many studies have short follow‐up, reported the early phase of the pandemic, or did not report bleeding or anticoagulant dosing.
Aims: We determined the incidence of symptomatic VTE and bleeding in patients admitted to hospital for COVID‐19 and their 3‐month risk of VTE post‐discharge.
Methods: All patients admitted for COVID‐19 at 5 regional hospitals were identified between January 1 and December 31, 2020. Data were collected from their hospital admission and for a minimum of 3 months post‐discharge. Re‐admissions during this period were considered as post‐discharge data of the index admission. Standard thromboprophylaxis for critical care and ward patients were enoxaparin 30 mg twice daily or 40 mg once daily. Post‐discharge thromboprophylaxis was not given. Patient consent was waived by the institutional research ethics board.
Results: A total of 565 patients were included. Baseline demographics are reported in Table 1. Median length‐of‐stay was 9.0 days (range 5–131). 178 patients (31.5%) required critical care support and 79 patients (14%) died during index admission. 25 patients (4.4%) had VTE during hospitalization, of which 17 occurred within first 2 weeks and none occurred in those on therapeutic anticoagulation. There were no fatal bleeds. 5 patients (0.88%) developed critical site bleeding. Patient characteristics, anticoagulant use and bleeding rates during hospitalization are reported in Table 2. Among 486 discharged patients, median length of follow‐up was 163 days (range 3‐600): 63.5% had at least 90 days of follow‐up data and 18.7% were lost to follow‐up. 5 patients (1.3%) had symptomatic VTE diagnosed within 3 months after discharge.
Conclusion(s): The in‐hospital incidence of VTE in COVID‐19 was lower but post‐discharge incidence was higher than other studies. Therapeutic anticoagulation appeared protective against symptomatic VTE.

PB0053
NET formation in patients with COVID‐19 and non‐COVID‐19 ARDS on venovenous ECMO
N. Buchtele1; M. Kollars2; H. Burgmann3; P. Kyrle2; T. Staudinger4; S. Eichinger 5; L. Traby6
1 Department of Medicine I,Intensivecare Unit 13i2, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Department of Medicine I, Clinical Division of Hematology and Hemostasis, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 3 Department of Medicine I, Clinical Division of Infectious Diseases and Tropical Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 4 Department of Medicine I, Intensivecare Unit 13i2, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria, 5 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 6 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: Venovenous extracorporeal membrane oxygenation (ECMO) is a cornerstone in the management of severe acute respiratory distress syndrome (ARDS) and causes hemostatic system activation and inflammation. COVID‐19 is known to cause thromboinflammation. Elucidation of the underlying pathomechanisms is of great importance.
Aims: To evaluate markers of NET formation in COVID‐19 and non‐COVID‐19 associated ARDS and ECMO and to explore the role of different NET parameters as markers of inflammation and coagulopathy.
Methods: We studied 31 adult COVID‐19 patients and 23 adult non‐COVID‐19 patients with severe ARDS requiring ECMO and 47 sex‐and age‐matched healthy controls. Blood was collected at time point A (ECMO day 0–4) and at time point B (ECMO day 7–17). Citrullinated histone H3 (citH3), cell‐free DNA (cfDNA), and plasma myeloperoxidase DNA (mpoDNA), as well as d‐Dimer, were evaluated. Values are given as median (25th, 75th percentile). Statistical testing was performed using unpaired t‐tests of logarithmized parameters.
Results: COVID‐19 and non‐COVID‐19 patients exhibited significantly higher levels of citH3, cfDNA, and mpoDNA than healthy controls (Table 1). Levels of citH3 decreased significantly from time point A to B in COVID‐19 patients. No comparable effect was identified for cfDNA and mpoDNA (Table 1). In non‐COVID‐19 patients, no differences in levels of citH3, cfDNA, and mpoDNA were found between timepoints A and B (Table 1). d‐Dimer increased from time point A to timepoint B in both groups (Table 1).
Conclusion(s): NET formation is comparably increased in patients on ECMO because of COVID‐19 and non‐COVID‐19 ARDS. Markers of NET formation could add information on immunothrombosis and the complex interplay of coagulation and inflammation in the context of ECMO.


PB0073
Matrix metalloproteinases are associated with poor prognosis in hospitalized patients with COVID‐19
M. Marcos‐Jubilar 1; M. Panizo2; A. Alfonso‐Piérola1; J. Orbe3; F. Alegre2; A. Argueta2; R. Troyas4; J. Del Pozo2; M. Lozano2; J. Páramo2; R. Lecumberri2
1 Clinica Universidad de Navarra, Pamplona, Navarra, Spain; 2 Clínica Universidad de Navarra, Pamplona, Navarra, Spain; 3 Centro de Investigación Médica Aplicada (CIMA), Universidad de Navarra, Pamplona, Navarra, Spain; 4 Universidad de Navarra, Pamplona, Navarra, Spain
Background: Patients with SARS‐CoV‐2 infection have highly variable presentations, from asymptomatic disease to severe bilateral pneumonia. Hyperinflammatory host response leads to lung and endothelial severe injury. Matrix‐metalloproteinases (MMPs) and their inhibitors (TIMPs), involved in tissue degradation and remodeling, are dysregulated in inflammatory processes and could play a role in the pathophysiology of COVID‐19.
Aims: To evaluate the association of MMPs/TIMPs with SARS‐CoV‐2 infection evolution and its role as prognosis biomarkers.
Methods: Retrospective study of SARS‐CoV‐2 patients that required hospitalization between March and December 2020. Upon admission, plasma sample were collected. We measured MMPs (1, 2, 7, 9 and 10) with MAP Human MMP panel 2 (Luminex) and tissue inhibitor of matrix metalloproteinases‐1 (TIMP‐1) with ELISA kit for TIMP‐1 (R&D Systems). The primary outcome was all‐cause death during hospitalization. Secondary endpoint included a composite of death, requirement of mechanical ventilation or intensive care unit (ICU) admission and venous/arterial thrombosis.
Results: We included 151 patients (mean age 62 ± 14 years). Overall, 9.9% patients died during hospitalization, 17.9% required transfer to the ICU and 7.9% developed a thrombotic event, despite the use of antithrombotic therapy in 99% of the patients. Statistically significant but weak positive correlations (R< 0.4 in all cases) between MMPs/TIMP‐1 and inflammatory biomarkers such as neutrophil count, CRP, Ferritin or IL‐6 were stablished. In the multivariate analysis, MMP‐10 was associated with the risk of death (odds ratio 6.05; 95% confidence interval, 1.80‐20.36; p = 0.004). Both, MMP‐10 and TIMP‐1 levels were independently associated with worse combined outcome (OR (95%CI) were 3.32 (1.41–7.83) and 4.38 (1.22–15.76) respectively). These OR were higher than those obtained for D‐Dimer (OR 2.41 (95%CI: 1.05–5.57)).
Conclusion(s): Baseline MMP‐10 and TIMP‐1 levels are predictors of unfavorable outcome in hospitalized COVID‐19 patients. Future studies should focus their use as potential therapeutic targets.
PB0074
Severe COVID‐19 disease presentation is associated with significantly decreased levels of coagulation factor XIII
S. Margetić 1; I. Džolan2; A. Salacan2; I. Ćelap2
1 Department of Clinical Chemistry, Sestre milosrdnice University Hospital Center, Vinogradska 29, 10 000 Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 2 University Hospital Center Sestre milosrdnice, Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia
Background: Coronavirus disease 2019 (COVID‐19) can be associated with severe hemostatic disorders due to profound changes in coagulation and fibrinolytic systems.
Aims: To investigate activities of coagulation factors II (FII), V (FV), VIII (FVIII), X (FX) and XIII (FXIII) in COVID‐19 patients with mild and severe disease presentation.
Methods: Activities of FII, FV and FX were measured using PT‐based coagulometric method (Innovin/factor deficient plasmas), FVIII using APTT‐based coagulometric method (Actin FS/FVIII deficient plasma) whereas FXIII was determined by chromogenic method (Berichrom FXIII Assay), all performed on BCSXP analyzer (Siemens Healthineers, Germany). The differences between groups were tested with Man‐Whitney test (statistically significant p‐values < 0.05).
Results: The study included 80 consecutive patients: 40 with mild disease admitted at general wards and 40 patients with severe disease admitted at intensive care units. Clinical data of the groups are presented in Table1. In contrast to other coagulation factors studied, acitivities of XIII were significantly decreased in severe COVID‐19 patients compared to patients with mild disease in which FXIII levels were within reference range (Table 2). Among patients with severe COVID‐19 disease, lower acitivites of FXIII in non‐survivors (n = 25) compared to survivors (n = 15) were observed, although without statistically significant difference (p = 0.056, Table 2).
Conclusion(s): Our results indicate an acquired FXIII deficiency in COVID‐19 patients with severe disease. Significant differences of FXIII levels between patients with mild and severe disease suggest that FXIII levels are related to disease severity and mortality. Simultaneously measurable differences with slight decrease of FII and FX acitivites in severe ill patients compared to those with mild desease suggest that consumption, as the consequence of previous coagulation activation, could be the cause of marked decrease of FXIII in severely ill patients. Further studies are required for elucidating the cause(s) of FXIII deficiency and its association with profound hemostatic disorders in critically ill COVID‐19 patients.

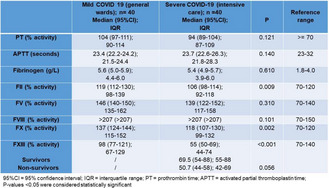
PB0075
High plasma levels of activated factor VII‐antithrombin complex indicate increased tissue factor expression/activation in patients with COVID‐19
N. Martinelli 1; N. Osti1; A. Rigoni2; S. De Marchi1; M. Montagnana1; A. Castagna1; P. Pattini1; S. Udali1; L. De Franceschi1; E. Tinazzi1; L. Delfino1; G. Sartori1; A. Azzini1; E. Tacconelli1; P. Van Dreden3; G. Lippi1; D. Girelli1; O. Olivieri1; S. Friso1; F. Pizzolo1
1 University of Verona, Verona, Veneto, Italy; 2 Verona Hospital, Verona, Veneto, Italy; 3 Diagnostica Stago, Gennevilliers, France., Gennevilliers, Ile‐de‐France, France
Background: The infection by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causal agent of coronavirus disease 2019 (COVID‐19), is associated with coagulation abnormalities, in which endothelial injury/dysfunction may be a key pathogenic mechanism. Endothelial dysfunction triggers tissue factor (TF) expression. Activated factor VII‐antithrombin (FVIIa‐AT) complex is a potential biomarker of prothrombotic diathesis reflecting FVIIa‐TF interaction.
Aims: To evaluate FVIIa‐AT plasma levels in subjects with COVID‐19 pneumonia.
Methods: FVIIa‐AT plasma levels were assessed in 40 subjects (30 males and 10 females; 64.8 ± 12.3 years) admitted for COVID‐19 pneumonia during the first pandemic wave in Italy (April 2020). FVIIa‐AT levels were compared with those of two sex‐ and age‐matched groups of hospitalized subjects without COVID‐19, with or without laboratory signs of systemic inflammation. The concentration of FVIIa‐AT was measured by ELISA on frozen citrate plasma samples. Data of coagulant activities of factor II (FII:c), factor V (FV:c), and factor VIII (FVIII:c) were available in a subgroup of subjects.
Results: Hospitalized COVID‐19 patients had FVIIa‐AT levels significantly higher than sex‐ and age‐matched no COVID‐19 subjects (Table 1), either with or without inflammation (p = 0.013 and p = 0.017 by ANOVA with Tukey post‐hoc comparison, respectively). No difference in FVIIa‐AT levels was observed between no COVID‐19 subjects with or without inflammation (p = 0.995). The association between COVID‐19 and FVIIa‐AT levels in the whole study sample remained significant by linear regression analysis adjusted for sex, age, C reactive protein, estimated glomerular filtration rate, fibrinogen, prothrombin time, and activated partial thromboplastin time (B coefficient 0.322 with standard error 0135, p = 0.019). In sub‐analysis COVID‐19 patients showed also lower FII:c, FV:c, and FVIII:c levels (Table 1).
Conclusion(s): Our results indicate that SARS‐CoV2 infection, at least during the first pandemic wave, was characterized by increased FVIIa‐AT levels, thereby suggesting an increased FVIIa‐TF interaction, which may be consistent with increased TF expression/activation due to SARS‐CoV2 ‐induced endotheliopathy.

PB0081
Impact of the COVID‐19 pandemic on anticoagulation control for patients using warfarin
L. O'Loughlin 1; S. Shah2; C. Hubbard2; G. Barnes3; M. Fang2; D. Kazi4; R. Patell4
1 Beth Israel Deaconess Medical Center, Brookline, Massachusetts, United States; 2 University of California San Francisco, San Francisco, California, United States; 3 University of Michigan, Ann Arbor, Michigan, United States; 4 Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States
Background: Patients on warfarin require regular international normalized ratio (INR) monitoring. The SARS‐CoV‐2 pandemic may have substantially affected in‐person medical visits and laboratory testing. However, it is not clear how warfarin monitoring practices have been affected by the pandemic, given the importance of tight INR control.
Aims: To assess whether chronic warfarin management, as measured by INR testing frequency and time in therapeutic range (TTR), differed during the COVID‐19 pandemic compared with the pre‐pandemic period.
Methods: We identified all patients enrolled in an anticoagulation clinic associated with an urban academic medical between January 1, 2019 and May 31, 2021 with at least 2 INRs checked during this time period. We calculated frequency of INRs checked per month and TTR based on individual INR goals and compared the pre‐pandemic (Jan 2019‐Feb 2020) and pandemic (March 2020‐May 2021) periods. INR frequency and TTR were modeled as a function of time period using Poisson and linear regressions respectively, accounting for repeated observations.
Results: Of the 1052 patients included, 43.9% were women and average age was 66.3 years. Pre‐pandemic, an average of 1.58 (95% CI 1.52‐1.64) INRs were checked per month, as compared with 1.09 (95% CI 1.03‐1.15) in the pandemic period (p< 0.001) (Figure 1). Average TTR per month was also calculated (Figure 2). On average, TTR was 56.8% (95% CI 54.9‐58.8%) pre‐pandemic compared to 39.4% (95% CI 37.5‐41.5%) during the pandemic period (p < 0.001).
Conclusion(s): There was a significant decrease in the frequency of INR measurements as well as TTR during the pandemic. A number of factors may have contributed to this, including regulatory and logistical constraints on clinic and laboratory visits and patient anxiety about visiting medical facilities. Larger scale studies are warranted to further characterize pandemic effects on warfarin use and monitoring, as well as clinically significant outcomes of thrombosis and hemorrhage.

PB0086
Haemostatic differences between SARS‐CoV‐2 PCR‐positive and ‐negative patients at the time of hospital admission
J. Remijn 1; M. Traets2; R. de Laat‐Kremers3; S. Verweij2; M. Ninivaggi4; E. Jong2; D. Huskens5; B. Blok2; G. Remme2; A. Miszta3; R. Nijhuis6; J. Herder7; R. Fijnheer2; M. Roest4; A. Fiolet2; B. de Laat8
1 Department of Clinical Chemistry, Meander Medical Center, Amersfoort, Amersfoort, Utrecht, Netherlands; 2 Department of Internal Medicine, Meander Medical Center, Amersfoort, Amersfoort, Utrecht, Netherlands; 3 Synapse Research Institute, Maastricht, Limburg, Netherlands; 4 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 5 Synapse Research Institute, Maastricht, Limburg, Netherlands; 6 Department of Medical Microbiology and Medical Immunology. Meander Medical Center, Amersfoort, Amersfoort, Utrecht, Netherlands; 7 Department of Pulmonology, Meander Medical Center, Amersfoort, Amersfoort, Utrecht, Netherlands; 8 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is associated with a prothrombotic phenotype with an increased risk for thrombosis.
Aims: To investigate whether COVID‐19 is associated with changes in coagulation parameters upon presentation at the emergency department and whether these changes are associated with the development of thrombotic complications in patients with SARS‐CoV‐2 infection.
Methods: A single centre, cross‐sectional cohort study: the MArkers in COVID‐19 And Relations to Outcomes in the Netherlands (MACARON) study was conducted. All patients suspected of SARS‐CoV‐2 infection referred to the emergency department of the Meander Medical Center between March‐May 2020 were included. 519 patients (26% PCR positive, median age 66 (range 19‐97 years), 52.2% male) were included from whom an oro‐ and nasopharyngeal swab was obtained for detection of SARS‐CoV‐2 by polymerase chain reaction (PCR). Blood samples for laboratory analysis were obtained from all patients. Thrombosis was defined as a clinical diagnosis of venous thromboembolism or atherothrombotic event based upon radiology and laboratory results.
Results: SARS‐CoV‐2 PCR positive patients had increased fibrinogen levels (5.41 g/L vs. 4.21 g/L, p < 0.001) and decreased levels of protein C (85.1% vs. 96.1%, p < 0.001) and α2‐macroglobulin (4.41 μM vs. 5.11 μM, p < 0.001) compared to the PCR negative patients. In addition, we found more acquired activated protein C resistance in PCR positive patients. Furthermore, we found that elevated levels of factor VIII (208% vs. 162%, p = 0.028) and von Willebrand Factor (208% vs. 186%, p = 0.038) and decreased ADAMTS‐13 levels (597 ng/ml vs. 691 ng/ml, p < 0.001) were associated with increased occurrence of thrombosis in PCR positive patients (thrombosis vs. non‐thrombosis).
Conclusion(s): We found that PCR positive patients had a more pronounced prothrombotic phenotype with endothelial activation upon hospital admission showing that coagulation tests may be considered useful to discriminate severe cases of COVID‐19 at risk for thrombosis.
PB0098
Long‐term persistence of anti‐PF4‐antibodies following vaccine‐induced thrombotic thrombocytopenia after first ChAdOx1 vaccination.
M. Engelen1; F. Mullier2; M. Jacquemin3; M. Debasse4; K. Peerlinck5; K. Devreese6; C. Vandenbriele7; P. Verhamme8; T. Vanassche 9
1 University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 2 Université Catholique de Louvain, CHU UCL Namur, Yvoir, Namur, Belgium; 3 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium; Department of Vascular Medicine and Hemostasis, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 4 Department of Vascular Medicine and Hemostasis, University Hospitals Leuven and National Coordinating Hemophilia Center, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 5 Center for Molecular and Vascular Biology, KULeuven, University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 6 Department of Laboratory Medicine, Department of Diagnostic Sciences, Ghent University Hospital, Ghent University, Ghent, Oost‐Vlaanderen, Belgium; 7 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KULeuven, University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 8 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 9 University Hospitals Leuven, KULeuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium
Background: Vaccine‐induced Thrombotic Thrombocytopenia (VITT) is a rare but potentially life‐threatening complication of the ChAdOx1 COVID‐vaccine, which involves binding of IgG antibodies against platelet factor 4 (PF4) to the platelet Fc‐gamma receptor. This causes platelet activation with thrombosis and thrombocytopenia. Because of the similarity with heparin‐induced thrombocytopenia (HIT), heparin is avoided in the acute treatment of VITT. There is limited information about the long‐term persistence of anti‐PF4‐antibodies and their clinical relevance.
Aims: To describe long‐term clinical and serological outcomes after VITT.
Methods: A case series of patients from Leuven University Hospitals with confirmed VITT and at least 6 months of follow‐up. All patients provided informed consent. Anti‐PF4 antibodies were measured via chemiluminescence (HemosIL® AcuStar HIT‐IgG(PF4‐H), Werfen) and enzyme‐linked immunosorbent assay (ELISA) with immobilised polyvinylsulfonate/PF4 complexes (PF4‐IgG Immucor, GTI Diagnostics); with an optical density (OD) cut‐off of 0.4. Aggregation of platelets after exposure to patient plasma with 0, 1 or 200 IU/ml of heparin was measured by whole‐blood impedance aggregometry (HIMEA) (Roche® multiplate analyser).
Results: Three middle‐aged women presented with thrombosis with thrombocytopenia and a positive anti‐PF4 ELISA 9 to 16 days after first ChAdOx1 vaccination. Their clinical presentation, lab results and treatment are summarised in Table 1. All patients recovered rapidly after non‐heparin anticoagulation with (case 2–3) or without (case 1) intravenous immunoglobulins. All patients received subsequent COVID‐vaccination with an mRNA‐based vaccine without thrombocytopenia or symptoms. Anti‐PF4‐antibodies remained elevated in two patients after 3 months and in one out of three after more than 6 months, but HIMEA results for all follow‐up tests became negative (Figure 1).
Conclusion(s): We report good short‐ and long‐term outcomes of three cases of VITT, including successful subsequent vaccination with an mRNA vaccine. Anti‐PF4‐antibodies can persist for at least several months. In contrast with the initial presentation, these persistent anti‐PF4‐antibodies did not trigger platelet activation in our patients.


PB0060
Hypercoagulability in COVID‐19: Is there an antiphospholipid syndrome?
K. Bliden1; A. Sadeghi‐Khomami2; K. Dier3; P. kundan1; U. Tantry4; Y. Alasadi5; P. Gurbel 6
1 SInai Center for Thrombosis Research and Drug Development, Baltimore, Maryland, United States; 2 Precision BioLogic, Dartmouth, Nova Scotia, Canada; 3 Corgenix, Broomfield, Colorado, United States; 4 Platelet and Thrombosis Research, Baltimore, Maryland, United States; 5 Sinai Center for Thrombosis Research and Drug Development, Baltimore, Maryland, United States; 6 Sinai Center for Thrombosis Research and Drug Development, Baltimore, MD, Maryland, United States
Background: Hypercoagulability has been established in COVID‐19 and has been linked to thrombotic risk. The existence of an antiphospholipid (aPL) syndrome in COVID‐19 remains controversial.
Aims: Determine if markers of aPL syndrome are elevated in COVID‐19 and associated with hypercoagulability and in‐hospital clinical events.
Methods: Blood, urine, clinical data, and outcomes were analyzed in patients hospitalized with COVID‐19 (n = 100) enrolled in the IRB approved TARGET‐COVID study and in healthy subjects (n = 131). aPL syndrome was assessed using using lupus anticoagulant assays (dRVVT and Hex LA, Precision BioLogic Inc.); aPL antibody (APA) profiling (IgA,IgM,IgG) against aB2GP1, anticardiolipin (aCL), and anti‐phosphotidyl serine (aPS) assays (Corgenix). Hypercoagulability, and coagulation markers (D‐Dimer, Factor‐V, VIII, XII, and Prekalikrein) were assessed using thromboelastography (TEG‐6s), ELISA, and standard coagulation assays, respectively.
Results: Mean age was 59 ± 19 years; predominately African American (65%), with a high prevalence of hypertension (74%), obesity (53%), and diabetes (45%). LA positivity was observed in 2%; and 32%, 23%, and 9% by aB2GP1, aCL, and aPS antibody testing, respectively (Figure 1). Hypercoagulability defined by platelet‐fibrin clot strength (MA ≥ 68 mm) was observed in 62% of the total group and was not associated with LA or APA positivity. Patients had lower FV, FXII, PK activity vs. healthy subjects (p < 0.05 for all). D‐dimer was higher in patients with aPL’s vs. negative patients (p = 0.03) but was not associated with thrombotic events (21% vs. 16%). Patients with positive aPS antibodies had higher mortality than aPS negative, and aCL positive, and aB2GP1 positive patients (44% vs. 18%, 10%, 7%; p< 0.05) respectively.
Conclusion(s): Based on LA assay, aPL syndrome is infrequent in COVID‐19. However, there is a high prevalence of aPL antibodies that correlate with D‐dimer with the greatest prevalence observed for aB2GP1. aPS positivity correlated with mortality and deserves further investigation as a biomarker of poor outcomes.

PB0057
Recovery after COVID‐19 and persisting endothelial cell activation and hypercoagulability—The prospective observational ROADMAP‐post COVID‐19 study
P. Van Dreden1; E. Terpos2; M. Politou3; M. a.Dimopoulos2; I. Elalamy4; G. Gerotziafas 5
1 Diagnostica Stago, Gennevilliers, France., Gennevilliers, Ile‐de‐France, France; 2 Department of Clinical Therapeutics, School of Medicine, National and Kapodistrian University of Athens, Athens, Attiki, Greece; 3 Hematology Laboratory—Blood Bank, Aretaieion Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Attiki, Greece; 4 Thrombosis and Haemostasis Centre, Biological Hematology Department, Hôpital Tenon, Assistance Publique‐Hôpitaux de Paris (AP‐HP), Sorbonne Université, Paris, France; Department of Obstetrics and Gynecology, I M Sechenov First Moscow State Medical University, Moscow, Russia, Paris, Ile‐de‐France, France; 5 Sorbonne Université, INSERM UMR_S938, Research Group « Cancer‐Hemostasis‐Angiogenesis », Centre de Recherche Saint‐Antoine, Institut Universitaire de Cancérologie, Paris, France; Thrombosis and Haemostasis Centre, Biological Hematology Department, Hôpital Tenon, Assistance Pu‐blique‐Hôpitaux de Paris (AP‐HP), Sorbonne Université, Paris, France, Paris, Ile‐de‐France, France
Background: Hypercoagulable state and endothelial cell activation are common alterations in patients with COVID‐19. Nevertheless, the hypothesis of persistent hypercoagulability and endothelial cell activation following recovery from COVID‐19 remains an unresolved issue.
Aims: To investigate the persistence of endothelial cell activation and hypercoagulability after recovery from COVID‐19. Patients/Methods. COVID‐19 survivors (n = 208) and 30 healthy individuals were enrolled in this study.
Methods: The following biomarkers were measured: procoagulant phospholipid‐dependent clotting time (PPL‐ct), D‐Dimer, fibrin monomers (FM), free Tissue factor pathway inhibitor (free‐TFP)I, heparinase, and soluble thrombomodulin (sTM). Antibodies against SARS‐CoV‐2 (IgG and IgA) were also measured.
Results: The median interval between symptom onset and screening for SARS‐CoV‐2 antibodies was 62 days (IQR = 22 days). Survivors showed significantly higher levels of D‐Dimers, FM, TFPI, and heparanase as compared to that of the control group. Survivors had significantly shorter PPL‐ct. Elevated D‐dimer was associated with older age. Elevated FM was associated with female gender. Elevated heparanase was independently associated with male gender. Decreased Procoag‐PPL clotting time was associated with female gender. One out of four of COVID‐19 survivors showed increase at least one biomarker of endothelial cell activation or hypercoagulability.
Conclusion(s): Two months after onset of COVID‐19, a significant activation of endothelial cells and in vivo thrombin generation persists in at least one out of four survivors of COVID‐19. The clinical relevance of these biomarkers in the diagnosis and follow‐up of patients with long COVID‐19 merits to be evaluated in a prospective clinical study.
PB0076
Antiphospholipid antibodies in patients with acute ischaemic stroke during the COVID‐19 pandemic
P. Mittal 1; M. Efthymiou2; R. Simister3; Z. Sayar4; A. Chandratheva3; D. Werring3; H. Cohen5
1 Department of Haematology, University College London Hospitals NHS Foundation Trust, London, UK; Haemostasis Research Unit, Department of Haematology, University College London, London, UK, LONDON, England, United Kingdom; 2 Haemostasis Research Unit, Department of Haematology, University College London, London, UK, London, England, United Kingdom; 3 Comprehensive Stroke Service, University College London Hospitals NHS Foundation Trust, London, UK; Stroke Research Centre, University College London Queen Square Institute of Neurology, London, UK, London, England, United Kingdom; 4 University College London Hospitals NHS Foundation Trust, London, UK; Whittington Hospital, London, UK, London, England, United Kingdom; 5 Department of Haematology, University College London Hospitals NHS Foundation Trust, London, UK; Haemostasis Research Unit, Department of Haematology, University College London, London, UK, London, England, United Kingdom
Background: COVID‐19 is associated with arterial thromboembolism, including acute ischaemic stroke (AIS). An association with antiphospholipid antibodies (aPL) has been noted; however, the prevalence of aPL and their clinical relevance in COVID 19‐associated AIS are undefined.
Aims: The aim was to assess the prevalence, subtypes and persistence of aPL in COVID‐19‐associated AIS.
Methods: We retrospectively reviewed AIS patients consecutively admitted to the Hyperacute Stroke Unit, University College London Hospitals, during local COVID‐19 admission waves (18‐Mar‐2020 to 31‐May‐2020 and 01‐Dec‐2020 to 24‐Feb‐2021). Electronic patient records were reviewed for relevant study data, including COVID‐19 and aPL status (in accordance with international consensus criteria).
Results: 380 patients with AIS were identified (median age 74 years, range 24‐99); 35/380 (9.2%) had active/recent COVID‐19 infection (median age 79 years, range 37–93). 132/380 patients were further analysed (those ≤65 years), including 11/132 with COVID‐19‐associated‐AIS. Overall, 105/132 (79.5% [including 31/32 (97.9%) patients < 50]), were screened for aPL, of which 26/105 (24.8% [including 7/31 (22.6%) patients < 50]) were aPL positive. In patients with AIS that were screened, aPL prevalence was significantly higher in those associated with COVID‐19 than those not associated with COVID‐19: 10/11 (90.9%) vs. 16/94 (17.0%), p< 0.05 (Fisher's exact test). Within the COVID‐19 AIS group, 8/10 aPL positive patients had an isolated lupus anticoagulant (LA); 1/10 was double aPL positive. Five of 10 patients with COVID‐19‐associated AIS underwent repeat aPL assessment: aPL were persistently positive beyond 12 weeks in 1/5, and transient in 4/5. In the non‐COVID‐19 AIS group, 7/16 underwent repeat aPL testing, with 4/7 (57.1%) demonstrating persistence. aPL subtypes are shown in Table 1.
Conclusion(s): Among AIS patients, aPL, mainly LA, are more frequent in those with COVID‐19 infection. Patients with AIS (with or without COVID‐19) found to have aPL should be retested for aPL persistence, potentially leading to a diagnosis of antiphospholipid syndrome

PB0070
Vaccine induced thrombotic thrombocytopenia post dose 2 ChAdOx1 nCoV19 vaccination: Less severe but not void of catastrophic outcomes
L. Clarke1; H. Tran2; J. Curnow3; J. Falconer4; S. Chunilal5; T. Brighton6; F. Passam7; C. Lee 8; D. Donikian6; M. Kondo6; V. Chen9
1 Department of Haematology, Concord Repatriation General Hospital; Australian Red Cross Lifeblood, Sydney Adventist Hospital, Concord, New South Wales, Australia; 2 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia; 3 Director Clinical Haematology WSLHD, Senior Staff Specialist, Westmead hospital Director Westmead Haemophilia Treatment Centre Clinical Assoc. Professor in Discipline of Medicine, Faculty of Medicine and Health, University of Sydney, Westmead, New South Wales, Australia; 4 Concord Repatriation General Hospital, Concord, New South Wales, Australia; 5 Monash Health, Melbourne, Victoria, Australia; 6 Department Haematology, New South Wales Health Pathology, Prince of Wales Hospital, Sydney, New South Wales, Australia; 7 Royal Prince Alfred Hospital; Haematology Research Group, The Heart Research Institute, Faculty of Medicine & Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia; 8 ANZAC Research Institute, University of Sydney, Sydney, New South Wales, Australia; 9 ANZAC Research Institute, University of Sydney; Department of Haematology, Concord Repatriation General Hospital, Sydney, New South Wales, Australia
Background: Clinical and pathological features of vaccine induced immune thrombotic thrombocytopenia (VITT) following first dose ChAdOx1 nCoV19 vaccination (AZD1222) are well described. VITT post 2nd dose AZD1222 is not widely recognised however its plausible undiagnosed platelet activating antibodies formed after dose 1 may be boosted upon subsequent exposure. (Greinacher et al).
Aims: We describe the clinicopathological features of suspected VITT post 2nd dose AZD1222 in Australia.
Methods: We conducted a prospective cohort study capturing sequential requests for confirmatory testing for suspected VITT after 2nd dose AZD1222. Testing was based upon key clinicopathological features: thrombosis within timeframe (4–42 days), thrombocytopenia, D‐Dimer >5xULN. High probability VITT (all criteria) underwent immunoassay Asserachrom HPIA IgG (Diagnostica Stago) and functional assay (serotonin release assay or procoagulant flow cytometry). Confirmed VITT cases were compared with those presenting after first dose AZD1222. Descriptive and comparative statistics were performed using SAS studio version 9.4.
Results: 35 high probability VITT cases presented at a median of 14 days (IQR 9,18) post 2nd dose with platelet count 116 × 109/L (IQR 92, 139) and 14.5 fold increase in D‐dimer (IQR 9.4,28.8) were tested. Median dose interval was 84 days (range 25–100), age 70 years (IQR 62, 78) with a male predominance of 66%. Platelet count and D‐dimer fold change were less severe compared to cases post 1st dose (Table 1). PF4 antibodies by ELISA were detected in 4 (11%) and antibody mediated platelet activation demonstrable in 19 (54%). These cases were classified as confirmed VITT. Three catastrophic cases including 2 fatalities occurred (Graph 2). Concomitant factors were present in all and influenced outcome severity.
Conclusion(s): We describe the largest cohort of suspected VITT post 2nd dose AZD1222. Confirmed cases are similar to those post D1 but platelet count and D‐Dimer changes were milder. Similarly, catastrophic cases occurred however concomitant factors were present in all including shorter dosing intervals.


PB0072
Predictors of venous thromboembolism in hospitalised COVID‐19 positive patients
P. MacCallum 1; S. Virdone1; S. Williams2; T. Bariana2; K. Pieper1; U. Maheshwari1; A. Kakkar1
1 Thrombosis Research Institute, London, England, United Kingdom; 2 Barts Health NHS Trust, London, England, United Kingdom
Background: Inpatients with COVID‐19 have a high rate of venous thromboembolism (VTE), yet those that are most unwell have been shown to exhibit excess bleeding following thromboprophylaxis. Risk profiling of those at highest thrombotic risk may therefore improve outcomes.
Aims: To derive and validate a risk assessment model for VTE in COVID‐19 inpatients.
Methods: Electronic health records were used to assess all patients admitted for ≥1 night with laboratory‐confirmed COVID‐19 between March 2020 and July 2021 to Barts Health NHS Trust in East London. The primary event of interest was VTE within 28 days from diagnosis. The study population was split into derivation (n = 4655) and validation sets (n = 1844). Potential predictors of VTE included demographic and lifestyle variables, clinical characteristics, and biomarkers. A logistic regression model was developed with predictors identified using least absolute shrinkage and selection operator (LASSO) methodology.
Results: The study population comprised 6499 patients (45% women, median age 60). 394 patients (6.1%) were diagnosed with ≥1 VTE event (30 DVT, 364 PE +/‐ DVT) within 28 days of diagnosis. D‐dimer on admission was the strongest predictor for VTE. The risk of VTE was associated with increasing D‐dimer up to 10 mg/L. Further rises in D‐dimer above this level did not confer additional risk. Chronic cardiac disease, chronic obstructive pulmonary disease, and oxygen flow rate were also independently associated with increased risk. High peripherally measured oxygen saturations, ischaemic heart disease and supraventricular arrhythmias were associated with a reduced risk of VTE (Figure 1). The risk assessment model offered a strong discriminatory value (c‐index 0.77) and achieved good calibration in both the derivation and validation set (Table 1).
Conclusion(s): The proposed model was robust in predicting VTE risk in successive waves of COVID‐19 infection (original, alpha and delta variants) and supports the use of the D‐dimer level for guiding thromboprophylaxis.


PB0083
Platelet activation and COVID‐19 mortality: Insights from coagulopathy, antiplatelet therapy and inflammation
A. Philippe 1; R. Chocron2; G. Bonnet3; N. Yatim4; W. Sutter5; J. Hadjadj6; O. Weizman7; C. L. Guerin8; T. Mirault9; C. Samama10; B. Planquette11; B. Terrier12; V. Waldmann13; M. Fontenay14; O. Sanchez15; J. Diehl15; P. Gaussem16; A. Cohen17; N. Gendron18; D. Smadja19
1 Université de Paris, Innovative Therapies in Haemostasis, INSERM, F‐75006, Paris, France, Paris, Ile‐de‐France, France; 24Department of Emergency, AP‐HP, Georges Pompidou European Hospital, Paris, France, Paris, Ile‐de‐France, France; 3 Paris Cardiovascular Research Center (PARCC), Paris Translational Research Center for Organ Transplantation, INSERM, UMR‐S970, Université de Paris, 75015 Paris, France., Paris, Ile‐de‐France, France; 4 Department of Internal Medicine, National Reference Center for Rare Systemic Autoimmune Diseases, AP‐HP Hôpital Cochin, Paris, France; Translational Immunology Lab, Department of Immunology, Institut Pasteur, Paris, France., Paris, Ile‐de‐France, France; 5 Paris Cardiovascular Research Center (PARCC), Paris Translational Research Center for Organ Transplantation, INSERM, UMR‐S970, Université de Paris, 75015 Paris, France, Paris, Ile‐de‐France, France; 6 Imagine Institute, Laboratory of Immunogenetics of Pediatric Autoimmune Diseases, INSERM UMR 1163, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 7 Centre Hospitalier Régional Universitaire de Nancy, 54511 Vandœuvre‐Lès‐Nancy, France., Paris, Ile‐de‐France, France; 8 Institut Curie, Paris, France, Paris, Ile‐de‐France, France; 9 HEGP, Paris, Ile‐de‐France, France; 10 Intensive Care Medicine and Reanimation Department, Assistance Publique Hôpitaux de Paris‐Centre (APHP‐CUP), Paris, France., Paris, Ile‐de‐France, France; 11 APHP, Paris, Ile‐de‐France, France; 12 Internal Medicine Department, Assistance Publique Hôpitaux de Paris‐Centre (APHP‐CUP), Paris, France, Paris, Ile‐de‐France, France; 13 Université de Paris, PARCC, INSERM, Paris, France; Adult Congenital Heart Disease Medico‐Surgical Unit, European Georges Pompidou Hospital, Paris, France; Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France, Paris, Ile‐de‐France, France; 14 Université de Paris, Institut Cochin, INSERM, Paris, France, Paris, Ile‐de‐France, France; 15 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 16 Department of Biological Hematology, European Hospital Georges Pompidou, AP‐HP, Innovative Therapies in Haemostasis, Paris University, INSERM U1140, Paris, France, Paris, Ile‐de‐France, France; 17 Cardiology Department Saint‐Antoine Hospital, Paris, France, Paris, Ile‐de‐France, France; 18 Hôpital européen Georges Pompidou, Inserm UMRS_1140, Paris, Ile‐de‐France, France; 19 HEGP, Inserm UMRS_1140, Paris, Ile‐de‐France, France
Background: Severe coronavirus disease 2019 (COVID‐19) is associated with inflammatory cytokine burst and coagulopathy. Platelets may contribute to microthrombosis development and be a target in COVID‐19 therapy.
Aims: To determine the significance of platelet activation and antiplatelet agents (APAs) treatment in COVID‐19 pathophysiology and mortality in two cohorts of patients with COVID‐19.
Methods: We explored two cohorts of COVID‐19 patients: cohort A (NCT04624997) included 208 ambulatory and hospitalized patients of different clinical severity with evaluation of soluble CD40 ligand (sCD40L) and P‐selectin (sP‐sel) plasma levels of within the first 48 hours following admission. Cohort B included 2878 patients initially admitted in medical ward with collection of clinical characteristics and outcomes (NCT04344327). In both cohorts, the primary outcome was in‐hospital mortality.
Results: In cohort A, circulating median levels of sCD40L and sP‐sel were significantly increased solely in critical patients with COVID‐19 (sP‐sel: 40059 pg/ml, IQR 26876−54678; sCD40L: 1914 pg/ml IQR 1410−2367; p < 0.001 for both), signaling platelet hyper‐activation. However, pre‐hospitalization APAs did not significantly modified sCD40L and sP‐sel levels. Admission sP‐sel levels were predictive in‐hospital mortality (Kaplan–Meier log‐rank p = 0.004), even after adjustment on CRP, while adjustment on D‐dimer abolished this relationship, suggesting that platelet activation is highly interrelated with coagulopathy. We confirmed this finding in a Cox model adjusted for age, sex, CRP and D‐dimer levels (Odds ratio 1.78, 95% CI 0.63–4.50). We confirmed in cohort B (2878 patients) that, among patients receiving APA before hospitalization, there was no significant difference in the proportion of death in a Cox model (Hazard ratio 1.0, IQR0.77–1.30) adjusted for demographic comorbidities.
Conclusion(s): Our findings highlight the critical role of coagulopathy, in contrast to platelet activation, in discriminating COVID‐19 severity and increased risk of in‐hospital mortality. We also confirm that APAs before hospitalization do not influence neither mortality nor platelet activation.
VPB0109
NLRP3 inflammasome activation and immunothrombosis in COVID‐19
A. Cambon 1; C. Guervilly2; C. Delteil3; R. Bachelier4; N. Potere5; E. Abdili6; J. Forel7; F. Dignat‐George8; S. Hraiech2; R. Lacroix9; L. Papazian10; G. Kaplanski11
1 Aix‐Marseille Univ, INSERM, INRAE, C2VN, Department of Internal Medicine, HIA SAINTE ANNE, Toulon, France, Toulon, Provence‐Alpes‐Cote d'Azur, France; 2 Aix‐Marseille Univ, APHM, Medical Intensive Care Unit, North Hospital ‐ Center for Studies and Research on Health Services and Quality of Life, EA3279 Research Unit, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Aix‐Marseille Univ, APHM, Institute of Legal Medicine, University Hospital La Timone, Marseille, France, Marseille, Rhone‐Alpes, France; 4 Aix‐Marseille Univ, INSERM, INRAE, C2VN, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 5 Department of Innovative Technologies in Medicine and Dentistry, "G. d'Annunzio" University of Chieti‐Pescara, Chieti, Italy. Chieti, Abruzzi, Italy; 6 APHM, Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 7 Aix‐Marseille Univ, APHM, Medical Intensive Care Unit, North Hospital ‐Center for Studies and Research on Health Services and Quality of Life, EA3279 Research Unit, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 8 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN ‐ Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France. Marseille, Provence‐Alpes‐Cote d'Azur, France; 9 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN, Laboratory of Hematology and Vascular Biology, University Hospital La Conception, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 10 Aix‐Marseille Univ, APHM, Medical Intensive Care Unit, North Hospital‐Center for Studies and Research on Health Services and Quality of Life, EA3279 Research Unit, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 11 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN, Department of Internal Medicine and Clinical Immunology, University Hospital La Conception, Marseille, France., Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: Immunothrombosis resulting from the crosstalk between activation of the innate immune system, coagulation and inflammation is thought to play an important role in severe COVID‐19 pathogenesis. Activation of the NLRP3 inflammasome inducing caspase‐1 activation and IL‐1b processing, constitutes an important step of the inflammatory cascade and may be central in COVID‐19 hyperinflammation and immunothrombosis.
Aims: We evaluated NLRP3 inflammasome activation in lung tissues and broncho‐alveolar lavage fluid (BALF) during severe COVID‐19 and studied its association with lung vasculopathy.
Methods: Postmortem lung biopsies from 40 patients deceased of acute respiratory distress syndrome (ARDS) complicating SARS‐Cov 2 infection were collected. Histological analysis and activated caspase‐1 expression by immunohistochemistry, were performed. In another cohort of 19 COVID‐19 patients with ARDS, soluble NLRP3, activated caspase‐1 and inflammatory cytokines were measured by ELISA in BALF collected within 48 h of admission and compared with BALF of patients with lung cancer.
Results: Among lung biopsies, 33 presented evidence of vasculopathy consisting in thrombo‐embolism (n = 9), thrombotic microangiopathy (n = 9), endothelitis (n = 15), intima fibrosis (n = 19) and intima edema (n = 11). Among them, 16 expressed activated caspase‐1, which appeared specifically located into endo‐alveolar macrophages. Higher concentrations of soluble NLRP3, activated caspase‐1, IL‐1B, and IL‐1Ra were observed in BALF from patients with COVID‐19 ARDS compared to controls (p < 0.0001).
Conclusion(s): We observed NLRP3 inflammasome activation in endo‐alveolar macrophages of COVID‐19 patients with ARDS and high inflammatory cytokine secretion associated with lung vasculopathy. These observations suggest that NRLP3 inflammasome may constitute a therapeutic target in severe COVID‐19.
VPB0111
Thrombocytopenia and thrombosis associated with AstraZeneca ChAdOx1 nCoV‐19 Vaccination—Results of Australian centralized clinical triage system and functional assay testing system
V. Chen 1; L. Clarke2; C. Lee3; F. Passam4; D. Donikian5; M. Kondo5; R. Bird6; P. Choi7; I. Tohidi‐Esfahani8; D. Pepperell9; C. TAN10; S. Chunilal11; H. Tran12; T. Brighton5
1 ANZAC Research Institute, University of Sydney; Department of Haematology, Concord Repatriation General Hospital, Sydney, New South Wales, Australia; 2 Department of Haematology, Concord Repatriation General Hospital; Australian Red Cross Lifeblood, Sydney Adventist Hospital, Concord, New South Wales, Australia; 3 ANZAC Research Institute, University of Sydney, Sydney, New South Wales, Australia; 4 Royal Prince Alfred Hospital; Haematology Research Group, The Heart Research Institute, Faculty of Medicine & Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia; 5 Department Haematology, New South Wales Health Pathology, Prince of Wales Hospital, Sydney, New South Wales, Australia; 6 Princess Alexandra Hospital, Woolloongabba, Queensland, Australia; 7 The Canberra Hospital, Garran, Australian Capital Territory, Australia; 8 University College London Hospital, London, England, United Kingdom; 9 Fiona Stanley Hospital, Perth, Western Australia, Australia; 10 Royal Adelaide Hospital, Adelaide, South Australia, Australia; 11 Monash Health, Melbourne, Victoria, Australia; 12 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia
Background: As a severe, though rare complication of COVID‐19 vaccination, vaccine induced immune thrombotic thrombocytopenia (VITT) emerged in 2021 as a public health issue affecting vaccine confidence. Australia pre‐emptively instituted a nationally coordinated system for diagnosis and management incorporating state based immunoassays (ELISA) for PF4 antibodies and national centralised functional testing using three functional assays.
Aims: To evaluate the triage strategy based upon clinical presentation, thrombocytopenia and D‐Dimer used to direct VITT testing.
Methods: Consecutive cases presenting between April 1 and June 11, 2021 referred for VITT testing in Australia were assessed regarding clinical classification, immune‐assay and functional assay results, thromboses and mortality according to triage category (Table 1). Functional testing proceeded for all triaged as “Probable VITT”, ELISA positive and unusual site thromboses positive patients if triaged “Less likely”, and only for quality assurance purposes if “Much less likely VITT”. Anti‐PF4 antibodies were measured by IgG‐specific ELISA (Asserachrom, Stago Diagnostics). Platelet‐activating antibodies were assayed using whole blood procoagulant platelet flow cytometry assay (Lee et al), PF4‐serotonin release assay or multiplate multiple electrode aggregation.
Results: 92% of 52 patients with a final diagnosis of VITT supported by a positive antibody assay (immunological or functional) were triaged prior to VITT specific testing into the high probability category. 100% ELISA positive individuals within the high and intermediate clinical probability groups were supported by detection of a platelet activating antibody using a functional assay and 0% in the low probability group. 16% of clinical VITT patients without confirmation of anti‐PF4 antibodies by ELISA had VITT diagnosis supported by functional assay in a final clinico‐pathological adjudication.
Conclusion(s): Triage of testing according to clinical probability of VITT based on clinical criteria and standard laboratory tests was helpful in directing testing in resource constrained period. Demonstration of platelet activating antibodies in functional assays significantly contributes to definition of the VITT syndrome.


VPB0119
Evaluation of thrombotic outcomes in patients in a large clinical enterprise following COVID‐19 vaccination
B. Greene 1; W. Patterson2; L. Tefera3; J. Bena4; A. Milinovich4; N. Mehta5; M. Chung6; S. Cameron3
1 Cleveland Clinic Foundation, Cleveland, Ohio, United States; 2 Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, Ohio, United States; 3 Department of Cardiovascular Medicine, Section of Vascular Medicine, Heart, Vascular & Thoracic Institute, Cleveland Clinic Foundation, Cleveland, Ohio, United States; 4 Quantitative Health Sciences, Cleveland Clinic Foundation, Cleveland, Ohio, United States; 5 Department of Medicine, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, Ohio, United States; 6 Department of Cardiovascular Medicine, Section of Cardiac Electrophysiology, Heart, Vascular & Thoracic Institute, Cleveland Clinic Foundation, Cleveland, Ohio, United States
Background: With widespread COVID‐19 immunization efforts, reports of vaccine‐induced thrombocytopenia and thrombosis (VITT) have emerged, particularly in association with adenoviral vector‐based vaccines (ChAdOx1 nCoV‐19 and Ad26.COV2.S). The incidence of VITT is considered to be extremely low, with the benefits of vaccination strongly outweighing associated risks. Despite the favorable safety profile of COVID‐19 vaccines, VITT has garnered the attention of and likely contributes to vaccine hesitancy among a persistently unvaccinated portion of the United States population.
Aims: We sought to characterize thrombotic events following COVID‐19 vaccination in a large clinical enterprise where mRNA‐based vaccines were mostly administered.
Methods: With institutional approval, medical records of 779,602 patients vaccinated against COVID‐19 (2 mRNA‐based vaccines: 61.2% BNT162b2, 36% mRNA‐1273, and adenovirus‐based Ad26.COV2.S, 2.7%) from 12/4/2020‐6/6/2021 at Cleveland Clinic Enterprise locations in Ohio and Florida were queried. A baseline complete blood count was available for 223,345 patients, of which 663 (0.3%) demonstrated thrombocytopenia—defined as ≥50% platelet decline 4‐28 days post‐vaccination—and were subject to chart review. Thrombotic events including deep vein thrombosis, pulmonary embolism, stroke/transient ischemic attack, myocardial infarction, cerebral venous sinus thrombosis, and splanchnic thrombosis were assessed. Thrombotic risk factors including medications, viruses, and malignancy, as well as platelet factor 4 antibody assays were recorded.
Results: Of 76 patients with thrombosis, 63 (82.9%) demonstrated clear etiologies. Thirty (39.5%) had malignancies (24 treated with chemotherapy associated with thrombosis risk). Seven (9.2%) were considered hypercoagulable, six (7.9%) had catheter‐related thrombosis, five (6.6%) had recent surgery, five (6.6%) had reduced mobility, five (6.6%) had cardiovascular risk factors, three (3.9%) had diagnosed/suspected immune thrombocytopenia, and two (2.6%) were septic. Of three patients with unprovoked thrombosis, one had findings concerning for VITT (Figure 1).
Conclusion(s): 76/223,345 (0.03%) patients demonstrated thrombosis following COVID‐19 vaccination, with one (0.0004%) case concerning for VITT. In a large clinical enterprise, VITT is exquisitely rare.

PB0077
Hematologic findings and complications of SARS‐COV‐2 in children and young adults
R. Graham1; D. Kaur2; C. Neunert 3; Z. Jin4; P. Satwani5
1 Department of Pediatric BMT/Children's Healthcare of Atlanta, Decatur, Georgia, United States; 2 Department of Pediatric Hematology/Columbia University Irving Medical Center, NY, New York, United States; 3 Columbia University Irving Medical Center, NY, New York, United States; 4 Columbia University, New York, New York, United States; 5 Department of Pediatric BMT/Columbia University Irving Medical Center, New York, New York, United States
Background: Thrombotic coagulopathy is a predictor of SARS‐CoV‐2 infection related mortality in adults (1‐4). Incidence of venous thromboembolic events (VTE) in children seems to be lower (5), but there remains paucity of data on this in children. The current study describes the coagulation and inflammatory disturbances occurring in children <21 years with SARS‐CoV‐2 infection and multisystem inflammatory syndrome in children (MIS‐C) and evaluates risk factors for hematologic complications.
Aims: 1. Report hematological and inflammatory abnormalities and hemorrhagic/thrombotic outcomes, in pediatric patients with SARS‐CoV‐2 or MIS‐C. 2. Identify risk factors for hematologic adverse outcomes with SARS‐CoV‐2 or MIS‐C.
Methods: Single‐institution, retrospective study of in children <21 years with SARS‐CoV2 acute infection or MIS‐C between March 1, 2020, and February 28, 2021. Laboratory parameters were collected at presentation, and during hospitalization. Bleeding was graded using the modified World Health Organizations (WHO) grading system. VTEs were diagnosed radiographically, or by strong clinical suspicion. Correlation studies were done to assess hematologic and inflammatory laboratory markers.
Results: In pediatric patients with SARS‐COV‐2 infection and MIS‐C (n = 127), we identified thrombotic and bleeding complications incidences at 6.3% and 6.3%, respectively. VTEs and bleeding events occurred mainly in females. All thrombotic events occurred in patients not previously on anticoagulation. No patients in our study received post‐discharge thromboprophylaxis; one VTE occurred 3 days after discharge for COVID‐19 pneumonia. Adolescent age (>13 years) and indwelling central venous‐access device (CVAD) increased thrombotic risk, while MIS‐C diagnosis did not. Obesity was not a risk factor for VTE but was associated with increased bleeding risk. Seven of eight bleeding events (5.5%) were WHO grade 3.
Conclusion(s): SARS‐CoV‐2 thrombotic complications in children are occur at lower rates than in adults. Older age, female sex, and CVADs increase thrombotic risk. With higher rates of bleeding events our study, daily risk‐assessment of the need for continued thromboprophylaxis is recommended.


PB0035
Increased thrombus formation via procoagulant platelets in vaccine induced thrombotic thrombocytopenia (VITT)
K. Althaus 1; A. Singh2; L. Pelzl1; J. Zlamal3; I. Marini4; T. Backchoul3
1 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 2 Institute for Clinical and Experimental Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany; 3 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 4 Transfusion Medicine, Medical Faculty of Tübingen, University Hospital Tübingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Vaccines against SARS‐CoV‐2 virus reduce morbidity and mortality of the pandemic. But with millions of people vaccinated in a short period of time, even very rare side effects like the clotting disorder vaccine‐induced thrombotic thrombocytopenia (VITT) became apparent. We recently identified an increase in procoagulant platelets in these patients.
Aims: Investigation of the impact of procoagulant platelets in thrombus formation.
Methods: 8 patients (4 female, 4 male) who were hospitalized with suspected thrombotic complications 5 to 16 days after ChAdOx1 nCoV‐19 vaccination were included in this study. The blood samples were analyzed by using enzyme immune assays, flow cytometry, ex vivo thrombus formation assay and heparin‐induced platelet aggregation assay.
Results: The median age was 38 years. All patients had thrombocytopenia at admission. Three had a fatal outcome and five were successfully treated. All sera from VITT patients contained high titer antibodies against platelet factor 4 (PF4) [OD: 3.0 ± 0.68] with the ability to activate platelets in the HIPA assay (8/8). Sera from VITT patients induced significant increase in procoagulant markers (P‐selectin [CD62P] and phosphatidylserine externalization) [% CD62P/PS positive PLTs: 40.82 ± 7.02] compared to COVID‐19 patients [% CD62P/PS positive PLTs: 15.71 ± 7.70]. The generation of procoagulant platelets was PF4 dependent. The formation of procoagulant platelets could be significantly reduced by use of the monoclonal IV.3 [% CD62P/PS positive PLTs: 1.05 ± 0.21]; p = 0.0001 antibody as well as IVIG [% CD62P/PS positive PLTs: 1.01 ± 0.36]; p = 0.0001. In thrombus formation model, IgGs from VITT patients induced increase platelet surface area (Mean % SAC ± SEM: 10.38 ± 1.30) compared to control IgG, which was inhibited by IVIG (4.08 ± 0.96), p = 0.001.
Conclusion(s): Our ex vivo microfluidic thrombus formation model supports the significance of procoagulant platelet in the pathogenesis of VITT. It may offer significant clinical implications and therapeutic options like evaluation of IVIG as a recommended therapy or other drugs for treatment of clinical picture of VITT.
PB0050
Evaluation and comparison of NETosis biomarkers in sepsis and COVID‐19 patients
J. Douxfils 1; L. Morimont2; C. David2; G. Constant3; J. Candiracci4; H. Haguet5; M. Dechamps6; P. Laterre7; J. De Poortere8; S. Horman9; C. Beauloye6; M. herzog4
11 Qualiblood s.a., 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS)., 5000, Namur, Belgium; 2 Qualiblood s.a., Namur, Namur, Belgium; 3 Department of pharmacy, Namur, Namur, Belgium; 4 Belgian Volition SPRL, Namur, Namur, Belgium; 5 University of Namur, department of pharmacy, Namur, Namur, Belgium; 6 Institut Cardiovasculaire Clinique Saint Luc, Bruxelles, Brussels Hoofdstedelijk Gewest, Belgium; 7 Cliniques Universitaires Saint‐Luc, Bruxelles, Brussels Hoofdstedelijk Gewest, Belgium, 8 nstitute of Experimental and Clinical Research (IREC), Louvain‐la‐neuve, Brabant Wallon, Belgium; 9 Université catholique de louvain, Louvain‐La‐Neuve, Brabant Wallon, Belgium
Background: Neutrophils are involved in the defense of the body against pathogens through the formation neutrophil extracellular trap, a mechanism called NETosis. These pathogens can be fungi, bacteria, viruses or other microorganisms. However, neutrophils through NETs have a double‐edged sword activity and can also be harmful.
Aims: This study aims to evaluate biomarkers of NETosis in two different patient populations admitted to the intensive care unit, i.e. COVID‐19 and sepsis patients. A control population of matched subjects have been included.
Methods: Among the individuals admitted to the ICU and included in this study, 46 were sepsis patients and 22 were COVID‐19 patients. 48 controls were included. Nucleosome histone H3.1, nucleosome citrullinated histone H3R8, free citrullinated histone (citrullinated at R2, R8 and R17), neutrophil elastase an myeloperoxidase were measured. Blood samples were taken at the admission to the intensive care unit. The different groups were compared using ordinary two‐way ANOVA with a Tukey’s multiple comparison on log‐transformed data.
Results: A significant difference in the levels of Nu.H3.1 and NE was observed between sepsis and COVID‐19 subjects. All NETosis parameters differs in ICU patients versus controls. A positive correlation was found between SOFA and APCHE‐II score and Nu.H3.1 in sepsis patients. No positive correlation was observed in COVID‐19 patients. Normalization of NETosis parameters according to the neutrophil count improves the sensitivity of Nu.H3.1 to discriminate between sepsis and COVID‐19 patients. The other parameters were also influenced but to a lesser extent. NE and Nu.H3.1 correlates well in sepsis patients while it is not the case in COVID‐19 patients.
Conclusion(s): Nu.H3.1 appears to be an interesting marker of NETosis in sepsis and COVID‐19 patients. Its correlation with NE in sepsis patients reveals that NE is important in generating circulating nucleosomes while its weak association in COVID‐19 suggest different patterns between the diseases.
PB0063
Coagulation disorders in pediatric patients hospitalized due to SARS‐CoV‐2 infection
A. Dettoraki1; A. Michalopoulou1; S. Thymianou1; L. Ioannidou 2; S. Saslis1; M. Mazarakis1; I. Stamati1; Z. Kapsimali1; H. Pergantou1
1 Haemophilia Centre/Haemostasis and Thrombosis Unit, AGHIA SOPHIA CHILDREN'S HOSPITAL, ATHENS, Attiki, Greece; 2 AGHIA SOPHIA CHILDREN'S HOSPITAL, ATHENS, Attiki, Greece
Background: Haemostatic impact of SARS‐CoV‐2 infection and multisystem inflammation syndrome (MIS‐C) in children is still under consideration.
Aims: To investigate COVID‐19 coagulopathy in hospitalized children due to SARS‐CoV‐2 infection.
Methods: A retrospective review on coagulation parameters of children with SARS‐CoV‐2 infection and MIS‐C hospitalized from August 2020 until December 2021 was conducted in our Haemostasis and Thrombosis Centre. Values of D‐dimers, fibrinogen, antithrombin, protein C, PT, APTT and the respective coagulation factors were recorded at hospital admission, in multiple time points during hospitalization and upon hospital discharge. Statistical analysis was performed with Mann‐Whitney test and t‐test.
Results: In total, 733 children (boys: 62.7%) of mean age 4.9 ± 5.4 years (0‐18) were investigated. Patients were categorized as follows: patients with mild/moderate (87.6%) or severe COVID‐19 (6.3%) and patients with MIS‐C (6.1%). Severe, in comparison with mild/moderate patients, had significantly increased admission D‐Dimer values (34.3 vs. 2.9 μg/ml), PT (25.2 vs. 14.3, sec), APTT (62.5 vs. 34.6, sec), fibrinogen (362.5 vs. 259.5, mg %), and factor VIII values (165.4 vs. 126.9, %). MIS‐C children had significantly increased admission values of fibrinogen (501 mg %), factor VIII (200.3, %) and statistically decreased factor VII values (38.2 vs. 56.6, %), antithrombin values (81.5 vs. 98.1, %) and protein C values (52.4 vs. 63.3, %) in comparison with mild/ moderate patients. Interestingly, MIS‐C children had markedly increased fibrinogen and factor VIII values even in comparison with severe patients. During hospitalization, PT, APTT, fibrinogen, factor VIII values were significantly decreased and factor VII values increased. Also, D‐Dimers values were markedly decreased but without statistical significance. No child died and thrombosis was noticed in three children, one of which was suffered from MIS‐C.
****
Conclusion(s): Our COVID19 hospitalized children showed a mild deterioration of coagulation parameters. However, in children with MIS‐C and severe COVID19, these coagulation parameters were found mainly affected.
VPB0108
Incidence of anti‐platelet factor4/polyanionic antibodies, thrombocytopenia, and thrombosis after COVID‐19 vaccination with ChAdOx1 nCoV‐19 in Thais
K. Boonyawat 1; T. Phojanasenee1; P. Noikongdee1; P. Police1; P. Chantrathammachart1; P. Niparuck1; T. Puavilai1; A. Phuphuakrat1; P. Angchaisuksiri2
1 Ramathibodi Hospital, Bangkok, Krung Thep, Thailand; 2 Mahidol University, Bangkok, Krung Thep, Thailand
Background: Prevalence of antiplatelet‐factor 4 (PF4)/polyanionic antibodies occurring after vaccination with ChAdOx1 nCoV‐19 was low. Most of these antibodies are weak and not associated with vaccine‐induced thrombotic thrombocytopenia. It remains unknown whether these antibodies are preexisting or occur after the vaccination.
Aims: In this study, we demonstrated the incidence of anti‐PF4/polyanionic antibodies, thrombocytopenia, and thrombosis after vaccination with ChAdOx1 nCoV‐19 in Thais.
Methods: We conducted a prospective study in health care workers and the general population who received COVID‐19 vaccination with ChAdOx1 nCoV‐19. Blood collection for complete blood count, D‐dimer, and anti‐PF4/polyanionic antibodies was performed before vaccination (day 0), day 10, and day 28 after vaccination. Anti‐PF4/polyanionic antibodies were detected using enzyme‐link immunosorbent assay (ELISA). Functional assay with platelet aggregation was performed for all positive anti‐PF4/polyanionic antibody ELISA tests.
Results: A total of 720 participants receiving the first, second, or third booster dose of ChAdOx1 nCoV‐19 were included in the study. Baseline characteristics are presented in Table 1. Three participants developed seroconversion. Therefore, the incidence of anti‐PF4/polyanionic antibodies was 0.42% (95% confidence interval 0.08, 1.23). However, these antibodies were low titer. Fourteen (1.9%) participants had preexisting anti‐PF4/polyanionic antibodies before the vaccination but the optical density of anti‐PF4/polyanionic antibodies did not significantly increase over time (Figure 1). None of the anti‐PF4/polyanionic positive sera induced platelet aggregation. Abnormal D‐dimer levels following the vaccination were not different among the positive and negative anti‐PF4/polyanionic groups (11.8% vs. 13.2%, p = 0.86). Thrombocytopenia occurred in one person with negative anti‐PF4/polyanionic antibodies. No clinical thrombosis occurred.
Conclusion(s): We found a low incidence of seroconversion of anti‐PF4/polyanionic antibodies after vaccination with ChAdOx1 nCoV‐19 in Thais. Most of the anti‐PF4/polyanionic antibodies are preexisting and did not significantly increase after vaccination with ChAdOx1 nCoV‐19. Some participants with anti‐PF4/polyanionic antibodies had elevated D‐dimer levels. However, no thrombocytopenia and thrombosis were observed.
VPB0136
Low incidence of thromboembolism in hospitalized COVID‐19 patients despite a very low thromboprophylaxis rate in Thailand
N. Tangcheewinsirikul 1; N. Uaprasert2; P. Rojnuckarin1
1 Chulalongkorn University, Division of Hematology, Department of Medicine, Faculty of Medicine, Bangkok, Thailand, bangkok, Krung Thep, Thailand; 2 Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Krung Thep, Thailand
Background: International guidelines recommend thromboprophylaxis for hospitalized coronavirus disease 2019 (COVID‐19) patients due to high prevalence of thromboembolism (TE). However, thromboprophylaxis is uncommonly prescribed in Thailand.
Aims: To determine the incidence of TE and the mortality rate of hospitalized COVID‐19 patients in Thailand.
Methods: We retrospectively reviewed medical records of COVID‐19 patients admitted to King Chulalongkorn Memorial Hospital between February 2020 and August 2021.
Results: There were 7452 hospitalized COVID‐19 patients with 460 (6.2%) intensive care unit (ICU) patients. The decision of thromboprophylaxis was based on the discretion of attending physicians. Only 85 (1.14%) patients received anticoagulants due to new TE without thromboprophylaxis (43; 0.58%), thromboprophylaxis (36; 0.48%) or preexisting conditions (6; 0.08%). Of 43 newly identified TE, there were 33 (0.44%) venous TE and 10 (0.14%) arterial TE. Among 43 TE, 29 were treated in ICU with an estimated incidence of 6.3% (29 of 460). The incidence of TE in non‐ICU patients was very low (0.2 %; 14 of 6992). Of 36 receiving standard‐dose enoxaparin thromboprophylaxis, 3 (8.3%) developed venous TE. The overall mortality rate was 1.7% (126 of 7452), while the mortality rate in patients with TE was as high as 41.3% (19 of 46). Compared to patients without TE, patients with TE had a substantially increased risk of death (odds ratio of 48.0, 95% confidence interval, 25.9, 89.0; p < 0.0001).
Conclusion(s): The incidence of TE in hospitalized COVID‐19 patients was lower than those reported from Western countries despite a very low thromboprophylaxis rate in Thailand. Routine thromboprophylaxis for hospitalized COVID‐19 Thais may not be cost‐effective. However, ICU patients were at higher risk of TE. Patients who developed TE were at greater risk for death
VPB0138
Diagnostic strategies for pulmonary embolism in patients hospitalized for COVID‐19: Role of clinical prediction rules
M. Vedovati; F. Balducci; C. Becattini
University of Perugia, Perugia, Umbria, Italy
Background: Diagnostic strategies for pulmonary embolism (PE) in patients already facing respiratory failure due to COVID‐19 is challenging. The use of the conventional diagnostic algorithm and clinical prediction rules (CPR) for PE is controversial.
Aims: To evaluate the accuracy of currently available diagnostic algorithms for the diagnosis of PE and CPRs to assess the risk for venous thromboembolism (VTE) in medically ill patients affected by COVID‐19.
Methods: Consecutive patients >18 years hospitalized for COVID‐19 (confirmed by molecular testing) at Perugia Hospital (Italy) from March 2020 to September 2021 were included if underwent chest‐CT angiography for suspicion of PE. The study outcome was the accuracy of currently available CPRs, for PE diagnosis (Wells and Geneva) and for VTE‐risk stratification in medically ill patients (IMPROVE, IMPROVEDD and Padua score), to predict PE.
Results: 74 patients were included (mean age 68 years, male 64.9%), 13 (17.6%) had PE confirmed. No significant differences were observed for comorbidities, antithrombotic treatment and mortality between the two groups. D‐dimer resulted significantly higher in patients with compared to patients without PE. Poor discrimination was observed for Wells and Geneva scores (AUC 0.596, 95% CI 0.413–0.779, and AUC 0.603, 95% CI 0.439–0.767, respectively), without substantial differences adding D‐dimer at conventional cut‐off (Table). The IMPROVEDD score had the highest discriminative power among CPRs for VTE (AUC 0.699, 95% CI 0.539‐0.860). Scores’ performance improved by increasing the D‐dimer cut‐off at level of 2000 ng/ml.
Conclusion(s): The accuracy of the currently used diagnostic and predictive scores for PE or VTE in COVID‐19 patients is poor. D‐dimer improves the diagnostic accuracy of these scores; most of all, it seems to allow a diagnostic strategy with a high negative predictive value, so we can rule out a consistent part of the patients with a low risk of PE.

PB0036
Clinical and serological follow up of VITT patients treated with heparin and alternative anticoagulation
K. Althaus 1; G. Uzun2; B. Luz3; M. Wolf4; H. Henkes5; T. Backchoul6
1 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 2 Center for Clinical Transfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 3 Institute of Transfusion Medicine, Klinikum Stuttgart, Stuttgart, Baden‐Wurttemberg, Germany; 4 Department of Neurology, Klinikum Stuttgart, Stuttgart, Baden‐Wurttemberg, Germany; 5 Department of Neuroradiology, Klinikum Stuttgart, Stuttgart, Baden‐Wurttemberg, Germany; 6 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Vaccine induced thrombotic thrombocytopenia (VITT) is a rare but severe complication following vaccination with ChAdOx1 nCoV‐19. Antibodies directed against platelet factor 4 (PF4) are thought to be responsible for platelet activation and subsequent thromboembolic events in these patients. Because of the similarities between heparin‐induced thrombocytopenia (HIT) and VITT heparin was avoided but the risk of thrombosis in VITT upon heparin administration remains unclear.
Aims: To assess the impact of heparin used as initial anticoagulants to treat VITT.
Methods: We prospectively analyzed follow up data from 4 patients with confirmed VITT patients (3 women and 1 men; median age, 44 years [range, 22–62 years]). ELISA and functional VITT testing was performed at each time point.
Results: The patients’ clinical symptoms started between days 4 and 17 after first vaccination with ChAdOx1 nCoV‐19. All patients presented with thrombocytopenia and thromboembolic events (amaurosis fugax and peripheral thrombosis and venous sinus thrombosis). The follow‐up duration ranged between 8 weeks and 9 months. No additional thromboembolic event or disease progression occured in any patient. A recovery in platelet count was monitored in all four patients within 10 days after starting treatment (heparin or alternative anticoagulation combined with IVIG). In both patients who were treated with heparin, anti‐PF4 antibodies were not detectable after 3 and 19 weeks respectively. All 4 patients received mRNA‐based vaccine as second vaccination against SARS CoV2. No significant drop in platelet count or new thromboembolic complication was observed.
Conclusion(s): In the treatment of VITT, early beginning of anticoagulation with close follow‐up of the platelet count and thrombosis signs seem to be more important than the choice of anticoagulant. Subsequent vaccination with an mRNA vaccine appears to be safe in VITT patients.
PB0087
Hyperresponsive platelets and a reduced platelet granule release capacity is associated with severity of COVID‐19 symptoms and with increased mortality
F. Garishah1; D. Huskens2; S. Pramudo3; D. Andriani4; M. Astrilia5; R. Sentosa6; A. van der Ven1; B. de Laat7; M. Gasem6; Q. de Mast8; M. Roest 9
1 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands; 2 Synapse Research Institute, Maastricht, Limburg, Netherlands; 3 Diponegoro University, Semarang, Jawa Barat, Indonesia; 4 KRMT Wongsonegoro Hospital, semarang, Jawa Barat, Indonesia; 5 KRMT Wongsonegoro Hospital, samarang, Jawa Barat, Indonesia; 6 Diponegoro University, Dr. Kariadi Hospital, Semarang, Jawa Barat, Indonesia; 7 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 8 Department of Internal Medicine, Radboud Center for Infectious Diseases, Radboud University Medical Centre, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 9 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: COVID‐19 is often associated with mild thrombocytopenia and increased platelet reactivity. The aim of the current study was to investigate if platelets of COVID‐19 patients were primed to hyper‐reactive platelets and if the circulating platelets become exhausted during COVID‐19 disease.
Aims: To investigate if platelets of COVID‐19 patients were primed to hyper‐reactive platelets and if the circulating platelets become exhausted during COVID‐19 disease.
Methods: Time dependent platelet activation is studied in whole blood by monitoring the ATP release kinetics from platelets upon stimulation with PAR1 receptor agonist in 41 critical ill COVID‐19 patients, 47 COVID‐19 patients who were hospitalized but were not critically ill and 30 healthy controls.
Results: Our study demonstrated that platelets of critical ill COVID‐19 patients were hyper‐responsive and had a reduced platelet granule release capacity, probably due to exhaustion. The platelet reactivity time of COVID‐19 patients admitted to the critical care unit was 2‐fold reduced if compared with the response time of healthy controls and 1.6‐fold reduced if compared with non‐critical COVID‐19 patients. Platelet responsiveness was also associated (spearman r; p‐value) with D‐dimer (0.5; <001), CRP (0.57; <001) and neutrophil‐lymphocyte ratio (0.54; <001). Moreover, an increased platelet responsiveness and reduced platelet granule release capacity were associated with an increased mortality (OR; 95% CI: 18.8; 6.1‐57.9 and 4.9; 1.5–16.3, respectively). These relationships remained significant after adjustment for age, sex, D‐dimer, CRP and neutrophil‐lymphocyte ratio.
Conclusion(s): Our findings show that platelet hyper‐reactivity and reduced platelet granule release capacity were associated with increased critical illness and increased mortality of COVID‐19 patients.


PB0095
High prevalence of anti‐PF4 antibodies among hospitalized pediatric COVID‐19 patients and the impact on morbidity and mortality
S. Hui1; S. Sartain2; K. Bruzdoski3; V. Kostousov1; A. Navaei1; L. Hensch1; J. Teruya 1
1 Texas Children's Hospitals/Baylor College of Medicine, Houston, Texas, United States; 2 Texas Children's Hospital/Baylor College of Medicine, Houston, Texas, United States; 3 Texas Childrens Hosptials, Houston, Texas, United States
Background: Presence of Anti‐PF4 ELISA antibodies (APF4) has been reported among patients with SARS‐CoV‐2 (COVID) infection and individuals who received COVID vaccines. These data are mostly limited to adults with sparse data among pediatric patients.
Aims: This study aimed to determine the prevalence and demographics of APF4 positivity among hospitalized COVID pediatric patients and its impact on morbidity and mortality including ICU admission, length of stay (LOS), requirement of mechanical ventilation (MV), thrombosis and heparin therapy (HT).
Methods: Seventy‐nine plasma samples from COVID positive (via PCR) patients (identified via in‐house COVID testing) were tested for APF4 using PF4 Enhanced assay (Immucor GTI Diagnostics, USA). Demographics and clinical data were collected via retrospective review of electronic medical records.
Results: Twenty patients of 79 (25%) COVID positive patients were found to have APF4 compared with 5% among COVID negative age and sex matched controls. There were no differences in age and gender between APF4 positive vs. APF4 negative COVID patients. Proportionally more Hispanic and Asian patients were APF4 positive (60% vs. 45% and 10% vs. 5% respectively). Table 1 highlighted the morbidity and mortality data of APF4 positive vs. negative patients. Among patients with thrombotic complications, only 3/8 (37%) patients had APF4. Lastly, there were no significant differences between APF4 positive vs. negative patients on HT in terms of thrombosis, percentage with thrombocytopenia as defined by platelet count <150,000/μl, ICU admission, LOS and MV.
Conclusion(s): There is a high prevalence of APF4 positivity among pediatric COVID patients. However, the presence of APF4 appears to have no significant impact on morbidity and mortality. In addition, the presence of incidental APF4 should not limit the use of heparin therapy in pediatric COVID patients.

PB0065
Sex‐ and age specific association of new‐onset atrial fibrillation with in‐hospital mortality in hospitalised COVID‐19 patients
L. Joosten 1; J. Offerhaus2; M. van Smeden3; M. Linschoten4; H. Bleijendaal2; R. Tieleman5; A. de Wilde2; F. Rutten3; G. Geersing3; C. Remme2
1 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 2 Department of Clinical and Experimental Cardiology, Amsterdam UMC, Heart Centre, Amsterdam Cardiovascular Sciences, University of Amsterdam, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 4 Department of Cardiology, Division of Heart and Lungs, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 5 Department of Cardiology, Martini Hospital, and University Medical Center Groningen, University of Groningen, Groningen, The Netherlands, Groningen, Groningen, Netherlands
Background: Coronavirus disease 2019 (COVID‐19) is a systemic disease with cardiovascular involvement, including cardiac arrhythmias. Notably, new‐onset atrial fibrillation (AF) and atrial flutter (AFL) during hospitalisation in COVID‐19 patients have been associated with increased mortality. However, how this risk is impacted by sex and age is still poorly understood.
Aims: The aim of this study was to explore the relation of AF and AFL to in‐hospital mortality, with specific attention for sex‐ and age‐related differences.
Methods: For this multicentre cohort study, we extracted demographics, medical history, occurrence of electrical disorders and in‐hospital mortality from the large international patient registry CAPACITY‐COVID. For each electrical disorder, prevalence during hospitalisation was calculated. Subsequently, we analysed the incremental prognostic effect of developing AF/AFL on in‐hospital mortality, using multivariable logistic regression analyses, stratified for sex and age.
Results: In total, 5,782 patients (64% male; median age 67) were included. Of all patients 11.0% (95% CI 10.2–11.8) experienced AF and 1.6% (95% CI 1.3–1.9) experienced AFL during hospitalisation. Ventricular arrhythmias were rare (< 0.8% (95% CI 0.6–1.0)) and a conduction disorder was observed in 6.3% (95% CI 5.7–7.0). An event of AF/AFL appeared to occur more often in patients with pre‐existing heart failure. After multivariable adjustment for age and sex, new‐onset AF/AFL was significantly associated with a poorer prognosis, exemplified by a two‐ to three‐fold increased risk of in‐hospital mortality in males aged 60–72 years, whereas this effect was largely attenuated in older male patients and not observed in female patients (Figure 1).
Conclusion(s): In this large COVID‐19 cohort, new‐onset AF/AFL was associated with increased in‐hospital mortality, yet this increased risk was restricted to males aged 60–72 years.

PB0069
Splanchnic vein thrombosis and Budd‐Chiari syndrome after SARS‐CoV‐2 vaccination—Incidence of VITT
M. Lauw 1; R. Maan2; A. Plessier3; L. China4; D. Patch5; A. Baiges6; J. Garcia‐Pagan7; V. Hernandez‐Gea6; M. Hilleret8; E. Tjwa9; B. Giguet10; A. Heurgue11; I. Ollivier‐Hourmand12; S. Darwish Murad2
1 Erasmus University MC, Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Gastroenterology and Hepatology, Erasmus University Medical Center, Rotterdam, Zuid‐Holland, Netherlands; 3 Université de Paris, Centre de recherche sur l'inflammation, Inserm, Paris, Ile‐de‐France, France; 4 Institute of Liver and Digestive Health, University College London, London, England, United Kingdom; 5 Hepatology and Liver Transplantation, Royal Free London NHS Foundation Trust, London, England, United Kingdom; 6 Barcelona Hepatic Hemodynamic Laboratory, Liver Unit, Hospital Clínic, Institut de Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), University of Barcelona, Barcelona, Catalonia, Spain; 7 Hepatic Hemodynamic Laboratory, Liver Unit, Hospital Clínic, IDIBAPS and CIBEREHD. University of Barcelona, Barcelona. Spain, Barcelona, Catalonia, Spain; 8 Service d'Hépato‐Gastroentérologie, CHU Grenoble Alpes, Grenoble, Auvergne, France; 9 Department of Gastroenterology and Hepatology, Radboud University Medical Center, Nijmegen, Nijmegen, Gelderland, Netherlands, 10 Liver disease department, CHU Rennes, Univ Rennes, Rennes, Bretagne, France, 11 Department of Hepato‐Gastroenterology, CHU Reims, Reims, Champagne‐Ardenne, France, 12 Department of hepatology and gastroenterology, University Hospital, Côte de Nacre, Caen, Caen, Basse‐Normandie, France
Background: Several studies have been published on a rare side effect of severe venous thrombosis at unusual sites and thrombocytopenia after vaccination against SARS‐CoV‐2, referred to as vaccine‐induced immune thrombocytopenia and thrombosis (VITT).
Aims: To identify new cases of acute splanchnic vein thrombosis (SVT) or Budd‐Chiari Syndrome (BCS) who presented following SARS‐CoV‐2 vaccination in the Vascular Liver Disease Group (VALDIG) network, and to evaluate the incidence of VITT.
Methods: We conducted a prospective international cohort study between May 1st, 2021 and January 10th, 2022, on consecutive patients with acute SVT or BCS who presented within 6 weeks following any type or dose of SARS‐CoV‐2 vaccination. Anonymous data were collected including baseline characteristics, risk factors, treatment and survival. Cases were identified as definite VITT, probable VITT or possible VITT or unlikely VITT as defined by Pavord et al (NEJM 2021).
Results: 25 patients with acute (N = 24) or recurrent (N = 1) SVT or BCS were collected from 14 centers in 4 countries (after ChAdOx1 nCoV‐19 N = 11, BNT162b2 N = 9, Ad26.COV2.S N = 1, mRNA‐1273 N = 1). Median time after vaccination to symptoms was 10 days (2‐40). Median age was 52.5 years (21‐66), 52% were female. Three patients (12%) fulfilled criteria for definite VITT, 6 (24%) for probable VITT, 2 (8%) for possible VITT, 14 (56%) for unlikely VITT. Thrombosis was located in the portal vein (N = 20), hepatic vein(s) (N = 9), mesenteric vein (N = 18) or splenic vein (N = 9). Concomitant extra‐abdominal thrombosis was seen in 5 patients (20%). Patients were treated with LMWH (60%), DOACs (24%) or VKA (40%). Six (2/3 with definite VITT) received IVIG. Thrombophilia was found in 5 patients and 3 had a myeloproliferative neoplasm.
Conclusion(s): 25 cases of acute SVT or BCS following SARS‐CoV‐2 vaccination were identified. Although definite VITT was rare (12%), no underlying disorder was identified in the majority of patients, contrary to ‘typical’ cases of SVT and BCS.
Diagnostics and OMICs
Biomarkers of Thrombosis and Hemostasis
PB0145
MRP‐4 («Multidrug Resistance Protein‐4) protein expression in patients with acute coronary syndrome (ACS) under chronic aspirin treatment
E. Schiera 1; M. Curreli1; V. Cesario1; F. De Felice2; G. Tocci1; F. Pulcinelli1
1 Sapienza University of Rome, Rome, Lazio, Italy; 2 San Camillo Forlanini Hospital, Roma, Lazio, Italy
Background: Aspirin reduces cardiovascular events. However, Aspirin causes «High‐on Aspirin Residual Platelet Reactivity» (HARPR) phenomenon. Some patients under chronic aspirin treatment present MRP‐4 overexpression and those who have high MRP4 levels correlate with HARPR, suggesting that platelet MRP‐4 overexpression is an additional CV risk factor.
Aims: To evaluate whether platelets over‐expression is an additional CV risk factor, we performed a study in which we investigated amounts of platelets MRP4 between the ACS population and control population (patients under chronic aspirin without ACS).
Methods: We enrolled patients with ACS on chronic aspirin therapy (75–100 mg/die) for at least 2 months (N = 51) and patients on chronic aspirin therapy (75–100 mg/die) for at least 2 months, in the absence of ACS (ASA; N = 30) from San Camillo Interventional Cardiology Unit and Platelet physiopatology Lab, Policlinico Umberto I respectively. Levels of platelets MRP4 expression were evaluated with WB analysis comparing the densitometric values of protein expression of each individual belonging ACS and ASA populations. Data were expressed as percentage of the densitometric mean of each sample compared to the average of the densitometric values obtained from three healthy volunteers (HV). Each gel data were normalized using the same HV samples.
Results: 1. ASA patients showed an increase of platelets MRP4 expression compared to HV subjects similarly to what was reported in previous work. 2. MRP4 levels in platelets obtained from in ACS patients are higher compared to ASA patients (52 ± 12% vs. 34 ± 6%).
Conclusion(s): Reduced sensitivity to aspirin by MRP‐4 could be one of the causes of SCA events. Platelet MRP4 levels may be a useful tool to identify patients less sensible to aspirin treatment and at high CV risk factor. In the context of precision medicine MRP4 platelet amount could be important to identify patients that required antiplatelet treatment variation, so as to create a personalized treatment.
PB0143
Interferences in the dosage of D‐dimer and variation between methodologies.
S. Lopes 1; R. Penteado2; J. Guerra2; T. Costa2; E. Welter2; V. de Aranda2; A. Villarinho2; B. Fogo2; A. dos Santos2; E. da Silveira2; D. Yamada2; C. Ito2; N. Constantino2; C. Mendes2
1 Hospital Israelita Albert Einstein, Suzano, Sao Paulo, Brazil; 2 Hospital Israelita Albert Einstein, São Paulo, Sao Paulo, Brazil
Background: The dosage of d‐dimer has been of great help in the management of patients at thrombotic risk. However, the greater flow of tests during the pandemic highlighted problems of specificity of the biomarker and differences between methodologies.
Aims: This project aims to identify interferents that cause variations in the results of d‐dimer dosages between different methodologies.
Methods: Samples were selected from patients who had their d‐dimer dosed in the ACL‐TOP® equipment (Werfen) by immunoturbidimetry, and confirmed in the Vidas3® equipment (Biomerieux) by ELFA. The samples were separated into two groups: Test Group, composed of 42 patients whose results showed significant discrepancies between ACL‐TOP® and Vidas3®; and Control Group, made up of 42 patients whose samples had similar dosages between the two devices. The FDPs and fibrinogen of all samples were measured in the ACL‐TOP®. Assays of rheumatoid factor were performed, as well as serial dilutions of d‐dimer.
Results: Through the dilutions and the determination of the rheumatoid factor, it was suggested that there was no interferent that would change the behavior of the samples during the immunoassays. Also, there wasn't significant presence of lipemia and hemolysis that could justify such alteration. When comparing the values of d‐dimer with those of FDP and fibrinogen, it was noted that there was no correlation between them when the d‐dimer was dosed by the ACL‐TOP®, only when it was dosed by Vidas3®.
Conclusion(s): The reason of the divergence, it was concluded, is possibly due to the nonspecificity of the antibodies, which have more affinity with high molecular weight fibrin degradation fragments than for d‐dimer. This conclusion is correlated with evidence found in the literature. These divergences may also be correlated with the patient's disease and further studies should be carried out for this purpose and to assess d‐dimer's relevance in the management of patients in different clinical conditions.


VPB0148
ADAMTS‐13 activity and silent brain infarction in patients with non‐ischemic dilated cardiomyopathy
R. Gómez De Antonio1; P. Martínez‐Legazpi2; E. Rodríguez González3; A. Fernández ávila3; G. Pérez Rus4; J. Díez Martín4; J. Bermejo Thomas3; C. Pascual Izquierdo 4
1 Deparment of Hematology, Hospital General Universitario Gregorio Marañón, Madrid, Spain., TORRELODONES, Madrid, Spain; 2 Department of Mathematical Physics and Fluids, Facultad de Ciencias, Universidad Nacional de Educación a Distancia, UNED, and CIBERCV, Madrid, Spain, Madrid, Madrid, Spain; 3 Department of Cardiology, Hospital General Universitario Gregorio Marañón, Facultad de Medicina, Universidad Complutense de Madrid, Instituto de Investigación Sanitaria Gregorio Marañón, and CIBERCV, Madrid, Spain, Madrid, Madrid, Spain; 4 Deparment of Hematology, Hospital General Universitario Gregorio Marañón, Madrid, Spain., Madrid, Madrid, Spain
Background: Von Willebrand factor levels (VWF) and ADAMTS‐13 activity have been recently described as predictors of thromboembolic events in patients with non‐valvular atrial fibrillation. Furthermore, left ventricular flow analysis by can predict mural thrombosis and cerebral microembolisms.
Aims: To investigate if hemostasis parameters combined with intraventricular flow analysis from echocardiography predict the silent brain infarction (SBI) in patients with non‐ischemic dilated cardiomyopathy (NIDCM).
Methods: This is a prospective and non‐interventional study. We included 80 patients with NIDCM in sinus rhythm and without history of thromboembolic episodes. Basal characteristics at inclusion were collected, including the hemostasis parameters evaluation, cerebral magnetic resonance (CMR) and intraventricular flow from echocardiography. Echocardiography images were post‐processed to determine the residence time of the blood (the time each blood particle remains inside the LV), and the shear stresses blood experience during its transit in the LV. The presence of SBI was evaluated using CMR images. We used Student's t‐test and the Spearman's test to compare variables and to conduct correlation analysis, respectively. Informed consent was obtained and this study was approved by a medical ethics committee.
Results: SBI was detected in 8 (12%) patients. None of the clinical factors and none of the hemostasis parameters analyzed correlated with the presence of SBI (Table 1). Blood shear stress in regions of high persistence (long residence time) in the LV was a good predictor of SBI (p < 0.001, ROC‐AUC (Bootstraped):0.81). ADAMTS13 activity and PT correlated with blood shear stress (p = 0.04, p = 0.01, respectively) (Figure1).
Conclusion(s): VWF levels, ADAMTS13 activity and other hemostasis parameters were not predictors of SCI. Blood shear stress was a good predictor of SSI. This variable has been related to incremental platelet activation in other series. ADAMTS13 activity and PT shown to be associated with blood shear stresses in the LV, allowing for further investigation.


PB0146
Obese patients show enhanced ex vivo collagen‐induced thrombus formation in flowing human blood
S. Troitiño 1; D. Fernández2; L. Hermida‐Nogueira1; S. Buján‐Garmendia3; D. López‐Fernández3; A. Sueiro4; Á. García1
1 Platelet Proteomics Group, Center for Research in Molecular Medicine and Chronic Diseases (CiMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain, Santiago de Compostela, Galicia, Spain; 2 Department of Biochemistry, CARIM, Maastricht University, 6200 MD Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 3 Santa Comba Primary Healthcare Center, Santa Comba, Spain, Santa Comba, Galicia, Spain; 4 Grupo de Endocrinología Molecular y Celular, Instituto de Investigación Sanitaria de Santiago (IDIS), Servicio de Endocrinología, Xerencia de Xestión Integrada de Santiago (XXS), Santiago de Compostela, Spain, Santiago De Compostela, Galicia, Spain
Background: We recently demonstrated that platelets from severely obese patients show higher surface expression levels of GPVI and CLEC‐2, and up‐regulated signaling pathways compared to lean‐matched controls, revealing that obesity induces an hyperactivation on platelets (Barrachina et al. ATVB; 2021;41:478–490).
Aims: To evaluate the impact of obesity in thrombus formation and platelet reactivity by using a novel ex vivo microfluidics assay on whole blood.
Methods: Severely obese patients (with no major comorbidities) and lean‐matched controls were recruited. Whole‐blood microfluidics assays evaluated the shear‐dependent thrombus formation process over collagen‐H in non‐coagulating conditions. Platelet adhesion and activation parameters (integrin activation, P‐selectin, and phosphatidylserine exposure) were recorded. Moreover, citrated blood was also perfused over a collagen surface to induce shear‐dependent thrombus and fibrin formation, where tissue factor (TF) was partly included to induce activation of the extrinsic coagulation pathway promoting thrombin generation.
Results: In obese patients, increasing thrombus size and volume was observed on collagen. Adhesion parameters were slightly higher in obese patients whereas no major differences between groups were observed in activation parameters. Obese patients showed a slightly more rapid fibrin formation following adhesion to collagen in the presence of TF. In the latter conditions, there were also increased levels in coagulation parameters, such as fibrin formation and phosphatidylserine exposure, in blood from obese patients in comparison to the lean‐matched subjects.
Conclusion(s): This is the first study evaluating the effect of obesity on ex vivo collagen‐induced thrombus formation in flowing blood under both coagulating and non‐coagulating conditions. Overall, we show that obesity enhances the platelet thrombotic phenotype to some extent. These results are in line with our previous studies on washed platelets and confirm an increased atherothrombotic risk in obese patients.
PB0147
Development of a microfluidic model to evaluate extracorporeal circuit material thrombogenicity
L. Gao1; J. Hong2; B. Akhavan3; A. Waterhouse 4
1 Heart Research Institute, The University of Sydney, Newtown, New South Wales, Australia; 2 School of Chemistry, The University of Sydney, Sydney, New South Wales, Australia; 3 School of Biomedical Engineering, The University of Sydney, Sydney, New South Wales, Australia; 4 The University of Sydney, Sydney, New South Wales, Australia
Background: Extracorporeal circuits, for example, extracorporeal membrane oxygenation, cardiopulmonary bypass, and dialysis circuits, require patients to receive systemic heparin to mitigate thrombosis caused by the circuit materials at low flow or stagnant regions such as tubing connectors. Despite the use of heparin and heparin coated circuits, thrombosis and severe bleeding events can still occur. Understanding how extracorporeal circuit materials cause thrombosis is critical to reduce thrombotic and bleeding events and our reliance on systemic heparin.
Aims: To develop a microfluidic model to evaluate extracorporeal circuit material thrombogenicity under flow.
Methods: Straight channel microfluidics incorporating control materials (glass and silicone) or the material of interest (polycarbonate (used for circuit connectors) and polyvinylchloride (PVC, used for circuit tubing)) were fabricated using standard photo and soft lithography. Whole blood anti‐coagulated with clinically relevant levels of heparin (0.5–5IU/ml) was flowed through the channels at a shear strain rate of 50‐2000s‐1. Platelet adhesion on materials was observed in real‐time using fluorescence microscopy.
Results: At a shear rate of 200s‐1, platelet adhesion was highest on PVC where platelets accumulated over time. Platelet adhesion was lower on silicone and polycarbonate compared to PVC, with platelets observed adhering and accumulating initially, then detaching over time. Platelet adhesion to glass was the lowest compared to all hydrophobic polymers. Platelet adhesion was reduced by blocking the materials with albumin prior to blood contact. The same trends were observed at shear rates from 50‐2000s‐1.
Conclusion(s): Real‐time visualisation of dynamic platelet events on extracorporeal circuit materials using clinically relevant shear rates and anticoagulation levels in a microfluidic model, revealed varying levels of platelet adhesion, accumulation, and detachment of different hydrophobic polymers. This model could be used to further understand platelet interactions during extracorporeal circuit thrombosis and for the development of anti‐thrombogenic materials for extracorporeal circuits.
VPB0149
Single nucleotide polymorphisms of the HIF1A gene are associated with sensitivity of glucocorticoid treatment in pediatric ITP patients
H. Gu 1; X. Xie2; J. MA3; L. Fu3; J. Ma3; R. Wu4; Z. Chen5
1 Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, Beijing, China (People's Republic); 2 Capital Medical University, Beijing, Beijing, China (People's Republic); 3 Hematology Oncology Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, Beijing, China (People's Republic); 4 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic); 5 Beijing Children's Hospital, Beijing, Beijing, China (People's Republic)
Background: Hypoxia‐inducible factor‐1α (HIF‐1α) plays a crucial role in both innate and adaptive immunity. Emerging evidence indicates that HIF‐1α is associated with the inflammation and pathologic activities of autoimmune diseases, suggesting that HIF1α may be involved in immune dysregulation in patients with immune thrombocytopenia (ITP).
Aims: The purpose of this study was to evaluate whether single nucleotide polymorphisms (SNPs) of the HIF1A gene are associated with susceptibility to ITP and its clinical prognosis including incidence of chronic immune thrombocytopenia (CITP) and glucocorticoid sensitivity.
Methods: This study involved 197 Chinese ITP pediatric patients (discovery cohort) and 220 healthy controls. The Sequenom MassArray system (Sequenom, San Diego, CA) was used to detect three SNPs genotypes in the HIF1A gene: rs11549465, rs1957757 and rs2057482. We also employed another ITP cohort (N = 127) to validate the significant results of SNPs found in the discovery cohort.
Results: The frequencies of the three SNPs did not show any significant differences between the ITP and healthy control groups. The CT genotype at rs11549465 was significantly higher in ITP patients sensitive to glucocorticoid‐treatment than in those insensitive to glucocorticoid‐treatment (p = 0.025). These results were validated using another ITP cohort (N = 127, p = 0.033). Moreover, the CC genotype was a risk factor for insensitive to GT the OR (95% confidence interval) was 5.96(5.23–6.69) in standard prednisone (PDN) (p = 0.0069) and 6.35(5.33–7.37) in high‐dose dexamethasone (HDD) (p = 0.04).
Conclusion(s): Although HIF1A gene polymorphisms were not associated with susceptibility to ITP, the CT genotype at rs11549465 was associated with the sensitivity to glucocorticoid‐treatment of ITP patients, suggesting that the rs11549465 SNP may contribute to the sensitivity of glucocorticoid treatment in pediatric ITP patients.

HIF1A SNP associated with sensitivity of glucocorticoid treatment in pediatric ITP patients
PB0142
Overall Haemostatic Potential (OHP) assay can risk stratify for venous thromboembolism recurrence in anticoagulated patients
J. Wang1; H. Lim2; R. Brook1; H. Nandurkar3; P. Ho 4
1 Northern Health, Melbourne, Victoria, Australia; 2 Northern Pathology Victoria, Department of Haematology, Northern Health, Epping, VIC, Epping, Victoria, Australia; 3 Alfred Health and the Australian Centre for Blood Diseases and Monash University, Melbourne, Victoria, Australia; 4 Northern Health, Victoria, Australia, Epping, Victoria, Australia
Background: Assessing the risk of recurrent venous thromboembolic (VTE) events, particularly when patients remain on anticoagulation, remains a major challenge largely due to lack of biomarkers.
Aims: We aimed to investigate the use of the OHP assay in newly diagnosed patients following VTE and explore its role in the risk stratification of VTE recurrence, including in patients whilst receiving anticoagulation.
Methods: Adult patients following VTE were recruited between January 2018 and September 2020. Platelet‐poor plasma was obtained whilst patients remained on therapeutic anticoagulation. Overall haemostatic potential (OHP) assay, which evaluates fibrin formation with and without tissue plasminogen activator (tPA), was performed on all plasma samples. Time‐to‐event analysis was performed with recurrent VTE or recurrent unprovoked VTE as endpoints.
Results: OHP assay results were obtained from 196 patients (52.6% male) with a mean age of 57.1 years. Compared to healthy subjects, VTE patients displayed significantly higher overall coagulation potential (without tPA) (39.6 v 34.5 units, p < 0.001) and OHP (with tPA) (9.3 v 6.4 units, p < 0.001) as well as lower overall fibrinolytic potential (OFP) (75.6 v 81.1%, p < 0.001). There were 16 VTE recurrences including 11 unprovoked, all of which occurred above an OCP cut‐off of 40th percentile (recurrence rate 4.32 per 100 patient‐years, 95% confidence interval (CI) 2.39–7.80, p = 0.002). Of 97 patients who subsequently ceased anticoagulation, all unprovoked VTE recurrences (n = 9) occurred above the 40th OCP percentile (recurrence rate 9.10 per 100 patient‐years, 95% CI 4.74–17.49, p = 0.005) and the 40th OHP percentile (recurrence rate 8.46 per 100 patient‐years, 95% CI 4.40–16.25, p = 0.009). OCP performed better than D‐dimer at predicting unprovoked VTE recurrence (AUC 0.72 vs. 0.43).
Conclusion(s): Our pilot study demonstrates that the OHP assay can detect a hypercoagulable and hypofibrinolytic state in anticoagulated VTE patients and may be able to risk stratify for VTE recurrence, allowing for more individualised targeting of long‐term anticoagulation.


PB0144
Expression of microRNAs related to platelet function and cardiovascular events in patients with stable coronary artery disease
O. Pedersen 1; P. Nissen2; L. Pasalic3; S. Larsen4; S. Kristensen5; E. Grove5; A. Hvas6
1 Aarhus University Hospital, Aarhus N, Midtjylland, Denmark; 2 Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark and Department of Clinical Medicine, Faculty of Health, Aarhus University; Aarhus, Denmark, Aarhus N, Midtjylland, Denmark; 3 Institute of Clinical Pathology and Medical Research and the Departments of Clinical and Laboratory Haematology, Westmead Hospital, Sydney, Australia and Westmead Clinical School, Faculty of Medicine, University of Sydney, Sydney, Australia, Sydney, New South Wales, Australia; 4 Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark, Aarhus N, Midtjylland, Denmark; 5 Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark and Department of Clinical Medicine, Faculty of Health, Aarhus University; Aarhus, Denmark, Aarhus N, Midtjylland, Denmark; 6 Department of Clinical Medicine, Faculty of Health, Aarhus University, Denmark, Aarhus N, Midtjylland, Denmark
Background: Despite antithrombotic therapy, many patients with coronary artery disease (CAD) suffer recurrent cardiovascular events. Hence, new biomarkers are warranted to help identify high‐risk patients. MicroRNAs (miR) may influence the risk of recurrent events in CAD by regulating platelet function.
Aims: We aimed to identify candidate miRs associating with platelet function in CAD patients receiving antithrombotic monotherapy with aspirin 75 mg daily and to investigate their utility in predicting future cardiovascular events.
Methods: We identified nine candidate miRs by performing a systematic review and a pilot study comparing 40 CAD patients with 20 healthy individuals. Subsequently, we investigated the expression of the identified candidate miRs and their relation to platelet function and cardiovascular events in a cohort of 749 CAD patients. Platelet function was analysed by impedance and optical aggregometry. Patients were followed for a median of 3 years. The primary endpoint was a composite of cardiovascular death, myocardial infarction (MI), stent thrombosis (ST) and ischaemic stroke. The secondary endpoint was a composite of MI and ST. Approvals were obtained from the Committee of Health Research Ethics.
Results: The expression of miR‐93, −126 and −150 correlated significantly with platelet function (p < 0.001, rho ranging from −0.13 to −0.36) in CAD patients. The combination of miR‐223 expression, miR‐150 expression and conventional risk factors (age, gender, body mass index, smoking, diabetes mellitus, prior myocardial infarction and creatinine) significantly increased the predictive value (AUC) of both the primary and secondary endpoint compared with clinical risk factors alone: 0.66 (0.59–0.72) vs. 0.57 (0.49–0.63), p = 0.01 and 0.75 (0.69–0.81) vs. 0.59 (0.51–0.68), p = 0.0002, respectively, Figure 1.
Conclusion(s): The expression of specific miRs may be important for platelet function and hence contribute to the risk of recurrent cardiovascular events. Adding the combination of miR‐223 and miR‐150 expressions to clinical cardiovascular risk factors improves risk assessment in CAD patients.

Blood Components and Management
PB0152
Changes in platelet functionality and morphology during storage of platelet concentrates
A. Khabirova1; L. Fatkhullina2; A. Peshkova3; I. Andrianova1; J. Weisel4; R. Litvinov 5
1 Institute of Fundamental Medicine and Biology, Kazan (Volga region) Federal University, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 2 Department of Blood Procurement, Interregional Clinical Diagnostic Center, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 3 Kazan (Volga region) Federal University, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 4 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 5 University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania, United States
Background: Platelet concentrates are used to prevent/stop bleeding in thrombocytopenias and/or thrombocytopathies. Stored platelets undergo changes that can reduce their hemostatic potential and increase the risk of post‐transfusion complications.
Aims: To study dynamic morphological and functional alterations in platelets during storage of platelet concentrates.
Methods: 150 samples of apheresis human platelet concentrates in a platelet additive solution SSP+ were analyzed within 1–7 days of storage at 20–24°C. Platelet functionality was assessed using flow cytometry by expression of P‐selectin, active integrin αIIbβ3, and phosphatidylserine before and after stimulation with the TRAP peptide. In addition, the mitochondrial transmembrane potential, platelet contractility (quantified as the extent of plasma clot shrinkage), collagen‐ and TRAP‐induced platelet aggregation were measured together with imaging of platelets by scanning electron microscopy.
Results: Expression of P‐selectin and phosphatidylserine in unstimulated platelets increased during storage, while the level of active αIIbβ3 remained low. The TRAP‐induced expression of P‐selectin, αIIbβ3, and phosphatidylserine was highest in fresh platelet concentrates and decreased gradually during storage. Platelet aggregation followed similar dynamics. The ability of platelets to contract plasma clots was almost unchanged in most samples and the mitochondrial transmembrane potential also remained high throughout the period of observation. Morphologically, the discoid quiescent platelets predominated in all samples; however, starting from the 1st day, a fraction of spindle‐like platelets appeared and increased progressively, and beginning from the 3rd day, spherical forms and platelet microaggregates were revealed.
Conclusion(s): The results obtained indicate that platelets in concentrates initially have a high functional potential, which declines progressively with time due to spontaneous activation and a simultaneous decrease in reactivity and structural alterations. Platelet contractility turns out to be stable up to 1 week. The results obtained substantiate the therapeutic efficacy of fresh platelet concentrates as hemostatic agents with their usefulness decreasing over time. Grant 21‐75‐00010 from the Russian Science Foundation.
PB0153
Comparative pharmacokinetics of coagulation factor VIII and IX products between independently developed and web‐based population pharmacokinetic models
D. Warad 1; J. Reid2; D. Grill3; A. Nageswara Rao1; V. Rodriguez4; A. Ashrani3
1 Mayo Clinic, Rochester MN, East Brunswick, New Jersey, United States; 2 Mayo Clinic, Rochester MN, Rochester, Minnesota, United States; 3 Mayo Clinic, Rochester, Minnesota, United States; 4 Nationwide Children's Hospital & The Ohio State University, Columbus, Ohio, United States
Background: Many variables affect factor replacement therapy for hemophilia patients. Population PK (PPK) modeling can facilitate dosing estimates even with reduced sampling schedule.
Aims: To compare pharmacokinetic (PK) estimates between PPK models for Factor VIII (FVIII) and Factor IX (FIX) products.
Methods: Retrospective review of hemophilia A or B patients. Comparison of PK estimates from in‐house Nonlinear Mixed Effects Modeling (NONMEM) model, online WAPPS‐Hemo, and expected age‐based mean product half‐lives (t1/2) in FDA prescriber labels (PL).
Results: A one‐compartment model with first‐order elimination was best fit for both cohorts. For hemophilia A (n = 51, males = 47, age 0.1–84.6 years, weight 3.5–151 kg), correlation coefficient (r) for t1/2 estimates between in‐house NONMEM and WAPPS‐Hemo models was 0.69 (95%CI 0.51, 0.81). Agreement between PL t1/2 values and WAPPS‐Hemo results was the best (mean bias 3.03; 95%CI 0.77, 5.29), while that with NONMEM was poorest (mean bias 6.48; 95%CI 3.44, 9.51). In‐house model t1/2 values demonstrated two distinct clusters (Figure 1). Confounding covariates were median age (p = 0.0010) and PK study type (peri‐procedural vs. prospective; p = 0.0222). For hemophilia B (n = 18, males = 17, age 5–79 years, weight 20.8–105 kg), correlation coefficient (r) for t1/2 estimates between in‐house NONMEM and WAPPS‐Hemo models was 0.89 (95%CI 0.78, 0.94). Agreement between WAPPS‐Hemo and NONMEM was lowest (mean bias 36.88; 95%CI 28.57, 45.20), while that between PL t1/2 and NONMEM was the best (mean bias 13.46; 95%CI 7.59, 19.33). Comparison of in‐house t1/2 with PL demonstrated two distinct clusters (Figure 2). Confounding covariates were product type (standard vs. extended t1/2; p< 0.0001), PK study type (peri‐procedural vs. prospective; p = 0.0003), and weight (p = 0.0296).
Conclusion(s): Despite differential comparative results with PL and WAPPS‐Hemo estimates, likely due to limited sample size and heterogeneity of factor products, an external validation of our independently built FVIII and FIX PPK models was possible and their performance characteristics were consistent with published literature.

Figure 1

Figure 2
PB0151
Automated genotyped matching of platelets to prevent alloimmunisation
N. Gleadall 1; R. Fenton2; D. Hart3; P. Winter4; K. Lee5; C. Booth6; L. Walker7; S. Stanworth8; B. Genomics Consortium9; W. Ouwehand10; W. Astle11; S. Sivapalaratnam12
1 University of Cambridge & NHS Blood and Transplant, Cambridge, England, United Kingdom; 2 Barts Health NHS Trust, Royal London Hospital, London, England, United Kingdom; 3 Royal London Hospital Haemophilia Centre, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom; 4 Department of Haematology, Barts Health NHS Trust, London, United Kingdom, London, England, United Kingdom; 5 Haematology Laboratory, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom; 6 Department of Haematology, Barts Health NHS Trust, London, United Kingdom and NHS Blood and Transplant, United Kingdom, London, England, United Kingdom; 7 Department of Haematology, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK., Cambridge, England, United Kingdom; 8 Oxford University Hospitals/NHS Blood and Transplant, Oxford, England, United Kingdom; 9 Cambridge University Hospitals, Cambridge Biomedical Campus, Cambridge, UK., Cambridge, England, United Kingdom; 10 Department of Haematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK. Department of Haematology, University College London Hospitals, London, NW1 2BU, UK, Cambridge, England, United Kingdom; 11 MRC Biostatistics Unit, Cambridge Institute of Public Health, University of Cambridge, Cambridge, UK, Cambridge, England, United Kingdom; 12 Barts Health NHS Trust, London, England, United Kingdom
Background: Immunisation against HLA antigens results in adverse healthcare outcomes and significantly increased costs for inherited platelet disorder (IPD) and haemato‐oncology patients requiring platelet transfusions. In a single tertiary London centre two of 17 patients with Glanzmann Thrombasthenia or Bernard Soulier Syndrome are refractory to random platelets because of HLA‐antibodies. Sourcing HLA‐matched platelets for these patients is not possible and costly treatments with activated recombinant‐Factor VII are required. These immunisations are avoidable with a sufficiently HLA‐typed donor pool.
Aims: To apply population‐scale genotyping for provision of HLA‐matched platelets.
Methods: The Blood transfusion Genomics Consortium (BGC) has developed an affordable genotyping array capable of typing all red cell, platelet (HPA) and HLA antigens at two‐field resolution. Validation has been performed using DNA samples from 14,000 donors from 7 BGC‐participating blood services. HPA and HLA types were inferred from the data using the bloodTyper and HLA:IMPUTE02 algorithms.
Results: Genotyping demonstrated concordance of 99.03% at two‐field resolution with clinically validated HLA typing results (Figure 1). Using the dense genotyping data for the donors in this study, we were able to identify 8 and 114 exact HLA matched donors for the two IPD patients with HLA‐antibodies, where through standard care none could be identified due to a lack of HLA‐typing data.
Conclusion(s): We present an affordable array‐based genotyping platform which has immediate value in increasing the availability and match quality of HLA‐matched platelet concentrates for IPD and Haemato‐Oncology patients. Currently, regulatory approval is sought and the technology has been introduced in several national blood services. In the near future full HLA and HPA antigen types will become available for millions of donors worldwide, providing the opportunity to implement a policy of genomics‐based precision platelet matching.

Cellular Therapies
PB0155
A potential use of leukocyte‐platelet rich fibrin secretome to improve wound healing and bone regeneration
L. Hermida‐Nogueira 1; J. Blanco2; J. Reseland3; Á. García1
1 Platelet Proteomics Group, Center for Research in Molecular Medicine and Chronic Diseases (CiMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain, Santiago de Compostela, Galicia, Spain; 2 Medical‐Surgical Dentistry Research Group (OMEQUI), Universidade de Santiago de Compostela (USC), Santiago de Compostela, Spain, Santiago de Compostela, Galicia, Spain; 3 Department of Biomaterial, Faculty of Dentistry, University of Oslo, Norway, Oslo, Oslo, Norway
Background: Leukocyte‐platelet rich fibrin (L‐PRF) is a bioproduct with platelets, leukocytes and a high‐density fibrin network. This biomaterial is very popular in dentistry for the treatment of oral wounds because of the growth factors released by platelets after activation. Recently, our group has characterized the whole secretome released by L‐PRF at different time points, finding changes over time that would benefit tissue regeneration (Hermida‐Nogueira et al. Sci Rep. 2020).
Aims: Therefore, our aim was to evaluate the effect of L‐PRF secretomes, collected at different time points, on several cell types related to the wound healing process.
Methods: The effect of the secretome was analysed on fibroblast proliferation and migration. Furthermore, the osteogenic potential of the secretome was evaluated in mesenchymal stem cells (MSC) measuring different bone formation biomarkers and cytokines.
Results: L‐PRF secretome collected at different time points showed differential effects on fibroblast migration and proliferation depending on the concentration applied. Low concentrations of secretomes collected at day 3 increased fibroblast proliferation and migration, however higher concentrations inhibited both processes. On the contrary, high concentrations of secretomes collected at day 7 showed a significant increase on fibroblast migration. Regarding the osteogenic potential, the levels of bone biomarkers OPG, DKK‐1 and calcium deposits were significant raised in MSC treated with L‐PRF secretomes. However, VEGF and MCP‐1 levels were also increased.
Conclusion(s): Differential effects on fibroblast migration and proliferation were found when the L‐PRF secretome collected at specific time points was applied at different concentrations. This result suggests a specific secretome protein contribution to wound healing. The L‐PRF secretome had also an effect on osteogenic differentiation of MSC, as well as a primary or secondary effect on other cell types such as endothelial cells, increasing the potential use of L‐PRF in other clinical scenarios. Funding: Intra‐Lock Iberia and Xunta de Galicia (predoctoral grant 2018)
PB0154
Lyophilized human platelets are superior to apheresis or fresh‐drawn platelets in their ability to accelerate thrombin production
L. Booth; M. Dickerson; K. Moskowitz
Cellphire Therapeutics, Inc, Rockville, Maryland, United States
Background: Platelet transfusions are used to treat bleeding or prophylactically increase platelet counts in thrombocytopenic patients. Unfortunately, the platelet supply is limited by many logistical challenges and presents a need for additional treatment options. One strategy to combat the danger of platelet shortages are Thrombosomes®, a shelf‐stable human platelet derived lyophilized hemostatic (LHP), currently in clinical trials for the treatment of thrombocytopenic bleeding. Direct comparisons of LHP’s hemostatic capabilities compared to apheresis platelet concentrates or fresh platelets needs additional investigation.
Aims: Compare the thrombin generation capacity of LHP to apheresis or fresh‐drawn platelets
Methods: Apheresis platelets units (APU) were sourced from a blood center and collected per AABB guidelines. Fresh‐drawn platelets were prepared from whole blood and used within four hours. Thrombin generation assay (TGA) using the calibrated automated thrombogram (CAT) or the Thrombodynamics analyzer, were used to determine thrombin generation in the presence of 2,000 LHP/μl or 20,000 LHP/μl versus 20,000/μl apheresis or fresh‐drawn platelets initiated by tissue factor in the absence of additional phospholipids.
Results: LHP (2000/μl) decreased the lag time (0.1 min vs. 0.6 min) and increased the maximum thrombin concentration (176 Units/L vs. 118 Units/L) compared to fresh platelets at 20,000/μl using the Thrombodynamics assay. Likewise, LHP decreased lag time to 6.5 min compared to 10‐fold amounts of fresh (9.1 min) or APU (24.7 min) using the TGA method. At equal concentrations, LHP increased the maximum thrombin peak height to 169 nM compared to fresh (80 nM) or apheresis (63 nM).
Conclusion(s): These in vitro results demonstrate that LHP produce a quicker thrombin burst and are more potent at thrombin generation compared to apheresis or fresh platelets. The data suggests that LHP are likely to have equal or better hemostatic benefits relative to APU. Additional in vivo confirmatory experiments are planned.
PB0156
Expression of exogenous proteins in donor platelets treated with lipid nanoparticles encapsulating mRNA
J. Leung 1; C. Strong2; K. Badior3; M. Robertson4; L. Juang4; E. Jan4; D. Devine4; P. Cullis4; C. Kastrup5
1 University of British Columbia, Richmond, British Columbia, Canada; 2 University of British Columbia, VANCOUVER, British Columbia, Canada; 3 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 4 University of British Columbia, Vancouver, British Columbia, Canada; 5 Medical College of Wisconsin, Milwaukee, Wisconsin, United States
Background: Platelets are transfused therapeutically for hemostasis, and are an integral part of hemorrhage management. However, transfusions can be ineffective in the most severe cases of hemorrhage. Platelets are also a potential cell therapy in other applications, but development has been hindered by inadequate methods to control which proteins are expressed by platelets. Currently, there are no methods to express exogenous proteins in transfusable platelets, which would expand their use to help treat the diseases they modulate. A method is therefore needed to modify transfusable platelets, and thus enhance their protein composition for specific applications.
Aims: To produce engineered, transfusable platelets to enhance their natural coagulability and functional repertoire by directly transfecting donor‐derived platelets with mRNA via lipid nanoparticle (LNP)‐mediated delivery. The recent advances through the COVID‐19 mRNA vaccines demonstrates the clinical safety and efficacy of LNP‐mediated gene therapy, and thus offers a promising strategy to effectively engineer modified platelets.
Methods: Donor‐derived platelets were washed and subsequently incubated with a systematic array of LNPs encapsulating Cy5‐labeled mRNA encoding for nanoluciferase in comparison to commercial transfection reagents. LNP uptake and platelet activation via CD62p levels was assessed following 4 hours by flow cytometry, while luciferase expression was assessed by normalizing the luminescence intensity to the total protein content.
Results: Platelets took up the mRNA through all conditions tested; nanoluciferase was only expressed, however, in platelets treated with LNPs and not commercial reagents. Systematically optimizing LNPs increased nanoluciferase expression nine‐fold relative to pre‐optimized LNPs. Exogenous protein expression did not appear to correlate with mRNA uptake nor platelet activation.
Conclusion(s): Platelets transfected with LNPs can express exogenous protein. Further optimization can eventually lead to the creation of a platform technology that in the long‐term will allow platelets to deliver therapeutic proteins and yield more effective platelet products.


PB0157
Platelets genetically modified with mRNA‐lipid nanoparticles are functionally similar to clinically transfused platelets in vitro
C. Strong 1; J. Leung2; K. Badior3; M. Robertson4; E. Jan4; P. Cullis4; D. Devine4; C. Kastrup5
1 University of British Columbia, VANCOUVER, British Columbia, Canada; 2 University of British Columbia, Richmond, British Columbia, Canada; 3 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 4 University of British Columbia, Vancouver, British Columbia, Canada; 5 Medical College of Wisconsin, Milwaukee, Wisconsin, United States
Background: Platelet transfusions are an essential treatment for attenuating bleeding but are often ineffective in cases of intractable hemorrhage. Although anucleate, mature platelets synthesize protein de novo, making them amenable to mRNA gene therapy; however, there remains to be an effective transfection technique. Advancements in lipid nanoparticle technology has enabled leading COVID vaccines and is an efficient method to deliver nucleic acids into target cells. Recently, we developed a LNP approach to successfully express exogenous protein in platelets [unpublished data], a first step towards demonstrating that donor platelet coagulability can be engineered. However, the effects of LNP treatment on platelet function has yet to be investigated.
Aims: To determine whether LNP‐treated donor platelets are functionally similar, or better, in vitro, than platelets currently transfused clinically as a next step to establish LNP engineered platelets as a new cell therapy.
Methods: The hemostatic profiles of LNP‐treated and clinical donor platelets were assessed using an adapted rotational thromboelastometry model of dilutional coagulopathy. LNP‐treated platelets were also stimulated with conventional platelet agonists to test if responsiveness is similar, or better than clinical platelets using flow cytometry.
Results: LNP‐treated platelets have a comparable hemostatic profile to clinically transfused platelets and significantly improved clotting dynamics when spiked into hemodiluted whole blood in an in vitro transfusion simulation. LNP‐treated platelets also respond comparably, and in some cases more potently to agonist simulation compared to untreated platelets as indicated by similar p‐selectin surface presentation.
Conclusion(s): LNPs are an effective way to deliver exogenous nucleic acids into platelets; they do not significantly change platelet coagulability or responsiveness to agonists. LNP platelet engineering is a promising new approach to load platelets with procoagulant protein to enhance their function.
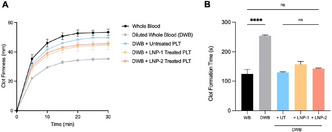

Epigenetics, OMICs and Bioinformatics
VPB0162
Protein expression in plasma after hemarthrosis differs among recombinant FVIII and FVIII‐Fc fusion replacement in FVIII‐deficient mice
J. Zhou 1; B. Chumappumkal Joseph2; C. Gonzalez3; D. Gonzalez3; T. Whisenant4; A. von Drygalski5
1 University of California San Diego, La Jolla, CA, USA, San Diego, California, United States; 2 Department of Medicine, Division of Hematology/Oncology, University of California San Diego, La Jolla, CA, USA, san Diego, California, United States; 3 Department of Pharmacology, University of California San Diego, CA, USA, San Diego, California, United States; 4 Department of Medicine, Center for Computational Biology and Bioinformatics, University of California San Diego, La Jolla, CA, USA, San Diego, California, United States; 5 Division of Hematology/Oncology, Department of Medicine, University of California San Diego, San Diego, California, United States
Background: Systemic protein responses after hemarthroses in hemophilia are unknown. Emerging evidence suggests FVIII‐Fc fusion protein containing the Fc‐portion of immunoglobulin may modulate immune responses.
Aims: To explore differences in plasma protein expression after hemarthroses in FVIII‐deficient mice treated with recombinant human Factor VIII (rhFVIII) compared to murine Fc‐FVIII (mFcFVIII).
Methods: Hemarthrosis was induced by needle puncture in mice treated with saline, 100 IU/kg mFcFVIII (Fc species specificity) or 120 IU/kg rhFVIII (account for t1/2 differences) administered intravenously 2hrs before and 6hrs after injury. Plasma samples collected at baseline, 3 days, 2 and 4 weeks after hemarthrosis (n = 40, Figure 1) underwent proteomics. Extracted protein was isobarically labeled, fractionated and subjected to liquid chromatography/mass spectrometry (LC/MS^3). Data were searched against Mus musculus database; identified protein intensities were normalized using a common “bridge” channel. Significance was determined by p < 0.05 and mean difference of log2 fold‐change > 0.5 or < −0.5. Saline‐treated groups were compared to rhFVIII and mFcFVIII treated groups and significant proteins were tested for enrichments using STRING database.
Results: RhFVIII and mFcFVIII prevented hemarthrosis. On day 3, both FVIII‐preparations downregulated acute phase responses with similar number of enriched proteins, participating in coagulation, complement, platelet activation and immune pathways. One protein was unique to rhFVIII (collagen alpha chain) and 14 proteins were unique to mFcFVIII (involved in complement, coagulation and immune system). At 2 weeks, numbers of proteins enriched for both FVIII‐preparations were similar, involved in platelet activation, extracellular matrix (ECM)/endothelial/actin cytoskeleton interactions, with few proteins unique to rhFVIII or mFcFVIII. Notably, responses continued to unfold 4 weeks after hemarthrosis. Both FVIII‐treatments affected proteins in tissue healing, however mFcFVIII elicited a pronounced immune response (Figure 2), with complement proteins upregulated and immunoglobulin components downregulated.
Conclusion(s): Hemarthrosis triggered lasting protein expression patterns, demonstrating FVIII‐type specific responses beyond hemostasis control, and notably late immune responses with mFcFVIII.


PB0158
AlphaFold and molecular dynamics structure predictions of mutations in serpins: Lessons from antithrombin
P. Garrido‐Rodríguez 1; M. Carmena‐Bargueño2; M. de la Morena‐Barrio1; C. Bravo‐Pérez3; B. de la Morena‐Barrio4; R. Cifuentes‐Riquelme1; V. Vicente1; M. Lozano1; H. Pérez‐Sánchez2; J. Corral1
1 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 2 Structural Bioinformatics & High Performance Computing Research Group (BIO‐HPC), Universidad Católica de Murcia (UCAM), Murcia, 30107, Spain, Murcia, Murcia, Spain; 3 Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 4 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain, Murcia, Murcia, Spain
Background: Serine protease inhibitors (serpins) include thousands of structurally conserved proteins playing a key role in many systems of diverse organisms, being antithrombin a key serpin anticoagulant. Mutations affecting serpins may disturb their stressed metastable native conformation leading to inactive, hyperstable relaxed conformations (latent form or polymers). Unfortunately, conformational consequences of mutations affecting serpins are difficult to predict.
Aims: Evaluate the predictions of AlphaFold (a novel artificial intelligence system) and molecular dynamics software for five SERPINC1 mutations causing antithrombin deficiency, the strongest congenital thrombophilia.
Methods: p.Arg79Cys; p.Pro112Ser, p.Met283Val; p.Pro352insValPheLeuPro, and p.Glu241_Leu242delinsValLeuValLeuValAsnThrArgThr variants were selected from a cohort of 350 unrelated patients based on functional, biochemical, and crystallographic evidence supporting a folding defect.
Results: AlphaFold gave an accurate prediction for the wild‐type antithrombin structure. Nonetheless, it only produced native structures for all variants evaluated, regardless of their complexity (from SNVs to insertion of 9 residues) and their in vivo conformational consequences (functional variant ‐p.Arg79Cys‐, latent ‐p.Met283Val‐, a particular relaxed structure ‐p.Glu241_Leu242delinsValLeuValLeuValAsnThrArgThr‐ or polymers ‐p.Pro112Ser and p.Pro352insValPheLeuPro‐). AlphaFold enlarged loops or created new helices to accommodate inserted residues into a native, stressed conformation (Figure 1). Molecular dynamics of up to 1000 ns at low (27°C), high (40°C) and extreme temperatures (100°C) that caused conformational transitions in serpins gave identical results, even for the more complex variant with available crystal structure (Figure 1).
Conclusion(s): Despite the fact that the crystal structure of human antithrombin is a native‐latent dimer, AlphaFold (a predictive tool of protein folding) and a computer simulation method for analyzing the physical movements of atoms and molecules (molecular dynamics) only force predictions into the native stressed conformation even at conditions known to cause conformational changes. The development of predictive strategies for serpins that consider the structural sensitivity of these molecules that can reach hyperstable conformations remains an unsolved problem. PI21/00174(ISCIII&FEDER).

VPB0161
Gene expression after hemarthrosis differs among recombinant FVIII and FVIII‐Fc fusion replacement in FVIII‐deficient mice
B. Chumappumkal Joseph 1; T. Whisenant2; E. J Cooke3; J. Zhou4; S. Ruiz5; D. Rattanamansuang5; A. von Drygalski6
1 Department of Medicine, Division of Hematology/Oncology, University of California San Diego, La Jolla, CA, USA, san Diego, California, United States; 2 Department of Medicine, Center for Computational Biology and Bioinformatics, University of California San Diego, La Jolla, CA, USA, San Diego, California, United States; 3 Department of Medicine, Division of Hematology/Oncology, University of California San Diego, La Jolla, CA, USA, San Diego, California, United States; 4 University of California San Diego, La Jolla, CA, USA, San Diego, California, United States; 5 Department of Biological Sciences, University of California San Diego, La Jolla, CA, USA, San Diego, California, United States; 6 Division of Hematology/Oncology, Department of Medicine, University of California San Diego, San Diego, California, United States
Background: Synovial molecular processes after hemarthrosis are poorly understood. Emerging evidence suggests that FVIII‐Fc fusion protein, containing the Fc‐portion of immunoglobulin, may mitigate joint inflammation.
Aims: To explore synovial differential gene expression (DGE) and molecular pathways after hemarthrosis in FVIIIKO mice when treated with recombinant human factor VIII (rhFVIII) compared to murine Fc‐FVIII (mFcFVIII).
Methods: Hemarthrosis was induced by needle puncture in mice treated with saline, 100 IU/kg mFcFVIII (Fc species specificity) or 120 IU/kg rhFVIII (accounting for t1/2 differences) administered intravenously 2hrs before and 6hrs after injury. Synovial RNA sequencing (day 3 or 14 post‐injury (n = 3–5)) was performed with Illumina NextSeq500, STAR alignment to mm10, RSEM gene quantification, Limma DGE analysis (model: ~0 + time + treatment; adjusted p‐value < 0.05), gprofiler enrichment analysis using KEGG and Reactome databases. Saline‐treated groups were compared to untreated and FVIII treated groups.
Results: RhFVIII and mFcFVIII prevented hemarthrosis. On day 3, 2349 differentially expressed genes (DEGs) were related to hemarthrosis/injury; furthermore, of these, 956 DEGs were affected by FVIII (387 rhFVIII‐specific, 106 mFcFVIII‐specific, 463 shared). Total DGE was lower on day 14 with 1606 related to injury (20 rhFVIII‐specific, 95 mFcFVIII‐specific, 40 shared). DEGs modulated by both FVIII preparations, unrelated to hemarthrosis/injury, shared pathways involved in tissue metabolism/repair. Other pathways were unique to each treatment. Notably on day 14, only mFcFVIII affected collagen/tissue repair, while both FVIII‐preparations affected immune regulatory pathways for the subset of DEGs unrelated to hemarthrosis/injury (“off‐target”), differing in number and type. “On‐target” injury‐related DEGs with mFcFVIII were important to protein composition via the Ribosome and Spliceosome pathways (Figures 1 and 2).
Conclusion(s): FVIII preparation‐specific molecular processes after hemarthrosis differed, suggesting that the type of FVIII preparation may have implications beyond hemostasis control.


PB0160
Circulating EV and platelet releasate proteomes mirror the hyperactive, pro‐inflammatory state of COVID‐19 patients and may be adjunctive markers of COVID‐19 severity
P. Szklanna1; L. Weiss 2; S. Comer1; L. Boehm1; S. Cullivan1; S. Kelliher1; K. Wynne3; C. Scaife4; F. Ní Áinle5; B. Kevane6; P. Maguire7
1 UCD Conway SPHERE Research Group, Conway Institute, University College Dublin, Ireland, Dublin, Dublin, Ireland; 2 UCD Conway SPHERE Research Group, Conway Institute, University College Dublin, Dublin, Ireland, Dublin, Dublin, Ireland; 3 Systems Biology Ireland, University College Dublin, Ireland, Dublin, Dublin, Ireland; 4 Mass Spectrometry Core Facility, Conway Institute, University College Dublin, Ireland, Dublin, Dublin, Ireland; 5 Department for Haematology, Mater Misericordiae University Hospital, Ireland, Dublin, Dublin, Ireland; 6 Mater Misericordiae University Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 7 University College Dublin, Ireland, Dublin, Dublin, Ireland
Background: COVID‐19 infection can present with a heightened inflammatory state and platelet hyperactivation, however the roles of circulating extracellular vesicles (cEVs; pro‐coagulant and pro‐inflammatory mediators of intercellular communication) and the platelet releasate (PR; cargo released from activated platelets) remain uncharacterised. We hypothesised that the cEVs and PR proteomic signatures are altered in COVID‐19 patients.
Aims: To characterise and contrast paired cEVs and PR proteomes from COVID‐19 patients and controls.
Methods: Ethical approval for this study was granted by the Mater Misericordiae University Hospital, Ireland. PR and platelet poor plasma (PPP) samples from COVID‐19 patients requiring intensive care (severe, n = 6) or hospital care (non‐severe, n = 6), and appropriate controls (n = 6 healthy controls and n = 5 COVID‐negative hospitalised patients) were obtained with informed consent. cEVs were enriched from PPP by ultrafiltration. Samples were analysed by mass spectrometry and immunoassays.
Results: Comparative statistical analysis revealed substantial overlap in the proteomic signatures of the cEVs and PR across COVID‐19 patients and controls. Profound increases in circulating levels of pro‐inflammatory proteins correlated with severity of infection, alongside significant dysregulation of proteins involved in blood coagulation including von Willebrand factor and fibrinogen. We also found evidence of ‘spent’ platelets in COVID‐19 patients, with reduced platelet factor 4 (PF4) levels in the PR and concomitant increase of PF4 in plasma. Strikingly, several platelet‐released proteins exhibited strong correlation with routinely obtained haematological parameters and together clearly differentiated between a severe and non‐severe hospitalised COVID‐19 patients.
Conclusion(s): These preliminary data suggest that severe COVID‐19 patients have a distinct cEV and PR profile and that proteomic signatures may be a useful tool in assessing the severity of COVID‐19 when characterized in larger sample sizes. Our findings may lay the foundations to provide healthcare professionals with an adjunctive and minimally invasive tool towards assessing COVID‐19 severity.
PB0159
Loss of function variant in SMIM1 is associated with reduced energy expenditure, weight gain and an increased risk for cardiovascular events
L. Stefanucci1; C. Moslemi2; A. Tome3; D. Vuckovic4; M. Frontini 5
1 Wellcome Sanger Institute, Cambridge, England, United Kingdom; 2 Department of Clinical Immunology, Zealand University Hospital, Køge, Roskilde University, Denmark., Roskilde, Sjelland, Denmark; 3 University of Cambridge, Cambridge, England, United Kingdom; 4 Imperial college London, London, England, United Kingdom; 5 University of Exeter, Exeter, England, United Kingdom
Background: Blood groups have been extensively studied because of their importance in transfusion medicine, and variants in the ABO group are also associated with a slightly increased risk of thrombotic and bleeding events. In sharp contrast, we describe a 17‐bp nonsense‐deletion in SMIM1 present in homozygosity in 1 in 5000 of the UK Biobank (UKB) participants (who thus lack the Vel blood group), that is associated with a significant gain in weight and an increased risk of cardiovascular events.
Aims: To characterise the metabolic and cardiovascular consequences of SMIM1 absence.
Methods: We performed logistic regression on the UKB (90 SMIM1−/−) to investigate genotype‐phenotype associations. Findings were replicated in English and Danish blood donor cohorts, as well as Smim1−/− mice. scRNA‐Seq was used to investigate possible mechanisms.
Results: In SMIM1−/− UKB participants we found an association with increased weight (=0.22, p=FDR=4.61e‐02), more prominent in women than men, of 4.6 and 2.4 kg on average respectively (Figure 1). This observation was replicated in the blood donor cohorts and in Smim1−/− mice. Analysis of UKB‐plasma biochemistry showed increased triglycerides, urate and liver‐enzymes levels. A clinical metabolic assessment showed reduced energy expenditure (Figure 1). Association analysis for cardiovascular events in UKB showed an increased odds ratio for cerebral bleeds (OR = 5.53, FDR = 6.88e‐04) and thrombotic stroke (OR = 3.46, FDR = 2.32e‐02). scRNA‐seq showed expression of SMIM1‐transcript in the pituitary and thyroid glands.
Conclusion(s): We showed that individuals lacking SMIM1 (Vel‐negative) exhibit altered lipid metabolism, due, at least in part, to reduced energy expenditure. These data also suggest mild hypothyroidism and an increased risk of cerebral bleeds and thrombotic stroke. Further studies are required to understand the role of this small transmembrane‐protein in the pituitary‐thyroid axis and whether thyroid hormone supplementation can reverse cardiometabolic risks associated with this condition.

Laboratory Diagnostics
PB1112
Optimising light transmission platelet aggregometry: A retrospective and quality improvement study
X. Wang 1; M. Al Moosawi2; N. Au3; R. Onell4; M. Bahmanyar4; S. Wong4; S. Jackson4; T. Smith5; H. Nicolson4
1 University of British Columbia, vancouver, British Columbia, Canada; 2 The University of British Columbia, Vancouver, British Columbia, Canada; 3 BC Children's and Women's Hospital, Vancouver, British Columbia, Canada; 4 St Paul's Hospital, Vancouver, British Columbia, Canada; 5 Vancouver General Hospital, Vancouver, British Columbia, Canada
Background: Light transmission aggregometry (LTA) assay can be challenging to standardize. Several international guidelines have attempted to optimise this assay. Following these guidelines can improve the assay performance and interpretation.
Aims: To review the platelet function abnormalities observed in patients tested at two tertiary centres in British Columbia, and to provide suggestions to improve the assay performance.
Methods: After ethics approval, we reviewed the LTA procedure and conducted a retrospective chart review of patients tested at two specialized coagulation laboratories in BC (pediatric and adult centres) between January 1, 2015, and July 30, 2021.
Results: Results from 34 pediatric patients and 54 adults (>18 years) were reviewed. The majority of patients were females (73.5% of pediatrics, 85.1% of adults). The median ages were 15 years (IQR: 8.8–16) and 40 years (IQR: 30–59), respectively. Bleeding history was not explicitly provided at the time of testing but was attained from the majority of patients’ charts (97.0% of pediatrics, 79.6% of adults). Most patients (94.1% of pediatrics, 98.1% of adults) had been investigated with standard coagulation testing (including factor VIII and von Willebrand factor) prior to LTA. Thrombocytopenia (platelets < 150 × 109/L) was present in 2.9% of pediatric and in 22.2% of adult patients. Platelet morphology was specifically reported in 20.5% of pediatric and 14.8% of adult patients. Overall, 44% of the analyses were reported as normal (50.0% of children, 40.7% of adults). Although both centres utilized platelet agonists that are compatible with the ISTH guidelines, variations were observed between the two laboratories (Table 1).
Conclusion(s): Preliminary results highlight areas in the pre‐analytical and analytical phase that can be improved, however additional evaluation will be required.

VPB1118
Application of prothrombin time clot waveform analysis to assessment of in vitro effects of anticoagulants on normal plasma
Y. Fujimori 1; M. Wakui2; Y. Ozaki3; E. Osada3; S. Oka3; Y. Kondo4; T. Nakagawa3; S. Nakamura3; H. Katagiri5; M. Murata2
1 Keio University Hospital, Tokyo, Tokyo, Japan; 2 Department of Laboratory Medicine, Keio University School of Medicine, Shinjuku, Tokyo, Japan; 3 Clinical Laboratory, Keio University Hospital, Tokyo, Tokyo, Japan; 4 Clinical Laboratory, Tokyo, Tokyo, Japan; 5 Clinical Laboratory, Keio University Hospital, Shinjuku, Tokyo, Japan
Background: Clot waveform analysis (CWA) has been reported to extend the interpretation of clotting time measurement. We have reported the usability of activated partial prothrombin time (APTT) measurement‐based clot waveform analysis (APTT‐CWA) for assessment of effects of various anticoagulants. Although prothrombin time (PT) measurement is also available for CWA, the usability of PT‐CWA for assessment of anticoagulant effects remains to be evaluated.
Aims: To evaluate the usability of PT‐CWA for the assessment of pharmacological characteristics of various anticoagulants.
Methods: Samples were prepared by spiking plasma with each of three antithrombin‐dependent (unfractionated heparin, low‐molecular‐weight heparin, and fondaparinux) and seven antithrombin‐independent anticoagulants (rivaroxaban, apixaban, edoxaban, dabigatran, argatroban, hirudin, and bivalirudin). PT measurement and PT‐CWA were performed using RecombiPlasTin 2G (Werfen, Spain) and automated blood coagulation analyzers, CN6000 (Sysmex, Japan). To compensate influence of fibrinogen on CWA parameters (maximum coagulation velocity, acceleration, and deacceleration), adjustment was performed according to the changes in transmittance of the optical system presenting clotting reaction curves. Both adjusted and non‐adjusted CWA parameters were used in the present study.
Results: PT and the adjusted or non‐adjusted PT‐CWA parameters were hardly changed by antithrombin‐dependent anticoagulants. While antithrombin‐independent anticoagulants also exhibited dose‐dependent PT prolongation, dose‐dependent decreases were observed in adjusted but not non‐adjusted PT‐CWA parameters. Rivaroxaban and bivalirudin exhibited rebounding elevation of adjusted PT‐CWA parameters at the highest concentration tested. These features were different from those observed in APTT‐CWA for the same drug, which have been reported previously. In addition, as suggested by the similarity in observations between rivaroxaban and bivalirudin, pharmacological categorization did not necessarily correspond to dose‐dependent changes in PT‐CWA parameters.
Conclusion(s): CWA should be interpreted in consideration of the differences in dose‐dependent anticoagulant effects between the intrinsic and extrinsic pathways. How best to use adjusted and non‐adjusted CWA parameters differently would be a concern to be addressed.
PB1090
A re‐visit of the Pre‐Kallikrein (Fletcher trait) assay to optimise for laboratory use
A. McCormick
Viapath Analytics, London, England, United Kingdom
Background: The glycoprotein Pre‐Kallikrein (PK) plays a role in the in vitro intrinsic coagulation pathway. The role of PK in vivo remains unclear and cases of deficiency are rare and largely asymptomatic. Diagnosis of PK deficiency is required to avoid the administration of unnecessary prohaemostatic therapy or delay to surgical procedures. Additionally there are occasional case reports of bleeding and cardiovascular co‐morbidities associated with PK deficiency which further necessitate the availability of a reliable and reproducible assay.
Aims: In response to frequent assay underperformance it was decided to investigate an amended Pre‐kallekrein protocol to improve assay functionality on the Sysmex CS and CN series analysers.
Methods: PK assay employs a one‐stage activated partial thromboplastin time (APTT) using PK (Fletcher trait) deficient plasma. Reference plasma or sample is diluted 1:10, mixed with PK deficient plasma and incubated with APTT reagent. Calcium Chloride is added to trigger the coagulation cascade and time to clot recorded by light transmission. The assay was reprogrammed to perform a broader range of calibrator values using initial dilutions of 1:10, 1:20 and 1:40. QC material and reference plasma was assayed at a range of dilutions to establish which protocol showed the best linearity and parallel recovery of PK concentrations.
Results: The extended protocol using 1:20 pre‐dilution gave the broadest range of calibrator curve values maintaining linearity (R 2 > 0.99). Figure 1 shows comparison of the existing and proposed calibration curves. Measurement of a range of quality control and reference plasma material showed much improved recoveries using the dilute protocol. PK recoveries from serially diluted reference plasma displayed non‐parallelism in the original assay. This was improved using the proposed protocol using 1:20 pre‐dilution (Table 1).
Conclusion(s): The revised protocol has been demonstrated to display improved PK assay parameters will be employed as the assay migrates to the next generation Sysmex CN analyser platform.


PB1060
Multicentre performance evaluation and reference range for the quantitative determination of factor IX activity on the cobas t 711 analyser
M. Büchsel 1; J. Creaghan2; U. Geisen3; P. Jilma4; R. Jones2; S. Kitchen5; G. Rozsnyai6; P. Quehenberger7
1 University Medical Center Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 2 Sheffield Haemostasis and Thrombosis Centre, Sheffield, England, United Kingdom; 3 Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 4 Department of Laboratory Medicine, Medical University of Vienna, Vienna, Wien, Austria; 5 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 6 Roche Diagnostics International Ltd, Rotkreuz, Zug, Switzerland; 7 Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: Factor IX (FIX), a vitamin K‐dependent plasma protease, plays an essential role in coagulation. A multicentre evaluation of an activated partial thromboplastin time (aPTT)‐type assay for quantitative determination of FIX activity was performed.
Aims: Evaluate the analytical performance of the one‐stage FIX assay on the high‐throughput cobas t 711 analyser (Roche Diagnostics International Ltd, Rotkreuz, Switzerland).
Methods: Analytical performance was evaluated at three sites (Freiburg, Sheffield, Vienna; October 2020–February 2021) using anonymised, ethically approved, 3.2% citrated, residual routine plasma samples. Five human plasma pools and two controls were used to determine assay repeatability (one run/site; n = 21 replicates/sample), intermediate precision and total reproducibility (five aliquots/sample/day for 5 days); coefficients of variation (CVs; samples >1.0 IU/dl FIX activity) and standard deviations (SDs; samples ≤1.0 IU/dl FIX activity [repeatability/intermediate precision only]) were calculated. Using three sets of ≥120 samples, method comparison (vs. Siemens Coagulation Factor IX Deficient Plasma [with Dade Actin FSL] on Sysmex CS‐5100 analyser) and lot‐to‐lot variability (three reagent lots) were evaluated using Passing‐Bablok and Deming regression, respectively; Pearson’s r was calculated. The reference range was determined using three reagent lots (Freiburg only) with fresh plasma samples from 200 healthy individuals aged ≥18 years (not receiving anticoagulants).
Results: Across the three sites, the FIX assay demonstrated repeatability (CV, 1.0–6.3%; SD, 0.0275–0.0303), intermediate precision (CV, 1.7–8.4%; SD, 0.0232–0.191), and total reproducibility (CV, 3.9–20.8%) within the prespecified acceptance criteria. The FIX assay also showed method comparison results (Pearson’s r, 0.993–0.996; Table 1) and lot‐to‐lot variability (Pearson’s r, 0.997–0.999; Table 2) within the prespecified acceptance criteria. The reference range for FIX activity in healthy adults was 69.6–146 IU/dl.
Conclusion(s): The robust analytical performance of the FIX assay on the cobas t 711 analyser supports routine clinical laboratory practice use. Study Funding: Roche Diagnostics International Ltd, Rotkreuz, Switzerland.


PB1086
A comparative in vitro study of the coagulation effects of branded versus generic preparations of rivaroxaban
K. Mangion 1; K. Vella2; A. Gatt3; D. Borg Aquilina4; N. Riva5
1 University of Malta, Kirkop, Malta; 2 Mater Dei Hospital, Department of Pathology, Msida, Malta, Msida, Not Applicable, Malta; 3 Department of Pathology, Faculty of Medicine and Surgery, University of Malta, Msida, Not Applicable, Malta; 4 National Blood Transfusion Service & Mater Dei Hospital Blood Bank, Msida, Not Applicable, Malta; 5 Faculty of Medicine and Surgery, University of Malta, Msida, Malta
Background: During the last few years, several generic versions of rivaroxaban were approved to be used interchangeably with their branded equivalent Xarelto®. However, there were no previous studies in the literature that directly compared the anticoagulant effects of branded and generic preparations of rivaroxaban on different coagulation assays.
Aims: The main aim of this in vitro study was to compare the anticoagulant effects of Xarelto® with two generic versions (Rivaroxaban Sandoz® and Rivarolto®) on a panel of coagulation assays.
Methods: The study was performed by first pooling normal plasma and spiking with different concentrations of the branded type and the two generic versions of rivaroxaban (concentration range 50–1000 ng/ml). Several coagulation assays were performed: Prothrombin time (PT), activated Partial Thromboplastin time (aPTT), Thrombin time (TT), Clauss Fibrinogen, Diluted Russell’s Viper Venom Test (dRVVT), Factor VII one‐stage assay, Factor VIII assays (one‐stage & chromogenic), Factor IX assays (one‐stage & chromogenic). The Pearson’s correlation test was utilized to check for correlation between the concentrations of the three different preparations of rivaroxaban and the selected assays. The One‐Way ANOVA test was utilized to identify any variabilities in their action on the selected assays.
Results: There was a positive correlation between the concentrations of the three preparations of rivaroxaban and PT, aPTT and dRVVT (Pearson's r > 0.93, p < 0.05 for all). There was a negative correlation between the concentrations of the three preparations and the one‐stage and chromogenic factor assays (Pearson's r < −0.83, p < 0.05 for all). TT did not correlate with any of the preparations (Table). No significant difference was identified in the anticoagulant effect of the three rivaroxaban formulations (p > 0.05 for all).
Conclusion(s): This study showed that the generic versions of rivaroxaban do not exert any different in vitro anticoagulant effect from Xarelto® on several coagulation assays.

VPB1120
Comparison between the DOAC Dipstick test in urine and chromogenic substrate methods in plasma on patients treated with direct oral anticoagulants
L. Papageorgiou1; J. Harenberg 2; S. Auge3; L. Tredler4; I. Elalamy5; G. Gerotziafas6
1 Hematology and Thrombosis Center, Tenon University Hospital, Sorbonne University Recent affiliation : Interdisciplinary Cancer Course Department (DIOPP),Gustave Roussy, PARIS, Ile‐de‐France, France; 2 Faculty of Medicine Mannheim and Clinic Mannheim, Heidelberg, Baden‐Wurttemberg, Germany; 3 Hematology and Thrombosis Center, Tenon University Hospital, Paris, Ile‐de‐France, France; 4 Hematology and Thrombosis Center, Tenon University Hospital, PARIS, Ile‐de‐France, France; 5 Head of the department of Hematology and Thrombosis Center, Tenon University Hospital, Sorbonne University, PARIS, Ile‐de‐France, France; 6 Head of the research group ''Cancer Hemostasis and Angiogenesis'', INSERM UMRS‐938 (Centre de Recherche Saint Antoine), Paris, Ile‐de‐France, France
Background: The efficacy and safety of direct oral anticoagulants (DOACs) in patients with venous thromboembolism (VTE) is closely related to DOAC presence in plasma. The medical device DOAC Dipstick was developed based on the rationale that DOACs are excreted, hence easily detected, in urine.
Aims: The aim was to compare this tool to a standard chromogenic‐substrate plasma assay.
Methods: A prospective observational cohort study was performed. Patients on DOACs were routinely assessed using STA®‐Liquid Anti‐Xa and STA®‐Liquid Anti‐IIa reagents (Diagnostica Stago, Asnieres, France). DOAC Dipstick test was performed on patients’ urine samples and visual evaluation of pads’ colors for oral direct factor ‐Xa (DXI) and thrombin inhibitors (DTI) by two trained staff members according to the instructions for use. Threshold plasma values for plasma concentrations inhibitors were set at >30 ng/ml and >20 ng/ml.
Results: The study assessed 124 patients (rivaroxaban n: 77, apixaban: 44, dabigatran n = 2) recruited from the anticoagulation clinic of a tertiary hospital center. Due to a low number of patients in the DTI group, statistical analysis was performed on the DXI group. At >30 ng/ml threshold sensitivity and accuracy were at 98%, a positive predictive value (PPV) at 89%, specificity at 13% and a negative predictive value (NPV) at 50%. At a >20 ng/ml plasma threshold sensitivity and accuracy were at 97% and PPV was 100%. In absence of false positive and false negative values, specificity and NPV could not be determined. There were no differences between observers (kappa index 1.0).
Conclusion(s): Sensitivity and accuracy at >20 ng/ml may be higher compared to >30 ng/ml for rivaroxaban and apixaban. Higher numbers of negative DOAC Dipstick results are needed to specify plasma DOAC threshold values for DXI and DTI.
PB1056
Evaluation of neutrophil lymphocytes ratio and platelets lymphocytes ratio in immune thrombocytopenic purpura
S. Mukry1; A. Arshad 1; T. Shamsi2
1 National Institute of Blood Diseases and BMT, KARACHI, Sindh, Pakistan; 2 National institute of blood diseases & Bone Marrow Transplantation, Karachi, Pakistan, Karachi, Sindh, Pakistan
Background: Immune thrombocytopenia (ITP) is an autoimmune disorder. The clinical biomarkers like neutrophils to lymphocytes ratio (NLR) and platelet to lymphocytes ratio (PLR) can be used as differential diagnostic tool in various diseases.
Aims: The current study was planned to evaluate utility of NLR and PLR in ITP diagnosis and their association with disease prognosis and response to treatment.
Methods: A case control study (1:1) was conducted from January 2015 to December 2017 with 111 ITP patients and 111 healthy controls. Peripheral blood was collected and CBC was recorded using Sysmex XN‐1000.The calculation of NLR and PLR was done using absolute value of neutrophils, lymphocytes and platelets counts. The significant difference (p =< 0.05) between ITP patients and healthy control groups was determined by Kruskal wallis test. Dunn’s test and spearman’s correlation test was performed to evaluate platelet count correlation with IPF using SPSS ver.23.
Results: Low hemoglobin and platelet counts with high total leucocyte count (TLC) and IPF were recorded in ITP patients as compared to healthy individuals (p =< 0.05).Among all groups of ITP patients, lowest platelet count was observed in ND‐ITP group (median(IQR): 2(3.8) × 109/L). The NLR correlated well with the persistence of disease and was found higher in P‐ITP. The PLR was significantly low in ND‐ITP, P‐ITP, C‐ITP, R‐ITP and compared to controls with p =< 0.001.
Conclusion(s): Hence, it can be concluded that NLR and PLR ratios can be used in predicting prognosis and response to treatment in ITP and to some extend the severity of disease.
PB1088
Results of the thrombin time test obtained with two different commercial reagents in real life patients treated with dabigatran
A. Bronic1; S. Margetic 2; E. Esmat3; B. Dejan4; C. Tomislav4; I. Bencic4; D. Vidovic4
1 Traumatology Clinic, Sestre milosrdnice University Hospital Center, Zagreb, Grad Zagreb, Croatia; 2 Department of Clinical Chemistry, University Clinical Hospital Center "Sestre Milosrdnice", Zagreb, Grad Zagreb, Croatia; 3 Traumatology Clinic, University Clinical Hospital Center "Sestre Milosrdnice", Grad Zagreb, Grad Zagreb, Croatia; 4 Traumatology Clinic, University Clinical Hospital Center "Sestre Milosrdnice", Zagreb, Grad Zagreb, Croatia
Background: Limited availability of dabigatran‐specific coagulation assay could complicate treatment decisions in emergencies like urgent surgical intervention, or anticoagulation reversal in patients requiring emergent surgery. Thrombin time (TT) test, as extremely sensitive to very low amount of dabigatran, may be elevated even in the presence of clinically insignificant levels of the drug (<30ng/ml), although response varies considerably by drug concentration and reagent.
Aims: The aim was to determine TT reagent specific cut off values that might help to rule out relevant drug induced anticoagulation.
Methods: Ninety citrated plasma samples from 17 patients taking dabigatran due to knee replacement were collected at the Traumatology Clinic, Sestre Milosrdnice University Hospital as a part of Croatian Science Foundation research project IP‐2016‐06‐8208. Samples were tested using two different TT reagents, Thromboclotin on Sysmex CS25000 (R1) and Thrombin time on Behring Coagulation System XP (R2), whereas dabigatran concentrations were measured using Innovance DTI assay (all Siemens Healthineers, Germany). Receiver operating characteristic analyses were used to determine sensitivity, specificity and reagent‐specific cut off values. Statistical analysis was performed using MedCalc Software, version11.5.1 (Ostend, Belgium).
Results: Range of obtained TT values for R1 and R2 was 15 to >120s, and 12.3 to >150s, respectively. Measured dabigatran concentrations and differences between values are presented in Table 1. R1 and R2 had only moderate linear relationship to dabigatran concentrations: ρ = 0.509 (95%CI: 0.337–0.649), p < 0.001 and ρ = 0.450 (95%CI: 0.263–0.604), p < 0.001, respectively. Results of sensitivity and specificity for the R1 and R2 at pre‐defined and reagent specific TT cut‐off values are presented in Table 2.
Conclusion(s): Balanced reagent‐specific threshold Thrombin Time assay studied in dabigatran treated patients ensure better sensitivity and negative predictive value for excluding clinically relevant drug concentrations in circulation and might help in emergency situations to support clinical decisions.


PB1096
Clot Time Ratio (CTR) in atrial fibrillation patients on rivaroxaban
L. Onelöv 1; E. Theodorsson2; M. Božič Mijovski3; A. Mavri4
1 Nordic Biomarker, R&D, Linköping, Ostergotlands Lan, Sweden; 2 Nordic Biomarker, Linköping, Ostergotlands Lan, Sweden; 3 University Medical Centre Ljubljana, Ljubljana, Ljubljana, Slovenia; 4 Department of Vascular Diseases, University Medical Centre Ljubljana, Ljubljana, Ljubljana, Slovenia
Background: There are clinical situations where information about the anticoagulant effects of rivaroxaban could be useful. Methods for measuring rivaroxaban concentrations are not available at all medical laboratories while the test MRX PT DOAC for measuring the functional effects of rivaroxaban, in CTR (Clot Time Ratio), can be available around the clock.
Aims: To investigate CTR in through and peak samples during rivaroxaban treatment and to correlate the findings to bleeding episodes.
Methods: 3 through‐ and 3 peak samples previously collected and investigated ([1]) from 60 patients (30 on 20 mg daily and 30 on 15 mg daily) were analysed with MRX PT DOAC. Patients had been followed for 20 months and bleeding and thrombotic events were documented. Non‐parametric t‐test was used for statistical analyses, when not otherwise specified (Sigma Plot 14.0). 1. Miklic, M., et al. Eur J Clin Pharmacol, 2019. 75(8): p. 1069‐1075.
Results: 178/180 through and 177/180 peak samples were available. Through CTR varied between 0.93–3.36 with median at 1.33 while peak CTR varied between 1.8–7.03 with median at 3.56 (p < 0.001). 28 patients (13 on 20 mg and 15 on 15 mg) had suffered bleeding. Peak CTR ranged between 1.89‐6.20 for patients on 20 mg and patients with bleeding(s) had higher mean‐CTR than patients without bleeding (p = 0.038, Student’s t‐test). Patients on the adjusted, lower dose of 15 mg had similar peak CTR ranging between 1.80–7.03 but there was no significant difference in mean‐CTR between patients with or without bleeding(s) (p = 0.678).
Conclusion(s): Clot time ratio (CTR) at peak was higher at group level (p < 0.05) in patients on 20 mg rivaroxaban who suffered from bleeding compared to patients who didn't. However, the difference found at the level of individual patients in the present study was not sufficient to clinically distinguish the patients at risk of bleeding and might warrant further, larger studies.


PB1101
Measurement of fibrinogen using two concentrations of Thrombin with patients receiving Argatroban or normal plasma spiked with Argatroban: A review of UK NEQAS (Blood Coagulation) exercises 2021
C. Reilly‐Stitt 1; S. Guy2; I. Jennings3; S. Kitchen4; I. Walker1
1 UK NEQAS Blood Coagulation, SHEFFIELD, England, United Kingdom; 2 Royal Hallamshire Hospital, Sheffield, England, United Kingdom; 3 UK NEQAS BC, Sheffield, England, United Kingdom, 4 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom
Background: Measurement of fibrinogen with the Clauss assay is a thrombin time‐based method that can be affected by Direct Thrombin Inhibitors. Argatroban is a direct thrombin inhibitor used in patients being treated for HIT and VITT.
Aims: To highlight the relative sensitivities of Clauss thrombin assays to argatroban.
Methods: A supplementary exercise for argatroban and fondaparinux included samples containing argatroban from a pool of treated patients and from a spiked normal plasma. Participants were requested to return Fibrinogen assay results. In addition, a regular Laboratory Programme exercise (survey 249) included a sample for fibrinogen assay from a patient undergoing plasma exchange and receiving argatroban for VITT.
Results: Samples distributed as part of an argatroban exercise (A21:01‐05) and as part of the laboratory programme (249 21:31) in 2021 are detailed in Table 1.
Conclusion(s): Commercial reagents for Fibrinogen quantitation have a range of thrombin concentrations and it is clear that the level of argatroban in patient or spiked samples can interfere with the measurement of fibrinogen. The data from the samples distributed highlight the fibrinogen underestimation with some assays in samples with levels of argatroban at therapeutic levels. Manufacturer’s inserts do not describe any assay limitation with regard to argatroban or other direct thrombin inhibitor.

PB1104
The effect of sample transportation on microparticle production measured in vitro using a procoagulant phospholipid activity assay
M. Sharp 1; J. Griffin2; S. MacDonald1
1 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 2 Norfolk and Norwich University Hospital, Norwick, England, United Kingdom
Background: Platelet microparticles are small membrane bound vesicles 0.1 μm–1 μm in size that are shed from the platelet membrane during platelet activation, stress or apoptosis. They contribute phosphatidylserine externalisation to provide a procoagulant surface. Platelet microparticles also express functional platelet receptors (including CD41) and other bioactive effector molecules. Microparticles produced via transportation of samples as whole blood have been shown to produce reduced DRVVT screen ratio results (Cox‐Morton, 2015). We investigated whether platelet microparticles rich in procoagulant phospholipids are released during sample transport manifesting as a reduction in the clot time using the Stago PPL kit.
Aims: Does sample transportation impact the generation of microparticles that may contribute to previously identified false negative results for phospholipd assays such as Lupus Anticoagulant.
Methods: A reference range was established on samples collected from 20 normal donors. Residual patient samples were obtained following completion of routine testing. Classification of transported or non‐transported was based upon available sample delivery information. PPL activity was measured using the Stago ProCoag‐PPL (Stago, Asniers, France) kit on the ACLTOP 750 analyser (Werfen, Bedford, USA). Statistical analysis was performed using Microsoft Excel (Microsoft Corporation, USA). Differences in means were calculated with the Welch's t‐test for unequal variances. As multiple significance tests were applied the Bonferroni correction was applied to set a significance level of 0.025.
Results: A statistically significant increase in PPL activity (reduced clot time) was associated with transported samples (mean 30.0 +/− 11.8 s) compared to patient samples taken in the adjacent clinical area (mean 50 +/− 5.88 s).
Conclusion(s): With the introduction of Hub and Satellite laboratories transport of samples is a significant pre‐analytical variable. Transportation of samples results in increased PPL activity from additional microparticle phospholipids being produced presumably due to platelet activation. This could be used in conjunction with dRVVT screen results to determine when transportation has affected lupus anticoagulant results.

VPB1119
Heparin monitoring during ECMO: Anti‐Xa assay with and without dextran
E. Hammami; L. Stiel; A. Debliquis; B. Drénou; i. Harzallah
Groupe Hospitalier Mulhouse et Sud Alsace, Mulhouse, Alsace, France
Background: Extracorporeal membrane oxygenation (ECMO) induces important shear stress that activates coagulation, rendering thromboprophylaxis mandatory. Unfractionated heparin (UFH) is commonly used for thromboprophylaxis and monitored with anti‐Xa assay. Some of the anti‐Xa’s reagents don't contain dextran. Studies comparing anti‐Xa assay with dextran (D‐anti Xa) and anti‐Xa assay without dextran during ECMO (ND‐anti Xa) have not been published.
Aims: The study’s aim was to compare D‐anti Xa and ND‐anti Xa during ECMO.
Methods: Patients requiring ECMO, between May and November 2021, in the intensive care unit, were included. Anticoagulation monitoring was performed using D‐anti Xa and ND‐anti Xa. Patients who did not benefit simultaneously from D‐anti Xa and ND‐anti Xa were excluded. Kappa statistics were performed to determine consistency among D‐anti‐Xa and ND‐anti Xa assays. The ROC curve led to a calculation of the area under the curve (AUC) for both variables.
Results: Fourteen patients were enrolled and 356 anti‐Xa assays were performed. Eight patients experienced bleeding and two experienced thrombotic events during ECMO (Table 1). There was a poor agreement between the D‐anti Xa and ND‐Anti Xa assays, κ = 0.007 (95% CI). The ROC curve of ND‐Anti Xa is represented by Figure 1. The AUC of D‐Anti Xa was 0.142 and 0.356 for ND‐Anti Xa.
Conclusion(s): The presence of dextran in anti‐Xa reagent seems to render the assay less sensitive. D‐Anti Xa and ND‐Anti Xa assays have poor consistency. This may be due to the release of platelet factor 4 during ECMO that binds to dextran, resulting in a false increase of heparin concentration. However, this comparison between two assays doesn’t tell us if ND‐anti‐XA is predictive of bleedings or thrombotic events. Further studies comparing anticoagulation monitoring assays and bleeding tendency on ECMO are needed.


PB1074
Influence of hemolysis, icterus and lipemia on Owren PT measured with a viscosity based detection coagulometer
M. Held 1; A. Hillarp2; A. Ståhlberg1
1 Halland County Hospital, Halmstad, Hallands Lan, Sweden; 2 Oslo University, Oslo, Oslo, Norway
Background: The presence of hemolysis (H), icterus (I) or lipemia (L) is reported to be a major cause of pre‐analytical errors [Salvagno GL et al., J Eval Clin Pract 2008]. It has been shown that H, I, L do not affect routine coagulation assays in a viscosity based detection coagulometer [Wolley A et al., Int J Lab Hem 2016]. However, only Quick PT assay was studied in this routine panel.
Aims: Evaluate the impact of H, I and L on Owren PT measured through mechanical clot detection.
Methods: Four different pools were prepared from non‐hemolyzed citrated blood samples. Each pool was divided into 4 parts, to induce different levels of hemolysis: no hemolysis (H0) and spurious hemolysis by 1, 3 or 5 aspirations through a thin needle (H1, H3, H5 respectively). Lipemic samples were created by adding Lipofundin S 20% (B.Braun SpA, Italy) in 4 different plasma pools at 0, 200, 500 and 1000 mg/dl respectively. Similarly, icteric samples were made by adding bilirubin (Sigma‐Aldrich, MO, USA) to 4 different plasma pools at concentrations of 0, 5, 20 and 40 mg/dl. All pools were different for H, I and L experiments. Hemoglobin, bilirubin and triglycerides levels of all samples were checked and samples were then analyzed using STA‐SPA+ on STA R Max analyzer (Diagnostica Stago, France).
Results: Results obtained are detailed in Table 1. Maximal observed deviation induced by H, I or L was a change of 0.07 on INR (from 1.44 to 1.51) for hemolysis, 0.08 on INR (from 1.70 to 1.78) for lipemia and 0.14 on INR (from 1.47 to 1.33). None of these differences proved to be clinically significant.
Conclusion(s): Consistent with the published observation on Quick PT, hemolysis, icterus and lipemia do not interfere with Owren PT results as measured with STA‐SPA+ reagent on STA R Max analyzer.

PB1095
The effect of aqueous Chromolaena odorata (Acheampong leaf) extract use on coagulation test parameters in vitro
D. Ofosu 1; A. Asamoah2; R. Tuekpe Mawuli2; A. Addai3; E. Takyi Kofi3; E. Opoku Addo3
1 Dept of Medical Laboratory Science (Basic and Applied Biology), University of Energy and Natural Resource, Sunyani, Brong‐Ahafo, Ghana; 2 Basic and Applied Biology Dept, University of Energy and Natural Resources, Sunyani, Brong‐Ahafo, Ghana; 3 Dept of Basic and Applied Biology, University of Energy and Natural Resource, Sunyani, Brong‐Ahafo, Ghana
Background: Medicinal plants, as sources of new medicines and herbal and nutritional supplements, continue to play an important role in healthcare. In Ghana, the use of Chromolaena odorata (Acheampong leaves) as a first‐aid material to control bleeding is a very common practice in rural areas. The efficacy of these abundant tropical leaves has not been thoroughly assessed though it is used for diverse interventions.
Aims: This study, therefore, sought to assess the coagulation effect of aqueous extract of C. odorata (Acheampong) leaves.
Methods: In this experimental study, freshly obtained C. odorata leaves were inspected and verified by an expert ecologist. The juice from the leaves was extracted using distilled water. Blood samples were taken from 197 non‐haemophiliacs who consented to participate in the study. For test samples, the extract was added to plasma whilst for paired control samples normal saline was added to the plasma. The samples were analyzed for the coagulation markers Prothrombin Time (PT) and activated partial thromboplastin time (aPTT)
Results: The study results showed that the use of aqueous C. odorata extracts significantly reduced the PT of the study participants (17.89 ± 3.06 s to 16.43 ± 2.51 s, p < 0.0001) but not the aPTT (38.83 ± 7.13 s against 39.12 ± 7.85, p = 0.709).
Conclusion(s):
C. odorata proved to be effective in promoting coagulation in vitro via Extrinsic Pathways. It is recommended that further studies could be done to identify, isolate and purify the active chemical compound in C. odorata leaves responsible for the prolonged effect on the Prothrombin Time.


PB1099
The validation of linear One‐Stage Clotting Assays (OSCA) using fully automated Multi‐Dilution Analysis (MDA) in the quantification of factor activity
K. Ram 1; S. Platton2; R. Narisada3
1 Sysmex UK, Harrow, England, United Kingdom; 2 Barts Health NHS Trust, London, England, United Kingdom; 3 Sysmex Corporation, Kobe, Hyogo, Japan
Background: When determining factor coagulant activities using One‐Stage Clotting Assays (OSCA), at least three dilutions of test plasma should be prepared to determine linearity and assess parallelism to the reference. The linear region of the reference curves and testing of plasma using Multi‐Dilution Analysis (MDA) results are essential in the assessment of parallelism using the slope ratio (SR) and linearity using the R‐value of the reference curve.
Aims: The validation of linear OSCA using fully automated MDA in the quantification of factor activity.
Methods: Linear MDA OSCA on a CN‐6000 were evaluated against point‐to‐point reference plot, single dilution analysis on a CS‐5100. The eight extrinsic and intrinsic pathway OSCAs were tested using Siemens Dade® Actin® FS and Innovin® reagents, respectively. Comparability of at least 156 samples for each assay was performed for all factors (1460 samples in total). On the CN‐6000, Lower limits of quantification (LLoQ) and linearity evaluations were performed using MDA and sample carryover was assessed by testing samples with high and low normal levels of the analytes concerned.
Results: Excellent correlation and LLoQ for all MDA OSCA were achieved; r 2 > 0.995 and < 0.9IU/dL, respectively. Acceptable between‐run imprecision was obtained using commercial controls with normal and abnormal activity for each analyte (%CV < 3%) for all eight assays using MDA, and no carryover was observed.
Conclusion(s): Linear OSCA using MDA allows for the detection of sample activation and presence of inhibition (Figure 1), presence of which can be easily missed using point‐to‐point interpolation and single dilutions, leading to misinterpretation of results. Inhibitory activity is commonly observed with interfering anticoagulants, antibodies to coagulation factors and lupus anticoagulants. Using point‐to‐point interpolation, loss of linearity below 10 IU/dl compromises differentiation between severe, moderate, and mild forms of factor deficiencies (Figure 2). This deterioration of precision justifies the need for linear OSCA using MDA as clinically imperative.


PB1058
Coag‐Lysis assay, an automated and standardized combined turbidity plasma clot formation and lysis assay
S. Fontaine1; O. Mathieu1; C. Vauthier1; M. Bourdin 2; F. Depasse3; G. Contant1
1 Diagnostica Stago, Gennevilliers, Ile‐de‐France, France; 2 Diagnostica Stago, Gennivilliers, Ile‐de‐France, France; 3 Diagnostica Stago, Asnières Sur Seine, Ile‐de‐France, France
Background: Clot properties are key in the development of thrombotic and bleeding complications. There is then an urgent need for a standardized clot turbidity and lysis assay to aid define healthy, hypo/hyper‐coagulation and fibrinolysis status, i.e. the coagulation–fibrinolysis balance, facilitate interlaboratory data comparisons and improve patient’s care [Pieters JTH2018; Longstaff JTH2017].
Aims: Develop a fully automated Coag‐Lysis Assay (CLA) for the measurement of the Coagulation‐Fibrinolysis balance.
Methods: CLA was automated on a STA‐R Max prototype using various concentrations of tissue factor (TF) (MP and PPP Reagents, Diagnostica Stago) with or without tissue Plasminogen Activator (h‐single‐chain tPA 0.10–0.30 μg/ml) and calcium in undiluted plasma. FXIa (0–200 pM) was added in the reagent for the Hemophilia A patients (HA). Heparin was inhibited by heparinase added in the reagent. Optical density deviation was recorded at 540 nm during 10–30 min at 37°C. Curve parameters were determined using a post‐processing software. Experiments were performed in 58 healthy volunteers samples (HV); normal pool plasma spiked with DOACs or tinzaparin; plasma samples from VTE patients (VTE) and HA spiked with FVIII replacement therapy; and quality controls (QC) of hypo/hyper‐coag/fibrinolysis.
Results: 0.13 μg/ml tPA was determined to obtain lysis parameters within 30 min. Addition of FXIa increased the assay sensitivity to low FVIII level. Within‐run (n = 21 measurements) and day‐to‐day (150 days) CVs determined using QC results were less than 6%–10% for coagulation and fibrinolysis. A 2SD normal range was established on HV plasmas. Coagulation parameters were shorten with increasing TF concentrations, in hyper‐coag QC or VTE plasma. CLA showed to be sensitive to DOAC concentration. Dabigatran enhanced clot susceptibility to fibrinolysis compared to other DOACs. Heparin neutralization using heparinase allowed CLA measurement in tinzaparin treated patients.
Conclusion(s): The fully automated CLA can be performed easily. It is available to assess bleeding or thrombotic tendency, based on plasma clot properties.
PB1073
Anti‐Xa and APTT stability in whole blood—A comparison of UFH & LMWH
P. Gajendra 1; C. Wigley1; E. Foxton2
1 Viapath, London, England, United Kingdom; 2 Viapath Analytics, Royston, England, United Kingdom
Background: Patients treated with unfractionated heparin (UFH) and Low molecular weight heparin (LMWH) may require monitoring of anti‐Xa activity in order to ensure adequate anticoagulation has been achieved. The release of platelet factor 4 (PF‐4) from platelets over time has a neutralising effect on anti‐Xa activity, leading to falsely reduced results and potential over‐anticoagulation.
Aims: To assess the stability of anti‐Xa activity and APTTr of patients samples anticoagulated with UFH & Daltaparin (LMWH) to determine the maximum viability of such samples, for local validation.
Methods: Blood was taken from normal donors into Sodium Citrate tubes, these tubes were spiked with UFH and Daltaparin to achieve 0.5 iu/L and 1.0 iu/L anti‐Xa activity. Whole blood was then aliquoted, centrifuged and analysed for APTTr & anti‐Xa activity at 1 h intervals over 10 h. Anti‐Xa activity testing was performed using the Biophen heparin LRT Kit (Hyphen Biomed) with drug specific calibrators and the APTTr was performed using Actin FS (Siemens) on the CN‐6000 analyser (Sysmex).
Results: APTTr results were stable up to 4 h for UFH samples, declining more significantly where the APTTr was more elevated (Figure 1). The APTTr of LWMH spiked samples were equivalent to baseline after 10 h. Anti –Xa activity results for UFH and LMWH at 0.5 IU/ml and 1.0 IU/ml concentration showed stability up to 8 h. A spurious low result was recorded at 5 h for UFH 1.0 iu/ml, Figure 2 shows this outlier.
Conclusion(s): Spiking studies suggest that a 4 h stability is recommended for determining APTTr on patients treated with UFH. This supports our local protocol. Our data suggests that the anti‐Xa activity for LMWH and UFH is stable for 8 h in whole blood samples held at room temperature, therefore the local time window for acceptance of anti‐Xa has been extended to 8 h.


PB1083
Accuracy of the hyphen Zymutest IgG ELISA assay for anti‐platelet factor 4 antibody detection after ChAdOx1 nCov‐19 vaccination in diagnosing vaccine‐induced immune thrombotic thrombocytopenia (VITT)
E. Leitinger 1; J. Clifford1; M. Parker1; A. Iacobelli1; P. Sung1; V. CHEN2; T. Brighton3; F. Passam4; L. Clarke5; H. Tran6; S. Chunilal1
1 Monash Health, Melbourne, Victoria, Australia; 2 ANZAC Research Institute, University of Sydney; Department of Haematology, Concord Repatriation General Hospital, Sydney, New South Wales, Australia; 3 Department Haematology, New South Wales Health Pathology, Prince of Wales Hospital, Sydney, New South Wales, Australia; 4 Royal Prince Alfred Hospital; Haematology Research Group, The Heart Research Institute, Faculty of Medicine & Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia; 5 Department of Haematology, Concord Repatriation General Hospital; Australian Red Cross Lifeblood, Sydney Adventist Hospital, Concord, New South Wales, Australia; 6 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia
Background: Vaccine‐induced Immune Thrombotic Thrombocytopenia (VITT) is a rare, anti‐platelet factor 4 (PF4) antibody‐mediated prothrombotic syndrome associated with the AstraZeneca (AZ) Covid‐19 vaccination. Diagnostic criteria include thrombosis, thrombocytopenia, appropriate timing post AZ vaccine, and demonstration of an anti‐PF4 antibody by enzyme‐linked immunosorbent assay (ELISA). ELISA methods have varying sensitivity and specificity for detecting anti‐PF4 antibodies, therefore, functional assays (modified serotonin release and flow cytometry) assist in diagnosis. In Australia, the primary assay for detection of anti‐PF4 antibodies is the Asserachrom HPIA IgG ELISA.
Aims: To assess the accuracy of an alternate ELISA method for suspected VITT compared to the gold standard (clinicopathological diagnosis).
Methods: 96 stored frozen samples from patients with suspected VITT, previously analysed by Asserachrom HPIA IgG ELISA (Stago, Melbourne, Australia), were tested using the Hyphen Biomed Zymutest HIA IgG ELISA (Haematex Research, Sydney, Australia). These patients had confirmed thrombosis within 42 days post first dose of AZ vaccine. Cases were classified as ‘serologically confirmed VITT’ if clinicopathological criteria were met, and initial ELISA testing and/or functional assays were positive. Results were interpreted as positive or negative for anti‐PF4 antibodies using the manufacturer’s cut‐offs derived for the diagnosis of Heparin‐Induced Thrombocytopenia.
Results: 96 patient samples were tested, including 35 classified as VITT (Table 1). The Hyphen assay had a moderate sensitivity (82%), including 2 probable false negatives on initial testing which were strongly positive by Hyphen assay. The specificity was 93%, including 5 probable false positives on initial ELISA which were negative by Hyphen assay (Table 2).
Conclusion(s): The Hyphen ELISA is suitably accurate for the diagnosis of VITT. These data highlight the limitations of using a single ELISA testing method for anti‐PF4 antibody detection. In cases with high clinical suspicion, a second ELISA method or functional assay should be considered.


PB1091
A potential suitable alternative to Triton‐X‐100 for use in sample preparation for platelet nucleotide analysis
A. McCormick; A. Culhane; E. Bromidge
Viapath Analytics, London, England, United Kingdom
Background: The nucleotides ATP and ADP present in two distinct pools within platelets. The metabolic pool accounts for approximately 40% of the nucleotide population maintained in a ratio of 8:1 ATP: ADP. The remaining 60% are found in the platelet dense/delta granules in a ratio of 2:3 ATP:ADP and play a role in activation and propagation of platelet aggregation in response to haemostatic challenge. Reduction, absence and/or altered relative ratio of ATP:ADP within the platelet delta granules are characteristic of platelet storage pool defects and require a robust, sensitive quantitative assay for diagnosis.
Aims: Preparation of platelet lysates for nucleotide analysis has relied on using 20% Triton‐X‐100 solution for membrane dissolution however this is now commercially unavailable due to it’s ecotoxicity. We have investigated the use of alternative detergents 20% tween 20 and 1% tergitol solutions to assess their potential as a viable replacement.
Methods: Platelet nucleotide analysis relies on direct measurement of ATP in extracted platelet lysates using luciferase enzyme reagent. Platelet ADP content is estimated following it's conversion to ATP, using pyruvate kinase, and subtracting the initial ATP concentration from the total ATP measured. Paired extracted platelet lysates from normal donor platelet rich plasma using 20% Triton‐X‐100 and either 20% tween 20 or 1% tergitol were prepared in parallel and assayed for ATP and ADP content.
Results: Figure 1 shows preliminary results from paired samples lysed and extracted using 20% triton‐X‐100 and 20% tween‐20 and show a large discrepancy in measured ATP. Comparison of samples extracted using 20% Triton‐X‐100 and 1% tergitol showed improved consistency of ATP and ADP results (Figure2).
Conclusion(s): The use of 1% tergitol as an alternative detergent to 20% Triton‐X‐100 has shown some promise in preliminary studies. It is proposed to expand this data to assess it's suitability for use in the routine diagnosis of platelet storage pool disorders.


PB1097
Can low‐molecular weight heparin calibrated anti‐FXa assays be used to estimate Factor Xa inhibiting Direct Oral Anticoagulant levels?
W. Pickering 1; M. Robinson1; C. Cogswell1; B. Poirier1; C. Solomon2; R. Sarode3
1 Labcorp Esoterix Colorado Coagulation, Englewood, Colorado, United States; 2 Octapharma AG, Lachen, Zurich, Switzerland; 3 University of Texas Southwestern Medical Center, Dallas, TX, Dallas, Texas, United States
Background: In patients presenting with major bleeds, or requiring emergency surgery Factor Xa inhibiting Direct Oral Anticoagulants (FXaI) plasma levels would guide appropriate therapeutic strategies. The principle of heparin and FXaI assays, apart from the calibrators used are identical.
Aims: To evaluate low‐molecular weight heparin (LMWH) anti‐Xa assays to estimate FXaI levels.
Methods: Pooled Normal Plasma (PNP) was spiked with Apixaban, Edoxaban or Rivaroxaban over the range 0–400 ng/ml. Measurement of levels was performed using commonly used anti‐Xa assays; STA® Compact (STA® Liquid Anti‐Xa), ACL TOP 700 (HemosIL® Liquid Anti‐Xa) and BCS®XP (INNOVANCE® Heparin) using LMWH calibration. Levels were also measured with FXai‐DOAC specific calibration using STA® Liquid Anti‐Xa on the STA® Compact. Manufacturer provided assay applications, calibrators and Quality Controls were used. FXaI specific and LMWH calibrated activity was correlated for each kit.
Results: The FXaI calibrated assays were linear over the whole calibration range but the response in IU/mL varied between kits. LMWH‐anti‐FXa calibrated activity correlated (r 2) strongly with Apixaban, Edoxaban and Rivaroxaban levels respectively, and was 0.842, 0.995, 0.992 (STA® Liquid Anti‐Xa), 0.994, 0.988, 0.990 (HemosIL® Liquid Anti‐Xa) and 0.988, 0.993 and 0.982 (INNOVANCE® Heparin). The relationship between FXaI levels determined by LMWH calibration was not linear for extrapolated results above the kit highest calibration point. FXaI levels determined by LMWH Calibration (STA® Liquid Anti‐Xa) required sample pre‐dilution in PNP for Apixaban and Rivaroxaban >75 ng/ml and Edoxaban >100 ng/ml; HemosIL® Liquid Anti‐Xa required pre‐sample dilution for Apixaban and Rivaroxaban >250 ng/ml, and Edoxaban >300 ng/ml; INNOVANCE® Heparin, Edoxaban and Rivaroxaban >150 ng/ml, and Apixaban >120 ng/ml.
Conclusion(s): This study showed a strong correlation between FXaI levels using LMWH calibration. There was variability between the anti‐FXa kits. These findings suggest for FXaI reversal LMWH assays can be used to assess FXaI levels over the clinically significant range of 50–150 ng/ml.
VPB1116
A novel material for the improvement of HIT detection
L. Chen 1; H. Nguyen2
1 Jena University; 2) Institute for Bioprocessing and Analytical Measurement Techniques, Heilbad Heiligenstadt, Thuringen, Germany, 2iba, Rosenhof 1, 37308 Heilbad Heiligenstadt, Germany, Heilbad Heiligenstadt, Thuringen, Germany
Background: Heparin‐induced thrombocytopenia (HIT) is a drug reverse disorder (happening in up to 5% of patients) that could lead to lethal consequences. The patient is confirmed with HIT if HIT antibodies (Abs) are present. Recently, HIT Abs were detected also in clinically ill Covid‐19 and VITT patients. Early detection of HIT is crucial, but the widely used immunoassays provide only ~50% accuracy while functional assays required fresh platelets, which can only be performed in limited laboratories. Thus, the development of a simple method but accurate detection for confirmation of HIT Abs is highly desired.
Aims: We aim to discover cell membranes as novel materials to improve the detection of HIT Abs.
Methods: We used the laser scanning confocal microscope (LSCM) to visualize and confirm the binding of HIT Abs (including monoclonal Abs and patient’s sera) on breast cancer cell line MDA‐MB‐231. Flow cytometry was used to quantify the binding of antibodies via bound PF4 or PF4/Heparin complexes on the cells. Optical density caused by bindings of HIT Abs to cells was detected by integrating the cells into the ELISA setup.
Results: We found that the MDA‐MB‐231 cell line allows bindings of HIT Abs via platelet factor 4 (PF4) or PF4/Heparin complexes. Bound PF4 on cells allowed better binding of HIT Abs than the bound PF4/H complexes. Heparin inhibits HIT antibody bindings to cells. Importantly, non‐HIT Abs also bind to the cells through PF4 but are much weaker than the binding of the HIT Abs. The different binding patterns between HIT and non‐HIT Abs could be clearly distinguished.
Conclusion(s): The MDA‐MB‐231 cell line has a great potential to distinguish HIT from non‐HIT Abs through PF4 but not PF4/H complexes.
VPB1122
Establishment of reference intervals for prothrombin time and international normalized ratio in Pakistani children using an indirect data mining approach
M. Shaikh; S. Ahmed; Z. Ahmed
Aga Khan University, Karachi, Sindh, Pakistan
Background: Reference intervals (RIs) help physicians in differentiating healthy from sick individuals. The prothrombin time (PT) and International normalized ratio (INR) assess the integrity of the extrinsic and common coagulation pathways. These may be prolonged or shortened in various conditions and have significant interlaboratory variability due to the instrument/reagent used.
Aims: Establishment of RIs using a direct approach is difficult in children. Therefore, we chose an indirect data mining method for the determining PT/INR RIs.
Methods: Prothrombin Time/INR measurements performed for both inpatients and outpatients aged birth‐18 years between January 2013 and December 2020, were retrieved from laboratory management system of the Aga Khan Hospital. Tests were analyzed on Sysmex instruments. Reference intervals were computed using an algorithm developed and validated by the German Society of Clinical Chemistry and Laboratory Medicine’s Working Group on Guide Limits. Assuming that, non‐pathologic samples follow a Gaussian distribution (after Box‐Cox transformation of the data), an elaborate statistical process was used to isolate distribution of physiological samples from mixed dataset and used to calculate RIs.
Results: A total of 56,712 and 52,245 values were retrieved for PT and INR respectively. After the exclusion of patients with multiple specimens obtained during the study period, RIs were calculated for 37,556 (PT) and 37,192 (INR) children with stratification into 9 age groups. A comparison of 2.5th and 97.5th percentile results with those of established RIs from SickKids Handbook of Pediatric Thrombosis and Hemostasis demonstrated good agreement in between different age groups (Figures 1 and 2).
Conclusion(s): This study supports data mining as an alternate indirect approach for establishing PT/INR RIs, specifically in resource‐limited settings. The results obtained are specific to studied population and instrument/reagent used. The study also allows understanding of fluctuations in coagulation pathways with increasing age. These intervals therefore, will assist in better clinical decision‐making based on PT and INR results.

PB1066
Discrimination between acquired hemophilia A and lupus anticoagulant using global coagulation assays
Y. Chikasawa 1; K. Amano2; K. Shinozawa3; Y. Harada3; A. Mitsuhashi4; R. Miyashita3; T. Yamaguchi3; Y. Kamikubo3; A. Ichiki3; M. Bingo5; R. Sekiya3; T. Muramatsu3; M. Yotsumoto6; T. Hagiwara6; I. Hiroshi3; E. Kinai6
1 Department of Laboratory Medicine, Tokyo Medical University, Japan, Tokyo, Tokyo, Japan; 2 Tokyo Medical University Hospital, Tokyo, Tokyo, Japan; 3 Tokyo Medical University, Shinjukuku, Tokyo, Japan; 4 Department of Laboratory Medicine, Tokyo Medical University, Tokyo, Tokyo, Japan; 5 Tokyo Medical University, Tokyo, Tokyo, Japan; 6 Tokyo Medical University, Shinjukuku Nishishinjuku, Tokyo, Japan
Background: Acquired hemophilia A (AHA) and lupus anticoagulant (LA) have opposing phenotypes of bleeding or thrombotic tendency. However, both can cause acquired activated partial thromboplastin time (aPTT) prolongation and show a non‐coagulation factor deficiency pattern in a cross‐mixing test.
Aims: To compare the usefulness of rotational thromboelastometry (ROTEM) with that of thrombin generation assay (TGA) and clot waveform analysis (CWA) in distinguishing AHA from LA.
Methods: Patients diagnosed with AHA or LA in Tokyo Medical University Hospital from November 2019 to September 2021 underwent ROTEM (NATEM, INTEM, EXTEM), TGA triggered by tissue factor, and CWA using Thrombocheck APTT‐SLA (Sysmex Corporation, Kobe, Japan). LA was defined as either a non‐coagulation factor deficiency pattern in the cross‐mixing test with an index of circulation anticoagulant (ICA) of >12.4 using Thrombocheck APTT‐SLA or a positive phospholipid neutralization assay using the aPTT or dilute Russell viper venom time. The Mann–Whitney U test was used for statistical analysis.
Results: Ten of 10 patients with AHA and 16 of 32 patients with LA showed an ICA of >12.4. The median aPTT in AHA vs. LA was 86.8 (interquartile range: 71.6–98.4) vs. 42.9 (37.4–49.4) seconds (p < 0.001). With ROTEM, the median clotting time of NATEM mode in AHA vs. LA was 3,600 (2768.5–3600) vs. 553 (452–624) seconds (p < 0.001). With TGA, the median peak thrombin in AHA vs. LA was 16.1 (13.6–25.7) vs. 173.8 (130.8–252.7) nM (p < 0.001). With CWA, the median |min1| (maximum velocity) in AHA vs. LA was 2.10 (1.69–3.14) vs. 6.73 (5.81–8.37) (p < 0.001). As shown in the figure, any test may be useful; in this study, however, only the clotting time of ROTEM in NATEM mode and peak thrombin of TGA completely distinguished between the two groups.
Conclusion(s): ROTEM in NATEM mode can clearly distinguish between AHA and LA.
Image


PB1085
Laboratory versus point‐of‐care INR testing in patients with lupus anticoagulant—A retrospective cohort analysis
A. Lubetsky 1; O. Cohen2; M. Misgav2; S. Lalezari2; G. Kenet3
1 Sheba Medical Center, Tel‐Hashomer, Not Applicable, Israel; 2 Sheba Medical Center, Tel‐Hashomer, HaMerkaz, Israel; 3 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel
Background: Patient self‐testing (PST) and self‐management (PSM) using point‐of‐care (POC) devices, was found to improve the quality of anticoagulation, reduce thromboembolic events and all‐cause mortality, and is recommended for VKA monitoring by various guidelines. Accumulated evidence supports the accuracy of LAC insensitive thromboplastins for INR testing in patients with lupus anticoagulant (LAC), however this may not be generalized for patients monitored by POC devices
Aims: To investigate the accuracy of POC devices for measuring INR in LAC positive and negative patient cohort followed at our outpatient clinic
Methods: We retrospectively analyzed INR results of consecutive VKA treated patients (with and without evidence of LAC in repeated testing) followed at our clinic and having had their INR checked simultaneously by both POC device (CoaguChek® XS) and our laboratory. We used linear models and analysis of covariance to evaluate the correlation between INR results from the POC device versus laboratory INR in adjusting for the presence of LAC
Results: We identified 510 paired samples from 130 patients of which 56 (43%) were LAC positive treated with VKAs mainly for venous and arterial thrombosis with a median age of 44 years. The ANCOVA full model analysis showed a very good correlation between INR results obtained by the POC device and laboratory INR results in both LAC negative and LAC positive patients (figure). The presence of LAC had no effect in the model on the correlation between POC and lab INRs (p = 0.378) while the POC INR results had a very significant effect (p < 0.001) and explained 79% of the variance in lab INR results
Conclusion(s): The correlation between INR results obtained by the POC device and the laboratory was comparable for both LAC positive and negative patients, suggesting POC devices may be used for PST and PSM regardless of LAC status

PB1098
Results in patients with heparin induced thrombocytopenia or vaccine induced thrombocytopenia with thrombosis confirm the unsuitability of using APTT ratio to monitor argatroban therapy
S. Platton 1; C. Dale2; M. Dave2; N. Yartey2; P. MacCallum3
1 Barts Health NHS Trust, London, England, United Kingdom; 2 Haemostasis Laboratory, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom; 3 Department of Haematology, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom
Background: The Summary of Product Characteristics for the direct thrombin inhibitor argatroban states monitoring should be by activated partial thromboplastin time (APTT), with a target range of 1.5‐3.0 times the patient’s baseline APTT. APTT may be influenced by coagulopathies, lupus anticoagulant and raised FVIII levels.
Aims: We investigated the APTT ratio in patients receiving argatroban for the treatment of Vaccine Induced Thrombocytopenia with Thrombosis (VITT) in April and May 2021 and compared it to the APTT ratio for those being treated for Heparin Induced Thrombocytopenia (HIT) from June 2020 to May 2021.
Methods: 146 samples from 5 patients with VITT and 165 samples from patients with HIT were analysed on Sysmex CS‐series analysers for APTT using Actin FS, and had argatrobran levels analysed using the Hyphen Biomed Hemoclot Thrombin Inhibitor reagents and Hyphen Biomed argatroban calibrators and controls.
Results: The APTT ratio was significantly shorter in VITT patients than in HIT patients, with a therapeutic level of argatroban (0.4–1.2 μg/ml) being equivalent to a median APTT ratio of 1.80 (95% confidence interval 1.31–2.60) in 106 HIT samples compared to 1.52 (1.07–2.86) in 67 VITT samples. An APTT ratio of 1.5–3.0 was equivalent to argatroban levels of 0.25–1.93 μg/ml in 125 HIT patients and 0.28–4.60 μg/ml in 82 VITT samples.
Conclusion(s): This study confirms the unsuitability of monitoring argatroban therapy by APTT ratio in either HIT or VITT.
PB1107
A specific threshold for ELISA detection of Von Willebrand alloantibodies in patients diagnosed as type 3 von Willebrand disease
E. Jeanpierre1; M. Desvages2; F. Lassalle2; S. Susen 3
1 Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 2 Lille University Hospital, Lille, Nord‐Pas‐de‐Calais, France; 3 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France
Background: The development of anti‐VWF antibodies can occur in two clinical situations: alloantibodies in patients with type 3 von Willebrand disease (VWD) and autoantibodies in several underlying conditions (e.g. lymphoproliferative syndrome) leading to acquired von Willebrand syndrome (AVWS. Both types of antibodies can be detected by an ELISA. The parameters of the home‐made ELISA for the detection of anti‐VWF antibodies in our laboratory have recently been modified. Plasma‐derived Von Willebrand factor (pVWF), previously used to coat the microplates, has been replaced by vonicog alfa, a recombinant VWF (rVWF Results are expressed as optical density (OD). The threshold of positivity, defined as mean + 2 standard deviation (SD), was calculated on 42 healthy donors. A signal is positive if the OD is greater than 0.230. Using this threshold, detection of anti‐VWF antibodies was slightly positive in a proportion of type 3 VWD patients, whereas it was previously negative with plasma VWF ELISA.
Aims: To evaluate the threshold of positivity for antibody detection in type 3 VWD patients
Methods: ELISA with rVWF was realized in a total of 41 samples from 20 patients (without any replacement therapy) diagnosed as type 3 VWD and well characterized. Briefly, rVWF is coated in microplates. After overnight at room temperature, the samples were dispensed and antibody was revealed by an anti‐IGg1 HRP. Mean, SD and threshold were calculated using the 41 values obtained.
Results: Mean and SD were respectively 0.237 and 0.12. A threshold of positivity of 0.470 was obtained (mean+ 2 SD) for patients with type 3 VWD, which is higher than that found in the healthy population.
Conclusion(s): A specific threshold of positivity was characterized and must be used for alloantibody detection in type 3 VWD patients. Thus, when prescribing an anti‐Willebrand antibody test, the context should be clarified in order to use the appropriate positivity threshold.
PB1109
Coaguchek® Pro II performance evaluation to assess direct oral anticoagulants action. The DOAC‐Check study
C. Legnani1; M. Cini1; S. Testa 2; A. Tosetto3; C. Dellanoce4; S. Bellesso3; G. Carli3; I. Nichele3; L. Lissandrini3; S. Zorzi1; E. Antonucci1; G. Palareti1
1 Arianna Anticoagulazione Foundation, Bologna, Emilia‐Romagna, Italy; 2 Cremona Hospital, Cremona, Lombardia, Italy; 3 Vicenza Hospital, Vicenza, Veneto, Italy; 4 Azienda Ospedaliera Istituti Ospitalieri di Cremona, Centro Emostasi e Trombosi, Cremona, Lombardia, Italy
Background: Direct oral anticoagulants (DOACs) do not require routine laboratory monitoring, which is suggested in specific conditions, especially emergencies. Specific tests for DOAC are available, though implemented in few laboratories. PT and aPTT may be useful for a rough evaluation of anticoagulation level but are performed in laboratory, and results are delayed by sample transportation and handling. Conversely, POCT for PT and aPTT measurements are currently used.
Aims: The DOAC‐CHECK Study, an observational, national, multicentre, no‐profit study, aimed to evaluate weather CoaguChek® Pro II (Roche Diagnostics) use can provide reliable information in DOAC treated patients. The study, promoted by Fondazione Arianna Anticoagulazione, was approved by medical ethics committees and informed consent was obtained from enrolled patients. The study was supported by Roche Diagnostics.
Methods: The study was carried out in two FCSA (Italian Federation of Thrombosis Centers) centers. PT and aPTT were performed by CoaguChek® Pro II using capillary blood; at the same time venous samples were collected for measuring DOAC levels by chromogenic assay (Diagnostica Stago). ROC curves were used to identify the PT and aPTT values (in seconds) that better discriminate DOAC levels >50 ng/ml, and CoaguChek® Pro II performance was than evaluated.
Results: 512 patients were enrolled; 222 received apixaban, 120 edoxaban, 111 rivaroxaban, and 59 dabigatran (see Table 1). The negative predictive values (NPVs) for PT and aPTT were high for edoxaban and rivaroxaban, but lower for dabigatran and apixaban. Taken altogether patients receiving edoxaban and rivaroxaban, a PT ≤ 12.7 s could exclude DOAC levels >50 ng/ml in about 95% of the cases [NPV 94.6% (95% CI: 88.1–97.6)].
Conclusion(s): From our data it seems that in patients receiving edoxaban or rivaroxaban the PT performed by CoaguChek® Pro II could be useful for ruling out values above 50 ng/ml.
PB1063
A sensitive and user‐friendly method for quantifying VWF collagen binding (VWF:CB) using a fiber optic—Surface plasmon resonance biosensor
B. Calcoen 1; M. Jacquemin2; C. Tersteeg3; K. Vanhoorelbeke3; S. De Meyer3
1 Laboratory for Thrombosis Research, KU Leuven campus Kulak Kortrijk, Kortrijk, West‐Vlaanderen, Belgium; 2 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium; Department of Vascular Medicine and Hemostasis, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 3 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium
Background: Determination of both von Willebrand factor (VWF) levels and functionality is crucial to classify patients with von Willebrand disease (VWD). VWF functionality primarily encompasses the quantification of both collagen (VWF:CB) and platelet binding of VWF (VWF:RCo) and can, if needed, be supplemented with more specialized tests. Fiber optic – surface plasmon resonance (FO‐SPR) biosensors allow fast evaluation of interactions between biomolecules via a simple plasma dip. We recently developed a FO‐SPR‐based VWF:Ag assay and developing similar assays for VWF:CB and VWF:RCo would further facilitate VWD classification.
Aims: Establishing a VWF:CB assay using FO‐SPR biosensors.
Methods: Gold‐coated optical fibers were made specific by coupling either collagen fibers type 3 or a synthetic triple‐helical‐peptide (THP) onto the carboxyl‐SAM layer. Next, normal human plasma (NHP) was added to allow binding of VWF to either the collagen fibers or THP. Finally, the end SPR signal was generated by adding biotinylated polyclonal anti‐VWF antibodies followed by anti‐biotin coated gold nanoparticles. Serial dilutions of NHP were used to establish a dose‐response curve. Assay sensitivity was determined using a VWD type 3 sample.
Results: Immobilization of collagen fibers type 3 to the optical fibers was inconsistent and variable in contrast to that of THP. Using the THP, antibody concentrations and buffer conditions in all assay steps were optimized and showed good reproducibility (CV < 20%). A calibration curve was established using NHP and an excellent non‐linear fitting (R² = 0.9920) was found. Detection and quantification limit were 0.36% and 2.56%, respectively. Further fine‐tuning is running and based on comparison studies with our in‐house VWF:CB ELISA.
Conclusion(s): We developed a FO‐SPR‐based VWF:CB assay using a synthetic THP which showed less variability than with the organic collagen source. In combination with our already developed FO‐SPR‐based VWF:Ag measurement, these FO‐SPR assays could be ideal candidates for a multiplex set‐up to determine VWF parameters simultaneously.
PB1077
Evaluation of automated mixing studies and calculation of index of circulating anticoagulant in samples with lupus anticoagulant or factor VIII inhibitors
S. Kamali 1; S. Platton2
1 Haematology and Blood Transfusion Laboratory, Newham University Hospital, Barts Health NHS Trust, London, England, United Kingdom; 2 Barts Health NHS Trust, London, England, United Kingdom
Background: Samples with prolonged clotting times often have mixing tests performed, but interpretation is often problematic. Software on Sysmex CN‐series analysers automates testing for immediate‐acting and delayed‐acting inhibitors, calculating index of circulating anticoagulant for immediate‐ (ICA) and delayed‐ (ICA_D) inhibitors, and compares them by division (ICA_D/ICA) and subtraction (ICA_D‐ICA).
Aims: To evaluate the software and identify which reagent is most suitable for detecting immediate‐ and delayed‐acting inhibitors.
Methods: Haemophilia patients with no detectable inhibitor (HPA) were used as controls. Three sample sets were analysed: congenital or acquired haemophilia A with inhibitors (INH); lupus anticoagulants by DRVVT and LA‐sensitive APTT (LA); on rivaroxaban (DOAC). Samples were tested neat and at 50:50 dilution with normal plasma immediately after mixing; the 50:50 mix was incubated for two hours and re‐tested along with neat samples and normal plasma. Samples were tested with five different APTT reagents: Actin FS (AFS); Actin FSL (FSL); Pathromtin SL (PSL); Cephen; and Cephen LS (CLS).
Results: ICA and ICA_D in LA samples were the same as or lower than in HPA samples with all reagents except CLS. ICA_D was higher than ICA in INH samples and higher than in HPA samples with all reagents, so ICA_D/ICA and ICA_D‐ICA both identify INH samples. DOAC samples had higher ICA and ICA_D than in HPA samples with all reagents. Based on cut‐offs derived from HPA controls, reagents had sensitivity/specificity for LA of 0.0%/79.2%, 11.1%/83.7%, 0.0%/76.9%, 0.0%/82.1% and 55.6%/78.4%; and for INH 76.5%/100.0%, 64.7%/100.0%, 86.7%/96.8%, 85.7%/100.0% and 71.4%/100.0% for AFS, AFSL, PSL, Cephen and CLS respectively.
Conclusion(s): The software is suitable for use to detect immediate‐ and delayed‐acting inhibitors. Of the reagents tested, Cephen LS was the best reagent to detect LA; all reagents identified INH. Care needs to be taken to exclude DOAC as a cause of a prolonged APTT to avoid misinterpretation.
PB1080
The validation of mixing test‐specific cut‐off in lupus anticoagulant mixing test interpretation for multiple reagents
O. Kumano 1; G. Moore2
1 HYPHEN BioMed, Neuville Sur Oise, Centre, France; 2 Cambridge University Hospitals, Bexley, England, United Kingdom
Background: Lupus anticoagulants (LA) are detected by prolongation of clotting times for activated partial thromboplastin time (APTT), dilute APTT (dAPTT) and dilute Russell’s viper venom time (dRVVT) screening tests. Mixing tests to evidence inhibition are recommended for LA detection, and interpretation via mixing test‐specific cut‐off (MTC) is recommended due to the high sensitivity in ISTH latest guidelines [1].
Aims: To investigate the sensitivity and specificity of APTT, dAPTT and dRVVT reagents and the distributions of LA‐positive and factor deficient groups in mixing test‐specific cut‐off for multiple reagents.
Methods: One hundred‐five samples from non‐anticoagulated LA‐positive patients, 52 factor deficient samples, and 25 non‐LA warfarin samples were employed. Samples were tested with 4 APTTs, 1 dAPTT and 2 dRVVT reagents, all elevated screen ratios were followed up with mixing tests and mix ratios were also calculated. The frequencies of elevated and non‐elevated results were calculated.
Results: The frequencies of screen ratio elevated results were 33.3–62.9%, 84.6–98.1% and 88.0–100% for APTT/dAPTT and 61.9–69.5%, 32.7% and 100% for dRVVT in LA‐positive, factor deficient and warfarin samples, respectively. The distributions of mix ratio in LA‐positive were wider than those of factor deficient and warfarin samples in all reagents. The frequencies of mix ratio elevated results were 45.5–83.6%, 0–88.0% and 12.0–80.0% for APTT/dAPTT and 72.3–80.8%, 35.3–94.1% and 88.0–96.0% for dRVVT, respectively.
Conclusion(s): The distributions of LA‐positivity were overlapped with those of factor deficient and warfarin samples in low values. The frequencies of screen ratio and mix ratio elevated results were dependent on the reagents. It is important to understand the characteristics of the reagents and the confirmatory tests should be performed in samples which are suspected in the mixing tests. [1] KMJ. Devreese et al. J Thromb Haemost. 2020; 18: 2828‐2839.
PB1055
“Does my patient have HIT? There should be an app for that”
F. Allemand; M. Stoll
Emosis, Illkirch‐Graffenstaden, France, Illkirch‐Graffenstaden, Alsace, France
Background: Heparin‐induced thrombocytopenia is a common, potentially lethal, clinical issue in intensive care setting. However, while HIT three‐step diagnostic work‐up is well defined, there is no way to easily quantify how likely a patient has HIT.
Aims: We developed an iOS and Android based app (NomoHIT) for quantifying HIT diagnosis probability at patient’s bedside, by fusing 4Ts score, immunoassay and functional test results.
Methods: The method is a digital equivalent of Fagan nomogram which is a graphical tool for quantifying the probability that a patient has the disease, on the basis of test performance and pre‐test probability of the condition. The Fagan nomogram belongs to the statistical field of Bayesian inference. It is however difficult to apply in practice, especially to a three‐step diagnostic workup. The app we developed consists in successive screens (Figures 1 and 2) with query fields to be sequentially completed with 4Ts score output, and the results of immunoassay and of functional assay, after having specified the sensitivity and specificity of the used assays. The app then applies the well‐known Bayes’ formula to compute the probability of positive diagnosis, by using each post‐test probability from a prior step as pre‐test probability for the following step.
Results: Results will be exemplified through an interactive interface. Interestingly enough, the app displays the intermediate probabilities, enabling the physician to assess the information gain she may expect from moving forward into the diagnostic workup.
Conclusion(s): Bayesian approach provides clinicians with a systematic framework for estimating the probability of HIT in their patients, to help in informing their medical decision making. The app we developed offers the lab specialists and physicians a user‐friendly tool to benefit from the Bayesian approach in practice.


PB1061
Multicentre performance evaluation and reference range for an immunoassay for the quantitative determination of factor XIII antigen on the cobas t 711 analyser
M. Büchsel 1; J. Creaghan2; U. Geisen3; P. Jilma4; R. Jones2; S. Kitchen5; G. Rozsnyai6; P. Quehenberger7
1 University Medical Center Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 2 Sheffield Haemostasis and Thrombosis Centre, Sheffield, England, United Kingdom; 3 Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 4 Department of Laboratory Medicine, Medical University of Vienna, Vienna, Wien, Austria; 5 Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 6 Roche Diagnostics International Ltd, Rotkreuz, Zug, Switzerland; 7 Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: Factor XIII (FXIII) is a protransglutaminase important in the final stage of blood coagulation and is vital in stabilising the haemostatic plug. FXIII deficiency is a rare bleeding disorder characterised by abnormal blood clotting. A multicentre evaluation of an immunoassay for the quantitative determination of FXIII antigen (subunit A) was performed.
Aims: Evaluate analytical performance of the FXIII immunoassay on the high‐throughput cobas t 711 analyser (Roche Diagnostics International Ltd, Rotkreuz, Switzerland).
Methods: Analytical performance was assessed at three sites (Freiburg, Sheffield, Vienna; April–August 2021) using 3.2% citrated, anonymised, ethically approved, residual routine plasma samples. Five human plasma pools and two controls were used to measure assay repeatability (one run/site; n = 21 replicates/sample), intermediate precision and total reproducibility (five aliquots/sample/day over 5 days); standard deviations (SDs) and coefficients of variation (CVs) were calculated. Using three sets of ≥120 samples covering the measuring range of the assay, method comparison (vs. HemosIL Factor XIII Antigen on IL/Werfen ACL TOP 700 analyser) and lot‐to‐lot variability (three reagent lots) were evaluated by Deming and Passing‐Bablok regression, respectively, and Pearson’s r calculated. The reference range was determined in fresh samples from healthy adults not receiving anticoagulants (Freiburg only; n = 200; three reagent lots).
Results: Across sites, the FXIII immunoassay met the acceptance criteria for repeatability (SD, 0.112–0.270; CV, 0.7–2.8%), intermediate precision (SD, 0.150–0.676; CV, 0.9–4.2%) and total reproducibility (CV, 3.3–9.9%). The FXIII immunoassay showed agreement with the comparator assay (Pearson’s r, 0.892–0.918; Table 1) and good lot‐to‐lot variability (Pearson’s r, 0.990–0.993; Table 2). The reference range of the FXIII immunoassay in healthy individuals was 68.0–159 IU/dL.
Conclusion(s): The FXIII immunoassay showed robust analytical performance on the cobas t 711 analyser, supporting its use in routine clinical laboratory practice. Study Funding: Roche Diagnostics International Ltd, Rotkreuz, Switzerland.
Table


PB1064
Exclusion of relevant concentrations of direct oral anticoagulants in blood by DOAC Dipstick—Proposal of a diagnostic algorithm for improvement of clinical decision‐making in emergencies
I. Ćelap 1; S. Margetić2; J. Periša3; M. Razum3; S. Šupraha Goreta4; A. Čajević Glojnarić3
1 University Hospital Center Sestre milosrdnice, Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 2 Department of Clinical Chemistry, Sestre milosrdnice University Hospital Center, Vinogradska 29, 10 000 Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 3 University Hospital Center Sestre milosrdnice, Zagreb, Grad Zagreb, Croatia; 4 Faculty of Pharmacy and Biochemistry, University of Zagreb, Zagreb, Grad Zagreb, Croatia
Background: Rapid and accurate determination of direct oral anticoagulants (DOACs) is still a major medical need in urgent clinical situations.
Aims: The aim of this study was to identify the feasibility of algorithm for rapid exclusion of clinically significant concentration of DOACs (>30 ng/ml) by data from in patients from cardiology and neurology departments.
Methods: The study included 128 paired plasma and urine samples from patients treated for non‐valvular atrial fibrillation of venous thromboembolism with an oral direct factor Xa inhibitor (DXI) (apixaban, n = 31, rivaroxaban, n = 53) and direct thrombin inhibitor (DTI) (dabigatran, n = 44) at trough drug levels. Plasma DOACs concentrations were determined by quantitative chromogenic substrate assays (Innovance anti‐FXa assay for rivaroxaban and apixaban and Innovance DTI assay for dabigatran) on BCSXP analyzer (Siemens Healthineers, Germany). Urine samples were evaluated by DOAC Dipstick test (DOASENSE GmbH, Heidelberg, Germany) to determine the presence or absence of DOACs. Parameters of diagnostic accuracy were determined by receiver operating curve (ROC) analysis. Comparison between DOAC Dipstick test results and plasma concentrations of DOACs were obtained using kappa statistics. The study was funded as a part of Croatian Science Foundation research project IP‐2016‐06‐8208.
Results: ROC analysis revealed threshold values of plasma concentrations for DXI ≥14 ng/ml and ≥19 ng/ml for DTI for detection of DXI and DTI by DOAC Dipstick, respectively. At cut‐off value ≥19 ng/mL for DTI there was no false negative or false positive results (kappa value = 1.0). For DXI at cut‐off ≥14 ng/ml one false positive result was obtained, κ = 0.92 (95 % CI 0.74‐1.00).
Conclusion(s): Application of an algorithm for exclusion of relevant plasma concentrations by DOAC Dipstick can be proposed by testing first DOACs in urine and if positive followed by quantitative DOAC assays if clinically required.

PB1067
Pre‐analytical screening and removal of Argatroban interference for haemostasis assays
S. Cox‐Morton; D. White; M. Sharp; E. Duff; M. Kirk; C. Moore; M. Robinson; E. Symington; M. Besser; W. Thomas; S. MacDonald
Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom
Background: Argatroban is a direct thrombin inhibitor licenced in the UK and USA for treatment of Heparin Induced Thrombocytopenia and has been utilised as alternative anticoagulation for critically ill COVID‐19 patients. Interferences in specialised haemostasis assays may be clinically important when bridging anticoagulant regimens or investigating thrombotic and bleeding complications common in critically ill patients.
Aims: • Assess effect of Argatroban on specialised haemostasis assays • Assess effectiveness of DOAC‐Remove (DR) in removing Argatroban interference
Methods: Argatroban calibration plasma, spanning concentrations of 0 μg/ml–2.08 μg/ml, was tested for Antithrombin (IIa activator), Factors IX, and XI, and dilute Russel’s viper venom time (dRVVT) to assess Argatroban interference. Samples were treated with DOAC‐Remove and re‐run for these assays. A p value of <0.05 was used to assess significance using paired t‐tests
Results: • Antithrombin results were significantly and linearly increased by increasing Argatroban concentrations (R" ‐ 0.99). • Factor IX and XI concentrations were significantly decreased by increasing Argatroban concentrations from 83.4 IU/dl at 0 μg/ml to 3.79 IU/dL at 2.07 μg/ml Argatroban • dRVVT screen results were significantly increased by increasing Argatroban concentrations (DRVVT ratio 1.07 (no Argatroban) to 3.79 at 2.07 ng/ml Argatroban) • Pre‐treatment of samples with DOAC‐Remove completely removes Argatroban interference to baseline.
Conclusion(s): Argatroban has significant effects on specialist haemostasis assays. Antithrombin overestimation was observed when IIa activator is used, a Xa activator should be considered in such patients. dRVVT screen ratios were significantly increased, which could lead to false positive Lupus anticoagulant results. Factors IX and XI results were significantly decreased. Similar results have been published for direct oral anticoagulants with the need for pre‐analytical screening, where anticoagulant status is unknown, becoming more important. For Argatroban, dilute thrombin time can be used as a pre‐analytical screening tool.


PB1079
Effect of anti ‐factor Xa direct oral anticoagulants (DOACs) on viscoelasticity measurements in External Quality Assessment (EQA) samples: UK NEQAS BC experience
D. Kitchen1; C. Reilly‐Stitt2; S. Kitchen 3; I. Jennings2; I. Walker4
1 UK NEQAS Blood Coagulation, Sheffield, England, United Kingdom; 2 UK NEQAS BC, Sheffield, England, United Kingdom, 3 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 4 UK NEQAS Blood Coagulation, SHEFFIELD, England, United Kingdom
Background: UK National External Quality Assurance Scheme for Blood Coagulation (UK NEQAS BC) have provided EQA samples for Rotem and TEG testing since 2014 and for the newer cartridge‐based system Rotem Sigma and TEG6 since 2019. Over this time, we have circulated samples covering normal, heparinised and more recently samples containing the DOACs edoxaban, rivaroxaban and apixaban.
Aims: To assess the effect of FX DOACs upon viscoelastic testing using EQA materials
Methods: A lyophilised sample is distributed together with a pre measured volume of distilled water and a disposable pipette in order to reconstitute the sample, and full instructions of how to process these samples. We require users to test for 30 minutes and record the CT (for Rotem devices) and R time (for TEG devices). We also ask users to supply a comment as to whether the values obtained would be normal or prolonged, and for the heparin component whether they consider the sample to have heparin present. Data is analysed to provide median values, %CV and range of results for all parameters and acceptable limits are set around the median.
Results: Results for Rotem devices are shown in table 1 and TEG devices in table 2 together with a mean of 3 normal samples also distributed through the EQA programme.
Conclusion(s): Results for all 3 DOACs tested here show some degree of prolongation of the CT and R times seen across all platforms. The interpretations of these results was considered “prolonged” and “not due to heparin present” in 68% or more with some tests and samples where all centres that provided an interpretation were in agreement (100%). however some centres did not indicate this prolongation,. Our data shows that EQA is an essential aspect of these point of care tests.


PB1094
Prothrombinase‐induced clotting time for the measurement of rivaroxaban, apixaban, and edoxaban drug concentrations: A cross‐sectional study
V. Sathanantham1; J. Studt2; A. Mendez3; L. Alberio4; P. Fontana5; W. Wuillemin6; A. Schmidt7; L. Graf8; B. Gerber9; G. Mäder10; C. Bovet10; T. Sauter10; M. Nagler 11
1 University of Bern, Bern, Bern, Switzerland; 2 Zurich University Hospital, Zurich, Zurich, Switzerland; 3 Cantonal Hospital Aarau, Aarau, Aargau, Switzerland; 4 Division of Hematology and Central Hematology Laboratory, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, Lausanne, Vaud, Switzerland; 5 Division of Angiology and Haemostatis and Laboratory of Haemostasis/Geneva University Hospitals, Genève, Geneve, Switzerland; 6 Cantonal Hospital of Lucerne, Lucerne, Luzern, Switzerland; 7 City Hospital Waid and Triemli, Zurich, Zurich, Switzerland; 8 Centre for Laboratory Medicine, St. Gallen, Sankt Gallen, Switzerland; 9 Oncology Institute of Southern Switzerland, Bellinzona, Ticino, Switzerland; 10 Inselspital, Bern University Hospital, Bern, Bern, Switzerland, 11 Inselspital, Bern University Hospital, and University of Bern, Bern, Bern, Switzerland
Background: The prothrombinase‐induced clotting time (PiCT) is proposed as a rapid and inexpensive laboratory test to measure direct oral anticoagulant (DOAC) drug levels.
Aims: We aimed to study the accuracy of PiCT to determine rivaroxaban, apixaban, and edoxaban drug concentrations and assessed whether clinically relevant drug levels could be predicted correctly.
Methods: This analysis was conducted as part of the Simple‐Xa study, a prospective multicenter cross‐sectional study including 932 patients treated with rivaroxaban, apixaban, or edoxaban. Citrated plasma samples were collected, and the Pefakit® PiCT was conducted on a Siemens Atellica Coag 360. Ultra‐high performance liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) was done to determine drug concentrations. Cut‐off levels were determined using receiver‐operating characteristics curves (ROC) and sensitivities regarding clinically relevant drug concentrations calculated (30 mg/L; 50 μg/L; 100 μg/L).
Results: Samples of 851 individuals were available for the current analysis (apixaban, n = 414; rivaroxaban, n = 373; edoxaban, n = 64). The median age was 76 (IQR 66 to 83), and 360 patients were female (42.7%). The correlation (Spearman’s correlation coefficient) between PiCT and drug concentrations was 0.85 in case of rivaroxaban (95% CI 0.82, 0.88), 0.66 in apixaban (95% CI 0.60, 0.71), and 0.78 in edoxaban (95% CI 0.65, 0.86). The sensitivity to detect clinically relevant drug concentrations was 85.1% in case of 30 μg/L (95% confidence interval [CI] 82.0, 87.7; specificity 77.9, 95%CI 72.1, 82.7), 85.7% in case of 50 μg/L (95% CI 82.4, 88.4; specificity 77.3, 95% CI 72.5, 81.5), and 85.1% in case of 100 μg/L (95% CI 80.9, 88.4; specificity 73.2%, 95% CI 69.1, 76.9). The distribution of PiCT in patients with and without clinically relevant concentrations is shown in Figure 1.
Conclusion(s): Overall, the association of PiCT with rivaroxaban, apixaban, and edoxaban drug concentrations was moderate, but the majority of clinically relevant drug levels were predicted correctly.

PB1108
Thrombin generation using the ST‐Genesia system in a cohort of healthy Asian adults: Absolute and normalized values
B. Fan1; M. Ang2; H. Tan 3; J. Boulaire4; T. Chai4; K. Ong5; V. Sampath5; C. SUM2
1 Department of Haematology, Tan Tock Seng Hospital, Singapore, Singapore, Singapore; 2 Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore, Singapore, Not Applicable, Singapore; 3 Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore, Singapore, Singapore; 4 All Eights (Singapore) Pte Ltd, Singapore, Not Applicable, Singapore; 5 Department of Haematology, Tan Tock Seng Hospital, Singapore, Singapore, Not Applicable, Singapore
Background: The thrombin generation assay (TGA) is a global haemostatic test widely utilized to investigate both diseases and drugs that cause thrombosis and bleeding.
Aims: To establish the reference intervals for TGA in healthy Asian adults and measure the variability of 3 standardised tests: STG‐BleedScreen, STG‐DrugScreen and STG‐ThromboScreen.
Methods: Whole blood was obtained from 125 healthy Asian adults aged 18 to 65 years (Chinese (71.2%, n = 89), Indians (8%, n = 10), Malays (15.2%, n = 19), Filipinos (4.8%, n = 6) and others (0.8%, n = 1)). 51.20% (n = 65) were women. TGA was performed on platelet poor plasma using the ST‐Genesia (STG) (Stago, Asnières‐sur‐Seine, France). 125 samples were assayed using the STG‐BleedScreen and STG‐ThromboScreen, with 62 samples measured using the STG‐DrugScreen. Subgroup analysis of sex and race was performed.
Results: Reference ranges were established with both normalized and absolute TG parameters (Table 1, Table 2). STG bleed screen (normalized) had a lag time ratio 1.07 ± 0.2, peak height 103.72 ± 31.4%, time to peak ratio 1.12 ± 0.1, ETP 101.59 ± 22.1%, Vel. Index 95.90 ± 37.5%, start tail ratio 0.99 ± 0.1 min. STG drug screen (normalized) had a lag time ratio 1.00 ± 0.17, peak height 96.52 ± 9.29%, time to peak ratio 1.01 ± 0.12, ETP 88.98 ± 12.72%, Vel. Index 99.51 ± 18.35%, start tail ratio 0.90 ± 0.08 min. STG Thromboscreen (without thrombomodulin) (normalized) had a lag time ratio 1.11 ± 0.19, peak height 84.19 ± 20.14%, time to peak ratio 1.23 ± 0.17, ETP 93.51 ± 15.06%, Vel. Index 73.30 ± 30.09%, start tail ratio 1.08 ± 0.15 min. STG Thromboscreen (with thrombomodulin) (absolute) had a lag time 2.42 ± 0.47 min, peak height 101.57 ± 46.51%, time to peak 4.31 ± 0.63 min, ETP 427.75 ± 189.04 nM.min, ETP Inhibition 64.85 ± 13.22%, Vel. Index 77.19 ± 40.66 nm/min, start tail 13.63 ± 0.97 min. The coefficient of variation for ETP was lower than the reported 23% and the ETP was decreased by 65% when TM was added. Intergender and interethnic variation were not statistically significant.
Conclusion(s): STG appears to be suitable for the accurate measurement of TG in healthy Asian adults.

TABLES 1 AND 2 Interindividual variability and reference intervals of thrombin generation parameters measured by ST Genesia in healthy Asian adults using the STG‐BleedScreen, STG‐DrugScreen and STG‐ThromboScreen assays.
PB1114
Precision of One‐Stage Clotting Factor Assays is improved by using fresh rather than stored calibration curves
N. Yartey 1; C. Dale1; M. Dave1; K. Lee2; S. Platton3
1 Haemostasis Laboratory, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom; 2 Haematology Laboratory, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom; 3 Barts Health NHS Trust, London, England, United Kingdom
Background: British Society for Haematology guidelines for laboratories state that ideally a fresh calibration curve should be carried out with each assay batch, and always when changing reagent lots. This laboratory used stored calibration curves until 2016, calibrating when changing reagent lots or when indicated by poor internal quality control or external quality assurance. Since 2016, a fresh calibration has been performed with every assay batch.
Aims: We evaluated the imprecision of the control results for all one‐stage clotting factor assays (OSCA) performed on one Sysmex CS‐2100 analyser for 4 years using stored curves and 4 years using fresh curves.
Methods: Normal and pathological internal quality controls were assayed with each batch of assays, with between 100 and 200 batches for each PT‐based OSCA and 200‐400 batches for each APTT‐based OSCA performed against both stored and fresh curves.
Results: For PT‐based OSCA, the mean CV for stored curves was 8.21% for normal controls and 8.8% for pathological controls; for fresh curves the CVs were 4.87% and 5.00% respectively. For APTT‐based OSCA the values were 7.23% and 8.75% for stored curves and 5.49% and 5.45% for fresh curves. These differences are statistically significant (see Table 1).
Conclusion(s): Although internal quality control and external quality assurance performance is acceptable whether using stored or fresh curves, precision is improved using fresh curves with each batch.

PB1054
Evaluation of platelet function in rare or undiagnosed bleeding disorders
P. Acuña 1; E. Monzón Manzano1; E. Arias Salgado1; E. García Pérez2; M. Alvarez‐Román3; M. Martín Salces1; M. Rivas Pollmar4; A. Dos Santos1; E. García Astudillo1; V. Jiménez Yuste5; N. Butta1
1 Hospital Universitario La Paz‐IdiPaz, Madrid, Madrid, Spain; 2 Hospital Universitario La Paz‐idiPaz, Madrid, Madrid, Spain; 3 Hospital Universitario La Paz, Madrid, Madrid, Spain; 4 Hospital Universitario La Paz‐Idipaz, Madrid, Madrid, Spain; 5 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain
Background: Qualitative and/or quantitative platelet defects promote bleeding and its identification requires a careful clinical evaluation and a rational use of diagnostic laboratory assays.
Aims: To study platelet function by different methods in patients with hemorrhagic symptoms who are suspected of thrombopathy to compare results obtained with each system and validate usefulness of methods employed.
Methods: We included 72 patients with bleeding suspected of thrombopathy, which in some cases was confirmed by genetic study (Table 1). Patients with coagulation disorders were excluded. We used the following methods to test platelet function: • Light aggregometry in platelet‐rich plasma (PRP) using ADP, collagen, epinephrine and arachidonic acid as stimulating agonists. • Flow cytometry in PRP using TRAP and ADP to induce platelet activation, and PAC1 monoclonal antibody (mAb) to recognize activated fibrinogen receptor, and anti P‐selectin and anti‐CD63 mAbs to test, respectively, release from alpha and dense granules. • PFA‐100® in whole citrated blood with collagen +ADP and collagen+Epinephrine cartridges. • Total Thrombus‐formation Analysis System (T‐TAS® 01, Zarcos, Japan) with T‐TAS® PL‐ chip (capillaries covered with collagen/under flow) in blood samples drawn in BAPA, a potent synthetic anticoagulant which inhibits Factor Xa and thrombin.
Results: Some bleeding due to platelet dysfunction was diagnosed by any of the methods used, but others can only be detected with some of the techniques tested (Table 2). This difference in the sensitivity of the methods may be due to the different experimental conditions used in each of them: type of sample used, platelets’ activators, and flow vs. static conditions. Forty % of undiagnosed bleedings did not seem to be related to platelet dysfunction.
Conclusion(s): All methods are complementary and their sensitivity depends on the pathway evaluated by the technique used and the mechanism involved in platelet dysfunction. Funding: Zacros, FUJIMORI KOGYO CO., LTD.


PB1065
Interpretation of coagulation mixing study results in the era of direct oral anticoagulants
J. Chen 1; M. Losos2
1 Houston Methodist Hospital, Houston, Texas, United States; 2 Cincinnati Children's Hospital, Cincinnati, Ohio, United States
Background: Coagulation mixing study can help distinguish the etiology of a coagulopathy. One of the challenges in the interpretation of the results is due to the increasing use of direct oral anticoagulants (DOACs) which the inhibitory pattern to PT and PTT can vary in individual patients.
Aims: In this retrospective study, we sought to assess the effects of DOAC and other factors on PT/PTT mixing studies.
Methods: We reviewed more than 1000 mixing studies performed in the last 5 years in our laboratory that serve mostly adults in a large metropolitan area. About 300 cases were excluded from analysis due to a lack of associated clinical information. At our laboratory, mixing study is only performed when initial PT/PTT is prolonged. The PTT mixing study is performed at 0‐min and after 60‐min incubation at 37°C. The presence of interfering factors was confirmed with additional laboratory testing, medication records, and medical history.
Results: All cases were separated into several groups according to correction patterns. (Table) As expected, most prolonged PT samples (93%) were corrected by mixing while 36 cases showed incomplete correction. Half of these 36 cases were due to DOAC use and 5 cases were due to factor V (FV) inhibitor. Another rare pattern is the prolongation of PTT mixing result after incubation, which is considered a characteristic of a specific factor inhibitor, most commonly, FVIII inhibitor. Only 12 out of 46 cases with PTTmix prolongation were confirmed as FVIII inhibitor and both heparin treatment and strong lupus anticoagulant (LA) also contributed to 12 cases. Nine cases were due to DOAC use. Among 309 cases with prolonged PTT, 295 (95%) samples showed incomplete correction after mixing with more than half were associated with concurrent positive LA.
Conclusion(s): Anticoagulant treatment and strong LA are the major causes of rare correction patterns of PT/PTT mixing studies.

PB1069
Utility of ISTH Bleeding Assessment Tool in identifying bleeders and predicting bleeding severity in inherited primary and secondary haemostasis defects
R. Dave; T. Geevar; J. Mammen; R. Vijayan; M. Gowri; S. Nair
Christian Medical College and Hospital, Vellore, Vellore, Tamil Nadu, India
Background: The ISTH‐Bleeding Assessment Tool(BAT) was devised to aid in identification of patients with bleeding diathesis by improving reproducibility of bleeding history. The utility of ISTH‐BAT in assessing bleeding severity is still elusive.
Aims: To assess the utility of ISTH‐BAT to identify probable bleeders with primary or secondary haemostasis defects and to differentiate between severe and non‐severe bleeders.
Methods: Participants referred for bleeding workup were recruited and grouped into “bleeders” and “non‐bleeders” based on established cut‐offs for abnormal BAT Score (males:>3, females:>5, children:>2). Patients were also grouped according to clinical bleeding severity into “severe bleeders” and “non‐severe bleeders”. Severe bleeders were defined as those with life‐threatening hemorrhages, multiple muscle hematomas, umbilical cord, gastrointestinal, CNS bleeding, >2 joints involvement by hemathroses or factor concentrate/transfusion requirement >5 times/year. Non‐severe bleeders were defined as those who bled only after trauma/surgery, bled less frequently, <2 joints involvement, less transfusion requirement, only minor symptoms like mucocutaneous bleeds. Detailed coagulation workup was performed including one‐stage clot‐based factor assays, light transmission aggregometry, ristocetin‐cofactor assay and von willebrand antigen. Participants with normal results were taken as controls.
Results: A total of 76 controls and 395 patients (primary haemostasis defects n = 244, secondary haemostasis defects n = 151) were recruited. Patients had ISTH‐BAT score ranging from 0 to 28 (Median:7, IQR:5‐11). Age of patients ranged from 6 days to 69 years (Median:11 years). ISTH‐BAT score had overall 98.21% sensitivity, 95% negative predictive value(NPV) to identify bleeders with primary haemostasis defects, and 93.3% sensitivity, 91.9% NPV to identify bleeders with secondary haemostasis defects. The sensitivity and NPV further increased to 100% for severe bleeders with Glanzmann Thrombasthenia, Bernard Soulier Syndrome, VWD and Severe Hemophilia A/B (Figure 1). There was significant overlap up to a score of 15 between clinically severe and non‐severe bleeders (Figure 2).
Conclusion(s): ISTH‐BAT is very useful screening tool to identify bleeders with primary/secondary haemostasis defects but may not predict bleeding severity.


PB1087
Rapid detection of exposure to direct oral anticoagulants (DOACs): A Qualitative Urinary Dipstick Point‐of‐Care Assay
M. Marchetti 1; C. Ambaglio2; E. Sanga2; L. Barcella2; F. Schieppati2; A. Falanga3; J. Harenberg4
1 ASST Papa Giovanni XXIII Bergamo, Italy, Dalmine, Lombardia, Italy; 2 ASST Papa Giovanni XXIII Bergamo, Italy, Bergamo, Lombardia, Italy; 3 ASST Papa Giovanni XXIII Bergamo and University of Milan Bicocca, Italy, Bergamo, Lombardia, Italy; 4 Ruprecht Karls University of Heidelberg, Heidelberg, Baden‐Wurttemberg, Germany
Background: Knowing whether a patient has been recently exposed to DOAC is a very important issue in the emergency setting for rapid clinical decisions. The DOASENSE Dipstick rapidly detects the presence of DOACs in urine, discriminating between direct factor Xa or thrombin inhibitors.
Aims: To evaluate the operativity of the DOASENSE Dipstick to implement its use in the Emergency Medicine Service at our Hospital.
Methods: Patients on DOAC referred to our thrombosis and hemostasis outpatient clinic were included. Comorbidities and concomitant therapies were recorded. Subjects not taking DOACs or on LMWH were included as controls. Venous blood and urine samples were collected at valley and/or peak of DOAC concentration. Urine qualitative detection of DOAC was performed using the DOASENSE Dipsticks and the DOASENSE Reader, while DOAC plasma concentration by specific tests on a Stago STA‐R Max® instrumentation.
Results: A group of 27 patients (21M/6F) with a median age of 69 years (range:22‐88) were studied: 9 were on rivaroxaban, 7 on dabigatran, 6 on edoxaban, and 5 on apixaban. In samples collected at valley (n = 19), the drug was clearly detected in urine of 18 patients, also in the presence of very low plasma drug concentration (range: 4–211 ng/ml). The urine of one patient on rivaroxaban tested negative and the corresponding plasma value was 0 ng/ml. Urine collected at peak (n = 18) were all positive for DOAC (plasma concentrations: 54–630 ng/ml). All plasma and urine samples from subjects not on DOACs tested negative, including those on LMWH. Results from the visual analysis of the pads were 100% in agreement with that provided by reader
Conclusion(s): Our study confirmed the reliability of the urine determination of DOACs by the DOASENSE tool, further supporting its utility at an emergency unit for screening patients with severe bleeding, and/or thrombotic events, or before urgent major surgical interventions.
PB1092
Results of samples spiked with chimeric monoclonal anti‐PF4 antibody 1C12 in ECAT external quality assessment surveys for heparin‐induced thrombocytopenia diagnosis
P. Meijer 1; J. Rollin2; G. Champier3; C. Vayne2; Y. Gruel2; C. Pouplard4
1 ECAT Foundation (External quality Control for Assays and Tests), Voorschoten, The Netherlands, Voorschoten, Zuid‐Holland, Netherlands; 2 University Hospital of Tours, Tours, Centre, France; 3 Arkab, Limoges, Centre, France; 4 University Hospital of Tours, Toiurs, Centre, France
Background: For the laboratory diagnosis of heparin‐induced thrombocytopenia (HIT), the majority of laboratories are using an immunological assay to detect the presence of anti‐PF4/heparin antibodies. Recently, chimeric monoclonal platelet factor 4 (PF4)‐specific antibodies with a human IgG1 Fc fragment have been developed, and they mimic immunological features of human HIT antibodies [1, 2].
Aims: In this study the response of the 1C12 monoclonal antibody in a wide range of frequently used HIT immunological tests was investigated in the HIT external quality assessment surveys of the ECAT Foundation.
Methods: Citrated plasma was spiked with different concentrations of 1C12 (5, 10 and 22 μg/ml). Samples were lyophilised and distributed by regular postal service to the participants (n = 465). Results, including details on the methods used, have ben submitted electronically to the ECAT Foundation.
Results: In semi‐quantitative methods, a positive response was found by 79%, 95% and 93% of the participants for samples with 5, 10 and 22 μg/ml antibodies, respectively. In quantitative methods, a positive response was reported by > 99% of the participants for any antibody concentration used. The table shows the average OD values or U/ml obtained for the different antibody concentrations used in the surveys. The between‐laboratory variation in test results varied between 10 and 25%, depending on the method used, and is comparable to the one previously observed in surveys using plasma samples from HIT positive patients.
Conclusion(s): On the basis of the results obtained in the ECAT surveys, it can be concluded that samples spiked with the monoclonal antibody 1C12 are suitable for the analytical performance assessment of immunological HIT assays. References: 1. Kizlik‐Masson, C., et al., J Thromb Haemost, 2017; 15: 2065‐2075. 2. Vayne, C., et al., Thromb Haemost, 2021; 121: 322‐331.
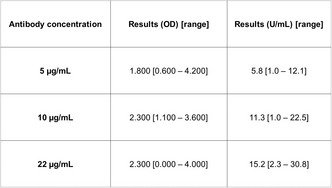
VPB1117
Diagnostic value of tromboelastography using Korean Society On Thrombosis and Haemostasis Criteria On Disseminated Intravascular Coagulation Induced Sepsis Patients
S. Fatonah 1; A. Fatoni2; H. Wiraharjanegara3; R. Adawiyah3
1 Universitas Brawijaya/ dr Saiful Anwar General Hospital, Kota Malang, Jawa Timur, Indonesia; 2 Brawijaya Universitiy, Malang, Jawa Timur, Indonesia; 3 Brawijaya Universitiy, Kota Malang, Jawa Timur, Indonesia
Background: Sepsis patients with DIC have a higher mortality, which is 43%, compared to sepsis patients without DIC which is 27%. Early diagnosis is important to support the success of DIC therapy. Conventional coagulation tests have many limitations. TEG is used to provide precise information to treat hemostasis disorders. This method can overcome several limitations in conventional coagulation tests.
Aims: To prove TEG can be used to diagnose sepsis patients with overt DIC at the Saiful Anwar General Hospital Malang Indonesia
Methods: This cross sectional study was carried out at the ICU of Saiful Anwar Hospital in the April‐September 2019 period. This study has been approved by the ethics committee of Saiful Anwar Hospital. Patients with a diagnosis of sepsis received blood samples for platelet count, PT, aPTT, Fibrinogen, D‐Dimer and TEG parameters. DIC scoring applies based on KSTH criteria as gold standard. Patients are considered to have overt DIC if the KSTH score ≥3 and non‐overt DIC if the KSTH score <3.The significance of difference among groups was tested using unpaired t‐test. ROC curve was made to produce optimal cutoff, sensitivity value, and specificity. Statistical analysis was performed with SPSS version 23.0.
Results: The number of study subjects was 46 people. 54,55% of sepsis group with overt DIC was male. The mean age in the DIC overt group was 53 years. Comparative test results were different (p < 0.05) in all parameters except age, fibrinogen and D‐Dimer. TEG parameter test results consisting of r time, k time, ⍺ angle, MA, and CI showed AUC 0,816; 0,874; 0,878; 0,899 and 0,935,respectively.
Conclusion(s): The diagnostic value of TEG in direct diagnosis of DIC shows good AUC, sensitivity and specificity, so it is important to note its use.


PB1062
Impact of the variability between normal pooled plasma batches on normalized ratio and mixing time reference cut‐off values in lupus anticoagulant testing
J. Cabo 1; R. Soleimani1; L. Morimont2; J. Baudar3; M. Guldenpfennig4; J. Douxfils2; F. Mullier5
1 Université catholique de Louvain, CHU UCL Namur, Yvoir, Namur, Belgium; 2 Qualiblood s.a., Namur, Namur, Belgium; 3 CHU UCL Namur, Yvoir, Namur, Belgium; 4 CHU UCL Namur, yvoir, Namur, Belgium; 5 Université catholique de Louvain, CHU UCL Namur, Namur Thrombosis and Hemostasis Center (NTHC), Hematology Laboratory, Namur Research Institute for Life Sciences (NARILIS), Yvoir, Belgium, Yvoir, Namur, Belgium
Background: Following ISTH guidelines for lupus anticoagulant testing, Normal Pooled Plasmas (NPPs) should be used for mixing studies and calculation of normalized ratios. The implementation of normalized ratios is assumed to reduce intra‐laboratory variability while mixing studies increase lupus anticoagulant diagnosis efficiency. Our current hypothesis is that different batches of a same commercial frozen NPP (Cryocheck®) may show significant variability in clotting times and therefore impact NPP‐related reference cut‐offs.
Aims: The objective of this study was to demonstrate that significant differences of clotting times between different batches of a same commercial frozen NPP (Cryocheck®) directly affect reference normalized ratios as well as reference mixing (1:1) clotting times.
Methods: Dilute Russell Viper Venom Time (DRVVT) and Activated Partial Thromboplastin Time (APTT) pathways were explored for lupus anticoagulant evaluation. Screening, mixing and confirm tests were performed with Stago® instruments and reagents. Two different batches (NPP 1291 and 1301 from Cryocheck®) were compared in the determination of reference cut‐off values with 60 healthy donors (plasmas stored less than 3 years at −80°C). Reference cut‐off values were defined as 99th percentile values according to a nonparametric approach. Differences between reference cut‐off values were assessed by Mann‐Whitney test.
Results: Reference cut‐off values obtained with two different NPP batches were significantly different for DRVVT system, reflecting the variability of NPPs clotting times. On the contrary, no significant difference was observed in APTT system (see Table 1).
Conclusion(s): This study has proved that differences between NPP batches may influence lupus anticoagulant reference values that directly depend on NPP clotting times such as normalized ratios and mixing studies. Comparing any new NPP batch to the former one would be useful in the decision of reviewing reference cut‐off values. Adequate cut‐off values, taking into account NPP clotting time, are important to provide accurate conclusion about the presence or absence of a lupus anticoagulant.

PB1070
Whole blood impedance aggregometry is inferior to lumiaggregometry in the diagnosis of platelet function defects amongst women with menorrhagia referred for surgical intervention
A. Delaney 1; M. Makris2; E. Kritsotakis3; R. Maclean4; K. Horner5; S. Kitchen6; J. Sedcole7; T. Baxter7; C. Samuelson2
1 Department of Haematology, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK, Sheffield, England, United Kingdom; 2 Sheffield Haemophilia and Thrombosis Centre, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 3 School of Health and Related Research, University of Sheffield; School of Medicine, University of Crete, Greece; 4 Sheffield Teaching Hospitals NHS Trust, Sheffield, England, United Kingdom; 5 Department of Coagulation, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 6 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 7 Department of Obstetrics & Gynaecology, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom
Background: There is clinical interest in alternative diagnostic methodology for platelet function disorders in contrast to the time consuming and labour intensive “gold standard” of light transmission or lumiaggregometry.
Aims: Within this prospective study, we aimed to determine the sensitivity of whole blood impedance aggregometry (WBIA) with the Multiplate® analyser (Roche) in detecting platelet function abnormalities amongst women referred for surgical management of severe menorrhagia.
Methods: Women aged ≥18 referred for surgical management of menorrhagia at our tertiary gynaecology centre were eligible for inclusion. Anti‐thrombotic therapy, known bleeding disorder or pelvic malignancy were exclusion criteria. After providing informed consent, participants underwent standardised ISTH‐BAT scoring and laboratory testing, including platelet function testing with both lumiaggregometry and WBIA (Multiplate® analyser, Roche). Those with abnormal lumiaggregometry results underwent repeated testing by both methods and were also referred for specialist haematology review.
Results: Fifty patients were recruited and underwent testing. Eight women (16%) were identified as having a platelet function defect based on reproducible abnormalities on lumiaggregometry. Of these, 3/8 had results consistent with a storage pool disorder; medication was suspected to be a contributory factor in a further 3/8 cases. WBIA results were normal in 3/8 women (37.5%) in whom lumiaggregometry detected platelet dysfunction, 2 of whom had a storage pool disorder. Only 2 patients with a platelet function abnormality detected on lumiaggregometry had persistently abnormal WBIA analysis, giving a test sensitivity of only 25%. Five patients had abnormal WBIA results despite normal lumiaggregometry results.
Conclusion(s): In conclusion, WBIA with the Multiplate® analyser (Roche) has poor sensitivity and specificity in comparison to gold standard lumiaggregometry in the detection of platelet function disorders amongst women with severe menorrhagia referred for surgical intervention. This study was supported by a grant from the Platelet Society and received local ethical approval.
PB1071
Routine measurement of direct factor Xa inhibiting anticoagulants
M. Dave1; C. Dale1; L. Fayehun‐Idowu 2; K. Mahadevan3; N. Yartey1; S. Platton4
1 Haemostasis Laboratory, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom; 2 Haematology Laboratory, Newham University Hospital, Barts Health NHS Trust, London, England, United Kingdom; 3 Haematology Laboratory, Whipps Cross University Hospital, Barts Health NHS Trust, London, England, United Kingdom; 4 Barts Health NHS Trust, London, England, United Kingdom
Background: The licensing of andexanet alfa for the reversal of rivaroxaban in bleeding patients with levels above 50ng/mL means direct factor Xa inhibitor (DFXaI) assays may be urgently required. Sysmex CS‐series analyser standard protocols for Hyphen‐Biomed Heparin LRT [HB‐LRT] reagents use different control and sample dilutions for each DFXaI (rivaroxaban 1/10; edoxaban 1/15; apixaban 1/20), making the assays uneconomical to run in laboratories with small numbers of DFXaI requests. Centralised testing in our Trust led to delays in DFXaI results, frequently >24 h.
Aims: A combined protocol for all DFXaI with a single set of controls for all assays was evaluated for its potential to make assays available at all sites, on demand.
Methods: HB‐LRT Heparin reagents were used with the standard protocols and the combined protocol, which uses a 1/15 sample dilution. Samples were measured on a Sysmex CS‐2100 using standard protocols, and on Sysmex CS‐5100 and CS‐2500 analysers using the combined protocol. Hyphen‐Biomed Rivaroxaban Controls 1 and 2 were tested five times a day for 5 days using the standard protocols for apixaban and edoxaban, to derive target values for use in the combined assay.
Results: Using drug‐specific calibrators in the combined protocol, the difference in optical density between highest and lowest calibrators was not significantly different from those in dedicated rivaroxaban or apixaban assays. Standard and combined protocols showed good correlation for patients samples. Standard and combined protocols showed comparable precision and accuracy for control samples. Target values for new batches of control are derived from parallel testing with the existing batch, with target SDs calculated from measurement uncertainty data. Performance in external quality assurance is acceptable for all analysers at all sites.
Conclusion(s): Rivaroxaban, apixaban and edoxaban assays are now available routinely, with the same turnaround time as all other routine clotting assays at Trust sites where the assay is available.
PB1105
In‐vivo mimicking platelet thrombus formation in a microfluidic system (Anysis): Assessment of P2Y12 inhibitors for cardiology patients
S. Shin 1; S. Kim2; C. Choi3; J. Piao1
1 Korea University, Seoul, Seoul‐t'ukpyolsi, Republic of Korea; 2 Rheomeditech Inc., Seoul, Seoul‐t'ukpyolsi, Republic of Korea; 3 Korea University Guro Hospital, Seoul, Seoul‐t'ukpyolsi, Republic of Korea
Background: Assessment of P2Y12 therapeutic responsiveness is vital to preventing thrombotic and hemorrhagic complications in patients with cardiovascular diseases. Although various P2Y12 tests are being used at POC, the concentration of agonist used for each test varies greatly depending on the test equipment, resulting in even the opposite diagnosis result. We introduced a microfluidic cartridge Anysis‐p2Y12 to assess P2Y12 inhibitor with minimum concentrations of ADP and PGE1.
Aims: This presentation describes the new Anysis‐P2Y12 assay performance comparing with VerifyNow‐P2Y12 in cardiac patients and analyzes the P2Y12 low‐response rates of the two devices.
Methods: In total, 125 samples were collected from cardiac patients referred for a P2Y12 antiplatelet response test. The test result of Anysis‐p2Y12 was the migration distance (MD) in the microfluidic system until the blood flow stops.
Results: The MDs without and with P2Y12 were 182 ± 30 and 264 ± 12mm, respectively (p < 0.0001). Compared to VerifyNow‐P2Y12, the positive percent agreement rate and negative percent agreement of Anysis‐200 were 96.8% and 88.7%, respectively. Cohen’s kappa coefficient between the two devices was 0.761. However, there was an apparent difference in the low‐response rate to P2Y12, which was 36.5% for VerifyNow and 5.9% for Anysis. The different results in the low‐response rate to P2Y12 was mainly due to the concentration of agonist used for each test. In fact, the concentrations of ADP in VerifyNow were 20 μM, which is the 10‐folds of that of Anysis‐P2Y12 (2 μM).
Conclusion(s): The microfluidic platelet function assay, Anysis‐P2Y12 adopted the recommended concentration of agonist and can help screen patients with abnormal P2Y12 non‐responsiveness consistent with clinical results.


PB1106
Microfluidic Thromboelastography (Micro‐TEG)
S. Shin
Korea University, Seoul, Seoul‐t'ukpyolsi, Republic of Korea
Background: Since blood viscoelastic characteristic during coagulation has been provided direct information on the hemostatic status, a measurement technique to quantify blood viscoelasticity is very important for the diagnosis of coagulation disorders at clinical environments.
Aims: The objective of the present study is to develop and evaluate an innovative thromboelastography (TEG), which can be easily used at clinical environments.
Methods: We introduce an innovative microfluidic thromboelastography (micro‐TEG) that measures the viscoelastic properties of coagulating blood by evaluating temporal oscillations.
Results: When periodic oscillatory pressure is applied, the anticoagulant blood periodically oscillates a constant distance. When blood coagulation begins, the oscillatory distance of blood is gradually decreased and yields a minimum oscillation. If accompanied by lysis, the oscillatory distance tends to re‐increase with time. In this micro‐TEGT system, we provide clotting times (R and K), maximum change in amplitude (MA), lysis time and Ly 1/2. These indexes show excellent agreement with those of a commercial TEG system.
Conclusion(s): The results of this study suggest that micro‐TEG is a useful tool that provides holistic hemostatic properties for blood coagulation studies and clinical diagnosis.

PB1082
Genomic screening of patients with bleeding tendency of unknown cause
E. Leinoe 1; A. Ørslev Rasmussen2; M. Rossing2
1 Rigshospitalet University Hospital, Copenhagen, Denmark, Gentofte, Hovedstaden, Denmark; 2 Dept. of Genomic Medicine, Rigshospitalet University Hospital, Copenhagen, Denmark, Copenhagen, Hovedstaden, Denmark
Background: Despite comprehensive laboratory work‐out, most patients referred for inherited bleeding disorders (IBD) receive an unspecified diagnosis: bleeding of unknown cause (BUC).
Aims: To identify plausible new genes and variants associated with specific bleeding phenotypes to generate a library of novel candidate genes. The long‐term aim is to improve the diagnostic outcome in this heterogenous group of patients suffering from BUC.
Methods: Following evaluation by standard coagulations test and bleeding assessment tool (BAT) score, patients with BUC and a highly significant BAT score >9 were enrolled. All patients were annotated according to their individual phenotype: Hypermobility (evaluated by Beighton score); Platelet dysfunction; Petechia; Hyperfibrinolysis (evaluated by thrombelastography). Germline DNA were subjected to whole‐genome‐sequencing (WGS) analysis and variant output were initially filtered for variants in an in‐house in silico gene panel comprising 113 IBD associated genes. Subsequently, for patients without a diagnostic finding among the 113 genes, we applied an extended in silico gene panel filtering for >2000 genes selected based on relevance and association to BUC, including hemostatic signaling pathways, hypermobility, co‐expression or protein‐interaction with known causal genes and genotype‐phenotype databases. Finally, variant enrichment analysis using Fishers exact test will be applied by grouping patients and variants according to phenotypic or laboratory characteristics, using Danish2k database as control. ACMG guidelines were used for variant classification.
Results: 102 patients were enrolled and assigned phenotypes distributed accordingly: Hypermobility: 47% (48/102); Platelet dysfunction: 40% (41/102); Petechia: 16% (16/102); Hyperfibrinolysis 19% (12/69). Output from the initial WGS analysis using the 113 IBD associated gene list resulted in a plausible genetic cause for 10% of the patients (10/102). Subsequently and currently we are evaluating the rare variants identified among the extended and pathway specific gene list
Conclusion(s): Further studies are needed to evaluate the generated library of candidate genes and to improve the diagnostic outcome in BUC
PB1089
Evaluation of the effect of freezing and thawing of citrated plasma on coagulation assays
K. Mayger 1; E. Foxton2; A. Drew3; A. McCormick4
1 Guys and St Thomas' NHS Trust, London, England, United Kingdom; 2 Viapath Analytics, Royston, England, United Kingdom; 3 Viapath, London, England, United Kingdom; 4 Viapath Analytics, London, England, United Kingdom
Background: Routine coagulation results can be affected by various pre analytical factors that includes the stability of the citrated plasma at room temperature. The current BSH guideline recommends that coagulation assays should be performed within 4 hours from sample collection, however this is not always possible.
Aims: To evaluate the impact of freezing and thawing of citrated plasma on routine coagulation assays.
Methods: Blood samples were collected into 3.2% sodium citrate tubes, spun and analysed for prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen, thrombin time (TT), reptilase time (RT) and D‐dimer. Plasma aliquots were then separated into screw‐top microvials and stored in a −80°C freezer for at least 2 weeks. The plasma aliquots were then thawed at 37°C for 4 min and re‐tested using the same reagents lots. All analyses were performed on the Sysmex CN6000 analysers using Siemens reagents (Siemens, Healthcare Diagnostics, Marburg, Germany). The difference between fresh and frozen sample results were determined using Wilcoxon test (p < 0.05 indicating statistically significant differences). Areas of bias were evaluated using Bland and Altman plots.
Results: Median (range) and p values for all assays are presented in table 1. The Wilcoxon analysis showed a statistically significant differences between fresh and frozen results for PT, TT, RT and D‐dimer.
Conclusion(s): We demonstrated that freezing and thawing conditions affect PT, TT, RT and D‐dimers with a statistical significance, however these differences are not considered to be clinically significant.

PB1102
UK NEQAS (Blood Coagulation) Genetics of Heritable Bleeding and Thrombotic disorders programme—A review of External Quality Assessment (EQA) Scheme exercises 2021
C. Reilly‐Stitt 1; I. Jennings2; M. Hill3; A. Page4; R. Wheeler5; M. Sutherland6
1 UK NEQAS Blood Coagulation, SHEFFIELD, England, United Kingdom; 2 UK NEQAS BC, Sheffield, England, United Kingdom; 3 Nottingham University Hospital NHS Trust, Nottingham, England, United Kingdom; 4 Royal Infirmary of Edinburgh, Edinburgh, Scotland, United Kingdom; 5 Viapath Analytics LLP, LONDON, England, United Kingdom; 6 Manchester University NHS FT, Manchester, England, United Kingdom
Background: The programme blends whole blood sample distribution, to assess laboratory methods for variant analysis, with paper exercises to scheme participants.
Aims: To provide vertical and horizontal auditing of laboratory methods for variant analysis.
Methods: Participants have wet and dry exercises annually to assess analytical platforms plus interpretation and reporting processes.
Results: For whole blood exercises, all centres correctly identified the genetic variant in case 1; marks were lost for omissions in the interpretative reports. In case 2, all participants investigating a F8 Intron 22 inversion achieved full marks. This demonstrates improvement in methodologies used to perform genetic analysis compared to an exercise in 2011 when 4/22 centres failed to identify the variant. For the paper exercises, the variant classification for F5 had 100% of participants reporting that the variant was either pathogenic or likely pathogenic for c.5044G>T and 80% made the same conclusion for c.6419G>A. For PROS1, 100% of participants reported the variant either pathogenic or likely pathogenic for c.1063C>T and 70% reported the variants either a disease associated polymorphism/variant of uncertain significance for c.1501T>C.
Conclusion(s): The scheme offers a blended approach to participants from genetics laboratories, testing platforms used for variant analysis and encouraging use of recommended guidelines for clinical reporting procedures.

VPB1115
State‐of‐the‐art for Coagulation Factors Activity Assays Evaluated from Results of an External Quality Assessment Program
C. Bon; R. Meley; M. Lopez; B. POGGI
PROBIOQUAL, LYON, Rhone‐Alpes, France
Background: Coagulation factors activity testing is necessary for the diagnosis and treatment of patients with hemostasis disorders and requires relevant analytical goals to promote accuracy and precision across multiple methods.
Aims: This work evaluates Prothrombin Time (ProT) and Activated Partial Thromboplastin Time (aPTT) differential factors (DF) assays state‐of‐the‐art, from results of an External Quality Assessment (EQA) program provided by ProBioQual, a french proficiency testing association.
Methods: Factors (F) II, V, VII, X, VIII, IX, XI, XII were measured each year on 20 lyophilized human citrated plasmas by participant laboratories (160 for FXII to 450 for FV in each survey) for the 2019‐2021 period. Statistical evaluation was performed according to the ISO13528:2015 guideline and Robust Algorithm A. The consensus value was calculated for all participants (AP) results and each peer group (PG). The imprecision of methods was evaluated by the inter‐laboratory 90th percentile coefficient of variation (CV90) for AP and each PG. Inaccuracy was quantified as 90th percentile bias (bias90) of laboratory results from AP or PG consensus value.
Results: ProT DF CVs do not change significantly with level and vary between 7 and 8% for FII and FX, 8 and 9% for FV and FVII. The AP and PG bias90 are very close and the lowest for FII and FX (around 12%). AP CVs are the highest for FVIII, FIX and FXII, respectively 14%, 17% and 13%, for a level close to 40%; PG CVs fall to 10%. AP bias90 is always higher than than PG bias90 for aPTT DF and reachs 20% at normal level, 22% at low level for FVIII and FIX; PG bias90 is respectively 14% and 17%.
Conclusion(s): Performance of ProT DF tests is satisfactory in routine practice. For aPTT DF assays, EQA survey results show a lack of standardization across multiple instrument‐reagent combinations.
PB1053
Immunophenotyping and cell sorting of human MKs from human primary sources or differentiated in vitro from hematopoietic progenitors
A. Acebes‐Huerta 1; P. Martínez‐Botía1; C. Martín Martín1; A. Bernardo2; A. R. Rodríguez3; M. Vicente‐Ayuso4; C. Benavente Cuesta5; L. Gutiérrez6
1 Instituto de Investigación Sanitaria del principado de Asturias (ISPA), Oviedo, Asturias, Spain; 2 HUCA, oviedo, Asturias, Spain; 3 University of Oviedo, Oviedo, Asturias, Spain; 4 Department of Hematology, University Hospital Severo Ochoa, Madrid, Madrid, Spain; 5 Department of Hematology, Instituto de Investigación Sanitaria San Carlos (IdISSC), Hospital Clínico San Carlos, Madrid, Madrid, Madrid, Spain; 6 University of Oviedo ‐ ISPA, Oviedo, Asturias, Spain
Background: Megakaryocyte (MK) differentiation encompasses a number of endomitotic cycles that result in a highly polyploid (>64N) and large (40–60 μm) cell. The characterization of megakaryopoiesis by flow cytometry is limited to the identification of mature MKs using lineage‐specific surface markers, while earlier MK differentiation stages remain unexplored.
Aims: To develop an immunophenotyping strategy for the staging of megakaryopoiesis.
Methods: Surface protein expression was evaluated by flow cytometry on human peripheral blood mononuclear cells (PBMC), bone marrow samples and in vitro PBMC‐derived megakaryocyte cultures using 7‐color panel mixes.
Results: Here, we present an immunophenotyping strategy that allows the identification of successive MK differentiation stages with a panel integrating MK specific and non‐specific surface markers (Table 1). We have seen that the combination of CD31/CD71 allows to set a number of gates which correspond to different stages towards MK differentiation. Further back‐gating with MK‐specific markers allows the separation of mature and immature MKs. Furthermore, in fresh samples, back‐gating to verify the presence of other markers used, or to place the populations in the Forward/Side Scatter axes, refines the assessment of MK differentiation stages and allows to discard other cell types that could be present on the same populations. These populations distribute distinctly in different hematological pathologies (example in Figure 1A), which will be discussed in detail. Ploidy analysis of these populations shows a positive correlation of ploidy status and maturation of MKs (Figure 1B). Despite its size and fragility, MKs can be immunophenotyped using the above‐mentioned panel and enriched by fluorescence‐activated cell sorting under specific conditions of pressure and nozzle diameter (Figure 1C) for further studies (i.e. multi‐Omics).
Conclusion(s): A better characterization of megakaryopoiesis by flow cytometry may pose fundamental in the diagnosis or prognosis of lineage‐related pathologies and malignancy and we believe our strategy eases that path.


PB1059
Next‐generation FVIII mimetic, Mim8, does not interfere with non‐factor VIII haemostasis assays
A. Bowyer 1; K. Hickey2; S. Kitchen3; M. Ezban4
1 Sheffield Teaching Hospitals NHSFT, Sheffield, England, United Kingdom; 2 Department of Coagulation, Sheffield Teaching Hospitals NHSFT, sheffield, England, United Kingdom, 3 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 4 Novo Nordisk A/S, Måløv, Hovedstaden, Denmark
Background: Mim8 is a next‐generation bispecific antibody that mimics the action of activated factor FVIII in patients with haemophilia A (HA). Mim8 complexes with factor X and factor IXa to accelerate factor X activation. Previous studies have assessed the effect of Mim8 on one‐stage and chromogenic assays of factor VIII activity (1).
Aims: To assess the influence of Mim8 on commonly used haemostasis assays that are not measuring factor VIII activity.
Methods: Severe HA plasma (HRF) was spiked with Mim8 0–20 μg/ml (Novo Nordisk) and tested on 3 days with the following tests. Prothrombin time (Innovin, Siemens and Recombiplastin, Werfen), Clauss Fibrinogen (Dade thrombin, Siemens), thrombin time (Thromboclotin, Siemens), Innovance D‐dimer (Siemens), Anti‐Xa (Hyphen Biomed), HIT (Acustar, Werfen), Protein C (chromogenic using Protac), free Protein S antigen (Werfen), Antithrombin (Berichrom, Siemens), VWF antigen (Siemens and Werfen), VWF activity (Siemens Innovance and Werfen VWF activity and VWF:RCo), one‐stage factors II, V, VII and X (Innovin and Precision Biologic), one‐stage factors IX, XI and XII (Actin FS, Siemens and Precision Biologic), Berichrom FXIII (Siemens), Rossix chromogenic FIX.
Results: Assay results are shown in table 1. A very minor increase in prothrombin time using Recombiplastin (1.3 s) and thrombin time (2.6 s) was observed between 0 and 20 μg/ml Mim8. Addition of 1 μg/ml Mim8 increased apparent factor IX, XI and XII activity by 1.3–2.4 fold. Non‐parallel assays were observed for factor IX and XI only.
Conclusion(s): Most commonly used haemostasis assays are not affected by the presence of Mim8 in plasma and can accurately be performed in the presence of this therapy. There were no clinically relevant differences between 0 and 20 μg/ml Mim8 in any assay with the exception of the one‐stage clotting factors IX, XI and XII. These latter APTT‐based assays are not suitable for testing in the presence of Mim8.

PB1078
TFTEM—A new reagent to detect direct oral factor Xa inhibitors on ROTEM devices
M. Schwaiger; A. Kholmukhamedov; R. Al Sawad; R. Bottenus
Werfen, San Diego, California, United States
Background: Direct oral factor Xa‐inhibitor (DOACs), do not require routine laboratory monitoring due to their predictable pharmacokinetics and pharmacodynamics. However, DOAC detection and monitoring are crucial in specific emergency situations. ROTEM systems are available in point‐of‐care settings for bleeding management and provide rapid in‐sights into patients’ coagulation status.
Aims: This study aimed to assess the diagnostic performance of the newly developed ROTEM TFTEM assay regarding the detection and monitoring of Xa inhibitors. Apixaban was chosen as a model for anti‐Xa DOACs as it has been known to be the most challenging DOAC for detecting at low levels by clotting assays.
Methods: TFTEM reagent consists of a diluted human recombinant tissue factor, calcium chloride, and a heparin inhibitor (polybrene). Different apixaban plasma concentrations (0 to 481 ng/mL) were prepared by using HemosIL Apixaban Calibrators (Werfen, USA). Some plasma samples were spiked with unfractionated heparin. All measurements were per‐formed on the ROTEM delta (Werfen, Germany). Reagents were reconstituted and re‐actions started by pipetting 300 μL apixaban containing plasma or donor blood to the lyophilized reagent. TFTEM clotting time (CT) was recorded.
Results: TFTEM CT reference range from 20 normal donors was 104.5 to 161 sec. The turn‐around time to detect apixaban plasma concentrations between 12 and 500 ng/ml was less than 20 min, with an excellent linear correlation (r = 0.9925). No interference with up to 2 IU/ml of unfractionated heparin occurred. Further data are shown in Figures 1 and 2.
Conclusion(s): The TFTEM assay provides quick results and is sensitive to even low apixaban plasma concentrations (12 ng/ml) and shows an excellent linear correlation to concentrations up to at least 481 ng/ml. TFTEM may be a valuable tool to detect and differentiate DO‐ACs in combination with other ROTEM assays.


PB1093
Diagnostic potential of rapid automated ADAMTS13 inhibitor screening and quantification
G. Moore 1; M. Griffiths2; N. Binder2
1 Cambridge University Hospitals, Bexley, England, United Kingdom; 2 Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH, Vienna, Wien, Austria
Background: Acquired ADAMTS13 deficiency due to inhibitory or clearing antibodies leads to thrombotic thrombocytopenic purpura (TTP). Rapid discrimination of TTP from similar disorders using laboratory assays improves clinical outcomes.
Aims: Evaluate rapid screening and quantification assays for inhibitory ADAMTS13 antibodies.
Methods: Technofluour ADAMTS13 FRET Activity (Technoclone) was performed on a Ceveron s100 (Technoclone) automate. The screen assayed ADAMTS13 activity on a 1:1 mixture of index:normal pooled plasma (NPP) to calculate the ADAMTS13 Inhibitor Mix (AIM) Ratio; [mix activity/(activity of 1:1 mix of NPP + ADAMTS13 depleted plasma)]. Instead of merely assessing if a mixing test returns to normal or not, which is not always reliable, AIM Ratio is a quantitative value derived from the mix result as a function of the measured activity of the NPP in which it is mixed, interpreted against an objective cut‐off. The FRET assay was used in a 30 min incubation Bethesda assay using heat treated index plasma, to quantify inhibitory antibodies. Results were compared to Technozym ADAMTS13 inhibitor ELISA (Technoclone), detecting both inhibitor types but not discriminating, and a Bethesda with Technozym ADAMTS13 activity ELISA (Technoclone). Four plasmas were tested with FRET‐based Bethesda assays at different incubation times.
Results: Table 1 shows comparative results from 13 acquired TTPs. Patients 4/10/12 had clearing antibodies and were thus AIM Ratio‐ and Bethesda‐negative. Correlations for FRET/ELISA Bethesdas, FRET Bethesda/ELISA Inhibitor, and FRET Bethesda/AIM Ratio were Pearson R 0.993/0.710/−0.870 respectively. Patients 2/13 had low Bethesdas relative to ELISA, suggesting presence of clearing and inhibitory antibodies. Patient 11 had borderline ELISA, negative Bethesdas, but weak‐positive AIM Ratio, suggesting the latter detects low titre inhibitory antibodies. The Bethesda incubation experiment suggests prolonged incubation is unnecessary (Table 2).
Conclusion(s): Fully automated ADAMTS13 activity and AIM Ratio assayed simultaneously provide immediate distinction between hereditary and acquired TTP through inhibitory antibodies. Prolonged incubation for subsequent Bethesdas is unnecessary.


PB1100
Vaccine induced immune thrombocytopenia and thrombosis (VITT) external quality assessment (EQA) scheme results and interpretations from a joint UKNEQAS BC, SSC and ECAT VITT Survey May 2021
C. Reilly‐Stitt 1; A. Lowe1; I. Jennings2; S. Kitchen3; P. Meijer4; M. de Maat5; T. Backchoul6; I. Walker1
1 UK NEQAS Blood Coagulation, SHEFFIELD, England, United Kingdom; 2 UK NEQAS BC, Sheffield, England, United Kingdom, 3 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 4 ECAT Foundation (External quality Control for Assays and Tests), Voorschoten, The Netherlands, Voorschoten, Zuid‐Holland, Netherlands; 5 Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: During the early part of 2021 cases of VITT a clinical phenomenon similar to Heparin Induced Thrombocytopenia (HIT) were encountered across Europe following the roll out of the Astra Zeneca ChAdOx1 vaccine.
Aims: Assays used for the diagnosis of HIT were available to help with the differential diagnosis of these two very similar conditions. In May 2021 UKNEQAS BC and ECAT sent samples from patients with a clinical diagnosis of VITT and sample from a healthy subject for HIT assays.
Methods: Lyophilized plasma samples for this exercise were distributed to 500 centres. The samples distributed from 4 subjects included: 3 samples from 1 patient with a clinical diagnosis of VITT (VITT01‐VITT 03)and one each from2 further patients with a diagnosis of VITT (VITT 04 and VITT05) for assay sensitivity and a sample from a healthy unvaccinated subject (VITT06)for specificity.
Results: A total of 485 returns were received. Table 1 is a summary of the results for sensitivity and specificity. The modifications to functional assays included different concentrations of PF4 and both low molecular weight and unfractionated heparin as co‐factors to serotonin release assays; heparin induced platelet aggregation assays and platelet aggregation assays.
Conclusion(s): From this study of HIT assay methods available the ELISA assays have better sensitivity compared to the recommended functional assays for diagnosis of VITT in our cases, even with functional assays specifically modified for detection of VITT. Non‐ELISA assays are unsuitable and should not be used for the diagnosis of VITT. Recommendations for VITT diagnosis and laboratory assays to employ recommend functional assays however the data captured in this exercise suggests that the guidance could be updated with reference to modifications of functional assays which may have been originally validated for HIT diagnosis.

PB1057
A novel, easy‐to‐use ADAMTS13 activity assay using a fiber‐optic surface plasmon resonance biosensor for diagnosis of thrombotic thrombocytopenic purpura
Q. Bonnez 1; E. Tellier2; G. Kaplanski3; C. Tersteeg4; S. De Meyer4; K. Vanhoorelbeke4
1 Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Kortrijk, West‐Vlaanderen, Belgium; 2 Aix Marseille University, INSERM 1263, INRAE, C2VN, Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Aix‐Marseille Univ, APHM, INSERM, INRAE, C2VN, Department of Internal Medicine and Clinical Immunology, University Hospital La Conception, Marseille, France., Marseille, Provence‐Alpes‐Cote d'Azur, France; 4 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium
Background: Determination of ADAMTS13 activity is crucial to differentiate thrombotic thrombocytopenic purpura (TTP) from other thrombotic microangiopathies. Current ADAMTS13 activity assays are complex and only performed in specialized laboratories, hampering fast diagnosis in non‐specialized centers. Hence, there is an unmet need to develop easy‐to‐use ADAMTS13 activity assays to allow swift diagnosis of TTP in both reference and non‐reference centers.
Aims: Develop an ADAMTS13 activity assay on the easy‐to‐use fiber‐optic surface plasmon resonance (FO‐SPR) biosensor.
Methods: Anti‐GST antibody was immobilized to gold‐coated optical fibers using EDC/NHS chemistry. After blocking, recombinant GST‐VWF73 was added. Addition of plasma ADAMTS13 triggers the cleavage of the Tyr‐Met bond within GST‐VWF73 after which the resulting decapeptide (DREQAPNLVY) is detected by mouse anti‐N10 antibody followed by signal amplification using goat anti‐mouse IgG‐labeled gold nanoparticles. Detection limit was determined by evaluating ADAMTS13 activity of heat‐inactivated plasma. The assay was validated by measuring ADAMTS13 activity of healthy donor (HD), acute TTP, sepsis and hemolytic uremic syndrome (HUS) patient samples and compared to ADAMTS13 activity determined in the FRETS‐VWF73 assay.
Results: By optimizing anti‐GST antibody, GST‐VWF73 and anti‐N10 detection antibody concentrations and selecting optimal buffer conditions, an FO‐SPR ADAMTS13 activity assay was developed. A calibration curve was established using pooled normal human plasma. The assay detection limit was 6.8% (%CV = 9.51). ADAMTS13 activity was undetectable in all TTP samples and above 10% in HD (51.83%–81.04%), HUS (27.91%–107.24%) and sepsis (14.18%–44.25%) samples. Significant correlation (Pearson r = 0.8544, p < 0.0001) was found between ADAMTS13 activity determined in both FO‐SPR and FRETS‐VWF73 assays.
Conclusion(s): An ADAMTS13 activity assay was successfully developed on the novel, easy‐to‐use FO‐SPR biosensor, allowing TTP patient discrimination in about 3 hours. Previously, ADAMTS13 antigen, conformation and anti‐ADAMTS13 autoantibody assays were developed on the FO‐SPR platform. Hence, simultaneous testing of all ADAMTS13 parameters is now available on the FO‐SPR biosensor which can ameliorate TTP diagnosis.
PB1072
Evaluation of the ultrasound sonorheometry based Quantra® System to monitor hemostasis in patients undergoing plasma exchange therapy
C. Wahl1; C. Cornillot2; M. Maison1; L. Lhotellier1; K. Yangaju1; M. Dalibard3; L. Macraigne1; V. Lopez1; A. Chhing1; C. Frere 4; S. Saheb1
1 Assistance Publique Hôpitaux de Paris, Paris, Ile‐de‐France, France; 2 Stago Biocare, Asnieres sur Seine, Ile‐de‐France, France, 3 a, Paris, Ile‐de‐France, France; 4 Sorbonne Université, Paris, Ile‐de‐France, France
Background: Plasma exchange therapy (PET) with albumin replacement results in coagulation factors and platelets depletion. A close and timely monitoring of hemostasis is therefore warranted before PET, particularly in patients at risk of bleeding or those planned to receive large volume exchanges. The Quantra® system is a coagulation POC device based on Sonic Estimation of Elasticity via Resonance (SEER) Sonorheometry equipped with a disposable QPlus® 4‐channel cartridge that performs 4 independent measurements with different combination of reagents. The output test results include Clot Time (CT), Clot Stiffness (CS), Fibrinogen Contribution to Clot Stiffness (FCS) and Platelet Contribution to Clot Stiffness (PCS).
Aims: To compare the Quantra® QPlus® parameters to routine laboratory parameters for near‐POC monitoring of hemostasis in patients undergoing PET.
Methods: This prospective, single‐center, observational study was conducted in the apheresis unit of a tertiary care center hospital from December 2021 to January 2022. Blood sampling was performed just before PET. SEER Sonorheometry was performed on a Quantra® analyzer with the QPlus® Cartridge (Stago BioCare, Asnières, France). Activated Partial Thromboplastin Time (aPTT) and functional fibrinogen level were determined on a STA‐R Max analyzer using commercially available reagents (Stago, Asnières, France). Platelet count was performed on a XN 9100 analyzer using the Cellpack reagent (Sysmex, Villepinte, France). Statistical analysis was performed using GraphPad Prism version 8.4.2 (GraphPad Software, La Jolla California USA).
Results: 33 patients were prospectively enrolled in the study. Significant correlations were observed between CT and aPTT (r = 0.62, p = 0.001), FCS and fibrinogen levels (r = 0.85, p < 0.0001), CS and fibrinogen levels (r = 0.85, p < 0.0001), and PCS and platelet count (r = 0.71, p < 0.0001). Result delivery was much faster with the Quantra® analyzer compared to conventional tests (mean time 35 vs. 74 min, p < 0.0001).
Conclusion(s): SEER sonorheometry demonstrates significant correlations with routine laboratory tests and provided prompt information to manage hemostasis in patients undergoing PET.
PB1075
Discrepant results for ADAMTS13 assays with different activity assays. Artefact or cause for concern?
I. Jennings 1; C. Reilly‐Stitt1; D. Kitchen2; A. Lowe1; S. Kitchen3; I. Walker1
1 UK NEQAS Blood Coagulation, SHEFFIELD, England, United Kingdom; 2 UK NEQAS Blood Coagulation, Sheffield, England, United Kingdom, 3 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom
Background: ADAMTS13 is a plasma metalloprotease which regulates the multimeric size of VWF by cleaving the protein at Tyr1605‐Met1606. ADAMTS13 deficiency (acquired or congenital) occurs in thrombotic thrombocytopenic purpura (TTP). This disease primarily requires a clinical diagnosis, but ADAMTS13 assays can provide confirmation, help characterise the phenotype of the deficiency, may be useful in monitoring treatment, and can be used to identify family members with ADAMTS13 deficiency.
Aims: Different assay methodologies are available for measurement of ADAMTS13 activity, which may give rise to differences in levels of ADAMTS13 depending on method used. Proficiency testing exercises may help identify differences in accuracy and precision of these methods.
Methods: We describe here data from a series of proficiency testing exercises for ADAMTS13 assays in 2020 and 2021, in which an average 25 UK and International centres performed assays on lyophilised plasma samples. All samples were obtained through plasma exchange from patients with suspected or confirmed TTP. Samples 2103 and 2104 were prepared from plasma from a patient with acquired TTP, to which normal plasma had been added.
Results: Results from 8 samples are shown in table 1. In samples with normal levels of ADAMTS13 activity, there was good agreement between results obtained by FRET, ELISA and chemiluminescence immunoassays (CLIA). For samples from donors with acquired TTP, and low levels of ADAMTS13 activity, results obtained by FRET‐based assay methods were significantly higher than those obtained by ELISA and CLIA methods.
Conclusion(s): It is unclear whether these findings are a consequence of plasma collection following plasma exchange, sample processing, related to presence of ADAMTS13 antibodies in the samples, or reflect a genuine difference in results obtained with these different methods. Further studies are required to elucidate the clinical relevance of these findings.

PB1068
Dilute thrombin time versus activated partial thromboplastin time to monitor Argatroban in critically ill patients
S. Cox‐Morton; D. White; E. Duff; M. Sharp; C. Bridgeman; M. Kirk; C. Moore; M. Besser; M. Robinson; E. Symington; W. Thomas; S. MacDonald
Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom
Background: Argatroban is a direct thrombin inhibitor currently licenced in the UK and USA for treatment of Heparin Induced Thrombocytopenia (HIT) and has been utilised as an alternative anticoagulant for critically ill COVID‐19 patients. UK and US guidelines recommend Argatroban monitoring via activated partial thromboplastin time (aPTT), in critically ill patients, recommending ratios of between 1.5–3 times patient’s baseline, without exceeding 100 s. This guidance is based on spiked pooled platelet poor plasma with increasing concentrations of Argatroban.
Aims: • Does the aPTT demonstrate suitable linearity for Argatroban monitoring? • Are readily available alternatives more useful to indicate extent of Argatroban anticoagulation?
Methods: From May to July 2021, 97 residual blood samples from 12 patients receiving Argatroban were processed for HemosIL APTT‐SS, Thrombin time, Argatroban, and dilute thrombin time using ACL‐TOP750 (Werfen, Bedford, USA). Performance was compared to commercial calibrators, across a therapeutic range from 0–2.08 μg/ml mimicking spiked plasma.
Results: • No linearity was observed between Argatroban concentration and aPTT ratio (R 2 value of 0.0778) in critically ill patient plasma compared to linearity for APTT ratios (R 2 value of 0.9346) in commercial calibrators • Thrombin time produced good linearity with Argatroban (R 2 value of 0.8473). Non‐specific clot curve kinetic issues and exceeding acquisition times were noted. • Dilute thrombin time produced very good linearity with Argatroban (R 2 value of 0.9443)
Conclusion(s): In critically ill patients, aPTT ratio lacks a demonstrable linear response, unlike spiked and commercial plasmas. Non linearity is most likely due to other effects on the aPTT than Argatroban alone. 10.3% of patients exceeded an Argatroban concentration of 2.08 μg/ml with an aPTT ratio within guideline quoted ranges. Thrombin time and dTT demonstrated linearity. However, the thrombin time had non‐specific clot curve kinetic abnormalities and some results exceeded acquisition time. No issues were seen with dilute thrombin time.


PB1076
Clinical utility of ISTH BAT and Thromboelastography in assessment of patients referred for evaluation of bleeding disorders
P. Kafley 1; S. Nair2; R. V3
1 Sikkim Manipal Institute of Medical sciences, East Sikkim, Sikkim, India; 2 Christian Medical College and Hospital, Vellore, Vellore, Tamil Nadu, India; 3 CMC Vellore, Vellore, Tamil Nadu, India
Background: Diagnosis of bleeding disorders still remains a challenge as bleeding is a symptom experienced by the healthy and diseased alike. A properly taken history is still considered the most important tool. Different bleeding assessment tools with standardized, guided questions have been devised, ISTH BAT is one such tool. The standard tests of coagulation are performed on platelet poor plasma which negates the contribution of red blood cells, platelets to hemostasis. A need for a test that simulates the steps of hemostasis invitro was long felt and the advent of Thromboelastography has addressed some of the issues mentioned. In our study we have seen the utility of these two tools in assessments of patients with bleeding disorders.
Aims: To determine the clinical utility of ISTH BAT and Thromboelastography in assessment of patients referred for evaluation of bleeding disorders.
Methods: A total of 223 patients who were referred for evaluation of bleeding disorders were enrolled into the study. ISTH BAT was administered in each patient and the final score was calculated. Thromboelastography(TEG) and other tests of coagulation was performed in each sample per protocol and the results were analyzed. Sensitivity, Specificity, Negative and Positive predictive value (NPV, PPV) were calculated for ISTH BAT bleeding score, TEG separately and taken together.
Results: Sensitivity, specificity, PPV, NPV for ISTH BAT were 92%, 63.3%, 75.9% and 86.4% respectively while for TEG it was 86%, 85.7%, 89.7% and 80.9% respectively. When both these modalities were taken together sensitivity, specificity, PPV, NPV was 97.5%, 56.6%, 76.3% and 94.4%.
Conclusion(s): Diagnosis of bleeding disorders is challenging and there are no tests that are considered as gold standards. As a result the tests performed are expensive and time consuming. ISTH BAT and TEG may serve as an effective screening tool in resource constrained setting owing to their high sensitivity and negative predictive value.
PB1103
Open‐Source robotic microfluidic blood analysis using Raspberry Pi
R. Sariyer 1; D. Hodge2; S. Needs1; N. Reis3; C. Jones4; A. Edwards1
1 Reading School of Pharmacy, University of Reading, Whiteknights, Reading, United Kingdom, Reading, England, United Kingdom; 2 Reading School of Biological Sciences, University of Reading, Whiteknights, Reading, United Kingdom, Reading, England, United Kingdom; 3 Department of Chemical Engineering, University of Bath, Claverton Down, Bath, United Kingdom, Bath, England, United Kingdom; 4 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: The rapid increase in the use of open‐source enables the development of new inexpensive, highly configurable, high‐performance haematological tests. We have described a low cost (< £300), open‐source, and customizable robotic microfluidic device for use in microfluidic blood analysis. Teflon® FEP microcapillary film (MCF) contains 10 parallel capillaries of 200μm diameter, which can be coated with platelet agonists or pro‐coagulant factors.
Aims: When dipped into whole blood (WB), the WB is drawn into the MCF capillaries. Changes in capillary rise can be used to measure platelet activation or coagulation.
Methods: We have automated the MCF dipping into WB and image capture to perform time‐resolved image analysis. The open‐source hardware used a servo motor, a camera, and a lightbox, controlled by the Raspberry Pi, as well as 3D printed parts held in a frame, that allows simultaneous measurement of capillary rise in 12 MCF strips totalling 120 individual capillaries and 180 images per capillary in 30 s.
Results: We obtained kinetic data of platelet activation and blood coagulation. Preliminary results show that WB rose rapidly up the capillaries in the first 8 seconds, but slowed and almost stopped at 30 seconds. The rate of rise was reduced in thrombin coated capillaries in a concentration dependant manner. The mean velocity of WB in the first 8 seconds was 0.25, 0.30, 0.37 and 0.40 cm/s for coating concentrations of 500, 158, 50 and 0 U/ml thrombin, respectively (p < 0.001).
Conclusion(s): Capturing high quality and time‐lapse images of capillary rise of blood in the capillaries enabled us to quantify haemostatic changes in WB. The simplicity of the design makes it suitable for adapting to different experimental needs and its open‐source‐based allows it to be easily accessed by other researchers. This low‐cost, high throughput imaging rig is a proof‐of‐concept demonstrating the potential for using open‐source hardware in platelet research.
PB1084
Comparability of anti‐Xa assessments using alternate calibration curves in patients receiving rivaroxaban and apixaban therapy
E. Leitinger; J. Clifford; W. Andrew; S. Chunilal
Monash Health, Melbourne, Victoria, Australia
Background: Oral anti‐factor Xa (anti‐Xa) inhibitors are widely used. Routine coagulation tests cannot exclude clinically significant drug activity, therefore an anti‐Xa assay using a drug‐specific calibrator is required. In the emergency setting, determining the specific drug can be challenging, however, most centres can perform a standard anti‐Xa assay with a low molecular weight heparin (LMWH) calibrator. While clinical data are limited, a rivaroxaban or apixaban level below 50 ng/ml appears safe for most procedures. Therefore establishing an anti‐Xa level with a LMWH calibrator that corresponds to a rivaroxaban or apixaban level below 50 ng/ml would be helpful.
Aims: 1. To measure the correlation between rivaroxaban and apixaban levels using drug‐specific and LMWH calibrators. 2. To establish the anti‐Xa level with a LMWH calibrator that correlates with trough levels of apixaban and rivaroxaban (<50 ng/ml).
Methods: 60 frozen samples from patients on rivaroxaban or apixaban (30 per drug) were selected and run in duplicate. Each 30 samples comprised: 10 samples with anti‐Xa levels 20‐100 ng/ml, 10 samples 100–200 ng/ml, 10 samples 200–300 ng/ml, and 2 data points at 0. They were analysed with a drug‐specific, and LMWH calibrator, using a one stage chromogenic assay (ACL TOP analyser, IL Liquid Anti‐Xa kit reagent). The mean anti‐Xa activity was determined for each sample, and a line of best fit established for each drug both separately and combined.
Results: We confirmed a strong, statistically significant correlation with apixaban and rivaroxaban drug levels using both calibrators (Table 1), and established a combined correlation curve (Figure 1). From this data, an anti‐Xa level of 0.35 U/ml corresponds to a drug level of 50 ng/ml.
Conclusion(s): There is a strong correlation between anti‐Xa levels for rivaroxaban or apixaban using a LMWH calibrator. This can establish an anti‐Xa cut‐off to exclude significant levels of either drug.


PB1111
A novel prothrombin R533Q variant in patient with recurrent venous thrombosis
C. Van Laer 1; K. Freson2; R. Lavend'homme2; V. Muczynski3; K. Peerlinck4; C. Thys2; M. Jacquemin5
1 Center for Molecular and Vascular Biology, University of Leuven; Clinical Department of Laboratory Medicine, University Hospitals Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 2 Center for Molecular and Vascular Biology, University of Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 3 Research Department of Haematology, University College London (UCL) – Cancer Institute, London, United‐Kingdom, London, England, United Kingdom; 4 University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 5 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium; Department of Vascular Medicine and Hemostasis, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium
Background: Specific genetic variants in F2 gene coding for prothrombin are known to increase risk for venous thrombosis (VT). The most common is prothrombin c.G20210A UTR variant. More recently heterozygous gain‐of‐function missense variants Arg596Leu/Gln/Trp have been described in patients with VT by causing increased resistance towards antithrombin [Girolami et al, 2018].
Aims: We studied the potential prothrombotic effect of a novel heterozygous F2 variant Arg533Gln (R533Q) detected in a patient with recurrent VT.
Methods: A 66‐year old male with recurrent pulmonary embolism and thrombophlebitis underwent screening using ThromboGenomics gene panel test [Downes et al, 2019]. This resulted in the detection of the common F2 c.G20210A variant and a second novel F2 variant R533Q. His 39‐year old daughter only carries the R533Q variant and has no history of thrombosis. Recombinant FII (rFII) wild type (WT) and R533Q proteins were produced in HEK Expi293TM cells. Thrombin generation assays (TGA) were performed with plasma samples treated with DOAC‐StopTM and with different dilutions of rFII in FII deficient plasma.
Results: The index patient and his daughter had normal FII activity levels of 96% and 100%, respectively. Plasma samples from both showed higher thrombin generation in comparison to a control plasma sample, which was however more pronounced for the index. TGA with rFII R533Q using a concentration of 100% FII activity showed a higher ETP, peak value and velocity index of 26%, 43% and 25%, respectively, compared to adding the same concentration of rFII WT (figure 1). Different tests such as a FII activation assay by the prothrombinase complex, activation of FVIII by rFIIa and ecarin‐based thrombin activity with rFII were carried out. None of these assays could explain the mechanism of the increased thrombin formation by the mutant.
Conclusion(s): The novel F2 R533Q variant is able to generate higher thrombin levels, which can be a risk factor for thrombosis.

PB1110
Buffy‐coat‐derived megakaryocytes for monoclonal antibody immobilization of platelet antigens (MAIMA) assay
G. Uzun 1; J. Lucic2; K. Althaus3; F. Rigoni4; F. Lyshy1; S. Nowak‐Harnau1; I. Marini4; U. Sachs5; T. Backchoul6
1 Center for Clinical Transfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 2 Department of Transfusion Medicine, Cellular Therapeutics and Hemostaseology, Clinic for Anesthesiology LMU University Hospital, Munich, Baden‐Wurttemberg, Germany; 3 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 4 Institute for Clinical and Experimental Tranfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 5 Institute for Clinical Immunology and Transfusion Medicine, Justus Liebig University, Giesen, Niedersachsen, Germany; 6 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Serologic detection and characterization of anti‐human platelet antigen (HPA) antibodies are essential for proper diagnosis and treatment in fetal and neonatal immune thrombocytopenia. The monoclonal antibody immobilization of platelet antigens (MAIPA) assay is the standard assay for the detection of anti‐HPA antibodies. For this assay, fresh platelets from HPA‐genotyped donors are required, which is a limitation for laboratories with no direct access to blood donors.
Aims: In the current study, the use of buffy coat‐derived, in‐vitro cultured megakaryocytes (MKs) instead of donor‐derived platelets in MAIPA for the determination of anti‐HPA antibodies was investigated.
Methods: We isolated CD34+ cells from buffy coats of blood donors using magnetic beads. CD34+ cells were differentiated for 28 days in vitro into megakaryocytes. Megakaryocyte cell lines were characterized with flow cytometry using CD41, CD61, CD42a and CD42d expression and DNA content. The performance of MAIPA with megakaryocytes was assessed using WHO reference sera und patients’ sera with well‐characterized anti‐HPA antibodies.
Results: Testing WHO standard sera in MAIPA with megakaryocytes (MAIMA) revealed similar reaction compared to standard MAIPA using platelets (OD HPA 1a:0.51 ± 0.13 vs. 0.52 ± 0.20, p = 0.99 and OD for HPA5b (0.15 ± 0.09 vs. 0.25 ± 0.07, p = 0.40, Figure 1). However, OD amplitudes were significantly lower in MAIMA than in MAIPA with donor platelets for HPA 3a (0.19 ± 0.03 vs. 0.53 ± 0.07, p = 0.02, Figure 1). OD amplitudes were similar with megakaryocytes and platelets in MAIPA using patients’ sera with anti‐HPA‐1a and anti‐HPA 5b antibodies (Figure 2).
Conclusion(s): Buffy‐coat‐derived megakaryocytes have the potential to replace donor platelets in MAIPA in the future. Further studies are needed for standardization and validation of this technique in routine laboratories.


PB1113
A method for measuring chromogenic factor VIII activity unbiased by the presence of the bispecific antibody Emicizumab
A. Weber; M. Billwein; A. Engelmaier; C. Zlabinger; G. Schrenk
Baxalta Innovations GmbH, Takeda group, Vienna, Wien, Austria
Background: Despite the administration of the bispecific antibody Emicizumab (Hemlibra) bleeding episodes can occur in Hemophilia A patients without inhibitors requiring the administration of factor FVIII (FVIII) concentrates to restore hemostasis. This could require the FVIII activity measurement, being achieved even in the presence of Emicizumab by using a chromogenic FVIII activity assay with bovine reagents.
Aims: Here, we report on another approach for measuring chromogenic FVIII activity in the presence of Emicizumab. Our method is based on the selective capture of FVIII by a monoclonal antibody so that accompanying sample matrix compounds including Emicizumab can be removed before running the chromonic assay.
Methods: Monoclonal anti FVIII IgG1 (GMA‐8024, Green Mountain Antibodies) specifically captured human FVIII, which was then measured with a chromogenic activity assay (Technoclone). A human plasma pool was spiked with therapeutically relevant Emicizumab concentrations (60, 200, and 600 nM) to determine any influence on the FVIII measurement.
Results: The assay calibration curve ranged from 3.03 to 97.0 mIU/ml. Increasing concentrations (60, 200 and 600 nM, representing 9 to 90 μg/ml) of Emicizumab added to human normal plasma did not influence the measured FVIII activity. FVIII recoveries of 98.8%, 104.4% and 101.7% rather reflect assay variability than an influence of Emicizumab. The dose‐response curves obtained were parallel to that of human plasma. In line with the assay principle, Emicizumab, measured in test buffer, essentially provided no signal.
Conclusion(s): The sequential combination of the antibody‐mediated capture of human FVIII and a chromogenic FVIII activity assay permits the measurement of FVIII activities in the presence of therapeutically relevant Emicizumab levels. Due to the assay principle, this approach is expected to work also for novel FVIII activity‐mimicking compounds, probably demonstrating no specific binding to human coagulation factors.


VPB1121
Application of next‐generation sequencing in the study of inherited platelet disorders
G. Ramil López; I. Tirado; L. Romero; N. Vilalta
Hospital de la Santa Creu i Sant Pau, Barcelona, Catalonia, Spain
Background: Inherited platelet disorders (IPDs) are a heterogeneous group of diseases where genetic alterations cause platelet malfunction and lead to a hemorrhagic tendency. Next‐generation sequencing (NGS) arises as an interesting diagnostic tool for these entities whose phenotypes are often ambiguous, allowing simultaneous analysis of multiple candidate genes.
Aims: To assess the utility of NGS in the diagnosis of IPDs.
Methods: Forty‐one patients with suspected IPDs were selected between 2019 and 2021 based on platelet function test findings. NGS was performed using a custom panel (Nonacus) that included 83 genes associated to diverse hemostatic disorders (Table 1) chosen according to current published evidence. Sequencing results were processed using Datagenomics platform (Imegen). Variants were classified following to ACMG criteria. Pathogenic variants were confirmed with Sanger sequencing.
Results: Our panel detected 17 variants in 10 genes of 17 patients (Table 2), 11 of them were classified as pathogenic, 2 likely pathogenic and 4 variants of uncertain significance (VUS). No pathogenic variants were found in 24 patients. NGS served as a confirmatory tool in 4 patients: 2 cases of Glanzmann thrombasthenia and 2 cases of Bernard Soulier Syndrome with variants affecting ITGA2B (GT) and GP1BA and GP9 (BSS). Other 2 suspected cases of GT carried variants in GP1BA classified as likely pathogenic. In 7 patients where functional studies were inconclusive, our panel detected pathogenic variants in NBEAL2, HPS1, MYH9 and TUBB1 and provided a diagnostic orientation. Four VUS were found in GP1BA, TBXAS and TBXA2R genes. Further studies are needed to elucidate their role in the pathogenesis of IPDs.
Conclusion(s): NGS is a feasible diagnostic test for IPDs. Our data reflect the importance of combining functional and genetic studies to provide an accurate diagnosis. Interpretation of genetic variants, especially VUS, can be challenging and functional protein models are necessary to determine their clinical significance.


PB1081
Optimizing variant curation guidelines to improve clinical genetic testing and diagnosis of von Willebrand disease
K. Lee 1; J. Ross1; P. Christopherson2; I. Corrales3; K. Desch4; J. Eikenboom5; K. Friedman6; I. Futchi1; W. Hankey1; M. Springer2; D. Hampshire7; J. Johnsen8; D. Lillicrap9
1 University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States; 2 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 3 Banc de Sang i Teixits; Vall d'Hebron Research Institute, Universitat Autònoma de Barcelona (VHIR‐UAB), Barcelona, Catalonia, Spain; 4 University of Michigan, Ann Arbor, Michigan, United States; 5 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 6 Versiti/Blood Center of Wisconsin, Milwaukee, Wisconsin, United States; 7 University of Hull, Hull, England, United Kingdom; 8 University of Washington; Bloodworks Northwest, Seattle, Washington, United States; 9 Department of Pathology and Molecular Medicine, Queen’s University, Kingston, Ontario, Canada
Background: Genetic testing can be pivotal to accurately diagnosing von Willebrand disease (VWD) to develop appropriate clinical management recommendations. Limiting the number of variants of uncertain significance (VUS) and increasing the number of rigorously curated variants in public databases is vitally important to ensure that all patients receive optimal care.
Aims: The American College of Medical Genetics and Association for Molecular Pathology (ACMG/AMP) developed a framework to assess variant pathogenicity; however, the guidelines were broadly written. The Clinical Genome Resource (ClinGen), funded by the National Human Genome Research Institute, convened the von Willebrand Disease Variant Curation Expert Panel (VWD VCEP) in June 2019 to develop rule specifications for curating variants in the VWF gene through the ClinGen FDA‐approved process.
Methods: The VWD VCEP members meet monthly to specify ACMG‐AMP rules for the VWF gene and discuss resulting variant assertions. Current work is focused on type 2 VWD (types 2A; 2B; 2M and 2N), and rules for types 1 and 3 will follow the completion of these rules. Each rule was assessed for appropriateness for the VWF gene and whether adding detailed criteria for its use and/or modifying its strength based on availability of evidence would be of benefit.
Results: Variant curation specifications were applied to 20 ACMG‐AMP rule criteria and 8 rules were deemed not applicable to the VWF gene. Criteria modifications included setting allele frequencies in control populations, defining phenotype criteria and conditions for adjusting rule strength. Fifteen VWF variants were chosen for a pilot study to test the rule specifications, comprising of 6 pathogenic/likely pathogenic, 6 VUS and 3 benign/likely benign variants.
Conclusion(s): Results of this effort will aid clinical variant interpretation of the VWF gene and help standardize variant curations across clinical laboratories. Expert level variant curation from this VCEP will be deposited in the ClinVar database for public access.
Nanotechnology and Novel Biomolecules
PB0163
In vitro monitoring of anti‐coagulation therapy in whole blood using magnetic nanoparticles and susceptometry
A. Harper1; A. Santana‐Otero2; N. Telling1; D. Ortega3; D. Cabrera 1
1 Keele University, Stoke on Trent, England, United Kingdom; 2 IMDEA Nanoscience, Madrid, Madrid, Spain; 3 University of Cádiz, Cádiz, Andalucia, Spain
Background: Alternating Current (AC) susceptometers measure an intrinsic property of magnetic nanoparticles called imaginary magnetic susceptibility (χ”). Imaginary susceptibility reveals variations in mobility and agglomeration of magnetic nanoparticles when these are dispersed in biological tissues such as human blood. By labelling human blood with magnetic nanoparticles, magnetic susceptometry could be used to measure blood coagulation, clot compaction and changes in internal viscosity of clots in real‐time.
Aims: To assess whether AC susceptometry can be used as a measure of whole blood coagulation and to assess the effect of anti‐coagulant drugs in altering this response in vitro.
Methods: AC susceptibility measurements were performed in citrated whole human blood, labelled with iron oxide nanoparticles (IONPs) coated with sucrose, and pre‐treated with different concentrations of bivalirudin, heparin or their carriers. After recalcification and stimulation with 125 μM adenosine diphosphate, susceptibility measurements of the blood samples were performed every 70 s for 22‐min at 400 Hz.
Results: AC susceptibility measurements in control samples (Figure 1AB‐Black dots) showed a decrease in the imaginary magnetic susceptibility χ” over time, reaching a slowly‐declining baseline within 5 minutes of stimulation. These observations are consistent with a rapidly diminishing mobility of IONPs over time due to blood coagulation and clot compaction. In contrast, the rapid fall in imaginary susceptibility over time seen in the control samples is significantly slowed in a dose‐dependent manner when blood is pre‐treated with bivalirudin (Figure 1A) and heparin (Figure 1B). This is consistent with reduced immobilisation of the IONPs within the clot due to the slowing of blood coagulation.
Conclusion(s): AC susceptometry measurements could provide an improved method to monitor blood coagulation and changes in the physical structure of blood clots. This could result in novel susceptibility‐based assays for diagnosis of bleeding disorders and monitoring efficacy of anti‐thrombotic drugs in patients.

PB0164
Anti‐inflammatory effect of archaeolipid nanovesicles on human endothelial cells
N. Charo 1; H. Jerez2; S. Tatti3; E. Romero4; M. Schattner5
1 Institute of Experimental Medicine, National Academy of Medicine‐CONICET, CABA, Buenos Aires, Argentina; 2 Center for Research and Development in Nanomedicines (CIDEN), National University of Quilmes, GBA, Buenos Aires, Argentina; 3 Department of Obstetrics and Gynecology, Clinical Hospital, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 4 Center for Research and Development in Nanomedicines (CIDEN), National University of Quilmes, La Plata, Buenos Aires, Argentina; 5 Institute of Experimental Medicine, caba, Buenos Aires, Argentina
Background: New generation of liposomes called nanoarchaeosomes (ARC) are nanovesicles (NVs) made of archaeolipids employed as drug delivery systems that exhibit high structural stability. So far there are no reports on the effects of ARC on human vascular endothelium, data crucial for their eventual clinical use.
Aims: The present study investigated the effects of ARC on human umbilical vein endothelial cells (HUVEC) in physiological conditions and under inflammatory conditions and compared it with conventional liposomes (LIP).
Methods: HUVEC were non‐stimulated or stimulated with LPS, Pam3CSK4, E. coli, TNF‐α or IL‐1β in the absence or presence of nanovesicles. We analyzed cell viability and apoptosis (acridine orange/ethidium bromide), cell proliferation (EdU assay), expression of ICAM‐1 and E‐selectin (cytometry), secretion of IL‐6 and von Willebrand Factor (vWF) (ELISA), mRNA expression of ICAM‐1 and vWF (RT‐qPCR), uptake of NVs (cytometry and confocal microscopy) and signaling pathways (cytometry and western blot). Results were analyzed by ANOVA followed by Bonferroni test (p < 0.05, n = 4–6).
Results: None of the NVs affected the viability, cell proliferation and expression of ICAM‐1 and E‐selectin under basal conditions, but ARC reduced the expression of both molecules and the secretion of IL‐6 induced by LPS, Pam3CSK4 or E. coli, an effect not observed with TNF‐α or IL‐1 beta (Figure 1). Interestingly, ARC significantly decreased basal vWF levels and those induced by all stimuli (Figure 1). None of these parameters were modified by the liposomes. Only ARC were endocytosed by HUVEC and reduced mRNA expression of ICAM‐1 and vWF via NF‐kB and ERK1/2 in LPS‐stimulated endothelial cells (Figure 2).
Conclusion(s): This is the first report on the anti‐inflammatory and anti‐thrombotic effect of ARC on endothelial cells. Loaded with anti‐inflammatory drugs, ARC could magnify their activity on inflamed endothelium, and therefore their research in vasculopathies is of special interest.


PB0165
Protein S Gla detects and attenuates early vascular calcification
A. Gentier 1; S. Agten2; L. Schurgers3; T. Hackeng3
1 Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 2 Department of BIochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 3 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands
Background: Early‐stage calcification (microcalcification) progresses atherosclerosis and is often related to severe pathological outcomes such as myocardial infarction and stroke. The danger of microcalcification is two‐fold in that it is undetectable with conventional imaging modalities, and no treatment currently exists to prevent or reduce it. An interesting source for development of new diagnostics and therapeutics against ectopic calcification is vitamin K‐dependent Gla‐domain of coagulation factors. These domains confer calcium ion‐binding properties to coagulation factors via γ‐carboxyglutamic acid (Gla) residues and have previously been shown to interact with ectopic calcium‐crystals. We selected the protein S Gla‐domain because of its 11 Gla‐residues and previous evidence of its calcification inhibiting effect.
Aims: To investigate the application of protein S Gla‐domain as tracer and treatment of microcalcification.
Methods: Protein S Gla‐domain (fully carboxylated) and negative control protein S Glu‐domain (uncarboxylated) were chemically synthesized and assessed for calcification inhibition and detection in non‐cellular crystallisation assays and primary human vascular smooth muscle cell (hVSMC)‐based calcification assays. Protein S Gla‐domain was also tested for ex vivo detection of microcalcification in atherosclerotic lesions of high‐fat diet fed ApoE‐/‐ mice. Interference with and detection of calcification was determined by a combination of quantitative calcium concentration measurements and fluorescence microscopy.
Results: In this setup, protein S Gla binding occurred already before any calcium salt deposits could be detected. Further, protein S Gla selectively binds to microcalcifications. Thus, protein S Gla interfers with both initiation and progression of hVSMC calcification in vitro. Equally, at low concentrations, protein S Gla‐domain could be used to follow progression of calcification time‐dependently without interfering with calcification process. Moreover, protein S‐Gla was used to detect microcalcifications in atherosclerotic lesions ex vivo.
Conclusion(s): Protein S Gla‐domain interferes and detects biological processes involved in VSMC driven calcium crystal formation and calcification progression.
Fibrinolysis and Proteolysis
Fibrinogen and Factor XIII
PB0620
Low molecular weight heparin slows the formation of clots from patients within twenty‐four hours of acute PE
S. Baker 1; G. Halliday2; R. Ariëns3
1 Wake Forest University, Winston Salem, North Carolina, United States; 2 University of Leeds, Leeds, England, United Kingdom; 3 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom
Background: Pulmonary embolism (PE) is a major cause of mortality and anticoagulation treatment with low molecular weight heparin (LMWH) is aimed at preventing short‐term recurrence. Eliciting differences in clot properties may facilitate a better understanding of the mechanisms underlying LMWH treatment in PE patients.
Aims: To investigate formation and lysis of paired plasma clots from patients with acute PE and 24 hours after initial treatment with LMWH.
Methods: Paired patient samples were used to determine plasma clot characteristics (n = 47). Clot formation and fibrinolysis was determined by turbidity (n = 44). Fully formed clot structural comparisons were determined using laser scanning confocal microscopy. Statistical evaluation included paired t‐test and the Wilcoxon signed‐rank test.
Results: Clots formed from plasma samples taken 24 h after treatment with LMWH had a longer lag phase and time to maximum absorbance in comparison to those formed prior to treatment. No structural differences in fully formed clots were observed using confocal microscopy following LMWH treatment compared to pre‐treatment samples.
Conclusion(s): LMWH changed clot formation and structure, which is in keeping with its anticoagulant mechanism of action. Lag time could be clinically important in future risk stratification studies alongside other prothrombotic properties to predict PE recurrence in patients receiving anticoagulation therapy. Predicting recurrence during anticoagulation would allow for personalization of treatment for each patient.
PB0619
Clot collapse in scanning electron microscopy (SEM)
S. Baker 1; C. Cai1; Z. de Lange‐Loots2; M. Pieters2; M. Guthold1
1 Wake Forest University, Winston Salem, North Carolina, United States; 2 North‐West University, Potchefstroom, North‐West, South Africa
Background: Preparation of blood clots for SEM imaging and analysis is technically challenging. Dehydration and drying steps may result in shrunken clots, fibers that are collapsed upon each other, and fibers having a ribbon‐like rather than a cylindrical appearance. Improper clot preparation may result in images that are a poor representation of the native clot structure.
Aims: Our aim was to evaluate current methods of clot preparation for SEM imaging and analysis and present a method that results in representative images of the overall 3D structure of native clots.
Methods: We compared hexamethyldisilazane (HMDS) and critical point drying preparation methods of blood clots. In vitro clots were made from fresh frozen plasma, purified fibrinogen and lyophilized fibrinogen. Resulting clots (>n = 5 for each method/sample) were imaged and compared qualitatively under SEM. Average fiber diameters were measured for each clot and preparation method.
Results: Standard HMDS drying methods resulted in clots that were more likely to be collapsed with larger average fiber diameters than those from clots dried using critical point drying with either ethanol or acetone.
Conclusion(s): Critical point drying to prepare clots for SEM imaging and analysis is more likely to produce clots that are un‐collapsed with smaller fibers than HMDS drying. HMDS preparation can still result in representative clots, similar to those from critical point drying; however extra precautions, like structurally supportive clot containers, may be needed.

PB0621
Virtually screening for novel small molecule inhibitors against the conformational landscape of coagulation factor XIII activation‐path
M. Islam1; S. Ramaraje Urs2; S. Singh3; H. Javed4; A. Paul George5; D. Imhof1; J. Oldenburg6; A. Biswas 2
1 University of Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Medical Faculty, University of Bonn, Germany, Bonn, Nordrhein‐Westfalen, Germany; 4 Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 5 BioSolveIT, Sankt Augustin, Nordrhein‐Westfalen, Germany; 6 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Although primarily known for the rare bleeding disorder its deficiency causes, coagulation Factor XIII (FXIII) is also known to play a major role in thrombosis making it a suitable and interesting pharmaceutical target for thrombotic events and directed inhibitor design. The activation of FXIII involves major structural/conformational changes, which provides a vast opportunity for computational screening for potential novel small molecule inhibitors against FXIII.
Aims: Our study aims to computationally screen FXIII activation‐path structures for potential small molecule inhibitors of FXIII.
Methods: The Chembl, Pubchem and Binding database were searched for small molecule binding to FXIII (Figure 1.). Binding efficiency charts, Ki, Kd, Ro5 and Partition coefficient values integrated within the small molecule details were used as filters to narrow down only pharmacologically suitable molecules. The crystal structures of zymogenic/activated FXIIIA and previously reported FXIIIA activation path transition state structure‐models (n = 2) were loaded onto the SeeSar virtual‐screening platform (BioSolveIT). Druggable pockets were identified for the structures on SeeSar and the family of small molecules screened from the medicinal chemistry databases were docked and poses within these binding pockets. The binding chemical space defined by the high affinity binding molecules/poses (nM binding affinity) were initially modified within the SeeSar to improve the molecules binding affinity or directly moved to the infiniSee chemical space navigation platform (BioSolveIT) to identify similar molecules across chemical spaces. Closest matches to target molecule were identified and re‐docked on SeeSar platform on the FXIIIA structures and the promising ligands binding to functional areas of FXIII were identified based on binding affinity (Hyde scores).
Results: A total of 53 small molecules against the activation‐path structures of FXIIIA.
Conclusion(s): We were able to identify a large number of small molecules binding with high affinity to FXIIIA. Their inhibitory capacity and specificity will now be tested on the bench.

PB0622
Determining the dominant‐negative causality of FXIII heterozygous mutations using a bi‐cistronic, bi‐tagged expression system
H. Javed 1; S. Singh2; S. Ramaraje Urs3; J. Oldenburg4; A. Biswas3
1 Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 4 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Mild Factor XIII (FXIII) deficiency (FXIII activity 20‐60%) is a phenomenon resulting from heterozygous mutations in either F13A1 or F13B genes coding for the plasma circulating coagulation FXIII. Mild FXIII deficiency is asymptomatic unless the individual is exposed to physical trauma i.e. peri‐operatively, dental extraction, etc. Earlier, we reported 23 missense mutations from mild FXIII deficient individuals. However, the “dominant‐negative” effect of these mutations in a heterozygous scenario can only be proved by studying the heterozygous variant species in isolation.
Aims: Our study aims to use a bi‐cistronic bi‐tagged expression system in which both mutant and wild‐type alleles of all 23 missense mutations can be simultaneously expressed followed by the isolation of heterozygous species for further analysis.
Methods: Two types of expression vector (Figure 1) were developed for this analysis: 1) A two promoter system with a CMV promoter separated by SV40 PA translation‐stop site in which the wildtype and the mutant are cloned after each promoter and 2) P2A vector system in which the cloned mutant and wild type are separated P2A self‐cleaving peptide. Both the mutant and wild type ORFs were tagged with His and Strep tags at the N and C terminal respectively to allow us to systematically segregate wild type, heterozygous, and mutant homozygous species post transient transfection onto HEK293t cells followed by upscaling in HEK293F suspension culture.
Results: Almost all mutations showed close to 50–80% of FXIII activity as well as antigenic levels corresponding to that of the wild type in concordance with the FXIII levels observed in the mild FXIII deficient individuals carrying these mutations. Further purification and functional results are awaited.
Conclusion(s): Both systems were able to successfully express the wild type as well as the mutant alleles as indicated by the carrier/ heterozygous activity levels of the mutant.

PB0623
Role of transglutaminase 2 in haemostasis and as a novel treatment option for acquired autoimmune Factor XIII deficiency
B. Li1; R. Suzuki1; M. Golomingi1; K. Hitomi2; V. Schroeder 1
1 University of Bern, Bern, Bern, Switzerland; 2 Nagoya University, Nagoya, Aichi, Japan
Background: Acquired autoimmune FXIII deficiency, due to autoantibodies against FXIII, is a rare but life‐threatening bleeding disorder that is extremely difficult to manage, that involves immunosuppressive therapy over a long time, and the outcome is poor or even fatal in many cases. Novel treatment options are needed. FXIII has a crucial function in clot formation and stabilisation, but it also contributes to extracellular matrix formation, wound healing and tissue regeneration, a function it shares with transglutaminase 2 (TG2). Whether TG2 may also have a role in haemostasis has not been studied in detail.
Aims: To study the role of TG2 in clot formation and haemostasis and explore TG2 as a potential novel treatment option in autoimmune FXIII deficiency.
Methods: We studied fibrin crosslinking by TG2 with gel electrophoresis and incorporation assays. Effects of TG2 on clot formation and lysis in plasma (FXIII‐depleted plasma and plasma from a patient with autoimmune FXIII deficiency) were assessed with a turbidimetric assay and rotation thrombelastometry (ROTEM). We used specific TG2 and FXIII substrates and inhibitors in our microvascular whole blood flow model to monitor TG2 during clot formation in realtime.
Results: TG2 crosslinks fibrin and exhibits transglutaminase activity towards fibrinogen/fibrin and small substrates in a purified and plasma environment. In plasma, TG2 promotes clot formation, stabilises clots and prolongs fibrinolysis. TG2 even supports clot formation and prolongs lysis in the presence of a FXIII autoantibody. Preliminary experiments with our microvascular whole blood flow model suggest that TG2 may be active during clot formation and incorporate a TG2‐specific substrate into the clot.
Conclusion(s): Our results suggest that TG2 can indeed support clot formation and haemostasis. Further experiments are underway to explore TG2 as a novel treatment option for patients with autoimmune FXIII deficiency.
PB0624
Comparison of fibrinogen concentrates to cryoprecipitate in restoring clot integrity and stability against fibrinolytic degradation
C. Whyte 1; A. Rastogi2; E. Ferguson3; M. Donnarumma1; N. Mutch1
1 University of Aberdeen, Aberdeen, Scotland, United Kingdom; 2 Univeristy of Aberdeen, Aberdeen, Scotland, United Kingdom; 3 NHS Grampian, Aberdeen, Scotland, United Kingdom
Background: Fibrinogen depletion is a critical feature in trauma induced coagulopathy (TIC). Cryoprecipitate or fibrinogen concentrates can be administered to restore levels of this clotting factor.
Aims: To compare equivalent fibrinogen concentrations of cryoprecipitate and the fibrinogen concentrates, RiaSTAP® (CSL Behring) and FibCLOT® (LFB Biotechnologies), in their ability to restore clot integrity in models of TIC.
Methods: Fibrinogen depleted plasma (FDP) and 40 % haemodilution were used to recapitulate TIC. FDP clot formation and tissue plasminogen activator‐mediated lysis were monitored as change in absorbance at 405 nm. Clot structure were observed using fluorescently‐labelled fibrinogen and confocal microscopy. The influence on the viscoelastic properties using both models was investigated using rotational thromboelastometry. Chandler model thrombi were used to establish changes to clot stability after haemodilution. Factor XIII (FXIII) and alpha2 antiplasmin (α2AP) were measured by ELISA.
Results: Supplementation with all fibrinogen sources (≥1 mg/ml) increased maximum absorbance in FDP clot lysis with cryoprecipitate and FibCLOT giving similar A405. Clots formed with RiaSTAP consisted of stunted fibres whereas cryoprecipitate and FibCLOT produced fibres closely representing those formed with normal plasma. Maximum clot firmness (MCF) showed a concentration dependent increase with both fibrinogen concentrates being superior to cryoprecipitate. Haemodilution reduced the MCF of a clot but this was normalised with supplementation ≥1 mg/ml of any fibrinogen source. Only FibCLOT significantly increased the lysis onset time as measured by ROTEM. Haemodilution reduced thrombus stability against fibrinolytic degradation but this was normalised by supplementation ≥1 mg/ml of any fibrinogen source. Cryoprecipitate contained α2AP whilst the fibrinogen concentrates contained minimal levels. Cryoprecipitate and FibCLOT contained approximately 3‐fold more FXIII/mg of fibrinogen than RiaSTAP.
Conclusion(s): Addition of 1 mg/ml fibrinogen, a clinically achievable concentration, restored adequate clot integrity. FibCLOT, which contained more FXIII, was superior to RiaSTAP in normalising clot structure and stabilising haemodiluted clots against fibrinolysis.
Fibrinolytic Factors and Inhibitors
VPB0639
Strong fibrinolysis with new fermented foods (Mung Bean Natto and Natto Miso)
H. Sumi 1; S. Naito1; C. Yatagai1; T. Mitsuo2
1 Kurashiki University of Science and the Arts, Kurashiki, Okayama, Japan; 2 Mitsuo Clinic, Shibuya, Tokyo, Japan
Background: Nattokinase, a thrombolytic enzyme contained in natto, is a single‐stranded polypeptide consisting of 275 residues, and increases fibrin degradation product (FDP) or tissue plasminogen activator (t‐PA) by oral administration even in vivo.
Aims: When Bacillus subtilis natto was bred on 11 different substrates, it was confirmed that mung bean showed particularly strong nattokinase activity and that it was also possible to make natto miso.
Methods: Mung bean natto was fermented at 41°C using mung bean and Bacillus subtilis natto in the usual manner. Natto miso was fermented at room temperature by mixing dried natto with the same amount of dried rice jiuqu and 0–25% saline solution.
Results: Mung beans had the highest fibrinolytic activity among the 11 types of substrates, and corn and brown rice had the lowest. The vitamin K content of mung bean natto was 44.1 μg, which is the amount of K1 per 100 g of dry weight, and 3.97 mg of K2 was newly produced by fermentation. After drying at 50°C, vitamin A (27 μg/100g), β‐carotene (160 μg/100 g), γ‐tocopherol (8.3 mg/100 g), etc. were detected together with nattokinase. Excellent natto miso was obtained by fermenting natto at room temperature for 6 months in the presence of rice jiuqu and saline solution. In addition, there was almost no activity without salt, and it was found that salt works to stabilize nattokinase. The final product had a nattokinase activity of 120,000 IU (urokinase equivalent)/100 g. The vitamin K2 content of natto miso was 526 μg or more per 100 g, which was strong enough to meet the daily requirement for adult males at 10 g.
Conclusion(s): Strong fibrinolytic enzymes were found in mung bean natto and natto miso. These fermented foods are excellent functional materials that Japanese people can easily eat anywhere, but what is noteworthy is that they are all inexpensive.
PB0626
Plasminogen deficiency: A case series of an ultra‐rare disease in a Spanish centre
O. Benítez Hidalgo 1; M. Huguet Mas2; A. de Jaureguizar Tesas2; M. Martínez García1
1 Universitary Vall d'Hebron Hospital, Barcelona, Catalonia, Spain; 2 Germans Trias i Pujol Hospital, Barcelona, Catalonia, Spain
Background: Plasminogen (PLG) deficiency is an ultra‐rare recessive disease with an estimated prevalence of 1,6 per million population. Patients with PLG deficiency may present a multi‐organ systemic disease that commonly manifests as extravasculars of fibrin‐rich pseudomembranes on mucous membranes. Ligneous conjunctivitis is the most common symptom disease (81% prevalence)
Aims: We describe four patients diagnosed with severe plasminogen deficiency who developed a heterogeneous clinical presentation in Vall d'Hebron Hospital in Spain
Methods: Retrospective data was collected from 4 patients with severe plasminogen deficiency. Study variables included gender, age, endogenous PLG activity and clinical presentation. Data were collected from medical records.
Results: Mean age were 34.5 years (Range 2–67) with one pediatric and 3 adults. All patients were female and were diagnosticated in their chilhood. Median endogenous PLG activity were 14.5% (Range 5–25%). Table 1 shows patients description.
Conclusion(s): Patients with PLG deficiency have multi‐organ involvement and to achieve a diagnosis can be hard. The mangange of this patients is complex and must be multidisciplinar (with hematologists, ophthalmologists, gynecologists, dentists…) to correctly identify and treat all the complications that may appear. A standard consensus for their treatment is still not clear. The treatment should be chosen individually. Although no specific commercial therapies are available


VPB0636
A comparative study of intravenous bolus versus bolus with continuous infusion of tranexamic acid to reduce blood loss in major surgical procedures
N. NEERU; V. ABRAHAM
Christian Medical College and Hospital, Ludhiana, Punjab, Ludhiana, Punjab, India
Background: The major surgical procedures are associated with excessive blood loss and necessitate the need for blood transfusion in the absence of blood conservation strategies. Allogeneic blood transfusion carries significant risks of immunological reactions, transmission of disease, hemolysis, coagulopathy, renal failure, admission to ICU and even death. Tranexamic acid (TXA) is a synthetic antifibrinolytic drug that competitively blocks the lysine‐binding sites of plasminogen, plasmin, and tissue plasminogen activator, thereby inhibiting fibrinolysis and blood clot degradation.
Aims: The aim of this study was to compare the efficacy of single intravenous bolus dose of tranexamic acid with bolus plus infusion dose of tranexamic acid in reducing intraoperative blood loss.
Methods: This study was conducted as prospective, randomized and controlled.120 patients of either sex, ASA grade I and II and aged between 18 to 65 years were included in the study. These patients were randomly divided into 3 groups i.e. Group A (bolus), Group B (bolus + infusion) and Group C(control). General anaesthesia was administered to the patient and the study drug was administered to the patient 10 min before the skin incision. Intraoperative blood loss and blood transfusions were noted.Data has been analysed using SPSS (Statistical Packages for Social Sciences, version 21.0. Armonk, NY: IBM corp.).
Results: The intra‐operative blood loss was least in the Bolus group and was maximum in the placebo group. The use of TXA significantly reduced the transfusion requirements in major surgeries with the least amount of transfusion needed in the Infusion group.
Conclusion(s): The use of TXA ( in bolus as well as the infusion) led to statistically significant reduction in blood loss and the requirement of transfusion. We would suggest the use of TXA in bolus dose pre‐operatively before a radical surgical procedure to minimize the intra‐operative blood loss and the requirement of transfusion.

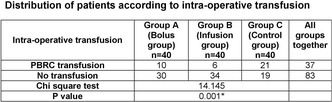
VPB0640
Vinegar produce t‐PA from human endothelial cells
H. Sumi 1; T. Mitsuo2; Y. Yanagisawa3; M. Imai1
1 Kurashiki University of Science and the Arts, Kurashiki, Okayama, Japan; 2 Mitsuo Clinic, Shibuya, Tokyo, Japan; 3 Chiba Institute of Science, Choshi, Chiba, Japan
Background: It has been speculated that in many alcoholic beverages, especially in Shochu, the first fraction other than alcohol is involved in fibrinolytic expression in the human body. Vinegar is also a brewed product made from the same raw materials as sake, and similar fermented products may be involved.
Aims: We investigated the amount of tissue plasminogen activator (t‐PA) released from human cells in vinegar.
Methods: Cell culture was performed by autoclaving vascular endothelial cells (ECV) in E‐MEM medium containing 10% fetal bovine serum (FBS) for 15 minutes using a 48‐well well and then filtering and sterilizing. To determine the fibrinolytic effect, after adding various samples to the culture medium, the t‐PA activity of the supernatant is measured by the fibrin plate method.
Results: Black vinegar natto showed the fibrin dissolving ability (199 mm²/0.03ml), but no ability was observed in the other two species (pear vinegar and balsamic vinegar). Secondly, the t‐PA release effect of each solution of pear vinegar and black vinegar natto on human cells is widespread when the concentration of the undiluted solution is high (1 × 1 or 1 × 2 dilution rate). It was found that it has the effect of increasing vinegar (252 ‐290 mm²/0.03ml). This is about 10 times higher activity than the control (10 mm² or less /0.03ml) that does not contain any undiluted solution, while balsamic vinegar was 3‐4 times lower in value, but t‐PA release effect was observed.
Conclusion(s): Vinegar was considered to have a t‐PA release mechanism similar to that of alcoholic beverages. Βeta‐phenethyl alcohol, which is an aroma component of alcohol, can release t‐PA and has the highest fibrinolytic activity‐enhancing effect at present. On the other hand, even when brandy or Chinese liquor is added, a weak t‐PA release effect is observed.
VPB0635
Attenuation of thrombotic biomarkers in a hyperlipidaemic sprague Dawley rats model
I. Kwaifa 1; K. Perumal2; H. Bahari3; K. Yong3; Z. Eshak4; S. Balan5; A. Abidin5; F. Ghadi3; L. Kong3; S. Noor3
1 University PUTRA MALAYSIA, Kuala Lumpur, Kuala Lumpur, Malaysia; 2 University Putra Malaysia, Malaysia, Selangor, Malaysia; 3 Universiti Putra Malaysia, Kuala Lumpur, Selangor, Malaysia; 4 Universiti institute Technology Mara, Malaysia, Kuala Lumpur, Selangor, Malaysia; 5 Management and Sciences University, Malaysia, Kuala Lumpur, Selangor, Malaysia
Background: Atherothrombosis is one of the major risk factors associated with the increased incidence of cardiovascular diseases, particularly in developed countries. Natural‐based products intervention has been suggested as one of the essential approaches for the management of atherothrombosis‐related cardiovascular diseases (CVDs). Phytochemical compounds, including gingerol, hesperidin, naringin and citric acid have been reported to exhibit antioxidant, antihyperlipidemic, anti‐inflammatory, antiplatelet, anticoagulant and antithrombotic properties.
Aims: The study determined the phytotherapeutic effects of herbal extracts; a combination of Zingiber officinale (ginger), Allium sativum (garlic), Citrus Limon (lemon), Malus Domestica (Apple)/ apple cider vinegar and Honey (ZACAH) on atherothrombosis‐related markers in SD rats fed with HCD.
Methods: Identification of the major related phytochemical compounds present in ZACAH extracts was performed by ultra‐high performance liquid chromatography‐mass spectrophotometry (UHPLC‐MS) profiling at both negative and positive models. Male SD rats were randomly divided into six groups, the normal diet (ND), HCD, treatment with simvastatin (TRTSM) at 10mg/kg of body weight, treatment with ZACAH extracts (TRT1) at 1mg, 3mg (TRT2), and 5mg (TRT3) per kilogram of body weight. At the end of the 2 weeks experiments, the animals were sacrificed and the activities of thrombosis‐related proteins were studied.
Results: The UHPLC‐MS profiling of the phytochemicals in ZACAH extracts yielded the identification of hesperidin (retention time (RT); 11.18), citric acid (2.18), gingerol (18.62) and naringin (10.07). Oral gavage supplementation with ZACAH extracts significantly attenuated the activities of plasminogen activator inhibitor‐1 (PAI‐1) and tissue factor (TF) while improving the levels of tissue‐type plasminogen activator (t‐PA) and nitric oxide (NO) in a dose‐dependent manner.
Conclusion(s): The findings suggested that identified phytochemicals available in ZACAH extracts could inhibit the progress of atherothrombotic plaque development through suppressing the expression of thrombotic marker activities induced by dietary high cholesterol intake and may represent novel therapeutic opportunities for the treatment of atherothrombosis‐related CVDs.

EXPRESSION OF ATHEROTHROMBOSIS‐RELATED PROTEINS

PB0625
Influence of centrifugation and residual platelet count on PAI‐1 plasma level, euglobulin clot lysis time and global fibrinolytic capacity of plasma
M. Bareille 1; T. Lecompte2; F. Mullier3
1 Université catholique de Louvain, CHU UCL Namur, Namur Thrombosis and Hemostasis Center (NTHC), Hematology Laboratory, Namur Research Institute for Life Sciences (NARILIS), Yvoir, Belgium, Ottignies, Brabant Wallon, Belgium; 2 University of Nancy, France, Nancy, Lorraine, France; 3 Université catholique de Louvain, CHU UCL Namur, Namur Thrombosis and Hemostasis Center (NTHC), Hematology Laboratory, Namur Research Institute for Life Sciences (NARILIS), Yvoir, Belgium, Yvoir, Namur, Belgium
Background: Coagulolytic assays with (i.e global fibrinolytic capacity of plasma (GFC)) or without exogenous fibrinolysis activator (i.e euglobulin clot lysis time (ECLT)) are often used as screening tools to explore fibrinolysis. Pre‐analytical conditions to obtain platelet‐depleted plasma (PPP) suffer from a lack of standardization, especially about centrifugation. Platelet α‐granules have been described as a circulating pool of PAI‐1, accounting for approximately 50 % of total circulating PAI‐1 activity due to platelets abundance. As ECLT and GFC are both sensitive to PAI‐1 plasma level, residual platelets in PPP could be a source of PAI‐1 which may interfere with GFC and ECLT results.
Aims: To study the influence of centrifugation scheme and residual platelet count on PAI‐1 level, euglobulin clot lysis time and global fibrinolytic capacity of plasma.
Methods: The influence of centrifugation scheme was assessed with plasma samples from healthy volunteers. The experimental design is summarized figure 1. The study was approved by the Local Ethics Committee (NUB: B0392020000042). Statistical analysis was performed in R, using one‐way ANOVA for repeated measures or Friedman test as appropriate.
Results: Good correlation between PAI‐1 plasma level and ECLT and GFC, with a Pearson coefficient of 0.63 (p < 0.0001) and 0.64 (p < 0.0001) respectively. Residual platelet counts differed according to the centrifugation scheme (p < 0.0001, Figure 2A), but not PAI‐1 levels (p = 0.75, fig. 2.B and 2.C) nor GFC (p = 0.18) and ECLT (p = 0.091).
Conclusion(s): Active PAI‐1 plasma level showed a statistically highly significant (p < 0.0001) correlation with ECLT and GFC. The centrifugation scheme, despite its expected influence on residual platelet count in plasma, does not seem to influence plasma PAI‐1 level nor ECLT and GFC. This could be explained by the fact that platelet PAI‐1 is less active than plasma PAI‐1, and approximately 40% of total platelet‐derived PAI‐1 remains anchored to the platelet membrane through the αIIbβ3 integrin.


VPB0634
Veno‐occlusive disease in pediatric patients after stem cell transplant is associated with impaired fibrinolysis
S. Hammer 1; V. Schneider2; M. Döring2; K. Althaus3; T. Backchoul4
1 Center for Clinical Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 2 Department I‐General Pediatrics, Haematology/Oncology, University Hospital Tuebingen‐Children’s Hospital, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 3 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 4 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Hepatic veno‐occlusive disease is a life‐threatening complication of hematopoietic stem cell transplantation (HSCT). Early diagnosis and prompt initiation of defibrotide ‐ the only approved therapy ‐ is important to a successful treatment of VOD. Because of the severity of this disease, an early diagnosis is important to start treatment of VOD as soon as possible. Currently, no predictive biomarker exists to monitor disease progression or therapy responsiveness.
Aims: In this prospective study, we systematically analyze the viscoelastic changes in coagulation system with a focus on plasmin resistance in HSCT patients.
Methods: 54 pediatric patients with leukemia or other hemato‐oncological disease who received HSCT with a high risk of VOD were included in this prospective study. Whole blood samples were investigated using a viscoelastic test system to assess coagulation and fibrinolysis. Additionally immunoassays for factors of the fibrinolysis system were performed.
Results: Significantly elevated resistance to clot lysis and longer time of lysis after adding tissue plasminogen activator were observed in blood samples from VOD patients compared to non‐VOD HSCT patients. Plasma levels of plasminogen activator inhibitor 1 are still in evaluation but seems to be significantly higher in plasma samples from clinical diagnosed VOD patients.
Conclusion(s): Our data support the hypothesis that VOD is associated with impaired fibrinolysis in blood. It’s assumed that fibrinolytic inhibitors are elevated leading to an increased resistance to clot lysis. Further investigations are following. Thromboelastography could be useful to investigate VOD early in patients after HSCT.
PB0629
The validation of new global fibrinolysis capacity assays in high tPA concentration situation and the comparison with thromboelastgraphy
O. Kumano 1; T. Tsuchida2; M. Hayakawa2
1 HYPHEN BioMed, Neuville Sur Oise, Centre, France; 2 Hokkaido University Hospital, Sapporo, Hokkaido, Japan
Background: Global fibrinolysis assay is the method which activates coagulation reaction with tissue‐type plasminogen activator (tPA) and detects fibrinolysis time of the clot. This assay is useful to investigate the global coagulation and fibrinolysis reactions simultaneously.
Aims: Recently, two kinds of global fibrinolysis assays of clot‐fibrinolysis waveform analysis (CFWA) and global fibrinolysis capacity (GFC) were developed. In this study, the validation of these two assays was performed and the comparison against thromboelastgraphy (TEG) was also investigated.
Methods: The fibrinolysis times of CFWA and GFC were detected as CFWA‐Lys and GFC‐Lys in the total 151 samples spiked with tPA at the concentration of 0, 100, 500 and 1000 ng/ml, respectively. The comparison was also investigated in the methods of extrinsic pathway and intrinsic pathway of TEG by 77 and 74 samples, respectively. Four parameters including clotting time (CT), amplitude after 10 min (A10), maximum clot firmness (MCF), and Lysis index after 30 min (LI30) in TEG were used for the comparison.
Results: CFWA‐Lys and GFC‐Lys were significantly shortened in tPA high concentration samples, in especially the concentration of 500 and 1000 ng/mL. The correlation coefficients against CT, A10, MCF and LI30 in TEG extrinsic pathway were 0.092, 0.620, 0.618, and 0.497 for CFWA‐Lys and 0.175, 0.731, 0.691, and 0.890 for GFC‐Lys, respectively. In the intrinsic pathway, these values were 0.263, 0.574, 0.627, and 0.481 for CFWA‐Lys, and 0.013, 0.745, 0.803, and 0.901 for GFC‐Lys, respectively.
Conclusion(s): Both global fibrinolysis assays showed shorter fibrinolysis times in high tPA concentration samples, and CFWA‐Lys and GFC‐Lys were highly correlated with the parameters of clot firmness like A10 and MCF. It was considered that these assays reflected tPA concentration and clot firmness in the assay and would be useful to investigate the fibrinolysis status.
PB0632
Activity of some protease inhibitors in patients with arterial hypertension of high risk
E. Shorikov 1; D. Shorikova1; P. Shorikov2; A. Esmaeil2
1 Bukovinian State Medical University, Chernivtsy, Chernivets'ka Oblast', Ukraine; 2 Bukovinian State Medical University, Chernivtsi, Chernivets'ka Oblast', Ukraine
Background: it is important to set the role of systems which can provide the protection of additional fibrinolysis in the development of thrombotic complication in patients with arterial hypertension of very high risk.
Aims: With this in mind, we investigated the activity of protease inhibitors (alpha‐2‐macroglobulin ‐ a‐2‐M and alpha‐1‐protease inhibitor ‐ a‐1‐PI) as universal blockers of fibrinolysis in patients with hypertension and concomitant type 2 diabetes.
Methods: We have studied 270 patients with arterial hypertension with stages II and III, 136 of them with concominant diabetes mellitus type 2. We have measured the activity of α‐2‐M and α‐1‐IP, and additionly the enzyme and non‐enzyme fibrinolitic activity.
Results: We have set that the difference in a‐2‐M according to the stage of hypertension was not detected (p > 0.05). In contrast, changes in the activity of α‐1‐IP were characterized by a differentiated dependence: it was greater in stage II than in stage IIII; (p < 0.05). The presence of diabetes contributes to additional increase (p < 0.05). The effects of inhibitory proteins on fibrinolysis in patients were similar: inhibition of enzymatic fibrinolysis was observed, as evidenced by negative correlations between them, the effect of α‐2‐M was more significant (t = ‐1.85; p = 0.06). The non‐enzymatic fibrinolysis was not significantly affected. The interaction with sum of fibrinolytic activity was manifested in different ways: weak negative but significant effect of α‐1‐IP (p = 0.027), no relationship of α‐2‐M (p = 0.096). Multiple R was 0.31 (p = 0.001), the coefficient of determination was only 0.096, so the direct inhibitory potential was not significally enough.
Conclusion(s): Decompensation of enzymatic fibrinolysis in patients with hypertension with concomitant diabetes mellitus type 2 is associated with increased coordinated activation of alpha‐2‐macroglobulin and alpha‐1‐protease inhibitor, but general influence of these systems on the fibrinolysis was low and need an additional learning.
VPB0633
Adipokines and insulin resistance in young individuals with various metabolic phenotypes
V. Chulkov 1; N. Vereina2; E. Gavrilova2; E. Lenets2; E. Pankova2; S. Martynov2; S. Sinitsin2
1 professor of Faculty Therapy Department South‐Ural state medical university, Russia, Chelyabinsk, Chelyabinsk, Sverdlovsk, Russia; 2 South Ural State Medical Universiry, Chelyabinsk, Sverdlovsk, Russia
Background: Dysregulation of certain adipokines can promote pathogenic conditions associated with insulin resistance. The contributions of individual adipokines to the pathophysiological features of cardiometabolic diseases remain controversial.
Aims: To evaluate the association of adipokines and insulin resistance in young individuals with various metabolic phenotypes.
Methods: A cross‐sectional study included 251 patients: group 1 – healthy metabolic profile with normal body weight (n = 62); group 2 – unhealthy metabolic profile with normal body weight (n = 57); group 3 ‐ healthy metabolic profile and obesity (n = 16); group 4 ‐ unhealthy metabolic profile and obesity (n = 116). Statistical significance was defined as p< 0.05.
Results: Individuals with unhealthy metabolic profile and obesity had the highest serum levels of leptin, plasminogen activator inhibitor‐1 (PAI‐1) and angiotensin II and the lowest serum levels of adiponectin. Overall, there was a significant relationship between the concentration of PAI‐1 and insulin (Spearman r = 0.345, p = 0.01), PAI‐1 and HOMA‐IR index (Spearman r = 0.251, p = 0.06) among metabolically unhealthy obese individuals. The most significant linear correlations among metabolically unhealthy individuals with normal body weight were obtained between PAI‐1 and angiotensin II (Spearman r = 0.484, p = 0.03), PAI‐1 and endothelin‐1 (Spearman r = ‐ 0.462, p = 0.04).
Conclusion(s): Understanding of the diverse effects of distinct adipokines and their interactions is still incomplete. Unraveling the pathophysiological roles of adipokines in young individuals with various metabolic phenotypes likely will result in a new personalized therapy strategy.
PB0628
The characterization of clot‐fibrinolysis waveform analysis and the investigation of usefulness in critical care patients with enhanced or suppressed fibrinolysis
O. Kumano 1; T. Tsuchida2; M. Hayakawa2
1 HYPHEN BioMed, Neuville Sur Oise, Centre, France; 2 Hokkaido University Hospital, Sapporo, Hokkaido, Japan
Background: A well‐controlled balance between pro‐coagulant, anti‐coagulant and fibrinolytic mechanisms is critical for maintain normal haemostasis in circulating blood. Especially, the fibrinolysis situation is changing in critical care patients, remarkably. It is useful to investigate the fibrinolysis situation with coagulation simultaneously in these patients.
Aims: Recently, clot‐fibrinolysis waveform analysis (CFWA) which is the coagulation and fibrinolysis global assay based on activated partial thromboplastin time with tissue‐type plasminogen activator was developed. In this study, the characteristics of CFWA was investigated by samples from enhanced or suppressed fibrinolysis patients in critical care.
Methods: The fibrinolysis times of CFWA were detected as CFWA‐Lys in the total 299 samples. These samples were divided into three groups based on the reference interval (RI) of CFWA: Group 1, less than RI; Group 2, within RI; Group 3, more than RI. The coagulation and fibrinolysis markers including fibrin/fibrinogen degradation products (FDP), D‐dimer, plasmin‐α2 plasmin inhibitor complex (PIC), fibrin monomer complex (FMC), Plasmin‐α2 Plasmin Inhibitor (α2PI), Plasminogen (Plg) and fibrinogen (Fbg) were investigated and compared among three groups. The informed consent was obtained from patients.
Results: The significant differences were recognized between group 1 and 3 in FDP, FMC, α2PI, Plg and Fbg. The values of FDP and FMC in group 1 were higher than those of group 3, the other parameters showed lower values in group 1 than group 3. Especially, the values of α2PI and Fbg became higher from the order of group 1, 2 and 3, significantly.
Conclusion(s): CFWA‐Lys was largely related to the values of α2PI and Fbg, and it was considered that CFWA‐Lys was prolonged in the samples with high concentration of plasmin inhibitor and large firmness clot derived from high concentration Fbg. CFWA‐Lys may be useful to investigate the fibrinolysis situation as the global assay.
VPB0637
Comparison between intermittent bolus versus continuous intravenous infusion of tranexamic acid in hematological disorders with thrombocytopenia (BRAVE Trial)
B. Poojitha 1; J. John2; A. Philips2; K. Modak2; P. DS2
1 Senior resident, Ludhiana, Punjab, India; 2 Christian Medical College Ludhiana, Ludhiana, Punjab, India
Background: Anti‐fibrinolytic agents like tranexamic acid (TXA) have been shown to be effective in preventing bleeding complications in a variety of hemostatic challenges. The elimination half‐life is approximately two hours and there are fewer studies comparing bolus and continuous infusions in bleeding disorders in hematology. This study focuses on comparing the intermittent bolus dosage and continuous infusions in different bleeding scenarios in hematology.
Aims: To compare the efficacy of continuous infusion of Tranexamic acid over intermittent bolus dosages in hematological disorders with thrombocytopenia. To study difference in the number of bleeding events, grade of bleeding, the number of platelet transfusions given, and duration of the hospital stay between the arms.
Methods: It was a prospective, randomized, open‐label, interventional study. Adult patients aged 18 years or more having a confirmed diagnosis of Acute Myeloid Leukemia, Acute Lymphoblastic Leukemia, Immune thrombocytopenia and Aplastic anemia with a platelet count below 20000/cu mm were taken as study subjects.
Results: A total 35 patients were included in the study. 17 (49%) patients were randomized to bolus arm and 18 (51%) patients were randomized to continuous infusion arm. Study population included 14 (40%) females, 21 (60%) males and were diagnosed to have 16 AML, 9 ALL, 7 ITP and 3 Aplastic anemia. The median age of the patients in the bolus arm was 40 (Q1,Q3, 32,49.5) and in continuous infusion arm was 35 (Q1,Q3, 49,72). Baseline characteristics were matched between the study arms with regard to gender, HB, TLC, PLT count, coagulation parameters. There were no significant difference between the two arms in terms of grade of bleeding (p = 0.29), duration of bleeding (p = 0.469), number of platelet transfusions (p = 0.65), PRBC transfusions (p = 0.407) and duration of hospital stay (p = 0.508).
Conclusion(s): Bolus dosing of tranexamic acid is as effective as continuous infusion for prophylactic use in thrombocytopenic patients with hematological disorders.


VPB0638
Effects of clot contraction on clot degradation: A mathematical and experimental approach
R. Risman 1; A. Abdelhamid2; J. Weisel3; B. Bannish4; V. Tutwiler2
1 Rutgers University, Cedar Grove, New Jersey, United States; 2 Rutgers University, New Brunswick, New Jersey, United States; 3 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 4 University of Central Oklahoma, Edmond, Oklahoma, United States
Background: The administration of tissue plasminogen activator (tPA) drives the enzymatic degradation (fibrinolysis) of fibrin in clots and thrombi. We have previously shown that clot contraction decreases the efficacy of fibrinolysis in experiments mimicking the external administration of tPA to preformed clots.
Aims: To understand how key structural changes (1. volume shrinkage, 2. redistribution of platelets‐fibrin to the periphery and erythrocytes to the core of the clot, and 3. fibrin densification, Figure 1A) that occur following clot contraction mechanistically drive the decrease in fibrinolysis of contracted blood clots.
Methods: We used a combination of mathematical modeling and experimental methodologies to characterize the process of external fibrinolysis. A 3D stochastic multiscale model of external fibrinolysis (Figure 1B) was used to determine how the structural changes that occur during the process of clot contraction influence the mechanism(s) of fibrinolysis. Turbidity and confocal microscopy experiments were performed based on modeling predictions using pooled human plasma activated with CaCl2 and thrombin, with external delivery of tPA to initiate lysis.
Results: Our model successfully simulates previous experimental results of reduced fibrinolysis rate in contracted clots compared to uncontracted clots (0.0050 vs. 0.022 fraction of fibrin degraded per second, p < 0.001). Analysis of additional model and experimental clot scenarios with varying structural changes indicates that fibrin densification (tight vs. loose) makes the most significant contribution to the rate of fibrinolysis (modeling: 0.0022 vs. 0.0069 fraction of fibrin degraded per second, p < 0.0001; experimentally: 0.000042 vs. 0.0004 optical density per second, p < 0.001, Figure 1C) when compared to the distribution of components (p < 0.05) and degree of compaction (ns).
Conclusion(s): These results suggest that high fibrin densities caused by clot contraction reduce fibrinolysis because they limit tPA diffusion. Our work has the potential to be used to improve on current therapeutics by optimizing timing and delivery of lytic agents.

PB0627
Hypofibrinolysis in patients with aortic stenosis is driven by overexpression of plasminogen activator inhibitor type 1 (PAI‐ 1) associated with elevated oxidized lipoproteins
M. Kopytek 1; A. Undas2; M. Ząbczyk2; J. Natorska2
1 Jagiellonian University Medical College, John Paul II Hospital, krakow, Malopolskie, Poland; 2 Jagiellonian University, Institute of Cardiology, John Paul II Hospital, Krakow, Malopolskie, Poland
Background: Aortic stenosis (AS) is associated with hypofibrinolysis, but the mechanism underlying this phenomenon is poorly understood.
Aims: We investigated whether hypofibrinolysis in AS is associated with plasminogen activator inhibitor type 1 (PAI‐1) overexpression and if oxidized (ox)LDL‐cholesterol affects this process.
Methods: We enrolled 75 patients with isolated severe AS (aged 66 ± 9 years) and 10 healthy well‐matched controls. Plasma PAI‐1 concentrations and oxLDL levels were assayed using commercially available ELISAs. Primary cultures of valve interstitial cells (VICs) obtained from AS valves were stimulated with oxLDL (300 μg/ml) or TNF‐α (50 ng/ml). Inhibitors of PAI‐1 (50 μM) or NF‐κB (10‐6 mol/l) were used to suppress PAI‐1 synthesis. After 7 days of culturing VICs were used for immunofluorescence, PCR and ELISAs. Clot lysis time (CLT) was measured in patients’ plasma and PAI‐deficient plasma supplemented with VICs supernatants. The Ethical Committee approved the study and all participants provided written informed consent in accordance with the Declaration of Helsinki.
Results: AS patients compared with controls had 51.2% higher oxLDL‐cholesterol levels (p = 0.027), 67% higher plasma PAI‐1 (p = 0.003) and 19% prolonged CLT (p = 0.000014). The expression of PAI‐1 was observed within stenotic but not control valves (24.6% vs. 0%, p = 0.00001). Valvular PAI‐1 expression strongly correlated with CLT (r 2 = 0.34, p = 0.00001). oxLDL‐cholesterol was weakly associated with plasma PAI‐1 (r 2 = 0.10; p = 0.007) and CLT (r 2 = 0.14, p = 0.0008). In vitro VICs expressed PAI‐1 independently of TNF‐α or oxLDL stimulation, which was downregulated by PAI‐1 (−33 ± 6.3%, p = 0.002), and NF‐kB (−29 ± 4.7%, p = 0.0026) inhibitors. Inhibition of PAI‐1 significantly downregulated SERPINE1 expression in VICs (all p < 0.001). TNF‐α stimulation prolonged CLT by 10.7% compared with VICs cultured in standard medium, while PAI‐1 inhibition shortened CLT by 8.9% (both p< 0.01).
Conclusion(s): In severe AS patients hypofibrinolysis is driven by local PAI‐1 overexpression associated with increased oxLDL. This work was supported by the Polish National Science Centre (UMO‐2018/29/B/NZ5/02629).
PB0630
Early systemic administration of a natural carboxypeptidase inhibitor potentiates tPA‐mediated thrombolysis: Evidence from real‐time intravital imaging analysis of microthrombi in mice
N. Mathews 1; Y. Suzuki2; N. Honkura2; H. Sano2; T. Urano2
1 Hamamatsu University School of Medicine, Hamamatsu, Japan, Hamamatsu, Shizuoka, Japan; 2 Department of Medical Physiology, Hamamatsu University School of Medicine, Hamamatsu, Shizuoka, Japan
Background: Fibrinolytic therapy using recombinant tissue‐type plasminogen activator (rt‐PA) is widely practiced in acute thrombotic disorders including myocardial infarction and ischemic stroke. It activates Glu‐plasminogen to plasmin on the surface of fibrin to promote clot lysis. However, its disadvantages include a narrow therapeutic window and bleeding complications.
Aims: To elucidate the functional role of activated thrombin‐activatable fibrinolysis inhibitor (TAFIa) and Glu‐plasminogen in vivo using labeled Glu‐plasminogen and a carboxypeptidase inhibitor from potato tuber (CPI), an inhibitor of TAFIa. Further, we aimed to demonstrate the effect of CPI on rt‐PA‐mediated thrombolysis in vivo.
Methods: Using multi‐photon excitation fluorescence microscopy, laser‐induced microthrombi were produced and imaged in real‐time in the mesenteric venules of Green Fluorescent Protein (GFP)‐expressing mice aged between 7–9 weeks. By measuring the changes in fluorescence intensity of labeled Glu‐plasminogen, microthrombus dynamics and thrombolysis patterns were examined following administration of epsilon aminocaproic acid (EACA), CPI, and rt‐PA.
Results: CPI promoted Glu‐plasminogen accumulation at the center of the microthrombus by inhibiting TAFIa, while EACA inhibited this process. Exogenous rt‐PA effectively triggered Glu‐plasminogen activation within the thrombus and promoted thrombolysis. Administration of CPI alone was not effective in clot lysis and CPI given together with rt‐PA did not show accelerated lysis. However, early‐phase systemic administration of CPI before thrombolytic therapy by rt‐PA accelerated clot lysis as evidenced by significantly faster time to reach peak Glu‐plasminogen fluorescence intensity and shorter time to achieve near‐complete clot lysis (p = 0.014 and p = 0.003 respectively).
Conclusion(s): Administration of the TAFIa inhibitor CPI potentiates rt‐PA‐mediated thrombolysis when given early in acute thrombotic events. CPI, and TAFIa inhibitors in general, show great promise in the management of thrombotic diseases. More studies are needed to assess the pharmacokinetic and pharmacodynamic profile of this group of drugs for potential use in thrombolysis or thromboprophylaxis.
PB0631
VhH anti‐thrombomodulin clone 1 inhibits TAFI activation and enhances fibrinolysis in human whole blood under flow
G. Poolen 1; M. van Moorsel2; C. Koekman1; S. Verhoef1; S. De Maat2; A. Barendrecht2; N. van Kleef1; J. Meijers3; R. Schiffelers4; C. Maas2; R. Urbanus5
1 University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 2 UMC Utrecht, Utrecht, Utrecht, Netherlands; 3 Department of Molecular Hematology, Sanquin Research, Amsterdam, the Netherlands, Department of Experimental Vascular Medicine, Amsterdam UMC, University of Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 CDL Research, University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 5 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: Thrombomodulin on endothelial cells can form a complex with thrombin. This complex has both anticoagulant properties, by activating protein C, and clot‐protective properties, by activating thrombin‐activatable fibrinolysis inhibitor (TAFI). Activated TAFI (TAFIa) inhibits plasmin‐mediated fibrinolysis. TAFIa inhibition is considered a potential antithrombotic strategy. So far, this goal has been pursued by developing compounds that directly inhibit TAFIa, but specific inhibition of TM‐mediated TAFI activation has not been attempted.
Aims: To develop variable domain of heavy‐chain‐only antibody (VhH) clones that inhibit TAFI activation by targeting human thrombomodulin.
Methods: Two Lama Glama were immunized and VhH anti‐thrombomodulin clones were selected with phage display. Affinity was determined with Surface Plasmon Resonance and binding to native thrombomodulin was confirmed with flow cytometry. Clone 1 was functionally assessed with competition‐, clot lysis‐ and thrombin generation assays. Lastly, the effect of clone 1 on fibrinolysis in human whole blood was investigated in a microfluidic fibrinolysis model.
Results: VhH anti‐TM clone 1 bound recombinant thrombomodulin with a binding affinity (KD) of 1.7 ± 0.4nM and showed binding to native thrombomodulin. Clone 1 competed with thrombin for binding to thrombomodulin (IC50 148.6 nM). Inhibitory effects of clone 1 on thrombomodulin‐dependent TAFI activation were confirmed in clot lysis assays: Clone 1 fully attenuated TAFI activation in a dose‐dependent manner when thrombomodulin was present, but not in its absence. In addition, clone 1 inhibited protein C activation in thrombin generation experiments in a dose‐dependent manner. In a microfluidic fibrinolysis model in which recalcified whole blood was perfused over a collagen, tissue factor and thrombomodulin surface in the presence of tissue plasminogen activator, clone 1 fully prevented TAFI activation.
Conclusion(s): We have developed VhH anti‐TM clone 1 which inhibits TAFI activation and enhances tPA‐mediated fibrinolysis under flow. Different from agents that directly target TAFIa, our strategy should preserve direct TAFI activation via thrombin.
Plasminogen Activation in the CNS and Immunity
PB0641
Alternative cleavage method of LPXTG motif in group A streptococcus by LPXTGase‐like protein has a higher selectivity towards aromatic fourth position residues at the sortase A consensus sequence
B. Readnour 1; Y. Ayinuola2; Z. Liang3; B. Russo3; V. Fischetti4; V. Ploplis5; F. Castellino5
1 University of Notre Dame, Mishiwaka, Indiana, United States; 2 University of Notre Dame, Notre Dame, Indiana, United States; 3 W.M Keck Center for Transgene Research, Mishawaka, Indiana, United States; 4 Rockefeller University, New York City, Indiana, United States; 5 W.M. Keck Center for Transgene Research, Notre Dame, Indiana, United States
Background: Group A Streptococcus (GAS) is a human specific gram‐positive bacterium responsible for several deadly infections, such as necrotizing fasciitis and streptococcal toxic shock syndrome. Plasminogen‐binding group A streptococcal M‐like protein (PAM) is the primary protein responsible for binding human plasminogen (hPg) in several Group A streptococcus (GAS) strains and is bound to the cell wall through cleavage and transpeptidation by Sortase A (srtA) interacting with the LPXTG motif.
Aims: Investigating an alternative cleavage site of the LPXTG motif in AP53 GAS cells characteristic of LPXTGase that shows an increased affinity for aromatic residues in the fourth position.
Methods: Peptides with mutations in the fourth position of the LPXTG motif containing FRET partners, Dabcyl and EDANS were synthesized (Dabcyl‐LPSXGEAA‐EDANS). Tyrosine at X had the lowest affinity for srtA. A mutant strain of AP53 containing PAM protein with a mutated LPSYG was made and tested to determine the levels of PAM within the cell wall and cell membrane. Cell membrane fractions were tested with the peptides to determine cleavage rates and mass spectrometry was performed to determine the cleavage sites for Dabcyl‐LPSTGEAA‐EDANS and Dabcyl‐LPSYGEAA‐EDANS.
Results: The LPSYG peptides showed reduced affinity for srtA protein. However, LPSYG‐mutated PAM protein was cleaved and transpeptidated at the same level as WT‐PAM. Interactions between the peptides and AP53 cell membrane fractions showed that Dabcyl‐LPSYGEAA‐EDANS was cleaved at rates higher than Dabcyl‐LPSTGEAA‐EDANS in both wild type and srtA membranes. Mass spectrometry results show an alternative cleavage site for LPSYGEAA.
Conclusion(s): LPSYGEAA was shown to be cleaved at rates far higher than the WT srtA consensus sequence in both the presence and absence of srtA. Mass spectrometry results show that the LPSYGEAA is cleaved at an alternative site C‐terminal to the glutamic acid which has been shown to be the cleavage site for another membrane protein in GAS, LPXTGase.


Thrombolytic Therapy
VPB0646
Thrombolytic therapy in pulmonary embolism patients with thrombocytopenia: A systematic review
F. Ata 1; W. H. Ibrahim1; M. Affas1; H. Ahmad Khan2; B. Daoudi1; H. Waqas Younas3; Z. Maat1; S. Mohamed1
1 Hamad Medical Corporation, Doha, Ad Dawhah, Qatar; 2 Nishtar Hospital Multan, Multan, Punjab, Pakistan; 3 NHS Forth Valley Royal Hospital, Larbert, Falkirk, Scotland, United Kingdom
Background: PE remains a common diagnosis in the emergency departments and requires urgent attention. Despite vast research on its management, there are still controversial management guidelines. More specifically, the role of thrombolysis in PE patients with relative contraindications is unclear. There are various reports of patients with PE who had thrombocytopenia and underwent thrombolysis.
Aims: This review aims to pool data from reports of thrombolytic therapy in patients with PE in the context of thrombocytopenia, focusing on the patients' demographics, methods of thrombolysis used, complications, and outcomes of these patients.
Methods: We searched English articles from PubMed, Scopus, and Google Scholar reporting patients with PE, who had thrombocytopenia and underwent thrombolysis. Demographics, clinical characteristics, management, and outcomes of patients were extracted and analyzed in Microsoft Excel 2020 and SPSS version 26 in the form of a systematic review.
Results: We added 11 case reports to this review. The average age of cases presenting with PE and thrombocytopenia who received thrombolysis was 50.5 years, with 54.5% females. Dyspnea (58.3%), Orthopnea (50%), and lower limb pain (25%) were the most commonly reported clinical findings. Most common thrombolysis method was systemic (58.3%) followed by catheter‐guided thrombolysis (41.7%). Most common thrombolytic agents used were alteplase (83%) and urokinase (8.3%). Out of 11 articles, one patient who had a segmental PE died due to aspiration pneumonia. Interestingly, no thrombocytopenia‐related complications were found. Complications were early infection in one case (8.3%), and aspiration pneumonia in one case (8.3%).
Conclusion(s): Thrombolytic therapy may have a mortality benefit with minimal bleeding risk even in patients with PE in the context of thrombocytopenia. More extensive studies on this specific subset are required to refine the guidelines on the use of thrombolysis in PE.

VPB0648
Combination thrombolytic therapy using electrohydraulic radial and focused shock waves in the animal model of embolic common carotid artery occlusion
H. Mehrad
Islamic Azad University, Tabriz Branch‐ Faculty of Basic Sciences, Tabriz, Azarbayjan‐e Sharqi, Iran
Background: A plaque may rupture with high risk of subsequent thrombus mediated acute clinical events such as myocardial infarction and stroke. The efficacy of intravenous thrombolytic agent recombinant tissue plasminogen (tPA) is limited owing to a relatively poor recanalization rate and incomplete function recovery in the majority of treated patients.
Aims: In this study, we developed an experimental combined electrohydraulic radial and focused shock wave generators, and investigated its effectiveness on the thrombus reduction in the rabbit common carotid artery.
Methods: Male New Zealand white rabbits were submitted to common carotid artery clot embolism. Then treatment group underwent combined electrohydraulic radial and focused shock wave therapy (Focused: V = 20 kv, F = 0.5 Hz, Impulses = 120 and Radial: V = 12 kv, F = 0.5 Hz, Impulses = 120) accompanied by tPA administration, wherein diagnostic B‐ mode ultrasound is combined with therapy system, with a goal of increased safety. Blood volume flow and blood mean velocity were measured by color Doppler ultrasonography. Moreover, percentage of luminal cross‐sectional area of stenosis were measured by B‐mode ultrasound at the occluded region after treatment.
Results: Results showed a significant reduction in the mean value for blood mean velocity and the percentage of luminal cross‐sectional area of stenosis and a significant increase in the mean value for blood volume flow in the treatment group compared with the other groups (p< 0.05).
Conclusion(s): Enhanced inertial cavitation‐ mediated thrombolytic effect of focused shock waves, induced by intravascular stable bubbles vibration effect of radial shock waves accompanied by anti‐ thrombotic effect of tPA, can cause to reduce the thrombus content and significantly dilate the luminal cross‐sectional area of stenosis. Combined radial and focused shock wave thrombolytic therapy may be a potential treatment for embolism.
VPB0647
DNase 1 unveils the presence and unlocks the thrombolytic potential of intraveneously administered t‐PA in large vessel occlusion acute ischemic stroke
L. Di Meglio 1; M. Solo Nomenjanahary2; M. BOURRIENNE3; V. Ollivier2; M. Mazighi4; B. Ho‐Tin‐Noé5; J. Desilles6
1 INSERM Unit 1148, PARIS, Ile‐de‐France, France; 2 INSERM UNIT 1148, PARIS, Ile‐de‐France, France; 3 Hôpital Beaujon (APHP) ; INSERM LVTS U1148 Université de Paris, CLICHY, Ile‐de‐France, France; 4 ROTHSCHILD FOUNDATION HOSPITAL PARIS, Paris, Ile‐de‐France, France; 5 INSERM, PARIS, Ile‐de‐France, France; 6 Rothschild Foundation Hospital, Paris, Ile‐de‐France, France
Background: DNase 1 has been shown to potentiate the thrombolytic activity of t‐PA added ex vivo to AIS thrombi. Nevertheless, it remains unknown whether extracellular DNA in AIS thrombi is responsible for intravenous thrombolysis (IVT) failure in AIS due to large vessel occlusion (LVO).
Aims: We investigated whether biologically relevant intrathrombus concentrations of t‐PA were achieved following IVT in patients with LVO‐related AIS, and whether extracellular DNA contributed to IVT failure.
Methods: In this cohort study, a total of 103 thrombi from AIS patients with LVO having received (n = 50) or not (n = 53) IVT prior to MT were analyzed. While 83 thrombi were compared for t‐PA content, a subset of 10 thrombi per patient group was subjected to ex vivo thrombolysis by addition of plasminogen, in the presence or absence of DNase 1.
Results: AIS thrombi from the IVT group contained more tPA than those from the non‐IVT group (0.093 vs. 0.209 μg/mg of thrombus, p < 0.0001). Thrombus Glu‐plasminogen content was similar in both groups. There were no correlations between thrombus t‐PA content of IVT‐treated patients and thrombus cell composition or site of occlusion. A highly significant negative correlation was found between thrombus t‐PA content and IVT to thrombectomy delay (p = 1.2 x 10‐5). Ex vivo thrombolysis assays revealed that, whereas addition of plasminogen alone did not induce lysis of AIS thrombi from either group of patients, it caused a significant decrease in thrombus weight in the IVT group in the presence of DNase 1.
Conclusion(s): These results indicate that intrathrombus t‐PA concentrations reached after IVT bear a therapeutic potential that is however impaired by DNA presence. Our results stress the interest of using DNase 1 as an adjuvant thrombolytic therapy to current IVT t‐PA regimens.
PB0642
The use of plasminogen activator inhibitor‐1 specific Affimers as a tool to modulate fibrin clot properties and thrombosis risk
R. Altalhi 1; C. Tiede1; F. Phoenix1; D. Tomlinson1; K. Naseem1; R. Ajjan1; N. Pechlivani2
1 University of Leeds, Leeds, England, United Kingdom; 2 University of Ledds, LEEDS, England, United Kingdom
Background: Hypofibrinolysis is associated with increased risk of thrombotic disease. Diabetes is characterized by hypofibrinolysis and elevated levels of plasminogen‐activator inhibitor (PAI)‐1 which is stabilised by binding to vitronectin. We hypothesise that inhibition of PAI‐1 activity using PAI‐1/vitronectin‐bound conformational peptides, named Affimers, improves hypofibrinolysis and consequently decreases the risk of thrombosis.
Aims: Isolate PAI‐1/vitronectin binding Affimers and characterise their role in modulating fibrinolysis.
Methods: A large phage display library of Affimers (>3 × 1010) was used to screen for PAI‐1/vitronectin binders. Functional analysis of Affimers was undertaken using turbidimetric and thromboelastometric assays employing plasma and whole blood systems.
Results: 104 PAI‐1/vitronectin Affimers were isolated of which 15 demonstrated distinct sequences. These Affimers were subcloned and expressed in E. coli and one Affimer, named RV8, reduced fibrin clot lysis time in plasma samples of patients with Type 2 diabetes (T2DM) without affecting fibrin network structure. RV8 decreased lysis time by 27% in T2DM pooled plasma when used at 5 μg/ml, and by 5‐27% when samples from 12 different T2D individuals were tested. Whole blood ROTEM analysis (n = 3 samples) revealed a 49% reduction in lysis time without affecting the maximum clot firmness (MCF) which remained similar to control.
Conclusion(s): Our data confirm the viability of this novel approach of isolating PAI‐1 binding Affimers, modulating fibrin clot lysis and in turn identifying potential therapeutic targets to reduce thrombosis risk. The effects of RV8 appears to be more pronounced in whole blood, possibly due to the contribution of platelet‐released PAI‐1 to modulation of fibrinolysis.
PB0644
Effect of novel thrombolytic Aptamer DTRI‐031 on microfluidic thrombolysis and VWF function dose‐response
S. Shea 1; R. Rassam1; K. Thomas2; C. Daniel3; P. Spinella4; S. Nimjee5
1 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 2 Department of Pediatrics, Washington University in St. Louis, St. Louis, Missouri, United States, 32. Department of Pediatrics, Washington University in St. Louis, St. Louis, Missouri, United States; 4 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA; Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States; 5 Department of Neurological Surgery, The Ohio State University Medical Center, Columbus, Ohio, United States
Background: The interaction between von Willebrand Factor (VWF) A1 domain and platelet GPIbα is essential for arterial thrombosis. Aptamer DTRI‐031 selectively inhibits VWF A1 and has potent thrombolytic properties. The ideal clinical assay to detect DTRI‐031 functional efficacy and the corresponding therapeutic range are unknown. We measured dose responsiveness of DTRI‐031 in a custom microfluidic model of thrombolysis and currently available clinical assays.
Aims: The aim of this study is to determine the capacity of clinical assays to reflect microfluidic thrombolysis relative to measurable inhibition induced by DTRI‐031.
Methods: Healthy human whole blood (WB, N = 10) was dosed with vehicle, 423 nM, 846 nM, 1692 nM, or 3384 nM DTRI‐031 to approximate an in vivo dose range of 0.25–2 mg/kg. VWF function was assayed via VWF:Ag, VWF:Ac (Siemens GP1bα‐interaction specific assay, unavailable in US), T‐TAS, VWF:RCo, ristocetin impedance aggregometry (RIA). WB was also stained with fluorescent CD41 antibody (platelet label) and perfused through a stenotic microfluidic chamber to induce partial thrombotic occlusion (25 mmHg pressure increase), followed by DTRI‐031 perfusion. Maximum reduction in thrombus surface was calculated using auto‐thresholding (Python v3.10) of CD41‐positive surface area yielding microfluidic lytic efficacy (MLE). One‐way ANOVA and multiple comparisons were used to assess dose response. Pearson correlation coefficients were calculated between assays.
Results: VWF assays were inhibited by DTRI‐031 (Figure 1) while VWF:Ag was, as expected, constant. MLE trended higher at the midrange dose. Correlation with dose was highest in VWF:Ac, VWF:RCo, and RIA, while MLE correlated best with T‐TAS and VWF:Ag (Figure 2).
Conclusion(s): Inhibition of GPIbα by DTRI‐031 is dose‐dependent and measurable on currently available clinical assays. In a thrombolysis microfluidic model, 3384 nM DTRI‐031 was less efficacious than 1692 nM DTRI‐031, perhaps indicating off‐target effects at the highest dose. MLE correlated best with T‐TAS, a function‐under‐flow assay. Further investigation of DTRI‐031 mechanisms will provide insight into discrepancies between static assays and flow‐based assays.


VPB0645
Modulation of plasmin inhibitor activities, using Affimer technology, improves the hypofibrinolytic environment in high vascular risk individuals
B. Alsayejh 1; N. Pechlivani2; C. Tiede3; F. Phoenix3; D. Tomlinson3; S. Ponnambalam3; R. Ajjan3
1 Division of Cardiovascular & Diabetes Research, Leeds Institute of Cardiovascular and Metabolic Medicine (LICAMM), University of Leeds, Leeds, UK., leeds, England, United Kingdom; 2 University of Ledds, LEEDS, England, United Kingdom; 3 University of Leeds, Leeds, England, United Kingdom
Background: Enhanced fibrin clot formation and/or suppression of fibrinolysis are associated with increased risk of vascular occlusion. Plasmin inhibitor (PI), a key anti‐fibrinolytic protein, is cross‐linked into fibrin networks with higher concentrations of PI documented in fibrin clots and plasma from high vascular risk individuals.
Aims: Use PI‐binding proteins, termed Affimers, for improving the hypofibrinolytic environment in high vascular risk individuals, including patients with cardiovascular disease (CVD) and those with type 1 and type 2 diabetes (T1D and T2D).
Methods: PI‐specific binders were isolated from a large library of Affimers using a phage display system. Turbidimetric assays and ROTEM were used to assess the effects of a PI‐binding Affimers on clot formation and lysis.
Results: Of n = 28 of PI binding Affimers, one (A11) reduced clot lysis time from 66.67 ± 5.78 to 28.40 ± 8.70 minutes in clots made from pooled human plasma (p < 0.05), and from 49.40 ± 25.79 to 15.93 ± 7.41 minutes (p < 0.05) in clots made from purified fibrinogen in the presence of PI and factor XIII. The effects of A11 was concentration‐dependent with maximum effects reached at 40:1 Affimer:PI molar ratio (107 μg/ml of Affimer in plasma and 319 μg/ml in purified). Moreover, Affimer A11 had consistent effect on clot lysis time in different populations of patients, using tPA at high or low concentrations (83 ng/ml or 21 ng/ml, respectively). Regardless of tPA concentration, and using individual plasma samples, A11 enhanced clot lysis time by 20‐40% in samples from CVD (n = 15), T1DM (n = 15) and T2DM patients (n = 15), as well as healthy controls (n = 15). In whole blood ROTEM, A11 also reduced from 25.72 ± 8.89 to 14.75 ± 5.19 minutes (n = 6 healthy controls) (p < 0.05) without affecting maximum clot firmness.
Conclusion(s): This work provides proof of concept that PI‐binding Affimers can alter protein function and may be a future tool to modulate fibrin clot lysis and reduce vascular thrombotic events in high risk populations.
PB0643
COVID‐19‐associated hemostasis alterations and outcomes in acute ischemic stroke patients treated with intravenous thrombolysis
L. Lóczi1; R. Orbán‐Kálmándi 2; I. Szegedi3; T. Árokszállási4; J. Tóth5; L. Oláh4; L. Csiba6; Z. Bagoly7
1 University of Debrecen; Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary; 2 University of Debrecen; Faculty of Medicine, Department of Laboratory Medicine, Division of Clinical Laboratory Sciences, Debrecen, Hajdu‐Bihar, Hungary; 3 University of Debrecen; Faculty of Medicine, Department of Neurology, Debrecen, Hajdu‐Bihar, Hungary; 4 University of Debrecen; Faculty of Medicine, Department of Neurology, Debrecen, Hajdu‐Bihar, Hungary; 5 University of Debrecen; Faculty of Medicine, Department of Laboratory Medicine, Debrecen, Hajdu‐Bihar, Hungary; 6 University of Debrecen; Faculty of Medicine, Department of Neurology and ELKH‐DE Neurodegenerative and Cerebrovascular Research Group, Debrecen, Hajdu‐Bihar, Hungary; 7 University of Debrecen, Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary
Background: Coronavirus disease‐2019 (COVID‐19) increases the risk of acute ischemic stroke (AIS). Hemostasis alterations and outcomes of reperfusion therapy (thrombolysis or thrombectomy) in COVID‐19‐positive AIS patients are not well studied, as yet.
Aims: We aimed to test hemostasis alterations in COVID‐19‐positive AIS patients receiving intravenous (i.v.) thrombolysis as compared to non‐infected AIS patients and to correlate results with therapy outcomes and safety.
Methods: In this prospective observational study, 110 AIS patients receiving i.v. thrombolysis (recombinant tissue plasminogen activator) with/without thrombectomy were enrolled (April 2020‐December 2021). Blood samples were taken on admission (within 4.5h of symptom onset), at 1h and 24h post‐event. SARS‐CoV‐2 RT‐PCR test performed on admission confirmed acute infection in 9 cases (COVID‐19+ group). Anti‐SARS‐CoV‐2 antibody test proved convalescence and/or vaccination in 48 patients (post‐COVID/post‐vaccination group). Markers of inflammation (CRP, ferritin, IL‐6), D‐dimer, fibrinogen, von Willebrand factor (VWF) antigen, factor VIII (FVIII) and factor XIII (FXIII) activity, clot‐lysis assay, thrombin generation, ROTEM and angiotensin convertase enzyme (ACE)1, ACE2 activities were analyzed. Stroke severity was determined by NIHSS. Therapy‐associated intracerebral hemorrhage was classified according to ECASSII criteria. Short‐ and long‐term outcomes were defined at 7 days and 3 months post‐event according to the change in NIHSS and the modified Rankin Scale, respectively.
Results: Stroke severity was significantly greater in the COVID‐19+ group. VWF antigen levels were markedly elevated in the COVID‐19+ group as compared to non‐infected and post‐COVID/post‐vaccination groups (323 ± 72% vs. 248 ± 75% and 222 ± 80%, respectively, p = 0.006). FVIII levels were parallel to VWF levels and showed significant elevation in the COVID‐19+ group. Short‐term outcomes of therapy and the occurrence of hemorrhagic transformation did not differ between groups.
Conclusion(s): Elevated FVIII and VWF levels in COVID‐19‐associated AIS seem to be linked to endothelial cell injury and are associated with more severe stroke. Efficacy of thrombolysis in COVID‐19+ AIS patients was similar to non‐infected patients in this cohort.
Hemophilia and Rare Bleeding Disorders
Acquired Hemorrhagic Coagulation Disorders
PB0167
Emicizumab use in acquired von Willebrand Disease: Single Case Report
M. Escobar 1; I. Aboshady2; N. Montanez2
1 University of Texas Health Science Center, Houston, Texas, United States; 2 The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, Texas, United States
Background: Acquired von Willebrand syndrome (AVWS) is a rare bleeding disorder characterized by functional or structural defect of von Willebrand factor (VWF) often secondary to autoimmune or lymphoproliferative disorders. Management concentrates on eradicating autoantibody and achieving hemostasis. Currently, there are no evidence‐based guidelines for management, however potential of emicizumab prophylaxis in the management of severe congenital von Willebrand disease has been reported.
Aims: Describe the use of emicizumab prophylaxis in the management of AVWS.
Methods: Chart review of 70 y/o Caucasian female diagnosed with AVWS in the setting of Rheumatoid Arthritis (RA) and Parkinson's disease following recurrent GI bleeding unresponsive to VWF replacement. Coagulation studies suggest severe von Willebrand disease (FVIII 5%, VWF: Ag 11%, VWF R:Co < 15%), full gene sequencing of the VWF (exons 1‐52) resulted in negative findings, with normal VWF GP1bM and VWF propeptide antigen assay. Suggesting markedly low VWF antigen is due to increased clearance of VWF without inhibition of function secondary to acquired inhibitor, likely resulting from active RA.
Results: Emicizumab standard prophylaxis (1.5 mg/kg/ once weekly) started following waning response to maintenance IVIG ~1000 mg/kg/dose every 4 weeks. Despite coagulation studies remaining at baseline (C: FVII 10%, VWF: Ag 12%, VWF R: Co < 15%) patient experienced no reported bleed events within the first 6 months, with increased Hemoglobin. Emicizumab equivalent FVIII levels averaged 28.6 μg/ml. Eradication of autoantibody remains unachievable lack of response to management of underlying disease mechanisms (RA) with Rituximab and Methotrexate.
Conclusion(s): In patients with AVWS, use of low dose IVIG maintenance therapy can achieve temporary favorable response to prevent bleeding symptoms with declining response noted ≥6 months. While the use of emicizumab prophylaxis in AVWS is very limited this case illustrates its efficacy and safety using standard dose prophylaxis in an AVWS patient with multiple comorbidities. Further studies in this disease are warranted.
PB1431
Acquired von Willebrand syndrome in essential thrombocythemia patients referred to special coagulation laboratory of Iranian Blood Transfusion Organization (IBTO)
A. Rajabi1; M. Ahmadinejad2; S. Ebrahimi3; M. Mojtabavi Naeini4; S. Riahi4; R. Shamriz4; P. Eshghi
1 Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 2 Blood Transfusion Research Center, High Institute For Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 3 Departmant of oncology, Loghman Hakim hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tehran, Tehran, Iran; 4 Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran
Background: Although acquired von Willebrand syndrome (AVWS) is rare, most cases are frequently underdiagnosed or misunderestimated. The disease is due to the quantitative or qualitative defects in von Willebrand factor (vWF) secondary to other medical conditions such as myeloproliferative neoplasms including essential thrombocythemia (ET). ET has a complicated condition of both thrombotic and hemorrhagic events. Aspirin will be prescribed for the prevention of thrombosis, but patients with extreme thrombocytosis (platelet counts >1000×109/L) and a ristocetin cofactor activity (RCo) < 30%, should not receive aspirin because of an excessive risk of bleeding.
Aims: We aimed to report cases of AVWS in ET patients who were referred to our laboratory during the last decade.
Methods: Three patients with diagnosed ET and the possibility of AVWS were referred to the reference coagulation laboratory of IBTO for coagulation testing during the last decade. All three cases were referred in the last 6 months. Standard von Willebrand panel coagulation tests were done and ISTH‐BAT was used for the assessment of bleeding scores.
Results: We describe three AVWS cases with ET, two females with 28 and 42 and one male with 49 years old. All patients had extreme thrombocytosis with no significant history of bleeding events. Laboratory results are shown in Table 1.
Conclusion(s): During the last decade, we have only three cases referred for the evaluation of AVWS and all were ET patients. It seems that there is much more than the world underestimation of the disease in our country. Raising awareness of practicing physicians about the need for this experience and conduction of facilities in specialized coagulation laboratories would be helpful for avoiding missing the AVWS cases.

PB0169
Proof of principle of a fast and fully automated FVIII functional inhibitor test
B. Verbruggen 1; N. Binder2; G. Moore3; R. Polenewen4
1 Hemostad, Nijmegen, Gelderland, Netherlands; 2 Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH, Vienna, Wien, Austria; 3 Cambridge University Hospitals, Bexley, England, United Kingdom; 4 Hemostad, Zevenaar, Gelderland, Netherlands
Background: Laboratory detection of Factor (F)VIII inhibitors is preferentially performed with the Nijmegen assay. It requires 2‐hours incubation of test sample/FVIII‐source mixture because of slow FVIII inactivation, due to its reversible binding to von Willebrand Factor (VWF) delaying inhibitor action. Extended incubation time results in non‐specific FVIII inactivation, that, together with complicated liquid handling, may contribute to the considerable variability (CV:30–40%) seen in inter‐laboratory surveys (e.g. ECAT, UKNEQAS). We hypothesize that testing in a VWF‐free assay matrix using recombinant (r)FVIII can dramatically lower incubation time that, together with full automation, will substantially improve standardisation.
Aims: Test proof of principle of a fast, fully automated FVIII inhibitor test using VWF‐free rFVIII and a dedicated analyser.
Methods: As in the original Nijmegen assay, test samples are heated for 90 min at 58°C and centrifuged for 10 min to destroy residual FVIII. The coagulation analyser employed must provide on board ability of three subsequent sample dilution steps and three reagent additions. An application was defined on a Ceveron s100 (Technoclone), as below. After loading the heat inactivated samples, sequential automated analytical steps occur as follows: 1. Predilution with heat inactivated FVIII/VWF deficient plasma (if needed) 2. Mixing with rFVIII (1.0 IU/mL) 3. Incubation for 5–10 min at 37°C 4. Dilution of incubated samples 1:10 with Imidazole buffer, pH 7.3. and analysis for residual rFVIII activity
Results: Two samples whose inhibitor activities with the original Nijmegen assay were 0 and 14 NBU, were analysed with the automated method resulting in activities of 0 and 11 NBU respectively. Incubation for 5 and 10 min yielded similar results.
Conclusion(s): Rapid, fully automated FVIII‐inhibitor testing can be performed with a dedicated coagulation analyser using rFVIII in a VWF‐free matrix. Automation and reduced assay time improve viability and availability of a normally protracted assay, permitting a more rapid and informed clinical response.
PB0166
Five cases of acquired haemophilia A (AHA) following COVID‐19 vaccination in Australia
C. Dix 1; B. Reardon2; J. Curnow2; R. Bird3; S. P'ng4; H. Tran5
1 The Alfred Hospital, Melbourne, Victoria, Australia; 2 Department of Haematology, Westmead Hospital, Sydney, New South Wales, Australia; 3 Princess Alexandra Hospital, Woolloongabba, Queensland, Australia; 4 Fiona Stanley Hospital, Perth, Western Australia, Australia; 5 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia
Background: Acquired Haemophilia A (AHA) is a rare but devastating acquired bleeding disorder, caused by autoantibodies against Factor VIII (FVIII). Up to 50% are associated with an underlying disorder including autoimmune disease, malignancy or pregnancy with the remainder considered idiopathic. There have been case reports of AHA occurring post vaccination, including following influenza and mRNA‐based COVID‐19 vaccination. Here we report on five cases of AHA following adenovirus‐vector COVID‐19 (ChAdOx1 nCoV‐19) vaccination in Australia.
Aims: Report on the clinical characteristics of five patients diagnosed with AHA following ChAdOx1 COVID‐19 vaccination
Methods: We collected clinical details of cases of AHA reported after COVID‐19 vaccination in Australia.
Results: Five patients were identified. All were aged >70 years and presented between 19‐ and 35‐days following ChAdOx‐1 vaccination. Table 1 outlines baseline and disease characteristics and outcomes. All presented with bleeding requiring hospitalisation; two subcutaneous, one oropharyngeal, one intramuscular and one presented with subcutaneous bleeding initially and subsequently had a large retroperitoneal haemorrhage. Three required bypassing agents (BPA) to control bleeding. No underlying aetiology was found despite investigation for malignancy and autoimmune disease. Four received first‐line dual immunosuppressive therapy (prednisolone with either cyclophosphamide or rituximab) and one received triple therapy (prednisolone, cyclophosphamide and rituximab). One also received intravenous immunoglobulin (IVIg). All achieved complete remission.
Conclusion(s): We report on five cases of AHA occurring following ChAdOx‐1 COVID‐19 vaccination. Autoimmune disorders are known to occur post vaccination, with postulated mechanisms including antigenic mimicry and non‐specific activation of quiescent autoreactive T and B cells. While there was a clear temporal relationship between all five cases of AHA and the ChAdOx‐1 COVID‐19 vaccination, all were aged >70 years, the age group more likely to be affected by AHA. Causation cannot be definitively proven, however these cases may highlight the risk of a rare adverse event following immunisation (AEFI).

PB0168
Acquired Hemophilia in four north European countries, a survey of 181 patients
R. Lindahl 1; A. Leithinen2; V. Nummi3; T. Szanto4; L. Hiltunen5; A. Olsson6; A. Glenthoej7; R. Chaireti8; I. Vaide9; E. Funding10; E. Zetterberg11
1 Lund University, Malmö, Skane Lan, Sweden; 2 Coagulation Disorders Unit Department of Hematology Comprehensive Cancer Center HUCH and University of Helsinki, Helsinki, Uusimaa, Finland; 3 Department of Hematology, Coagulation Disorders Unit, Comprehensive Cancer Center, Helsinki University Hospital, Helsinki, Uusimaa, Finland; 4 Coagulation Disorders Unit, Comprehensive Cancer Center, Helsinki University Hospital, Helsinki, Uusimaa, Finland; 5 Fimlab Laboratoriot Oy Ltd. Hemostasis and Platelet Laboratory, Vantaa, Uusimaa, Finland; 6 Sahlgrenska University Hospital, Department of Medicine, Gothenburg, Vastra Gotaland, Sweden; 7 Copenhagen University Hospital/Copenhagen University, Copenhagen, Hovedstaden, Denmark, 8 (1) Department och Molecular Medicine and Surgery, Karolinska Institute och (2) Department of Haematology, Karolinska University Hospital, Stockholm, Stockholms Lan, Sweden; 9 University of Tartu, Department of Hematology and Oncology, Tartu, Tartumaa, Estonia, 10 Rigshospitalet, Copenhagen, Hovedstaden, Denmark, 11 Department of Hematology, oncology and radiation physics, Coagulation Unit, Skane University Hospital, Malmö, and Lund University, Sweden, Malmö, Skane Lan, Sweden
Background: Acquired haemophilia A (AHA) is a rare bleeding disorder due to antibodies against coagulation factor VIII. Treatment involves the use of coagulation concentrates as well as immunosuppressive treatment (IST). Current guidelines on AHA are based on retrospective data, derived from studies conducted before 2015. No studies have been performed in the Nordic countries in recent times.
Aims: To collect retrospective data on patients diagnosed with AHA in the Nordic countries 2006‐2018 and compare demographic data and clinical outcomes with previously published reports.
Methods: In the Nordic Acquired Haemophilia A study, data was collected in 2020‐2021 by six haemophilia centers: three Swedish, one Finnish, one Danish and one Estonian. Data on demographics, underlying conditions, bleeding episodes, haemostatic and immunosuppressive therapy (IST), response to therapy, adverse events, relapse and death, were collected.
Results: The study included 181 patients. Median age at diagnosis was 76 years, with even sex distribution, 48% females and 52% males. Type of and severity of bleeding was comparable to the large EACH2 study (Table 1). Bleedings were primarily treated with recombinant FVIIa (rFVIIa) and activated prothrombin complex concentrate (APCC). Treatment of bleeds with rFVIIa was less efficient than in the EACH2 study (55% vs. 91% of treatments considered efficient). As IST, most patients were treated with corticosteroids as monotherapy, but the highest success rate was seen in patients treated with rituximab and corticosteroids in combination (82%) (Table 2).
Conclusion(s): Data on 181 patients collected from 6 centers in 4 north European countries show similar demographic and clinical features as in previous studies on AHA. However, the success rate of rFVIIa in treatment of bleeds was lower than in previous studies. Rituximab based regimens were the most efficient IST.


VPB0170
Correlation of factor VIII activity measured by one‐stage clotting and chromogenic substrate assays in a phase III study of emicizumab prophylaxis for acquired hemophilia A (AGEHA)
K. Yoneyama 1; K. Tokuda2; R. Ozaki1; R. Kobayashi1; Y. Oguchi1; A. Kiialainen3; K. Amano4; M. Shima5
1 Chugai Pharmaceutical Co., Ltd., Tokyo, Tokyo, Japan; 2 Chugai Pharmaceutical Co., Ltd., Kamakura, Kanagawa, Japan; 3 F. Hoffmann‐La Roche Ltd., Basel, Basel‐Stadt, Switzerland; 4 Tokyo Medical University Hospital, Tokyo, Tokyo, Japan; 5 Nara Medical University, Kashihara, Nara, Japan
Background: Emicizumab is a bispecific antibody mimicking the cofactor function of activated factor (F) VIII. In a phase III study of emicizumab in patients with acquired hemophilia A (PwAHA) (AGEHA), endogenous FVIII activity was monitored and restoration to >50 IU/dL was employed as a criterion for guiding completion of emicizumab prophylaxis. A one‐stage clotting assay with emicizumab neutralization by adding anti‐emicizumab antibodies (OSAwEN) was used for the measurement.
Aims: To assess whether chromogenic substrate assays using bovine or human coagulation factors (bCSA or hCSA) can be alternative methods for OSAwEN to measure FVIII activity for guiding completion of emicizumab prophylaxis in PwAHA.
Methods: FVIII activity was measured by OSAwEN, bCSA, and hCSA in AGEHA, and their correlations were evaluated. AGEHA was conducted in accordance with relevant ethical standards.
Results: When >50 (I)U/dL was defined as a positive result, bCSA and hCSA provided positive, negative, and overall percent agreements of ≥80% with OSAwEN (Table). A linear simple regression estimated a slope slightly above 0.9 for OSAwEN values against bCSA values. Given the reactivity of emicizumab in hCSA, a linear multiple regression was performed incorporating plasma emicizumab concentrations (Cemi) in addition to OSAwEN values as predictor variables against hCSA values, estimating a slope slightly below 0.9 for OSAwEN values. bCSA and hCSA were predicted to have Pearson’s correlation coefficients of ≥0.9 with OSAwEN and to derive 51.8 and 53.7 U/dl, respectively, corresponding to 50 IU/dl for OSAwEN in the absence of emicizumab. Add‐on effect of emicizumab in hCSA was predicted to correspond to approximately 15 U/dl with the observed mean steady‐state trough Cemi slightly above 35 μg/ml in AGEHA (Figure).
Conclusion(s): bCSA and hCSA showed good correlations with OSAwEN and may be applicable to measure FVIII activity for guiding completion of emicizumab prophylaxis in PwAHA. However, add‐on effect of emicizumab should be considered when interpreting hCSA values.


Disseminated Intravascular Coagulation
VPB0171
The effect of anti‐coagulant agents against in vitro hemophilia A plasma coagulation and fibrinolysis potential in the presence of emicizumab
T. Onishi 1; S. Harada2; H. Shimo3; Y. Tashiro2; T. Soeda2; K. Nogami4
1 Pediatrics/Nara Medical University, Kashihara, Nara, Japan; 2 Chugai Pharmaceutical Co., Ltd., Kamakura, Kanagawa, Japan; 3 Pediatrics/Nara Medical University, Kashihara, Nara, Japan; 4 Nara Medical University, Kashihara, Nara, Japan
Background: Emicizumab is a factor (F) VIII‐function mimetic antibody for the treatment of people with hemophilia A (PwHA) with or without inhibitors. Infection can cause varying degrees of hemostatic dysfunction, especially bacterial infection, which can lead to a pro‐coagulant state. There is limited information about coagulation and fibrinolysis status in infected PwHA treated with emicizumab and the use of anti‐coagulant agents in such cases.
Aims: In this study, we investigated how anti‐coagulant agents affect the balance of coagulation and fibrinolysis in PwHA plasma spiked with emicizumab.
Methods: PwHA plasma was supplemented with 50 μg/ml of emicizumab and anti‐coagulant agents (heparin, nafamostat mesylate (NM), recombinant thrombomodulin(rTM)). PwHA plasma spiked with 100 IU/dl of FVIII was used as a normal reference. The coagulable and fibrinolytic status of plasma was measured by Thrombin/Plasmin‐Generation Assay (T/P‐GA) and Clot‐Fibrinolysis Waveform Analysis (CFWA).
Results: In T/P‐GA and CFWA, spiking PwHA plasma with emicizumab improved coagulation function but had no effect on fibrinolytic function. Adding heparin, NM, and rTM to normal reference plasma inhibited both the peak thrombin (Th‐Peak) and the peak plasmin (Plm‐Peak) in T/P‐GA. In CFWA, heparin, NM, and rTM also reduced the maximum coagulation velocity (|min1|) and the maximum fibrinolytic velocity (|FL‐min1|). The addition of heparin, NM, and rTM to PwHA plasma spiked with emicizumab suppressed the Th‐Peak and the Plm‐Peak of T/P‐GA. In CFWA, heparin, NM, and rTM also reduced the |min1|and the |FL‐min1|. These results showed a tendency similar to the inhibitory effect of anti‐coagulant agents on coagulation and fibrinolysis function in normal reference plasma.
Conclusion(s): Anti‐coagulant agents exhibit inhibitory effects on the coagulation and fibrinolytic function in PwHA plasma spiked with emicizumab similar to those in normal reference plasma.
Hemophilia ‐ Basic
PB0182
Results of the implementation of home management in times of the covid‐19 pandemic in patients with hemophilia A in Colombia
S. Sarmiento1; G. Diaz Mosquera 2; J. cortes3; C. Agudelo Rico4; J. Peña Siado2; N. Ramirez Plazas2; A. Parrado Rey2; F. Meza Cadavid2; C. Montaño Mejía5; A. Maza Villadiego2; I. Perdomo Amar5
1 Integral Solutions sd, Bogota, Distrito Capital de Bogota, Colombia; 2 Integral Solutions SD, Bogota, Distrito Capital de Bogota, Colombia; 3 Integral Solution SD, Bogota, Distrito Capital de Bogota, Colombia; 4 Integral Solutions Research, Bogota, Distrito Capital de Bogota, Colombia; 5 Integral solutions SD, Bogota, Distrito Capital de Bogota, Colombia
Background: On March 11, 2020, the World Health Organization declared COVID‐19 a pandemic, generating impediments in the follow‐up and treatment of patients with hemophilia, altering the provision of services.
Aims: To determine the number of bleeding episodes, hospitalizations and infection by COVID‐19 after implementing a comprehensive management program adapted to the requirements of the pandemic in the framework of the provision of health services in patients with hemophilia A (HA).
Methods: Descriptive, retrospective study in a cohort of 107 patients with HA in a health center (Integral Solutions SD), between March‐2020 and December‐2021, based on the analysis of the variables: Annual bleeding rate (ABR), Hospitalization, cases by covid‐19 in patients with HA in the face of the implementation of strategies such as: education, implementation of biosafety processes as well as facilitating treatment at the patients' homes within a comprehensive management program in times of pandemic.
Results: Of the 107 patients, 75.7% presented severe HA, all maintained their home prophylaxis, with an ABR of 0.52, of these bleeds, 99.1% had home management and 0.9% required hospitalization for one day. 1 patient confirmed by covid‐19 and 5 with symptoms without diagnostic confirmation were reported, all recovered without needing hospitalization. Regarding immunization, 56.1% (n = 60) were vaccinated, among whom the most frequent adverse event was pain where they received the vaccine (61.6%) followed by general malaise (25%).
Conclusion(s): During the COVID‐19 pandemic, the personalized comprehensive management program in patients with HA, together with the implementation of home management and the preventive measures adopted, showed a trend in the reduction of ABR compared to national reports from previous years. On the other hand, the proportion of patients with Covid‐19 infection or symptoms in the analyzed population was lower than the general population. All the patients who were vaccinated did not present adverse events different from the general population.
PB0188
World Bleeding Disorders Registry (WBDR): Congenital haemophilia, Ampang Hospital experience
S. Nasution 1; T. Kunju2; S. Edmund3; V. Selvaratnam4; J. Sathar4
1 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Kuala Lumpur, Kuala Lumpur, Malaysia; 2 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Selangor, Malaysia; 3 Clinical Research Centre, Ampang Hospital, Ministry of Health Malaysia, Ampang, Selangor, Malaysia; 4 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Ampang Jaya, Selangor, Malaysia
Background: Ampang Hospital (AH) is a Haemophilia Comprehensive Care Centre (CCCH) in the central region of Malaysia and national referral centre for bleeding disorders. Our centre has one of the highest numbers of patients with haemophilia (PWH) in Southeast Asia. AH uses the WBDR; a web‐based system to gather data of PWH.
Aims: To describe the epidemiology of congenital haemophilia, evaluate & improve the quality of care of PWH in AH.
Methods: A total of 232 PWH which represent 16% of total PWH in Malaysia were registered into the WBDR from April 1, 2019 until May 20, 2020. Clinical records were derived retrospectively from electronic Hospital Information System (eHIS), 2006‐2020. Inclusion criteria; PWH in AH with congenital Haemophilia A/B. Acquired haemophilia and other congenital bleeding disorders were excluded.
Results: Most PWH were diagnosed at 0‐12 months of age. The median age for PWH was 33 years old. Severe haemophilia represents the majority of cases (75%) followed by mild 13.8% and moderate 11.2%. 6% of 188 haemophilia A (HA) & 1.3% haemophilia B (HB) developed inhibitors. All 17 PWH with inhibitors being severe haemophilia; 9 of them needing bypassing agents (4 on FEIBA and 5 on NovoSeven). 76% non‐inhibitor patients with HA require factor VIII concentrates (75.5% on prophylaxis). As for HB, 77.3% require factor IX concentrates (59.1% on prophylaxis). Haemarthrosis was the most common breakthrough bleed. Bleeding rate was the highest in the severe group (78.7%), followed by moderate & mild group (16.7%). Mean annual bleeding rate was 2.9 in prophylaxis and 3.8 in on‐demand group.
Conclusion(s): Mean annual bleeding rate was higher in on‐demand group. Bleeding rate was high in the severe group despite the introduction of prophylaxis regime. Prophylaxis infusion should be started earlier amongst severe PWH. The introduction of myWBDR mobile application may optimise treatment outcome & improve PWH quality of life.

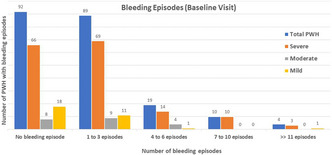
VPB0208
Coagulant potential of emicizumab in child hemophilia A patients
M. Takeyama 1; N. Matsumoto2; H. Abe2; S. Harada2; K. Ogiwara3; S. Furukawa3; T. Soeda2; K. Nogami1
1 Nara Medical University, Kashihara, Nara, Japan; 2 Chugai Pharmaceutical Co., Ltd., Kamakura, Kanagawa, Japan; 3 Department of Pediatrics, Nara Medical University, Kashihara, Nara, Japan
Background: Emicizumab prophylaxis reduces bleedings in the patients with hemophilia A (PwHA). A clinical study (HAVEN 7) including PwHA without inhibitors up to 12 months of age is currently ongoing, but it is not yet clear whether emicizumab affects infant PwHA.
Aims: To examine the coagulation potential of emicizumab in vivo or ex vivo in plasma collected from 0 to 42‐month‐old child PwHA (child‐HA).
Methods: This study was approved by two institutional review boards and informed consent was obtained. Plasma from 27 child‐HA patients up to 42 months of age, receiving emicizumab, a factor (F) VIII agent, or neither emicizumab nor FVIII agent (median age; 19 months) were enrolled. FVIII activity in PwHA receiving FVIII agents was reduced to < 1 IU/dL by the addition of anti‐FVIII A2 monoclonal antibody. Samples of emicizumab‐spiked plasma (ex vivo) or emicizumab‐treated plasma (in vivo) were analyzed. Untreated plasma or emicizumab‐treated plasma supplemented with anti‐emicizumab antibody were used as reference samples, respectively. Global coagulation activity was measured and adjusted for maximum coagulation velocity (Ad|min1|) in a clot waveform analysis (CWA) and peak thrombin was measured using thrombin generation assay (TGA).
Results: Ad|min1| in 24 of the 27 cases was improved in the presence of emicizumab. The 3 child‐HA which did not respond were 1, 23, and 31 months of age. Although 20 of the 27 cases showed an age‐dependent increase in peak thrombin with emicizumab, 7 cases (0, 1, 1, 2, 8, 19, and 36 months) did not. An emicizumab‐dependent increase in coagulant potential was shown in 18 cases by both Ad|min1| and peak thrombin, and in 8 cases by one parameter but not the other. Only 1 case (1 month of age) did not respond with either Ad|min1| or peak thrombin.
Conclusion(s): In general, emicizumab improved coagulant potential in child hemophilia A plasma evaluated by global coagulation assays.
PB0174
Activated factor XI as activator of thrombin generation to increase assay sensitivity
R. Arisz 1; T. van Lienden2; R. de Laat‐Kremers3; J. Eikenboom4; S. Schols5; M. de Maat6
1 Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands., Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands., Rotterdam, Zuid‐Holland, Netherlands; 3 Synapse Research Institute, Maastricht, Limburg, Netherlands; 4 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 5 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands; 6 Erasmus University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Haemophilia A is an X‐linked genetic disorder, leading to a reduced factor VIII (FVIII) activity. FVIII activity levels are often used to classify disease severity. However, FVIII activity levels do not always correlate with bleeding tendency. The thrombin generation assay (TGA) may be a robust assay to predict interindividual bleeding risk, but the sensitivity for low FVIII levels is limited when activated with tissue factor (TF). Activated factor XI (FXIa) has been proposed to increase the sensitivity of the fluorescent signal in the TGA.
Aims: To evaluate if TGA is more sensitive for low FVIII activity levels with FXIa as activator, when compared to activation with TF.
Methods: Platelet poor normal pooled plasma, FVIII deficient plasma (Siemens) or a mix of both plasma’s were used to obtain 0%, 1%, 5%, 10% and 100% FVIII plasma concentrations. A low concentration of tissue factor (1pM) or 0.22 nM activated FXIa, together with calcium and phospholipids, were used to activate the TGA using the calibrated automated thrombogram (CAT; Stago). Results were analysed with the Thrombinoscope software.
Results: Activation with 0.22 nM FXIa resulted in 196%, 335%, 376%, and 455% higher peak heights, compared to activation with 1 pM TF for plasma samples with 1%, 5%, 10%, and 100% FVIII, respectively. The endogenous thrombin potential (ETP) and the lag time did not change despite the variation in plasma FVIII levels after activation with either TF or FXIa.
Conclusion(s): In conclusion, activation with FXIa leads to an increased signal in the TGA compared to activation with TF, especially for FVIII activity levels < 5%. With this alternative activator, the TGA is a promising tool to measure thrombin generation parameters in severe haemophilia A patients. More accurate predictors of bleeding will contribute to personalised treatment for these patients.


VPB0203
Prevalence of inhibitors in haemophilia patients: A study of 92 patients at the Annaba University Hospital Center in Algeria
H. Brouk; A. Amireche
Faculty of Medicine, University of Badji Mokhtar of Annaba, Annaba, Annaba, Algeria
Background: Hemophilia is a hereditary, life‐threatening bleeding disorder characterized by a deficiency or dysfunction of clotting factors VIII (hemophilia A) or IX (hemophilia B), essential coagulation elements in the complex blood clotting cascade. One of the greatest complications in hemophilia is the development of inhibitor (alloantibodies) that inhibit FVIII or FIX activity.
Aims: We aimed to obtain data that are needed on the burden of inhibitors in our region to aid in prevention efforts.
Methods: Blood specimens were obtained from hemophilia subjects to be tested for FVIII and FIX antibodies. Inhibitor analysis was performed in the hemobiology‐blood transfusion service at the Ibn Rochd hospital using a Bethesda assay.
Results: A total of 92 males with hemophilia A (HA) or hemophilia B (HB) were enrolled in the study. Data with complete baseline information were analyzed. Mean age was 28.24 (standard deviation (SD) = 14.7) years, 69.54% were adults, 73.91% of patients were HA and 26.08% were HB. Approximately half of the patients (56%) have severe hemophilia. During the study period there were 10 cases of elevated inhibitor titers among 68 HA which represent 14.70% of cases and no case among HB participants. From the 10 patients who developed FVIII inhibitors, 6 have a high titer and 4 a low titer, two of whom had an inhibitor titer less than 1BU/ml. Two of the low‐titer inhibitors were transient.
Conclusion(s): Our observed prevalence (14.7%) of Bethesda positive subjects is consistent with other studies performed on the same topic. These data will be useful in targeting populations for research and evaluating the effectiveness of public health prevention programs.
PB0172
Results of thromboelastometry and thrombin generation assays for emicizumab prophylaxis monitoring
S. Arcudi 1; M. Clerici2; E. Scalambrino3; R. Gualtierotti4; C. Novembrino5; A. Truma6; A. Ciavarella7; S. Siboni3; E. Biguzzi2; A. Tripodi2; F. Peyvandi8
1 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, MILAN, Lombardia, Italy; 2 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 4 Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 5 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Lombardia, Italy; 6 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Lombardia, Italy; 7 Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 8 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: The ability of coagulation assays to monitor severe hemophilia A (HA) patients on emicizumab prophylaxis has been poorly investigated.
Aims: To report the results of non‐activated rotational thromboelastometry (NATEM), thrombin generation assay (TGA) and to correlate findings with plasma emicizumab concentration (EmiC).
Methods: Consecutive HA patients on emicizumab prophylaxis at steady state were enrolled in an observational prospective study. TGA was assessed with a homemade method upon addition to plasma of tissue‐factor (1 pM) and synthetic phospholipids (1 μM). NATEM was measured for whole blood after addition of CaCl2 (100 mM). Emicizumab plasma concentrations (EmiC) were measured by the modified calibrated one stage FVIII assay. We considered for TGA lag‐time (LT) endogenous thrombin potential (ETP) and peak‐thrombin (PH); for NATEM clotting‐time (CT), clotting‐firmness‐time (CFT), time‐to‐maximum‐clotting‐firmness (MCF‐t). Spearman’s Rho coefficient was used to assess the correlation between variables.
Results: Sixteen patients on emicizumab prophylaxis (aged 17‐64 years) were included in the study. Three were positive for FVIII inhibitors. Results for NATEM and TGA are reported in Table 1. When assessing the correlation with EmiC, NATEM‐CT, NATEM‐CT+CFT and NATEM‐MCF‐t were inversely correlated with EmiC (CT rho = −0.536, p < 0.05; CT+CFT rho = −0.515, p< 0.05; MCF‐t rho = −0.561, p < 0.05). TGA‐ETP and TGA‐PH were positively correlated with EmiC (ETP rho = 0.507, p < 0.05; TGA‐PH rho = 0.643, p < 0.01). TGA and NATEM were inversely correlated (CT vs. ETP, rho = −0.771, p < 0.001; CFT vs. ETP, rho = −0.765, p < 0.001; CT+CFT vs. ETP rho = −0.805, p < 0.001), Figure 1.
Conclusion(s): Our analysis of 16 patients on emicizumab prophylaxis shows that NATEM and TGA correlate with each other and with the concentration of emicizumab. Dose‐adjustment of emicizumab based on laboratory testing is currently not required. However, our preliminary data suggest that NATEM and TGA might be useful to help managing special situations requiring monitoring with a global test, such as surgeries or concomitant treatment with bypassing agents.


PB0192
Bleeding rates in severe non‐inhibitor haemophilia A patients across US, EU4&UK and Japan
A. Amirah1; I. Palau 2; A. Rosli1; N. Allie2
1 Ipsos Sdn Bhd., Kuala Lumpur, Kuala Lumpur, Malaysia; 2 Ipsos, London, England, United Kingdom
Background: Bleeding events can impact the health status of patients living with haemophilia.
Aims: This study aims to investigate bleeding occurrences among severe Haemophilia A patients without inhibitors in US, EU&UK (Germany, UK, France, Italy, Spain) and Japan.
Methods: The Ipsos Hemophilia Therapy Monitor is a multi‐country, multi‐center online medical chart review study of patients with hemophilia A and B (HA & HB). Recruited from a large access panel, 295 treating physicians in US (n = 70), EU&UK (n = 180; ~equal split across countries) and Japan (n = 45) were screened for duration of practice in their speciality and caseload, and provided data on 1958 HA pts (EU&UK = 984, US = 355, JP = 85), of which 1424 were non‐inhibitor (NI) and had severe HA. Data collection between June to August 2021.
Results: In EU&UK, 59% of the 984 reported severe NI HA patients experienced a bleed in the last 12 months, significantly higher than 48% of reported US sample (n = 171 of 355) and 40% of reported Japan sample (n = 34 of 85) (p < 0.01). Of the reported severe NI HA pts experiencing a bleed in the last 12 months (EU&UK = 579, US = 171, JP = 34), patients in EU&UK and US were younger than in Japan (mean age of 27, 26 and 40, respectively). In Japan, 41% (n = 14) of reported patients are less than 41 years compared to 85% (n = 491) in EU&UK and 82% (n = 140) in US. The percentage of reported patients that switched treatment since diagnosis is similar in EU&UK and Japan (68.4%, n = 394 and 67.7%, n = 23, respectively). In EU&UK, it is significantly higher compared to the US (53%, n = 91) (p < 0.01).
Conclusion(s): Results highlight presence of regional variation across age and switch rates for severe NI HA patients who experienced bleeding in the last 12 months. These variations can be considered when reviewing or developing regionally specific clinical guidelines. Further investigation using comparator cohort is warranted.
PB0175
Monitoring emicizumab with global coagulation assays
D. Bertaggia Calderara 1; A. Batista Mesquita Sauvage2; F. Gomez2; M. Zermatten2; A. Aliotta2; L. Veuthey2; C. Pereira Portela2; L. Alberio2
1 University Hospital Lausanne CHUV and University of Lausanne, Switzerland, Lausanne, Vaud, Switzerland; 2 Division of Hematology and Central Hematology Laboratory, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, Lausanne, Vaud, Switzerland
Background: Patients with Hemophilia A (PwHA) might benefit from treatment with emicizumab, a bispecific antibody mimicking the activity of the missing coagulation factor VIII (FVIII). Unlike FVIII, emicizumab does not require activation by thrombin and is immediately effective. Conventional aPTT‐based FVIII assays are inadequate to monitor emicizumab efficacy, since even small concentrations shorten the aPTT, which does not reflect the real degree of coagulation correction achieved by emicizumab in vivo. The development of new tools able to predict the biological phenotype in patients under emicizumab treatment would have a high clinical impact.
Aims: To investigate the utility of global coagulation assays (GCA) in monitoring emicizumab.
Methods: Six PwHA received a weekly dose of Hemlibra® (Roche Pharma, Switzerland) (3 mg/kg per body weight W1‐4, 1.5 mg/kg from W5 onwards). Thrombin generation (TG) was measured with Calibrated‐Automated‐Thrombogram (Stago, Asnières‐sur‐Seine, France). Fibrin clot formation (FCF) was measured with Thrombodynamics‐Analyzer (Hemacore, Russia). Response to treatment was monitored weekly for 2 months. TG and FCF were compared to patient baseline values and to healthy controls (C).
Results: ‐Mean concentration of emicizumab showed a large variation among six PwHA; ‐Compared to baseline values, TG and FCF significantly increased in PwHA after completion of the loading period (W5), reaching a plateau that lasted until the end of the monitoring; ‐TG and FCF during the plateau phase remained at the lower limits of reference values measured in C; ‐Patients having similar plasmatic plateau concentrations of emicizumab, showed different degree of TG and FCF improvement, which correlated with their clinical response.
Conclusion(s): In these six PwHA, emicizumab improved and maintained the hemostatic potential during monitoring. However, despite a similar dosage, patients showed individually variable responses to emicizumab. GCA appear to be a promising tool for evaluating the degree of coagulation correction exerted by emicizumab and to personalize patient therapy. Further clinical investigation is needed.
PB0177
Detection of IgG4 anti‐FVIII levels as complementary test to define the ITI outcome in patients with hemophilia A: Results from the BrazIT Study
D. Chaves 1; B. Ayala2; R. Camelo3; L. Etto4; A. De Oliveira5; V. Franco6; M. Roberti7; R. Ribeiro8; M. De Cerqueira9; F. Callado10; T. Anegawa11; M. Cerqueira12; S. Rezende2
1 Fundação Hemominas, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 2 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 3 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 4 Centro de Hematologia e Hemoterapia da Paraíba (HEMOÍBA), João Pessoa, Paraiba, Brazil; 5 Fundação HEMOMINAS, Belo Horizonte, Minas Gerais, Brazil; 6 Centro de Hematologia e Hemoterapia de Santa Catarina (HEMOSC), florianópolis, Santa Catarina, Brazil; 7 Centro de Hematologia e Hemoterapia de Goiás (HEMOGO), Goiânia, Goias, Brazil; 8 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaleza, Ceara, Brazil; 9 Centro de Hematologia e Hemoterapia do Piauí (HEMOPI), Teresina, Piaui, Brazil, 10 Fundação de Hematologia e Hemoterapia de Pernambuco (HEMOPE), Recife, Pernambuco, Brazil, 11 Centro Regional de Hematologia e Hemoterapia de Londrina, Londrina, Parana, Brazil, 12 Instituto de Hematologia do Estado do Rio de Janeiro (HEMORIO), Rio de Janeiro, Rio de Janeiro, Brazil
Background: Immune tolerance induction (ITI) is the treatment of choice to eradicate inhibitors in patients with inherited hemophilia A (PwHA). In addition to the Bethesda test, ELISA is an important assay to detect anti‐factor VIII (FVIII) antibodies.
Aims: This study aimed to detect plasma levels of anti‐FVIII IgG4 and to evaluate the agreement of the ELISA by using two different classes of FVIII to coat the plates ‐ plasma‐derived (pd) and recombinant (r) FVIII in PwHA after ITI.
Methods: We included PwHA who completed ITI from nine hemophilia centers. ITI outcome was defined as total/partial success and failure, based on inhibitor levels and FVIII pharmacokinetics. Comparison of IgG4 anti‐FVIII levels was performed using paired and unpaired t‐test. ROC curve analysis was performed to calculate sensitivity/specificity of the assays. Kappa coefficient of agreement was used to compare the assays. The study was approved by Ethical Committees and all participants/guardians signed a consent form.
Results: A total of 79 PwHA who completed ITI were included. A total of 56 (70.9%) and 23 (29.1%) patients had complete/partial success and failure, respectively. The majority of PwHA (73.4%) used pdFVIII during ITI. Median OD of anti‐FVIII ELISA for PwHA who failed ITI was 2.37 (IQR 2.17–2.54) with rFVIII and 2.32 (IQR 2.04–2.45) with pdFVIII (p = 0.002). Median OD for PwHA who had complete/partial response to ITI was 0.11 (IQR 0.06–0.43) with rFVIII and 0.12 (IQR 0.04‐0.40) with pdFVIII (p = 0.254). Samples were considered positive for IgG4 anti‐FVIII in assays with rFVIII and pdFVIII when the OD value was above 0.17 (sensitivity = 76.9%/specificity = 81.5%) and 0.25 (sensitivity = 71.2%/specificity = 77.8%), respectively. The Kappa coefficient of agreement of anti‐FVIII IgG4 between the two reagents was 0.85 (95% CI 0.73–0.96).
Conclusion(s): Either pdFVIII or rFVIII are useful on ELISA to detect IgG4 anti‐FVIII in complementary test to define the ITI outcome in PwHA.
PB0195
Prevalence of sporadic haemophilia A: An updated analysis of two cohort studies
S. Rezende1; D. Chaves 2; L. Jardim3; R. Camelo4; L. ZUCCHERATO5; M. Santana5
1 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 2 Fundação Hemominas, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 3 Faculty of Medicine, Universidade Federal de Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil; 4 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 5 Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Background: Haemophilia A (HA) is an inherited bleeding disorder caused by mutations in the factor VIII (FVIII) coding gene (F8). In Brazil, family structure has undergone great changes in the last decades resulting in reduced childbirth rates with longer life expectancy. Therefore, we have hypothesised that the prevalence of familial HA might have changed over the years.
Aims: The aim of this study was to evaluate the proportion of sporadic and familial HA in two Brazilian studies.
Methods: The HEMFIL Study is a multicentric prospective cohort of previously untreated patients (PUPs) with HA which aims to identify risk factors for inhibitor development. The BrazIT Study is a multicentric cohort study of patients with HA on immune tolerance induction (ITI) which aims to identify predictors of response of ITI. We included patients with HA (PwHA) with FVIII <2%. We assessed clinical, laboratory and F8 genotype by validated questionnaires and analysis of medical records. We also investigated the inheritance status by assessing the history of HA of each patient’s families. For statistical analysis of non‐parametric variables, we used Fisher Exact Test. The participants signed informed consent and the study was approved by the ethics committee.
Results: A total of 264 PwHA were included. Median age was 15 years. The overall prevalence of sporadic HA was 44,3%, without difference between studies (46.5% HEMFIL vs. 43.0% BrazIT; p = 0,37). To verify whether the prevalence of sporadic HA cases has changed over time, we stratified PwHA into five age ranges (≤10; 10–19,9; 20–29,9; 30–39,9 and ≥ 40 years). The frequency of sporadic PwHA increased from 18,8% in patients ≥ 40 years to 49,7% in PwHA ≤ 10 years (p = 0.006).
Conclusion(s): The prevalence of sporadic HA increased about 3 times within the last 40 years in Brazil. This likely reflects the changes in family structure in the country.
PB0187
FIX binding to collagen IV generates thrombin via the intrinsic tenase
G. Morrow 1; M. Laffan2; N. Curry3
1 University of Oxford, Aberdeen, Scotland, United Kingdom; 2 Centre for Haematology, Imperial College London, London, England, United Kingdom; 3 University of Oxford, Oxford, England, United Kingdom
Background: Haemophilia B is a blood clotting disorder caused by deficiency in coagulation Factor IX (FIX). Patients are treated by infusion of FIX concentrates based on their plasma concentration. FIX binds reversibly to collagen IV in the basal lamina of blood vessels and may be the site of an extravascular store.
Aims: To determine if the FIX‐collagen IV complex participates in coagulation.
Methods: Flat‐bottomed plates were coated with collagen IV prior to application of recombinant FIX. Wells were blocked and washed to prevent unspecific binding and remove unbound protein. Thrombin generation (TG) was performed in FIX deficient plasma (FIX‐DP) or pooled normal plasma (PNP) and measured using the Calibrated Automated Thrombogram. In some cases, 2 × 108 plt/mL isolated from a healthy donor, 1 pM tissue factor (TF) or 600 nM corn trypsin inhibitor (CTI) were incorporated. FIX activity was measured using a commercial chromogenic assay.
Results: No thrombin was generated by FIX‐DP ± platelets on uncoated or collagen coated surfaces. However, on surfaces with the FIX‐collagen complex present, FIX‐DP + platelets generated a peak thrombin concentration comparable to PNP; 139 vs. 162 nM, respectively. Addition of 1 pM TF to FIX‐DP + platelets shortened the lag time by 24‐fold and increased the peak. Inhibition of the contact pathway by an excess of CTI confirmed that thrombin generation was not taking place via contact activation. FIX activity while in complex with collagen was confirmed by chromogenic assay to be approximately 33 μM.
Conclusion(s): Our data suggest that collagen may induce activation of FIX and raises the possibility that the intrinsic tenase may assemble on the collagen surface. Collagen IV binding of FIX may provide a long‐lasting extravascular reservoir of FIX at a haemostatic and functional location, such as the joints.
PB0199
Budget impact of increasing rVIII‐SingleChain use in patients in Italy with moderate and severe hemophilia A
S. Yan1; R. Tomic 2; M. Panebianco2; E. Dlotko3; L. Stern3; R. Marino4
1 CSL Behring, King of Prussia, Pennsylvania, United States; 2 CSL Behring, Milan, Lombardia, Italy; 3 Certara USA Inc, New York, New York, United States; 4 University Hospital of Bari, Bari, Puglia, Italy
Background: rVIII‐SingleChain is a recombinant factor VIII (rFVIII), the safety and efficacy of which has been demonstrated in clinical trials and real‐world studies in patients with hemophilia A.
Aims: To estimate the potential budget impact of increasing rVIII‐SingleChain usage over a 3‐year period in Italy.
Methods: Patients with moderate and severe hemophilia A receiving prophylaxis or episodic treatment were included. Two analyses were conducted. First, comparing two scenarios where rVIII‐SingleChain share is constant (basecase) or increases over time by taking from other rFVIII products (alternative), considering the list prices of rFVIII products in Italy. Assumptions included the anticipated patient share estimates of rVIII‐SingleChain and competitor products, while current list prices were kept constant (over 3‐year period). For each scenario, the total cost of rFVIII products used for prophylaxis and in the treatment of bleeds was calculated. Mean product consumption and mean annual bleeding rate for rVIII‐SingleChain, rFVIIIFc, octocog alfa and BAY 81‐8973 were based on pooled real‐world data from Italy and Germany. Consumption and bleeding rates for other products were derived from the product labels. Second, a sensitivity analysis considered the current regional product acquisition costs in the basecase and alternative scenarios, while all the other parameters remained the same.
Results: If the patient share of rVIII‐SingleChain increases from 9% (basecase, constant over the 3 years) in first year to 30% in the third year in the alternative scenario, there is expected to be a decrease in total expenditure for rFVIII products of €2.9 million in year 1, €7.2 million in year 2 and €11.3 million in year 3. These findings remained consistent when regional acquisition costs were used instead of list prices.
Conclusion(s): Increasing utilization of rVIII‐SingleChain in the treatment of patients with hemophilia A may lead to cost‐savings as a result of reduced FVIII consumption.
PB0202
Molecular characterization of the F9 missense variants
B. Zulfikar 1; S. Genç2; M. Ozbil3; B. Koc4; V. Okan5; K. Kavaklı6; B. Antmen7; F. Sahin8; A. Meral Gunes9; Y. Ay10; C. Albayrak11; E. Unal12; M. Dagli13; E. Berber14
1 Istanbul University Oncology Institute, Istanbul, Istanbul, Turkey; 2 Yıldız Teknik University, İstanbul, Istanbul, Turkey; 3 Gebze Tecnical University, İstanbul, Istanbul, Turkey; 4 Istanbul University, Oncology Institute, İstanbul, Istanbul, Turkey; 5 Gaziantep University, Medical Faculty, Department of Hematology, Gaziantep, Gaziantep, Turkey; 6 Ege University Children Hospital, İzmir, Izmir, Turkey; 7 Department of Pediatric Hematology/Oncology and Bone Marrow Transplantation Unit, Faculty of Medicine, Acibadem University, Adana Hospital, Adana, Adana, Turkey; 8 Ege University, Department of Hematology, Ege Adult Hemophilia and Thrombosis Center, İzmir, Izmir, Turkey; 9 Bursa Uludag University Pediatric Hematology, Bursa, Bursa, Turkey,; 10 Pamukkale University Faculty of Medicine, Denizli, Denizli, Turkey; 11 Ondokuz Mayıs University Medical Faculty Haed of pediatric bone marrow unit, Samsun, Samsun, Turkey; 12 Erciyes University Faculty of Medicine, Kayseri, Kayseri, Turkey; 13 Selcuk University, Faculty of Medicine, Konya, Konya, Turkey, 14 Istanbul Arel University, İstanbul, Istanbul, Turkey
Background: Genotyping in Hemophilia B (HB) is important for genetic counseling, patient management, carrier determination since carrier women also have bleeding risk. Functional characterization of gene variants is important to establish genotype‐phenotype correlation. However, minority of F9 mutations were explored through in vitro expression to determine the pathogenicity mode.
Aims: The aim is to genotype F9 gene variations in HB patients in Turkey and to functionally characterize the selected F9 missense variants by in vitro expression and molecular dynamics simulation studies.
Methods: 140 HB patients in Turkey (66 Severe, 39 Moderate, 18 Mild, 19 Unknown), have been being screened for a F9 gene variation by HRM analysis and the variants were identified by direct DNA sequencing. Molecular dynamics simulations for the F9 gene variants were performed by using the GROMACS 5.1.4 software to analyze the conformational effects of the changes. Ethical approval for this study was obtained from the ethical committee of Istanbul Arel University.
Results: HRM analysis and direct DNA sequencing revealed that 60 of the patients had 27 different F9 gene variants. The most common type was missense variation with 59.25% frequency, including one novel variation (p.166Asn>Lys). The most common missense variant was p.194Thr>Ala. Among the patients having p.194Thr>Ala, 9 were severe, 11 were moderate, 4 were mild HB patients. Molecular dynamics simulations for p.194Thr>Ala, p.226 Arg>Gln and p.318 Lys>Arg revealed that p.194Thr>Ala, p.226 Arg>Gln changes caused significant alterations, compared to WT structure while p.318 Lys>Arg did not.
Conclusion(s): The preliminary data demonstrated that the most common F9 variation in the HB population in Turkey is p.194Thr>Ala (50%). Polyphen analysis revealed that it is a benign alteration. However, ESE finder analysis revealed that it creates additional SR protein binding. Molecular dynamics simulations demonstrated that it severely affects F9 protein structure. In vitro expression studies will demonstrate its effect in a heterologous expression system.
PB0173
Global coagulation assays in hemophilic patients on emicizumab prophylaxis, with and without partially neutralizing anti‐drug antibodies
S. Arcudi 1; M. Clerici2; E. Scalambrino3; R. Gualtierotti4; G. Nicolò5; G. Cafasso6; E. Biguzzi2; C. Novembrino7; C. Valsecchi2; A. Tripodi2; F. Peyvandi8
1 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, MILAN, Lombardia, Italy; 2 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 3 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico di Milano, Italy, Milano, Lombardia, Italy; 4 Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 5 Department of Healthcare Professions, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico di Milano, Milan, Italy, Milan, Lombardia, Italy; 6 Division of Hematology, University of Milan, Milan, Italy., Milan, Lombardia, Italy; 7 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Lombardia, Italy; 8 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: The development of partially neutralizing anti‐drug antibodies (ADA) might reduce emicizumab efficacy, but the effect on global coagulation assays is unknown.
Aims: To investigate the ability of non‐activated thromboelastometry (NATEM) and thrombin generation assay (TGA), to reflect the biological effect of the presence of ADA.
Methods: We performed a cross‐sectional study in patients with severe hemophilia A (HA) on emicizumab prophylaxis with and without partially neutralizing ADA. TGA was assessed with a homemade method upon addition of tissue‐factor (1pM) and synthetic phospholipids (1 μM) to plasma. Emicizumab plasma concentration (EmiC) was measured by a modified calibrated one stage FVIII assay, ADA were searched for with western blot method. NATEM was measured on citrated whole blood after addition of CaCl2 (100 mM). Student’s T test for unpaired samples was used.
Results: Two patients aged 10 and 79, with persistent partially neutralizing ADA occurring after their 9th exposure, and 14 patients without ADA were enrolled. After 6 months of follow‐up, the two patients with ADA and decreased EmiC reported no bleeding. Mean EmiC was 62.6 μg/ml in patients without ADA and 25.9 μg/ml in patients with ADA. When analyzing the coagulation with global assays in ADA patients, NATEM showed a delay in clot activation but the clotting formation was sustained and within the control range, as reported in Table 1 and Figure 1. When the TGA was carried out, the lagtime was not prolonged in comparison to controls, and again the endogenous thrombin potential and peak height were within control range, as shown in Table 1.
Conclusion(s): Our results show that despite decreased emicizumab level and prolonged aPTT, the coagulation of our patients was comparable to the controls when assessed with global coagulation tests, reflecting the absence of bleedings in vivo in two patients with ADA.


PB0183
The effect of regulatory T cell functions and cytokines on the development of inhibitor
S. Genc 1; E. Akkaya2; B. Koc3; B. Zulfikar4
1 Acibadem Hospital, Maslak, Istanbul, Istanbul, Turkey; 2 Istanbul University, Istanbul Faculty of Medicine, Istanbul, Istanbul, Turkey; 3 Istanbul University, Oncology Institute, İstanbul, Istanbul, Turkey; 4 Istanbul University Oncology Institute, Istanbul, Istanbul, Turkey
Background: The most important complication in hemophilia A(HA) is the development of inhibitors, which interfere FVIII function.
Aims: The purpose of this study was to investigate the roles of CD4+, CD4+CD25+ T cells, and the cytokines secreted from Tcells in the pathogenesis of inhibitor development in the patients with inhibitor and no inhibitor.
Methods: The study consisted 20patients, who were previously diagnosed and followed up in Istanbul University Oncology Institute and 10 healthy subjects. The patients were classified in two groups depending on presence of inhibitor[INH(+), INH(‐)]. FVIII was measured by one‐stage clotting assay using SiemensCS‐2500. CD4+ and CD4+CD25+T cells were isolated from the PBMCs, cultured for 72 to 120h, and induced with rFVIII(iFVIII)/phytohemagglutinin(i‐PHA), or not‐induced(NI). TNF‐α, IFN‐ϒ, IL‐2, IL‐10, IL‐13, TGF‐β concentrations were measured by ELISA method in supernatants of cell culture.
Results: Various changes were obtained in CD4+, CD4+CD25+ T cells and Tregs in INH(+) group compared with control and INH(−) group. These differences were significant in CD4+CD25+ T cells of INH(+) compared with INH(−)group in NI and i‐FVIII conditions (p = 0.023,p = 0.028), and in CD4+ and Tregs in INH(+) group compared with control in NI condition (p = 0.028, p = 0.050). Tregs, CD4+T cells in NI group (p = 0.033, p = 0.023) and CD4+,CD4+CD25+ in i‐FVIII group were also significantly different (p = 0.011,p = 0.013) between INH(−) and control group. Increased IL‐10, IL‐13 levels in i‐FVIII and decreased TNF‐α in NI were obtained compared with control (p = 0.05, p = 0.017, p = 0.032), however only IL‐10 was significantly high between INH(−) and controls. Also,significant alterations were found in TNF‐α and TGF‐β between INH+ and INH− in NI condition.
Conclusion(s): Our findings revealed the role of CD4+D25+ and Tregs in inhibitor development in patients with HemophiliaA. In particular, the changes in TNF‐α and TGF‐β levels and the increase in IL‐10, which shows exacerbation of the anti‐inflammatory effect with rFVIII stimulation, show that the immune system controlling effect of IL‐10 is abolished with rFVIII treatment.


PB0190
Platelet‐dependent factor VIII activity is more susceptible to inhibitor antibodies than phospholipid vesicle‐dependent factor VIII activity
G. Gilbert1; V. Novakovic 2; M. Chatterjee2
1 Harvard Medical School, WINCHESTER, Massachusetts, United States; 2 VA Boston Healthcare System, West Roxbury, Massachusetts, United States
Background: Inhibitor antibodies remain a major complication of hemophilia A. However, clinical assays do not adequately predict bleeding risk. We recently demonstrated that factor VIII activity (fVIII:C) supported by activated platelets is inhibited by anti‐C2 mAbs in a manner that has no significant correlation with the degree of inhibition in a standard aPTT‐based assay. These findings suggest that a platelet‐based assay may better assess bleeding risk.
Aims: To measure residual fVIII:C in the presence of patient inhibitors utilizing the activated platelet time (aPT) adapted for the clinical laboratory.
Methods: Cryopreserved platelets were thawed at 37˚C and purified on a density gradient. The aPT was initiated by combining i) platelets reconstituted in fVIII‐deficient plasma, ii) a fVIII sample in 10% plasma (with or without inhibitory antibodies and using either fVIII‐deficient plasma or inhibitor patient plasma), and iii) an activation mix (factor XIa, PAR1/4 agonist peptides, Ca++). Time to fibrin polymerization was measured using viscosity‐based clot detection (STA Compact Max, Diagnostica Stago).
Results: A 3rd‐order relationship between time to fibrin formation and the log of fVIII concentration was reliable over a 3.5‐log range (Figure 1A). The assay was robust over platelet concentrations of 300,000‐10,000,000 platelets/mL and 2‐60 pM fXIa. A panel of anti‐fVIII‐C2 antibodies caused increased inhibition of the aPT vs. the aPTT (Figure 1B) consistent with prior results (Figure 1C). Out of 6 patient inhibitor plasmas, 5 showed lower factor VIII residual activity when measured using the aPT assay (Figure 2). For three samples, the aPT measured fVIII:C ≤ 1% while the aPTT‐based assay indicated fVIII:C ≥ 5% for all samples.
Conclusion(s): The aPT, refined for clinical use, measures inhibitor‐suppressed fVIII:C low enough to rationalize a high bleeding risk for some patients. Further studies will be needed to indicate whether the aPT more accurately predicts bleeding risk than current clinical assays.


PB0196
In silico structural analysis of FVIII molecular changes from missense pathogenic variants in non‐severe hemophilia A patients displaying FVIII:C discrepancy between one‐stage vs. chromogenic assays
L. Rossetti 1; B. Ziegler2; M. Arias3; R. Sueldo4; V. Marchione5; P. Radic6; M. Abelleyro7; A. Baqués8; C. De Brasi9
1 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital Dr. César Milstein, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 4 Laboratorio de Hematología. Hospital Dr. César Milstein, Hospital de Pediatría Juan P. Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Instituto de Medina Experimental (IMEX)‐CONICET‐ Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 6 Instituto de Medicina Experimental (IMEX)‐CONICET‐Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 7 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 8 Servicio de Hemofilia. Hospital Dr. César Milstein., Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 9 Instituto de Medicina Experimental (IMEX)‐CONICET‐Academia Nacional de Medicina (ANM)/Instituto de Investigaciones Hematológicas (IIHEMA)‐ANM, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: Hemophilia A (HA) associates with a qualitative or quantitative deficiency in the coagulation factor VIII (FVIII). About one‐third of patients with non‐severe HA carry particular missense variants, which show discrepant results between FVIII activity levels (FVIII:C) measured by one‐stage‐assay (OSA) or chromogenic‐substrate‐assays (CSA).
Aims: Analyze possible mechanisms underlying OSA vs. CSA FVIII:C discrepancy in five patients with HA‐causative missense variants by in silico structural analysis.
Methods: Five patients with non‐severe HA from four families associated with discrepant results in samples (OSA, ranging mild‐moderate, higher than CSA levels) were included. OSA and CSA FVIII:C were determined using two reagent/coagulometer systems, IL/ACL Top 300 and Siemens/Sysmex CS‐2500. Genotyping: leukocyte‐extracted genomic‐DNA was mutational screened by PCR amplification of all coding and regulatory regions of F8 followed by CSGE and selected‐amplimers were characterized by Sanger sequencing. Pathogenicity of F8‐variants was classified according the ACMG criteria using the International F8‐variant database EAHAD (European Association for Hemophilia and Allied Disorders). 3D‐structural analysis of missense variants were performed in silico using publicly available resources (Figure).
Results: Four HA‐causative missense variants were identified in all 5 patients from Argentina: ‐one located in FVIII‐C1‐Domain and 4 in FVIII‐A3‐Domain. None of them was previously associated with assay discrepancy in EAHAD. In silico analysis revealed several types of protein structural damages in the missense variants (Figure), which support their differential impact in the FVIII miss function and region changes accounting for the observed OSA>CSA level discrepancy.
Conclusion(s): Our results points the utility to complement the genetic studies of missense pathogenic variants in HA affected patients showing FVIII:C assay discrepancy (OSA vs. CSA) with in silico structural analysis of the molecular impact in the FVIII protein to improve diagnosis, interpretation of FVIII:C assay discrepancy and the correct assessment of the HA severity.

VPB0206
Characterization of anti‐emicizumab antibodies using repository samples obtained in clinical studies of emicizumab conducted in Japan
N. Matsumoto 1; H. Abe1; R. Kawasaki1; Y. Tashiro1; M. Noguchi1; S. Harada1; K. Yoneyama2; T. Niino2; T. Soeda1; Y. Yoshimura1
1 Chugai Pharmaceutical Co., Ltd., Kamakura, Kanagawa, Japan; 2 Chugai Pharmaceutical Co., Ltd., Tokyo, Tokyo, Japan
Background: Emicizumab is a factor (F) VIII function‐mimetic anti‐FIXa/X humanized bispecific monoclonal antibody for use in hemophilia A patients with or without inhibitors. Although the incidence of anti‐drug antibodies (ADAs) to emicizumab is low, caution should be exercised in loss of efficacy due to ADAs. Further characterization of the ADAs (e.g. epitope analysis) would be useful.
Aims: To characterize ADAs using repository samples from ADA‐positive subjects/patients in phase I, phase I/II and bioavailability studies conducted in Japan.
Methods: This research was conducted in accordance with relevant ethical standards. Neutralizing activity of ADAs against emicizumab was measured in 10 subjects/patients who tested positive for ADAs in the clinical studies, and epitope analysis was performed in 8 of these subjects/patients. Neutralizing activity was assessed by a modified Bethesda assay using FVIII‐deficient plasma spiked with emicizumab, and epitope analysis was assessed by an electrochemiluminescence immunoassay.
Results: Neutralizing activity was observed in 3 subjects of 10 subjects/patients, and all epitopes were on the common light chain of emicizumab. ADAs developed in these 3 subjects were associated with decreased emicizumab exposure. Neutralizing activity could not be measured in 7 subjects/patients, including 1 subject who had decreased exposure, because ADA titers were low or the assay was affected by coexisting emicizumab. In 5 of the 7 subjects/patients for whom epitope analysis could be performed, ADAs recognized the Fab‐regions (FIXa/FX‐arms or FIXa‐arm only) or the common light chain.
Conclusion(s): This research confirmed the neutralizing activity of ADAs in 3 subjects of 10 ADA‐positive subjects/patients. The epitopes of ADAs in these 3 subjects included regions of the common light chain of emicizumab that is important to exert the pharmacological activity.
PB0181
Bleeding according to FVIII activity in 641 children with non‐severe haemophilia—Data from the PedNet study group
M. de Kovel 1; C. Königs2; S. Ranta3; C. Escuriola Ettingshausen4; K. Fischer5
1 PedNet Haemophilia Research Foundation, Utrecht, Utrecht, Netherlands; 2 University Hospital Frankfurt, Goethe University, Frankfurt, Hessen, Germany; 3 Department of Women's and Children's Health, Karolinska Univeristy Hospital and Karolinska Institute, Stockholm, Stockholms Lan, Sweden; 4 Hämophilie Zentrum Rhein Main (HZRM), Mörfelden‐Walldorf, Germany, Moerfelden‐Walldorf, Hessen, Germany; 5 University Medical Center Utrecht, University Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands
Background: Novel therapies, including modified replacement therapy and gene therapy, provide opportunity to substantially increase baseline FVIII activity levels, or (partially) correct haemostasis. Information on bleeding phenotype in non‐severe haemophilia provides the best possible information regarding optimum target for prophylactic treatment.
Aims: To assess bleeding according to baseline FVIII activity in children with non‐severe haemophilia A (HA).
Methods: HA patients in the PedNet Registry (Clin.gov.trial NCT02979119) by January 1st, 2020 with baseline factor FVIII activity between 1%‐25% (0.01 UI/mL to 0.25 UI/mL) were included. Patients were followed from diagnosis to: January 1st, 2020, 18 years of age, start prophylaxis, or inhibitor diagnosis. Onset of bleeding and annual (joint) bleeding rate (ABR/A(J)BR) were compared according to categories of baseline FVIII level: 1‐2%, 3‐5%, 6‐10%, 11‐15%, 16‐20% and 21‐25%. ABR and A(J)BR were established by negative binomial modelling. Onset of bleeding was analysed using Kaplan‐Meier survival.
Results: 641 non‐severe HA patients were included and were followed until a median age of 9.7 (IQR 6.0–13.8) years and for 5249 patients years in total. Median age at first joint bleed was higher with increasing FVIII activity: from 1.4 (for 1–2%), to 2.0 (for 3–5%), to 4.8 (for 6–10%), and 12.6 years for patients with 16–20% FVIII (Figure 1). Overall ABR was low, with 0.3 for all bleeds and 0.1 for joint bleeds (AJBR). A(J)BRs decreased with increasing baseline FVIII activity (Table 1), ABR from 1.6 (for 1–2%) to 0.1 for patients with 16–25% FVIII, AJBR was 0.5 for 1–2% and approached zero in patients with FVIII levels >6%.
Conclusion(s): Children with non‐severe HA have a low bleeding tendency. Onset of (joint) bleeding was delayed and bleeding decreased with increasing FVIII levels. In A(J)BR a steep decrease is observed in patients with FVIII activities >2%. Bleeding rates approached zero at 16% for all bleeds, and at 6% for joint bleeds.


PB0186
Production of a bispecific monoclonal antibody that enhances canine fixa activation of canine FX
D. Monroe 1; J. Chang2; M. Caughey3; E. Merricks4; T. Nichols5
1 UNC Blood Research Center, Chapel Hill, North Carolina, United States; 2 UNC School of Medicine, Chapel Hill, North Carolina, United States; 3 UNC Biomedical Engineering, Chapel Hill, North Carolina, United States; 4 UNC Department of Pathology and Lab Medicine (FOBRL), Chapel Hill, North Carolina, United States; 5 Department of Medicine and Pathology and Lab Medicine, UNC, Chapel Hill, North Carolina, United States
Background: Therapy for hemophilia A is replacement of FVIII. Up to 40% of patients who receive FVIII develop inhibitory antibodies to FVIII. Likewise, dogs with hemophilia A develop inhibitory antibodies to canine FVIII providing a model to identify mechanisms of inhibitor formation and to develop and test novel approaches to prevent and reverse inhibitor formation. However these inhibitor dogs, like humans with inhibitors, need effective hemostatic therapy. Emicizumab does not bind effectively to canine FIXa or FX.
Aims: To develop a bispecific monoclonal antibody that will bind canine coagulation FIXa and FX and prevent bleeding in hemophilia A dogs with and without inhibitory antibodies to canine FVIII.
Methods: Starting with the bispecific antibody emicizumab that binds human FIXa and FX, we assayed its ability to enhance FIXa activation of FX. To create a canine therapeutic, the antigen binding regions of emicizumab were transferred to canine IgG4 constant regions. To create a bifunctional antibody on this canine IgG4 framework (k‐IX‐X), positive charges were introduced on the FIXa binding arm (L323K and L325K) and negative charges on the FX binding arm (K237E, L323E, and L325E). Residues in complementarity determining regions were mutated and activity of the mutations was assessed by activity assay.
Results: Emicizumab activity with individual factors was probed by mixing human and canine factors. Binding is reduced ~10‐fold each for canine FIXa and FX (Figure 1). Moving the antigen binding regions of emicizumab to the k‐IX‐X framework did not change the activity. Selected mutations in k‐IX‐X increased the activity relative to emicizumab (Figure 2).
Conclusion(s): Improvements in binding to canine factors by introducing mutations into the antigen binding regions could enhance the activity of the canine bispecific monoclonal antibody into the range where it is a safe therapeutic product that could reduce bleeding events in hemophilia A dogs.


PB0193
Dose optimization for prophylaxis using a pharmacokinetic model for factor IX products in severe hemophilia B
B. Vandewalle1; G. Castaman2; M. Alvarez‐Román3; C. Escuriola Ettingshausen4; L. Nemes5; R. Tomic6; P. Martins1; J. Rodrigues7; K. Pinachyan 8
1 Exigo Consultores, Lisbon, Portugal, Lisbon, Lisboa, Portugal; 2 Careggi University Hospital, Florence, Toscana, Italy; 3 Hospital Universitario La Paz, Madrid, Madrid, Spain; 4 Hämophilie Zentrum Rhein Main (HZRM), Mörfelden‐Walldorf, Germany, Moerfelden‐Walldorf, Hessen, Germany; 5 National Haemophilia Centre and Haemostasis Department, Medical Centre of Hungarian Defence forces, Budapest, Hungary, Budapest, Budapest, Hungary; 6 CSL Behring, Milan, Lombardia, Italy; 7 CSL Behring, Marburg, Hessen, Germany; 8 CSL Behring Europe, Mechelen, Antwerpen, Belgium
Background: Trough factor IX (FIX) levels have been used as a proxy for bleeding control in patients with hemophilia B. However, there is some debate about the exact correlation between trough levels and bleeding phenotype and which trough levels are optimal for effective, manageable treatment. Understanding the pharmacokinetic (PK) properties under prophylactic utilization of FIX products is imperative for achieving and maintaining desired FIX trough levels, improving patients’ quality of life, and managing treatment resources.
Aims: This population PK model‐based analysis aimed to assess optimal dosing and administration frequency to achieve predefined FIX activity trough levels for a range of different FIX replacement products in patients with severe hemophilia B.
Methods: The model used a hypothetical simulated 10,000 patient population and employed published population PK models to compare extended half life factor FIX products available in Europe (rIX‑FP, rFIXFc, N9‐GP), considering posologies based on their respective product labels.
Results: The capacity of FIX products in achieving predefined trough levels was effectively estimated by the model, predicting the optimum dosing and administration frequency required (Table 1). This provides a greater understanding of PK and consumption of FIX products, allowing for a more individualized, patient‐clinician shared decision making approach, focused on reducing treatment burden, increasing trough levels and improving bleeding control. The model indicates that some FIX products were unable to reach certain trough levels (or for a low proportion of patients only). For those products that did consistently reach target trough levels, a reduced treatment burden and consequent lower FIX consumption was observed. This may also improve resource utilization and treatment costs, whilst maintaining or reducing the bleeding risk.
Conclusion(s): This model‐based assessment should help broaden the understanding of different FIX product capabilities and provide optimal dosing strategies, facilitating individualization of care.

VPB0205
Predictors of body mass index in persons with hemophilia over the life course: Multisite, cross‐sectional study (n = 691)
M. Glenzer 1; R. Barnes2; A. von Drygalski3
1 University of California, San Diego, San Diego, California, United States; 2 University of California San Diego, Dept of Medicine, San Diego, USA, San Diego, California, United States; 3 Division of Hematology/Oncology, Department of Medicine, University of California San Diego, San Diego, California, United States
Background: It is assumed that persons with hemophilia (PWH) may be heavier compared to the general population inherent to a less active lifestyle due to bleed‐induced arthropathy. In turn, heavy body habitus may contribute to arthropathic progression.
Aims: To examine factors that influence body mass index (BMI) in PWH, and to compare their distribution of BMI to the general male population.
Methods: Cross‐sectional data from adult PWH (≥18 years) were extracted from medical records at four Hemophilia Treatment Centers between 2003 and 2014, including BMI, age, race, hemophilia type and severity, and history of Hepatitis C (HCV), human immunodeficiency virus (HIV), and inhibitor status. A matched sample from the general population was derived from a combined dataset of National Health and Nutrition Examination Surveys (NHANES) from 2003 to 2016 (5:1 NHANES:PWH).
Results: 691 PWH were included (median age 39 [IQR 28‐52]). PWH demonstrated a leaner distribution of BMI than the general male population (median BMI 26.2 vs. 27.3 kg/m2; p < .0001). In PWH, prevalence of HCV infection and coinfection with HCV and HIV (only present ≥age 27) was 63% and 18.4%, respectively. HIV infection alone was rare (n = 5). Coinfection was a major contributor to leanness; coinfected PWH were 3–5 kg/m2 lighter than uninfected PWH. HCV infection alone was not a prominent predictor of BMI in PWH, nor was hemophilia type or history of inhibitor. Adjusted BMI among Hispanic PWH was 2–3 kg/m2 higher than among Whites. Prevalence of obesity was higher for moderate severity than for mild or severe hemophilia (36.9% vs. 26.2% and 17.5%, respectively).
Conclusion(s): Contrary to prevailing assumptions, adult PWH were leaner than the general male population, in part explained by a high rate of coinfection with HCV and HIV. Improved understanding of PWH‐specific weight trends should inform lifestyle interventions to advance hemophilia health outcomes.


PB0176
Gene expression profile in severe Hemophilia A patients developing anti‐Factor VIII neutralizing alloantibodies
A. Cairo 1; S. Spena1; E. Pappalardo2; M. Mortarino3; I. Garagiola4; F. Peyvandi5
1 Angelo Bianchi Bonomi Haemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milano, Lombardia, Italy; 2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca’Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 4 Angelo Bianchi Bonomi Haemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milan, Lombardia, Italy; 5 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Approximately 30% of severe hemophilia A (HA) patients develop anti FactorVIII (FVIII) neutralizing alloantibodies in response to replacement therapy that decrease the efficacy of the treatment. Understanding the etiology of inhibitor development and the biological mechanisms involved is need.
Aims: To investigate differentially expressed genes in severe HA patients who developed anti‐FVIII neutralizing alloantibodies after exposure to FVIII.
Methods: Twenty‐nine severe HA patients from the SIPPET study (15 with and 14 without alloantibodies) were analyzed. All patients had a FVIII:C< 1% and were treated with recombinant FVIII products. RNA was collected after alloantibodies development and extracted using PAXgene Blood RNA Kit. Libraries were prepared with TruSeq Stranded Total RNA Library Prep Kit and sequenced on Illumina NovaSeq. Reads were aligned with STAR and counted with RSEM; DESeq2 and edgeR were used to perform differential expression analysis. Validation of identified genes was conducted by qRT‐PCR (TaqMan assays). Pre‐ranked pathway analyses were performed with GSEA on Hallmark dataset starting from DESeq2 and edgeR results.
Results: Three genes (OTOF, TNFAIP6 and IGLC7) were downregulated in HA patients with inhibitor (Table1). Technical validation confirmed a reduced expression of OTOF and TNAFIP6, whereas IGLC7 validation has not completed yet. TNFAIP6 plays an anti‐inflammatory role and can be secreted after proinflammatory stimuli and it has already been reported to be upregulated in the spleen of HA mice in response to FVIII infusion. OTOF is an interferon inducible gene reported to be associated with systemic lupus erythematosus. IGLC7 product is an immunoglobulin involved in the activation of immune system. GSEA analyses revealed a downregulation of interferon gamma/alpha response pathways in patients with alloantibodies in both approaches used.
Conclusion(s): We identified three downregulated genes related to inflammatory response in severe HA patients with alloantibodies suggesting that inflammation induced by treatment could have a role in inhibitor’s development.


PB0179
Establishment of a monolayer‐based LSEC‐like endothelial cell in vitro system from induced pluripotent stem cells
P. Chawla 1; M. Rath2; J. Oldenburg3; H. Singer4
1 IHT, UKB, Bonn, Nordrhein‐Westfalen, Germany; 2 IHT,UKB, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 4 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Liver sinusoidal endothelial cells (LSECs) are the major source of FVIII expression and secretion. Maintenance of these cells in vitro remains challenging as primary LSECs tend to rapidly lose their specific properties, including the expression of LSEC markers and the presence of fenestrae. In contrast IPS cells provide an alternative source to generate organ‐specific ECs with high proliferative potential. Currently, embryoid body generation with a combination of adrenomedullin pathway activation, TGF‐ß inhibition and hypoxia comprise recent strategies to derive LSEC‐like EC from IPS.
Aims: To establish a 2D monolayer‐based protocol from IPS to LSEC‐like cells and track intracellular fate of wild type FVIII and FVIII variants in HA‐patients.
Methods: Monolayer IPS single cells were differentiated for 3 days into mesoderm using small molecule CHIR and Bmp4. CD34+ cells were MACS isolated after 2 days of treatment with Forskolin, GSI and VEGF‐A. Subsequently CD34+ angioblasts were maintained under hypoxic conditions for 4 days in StemPro34 containing low VEGF‐A. Induction of LSEC‐like cells was implemented by adding small molecules SB431542 and 8‐Br‐cAMP. Venous, arterial and LSEC specific markers FCGR2b, Stab2 & F8 were analyzed by rtPCR. Finally, intracellular FVIII protein was visualized and co‐localized by immunofluorescence using cellular organelle marker for Calreticulin, COPII (Sec31a) and Golgi‐trans (TGN46)
Results: Early angioblasts (day 6) show an increase of venous marker COUP‐TF and NT5E after 4 days under low VEGF‐A conditions (Day10). During LSEC induction (Day10 ‐ 16) LSEC markers show clear increase compared to HUVEC: FCGR2b (6‐10 fold), Stab2 (7‐14 fold) and F8 (8‐13 fold). IF‐staining presents strong co‐localization of FVIII with classical cellular secretion marker CALR, COPII and Golgi.
Conclusion(s): This LSEC‐like cellular model provides 8‐fold higher F8 expression compared to a normal vascular EC in vitro system. The additional presence of LSEC specific receptors enables a more native and precise disease modeling for HA research and therapy.
PB0189
Low normal levels of factor V enhance thrombin generation in hemophilia A
D. Monroe1; C. Baird2; J. Peterson3; A. Fogelson4; K. Leiderman5; S. Sindi6; M. Stobb7; A. Mast3; M. Manco‐Johnson8; K. Neeves 2
1 University of North Carolina Blood Research Center, Chapel Hill, NC, USA., Chapel Hill, North Carolina, United States; 2 University of Colorado Denver, Aurora, Colorado, United States; 3 Versiti, Milwaukee, Wisconsin, United States; 4 University of Utah, Salt Lake City, Utah, United States; 5 Colorado School of Mines, Golden, Colorado, United States; 6 University of California Merced, Merced, California, United States; 7 Coe College, Cedar Rapids, Iowa, United States; 8 University of Colorado and Hemophilia and Thrombosis Center, Aurora, Colorado, United States
Background: We previously reported that a mathematical model of coagulation predicted low normal levels (50%‐75%) of factor V (FV) enhance thrombin generation in hemophilia A (Link, JTH, 18, 2020). The study suggested that FV and FVIII compete for cleavage by FXa during the initiation of coagulation.
Aims: To test the hypothesis that substrate competition for FXa is responsible for enhanced thrombin generation in hemophilia A plasma with low amounts of FV.
Methods: 1) A purified system of FV, FVIII, FXa and phospholipids examined the biochemical feasibility of the substrate competition mechanism, wherein the generation of FVIIIa was the output. 2) Tissue factor (TF) initiated thrombin generation was measured in synthetic plasma containing the zymogens and co‐factors of the extrinsic pathway and anticoagulants (ATIII, +/‐TFPI) at varying levels of FV (25%–150%). 3) A mixture study of FVIII‐deficient (5%) human plasma with normal FV and immunodepleted of FV was performed using calibrated automated thrombography (CAT). 4) CAT initiated with 1 or 5 pM TF was used to measure thrombin generation in 75 severe to mild hemophilia A plasmas. Prothrombin, FV, FVIII, total TFPI, and TFPIα levels were measured in these plasma samples and Pearson correlation coefficients were calculated against thrombin generation metrics.
Results: In the purified system, there was an inverse relationship between FV concentration and the amount of FVIIIa generated, indicating competition for FXa between the two substrates. In both synthetic and human plasma, there was an inverse relationship between FV concentration and thrombin generation metrics (Figure 1). There was negative correlation between FV concentrations and these thrombin generation metrics in hemophilia A plasmas (p < 0.01).
Conclusion(s): Four different biochemical systems support the hypothesis that low normal levels (50%‐75%) of FV enhance thrombin generation in hemophilia A plasma. These experiments support the substrate competition mechanism for FXa predicted by our mathematical model.

PB0201
Pathogenic variants in F8 gene—Results from the Brazilian Immune Tolerance (BrazIT) Study
L. Zuccherato 1; R. Camelo2; L. Jardim3; M. Moreira Dias4; L. de Magalhães5; A. De Oliveira6; R. Ribeiro7; V. Franco8; M. Roberti9; F. Callado10; L. Etto11; M. De Cerqueira12; M. Cerqueira13; C. Lorenzato14; I. Pinto15; A. Garcia16; D. Neves17; D. Tan18; D. Chaves19; S. Rezende20
1 Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 2 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 3 Faculty of Medicine, Universidade Federal de Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil; 4 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 5 Department of Clinics, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 6 Fundação HEMOMINAS, Belo Horizonte, Minas Gerais, Brazil; 7 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaleza, Ceara, Brazil; 8 Centro de Hematologia e Hemoterapia de Santa Catarina (HEMOSC), florianópolis, Santa Catarina, Brazil; 9 Centro de Hematologia e Hemoterapia de Goiás (HEMOGO), Goiânia, Goias, Brazil; 10 Fundação de Hematologia e Hemoterapia de Pernambuco (HEMOPE), Recife, Pernambuco, Brazil; 11 Centro de Hematologia e Hemoterapia da Paraíba (HEMOÍBA), João Pessoa, Paraiba, Brazil; 12 Centro de Hematologia e Hemoterapia do Piauí (HEMOPI), Teresina, Piaui, Brazil; 13 Instituto de Hematologia do Estado do Rio de Janeiro (HEMORIO), Rio de Janeiro, Rio de Janeiro, Brazil; 14 Hemocentro do Paraná (HEMEPAR), Curitiba, Parana, Brazil; 15 Fundação HEMOPA, Belém, Para, Brazil; 16 Centro de Sangue de São José do Rio Preto, São José do Rio Preto, Sao Paulo, Brazil; 17 Fundação Hemocentro de Rondônia (FHEMERON), Porto Velho, Rondonia, Brazil; 18 Department of Pediatric Onco‐hematology, Faculdade de Medicina de Marília, Marília, Sao Paulo, Brazil; 19 Fundação Hemominas, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 20 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil
Background: Hemophilia A (HA) occurs due to deleterious mutations in the factor VIII (FVIII) gene (F8). Patients with HA (PwHA) can develop inhibitors against FVIII. F8 null mutation is a risk factor for inhibitors. However, most molecular studies have small sample size and/or included European/North American PwHA with and without inhibitors.
Aims: To evaluate the spectrum of F8 mutations in PwHA with inhibitors who underwent immune tolerance induction (ITI).
Methods: We included PwHA undergoing ITI from the BrazIT Cohort Study. This study aimed at evaluating predictors of ITI response. F8 sequencing was performed using an Ampliseq for Illumina Custom DNA Panel and sequenced on a MiSeq System. Inversion of introns 22 (Inv22) and intron 1 (Inv1) were detected by inverse shifting and allelic specific PCRs. Pathogenic variants were assessed with Varsome (https://varsome.com/). All patients/guardians signed a consent form. Study was approved by ethical committees.
Results: A total of 177 PwHA were included. We found deleterious mutations in 98% of PwHA, of which Inv 22 was the most prevalent (48.6%; 86/177), followed by frameshift (15.8%; 28/177), nonsense (14.7%; 26/177), large deletions (8.5%, 15/177), splice‐site (3.9%, 7/177), missense (3.9%, 7/177) and Inv1 (2.2%, 4/177). Four patients (2.2%) showed no detectable pathogenic mutation. Nonsense, frameshift, missense and splice‐site variants were mainly found in the heavy chain of FVIII (57.0%), mainly A1 and B domains. Most of large deletions (14/15) encompasses FVIII heavy chain. The prevalence of null variants (inversions, large deletions, nonsense and frameshifts) represents the highest percentage of F8 deleterious alleles (89.8%, 159/177).
Conclusion(s): In this population of admixed patients with PwHA with inhibitors on ITI, there is a high prevalence of F8 null variants. To date, this is the largest study assessing the spectrum of F8 variants in a population of PwHA under ITI.
VPB0209
Usefulness of the human‐factor based chromogenic substrate assay for evaluating coagulability of Emicizumab
T. Yamaguchi 1; K. Shinozawa1; I. Hiroshi1; A. Mitsuhashi1; Y. Harada1; R. Miyashita1; Y. Kamikubo1; A. Ichiki1; Y. Chikasawa2; M. Bingo2; R. Sekiya1; T. Muramatsu1; M. Yotsumoto3; T. Hagiwara3; K. Amano4; S. Nagatoishi5; K. Tsumoto5; E. Kinai3
1 Tokyo Medical University, Shinjukuku, Tokyo, Japan; 2 Tokyo Medical University, Tokyo, Tokyo, Japan; 3 Tokyo Medical University, Shinjukuku Nishishinjuku, Tokyo, Japan; 4 Tokyo Medical University Hospital, Tokyo, Tokyo, Japan; 5 The Institute of Medical Science, The University of Tokyo, Minatoku, Tokyo, Japan
Background: Emicizumab is a bispecific antibody mimicking activated factor VIII (FVIII) and increasingly used because of its practicality. In patients receiving emicizumab, since activated partial thromboplastin time (aPTT) is excessively shortened, it is an urgent issue to establish an appropriate and easy‐to‐access coagulation assay.
Aims: The aim of this study is to assess the usefulness of the human factor‐based chromogenic substrate assay (hCSA) with reference to the thrombin generation assay (TGA), the established coagulation assay for emicizumab.
Methods: The coagulability of spiked emicizumab and recombinant FVIII (rFVIII) were measured and compared among TGA using extrinsic and intrinsic pathway triggers, hCSA, one‐stage clotting assay (OSA), and clot waveform analysis (CWA). We also investigated the additive effect of 340 nM emicizumab in combination with rFVIII by measuring in the same assays.
Results: Both the peak thrombin by TGA and FXa generation by hCSA consistently showed a linear relationship between emicizumab and rFVIII concentration in wide range of emicizumab concentration (10‐2000 nM). While TGA showed a plateau in higher emicizumab concentration, hCSA did not at any concentration. FVIII‐equivalent activity of emicizumab in hCSA can be approximated by halving to that in TGA triggered by the extrinsic pathway reagent (27.3 IU/dL vs. 13.9 IU/dL) in steady state (340 nM) of emicizumab. The additive effect in combination of 340 nM of emicizumab plus rFVIII gradually diminished at higher range of rFVIII in TGA, hCSA and CWA.
Conclusion(s): Reported FVIII activity of emicizumab in hCSA can be easily approximated to TGA, and the additive effect of FVIII on emicizumab is diminished in higher doses of FVIII. For clinical applicability of hCSA, further investigation is needed. This research was supported by Chugai Pharmaceutical Co., Ltd, and emicizumab used in this study was provided by Chugai Pharmaceutical.


PB0180
Laboratory evaluation of extendend‐half‐life recombinant FVIII products (EHL‐FVIIIs): One stage coagulation assay or chromogenic assay?
R. De Cristofaro 1; S. Lancellotti2; M. Sacco1; M. Tardugno1
1 Catholic University of Sacred Heart, Translational Medicine and Surgery Department, Rome, Lazio, Italy; 2 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Rome, Lazio, Italy
Background: The EHL‐FVIIIs have been introduced to improve the FVIII pharmacokinetic profile reducing the infusions number in hemophilic patients. These products are structurally and functionally heterogeneous, leading to several discrepancies in FVIII coagulant (FVIII:C) by chromogenic substrate assay (CSA) and one‐stage clotting assay (OSA), and inducing an altered FVIII recovery with an over/under‐estimating by more than 30%. Similar discrepancies were observed in patients receiving ReFacto® (B‐domain‐deleted FVIII) a decade ago and has been resolved with the use of specific ReFacto Laboratory Standard.
Aims: The aim of the study is to evaluate FVIII:C and recovery of EHL FVIIIs in spiked FVIII‐deficient human plasma studies with four different type of assay.
Methods: The EHL‐FVIII products tested were: Damoctocog alpha‐pegol, Rurioctocog alpha‐pegol, Turoctocog alpha‐pegol and Efmoroctocog alfa. All EHL‐FVIIIs were reconstituted according to the manufacturer's instructions and serial dilutions (1/0.5/0.1/0.05/0.025 U/ml) were obtained by using HemosIL® FVIII Deficient Plasma (Werfen). For each product, FVIII:C was then tested through OSA (Werfen), EHL‐FVIII‐calibrated OSA methods and two different commercially available CSA (Chromogenix Coamatic® Factor VIII and Trinichrom FVIII Stago).
Results: The classical OSA assay could show significant discrepancies between the nominal and real EHL‐FVIII concentrations. The determination of Efmoroctocog alfa by OSA provides a value two‐fold higher than the expected level. The calibrated‐OSA assay is very accurate in determining the values of all EHL‐FVIIIs, showing in the spiked experiments a drug recovery from 92% to 101%. On the contrary, the CSA, despite having been used to determine the potency of all products, reported very high CV (>30%). Noteworthy, Jivi levels were significantly underestimated by one of the CSA tested, underestimating the nominal value up to 50%.
Conclusion(s): In this scenario, a standard calibration curve for each EHL‐FVIII product could avoid inaccurate laboratory results, which might heavily impact on patient management.
PB0184
FVIII‐specific immune response in hemophilia A mice upon infection
B. Jurado‐Mestre 1; V. Kotov2; A. Maione2; J. Oldenburg3; C. Kurts2; J. Becker‐Gotot2
1 IMMEI, Bonn, Germany, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Molecular Medicine and Experimental Immunology, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Hemophilia A (HemA) is the most common inherited bleeding disorder, caused by deficiency of coagulation factor VIII (FVIII). Severe affected individuals follow a prophylactic treatment with intravenous replacement therapy of recombinant or plasma derived FVIII. Unfortunately, up to 30% of patients develop inhibitory antibodies (inhibitors) against this protein. Eradication of these inhibitors can be accomplished via immune tolerance induction (ITI) through frequent high‐dose administration of FVIII, but tolerance is only achieved in about 70% of patients. The molecular mechanism as well as environmental factors which disrupt this process are mainly unknown.
Aims: The aim of this study is to identify potential environmental effects on tolerance induction within Hemophilia A mice. In detail, the stability of tolerance is analyzed upon an influenza A infection.
Methods: To resemble the ITI process in hemophilic mice, HemA were injected with rFVIII twice a week in contrast to the control group which receives weekly injections. To study the effect of external factors, we additionally induced an infection with influenza in the twice a week treated group.
Results: FVIII immunization induces a robust immune response in HemA mice with FVIII‐specific IgG formation and low amounts of active FVIII in serum. In the ITI group a FVIII‐tolerogenic phenotype is initiated by increasing the amounts of active FVIII and apoptosis rate of FVIII‐specific B cells. In correlation, PD‐1 expression on FVIII+ B cells is enhanced too. Moreover, there is an increase in the FVIII‐specific amount of FVIII‐specific regulatory T cells. On top of that, preliminary results after viral infection during ITI treatment suggest a decrease in tolerance to FVIII, displayed as less active FVIII and significant reduction of FVIII‐specific Tregs.
Conclusion(s): The established high dose protocol allows the analysis of molecular mechanisms of tolerance induction. This process is mediated by FVIII‐specific Tregs and is altered upon an infection.
PB0194
Race, ethnicity and F8 mutation type influence inhibitor risk: Analysis of the “My Life Our Future” Hemophilia Database
K. Pratt 1; A. Ahmed2
1 Uniformed Services University, Bethesda, Maryland, United States; 2 Uniformed Services University of the Health Sciences, Bethesda, Maryland, United States
Background: Inhibitors (anti‐FVIII neutralizing antibodies) afflict ~1/3 of severe hemophilia A (HA) patients. Several studies have suggested that Black and Hispanic patients suffer higher inhibitor incidences than their White counterparts. The possible influence of non‐synonymous single nucleotide polymorphisms (ns‐SNPs) in the F8 gene sequence on inhibitor development has been proposed as a possible race‐associated contributing factor, although this hypothesis has been controversial.
Aims: The “My Life Our Future” database contains demographic, clinical and F8 sequence data from >6,000 HA participants, allowing for robust statistical analyses of associations between race/ethnicity, F8 mutation and inhibitors. Aims were to evaluate possible associations between race/ethnicity, F8 sequence variants, and clinical and demographic variables and inhibitors.
Methods: Adjusted regression analysis of data from 2,417 severe and 1,752 mild+moderate HA subjects.
Results: Among mild+moderate HA subjects, inhibitor risk was up to 4X lower in those who expressed higher endogenous, hemophilic FVIII (FVIII:C 6–15% and 16‐49% vs. 1–5%, aOR, 0.47, 95%CI: 0.31–0.71 and aOR, 0.25, 95%CI: 13–.48, p < 0.0001). Severe HA due to frameshift or missense mutations carried a lower inhibitor risk than intron‐22 inversions (aOR, 0.71, 95%CI: 0.54‐0.92 and aOR, 0.22, 95%CI: 0.16–0.32, p < 0.0001). In contrast to some earlier studies, there was no difference in inhibitor risk among subjects with severe HA due to an intron‐22 inversion, large structural change or nonsense mutation. Black and Hispanic severe HA subjects had a higher inhibitor risk than Non‐Hispanic Whites (aOR, 1.85, 95%CI: 1.40–2.45 and aOR, 1.63, 95%CI: 1.21–2.20), confirming this racial/ethnic/medical disparity; however, F8 ns‐SNPs were not associated with inhibitor development.
Conclusion(s): African American and Hispanic severe HA patients are at increased risk of developing inhibitors compared to White nonHispanic patients; race‐associated F8 haplotypes were not a significant risk factor. Intron 22 and Intron 1 mutations were associated with similar inhibitor risk to other F8 mutations that preclude intact FVIII protein expression.
PB0197
FVIII stimulation causes a variable response in FVIII‐specific third generation chimeric antigen receptor (CAR)‐transduced T cells
S. SCATIGNA 1; S. Schultze‐Strasser2; C. Königs3; A. Schmidt2
1 University Hospital Frankfurt Am Main, Sachsenhausen, Frankfurt am Main, Hessen, Germany; 2 University Hospital Frankfurt am Main, Frankfurt am Main, Hessen, Germany; 3 University Hospital Frankfurt, Goethe University, Frankfurt, Hessen, Germany
Background: About one third of severe HA patients develop inhibitory antibodies when treated by protein replacement therapy with FVIII due to a lack of tolerance to FVIII. Solutions to avoid or reverse inhibitor formation are urgently needed.
Aims: The main aim of this study is to develop and characterise third‐generation FVIII‐specific CAR constructs and compare them to FVIII‐specific second‐generation CARs, which have been shown to suppress T and B cell responses to FVIII, when transduced into regulatory T cells (Tregs).
Methods: Naïve CD4+ T cells and Tregs were purified from PBMCs by magnetic isolation. The designed second‐ and third‐generation FVIII‐specific CAR constructs have been cloned into lentiviral vectors to infect naïve CD4+ T cells and Tregs. CAR protein surface expression was studied in both cell types. Activation marker expression upon FVIII stimulation was analysed in transduced cells to verify a FVIII‐specific response. Comparison between second‐ and third‐generation CAR transduced cells was based on their staining profiles.
Results: Surface expression of the second‐generation CAR was highest while third‐generation constructs differed considerably in their amount of CAR extracellular expression. To check whether low surface expression of CARs was caused by surface trafficking problems we measured intracellular CAR retainment in transduced Jurkat cells, but no intracellular CAR was detected. Next, we investigated the response of transduced cells to FVIII. Activation marker expression was diverse in transduced cells with different CAR constructs, showing a specific but variable response towards FVIII. This might be a consequence of variable CAR surface expression or the combination of costimulatory domains that impact antigen‐specific responses.
Conclusion(s): Our studies showed large differences in terms of surface expression of the CAR proteins and cellular responses towards FVIII. Further experiments will be needed to identify CAR characteristics associated with a robust FVIII‐specific response.
PB0178
High plasma levels of IgG4 anti‐FVIII is the hallmark of immune tolerance failure in patients with hereditary hemophilia A: Results from the BrazIT Study
D. Chaves 1; R. Camelo2; B. Ayala3; L. Etto4; A. De Oliveira5; V. Franco6; M. Roberti7; M. De Cerqueira8; R. Ribeiro9; F. Callado10; T. Anegawa11; M. Cerqueira12; S. Rezende3
1 Fundação Hemominas, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 2 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 3 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 4 Centro de Hematologia e Hemoterapia da Paraíba (HEMOÍBA), João Pessoa, Paraiba, Brazil; 5 Fundação HEMOMINAS, Belo Horizonte, Minas Gerais, Brazil; 6 Centro de Hematologia e Hemoterapia de Santa Catarina (HEMOSC), florianópolis, Santa Catarina, Brazil; 7 Centro de Hematologia e Hemoterapia de Goiás (HEMOGO), Goiânia, Goias, Brazil; 8 Centro de Hematologia e Hemoterapia do Piauí (HEMOPI), Teresina, Piaui, Brazil; 9 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaleza, Ceara, Brazil; 10 Fundação de Hematologia e Hemoterapia de Pernambuco (HEMOPE), Recife, Pernambuco, Brazil; 11 Centro Regional de Hematologia e Hemoterapia de Londrina, Londrina, Parana, Brazil; 12 Instituto de Hematologia do Estado do Rio de Janeiro (HEMORIO), Rio de Janeiro, Rio de Janeiro, Brazil
Background: Immune tolerance induction (ITI) eradicates anti‐factor VIII (FVIII) inhibitors in about 70% of patients with inherited hemophilia A (PwHA). Of all immunoglobulins (Ig), anti‐ FVIII IgG4 is known to correlate with inhibitor titre. Therefore, we hypothesized that it could be a biomarker of ITI outcome.
Aims: To evaluate the association of IgG4 anti‐FVIII levels with the outcome of ITI in patients with severe hemophilia A (FVIII < 2%) with high response inhibitors.
Methods: We included PwHA who completed ITI from nine hemophilia centers. ELISA was used to detect specific IgG4 anti‐FVIII in plasma samples from all patients after ITI conclusion. Most patients (66.7%) were treated with a low‐dose regimen (50 international units [IU] of FVIII concentrate per kilogram 3 times weekly). ITI outcome was defined as total/partial success and failure, based on inhibitor levels and FVIII pharmacokinetics. Comparison of IgG4 anti‐FVIII levels was performed using Mann Whitney test. ROC curve analysis was performed to calculate sensitivity and specificity of the ELISA. The study was approved by Ethical Committees and all participants/guardians signed a consent form.
Results: A total of 50 PwHA who completed ITI were included. Levels of IgG4 anti‐FVIII were significantly higher in patients who failed ITI (median OD, 2.18; IQR 1.69–2.44) compared with those who had partial (median OD, 0.15; IQR 0.05–0.52) and complete success (median OD, 0.05; IQR 0.00–0.14) (p < 0.0001 for both). There was no difference of anti‐FVIII IgG4 levels in patients with total and partial success (p = 0.07). Considering the OD cut‐off of 0.62, the test had a sensitivity of 93.8% and a specificity of 82.4% for ITI failure.
Conclusion(s): PwHA who had total and partial success on ITI presented similar levels of IgG4 anti‐FVIII in plasma. High levels of plasma IgG4 anti‐FVIII is a hallmark of ITI failure.
PB0185
Exploring red blood cells as a novel tolerogenic approach for factor VIII inhibitors employing immuno‐dominant FVIII derived peptides presented on MHC class II
M. Miranda 1; E. Brandsma2; P. Kaijen1; F. van Alphen3; R. van Bruggen4; K. Fijnvandraat5; S. Lacroix‐Desmazes6; J. Voorberg1
1 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Hematopoiesise, Sanquin Research, Amsterdam, The Netherlands, amsterdam, Noord‐Holland, Netherlands; 3 Sanquin Research, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Molecular Hematology, Sanquin Research, University of Amsterdam, Amsterdam, The Netherlands, Uithoorn, Noord‐Holland, Netherlands; 5 Department of Pediatric Hematology, Amsterdam UMC, University of Amsterdam, Emma Children’s Hospital, Amsterdam, Noord‐Holland, Netherlands; 6 INSERM, Paris, Ile‐de‐France, France
Background: The main complication of hemophilia A treatment is the development of neutralizing antibodies (inhibitors) against factor VIII (FVIII). The eradication of anti‐FVIII antibodies relies on immune tolerance induction (ITI).
Aims: Since ITI is efficient in only 60–80% of cases, within the EDUC8‐consortium we are developing innovative methods to reduce biotherapeutics immunogenicity. Here we employed bioinformatics and advanced peptide presentation assays to identify promiscuously presented FVIII peptides to use in pioneering immuno‐tolerogenic approaches. To this end, we are exploring red blood cells (RBCs) as innovative antigen delivery system to induce tolerance.
Methods: A database of FVIII peptides presented on different MHC class II alleles was assembled and compared to predicted MHC class II presented epitopes using the ‘Immune Epitope Database (IEDB)’ website. An additional data‐set of naturally processed FVIII peptides was generated by incubating human FVIII with immature monocytes‐derived DCs from HLA‐typed healthy donors. Specific attention was on the identification of FVIII peptides presented on HLA‐DP4, since these alleles are highly prevalent in the Caucasian population.
Results: The IEDB website identified a large number of FVIII core peptides that were shown to be presented by multiple MHC class II alleles. To supplement this data‐set we successfully developed a protocol to study HLA‐DR and HLA‐DP antigen presentation utilizing specific monoclonal antibodies. Using this novel approach, approximately 4000 HLA‐DR‐associated peptides were identified. We detected over 100 HLA‐DR and 5 HLA‐DP4 presented FVIII peptides. An HLA‐DR presented FVIII peptide was fused to a cell‐penetrating peptide and incubated with RBCs. FACS analysis revealed its internalization in a dose‐dependent manner. We are currently exploring whether FVIII peptide‐treated RBCs phagocytosis by macrophages results in tolerogenic presentation of immunodominant FVIII peptides on MHC class II.
Conclusion(s): Our data provide an inventory of promiscuously presented FVIII‐derived peptides which will guide the development of novel tolerogenic approaches for FVIII inhibitors employing RBCs as carrier.
PB0191
DNA extracellular traps as potential biomarker of chronic haemophilic synovitis and therapeutic perspective in patients treated with PRP
P. Oneto 1; M. Landro2; C. Daffunchio2; A. Douglas Price3; E. Carrera SIlva1; H. Caviglia2; J. Etulain4
1 IMEX‐ANM‐CONICET, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Department of Orthopedic Surgery and Traumatology, Dr. Juan A. Fernández Hospital, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Department of Orthopedic Surgery and Traumatology, Dr. Juan A. Fernández Hospital, Buenos aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 IMEX‐ANM‐CONICET, Buenos Aires, Cordoba, Argentina
Background: Haemophilia‐associated hemarthrosis cause chronic haemophilic synovitis (CHS). Although neutrophils are major immune blood cells infiltrating joints after bleeding, their role on the pathogenesis of CHS is unknown. Neutrophils release extracellular DNA traps (ETs), structures of DNA with bound granular enzymes (including elastase) that were associated with tissue damage.
Aims: To evaluate the presence of ETs as pathogenic biomarkers, and the protective effect of intraarticular injection of platelet‐rich plasma (PRP) in patients with CHS.
Methods: Synovial fluids (SF) and plasma/PRP were obtained from 21 patients with CHS (28 ± 11 years old, 19 type A, 2 type B). 22 joints (1 ankle and 21 knees) were evaluated for Hemophilia Joint Health Score (HJHS). Synovial and plasmatic ETs were indirectly quantified by fluorometry (DNA) and directly by ELISA (DNA‐Elastase complexes). Pearson or Spearman correlations with clinical parameters were calculated.
Results: DNA (0.37 ± 0.06 μg/ml) and DNA‐Elastase (0.27 ± 0.03 OD) were detected in SF of patients with CHS and positively correlated with HJHS (r > 0.5–0.7, p < 0.05). While DNA or DNA‐Elastase were not detected in plasma of healthy donors, they were found in the plasma of CHS patients (DNA = 0.17 ± 0.01μg/ml; DNA‐Elastase = 0.16 ± 0.03OD) and showed a strong positive correlation (r = 0.7–0.8, p > 0.05) with the synovial levels of both parameters. Remaining ETs‐inducer factors were present in SF, as this fluid induced the in vitro release of ETs from blood‐isolated neutrophils. This phenomenon was impaired by adding plasma or PRP. Finally, promising preliminary data with 5 patients with CHS indicate that levels of DNA‐Elastase and joint damage decreased after 2 weeks of receiving intra‐articular injection of PRP.
Conclusion(s): The synovial and plasma levels of DNA‐Elastase correlated with joint damage suggesting that ETs formation could be a biomarker and potential therapeutic target for CHS. The intraarticular injection of PRP underlined a new potential alternative therapy, decreasing ETs formation in synovia of patients with CHS.


PB0200
Molecular algorithm for genetic diagnosis of women with hemophilia A in Argentina
B. Ziegler 1; L. Rossetti2; M. Abelleyro3; V. Marchione4; D. Neme5; E. Medina‐Acosta6; C. De Brasi7; P. Radic8
1 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Instituto de Medina Experimental (IMEX)‐CONICET‐ Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Fundación de la Hemofilia de Buenos Aires, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 6 Laboratory of Biotechnology, Universidade Estadual do Norte Fluminense Darcy Ribeiro/Molecular Identification and Diagnostics Unit, Hospital Escola A´lvaro Alvim, Campos dos Goytacazes, Rio de Janeiro, Brazil; 7 Instituto de Medicina Experimental (IMEX)‐CONICET‐Academia Nacional de Medicina (ANM)/Instituto de Investigaciones Hematológicas (IIHEMA)‐ANM, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 8 Instituto de Medicina Experimental (IMEX)‐CONICET‐Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: Hemophilia A (HA) is an X‐linked recessive coagulopathy caused by pathogenic variants in FVIII gene (F8), typically expressed in males but rarely in females. Recently a new nomenclature defines hemophilia carrier women: women/girls with severe (FVIII < 0.01 IU/ml), moderate (0.01–0.05 IU/ml) or mild HA (0.05–0.40 IU/ml), symptomatic and asymptomatic HA carriers (HAC) (FVIII ≥ 0.40 IU/ml) with and without a bleeding phenotype, respectively. HA expression in females associates with different causes: in most cases, heterozygous‐F8 variant and skewed X‐chromosome inactivation (XCI) silencing the normal F8, and rarely: combining two F8‐variants (in homozygous or compound heterozygous cases) or F8‐hemizygosity associated with female phenotype.
Aims: Analyze the molecular basis of HA expression in women through a simple algorithm.
Methods: 150 women suspected of being carriers of HA were studied. In cases with a known HA‐causative familiar variant: point‐mutations and indels were carrier diagnosed by Sanger‐sequencing, gross‐deletions by breakpoint‐specific‐PCR, or F8‐inversions (Inv22/Inv1) by inverse‐shifting‐PCR. In cases with no history of hemophilia or an unknown familial variant, the complete F8‐analysis algorithm was applied: ‐inverse‐shifting‐PCR for Inv22/Inv1, small‐variant screening by CSGE (conformation‐sensitive‐gel‐electrophoresis) on all relevant F8 amplicons (38 PCR‐products), and Sanger‐sequencing. Large deletions were investigated in suspected cases by MLPA or qPCR dosage. Variant pathogenicity was evaluated following ACMG criteria (American College of Medical Genetics). The XCI status was investigated in peripheral blood by XCI‐sensitive markers at AR (Xq) and RP2 (Xp).
Results: 105 women resulted HACs, including severe, moderate, mild and non‐symptomatic carriers. 107 F8‐variants were found. 102 HACs resulted heterozygous, one hemizygous (X0), one homozygous and a compound heterozygous (Figure 1).
Conclusion(s): Causes of bleeding phenotype were stated in eight HACs. Full F8 genotyping of HA‐causative variants and XCI patterns allow understanding the molecular basis of FVIII:C levels and clinical expression in HAC. The cost‐effectiveness of our approach makes it suitable also for developing countries

VPB0207
Factor VIII K1813A has high affinity for activated factor IX and enhances global coagulation function
Y. Nakajima 1; M. Takeyama2; A. Oda3; K. Nogami2
1 Nara medical University, Kashihara, Nara, Japan; 2 Nara Medical University, Kashihara, Nara, Japan; 3 Department of Pediatrics, Nara medical university, Kashihara, Nara, Japan
Background: Factor (F) VIII functions as a cofactor in the tenase complex responsible for phospholipid surface‐dependent conversion of FX to activated FX (FXa) by FIXa. Previous studies demonstrated the 1811‐1818 region in FVIII A3 domain contributed to FIXa binding and that the binding affinity of K1813A/K1818A for FIXa was increased. We hypothesized that FVIII mutants with higher affinity for FIXa could enhance procoagulant activity.
Aims: To create gain‐of‐function FVIII mutant.
Methods: We prepared several FVIII mutants (K1813A, K1818A, and K1813A/K1818A) by BHK cells and evaluated these mutants by SPR‐based assay, FVIII activity, thrombin generation assay (TGA), rotational thromboelastmetry (ROTEM) and hemophilia A mouse.
Results: The binding affinities of K1818A (Kd; 5.1 ± 1.8 nM) and K1813A/K1818A (Kd; 4.7 ± 1.2 nM) for FIXa on SPR‐based assays were similar to wild type (WT) FVIII (Kd; 6.3 ± 0.3 nM), however, that of K1813A (Kd; 3.9 ± 0.7 nM) was increased compared to WT. Peak FVIII activity of K1818A and K1813A/K1818A activated by thrombin was ~30% of WT. On the other hand, activation of K1813A by thrombin was comparable to those of WT, and decay rates of K1813A after peak thrombin activation was 2‐fold slower than that of WT. As for FVIII stability at 55°C, the decay rate for K1813A was 2.5‐fold improved relative to WT, indicating that K1813A was reduced in FVIII A2 dissociation. Tissue factor‐triggered TGA revealed that 0.5 nM of K1813A showed similar peak thrombin and endogenous thrombin potential in 1 nM of WT. The ex vivo addition of K1813A (0.5nM) to whole blood from the patient with hemophilia A also showed almost same coagulation potential as that of WT (1nM) in ROTEM. In the tail clip assay of hemophilia A mouse, K1813A showed approximately 4‐fold increased procoagulant function relative to WT.
Conclusion(s): K1813A enhanced global coagulation potential due to stability and the higher FIXa affinity.
VPB0204
Association between platelet receptor genotypes and the bleeding tendency of hemophilia patients
L. Bury 1; H. Kuchi Bhotla2; G. Piroli2; E. Cesari2; E. Falcinelli2; G. Guglielmini2; R. De Cristofaro3; B. Zieger4; P. Gresele2
1 University of Perugia, Perugia, Umbria, Italy; 2 University of Perugia, Department of Medicine and Surgery, Section of Internal and Cardiovascular Medicine, Perugia, Umbria, Italy; 3 Catholic University of Sacred Heart, Translational Medicine and Surgery Department, Rome, Lazio, Italy; 4 Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology and Oncology, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany
Background: Hemophilia bleeding severity is classified by residual factor activity as mild, moderate and severe. This classification, however, is not an entirely reliable predictor of the bleeding phenotype of individual patients. Little information is available on the contribution of platelets to the severity of the bleeding phenotype of patients with haemophilia, but some observations suggest a possible compensating function of increased platelet reactivity. Common single nucleotide variants (SNVs) of platelet receptors have been shown to account for interindividual differences in platelet reactivity in normal individuals and the bleeding and thrombotic risk in some chronic disorders. These variants may also play a role in determining clinical disease severity in hemophilia.
Aims: Aim of our work was to investigate if common SNVs influencing platelet reactivity have a different distribution in hemophiliacs with similar residual factor levels but with different bleeding phenotypes.
Methods: 55 patients with haemophilia A or B were enrolled and their bleeding score (BS) was calculated using the ISTH‐BAT. Blood was collected in sodium citrate and DNA was extracted. SNVs of ITGB3 (rs5918), ITGA2B (rs5911), ITGA2 (rs1126643 and rs1801106), GP6 (rs1613662), SELP (rs6136), ADRA2A (rs553668) and FCGR2A (rs1801274) were analyzed by PCR and Sanger sequencing.
Results: BS was significantly higher in hemophiliacs carriers of the SELP rs6136 A/C (BS A/A = 8 ± 7 vd A/C = 12 ± 5) and the FCGR2A rs1801274 G/G and A/G (BS A/A = 7.5 ± 3.5 vs. A/G+G/G = 13 ± 6.6), previously associated with decreased platelet reactivity, despite similar residual factor levels. A combination of “platelet inhibiting” genotypes (FCGR2A rs1801274 A/G+G/G; SELP rs6136 A/C; ITGB3 rs5918 T/T) had a significantly higher prevalence in high bleeders and was associated with higher bleeding score (OR = 4.9; 95% CI 1.1733 to 20.4798, p = 0.029).
Conclusion(s): These results show for the first time that common genetic variants modulating platelet reactivity influence the bleeding phenotype of haemophilic patients, suggesting that platelet genotyping of hemophiliacs may help to formulate prognostic predictions.
PB0198
Stable binding to phosphatidylserine‐containing membranes requires conserved arginine residues in Tandem C domains of blood coagulation factor VIII
S. Peters; K. Childers; C. Mitchell; S. Reese; S. Wo; C. Swanson; C. Brison; P. Spiegel
Western Washington University, Bellingham, Washington, United States
Background: Blood coagulation factor VIII (fVIII) is activated by thrombin and binds to activated platelet surfaces with activated factor IX to form the intrinsic ‘tenase’ complex and promote blood coagulation. Previous structural and mutational studies of fVIII have identified the C1 and C2 domains in binding to negatively charged membrane surfaces through β‐hairpin loops with solvent‐exposed hydrophobic residues and a ring of positively charged basic residues. Several hemophilia A‐associated mutations within the C domains are suggested to disrupt lipid binding, preventing formation of the intrinsic tenase complex.
Aims: In this study, we have aimed to generate recombinant C1, C2, and C1C2 constructs, along with hypothesis‐driven and hemophilia A‐associated mutants, to examine the membrane binding behavior for each domain. Additionally, we focused on elucidating the structural nature of phosphatidylserine binding at atomic resolution.
Methods: We devised a novel expression platform for generating recombinant C1, C2, and C1C2 domain constructs and performed site‐directed mutagenesis of several charged residues proximal to the putative membrane binding region of each C domain. Membrane binding was measured with established ELISA methods, biolayer interferometry (BLI) with affinity‐labeled lipid nanodiscs and a liposome sedimentation assay. Structure determination was performed with X‐ray crystallography.
Results: Mutations to basic residues adjacent to the surface‐exposed hydrophobic regions of C1 and C2 differentially disrupted membrane binding, with abrogation of binding occurring for mutations to conserved arginine residues in the C1 (R2163) and C2 (R2320) domains. We also determined the high‐resolution X‐ray crystal structure of the porcine fVIII C2 domain bound to o‐phospho‐L‐serine, the polar headgroup of phosphatidylserine, which binds to a basic cleft and makes charge‐charge contact with R2320.
Conclusion(s): We conclude that fVIII binds to phosphatidylserine‐containing membranes at similar basic clefts containing conserved arginine residues through C domain modularity, where each C domain possesses modest electrostatic‐dependent affinity, and tandem domains are required for high affinity binding.
Hemophilia – Clinical
PB0658
Improvement in school absence after factor replacement in students with haemophilia in Upper Assam, India
A. Dutta; T. Dutta; D. Boruah
Assam Medical College and Hospital, Dibrugarh, Assam, India
Background: Inadequately treated haemophilia patients experience complications related to repeated bleeding episodes caused by low levels of clotting factor VIII or IX in blood, that impacts day‐to‐day life, including active participation and performance in school in children with haemophilia (CwH). This complications can be prevented by factor replacement therapy, but access to factor has been challenging in low‐resource settings such as Northeast India. This study shows the impact of this therapy on bleeding episodes, joint complications and school absence among CwH in this setting.
Aims: To evaluate the impact of factor replacement therapy on school absence in a tertiary care center of upper Assam, in North East India.
Methods: Inadequately treated haemophilia patients experience complications related to repeated bleeding episodes caused by low levels of clotting factor VIII or IX in blood, that impacts day‐to‐day life, including active participation and performance in school in children with haemophilia (CwH). This complications can be prevented by factor replacement therapy, but access to factor has been challenging in low‐resource settings such as Northeast India. This study shows the impact of this therapy on bleeding episodes, joint complications and school absence among CwH in this setting.
Results: Thirty‐eight CwH were eligible for the study; 26(68.4%) were on prophylactic‐therapy and 12(31.5%) received on‐demand therapy. Findings at the end of the study period were compared to that at the beginning. The mean (+SD) ABR in prophylactic therapy was 5.8(+4.6) (p < 0.001), HJHS was 4.7 (+4.6) and FISH score was 27.9 (+3.3) (p < 0.001), all suggested significant improvement. Prophylaxis showed better (but insignificant) results in comparison to on‐demand therapy.
Conclusion(s): Factor replacement therapy significantly reduces school absence in CwH and correlates strongly with joint health and functional improvement, with the effect slightly better with prophylaxis than on‐demand therapy.


VPB1167
Frequency and quantification of inhibitors in congenital coagulation disorder patients—An experience at Tertiary Care Paediatric Hospital
N. Mazhar 1; J. Fatima1; S. Rafi2; S. Farhan3; N. Ahmed4
1 The Children’s Hospital Lahore, lahore, Punjab, Pakistan; 2 the children s hospital lahore, lahore, Punjab, Pakistan; 3 the children's hospital lahore, lahore, Punjab, Pakistan; 4 the children's hospital lahore, lahore, Punjab, Pakistan
Background: Congenital coagulation disorders are found in all racial groups and have a worldwide distribution. Inhibitors can develop in congenital coagulation factor deficiencies. An inhibitor is a polyclonal high affinity immunoglobulin G (IgG) antibody that specifically neutralizes the procoagulant activity of the relevant clotting factor. Bethesda assay is used worldwide as a laboratory investigation to quantify these inhibitors.
Aims: To quantify and calculate the frequency of inhibitors in congenital coagulation disorder patients by using Bethesda assay.
Methods: A cross sectional study carried out in the department of Haematology, Children’s Hospital Lahore, Pakistan for duration of 6 months (from 7th September 2016 to 7th March 2017). Blood samples were taken in Na‐citrate (blue top) vials from the study population for PT, APTT, inhibitor screening and quantification by Bethesda assay.
Results: Total 350 children were enrolled in the study out of which 277 (79.1%) were males and 73 (20.9%) were females. The age of these children ranged from 1–15 years—127 (36.3%) patients out of 350 were diagnosed as Hemophilia A, 73 (20.9 %) had Hemophilia B, 38 (10.9%) had rare bleeding disorders &112 (32%) had Von Willebrand disease, 16 (5.77%) patients out of 350 developed inhibitors after getting treatment. All of the 16 patients were from 1–4 years of age and were male. Quantifications of inhibitors in these patients was done by Bethesda Assay.
Conclusion(s): Coagulation factor inhibitors can develop against any coagulation factor although the most common are against FVIII (Haemophilia‐A). Coagulation factor inhibitor studies includes Bethesda assay to measure inhibitor titre which further guides about treatment.
VPB0680
Evaluating factors that influence the joint health status of Nigerians with moderate and severe Haemophilia (WBDR Research Support Program)
T. Nwagha 1; H. Okoye2; N. Iloanusi2; A. Nnamani3; N. Chigbo3; C. Kela‐eke4; O. Obodo3; N. Ogbodo5; I. Uwaegbuonu3; C. Ojinnaka3; A. Ilo3
1 College of Medicine University of Nigeria/University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria; 2 College of Medicine University of Nigeria Ituku Ozalla, Enugu, Enugu, Nigeria; 3 University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria; 4 University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria; 5 University Teaching Hospital Ituku Ozalla, Enugu, Enugu, Nigeria
Background: Joint bleeds is a major hallmark of Haemophilia. The resultant joint damage lead to physical and functional defects which affects the overall quality of life
Aims: This study examined whether age, age of first joint bleed, annualized bleeding rates (ABR) and Qol influenced joint health status in moderate and severe haemophilia
Methods: A cross sectional single‐center study involving persons with moderate(MH) and severe Haemophilia (SH) in Enugu Southeast Nigeria. Joint health status was evaluated using HJHS ( 0‐124, optimum score 0) Pettersson score (which grades findings on the radiograph, max 13 points), and Universal simplified ultrasound system(us‐us) (which grades findings on ultrasonography, max 14 points). Qol was assessed using ED ‐5D‐5L questionnaire, medical notes, and WBDR were also reviewed.
Results: We report 31 patients, mean age 10 years (SD 2, range 1‐39). The majority, 97% were HA. Sixty‐five percent were MH and SH was 35%. Age at diagnosis was 3 years (SD 2, range 3–15), age at first joint bleed was 20 months (SD 3, range 1–190 months) and the mean ABR was 9 (SD 4, range 1–40). The mean us‐us score was 3 (SD 2 range 1–13). The total mean HJHS was 16 (SD 2, range 3–56) and Pettersson's score was 8 (SD 1 range 4–18) with a significant moderate correlation between them (r = 0.44 p 0.01). The mean EQ‐5D‐5L score was 2 (SD 2 range 1–7) with a significant moderate correlation with HJHS (r = 0.5 p, 0.03). Higher HJHS was seen in MH (p 0.04). Age of PWH and the ED‐5D‐5L score were predictors of haemophilia joint health score.
Conclusion(s): The current joint health status for Nigerians with MH and SH was of moderate severity. Age of PWH and EQ‐5D‐5L score were predictors of HJHS. We suggest early routine joint assessment for persons with moderate to severe haemophilia.


VPB0682
Adherence to on demand treatment regimen and bleeding outcome (a multicenter Nigerian study)
T. Nwagha 1; H. Okoye2; S. Yuguda3; C. Udo4; D. Gwarzo5; M. Ogunfemi6; N. Osuji4
1 College of Medicine University of Nigeria/University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria; 2 College of Medicine University of Nigeria Ituku Ozalla, Enugu, Enugu, Nigeria; 3 Federal Teaching Hospital Gombe/Gombe State University, Gombe, Gombe, Nigeria; 4 National Hospital Abuja, Abuja, Federal Capital Territory, Nigeria; 5 Ahmad Bello Teaching Hospital, Kano, Kano, Nigeria; 6 University of Ilorin Teaching Hospital, Ilorin, Kwara, Nigeria
Background: Given the disparate access to coagulation factor concentrates (CFC) in resource‐constrained countries and individual preferences, on‐demand therapy remains a treatment option for the management of haemophilia. Non‐adherence can delay desired treatment outcomes There is a dearth of evidence on adherence to on‐demand therapy among PWH in sub–Saharan Africa
Aims: To evaluate self‐reported/parent‐reported adherence to on‐demand therapy among PWH and establish the associations between adherence and presence of target joints and annualized bleed rate
Methods: A cross‐sectional survey of 55 participants on episodic treatment recruited during outpatient appointments in 5 haemophilia treatment centers (HTC) across Nigeria using the validated Haemophilia Regimen Treatment Adherence Scale‐ Prophylaxis (VERITAS ‐PRN), 24 questions on six (four‐item) subscales (treat, time, dose, plan, remember, and communicate). The options of VERITAS ‐PRN were represented in 5 Likert scale and possible subscale ranged from 4 points (most adherent)to 20 points (least adherent) and possible total score ranging from 24 (most adherent) to 120 (least adherent ) the cut off for overall adherence put at >61 to indicate non‐adherence.
Results: Of the 55 participants, 94.1% both had haemophilia A and target joints. The majority, 51(92.7%) had scores indicating non adherence. The mean age of non‐adherent and adherent participants were 19(13.9) years and 24(13.23) years, respectively. For the non‐adherent, the overall mean score was 68.05(8.54) Subscale scores range from 9.38 (treat) to 15.00 (remember) There was a significant difference in the overall adherence scores and subscale scores between adherent and non‐adherent participants with p value < 0.05. There was a significant association between mean log of the number of target joints and the communicating subscale for the non‐adherent group, r‐0.61 p value < 0.05.
Conclusion(s): Adherence to on‐demand therapy is generally poor. Most were non intentionally skipping their doses. The association between the communication subscale and the number of target joints was significant.


PB1131
Adherence and cost effectively of home based prophylaxis over institutionalised based prophylaxis in patients with Hemophilia (PwH) in Upper Assam
A. Dutta; D. Boruah; A. Boruah
Assam Medical College and Hospital, Dibrugarh, Assam, India
Background: Home care management in Hemophilia is recommended by WFH as the standard care for patients with haemophilia (PwH) as it facilitates strict adherence to prophylaxis regime which in turn can reduces the emergency bleeding episodes, hospitalization rates, economic burden of factor infusion and travelling difficulties during active bleeding.
Aims: The Aim of the study is to study the impact of implementation of home therapy and its benefits in PwH registered under Hemophilia Treatment Centre (HTC) of Assam Medical College and Hospital conducted for over a period of 5 years.
Methods: In this retrospective study conducted over a period of 5 years, PwH and the parents of Children with haemophilia (CwH) were trained for home infusions. Appropriate documentation and records of factor dosage, correct storage and handling of the products, careful preparation of factor concentrate were also taught.Data collected based on a questionnaire for doses of factor used by them for prophylaxis in HTC and at home or local medical centres. Bleeding rates, cost of transportation per prophylaxis treatment in HTC were also recorded. Transportation cost was calculated by taking into account the cost of transport per unit.
Results: During the study period of 5 years, a shift from 0% to 77% was seen for self/home infusion. Most children preferred treatment by family members rather than medical staff. Use of Factor concentrates on time reduced the risk of emergency hospitalization. Home therapy also reduced the expenditure cost, time and potential risk of health of the PwH belonging to distant area.
Conclusion(s): Use of home based factor concentrates reduced the time of treatment during emergency bleeds thus reducing the risk of emergency hospitalization and improving the quality of life in PwH, experiencing less pain and greater flexibility in daily activities and less absenteeism from school and work.


VPB1169
The incidence of FV Leiden and MTHFR gene mutations in patients with hemophilia A and B in the North‐Western region of Russia
V. Soldatenkov 1; O. Soldatenkova2; K. Komissarov2; V. Burakov2; N. Saltykova2; L. Papayan3; N. Silina2; S. Kapustin1; O. Smirnova4; A. Titov2
1 Russian Research Institute of Hematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 2 Russian Scientific Research Institute of Hematology and Transfusiology, Saint‐Petersburg, Saint Petersburg City, Russia; 3 Russian Research Institute of Haematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 4 Russian Research Institute of Hematology and Transfusiology, Saint‐Petersburg, Saint Petersburg City, Russia
Background: The role of prothrombotic markers in patients with hemophilia is still unclear. On the one hand, procoagulatory changes may have protective effect on the clinical course. On the other hand, novel therapy approaches and high‐dose perioperative factor prophylaxis may be complicated by thrombosis. So all the risk factors should be considered before the drug prescription.
Aims: To study the incidence of genetic thrombophilia markers (FV Leiden, MTHFR) in patients with hemophilia A and B and compare it to the average for population in the North‐Western region of Russia.
Methods: 98 case‐records of patients with hemophilia A (85 patients) and B (13 patients), who underwent treatment in Russian Scientific Research Institute of Hematology and Transfusiology in 2020‐2021, were studied. The results of molecular genetics testing for thrombophilic mutations were analyzed.
Results: 1) The incidence of heterozygous FV Leiden mutation in the studied group comprised 4.08%. The difference between this value and the average for the population (4.4%) was not statistically significant. 2) The difference between incidences of the heterozygous FV Leiden mutation in patients with hemophilia A and B was not statistically significant. 3) The incidence of heterozygous MTHFR gene mutation comprised 51,22%, which exceeds the average for the population (39.5%). But the further statistical analysis showed that the difference was not statistically significant. 4) The incidence of homozygous MTHFR gene mutation comprised 8,54%. The difference between this value and the average for the population (10.1%) was not statistically significant.
Conclusion(s): The incidence of FV Leiden and MTHFR mutation in hemophiliacs of the North‐Western region of Russia does not statistically significant differ from the average population which means similar risks in terms of the correction of coagulation defect. The study will be proceeded to estimate other prothrombotic markers and their impact on clinical course.
PB0649
Real world experience using rIX‐FP prophylaxis in patients with haemophilia B: Experience from a Spanish centre
O. Benítez Hidalgo 1; A. de Jaureguizar Tesas2; J. Juarez Giménez1
1 Universitary Vall d'Hebron Hospital, Barcelona, Catalonia, Spain; 2 Germans Trias i Pujol Hospital, Barcelona, Catalonia, Spain
Background: rIX‐FP, a recombinant factor IX linked with recombinant human albumin, has shown good efficacy for prophylaxis in patients with haemophilia B (PWHB)
Aims: This study reports real‐world experience of managing rIX‐FP prophylaxis in a Spanish reference centre
Methods: Retrospective data was collected from PWHB who switched from standard half‐life (SHL) FIX products to rIX‐FP. Data was also collected from one previously untreated patient (PUP) who began prophylaxis with rIX‐FP Data was collected from medical records for the 12 months prior to and 12 months after switching to rIX‐FP, including age, infusion frequency and dose, and annualised bleeding rates (ABR). A pharmacokinetic analysis was conducted via the WAPPS‐Hemo platform
Results: Five patients with severe haemophilia B were initiated on rIX‐FP prophylaxis. The mean age was 25 years old, (range 1–60). 4 of these patients (3 adults and 1 paediatric) were previously treated with twice a week prophylaxis with SHL‐FIX products (3 with recombinant and one with plasma‐derived). One was a paediatric previously untreated patient. All patients began prophylaxis with rIX‐FP every 7 days. After being well‐controlled on this regimen, two previously treated patients (one adult and one paediatric) switched to dosing every 14 days. Two of the adult previously treated patients further extended dosing to every 21 days. In all previously treated patients (PTPs), annualised spontaneous bleeding rates were maintained at 0.00, when compared to their previous regimen. In PTPs across all dosing intervals with rIX‐FP prophylaxis: mean FIX levels at Day 7 post‐infusion were 19.2% No adverse events were reported after switching to or initiating rIX‐FP
Conclusion(s): Patients with haemophilia B who switched to rIX‐FP from a SHL‐FIX were able to reduce their infusion frequency and factor consumption, whilst maintaining bleed control and increasing their FIX trough level
PB0666
A new FVIII chromogenic assay with bovine FX is sensitive enough for the diagnosis of severe haemophilia A
M. Jacquemin 1; J. Toelen2; K. Peerlinck2; P. Verhamme3; C. Van Laer4
1 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium; Department of Vascular Medicine and Hemostasis, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 2 University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 3 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 4 Center for Molecular and Vascular Biology, University of Leuven; Clinical Department of Laboratory Medicine, University Hospitals Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium
Background: The development of extended half‐life recombinant factor VIII (rFVIII) and of the bispecific monoclonal antibody that binds to both factor IXa and factor X (emicizumab) has considerably increased the complexity of the monitoring of FVIII replacement therapy in patients with haemophilia A. To allow the monitoring of FVIII substitution therapy in patients treated with emicizumab, a novel FVIII chromogenic assay with bovine Factor X and human Factor IXa has recently been developed by Hyphen Biomed.
Aims: We determined whether the sensitivity of this novel assay was sufficient for the diagnosis of severe haemophilia A.
Methods: The tests were carried out with the BIOPHEN FVIII variant (emicizumab insensitive) chromogenic assay (Hyphen BioMed, Neuville‐Sur‐Oise) according to the manufacturer’s instructions. Three calibration curves were generated by diluting the calibrator plasma in diluent buffer provided with the kit, in FVIII deficient plasma and in pooled normal plasma inactivated at 58°C for 30 min.
Results: There was a significant difference between the calibration curve generated with different diluents (Figure). The optical density recorded for 0% FVIII when using diluent buffer only corresponds to more than 1% FVIII when it is read on the calibration curves generated by dilution in severe haemophilia A plasma or inactivated plasma. Accordingly, the use of the calibration curves generated with diluent buffer is not appropriate to measure low FVIII levels as the bias would result in the measurement of more than 1% FVIII in plasma of patients with severe haemophilia A.
Conclusion(s): The assay can be used for the diagnosis of severe haemophilia A when the calibration curve is generated in FVIII‐deficient plasma.

PB1161
Retrospective analysis of treatment individualization on hemophilia B patients from two Spanish centers who switched from SHL‐FIX products to rIX‐FP
A. Tugues 1; E. Vicente1; R. Santoja2; A. Marco3; P. Marco Vera4; M. Gilabert5; J. Rius5; I. Mangues5; I. Delgado2; A. Ruiz6; A. Garcia6; C. Chávez6; M. Teixidó7; E. Rivero2; A. Luaña7; A. Ferrero2; T. Garcia7; C. Marzo8
1 Hospital Universitari Arnau de Vilanova de Lleida, Departamento de Trombosis y Hemostasia, Servicio de Hematología y Hemoterapia., Lleida, Catalonia, Spain; 2 Hospital Universitari Arnau de Vilanova de Lleida, Servicio de Hematología y Hemoterapia., Lleida, Catalonia, Spain; 3 Hospital General Universitario de Alicante, Departamento de Trombosis y Hemostasia, Servicio de Hematología y Hemoterapia., Alicante, Comunidad Valenciana, Spain; 4 Departamento de Medicina Clínica. Universidad Miguel Hernández, Jefe de Servicio de Hematología y Hemoterapia, Hospital General Universitario de Alicante, Alicante, Comunidad Valenciana, Spain; 5 Hospital Universitari Arnau de Vilanova de Lleida, Departamento de Farmacia, Lleida, Catalonia, Spain; 6 Hospital Universitari Arnau de Vilanova de Lleida, Servicio de Hematología y Hemoterapia., lleida, Catalonia, Spain; 7 Hospital Universitari Arnau de Vilanova de Lleida, Departamento de Trombosis y Hemostasia, Servicio de Hematología y Hemoterapia., lleida, Catalonia, Spain; 8 Hospital Universitari Arnau de Vilanova de Lleida, Departamento de Trombosis y Hemostasia, Servicio de Hematología y Hemoterapia., Lérida, Catalonia, Spain
Background: rIX‐FP is an extended half‐life (EHL‐rFIX) fusion protein of coagulation factor IX with recombinant human albumin, indicated for on‐demand and prophylactic treatment in patients with hemophilia B (PWHB) of all ages. EHL‐rFIX products allow PWHB for prolonged dosing intervals, individualize their treatment and so reduce their treatment burden.
Aims: Analyse the switch to rIX‐FP from 4 PHWB and their treatment individualization.
Methods: Retrospective data was collected for 4 patients from two Spanish centers who switched from SHL‐FIX products to rIX‐FP Patient demographic data, pharmacokinetics,adherence and consumption have been compiled. A pharmacokinetic (PK) analysis was done with the WAPPS‐Hemo platform.
Results: Four PWHB, two pediatric (ages 5,7) and two adults (17,49) with severe haemophilia B, began prophylaxis with rIX‐FP. Both pediatric were previously treated on 1x/week prophylaxis with recombinant FIX (2000 and 1500 UI weigh adjusted ) and switch to rIX‐FP with same dose every 10 days and further extended dosing to 14 days given mean trough levels > 8.5% at day 10 days post‐administration. The adult patients were on 2x/week prophylaxis with plasma‐derived FIX and switch to rIX‐FP every 7 days for 2 months and extended the dosing interval to 10 (teenager) and 14 days (adult) with mean FIX levels at day 7 post‐infusion of 13.9% and 19.7% respectively (based on balanced prediction). This treatment individualization has allowed reduction on 28.7–78.61% number of infusions/Year and consumption by 54% (34–75%) from 1500 UI ‐18.000 UI/month No bleeding or adverse events were reported following the switch to rIX‐FP
Conclusion(s): In summary, rIX‐FP is an excellent choice for the prophylactic treatment of any PWHB and allows treatment individualization to the patient needs, providing benefits in terms of efficacy, adherence and quality of life.


PB0664
Demographic and baseline clinical characteristics of patients with hemophilia A in China: Real‐world data from the AHEAD International Study
R. Wu1; Z. Li2; J. Sun3; X. Du4; X. Zhang5; Y. Wang6; Q. Hu7; R. Zhou8; J. Gu9; R. Guerra 9; L. Tang10; R. Yang11
1 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic); 2 The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China (People's Republic); 3 Nanfang Hospital, Nanfang Medical University, Guangzhou, Guangdong, China (People's Republic); 4 Shenzhen Second People's Hospital, The First Affiliated Hospital of Shenzhen University, Shenzhen, Guangdong, China (People's Republic); 5 Shandong Blood Center, Jinan, Shandong, China (People's Republic); 6 Shenzhen Children's Hospital, Shenzhen, Guangdong, China (People's Republic); 7 Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei, China (People's Republic); 8 Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China (People's Republic); 9 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States; 10 Takeda Pharmaceuticals International AG, Zürich, Zurich, Switzerland; 11 State Key Laboratory of Experimental Hematology, National Clinical Research Center for Hematological Disorders, Institute of Hematology & Blood Diseases Hospital, Tianjin Laboratory of Blood Disease Gene Therapy, CAMS Key Laboratory of Gene Therapy for Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, Tianjin, Tianjin, China (People's Republic)
Background: The international AHEAD study (NCT02078427, started in 2011) is assessing the long‐term effectiveness and safety of octocog alfa and rurioctocog alfa pegol in patients with hemophilia in real‐world clinical practice.
Aims: To report baseline demographic and clinical characteristics of patients from the AHEAD China subgroup.
Methods: AHEAD is a prospective, non‐interventional, multicenter study conducted in 22 countries. Patients of any age with hemophilia A are eligible for inclusion. The primary endpoint is joint health outcomes evaluated by Gilbert score (pain, bleeding, physical exam). Secondary endpoints include annualized bleeding rates (ABRs), annualized joint bleeding rates (AJBRs), Hemophilia Joint Health Score (HJHS), and safety. Ethics committee approval and informed consent were obtained. Patient enrollment in China commenced in June 2020. Data were extracted from the 8th interim read‐out (data cutoff: July 01, 2021).
Results: The AHEAD China subgroup included 141 patients (mean [SD] age, 20.3 [12.0] years) who received treatment with octocog alfa: 95 (67.4%) received prophylaxis (mean [SD] age, 17.2 [11.3] years) and 46 (32.6%) received on‐demand treatment (mean [SD] age, 26.8 [10.9] years). All patients were male and of Asian ethnicity. Hemophilia A severity, history of FVIII inhibitor detection, and number of target joints at baseline are shown in Table 1. At screening, 72 prophylaxis patients (75.8%) and 39 on‐demand patients (84.8%) had experienced bleeding events, which occurred up to 12 months prior to screening. Mean ABRs and AJBRs at baseline were numerically lower in patients receiving prophylaxis compared with on‐demand treatment (Table 2). Gilbert scores and HJHS: Global Gait Scores at baseline were consistent across treatment groups (Table 2).
Conclusion(s): These real‐world data from the AHEAD China subgroup demonstrate lower bleeding rates in patients receiving prophylaxis compared with on‐demand treatment at baseline. These data will provide a basis for future analyses observing the clinical outcomes of patients and standard of care in China.


VPB0695
A study of mood state using POMS2 in hemophilia patients
A. Nakao 1; J. Yamanouchi1; K. Kawabe2; T. Hato1; K. Takenaka2
1 Ehime University Hospital, Toon, Ehime, Japan; 2 Ehime University Graduate School of Medicine, Toon, Ehime, Japan
Background: Advancements in treatment have extended the life expectancy of patients with hemophilia. There are patients who are already living with the pain and inconvenience because of arthropathy, or who have complications from aging, such as malignancy or lifestyle‐related diseases. Then, patients also suffer from anxiety related to the disease and mental stress caused by stigma from others.
Aims: This study reports on the psychological issues of hemophilia patients, especially their mood state.
Methods: We evaluated 28 adult patients with hemophilia (median age of 35 (range: 19–76)). A clinical psychologist had interviews with patients independently of doctor’s medical examination, measured mood and emotion state by POMS2 (Profile of Mood States Second Edition), and assessed the Total Mood Disturbance (TMD) scores and each factors of POMS2.
Results: The TMD score of the patients was 43.6. This was in the “average” TMD score group of the general male population in their 30s. On the other hand, one patient (4%) was in the "high" TMD score group, which was less than the "high" TMD score group of the general male population (16%). Each factors of POMS2 showed the healthy profile, with low scores on negative mood states and high scores on positive mood states. However, five patients had high scores on negative mood states or low scores on positive mood states. Patients who had inhibitors had low scores on positive mood state.
Conclusion(s): We found that few hemophilia patients were high scores more than average TMD scores. This was less than the general male population. However, hemophilia patients have the various kind of mental stress. It is necessary to establish a support system by interdisciplinary approach. We would like to continue to provide supports for the psychosocial issues.
PB1132
The new era of Haemophilia in a Comprehensive Hemophilia Treatment Center in Mexico
A. Gonzalez1; T. Garcia‐Lee 2; G. Ignacio1; S. Quintana3
1 IMSS, CDMX, Distrito Federal, Mexico; 2 Instituto Mexicano del Seguro Social, CDMX, Distrito Federal, Mexico, 39011757, CDMX, Distrito Federal, Mexico
Background: Haemophilia is a chronic condition, that requires specialized treatment. It´s recommended that the People with Haemophilia (PwH) be managed in a Comprehensive Hemophilia Center (CHC).
Aims: The aim of this study is to assess the current haemophilia situation in our CHC.
Methods: Electronic records from the last 2 years. of PwH were reviewed at the CIHT (Centro Integral en Hemostasia y Trombosis) in Regional Hospital No. 1, in Mexico City, Mexico
Results: The total population of PwH in our center is 120 patients; of which Haemophilia A (HA) are 103 (85.9%) & Haemophilia B (HB) 17 patients (14.1%). The median (range) age was 31 (1‐86) years; the pediatric population is 17, and PwH and inhibitors are 9 (8.7%), all of them with HA and high response inhibitors. 80% of the total have arthropathy, of which those with HA are 81 (78.5%), and with HB are 15 (88.2%). 21 patients have >45 years old and 62% of the them have comorbidities (Graph 2). Transfusion related infections were present in 27 PwH. Treatment regimen: Most patients with severe hemophilia were treated with prophylaxis 76/82 (93%). All children with severe haemophilia received prophylaxis; and 8/11 children had arthropathy. All patients with HA received rFVIII‐SHL (recombinant FVIII of Standard Half‐Life), and pdFIX (plasma‐derived FIX) for HB. The frequency of prophylaxis is according with the guidelines. 8/9 patients with HA and high response inhibitors are treated with prophylaxis with emicizumab. Only 1 patient is treated with ITI.
Conclusion(s): In this new era where Clotting Factor Concentrates are a reality in our center, all patients with severe haemophilia should be managed with a strategy of bleeding prevention and we need to focus on the prevention of arthropathy. Indeed is essential the comprehensive treatment for the PwH.


VPB0698
Analysis of low‐ and intermediate‐dose prophylaxis of 30 adult patients with severe hemophilia A in a single center in Nanjing
R. Zhou 1; Y. Yuan2; Y. Xu1
1 Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China (People's Republic); 2 Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China (People's Republic)
Background: Limited data exist on the impact of prophylaxis (routine FVIII replacement) on adults with severe hemophilia A and pre‐existing joint disease although prophylaxis has been recommended as the optimal therapy protocol for children.
Aims: To evaluate the clinical effects of low‐ and intermediate‐dose factor VIII (FVIII) prophylaxis on adult patients with severe haemophilia A in a single HTC from Nanjing.
Methods: Data were retrospectively analyzed from 30 adults severe haemophilia A on regular low‐dose (20 cases) or intermediate‐dose (10 cases) prophylaxis. After a median follow‐up of 48 months, the annual bleeding rate (ABR), annual joint bleeding rate (AJBR), the number of target joints, joint imaging scores (ultrasound HEAD‐US score), Functional Independence Score in Hemophilia (FISH), quality of life score and health status score (SF‐36) were compared between the two groups before and after prophylaxis.
Results: Compared with on‐demand treatment before prophylaxis, low‐ and intermediate‐dose prophylaxis significantly reduced ABR, AJBR and the number of target joints (p < 0.05), and the improvement of intermediate‐dose prophylaxis group was better than that of low‐dose prophylaxis group (p < 0.05). Compared with on‐demand treatment, FISH score, quality of life score and SF‐36 score were significantly improved (p < 0.05), but there was no significant difference between low‐dose prophylaxis group and intermediate‐dose prophylaxis group (p > 0.05). 11 patients received ultrasound HEAD‐US score. 1 patient had significant improvement after right knee arthroplasty, 3 patients had no change in ultrasound score, and ultrasound score of the other 7 patients showed that the joint damage was still slowly aggravating after prophylaxis.
Conclusion(s): In adults with severe hemophilia A, low‐ and intermediate‐dose prophylaxis can significantly reduce the bleeding frequency, delay the progress of joint lesions and improve the quality of life of patients as compared with on‐demand treatment. The improvement of clinical bleeding in intermediate‐dose prophylaxis was better than the low‐dose prophylaxis.
PB1126
Safety and efficacy of simoctocog alfa in patients with haemophilia A in routine clinical practice (GENA‐99)
C. Berger 1; M. Sol Cruz2; S. Carolina Oliva3; A. Molina Pantoja4; M. Mathias5; E. Zanon6; S. Siragusa7; B. Pollio8; H. Glosli9; A. Batorova10; E. Dmitriev11; M. Janbain12; J. Martinez13; S. Saulyte Trakymiene14; M. Penka15; P. Radossi16; S. Werner17; G. Pezeshki17; G. Di Minno18
1 Hemophilia CRC Hospital, INSERM, U 1059, Jean Monnet University, Saint‐Etienne, Rhone‐Alpes, France; 2 Centro Diabetologico del Norte (CEDINOR), Salta, Salta, Argentina; 3 PEDIAS INC, Guatemala City, Alta Verapaz, Guatemala; 4 Teodoro Maldonado Carbo Specialty Hospital, Guayaquil, Guayas, Ecuador; 5 Great Ormond Street Hospital for Children, NHS Foundation Trust, London, England, United Kingdom; 6 Medicina Generale, Azienda Ospedaliera di Padova, Padova, Veneto, Italy; 7 AOUP Policlinico Giaccone, Palermo, Sicilia, Italy; 8 Regina Margherita Children Hospital Azienda Ospedaliero‐Universitaria Città della Salute e della Scienza, Torino, Piemonte, Italy; 9 Oslo University Hospital, Oslo, Oslo, Norway; 10 National Haemophilia Center, Bratislava, Bratislava, Slovakia; 11 Belarussian Research Centre for Paediatric Oncology, Haematology and Immunology, Minsk, Minskaya Voblasts', Belarus; 12 Tulane University, New Orleans, Louisiana, United States; 13 University of Cova da Beira, Centro Hospitalar Universitário Cova da Beira, EPE, Colviha, Castelo Branco, Portugal; 14 Clinic of Children’s diseases, Faculty of Medicine, Vilnius University, Vilnius University Hospital Santaros Klinikos, Vilnius, Vilniaus Apskritis, Lithuania; 15 Teaching Hospital of Faculty of Medicine of Masaryk University, Brno, Jihomoravsky kraj, Czech Republic; 16 Istituto Oncologico Veneto, IRCCS, Castelfranco Veneto, Veneto, Italy; 17 Octapharma USA, Paramus, New Jersey, United States; 18 Azienda Ospedaliera Universitaria "Federico II", Napoli, Campania, Italy
Background: Clinical studies have demonstrated the efficacy and safety of simoctocog alfa in patients with severe haemophilia A. GENA‐99 (NCT02962765) was a prospective, multinational, non‐interventional study that assessed the immunogenicity, safety and effectiveness of simoctocog alfa in routine clinical practice.
Aims: To generate real‐world data on the use of simoctocog alfa for the routine care of previously treated patients (PTPs) with haemophilia A.
Methods: Male PTPs (≥150 exposure days [EDs] to FVIII) with haemophilia A (FVIII:C ≤2%) of any age/ethnicity were enrolled in 13 countries and followed for 100 EDs. The study was approved by independent ethics committees and institutional review boards, with written informed consent obtained before any study‐related activities. Adverse drug reactions (ADRs), including hypersensitivity reactions, FVIII inhibitor development and thromboembolic events, were monitored throughout the study. The effectiveness of simoctocog alfa in prevention of bleeds, and treatment of bleeding episodes (BEs) and surgery was assessed.
Results: Seventy‐eight PTPs (Table 1) were enrolled and treated with a total of 14,628,000 IU of simoctocog alfa. No patient experienced an ADR, and no inhibitors were reported. Of the 77 patients who received prophylaxis, 32 (41.6%) patients received a 3x/week regimen, 18 (23.4%) every‐other‐day, and 17 (22.1%) 2x/week. In 74 patients who received ≥3 months of prophylaxis, mean duration was 10.2 months (maximum 21.2 months). Median ABR was comparable to those in the clinical registration studies GENA‐08 and GENA‐03 (Table 2). In the 77 patients who received prophylaxis, 246 BEs occurred and were treated with simoctocog alfa. BEs were rated with excellent (67.9%) or good (20.3%) treatment efficacy, for a success rate of 88.2%. Efficacy of all 5 rated surgeries was rated excellent.
Conclusion(s): These data indicate that the long‐term safety and efficacy of simoctocog alfa in the real‐life clinical setting are comparable to those in clinical registration studies.

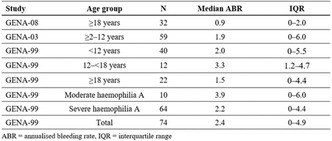
PB1147
Sexism in hemophilia? Identifying disparities in care of symptomatic hemophilia carriers in a comprehensive Hemophilia Treatment Center
E. Palmer 1; S. Dugan2; L. Malec3
1 Versiti Comprehensive Center for Bleeding Disorders and Diagnostic Laboratories, Versiti Blood Center of Wisconsin, Milwaukee, Wisconsin, United States; 2 Comprehensive Center for Bleeding Disorders and Diagnostic Laboratories, Versiti Blood Center of Wisconsin, Milwaukee, Wisconsin, United States; 3 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States
Background: In 2021, an expert consensus publication about hemophilia in women and girls defined new nomenclature and highlighted the need for a more inclusive classification system to enhance awareness and improve clinical diagnosis and care. As a quality improvement measure, we sought to evaluate the cadence of clinical care for and classification of women and girls with hemophilia (W/GH) and hemophilia carriers (HC) at our hemophilia treatment center (HTC). We currently recommend clinic visits at least every 2 years for these female patients regardless of baseline factor level, as compared to every 6 months or yearly for males with severe or moderate/mild hemophilia, respectively.
Aims: We aimed to quantify baseline cadence of care for W/GH and symptomatic HC at our HTC prior to a strategic initiative to improve clinical follow up of these patients.
Methods: A retrospective analysis of our HTC’s medical database was performed to assess timing of clinical care, age, factor activity, and bleeding symptoms of W/GH and symptomatic HC. Logistic regression analysis was performed to identify factors associated with compliance with recommended 2‐year follow up.
Results: 79 symptomatic HC were identified in the medical database (clinical data summarized in Table 1). 69.6% of symptomatic HC have HTC clinic visit history outside of recommended intervals: 43% of HC have not attended clinic in more than 2 years, 26.6% of HC have never attended clinic. Logistic regression of clinic visit attendance in all HC revealed a weak negative correlation with age (p = 0.021, r = −0.036).
Conclusion(s): Improving the experience and outcomes of W/GH and symptomatic HC may require institutional identification of gaps in care. The striking finding that over two‐thirds of carriers at our HTC are not receiving care within the recommended interval calls for further investigation to ensure adequate engagement and reduce disparities in care of this population.

VPB0696
No difference in the management of Covid‐19 hemophilic and non‐hemophilic patients
G. Sottilotta 1; G. Nicolò2; C. Mangano3; F. Luise4; A. Piromalli4
1 Hemophilia Centre ‐ Thrombosis and Hemostasis Service. Great Metropolitan Hospital of Reggio Calabria (Italy), REGGIO CALABRIA, Calabria, Italy; 2 Hemophilia Centre ‐ Thrombosis and Hemostasis Service. Great Metropolitan Hospital of Reggio Calabria (Italy), Reggio Calabria, Calabria, Italy; 3 ASP Reggio Calabria (Italy), Reggio Calabria, Calabria, Italy; 4 Analysis Laboratory. Great Metropolitan Hospital of Reggio Calabria (Italy), Reggio Calabria, Calabria, Italy
Background: Several guidelines specifically suggest how to manage hemophilic patients (PWH) with COVID‐19, but recent studies have shown that Covid‐19 PWH management does not differ from the general population
Aims: To evaluate our COVID‐19 PWH to confirm if this trend is also present in our Centre
Methods: We retrospectively evaluated data about 8 Covid‐19 PWH (4 adults and 4 pediatrics) during 2020‐22.Median age was 28.7 (range 10‐61).5 were hemophilic A (2 severe and 3 moderate), 3 were severe B (one inhibitor). At the moment of observation 6 they had not yet been vaccinated, two had received the second and third doses respectively
Results: Only one 56‐year‐old patient needed hospitalization: he presented diabetes, hypertension, hepatitis C and prostatic hypertrophy and was admitted to the hospital because of a worsening dyspnea, and was treated with noninvasive ventilation. Heparin was avoided because of the hemorrhagic risk and the other therapies were administered orally or intravenously. He was discharged after 7 days. Of the other adults, a 27‐year‐old man manifested mild dyspnea and diarrhea without needing hospitalization.The 34‐year‐old who had received the two doses of vaccine scheduled at that time did not show any symptomatology. The 61‐year‐old with three doses of vaccine, developed mild symptoms (low grade fever, sore throat, mild muscle pains). All 4 pediatric patients were successfully treated at home:only the inhibitor patient had an episode of nosebleed treated with single by‐passant treatment; the pediatric PWH reported mild symptoms: low‐grade fever, asthenia, headache, cold and cough; one had a temporary anosmia and ageusia. Results are resumed in table 1
Conclusion(s): Our experience showed no difference between PWH and the general population: the only adult who needed hospitalization was the one with comorbidities and for whom the application of Guidelines for Covid‐19 PWH avoided bleedings.The pediatric patients and the vaccinated adults were pauci‐symptomatic and were cured at home

PB1137
Intracranial haemorrhage in children with haemophilia in Turkey
M. Sezgin Evim1; C. Albayrak2; E. Zengin3; E. Yılmaz4; N. Özdemir5; M. Ertekin6; G. Özdemir7; A. Bahadır8; B. Yılmaz7; S. Akbayram9; F. Küpesiz10; H. Tokgöz11; B. Tatlı Güneş6; Y. Aral12; C. Acıpayam13; F. Belen Apak14; A. Unuvar15; S. Karaman 16; H. Ören17
1 Bursa Uludag University Pediatric Hematology, Bursa, Bursa, Turkey; 2 Ondokuz Mayıs University Medical Faculty Haed of pediatric bone marrow unit, Samsun, Samsun, Turkey; 3 Pediatric Hematologist, Kocaeli, Kocaeli, Turkey; 4 Pediatric Hematologist, Kayseri, Kayseri, Turkey; 5 Pediatric Hematologist, izmir, Izmir, Turkey; 6 Pediatric Hematologist, İzmir, Izmir, Turkey; 7 Pediatric Hematologist, İstanbul, Istanbul, Turkey; 8 Pediatric Hematologist, Trabzon, Trabzon, Turkey; 9 Pediatric Hematologist, Gaziantep, Gaziantep, Turkey; 10 Pediatric Hematologist, Antalya, Antalya, Turkey; 11 Pediatric Hematologist, Konya, Konya, Turkey; 12 Pediatric Hematologist, Aydın, Aydin, Turkey; 13 Pediatric Hematologist, Kahramanmaraş, Kahramanmaras, Turkey; 14 Baskent University Medical Faculty Department of Pediatric Hematology and Oncology, Ankara, Ankara, Turkey; 15 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, ISTANBUL, Istanbul, Turkey; 16 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, Istanbul, Istanbul, Turkey; 17 Dokuz Eylül University Faculty of Medicine, İzmir, Izmir, Turkey
Background: Intracranial haemorrhage (ICH) is a rare but life‐threatening complication.
Aims: To evaluate risk factors for ICH, mortality rate, long‐term morbidity and affecting factor of sequelae in patients with haemophilia.
Methods: We contacted pediatric hematologist by e‐mail and information about children who had intracranial bleeding was requested.
Results: In total 86 patients, haemophilia A/B ratio was 65/21. The numbers of severe, moderate and mild haemophilia were 65, 15, and 6, respectively. Nine patients had no complaints. Seizure was the most common complaint and was present in 34 patients, followed by lost of consciousness (n: 21) and nausea/vomiting (n: 10). Other complaints were focal neurological findings (n: 4), headache (n: 3), arrest (n: 2), irritability (2) and hypotonia (1). Thirteen (15%) patients who bleed in the neonatal period, Thirty‐three of the remaining 73 patients were not known to have hemophilia. Haemophilia diagnosis of 40 patients was known before ICH, only 8 of them were under prophylaxis. The most common bleeding locations were parenchymal (29/86), combined (21/86) and subdural (18/86) haemorrhages. Other bleeding sites were epidural (9/86), subarachnoid (5/86) and ventricular (4/86). After the neonatal peiod, parenchymal bleeding was common in the diagnosed group (17 vs. 7) and combined bleeding was common in the undiagnosed group (14 vs. 4). The undiagnosed group was operated on more frequently in the first 24 hours (18 vs. 6). Five patients died during the first ICH. The rate of loss in the neonatal period was higher compared to other periods (15% (2/13) vs. 4%(3/73)). The rate of neurological sequelae was 25/81 (30%). In the multiple regression analysis, only shift was found to be significant (OR: 4.464, CI 95% 1.151‐ + 17.309. (p 0.031)).
Conclusion(s): ICH are an important cause of mortality and morbidity, sequelae can be reduced by increasing compliance to prophylaxis.


PB1141
Congenital Haemophilia A with very low titre inhibitor, undetectable by routine methods and presenting with discrepant factor VIII assays post treatment with recombinant FVIII
D. White; A. Kelly; J. Fox; J. Crowe; S. MacDonald
Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom
Background: An 8 week old male presented with a subdural haemorrhage and was diagnosed with severe Haemophilia A (SHA). He was managed conservatively with 250IU recombinant FVIII (Advate) bi‐daily (BD). Factor VIII recovery was disappointing leading to suspicion of an inhibitor being developed.
Aims: Assess the use of the Anti‐FVIII ELISA for low level inhibitor detection Report of findings of discrepant FVIII assay results in post treatment dose samples
Methods: Chromogenic (CFVIII) (Hyphen Biomed, Neuville‐Sur‐Oise, France) and one‐stage (OS) FVIII were performed on the ACL TOP 750 (Werfen, Bedford, USA). Anti‐FVIII ELISA (Hyphen Biomed) and Bethesda assays were performed on heat treated plasma.
Results: From day 18 of admission, the patient displayed reduced half‐life and increments, with post dose levels only reaching 0.42 IU/ml. This prompted dose escalation to 250IU 6 hourly. OS FVIII and CFVIII results became discrepant, with results of the CFVIII being double that seen in the one stage assay. The Bethesda assay was positive on one occasion. However, by Anti‐FVIII ELISA, one sample was positive and one borderline. By day 33, increments hadn’t improved and the patient was immune‐tolerated with 500 IU rFVIII BD. The inhibitor was eradicated as determined by both methods and factor assays were in agreement.
Conclusion(s): In severe Haemophilia A, if suspected, timely identification of an inhibitor is essential. In this case, the Anti‐FVIII ELISA was more sensitive to the low‐level inhibitor and better reflected the clinical picture of the patient. The factor assay discrepancy also appeared to correlate with clinical progression and resolved following immune‐tolerance. Parallel testing of FVIII is not routine practice so it is unknown whether this is a common finding but further investigation is merited. Possible mechanisms may be the prolonged incubation in the chromogenic assay, different dilutions, as well as the supraphysiological factor concentrations present in chromogenic reagents (Marlar 2020).

PB1148
Real‐world usage of extended half‐life FIX in a Portuguese Haemophilia Centre—Prophylaxis must be for moderate and severe hemophilia B patients
C. Peixoto 1; F. Rodrigues2; A. Pereira2; S. Campaniço1; C. Correia1; P. Afonso1; J. Pestana3; L. Moura4; L. Parusnikova1; C. Catarino5
1 Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisboa, Lisboa, Portugal; 2 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisboa, Lisboa, Portugal; 3 Hospital do Espirito Santo ‐ Évora, Évora, Evora, Portugal; 4 Centro Hospitalar Tondela ‐ Viseu, Hospital São Teotónio, Lisboa, Lisboa, Portugal; 5 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisbon, Lisboa, Portugal
Background: Extended half‐life factor IX (EHL‐FIX) products promise less frequent dosing while improving protection, compliance and outcomes. All our moderate and severe hemophilia B (HB) patients, were offered prophylaxis with recombinant FIX Fc fusion (rFIXFc).
Aims: To compare prophylaxis regimens and outcomes before and after switching from a standard recombinant half‐life FIX product (SHL) to EHL rFIXFc.
Methods: Data on patients’ characteristics, previous and actual regimen, pharmacokinetics (PK), annual bleeding rate (ABR) and factor consumption (FC) were reviewed.
Results: Ten patients (9adults and 1child; aged 8–69 years) with HB (5 severe, 5 moderate), are now on prophylaxis with rFIX‐Fc. Five severe HB patients were already on prophylaxis (4 on a twice and 1 on a weekly regimen). Five moderate patients, previously on demand (OD) started prophylaxis with rFIX‐Fc. These patients have been treated with rFIX between 1 to 21 months. After evaluation of PK, 9/10 patients were put on a fortnight dosing regimen, aiming FIX levels >3–5 IU/dl. 3 patients were later switched to a weekly regimen as higher through levels and better protection were needed. A 10‐day regimen was considered safer for a previously untreated boy (PUP). Dosage per infusion (31–45 UI/kg) did not change in the patients previously on prophylaxis. In these patients a marked decrease in the number of annual infusions (61%) and FC (58,2%) was observed. Mean ABR decreased from 15 to 5, while traumatic and spontaneous joint ABR decreased from 6 to 1 and from 4 to 0, respectively. More patients had zero joint bleeds after starting prophylaxis with the EHL FIX product (50% vs. 10%). More important, in the last 12 months, no patient had a spontaneous joint bleed. No patient developed inhibitors to rFIXFc.
Conclusion(s): Prophylaxis with rFIX‐Fc allowed a significant reduction in the frequency of infusions and a lower bleeding rate but also an improved protection and QoL.

PB0667
Results of thromboelastography and thrombin generation assays for pediatric severe hemophilia A patients are highly variable and not predictive of clinical phenotypes
N. Mathews1; F. Pluthero2; M. Rand3; A. Stain2; M. Carcao4; V. Blanchette3; W. Kahr 3
1 Hospital for Sick Children, Hamilton, Ontario, Canada; 2 Hospital for Sick Children, Toronto, Ontario, Canada; 3 Hospital for Sick Children/University of Toronto, Toronto, Ontario, Canada; 4 Division of Hematology/Oncology, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada
Background: Severe hemophilia A (SHA) patients vary in clinical traits including severity of bleeding, arthropathy and requirements for replacement factor VIII (FVIII). Hemostasis can be assessed in vitro via thromboelastography (TEG) using blood, and calibrated automated thrombography (CAT) using platelet‐rich (PRP) or platelet‐poor (PPP) plasma.
Aims: Use CAT and TEG to assess baseline hemostatic activity in a cohort of thirty pediatric SHA patients with factor requirements varying from on demand to 3 doses/week, for whom clinical data were available, and examine: 1) variability within the cohort of assay results using different tissue factor concentrations; 2) correlations among assay results and between them and clinical parameters.
Methods: Key clinical parameter was FVIII dosing regimen; others included Von Willebrand factor level (VWF:Ag) and Pettersson arthropathy score. Blood samples were obtained after factor washout (treated with corn trypsin inhibitor), subjected to TEG and used to prepare PRP and PPP for CAT assays. Varying concentrations of tissue factor (TF) were used. Statistical analysis examined relationships between assay results, and between them and clinical parameters.
Results: TEG data and CAT results with PPP and PRP all indicated weak hemostatic function in samples at low TF levels, and many but not all samples approached normal assay results at high TF concentrations (Figure 1). A significant positive correlation was observed between TEG and CAT‐PRP results. Correlations were not detected between assay results and clinical parameters (Figure 2).
Conclusion(s): In vitro hemostatic assay results indicated considerable heterogeneity among patients at all TF concentrations used. Correlation was observed among some assay results, but not between those results and FVIII requirements or other clinical parameters. We conclude that patient heterogeneity is likely influenced by factors other than baseline hemostatic activity.


PB0668
Inhibitor development is associated with decreased quality of life in patients with hemophilia
M. Karimi 1; S. Haghpanah2; M. Naderi3; S. Kamalian Fard2; H. Tavoosi4; S. Parand2; N. Javanmardi2
1 Hematology Research Center Shiraz University of Medical Sciences, Shiraz, Iran, Shiraz, Fars, Iran; 2 Shiraz University of Medical Sciences, Shiraz, Fars, Iran; 3 Zahedan University of Medical Sciences, Zahedan, Sistan va Baluchestan, Iran; 4 Shiraz University of Medical Sciences, Shiraz, Fars, Iran
Background: Inhibitor development leads to a higher rate of disease related complications in patients with hemophilia.
Aims: To assess the Health‐related quality of life (HRQoL) in patients with hemophilia with and without inhibitor.
Methods: In this multi‐center cross‐sectional study, 41 male patients with hemophilia were investigated between May and October 2021 in Iran. HRQoL were evaluated by the Short Form‐36 (SF36) questionnaire. The relationship of inhibitor and disease severity with Physical Health (PH) and Mental Health (MH) dimensions and the total SF36 was assessed by appropriate tests using SPSS software.
Results: The mean age ± SD of the patients was 36.9 ± 13.2 (range: 18–76) years. Approximately half of the patients (51.2%) had severe hemophilia (<1% factor activity) and 26.8% were inhibitor positive. Nine patients (22%) had at least one type of blood‐borne infections. Patients with severe hemophilia showed significantly lower scores in Physical Function (PF) (p < 0.001), PH dimension (p = 0.018), and total SF36 (p = 0.031) than patients with mild and moderate hemophilia. Moreover, PF, MH dimension, and total SF36 scores were significantly lower in the inhibitor‐positive compared to the none‐inhibitor groups (p < 0.001, p = 0.045, and p = 0.035 respectively) (Table 1). Patients who were complicated by blood‐borne infections showed a significantly lower median score in PH dimension than the patients without blood‐borne infection (p = 0.038). In addition, annual bleeding rate showed significant negative correlation with PH dimension (rs = −0.609, p < 0.001), MH dimension (r = −0.317, p = 0.044) and total SF36 (r = −0.455, p = 0.003) scores.
Conclusion(s): Reduced HRQoL was observed in hemophilia patients with inhibitor, severe disease and patients with blood‐born infections that warrants further attention to these subgroups of patients. Moreover, precise attention should be considered in the prevention and management of bleeding symptoms. On the other hand, improving the social environment of these patients by providing appropriate employment status can improve Physical and Mental health status in these patients.

VPB1164
Outcome of emicizumab in management of Egyptian children and adolescents with Hemophilia A with inhibitors: A 2 year single center prospective study
M. Abdelwahab 1; M. Elghamrawy2; H. Seifeldeen2; N. Fathi3
1 Cairo University Pediatric Hospital, Social and Preventive medicine center, Kasr Alainy hospital, Cairo, Al Qahirah, Egypt; 2 Cairo University Pediatric Hospital, Cairo, Al Qahirah, Egypt; 3 National Insurance Hospital, Cairo, Al Qahirah, Egypt
Background: Emicizumab (ACE900) has been recently extensively approved for the prophylaxis of HA patients with FVIII‐inhibitors of all ages. Though few data(Phase 3 clinical trials and case series) are currently available in children they showed significantly reduced bleeding rates compared to previous treatments and had beneficial effects on health‐related quality of life, However real‐world patient studies are required to evaluate the long‐term outcome of Emicizumab prophylaxis especially in HA children with inhibitors.
Aims: So our study describes real‐world experience of Egyptian HA children and adolescents( PwHAs) with high titre inhibitors started on biweekly subcutaneous Emicizumab 3 mg/kg, following a 4‐week induction
Methods: Outcome measures of Emicizumab in PwHAs included bleeding rate, ISTH ‐BAT and objective measures as joint physical examination (WFH musculoskeletal score) and joint imaging (HEAD‐ US and MRI Denver score of target joints) and HRQOL. Assessment was done initially and every 6 months thereafter over a 2 year period
Results: 14 severe HA male patients with a mean age of 9.2 years were enrolled .35.7% are of a consanguineous marriage and 9(64.2%) have a positive family history. Our enrolled cohort usually had 3 to 4 target joints. On baseline assessment the bleeding rate ranged from 6–8 events/year and the ISTH –BAT ranged from 6 to 29 and both dropped to zero bleeds after 6 months on biweekly prophylactic Emicizumab in 11 (78.6%) patients. Only 3 patients experienced at least one breakthrough bleed but none into target joints. The mean musculoskeletal score was 17 on initial assessment and dropped to 4 on treatment with Emicizumab. HRQOL is a patient‐reported outcome that showed significant improvement. Radiological changes on HEAD US and Denver MRI scores were stationary
Conclusion(s): Long term outcome of Emicizumab in our described cohort of PwHAs with inhibitors showed significant improvements in bleeding rate, joint status and HRQOL.
PB0686
Real‐world use of recombinant factor IX albumin fusion protein (rIX‐FP) in patients with hemophilia B: A prospective, multicenter, non‐interventional post‐marketing surveillance study in France
B. Pan‐Petesch1; M. Fouassier2; B. Guillet3; A. Harroche4; R. d'Oiron5; Y. Dargaud6; S. Susen 7
1 CHU Hôpital Morvan, CRC‐MHC, Brest, France, Brest, Bretagne, France; 2 Haemostasis Consultations, CRC‐MHC, CHU Hôtel‐Dieu, Nantes, France, Nantes, Pays de la Loire, France; 3 CRC‐MHC, CHU de Rennes – Hôpital Pontchaillou, Rennes, France, Rennes, Bretagne, France; 4 CRC‐MHC Hôpital Necker‐Enfants malades, Paris, France, Paris, Ile‐de‐France, France; 5 CRC‐MHC, Hôpital de Bicetre AP‐HP, Le Kremlin Bicetre, France, Le Kremlin Bicêtre, Ile‐de‐France, France; 6 CRC‐MHC, Hôpital Edouard Herriot Pavillon, Lyon, France, Lyon, Rhone‐Alpes, France; 7 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France
Background: rIX‐FP is a long‐acting fusion protein genetically linking recombinant human coagulation factor IX (FIX) with recombinant human albumin. In clinical trials, rIX‐FP provided low annualized bleeding rates with 7‐, 14‐ and 21‐day prophylaxis and robust efficacy in bleed treatment and surgery. Long‐term data is needed on the effectiveness and safety of rIX‐FP in routine clinical practice.
Aims: This post‐marketing surveillance study (OrPHEe; Clinicaltrials.gov identifier: NCT05086575) aims to collect real‐world effectiveness and safety data on the use of rIX‐FP in real‐world clinical practice in France.
Methods: The study is being conducted across up to 25 specialist hemophilia centers in France. The study cohort includes patients with hemophilia B, regardless of severity, who are currently or previously receiving rIX‐FP for long‐term prophylaxis, for short‐term prophylaxis to cover periods with a high risk of bleeding, for on‐demand treatment, or for treatment during surgery. Patients (N = 100) satisfying the inclusion criteria will be enrolled for a period of 2 years and each patient will be followed up for 3 years. Data on safety, efficacy and health‐related quality of life will be gathered, in addition to disease and treatment (in the year prior to rIX‐FP initiation) history.
Results: Recruitment to the study began in December 2021. Study methodology will be described in detail, and the interim data analysis will be provided by the time of congress presentation.
Conclusion(s): This prospective, non‐interventional, multicenter study will provide data on the real‐world use of rIX‐FP in France to confirm the favorable efficacy and safety of rIX‐FP seen in clinical development studies.
PB1136
An observational study to evaluate the impact and disease burden in moderate and severe hemophilia from nursing and patient perspective (Study Nurses‐Who‐Care/ML42270)
S. García Barcenilla1; E. Álvarez‐Martínez2; F. Jaime 3; Y. Echarte‐Buil4; B. Gago‐Caballero5; m. Coca6; I. Guerra6; A. Torres‐Ortuño7
1 Hospital Universitario La Paz‐IdiPAZ, Madrid, Madrid, Spain; 2 Hospital Universitario Vall d` Hebron, Barcelona, Catalonia, Spain; 3 Hospital Universitario Regional de Málaga, Málaga, Andalucia, Spain; 4 Hospital Universitario Miguel Servet, Zaragoza, Aragon, Spain; 5 Hospital Universitario Regional de Málaga, Malaga, Andalucia, Spain; 6 Roche Farma, Madrid, Madrid, Spain; 7 Universidad de Murcia, Murcia, Murcia, Spain
Background: The quality of life in persons with hemophilia A (PwHA) could be directly influenced by the hemophilia nurse care
Aims: To evaluate the preoccupation and disease burden in moderate and severe PwHA and the impact of the nurse care
Methods: Nurses‐Who‐Care (ML42270) is a two‐phase non‐interventional study: 1) ad hoc questionnaire was completed by nurses to describe their role concerning the work they perform with PwHA; 2) the preoccupation and disease burden in PWHA and the impact of the nurse care will be evaluated. Study design in Figure 1. Patients signed the informed consent and were asked to complete the CATCH questionnaire and a study specific questionnaire, during one routine visit with their clinical nurse (principal investigator). Patients ≥12 year old, moderate and severe PwHA were included. This Study was carried out according to the ERB and current legislation in Spain
Results: Study is in progress, 15 Hospitals across Spain have been included to obtain a representative sample of the Spanish population. Preliminary data phase 1 have showed the crucial role of nurses in PWHA throughout the disease. (Table 1). It has been shown that 70% nurses spend most of their time in coagulopathies. Also, the 100% of nurses promote and participate in the learning of patients mainly by taking part in the disease journey, teaching techniques related home treatment, encouraging adherence and compliance, and positively influencing their perception of the disease and motivating the achievement of objectives
Conclusion(s): This first study highlights the key role of nurses providing relevant information related to self‐management of PwHA and their contribution to improving clinical practice and patient care of Haemophilia in Spain. Data from the next phase will allow knowing the burden of the disease of PwHA and the influence of the work of nurses

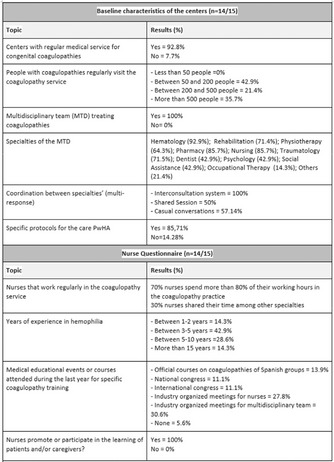
PB1151
Mild bleeding phenotypes in severe hemophilia A with transfusion dependent alpha‐thalassemia
P. Pongphitcha 1; W. Sasanakul2; P. Kadegasem2; D. Songdej3; A. Chuansumrit4; N. Sirachainan5
1 Faculty of Medicine Ramathibodi Hospital, Mahidol University, Rachatewi, Krung Thep, Thailand; 2 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Rachatewi, Krung Thep, Thailand; 3 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Krung Thep, Thailand; 4 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Nakhon Pathom, Thailand; 5 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Background: Approximately 10–15% of patients with severe hemophilia A have paradoxically mild bleeding phenotypes. Prothrombotic gene mutations were found in 50% of those patients. Moreover, thalassemia disease is known to have a lifelong hypercoagulable state, but bleeding phenotypes in severe hemophilia A with thalassemia disease has never been reported.
Aims: To demonstrate bleeding phenotypes in severe hemophilia A with alpha‐thalassemia disease.
Methods: Case description of severe hemophilia A with alpha‐thalassemia disease. DNA was sent for whole exome sequencing (WES). Then, the sequencing data was analyzed via the commercial software Sophia DDM v5.10.12 (Sophia Genetics™) and focused on genes based on the Human Phenotype Ontology HP:0001892 (abnormal bleeding) and HP:0001977 (abnormal thrombosis).
Results: A 6‐year‐old boy with alpha‐thalassemia disease ((‐‐SEA/αCSα), hemoglobin H with Constant Spring) receiving regular transfusion every 4 weeks since the age of 3 years. He first presented to the clinic with easy bruising after trauma. There was no history of joint or muscle bleeding. The Thai Version of the Pediatric Bleeding Assessment Tool noted a positive score of 4 (N≤3). Physical examinations revealed thalassemic faces and hepatosplenomegaly without evidence of joint bleeding. Activated partial thromboplastin time was 126 seconds (N: 22–33), and factor VIII activity by one stage and chromogenic assay <1%. Additionally, ultrasound of both elbows, knees, and ankles showed no recent or remote joint bleeding features. Before transfusion and initiation of factor prophylaxis, thrombin generation using 5 pM of tissue factor demonstrated an endogenous thrombin potential of 1051.4 nM × min (N: 1393.7, (1175.1–1612.3)) which was 75% of normal. WES could not identify any additional variant, including prothrombotic genes. Therefore, the alpha‐thalassemia disease was the contributing factor to mild bleeding phenotypes in this patient. At present, the evaluation of the F8 inversion is being performed.
Conclusion(s): The hypercoagulable state caused by alpha‐thalassemia may affect bleeding phenotypes of severe hemophilla A.
PB1158
MOdern Treatment of Inhibitor‐PositiVe PATiEnts with Haemophilia A – MOTIVATE (www.motivate‐study.com)
R. Sidonio 1; C. Escuriola Ettingshausen2
1 Aflac Cancer and Blood Disorders Center, Emory University, Atlanta, Georgia, United States; 2 HZRM Hämophilie Zentrum Rhein Main, Mörfelden‐Walldorf, Hessen, Germany
Background: A number of treatment options are available for haemophilia A patients with inhibitors. While emicizumab and bypassing agents may control bleeding in patients with inhibitors, immune tolerance induction (ITI) is the only method proven to eradicate inhibitors.
Aims: The investigator‐initiated, international MOTIVATE study (NCT04023019; EudraCT 2019‐003427‐38) is investigating the management of haemophilia A patients with inhibitors in real‐life clinical practice. The objectives are to capture the different management approaches for haemophilia A patients with inhibitors, document current ITI approaches, and evaluate the efficacy and safety of ITI, emicizumab prophylaxis, and ITI with emicizumab prophylaxis.
Methods: Patients of any age with haemophilia A of any severity and inhibitors will be observed for up to 5 years. The treatment approaches evaluated are standard ITI with FVIII, FVIII ITI with emicizumab prophylaxis and prophylaxis with emicizumab or bypassing agents (Figure 1). Participants are not randomised, and treatment decisions are at the discretion of the investigators. Primary endpoints are ITI success and annualised bleeding rates. Optional sub‐studies will explore factors that influence ITI outcome and the impact of different treatment approaches on patient health, including joint and bone health and the risk of thrombotic events.
Results: Target enrolment is 120 patients. As of December 2021, 29 patients have been recruited at 13 study sites in Croatia, France, Germany, Norway, Spain, Sweden, Switzerland, UK and the US. A total of 48 sites have been selected in Canada, Colombia, Croatia, Finland, France, Germany, Guatemala, Italy, Norway, Spain, Sweden, Switzerland, UK, Ukraine and the US. Data on enrolment by country and baseline characteristics as well as demographics of enrolled patients will be presented.
Conclusion(s): The MOTIVATE study is collecting real‐world data on current approaches for management of patients with haemophilia A and inhibitors with the goal of informing decision making for the optimal management of such patients.

PB0669
The interobserver and intraobserver agreements on HEAD‐US in the assessment of hemophilic arthropathy
E. Yılmaz1; Z. Kaya 1; H. Satış2; İ. Akdulum3; M. Yazol3; A. Tufan4; Ş. Haznedaroğlu2; O. Konuş Boyunaga3; S. Kirkiz1; Ü. Koçak1
1 Gazi University Faculty of Medicine, Department of Pediatric Hematology, Ankara, Ankara, Turkey; 2 Gazi University Faculty of Medicine, Department of Rheumatology, Ankara, Ankara, Turkey; 3 Gazi University Faculty of Medicine, Department of Radiology, Ankara, Ankara, Turkey; 4 Gazi University Faculty of Medicine, Department of Rheumatology, ANKARA, Ankara, Turkey
Background: Few data are available in the evaluation of hemophilic arthropathy with HEAD‐US (hemophilia early arthropathy detection with ultrasound) by different caregivers.
Aims: The purpose of this study was to evaluate the interobserver and intraobserver agreements in the assessment of hemophilic arthropathy on HEAD‐US by different caregivers.
Methods: A total of 28 patients with severe hemophilia A and B were examined 6 months intervals at two different time points (T1 and T2). The bleeding episodes and Hemophilia Joint Health Score (HJHS) were recorded in all patients. The interobserver and intraobserver agreements on HEAD‐US were assessed in six joints (elbow, knee, and ankle) of each hemophilic patient by different caregivers (radiologist and rheumatologist). Kappa (Kw) statistics (Kw >0.8‐excellent, 0.61–0.80‐good, 0.41–0.60‐moderate, 0.21–0.40‐fair and <0.2 poor agreements) were used in SPSS 15.0 program.
Results: The number of joint bleedings in the right knee and ankle joints was significantly higher at the T2 than at the T1 (p < 0.05). The score of HJHS in the right ankle joint was significantly higher at T1 than at T2 (p < 0.05). Intraobserver agreement of radiologists was ranged from moderate (Kw = 0.46) to good (Kw = 0.75) for scoring synovitis, cartilage, and bone damages in 14 (78%) of 18 joints. Intraobserver agreement of rheumatologists was ranged from moderate (Kw = 0.43) to excellent (Kw = 0.84) for scoring synovitis, cartilage, and bone damages in 13 (72%) of 18 joints. Interobserver agreement between radiologists and rheumatologists at T1 was ranged from moderate (Kw = 0.42) to good (Kw = 0.78) for scoring synovitis, cartilage, and bone damages in 10 (56%) of 18 joints. Interobserver agreement between radiologists and rheumatologists at T2 was ranged from fair (Kw = 0.38) to good (Kw = 0.73) for scoring synovitis, cartilage, and bone damages in 10 (56%) of 18 joints.
Conclusion(s): Our study indicates that intraobserver agreement was better than interobserver agreement in the assessment of hemophilic arthropathy by HEAD‐US.
PB0673
A post‐authorization safety surveillance study to report clinical experience with purified FIX concentrate in pediatric patients with hemophilia B
Z. Igrutinović1; L. Hooimeijer2; K. Kentouche3; J. Botha4; P. Turecek5; M. Kokot‐Kierepa 6; H. Gazda7
1 Clinic of Pediatrics, Department of Hemato‐oncology, University Clinical Center of Kragujevac; Faculty of Medical Sciences, Department of Pediatrics, University of Kragujevac, Kragujevac, Kosovo, Serbia; 2 Pediatric Hematology, Beatrix Children’s Hospital, University Medical Center Groningen, Groningen, Groningen, Netherlands; 3 Klinik für Kinder‐ und Jugendmedizin, Universitätsklinikum Jena, Jena, Thuringen, Germany; 4 Takeda Pharmaceuticals International AG, Zurich, Zurich, Switzerland; 5 Baxalta Innovations GmbH, a Takeda company, Vienna, Wien, Austria; 6 Takeda Pharmaceuticals International AG, Glattpark, Zurich, Switzerland; 7 Takeda Pharmaceutical Company Limited, Lexington, Massachusetts, United States
Background: The safety and efficacy of purified factor IX (FIX) concentrate (Baxter Innovations GmbH, Vienna, Austria) have been demonstrated in clinical trials in adult and pediatric patients with hemophilia B (HB).
Aims: To document clinical experience with purified FIX concentrate in routine practice for pediatric patients with HB.
Methods: This was a prospective, uncontrolled, open‐label, post‐authorization safety surveillance study. Patients aged ≤6 years with moderate or severe HB (baseline FIX ≤5%) who were prescribed purified FIX concentrate were eligible for inclusion. The observation period was either 12 months or ≥50 exposure days, whichever occurred first. The treatment regimen was determined by the treating physician. The primary endpoint was the occurrence of treatment‐related adverse events (AEs) and serious AEs (SAEs), and inhibitor development. Secondary endpoints included hemostatic efficacy and number of infusions required to achieve bleed resolution.
Results: In total, 13 patients enrolled and received ≥1 treatment with purified FIX concentrate. All patients were male and mean (SD) age at baseline was 3.80 (1.76) years. During the study, 11 patients received prophylaxis for mean (SD) 73.6 (37.0) days and 6 received on‐demand treatment for mean (SD) 3.7 (1.0) days. Two patients received prophylaxis for surgery. In total, 28 non‐serious AEs in 6 patients and 4 SAEs in 3 patients were reported (Table 1). No AEs were considered related to study treatment. No patients (n = 10) developed inhibitory antibodies. Inhibitor testing was not done for 3 patients. Overall hemostatic efficacy ratings and annualized bleeding rates are shown in Table 2. In total, 18 bleeding episodes reported in 6 patients were treated with purified FIX concentrate. Mean (SD) number of infusions per bleeding episode was 1.18 (0.57).
Conclusion(s): Purified FIX concentrate was not associated with any treatment‐related AEs and was shown to be effective in treating and preventing bleeding episodes in pediatric patients aged ≤6 years with HB.


VPB0681
Adherence to prophylaxis and bleeding outcome in haemophilia: A multicentre Nigerian study
T. Nwagha 1; H. Okoye2; S. Yuguda3; C. Udo4; M. Ogunfemi5; D. Gwarzo6; O. Nnamdi Joel4
1 College of Medicine University of Nigeria/University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria; 2 College of Medicine University of Nigeria Ituku Ozalla, Enugu, Enugu, Nigeria; 3 Federal Teaching Hospital Gombe/Gombe State University, Gombe, Gombe, Nigeria; 4 National Hospital, Abuja, Nigeria., Abuja, Federal Capital Territory, Nigeria; 5 Department of Haematology and Blood Transfusion, University of Ilorin, Kwara State, Nigeria, Ilorin, Kwara, Nigeria; 6 Department of Haematology and Blood Transfusion, Aminu Kano Teaching Hospital, Kano, Nigeria, Kano, Kano, Nigeria
Background: Low‐dose prophylaxis is the standard of care as it reduces bleeding, development of target joints, arthropathy, and improvement of quality of life. Non‐adherence or poor adherence can prevent the achievement of these outcomes. The levels and determinants of (non‐)adherence among people with haemophilia (PWH) in Sub Saharan Africa have not been evidenced.
Aims: To evaluate self‐reported adherence among PWH, provide evidence of determinants/predictors of adherence, and establish the associations between non‐adherence and presence of target joints and annualized bleed rate (ABR).
Methods: A cross‐sectional survey of 42 participants on low‐dose prophylaxis recruited from five haemophilia treatment centers in Nigeria using validated Haemophilia Regimen Treatment Adherence Scale ‐ Prophylaxis (VERITAS‐Pro). Information on the presence of target joints, the number of target joints, and ABR were collected from medical files.
Results: The mean age of the participants was 9.79 (6.29) years, with 96.6% having hemophilia A and 79.3% having target joints. Overall adherence to the prophylaxis regimen was 81.0%. The mean total VERITAS‐Pro for the adherent group and the non‐adherent group was 37.35 ± 9.08 and 63.0 ± 6.37, respectively. The mean subscale scores for the adherent group ranged from 0.67 (communication) to 8.68 (planning), while for the non‐adherent group the mean subscale scores range from 1.0 communication to 13.88 (planning). The mean difference of all except the dosing subscale was statistically significant with p < 0.05. Only the skipping subscale showed a statistically significant positive correlation with ABR in the non‐adherent group p = 0.02.
Conclusion(s): The findings indicate that adherence was very good, and most were in communication with their treatment centers. The skipping sub‐scale was significantly associated with ABR for the non‐adherent group. Interventions aimed at improving adherence are the key to better treatment outcomes. A multicenter study is needed to assess the reason for poor adherence. We acknowledge Natalie Duncan for Indiana Haemophilia and Thrombosis Center for sharing VERITAS‐PRO.
PB1123
Annualised bleeding rate and joint health: Second interim analysis of the real‐world HEM‐POWR study evaluating the effectiveness of damoctocog alfa pegol in patients with haemophilia A
M. Reding1; M. Alvarez‐Román 2; M. Sanabria3; G. Castaman4; M. Janbain5; T. Matsushita6; K. Meijer7; K. Schmidt8; J. Oldenburg9
1 Center for Bleeding and Clotting Disorders, University of Minnesota, Minneapolis, Minnesota, United States; 2 Hospital Universitario La Paz, Madrid, Madrid, Spain; 3 Bayer, Basel, Basel‐Stadt, Switzerland; 4 Careggi University Hospital, Florence, Toscana, Italy; 5 Tulane University, New Orleans, Louisiana, United States; 6 Department of Transfusion Medicine, Nagoya University Hospital, Nagoya, Aichi, Japan; 7 University Medical Center Groningen, Groningen, Groningen, Netherlands; 8 Bayer, Berlin, Berlin, Germany; 9 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Damoctocog alfa pegol is an extended half‐life PEGylated recombinant Factor VIII product for previously treated patients (PTPs) aged ≥12 years with haemophilia A. Efficacy and safety of damoctocog alfa pegol was demonstrated in the pivotal Phase 3 PROTECT VIII trials and their extensions.
Aims: HEM‐POWR (NCT03932201) is an ongoing, multicentre Phase IV study to demonstrate the real‐world effectiveness and safety of damoctocog alfa pegol in PTPs aged ≥12 years with haemophilia A. Second interim effectiveness analyses are presented.
Methods: PTPs aged ≥12 years with haemophilia A receiving damoctocog alfa pegol with any kind of treatment modality (prophylaxis, intermittent prophylaxis or on‐demand) are eligible. Primary endpoints are total bleeding events and annualised bleeding rate (ABR). Data were recorded electronically and patients provided informed consent. Ethical approval was obtained for all sites.
Results: At data cut‐off (31 August 2021), 162 patients were enrolled, with 78 patients included in the full analysis set (FAS; 3 mild, 9 moderate, 66 severe disease). In the FAS, mean (standard deviation [SD]) age at enrolment was 35.4 (13.6) years; 71/78 (91.0%) received prophylaxis before enrolment. The median (mean, SD) difference in ABR between the observation period and prior to damoctocog alfa pegol initiation was −0.11 (−1.01, 9.94). Additional data are summarised in Table 1. Joint health was assessed by the number of pre‐treatment joint bleeds compared with the number of bleeds at the first follow‐up. The number of patients with any affected joint decreased from 37/62 (59.7%) to 8/56 (14.3%). Additional data are presented in Figure 1.
Conclusion(s): These second interim data support the conclusions from the PROTECT clinical trials, demonstrating the effectiveness of damoctocog alfa pegol for reducing ABR and improving joint health in patients with mild, moderate and severe haemophilia A. This study was funded by Bayer.


PB1128
Challenges in the management of gynecological bleedings and pregnancies in a woman with severe haemophilia A
E. Cruz 1; M. Coutinho2; F. Leite2; L. Moreira2; N. Pinho2; N. Seidi2; S. Morais3
1 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 2 Thrombosis and Haemostasis Unit, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 3 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto, Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal
Background: Severe hemophilia A is a serious condition and is even more critical in women. Besides common haemorrhagic symptoms (hemarthrosis and/or hematomas), women may have increased risk of developing gynaecological bleedings. Also challenging is the management of gestation and delivery.
Aims: Here we describe the clinical case of a severe haemophilia A female.
Methods: Sporadic severe haemophilia A female, homozygous for the F8 intron 22 inversion resulting from a maternally inherited distal inversion and a paternally inherited de novo, also distal inversion. Furthermore, the maternally inherited F8 was transcriptionally inactive.
Results: Since the diagnosis, she had several bleeding episodes, including hemarthrosis (knees and elbows) and muscle hematomas. She started regular prophylaxis at age of 11 before menarche to prevent gynecological bleedings. Besides symptoms of severe haemophilia, she also had life‐threatening gynaecological bleeding (ovarian hemorrhagic cysts that resulted in right oophorectomy and annexectomy). When she decided to be a mother, she was oriented to preimplantation diagnosis with gender selection and transfer of female embryos. After two unsuccessful procedures, she decided accepting the risk of giving birth to an affected child. She had two pregnancies without bleeding complications, under 3x/week prophylaxis in the first, and 2x/week in the second pregnancy, and was submitted to two caesareans. Pharmacokinetic (PK) studies performed at 28‐years‐old revealed a FVIII half‐life of 15.1 h and FVIII in‐vivo recovery of 3.2 (IU/dl per IU/kg). During first pregnancy, PK studies, at 12, 31 and 35 weeks of gestation, revealed FVIII half‐lives of 17.8, 19.9 and 17.7 h and FVIII in‐vivo recovery of 2.36, 3.58 and 3.26 (IU/dl per IU/kg), respectively.
Conclusion(s): This report underlines the difficulties in management of gynecological bleedings in women with severe haemorrhagic disease and the management of successful pregnancies and deliveries with a multidisciplinary approach. Additionally it shows an increase of FVIII half‐life, during pregnancy.
PB1129
Experience in rural areas in the evaluation of the joint health status of patients with non‐severe hemophilia A not treated with prophylaxis
B. Diaz Jordan; J. Herrero Reyes
Hospital General de Valdepenas, Valdepenas, Castilla‐La Mancha, Spain
Background: Although clinical and/or radiological joint involvement is clearly documented in moderate or severe forms of hemophilia, its hidden presentation makes its presence underestimated in mild forms.
Aims: The objective is to document the presence of clinical and/or radiological alterations in mild hemophiliac patients without prophylactic treatment.
Methods: Prospective study that analyzed 5 patients diagnosed with mild hemophilia A (FVIIIc: 10‐40%) and asymptomatic from the joint clinical point of view and without the need for prophylactic treatment. The radiological assessment was carried out by ultrasound using the HEAD‐US scale.
Results: The median age was 11 years (range: 5–32 years) and FVIIIc levels of 23% (range: 20%–40%) without previous exposure to treatment and with physical activity according to age (even 40% patients performed physical activity with high joint impact, agriculture for example). In this same population, joint damage was evidenced through the HEAD‐US scale (minimal synovitis of the left ankle in the first patient and in the second patient there was established bilateral knee synovitis + osteochondral involvement <50% in the right knee). This same population reports presenting more than one episode of joint pain per year (sometimes disabling).
Conclusion(s): 40% of the sample, presented radiological alterations compatible with hemophilic arthropathy, so it is essential to jointly evaluate mild hemophiliac patients (especially those who perform physical activity with great joint impact) to optimize their therapeutic management, regardless of the quantification of FVIIIc levels.
PB1143
The Thrombin Generation Test represents a better tool than Factor VIII levels to assess the risk of bleedings in patients with hemophilia A
A. Bernardo1; I. Soto2; L. Gutiérrez3; A. Caro2; D. Martínez‐Carballeira2; J. Corte Buelga4; S. Vázquez2; C. Palomo‐Antequera5; A. Andreu6; A. Fernández‐Pardo7; J. Oto8; P. Medina 7
1 HUCA, oviedo, Asturias, Spain; 2 Department of Hematology, Central University Hospital of Asturias, Oviedo, Asturias, Spain; 3 University of Oviedo ‐ ISPA, Oviedo, Asturias, Spain; 4 HOSPITAL UNIVERSITARIO CENTRAL DE ASTURIAS, Oviedo, Asturias, Spain; 5 Department of Internal Medicine, Central University Hospital of Asturias, Oviedo, Asturias, Spain; 6 Department of Pharmacology, University of Navarra, Pamplona, Navarra, Spain; 7 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 8 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain
Background: Hemophilia A (HA) is a rare bleeding disorder caused by factor VIII (FVIII) deficiency due to mutations in the F8 gene. Disease severity inversely correlates with plasma FVIII (FVIII:C) concentration. Unaccountably, a subgroup of severe HA patients scarcely bleed and do not require prophylactic FVIII administration, while moderate HA patients bleed frequently, despite their FVIII:C levels.
Aims: To evaluate the capacity of the ST Genesia® Thrombin Generation System (TGT) to ascertain the hemostatic status of the entire cohort of HA outpatients, treated and non‐ bleeding, in Asturias (Spain) and to compare it with FVIII performance.
Methods: We recruited 55 HA patients (18 severe, 7 moderate and 30 mild; FVIII:C< 40%) following treatment and without inhibitors, and 25 healthy controls. HA patients were monitored assessing FVIII activity (and vWF levels) at diagnosis and follow‐up, and screened for F8 mutations. The TGT BleedScreen was evaluated during follow‐up.
Results: Patients with severe/moderate HA had lower FVIII:C at diagnosis and a higher annual bleeding rate (ABR) than mild HA patients (p < 0.001) (Table 1). Importantly, on the day of the TGT and despite having lower FVIII:C, severe/moderate patients had a similar thrombin potential than mild patients, reflecting better the non‐bleeding phase of our patient cohort (Figure 1). Additionally, a correlation of the F8 mutation type was observed with HA severity, ABR and hemostatic status.
Conclusion(s): The hemostatic status of HA patients measured with ST Genesia® correlates more precisely with their bleeding risk than classical FVIII:C. The F8 mutation type may indicate a worse prognosis, higher ABR and worse hemostatic status. The TGT may represent a more suitable tool than classical FVIII:C determination to identify HA patients that may require a closer follow‐up and a tailored therapeutic adjustment in high‐risk situations. MINECO (RYC‐2013‐12587), MICIU (SAF2017‐85489‐P), Roche (SP200221001), GVA (ACIF/2017/138) and Sociedad Española de Trombosis y Hemostasia.


PB1145
Real World Experience of perioperative haemostasis with recombinant factor VIII Fc fusion protein (rFVIIIFc) in people with haemophilia A (PWHA)
M. Murray 1; S. Bocchinfuso2; M. O'Donovan3; E. Singleton4; J. Benson3; N. Larkin5; M. Byrne6; M. McGowan3; S. Roche3; K. Ryan4; N. O'Connell7
1 St. James's Hospital, Dublin, Ireland, Dublin 16, Dublin, Ireland; 2 Trinity College Dublin, The University of Dublin, Dublin, Dublin, Ireland; 3 St James's Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 4 National Coagulation Centre, St James's Hospital,Dublin8, Dublin, Dublin, Ireland; 5 St James's Hospital,Dublin8, Dublin, Dublin, Ireland; 6 National Coagulation Centre, St James’s Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 7 St. James Hospital, Dublin, Ireland
Background: In Ireland, since 2018, recombinant factor VIII Fc fusion protein (rFVIIIFc) is used exclusively for perioperative haemostasis in people with haemophilia A (PWHA).
Aims: To evaluate real‐world surgical outcomes in PWHA (≥18 years) who received rFVIIIFc for surgical haemostasis.
Methods: Single centre, retrospective review of all PWHA who underwent major and minor surgical procedures between January 2018 and September 2021 and who received rFVIIIFc perioperatively for haemostasis.
Results: 24 major and 225 minor procedures were managed with rFVIIIFc in 105 PWHA (96 male and 9 female). Major procedures included 16 orthopaedic and 8 other procedures which were undertaken in people with severe (n = 7), moderate (n = 5) and mild (n = 9) haemophilia. The median pre‐operative dose was 46.5 IU/kg (range 21–58 IU/kg), resulting in a median pre‐operative FVIII:C level of 1.2 IU/ml (range 0.8–1.98 IU/ml). A second infusion on the day of surgery was given if the postoperative FVIII:C level was <0.9 IU/ml (8/24 procedures). Median number of perioperative infusions was 8 (range 3–22) and the total median rFVIIIFc consumption in severe, moderate and mild HA was 388 IU/kg (194–654 IU/kg), 250 IU/kg (165‐257 IU/kg) and 93 IU/kg (range 58–247 IU/kg), respectively. Haemostasis was rated as excellent in 23/24 major procedures and good in one total knee replacement due to excess postoperative blood loss, though not requiring red cell transfusion. 225 minor procedures were undertaken in people with severe (n = 38), moderate (n = 11) and mild (n = 42) haemophilia. These included 48 dental, 52 orthopaedic, 59 endoscopic, and 66 non‐categorised procedures. Most minor procedures required a single infusion of rFVIIIFc, with bleeding complications in <1% of minor procedures. A transient inhibitor (< 0.5 BU) was detected in one patient 4 weeks post procedure but was not present on repeat testing.
Conclusion(s): Perioperative surgical haemostasis with rFVIIIFc is effective and safe in real‐world conditions in PWHA.
VPB1168
New technologies such as virtual reality to increase adherence in hemophilia treatment
A. Santamaria 1; T. Lobez2; M. Sanchez3; S. García Barcenilla4; J. Farriol5; L. Pérez6; D. García6
1 University Hospital Vinalopo, Alicante, Comunidad Valenciana, Spain; 2 Nixi for Childrem, Barcelona, Catalonia, Spain; 3 Spanish Federation of Hemophilia (FEDHEMO), Madrid, Madrid, Spain; 4 Hospital Universitario La Paz‐IdiPAZ, Madrid, Madrid, Spain; 5 Nixi for Children, Barcelona, Catalonia, Spain; 6 Spanish Federation of Haemophilia, Madrid, Madrid, Spain
Background: Throughout the life of people with Hemophilia, there are critical moments such as self‐treatment, which involves intravenous administration of medication.
Aims: We have launched a pioneering project in the world of congenital coagulopathies using new technologies such as virtual reality as a training tool for children with congenital coagulopathies to improve the adherence and empowerment of children with those pathologies.
Methods: Since September 2020 until October 2021 we started this project to creat a virtual reality experience in self‐treatment including children, aged between 7 and 12 years old with hemophilia in collaboration with Nixi for Children’s, as VR designers, and a multidisciplinary focus group with hematologist, nurse, Fedhemo´s social workers and psychologist, and families with children with haemophilia at different ages. Objectives are to assess adherence, the empowerment of children and families with Haemophilia and quality of life
Results: The script was written by Nixi for Children’s team and reviewed by Fedhemo’s psychologists and families. Production of the VR experience was done mixing 360º real environments and 3D animation. Production of the VR experience was done mixing 360º real environments and 3D animation. Inside the VR experience, Nixi, a 3D animated character, interacts with “Nacho”, a 17 years‐old boy with Hemophilia. Nixi and the spectator will travel back in time to discover how "Nacho" administered himself the medication for the first time when he was 8 years old. In order to assess the effectiveness and psychological aspects, we will perform 3 questionnaires,
Conclusion(s): New technologies such as virtual reality can be an example of how it could improve empowerment in self‐treatment on that journey to autonomy in a chronic disease as well as redefine the role of the family in its accompaniment of the journey of people with hemophilia.


PB1155
Cost of factor concentrates in the Brazilian Protocol of Immune Tolerance Induction: preliminary results of the Co$tIT Study
S. Rezende 1; V. Franco2; J. Angela da Silva3; M. Moreira Dias4; A. De Oliveira5; A. Vilela De Oliveira Santos6; R. Ribeiro7; N. Martins Beserra8; C. Lorenzato9; J. Álvares‐Teodoro10; R. Camelo11
1 Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 2 Centro de Hematologia e Hemoterapia de Santa Catarina (HEMOSC), florianópolis, Santa Catarina, Brazil; 3 Centro de Hematologia e Hemoterapia de Santa Catarina ‐ HEMOSC, Florianópolis, Santa Catarina, Brazil; 4 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 5 Fundação HEMOMINAS, Belo Horizonte, Minas Gerais, Brazil; 6 Fundação HEMOMINAS, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 7 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaleza, Ceara, Brazil; 8 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaleza, Brazil, Fortaleza, Ceara, Brazil; 9 Hemocentro do Paraná (HEMEPAR), Curitiba, Parana, Brazil; 10 Federal University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 11 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil
Background: Immune tolerance induction (ITI) is the standard treatment for eradicating inhibitors in haemophilia A (HA), but is costly. The Brazilian Ministry of Health purchases factor concentrates (FC) in a centralized manner. Therefore, the prices tend to be one of the lowest in the world market of hemophilia. However, assessment of cost on expenditure of FC in ITI is still missing in Brazil.
Aims: To access the costs of FC used during ITI in patients with HA (PwHA) using a national ITI protocol.
Methods: Co$tIT is a subset of the BrazIT, a Brazilian Cohort Study aimed at evaluating the predictors of ITI response. The protocol was based on a low‐dose ITI regimen. For this study, we collected number of bleeding episodes, duration/outcome of ITI, and cost of FC use during ITI of PwHA from four hemophilia treatment centers in Brazil. We analysed the costs according to the outcome of ITI, which was defined as complete/partial success, and failure. All patients/guardians signed informed consent. The study was approved by ethical committees.
Results: We evaluated 62 severe (FVIII < 1%) PwHA on ITI. Total/partial success was achieved in 42 (68%), and 20 (32%) failed. Duration of ITI was longer (37 months vs. 24 months) and there were more bleeding episodes (16.5 vs. 4.5) in patients who failed compared with total/partial success, respectively. During ITI, the median total cost with FVIII (US$379,448.25 vs. US$138,180.20), activated prothrombin complex concentrate (US$191,420.25 vs. US$28,280.00) and recombinant activated factor VII (US$580,720. 25 vs. US$0.00) was higher in patients who failed compared with total/partial success, respectively.
Conclusion(s): ITI represents a substantial economic impact for the Brazilian Public Health System, especially when failure occurs. Predictors of response to ITI are awaited once could be used to focus on non‐responders PwHA and drive management to other therapeutic strategies.
VPB1166
Eradication of FIX inhibitor in haemophilia B children: A retrospective study from haemophilia pediatric comprehensive care center of China
Z. Li 1; G. Liu2; W. Yao3; Z. Chen1; M. Poon4; R. Wu5
1 Beijing Children's Hospital, Beijing, Beijing, China (People's Republic); 2 Beijing Children’s Hospital, Capital Medical University, Bejing, Beijing, China (People's Republic); 3 Beijing Children’s Hospital, Capital Medical University, Beijing, Beijing, China (People's Republic); 4 University of Calgary, Calgary, Alberta, Canada; 5 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic)
Background: Development of hemophilia B inhibitors (HBI) results in ineffectiveness of FIX replacement therapy. Inhibitor eradication by immune tolerance induction (ITI) is therefore necessary. In HBI, ITI even at high FIX dose is less effective and has a higher risk of severe complications.
Aims: To characterize the clinical features and outcome of ITI in HBI.
Methods: This is a single Chinese pediatric hemophilia center retrospective study. We used low‐dose ITI (25‐50 FIX IU/kg/three‐times‐weekly to every‐other‐day) with domestic prothrombin complex concentrate, combined with two successive immunosuppressive (IS) regimens.
Results: Sixteen HBI children, representing 5.7% of all and 14.4% of our severe registered HB patients, were enrolled. Seven cases reported allergic reactions proximal to inhibitor development. The historic peak inhibitor titer was median 54.2 (range 4.7–512) BU, and 15 (93.8%) had high‐titer inhibitors. Twelve patients adherent to ITI were analyzable. Of the nine ITI patients who received rituximab/prednisone (IS Regimen‐1), 4 achieved tolerization in 1.4‐43.3 months. Two subsequently relapsed but re‐tolerized after a second course of IS Regimen‐1. During ITI, the median treated bleed was 0.39/month (82.7% bleed reduction from before ITI), and the incidence of allergic reaction and nephrotic syndrome complications was each at 22% (2/9). Three ITI patients received modified “Beutel” protocol (IS Regimen‐2) using multiple‐IS‐drugs, and two had rapid tolerization (0.8 and 1.8 months).
Conclusion(s): Inhibitor eradication could be achieved by this low‐dose ITI protocol using PCC combined with IS. Larger studies are needed to confirm if ITI with the IS‐protocols is more effective with less complications.


1. PB0661
1.1. Emicizumab in haemophilia A with inhibitors and end‐stage renal disease
E. Funding 1; M. Rix2
1 Rigshospitalet, Copenhagen, Hovedstaden, Denmark; 2 Department of Nephrology, Rigshospitalet, Copenhagen, Hovedstaden, Denmark
Background: In severe haemophilia A with inhibitors, emicizumab has emerged as an effective prophylactic treatment for prevention of bleeding. Meanwhile, patients with renal failure were excluded from the clinical trials, and there are limited experience with emicizumab in chronic renal failure and haemodialysis. Furthermore, it has been speculated that renal failure with proteinuria could affect emicizumab plasma levels.
Aims: We report emicizumab plasma levels and clinical efficacy of continued emicizumab prophylaxis in a case of severe haemophilia A with inhibitors and progressive renal failure with substantial proteinuria due to AA‐amyloidosis, developing end‐stage renal disease necessitating chronic intermittent haemodialysis.
Methods: Emicizumab plasma levels (Haemochrom Diagnostica), urinary protein and urine output were measured regularly in the two years leading up to haemodialysis. Emicizumab plasma levels were measured before and after the dialysis procedure on four occassions. Bleeding episodes and treatment with by‐passing agents were recorded, as a measure of emicizumab efficacy.
Results: Emicizumab levels remained stable during progression of renal disease and haemodialysis, with mean level 70 mg/L (95% CI 64–76) versus 72 mg/L (95% CI 68–75) before and after initiation of dialysis. Marked proteinuria did not affect emicizumab levels in this case. Bleeding pattern did not change, and break through bleeds were succesfully treated with recombinant FVIIa in similiar doses as before haemodialysis.
Conclusion(s): Emicizumab in standard dosing remained stable and effective as prophylaxis during progressive renal failure and haemodialysis.
2. PB0665
2.1. A case of discrepant one‐stage and chromogenic factor VIII activity assay in a patient with a hemizygous Arg550His mutation in the F8 gene
J. Hancock 1; E. Li2; J. Larson1; N. Montanez2; M. Escobar3
1 The University of Texas Health Science Center at Houston, Houston, Texas, United States; 2 The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, Texas, United States; 3 University of Texas Health Science Center, Houston, Texas, United States
Background: Missense mutations in the F8 gene are known to cause discrepant one‐stage and chromogenic assays of Factor VIII (FVIII) activity. Lower FVIII activity (>2‐fold difference) when measured with the chromogenic substrate assay compared to the one‐stage assay has been described with mutations in the A1‐A2‐A3 domains of F8. These mutations are thought to disrupt FVIII heterodimer stability and FVIIIa heterotrimer stability.
Aims: We describe a clinical case of discrepant one‐stage and chromogenic substrate assay for factor VIII activity in a patient with mild Hemophilia A with a c.1649G>A(p.Arg550His) mutation in the F8 gene.
Methods: The electronic medical record was retrospectively reviewed.
Results: A 50‐year‐old man with prior history of hemarthrosis, recurrent epistaxis, prolonged bleeding with skin lacerations and prolonged bleeding after tooth extraction presented to our center for evaluation. He had a normal INR of 1.16 and mild prolongation in PTT of 43.8 that fully corrected on mixing study. He had a normal Thrombin Time. He had multiple prior one‐stage assays of FVIII activities that ranged from 57% to 71%. At the time of evaluation, his one‐stage FVIII assay was 57%. A chromogenic FVIII activity was checked and found to be 27%. The patient was given a diagnosis of mild Hemophilia A and genetic testing was ordered. He was found to have a hemizygous missense mutation for a c.1649G>A (p.Arg550His) in exon 11 of the F8 gene, consistent with mild Hemophilia A.
Conclusion(s): The c.1649G>A (p.Arg550His) mutation in F8 has been described to cause discrepant results between one‐stage and chromogenic substrate assays along with other missense mutations in the A1‐A2‐A3 domain interfaces, and chromogenic assays should be considered in patients with high clinical suspicion for Hemophilia A even with a normal one‐stage FVIII activity assay.
3. VPB0690
3.1. Dose optimisation in children with severe haemophilia A on long‐term octanate® prophylaxis
E. Dmitriev
Belarussian Research Centre for Paediatric Oncology, Haematology and Immunology, Minsk, Minskaya Voblasts', Belarus
Background: Prophylaxis with FVIII at a standardised dose is the standard of care for people with severe haemophilia A. However, there is increasing interest in personalised prophylactic dosing regimens based on individual pharmacokinetic parameters.
Aims: To investigate the potential of dose optimisation based on the time to reach FVIII activity of 1% in children with severe haemophilia A on FVIII prophylaxis.
Methods: Data were analysed for 41 patients (aged 4–16 years) included in the Belarus Register of Patients with Congenital Clotting Disorders who had received regular prophylaxis with the plasma‐derived FVIII concentrate octanate® over a two‐year period. The prophylactic regimen was up to 50 IU/kg over a fixed time interval (48, 72 or 96 h). A pharmacokinetic assessment was performed on the day of administration of the scheduled prophylactic octanate® dose.
Results: Four groups were defined based on the dosing interval and the presence or absence of bleeding episodes (BEs; spontaneous or associated with increased physical activity) in the previous year (Table 1). The estimated time to 1% FVIII activity for children on a 72‐h dosing interval was 78 h in those with no previous BEs (Group 1) and 70 h in those with previous BEs (Group 2). For patients on a 96‐h dosing interval, the values were 116 h in children without previous BEs (Group 3) and 111 h in those with previous BEs (Group 4). The time to 1% FVIII activity was longer than the dosing interval for children in Groups 1, 3 and 4, suggesting that the dose could be decreased, whereas it was shorter than the dosing interval for children in Group 2, suggesting that the dose should be increased in these patients.
Conclusion(s): Determination of time to 1% FVIII could be considered to optimise the dosing of octanate® prophylaxis in patients with haemophilia A.

4. PB1133
4.1. COVID‐19 vaccination in patients with hemophilia: Experience from a single center
C. Suffritti1; R. Gualtierotti 2; E. Scalambrino3; M. Clerici4; A. Lecchi5; C. Novembrino6; E. Biguzzi7; S. Siboni3; S. Arcudi8; A. Ciavarella9; P. Bono10; F. Ceriotti10; F. Peyvandi11
1 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Milan, Italy, Milano, Lombardia, Italy; 2 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 4 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 5 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milan, Lombardia, Italy; 6 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Lombardia, Italy; 7 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 8 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, MILAN, Lombardia, Italy; 9 Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy, 10 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Milan, Italy, Milan, Lombardia, Italy, 11 Fondazione IRCCS Ca' Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Coronavirus disease‐2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has resulted in an ongoing world‐wide pandemic. Vaccination is the key countermeasure of the COVID‐19 pandemic. Data on the efficacy, safety and immunogenicity of COVID‐19 vaccination in patients with hemophilia ‐ and in particular in those with HIV ‐ are still scarce.
Aims: The aim of our study was to characterize the immunogenicity and biomarkers of coagulation and endothelial perturbation after mRNA‐COVID‐19 vaccination in HIV‐positive hemophilic patients.
Methods: We collected blood from 24 adult HIV‐positive hemophilic patients followed at our centre (19 with hemophilia A, 5 with hemophilia B) before and two weeks after the administration of the complete vaccination schedule with mRNA‐1273 (Moderna Biotech). Most patients had severe hemophilia (n = 21). We measured antibodies to SARS‐CoV‐2 spike protein by Elecsys (Roche) to assess immunogenicity and we evaluated protein C, VWF, D‐dimer plasma levels as biomarkers of coagulation and endothelial perturbation. Anti‐Platelet Factor 4 (PF4) antibodies were also measured.
Results: Before vaccination, three patients out of 24 showed anti‐Spike IgG levels >0.8 U/ml (cut‐off value). Two weeks after completing the vaccination schedule, all patients had high values of anti‐Spike IgG (min–max 2,387–12,500 U/ml). Mean (standard deviation) basal values of protein C, VWF and D‐dimer (106 ± 21%, 171 ± 45%, 593 ± 692 ng/ml respectively) were not significantly different from values measured two weeks after the second dose of vaccine (103 ± 20%, 162 ± 43%, 583 ± 531 ng/ml). Anti‐PF4 antibodies were detected in three patients with no associated clinical manifestations. None of the patients reported bleeding in the site of inoculation nor serious adverse events after the vaccination.
Conclusion(s): Since immune abnormalities can occur in HIV‐positive patients, it is important to collect data on COVID‐19 vaccination immunogenicity. We demonstrated that hemophilic HIV‐positive patients have a normal antibody response against SARS‐CoV‐2 spike protein. In addition, mRNA‐1273 had no effect on coagulation and endothelial perturbation.
5. PB1134
5.1. Practical utilisation of Octapharma FVIII concentrates in previously untreated and minimally treated haemophilia A patients entering routine clinical treatment – The Protect‐NOW Study
S. Halimeh 1; J. Oldenburg2; R. Klamroth3; M. Mathias4; G. Hall5; P. Marco Vera6; M. Jansen7
1 Gerinnungszentrum Rhein‐Ruhr, Duisburg, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Vivantes Klinikum, Friedrichshain, Berlin, Berlin, Germany; 4 Great Ormond Street Hospital for Children, NHS Foundation Trust, London, England, United Kingdom; 5 Children's Hospital Oxford, Oxford Haemophilia and Thrombosis Comprehensive Care Centre, Oxford University Hospital NHS Foundation Trust, Oxford, England, United Kingdom; 6 Departamento de Medicina Clínica. Universidad Miguel Hernández, Jefe de Servicio de Hematología y Hemoterapia, Hospital General Universitario de Alicante, Alicante, Comunidad Valenciana, Spain; 7 Octapharma Pharmazeutika Produktionsgesellschaft m.b.H, Vienna, Salzburg, Austria
Background: For previously untreated patients (PUPs) and minimally treated patients (MTPs) with severe haemophilia A it is important to balance the need to minimise the risk of inhibitor development while providing all‐around bleed prevention. The efficacy, safety and immunogenicity of Octapharma FVIII products in PUPs have been demonstrated in prospective clinical trials. The incidence of high‐titre inhibitors was 16%, 8% and 11% in trials with Nuwiq®, octanate® and wilate®, respectively.
Aims: The aim of the ongoing, non‐interventional, prospective and retrospective Protect‐NOW study (NCT03695978) is to investigate the effectiveness, safety, immunogenicity and utilisation of Nuwiq®, octanate® and wilate® in routine clinical practice in PUPs and MTPs with severe haemophilia A. Optional sub‐studies will assess risk factors associated with inhibitor development.
Methods: 140 PUPs and MTPs (<5 previous exposure days [EDs] with other FVIII products) of any age and ethnicity will be followed for 100 EDs or up to 3 years after ED1. The primary outcome measures are the annualised rate of breakthrough bleeds to assess prophylactic efficacy and the incidence of adverse drug reactions to assess safety. Measurement of inhibitor activity is recommended every 3–4 EDs until ED20 and every 10–12 EDs thereafter. Optional sub‐studies include F8 gene analysis, measurement of non‐neutralising anti‐FVIII antibodies, and epitope mapping. Patients on concomitant emicizumab prophylaxis may be included in the study.
Results: As of January 2022, 51 patients have been enrolled in the study from 29 sites in 14 countries. Recruitment is ongoing, and additional sites are being initiated.
Conclusion(s): Protect‐NOW is collecting real‐world data on the effectiveness and safety of Octapharma FVIII products in routine clinical practice in PUPs and MTPs.
6. PB1170
6.1. Evaluation of nonacog beta pegol recovery in spiked samples using different APTT reagents/detection system available in Argentina
R. Sueldo 1; M. Arias2; C. Rosa3; M. Zirpoli3; J. Ceresetto4; C. Duboscq4
1 Laboratorio de Hematología. Hospital Dr. César Milstein, Hospital de Pediatría Juan P. Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Hospital Dr. César Milstein, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 3 Laboratorio del Hospital Universitario Austral, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 4 Servicio de Hematología y Hemoterapia. Hospital Británico de Buenos Aires, CABA, Ciudad Autonoma de Buenos Aires, Argentina
Background: Nonacog beta pegol (N9‐GP, Refixia) is a glycoPEGylated extended half‐life (EHL) recombinant factor IX developed for prophylaxis and treatment in hemophilia B patients. A great variability has been reported when one stage clotting assay for FIX:C (OSA) was used to measure this recombinant FIX product. Chromogenic FIX assay (CSA) has not yet been approved in Argentina.
Aims: To investigate in three laboratories the recovery of N9‐GP by OSA with 10 different aPTT reagents and three different clot detection systems in four different levels of N9‐GP.
Methods: FIX:C by OSA were performed with10 different aPTT reagents: Diagnostica Stago (Cephascreen: poliphenol, C.K. Prest: kaolin, Triniclot aPTT S, Silica), Instrumentation Laboratory (APTT‐SP and Synthasil: colloidal silica), Siemens Healthineers (Actin and Actin FSL: ellagic acid, Pathromtin SL: silicon dioxide) and Wiener Lab (APTTest: ellagic acid). Three coagulometer were used: ACL TOP 500, Sysmex CS‐2500 and STA Compact Max. Calibration curves were performed with conventional plasma calibrator (cpCal, validated with internal control) and with a home‐made specific product calibrator (hmspCal, 1.00 IU/ml). Samples: FIX immunodepleted plasma was spiked with N9‐GP at four target levels: 0.05, 0.13, 0.50 and 1.00 IU/ml. Spiked samples were aliquoted and frozen at −80°C until analysis. Mean %Rec is the average recovery of all levels studied. Acceptable recovery: ±20% (Accep), Overestimation: greater than 20% (Over), Subestimation: less than 20% (Sub).
Results: Table 1.
Conclusion(s): There were differences of FIX:C in OSA when cpCal was used, some reagents overestimated and did so at all levels. According to the %Rec only cephascreen, actin FSL and APTTest ellagic (aPTT reagent used in Latinoamerican region) could be used for monitoring of treatment with N9‐GP in the platforms studied. If a hmspCal was used, FIX:C by OSA were closer to the expected values for all reagent/detection system pairs used, this would be an alternative when CSA is not available.

7. PB0660
7.1. Impact of a product‐specific reference standard for the measurement of a PEGylated rFVIII activity: An ex‐vivo study
L. Bounaix1; O. Bulla2; P. Fontana 2
1 Division of Angiology and Haemostatis and Laboratory of Haemostasis/Geneva University Hospitals, Geneva, Geneve, Switzerland; 2 Division of Angiology and Haemostatis and Laboratory of Haemostasis/Geneva University Hospitals, Genève, Geneve, Switzerland
Background: Monitoring activity of factor VIII (FVIII:C) for hemophiliac patients treated extended half‐life products remains challenging. We previously demonstrated that, using FVIII‐deficient plasma spiked with PEGylated rFVIII (Adynovi®), product‐specific reference standards (SS) with a chromogenic assay (CA) was the most reliable method to evaluate FVIII:C.
Aims: The aim of this study was to compare different methods to evaluate FVIII:C in plasma samples of patients treated with PEGylated rFVIII (Adynovi®).
Methods: We used 73 samples of 8 patients treated by Adynovi® at different time points. We compared FVIII:C measured with one‐stage clotting assay (OSA) or CA with local reference standard (LS) or SS and compared the results with CA and SS, our reference assay.
Results: Compared to our reference assay, FVIII:C levels correlated well using OSA with LS (r 2 = 0.96, p < 0.001), OSA with SS (r 2 = 0.96, p < 0.001) or CA with LS (r 2 = 0.98, p < 0.001). When the results obtained with our reference assay were categorized into quartiles, there was only minimal differences between assays (Figure 1), with the largest mean difference in quartile #4 (‐12%, p = 0.01 with OSA with LS).
Conclusion(s): Unlike our previous study, this ex‐vivo study shows a good correlation between the different FVIII:C assays. Although this study is based on a limited number of patients, it suggests that results of studies using spiked deficient plasma should be interpreted with caution.

8. PB1135
8.1. Hemophilia Registry in a province in northern Spain
P. Lopez1; I. Hernández De Castro 2; A. Porro1; J. Iglesias1; G. Florez Meana3; C. Rodriguez de Miñon3; D. Martinez Carballeira3; J. Corte Buelga3; A. Bernardo1; D. Mateos Jimenez3; I. Soto1
1 HUCA, oviedo, Asturias, Spain, 2 hematology, oviedo, Asturias, Spain; 3 Hospital Universitario Central De Asturias, Oviedo, Asturias, Spain
Background: In 2018, the World Federation of Hemophilia (WFH) established the need to create a World Bleeding Disorders Registry (WBDR), a database which aim was to unify the network of hemophilia treatment centers, in order to collect standardized data on hemophiliac patients and guide medical practice. In 2020 the WBDR has registered 7,208 patients with hemophilia (84% A; 15% B) in 86 treatment centers distributed in 33 countries, with no representation from Spain. The Spanish Ministry of Health has not yet published any hemophilia registry.
Aims: To present a hemophiliac patients' registry in the Principality of Asturias, first community in Spain to develop such record.
Methods: The Redcap web tool was introduced to create a shared environment that would allow working both with the information already available and with new variables. Such information was loaded and verified from local files to the web registry.
Results: Asturias' population, according to the latest census of the Spanish National Institute of Statistics, is 1,011,792 inhabitants. Of the 66 hemophiliac patients, with age ranging from 3 to 86 (median 35), 92.4% are type A and 7% type B. 50% of the cases are mild; 6.1% are moderate and 43.9% are severe. 45.5% of them receive prophylactic treatment while 54.5% receive it on demand. The most used ones are DDAVP, Elocta, Nuwiq and Hemlibra, according to the distribution of graph 1. Regarding the development of inhibitor, it was 5% in hemophilia A and 1.5% in hemophilia B.
Conclusion(s): To create a WBDR is not only a mandate from the WFH, but is also necessary to estimate the social and health impact on this population. It aims to improve hemophiliac patients' quality of life. This is the first registry of hemophilia in the Spanish state, which is currently being extended to the rest of congenital coagulopathies.

9. PB0650
9.1. To predict the impact of low dose prophylaxis versus on demand treatment in severe Hemophilia A patients in Pakistan
M. Borhany 1; M. Abid1; R. Ahmed2; A. Jamal2; S. Zafar1; T. Shamsi3
1 National Institute of Blood Diseases and Bone marrow Transplantation, Karachi, Sindh, Pakistan; 2 Hemophilia Welfare society, Karachi, Sindh, Pakistan; 3 National Institute of Blood Diseases and BMT, KARACHI, Sindh, Pakistan
Background: A gold standard Prophylactic application of clotting factor concentrates particularly in children with hemophilia. However, prophylaxis is not practiced in Pakistan due to ill‐affordability of clotting factor concentrates.
Aims: To determined the impact of low dose prophylaxis versus on demand treatment in severe Hemophilia A (HA) patients.
Methods: A prospective observational study conducted from 2018 to 2021. Severe HA patients were sub‐grouped into prophylaxis and on‐demand treatment with administration of FVIII of 15 IU/kg twice weekly in the prophylaxis group. Comparison was evaluated between both the groups with (Hemophilia Joint Health Score (HJHS) and Functional Independence Score (FISH)).
Results: A total of 50 patients were recruited in the study; 25 patients in each group with median (IQR) age on demand patients were 4(6.2) while 6(4.5) years in prophylaxis group. Overall Joint assessment scoring of patients is mentioned in Table 1. Significant association of annual bleeding rate (ABR) between on demand and prophylaxis group includes bruising 21(72.4) vs. 8(27.6), joint pain 14(66.7) versus 7(33.3), with p‐value = 0.001. However, comparison of outcomes of on demand versus prophylaxis showed insignificant differences in days of hospitalization i.e. lower in the prophylaxis group than on demand group (44% vs. 55.6; p = 0.769), no inhibitor was reported in the prophylaxis while 2(100%) patients in the on‐demand group (Figure 1). Estimated given dose of factor was about 477,231 IU/kg/year and 593562 IU/kg/year in on demand and prophylaxis group respectively. 18(36) showed significant absenteeism from the school in which 16(88.6) were from on demand whereas 2(11.1) patients from the prophylaxis group i.e. (p‐value = 0.001) due to bleeding episodes.
Conclusion(s): This study showed that FISH and HJHS had positive impact on the prophylactic group than on the demand group. Overall health of the patients improved after receiving prophylaxis with less bleeding events and better social lives.


10. PB0654
10.1. Burden of treatment for adult and adolescent people with haemophilia (PwH): Concept elicitation results supporting the development of the hemophilia treatment experience measure (Hemo‐TEM)
M. Brod 1; L. Waldman2; J. Skov Neergaard3; A. Busk3
1 The Brod Group, Mill Valley, California, United States; 2 The Brod Group, San Diego, California, United States; 3 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark
Background: Currently available treatments for PwH have resulted in significant improvements in quality of life. However, PwH may still experience considerable treatment burden due to technical, physical, logistical, and emotional aspects of self‐administering intravenous infusions. Most research to date has focused on disease burden rather than treatment burden.
Aims: This study's purpose was to investigate treatment burden among adult and adolescent PwH and collect data to support the content validity of a new disease‐specific patient‐reported outcome measure of treatment burden.
Methods: Following U.S. Food and Drug Administration guidelines, interviews were conducted with 30 adult males with moderate or severe haemophilia, with or without inhibitors, and who self‐infused prophylactically or on demand. Interviews were analysed and coded using an adapted grounded theory approach to determine overarching themes and concepts. A theoretical model of treatment burden was then developed, and items for the new Hemo‐TEM measure were generated and cognitively debriefed in a total of 35 PwH (15 adults and 20 adolescents).
Results: Qualitative analysis revealed that saturation of concepts was reached with 5 treatment burden domains: injection difficulties; adherence; emotional burden; physical burden; and interference with daily life. Sub‐concepts endorsed by patients included inserting the needle correctly (53%) and fitting treatment into daily schedule (43%; injection difficulties); missed (53%) and delayed (40%) treatments (adherence); worry (47%) and anxiety/nervousness (27%) (emotional burden); blown veins (50%) and pain (50%) (physical burden); and travel (73%) and work (40%) (interference with daily life) (Table 1). The cognitive debriefing resulted in a draft measure of 30 items, which were found to be understandable and relevant.
Conclusion(s): The content validity of the Hemo‐TEM is supported by these qualitative findings. The validation‐ready Hemo‐TEM version contained items on treatment difficulties, adherence, emotional burden, physical burden, and interference with daily life and was found acceptable by adult and adolescent patients.

11. PB0659
11.1. Medically recorded bleeds and healthcare resource use in the United States among adult males with hemophilia A
L. Markson1; L. Young2; L. Ban3; Y. Chen1; P. Fogarty 1
1 Pfizer Inc., Collegeville, Pennsylvania, United States; 2 Pfizer Ltd., Walton Oaks, England, United Kingdom; 3 PPD Ltd., Beijing, Beijing, China (People's Republic)
Background: Repeated bleeding in muscles and joints are major causes of morbidity and disability in people with hemophilia A (PwHA).
Aims: Examine all‐cause and arthropathy‐related healthcare resource use (HRU) and comorbidities in PwHA with medically recorded bleeds (MRB).
Methods: PwHA (adult males 19–64 years of age) prescribed factor VIII replacement therapy were identified from US PharMetrics Plus® health insurance claims data from 01/01/2010 to 12/31/2019. This analysis was sponsored by Pfizer. Exclusions included moderate or severe liver disease diagnoses as well as evidence of inhibitor. MRB included hemarthrosis, intracerebral hemorrhage, gastrointestinal bleeding, and other symptomatic bleeds that could be identified using relevant diagnostic codes. Poisson (or negative binomial) regression was used to estimate rates and rate ratios [RR] of all‐cause inpatient admissions and emergency room (ER) visits, and joint surgery between PwHA with and without MRB. Hemophilia severity was not coded in the data.
Results: PwHA with MRB (n = 1045) were more likely to have significant comorbidities (eg, mild chronic liver disease, anxiety, and depression) compared with PwHA without MRB (n = 916). The number of all‐cause inpatient admissions in PwHA with MRB was 14.6 per 100 person‐years, nearly 3 times higher than PwHA without MRB after adjustment for demographic and clinical characteristics, including major comorbidities (adjusted RR: 2.6, 95% CI: 2.0–3.3). The number of all‐cause ED visits in PwHA with MRB was 61.6 per 100 person‐years, more than 3 times higher than PwHA without MRB (adjusted RR: 3.1, 95% CI: 2.6–3.7). The number of joint surgeries in PwHA with MRB was 7.4 per 100 person‐years, nearly 4 times higher than PwHA without MRB (adjusted RR: 3.7, 95% CI: 2.5–5.4).
Conclusion(s): Significant disease burden is incurred by adult PwHA experiencing bleeding events, underscoring the need for treatment advances in this population.
12. PB0675
12.1. Pain as a determinant of the quality of everyday functioning of persons with haemophilia
M. Marinić 1; S. Rihtar2; A. Boban3
1 Institute of Social Sciences Ivo Pilar, Zagreb, Croatia/Croatian Haemophilia Society, Zagreb, Grad Zagreb, Croatia; 2 Institute of Social Sciences Ivo Pilar, Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 3 University Hospital Centre Zagreb, University of Zagreb School of Medicine, Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia
Background: Pain has been described commonly in persons with haemophilia, however, the effect of pain on various aspects of their everyday functioning is still underexplored.
Aims: The aim of this study was to analyse the frequency and intensity of pain among persons with haemophilia, its effect on daily activities, and correlation with disease severity and sociodemographic and psychological characteristics.
Methods: The data were obtained by a survey conducted in 2021 among adults with haemophilia in Croatia. The questions included sociodemographic data (age and education), details on haemophilia (type, severity, and frequency of bleeding), pain scales evaluating frequency, intensity, and the effect of pain on daily activities, and psychological characteristics (depression, optimism, happiness, and feeling of physical safety).
Results: A total of 98 patients responded to the survey. 24.7% of them reported that they suffer from pain daily, and 25.8% frequently. Pain was reported as moderately intensive in 45.4%, intensive in 13.4% and very intensive in 2.1% of the respondents. The influence of pain on daily activities demonstrated interaction with recreational activities (M = 2.73), walking (M = 2.63), working (M = 2.42), and sleeping (M = 2.16), when using the scoring scale (range 1–5). Pain significantly correlated with haemophilia severity, frequency of bleeding, age, and was related to depression, optimism, and feelings of happiness and physical safety. Regarding the concurrent effect of pain intensity and/or frequency on these psychological variables, regression analysis shows that in both cases pain is a significant predictor only if it has a negative impact on daily activities as a mediator.
Conclusion(s): Pain is present in many persons with haemophilia, particularly the older ones with severe disease, and affects various aspects of their everyday functioning. It is necessary to raise awareness of this problem, to continue working on pain prevention and treatment, and to provide effective mechanisms of psychological support.
13. PB0676
13.1. Risk factors for bleeding in people living with Hemophilia A and B treated with regular prophylaxis: A systematic review of the literature
F. Germini1; N. Noronha2; A. Binu2; O. Olasupo2; D. Pete2; T. Navarro2; A. Keepanasseril1; D. Matino 3; K. de Wit4; S. Parpia2; A. Iorio1
1 McMaster University Medical Centre, Hamilton, Ontario, Canada; 2 McMaster University, Hamilton, Ontario, Canada; 3 Division of Hematology & Thromboembolism, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 4 Queen's University, Kingston, Ontario, Canada
Background: Knowledge about the risk for bleeding in patients with hemophilia (PWH) would be relevant for patients, stakeholders, and policymakers.
Aims: Our objectives were to perform a systematic review of the literature on 1) risk assessment models (RAMs) and 2) risk factors for bleeding in PWH on regular prophylaxis.
Methods: We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews from inception through August 2019. In duplicate, reviewers screened the articles for inclusion, extracted data, and assessed the risk for bias using the QUIPS tool. A qualitative synthesis of the results was performed due to high heterogeneity in risk factors, outcomes definition and measurement, and statistical analysis of the results.
Results: From 1843 search results, 10 studies met the inclusion criteria. The characteristics of the included studies are reported in Table 1. No RAM for the risk for bleeding in PWH was found. Most studies included only PWH A or both PWH A and B and were conducted in North America or Europe. Only one study had a low risk for bias in all the domains. The risk factors, outcome definitions, risk estimates, and confounders considered in the analyses are reported in Table 2. Eight categories of risk factors were identified. The risk for bleeding was increased when factor levels were lower and in people with a significant history of bleeding or who engaged in physical activities involving contact.
Conclusion(s): Increased plasma factor levels, history of previous bleeds, and physical activity are relevant risk factors for bleeding. These risk factors should be included while building a RAM, and the role of other risk factors should be explored.


14. PB1144
14.1. Outcomes with perioperative factor utilization and monitoring strategies in hemophilia A
T. Kartika; S. Gin; A. Mohinani; K. Leong; J. Shatzel; S. Olson
Oregon Health & Science University, Portland, Oregon, United States
Background: Factor activity levels are commonly monitored in the post‐operative setting for persons with hemophilia (PWH). The clinical relevance of this remains unexplored and it is unknown how often the levels effect changes to factor dosing.
Aims: To describe real‐world experience of modern perioperative management in hemophilia A and observe the clinical relevance of factor level monitoring.
Methods: We conducted a retrospective observational study of surgical procedures of adults (age >18) living with hemophilia A performed at our institution from January 2015 to July 2021. Data collected includes patient demographics, factor administration strategy, dose adjustments and outcomes. Factor levels were also captured and defined as therapeutic if >60 IU/dl on post‐operative days 1–3. Major bleeding was defined according to the standard ISTH definition.
Results: Eighty surgical procedures were identified (44 on patients with severe hemophilia, 34 with mild and 2 with moderate disease). There were 9 patients on active emicizumab therapy and 4 patients with inhibitor. Thirty‐eight (47.5%) cases utilized a continuous factor infusion. The drip rate was decreased in 15 (39.5%) cases and increased in 3 (7.9%). The starting infusion rates for the latter were <4 units/kg. None had subtherapeutic factor levels or major bleeding. Of patients who underwent bolus dosing only (52.5%), the dose was decreased in 3 cases (7.1%) and increased in 2 (4.8%). In total, 13 (16.3%) hospitalizations had subtherapeutic factor levels in the post‐operative setting. Complications were rare. Four cases had major bleeding (three intraoperative), no patients required packed red blood cell transfusion and there were no thrombotic complications.
Conclusion(s): For PWH undergoing surgery, factor monitoring was more likely to lead to a decrease in rate of continuous infusions rather than an increase. Subtherapeutic factor levels resulting in the increase in factor dosing is uncommon and with minimal clinical consequence.
15. PB1149
15.1. Pooled analysis of long‐term efficacy and safety of simoctocog alfa in previously treated patients with haemophilia A
M. Mathias1; A. Borchiellini2; F. Peyvandi3; A. Chistolini4; R. Marino5; A. Rocino6; M. Sol Cruz7; S. Carolina Oliva8; A. Molina Pantoja9; C. Grimley10; S. Bayart11; A. Klukowska12; T. Lissitchkov13; S. Halimeh14; S. Werner15; G. Pezeshki 15; S. Knaub16; C. Kessler17
1 Great Ormond Street Hospital for Children, NHS Foundation Trust, London, England, United Kingdom; 2 A.O.U. Città della Salute e della Scienza di Torino, Torino, Piemonte, Italy; 3 Fondazione IRCCS Ca' Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy; 4 Department of Translational and Precision Medicine, Sapienza University, Rome, Lazio, Italy; 5 Haemophilia and Thrombosis Centre, University Hospital of Bari, Bari, Puglia, Italy; 6 Ospedale del Mare Hospital, Napoli, Campania, Italy; 7 Centro Diabetologico del Norte (CEDINOR), Salta, Salta, Argentina; 8 PEDIAS INC, Guatemala City, Alta Verapaz, Guatemala; 9 Teodoro Maldonado Carbo Specialty Hospital, Guayaquil, Guayas, Ecuador, 10 Nottingham Haemophilia Comprehensive Care Centre, Nottingham, England, United Kingdom, 11 Hôpital Pontchaillou, Rennes, Bretagne, France, 12 Department of Pediatrics, Hematology and Oncology, Warsaw Medical University, Warsaw, Mazowieckie, Poland, 13 Blood Diseases at Clinical Hematology Clinic, Sofia, Sofiya, Bulgaria, 14 Gerinnungszentrum Rhein‐Ruhr, Duisburg, Nordrhein‐Westfalen, Germany, 15 Octapharma USA, Paramus, New Jersey, United States, 16 Octapharma AG, Lachen, Schwyz, Switzerland, 17 Georgetown University Medical Center, Washington, District of Columbia, United States
Background: Simoctocog alfa (Nuwiq®) is a human cell line‐derived recombinant human FVIII that was developed with the aim to provide a low immunogenicity product with excellent haemostatic efficacy for people with haemophilia A. The efficacy and safety of Nuwiq® have been demonstrated in clinical trials of previously treated patients (PTPs) and previously untreated patients with severe haemophilia A.
Aims: To assess the long‐term immunogenicity, safety and efficacy of Nuwiq® in a pooled analysis of PTPs with haemophilia A.
Methods: Data from patients who received Nuwiq® prophylaxis for ≥100 exposure days in two observational and four interventional post‐authorisation studies were pooled. These studies included male PTPs with severe haemophilia A (FVIII:C <1%); one study included patients with FVIII:C ≤2%. Cut‐off dates were used for studies ongoing at the time of database lock.
Results: Demographics of the 216 patients included in the analysis are shown in Table 1. There were no reports of inhibitor development in any patient. Twelve adverse drug reactions occurred in 8 patients; one was serious (mild pyrexia requiring hospitalisation) but resolved and treatment was continued. No thromboembolic events occurred. The median (interquartile range [IQR]) duration of prophylaxis was 16.8 months (9.4–24.4); 108 (50.0%) patients experienced no spontaneous bleeding episodes (BEs) during prophylaxis. Median (IQR) annualised bleeding rates were 0.11 (0–1.45), 0.50 (0–1.95) and 1.78 (0–4.24) for spontaneous, traumatic and all BEs, respectively. A total of 1011 BEs in 152 patients were treated with Nuwiq®. Most BEs (860 [85.1%]) resolved with ≤2 infusions of Nuwiq®; treatment efficacy was rated excellent or good for the majority (84.3%) of BEs. Of 36 surgeries with a postoperative rating by a haematologist, 31 (86.1%) were rated successful.
Conclusion(s): These pooled long‐term data from PTPs confirm the safety and efficacy of Nuwiq® demonstrated previously in clinical trials.

16. PB0663
16.1. Hemophilia joint health score (HJHS) and hemophilia early arthropathy detection with ultrasound (HEAD‐US) score in patients with hemophilia: Experience from a single comprehensive care center
R. Gualtierotti 1; A. Truma2; V. Begnozzi3; E. Biguzzi3; S. Siboni4; S. Arcudi5; A. Ciavarella6; E. Boccalandro3; F. Peyvandi7
1 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 2 Department of Pathophysiology and Transplantation, University of Milan, Milan, Lombardia, Italy; 3 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 4 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 5 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, MILAN, Lombardia, Italy; 6 Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 7 Fondazione IRCCS Ca' Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Recurrent joint bleeding leads to chronic arthropathy. The hemophilia joint health score (HJHS) is used to assess the joint health based on physical examination. More recently, musculoskeletal ultrasound (MSKUS) assessment has been introduced in the clinical practice and the hemophilia early detection with ultrasound (HEAD‐US) score has been proposed.
Aims: This study aims to evaluate the correlation between the HJHS and the HEAD‐US in evaluating the joint status and stage of arthropathy of patients with hemophilia.
Methods: In a cross‐sectional study we obtained the HJHS and the HEAD‐US in 100 patients with hemophilia A and B with different severity. The HJHS was based on the physical examination of the six index joints (elbows, knees and ankles) and gait assessment by a trained physiotherapist and the HEAD‐US was performed by an expert rheumatologist with experience in MSKUS in order to evaluate synovitis and osteochondral damage on the same joints. Spearman's rho was used to assess the correlations, significance was set at a p < 0.0001 (**).
Results: The mean age was 41 ± 19 years. The median score of HJHS 2.1 was 11 (3.75–17.25), the median score of HEAD‐US was 8.5 (2–17.25). Based on ultrasound examination, 44% of patients had at least one joint with synovitis (HEAD‐US score ≥1). There was a good correlation between HEAD‐US and HJHS score (rho = 0.74**). Furthermore, we observed a moderate to strong correlation between the joint total score of the HJHS and the total synovitis, cartilage and bone damage domains of the HEAD‐US score (0.55**, 0.78**, 0.72**, respectively). Notably, the HJHS total pain domain correlated weakly with the HEAD‐US cartilage and bone domains (0.33** and 0.30** respectively).
Conclusion(s): Our results show a good correlation and a complementarity between HJHS and HEAD‐US scores. The routine assessment of both tools helps clinicians define the stage of arthropathy and inform personalised management.

17. PB0677
17.1. Low level mosaicism in a boy with mild haemophilia A excludes his mother as a carrier
M. Mitchell 1; R. Wheeler2; J. Cutler2; S. Mangles3
1 Viapath Analytics LLP, Guy's & St. Thomas' NHSFT, LONDON, England, United Kingdom; 2 Viapath Analytics LLP, LONDON, England, United Kingdom; 3 North Hampshire NHS Trust, Basingstoke, England, United Kingdom
Background: Haemophilia A is an X‐linked bleeding disorder caused by variants in F8. Due to the possibility of mosaicism, mothers of sporadic cases of haemophilia cannot be excluded as carriers, even if the causative variant is absent in their somatic cells. This can lead to difficult conversations with these mothers explaining that while not confirmed as a carrier, the possibility of her having a second affected son cannot be excluded.
Aims: The definitive resolution of carrier status.
Methods: A 2 year old boy with sporadic mild haemophilia A (FVIII 15 IU/dl) was referred for analysis of F8 to identify the causative variant. His mother was subsequently analysed for the F8 variant identified in her son. Analysis was performed by PCR amplification and Sanger sequencing of the 26 exons and immediate flanking regions of F8. An allele specific assay was designed to allow the amplification of either the wild‐type or variant sequence.
Results: The affected child was initially shown to be hemizygous for a c.1733_1738dupTAGATC in exon 11 of F8. This duplication predicts the in‐frame insertion of two amino acids in to the Factor VIII protein, p.(Asp579_Gln580insLeuAsp). Analysis of the mother showed her to have only the normal sequence at this position. Review of the sequence data for the affected child showed the presence of a low level of normal sequence under the variant sequence. An allele specific assay demonstrated that both wild‐type and variant sequences were present, confirming mosaicism in the child.
Conclusion(s): The demonstration of low level mosaicism in the affected child allowed the conclusion that the pathogenic variant had arisen post fertilisation and had not been inherited from the maternal germ cell. This in turn allowed the mother to be informed that she did not carry the variant and that subsequent pregnancies would be unaffected by this variant.
18. PB0687
18.1. Real‐world efficacy of extended half‐life Factor VIII in patients with haemophilia A on prophylaxis
K. White 1; J. Alamelu2; G. Ling2
1 Barts Health NHS Trust, London, England, United Kingdom; 2 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom
Background: Standard half‐life Factor VIII (FVIII) products were previously the standard of care as prophylaxis in Haemophilia A with requiring alternate day administration. Recently, extended half‐life (EHL) FVIII has been licensed to improve adherence and convenience for patients. Two products available in the United Kingdom are 1. Esperoct (turoctocog alfa pegol), a PEGylated recombinant FVIII, and 2. Elocta (efmoroctocog alfa), a rFVIII‐Fc fusion protein. While the ASPIRE trial (Elocta) and Pathfinder (Esperoct) trial demonstrated ABR of <1 and 1.2 respectively, evidence in the real‐world setting are useful to validate the findings.
Aims: To review the management, real‐world outcomes and concordance of patients receiving EHL FVIII outside of the clinical trial setting.
Methods: A single centre, retrospective audit of clinical notes and laboratory results for all patients (children and adults) currently receiving EHL FVIII. Data were collected on patient demographics, annualised bleed rates (ABR), surgery and the presence of FVIII inhibitors.
Results: 44 patients are on EHL FVIII at St Thomas' Hospital Haemophilia comprehensive care centre, which treats a total of 370 patients with Haemophilia A of any severity. 18 patients use Esperoct and 26 use Elocta with an average age of 41.5 years and 21 years respectively. Treatment time on Esperoct was 25 months (stdev 25.8) and Elocta 45.2 months (stdev 25.9), with the majority infusing twice weekly. The ABR for Esperoct was 1.2 and for Elocta 0.92. Major and minor surgical procedures were performed with no bleeding. Pharmacokinetic studies have been difficult during the pandemic but 14/18 (77.8%) troughs were done with Esperoct and 26/26 with Elocta. No inhibitors were identified with either EHL FVIII.
Conclusion(s): In a large cohort, EHL FVIII have demonstrated good efficacy data at minimising ABRs in the real‐world setting, providing adequate haemostasis during surgical procedures whilst reducing the infusional burden for patients.
19. PB1159
19.1. The Atlanta study: Emicizumab and simoctocog alfa in previously untreated/minimally treated patients and in patients with inhibitors
R. Sidonio 1; M. Callaghan2; M. Manco‐Johnson3; S. Meeks4; A. Shapiro5
1 Aflac Cancer and Blood Disorders Center, Emory University, Atlanta, Georgia, United States; 2 Wayne State University and Children's Hospital of Michigan, Detroit, Michigan, United States; 3 University of Colorado and Hemophilia and Thrombosis Center, Aurora, Colorado, United States; 4 Emory University and Aflac Cancer and Blood Disorder Center, Children's Healthcare of Atlanta, Atlanta, Georgia, United States; 5 Indiana Hemophilia and Thrombosis Center, Indianapolis, Indiana, United States
Background: FVIII remains the first choice to treat bleeding events in hemophilia A. It is important to keep patients free of FVIII inhibitors, either by reducing the risk of inhibitor development or by eradicating inhibitors should they develop. Concomitant use of simoctocog alfa, a human cell‐line derived recombinant FVIII, with emicizumab may be an effective approach to provide effective bleed prevention while establishing FVIII tolerance.
Aims: Part A: To evaluate the immunogenicity of low‐dose FVIII given concomitantly with emicizumab in patients with little to no previous exposure to FVIII. Part B: to assess the efficacy and safety of concomitant emicizumab prophylaxis with FVIII for immune tolerance induction (ITI) in patients with inhibitors.
Methods: The Atlanta Study (NCT04030052) is a two‐part, prospective, investigator‐initiated, US multi‐center study in patients with hemophilia A (≤2% FVIII:C). Part A enrolls previously untreated patients (PUPs) or minimally treated patients (MTPs; ≤2 exposure days [EDs] to FVIII) <3 years of age and with no inhibitors. Patients receive standard emicizumab prophylaxis and 25 ± 5 IU/kg simoctocog alfa prophylaxis every 7–14 days until 50 EDs or 3 years, whichever comes first. The primary endpoint is the cumulative incidence of FVIII inhibitors. Part B enrolls patients <21 years of age with inhibitors (>0.6 BU/ml). Patients receive standard emicizumab prophylaxis and simoctocog alfa ITI (100 IU/kg, 3x/week). The primary endpoint is the rate of ITI success. Secondary endpoints include bleeding rates, safety, and treatments used for breakthrough bleeds.
Results: The study is recruiting patients, with eight active sites across the US. Target enrolment is 40 participants for Part A, and 20 participants for Part B.
Conclusion(s): Results from the Atlanta Study may offer guidance to healthcare professionals on how to provide PUPs, MTPs, and patients with inhibitors with effective prophylaxis while striving to establish FVIII tolerance.
20. PB0685
20.1. A retrospective database analysis study to evaluate disease burden and treatment patterns among patients with hemophilia A in China
X. Song1; W. Liu2; F. Xue2; Y. Yang2; S. Sun 3; J. Zhong4; Y. Liu2; J. Shi2; L. Zhang2; E. Wu4; R. Yang2
1 State Key Laboratory of Experimental Hematology, National Clinical Research Center for Hematological Disorders, Institute of Hematology & Blood Diseases Hospital, Tianjin Laboratory of Blood Disease Gene Therapy, CAMS Key Laboratory of Gene Therapy for Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin; Department of Hematology, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, Tianjin, Tianjin, China (People's Republic); 2 State Key Laboratory of Experimental Hematology, National Clinical Research Center for Hematological Disorders, Institute of Hematology & Blood Diseases Hospital, Tianjin Laboratory of Blood Disease Gene Therapy, CAMS Key Laboratory of Gene Therapy for Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, Tianjin, Tianjin, China (People's Republic); 3 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States; 4 Analysis Group, Inc., Beijing, Beijing, China (People's Republic)
Background: The clinical and economic burden of hemophilia A (HA) in China is not well characterized. A greater understanding of this patient population should help to improve quality of care and optimize patient outcomes.
Aims: To describe patient characteristics, treatment patterns, and the clinical and economic burden among patients with HA in China.
Methods: This retrospective database analysis utilized data from the hospital‐based National Hemophilia Registry (NHR) and healthcare resource utilization data from the Institute of Hematology and Blood Disease Hospital (IHBDH). Male patients with HA who had been enrolled in the NHR database since January 01, 2015 and had complete patient characteristic information were selected for evaluation of patient characteristics, treatment patterns, and clinical burden (Group 1). Data cutoff: December 31, 2019. Patients with HA who had been enrolled in the NHR database since January 01, 2015, who, between 2017 and 2019, were continuously enrolled for ≥1 year in a special disease health plan in Tianjin and incurred ≥1 inpatient or outpatient visit at the IHBDH were selected for evaluation of economic burden (Group 2).
Results: Group 1 included 3164 patients. Mean (SD) age at enrollment was 21.5 (15.5) years and 58.9% had severe HA (factor VIII <1 IU/dl). Mean (SD) time from initial diagnosis to replacement therapy was 2.1 (5.7) years (n = 1340). During the study period, 30.1% of patients received prophylaxis; at the index visit, 71.1% were only receiving on‐demand treatment (Table 1). In total, 2373/3045 patients (77.9%) experienced joint bleeding over the course of the disease. Annual bleeding rates are presented in Table 1. Group 2 included 118 patients. Mean (SD) total outpatient and hospitalization costs/patient/year were ¥56,736.8 (¥79,517.9) and ¥702.6 (¥1738.4), respectively.
Conclusion(s): These findings highlight the burden of illness and varied disease management experienced by patients with HA in China.

21. VPB0691
21.1. 10 Years comparative hemophilia prophylaxis across São Paulo State—Brazil
A. Guersoni 1; E. M. Trindade2; P. R Villaça3; O. Luiz4
1 PhD student ‐ University of São Paulo, sÃO pAULO, Sao Paulo, Brazil; 2 Faculty of Medicine of the University of São Paulo, São Paulo, Sao Paulo, Brazil; 3 University of São Paulo, São Paulo, Sao Paulo, Brazil; 4 Department of Preventive Medicine ‐ University of São Paulo, São Paulo, Sao Paulo, Brazil
Background: According to the World Federation of Hemophilia, Brazil has the 3rd largest population of hemophiliacs. Primary prophylaxis was implemented in Brazil from 2009 onwards.
Aims: To compare demographic and clinical Hemophilia A and B patients' characteristics across São Paulo State.
Methods: São Paulo State Hemovida Web – Coagulopathies registry from 2009 to 2018 quantitative and temporal series analysis.
Results: The prophylaxis program started in 2009. In 10 years, 2045 Hemophilia A (HA) or B (HB) patients were followed and registered at hemophilia centers in São Paulo State. Patients registered increased an average 3.2% per year, and 2015 had the largest number of new patients. Hemovida registered patients are 82.4% HA and 17.6% HB. Age stratum 20 to 39 years had the highest hemophilia prevalence. Overall, 88.9% participated in the long‐term primary or secondary prophylaxis program and 6% are only on‐demand treatment. Of these, 3.7% registered patients developed immunotolerance. Standardized mortality rates were 2.94 amid the cohort anteceding the program mainly at the 30–39 age stratum and 1.24:1,000 among those currently under prophylaxis, mainly over 60 years.
Conclusion(s): In Brazilian São Paulo State, similar to other countries, HA and HB regular prophylaxis treatment schedule has been, gradually, personalized and decreased morbidity and mortality.


22. VPB0697
22.1. Efficacy, safety and pharmacokinetics of recombinant human coagulation factor VIII (SCT800) in previously treated children with severe hemophilia A
R. Wu 1; X. Wang2; X. Zhao3; Y. Chen4; Z. Zhou5; J. Sun6; M. Xu7; W. Li8; J. Xiao9; F. Yang10; X. Chen11; W. Xu12; J. Huang13; C. Ma14; W. Gai14; L. Xie14; R. Yang15
1 Beijing Children's Hospital, Beijing, Beijing, China (People's Republic); 2 Beijing Children's Hospital, Capital Medical University, Beijing, China, Beijing, Beijing, China (People's Republic); 3 Xiangya Hospital Central South University, Changsha, Henan, China (People's Republic); 4 Shanxi provincial children's hospital, taiyuan, Shanxi, China (People's Republic); 5 The Second Affiliated Hospital of Kunming Medical University, kunming, Yunnan, China (People's Republic); 6 Nanfang Hospital Affiliated to Southern Medical University, guangzhou, Guangdong, China (People's Republic); 7 Chengdu Women’s & Children's Central Hospital, chengdu, Sichuan, China (People's Republic); 8 Qinghai Provincial People's Hospital, xining, Qinghai, China (People's Republic); 9 The Affiliated Children's Hospital of Chongqing Medical University, chongqing, Chongqing, China (People's Republic), 10 Fujian Medical University Union Hospital, fuzhou, Fujian, China (People's Republic), 11 Jinan Central Hospital, Jinan, Shandong, China (People's Republic), 12 The Affiliated Children's Hospital of Zhejiang Medical University, hangzhou, Zhejiang, China (People's Republic), 13 The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China (People's Republic), 14 Sinocelltech Ltd., Beijing, Beijing, China (People's Republic), 15 Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, tianjin, Tianjin, China (People's Republic)
Background: SCT800 is the first domestic recombinant factor VIII (rFVIII) approved in China for treatment of hemophilia A in adult patients, and is currently under investigation for pediatric indications.
Aims: The phase III study was designed to investigate the efficacy, safety and pharmacokinetics (PK) of SCT800 for prophylaxis and treatment of bleeding episodes in severe pediatric hemophilia A patients who have been previously treated with factor VIII (FVIII)
Methods: Study patients include boys <12 year with severe hemophilia A (FVIII:C <1%) and no history of FVIII inhibitors. For patients 0–5 year, the previous FVIII treatment should be >50 exposure days (ED); for those aged 6–11 year, ED should be >150. Eligible patients received SCT800 25–50 IU/kg every other day or three times a week for approximately 24 weeks. PK was evaluated after single injections of 50 IU/kg. The primary efficacy endpoint was annualized bleeding rate (ABR).
Results: Sixty‐eight (68) patients were enrolled (34 each for age group of 0–5 year and 6–11) and the mean exposure to SCT800 was 78.9 days. The median ABRs of the two groups were similar, 2.21 vs 2.17 (0–5 year vs 6–11 year). The median ABRs for spontaneous bleeds were 0.00 for both groups. Hemostatic efficacy was rated as “excellent” or “good” for 92.1% of bleeds. 83% of 127 reported bleeds were treated with ≤2 injections. Detailed ABR data for each group were included in the table below. Drug‐related adverse events (AEs) occurred in 2.9% of the patients. Only one 2‐year‐old patient developed inhibitors and was successfully treated with high dose SCT800 using an immune tolerance induction (ITI) scheme. The higher clearance and shorter t1/2 were observed in patients 0–5 year compared to those of 6–11 year.
Conclusion(s): The data indicate that SCT800 is effective and safe for the prophylaxis and treatment of bleeds in previously FVIII‐treated severe pediatric hemophilia A patients.

23. PB1127
23.1. Novel accreditation of European haemophilia treatment and gene therapy centres
A. Boban 1; F. Baghaei Borzabadi2; K. Fijnvandraat3; R. Klamroth4; W. Miesbach5; D. Stephensen6; M. Kavanagh7; D. Noone8; M. Crato9; F. Peyvandi10
1 University Hospital Centre Zagreb, University of Zagreb School of Medicine, Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 2 Sahlgrenska University Hospital, Gothenburg, Vastra Gotaland, Sweden; 3 Department of Pediatric Hematology, Emma Children's Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 Vivantes Klinikum, Friedrichshain, Berlin, Berlin, Germany; 5 Goethe University Hospital, Frankfurt, Hessen, Germany; 6 East Kent Hospitals University NHS Trust, Faculty of Health and Wellbeing, Canterbury Christ Church University, Canterbury, England, United Kingdom; 7 Children's Health Ireland at Crumlin, Dublin, Dublin, Ireland; 8 European Haemophilia Consortium, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 9 European Haempophilia Consortium, Montijo, Setubal, Portugal, 10 Fondazione IRCCS Ca' Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: The international accreditation of haemophilia centres in Europe is run by the EAHAD and EHC since 2013. The centres are designated as European Haemophilia Comprehensive Care Centres (EHCCC) or European Haemophilia Treating Centres (EHTC), based on the specific requirements which evaluate centres' ability to provide care for patients with haemophilia and allied disorders.
Aims: The development of a novel accreditation for gene therapy and other novel treatment approaches for management of haemophilia by EAHAD and EHC.
Methods: To facilitate the realization of these changes, EAHAD, in collaboration with EHC, has taken the steps to define necessary measures to safeguard quality and improvement of bleeding disorders care throughout Europe, to build a novel model for implementation of gene therapy and to provide a new Auditing and Certification protocol.
Results: The haemophilia centres are organized to offer multi‐disciplinary integrated comprehensive care and equal access to all treatment possibilities, including gene therapy, to all patients. A tight network of centres structured along a hub‐and‐spoke‐model, will address all aspects of gene therapy, from dosing, surveillance of the immediate post‐infusion period, to the long‐term follow‐up. We are presently designing the content and logistics of the multidisciplinary Auditing and Certification procedures and expect to start with our auditing process in 2023.
Conclusion(s): The new EAHAD audit and accreditation process has a challenging task to help haemophilia centres to improve the delivery of care by multi‐disciplinary integrated comprehensive care model and equal access to all novel therapies, including gene therapy as well as multidisciplinary services to all patients.
24. PB1146
24.1. Determinants of successful immune tolerance induction in persons with hemophilia A: Systematic review and meta‐analysis
I. Oomen 1; R. Camelo2; S. Rezende3; J. Voorberg4; M. Mancuso5; J. Oldenburg6; M. Carcao7; D. Matino8; D. Lillicrap9; K. Fischer10; K. Fijnvandraat1; S. Gouw11
1 Department of Pediatric Hematology, Emma Children's Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 3 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 4 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 5 IRCCS Humanitas Research Hospital, Milan, Lombardia, Italy; 6 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 7 Division of Hematology/Oncology, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada; 8 Division of Hematology & Thromboembolism, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 9 Department of Pathology and Molecular Medicine, Queen's University, Kingston, Ontario, Canada, 10 University Medical Center Utrecht, University Utrecht, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands, 11 Department of Pediatric Hematology, Emma Children's Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Immune tolerance induction (ITI) is the only therapy to eradicate anti‐factor VIII (FVIII) antibodies (inhibitors) in persons with hemophilia A (PwHA). However, this burdensome treatment fails in 10%–40% of patients. In order to predict the ITI outcome is it important to identify predictors of ITI success.
Aims: To summarize the current evidence on factors associated with ITI outcome in PwHA.
Methods: On February 15, 2021, a comprehensive literature search was conducted, which was updated on December 29, 2021. Randomized controlled trials (RCT), cohort ‐, or case‐control studies reporting on predictors for ITI outcome in PwHA were eligible for inclusion. ITI success was defined according to the methods of the investigators of the original studies. Data were extracted on study characteristics, outcome definitions, and predictors of ITI outcome, using a data collection form. Methodological quality was assessed by using an adapted Joanna Briggs Institute checklist for cohort studies. Studies were rated as high quality if ≥11/13 criteria were met.
Results: The literature searches yielded 1,079 unique papers of which 27 studies were included. We included 1 (4%) RCT and 26 (96%) cohort studies, involving a total of 1,734 PwHA. Six studies were rated as high quality (22%). ITI success was predominantly defined as negative inhibitor titer (<0.6 BU/ml), FVIII recovery ≥66% of expected, and FVIII half‐life ≥6 h (16/27; (59.3%)). Historical peak titer <100 BU/ml, pre‐ITI titer <10 BU/ml, and peak titer during ITI <100 BU/ml were associated with ITI success with pooled odds ratios of 1.50 (95% CI, 1.19–1.89), 1.68 (95% CI, 1.33–2.13), and 2.43 (95% CI, 1.58–3.74), respectively.
Conclusion(s): This systematic review and meta‐analysis summarized and analyzed all available evidence of single variables that influence ITI outcome. However, the effects of multiple variables on ITI outcome were not investigated in this review.
25. PB1156
25.1. Desmopressin in hemophilia A carriers: Determinants and duration of FVIII response
L. Romano 1; T. den Hertog2; G. Mulders‐van der Meer1; M. Cnossen3; F. Leebeek4; M. Kruip5
1 Erasmus MC, Erasmus University Medical Center., Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Hematology, Erasmus MC, Erasmus University Medical Center., Rotterdam, Zuid‐Holland, Netherlands; 3 Department of Pediatric Hematology, Sophia Children's Hospital, Erasmus University Medical Center, Erasmus University Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 5 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Desmopressin is a synthetic derivative of vasopressin often utilized in hemophilia A carriers to treat or prevent bleeding as it increases von Willebrand factor (VWF) and endogenous factor VIII (FVIII). Despite its worldwide use, determinants of FVIII response in carriers are largely unknown. Improved insight may increase its therapeutic use which has several advantages.
Aims: To assess the determinants and duration of desmopressin response (FVIII) in hemophilia A carriers.
Methods: Hemophilia A carriers with a historically lowest FVIII < 0.50 IU/ml who received desmopressin (0.3 μg/kg intravenously or subcutaneously) were included. Desmopressin response (FVIII) was measured at 0, 1, 3, 5 and 24 h (T0‐T24) after administration. F8 mutations were categorized according to F8 domain with severity based on male relatives with hemophilia A (severe/non‐severe). Desmopressin response was defined as: complete (CR) if measured FVIII level >0.5 IU/ml, partial (PR) if 0.3–0.5 IU/ml and non‐responder (NR) if < 0.3 IU/ml. Multiple linear regression was used to analyze associations between FVIII peak response (T1) and historically lowest FVIII level corrected for F8 mutation severity and historically lowest measured VWF:Ag.
Results: Fifty‐four hemophilia A carriers were included (Table 1). The mean historically lowest FVIII:C measured was 0.36 (SD 0.10 IU/ml). All women showed complete response at T1. At T5 (n = 40), 36 carriers (90%) retained CR, and four (10%) PR (Figure 1). The most important determinant for FVIII response was historically lowest FVIII: women with higher historically lowest FVIII (n = 37) presented with a higher absolute peak FVIII response (β = 0.454, CI 95% [0.29, 3.14] after correction).
Conclusion(s): All hemophilia A carriers had a complete response at T1 after desmopressin administration and retained at least a partial response at T5, with higher absolute peak FVIII response with higher historically lowest FVIII levels. Therefore, desmopressin is treatment of choice in hemophilia A carriers.


26. PB1153
26.1. Interim analysis from B‐MORE, a 24‐month prospective, multicentre, non‐interventional study on effectiveness and usage of recombinant factor IX Fc (rFIXFc) in haemophilia B
H. Glosli1; S. Ranta 2; D. Allsup3; I. Ricca4; M. Saleh5; Å. Carlsheimer6; A. Falk6; E. Santagostino7
1 Oslo University Hospital, Oslo, Oslo, Norway; 2 Karolinska University Hospital, Stockholm, Stockholms Lan, Sweden; 3 Hull University Teaching Hospital Trust And Centre for Atherothrombosis and Metabolic Disease, Hull, England, United Kingdom; 4 AOU Città della Salute e della Scienza di Torino, Torino, Piemonte, Italy; 5 King Faisal Specialist Hospital and Research Centre, Riyadh, Ar Riyad, Saudi Arabia; 6 Swedish Orphan Biovitrum AB, Stockholm, Stockholms Lan, Sweden; 7 Sobi, Stockholm, Stockholms Lan, Sweden
Background: The extended half life rFIXFc has a well‐established safety and efficacy profile demonstrated by phase 3 trials in patients with haemophilia B (HB; ≤2 IU/dl FIX) across all age‐groups. The B‐MORE study is prospectively evaluating real‐world effectiveness and usage of rFIXFc in HB patients in Europe and Middle East.
Aims: To describe interim data for patients treated with rFIXFc in the ongoing B‐MORE study.
Methods: This 24‐month prospective, multicentre, non‐interventional study includes patients prescribed with prophylactic or on‐demand rFIXFc treatment prior to or at study enrolment. Baseline characteristics, 12‐month retrospective data on previous FIX treatment prior to rFIXFc switch, rFIXFc prescribed dose and annualised bleeding rate (ABR) are reported.
Results: At interim data cut‐off (22nd October 2021), 117 patients (2 female) from 25 centres were included in the effectiveness analysis. Baseline patient characteristics are shown in Table 1. Sixty patients were aged <18 years (median 8.3, range 1–18 years). Patients with ≥6 months documented prophylaxis with standard half‐life FIX prior to rFIXFc had a median (IQR) of 2.0 injections with FIX per week (2.0–2.0) (n = 69), with a median (IQR) factor consumption of 74.9 (58.4–92.1) IU/kg/week (n = 65) and a median (IQR) overall ABR of 1.0 (0.0–2.0) (n = 69). Mean (range) overall duration of rFIXFc treatment from initiation to data cut‐off was 824 (42–3650) and 457 (15–1207) days for prophylactically (n = 106) and on‐demand treated subjects (n = 11), respectively. Dosing and ABR on rFIXFc prophylaxis are shown in Table 2.
Conclusion(s): Interim data from the ongoing B‐MORE study indicate that rFIXFc prophylaxis can provide and maintain excellent bleed protection in this HB population including a large number of paediatric patients. These real‐world data show a median ABR close to zero on rFIXFc prophylaxis with a low factor consumption mainly based on a once‐weekly regimen.


27. PB0653
27.1. Validation of the hemophilia treatment experience measure (Hemo‐TEM): A new haemophilia‐specific patient‐reported outcome measure
M. Brod 1; D. Bushnell2; J. Skov Neergaard3; A. Busk3
1 The Brod Group, Mill Valley, California, United States; 2 Evidera | PPD, Mountlake Terrace, Washington, United States; 3 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark
Background: The Hemo‐TEM is a patient‐reported outcome (PRO) measure developed based on U.S. Food and Drug Administration (FDA) guidance to assess the burden of treatment on people with haemophilia. The measure is currently being administered in ongoing concizumab phase 3 trials.
Aims: The objective of this study was to validate the psychometric properties of the Hemo‐TEM for adolescents and adults.
Methods: Patients from 3 clinical trials (NN7170‐4213, NN7415‐4255, NN7415‐4310) currently taking an injection for haemophilia (n = 88) completed a validation battery (demographics and PRO measures needed for the cross‐sectional validation analysis) at a screening visit, at baseline (retest), and at 24 weeks post‐baseline (n = 56, sensitivity to change). Psychometric testing, including of the measurement model, reliability, validity, sensitivity to change, and meaningful change, followed FDA guidelines for PRO measure validation.
Results: Item Reduction dropped 4 items resulting in a final 26‐item measure (Figure 1). Factor analysis generated 5 domains in the Hemo‐TEM [injection difficulties (3 items), physical impact (6 items), treatment bother (7 items), interference with daily life (4 items), and emotional impact (6 items)] and a total score. All scores were reliable [internally consistent (0.84 to 0.88) and reproducible (0.80 to 0.92)]. A‐priori hypothesized associations for validity of the Hemo‐TEM domains were confirmed. Preliminary estimates of sensitivity to change were seen with effect sizes between −0.30 and −0.70. The meaningful change thresholds ranged from 6 points (physical impact and emotional impact) to 10 points (treatment bother) with 8 points for the Hemo‐TEM total score. The measure took approximately 5 min to complete suggesting minimal administration burden.
Conclusion(s): The Hemo‐TEM can be considered a well‐designed, valid, and reliable measure of the burden of haemophilia treatment on patients. This measure should prove useful to assess impacts related to haemophilia as well as to clinicians in tailoring treatments to patient characteristics and situations.

28. VPB1140
28.1. Role of activated platelets in compensation of bleeding severity in hemophilia A
C. Laha Roy 1; S. Maharana1; K. Kishor2; R. Ranjan2; M. Mahapatra3; R. Saxena4; M. Kannan1
1 Blood and Vascular Biology Research Lab, Department of Life Sciences, School of Life Sciences, Central University of Tamil Nadu, Thiruvarur, Tamil Nadu, India; 2 All India Institute of Medical Sciences, New Delhi, Delhi, India; 3 All India Institute of Medical Sciences, Delhi, Delhi, India; 4 The Medicity Hospital Gurgaon, gurgaon, Haryana, India
Background: Hemophilia A (HA) is an X linked bleeding disorder which is due to the deficiency of factor VIII (FVIII). Severity of the disease is categorized based on the FVIII level and the HA patients with factor level <1% are characterized ad severe. Majority of these patients tend to have major hemorrhages. However, in general, 10% of severe patients compensate major bleeding. We hypothesize that activated platelets may have a role in compensation of bleeding severity in these patients.
Aims: To investigate the role of activated platelets in severe HA with mild hemorrhages.
Methods: Twelve severe HA patients and equal number of age matched normal were included after obtaining informed consent. Clinical details such as bleeding type, frequency and number of transfusions were recorded. 10 ml whole blood was collected and subjected for flow cytometry analyses to study the activated platelets. For this purpose, platelets were incubated with CD62P, PAC‐1 and Annexin V and a minimum of 10,000 events were recorded for the analysis.
Results: All HA patients had <1% FVIII and their age ranged between 7 and 39 years. Two of these patients never had muscle bleed and FVIII was infused to them only on demand. In addition to this, one of these two patients never developed any joint bleed. Based on bleeding and FVIII infusion, these patients were categorized as mild bleeders. When examined their platelets using flow cytometry, the PAC‐1 binding were higher in both the patients (3.64% and 2.91% respectively) compared to that of normal (0.95%). However, the CD62P and AV binding in these patients were within the normal.
Conclusion(s): Patients with mild bleeding had higher expression of integrin receptor complex αIIbβ3 which indicates the activated status of platelets in them. Thus, it is possible that activated platelets may contribute to mild hemorrhage in these patients.


29. PB0651
29.1. Health‐related quality‐of‐lives in hemophilia A patients on prophylactic therapy of Emicizumab (Hemlibra)
M. Borhany 1; M. Abid1; S. Zafar1; R. Ahmed2; A. Jamal2; R. Nadeem2
1 National Institute of Blood Diseases and Bone marrow Transplantation, Karachi, Sindh, Pakistan; 2 Hemophilia Welfare society, Karachi, Sindh, Pakistan
Background: Hemophilia A (HA) is a congenital bleeding disorder. Understanding the impact of the disease is critical, and improvement in the quality of life from treatments that significantly reduce bleeds is of high interest.
Aims: The following study was conducted to determine the outcome of Emicizumab (Hemlibra) prophylaxis on the Health‐Related Quality‐Of‐Lives in HA patients.
Methods: This study was conducted from January to December 2021. Hemlibra dose was 3 mg/kg in the first 4 weeks and the maintenance dose was started at week 5 at 6 mg/kg/month. Quality of life and disability in these patients was assessed using EQ‐5D‐5L and FISH questionnaires.
Results: A total of 36 male HA patients with ABR >8 were enrolled in the study, among them 14(38.8%) patients had positive inhibitor. Median age of the patients was 18 years (IQR‐18) with history of consanguinity in 30(83.3%) and family history of bleeding in 29(80.5%) patients. Health related quality of life (Figure 1) was assessed at different time points i.e. baseline, at 6 months and at 12 months after prophylactic treatment. The scores marked by the patients after treatment using visual analog scale (VAS) indicated <60% of health status of 3(8.3%), 60%–80% of 28(77.8%) and >80% of 5(13.9%) patients. Frequency of bleeding symptoms; hemarthrosis, gums bleeding and bruising were also reduced during the follow‐up (up to 8.3% vs 2.8 vs 2.8%). Moreover, FISH was also assessed to measure the disability (Table 1). Median (IQR) FISH scores noted were 8.0(5) vs 12.0(16) vs 30(2) with significant change (p value = 0.000) in functional independence after Hemlibra. The treatment frequency was observed in 78% patients at baseline which was reduced to 22.8% at 6 months and 8.3% at 12 months.
Conclusion(s): Our results suggest that patients on prophylactic treatment with Hemlibra were less restricted and had improved quality of life, especially improved health and social lives.


30. PB0678
30.1. Changing recombinant factor VIII for plasma‐derived FVIII during immune tolerance induction: Results from the Brazilian Immune Tolerance (BrazIT) study
S. Rezende1; M. Moreira Dias 2; L. de Magalhães3; L. Jardim4; A. De Oliveira5; R. Ribeiro6; V. Franco7; M. Roberti8; F. Callado9; L. Etto10; M. De Cerqueira11; M. Cerqueira12; C. Lorenzato13; I. Pinto14; É. Serafim15; A. Garcia16; T. Anegawa17; D. Neves18; D. Tan19; R. Camelo20
1 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 2 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 3 Department of Clinics, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 4 Faculty of Medicine, Universidade Federal de Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil; 5 Fundação HEMOMINAS, Belo Horizonte, Minas Gerais, Brazil; 6 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaleza, Ceara, Brazil; 7 Centro de Hematologia e Hemoterapia de Santa Catarina (HEMOSC), florianópolis, Santa Catarina, Brazil; 8 Centro de Hematologia e Hemoterapia de Goiás (HEMOGO), Goiânia, Goias, Brazil; 9 Fundação de Hematologia e Hemoterapia de Pernambuco (HEMOPE), Recife, Pernambuco, Brazil, 10 Centro de Hematologia e Hemoterapia da Paraíba (HEMOÍBA), João Pessoa, Paraiba, Brazil, 11 Centro de Hematologia e Hemoterapia do Piauí (HEMOPI), Teresina, Piaui, Brazil, 12 Instituto de Hematologia do Estado do Rio de Janeiro (HEMORIO), Rio de Janeiro, Rio de Janeiro, Brazil, 13 Hemocentro do Paraná (HEMEPAR), Curitiba, Parana, Brazil, 14 Fundação HEMOPA, Belém, Para, Brazil, 15 Centro de Hematologia e Hemoterapia do Rio Grande do Norte (HEMONORTE), Natal, Rio Grande do Norte, Brazil, 16 Centro de Sangue de São José do Rio Preto, São José do Rio Preto, Sao Paulo, Brazil, 17 Centro Regional de Hematologia e Hemoterapia de Londrina, Londrina, Parana, Brazil, 18 Fundação Hemocentro de Rondônia (FHEMERON), Porto Velho, Rondonia, Brazil, 19 Department of Pediatric Onco‐hematology, Faculdade de Medicina de Marília, Marília, Sao Paulo, Brazil, 20 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil
Background: The main complication of factor VIII (FVIII) replacement in people with haemophilia A (PwHA) is the development of neutralising alloantibodies (inhibitors) against FVIII. Immune tolerance induction (ITI) is the only treatment for eradicating inhibitors.
Aims: We evaluated ITI outcome after changing the product from recombinant FVIII (rFVIII) to plasma‐derived FVIII (pdFVIII) among PwHA who were not responding to rFVIII as a first‐line therapy.
Methods: PwHA on ITI were enrolled at 15 haemophilia treatment centres. For this study, we included those who completed ITI, received only pdFVIII or changed from rFVIII to pdFVIII (n = 104). PwHA started ITI on a low‐dose FVIII regimen (50 international units [IU]/kg 3×/week). Upon lack of response (no decline of inhibitor of at least 20% of peak levels 6 months after ITI start), high‐dose FVIII regimen (100 IU/kg/day) and/or changing to pdFVIII for those who started ITI with rFVIII. Outcome was based on inhibitor titre and FVIII pharmacokinetics.
Results: A total of 8/104 patients (8%) changed from rFVIII into pdFVIII due to unresponsiveness to the former. Median age at inhibitor detection and ITI start were 4.6 years (interquartile range [IQR], 2.0–12.8) and 13.2 years (IQR, 5.1–25.5), respectively. While PwHA who received only pdFVIII had 68% of success, 100% of PwHA who changed from rFVIII to pdFVIII failed. Pre‐ITI inhibitor peak, inhibitor titre immediately before ITI start, and peak during ITI were higher among PwHA who changed from rFVIII to pdFVIII in comparison with the ones who received exclusively pdFVIII during ITI.
Conclusion(s): In this study, all PwHA who received rFVIII as first‐line product and changed to pdFVIII due to unresponsiveness to rFVIII, failed ITI. High inhibitor titres before and/or during ITI were higher among PwHA who changed from rFVIII to pdFVIII in comparison with the ones who received exclusively pdFVIII during ITI.
31. PB0683
31.1. Current landscape of prophylaxis and tolerisation in patients with haemophilia A in Australia
S. Parikh 1; S. P'ng2; S. Brown3; C. Barnes4; C. Tan5; T. Carter6; H. Tran7
1 Australian Haemophilia Centre Director's Organisation, Malvern East, Victoria, Australia; 2 Fiona Stanley Hospital, Perth, Western Australia, Australia; 3 Queensland Children's Hospital, Brisbane, Queensland, Australia; 4 The Royal Children’s Hospital, Melbourne, Victoria, Australia; 5 Royal Adelaide Hospital, Adelaide, South Australia, Australia; 6 Perth Children's Hospital, Perth, Western Australia, Australia; 7 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia
Background: Prescribing prophylaxis in patients with Haemophilia A (HA) is a well‐established standard of care, helping to reduce or abolish frequency of bleeds, maintain joint health and improve quality of life. Emerging trends and availability of various factor (F8), including Standard Half‐Life (SHL) and Extended Half‐Life (EHL), and non‐factor replacement (emicizumab) therapies provide an opportunity to assess changing landscape of prophylaxis and tolerisation in the Australian cohort.
Aims: To characterise current practice in HA patients prescribed prophylaxis and/or Immune Tolerance Therapy (ITT) in Australia.
Methods: Data was derived from the Australian Bleeding Disorder Registry (ABDR) on consented HA patients. Data was obtained on patient diagnosis, severity, age group, treatment product, treatment regimen and inhibitor status. Patient demographics and results presented are based on ABDR data up until December 2021.
Results: There are currently 2346 active HA patients in the ABDR. 87.1% (637/731) of severe HA patients and 35.7% (86/241) of moderate HA patients are prescribed prophylaxis. Breakdown of severe and moderate HA patients on prophylaxis are 61.6%, 21.3% and 17.1% for using emicizumab, EHL & SHL respectively. 9 non‐inhibitor HA patients (all severe) are prescribed both, F8 and emicizumab. A significant proportion (76.2%) of paediatric patients with severe HA are prescribed emicizumab as prophylaxis compared to 48.1% of severe adult HA population with the majority of all patients on fortnightly dosing. Among the 70 patients with F8 inhibitors, 17 are on‐demand treatment, 3 are on ITT using F8 only and 50 are on emicizumab (including 4 that are also prescribed F8). There were no serious adverse events reported in the ABDR for Year 2021 caused by any F8 product or emicizumab.
Conclusion(s): Improved availability of various treatment options has led to an increased uptake of HA patients prescribed prophylaxis in the last few years. Further information is required to examine treatment guidelines and clinical outcomes.
32. PB1139
32.1. Annualized bleeding rates in severe hemophilia on prophylaxis in a real‐world setting
D. Kraemmer 1; B. Bauer2; C. Ay2; I. Pabinger2
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Therapeutic options for hemophilia have expanded considerably with the introduction of extended half‐life products (EHL).
Aims: We investigated prophylaxis with standard (SHL) and EHL in a real‐world setting.
Methods: In our center, patient diaries used to self‐document infusions and bleeding episodes are collected within the framework of the Austrian Haemophilia Registry. We included all recorded infusions of adult patients with hemophilia A (HA) or B (HB) receiving prophylaxis with SHL or EHL (albutrepenonacog‐alfa, damoctocog‐alfa‐pegol, efmoroctocog‐alfa, eftrenonacog‐alfa, lonoctocog‐alfa, nonacog‐beta‐pegol, rurioctocog‐alfa‐pegol) for ≥6 months between 2018‐01‐01 and 2020‐12‐31. Since switching between products could occur, we analyzed the data using mixed‐effects models allowing for random intercepts and adjusting for age and hemophilia type.
Results: Fifty HA (median age 35 years; interquartile range 25–41) and 7 HB patients (42 years; 29–59) recorded a total of 31.3 million IU over 13,820 infusions for a median of 730 days (range 190–1,095). Median annualized bleeding rate (ABR) and joint ABR of the whole cohort was 4.74 (0.67–14.15) and 3.01 (0–8.36), respectively. Twenty‐five patients were treated with EHL, eight of which documented a switch during the observation period. Summary statistics of ABR, joint ABR, infusion frequency, and factor consumption are presented in Table 1. Prophylaxis with EHL was associated with an incidence total and joint ABR ratio of 0.55 (95% CI 0.42, 0.73) and 0.73 (95% CI 0.53, 1.01), respectively; and a difference in weekly factor usage of 386.6 IU (95% CI −480.9, 1,254.1). Weekly infusions were lower on EHL (−0.43; 95% CI −0.76, −0.08).
Conclusion(s): ABR and joint ABR during prophylaxis with EHL, albeit lower when compared to SHL, were still high overall, which might partly be explained by preferential switching of patients with more complicated bleeding phenotypes. Patients with high bleeding rates despite prophylaxis require an individualized approach such as an increase in their infusion frequency, a switch to non‐factor treatments, or gene therapy.

33. PB1142
33.1. Canadian patient experience on switching from octocog alfa to extended half‐life FVIII damoctocog alfa pegol in patients with severe hemophilia A
D. Matino 1; K. Decker2; E. Iserman3; A. Keepanasseril3; F. Germini3; A. Chan4; L. Walsh3; A. Iorio3
1 Division of Hematology & Thromboembolism, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 2 Hamilton Health Sciences, McMaster University Medical Centre, Hamilton, Ontario, Canada; 3 McMaster University Medical Centre, Hamilton, Ontario, Canada; 4 McMaster University, Hamilton, Ontario, Canada
Background: In Canada, as yet, there are no reports on real‐world safety, effectiveness and patient satisfaction outcomes when switching from a previous factor (F)VIII product to the extended half‐life recombinant FVIII, damoctocog alfa pegol (BAY 94‐9027/Jivi®).
Aims: To report on changes in effectiveness, utilization, and patient satisfaction when switching to damoctocog alfa pegol prophylaxis from previous standard half‐life octocog alfa (BAY 81‐8973/Kovaltry®) treatment in patients with severe hemophilia A aged ≥12 years in a Canadian real‐world setting.
Methods: This observational, retrospective, intra‐patient comparison study uses real‐world data collected at the Hamilton‐Niagara Regional Hemophilia Treatment Centre. Patients with severe hemophilia A (FVIII:C <0.01 IU/ml) aged ≥12 years receiving octocog alfa prophylaxis for ≥9 months and willing to switch to damoctocog alfa pegol were included and were observed for 9 months pre‐ and post‐switch. Clinical outcomes included annualized bleeding rate and quality‐of‐life (QoL) evaluations (Patient Reported Outcomes, Burdens and Experiences [PROBE]).
Results: Clinical outcomes data were available for 18 patients. Median annualized bleeding rates following the switch to damoctocog alfa pegol are shown in Table 1. For damoctocog alfa pegol vs octocog alfa, the median (Q1;Q3) number of infusions per week was 2.20 (2.01;2.98) vs 2.67 (2.09;2.93), respectively and the median annualized recorded FVIII utilization was 3819.6 (2980.9;4564.8) vs 4348.7 (3769.0;5156.5) IU/kg/year, respectively. QoL was maintained following the switch: median (Q1;Q3) improvement in PROBE score was 0.02 (−0.08;0.07, n = 12).
Conclusion(s): These data provide real‐world evidence supporting the use of damoctocog alfa pegol as an effective alternative therapy for patients with severe hemophilia A currently receiving octocog alfa. Improved clinical outcomes were observed following this switch even when patients’ QoL and bleeding control were well‐maintained with octocog alfa. Long‐term observation of patients on damoctocog alfa pegol prophylaxis may reveal progressive improvement in clinical outcomes and/or QoL. Study sponsored by Hemalytic, Inc.

34. PB1162
34.1. Safety of non‐steroidal anti‐inflammatory drugs in hemophilia: A systematic review reporting on adverse bleeding and cardiovascular events
E. van Bergen 1; M. Monnikhof2; R. Schutgens3; F. Lafeber2; S. Mastbergen2; L. van Vulpen4
1 Van Creveldkliniek, UMC Utrecht, Utrecht, Netherlands; 2 UMC Utrecht, Utrecht, Netherlands; 3 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands; 4 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, University Utrecht, Utrecht, the Netherlands
Background: Hemophilic arthropathy (HA) caused by recurrent joint bleedings leads to major morbidity. Non‐steroidal anti‐inflammatory drugs (NSAIDs) are effective for musculoskeletal related pain and may also have anti‐inflammatory and thus joint‐protective effects. However, fear for increased bleeding risk during the use of non‐selective cyclooxygenase (COX) inhibitors and cardiovascular events in COX‐2 inhibitors restrains prescription within this population.
Aims: This systematic review aimed to give a complete overview of all publications reporting on safety of NSAIDs in patients with hemophilia (PWH).
Methods: A systematic search in PubMed, EMBASE and Cochrane till March 2021 was performed, including conference abstract from 2017 to search date. Two authors reviewed all studies independently on title/abstract and included studies reporting about adverse bleeding or cardiovascular events in PWH on NSAIDs. Articles in non‐English, in vitro/animal experiments only, expert opinions, case reports (n < 3) and articles without full‐text availability were excluded.
Results: Our search resulted in 1476 studies of which 19 studies could be included for this review. Selective COX‐2 inhibitors showed no evident risk for adverse bleeding events or increased factor use. Also, no thrombotic cardiovascular events were reported. Most of the studies reporting about traditional NSAIDs showed no statistically significant differences in hemorrhages compared to placebo. However, patients with comorbidity (e.g. hepatic decompensation, H. pylori presence) may have risk for additional bleeding episodes.
Conclusion(s): Use of NSAIDs in PWH is safe based on the current literature. However, most studies are of low quality with a high risk of bias, included specific or small patient populations or used self‐reported registrations. Causality is difficult to examine as there are several other factors that can influence bleeding tendency in PWH and follow‐up is relatively short. Future (randomized controlled trial) studies with longitudinal follow‐up in large patient populations should be performed to confirm safe use of these cheap, easily accessible and potentially joint protective drugs.
35. PB0652
35.1. Transition readiness among Dutch adolescents and young adults with haemophilia: A questionnaire study
M. Brands 1; E. Janssen2; C. Smit3; L. van Vulpen4; J. Eikenboom5; E. Beckers6; L. Hooimeijer7; M. Coppens8; S. Schols9; F. Leebeek10; M. Driessens11; F. Rosendaal12; M. Cnossen13; P. Van der Valk14; B. Laros15; J. van der Bom16; K. Fijnvandraat17; S. Gouw18
1 Emma Children’s Hospital, Amsterdam UMC location AMC, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam UMC location AMC, University of Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 4 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, University Utrecht, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 5 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 6 Department of Hematology, Maastricht University Medical Center, Maastricht University, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 7 Pediatric Hematology, Beatrix Children’s Hospital, University Medical Center Groningen, Groningen, Groningen, Netherlands; 8 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 9 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands, 10 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 11 Netherlands Hemophilia Patient Society (NVHP), Nijkerk, the Netherlands, Nijkerk, Gelderland, Netherlands, 12 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands, 13 Department of Pediatric Hematology, Sophia Children’s Hospital, Erasmus University Medical Center, Erasmus University Rotterdam, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands, 14 Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands, 15 Department of Hematology, Radboud University Medical Center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands, 16 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, the Netherlands; Center for Clinical Transfusion Research, Sanquin‐Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands, 17 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands, 18 Department of Pediatric Hematology, Emma Children’s Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Around the age of 18 years, care for patients with a chronic disease, including haemophilia, is transferred from paediatric to adult care. This phase is often associated with a decrease in treatment adherence. To improve adherence and transition readiness, transition programmes were developed. These help guide adolescents through transition in a structured, comprehensive way, focussing on increasing self‐management. The first Dutch transition programme was implemented in haemophilia care.
Aims: To evaluate how adolescents and young adults with haemophilia in the Netherlands perceived their self‐reported readiness to transfer to adult care, and to determine associated factors.
Methods: In 2019, people with haemophilia evaluated various aspects of their life using a nationwide questionnaire. Adolescents aged 12–17 years and young adults aged 18–25 years completed age‐specific questionnaires on their transition readiness and preparation. Possible determinants of readiness were assessed using validated questionnaires, including quality of life (CHO‐KLAT), self‐efficacy (HSES) and treatment adherence (VERITAS‐PRO).
Results: Data of 45 adolescents and 84 young adults with haemophilia (n = 60, 46.5% with severe haemophilia; n = 64, 49.6% using prophylaxis) were analysed. Of adolescents aged 12–14 years, 38.5% (10/26) reported to feel ready or almost ready to transition, which rose to 63.2% (12/19) for adolescents aged 15–17 years. Of young adults, only one participant (1.2%) reported he had not been ready to transition, and six (7.1%) did not know. Still, 13 young adults (15.5%) would have liked to receive more information on which professionals they would encounter in adult care, and 10 (11.9%) on healthcare costs. Prophylaxis use, joint bleeding rate and family history of haemophilia were not associated with readiness. Neither were quality of life, self‐efficacy and treatment adherence, although positive trends were identified.
Conclusion(s): Self‐reported transition readiness in adolescents and young adults with haemophilia is relatively high and increases with age. Nevertheless, several improvements were suggested to further personalize and improve transitioning.
36. VPB0692
36.1. Enhanced pharmacokinetics and improved clinical outcomes in young hemophiliac boys after the switch to BAY 81‐8973 from other standard half‐life FVIII concentrates
K. Huang 1; Y. Zhen2; G. Li2; X. Wu1; Z. Chen2; R. Wu3
1 Beijing Children's Hospital, Capital Medical University, Beijing, Beijing, China (People's Republic); 2 Beijing Children's Hospital, Beijing, Beijing, China (People's Republic); 3 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic)
Background: BAY81‐8973(Kovaltry, Bayer, US) was reported with enhanced pharmacokinetic (PK) profiles compared with some other standard half‐life (SHL) FVIII concentrates. Limited head‐to‐head comparative studies were conducted in a real‐world setting.
Aims: To make head‐to‐head comparisons of PK and clinical outcomes between Kovaltry and the other three SHL FVIII concentrates.
Methods: Forty‐seven hemophiliac boys were enrolled and divided into three groups according to their previously used FVIII concentrates (Kogenate FS, N = 22; Advate, N = 14; GreenMono, N = 11). Two separate PK tests were conducted in each patient with a five‐point assay during the study period from 6 months pre‐switch to 6 months post‐switch. FVIII levels were detected by one‐stage assay and PK profiles were calculated by Non‐Compartment Assay. Annualized bleeding rates were collected through patients’ bleeds logs during the one‐year study period.
Results: Longer half‐life time (t1/2)[Kogenate FS group: 14.36 ± 1.92 vs. 11.92 ± 2.41 h, p < 0.0001); Advate group (13.41 ± 1.44 vs. 9.67 ± 1.74 h, p < 0.0001); GreenMono group (15.07 ± 3.36 vs. 10.70 ± 1.66 h, p < 0.001)] and lower clearance [Kogenate FS group: 3.26 ± 1.06 vs. 3.92 ± 1.16 ml/kg/h, p < 0.01; Advate group: 3.70 ± 1.20 vs. 5.91 ± 2.22 ml/kg/h, p < 0.01; GreenMono group, 3.02 ± 1.24 vs. 5.07 ± 1.55 ml/kg/h, p < 0.01] were observed in Kovaltry as well as longer mean residential time (p < 0.01) and higher area under curve (p < 0.01). No statistical difference was found in in‐vivo recovery between Kovaltry and other FVIII products. Patients with higher VWF:Ag levels or blood group non‐O had a longer augment of t1/2 during the switch in all three groups. Significantly reduced annualized bleeding rates (ABR) were observed in all three groups (p < 0.05) as well as improved annualized joint bleeding rates (AJBR) and annualized spontaneous bleeding rates (ASBR) in the Advate group and GreenMono group. Despite the P‐values did not reach a statistical difference in the Kogenate FS group, the decreased mean value of AJBR (1.55 vs. 2.18) and ASBR (0.86 vs. 1.54) indicated the improvements.
Conclusion(s): Compared with Kogenate FS, Advate, and GreenMono, Kovaltry have enhanced PK profiles, which contributed to later‐observed reduced bleeding rates.


37. PB1150
37.1. Improvement in the musculoskeletal health and quality of life‐outcomes among patients with severe hemophilia A with inhibitor after 2 years of personalised physiotherapy
M. Podolak‐Dawidziak 1; J. Zawilski2; M. Bober2; E. Stefańska‐Windyga3; A. Buczma3; M. Biernat4
1 Wroclaw Medical University, Department of Haematology, Blood Neoplasms and Bone Marrow Transplantation, Wroclaw, Dolnoslaskie, Poland; 2 INTERLAB, Poznan, Wielkopolskie, Poland; 3 Institute of Hematology and Blood Transfusion, Warsaw, Mazowieckie, Poland; 4 Wroclaw Medical University, Department of Haematology, Blood Neoplasms and Bone Marrow Transplantation, Wrocław, Dolnoslaskie, Poland
Background: In people with hemophilia with inhibitors (PWHI) it is not easy to stop some bleeding episodes, therefore arthropathy occurs more often. Till now there are no recommendations for safe and effective physiotherapy in PWHI.
Aims: The aim of the study was to improve the musculoskeletal health and quality of life in PWHI due to the personalised rehabilitation.
Methods: Joint status was assessed using the Hemophilia Joint Health Score (HJHS) and Health‐Related Quality of Life (HR‐QoL) was assessed using the EQ‐5D scale (EuroQoL). PWHI participated weekly in 60–90‐min rehabilitation sessions at home, and after 6, 12, 18 and 24 months of the programme they attended four 5‐day rehabilitation camps of 5 h per day held in Poznań. Rehabilitation was conducted only in patients taking regular aPCC prophylaxis, provided that the aPCC dose was always administered 1 h before the start of the session.
Results: Twenty‐four subjects (mean age 42.1 years, median 42 years) receiving a prophylactic regimen three times a week with aPCC 85 ± 15 IU/kg were included. 14 subjects took part in the entire project, as 10 had since switched to emicizumab prophylaxis. These were aged between 28 and 69 years (mean age 47.36 years, median 45.5 years). Thus, the mean age of PWHI increased by 5.26 years and the median by 3.5 years, as mainly younger people withdrew from the project. Figure 1 shows joints mobility improvement that was confirmed in the HJHS scores. Figure 2 presents the clear positive impact of the physiotherapy programme on HRQoL.
Conclusion(s): In adult patients with severe haemophilia A with an inhibitor, regular aPCC prophylaxis and an individualised, once‐weekly rehabilitation programme were safe and led to significant improvements in both physical condition and health‐related quality of life.
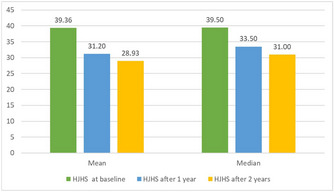

38. PB0655
38.1. Minor surgical procedures and the outcome of immunotolerance induction: An analysis of the BrazIT Study
R. Camelo 1; M. Moreira Dias2; L. de Magalhães3; L. Jardim4; A. De Oliveira5; R. Ribeiro6; V. Franco7; M. Roberti8; F. Callado9; L. Etto10; M. De Cerqueira11; M. Cerqueira12; C. Lorenzato13; I. Pinto14; É. Serafim15; A. Garcia16; T. Anegawa17; D. Neves18; D. Tan19; S. Rezende20
1 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 2 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 3 Department of Clinics, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 4 Faculty of Medicine, Universidade Federal de Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil; 5 Fundação HEMOMINAS, Belo Horizonte, Minas Gerais, Brazil; 6 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaleza, Ceara, Brazil; 7 Centro de Hematologia e Hemoterapia de Santa Catarina (HEMOSC), florianópolis, Santa Catarina, Brazil; 8 Centro de Hematologia e Hemoterapia de Goiás (HEMOGO), Goiânia, Goias, Brazil; 9 Fundação de Hematologia e Hemoterapia de Pernambuco (HEMOPE), Recife, Pernambuco, Brazil, 10 Centro de Hematologia e Hemoterapia da Paraíba (HEMOÍBA), João Pessoa, Paraiba, Brazil, 11 Centro de Hematologia e Hemoterapia do Piauí (HEMOPI), Teresina, Piaui, Brazil, 12 Instituto de Hematologia do Estado do Rio de Janeiro (HEMORIO), Rio de Janeiro, Rio de Janeiro, Brazil, 13 Hemocentro do Paraná (HEMEPAR), Curitiba, Parana, Brazil, 14 Fundação HEMOPA, Belém, Para, Brazil, 15 Centro de Hematologia e Hemoterapia do Rio Grande do Norte (HEMONORTE), Natal, Rio Grande do Norte, Brazil, 16 Centro de Sangue de São José do Rio Preto, São José do Rio Preto, Sao Paulo, Brazil, 17 Centro Regional de Hematologia e Hemoterapia de Londrina, Londrina, Parana, Brazil, 18 Fundação Hemocentro de Rondônia (FHEMERON), Porto Velho, Rondonia, Brazil, 19 Department of Pediatric Onco‐hematology, Faculdade de Medicina de Marília, Marília, Sao Paulo, Brazil, 20 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil
Background: Inhibitors against factor VIII (FVIII) are associated with increased bleeding episodes, worse musculoskeletal outcomes, lower quality of life, and higher mortality in people with hemophilia A (PwHA). Immune tolerance induction (ITI) is the treatment of choice to eradicate inhibitors and recover the hemostatic activity of FVIII. Surgeries may induce immunological imbalances, perpetuating the stimuli to inhibitor maintenance.
Aims: Herein, we performed an analysis of the Brazilian Immunotolerance (BrazIT) Study to evaluate the impact of surgical procedures during ITI on its outcome.
Methods: PwHA on ITI (n = 207) were enrolled in 15 Brazilian hemophilia treatment centers. PwHA were treated according to the Brazilian ITI Protocol, which consists of starting with a low dose of FVIII (50 international units [IU]/kg 3×/week). High‐dose ITI (100 IU/kg/day) was started if inhibitor titers did not reduce at least 20% of peak levels after 6 months. ITI outcomes were defined as (i) complete success: negative inhibitor titer (<0.6 BU/ml) and a normal FVIII pharmacokinetics (recovery ≥66% of expected values, and half‐life ≥6 h) (ii) partial success: inhibitor titer ≤2 BU/ml and/or abnormal FVIII pharmacokinetics; and (iii) failure: success criteria was not achieved after 33 months of ITI. Clinical, laboratory, and therapeutic data were collected from medical files using a standardized form.
Results: A total of 161 (77.8%) of PwHA who completed ITI provided information on surgical procedures. The overall success rate of ITI was 68.9%. Forty‐nine surgical procedures were reported for 31 (19.3%) PwHA during ITI. All the described procedures were minor surgeries. Central line insertion/removal was the most frequent (34.7%). The success rates were similar between PwHA who were submitted to surgeries during ITI (71.0%) and those who were not (68.5%; p > 0.05).
Conclusion(s): We conclude that minor surgical procedures during ITI do not alter its outcome.
39. PB0656
39.1. Comparability of bleeding outcomes by prophylactic FVIII replacement intensity: A post‐hoc analysis of a non‐interventional study of men with severe hemophilia A
C. Camp 1; C. Hawes1; D. Hinds2; S. Hawley2; D. Reddy2; V. Newman1; E. Goodman2
1 BioMarin Europe Ltd, London, United Kingdom; 2 BioMarin Pharmaceutical, Inc., Novato, California, United States
Background: Study BMN 270‐902 collected real‐world data on outcomes of a global cohort of people with severe hemophilia A (SHA) receiving regular exogenous factor VIII (FVIII) prophylaxis. Intensity of prophylactic treatment varied widely and may have impacted the findings.
Aims: To verify consistency in bleeding outcomes between those receiving high‐ and low‐intensity prophylactic FVIII replacement in study BMN 270‐902.
Methods: Post‐hoc analysis of adult males with SHA (FVIII ≤1 IU/dl) receiving prophylactic FVIII replacement enrolled in BMN 270‐902, a non‐interventional Phase 3 lead‐in study (providing 6 months of retrospective data at baseline and up to 12 months prospective data). Adeno‐associated virus serotype 5 total antibody negative participants with ≥6 months follow‐up were stratified by annualized FVIII utilization for prophylaxis per the third edition of the World Federation Hemophilia treatment guidelines (low‐dose, <4000 IU/kg/year; high‐dose, ≥4000 IU/kg/year) at baseline. Mean annualized bleeding rate (ABR, treated and all bleeds) and proportion of participants with zero bleeds (treated and all bleeds) were compared.
Results: Among 194 participants, mean (SD) baseline annualized FVIII utilization was 5499.6 (1751.4) and 2838.0 (705.7) IU/kg/year and mean annualized FVIII infusion rate was 146.7 (42.0) and 120.2 (46.0) infusions per year for high‐dose (n = 78) and low‐dose (n = 116) intensity cohorts, respectively (Table 1). Median (range) follow‐up time for bleeding outcomes was 223 (171–469) days. Throughout follow‐up, mean annualized FVIII utilization was 5236.5 (1665.9) and 3065.5 (1188.4) IU/kg/year and mean calculated ABR for treated bleeds was 5.09 (7.08) and 4.38 (6.52) in the high‐ and low‐dose cohorts, respectively; absolute difference in mean ABR for treated bleeds was 0.71 (p = 0.4761). Absolute difference in proportion of participants with zero treated bleeds was 0.7% (34.6% vs 35.3%; p = 0.7708). Similar trends were observed for all bleeds (Table 2).
Conclusion(s): Intensity of prophylaxis did not impact bleeding outcomes in study BMN 270‐902. Funded by BioMarin.

40. PB0662
40.1. Clinical study of adenovirus‐associated viral vector‐mediated gene therapy of human factor VIII in severe and moderately severe hemophilia A
G. Gonen‐Yaacovi 1; S. Pipe2; K. Stine3; G. Kenet4; A. von Drygalski5; O. Segurado1
1 ASC Therapeutics, Milpitas, California, United States; 2 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States; 3 ARKANSAS CHILDREN’S HOSPITA, Arkansas, Arkansas, United States; 4 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 5 Division of Hematology/Oncology, Department of Medicine, University of California San Diego, San Diego, California, United States
Background: Hemophilia A is a rare congenital, monogenic, X‐linked bleeding disorder caused by mutations in the coagulation factor VIII (fVIII) gene. Standard of care for hemophilia A includes prophylactic fVIII replacement therapy primarily using plasma‐based or recombinant fVIII concentrates as an intravenous (IV) bolus or as treatment for bleeding episodes. Despite treatments available, patients may experience progressive joint disease due to repeated hemarthroses as well as the development of fVIII inhibitors, that render replacement therapy ineffective. As hemophilia has a well‐known genetic background, it became an ideal target for gene therapy.
Aims: ASC Therapeutics has developed ASC618, a highly potent and minimally sized vector containing a hepatic combinatorial bundle promoter, liver‐specific codon optimization, and a highly expressing bioengineered human fVIII (ET3) transgene, designed to express fVIII protein for the treatment of patients with severe and moderately severe hemophilia A, potentially providing a long‐term solution or even functional cure for people living with this disease (Pipe, 2021; https://www.tandfonline.com/doi/full/10.1080/14712598.2022.2002842).
Methods: In the prospective clinical study (NCT04676048; https://www.asctherapeutics.com/en/company-news/asc-therapeutics-receives-ind-clearance-from-the-u-s-food-and-drug-administration-for-asc618-second-generation-gene-therapy-for-hemophilia-a/), the safety, tolerability and preliminary efficacy of ASC618 will be assessed (see Figure 1). The decision of dose escalation versus dose expansion in each cohort will be based on fVIII levels and safety evaluations such as Liver Function Tests, enzyme‐linked immunospot (ELISpot), bleeding episodes and AEs. Following the infusion, patients will be closely monitored for fVIII levels and ALT/AST for up to 5 years post‐infusion.
Results: Preclinical work demonstrated that in the C57Bl/6 murine model, the cynomolgus monkey, and the humanized liver mouse model (FRG‐KO), ASC618 was well tolerated at all doses evaluated with no toxicologically significant microscopic findings (Veselinovic 2020).
Conclusion(s): ASC618 will be evaluated in this clinical program for its potential to provide durable therapeutic benefit, reduce treatment burden on patients and significantly improve their quality of life.

41. PB0674
41.1. Annualized bleed rates in severe hemophilia A after switch to emicizumab based on CHESS II data, including an oversample of emicizumab patients
M. Mancuso 1; F. Nissen2; H. Zhang2; E. Ferri‐Grazzi3; J. O’Hara4; R. Ofori‐Asenso5; K. Moreno2; T. Burke4
1 IRCCS Humanitas Research Hospital, Rozzano, Milan, Lombardia, Italy; 2 F. Hoffmann‐La Roche Ltd., Basel, Basel‐Stadt, Switzerland; 3 HCD Economics, Daresbury, United Kingdom; 4 HCD Economics and Faculty of Health and Social Care, University of Chester, Daresbury, United Kingdom; 5 Roche Products Ltd., Welwyn Garden City, United Kingdom
Background: Hemophilia A, a bleeding disorder characterized by deficiency of factor (F)VIII, is associated with substantial morbidity. Emicizumab, a bispecific monoclonal antibody that substitutes for deficient activated FVIII, has proven efficacious for bleeding prevention in clinical trials in people with hemophilia A (PwHA) with and without FVIII inhibitors.
Aims: Here, we examine the effectiveness of emicizumab in a real‐world setting.
Methods: This analysis utilizes data on all bleeds from Cost of Haemophilia in Europe: a Socioeconomic Survey II (CHESS II), a retrospective, burden‐of‐illness questionnaire‐based study, including the base study and an increased selection (oversample) of patients who initiated emicizumab. Safety data were not captured. Informed consent and ethics approval were obtained.
Results: Overall, 146 PwHA with severe disease (Italy n = 81; Germany n = 26; Spain n = 26; France n = 10; UK n = 3), 22 from the base study and 124 from the oversample, were included in the analysis. Of these, 78 adults received emicizumab for ≥12 months, including 49 (62.8%) previously treated with FVIII prophylaxis, 28 (35.9%) treated on demand, and 1 who previously received gene therapy; 22/78 (28.2%) had either a history of, or current, FVIII inhibitors (Table 1). Mean all‐bleeds annualized bleed rate (ABR) in the 78 participants decreased from 3.37 at last treatment pre‐emicizumab to 1.42 post‐emicizumab. Median ABR decreased from 3.00 to 1.19. In the 49 participants previously on prophylaxis, mean ABR decreased from 3.49 to 1.40 after switching to emicizumab (Figure 1). Median ABR decreased from 3.00 to 1.09. A sensitivity analysis including 123 PwHA who received emicizumab for ≥6 months showed a decrease in mean ABR from 4.33 pre‐emicizumab to 1.91 post‐emicizumab; median ABR decreased from 3.00 to 1.33 (all p < 0.001).
Conclusion(s): ABRs decreased in all groups after switching to emicizumab based on physician‐reported data from the CHESS II study, both in PwHA previously on FVIII prophylaxis and those previously treated on demand.


42. VPB0693
42.1. First year report of the multicenter prospective cohort study on ischemic events in adult patients with hemophilia (ADVANCE Japan)
A. Nagao 1; Y. Chikasawa2; A. Sawada3; T. Kanematsu4; N. Yamasaki5; H. Takedani6; M. Nojima6; T. Fujii5; N. Suzuki4; T. Matsushita7; S. Higasa8; K. Amano9; T. Suzuki10
1 Ogikubo hospital, Tokyo, Tokyo, Japan; 2 Tokyo Medical University, Tokyo, Tokyo, Japan; 3 Hyogo College of Medicine, Hyogo, Hyogo, Japan; 4 Nagoya University Hospital, Nagoya, Aichi, Japan; 5 Hiroshima University hospital, Hiroshima, Hiroshima, Japan; 6 IMSUT Hospital, The Institute of Medical Science, Tokyo, Tokyo, Japan; 7 Department of Transfusion Medicine, Nagoya University Hospital, Nagoya, Aichi, Japan; 8 Hyogo College of Medicine Hospital, Nishinomiya, Hyogo, Japan; 9 Tokyo Medical University Hospital, Tokyo, Tokyo, Japan, 10 ogikubo hospital, tokyo, Tokyo, Japan
Background: Although advancements in treatment have extended the life expectancy of patients with hemophilia, age‐related complications, including cardiovascular disease (CVD), have become a rising concern. In 2016, we conducted a retrospective study on 711 patients with hemophilia and observed that only 2 patients experienced myocardial infarction.
Aims: We initiated a multicenter prospective cohort study in 2019 for investigating the incidence and treatment status of CVD, lifestyle‐related diseases, malignancies, and other parameters in Japanese patients with hemophilia (Figure 1). The preliminary data obtained from the first year of the study are presented herein.
Methods: Patients with congenital hemophilia A/B, aged ≥40 years, will be included in the study, which aims to enroll 600 patients, to be followed‐up for 10 years.
Results: As of December 2019, 286 patients with hemophilia were enrolled from 13 centers. Their median age was 51.0 years (range 40–89), and 86.0% and 14.0% of patients had hemophilia A and B, respectively. Breakdown of disease severity revealed that 69.2%, 13.6%, and 17.1% of patients had severe, moderate, and mild disease, respectively, and 74.8% of patients received prophylaxis. Past medical history revealed that 4 patients experienced cerebral infarction. Analysis of the ischemic events in 2019 revealed 1, 1, and 2 cases of angina pectoris, transient ischemic attack, and atherosclerosis obliterans, respectively. Three patients were on antiplatelet therapy. The prevalence of comorbidities and risk factors is depicted in Fig 2. The median QRISK3 and Suita scores were 7.1 (interquartile range [IQR]: 4.2–13.8) and 42 (IQR: 35–46), respectively.
Conclusion(s): Four cases with CVD complications were registered in the first preliminary report. Based on QRISK3 and Suita scores during registration, there is a concern about the possible development of CVD within 10 years, which highlights the importance of continual registration and analyses of such cases.


43. VPB0694
43.1. Analysis of emicizumab discontinuation in 10 patients with hemophilia A
A. Nagao 1; T. Yamaguchi2; M. Bingo3; K. Fukutake1
1 Ogikubo hospital, Tokyo, Tokyo, Japan; 2 Tokyo Medical University, Shinjukuku, Tokyo, Japan; 3 Tokyo Medical University, Tokyo, Tokyo, Japan
Background: The number of patients receiving emicizumab subcutaneous injections with longer dosing intervals is rapidly increasing. Although emicizumab efficacy is widely reported, little is known about discontinuation by patients in real‐world settings.
Aims: To evaluate factors contributing to emicizumab discontinuation and promoting its safe use.
Methods: We collected data from medical records of patients administered emicizumab at our hospital until the analysis in September 2021.
Results: Of 64 patients (including 9 inhibitors), 10 discontinued emicizumab (median, 23 months until discontinuation). The mean age of these 10 was 7–69 years (table); all were non‐inhibitors. Thereafter, only non‐inhibitors were examined because the bleeding nature is different between inhibitors and non‐inhibitors. The severity was severe (43 continued, 8 discontinued), moderate (2 continued, 1 discontinued), and mild (1 discontinued). Adherence to prophylaxis before emicizumab (continuous group [mean 89% ± 16], discontinued group [mean 94% ± 17]) and change in the annual bleeding rate during emicizumab are shown in Fig. No patient in the discontinued group achieved zero bleeding. Reasons for discontinuation included poor bleeding control (5 patients), possible side effects (2 patients), prolonged dosing intervals due to poor understanding of the treatment (1 patient), and concerns about medical costs (1 patient). Four patients with poor bleeding control experienced frequent joint pain and bleeding sensation, and hemorrhagic synovitis was detected using joint ultrasound. Emicizumab blood levels were measured in several patients; none had low emicizumab levels. Six patients initiated prophylaxis with standard half‐life products (SHLs), two with extended half‐life products (EHLs), and two with SHLs before switching to EHLs.
Conclusion(s): Patients who discontinued emicizumab had experienced more bleeding before and during emicizumab use. It is important to educate patients before switching to alternate treatments.

44. PB1138
44.1. Obesity, fibrinolysis, and bleeding in patients with non‐severe hemophilia
O. Königsbrügge 1; J. Rejtö2; D. Kraemmer2; G. Schuster3; C. Feistritzer4; J. Gebhart1; C. Ay5; I. Pabinger5
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Medical University of Vienna, Vienna, Wien, Austria; 3 Upper Austrian Red Cross, Linz, Oberosterreich, Austria; 4 Internal Medicine V ‐ Hematology and Oncology, Medical University of Innsbruck, Vienna, Wien, Austria; 5 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Obesity leads to increased strain on muscles and joints and could be a contributing factor to the burden of disease in patients with non‐severe hemophilia. Fatty tissue, however, also plays an active part in the production of tissue‐type plasminogen activator (t‐PA) and plasminogen activator inhibitor 1 (PAI‐1) and therefore balance of fibrinolysis.
Aims: We aimed to investigate the effects of obesity on the frequency of bleeding and fibrinolysis in hemophilia.
Methods: Persons with non‐severe hemophilia A or B (PwH) were recruited into a cross‐sectional cohort study after informed consent. Frequency of bleeding in the past 5 years was retrospectively recorded. Body‐mass index (BMI), FVIII or IX activity, t‐PA antigen and activity, and PAI‐1 activity were measured.
Results: The cohort includes 90 PwH (74 hemophilia A, 16 hemophilia B) with median age 49.6 years (25th to 75th percentile 34.5–59.9), and median FVIII/FIX level 12% (8–19%). The median BMI was 25.7 kg/m2 (23.3–28.3), and 14 patients (15.5%) had BMI >30. The median number of bleeding episodes in the past 5 years was 0 (0–1). In age‐adjusted negative binomial regression, factor VIII/IX level (incidence rate ratio [IRR] 0.93 per 1% increase, 95% confidence interval [CI] 0.88–0.98, p < 0.001), and BMI (IRR 0.82 per 1 kg/m2 increase, 95% CI 0.74–0.91, p < 0.001) were independently associated with frequency of bleeding episodes. Factor VIII/IX levels (IRR 0.88, 95% CI 0.80–0.96, p = 0.005) and BMI (IRR 0.68, 95% CI 0.55–0.82) were also independently associated with the frequency of joint and muscle bleeding. BMI correlated with PAI and t‐PA antigen levels (rho 0.241, p = 0.025 and 0.257, p = 0.017) and correlated inversely with t‐PA activity (rho −0.240, p = 0.026).
Conclusion(s): Increasing BMI decreased the number of bleeding episodes and joint and muscle specific bleeding episodes in persons with non‐severe hemophilia. The bleeding severity was potentially mitigated by increasing PAI levels and decreasing t‐PA activity.
45. PB1154
45.1. Impact of emicizumab on anticoagulant drugs at therapeutic concentrations using thrombin generation assay
B. Leroy1; B. Gillet1; M. Brionne Francois1; Y. Repesse 2
1 Caen University Hospital, Haematology Laboratory, Caen, Basse‐Normandie, France; 2 Caen University Hospital, CAEN, Basse‐Normandie, France
Background: Emicizumab is a non‐substitutive prophylactic treatment approved for patients with severe haemophilia A (HA). Emicizumab is a bispecific monoclonal antibody mimicking the activity of factor VIII (FVIII) that binds to factors IXa and factor X. Studies have shown that emicizumab can also bind to factor Xa. Under particular clinical situations, even in patients with HA, an anticoagulant therapy is needed.
Aims: The potential interference of emicizumab on the anticoagulant effect of therapeutic drugs is poorly evaluated.
Methods: FVIII‐deficient plasma (Siemens®) and a pool of normal plasma (PNP) were used to assess the thrombin generation in the presence of anticoagulant drugs (UFH, LMWH, fondaparinux, rivaroxaban, apixaban, dabigatran) at therapeutic doses alone or in presence of emicizumab. Thrombin generation was evaluated on the ST‐Genesia (Stago®) with the STG‐BleedScreen reagent (Stago®). The anticoagulant effect of heparins or DOACs in the absence or presence of emicizumab (50 μg/ml) was assessed using the inhibition of ETP and the peak‐thrombin and the increase of lag‐time.
Results: Addition of emicizumab (50 μg/ml) to FVIII‐deficient plasma significantly increased thrombin generation parameters (equivalent to a plasma containing 0.1 to 0.2 IU/ml of FVIII). Under our experimental conditions, the different anticoagulant‐drug tested (UFH 0.3–0.6 IU/ml, LMWH 0.5–1.5 IU/ml, DOACs 20 to 500 ng/ml) significantly inhibited thrombin generation. The addition of emicizumab (50 μg/ml) did not alter the anticoagulant action of tested drugs. The inhibition of ETP and peak‐thrombin and the increase of lag‐time were not significantly different in presence of emicizumab.
Conclusion(s): To our knowledge, this is the first study evaluating the interference of emicizumab on the action of different anticoagulant drugs including heparins and DOACs. These results suggest the absence of impact of emicizumab, at a therapeutic dose, on the anticoagulant effect of heparins and DOACs. These data need to be confirmed in samples from severe HA A patients treated with emicizumab.
46. PB1157
46.1. Molecular analysis of inherited bleeding disorders in North Indian cohort
R. Sharma 1; M. Jamwal1; H. Senee1; C. Hans2; D. Bansal3; A. Trehan3; P. Malhotra1; N. Kumar2; J. Ahluwalia4; R. Das1
1 PGIMER Chandigarh, Chandigarh, Chandigarh, India; 2 Post Graduate Institute of Medical Education and Research, Chandigarh, India, Chandigarh, Chandigarh, India; 3 Postgraduate Institute of Medical Education and Research, Chandigarh, India, Chandigarh, Chandigarh, India; 4 Postgraduate Institute of Medical Education and Research, Chandigarh, India., Chandigarh, Chandigarh, India
Background: Inherited bleeding disorders (IBDs), a heterogeneous group of disorders characterized by lifelong bleeding episodes. Diagnosis includes assessment of bleeding phenotype, laboratory investigations followed by genetic analysis.
Aims: We aimed to determine the genetic spectrum of IBDs using various DNA‐based techniques.
Methods: A total of 588 families (635 cases) of IBDs were recruited, including 400 Hemophilia A (HA), 90 Hemophilia B (HB), 40 von Willebrand Disease (VWD), 35 FVII deficiency, 16 FXIII deficiency, and 7 Glanzmann Thrombasthenia (GT) based on laboratory investigations. Genetic workup for HA included intron 22 (INV22) and intron 1 inversion (INV1), conformation sensitive gel electrophoresis (CSGE) followed by targeted Next‐generation sequencing (NGS). Molecular characterization of HB cases was done using Sanger sequencing. HaloPlexHS Target Enrichment System for Illumina and AmpliSeq Custom Panel for Illumina were used for library preparation. SureCall, Local Run Manager, and Variant Interpreter were used for data analysis. Pathogenicity of shortlisted variants was predicted and validated by Sanger sequencing.
Results: In HA cases, 35.5% had INV22, INV1 was in 2.3%, and in 5.2% cases, large deletions were found. The molecular characterization using CSGE, targeted NGS, and Sanger sequencing in 153 HA cases revealed probably pathogenic variants in 73.8%. Targeted NGS detected the variants in 87.5% VWD, 68.8% of FXIII deficiency, 74.3% FVII deficiency, 100% in GT and HB (using Sanger sequencing). Hot spots of genetic variants were found in F8, F9, F7, F13A1, and VWF genes. Overall 54% missense, 20.7% frameshift, 15.3% nonsense and 9.5% was splice site variants. Fifty‐six variants were novel.
Conclusion(s): This is the largest cohort of IBDs diagnosed using targeted NGS from north India. Multiple DNA‐based techniques were able to determine pathogenic variants in 74.5% of cases. Hot spot variants can be screened as the first step for genetic diagnosis. This molecular analysis has helped us in genetic counseling, carrier screening, and prenatal testing.
47. PB1163
47.1. Real‐world unmet needs of patients with haemophilia A/B with or without inhibitors: Historical haemophilia characteristics from patients entering a non‐interventional study
J. Windyga 1; H. Tran2; T. Fujii3; C. Lyu4; L. Villarreal Martinez5; J. Sathar6; O. Stasyshyn7; N. Zozulya8; R. Brown Frandsen9; C. Eskelund10; S. Apte11; J. Mahlangu12
1 Institute of Hematology and Transfusion Medicine, Warsaw, Mazowieckie, Poland; 2 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia; 3 Hiroshima University hospital, Hiroshima, Hiroshima, Japan; 4 Department of Pediatrics, Yonsei University College of Medicine, seoul, Seoul‐t'ukpyolsi, Republic of Korea; 5 Hospital Universitario Dr. José Eleuterio González, Monterrey, Nuevo Leon, Mexico; 6 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Ampang Jaya, Selangor, Malaysia; 7 Institute of Blood Pathology and Transfusion Medicine, Lviv, Ukraine, Lviv, L'vivs'ka Oblast', Ukraine; 8 National Research Center for Hematology, Moscow, Moskva, Russia; 9 Novo Nordisk A/S, Søborg, Denmark, Søborg, Hovedstaden, Denmark, 10 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark, 11 Sahyadri Speciality Hospital, Pune, India, Pune, Maharashtra, India, 12 University of the Witwatersrand and NHLS, Johannesburg, South Africa
Background: Many patients with haemophilia A (HA) and B (HB) continue to have unmet needs in disease management and treatment burden. Real‐world evidence may help to further describe these unmet needs, ultimately improving haemophilia management.
Aims: To describe real‐world unmet medical needs of patients with HA/HB with or without inhibitors using historical data.
Methods: Historical data were collected from patients entering the non‐interventional study, explorer6 (NCT03741881). Male patients ≥12 years old with severe HA (FVIII activity <1%), severe/moderate HB (FIX activity ≤2%) or HA/HB with inhibitors of any severity were included. Patients with previous/current concizumab or emicizumab treatment were excluded. Each patient was treated according their country’s standard‐of‐care (SoC). Historical data including demographics and treatment regimen were collected, analysed and described.
Results: In total, data from 231 patients (Figure 1) from 109 centres were collected. Median age was 28 years (range, 12–78 years). For the prophylaxis (PPX) group, the mean (median) annualised bleeding rates (ABR) for patients without inhibitors were HA 6.6 (2.0), HB 5.4 (2.0); and for patients with inhibitors HA 22.0 (9.0), HB 18.0 (10.0). For the episodic treatment group, the mean (median) ABRs for patients without inhibitors were HA 30.3 (24.0), HB 19.3 (12.0); and for patients with inhibitors HA 25.2 (14.0), HB 17.9 (18.0) (Table 1). At baseline, 65.6% of patients on episodic and 34.1% of patients on PPX had ≥1 target joint.
Conclusion(s): Here we present data from >30 countries, describing the historic haemophilia characteristics of patients with HA/HB, with or without inhibitors. Many patients had a high ABR and target joints despite receiving their country’s SoC treatment (episodic or PPX). This reflects a high unmet medical need, particularly for those receiving episodic treatment or those with inhibitors. Emerging therapy options could help improve health outcomes in these patients.

48. PB1171
48.1. Comprehensive genotype and phenotype characterization in non‐severe Hemophilia A patients on assay discrepancies
R. Sueldo 1; L. Rossetti2; B. Ziegler3; V. Marchione4; P. Radic5; M. Abelleyro6; C. De Brasi7; A. Baques8; M. Arias9
1 Laboratorio de Hematología. Hospital Dr. César Milstein, Hospital de Pediatría Juan P. Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Instituto de Medina Experimental (IMEX)‐CONICET‐ Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Instituto de Medicina Experimental (IMEX)‐CONICET‐Academia Nacional de Medicina (ANM), Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 6 Instituto de Medicina Experimental (IMEX)‐CONICET/Academia Nacional de Medicina (ANM), Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 7 Instituto de Medicina Experimental (IMEX)‐CONICET‐Academia Nacional de Medicina (ANM)/Instituto de Investigaciones Hematológicas (IIHEMA)‐ANM, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 8 Hospital Dr. César Milstein, Buenos Aires, Alabama, United States; 9 Hospital Dr. César Milstein, CABA, Ciudad Autonoma de Buenos Aires, Argentina
Background: One‐third of patients with non‐severe Hemophilia A (nsHA) presents a discrepancy between the results of FVIII activity (FVIII:C) measured by one‐stage‐assay (OSA) and chromogenic‐substrate‐assays (CSA). It is observed that this situation can lead to misunderstanding the diagnostic in Hemophilia A (HA) or even worst to get a wrong classification regarding the severity of the disease.
Aims: Consider the impact of the discrepancies between OSA and CSA, to face the diagnostic and classification of Patients with Hemophilia and also evaluate the F8‐genotype and phenotype.
Methods: 111 patients having bleeding disorders with a diagnostic of HA between 2012 and 2021 were evaluated. We performed laboratory hemostasis studies in all samples (Prothrombin time, Activated Partial thromboplastin time (aPTT), Thrombin Time (TT), aPTT 1:1 mix, OSA, CSA, vWF:Ag, vWF:CoR, Siemens Healthineers; Sysmex CS‐2500). The Initial classification of the disease was performed with OSA (Severe Hemophilia A: sHA <0.01 IU/ml, Moderate Hemophilia A: modHA 0.01–0.05 IU/ml, Mild Hemophilia A: miHA 0.05–0.40 IU/ml). FVIII discrepancy was defined as OSA/CSA ≤0.6 or CSA/OSA ≥ 1.5, correlating it with the clinical phenotype. Genotyping: PCR‐amplification method followed by conformation sensitive gel electrophoresis (CSGE) and Sanger sequencing.
Results: 81 patients were initially classified as sHA, and 30 patients were nsHA. Six patients with nsHA showed discrepancies between the methods, F8‐genotype was analyzed in the 6 cases. A comprehensive analysis of the correlation of genotype and phenotype is showed in Table 1 and Figure 1.
Conclusion(s): 20% of patients with nsHA showed discrepancies. No discrepancies were found in patients with sHA. We conclude that non‐severe haemophilia patients could benefit if chromogenic assay is included in diagnosing and classification of severity haemophilia, and it also could help to individualize group of patients that could receive early replacement treatment.


49. PB0671
49.1. Analysis of F8 genotypes and pharmacodynamic and safety biomarkers in people with moderate or mild hemophilia A receiving emicizumab in the HAVEN 6 trial
A. Kiialainen 1; C. Negrier2; S. Pipe3; B. Beckermann1; O. Catalani1; C. Schmitt1; C. Hermans4; J. Oldenburg5; M. Lehle1
1 F. Hoffmann‐La Roche Ltd., Basel, Basel‐Stadt, Switzerland; 2 Louis Pradel University Hospital, Claude Bernard University Lyon 1, Lyon, Auvergne, France; 3 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States; 4 Cliniques Universitaires Saint‐Luc, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 5 University Clinic Bonn, Institute of Experimental Haematology and Transfusion Medicine, Bonn, Germany, Bonn, Berlin, Germany
Background: Emicizumab, a bispecific antibody that substitutes for missing or deficient activated factor (F)VIII, demonstrated a favorable safety and efficacy profile in people with moderate/mild hemophilia A (HA) in the HAVEN 6 trial (Negrier, et al. Blood 2021).
Aims: To present: a F8 genetic analysis of people with moderate/mild HA; pharmacodynamics (PD) of emicizumab; and exploratory safety biomarkers in people receiving emicizumab, in HAVEN 6 (NCT04158648).
Methods: Informed consent and ethics committee approval were obtained. F8 coding regions and exon/intron boundaries were sequenced (Sanger sequencing) and rearrangements analyzed using inverse‐shifting PCR. Variants were mapped against the EAHAD F8 database, ClinVar, and gnomAD. For PD, a chromogenic FVIII activity assay containing human factors, two thrombin generation (TG) assays (triggered by FXIa and tissue factor [TF]), and activated partial thromboplastin time (aPTT) were used. Endogenous FVIII was measured using a chromogenic FVIII activity assay containing bovine factors, and FVIII antigen concentration using ELISA. Plasma levels of the safety biomarkers fibrinogen, prothrombin fragment 1.2, and D‐dimer were determined.
Results: F8 genetic analysis was conducted in 67 participants. Overall, one rearrangement and 64 unique short variants were identified: 13 benign, 42 likely pathogenic/unclassified, and nine novel. After excluding benign variants, most participants (81%) had one missense F8 variant. PD and safety biomarkers were assessed in 72 participants throughout emicizumab exposure (median 54.3 weeks; data cut‐off 30 October 2021). PD markers demonstrated emicizumab activity in addition to endogenous FVIII. Mean FVIII‐like activity and TG peak height increased during loading and were maintained throughout exposure, but were below normal ranges. aPTT was normalized after first emicizumab dose. Endogenous FVIII activity, antigen levels, and safety biomarker concentrations were unaffected by emicizumab.
Conclusion(s): Identified F8 mutations were consistent with mutations in people with moderate/mild HA. Emicizumab improved coagulation parameters without showing abnormal signs of interaction with endogenous FVIII or affecting safety biomarkers.
50. VPB1165
50.1. IDEAL study: A real‐world assessment of pattern of use and clinical outcomes with recombinant factor IX albumin fusion protein (rIX‐FP) in patients with haemophilia B in Italy
G. Castaman 1; A. Tagliaferri2; A. Molinari3; F. Peyvandi4; A. Coppola5; A. Finardi6; I. Schiavetti7; D. Vaccari8; A. Rocino9
1 “Careggi” University Hospital, Hemorrhagic and Coagulation Disease Unit, Department of Oncology., Florence, Toscana, Italy; 2 Regional Reference Center for Inherited Bleeding Disorders, University Hospital of Parma, Parma, Emilia‐Romagna, Italy; 3 Regional Reference Center for Hemorrhagic Diseases, G. Gaslini Children's Hospital, Genova, Liguria, Italy; 4 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi, Hemophilia and Thrombosis Center, and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milano, Lombardia, Italy; 5 Regional Reference Centre for Inherited Bleeding Disorders, University Hospital of Parma, Parma, Emilia‐Romagna, Italy; 6 CSL Behring, Milano, Lombardia, Italy; 7 Department of Health Sciences, University of Genoa, Genova, Liguria, Italy; 8 Hippocrates Research, Genova, Liguria, Italy; 9 Ospedale del Mare Hospital, Napoli, Campania, Italy
Background: Factor IX replacement therapy is used for treatment and prophylaxis of bleeding in hemophilia B. rIX‐FP is a long‐acting albumin‐fusion protein, which, in clinical studies, has demonstrated prolonged dosing intervals up to 21 days for routine prophylaxis, providing therapeutic benefit.
Aims: To describe dosing frequency and consumption (primary endpoint), efficacy and safety of rIX‐FP treatment during routine clinical practice in Italy.
Methods: Patients with moderate/severe hemophilia B on prophylaxis with rIX‐FP for ≥6 months, providing written informed consent, were enrolled in this observational study from March 2017 to February 2019 and followed‐up for two years. Descriptive analysis included prospective and retrospective data (12 months prior to switching to rIX‐FP). The study was approved by competent Ethics Committees.
Results: Data were collected from 59 male patients (median age 30.1 years) enrolled by 23 Italian centers. Of them, 50 were on prophylaxis during the entire observation period and completed the study. The infusion frequency changed from every 2–3 times/week in 86.0% of patients with previous treatment, to less than once a week in 84.0% of patients treated with rIX‐FP at the second‐year follow‐up. Annual number of infusions decreased of about 70%, however, mean FIX activity trough level increased from 3.8% to 14.4% (Table 1). Median Annualized Bleeding Rate of 0.00 was achieved across all prophylaxis regimens (Table 2). Subjects with zero bleedings increased with rIX‐FP from 66.0 % to 78.0%. In the 2‐year follow‐up, the rate of patients with chronic joint pain decreased from 44.0% at baseline to 16.0%, while that of patients with target joints from 30.0% to 8.0%. No safety concerns were identified, no patient developed inhibitors.
Conclusion(s): Treatment with rIX‐FP reduced infusion frequency, while providing higher FIX trough levels, reduction of target joints and chronic pain, with a good safety profile.


51. PB0688
51.1. Effect of PK assessment on clinical outcomes for patients with moderate to severe hemophilia A
G. Young 1; M. Callaghan2; V. Balasa3; A. Soni4; S. Ahuja5; J. Roberts6; M. Simpson7; A. Frick8; A. Mokdad9; S. Xing10; J. Caicedo10
1 Hemostasis and Thrombosis Center, Cancer and Blood Diseases Institute, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, California, United States; 2 Children’s Hospital of Michigan, Detroit, MI, USA; 3 Valley Children's Healthcare, Madera, CA, USA, Madera, California, United States; 4 Center for Inherited Blood Disorders, Orange, CA, USA, Orange, California, United States; 5 Rainbow Babies and Children's Hospital, Cleveland, OH, USA; 6 Bleeding and Clotting Disorders Institute, Peoria, IL, USA, Peoria, Illinois, United States; 7 Rush University Medical Center, Chicago, IL, USA; 8 Trio Health Inc., Louisville, CO, USA, Sudbury, Massachusetts, United States, 10 Takeda Development Center Americas, Inc., Cambridge, MA, United States
Background: Individualized pharmacokinetic (PK)‐guided prophylaxis has been shown to improve outcomes in patients with hemophilia A (PWHA) in clinical trials; however, PK‐guided testing in real‐world settings is poorly understood.
Aims: This retrospective chart review examined clinical outcomes for patients before and after PK testing in US hemophilia treatment centers (HTC).
Methods: This study included 132 male PWHA from 7 HTCs with moderate or severe disease, ≥3 months prior FVIII prophylaxis and up to 12 months FVIII prophylaxis after PK‐guided prescription change (index date), no inhibitors prior to index date, and no concurrent participation in clinical trials. Change in mean annualized bleed rate (ΔABR) and confidence intervals (CIs) were calculated using patient bleed logs.
Results: Of the 132 patients (age: median = 13, range: 2–43; severe disease: 93.2%), 58 (44%) remained on the same FVIII (non‐switchers) and 74 (56%) switched FVIII therapy (switchers) after PK assessment. The majority (95%) of switchers had transitioned from a standard half‐life (SHL) to extended half‐life (EHL) product. ABR decreased for the entire cohort post‐index [−0.97 (95% CI: −1.54, −0.42)], driven by a decrease in joint bleeds [−0.77 (−1.23, −0.34)]. Decrease in ABR was larger among non‐switchers [−1.93 (−3.14, −0.78)], similarly driven by a decrease in joint bleeds [−1.43 (−2.45, −0.54)]. Although switchers did not have a significant decrease in ABR [−0.35 (−0.78, 0.06)], switchers had lower ABRs before PK‐guided prophylaxis vs non‐switchers (1.23 vs 3.85, respectively) and maintained lower ABRs after index date (0.88 vs 1.93).
Conclusion(s): Individualized pharmacokinetic‐guided prophylaxis was associated with an overall reduction in ABR in both FVIII switchers and non‐switchers, with results more pronounced among non‐switchers. Dose adjustments based on PK testing could be a useful strategy for optimizing treatment for those remaining on same SHL or EHL therapy and maintaining proper trough levels in patients switching from SHL to EHL.


52. PB0657
52.1. Up to 6 years of once‐weekly prophylactic dosing of nonacog beta pegol (N9‐GP) was associated with high adherence, with a low incidence of spontaneous bleeds in children with hemophilia B
A. Chan 1; C. Barnes2; A. Chuansumrit3; P. Nielsen4; H. Holst4; G. Young5
1 McMaster University, Hamilton, Ontario, Canada; 2 The Royal Children’s Hospital, Melbourne, Victoria, Australia; 3 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Nakhon Pathom, Thailand; 4 Novo Nordisk A/S, Søborg, Hovedstaden, Denmark; 5 Hemostasis and Thrombosis Center, Cancer and Blood Diseases Institute, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, California, United States
Background: Nonacog beta pegol (N9‐GP) is a recombinant factor IX (FIX) with an extended half‐life that minimizes treatment burden by reducing dosing frequency while maintaining high FIX trough levels with once‐weekly dosing.
Aims: To report updated data from the ongoing main and extension phases of paradigm6 (NCT02141074), an open‐label phase III trial evaluating N9‐GP prophylaxis in previously untreated children with hemophilia B.
Methods: The study included previously untreated males aged < 6 years with hemophilia B (FIX activity level ≤2%); up to three exposure days (EDs) to FIX‐containing products prior to enrollment. Patients received N9‐GP 40 IU/kg once weekly (prophylaxis) with an optional on‐demand or slow start‐up of prophylaxis up to 24 months of age or 20 EDs, whichever came first (pre‐prophylaxis). Key treatment outcomes were incidence of anti‐FIX inhibitory antibodies (≥0.6 Bethesda units), annualized bleeding rates (ABRs), N9‐GP consumption, hemostatic response for treatment of bleeds, FIX trough levels, and patient adherence (defined as percentage of all administered prophylactic doses taken within 6–8 days of previous prophylactic dose).
Results: Figure 1 shows numbers of patients at each trial stage. The inhibitor incidence rate was 8%. Adherence to N9‐GP prophylaxis was high (96.1%). The overall hemostatic success rate was high (96.4%). Table 1 summarizes further key efficacy outcomes for patients on N9‐GP prophylaxis. Notably, both overall and spontaneous ABRs were low (medians of 0.25 and 0.00, respectively), and most bleeds (88.3%) required only one injection. Furthermore, mean steady‐state FIX trough levels (15.6 IU/dl) were within the range of mild hemophilia; no patients developed target joints.
Conclusion(s): This updated analysis provides additional data on the long‐term (up to 6.11 years) outcomes following N9‐GP prophylaxis. The low inhibitor incidence, favorable ABRs for prophylactic treatment, no target joint development, and high adherence demonstrate the continued efficacy of once‐weekly N9‐GP in children with hemophilia B.


53. PB0670
53.1. Bleed outcomes in the moderate and mild hemophilia A population without prophylactic treatment in CHESS II and CHESS PAEDs
K. Khair 1; F. Nissen2; E. Ferri‐Grazzi3; J. O’Hara4; R. Ofori‐Asenso5; M. Aizenas2; H. Zhang2; K. Moreno2; T. Burke4; B. Nolan6
1 Haemnet, London, England, United Kingdom; 2 F. Hoffmann‐La Roche Ltd., Basel, Basel‐Stadt, Switzerland; 3 HCD Economics, Daresbury, England, United Kingdom; 4 HCD Economics and Faculty of Health and Social Care, University of Chester, Daresbury, England, United Kingdom; 5 Roche Products Ltd., Welwyn Garden City, England, United Kingdom; 6 Children's Health Ireland at Crumlin, Dublin, Dublin, Ireland
Background: Hemophilia A (HA) is a congenital bleeding disorder caused by a deficiency in clotting factor (F)VIII and can cause uncontrolled bleeding and musculoskeletal dysfunction. Published data on the unmet clinical need in moderate and mild HA are limited.
Aims: To assess bleed outcomes in people with moderate or mild HA.
Methods: CHESS II and CHESS PAEDs are retrospective, burden‐of‐illness studies. CHESS II was conducted in adults (≥18 years), with hemophilia of any severity, across eight European countries. CHESS PAEDs focused on children (0–17 years), with moderate or severe hemophilia, across five European countries. Both studies obtained informed consent and ethical approval for data use. Data were captured by specialists via web‐based forms. Twelve months of retrospective data on all bleeding episodes, target joints and pain were examined.
Results: In CHESS II, 174/190 adults (91.6%) with moderate HA and 92/94 adults (97.9%) with mild HA did not receive routine prophylaxis (Table 1). Of those not receiving prophylaxis: 147/174 (84.5%) with moderate HA had ≥1 bleed/year, including 60/174 (34.5%) who had ≥3 bleeds/year; 69/92 (75.0%) with mild HA had ≥1 bleed/year, including 7/92 (7.6%) who had ≥3 bleeds/year. In CHESS PAEDs, of the 282 children with moderate HA included, 146/282 (51.8%) did not receive routine prophylaxis (Table 2); of those, 108/146 (74.0%) had ≥1 bleed/year, including 59/146 (40.4%) who had ≥3 bleeds/year. In those not receiving prophylaxis, the number of target joints and pain severity increased with a higher number of bleeds.
Conclusion(s): Most adults with moderate or mild HA in CHESS II and more than half of children with moderate HA in CHESS PAEDs did not receive routine prophylaxis; more than one third of adults and ~40% of children with moderate HA not receiving prophylaxis experienced ≥3 bleeds/year. Additional research is warranted to investigate potential reasons for limited use of prophylaxis in these populations.


54. PB0672
54.1. Safety and pharmacokinetics of a subcutaneous recombinant FVIII (OCTA101) in adult patients with severe haemophilia A
T. Lissitchkov1; M. Jansen2; J. Bichler3; S. Knaub 3
1 Blood Diseases at Clinical Hematology Clinic, Sofia, Sofiya, Bulgaria; 2 Octapharma Pharmazeutika Produktionsgesellschaft m.b.H, Vienna, Salzburg, Austria; 3 Octapharma AG, Lachen, Schwyz, Switzerland
Background: Regular, prophylactic intravenous FVIII is the standard of care for severe haemophilia A, but can be challenging for some patients. Subcutaneous FVIII administration could provide an alternative treatment option without the complications associated with venous access.
Aims: This phase I/II study (NCT04046848) assessed the safety, pharmacokinetics (PK), and bioavailability of subcutaneous OCTA101, a recombinant FVIII with a recombinant von Willebrand factor fragment dimer.
Methods: Previously treated (≥150 exposure days to FVIII) male patients (≥18 years) with severe haemophilia A were eligible for this single‐centre, prospective, open‐label, dose‐escalation study. Patients in the dose‐escalation cohorts received a single injection of OCTA101 for PK assessment followed by 3‐month daily prophylaxis, with assessment by an independent Data Monitoring Committee (DMC) before the next higher dose. Primary outcome measures included FVIII and VWF fragment PK, dose‐linearity, adverse events (AEs), dose‐limiting toxicities, thromboembolic events, local injection site reactions, and FVIII inhibitor formation.
Results: Twenty patients were enrolled in the PK part of the study. Half‐life and recovery were as expected from pre‐clinical studies, and trough plasma FVIII levels ≥10% were achieved. Two patients developed inhibitory antibodies to OCTA101, and the trial was put on hold during discussion with the DMC. The trial resumed with an amended protocol, including a lower dose and integration of additional safety steps, and ten patients started OCTA101 treatment. Another two patients subsequently developed inhibitors while on daily prophylaxis. In accordance with the amended protocol and in agreement with the DMC, the study was immediately terminated. Other than the development of FVIII inhibitors, no serious treatment‐related AEs were reported.
Conclusion(s): The occurrence of inhibitors despite dose reduction suggests that their development may be related to the subcutaneous route of administration. Similarly, development of another subcutaneous recombinant FVIII product (turoctocog alfa pegol) was terminated due to antibody development in previously treated patients.
55. PB0684
55.1. Clinical outcomes in patients with hemophilia A in Brazil: 3‐years of follow‐up of the AHEAD International Study
M. Cerqueira1; C. Ferreira2; C. Lorenzato3; L. Mesquita Carvalho4; L. Correa Oliveira5; I. Pinto6; A. Prezotti 7; J. Gu8; R. Guerra8; L. Tang9; M. Ozelo10
1 Instituto de Hematologia do Estado do Rio de Janeiro (HEMORIO), Rio de Janeiro, Rio de Janeiro, Brazil; 2 Hemocentro de Porto Alegre (HEMORGS), Porto Alegre, Rio Grande do Sul, Brazil; 3 Hemocentro do Paraná (HEMEPAR), Curitiba, Parana, Brazil; 4 Centro de Hematologia e Hemoterapia do Ceará (HEMOCE), Fortaliza, Ceara, Brazil; 5 Hemocentro Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Sao Paulo, Brazil; 6 Fundação HEMOPA, Belém, Para, Brazil; 7 Centro de Hemoterapia e Hematologia do Espírito Santo (HEMOES), Vitoria, Espirito Santo, Brazil; 8 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States; 9 Takeda Pharmaceuticals International AG, Zürich, Zurich, Switzerland, 10 Hemocentro UNICAMP, Department of Internal Medicine, School of Medical Sciences, University of Campinas, Campinas, Sao Paulo, Brazil
Background: The ongoing international AHEAD study (NCT02078427) evaluates the long‐term effectiveness and safety of patients with hemophilia A (HA) receiving octocog alfa or rurioctocog alfa pegol in real‐world clinical practice.
Aims: To report clinical outcomes from 3 years of follow‐up from a subgroup of patients in Brazil with HA receiving octocog alfa using data from the AHEAD 6‐year interim read‐out (data cutoff: July 15, 2019).
Methods: AHEAD is a prospective, non‐interventional, multicenter study including patients of any age with HA. The primary endpoint is joint health, assessed by Gilbert score (pain, bleeding, physical exam). Hemophilia Joint Health Score (HJHS), annualized bleeding rates (ABRs), annualized joint bleeding rates (AJBRs), quality of life, and safety were also recorded. Patients’ informed consent and ethics committee approval were obtained.
Results: A total of 203 patients were enrolled; 166 (81.8%) had severe HA (factor VIII < 1%) and 37 (18.2%) had moderate HA (factor VIII 1–5%). 190 patients received prophylaxis, 2 received on‐demand treatment, and 11 received immune tolerance induction. Patient demographics stratified by HA severity and treatment regimen are shown in Table 1. Average Gilbert score, ABRs, and AJBRs were higher in patients with severe HA than those with moderate HA (Table 2). Mean ± SD total HJHS score in patients receiving prophylaxis was 16.8 ± 20.8 at year 1 (n = 67), 12.0 ± 15.2 at year 2 (n = 52), and 25.7 ± 30.9 at year 3 (n = 6). 210 adverse events (AEs) were experienced by 81 patients (39.9%) and 18 serious AEs by 16 patients (7.9%). Of these, 6 (3%) AEs were treatment related, none of which were classified as serious.
Conclusion(s): The majority of patients were treated with prophylaxis, regardless of HA severity. Joint health was well maintained, and ABRs and AJBRs remained consistent over the 3‐year observation period in patients with both moderate and severe HA treated with prophylaxis.

56. PB1125
56.1. Musculoskeletal health and health‐related quality of life in patients switching to emicizumab prophylaxis
S. Arcudi 1; R. Gualtierotti2; V. Begnozzi3; A. Ciavarella4; G. Nicolò5; S. Siboni6; E. Biguzzi3; E. Boccalandro3; F. Peyvandi7
1 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, MILAN, Lombardia, Italy; 2 Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 3 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 4 Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 5 Department of Healthcare Professions, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico di Milano, Milan, Italy, Milan, Lombardia, Italy; 6 Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 7 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Emicizumab is effective as a non‐replacement prophylaxis for patients with severe hemophilia A, but its effect on musculoskeletal health is still under debate.
Aims: To explore the effect of emicizumab prophylaxis on musculoskeletal health and health‐related quality of life (HRQoL) in patients affected with severe hemophilia A (HA) from the Milan cohort.
Methods: We collected hemophilia joint health score (HJHS), the five‐level‐EQ‐5D (EQ‐5D‐5L) and hemophilia early arthropathy detection with ultrasound (HEAD‐US) in patients with HA switched to emicizumab in the post‐marketing era, at baseline before switching and after a follow‐up of at least 8 months. The HJHS was performed by a trained physiotherapist and the HEAD‐US was performed on the same joints by an expert rheumatologist. Paired sample T‐test was used to assess differences before and after switching.
Results: We evaluated 8 consecutive adults at baseline and 8–12 months after switching to emicizumab prophylaxis. Mean age was 43.3 years (SD 17.1). Three out of 8 patients had one or more prosthetic joints. Mean±SD HEAD‐US total score was stable (13 ± 9.6 to 12.5 ± 8.7, p‐value 0.5), with a slight improvement of 1 to 2 points in total synovitis domain in 3 out of 8 patients. Mean ± SD HJHS decreased from 9.6 ± 9.2 to 7.6 ± 8.1 (p‐value < 0.05). When analyzing the pain/discomfort domain of the EQ‐5D‐5L, an improvement was reported in five out of eight patients from “slight problems” to “no problems” after switching, two patients remained at the same level and one patient improved from “severe” to “moderate problems”.
Conclusion(s): Our preliminary results showed improved or stable musculoskeletal health in patients switched to emicizumab. As expected, the cartilage and bone damage domains of the HEAD‐US score do not seem to be influenced by the therapy switch, whereas an improvement in synovitis, HJHS score and HRQoL was observed.
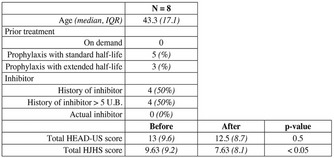

57. PB1160
57.1. WAPPS‐Hemo as a tool for optimizing prophylaxis with extended half‐life FIX in patients with severe or moderate hemophilia B
T. Szanto 1; R. Lassila2; V. Nummi1; A. Iorio3; A. Leithinen4
1 Coagulation Disorders Unit, Comprehensive Cancer Center, Helsinki University Hospital, Helsinki, Uusimaa, Finland; 2 Helsinki University Hospital, and University of Helsinki, Finland, Helsinki, Uusimaa, Finland; 3 McMaster University Medical Centre, Hamilton, Ontario, Canada; 4 Coagulation Disorders Unit Department of Hematology Comprehensive Cancer Center HUCH and University of Helsinki, Helsinki, Uusimaa, Finland
Background: The Web‐Accessible Population Pharmacokinetic Service—Hemophilia (WAPPS‐Hemo) is a web‐based database application developed to individualize coagulation factor replacement therapy.
Aims: The impact of switching from standard half‐life (SHL) rFIX product (Nonacog alfa, Benefix) to extended half‐life (EHL) rFIX‐Fc (Eftrenonacog alfa, Alprolix) or N9‐GP (Nonacog beta pegol, Refixia) was evaluated on FIX pharmacokinetics (PK), weekly dosage and prophylactic infusion frequency.
Methods: FIX activity was measured by one‐stage assay at least twice between two doses during regular EHL prophylaxis. PK analyses were performed using the WAPPS‐Hemo application and dosing estimations aimed to maintain trough levels of 5 IU/dl.
Results: Thirteen patients with hemophilia B were switched to EHL‐FIX, six to rFIX‐Fc (2 severe and 4 moderate phenotypes; median age 25.5 year, range 18–60 year) and seven to N9‐GP (3 severe and 4 moderate; median age 53 year, range 40–74 year). Median dose of FIX concentrate for the PK analysis was 46 IU/kg for rFIX‐Fc and 52 IU/kg for N9‐GP. The median half‐life was extended to 124–125 h for both FIX‐Fc and N9‐GP compared with 22–38 h using rFIX (Benefix). The median time to maintain trough levels at least 5 IU/dl was extended by 4.9 days for rFIX‐Fc (p = 0.0007), but 13 days for N9‐GP (p = 0.0017). After switching to EHL‐FIX, the median weekly dosage decreased by 10% for rFIX‐Fc (p = 0.7) while by 66% for N9‐GP (p = 0.002). Prophylactic infusion frequency could be reduced in almost all: 5/6 (83%) for rFIX‐Fc and 6/7 (86%) for N9‐GP. In the rFIX‐Fc group, 3 patients currently infuse once every 2 weeks. In the N9‐GP group, 5 patients infuse once every 2 weeks and 1 patient once every 23 days.
Conclusion(s): Switch from SHL to N9‐GP resulted in more significant reduction in weekly dosage and infusion frequency in comparison with rFIX‐Fc. The clinical effects of rFIX‐Fc and N9‐GP will be evaluated in the future.

58. PB0679
58.1. Local experience of monitoring and effectiveness of Emicizumab in severe Haemophilia A
J. Needham; S. Mangles; L. Oyesiku; H. Ma Cordial
Hampshire Hospitals NHS Foundation Trust, Basingstoke, England, United Kingdom
Background: Development of Emicizumab (Hemlibra) a bispecific monoclonal antibody that substitutes for activated factor VIII by directly binding activated FIX to FX, initially for haemophilia A patients with inhibitors, it is now a treatment option for all severe haemophilia A patients, bringing potential for improved clinical outcomes and quality of life.
Aims: To assess stability of circulating levels of Emicizumab and clinical effectiveness of prophylactic treatment in bleed prevention in local cohort of severe haemophilia A patients without inhibitors.
Methods: Citrated plasma samples were collected from 19 severe haemophilia A patients on Emicizumab having commenced maintenance dose at week 5 then 3, 6, 9 and 12 months as part of routine care, 7 patients with complete time points. Dosing was fortnightly, according to manufacturer’s recommendations. Emicizumab levels were measured using a specific one‐stage assay with R2 Diagnostic calibrators and controls, performed on STAR MAX2 (Stago). Thrombin generation using CAT analyser (Thermo) and Thrombinoscope reagents was measured at week 5. ANOVA and descriptive statistics were applied.
Results: Circulating levels of Emicizumab for all patients mean = 50.5 μg/ml, median = 50.4 μg/ml, with no statistically significant difference p = 0.924 across time points (see Table 1). Comparison between individual patients range 22.2– 66.7 μg/ml showed statistically significant difference p = <0.001. Endogenous thrombin potential (ETP) mean = 1225.7 nM/mins and Peak thrombin mean = 117.9 nM with 92.3% and 23.1% respectively within normal range. There was no correlation between Emicizumab level and ETP or Peak thrombin R 2 = 0.03, 0.16 respectively. There were 6 spontaneous bleeds recorded with 66.6% in patients with levels < 50 μg/ml.
Conclusion(s): Local experience of self‐administered Emizicumab, treatment showed good circulating stability in all haemophilia A patients consistent with trial findings. Results support 50 μg/ml threshold but in the future, perhaps dose adjustment could be used with monitoring to guide optimise clinical effectiveness and minimise bleeds.

59. PB1130
59.1. Real‐world evidence on emicizumab in 119 Hemophilia A patients with inhibitors from the French FranceCoag cohort
A. Harroche1; Y. Repesse2; A. Rauch3; F. Hadim4; A. Gaude5; D. Pau6; S. Pibre6; R. d'Oiron 7
1 CRC‐MHC Hôpital Necker‐Enfants malades, Paris, France, Paris, Ile‐de‐France, France; 2 Department of Hemostasis, University Hospital of Caen, Caen, Basse‐Normandie, France; 3 University of Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐EGID, France, Lille, Nord‐Pas‐de‐Calais, France; 4 ROCHE FRANCE, Boulogne Billancourt, Ile‐de‐France, France; 5 Chugai France, La défense, Ile‐de‐France, France; 6 Roche S.A.S, Boulgone Billancourt, Ile‐de‐France, France; 7 CRC‐MHC, Hôpital de Bicetre AP‐HP, Le Kremlin Bicetre, France, Le Kremlin Bicêtre, Ile‐de‐France, France
Background: Emicizumab has demonstrated significant reductions in bleeding episodes in the HAVEN program for Hemophilia A with inhibitors patients (HAwI). However, limited data are available in a real‐life setting.
Aims: This study aimed to describe treated bleeds during emicizumab treatment in HAwI, patient and disease characteristics, and emicizumab tolerability.
Methods: In this non‐interventional secondary data use study, data of HAwI having initiated emicizumab in France from June 2017 to December 2020 were extracted from the national FranceCoag database in October 2021. The primary endpoint was the treated bleeds' rate under emicizumab. Descriptive analysis was performed. Treated Bleeding‐Free Survival (TBFS) was described using Kaplan‐Meier method.
Results: Among a total of 119 eligible patients analyzed, 112 (94.1%) were severe, 5 (4.2%) moderate, and 2 (1.7%) minor HAwI. Patient age distribution at emicizumab initiation was <2 yo (10.9%)/[2–18](33.6%)/[18–65](50.4%)/≥65 yo (5.0%); their median historical peak of inhibitor titer was 301 UB/ml (IQR 48–1,780) and inhibitor peak was 2.6 (0.5–10.0) over the 3 months before emicizumab initiation. 102 patients with ≥6 months treatment duration were retained for effectiveness analysis. Over follow‐up (median: 96.5 weeks, IQR 67.9–131.7), patients received emicizumab during 89.1 weeks (IQR 54.2–127.1). Under emicizumab, 78 patients (76.5%) experienced no bleeds; 24 (23.5%, 95% CI: [15.7%–32.9%]) had 37 bleeds (3 patients presented bleed(s) during the loading dose), including 17 patients with 1 event and 7 patients with 2 or more events. 16 joint bleeds occurred in 12 patients (11.8%, 95% CI: [6.2%–19.7%]), 2 cerebral hemorrhages in 1 (1.0%, 95% CI: [0.02%–5.34%]) and/or 1 life‐threatening retroperitoneal hemorrhage in 1 (1.0%, 95% CI: [0.02%–5.34%]). The overall mean annual treated bleeds’ rate under emicizumab was 0.23 ± 0.79 (joint bleeds 0.16 ± 0.46). TBFS at 26, 52 and 104 weeks was 95%, 84% and 73% respectively. No safety signal was identified.
Conclusion(s): Using FranceCoag prospective cohort, this real‐world study allows updating emicizumab knowledge in a large and non‐selected HAwI population.
60. PB0689
60.1. Effects of PK assessment on FVIII consumption and estimated costs for patients with moderate to severe hemophilia A
G. Young 1; M. Callaghan2; V. Balasa3; A. Soni4; S. Ahuja5; J. Roberts6; M. Simpson7; A. Frick8; A. Mokdad9; S. Xing10; J. Caicedo10
1 Hemostasis and Thrombosis Center, Cancer and Blood Diseases Institute, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, California, United States; 2 Children’s Hospital of Michigan, Detroit, MI, USA, Detroit, Michigan, United States; 3 Valley Children's Healthcare, Madera, CA, USA, Madera, California, United States; 4 Center for Inherited Blood Disorders, Orange, CA, USA, Orange, California, United States; 5 Rainbow Babies and Children's Hospital, Cleveland, OH, USA, Cleveland, Ohio, United States; 6 Bleeding and Clotting Disorders Institute, Peoria, IL, USA, Peoria, Illinois, United States; 7 Rush University Medical Center, Chicago, IL, USA, Chicago, Illinois, United States; 8 Trio Health Inc., Louisville, CO, USA, Louisville, Colorado, United States; 9 Takeda, Sudbury, Massachusetts, United States, 10 Takeda Development Center Americas, Inc., Cambridge, MA, Cambridge, Massachusetts, United States
Background: Individualized pharmacokinetic (PK)‐guided prophylaxis has been shown to improve outcomes in patients with hemophilia A (PWHA) in clinical trials; however, the impact of PK‐guided prophylaxis on FVIII consumption in real‐world settings is poorly understood.
Aims: This retrospective chart review examined FVIII consumption and cost of treatment before and after PK‐guidance in US hemophilia treatment centers (HTC).
Methods: This study included 132 male PWHA from 7 HTCs with moderate or severe disease, ≥3 months prior FVIII prophylaxis and up to 12 months FVIII prophylaxis after PK‐guided prescription change (index date), no inhibitors prior to index date, and no concurrent participation in clinical trials. Changes in annualized bleed rate (ΔABR) and confidence intervals (CIs) were evaluated. Changes in mean weekly consumption of FVIII prophylaxis (ΔMWC; IU/kg/week) were estimated based on physician prescription with costs of prophylaxis estimated using Average Sales Price (Q3 2021) and annualized.
Results: Of the 132 patients (age: median = 13, range: 2–43; severe disease: 93.2%), 58 (44%) remained on the same FVIII (non‐switchers) and 74 (56%) switched FVIII therapy (switchers) after PK assessment. Most switchers (95%) transitioned from standard half‐life product (SHL) to extended half‐life product (EHL), and 39/58 of non‐switchers (67%) were on a SHL. ABRs decreased among all patients [−0.97 (95% CI: −1.54, −0.42)], with decrease more pronounced in non‐switchers [−1.93 (−3.14, −0.78)]. MWC decreased among patients switching to EHL. While annual cost of therapy increased on average (related to higher per unit cost with EHL vs. SHL; Table), 32% of patients had a decrease in annualized cost (Figure). Non‐switchers had no significant change in MWC or cost of therapy.
Conclusion(s): PK‐guided prophylaxis was associated with an overall ABR reduction in all patients. Many patients had similar or improved effectiveness without any increase in cost of therapy. PK guidance could be a useful and cost‐effective strategy in the management of PWHA.


61. PB1152
61.1. Fitusiran, an investigational siRNA therapeutic targeting antithrombin: Analysis of antithrombin levels and thrombin generation from a phase 3 study in people with hemophilia A or B with inhibitors
S. Rangarajan 1; A. Srivastava2; B. Zulfikar3; E. Cockrell4; S. Kichou5; Z. Qiu6; S. Andersson7; B. Mei7; G. Young8
1 University of Southampton, Southhampton, England, United Kingdom; 2 Christian Medical College, Vellore, India, Vellore, Tamil Nadu, India; 3 Istanbul University Oncology Institute, Istanbul, Istanbul, Turkey; 4 BayCare Medical Group, Pediatric Hematology Oncology at St. Jospeh’s Children’s Hospital, Tampa, Florida, USA, Tampa, Florida, United States; 5 Sanofi Genzyme, Paris, France, Paris, Ile‐de‐France, France; 6 Sanofi, Bridgewater, NJ, USA, Bridgewater, New Jersey, United States; 7 Sanofi, Cambridge, MA, USA, Cambridge, Massachusetts, United States; 8 Hemostasis and Thrombosis Center, Cancer and Blood Diseases Institute, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, California, United States
Background: Hemophilia is characterized by deficiency of FVIII or FIX resulting in impaired thrombin generation (TG) and ineffective clot formation. Fitusiran is an investigational siRNA therapeutic which targets antithrombin to enhance TG and rebalance hemostasis in people with hemophilia A or B, irrespective of inhibitor status.
Aims: Longitudinal assessment of changes over time in antithrombin activity levels and TG in people with hemophilia A or B with inhbitors (PwHI) with fitusiran prophylaxis to prevent bleeding.
Methods: This analysis of a Phase 3, multinational, randomised, open‐label study (NCT03417102) included males aged ≥12 years with severe hemophilia A or B with inhibitors, previously treated on‐demand with bypassing agents (BPA). Participants were randomized 2:1 to receive once‐monthly 80 mg subcutaneous fitusiran prophylaxis (fitusiran arm) or on‐demand BPA (BPA arm) for 9 months. The primary endpoint was annualized bleeding rate (ABR). Exploratory endpoints included changes in antithrombin levels and TG over time.
Results: Overall, 60 participants were included in the exploratory analysis. On day 15, there was a 76.8% mean reduction from baseline in antithrombin activity levels in the fitusiran arm, with a further reduction to 84.1% on day 29 and maintained at 87.6%–89.9% from day 43 onwards (Figure 1). There was a mean increase in Peak TG of 21.9 nM from baseline in the fitusiran arm on day 15, with a further increase to 30.8 nM on day 29 and maintained at 36.8–47.5 nM from day 43 onwards (Figure 2). These results translated into a 90.8% reduction in estimated ABR with fitusiran prophylaxis vs on‐demand BPA.
Conclusion(s): Fitusiran gradually reached target pharmacodynamic effect of antithrombin lowering and increased TG by day 29 and demonstrated a sustained effect over the duration of the study. These findings and the significant reduction in ABR suggest fitusiran has the potential to rebalance hemostasis and provide consistent bleed protection in PwHI.


61.2. Hemophilia Gene Therapy
62. PB0212
62.1. Collaboration between the world federation of hemophilia and national registries for the long‐term follow‐up of people with hemophilia treated with gene therapy
M. Naccache1; B. Konkle2; D. Coffin 1; C. Clark3; L. George4; A. Iorio5; W. Miesbach6; D. Noone7; F. Peyvandi8; S. Pipe9; M. Recht10; M. Skinner11; L. Valentino12; J. Mahlangu13; G. Pierce1; F. Leebeek14
1 World Federation of Hemophilia, Montreal, Quebec, Canada; 2 Washington Center for Bleeding Disorders and the University of Washington, Seattle, Washington, United States; 3 International Society on Thrombosis and Haemostasis, Carrboro, North Carolina, United States; 4 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 5 McMaster University Medical Centre, Hamilton, Ontario, Canada; 6 Goethe University Hospital, Frankfurt, Hessen, Germany; 7 European Haemophilia Consortium, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 8 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy; 9 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States, 10 American Thrombosis and Hemostasis Network, Rochester, New York, United States, 11 Institute for Policy Advancement Ltd., Washington, District of Columbia, United States, 12 National Hemophilia Foundation, New York, New York, United States, 13 University of the Witwatersrand and NHLS, Johannesburg, South Africa, 14 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Registries, with international collaboration between countries, are an effective way to pool enough data to enable robust evaluation of rare safety events and durability of gene therapy. Linking registries is an extensive process involving many steps and collaboration among multiple partners.
Aims: To maximize the utility of current data collection systems, the World Federation of Hemophilia (WFH) Gene Therapy Registry (GTR) is collaborating with national registries to promote international data integration.
Methods: The technical ease of linking registries relies on meticulous preparatory work between registries, to minimize data integration barriers. Barriers include poor interoperability due to the complexity and heterogeneity of data fields, lack of standard terms, data descriptions, and measurement methods. A credible database partner, with technical capability and strong security systems has been selected and is central to a successful registry and data integration. De‐identified data will be transferred from partner registries to the GTR on a regular basis, without access to any identifying information other than a unique patient identifier.
Results: The GTR has begun a collaboration with the American Thrombosis and Hemostasis Network (ATHN) (USA), HemoNed (Netherlands), and the Canadian Bleeding Disorders Registry (CBDR) (Canada). Common data definitions and careful comparison of data field measurements, such as ensuring common ontology terms, units of measurement and date formats, as much as possible, was conducted to facilitate the technical mapping of data fields between the GTR and partner registries. Discussions on potential integrations with other national registries are ongoing. The GTR will be available for data entry and linkage by mid‐2022.
Conclusion(s): The GTR is collaborating with ATHN, HemoNed, and the CBDR to ensure that rare adverse events, in a small patient population over a large geographical area, will be detected. The GTR and partner registries will also help establish long‐term follow‐up as the standard of care for gene therapy.
63. PB0213
63.1. Results from B‐LIEVE, a phase 1/2 dose‐confirmation study of FLT180a AAV gene therapy in patients with hemophilia B
G. Young 1; P. Chowdary2; S. Barton3; D. Yee3; F. Ferrante3
1 Hemostasis and Thrombosis Center, Cancer and Blood Diseases Institute, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, California, United States; 2 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital, London, England, United Kingdom; 3 Freeline, Stevenage, England, United Kingdom
Background: FLT180a (verbrinacogene setparvovec) is an investigational gene therapy for the treatment of hemophilia B that uses a rationally designed AAVS3 capsid carrying a gain‐of‐function factor IX (FIX) Padua (R338L) transgene. B‐AMAZE was a Phase 1/2 dose‐finding study of FLT180a in 10 patients with hemophilia B (FIX ≤2%) who also received prophylactic immune management with corticosteroids ± tacrolimus. FIX expression was dose dependent, and FIX levels in the normal range (50%–150%) were observed in 5 of 10 patients at most recent follow‐up. B‐LIEVE is a Phase 1/2 study of FLT180a manufactured with a commercial process that began enrollment in early 2022 and is intended to confirm the dose and immune management regimen for Phase 3.
Aims: Present safety and FIX activity data for patients treated with FLT180a in B‐LIEVE.
Methods: Males aged 18–65 who have hemophilia B (FIX ≤2%) and are receiving FIX prophylaxis are eligible to participate in B‐LIEVE. Patients must be negative for AAVS3 neutralizing antibodies and have no history of FIX inhibitors. The dose for the first cohort of three patients in B‐LIEVE is 7.7e11 vg/kg with a dose cap at 90 kg. Prophylactic immune management to prevent vector‐related transaminitis consists of a tapering course of corticosteroids concurrent with a short course of tacrolimus. Up to six additional patients can be treated based on dose‐adjustment requirements.
Results: Safety data and FIX levels for the first cohort treated in B‐LIEVE will be presented.
Conclusion(s): Sustained FIX expression in the normal range is an important therapeutic goal for hemophilia B gene therapy. We will present results from B‐LIEVE, a Phase 1/2 study of FLT180a designed to confirm the dose and immune management approach that consistently enables FIX expression within the normal range.
64. PB0210
64.1. Blood biodistribution and vector shedding of valoctocogene roxaparvovec in people with severe hemophilia A: Results from the phase 3 GENEr8‐1 trial
S. Agarwal 1; K. Obrochta1; M. Vora1; A. Bowen1; B. Bunch1; J. Holcomb1; D. Guo1; T. Robinson2; K. Jayaram1; C. Russell1; C. Vettermann1; J. Henshaw1
1 BioMarin Pharmaceutical Inc., Novato, California, United States; 2 BioMarin Pharmaceutical, Inc., Novato, California, United States
Background: Valoctocogene roxaparvovec is a recombinant adeno‐associated virus serotype 5 (AAV5) gene therapy vector that enables steady, endogenous factor VIII expression in people with severe hemophilia A.
Aims: To characterize vector DNA biodistribution and shedding following valoctocogene roxaparvovec administration.
Methods: Adult AAV5‐negative men (N = 134) with severe hemophilia A received a single 6E13 vg/kg valoctocogene roxaparvovec infusion in the phase 3 GENEr8‐1 trial (NCT03370913). Informed consent and all relevant ethics approvals were obtained. Total vector DNA was quantified in blood, saliva, stool, semen, and urine with a validated quantitative (q)PCR assay. Encapsidated vector DNA was measured in plasma and semen with an immunoprecipitation‐coupled qPCR assay. The contiguity of vector genomes was measured in whole blood, plasma, peripheral blood mononuclear cells (PBMCs), and red blood cells (RBCs) using a drop‐phase droplet‐digital (dd)PCR assay. Vector genome inverted terminal repeat (ITR) fusions were measured in whole blood and PBMCs using ddPCR and primers directed outward from the 5′ and 3′ ends of the linear vector genome.
Results: Median peak vector DNA levels were observed 1–8 days after dosing and were highest in blood followed by saliva, semen, stool, and urine; concentrations then declined steadily. Encapsidated vector DNA was cleared more rapidly than total vector DNA, with maximum time to clearance ≤12 weeks in both plasma and semen. Vector genomes transitioned from initial non‐contiguous forms into full‐length forms over time; fraction of ITR fusions, indicating formulation of circularized episomes, steadily increased. Full data from 2 years of follow‐up will be shared at ISTH 2022 Congress.
Conclusion(s): Vector DNA and vector capsids were steadily cleared from the blood and shedding matrices of people with severe hemophilia A treated with valoctocogene roxaparvovec. The replication‐incompetent nature of valoctocogene roxaparvovec and the rapid clearance of encapsidated vector make the risk of transmission to untreated individuals extremely low.
65. PB0211
65.1. A translational analysis of immune components in peripheral blood from severe hemophilia A patients treated with TAK‐754, an AAV8 vector with a codon‐optimized B‐domain–deleted factor VIII transgene
J. Chapin; M. Ayash‐Rashkovsky; J. Kenniston; M. Wagoner; Q. Wang
Takeda Development Center Americas, Cambridge, Massachusetts, United States
Background: Immune responses to gene therapies have been associated with infusion reactions, transaminase elevation, and loss of transgene expression. Here, we describe an immunogenicity analysis in peripheral blood from three hemophilia A (HA) patients following infusion of TAK‐754, an AAV8‐based FVIII gene therapy (NCT 03370172).
Aims: (1) To report clinical biomarkers of immunogenicity from patients treated with TAK‐754. (2) To present an immunogenicity transcriptomics analysis from peripheral blood.
Methods: HA patients received TAK‐754 in two cohorts: 2 × 1012 and 6 × 1012 capsid particles (cp)/kg. Serum cytokines, complete blood counts (CBC), anti‐capsid immunoglobulin assays, and enzyme linked immunospot (ELISpot) assays were collected. Bulk mRNA sequencing was performed on whole blood compared to healthy volunteers. This study was approved by local ethics committees, and patients provided informed consent.
Results: No changes in cytokines were observed within 24 h of infusion. Transaminase elevation and decline of FVIII expression occurred 4–9 weeks post‐infusion despite use of glucocorticoids. No significant changes in complete blood count populations were observed. ELISpot assays did not correspond with loss of FVIII transgene expression or transaminase elevation. Bulk mRNA transcriptomic analysis did not demonstrate significant changes in NK cell, dendritic cell, NF‐kB, IL‐6, TLRs 1‐8, or T cell pathway signals. A small transient increase in TLR9, TNF‐a, CCL5, and IRF7 signals occurred 8 h after infusion without activating a Type 1 IFN response.
Conclusion(s): TAK‐754 evoked a limited immune response as measured in blood with highly sensitive methodology, demonstrating that such methods have limited value for clinical decision‐making or post hoc analysis. Efforts to optimize AAV gene therapy should focus on target tissue‐specific analysis and improving preclinical immunogenicity models.
65.2. Management of Bleeding and Trauma
66. PB1187
66.1. Safety and efficacy of emicizumab during dental extraction in haemophilia patient
F. Wijaya; J. Harvery
Maranatha Christian University ‐ Department of Dental Medicine, Bandung, Jawa Barat, Indonesia
Background: Emicizumab is a recombinant humanized bispecific monoclonal antibody mimicking the cofactor function of activated factor VIII. It is approved to prevent bleeding in patients with haemophilia A (HA). However, few studies have assessed the safety and efficacy of emicizumab during dental extraction in patients with haemophilia A (HA).
Aims: To systematically review the literature on efficacy and safety of emicizumab during dental extraction in patients with haemophilia A (HA).
Methods: Science direct, Pubmed, Google Scholar, Europe PMC, and Cochrane Library were searched. Searches were performed until December 27, 2021. Snowballing of potential literature was also carried out. The studies included patients with any age with a diagnosis of haemophilia with and without inhibitors. Patients were also required to have received a minimum of four loading doses of emicizumab. All study designs were included in this review.
Results: There were few adverse events were reported in all patients having emicizumab prophylaxis in all procedures, including tooth extraction. Adverse effects most frequently occured in patients with inhibitors. There were no thromboembolic events or thrombotic microangiopathy. All patients experienced minimal blood loss perioperatively. Few patients experienced no treated bleeding events.
Conclusion(s): Evidence are strong enough to determine that emicizumab is effective and safe for patients with haemophilia A during dental extraction.
67. VPB1189
67.1. ATHN 16: Safety of coagulation factor VIIa (recombinant)‐jncw for the treatment of bleeding events in patients with congenital hemophilia A or B with inhibitors, with or without prophylactic treatment
T. Chrisentery‐Singleton 1; W. Alexander2; A. Al‐Sabbagh3; D. Bonzo4; M. Callaghan5; M. Escobar6; A. Giermasz7; M. Hirsh8; J. Journeycake9; S. Nasr10; R. Pruthi11; D. Quon12; A. Rafique13; M. Reding14; s. Sullivan15; M. Recht16
1 Mississippi Center For Advanced Medicine, Slidell, Louisiana, United States, 2 unknown, Louisville, Kentucky, United States; 3 LFB, Norwood, Maryland, United States; 4 LFB, Norwood, Massachusetts, United States; 5 Central Michigan University SOM, Children’s Hospital of Michigan, etroit, Michigan, United States; 6 University of Texas Health Science Center, Houston, Texas, United States; 7 University of California Davis Medical Center, Sacramento, California, United States; 8 American Thrombosis and Hemostasis Network (ATHN), Rochester, New York, United States; 9 University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States, 10 Gloval, Broomfield, Colorado, United States, 11 Mayo Clinic, Rochester, Minnesota, United States, 12 Orthopaedic Hemophilia Treatment Center, Los Angeles, California, United States, 13 Louisiana Comprehensive Hemophilia Center; Tulane University Medical Center, New Orleans, Louisiana, United States, 14 Center for Bleeding and Clotting Disorders, University of Minnesota, Minneapolis, Minnesota, United States, 15 Mississippi Center For Advanced Medicine, Madison, Mississippi, United States, 16 American Thrombosis and Hemostasis Network, Rochester, New York, United States
Background: A new recombinant activated factor VII, eptacog beta (SEVENFACT®, rFVIIa‐jncw) has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of bleeding events (BEs) in individuals >12 years of age with hemophilia A or B (HAB) with inhibitors. To date, no studies designed to assess safety of treatment of breakthrough BEs in people on emicizumab with eptacog beta have been performed.
Aims: We plan to evaluate the safety of eptacog beta when used to treat BEs.
Methods: ATHN 16 (NCT04647227) is a phase IV, United States‐centric, multi‐center, open‐label safety study enrolling participants with hemophilia A or B (HAB) with inhibitors, aged 12 to 65 years, who may need to control a BE. The maximum study duration for any participant is up to 2 years from the time of enrollment. We plan to collect data on 100 BEs.
Results: At the time of abstract submission, ATHN 16, having received central IRB approval, is being rolled out across the United States, at the American Thrombosis and Hemostasis Network (ATHN), ATHN‐affiliated hemophilia treatment centers. There are 22 participating sites. Accrual has been initiated, two participants provided informed consent and were enrolled. Reporting will include BE details, and safety data for 28 to 55 participants. There are no pre‐specified efficacy endpoints.
Conclusion(s): ATHN 16 will explore the safety of eptacog beta as therapy for BEs in participants with HAB complicated by inhibitors with or without concurrent prophylactic treatment. As the first interventional study sponsored by ATHN, ATHN 16 represents a crucial step forward in ATHN’s clinical research capabilities.
68. VPB1192
68.1. A teenage boy with severe hemophilia A with inhibitors on Emicizumab prophylaxis presenting with hyphema
J. He 1; Z. Khademi2; N. Rodriguez3
1 The University of Texas MD Anderson Cancer Center, Houston, Texas, United States; 2 The University of Texas Health Science Center at Houston, Houston, Texas, United States; 3 The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, TX, United States, Houston, Texas, United States
Background: Hyphema is an uncommon phenomenon in patients with hemophilia A which is associated with significant morbidity including blindness. Management of hyphema can be challenging due to a significant rebleeding rate even in individuals without bleeding disorders. To our knowledge, there is currently no data on the management of traumatic hyphema in severe hemophilia A with inhibitors on Emicizumab prophylaxis.
Aims: To describe a case of traumatic hyphema in a pediatric patient with severe hemophilia A with inhibitors on Emicizumab.
Methods: Case report.
Results: 13‐year‐old male with severe hemophilia A and low titer inhibitor (peak 3.5 BU) on weekly Emicizumab prophylaxis (1.5 mg/kg) with good compliance presenting with left eye pain and vision loss 12 h after left eye trauma from a Nerf gun bullet. Emicizumab prophylaxis was given 6 days prior. Patient was not infused with bypassing agent after the injury since he was asymptomatic. About 14 h after trauma, patient became symptomatic and received rFVIIa (~100 mcg/kg) at home followed by another dose 4 h later in the hospital. Ophthalmology evaluation revealed increased left eye intraocular pressure with grade IV hyphema requiring emergency anterior chamber washout and removal of anterior chamber fibrin clot via anterior vitrectomy (refer to Picture 1). The procedure was performed within 4 h of hospital arrival and 20 h after trauma. Aggressive management with rFVIIa was initiated resulting in excellent bleeding control and no rebleeding complications (refer to Table 1). Patient was discharged on daily rFVIIa on post‐op day 4. Outpatient follow up 3 days after discharge showed normal intraocular pressure, no evidence of rebleeding and improving eye exam.
Conclusion(s): Timely management with surgery and aggressive use of bypassing therapy for trauma‐related total unilateral hyphema successfully restored vision and prevented rebleeding in a pediatric patient with severe hemophilia A with inhibitors on weekly Emicizumab prophylaxis.


69. VPB1193
69.1. Easy and efficient method for purification of human thrombin, for use as a component of fibrin sealants
R. Lombardi 1; F. Farias2; H. Guglielmone2; G. Rodriguez1; M. Bernardi1
1 UNC HEMODERIVADOS, Córdoba, Cordoba, Argentina; 2 UNC HEMODERIVADOS, Cordoba, Cordoba, Argentina
Background: Thrombin (Tb) is a serine protease generated by activation of its inactive precursor, prothrombin. One of its functions is the conversion of fibrinogen to fibrin. In addition, Tb is used with therapeutic purposes in fibrin sealants, where it acts as a local hemostatic agent.
Aims: To develop a method of purification of Tb, using human plasma as raw material.
Methods: The procedure consists of the activation of prothrombin to Tb by diluting the plasma with distilled water and adjusting the pH to 5.3. The resulting precipitate is separated and dissolved with buffer, the pH is raised to 6.5, CaCl2 is added and the fibrinogen that forms is separated by filtration. The Tb solution is subjected to ultrafiltration with a 10 KD membrane, and diafiltered to bring the sample to the conductivity conditions necessary for chromatography. The second step is cation exchange chromatography using a column with SPXL and connected to an AKTA Purifier equipment. The Tb contained in the solution binds to the matrix and is eluted by increasing the ionic strength of the buffer. The purity of the product obtained is evaluated by SDS‐PAGE. The specific activity is assessed by measuring thrombin activity by a coagulometric method using an international standard (NIBSC 01/580) as reference. Total proteins are measured by the bicinchoninic acid method.
Results: The Tb eluates from three independent processes have an average Tb activity of 142 IU/ml, with a yield of 81% and a specific activity of 415 IU/mg. The analysis of the process samples by SDS PAGE shows a band of 37 kDa corresponding to α Thrombin, which represents the most preponderant protein band.
Conclusion(s): The results obtained show that the developed method is reproducible and that purified Tb could be used as a component of fibrin sealants since it meets the quality requirements of the European Pharmacopoeia.
70. PB1183
70.1. Performance of a chromogenic factor IX activity assay in the recovery of factor IX replacement therapies
T. Quinton1; J. Fraser2; K. Black2; A. Sadeghi‐Khomami 2
1 Precision BioLogic, Bedford, Nova Scotia, Canada; 2 Precision BioLogic, Dartmouth, Nova Scotia, Canada
Background: The standard treatment for patients with hemophilia B without inhibitors is intravenous FIX replacement therapy with recombinant FIX or plasma‐derived FIX concentrates. Accurate measurement of factor concentrates is necessary to ensure correct dosing. For some modified rFIX products, one‐stage clotting assay methods can result in different potencies depending upon the activated partial thromboplastin time (APTT) reagent. Chromogenic FIX activity assays have shown useful in monitoring select extended half‐life coagulation factor replacements although limited data exists.
Aims: To evaluate the recovered activity of seven FIX replacement products including AlphaNine SD, Alprolix, BeneFIX, Idelvion, Rebinyn, Ixinity and Rixubis by a new chromogenic FIX assay.
Methods: Each replacement product was reconstituted according to the manufacturer’s instruction, then diluted with congenital FIX deficient plasma to prepare seven levels (0.05, 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 IU/ml) based on labelled potencies. Ten replicates of each level were measured fresh after preparation using a new Chromogenic Factor IX assay (Precision BioLogic) on an IL ACL TOP 700 CTS analyzer. Linear regression analysis of dose‐dependent recovery was performed.
Results: Using acceptance criteria of 100 ± 25 percent recovery, the Chromogenic Factor IX assay accurately quantified 6/7 products across all levels including AlphaNine, Alprolix, BeneFIX, Rebinyn, Ixinity and Rixubis. The mean FIX recovery was 96, 116, 93, 82, 117 and 102% respectively, relative to the theoretical target across all tested levels. There was an over‐recovery of Idelvion (albumin fusion recombinant FIX) with a mean recovery of 153%.
Conclusion(s): Our Chromogenic Factor IX assay can be used to accurately measure FIX activity in plasma samples with AlphaNine, Alprolix, BeneFIX, Ixinity, Rebinyn and Rixubis. However, there was an overestimation of Idelvion, suggesting a chromogenic method may not be suitable for monitoring this product.
71. PB1177
71.1. Performance of FVIII deficient plasma with VWF in the activity measurement of FVIII replacement products in plasma samples using an OSC assay
N. Kesavan 1; M. Rahman1; A. Sadeghi‐Khomami2; K. Black2; A. Wood3
1 Precision Biologic Inc., Dartmouth, Nova Scotia, Canada; 2 Precision BioLogic, Dartmouth, Nova Scotia, Canada; 3 Precision Biologic, Dartmouth, Nova Scotia, Canada
Background: Factor VIII (FVIII) replacement therapy is the standard of care for persons with hemophilia A. One‐stage clotting (OSC) assays are commonly used to measure FVIII activity in plasma samples. FVIII depleted plasma, used as a substrate in OSC assays, may be deficient in von Willebrand Factor (VWF). FVIII depleted plasma with normal levels of VWF is a better alternative, mimicking congenital hemophilia A plasma.
Aims: To evaluate the recovered activity of six FVIII replacement products – ADVATE®, AFSTYLA®, ELOCTATE®, Jivi®, Novoeight®, & Wilate® – in plasma samples by an OSC assay using FVIII Deficient Plasma with VWF.
Methods: Each FVIII replacement product was reconstituted according to the manufacturer’s instruction and diluted with congenital FVIII deficient plasma to prepare seven levels of FVIII activities (0.05, 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 IU/ml) based on labeled potency. Five replicates of each level were measured on an IL ACL TOP 700 CTS analyzer freshly after preparation using HemosIL aPTT SynthASiL and Factor VIII Deficient Plasma with VWF (Precision BioLogic Inc.). Linear regression analysis of dose‐dependent recoveries was performed.
Results: Using acceptance criteria of 100 ± 25% recovery, 5 out of 6 products across all levels were assayed accurately. Mean FVIII% recoveries across all levels of Advate, Eloctate, Jivi, Novoeight and Wilate were 93, 95, 101, 113 and 95% respectively. The percent recoveries of Afstyla, after x2 conversion, fell within the acceptance criteria for levels of ≥ 0.1 IU/ml but over‐estimated at 149.76% at the lowest level of 0.05 IU/ml.
Conclusion(s): Factor VIII Deficient Plasma with VWF correctly supports quantification of FVIII activity in plasma samples containing Advate, Eloctate, Jivi, Novoeight and Wilate in the range of 0.05–1.0 IU/ml. Afstyla doses lower than 0.1 IU/ml were over‐estimated by our assay.
72. PB1185
72.1. Novel miniaturized chemiluminescent‐based factor VIII activity assay
D. Steeghs; M. van Geffen; W. van Heerde; C. van 't Veer
Enzyre, Nijmegen, Gelderland, Netherlands
Background: Patients with severe hemophilia A require frequent injections with Factor VIII (FVIII). Due to the variability in the clearance of FVIII, these injections do not guarantee an adequate hemostatic response. A self‐test would allow patients to monitor their FVIII levels. Luminescent‐based assays can be incorporated in a handheld device without the need for a light source or filters, providing a linear signal over several orders of magnitude.
Aims: To develop a chemiluminescent FVIII activity assay to measure FVIII activity levels in small volumes.
Methods: A chemiluminescent‐based FVIII activity assay (FVIIIlum) was constructed based on tenase complex formation. The activation of FX is made limited by FVIII by adding the other components of the Xase‐complex in excess. FVIII activity was determined by measuring the amount of Factor Xa (FXa) formed using a FXa‐specific substrate, consisting of a tetrapeptide coupled to a luciferin‐moiety. Upon production of FXa, the specific substrate is cleaved, releasing aminoluciferin. Free aminoluciferin is converted by luciferase to oxyluciferin and a light quant. The Michaelis‐Menten constant and Vmax were determined using 1.6 nM FXa. The assay was optimized to differentiate between 0.01 and 1.0 IU/ml FVIII activity in a total reaction mixture of 30 μl. FVIIIlum was compared to the FVIII:C BIOPHEN assay using samples from ECAT surveys.
Results: The FXa‐specific substrate showed low cross‐reactivity (<10%) for thrombin and FIXa at physiological concentrations. The Michaelis‐Menten kinetics demonstrated a Km of 535 μM. FVIIIlum correlated well with the FVIII:C BIOPHEN with a slope of 0.819 and an R2 of 0.943, tested in ECAT samples (n = 8) within the range of <1% to 67%.
Conclusion(s): A chemiluminescent‐based assay sensitive for FVIII activity was developed for use in small plasma volumes. Furthermore, a good correlation was demonstrated with the standard chromogenic FVIII:C assay. As the FVIIIlum demonstrated high RLU signals, this technique is suitable for further miniaturization.
73. PB1181
73.1. Post‐menopausal bleeding and arterial thrombosis in biological females with inherited bleeding disorders
G. Moyer 1; M. Manco‐Johnson2
1 University of Colorado, Centennial, Colorado, United States; 2 Children's Hospital Colorado, Aurora, Colorado, United States
Background: Increasing numbers of biological females with inherited bleeding disorders (IBD)s are seeking care at Hemophilia Treatment Centers (HTC)s. Clinicians face a growing need for guidance in the use of hemostatic therapies in such patients, particularly those with competing age‐associated vascular risk factors.
Aims: This study will report the frequency of bleeding and arterial vascular events in post‐menopausal biological females with IBDs to inform practitioners who make treatment decisions for this population.
Methods: This study was funded by a Women’s Health Research Seed grant and was approved by institutional IRB. Biological females over 45 years who had undergone menopause or had an FSH of >30 mlU/ml were recruited from the University of Colorado’s HTC. Consenting subjects participated in a guided telephone survey conducted by the primary investigator.
Results: Thirty‐five biological females with an average age of 64.29 years participated. The most common inherited bleeding disorder diagnoses were von Willebrand Disease (N = 13), heterozygous hemophilia A (N = 10), and heterozygous hemophilia B (N = 6). Eight biological females (22.86%) experienced post‐menopausal vaginal bleeding. Over two thirds of subjects (N = 27, 77.14%) reported non‐vaginal bleeding after menopause, with bleeding associated with invasive procedures being most common (N = 18, 66.67%; Table 1). Three subjects experienced a stroke or myocardial infarction. Four subjects each were treated with aspirin and/or anticoagulation. All patients treated with anticoagulation experienced an adverse bleeding event while on treatment (Table 2).
Conclusion(s): Although this data is limited by a small sample size and biased towards patients with more severe phenotypes, it is notable that post‐menopausal bleeding, in particular surgical bleeding, remains common despite the competing vascular risk factors and rising von Willebrand Factor and Factor VIII activity levels that accompany increasing age. These results underscore the need for strategies to individualize hemostatic therapies in older biological females with IBDs.


74. PB1184
74.1. Dose‐effect analyses of FEIBA in low volume plasma samples of inhibitor patients using a newly defined chemiluminescent‐based TGA
D. Steeghs 1; C. van de Ridder2; M. van Geffen1; C. van 't Veer1; W. van Heerde1
1 Enzyre, Nijmegen, Gelderland, Netherlands; 2 Employee, Nijmegen, Gelderland, Netherlands
Background: The main adverse effect of FVIII administration is the development of neutralizing antibodies, rendering replacement therapy less effective in patients with hemophilia A. These inhibitor patients are optionally treated with bypassing agents such as activated prothrombin complex concentrate (aPCC/FEIBA). Previous studies demonstrated the correlation between the characteristic parameters of the thrombin generation assay (TGA) with the administration of FEIBA. Here we present a new method to monitor FEIBA in plasma samples.
Aims: Evaluation of a newly developed chemiluminescent‐based TGA (TGAlum) with spiked FEIBA samples spanning the medically relevant interval.
Methods: The TGAlum detects thrombin generation with a thrombin‐specific substrate, consisting of a tripeptide coupled to a luciferin‐moiety. Upon activation of thrombin, the specific substrate is cleaved, releasing the aminoluciferin. Luciferase converts the free aminoluciferin to oxyluciferin and a light quant. The chemiluminescent TGA was performed in citrated plasma using 1 pM Tissue Factor, 8.3 μM phospholipids, and 16.7 mM calcium with a total volume of 60 μl. Different concentrations of FEIBA were spiked in FVIII deficient plasma, without and with inhibitor (0.9 and 80 NBU), with low (0.01 IU/ml), mid (1.05 IU/ml), and high (2.0 IU/ml) FEIBA levels. The technical validation was performed according to CLSI protocol EP10‐A3‐AMD.
Results: Five parameters of the TGAlum were analyzed, including peak height (PH), lag‐time (LT), time to peak thrombin (TPT), area under the curve (AUC), and the velocity index (VI). Three of these parameters, PH, AUC, and VI, demonstrated a clear correlation with the different FEIBA concentrations (R 2 > 0.912). The inter‐assay variations for these three parameters were all <20%.
Conclusion(s): The developed miniaturized chemiluminescent TGA is suitable to monitor FVIII bypassing agent therapy, even in small volumes, making it suitable for near‐patient testing.
75. VPB1191
75.1. Bleeding of unknown cause or unclassified bleeding disorder: Single center experience in west Algeria
B. Driss 1; M. Moueden2
1 Faculty of medicin university Oran 1 Algeria, MOSTAGANEM, Mostaganem, Algeria; 2 University Hospital Center of Oran, Oran, Oran, Algeria
Background: Patients with unclassified bleeding disorder or Bleeding of unknown cause, sometimes present with significant bleeding symptoms despite the presence of normal haemostatic tests. The management of this bleeding episode is not standardized.
Aims: To assess the frequency and impact of bleeding measured by ISTH‐BAT.
Methods: Retrospective cohort analysis of patients with unclassified bleeding disorder betwene 2007 and 2020 at haemobiology departement of university Hospital of Oran in west Algeria. The inclusion criterion is the presence of a significant bleeding symptom ISTH‐BAT greater than 3 and the presence of normal haemostatic exploratory tests.
Results: Out of 3,321 patients referred for exploration of a hemorrhagic syndrome over a period of 13 years, 61 patients with unclassified bleeding disorder were identified, therefore an incidence of 1.83%. 42 (69%) were female and 19 (31%) male. Mean age at diagnosis was 27 years (range 13–71 years). There was 13 case of consanguinity. Leading presenting symptoms in women were heavy menstrual bleeding (69%) and epistaxis (26%). Most frequent symptoms leading to diagnosis in men were epistaxis (93%) and bleeding after tooth‐extraction (10%). Incidence of anaemia was 48% (range 8.5–11.5 g/dl). Mean ISTH‐BAT score was 3.56 (range 3–7) and in women mean score was 5.2. 11 patients underwent 16 majors and minors surgeries. Haemostatic cover was administered in 9 cases; Blood and platelet transfusion in 02 patients.
Conclusion(s): Patients with an Unclassified Bleeding Disorder suffer from various bleeding symptoms and are at risk of bleeding when undergoing surgical procedures. Women are more exposed to hemostatic challenge, especially due to monstrual bleeding. There is a need for standardization of diagnosis and management guidelines for these patients.
76. PB1178
76.1. Praxbind for reversal of Dabigatran: Experience in a tertiary hospital
A. Lubetsky 1; S. Lalezari2; G. Kenet3; M. Misgav2
1 Sheba Medical Center, Tel‐Hashomer, Not Applicable, Israel; 2 Sheba Medical Center, Tel‐Hashomer, HaMerkaz, Israel; 3 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel
Background: Praxbind is an antibody fragment that can effectively reverses the anticoagulant effect of Dabigatran in patients with bleeding and facing urgent surgery. The 5 g dose used was planned to reverse the 99 percentile of dabigatran levels, however lower doses can also neutralize dabigatran effectively
Aims: To evaluate the use and effectiveness of Praxbind administered to bleeding and surgical patients at our center and study the effect of Praxbind dose.
Methods: A retrospective cohort of consecutive patients treated with Praxbind between 01/2016 to 10/2021 for major bleeding or before urgent invasive procedures. Praxbind dose (5 g ‐ HD, 2.5 g ‐ LD) and the decision for repeated administration was at the discretion of the treating physician. In some patients dabigatran blood levels was taken immediately before and after (30 min) administration. Response to treatment was graded as adequate, fair or non‐responding.
Results: Thirty one patients were included (14 females, median age 82 years). Dabigatran indication was atrial fibrillation in all patients. In 26% of patients (8/31) Praxbind was administered at LD. Median pre Praxbind blood levels were 117 ng/ml (IQR 323) while post administration median levels was 1 ng/ml (IQR 2). In 28 patients response to Praxbind was graded adequate. Eight patients died (mainly infection) and a single patient died from GI bleeding. In 34% of patients Dabigatran treatment was resumed pre‐discharged.
Conclusion(s): In our study cohort lower dose Praxbind seemed to be effective in achieving adequate hemostasis in most of our bleeding and surgical patients. This should be confirmed in further studies.

77. PB1180
77.1. The ex vivo effects of varying factor VIII concentration on clot formation and structure in Haemophilia A patients
C. Morris 1; J. Bester2; J. Potgieter2
1 University of Pretoria, Johannesburg, Gauteng, South Africa; 2 University of Pretoria, Tshwane, Gauteng, South Africa
Background: Haemophilia A (HA) is an X‐linked inherited bleeding disorder, caused by a deficiency of coagulation factor VIII (FVIII). Patients are not able to produce a stable fibrin clot in the propagation phase as they do not generate sufficient thrombin on platelets. The mainstay of treatment for HA in South Africa remains replacement therapy with standard half‐life FVIII clotting factor concentrate (CFC).
Aims: The study aims to examine the kinetics of blood clot formation and clot architecture at varying concentrations of FVIII in adult patients with moderate to severe HA
Methods: Whole blood of 20 HA patients with native FVIII deficient levels, and with ex vivo addition of factor VIII CFC to 10–15 IU/dl and to 30–35 IU/dl were examined using scanning electron microscopy and thromboelastography® and compared to whole blood from 10 healthy volunteers. The 10–15 IU/dl factor VIII target level was chosen as equivalent for emicizumab 50 μg/ml concentration and the presumptive level at which all bleeds were prevented in patients on prophylaxis.
Results: Scanning electron microscopy revealed distinct differences between the comparative groups. Haemophilia patients have disorganised fibrin networks with loose fibers and crosslinking. The fibrin network improved with an increase in factor VIII concentration. Blood plasma spiked to a 10–15 IU/dl factor VIII concentration, showed improvements in fibrin concentration and organisation of the fibrin network. Blood plasma spiked to a 30–35 IU/dl FVIII concentration showed an organised, stable fibrin network, comparable with the control group. Thromboelastography® showed prolonged clot formation with decreased clot strength in HA patients. When spiked with FVIII concentrate, the overall clot profile improved. While the quality of the clot improved at 30–35 IU/dl FVIII concentration, the only parameters that normalised were the R‐time and alpha angles.
Conclusion(s): FVIII concentrations of 10–15 IU/dl improved the quality of the clot, which was further enhanced by increases to 30–35 IU/dl.
78. PB1186
78.1. A novel chemiluminescent‐based Thrombin Generation Assay for near‐patient monitoring
L. van Engelshoven 1; C. van de Ridder2; D. Steeghs1; M. van Geffen1; C. van 't Veer1; W. van Heerde1
1 Enzyre, Nijmegen, Gelderland, Netherlands; 2 Employee, Nijmegen, Gelderland, Netherlands
Background: Patients with hemophilia A may benefit from self‐monitoring their coagulation status and adjusting their medication real‐time based on their physiological status without a long laboratory turnaround time. Despite Factor VIII (FVIII) levels being above 1% trough levels, this does not guarantee a proper hemostatic response. The Thrombin Generation Assay (TGA) provides a global and overall understanding of the hemostatic balance of a patient, however, it requires specific lab equipment, as well as relatively large quantities of plasma. The use of a chemiluminescent‐based TGA would allow for assay volume reduction as well as monitoring non‐replacement and FVIII bypassing agents.
Aims: Developing a chemiluminescent‐based assay for real‐time TGA analyses applicable for near‐patient platform technology.
Methods: A chemiluminescent‐based TGA was constructed using an aminoluciferin‐coupled thrombin substrate which releases aminoluciferin upon cleavage by formed thrombin. Almost instantaneously luciferase oxidizes aminoluciferin (in the presence of ATP and Magnesium) resulting in the generation of photons. The created TGA uses recalcified plasma and the addition of 1 pM Tissue Factor. Different plasma mixtures with FVIII activity (ranging 0–100%) were measured to determine the correlation between FVIII and TGA parameters, analysed with the Pearson correlation coefficient.
Results: The chemiluminescent‐based TGA displays the typical TGA characteristics; lag‐time, propagated thrombin formation, and inhibition of formed thrombin. The assay readout is real‐time without the need for mathematical derivation as is required in assays with fluorogenic substrates. Concerning FVIII dependency, peak height ranges from 1.5*106–6.2*106 Relative Light Units and showed a strong correlation with FVIII levels (r = 0.97). Strong correlations were also found for the velocity index (r = 0.99), and time to peak (r = −0.92).
Conclusion(s): This chemiluminescent‐based TGA allows for FVIII dependent thrombin generation and can therefore be used for personalized hemophilia A near‐patient test application. The dynamic range and signal intensity offer the potential to miniaturize the assay to a volume off less than 1 μl.
79. VPB1190
79.1. Efmoroctocog alfa and invasive procedures in patients with hemophilia A
S. Desage 1; C. Nougier2; S. Meunier3; Y. Dargaud4; A. Lienhart3
1 Hôpital cardiologique Louis Pradel, Hospices Civils de Lyon, LYON, Rhone‐Alpes, France; 2 Laboratoire d'Hématologie, Groupement Hospitalier Est, Hospices Civils de Lyon, Lyon, France, lyon, Rhone‐Alpes, France; 3 Unité d'hémostase clinique, Hôpital cardiologique Louis Pradel, Hospices Civils de Lyon, LYON, Rhone‐Alpes, France; 4 GEMMAT, Groupe d'Etude Multidisciplinaire en Maladies Thrombotiques, Lyon, France4 Service de Médecine Intensive Réanimation, Hôpital Edouard Herriot, Lyon, France; Unité d'Hémostase Clinique Hôpital Cardiologique Louis Pradel, Lyon, France; UR4609 Hémostase & Thrombose, Université Claude Bernard Lyon 1, Lyon, LYON, Rhone‐Alpes, France
Background: Efmoroctocog alfa (rFVIIIFc) is an extended half‐life FVIII.
Aims: We report herein our experience in managing 70 surgeries in patients with hemophilia A (HA), using rFVIIIFc.
Methods: Patients with HA followed at the Lyon Comprehensive Hemophilia Care Center who underwent a surgery with rFVIIIFc were included in this retrospective analysis. Considering the large variability in pharmacokinetics (PK), patients underwent a preoperative PK evaluation. Plasma factor VIII clotting activity (FVIII:C) was evaluated using both one‐stage (FVIII:Cos) and chromogenic (FVIII:Cch) assays for preoperative‐PK and daily monitoring of FVIII:C during the post‐operative period. The results were compared using Mann Whitney U test and p < 0.05 was considered statistically significant.
Results: 39 major surgeries and 31 minor surgeries were performed in 49 patients (33 severe, 2 moderate and 14 mild HA). The preoperative dose of rFVIIIFc was 66.1 ± 16.5 IU/kg (mean ± SD) and 45.5 ± 7.4 IU/kg, for major and minor surgeries respectively. An additional infusion of rFVIIIFc 28.04 ± 1.62 IU/kg was administered 12 h after the first infusion in 8 major surgeries. During the first postoperative week, the rFVIIIFc consumption was 38.95 ± 10.29 IU/kg/day with a total of 6.51 ± 1.68 rFVIIIFc infusions per major surgery, whilst it was 3.06 ± 1.86 infusions per minor surgery with a rFVIIIFc consumption of 36.01 ± 11.20 IU/kg/day (p = 0.03). When available, the peroperative blood loss was estimated at 310.34 ± 350.76 ml for major surgeries. No red blood cell transfusions were required in none of the surgeries. During the postoperative period, rFVIIIFc doses were adjusted according to FVIII:Cch results. According to our local guideline, we compared rFVIIIFc consumption when dosages are calculated based on the chromogenic and one‐stage‐FVIII:C assays. Using FVIII:Cch, rFVIIIFc consumption is decreased compared to FVIII:Cos. Considering the high cost of the molecule, the use of FVIII:Cch in our patients was associated with cost saving.
Conclusion(s): rFVIIIFc is effective, safe and well tolerated in patients with HA undergoing invasive procedures.
80. PB1182
80.1. Post‐operative bleeding and thrombosis in biological females with inherited bleeding disorders: A cross‐sectional analysis from the community counts registry
G. Moyer 1; M. Manco‐Johnson2
1 University of Colorado, Centennial, Colorado, United States; 2 Children's Hospital Colorado, Aurora, Colorado, United States
Background: Providers are in dire need of information to guide hemostatic therapy planning in the peri‐procedural interval for biological females with inherited bleeding disorders (IBD). This is most evident in the treatment of older patients in whom the risk of bleeding is thought to be entangled with age‐associated venous and arterial vascular disease risks.
Aims: This study aims to report the frequency of post‐operative bleeding and thrombotic events in older biological females with IBDs.
Methods: With institutional IRB approval, we performed a cross‐sectional analysis of the Center for Disease Controls’ Community Counts registry which records clinical information from individuals with IBD during each clinical visit. Information was requested for all biological females older than 44 years of age seen between the years 2000 and 2022. Results are provided as descriptive statistics and odds ratios.
Results: Out of 2,032 clinical visits, 578 surgeries were reported (Table 1) and 216 (37.3%) of these were associated with adverse bleeding. Twenty‐nine subjects (5%) experienced 34 arterial or venous thrombotic events in association with a surgical procedure (Table 2). Individuals with a venous or arterial thrombotic event were 30% less likely to have received hemostatic therapy in association with their procedure (OR = 0.7).
Conclusion(s): This is the first study to report the frequency of bleeding and thrombotic events associated with surgical intervention in older biological females with IBDs. These results do not support the hypothesis that hemostatic therapies increase the risk of post‐operative thrombotic complications in this population; though, the limitations of our methodological approach restricted our ability to properly assess this association. Despite an age‐associated increased risk of venous and arterial thrombosis, older biological females with IBDs remain at risk for surgical‐associated bleeding and additional investigation is needed to incorporate this information into individualized risk stratification assessments to guide the use of hemostatic therapies.


81. PB1179
81.1. Efficacy of subcutaneous versus intravenous administration of desmopressin in patients with von Willebrand disease and hemophilia A in need of COVID‐19 vaccination
D. Maas 1; J. van Wanroij2; N. Blijlevens3; C. Kramers4; B. Laros‐van Gorkom2; D. Meijer5; W. van Heerde6; S. Schols7
1 Radboud university medical center, Nijmegen, The Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, The Netherlands, Nijmegen, Gelderland, Netherlands; 2 Department of Hematology, Radboud university medical center, Nijmegen, The Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, The Netherlands, Nijmegen, Gelderland, Netherlands; 3 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 4 Department of Internal Medicine, Radboud university medical center, Nijmegen, The Netherlands; Department of Pharmacology‐Toxicology, Radboud university medical center, Nijmegen, The Netherlands; Department of Clinical Pharmacy, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands, Nijmegen, Gelderland, Netherlands; 5 Department of Laboratory Medicine, Laboratory of Hematology, Radboud university medical center, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 6 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, the Netherlands; Enzyre BV, Novio Tech Campus, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 7 Department of Hematology, Radboud university medical center, Nijmegen, the Netherlands; Hemophilia Treatment Center Nijmegen‐Eindhoven‐Maastricht, the Netherlands, Nijmegen, Gelderland, Netherlands
Background: Subcutaneous desmopressin (DDAVP) can be more easily administered than intravenous DDAVP and may be an efficacious alternative for the currently unavailable intranasal DDAVP to treat mild bleedings or for minor invasive procedures in von Willebrand disease (VWD) and hemophilia A.
Aims: To compare the one‐hour response to subcutaneous and intravenous DDAVP in patients with VWD or hemophilia A.
Methods: Patients with hemophilia A (FVIII ≤10 IU/dl) or VWD (VWF activity ≤10 IU/dl) whose treatment plans include DDAVP and who were to receive a COVID‐19 vaccination were eligible to participate. For COVID‐19 vaccination, FVIII or VWF activity target levels of >10 IU/dl were pursued according to international guidelines (ISTH). DDAVP was administered subcutaneously 1.5 h before vaccination. FVIII (in hemophilia and VWD) and VWF activity levels (in VWD) were determined prior to (t = 0) and 1 h after DDAVP (t = 1). All patients had a positive historical routine challenge test with intravenous DDAVP. For each participant, absolute and relative changes of FVIII and VWF activity levels 1 h after subcutaneous and intravenous DDAVP (both 0.3 μg/kg) were compared.
Results: Eleven patients were included: six with hemophilia A, three with VWD type 2M and two with VWD type 2A. Both intravenous and subcutaneous DDAVP increased FVIII and VWF activity levels in all patients. In hemophilia patients, intravenous and subcutaneous DDAVP increased FVIII levels by an average of 3.8‐fold and 3.4‐fold respectively. Peak FVIII activity levels at t = 1 ranged from 25–62 IU/dl and 29–51 IU/dl. In VWD patients, intravenous and subcutaneous DDAVP was associated with a 11.4‐fold and 5.1‐fold mean increase in VWF activity levels respectively. Corresponding peak VWF activity levels ranged from 18–100 IU/dl and 28–74 IU/dl. No bleeding after vaccination was reported.
Conclusion(s): Subcutaneous DDAVP appears to be an effective alternative for intravenous DDAVP. Moreover, like intranasal DDAVP, subcutaneous DDAVP allows the possibility of self‐administration at home.


82. PB1172
82.1. Impact of inhibitors on the procoagulant efficacy of bypass agents or hemostatic factors in combination with emicizumab
E. Arias Salgado 1; A. Dos Santos1; E. Monzón Manzano1; P. Acuña1; M. Alvarez‐Román2; M. Martín Salces1; M. Rivas Pollmar3; S. García Barcenilla4; N. Butta1; V. Jiménez Yuste5
1 Hospital Universitario La Paz‐IdiPaz, Madrid, Madrid, Spain; 2 Hospital Universitario La Paz, Madrid, Madrid, Spain; 3 Hospital Universitario La Paz‐Idipaz, Madrid, Madrid, Spain; 4 Hospital Universitario La Paz‐IdiPAZ, Madrid, Madrid, Spain; 5 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain
Background: Hemophilia A patients (PwHA) treated with Emicizumab, exhibit mild‐moderate bleeding phenotypes, thus FVIII or bypassing agents (BPAs) are required to control breakthrough/perioperative bleeds. Increments of FIX levels also induce emicizumab procoagulant effects similarly to FVIII or BPAs.
Aims: To elucidate the impact of FVIII inhibitors in the efficacy of this hemostatic response, we compared in vitro procoagulant effects of BPAs and FIX products in samples from emicizumab‐treated patients with or without inhibitors.
Methods: Blood from 21 emicizumab‐treated patients, 8 with inhibitor, was collected in CTI. Therapeutic doses of rFVIII (100IU/dl octacog‐alfa), BPAs (1μg/ml rFVIIa; 5IU/dl aPCC) or 100IU/dl of plasma‐derived FIX (pFIX), two standard‐half‐life‐rFIX (Nonacog‐alfa and Nonacog‐gamma), and extended‐half‐life‐rFIX (albutrepenonacog‐alfa) were tested. Clotting time (CT) was evaluated by Rotational Thromboelastometry (ROTEM) and Thrombin Peak by thrombin generation test (TGT) using low tissue factor concentration.
Results: Emizicumab‐treated samples showed prolonged CT (Fig‐1A) and lower thrombin peak values (Fig‐1B) than healthy controls and no significant differences were observed between samples with and without inhibitors. Addition of 100IU/dl of all FIX products normalized the CT values similarly to the same dose of rFVIII. Nonacog‐alfa was more efficient producing similar effects than the addition of 1 μg/ml rFVIIa. aPCC response was stronger. Reduction of CT was similar in samples from PwHA without or with inhibitors in all assayed conditions (Fig‐1A). Higher thrombin peak values were obtained by the addition of all FVIII/FIX factors or BPAs tested by TGT but the procoagulant effect of all the recombinant FIX was significantly higher in samples with inhibitors (Fig‐1B).
Conclusion(s): Procoagulant effect of BPAs with emicizumab was similar with or without inhibitors whereas thrombin generation induced by rFIX was enhanced in the presence of inhibitors. These results provide a rationale for investigating the concomitant use of rFIX and emicizumab in PwHA with inhibitor in breakthrough bleeding and surgery. Funding: ISCIII‐FEDER(PI19/00631); CSL Behring; Cátedra UAM‐Roche.

83. PB1175
83.1. Norwegian Real‐World Experience with recombinant factor IX Fc (rFIXFc) in Haemophilia B (HB) Patients
P. Holme 1; H. Glosli2; J. Thanner3; K. Sennfält3
11Department of Haematology, Oslo University Hospital 2Research Institute of Internal Medicine, Oslo University Hospital 3Institute of Clinical Medicine, University of Oslo, Oslo, Norway, Oslo, Oslo, Norway; 2 Oslo University Hospital, Oslo, Oslo, Norway, 36Swedish Orphan Biovitrum AB, Stockholm, Sweden, Stockholm, Stockholms Lan, Sweden
Background: In Norway, patients with haemophilia are provided centralized care at Oslo University Hospital and are registered in the national haemophilia registry. The current standard of care for moderate and severe HB patients is prophylaxis with factor IX (FIX).
Aims: To use real‐world data to evaluate treatment outcomes in prophylactically treated HB patients switching from standard half‐life (SHL) FIX to extended half‐life rFIXFc and report long‐term experience of rFIXFc treatment.
Methods: Prospective cohort study of treatment outcomes in moderate and severe HB patients switching from SHL FIX to rFIXFc treated prophylactically pre‐and post‐switch for ≥12 months. Longitudinal analysis of ongoing rFIXFc prophylaxis over 3 years.
Results: No patients had detectable inhibitors during 365 days before inclusion or developed inhibitors at data cut‐off (January 26, 2022). Mean (SD) age of the switch cohort (n = 11) was 38.1 (20.9) years and 72.7% had severe HB. Median (IQR) follow‐up time was 392.0 (365.0–582.3) days on SHL FIX and 1415.0 (1319.2–1537.2) days on rFIXFc. Median (IQR) prescribed prophylactic dose decreased by 39.5% from 42.3 (36.7–74.4) to 25.6 (23.5–29.4) IU/kg/week after switching from SHL FIX to rFIXFc; median (IQR) number of injections/week decreased from 2.5 (2.0–3.0) to 0.7 (0.7–1.0); median (IQR) annualized bleeding rate was 0.0 (0.0–1.0) before and 0.0 (0.0–0.0) after switch (Table 1). For the cohort of rFIXFc patients followed over 3 years, median (IQR) prescribed prophylactic dose was 34.9 (20.9–38.9) IU/kg/week and median (IQR) injection frequency/week was 1.0 (0.7–1.0) after 3 years (n = 12). Overall, 52.9% of rFIXFc patients experienced zero spontaneous bleedings after 1 year (n = 17) versus 91.7% after 3 years (n = 12) (Table 2).
Conclusion(s): Norwegian real‐world data suggest that HB patients on prophylaxis switching from SHL FIX to rFIXFc reduced factor consumption and injection frequency with a similar level of bleeding outcomes. Prophylactic treatment with rFIXFc resulted in high levels of bleed protection over 3 years.


84. PB1176
84.1. Norwegian real‐world experience with recombinant factor VIII Fc (rFVIIIFc) in haemophilia A (HA) patients
P. Holme 1; H. Glosli2; J. Thanner3; K. Sennfält3
1 Department of Haematology, Oslo University Hospital 2Research Institute of Internal Medicine, Oslo University Hospital 3Institute of Clinical Medicine, University of Oslo, Oslo, Norway, Oslo, Oslo, Norway; 2 Oslo University Hospital, Oslo, Oslo, Norway, 36Swedish Orphan Biovitrum AB, Stockholm, Sweden, Stockholm, Stockholms Lan, Sweden
Background: In Norway, patients with haemophilia are provided centralized care at Oslo University Hospital and are registered in the national haemophilia registry. The current standard of care for moderate and severe HA patients is prophylaxis with factor VIII (FVIII).
Aims: To use real‐world data to evaluate treatment outcomes in prophylactically treated HA patients switching from standard half‐life (SHL) FVIII to extended half‐life rFVIIIFc and report long‐term experience of rFVIIIFc treatment.
Methods: Prospective cohort study of treatment outcomes in moderate and severe HA patients switching from SHL FVIII to rFVIIIFc treated prophylactically pre‐and post‐switch for ≥12 months. Longitudinal analysis of ongoing rFVIIIFc prophylaxis over 3 years.
Results: No patients had detectable inhibitors during 365 days before inclusion or developed inhibitors at data cut‐off (January 26, 2022). Mean (SD) age of the switch cohort (n = 20) was 19.6 (16.6) years and 85.0% had severe HA. Median (IQR) follow‐up time was 437.0 (365.0–577.5) days on SHL FVIII and 1430.0 (1253.0–1516.2) days on rFVIIIFc. Median (IQR) prescribed prophylactic dose decreased from 71.8 (51.9–81.0) to 58.7 (47.8–76.3) IU/kg/week after switching from SHL FVIII to rFVIIIFc; median (IQR) number of injections per week decreased from 3.0 (2.0–3.0) to 2.0 (2.0–2.7); median (IQR) annualized bleeding rate was 0.5 (0.0 ‐ 1.0) before and 0.0 (0.0–2.0) after switch (Table 1). For the cohort of rFVIIIFc patients followed over 3 years, median (IQR) prescribed prophylactic dose was 67.7 (59.2–77.3) IU/kg/week and median (IQR) injection frequency/week was 2.0 (2.0–2.3) after 3 years (n = 19). Overall, 84.2% of rFVIIIFc patients experienced zero spontaneous bleedings after 3 years (n = 19) (Table 2).
Conclusion(s): Norwegian real‐world data suggest that HA patients on prophylaxis switching from SHL FVIII to rFVIIIFc reduced factor consumption and injection frequency with an improved level of bleeding outcomes. Prophylactic treatment with rFVIIIFc resulted in high levels of bleed protection over 3 years.

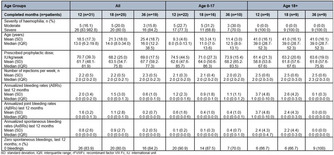
85. PB1174
85.1. Eptacog beta for resolution of bleeding episodes in patients with hemophilia A or B with inhibitors: Efficacy and safety according to inhibitor titer
D. Hart 1; R. Sidonio2; Y. Abajas3; S. Ahuja4; M. Alvarez‐Román5; M. Carcao6; Y. Dargaud7; M. Escobar8; C. Kessler9; O. Khan10; C. Leissinger11; P. Maes12; M. Mancuso13; A. Majluf Cruz14; W. Miesbach15; M. Simpson16; M. Wang17; C. Macie18; H. Wang19; A. Srivastava20
1 The Royal London Hospital Haemophilia Centre, Barts and The London School of Medicine and Dentistry, London, England, United Kingdom; 2 Aflac Cancer and Blood Disorders Center, Emory University, Atlanta, Georgia, United States; 3 UNC Hemophilia and Thrombosis Center; University of NC at Chapel Hill, Chapel Hill, North Carolina, United States; 4 Rainbow Babies and Children's Hospital, Cleveland, OH, USA, Cleveland, Ohio, United States; 5 Hospital Universitario La Paz, Madrid, Madrid, Spain; 6 Division of Hematology/Oncology, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada; 7 GEMMAT, Groupe d'Etude Multidisciplinaire en Maladies Thrombotiques, Lyon, France4 Service de Médecine Intensive Réanimation, Hôpital Edouard Herriot, Lyon, France; Unité d'Hémostase Clinique Hôpital Cardiologique Louis Pradel, Lyon, France; UR4609 Hémostase & Thrombose, Université Claude Bernard Lyon 1, Lyon, LYON, Rhone‐Alpes, France; 8 University of Texas Health Science Center, Houston, Texas, United States; 9 Georgetown University Medical Center, Washington, District of Columbia, United States, 10 University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States, 11 Tulane University, New Orleans, California, United States, 12 Antwerp University Hospital, Egedem, Antwerpen, Belgium, 13 IRCCS Humanitas Research Hospital, Milan, Lombardia, Italy, 14 Unidad de Investigación Medica de Trombosis, Hemostasia y Aterogénesis. Instituto Mexicano del Seguro Social, Mexico City, Distrito Federal, Mexico, 15 Goethe University Hospital, Frankfurt, Hessen, Germany, 16 Rush University Medical Center, Chicago, IL, USA, Chicago, Illinois, United States, 17 University of Colorado Hemophilia and Thrombosis Center, Aurora, Colorado, United States, 18 HEMA Biologics, Louisville, Kentucky, United States, 19 LFB‐USA, Framingham, Massachusetts, United States, 20 Christian Medical College, Vellore, India, Vellore, Tamil Nadu, India
Background: Eptacog beta (LFB/HEMA Biologics) is a new bypassing agent approved in the US for treatment of bleeding episodes (BEs) in patients with hemophilia A/B with inhibitors (PwHABI) aged ≥12 years.
Aims: To evaluate eptacog beta for treatment of BEs according to low (<5 BU) or high (≥5 BU) inhibitor titer.
Methods: This analysis pertains to PERSEPT 1, a phase 3 study performed by LFB in PwHABI aged ≥12 years (NCT02020369). IEC/IRB approvals and informed consent were obtained. PwHABI received eptacog beta for treatment of BEs at an initial dose of 75 or 225 μg/kg, followed by 75 μg/kg at predefined intervals over 24 h. The primary endpoint (EMA definition) was the proportion of successfully treated BEs (i.e., ‘good’/‘excellent’ response), irrespective of BE severity, 12 h after initial dose.
Results: Twenty‐seven patients with 468 BEs were analyzed (low‐titer subgroup, 218 BEs; high‐titer subgroup, 250 BEs) (Table 1). The proportion (95% CI) of successfully treated BEs at 12 h with 75 and 225 μg/kg initial dose regimens (IDRs), respectively, was 75.2% (63.6–86.8) versus 87.1% (79.8–94.5) in the low‐titer subgroup (p = 0.006), and 94.6% (88.2–100) versus 100% (100–100) in the high‐titer subgroup (p = 0.034) (Figure 1). Corresponding results at 24 h were 96.4% (91.5–100) versus 99.0% (97.1–100) in the low‐titer subgroup (p = 0.118), and 96.9% (92.4–100) versus 100% (100–100) in the high‐titer subgroup (p = 0.125). Two patients in the low‐titer subgroup experienced seven treatment‐related adverse events (infusion site discomfort [n = 4]; infusion site hematoma [n = 2]; body temperature increase [n = 1]). None occurred in the high‐titer subgroup. No thromboembolic events, hypersensitivity reactions, or deaths were reported. No eptacog beta neutralizing antibodies were detected.
Conclusion(s): Both eptacog beta IDRs achieved successful resolution of BEs in PwHABI irrespective of inhibitor titer with a higher response rate for 225 μg/kg IDR at 12 h. No safety issues were noted.


86. PB1188
86.1. Efficacy and safety of eptacog beta for resolution of spontaneous versus traumatic bleeding episodes in patients with hemophilia A or B with inhibitors
J. Windyga 1; S. Meeks2; S. Acharya3; L. Boggio4; S. Bonanad5; G. Castaman6; A. Harroche7; J. Journeycake8; J. Mahlangu9; L. Malec10; C. McGuinn11; U. Nowak‐Göttl12; A. Rafique13; M. Reding14; C. Macie15; H. Wang16; C. Hermans17
1 Institute of Hematology and Transfusion Medicine, Warsaw, Mazowieckie, Poland; 2 Emory University and Aflac Cancer and Blood Disorder Center, Children's Healthcare of Atlanta, Atlanta, Georgia, United States; 3 Rainbow Babies & Children’s Hospital, Cleveland, Ohio, United States; 4 Rush University Medical Center, Chicago, Illinois, United States; 5 Hematology Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 6 Careggi University Hospital, Florence, Toscana, Italy; 7 CRC‐MHC Hôpital Necker‐Enfants malades, Paris, France, Paris, Ile‐de‐France, France; 8 University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States; 9 University of the Witwatersrand and NHLS, Johannesburg, South Africa, 10 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States, 11 New York Weill Cornell Center; Reg Comp Hemophilia Diag & Trmt Center, New York, New York, United States, 12 University Hospital Schleswig‐Holstein, Department for Hemostasis and Thrombosis, Institute for Clinical Chemistry, Kiel, Schleswig‐Holstein, Germany, 13 Louisiana Comprehensive Hemophilia Center; Tulane University Medical Center, New Orleans, Louisiana, United States, 14 Center for Bleeding and Clotting Disorders, University of Minnesota, Minneapolis, Minnesota, United States, 15 HEMA Biologics, Louisville, Kentucky, United States, 16 LFB‐USA, Framingham, Massachusetts, United States, 17 Cliniques Universitaires Saint‐Luc, Brussels, Brussels Hoofdstedelijk Gewest, Belgium
Background: Prophylaxis with non‐factor replacement therapy has significantly reduced the incidence of spontaneous bleeds in patients with hemophilia. However, bypassing agents remain the first line to treat traumatic bleeding episodes (BEs). Eptacog beta is a new bypassing agent approved in the US for BE treatment in patients with hemophilia A or B with inhibitors (PwHABI) aged ≥12 years.
Aims: To evaluate eptacog beta efficacy and safety for treatment of spontaneous versus traumatic BEs in PwHABI.
Methods: PERSEPT 1 was a randomized, cross‐over, phase 3 trial (NCT02020369, LFB funded) in which PwHABI aged ≥12–75 years received eptacog beta for treatment of BEs using an initial dose regimen (IDR) of either 75 or 225 μg/kg followed by 75 μg/kg at predefined intervals over 24 h. The primary endpoint, per EMA definition, was the proportion of successfully treated BEs (i.e., ‘good’/‘excellent’ response), irrespective of BE severity, 12 h after the initial dose. IEC/IRB approval was granted, and all patients (or parents/legal guardians) provided informed consent.
Results: Among the 27 patients in PERSEPT 1, 27 had spontaneous BEs (197 BEs treated with 75 μg/kg IDR and 184 BEs treated with 225 μg/kg IDR) and 21 had traumatic BEs (53 BEs treated with 75 μg/kg IDR and 32 BEs treated with 225 μg/kg IDR). The location of BEs is presented in Figure 1. A similarly high proportion of spontaneous and traumatic BEs achieved successful resolution at 12 and 24 h (Table 1). Two patients experienced seven treatment‐related adverse events [infusion site discomfort (n = 4), infusion site hematoma (n = 2) and body temperature increase (n = 1)] during 2 spontaneous and 3 traumatic BEs. There were no thromboembolic events, hypersensitivity reactions, or deaths; no neutralizing antibodies to eptacog beta were detected.
Conclusion(s): Both eptacog beta IDRs achieved successful and safe resolution of spontaneous and traumatic BEs at 12 and 24 h in adolescent and adult PwHABI.


86.2. Novel Biotherapeutics in Hemophilia
87. PB0215
87.1. Efficacy, effectiveness, and safety of emicizumab for the treatment of people with hemophilia A: A systematic review
R. Muniz1; R. Camelo 2; J. Álvares‐Teodoro1; M. Barbosa1; M. Araujo3
1 Federal University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 2 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 3 Federal University of Minas Gerais, Juiz de Fora, Minas Gerais, Brazil
Background: Hemophilia A (HA) is a rare bleeding disorder due to reduced or complete absence of the clotting activity of the factor VIII (FVIII). Replacement of FVIII to treat (episodic) or prevent (prophylaxis) bleeding is the main therapeutic recommendation for people with HA (PwHA) and a hemorrhagic phenotype. However, up to 30% of these PwHA may develop anti‐FVIII alloantibodies (inhibitors), neutralizing the clotting activity of FVIII and requiring bypassing agents. Emicizumab is a monoclonal antibody recently approved for prophylaxis of both PwHA without or with inhibitors.
Aims: We are performing a systematic review to evaluate efficacy, effectiveness, and safety of emicizumab as prophylactic therapy of PwHA.
Methods: The protocol for this systematic review was published on PROSPERO (CRD42021282088) and conducted following PRISMA 2020 statement. Electronic bibliographic databases were used to search publications, followed by validation. No language nor publication period were used as restrictions. We included randomized controlled trials and observational studies (prospective cohort, retrospective cohort, and cross‐sectional) comparing emicizumab with FVIII or bypassing agents as prophylaxis. Two independent reviewers will select the articles and extract the data using a standardized form. Risk of bias assessments and grading the quality of evidence will be performed for the evaluation (image 1).
Results: Our search identified 1,436 publications from the databases. After removal of 411 duplicates and 924 articles by title/abstract screening, 101 publications will be evaluated as full‐text for selection (image 2). We hope this systematic review will be complete by the ISTH congress.
Conclusion(s): It is expected that this systematic review will help to understand the aspects of efficacy, effectiveness, and safety regarding the treatment of PwHA with emicizumab.


88. PB0216
88.1. Efficacy, effectiveness, and safety of extended half‐life factor VIII products for the treatment of hemophilia A: Protocol of a systematic review
M. Araujo1; R. Camelo 2; M. Barbosa3; R. Muniz3; J. Álvares‐Teodoro3
1 Federal University of Minas Gerais, Juiz de Fora, Minas Gerais, Brazil; 2 Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, The Netherlands, Belo Horizonte, Minas Gerais, Brazil; 3 Federal University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Background: The treatment of people with hemophilia A (PwHA) consists of replacing the deficient clotting factor with factor VIII (FVIII) products, intravenously infused either as prophylaxis (to avoid bleedings) or on‐demand (after a bleeding episode). Until recently, frequent infusions were required to reach and maintain the desirable plasma FVIII‐clotting activity, due to its pharmacokinetics. Extended half‐life (EHL) products were developed to provide a more flexible posology.
Aims: To evaluate the efficacy, effectiveness, and safety of EHL‐FVIII for the treatment of PwHA.
Methods: The systematic review was approved by PROSPERO (CRD42021281642) and is being conducted using the PRISMA 2020 statement. Structured search strategies were performed in the PubMed, Embase, Cochrane Library, LILACS, and CRD databases, validated in sequence through the Epistemonikos database and Google Scholar. No language nor publication period were used as restrictions. Randomized controlled trials, non‐randomized controlled trials, and observational studies (prospective cohort, retrospective cohort, and cross‐sectional) that evaluated the prophylaxis with EHL‐FVIII in PwHA compared with standard half‐life FVIII or other EHL‐FVIII are being included. Two independent reviewers will select the articles and extract the data using standardized forms. Disagreement will be resolved by a third reviewer. The methodological quality assessment tools will be adopted according to the specific research design of each included study. Likewise, the certainty of the evidence will also be evaluated, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool (Figure 1).
Results: A total of 6,068 articles were retrieved in the initial search. After excluding duplicates, 4,999 articles are under evaluation. We expect this review will be complete by the date of the congress.
Conclusion(s): It is expected that our findings will contribute to filling a knowledge gap regarding the EHL‐FVIII products for prophylaxis in PwHA.

89. VPB0228
89.1. Investigation of properties of macropore silica sorbents and active dyes as ligands suitable for purification of factor VIII coagulation
N. Shurko; T. Danysh; I. Yurchyshak; S. Miliashkevych; V. Novak
State Institution “Institute of Blood Pathology and Transfusion Medicine NAMS of Ukraine”, Lviv, L'vivs'ka Oblast', Ukraine
Background: A number of synthetic dyes, as they react with a wide variety of biological materials could be used as affinity ligands. The commercial active dyes contain various impurities which may affect their biochemical and related use. The purification of FVIII is generally required for the treatment Haemophilia A or von Willebrand’s disease and heavy loss of blood, requires relatively high purity for medical use.
Aims: to investigate of properties macropore silica and active dyes as ligands.
Methods: chemical synthesis of macropore silica sorbents with active dyes by the method of "salt inclusion"; ultradiafiltration; spectrophotometric.
Results: We used in the work: Diasorb‐aminopropyl 800/70, fraction 0.25–0.50 μm, Cibacron Brilliant Yellow 3GP, Procion Blue HB, Procion Blue MXR, Procion Yellow HE3G, Procion Gelb M4R, Procion Red MX5B, Reactive Brown 10, Reactive Red 120, Reactive Green 5, Reactive Green 19, Active bright orange KH, Active violet 4K, Active bright blue КН, Active bright red 5SН, Active burgundy 4SG and Active purple 4GT. The chemical stability of sorbents was evaluated under the influence of high ionic strength of the solution, polar solvents, changes in pH and high temperature. The bond strength was assessed by detecting the free dye in the light‐absorbing solution at the appropriate wavelength for each dye, which was measured on a DS‐11FX spectrophotometer (DeNovix, England). The appearance of free dyes in the supernatant at high concentrations of NaCl (5.0 M) or isopropanol (50%) indicates that a small amount of dyes was bound to the matrix due to non‐covalent interaction. The release of the dye into solution at pH 10.0 is due to the partial hydrolysis of the silica matrix.
Conclusion(s): Characteristics and advantages in practical application of affinity sorbents are determined.
90. PB0219
90.1. Thrombin generation measurement in hemophilia A patients with inhibitors receiving emicizumab prophylaxis
Y. Dargaud 1; C. Escuriola Ettingshausen2; S. Desage3; S. Meunier4; A. Lienhart4
1 University Lyon 1, Lyon, Rhone‐Alpes, France; 2 Hämophilie Zentrum Rhein Main (HZRM), Mörfelden‐Walldorf, Germany, Moerfelden‐Walldorf, Hessen, Germany; 3 Hôpital cardiologique Louis Pradel, Hospices Civils de Lyon, LYON, Rhone‐Alpes, France; 4 Unité d'hémostase clinique, Hôpital cardiologique Louis Pradel, Hospices Civils de Lyon, LYON, Rhone‐Alpes, France
Background: Emicizumab is a recombinant, humanized, bispecific, monoclonal antibody that bridges activated factor IX and factor X to restore the function of deficient factor VIII. Bypassing agents (BPA) are used for the management of breakthrough bleeds in patients with hemophilia A patients and inhibitors (PWHAI) on prophylaxis with emicizumab, with concerns about the risks of combining two procoagulant drugs. Thrombin generation assay (TGA) might be used to support decision‐making in patients on emicizumab undergoing major surgery or experiencing breakthrough bleeds, to both predict efficacy and to potentially minimize the risk of thrombotic events.
Aims: The aim of this study was to compare different analytical conditions in order to make TGA more sensitive to the procoagulant effect of emicizumab.
Methods: TG was measured in platelet rich (PRP) and platelet poor‐plasma (PPP) samples from PWIHAI receiving emicizumab. Coagulation was triggered with tissue factor (TF) 1pM or FXIa + TF; results were compared to normal values determined with the same activators. Increasing concentrations of aPCC 0–12.5–25–50 U/kg; rFVIIa 90–180–200–270 μg/kg and recombinant porcine FVIII (r‐pFVIII) 6–12.5–25–50–100–200 IU/dl were tested.
Results: The mean endogenous thrombin potential (ETP) of PPP samples from PWIHAI receiving emicizumab, was very low 371 ± 114 nM.min when coagulation was triggered with TF1pM, while ETP was almost normalized (1014 ± 363 nM.min) in PRP [normal values 1432 ± 229 nM.min]. In PPP, we observed significantly improved ETP (1975 ± 268 nM.min), when coagulation was triggered with TF + FXIa [normal values 2110 ± 737 nM.min]. Using TF + FXIa as trigger, the addition of BPA improved TG in all plasma samples with a certain variability according to patient’s individual responses.
Conclusion(s): TG measurement in PRP or the use of TF + FXIa with PPP may be more appropriate for evaluating the procoagulant effect of emicizumab, compared to TF 1pM. Using appropriate analytical conditions, TG assay may help to monitor the efficacy of BPA and r‐pFVIII in PWIHAI receiving prophylaxis with emicizumab.
91. PB0224
91.1. Evaluation of thrombin generation and emicizumab concentration in a cohort of severe Hemophilia A patients with and without inhibitor during emicizumab prophylaxis
M. Mormile1; F. Barone2; E. Baldacci1; A. Ferretti2; M. Chavez Orellana2; G. Maestrini2; A. Serrao3; F. Viola2; A. Chistolini2; C. Santoro 1
1 Hematology, University Hospital Policlinico Umberto I, Rome, Rome, Lazio, Italy; 2 Hematology, Sapienza University of Rome, Rome, Lazio, Italy; 3 Hematology, Sapienza University, Rome, Italy, Rome, Lazio, Italy
Background: Although emicizumab is administered at fixed dose, its measurement could be occasionally required. Thrombin generation assay (TGA) can be used to monitor the global hemostatic response in emicizumab treated patients.
Aims: To measure emicizumab plasma levels and TGA in severe hemophilia A (HA) patients without and with inhibitors.
Methods: A modified one‐stage FVIII clotting assay has been used to measure emicizumab concentration. The assay is calibrated against emicizumab using a dedicated plasma emicizumab calibrator and two levels of control. Thrombin generation was determined by Calibrated Automated Thrombography (CAT) using PPP‐Reagent (phospholipids [4 μM], TF [5 pM]).
Results: 10 patients (non‐inhibitor 6; inhibitor 4) received emicizumab. At start, six were without and four with inhibitor. No spontaneous bleedings occurred. Two patients experienced traumatic bleedings. One was treated with rFVIII concentrate (14th dose of life) and subsequently developed a high titer inhibitor. At the time of blood drawn he was considered an inhibitor patient. Emicizumab plasma levels increase during loading phase; median concentration at steady state is 42.2 μg/ml (25.3–75). Lag time is in normal range after the first dose; ETP is variable among patients, almost always below normal range, and does not significantly differ between loading and steady state phase (Table 1). Comparing non inhibitor and inhibitor patients (Table 2), at steady state median emicizumab concentration is 58.4 μg/ml (46.1–70.7) and 34.7 μg/ml (25.3–75), respectively. In non‐inhibitor patients, median ETP and peak are higher than in inhibitor patients: ETP 1073.14 nMxmin (562.17–1412.23), peak 92.67 nM (38.43–107.11) versus ETP 688.64 nMxmin (369.64–895.24), peak 43.065 nM (22.99–56.66).
Conclusion(s): We observed variability among patients as regards emicizumab concentration and TGA, but the drug was efficacious in all. It has to be explored if variables such as inhibitors, BMI, patients age, adherence to treatment, time period from the last infusion, influences emicizumab concentration and/or TGA.

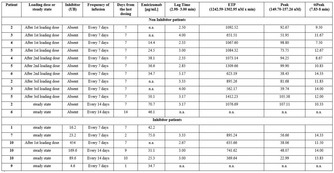
92. PB0220
92.1. Evaluation of four different reagents on the coagulation analyzers STAR MAX® and ATELLICA® COAG 360 for Emicizumab concentration measurement in severe hemophilia A patients
A. Launois1; E. de Raucourt2; I. Martin‐Toutain1; F. Samdjee3; S. Le Dore3; E. Ferre3; C. Flaujac 4
1 Laboratoire de Biologie Médicale, Hôpital André Mignot, Le Chesnay, Ile‐de‐France, France; 2 Centre de ressources et compétences des Maladies Hémorragiques Constitutionnelles rares, centre hospitalier de Versailles (André Mignot), Le chesnay, Ile‐de‐France, France; 3 Hôpital André Mignot, Le Chesnay, Ile‐de‐France, France, 4 laboratoire de biologie médicale‐ secteur hémostase ‐ André Mignot hospital, Versailles, Le Chesnay, Ile‐de‐France, France
Background: Emicizumab is a bispecific antibody restoring part of coagulation and indicated in prophylaxis in severe hemophilia A patients. Biological monitoring is a challenge because emicizumab interferes with coagulation tests.
Aims: The objective of this study is to evaluate the reliability of the CK‐Prest®, Cephasceen® and STA‐PTT Automate® reagent (Stago) on the analyzer STAR MAX® (Stago) and Actin FS® (Siemens) on the analyzer ATELLICA® COAG 360 (Siemens) for emicizumab dosing in real life conditions.
Methods: 21 patients (mean age = 33 years [7–55]), had a dosage before emicizumab treatment, and at day 7, 14 and 21. 83 samples were analyzed with APPT (CK‐Prest®) and FVIII:C dosage with one stage chronometric assay. Emicizumab dosage were calibrated with r 2 diagnostics reagents, on a FVIII derived methodology using CK‐Prest®, Cephascreen®, STA‐PTT Automate® or Actin FS®. Inter‐assay variability was evaluated by normal and pathologic controls (CN;CP).
Results: At baseline, emicizumab was <2 μg/ml, except for 4 patients with FVIII:C >0.15 IU/ml. APTT normalizes at day 7 with a FVIII:C mean concentration at 1.80 IU/ml [1.10–3.00]. The mean emicizumab concentration was around 21 μg/ml at day 7, 37 μg/ml at day 14 and 50 μg/ml at day 21, with a mean FVIII:C >4.0 IU/ml for all patients except one (3.11 IU/ml) which had the lowest emicizumab concentration. Inter‐assay variabilities were, respectively for CP and CN, 7.0% and 7.2% with CK‐Prest®, 6.6% and 6.4% with Cephascreen®, 5.9% and 5.0% with STA‐PTT Automate® and 1.9% and 5.0% with Actin FS®. Emicizumab assays showed a good correlation and a mean bias under 10% for the four reagents [2.7%;‐8.4%] compared to CK‐Prest®.
Conclusion(s): In real life conditions, our evaluations show that the four techniques seem to be acceptable for the measurement of emicizumab on the coagulation analyzers STAR MAX® and ATELLICA® COAG 360. This must be confirmed by further evaluation.


93. PB0229
93.1. Emicizumab: In‐house method validation
R. Sueldo 1; M. Hepner2; J. Frontroth3; E. Annetta4; C. Cervio3; M. Bianco5; G. Sciuccati5
1 Laboratorio de Hematología. Hospital Dr. César Milstein, Hospital de Pediatría Juan P. Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Laboratorio de Hemostasia y Trombosis. Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina, 4 Hospital de Pediatría Prof Dr JP Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: Emicizumab (Hemlibra®, ROCHE), is effective in the prevention of bleeding in severe Hemophilia A patients with (sHAinh) or without (sHA) inhibitors. To date, emicizumab plasma quantification (Epq) is not routinely recommended but, could be required to verify treatment adherence, suspected anti‐Emicizumab antibodies. Epq method, calibrators and quality controls are not yet available.
Aims: To validate in‐house Epq method using a modified one‐stage assay (modOSA).
Methods: modOSA: 1:80 pre‐diluted sample with Owren Koller Buffer (OKB). Calibration Curve: 1:1 (1:80 pre‐diluted home‐made calibrator (hmCal) with OKB), 1:2, 1:4, 1:8, 1:16, 1:32. hmCal (150 μg/ml of Emicizumab): FVIII deficient plasma (FVIIIdp) was spiked with Emicizumab. Home‐made Controls: level 1 = 25μg/ml (hmL1), level 2 = 75μg/ml (hmL2). Reagents: Immunodef VIII, C.K. Prest, CaCl2 0.025 M. STA Compact Max2, Diagnostica Stago. Repeatability (CVr) = hmL1 and hmL2: 20 times in the same run. Within‐laboratory Imprecision(CVwl) = hmL1 and hmL2 in triplicate for 5 days. Calibration curve: 6 levels in duplicate (range: 5–150 μg/ml). Linearity: 11 levels in triplicate, using hmCal and FVIIIdp (0 μg/ml). In addition, aPTT, One Stage FVIII Assay (OSA), FVIII Chromogenic Assay (CSA, Bovine reagent, Chromogenix) and OSA/Epc were measured in the 9 levels. Limit of quantification (LoQ): FVIIIdp, sHAinh and sHA samples were evaluated. Bias estimation: from an external quality assessment program (UK NEQAS). Interference: mixtures of Emicizumab with FVIII were evaluated (Unicalibrator, Diagnostica Stago as a source of FVIII). EP evaluator was used for statistical analysis.
Results: hmL1: CVr 4.8% (27 μg/ml); CVwl 5.7% (26 μg/ml), hmL2: CVr 3.1% (73 μg/ml); CVwl 4.1% (74 μg/ml). Calibration curve: r: −0.999, r 2: 0.998. Linearity: Table1, r 2: 0.997 (linear range 5–150 μg/ml), Slope: 1.011, Intercept: 0.52. LoQ: 5 μg/ml. Bias: −4.0%. Interference: Table 2.
Conclusion(s): modOSA showed a good performance in the Epq. aPTT was below the reference range and CSA did not interfere in the evaluated range of 5–100 μg/ml of Epq. FVIII surrogate activity is appoximately 7 times the level of Epq. In vitro FVIII acts as an interference in the method.


94. PB0222
94.1. Assay of modified factor VIII is modulated by von Willebrand factor. What should we measure ?
C. Kluft; M. Verhoek
Good Biomarker Sciences, Leiden, Zuid‐Holland, Netherlands
Background: Factor VIII circulates in a reversible complex with von Willebrand factor (VWF), usually complexed for >95%. It can be calculated that this complex remains highly associated up to very low factor VIII levels. It is secured as complex in the One‐stage Clotting Assay (OCA) by a final dilution before assay of the sample 1:1 with vWF containing factor VIII deficient plasma. Consequently, it is the activity of the complex that is assayed in the OCA.
Results: For normal plasma FVIII and Advate, clotting activity of free and complexed FVIII is similar. However, for modified FVIII (Refacto, Eloctate, Adynovi) added VWF (Wilfactin) inhibits their coagulation activities. For Eloctate and Refacto this concerns a maximal inhibition of 74% and 52%. Adynovi showed maximal inhibition of 27%. The optimal inhibitory effect is reached at 50% of added VWF. Thus, also for these preparations in the OCA the activity of the complex will be recorded. This is different for the chromogenic assay (CA) which records factor VIII independent of VWF. Comparing five preparations with labeled activity in the OCA and CA, both calibrated with normal plasma, showed especially for Eloctate a discrepancy with 0.44 x lower activity in the OCA as expected from VWF inhibition. For Advate and Beriate activities were similar in both assays. In a VWF free APTT spiking of Eloctata and Advate activities were similar as expected. The labeling of the five preparations showed for three agreements with our analysis with CA, but was fourfold lower for Advate and Beriate.
Conclusion(s): Normal factor VIII can be measured and labeled with both CA and OCA; our results indicate that the preferred method for modified FVIII is the OCA. In patients treated with modified FVIII, the OCA method should be used as pharmacodynamic method. The labeling of preparations needs to be reconsidered.
95. PB0217
95.1. Impacts of Mim8 on routine coagulation assays, on intrinsic pathway coagulation factor assays and on thrombophilia coagulation tests
L. Fauconnier1; N. Roland1; A. Carlo 2; T. Hervé3; F. Depasse2; M. Ezban4; L. Nicoud1; N. Martineau1
1 Diagnostica Stago, Gennevilliers, Ile‐de‐France, France; 2 Diagnostica Stago, Asnières Sur Seine, Ile‐de‐France, France; 3 Diagnostica Stago, Asnières sur Seine, Ile‐de‐France, France; 4 Novo Nordisk A/S, Måløv, Hovedstaden, Denmark
Background: Mim8 is a new bispecific antibody that mimics the cofactor function of activated factor VIII (FVIIIa) by bridging activated factor IX and factor X, enhancing the activation of Factor X. Mim8, which is currently in clinical trials, is aimed for prophylactic treatment of haemophilia A patients, with or without inhibitors. Like emicizumab, it has been observed, that these new drugs can impact the results of clotting assays.
Aims: Evaluate Mim8 effects on routine coagulation assays (PT and aPTT), on one‐stage and chromogenic intrinsic pathway coagulation factor assays and on thrombophilia coagulation tests using Stago reagents.
Methods: A Factor VIII deficient plasma (Stago, Asnières/Seine, France) was spiked with 1.7, 5 and 50 μg/ml of Mim8. All samples were tested once, in triplicate, in a single experiment with Stago reagents on STA‐R Max analyzer.
Results: aPTT clotting times using different aPTT reagents were significantly shortened with Mim8 at all concentrations tested. Accordingly, the activity measurements of the clotting factors VIII, IX, XI and XII with a one‐stage clotting assay were also significantly affected by the presence of Mim8. It was also observed that Mim8 interferes slightly on FVIII measurement with a chromogenic assay (TriniCHROM VIII:C) (Table 1). Mim8 has a limited impact on PT measurement and no impact on the chromogenic measurement of protein C (PC) activity and on immunoturbidimetric measurement of free protein S (PS). In contrast, PC and PS activities measured with clotting assays are significantly affected by Mim8 (Table 2).
Conclusion(s): As it has been described for emicizumab, Mim8 interferes with aPTT and aPTT based one‐stage factor VIII, IX, XI and XII assays. Mim8 has a limited effect on PT measurement. Regarding PC and PS measurement, chromogenic and immunoturbidimetric assays remain unaffected by Mim8 while PC and PS levels decrease artefactually with clotting‐based assays.


96. VPB0230
96.1. Emicizumab treatment of 4 patients with severe hemophilia A and inhibitors in pediatric age—Resource management to minimize economic costs while maintaining the quality of clinical follow‐up
M. Urbano 1; F. Machado2; B. Marques1; C. Guedes3; T. Brites3; O. Lavrukhina3; S. Silva2; J. Feio2; C. Geraldes1; R. Salvado3; J. Tomaz3
1 Clinical Hematology Department, Centro Hospitalar e Universitário de Coimbra, Coimbra, Coimbra, Portugal; 2 Pharmacy Department, Centro Hospitalar e Universitário de Coimbra, Coimbra, Coimbra, Portugal; 3 Blood and Transfusion Medicine Department, Hemophilia Reference Centre, Centro Hospitalar e Universitário de Coimbra, Coimbra, Coimbra, Portugal
Background: People with hemophilia lack certain proteins (clotting factors) necessary for hemostasis. Its treatment consists in the replacement of these factors. The development of antibodies (inhibitors) that eliminate the administered factor, nullifying its function, is the main therapeutic complication of hemophilia. Hemophiliacs with inhibitors were usually treated with bypass factors, but we now have new therapeutic resources. Emicizumab is a bispecific monoclonal antibody with antigen‐binding fragments recognizing both FIXa and FX, partially simulating FVIII cofactor role, reconstituting the intrinsic factor Xase reaction despite the presence of FVIII inhibitor antibodies.
Aims: To study the benefits obtained with a careful management strategy of emicizumab simultaneous administration to pediatric patients with severe hemophilia A who developed inhibitors, without neglecting clinical follow‐up quality.
Methods: We analyzed retrospective results of in‐hospital scheduled emicizumab treatment of 4 children with hemophilia A with inhibitors, over 21 months. Enrolled children were summoned to come to our Center on the same day (monthly), enabling optimal usage of required vials. Emicizumab regimen: Loading dose 3 mg/kg weekly (4 weeks); maintenance dose 6 mg/kg every 4 weeks, for reduced administrations frequency.
Results: In all patients a strict clinical follow‐up was confirmed. No bleeding episodes were recorded any patient included in this study. 21 months of simultaneous emicizumab in‐hospital administration to our 4 pediatric patients resulted in overall saving of 2520 mg, translating into a 25% cost reduction during this period (which, in our case, meant saving 200 133.13€ (116 020.8€ savings per year))
Conclusion(s): Emicizumab therapy requires implementation of a careful management strategy. Our intra‐hospital, monthly, simultaneous administrations allowed us to reduce resources consumption, particularly in pediatrics due to significant weight variations, without neglecting appropriate clinical follow‐up with more frequent clinical control. Although there are publications recommending shorter intervals administration regimens as more efficient, in our series no bleeding episodes were recorded, thus equaling the effectiveness of intensive regimens.


97. PB0218
97.1. Adaptative methods using one‐stage and chromogenic substrate assays for the quantitative measurement of Mim8 in human plasma
N. Martineau1; L. Fauconnier1; N. Roland1; A. Carlo 2; T. Hervé3; F. Depasse2; M. Ezban4; L. Nicoud1
1 Diagnostica Stago, Gennevilliers, Ile‐de‐France, France; 2 Diagnostica Stago, Asnières Sur Seine, Ile‐de‐France, France; 3 Diagnostica Stago, Asnières sur Seine, Ile‐de‐France, France; 4 Novo Nordisk A/S, Måløv, Hovedstaden, Denmark
Background: Mim8 is a new bispecific antibody that mimics the cofactor function of activated factor VIII (FVIIIa) by bridging activated factor IX (FIXa) and factor X, and enhancing the proteolytic function of FIXa to activate FX. It is currently in phase 3 clinical development for prophylaxis in patients with haemophilia A (HA), independent of inhibitor status. Measurement of the drug concentration in a plasma sample may be useful to follow up Mim8 therapy.
Aims: Evaluate the use of Stago modified one stage assay (OSA) and chromogenic substrate assay (CSA) to determine plasma levels of Mim8.
Methods: A severe haemophilia A plasma was spiked with Mim8 at concentrations ranging from 0 to 30 μg/ml. The concentration of Mim8 samples is determined with modified FVIII:C OSA using STA‐PTT Automate/STA‐Deficient FVIII or STA‐CK Prest/STA‐ImmunoDef FVIII and a modified FVIII:C CSA using TriniCHROM Factor VIII:C (all reagents Stago, Asnières sur Seine, France) on a STAR Max analyzer (Stago). Mim8 reference material from Novo Nordisk A/S (Malov, Denmark) was used as calibrator.
Results: Recovery of measured Mim8 concentration compared to the expected target concentration is similar for the different reagents tested with modified FVIII:C OSA and modified FVIII:C CSA, with a relative difference lower than 10% in most cases (12% for the lowest Mim8 concentration tested with STA‐CK Prest and STA‐ImmunoDef VIII) (Table 1 and Figure 1).
Conclusion(s): Modified method using either aPTT or chromogenic reagents from Stago can be used to quantify Mim8 concentration in a hemophiliac plasma sample using Mim8 reference material as calibrator.

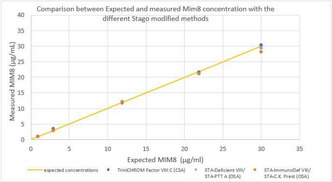
98. PB0221
98.1. Neutralizing antidrug antibody to emicizumab: Detailed laboratory evaluation and review of the literature
H. Kizilocak 1; J. Brown2; G. Young3
1 Children's Hospital Los Angeles Hemostasis and Thrombosis Center, Los Angeles, California, United States; 2 Children's Hospital Los Angeles, Los Angeles, California, United States; 3 Hemostasis and Thrombosis Center, Cancer and Blood Diseases Institute, Children's Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, California, United States
Background: Emicizumab is a monoclonal bispecific antibody which has been shown to be safe and effective in patients with hemophilia A (HA) with and without inhibitors. Treatment with monoclonal antibodies can result in the formation of antidrug antibodies (ADA).
Aims: To report 5 persons with HA (PwHA) treated with emicizumab who developed ADA (some previously published).
Methods: A literature review was conducted to identify previously reported cases of anti‐emicizumab ADA from around the world and these cases were supplemented by additional patients tested in our specialized laboratory by methods we previously published (see figure). Essentially, we developed a Bethesda‐like assay using the human chromogenic FVIII activity (humCHR) to determine the presence of ADA from patient plasma with a known concentration of emicizumab in control plasma.
Results: Five PwHA with and without inhibitors with a median age of 7 (2–62) have been identified with anti‐emicizumab ADA (see table). According to the manufacturer, ~12,000 individual PwHA have been treated with emicizumab. The presentation was unexpected/excessive bleeding and a prolonged aPTT in all cases. Four of the 5 cases had FVIII inhibitors and one did not. Four of the 5 were found to have a neutralizing ADA and one had a non‐neutralizing ADA which resulted in increased/rapid clearance of emicizumab. Interestingly, all the cases presented in the first year and 4/5 in the first 24 weeks after emicizumab initiation.
Conclusion(s): Emicizumab is very effective at bleed prevention, however, albeit rare, ADA can still occur. Breakthrough bleeding and aPTT prolongation are the first signs of ADA to emicizumab which should prompt further evaluation. There currently are no commercially available assays to determine ADA to emicizumab. Suspected cases can be identified using a modified Bethesda assay with commercially available reagents and an approximate level of ADA can be calculated.


99. PB0226
99.1. Assay of the factor VIIIa mimetic, Mim8, using one‐stage clotting assay requires a product‐specific reference material
H. Wilmot 1; M. Ezban2; E. Gray3
1 MHRA‐NIBSC, Potters Bar, England, United Kingdom; 2 Novo Nordisk A/S, Måløv, Hovedstaden, Denmark; 3 National Institute for Biological Standards and Control, Medicines and Healthcare products Regulatory Agency, Potters Bar, England, United Kingdom
Background: The human bispecific antibody, Mim8, is in clinical trials for treatment of haemophilia A. Mim8 binds factor (F) X and FIXa, enabling activation of FX and bypassing the need for FVIII.
Aims: This study investigates the suitability of FVIII one‐stage clotting (OSC) assays for measuring Mim8.
Methods: Mim8 reference material (RM) and drug product (DP) (both 200 μg/ml) were diluted to 20 μg/ml in imidazole buffer, followed by 1/10 in FVIII‐deficient plasma and 1/3 in buffer. For comparison, the FVIII plasma International Standard (IS), diluted 1/3 in buffer, and the calibrator for emicizumab (a licensed FVIII mimetic), diluted 1/10 in deficient plasma and 1/3 in buffer (~3 μg/ml) were also included. All materials were assayed (n ≥ 3) using a range of activated partial thromboplastin time (APTT) reagents and data were analysed by parallel line bioassay. Linear portions of the responses were used for calculations. Parallelism was assessed using the ratio of the standard and test slopes (acceptability 0.95–1.07).
Results: A representative result is shown in Figure 1, using APTT reagent Actin FS. The Mim8 DP was not parallel to the FVIII IS or the emicizumab calibrator (ratio of slopes for DP 1.15 and 0.84, respectively). However, the Mim8 DP was parallel to the RM (ratio of slopes 1.02), indicating a valid comparison could be made. The same trend for non‐parallelism of Mim8 to the IS or emicizumab calibrator was observed with other APTT reagents. Using RM as the assay standard, 100% recovery of DP was achieved across the 6 APTT reagents studied (Figure 2), with no assay discrepancy observed.
Conclusion(s): In conclusion, Mim8 should not be assayed against the FVIII IS nor the emicizumab calibrator. For accurate measurement of Mim8, a specific reference material must be used. With this, no APTT reagent discrepancy was observed, therefore inter‐laboratory discrepancy would be reduced.


100. VPB0227
100.1. Laboratory monitoring in haemophilia A patients with factor VIII inhibitor treated with emicizumab
C. Novembrino 1; M. Boscolo‐Anzoletti2; E. Galbiati2; F. Peyvandi3
1 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Lombardia, Italy; 2 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Milan, Lombardia, Italy; 3 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Emicizumab, a humanized bispecific antibody for haemophilia A (HA) treatment, is known to affect the activated partial thromboplastin time (aPTT) making not reliable the aPTT‐based tests, such as the “classic” Nijmegen‐Bethesda assay (clot‐based) for measuring FVIII inhibitor titre. The use of chromogenic assay with bovine regents (insensitive to Emicizumab) can avoid misinterpretation of results. A mixture of two recombinant anti‐idiotype monoclonal antibodies (anti‐Emi; Chugai and Nara Medical University) for preventive neutralization of the drugs could be an option when the chromogenic assay is not available.
Aims: To evaluate the efficacy of neutralizing anti‐Emi by comparing the results obtained with aPTT‐based and chromogenic assays on samples from HA patients treated with emicizumab.
Methods: Fifty plasma samples of HA patients with inhibitor were analysed for FVIII inhibitor titre using Nijmegen‐Bethesda assay measuring residual FVIII both with the one‐stage (Actin FS‐Siemens, Germany) and the chromogenic assay (with bovine regents, Siemens). Emicizumab concentration was measured by using a modified one‐stage assay calibrated with a specific Calibrator (r2 Diagnostics Inc., US), before and after the addition of anti‐Emi (1:20). All the tests were performed on Sysmex CS‐2400 (Sysmex, Kobe, Japan).
Results: The results are reported in Table as mean [range]. Emicizumab concentration, ranging from 100.4 to 0.9 μg/ml before neutralization, was <0.5 μg/ml in all the samples measured after the neutralization. Inhibitor titre was <0.5 UB in almost all the samples at medium and low inhibitor titre when measured with the one‐stage before neutralization. Good agreement was found between inhibitor titre measured with chromogenic assay and with one‐stage assay after neutralization, as shown by Bland‐Altman plot (Figure 1)
Conclusion(s): These results showed the efficacy of the anti‐Emi that could be used in the laboratory monitoring of inhibitor titre in HA patients treated with emicizumab when the one‐stage is the only available assay.


101. PB0214
101.1. Snake venom‐based hemocoagulase restores in vivo hemostasis and limits bleeding in murine model of hemophilia A
N. Rhoads1; L. Stanger1; M. Wong2; B. Li2; M. Holinstat1; R. Adili 3
1 Department of Pharmacology, University of Michigan, Ann Arbor, Michigan, United States; 2 Lee’s Pharmaceutical Holdings Ltd., Shatin, Not Applicable, Hong Kong, 3 University of Michigan, ANN ARBOR, Michigan, United States
Background: Hemophilia A is a hereditary life‐long bleeding disorder and FVIII replacement has been the mainstay of treatment. However, there is an unmet need for novel therapeutics to prevent and treat bleeding. We recently reported that slounase, a thrombin‐like enzyme batroxobin from a snake venom containing activated Factor X, enhances platelet‐fibrin clot formation in vivo in heparin‐anticoagulated mice, suggesting slounase may restore hemostasis by bypassing coagulation.
Aims: To determine the effect of slounase treatment on hemostatic clot formation in FVIII−/− mice with and without inhibitors in vivo and in vitro and confirm slounase restores hemostasis in hemophilia.
Methods: The effect of slounase on hemostasis and bleeding was determined in FVIII−/− mice, with and without inhibitors, using intravital microscopy hemostatic models, thromboelastography and a tail‐bleeding assay.
Results: FVIII−/− mice are unable to form hemostatic clots in vivo due to a severe defect in platelet accumulation and absence of fibrin formation at the site of vascular injury, as confirmed in micro and macro hemostatic models under real‐time intravital microscopy. Prophylactic intravenous treatment of 1U/kg slounase in FVIII−/− mice significantly enhanced platelet activation, accumulation, and fibrin formation in response to vascular injury resulting in stable hemostatic clot formation in the cremaster artery and saphenous vein laser ablation hemostasis models. Platelet‐fibrin hemostatic clots also formed following ferric chloride injury to the carotid artery in FVIII−/− mice pretreated with slounase without reaching vessel occlusion. Importantly, in vivo hemostatic enhancement persisted in FVIII−/− mice intravenously treated with anti‐FVIII antibodies and slounase. Furthermore, the hemostatic effect of slounase was also confirmed by in vitro thromboelastography and in vivo tail‐bleeding assays.
Conclusion(s): Our data indicates slounase is a novel bypassing agent that promotes platelet procoagulant activity and fibrin formation, to restore hemostasis and limit bleeding in hemophilia A, with and without inhibitors. This is a collaborative research study funded by Lee’s Pharm.
102. PB0223
102.1. Mim8 does not affect FVIII function in hemophilia A
J. Lund; K. Jensen; M. Ezban
Novo Nordisk A/S, Måløv, Hovedstaden, Denmark
Background: Mim8 is a factor IXa/factor X (FIXa/FX) bispecific antibody (BiAb) that demonstrates superior hemostatic properties in vitro and in hemophilia A (HA) mouse models compared with emicizumab. HA patients without inhibitors treated with a BiAb may require concomitant administration of factor VIII (FVIII), for treatment of breakthrough bleeds or surgeries. Both molecules compete for the same substrates; therefore, to evaluate the hemostatic potential of Mim8 and FVIII combined, we performed the thrombin generation test (TGT) with different activators.
Aims: To evaluate the effect of Mim8 + FVIII over a broad range of concentrations in severe HA plasma, using tissue factor (TF) and factor XIa (FXIa) as activators in TGT.
Methods: Thrombin generation was evaluated in severe HA plasma with added FVIII and Mim8 at concentrations between 0–2 IU/ml and 3–100 nM, respectively. TGT was initiated by 0.001 and 0.008 U/ml FXIa, or 1 pM TF.
Results: When evaluating the concentration response for FVIII or Mim8 in TGT, the amount of thrombin generated appeared dependent on the amount of FIXa generated by the activator. The effect of combining Mim8 and FVIII was similar across all respective activation conditions tested. For all Mim8 concentrations tested, an additive effect was observed for thrombin generation when combining with up to 0.3 IU/ml FVIII. At higher FVIII concentrations, thrombin generation was similar or slightly lower compared to samples without Mim8 (Figure 1).
Conclusion(s): Mim8 does not affect FVIII function in hemophilia A, thus per‐label FVIII doses can be used with Mim8 treatment, when necessary. Our TGT data suggest that patients with FVIII plasma levels up to 0.3 IU/ml may benefit from supplemental treatment with Mim8. Above 0.3 IU/ml FVIII, the added effect of Mim8 was limited and at very high concentrations of Mim8 and FVIII there was minimal competition for substrate.

103. PB0225
103.1. An engineered factor X variant as a novel by‐passing agent for hemophilia
E. Tonetto 1; M. Testa1; R. Tarantino2; L. Peretto1; M. Pinotti1; A. Branchini1
1 Department of Life Sciences and Biotechnology, University of Ferrara, Ferrara, Italy, Ferrara, Emilia‐Romagna, Italy; 2 University of Ferrara, Ferrara, Emilia‐Romagna, Italy
Background: In hemophilia with inhibitors, by‐passing agents are aimed at increasing thrombin generation efficiency by restoring or boosting the activation of factor X (FX). However, although activated FX (FXa) should be the ideal therapeutic molecule, it is rapidly inhibited and displays very short half‐life in vivo. In this context, the availability of a by‐passing FX zymogen molecule would be of great relevance, with potentially low immunogenicity due to the presence of circulating FX in patients.
Aims: To develop a novel hyper‐activatable engineered FX variant (FXHA‐BP, patent application in progress) able to by‐pass the defective intrinsic pathway in hemophilia plasma.
Methods: The expression plasmid for the FXHA‐BP variant was produced by site‐directed mutagenesis of wild‐type FX (FXwt) cDNA and transiently transfected into HEK293 cells. Secreted FX protein levels were evaluated by ELISA. Activity was assessed by PT‐based (FX‐deficient plasma) and aPTT‐based (hemophilia A plasma) assays.
Results: The FXHA‐BP variant was secreted at appreciable levels from transiently transfected HEK293 cells. As compared to FXwt, the specific activity of FXHA‐BP was slightly increased in FX‐deficient plasma (1.5 ± 0.4 fold; p = 0.0414) in PT‐based assays. Importantly, upon intrinsic activation in aPTT‐based assays, addition of 500 ng/ml FXHA‐BP to hemophilia A plasma significantly shortened coagulation times (105 ± 2 s) over the baseline (118 ± 4 s; p < 0.0001), whereas addition of an equivalent amount of FXwt, used as control, resulted in negligible effects (117 ± 4 s; p < 0.0001). These data demonstrated the by‐passing activity of the FXHA‐BP variant even at the relatively low concentrations obtained after transient expression.
Conclusion(s): Through rational engineering we created a novel improved FX variant acting as a by‐passing agent in hemophilia plasma. The FXHA‐BP molecule will be further engineered to prolong its half‐life, with the aim of developing an extended half‐life by‐passing variant that might represent a novel therapeutic tool for hemophilia with inhibitors.
103.2. Rare Bleeding Disorders
104. VPB0723
104.1. Hematological orphan diseases and health literacy in sick people: State of the art
N. Perdomo Olarte
Universidad del Tolima, Vihonco IPS, Medicarte SAS, IPS Especializada, Ibagué, Tolima, Colombia
Background: This article presents an analysis of the current state of studies carried out in the world and in the country on orphan and hematological diseases and their relationship with health literacy.
Aims: Its objective is to analyze the situation of hematological orphan diseases at the international, national and local level, in order to build bases for new lines of research and design of strategies that allow progress in care models and health literacy levels.
Methods: It was established that a high number of studies aim to make visible and analyze the reality in the care of orphan diseases and the efforts to improve the quality of life of the patient and her family.
Results: The review of documents and the analysis of their approaches, methodologies and theoretical frameworks, indicates that the research trend is quantitative, with descriptive analysis and the use of general care guidelines as a result of the consensus of experts or of public consultations carried out.
Conclusion(s): It was found that government entities generate the directives and non‐governmental entities support activities aimed at improving comprehensive care for patients with orphan diseases. Documents related to hematological orphan diseases and health literacy are scarce and therefore become a source of interest for future research.
105. PB0708
105.1. A case of factor VII deficiency presented with repeated rectal bleeding
Ş. Şahin1; S. Khudiyeva2; A. Unuvar3; D. Tugcu4; G. Tanyildiz4; M. Bilici4; Z. Karakas4; S. Karaman 4
1 Istanbul University Faculty of Medicine, Istanbul, Istanbul, Turkey; 2 Istanbul University Faculty of Medicine, ıstanbul, Istanbul, Turkey; 3 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, ISTANBUL, Istanbul, Turkey; 4 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology & Oncology, Istanbul, Istanbul, Turkey
Background: Signs of bleeding include menorrhagia, epistaxis, hematuria, melena, easy bruising, soft tissue hematoma, hemarthrosis, and intracranial hemorrhage.
Aims: In this case, we presented a patient diagnosed in the neonatal period and hospitalized with recurrent rectal bleeding episodes.
Methods:; It is aimed to draw attention to the importance of treatment compliance and that factor VII deficiency may cause level‐independent bleeding.
Results: A 13‐year‐old female patient applied to the emergency service with the complaints of bloody stools and bruises on her legs this morning. In the patient's history, it was learned that she was taking rFVIIa (Novoseven) twice a week with Factor VII deficiency. The patient had rectal bleeding accompanying abdominal pain at the age of 7 years. Novoseven treatment was given for 3 days. There was no pathology in intestinal examinations for rectal bleeding. At the age of nine, she presented to the emergency department with rectal bleeding again. Factor VII treatment was given at 30 μg/kg every 4 h on the first day, every 6 h on the 2nd day, every 8 h on the 3rd day, every 12 h (5 days) on the following days, once a day (5 days). Gastroscopy revealed 3 ulcerated lesions in the distal sigmoid and rectum, and a white granulation tissue‐like lesion in the ileum. Meckel scintigraphy were normal. Follow‐up was planned in consultation with pediatric gastroenterology and pediatric surgery. It was controlled with a proton pump inhibitor and intravenous hydration. She was discharged with prophylaxis treatment 3 days a week.
Conclusion(s): Each patient should be evaluated individually, taking into account the clinical history and the needs of the patient. Especially in patients with recurrent bleeding episodes, close follow‐up and treatment plan become important.

106. VPB0721
106.1. A rare case of factor XII deficiency manifesting as bleeding disorder
A. Kessira; A. Amireche; H. Brouk
Faculty of Medicine, University of Badji Mokhtar of Annaba, Annaba, Algeria
Background: Factor XII, also known as Hageman factor, is a plasma serine protease implied in the intrinsic pathway of coagulation. FXII deficiency is a rare, autosomal recessive disorder, with an incidence of approximately 1 in a million. Asymptomatic prolongation of aPTT is the most commonly reported manifestation of factor XII deficiency, and it is very rarely associated with bleeding diathesis and hemorrhage.
Aims: The aim of this case report is to describe a rare case of unusual bleeding secondary to Factor XII deficiency in an adult patient.
Methods: A 30‐years‐algerian female patient presented following a history of abundant hemorrhage after surgical intervention for wisdom tooth removal, no other history of bleeding was described and her general physical examination was unremarkable.
Results: Laboratory investigation showed normal complete blood count, liver functions and serum electrolytes were normal. Baseline coagulation studies showed isolated prolonged aPTT = 64s. Absence of any inhibitors with a correction of mixing test after incubation at 37°C. Coagulation factor assay panel for intrinsic pathway showed deficiency of factor XII being 2%. No other coagulation abnormality was detected. Hemorrhagic manifestations caused by FXII deficiency are rarely described in the literature. Theoretically, FXII deficiency may lead either bleeding risk or a risk of thrombosis. Bleeding caused by FXII deficiency, if described, is very mild, such as epistaxis or skin abrasions. In our case, we were able to link the bleeding disorder to the factor XII deficiency.
Conclusion(s): FXII deficiency is a rare disorder, treatment for this disorder is usually not necessary since bleeding abnormalities are only mild or nonexistent.
107. PB0716
107.1. Prospective, phase III study of the efficacy, safety, and pharmacokinetics of a human antithrombin III concentrate in congenital antithrombin deficiency during surgery or childbirth
C. Solomon 1; M. Jansen2; S. Knaub3; C. Kessler4
1 Octapharma AG, Lachen, Zurich, Switzerland; 2 Octapharma Pharmazeutika Produktionsgesellschaft m.b.H, Vienna, Salzburg, Austria; 3 Octapharma AG, Lachen, Schwyz, Switzerland; 4 Georgetown University Medical Center, Washington, District of Columbia, United States
Background: Congenital antithrombin deficiency (CAD) is a rare genetic blood disorder associated with spontaneous thrombotic events (TEs) and thromboembolic events (TEEs) in connection with surgery and childbirth. Human plasma‐derived or recombinant antithrombin replacement therapy can be used for the prevention of TEs/TEEs in these settings.
Aims: To evaluate the efficacy, safety, and pharmacokinetics (PK) of a high‐purity, double virus inactivated, lyophilized human antithrombin III concentrate (Atenativ®) in patients with CAD undergoing surgery or delivery.
Methods: The ATN‐106 study (NCT04918173, Sponsor: Octapharma) is a multicenter, prospective, single‐group, open‐label, Phase III study in male and female patients (12–80 years) with documented CAD (plasma antithrombin ≤60%), and personal or family history of TEs or TEEs. For the PK phase, ~14 non‐pregnant adult and 4 adolescent patients will receive one 60 IU/kg intravenous (IV) infusion of antithrombin concentrate. The treatment phase will enrol ~20 patients who are either non‐pregnant and scheduled to undergo elective surgery associated with high risk of TEs or TEEs, or pregnant and scheduled for caesarean section or delivery. A single IV dose targeting an antithrombin level of 120% of normal will be administered prior to surgery/delivery followed by maintenance doses every 24 h for ~2–7 (surgical patients) or ~5 (delivery patients) days targeting an antithrombin level of 80–120% of normal (Figure 1). The primary outcome measure will be the incidence of composite TEs and TEEs with secondary outcome measures including PK measurements, safety and tolerability (number of adverse events).
Results: Enrollment is estimated to start in Q1 2022 and end in Q3 2024.
Conclusion(s): The results from this pivotal registration study are expected to confirm and strengthen available evidence indicating that human plasma‐derived antithrombin concentrate is effective in preventing TEs and TEEs in patients with CAD in surgery and delivery, with a favorable safety profile.

108. VPB0722
108.1. Health education needs in hematological orphan diseases for professionals, sick people and caregivers in Ibagué, Tolima
N. Perdomo Olarte
Universidad del Tolima, Vihonco IPS, Medicarte SAS, IPS Especializada, Ibagué, Tolima, Colombia
Background: Establishing the educational needs of health professionals, caregivers and patients diagnosed with hereditary coagulopathies, taking into account deficiencies, activities and their participation in the social context, allows to propose educational strategies that are in accordance with the individual and collective reality.
Aims: Assess the educational needs of professionals and the level of digital information health literacy of people with orphan hematological diseases and their caregivers, as the basis for a proposal for an educational strategy that promotes self‐care, adherence, comprehensive care and humanization of the service.
Methods: Documentary review of the national context according to the epidemiological bulletin "Behavior of notification to Sivigila of orphan diseases ‐ Rare, Colombia, 2016 to EW 04 of 2021", 42,560 cases of orphan diseases have been notified to Sivigila in the In the country, the most important are diseases of the central nervous system followed by diseases of the blood and hematopoietic organs, with congenital deficiency of factor VIII with 1,801 and von Willebrand disease with 1,726 with the highest prevalence
Results: There is significant inequality between people with orphan diseases who are disadvantaged compared to the general population and people with common chronic diseases. This can be attributed to "the challenges in accessing diagnoses, medical information, treatment, psychosocial support, and coping with stigma and uncertainty"; demonstrates the need for a global approach based on efforts by both health care institutions, as well as the community and patients.
Conclusion(s): It is a priority to propose strategies in health education aimed at the population with hereditary coagulopathies, professionals and caregivers based on the results of instruments that allow determining educational needs with the measurement of the rate of literacy in health and digital health in people diagnosed with diseases orphan hematological diseases and their caregivers, the level of understanding and educational needs of professionals part of health care.
109. PB0712
109.1. The utility of bleeding assessment tools in fibrinogen deficiencies
S. Mohsenian 1; O. Seidizadeh2; R. Palla3; M. Jazebi4; A. Azarkeivan5; S. Moazezi4; M. baghaipour4; M. Menegatti6; F. Peyvandi7
1 Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Lombardia, Italy, 21. Fondazione IRCCS Ca’Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy 2. Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, Milan, Lombardia, Italy; 3 Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 4 Iranian Comprehensive Hemophilia Care Center, Tehran, Tehran, Iran; 5 Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Tehran, Iran; 6 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 7 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Bleeding‐assessment‐tools (BAT) or bleeding‐score‐systems (BSS) are developed to address the challenges associated with evaluation of patients. A small number of patients with congenital fibrinogen deficiencies (CFD) are evaluated with these tools without definitive results.
Aims: To compare the results of the ISTH‐BAT with the EN‐RBD‐BSS in patients with CFD. We further evaluated the correlation between bleeding scores (BS) and fibrinogen levels.
Methods: In total, 100 (55% females) Iranian patients with CFD were included. Routine coagulation and fibrinogen‐specific tests including antigen (Fg:Ag) and its functional activity (Fg:C) were performed. Patients were classified using Fg:C/Fg:Ag ratio and they were divided into four categories of bleeding severity (asymptomatic, grade I, grade II, grade III). The ISTH‐BAT and the EN‐RBD‐BSS were used to assess the BS of all patients. Pearson’s correlation was performed using IBM‐SPSS Statistics 26.
Results: Patients’ characteristics are summarized in Table 1. The median (range) of the ISTH‐BAT and EN‐RBD‐BSS were 4 (0–16) and 2.21 (−1.49–6.71), respectively. In general, a moderate positive correlation (r = 0.597, p < 0.001) was found between the ISTH‐BAT score and EN‐RBD‐BSS (Figure 1‐A). The correlation between Fg:Ag and the ISTH‐BAT scores was weak negative (r = −0.410, p < 0.001), comparable to the correlation between Fg:C and ISTH‐BAT scores (r = −0.419, p < 0.001). There was a weak negative correlation between Fg:Ag and EN‐RBD‐BSS (r = −0.460, p < 0.001), and between Fg:C and EN‐RBD‐BSS scores (r = −0.405, p < 0.001, Figure 1‐B). Hypodysfibrinogenemic patients had the strongest correlation between the EN‐RBD‐BSS and ISTH‐BAT (r = −0.916, p = 0.001). Excluding dysfibrinogenemic patients, there was a significant association between bleeding severity and Fg:C (r = −0.399, p < 0.001), and Fg:Ag (r = −0.322, p = 0.005).
Conclusion(s): Based on the good correlation between the two systems, it appears that in addition to the ISTH‐BAT score, the EN‐RBD‐BSS could also be useful in CFD patients. The BS showed a significant negative correlation with fibrinogen antigen/activity levels.


110. PB0703
110.1. Prophylaxis with human coagulation factor X in an adolescent girl with hereditary major Stuart factor deficiency
F. Zekre1; F. Tardy2; A. Biguet Petit Jean3; S. Thouvenin3; C. Legendre3; P. Noyel3; P. de Mazancourt4; B. Tardy3; C. Berger 5
1 Chu Saint Etienne, Saint‐Etienne, Rhone‐Alpes, France; 2 CHU Saint‐Etienne, Saint‐Etienne, Rhone‐Alpes, France; 3 CHU Saint‐Etienne, Saint‐Etienne, Rhone‐Alpes, France; 4 Laboratoire de biologie moléculaire, Hôpital Ambroise Paré (APHP), UMR1179 Paris Saclay University, Boulogne‐Billancourt, Ile‐de‐France, France; 5 Hemophilia CRC Hospital, Inserm, U 1059, Jean Monnet University, Saint‐Etienne, Rhone‐Alpes, France
Background: FX or Stuart Factor deficiency is a rare constitutional hemorrhagic disorder of autosomal recessive transmission. Its incidence is estimated at 1/1,000,000 inhabitants. The hemorrhagic phenotype is variable. Twenty‐seven cases are listed in the France Coag registry (inclusion criteria: FX <10%) in 2021.
Aims: To describe prophylaxis with a specific human product.
Methods: We report the case of a young girl, 18 years old, with a major factor X deficiency.
Results: She presented with rectal bleeding at 15 days of age, which prompted a coagulation assay showing a prothrombin index <5%, APTT 102 s, factor X <1%, and factor VII 20%. Factor V and VII normalized with age. Parents were first‐degree relatives of Algerian origin, both had respectively factor X 46% and 59%, and the father also had heterozygous factor VII deficiency (55%). Our case subject did not identify the genetic mutation (NGS and MLPA). At the age of 4, she presented twice with hemarthrosis of the left knee, motivating the introduction of prophylaxis with human prothrombin complex (HPC) (20 IU/kg once a week). The recuperation rate was 1.9%. Later, her first menstrual period leads to anemia, requiring tranexamic acid and iron supplementation. At 16, we suggested switching to COAGADEX® prophylaxis (plasma‐derived blood coagulation factor X concentrate) 25 IU/kg once a week. We performed a pharmacokinetics assay; the peak 15 min later was 65% of factor X, maintained at 63% at T1 hour, and residual level one week later was 2%. The recuperation rate was 2.5 %. For 18 months, this young girl has been pleased with this product. Injection time was faster than with HPC (1 min 30 s versus 20 min), and she did not have any recurrence of anemia.
Conclusion(s): COAGADEX®, pure human coagulation factor X, could be an exciting alternative for prophylaxis in factor X deficiency.
111. VPB0720
111.1. Hereditary severe factor XIII deficiency in northern region of Turkey
C. Albayrak 1; D. Albayrak2
1 Ondokuz Mayıs University Medical Faculty Haed of pediatric bone marrow unit, Samsun, Samsun, Turkey; 2 Samsun Medicalpark Hospital, Samsun, Samsun, Turkey
Background: Severe inherited Factor XIII deficiency is a rare autosomal recessive disorder of hemostasis. Normal screening tests may complicate the diagnosis.
Aims: The aim of our study is to determine the clinical, laboratory characteristics and genetic results of severe hereditary Factor XIII deficiency patients diagnosed in our clinic.
Methods: Six patients diagnosed and treated in our hospital were evaluated retrospectively.
Results: PT and APTT tests were within normal limits in all of our patients, and the activity of Factor XIII in plasma was less than 1%. Six patients, (four boys) were diagnosed. The median age at diagnosis was 3.5 years (range 6 months‐ 23 years). The parents of all patients were relatives. All of them had umbilical hemorrhage in newborns. Therefore, they applied to the hospital, but the screening tests were found to be normal and they were not diagnosed during this period. Four patients had intracranial hemorrhage, one had cerebral hemorrhage twice at the age of eight and ten years. One patient was followed up with the diagnosis of cerebral palsy after a cerebral hemorrhage in the neonatal period. The patient, who could not walk and could not speak, came to the hospital urgently with oral bleeding from time to time, returned home with erythrocyte suspension, and no further examination was performed. A female patient, 11 years old, was admitted with iliopsoas hematoma. She was diagnosed preoperatively and was operated with fresh frozen plasma. There are three patients who received prophylaxis with fresh frozen plasma after cerebral hemorrhage. Genetic analysis identified different homozygous mutations, one novel and the other known, in the A subunit of factor XIII in two patients.
Conclusion(s): Severe hereditary Factor XIII deficiency is a rare bleeding disorder, is particularly associated with life‐threatening or disabling intracranial hemorrhages. In patients presenting with umbilical bleeding, the diagnosis should be made with further investigations.
112. VPB0724
112.1. Online survey of Chinese patients with von Willebrand disease and the impact of COVID‐19
Z. Zeping1; W. Yang 2
1 The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China (People's Republic); 2 Kunming Medical University, Kunming, Yunnan, China (People's Republic)
Background: As of November 12, 2021, the China Hemophilia Information Registry has recorded 39,030 cases of hemophilia, of which only 482 cases of Von Willebrand disease (VWD). Obviously, this data is far from reaching the true number of VWDs in China. Therefore, the analysis of clinical characteristics of Chinese VWD patients needs to be resolved urgently.
Aims: This study described the clinical features, diagnosis and treatment plan of 96 Chinese patients with VWD in detail, and also analyzed the von Willebrand factor (VWF) gene mutation in some patients. In addition, the impact on patients with VWD during COVID‐19 was collected.
Methods: The study conducted an online questionnaire survey of patients in a VWD communication group, and the data were analyzed in SPSS version 25.0.
Results: The results showed that 80% of patients had their first bleeding before the age of 5 years. 58% of the first bleeding site was epistaxis. 46% were misdiagnosed on first visit, such as Hemophilia, Epistaxis, Pseudohemophilia, Thrombocytopenia, Platelet weakness, Functional uterine bleeding, Coagulation dysfunction. There were differences between age at diagnosis and sex (p = .014) and VWD classification (p = .034). Cryoprecipitation (38%) is currently the most commonly used treatment for von Willebrand disease in China. Only 19% of patients were using prophylaxis. Drug shortages (68%) were the biggest problem patients faced during the pandemic. At present, the biggest appeal of VWD patients (64%) is to hope that the country can independently develop or allow the import of foreign recombinant VWF drugs. We found 21 VWF gene mutation sites in 14 patients, of which 18 were reported for the first time in the world. (p.H1536N, p.R507G, p.C2389R, p.Q393X, p.W2444X, p.W974X, p.E1015X, c.4413delC /4414delG, c.6085‐6091delGTCTCTG, c.6496‐6497insTCCT, c.2501‐2502insA, c.6949dupG, c.2186+1G>A, c.5842+5G>A, c.2684A>G, Exon17del, Exon4‐Exon52 del, Exon11‐Exon43del).
Conclusion(s): Our findings give Chinese clinicians provides guidance on early identification of abnormal bleeding in VWD, and expands the international VWF gene database.

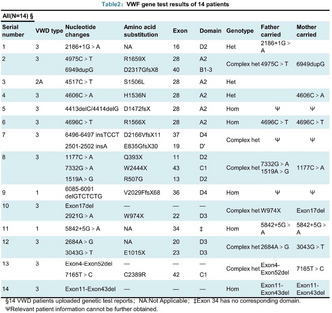
113. PB0709
113.1. Bleeding story of an adolescent: Factor VII deficiency and repeat iliopsoas bleeding
S. Karaman 1; A. Unuvar2; ş. şahin3; C. Ercan4; g. polat4; M. Bilici1; D. Tugcu1; G. Tanyildiz1; Z. Karakas1
1 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, Istanbul, Istanbul, Turkey; 2 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, ISTANBUL, Istanbul, Turkey; 3 Istanbul University Faculty of Medicine, Istanbul, Istanbul, Turkey; 4 Istanbul University Faculty of Medicine, ıstanbul, Istanbul, Turkey
Background: Factor VII deficiency is the most common of the rare factor deficiencies. The bleeding clinic is independent of the factor level and the level required for effective hemostasis varies from patient to patient.
Aims: It is aimed to draw attention to the importance of treatment compliance.
Methods: This case described to draw attention to rare bleeding disorders.
Results: Case: A sixteen‐year‐old male patient; applied to the emergency department with the complaint of pain in the left groin. He had strained his leg and had pain while playing basketball 5 days before. He was examined for prolonged bleeding and recurrent nosebleeds after circumcision, and was diagnosed with factor VII deficiency (F VII level was between 27% and 53%). He was also under follow‐up from rheumatology due to the diagnosis of FMF and was using colchicine and anakinra. In the abdominal tomography, a 17 mm thick hematoma was observed in the left iliac fossa, near the ileopsoas muscle. The patient was started on rFVIIa (Novoseven) therapy. The patient, who received inpatient treatment for a total of 14 days, received 30 μgr/kg every 6 h in the first two days, rFVIIa was administered every 12 h between days 3–5 and once a day for the next 5 days. Novoseven was continued 3 days in a week. However, the patient applied to our outpatient clinic with pain in the left leg again one month later. In the USG performed after the factor was applied, it was observed that the hematoma, approximately 7 cm in size, recurred in the same place. The hematoma, regressed to 1 cm after 10 days. The patient, who was discharged, receives factor therapy three times a week and continues stretching exercises.
Conclusion(s): In the literature, no case of FVII deficiency with iliopsoas hemorrhage, who had a mild factor level as in our patient, was found.

114. PB0714
114.1. Genetic and clinical characterization in 106 patients with congenital quantitative and qualitative fibrinogen deficiencies: A Slovak centre experience
T. Simurda 1; J. Zolkova2; M. Sterankova3; Z. Kolkova4; D. Loderer4; M. Dobrotova2; I. Plamenova2; P. Holly2; J. Hudecek2; I. Skornova2; M. Brunclikova2; M. Grendar4; Z. Lasabova5; J. Stasko2; P. Kubisz2
1 Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, University Hospital Martin, Martin, Zilina, Slovakia; 2 National Centre of Hemostasis and Thrombosis, Department of Hematology and Transfusiology, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin and University Hospital in Martin, Slovakia, Martin, Zilina, Slovakia; 3 Comenius University, The Jessenius Faculty of Medicine in Martin, Martin, Zilina, Slovakia; 4 Biomedical Center Martin, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Slovakia, Martin, Zilina, Slovakia; 5 Department of Molecular Biology and Genomics, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Slovakia, Martin, Zilina, Slovakia
Background: Congenital fibrinogen disorders (CFD) are considered as rare disorders of hemostasis. In afibrinogenemia and hypofibrinogenemia, most mutations of the FGA, FGB, or FGG fibrinogen encoding genes are null mutations. Dysfibrinogenemia is caused mainly by missense mutations. Patients with CFD are usually associated with bleeding phenotype or thrombosis, but many of them are asymptomatic. Aim of the study was to characterize the clinical manifestations and genetic background in patients with CFD presenting at our centre.
Aims: To identify genetic basis and associated clinical and laboratory phenotypes in cross‐sectional survey of 106 patients from 53 unrelated families with congenital fibrinogen disorder using basic laboratory screening tests and genetic analysis of FGA, FGB, and FGG genes.
Methods: In 106 patients from 53 unrelated families (mean [SD] age, 38 [18.8] years; 28.0% men) there were realized standard coagulation tests, fibrinogen activity and antigen (Clauss method, LIA). Genetic analysis of all three fibrinogen genes was performed by high‐throughput sequencing with confirmation of mutations by Sanger sequencing method.
Results: The clinical phenotype consisted of bleeding in 32/106 (30.2%), thrombotic events 14/106 (13.2%) patients, additional other patients were asymptomatic. The following CFD were identified: 1A afibrinogenemia, n = 1; 2A severe hypofibrinogenemia, n = 3; moderate hypofibrinogenemia, n = 1; 2C mild hypofibrinogenemia, n = 20; 3A dysfibrinogenemia, n = 80; 4C mild hypodysfibrinogenemia, n = 1. Hotspot mutations in FGA (exon 2) were identified in 64 dysfibrinogenemic patients. In other patients there were identified 1 mutation in FGA, 7 mutations in FGB and 10 mutations in FGG gene.
Conclusion(s): In our cross‐sectional study there were 56.6% asymptomatic patients with CFD. The remainder of the patients with CFD had either bleeding or thrombosis. Clinical manifestations showed similar features irrespective of genotype. Nevertheless, results from genetic and clinical studies seems to yield valuable information on the structure and function of the fibrinogen molecule.
115. PB0718
115.1. The management of craniopharyngioma in a child with severe factor XI deficiency
A. Unuvar 1; S. Karaman2; O. Kuleli2; A. Sencer3; D. Demirkol4; G. Tanyildiz2; M. Bilici2; G. Gencay5; Z. Karakas2; D. Tugcu2
1 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, ISTANBUL, Istanbul, Turkey; 2 Istanbul University, Istanbul School of Medicine, Division of Pediatric Hematology&Oncology, Istanbul, Istanbul, Turkey; 3 Istanbul University, Istanbul School of Medicine, Department of Neurosurgery, Istanbul, Istanbul, Turkey; 4 Istanbul University, Istanbul School of Medicine, Division of Intensive Care Unit, Istanbul, Istanbul, Turkey; 5 Istanbul University, Istanbul School of Medicine, Division of Pediatric Intensive Care Unit, Istanbul, Istanbul, Turkey
Background: The bleeding phenotype in patients with severe factor XI deficiency (SFXID) is not correlated with FXI levels. Therefore, there is no standard protocol for perioperative management.
Aims: We present the management of a child with SFXID and diabetes insipidus (DI) who underwent surgery for craniopharyngioma. To our best knowledge, this is the first report of neurosurgery of SFXID.
Methods: A 12‐year‐old boy presented with headaches. He had a history of surgery for craniopharyngioma with residual mass, 4 years ago. We were unable to obtain the hospital records despite our best efforts, but the family reported uneventfully (inspite of 1‐month stay in ICU). The patient had been diagnosed as SFXID during that admission. Radiotherapy had been given, also.
Results: The patient had headaches and recurrent vomiting, 4years later. The suprasellar mass was increased in size, surgery was planned. FXI inhibitor was negative, FXI level was 0.6%. He had no bleeding history. Tranexamic acid (TXA) was given orally, one day before surgery. FXI concentrate is not available in Turkey. Therefore, FFPs was started for prophylactic replacement the day before surgery. FFPs were administered with close follow‐up due to DI, desmopressin doses were adjusted. One additional FFP was given immediately before surgery. The desired FXI level immediately before surgery was achieved. In addition, TXA was administered intravenously. Intraoperative bleeding was within normal range. However, one unit of FFP was administered at the surgeon’s recommendation. Moreover, rVIIa (1 mg) was administered at the end of surgery due to superficial mildly increased bleeding during suturing. FXI levels were maintained above 45% with FFPs for 10 days (&TXA). No bleeding or thrombosis was observed. Postoperative cranial MRI were performed twice, revealed a near‐total excision of the tumor and no bleeding.
Conclusion(s): Perioperative management and effective hemostasis is very challenging in patients with severe FXI deficiency. If FXI concentrate is not available, effective hemostasis can be achieved by using FFP and TXA. If the patient has excessive bleeding in spite of these interventions, one dose of rFVIIa (with consent for off‐label use) may help control bleeding, but this matter warrants further research.
116. PB0702
116.1. Usefulness of rotational plasma thromboelastometry in severe inherited FXIII deficiency
I. Ben Salah 1; A. Launois2; C. Flaujac3; C. Lavenu‐Bombled4; P. De Mazancourt5; I. Martin‐Toutain2; M. Bourrienne6; E. de Raucourt7
1 Service Hématologie Biologique, Hôpital Beaujon (APHP), Clichy, Ile‐de‐France, France; 2 Laboratoire de Biologie Médicale, Hôpital André Mignot, Le Chesnay, Ile‐de‐France, France, 3 laboratoire de biologie médicale‐ secteur hémostase ‐ André Mignot hospital, Versailles, Le Chesnay, Ile‐de‐France, France; 4 Laboratoire Hématologie, Hôpital Bicêtre (APHP) ‐ HITh, UMR_S1176, INSERM, Université Paris‐Saclay, Le Kremlin‐Bicêtre, Ile‐de‐France, France; 5 Laboratoire de biologie moléculaire, Hôpital Ambroise Paré (APHP), UMR1179 Paris Saclay University, BOULOGNE‐BILLANCOURT, Ile‐de‐France, France; 6 Hôpital Beaujon (APHP); INSERM LVTS U1148 Université de Paris, CLICHY, Ile‐de‐France, France; 7 Centre de ressources et compétences des Maladies Hémorragiques Constitutionnelles rares, centre hospitalier de Versailles (André Mignot), Le chesnay, Ile‐de‐France, France
Background: FXIII deficiency is one of the rarest bleeding disorders, characterized, in severe cases, by neonatal umbilical hemorrhage and spontaneous intracranial hemorrhage. Severe FXIII deficiency (<5 UI/dl) is usually diagnosed in early childhood and requires lifelong prophylaxis with FXIII concentrate. Diagnosis and monitoring of FXIII deficiency remains challenging as standard coagulation tests are insensitive, and specific assays remain difficult to perform. Plasma thromboelastometry (ROTEM) has been reported to be affected by FXIII deficiency.
Aims: Herein, we report the case of a children referred to our center for bleeding tendency.
Methods: Plasma ROTEM assay was used at diagnosis and after to monitor substitution with FXIII concentrates.
Results: An eight‐ year‐ old boy was referred for delayed post‐ traumatic bleedings and spontaneous ecchymosis observed for several months. There was no personal or familial bleeding history and no consanguinity. All standard coagulation assays, von Willebrand factor levels, both platelet count and function were normal. EXTEM Parameters with ROTEM used on plasma showed abnormalities, particularly decreased MCF (Figure A) despite normal fibrinogen level. FXIII specific assay revealed a severe FXIII deficiency (FXIII <1 UI/dl). As no bleeding tendency had been reported in early childhood an acquired FXIII deficiency was suspected. After FXIII concentrate infusion, bleeding tendency improved, ROTEM parameters normalized (Figure B) and remained within normal range at Day 7, 14 and 28 ruling out an inhibitor. Specific assay confirmed the absence of FXIII inhibitor. ROTEM was normal in both parents who exhibited FXIII level at 55 IU/dl and 66 IU/dl, respectively (figure C‐D). Constitutive FXIII deficiency was confirmed, with composite heterozygosity for the F13A1 variants c.1984C>T in exon 14 (p.Arg662*) and c.1504G>A in exon 12 (p.G502R), inherited by his father and mother, respectively.
Conclusion(s): ROTEM/thromboelastometry used on plasma is sensitive to severe FXIII deficiency and may help to monitor replacement therapy with FXIII concentrate.

117. PB0711
117.1. A novel homozygous KLKB1 missense variant causative of severe Prekallikrein deficiency
M. Mitchell 1; R. Wheeler2; J. Cutler2; G. Ling3
1 Viapath Analytics LLP, Guy's & St. Thomas' NHSFT, LONDON, England, United Kingdom; 2 Viapath Analytics LLP, LONDON, England, United Kingdom; 3 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom
Background: Prekallikrein deficiency is a rare autosomal disorder caused by variants in KLKB1. It is generally not associated with a bleeding phenotype so is probably under reported, usually detected incidentally following an abnormal coagulation screen. Detection of Prekallikrein deficiency, in the presence of a prolonged APTT, is beneficial as it gives definitive explanation excluding other potential causes.
Aims: Genetic confirmation of Prekallikrein deficiency and identification of causative variant.
Methods: A 52 year old woman with Primary Sjögren’s syndrome was referred for investigation of ‘unexplained bruising to limbs’. Coagulation assays were performed as standard on a Sysmex analyser. Prekallikrein was measured using an APTT‐based factor assay, also on a Sysmex analyser. NGS analysis was performed for the R90 Bleeding and Platelet disorder panel. Analysis was performed using a Twist Bioscience custom design panel run on a NovaSeq and the data analysed using Congenica. Putative pathogenic variants were confirmed by Sanger sequencing.
Results: Initial coagulation studies showed a normal INR but raised APTT. This was initially considered to be ‘consistent with the presence of a lupus anticoagulant’. Factor analysis showed a severe Prekallikrein deficiency (PK <1 IU/dl). NGS analysis showed her to be homozygous for a c.1739G>T; p.(Gly580Val) missense variant in KLKB1 that scores as ‘likely pathogenic’ using ACGS Guidelines.
Conclusion(s): The identification of the homozygous p.(Gly580Val) missense variant confirms a diagnosis of congenital Prekallikrein deficiency. This explains the prolonged APTT and excludes its being an inhibitory antibody effect, in a patient with an autoimmune condition. To date only 15 KLKB1 variants have been reported associated with Prekallikrein deficiency (HGMD Professional 2021.4), only 6 of which are missense variants. The identification of this novel missense variant adds to our knowledge of KLKB1/Prekallikrein deficiency.
118. PB0717
118.1. In vitro rescue of a nonsense mutation responsible for severe coagulation factor V deficiency using ribosome readthrough agents
A. Todaro 1; M. Ciccone2; M. Serino2; D. Gemmati2; A. Cuneo2; T. Hackeng1; F. Bernardi3; E. Castoldi4
1 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 2 Department of Medical Sciences, Section of Haematology, Sant’Anna Hospital, Ferrara University, Ferrara, Emilia‐Romagna, Italy; 3 Department of Life Sciences and Biotechnology, University of Ferrara, Italy., Ferrara, Emilia‐Romagna, Italy; 4 CARIM, Maastricht University, Maastricht, Not Applicable, Netherlands
Background: Severe coagulation factor V (FV) deficiency is a rare autosomal recessive bleeding disorder. Fresh‐frozen plasma is the mainstay of treatment, but patients with nonsense mutations might benefit from emerging molecular therapies with ribosome readthrough agents.
Aims: We investigated a 28‐year‐old male with FV:C <1% and a moderate bleeding tendency due to a homozygous F5 nonsense mutation. Ribosome readthrough agents were tested for their ability to restore functional FV expression in an in vitro model of the patient’s mutation.
Methods: Following written informed consent, the patient and his parents were investigated at the DNA, mRNA and plasma level (thrombin generation). COS‐1 cells transfected with wild‐type or mutant FV cDNA were treated with 0–500 μM G418 (Geneticin), ELX‐02, PTC‐124 (Ataluren), 2,6‐diaminopurine (DAP) or Amlexanox. After 48 h, conditioned media of untreated and treated cells were assayed for FV activity by a prothrombinase‐based assay.
Results: Thrombin generation was hardly detectable in the patient’s platelet‐poor plasma, but measurable in platelet‐rich plasma. The patient was homozygous (and his parents heterozygous) for the F5 c.3481C>T mutation, which introduces a TGA stop codon in exon 13, causing nonsense‐mediated decay of the FV mRNA and predicting premature termination of translation (p.Arg1161Ter). The mutation was linked to FV Leiden on both alleles. Expression of the p.Arg1161Ter mutation in COS‐1 cells resulted in minimal FV activity in the medium (0.8 ± 0.3% of wild‐type), mimicking the patient’s undetectable FV level. Treatment with G418, ELX‐02 and DAP increased FV activity in a dose‐dependent manner, up to 4.7 (G418), 2.8 (ELX‐02) and 13.6 times (DAP) compared to untreated cells. Differently, PTC‐124 and Amlexanox (alone or in combination) did not improve FV expression.
Conclusion(s): This study provides in vitro proof‐of‐principle for the potential of some ribosome readthrough agents to partially restore functional FV synthesis and secretion impaired by the p.Arg1161Ter mutation.
119. PB0701
119.1. Comparison between self‐administered and clinician‐administered ISTH bleeding assessment tool in RUNX1 patient population
E. Andrews 1; H. Choo‐Wosoba2; S. Kalsi3; M. Merguerian4; M. Ring5; J. Davis6; S. Steinberg7; P. Liu8; L. Cunningham8
1 National Institutes of Health, North Bethesda, Maryland, United States; 2 National Cancer Institute, National Institutes of Health, Bethesda, Maryland, United States; 3 National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, United States; 4 Johns Hopkins University School of Medicine, Department of Pediatrics, Bethesda, Maryland, United States; 5 Biomedical Translational Research Information System, National Institutes of Health, Bethesda, Maryland, United States; 6 National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, United States; 7 Office of the Clinical Director, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, United States; 8 National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland, United States
Background: RUNX1 is a master regulator of hematopoiesis. Pathogenic germline RUNX1 variants cause Familial Platelet Disorder with associated Myeloid Malignancy (FPDMM), which is characterized by abnormal bleeding and a 35%–45% lifetime risk of hematologic malignancy. The ISTH Bleeding Assessment Tool (BAT) questionnaire quantifies bleeding symptoms but has not been studied in patients with germline RUNX1 variants.
Aims: To evaluate the agreement between self‐administered and clinician‐administered BAT questionnaires in patients with deleterious RUNX1 germline variants.
Methods: Thirty‐eight patients consented on the NIH IRB‐approved protocol 19‐HG‐0059 completed the questionnaire. The clinician administered the questionnaire without knowledge of the self‐administered questionnaire results. The McNemar test assesses how similarly these two versions agreed in classifying abnormal or normal status per criteria. A Bland–Altman plot of differences versus averages compares the self‐ and clinician‐administered questionnaire score results.
Results: Self‐ and clinician‐administered BAT score classifications of normal or abnormal agreed for 31 of 38 (82%) patients, with a p‐value of 0.12 (Table 1). Six patients self‐classified their symptoms as abnormal, in contrast to the clinician’s classification as normal. One patient self‐classified their symptoms as normal, in contrast to the clinician’s classification as abnormal. Comparison of the self‐ and clinician‐administered BAT scores shows that all but one of the differences are between or very close to the lower and upper limits of agreement, with no distinctive pattern by sex (Figure 1).
Conclusion(s): In patients with germline RUNX1 mutations, self‐ and clinician‐ administered ISTH‐BAT questionnaire score classifications agreed at a high rate. For most discordant classifications, patients self‐classified their bleeding symptoms as more severe than did the clinician. In the future, a larger sample size will improve the statistical confidence in the rate of agreement, and the BAT can be further analyzed with data on platelet count and function in this unique patient population.


120. PB0699
120.1. Bevacizumab for refractory hereditary hemorrhagic telangiectasia
C. Almeida 1; A. Sarmento1; S. Carvalho2; G. Ferreira2
1 Centro Hospitalar Baixo Vouga, Aveiro, Aveiro, Aveiro, Portugal; 2 Centro Hospitalar Baixo Vouga, Aveiro, Aveiro, Portugal
Background: Hereditary Hemorrhagic Telangiectasia(HHT) is a genetic disorder characterized by an uncontrolled multisystem angiogenesis and a variable clinical presentation. There’s many treatments that prevent life‐threatening complications. More recently, bevacizumab was shown to significantly reduce severe anemia related to epistaxis and/or gastrointestinal bleeding.
Aims: To provide real world data of the management of refractory HHT.
Methods: We report the case of two siblings with refractory HHT who showed good and sustained response to bevacizumab.
Results: A 79‐yo male and a 71‐yo female with HHT displayed different features: he presented with spontaneous epistaxis, mucocutaneous telangiectasias and transfusion‐dependent anemia (6.5 g/dl); she presented with spontaneous epistaxis, hemoptysis and transfusion‐dependent anemia (7.5 g/dl). An extensive study was performed which determined the presence of 1) mucocutaneous + gastrointestinal telangiectasias(brother); 2) mucocutaneous telangiectasias + pulmonary AVMs(sister). Apart from the fact that the sister performed an embolization of one pulmonary AVM, both of them were treated similarly and with comparable responses. They did intravenous iron and transdermal estrogens with no response. They received thalidomide 50–200 mg id (through patient’s tolerance), 6 cycles, with increase in hemoglobin until 11–12.6 g/dl. Due to onset of peripheral neuropathy, thalidomide was discontinued. They received lenalidomide 5–15 mg id (through patient’s tolerance) which was suspended 2 months later as they needed to resume blood transfusion. In 2018 they initiated intravenous bevacizumab (1 mg/kg every 3–9 weeks), with a significant reduction of nosebleeds/hemoptysis, hemoglobin values 11.4–14 g/dl, ferritine >22 ng/ml. No adverse events were observed, namely, renal function deterioration or hypertension.
Conclusion(s): Treatment options in HHT still greatly rely on individual experience and expert opinions. Given the lack of uniformity when approaching it, it would be desirable the performance of prospective cohort studies that allow the implementation of evidence‐based guidelines. Until then, bevacizumab provides an important treatment option in patients with refractory HHT as it has shown a long‐term good response and a favorable safety profile in these two siblings.
121. PB0706
121.1. Pregnancy in women with factor XI deficiency (<60 UI/dl) and use of neuraxial analgesia: Real life data in a French cohort of 314 pregnancies
C. Flaujac 1; C. Cavalie2; N. Drillaud3; C. Lavenu‐Bombled4; S. Timsit5; P. Billoir6; C. Desconclois7; L. Touzet8; A. Lebreton9; I. Diaz‐Cau10; R. d'Oiron11; M. Giansily‐Blaizot10; M. Fretigny12; S. Desage13; B. Wibaut14; P. Beurrier15; F. Volot16; L. Rugeri17; V. Roussel‐Robert18; E. de Raucourt19
1 laboratoire de biologie médicale‐ secteur hémostase ‐ André Mignot hospital, Versailles, Le Chesnay, Ile‐de‐France, France; 2 Service d’hématologie biologique ‐ AP‐HP. Centre‐Université de Paris, hôpital Bichat, Paris, Ile‐de‐France, France; 3 University Hospital of Nantes, Nantes, Pays de la Loire, France; 4 Service d’hématologie biologique, Hôpital Bicêtre, APHP, Université Paris‐Saclay, Le Kremlin‐Bicêtre, Ile‐de‐France, France; 5 Service d’hématologie biologique, AP‐HP. Centre‐Université de Paris, hôpital Necker, Paris, Ile‐de‐France, France; 6 Normandie Univ, UNIROUEN, INSERM U1096, Vascular Hemostasis Unit, Rouen University Hospital, France, Rouen, Haute‐Normandie, France; 7 CHU BECLERE APHP, Clamart, Ile‐de‐France, France; 8 Laboratoire de biologie médicale, centre hospitalier de Valencienne, Valencienne, Nord‐Pas‐de‐Calais, France; 9 Service d’hématologie biologique, CHU Clermont‐Ferrand, Clermont‐Ferrand, France, Clermont‐Ferrand, Rhone‐Alpes, France, 10 Service d’hématologie biologique, Centre hospitalier universitaire de Montpellier, Montpellier, Languedoc‐Roussillon, France, 11 CRC‐MHC, Hôpital de Bicetre AP‐HP, Le Kremlin Bicetre, France, Le Kremlin Bicêtre, Ile‐de‐France, France, 12 Hospices Civils de Lyon. ‐ Unité d'Hémostase Clinique, Hôpital Cardiologique, Bron, Rhone‐Alpes, France, 13 Hôpital cardiologique Louis Pradel, Hospices Civils de Lyon, LYON, Rhone‐Alpes, France, 14 Lille University Hospital, Lille, Nord‐Pas‐de‐Calais, France, 15 Centre de ressources et compétences des Maladies Hémorragiques Constitutionnelles rares, Centre hospitalier universitaire d’Angers, Angers, Pays de la Loire, France, 16 Centre de ressources et compétences des Maladies Hémorragiques Constitutionnelles rares, Centre hospitalier universitaire de Dijon, Dijon, Bourgogne, France, 17 Hospices Civils de Lyon. Centre de Référence de l’Hémophilie et des Maladies Hémorragiques Constitutionnelles rares ‐ Unité d'Hémostase Clinique, Hôpital Cardiologique, Bron, Rhone‐Alpes, France, 18 Centre de Ressources et de Compétences des Maladies Hémorragiques Constitutionnelles rares, AP‐HP. Centre‐Université de Paris, Hôpital Cochin, Paris, Ile‐de‐France, France, 19 Centre de ressources et compétences des Maladies Hémorragiques Constitutionnelles rares, centre hospitalier de Versailles (André Mignot), Le chesnay, Ile‐de‐France, France
Background: Factor XI (FXI) deficiency is a rare bleeding disorder with a poor correlation between bleeding tendency and FXI level. Management of pregnant women with FXI deficiency is not established, especially regarding neuraxial analgesia (NA). In 2018, the CoMETH group conducted a national survey among French physicians (32 centers) showing heterogenous practices regarding the FXI threshold to allow NA, ranging from 30 to 60 UI/dl.
Aims: Here, we report a new study of a large French cohort of pregnant women with FXI deficiency (FXI <60 UI/dl), in order to assess FXI levels used to achieve NA in real life practice.
Methods: An on‐line questionnaire was sent to French haemostasis centres. The objectives were to report (i) FXI levels before pregnancy and at term of delivery in women with FXI deficiency who underwent NA, (ii) delivery management modalities and (iii) possible complications related to the NA.
Results:: 314 pregnancies with FXI deficiency were included (from 20 centres), 200 NA have been completed (137 epidural and 61 spinal, one had both). The period of childbirth was mostly from 2014 to 2020 (89.5%). Congenital FXI deficiency was confirmed in 32.8% (n = 103; 27/103 genotyped). Previous bleedings were described in 19.75% (49.2% cutaneous, 33.9% gynaecological, 17% post‐surgical). FXI level at delivery and mode of delivery are presented in Table 1. Median FXI levels in the epidural and spinal group are not significantly different. 18 deliveries had a NA with FXI< 30 UI/dl, of which 6 women received FXI concentrates. 42 deliveries had NA with FXI [30–40 UI/dl] and 119 with FXI [40–60 UI/dl]. There were no complications related to NA.
Conclusion(s): We describe here the largest cohort in FXI deficiency pregnant women's, to our knowledge. The results confirm that a 30UI/dl FXI threshold is safe for NA. This is consistent with the French recommendations published in august 2021 (PNDS 2021).

122. PB0713
122.1. Extensive genetic screening of Iranian FVII deficient individuals with severe phenotype unraveled several novel mutations
T. Shahani 1; S. Ravanbod2; M. Faranoush3; H. Rokni‐Zadeh4
1 School of medicine, zanjan university of medical sciences (zums), Zanjan, Zanjan, Iran; 2 Iranian Hemophilia and Thrombophilia Association (MAHTA), Tehran, Tehran, Iran; 3 Iran university of medical sciences, Tehran, Tehran, Iran; 4 Zanjan University of Medical Sciences (ZUMS), Zanjan, Zanjan, Iran
Background: Coagulation factor VII (FVII) deficiency (OMIM: 227500) is the most common rare bleeding disorder characterized by low or undetectable plasma levels of FVII, a key player in the initiation of blood coagulation. Mutations that result in the complete absence of active protein are associated with early onset pathologies such as neonatal mortality or irreversible organ damage such as intracranial hemorrhage (ICH), defined as severe phenotype. In several cases, a discrepancy between the FVII activity (FVII:C) levels and the observed phenotype has been reported while the underlying reason is largely elusive.
Aims: The aim of the present study was to identify molecular defects that might be related to neonatal and infantile bleeding in phenotypically severe FVII deficient patients.
Methods: 24 independent pedigrees with at least one individual presenting severe phenotype are recruited for genetic analysis using standard PCR‐Sanger sequencing. For a few cases, whole‐exome sequencing was also performed for in‐depth genetic analysis. The study was conducted in accordance with the Helsinki Declaration and approved by Zanjan University of Medical Sciences Ethics Committee (IR.ZUMS.REC.1398.280)
Results: 9 homozygote mutations were identified in 24 individuals with severe phenotype of which c.1A>G (Met1Val), c.634C>T (Arg212Stop) and c.1285G>A (Ala429Thr) were previously reported in heterozygotes. Homozygous c.790delC was identified in 8 independent pedigrees of similar ethnicity. Presence of a founder effect is therefore suggested for this gene alteration.
Conclusion(s): The mutations we report here not only enrich the current database of FVII patients and mutations (https://f7-db.eahad.org/), but clarify the critical role of certain amino acids in FVII protein seq. which their homozygote modification will be disastrous.

123. PB0705
123.1. East Texas bleeding disorder: A novel bleeding disorder
M. Escobar 1; N. Rodriguez2; I. Aboshady3; E. Li3; N. Montanez3
1 University of Texas Health Science Center, Houston, Texas, United States; 2 The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, TX, United States, Houston, Texas, United States; 3 The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, Texas, United States
Background: A large kindred from east Texas, USA was reported with a novel moderately severe bleeding disorder affecting both males and females given the autosomal dominant trait from a gain‐of‐function mutation in the F5 gene. The truncated FV, termed FV‐short, forms a high‐affinity complex with tissue factor pathway inhibitor (TPFI) generating a significant increase in its level, thus inhibiting coagulation. Clinical characteristics include easy bruising, epistaxis, menorrhagia, and bleeding related to surgical procedures and significant trauma.
Aims: Diagnosis and treat affected family members of the east Texas cohort.
Methods: All members of the family were invited to Gulf States Hemophilia and Thrombophilia Center for a multidisciplinary evaluation, including ISTH‐SCC‐ Bleeding Assessment (BAT), coagulation studies, and targeted variant testing for the F5 gene c.2350A>G, p. Ser784Gly mutation.
Results: Four generations, a total of 31 patients, were assessed resulting in 22 affected family members with positive variant findings for c.2350A>G, p. Ser784Gly mutation. Forty percent of the cohort was found to have at least one abnormal routine coagulation study, with forty percent displaying both prolonged PT and PTT, and twenty percent resulting in unaffected coagulation studies. Highest BAT scores within the cohort were noted in both the female and male population ≥18 years of age (Highest Female ISTH BAT‐26, Highest Male ISTH BAT‐24). Children <18 years of age were noted to have the lowest BAT scores between 0 and 8.
Conclusion(s): Given rarity of the disease and management challenges a multidisciplinary approach to care is recommended, ideally at a Hemophilia Treatment Center (HTC). Access to a team of experts with specialized knowledge and skill is essential in providing ongoing patient education including prevention, recognition and treatment of bleeding. As TFPI levels are not readily accessible and routine coagulation studies may be unaffected, genetic testing should be pursued for diagnosis.
124. PB0710
124.1. Clinical manifestations and bleeding scores in inherited factor VII deficiency; The first national report from Iran
A. Khosravi1; M. Paridar2; M. Karimzadeh3; V. Takhviji4; A. Kordian5; O. Kiani Ghalehsardi6; A. Kalantari 7
1 *Transfusion Research center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 2 Ministry of health and medical edjucation, Tehran, Iran, Tehran, Tehran, Iran; 3 Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tehran, Tehran, Iran; 4 Transfusion Research center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 5 Ahvaz University of Medical Sciences, Ahvaz, Iran, Tehran, Tehran, Iran; 6 Iran University of Medical Sciences, Tehran, Iran, Tehran, Tehran, Iran; 7 Department of Anesthesiology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Khuzestan, Iran
Background: Factor VII deficiency is the most frequent disorder among “rare inherited bleeding disorders” and is considered as a heterogeneous disorder, on the phenotype and genotype levels. The majority of studies have been focused on the genetic characteristics.
Aims: The present study investigates the bleeding patterns and their severity attitude regarding multiple clinical, laboratory, and demographic properties in Factor VII (FVII) deficient patients.
Methods: Demographic characteristics, clinical manifestations, family history, and treatment history were recorded, along with specific tests. By recording 12 bleeding symptoms in the year prior to inclusion, the severity of those symptoms was assessed using ISTH Bleeding Scoring.
Results: Data revealed a varied pattern in bleeding symptoms (mild to life‐threatening) and FVII level in sex, age of manifestation, and the level of FVII (severe: 7.4%, moderate: 35%, mild: 52.6%). Bleeding tendency was significantly associated with FVII deficiencies. In fact, the odds of bleeding doubled with decreasing FVII levels, even after adjusting for other factors. Moreover, there were significant correlations between all the symptoms and their severities and sex and age subgroups. Life‐threatening symptoms (CNS and GI) were more prevalent in pediatrics than adults that showed significantly higher cutaneous bleeding types. The incidence of all symptoms was significantly higher in women especially mucocutaneus bleedings.
Conclusion(s): The assessment of bleeding tendency and severity in FVII deficiency should not be limited to ISTH scores, but rather based on clinical manifestations, laboratory results, and demographic factors.

125. PB0704
125.1. Hemizygous FGG p.Ala108Gly in a hypofibrinogenemic patient with a heterozygous 14.8 Mb deletion encompassing the entire fibrinogen gene cluster
A. Couzens 1; A. Lebreton2; F. Masclaux3; M. Guipponi3; C. Pebrel‐Richard4; F. Laffargue5; P. Gembara2; A. Casini6; M. Neerman‐Arbez7
1 University of Geneva, Geneva, Switzerland, Geneva, Geneve, Switzerland; 2 Service d’hématologie biologique, CHU Clermont‐Ferrand, Clermont‐Ferrand, France, Clermont‐Ferrand, Rhone‐Alpes, France; 3 Medical Genetics Service, University Hospitals of Geneva, Geneva, Switzerland, Geneva, Geneve, Switzerland; 4 Service de cytogénétique médicale, CHU Clermont‐Ferrand, Clermont‐Ferrand, France, Clermont‐Ferrand, Rhone‐Alpes, France; 5 Service de génétique médicale, CHU Clermont‐Ferrand, Clermont‐Ferrand, France, Clermont‐Ferrand, Rhone‐Alpes, France; 6 Division of Angiology and Hemostasis, University Hospitals of Geneva, Geneva, Switzerland, Geneva, Geneve, Switzerland; 7 Department of Genetic Medicine and Development, Faculty of Medicine, University of Geneva, Geneva, Switzerland, Geneva, Geneve, Switzerland
Background: Congenital hypofibrinogenemia is a quantitative congenital fibrinogen disorder (CFD) characterised by concordantly low levels of antigenic and functional fibrinogen. Most cases are due to heterozygous variants which in homozygosity would lead to afibrinogenemia (no detectable fibrinogen). Here we present the case of a patient with severe hypofibrinogenemia (antigenic fibrinogen 0.6 g/L; functional fibrinogen 0.5–0.7 g/L Clauss) and developmental delay. CGH array and FISH analysis identified an interstitial de novo 14.8 Mb deletion on chromosome 4, causing the loss of one copy of the entire fibrinogen cluster (genes, FGA, FGB and FGG).
Aims: The fibrinogen levels observed appeared too low to be explained by the large deletion alone. We investigated the hypothesis that a second mutation was present using whole exome sequencing (WES).
Methods: WES was performed at the Health 2030 Genome Centre, Geneva using IDT reagents and protocols. WES read mapping, variant calling and copy number variation detection was performed using an in‐house read depth‐based algorithm. This study was performed with institutional review board approval and written informed consent from all participants (Declaration of Helsinki).
Results: We identified variant rs148685782 (FGG exon 4 c.323C>G p.Ala108Gly) on the non‐deleted allele which has previously been associated with hypofibrinogenemia and predicted to destabilise fibrinogen assembly. Due to the hemizygosity of FGG in this patient the effect of the variant is ‘homozygous‐like’ but does not result in afibrinogenemia. Sanger sequencing revealed that the patient’s asymptomatic mother with a fibrinogen concentration of 2.2 g/L (Clauss) was heterozygous for the FGG p.Ala108Gly variant.
Conclusion(s): We identified a patient with a large de novo deletion of the entire fibrinogen gene cluster and a hemizygous variant in FGG that results in a severe hypofibrinogenemia phenotype. This case emphasizes the value of further probing cases for which the fibrinogen levels appear too low to be explained by a single mutation.
126. PB0707
126.1. An integrated clinical and pathological approach to rare bleeding disorders
R. Hargreaves 1; P. Thwin1; A. Riddell2; T. Yee1
1 Royal Free London NHS Foundation Trust, London, England, United Kingdom; 2 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free London NHS Foundation Trust, London, UK, London, England, United Kingdom
Background: Prompt recognition of rare acquired bleeding disorders is imperative, especially amongst older patients who often present with life‐threatening bleeds.
Aims: To highlight the diagnostic challenges and working relationship between clinicians and biomedical scientists in making a timely diagnosis.
Methods: We present three cases of rare acquired coagulation disorders.
Results: Patient 1 is a 77 year old man presenting with extensive bruising over the chest and arm and a haemoglobin of 60g/L. Normal Clotting screen (PT, APTT and Fibrinogen) prompted the lab staff to discuss immediately with the clinician and test factor XIII levels, diagnosing acquired factor XIII deficiency. Patient 2 is a 99 year old man on long term warfarin for arrhythmia, presenting to another hospital several times with bruises over the arms, especially at venepuncture sites. INR checked and given vitamin K. Eventually, when tested in our laboratory both the PT and APTT were performed for this warfarinised patient. The APTT was disproportionately prolonged relative to the PT enabling the diagnosis of acquired haemophilia A. Patient 3 is an 84 year old lady who visited casualty of another hospital several times with GI bleeds necessitating blood transfusions and upper/lower GI endoscopies. Referred to our hospital for double balloon pushed enteroscopy where a clotting screen showed a prolonged APTT with no 50/50 correction. Von Willebrand disease (VWD) screening was conducted in addition to factor VIII levels, confirming acquired VWD which is strongly associated with angiodysplasia.
Conclusion(s): The unique working relationship between clinical and laboratory staff at our UK tertiary haemostasis centre is exemplified by the following: Weekly laboratory results and MDT meetings; co‐location of the haemostasis laboratory and clinical area including a shared common room; an inclusive working environment where all staff feel empowered to contribute positively to patient care. We have highlighted how this effective partnership can streamline diagnostics and enhance patient care.
127. PB0700
127.1. Evaluation of pharmacological enhancers of mutated factor VII activity ex vivo
M. Andresen 1; E. Andersen1; M. Mowinckel1; B. Stavik2; P. Sandset3; M. Chollet4
1 Oslo University Hospital, Oslo, Oslo, Norway; 2 Oslo university hospital, Oslo, Oslo, Norway; 3 University of Oslo, Oslo, Oslo, Norway; 4 Oslo University Hospital
Background: As replacement therapy requiring intravenous injection is the mainstay of treatment for congenital factor (F) VII deficiency, there is an unmet need for new therapeutic strategies using less invasive delivery mechanisms. Drug repurposing allows for rapid, more cost‐effective discovery and implementation of new treatments, and identification of pharmacological enhancers of FVII activity would be of clinical importance. High‐throughput screening of >1500 FDA‐approved drugs identified the orally available histone deacetylase inhibitor abexinostat and the inhaled surfactant tyloxapol as enhancers of FVII p.Q160R variant activity in patient’s plasma in an ex vivo pilot study.
Aims: To investigate the dose‐response effect of the clinically approved drugs abexinostat and tyloxapol ex vivo in patient’s plasma.
Methods: Plasma samples from seven patients carrying FVII p.Q160R mutation were incubated with eleven different concentrations of abexinostat and tyloxapol (0.1–10 μM) for 1.5 h at 37°C. Equal amounts of FVII antigen of each sample were loaded onto the assay plates. FVII activity was analyzed using Biophen FVII assay and absorbance measured at 405nm for 2h, 37°C. Data were analyzed using KNIME software.
Results: The initial velocity (V0) and absorbance (A405) at 2h were calculated. Both treatments showed a dose‐response effect in patient’s plasma. Abexinostat showed an EC50 value of 2,87μM (SEM ±0.554) in six patients. The change of magnitude in FVII activity was approximately 19% (SEM ±1.885). Tyloxapol demonstrated an EC50 of 2,43μM (SEM ±0.320) and the change of magnitude in FVII activity was 21,64% (SEM ±3.14).
Conclusion(s): These results showed that treatment with the FDA approved drugs abexinostat and tyloxapol increased FVII activity about 20% in patient’s plasma ex vivo. It is of importance that a modest increase of FVII activity can ameliorate the bleeding phenotype in patients. This proof‐of‐concept study demonstrates that drug repurposing may be feasible for novel treatment of FVII deficiency.
128. PB0715
128.1. Exploring diverse coagulation Factor XIII subunit expression datasets: A bioinformatic analysis
S. Singh1; M. Jamil 1; O. El‐maarri1; J. Oldenburg2; A. Biswas3
1 Uniklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 2 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Coagulation Factor XIII (FXIII) circulates in plasma as a pro‐transglutaminase complex composed of the catalytic FXIIIA and carrier/regulatory FXIIIB subunits. It functions by crosslinking fibrin clot making it resistant to premature fibrinolysis. The FXIIIA subunit in plasma is expressed from resident macrophages, while the FXIIIB subunit is contributed by hepatocytes. Very little is known on the intracellular regulation of these two subunits.
Aims: The current study uses a purely bioinformatics approach to understand the intracellular regulatory mechanism underlying both FXIIIA and FXIIIB subunits.
Methods: Expression data were extracted from NCBI GEO (GSE128303, GSE98324, GSE39157, and GSE41571) for macrophages (time‐series), hepatic progenitor, hepatocytes and human embryonic stem cells derived hepatoblasts. Data were background corrected, normalized, and filtered based on the array platform using R. Bioinformatics tools (Qlucore Omics Explorer, TRANSFAC, and Cytoscape) were used for identifying transcriptional regulation of the differentially expressed genes between cell types and generating networks for visualization. Ingenuity pathway analysis (IPA) was used to identify upstream regulators of differentially expressed genes (Figure 1).
Results: Genes correlated with F13A1 and F13B genes are linked to several biological processes including immune response. Both FXIII subunits show similarity in transcription factor binding suggesting the possibility of inter‐regulation (e.g. HES2, MXD1, HES6). On profiling the array data from M1 and M2 macrophages a strong expression of F13A1 in M2 was observed, the time‐based array indicates that FXIII‐A most likely contributes towards macrophage polarization. No differential expression of F13A1 in resident macrophages derived from ruptured vs vulnerable plaques was observed, indicating that its constitutive expression of F13A1 is not responsible for plaque initiation/progression, but maintenance.
Conclusion(s): Bioinformatic expression profiling reveals that both FXIIIA and FXIIIB subunit have roles beyond coagulation, most likely in immune response. Sharing of transcription factor binding elements suggests the possiblity of interregulation between the FXIIIA and FXIIIB subunits.

129. PB0719
129.1. The novel bispecific antibody HMB‐001 enhances the haemostatic response in models of Glanzmann Thrombasthenia by targeting FVIIa to activated platelets
M. Zivkovic 1; P. Gandhi2; H. Ostergaard2; E. Olsen2; C. Rea3; B. Sorensen4; J. Faber2; R. Schutgens1; S. Bjorn2; R. Urbanus5
1 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 2 Hemab Therapeutics, Copenhagen, Hovedstaden, Denmark; 3 Hemab Therapeutics, London, England, United Kingdom; 4 Hemab Therapeutics, Cambridge, Massachusetts, United States; 5 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: Glanzmann Thrombasthenia (GT) is a severe platelet disorder caused by lack of functional integrin αIIbβ3. Acute bleeds in GT can be managed with recombinant FVIIa (rFVIIa), which enhances thrombin generation in a platelet‐dependent manner. HMB‐001 is a bispecific antibody that accumulates endogenous FVIIa and recruits it to the surface of activated platelets by binding FVIIa with one arm and the platelet TREM‐like transcript 1 (TLT‐1) receptor with the other. It thereby aims to restore clot formation in GT and enable long‐term bleed prevention.
Aims: To evaluate the potentiation of FVIIa activity by HMB‐001 on platelets in GT.
Methods: Platelet TLT‐1 expression and plasma FVIIa levels were evaluated in GT patients and healthy controls. Effects of HMB‐001 were evaluated with light transmission aggregometry in αIIbβ3‐inhibited (GT‐like) washed platelets with purified coagulation factors and in GT‐like whole blood in a microfluidic flow chamber. Results were confirmed in blood of GT patients. Ethical committee approval was obtained.
Results: TLT‐1 expression on activated GT platelets was normal and plasma FVIIa levels in GT patients and healthy controls were similar. Aggregation of activated GT‐like platelets was absent, but aggregation occurred when fibrin formation was initiated with rFVIIa. Aggregation onset shortened with increasing FVIIa concentrations. HMB‐001 lowered FVIIa requirements for aggregation 10‐fold but had no effect in absence of FVIIa. Similar results were obtained with GT platelets. Addition of excess soluble TLT‐1 attenuated the effects of HMB‐001, confirming that HMB‐001 is TLT‐1‐dependent. Perfusion of recalcified GT‐like whole blood over a collagen surface at a shear rate of 300 s‐1 resulted in platelet adhesion, but not in fibrin formation. Addition of rFVIIa caused a dose‐dependent increase in fibrin formation on adhered GT‐like platelets. HMB‐001 strongly potentiated FVIIa‐dependent fibrin formation on adhered platelets in flowing blood.
Conclusion(s): HMB‐001 potentiates FVIIa‐dependent fibrin formation on platelets in GT.
130. Hemostatic System in Cancer, Inflammation and Immunity
130.1. Coagulation Proteins Beyond Hemostasis
131. PB0733
131.1. Frequency of Factor II, Factor V Leiden and MTHFR mutations in children with cancer
J. Roganovic
Clinical Hospital Centre Rijeka, Rijeka, Primorsko‐Goranska, Croatia
Background: Thrombosis is an increasingly recognized complication of paediatric malignancy, occurring in up to 20% of children with cancer. The pathogenesis is complex, including multiple genetic and acquired factors. There is significantly less knowledge about thrombosis in paediatric cancer population compared to adults.
Aims: The aim of the study was to analyse the role of inherited thrombophilic defects in paediatric cancer‐related thrombosis.
Methods: This retrospective study included 47 children with cancer (36 haematological malignancies and 11 solid tumours), treated at the Division of Paediatric Haematology and Oncology, Clinical Hospital Centre Rijeka. The frequency of Factor V Leiden, prothrombin G20210A mutation and MTHFR C677T mutation in children, the frequency of thrombotic events, and a possible role in cancer‐associated thrombosis in children was examined.
Results: The median age of patients was 8.8 years (range 0.4 – 19.3 years). Among 47 cancer patients, 8.5% were heterozygous for Factor V Leiden, 6.4% were heterozygous for prothrombin G20210A mutation, 6.4% were homozygous for MTHFR C677T mutation, and 10.6% had combined thrombophilic defects. The prevalence of thrombophilic defects was in accordance with the prevalence in general population. There was no difference between boys and girls, and between patients with haematological malignancies and solid tumours. The rate of thrombosis was 8.5%. Three patients had upper extremity deep venous thrombosis and 1 had right atrial thrombosis. In half (2/4) of patients combined Factor V Leiden and MTHFR C677T heterozygosity were identified.
Conclusion(s): Our data suggest that children with cancer have an increased risk for thrombosis. Inherited thrombophilia may play a role in the pathogenesis of cancer‐associated thrombosis. Routine thrombophilia screening in children with newly diagnosed cancer could be useful. A better understanding of the interactions between genetic and non‐genetic causes will help to improve the prophylactic and treatment strategies for thrombosis in this patient population.
132. PB0726
132.1. (Factor VIII/Factor V) ratio as a new prognostic marker for decompensated liver cirrhosis
M. El Horri 1; N. Feghoul1; F. El Horri2; I. Khachaa1; A. Chikh Khelifa3; M. Baghdadi1; A. Benglia1; K. Benbouhadi1; H. Mimene1; K. Kebir1; K. Benhalima1; L. Benmahdi1; H. BROUK4
1 Regional Military University Hospital of Oran, Oran, Oran, Algeria; 2 Mixed Hospital of Ras elma, Sidi Bel Abbes, Sidi Bel Abbes, Algeria; 3 Regional Military University Hospital of Oran, Saida, Saida, Algeria; 4 Faculty of medicine, University of Badji Mokhtar of Annaba, Annaba, Annaba, Algeria
Background: Factor VIII is synthesized by the liver but also by endothelial cells. Thus, its concentration does not decrease with liver cirrhosis and may even be increased due to endothelial dysfunction. But this increase does not correlate with the worsening of the disease due to the deterioration of liver function. Therefore we hypothetically assumed that the expression of factor VIII according to factor V could fill this gap.
Aims: To assess the prognosis value of the RATIO (Factor VIII/Factor V) in predicting mortality in decompensated hepatic cirrhosis.
Methods: A prospective cohort was conducted on 60 decompensated cirrhotic patients. All these patients got a measurement of factor VIII and factor V and the ratio was calculated. The endpoint was the occurrence of death.
Results: Patients were followed for a median of 15 months, after which 38% of death cases were noticed and we recorded one tracking lost. The ratio (FVIII/FV) was significantly higher in deceased patients compared to survivors: 5.79 [CI: 4.36–7.21] versus 3.49 [CI: 2.91–3.98]; p = 0.001. The threshold value of 4.76 for our ratio which made it possible to predict death with a sensitivity of 56.52% and a specificity of 89%. Patients with a ratio < 4.76 (group I): had a mean survival of 17.3 months and a survival probability of 75.5% at 12 months. Those whose ratio exceeded 4.76 (group II): had a mean survival of 4.6 months with a survival probability of 60.8% at 12 months. Patients in group II had higher risk of mortality than those in group I, with a HR = 4.5 [IC: 1,95 – 10,35].
Conclusion(s): The ratio (FVIII/FV) is a good prognosis marker allowing the prediction of mortality in decompensated cirrhotic patients, independently of the Child Pugh and MELD score. The results found are promising and should arouse the interest of researchers in this field.


133. VPB0736
133.1. Prothrombin can modulate its expression in colon cancer cells
M. Cumbo 1; S. Dunjic Manevski2; B. Tomic2; M. Gvozdenov2; V. Djordjevic2
1 Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Belgrade, Serbia, Belgrade, Not Applicable, Serbia; 2 Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Belgrade, Serbia, Belgrade, Vojvodina, Serbia
Background: Prothrombotic disorders are the main comorbidity of cancer and can significantly contribute to its morbidity and mortality. Thrombin, pivotal regulator of the coagulation cascade, influences cancer growth and invasion through the activation of protease‐activated receptors (PARs). Prothrombin, precursor of thrombin, is predominantly synthesized in the liver and secreted into the bloodstream, but it can also be expressed in the cancer cells. Recent data show that prothrombin binds to the transmembrane receptors, which activate migration and invasion and which are up‐regulated in cancers. Even though there is a vast amount of evidence for the effects of thrombin in cancer progression, there are little data of its activation and effects of its precursor ‐ prothrombin, on cancer cells.
Aims: Evaluate the effects of exogenous prothrombin treatment on the prothrombin expression in cancer cell lines.
Methods: Permanent colon cancer cell lines (Caco2, SW480, SW620, HT29 and HCT116) were treated with commercial human prothrombin. Real‐Time PCR (qPCR) for relative quantification of prothrombin mRNA expression was performed by TaqMan Gene Expression Assays using cDNA from cell lines as a template. Expression pattern was analyzed 24h after the treatment. Data were compared with results of untreated cell lines.
Results: This study confirms that analyzed colon cancer cell lines express prothrombin. The detected effect of the treatment with exogenous prothrombin differed between cell lines. There was significantly lower level of prothrombin expression in treated Caco2, SW620 and HCT116 cell lines in comparison to untreated.
Conclusion(s): Our results indicate that prothrombin (originated from circulation or expressed by cancer cell), although inactive form, can regulate its expression in colon cancer cells.
134. PB0725
134.1. Role of von willebrand factor antigen as non‐invasive biomarker for the prediction of portal hypertensive gastropathy in patients with liver cirrhosis
M. El Horri 1; F. El Horri2; A. Chikh Khelifa3; M. Chekkal4; K. Benbouhadi1; H. Mimene1; K. Kebir1; K. Benhalima1; A. Benglia1; L. Benmahdi1; H. BROUK5
1 Regional Military University Hospital of Oran, Oran, Oran, Algeria; 2 Mixed Hospital of Ras elma, Sidi Bel Abbes, Sidi Bel Abbes, Algeria; 3 Regional Military University Hospital of Oran, Saida, Saida, Algeria; 4 University Hospital Establishment of Oran, Oran, Oran, Algeria; 5 Faculty of medicine, University of Badji Mokhtar of Annaba, Annaba, Annaba, Algeria
Background: Recently, Von Willebrand factor antigen (vWF‐Ag) has been identified as a new marker of portal hypertension (PH) and its complications, but untill now, no published study discuss its predictive value for portal hypertensive gastropathy (PHG). This marker is considered as a non‐invasive approach, In order to avoid the endoscopic burden, cost, drawbacks, unpleasant and repeated examinations for patients.
Aims: To evaluate the ability of vWF‐Ag in the prediction of PHG, which is diagnosed by endoscopic tools.
Methods: One hundred twenty‐four patients with liver cirrhosis were included in this study. Patients with no history of bleeding who underwent screening endoscopy for PH related complications like: esophageal varices (EVs) and PHG. vWF‐Ag testing was performed by immunoturbidimetric technique. The diagnostic performance of our marker was assessed using sensitivity, specificity, positive predictive value, negative predictive value, accuracy and receiver operating characteristic curves.
Results: Screening endoscopy revealed the presence of 20.2% PHG cases, and 83.1% EVs cases. vWF‐Ag levels, were significantly increased in patients with PHG compared to those who have not: 441% [CI: 375 – 506], versus 279% [CI: 253 – 304], respectively (p < 0.0001). vWF‐Ag was a good predictor for the presence of PHG. With a cut‐off value of 320% and an AUC of 0.824, vWF‐Ag had an 84% sensitivity, 74% specificity, 44.7% positive predictive value, 94.8% negative predictive value and 75.8% diagnostic accuracy.
Conclusion(s): vWF‐Ag is a good non‐invasive affordable marker for excluding the presence of PHG in patients with liver cirrhosis. Using this marker as part of a selective screening strategy, might reduce the coast of patient care and the need for endoscopic screening.
135. PB0730
135.1. Circulating activated protein C levels are reduced in cancer patients, a novel contribution to tumorigenesis?
J. Oto 1; R. Herranz1; E. Plana2; F. Cana3; J. Amaya4; C. Vera‐Donoso5; M. Martínez‐Sarmiento5; S. Bonanad6; P. Medina3
1 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 2 Angiology and Vascular Surgery Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 3 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 4 Orthopaedics and Traumatology Service. La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 5 Urology Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 6 Hematology Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain
Background: Cancer growth develops a subclinical hypercoagulable state and hypercoagulability is also a key contributor in cancer progression beyond cancer‐associated thrombosis. Increasing evidence shows that coagulation proteins have multiple functions in tumor progression and metastasis, however the role of natural anticoagulants in cancer remains barely unexplored. Activated protein C (APC) displays anticoagulant and cytoprotective functions and its dysregulation may prompt cancer growth.
Aims: To ascertain whether circulating APC levels are dysregulated in cancer patients.
Methods: We obtained heparinized plasma from 88 cancer patients (45 bladder, 15 sarcoma, 9 renal and 19 prostate) and 14 controls, namely patients with a cancer suspicion anatomopathologically discarded. Circulating APC was quantified with an in‐house sandwich ELISA, based on the interaction between APC and its main natural inhibitor PCI. Briefly, heparin forces APC:PCI complexation and these complexes are measured using an anti‐PC primary antibody and an anti‐PCI secondary antibody. Differences in circulating APC between groups was assessed with a Mann‐Whitney test using GraphPad (v.8.0.1).
Results: Compared to controls, cancer patients showed a 16% reduction in circulating APC concentration (p = 0.0210) (Figure 1). Attending to the different cancer types, bladder cancer patients showed a 23% APC decrease (p = 0.0038) and sarcoma patients showed a 14% decrease (p = 0.0116), whereas renal and prostate cancer patients did not show a significant decrease.
Conclusion(s): Circulating APC levels are significantly decreased in cancer patients. Differences among cancer types may be due to differential contribution of APC in tumorigenesis or by the regulation driven by cancer cells on APC levels. The reduction in circulating APC may further enhance hypercoagulability in cancer patients while reducing its cytoprotective functions, thus contributing to tumorigenesis. ISCIII‐FEDER (PI17/00495, PI20/00075, FI21/00171), GVA (ACIF/2017/138) and SETH.

136. PB0735
136.1. Understanding fate of circulating von Willebrand factor following interaction with polymorphonuclear leukocytes
A. Kuanyshbek1; H. Yadegari 2; J. Oldenburg3
1 University Clinic Bonn Institute of Experimental Hematology and Transfusion Medicine, Bonn, Nordrhein‐Westfalen, Germany, 2 University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany
Background: Von Willebrand factor (VWF) is best known for its crucial functions in hemostasis. Additionally, the involvement of VWF in inflammation has been suggested but not well studied. It is previously proved that anchored VWF on the endothelium binds to the polymorphonuclear leukocytes (PMNs) through the β2 integrin.
Aims: In this study, we intend to investigate the intraction of free VWF with PMNs, and its fate following binding into the PMNs.
Methods: PMNs were isolated from whole blood using EasySep™ Direct Human Neutrophil Isolation Kit. The purity of the isolated cells was determined by flow cytometry (CD66+ CD16+) on the CD45‐positive cells. The isolated PMNs either were left unstimulated, or were activated by treatment of the cells with either PMA, TNFα, or IL‐8, in presence of the purified plasma‐derived VWF. Further, the native/or activated PMNs were immune‐stained to visualize VWF, CD11b, EEA1, and Rab7. The Z‐series images were acquired by Carl Zeiss Apotome.2 microscope. The Mean Intensity Value (MIV) of the VWF signal and co‐localization of VWF with PMNs markers were calculated.
Results: The immunofluorescence image analysis demonstrated that the MIV of VWF signal was significantly increased after activation of PMNs with PMA (10 ng/ml), TNFα (5ng/ml), or IL8 (0.7 ng/ml), compared to the resting cells. The images indicated the elevated VWF signals after activation of PMNs were associated with increased colocalization of VWF with PMNs β2 integrin receptor marker, CD11b. Furthermore, we showed that colocalization (indicated by Pearson's correlation coefficient) of VWF with early endosome (EEA1) and late endosome (Rab7) markers was increased with incubation time.
Conclusion(s): Our data showed that circulating VWF interacts/binds to PMNs upon activation with inflammatory modulators, and we confirmed that VWF is internalized following interaction with PMNs. The impact of VWF/PMNs interaction on the function of the PMNs remains to be studied.
137. PB0732
137.1. Fibrin promotes glioblastoma progression via focal adhesion kinase
L. Knowles1; C. Wolter2; R. Ketter3; S. Urbschat3; S. Linsler3; S. Müller3; A. Müller4; X. Zhou5; B. Qu6; E. Hermann7; J. Pilch 8
1 Institute of Clinical Hemostaseology and Transfusion Medicine, Homburg, Saarland, Germany; 2 Saarland University, Homburg, Saarland, Germany; 3 Departement of Neurosurgery, Homburg, Saarland, Germany; 4 Departement of Radiology, Homburg, Saarland, Germany; 5 Biophysics, Homburg, Saarland, Germany; 6 Biophysics and INM‐Leibniz Institute for New Materials, Homburg, Saarland, Germany; 7 Universitätsklinikum des Saarlandes, Homburg/Saar, Saarland, Germany; 8 Saarland University Medical Center, Homburg, Saarland, Germany
Background: Glioblastoma (GBM) is a highly aggressive brain tumor characterized by invasive growth and the leakage of a fibrin‐rich edema into tumor interstitial spaces from poorly organized tumor blood vessels.
Aims: To test if clotted plasma supports GBM progression.
Methods: We assessed fibrin formation in astrocytoma samples from patients using immunohistochemistry and examined the effect of plasma clot on glioblastoma growth in mice in vivo. Glioblastoma cells embedded in 3‐D fibrin were analyzed for invasion and growth using live cell imaging and deconvolution microscopy. In order to assess clinical relevance, we analyzed primary cells and tissue arrays.
Results: We demonstrate a marked upregulation of clot formation in the interstitial spaces of astrocytoma patients while tumor‐free brain is essentially devoid of fibrin. This is relevant as we detected a delay of glioblastoma development in clotting‐deficient hemophilia mice and accelerated tumor growth in nude mice after implanting glioblastoma cells in conjunction with clotted plasma. This growth promoting effect was reproducible when we cultured GBM cells in a 3‐dimensional matrix of clotted plasma in vitro. The pro‐growth effect of the clotted plasma resulted from adhesive interactions with integrins on GBM cells, which in turn promoted the activation of focal adhesion kinase and p42/Erk. In addition, engagement of integrins promoted the formation of actin‐rich invadopodia and subsequent locomotion of GBM cells. Invadopodia formation in clotted plasma was also evident in primary tumor cells from patients with astrocytoma 2 and 3 but only primary GBM cells were able to grow sufficiently in 3D clot. Finally, overexpression of focal adhesion kinase‐activating integrins in glioma tissues from patients was associated with a significantly decreased overall survival.
Conclusion(s): The deposition of clotted plasma in tumor interstitial spaces promotes infiltration and proliferation of glioblastoma cells and, therefore, may provide a therapeutic target to combat primary brain tumors.
138. PB0727
138.1. The induction of Tregs by aPC depends on metabolic reprogramming and reduced αKG levels
D. Gupta 1; A. Elwakiel2; S. Ranjan2; M. Pandey2; S. Krishnan3; R. Henschler4; S. Kohli1; B. Isermann1
1 Institute of laboratory medicine, clinical chemistry and molecular diagnostics, Leipzig University, Leipzig, Sachsen, Germany; 2 Institute for Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, Leipzig University, Leipzig, Sachsen, Germany; 3 Institute of Experimental Internal Medicine, Otto von Guericke University, Magdeburg, Sachsen‐Anhalt, Germany; 4 Institute for Transfusion Medicine, Leipzig University, Leipzig, Sachsen, Germany
Background: Activated protein C (aPC) is a coagulation protease known to regulate cellular signaling in innate as well as adaptive immunity. We and others have previously shown that aPC ameliorates graft versus host disease (GvHD). aPC induced regulatory T cells (Tregs) from T‐effector (Teff) cells. Interestingly, cellular metabolism is known to regulate T‐cell plasticity. Accordingly, we hypothesized that the coagulation protease aPC regulates T cell metabolism and plasticity.
Aims: We aim to investigate whether aPC induces Tregs via metabolic reprogramming of Teff cells.
Methods: Activation of T cells was determined by in vitro proliferation using the MLR (mixed lymphocyte reaction model: stimulation of Teff cells with AgPC, ratio 1:3) or plate‐bound anti‐CD3 and CD28 stimulation. Teff and Treg cell markers were measured by FACS. 5‐methyl cytosine(5mC) pattern was analyzed by dot blot and FACS. Oxygen consumption rate (OCR) was measured by Seahorse and TCA metabolites by LC/MS. Steady‐state T‐cell phenotyping was done by analyses of T‐cells from APChigh and WT mice. GvHD was induced in BALB/c mice by transplanting 2x106 T cells with 5–6x106 bone marrow cells from C57BL/6 mice.
Results: aPC treatment suppressed the global 5mC methylation in Tregs. Furthermore, induction of Tregs from Teff cells was associated with Foxp3 promoter demethylation and increased Foxp3 expression in vitro suggesting that T‐cell plasticity is associated with epigenetic changes. These epigenetic changes were associated with metabolic reprogramming in T‐cells, reflected by reduced OCR and alteration in TCA cycle metabolites upon aPC treatment. In particular α‐ketoglutarate (αKG) was reduced in aPC‐pretreated Teff cells, which was associated with reduced expression of the glutamine transporter Snat1 and Asct2 and reduced glutamate levels. These effects of aPC were reversed by addition of α‐KG and glutamine resulting in increased proliferation and decreased Foxp3 expression.
Conclusion(s): These data demonstrate that aPC metabolically re‐programs T‐cells and induces epigenetic changes, favoring a Treg phenotype.
139. PB0728
139.1. Bladder cancer patients have increased NETosis and impaired DNaseI‐mediated NET degradation that can be therapeutically restored in vitro
R. Herranz 1; J. Oto1; M. Hueso2; E. Plana3; F. Cana2; A. Fernández‐Pardo2; S. Bonanad4; C. Vera‐Donoso5; M. Martínez‐Sarmiento5; P. Medina2
1 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 2 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 3 Angiology and Vascular Surgery Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 4 Hematology Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 5 Urology Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain
Background: An increased release of neutrophil extracellular traps (NETs), through a process called NETosis, occurs in cancer. This enhances tumor growth and metastasis, which may be mediated by impaired NET degradation. However, this has never been evidenced in bladder cancer (BC).
Aims: To evaluate the increase in NETosis in plasma of BC patients, to ascertain whether it is mediated by DNaseI and to in vitro explore therapeutic interventions. To compare the ability of plasma from patients with BC and from healthy volunteers (controls) to degrade NETs. Also, we aimed to evaluate the effect of the addition of Dornase Alfa (a recombinant human DNaseI used in clinical practice for management and treatment of cystic fibrosis) in NET degradation.
Methods: NETs markers were measured in plasma samples of 25 BC patients and 23 controls. Plasma DNaseI activity was measured with the SRED assay. Neutrophils were isolated from a healthy volunteer, seeded and activated with phorbol myristate acetate (PMA) to prompt NETosis. NETs were incubated with 5 μl plasma from each participant. In parallel, plasma from BC patients was supplemented with Dornase Alfa (Pulmozyme, Roche), control pooled plasma or vehicle (PBS 1X + 0.1%BSA).
Results: BC patients had significantly increased NETs markers in plasma compared to controls (p < 0.0001) and lower DNaseI activity (p < 0.0001) (Figure 1). In vitro NET degradation was impaired when incubated with BC plasma compared to control plasma (p < 0.0001) (Figure 2). Addition of DNaseI restored NET degradation up to the level of healthy controls (p < 0.0001) (Figure 2).
Conclusion(s): BC patients have increased NETosis in part mediated by a reduced DNaseI activity. In vitro addition of exogenous DNaseI, an approved treatment for cystic fibrosis, restores NET degradation in BC patients, thus becoming a potential therapeutic tool to reduce the risk of cancer‐associated thrombosis and metastasis. ISCIII‐FEDER (PI17/00495, PI20/00075, FI21/00171), GVA (ACIF/2017/138) and SETH.

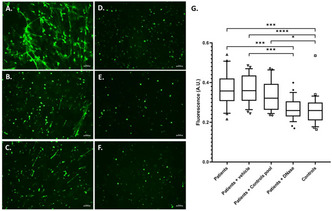
140. PB0729
140.1. Development of a viral vector for hemopexin protein expression for gene therapy in disorders associated with increased levels of free extracellular heme
F. Lima 1; B. Hounkpe1; C. Peachazepi de Moraes2; I. Borba3; F. Costa1; E. de Paula4
1 University of Campinas, Campinas, Sao Paulo, Brazil; 2 University of Campinas, Campinas, South Carolina, United States; 3 University of Campinas, CAMPINAS, Sao Paulo, Brazil; 4 School of Medical Sciences of the University of Campinas, Campinas, Sao Paulo, Brazil
Background: Extracellular heme induces tissue factor‐dependent coagulation activation and tissue damage. Hemopexin (HPX), responsible for removing free heme from the circulation, has been shown to be depleted in hemolytic diseases. Gene therapy with adeno‐associated viral vectors (AAV) has been applied to replenish circulating proteins in hemophilia with promising results
Aims: Development of an adeno‐associated virus‐based vector for HPX expression
Methods: An AAV‐8 vector was produced containing the full cDNA sequence of human HPX (hHPX). Mice were injected with escalating doses of 2x1012, 1x1013 and 2x1013 vector genomes/kg, while a control group received an empty vector. Blood was obtained every two weeks for HPX ELISA. Expression was also confirmed in liver samples obtained after 6 weeks, and functional activity assessed by heme challenge and phenylhydrazine‐induced hemolysis.
Results: Liver hHPX expression was confirmed by RT‐PCR and western blot. Sustained expression of hHPX were observed up to 58 weeks in plasma. Expression was dose‐dependent and not associated with clinical signs of toxicity. Levels of hHPX were significantly reduced by heme infusions and phenylhydrazine‐induced hemolysis. No clinical toxicity or laboratory signs of liver damage were observed in preliminary short term studies of heme challenge.
Conclusion(s): Long‐term expression of hHPX can be achieved with AAV vectors in mice. Additional studies are required to confirm the biological effect of the transgenic protein and its effect in animal models of chronic hemolysis.

141. PB0731
141.1. Neutrophil activation markers as novel biomarkers for advanced stages of high‐grade serous ovarian cancer
J. Oto 1; B. Mc Cormack2; S. Tomás‐Pérez2; A. Fernández‐Pardo3; E. González‐Cantó2; F. Cana3; C. Aghababyan4; R. Herranz1; S. Cañete‐Mota5; A. Cuadros‐Lozano4; L. Martínez‐Fernández4; A. Aranda‐Borreda2; A. Arroyo‐Álvarez2; N. Santonja‐López6; R. Ramírez‐Belloch7; A. Llueca‐Abella8; J. Marí‐Alexandre J2; J. Gilabert‐Estellés9; P. Medina3
1 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 2 Research Laboratory in Biomarkers in Reproduction, Gynaecology and Obstetrics, Research Foundation of the General University Hospital of Valencia, Valencia, Comunidad Valenciana, Spain; 3 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 4 Obstetrics and Gynaecology Service, General University Hospital of Valencia Consortium, Valencia, Comunidad Valenciana, Spain; 5 Obstetrics and Gynaecology Service, General University Hospital of Castellón, Castellón, Comunidad Valenciana, Spain; 6 Pathological Anatomy Service, General University Hospital of Valencia Consortium, Valencia, Comunidad Valenciana, Spain; 7 Medical Oncology Service, General University Hospital of Valencia Consortium, Valencia, Comunidad Valenciana, Spain; 8 Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery (MUAPOS), General University Hospital of Castellón, Valencia, Comunidad Valenciana, Spain; 9 Departament of Paediatrics, Obstetrics and Gynaecology, University of Valencia, Valencia, Comunidad Valenciana, Spain
Background: In the tumor environment, NETosis participates in immunothrombosis, tumor progression, metastasis, and immune evasion. NETosis has been only evidenced in the initial metastasis of high‐grade serous ovarian cancer (HGSOC).
Aims: To ascertain the contribution of NETosis in advanced stages of HGSOC and as a diagnostic biomarker.
Methods: In paired plasma and peritoneal fluid (PF) samples from women with advanced HGSOC (n = 28) and controls (n = 16), we quantified as NETosis markers: circulating DNA (cfDNA, Quant‐iT PicoGreen dsDNA kit), nucleosomes (Cell Death Detection ELISAPLUS kit), calprotectin (Human Calprotectin ELISA kit) and myeloperoxidase (MPO, Human MPO ELISA kit). We estimated their differences with SPSS software (v.21).
Results: Patients with HGSOC presented a higher concentration of cfDNA in plasma than controls (p < 0.001) and an increase in all 4 NETosis markers in PF (Figure 1). All markers correlated pairwise in both biofluids (p < 0.032) and with plasma neutrophil count (p < 0.001). Remarkably, plasma cfDNA could distinguish patients from controls (AUC = 0.842). Interestingly, among HGSOG patients, those who previously received neoadjuvant treatment (n = 7) presented decreased levels of NETosis markers, predominantly in plasma and not in PF (Figure 2).
Conclusion(s): For the first time, we describe an increase in NETosis markers in PF of advanced HGSOC, which could reflect the contribution of NETosis in its development in the pelvic microenvironment. Plasma cfDNA could represent a minimally invasive diagnostic biomarker for HGSOC that could reduce the pre‐surgical rate of false positives. Our observation that NETosis biomarkers mainly decrease in plasma as consequence of neoadjuvant treatment might involve that, at least for NETosis process, the intravenous administration of chemotherapy would produce more changes at the systemic level (plasma) than in the peritoneal tumor environment (PF). This could deserve a reflection on the role of intraperitoneal chemotherapy in the management of HGSOC patients. ISCIII‐FEDER (PI17/01945, PI20/00075), GV/2020/200, Premio FIHGUV 2019 and 2020, APOSTD/2019/087, ACIF/2020/216, SETH.


142. VPB0737
142.1. Prevalence and impact of antiphospholipid antibodies on thrombotic events in ambulatory cancer patients
C. Kansuttiviwat 1; P. Niprapan2; L. Norasetthada2; E. Rattaritamrong2; A. Tantiworawit2; P. Piriyakhuntorn2; S. Hantrakool3; N. Hantrakun2; T. Punnachet2; C. Chai‐Adisaksopha2
1 Faculty of Medicine, Chiang Mai University, Thailand, Chiang Mai, Thailand; 2 Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand, Sri Phum, Muang, Chiang Mai, Thailand; 3 Division of Hematology, Department of Internal Medicine, Chiang Mai University, Sri Phum, Muang, Chiang Mai, Thailand
Background: Despite the conflicting data, the positivity of antiphospholipid antibodies (APLA) in cancer patients may be associated with an increased risk of thrombosis.
Aims: To identify the prevalence of APLA and impact of APLA on arterial and venous thrombosis in ambulatory cancer patients
Methods: This was a prospective cohort study. We enrolled newly diagnosed ambulatory cancer patients receiving chemotherapy. Controls were enrolled from age‐ and sex‐matched non‐cancer, non‐autoimmune disease population. Participants were evaluated for APLA (lupus anticoagulant (LA), anti‐cardiolipin (aCL) and anti‐β2 glycoprotein 1 (anti‐β2‐GPI). Primary outcome was the prevalence of APLA positivity. Secondary outcomes were the prevalence of arterial and venous thrombosis and all‐cause mortality at 6‐month follow‐up duration.
Results: There were 137 cases and 137 controls with mean age of 56.0 ± 12.3 and 55.5 ± 12.1 years, respectively. Cancer patients were more likely to have APLA positivity compared to controls, with risk difference of 2.9% (95% CI 0.1 to 5.7) for dual LA positivity, 5.8% (95% CI 0.2 to 9.7) for aCL IgM or IgG and 6.6% (−0.7 to 13.1) for anti‐β2‐GPI IgM or IgG (Table 1). Composites of venous and arterial thrombosis occurred in 10 (7.3%) in cancer patients and 2 (4.5%) in controls. Death occurred in 19 (13.9%) in cancer patients and 0% in controls. In cancer patients who had any APLA positivity were associated with higher risk of composites of venous and arterial thrombosis compared to those without (RR 3.6, 95% CI 1.0–12.4). Cancer patients with LA positivity were associated with higher risk of arterial or venous thrombosis (RR 5.3 95% CI 1.3–21.0), whereas patients with anti‐β2‐GPI positivity were associated with higher risk of venous thrombosis (RR 4.7, 95% CI 1.1–19.2), Table 2.
Conclusion(s): APLA was more prevalent in patients with active cancer than non‐cancer patients. Positive APLA in cancer patients was associated with arterial or venous thrombosis.


143. PB0734
143.1. Visualization of gliomas with clot‐binding peptides
C. Wolter 1; L. Knowles2; S. Linsler3; S. Müller3; M. Jung4; E. Hermann5; J. Pilch6
1 Saarland University, Homburg, Saarland, Germany; 2 Institute of Clinical Hemostaseology and Transfusion Medicine, Homburg, Saarland, Germany; 3 Departement of Neurosurgery, Homburg, Saarland, Germany; 4 Medical Biochemistry and Molecular Biology, Homburg, Saarland, Germany; 5 Universitätsklinikum des Saarlandes, Homburg/Saar, Saarland, Germany; 6 Saarland University Medical Center, Homburg, Saarland, Germany
Background: Glioblastoma (GBM) is a highly aggressive brain tumor characterized by necrosis, hemorrhage and thrombosis. The outcome of GBM correlates with the extent of surgical tumor removal.
Aims: To test if the formation of a fibrin‐rich extracellular matrix provides binding sites for clot‐binding peptides that support fluorescent‐guided recognition and surgical removal of astrocytomas.
Methods: We analyzed fibrin formation in astrocytoma samples from patients using immunohistochemistry. To assess the uptake of clot‐binding peptides into brain tumors, we injected fluorescein‐labeled peptides intravenously in GBM‐bearing mice. Two‐four hours later the brain and control organs were isolated and tissue sections were evaluated by fluorescence microscopy. In addition, we assessed peptide uptake into intracranial brain tumors in intact mice using a fluorescence endoscope in situ. The role of clotting was tested in GL‐261 glioblastomas grown in transgenic hemophilia A mice.
Results: We demonstrate a marked upregulation of clot formation in the interstitial spaces of astrocytoma patients while tumor‐free brain is essentially devoid of fibrin. Five different peptides with affinity for clot or brain injury (CLT1, CLT1‐IK, CLT2, CREKA, CAQK) were injected into GBM‐bearing mice to visualize fibrin accumulation. Subsequent analysis showed the strongest fluorescence after injection of CLT1 while two unspecific control peptides did not generate any fluorescence. The fluorescent label of CLT1 was specific for clot in brain tumor tissue whereas no fluorescence was detectable in tumor tissue in absence of clotting activity or in normal brain tissue. Using fluorescence endoscopy, specific fluorescence by the clot‐binding peptide was detectable in brain tumors of intact mice in situ.
Conclusion(s): Our data demonstrate stage‐dependent fibrin deposition in astrocytomas that can be visualized with the clot‐binding peptide CLT1 in vivo. Moreover, our data suggest that CLT1 can improve fluorescent‐guided detection and removal of astrocytoma in situ.
143.2. Complement and Hemostatic System
144. VPB0741
144.1. Vasculopathy as an atypical manifestation of paroxysmal nocturnal hemoglobinuria: A case report
S. Candrianita; L. Hamijoyo; S. Prodjosoewojo; E. Martanto
Hasan Sadikin General Hospital, Bandung, Indonesia, Bandung, Jawa Barat, Indonesia
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare stem cell disorder which manifests typically as non‐immune hemolytic anemia, haemoglobinuria, thrombosis, and renal impairment.
Aims: Misdiagnosis commonly found due to burdensome diagnostic approach, particularly in developing countries. This case illustrated an atypical manifestation of PNH appearing as vasculopathy.
Methods: A 29‐year‐old male patient with sudden‐onset right leg pain preceded by non‐immune hemolytic anemia in the previous month. At first the patient was suspected as having septic arthritis, however vasospasm developed in the whole leg. Thus, vascular Doppler ultrasound was indicated.
Results: A positive Ham test was obtained from the patient's blood, supporting the diagnosis of PNH. However, there was no thrombus found albeit multiple times of Doppler ultrasound examination, supporting vasculopathy in this patient. Flow cytometry examination should have been utilised, as well as the treatment using Eculizumab. However, due to lack of resources, we could not do further examinations for this patient.
Conclusion(s): This case highlights atypical manifestation of PNH, the diagnostic approach, and what should have been done for the patient; considering the limited resources in the developing countries.
145. PB0739
145.1. Interaction of antiplatelet and anticoagulant APAC with complement proteins attenuates complement activation via C1q and factor H
J. Kotimaa1; M. Rezola1; A. Jouppila 2; S. Meri1; R. Lassila3
1 University of Helsinki, Department of Bacteriology and Immunology, Translational immunity research program (TRIMM), Helsinki, Finland, Helsinki, Uusimaa, Finland; 2 Clinical Research Institute HUCH, Helsinki, Finland, Helsinki, Uusimaa, Finland; 3 Helsinki University Hospital, and University of Helsinki, Finland, Helsinki, Uusimaa, Finland
Background: Antiplatelet, anticoagulant APAC, conjugate of human albumin and unfractionated heparin (UFH) differs from UFH as superior antithrombotic in vascular injury models. APAC targets vascular injury sites by interacting with extracellular proteins. Unlike UFH, APAC inhibits collagen‐ and thrombin‐induced platelet activity and is a stronger anticoagulant. Heparin and heparan sulphates interact with complement system prominently involved in injury responses, by inducing apoptosis, promoting local inflammation, and recruiting inflammatory cells to the injury site.
Aims: Our aim was to compare the effects of APAC with UFH in complement activation and its regulation in solution and on cell surfaces.
Methods: Pathway‐specific functional assays were used to determine complement activities in plasma. Binding of complement regulators C1q and factor H (FH) to APAC‐ and UFH‐coated on polystyrene surfaces was analyzed by ELISAs. Interactive partners were evaluated by serum pulldowns with biotinylated APAC, with follow‐up western‐blot detection of C1q and FH. APAC interaction and inhibitory capability on cell surfaces were assessed with activation assays with zymosan and cells.
Results: APAC (1–30 μg/ml) is more potent (1.3–3‐fold) anticoagulant than UFH in plasma and whole blood. In comparison to UFH, APAC (100–300 μg/ml) potently inhibits classical (CP) and alternative (AP) pathways. Serum pulldown showed selective binding between APAC and CP initiator C1q and AP regulator FH. On APAC‐coated surfaces, C1q and FH have detectable binding already at 0.01% serum dilution, whereas 1% serum is needed for binding to UFH‐coating. APAC binds to necrotic cells, and at the level of C5b‐9 activation, APAC decreases complement activation on zymosan up to 90%.
Conclusion(s): Overall, APAC may modulate fluid phase and surface‐localized complement activation through combined effects of regulators C1q and FH. Compared to UFH, APAC has more potential to regulate the complement system. As a net effect on APAC targeted sites inflammatory response may be reduced and phagocytic clearance of debris promoted.
146. VPB0740
146.1. Unique protein profiles with inflammatory, complement, and endothelial activation signature identified patients with alcohol‐associated and non‐alcoholic steatohepatitis associated cirrhosis
C. Allen; A. Butt; X. Zhao; F. Li; P. Bahel; C. Chang; H. Chun; S. Gu; J. Hwa; S. Jakab; M. McConnell; H. Rinder; U. To; A. Lee; A. Pine
Yale School of Medicine, New Haven, Connecticut, United States
Background: Cirrhosis‐related coagulation derangements result in altered hemostasis, and hypercoagulability markers such as von Willebrand factor (VWF) antigen and the factor VIII‐to‐protein C ratio (FVIII/PC) have prognostic significance. We sought to use plasma proteomic profiling to characterize plasma protein signatures in alcoholic versus non‐alcoholic cirrhosis, and to identify protein biomarkers of prognostic significance associated with hypercoagulability of alcohol‐associated cirrhosis.
Aims: To characterize and differentiate plasma protein signatures in alcohol versus non‐alcoholic cirrhosis, with a focus on VWF and FVIII/PC in alcohol‐associated cirrhosis.
Methods: Thirteen patients with alcohol‐associated cirrhosis (4 Child‐Pugh Class A, 9 Class C), 7 with non‐alcoholic steatohepatitis (NASH) cirrhosis (4 Class A, 3 Class C), and 5 controls without cirrhosis were recruited between April and June 2021 with informed consent and Institutional Review Board approval. Blood was drawn and plasma sent for proteomic profiling (Eve Technologies). Proteomic profiles were compared using pairwise t‐test and Wilcoxon rank sum test, identifying proteins that best clustered groups with linear discriminant analysis using Wilk’s Lambda criteria. Boruta feature selection was performed to identify candidate proteins associated with VWF antigen or FVIII/PC in alcohol‐associated cirrhosis. Statistical analysis was performed using R (v4, R Core Team); P‐values < 0.05 were considered statistically significant.
Results: Of study patients, 9 were female; 17 had cardiovascular risk factors and 3 chronic kidney disease. Eleven proteins involved in inflammation, complement activation, or endothelial activation uniquely segregated alcohol‐associated cirrhosis, NASH cirrhosis, and controls (Figure 1). Several plasma proteins were significantly associated with VWF antigen or FVIII/PC in alcohol‐associated cirrhosis (Figure 2).
Conclusion(s): Plasma proteomic profiling identifies unique complement, inflammation, and endothelial activation signatures in alcohol‐associated versus NASH cirrhosis. Endotheliopathy may contribute to the prognostic value of VWF antigen and FVIII/PC in alcohol‐associated cirrhosis. Further investigations may highlight the interconnection of complement and inflammation with the endothelium in the pathogenesis and coagulopathy of cirrhosis.
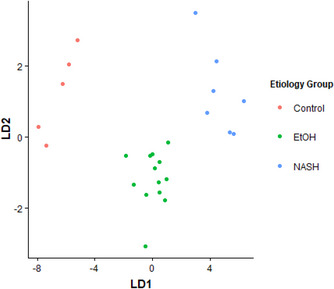
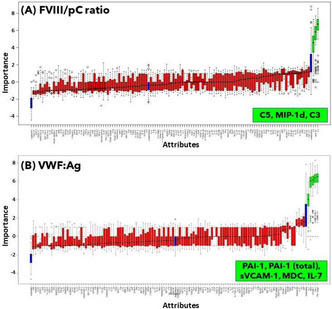
147. PB0738
147.1. Complement and haemostasis: Effects of complement inhibitors in a microfluidic bleeding model
M. Golomingi 1; J. Kohler1; C. Lamers2; R. Pouw2; E. Hardy3; D. Ricklin2; W. Lam3; V. Schroeder1
1 University of Bern, Bern, Bern, Switzerland; 2 University of Basel, Basel, Basel‐Stadt, Switzerland; 3 Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, USA & Department of Pediatrics, Division of Pediatric Hematology/Oncology, Aflac Cancer Center and Blood Disorders Center of Children’s Healthcare of Atlanta, Emory University School of Medicine, Atlanta, USA, Atlanta, Georgia, United States
Background: Even though the coagulation cascade is responsible for the formation of clots, other physiological systems, such as the complement system, can influence it. There have been several studies on the interactions between the complement system and clot formation, mainly in the context of atherosclerosis, inflammation, and thrombosis. To investigate a potential role of the complement system in the haemostatic response upon vessel injury, we have established a microvascular endothelialised whole blood flow model. This model allows the imaging of clot formation after vessel injury in real‐time by confocal microscopy.
Aims: To investigate the role of the complement system during clot formation upon vascular injury in a microfluidic bleeding model.
Methods: Endothelial cells were used to create a synthetic vessel in a polydimethylsiloxane mould. Whole blood was then flown through the vessel and an injury was created by rupturing a collagen pouch adjacent to the vessel. The formation of the clot was monitored in real‐time with a confocal microscope, fluorescently labelled fibrinogen and antibodies against P‐Selectin (CD62P). The fluorescence signal intensity over time was then compared with and without the following inhibitors targeting complement activation on different levels: inhibitory antibodies anti‐C1s, anti‐FD and anti‐C5, the C3 inhibitor Cp20, and the MASP‐1 inhibitor SGMI‐1.
Results: We could show so far that inhibition of MASP‐1 prolonged the bleeding time after vessel injury, and platelet activation (P‐Selectin signal) was lower compared with the control. Preliminary experiments with C1s and FD inhibitory antibodies showed a lower fibrinogen/fibrin signal at the injury site compared with the controls. Experiments with the other inhibitors are underway.
Conclusion(s): Both the bleeding time and markers of clot formation at the injury site suggest an anticoagulant effect of complement inhibition on the clotting process. Further experiments are underway to confirm and extend our results.
147.2. Infection and Hemostatic Factors
148. PB0745
148.1. NETosis in sickle cell anemia
A. Tombak 1; G. Hatem2; L. Tamer2; N. Tiftik2; N. Yilmaz2
1 Mersin University Medical Faculty, MERSIN, Mersin, Turkey; 2 Mersin University, Mersin, Mersin, Turkey
Background: Immune regulatory functions of neutrophils include phagocytosis, generation of reactive oxygen species, degranulation, formation of neutrophil extracellular traps (NETs). Most common cause of morbidity and mortality in sickle cell anemia (SCA) is vascular occlusion. Neuthrophil activation plays an important role in vaso‐occlusive events. Detection of NETs formation in these patients may play an important role in determining morbidity and mortality.
Aims: Study aimed to demonstrate the increased inflammation in SCA by demonstrating the activity of NETosis and to regulate the prophylaxis necessary for life‐threatening thrombosis, which may occur at the end of this process.
Methods: Blood samples of patients and healthy persons were obtained. Twenty five patients with SCA and 25 healthy individuals were included in the study. Two millilitre venous blood samples of patients and healthy individuals, EDTA tube for neutrophil elastase enzyme level received. Blood samples taken into EDTA tube for neutrophil isolation were centrifuged at 4000 rpm for 10 min and stored at +4°C. Neutrophils obtained using MACSxpress® Neutrophil Isolation Kit Human were used for NETs formation. In the NETosis ASSAY Kit stage, cells were suspended with NET assay buffer and incubated, then centrifuged to collect neutrophil elastase enzyme activity. Supernatants were incubated with the addition of NET Assay Neutrophil Elastase Substrate and the film on the plate was opened at 405 nm. Concentration absorbance graph was drawn by utilizing standards. Blood samples from patient and control groups were prepared for appropriate fluorescence and scanning electron microscope images and prepared with appropriate protocols.
Results: Although no statistically significant difference was found, we showed that NETosis formation increases SCA compared to healthy subjects (p = 0,329). We also showed that NETosis formation increses with increasing number of painfull crisis in these patients.
Conclusion(s): In this study, it has been shown that NETosis formation increases in patients with SCA compared to healthy individuals.


149. VPB0747
149.1. The prospective advances of von willebrand factor, ADAMTS13, and factor VIII in severe malaria: A systematic review
B. Putra 1; F. Putra2
1 Private Practice, Blitar, Jawa Timur, Indonesia; 2 Faculty of Medicine Universitas Airlangga, Surabaya, Jawa Timur, Indonesia
Background: Endothelial activation contributes to severe malaria progression and is a common condition in critically ill patients. The von Willebrand Factor (VWF), a disintegrin‐like and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13), and factor VIII (FVIII) are endothelial activation and regulator markers.
Aims: This study aims to evaluate the impacts of altered VWF, ADAMTS13, and FVIII in malaria patients.
Methods: Comprehensive literature searching using predefined keywords was performed through online databases Pubmed, EMBASE, ScienceDirect, and The Cochrane Library. This study followed the PRISMA guideline. The included and extracted studies were all relevant clinical studies that provide the impacts of altered VWF, ADAMTS13, or FVIII on pathogenesis and severity in malaria patients. The quality of studies was accessed by using the Newcastle‐Ottawa Scale.
Results: Thirteen clinical studies matched the inclusion criteria. The median concentration of VWF and propeptide levels were higher in cerebral malaria (CM) than in uncomplicated malaria patients. The VWF concentration showed significant correlation with circulating parasite biomass and significant association with the acute kidney injury incidence in CM patients. The VWF also showed significant correlation with peripheral parasitemia in vivax malaria. A post‐mortem study revealed incremental VWF alveolar edema staining in malaria‐associated acute respiratory distress syndrome. Both ADAMTS13 antigen and activity concentrations significantly correlated with severity and parasitemia in falciparum malaria and the microvascular reactivity in vivax malaria. Single nucleotide polymorphism study of ADAMTS13 suggested significant association of protection against CM. The FVIII concentration is significantly increased in severe falciparum malaria.
Conclusion(s): The VWF, ADAMTS13, and Factor VIII might provide prospective advances in severe malaria. Nevertheless, further clinical studies are warranted to confirm the importance.
150. PB0743
150.1. Cell‐free DNA is increased in plasma of immunothrombotically dysregulated patients with periprosthetic joint infection in part due to lower DNaseI activity
R. Herranz 1; J. Oto1; M. Fuertes2; E. Plana3; F. Cana4; C. De La Calva2; M. Angulo2; F. Arguelles2; J. Baeza2; I. Baixauli2; F. Baixauli2; J. Amaya2; P. Medina4
1 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 2 Orthopaedics and Traumatology Service. La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 3 Angiology and Vascular Surgery Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 4 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain
Background: Bacterial infection activates neutrophil activation through immunothrombosis. Neutrophils release neutrophil extracellular traps (NETs) in bacterial biofilm of periprosthetic joint infections (PJI). We previously evidenced an increase in NETs markers in PJI plasma and that plasma cell‐free DNA (cfDNA) levels may improve PJI diagnosis. In some disorders like thrombotic microangiopathies, increased plasma cfDNA is mediated by impaired plasma DNaseI activity.
Aims: To ascertain whether increased plasma cfDNA in PJI patients may be mediated by lower DNaseI activity.
Methods: We obtained citrated plasma, in the preoperative period of prosthetic surgery, from 94 patients prospectively recruited. PJI was confirmed in 50 of these patients and 44 had no infection. cfDNA was quantified with PicoGreen (Life Technologies) and DNase I activity was measured with a single radial enzyme‐diffusion (SRED) assay. The statistical analysis was performed with GraphPad (v.8.0.1).
Results: Plasma cfDNA levels were significantly higher in PJI patients (median 1403 ng/ml, 1st‐3rd quartile: 1062–1617) compared to patients undergoing prosthetic surgery due to non‐septic causes (920.5, 836.2–1361) (p < 0.0001). Interestingly, DNase I activity was significantly decreased in PJI (0.42 cm2, 0.38–0.51) than in non‐PJI (0.55, 0.49–0.61) (p < 0.0001) (Figure 1).
Conclusion(s): The increase in plasma cfDNA in PJI patients may be due to a combination of increased NETosis and an impairment of DNaseI activity. Further in vitro studies are needed to clarify whether increased NETosis is beneficial or detrimental to fight PJI. ISCIII‐FEDER (PI17/00495, PI20/00075, FI21/00171), GVA (ACIF/2017/138), Zimmer Biomet and SETH.

151. PB0744
151.1. Coagulation markers improve the diagnosis of periprosthetic joint infection: Involvement of immunothrombosis
J. Oto 1; R. Herranz1; A. Fernández‐Pardo2; M. Fuertes3; E. Plana4; A. Cañada5; F. Cana2; C. De La Calva3; M. Angulo3; F. Arguelles3; J. Baeza3; I. Baixauli3; F. Baixauli3; J. Amaya3; P. Medina2
1 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 2 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 3 Orthopaedics and Traumatology Service. La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 4 Angiology and Vascular Surgery Service, La Fe University and Polytechnic Hospital, Valencia, Comunidad Valenciana, Spain; 5 Biostatistics Unit, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain
Background: Bacterial infection activates coagulation through immunothrombosis. Neutrophils may be involved in periprosthetic joint infections (PJI) by releasing neutrophil extracellular traps (NETs) present in the bacterial biofilm. As PJI diagnosis is often uncertain due to lack of compliance with some criteria, we previously obtained a model to estimate the pre‐surgical PJI risk using markers of NETs and thrombin generation test (TGT).
Aims: To improve the performance of our model including additional coagulation markers and to validate its diagnostic capacity of PJI.
Methods: We obtained citrated plasma, in the preoperative period of prosthetic surgery, from 111 patients prospectively recruited. PJI was confirmed in 50 of these patients, 46 had no infection, while PJI was uncertain in 15 patients, which is relatively frequent as above mentioned. Besides NETs markers (cell‐free DNA, nucleosomes, myeloperoxidase, calprotectin, elastase) and the TGT, we analyzed PT, APTT, TT, fibrinogen, D‐dimer, procalcitonin, α‐defensins and IL‐6. We performed a multivariable logistic regression model penalized with elastic net using R (v3.5.0).
Results: cfDNA, calprotectin, elastase, starttail of TGT, fibrinogen α‐defensins and IL‐6 were significantly increased in PJI patients compared with patients undergoing prosthetic surgery due to non‐septic causes. The new coagulation markers studied did not improve our predictive model of PJI containing markers of NETs (cfDNA and calprotectin) and the starttail of TGT; thus, we validated it achieving an AUC = 0.77 (95% CI [0.58, 1]; p = 0.019).
Conclusion(s): An activation of coagulation and immunothrombosis seems to occur in patients with PJI. Markers of NETs and TGT seem to have diagnostic utility of PJI before surgery. Our model could reinforce current clinical criteria to reduce the number of uncertain diagnoses and thus be able to make an early and effective diagnosis and treatment to minimize side effects of the PJI such as tissue damage, bone degradation and replacement surgery. ISCIII‐FEDER (PI17/00495, PI20/00075, FI21/00171), GVA (ACIF/2017/138), Zimmer Biomet and SETH.
152. VPB0746
152.1. Alterations in coagulation profiles and bleeding rates in patients with acute myeloid leukemia/myelodysplastic syndrome on prophylactic anti‐bacterials
C. Allen; S. Gautam; A. Pine; A. Zeidan; A. Lee; R. Shallis
Yale School of Medicine, New Haven, Connecticut, United States
Background: Patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) often receive anti‐bacterial prophylaxis. Anti‐bacterials are shown in other populations to interfere with vitamin K metabolism and induce elevations in international normalized ratio (INR). The impact these agents have on the coagulation profiles and bleeding risk in patients with AML/MDS, who are subject to additional acquired coagulopathy and disease‐related challenges, is unknown.
Aims: To compare the coagulation profiles and bleeding rates of patients with AML/MDS on and off prophylactic anti‐bacterials.
Methods: With institutional review board approval, we conducted a retrospective cohort study of adult patients with active AML/MDS and were admitted to Yale‐New Haven Hospital between 2015 and 2019. The cohort was divided into patients on and off prophylactic anti‐bacterials. Patients with acute/chronic liver disease, with disseminated intravascular coagulation, on anticoagulation or current/recent therapeutic anti‐bacterials were excluded. Demographic/admission laboratory parameters and bleeding rates were extracted. Pearson’s χ2 tests and Wilcoxon rank tests were used for bivariate analyses.
Results: Among 759 unique patient hospital encounters, 28 occurred while on and 99 were off anti‐bacterial prophylaxis (Table 1). Median duration of pre‐admission anti‐bacterial exposure was 3 (range: 0.06–24) months. Patients on anti‐bacterial prophylaxis had higher INR (p = 0.0001), and rates of partial thromboplastin time (PTT) prolongation (60.7% vs. 12.1%, p = 0.0001); isolated INR elevation was also more common (46.4% vs. 9.1%, p < 0.0001). Patients off anti‐bacterial prophylaxis had higher rates of bleeding (26.3% vs. 7.1%, p = 0.031), including a numerically higher rate of major/clinically relevant bleeding (Table 2).
Conclusion(s): Patients with AML/MDS on anti‐bacterial prophylaxis were more likely to have an abnormal coagulation profile when compared with their counterparts not on prophylaxis. However, rates of bleeding were higher in patients not on prophylaxis and this requires further study. These data demonstrate that recommended anti‐bacterials do not appear to increase the bleeding risk in patients with AML/MDS.


153. PB0742
153.1. Functional changes in platelets and coagulation during asexual and sexual parasitemia in a controlled human malaria infection
S. Huang1; W. van der Heijden2; I. Reuling3; J. Wan4; Q. Yan4; R. de Laat‐Kremers 5; A. Van der Ven2; P. de Groot4; M. McCall6; R. Sauerwein6; T. Bousema6; M. Roest7; M. Ninivaggi7; Q. de Mast8; B. de Laat1
1 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 2 Department of Internal Medicine, Radboud Center for Infectious Diseases, Radboud University Medical Centre, Nijmegen, the Netherlands;, Nijmegen, Gelderland, Netherlands; 3 Department of Medical Microbiology, Radboud University Medical Centre, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands; 4 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 5 Synapse Research Institute, Maastricht, Limburg, Netherlands; 6 Department of Medical Microbiology, Radboud University Medical Centre, Nijmegen, the Netherlands;, Nijmegen, Gelderland, Netherlands; 7 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 8 Department of Internal Medicine, Radboud Center for Infectious Diseases, Radboud University Medical Centre, Nijmegen, the Netherlands, Nijmegen, Gelderland, Netherlands
Background: Asexual Plasmodium falciparum parasitemia in a human malaria infection is associated with a drop in platelet number. It remains unknown whether this is related to acute and/or long‐term functional changes in platelets and coagulation. Additionally, the impact of the malaria gametocytemia stage on platelets and coagulation has not yet been clarified.
Aims: We aimed to assess changes in the number and function of platelets, total and active VWF, and the coagulation system during early asexual parasitemia, subsequent gametocytemia and convalescence.
Methods: Sixteen individuals were enrolled in a controlled human malaria infection (CHMI) designed to induce gametocytemia. Blood samples were collected at baseline, at the peak of asexual blood stage parasitemia, during gametocytemia and at day 64. Platelet activation and reactivity was determined by flow cytometry and the activation of the coagulation system was quantified by coagulation factor levels, thrombin generation, and thrombin dynamics analysis.
Results: Platelet count was reduced during asexual blood stage infection but normalized during gametocytemia. Platelet P‐selectin expression was slightly increased during asexual parasitemia, gametocytemia and on day 64, and platelets showed a marked decrease in reactivity to low dose collagen‐related peptide‐XL. During asexual parasitemia, the TG lag time is prolonged (+19.5%; p < 0.001). Thrombin dynamics analysis revealed a decreased prothrombin conversion (−12.9%, p = 0.039) and a trend towards decreased prothrombin levels (−3.7%). At the time of gametocytemia and day 64, the thrombin generation ETP, peak height and velocity index were increased. Finally, concentrations of von Willebrand factor (VWF) and active VWF were markedly increased during asexual parasitemia, but normalized during gametocytemia.
Conclusion(s): We show that the early decrease in platelet count during asexual parasitemia is not associated with increased platelet reactivity but with endothelial activation leading to increased levels of active VWF which are able to form spontaneous VWF‐platelet complexes and thereby increased platelet clearance.
153.2. Platelets and Cancer
154. VPB0761
154.1. Severe hemorrhage complicated with acute multiple cerebral infarction in a patient with thrombocytopenia caused by chemotherapy
R. Zhou; D. Zhou
Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China (People's Republic)
Background: Severe hemorrhage complictated with acute cerebral infraction in a ptaient with thrombocytopenia is rare.
Aims: To understand possible mechanisms in a patient with thrombocytopenia why cerebral infarction occurs when there is severe bleeding.
Methods: To review retrospectively the patient's past history, related test results and to discuss the possible mechanisms combined with the relevant literature
Results: The 46‐year‐old woman was admitted to hospital for ecchymosis for 3 days. One year before, she was diagnosed as sigmoid adenocarcinoma and removed with Dixon method. Subsequently, she was treated with chemotherapy. After that, she had Grade IV myelosuppression. She was treated with TPO‐RA, Glucocorticoid and gamma globulin successionly. The amount of menstruation increased significantly. On admission, she couldn't walk on herself. Body examination suggested she had nasal bleeding and petechia and ecchymosis on lower limb. Muscle strenth of lower limb was Grade 1 with tactile hypoesthesia. Blood test showed Hb 52 g/L, platelet 6x109/L, fibrinogen 0.9g/L and D‐dimer 2.48mg/L. Antiphospholipid antibodies were negative. Head and chest CT scan showed subarachnoid hemorrhage, multiple nodules and masses in both lungs. She was given albumin and fibrinogen infusion, RBC and platelet transfusion. Brain MRI on day 3 revealed multiple acute cerebral infarction in the left basal ganglia, radiation crown, bilateral semi‐oval center and parietal occipital lobe. PET/CT on day 8 showed that there were nodules near the sigmoid anastomosis, multiple lymph nodes near the left psoas major muscle, left presacral and left iliac, bilateral multiple masses and nodules. Because of platelet lower than 10x109/L, Avatrombopag was used again. On day 12, she was discharged from hospital with petechial regression, muscle strength Grade IV and tactile sensation recovered. The possible mechanism and treatment was discussed combined with related literature.
Conclusion(s): Patient with thrombocytopenia may has bleeding and thrombosis simultaneously, which needs weighing the pros and cons and gives appropriate treatment.
155. PB0749
155.1. Significance of platelet count at diagnosis and its association with survival in myelodysplastic syndrome patients; an experience from Pakistan
N. Anwar 1; N. Fatima2; A. Arshad3; L. Majeed2; M. Borhany4
1 National Institute of Blood Disease and Bone Marrow Transplantation, special plot d3 block 6 PECHS, Karachi, Karachi, Sindh, Pakistan; 2 National Institute of Blood Diseases and Bone Marrow Transplantation, Karachi, Sindh, Pakistan; 3 National Institute of Blood Diseases and BMT, KARACHI, Sindh, Pakistan; 4 National Institute of Blood Diseases and Bone marrow Transplantation, Karachi, Sindh, Pakistan
Background: Myelodysplastic syndromes (MDS) are heterogeneous group of clonal hematopoietic disorder characterized by dysplastic progenitors, peripheral cytopenias and tendency to evolve into acute myeloid leukaemia. Thrombocytopenia is a significant consequence of ineffective hematopoiesis with decreased overall survival. However, there is a scarcity of data with none of the study reported in Pakistan.
Aims: To observe the association of overall survival of MDS patients presenting with or without thrombocytopenia at diagnosis.
Methods: A retrospective cohort study was conducted at NIBD PECHS campus where MDS patients were recruited from 2018‐ 2021. Kruskal Wallis test was applied to observe the difference in survival days and Kaplan Meier Survival analysis was performed to observe the survival in each platelet category. P‐value of < 0.05 was considered to be statistically significant.
Results: A total of 65 patients were analyzed. Median age (IQR) of patients was 60 (37) years with male predominance 41(63%). 18(28%) patients were MDS‐EB1 and majority of patients were Intermediate risk IPSS. Overall median (IQR) hemoglobin (Hb) g/dl, total leucocyte count x109/L and platelet count (PLT) x109/L at diagnosis was 8(3.1), 4.2 (4.0) and 44 (101) respectively. Overall survival in patients with PLT < 25 and with PLT 51–100 was 57%, with PLT 25–50 was 70% and with PLT > 100 was 91%. Median (IQR) survival days with < 25 PLT was 79 (331), with 25–50 PLT was 66(577), 51–100 was 210 (301) and with > 100 it was 343 (498) days. The difference in mortality and survival days was not found significant between platelet categories (P‐value >0.05).
Conclusion(s): The median difference in survival days was higher in patients who were not presented with thrombocytopenia at diagnosis however it was not found significant. Further studies with large sample size are needed to evaluate the significance of thrombocytopenia presentation at baseline and its impact on survival in our patients.
156. VPB0758
156.1. JAK2 V617F confers aspirin resistance in patients with myeloproliferative neoplasms
K. Rizzo 1; K. Vella2; M. Cini Masini2; L. Camilleri3; N. Riva4; A. Gatt5
1 University of Malta, Msida, Malta, Msida, Malta; 2 Mater Dei Hospital, Department of Pathology, Msida, Malta, Msida, Not Applicable, Malta; 3 Department of Statistics & Operations Research, University of Malta, Msida, Malta, Msida, Not Applicable, Malta; 4 Department of Pathology, Faculty of Medicine and Surgery, University of Malta, Msida, Malta, Msida, Not Applicable, Malta; 5 University of Malta, Department of Pathology, Msida, Malta, Msida, Not Applicable, Malta
Background: Myeloproliferative neoplasms (MPNs) are a group of clonal disorders associated with an overproduction of differentiated haematopoietic stem cells (HSCs). Affected patients are at an increased risk of thrombosis. Despite treatment with aspirin, some patients still develop thrombotic phenomena whilst others develop major bleeding especially when subjected to surgical interventions.
Aims: Compare platelet aggregometry (PA) by Arachidonic Acid (AA) results of MPNs to healthy controls (HC) and to controls on aspirin (COA) therapy and assess the pharmacological response to aspirin by measuring the levels of thromboxane B2 (TXB2) in blood.
Methods: A total of 36 MPNs were recruited (17 JAK2 V617F positive and 19 JAK2 V617F negative), 22 HCs and 21 COAs. All subjects on aspirin were subjected to a 24 h wash‐out period. PA was performed using the PAP‐8E Platelet Aggregation Profiler®. TXB2 was performed by an enzyme‐linked immunosorbent assay (Cayman Chemical©).
Results: A statistical significant difference was detected between all 3 cohorts in AA maximum aggregation (MA) and TXB2. AA MA is slightly more supressed in COAs (mean 7.10) when compared to MPNs (mean 9.73), but both groups on aspirin have limited platelet function in response to AA when compared to HCs (mean 71.41). TXB2 levels were higher in MPNs (6469.71 pg/ml) when compared to the COA (445.08 pg/ml). When grouped by JAK2 status, no statistically significant differences were observed between JAK2+ve and JAK2‐ve in AA MA, however differences were observed in TXB2, showing a higher mean in JAK2+ve (9605.94 pg/ml) when compared to JAK2‐ve (3124.39 pg/ml).
Conclusion(s): JAK2 positivity confers aspirin resistance in MPNs, giving higher TXB2 values than JAK2‐ve. This might in part explain the pathogenesis of higher risk of thrombosis in patients with JAK2 positivity.


157. PB0755
157.1. Synthetic Bcr‐Abl tyrosine kinase inhibitors; their profile towards antiplatelet activity and cancer cell functionality
L. Pechlivani1; N. Ntemou2; P. Boulgari2; D. Pantazi1; K. Skobridis2; A. Tselepis 1
1 Atherothrombosis Research Centre, Department of Chemistry, University of Ioannina, Ioannina, Ioannina, Greece; 2 Section of Organic Chemistry and Biochemistry, Department of Chemistry, University of Ioannina, Ioannina, Ioannina, Greece
Background: Bcr‐Abl tyrosine kinase inhibitors are used to treat various malignancies and we had previously demonstrated that they also exhibit antiplatelet activity (Pantazi D, et al. DDDT. 2019;13:4225–38).
Aims: We synthesized new inhibitors and evaluated their profile towards cancer cell functionality and transition, as well as their antiplatelet potency.
Methods: Three novel Bcr‐Abl tyrosine kinase inhibitors were designed, synthesized and fully characterized. They differentiate at their final phenyl ring, having a halogen atom, F or Cl at the 4th position (Figure). Light Transmittance Aggregometry in the presence of these inhibitors was performed in platelet‐rich plasma activated with Arachidonic acid (AA). Confluent hepatoma HepG2 cells in culture were incubated for 48h with the synthetic compounds and the cell apoptosis was studied by Flow Cytometry after cell staining with Propidium iodide and Annexin‐V. To evaluate the epithelial–mesenchymal transition (EMT) we quantified the expression of adhesion proteins, E‐cadherine and N‐cadherine by Western blot, while the cell functionality and migration was studied using the Boyden chamber assay.
Results: Compound‐1, ‐2 and ‐3 significantly inhibited platelet aggregation induced by AA to a similar extent and the IC50 values were 1.50–1.08 μΜ, 0.94–0.66 μΜ and 1.73–0.73 μΜ, respectively. In HepG2 cells, the compound‐3 exhibited the strongest apoptotic effect (Table). Furthermore, all compounds decreased the expression of E‐cadherine by 51.78%, 25.07% and 82.73%, and N‐cadherine by 32.16%, 65.24% and 48.17%, respectively and inhibited the migration of HepG2 cells by 25.42%, 17.37% and 25.98%, respectively.
Conclusion(s): Compounds‐1 and ‐3 had potent antiplatelet activity and similar inhibitory effects towards cancer cell functionality and transition. Their structure could set the example to develop new Bcr‐Abl tyrosine kinase inhibitors in order to efficiently treat cancer and effectively prevent cancer‐associated thrombosis.


158. VPB0760
158.1. Heightened procoagulation after thromboprophylaxis completion inorthopaedic surgery patients with metastatic bone disease: Outcome of a pilot study
E. Agbani1; L. Yamaura 2; D. Young2; M. Monument2; L. Skeith2; A. Dufour2; P. Skeith2
1 University of Calgary, CALGARY, Alberta, Canada; 2 University of Calgary, Calgary, Alberta, Canada
Background: Patients with metastatic bone disease (MBD) often require surgical stabilization of painful or unstable bone lesions. Hypercoagulability is associated with malignancy, and venous thromboembolism risk is 7‐fold higher in patients with MBD. Platelet‐tumour cell interactions can promote metastasis, and platelets’ role in the hypercoagulability after surgical intervention for MBD is ill‐defined.
Aims: To characterise platelet activation and procoagulation mechanisms in patients with MBD before and after surgical interventions.
Methods: Healthy participants and patients with various primary cancers (lung, breast, pancreatic, renal, multiple myeloma, and colorectal) provided informed consent (n = 8; 67 ± 9years; HREBA.CC‐20–0147). We examined patients’ blood at 7‐timepoints (pre‐operatively; and postoperative days 1, 3, 5, 14, 42, and 90). We utilized high‐resolution fluorescence imaging to visualize platelets in plasma reconstituted to contain leucocytes, followed by a systematic analysis of procoagulant membrane dynamics. We then coupled our findings to biochemical and proteomics studies in these patients.
Results: Platelet activation and membrane P‐selectin expression increased 1‐ to 2‐fold after completion of thromboprophylaxis or VTE treatment in all patients except for our myeloma patient, in which platelet activation increased dramatically post‐operatively, but normalized after thromboprophylaxis completion. Expectedly, platelet membrane phosphatidylserine externalization and thrombin generation followed a similar temporal pattern. We visualized microthrombi (aggregate of ≥5 platelets) and platelet‐leucocyte aggregate in the fresh plasma from all patients, but not healthy participants. Both aggregates increased rapidly after the complexion of thromboprophylaxis or the discontinuation of VTE treatment in patients with solid primary cancers in particular. We associated the amplified procoagulation with increased phosphorylation of spleen tyrosine kinase (Syk:Tyr‐525/Tyr‐526) in platelets, and a plasma milieu enriched with vWF, FAM65B, and the Afamin‐AFM and Attractin‐ATRN inflammatory mediators.
Conclusion(s): Procoagulation continued beyond the duration of thromboprophylaxis’ in surgical patients with MBD. On‐going study will provide further mechanistic insight into hypercoagulability and aims to identify novel drug targets for effective thromboprophylaxis in MBD patients.
159. PB0753
159.1. Platelet count as a novel diagnostic marker for early lung cancer diagnosis: An epidemiological study
M. Barlow1; G. Pula 2; W. Hamilton1; S. Bailey1
1 University of Exeter, Exeter, England, United Kingdom; 2 University Medical Center Eppendorf Hamburg (UKE), Hamburg, Hamburg, Germany
Background: Two‐thirds of lung cancer patients are diagnosed at an advanced stage, often following the onset of symptoms such as haemoptysis. Thrombocytosis is a positive predictive marker for lung cancer and is often present before other symptoms.
Aims: To determine when the platelet count begins to rise before a lung cancer diagnosis, and to ascertain whether thrombocytosis is equally indicative of its three main histological subtypes: adenocarcinoma (ADC), squamous cell carcinoma (SCC), and small cell lung cancer (SCLC).
Methods: We designed a matched cohort study to analyse primary care records from the Clinical Practice Research Datalink (CPRD) with linkage to the cancer registry. There were 4,720 lung cancer patients (2,355 ADC, 1,546 SCC, and 819 SCLC) matched on age, sex, and practice at a ratio of 1:5 to 23,076 healthy controls. Fractional polynomial regression predicted the rise in platelet count in the days preceding lung cancer diagnosis, and the z‐test for independent proportions compared pre‐diagnostic thrombocytosis occurrence across subtypes.
Results: The platelet count began to rise 300 days before lung cancer diagnosis, with SCC patients experiencing a significantly faster rise than ADC and SCLC patients. At diagnosis, 22.6% of SCC patients had thrombocytosis (95% CI: 20.5%–24.7%) which was significantly higher than 15.5% of ADC (95% CI: 14.1%–17.0%, p < 0.0001) and 16.1% of SCLC patients (95% CI: 13.6%–18.6%, p < 0.001).
Conclusion(s): Thrombocytosis is more common in SCC than other subtypes. The steady rise in platelet count leading up to a lung cancer diagnosis may indicate that GPs should consider lung cancer in patients with an otherwise unexplained increase in platelet numbers, even below the thrombocytosis threshold. As stimulation of thrombopoiesis occurs at least 300 days before a lung cancer diagnosis, these unknown stimulatory factors could serve as novel biomarkers to significantly expedite lung cancer diagnosis.


160. PB0756
160.1. Proteomic and functional profile of platelet‐derived extracellular vesicles released under physiological and tumour‐associated conditions
M. Vismara 1; M. Manfredi2; M. Zarà3; L. Galgano4; S. Trivigno4; A. Piotti5; I. Canobbio1; M. Torti5; G. Guidetti1
1 University of Pavia, Pavia, Pavia, Lombardia, Italy; 2 University of Piemonte Orientale, Novara, Piemonte, Italy; 3 Centro Cardiologico Monzino IRCCS, Milano, Milano, Lombardia, Italy; 4 University School for Advanced Studies IUSS, Pavia, Pavia, Lombardia, Italy; 5 University of Pavia, Pavia, Lombardia, Italy
Background: Platelet‐derived extracellular vesicles (PEVs) contribute to platelet‐cancer crosstalk and regulate cancer progression by delivering bioactive molecules to target cells. We have recently demonstrated that breast adenocarcinoma cells are able to stimulate the release of PEVs with an efficacy comparable to the physiologic agonist thrombin. However, it is not known whether cancer cell‐induced PEVs differ in composition and function from those released by thrombin‐stimulated platelets.
Aims: This study aims to provide a comparative analysis of protein cargo and functional effects of PEVs released from platelets stimulated with thrombin (PEVs‐THR) or stimulated with the breast cancer cell line MDA‐MB‐231 (PEVs‐BCC).
Methods: PEVs were isolated by differential centrifugation from platelets incubated with thrombin (0.2 U/ml) or with MDA‐MB‐231 cells (5x104 cell/ml) for 30 min. The proteomic analysis was performed by liquid chromatography‐tandem mass spectrometry. Migration of Jurkat cells was evaluated by trans‐well assays. PEVs‐induced apoptosis of MDA‐MB‐231 cells was investigated by flow cytometry.
Results: A total of 937 proteins were identified, with 431 found in both PEVs populations, 86 exclusively in the PEVs‐THR and 420 only in the PEVs‐BCC. Bioinformatic analyses indicated that the proteomic composition of the two populations may be associated to differential regulation of extravasation signalling and calcium‐induced apoptosis in target cells. Both PEVs populations were equally able to potentiate invasiveness of MDA‐MB‐231 cells, but PEVs‐BCC displayed a more pronounced proapoptotic effect on MDA‐MB‐231 cells compared to PEVs‐THR. Interestingly, PEVs‐THR and PEVs‐BCC were also able to downregulate migration of Jurkat cells, suggesting a possible novel function of PEVs in the regulation of leukocyte mobility.
Conclusion(s): This study revealed that PEVs released under physiological or cancer‐associated conditions differ in composition and display peculiar biological functions, suggesting that cancer cell‐induced PEVs may play unique roles in the platelet‐cancer crosstalk.
161. VPB0757
161.1. TREGs‐induced immune thrombotic thrombocytopenia in polycythemia Vera
R. Cacciola 1; V. Vecchio2; E. Gentilini Cacciola3; E. Cacciola1
1 Università di Catania, Catania, Sicilia, Italy; 2 School of Medicine University of Catania, Catania, Sicilia, Italy; 3 Sapienza University of Rome, Rome, Lazio, Italy
Background: T‐regulatory cells (Tregs) defined as CD4+, CD25high, CD127low cells prevent autoimmunity. The Ph1‐negative myeloproliferative neoplasms (MPNs) show a JAK/STAT‐mediated reduction of Tregs.
Aims: We investigated Tregs and platelet and coagulation activation in Polycythemia Vera (PV) and thrombosis.
Methods: We enrolled 60 WHO‐defined PV patients (30 men, 30 women; mean age 45 ± 10 years) without cardiovascular risk factors or thrombotic history having poplitea deep vein thrombosis (40/60) and pulmonary embolism (20/60) on lower‐limb ultrasonography and computed tomography angiography. 60 healthy age‐ and sex‐matched were controls. All patients were evaluated for Tregs, PF4‐dependent antibodies, platelets, prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (Fib) and D‐dimer (DD). Tregs were measured by flow cytometry, PF4‐dependent antibodies by PF4‐dependent EIA, platelets by automated analyzer, PT and APTT by coagulometric test, Fib using Clauss method, and DD using ELISA. Complete blood hemostasis was studied by PFA‐100 on Collagen/ADP (CT‐ADP) and Collagen/Epinephrine (CT‐EPI) cartridges and Thromboelastometry method on Clotting Time (CT), Clotting Formation Time (CFT), Maximum Clot Firmness (MCF), and clot lysis at 30 min (LY‐30).
Results: All patients had low Tregs (1,5 ± 0,5% vs 5 ± 1%) thrombocytopenia (70 ± 5x109/L vs 230 ± 20x109/L), longer PT (30 ± 10 s vs 14 ± 2 s) and PTT (60 ± 10 s vs 30 ± 5 s), lower Fib (90 ± 20 mg/dl vs 180 ± 20 mg/dl), higher DD (650 ± 100 μg/L vs 150 ± 50 μg/L) and shorter C/ADP and C/EPI (C/ADP, n.v. 68–121 s (40 ± 10 s vs 80 ± 20 s) and C/EPI n.v. 84–160 s (35 ± 5 s vs 95 ± 10 s). All patients had shorter CT (INTEM 35 ± 20 s vs 180 ± 20 s, EXTEM 20 ± 10 s vs 52 ± 10 s), shorter CFT (INTEM 15 ± 10 s vs 90 ± 10 s, EXTEM 20 ± 10 s vs 70 ± 30 s), longer MCF (INTEM 130 ± 10 mm vs 60 ± 10 mm, EXTEM 120 ± 10 mm vs 62 ± 10 mm), and lower LY‐30 (INTEM 0.9% vs 15%, EXTEM 0.8% vs 15%).
Conclusion(s): These findings shed new light on thrombotic pathogenesis in patients with PV.
162. PB0748
162.1. Diminished platelet CLEC‐2 sensitivity in kaposiform hemangioendothelioma identified with novel CLEC‐2 antagonists
A. Martyanov1; I. Tesakov2; O. An 1; A. Ignatova2; G. Svidelskaya3; L. Khachatryan3; J. Eble4; D. Kalinin5; A. Sveshnikova3
1 Center for Theoretical Problems of Physicochemical Pharmacology, Moscow, Moskva, Russia; 2 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 3 Dmitry Rogachev National Medical Research Centre of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 4 Institute of Physiological Chemistry and Pathobiochemistry, University of Münster, Munich, Bayern, Germany; 5 Institute of Pharmaceutical and Medicinal Chemistry, University of Münster, Munster, Nordrhein‐Westfalen, Germany
Background: Kaposiform hemangioendothelioma (KHE) is a rare vascular tumor. 70% cases of KHE are associated with Kasabach‐Merritt phenomenon (KMP) which includes thrombocytopenia and coagulopathy. Platelets are involved in KHE/KMP pathogenesis: KHE is enriched with podoplanin, which induces platelet adhesion and activation via CLEC‐2 receptor. While applicability of conventional anti‐platelet agents in KHE/KMP patients is controversial, novel anti‐CLEC‐2 agents could be beneficial.
Aims: The evaluation of CLEC‐2‐induced platelet activation in KHE/KMP.
Methods: 7 pediatric patients with KHE/KMP were enrolled in the study and compared with age‐matched healthy donors (HDs). Platelets of the KHE/KMP patients were analyzed by flow cytometry and low‐angle light‐scattering aggregometry. The study was approved by the independent ethics committee of CTP PCP RAS and performed according to the Declaration of Helsinki. Mann‐Whitney and Wilcoxon criteria were used for the data analysis.
Results: Quiescent platelet cytosolic calcium as well as platelet responsiveness to ADP, TRAP‐6 and CRP was within normal ranges. Pre‐incubation of platelets with CLEC‐2 antagonists 2CP and HB125 resulted in mild increase in cytosolic calcium in HD platelets, but not in those of patients. CLEC‐2 agonist rhodocytin‐induced (200 nM) calcium and fibrinogen responses were diminished in KHE/KMP. CLEC‐2 antagonists; 2CP and HB125, inhibited rhodocytin‐induced platelet activation in both patients and HDs. Neither 2CP nor HB125 affected platelet responses to ADP or TRAP‐6. However; 2CP inhibited CRP‐induced platelet activation. Similar results were obtained in the aggregation assays.
Conclusion(s): CLEC‐2‐induced platelet signaling in KHE/KMP is significantly impaired. 2CP and HB125 decreased CLEC‐2 induced calcium mobilization in both patients and HDs. Presumably, the high podoplanin expression in KHE cause consumption of platelets with normal and high CLEC‐2 expression and thus enrich platelets with lower CLEC‐2 in these patients. The study was supported by the ICCR of University of Münster (IZKF grant: Ebl4/009/21) and the RFBR Grants 17‐00‐00138 and 21‐51‐10005 (together with the RS of London).
163. PB0750
163.1. Changes to platelet quality and function in Waldenstrӧm Macroglobulinaemia
S. Brysland 1; D. Talaulikar2; E. Gardiner3
1 John Curtin School of Medical Research, Australian National University, Canberra, Canberra, Australian Capital Territory, Australia; 2 The Canberra Hospital, Canberra, Australian Capital Territory, Australia; 3 John Curtin School of Medical Research, The Australian National University, Canberra, Australian Capital Territory, Australia
Background: Platelet glycoprotein (GP) receptors are essential for initiating platelet adhesion, activation and aggregation. Reduced levels can occur when thrombopoiesis is disturbed, or coincident with bleeding in patients with thrombocytopenia, trauma or haematological malignancies. Both GPVI and GPIbα are metalloproteolytically shed from activated or aged platelets. Waldenström Macroglobulinaemia (WM) is a B‐cell lymphoma with common symptoms including bleeding/bruising and thrombocytopenia. WM is treated with Bruton’s tyrosine kinase inhibitors (BTKis) and chemotherapies, which can exacerbate bleeding. Platelet function and quality are under‐investigated in WM.
Aims: To evaluate platelet quality and function, and clotting potential in WM.
Methods: Relative platelet receptor levels, reticulated platelets and platelet activation were measured by whole blood or PRP flow cytometry in 16 WM patients compared with 66 healthy donors (HD). Blood was enumerated by automated analyser; metalloproteolytically‐shed soluble GPVI was measured by ELISA; whole blood clotting potential was evaluated using ROTEM.
Results: WM blood displayed significantly reduced platelets (**p < 0.01) bearing less GPVI (**p < 0.01), GPIbα (*p < 0.05) and reticulation (*p < 0.05), and increased tetraspanin CD9 (***p < 0.001) and P‐selectin (*p < 0.05) compared with HDs. Plasma soluble GPVI was within HD ranges. Samples from WM patients displayed normal ROTEM parameters in intrinsic and extrinsic clotting assays but markedly reduced thrombus size (****p < 0.0001) compared to HD values in FIBTEM. When recalcified (NATEM), WM patient blood formed thrombi 58% faster (****p < 0.0001), that were enlarged 22% (****p < 0.0001) over HDs.
Conclusion(s): WM patient platelets bore reduced levels of key receptors governing platelet function. Reductions may be due to changes in platelet maturation/production as GPVI shedding was not evident and reticulated platelets were reduced. Global clotting capacity was normal, however the relative contributions of platelets and plasma components were abnormal, consistent with deranged haemostatic regulatory capacity in WM. Future work will assess the utility of these measurements in stratifying patients for bleeding risk.
164. PB0752
164.1. Platelet and immune cell cloaking of circulating tumour cells [CTCs] in breast and ovarian cancer
M. Ward1; L. Kane2; S. Lochrin3; C. Ovaere4; C. O’Gorman4; R. O'Connor5; T. Kelly5; B. Mohamed5; M. Bates5; M. Carter5; J. Kennedy3; A. Tierney3; J. Beirne6; W. Kamran4; F. Abu Saadeh7; K. Cadoo8; L. Norris 9; C. Martin10; S. O'Toole11; J. O'Leary12
1 Department of Histopathology, Trinity College Dublin, Trinity St James’s Cancer Institute, St James’s Hospital, Ireland, Dublin, Dublin, Ireland; 2 Trinity College Dublin, Dublin, Dublin, Ireland; 3 The Haematology, Oncology and Palliative Care (HOPe) Directorate, St James’s Hospital, Dublin 8, Ireland., Dublin, Dublin, Ireland; 4 Division of Gynaecological Oncology, St James’s Hospital, Dublin 8, Ireland., Dublin, Dublin, Ireland; 5 Department of Histopathology, Trinity College Dublin, Ireland., Dublin, Dublin, Ireland; 6 St James's Hospital, Dublin, Dublin, Ireland; 7 Dept of Gynae‐Oncology, Trinity St. James's Cancer Institute, Dublin 8, Ireland, Dublin, Dublin, Ireland; 8 Trinity St James’s Cancer Institute, St James’s Hospital, The Haematology, Oncology and Palliative Care (HOPe) Directorate, St James’s Hospital, Dublin 8, Ireland., Dublin, Dublin, Ireland; 9 Trinity St. James's Institute, Dublin 8, Dublin, Ireland, 10 Department of Histopathology, Trinity College Dublin, Dublin, Dublin, Ireland, 11 Dept of Histopathology & Dept of Obstetrics and Gynaecology, Trinity St. James's Cancer Institute, St. James's Hospital, Dublin 8, Dublin, Dublin, Ireland, 12 Dept of Histopathology, Trinity St. James Cancer Institute, St. James's Hospital, Dublin 8, Dublin, Dublin, Ireland
Background: Circulating tumour cells [CTCs] are rare, silent precursors of metastatic disease that survive in the blood circulation and metastasise to distal sites. CTCs are highly heterogeneous and often undergo EMT. Platelets and immune cells can ‘cloak’ cancer cells, aiding their survival in circulation. However, the clinical significance of platelet and immune cloaked CTCs remains unclear.
Aims: Assess the influence of platelets/neutrophils in the characterisation of CTCs from metastatic breast [MBC] and ovarian [OC] cancer patients
Methods: Platelets and neutrophils were isolated from healthy donors and co‐cultured with cell lines for 24h. Markers of EMT and immune evasion were assessed by Taq‐Man PCR and flow cytometry. 7.5 ml peripheral blood specimens were collected prospectively from patients for CTC isolation using Parsortix; MBC [n = 20] and OC [n = 23], with a subset of patients [n = 10] analysed by CellSearch. Isolated CTCs were enumerated using a cancer specific CTC‐ID by IF microscopy.
Results: Platelets and neutrophils altered EpCAM, TWIST1, PAI‐1 and PD‐L1 gene expression in OC and MBC cell lines. Platelets altered PLEK2 and CCL2 expression in MBC cells. 85% of MBC patients had >1 CTC [1–220 cells/7.5ml] while 62% of OC patients had >1 CTC [1–19 cells/7.5 ml] in blood samples. MBC CTCs clusters had immune cell cluster infiltrates, while 43% of OC CTCs were CD42b+. Follow up of CTC immunophenotyping with treatment response and survival is ongoing.
Conclusion(s): Platelet and neutrophils can alter the expression of markers of CTC detection in vitro and promote immune evasion through the up‐regulation of PD‐L1 and induction of EMT. CTCs isolated from patients were found to be cloaked with platelets, while CTC clusters were found to be associated with immune cells. Platelet and immune cell cloaked CTCs may be a predictive biomarker of aggressive disease in patients and aid in identifying patients with poor prognosis which may benefit from more aggressive treatment.

165. VPB0759
165.1. Cancer‐associated fibroblasts promote venous thrombosis through C‐type lectin‐like receptor 2/podoplanin in 3LL lung cancer mouse model
T. Shirai 1; T. Sasaki2; N. Tsukiji2; S. Ohishi2; R. Yokomori2; K. Takano2; K. Suzuki‐Inoue3
1 Yamanashi University, Japan, Chuo, Yamanashi, Japan; 2 Department of Clinical and Laboratory Medicine, Faculty of Medicine, Yamanashi University, Japan, Chuo, Yamanashi, Japan; 3 University of Yamanashi, Chuo, Yamanashi, Japan
Background: Platelets play pathophysiological roles in cancer‐associated thrombosis (CAT). One of the underlying mechanisms is the membrane protein podoplanin. Some types of tumor cells expressing podoplanin can induce platelet aggregation via the platelet activation receptor C‐type lectin‐like receptor 2 (CLEC‐2). In addition to tumor cells, cancer‐associated fibroblasts (CAFs) also express podoplanin. CAFs are the most abundant cancer‐stromal cells; they promote cancer progression through cell‐cell interactions and release extracellular vesicles (EVs). However, the relationship between CAFs and platelets in CAT remains unstudied.
Aims: We investigate whether CAFs and their EVs potentially promote CAT in 3LL mouse lung cancer model.
Methods: Podoplanin expression in EVs purified from NIH/3T3 cell‐conditioned medium and 3LL tumor was analyzed by immunoblotting. Mouse platelet aggregation was induced by NIH/3T3 cells and their EVs. Plasma podoplanin level was compared between healthy mice and 3LL‐bearing mice. At the femoral vein, the time to occlusion after ferric chloride injury was measured in healthy mice and 3LL‐bearing mice. Tumor‐bearing mice were treated with rat anti‐mouse CLEC‐2 monoclonal antibody 2A2B10 or control rat IgG four days before ferric chloride injury.
Results: EVs purified from NIH/3T3 cell‐conditioned medium and 3LL tumor presented podoplanin. NIH/3T3 cells and their EVs induced platelet aggregation in a CLEC‐2 dependent manner. The 3LL‐bearing mice showed increased podoplanin levels in plasma and a shortened time to occlusion compared to healthy mice. In the tumor‐bearing state, antibody‐induced CLEC‐2 depletion extended the time to occlusion compared to control IgG‐treated mice.
Conclusion(s): Podoplanin‐expressing EVs released from CAFs impact circulating podoplanin levels, which promote venous thrombosis. Antiplatelet therapy targeting CLEC‐2 may be effective in treating CAT mediated by podoplanin derived from CAFs as well as tumor cells.
166. PB0751
166.1. Platelets are integral components of the tumor microenvironment whose regulatory actions vary with the tumor type
O. Le Chapelain 1; S. Jadoui2; V. Ollivier2; B. Ho‐Tin‐Noé3
1 INSERM, PARIS, Ile‐de‐France, France; 2 INSERM UNIT 1148, PARIS, Ile‐de‐France, France; 3 INSERM, PARIS, Ile‐de‐France, France
Background: Whereas platelets are well known to promote the metastatic process, there is little evidence of their presence in the microenvironment (TME) of solid tumors. In addition, there are controversial data regarding the impact of platelets on primary tumor growth and it remains unclear whether platelets regulate immune cell infiltration in solid tumors as they do in other inflammatory sites.
Aims: We investigated whether platelets are present in the TME of solid tumors and participate in the regulation of its vascular and immune components.
Methods: The participation of platelets in the TME was assessed in 2 different mouse models of solid cancers (PyMT AT‐3 breast cancer and B16F1 melanoma). The microenvironment of both types of solid tumor was characterized by immunohistology and semi‐quantitative proteome profiler analysis. Platelet presence in the TME was investigated by immunohistology and intravital microscopy. The contribution of platelets to tumor growth, vessel density and integrity, and immune cell infiltration was determined using a model of chronic and profound thrombocytopenia based on the injection of a platelet‐depleting antibody in mpl−/− mice.
Results: AT‐3 mammary tumors were much more inflammatory and displayed a higher vessel density as compared to B16F1 melanoma. Firmly adherent platelets lining tumor vessels and perivascular platelets were systematically found in both types of tumor. “Platelet‐like” cells displaying only a subset of platelet markers were additionally found in the stroma of AT‐3 mammary tumors. Chronic and profound thrombocytopenia was associated with signs of severe endothelial degeneration and increased vascular leakage and bleeding only in AT‐3 mammary tumors. Immune cell infiltration was increased in thrombocytopenic mice in both models of tumors while vascular density was unchanged between thrombocytopenic and WT mice.
Conclusion(s): Our findings indicate that platelets are integral components of the TME whose impact on tumor growth and TME modeling varies with the tumor type.
167. PB0754
167.1. Treatment of hepatocellular carcinoma with autologous platelets encapsulating sorafenib or lenvatinib: A novel therapy exploiting tumor‐platelet interactions
H. Tanaka 1; K. Horioka2
1 Asahikawa Medical University, Asahikawa, Hokkaido, Japan; 2 Karolinska Institutet, Stockholm, Stockholms Lan, Sweden
Background: Hepatocellular carcinoma (HCC) activates platelets through the action of adjacent sinusoidal cells. Activated platelets bind to tumor‐associated endothelial cells and release growth factors that promote tumor progression. We hypothesized that platelets encapsulated with tumor inhibitors would function as drug carriers for tumor therapy.
Aims: We propose a therapeutic strategy for HCC using autologous platelets encapsulating multiple tyrosine kinase inhibitors in a rat chemically‐induced HCC model.
Methods: Sorafenib or lenvatinib was encapsulated in platelets isolated from tumor‐bearing rats in vitro. The rats were divided into groups that received repeated intravenous injections (twice a week for 10 weeks) of the following materials: placebo, sorafenib (SOR), lenvatinib (LEN), autologous platelets, autologous platelets encapsulating sorafenib (SOR‐PLT), and autologous platelets encapsulating lenvatinib (LEN‐PLT). The therapeutic effect was then analyzed by ultrasonography (US) and histopathological analysis.
Results: Histopathological and US analysis demonstrated extensive tumor necrosis in the tumor tissue of SOR‐PLT or LEN‐PLT, but not in other experimental groups. By liquid chromatography‐mass spectrometry, more abundant sorafenib was detected in tumor tissues after SOR‐PLT administration than in surrounding normal tissues, but no such difference in sorafenib level was observed with SOR administration.
Conclusion(s): The use of autologous platelets encapsulating drugs might be a novel therapeutic strategy for HCC.
167.2. Platelets and Infection
168. PB0764
168.1. Impact of Puumala Hantavirus infection on primary haemostasis: Potential mechanisms of thrombocytopenia
A. Schmuckenschlager 1; A. Assinger2; J. Wigren Byström3; A. Fors Connoly3; C. Ahlm3; M. Forsell3; W. Schrottmaier2
1 Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Wien, Austria; 2 Medical University of Vienna, Vienna, Wien, Austria; 3 Department of Clinical Microbiology, Umeå University, SWE, Umea, Vasterbottens Lan, Sweden
Background: Viral hemorrhagic fevers caused by RNA virus families are associated with impaired haemostasis, leading to a clinical picture of increased bleeding and decreased platelet counts (thrombocytopenia). In Europe a mild form of viral hemorrhagic fever frequently occurs, which is caused by Puumala Hantavirus (PUUV). Thrombocytopenia during PUUV infection may affect not only haemostasis but also platelet‐mediated immunomodulatory functions. Therefore, platelets are thought to play a pivotal role in disease manifestations of PUUV infections, but the underlying mechanism is yet unknown.
Aims: Thus, we investigated the effects of PUUV‐associated thrombocytopenia on platelets in vitro by evaluating the formation of platelet‐neutrophil/monocyte aggregates, platelet‐induced leukocyte activation and platelet reactivity in response to various agonists at different platelet densities by flow cytometry. Further, the underlying mechanism of PUUV‐induced thrombocytopenia was investigated, which could be caused by e.g. platelet desialylation, which we investigated by flow cytometry, or endothelial adhesion. Therefore, direct and indirect effects (e.g. via infected endothelial cells) of PUUV on platelet adhesion under capillary shear stress were analyzed by immunofluorescence microscopy.
Methods: Statistics were performed using GraphPad Prism 8.0 according to general guidelines using ANOVA, parametric t‐test or Wilcoxon test. p‐values of 0.05 or less were considered significant.
Results: Platelet function was only mildly affected by platelet density. No direct effects of PUUV on platelet sialylation nor adhesion were observed, suggesting that platelet dysfunction and thrombocytopenia are probably multifactorial and indirectly induced by PUUV. This was further supported by the observation that platelets adhered more frequently to PUUV infected HUVECs, which could represent a potential indirect mechanism of thrombocytopenia.
Conclusion(s): Taken together, we could shed light on the mechanism of PUUV‐mediated thrombocytopenia and its consequences on platelet function. A better understanding of the underlying processes could path the way for urgently needed treatment options for haemorrhagic complications these patients.
169. PB0766
169.1. Essential oils and their sesquiterpenes reduce DENV‐2‐induced platelet activation
L. Silva 1; R. Gonçalves2; P. T. Bozza2; P. Rondón3; R. Ocazionez1; E. Stashenko1
1 Universidad Industrial de Santander, Bucaramanga, Santander, Colombia; 2 Instituto Oswaldo Cruz, Rio de Janeiro, Rio de Janeiro, Brazil; 3 Universidad de Santander, Bucaramanga, Santander, Colombia
Background: Dengue virus (DENV) infection causes dengue illness, leading sometimes to serious disease and death. DENV‐driven exacerbate platelet activation, and DENV nonstructural protein‐1 (NS1) protein contribute to inflammatory amplification and thrombocytopenia during dengue. Since there are no effective drugs for the treatment, essential oils (EOs) could be good resource for developing drugs that prevent dengue from progressing to severity.
Aims: To evaluate the in vitro inhibitory effect of EOs and their sesquiterpenes on human platelet activation induced by DENV‐2 and NS1 protein.
Methods: EOs were extracted by MWHD from plants grown in Colombia. GC/MS was used to determine the chemical composition of Lippia origanoides (phellandrene chemotype), Turnera diffusa (EO), Lippia alba EOs (citral, carvone chemotypes) and three sesquiterpenes (trans‐β‐caryophyllene, caryophyllene oxide and germacrene D). Molecular interactions with DENV/platelets proteins were predicted with an in silico analysis. Platelets isolated from healthy donors blood and purified by CD45 depletion were exposed to DENV‐2 (MOI = 1) or recombinant NS1 (5μg/ml) for 4h and 3h, respectively, in the presence of non‐cytotoxic sample concentrations. Platelet activation was determined through flow cytometry and detection of activation markers in the supernatant.
Results: Twenty‐nine compounds in the EOs studied were identified; some of them (trans‐β‐caryophyllene, caryophyllene oxide, germacrene D, α‐humulene, and α‐selinene) were capable of strongly binding to proteins important to the DENV‐platelet interaction (DC‐SIGN, HSP70, TLR4, and TLR2) (Figure 1). EOs exposition for 4h did not affect platelet viability (Figure 2A). In addition, treatment with EOs could reduce the platelet surface expression of activation markers and secretion of α‐granule products induced by exposure to DENV and NS1. Lippia alba‐carvone EO reduced CD62p and CD63 surface expression, PF4/CXCL4 and RANTES/CCL5 secretion. trans‐β‐Caryophyllene and caryophyllene‐oxide reduced CD62p expression, PF4/CXCL4 and RANTES/CCL5 secretion (Figure 2B and C).
Conclusion(s): The EOs and sesquiterpenes reduce platelet activation induced by DENV‐2 and NS1 protein and may act as potential therapeutic agents.


170. PB0765
170.1. Impaired platelet function caused by Vibrio cholera outer membrane vesicles
W. Schrottmaier 1; A. Pirabe2; U. Resch2; J. Santol2; S. Frühwirth1; K. Aung3; M. Dongre3; A. Digruber4; B. Jilma5; A. Kirschner1; M. Ehling‐Schulz4; S. Wai3; M. Forsell6; A. Assinger1
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Institute of Vascular Biology and Thrombosis Research, Medical University of Vienna, Vienna, Wien, Austria; 3 Umeå University, Umeå, Vasterbottens Lan, Sweden; 4 University of Veterinary Medicine Vienna, Vienna, Wien, Austria; 5 Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Wien, Austria; 6 Department of Clinical Microbiology, Umeå University, SWE, Umea, Vasterbottens Lan, Sweden
Background: Haemorrhagic complications in cholera disease are associated with bacteraemia and increased mortality. Vibrio cholerae, the causative pathogen of cholera, sheds outer membrane vesicles (OMV) to modulate and evade host immunity. However, given the immune‐sensing capacity of platelets, these OMV may also affect platelet function.
Aims: We aimed to investigate whether V. cholerae‐derived OMV may modulate haemostatic/pro‐thrombotic platelet function.
Methods: OMV were isolated in the late logarithmic growth phase. Effects of OMV on platelet function were analysed in vitro by pre‐incubating platelets with OMV from V. cholerae before assessing platelet activation using flow cytometry, light transmission aggregometry and Western Blot in response to various agonists. Platelet spreading and cytoskeletal remodelling were assessed by (super‐resolution) fluorescence microscopy. In vivo relevance was tested in a model of FeCl3‐induced thrombosis using mice transfused with OMV‐treated platelets. To clarify species specificity OMV effects on platelet degranulation and GPIIb/IIIa activation was compared to OMV from different bacteria via flow cytometry.
Results: V. cholerae‐derived OMV significantly reduced platelet degranulation (measured by surface P‐selectin) and almost abolished GPIIb/IIIa activation independently of the agonist. Investigation of intracellular signalling events revealed profound impairment of AKT phosphorylation, whereas phosphorylation of p38 and vasodilator‐stimulated phosphoprotein (VASP) were unaffected. OMV also diminished platelet aggregation and curtailed platelet spreading, which involved aberrant rearrangement of the actin cytoskeleton. In vivo, mice transfused with OMV‐treated platelets were protected from FeCl3‐induced thrombosis. Similar effects of OMV on platelet CD62P exposure and GPIIb/IIIa activation were also observed for other Vibrio strains.
Conclusion(s): Our results show that OMV from V. cholerae strongly impair pro‐thrombotic platelet function in vitro and in vivo, mediated by inhibition of the central signalling hub AKT and dysregulation of the actin cytoskeleton. Thereby, OMV‐mediated platelet inhibition may represent an underlying mechanism that contributes to haemorrhagic complications in cholera patients.
171. PB0762
171.1. Ticagrelor prevents infective endocarditis by mitigating Staphylococcus aureus virulence
N. Jacques 1; S. Meyers2; K. Leeten1; M. Lox3; M. Debuisson1; P. Delvenne4; A. Nchimi1; M. Kuijpers5; T. Vanassche6; K. Martinod7; P. Verhamme3; P. Lancellotti8; C. Oury1
1 Laboratory of Cardiology, GIGA Institute, University of Liège, Liège, Belgium, Liege, Liege, Belgium; 2 Center for Molecular and Vascular Biology, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 3 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 4 Department of Pathology, CHU of Liège, Liège, Belgium; Laboratory of Experimental Pathology, GIGA Institute, University of Liège, Liège, Belgium, Liège, Liege, Belgium; 5 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 6 University Hospitals Leuven, KULeuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 7 KU Leuven, Leuven, Vlaams‐Brabant, Belgium; 8 Laboratory of Cardiology, GIGA Institute, University of Liège, Liège, Belgium; Gruppo Villa Maria Care and Research, Maria Cecilia Hospital, Cotignola, and Anthea Hospital, Bari, Italy, Liege, Liege, Belgium
Background: Infective endocarditis (IE) is a deadly disease mainly caused by the gram‐positive and highly virulent bacteria Staphylococcus aureus (SA). Due to lack of efficacy of current antibiotic therapy, there is an urgent need to discover new strategies that could prevent this disease.
Aims: To assess the ability of the antiplatelet drug ticagrelor, which also displays antibacterial activity against gram‐positive bacteria, to prevent SAIE.
Methods: We used a mouse model of infective endocarditis induced by a SAIE clinical isolate. Ticagrelor was administered orally at a conventional dosage (3mg/kg, single dose) prior to infection and local histamine infusion on the aortic valve. Infected vegetation presence was determined by Gram staining on heart sections after three days. Antibacterial effect of ticagrelor at dosages equivalent to plasma levels achieved in patients (0.75–1.25mg/L) was assessed in vitro in human blood.
Results: Ticagrelor significantly prevented the formation of infected vegetation, with IE in only 14.3% of ticagrelor‐treated mice (n = 21) compared to 52.3% of vehicle‐treated mice (n = 37). Ex vivo ADP‐induced platelet aggregation assays confirmed rapid reversibility of antiplatelet activity (4 h), which made it unlikely that solely the antiplatelet effect would explain IE prevention by ticagrelor. Bacterial survival in blood was not diminished three days post‐infection. We therefore assessed whether ticagrelor could affect bacterial virulence. We found that growing SA in the presence of 1.25mg/L ticagrelor inhibited alpha‐toxin RNA expression and protein secretion, which was corroborated with drastically reduced hemolysis and platelet aggregation induced by bacterial supernatants. Ticagrelor‐treated bacteria could no longer adhered to fibrinogen, and in whole blood perfusion experiments, flow‐dependent bacterial adhesion on activated endothelial cells was severely impaired.
Conclusion(s): Our study demonstrates the unprecedented ability of ticagrelor to prevent IE by a novel mechanism of directly mitigating bacterial virulence. Hence, clinical trials using ticagrelor as adjunct therapy to antibiotics in patients at risk for IE are warranted.
172. PB0763
172.1. The mTOR pathway in platelets contributes to the pathophysiology of experimental cerebral malaria
I. Portier 1; F. Denorme1; K. Queisser1; Y. Kosaka1; A. Petrey1; G. Zimmerman1; C. Morrell2; R. Campbell1; M. Rondina1
1 University of Utah, Salt Lake City, Utah, United States; 2 University of Rochester, Rochester, New York, United States
Background: Cerebral malaria remains a major public health concern in Sub‐Saharan Africa. The pathogenesis arises from damaged vascular endothelium and a maladaptive immune response. However, growing evidence suggests a crucial role for platelets in malaria.
Aims: Determine if the mammalian target of rapamycin (mTOR) in platelets contributes to the pathogenesis of malaria.
Methods: Platelet‐specific mTOR knock out mice (KO; mTORfl/fl‐Pf4‐cre) and littermate controls (WT; mTORfl/fl) were subjected to a well‐established model of experimental cerebral malaria (ECM) using Plasmodium berghei ANKA (PbA).
Results: The mTOR pathway was activated in platelets from PbA‐infected C57Bl/6J mice, based on phosphorylation of mTOR and its downstream effector; 4E‐BP1. Consistent with mTOR activation, we also observed increased protein translation in platelets from PbA‐infected mice compared to uninfected controls. To study the role of platelet mTOR during ECM, KO mice and WT controls were infected with PbA. Platelet mTOR‐deficient mice had significantly increased survival compared to WT mice (p = 0.004). Increased survival was independent of parasitemia, thrombocytopenia and platelet turnover. Furthermore, KO mice had significantly reduced brain vascular permeability (p = 0.007), which was associated with improved neurological outcomes. Interestingly, platelet adhesion to the brain vasculature was reduced in KO mice during late‐stage ECM. As platelet adhesion to the vasculature is often accompanied by monocyte recruitment, we next assessed the number of monocytes in the brain. Interestingly, the number of inflammatory monocytes was significantly reduced in the brains (p = 0.04) of platelet‐deficient mTOR mice during ECM. Taken together, these results suggest that platelets assist in the recruitment of leukocytes to the brain vasculature during ECM, which is impaired when mTOR is ablated.
Conclusion(s): Our data demonstrates that the mTOR pathway in platelets plays a significant role in malaria pathogenesis. Deletion of platelet mTOR reduces vascular permeability and prolongs survival during ECM. We hypothesize that altered platelet‐inflammatory monocyte interactions drive this phenotype.
172.2. Platelets and Inflammation
173. PB0767
173.1. The relevance of thromboinflammatory biomarkers and their relationship with circulating glycosaminoglycans in end‐stage renal disease patients
M. Allen 1; V. Bansal2; F. Siddiqui2; D. Hoppensteadt3; E. Krupa2; B. Kantarcioglu4; J. Fareed3
1 Loyola University Medical Center, Naperville, Illinois, United States; 2 Loyola University Medical Center, Maywood, Illinois, United States; 3 Loyola University Chicago, Maywood, Illinois, United States; 4 Loyola University Chicago, Oak Park, Illinois, United States
Background: End stage renal disease (ESRD) is a complex progressive medical condition that affects multiple organ systems. Given the high risk of morbidity and mortality of ESRD as well as its rising incidence, it is critical to understand the relevance of thromboinflammatory biomarkers in disease development and progression of kidney dysfunction.
Aims: The purpose of this study is to profile the levels of thromboinflammatory biomarkers in ESRD patients including D‐Dimer, C‐reactive protein (CRP), von Willebrand factor (vWF), plasminogen activator inhibitor 1 (PAI‐1), and thrombin activatable fibrinolysis inhibitor (TAFI). In addition, the levels of anti‐PF4 IgG and endogenous circulating glycosaminoglycans (GAGs) were measured.
Methods: Citrated plasma samples were collected from seventy‐three ESRD patients. Control plasma samples (NHP) from healthy, non‐smoking adults aged 19 to 53 were obtained commercially. Validated ELISA methods have been used to profile each of the biomarkers. The levels of endogenous GAGs were determined (Redprobes UG, Germany). To compare the levels of thromboinflammatory biomarkers, anti‐PF4 IgG, and endogenous GAGs in different groups, appropriate statistical methods included Mann‐Whitney U, t‐tests, Kruskal‐Wallis ANOVA and experiment correlation analysis methods were performed.
Results: All of the biomarkers and GAGs were significantly elevated, with the exception of TAFI, in ESRD patients. The ESRD patients exhibited varying levels of increase in the D‐Dimer, CRP, vWF, PAI‐1, anti‐PF4 IgG, and GAGs as shown in Figure 1 (p < 0.05). D‐Dimer showed the most pronounced increase (1075%) followed by PAI‐1 (361.31%), anti‐PF4 IgG (209.78%), CRP (101.77%) and endogenous GAGs (17.29%). The correlation analysis revealed varying degrees of association among these biomarkers (Figure 2).
Conclusion(s): These results suggest that thromboinflammatory biomarkers offer the potential utility of identifying inflammation in end‐stage renal disease. Marked increase in thromboinflammatory mediators due to endothelial damage may result in the upregulation of glycosaminoglycans and anti‐PF4 IgG antibodies in the ESRD patients.


174. PB0772
174.1. Platelet‐leukocyte aggregates as markers of (pro)thrombotic inflammation
A. Peshkova1; K. Sounbuli2; Y. Selivanova2; I. Andrianova2; R. Litvinov 3; J. Weisel4
1 Kazan (Volga region) Federal University, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 2 Institute of Fundamental Medicine and Biology, Kazan (Volga region) Federal University, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 3 University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania, United States; 4 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: Platelet‐leukocyte aggregates are formed in the blood of patients with inflammatory diseases as a result of pathological activation of blood cells. They reflect relationships between hemostasis and immunity, but the mechanisms of the interactions between platelets and leukocytes and their pathophysiological significance are not fully understood.
Aims: The aim of this work was to study the conditions of formation and morphological characterization of platelet‐leukocyte aggregates in the blood in vitro.
Methods: Flow cytometry was used to identify and quantify platelet‐leukocyte aggregates in whole human blood. Gating of different cell types and cellular aggregates was performed using fluorescently labeled antibodies against specific surface antigens, namely CD45 (all leukocytes), CD16 (neutrophils), CD14 (monocytes) and CD41 (platelets). Phorbol‐12‐myristate‐13‐acetate (PMA), lipopolysaccharide (LPS), thrombin receptor‐activating peptide (TRAP), and adenosine diphosphate (ADP) were used as cellular activators. For confocal fluorescent microscopy, cells were labeled with DiI and DAPI.
Results: After adding PMA to citrated blood, about 36% of neutrophils and 67% of monocytes were found within platelet‐neutrophil and platelet‐monocyte aggregates, respectively. ADP and TRAP had similar effects and caused incorporation of ~30% of neutrophils and 58%–76% of monocytes into aggregates with platelets. Unlike other stimulants studied, LPS did not induce platelet co‐aggregation with either neutrophils or monocytes, likely as a relatively weak platelet stimulant. Microscopy of the platelet‐leukocyte aggregates showed that the prevailing and roughly equal platelet/neutrophil and platelet/monocyte ratios were 1:1 and 2:1 for both types of heterotypic aggregates.
Conclusion(s): Activation of blood cells with PMA, ADP, or TRAP promotes the formation of platelet‐neutrophil and platelet‐monocyte aggregates, whereas LPS does not cause platelet activation sufficient for expression of adhesive molecules needed for the heterotypic cellular aggregation. Therefore, platelet‐leukocyte aggregates are induced by some, but not all, inflammatory mediators and metabolites and can be used as markers of (pro)thrombotic conditions associated with systemic inflammation. Funding: the "Priority‐2030" Program at KFU.

175. PB0770
175.1. High levels of TLT‐1+, but not Gp1b+, microparticles are indicative of poor outcomes in acute respiratory distress syndrome
A. Gibson 1; Z. Bayron‐Marrero2; J. Warrick1; A. Washington3
1 Maryville College, Maryville, Tennessee, United States; 2 University of Puerto Rico, San Juan, Puerto Rico, United States; 3 Oakland University, Rochester, Michigan, United States
Background: Microparticles (MPs) are cellular fragments that range in size from 50 to 1000 nm. MPs have been shown to mediate cellular communication, as well as thrombosis, in disease states. In the vasculature, MPs are derived from each of the cellular components (platelets, granulocytes, endothelial cells, monocytes, and red blood cells). Several studies have evaluated the prominence of MPs during Acute Respiratory Distress Syndrome (ARDS) and have identified correlations with disease severity and the presence of alveolar endothelial cell MPs, particularly those that contain the coagulation initiator, tissue factor (TF). Oddly, no associations have been reported with platelet‐derived MPs, even though platelets are predicted to produce an estimated 80% of circulating MPs. Recently, our group has found that increased levels of the soluble TREM – like transcript‐1 (TLT‐1) is associated with increased mortality and decreased ability to obtain unassisted breathing and is thus a prognostic indicator for ARDS. Full‐length TLT‐1 binds fibrinogen and is a platelet‐specific receptor that is stored in platelet α‐granules and brought to the surface upon platelet activation.
Aims: In this study we hypothesized the existence of TLT‐1 positive microparticles and that their presence would correlate with disease severity in ARDS.
Methods: We retrospectively quantified TLT‐1, Gp1b, a2bb3, or P‐selectin positive microparticles using flow cytometry in plasma samples from the ARDS Network clinical trials and correlated the data with clinical outcomes.
Results: No correlations were found with Gp1b+ or P‐selectin+ MPs. When divided into quartiles, correlations were found with coagulation failure and survival with a2bb3+ and TLT‐1 + MPs (chi sq p < 0.001). Only TLT‐1+ MPs correlated with MSOF (multi‐system organ failure), APACHE II scores and unassisted breathing (p < 0.001).
Conclusion(s): While both fibrinogen binding+ microparticles (α2bβ3, TLT‐1) correlated with survival, TLT‐1+ MPs demonstrated stronger correlations with APACHE II scores, unassisted breathing, and MSOF.
176. PB0774
176.1. The role of platelets in chemotherapy‐associated steatohepatitis
P. Spitzenstätter1; J. Sturm1; M. Derler1; D. Pereyra2; P. Starlinger3; A. Assinger4; M. Mussbacher 1
1 University of Graz, Graz, Steiermark, Austria; 2 Division of Visceral Surgery, Medical University of Vienna, Vienna, Wien, Austria; 3 Medical University Vienna, Vienna, Wien, Austria; 4 Medical University of Vienna, Vienna, Wien, Austria
Background: The use of anti‐tumoral drugs such as irinotecan or 5‐fluorouracil is linked to causing chemotherapy‐associated steatohepatitis (CASH) during neoadjuvant chemotherapy for colorectal carcinoma. CASH is characterized by inflammatory and metabolic adaptations of the liver restricting effective recovery. Preliminary data indicate that antiplatelet medication such as acetylsalicylic acid or ticagrelor might reduce CASH severity and point to a role for platelets in the development of CASH.
Aims: The aim of this project is to investigate the role of platelet in the development of CASH and associated inflammatory processes.
Methods: Mice were treated with the anti‐tumoral drug irinotecan in the absence and presence of antiplatelet medication. Plasma and liver samples were analyzed for platelet activation and lipid accumulation, respectively. Moreover, an in vitro CASH cell culture model was established using the hepatocyte cell line AML‐12 and the anti‐tumoral drugs irinotecan and 5‐fluorouracil, which were both coincubated with platelet lysates.
Results: In vivo experiments showed significantly elevated plasma levels of the platelet degranulation marker platelet factor 4 as well as increased hepatic apoptosis and inflammation in platelet‐inhibited, irinotecan‐treated mice. In vitro co‐incubation of AML12 cells with platelets reduced the degree of lipid accumulation in irinotecan‐treated cells.
Conclusion(s): Platelets become activated during the development of CASH and potentially contribute to disease development in a dual manner.
177. PB0769
177.1. Platelet activation and expression of CAR on cardiomyocytes and platelets: Implication on inflammation in patients with dilated cardiomyopathy
G. Elena 1; L. Buryachkovsaya1; N. Mironova1; P. Chumachenko1; S. Golitsyn1; M. Othman2
1 National Medical Research Center of Cardiology, Moscow, Moskva, Russia; 2 Queen's University, Kingston, Ontario, Canada
Background: Coxsackie‐adenovirus receptor (CAR), a tight junction protein, normally expressed in myocardial intercalated disc, is involved in the pathogenesis of virus‐mediated myocarditis leading to dilated cardiomyopathy (DCM). CAR is also expressed on platelets; the cells known to contribute to systemic inflammation.
Aims: To evaluate the relationship of CAR expression on platelets and cardiomyocytes with local and systemic inflammation in patients with DCM.
Methods: 38 patients (mean age 39.5 ± 11.3 years, 20 male) with DCM and clinical indications for endomyocardial biopsy (EMB) and 30 healthy subjects (HS) were recruited for the study. CAR expression in EMB samples was determined by immunohistochemistry and compared to the histological grade of myocardial inflammation. Platelet activity was analyzed by light transmission (LTA) aggregation, platelet CAR expression was assessed by immunofluorescence and platelet morphology was examined by SEM. Systemic inflammation was assessed by serum levels of tumor necrosis factor alpha (TNF α), Interleukin 6, using ELISA.
Results: CAR expression in EMB samples was not related to the intensity of inflammation. It was maximal in patients with increased IL‐6 level (Figure 1A,B). Platelets of DCM patients were activated as shown by enhanced spontaneous and/or ADP induced platelet aggregation (Figure 2C) and formed microaggregates and leukocyte‐platelet aggregates in blood (Figure 2A,B). Platelets’ CAR expression was >4 fold higher in DCM than HS (8.0 % vs 1.75%, p = 0.0002) and was observed predominantly at sites of intercellular communications (Figure 1C,D). CAR‐positive patients showed significantly higher TNF‐α and IL‐6 serum levels (4.94 vs. 0 pg/ml, p = 0.024) and (3.76 vs 0.19 pg/ml, p = 0.02) in CAR‐negative patients.
Conclusion(s): CAR expression in myocardium is associated with systemic ‐not local‐ inflammation in DCM patients. Activated platelets express CAR at sites of intercellular communications in mircoaggregates resembling the pattern of intercalated discs of the myocardium. Platelets contribute to the inflammation seen with DCM.


178. PB0777
178.1. Adenovirus‐based but not mRNA‐based vaccines transiently alter platelet count homeostasis in healthy subjects
L. lombardi1; N. Scafa2; S. Cesaroni1; R. Marrapodi3; F. Maiorca4; R. Cangemi1; M. Visentini1; G. Romiti1; B. Corica1; F. Pulcinelli3; M. Venneri1; S. Basili1; L. Stefanini 3
1 Sapienza University of Rome, roma, Lazio, Italy; 2 Sapienza University of Rome, Roma, Lazio, Italy; 3 Sapienza University of Rome, Rome, Lazio, Italy; 4 Sapienza University of Rome, ROMA, Lazio, Italy
Background: Nuclei acid‐based COVID‐19 vaccines have proved highly effective in reducing the risk of hospitalisation and death. As they were distributed for the first time on a large‐scale population, the adenovirus‐based vaccines were linked to a very rare thrombosis with thrombocytopenia syndrome and the interplay between vaccination and platelet activation gained increasing attention.
Aims: To compare the effect of mRNA‐based and adenovirus‐based vaccines on platelets of young healthy adults.
Methods: We prospectively enrolled 15 healthy volunteers (53% females) who received two doses of the mRNA‐based vaccine BNT162b2, 21 days apart, and 25 healthy volunteers (64% females) that received one dose of the adenovirus‐based vaccine, AZD1222, followed by one dose of BNT162b2 and we studied their platelet response before and after each dose of the vaccine (3 and 10 days post‐injection).
Results: Subjects receiving the AZD1222 vaccine experienced a transient but significant 20% decrease of the platelet count 3 days after the first injection, which was not detected after the first dose of BNT162b2. The BNT162b2, but not the AZD1222, vaccine was followed by increased plasmatic thrombopoietin concentration and mean platelet volume, indicative of higher platelet turnover. Three days after the AZD1222 injection, basal platelet integrin activation was elevated, but P‐selectin exposure was unchanged. Conversely, the BNT162b2 vaccine induced a gradual increase in platelet P‐selectin exposure and platelet‐leukocyte aggregate formation, which correlated with the ability of the vaccines to evoke neutralizing antibodies against the Sars‐COV‐2 spike protein. Moreover, three days after the AZD1222 injection we detected a transient 10‐fold increase of the plasmatic concentration of IFN‐gamma, while BNT vaccination induced a progressive increase of IL‐1beta.
Conclusion(s): Based on these observations we propose that the adenovirus‐based vaccines, not the mRNA‐based vaccines, transiently impair platelet count homeostasis. Future studies will investigate how these distinct vaccine vectors and inflammatory profiles affect platelet consumption and platelet production.
179. VPB0778
179.1. Platelet proteomics reveals an inflammatory phenotype of platelets in type 2 diabetes
Y. Kong 1; R. Rehan2; J. Weaver3; C. Houlahan4; B. Johnston4; K. Peng4; M. Cielesh4; M. Larance5; F. Passam6
1 Royal Prince Alfred Hospital, Sydney; Central Clinical School, University of Sydney; Haematology Research Group, the Heart Research Institute, CAMPERDOWN, New South Wales, Australia; 2 Cardiology Dept, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; 3 Department of Cardiology, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; 4 Haematology Research Group, The Heart Research Institute, University of Sydney; Faculty of Medicine & Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia; 5 Charles Perkins Centre, School of Life and Environmental Sciences, Faculty of Science, University of Sydney, New South Wales, Australia, Sydney, New South Wales, Australia; 6 Royal Prince Alfred Hospital; Haematology Research Group, The Heart Research Institute, Faculty of Medicine & Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia
Background: Platelets in patients with type 2 diabetes are “hyperactive” resulting in decreased efficacy of antiplatelet agents in the prevention of cardiovascular events [1]. Platelet proteomics allows quantitative analysis of mediators of platelet hyper‐reactivity. However, a dedicated study of the platelet proteome in patients with diabetes and cardiovascular disease (CVD) has not been performed previously.
Aims: To identify proteomic differences in the platelets of patients with or without diabetes with, or at risk of, coronary artery disease and to correlate proteomic changes with functional platelet assays.
Methods: Forty‐two patients with type 2 diabetes and 34 patients non‐diabetic patients, matched by sex and age, were recruited through the Coronary catheterization lab of Royal Prince Alfred Hospital, Sydney (Ethics X20‐0085) [2]. Platelets were isolated from whole blood and separated into platelet lysate and releasate fractions (with or without pre‐activation with low dose thrombin 0.025 U/ml). Platelet function was assessed by platelet aggregation and by the mobilization of P selectin and PAC‐1 after stimulation with a panel of agonists. Releasate and lysate proteins were identified by LC‐MS/MS using a Thermo Lumos Tribrid Orbitrap instrument [3].
Results: Platelets from patients with or without diabetes had similar surface P‐selectin and PAC‐1 expression by flow cytometry. Platelets from patients with diabetes showed increased aggregation to 5 μM ADP and 1 μM U44619. There was a decrease in cardioprotective proteins, e.g. superoxidase dismutase 2 and myeloid derived growth factors released by platelets from patients with diabetes after thrombin stimulation. Secreted chemokines, e.g. CXCL3 and CXCL5, were significantly increased in the releasate in patients with diabetes, and was also increased in male compared to female patients regardless of diabetic status.
Conclusion(s): These studies identify platelets as mediators of the inflammatory status in type 2 diabetes. Platelet proteomics is a promising tool for the identification of platelet biomarkers in CVD.
180. PB0776
180.1. Using a multi‐omics approach to understand endothelial dysregulation of platelet activation in inflammatory conditions
C. Schönichen 1; I. Provenzale2; A. Ludt3; F. Solari4; F. Marini5; A. Cleuren6; K. Jurk7; M. Stoll8; A. Sickmann4; M. Kuijpers9; J. Heemskerk10
1 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Center for Thrombosis and Hemostasis, University Medical Center of the Johannes Gutenberg‐University of Mainz, Germany, Maastricht, Limburg, Netherlands; 2 Department for Biochemistry, CARIM School for Cardiovascular Diseases, Maastricht University, The Netherlands; Institute for Cardiovascular and Metabolic Research (ICMR), School of Biological Sciences, University of Reading, Reading (UK), Maastricht, Limburg, Netherlands; 3 Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), University Medical Center of the Johannes Gutenberg‐University, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 Leibniz‐Institut für Analytische Wissenschaften – ISAS – e.V. Dortmund, Germany, Dortmund, Nordrhein‐Westfalen, Germany; 5 Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), University Medical Center of the Johannes Gutenberg‐University, Mainz, Germany; Virchow Fellow, Center for Thrombosis and Hemostasis Mainz (CTH), Germany, Mainz, Rheinland‐Pfalz, Germany; 6 Oklahoma Medical Research Foundation, Oklahoma City, OK, USA, Oklahoma City, Oklahoma, United States; 7 Center for Thrombosis and Hemostasis, University Medical Center of the Johannes Gutenberg‐University of Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 8 Departments of Biochemistry and Genetic Epidemiology and Statistical Genetics, CARIM School for Cardiovascular Diseases, Maastricht University, The Netherlands, Maastricht, Limburg, Netherlands; 9 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands, 10 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands
Background: Endothelial cells (EC) suppress platelet activation and coagulation to prevent blood clotting. In inflammatory conditions, this negative regulation can be hampered and lead to thrombotic complications, but the mechanism is largely unknown.
Aims: Unravel the pathways by which (inflammatory) EC influence platelet signaling using vessel‐on‐a‐chip and multi‐omics approaches.
Methods: The transcriptomes of control‐, TNFalpha‐ or LPS‐treated Human Umbilical Venous EC (HUVEC) were characterized by RNA‐sequencing. Platelet activation and thrombus formation on sub‐confluent EC were monitored in whole‐blood and high shear conditions. Changes in phosphorylation levels of platelets exposed to (treated) EC were assessed by TMT phosphoproteomic analysis. Platelet integrin activation (PAC1 mAb) in these conditions were measured by flow cytometry.
Results: Out of >18k robustly detectable genes, the HUVEC transcriptome showed ~0.7k and ~6.5k expression changes (LPS or TNF‐treatment vs. Control respectively, adjusted p‐value >0.01), in particular of inflammatory pathways and secretory mediators, after TNFalpha >> LPS treatment. Pathway analysis via ClusterProfiler and topGO revealed distinct inflammatory footprints of the treated EC. In high shear flow conditions on a collagen/tissue factor surface, the presence of sub‐confluent EC potently suppressed platelet adhesion, thrombus and fibrin formation. When pre‐treated with TNFalpha, the thrombo‐protective and anticoagulant effect of the EC was significantly reverted. Flow cytometry revealed a ~80% reduction in agonist‐induced integrin activation of platelets exposed to the control EC. This effect was half as strong after TNFalpha treatment, but not after LPS treatment. Key changes in the phosphoproteome (3.0k phospho‐sites) in EC‐exposed platelets are currently validated to uncover most relevant signaling alterations due to TNFalpha or LPS treatment of the cells.
Conclusion(s): The combined use of OMICs and microfluidic approaches reveals a high complexity of inflammation‐modulated interactions between EC and platelets. This project is funded from the EU Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement No 813409 (TICARDIO).
181. PB0768
181.1. The role of platelets in ER stress‐induced hepatic steatosis
M. Derler 1; W. Schrottmaier2; M. Salzmann2; G. Schoiswohl1; J. Schmid3; A. Assinger2; M. Mussbacher1
1 University of Graz, Graz, Steiermark, Austria; 2 Medical University of Vienna, Vienna, Wien, Austria; 3 Medical University of Vienna, Wien, Wien, Austria
Background: Besides their hemostatic functions, platelets play an important role in liver diseases by interacting with circulatory and liver‐resident (immune) cells such as hepatocytes, Kupffer cells, and stellate cells. The most common chronic liver disease is non‐alcoholic fatty liver disease (NAFLD), which is characterized by excessive accumulation of hepatic triglycerides and eventually progresses into non‐alcoholic steatohepatitis (NASH) and liver fibrosis/cirrhosis. Platelets accumulate in steatotic livers of NAFLD patients and potentially contribute to hepatic inflammation and endoplasmic reticulum (ER) stress.
Aims: The central aim of this project is to elucidate the role of platelets in the development of hepatic ER stress, lipid accumulation, and inflammation.
Methods: Mice were treated with tunicamycin to induce hepatic ER stress and depleted of platelets using an anti‐GPIb‐antibody. Hepatic tissue was analyzed on mRNA, protein, and lipid levels. Furthermore, immune cell influx was determined by flow cytometry and histological stainings. Additionally, in vitro studies were performed by coincubation of platelets with murine hepatocytes.
Results: Tunicamycin led to a significant increase of hepatic triglycerides, which was associated with elevated hepatic expression of very‐low‐density lipoprotein receptor (VLDLR). Depletion of platelets alleviated hepatic steatosis and lowered plasma levels of alanine transaminase (ALT) and aspartate transaminase (AST). In addition, the absence of platelets significantly decreased markers of ER stress and mRNA levels of interleukin‐1b. In contrast to the in vivo data, in vitro co‐incubation of platelets with hepatocytes did not alter ER stress and only mildly affected lipid accumulation, indicating the need for an additional (immune) cell type.
Conclusion(s): Platelets exert detrimental effects on ER stress‐induced hepatic steatosis by interacting with (liver‐resident) immune cells.
182. PB0771
182.1. Platelets promote neutrophil extracellular traps mediated thrombo‐inflammation and glomerular endothelial dysfunction in diabetic kidney disease
A. Gupta 1; K. Singh1; R. Younis1; S. Krishnan2; S. Fatima2; S. Ambreen2; H. Mai2; N. Hoffmann2; R. Biemann2; K. Shahzad2; S. Zimmermann2; B. Isermann3; S. Kohli3
1 Institute for Laboratory Medicine, Leipzig University, Leipzig, Sachsen, Germany; 2 University of Leipzig, Leipzig, Sachsen, Germany; 3 Institute of laboratory medicine, clinical chemistry and molecular diagnostics, Leipzig University, Leipzig, Sachsen, Germany
Background: Diabetic kidney disease (DKD) is a major cause of end‐stage renal failure and is associated with endothelial dysfunction, platelet‐hyperactivity, immune‐cell infiltration and dysfunctional glomerular filtration barrier. Mechanistic insights into the role of platelets for DKD progression are limited.
Aims: We aim to scrutinize the mechanistic interplay between platelets and neutrophil extracellular traps (NETs) and ensuing renal thrombo‐inflammation in DKD.
Methods: Renal function (albuminuria, fractional mesangial area), platelet activation and NET formation was evaluated in a mouse diabetes model. Therapeutic interventions (Aspirin, Anakinra, Solulin, GSK484) were performed in sub‐groups of mice with drugs starting after 16 weeks of diabetes until 24 weeks to study disease reversal. In vitro studies were performed using glomerular endothelial cells (GENCs), platelets and neutrophils exposed to hyperglycaemia under static and flow conditions.
Results: Activated platelets (CD62P) and neutrophil extracellular traps (NETs; CitH3, NE, PAD4) were readily detectable in glomeruli of diabetic mice. Expression of inflammasome markers (NLRP3, IL1β) were positively correlated, reduced thrombomodulin (TM) expression was negatively correlating with NETs. In vitro, platelets exacerbate high glucose and NET induced endothelial dysfunction (p‐eNOS, KLF2, KLF4 and TM), sterile inflammation (IL1β, NLRP3), cell death (TUNEL, Caspase‐3) and glomerular filtration barrier disruption (enhanced FITC‐albumin leakage, disoriented VE cadherin). Under flow conditions, platelets promoted hyperglycaemia induced NET formation on GENCs. Inhibition of platelet activation (Aspirin), amelioration of NETs by inhibition of histone citrullination PAD4 inhibition (GSK484), IL‐1 receptor inhibition (anakinra) or restoring TM expression (solulin) ameliorated these effects in vitro and resulted in diabetic kidney disease reversal in vivo.
Conclusion(s): Hyperglycaemia promotes platelet‐neutrophil interactions resulting in NETs, hypercoagulability, endothelial sterile inflammation, glomerular endothelial dysfunction, barrier disruption and cell death. These changes aggravate the course of DKD. Inhibition of platelets or NETs is a promising therapeutic strategy for DKD.
183. PB0773
183.1. Platelet number and function alterations in preclinical models of sterile inflammation and sepsis patients: Implications in the pathophysiology and treatment of inflammation
M. Villa‐Fajardo1; M. Yáñez Palma2; A. Acebes‐Huerta3; P. Martínez‐Botía 4; M. Meinders5; M. Nolte6; C. Benavente Cuesta7; J. Eble8; J. del Castillo2; F. Martín‐Sánchez2; L. Gutiérrez9
1 Department of Hematology, Instituto de Investigación Sanitaria San Carlos (IdISSC), Hospital Clínico San Carlos, Madrid, Madrid, Spain; 2 Department of Emergency, Instituto de Investigación Sanitaria San Carlos (IdISSC), Hospital Clínico San Carlos (HCSC), Madrid, Madrid, Spain; 3 Instituto de Investigación Sanitaria del principado de Asturias (ISPA), Oviedo, Asturias, Spain; 4 Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Oviedo, Asturias, Spain; 5 Dept. of Blood Cell Research, Sanquin Research and Landsteiner Laboratory, Academic Medical Centre, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 6 Dept. of Hematology, Sanquin Research and Landsteiner Laboratory, Academic Medical Centre, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 7 Department of Hematology, Instituto de Investigación Sanitaria San Carlos (IdISSC), Hospital Clínico San Carlos, Madrid, Madrid, Madrid, Spain; 8 Institute of Physiological Chemistry and Pathobiochemistry, University of Münster, Munich, Bayern, Germany; 9 University of Oviedo ‐ ISPA, Oviedo, Asturias, Spain
Background: Platelet transfusions are given to thrombocytopenic sepsis patients at risk of bleeding, which pose an increased risk of transfusion related adverse events including acute lung injury (TRALI). Emerging evidence points to platelets and neutrophils as major contributors to TRALI, as targeting platelet function ameliorates its risk in preclinical models. However, current anti‐platelet agents would also compromise their hemostatic function and therefore, are not suitable as treatment in this context.
Aims: To characterize platelet function in the context of inflammation (sepsis).
Methods: We have characterized the platelet aggregation capacity in a receptor‐wise manner in murine preclinical models of acute (LPS‐induced) and chronic (CD70Tg) sterile inflammation, and in a cohort of sepsis patients. The study was approved by the respective Ethical Committees. Flow cytometry‐based platelet aggregation assays (FCA) were done.
Results: Platelets from day 5 LPS‐treated mice (i.e., when a new batch of platelets has been produced under inflammatory conditions) displayed an aggretin A (CLEC2)‐ and collagen (α2β1/GPVI)‐induced aggregation defect, similarly to platelets from CD70Tg mice, which also displayed a PMA (αIIbβ3)‐associated impairment (Figure 1). A reduced response was observed in human sepsis platelets, stratified by count, with aggretin A, convulxin (GPVI) and ristocetin (vWF receptor) (Figure 2A). The plasma from sepsis patients displayed a tendency to alter platelet quiescence and to inhibit healthy‐donor platelet aggregation (Figure 1B).
Conclusion(s): While the platelet aggregation signature of inflammation differed inter‐species, our results suggest a specific “shutdown” of the non‐hemostatic or immune receptors (CLEC2, GPVI), which occurs at the platelet production level and is tuned or primed further in the circulation. These results led us to hypothesize that “inflammation‐conditioned” platelets are produced with a specific receptor function profile to avoid an exacerbated inflammation response that could represent danger to the body’s well‐being. Our results have implications in the treatment and in the indication of platelet transfusions in sepsis patients.


184. PB0775
184.1. Disruption of the interaction of platelets with leukocytes by a novel short synthetic peptide targeting P‐selectin
S. Wallis1; N. Wolska2; M. Posner3; A. Upadhyay4; T. Renné5; I. Eggleston6; S. Bagby1; G. Pula 7
1 University of Bath, Bath, England, United Kingdom; 2 University Medical Center Hamburg Eppendorf, Hamburg, Hamburg, Germany; 3 Manchester Metropolitan University, Manchester, England, United Kingdom; 4 University of Bath, BATH, England, United Kingdom; 5 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 6 University Of Bath, Bath, England, United Kingdom; 7 University Medical Center Eppendorf Hamburg (UKE), Hamburg, Hamburg, Germany
Background: The interaction of platelets and leukocytes plays an important role in inflammation and thrombosis. The discovery of novel pharmacological tools able to reduce the formation of platelet‐leukocyte complexes is therefore a promising yet unexplored aspect of cardiovascular research.
Aims: Based on previous studies showing that the N‐terminal domain of S. aureus extracellular fibrinogen‐binding protein (Efb) binds to P‐selectin and interferes with platelet‐leukocyte aggregate formation, here we aimed to identify the minimal Efb motif required for binding platelets and to investigate its effect on the interaction of platelets with leukocyte.
Methods: Using a library of synthetic peptides, we mapped the platelet‐binding region of the N‐terminal domain of Efb using dot blots, pull‐down assays and flow cytometry. The effect of the peptide on platelets was tested by aggregometry, flow cytometry, immunoblotting, and in vitro thrombus formation assays. The effect on platelet‐leukocyte interactions was tested by flow cytometry and cell imaging.
Results: Efb68‐87 is a 20 amino acid‐long peptide identified for its ability to bind to resting and, to a greater extent, thrombin‐stimulated platelets in the absence of fibrinogen. Competitive binding experiments utilising P‐selectin glycoprotein ligand‐1 (PSGL‐1) identified P‐selectin as the cellular docking site mediating platelet binding of Efb68‐87. Accordingly, Efb68‐87 did not bind to other blood cells and captured platelets from human whole blood under low shear stress conditions. Efb68‐87 did not affect platelet activation, but inhibited the formation of platelet‐leukocyte aggregates (PLAs). In addition, Efb68‐87 also abolished the platelet‐dependent stimulation of neutrophil extracellular traps (NETs), which have been shown to contribute to the vascular complications of acute and chronic inflammation, and sepsis.
Conclusion(s): We have identified Efb68‐87 as a peptide selectively binding P‐selectin and inhibiting the interaction of platelets with leukocytes. Efb68‐87 is therefore an exciting candidate for the development of novel selective antagonists of the proinflammatory activity of platelets.


184.2. Proteases and Cancer
185. PB0781
185.1. Prelatent and native antithrombin effect on cell cycle and proliferation of glioblastoma multiforme cells
J. Peñas‐Martínez1; D. Zaragoza Huesca2; E. Navarro Mazano3; E. Cuenca Zamora3; S. Espín4; V. Vicente5; A. Carmona Bayonas6; I. Martínez Martínez 7
1 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain, Murcia, Murcia, Spain; 2 Hospital General Universitario Morales Meseguer, Centro Regional de Hemodonación, University of Murcia, IMIB‐Arrixaca, Murcia, San Javier, Murcia, Spain; 3 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia., Murcia, Murcia, Spain; 4 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain, murcia, Murcia, Spain; 5 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 6 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain., Murcia, Murcia, Spain; 7 IMIB‐Arrixaca, CIBERER, Universidad de Murcia, Murcia, Spain., Murcia, Murcia, Spain
Background: Besides antithrombin’s (AT) key role on hemostasis, two AT’s conformations, native and prelatent, have the anti‐tumour functions. We have recently shown that prelatent AT reduces migration and invasion of glioblastoma multiforme cells U‐87 MG (U87) and inhibits the expression of VEGFA, showing a potential anti‐angiogenic effect. Prelatent AT also reduces pSTAT3 (Tyr705) and pERK1/2 (Thr202/Tyr204) phosphorilation, and STAT3 levels, which have been associated with less resistance to treatment.
Aims: To investigate the effect of prelatent and native AT on glioblastoma multiforme cells.
Methods: Native and prelatent AT were purified from healthy donor’s plasma. A transcriptome analysis was carried out using Human Clariom D chip. The results were analyzed with Partek Genomics Suite software. Differentially expressed genes had >1.5 fold‐change. U87 and U‐251 MG (U251) cells were treated with native or prelatent AT (2.16 μM) or phosphate‐buffered saline for 12 h before each experiment. Cell cycle and proliferation were assessed by measuring 7‐AAD and BrdU incorporation by flow cytometry. Differences in proteins expression were validated by western blot.
Results: After U87 treatment with prelatent AT, 2467 transcripts were overexpressed and 6738 underexpressed. Remarkably, expression of cyclins and regulatory proteins involved in cell cycle were significantly inhibited. We observed ~10% more events in G0‐1 phase, a halfway inhibition of S phase, and a 40.63% cell proliferation reduction after U87 treatment with native AT. However, we did not observe significant results in U251 (Figure 1). Western blot analysis validated the inhibition of the expression of CDK4 after U87 treatment with prelatente AT, but not with the native form (p = 0.020) (Figure 2).
Conclusion(s): Native and prelatent AT has versatile anti‐tumor properties on U87 cells. In particular, we have demonstrated that AT is able to induces cell cycle arrest, but not inhibiting cell cycle cyclins. These results support the potential therapeutic role of AT in glioblastoma multiforme.


186. PB0783
186.1. Suramin is a Hepsin inhibitor which reduces its protumor and prothrombotic effects in colorectal cancer cells
D. Zaragoza Huesca 1; M. Ródenas2; J. Peñas‐Martínez2; I. Pardo Sánchez3; C. Ortega Sabater4; J. Peña García5; S. Espín6; G. Ricote Sánchez2; S. Montenegro Luis2; F. Ayala de la peña2; G. Luengo Gil7; A. Nieto Olivares8; F. García Molina9; V. Vicente10; F. Bernardi11; V. Mulero3; H. Pérez‐Sánchez12; A. Carmona Bayonas13; I. Martínez Martínez14
1 Hospital General Universitario Morales Meseguer, Centro Regional de Hemodonación, University of Murcia, IMIB‐Arrixaca, Murcia, San Javier, Murcia, Spain; 2 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain, Murcia, Murcia, Spain; 3 Department of Cell Biology, Faculty of Biology, Universidad de Murcia. CIBERER, IMIB‐Arrixaca, Murcia, Murcia, Murcia, Spain; 4 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain., murcia, Murcia, Spain; 5 Structural Bioinformatics and High Performance Computing Research Group (BIOHPC), Computer Engineering Department, UCAM Universidad Católica de Murcia, 30107 Guadalupe, Spain, Murcia, Murcia, Spain; 6 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain, murcia, Murcia, Spain; 7 Clinical Analysis and Pathology Department, Group of Molecular Pathology and Pharmacogenetics, IMIB‐Arrixaca, Hospital Universitario Santa Lucía, 30202 Cartagena, Spain., Murcia, Murcia, Spain; 8 Department of Pathology, Hospital Universitario Morales Meseguer, Murcia, Spain., Murcia, Murcia, Spain; 9 Department of Pathology, Hospital Universitario Reina Sofía, Murcia, Spain., Murcia, Murcia, Spain, 10 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain, 11 Department of Life Sciences and Biotechnology, University of Ferrara, Italy., Ferrara, Emilia‐Romagna, Italy, 12 Structural Bioinformatics & High Performance Computing Research Group (BIO‐HPC), Universidad Católica de Murcia (UCAM), Murcia, 30107, Spain, Murcia, Murcia, Spain, 13 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain., Murcia, Murcia, Spain, 14 IMIB‐Arrixaca, CIBERER, Universidad de Murcia, Murcia, Spain., Murcia, Murcia, Spain
Background: Hepsin (HPN) is an extracellular serine‐protease which dysregulation leads to tumor invasion and malignancy. Our group previously described HPN increases protumor and prothrombotic phenotypes in colorectal cancer (CRC) cells, and promotes invasion in zebrafish models. We also identified higher HPN plasma levels in metastatic vs. localized CRC patients, and in patients with thrombosis vs. no thrombosis.
Aims: To identify new HPN inhibitors that reduced its protumor and prothrombotic effects in CRC cells.
Methods: We performed a virtual screening to identify potential compounds that blocked HPN active site. The most outstanding compounds were tested in a fluorogenic assay that measured HPN proteolytic activity. Then, we measured HPN inhibitors effect on CRC cell line Caco‐2 transfected with HPN‐expression vector (Caco‐2‐HPN; HPN‐overexpression) and negative‐control vector (Caco‐2; HPN‐endogenous expression). We conducted wound confluence, gelatin matrix, cell cytometry and thrombin generation assays (TGA) for measuring migration, invasion, proliferation and procoagulant cell phenotypes, respectively. Finally, zebrafish models were used to test whether HPN inhibitors interfered with distant tumor dissemination.
Results: Virtual screening highlighted suramin as the most effective HPN inhibitor, with a docking score = −12 Kcal/mol. Fluorogenic assay confirmed suramin inhibited HPN in an irreversible and dose‐dependent way (IC50 = 0.66 μM; 95%IC = 0.42–1.32). In Caco‐2 and Caco‐2‐HPN cells, suramin reduced migration and, significantly, invasion phenotypes (Figure 1A,B). These effects were more pronounced in Caco‐2‐HPN cells. Also in both cell types, suramin increased lag time and reduced endogenous thrombin potential and thrombin peak in TGA. Moreover, suramin increased time to peak and reduced velocity to peak in Caco‐2‐HPN cells (Figure 2A). Finally, suramin reduced Caco‐2‐HPN invasion to Caco‐2 levels in zebrafish (Figure 2B).
Conclusion(s): Suramin is a HPN inhibitor that reduces invasive, migratory and procoagulant characteristics of CRC cells in vitro. In addition, suramin reduces the invasion phenotype in a zebrafish model. These results point suramin as a potential therapeutic agent in CRC.


187. PB0782
187.1. Hepsin is a risk factor for thrombosis and metastasis in localized colorectal cancer patients
D. Zaragoza Huesca 1; A. Nieto Olivares2; F. García Molina3; G. Ricote Sánchez4; M. Sánchez Cánovas5; A. Martínez2; P. Garrido‐Rodríguez6; J. Peñas‐Martínez4; S. Montenegro Luis4; V. Vicente6; A. Carmona Bayonas7; I. Martínez Martínez8
1 Hospital General Universitario Morales Meseguer, Centro Regional de Hemodonación, University of Murcia, IMIB‐Arrixaca, Murcia, San Javier, Murcia, Spain; 2 Department of Pathology, Hospital Universitario Morales Meseguer, Murcia, Spain., Murcia, Murcia, Spain; 3 Department of Pathology, Hospital Universitario Reina Sofía, Murcia, Spain., Murcia, Murcia, Spain; 4 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain, Murcia, Murcia, Spain; 5 Centro Regional de Hemodonación, Department of Haematology and Medical Oncology, Hospital General Universitario Morales Meseguer, University of Murcia, IMIB‐Arrixaca, 30003 Murcia, Spain., Murcia, Murcia, Spain; 6 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain; 7 Department of Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, CIBERER, Universidad de Murcia, IMIB‐Arrixaca, Murcia, Spain., Murcia, Murcia, Spain; 8 IMIB‐Arrixaca, CIBERER, Universidad de Murcia, Murcia, Spain., Murcia, Murcia, Spain
Background: Hepsin (HPN) is a transmembrane serine‐protease which functions include extracellular matrix degradation and factor VII activation. In cancer, HPN dysregulation leads to tumor invasion by proteolysis of pericellular components. Colorectal cancer (CRC) relates to high thrombotic and metastatic risk, but HPN role is unknown.
Aims: To study correlations between primary tumor HPN expression and clinical‐histopathological variables of localized and metastatic CRC patients.
Methods: We recruited 287 patients, 169 with localized and 118 with metastatic CRC at diagnosis. Two‐millimeter diameter sections from primary tumor diagnostic biopsies were obtained to prepare tissue microarrays (TMAs), which were stained with an anti‐HPN antibody. HPN expression levels were determined by two independent pathologists, and finally, the association with clinical‐histopathological variables was assessed by chi‐square tests, One‐way Anova models and Kruskal‐Wallis tests. Time‐to‐event results were estimated using Kaplan‐Meier or competing risk‐sensitive estimators.
Results: In localized CRC patients, elevated HPN levels independently increased the incidence of thrombosis and metastasis (Figure 1). By pairwise comparison, differences were significant between high and low HPN levels for both thrombosis and metastasis (p‐value = 0.006 and 0.008, respectively). Five‐year thrombosis‐free survival was 76% (64%–89.4%, 95% CI), 89% (80%–98.6%, 95% CI) and 100% (100%–100%, 95% CI) for patients with high, medium and low HPN levels, respectively. Five‐year metastasis‐free survival was 52.9% (39.5%–71%, 95% CI), 62.6% (49.3%–79.6%, 95% CI) and 78.2% (61.8%–99%, 95% CI) for patients with high, medium and low HPN levels, respectively. Regarding metastatic CRC patients, we found HPN levels significantly increased (p‐value = 2.55E‐06) when tumor reached a moderate differentiation respecting healthy tissue, but this expression was dramatically lost when cancer reached its maximum differentiation (poorly differentiated) (Figure 2), which could indicate that HPN is necessary just for early differentiation steps.
Conclusion(s): HPN constitutes a potential biomarker for thrombosis and metastasis in localized CRC patients. Validation in a larger series could help to assess these patients.


188. PB0780
188.1. Patients with multiple myeloma have an increased prevalence of acquired platelet secretion defects and a disbalanced whole blood thrombin generation profile
M. Roest1; Y. Sang2; L. Li3; J. Remijn4; R. Fijnheer5; R. Urbanus6; D. Huskens7; J. Wan2; B. de Laat8; J. Konings 9
1 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 2 Blood Research Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA, Chapel Hill, North Carolina, United States; 3 Department of Platelet pathophysiology, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 4 Department of Clinical Chemistry. Meander Medical Center, Amersfoort, Amersfoort, Utrecht, Netherlands; 5 Meander Medical Center, Amersfoort, The Netherlands, Amersfoort, Utrecht, Netherlands; 6 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands, 7 Synapse Research Institute, Maastricht, Limburg, Netherlands; 8 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 9 Synapse Research Institute, Maastricht, the Netherlands, MAASTRICHT, Limburg, Netherlands
Background: Multiple myeloma (MM) is associated with a mild thrombocytopenia and/or anemia as well as an increased risk of thrombo‐embolism.
Aims: To determine if the disbalanced platelets and erythrocyte state play a role in the high thrombosis risk in patients with MM.
Methods: To study platelet and erythrocyte dependent haemostasis in MM patients, we conducted a case‐control study in 21 MM patients and 21 related controls and measured thrombin generation in whole blood (WB‐TG) and platelet poor plasma (PPP‐TG), and the platelet activation profiles and platelet vitality in WB. Mann‐Whitney U test was used to compare the groups. The study was approved by the Medical Ethical Committee of Maastricht University Medical Center, and patients and volunteers gave full informed consent according to the Helsinki declaration.
Results: In WB‐TG, we observed that the median time to the thrombin Peak was 52% longer in MM patients than in controls, while the median endogenous thrombin potential until the Peak (ETPp) was 39% higher in MM‐patients than in controls (Figure 1). In line with these findings, platelet response to agonists, agonist induced platelet granule release, as well as the whole blood cell counts (platelets, erythrocytes and white blood cells) were decreased in MM patients compared to controls. The plasma TG experiments showed no differences between MM‐patients and controls.
Conclusion(s): Our results show that patients with MM have a disturbed blood cell count, a disturbed platelet phenotype and a disbalanced WB‐TG profile. This disbalance may explain the paradoxical high prevalence of thrombosis in MM patients, despite low platelet counts and impaired function.
189. PB0779
189.1. Low expression of coagulation inhibitors in the immune microenvironment in precursor stages of multiple myeloma
X. Cui 1; K. Yang2; J. Myklebust3; L. Munthe3; F. Schjesvold4; G. Tjønnfjord4; B. Stavik2; P. Sandset5
1 Oslo University Hospital, OSLO, Oslo, Norway; 2 Oslo university hospital, Oslo, Oslo, Norway, 3 university of Oslo, Oslo, Oslo, Norway; 4 Oslo University Hospital, Oslo, Oslo, Norway; 5 University of Oslo, Oslo, Oslo, Norway
Background: High risk of venous thromboembolism has been observed among patients with a pre‐neoplastic phase of multiple myeloma (MM), monoclonal gammopathy of undetermined significance (MGUS), which indicates that the procoagulant state exists already before the onset of MM. Moreover, the expression of tissue factor, which is the initiator of coagulation and a key molecule in cancer‐associated thrombosis, is absent in MM cells. Altogether, this indicates that other aberrant microenvironmental cells than MM cells alone are the main trigger for MM‐associated thrombosis. Microenvironmental cells in the bone marrow (BM) play an important supportive role in regulating the pathological progression of MM. However, the role of microenvironmental cells in thrombogenesis of MM has not been explored.
Aims: To explore the potential involvement of BM microenvironmental cells in the thrombogenicity in precursor stages of MM.
Methods: The single‐cell sequencing data from Zavidij et al. (Nat Cancer, 2020) was reused. By utilizing Seurat software, we explored and compared the expression level of coagulation‐related factors in the CD45+ cells from the BM of patients with MGUS, smouldering MM (SMM), and MM as well as healthy controls.
Results: At single‐cell resolution, we found that the mRNA expression of tissue factor pathway inhibitor (TFPI) and thrombomodulin was significantly decreased in the CD45+ microenvironmental cells in the BM of MGUS, SMM and MM compared to normal BM. No difference was found between MGUS, SMM and MM. Tissue factor (TF) expression was undetectable in the dataset. Factor V expression was repressed only in SMM.
Conclusion(s): Coagulation inhibitors were transcriptionally repressed in the BM microenvironment in the very early stages of MM, which is consistent with the high risk of thrombosis observed in patients with MGUS. We suggest that procoagulant BM microenvironment could underlie the thrombogenesis in the early stages of MM.

190. Nurses and Allied Health
190.1. Nurses and Allied Health
191. VPB0234
191.1. The use of mixing tests on some prolonged coagulation tests results generated from haematology core laboratory of two tertiary hospital in Calabar. Cross River State, Nigeria
D. Okpokam 1; H. Ina1; E. Iyamah2; U. Udofia1; A. Emeribe1
1 University of Calabar, Calabar, Cross River, Nigeria; 2 University of Benin Teaching Hospital, Benin, Edo, Nigeria
Background: Mixing studies performed on prolonged samples is a very important laboratory approach to the investigation of coagulation disorders
Aims: The aim is to assess some prolonged coagulation profile samples using mixing studies.
Methods: Citrated prolonged PT/APTT samples (citrated) collected and mixed test performed by measuring the PT and APTT. Mixing an equal volume of the patient's plasma and normal pooled plasma (NPP), and repeating the PT and APTT test immediately (immediate mix) and after 1 h incubation (incubation mix) was done using Quick one‐staged method.
Results: The demographic profile shows that UCTH (54%) has the highest percentage of prolonged samples, while GHC has 46%. Male samples (50.7%) were more than females (49.3%), and the percentage age range of 51 years and above (46.7%) comprised the highest percentage followed by 26 – 50 years (39.3%) and 4 – 25 years (14%). From the samples collected, initial prolongation of PT, APTT and both were 117, 125 and 93 respectively. Therefore, out of 117 prolonged PT, 23 (19.7%) were normalized, 94 (80.3%) were still prolonged. while in APTT out of 125 initial prolonged samples, 30 (24%) were normalized, and 95 (76%) was still prolonged for immediate mix method. Whereas for the incubated mix method, out of the 117 initial prolonged PT, 75 (64.1%) were normalized, 42 (35.9%) was still prolonged. while in APTT out of 125 initial prolonged samples, 58 (46.4%) were normalized, and 67 (53.6%) was still prolonged.
Conclusion(s): Mixing test should therefore be requested by the clinicians after an initial prolonged PT or APTT and rather included in the routine laboratory coagulation investigations by the hospital management. This will help clinicians find out the cause of prolongation before commencing treatment to patients.


192. VPB0233
192.1. Development of clinical pharmacist led Anticoagulation stewardship program in cardiac care setting in developing country
S. Khan 1; M. Qureshi2; A. Jahangir2
1 Tabba heart Institute, karachi, Sindh, Pakistan; 2 Tabba Heart Institute, karachi, Sindh, Pakistan
Background: In cardiac care hospital, several medications are used that falls in category of High Alert Medications. Anti‐coagulants are drugs that are included in this list because of various drug‐drug interactions, therapeutic drug monitoring and adverse drug event associated with the use of these drugs. Upon reviewing literature, it is also evident that anticoagulants are class of medications most frequently leads to emergency room visits and hospital admissions. Pharmacist involvement in such program can significantly reduce harm to patients.
Aims: The program plan to achieve the following aims: o To ensure the safe and effective use of anticoagulants use in hospital. o To minimize the adverse drug events reported using these drugs. o To minimize the Readmissions after the inappropriate use of anticoagulants. o To improve Monitoring and education of patients on warfarin.
Methods: The data of INR from Jan2020 – June 2020 (six month) was collected from laboratory. Which shows 65% patients reported with sub therapeutic < 1.9 INR, 11% patients were identified with supra therapeutic INR > 4 and 24% population were within therapeutic range. 8 patients were admitted in Emergency due to warfarin toxicity.
Results: Based on above data a quality improvement project on development of anticoagulation stewardship program was proposed to pharmacy and therapeutic committee. The clinical pharmacist led ASP approved, in this program during admission pharmacist will review the medications for appropriateness, at the time of discharge pharmacist will be responsible for warfarin teaching and then follow up in outpatient clinic for INR monitoring. Pharmacist are closely working with cardiologist, in case of emergency contact the primary physician.
Conclusion(s): Due to the complexity of warfarin management, clinical pharmacist led ASP program will play major role in INR monitoring, reduce emergency admissions and enhancing medication compliance.
193. PB0232
193.1. Occult cancer assessment in a nurse‐led DVT clinic
M. Rees 1; R. Cloudsdale2; S. Bladen2; R. Alikhan2
1 Cardiff & Vale University Health Board, Cardiff, Wales, United Kingdom; 2 Cardiff & Vale UHB, Cardiff, Wales, United Kingdom
Background: 1 in 20 cases of unprovoked VTE are associated with and maybe the first manifestation of an occult malignancy. Current UK guidelines for patients with unprovoked VTE no longer recommend routine screening for cancer and instead advise targeted investigations based on history taking, base line investigations and basic radiology. Physical examination in the context of our outpatient DVT clinic has historically been performed by physicians, supported by clinical nurse specialists. The COVID‐19 pandemic has necessitated changed ways of working in the DVT service due to clinical pressures and redeployment of medical staff. With less in‐person medical cover, a need to develop a nurse‐led pathway for cancer assessment without routine physical examination was identified.
Aims: To develop a cancer assessment tool for history taking in the nurse‐led DVT clinic
Methods: National guidance “Suspected cancer: recognition and referral” (NICE NG12) www.nice.org.uk/guidance/ng12 reviewed to identify signs and symptoms warranting investigation and referral for suspected cancer. Grouped according to body systems (e.g. respiratory, gastrointestinal) to facilitate ease of use in a structured assessment questionnaire used by clinical nurse specialists in the DVT clinic. The tool was then incorporated into modified clerking paperwork as part of the patient’s systems review. A pathway was established to ensure appropriate referral or investigation if cancer suspected, including weekly discussion of all cases in a DVT clinic MDT meeting, arranging medical review for physical examination if indicated and regular review of the outcome of investigations.
Results: A pragmatic, nurse‐led cancer assessment tool has been successfully implemented as part of routine patient assessment for unprovoked DVT in an outpatient clinic. Ongoing audit and review is planned to ensure a high standard of patient care
Conclusion(s): The COVID‐19 pandemic has precipitated changes in clinical working practice that we as nurses have embraced to fimplement a new occult cancer assessment pathway for patients with unprovoked VTE.
194. PB0231
194.1. Improving the provision of patient information regarding venous thromboembolism prevention at King's College Hospital NHS Foundation Trust, London
G. Giron 1; E. Gee1; L. Georgiou2
1 King's College Hospital NHS Foundation Trust, London, England, United Kingdom; 2 Princess Royal University Hospital, London, England, United Kingdom
Background: All patients assessed as increased risk of Venous Thromboembolism (VTE) during hospital admission should be given verbal and written information, as recommended by NICE guidelines (National Institute for Health Care Excellence, 2018). Whilst contributing to a national VTE prevention survey (Getting it Right First Time, October 2019), sub‐optimal documentation regarding provision VTE prevention information was noted (34.4%). This prompted a quality improvement initiative to drive improvement.
Aims: The project aimed at improving provision of VTE information to patients admitted to the hospital.
Methods: A further hospital‐wide audit was undertaken in March 2020. A patient questionnaire was also designed to explore their knowledge of VTE prevention. The main actions were: implementation of a hospital‐wide training program, creation of an alert in the electronic patient record to highlight when the VTE information leaflet had not been given, active involvement in patient safety forums, hospital‐wide information dissemination (e.g. newsletter, virtual posters, VTE information stands, VTE virtual quiz), and regular surveillance of ward areas to ensure sufficient information leaflet supplies.
Results: Baseline audit revealed that 80% (n = 422/530) of patients had an electronic order for the VTE information leaflet but only 28% (n = 151/530) had documentation confirming the patient had been provided with VTE information. Patient questionnaires showed only 51% (n = 104/205) were aware of the measures to help reduce their risk of developing VTE. Repeat audit in January 2022 showed an increase in prescription of VTE patient information at 93% (n = 2771/2985) as shown in figure 1, and in delivery of patient information at 50% (n = 1500/2985) as shown in figure 2.
Conclusion(s): Regular teaching, automated electronic medical record alerts and an education program had a positive impact on the provision of VTE patient information. More work is needed to ensure all hospitalised patients receive VTE information.


195. Pediatrics
195.1. Bleeding in Neonates and Children
196. PB0236
196.1. Coinheritance of combined factor V and factor VII deficiency is associated with a mild bleeding phenotype in patient with non‐transfusion dependent thalassemia
O. Alshareef 1; B. Allahyani2; E. Alnasser2
1 King Abdulaziz Hospital, JEDDAH, Makkah, Saudi Arabia; 2 King Abdulaziz Hospital, Jeddah, Makkah, Saudi Arabia
Background: The coinheritance of FV and FVII is extremely rare. FV and FVII deficiency was common in the Saudi population. However, double heterozygous have not been described in hypercoagulable states such as thalassemia. This is a case of combined FV and FVII deficiency in a non‐transfusion‐dependent thalassemic presented with hemarthrosis.
Aims: This report aims to describe a case of combined coagulation factor V and factor FVII deficiency in beta thalassemia trait child who was presented with painful knee swelling post minor trauma.
Methods: We conducted a retrospective chart review.
Results: An 8‐year‐old boy known to have a beta‐thalassemia trait presented with painful knee swelling after mild trauma. He was pale, jaundiced, with no palpable hepato‐splenomegaly. Soft tissue ultrasound suggested hemarthrosis. Coagulation factors assays showed normal results with a notably combined deficiency of coagulation factors V and VII. During infancy, he had a history of prolonged oozing after circumcision. Since then, the child has had a history of multiple bleeding episodes post minor trauma that was controlled by fresh frozen plasma (FFP) transfusion. Moreover, with a significant history of traumatic subgaleal hematoma, he was admitted and received FFP and packed red blood cells (PRBCs). The child's parents were consequence with maternal positive beta‐thalassemia trait.
Conclusion(s): We have identified a previously unreported mild phenotypic combined FV and FVII deficiency in patients with hemoglobinopathy disorder. Hypercoagulable state in hemoglobinopathies might mitigate the bleeding phenotype of factor deficiency, though bleeding history should be thoroughly investigated in such cases.
197. VPB0252
197.1. Evaluation of guidelines for primary immune thrombocytopenia
J. Zhang
Beijing Children's Hospital, BeiJing, Beijing, China (People's Republic)
Background: Primary immune thrombocytopenia (ITP) is the most common clinical bleeding disease in children. Scientific and standardized diagnosis and treatment are of great clinical significance for children with ITP. High‐quality evidence‐based guidelines have important guiding significance for the standardized diagnosis and treatment of clinical childhood ITP.
Aims: To evaluate the quality of clinical practice guidelines related to the diagnosis and treatment of childhood primary immune thrombocytopenia.
Methods: Guided by the ADAPTE method of adapting the guideline, we searched PubMed, Embase, CNKI, Wanfang Database, VIP Database, China Biomedical Literature Database and Medlive (http://guide.medlive.cn), UpToDate (https://www.uptodate.com/contents/search), Practice Guideline Registry Platform (http://www.guidelines-registry.cn). The search time limit is from the establishment of the database to May 2020. Apply the Clinical Guideline Research and Evaluation System II (AGREE II) to evaluate the quality of the included ITP guidelines.
Results: 19 guides in the past 10 years were included and evaluated by 2 researchers using AGREE II tools. The overall quality of the 19 guides is not high. The domain called Clarity of Presentation has the highest average score of 79.68% in each domain, while the " Stakeholder Involvement" domain has the lowest average score of 23.68%. Three of the guidelines have high overall quality and scores higher than 70% in the domain called Rigour of Development.
Conclusion(s): The average quality of the included guidelines is low. At present, there is a lack of high‐quality evidence‐based guidelines for the diagnosis and treatment of childhood ITP in China.
198. PB0241
198.1. Acquired inhibitors: Two case studies in pediatrics
J. Jain 1; M. de La Maza2; L. Truscott2
1 Banner University Medical Center Tucson/University of Arizona College of Medicine, Tucson, Arizona, United States; 2 University of Arizona College of Medicine, Tucson, Arizona, United States
Background: Acquired inhibitors are rare in pediatrics and management is complex due to limited literature. Acquired inhibitors are due to post‐infectious, autoimmune, malignant, drug or idiopathic conditions and treatment options include prednisone, cyclophosphamide, rituximab and IVIG.
Aims: Report management of two cases of acquired inhibitors in pediatric patients
Methods: Literature review
Results: Case 1: A previously healthy 4‐year‐old boy presented with ecchymoses and mobility restricting edema of left extremities without hemarthrosis. His lab workup showed: hemoglobin 5.4g/dl, PTT 114s, and Factor VIII < 1% with a high titer inhibitor (35.2 Bethesda units). A secondary cause was not found. He received supportive treatment with FVIII and recombinant FVIIa. He was treated with prednisone 1mg/kg/day with normalization of his PTT and FVIII level. Our patient is now 5 months in remission off steroids. Case 2: A 12‐year‐old female previously diagnosed with refractory chronic ITP with an inconclusive autoimmune workup presented with a left psoas hematoma. Her Hgb was 7.5 g/dl, platelet 92 K/μl, PT 39s, INR 3.4, PTT 142s and mixing studies demonstrated an inhibitor. Additional workup was significant for a factor II level of 9%, elevated lupus anticoagulant and positive ANA and dsDNA. She was treated with FFP, recombinant FVIIa and Vitamin K for acute bleeding then started on prednisone 2mg/kg/day for lupus anticoagulant hypoprothrombinemia syndrome. As an outpatient, she was started on hydroxychloroquine. She is currently on a prednisone wean, now at 30mg daily with stable PTT and normal factor II. She has improving strength and sensation of her left hip and extremity and decreasing size of the hematoma 2 months after her initial presentation.
Conclusion(s): Diagnosis and management of acquired inhibitors in children is challenging due to their rarity. Management includes treating and preventing hemorrhagic complications using high dose steroids. These cases demonstrate the complexity of diagnosis and management of acquired inhibitors in the pediatric population.
199. PB0239
199.1. Pediatric primary immune thrombocytopenia: Observation in Armenia
H. Grigoryan 1; A. Avagyan2; C. Stepanyan3; S. Hovsepyan4; L. Vagarshakyan5; G. Tamamyan6; L. Hambardzumyan
1 Yerevan State Medical University after M. Heratsi, Pediatric Oncology and Hematology, Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 2 Yerevan State Medical University after M. Heratsi, Pediatric Oncology and Hematology, Yerevan, Armenia, Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 3 Yerevan State Medical University after M. Heratsi, Pediatric Oncology and Hematology, Yerevan, Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 4 Yerevan State Medical University after M. Heratsi, Pediatric Oncology and Hematology, Yerevan, Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Gyumri, Shirak, Armenia; 5 Yerevan State Medical University after M. Heratsi, Pediatric Oncology and Hematology, Yerevan, Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 6 Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Department of Pediatric Oncology and Hematology, Yerevan State Medical University, Yerevan, Armenia, Yerevan, Yerevan, Armenia
Background: Primary immune thrombocytopenia (ITP) is the most common form of thrombocytopenia in children. The incidence is 50 cases per 1,000,000 children per year.
Aims: The endpoint of this study is to identify the incidence of ITP among children receiving treatment at the Hematology center after Prof. Yeolyan, only center in Armenia, between January 2016 and December 2020.
Methods: We collected and analyzed the data of 176 children (≤18y/o) of whom 96 (54.5%) were male. The median age was 3.75 years. In the age groups less than 1, 1–5, and older than 5 years there were 31 (17.6%), 78 (44.3%) and 67 (38.1%) patients, respectively.
Results: The median platelet count was 6x10 9/L. Chronic ITP was observed in 31 (17.6%) children, persistent ITP in 14 (8%) and newly diagnosed in 131 (74.4%) patients. In the < 1‐year‐old age group newly diagnosed, persistent and chronic ITP were 28 (90.3%), 3 (9,7%) and 0 children, 1–5 years old patients 64 (82.1%), 4 (5.1%), 10 (12.8%), and >5‐year‐old patients 39 (58.2%), 7 (10.5%), 21 (31.3%), respectively. First‐line treatment was corticosteroids for 148 (84.1%) of whom 60 received Prednisolone (4 mg/kg 4–7 days) and 89 Prednisolone (2 mg/kg 2–3 weeks). Three patients received IVIG (one of them with glucocorticoids), four didn’t received any treatment and 22 (11.5%) received only symptomatic treatment (Etamsylate, Ascorbic acid). After the first course of glucocorticoids 106 patients platelet count increased (>100 × 109/L). Four patients with chronic ITP underwent splenectomy, results were satisfying and platelet count increased.
Conclusion(s): Although the cohort was retrospective, our data showed the incidence of ITP corresponds to the range reported in the literature.


200. VPB0253
200.1. An escalating treatment strategy for children with chronic immune thrombocytopenia
J. Zhang
Beijing Children's Hospital, BeiJing, Beijing, China (People's Republic)
Background: Immune thrombocytopenia (ITP) is the most common hemorrhagic disease in children, which is mainly caused by increased destruction and decreased production of autologous platelets due to abnormal autoimmune tolerance. The current international guidelines recommend that the second‐line therapy drugs for children with chronic ITP are thrombopoietic agents (TRAs) and rituximab. Efficacy of combination therapy has been reported. Our center used the escalating treatment strategy, including three steps: Step I (high‐dose dexamethasone), Step II (rituximab), and Step III (eltrombopag).
Aims: The purpose of this study was to confirm the efficacy of escalating treatment strategy in the treatment of childhood CITP.
Methods: This was a single‐center, retrospective cohort study. Chidren were divided into 2 groups according to the treatment regimen: the group A (escalating treatment strategy) and the group B (eltrombopag). We evaluated the therapeutic effect after 12 months of follow‐up.
Results: A total of 58 cases (30 males and 28 females) were included. Overall response was achieved in 72.41% of patients in the group A vs 68.97% in the group B, and the complete response (CR) rate was 34.48% in the group A compared with 20.69% in the group B. There was no difference between two groups (p>0.05).
Conclusion(s): This study suggests that escalating treatment strategy can achieve the similar efficacy as TRAs. It is an effective, safe and second‐line treatment scheme suitable for Chinese patients. It is still necessary to further evaluate the feasibility of shortening the course of treatment and reducing the burden of treatment.
201. VPB0249
201.1. Persistent autoantibodies against Factor XI in a girl with an inborn error of immunity
E. Annetta 1; M. Hepner2; J. Frontroth3; C. Cervio3; R. Sueldo4; V. Goris5; M. Oleastro6; G. Sciuccati7
1 Hospital de Pediatría Prof Dr JP Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Laboratorio de Hemostasia y Trombosis. Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Laboratorio de Hematología. Hospital Dr. César Milstein, Hospital de Pediatría Juan P. Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Laboratorio de Biologia Molecular Inmunologia. Hospital de Pediatría Prof Dr JP Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 6 Servicio de Inmunologia y Reumatologia. Hospital de Pediatría Prof Dr JP Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 7 Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: FXI inhibitor in patients without congenital deficiency is a rare acquired disorder, characterized by the presence of neutralizing autoantibodies, usually IgG, targeting functional epitopes of FXI. They are mainly associated with autoimmune diseases.
Aims: To describe a persistent autoantibody against FXI in a girl with a probable inborn error of immunity (IEI)
Methods: A 6‐year‐old girl with an IEI under evaluation was referred to the Hematology Department because of an abnormal aPTT detected during pre‐operative coagulation screening for an elective tonsillectomy. The patient had history of recurrent infections and non‐malignant lymphoproliferation. She is the only‐child of healthy unrelated parents, with neither personal nor family history of bleeding. Laboratory evaluation included complete blood count, coagulation screening tests, lupus anticoagulant (LA), coagulation factors measurement, mixing studies, Bethesda inhibitor assay and plasma IgG adsorption with protein A Sepharose (pAS).
Results: Haemostasis screening showed a markedly prolonged aPTT (ratio:2.53). The index of circulating anticoagulant (29.6%) revealed the presence of an inhibitor. LA was negative, FXI:< 1%, Bethesda titer for FXI inhibitor:12.4BU/ml. Plasma IgG adsorption with pAS fully removed detectable inhibitor (<0.6 BU/ml) (Table 1) Parental coagulation profiles were normal. The patient received a single dose of rFVIIa 30 μg/kg before tonsillectomy and tranexamic acid before and after this procedure. No bleeding complications were observed. FXI persisted <1% during the three years follow‐up and a novel CARD11 heterozygous variant was identified (c.746A>C) (functional analyses in progress)
Conclusion(s): We report a child with acquired factor XI deficiency probably related to an IEI. To our knowledge, there are no pediatric reports of long‐lasting neutralizing anti‐FXI antibodies in patients with an IEI. This scenario may be an expression of the underlying immune dysregulation. Results of adsorption with pAS strongly suggest that these antibodies are IgG isotype. The identification of a neutralizing FXI inhibitor allowed for an accurate management of the surgical procedure.

202. PB0245
202.1. Series review of fetal/neonatal alloimmune thrombocytopenia diagnosis, management and outcome in a tertiary care centre
C. Santos 1; L. Costa2; D. Leão2; O. Graça2; F. Monteiro3; F. Correia2; C. Koch4
1 Centro Hospital e Universitário São João, Porto, Porto, Portugal; 2 Center of Thrombosis and Haemostasis, Department of imunohemotherapy, Centro Hospital e Universitário São João, Porto, Porto, Portugal; 3 Center of Thrombosis and Haemostasis, Department of imunohemotherapy, Centro Hospital e Universitário São João, Porto, Portalegre, Portugal; 4 Center of Thombosis and Haemostasis, Department of Immunohemotherapy, Centro Hospitalar e Universitário São João, Porto, Porto, Portugal
Background: Fetal/neonatal alloimmune thrombocytopenia (FNAIT) is due to passively acquired maternal antibodies directed against paternal human platelet antigens (HPA) inherited by the newborn, affecting 1:1000 neonates. Intracranial haemorrhage is a major concern (1:10.000 neonates). In Caucasians, alloimmunization to HPA‐1a is involved in 85% of FNAIT. Other antibodies against HPA are rare and its frequencies varies in different ethnic populations.
Aims: Our aim was to diagnose neonatal alloimmune thrombocytopenia and evaluate treatment options and outcomes.
Methods: FNAIT was suspected in 12 cases of fetal intracranial hemorrhage, and/or neonatal nadir platelet count <100.000/μl at birth or within seven days after birth. Antibodies against platelet in maternal serum/plasma were screened. Genetic polymorphisms for HPA‐1,‐2,‐3,‐5 were studied using in house real‐time PCR. Maternal thrombocytopenia was excluded in all cases. Therapeutic interventions and outcomes were also evaluated.
Results: Our results confirmed FNAIT in 5/12 of suspected cases. Results are depicted in Table 1. As expected, the majority were due to anti‐HPA‐1a (n = 3), one of which presented both anti‐HPA‐1a and anti‐HPA‐2b. We found two cases of rare antigen discrepancies: anti‐HPA‐1b (n = 1) and anti‐HPA‐2b (n = 1). The HPA‐2b discrepancy was found in an African‐descendent family. Neonates were treated with human immunoglobulin and random donor platelets transfusions when needed (Table 1). All of them had favorable outcomes.
Conclusion(s): FNAIT is an infrequent entity, probably underdiagnosed. Despite its small size, this series highlights the relevance of a prompt diagnosis and intervention. Correct diagnosis impacts the subsequent couples’ offspring as it allows an adequate future pregnancy planning.

203. PB0244
203.1. Current practice of monitoring joint health in children and adolescents with hemophilia in Germany, Austria and Switzerland (GAS)
M. Reschke 1; C. Königs2; N. Marquardt3; M. sigl‐Krätzig4; S. Holzhauer5
1 University Medicine, Charite, Berlin, Germany, Berlin, Berlin, Germany; 2 University Hospital Frankfurt, Goethe University, Frankfurt, Hessen, Germany; 3 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Medical Faculty, University of Bonn, Germany, Bonn, Nordrhein‐Westfalen, Germany; 4 IPFW (Institute for Paediatric Research and Further Education) and, Haemophilia Treatment Centre for Children and Adolescents, Blaubeuren, Baden‐Württemberg, Germany, Blaubeuren, Baden‐Wurttemberg, Germany; 5 Charité University Medicine, Charite, Berlin, Germany, Berlin, Berlin, Germany
Background: Treatment options for children with hemophilia have greatly improved, new treatment options, including gene therapy, emerged throughout the last years protecting from premature arthropathy and allowing an active life. Ultrasound scoring systems have been established1,2,3 as point of care methods for routine assessment of joint health to guide therapy or assess outcomes in clinical trials.
Aims: To gain information on techniques currently available and used to assess joint health including standardized physiotherapeutic examinations, MRI or ultrasound. To understand what prevents physicians to use ultrasound to regularly screen for arthropathy.
Methods: We electronically sent 17 questions to all members of the Pediatric working group of the Society of Thrombosis and Hemostasis (GTH).
Results: 24 physicians of at least 19 different pediatric hemophilia centers answered all questions. All physicians used several examination methods to assess joint health, 54% used standardized physiotherapist exams, 50% performed ultrasound and/or MRI. Most physicians performed those examinations based on clinical symptoms (n = 13; 54%), not on a regular basis. The most commonly used ultrasound score was HEADUS (n = 10/12 performing ultrasound; 83%). 50% of physicians do not use standardized ultrasound examinations. Ultrasound examinations are mostly carried out by radiologists or treating physicians (n = 12; 50%) and (n = 11; 46%) respectively. Reasons limiting use of ultrasound were incomplete training and limited resources (time, space, physicians). 50% of physicians see a need for more education on the subject. Symptoms were rated most influential for making therapy decisions, ultrasound and MRI examinations were deemed less important.
Conclusion(s): Standard of care to monitor joint health in children with hemophilia in GAS is still physical examination. Up to now, implementation of ultrasound as a regular screening tool is hampered by lack of training.
204. PB0247
204.1. Clinical report of five young patients (3–26 months of age) with moderate or severe hemophilia A receiving emicizumab
I. Wieland 1; L. Hinze2; K. Lambeck1
1 Hanover Medical University, Hannover, Niedersachsen, Germany; 2 Medizinische Hochschule Hannover, Hannover, Niedersachsen, Germany
Background: Emicizumab is the first approved non replacement therapy for bleeding prophylaxis in hemophilia A (HA) patients. Although emicizumab was approved for all age groups, data is limited for young children (< 2 years).
Aims: Experience in young children
Methods: Here, we present the clinical course of 5 patients with HA treated with emicizumab.
Results: Four patients with a severe HA started with emicizumab treatment in an age range of 3 to 12 months. Three of them were minimal treated patients with 2 to 7 factor VIII (FVIII) exposure days (EDs) prior start of emicizumab. One patient switched to emicizumab after having previously received 29 EDs of FVIII during prophylaxis. The follow up time of our patients was 1 to 23 months. No spontaneous bleedings occurred during this time. For bleeding prevention due to head injuries, or for factor VIII immune tolerance purposes, these patients received additional single doses of FVIII (9–16 EDs). Of note, the patients did not develop an inhibitor after 2 to 53 doses of FVIII. By contrast, the fifth patient was a 28 months old boy with moderate HA (FVIII 2%–4%) and reduced von Willebrand parameters (39%–50%). Due to several traumatic bleeding requiring treatment with 15 FVIII substitutions within 16 months, we first decided to start a standard prophylaxis. Due to a complicated venous access, a prophylaxis with this regimen was not possible. We thus requested an off‐label use of emicizumab, and received the approval for treatment. After start of emicizumab, no additional FVIII substitutions were necessary.
Conclusion(s): To conclude, in four patients with severe HA and one patient with moderate HA, emicizumab was well tolerated and safe. There was no occurrence of spontaneous bleedings. All FVIII substitutions were performed following head injuries as single doses, or were given for FVIII immune tolerance. The parents’ satisfaction upon emicizumab treatment was high.
205. PB0237
205.1. Bleeding assesment in children with Noonan syndrome
A. Barg 1; E. Avishai2; S. levy1; y. yeshayahu1; T. Livnat2; G. Kenet3
1 Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 2 Sheba medical center, Tel Aviv University, Ramat Gan, HaMerkaz, Israel; 3 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel
Background: Noonan syndrome (NS) is an autosomal dominant disease involving multisystem clinical aspects including a bleeding tendency. The exact nature of the hemostatic impairment in NS has not been elucidated. Various hematological abnormalities have reported including impairment of platelet number and function as well as clotting factor deficiencies and von Willebrand disease. Hemostatic evaluation by global coagulation assays has not been reported.
Aims: To assess clinical aspects and laboratory parameters of hemostasis in a cohort of children diagnosed with NS, including application of thromin generation (TG) assays.
Methods: Clinical bleeding tendency was evaluated by the ISTH bleeding assessment tool (BAT). Hemostatic assessment included platelets number, Platelets function by light transmission aggregometry (LTA). PT, aPTT, Fibrinogen, Von Willebrand factor antigen, Ristocetin Cofactor, FVIII levels and TG parameters (peak height and endogenous thrombin potential‐ ETP in platelet rich plasma (PRP).
Results: Twenty patients (10 males) whose median age at evaluation was 6.5 years IQR (3–13) years composed our NS cohort. The most common pathogenic genetic variants were in PTPN11 and KRAS. Four patients (20%) had an elevated BAT score. Platelet number, PT and aPTT were within normal range for the entire cohort. Five patients had lower than normal Fibrinogen levels. Reduced levels of VWF antigen and activity were detected in three patients, two of which had pulmonic stenosis. Platelet aggregation studies as evaluated by LTA were impaired in half of the cohort mostly in response to Epinephirine. Median peak height 136 nM IQR (108–153) and median ETP 1657 (nM × min) IQR (1511–2026) levels were lower as compared to healthy controls.
Conclusion(s): Impaired hemostasis may commonly be encountered among individuals with NS. The most common abnormality is impaired platelet aggregation. NS patients especially, those with pulmonic stenosis, should be evaluated for VWD. The clinical association of TG with patients' bleeding phenotype should be further investigated.
206. PB0242
206.1. Monitoring of hemostasis and heparinization of neonates undergoing cardiac surgery (CS) using viscoelastic testing (VT) and standard laboratory test (SLT)
M. Lopez 1; P. Rossi2; T. Vainstein2; B. Diaz3; L. Barrera3; M. Martinuzzo4
1 Hematology and hemostasis division, Central Laboratory, Hospital Italiano de Buenos Aires, Argentina., Ciudad Autonoma de buenos aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Anesthesia department, Hospital Italiano de Buenos Aires, Argentina, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hematology and hemostasis division, Central Laboratory, Hospital Italiano de Buenos Aires, Argentina., Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Hematology and hemostasis division, Central Laboratory, Hospital Italiano de Buenos Aires, Argentina., Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: Adequate management of heparinization and hemostasis in neonates undergoing CS with cardiopulmonary bypass is challenging.
Aims: To track the effect of heparin and CS in neonates weighing <5 kg using VT and SLT.
Methods: Neonates weighing <5 kg undergoing CS were included. Samples were obtained at the start of surgery (Basal), before separation from bypass (Bypass) and after heparin reversal (Postprotamine). VT performed by ROTEM delta (Instrumentation Laboratory, IL). SLT: PT% activity (PT%), APTT, thrombin time (TT), antithrombin (AT) and anti‐factor Xa activity (AntiXa) by IL reagents in ACL TOP coagulometer. HemoCell CXH800 (Beckman Coulter) for PLT. Cumulative 12 h chest tube drainage (CTD) was assessed for postsurgical bleeding.
Results: 32 patients were included. Median (IRQ) age was 19 days (6–25), median weight was 3380 g (3082–3785). Bypass samples showed significantly prolonged EXTEM, INTEM and FIBTEM CFT and CT, as well as lower amplitude for A5, A10 and MCF when compared to Basal and Postprotamine. PT% decreased, and APTT increased in Postprotamine compared to Basal. PLT were lowest during bypass. Median basal AT was 0.56 IU/ml. AntiXa median (IQR) was 5.3 (4.1–6.9) during Bypass. Postprotamine AntiXa was 0.1 (0.03–0.33), 9/32 patients with >0.2 U/ml. AntiXa correlated with EXTEM‐CT and ACT during bypass, and with TT in Postprotamine. Postprotamine ACT correlated with PT, APTT, INTEM and HEPTEM CTs, and HEPTEM/INTEM CT ratio (Table 1). Postprotamine AntiXa and ACT did not correlate. HemoCell CXH800 (Beckman Coulter) for PLT. Median CTD was 50 ml (28–91). CTD correlated with Postprotamine AntiXa and TT, and with total heparin dose administered (Table 2).
Conclusion(s): ROTEM results were most affected during bypass, when hemodilution and consumption of procoagulant factors, fibrinogen and platelets are expected to occur. AntiXa was the only test that tracked heparinization at all stages of surgery. Residual heparin measured by AntiXa correlated with postoperative bleeding.


207. VPB0251
207.1. An analysis of factors influencing survival of bleeding patients during first two years of life
S. Sharma 1; D. Padhi2; D. Sahu2; R. Sharma2; A. Goel3
1 Transfusion Medicine, Raipur, Chhattisgarh, India; 2 AIIMS Raipur, Raipur, Chhattisgarh, India; 3 AIIMS raipur, Raipur, Chhattisgarh, India
Background: Plasma, platelet transfusion threshold are difficult to interpret in early childhood due to developmental differences and pathogenesis of disease as compared to adults. Clinical diagnosis such as ongoing sepsis can interfere with transfusions and subsequent patient prognosis.
Aims: Aims of present study were as follows: 1. Gestational age and APGAR score effect patient survival in Plasma and/or Platelet transfused < = 2‐year hospital admissions. 2. Children (< = 2years) with sepsis have an adverse prognosis with platelet and/or plasma transfusions.
Methods: All Calculations were performed on 2021 Minitab software. Patient age, weight, gestational age; Length of stay (LOS) were correlated with death. Gestational age (< = 34 weeks;>34 weeks) (n = 72) and APGAR score 5 min (< = 7;>7) (n = 62); Preterm; term children and sepsis with remaining diagnoses for death were evaluated by Binary logistic regression (Odds ratio).
Results: In this observational study (n = 104), median age (SD) months 1.0(IQR 6.9); (< = 1 month, n = 60, >1 month, n = 44); median LOS 11.0d (16.0d). Preterm {0.23(IQR 0.67 months);(n = 33)};term {3.0 (IQR 11.7 months) (n = 73)}; median gestational age 38.0 (n = 72){(IQR 6.0weeks);(< = 34 weeks, n = 23);(>34 weeks, n = 49)} respectively. A total of 47 deaths with 57 survivors. Median LOS was 11.0(IQR 16.0)days. Age, weight were not significant in correlation (−0.031; −0.219; p = 0.74; p = 0.19); gestational age < = 34 weeks (−0.23; p = 0.013); Apgar score 5 min (−0.35; p = 0.005) and LOS (−0.23; p = 0.018) correlated significantly with death. Odds ratio of Gestational age (< = 34 wk; >34) 2.21; CI (0.7 – 6.4), p = 0.14; APGAR score 5 min (< = 7; >7) 12.3; CI (0.9; 160.4); p = 0.054 and Preterm at birth was 2.2(0.8–5.4); p = 0.087 with death. Sepsis compared to remaining diagnosis had Odds ratio of 10.3; CI (3.8, 27.8); p < 0.01 for death during hospital stay.
Conclusion(s): 1. Prognosis of plasma and platelet transfusions in newborn is influenced by gestational age and Apgar score. 2. Sepsis patients are resistant to platelet and/or plasma therapy with worse prognosis as compared to patients without clinical sepsis.

Compar
Probability Plot Gestational Age and APGAR (5 min)

208. PB0238
208.1. Tapering of trombopoietin receptor agonist in pediatric patients with chronic ITP. Is it posible?
R. Berrueco 1; M. Solsona2; E. Sebastián3; A. Cervera4; A. Sastre5; I. Astigarraga6; B. Argiles7; M. Dasí7; J. Dapena8; E. Monteagudo7
1 Hospital Sant Joan de Déu, Esplugues Llobregat, Catalonia, Spain; 2 Hospital Sant Joan de Déu, Esplugues de Llobregat, Catalonia, Spain; 3 Hospital Universitario Niño Jesús., Madrid, Madrid, Spain; 4 Hospital Universitario de Móstoles, Madrid, Madrid, Spain; 5 Hospital La Paz, Madrid, Madrid, Spain; 6 Hospital Universitario Cruces, Bilbao, Pais Vasco, Spain; 7 Hospital Universitario La Fe, Valencia, Comunidad Valenciana, Spain; 8 Hospital Sant Joan de Déu, Barcelona, Catalonia, Spain
Background: Treatment approach to chronic immune thrombopenia (ITPc) in pediatric patients has changed during the last decade thanks to the development of thrombopoietin receptor agonist (ar‐TPO). During the last decade, series in adult patients have described that up to 30% of patients with ITPc on ar‐TPO treatment may have a sustained platelet response after drug withdrawal, but there is still scarce information in children.
Aims: To describe tapering of ar‐TPO in ITPc pediatric patients treated in Spanish hospitals.
Methods: A multicenter retrospective observational study was performed in children with chronic immune thrombopenia (ITPc) to describe ar‐TPO tapering and withdrawal in patients who had achieved a sustained complete response to ar‐TPO.
Results: Ten pediatric patients with ITPc were included. Table 1 resumes patients characteristics and information regarding ar‐TPO tapering. None of them required relevant dose increasing during this process. One case had an asymptomatic relevant thrombopenia (< 30x109/L), but platelet count increased progressively without the need of dose escalation. After a median time of 24 months (SD 8.7), two patients relapsed (figure 1). One of them achieved a new complete response with ar‐TPO. The other was refractory to ar‐TPO and immunosuppressants but responded to rituximab. Three patients had a transient decrease of total platelet count (34–72 × 109/L) that improved without treatment administration.
Conclusion(s): This series shows that tapering and withdrawal of ar‐TPO treatment can be considered in pediatric patients with ITPc who have achieved a sustained complete response. Our experience suggests that discontinuation should be progressive and slow.


209. PB0235
209.1. Diagnosis, predisposing factors, and management of disseminated intravascular coagulation in neonatal intensive care unit in a tertiary care hospital
R. Akbar
Cantonment General Hospital Rawalpindi Pakistan, Rawalpindi, Punjab, Pakistan
Background: Neonates are at a greater risk of developing Disseminated Intravascular Coagulation (DIC) because they are born with slightly altered hemostasis due to developmental coagulopathy. Because of the serious nature of this disease, it is important to diagnose and manage DIC in a timely manner.
Aims: This study was aimed at determining the diagnosis, predicting factors, and management of DIC in neonatal population admitted to NICU at our tertiary care hospital.
Methods: Neonates admitted to NICU at Cantonment General Hospital (CGH) from Jan 2019 to Dec 2021 were included in the study. The standard method of diagnosing DIC was based on sequential testing of the components of the ISTH scoring system (ie, prothrombin time [PT], platelet count, fibrinogen, and D‐dimer). Patients' clinical notes were checked for predisposing factors and information was recorded on the proformas. Logistic regression analysis was done to assess the ability of the independent variables, such as PT, D‐dimer, platelet count, and fibrinogen, to predict overt DIC according to the ISTH scoring system.
Results: Out of a total of 1365 neonates admitted to NICU at CGH, 180 (13%) were diagnosed as having DIC with a score of ≥5 based on ISTH criteria of diagnosing overt DIC. DIC in neonates was caused by prenatal risk factors, such as placental abruption (5%), pregnancy‐induced hypertension (13%), and neonatal factors such as sepsis (51%), prematurity (20%), and asphyxia (11%). Treatment of DIC was mostly carried out by administering fresh frozen plasma (FFP), platelet transfusion, and treatment of underlying cause including administering antibiotics. Despite rigorous treatment, 29 patients ( 16%) died due to uncontrolled DIC.
Conclusion(s): In conclusion, the present study demonstrates that various factors are associated with neonatal DIC at birth, with sepsis among the most significant of these factors. Furthermore, FFP and platelet transfusion therapy are effective for treating neonatal DIC.

210. PB0240
210.1. Improving discussion of menstrual cycle and bleeding during well‐child visits at a pediatric residency clinic
A. Jackson 1; A. Yamasaki2; E. Parker3; A. Close2
1 Spectrum Health/Helen DeVos Children’s Hospital/ Michigan State University, Grand Rapids, Michigan, United States; 2 Spectrum Health/ Helen DeVos Children’s Hospital/ Michigan State University, Grand Rapids, Michigan, United States; 3 Spectrum Health/ Helen DeVos Children’s Hospital/Michigan State University, Grand Rapids, Michigan, United States
Background: Menstrual health is not consistently documented by pediatric residents in well‐child examinations. Average cycle blood loss is 30–40 ml or use of 3–6 soaked pads or tampons per day. Abnormal uterine bleeding (AUB) is defined as any aberration of menstrual volume, regulation, duration and/or frequency in a non‐pregnant woman. The leading cause of AUB is immature hippocampal‐pituitary axis followed by bleeding disorders in adolescents. Literature notes that pediatricians document screening for coagulopathy in patients with AUB with one follow‐up question during 26% of encounters.
Aims: Improve menstrual cycle screening in pediatric well‐child visits.
Methods: Pediatric residents were surveyed to assess menstrual screening of cycle regularity, duration, and heavy bleeding. Responses were recorded on likert scale; 1 (never) to 5 (always). A screening tool for AUB was integrated into the EMR template for well‐child visits to document cycle regularity, duration, and frequency of product change. Residents received education on menses and AUB. Surveys were repeated at 3 and 6 months to assess retention. One‐sided Mann‐Whitney U tests with a Bonferroni correction for multiple comparisons were used to test for improvements in scores for each question comparing baseline to 3‐month and to 6‐month.
Results: Reported frequency of asking cycle regularity increased over the 6‐month period (p = 0.0118) with the median rating increasing from 4 (3, 5) to 5 (4, 5). Reported asking last menstrual period (LMP) also improved from 4 (3, 4.5) to 5 (4, 5)(p = 0.0049). Reported asking about clots/gushing improved over 6 month period from 2.5 (2, 3) to 3 (3, 4)(p = 0.0208) and improvement in asking frequency of menstrual product change from 3 (3, 4) to 4 (4, 5) (p = 0.0181).
Conclusion(s): Utilizing a menstrual screener in note templates improves resident discussion of cycle regularity, LMP, clots, and product usage during well‐child visits.


211. PB0246
211.1. Validation of Thai pediatric bleeding assessment tools mobile application for diagnosis of pediatric bleeding disorders
A. Sermcheep 1; N. Sirachainan2; D. Songdej3; A. Chuansumrit4; P. Wongwerawattanakoon5
1 Ramathibodi hospital, Mahidol university, Prawet, Krung Thep, Thailand; 2 Ramathibodi hospital, Mahidol university, Ratchathewi, Krung Thep, Thailand; 3 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Krung Thep, Thailand; 4 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Nakhon Pathom, Thailand; 5 Ramathibodi hospital, Mahidol university, Bangkok, Krung Thep, Thailand
Background: Mild bleeding disorders such as von Willebrand disease (vWD) and other platelet disorders are usually underdiagnosed due to minor signs and symptoms. In 2017, the Thai‐pediatric bleeding assessment tool (Thai‐pediatric BAT) was translated and validated. It is now used for screening bleeding disorders. Its limitation is that the BAT is designed for medical personnel; therefore, it requires additional time during patients' visits. Mobile application for self‐screening Thai‐pediatric BAT may be useful for screening, especially in community.
Aims: To develop and validate self‐screening mobile application for bleeding disorders in children.
Methods: After inform consent, Thai pediatric‐BAT mobile application was firstly developed. Children with known bleeding disorders were interviewed using the paper‐based BAT and mobile application at least two weeks apart. Pearson correlation coefficient was used for analysing relations between paper‐based BAT and mobile application. The cut‐off bleeding score of mobile application was calculated to predict bleeding disorders.
Results: After mobile application was developed by using simple sentences with additional video, pictures, and auto‐calculation. The Thai pediatric‐BAT mobile application showed a strong correlation with paper‐based BAT. The correlation coefficient (r) is 0.956 (P < 0.001) in PBQ and 0.970 (P < 0.001) in ISTH scoring system. The score equal or more than three (≥3) was considered abnormal. The PBQ bleeding score ≥3 had a sensitivity of 90.9%, a specificity of 94.7%, a positive predictive value of 94.6% and a negative predictive value of 87% (P < 0.001). The ISTH bleeding score ≥3 had a sensitivity of 90.9%, a specificity 93.93%, a positive predictive value of 93.3% and a negative predictive value of 93.3% (p < 0.001).
Conclusion(s): Thai pediatric‐BAT self‐screening mobile application was proven to have a strong relation with the paper‐based BAT version. Further study regarding the effectiveness of mobile application is warranted. Therefore, this application is a convenient and effective self‐screening tool for increasing diagnosis of pediatric bleeding disorders.
212. PB0248
212.1. Prevalence of iron deficiency anemia in children with hemophilia
S. Zarb 1; M. Bhatt2; K. Decker3; J. Ewusie4; A. Chan4
1 McMaster University, Toronto, Ontario, Canada; 2 McMaster Children's Hospital, Hamilton, Ontario, Canada; 3 Hamilton Health Sciences, McMaster University Medical Centre, Hamilton, Ontario, Canada; 4 McMaster University, Hamilton, Ontario, Canada
Background: Prevalence of iron deficiency (ID) and iron deficiency anemia (IDA) in children is 16%–20% and 3%–5%, respectively. While several risk factors have been associated with increased risk, prevalence of ID/IDA has not been studied in children with hemophilia.
Aims: To determine prevalence of ID/IDA in children with hemophilia and analyze whether hemophilia type/severity or bleeding frequency are associated with increased risk.
Methods: Retrospective chart review of children (< 18 years) with hemophilia from 2016 to 2021. Data collection included: demographics, hemophilia type/severity, annualized bleeding rate (ABR), and bloodwork for ID/IDA. Ferritin < 30μg/L was classified as ID and < 15μg/L as severe ID. Unadjusted and adjusted odds ratios with 95% CI were calculated for predictors using generalized linear‐mixed model.
Results: Among 90 patients included, 73(81.1%) had hemophilia A and 17(18.9%) hemophilia B. Severity was mild in 33(36.7%), moderate in 8(8.9%), and severe in 49(54.4%). Ferritin was checked in 76(84.4%) patients. Among these, 62(81.6%) and 56(73.7%) had ID and IDA, respectively, after median follow‐up of 4 years (range: 1–14 years). Prevalence of severe ID and IDA was 36(47.4%) and 30(39.5%), respectively. For ID, age < 6 years (OR 3.83, 95% CI 1.88–7.82) was associated with higher prevalence in unadjusted and adjusted analysis (Figure 1). Hemophilia type/severity and preceding year ABR were not associated with ID. For IDA, age < 6 years (OR 33.52, 95% CI 10.09‐>99.9), age 6‐< 12 years (OR 4.69, 95% CI 1.50–14.68), and ABR in preceding year (OR 1.41, 95% CI 1.15–1.73) were associated with higher prevalence in unadjusted analysis, while only age remained significant in the adjusted analysis (Table.1). Hemophilia type/severity were not associated with IDA.
Conclusion(s): Prevalence of ID/IDA is increased in children with hemophilia. Younger age and ABR are associated with increased risk. Further prospective studies are required to validate these findings and determine whether screening for ID/IDA would be helpful in some or all children with hemophilia.

213. PB0243
213.1. Validation of the PLTEMA10 in Cardiac Surgery (CS) neonatal population weighing less than 5 kg
M. Lopez 1; F. Mileo2; P. Rossi2; T. Vainstein2; L. Barrera3; M. Martinuzzo4
1 Hematology and hemostasis division, Central Laboratory, Hospital Italiano de Buenos Aires, Argentina., Ciudad Autonoma de buenos aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Anesthesia department, Hospital Italiano de Buenos Aires, Argentina., Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hematology and hemostasis division, Central Laboratory, Hospital Italiano de Buenos Aires, Argentina., Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Hematology and hemostasis division, Central Laboratory, Hospital Italiano de Buenos Aires, Argentina., Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: PLTEM is a useful parameter to directly assess the platelet component in clot formation in CS patients. In previous study, PLTEM cut‐off values were calculated for platelet counts (PLT) of 50000 and 100000/mm3 in pediatric patients.
Aims: To validate cut‐off values, sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively) of the PLTEM in neonates weighing <5 kg undergoing CS for PLT of 50000 and 100000/mm3.
Methods: Population: consecutive neonate CS patients (period: October 2020‐September 2021). Samples were drawn at Basal (B), before termination of extracorporeal circulation (ECC) and after administration of protamine (PostProtamine). Thromboelastometry ROTEM® delta (Instrumentation Laboratory, IL) results were used to calculate PLTEM: EXTEM amplitude at 10 min (A10) minus FIBTEM A10. PLT: HemoCell CXH800 (Beckman Coulter) Statistical evaluation: SPSS 23 software.
Results: 32 patients. Age: median (IQR) 19 (6–26) days; Weight: median 3350 (3055–3785) g. Significant positive correlation between PLTEMA10 and PLT was observed at all surgical stages (Table 1), reaching a plateau at normal PLT (Image 1). This correlation was strongest at lower PLT. Sensitivity, specificity, PPV and NPV were calculated for two different PLT cut‐off values: <100000/mm3 and <50000/mm3 considering two levels of fibrinogen (FIBTEMA10 <10 and >10 mm), all patients with hematocrit >30% (Table 1). Very high NPV (all >95%) were observed regardless of PLT or FIBTEMA10, while for PPV, higher values were observed when PLT <100000/mm3, and slightly lower for PLT <50000/mm3 (Table 1).
Conclusion(s): Previously established PLTEMA10 cut‐off for the pediatric population was validated in <5 kg neonates undergoing CS. Results show that PLTEM is useful to estimate patient's PLT and detect thrombocytopenia during CS, with good sensitivity, specificity and NPV. Furthermore, the performance is better in more severe thrombocytopenia (PLT <50000/mm3). Timely detection of thrombocytopenia may allow early intervention as well as avoidance of unnecessary platelet transfusion.


214. VPB0250
214.1. Heme regulates inflammatory signaling pathways in human choroid plexus epithelial cells after preterm intraventricular hemorrhage
Z. Fejes 1; M. Pócsi2; Á. Gyetvai2; S. Póliska2; A. Tóth2; E. Balogh2; Á. Rusznyák2; F. Fenyvesi2; V. Jeney2; J. Kappelmayer2; B. Nagy2
1 Faculty of Medicine, University of Debrecen, Debrecen, Hungary, Debrecen, Hajdu‐Bihar, Hungary; 2 University of Debrecen, Debrecen, Hajdu‐Bihar, Hungary
Background: Upon preterm intraventricular hemorrhage (IVH), released heme can induce inflammation, cell activation, and dysfunction of choroid plexus epithelial cells of the ventricles. We have recently reported that coculturing of cerebrospinal fluid samples derived from these patients with human choroid plexus epithelial cells (HCPEpiCs) provoked inflammatory cellular response in vitro causing altered expression of some selected miRNAs.
Aims: In this study, our aim was to further investigate the genetic background of heme‐induced inflammatory mechanisms in HCPEpiCs.
Methods: To observe the direct effect of hemorrhage, HCPEpiCs were exposed to heme (25 μM) or TNF‐α (100 ng/ml) as a classic pro‐inflammatory agonist for 24 h. The consequence of heme treatment on mRNA expression was investigated by RNA‐seq. In parallel, we profiled miRNAs using TaqMan OpenArray panels and Cytoscape‐ClueGO was used for functional enrichment analysis. Concentrations of released proteins in cell cultures were measured by ELISA, while the activation of NF‐κB pathway was observed by fluorescence microscopy.
Results: Similarly to TNF‐α, heme induced the NF‐κB pathway via nuclear translocation of the p65 subunit. As a result, there were 231 significantly upregulated, and 106 attenuated mRNAs in heme treated HCPEpiCs compared to untreated cells. Among of the top 50 differentially expressed genes HMOX1, IL8, IL1B and ICAM1 mRNAs were upregulated in association with several mechanisms, such as cell response to cytokine activity and chemical stress, TLR4 mediated signaling and regulation of apoptosis based on ClueGO analysis. Furthermore, significantly elevated concentrations of IL‐8 and ICAM‐1 were detected in cell culture supernatants. Out of 358 detected miRNAs, 25 significantly decreased (e.g. miR‐223, miR‐215 and miR‐9), and 10 were upregulated (e.g. miR‐181c) in stimulated cells, which participate in the development of oxidative stress‐induced cell death.
Conclusion(s): In preterm IVH, heme has important regulatory functions in choroid plexus epithelial cells that may contribute to the development of clinical complications in affected infants.
214.2. Thrombosis in Neonates and Children
215. VPB0283
215.1. Deep venous thrombosis in a child with Down syndrome as a presenting manifestation of acute lymphoblastic leukemia
A. Jovanovska 1; K. Martinova2; S. Kocheva2; Z. Trajkova‐Antevska2; K. Kuzevska‐Maneva2; B. Gjurkova‐Angelovska2
1 University Children's Hospital, Medical Faculty, University„Ss. Cyril and Methodius“ Skopje, Macedonia, Skopje, Skopje, Macedonia; 2 University Children's Hospital, Medical Faculty, University „Ss. Cyril and Methodius“ Skopje, Macedonia, Skopje, Skopje, Macedonia
Background: Venous thrombosis is a well‐recognized and serious complication of acute lymphoblastic leukemia (ALL) in childhood. The etiology is multifactorial and may include inherited thrombophilia risk factors, hypercoagulability resulting from the leukemia itself, the presence of central venous line and the contribution of chemotherapy.
Aims: This case report aims to describe the clinical course and management of deep venous thrombosis as a presenting manifestation of ALL in a 5‐year‐old boy with Down syndrome.
Methods: case report
Results: A 5‐year old boy with Down syndrome presented with one‐day history of left leg pain, swelling in the thigh and calf and refusal of walking. Complete blood count on admission was normal. His coagulation studies revealed a shortened APTT (22.2 s) and high levels of D‐dimer (9077 ng/ml). Doppler ultrasound exam of lower extremity showed occlusive thrombus in the left common femoral and popliteal vein. Anticoagulant therapy with low‐molecular‐weight heparin (LMWH) was initiated. After 3 weeks at the follow‐up examination it was observed signs of complete recanalization of the popliteal vein. Common femoral vein was still thrombosed and incompressible. Over the following days his condition has worsened experiencing fever, irritability, lower back and legs pain. Blood counts showed mild anemia and thrombocytopenia. Bone marrow aspiration revealed precursor – B cell ALL. Induction chemotherapy was started according to the ALL IC BFM 2002 along with concomitant application of LMWH. Complete remission on day 33 was achieved and thrombosis was resolved. The patient continued the protocol along with concurrent LMWH prophylaxis and the outcome was favorable without clinical symptoms or signs related to recurrent thrombosis. Thrombophilia screening showed heterozygous mutation for Factor II, MTHFR C677T, MTHFR A1298C and B‐Fibrinogen.
Conclusion(s): Thrombosis may be a presenting feature of childhood ALL. Prompt anticoagulation with LMWH is mandatory to prevent extension or recurrence of VTE and save in the setting of ALL treatment.
216. VPB0289
216.1. T cells predict the response of high‐dose dexamethasone in pediatric newly dignosed immune thrombocytopenia
W. Zhifa 1; R. Wu2
1 National Key Discipline of Pediatrics (Capital Medical University), Xi’an, Beijing, China (People's Republic); 2 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic)
Background: Immune thrombocytopenia (ITP) is a disease characterized by isolated thrombocytopenia. In our previous studies, we also found that High‐dose dexamethasone (HDD) provides a high rate of remission as a frst‐line treatment for children previously untreated primary ITP, with better tolerance than conventional PDN.
Aims: The present study aimed to explore the predictive value of ‐T cells on HDD sensitivity in patients with ITP and their effect on the pathogenesis of ITP.
Methods: We retrospectively analyzed the response of High‐dose dexamethasone in pediatric newly dignosed immune thrombocytopenia from February 2018 to August 2021. The distribution of age, gender, and measured CBC parameters, including PLTs, white blood cells (WBCs), absolute lymphocyte count (ALC) and Hemoglobin (Hb) among the study were recomed and compared. Hematological indices including CD3+CD4+CD25+FOXP3+, CD4+, CD8+, CD4+/CD8+, αβ‐T, γδ‐T, DNT‐T cells were measured and compared between the two groups.
Results: At 1 week, the children were divided into two groups based on their respond to HDD. There were no significant differences in the absolute number of CD3+, CD8+, CD8‐, CD4+CD25+FOXP3+, CD4‐CD8‐, αβ‐T, DNT T cells in BM between CR+R group and NR group (p > 0.05). However, the absolute number of CD4+, the CD4:CD8 ratio and the γδ‐T cells was higher in the patents of CR +R group when compared the NR group (p < 0.05).
Conclusion(s): This is the first study to report the association between the γδ‐T cells in peripheral blood with the ITP patients. The CD4+/CD8+ rate and the γδ‐T cells maybe the reliable factors for predicting the early response of HDD therapies.
217. VPB0284
217.1. The potentiality of thromboelastographic examination of newborns with venous thrombosis
A. Ilyina1; A. Mishchenko2; L. Vorona1; A. Martynov 1; E. Akhalova1; A. Rogova1; L. Doronin1; E. Akkuzina1
1 Pirogov Russian National Research Medical University, Moscow, Moskva, Russia; 2 I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Moskva, Russia
Background: An extended hemostasiological examination in newborns with thrombosis allows operatively detect an increased coagulation potential.
Aims: To determine main indicators of an extended hemostasiological examination for early thrombotic events detection in newborns.
Methods: The research group includes newborns (30) with thrombotic risk factors (hemostasis system genetic polymorphisms, intrauterine hypoxia and infection) and realized venous thrombosis. The main diagnostic methods were: anamnestic, clinical, laboratory and instrumental examinations (ultrasound to visualize blood clots and signs of perinatal hypoxia, PCR‐tests to detect hemostasis system genes polymorphisms and infections, thromboelastographic analyse and comparative platelet‐rich plasma [PRP] and platelet‐poor plasma [PPP] tests, and aggregation and functional platelets activity determining methods.
Results: All patients have thrombotic and aggravated obstetric anamnesis. All patients were born with hypoxic‐ischemic brain injury and realized intrauterine infection (congenital pneumonia). Every third child (27.2%) was born prematurely. Ultrasound visualized thrombosis in ramus sinister venae portae in all cases. Neurosonographic examination determined hypoxic‐ischemic brain injury entire research group. Among the research group most frequently detected hemostasiс system genetic polymorphisms were homozygous variant PAI‐1 (nucleotide substitution c.‐820_‐820insG, 80.0%), heterozygous variant MTHFR (c.665C>T, 60%), heterozygous MTRR variant (c.66A>G, 50.0%). Identified infection thrombogenic risk factors were: Klebsiella pneumoniae (20.0%), Staphylococcus haemolyticus (26.6%), Escherichia coli (20.0%) (PCR‐test). During a hemostasiological examination in children with thrombosis were noted: chronometric hypercoagulation (70.0%), an increase in the ∠α (76.6%), a decrease in the functional and aggregation activity of platelets (76.6%, respectively) and an increase in their coagulant activity at the same time (76.6%).
Conclusion(s): The main extended hemostasiological examination indicators, indicating coagulopathy, strong hypercoagulability and a high thrombosis risk in newborns, are: chronometric hypercoagulation, an increase of the ∠α, a decrease of functional and aggregation platelets activity, and an increase of coagulant platelets activity.
218. PB0268
218.1. Aortic thrombosis presenting as myocardial infarction in a child
K. Nelson McMillan 1; D. Patel2; N. Ikeda2; S. Kane3; C. Kriesberg2; V. Dorsey2; F. Yousaf2; N. Hibino4; C. El‐Zein2; L. Vricella5
1 Advocate Children's Heart Institute, University of Chicago, Valparaiso, Indiana, United States; 2 Advocate Children's Heart Institute, Oak Lawn, Illinois, United States; 3 Advocate Children's Heart Insitute, Oak Lawn, Illinois, United States; 4 Advocate Children's Heart Institute/University of Chicago, Chicago, Illinois, United States; 5 Advocate Children's Heart Instiute/University of Chicago, Chicago, Illinois, United States
Background: Ascending aortic thrombus is rare in children without history of trauma, hypercoagulable condition or vascular disease and carries a high mortality risk necessitating rapid identification and management.
Aims: We aim to present the clinical course for a rare pediatric case.
Methods: We reviewed the medical record for a child with recurrent life‐threatening thrombi.
Results: A 12‐year old previously healthy male presented with chest pain. ECG revealed ST segment elevation. Echocardiography revealed an ejection fraction of 25% and a mobile mass (10 × 20 mm) in the ascending aorta. COVID testing was negative. Troponin‐I was elevated. He was emergently placed on cardiopulmonary bypass where a large organized thrombus was removed. The left anterior descending coronary artery was occluded. He underwent intracoronary tPA, aspiration thrombectomy and balloon angioplasty. Hypercoagulable and autoimmune work‐up revealed elevated factor 8 activity, von willebrand factor (vWF) activity and thrombocytosis with increased function by viscoelastic testing (ROTEM). Myocarditis, cardiogenetics and genetic testing for thrombophilia were negative. He was discharged on heart failure therapy, triple anti‐platelet therapy (aspirin, clopidogrel, dipyridamole) and apixiban. He underwent a heart transplant 5 months later. Three weeks post‐transplantion, he was incidentally found to have a large left atrial thrombus. At this time, he was only on aspirin. Factor 8 activity at time of transplant and second thrombus discovery was >400%. vWF activity and platelet count were also elevated. ROTEM revealed elevated platelet and fibrinogen activity. He underwent left atrial thrombectomy and was restarted on triple antiplatelet therapy and apixiban. He has not had recurrence on this regimen for 8 months.
Conclusion(s): Thrombocytosis and elevated pro‐inflammatory coagulation factors may predispose to development of potentially fatal thrombi. Besides inflammation, etiology may be unknown, particularly in apparently healthy children, prompting additional research into potentially genetic conditions in these complex pathways to further elucidate patients at risk.


219. PB0258
219.1. Plasma Lipoprotein(a) and factor VIII levels after pediatric renal transplantation: A 10‐year experience from a tertiary center in Brazil
B. Blanco 1; M. Garanito1; D. Celeste1; C. Ortega2; A. Watanabe2; V. Koch2; J. Carneiro1
1 Division of Pediatric Hematology, São Paulo University, São Paulo, Sao Paulo, Brazil; 2 Pediatric Nephrology Unit, São Paulo University, São Paulo, Sao Paulo, Brazil
Background: Renal transplantation is the treatment of choice for children with end‐stage renal disease (ESRD). Graft thrombosis represents 2 to 18% of early allograft losses in pediatric patients. Several factors may be involved in the pathogenesis of graft thrombosis. Some transplant recipients may have acquired prothrombotic risk factors as factor VIII (FVIII) and lipoprotein A [Lp(a)] elevation, and the impact and pharmacological approaches of these conditions should be considered.
Aims: To evaluate serum levels of Lp(a) and FVIII before and after pediatric kidney transplantation.
Methods: Retrospective chart review of patients aged < 18 years, submitted to renal transplantation at a single center in Brazil, from 2011 to 2021. We evaluated FVIII and Lp(a) levels before the surgical procedure and after a median time of 12 months. Exclusion criteria: immediate postoperative graft thrombosis and/or plasmapheresis before Lp(a) and FVIII levels.
Results: Of 101 patients, six were excluded (immediate postoperative graft thrombosis). We analyzed 95 patients. The median age was 9.4 years (range: 2.7–17.4), 62% were males (Table 1). The most prevalent thrombophilias were FVIII ≥150 IU/dl and Lp(a) >75 nmol/L elevation. Prior to the transplantation Lp(a) and FVIII levels were elevated in 70.5%, and 50,5% of the subjects, respectively. Post‐transplant, the proportion of elevated FVIII reduced to 26.3% (p < 0.001), and elevated Lp(a) was noted in 29.4% recipients, with a significant reduction in the median levels (p ≤ 0.001) (Table 2). All patients received pharmacological prophylaxis with heparin, followed by aspirin for one year, according to institutional guideline for kidney transplant recipients, and no long‐term graft thrombosis was observed.
Conclusion(s): Lp(a) and FVIII levels are raised in pediatric patients with ESRD, and it can be reversed by kidney transplantation. The risk of early allograft losses associated with these acquired abnormalities is unknown. Future prospective studies are needed to understand these conditions and the management of recipients.

220. PB0269
220.1. Single center case series & literature review: Deficiency of Adenosine Desaminase (DADA2) causes stroke, livedo racemosa, fever, lymphoproliferation, immunoglobulin deficiency&pancytopenia in children
M. Reschke 1; H. Bernuth2; S. Holzhauer3; T. Kallinich4
1 University Medicine, Charite, Berlin, Germany, Berlin, Berlin, Germany; 2 Charité ‐ Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt‐Universität zu Berlin, and Berlin Institute of Health, Pediatric Respiratory Medicine, Immunology and Critical Care Medicine, Berlin, Berlin, Germany. Labor Berlin GmbH, Immunology, Berlin, Berlin, Germany., Berlin, Berlin, Germany; 3 Charité University Medicine, Charite, Berlin, Germany, Berlin, Berlin, Germany; 4 Charité ‐ Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt‐Universität zu Berlin, and Berlin Institute of Health, Pediatric Respiratory Medicine, Immunology and Critical Care Medicine, Berlin, Berlin, Germany. Deutsches Rheuma‐Forschungszentrum (DRFZ), an Institute of the Leibniz Association, Rheumatology, Berlin, Berlin, Germany., Berlin, Berlin, Germany
Background: Cryptogenic pediatric stroke is rare. DADA2 has recently been identified as a possible cause. The monogenetic (ADA21), multisystemic, autoinflammatory disease manifests mainly as cytopenia, antibody deficiency or localized vasculitis causing ischemic strokes, often in the lacunar brain stem area5. TNF α is highly efficacious2, 5 in suppressing symptoms; only stem cell transplantation is curative3. Most common differential diagnosis are “CVID, ALPS ‐ like, pancytopenia, or low IgG”.
Aims: Raising awareness for rare causes of pediatric stroke.
Methods: Collecting and analyzing data on all children who have been diagnosed with DADA2 at our center, focusing on incidence of cerebral ischemia, first symptoms and treatment success.
Results: In six children DADA2 was genetically confirmed at our center (4x compound heterozygous, 2x homozygous variation in ADA2). Median time to diagnosis: 3.5 years. Symptoms at presentation were typical4: livedo, immunoglobin‐deficiency, cytopenia, fever of unknown origin, panniculitis/myositis, lymphoproliferation and failure to thrive (n = 6,4,4,3,3,1,1 respectively). Lacunar stroke as first symptom occurred twice in two patients, causing hemiparesis, diplopia, coma, vertigo, cranial nerve paralysis and aphasia. All were treated with a TNF α inhibitor. Median time of follow up is: 13,5 months. Lost to follow‐up: 2/6 (siblings). After starting treatment, no further ischemic cerebral events or other symptoms were observed. Persisting laboratory abnormalities: mild leukopenia and immunoglobulin deficiency (n = 2,3 respectively).
Conclusion(s): DADA2 is a treatable, potentially life threatening disease that can cause recurrent stroke and other dangerous complications; even in young children. Pediatric patients with stroke should be screened for ostensibly unrelated symptoms in order to determine if genetic analysis is indicated.

221. PB0255
221.1. Efficacy of normal saline versus heparin locking in the prevention of non‐valved peripherally inserted central catheter associated complications: A systematic review
S. Afraz 1; G. Zhao2; P. Tieu3; M. Bhatt4; N. Samji4; J. Donnellan5; A. Chan3
1 University of Texas Southwestern Medical Center, Dallas, Texas, United States; 2 University of Toronto, Toronto, Ontario, Canada; 3 McMaster University, Hamilton, Ontario, Canada; 4 McMaster Children's Hospital, Hamilton, Ontario, Canada; 5 McMaster Children’s Hospital, Hamilton, Ontario, Canada
Background: Varying types of peripherally inserted central catheters (PICCs) are widely used. Cuffed, non‐valved PICCs (NVPICCs) are preferred type of PICCs for pediatric patients in our institution due to less risk of line displacement and fewer associated complications. Intermittent locking of PICCs with either heparin or normal saline (NS) is required to maintain its patency. Catheter maintenance practice varies among institutions, and NVPICCs require daily heparin flush per our institution policy. However, the use of heparin is questionable, as it is costly and is not risk‐free particularly in pediatrics.
Aims: To compare the effectiveness of NS versus heparin in prevention of complications associated with NVPICCs
Methods: A systematic literature review was conducted using PubMed, MEDLINE, EMBASE and Cochrane library (Figure 1). Studies reporting use of NS or heparin lock/flush for NVPICCs in adult or pediatric patients that investigated catheter‐associated complications were included. The primary and secondary outcomes were rates of all‐cause complications and premature catheter removal, respectively.
Results: Systematic search retrieved 335 unique articles. After abstract and full text screening, 7 studies were included (Table.1). Six trials involved adults and one involved neonates. Four studies utilized NS and 3 used heparin as locking solution. No studies directly compared the efficacy of NS versus heparin in NVPICCs. Although NS was associated with higher complication rate per line compared to heparin (21.3% vs. 13.5%), overall rate of complication per catheter‐days and premature removal were lower with NS (1.3 vs. 5.3 per 1000 catheter‐days and 5.5% vs. 11.3%, respectively).
Conclusion(s): Evaluation of 7 heterogenous studies did not show inferiority of NS compared to heparin with regards to prevention of complications associated with NVPICCs. There is a need for a randomized controlled trial to specifically assess the efficacy of NS versus heparin in NVPICCs in pediatric patients.


222. PB0256
222.1. D dimer levels in children with Kawasaki disease correlate with disease activity
S. Rajasekaran1; A. Jindal2; N. Kumar3; V. Uppal4; S. Singh5; J. Ahluwalia 5
1 Postgraduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India; 2 Postgraduate Institute of Medical Education and Research, Chandigarh, India, Chandigarh, Chandigarh, India; 3 Post Graduate Institute of Medical Education and Research, Chandigarh, India, Chandigarh, Chandigarh, India; 4 Postgraduate Institute of Medical Education and Research, Chandigarh, India, Chandigarh, Chandigarh, India; 5 Postgraduate Institute of Medical Education and Research, Chandigarh, India., Chandigarh, Chandigarh, India
Background: Kawasaki disease (KD) is the commonest childhood vasculitis. Coronary artery aneurysms (CAA) develop in 20%–25% of untreated children. CAA may lead to morbidity and early death. Early diagnosis and treatment with IVIg significantly reduces the risk of development of CAA. IVIg resistance (IVIgR) seen in 10%–20% cases, needs alternative treatment. IVIgR and CAA development are difficult to predict at the outset or on admission.
Aims: To assess the role of D dimer levels in children with KD with regards to IVIg resistance and development of coronary artery aneurysms.
Methods: D dimer levels from peripheral blood were estimated in 32 newly diagnosed KD children aged ≤15 years. Follow‐up D dimer levels were available in 22 children. A separate group of 26 known cases of KD were recruited and D‐dimer was assessed once anytime during their follow up visits. The assay was performed using the HemosIL D dimer HS kit on the ACL TOP coagulation analyser. The D dimer levels were correlated with disease activity, development of CAA or IVIgR.
Results: D dimer levels were significantly higher with disease activity (median‐ 536.5 ng/ml) vs. in inactive phase during follow up ( median‐107.5 ng/ml), p < 0.001. D dimer level of 190.5 ng/ml could predict activity with a sensitivity of 81% and specificity of 83%. Though D dimer levels were higher in active KD patients with IVIg resistance (median‐ 860 ng/ml vs. median‐531ng/ml in IVIg responsive patients) and in those with CAA (median‐ 1227.5 ng/ml) vs. those without (median‐ 457 ng/ml), statistical significance was not obtained (p values .583 and .134 respectively).
Conclusion(s): D dimer is a reliable predictor of disease activity in KD. Though D dimer levels at admission were increased in patients with CAA and IVIgR, their clinical utility in predicting the same is uncertain and needs validation in a larger cohort.
223. PB0278
223.1. To load or not to load: Safety and efficacy of warfarin dosing in pediatric cardiology patients
J. Branstetter1; H. Zaki1; G. Woods 2; M. Allard3
1 Children's Healthcare of Atlanta, Atlanta, Georgia, United States; 2 Children's Healthcare of Atlanta/Emory University, Atlanta, Georgia, United States; 3 Children's Healthcare of Atlanta, atlanta, Georgia, United States
Background: Warfarin (Coumadin®) is a vitamin K antagonist that may take 72–96 h to exhibit peak anticoagulation effects. Some current guidelines recommend initiating pediatric patients with a loading dose (LD) of 0.2 mg/kg per dose. Due to the paucity of data in regard to administering a LD, this study evaluated the utility of utilizing a LD in pediatric cardiology patients requiring warfarin initiation.
Aims: 1. Compare the rates of therapeutic, supratherapeutic, and subtherapeutic INR within the first 7 days after warfarin initiation with a LD compared to no loading dose (NLD) 2. Evaluate the safety and efficacy of warfarin initiation with a LD versus NLD
Methods: A retrospective chart review evaluated patients 0 to 18 years of age admitted to the heart center for warfarin initiation between 01/01/2018 and 12/31/2020. The two groups compared are an initial LD of warfarin (>0.15 mg/kg/dose) versus NLD (<0.15 mg/kg/dose).
Results: Twenty‐one patients were included for final analysis with 14 in the LD group and 7 in the NLD group. Baseline characteristics were similar amongst both groups except patients in the NLD group were significantly older (66 months +¬ 8 vs 139 months + 72) and weighed significantly more (15.8 kg +¬ 8 vs 44.1 kg + 18). There was no difference in the rate of therapeutic (5, 36% vs 1, 14%), subtherapeutic (1, 7% vs 0, 0%), or supratherapeutic (8, 57% vs 6, 86%) INR between the LD and NLD group respectively. There were no difference in minor bleeding rates (0, 0% vs 1, 14%) and no major bleeding events occurred.
Conclusion(s): There was no difference in target INR achievement in patients in the LD and NLD groups. There was a high overall rate of supratherapeutic INR values in both groups; however, very low rates of bleeding. Pediatric cardiology patients may not require warfarin loading doses.
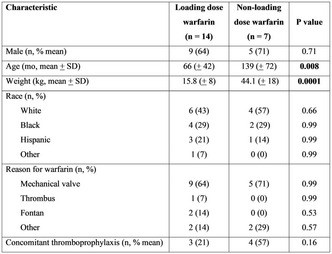

224. VPB0286
224.1. Incidence, risk factors, and outcome of thrombotic events in pediatric cancer patients
R. Çeçen1; D. Kızmazoğlu1; Ö. Tüfekçi2; Ş. Yılmaz2; D. İnce1; N. Olgun1; H. Ören 3
1 Dokuz Eylül University Oncology Institute, Izmir, Izmir, Turkey; 2 Dokuz Eylül University Faculty of Medicine, Izmir, Izmir, Turkey; 3 Dokuz Eylül University Faculty of Medicine, İzmir, Izmir, Turkey
Background: Thrombosis can occur commonly in children with cancer due to presence of different risk factors.
Aims: We aimed to evaluate the incidence, clinical characteristics, risk factors, and outcomes of patients with cancer and thrombosis and to examine the similarities and differences of thrombotic events in patients with leukemia and solid tumors.
Methods: A retrospective study of cancer patients who were diagnosed and treated between January 1, 2016 and January 1, 2021 was conducted. Data of patients who had thrombosis at diagnosis or in the course of their treatments were analysed.
Results: Thrombosis occurred in 15 out of 366 (4%) cancer patients. The incidence of thrombosis in patients with leukemia was 9.3% (7/75). The most common risk factor was the presence of a port catheter (5/7). The majority of thrombosis were located in the upper venous system. Three patients had intracardiac thrombosis. All thrombotic events resolved completely with low molecular weight heparin (LMWH). Recurrent thrombosis developed in one patient. The incidence of thrombosis in patients with lymphoma and solid tumors was 2.7% (8/291; cancer type: neuroblastoma in 2, Wilms tumor in 2, osteosarcoma in 2, and carcinoma in 1). The location of thrombosis was renal vein, vena cava inferior, jugular vein, portal vein, pulmonary vein, and sagittal sinus. Most patients (6/8) with solid tumors had thrombosis at diagnosis. Port‐related thrombosis developed in one patient. No inherited thrombophilic risk factors could be defined. Six patients received LMWH, complete resolution was achieved in all.
Conclusion(s): The incidence of thrombotic events was high in patients with leukemia. More than half of solid tumor patients had thrombosis at diagnosis, while leukemia patients developed thrombosis during chemotherapy and after catheter insertion. This finding indicates that development of thrombosis is mostly associated with the disease itself in solid tumors, while it is mostly related to treatment and/or catheter in leukemia patients.
225. PB0270
225.1. Rivaroxaban for the treatment of thrombosis in pediatric patients with leukemia. Results of a pilot study
S. Rodriguez Miranda 1; J. Colunga Pedraza2; L. Villarreal Martinez3; M. Zazueta Martinez4; S. Ramirez Cortinas4; L. Hernandez Torres4; S. Fierro Saenz4; H. Ramirez Duran4; G. Aliaga Orellana4
1 Universidad Autonoma de Nuevo Leon, San Nicolas De los Garza, Nuevo Leon, Mexico; 2 Universidad Autonoma de Nuevo Leon, Monterrey, Nuevo Leon, Mexico; 3 Hospital Universitario Dr. José Eleuterio González, Monterrey, Nuevo Leon, Mexico; 4 UANL, Monterrey, Nuevo Leon, Mexico
Background: Venous thromboembolism (VTE) in children with acute lymphoblastic leukemia (ALL) is a multifactorial entity whose timely diagnosis and treatment are vital to avoid sequelae. Factors associated with the presence of a prothrombotic state include the underlying disease, catheter placement, immobilization, obesity, and drugs such as steroids and L‐asparaginase. In children thrombosis treatment with rivaroxaban resulted in a similarly low recurrence risk without increased bleeding, as compared with standard anticoagulants. It is also an affordable therapy and easier to use.
Aims: To describe the results of the treatment regimen with enoxaparin for 7 days and then rivaroxaban for a minimum of 3 months in pediatric patients with ALL whom development of VTE.
Methods: Pediatric patients with ALL who developed VTE from 2020 to 2021 were included. Patients received treatment with enoxaparin for 7 days and then received a bodyweight‐adjusted 20 mg equivalent dose of rivaroxaban, given once‐daily (≥30 kg) or twice‐daily (12–30 kg) for a minimum of 3 months. Weekly clinical follow‐up was performed, bleeding events were recorded.
Results: Symptomatic thrombotic events were documented in 6 of 77 included patients diagnosed with ALL (7.8%). The mean age of the patients who presented thrombosis was 9.6 years (1–16). One patient was obese (16%). Three patients (50%) were receiving L‐asparaginase and steroids and 2 (33%) had central venous access. After 90 days no child present symptomatic recurrent VTE or bleeding events. Complete clinical resolution occurred in 66%. Adverse events were not reported.
Conclusion(s): We found a frequency of thrombosis of 7.8% in our population; the VTE treatment regimen with rivaroxaban was effective with no significant toxicities. Although it is a very small group, we found it as an attractive therapeutic option.

226. PB0265
226.1. Prevention of upper extremity PICC‐associated thrombosis using squeeze balls in the inpatient oncology setting: Preliminary findings from a prospective single‐arm non‐randomized investigation
E. Harris 1; J. Finkelstein2; B. Esty2; O. Halyabar2; C. Cherella2; C. Parsons1; R. Rosen3; D. Morhardt4; B. Aiyanyor1; D. O'Meara1; S. Ferrell2; D. Ciccolini2; E. Lambrinos2; E. Drake5; K. Humphrey2; R. Kumar6
1 Boston Children's Hospital/Harvard Medical School, Boston, Massachusetts, United States; 2 Boston Children's Hospital, Boston, Massachusetts, United States; 3 Boston Combined Residency Program, Boston, Massachusetts, United States; 4 Geisel School of Medicine, Dartmouth, Lebanon, New Hampshire, United States; 5 Division of Hematology and Oncology, Boston Children's Hospital and Harvard Medical School, Boston, Massachusetts, United States; 6 Harvard Medical School, Boston, Massachusetts, United States
Background: The incidence of venous thrombo‐embolism (VTE) in children has increased by 70%–200% over the last two decades with ~80% of thrombosis occurring secondary to central venous catheters (CVCs). Among CVCs, peripherally inserted central catheters (PICCs) have the highest risk of VTE. Sequential compression devices reduce venous stasis in lower extremities, but similar devices are unavailable for upper extremities (UE).
Aims: In this prospective, single‐arm study we investigated the use of squeeze balls to encourage UE mobilization in pediatric oncology patients with PICCs to reduce VTE risk.
Methods: The study was approved by the research ethics board. Children (≥4 years) with upper extremity PICCs who met a‐priori eligibility criteria were asked to participate. Informed assent was obtained. Enrolled patients were instructed to squeeze the ball 10 times/hour (while awake) with the ipsilateral hand. Patients were followed through their hospitalization. Standard statistical methods were used.
Results: Patient enrollment started in 09/2020. At the time of preliminary analysis (01/2022), 94 patients were screened (Figure 1). 33 patients (15 female gender) participated in the study (Table 1). Median age at participation was 13 (range: 4–22) years. The most common admission diagnosis was leukemia (n = 23; 70%). Most patients had a single lumen PICC (n = 20, 60%). Five patients received prophylactic enoxaparin during admission, none of whom developed VTE. Seven (21%) patients had UE ultrasound performed during admission given clinical concerns for VTE. Four (12%) patients developed VTE: two in the ipsilateral arm as PICC placement, and one in the contralateral arm. Two patients had pulmonary embolism. In our preliminary analysis, age, gender, diagnosis (leukemia versus solid tumor), laterality, or prophylactic anticoagulation were not significantly associated with VTE (p‐value >0.05).
Conclusion(s): In this preliminary analysis, 2/33 (6%) of pediatric oncology patients with an upper extremity PICC using a squeeze ball regimen developed a PICC‐associated thrombosis. We continue to actively enroll patients.
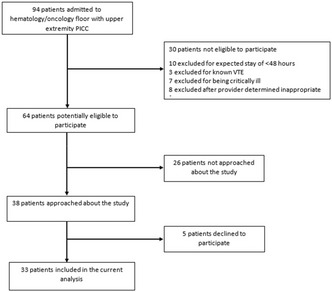

227. VPB0285
227.1. Pediatric thromboprophylaxis of large coronary artery aneurysm using rivaroxaban
N. Menon; N. Montanez
The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, Texas, United States
Background: Giant or large coronary artery aneurysms (CAA) are a rare occurrence in the pediatric population, most often secondary to Kawasaki disease. Anticoagulation therapy is recommended for prevention of thromboembolic events with either warfarin or low molecular weight heparin (LMWH). There are no currently published reports on the use of a direct oral anticoagulant (DOAC) in the pediatric population for this indication.
Aims: Describe the anticoagulation management of a pediatric patient with large coronary artery aneurysm secondary to Kawasaki disease using rivaroxaban, a DOAC.
Methods: Chart review of an 8‐year‐old male diagnosed with a persistently dilated right coronary artery aneurysm secondary to Kawasaki disease at age 4 years.
Results: A large to giant aneurysm (6–8 mm) in the distal right coronary artery was noted one month following Kawasaki diagnosis. Patient was initially treated with high dose aspirin therapy with reduction to low dose aspirin and addition of low molecular weight heparin (LWMH) at the time of imaging findings. Therapy was complicated by multiple bleeding events, in the form of calf hematomas, prompting a switch from LMWH to warfarin. Due to labile INR levels on warfarin therapy, he was transitioned to rivaroxaban therapy (5 mg twice daily per weight‐based dosing used in a phase III randomized controlled trial in children) two years following initial diagnosis of CAA. CAA has remained stable for greater than one year on dual anticoagulation with rivaroxaban and low dose aspirin without any thrombotic event or bleeding complication.
Conclusion(s): The use of rivaroxaban as an alternative to tradition vitamin K antagonist or LMWH appears to be safe and effective in the prevention of thrombosis in a pediatric patient with large to giant CAA.
228. PB0271
228.1. Thrombosis risk in children with acute lymphoblastic leukimia during the therapy
E. Seregina 1; L. Zharikova2; N. Trubina2; M. Korsantiya2; M. Gracheva2; A. Poletaev2; T. Vuimo2; J. Rumyantseva2; F. Ataullakhanov3; A. Karachunsky2
1 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 2 Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 3 Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia
Background: Thrombosis is a common complication in cancer patients. Сутекфд venous catheters (CVC), L‐asparaginase, steroids, as well as the presence of inherited thrombophilic disorders, are known risk factors for thrombosis in children with acute lymphiblastic leukemia (ALL).
Aims: The aim was to identify the thrombosis risk‐group in children with ALL.
Methods: The hemostasis status of 117 patients (74 boys, 43 girls, 1–17 years, median 5 year) with ALL treated according to ALL‐MB‐2015 protocol was assessed using APTT, fibrinogen, ATIII, D‐dimer levels, Thromboelastography (TEG), Thrombodynamics (TD), thrombomodulin (TM) and endothelin‐1(ET‐1) during the treatment.
Results: 58 patients (56%) had thrombotic complications during the treatment: 6% were symptomatic . TEG parameter R was mostly normal in 91% of points while MA were in hypocoagulation in 35% of patients due to low platelets count. Fibrinogen levels were decreased in nearly 50%. ATIII were decreased in 76%. TD clot growth rate revealed hypercoagulation in 81% while APPT was prolonged in 52%. D‐dimer levels were increased in 36%. Multidirectional changes in different tests revealed the imbalance in procoagulation and anticoagulation systems. If patients had hypercagulation by clot growth rate and no D‐dimer increases they had nearly 50% of thrombotic complictations despite ATIII levels. If patients had increased D‐dimer levels the percentage of thrombosis was much lower (nearly 12%). This may be related to lysis system function: if it was damaged during the treatment there can’t be D‐dimer levels increased and hypercoagulation leads to thrombosis. No patients with normal clot growth rate had thrombosis. There were high TM and ET‐1 levels in thrombosis group that can prooved the endothelium dysfunction in ALL.
Conclusion(s): The lysis system may be damages during ALL treatment that can lead to thrombotic complications. Endothelium dysfunction was proved by high markers in thrombosis group. Lysis dysfunction and endothelium damage can help to reveal the thrombosis high‐risk group in children with ALL.
229. PB0264
229.1. Composition of thrombi in pediatric ECMO circuits
J. Drop 1; L. Verhage2; E. Wildschut3; M. De Hoog3; C. van Ommen1; H. van Beusekom2
1 Erasmus Medical Center‐Sophia Children’s, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Cardiology, Erasmus MC, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 3 Department of Intensive care and pediatric surgery, Erasmus University Medical Center – Sophia Children’s Hospital, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: Extracorporeal membrane oxygenation (ECMO) is a treatment option for children with severe cardiac and/or respiratory failure. Despite anticoagulant therapy, thrombi regularly develop inside ECMO circuits of children. Thrombi that necessitate change of ECMO circuits can also embolize to the patients. The composition of thrombi inside ECMO circuits is unknown and scarcely investigated. Knowledge of thrombus composition might be helpful in developing targeted antithrombotic strategies to prevent thrombus formation.
Aims: In this pilot study, we aim to investigate the composition of thrombi inside ECMO circuits after clinical use in children.
Methods: After clinical use in pediatric patients (< 18 years), ECMO circuits were rinsed with buffer solution, visually inspected for thrombi and the location of thrombi was recorded. Thrombi were obtained from connector sites of pump, oxygenator, and oxygenator fibers and processed for paraffin embedding. Thrombi were stained with hematoxylin‐eosin and examined via immunohistochemistry for presence of platelets (CD61) and Von Willebrand Factor (VWF). Scanning electron microscopy (SEM) was used to investigate the structures adherent to different surfaces of the circuit.
Results: Fourteen circuits from 11 patients (median age 1 day, 9 neonates, 12 veno‐arterial ECMO) were studied. All circuits exhibited thrombi, especially in oxygenator and connection sites. CD61 staining showed platelet rich thrombi at the connection site (Figure 1); SEM showed activated platelets in the oxygenator and protein deposits at connector sites.
Conclusion(s): Despite anticoagulation, platelet rich thrombi develop in all ECMO circuits of pediatric patients, especially at connector sites and in the oxygenator. In this pilot study, a method has been developed to perform several techniques to evaluate the composition of those thrombi. These methods will be extended to VWF staining on additional slides. Pilot data suggest that antiplatelet therapy might be a valuable addition to current anticoagulant therapy.

230. PB0261
230.1. Extensive thrombosis from cranial venous sinuses to superior vena cava in an infant with Lemierre syndrome with successful management
J. Connors 1; N. Montanez2; L. Srivaths3
1 UT Houston MD Anderson Cancer Center, Houston, Texas, United States; 2 The University of Texas Health Science Center at Houston, McGovern Medical School, Gulf States Hemophilia and Thrombophilia Center, Houston, Texas, United States; 3 UTHealth McGovern Medical School/Thrombosis Sub‐Committee, Women and Girls with Blood Disorders ‐ Learning Action Network, Houston, Texas, United States
Background: Lemierre syndrome is rare disease entity characterized by infection of the oropharynx and septic thrombosis of the internal jugular vein, which can progress to septicemia and septic pulmonary emboli. Extension of thrombosis to cranial venous sinuses is rarely reported and simultaneous extension to cranial venous sinuses and superior vena cava (SVC) has not been previously reported.
Aims: We present the first case of an infant with Lemierre syndrome with simultaneous thrombus extension to cranial venous sinuses and SVC, managed successfully.
Methods: Retrospective review of electronic medical record of an infant with Lemierre syndrome and collection of case details including laboratory/imaging results, management and outcome.
Results: A twelve‐month‐old male presented with two weeks history of fever, otitis media, and right‐sided facial/neck swelling. Clinical findings and imaging revealed extensive suppurative abscesses from neck to the mediastinum, extensive thrombophlebitis and thrombosis extending from sigmoid and transverse sinuses, through right facial and internal jugular veins, terminating in the SVC. Antibiotics were started and patient underwent incision and drainage. Cultures of the purulent material grew MRSA. Post‐operatively, patient was started on therapeutic unfractionated heparin, and transitioned to therapeutic enoxaparin after four days. Patient completed six months of anticoagulation outpatient with clinical improvement, and radiologic thrombus resolution.
Conclusion(s): Only few cases of Lemierre syndrome have been described in children with thrombus extension to cranial venous sinuses and only one report with SVC thrombosis shown at autopsy. Simultaneous thrombus extension to the cranial venous sinuses and SVC is a rare, serious complication of Lemierre syndrome as seen in our patient, not described previously. Our case illustrates the severity of Lemierre syndrome, and the successful management with prompt institution of appropriate antibiotics and therapeutic anticoagulation.


231. VPB0287
231.1. Relationship of hemostasiological properties of blood in children of different age groups
A. Popovicheva
FSBEI HE PRMU of Ministry of Health of Russia, nizny novgorod, Nizhegorod, Russia
Background: A significant role in disorders of blood microcirculation is played by changes in the functional activity of the hemostasis system, the determination of which is especially important in the prepubertal, pubertal and postpubertal periods regardless of the sex of the child.
Aims: To study the functional properties of platelets and hemostasis in practically healthy children of different age groups.
Methods: The blood of age groups was used: 10 children ‐ up to 6 years old inclusive, 10 children – 7–11 years old, 10 children – 12–16 years old, 35 people ‐ 17 years old and older. In children’s blood were determined: platelet aggregation and activation, content of SFMC, activity of VWF, thromboelastography. Spearman's correlation was used. The results of study were processed using the Mann‐Whitney criteria.
Results: A trend of increased flow‐induced aggregation and activation was revealed with increasing age. Children under of 6 years and 7–11 years had the lowest values of the degree and rate of these processes (40% decrease compared to 17 years, p < 0.05 and p < 0.001, respectively). The degree and rate of these processes in 12 years did not differ from parameters in 17 years. At the age to 11 years old had the lowest values of the RFMC level and VWF activity (a decrease of 30% compared to 17 years, p < 0.05). Сorrelations between the studied indicators only at 17 years fixed – between the level of SFMC and the degree of platelet activation (r = −0.81) and with the rate of their aggregation (r = 0.60), between the activity of VWF and the parameter R of the thromboelastogram (r = −0.90).
Conclusion(s): Thus, the functional properties of platelets and plasma hemostasis change in healthy children during ontogenesis.
232. PB0277
232.1. Rate of recurrent venous thromboembolism in infants with unprovoked venous thromboembolism
H. Whitworth 1; R. Hubbard2; C. Leonard2; C. Witmer3; L. Raffini1
1 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 2 Department of Biostatistics, Epidemiology & Informatics Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 3 Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, United States
Background: Unprovoked venous thromboembolism (VTE) is rare in infants and few studies have evaluated the incidence of recurrent VTE in this population.
Aims: Describe the characteristics and management of infants with unprovoked VTE and determine the incidence of recurrent VTE.
Methods: We performed a single center, retrospective study including children age <1 year with unprovoked VTE between 1/1/2003–8/1/2021. Patients were followed from index VTE until recurrent VTE or censoring (death or date of last contact). Analysis included descriptive statistics and estimated incidence of recurrent VTE with 95% confidence interval (CI). The Institutional Review Board exempted this study and the National Institutes of Health support HW.
Results: 43 infants met inclusion criteria (Table 1). We identified one recurrent VTE in 240.45 person‐years (incidence rate = 4.16 [95% CI: 0.59–29.53] per 1000 person‐years). This event was in a different anatomic location, 16.2 years after the index event in a patient with double inherited thrombophilia. The event occurred during an acute illness and anticoagulation dose reduction. Therapeutic anticoagulation is summarized in Table 1. Documented reasons for not starting anticoagulation include concomitant or high risk for bleeding (13/23), unilateral renal vein thrombosis (2/23), minimal or no acute symptoms and/or thought to have occurred in‐utero (8/23). Four patients remained on anticoagulation at the end of the study period or at censoring. Two patients were transitioned to low‐dose anticoagulation for secondary prophylaxis; both had an inherited thrombophilia. The remaining seven patients with inherited thrombophilia did not receive long‐term anticoagulation. No patients experienced major bleeding on anticoagulation, however two patients experienced clinically relevant non‐major bleeding.
Conclusion(s): We demonstrated a low rate of recurrent VTE in children with a history of unprovoked VTE < 1 year old, with a median follow‐up of 4.61 years (IQR: 1.34–8.88). Anticoagulation management varied and there were no observed, recurrent events after anticoagulation discontinuation.

233. PB0262
233.1. Cerebral sinovenous thrombosis in Greek children: A single centre experience
A. Dettoraki 1; A. Michalopoulou1; M. Gavra2; Z. Kapsimali1; S. Thymianou1; S. Vassilopoulou3; A. Kattamis4; C. Kanaka‐Gantenbein5; H. PERGANTOU1
1 Haemophilia Centre/Haemostasis and Thrombosis Unit, Aghia Sophia Children's Hospital, Athens, Attiki, Greece; 2 Department of CT and MRI, Aghia Sophia Children's Hospital, Athens, Attiki, Greece; 3 Eginition Hospital, National And Kapodistrian University Of Athens Department of Neurology, Stroke Unit, ATHENS, Attiki, Greece; 4 Hospital, First Department of Pediatrics, National and Kapodistrian University of Athens, AGHIA SOPHIA CHILDREN'S HOSPITAL, ATHENS, Attiki, Greece; 5 Division of Endocrinology, Diabetes and Metabolism, First Department of Pediatrics, Medical School, National and Kapodistrian University of Athens, Aghia Sophia Children’s Hospital, ATHENS, Attiki, Greece
Background: Cerebral sinovenous thrombosis (CSVT) in children is a rare, often underdiagnosed, serious event.
Aims: To investigate CSVT characteristics in a reference paediatric centre for thrombosis.
Methods: Data were retrospectively collected for children with CSVT, referred between 2010 and 2021. The categorical variables were described by frequency distributions and compared with x2‐test.
Results: Sixty‐eight patients (57% males, mean age 6.0 ± 3.9 years) were included. The commonest presenting symptoms were headache (44%), decreased consciousness (32%), vomiting (15%), seizures (16%), diplopia (7%) and torticollis (4%). Papilledema was found in 17 children. Infection (74%) was the main cause with acute otitis media with/without mastoiditis (37% and 18% respectively) being the most frequent one. For diagnostic evaluation MRI/MRV was performed in 68% and CT in 24% of children. Thrombosis was detected in 44% on left side and in 37% on right side. The most frequent site of thrombosis was deep venous system (74%) with multiple localizations in 59% of patients. Sigmoid sinus thrombosis (60%) was predominantly observed, followed by transverse sinus (57%), while extension to ipsilateral jugular vein occurred in 38%. Simultaneous localization in transverse and sigmoid sinuses had an increased probability of papilledema occurrence (p = 0.04). One or more prothrombotic risk factors were found in 28% of children. Heterozygosity for FV Leiden and FII20210A mutations were found in 7% and 4% of patients, respectively, and homozygosity for MTHFR‐C776T in 15%. All children received anticoagulation (59% coumarin anticoagulants, 41% Low Molecular Weight Heparin) for a mean duration of 8.5 ± 4.8 months and 21 ± 5.5 months respectively. One third of patients showed complete recanalization after 11 ± 16 months and another one third partial recanalization in 9 ± 12 months at MRV. No child died or had persisting neurological sequelae.
Conclusion(s): Physicians should be suspicious of CSVT in children with infections, when referred for neurological signs.
234. PB0266
234.1. Cerebral venous sinus thrombosis in pediatric acute lymphoblastic leukemia: Risk factors and long‐term outcomes
L. Johnson‐Bishop 1; D. Pehlivan1; C. Cohen1; C. Karakas2; S. Bhar1
1 Baylor College of Medicine, Houston, Texas, United States; 2 Norton Children’s Hospital University of Louisville, Louisville, Kentucky, United States
Background: Cerebral venous sinus thrombosis (CVST) has been identified as a complication in 1%–2% of patients treated for pediatric acute lymphoblastic leukemia (ALL), with subsequent risk of neurological morbidity and mortality rate of 8%–13%. Multiple risk factors have been identified in acquisition of CVST, including active malignancy, inherited thrombophilia, infection, or pharmacologic side effects.
Aims: To describe the incidence, risk factors, and long‐term outcomes of CVST in children with ALL at a tertiary pediatric hospital.
Methods: A retrospective cohort study comprised of pediatric patients (ages 1–18 years) with newly diagnosed or first‐relapse ALL enrolled on Children’s Oncology Group and four Pediatric Oncology Group protocols from 2002 to 2021. Included were patients diagnosed with CVST and had confirmed computed tomography/venography or magnetic resonance imaging/venography. Patients were excluded if they developed extracerebral or isolated jugular vein thrombosis. Long‐term neurologic status was evaluated based on clinic notes from most recent follow up neurologic exam. Baylor College of Medicine institutional review board approval was obtained for the study.
Results: Nineteen cases of CVST have been identified in pediatric ALL patients at our center, an incidence of 8.8% of patients with CVST (N = 215). In regards to etiology of CVST, 68% (N = 13) of cases occurred during induction, followed by consolidation (16%, N = 3) and maintenance therapy (16%, N = 3). Presenting symptoms, therapeutic intervention and neurologic outcomes varied across the cohort (See Table 1), from normal neurologic exams at five years post diagnosis of CVST, to patients with persisting neurologic deficits including decreased strength, cranial nerve palsy, and seizure disorders.
Conclusion(s): CSVT is a notable complication of pediatric ALL therapy. In our cohort, significant risk factors identified for CSVT included immunophenotype, age and side effects of chemotherapy. However, due to the rarity and variability of cases, there is no universally accepted consensus on identified high‐risk variables that warrant implementation of preventative thromboprophylaxis.

235. PB0275
235.1. Successful off‐label use of intra‐arterial eptifibatide followed by ticagrelor in a pediatric acute arterial ischemic stroke patient
M. Torres 1; A. Schenk2; M. Fiesta3; J. Tilley2; M. Trang2; R. Herring2
1 Cook Children’s Medical Center, Fort Worth, Texas, United States; 2 Cook Children's Medical Center, Fort Worth, Texas, United States; 3 Radiology Associates of North Texas, P.A., Fort Worth, Texas, United States
Background: Standard antiplatelet therapy for pediatric arterial ischemic stroke (AIS) is limited to clopidogrel and acetylsalicyclic acid (ASA). In adult AIS, eptifibatide is used for catheter‐directed therapy and ticagrelor is Food and Drug Administration approved for the prevention of recurrent stroke/transient ischemic attack (TIA). We successfully treated a pediatric AIS with eptifibatide and ticagrelor after clinical deterioration despite clopidogrel and ASA therapy.
Aims: To present additional antiplatelet therapy options following pediatric AIS.
Methods: Single subject case report.
Results: A 14‐year‐old male presented to the ED with left hemiplegia beginning 20 h prior. Pediatric National Institutes of Health Stroke Scale (PedsNIHSS) was 9 upon arrival and brain magnetic resonance imaging confirmed an acute right middle cerebral artery infarction. Endovascular thrombectomy/thrombolysis was performed, during which the artery was noted to quickly re‐occlude. Catheter‐directed eptifibatide was successful followed by intravenous eptifibatide. PedsNIHSS post‐procedure was 11, 6 after 8 h, and 4 the next morning. He received 30 h of eptifibatide drip with one recurrent left hemiplegia episode that resolved within 20 min. ASA and clopidogrel were given 4 h prior to stopping eptifibatide. Five hours after drip stopped, left hemiplegia recurred. Repeat imaging showed no new stroke/extension. Unfractionated heparin (UFH) was added, but stuttering hemiplegia persisted with the last episode lasting over 2 h. Course suggested clopidogrel and/or ASA clinical resistance. UFH and clopidogrel were discontinued and ticagrelor initiated. No further hemiplegic episodes occurred. P2Y12 platelet reactivity units (PRU) were 33 after 24 h, indicating successful inhibition. After 10 days, he was discharged on ticagrelor and ASA with a PedNIHSS score of 0 and no evidence of hemorrhagic conversion.
Conclusion(s): We successfully used eptifibatide and ticagrelor in a pediatric AIS case with no adverse events and resolution of stuttering episodes of hemiplegia. Further research into the use of novel antiplatelet agents in the pediatric population is needed.
236. PB0257
236.1. Benefits of prolonged anticoagulant treatment in a series of cerebral venous thrombosis in pediatric patients
R. Berrueco 1; A. Faura2; A. Ruiz‐Llobet3; M. Mesegué4; M. Solsona3; E. González‐Forster3
1 Hospital Sant Joan de Déu, Esplugues Llobregat, Catalonia, Spain; 2 Sant Joan de Déu Hospital, Espluges de Llobregat, Catalonia, Spain; 3 Hospital Sant Joan de Déu, Esplugues de Llobregat, Catalonia, Spain; 4 Hospital Sant Joan de Déu, Espluges de Llobregat, Catalonia, Spain
Background: Cerebral sinus venous thrombosis (CSVT) is a rare complication in children, usually secondary to an underlying condition. Recent guidelines recommend treating all pediatric patients with CSVT, but also suggest that treatment longer than three months should not be justified in provoked cases in which the risk factor has disappeared.
Aims: To evaluate the efficacy of prolonged antithrombotic treatment in a pediatric series of CSVT while describing clinical presentation, radiological findings, and outcome.
Methods: Retrospective observational study analyzing data of CSVT in children (1–18 years‐old) admitted in a single hospital. Demographic data, clinical presentation, etiology, extension, treatment and outcome were evaluated.
Results: Thirty‐one patients had a confirmed CSVT. Sixteen were girls, mean age was 7.3 years (SD 4.39). Sigmoid sinus (n = 17) and transverse sinus (n = 14) were the most frequent sinuses affected. Multiple sinus thrombosis was found in 20 patients (64.5%). Underlying condition was described in 29 cases: infectious disease (n = 20), major head trauma (n = 4), chemotherapy (n = 3), arteriovenous malformation (n = 2). More frequent symptoms were: headache (n = 15), decreased level of consciousness (n = 5), seizures (n = 3), focal neurological deficit (n = 2), papilledema (n = 2), diplopia (n = 2), vision impairment (n = 1), and coma (n = 4). All patients received anticoagulant treatment. Complete vein recanalization after 3 months was achieved in 53.3% of the cases. Treatment was prolonged up to 6 months in those patients with partial or no response (n = 14). Seven of them improved (Table 1). Complete and partial vein recanalization were reported in 73.3% and 20% of the patients.
Conclusion(s): Anticoagulation was useful to achieve complete vein recanalization in this series. Extending anticoagulation up to 6 months could be useful to improve outcome of pediatric patients with CSVT in selected cases.

237. PB0267
237.1. Predictors of catheter‐related arterial thrombosis in pediatric patients after cardiac catheterization
R. Natesirinilkul 1; S. Sethasathien2; N. Lawtrakulngam2; S. Silvilairat2; S. Manowong3; P. Tuntivate3
1 Faculty of Medicine Chiang Mai University, Chiang Mai, Thailand, Chiang Mai, Chiang Mai, Thailand; 2 Faculty of Medicine, Chiang Mai university, Chiangmai, Chiang Mai, Thailand; 3 Faculty of Medicine, Chiang Mai University, Chiangmai, Chiang Mai, Thailand
Background: Cardiac catheterization is one of the important modalities for diagnosis and treatment in patients with congenital heart disease. Femoral artery thrombosis is the most frequent complication after this procedure. Previous several studies reported that some biomarkers including D‐dimer, thrombin‐antithrombin complex (TAT), factor VIII clotting activity (FVIII:C) and microparticles could predict vascular thrombosis.
Aims: This study was to determine the association between biomarkers and vascular thrombosis in pediatric patients after cardiac catheterization. Also, it was to determine the risk factors for vascular thrombosis after cardiac catheterization.
Methods: Seventy‐five consecutive patients with weight of more than 8 kg and age between 6 months and 18 years old who underwent cardiac catheterization during June 2020 ‐ September 2021 were evaluated. Patients who stopped taking warfarin less than 48 h or who had arterial and venous thrombosis at lower limbs before the catheterization were excluded. Blood tests for biomarkers and Doppler ultrasound were performed before and 24 h after the cardiac catheterization.
Results: Seventy‐five patients (37 male) were classified into two groups: thrombosis and non‐thrombosis group. Five patients were identified to have the femoral artery thrombosis using the Doppler ultrasound. Patients with femoral artery thrombosis were age <5.7 years, weight <11.5 kg, procedure time >70 min, and size of sheath/BSA >9.3. In multivariate logistic regression analysis, weight <11.5 kg was the only significant risk factor for femoral artery thrombosis [odd ratio 10.9 (95% CI 1.1–108.6)]. However, biomarkers including D‐dimer, TAT, FVIII:C, and microparticles were not statistically significantly different between the two groups at the pre‐and post‐cardiac catheterization.
Conclusion(s): The biomarkers including D‐dimer, TAT, FVIII, and microparticles could not predict arterial thrombosis after cardiac catheterization. The patients with weight <11.5 kg were a significant risk factor for femoral artery thrombosis.
238. PB0272
238.1. The role of procoagulant microparticles in hemostasis of patients with hemolysis
E. Seregina 1; E. Lipets1; A. Poletaev1; A. Ignatova1; Y. Chuiko2; T. Vuimo3; F. Ataullakhanov4; N. Smetanina4
1 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 2 Pediatric Department of Pirogov Russian National Research Medical University, Moscow, Moskva, Russia; 3 Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 4 Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia
Background: Patients with hemolytic anemias are characterized by the increased risk for thrombosis. More and more studies have reported about procoagulant microparticles derived from the cells during hemolysis.
Aims: The aim of this study was to assess the contribution of procoagulant microparticles to the hypercoagulation in patients with hemolytic anemias.
Methods: The hemostasis of 5 children (boys, age: 4–12 years, girl 5 yr) with hereditary spherocytosis (HS) and 8 children with β‐thalassemia major (β‐TM) (2 girls 8 and 17 yrs, and 6 boys 2–15 years) was described using clotting times (APTT, PT), fibrinogen and D‐dimer levels and thrombodynamics (TD) global hemostasis assay. The count of microparticles was assessed by flow cytometry. Five healthy donors were enrolled as a control group.
Results: Clotting times were normal in all patients but TD clot growth rate was increased in 7 children compared to normal range (59 ± 2 μm/min for TD initial clot growth rate with normal range 38–56 μm/min). All patients with hypercoagulation were in severe condition: 3 patients with β‐TM have no remission after splenectomy. The patients with high clot growth rates have the highest concentrations of phosphatidylserine‐exposing microparticles (PS+MP) identified as procoagulant (1747 ± 631 PS+MP/μl with normal range 306–606 PS+MP/μl). The patients without hypercoagulation have nearly 2 times lower counts of the procoagulant microparticles (740 ± 378 PS+MP/μl). Glycophorin‐exposing(CD235a+MP) microparticles were identified as erythrocyte‐derived. Erythrocyte‐derived microparticles were the highest in patients with the changes in the spatial dynamics of clot growth: an increase in stationary clot growth rate measured after fibrin layer was closed the activator surface with tissue factor.
Conclusion(s): Hypercoagulation was observed in patients with hemolysis in severe condition. The highest counts of procoagulant microparticles were in patients with hypercoagulation by clot growth rates. The highest counts erythrocyte‐derived microparticles were revealed in patients with the disturbance of spatial dynamics of clot growth.
239. PB0273
239.1. Dealing with pediatric cancer associated thrombosis: A monocentric retrospective cohort study
E. Campello 1; A. Francavilla1; S. Sartori1; C. Forestan1; S. Sebellin1; m. Pelizza2; M. Nosadini1; J. Pin1; A. Biffi1; D. Gregori1; G. Lorenzoni1; M. Martinato1; P. Simioni3
1 University of Padova, Padova, Veneto, Italy; 2 University of Padova, Italy, Veneto, Italy; 3 Padua University Hospital, Padua, Veneto, Italy
Background: Little is known about cancer‐associated thrombosis (CAT) in children.
Aims: In this retrospective cohort study we aim to describe clinical presentation, management and outcomes of pediatric CAT.
Methods: All consecutive patients admitted at the Pediatric Department of the Padova University Hospital between January 2007 and December 2021 with a thrombotic event were retrospectively enrolled. Inclusion criteria were age >28 days, acute venous or arterial thrombosis confirmed by objective tests (ultrasound, magnetic resonance imaging, magnetic resonance angiography, computed tomography, computed tomography angiography, angiography, ventilation‐perfusion scan), cancer among risk factors. Clinical outcomes including thrombosis‐related death, recurrence and bleeding complications were recorded.
Results: During the study period, 59 pediatric patients with CAT were considered (Table 1). The most involved neoplasms were hematological malignancies. 24 events (40.6%) were cerebral arterial thrombosis; 35 (59%) were venous thrombosis (VTE, 8 cerebral vein, 25 systemic vein thrombosis and 2 pulmonary embolism). Of 27 VTE, 15 (55%) were CVC‐related, 19 (70%) were symptomatic. Main signs/symptoms were 4 catheter malfunction, 2 dyspnea and 13 limb edema and pain. Antithrombotic therapy was administered in 51/59 (86%) of patients, mainly heparin (98%) (low molecular weight heparin [mean dosage 162.25 U/kg/die] or unfractionated heparin [mean dosage 19.7 U/kg/h]). One patient was treated with warfarin (2%). As for the outcomes, 2 patients died of CAT‐related death (3.4%), 6 patients (10.1%) underwent CAT recurrence after a mean period of 540 weeks [IQR 428‐652]. Recurrence events were 3 cerebral vein and 3 systemic vein thrombosis. As for bleeding complications only 3 minor events were reported (5%).
Conclusion(s): Pediatric CAT characteristics and outcome resemble those of adult CAT. Particularly, a high incidence of both arterial and venous events was reported; hematological neoplasms and CVC are the major risk factors; heparin the only used therapeutic option; a high incidence rate of recurrence was detected.

240. VPB0282
240.1. Using an EMR patient portal for warfarin self‐management: Exploring children and families’ experiences
S. Jones 1; J. Hibbard2; J. Hislop3; H. Gilmore3; A. Greenway3; F. Newall4
1 The University of Melbourne, Melbourne, Victoria, Australia; 2 Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; 3 Royal Children's Hospital, Melbourne, Victoria, Australia; 4 Royal Children's Hospital, Melbourne, Tasmania, Australia
Background: Many children taking warfarin perform their International Normalised Ratio (INR) at home, with results phoned to a clinician who instructs warfarin‐dosing. International data suggests parents can be supported to make warfarin‐dosing decisions themselves, a process known as patient self‐management (PSM).
Aims: This study explored patient and family confidence and competence with warfarin PSM in children using an Electronic Medical Record (EMR) Patient Portal.
Methods: Children currently performing INR self‐testing were eligible. Participation involved attending an individualised education session, adherence to the warfarin PSM program and participation in phone interviews. Clinical outcomes (INR time in therapeutic range and safety outcomes) were assessed and family confidence and competence in PSM was explored. The hospital human research ethics committee approved the study and consent was obtained from parents.
Results: 24 families undertook PSM. Children’s median age was 11 years and all children had underlying congenital heart disease. Pre PSM the median time the INR was in therapeutic range was 72%; this increased to 81% during PSM (p < 0.001). There were no adverse events. Eight families participated in a phone interview. The major theme identified was empowerment; all parents commented that PSM empowered them and/ or gave them some control over their child’s health. Minor themes that emerged included gaining knowledge;, trust and responsibility builds confidence;, saving time; and resources as a safety net (Figure 1).
Conclusion(s): This study demonstrates that communication via an EMR Patient Portal is satisfactory to families, offering a safe and efficacious option for PSM for children. PSM empowered and built confidence in families to manage their child’s health. Using the patient portal for PSM transformed an existing therapeutic relation between clinicians and family; families were empowered to manage their child’s warfarin but still connected to clinicians remotely. This study represents diversification of how the patient/ clinician relationship can exist in the digital age.

241. PB0274
241.1. Effect of SARS‐CoV‐2 mRNA vaccination on thrombin generation in children with inflammatory bowel disease
V. Stercel 1; L. Lóczi2; O. Kadenczki1; É. Nemes1; R. Hodossy‐Takács3; T. Szabó1; Z. Bagoly4
1 University of Debrecen, Faculty of Medicine, Department of Pediatrics, Debrecen, Hajdu‐Bihar, Hungary; 2 University of Debrecen; Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary; 3 University of Debrecen, Faculty of Medicine, Division of Clinical Laboratory Sciences, Debrecen, Hajdu‐Bihar, Hungary; 4 University of Debrecen, Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary
Background: Inflammatory bowel disease (IBD) including Crohn’s disease (CD) and ulcerative colitis (UC), is associated with higher thrombotic risk and enhanced thrombin generation (TG) in adults. IBD patients were underrepresented in SARS‐CoV‐2 mRNA vaccine trials. Case reports indicated that adverse events post‐vaccination, including IBD flare, were more common among children, and those with prior COVID‐19.
Aims: To find out whether TG is increased in children with IBD as compared to healthy controls and whether TG parameters show significant changes following SARS‐CoV‐2 mRNA vaccination.
Methods: In this observational case‐control study, 37 children with IBD (CD:16, UC: 21) aged 12–18 years and 55 healthy age‐matched children were enrolled. Blood was collected before and 2–4 weeks after the second dose of BNT162b2 (Pfizer‐BioNTech) vaccine dose. Whole blood count, fibrinogen, inflammatory markers (CRP, ferritin), anti‐SARS‐CoV‐2 antibody levels were investigated, TG assay was carried‐out using platelet‐poor plasma. Lag time, endogen thrombin potential (ETP), peak thrombin, time‐to‐peak were calculated. Detailed clinical parameters including post‐vaccination symptoms, COVID‐19 history, disease activity scores (PUCAI, Mayo score, PCDAI) were registered.
Results: CRP was significantly elevated in children with IBD and showed a positive correlation with ETP (CD: r = 0.700; p = 0.003 and CU: r = 0.501; p = 0.020). TG parameters did not differ between patients and controls pre‐ or post‐vaccination. TG parameters remained unaltered post‐vaccination in both groups. IBD disease flare was not observed post‐vaccination, but reduced anti‐SARS‐CoV‐2 antibody titers were found in 4 patients receiving immunosuppressive therapies. Previous COVID‐19 infection had no effect on TG levels.
Conclusion(s): Although TG parameters correlated with the level of inflammation in children with IBD, the extent of TG was not significantly different from healthy controls. TG parameters and IBD disease activity scores did not increase significantly following mRNA vaccination. Our results support the safety of SARS‐CoV‐2 mRNA vaccination in children with IBD, highlighting observations of lower antibody titers in immunosuppressed children. Funding: NKFI‐FK128582.
242. VPB0279
242.1. Anti‐factor Xa‐based risk stratification of children receiving therapeutic enoxaparin is associated with increased risk of recurrent/progressive thrombosis and hemorrhage: A retrospective cohort study
W. Bourgeois 1; E. Drake2; J. Duzan2; N. Chen3; W. London4; N. Ramesh5; R. Kumar6
1 Dana‐Farber/Boston Children’s Cancer and Blood Disorders Center, Harvard Medical School, Boston, Massachusetts, United States; 2 Division of Hematology and Oncology, Boston Children's Hospital and Harvard Medical School, Boston, Massachusetts, United States; 3 Dana‐Farber/Boston Children’s Cancer and Blood Disorders Center, Boston, Massachusetts, United States; 4 Dana‐Farber/Boston Children’s Cancer and Blood Disorders Center, Harvard Medical School, Boston, MA, Boston, Massachusetts, United States; 5 Boston Children's Hospital, Boston, Massachusetts, United States; 6 Harvard Medical School, Boston, Massachusetts, United States
Background: Despite recent regulatory approval of direct oral‐anticoagulants, enoxaparin remains a frequently prescribed anticoagulant in children. Patients at our quaternary‐care center requiring therapeutic enoxaparin have been historically stratified to aim for high‐risk (HR; target anti‐factor Xa: 0.4–0.6 IU/ml) and standard‐risk (SR; anti‐factor Xa: 0.5–1 IU/ml) enoxaparin levels.
Aims: Principal objective of this observational study was to compare rates of recurrent/progressive thrombosis and hemorrhage (major/clinically‐relevant non‐major bleeding) for HR versus SR cohorts.
Methods: Permission from the research ethics board was obtained. Pediatric patients (≤18 years) diagnosed with venous‐thrombosis between 06/01/2009 ‐ 06/30/2020, who received therapeutic enoxaparin were eligible. Demographic data were abstracted from medical records. Assignment to HR or SR cohorts was at the treating physician’s discretion. Recurrent/progressive thrombosis and hemorrhage were defined per International Society on Thrombosis and Haemostasis guidelines. Standardized statistical methods were used to summarize data, Fisher’s exact test was used to compare variables between HR and SR cohorts.
Results: 881 patients received enoxaparin during the study‐period of whom 315 did not meet eligibility criteria and were excluded. 565 patients (288 female) were analyzed (Figure 1). Mean (± SD) age at VTE diagnosis was 9.4 (±6.6) years. Mean duration of therapeutic anticoagulation for the entire cohort was 4.2 (±4.4) months. Demographic data are summarized in Table 1. 99 (17.5%) patients were initially allocated to the HR cohort. Rate of recurrent/progressive thrombosis was 21 (21.2%) in the HR cohort, compared to 48 (10.3%) in the SR cohort (p = 0.003). Rate of major/CRNM bleeding was 20 (20.4%) in the HR cohort, compared to 38 (8.2%) in the SR cohort (p < 0.001).
Conclusion(s): Risk stratification of patients receiving therapeutic enoxaparin appears to be associated with a higher risk of both thrombus recurrence/progression and hemorrhage in patients allocated to a lower target anti‐factor Xa (HR cohort). Analysis is ongoing to investigate additional predictors of thrombus recurrence/progression and hemorrhage.


243. PB0259
243.1. Thrombolytic therapy in neonates and children: A twelve‐year prospective registry of a single tertiary pediatric center in Argentina
C. Cervio 1; M. Hepner2; M. Bianco3; R. Escobar4; G. Pieroni2; E. Annetta5; J. Frontroth1; G. Sciuccati3
1 Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Laboratorio de Hemostasia y Trombosis. Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr JP Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina, 5 Hospital de Pediatría Prof Dr JP Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: Thrombolytic therapy (TT) indications are limited to acute or subacute occlusive organ‐ limb‐ or life‐threatening thrombosis. Thorough case‐by‐case patient selection is warranted and there is no agreement on the optimal TT regimen in children.
Aims: To describe clinical characteristics, management and outcomes of neonates and children treated with TT at a single tertiary pediatric center. To describe patients' characteristics according to clot resolution.
Methods: Jan/2010‐Dec/2021, consecutive children < 18 years old with organ‐ limb‐ or life‐threatening non‐stroke venous or arterial thrombosis treated with TT were registered. Demographic and clinical data, treatment and outcomes were prospectively collected. Results are expressed as: median (range). Protocol: systemic rtPA, 0.1–0.5 mg/kg/h for 6 h, 1–6 days; infants< 6 months of age received fresh frozen plasma transfusion before each rtPA infusion; heparin was indicated in‐between rtPA doses. Complete, partial or no clot resolution was daily evaluated with imaging methods. Bleeding was classified following the ISTH bleeding scale.
Results: 40pts received rtPA, 25(62.5%)males, age:3.5 months (11 days‐17.9 years). Four patients presented with an unprovoked thrombosis, of whom 2 were diagnosed with hereditary antithrombin deficiency during follow‐up. Mean dose of rtPA was 0.3mg/kg/h (0.1–0.5 mg/kg/h) and treatment duration, 2days (1–6 days). Lag time from diagnosis to TT was 2 days (6hs‐34 days): < 14days in 34pts. All‐cause mortality, 12 pts (30%); death attributable to thrombosis, 3/12pts. There was no mortality due to TT. Patients' characteristics, TT indications and outcomes are depicted in Table1. Clot resolution was observed in 26/40pts (65%, CI 95%: 50.2–79.8%): 11/14(78.6%, CI 95%: 57.1–100%) neonates and 15/26(57.7%, CI 95%: 38.7–76.7%) children. Patients' characteristics according to clot resolution is shown in Table2.
Conclusion(s): In this pediatric registry, most patients achieved successful clot resolution with a low bleeding rate. No differences were observed in the variables analyzed between patients who achieved recanalization versus those who did not, probably due to the limitation of the sample size. Thrombolytic agents could be considered in patients with high‐risk thrombosis to improve outcomes.


244. PB0263
244.1. Whole blood microfluidics to assess direct oral anticoagulants (DOACs) activity in neonatal cardiac patients
T. Diacovo 1; M. Decortin2; S. Diamond2
1 University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 2 University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: VTEs increase morbidity and mortality in neonates with complex congenital heart disease (CCHD). Current prophylaxis consists of UFH or LMWH, requiring repeated testing to ensure therapeutic dosing. DOACs (e.g. Apixaban) are attractive alternatives due to predictable pharmacokinetics, a wide therapeutic window, and limited drug–drug interactions. Although a therapeutic range is established for adults, drug concentrations needed to mitigate clot formation in neonates have not been determined.
Aims: To determine the effects of Apixaban on platelet/fibrin deposition and clot stability using a low volume, multi‐channel microfluidic device on whole blood from neonatal cardiac patients.
Methods: This IRB approved study was conducted at Children’s Hospital of Pittsburgh. A microfluidic device was used to study coagulation during corn trypsin inhibitor‐treated whole blood perfusion (200 s‐1, 15 min) over surface‐immobilized tissue factor and collagen. Blood was drawn immediately before and 24h after surgery when thrombophrophylaxis is typically started. Platelet and fibrin deposition were monitored by fluorescence microscopy (± Apixiban).
Results: 31 neonates were enrolled. Demographics: weight 3.25 ± 0.6 kg; GA at birth 39.1 ± 1 weeks; age at surgery 6.9 ± 3.6 days. Most participants had double ventricle physiology (26/31), required bypass (26/31) and intraoperative blood products (26/31). There were no significant differences (P>0.05) in Hb/Hct, platelet count, or fibrinogen concentrations pre‐ vs post‐surgery. Time to clot‐induced channel occlusion and platelet/fibrin deposition were similar in post‐operative vs adult participants (7.6 ± 3.1min vs 7.9 ± 1.5min, and Figure 1A), the former prolonged (14.2 ± 1.9min) and the latter reduced in pre‐operative neonates. Although Apixaban had a similar effect on platelet/fibrin deposition (Figure 1B), it significantly increased channel occlusion time for of blood from post‐operative neonates vs adults (11.1 ± 4.1min vs 7.9 ± 1.5min, respectively, p < 0.001; 0.25 μM).
Conclusion(s): Majority of post‐operative neonates with CCHD are hypercoaguable compared to the preoperative state and appear to be more sensitive to Apixiban inhibition than adults based on occlusion times.

245. PB0254
245.1. Effects of apixaban versus vitamin K antagonists and low molecular weight heparin in pediatric cardiac patients: Exploratory biomarker analysis from SAXOPHONE a multicentre randomized clinical trial
G. Aborkhees 1; Z. Wang2; C. Yao2; A. Reedy3; R. Payne4; C. Male5; A. Glatz6; J. Dyme7; A. Barr1; A. He2; P. Monagle8; K. Burns9; P. Schafer2; L. Mitchell10
1 University of Alberta, Edmonton, Alberta, Canada; 2 Bristol Myers Squibb, Lawrence Township, New Jersey, United States; 3 Bristol Myers Squibb, Inc, Lawrence Township, New Jersey, United States; 4 Indiana University School of Medicine, Dept of Pediatrics (Cardiology), Indianapolis, Indiana, United States, 5 Medical University of Vienna, Austria, Vienna, Wien, Austria; 6 Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 7 Bristol Myers Squibb, Inc., Lawrence Township, New Jersey, United States; 8 University of Melbourne, and Royal Children's Hospital, Melbourne, Victoria, Australia; 9 Heart Development and Structural Diseases Branch/Division of Cardiovascular Sciences, Bethesda, Maryland, United States, 10 Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada
Background: The safety and efficacy of apixaban for venous thromboembolism (VTE) prophylaxis was assessed in pediatric patients with congenital or acquired heart disease in a multinational trial (SAXOPHONE). Patients in need of extended thromboprophylaxis were randomized 2:1 to receive either apixaban or standard of care (SOC) which was either VKA or LMWH.
Aims: An exploratory biomarker analysis was conducted to better understand mechanisms of the anticoagulation effect in pediatric patients.
Methods: Plasma samples were obtained from study participants at baseline (screening or pre‐dose on Day1), after 2 weeks and 6 months of treatment. Samples were analyzed for FVIII, Fibrinogen, Protein C, Protein S, and D‐Dimer by ELISA. Ex‐vivo thrombin generation was measured using Technoclone TGA. Data from 182 participants were included [apixaban (n = 123) and SOC (n = 59)].
Results: Fibrinogen and FVIII levels showed no difference between treatment groups. Protein C and Protein S were statistically significantly increased in apixaban‐treated patients when compared to those who received SOC (p < 0.005, Table 1). D‐Dimer showed a trend to decrease at Month 6 in both groups without significant treatment differences. Endogenous thrombin potential and peak thrombin levels were higher in the apixaban group compared to SOC. The lag time and time to peak were significantly increased in the apixaban arm compared to SOC (Figure 1).
Conclusion(s): D‐Dimer trended toward a decrease in both apixaban and SOC groups at Month 6 suggesting a reduction in thrombin activation. Protein C and S levels increased in the apixaban group compared to SOC. Thrombin generation patterns differed between the two groups, with apixaban showing more prominent prolongation of lag time and time to peak compared to SOC. No difference was observed in the rate of thrombin generation between groups. SOC treatment reduced the thrombin generated and decreased endogenous thrombin potential compared with apixaban. Additional subgroup analyses based on clinical characteristics are in progress.


246. VPB0288
246.1. Improving thromboprophylaxis in children admitted to a quaternary care children’s hospital with COVID‐19
A. Yan 1; C. Parsons2; G. Caplan3; D. Kelly4; J. Duzan5; E. Drake5; R. Kumar6
1 Boston Children's Hospital and Harvard Medical School, Brookline, Massachusetts, United States; 2 Boston Children's Hospital/Harvard Medical School, Boston, Massachusetts, United States; 3 Boston Children’s Hospital Program for Patient Safety and Quality, Boston, Massachusetts, United States; 4 Division of Medical Critical Care, Boston Children's Hospital and Harvard Medical School, Boston, Massachusetts, United States; 5 Division of Hematology and Oncology, Boston Children's Hospital and Harvard Medical School, Boston, Massachusetts, United States; 6 Harvard Medical School, Boston, Massachusetts, United States
Background: The incidence of venous thrombo‐embolism (VTE) in hospitalized children has increased by 70%–200% over the last 2‐decades. Given this increase, many pediatric centers have initiated electronic clinical decision supports (ECDS) to prognosticate VTE risk and recommend appropriate prophylaxis. COVID‐19 is a risk factor for VTE, however ECDS algorithms developed before the COVID‐19 pandemic may not accurately prognosticate VTE risk in children with COVID‐19.
Aims: To identify areas for improvement of thromboprophylaxis recommendations for children admitted to hospital with COVID‐19.
Methods: Inpatients with a positive COVID‐19 PCR test on admission (or within 24 h) were identified at a quaternary‐care pediatric center between March 1st 2020 and January 20th 2022. The results of the institution’s automated thromboprophylaxis recommendations were compared to institutional best practice guidelines for COVID‐19 thromboprophylaxis and to the thromboprophylaxis actually received by the patient. Using this data, a quality improvement (QI) initiative to improve adherence to COVID‐19 thromboprophylaxis recommendations through ECDS optimization was implemented. This QI study was exempt from ethics approval.
Results: Of the 375 inpatients with COVID‐19 who underwent thromboprophylaxis screening, 43 were excluded as their COVID‐19 was performed >24 h after admission and 5 were excluded for having incomplete data. Table 1 shows the characteristics of the final cohort. 179 (54.4%) patients had a D‐dimer performed during their admission. The number of patients that met criteria for chemo‐prophylaxis via each screening modality is shown in Figure 1. Five inpatients developed VTE; three had VTE symptoms at presentation, two were identified as high‐risk for VTE by both the automated and best practice assessments but were not started on chemoprophylaxis due to family preference or a contraindication to anticoagulation.
Conclusion(s): Automated thromboprophylaxis recommendations developed prior to the COVID‐19 pandemic may not identify COVID‐19 patients needing chemoprophylaxis. Existing ECDS tools need to be updated to reflect COVID‐19 specific risk factors for VTEs.


247. PB0260
247.1. Comparison of heparin‐induced thrombocytopenia workup and clinical characteristics between pediatric and adult patients
O. Cohen 1; K. Lange2; I. Budnik3; A. Lubetsky1; I. Tamarin1; N. Rosenberg1; G. Kenet4; S. Levy‐Mendelovich1
1 Sheba medical center, Tel Aviv University, Ramat Gan, HaMerkaz, Israel; 2 Tel Aviv University, Tel Aviv, HaMerkaz, Israel; 3 Sechenov First Moscow State Medical University, Moscow, Moskva, Russia; 4 Sheba medical center& Tel Aviv University, Tel Aviv, Tel Aviv, Israel
Background: Heparin‐induced thrombocytopenia (HIT) is rare among pediatric patients. The diagnosis of HIT depends upon clinical decision tools to assess its pre‐test probability, supported by laboratory evidence of anti‐PF4/heparin antibodies detected by either immunoassays or functional platelet activation assays.
Aims: To compare the use of the 4T score clinical decision tool, clinical characteristics and laboratory findings between pediatric and adult patients with suspected HIT.
Methods: We compiled all pediatric patients in our center for whom HIT testing was performed during the years 2014–2020. These were compared 1:3 with a cohort of adult patients. Clinical and laboratory data were obtained from the computerized medical records. Laboratory diagnosis of HIT was performed with either particle gel immunoassay (PaGIA), antibody detection by the automated ACL‐TOP 750® or a functional heparin activation test (HAT). Statistical analysis was performed using GraphPad Prism (version 7.0), USA.
Results: The cohort included 34 children (under 18 years) and 105 adults. Adults mostly received heparins for thromboembolism prophylaxis and treatment (72.4%, n = 76), and were more frequently treated with LMWH. Children were mostly exposed during cardiopulmonary bypass and ECMO (61.8%, n = 21), and were more frequently treated with UFH. Compared with adults, children had significantly higher 4T scores (Figure 1). Nevertheless, adults had a slightly higher rate of a positive diagnosis of HIT (Figure 2). Six out of 16 adult patients with a positive HIT test presented with thrombosis (37.5%) whereas all 3 pediatric patients with positive HIT test presented with thrombosis (p value 0.087).
Conclusion(s): In children laboratory tests for HIT are performed in accordance with the 4T score more often than in adults. Despite this, a positive diagnosis of HIT in children remains rare. A potentially higher incidence of thrombosis in children may be attributable to the severity of underlying illness.


248. PB0276
248.1. Real world data of pediatric thromboembolic disease by the international pediatric thrombosis network
C. van Ommen 1; M. Albisetti2; M. Bhatt3; B. Branchford4; A. Chan5; N. Goldenberg6; S. Holzhauer7; C. Male8; P. Monagle9; U. Nowak‐Gottl10; S. Revel‐Vilk11; G. Sciuccati12; N. Sirachainan13
1 Erasmus Medical Center‐Sophia Children’s, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 University Children’s Hospital, Zürich, Switzerland, Zürich, Zurich, Switzerland; 3 McMaster Children's Hospital, Hamilton, Ontario, Canada; 4 Versiti, Milwaukee, Wisconsin, United States; 5 McMaster University, Hamilton, Ontario, Canada; 6 John Hopkins All Children's Hospital, St Petersburg, Florida, United States; 7 Charité University Medicine, Charite, Berlin, Germany, Berlin, Berlin, Germany, 8 Medical University of Vienna, Austria, Vienna, Wien, Austria; 9 University of Melbourne, and Royal Children's Hospital, Melbourne, Victoria, Australia, 10 University Hospital Schleswig‐Holstein, Kiel, Hamburg, Germany, 11 Shaare Zedek Medical Center, Jerusalem, Yerushalayim, Israel, 12 Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina, 13 Department of Pediatric, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Background: Pediatric thromboembolism (TE) is a rare disease. In 2017, we established an International Pediatric Thrombosis Network (IPTN) to improve the care of children with TE. The IPTN currently consists of 92 pediatric thrombosis centers in 27 countries. Through the IPTN, we established a prospective disease registry, the Throm‐PED registry, that brings together real world data on arterial (ATE) and venous TE (VTE) in children of all age groups globally.
Aims: To report the epidemiology, risk factors and management of children enrolled in the Throm‐PED registry.
Methods: An international, multicentre, prospective observational cohort study of children (< 18 years) with TE. The following data are prospectively collected in the basic registry: age at diagnosis, gender, TE type, location, risk factors, and treatment. In addition, the Throm‐PED registry contains four sub‐registers: safety and efficacy of DOACs, neonatal renal vein thrombosis, catheter‐related ATE, and adolescent TE.
Results: The number of included children increased from 148 in 2019 (5 centers) to 895 in January 2022 (15 centers). Of 895 children, 795 had VTE (88.8%) and 100 had ATE (11.2%) (Table 1). Age distribution of VTE was bimodal with peaks in neonates and infants < 1 year and teenagers. Almost 80% of ATE children were younger than 8 years. Catheters, surgery, and congenital heart disease were more frequent in younger children with VTE, whereas thrombophilia, obesity, malignancy and immobility were more common in teenagers. Unprovoked VTE and infection were more common in males than females, whereas obesity and oral contraceptives were more frequent in females. DOACs were increasingly used, especially in teenagers.
Conclusion(s): The Throm‐PED registry is an important tool to collect real world prospective data of pediatric TE which can provide post marketing surveillance data to validate previous clinical trials, identify geographic variations in clinical care, and assist in identifying optimal questions for future clinical trials.

249. VPB0280
249.1. Retrospective case series study of patients with may‐Thurner syndrome in a single tertiary pediatric center
J. Brauner; R. Aneja; S. Yilmaz; J. Cooper; F. Xavier
University of Pittsburgh, Pittsburgh, Pennsylvania, United States
Background: The 2018 ASH Pediatric Guidelines for Management of Venous Thromboembolism suggests against using thrombolysis in symptomatic deep venous thrombosis (DVT) patients (conditional recommendation).
Aims: Here we present a single center case series of 25 pediatric patients with May‐Thurner Syndrome. Patients underwent thrombolysis followed by anticoagulation with little complications and no long‐term significant post‐thrombotic syndrome (PTS), challenging current guidelines. Current data using anticoagulation only demonstrates up to 80% PTS in this age group, suggesting alternative treatment regimens should be explored.
Methods: Deidentified data were extracted from outpatient records under institutional IRB exempt STUDY21050204, from the last 10 years. Thrombolysis consisted of institutional protocol using t‐PA (0.03mg/kg/h), AngioJet (Boston Scientific) and unfractionated heparin infusion (10 u/kg/h). All patients received outpatient anticoagulation. Diagnosis of May‐Thurner Syndrome was confirmed by venogram. Anticoagulation was started immediately after thrombolysis for 3 months followed by prophylaxis. PTS was assessed using modified Villalta Score (VS) and Manco‐Johnson Instrument (MJI).
Results: Of 25 patients, 23 had analyzable data. Median age: 16 years old (80% females, 80% white). Presentation of symptoms to procedure: 4 days average. 78% with complete occlusion of, at least, one deep vein (Femoral, External Iliac, and Common Iliac). 46% of patients received a 2‐day procedure and 81% received stent placement. Complications: 4 patients dropped ≥ 2g/dl hemoglobin, none required red cell transfusions, there were no bleeding in critical areas or compartment syndrome. Only 2 patients had readmissions for a second procedure due to recurrence of thrombosis. No patient scored moderate or higher in the VS nor Physically and Functionally Significant PTS in the MJI.
Conclusion(s): Thrombolysis in pediatric patients with May‐Thurner Syndrome can be safe and effective, and may be considered in experienced pediatric tertiary centers. Further data is urgently needed to standardize care and avoid PTS is this young healthy population.
250. VPB0281
250.1. The use of Apixaban for thromboprophylaxis and treatment in children with cardiac disease younger than 6 months of age
P. Esteso 1; R. Kobayashi2; R. Williams3; C. Ventresco2; B. Hawkins2; M. Cetatoui2; J. Esch1; C. VanderPluym4
1 Boston Children's Hospital, Harvard University, Boston, Massachusetts, United States; 2 Boston Children's Hospital, Boston, Massachusetts, United States; 3 Mattel Children’s Hospital at UCLA, Los Angeles, California, United States; 4 Children’s Hospital, Harvard University, Boston, Massachusetts, United States
Background: Antithrombosis for children with congenital heart disease (CHD) is complicated by developmental hemostasis, difficult administration and monitoring. The evaluation of novel anticoagulation strategies is critical. To our knowledge, we report the first use of Apixaban in this age group with commercially available dosing.
Aims: Evaluate safety (clinically relevant non‐major (CRNM) or major bleeding; thrombotic events) and efficacy (thrombosis improvement and resolution) of Apixaban in children with CHD.
Methods: Retrospective single‐center analysis of children < 6 months old with CHD treated with Apixaban.
Results: From 1/2020–12/2021, 24 children < 6 months received Apixaban, median age 4.7 months (0.4–5.9), median weight 5.2 kg (2.9–6.5), for 2815 total days (1–396). Single ventricle CHD accounted for 14/24. Eight required thromboprophylaxis, 16 treatment (8 arterial, 1 intracardiac, 10 venous; 4 multifocal). Over half (13) received concomitant aspirin. Smallest dose possible using 2.5 mg tablets was 0.625 mg BID (21/24 patients) with median dose of 0.15 mg/kg/dose, median peak level (Apixaban chromogenic anti‐Xa assay, HE‐Stago 2) of 205 ng/ml (66–396, n = 21). Based on level and indication, 10 patients required 1.25 mg BID (0.22 mg/kg/dose). Those requiring 0.625 mg BID were smaller (4.65 vs 5.5 kg). There was no major bleeding and 2 patients had CRNM bleeding (blood‐streaked stools) prompting discontinuation of Apixaban. One received concurrent aspirin. Supratherapeutic level of >400 ng/ml at minimum dose in 1 patient, in the absence of any symptoms, prompted discontinuation as a precautionary measure. One patient was transitioned to daily dosing without difficulty in achieving goal peak and trough levels. Two patients died during the study period due to progression of their underlying disease, for reasons unrelated to anticoagulation. Apixaban was associated with thrombus improvement or resolution in 11/16 (69%) patients.
Conclusion(s): Apixaban administration with commercially available tablets was feasible in young children with low rate of bleeding events.
251. Platelet Disorders, von Willebrand Disease and Thrombotic Microangiopathies
251.1. Acquired Thrombocytopenias
252. VPB1220
252.1. A rare case of chronic immune thrombocytopenia with autoantibodies inhibiting platelet aggregation. Effects of romiplostim
N. Tsvetaeva1; O. Nikulina1; S. Vasiliev2; S. Khaspekova3; A. Mazurov 3
1 National Medical Research Center for Hematology, Russian Ministry of Health, Moscow, Moskva, Russia; 2 National Medical Research Center for Hematology, Russian Ministry of Health, Moscow, Moskva, Russia; 3 National Medical Research Center for Cardiology, Russian Ministry of Health, Moscow, Moskva, Russia
Background: Immune thrombocytopenia (ITP) is the common cause of impaired platelet hemostasis. Autoantibodies suppressing platelet function might increase the risk of hemorrhagic complications in ITP patients.
Aims: We described a rare case of chronic ITP with the production of autoantibodies against glycoprotein (GP) IIb‐IIIa, inhibiting platelet aggregation
Methods: Platelet‐associated IgG (PA‐IgG) were measured by binding of anti‐human IgG 125I‐labelled antibodies, and circulating autoantibodies by interaction with donor platelets using ELISA. Glycoproteins (GP) IIb‐IIIa and Ib were evaluated by binding of radio‐ or fluorescently labeled monoclonal antibodies (monoAB). Platelet aggregation was assessed by light transmission aggregometry.
Results: Patient S. (female) applied to the clinic for the first time in 2008 (age 32) due to hemorrhagic syndrome (petechiae, bruises, nosebleeds). Medical history included menorrhagia during adolescence, normal pregnancy and delivery. Platelet count was normal (215x10^9/L), but no ADP‐induced aggregation and no binding of anti‐GP IIb‐IIIa monoAB (with normal binding of anti‐GP Ib monoAB) were detected. Glanzmann's thrombasthenia was suspected and tranexamic acid was prescribed. In 2009 the patient applied to the clinic with hemorrhages and deep thrombocytopenia (< 10x10^9/L). At that time ITP was diagnosed due to PA‐IgG increase and presence of circulating antiplatelet autoantibodies. The patient's plasma inhibited ADP‐induced aggregation and binding of anti‐GP IIb‐IIIa monoAB. For the next two years, the patient received glucocorticosteroids followed by rituximab, and splenectomy was performed. Treatment caused a short‐term slight increase of platelet count (27–63 × 109/L) with a rapid loss of response and recurrence of hemorrhages. Romiplostim therapy was started in 2011 and continued up to the present time. Despite still slightly increased PA‐IgG romiplostim treatment resulted in normalization of platelet count and aggregation, and the absence of significant hemorrhages.
Conclusion(s): Autoantibodies against GP IIb‐IIIa, blocking platelet aggregation, complicated the course of chronic ITP. Romiplostim supported normal platelet count, restored platelet aggregation, and prevented bleedings. Support RFBR # 20‐015‐00246
253. VPB1219
253.1. The relationship between platelet specific antibodies and the severity of newly diagnosed pediatric ITP
S. Dong
Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, Beijing, China (People's Republic)
Background: Background: Immune thrombocytopenia (ITP) is an autoimmune mediated hemorrhagic disease characterized by thrombocytopenia. Platelet specific antibodies play a key role in platelet destruction.
Aims: This study was aimed to determine whether platelet specific antibodies (including anti‐GPIIb/IIIa, anti‐GPIb/IX and anti‐GPIa/IIa antibodies) are related to the severity of newly diagnosed pediatric ITP.
Methods: This study retrospectively analyzed 154 newly diagnosed ITP patients from October 2016 to September 2021 in Beijing Children's Hospital. Children who used only glucocorticoids during hospitalization were defined as Severity 1. Children who used glucocorticoids combined with intravenous immunoglobulin (IVIG) and/or single rhTPO were defined as Severity 2. Platelet specific antibodies were detected by PAKAUTO.
Results: When the antibody levels were divided into positive and negative, children with positive anti‐GPIIb/IIIa antibody are more severe than children with negative anti‐GPIIb/IIIa antibody in newly diagnosed ITP (2 = 3.969, p = 0.046). When the antibody titer ratio was used to reflect the antibody levels, there was no significant difference between platelet specific antibodies and the severity of newly diagnosed pediatric ITP.
Conclusion(s): Anti‐GPIIb/IIIa antibody is a sign of higher severity in newly diagnosed pediatric ITP. anti‐GPIb/IX and anti‐GPIa/IIa antibodies were not found to be related to the severity of ITP.
254. PB1216
254.1. The association between vitamin D and disease activity in adult patients with chronic primary immune thrombocytopenia
K. Van Eeden 1; W. van Dijk2; R. Schutgens3
1 Utrecht University/UMC Utrecht, Utrecht, Utrecht, Netherlands; 2 UMC Utrecht, Utrecht, Utrecht, Netherlands; 3 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: Vitamin D deficiency is associated with several autoimmune diseases and increases the autoimmune response. For immune thrombocytopenia (ITP), vitamin D deficiency was found to be associated with disease activity in children, but has never been assessed in adults.
Aims: To evaluate the association between vitamin D levels and disease activity in adults with chronic primary ITP.
Methods: This is a single‐center cross‐sectional study, in which patients with a confirmed diagnosis of chronic primary ITP with current platelet counts of < 100x10^9/L or with a current treatment for chronic primary ITP were included. Serum 25‐OH‐vitamin D and platelet count were determined in patients using blood samples. Vitamin D deficiency was defined as serum 25‐OH‐vitamin D < 50nmol/L. High disease activity was defined as platelet counts < 30x10^9/L or current treatment for ITP. Logistic regression was used to assess the relation between vitamin D deficiency and disease activity.
Results: 57 patients (61% female, mean age 45 (range 17–82)) were included, of whom 23 (40%) had high disease activity and 28 (49%) had a vitamin D deficiency. 13 out of 28 (46%) patients with a vitamin D deficiency had high disease activity compared to 10 out of 29 (34%) in the patients without a vitamin D deficiency ( odds ratio 1,65 (95% CI 0.57–4.78; p = 0.36)).
Conclusion(s): In this study, no significant association was found between vitamin D deficiency and disease activity in adult patients with chronic primary ITP.
255. VPB1221
255.1. A retrospective analysis of 45 cases of HIV‐associated thrombocytopenia in a single center over ten years
Y. Tan 1; L. Che1; H. Bi1; S. Fan2; H. Min2; Z. Zeping3
1 The Second Affiliated Hospital of Kunming Medical University, kunming, Yunnan, China (People's Republic); 2 Yunnan Infectious Diseases Specialist Hospital, kunming, Yunnan, China (People's Republic); 3 The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China (People's Republic)
Background: Thrombocytopenia is one of the most common hematological complications of HIV infection. Prior to the advent of highly active antiretroviral therapy (HAART), the incidence of HIV‐associated thrombocytopenia was estimated at 10–30%.
Aims: To analyze the clinical characteristics and treatment effects of patients with HIV infection combined with thrombocytopenia.
Methods: To retrospectively analyze the clinical manifestations and medical records of 45 patients diagnosed with HIV/AIDS combined with thrombocytopenia in the Department II of Infectious Diseases of Yunnan Infectious Diseases Specialist Hospital between January 2010 and December 2020. Comparing the therapeutic effects of different treatment and exploring the factors affecting platelet counts in HIV patients.
Results: (1)The median platelet count at admission was 10 (0–64 × 109/L) in 45 cases, and 31 patients had a platelet count < 20×109/L. (2) 1 month after treatment, the median platelet count of patients was 37 (4–309) × 109/L, and the median time to reach R was 26 (12–44) days. The total follow‐up time was 79 (14–368) days, 18 patients did not respond to treatment, 12 patients experienced relapse during the follow‐up period, and the median platelet count of 14 patients was 148 (104–230) × 109/L as of the last follow‐up time, different protocols have different treatment responses (Figure 1). (3) The number of CD4+ T cells at the initial diagnosis had no significant effect on platelet count, treatment effect, nor relapse rate (Z = −0.104, p = 0.917; χ2 = 0.015, p = 0.901; χ2 = 1.361, p = 0.243). (4) Bleeding score was significantly lower in hepatitis B patients compared with uninfected patients (Z = −2.504, p = 0.011). Platelet count was significantly lower in hepatitis C positive patients compared with uninfected patients (Z = −2.855, p = 0.003). The risk of bleeding was less in HIV patients than in uninfected patients when platelets were reduced (Figure 2).
Conclusion(s): Patients with HIV combined with thrombocytopenia have a low response rate to treatment and tend to relapse. Hepatitis C virus infection was significantly associated with thrombocytopenia.


256. PB1203
256.1. Fostamatinib for the treatment of immune thrombocytopenia in a real‐world setting: A case series of five patients
R. Grantab1; V. Markovtsov 2; C. Hsia3
1 Medison Canada, Toronto, Ontario, Canada; 2 Rigel Pharmaceuticals, Inc., South San Francisco, California, United States; 3 London Health Sciences Centre, Victoria Hospital, London, Ontario, Canada
Background: Immune thrombocytopenia (ITP) is a heterogenous, immune‐mediated disorder characterized by an increased risk for bleeding due to low platelet numbers. Standard treatment guidelines are not always applicable in real‐world settings, particularly in the elderly population, making consideration of patient‐centric factors critical for successful ITP management. Based on specific patient factors such as age, cardiovascular risk factors, suitability for splenectomy, and patient therapy preference (including awareness of likely treatment‐related adverse events [AEs]), five patients were treated with fostamatinib, an oral spleen tyrosine kinase (SYK) inhibitor that averts platelet destruction.
Aims: This case series describes fostamatinib treatment for patients with different severities of ITP in a real‐world setting.
Methods: ITP patients prescribed fostamatinib were identified through a retrospective chart review.
Results: Five patients (mean age: 76 years, 3 male) were identified, all with underlying cardiovascular risk due to comorbidities or age. The mean (±SD) platelet count at diagnosis was 16,400/μl (±8142). All patients had previously received corticosteroids, with 2 patients on prior thrombopoietin receptor agonists. Three patients could not undergo splenectomy due to cardiovascular risk. Fostamatinib was prescribed as a second‐line therapy to 3 patients who had received only prior corticosteroids and/or IVIg and to 2 patients with persistent ITP (see Table). All patients had a platelet response (≥50,000/μl) following fostamatinib initiation (4/5 on 100 mg BID and 1/5 on 150 mg BID). As of November 15, 2021, all patients were continuing with fostamatinib (treatment period range: 2–6 months). Fostamatinib was prescribed to 4 patients >65 years old. No AEs or bleeding events were reported in any patient.
Conclusion(s): In this case series of 5 patients with ITP, factors such as age and cardiovascular risk factors influenced choice of treatment. Fostamatinib elicited a platelet response and was well‐tolerated in all patients demonstrating treatment benefit in different clinical settings in the real‐world management of ITP.

257. PB1208
257.1. Discontinuation of thrombopoietin receptor agonists: A 10 year real‐world experience from an academic hospital in Madrid
A. Ramírez López 1; M. Alvarez‐Román2; M. Martín Salces1; M. Rivas Pollmar3; E. Monzón Manzano1; E. Arias Salgado1; P. Acuña1; V. Jiménez Yuste4; N. Butta1
1 Hospital Universitario La Paz‐IdiPaz, Madrid, Madrid, Spain; 2 Hospital Universitario La Paz, Madrid, Madrid, Spain; 3 Hospital Universitario La Paz‐Idipaz, Madrid, Madrid, Spain; 4 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain
Background: Thrombopoietin receptor agonists (TPO‐RAs) have become the second line treatment for immune thrombocytopenia (ITP) patients. Despite TPO‐RAs are currently indicated for continuous treatment, some patients can achieve a sustained response that allows tapering and discontinuation. Predictors of responses and relapses after discontinuation are unknown factors that are being under investigation.
Aims: We aimed to determine the proportion of patients achieving long‐term remission after TPO‐RAs discontinuation.
Methods: This is a retrospective study approved by Hospital Universitario La Paz Ethical Committee. In a preliminary analysis, we described our experience of 10 years in discontinuation of TPO‐RAs and relapses in the ITP patient cohort from Hospital Universitario La Paz in Madrid.
Results: One‐hundred‐fifty‐one ITP patients were treated with TPO‐RAs between 1st January 2011 and 31st December 2020. We excluded those without complete information, analyzing a total of 133 patients. Thirteen received Romiplostim, 7 men and 4 women, with a median age of 66y (range 3–98). All of them achieved a clinical response according to standard criteria and in four it was possible to discontinue TPO‐AR, with no need of retreatment. Seventy‐five patients were treated with Eltrombopag, 35 men and 49 women, with a median age of 49y (range 1–98). Seventy‐one patients got a clinical response, which allows discontinuation of TPO‐AR in 43 of them, with 25 sustained responses. We also included 45 ITP patients, 21 men and 24 women, median age of 53y (range 2–93), who received both TPO‐AR because of lack of response or intolerance with the first (4 Romiplostim and 41 Eltrombopag). Among the 34 patients who achieved a response, 17 were able to discontinue TPO‐ARs and 7 needed retreatments. Globally, 39 patients discontinue TPO‐RAs with no need of further ITP treatment. Results are summarized in Table 1.
Conclusion(s): Twenty‐nine% (39/113) of ITP patients who were treated with TPO‐RAs could discontinue this treatment without relapses.

258. PB1195
258.1. Optimized protocol for measurement of platelet desialylation level allows to determine immune thrombocytopenia patients’ responsiveness to first‐line therapy
O. An 1; A. Martyanov1; A. Ignatova2; M. Pankrashkina3; V. Ptushkin3; F. Ataullakhanov1; M. Panteleev1; A. Sveshnikova2
1 Center for Theoretical Problems of Physicochemical Pharmacology, Moscow, Moskva, Russia; 2 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 3 Moscow City Center for Hematology, Botkin Hospital, Moscow, Moskva, Russia
Background: The removal of sialic acid residues (SA) from platelet glycoproteins is among the most important markers for platelet clearance. Decreased amount of SA was observed in patients with immune thrombocytopenia (ITP), an acquired autoimmune bleeding disorder. However, the existing approaches to platelet desialylation assessment provide controversial results, those limit their applicability for routine ITP diagnostics.
Aims: Evaluation of different protocols for the characterization of platelet desialylation.
Methods: Whole blood of 10 healthy donors and 13 ITP patients was collected into sodium citrate‐containing tubes in accordance with the declaration of Helsinki. Platelets were washed using three sequential centrifugations and resuspended in Tyrode’s buffer in the presence/absence of BSA and calcium. Desialylation was measured using lectins from Erythrina cristagalli and Ricinus communis in normal or in 2% PFA fixed samples. The desialylation level was scaled to the desialylation maximum, obtained upon platelet incubation with neuraminidase from Cl.perfringens. Alpha‐ and dense granule release was monitored by anti‐CD62p and mepacrine, correspondingly. Samples were analyzed by flow cytometry. Mann‐Whitney test was used for statistical analysis.
Results: Lectin binding to platelets achieved saturation after 20 min of incubation at 37°C. Platelet stimulation with ADP (10 μM), SFLLRN (4.5 μM) or AYGPKF (4.5 μM) resulted in a detectable increase in the desialylation level only in presence of calcium. Furthermore, platelet desialylation correlated with P‐selectin exposure and dense granule release (r = 0.935 and r = 0.503). Finally, fixation with PFA caused platelet desialylation even without platelet activation. The developed protocol was applied to blood samples from ITP patients. Refractory to first‐line therapy patients had significantly increased platelet desialylation (p = 0.0061).
Conclusion(s): We conclude that the measurement of platelet desialylation should be performed in the presence of calcium, while fixation should be avoided. The protocol allowed to distinguish ITP patients, refractory to first‐line therapy. Study was supported with RSF grant 21‐45‐00012.
259. PB1197
259.1. Do regulatory T cells reemerge with resolution of childhood immune thrombocytopenia?
P. Bangalore Parthasarathy 1; M. Mundell2; B. Race2; P. Rayman2; M. Diaz2; S. Swaidani2; R. Talati3; K. McCrae4
1 Cleveland Clinic Children's Hospital, Rocky River, Ohio, United States; 2 Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, United States; 3 Cleveland Clinic Children's Hospital, Cleveland, Ohio, United States; 4 Taussig Cancer Institute, Cleveland Clinic, Cleveland, Ohio, United States
Background: Escaping surveillance from regulatory T cells (Tregs) may contribute to the pathogenesis of immune dysregulation leading to Immune Thrombocytopenia (ITP). Deficiency in this Treg compartment of the CD4+T cells may lead to loss of tolerance. A reemergence of Tregs would therefore be expected with clinical resolution of ITP.
Aims: To quantify Tregs in pediatric patients with ITP at diagnosis and at resolution or persistence of ITP, followed prospectively through evolution of disease.
Methods: Two patients were enrolled on the protocol and informed consent approved by the Institutional Review Board (IRB). Peripheral blood samples were collected at diagnosis, resolution in the first, and during persistent ITP in the second patient. Peripheral Blood Mononuclear Cells (PBMCs) were analyzed at each time point. Flow cytometry for CD4+/CD25hi/CD127lo Tregs was performed on a Becton Dickenson FACSFortessa and analyzed using FlowJo software after appropriate staining.
Results: Both previously untreated males with newly diagnosed ITP presented with a platelet count of 4 × 109/L. The first was a 20‐year‐old with bruising and received two intravenous immunoglobulin (IVIg) doses and prednisone with subsequent resolution within three months. Tregs were 0.56% at diagnosis and 0.064% at resolution (Figure 1). The second was a 17‐year‐old presenting with epistaxis, who received the same treatment as the former, developed persistent ITP requiring weekly romiplostim. Treg measurement was 0.4% at diagnosis and 0.066% five months later with persistence of disease (Figure 2).
Conclusion(s): In this small study, Tregs were not found to improve with resolution of disease or with the use of a thrombopoietin receptor agonist, arguing against a critical role for Tregs in ITP resolution. The function of reduced Tregs in ITP on T‐effector cells and autoreactive B cells needs further characterization by transcriptomic methods to better illustrate immune dysregulation in ITP before and after therapy. Funding was received from the Ellen and Steven Ross Research Award.


260. PB1205
260.1. Clinical and laboratory characteristics of adults with immune thrombocytopenia in a contemporary cohort in Austria
C. Ungerböck1; T. Schramm2; D. Mehic 3; M. Fillitz4; B. Dixer4; C. Ay5; I. Pabinger5; J. Gebhart2
1 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 3 Medical University of Vienna, Vienna, Wien, Austria; 4 Department of Internal Medicine, Hanusch Krankenhaus, Vienna, Austria, Vienna, Wien, Austria; 5 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Due to the rarity of primary immune thrombocytopenia (ITP), clinical data on adult patients with primary ITP are scarce.
Aims: The Vienna ITP biobank aims to capture longitudinal clinical data to further characterize Austrian ITP patients.
Methods: The Vienna ITP biobank is an ongoing dual centre biobank (Medical University of Vienna; Hanusch Hospital) that prospectively collects data of patients diagnosed with primary ITP (EC 1843/2016). Patients aged > 18 years are included. At each visit blood is taken for laboratory analyses and the individual medical and bleeding history of each patient is recorded. Bleeding severity is assessed using the ITP ISTH bleeding assessment tool (SMOG BS) and Vicenza BS.
Results: Of 137 included patients, 2 withdrew consent, and 6 were uncovered as secondary ITP after recruitment. Thus, the study cohort comprises a total number of 129 patients. Patients’ and ITP related characteristics are shown in Tables 1 & 2. In total, 27 patients had acute, 13 persistent and 88 chronic ITP at study inclusion. The majority of patients were female, and the mean age was 44.8 years. Half of the patients were under ITP treatment at the time of recruitment and 17 (13.2%) were splenectomized. The median (range) bleeding score was 1.50 (0.0–14.0), 49 patients (38.0%) had no bleeding. Anti‐platelet antibodies were detected in 40 patients (31.0%), most of them directed against GP IIb/IIIa. 18 patients (19.8%) had positive antiphospholipid antibodies (APLA), of whom 12 had APLA above the relevant cut‐off and 2 fulfilled the diagnosis of antiphospholipid syndrome. Seven patients (7.7%) were double positive, 4 patients (4.4%) had triple positivity.
Conclusion(s): Our cohort of ITP patients shows typical characteristics in respect to clinical and laboratory factors such as age distribution, female predominance, or antibody positivity. We detected a high rate of APLA positivity whose persistence and clinical implications needs to be proven.


261. PB1211
261.1. Psychological support for patients with Vaccine‐induced Immune Thrombotic Thrombocytopenia (VITT) in Germany
L. Schönborn 1; M. Braune‐Krickau2; L. Stiefel2; A. Greinacher3; H. Grabe2
1 Universitätsmedizin Greifswald, Greifswald, Germany, Greifswald, Mecklenburg‐Vorpommern, Germany; 2 Department of Psychiatry and Psychotherapy, University Medicine Greifswald, Greifswald, Germany, Greifswald, Mecklenburg‐Vorpommern, Germany; 3 Universitätsmedizin Greifswald, Greifswald, Mecklenburg‐Vorpommern, Germany
Background: In Germany, 68 patients with laboratory‐confirmed vaccine‐induced immune thrombotic thrombocytopenia (VITT) are enrolled in a follow‐up study of their anti‐PF4‐antibody response. During contact to the respective patients, it became evident, that beside a great variability of physical sequelae some of them also suffer from psychological distress.
Aims: We offered psychological support to patients who suffer from mental stress due to their experiences in connection with VITT. A particular challenge was that the patients are located throughout Germany with long distances to Greifswald. Therefore, both diagnostics and therapy were carried out telemedically.
Methods: VITT patients were offered to send in a brief symptom inventory (BSI‐18) and provide informed consent, if they wanted to be contacted by specialized psychologists. To assess amount and severity of their symptoms, standardized questionnaires were sent (Table 1). Additionally, contacts between patients were realized as group video calls.
Results: Of 68 patients enrolled into the follow‐up study, 20 sent back the first BSI‐18 questionnaire and showed interest to be contacted by a psychologist; 14 completed all questionnaires (females n = 10; median age 33.5y, range 23–74 years). After first telephone call, ten patients expressed interest in further psychological treatment (14.7%). Most of these patients were in good mental and physical state before vaccination. Patients showed a wide range in the level of the regained functioning. In the IES‐R‐5, six patients described symptoms that signify the likely presence of a post‐traumatic stress disorder, especially those with delayed diagnosis due to the novelty of VITT. Nine patients suffered from fatigue, six severely. VITT complications following vaccinations against COVID‐19 were associated with great anxiety and sometimes trauma related physical symptoms.
Conclusion(s): Clinicians should be aware that psychological disorders can appear after VITT. Patients benefit from psychological support to cope with impairments of daily life, increased fatigue and increased anxiety in regard to decision making for booster vaccinations.

262. PB1199
262.1. Changing treatment paradigm in second line treatment of adult ITP patients with the availability of TPO agonists: A retrospective study from a tertiary care center in India
N. Gupta1; R. Balasubramanian2; Y. Sivaprakasm2; D. Gupta 2
1 Ganga Ram Institute for Postgraduate Medical Education and Research, New Delhi, Delhi, India; 2 Sir Ganga Ram Institute for postgraduate medical education and research, New Delhi, Delhi, India
Background: Optimal treatment of adults with immune thrombocytopenia who do not respond to steroid or respond transiently is not known. Second line treatment options include immunomodulatory drugs, rituximab, emergency splenectomy or thrombopoietin (TPO) receptor agonists. Here we report our experience of treating these patients from a tertiary care center in India.
Aims: To evaluate treatment pattern and response in steroid refractory acute and persistent severe ITP patients.
Methods: Retrospective review of medical records of patients with ITP who did not respond to initial steroid therapy or who showed only a transient response was done from January 2017 to December 2021. Outcome data was analyzed for 2 line and subsequent treatment.
Results: A total of 56 patients (male 28: female 28) were identified. Eltrombopag was our preferred second line therapy and was given alone to 28 patients with 19 (75%) responded. Dapsone alone was given to 23 patients and lead to a response in 7 (30%) patients. Azathioprine alone was used in 11 patients and 3(27%) responded. Romiplostim alone was used in 7 patients with 2 (28%) response. TPO agonists were used in combination therapy in 11 patients (eltrombopag with Azathioprine in 4, or eltrombopag with dapsone in 3 patients) with 100% response which were maintained on withdrawal of eltrombopag. Similarly, romiplostim was used with azathioprine in 4 patients and 3 showed and complete response. Rituximab was given in 9 patients with 6(66%) response. Two patients underwent splenectomy and both responded. Of note three lines of therapies were given in 12/56 (22%) and 4 lines in 6/56 (11%) patients.
Conclusion(s): Outcome to steroid refractory acute and persistent adult ITP patients have improved with the availability of TPO agonists. Older therapies like dapsone or azathioprine still had a role to play in a resource constraint setting. TPO agonists can be safely and effectively combined with immunomodulatory drugs.
263. VPB1224
263.1. Autoimmune mediated hematological disease associated with COVID‐19 disease or vaccination: A multi‐centre case series
C. Wynick; H. Sun
University of Alberta, Edmonton, Alberta, Canada
Background: Case reports of autoimmune hematological diseases secondary to COVID‐19 infection or vaccination are emerging. However, there are limited case series that analyze patterns of presentation, treatment patterns and responses. Most reports have limited follow‐up duration, occurred prior to the incorporation of corticosteroid in COVID‐19 treatment, and did not report on vaccine re‐challenge.
Aims: To characterize case presentations, severity, treatment courses and response of immune thrombocytopenia (ITP), autoimmune hemolytic anemia (AIHA) and thrombotic thrombocytopenic purpura (TTP) associated with COVID‐19 infection, or vaccination.
Methods: In this multi‐centre case series, adults (>18) diagnosed with de novo or relapsed ITP, AIHA and TTP associated with COVID‐19 infection or vaccination (January‐December 2021) were recruited from hematologists at two academic hospitals in Edmonton, Alberta (population 1.5 million). The Brighton Collaboration criteria was used to adjudicate diagnostic certainty. Research ethics approval and informed consent were obtained.
Results: Thirteen patients presented with 14 autoimmune hematological complications (Tables 1–2). Following the BNT162b2 (n = 7) or mRNA‐1273 (n = 2) vaccine, we diagnosed 6 cases of ITP (5 de novo) and 3 AIHA (1 de novo). A greater proportion of hematological disorders developed after second‐doses of vaccine compared to first (66 vs 33%). Three individuals received subsequent doses of COVID‐19 vaccine without disease relapse. In addition, 5 patients developed ITP (3), AIHA (1) and relapsed TTP (1) precipitated by COVID‐19 infection. Among 8 patients with de novo autoimmune hematological disorders, the majority (75%) achieved complete or partial response at a median follow‐up of 4.6 months, only three individuals had relapses requiring subsequent treatment. All patients were managed with glucocorticoids, 3 received IVIG and 4 required second‐line therapy.
Conclusion(s): Autoimmune hematological conditions due to COVID‐19 infection or vaccination present, and are successfully managed similarly to cases unrelated to COVID‐19. Among vaccine‐associated hematological disorders, repeat doses of vaccine may be safely administered.


264. PB1214
264.1. Effect of eltrombopag on platelet receptor expression and activation in patients with immune thrombocytopenia
W. van Dijk1; G. Poolen2; H. Koene3; R. Fijnheer4; N. Thielen5; E. van Bladel6; K. van Galen7; R. Schutgens 8; R. Urbanus9
1 UMC Utrecht, Utrecht, Utrecht, Netherlands; 2 University Medical Center Utrecht, Utrecht, Utrecht, Netherlands; 3 St. Antonius Hospital, Utrecht, Utrecht, Netherlands; 4 Meander Medical Center, Amersfoort, The Netherlands, Amersfoort, Utrecht, Netherlands; 5 Diakonessenhuis, Utrecht, Utrecht, Netherlands; 6 Slingeland Hospital, Doetinchem, Gelderland, Netherlands, 7 van Creveldkliniek, UMC Utrecht, Utrecht, Utrecht, Netherlands; 8 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 9 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: Alterations in platelet function may explain the increased rate of thrombosis associated with eltrombopag treatment in immune thrombocytopenia (ITP).
Aims: To assess the effect of eltrombopag on platelet function.
Methods: This prospective multicenter cohort study included adult (age ≥16) primary ITP patients who started eltrombopag treatment. Flow cytometry in whole blood was performed before start of eltrombopag (timepoint A) and 2–3 weeks after initiation (timepoint B) and evaluated the following parameters: in vivo expression of glycoprotein (GP) Ib, GPVI, α2β1, αIIbβ3 (activated and unactivated), and p‐selectin, and activated αIIbβ3 and p‐selectin expression after stimulation by adenosine diphosphate (ADP; dose 60/30/15 μM) and thrombin receptor agonist peptide (TRAP; dose 25/12.5/6.25 μM), respectively. The effect of eltrombopag on each platelet parameter was assessed by linear regression, corrected for baseline values. An interaction term assessed whether the relation differed depending on response after 2–3 weeks (defined as platelets ≥50*109/L), and eltrombopag dose at start and timepoint B, respectively.
Results: 17 patients were included (mean age 53 ± 14, 41% female, ITP duration 4 years (interquartile range 2–13), and 12% had a splenectomy), of whom one was excluded because the severe thrombocytopenia caused unreliable data. Of all platelet parameters, only GPVI significantly increased during eltrombopag treatment (β 1204.5 (95%‐confidence interval (CI) 77.7–2331.3), p = 0.04). There was no significant difference in the effect of eltrombopag between responders/nonresponders and between different start doses (25 mg every other day, 25 mg/day, or 50 mg/day). A higher eltrombopag dose at timepoint B was associated with a higher increase in activated αIIbβ3‐expression upon stimulation with 6.25 μM TRAP (17.0 (95%‐CI 0.0–33.9), p = 0.05), but none of the other responses to agonists were significant. None of the findings remained significant after correction for multiple testing.
Conclusion(s): In this small study population, eltrombopag treatment may increase GPVI expression without significant alterations in platelet function.
265. PB1204
265.1. Paradigm shift in ITP treatment patterns in the era of the COVID‐19 pandemic ‐ opportunity to study real world outcomes from an established platform: Update from UK Adult ITP registry
H. Miah1; A. Miah2; Q. Hill3; N. Cooper4; A. Newland1; D. Provan2; C. Bradbury5; M. Makris6; V. McDonald 7
1 Queen Mary University London, London, England, United Kingdom; 2 Royal London Hospital, London, England, United Kingdom; 3 The Leeds Teaching Hospitals NHS Trust, Leeds, England, United Kingdom; 4 Hammersmith Hospital, London, England, United Kingdom; 5 University of Bristol, Bristol, England, United Kingdom; 6 University of Sheffield, Sheffield, England, United Kingdom; 7 Barts Health NHS Trust, The Royal London Hospital, London, England, United Kingdom
Background: The SARS‐CoV‐2 pandemic has led to changes in the way we manage autoimmune disease such as immune thrombocytopenia (ITP). Immunosuppression is a risk factor for severe COVID‐19 and also leads to reduced vaccine responses. Medical therapy for ITP is broadly divided into immunosuppressive or that which stimulates megakaryopoeisis.
Aims: To assess changes in medical therapy for ITP during the SARS‐CoV‐2 pandemic using data from the UK adult ITP registry
Methods: The UK registry of primary adult ITP (https://www.qmul.ac.uk/itpregistry/), one of the largest internationally, produces annual treatment reports on therapy use, trends and to identify cohorts for study. We reviewed data from annual reports to assess treatment changes.
Results: At the time of analysis, there were 4503 patients in the registry: 224 diagnosed with ITP in 2019, 105 in 2020 and 97 in 2021 (median age 57y, 59y and 55y respectively). Table 1 shows changes in treatment 2019–2021. Most patients still received steroids as part of the treatment for ITP acutely. IVIG use remained static between 2019 and 2021. For diagnoses made between 2019 and 2021 all patients received at least 1 treatment. Immunosuppressive therapy use reduced from pre‐2019 levels (data not tabulated) where almost 23% patients received rituximab and 18% MMF to 0% and 3% respectively by 2021. Use of TPO‐RA increased, from 33% in 2019 to 43% in 2021. Median time to starting TPO‐RA was 3.76 months (IQR 1.317,9.225) in 2019 reducing to 0.985 months (IQR 0.58,1.465) in 2021. Platelet response criteria will undergo analysis once additional data entry has taken place.
Conclusion(s): Steroids continue to be used acutely for most ITP patients but TPO‐RA are being used ahead of other immunosuppressive therapy in line with interim NHSE pandemic policy. Most patients entered received at least one line of treatment – likely reflecting those frequently attending sites to access healthcare.

266. VPB1218
266.1. The predictive value of platelet specific antibodies for the first‐line treatment effect in newly diagnosed pediatric ITP
S. Dong
Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, Beijing, China (People's Republic)
Background: Immune thrombocytopenia (ITP) is an autoimmune mediated hemorrhagic disease characterized by thrombocytopenia. Platelet specific antibodies play a key role in platelet destruction.
Aims: This study was aimed to determine whether platelet specific antibodies (including anti‐GPIIb/IIIa, anti‐GPIb/IX and anti‐GPIa/IIa antibodies) are related to the first‐line treatment effect of newly diagnosed pediatric ITP.
Methods: This study retrospectively analyzed 154 newly diagnosed ITP patients from October 2016 to September 2021 in Beijing Children's Hospital. These patients were treated with glucocorticoids and/or intravenous immunoglobulin (IVIG). And platelet counts were detected one week and one month after treatment. Platelet specific antibodies were detected by PAKAUTO.
Results: When the antibody levels were divided into positive and negative, children with positive anti‐GPIb/IX antibody responded worse than those with negative anti‐GPIb/IX antibody at both one week and one month (Z = −2.565, p = 0.010; Z = −2.579, p = 0.010). When the antibody titer ratio was used to reflect the antibody levels, there was no significant difference between platelet specific antibodies and the first‐line treatment effect in newly diagnosed pediatric ITP.
Conclusion(s): Anti‐GPIb/IX antibody is a sign of worse response in newly diagnosed pediatric ITP.
267. PB1207
267.1. Effect of anti‐nuclear antibody positivity on adult newly diagnosed immune thrombocytopenia prognosis
N. Nguyen 1; T. Nguyen2; S. Thanh‐Thanh3; T. Tran3
1 CHo Ray Hospital, Hematology Department, ho Chi Minh city, Ho Chi Minh, Vietnam; 2 Cho Ray Hospital, ho chi minh city, Ho Chi Minh, Vietnam; 3 Cho Ray Hospital, Ho Chi Minh city, Ho Chi Minh, Vietnam
Background: Idiopathic thrombocytopenia (ITP) is an acquired autoimmune disease that may be associated with other autoimmune disorders. Patients with primary ITP are occasionally found to have a positive Antinuclear Antibody (ANA).
Aims: Our objective was to study the effect of positive ANA on ITP clinical course.
Methods: This was a retrospective descriptive study of adult patients with newly diagnosed ITP, treated at Cho Ray Hospital from February 2020 till July 2021. Baseline characteristics regarding age, sex, bleeding grade, hemoglobin level, platelets count at presentation, hospital length of stay, in‐hospital amounts of blood products transfused, corticosteroid responsiveness, sustained response (SR) were recorded.
Results: 152 patients fulfilled the inclusion criteria. ANAs were positive in 41 (27%) of the included patients, 3.3% had “uncertain” results with ELISA test. Patients with positive ANA had higher mean severe bleeding grade according to IWG criteria, and lower hemoglobin level at presentation than patients with negative ANA ( 10.63 g/dl vs 11.83 g/dl) (p < 0.01). The incidence of overall response (R) and complete response (CR) after 2 weeks of steroids in ANA‐positive patients was significantly lower than that in ANA negative patients (overall response: 52.6% vs 70.4%, p < 0.05). This difference between positive and negative ANA patients remained significant after adjusting for sex, C3, C4, antiDsDNA (p < 0.05). Patients with a positive ANA were 2.5 times (95% CI:1,01–6,2) more likely of not achieving a response defined as a platelet count of 30 × 10e9/ml or more after 2 weeks of steroids. However, sustained response (SR) rates in ANA‐positive patients at 12 and 18 months were the same as ANA‐negative patients (p > 0.05). ANA were also associated with persistent disease (OR: 3.68, 95% CI: 1.14–11.83).
Conclusion(s): Patients with positive ANA had more severe bleeding, more likely to become steroid‐resistant, had a higher risk of developing persistent ITP, and presented with lower Hemoglobin level.


268. PB1213
268.1. Possible targets to reduce fatigue in chronic immune thrombocytopenia patients: An explorative study
W. van Dijk1; M. Nap‐van der vlist2; H. Knoop3; R. Schutgens 4
1 UMC Utrecht, Utrecht, Utrecht, Netherlands; 2 Social Pediatrics, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands, Utrecht, Utrecht, Netherlands; 3 Department of Medical Psychology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam Public health research institute, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: Fatigue in immune thrombocytopenia (ITP) is frequent and burdensome, but we lack knowledge on how to help these patients.
Aims: To explore the role of disease activity in fatigue and identify potentially modifiable factors.
Methods: This cross‐sectional study included adult chronic ITP patients. Univariate linear regression (corrected for confounders) was used to determine the relation between fatigue (Checklist Individual Strength fatigue subscale) and disease activity (high activity: platelet count < 30*10^9/L or current ITP treatment), disease‐specific factors (bleeding symptoms (ITP‐bleeding assessment tool), ferritin), and transdiagnostic (i.e. non‐disease‐specific) factors (physical, functional, emotional, and social well‐being (Functional Assessment of Cancer Therapy‐General (FACT‐G) subscales), activity level, and 25‐OH‐vitamin‐D). Several models with clustered sets of variables were used to compare the proportion of explained variance of fatigue (adjusted R2).
Results: We included 59 patients (mean age 45 ± 18,4, median platelet count 61 (interquartile range 39–92), 61% was female, and 24% received current ITP treatment). Significant relations with fatigue with the largest effect size (>0.50) were found for physical and functional well‐being, and activity. Other significant relations (effect size 0.30–0.47) included skin and organ bleeding, emotional and social well‐being, vitamin D, and disease activity (Table I). Notably, the two clustered models with disease activity and disease‐specific factors explained < 20% of the variance in fatigue, while the two clustered models with FACT‐G subscales and activity level explained >50% (Table II). Vitamin D alone explained 12%.
Conclusion(s): Transdiagnostic (non‐disease‐specific) rather than disease‐specific factors explained a large part of ITP‐related fatigue. Many factors related to fatigue are potentially modifiable and should be investigated as potential targets for interventions.


269. PB1196
269.1. Antioxidative capacity of platelets in the pathogenesis of immune thrombocytopenia; Finding the forest for trees
A. Arshad; S. Mukry; T. Shamsi
National Institute of Blood Diseases and BMT, KARACHI, Sindh, Pakistan
Background: Immune thrombocytopenic purpura (ITP) is an autoimmune disorder. The exact triggering factor is still elusive. The peripheral destruction of platelets is result of various factors. One of the factor is oxidative stress which plays significant role not only damaging RBCs but also platelets.
Aims: The current study was planned to assess antioxidative capacity of platelets in the pathogenesis of ITP patients in Pakistan.
Methods: A case control study (1:1) was conducted from January 2015 to July 2017 with 111 ITP patients and 111 age matched controls. ITP patients were grouped as newly diagnosed/persistent ITP and chronic/refractory ITP patients. Peripheral blood was collected and CBC was recorded using XN 1000. The redox state of platelets was observed flow cytometrically in PRP using DCF and H2O2. The statistical analysis was determined by Kruskal Wallis, dunn’s test and Spearman correlations test using SPSS ver.23.
Results: A total of 111 ITP patients and healthy participants were included. The ITP patients were categorized as 38(34%) C‐ITP, 32(29%) ND‐ITP, 31(28%) P‐ITP and 10(09%) R‐ITP. The redox state of platelets was significantly deranged (p≤0.05) in all patients groups with raised MFI values in ND‐ITP 462(213) followed by R‐ITP 547(123), C‐ITP 450(186), P‐ITP 412(162) as compared to controls 389(188) revealing reduced AOC of platelets in ITP patients. There was significant (≤0.05) positive correlation between platelets and AOC in ND‐ITP r = 0.874**and C‐ITP r = 0.984**.
Conclusion(s): ITP is a condition with peripheral destruction of platelets with unknown specific triggering factor. AOC may also be included in early diagnostic model for better treatment option which will reduce drastic damage caused by OS.
270. PB1210
270.1. Safety and efficacy of splenectomy for the treatment of chronic immune thrombocytopenia (ITP)
A. Saldanha 1; F. Orsi2; E. Okazaki2; C. Rothschild2; P. Prestes2; B. Stefanello2; L. Alves2; V. Rocha2; P. Villaca2
1 Hospital das Clinicas of the University of Sao Paulo Medical School, São Paulo, Sao Paulo, Brazil; 2 Clinics Hospital of the University of São Paulo Medical School, São Paulo, Sao Paulo, Brazil
Background: Although splenectomy has high rates of durable response, its indication has decreased due to concerns on short and long‐term complications.
Aims: To evaluate the short/long‐term safety and efficacy of splenectomy for chronic ITP treatment.
Methods: A cohort of ITP patients who underwent splenectomy was included and followed for up to 10 years. Descriptive analyses of complications (related to the procedure, infections, and thrombosis) and response to treatment were performed.
Results: Out of 87 patients included, 80.5% were women, the median age at diagnosis was 33 years (IQR 24 – 50), 28.7% had hypertension and 20.7% had antiphospholipid antibodies. Median time from diagnosis to splenectomy was 19 months (IQR 6.0 – 50.0) and of follow‐up duration, 61 months (27 – 108). Post‐procedure complications, such as fistula, surgical site infection and bleeding requiring transfusion, occurred in 6 (7%) patients and hospital‐associated thrombosis (thrombosis occurring within 90 days after splenectomy) in 3 (3.4%). Two patients had abdominal thrombosis and 1 had abdominal and deep vein thrombosis. During follow‐up, 3 patients had infections requiring hospitalization, resulting in a 5‐year cumulative risk of infections of 3.4% (0.9 – 9.1) (Table1). Sixty days after splenectomy, 72 patients (82.5%) had an overall response, of which 55 were complete (63.2%) and 17 partial responses (19.3%). Median time for relapse was 12.5 months (IQR 4 ‐ 45.3) and the 5‐year treatment free survival was 55.3% (95% CI 45.8 – 66.4) (Figure 1). At the end of the follow‐up, 77 patients (88.5%) had partial or complete response and 2 (2.3%) had died.
Conclusion(s): Splenectomy is an efficacious well‐tolerated treatment. The response rate is high, even for those requiring further ITP treatment, suggesting that splenectomy modulates future therapy response. In conclusion, splenectomy‐associated rates of efficacy outweigh its rates of complications, which underscores that splenectomy should be regarded as one of the main approach for long‐term ITP remission.


271. PB1215
271.1. Exacerbation of immune thrombocytopenia following initial and booster vaccination with pfizer‐BioNTech COVID‐19 vaccine
A. Leader1; M. Aharoni2; P. Raanani3; G. Spectre 3
1 Rabin Medical Center, Petah Tikva, HaMerkaz, Israel; 2 Sackler School of Medicine, Tel Aviv University, Tel aviv, HaMerkaz, Israel; 3 Institute of Hematology, Davidoff Cancer Centre, Rabin Medical Centre, Petah Tikva, HaMerkaz, Israel
Background: There is emerging data on immune thrombocytopenia (ITP) exacerbation after initial Covid‐19 vaccination. The rate of ITP exacerbation after booster vaccines is unknown.
Aims: Explore rates of ITP exacerbation after first, second and booster Pfizer‐BioNTech COVID‐19 vaccines in adult ITP patients.
Methods: Retrospective study of ITP patients, receiving 1–3 doses of Pfizer‐BioNTech vaccine. The primary outcome was clinical ITP exacerbation defined as platelet count decrease requiring initiation or escalation of ITP treatment and/or a new medical attention due to bleeding, within 3 months. Secondary outcome was any clinically‐relevant platelet decrease during the 3 months post‐vaccination compared to pre‐vaccine count, defined as platelet decrease to ≤30×109/L or thrombocytopenia with >50% decrease from baseline.
Results: The study included 93 ITP patients (83 with chronic ITP) receiving 1 (n = 2), 2 (n = 22) or 3 (n = 69) vaccine doses. ITP exacerbation occurred in 2/93 (2.2%) patients following first/second dose, and 3/69 (4.3%) following booster dose. Clinically‐relevant platelet decreases after initial doses occurred in 8/72 (11.1%) patients with platelet counts available, and in 8/39 (20.5%) after the booster, within a median 18 and 20 days. Table 1 shows clinical data and platelet counts in patients experiencing primary or secondary outcomes. Clinical ITP exacerbation after booster doses was preceded by a clinically‐relevant platelet decrease after the initial doses in 1 of 3 patients, but not by clinical exacerbations. Four of 8 patients (50%) with clinically‐relevant platelet decreases after booster doses also had clinically‐relevant decreases following initial vaccination.
Conclusion(s): Clinical ITP exacerbation in chronic ITP patients is infrequent following Pfizer‐BioNTech COVID‐19 vaccine. Clinical exacerbation after booster doses was not preceded by clinical exacerbation after initial doses in this study. Clinically‐relevant platelet decreases after booster doses occur frequently in patients with clinically relevant decreases after initial doses. This suggests monitoring of platelet counts in ITP patients receiving COVID‐19 vaccines.

272. VPB1217
272.1. Association of platelet desialylation and circulating follicular helper T cells in patients with thrombocytopenia
Y. Chen 1; L. Luo1; Q. Zhen1; S. Kalayu Yirga1; Q. Shi2; J. Hu1
1 Fujian Institute of Hematology, Fujian Medical University Union Hospital, Fuzhou, Fujian, China (People's Republic); 2 Medical College of Wisconsin, Blood Research Institute, Children's Research Institute, Milwaukee, Wisconsin, United States
Background: Thrombocytopenia is a multifactorial condition that frequently involves concomitant defects in platelet production and clearance. Sialylation on platelet membrane glycoprotein and follicular helper T cells (TFHs) are thought to be the novel platelet clearance pathways.
Aims: We aimed to evaluate the associations of platelet desialylation and circulating TFHs in the pathophysiology of immune thrombocytopenia (ITP) and non‐ITP thrombocytopenia.
Methods: A cohort of 190 patients with ITP and 94 patients with non‐ITP‐related thrombocytopenia including case of aplastic anemia (AA) and myelodysplastic syndromes (MDS) were enrolled. Healthy volunteers were included as controls (n = 110). Desialylated platelets binding with Erythrina cristagalli lectin (ECL) and Ricinus communis agglutinin I (RCA‐I), apoptotic platelets and circulating TFHs were measured. CXCL13 (a key chemokine produced by TFHs) and platelet autoantibodies were assessed.
Results: In comparison to healthy controls, we observed at least a 2.0‐fold increase in desialylated platelet levels and circulating TFHs from patients with ITP or non‐ITP‐related thrombocytopenia in the AA and MDS cohorts. Desialylated platelets were negatively correlated with platelet count (r = −0.1867, p = 0.0267 vs. ECL; and r = −0.3587, P < 0.0001 vs. RCA‐I, respectively), and positively correlated with TFHs proportion (r = 0.2184, p = 0.0161 for CD4+CXCR5+PD1+ TFHs vs. ECL; r = 0.2484, p = 0.0052 for CD4+CXCR5+ TFHs vs. ECL; r = 0.2281, p = 0.0122 for CD4+CXCR5+PD1+ TFHs vs. RCA‐I). Patients bearing anti‐platelet autoantibodies displayed 5.9‐fold higher levels of TFHs than those without autoantibodies (p = 0.0385). Moreover, CXCL13 and apoptotic platelets were significantly upregulated, and pro‐apoptotic proteins and MAPK/mTOR pathway were markedly activated. Responders after treatment presented significantly greater reduction in platelet desialylation levels than non‐responders did (ECL: p = 0.0946; RCA‐I: p = 0.0024).
Conclusion(s): Platelet desialylation and circulating TFHs may become the potential biomarkers and therapeutic targets for thrombocytopenia in patients with ITP and non‐ITP.
273. PB1206
273.1. Effect of agonists to thrombopoietin receptors on platelet glycome of patients with immune thrombocytopenia
E. Monzón Manzano 1; M. Alvarez‐Román2; P. Acuña1; E. Arias Salgado1; A. Ramírez López1; M. Martín Salces1; M. Rivas Pollmar3; B. Guerra Villena1; V. Jiménez Yuste4; N. Butta1
1 Hospital Universitario La Paz‐IdiPaz, Madrid, Madrid, Spain; 2 Hospital Universitario La Paz, Madrid, Madrid, Spain; 3 Hospital Universitario La Paz‐Idipaz, Madrid, Madrid, Spain; 4 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain
Background: Platelet destruction and suppression of platelet production are involved in immune thrombocytopenia (ITP). Recently, attention has been drawn to the composition of platelet glycome in etiopathogenesis of ITP. We have previously reported that exposure of glycosides that might be involved in platelet destruction are increased in platelets from ITP patients (Table 1 and Figure 1A). Agonists to thrombopoietin receptors (TPO‐RAs) have been shown to produce sustained platelet responses in ITP. Nevertheless, little is known about the effect of TPO‐RAs on platelet’s features.
Aims: We aimed to study whether the treatment with TPO‐RAs modified platelet function and glycoside composition of platelets from ITP patients.
Methods: ITP patients were studied before and after achieving a response (defined as a platelet count ≥30×109 platelets/L and at least two fold increases over the baseline count) to TPO‐RA therapy. Platelet activation markers (activation of fibrinogen receptor determined through PAC1‐binding, and TRAP‐induced P‐selectin and CD63 exposure) and active caspase‐3, ‐7, ‐8 or ‐9 were evaluated. Platelet surface glycan exposure was analysed by determining the binding of lectins to specific glycosidic residues (Table 1). Samples were analyzed by flow cytometry. Results were expressed as the ratio between values obtained after responding/before treatment with TPO‐RA.
Results: 16 patients were included [53.8% women, 58 (22–81) years, 46.2% with eltrombopag, 53.8% with romiplostim]. Increment of platelet count was variable and was accompanied by the recovery of platelet's ability to be stimulated, especially the activation of fibrinogen receptor. TPO‐RA treatment seemed to have a negligible effect on caspase activity. Regarding glycoside residues, exposure of ɑ‐mannose, galactose and ß‐GlcNAc showed a tendency to decrease (Figure 1).
Conclusion(s): Our results showed that TPO‐RA treatment appeared to recover platelet function and to restore a glycome composition similar to that found in a healthy state. Funded by ISCIII‐Fondos FEDER PI19/00772 and Platelet Disorder Support Association


274. PB1201
274.1. Clinical characteristics and maternal/neonatal outcomes in pregnant women with immune thrombocytopenic purpura: A systematic review
S. Khaliq
Foundation University Islamabad Pakistan, Islamabad, Islamabad, Pakistan
Background: Immune thrombocytopenic purpura (ITP) occurs in 1 to 2 of every 1,000 pregnancies. Management of ITP in pregnancy can be a complex and challenging task and requires multidisciplinary approach.
Aims: Systematic review of literature to evaluate the clinical characteristics and maternal/neonatal outcomes in pregnant women with ITP.
Methods: Literature search (1959‐ 2021) on ITP in pregnancy was performed using PubMed, Embase and Cochrane. Pooled estimates expressed as percentage (%) with 95% confidence interval (CI) were generated using inverse‐variance weighted method in random‐effect models.
Results: 81 articles met our inclusion criteria (35 case reports, 16 case series, 19 observational studies and 11 review articles). 1169 pregnant females aged 16–45 years had ITP. (44%, 95% CI = 33–64) had ITP diagnosed before pregnancy and (56%, 95% CI = 39–68) had ITP diagnosed during pregnancy. The commonest maternal complication was postpartum haemorrhage (PPH) (16%, 95% CI = 11–23). Vaginal delivery was done in (60%, 95% CI = 39–70) while (40%, 95% CI = 27–61) had caesarean section. There was no maternal death. Management during pregnancy included glucocorticoids (GC) (46%, 95% CI = 35–68), GC + Intravenous immunoglobulins (IVIg) (31%, 95% CI = 21–55), Eltrombopag (0.34%, 95% CI = 0.13–0.8), Rituximab (0.25%, 95% CI = 0.05–0.75), splenectomy (0.76%, 95% CI = 0.28–0.95) and anti‐RhD Immunoglobulin (0.16% 95% CI = 0.01–0.4) Neonatal outcomes were: (09%, 95% CI = 05–13) preterm births, (0.68%, 95% CI = 0.21–1.21) intracranial haemorrhage, (0.76%, 95% CI = 0.33–0.98) intrauterine fetal death, (0.25% 95% CI = 0.06–0.67) still births, (0.17% 95% CI = 0.02–0.35) infantile deaths, (2.5% 95% CI = 1.8–3.7%) low birth weight babies and (0.8%, 95% CI = 0.3–1.0) fetal anomalies. (41%, 95% CI = 29–61) had neonatal thrombocytopenia out of which (21%, 95% CI = 17–30) neonates required treatment.
Conclusion(s): Pregnant patients with ITP usually have a good maternal/neonatal outcome. However, there is a higher risk of PPH which requires close monitoring. This systemic review constitutes an essential step to identify gaps in knowledge of ITP in pregnancy.
275. PB1202
275.1. Monitoring the persistence of vaccine‐induced immune thrombotic thrombocytopenia (VITT) antibodies and their pathogenicity
C. Lee 1; L. Clarke2; T. Brighton3; S. Chunilal4; E. Favaloro5; D. Donikian3; M. Kondo3; H. Tran6; V. CHEN7
1 ANZAC Research Institute, University of Sydney, Sydney, New South Wales, Australia; 2 Department of Haematology, Concord Repatriation General Hospital; Australian Red Cross Lifeblood, Sydney Adventist Hospital, Concord, New South Wales, Australia; 3 Department Haematology, New South Wales Health Pathology, Prince of Wales Hospital, Sydney, New South Wales, Australia; 4 Monash Health, Melbourne, Victoria, Australia; 5 Charles Sturt University, NSW Health Pathology, Sydney Centres for Thrombosis and Haemostasis, Sydney, New South Wales, Australia; 6 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia; 7 ANZAC Research Institute, University of Sydney; Department of Haematology, Concord Repatriation General Hospital, Sydney, New South Wales, Australia
Background: The putative mechanism for vaccine‐induced immune thrombotic thrombocytopenia (VITT) following ChAdOx1 nCoV‐19 vaccination involves pathological anti‐PF4 platelet‐activating antibodies driving thrombosis and thrombocytopenia. Reports suggest platelet‐activating antibodies decline within 12 weeks despite persistence of anti‐PF4 by ELISA (Schönborn et al). Optimal duration of anticoagulation remains unclear. The Australian‐New Zealand guidance recommends repeat testing of patients with serologically‐confirmed VITT at 3, 6 and 12 months.
Aims: To determine rate of persistence of anti‐PF4 ELISA compared with functional platelet‐activating tests in VITT.
Methods: We conducted a prospective study of clinicopathological adjudicated VITT patients (thrombocytopenic with D‐dimer levels >5x ULN and thrombosis within 4–43 days of ChAdOx1 nCoV‐19 vaccination) with a positive functional assay at diagnosis who had follow‐up VITT testing. Anti‐PF4 antibodies were measured by IgG‐specific ELISA (Asserachrom, Stago Diagnostics). Platelet‐activating antibodies were assayed using whole blood procoagulant platelet flow cytometry assay (Lee et al) or PF4‐serotonin release assay.
Results: 14 confirmed VITT patients (7 females, 7 males; median age 62.5 years [range, 40–80]) underwent follow‐up testing, median follow‐up of 12 weeks (range, 3–24). Median time to return a negative functional test was 12 weeks (range, 4–16) with platelet‐activating antibodies persistent at 24 weeks in two patients. Median time to negative ELISA was not reached. During follow‐up, 9 of 14 patients became negative by functional assay while anti‐PF4 antibodies persisted in 8 of 12 (Figure 1). 93% of patients received a documented second vaccination (Comirnaty, median interval 16 weeks [range, 11–28]) and 71% have received an mRNA booster with no reported adverse events.
Conclusion(s): Platelet‐activating antibodies were persistent in 54% of tested patients at 12 weeks and anti‐PF4 ELISA remained positive in the majority. Anti‐PF4 antibodies persist longer than functional platelet‐activating antibodies in VITT. Whether the decline in platelet‐activating antibodies allows withdrawal of anticoagulation in these patients is unclear but could be a useful guide.

276. PB1212
276.1. Platelet desialylation status in adults with primary immune thrombocytopenia
T. Schramm 1; C. Ungerböck2; M. Fillitz3; B. Dixer3; D. Mehic4; T. Backchoul5; I. Marini6; J. Zlamal5; C. Ay7; I. Pabinger7; J. Gebhart1
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 3 Department of Internal Medicine, Hanusch Krankenhaus, Vienna, Austria, Vienna, Wien, Austria; 4 Medical University of Vienna, Vienna, Wien, Austria; 5 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 6 Transfusion Medicine, Medical Faculty of Tübingen, University Hospital Tübingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 7 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: In primary immune thrombocytopenia (ITP), patients express heterogeneous bleeding phenotypes and partially increased thromboembolic risk. Autoantibodies (AAbs) can induce platelet desialylation, leading to platelet clearance. A high level of platelet desialylation is observed in refractory ITP, whereas its impact on bleeding phenotype and thrombosis incidence is not completely elucidated yet.
Aims: To identify the effect of desialylated platelets on the clinical phenotype in respect to bleeding or thrombosis in adults with primary ITP.
Methods: Samples of a characterized cohort of 86 adult primary ITP patients and 20 healthy controls (HC) from the Vienna ITP Biobank (EC 1843/2016) were investigated. Platelet desialylation was assessed via a lectin binding assay: washed platelets from healthy individuals were incubated with patient or HC serum and stained with erythrina cristagalli lectin (ECL) or ricinus communis agglutinin (RCA) followed by flow cytometric analysis. Mean fluorescence intensity (MFI) cutoffs (ECL: MFI = 1.43; RCA: MFI = 1.10) were calculated based on HCs.
Results: 86 ITP patients were investigated in this study. 9 patient sera (10.5%) induced significant platelet desialylation with increased binding of ECL and/or RCA to platelets (3 ECL only, 4 RCA only, 2 ECL and RCA, Figure 1). Subsequent analysis of ITP AAb glycoprotein specifities revealed that 1 patient presented with AAbs against platelet GPIIb/IIIa and 5 without detectable ITP AAbs. The AAb status was not known in 3 patients. The 2 patients with binding of both lectins presented with chronic ITP and did not have detectable AAbs against GPIIb/IIIa or GPIb/IX. There were no significant differences in relevant clinical characteristics, bleeding severity, or previous thrombosis history between patients with and without platelet desialylation (Table 1).
Conclusion(s): Desialylation was observed in 10.5% of the investigated patient population and mainly restricted to patients with chronic ITP. No association of platelet desialylation with multi‐refractory ITP, the bleeding phenotype or thrombosis history was detected.


277. PB1209
277.1. Glycoprotein‐specific platelet autoantibodies predict lower platelet counts in thrombocytopenic patients
U. Sachs 1; M. Reich2; D. Qiu3; N. Cooper4; G. Bein4
1 Giessen University Hospital, Giessen, Hessen, Germany; 2 Dept. of Thrombosis and Hemostasis, Giessen University Hospital, Giessen, Hessen, Germany; 3 IT Department, Giessen University Hospital, Giessen, Hessen, Germany; 4 Center for Transfusion Medicine and Haemotherapy, Giessen and Marburg University Hospital, Giessen, Hessen, Germany
Background: Autoantibodies are a major pathophysiologic mechanism in immune thrombocytopenia (ITP). Testing for glycoprotein‐specific platelet autoantibodies (G‐PA) is an attractive diagnostic tool when evaluating thrombocytopenic patients. Various studies assessing diagnostic tests for ITP are most likely confounded because the diagnosis of ITP relies on clinical criteria and no gold standard is available.
Aims: We hypothesized that if an assay identifies clinically relevant G‐PA in a patient, antibody level and number of targeted glycoproteins should be associated with the extent of thrombocytopenia if the identified G‐PA truly mediates a major pathophysiologic mechanism in ITP.
Methods: We performed a retrospective cohort study by searching for patients for whom G‐PA has been ordered from our laboratory. G‐PA were identified using the direct monoclonal antibody–specific immobilization of platelet antigen (DM) assay for platelet glycoproteins (GP) IIb/IIIa and Ib/IX. Antibody levels were recorded semiquantitatively.
Results: We included 3,548 children and 15,031 adults. In children, the median platelet count was 98 G/L (interquartile range, 58–154) for DM negative and 39 G/L (20–79) for DM positives (p < 0.0001). For adults, the median platelet count was 88 G/L (55–124) for DM negative and 48 (21–86) for DM positives (p < 0.0001). In children, presence of two antibodies had no additive effect (48 G/L (26–93) vs. 64 G/L (32–100), p = n.s.). In contrast, double positivity in adults was associated with significantly lower platelet counts (47 G/L (26–81) vs. 62 G/L (32–99), p = 0.0009). Antibody levels correlated well with the platelet count for the two antibody entities both in children and adults.
Conclusion(s): The presence of autoantibodies against GP IIb/IIIa or GP Ib/IX is associated with a lower platelet count in thrombocytopenic patients. Increased antibody levels and more glycoproteins targeted predict lower platelet counts in adults. We conclude that detecting G‐PA is diagnostic for pathological antibodies and useful when evaluating patients suspected of suffering from ITP.
278. PB1194
278.1. Platelet response to avatrombopag following switch from eltrombopag or romiplostim in primary versus secondary immune thrombocytopenia: A multicenter study of U.S. ITP Referral Centers
H. Al‐Samkari 1; D. Jiang2; T. Gernsheimer2; H. Liebman3; S. Lee3; C. Bernheisel4; S. Kolodny4; M. Wojdyla4; M. Vredenburg4; A. Cuker5
1 Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, United States; 2 University of Washington, Seattle, Washington, United States; 3 University of Southern California‐Keck School of Medicine, Los Angeles, California, United States; 4 Sobi, Inc., Durham, North Carolina, United States; 5 University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, United States
Background: Avatrombopag is an oral thrombopoietin receptor agonist (TPO‐RA) approved for treatment of immune thrombocytopenia (ITP) without food‐type restrictions. Data comparing effectiveness of avatrombopag following treatment with other TPO‐RAs in primary ITP (pITP) and secondary ITP (sITP) is limited.
Aims: Evaluate avatrombopag response in pITP and sITP after switching from other TPO‐RAs.
Methods: We retrospectively evaluated all adults with ITP switched from eltrombopag or romiplostim to avatrombopag at four U.S. centers from July 2019 through December 2020. Reasons for switch included effectiveness (n = 14), convenience (n = 23) and adverse events (n = 7). Response was defined as a platelet count (PC) ≥30,000/μl (R30) and ≥50,000/μl (R50) with loss of response as two consecutive PCs, at least 7 days apart, < 30,000/μl and <50,000/μl respectively. The proportion of time with a defined platelet response while on avatrombopag was measured.
Results: 44 patients were included, 25 (57%) with pITP and 19 (43%) with sITP (Table 1). Causes of sITP included hepatitis C, chronic lymphocytic leukemia, systemic lupus erythematosus (n = 3 each), autoimmune hepatitis, B‐cell lymphoma, Evan’s syndrome, HIV, CREST syndrome, rheumatoid arthritis, relapsing polychondritis, antiphospholipid antibody syndrome, CVID, not listed (n = 1 each). 42/44 (96%) achieved an R30 with avatrombopag including 23/25 (92%) pITP patients and 19/19 (100%) sITP patients. pITP patients and sITP patients maintained an R30 91.5% and 85% of their time on avatrombopag, respectively. Table 2 shows additional durability of response data with avatrombopag.
Conclusion(s): In a heavily pretreated, chronic ITP population switched from another TPO‐RA to avatrombopag, nearly all (96%) patients achieved a platelet response. Response and proportion of time with response in the observation period were similar, regardless of primary or secondary ITP diagnosis. This represents the first evidence reported regarding durability of response with avatrombopag in secondary ITP; more data on the effectiveness and safety of avatrombopag in patients with secondary ITP are needed.


279. VPB1222
279.1. High frequency of bone marrow evaluations but low diagnostic yield in relapsed or refractory immune thrombocytopenia
K. Taparia; E. Wall; H. Sun
University of Alberta, Edmonton, Alberta, Canada
Background: The diagnosis of primary immune thrombocytopenia (ITP) is one of exclusion. While consensus guidelines recommend against routine bone marrow examination (BME) in typical ITP, the role of BME in relapsed/refractory ITP has not been studied.
Aims: 1. To examine the prevalence and predictors of BME in relapsed/refractory ITP. 2. To assess the prognostic impact of abnormal bone marrow on management.
Methods: This multi‐centre retrospective cohort study included all chronic ITP patients ≥18 years who received second‐line therapy (rituximab, thrombopoietin receptor agonists [TPO‐RA], splenectomy) between 2012–2019 in Alberta, Canada. We calculated the frequency of BME and the rate of abnormal marrow findings. Logistic regression analyses were conducted to assess BME predictors such as age, sex, rural residence, ITP etiology, laboratory parameters, and response to second‐line therapies. Research ethics approval was obtained.
Results: We identified 324 patients who received second‐line therapy. 181 (56%) patients underwent a BME, at a median age of 61. We observed a marked decline in the rates of BME among patients >60 years over the past decade, from 86% (2008–2010) to 30% (2017–2019), but not in patients under 60 years (Figure 1). On univariate logistic regression, older age (OR 1.02, p < 0.001), male sex (OR 1.7, p = 0.02), splenectomy (OR 2.1, p = 0.002) or TPO‐RA (OR 1.9, p = 0.006) were significant predictors of undergoing BME (Table 1), while response to splenectomy (OR 0.25, p = 0.005) and rituximab (OR 0.5, p = 0.03) were associated with lower odds of BME. Of the 181 patients who underwent BME, only 8 (4%) had abnormal marrow findings and of those, 4 experienced changes in clinical management.
Conclusion(s): While BME is frequently performed in relapsed/refractory ITP, abnormal findings are uncommon in most patients. Analysis examining predictors of abnormal marrow findings is ongoing and may help identify a subgroup of relapsed/refractory ITP who may benefit from BME.


280. VPB1223
280.1. Platelet‐specific CD8+ T cells in patients with immune thrombocytopenia
J. Vrbensky 1; I. Nazy1; J. Kelton1; M. Larché2; R. Clare1; H. Bhakta1; M. Roussakis1; V. Rybenchuk1; D. Arnold1
1 Platelet Immunology Laboratory at McMaster University, Hamilton, Ontario, Canada; 2 McMaster University, Hamilton, Ontario, Canada
Background: Immune thrombocytopenia (ITP) is a bleeding disorder characterized by a low platelet count. T cells have been implicated in the pathogenesis of ITP, but evidence of platelet‐specific CD8+ T cells is lacking.
Aims: Detect platelet‐specific CD8+ T cells in ITP patients. Characterize CD8+ T cell regulation and activity in ITP.
Methods: Peripheral blood mononuclear cells from ITP patients (n = 11) and healthy individuals (n = 10) were labelled with carboxyfluorescein succinimidyl ester (CFSE) to detect platelet‐specific T cell proliferation in response to platelet autoantigens. CD8+ T cell proliferation was assessed by flow cytometry after 9 days of stimulation with GP IIbIIIa or Ibα. Anti‐GP IIbIIIa and anti‐GP IbIX were measured with the direct antigen capture assay. Plasma TGF‐β, IL‐6, GrA, GrB, and sCD137 were assessed by multiplex (Eve Technologies) in ITP patients, healthy individuals, and non‐ITP patients (n = 5). p values < 0.05 were considered significant. Informed consent was obtained, and the study was approved by the Hamilton Integrated Research Ethics Board.
Results: After stimulation with GP IIbIIIa, 5 of 11 ITP patients and 6 of 10 healthy individuals showed a CD8+ T cell proliferative response. After stimulation with GP Ibα, 9 of 11 ITP patients and 7 of 10 healthy individuals showed a CD8+ T cell proliferative response (Figure 1). Platelet counts and autoantibody levels were comparable in patients with or without platelet‐specific T cells. Plasma TGF‐β was significantly lower in ITP patients, while IL‐6, GrA, GrB, and sCD137 were significantly elevated in ITP patients compared to healthy individuals and non‐ITP patients (Figure 2).
Conclusion(s): Platelet‐specific CD8+ T cells can be found in ITP and in healthy individuals, and CD8+ T cell activity is dysregulated in ITP patients. Platelet‐specific CD8+ T cells should be isolated are characterized for their ability to promote platelet destruction and underproduction in future investigations.


281. PB1200
281.1. Factors influencing health‐related quality of life in patients with immune thrombocytopenia
C. Ittikamonchok1; S. Kaewsuwan 2; P. Jewprasertpan2; T. Wilailak2; K. Sophonwatanagit2; P. Niprapan3; N. Hantrakun3; C. Chai‐Adisaksopha3
1 Chiang Mai University, Sri phum, Muang, Chiang Mai, Thailand; 2 Chiang Mai University, Sri phum, Muang, Chiang Mai, Thailand; 3 Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand, Sri Phum, Muang, Chiang Mai, Thailand
Background: Immune thrombocytopenia (ITP) and its treatment directly impact patients’ live, including physical health, well‐being and health‐related quality of life (HRQoL).
Aims: This study aimed to investigate the HRQoL of patients who were diagnosed with ITP.
Methods: This was a cross‐sectional study conducted at Hematology Clinic, Chiang Mai University Hospital, Thailand between April and December 2021. We enrolled consecutive patients who were diagnosed with ITP. Controls were age‐ and sex‐matched, non‐ITP participants. The Immune Thrombocytopenic Purpura Patient Assessment Questionnaire (ITP‐PAQ) and the 5‐level EQ‐5D version (EQ‐5D‐5L) were used to evaluate HRQoL. ITP patients were assessed for bleeding using the International Working Group bleeding assessment tool. We compared the EQ‐5D‐5L responses and utility score between 2 groups. The factors influenced the HRQoL were assessed by ITP‐PAQ, using a linear regression model.
Results: We included 130 ITP patients and 130 controls with mean age of 49.9 (SD 15.3) and 49.8 (SD 14.9) years, respectively. Seventy‐nine percent of participants were female. Among ITP patients, 82.3% were primary ITP. Median time of ITP diagnosis was 59.5 months (IQR 18.7–124.9). When compared to controls, ITP patients had significant lower mean EQ‐5D‐5L utility score indicating that they had poorer HRQoL, 0.95 (SD 0.01) vs 0.88 (SD 0.02), mean difference 0.64 (95% CI, 0.10–0.27; p < 0.001) and had lower mean utility score associated with usual activities and pain/discomfort, Table 1. Univariable analysis revealed that higher bleeding score, platelet count <50 × 109/L, and prednisone dose >10 mg/day were associated with lower ITP‐PAQ total score, Table 2. Multivariable analysis revealed that bleeding score but not platelet count was associated with poor HRQoL.
Conclusion(s): ITP patients had significant poor HRQoL when compared to non‐ITP individuals. Bleeding score but not number of platelets was associated with poor HRQoL. Physicians should be focusing on bleeding symptoms rather than platelet number in long‐term care of ITP patients.


282. PB1198
282.1. ITP before and after COVID‐19 vaccinations: A national cohort study
P. Choi 1; D. Hsu2; J. Curnow3; C. TAN4; A. Enjeti5; H. Tran6; V. CHEN7; A. Yong8; F. Roncolato9; J. Simpson10; D. Pepperell11; R. Bird12
1 The Canberra Hospital, Garran, Australian Capital Territory, Australia; 2 Liverpool Hospital, Sydney, New South Wales, Australia; 3 Department of Haematology, Westmead Hospital, Sydney, New South Wales, Australia; 4 Royal Adelaide Hospital, Adelaide, South Australia, Australia; 5 Calvary Matery Hospital, Newcastle, New South Wales, Australia; 6 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia; 7 ANZAC Research Institute, University of Sydney; Department of Haematology, Concord Repatriation General Hospital, Sydney, New South Wales, Australia; 8 Royal Perth Hospital, Perth, Western Australia, Australia; 9 St George Hospital, Sydney, New South Wales, Australia, 10 Mid North Coast Haematology, Port Macquarie, New South Wales, Australia, 11 Fiona Stanley Hospital, Perth, Western Australia, Australia, 12 Princess Alexandra Hospital, Woolloongabba, Queensland, Australia
Background: Immune thrombocytopenia (ITP) has been reported following COVID‐19 vaccination. From a population of over 20 million eligible vaccine recipients in Australia, over 32 million doses have been administered: 19,600,000 Pfizer BNT162b2 (BNT), 12,600,000 AstraZeneca ChAdOx1 nCoV‐19 (ChAd), and 397,000 Moderna mRNA‐1273.
Aims: Describe a comprehensive series of ITP after vaccination with clinical outcomes.
Methods: After obtaining IRB approval (2021/ETH00723), we collected data on all ITP cases diagnosed by haematologists in Australia within six weeks of any COVID‐19 vaccination. We analysed their outcomes using international consensus definitions of responses and WHO bleeding.
Results: Demographics (n = 50), treatments, and platelet outcomes (Figures A and B). Bleeding was mostly minor: 35/50 (70%) WHO score <2. Compared to relapses of prior ITP, new presentations of ITP were significantly associated with ChAd over BNT (OR 7.1: 95% CI 1.7 to 25.7, p = 0.0124*). Most patients responded quickly and deeply: median TTR 4 days (IQR 2–7), median TTCR 7 days (IQR 4–19), overall RR 45/47 (96%), and CR 40/45 (89%). Gender, age, antecedent influenza vaccination and severity of thrombocytopenia had no significant impact on: bleeding at presentation, response rates, relapse rates, time to response, or the need for ongoing treatments at day 90. No patients presented with thrombosis. PF4 ELISA was positive in one of 18 cases after ChAd (functional testing was negative).
Conclusion(s): We diagnosed ITP more frequently after ChAd than BNT vaccination, occurring de novo after 1st doses. Ascertainment bias cannot be excluded due to greater scrutiny for platelet related complications, but almost all patients in this cohort needed treatment. Standard first‐line treatments for ITP are highly effective for both de novo and prior ITP (96%), but second‐line therapies are often required (34%). Our data reaffirms the safety of vaccinating patients with pre‐existing ITP, as bleeding is mild (92% WHO < 2) and platelets respond quickly (TTCR 5 days).


282.2. ADAMTS13 and TTP
283. VPB0315
283.1. A rare case of thrombosis with ITP
L. Hambardzumyan; N. Sargsyan1; G. Tamamyan2; A. Harutyunyan3; I. Karapetyan4; N. Yeghiazaryan5; L. Minasyan5; A. Voskanyan6; H. Khachatryan6
1 Hematology center after prof. R.H. Yeloyan Armenian Hemophilia and Thrombosis Center Yerevan State Medical University, Department of Pediatric Oncology and Hematology, Yerevan, Yerevan, Armenia; 2 Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Department of Pediatric Oncology and Hematology, Yerevan State Medical University, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 3 Yerevan State Medical University, Depatment of Obstetrics and Gynecology, Yerevan, Yerevan, Armenia; 4 Yerevan State Medical University, Department of Obstetrics and Gynecology, Yerevan, Yerevan, Armenia; 5 Erebuni Medical Center, Yerevan, Armenia, Stroke Center, Yerevan, Yerevan, Armenia; 6 Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Yerevan, Yerevan, Armenia
Background: Immune hemolytic anemia (AIHA) and thrombocytopenia (ITP) may rarely coexist with autoimmune disorders. Primary IHA and ITP usually respond to steroids and intravenous immunoglobulins. However, IHA+ITP may be difficult to treat when associated with autoimmune disorders.
Aims: We report a case of a 57‐year‐old man diagnosed with AIHA and ITP and also found to have thyroiditis.
Methods: A retrospective review of the patient’s medical history was performed.
Results: A 57‐year‐old‐woman with bruises, weakness, fatigue, and dizziness was admitted to the hematology center. This time CBC test was revealed hyperchromic macrocytic anemia with anisocytosis, thrombocytopenia, lymphocytosis and high level of ESR. Coomb's reactions were positive. The patient had been on autoimmune thyroiditis for about 5 years and was receiving 50 mg of L‐thyroxin. Laboratory testing revealed only slightly elevated LDH level in serum and no other significant abnormalities. Instrumental examination were also normal. Our differential diagnosis included TTP, Evans and marrow infiltrative disorders. Direct antiglobulin test positive (IgG, IgG + C3d). We made a diagnosis of immune hemolytic anemia and thrombocytopenia and started treatment with Prednisolone 65mg per day. In about six months Duplex scan showed acute thrombosis of right external iliac vein. October 2021. The patient was diagnosed with COVID‐19, Bilateral interstitial pneumnonia. On the way home ischemic stroke occurred. Thrombectomy ‐ within 12 h
Conclusion(s): ITP can rarely coexist with thyroiditis and Thrombosis episode. In such cases, we involuntarily deviate from the contraindications of standard anticoagulant therapy, and, regardless of the low platelet count, administer anticoagulant therapy and combine it with steroid medication. The clinical case is evidence that the treatment of concomitant immune system disorders improves the course of treatment for all pathologies but poses a thrombotic complication.

284. VPB0317
284.1. Experience in diagnosis and treatment of thrombotic thrombocytopenic purpura: Case reports
T. Talako 1; D. Tsvirko2; K. Kabaeva3; I. Iskrov2; Y. Statsenko4; A. Avadok5; I. Lendina6
1 Belarusian Medical Academy of Postgraduate Education, Minsk, Minskaya Voblasts', Belarus; 2 Belorusian Medical Academy of Postgraduate Education, Minsk, Minskaya Voblasts', Belarus; 3 Belorusian Medical Academy of Postgraduate Education, MInsk, Minskaya Voblasts', Belarus; 4 United Arab Emirates University, Al Ain, Abu Dhabi, United Arab Emirates; 5 Minsk Sate Scientific and Practical Center for Surgery, Transplantology and Hematology, MInsk, Minskaya Voblasts', Belarus; 6 Minsk Sate Scientific and Practical Center for Surgery, Transplantology and Hematology, Minsk, Minskaya Voblasts', Belarus
Background: Thrombotic thrombocytopenic purpura (TTP) has high lethal rate what justified its early diagnosis and beginning of treatment.
Aims: To present the diagnosis and management of cases of patients with TTP.
Methods: We report 6 cases of patients from 23 to 46 years old with TTP. Hematological and biochemical tests were performed; activity of ADAMTS 13 was measured.
Results: All patients had sudden onset of the disease that manifested with neurologic symptoms: headache, speech impairment, numbness of a limb accompanied by progressive thrombocytopenia with hemorrhage and microangiopathic hemolytic anemia (MAHA). Nobody had all sings from TTP pentad. On admission all patients had hemoglobin (HGB) <90 g/dl, thrombocytes <26 × 109/L, reticulocytosis >15%, mean leukocytes count ‐ 10 ± 2.2 × 109/L. Schistocytes were described in 3 persons. Markers of MAHA were revealed with elevation of lactate dehydrogenase ‐ 5703 ± 2400 IU/ml, decline of haptoglobine – 2.1 ± 0.5 mg/dl. ADAMTS 13 activity was measured in 3 patients and appeared to be 0%. The diagnosis of TTP set up in them and suspected in others patients. Pulse‐therapy with steroids for 5 days and plasmapheresis ‐ 18 procedures for each patient ‐ were started immediately in 4 patients and stopped when thrombocytes were ≥ 120 × 109/L and decrease of MAHA intensity was reached. Stable remission was achieved in them. Other 2 patients had 4‐day delay in the beginning of plasmapheresis. Their HGB level and thrombocytes started to rise only after 25th cycle of plasmapheresis. Rituximab once a week for 4 weeks was added. Unfortunately one patient developed hemorrhagic stroke and the other – secondary hemolytic‐uremic syndrome and polyorgan failure. Four patients are followed up for a year now, no signs of relapse are found.
Conclusion(s): Setting up the diagnosis of TTP early is crucial for its treatment with plasmapheresis playing the mail role. It delay can significantly decrease survival rate.
285. VPB0312
285.1. Thrombotic thrombocytopenic purpura in adult‐onset Still's disease
R. Ben Salah 1; Y. Bouattour2; A. Ben Mefteh3; M. Chaari4; Z. Bahloul5
1 Hedi Chaker Hospital, Sfax, Sfax, Tunisia; 2 Departement of internal medicine, Hédi Chaker Hospital, Sfax, Sfax, Tunisia; 3 Sfax University Tunisia, sfax, Sfax, Tunisia, 4 hematology department, sfax, Sfax, Tunisia; 5 University of Sfax, SFAX, Sfax, Tunisia
Background: Coexistence of TTP and adult‐onset Still’s disease is extremely rare.
Aims: There is increasing evidence that this association could be more than just coincidental.
Methods: Hereby, we present a case of TTP occurring in patient with a known ASD and the successful outcome after plasma exchanges.
Results: A 24 year‐old Tunisian woman was referred to our department for the first time in February 2006. The diagnosis of AOSD was established and the patient was treated first with high doses of corticosteroids and all her symptoms resolved. At the age of 35 year‐old, she was admitted again after one month of intense arthralgias involving her shoulders, elbows and wrists. She had also recurrent nosebleed episodes. The physical examination was remarkable for multiple ecchymosis on the lowers extremities. Blood smear showed frequent schistocytes (10%) and increased reticulocytes rate 286 000in addition to hemogram disorders: low circulating platelet count 6000, haemolytic anemia 7,4g/dland increased white cell count at 13000 Elts/mm3. Electrolytes, kidney function test, liver enzymes were within normal range. There was no evidence of an evolutive infection or neoplasia. The diagnosis of TTP based on microangiopathic hemolytic anemia and thrombocytopenia that cannot be explained otherwise. Of note, blood tests for ADAMTS13 activity and inhibitor were not available in the hospital. To reduce bleeding frequency, the patient received platelets transfusion and high‐dose corticosteroid therapy. Then, intravenous immunoglobulin treatment was added but with no significant improvement. Finally, within the application of plasmapheresis regimen, her symptoms began to resolve: there have been no further arthralgias or hemorrhage signs. The patient was discharged on prednisone therapy with plans for extended taper.
Conclusion(s): The purpose of this case is to insist on the importance of searching for a TTP once clinical evocative symptoms are reported by a patientwith AOSD.
286. PB0300
286.1. Refractory acquired thrombocytopenic thrombotic purpura with genetic polymorphism complement system
E. Fernandes de Figueiredo 1; E. Marín2; S. Ríos2; H. Sarmiento1; F. Tarín1; L. Hernández3
1 HGUA, Alicante, Comunidad Valenciana, Spain; 2 HGUA, ALICANTE, Comunidad Valenciana, Spain; 3 HGUA, Tibi, Comunidad Valenciana, Spain
Background: Acquired thrombotic thrombocytopenic purpura (aTTP) is caused by autoantibody‐mediated inhibition of the protease ADAMTS13. Lack of functional protease leads to ultralarge von Willegrand Factor multimers and platelet activation, with systemic thrombi. The role of complement dysregulation, well identified in other syndromes, has been discussed in TTP. This leads to the concept of a variety of mixed clinical presentations and outcomes, in which pathological mechanisms are intertwined. Novel therapies for refractory TTP include bortezomib and, anti‐VWF nanobody, and eculizumab.
Aims: We present a case of refractory TTP, in which variants in complement genes were observed.
Methods: ADAMSTS13 activity was analyzed by a chromogenic test measured with ELISA (normal >40%). Quantification of antibodies IgG against ADAMTS13 was obtained by ELISA with IMUBIND, and defined as positive > = 9.6 U/ml. Sanger sequencing of the whole ADAMTS13 gene was performed (cDNA sequence ENST00000371929). Genetic study of complement was made by NGS.
Results: A 19‐year‐old pregnant presented at delivery with anemia (6.6 g/dl), thrombocytopenia (7000/μl), schistocytes. aTTP was diagnosed (0% ADAMTS13 activity). Plasma exchange (PEX), methylprednisolone iv (500 mg daily × 3, tapering), and rituximab (375 mg/m2 × 4) were given, with no response. Therapy was intensified: corticoids (1 g/24 h × 3), PEX twice a day, vincristine (1.6 mg three doses). On day +20 caplacizumab was started. Platelet count rose until normal levels, ADAMTS13 activity persisted 0%. Anti‐ADAMTS13 antibodies were negative. ADAMTS13 gene sequencing was normal. After three cycles of bortezomib (1.3 mg/m2 × 4) ADAMTS13 activity was 24%, on day +209. ADAMTS13 activity was 58.6% on day +291, remaining >40% since then. Genetic study of complement was performed, finding a carrier status for atypical Hemolytic Uremic Syndrome in heterozygosis (MCPggaac/MCPaaggt).
Conclusion(s): Bortezomib is effective in refractory aTTP. Caplacizumab is a safe therapy even in long‐term scheduling. Severe refractory cases may involve associated polymorphisms in the complement system.


287. VPB0311
287.1. Thrombotic thrombocytopenic purpura mimicking acute ischemic stroke
A. Aribandi 1; C. Ranjith2; K. Ashok1; R. Gottipati3
1 American Oncology Institute, Hyderabad, Telangana, India; 2 Amerian Oncology Institute, Hyderabad, Telangana, India; 3 American Oncology Insttute, Vijayawada, Andhra Pradesh, India
Background: Thrombotic thrombocytopenic purpura (TTP) is an autoimmune disorder characterized by thrombocytopenia, hemolytic anemia, fluctuating neurological deficits, fever and renal impairment. Differentiation of TTP from ischemic stroke is extremely important as management is entirely different. Here we report a series of patients with TTP who presented with expressive aphasia, hemiparesis and altered sensorium.
Aims: TTP and acute ischemic stroke need to be differentiated in view of differences in management, complex management strategy for TTP and high morbidity and mortality due to delays in diagnosis with TTP.
Methods: Here we present the data of 3 patients who were admitted with symptoms mimicking an acute stroke. Data was collected from hospital case records and tabulated.
Results: The profile is depicted in the table below.
Conclusion(s): TTP is a life threatening condition and has to be one of the differential diagnosis when a patient presents with altered sensorium and neurological features like ischemic stroke. Early identification and initiation of Plasmapheresis is extremely important Delay in treatment increases mortality significantly. Simple peripheral smear examination will give a clue to excluding or suspecting TTP.

288. VPB0313
288.1. Treatment and prognosis of children with inherited TTP: A single‐center retrospective clinical study
L. Fu 1; J. Ma2; R. Wu3; Y. Wei4
1 Beijing Children Hospitlal, beijing, Beijing, China (People's Republic); 2 Hematology Oncology Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, Beijing, China (People's Republic); 3 Beijing Children’s Hospital, Beijing, Beijing, China (People's Republic); 4 Beijing Children Hospital, beijing, Beijing, China (People's Republic)
Background: As a rare disorder, congenital thrombotic thrombocytopenic purpura (cTTP) is often severe in childhood and requires preventive treatment to avoid long‐term adverse prognosis.
Aims: The purpose of this study was to understand the relationship between cTTP treatment and prognosis in China through single‐center data analysis, and to provide a basis for standardized diagnosis and treatment.
Methods: Clinical data of children diagnosed with cTTP in our center were retrospectively collected, including onset age, treatment mode, acute episodes, renal and nervous system prognosis.
Results: There were 7 children, including 1 male and 6 female, with onset age ranging from several hours after birth to 28 months. The median follow‐up time was 5.2 years (0.6–6.9) years, and the median follow‐up was 8 times (4–35 times). All patients were treated with fresh frozen plasma for replacement therapy, and 2 patients received preventive therapy. The average annual frequency of acute episodes was 1.05 (0.7–1.4) times, and no cerebral infarction or renal dysfunction occurred. Five patients were treated on demand, with an average annual acute episodes of 1.7 (1–6) times, and 3 patients had recent episodes of urinary protein (+). In case 3, the disease was poorly controlled, with the frequency of acute episodes as high as 9 times a year, and proteinuria and hematuria gradually appeared. The creatinine clearance rate decreased by 84ml/min and blood pressure increased, which could be controlled within the normal range by oral antihypertensive drug therapy. The annual frequency of episodes in children older than 10 years was lower than in all children younger than 10 years (1.05 vs 3.32). The average number of episodes per year was lower with prophylactic treatment than with on‐demand treatment (1.05 vs 3.32). There was no significant difference in the severity of the episodes, which were all in grade 1.
Conclusion(s): Prophylactic treatment is expected to reduce the number of acute episodes.
289. PB0292
289.1. Evaluation of a rapid screening test as an effective tool in decentralizing ADAMTS13 activity testing ‐ report from the rapid assessment of plasma in microangiopathic thrombocytopenia (RAPMAT) study
G. Gilmore1; R. Baker 2; J. Tiao1
1 WA Centre of Thrombosis and Haemostasis (WACTH) Centre for Computational and Systems Medicine, Murdoch University; Perth Blood Institute, Perth, Western Australia, Australia; 2 WA Centre of Thrombosis and Haemostasis (WACTH) Centre for Computational and Systems Medicine, Murdoch University; Perth Blood Institute., Perth, Western Australia, Australia
Background: Thrombotic thrombocytopenia purpura (TTP) is a rare life‐threatening disease and timely diagnosis is critical. An ADAMTS13 activity level of < 10 IU/dl is used to differentiate TTP from other thrombotic microangiopathic thrombocytopenia (TMAT) diagnosis. Current test iterations based on FRET, ELISA or chemiluminescence assays are time consuming, have long turnaround times or are not readily available.
Aims: To evaluate and compare a rapid flow through screening test (Technoclone, Vienna) against a VWF73 ADAMTS13 activity ELISA (Technoclone, Vienna) and an ADAMTS13 automated chemiluminescence method (Acustar ‐ Werfen).
Methods: De‐ identified suspect and confirmed TTP plasmas (n = 120) from 6 diagnostic laboratories in Australia and New Zealand were collected. Study was approved by MU HREC for assay development and calibration (Permit No. 2013/131). Local and central measurement of ADAMTS13 activity level comparison was made with the flow through test and functional ELISA assay. Additionally, the central study site also included a chemiluminescence method. The semi quantitative screening test assigns ADAMTS13 to one of four level indicator guides: 0, 10, 40 and 80 IU/dl.
Results: ADAMTS13 activity levels from local and central study sites were comparable, (r 2 = 0.78) as was the correlation with the chemiluminescence and ELISA methods (r 2 = 0.86). All but 1 of the rapid flow through results confirmed the quantitative ELISA results of <10 IU/dl, the TTP diagnostic threshold. There were 2 results of 40 IU/dl that were <10 IU/dl by the ELISA assay and no results at 80 IU/dl resulted in activity levels less than <10 IU/dl by the ELISA assay.
Conclusion(s): The rapid flow through test provides an initial time critical semi‐quantitative ADAMTS13 evaluation. A semi quantitative value of 80 IU/dl does not require an urgent ADAMTS13 assay. The point of care test makes it suitable for all laboratories, especially regional laboratories where quantitative levels are not performed.
290. VPB0318
290.1. Efficacy and safety of single, low‐dose rituximab‐contained regimen for Refractory/Relapsed thrombotic thrombocytopenic purpura: A retrospective study of 7 cases
Y. Tan 1; Q. Zhu1; H. Bi1; Z. Zeping2
1 The Second Affiliated Hospital of Kunming Medical University, kunming, Yunnan, China (People's Republic); 2 The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China (People's Republic)
Background: The use of rituximab to treat TTP has been described as second‐line treatment for relapsed or refractory patients, but single, low‐dose rituximab‐contained regimen for TTP is currently not reported.
Aims: The purpose of this study was to retrospectively analyze the efficacy and safety of single, low‐dose rituximab (600 mg)‐contained regimen for the treatment of refractory/relapsed TTP.
Methods: Seven patients with refractory/relapsed TTP admitted to The Second Affiliated Hospital of Kunming Medical University from February 2016 to December 2020 were included in the study. The medical records of 7 patients treated by single, low‐dose rituximab (600 mg)‐contained regimen were collected and analyzing.
Results: (1)A total of 7 patients were enrolled in the study, there were 5 women, and the median age was 57(27–66) years. Five patients were enrolled during new diagnosis of TTP. (2)The median platelet count was 4 (range, 3–49 × 109/L), the median LDH was 626 (414~2117) U/L in all 7 patients. ADAMTS13 activity was detected in 3 patients at 0.73%, 0.9%, and 2.28%, respectively, and two of them tested positive for ADAMTS13 inhibitor.(3) All seven TTP patients were treated with glucocorticoid combined with plasma exchange within 24 h after admission. The first dose of rituximab was 9 (5–47) days after admission. The median number of plasma exchange was 11 (6–35) times, and the median amount of exchanged plasma was 17710 (12150–66900) ml. 6 patients achieved CR after treatment, and the median time to CR was 10 (6–25) days after the first dose of rituximab. The median follow‐up time for all patients was 523 (8–984) days, 1 patient died of intracranial hemorrhage on the eighth day after admission, and 1 patient relapsed on the 8th month after rituximab infusion, the relapse‐free survival at 1 years was 68.57% (Figure 1).
Conclusion(s): Single, low‐dose rituximab has a good effect on refractory/relapsed thrombotic thrombocytopenic purpura, and the risk of infection with rituximab will increased.


291. PB0306
291.1. Biomarkers of HIV‐associated thrombotic thrombocytopaenic purpura (HIV‐TTP)
S. Louw1; M. Gededzha2; A. Mayne3; E. Mayne 4
1 National Health Laboratory Service (NHLS)/University of the Witwatersrand (Wits), Johannesburg, Gauteng, South Africa; 2 National Health Laboratory Service (NHLS), Johannesburg, Gauteng, South Africa; 3 University of the Witwatersrand, Johannesburg, Gauteng, South Africa; 4 Department of Pathology, Faculty of Health Sciences, University of Cape Town and National Health Laboratory Service, Cape Town, Western Cape, South Africa
Background: Secondary thrombotic thrombocytopaenic purpura (TTP) is a complication of infection with human immunodeficiency virus (HIV) with platelet‐rich microvascular thrombosis and end‐organ failure. TTP is caused by an absolute or relative deficiency of the von‐Willebrand factor (VWF)‐cleaving protease, a‐disintegrin‐and‐metalloproteinase‐with‐thrombospondin‐motifs 13 (ADAMTS‐13). Controversy exists regarding the pathogenesis of HIV‐associated TTP with both complement activation and endothelial dysfunction secondary to inflammation being postulated. TTP should be distinguished from other thrombotic microangiopathies notably disseminated intravascular coagulation (DIC) as treatment differs with plasma therapy being the mainstay of TTP treatment. Disease related biomarkers may be useful to assist in differentiating these conditions.
Aims: Investigate the derangement in biomarkers of inflammation and of activation of the coagulation system and endothelium in patients with HIV‐ associated TTP.
Methods: Venous blood was collected from 35 patients with HIV‐associated TTP prior to commencement of therapeutic plasma exchange after informed signed consent was obtained (University of the Witwatersrand ethics approval number: M160134). Levels of the pro‐inflammatory cytokine interleukin‐6 (IL‐6), fibrin degradation products (D‐dimers) and the endothelial activation marker, Intercellular Adhesion Molecule‐1 (ICAM‐1), were measured on the samples. The median results of these biomarkers were compared with published results in HIV‐infected cohorts without TTP to determine the significance of the changes in HIV infected patients with TTP. A p‐value of <0.05 was considered significant.
Results: IL‐6, D‐dimer and ICAM‐1 levels were significantly higher in patients with HIV‐associated TTP versus HIV infected individuals without TTP irrespective of exposure to anti‐retroviral treatment (ART) (Table 1).
Conclusion(s): Patients with HIV‐associated TTP had significantly elevated biomarkers of inflammation as well as of activation of the coagulation system and endothelium compared with published data in HIV infected cohorts without TTP. The pathogenesis of HIV‐associated TTP therefore probably involves endothelial damage and local activation of the coagulation cascade and excessive release of VWF resulting in a relative deficiency of ADAMTS‐13.

292. PB0293
292.1. Cattle‐FRETS71, a novel fluorogenic substrate with broad applicability in characterizing ADAMTS13 properties and function
J. Barton 1; C. Anderson2; F. Miranda2; R. Kelley3; J. Kremer Hovinga4; D. Terrell5; S. Vesely5; J. George6; J. Muia2
1 Oklahoma State University Center for Health Sciences, Broken Arrow, Oklahoma, United States; 2 Oklahoma State University Center for Health Sciences, Tulsa, Oklahoma, United States; 3 University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States; 4 Department of Hematology and Central Hematology Laboratory, Bern University Hospital, University of Bern, Bern, Bern, Switzerland; 5 University of Oklahoma Health Sciences Center, Hudson College of Public Health, Oklahoma City, Oklahoma, United States; 6 University of Oklahoma Health Sciences Center, College of Medicine, Oklahoma City, Oklahoma, United States
Background: Thrombotic thrombocytopenic purpura (TTP) is a life‐threatening microangiopathic condition caused by an autoimmune or a genetic deficiency of ADAMTS13. Many ADAMTS13 activity assays are available that aid TTP diagnosis but their sensitivity and applicability are limited. Cattle‐FRETS71 (C‐71) is a bovine von Willebrand factor (VWF) domain A2‐derived fluorogenic substrate that is equally cleaved by plasma ADAMTS13 of different species of research interest (e.g., mouse, rat, sheep, cattle). Therefore, the C‐71 substrate would be ideal for both clinical and research applications within the same settings.
Aims: The objective of the current work was to validate the performance of C‐71 substrate compared to FRETS‐rVWF71 (H‐71), an optimized ADAMTS13 substrate derived from the human VWF A2 domain but lacking additional C‐71 advantages. Comparison with FRETS‐VWF73 (H‐73), the common commercial substrate also was sought.
Methods: Internal validation was performed using heparinized plasma samples previously analyzed by H‐71 (n = 89). External validation was a blinded study using serum samples (n = 118) from the Oklahoma TTP‐HUS registry that had been assayed by H‐73 within 1 year of collection, in 2004–2014.
Results: In the 89 plasma samples (Figure 1A), the correlation between C‐71 and H‐71 was excellent (r = 0.95). There was 100% agreement (Kappa = 1.0) between C‐71 and H‐71 when classifying patients into low (<10%) and normal (≥10) groups. In the 118 serum samples from the Registry (Figure 1B), the correlation between C‐71 and H‐73 was good (r = 0.81). There was 86% agreement (kappa = 0.60) between C‐71 and H‐73 classifying patients as <10% and ≥10%. Of the 17 misclassified patients, 15 were only <10% on the C‐71 assay and 2 were only <10% on the H‐73 assay.
Conclusion(s): Results were similar among C‐71 and H‐71 and classification into low/normal groups was the same. Less agreement between C‐71 and H‐73 may be caused by decreased ADAMTS13 activity during frozen storage.

PB0302
Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura: comparative real‐world multicenter study
E. Gavriilaki 1; E. Koravou2; S. Besikli2; T. Chatziconstantinou2; E. Nikolousis3; A. Mpanti4; C. Pontikoglou5; C. Kalpadaki5; A. Bitsani6; I. Tassi7; T. Touloumenidou2; M. Papathanasiou2; A. Syrigou2; G. Kaiafa8; E. Kapsali7; E. Papadaki5; A. Anagnostopoulos2; C. Lalayanni2; I. Sakellari2
1 G Papanicolaou Hospital, Thessaloniki, Greece, Thessaloniki, Thessaloniki, Greece; 2 G Papanicolaou Hospital, Thessaloniki, Thessaloniki, Greece; 3 Athens Medical Center, Athens, Attiki, Greece; 4 Papageorgiou Hospital, Thessaloniki, Thessaloniki, Greece; 5 University of Crete, Heraklion, Iraklion, Greece; 6 Laiko University Hospital, Athens, Attiki, Greece; 7 University Hospital of Ioannina, Ioannina, Ioannina, Greece; 8 Aristotle University of Thessaloniki, Thessaloniki, Thessaloniki, Greece
Background: Caplacizumab is the first approved targeted therapy of acquired thrombotic thrombocytopenic porphyra (aTTP).
Aims: We designed this multicenter real‐world study to assess safety and efficacy of caplacizumab compared to historic controls.
Methods: We recorded clinico‐biological data from consecutive adult aTTP patients that were treated with corticosteroids and plasma exchange over the last decade (2011–2021) according to international guidelines. Diagnosis was confirmed with ADAMTS13 activity. Results are presented as median (range).
Results: We studied 63 patients [aged 48 (19–75) years]: 16 in the caplacizumab and 47 in the historic control group. The latter had a higher rate of neurological involvement (p = 0.017). Rituximab was given in the majority of patients for refractory disease (2/3 patients with refractory disease in the caplacizumab group versus 15/16 in controls, p = 0.161) or as pre‐emptive treatment (12/13 in caplacizumab versus 15/29 in controls, p = 0.011). No other significant differences were observed in patient or disease characteristics between groups. Caplacizumab was given at a median day 4 (1–32) from diagnosis of acute phase (10 mg daily) for 31 (6–40) doses. There were no major hemorrhages or other complications. Plasma exchange sessions were 12 (9–21) in caplacizumab versus 14 (6–32) in controls. ADAMTS13 activity normalized after treatment in all caplacizumab patients and no relapses have been observed. Control patients presented a median of 1 (0–14) relapse. With 15.4 (2.2–320) months from disease diagnosis, there were 4 deaths (only from the control group, p‐0.310, Figure).
Conclusion(s): Given the limited international experience, our study confirms safety and efficacy of caplacizumab in aTTP. Modifications of dosing compared to the clinical trial setting seem to have non‐inferior outcomes in the real‐world setting. Since ADAMTS13 remission has emerged as an important marker of long‐term results, further studies in large real‐world populations with longer follow‐up are needed to highlight the value of caplacizumab.

VPB0314
Relationship between recurrence of congenital and immune‐mediated thrombotic thrombocytopenic purpura and mRNA‐based COVID‐19 vaccination: A questionnaire survey in a Japanese cohort
E. Hamada 1; M. Kubo2; K. Sakai2; S. Yamada1; M. Hayakawa2; M. Matsumoto2.
1 Nara Medical University, Kashihara, Nara, Japan; 2 Department of Blood Transfusion Medicine, Nara Medical University, Kashihara, Nara, Japan
Background: Patients with thrombotic thrombocytopenic purpura (TTP) are theoretically at high risk for thrombosis due to COVID‐19, which is attributed to the increase in plasma von Willebrand factor levels and decrease in plasma ADAMTS13 activity with disease severity of COVID‐19. So far, one case of a flare of congenital TTP (cTTP) and some cases of de novo immune‐mediated TTP (iTTP) have been reported following COVID‐19 vaccination worldwide.
Aims: This study aimed to investigate whether mRNA‐based COVID‐19 vaccines could induce TTP episodes in patients with a history of TTP.
Methods: A questionnaire was administered to Japanese patients with cTTP and iTTP in October 2021 regarding the adverse events they experienced after receiving an mRNA‐based COVID‐19 vaccine. Only patients aged ≥12 years were included in the study. The questionnaire was sent to physicians in‐charge of managing patients with TTP. This study was limited to analyzing the risk of TTP recurrence.
Results: A total of 42 patients with cTTP and 33 patients with iTTP were included in the study. Among the patients with cTTP, 38 were fully vaccinated (Table 1), and four were unvaccinated. No severe adverse effects of vaccination, especially recurrence of cTTP, were observed in all patients. Moreover, the platelet counts of the patients remained unchanged. Those receiving fresh frozen plasma infusion were vaccinated within two weeks of plasma infusion. Out of the 33 patients with iTTP, 26 were fully vaccinated (Table 2), and seven patients were unvaccinated. No recurrence was observed in patients with iTTP. Likewise, the platelet counts of the patients remained unchanged.
Conclusion(s): We believe that all patients with cTTP and iTTP should receive COVID‐19 vaccination, given its benefits. Careful observation for any adverse events should be after vaccination.


PB0308
Method discrepant ADAMTS‐13 activity results in first presentation query TTP subjects
T. Pitchford 1; S. Kitchen2; J. Vanveen3; R. Maclean3; R. Fretwell3
1 Sheffield Teaching Hospital NHS Foundation Trust, Sheffield, England, United Kingdom; 2 Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom; 3 Sheffield Teaching Hospitals NHS Trust, Sheffield, England, United Kingdom
Background: Thrombotic thrombocytopenia purpura (TTP) is a rare life‐threatening disease caused by severe deficiency of the von Willebrand factor cleaving protease ADAMTS13. A rapid automated chemiluminescent method (ACL Acustar) has recently been introduced. We have identified a small number of subjects who have Acustar results <10 IU/dL that do not match the classical presentation of either immune mediated acquired TTP (iTTP) or congenital TTP (cTTP) and often demonstrate method discrepancy when compared to ADAMTS13 activity by an ELISA method.
Aims: To identify and characterize potential method discrepancy subjects within first presentation query TTP subjects.
Methods: Data was examined on 401 first presentation patients tested for ADAMTS13 activity since 9/2019. Subjects with ADAMTS13 activity <15 IU/dL (Acustar) and negative ADAMTS13 antibody (technozyme) were tested for ADAMTS13 activity with (technozyme) ELISA method.
Results: Method discrepancy was identified in 1.5–2.5% of first presentation? TTP subjects tested and 17.5–20% of all 1st presentation? TTP subjects with Acustar activity <10 IU/dL.
Conclusion(s): ADAMTS 13 activity >10 IU/dL by Acustar could be safely used to exclude TTP. We identified two distinct groups among subjects with ADAMTS13 results of < 10 IU/dL by Acustar. New iTTP subjects are unlikely to present with results of >2.0 and subjects with results between 2–10 IU/dL by Acustar are unlikely to be ADAMTS13 antibody positive and were typically found to demonstrate method discrepancy. The reason for the method discrepancy is currently unclear and was not described during verification studies of the Acustar Adamts13 activity method. Knowledge of the small but clinically significant group of method discrepant subjects alongside the typically pattern of new iTTP subjects seen on the Acustar is important for clinical and laboratory teams using the Acustar.
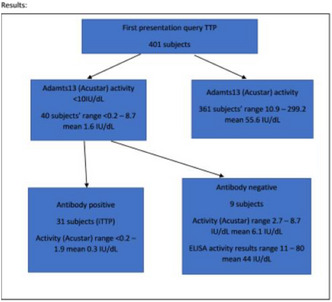
PB0298
A systematic review of the current epidemiology of immune‐mediated and congenital thrombotic thrombocytopenic purpura caused by severe ADAMTS13 deficiencies
P. Du 1; T. Cristarella2; Y. Moride3
1 Takeda Development Center Americas, Inc., Hershey, Pennsylvania, United States; 2 YolaRX Consultants Inc., Montreal, Quebec, Canada; 3 YolaRX Consultants Inc., Montreal, Quebec; Rutgers, The State University of New Jersey, New Brunswick, NJ, USA, Montreal, Quebec, Canada
Background: Immune‐mediated (iTTP) and congenital (cTTP) thrombotic thrombocytopenic purpura are rare clotting disorders caused by either an acquired or an inherited deficiency in the von Willebrand factor‐cleaving enzyme ADAMTS13.
Aims: To synthesize knowledge on the epidemiology of iTTP and cTTP worldwide.
Methods: A systematic review (PROSPERO CRD42020172273) of observational studies published from January 01, 2010 to February 06, 2020 was conducted following the PICOTS (population, intervention/exposure, comparator, outcomes, time period, study type) approach. Target population was overall TTP, iTTP, and cTTP. Outcomes of interest included incidence, prevalence, patient characteristics at diagnosis, and mortality. Information sources were literature searches (Ovid® MEDLINE and Embase), pragmatic searches (Google, Google Scholar, congresses, clinical societies, and patient organizations), and snowballing. Case reports, case series, clinical trials, and non‐clinical/experimental studies were excluded. Study selection and data extraction were conducted independently by 2 reviewers.
Results: A total of 153 eligible sources, mainly from Europe and North America, were included in the review (iTTP specific, n = 47; cTTP specific, n = 11; iTTP and cTTP, n = 2; unspecified, n = 93). Table 1 shows overall prevalence and incidence of TTP. Median age at cTTP diagnosis ranged from 16.7 to 18.7 years in two multinational studies, while in another study disease onset generally occurred either during early childhood (median age at diagnosis 3.5 years) or in adulthood (median age at diagnosis 31 years). For iTTP, patients typically experienced their first episode in their 40s (9 sources). Mortality associated with cTTP was reported between 0 and 8.3% with different populations and follow‐up periods in 7 studies, mainly involving European countries. Mortality associated with iTTP was highly varied across 29 studies, with estimates up to 15.2% in France, 12.8% in the US, and 30% in China.
Conclusion(s): Data on the epidemiology of cTTP are limited. In addition, data from Asia and the Middle East are limited for both iTTP and cTTP.

PB0305
Method and laboratory specific clinical cut‐offs support appropriate interpretation of ADAMTS13 activity assays
S. MacDonald 1; P. Murphy2; R. Fretwell3; C. Downey4; P. Baker5; E. Foxton6; C. Lawrence7; A. Ali8; V. Jenkins9; E. Vilar10; M. Besser1; E. Symington1; M. Robinson1; M. Desborough5; W. Thomas1; J. Yong4; T. Dutt4
1 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 2 THE NEWCASTLE UPON TYNE HOSPITALS NHS FOUNDATION TRUST, Newcastle, England, United Kingdom; 3 Sheffield Teaching Hospitals NHS Trust, Sheffield, England, United Kingdom; 4 Liverpool University Hospitals NHS Foundation Trust, Liverpool, England, United Kingdom; 5 Oxford University Hospitals, Oxford, England, United Kingdom; 6 Guy's and St. Thomas's NHS Foundation Trust, London, England, United Kingdom; 7 Glasgow Royal Infirmary, Glasgow, Scotland, United Kingdom; 8 Edinburgh Royal Infirmary, Edinburgh, Scotland, United Kingdom; 9 Cardiff and Vale University Health Board, Cardiff, Wales, United Kingdom, 10 Univeristy of Hertfordshire, Hatfield, England, United Kingdom
Background: Analytical and clinical equivalence are influenced by choice of methods used to analyze verification data. Numerical equivalence is often evidenced with correlation, regression and Bland–Altman analysis, whereas qualitative performance is determine by metrics derived from classification of results as above or below a clinical cut‐off, often not locally verified for clinical utility.
Aims: Does the method of verification data analysis impact clinical interpretation of ADAMTS13 activity assays? Are method specific local cut‐offs required to achieve published performance specifications and improve diagnostic interpretation?
Methods: Here we present comparative multicentre verification data from 9 sites in the UK for implementation of rapid ADAMTS13 assays. The HemoSIL chemiluminescent (Acustar, Werfen, Bedford, USA) and Technofluor FRET (Technoclone, Austria) assays are recently available rapid turnaround methods whose performance was determined by comparison with the established methods at each site independently (Technozym, Technoclone, Austria of in‐house FRET).
Results: Passing‐Bablok underestimates systematic error compared to Deming Regression similar to that seen with ordinary least squares. Proportional bias is underestimated using Passing‐Bablok. Agreement is improved in Technofluor (Kappa ‐ 0.8 to 0.9) by applying a cut‐off of 12.5 IU/dL and sensitivity is increased from 0.72 to 0.9 by the same change. There is no impact on specificity (0.98). Contrastingly, for HemoSIL Acustar reducing cut‐off from 10 to 8 IU/dL improves agreement (Kappa 0.78 to 0.85). Specificity is improved to 0.97 from 0.90 with sensitivity remaining at acceptable levels of 0.95. Differences between methods is comparison specific and correlates with sample size.
Conclusion(s): Deming regression accounts for method specific differences in measurement uncertainty. This is essential for substantially different measurement methods. Applying a local cut off for TTP diagnosis supports consistent clinical interpretation. Performance specifications can be standardized and achieved between centres. The cut‐off must also consider sample size used for the verification to represent measurement uncertainty at the decision level.


PB0310
Longitudinal kinetics of B‐cell subsets in immune thrombotic thrombocytopenic purpura patients: Relapses vs remission
M. Subhan 1; J. Shin2; B. Craven1; R. de Groot1; Y. Guo3; G. Cambridge1; M. Scully2; M. Thomas4
1 University College London, London, England, United Kingdom; 2 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom; 3 Univsersity College London, London, England, United Kingdom; 4 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom
Background: Risk of relapse in immune‐mediated Thrombotic Thrombocytopenic Purpura (iTTP) is 30–50%. B‐cell return following rituximab is not temporally associated with disease relapse.
Aims: We prospectively investigated changes in B‐cell subsets in relation to clinical and laboratory parameters in iTTP patients longitudinally after anti‐CD20 therapy for an acute iTTP episode or ADAMTS13 relapse (ADAMTS13 activity <15%) until subsequent clinical or ADAMTS13 relapse, and compared to patients who remained in remission over an equivalent period.
Methods: Heparinized blood samples were collected from iTTP patients consented to the UK TTP Registry at presentation of acute episode or prior to elective rituximab, and thereafter at 3–6 monthly intervals. Flow cytometry on isolated PBMCs was performed on a Cytoflex‐S cytometer using the mature B‐cell ‘Bm1–Bm5’ classification to identify B‐cell subsets based on co‐expression of IgD and CD38 and analyzed using FlowJo software.
Results: There were 10 patients in the relapse cohort (3 followed after an acute episode, 7 after elective rituximab) and 10 in the remission cohort (4 acute and 6 elective episodes at t = 0). Median age was 51y (range 38–81) and 45.5y (21–68) respectively. One patient in each group did not achieve B‐cell depletion (CD19+ count<0.005x10^9/L). Time to B‐cell return was similar: 8 months (4–13) in relapses vs 9 months in remission (5–15.5). All subsequent relapses were ADAMTS13 relapses and occurred at a median of 16 months (9–25). Longitudinal analysis showed that in both groups B‐cell return after rituximab develops along normal B‐cell ontogeny. The kinetics of naïve, transitional, memory B‐cells and plasmablasts did not differ significantly between patients who went on to relapse vs those who remained in remission.
Conclusion(s): No alterations in B‐cell subsets were identified prior to a relapse, suggesting temporal relationships between B sub‐populations cannot be used as a biomarker to better predict relapses.

PB0307
Results of a single‐centre targeted screening approach to identify potential occult cases of pregnancy‐associated thrombotic thrombocytopenic purpura (TTP)
L. Neave 1; M. Thomas2; C. Vendramin3; K. Maksym4; R. Spencer5; A. David6; A. Doyle7; M. Whitten8; M. Scully9
1 UCLH Hospitals NHS Trust /Hemostasis Research Unit, University College London, London, England, United Kingdom; 2 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 3 University College London, London, England, United Kingdom; 4 Fetal Medicine Unit, UCLH Hospitals NHS Trust/Institute for Women's Health, University College London, London, England, United Kingdom; 5 Elizabeth Garrett Anderson Institute for Womens Health, University College London/Discovery and Translational Science Deprtment, Univeristy of Leeds, Leeds, England, United Kingdom; 6 Elizabeth Garrett Anderson Institute for Women's Health, Univerisity College London, London, England, United Kingdom; 7 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 8 Elizabeth Garrett Anderson Institute for Women's Health, London, England, United Kingdom; 9 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom
Background: Pregnancy‐associated TTP can be associated with hypertension, proteinuria, fetal growth restriction and fetal loss, and cases may be initially diagnosed as preeclampsia +/− HELLP syndrome. Meanwhile, a subset of cases of ‘unexplained’ fetal growth restriction, stillbirth, or thrombocytopenia in pregnancy may represent undiagnosed TTP. Given that effective treatment of TTP drastically improves fetomaternal outcomes, it is vital that cases are not missed.
Aims: We sought to screen for TTP in women with obstetric complications possibly compatible with underlying TTP.
Methods: Women with a current or previous history of unexplained fetal growth restriction or stillbirth (+/− suspected uteroplacental insufficiency), preeclampsia, HELLP syndrome, or new onset thrombocytopenia (nadir < 75 x109/L) were recruited at a regional centre. In cases with additional features ‘suspicious’ for TTP (1+ of: (i) >25% fall in platelet count; (ii) features of haemolysis (iii) features of uteroplacental insufficiency and (iv) additional adverse obstetric history), ADAMTS13 activity (FRETS‐VWF73 assay) was measured. ADAMTS13 activity >10 iu/dL excluded TTP. ADAMTS13 activity was also measured in all cases of severe early onset fetal growth restriction recruited to the multicentre prospective EVERREST study. Regional ethics committee approval was obtained (18/NW/0552, 13/LO/1254) and informed consent sought in accordance with the Helsinki Declaration.
Results: Of a total of 218 women recruited, 211 met the criteria for ADAMTS13 activity measurement. One case initially presenting with severe early onset fetal growth restriction and preeclampsia was subsequently diagnosed with TTP. Figure 1 summarizes the results.
Conclusion(s): Fetal growth restriction may in some cases be the initial presenting feature of pregnancy‐associated TTP and there is potential for misdiagnosis as preeclampsia/HELLP. Whilst larger scale screening for TTP in complicated pregnancies may not be feasible/justified, education of healthcare professionals to raise awareness of TTP is of paramount importance in reducing missed cases.

PB0296
In vitro characterization of a novel Arg102 mutation in the metalloprotease domain of ADAMTS13
L. De Waele 1; L. Vermeersch1; C. Tersteeg2; S. De Meyer2; K. Pavenski3; K. Vanhoorelbeke2
1 Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 2 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 3 Departments of Medicine and Laboratory Medicine & Pathobiology, St. Michael's Hospital, Toronto and University of Toronto, Toronto, Canada, Toronto, Ontario, Canada
Background: We recently identified a novel p.R102S mutation in the metalloprotease (MP) domain of ADAMTS13 in a congenital thrombotic thrombocytopenic purpura (cTTP) patient with decreased ADAMTS13 antigen (32 IU/dL) and undetectable ADAMTS13 activity. The in vitro effect of the mutation on ADAMTS13 secretion and activity has not been studied yet. Whether the mutation, which eliminates the H‐bond of R102 with P297 in the loop that connects the MP with the disintegrin‐like (D) domain, thereby affects preactivation of the MP domain is also unknown.
Aims: We aimed to investigate the in vitro effect of the p.R102S mutation on ADAMTS13 secretion, activity and preactivation.
Methods: CHO K1 or HEK293‐T cells were transfected with wild‐type (WT) or p.R102S mutant expression plasmids. ADAMTS13 antigen levels in expression medium were measured in ELISA. WT and p.R102S mutant were further purified by Zn2 + −affinity chromatography. Preactivation of the MP domain was studied using anti‐CUB1 monoclonal antibody (mAb) 17G2 which induces a 2‐fold increase in ADAMTS13 activity in the FRETS‐VWF73 assay, and exposes a cryptic epitope in the MP domain which is recognized by mAb 6A6 in ELISA.
Results: Secretion of the p.R102S mutant was impaired to 35% of WT secretion. ADAMTS13 activity of purified p.R102S mutant was severely reduced to 12% of WT activity. Although the activity of the p.R102S mutant was low, it could still be 2‐fold activated by 17G2. In line with this, the 6A6 epitope was accessible when the p.R102S mutant was preincubated with 17G2.
Conclusion(s): The p.R102S mutation impairs ADAMTS13 secretion and severely reduces ADAMTS13 activity explaining the patient's phenotype. However, the mutation did not impair preactivation of the MP domain. Molecular modeling studies are ongoing to further understand how the mutation affects ADAMTS13 activity.
VPB0319
Efficacy and safety of two different low‐dose rituximab regimens for Chinese children patients with primary immune thrombocytopenia
X. Zhu
Capital Medical University, Beijing, Beijing, China (People's Republic)
Background: Rituximab is one of the second‐line treatments for ITP. At present, there are few studies on low‐dose rituximab, lacking of a large number of prospective and randomized trials to support the efficacy and safety of low‐dose rituximab, especially in children's ITP. Influenced by COVID‐19, we used two low‐dose rituximab regimens before and after March 2020 for second‐line treatment of children's ITP.
Aims: To compare the efficacy and safety of two different regimens for low‐dose rituximab of children patients with chronic/refractory ITP, so as to provide basis for clinical treatment.
Methods: 83 children patients were enrolled in this study and non‐randomly assigned to receive 100 mg/200 mg (body weight 30 kg) rituximab weekly for 4 weeks (group A, 53 cases) or a single dose of 375 mg/m2 rituximab (group B, 30cases). The study was follow‐up for at least half a year.
Results: The baseline data of group A and B were the same. For group A: Overall and complete response (OR and CR) rates were 35.8% and 15%, respectively; the side effects rate is 3.8%. In responders, the median time to response was 4 (1–12) weeks, with a median follow‐up time of 36 (14 ~ 46) months, 6 of 19 responders (31.6%) relapsed. For group B: OR and CR rates were 36.7% and 23%, respectively; the side effects rate is 10%. In responders, the median time to response was 1 (1 ~ 4) weeks, with a median follow‐up time of 12 (6 ~ 19) months, 4 of 11 responders (36.4%) relapsed. No significant difference in the OR, NR, relapse free survival and incidence of side effects was observed in patients between the two groups.
Conclusion(s): The two low‐dose rituximab regimens in the treatment of ITP in children both are safe and effective; The single‐agent scheme is more recommended because of easier use and not increasing safety events.


PB0290
Caplacizumab for the management of acute immune‐mediated thrombotic thrombocytopenic purpura: real world data from the Milan TTP Registry
P. Agosti 1; P. De Leo2; M. Capecchi3; A. Artoni4; I. Mancini5; M. Biganzoli6; B. Ferrari7; S. Gattillo8; S. Trisolini9; E. Rinaldi10; G. Podda11; L. Prezioso12; S. Prassede13; L. Facchini14; D. Caramazza15; G. Tolomelli16; F. Peyvandi17
1 Università degli Studi di Milano and Fondazione Luigi Villa, Milan, Italy, Milano, Lombardia, Italy; 2 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 3 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Università degli Studi di Milano, Department of Biomedical Sciences for Health, Milan, Italy, Milan, Lombardia, Italy; 4 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Milan, Italy, Milano, Lombardia, Italy; 5 Università degli Studi di Milano, Milan, Italy, Milano, Lombardia, Italy; 6 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milano, Lombardia, Italy; 7 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 8 Immuno‐Hematology and Transfusion Medicine Unit, San Raffaele Hospital, 20,132 Milan, Italy, Milano, Lombardia, Italy; 9 Hematology, Department of Translational and Precision Medicine, “Sapienza” University of Rome, Rome, Italy, Roma, Lazio, Italy, 10 Hematology Unit, A. Perrino Hospital, 72,100 Brindisi, Italy., Brindisi, Puglia, Italy, 11 U.O. Medicina III, ASST Santi Paolo e Carlo, Dipartimento di Scienze della Salute, Università degli Studi di Milano, 20,142 Milan, Italy., Milano, Lombardia, Italy, 12 Haematology and BMT Unit, Azienda Ospedaliero‐Universitaria di Parma, Parma, Italy., Parma, Emilia‐Romagna, Italy, 13 Ospedale Civile “Spirito Santo,” Pescara, Pescara, Abruzzi, Italy, 14 Hematology Unit, Azienda Unità Sanitaria Locale‐IRCCS di Reggio Emilia, Reggio Emilia, Italy, Reggio Emilia, Emilia‐Romagna, Italy, 15 Hematology, University Hospital “Ospedale di Circolo e Fondazione Macchi” ‐ ASST Sette Laghi, Viale L. Borri 57, 21,100, Varese, Italy, Varese, Lombardia, Italy, 16 Unit of Hematology, Rimini Hospital “Infermi”, Rimini, Italy, Rimini, Emilia‐Romagna, Italy, 17 Fondazione IRCCS Ca′ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Acute immune‐mediated thrombotic thrombocytopenic purpura (iTTP) is a rare but life‐threatening thrombotic microangiopathy. In 2018 caplacizumab, an anti‐von Willebrand factor nanobody, was approved by the European Medicines Agency for the treatment of acute iTTP. To date, data stemming from real‐world experience are limited.
Aims: To describe the efficacy and safety endpoints of caplacizumab treatment in Italian iTTP patients from a real‐world clinical setting.
Methods: In this observational retrospective study, 26 patients from the Milan TTP Registry receiving caplacizumab treatment for an acute iTTP episode between 2018 and August 2021 were enrolled.
Results: The main patient characteristics and acute event features are shown in table 1. All patients were treated with plasma‐exchange (PEX), corticosteroids and caplacizumab, except for one patient with mild thrombocytopenia (113,000/μL) treated with steroids and caplacizumab. Rituximab was used in 16/24 patients (66.7%). Caplacizumab was started a median of 1 day after PEX initiation (table 2). Thirty percent of patients started caplacizumab 7 days or more after PEX start to treat refractory TTP or exacerbation. All patients achieved clinical remission. In patients treated with caplacizumab within 24 hours after PEX start, the median time to platelet count normalization was 3.5 days (3.0–4.3), with a median of 6 days of PEX (4.3–7.8) to achieve clinical response. One patient experienced acute TTP exacerbation 11 days after stopping caplacizumab. Two caplacizumab‐related adverse events leading to drug discontinuation were observed, an episode of hemoptysis treated with a plasma‐derived von Willebrand factor/factor VIII‐containing concentrate and artery embolization, and a gastrointestinal bleeding requiring blood transfusion in a patient with several conditions predisposing to bleeding.
Conclusion(s): With a median of 4 days to achieve platelet count normalization, caplacizumab proved effective and safe for the treatment of acute iTTP in the real‐world clinical setting of the Milan TTP Registry, consistently with the results of randomized clinical trials.


PB0291
Citrullination of plasma proteins in immune‐mediated thrombotic thrombocytopenic purpura and cancer‐related thrombosis
T. Arfman 1; P. Kaijen2; G. Nicolaes3; J. Voorberg2
1 Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Maastricht University, Maastricht, Limburg, Netherlands
Background: Peptidyl Arginine Deiminase‐4 (PAD4) mediated citrullination affects coagulation and fibrinolysis and is a known driver of autoimmune disease. Importantly, PAD4 is released in large quantities from neutrophil extracellular traps (NETs), which have been linked to autoimmune diseases as well as thrombo‐inflammatory disorders. Citrullinated ADAMTS13 has been found in sepsis patients as well as in patients with cardiovascular comorbidities.
Aims: In this project, we investigate plasma protein citrullination in immune‐mediated thrombotic thrombocytopenic purpura (iTTP) and sepsis as a link between inflammation and iTTP.
Methods: Citrulline‐specific labeling of proteins is possible by targeting the modified guanidinium group with phenylglyoxal (PG) and PG derivatives. We are developing mass‐spectrometry based methods for the assessment of plasma protein citrullination. Using Biotin coupled PG we enrich for citrullinated proteins to determine target proteins in patient plasma. Furthermore we employ mass‐spectrometry to determine the specific citrullination sites of plasma proteins after PG labeling to gain insight on protein structure–function effects.
Results: Neutrophil proteins are expected citrullinated targets in sepsis and iTTP patient samples. We have successfully established a protocol for producing NETs in vitro using Ionomycin as a stimulus. NET‐formation coincided with citrullination of histone H3 and vimentin. We are currently optimizing our Biotin‐PG assay for the analysis of the plasma protein citrullination. Initial results show citrullination of coagulation pathway proteins and complement components. We successfully validated the labeling specificity of PG by labeling synthetic peptides containing citrullines and arginines.
Conclusion(s): We have successfully established assays to monitor PAD4 mediated citrullination of histone H3 and vimentin during in vitro formation of NETs. Determining targets for citrullination in patient plasma is facilitated by biotin‐PG. Using PG, we selectively label citrulline residues and detect the modification using mass spectrometry. We will further establish these methods and utilize them to assess citrullination of plasma proteins in patients with sepsis and iTTP.
PB0294
Adolescent thrombotic thrombocytopenic purpura is a serious disorder requiring intense therapies with significant morbidity and relapse
C. Cohen 1; M. Zobeck2; T. Olmsted Kim2; S. Sartain2; L. Raffini3; L. Srivaths4
1 Baylor College of Medicine, HOUSTON, Texas, United States; 2 Baylor College of Medicine, Houston, Texas, United States; 3 Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 4 UTHealth McGovern Medical School/Thrombosis Sub‐Committee, Women and Girls with Blood Disorders ‐ Learning Action Network, Houston, Texas, United States
Background: Thrombotic thrombocytopenic purpura (TTP) is a rare hematologic condition with a paucity of data in adolescents.
Aims: To study the clinical characteristics, treatments, and outcomes in adolescents diagnosed with acquired TTP between 2009–2020 using the Pediatric Health Information System (PHIS) database.
Methods: The PHIS database was queried to identify adolescents ages 10–21 years with the Internal Classification of Diseases (ICD‐9 and ‐10) codes for thrombotic microangiopathy from 2009–2020. Inclusion criteria included the ICD lab test code for ADAMTS‐13 and procedure codes for therapeutic plasmapheresis, consistent with treatment for acquired TTP. Exclusion criteria included ICD procedure codes for hemodialysis and peritoneal dialysis, diagnostic code for hemolytic‐uremic syndrome, and drug code for eculizumab. Primary outcomes evaluated were mortality and relapse, defined as hospital readmissions requiring plasmapheresis. Patient demographics, clinical characteristics, treatment, and outcome data were collected. Statistical analysis included the use of Fisher's exact, Chi‐squared, and Wilcoxon rank sum tests.
Results: A total of 99 patients with 123 hospitalizations for TTP were identified. Patient demographics are noted in Table 1. Hospitalization and patient outcome characteristics are noted in Table 2. Second‐line therapies were required in 76 (62%), ICU admissions in 85 (69%), mechanical ventilation in 33 (27%), and ECMO in 2 (1.6%) of hospitalizations. Twenty patients (20%) had at least one TTP relapse requiring subsequent hospitalization and plasmapheresis. The median time to relapse from initial hospitalization was 33 days (IQR 15, 92). There were 12 hemorrhagic, 4 thrombotic complications and 6 deaths (6%). No statistically significant differences were noted when comparing demographics, time‐period of diagnosis, and the presence of comorbidities of patients with and without mortality or relapse.
Conclusion(s): Adolescent TTP is a serious disorder requiring intense therapies, with significant morbidity and apparent higher relapse when compared to adults. Multicenter studies are needed to develop optimal treatment strategies to improve the outcome of adolescent TTP.


PB0304
Low ADAMTS13 activity rapid assay results in patients with low clinical probability of Thrombotic Thrombocytopaenic Purpura (TTP) – the United Kingdom experience
S. MacDonald 1; P. Murphy2; R. Fretwell3; C. Downey4; P. Baker5; E. Foxton6; C. Lawrence7; A. Ali8; V. Jenkins9; E. Vilar10; M. Besser1; E. Symington1; M. Robinson1; M. Desborough5; W. Thomas1; J. Yong4; T. Dutt4
1 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom; 2 The Newcastle Upon Tyne Hospitals Nhs Foundation Trust, Newcastle, England, United Kingdom; 3 Sheffield Teaching Hospitals NHS Trust, Sheffield, England, United Kingdom; 4 Liverpool University Hospitals NHS Foundation Trust, Liverpool, England, United Kingdom; 5 Oxford University Hospitals, Oxford, England, United Kingdom; 6 Guy's and St. Thomas's NHS Foundation Trust, London, England, United Kingdom; 7 Glasgow Royal Infirmary, Glasgow, Scotland, United Kingdom; 8 Edinburgh Royal Infirmary, Edinburgh, Scotland, United Kingdom; 9 Cardiff and Vale University Health Board, Cardiff, Wales, United Kingdom, 10 Univeristy of Hertfordshire, Hatfield, England, United Kingdom
Background: Severe ADAMTS13 deficiency <10 IU/dL is considered diagnostic for TTP. 2020 ISTH guidelines recommend that where the clinical suspicion for TTP is high, treatment should be initiated urgently whilst ADAMTS13 testing is undertaken. Access to ADAMTS13 testing is variable and quantification of ADAMTS13 activity can vary based on the method used. Traditional assays take a few hours to perform. A rapid ADAMTS13 assay is now available, validated in patients with confirmed TTP. For patients where there is a low probability of TTP, the rapid ADAMTS13 assay in early diagnosis requires further evaluation.
Aims: To evaluate the role of the rapid ADAMTS13 assay in acutely unwell patients where the clinical suspicion of TTP is low.
Methods: This was a real time, multicentre study of ADAMTS13 activity testing using the rapid methodology (Acustar, Werfen, Bedford, USA) and ELISA (Technozym, Technoclone, Austria) in parallel in patients referred to regional centres with suspected TTP. Routine clinical and laboratory parameters were collected for each patient at presentation.
Results: Nine UK regional reference laboratories participated in data collection during 2020 and 2021. Twenty patients referred with suspected TTP but felt to have a low clinical probability of TTP were noted to have unexpectedly low ADAMTS13 activity (<20 IU/dL). Secondary testing using the ELISA revealed normal range ADAMTS13 activity levels.. Von Willebrand Factor (vWF) and D‐Dimer concentrations were found to be significantly raised, beyond published assay limitations.
Conclusion(s): For patients with a low clinical probability of TTP, discrepant results are low prevalence and appear to occur in acutely unwell patients with disease processes including cancer, sepsis and DIC. Raised vWF and D‐Dimer levels suggest the presence of an unmeasured confounding measurand that reduces ADAMTS13 activity in rapid assays. Pending further study of the rapid ADMATS13 assay, a suggested approach for clinical and laboratory teams is provided to guide decision‐making.


PB0297
The use of obinutuzumab and ofatumumab in the treatment of immune thrombotic thrombocytopenic purpura
A. Doyle 1; M. Stubbs2; W. Lester3; W. Thomas4; J. Westwood2; M. Thomas2; C. Percy3; N. Prasannan2; M. Scully2
1 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 2 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 3 University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 4 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom
Background: Immune Thrombotic Thrombocytopenic Purpura (iTTP) is an autoantibody‐driven condition targeting the ADAMTS13 protein. Eradication of B‐cell lymphocytes with rituximab has proven to be efficacious in patients with iTTP at disease presentation and relapse. However, a small number of patients are unable to receive rituximab due adverse drug reactions including rituximab‐induced serum sickness (RISS).
Aims: We aimed to review cases of patients with iTTP who received the alternative humanized anti‐CD20 monoclonal antibodies, ofatumumab and obinutuzumab for treatment response and adverse events.
Methods: Data from the UK TTP Registry was reviewed for clinical details, disease relapse and treatment response of patients with iTTP receving ofatumumab and obinutuzumab.
Results: 15 patients were identified with 26 treatment episodes with either ofatumumab (n = 18) or obinutuzimab (n = 8). 21/26 episodes were pre‐emptive treatment in ADAMTS13 relapses, 4/26 episodes were as part of treatment for clinical relapses and 1/26 episode was during the acute presentation period. All patients had previously received rituximab but was clinically felt inappropriate for further treatments ‐ 12/15 had rituximab‐induce serum sickness, 1/15 had acute drug intolerance and 2/15 had short duration of response to rituximab. Other immunosuppressive agents had been used in 5/15 with poor/non‐sustained responses. All patients achieved a remission following treatment with ofatumumab or obinutuzimab – 24/26 episodes achieved complete remission (ADAMTS13 activity =/>60%) and 2/26 achieved partial remission (ADAMTS13 activity 30–50%). The median time to complete remission was 15 days (interquartile range 11.5–32.5 days). There were 4/26 (15%) episodes of adverse events ‐ two infections and two atopic episodes.
Conclusion(s): Our data shows that ofatumumab or obinutuzimab should be considered as an option in patients with iTTP unable to have rituximab as part of treatment but have previously been responsive.
PB0301
Multi‐centre performance evaluation of ADAMTS13 activity quantification using three different measurement technologies
E. Foxton 1; B. Brannan2; P. Murphy3; S. MacDonald4
1 Viapath Analytics, Royston, England, United Kingdom; 2 Viapath Analytics, London, England, United Kingdom; 3 The Newcastle Upon Tyne Hospitals Nhs Foundation Trust, Newcastle, England, United Kingdom; 4 Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom
Background: ADAMTS13 activity (ADAMTS13:Ac) measurement is an important diagnostic marker of acute thrombotic thrombocytopenic purpura (TTP), defined as levels <10%. As delay to, or incorrect treatment in TTP is detrimental, testing is often urgent. Increasing numbers of commercial assays are available for diagnostic purposes using differing technology. Enzyme linked immunosorbent assay's (ELISA) are frequently used, but are laborious, with turnaround times of 3‐4 hrs. Rapid ADAMTS13:Ac assays with ~30 min turnaround time are now commercially available and warrant evaluation.
Aims: Method comparison of two rapid ADAMTS13:Ac assays against current ELISA methodology at three regional TTP centres (UK).
Methods: Method comparison performed using 110 samples from patients with clinical suspicion of TTP or undergoing TTP treatment. Samples were tested using rapid Technofluor fluorescence resonance energy transfer (FRET) (Technoclone) and HemosIL AcuStar chemiluminescent immunoassay (CIA) (Werfen) assays, according to manufacturer's instructions. Results were compared to the Technozym ELISA assay (Technoclone). An estimation of bias was obtained by Bland Altman analysis.
Results: ADAMTS13:Ac analysis by FRET displayed a positive bias strengthening with rising values in comparison to ELISA. Method comparison by Bland Altman's analysis highlighted a mean difference of 10.2% between FRET and ELISA. The CIA showed a mean difference of −2.3% from ELISA (Figure 1). 17 results were consistent with a diagnosis of TTP (<10%) by all methods. 7 results were < 10% by two methods, and 2 were < 10% by CIA only.
Conclusion(s): Real‐world patient testing demonstrates significant variability between ADAMTS13:Ac methods, impacting TTP diagnosis, follow up and treatment decisions. Although good concordance was seen at levels of <10% across all methods, increased variability was observed for levels of 10–50%. At these levels increased patient monitoring and subsequent treatment may be indicated to avoid frank relapse. ADAMTS13:Ac results should be obtained from consistent methodology, and where cross‐site patient management occurs variation should be estimated.

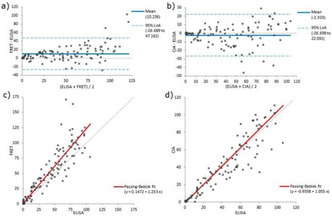
PB0309
Immunoprofiles and open ADAMTS13 in Japanese patients with immune‐mediated thrombotic thrombocytopenic purpura
K. Sakai 1; M. Matsumoto2; L. De Waele3; C. Dekimpe3; C. Tersteeg4; S. De Meyer4; E. Hamada5; M. Kubo2; K. Vanhoorelbeke4
1 KU Leuven Campus Kulak, Kortrijk, Belgium, 8500, West‐Vlaanderen, Belgium; 2 Department of Blood Transfusion Medicine, Nara Medical University, Kashihara, Nara, Japan; 3 Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 4 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 5 Nara Medical University, Kashihara, Nara, Japan
Background: Immune‐mediated thrombotic thrombocytopenic purpura (iTTP) is caused by an autoantibody (autoAb) induced ADAMTS13 deficiency. Immunoprofiles of anti‐ADAMTS13 autoAbs have been well‐studied in mainly the Caucasian population. In the same population, ADAMTS13 with an open conformation was identified as a novel biomarker for iTTP. However, this information is not available for other ethnic cohorts.
Aims: To study the immunoprofile of anti‐ADAMTS13 autoAbs and the presence of open ADAMTS13 in a Japanese cohort of acute iTTP patients.
Methods: Immunoprofiling was performed on citrated plasma samples of 195 Japanese iTTP patients in ELISA using M, DT, CS, T2‐T5, T6‐T8 and CUB domains. The open conformation of ADAMTS13 was studied in ELISA using monoclonal Ab 1C4 which recognizes a cryptic epitope in the S domain of ADAMTS13.
Results: In the Japanese cohort, anti‐CS autoAbs were present in 73.0%, anti‐T2‐5 in 53.5%, anti‐CUB in 51.8%, anti‐T6‐8 in 45.9%, anti‐M in 25.8% and anti‐DT autoAbs in 22.6% of the patients. We identified 28 different immunoprofiles of which only one was dominant, the one characterized by only CS autoAbs (in 28.8% of the patients). When comparing the 3 most prevalent profiles with the other profiles, the former were linked with better clinical outcome. Finally, in 77 of the 195 samples enough ADAMTS13 antigen was present to determine the ADAMTS13 conformation, which was open in 74 of the patients (96%).
Conclusion(s): The immunoprofile with only‐anti‐CS autoAbs was the most common immunoprofile in the Japanese iTTP cohort while 3 dominant profiles were identified in the Caucasian cohort. Whether the differences in the prevalence and types of the other immunoprofiles between cohorts is related to ethnic background remains to be determined. Validation of open ADAMTS13 as a common biomarker in iTTP in the Japanese cohort further supports the use of this biomarker in the diagnosis of iTTP.
VPB0316
Anti‐ADAMTS13 IgG in congenital TTP patients
I. Mancini 1; M. Biganzoli2; A. Cairo3; M. Capecchi4; A. Artoni5; B. Ferrari2; S. Trisolini6; L. Facchini7; G. Giuffrida8; A. De Fanti9; P. Agosti10; F. Peyvandi11
1 Università degli Studi di Milano, Milan, Italy, Milano, Lombardia, Italy; 2 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 3 Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milano, Lombardia, Italy; 4 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Università degli Studi di Milano, Department of Biomedical Sciences for Health, Milan, Italy, Milan, Lombardia, Italy; 5 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Milan, Italy, Milano, Lombardia, Italy; 6 Hematology, Department of Translational and Precision Medicine, “Sapienza” University of Rome, Rome, Italy, Roma, Lazio, Italy; 7 Hematology Unit, Azienda Unità Sanitaria Locale‐IRCCS di Reggio Emilia, Reggio Emilia, Italy, Reggio Emilia, Emilia‐Romagna, Italy; 8 Hematology Division, Department of Clinical and Molecular Biomedicine, University of Catania, Catania, Italy, Catania, Sicilia, Italy; 9 Departmental Simple Unit of Pediatric Rheumatology, AUSL‐IRCSS Reggio Emilia, Reggio Emilia, Italy, Reggio Emilia, Emilia‐Romagna, Italy, 10 Università degli Studi di Milano and Fondazione Luigi Villa, Milan, Italy, Milano, Lombardia, Italy, 11 Fondazione IRCCS Ca′ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Congenital thrombotic thrombocytopenic purpura (cTTP) is an autosomal recessive disease due to mutations in the ADAMTS13 gene. cTTP manifests as acute episodes of thrombotic microangiopathy characterized by ADAMTS13 activity <10 IU/dL and absence of anti‐ADAMTS13 antibodies, which are treated by plasma infusion to replenish the deficient protease. cTTP patients with anti‐ADAMTS13 antibodies were rarely described.
Aims: To investigate anti‐ADAMTS13 IgG in our cTTP cohort.
Methods: We performed a cross‐sectional study of cTTP patients with homozygous or compound‐heterozygous ADAMTS13 mutations, enrolled in the Milan TTP registry from 2002 to 2018. Plasma samples collected during the acute or remission phase were tested for anti‐ADAMTS13 IgG using an in‐house western blot or ELISA‐based assay or the Technozym ADAMTS13 Inhibitor ELISA kit (Technoclone). Neutralizing activity of anti‐ADAMTS13 antibodies was measured using a FRET‐based Bethesda‐like assay.
Results: Ninety‐one samples from 27 cTTP patients (52% females, median age at onset 6.5 years [interquartile range 1.2–20.1]) were tested for anti‐ADAMTS13 IgG (samples per patient, median [range]: 2 [1–13]). Four acute phase samples from four cTTP patients were positive by at least two anti‐ADAMTS13 IgG assays. In one case, non‐neutralizing anti‐ADAMTS13 IgG (83 U/mL) developed during ticlopidine treatment, considered the trigger of the acute event. Two patients presented non‐neutralizing anti‐ADAMTS13 IgG (33 and 19 U/mL) after infusion of four units of fresh frozen plasma over 33 and 59 days, respectively. The last case presented extremely high titers of anti‐ADAMTS13 IgG (8800 U/mL) with neutralizing activity (295 BU/ml) after being misdiagnosed with acquired TTP due to baseline low titer anti‐ADAMTS13 IgGs (17 U/mL) and treated with plasma‐exchange every other day for nine months, rituximab, vincristine, cyclophosphamide and splenectomy.
Conclusion(s): Neutralizing anti‐ADAMTS13 IgG could develop very rarely in cTTP patients after prolonged exposure to plasma, exogenous source of ADAMTS13. Monitoring of anti‐ADAMTS13 antibodies in cTTP patients with a reduced therapeutic response is recommended.
PB0299
Von Willebrand factor (VWF) multimer (MM) pattern in autoimmune thrombotic thrombocytopenic purpura (iTTP) during acute episodes and in remission
T. Falter 1; H. Roßmann2; C. Dekimpe3; L. de Waele4; E. Roose5; C. von Auer6; A. Degreif2; D. Marandiuc7; X. Messmer8; K. Lackner9; S. de Meyer10; K. Jurk11; K. Vanhoorelbeke10; B. Lämmle8
1 University Medical Center of the Johannes Gutenberg University, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 2 Institute of Clinical Chemistry and Laboratory Medicine, University Medical Center of the Johannes Gutenberg University, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 3 Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 4 Laboratory for Thrombosis Research, IRC, KU Leuven Campus Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 5 Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium., Leuven, Vlaams‐Brabant, Belgium; 6 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, Mainz, Germany, Mainz, Guatemala, Germany; 7 Transfusion Center, University Medical Center of Johannes Gutenberg‐University, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 8 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 9 Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, mainz, Rheinland‐Pfalz, Germany, 10 Laboratory for Thrombosis Research, IRC, KU Leuven Campus Kortrijk, Belgium, Leuven, West‐Vlaanderen, Belgium, 11 University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: iTTP is caused by autoantibody‐mediated severe ADAMTS13 deficiency causing insufficient proteolytic processing of VWF‐MM. The (unusually)‐large VWF‐MM lead to microvascular platelet thrombi and organ ischemia. Elimination of autoantibodies leads to normalization of ADAMTS13 and clinical remission. Recurrence of acute iTTP is associated with reappearance of ADAMTS13 deficiency and ULVWF‐MM. Some patients remain in remission despite recurring/persisting severe ADAMTS13 deficiency.
Aims: We repeatedly investigated VWF‐MM and ADAMTS13 in iTTP patients at acute episode‐onset and in remission over 2 years.
Methods: Total cohort of 78 iTTP patients: 10 suffered from 16 acute iTTP episodes, samples before plasmapheresis were available. For 11 instances a sample was available before the acute iTTP. 68 patients had no acute iTTP during follow‐up, 15 with ADAMTS13 < 10%, 53 with ADAMTS13 > 10%. A new VWF‐MM ratio (HMW/LMW) based on modified evaluation of Sebia electrophoresis gels was compared to ADAMTS13 activity.
Results: VWF ratio was significantly higher (p < 0.0025) in remission with <10% as compared to remission with >10% ADAMTS13 activity. 16 samples of acute iTTP showed significantly lower VWF ratio despite <10% ADAMTS13 in 15. Eleven samples obtained 39 days (IQR9‐42) before acute iTTP (ADAMTS13 < 10% in 6, 10–26% in 5) had VWF ratios significantly higher than those from 15 patients in continuing remission with ADAMTS13 < 10% (p < 0.05).
Conclusion(s): The VWF ratio is not dependent exclusively on ADAMTS13 activity. ADAMTS13 regulates the size of VWF‐MM as evidenced in patients with continuing remission with <10% vs. >10% ADAMTS13 activity. The disappearance of HMW‐VWF‐MM in acute iTTP resulting in a low VWF ratio may be explained by consumption of larger VWF‐MM in the iTTP process. The very high VWF ratio preceding acute iTTP recurrence with ADAMTS13 between <10% and 26% suggests that VWF processing is hampered more than in similarly ADAMTS13 deficient patients remaining in remission.

PB0295
A Novel Assay to Investigate Thrombogenesis in TTP Patients
A. Constantinescu‐Bercu 1; R. de Groot1; C. Vendramin1; R. Sivera1; C. Capelli1; K. Vanhoorelbeke2; M. Scully3
1 University College London, London, England, United Kingdom; 2 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 3 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom
Background: Thrombotic thrombocytopenic purpura (TTP) patients with sustained low ADAMTS13 activity could benefit from a new assay to better monitor risk of relapse.
Aims: To develop a novel dynamic assay that better resembles the conditions in TTP, for improved monitoring of TTP patients at risk of relapse.
Methods: Microfluidic channels were coated with collagen or an antibody against VWF‐A3 domain (82D6A3). Blood was collected in citrate or lithium heparin from healthy volunteers, congenital cTTP, or immune iTTP patients with normal platelet counts. Informed consent and ethical approval were obtained. Fluorescently‐labeled blood was perfused at a shear of 1800s‐1. Platelet binding was recorded in real‐time and analyzed using software developed in‐house.
Results: Twenty‐one patient samples (13 cTTP and 8 iTTP) and ten controls were analyzed. Time‐dependent platelet capture was observed in all conditions, forming extensive thrombi on collagen, and small aggregates on anti‐VWF A3 antibody. After 3 minutes of flow, platelet surface coverage on anti‐VWF A3 antibody was significantly increased in TTP samples with low ADAMTS13 activity (Figure 1). On collagen, thrombi adopted different geometries, with elongated thrombi in patient samples with low ADAMTS13 activity as indicated by FRETS analysis, and compact thrombi in controls (Figure 2). These differences were most evident in heparin samples and indicate the presence of high ultra‐large VWF levels. Patients receiving Caplacizumab exhibited no platelet binding on either collagen or anti‐VWF A3 antibody.
Conclusion(s): We present a new method to investigate VWF‐dependent platelet recruitment under flow using an antibody against VWF‐A3 domain to capture plasma VWF. This removes platelet activation by collagen, being more representative for TTP. The assay discriminates between TTP samples and controls, exhibiting significantly higher platelet coverage in patient samples anticoagulated with either citrate or heparin. This assay could represent a new rapid and sensitive diagnostic tool to monitor TTP patients, and a new research tool to study thrombogenesis.


PB0303
Alternate‐day dosing of caplacizumab for immune‐mediated thrombotic thrombocytopenic purpura
L. Kühne1; J. Kaufeld2; L. Völker1; R. Wendt3; U. Schönermarck4; D. Eichenauer5; V. Buxhofer‐Ausch6; J. Menne2; P. Brinkkoetter1; P. Knöbl 7
1 Department II of Internal Medicine and Center for Molecular Medicine Cologne (CMMC), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany, Köln, Nordrhein‐Westfalen, Germany; 2 Department of Nephrology and Hypertension, Medical School Hannover, Hannover, Germany, Hannover, Niedersachsen, Germany; 3 Department of Nephrology and Kuratorium for Dialysis and Transplantation Renal Unit, Hospital St. Georg, Leipzig, Germany, Leipzig, Sachsen, Germany; 4 Klinikum der Universität München – Medizinische Klinik und Poliklinik IV, Nephrologisches Zentrum, Munich, Germany, München, Bayern, Germany; 5 Department I of Internal Medicine, Center for Integrated Oncology, Aachen Bonn Cologne Dusseldorf, University of Cologne, Cologne, Germany, Köln, Nordrhein‐Westfalen, Germany; 6 Department of Internal Medicine I with Hematology, Stem Cell Transplantation, Hemostaseology and Medical Oncology, Ordensklinikum Linz Elisabethinnen, Linz, Austria, Linz, Oberosterreich, Austria; 7 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: The anti‐von Willebrand factor (VWF) nanobody caplacizumab has found its way into current standard of care for immune‐mediated thrombotic thrombocytopenic purpura (iTTP), in addition to plasma exchange (PEX), steroids and rituximab. However, real‐world use of caplacizumab remains heterogenous, including alternate‐day dosing of caplacizumab.
Aims: The aim of this study is to better characterize the use of an alternate‐day dosing regimen for caplacizumab in iTTP patient management. In detail, we aim to identify patients at risk for exacerbations or relapses during alternate‐day caplacizumab treatment, to determine the optimal timing for this treatment modification and to analyze the socioeconomic benefits of this regimen.
Methods: This study is conducted as a retrospective observational study. iTTP patients treated with an alternate‐day regimen of caplacizumab were identified from a large cohort of iTTP patients treated at different medical centers in Austria and Germany since 2018.
Results: All patients were initially treated according to labeling with daily caplacizumab injections and additionally received rituximab and steroids (n = 25). Median time of daily, post‐PEX caplacizumab treatment until introduction of alternate‐day dosing in this cohort was 11 days. Thereafter, alternate‐day dosing of caplacizumab led to persisting normal platelet counts in the majority of patients in this cohort. Exacerbations and relapses occurred only in five patients, and all instances could be managed solely by intensification of caplacizumab treatment to daily dosing. Based on the current market price (September 2021) for a single caplacizumab dose of 4396 € (7724 USD), this treatment modification led to cost savings of 1,622,124 € (2,850,156 USD) in this cohort.
Conclusion(s): Extension of caplacizumab application intervals from daily to alternate‐day dosing may be safely considered in selected patients after 3 to 4 weeks of daily treatment. Earlier modifications may be discussed in low‐risk patients but require close monitoring for clinical and laboratory features of thrombotic microangiopathy.
Antiplatelet Therapy
PB0320
Estimation of prevalence and factors affecting Aspirin / Clopidogrel resistance in Indian patients evaluated by light transmission aggregometry and VerifyNow
R. Dave; T. Geevar; S. Aaron; J. Mammen; G. Chellaiya; R. Vijayan; A. Samuel; S. Nair.
Christian Medical College and Hospital, Vellore, Vellore, Tamil Nadu, India
Background: Antiplatelet resistance may be a major cause of recurrent arterial thrombosis in patients on Aspirin or Clopidogrel secondary prophylaxis. Hence identification of antiplatelet responsiveness by measuring platelet function is important.
Aims: To determine the prevalence of Aspirin and Clopidogrel resistance in Indian patients with previous arterial thrombosis, compare the performance characteristics of VerifyNow to the Gold Standard Light Transmission Aggregometry(LTA) to identify antiplatelet resistance and determine the association between antiplatelet resistance and clinical characteristics.
Methods: Platelet function testing was performed by LTA and VerifyNow in patients with arterial thrombosis on Aspirin (75 mg OD, n = 54) and/or Clopidogrel (75 mg OD, n = 27) secondary prophylaxis. Aspirin resistance on LTA was defined as >20% platelet aggregation response to 1 mM Arachidonic Acid. Clopidogrel resistance on LTA was defined as >30% platelet aggregation response to 2 μM Adenosine Diphosphate. VerifyNow results for Aspirin and Clopidogrel response were expressed as Aspirin Reaction Units (ARU) and P2Y12 Reaction Units (PRU) respectively. The cut‐off for aspirin and clopidogrel resistance was >550 ARU and > 208 PRU respectively. Fisher's Exact Test was used to estimate association between antiplatelet resistance and various clinical characteristics.
Results: The prevalence of Aspirin resistance was 20.3% using LTA and 19.6% using VerifyNow. The prevalence of Clopidogrel Resistance was 25.9% using LTA and 29.6% using VerifyNow. Considering LTA as the gold standard, VerifyNow had 79.5% sensitivity, 18.2% specificity for aspirin resistance (using >550 ARU cut‐off) and 80% sensitivity, 57.1% specificity for clopidogrel resistance (using >208 PRU cut‐off). There was no significant association between Aspirin/Clopidogrel resistance and history of ischemic stroke, coronary artery disease, recurrent thrombosis, smoking, alcohol, hypertension, diabetes, dyslipidemia or homocysteinemia (Table 1).
Conclusion(s): Although VerifyNow had 80% sensitivity, the specificity for identifying aspirin and clopidogrel resistance using the recommended cut‐offs >550 ARU and > 208 PRU respectively was low. Further studies may be required to identify relevant cut‐offs suitable for each population.


PB0785
Comparison of antiplatelet effects of small phenolic compounds
M. Hrubša 1; S. Parvin2; P. Mladěnka3
1 Faculty of Pharmacy in Hradec Králové, Hradec Králové, Kralovehradecky kraj, Czech Republic; 2 Department of Pharmacognosy, Faculty of Pharmacy in Hradec Králové, Hradec Králové, Kralovehradecky kraj, Czech Republic; 3 Department of Pharmacology and Toxicology, Faculty of Pharmacy in Hradec Králové, Hradec Králové, Kralovehradecky kraj, Czech Republic
Background: Metabolites of polyphenolic compounds formed by human gut microbiome have recently been found to reach significantly higher plasma levels than their parent compounds in both humans and animals. As they also possess anti‐platelet properties, they may therefore contribute to beneficial cardiovascular effects of polyphenol rich diet.
Aims: To assess the effect of a series of phenolic compounds related to a potent anti‐platelet compound, 4‐ methylcatechol (4‐MC), on human platelet aggregation and elucidate their mechanism of action.
Methods: Anti‐platelet activity was assessed using impedance aggregometry and various aggregation inducers and inhibitors. Mechanism of action of these compounds was further explored using ELISA ‐ based spectroscopic methods. Both whole human blood and human platelet rich plasma (PRP) was used for these experiments. Informed consent was obtained from all subjects involved in the study. The protocol was approved by the Ethics Committee of the Faculty of Pharmacy, Charles University on May 31, 2019.
Results: Several tested compounds related to a polyphenol metabolite, 4‐MC were shown to be more efficient than the clinical standard, acetylsalicylic acid. Insights into the mechanism of action of these compounds were also obtained.
Conclusion(s): Phenolic compounds, their metabolites and derivatives can be considered a viable strategy to develop potential anti‐platelet drugs. Further experiments elucidating their mechanism of action, safety and clinical efficacy are however required. This work was funded by the Charles University grant GA UK 1322120‐C3. Authors declare no conflict of interest.
VPB0790
Meta‐analysis of the frequency of platelet hyperreactivity after antiplatelet treatment in patients with diabetes and hypertension, undergoing percutaneous coronary intervention, 2005–2021
X. Gutierrez Cadavid 1; D. Rúa Molina2
1 Laboratorio Medico de Referencia, Medellin, Antioquia, Colombia; 2 Universidad de antioquia, Medellin, Antioquia, Colombia
Background: Dual therapy with aspirin and clopidogrel has shown efficacy in the prevention of ischemic events in patients undergoing percutaneous coronary intervention (PCI). Several studies have reported heterogeneity in the response to antiplatelet agents, causing a state of post‐treatment platelet hyperreactivity (HRP). Additionally, it has been documented that patients with hypertension and diabetes mellitus undergoing PCI have a prothrombotic state and a decrease in the antiplatelet response causing HRP, associated with increased risk of vascular and thrombotic events.
Aims: To meta‐analyze the frequency of platelet hyperreactivity after antiplatelet treatment in patients with diabetes and hypertension, undergoing PCI.
Methods: Systematic review with meta‐analysis using 40 searches in three multidisciplinary databases (PubMed, Science Direct and Scielo), following the phases of the PRISMA guide. Reproducibility and evaluation of methodological quality by two researchers were guaranteed using the STROBE guide. Analysis was based on frequencies and odds ratio meta‐analyses, using Epidat software with a 95% confidence interval.
Results: 22 studies mainly conducted in Italy (n = 8) and South Korea (n = 5) were included, the frequency of HRP was for aspirin from 18.8% to 81.8%, and clopidogrel from 18.2% to 71.3%, which denotes a significant heterogeneity, it was observed that the frequency of PRH in patients with diabetes varied from 21% to 78.7% and in hypertensive patients from 17.6% to 76.8%, and Finally, it was observed that the HRP was 1.38 times higher in patients with diabetes than in those who were not diabetic and 1.23 times higher in hypertensive patients than in those who were not hypertensive.
Conclusion(s): diabetes and hypertension as underlying pathologies in patients undergoing PCI, are associated with the presence of HRP, and HRP (as previous studies have shown), with the development of long‐term vascular events, therefore a subsequent assessment to the procedure with platelet aggregometry tests would be potentially useful in the context of personalized therapy.


PB0787
Antithrombotic and prohemorrhagic actions of different concentrations of apixaban in patients exposed to single and double antiplatelet regimens
J. Martinez‐Sanchez 1; L. Castrillo2; D. Jerez3; S. Torramade‐Moix3; M. Palomo1; G. Mendieta4; U. Zafar5; J. Badimon6; M. Roque4; G. Escolar3; M. Diaz‐Ricart7
1 Josep Carreras Leukemia Research Institute, Hospital Clínic de Barcelona, Universitat de Barcelona, Barcelona, Spain, Barcelona, Catalonia, Spain; 2 Department of Cardiology, Hospital Clínic de Barcelona, IDIBAPS, Universitat de Barcelona, Barcelona, Spain, Barcelona, Catalonia, Spain; 3 Hematopathology, Department of Pathology, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain, Barcelona, Catalonia, Spain; 4 Department of Cardiology, Hospital Clinic de Barcelona, IDIBAPS, Universitat de Barcelona, Barcelona, Spain, Barcelona, Catalonia, Spain; 5 Department of Medicine, AtheroThrombosis Research Unit (ATRU), Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, USA, New York, New York, United States; 6 Department of Medicine, AtheroThrombosis Research Unit (ATRU), Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, USA, New York, New York, United States; 7 Hospital Clinic, IDIBAPS, University of Barcelona, Spain, Barcelona, Catalonia, Spain
Background: Treatment with triple antithrombotic therapies may increase bleeding risks in patients.
Aims: We evaluated antithrombotic and prohemorrhagic actions of the combination of different concentrations of apixaban (APIX) in an exploratory study in patients exposed to current antiplatelet regimens.
Methods: A total of 25 healthy subjects and 53 patients treated with aspirin (ASA, n = 21), ASA and clopidogrel (ASA + CLOPI, n = 11), or ASA and ticagrelor (ASA + TICA, n = 21) participated in the study. Studies were carried out ex vivo spiking blood samples from participants with 0 (APIX0), 40 (APIX40) and 160 ng/mL (APIX160). Specific tests were performed to detect high on‐treatment platelet reactivity (HPR). We assessed the inhibitory effects of APIX on: 1) clot formation, by ROTEM thromboelastometry; 2) thrombin generation primed by platelets; and 3) platelet and fibrin interactions onto a thrombogenic surface, in an experimental microfluidic model with circulating blood.
Results: No evidence of HPR was detected in this study. Incubation with APIX caused a dose‐related prolongation of the clotting time with minimal impact on other ROTEM parameters. Thrombin generation was significantly inhibited by APIX 160 ng/mL and moderately affected by the antiplatelet therapies being more intense in patients under ASA + TICA and decreasing slightly for ASA + CLOPI and ASA treated populations. Confocal analysis of microfluidic studies revealed a progressive antithrombotic activity for APIX and antiplatelet therapies. APIX 160 ng/mL was more potent to suppress platelet and fibrin interactions. APIX 40 ng/mL demonstrated a consistent antithrombotic action reducing platelet and fibrin interactions, but proved more respectful at preserving hemostatic parameters with all antiplatelet regimens.
Conclusion(s): APIX potentiated the antithrombotic effects of current antiplatelet regimens. At 40 ng/mL APIX shows an enhanced antithrombotic action in combination with the different antiplatelet regimens, but seems more conservative for hemostasis than the concentrations of 160 ng/mL that assimilates to the Cmax reached after the recommended dose for thromboprophylaxis.
PB0789
Impact of age on in vitro metabolism of clopidogrel: a potential explanation for high on‐treatment platelet reactivity in elderly?
A. Pontis 1; X. Delavenne2; M. Verdier3; S. Hodin4; A. Andriamaharo5; P. Gueret6; F. Nedelec‐Gac6; P. Gaussem7; C. Bachelot‐LOZA8; I. Gouin1
1 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, Institut de recherche en santé, environnement et travail ‐ IRSET, Inserm UMR_S 1085, Univ Rennes, CHU Rennes, France, Rennes, Bretagne, France; 2 Clinical Pharmacology Department, University Hospital of Saint‐Etienne, INSERM, U1059, Vascular Dysfunction and Hemostasis, F‐42023 Saint‐Etienne, France, Saint Etienne, Rhone‐Alpes, France; 3 Laboratory of Clinical Pharmacology, University Hospital of Rennes, EHESP, Irset (Institut de Recherche en Santé, Environnement et Travail), UMR_S 1085, University of Rennes, France, Rennes, Bretagne, France; 4 INSERM U1059, Jean Monnet University of Saint‐Etienne, France, Saint Etienne, Bretagne, France; 5 University of Rennes, France, Rennes, Bretagne, France; 6 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, France, Rennes, Bretagne, France; 7 Department of Biological Hematology, European Hospital Georges Pompidou, AP‐HP, Innovative Therapies in Hemostasis, Paris University, INSERM U1140, Paris, France, Paris, Ile‐de‐France, France; 8 Innovative Therapies in Hemostasis, Paris University, INSERM U1140, Paris, France, Paris, Ile‐de‐France, France
Background: High on‐treatment platelet reactivity (HTPR) exits in about 30% of patients on clopidogrel and has been associated with an increased risk of cardiovascular events. Interestingly, this HTPR reaches 50% in elderly patients (≥70), but little is known on the mechanism of this resistance. One hypothesis is an age‐related impaired hepatic metabolism of the prodrug clopidogrel, leading to a lower formation of its active metabolite (clopidogrel‐AM).
Aims: To compare levels of clopidogrel‐AM formed in vitro using “old” and “young” human liver microsomes (HLMs) and their consequences on platelet functions.
Methods: An in vitro model was developed using “young” (mean age = 51.2–8.5y) and “old” (mean age = 73.6–2.3y) HLMs. “Young” or “old” HLMs, in an incubation/reaction mixture, were added to a citrated platelet‐rich plasma from healthy donors (1:1) in the presence or not of clopidogrel (50 μM) and incubated at 37°C for 30 (T30) and 45 min (T45). Clopidogrel‐AM was quantified by a validated LC MS/MS method (lower limit of quantification: 0.8 ng/mL). Platelet aggregation was performed by light transmission aggregometry (LTA) at each incubation time.
Results: The generation of clopidogrel‐AM was time‐dependant and concentrations were comparable to those observed in treated patients. At T30, the mean concentration of clopidogrel‐AM was significantly higher with “young” HLMs 8.56 μg/L, 95%CI [5.87–11.24] than with “old” HLMs 7.64 μg/L, 95%CI [5.14–10.14] (p = 0.002) (n = 21). Similar differences were found at T45: 11.40 μg/L, 95%CI [7.57–15.22] and 10.63 μg/L, 95%CI [7.10–14.15] with “young” and “old” HLMs (p = 0.016), respectively (figure 1). Despite a significant inhibition of platelet aggregation, no significant difference was found in LTA (ADP 10 μM) after clopidogrel metabolism by “old” or “young” HLMs, probably due to a low sensitivity of the method to detect significant but small variations of clopidogrel‐AM.
Conclusion(s): In this original model combining metabolic and functional approaches, less clopidogrel‐AM was systematically produced with older HLMs. This provides support for a decreased in CYP450 activity that may contribute to HTPR in elderly patients.

PB0786
Dual anti‐platelet therapy‐associated bleeding is exacerbated by platelet counts <100 x 10^9/L in mice due to impaired initial hemostatic plug formation
R. Lee; A. Ballard; T. Rudran; T. Kawano; N. Mackman; G. Stouffer; W. Bergmeier.
University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States
Background: Dual antiplatelet therapy (DAPT; aspirin + P2Y12 inhibitor) is a safe treatment for secondary prevention of arterial thrombotic events. However, due to risk of major hemorrhage, patients with thrombocytopenia (TP) are typically excluded from major clinical trials and the appropriate use of DAPT in these patients remains unclear.
Aims: To investigate the impact of thrombocytopenia on bleeding in the setting of DAPT.
Methods: To study how TP alters bleeding risk associated with DAPT, we assessed hemostatic plug formation by intravital microscopy following laser injury to the murine saphenous vein. Mice were treated, or not, with DAPT (aspirin 20 mg/kg; clopidogrel 25 mg/kg) and platelet counts were adjusted to >200, 100–200, or < 100 x 10^9/L. Time to initial hemostasis, re‐bleeding events, and platelet accumulation were monitored. 3D reconstructions of platelet plugs were rendered from spinning disk confocal Z‐stacks.
Results: WT mice with normal platelet counts rapidly achieved hemostasis with robust platelet accumulation and no rebleeding. Reducing the platelet count did not significantly affect hemostasis time or platelet accumulation. In contrast, DAPT treatment did not affect initial hemostasis times but led to significantly more re‐bleeding, and platelet accumulation was reduced. Interestingly, reducing the platelet count in DAPT‐treated mice down to 100–200 x 10^9/L did not further impair hemostasis, although the total bleeding time trended towards being longer. Significant changes were only observed in DAPT‐treated mice with platelet counts below 100 x 10^9/L. Intravital imaging revealed instability of the center of the platelet plug, and initial hemostasis was never achieved at many of the injury sites.
Conclusion(s): Conclusions: DAPT‐associated bleeding was exacerbated by low platelet counts, mainly due to loss of platelet mass at the site of injury. These results suggest that DAPT treatment could further increase bleeding risk in patients with platelet counts below 100 x 10^9/L. This study was supported by ASH and NBF.
PB0784
Arginine‐containing peptides exhibit antiplatelet effects in experimental type‐2 diabetes mellitus and metabolic syndrome
M. Grigorjeva; T. Obergan; T. Shubina; L. Lyapina.
Lomonosov Moscow State University, Moscow, Moskva, Russia
Background: Increased platelet aggregation is observed in hyperglycemia, metabolic syndrome, atherosclerosis, other cardiovascular diseases and metabolic disorders. This can complicate the course of the disease and lead to hypercoagulation and thrombotic complications in the organism. The use of antiplatelet drugs can reduce pathologically elevated platelet aggregation. We have previously shown that proline‐ and glycine‐containing peptides exhibit antirhrombotic effect in the organism.
Aims: Study of the effects of peptides Pro‐Arg‐Gly (PRG) and Arg‐Pro‐Ply‐Pro (RPGP) on ADP‐induced platelet aggregation (PA) in experimental type‐2 diabetes mellitus (T2DM) and metabolic syndrome (MS) in rats.
Methods: The experiments were carried out on male Wistar rats in accordance with the Helsinki Declaration. T2DM was induced by prolonged administration of 40% glucose solution (intragastrically, 2 g/kg, 15 days). MS was caused by a high‐calorie diet and 10% glucose ad libitum (6 weeks). After that peptides were intranasally applied to rats for 5–7 days. Control rats received saline. Blood for the ADP‐induced PA study was taken 20 h after the last peptides application.
Results: The development of T2DM and MS in rats led to disturbances in vascular‐platelet hemostasis, which was accompanied by an increase in aggregation by 1.5–2 times vs. normal rats. Animals treatments by all used peptides lead to PA reduce in all experiments. However, with the development of T2DM, PA decreased by 43–57% vs. control after the use of both peptides, whereas in MS conditions ‐ by 27% (PRG) and 52% (RPGP). The maximum effect was possessed by RPGP, the antiplatelet effect of which was manifested in both pathologies.
Conclusion(s): Thus, the studied peptides in the development of T2DM and MS in rats exhibit antiplatelet effect. We believe that proline‐ and glycine‐containing peptides with the addition of arginine to their molecule can be used as antiplatelet agents in conditions of increased functional platelets activity and tendency to thrombosis.
VPB0791
Circulating miRNAs release predicts suboptimal response to aspirin in patients at high cardiovascular risk with and without type 2 diabetes mellitus
R. Liani 1; P. Simeone2; S. Ciotti3; R. Tripaldi1; A. Boccatonda4; D. D'Ardes1; A. Recchiuti5; M. Romano5; f. Cipollone1; F. Santilli6
1 Department of Medicine and Aging, Center for Advanced Studies and Technology (CAST), Chieti, Italy;, Chieti, Abruzzi, Italy; 2 Department of Medicine and Aging, and Center for Advanced Studies and Technology (CAST), Via Luigi Polacchi, Chieti, Italy, Pescara, Abruzzi, Italy; 3 Center for Advanced Studies and Technology (CAST) ‐ Chieti University, Chieti, Italy, Chieti, Abruzzi, Italy; 4 Internal Medicine – Chieti University, Chieti, Italy, Chieti, Abruzzi, Italy; 5 Department of Medical and Oral Sciences and Biotechnologies, and Center for Advanced Studies and Technology (CAST), Chieti, Italy, Chieti, Abruzzi, Italy; 6 Department of Medicine and Aging, Center for Advanced Studies and Technology (CAST), Chieti, Italy;, Pescara, Abruzzi, Italy
Background: The recovery rate of platelet COX‐1 activity during the 12 to 24 h dosing interval of aspirin administration, in aspirin‐treated subjects, is characterized by substantial interindividual variability. Circulating myeloid‐related protein (MRP)‐8/14 is an inflammatory protein associated with residual thromboxane (TX)‐dependent platelet activation in aspirin‐treated patients with acute coronary syndrome.
Aims: To identify any circulating miRNAs associated with a suboptimal ASA response in patients at high cardiovascular (CV) risk.
Methods: Two‐hundred high CV risk patients (100 with type 2 diabetes mellitus (T2DM)) in chronic treatment with ASA (100 mg/day), for cardiovascular prevention, were enrolled. Blood sampling was performed at 10 (T10) and 24 hours (T24) after a witnessed ASA administration. Patients were stratified in tertiles according to serum TXB2 slope. First vs. third tertile were compared. Circulating miRNAs custom array cards were applied to assay the expression levels of 14 miRNAs in plasma. We also measured plasma myeloid‐related protein (MRP)‐8/14 as an inflammatory index and predictor of cardiovascular events.
Results: miRNA‐21‐5p (p = 0.017), 22–5p (p = 0.026), 24‐3p (p = 0.020), 150‐5p (p = 0.026), 155‐5p (p = 0.007), 181b‐5p (p = 0.011), 223‐5p (p = 0.021) were significantly lower in first vs. third tertiles at 24 hours after ASA administration in all patients (Figure 1). MRP‐8/14 were higher in third vs. first sTXB2 slope tertile in all patients. MRP‐8/14 was directly correlated with miRNA‐21‐5p (rho = 0.279, p = 0.008), 22–5p (rho = 0.264, p = 0.012), 24‐3p (rho = 0.239, p = 0.023), 150‐5p (rho = 0.236, p = 0.025), 155‐5p (rho = 0.270, p = 0.011), 181b‐5p (rho = 0.240, p = 0.023) and 223‐5p (rho = 0.244, p = 0.030) in all patients.
Conclusion(s): MRP 8/14 may contribute to circulating miRNA release and response variability to ASA. Vice versa, shorter duration of aspirin effect at 24 hours in third sTXB2 slope tertile patients translates into higher degree of TX‐dependent platelet activation, possibly promoting the release of both circulating MRP8/14 and miRNAs. Reduced levels of circulating miRNAs may be a potential biomarker for predicting response to ASA treatment in high‐risk cardiovascular patients.

PB0788
Chloroacilhidroquinone AR‐5820 Reduces the Platelet Activity by Inhibition of Mitochondrial Bioenergetics
E. Fuentes1; D. Mendez 2; F. Urra3; S. Fuentes‐Retamal3; C. Palominos3; J. Millas‐Vargas4; H. Montecino; I. Palomo2; M. Alarcón2; A. Trostchansky5; R. Araya‐Maturana2
1 Thrombosis Research Center, University of Talca, Talca, Maule, Chile; 2 University of Talca, Chile, Talca, Maule, Chile; 3 University of Chile, Santiago, Chile, Santiago, Region Metropolitana, Chile; 4 University of Talca, Chile, Santiago, Region Metropolitana, Chile; 5 University of the Republic, Montevideo, Uruguay, Montevideo, Montevideo, Uruguay
Background: Cardiovascular diseases are the leading cause of death in the world. Platelets play a major role in cardiovascular events, as they bind to the damaged endothelium, becoming activated and forming thrombi. We evaluated the effect of a hydroquinone derivative (AR‐5820) on human platelet function.
Aims: ‐Evaluate the effect of Chloroacylhydroquinone AR‐5820 on platelet function ‐Identify the antiplatelet mechanism of AR‐5820.
Methods: AR‐5820 (Figure 1) lacked cytotoxicity on platelet viability and inhibited the platelet aggregation with IC50 values of 3.99 ± 1.43 μM (TRAP‐6) and 7.16 ± 0.9 μM (PMA). To evaluate the mechanism of action of AR‐5820, we analyzed changes in several markers of platelet activation, ROS, mitochondrial membrane potential, and intracellular calcium levels by flow cytometry; platelet spreading by fluorescence microscopy; liquid chromatography‐mass spectrometry for metabolites of arachidonic acid; intracellular and secreted ATP levels by luminescence; NADH in real‐time by fluorimetry. Moreover, the effect of this compound on CAMKK‐AMPK‐ACC signaling, clot retraction in vitro and closure time by PFA‐200 System was evaluated.
Results: Concentrations close to the IC50 obtained in platelet aggregation were used. Our results indicate that compound AR‐5820 decreases the expression of P‐selectin, CD63, and PAC‐1, decreases the TxB2 production and platelet spreading. It is an inhibitor of electron transport chain, decreasing the mitochondrial NADH oxidation, oxygen consumption rate, and producing a drop of ATP and mitochondrial membrane potential. Western blot assays show that the antiplatelet activity induced by compound AR‐5820 is mediated by CAMKK‐AMPK‐ACC signaling, in response to altered mitochondrial function, increased intracellular calcium and decreased ATP levels. In addition, it does not significantly alter clot retraction (stimulated by Thrombin) and does not prolong the closure time generated by collagen/Epinephrine and Collagen/ADP.
Conclusion(s): Collectively, our data indicate that compound AR‐5820 decreases platelet activation by inhibiting mitochondrial bioenergetics, which activates CAMKK‐AMPK‐ACC signaling; and has a low risk of bleeding (in vitro).

HIT
PB0323
Hemorrhagic clinic in an immune‐mediated and prothrombotic entity: heparin‐induced thrombocytopenia (HIT). Case report
I. Hernández De Castro1; A. Bernardo2; J. Iglesias2; P. Lopez2; A. Porro 2; I. Soto2
1 hematology, oviedo, Asturias, Spain; 2 HUCA, oviedo, Asturias, Spain
Background: Heparin‐induced thrombocytopenia (HIT) is a rare immune‐mediated adverse reaction. Bleeding is rare and is typically associated with thromboembolic complications.
Aims: The purpose is to analyze a case of a 65‐year‐old female patient, who experiences both possible complications secondary to a picture of HIT: pulmonary thromboembolism and extensive intracranial bleeding.
Methods: On the subject of a case at the Central University Hospital of Asturias in 2021. HIT test is performed by automated chemiluminescience that detects the existence of IgG‐type antibodies.
Results: Female patient with no medical history. After a hip surgical intervention, it was started low molecular weight heparines (LMWH) at prophylactic doses. Two weeks later, she started with dyspnea and pleuritic pain. Analytically it was observed elevation of D‐dimer. An angio‐CT scan was performed that described an acute pulmonary thromboembolism in the middle lobe and right pulmonary artery. In this moment it was increased LMWH at therapeutic doses. Subsequently in successive analytical controls, there were a progressive thrombopenia up to 56,000/ mm3 (Graph 1), without anemia and with normal smears. With this count of platelets she experienced a sudden neurological picture with disorientation, aphasia, and hemiparesis and with a radiological finding of an extensive left hemispheric hematoma, not candidate for surgical intervention. The transfusion yield was null. Therefore, due to the high suspicion, a HIT test was requested, which was positive and a high titer (IgG of 5.83 U/mL), stopping the transfusion support. The evolution was poor and in a few hours the patient died.
Conclusion(s): In immune‐mediated pathologies, the possibility of overlapping and/or transformation of some complications into others must be taken into account. It is essential to suspect this entity in patients with thrombopenia and with a history of onset of LMWH 5–10 days before or immediately if they received it in the previous 100 days.

PB0324
Alternative anticoagulant treatment in patients with Heparin Induced Thrombocytopenia (HIT). Experience in a high complexity center according to anticoagulants local availability
V. Privitera 1; M. Garbarini2; F. Chuliber3; M. Villagra Iturre4; D. Mezzarobba4; D. Penchasky4; E. Viñuales4; J. Arbelbide4; M. Martinuzzo5
1 Hospital Italiano de Buenos Aires, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Hospital Italiano de Buenos Aires, Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital ItaIiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Hospital Italiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Central Laboratory, Hospital Italiano de Buenos Aires, Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: HIT is a life‐threatening condition secondary to heparin exposure. Prompt recognition, diagnosis, and alternate anticoagulation are essential. Parenteral non‐heparin anticoagulants are unavailable in our country since mid‐2017.
Aims: To describe characteristics and treatment of patients with HIT suspicion and positive anti‐FP4/heparin antibodies (aFP4/H) and compare patients who received or not appropriate treatment according to international guidelines.
Methods: Descriptive retrospective cohort study including all adult patients with positive aFP4/H (HemosIL HIT‐Ab, Instrumentation Laboratory), in a high complexity general hospital between 2017–2022.
Results: 43 patients, 52% women, mean age 69 y.o. 4Ts Score: intermediate‐ or high‐probability in 95% of patients. Median (IQR) platelets 51,900 (40150–64,500)/mm3. Surgical admissions in 38%, the most frequent cardiovascular surgery. UFH was given to 35 patients. Thrombotic events: 21 patients, being more frequent lower limb DVT (52%). Patients with thrombotic events had higher 4Ts score (p 0.0001) and a tendency to higher median antibodies title (5.7 vs 3.2 p 0.19) Two patients continued on heparin treatment, in one HIT diagnosis dismissed and another on ECMO support and intravenous immunoglobulin treatment. In 67% alternative anticoagulants were started: 7 Bivalirudina, 6 Rivaroxaban, 4 Apixaban and 10 dicumarinics. Two patients receiving Rivaroxaban on hemodialysis (one on ECMO) needed supra‐therapeutic doses to achieve optimal drug plasma levels monitored by Rivaroxaban calibrated anti‐Xa assay. Only 34% of patients received proper treatment according to ASH 2018 guidelines. Inferior vena cava filter was used in 5 patients, 4 patients received intravenous immunoglobulin. Non‐significant differences were found in bleeding and days with thrombocytopenia (<150,000/mm3) between patients with or without appropriate treatment. Eighteen patients died during follow‐up, 72% did not receive appropriate treatment without significant difference.
Conclusion(s): In our experience, the treatment of patients with HIT was difficult due to unavailability of non‐heparin parenteral anticoagulants, a situation that may worsen the mortality rate. Guidelines based on local availability are needed.
VPB0328
Contactless dielectric spectroscopy for detection of platelet‐activating antibodies
N. Khan 1; D. Martin2; U. Pliquett2; Y. ZAIKOU2; T. Nacke3; H. Nguyen4
1 Institute for Bioprocessing and Analytical Measurement Techniques (iba) (IBA), Heilbad Heiligenstadt, Thuringen, Germany; 2 IBA, Heilbad Heiligenstadt, Thuringen, Germany; 3 IBA, heilbad Heiligenstadt, Thuringen, Germany, 4 iba, Rosenhof 1, 37,308 Heilbad Heiligenstadt, Germany, Heilbad Heiligenstadt, Thuringen, Germany
Background: Heparin‐induced thrombocytopenia (HIT) is a severe autoimmune disorder, that occurs in approximately 3% of patients receiving heparin. HIT causes significant morbidity and mortality. Current diagnostics to detect HIT have their disadvantages such as ELISA having only ~50% specificity while functional tests offer higher accuracy but those methods require fresh platelets and trained personnel. Improvement of detection method for HIT has a high impact on the decision of the doctor on how to further treat the patients.
Aims: Development of a high‐frequency microwave resonator to detect HIT antibodies (Abs)‐induced platelet aggregation.
Methods: We developed a high‐frequency microwave resonator to detect HIT Abs using a contactless and cost‐effective mannersensor. The microwave sensor was mounted under an applied electrical field and connected with an inlet and outlet. Adding samples to the sensor caused the damping of particles leading to changes in the resonant frequency. As a model, we used murine non‐HIT Abs (RTO) and HIT Abs (KKO) to track the changes induced by platelet aggregation caused by KKO. Platelet aggregates were formed in a 96‐well plate by stirring the mixture of platelet and antibodies for 45 minutes and then the sample was pumped in the tube, which serves as Material Under Test (MUT) and the reflection coefficient (S11), as well as frequency shift, were measured.
Results: The shift of resonant frequency was observed in the platelet samples containing both RTO and KKO. However, an additional frequency shift was observed in the case of the sample containing KKO. As KKO is known to induce platelet aggregation while the RTO does not, the frequency shift in the sample with KKO indicates a possibility to differentiate between these two Abs.
Conclusion(s): The contactless contactless sensor allowed to distinguish platelet‐activating KKO antibody from the non‐platelet activating RTO antibody based on the shift in resonant frequency.
PB0322
Comparison of 2 functional assays (HIMEA and HIPA) for the diagnosis of vaccine‐induced immune thrombocytopenia and thrombosis (VITT): a franco‐greek collaborative study
D. Faille 1; A. Kotsiafti2; M. Bourrienne3; C. Achard4; A. Perrier‐Cornet5; C. Matsouka2; N. Ajzenberg6; V. Gkalea2
1 Université de Paris ‐ APHP, PARIS CEDEX 18, Ile‐de‐France, France; 2 Alexandra General Hospital, ATHENS, Attiki, Greece; 3 Hôpital Beaujon (APHP); INSERM LVTS U1148 Université de Paris, CLICHY, Ile‐de‐France, France; 4 Université de Paris ‐ APHP, PARIS, Ile‐de‐France, France; 5 APHP, PARIS, Ile‐de‐France, France; 6 APHP, Paris, Ile‐de‐France, France
Background: Vaccine‐induced immune thrombosis and thrombocytopenia (VITT) has been recognized as a rare thrombotic complication of adenovirus‐based vaccines against COVID‐19. While VITT presents some common features with heparin‐induced thrombocytopenia (HIT), VITT antibodies differ from typical HIT antibodies by their ability to induce spontaneous platelet aggregation in the absence of heparin. In this context, reliable functional tests are of crucial importance to confirm VITT diagnosis.
Aims: To compare the performances of Heparin‐Induced Multiple Aggregometry (HIMEA) and Heparin‐Induced Platelet Activation assay (HIPA) for VITT diagnosis.
Methods: From April to October 2021, 7 patients meeting the 5 following criteriae of definite VITT were included: onset of symptoms 5–42 days after vaccination, thrombosis, thrombocytopenia, D‐dimers >4000 ng/mL and positive anti‐PF4 IgG (Zymutest HIA IgG and/or Lifecodes PF4 IgG). HIMEA was performed on whole blood from healthy donors in the hematology laboratory of Alexandra General Hospital. HIPA was performed on washed platelets from healthy donors in the hematology laboratory of Bichat Hospital.
Results: 4 patients showed a characteristic pattern that was concordant between the two methods. HIMEA showed a sigmoid curve with elevated area under the curve (mean AUC 189+/‐78 AU) in saline, that was lower (mean 148+/‐51 AU) in the presence of heparin 1 IU/mL and further decreased (mean 34+/‐28 AU) in the presence of heparin 100 IU/mL. HIPA showed positive aggregation in the presence of saline and heparin 0,2 IU/mL but no aggregation with heparin 50 IU/mL. 2 patients who showed lower AUC in HIMEA saline (109 and 60 AU) had weakly positive or negative results with HIPA. In these 2 cases, blood was drawn after intravenous immunogloblulin (IVIG) initiation. 1 patient showed discrepant results with typical positive HIMEA but negative HIPA patterns.
Conclusion(s): VITT antibodies show heterogenous reactivity patterns that can be highlighted only by using different functional assays such as HIMEA and HIPA. IVIG treatment can be reponsible for lower antibody reactivity.
PB0325
Heparin‐induced thrombocytopenia (HIT) in a COVID‐19 patient on extracorporeal membrane oxygenation (ECMO) support: case report experience with rivaroxaban
V. Privitera 1; M. Garbarini2; D. Mezzarobba3; F. Chuliber4; M. Lopez5; D. Penchasky3; M. Villagra Iturre3; E. Viñuales3; J. Arbelbide3; M. Martinuzzo6
1 Hospital Italiano de Buenos Aires, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Hospital Italiano de Buenos Aires, Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital Italiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Hospital ItaIiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Central Laboratory, Hospital Italiano de Buenos Aires, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 6 Central Laboratory, Hospital Italiano de Buenos Aires, Ciudad Autonoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: HIT is an intensely procoagulant disorder and in patients on ECMO it is associated with a high thrombotic morbidity and mortality, thus is crucial to intervene early.
Aims: To present a case of a COVID‐19 patient on ECMO who was diagnosed HIT. To describe evolution on Rivaroxaban treatment due to the unavailability of parenteral non‐heparin anticoagulants in our country.
Methods: not applicable.
Results: A 60‐year‐old female patient was admitted to our hospital for ECMO support. She was diagnosed with SARS‐CoV2, her condition quickly worsened with pneumothorax and refractory hypoxemia hence she was referred to our Institution. ECMO and hemodialysis were required. Unfractionated heparin (UFH) was given to achieve goal anti‐Xa 0.3–0.7 units/mL. Platelet count (PLT) was 278,000 /mm3 and D dimer 1844 ng/mL FEU. Next day UFH was stopped because of haemothorax. Five days after restart anticoagulation an increased transmembrane pressure and a trombi was observed in the system. PLT were 12,000/mm3 and Ddimer 8500 ng/mL FEU. HIT was suspected; 4Ts score = 7 and anti‐FP4 antibodies positive (5.7 UA/mL). UFH was stopped and due to inaccessibility to other intravenous anticoagulant rivaroxaban 15 mg twice daily was started. Rivaroxaban calibrated anti‐Xa assay was twice daily performed to monitor trough and peak levels, 30 mg twice daily was necessary to achieve therapeutic plasma concentration. Patient's PLT recovered after 7 days. No thrombotic event was recorded during rivaroxaban use. After ECMO was weaning‐off rivaroxaban dose was reduced to 10 mg/day. Ten days later she had an upper gastrointestinal bleeding because gastric ulcer and rivaroxaban was stopped. With clinical stability and non evidence of bleeding, thromboprophylaxis with rivaroxaban was restarted and continued until discharged.
Conclusion(s): Our case highlights the difficulties on management of HIT in patients on ECMO support and the need for consented guidelines in this specific situation, particularly for countries without access to parenteral non‐heparin anticoagulants.
PB0321
Fibrin monomers: a new useful tool to monitor argatroban in patients with Heparin‐Induced Thrombocytopenia – A single‐center retrospective study
E. TOUBOUL1; M. BOURRIENNE2; J. GAY3; C. ACHARD3; A. PERRIER‐CORNET4; N. Ajzenberg5; D. Faille 6
1 Université de Paris, PARIS, Ile‐de‐France, France; 2 Hôpital Beaujon (APHP); INSERM LVTS U1148 Université de Paris, CLICHY, Ile‐de‐France, France; 3 Université de Paris ‐ APHP, PARIS, Ile‐de‐France, France; 4 APHP, PARIS, Ile‐de‐France, France; 5 APHP, Paris, Ile‐de‐France, France; 6 Université de Paris ‐ APHP, PARIS CEDEX 18, Ile‐de‐France, France
Background: Due to its short half‐life and hepatic metabolism, argatroban is an alternative anticoagulant of choice in patients with suspected or confirmed heparin‐induced thrombocytopenia (HIT) and renal failure, risk of bleeding or possible surgery. APTT prolongation and anti‐IIa activity being weakly correlated, the monitoring of argatroban remains difficult and the therapeutic range is not clearly defined.
Aims: To determine whether soluble fibrin monomers (FM), indirect markers of in vivo thrombin generation, could represent a new strategy for monitoring argatroban therapy.
Methods: 36 patients treated with argatroban at Bichat Hospital from April 2013 to July 2021 who beneficiated from a measurement of plasma FM (FM Liatest, Diagnostica Stago) on day 0 (D0) and day 3 (D3) of argatroban treatment were included.
Results: 20 patients (56%) had positive FM (>65 μg/mL) on D0. Among them, 13 (65%) had negative FM on D3. Patients in whom FM remained positive on D3 (n = 7; 35%) had a higher body mass index (33+/−6 versus 27+/−5 kg/m2, p = 0.04) and benefited more often from extracorporeal membrane oxygenation (ECMO) (86% versus 23%, p = 0.007) than patients in whom FM became negative. The mean anti‐IIa activity between D0 and D3 seemed lower in patients in whom FM remained positive, but this difference was not significant (0.74+/−0.5 versus 1.2+/−0.9 μg/mL, p = 0.2). The negativation of FM between D0 and D3 was associated with a significantly higher platelet count at D5 (212+/−107 versus 90+/−52G/L, p = 0.01), except for patients receiving ECMO. The negativation of FM under argatroban therefore seems to precede the rise of platelets.
Conclusion(s): FM could be an early biomarker of argatroban efficacy in HIT patients without ECMO. The persistence of positive FM in patients receiving ECMO might be related to their underlying pathology rather than to the inefficacy of argatroban. These results deserve to be validated in a prospective multicenter study on a larger number of patients.
PB0326
Rapid immunoassay for platelet factor‐4 in the diagnosis of vaccine‐induced immune thrombotic thrombocytopenia after vaccination with ChAdOx1 nCoV‐19
G. Uzun 1; K. Althaus2; S. Hammer3; Y. Wanner1; S. Nowak‐Harnau1; S. Enkel1; T. Backchoul4
1 Center for Clinical Transfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 2 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 3 Center for Clinical Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 4 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Vaccine‐induced immune thrombotic thrombocytopenia (VITT) is characterized by the presence of platelet activating antibodies against platelet factor 4 (PF‐4). The prompt diagnosis is of paramount importance in the management of VITT. First step in the laboratory diagnosis of VITT is the detection of anti‐PF4 antibodies. Although recently published expert opinions recommend against the use of rapid immunoassays, their diagnostic performance have not been investigated systematically before.
Aims: To evaluate the performance of rapid immune assay in the dignosis of VITT.
Methods: In this retrospective single‐center study, we evaluated the correlation between rapid immunoassay, enzyme‐linked immunosorbent assay (ELISA) and modified heparin induced platelet aggregation assay (HIPA) in patients with findings suggestive of VITT. A commercially available PF4 rapid immunoassay (ID PaGIA H/PF4, Diamed) and an anti PF4/Heparin ELISA (Zymutest HIA IgG, Hyphen) were used according to manufacturer's instructions. A sample was considered reactive in ELISA if the optical density (OD) was ≥0.500. Modified HIPA was accepted as the gold standard test.
Results: Between March 8th and May 20th, 21 samples from clinically well‐characterized VITT patients were analyzed with PF4 rapid immunoassay. Of these sera 8 revealed positive results and 13 tested negative in rapid immunoassay. In contrast, all sera showed positive results in ELISA and modified HIPA. Sensitivity and specificity of rapid immunoassay were 50% and 69%, respectively. On the other hand, sensitivity and specificity of ELISA were 88% and 92%, respectively.
Conclusion(s): Particle gel immunoassay is not reliable in the detection of anti‐PF4 antibodies in patients suspected of VITT.
VPB0329
Propensity Matched Comparison of Incidence and Outcomes of Heparin‐Induce Thrombocytopenia in Patients Undergoing Percutaneous Cardiac Intervention vs Coronary Artery Bypass Grafting
K. Park 1; P. Aung2
1 Memorial Healthcare System, Miramar, Florida, United States; 2 Memorial Healthcare System, Pembroke Pines, Florida, United States
Background: Heparin‐induced thrombocytopenia (HIT) is a well‐established, devastating complication for patients undergoing cardiac surgery. However, there is no data comparing the risk of HIT between percutaneous cardiac intervention (PCI) and coronary artery bypass graft surgery (CABG).
Aims: We sought to determine the impact of the procedure modality (PCI vs CABG) on the development and outcomes of HIT.
Methods: We conducted a retrospective analysis of the 2016 to 2018 Nationwide Inpatient Sample. Adult patients undergoing PCI or CABG (age ≥ 18) were selected using the ICD‐10 procedure and diagnosis codes. Discharge‐level weight analysis was used to produce a national estimate. Propensity score matching (1:1) with age, female and comorbidity burden was performed and outcomes were compared between matched cohorts.
Results: During the study period, 2,008,294 patients were admitted for PCI (70.8%) or CABG (29.2%). PCI patients tended to be younger (65.2 SE 0.02 vs 66.2 SE 0.03) and female (33.0% vs 25.0%) with lower proportion of chronic kidney disease (10.2% vs 11.2%). The overall incidence of HIT was 0.19%. In a propensity matched cohort, PCI was associated with lower risk of HIT (OR 0.99; 0.99–0.99; p = 0.001). Among those who developed HIT, PCI decreased the risk for deep vein thrombosis (OR 0.91; 0.86–0.96; p = 0.001), pulmonary embolism (OR 0.95; 0.92–0.98; p = 0.003) and acute kidney injury (OR 0.89; 0.82–0.96; p = 0.003). When we limited it to patients who underwent three‐vessel PCI or CABG, PCI still decreased the risk of HIT (OR 0.99; 0.99–0.99; p = 0.001). There was no significant difference in HIT related in‐hospital mortality in both groups (p = 0.8).
Conclusion(s): Incidence of HIT was extremely low in patients undergoing cardiac procedure. CABG was associated with higher risk for development of HIT and HIT/Thrombosis and acute kidney injury when it compared to PCI. Further studies are needed to investigate procedure‐related risk for HIT.


PB0327
Antibody‐induced procoagulant platelets cause increased thrombus formation in heparin‐induced thrombocytopenia
J. Zlamal 1; H. Jaffal2; A. Singh3; L. Pelzl4; K. Weich4; K. Althaus4; T. Backchoul1
1 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany; 2 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, tuebingen, Baden‐Wurttemberg, Germany; 3 Institute for Clinical and Experimental Transfusion Medicine, University Hospital of Tuebingen, Tuebingen, Germany; 4 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany
Background: Thromboembolic events are life‐threatening complication frequently observed in patients with heparin‐induced thrombocytopenia (HIT). The interplay of platelets (PLTs) and different actors of the adaptive and innate immune system are increasingly identified to cause a procoagulant state in HIT. However, the impact of antibody (Ab)‐induced Fc‐gamma‐RIIA (FcγRIIA) mediated procoagulant PLTs and their role regarding thrombus formation in HIT remains elusive.
Aims: In this study, the ability of HIT Abs to induce procoagulant PLTs and their potential to propagate thrombus formation was investigated.
Methods: PLTs from healthy individuals were incubated with IgG fractions from well characterized HIT patients or healthy controls (HCs) in the presence of low or high dose heparin. Changes in the expression of CD62P (P‐selectin) and phosphatidylserine (PS) were assessed using a flow‐cytometric procoagulant PLT‐assay. To investigate whether HIT Ab‐induced procoagulant PLTs affect thrombus formation, an ex‐vivo model of thrombosis was used under flow conditions.
Results: HIT IgG fractions induced significant increase in the formation of CD62P/PS positive procoagulant PLTs compared to buffer and HC IgG (38.87% ± 1.99 vs. 4.43% ± 3.25, p = 0.001; and vs. 1.43% ± 0.83, p = 0.050, respectively, Figure 1A). Of note, HIT IgG incubated PLTs reconstituted into autologous whole blood induced marked increase in thrombus formation on collagen whereas these changes were nearly absent in HCs (Figure 1B). Interestingly, and of potential therapeutic interest, Ab‐induced procoagulant PLT formation as well as increased thrombus formation were significantly reduced in the presence of the prostacyclin derivate Iloprost, an elevator of PLTs' intracellular cAMP (Figure 1A).
Conclusion(s): Findings of our study indicate that HIT Abs harbor the ability to induce procoagulant PLTs via PLT FcγRIIA mediated signaling pathways. The observation that Iloprost showed the potential to reduce the formation of procoagulant PLTs and most importantly inhibits thrombus formation indicates a potential therapeutic use.

HUS
PB0792
Early use of eculizumab in patient with pregnancy‐associated atypical haemolytic‐uraemic syndrome
J. Gumulec; O. Šimetka; A. Piegzová; R. Špaček
University Hospital Ostrava and Faculty of Medicine University of Ostrava, Ostrava, Moravskoslezsky kraj, Czech Republic
Background: Pregnancy‐associated atypical haemolytic uraemic syndrome (p‐aHUS) is a rare thrombotic microangiopathy (TMA) affecting one in 25,000 pregnancies. The most common disorders with features of TMA in pregnancy and postpartum are preeclampsia (PE) and HELLP syndrome. Other possible causes included thrombotic thrombocytopenic purpura (TTP) and p‐aHUS. Differentiating PE, HELLP syndrome, TTP and p‐aHUS is critical because the first‐line treatment for p‐aHUS is complement blockade with eculizumab. Corticosteroids and/or plasmapheresis are often attempted as first‐line agents. These treatments are less effective for p‐aHUS, and their use may increase maternal risk by delaying appropriate care.
Aims: We present a case of the 39‐year old woman who was admitted to the hospital in the 37th week of pregnancy with gestational hypertension and foetal growth restriction. Her pregnancy was terminated by acute caesarean section due to imminent foetal hypoxia and placental abruption. Early after caesarean section, she developed acute respiratory distress syndrome, severe impairment of consciousness, anuric acute kidney injury, thrombocytopenia and microangiopathic haemolytic anemia. Due to severe hemodynamic instability, eculizumab treatment and comprehensive supportive care, including continuous hemodialysis, were initiated immediately. Unfortunately, despite early treatment, she developed an end‐stage renal disease. Additional genetic testing revealed inherited abnormalities in complement proteins ‐ decreased expression of membrane cofactor protein (MCP, including in our case MCPggaac risk haplotype) and combination of variants within risk haplotype CFH‐H3. Application of complement inhibitors continued, and 22 months later she underwent kidney transplantation.
Methods: not applicable.
Results: not applicable.
Conclusion(s): Our case presents the importance of interdisciplinary cooperation in pregnancy‐associated TMA. Early administration of eculizumab probably saved our critically ill patient, and maintenance treatment contributed to the success of kidney transplantation.
VPB0795
Reviewing atypical Hemolytic Uremic Syndrome in a portuguese terciary hospital
M. Afonso; R. Almeida Dias; A. Barbosa; A. Marques
Hospital de Braga, Braga, Braga, Portugal
Background: Atypical Hemolytic‐uremic syndrome (aHUS) is a rare clinical syndrome whose diagnosis is based on the combination of clinical evaluation, biochemical findings and genetic testing. It develops with thrombocytopenia, microangiopathic hemolytic anemia, renal injury and non‐specific symptoms. Nowadays, Eculizumab is an specific treatment for aHUS that should be considered in case of renal failure and after exclusion of other Thrombotic Microangiopathies (TM), namely Thrombotic Thrombocytopenic Purpura and infection by Shiga‐Toxin producing Escherichia Coli.
Aims: This review intends to characterize the management of aHUS at our center aiming the optimization of early recognition, diagnosis and treatment of aHUS.
Methods: This retrospective study based on the evaluation of all the patents coded as aHUS at our hospital between 2017 and 2021.
Results: Five presumed cases were identified – three females and two males, aged between 33–75 (mean of 48 years). In all cases the onset was abrupt, mainly identified by analytical changes and few clinical relavants signs, despite fluctuating neurological symptoms in two patients. One patient had a previous asymptomatic Coronavirus Disease 2019. Platelets ranged from 30.000–135.000/microliter, hemoglobin ranged from 7,7–12,8 grams per deciliter and renal dysfunction occurred with creatinine levels between 2.8–6.9 miligram/deciliter. The therapeutic approach varied according to disease's severity and patient outcome. Three patients were submitted to Therapeutic Plasma Exchange (TPE). The number of TPE varied from 3–12 sessions. Eculizumab was administered in two patients, as the other three had substantial clinical improvement before molecular results that led to Eculizumab request. This drug availability has few obstacles at our center. No patient had died, however two patients are now hemodialysis dependent.
Conclusion(s): Further characterization of future cases is imperative to better understand this condition and it urges the implementation of protocols to optimize general practice in TM assistance and to avoid misdiagnosis of such rare diseases that need a specific approach.
PB0793
Preference for ravulizumab over eculizumab: a real‐world patient preference and life impact study on the treatment of atypical hemolytic uremic syndrome
T. Mauch1; M. Chladek2; S. Cataland3; S. Chaturvedi4; B. Dixon5; K. Garlo6; C. Gasteyger6; A. Java7; J. Leguizamo 8; L. Lloyd‐Price9; T. Pham10; T. Symonds9; I. Tomazos6; Y. Wang6
1 University of Nebraska, Omaha, Nebraska, United States; 2 Clinical Outcomes Solutions, LLC (at the time of the study), Chicago, Illinois, United States; 3 Wexner Medical Center, Ohio State University, Columbus, Ohio, United States; 4 Johns Hopkins University, Baltimore, Maryland, United States; 5 University of Colorado School of Medicine, Aurora, Colorado, United States; 6 Alexion, AstraZeneca Rare Disease, Boston, Massachusetts, United States; 7 Washington University School of Medicine, St Louis, Missouri, United States; 8 Georgia Cancer Specialists/Northside Hospital Cancer Institute, Atlanta, Georgia, United States; 9 Clinical Outcomes Solutions Ltd., Folkestone, England, United Kingdom, 10 Clinical Outcomes Solutions, LLC, Chicago, Illinois, United States
Background: Terminal complement inhibitors eculizumab and ravulizumab are approved treatments for atypical hemolytic uremic syndrome (aHUS). Ravulizumab was engineered from eculizumab to reduce dosing frequency from every 2 weeks to every 4–8 weeks (bodyweight dependent).
Aims: To evaluate treatment preference for eculizumab and ravulizumab, and treatment impact on daily life, in adults with aHUS.
Methods: Eligible US adults (aged ≥18 years) with a confirmed diagnosis of aHUS who had previously received eculizumab and ≥3 doses of ravulizumab were included. Treatment preference and impacts were evaluated using a web‐based survey that included questions on demographics, aHUS disease and management history, and ravulizumab versus eculizumab preference. Variables were compared using paired t‐tests (continuous) or McNemar‐Bowker tests (dichotomous) at a significance level of p < 0.05 (descriptive purposes only).
Results: Fifty patients completed the survey (mean [standard deviation] age, 46.5 [13.9] years; 80% female). Mean (standard deviation) duration of eculizumab and ravulizumab treatment was 46.9 (33.4) and 12.9 (6.0) months, respectively. Overall, 94% of patients preferred ravulizumab (6% either had no preference or preferred eculizumab; p < 0.0001). Regarding factors related to daily life, patients showed significant preference for ravulizumab over eculizumab (Figure 1); when considering infusion frequency, all patients preferred ravulizumab. When asked to select up to five factors that were most important when deciding overall treatment preference, 80% of patients selected infusion frequency. Significantly more patients (p ≤ 0.05) responded that they were able to enjoy life more with ravulizumab than with eculizumab, and that ravulizumab infusion frequency had minimal impact on daily life and work (Figure 2). When considering factors such as treatment effectiveness, safety and financial wellbeing, there were no significant differences between treatment preferences.
Conclusion(s): Survey results suggest that less frequent infusions strongly influences patient preference for ravulizumab over eculizumab, and demonstrates the benefit of ravulizumab treatment on patients' quality of life and completion of daily activities.


PB0794
Classification of Japanese patients with aHUS treated with eculizumab by a diagnostic scoring system
H. Wada 1; S. Maruyama2; N. Kato2; H. Teranishi3; M. Matsumoto4
1 Mie Prefectural General Medical Center, Yokkaichi, Mie, Japan; 2 Nagoya university, Nagoya, Aichi, Japan; 3 Alexion Pharma GK, Minato‐ku, Tokyo, Japan; 4 Department of Blood Transfusion Medicine, Nara Medical University, Kashihara, Nara, Japan
Background: Atypical hemolytic uremic syndrome (aHUS) is generally diagnosed by exclusion from other disorders of thrombotic microangiopathy (TMA) requiring considerable knowledge, and experience. Currently there are no definitive diagnostic makers available in clinical practice. Recently, a ‘TMA scoring system’ was developed along with an associated ‘aHUS score’ as a diagnostic tool (Wada et al. 2020).
Aims: We modified this system according to the variables recorded during a Japanese post‐marketing surveillance (PMS) study and assessed the usefulness of the modified aHUS score using PMS data.
Methods: In PMS, 188 Japanese patients who were clinically diagnosed with aHUS and treated with eculizumab from 2013 to 2018 were registered. The treatment response was evaluated using 3 criteria: Criterion 1, partial hematologic response or renal improvement; Criterion 2, complete hematologic response or renal improvement; and Criterion 3, complete hematologic response and renal improvement. The relationship between the modified aHUS score and treatment response was investigated.
Results: A modified aHUS score value of 5 corresponded to the cutoff value from the previous report; 98% of patients met ≥5 points. In patients who had medical record after eculizumab treatment, 96% of patients fulfilled Criterion 1, 83% of patients fulfilled Criterion 2, and 30% of patients fulfilled Criterion 3. Higher percentages of patients with impaired renal function, younger onset and no underlying diseases were observed in the population who fulfilled Criterion 3. From Receiver Operating Characteristic curve analysis, the estimated cutoff value of modified aHUS score was approximately 10. In contrast, the odds ratio was highest at 5 points and 96% of patients with ≥5 points met the Criterion 1.
Conclusion(s): Almost all patients in PMS were classified as aHUS by modified aHUS score, which can be developed as a tool for predicting the response to eculizumab treatment in patients with aHUS.
Inherited Thrombocytopenias
PB0330
Romiplostim (Nplate) Use for Thrombocytopenia at a Large Academic Medical Center
A. Wong; E. Xiang; F. Cirrone; J. Papadopoulos; T. Ahuja
NYU Langone Health, New York, New York, United States
Background: Romiplostim (Nplate®) is an injectable thrombopoietin (TPO) peptide mimetic used for immune thrombocytopenia (ITP) dosed at 1 mcg/kg, with dose adjustments made in 1 mcg/kg/week increments. It is indicated for ITP for ≥3 months and who are corticosteroid dependent and or unresponsive. In practice, romiplostim is often initiated at higher doses. Furthermore, doses may be administered within a 3–5 day period interval and/or increased at 1–2 mcg/kg increments. Romiplostim is used off‐label in: hematopoietic stem cell transplant (HSCT), chemotherapy induced thrombocytopenia (CIT), and cirrhosis.
Aims: To analyze the utilization of romiplostim in patients with ITP or off‐label.
Methods: This was a retrospective review of romiplostim at NYU Langone Health from January 2019 to July 2021. The primary outcome was percent of patients achieving platelet counts ≥50 by week 1 and maintaining platelet counts ≥50 by end of each week. Secondary outcomes included tolerability.
Results: There were a total of 150 romiplostim administrations in 84 patients. The top three romiplostim indications include: ITP, HSCT, and CIT. The majority of patients receiving romiplostim for ITP adjunctive received corticosteroids and/or IVIG. Baseline platelet values may be confounded by concomitant platelet transfusions. A median dose of 2.4 mcg/kg was sufficient to achieve goal platelets by week 1, however providers at NYULH initiate romiplostim at a median dose of 3.8 mcg/kg (250 mcg) for the purposes of dose rounding. Majority of patients were not re‐dosed within a week, however many had >1 mcg/kg dose increases from week to week. There were two thrombotic adverse events; however, not attributed to romiplostim use.
Conclusion(s): Higher than package label doses of romiplostim do not seem to be associated with an increased incidence of adverse events, and were well tolerated. A flat dosing policy rounding to the nearest vial size should be implemented in order to minimize drug waste, given tolerability.
PB0338
Efficacy comparison and safety analysis of megadose recombinant human thrombopoietin(rhTPO) in the treatment of immune thrombocytopenia (ITP)
X. Wang
Department of Hematology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China (People's Republic)
Background: The conventional rhTPO dose was 300 U/Kg,the clinical response rate was not satisfactory. So we explored the efficacy and safety of increasing dose.
Aims: To compare the efficacy and safety of recombinant human thrombopoietin (rhTPO) 15,000 U and 30,000 U in ITP.
Methods: ITP admitted to hematology Department of the Second Affiliated Hospital of Kunming Medical University from 2016 to 2021 were collected in A retrospective study. The patients were divided into group A (15 000 U) and Group B (30 000 U). The efficacy of the two groups were compared.
Results: The dose of rhTPO in group A and group B was (19,830 ± 3427) U/Kg vs (19,652 ± 2837) U/Kg according to the traditional dose. The total effective rate of group B vs group A (91.80% vs 70.45%, p < 0.01). On Day 7,Day 14,Day 21,and Day 28 of treatment, group B had better results (p < 0.001, p < 0.01, p > 0.05, p > 0.05). In fact, in our study, group B increased the dose of rhTPO and the efficacy was significantly improved. Group B demonstrated shorter onset of action, longer maintenance of efficacy, and better salvage therapy for relapsed patients (Table I). Monotherapy were not different between the two groups, but high‐dose rhTPO combined with hormone therapy were statistically significant (p < 0.01). There were no significant difference in the onset time of single drug therapy and combined glucocorticoid therapy. But, In group A, the onset time of monotherapy was faster than that of combined hormone therapy (p < 0.05), which may be due to the small number of cases (TableII). Platelet counts increased significantly in both groups after treatment. Treatment‐related adverse events were reported in 6 (9.8%) and 6 (13.6%) patients in the group A and group B, respectively, all being mild and transient in nature.
Conclusion(s): Increasing the dose of rhTPO were effective and safe in the treatment of immune thrombocytopenia. Combination with glucocorticoids may be more effective.


PB0331
Bleeding tendency in thrombocytopenic patients due to impaired defects in primary and secondary hemostasis
N. Buntsma 1; A. Gasecka2; S. Talsma3; J. Rytel4; K. Mądry4; G. Basak4; E. van der Pol5; R. Nieuwland6
1 Amsterdam UMC, Amsterdam, Noord‐Holland, Netherlands; 2 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 3 Laboratory of Experimental Clinical Chemistry, Amsterdam UMC, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Hematology, Oncology and Internal Medicine, Medical University of Warsaw, Warsaw, Poland, Warsaw, Mazowieckie, Poland; 5 University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 6 Laboratory of Experimental Clinical Chemistry, Vesicle Observation Center, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: It is unknown why a bleeding tendency occurs at or below a critical platelet concentration of 50 million platelets/mL.
Aims: We hypothesize that below this critical platelet concentration, not only primary hemostasis is inhibited by reduced platelet–platelet interaction, but also secondary hemostasis by an inhibited release of platelet‐derived extracellular vesicles (PEVs).
Methods: Citrate‐anticoagulated blood was collected from immune thrombocytopenia (ITP) patients (n = 10) and healthy individuals (n = 4) to study aggregation by Multiplate and PEV release in blood plasma (not stirred) by calibrated flow cytometry (Apogee A60‐Micro, scatter triggering, EV diameter ≥ 160 nm). In all experiments, platelets were activated by 30 μM thrombin‐receptor activating peptide (TRAP), and saline was used as a control. The Mann–Whitney U test was used to test for significant differences.
Results: Addition of TRAP to undiluted blood of healthy individuals induced aggregation (p = 0.029) and increased the PEV concentration in 2 out of 4 individuals. In contrast, no aggregation and no PEV release were observed in blood from ITP patients (Figure 1).
Conclusion(s): Below a critical platelet concentration, activated platelets neither aggregate nor release PEVs. Thus, the bleeding tendency of ITP patients may be caused by a combined effect of impaired primary and secondary hemostasis.
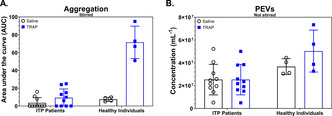
PB0335
The GFI1B c.503G > T; p.(Cys168Phe) variant – a diagnostic conundrum
M. Mitchell 1; R. Wheeler2; J. Cutler2
1 Viapath Analytics LLP, Guy's & St. Thomas' NHSFT, LONDON, England, United Kingdom; 2 Viapath Analytics LLP, LONDON, England, United Kingdom
Background: Congenital thrombocytopenia is a highly heterogeneous disorder in both genetic cause and clinical phenotype. Confirmation of congenital thrombocytopenia is clinically important both for exclusion of ITP or other cause and for family studies. GFI1B is one of many genes associated with thrombocytopenia and codes for a zinc‐finger containing transcription factor, primarily expressed in hematopoietic cells. A number of variants have been reported in GFI1B associated with thrombocytopenia.
Aims: Clarification of the role of GFI1B p.(Cys168Phe).
Methods: Almost 100 patients referred with thrombocytopenia /macrothrombocytopenia were analysed for the R90 Bleeding and Platelet disorder panel. Analysis was performed using a Twist Bioscience custom design panel run on a NovaSeq and the data analysed using Congenica. Putative pathogenic variants were confirmed by Sanger sequencing.Results: Our laboratory has identified the c.503G > T; p.(Cys168Phe) variant in 15 index cases, including two homozygotes, from 12 families. This variant has been reported with a minor allele frequency of 0.4% in the South Asian population. The platelet count in this cohort was between 80–100 x 10^9/L, with large platelets observed in some patients.
Conclusion(s): Following the ACGS/ACMG guidelines this variant scores as a variant of uncertain significance, due to its high frequency in the South Asian population. This would not normally merit reporting as a diagnostic finding. However, our data confirms that this variant is significantly more common in individuals with mild thrombocytopenia than the ‘normal’ population. Given the reported MAF of 0.4%, 3000 individuals of South Asian heritage would require screening to identify 12 affected families. Rabbolini (2017) reported that this variant may be associated with a macrothrombocytopenia phenotype with no alpha‐granule deficiency or bleeding symptoms. It is possible that this variant explains a mild thrombocytopenia but would not be sufficient to explain a significant bleeding phenotype. As such it should be reported in context.
PB0332
The role of thrombopoietin receptor agonists in preventing surgical bleeding in patients with inherited thrombocytopenia
M. Carneiro 1; E. Silva1; A. Ferreira2; M. Oliveira1; M. Coutinho1; E. Cruz3; S. Morais4
1 Thrombosis and Hemostasis Unit, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 2 Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 3 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 4 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto, Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal
Background: Inherited thrombocytopenia (IT) are heterogeneous group of platelet disorders, characterized by diminished platelet count (PLT) and increased bleeding potential. Recent advances in diagnostic methods allowed the identification of an increasing number of IT. In contrast, the therapeutic options of IT patients remained unchanged and based mainly on PLT transfusion. Recently, thrombopoietin receptor agonists (TPO‐RA) were introduced in the treatment of patients with IT.
Aims: To evaluate the efficacy of a TPO‐RA (Eltrombopag) in preventing surgical bleeding in patients with IT.
Methods: Four patients; 3 M/1F, median age 45 years‐old (min‐max 12–60) with different IT (MYH9‐RD, ANKRD26‐RT, ACTN1‐RT and ITGA2B/ITGB3‐RT), achieved five treatments with Eltrombopag (50 mg/day) prior to surgical procedures, between 2017 and 2020
Results: The procedures were tympanoplasty, two hydrocelectomies, a mitral valvuloplasty with tricuspid annuloplasty and a biopsy of pancreatic lesion. The median PLT was 43x103/μL (min‐max 26–83x10^3/μL) at baseline, and 103x10^3/μL (min‐max 56‐211x10^3/μL) after 24 days of treatment in average (min‐max 19–26). PLT increase was greater than 100% in 4 of the treatments and was only marginal (30%) in one treatment. No additional measures to improve hemostasis were needed and all procedures were performed without increase of the expected bleeding. Only one treatment was interrupted early, soon after surgery, due to severe headaches. The patient who underwent cardiac surgery had an acute coronary syndrome considered secondary to the surgery.
Conclusion(s): Treatment with 50 mg/day of Eltrombopag was confirmed to be effective in preventing haemorrhagic events in patients with IT. The limited duration of treatment allows to avoid potential adverse effects such as thrombosis or bone marrow fibrosis. A patient with ACTN1‐RT, an IT associated with defects in cytoskeletal proteins, had an excellent response to treatment. This was the first time that TPO‐RA treatment had been reported in a patient with this diagnosis.
PB0336
Phenotypic and genetic features of thirteen families with variants in GP1BA, GP1BB and GP9 genes
C. Monteiro 1; M. Pereira2; M. Gonçalves3; A. Gonçalves4; C. Lau3; E. Cruz5; S. Morais6; R. Santos7
1 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, CHUPorto; Unidade Multidisciplinar de Investigação Biomédica, Instituto de Ciências Biomédicas (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 2 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 3 Unidade de Diagnóstico Hematológico Margarida Lima, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 4 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 5 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal, Porto, Porto, Portugal; 6 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto, Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 7 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal
Background: Variants in GP1BA, GP1BB or GP9 affecting the assembly of GPIbα, GPIbβ and GPIX in the GPIb‐IX‐V complex, can cause two entities, monoallelic Bernard‐Soulier syndrome (BSS) and biallelic BSS, related with loss‐of‐function (LoF) variants. Alternatively, gain‐of‐function (GoF) genetic alterations are linked to Platelet‐type von Willebrand Disease (PT‐VWD). DiGeorge syndrome, a deletion on chromosome 22q11.2, can also involve GP1BB.
Aims: To report clinical and laboratory manifestations in a cohort of 24 patients with variants in GP1BA, GP1BB and GP9 genes.
Methods: From a cohort of patients with presumed inherited thrombocytopenias, eleven index cases were suspected of having GP1BA, GP1BB or GP9‐related thrombocytopenia, based on clinical data (family history, bleeding score), platelet counts and indexes [mean platelet volume (MPV) and immature platelet fraction (IPF)] and functional studies (aggregometry and glycoprotein quantification by flow cytometry). Genetic analyses were performed by NGS and/or Sanger sequencing. In two other index cases, the suspicion resulted from NGS studies. Variants were recognized as clinically relevant based on allele frequency, bioinformatics predictions and disease/familiar co‐segregations analysis.
Results: From the 13 families, 9 were diagnosed as biallelic (4) or monoallelic (5) BSS, according to the findings of phenotypic and genetic studies, with impaired clinical and laboratory results more pronounced in biallelic. In contrast, 2 additional families failed to achieve a solid phenotypic and genetic identification. Furthermore, a new GoF GP1BA variant was identified in a single case recognized as PT‐VWD. Additionally, through Multiplex Ligation‐dependent Probe Amplification (MLPA), one DiGeorge case was found. Overall, 7 new variants, in GP1BA (6) and GP1BB (1), were recognized and classified as variants of unknown significance (3) or Likely‐Pathogenic/Pathogenic (4). [1].
Conclusion(s): Using an adequate diagnostic workflow, genotype–phenotype correlations and segregation studies, we were able to confirm or reach a suspected diagnosis in 11 families. Future studies are necessary to address the pathogenic status of the novel variants.
PB0333
Quantification of platelet Filamin A by flow cytometry and immunofluorescence does not support the pathogenicity of FLNA missense compared to nonsense variants
M. Ibrahim‐Kosta 1; M. Poggi2; V. Sbarra2; C. Falaise3; N. Hezard4; G. Mourey5; F. NEDELEC‐GAC6; M. Fiore7; N. Saut8; E. Tomeï9; M. Alessi2; P. Saultier1
1 Aix Marseille Univ, INSERM 1263, INRAE 1260, C2VN, APHM, National Reference Center on Constitutional Platelet Disorders (CRPP), Marseille, Provence‐Alpes‐Cote d'Azur, France; 2 Aix Marseille Université, Marseille, Provence‐Alpes‐Cote d'Azur, France; 3 Aix APHM, National Reference Center on Constitutional Platelet Disorders (CRPP), Marseille, Provence‐Alpes‐Cote d'Azur, France; 4 APHM, Marseille, Provence‐Alpes‐Cote d'Azur, France; 5 EFS, Besançon, Bourgogne, France; 6 Department of Biological Hematology, Pontchaillou, University Hospital of Rennes, France, Rennes, Bretagne, France; 7 Laboratory of Hematology, University Hospital Center of Bordeaux, Pessac, Aquitaine, France; 8 Laboratory of Hematology, La Timone Hospital, Marseille, France, marseille, Provence‐Alpes‐Cote d'Azur, France; 9 Laboratory of Hematology‐APHM, Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: Filamin A (FLNA) variants are responsible for a rare X‐linked dominant syndromic macrothrombocytopenia mainly associated with nodular brain heterotopia. Absence of FLNA within platelets may help to establish the pathogenicity of candidate variants.
Aims: To assess the value of platelet FLNA quantification in 10 patients (9 females) carrying 8 novel FLNA variants (3 nonsense and 5 missense) and referred for thrombocytopenia and/or dense granule defect.
Methods: Clinical features, platelet count and size, results of platelet aggregation, dense granules analysis, and glycoprotein levels were collected. Intra‐platelet FLNA was quantified by whole blood flow cytometry (FC) and immunofluorescence. Genetic variants were identified using a platelet disorders gene sequencing panel.
Results: The three nonsense variants were: c.133C > T (p.Gln45Ter), c.1056delG (p.Thr353LeufsTer32) and c.1120_1125delinsTCTTG (p.Val374SerfsTer2). All patients (n = 5) exhibited macrothrombocytopenia (83‐130x109/L, 5–10% macroplatelets) associated with extra‐hematological manifestations (nodular brain heterotopia in all, ligamentous hyperlaxity and cardiac malformations). In all patients, a FLNA‐negative macroplatelets subpopulation (30–35%) was identified using FC and immunofluorescence (Figure.1A). The five missense variants were: c.1354G > A (p.Gly452Ser), c.3755C > T (p.Ala1252Val), c.6791G > A (p.Arg2264Gln), c.568C > T (p.Arg190Trp), and c.5053A > G (p.Thr1685Ala). All variants were predicted deleterious using multiple prediction tools. Two variants were absent from general population databases and three were very rarely reported (GnomAD exome frequency: 1.69x10‐5‐5.65x10‐6). Thrombocytopenia (36‐133x109/L) was reported in 4/5 patients. One patient had an isolated dense granule defect. Macroplatelets were detected at a variable level (0–50%). Two patients had associated organ defects (psychomotor delay, cardiac defect, hyperlaxity, liver cytolysis). Brain magnetic resonance imaging, performed in three patients, was normal. The patient carrying the p.Ala1252Val was finally diagnosed with telomeropathy (TERT variant). FC and immunofluorescence analysis did not reveal any negative FLNA platelet subpopulation (Figure.1B).
Conclusion(s): Our results highlight the value of FLNA platelet quantification to investigate the pathogenicity of nonsense FLNA variants. In contrast, quantification of platelet FLNA may not be informative regarding missense variants.

PB0337
Identification and characterization of ANKRD26‐related thrombocytopenia in patients from the GAPP cohort
H. Vyas 1; G. Lowe2; N. Morgan1
1 University of Birmingham, Birmingham, England, United Kingdom; 2 University Hospitals Birmingham NHS Trust, Birmingham, England, United Kingdom
Background: ANKRD26‐related thrombocytopenia is an inherited platelet disorder characterized by mild thrombocytopenia, normal platelet size and a mild bleeding diathesis. It results from point mutations in the highly conserved 5’UTR of the ANKRD26 gene; a known binding site for transcription factors RUNX1 and FLI1. Patients have a 24‐fold increased risk of developing myeloid malignancy, and knowledge of the condition may influence monitoring strategies and impact clinical management at the time of a cancer diagnosis. Although over 250 cases of ANKRD26‐related thrombocytopenia have now been described, its incidence is unknown.
Aims: To identify undiagnosed cases of ANKRD26‐related thrombocytopenia from a pre‐defined patient cohort with unexplained bleeding diathesis or thrombocytopenia, and further characterize their protein and RNA expression.
Methods: We identified 133 patients with a documented history of bleeding diathesis, or thrombocytopenia from the GAPP cohort. Platelet DNA was assessed following Sanger sequencing. Protein analysis and RNA sequencing is currently in process.
Results: Of the 133 patients in whom samples were available for analysis, 11 patients have single point mutations in the 5’UTR which have previously been described as pathogenic. Three variants were identified among the cohort: C.‐140C > G (7 patients), C.‐116 C > T (3 patients) and C.‐126 T > G (1 patient).
Conclusion(s): The C.‐140 C > G mutation identified has previously been described as a pathogenic variant causing thrombocytopenia in eight patients of which one developed AML. However, given its frequency, and on comparison to population databases, it is possible that this may in fact represent a benign variant. Ongoing protein analysis may help determine the significance of this variant. The C.‐116 C > T mutation lies within the RUNX1 binding site of 5’UTR and has been described in ten previous patients, with one of them going on to develop CMML. Four C.‐126 T > G variants have been described previously with ANKRD26‐related thrombocytopenia.
PB0340
Treatment by Line in Patients With Persistent and Chronic Immune Thrombocytopenia, A Real‐World Point‐In‐Time Study
M. Cooney1; S. Chang2; M. Isaila 3; I. Leunikava4; J. DeCourcy5; K. Lewis5; S. Libby5; S. Zalpuri6
1 UCB Pharma, Morrisville, North Carolina, United States; 2 UCB Pharma, Atlanta, Georgia, United States; 3 UCB Pharma, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 4 UCB Pharma, Monheim, Nordrhein‐Westfalen, Germany; 5 Adelphi Real World, Bollington, England, United Kingdom; 6 UCB Pharma, Breda, Noord‐Brabant, Netherlands
Background: Primary immune thrombocytopenia (ITP) is an autoimmune, heterogenous hematological disorder characterized by a transient or persistent decrease in platelets without other underlying causes. Patients with chronic or persistent disease can receive multiple lines of treatment with potential adverse effects.
Aims: To explore real‐world trends in ITP treatment, focusing on which treatments are used as first‐, second‐ or subsequent lines of therapy, and the reasons for their choice.
Methods: Data were drawn from the Adelphi ITP Disease Specific Programme™ (DSP), a real‐world point‐in‐time study. Physicians were asked to complete attitudinal questionnaires and patient record forms for their next 1–5 consulting patients. Data collection occurred in six countries between August and September 2020. This analysis summarizes treatment history and treatment rationale in patients with persistent (3–12 months) and chronic (>12 months) ITP.
Results: A total of 124 physicians were surveyed, with data collected for 405 persistent and chronic patients. Of these (mean age: 52 years; 54% male; mean time since diagnosis [n = 377]: 35.5 months), 307 (75.8%) were currently undergoing maintenance (chronic) therapy. Including both maintenance and acute (i.e., short‐term treatment for a specific purpose) therapies, first‐line treatment had been received by 389 patients, second‐line by 271 patients, and third‐line by 85 patients. Sixteen patients were not treated. Prednisolone was the most commonly prescribed first‐line therapy (65%, n = 253); eltrombopag was the most commonly prescribed second‐line (36%, n = 98) and third‐line (41%, n = 35) therapy (Figure 1). Efficacy was the main reason for treatment choice in first‐ and second‐line treatments, specifically ‘improved platelet counts’ (n = 188, 49%; n = 150, 56%). At third‐line, tolerability was most stated, specifically ‘to spare patients from steroids’ (n = 39, 47%) (Figure 2).
Conclusion(s): Three quarters of persistent or chronic patients were receiving maintenance therapy. When progressing treatment lines, tolerability became an increasingly important reason for therapy choice. Adelphi ITP DSP is part‐sponsored by UCB Pharma.


PB0339
Interference with dendritic cells by siRNA‐CD83 can reverse the polarization of Th1/Th2 and Treg/Th17 in ITP patients
X. Wang
Department of Hematology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China (People's Republic)
Background: Dendritic cells (DCs) are closely related to the pathogenesis of immune thrombocytopenia (ITP), but the details are not unclear. We for the first time investigated the biological characteristics and functions of CD83 in DCs.
Aims: To explore the relationship between CD83 and CD4 + T cell subsets and clarify the role of CD83 in ITP autoimmunity regulation.
Methods: The expression of CD4 + 、CD25 + 、FoxP3 and CD83+ on CD4 + T cells was detected by flow cytometry. CD83, GATA‐3, TGF‐β, IL‐10, FoxP3, TLR4, T‐bet were detected by QPCR. The concentration of sCD83 was determined by ELISA. DCs were transfected with siRNA‐CD83, NC (negative control); and plasmid overexpressed DC‐CD83 (pcDNA),pEX‐1 (negative control); Blank control. The DCs was co‐cultured with CD4 + T cells 1:5 and 1:10 for 72 hours. The concentrations of IFN‐γ, IL‐17, TGF‐β and IL‐10 were detected by ELISA. The DCs was co‐cultured with CD4 + T cells 1:10 to detect Tregs.
Results: The expression levels of CD4 + CD25 + FoxP3 and CD83 + CD4 + CD25 + FoxP3 were higher in the control group (p > 0.05). The expression of CD4 + CD83+ was higher in ITP group (p > 0.05). CD83 and TLR4 in ITP group were higher than those in control group, while GATA‐3, IL‐10 and FoxP3 were lower than those in control group, with statistical significance. TGF‐β and T‐ bet had no significant difference between the two groups. sCD83 was higher in ITP group (p = 0.001). siRNA inhibited the proliferation of CD4 + T cells, while pcDNA promoted the proliferation of CD4 + T cells (Figure 1). The IFN‐γ and IL‐17 levels in pcDNA group were higher than those in siRNA group. Both IL‐10 and TGF‐β were lower than those in siRNA group (Figure 2). Overexpression of CD83 promoted the up‐regulation of Tregs, pcDNA vs siRNA in ITP group (p < 0.0001). Control group pcDNA vs siRNA (p < 0.0001).
Conclusion(s): siRNA‐CD83 inhibits CD4+T cell proliferation by interfering with dendritic cells, reverse the imbalance of Th1/Th2 and Th17/Treg in ITP patients.


A novel R1162S variant in MYH9 alters the biochemical interactions and dynamics of NMMHC‐IIA, induces severe macrothrombocytopenia and perturbs leukocyte function and cellular coherence.
C. Llorente‐González1; M. Millán‐Salanova1; A. Marin‐Quilez 2; L. Díaz‐Ajenjo2; S. Santos‐Mínguez2; C. Miguel‐García2; R. Benito2; J. González‐Porras3; J. Rivera4; M. Vicente‐Manzanares1; J. Bastida5
1 IBMCC, CIC, CSIC‐Universidad de Salamanca, Salamanca, Spain, Salamanca, Castilla y Leon, Spain; 2 IBSAL, CIC, IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain, Salamanca, Castilla y Leon, Spain; 3 Department of Hematology, Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain, Salamanca, Castilla y Leon, Spain; 4 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Murcia, Murcia, Spain; 5 Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Salamanca, Castilla y Leon, Spain
Background: Patients with MYH9‐related disorders (MYH9‐RD) show a strong genotype–phenotype correlation based on thrombocytopenia, renal disease, deafness and/or cataracts. Beyond platelets, the cellular effects, and underlying biochemical mechanism, of MYH9 variants which affect to the heavy chain of non‐muscle myosin IIA (NMMHCII‐A) are poorly characterized. Recurrent infections have not been previously described.
Aims: To characterize the biochemical and dynamic behavior of the MYH9 variant c.3486G > C [p.R1162S] and assess its correlation with alterations observed in leukocytes and recurrent infections.
Methods: MYH9‐RD was diagnosed in five patients from the same family with recurrent infections since early age (Table 1). The behavior of neutrophils, activated T cells and monocyte‐derived dendritic cells was examined in adhesion and migration assays. NMMHCII‐A 1162S mutant was expressed as GFP‐coupled protein in a Myh9‐deficient cell line for cellular and biochemical characterization.
Results: Leukocyte anomalies consisted of morphology and migratory defects in neutrophils, T lymphoblast and monocyte‐derived dendritic cells (mDCs). Upon adhesion to fibronectin, neutrophils showed striking, highly‐organized and stack‐like NMMHCII‐A structures consistent with increased filament stability (Figure 1A). Neutrophils bearing this variant also showed decreased fMLP‐dependent migration through Boyden chambers (Figure 1B). T lymphoblasts adhered to fibronectin displayed increased polarization and spreading area (Figure 1C) whereas the adhesion capability of mDCs was impaired (Figure 1D). Further analysis of the migratory ability of mDCs on 3D substrates showed an aberrant CCL19‐dependent migration in collagen gels (Figure 1E). Surprisingly, mDCs carrying this variant displayed decreased cellular coherence and increased release of cytoplastic fragments when navigating through collagen fibers (Figure 1F). Expression of GFP‐NMMHCIIA R1162S in a MYH9‐deficient cell line revealed assembly into abnormally stable mini‐filaments dynamically distinct from wild type filaments (Figure 1G).
Conclusion(s): The MYH9 variant p.R1162S led to specific perturbations of the biochemical interactions and dynamics of mutant NMMHCII‐A protein. The leukocyte alterations reported here could underlie the recurrent infections observed in patients carrying this variant.


Non HUS/TTP Microangiopathies
PB0341
Impact of SARS‐CoV‐2 vaccination in thrombotic thrombocytopenic purpura patients
M. Capecchi1; P. De Leo2; M. Biganzoli2; I. Mancini3; P. Agosti4; B. Ferrari5; R. Gualtierotti6; A. Artoni 7; F. Peyvandi8
1 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milano, Lombardia, Italy; 2 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milano, Lombardia, Italy; 3 Università degli Studi di Milano, Milan, Italy, Milano, Lombardia, Italy; 4 Università degli Studi di Milano and Fondazione Luigi Villa, Milan, Italy, Milano, Lombardia, Italy; 5 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 6 Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy, Milan, Lombardia, Italy; 7 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, Milano, Lombardia, Italy; 8 Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Thrombotic thrombocytopenic purpura (TTP) has occasionally been described after vaccination. Since the availability of anti‐SARS‐CoV‐2 vaccines, 12 cases have been described on a possible association with TTP onset.
Aims: This study aims to evaluate the relapse rates in patients affected by TTP undergoing anti‐SARS‐CoV‐2 vaccination.
Methods: All consecutive TTP patients undergoing anti‐SARS‐CoV‐2 vaccination from March to May 2021 were enrolled. Blood samples were collected before vaccination (T0), 2 weeks after the first (T1) and the second dose (T2) to evaluate ADAMTS13 activity and anti‐ADAMTS13 antibody titer.
Results: A total of 49 TTP patients were enrolled (48 acquired and 1 congenital), all vaccinated with an mRNA vaccine. No patients had a clinical TTP relapse, with an ADAMTS13 relapse rate of 1.36% per month. Mean levels of ADAMTS13 activity were stable among the three timepoints (Figure). In only two patients a significant drop in ADAMTS13 levels occurred after the first dose (from 28% to <3% and from 101% to 82%), and both remained stable after the second dose, with negative anti‐ADAMTS13 antibodies. Due to a stable undetectable ADAMTS13, the first patient was treated with 4 doses of weekly 375 mg/m2 rituximab with a rapid ADAMTS13 response. One patient had positive basal anti‐ADAMTS13 antibodies with a titer remaining stable after the two vaccine doses, while in another patient anti‐ADAMTS13 antibodies became detectable after the first dose, with no corresponding drop in ADAMTS13 levels and a stable titer after the second dose.
Conclusion(s): The result of our study prospectively evaluating the effect of anti‐SARS‐CoV‐2 vaccination on the risk of relapse in a large cohort of patients with TTP in Milan showed a lower than reported relapse rate (1.36% vs 2.6%) with an observed to expected incidence rate ratio of 0.52, confirming the safety of mRNA‐based anti‐SARS‐CoV‐2 vaccination in TTP patients.

VPB0342
Effect of high molecular weight polyethylene oxide on thrombosis under high shear blood flow conditions
D. Kim 1; L. Herbertson2; R. Natu2; R. Malinauskas2; J. Baek3; P. Buehler4; J. Pinto5; X. Feng5; H. Qu5; X. Xu5
1 FDA/Center for Devices and Radiological Health (CDRH), Center for Drug Evaluation and Research (CDER), Silver Spring, Maryland, United States; 2 Center for Devices and Radiological Health (CDRH) / FDA, Silver Spring, Maryland, United States; 3 Center for Biologics Evaluation and Research (CBER) / FDA, Silver Spring, Maryland, United States; 4 Department of Pathology/University of Maryland School of Medicine, Baltimore, Maryland, United States; 5 Center for Drug Evaluation and Research (CDER) /FDA, Silver Spring, Maryland, United States
Background: Opioid abuse is a major public health issue. To combat this problem, opioid tablets include excipients such as polyethylene oxide (PEO) to deter crushing and snorting. Yet, these deterrents do not protect against unintended intravenous injection. Intravenously injected PEO‐containing opioids have led to complications such as hemolytic anemia, thrombocytopenia, and renal failure consistent with clinical features of thrombotic microangiopathy (TMA). In vivo and in vitro models have been used to demonstrate the hemolytic effect of high molecular weight (HMW) PEO above 4 MDa, but the mechanism by which PEO causes TMA‐associated events is not well understood.
Aims: The aim of this study is to assess the thrombogenicity of PEO by measuring clotting time and visualizing thrombus formation in an in vitro microfluidic model.
Methods: PEO solutions were prepared by dissolving PEO powder into phosphate buffered saline and then gently mixing with ACDA‐anticoagulated porcine blood for 5 min. The blood‐PEO mixtures were then recalcified and heparinized. The effects of PEO concentration and molecular weight on blood coagulation were assessed using a Hemochron ACT+ system (Figure 1). To model thrombosis, blood‐PEO mixtures were also perfused through a 100 x 200 μm microfluidic model at 0.1 ml/min (shear rate of 5000 s‐1) for 20 min and monitored for thrombotic events via microscopy.
Results: Increasing PEO concentration and molecular weight caused significantly shorter blood clotting times (Figure 1), especially for HMW PEO formulations above 4 MDa. In the microfluidic model, no thrombosis was observed in the control microchannel, but thrombi were observed when HMW PEO was introduced (Figure 2). Thrombosis was more pronounced in the upstream region of the channel and appeared white in color, indicating the thrombi consisted mostly of platelets, not red blood cells.
Conclusion(s): Clotting occurred faster when HMW PEO was introduced into the blood, and white thrombi formed in an in vitro microfluidic model under high shear flow.


Platelet Antagonists and Novel Therapeutics
VPB0350
Curcumin potentiates an inhibitory effect of cangrelor on platelets
N. Rukoyatkina1; V. Shpakova 2; S. Gambaryan3
1 Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, Saint‐Petersburg, Saint Petersburg City, Russia; 2 Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, Saint‐Petesburg, Saint Petersburg City, Russia; 3 Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, Saint Petersburg, Saint Petersburg City, Russia
Background: Curcumin is a natural polyphenol derived from turmeric plant (Curcuma longa) that has many beneficial effects including inhibition of platelet activation and thrombus formation. Previously, we showed that curcumin by activation of adenosine A2A receptor stimulates AC/cAMP/PKA inhibitory pathway in platelets. We hypothesized that curcumin could potentiate anti‐thrombotic effect of P2Y12 receptor inhibitor.
Aims: The aim of this study was to elucidate whether curcumin could potentiate the inhibitory effect of cangrelor (AR‐C69931) on platelet aggregation induced by ADP.
Methods: Washed platelets from healthy volunteers were incubated with curcumin and aggregation was initiated by ADP. Laser diffraction method was applied for analysis of platelet aggregation. Activation of AC/cAMP/PKA inhibitory system of platelets was analyzed by Western blotting.
Results: Curcumin alone inhibited 1 μM ADP‐induced platelet aggregation started from 50 μM which correspond to strong VASP phosphorylation and formation of procoagulant platelets. However, low curcumin concentrations (5–10 μM) did not significantly inhibit platelet aggregation and only slightly increased VASP phosphorylation. Preincubation with AR‐C69931 (1 nM) prevented (54 ± 14%) ADP‐induced aggregation. Combination of curcumin (5 μM) with AR‐C69931 (1 nM) inhibited aggregation up to 86 ± 4.9%. Both substances alone only slightly increased VASP phosphorylation, whereas their combination induced strong phosphorylation of VASP which correlate with a strong inhibition of aggregation.
Conclusion(s): In summary, we showed that curcumin at low concentration potentiated inhibitory effect of cangrelor in ADP‐stimulated platelet aggregation and VASP phosphorylation. Curcumin alone or in combination with other drugs is often used in clinical practice and presented data illustrated that low concentrations of curcumin, in combination with P2Y12 receptor antagonists, could be used as supplementation to prevent platelet activation.
PB0344
Use of romiplostim in pediatric patients with immune thrombocytopenic purpura (ITP)
A. Carranco Falcón1; B. Díaz Roldán 2; J. Domínguez Rodríguez2; P. Velarde López Ayala2
1 Juan Ramón Jiménez Universitary Hospital, Huelva, Andalucia, Spain; 2 HU Juan Ramón Jiménez, Huelva, Andalucia, Spain
Background: Immune Thrombocytopenic Purpura (ITP) disease destroys platelets with an autoimmune process. During this one, the count of platelets reduces increasing at the same time the risk of bleeding. Pediatric classic treatmets for ITP includes corticosteroids, gamma globulins, splenectomy and immunosuppresive drugs like Rituximab. Currently, the possibility of new treatments such us thrombopoietin (TPO) receiver analogues (Romiplostim y Eltrombopag) have been used as second row treatments instead of splecnetomy and Rituximab due to a better security profile for the patient.
Aims: Describe our experience with Romiplostim in pediatric patients with refractory Immune Thrombocytopenic Purpura (ITP) as first row treatment.
Methods: Data analysis of pediatric patients with ITP and Romiplostim as first row treatment from 2018 to 2021.
Results: All the patients treated with Romiplostim have achieved the complete remission (CR) without any side effects. In one of the patients, after the complete remission it was possible to stop the Romiplostim treatment.
Conclusion(s): Based in our experience with it, Romiplostim is a real alternative for patients where first row treatments have not been effective; furthermore, it has a good security profile without any side effects.

PB0343
N‐Acetyl Cysteine Prevents Occlusive Arterial Thrombosis in a Dose‐Dependent Manner
C. Bresette 1; K. Ashworth2; J. Di Paola3; D. Ku4
1 Georgia Institute of Technology, Atlanta, Georgia, United States; 2 Washington University School of Medicine in St. Louis, St Louis, Missouri, United States; 3 Washington University in St. Louis, St. Louis, Missouri, United States; 4 Georgia Institute of Technology, Decatur, Georgia, United States
Background: Platelet‐rich thrombi formed with elongated VWF can cause occlusions of arteries causing fatal infarcts. N‐acetyl cysteine (NAC) has been shown to cleave vWF and promote clot lysis previously. In this study we investigate the efficacy of NAC to prevent thrombi from forming under high shear stress conditions.
Aims: Prevention of arterial thrombosis due to NAC is assessed. A dose–response curve for occlusion time in NAC‐treated human blood is assessed in a microfluidic assay. Efficacy is shown in a murine model.
Methods: Human whole blood incubated with 3, 5 or 10 mM NAC for 30 minutes was run through a microfluidic device of a stenosis. Occlusion time (OT) and platelet intensity were measured. Subsequently, a Modified‐Folts model of a carotid stenosis in 13 mice were treated with either 200, 400 mg/kg NAC or vehicle 45 minutes prior to the carotid injury.
Results: Human blood treated with 3 mM or 5 mM NAC had occlusion times of 255 s and 457 s respectively while the control OT was 124 s (Figure 1). Blood treated with 10 mM NAC did not occlude and no platelet aggregation was detected during the experiment. Platelet intensity measurements show the increase in occlusion time is due to a decreased platelet aggregation rate. Mice treated with 200 mg/kg NAC formed occlusive thrombi in 18.0 m versus 9.4 m for the control (Figure 2). 80% of the control clots were stable and remained occlusive, while all 200 mg/kg clots were unstable, breaking and reforming. No clots were formed in the 400 mg/kg mice.
Conclusion(s): NAC reduces the rate of platelet aggregation and increases the time to form occlusion with concentrations between 3 mM and 10 mM. At 10 mM NAC completely prevented platelet aggregation. This work demonstrates the dose–response curve for NAC preventing arterial thrombosis with human and murine blood, and provides evidence that NAC can be repurposed into an anti‐thrombotic therapeutic.


PB0348
Small‐molecule cyclophilin inhibitors as a potential therapeutic application to limit thrombosis
J. Van Bael 1; A. Vandenbulcke2; A. Ahmed‐Belkacem3; J. Guichou4; J. Pawlotsky5; S. De Meyer6; K. Vanhoorelbeke6; C. Tersteeg6
1 Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, Kortrijk, West‐Vlaanderen, Belgium; 2 Laboratory for Thrombosis Research, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 3 INSERM U955, Team Viruses, Hepatology Cancer, Créteil, Ile‐de‐France, France; 4 Centre de Biologie Structurale (CBS), INSERM U1054, CNRS UMR5048, Université de Montpellier, Montpellier, Languedoc‐Roussillon, France; 5 INSERM U955, Team Viruses, Hepatology Cancer & National Reference Center for Viral Hepatitis B, C and Delta, Department of Virology, Hôpital Henri Mondor, Université Paris‐Est, Créteil, Ile‐de‐France, France; 6 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium
Background: Cyclophilins (CyP) are involved in various pathways regulating platelet function. CyPA is a Ca2+ regulator and is involved in integrin αIIbβ3 bidirectional signaling, while CyPD mediates opening of the mitochondrial permeability transition pore resulting in procoagulant platelet formation. Platelet CyP has therefore been suggested as a therapeutic target to limit thrombosis. Many studies utilize the immunosuppressant Cyclosporin A (CsA) or derivatives. However, these have several disadvantages including side‐effects unrelated to CyP inhibition. Among others, CsA was shown to enhance platelet aggregation, which was suggested to be responsible for the thrombotic complications observed in patients treated with CsA. Therefore, potent CyP inhibitors unrelated to CsA are needed.
Aims: Test the effect of F759, a novel non‐peptidic small‐molecule CyP inhibitor (SMCypI), on platelet activation, aggregation and procoagulant platelet formation, in comparison with CsA.
Methods: Washed human platelets were incubated with 4 μM CsA or 125 μM F759 prior to activation. Procoagulant platelet formation was measured using flow cytometry, by analyzing AnnexinV binding and P‐selectin expression, as well as tetramethylrhodamine methyl ester staining demonstrating an intact mitochondrial membrane potential. P‐selectin expression and activated integrin αIIbβ3 were measured using flow cytometry. Platelet aggregation was measured using light transmission aggregometry.
Results: Addition of F759 resulted in a decrease in AnnexinV+/P‐selectin+ platelets upon dual‐agonist activation, comparable to the inhibition observed with CsA. Similarly, F759 prevented the dual‐agonist induced loss of mitochondrial membrane potential analogous to CsA. No effect was observed of F759 and CsA on P‐selectin expression and integrin αIIβ3 activation after thrombin, collagen related peptide or ADP activation. F759 induced a moderate inhibition of sub‐optimal concentrations of thrombin‐, collagen‐ and ADP‐induced platelet aggregation, while a significant increased aggregation was observed with CsA.
Conclusion(s): A novel non‐peptidic SMCypI reduced procoagulant platelet formation and moderately reduced platelet aggregation. Further experiments are needed to demonstrate its potential therapeutic application to limit thrombosis.
VPB0349
ML355 modulates platelet activation and prevents ABT‐737 induced apoptosis in platelets
V. Shpakova 1; S. Gambaryan2; N. Rukoyatkina3
1 Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, Saint‐Petesburg, Saint Petersburg City, Russia; 2 Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, Saint Petersburg, Saint Petersburg City, Russia; 3 Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, Saint‐Petersburg, Saint Petersburg City, Russia
Background: 12‐lipoxigenase (12‐LOX) is implicated in regulation of platelet activation processes and can be a new promising target for antiplatelet therapy. However, investigations of 12‐LOX were restricted by the lack of specific and potent 12‐LOX inhibitors. A novel specific 12‐LOX inhibitor ML355 was shown to inhibit platelet aggregation without adverse side effects on hemostasis, however, the molecular mechanisms of its action on platelets are poorly understood.
Aims: The aim of our study was to investigate effects of the selective 12‐LOX inhibitor ML355 on platelet functions and apoptosis.
Methods: Washed platelets from healthy volunteers were incubated with ML355 and analyzed by flow cytometry or Western blotting. Phosphatidylserine (PS) surface expression (Annexin V‐PE), αIIbβ3 integrins activation (Fibrinogen‐Alexa‐647) and P‐selectin exposure (CD62P‐PE) were assessed by flow cytometry. Phosphorylation and activation of platelet proteins was analyzed by Western blotting.
Results: ML355 (10–50 μM) impaired platelet αIIbβ3 integrins activation and α‐granule secretion induced by thrombin or TxA2, but not by collagen‐related peptide (CRP‐XL). ML355 inhibited PKB, Erk1/2 and PI3K but not p38, Syk or PLCγ2 phosphorylation. Furthermore, 50 μM of ML355 even potentiated Syk or PLCγ2 phosphorylation. These data indicate that ML355 does not block platelet activation induced by GPVI signaling. ML355 at high dose (50 μM) induced Vasodilator‐stimulated phosphoprotein (VASP) phosphorylation, hence its inhibitory effect on platelets can be mediated by activation of PKA or PKG. We showed that VASP phosphorylation was blocked by inhibitor of adenylate (SQ22563), but not guanylate (ODQ) cyclase. Additionally, ML355 itself did not affect platelet viability but surprisingly at high dose (50 μM) even blocked PS exposure and caspase‐3 activation, induced by a high affinity BCL‐XL inhibitor ABT‐737.
Conclusion(s): In summary, we showed that ML355 inhibits platelet activation induced by thrombin or TxA2 through AC/cAMP/PKA inhibitory pathway activation, but this compound does not modulate GPVI activation signaling events. Furthermore, ML355 blocks ABT‐737‐induced platelet apoptosis.
PB0345
Targeting platelet Glycoprotein VI with Glenzocimab: a novel mechanism of inhibition
P. Billiald1; A. Slater2; M. Pugnière3; I. Jiacomini4; M. Welin5; N. Rose5; E. Toledano6; D. François6; S. Watson7; M. Jandrot‐Perrus 8
1 LVTS, UMR_S1148 INSERM, Paris, France, Paris, Ile‐de‐France, France; 2 University of Birmingham, Birmingham, England, United Kingdom; 3 Inserm U1194 ‐ Université de Montpellier, France, Montpellier, Midi‐Pyrenees, France; 4 Laboratório de Imunoquímica, Departamento de Patologia Básica, Universidade Federal do Paraná, Brazil, Curitiba, Parana, Brazil; 5 SARomics Biostructures, Lund, Sweden, Lund, Skane Lan, Sweden; 6 Acticor‐Biotech SAS, Paris, France, Paris, Ile‐de‐France, France; 7 University of Birmingham, Birmingham, UK, Birmingham, England, United Kingdom; 8 LVTS, UMR_S1148 INSERM, Université de Paris, France, PARIS, Ile‐de‐France, France
Background: Platelet glycoprotein VI (GPVI) receives interest when developing new antiplatelet drugs with low bleeding risk. GPVI interactions with collagen initiates thrombus formation and GPVI binding to fibrin(ogen) promotes thrombus growth and stability. To be considered to have the greatest efficacy, GPVI antagonists are expected to inhibit the interaction of GPVI with its main ligands. Glenzocimab is a clinical grade anti‐GPVI humanized antibody fragment that inhibits GPVI interaction with collagen and fibrin (ogen).
Aims: To determine the mechanism of the glenzocimab's inhibitory effect.
Methods: The extracellular domain of GPVI expressed as a monomer (GPVIex) or a dimer (GPVI‐Fc) and GPVI‐Fc des129‐136, were produced and their interaction with glenzocimab analyzed by SPR and solid phase assays. A co‐crystal of glenzocimab with GPVIex was obtained and the 1.9 Å structure compared to known GPVI structures (PBB ID: 2GI7 and 5OU7) and GPVI‐CRP complex structures (PDB ID: 5OU8 and 5OU9).
Results: Glenzocimab binds to the D2 domain of GPVI. Rearrangements within this domain prevent D2 homotypic interactions and formation of GPVI dimers of high affinity for collagen and fibrin. Glenzocimab induces allosteric modifications within the D1 domain with alterations of the betaC and betaF strands in the CRP binding groove. A shift of the betaC strand results in movement of R38, a key residue into the CRP binding channel and to a direct clash with CRP. Moreover, the light variable region of the GPVI‐bound Fab causes steric hindrance which prevents the CRP/collagen elongated chains to extend out of their binding site. Truncation of D2 residues 129–136 blocked binding to glenzocimab, validating the epitope localization.
Conclusion(s): This study demonstrates that altogether, the inhibition of GPVI‐dimerization, allosteric modifications of the collagen‐binding groove and steric hindrance by glenzocimab allow the inhibition of GPVI interactions with its major ligands.


PB0347
Efgartigimod: A Novel FcRn Antagonist in the Treatment of Autoimmune Diseases
V. McDonald 1; H. de Haard2; W. Parys2; P. Ulrichts2; B. Wittlin2; A. Guglietta2; J. Ayguasanosa2
1 Barts Health NHS Trust, The Royal London Hospital, London, England, United Kingdom; 2 argenx, Ghent, Oost‐Vlaanderen, Belgium
Background: Immunoglobulin G (IgG) autoantibodies play a key role in the pathogenesis of immune thrombocytopenia (ITP) and other autoimmune diseases, such as myasthenia gravis (MG), chronic inflammatory demyelinating polyneuropathy (CIDP), pemphigus vulgaris (PV) and foliaceus (PF), bullous pemphigoid (BP), and myositis. The neonatal Fc receptor (FcRn) is the central regulator of IgG homeostasis, rescuing IgG (including pathogenic autoantibodies) and albumin from lysosomal degradation and is responsible for the long half‐life of IgGs.
Aims: Therapeutic blocking of FcRn has been shown to lead to reduction of all IgG subtypes without reducing other immunoglobulin types, making it a rational target for treatment of autoimmune disorders while maintaining normal immunological responses.
Methods: Efgartigimod (EFG), an FcRn antagonist, is a human IgG1‐derived Fc‐fragment that outcompetes endogenous IgG binding, reduces IgG recycling, and increases IgG degradation. In healthy volunteers, EFG reduced IgG by 50–75%.
Results: In a Phase 3 study in MG (ADAPT), EFG rapidly reduced IgG without impacting IgM, IgA, or albumin levels while participants recorded statistically significant functional improvements. In a Phase 2 trial in ITP, EFG dosed at 5 and 10 mg/kg weekly for 4 weeks reduced IgG by ~60%, leading to clinically relevant increases in platelet counts (46% of EFG patients vs 25% on placebo achieved a platelet count of ≥50 × 109/L on ≥2 occasions). Phase 2/3 studies in CIDP (ADHERE), PV/PF (ADDRESS), BP (BALLAD), and myositis (ALKIVIA) are ongoing. In all studies to date, EFG was well tolerated, and adverse events were mainly mild to moderate. EFG is approved in the US and Japan for treatment of generalized MG in adult patients. Phase 3 studies in ITP (ADVANCE and ADVANCE SC) are ongoing.
Conclusion(s): FcRn inhibition by EFG is a promising potential therapeutic option for several autoimmune diseases mediated by pathogenic IgG autoantibodies.
PB0346
Inhibition of PI3KC2alpha selectively reduces platelet recruitment after vascular injury while preserving thrombus architecture
P. Larsson 1; N. Setiabakti1; V. Tarlac2; A. McGovern3; J. Nunez‐Iglesias4; K. Tunströmer5; J. Hamilton2; N. Boknäs6
1 Monash University, Melbourne, Victoria, Australia; 2 Australian Centre for Blood Diseases, Monash University, Melbourne, Victoria, Australia; 3 Department of Anatomy and Developmental Biology, Monash University, Melbourne, Victoria, Australia; 4 Anatomy and Developmental Biology, Monash University, Melbourne, Victoria, Australia; 5 University of Linkoping, Linkoping, Ostergotlands Lan, Sweden; 6 Region Östergötland, Linkoping, Ostergotlands Lan, Sweden
Background: We have recently examined the class II PI3‐Kinase enzyme PI3KC2alpha (C2a) as a potential therapeutic target for antithrombotic therapy. Interestingly, despite being anti‐thrombotic in whole blood flow assays in vitro and in vivo, we have failed to detect any effect of C2a deletion on platelet function or traditional readouts of platelet activation. Thus, the mechanism by which C2a inhibition inhibits thrombosis is still unknown.
Aims: To investigate the effects of pharmacological C2a inhibition on thrombus formation in vivo.
Methods: We combined a novel intravital microscopy thrombosis model with automatized segmentation and tracking algorithms to track the positions, activation states and movements of a large number of platelets during thrombus formation. Labeled donor platelets were injected into recipient mice treated with our novel C2a inhibitor MIPS‐21335, or vehicle. 4D confocal imaging of thrombus formation was performed for 10 min after laser injuries to mesenteric veins. A neural network trained for platelet segmentation was used to analyze the resulting time‐lapse data.
Results: Our analysis revealed no significant effects of MIPS‐21335 treatment on the acute early phase of thrombus build‐up in vivo. However, starting at 3 min after injury there was a significant reduction of new platelets being recruited to the thrombus, specifically to the outer lateral parts that experience elevated shear gradients. Total platelet numbers, packing density and stability in the injury zone were not affected by C2a inhibition indicating that this treatment did not affect the structural integrity of the thrombus core.
Conclusion(s): Pharmacological inhibition of PI3KC2alpha maintains the structure and stability of the developing thrombus core while selectively reducing platelet accumulation to the outer parts of the thrombus. Thus PI3KC2alpha appears to be a promising anti‐thrombotic target that can potentially show selectivity against pathological expansion of a thrombus while maintaining its haemostatic function.

Platelet Function Disorders, Acquired
VPB1231
Clinical efficacy of rituximab in the treatment of adult immune thrombocytopenia: a single center data analysis
M. Yang 1; Z. Zeping2
1 The Second Affiliated Hospital Of Kunming Medical University, Kunming, Yunnan, China (People's Republic); 2 The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China (People's Republic)
Background: Rituximab is a Second‐line treatment for immune thrombocytopenia. Rituximab is expensive and patients in Yunnan have different understanding of the drug and affordability of the economy, and selection of rituximab requires a combination of patient characteristics, availability of the drug, and treatment experience at a single center.
Aims: This study performed a retrospective approach to analyze the use of rituximab in patients with ITP to summarize the treatment experience and provide a single center data analysis.
Methods: The clinical materials of patients who were admitted to the Department of Hematology the Second Affiliated Hospital of Kunming Medical University from January 2014 to July 2020 were collected.
Results: For group low‐dose rituximab therapy: The median age was 45 (23 ~ 66) years, median disease duration was 13.5 (1 ~ 136) months, median follow‐up time was 27 (6 ~ 79) months. In the 15 patients with OR (50%), the median efficacy maintenance time was 12 (3 ~ 40) months, early response in 13 patients, initial response in 15 patients, and sustained response in 12 patients, the response time more than 1 month in 14 patients and the response time less than 1 month in 2 patients. The 1‐year response rate for all adults treated with rituximab was 40.9% and the 2‐year response rate was 18.7%. The correlation between bleeding score and age, course of disease and platelet count before treatment was not statistically different (r = −0.04, p = 0.985; r = 0.073, p = 0.701; r = −2.51, p = 0.181). There was no significant difference in gender, age and disease stage between group NR and group OR.
Conclusion(s): The results showed that rituximab is an effective second‐line treatment in the treatment of adult ITP, with mild adverse reactions, and no index can be used as a predictor of the efficacy of rituximab.
VPB1230
Comparison of platelet function with different tyrosine kinase inhibitors treatment in chronic myeloid leukemia, in vivo and ex vivo studies
Y. WU 1; C. SHEN2; T. WANG2; C. LIU3
1 Tzu Chi University, Hualien, Hualien, Taiwan (Republic of China); 2 Department of Hematology and Oncology, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, HUALIEN, Hualien, Taiwan (Republic of China); 3 College of Medicine, Tzu‐Chi University, HUALIEN, Hualien, Taiwan (Republic of China)
Background: Tyrosine kinase inhibitors (TKI), including imatinib, nilotinib, and dasatinib, have revolutionized the treatment of chronic myeloid leukemia (CML). However, the treatment is associated with serious adverse events including both bleeding and thromboembolism. Previous studies have shown that TKIs can cause platelet dysfunction.
Aims: In this study, we used flow cytometry to compare the effect of platelet function in vivo and ex vivo studies between three TKIs treatment and healthy donors.
Methods: In vivo, a total of 23 patients, including imatinib (N = 8), nilotinib (N = 7), dasatinib (N = 8), and healthy donors (N = 21) were enrolled in this study. Platelets treated with ADP and TRAP had surface GPIbα, activated GPIIb‐IIIa, and P‐selectin levels measured using flow cytometry. In ex vivo, normal platelets were collected from healthy donors (N = 6) and incubated with TKIs. Then they were treated with ADP and TRAP as in vivo. The differences were analyzed by ANOVA. The studies were approved by IRB (IRB109‐049‐B and IRB109‐080‐B).
Results: Figure 1A showed a study in vivo that the expression of PAC‐1 (GPIIbIIIa) in TKIs was significantly lower than healthy donors in ADP (p = 0.024) and TRAP (p = 0.023), especially in Dasatinib. P‐selectin showed no significance in TKIs and healthy donors. Figure 1B showed the results from ex vivo. PAC‐1 (GPIIbIIIa) in TKIs was significantly lower than control in ADP (p = 0.012) and TRAP (p = 0.009), especially in Dasatinib, but not P‐selectin.
Conclusion(s): CML patients under TKIs treatment showed platelet dysfunction in vivo and ex vivo studies, but only in GPIIbIIIa (PAC‐1), not in P‐selection. Dasatinib had the most serious effect on platelet function in vivo and ex vivo studies.

PB1225
Comparison of platelets function in patients with JAK2V617F or CALR mutated myeloproliferative neoplasms
A. Guy 1; M. Fiore2; K. Helzy3; O. Mansier2; J. Bordet4; E. Riviere5; C. James6
1 University Hospital Center of Bordeaux, Pessac, Aquitaine, France; 2 Laboratory of Hematology, University Hospital Center of Bordeaux, Pessac, Aquitaine, France; 3 Laboratory of Hematology, University Hospital Center of Bordeaux, Bordeaux, Aquitaine, France; 4 Laboratory of Hematology, University Hospital Center of Lyon, Lyon, Rhone‐Alpes, France; 5 Department of Internal Medicine, University Hospital Center of Bordeaux, Pessac, Aquitaine, France; 6 Laboratory of Hematology, University Hospital Center Bordeaux, Bordeaux, Aquitaine, France
Background: Myeloproliferative neoplasms (MPN) includes polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF). Mutations in the calreticulin (CALR) gene were identified in patients with TE and PMF. Calreticulin, a chaperone protein, is involved in calcium homeostasis. CALR+ patients have fewer thrombotic complications than JAK2V617F+ patients.
Aims: As calcium plays a major role in platelet activation, we hypothesized that CALR+ patients have impaired platelet functions compared with JAK2V617F+ patients.
Methods: To assess platelet functions, we analyzed platelet aggregation, αIIbβ3 and GPIb expression, ATP secretion, fibrinogen binding, dense and alpha granule content, and phosphatidylserine (PS) expression. We used ELISA to measure two plasmatic platelets activation markers, soluble P‐selectin (sCD62P) and CD40‐ligand (CD40L).
Results: 33 MPN patients were included: 16 CALR+ and 17 JAK2V617F+ patients. None of them had antiplatelet therapy. We first observed a decreased aggregation in response to arachidonic acid in all MPN patients and a decreased aggregation in the presence of epinephrine and TRAP in CALR+ patients only. Expression of αIIbβ3 and internalization of GPIb was decreased in JAK2V617F+ and CALR+ patients compared to controls. Fibrinogen binding was also similarly decreased in CALR+ and JAK2V617F+ patients. Platelets P‐selectin expression was decreased in JAK2V617F+ and CALR+ patients, as was the dense granule content. Interestingly, we observed PS overexpression in JAK2V617F+ patients, in favor of a procoagulant potential associated with JAK2V617F mutation. Together with this observation, we reported decreased PS exposure in hydroxyurea‐treated patients compared to non‐treated patients. Finally, plasmatic concentrations of sCD62P and CD40L were increased without difference between JAK2V617F+ and CALR+ patients.
Conclusion(s): We here report significantly altered platelet functions in CALR+ and JAK2V167F+ patients, but no difference between CALR+ and JAK2V167F+ patients. The presence of increased plasmatic concentrations of CD40L and sCD62 is in favor of in vivo platelet preactivation.
PB1227
Platelet transfusion better preserves ROTEM clot strength and thrombin generation: a case–control study
A. Rossetto 1; P. Vulliamy2; L. Green3; R. Davenport4
1 Queen Mary Univeristy of London, UK, London, England, United Kingdom; 2 Queen Mary University of London, UK, London, England, United Kingdom; 3 Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom; 4 Centre for Trauma Science, Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom
Background: Platelet transfusion improve clinical hemostasis and reduce mortality due to bleeding, yet the mechanism of how this is achieved remains unknown. ¬.
Aims: Determine the haemostatic benefit of current platelet transfusion in bleeding trauma patients.
Methods: Secondary analysis of previously collected data from adult trauma patients enrolled into an ongoing prospective cohort study (ISRCTN12962642) at a UK major trauma center between 2008 and 2020. Blood samples were drawn in the emergency department and at intervals after 4, 8 and 12 units of red blood cells. Tissue factor‐activated rotational thromboelastometry with cytochalasin D (FIBTEM) and without (EXTEM) were performed with fresh whole blood. Plasmin‐antiplasmin complex levels (PAP) were quantified by enzyme‐linked immunosorbent assay in stored plasma. Resuscitation intervals during which platelets were and were not transfused were matched 1:1 based on interval type (0 hr‐4 units, 4 units‐8 units, 8 units‐12 units), interval duration, prior platelet transfusions, and amount of co‐administered fresh frozen plasma (FFP) and cryoprecipitate.
Results: 313 resuscitation intervals from 223 trauma patients were analyzed, 207 with and 106 without platelet transfusion. Platelet‐transfused intervals had wider time gap from injury (p < 0.001), lasted longer (p < 0.001) and were characterized by a larger co‐administration of other pro‐haemostatic components (FFP, p < 0.001; cryoprecipitate, p < 0.001). Following case–control matching, 40 intervals in each group were analyzed. Hemostasis was better persevered at the end of platelet transfusion intervals as measured by EXTEM – FIBTEM A5 (28 vs 22 mm, p = 0.021), EXTEM A5 (37 vs 28 mm, p = 0.020), EXTEM clot formation time (110 vs 182 sec, p = 0.022) and EXTEM maximum velocity (12 vs 8 mm*min, p = 0.015) (Figure 1A‐B‐D‐E). There was no difference in EXTEM maximum lysis (p = 0.449), PAP levels (p = 0.190) and platelet count (p = 0.199).
Conclusion(s): Platelet transfusion is associated with a larger platelet contribution to early clot strength and thrombin generation, but not platelet count or decreased fibrinolysis.

PB1228
Increased platelet aggregation in patients with decompensated cirrhosis indicates higher risk of further decompensation and death
E. Campello1; A. Zanetto2; C. Bulato2; S. Gavasso2; F. Farinati2; F. Russo2; D. Tormene3; P. Burra2; M. Senzolo4; P. Simioni 5
1 University of Padova, Padova, Veneto, Italy; 2 Unviersity of Padova, Padova, Veneto, Italy; 3 Padova university hospital, Padova, Veneto, Italy; 4 Multivisceral Transplant Unit, University Hospital of Padua, Padua, Italy, Padova, Veneto, Italy; 5 Padua University Hospital, Padua, Veneto, Italy
Background: Studies on platelet aggregation in cirrhosis are controversial because interpretation of platelet function is challenged by thrombocytopenia.
Aims: We conducted a prospective study to investigate whole blood platelet aggregation in cirrhosis and its association with liver‐related outcomes.
Methods: Platelet aggregation was assessed by whole blood aggregometry (Multiplate®). To overcome the influence of platelet count and compare cirrhosis with thrombocytopenia versus controls with normal platelet count, we calculated a ratio between platelet aggregation and platelet count (PLT ratio). Then, we prospectively followed patients with cirrhosis and ascertained predictors of decompensation, transplantation, and death. Informed consent was obtained and the study was approved by the local medical ethics committee.
Results: Two‐hundred and three cirrhosis patients were prospectively recruited (77% decompensated). PLT ratio was significantly higher in cirrhosis than in chronic hepatitis and healthy subjects (0.44 vs. 0.25 and 0.26, respectively; p < 0.0001). In cirrhosis, the ratio increased with disease severity (Child‐Pugh class C > B > A) and was particularly elevated in decompensated patients with severe thrombocytopenia. Among decompensated patients, 65 had further decompensation, underwent transplantation, or died during a 6‐month follow‐up. On multivariate analysis, PLT ratio (OR: 1.87, 95%CI: 1.23–2.84; p = 0.003) and MELD score (OR: 1.05, 95%CI: 1.01–1.08; p = 0.01) were independently associated with outcome. The relative risk of events was 7.5‐fold higher in patients with PLT ratio >0.75 versus patients with PLT ratio <0.25 (RR: 7.5, 95% CI: 2.5–21.9; p = 0.003) (Figure 1). The increased PLT ratio, its discriminative ability for composite outcome, and the prognostic value of PLT ratio >0.75 were validated in an independent cohort of hospitalized patients with decompensated cirrhosis (n = 41).
Conclusion(s): Patients with cirrhosis, particularly when decompensated, exhibit significantly increased whole blood platelet aggregation. Decompensated patients with PLT ratio >0.75 have >80% probability of further decompensation, transplantation, or liver‐related death within 6 months.

PB1226
Hyperglycaemia enhances platelet degranulation and microparticle shedding: a novel mechanism underlying thrombotic complications in diabetes patients
N. Wolska1; R. Haghiri Limoudehi1; M. Kuhr2; G. Pula 2
1 University Medical Center Hamburg‐Eppendorf (UKE), Hamburg, Hamburg, Germany; 2 University Medical Center Eppendorf Hamburg (UKE), Hamburg, Hamburg, Germany
Background: Platelet hyperactivity and increased thrombosis risk are typical of type 2 diabetes mellitus patients, for whom cardiovascular diseases represent the most common cause of death. The cellular and molecular causes for the increased thrombotic risk in diabetes remain to be elucidated, although platelet hyperactivity has been observed in these patients.
Aims: In order to understand the effect of diabetes on platelets, we studied the effect of hyperglycaemia on platelet responsiveness in vitro. Platelet phenotypes and underlying molecular mechanisms identified in vitro were validated in clinical samples from diabetes patients.
Methods: In vitro studies were performed on peripheral blood from healthy volunteers treated for up to 48 hours with different concentrations of glucose. Clinical samples from diabetes patients were obtained from the University Medical Center Eppendorf (Hamburg) and were selected based on glycated hemoglobin (HbA1c): healthy control with HbA1c < 6.0%, diabetes patients with HbA1c > 7.0%. Further patient stratification was applied based on age, sex, cardiovascular comorbidity, and renal comorbidity. Platelet activation, secretion, procoagulant activity, microparticle release and leukocyte complexation were assessed by flow cytometry. The intracellular signaling underlying platelet phenotypes was investigated by immunochemistry and in vitro pharmacology.
Results: Supraphysiological glucose concentrations in vitro increased basal and primary agonist‐induced platelet degranulation. The increased degranulation was not associated with an increase in integrin activation and platelet aggregation, which suggested a selective glucose‐dependent control of granule secretion rather than a generalized effect on platelet responsiveness. Platelet degranulation in supraphysiological glucose was accompanied by a release of platelet‐derived microparticles and procoagulant microparticles, which was confirmed in the plasma of diabetes patients. The microparticles from diabetes patients enhanced platelet responses and coagulation cascade in vitro.
Conclusion(s): In this study, we describe a potential new mechanism linking the secretory properties of platelets with the increase cardiovascular risk of diabetes mellitus patients.


PB1229
Frequency of reported thrombotic adverse events and clinical outcomes associated with three COVID‐19 vaccines
M. Tobaiqy 1; K. MacLure2; H. Elkout3; D. Stewart4
1 College of Medicine, University of Jeddah, Jeddah, Kingdom of Saudi Arabia, Jeddah, Makkah, Saudi Arabia; 2 Independent Research Consultant, Aberdeen, Scotland, United Kingdom; 3 Department of Family and Community Medicine, Medical Faculty, University of Tripoli, Tripoli, Tripoli, Libya; 4 College of Pharmacy, QU Health, Qatar University, Doha, Ad Dawhah, Qatar
Background: During the first quarter of 2021, several European countries suspended the use of the Oxford–AstraZeneca vaccine amid reports of blood clot events and the death of a vaccinated person. This was followed by several reports of fatalities related to pulmonary embolism and other thrombotic events including thrombocytopenia which has been referred to as vaccine‐induced immune thrombotic thrombocytopenia (VITT).
Aims: This study investigated the occurrence of thrombotic adverse events and their clinical outcomes of the three approved and most used COVID‐19 vaccines namely Moderna, Pfizer and Oxford‐AstraZeneca, using one of the largest spontaneous adverse events databases, namely EudraVigilance.
Methods: A retrospective descriptive analysis was conducted of spontaneous reports for Moderna, Pfizer and Oxford‐AstraZeneca COVID‐19 vaccines submitted to the EudraVigilance database in the period from 17 February to 14 June 2021.
Results: There were 729,496 adverse events for the three vaccines, of which 3420 were thrombotic, mainly Oxford‐AstraZeneca (n = 1988, 58·1%) followed by Pfizer (n = 1096, 32·0%) and Moderna (n = 336, 9·8%). As serious adverse events, there were 705 reports of pulmonary embolism for the three vaccines, of which 130 reports (18·4%) were for Moderna, 226 reports (32·1%) for Pfizer and 349 (49·5%) for Oxford‐AstraZeneca vaccines. The occurrence of pulmonary embolism is significantly associated with a fatal outcome (p = < 0·001). Sixty‐three fatalities were recorded (63/3420, 1.8%), of which Moderna (n = 6), Pfizer (n = 25) and Oxford‐AstraZeneca (n = 32).
Conclusion(s): Thrombotic adverse events reported for the three vaccines remains extremely rare with multiple causative factors reported elsewhere as precipitating these events. Practicing vigilance and proper clinical management for the affected vaccines, as well as continuing to report adverse events, are essential. More than 4·89 billion doses of different COVID‐19 vaccines have been administered across the globe. On the basis of scientific evidence showing that benefit outweighs risk, people continue to be urged to accept the vaccination when offered.


Platelet Function Disorders, Hereditary
VPB1249
A case report of Inherited platelet function disorders caused by double site mutation of RASGRP2 gene and pedigree analysis
Q. Bian 1; X. Yang2; D. Wang1; X. Zhou1
1 Affiliated Hospital of Guizhou Medical University, GUIYANG, Guizhou, China (People's Republic); 2 Department of Pediatric Hematology, Affiliated Hospital of Guizhou Medical University, guiyang, Guizhou, China (People's Republic)
Background: Platelet function is closely related to platelet membrane, platelet storage granules, platelet signal transduction and platelet coagulation activity. RASGRP2 defect can lead to platelet dysfunction. In this study, we found a case of bleeding caused by platelet dysfunction caused by a new mutation of RASGRP2 gene, and analyzed its family.
Aims: To investigate the clinical characteristics of a case of Inherited platelet function disorders (IPFD) caused by dual site mutation of RASGRP2 gene, and to analyze the molecular pathogenesis of IPFD, so as to provide evidence for early diagnosis and treatment of IPFD.
Methods: A case of epistaxis with hemorrhagic anemia in the Affiliated Hospital of Guizhou Medical University was selected. Through the analysis of clinical manifestations, laboratory examination and treatment process, high‐throughput sequencing technology was used to detect pathogenic genes to find out the cause, and the clinical characteristics and gene sequencing results of his family were retrospectively analyzed.
Results: The results of high‐throughput sequencing showed that there were double heterozygous mutations of RASGRP2 gene on chromosome 11 in children. The father detected the mutation at the same site as the RASGRP2 gene of the child; Mother also detected the mutation at the same site of RASGRP2 gene and was a heterozygous carrier. RASGRP2 gene mutation was detected in both (maternal) grandma and (paternal) grandma related mutant genes were detected in children's grandfathers, grandfathers, aunts 1 and 2.
Conclusion(s): The double heterozygosity mutation at position 6,497,534 [C.1545c > a, P.515c > x] and position 64,509,585 [C.74‐1 g > c] is a new mutation that has not been reported, enriching the mutation spectrum of IPFD gene. The defect of RASGRP2 can affect the function of platelet aggregation and adhesion, leading to hemorrhagic diseases. High‐throughput sequencing can diagnose this kind of complicated hemorrhagic diseases more accurately and effectively, and improve clinicians' understanding of IPFD.

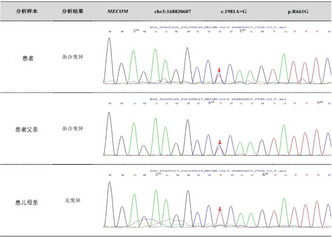
PB1233
Association of Genetic Variability in MRVI1Gene in Patients With Arterial Thrombosis and Platelet Hyperaggregability
M. Brunclikova 1; J. Ivankova2; M. Skerenova2; T. Simurda3; M. Dobrotova1; P. Holly2; L. Stanciakova2; M. Sterankova2; I. Skornova1; P. Kubisz1; J. Stasko1
1 National Centre of Hemostasis and Thrombosis, Department of Hematology and Transfusiology, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin and University Hospital in Martin, Slovakia, Martin, Zilina, Slovakia; 2 Comenius University, The Jessenius Faculty of Medicine in Martin, Martin, Zilina, Slovakia; 3 Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, University Hospital Martin, Martin, Zilina, Slovakia
Background: The thrombotic thrombocytopathy ‐ Sticky Platelet Syndrome (SPS) can predispose to thrombotic occlusions, especially in the arterial system. SPS has a clear autosomal pattern of inheritance, although the exact genetic cause has not been identified yet. Murine retrovirus integration site 1 homolog (MRVI1), has prior evidence of functions in platelet aggregation. In mice, MRVI1 plays a direct role in the inhibition of platelet aggregation and in vivo thrombosis.
Aims: The aim of this study was to evaluate the genetic variability of the selected single nucleotide polymorphisms (SNPs) in the MRVI1 gene in patients with SPS who developed occlusions in arterial system and examine the association between these SNPs and risk for development of arterial thrombosis in these patients.
Methods: We examined 50 patients with SPS and arterial thrombosis in various sites. The target population of our analysis consisted of patients, who developed arterial thrombotic occlusion at younger age (up to 40 years) than the general population, which draws the attention rather to genetic than to acquired risk factors. The control group consisted of 50 healthy blood donors. In all subjects we performed genetic testing of SNPs within MRVI1 gene.
Results: We assume that SPS has probably a polygenic mode of inheritance. Detailed results will be published in poster.
Conclusion(s): The platelet hyperaggregability can be a serious risk factor for the development of thrombotic events. Accurate determination of genetic changes in SPS and their relationship to the clinical phenotype can help physicians better understand the etiopathogenesis of the defect and help predict the clinical manifestation of the syndrome.
PB1239
A Familial Case of MYH9 Gene Mutation Associated with Impaired Platelet Functionality and Structural Alterations
S. Safiullina1; N. Evtugina1; I. Andrianova1; R. Khismatullin1; O. Kravtsova1; A. Khabirova1; C. Nagaswami2; A. Daminova1; A. Peshkova3; J. Weisel4; R. Litvinov 5
1 Institute of Fundamental Medicine and Biology, Kazan (Volga region) Federal University, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 2 Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 3 Kazan (Volga region) Federal University, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 4 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 5 University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania, United States
Background: Mutations in the MYH9 gene disrupt the cytoskeletal dynamics in megakaryocytes, causing macrothrombocytopenia, often associated with bleeding via unknown mechanism (s) related to defective platelets.
Aims: To study the platelet structure and function in a family with a MYH9 gene mutation.
Methods: The proband, her sister and their mother had one of the closely related MYH9‐related disorders: May‐Hegglin anomaly or Sebastian syndrome. All the patients had a characteristic heterozygous R1933X mutation in the MYH9 gene. Examination included CBC, blood smear, flow cytometry of platelets (expression of P‐selectin and active integrin αIIbβ3 before and after TRAP‐induced activation), kinetics of blood clot contraction, scanning/transmission electron microscopy of platelets.
Results: Despite thrombocytopenia (36x109/l in the proband, 55x109/l in the sister, 83x109/l in the mother), at the time of the examination, none of the patients had hemorrhages, although they had a history of heavy menstruation, spontaneous ecchymosis, and postpartum hemorrhage. The extent of clot contraction was normal or moderately reduced (33% in the proband, 39% in the sister and 42% in the mother, reference values >41%). Flow cytometry revealed the background activation of unstimulated platelets assessed by overexpression of P‐selectin and active αIIbβ3 integrin. After stimulation, the fraction of P‐selectin‐expressing platelets in the proband and her sister was lower than control, indicating partial platelet refractoriness. Electron microscopy revealed an increase in platelet size and ultrastructural alterations, such as multiple filopodia and huge dilatation of the open canalicular system, containing filamentous and vesicular inclusions.
Conclusion(s): The mutation of MYH9 gene, even heterozygous, is associated not only with thrombocytopenia, but also with qualitative structural and functional defects in platelets. The signs of background platelet activation are combined paradoxically with partial refractoriness and impaired contractility, which may contribute to the bleeding tendency in this MYH9‐related disorder. Informed consent and approval from the KFU Ethics Committee were obtained.

PB1248
Integrated diagnostic workflow in two siblings with albinism and suspected Hermansky‐Pudlak syndrome
V. Palma‐Barqueros1; N. Fernández‐Mosteririn2; C. Zaninetti3; N. Pardiñas‐ Barón4; A. Zamora‐Canovas 5; A. Sánchez‐Fuentes6; N. Revilla7; A. Rodríguez‐Alen8; A. Marin‐Quilez9; L. Díaz‐Ajenjo10; A. Torrecillas6; N. Bohdan6; J. Padilla11; C. Miguel‐García9; V. Vicente12; M. Lozano12; A. Greinacher13; J. Bastida14; J. Rivera15
1 Centro Regional de Hemodonación‐ IMIB, Mur, Murcia, Spain; 2 Hospital Miguel Servet, Zaragoza, Aragon, Spain; 3 Greifswald University, Greifswald, Mecklenburg‐Vorpommern, Germany; 4 University of Zaragoza, Zaragoza, Aragon, Spain; 5 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain, Murcia, Murcia, Spain; 6 Centro Regional de Hemodonación‐IMIB, Murcia, Murcia, Spain; 7 Hospital Universitario Ramón y Cajal, Madrid, Spain, Madrid, Madrid, Spain; 8 Hospital Virgen de la Salud, Toledo, Spain, Toledo, Castilla‐La Mancha, Spain; 9 IBSAL, CIC, IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain, Salamanca, Castilla y Leon, Spain, 10 University of Salamanca, Salamanca, Castilla y Leon, Spain, 11 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER, Murcia, Spain, Murcia, Murcia, Spain, 12 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain, 13 Universitätsmedizin Greifswald, Greifswald, Mecklenburg‐Vorpommern, Germany, 14 Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Salamanca, Castilla y Leon, Spain, 15 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Murcia, Murcia, Spain
Background: Hermansky‐Pudlak syndrome (HPS) comprises eleven autosomal recessive multisystem disorders sharing bleeding diathesis and albinism, due to abnormalities in genes (HPS1 to HPS11) essential for lysosome‐related organelles. Knowing the HPS subtype is important for prognosis and clinical management, since other relevant organ manifestations may be present depending on the affected gen.
Aims: Assessing the platelet phenotype and genetic diagnosis in two siblings with mild bleeding symptoms and albinism.
Methods: A 25 yr old woman (IC) with oculocutaneous albinism, macular hypoplasia in both eyes, iris transillumination, long‐life frequent epistaxis and irregular menses (ISTH‐BAT = 4), was enrolled in the Spanish Project of Inherited Platelet Disorders. Her sister has milder albinism and occasional epistaxis (ISTH‐BAT = 1). They showed no other clinically relevant features. We assessed full blood count, blood smears immunostaining of platelets proteins (P‐IF), platelet aggregation, and glycoproteins (GPs) expression, agonist‐induced P‐selectin and CD63 secretion by flow cytometry, and whole mount electron microscopy (WMEM). DNAs were analyzed by HTS‐gene panel and CNV, and candidate variants segregated by Sanger.
Results: Patients showed normal platelet counts and volume. Platelet aggregation was impaired (20–25%) with arachidonic acid, TRAP and collagen, but normal with ADP and ristocetin. Platelets showed normal GPs levels (Ib/IX, IIb/IIIa, Ia, VI), mildly reduced (10–50%) P‐selectin secretion and no CD63 release. P‐IF in IC showed no CD63 staining, but normal CD62 or Lamp2 (Figure 1A). WMEM demonstrated a severe dense granule defect in both sisters. DNA analysis identified the heterozygous candidate variant c.632G > C [p.Gly211Ala] in HPS6 in the siblings, but not in their unaffected half‐brother.
Conclusion(s): Platelet phenotyping and HTS are valuable for diagnosis of patients with albinism. P‐IF can be used to screen patients with albinism and mild bleeding symptoms; if CD63 staining is absent, HPS should be considered. The HPS6 missense variant c.632G > C [p.Gly211Ala] is rather frequent (MAF = 0.0052), and has therefore a higher likelihood to occur homozygously.

PB1238
Effect of constitutive activation of the calcium sensor STIM1 on hemostasis
E. García Pérez 1; E. Arias Salgado2; E. Monzón Manzano2; L. Del Pino Molina3; Y. Soto Serrano4; P. Acuña2; M. Alvarez‐Román5; M. Martín Salces2; M. Rivas Pollmar6; A. Dos Santos2; V. Jiménez Yuste7; N. Butta2
1 Hospital Universitario La Paz‐idiPaz, Madrid, Madrid, Spain; 2 Hospital Universitario La Paz‐IdiPaz, Madrid, Madrid, Spain; 3 Center for Biomedical Network Research on Rare diseases (CIBERER U767); Lymphocyte Pathophysiology in Immunodeficiencies Group, IdiPaz, Madrid, Madrid, Spain; 4 Lymphocyte Pathophysiology in Immunodeficiencies Group, IdiPaz, Madrid, Madrid, Spain; 5 Hospital Universitario La Paz, Madrid, Madrid, Spain; 6 Hospital Universitario La Paz‐Idipaz, Madrid, Madrid, Spain; 7 Hospital Universitario La Paz, Autónoma University, Madrid, Madrid, Spain
Background: Quiescent platelets maintain a low cytosolic (cyt) Ca2+ concentration that increases upon platelet activation partially due to depletion of intracellular stores. Stromal interaction molecule 1 (STIM1) is the main Ca2+ sensor on endoplasmic reticulum that regulates Ca2+ entry from the extracellular space. Stormorken syndrome is a rare autosomaldominantdisease caused by gain‐of‐function mutations that induce excessive extracellular Ca2+ entry withmild bleeding tendency.
Aims: To study effect of constitutive activation of STIM1 on hemostasis.
Methods: A16 years old male patient with Stormorken syndrome (STKP), with asplenia, myopathy, mild muscle weakness, and repeated bleeding, was studied after obtaining his signed consent. Three healthy controls were also recruited. Platelet activation markers (activity of fibrinogen receptor and surface exposure of P‐selectin) were determined in platelet rich plasma (PRP). Platelet annexin V binding to detect phosphatidylserine was assayed in washed platelets. Samples were analyzed by flow cytometry. Thrombin generation (TG) was determined by calibrated automated thrombogramin PRP adjusted to 290x109platelets/L with 1pM tissue factor (PRP reagent). TG in plasma was triggered with PRP and MP‐reagents to detect TG associated, respectively, to phospholipids and tissue factor of microparticles.
Results: Sequencing of STKP showed a previously described mutation in exon 3 of the STIM1 gene, obtaining a variant c.326A > G (p.His109Arg) in heterozygosis. Platelets from STKP were basally activated and their ability to respond to agonists was diminished, mainly affecting the activation of the fibrinogen receptor. Exposure of phosphatidylserine was higher than in controls (Table 1). TG dependant on PRP and microparticles were increased (Table 2).
Conclusion(s): Constitutive activation of STIM1 gene induced basal activation of platelets and increased surface‐phosphatidylserine which seemed to be functional for anchoring more pro‐thrombinase complex to generate thrombin. In accordance to platelet activation, more microparticles seemed to be released increasing TG. Bleeding in STKP seems to be related to an anomalous primary hemostasis and not to coagulation impairment.

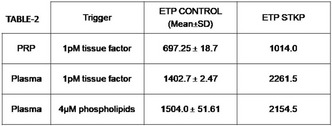
PB1247
High bleeding rates in delta‐storage pool disease
E. Van Heerwaarde 1; R. Schutgens2; A. Huisman1; I. Kremer Hovinga3
1 UMC Utrecht, Utrecht, Utrecht, Netherlands; 2 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands, 3 van Creveldkliniek, UMC Utrecht, Utrecht, Utrecht, Netherlands
Background: Δ‐Storage pool disease (δ‐SPD) is a heterogenous group of platelet function disorders caused by deficiency or reduced secretion of platelet dense granules. It is characterized by an increased mucocutaneous bleeding tendency and hemorrhage after surgery or delivery. The optimal strategy to prevent bleeding in δ‐SPD is unknown.
Aims: To evaluate the efficacy of current preventive treatment in a cohort of patients with δ‐SPD.
Methods: In a single center study, we retrospectively reviewed medical files of patients diagnosed with δ‐SPD from 2016–2021. δ‐SPD was confirmed using by determining platelet ADP concentrations. Data collection included all medical interventions around invasive diagnostic procedures, surgeries (minor and major) and deliveries (vaginal or caesarean section). Definition of bleeding complications were according to the ISTH bleeding scale criteria. Data on drug use and platelet transfusion were documented.
Results: A total of 85 procedures in 23 patients were included. Mean age of patients at inclusion was 39.8 (IQR 28–50), 15 (65.2%) were female. The average ADP measurement was 0.93 μmol/10^11 platelets (IQR 0.6–1.2). The most frequent procedures among 44 minor interventions were dental extractions (52.3%) and tonsillectomy (13.6%). Among 27 major interventions mostly abdominal (29.6%) and neurological surgery (22.2%) were documented. Bleeding occurred in 60% of the interventions in which no prophylaxis was given and in 22% of the cases in which any type of treatment was given. All bleeds were classified as non‐major. 10 vaginal deliveries and 4 caesarean sections are documented. Five postpartum bleeding events occurred in 9 cases of vaginal delivery in which patients did not receive preventive treatment. In all 4 cases of caesarean section platelet transfusions were given and no bleeding was described.
Conclusion(s): In the majority of δ‐SPD patients that received no preventive treatment strategy bleeding occurred during surgery. In addition, without prophylaxis, the incidence of postpartum hemorrhage was very high.

PB1235
Clinical and mutational spectrum of inherited thrombocytopenia related to cytoskeleton protein defects
S. Morais1; C. Monteiro2; M. Pereira3; A. Gonçalves4; M. Gonçalves5; C. Lau5; R. Santos6; E. Cruz 7
1 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto, Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 2 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, CHUPorto; Unidade Multidisciplinar de Investigação Biomédica, Instituto de Ciências Biomédicas (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 3 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 4 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 5 Unidade de Diagnóstico Hematológico Margarida Lima, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 6 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 7 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal
Background: Inherited thrombocytopenia (IT) related to cytoskeletal protein defects (CPD) caused by variants in genes encoding for components of the acto‐myosin cytoskeleton (MYH9‐RD, TUBB1‐RT, ACTN1‐RT, or DIAPH1‐RD, among others) are increasingly recognized, and altogether, are the most frequent in our cohort of patients.
Aims: To characterize the platelet phenotype and genetic defects in 26 unrelated families with CPD‐related IT, from a Portuguese unique centre.
Methods: Sixty‐two patients from 26 families from the north of Portugal (9 with TUBB1‐RT, 9 with ACTN1‐RT, 7 with MYH9‐RD and 1 with DIAPH1‐RD) were included. Platelet counts and indexes [mean platelet volume (MPV) and immature platelet fraction (IPF)] were evaluated. Patients DNA were analyzed by high‐throughput sequencing (HTS) using a 91‐gene panel related to Hemostasis, or by Sanger sequencing. Variants classification was performed according to ACMG recommendations.
Results: Globally patients had irrelevant bleedings. Twenty‐five patients from 9 families with identified variants in TUBB1, had median platelet counts 120x109/L, MPV 13.5 fL and IPF 15.2%; the variants were classified as likely‐pathogenic/pathogenic in five, variants of uncertain significance (VUS) in two, and likely‐benign/benign in other two families. Twenty‐two patients from 9 families with identified variants in ACTN1, had median platelet counts 101x109/L, MPV 14.6 fL and IPF 26.4%; the variants were classified as likely‐pathogenic/pathogenic in six families, VUS in other two families and one benign. Regarding MYH9, 14 patients from 7 families, had median platelet counts 25x109/L, MPV 23 fL and IPF 62.1%; the identified variants were classified as likely‐pathogenic/pathogenic in five families, VUS in another and one likely‐benign. The patient with DIAPH‐RD had a causal variant already known. In total, five variants were identified for the first time.
Conclusion(s): In the presence of macrothrombocytopenia without bleeding symptoms and platelet dysfunction, CPD‐related IT should be suspected. Although some platelet indexes may suggest different disorders, only genetic tests confirm the diagnosis.
PB1240
Inherited Platelet Disorders Associated With Platelet Membrane Glycoproteins Abnormalities: A Portuguese Centre Experience
P. Martinho 1; C. Pinto2; J. Cabral3; J. Azevedo2; A. Roque2; O. Lavrukhina4; C. Catarino5; F. Rodrigues6; E. Antunes7; S. Batalha8; S. Nobre Fernandes9; M. Lopes10; C. Geraldes11; T. Fidalgo12
1 Unidade Funcional de Hematologia Moleculat, Serviço de Hematologia Clínica, CHUC, Coimbra, Coimbra, Portugal; 2 Unidade Funcional de Hematologia Molecular, Serviço de Hematologia Clínica, CHUC, Coimbra, Coimbra, Portugal; 3 Serviço Imunohemoterapia, Hospital de Braga, Braga, Braga, Portugal; 4 Blood and Transfusion Medicine Department, Hemophilia Reference Centre, Centro Hospitalar e Universitário de Coimbra, Coimbra, Coimbra, Portugal; 5 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisbon, Lisboa, Portugal; 6 Serviço de Imunohemoterapia, Hospital Santa Maria, Lisboa, Lisboa, Portugal; 7 Serviço de Imunohemoterapia, Hospital São José, Lisboa, Lisboa, Portugal; 8 Serviço de Pediatria, Hospital Dona Estefania, Lisboa, Lisboa, Portugal; 9 Immunohemotherapy Service, Hospital de S. João, Porto, Porto, Portugal, 10 Serviço de Imunohemoterapia, Hospital São João, Porto, Porto, Portugal, 11 Clinical Hematology Department, Centro Hospitalar e Universitário de Coimbra, Coimbra, Coimbra, Portugal, 12 Unidade Funcional de Hematologia Molecular, Serviço de Hematologia Clínica, Coimbra, Coimbra, Portugal
Background: Inherited platelet disorders, affecting either platelet functions or platelet count are a heterogeneous group of rare inherited disorders, and a molecular diagnosis is especially relevant to proper clinical management. The most frequent molecular abnormalities in genes coding platelet membrane glycoproteins are: i) GP1BA, GP1BB and GP9 (Bernard‐Soulier Syndrome (BSS) or dominantly inherited macrothrombocytopenia (monoallelic BSS); ii) ITGA2B and ITGB3 (Glanzmann thrombasthenia (GT), GT‐like syndrome (GT‐like) and autosomal dominant macrothrombocytopenia (ATMD).
Aims: Improve the molecular diagnosis of patients with suspected glycoprotein abnormalities and/or haemorrhagic diathesis of unknown cause by next generation sequencing (NGS).
Methods: Twenty‐two unrelated patients [median age 39 years (4–70), 17F] and 10 relatives were analyzed. The molecular diagnosis was done using a custom panel for NGS (43 genes). Library preparation and sequencing was done using IonS5 protocol (Thermo Fisher Scientific).
Results: We have identified 23 different variants (pathogenic, potentially pathogenic, and unknown significance variant) in GP1BA (n = 3), GP9 (n = 3), ITGA2B (n = 8) and ITGB3 (n = 9) genes, including eight new variants. The patients diagnosed as BSS (n = 3) and GT (n = 5) were homozygous or compound heterozygous for 2 variants in GP9, 1 in GP1BA, 5 in ITGB3 and 1 in ITGA2B. These patients had more severe haemorrhagic manifestations (BS: 8–14). The remaining 14 heterozygous patients were diagnosed as GT‐like (n = 7; BS:2–11) monoallelic BSS (n = 2; BS:0–2), and ATMD (n = 5; BS:0–3). Some variants were previously reported in association with monoallelic BSS, GT‐like syndrome and ATMD (Table 1).
Conclusion(s): A phenotype–genotype correlation was observed in all patients, not only in the more severe forms (BSS and GT), but also, in the milder ones (monoallelic BSS, GT type and ATMD). Our work broadens the spectrum of variants identified in BSS and GT, underlying the importance of genetic testing in recognizing both monoallelic and biallelic forms of these disorders, allowing a better clinical care, prognosis, and preventive treatments.
https://778c1607566f28c5e8fd-e6db6de54823ad7fd298e0f6ff75b72a.ssl.cf1.rackcdn.com/ISTH_2941_MFNLWBDK_247_SubmissionID1202558_PosterUpdatedImage_April.docx. Table 1. Patients with Platelet Disorders Associated With Platelet Membrane Glycoproteins Abnormalities.
PB1244
Development of Consensus on Standardized Nomenclature for PT‐VWD‐ Joint Project from the ISTH SSCs on Platelet Physiology, VWF and Genomics in thrombosis and Hemostasis
M. Othman 1; M. Lavin2; R. Li3; A. Elsebaie1; P. Gresele4
1 Queen's University, Kingston, Ontario, Canada, 2 St James Hospital, Dublin, Dublin, Dublin, Ireland; 3 Aflac Cancer and Blood Disorders Center, Children's Healthcare of Atlanta, Department of Pediatrics, Emory University School of Medicine, Atlanta, USA, Atlanta, Georgia, United States; 4 University of Perugia, Department of Medicine and Surgery, Section of Internal and Cardiovascular Medicine, Perugia, Umbria, Italy
Background: The issue of nomenclature in PT‐VWD was discussed on several occasions. Several names were proposed: Pseudo‐VWD, PT‐VWD, ‘VWD‐mimic’ disorder, and Platelet type pseudo VWD. Of all, platelet‐type VWD has gained the widest acceptance. This name may be problematic since the diagnostic pathway of patients often starts with VWF assays, but the disorder is due to a gain‐of‐function mutation in the platelet GP1BA gene and thus the therapeutic approaches are quite different than VWD.
Aims: To re‐visit and standardize the disease’ nomenclature in consultation with the ISTH members and scientific community.
Methods: A working group was formulated under the auspice of 3 ISTH subcommittees, explored issues around nomenclature and proposed possible names with input from a panel of 15 worldwide experts. A focused 4‐questions survey proposed 8 names with justifications for each, was administered online via ISTH REDCap and emailed to ISTH members of the three SSCs between JUL‐AUG 2021. Participants were asked to rank their top three choices.
Results: We received 155 responses (VWF SSC members: 74%, PLT: 45%, Genomics: 28%). 103 (68%) respondents supported the need for name change. “Platelet GP1BA gain‐of‐function disorder” received the highest support (28%) followed by “Platelet type‐Von Willebrand disease” (24%) and “Platelet type‐pseudo‐Von Willebrand disease” (13%). Of the 43 respondents who contributed to the top choice (Figure 1), 53% were members at the VWF SSC, 25% from Platelet SSC and 22% from Genomics SSC. The new reporting system for genetic variants (starting from Met) was supported by 31%. 91% recommended the disease be discussed jointly by all three ISTH SSCs.
Conclusion(s): There is international awareness of the need for standardization of the nomenclature for PT‐VWD. The terminology “Platelet GP1BA gain‐of‐function disorder” gathers most support, and reflects the protein and gene involved and highlights the pathologic nature of the functional defect.

PB1245
Uncommon arterial and venous thrombotic complications in Bernard Soulier Syndrome and Glanzmann Thrombasthenia patients
A. Sánchez‐Fuentes1; F. García‐Candel2; A. Nicolá‐Sandoval1; M. Varo3; A. Zamora‐Canovas4; V. Palma‐Barqueros5; N. Revilla6; A. Rodríguez‐Alen7; A. Marin‐Quilez8; L. Díaz‐Ajenjo8; A. Torrecillas1; V. Vicente9; M. Lozano9; J. Rivera 10; J. Bastida11
1 Centro Regional de Hemodonación‐IMIB, Murcia, Murcia, Spain; 2 Hospital Clínica Universitario Virgen de la Arrixaca, Murcia, Murcia, Spain; 3 Hospital General Universitario de Albacete, Albacete, Castilla‐La Mancha, Spain; 4 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain, Murcia, Murcia, Spain; 5 Centro Regional de Hemodonación‐ IMIB, Mur, Murcia, Spain; 6 Hospital Universitario Ramón y Cajal, Madrid, Spain, Madrid, Madrid, Spain; 7 Hospital Virgen de la Salud, Toledo, Spain, Toledo, Castilla‐La Mancha, Spain; 8 IBSAL, CIC, IBMCC, Universidad de Salamanca‐CSIC, Salamanca, Spain, Salamanca, Castilla y Leon, Spain; 9 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer‐Centro Regional de Hemodonación, Universidad de Murcia, IMIB, CIBERER Spain, Murcia, Murcia, Spain, 10 Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, IMIB‐Arrixaca, CIBERER‐U765, Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Murcia, Murcia, Spain, 11 Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Salamanca, Castilla y Leon, Spain
Background: Bernard Soulier Syndrome (BSS) and Glanzmann Thrombasthenia (GT) patients present frequent and relevant bleeding complications due to remarkable platelet dysfunction, while thrombotic events are very rare.
Aims: To report thrombotic events and their management in two unrelated patients with BSS and GT.
Methods: We reviewed clinical records of two unrelated sibling pairs of the Spanish Project of Inherited Platelet Disorders. Cases A1 (female, 61 yr) and A2 (male, 59 yr) were diagnosedwith BSS due to the homozygous GP9 variant c.182A > G [p.Asn61Ser]. Cases B1 (female, 23 yr) and B2 (male, 16 yr) had type II GT caused by homozygous ITGA2B variant c.2113 T > C [p.Cys705Arg]).
Results: Mucocutaneous bleeding is the lifelong relevant, almost exclusive, clinical feature in A1 (ISTH‐BAT = 12) and B1 (ISTH‐BAT = 4), while less severe in A2 (ISTH‐BAT = 2) and B2 (ISTH‐BAT = 2). Case A2, at age of 58 yr, was admitted at the hospital due to angor pectoris and underwent coronary angiography revealing severe disease in right and circumflex coronary arteries. Percutaneous coronary angioplasty (PCA) was performed with implantation of five drug‐eluting stent (DES). He was discharged on standard aspirin and clopidogrel treatment. Case B1, was admitted to hospital at age 21 for dyspnea and fever. Laboratory findings showed low hemoglobin and elevated D‐Dimer. Computerized tomographic pulmonary angiography showed lingular artery thrombosis. She was withdrawn from oral contraceptives and started full dose LMWH which lasted 5 months. Thrombophilia studies (antithrombin, proteins C and S, lupus anticoagulant and cardiolipin antibodies, FV Leiden and G20210 prothrombin) were negative. Two months after the thrombotic episode she was diagnosed with uncomplicated SARS‐Cov‐2 infection.
Conclusion(s): These patients illustrate that platelet dysfunction in BSS and GT does not exclude for thrombotic complications, which may be triggered by individual genetic and environmental factors, requiring individualized antithrombotic treatment.
PB1236
Carrier detection of Glanzmann Thrombasthenia by Flow Cytometry
R. Dave; T. Geevar; J. Mammen; G. Chellaiya; R. Vijayan; A. Samuel; M. Gowri; S. Nair
Christian Medical College and Hospital, Vellore, Vellore, Tamil Nadu, India
Background: Glanzmann Thrombosthenia (GT) has autosomal recessive inheritance. Carrier detection in parents and siblings of GT patient is essential to reduce the burden of this disease. Flow cytometry can be a rapid and cost‐effective method for carrier detection.
Aims: To assess the utility of percentage and median fluorescence intensity (MFI) of CD41 expression by flow cytometry to differentiate between GT patients, carriers and healthy controls.
Methods: A total of 105 Healthy controls, 105 GT patients and 126 parents (59 Dads, 67 Moms) of GT patients were recruited after informed consent. GT was diagnosed based on a classical Light Transmission Aggregometry pattern of absent response to all the agonists (ADP, Collagen, Arachidonic acid, Epinephrine) with reversible response to ristocetin (1.5 mg/mL) further confirmed by absent CD41 expression on flow cytometry. Percentage and Median Fluorescence Intensity (MFI) of CD41 was assessed by whole‐blood flow cytometry. Kruskal‐Wallis test followed by post hoc analysis by Dunnett's Multiple Comparison was performed to compare the statistical difference between the percentage and MFI of CD41 among GT patients, carriers and controls. Receiver operating characteristic (ROC) curve was used to identify the best cut‐off for CD41 MFI to differentiate between carrier and control.
Results: Patients with GT showed absent expression of CD41 (Median MFI:0.287,IQR:0.268–0.324), while carriers had lower CD41 expression (Median MFI:20.1,IQR:17.45–23.4) as compared to controls (Median MFI:29.6,IQR:25.6–33.6). There was statistically significant difference (p < 0.0001) between the CD41 MFI of carriers compared to patients and controls (Figure 1). There was no significant difference (p = 0.821) between the CD41 percentage expression in carriers and controls (Figure 2). The CD41 MFI < 24.6 had 81.75% sensitivity and 80.95% in differentiating GT Carrier from control.
Conclusion(s): The MFI of CD41 by flow cytometry can be a cost‐effective prenatal screening tool to identify carrier status in siblings/parents of GT patients.


PB1241
Rotational thromboelastometry (ROTEM) in patients with bleeding of unknown cause: evidence for hemostatic imbalance
D. Mehic 1; B. Jilma2; C. Schörgenhofer2; M. Colling1; C. Ay3; I. Pabinger3; J. Gebhart4
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Wien, Austria; 3 Department of Medicine I, Clinical Division of Hematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 4 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: In up to 75% of patients with a mild to moderate bleeding disorder (MBD) an underlying cause cannot be found despite extensive laboratory investigations (bleeding of unknown cause, BUC). Patients with BUC do not differ in their bleeding phenotype from patients with an established MBD. Whole‐blood thromboelastometry (ROTEM) measures diverse aspects of coagulation including fibrinolysis and captures the influence of erythrocytes, leucocytes and platelets on hemostasis.
Aims: To analyze clinical factors influencing ROTEM measurements in BUC patients and to compare the hemostatic state assessed by ROTEM between patients with BUC and an established MBD.
Methods: Patients from the Vienna bleeding biobank (VIBB, EC no. 603/2009) were analyzed (Gebhart et al, Hemophilia, 2018). Viscoelastic clot measurement was performed with non‐activated ROTEM® (EM International, Munich, Germany) and the following parameters were analyzed: clotting time (CT, sec, time from analysis start until start of clot formation), clot formation time (CFT, sec, time needed to form a clot), maximum clot firmness (MCF, mm, peak amplitude of the clot) and maximal lysis (ML, %, expression of clot lysis in % of MCF).
Results: ROTEM data were available of 513/714 (71.8%) patients included in VIBB. The mean (SD) age was 41.4 (15.0) years, 425 (80.3%) were women and 252 (47.6%) patients had blood group O. In BUC patients ROTEM measurements were significantly associated with age, sex, blood group O (vs. non‐O) and the bleeding score in multivariable linear regression (Table 1). BUC patients had similar ROTEM measurements than patients with platelet function defects or von Willebrand disease, whereas patients with clotting factor deficiency had longer CT and CFT, a lower MCF and higher ML (Table 2).
Conclusion(s): Hemostatic capacity measured by ROTEM is similar in patients with BUC and those with mild primary hemostatic bleeding disorders. Our data support the hemostatic imbalance in patients with BUC.


PB1242
The phenotypic and genetic heterogeneity of Hermansky‐Pudlack Syndrome
F. Santos1; M. Coutinho1; C. Monteiro 2; M. Pereira3; R. Matos1; A. Gonçalves4; E. Cruz5; R. Santos6; S. Morais7
1 Thrombosis and Hemostasis Unit, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 2 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, CHUPorto; Unidade Multidisciplinar de Investigação Biomédica, Instituto de Ciências Biomédicas (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 3 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 4 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 5 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 6 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto, Porto, Portugal, Porto, Porto, Portugal; 7 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto, Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal
Background: Hermansky‐Pudlak syndrome (HPS) is a group of 11 autosomal recessive multisystemic disorders. All individuals exhibit oculocutaneous albinism (OCA) and a bleeding diathesis; additional features, like pulmonary fibrosis, granulomatous colitis, and immunodeficiency, can occur in some subtypes. Identification of the HPS subtype is important for prognosis and clinical management, making early molecular diagnosis important.
Aims: To assess the clinical and platelet phenotype and genetic defects in nine patients with suspected HPS, from 6 unrelated families from a Portuguese unique centre.
Methods: Nine patients from one consanguineous and five non‐consanguineous families, from the north of Portugal, were included. Bleeding was evaluated by ISTH‐BAT score (BS), patient platelets were evaluated by functional studies (lumiaggregometry and flow cytometry) and by electron microscopy (EM). Patients DNA were analyzed by high‐throughput sequencing (HTS) using a 92‐gene panel related to Hemostasis. Sequence variants classification was performed according to ACMG recommendations.
Results: All patients [3 M/6F, median age 7 years (min‐max 1–56)] had confirmed OCA and platelet disfunction (impaired platelet aggregation and ATP release, and absent/reduced dense granules by EM). The median BS was 8 (min‐max 6–11). Molecular diagnosis revealed in two non‐related and non‐consanguineous families, a nonsense variant in exon 5 of DTNBP1, and in another family, a five base pair duplication in the single exon of HPS6: these three families were previously reported (Bastida JM, Morais S et al. 2019). In the fourth family, a nonsense homozygous variant in HPS6 and in the fifth family, a nonsense variant in exon 13 and a frameshift variant in exon 2 of HPS3 in compound heterozygous, were identified. Interestingly, in a sixth family, four heterozygous missense variants in HPS1, HPS3, LYST, and SLC45A2 were identified.
Conclusion(s): The HTS technology simplified the molecular diagnosis of HPS, and at same time, underline the heterogeneously and complexity of the phenotype/genotype correlations.
PB1237
Bleeding diathesis in a girl with biallelic variants in RASGRP2 gene affecting platelet CalDAG‐GEFI function
J. Frontroth 1; M. Hepner2; K. Downes3; C. Cervio1; S. Annetta4; R. Sueldo5; B. Bianco6; G. Sciuccati7
1 Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Laboratorio de Hemostasia y Trombosis. Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Cambridge Genomics Laboratory, Cambridge University Hospital NHS Foundation Trust, Cambridge, UK, Cambridge, England, United Kingdom; 4 Laboratorio de Hemostasia y Trombosis. Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P GarrahanLaboratorio de Hemostasia y Trombosis. Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 Laboratorio de Hematología. Hospital Dr. César Milstein, Hospital de Pediatría Juan P. Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 6 Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P GarrahanLaboratorio de Hemostasia y Trombosis. Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 7 Servicio de Hematología y Oncología. Hospital de Pediatría Prof Dr Juan P Garrahan, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: The RASGRP2 gene encodes the Ca2+ and DAG‐regulated guanine nucleotide exchange factor (CalDAG‐GEFI). Variants of this gene lead to BDPLT‐18 disorders (OMIM 615888). Platelet granules release dysfunction was reported with variable responses.
Aims: To report a compound heterozygous RASGRP2 variants and phenotypic findings in a girl with severe bleeding symptoms.
Methods: Screening coagulation tests plus VWF:Ag and VWF:RCo were performed. Blood smears were analyzed by optic microscopy. Platelet glycoprotein expressions were quantified by flow cytometry. Platelet aggregation in response to ADP, collagen, arachidonic acid, epinephrine and ristocetin were investigated by LTA. ATP release was determined by Lumi‐aggregometry. Patient's DNA was analyzed by next‐generation sequencing (NGS), of known platelet function genes (Thrombo Genomics, Cambridge, UK).
Results: A five‐year‐old girl presented with severe and recurrent epistaxis requiring frequent hospitalizations and blood transfusions (ISTH‐BAT score = 7). Routine coagulation profile, VWF:Ag and VWF:RCo were normal. Blood smear examination showed large platelets with some vacuolated ones. Flow cytometry showed normal expression of αIIbβ3 and GPIb. Platelet aggregation in response to ADP, epinephrine, arachidonic acid, ristocetin and low collagen concentration was impaired. Collagen at high concentration showed a normal profile. ATP release was defective in response to all agonists assessed. A known missense variant (NM_153819.1, p.Gly305Asp) and a novel intronic splice site deletion (c.814‐16_814‐5delTGTCCTTTGTCC) in RASGRP2 were identified using NGS.
Conclusion(s): We identified compound heterozygous RASGRP2 variants affecting CalDAG‐GEFI function and leading to BDPLT‐18 disorder in a patient with severe bleeding symptoms. To our knowledge, only a few patients with BDPLT‐18 have been described so far. We could not assign the impaired ATP release to any variant tested in the NGS panel, so we hypothesized an unrevealed mechanism in which CalDAG‐GEFI dysfunction affects platelet granules release.
PB1243
ITGB3/ITGA2B‐related platelet disorders: phenotypic and genetic aspects of a series of 20 families evaluated in a single centre
C. Monteiro 1; A. Gonçalves2; M. Pereira3; M. Gonçalves4; C. Lau4; E. Cruz5; R. Santos6; S. Morais7
1 Unidade de Trombose e Hemostase, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, CHUPorto; Unidade Multidisciplinar de Investigação Biomédica, Instituto de Ciências Biomédicas (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 2 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 3 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 4 Unidade de Diagnóstico Hematológico Margarida Lima, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto; Unidade de Investigação Biomédica (UMIB/ICBAS/UP) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 5 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal, Porto, Porto, Portugal; 6 Unidade de Genética Molecular, Centro de Genética Médica Doutor Jacinto Magalhães, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal; 7 Unidade de Trombose e Hemostase, Serviço de Hematologia Clínica, Centro Hospitalar Universitário do Porto, Porto, Portugal; Unidade de Investigação Biomédica (UMIB/ICBAS) e Laboratório para a Investigação Integrativa e translacional em Saúde Populacional (ITR), Universidade do Porto, Porto, Portugal, Porto, Porto, Portugal
Background: Pathogenic variants in ITGB3 and ITGA2B, the genes coding for integrin αIIbβ3 have been linked to Glanzmann thrombasthenia (GT) and, most recently, to an inherited macrothrombocytopenia entity, designated as ITGB3/ITGA2B‐related thrombocytopenia (ITGB3/ITGA2B‐RT).
Aims: To describe 20 families with variants in either ITGA2B and ITGB3 genes linked to phenotypes of both Glanzmann Thrombasthenia and ITGA2B/ITGB3‐related thrombocytopenia.
Methods: Clinical data (bleeding scores and family history), platelet (PLT) count and morphology, and functional studies (lumi‐aggregometry, glycoprotein expression by flow cytometry and integrin activation evaluation), were assessed. Genetic analysis of ITGB3 and ITGA2B was performed by NGS gene panel and/or direct Sanger sequencing.
Results: Patients with ITGB3/ITGA2B‐RT had absent to moderate bleeding, autosomal dominant macrothrombocytopenia associated with impaired platelet aggregation/ATP release and low αIIbβ3 expression and activation. In contrast, patients with GT had an autosomal recessive disorder, with severe bleeding and PLT dysfunction (defective PLT aggregations and diminished/absent platelet αIIbβ3 expression and activation). Besides the previously reported 10 families with ITGB3/ITGA2B‐RT (Morais et al. 2020), we describe 3 new families with heterozygous variants in ITGB3/ITGA2B, in a total of 13 families and additional 2 novel variants. Furthermore, 7 families with GT, including 3 novel homozygous variants, were identified and presumed as causative. The novel variants were established as clinically relevant based on allele frequency, bioinformatics predictions, disease correlation and familiar co‐segregations analysis.
Conclusion(s): Our study details the phenotypic, functional, and genetic defects observed in ITGA2B/ITGB3‐RT and GT and expands the number of the associated αIIbβ3 variants. The high number of families with ITGA2B/ITGB3‐RT found in our Centre is probably due to a systematic screening for αIIbβ3 deficiency in patients with familial macrothrombocytopenia. The relatively high number of families with variants in ITGB3 or ITGA2B suggests that the frequency of these entities may be higher than previously thought. Future studies are necessary to proceed with variants' evaluation of pathogenicity.
PB1246
Platelet Dysfunction in Inherited Thrombocytopenias with Predisposition to Hematologic Malignancies and its Correlation with Bleeding: Analysis of 23 Patients with RUNX1, ANKRD26, and EVT6 Mutations
I. Tesakov 1; D. Fedorova1; G. Ovsyannikova1; A. Martyanov2; A. Ignatova1; E. Ponomarenko1; A. Pavlova1; E. Raykina1; P. Zharkov1; N. Smetanina1; M. Panteleev2; A. Sveshnikova1
1 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 2 Center for Theoretical Problems of Physicochemical Pharmacology, Moscow, Moskva, Russia
Background: Inherited thrombocytopenias (ITs) with predisposition to MDS/AML are commonly associated with hemorrhage.
Aims: To investigate the mechanisms of platelet dysfunction in ITs and its correlation with bleeding tendency.
Methods: Pediatric patients with ITs were included in the study. Bleeding severity was assessed with PBQ score. Aggregometry (LTA for PRP) and flow cytometry were performed to study platelet responsiveness. Healthy age‐matched children were used as controls. Data were analyzed using Mann–Whitney U and Spearman correlation tests.
Results: 23 patients with germline RUNX1 (n = 14), ANKRD26 (n = 8), and ETV6 (n = 1) mutations were included in the study. All patients had mild to moderate thrombocytopenia (Figure 1). In patients with RUNX1 and ANKRD26 mutations, we observed diminished platelet aggregation upon stimulation with epinephrine, PAR1‐AP (no secondary wave for RUNX1), collagen (only RUNX1), ADP (only ANKRD26). In patients with RUNX1 mutations, flow cytometry revealed reduced dense granule content and diminished platelet necrosis after double stimulation with CRP and PAR1‐AP. In patients with ANKRD26 and ETV6 mutations, we observed impaired GPIb shedding. Total GPIIb/IIIa density on activated platelets was increased in patients with ANKRD26 and decreased in the patient with ETV6 mutation. We observed increased quiescent platelet cytosolic calcium concentration in patients with RUNX1 and ANKRD26 mutations. Impaired calcium mobilization and diminished fibrinogen binding upon stimulation with ADP or PAR1‐AP were also observed. We found no correlation between platelet count and bleeding in patients with RUNX1 mutations. However, we observed strong correlations between PBQ score, percentage of necrotic platelets (r = −0.64, p = 0.02) and GPIb expression (r = −0.63, p = 0.02) upon stimulation.
Conclusion(s): Platelet functional abnormalities are different in patients with ITs, and significantly correlate with bleeding. These findings emphasize the role of flow cytometry in diagnosis of these rare disorders and evaluation the prognosis of hemorrhage. The study was supported by Russian Science Foundation grant 21–74‐20,087.

PB1232
Hermansky‐Pudlak syndrome: Identification of novel variants in the genes HPS3, HPS5, and DTNBP1 (HPS‐7) and analysis of lymphocyte cytotoxicity for the HPS‐7 patient
D. Boeckelmann 1; M. Wolter2; K. Neubauer2; L. Weiss3; H. Schulze4; F. Sobotta2; A. Lenz2; H. Glonnegger2; B. Käsmann‐Kellner5; J. Mann6; S. Ehl6; B. Zieger2
1 Division of Pediatric Hematology and Oncology, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 2 Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology and Oncology, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Baden‐Wurttemberg, Germany; 3 University Hospital Wuerzburg, Würzburg, Bayern, Germany; 4 Institute of Experimental Biomedicine, Chair I, University Hospital Würzburg, Würzburg, Bayern, Germany; 5 Department of Ophthalmology, Saarland University Medical Center, Homburg, Saarland, Germany; 6 Institute for Immunodeficiency, Center for Chronic Immunodeficiency (CCI), Medical Center – University of Freiburg, Faculty of Medicine, Freiburg, Baden‐Wurttemberg, Germany
Background: Hermansky‐Pudlak syndrome (HPS) is characterized by oculocutaneous albinism (OCA) and bleeding diathesis (due to a defect regarding melanosomes and platelet δ‐granule secretion). The disease‐associated genes encode either for multi‐protein complexes BLOC‐1 ‐ BLOC‐3 (biogenesis of lysosome‐related organelles complex) or for AP‐3 (adaptor protein‐3). Patients with HPS type 2 (HPS‐2) or HPS‐10 present additionally with an immunological defect. BLOC‐2 deficiencies (genes: HPS3, HPS5, HPS6) seem to have milder phenotypes especially regarding albinism.
Aims: To characterize the platelet defect of three patients (Pt.1–3) who suffer from bleeding diathesis. Only patient 3 showed apparent OCA.
Methods: Platelet count, platelet aggregometry (LTA), and flow cytometry (FC). Mepacrine analysis and whole mount‐transmission electronmicroscopy (WM‐TEM) (Pt.1, Pt.3). Panel next‐generation sequencing (NGS). Western blot analysis and analysis of lymphocyte cytotoxicity (Pt.3).
Results: Platelet aggregometry showed impaired platelet function. FC revealed a severely reduced CD63 expression (hinting to δ‐granule defect). Mepacrine uptake was profoundly impaired in the investigated patients implying lack of δ‐granules as confirmed by WM‐TEM. NGS identified a homozygous deletion of exon 6 in DTNBP1 for Pt.3. Western blot analysis confirmed the absence of the encoded protein dysbindin leading to the diagnosis HPS‐7 (BLOC‐1). Interestingly, this patient reported additionally recurrent bacterial infections. Analysis of lymphocyte cytotoxicity showed a slightly reduced NK degranulation previously documented in a more severe form in patients with HPS‐2 or HPS‐10. Pt.1 is carrier of compound heterozygous variants in the HPS3 gene (c.65C > G and c.1193G > A). A homozygous variant in HPS5 (c.760G > T) was identified in Pt.2. The novel missense variants were classified as variants of uncertain significance according to ACMG guidelines. For Pt.1, a specialized ophthalmological examination showed ocular albinism.
Conclusion(s): NGS can facilitate the process of identifying the genetic defects regarding the different HPS subtypes (even with mild clinical phenotype). Analysis of lymphocyte cytotoxicity in a patient with BLOC‐1 deficiency showed a slightly reduced NK degranulation.
VPB1251
Identification and evaluation of novel variants associated with platelet function disorders by NGS‐based high‐throughput sequencing
H. Schulze 1; A. Borst2; E. Klopocki2; O. Andres3
1 Institute of Experimental Biomedicine, Chair I, University Hospital Wuerzburg, Centre of Inherited Blood Cell Disorders, University Hospital Wuerzburg, Würzburg, Bayern, Germany; 2 Institute of Human Genetics, University of Würzburg, Würzburg, Bayern, Germany; 3 Department of Pediatrics, University Hospital Wuerzburg, Centre of Inherited Blood Cell Disorders, University Hospital Wuerzburg, Wuerzburg, Bayern, Germany
Background: Inherited platelet disorders (IPD) are classified as rare diseases. New IPD‐associated genes and variants are continuously reported to databases, but strong evidence is sometimes lacking. Coherent classification of the underlying genetic cause is of high relevance for appropriate prognosis and genetic counseling.
Aims: To diagnose patients with IPD. Clinical signs and platelet function analyses were complemented by next generation sequencing (NGS).
Methods: NGS‐based analysis using an in‐silico panel applied to whole‐exome data (Nextera XT‐DNA‐Library Kit, Illumina; Nextera‐xGen‐Exome‐Research‐Panel). The ISTH Platelet Disorder TIER1 genes (v.ISTH_2020.1) are included in our analysis. Data analysis was performed by GensearchNGS (PhenoSystems). Genetic variants were called for minor allele frequency <0.02, exon distance (±20 bp into intron), and quality (variant frequency <0.15, coverage ≥10X). Fourty‐one patients that were evaluated in 2020 (n = 28) and 2021 (n = 13) in specialized centers due to a bleeding diathesis and considered having a familial, syndromal or functionally diagnosed platelet disorder with early onset, were included.
Results: In 15 patients we detected single nucleotide variants in: ACTN1; ANKRD26; GFI1B; GP1BA; FLNA; ITGA2B; ITGB3; JAK2; NBEAL2; WASP; and THPO. According to ACMG criteria three variants were defined as pathogenic, three as likely pathogenic, and six as uncertain significance (VUS). In three cases segregation analyses allowed us to classify so far unreported variants as likely pathogenic. In the remaining six VUS, segregation analyses and functional studies were suggested and are still pending. A special constellation was detected in one patient carrying two heterozygous VUS in JAK2 and THPO, respectively. The JAK2 missense variant is inherited from the mother and the THPO missense variant from the father. These two variants in combination could contribute to the patient's phenotype, however, further characterization is still on‐going.
Conclusion(s): We identified the genetic cause of a broad range of clinically conspicuous platelet disorders by NGS with a positive testing ratio of 37%.
PB1234
Novel Pathological GATA1 Variant Leads to Defective Platelet Biogenesis via Impaired Transcriptional Regulation
K. Butov 1; Z. Kondrashova2; S. Obydennyi1; A. Ignatova3; N. Podoplelova1; M. Kurnikova1; E. Raykina3; E. Osipova1; G. Novichkova1; E. Donyush2; K. Machlus4; M. Panteleev5
1 Dmitry Rogachev Pediatric Hematology and Immunology Hospital, Moscow, Moskva, Russia; 2 Pirogov Russian National Research Medical University, Moscow, Moskva, Russia; 3 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 4 Vascular Biology Program, Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts, United States; 5 Center for Theoretical Problems of Physicochemical Pharmacology, Moscow, Moskva, Russia
Background: Mutations in GATA1 are associated with anemia and thrombocytopenia with defective platelet function and granule content. We discovered a novel uncharacterized hemizygous missense mutation in GATA1 (с.616A > C; p.Asp206His) in a 2‐year‐old male patient.
Aims: We sought to characterize patient‐derived megakaryocytes (MKs) and platelets and their function. We hypothesized that thrombocytopenia and granular defects in our patient may be due to impaired transcriptional control by the novel GATA1 variant.
Methods: MKs were cultured from patient‐derived bone marrow. Flow cytometry, confocal and transmission electron microscopy, qPCR, and next‐generation sequencing (NGS) were used to characterize patient‐derived platelets and MKs. Molecular cloning and mRNA transfection of His‐tagged GATA1 protein variants were used to reproduce mutation effects in Meg‐01 cells.
Results: NGS analysis revealed the novel hemizygous variant GATA1 с.616A > C; p.Asp206His, confirmed by Sanger sequencing. Mepacrine uptake assays revealed decreased dense granules (38% of normal) in resting platelets with impaired secretion after activation (8% of normal), although the CD62p expression level was not affected. Confocal microscopy of platelets for CD62p, vWF, LAMP2, and CD63 showed diffuse staining with no visible granules. Profound alpha‐ and dense‐ granule deficiency was directly confirmed with transmission electron microscopy. Differentiated MKs were largely unable to form proplatelets. Fibrinogen loading assays showed no significant changes in MK granule content after a 24‐hour incubation, despite a 60% decrease in NBEAL2 expression revealed by qPCR. Overexpression of mutated GATA1 in Meg‐01 also resulted in a similar decrease in NBEAL2 mRNA levels compared to overexpression of the normal variant, with a slight increase in FLI1 and Rab27b expression.
Conclusion(s): Our results reveal that a novel mutation in the GATA1 N‐terminal zinc finger impairs late MK development and platelet formation via transcriptional dysregulation of crucial MK genes. Further, we have developed a model that allows for future studies of MK development, thereby reducing the need for limited patient‐derived material.
von Willebrand Factor Biology
PB0797
Restoration of VWF in Von Willebrand Disease type 3 canine ECFCs through CRISPR‐Cas9 mediated gene editing
A. Barraclough 1; A. D'Amico2; R. Leite de Oliveira3; R. Bierings4; F. Leebeek5; J. Eikenboom6; S. De Meyer7; K. Vanhoorelbeke7; K. Fijnvandraat8; J. Voorberg9
1 Amsterdam UMC, University of Amsterdam, Emma Children's Hospital, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Sanquin Research and Landsteiner Laboratory, Amsterdam, Noord‐Holland, Netherlands; 3 CRISPR Expertise Center, Cancer Center Amsterdam, Amsterdam University Medical Cente, Amsterdam, Noord‐Brabant, Netherlands; 4 Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 5 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 7 Laboratory for Thrombosis Research, KU Leuven Campus Kulak, Kortrijk, Belgium, Kortrijk, West‐Vlaanderen, Belgium; 8 Department of Pediatric Hematology, Emma Children's Hospital, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 9 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Von Willebrand Disease (VWD) is a common and complex bleeding disorder due to the large variance in pathogenic mutation distributions between patients and families. The most severe form is type 3 which is characterized by the inability to produce Von Willebrand Factor (VWF). Robust gene editing technologies such as CRISPR‐Cas9 can provide treatment options to patients with all forms of VWF. Presented here is a previously identified type 3 canine VWD ECFC (endothelial colony forming cell) model. This model can demonstrate gene correction for VWD type 3 in‐vitro, which could be further translated to a canine in‐vivo model.
Aims: To deploy CRISPR‐Cas9 gene correction technologies to permanently correct the pathogenic homozygous G > A mutation present in VWD type 3 canine ECFCs and restore normal VWF.
Methods: Canine ECFCs previously identified as type 3 are subjected to CRISPR‐Cas9 gene correction utilizing adenine base editing through viral and non‐viral delivery; and homology directed repair (HDR) via a ribonucleoprotein. Qualitative and quantitative phenotypic confirmation of VWF produced by clonal populations of transformed cells can be monitored via VWF:Ag (antigen) and immunostaining to confirm abundance and localisation of VWF in the Weibel‐Palade‐bodies. Glycoprotein Ib binding assays (VWF:GPlbM) will determine if the produced VWF is functional.
Results: Canine ECFCs have been isolated and their phenotypes confirmed. Non‐viral delivery systems for base editing are being deployed to transfect ECFCs. For the HDR, sgRNAs are currently being tested for efficiency.
Conclusion(s): Gene editing is a powerful tool for treating patients with varying forms of VWD and can be tailored to each individual. A larger screening of the population to identify common pathogenic mutations could open gene editing as wider and more accessible treatment. This research received funding from the Netherlands Organization for Scientific Research (NWO), Domain Applied and Engineering Sciences (TTW), ‘Connecting Innovators’ Open Technology Programme, Project#18712.
PB0801
Conformational dynamics of plasma‐derived von Willebrand factor recapitulate isolated A1 domain and autoinhibitory module
E. Legan 1; M. Wilson2; N. Arce1; E. Parker2; P. Lollar2; R. Li1
1 Aflac Cancer and Blood Disorders Center, Children's Healthcare of Atlanta, Department of Pediatrics, Emory University School of Medicine, Atlanta, USA, Atlanta, Georgia, United States; 2 Emory University, Atlanta, Georgia, United States
Background: Our lab has recently provided detailed characterizations of the autoinhibitory module (AIM), the discontinuous flanking regions that cooperatively shield the A1 domain and regulate its binding to cognate platelet receptor glycoprotein (GP) Ibα. While our recent analyses have described the mechanical and biochemical properties of AIM‐A1 as an independent functional unit, the impact of the neighboring D′D3 and A2 domains on AIM dynamics remain unclear.
Aims: We aim to evaluate whether adjacent domains to the VWF A1 domain impact protection of the GPIbα binding site through further stabilization of the autoinhibitory module.
Methods: Conformational dynamics of purified plasma‐derived VWF were compared to purified mammalian‐derived protein fragments with the A1 domain using hydrogen‐deuterium exchange mass spectrometry.
Results: AIM‐A1 has slightly faster rates of HDX in both regions of the AIM compared to longer VWF fragments and pdVWF as well as the secondary GPIbα binding site proximal to residue Arg1341, which we have previously shown to form a complex with the N‐terminal AIM through X‐ray crystallography. Additionally, we observe similar HDX rates and relative fractional uptake of deuterium in the secondary GPIbα binding sites, suggesting that additional protection of the GPIbα binding sites is not conferred through the D'D3 or A2 domains.
Conclusion(s): These data suggest that adjacent D'D3 and A2 domains marginally alter the conformational dynamics of the AIM in solution, likely by constraining flexibility of the module. Nonetheless, regulation of AIM function appears to be primarily influenced by local AIM‐A1 dynamics.
PB0798
Evaluation of thrombin generation capacity of plasma‐derived Factor VIII/von Willebrand Factor in the Treatment of von Willebrand Disease
P. Lafuente1; M. Bravo 2; M. Costa2; T. Barnett3; T. Willis3
1 Grifols, Los Angeles, California, United States; 2 Grifols, Parets del Vallès, Catalonia, Spain; 3 Grifols, Research Triangle Park, North Carolina, United States
Background: The efficacy and safety of plasma‐derived Factor VIII/von Willebrand Factor (pdFVIII/VWF) concentrates for the treatment of von Willebrand Disease (VWD) are supported by several clinical and real‐world evidence studies. However, some concerns still remain related to a possible higher thrombotic risk due to secondary FVIII elevation.
Aims: To evaluate the in vitro procoagulant effect of two pdFVIII/VWF concentrates with different FVIII content (Fanhdi® and Humate‐P®) in plasma from VWD patients by thrombin generation (TG) assay.
Methods: Plasma samples from patients with VWD type 1 (VWD1); 2A (VWD2A); 2B (VWD2B) and 3 (VWD3) (George King Bio‐Medical, Inc.) were spiked with increasing concentrations of pdFVIII/VWF concentrates (25 to 200 FVIII IU/dL). Samples were analyzed by TG assay (Technothrombin® TGA, Technoclone), using RC High reagent and measuring the thrombin peak (TP); TP normal range: mean (SD) 597.0 (225.7) nM.
Results: TP levels increased dose‐dependently with increasing FVIII concentrations and were similar between Fanhdi® and Humate‐P® (Figure 1). VWD plasmas with endogenous FVIII (VWD1, VWD2A and VWD2B) needed lower doses of pdFVIII/VWF concentrate to reach normal TP range, while in FVIII‐deficient samples (VWD3), TP was restored within the normal range at a higher dose (200 IU/dL). The highest TP was observed with endogenous FVIII (VWD1) samples spiked with Fanhdi® (584.6 [55] nM) and Humate® (586 [79.7] nM) at the highest FVIII dose, although TP levels were within normal range. FVIII estimated activity for each condition before and after Fanhdi® and Humate‐P® is shown in Table 1.
Conclusion(s): Thrombin generation was dependent on FVIII activity in plasma samples of VWD patients. At supra‐therapeutic VWF:RCo doses (>150 IU/dL), the procoagulant effect of pdFVIII/VWF concentrates did not exceed TG normal range in VWD plasma. These results validated the safety recommendation of not exceeding hemostatic FVIII activity (150 IU/dL) and suggested a low thrombogenicity risk of pdFVIII/VWF concentrates in VWD.


PB0802
The VWF Propeptide Is A Novel Component Of Venous Thrombi In A Mouse Model Of DVT
O. Rawley 1; C. Dwyer1; K. Nesbitt1; C. Notley1; A. Michels1; D. Lillicrap2
1 Queen's University, Kingston, Ontario, Canada; 2 Department of Pathology and Molecular Medicine, Queen's University, Kingston, Ontario, Canada
Background: The essential roles of the von Willebrand Factor propeptide (VWFpp) in mediating VWF multimerization and trafficking to Weibel‐Palade are well documented. However, whether plasma VWFpp exerts haemostatic activity or contributes to thrombus formation has not been determined.
Aims: To investigate whether VWFpp contributes to venous thrombus formation in vivo.
Methods: The inferior vena cava (IVC) stenosis model of deep vein thrombosis (DVT) was used. Anesthetized male normal or VWF−/− mice on a C57Bl/6 background underwent a midline laparotomy to expose the IVC. The vessel was ligated around a blunted 30‐gauge needle which was subsequently removed to create a 90% stenosis. Mice were recovered for 24 hours at which point the IVC was removed. Thrombi were dissected from the IVC wall and were formalin‐fixed, paraffin‐embedded. Longitudinal 5 μm sections were prepared and stained. Images were captured using an Aperio ScanScope CS slide scanner and image analysis was performed in ImageJ. Additional experiments involving VWFpp overexpression were carried out via hydrodynamic injection of murine VWFpp cDNA in normal mice one week prior to IVC stenosis.
Results: Thrombus development was observed in 70% of normal C57Bl/6 mice with a mean thrombus weight of 14.8 ± 1.4 mg versus an incidence of 30% in VWF−/− mice with a mean thrombus weight of 6.9 ± 0.3 mg. Immunofluorescent analysis carried out using a polyclonal anti‐VWFpp antibody revealed distinct VWFpp staining in normal thrombi compared with VWF−/− thrombi. Fluorescent VWFpp staining partially co‐localized with VWF staining. Dense VWFpp staining was observed at the thrombus tail, the region of active thrombus growth and resolution. Comparative analysis with H&E‐stained sections also showed concentration of VWFpp in white thrombus regions. Subsequent overexpression of VWFpp had no effect on the incidence of thrombus formation, thrombus size or amount of VWFpp incorporated into the thrombus.
Conclusion(s): VWFpp constitutes a novel component of venous thrombus architecture in a mouse model of DVT.
PB0804
A new type 1 von Willebrand Disease (VWD) characterized by increased clearance of von Willebrand Factor (VWF) due to the heterozygous p.P1127S mutation: clinical phenotype and pathogenic mechanisms
M. Tardugno 1; M. Sacco1; R. De Cristofaro1; S. Lancellotti2; F. Bernardi3; M. Pinotti3; A. Branchini4; B. Giusti5; G. Castaman6
1 Catholic University of Sacred Heart, Translational Medicine and Surgery Department, Rome, Lazio, Italy; 2 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Rome, Lazio, Italy; 3 Department of Life Sciences and Biotechnology, University of Ferrara, Italy., Ferrara, Emilia‐Romagna, Italy; 4 Department of Life Sciences and Biotechnology, University of Ferrara, Ferrara, Italy, Ferrara, Emilia‐Romagna, Italy; 5 University of Florence, Department of Clinical and Applied Medicine, Florence, Toscana, Italy, 6 “Careggi” University Hospital, Hemorrhagic and Coagulation Disease Unit, Department of Oncology., Florence, Toscana, Italy
Background: A 21‐year‐old Italian woman with a large thigh hematoma, appeared after a minor trauma, showed a proportional decrease of both VWF antigen (VWF:Ag = 34.3 IU/dL) and activity (VWF:GpIbR = 32.8 IU/dL), and low levels of factor VIII (FVIII:C = 55.3 IU/dL). She inherited, from her 51‐year‐old mother (VWF:Ag ≈ 60 IU/dL), a new VWF heterozygous missense mutation c.C3379 > T in the exon 25, causing the p.P1127S substitution in the D'D3 domain. Both patients signed an informed consent.
Aims: Because of VWD type 1 phenotype is extremely heterogeneous, low VWF levels are caused by different molecular mechanisms: alteration of secretion; intracellular retention; accelerated plasmatic clearance. This study focused on clinical/biochemical characterization of this new VWF gene mutation.
Methods: VWF:Ag and VWF:GpIbR were measured by chemiluminescence assays, FVIII:C by chromogenic assay; the VWF‐FVIII binding (VWF:VIIIB) and pro‐peptide levels (VWF:pp) by ELISA assays; ADAMTS‐13:activity by FRET‐based‐assay; the VWF multimeric pattern (mVWF) by SDS‐agarose‐gel electrophoresis; ristocetin‐induced platelet aggregation by Born‐assay. Molecular modeling was performed by using I‐TASSER software. Recombinant expression of VWF WT and p.P1127S‐mutant variants was obtained by HEK‐293 cells, while their interaction with the low‐density lipoprotein receptor‐related protein‐1 (LRP1) was analyzed by fluorescence assay.
Results: The heterozygous p.P1127S mutation was clinically associated with a proportional mild decrease of both VWF:Ag and VWF:activity. Although 0.3 μg/Kg‐BW desmopressin infusion normalized the patient's VWF levels, the drug clearance resulted enhanced (t1/2 = 6.7 h) than in normal subjects (t1/2 = 12 ± 0.7 h), (Figure 1). The VWF:VIIIB and ADAMTS‐13:activity were normal, as well as the ristocetin‐induced platelet aggregation and the mVWF pattern. The p.P1127S‐mutant variant was normally synthesized and secreted by HEK‐293 cells. Molecular modeling predicted p.P1127S‐mutant conformational changes, showing an increased binding affinity for the VWF scavenging‐receptor LRP1 (Figure 2), confirmed by in vitro studies.
Conclusion(s): The VWF p.P1127S mutation causes conformational rearrangements, due to multiple missing van der Waals contacts, accelerating the VWF clearance, but not its synthesis and secretion.


PB1433
Analysis of uncharacterised Von Willebrand's Disease causing mutations located in the D4 and C‐domains: identification of a novel type 2A variant and gain‐of‐function VWF
G. Mobayen 1; M. Appiah2; M. Laffan3; T. McKinnon4
1 Imperial College, London, England, United Kingdom; 2 Imperial College London, london, England, United Kingdom; 3 Centre for Hematology, Imperial College London, London, England, United Kingdom; 4 Imperial College London, London, England, United Kingdom
Background: Von Willebrand's disease is a common autosomal inherited bleeding disorder characterized by a quantitative (type 1 and type 3) or qualitative (type 2) deficiency of Von Willebrand Factor (VWF). While most of the repeating domains in the VWF molecule have ascribed roles, the functional role of the D4 and C‐domains remain poorly understood, although we have previously shown that deletion of various C‐domain regions abolishes VWF secretion. Analysis of the VWD database highlighted several putative type 1 and type 3 mutations in the D4 and C‐domains that are yet to be characterized.
Aims: To characterize the impact of ten D4 and C‐domains VWD mutations; C1950Y, G2044D, C2174G, E2233Q, R2287W, C2340R, G2343V, R2379C, S2497P and C2693Y.
Methods: Mutations generated by site‐directed mutagenesis were expressed in HEK293/T cells alone or alongside wild‐type (wt) VWF or VWF lacking the A1 domain. Expression was analyzed by ELISA and multimer gels and cellular retention investigated by endo H digestion and immunofluorescence staining. Flow assays were performed to determine protein function.
Results: Homozygous transfections resulted in abolished or reduced secretion for all mutants which was rescued by co‐transfection with wtVWF; however, for mutations C1950Y, C2174G, E2233Q, C2340R and R2379C the mutant monomer was not expressed. Endo‐H digests confirmed endoplasmic‐reticulum processing and immunofluorescent imaging showed most mutants formed pseudo Weibel‐Palade bodies, albeit to a lesser extent than wtVWF. The R2379C mutation was shown to prevent dimer and subsequent multimer formation and accordingly could not support platelet capture under shear stress. Interestingly, although the R2287W mutation showed significantly reduced expression, its platelet capture function was enhanced under shear stress indicating gain‐of‐function activity.
Conclusion(s): Mutations in the D4 and C‐domain affect VWF secretion indicating a critical role for the D4 and C‐domains for correct VWF folding and multimer formation, however mutations in this region can also enhance function under flow conditions.
PB0803
The role of VWF‐ADAMTS13 axis in cisplatin‐induced acute kidney injury in mice
S. Senzaki 1; A. Kakiwaki2; A. Sawa2; N. Nishimura2; R. Yoneima2; H. Kawashima2; S. Ono2; K. Yoshimoto2; A. Sakata3; K. Tatsumi4; M. Sugimoto2; M. Shima5; K. Nishio2
1 Department of General Medicine/Nara Medical University, Kashiharashi, Nara, Japan; 2 Department of General Medicine/Nara Medical University, Kashihara, Nara, Japan; 3 Medicinal biology of thrombosis and hemostasis/Nara Medical University, Kashihara, Nara, Japan; 4 Advanced Medical Science of Thrombosis and Hemostasis/Nara Medical University, Kashihara, Nara, Japan; 5 Nara Medical University, Kashihara, Nara, Japan
Background: Cisplatin (CDDP) is known as an effective anticancer agent. However, approximately 20% of CDDP‐treated patients develop acute kidney injury (AKI), the mechanisms of which are assumed to direct injury of the kidney epithelial cells and subsequent inflammation and vascular endothelial injury.
Aims: We hypothesized that CDDP‐induced AKI are exacerbated by the activation of VWF‐platelet axis and ameliorated by VWF‐cleaving enzyme, ADAMTS13.
Methods: After 6 hours fasting, CDDP were intraperitoneally administered to male wild‐type (WT) and ADAMTS13‐knockout (KO) mice (C57BL/6 background) at the dose of 20 mg/kg body weight. Five days after the administration, vital signs (blood pressure; BP, heart rate; HR) and renal blood flow were measured, then blood and kidney samples were collected. Blood cell counts and biochemical analysis were performed using the blood samples, and histological analysis and gene expression analysis by qPCR were conducted using the kidney samples.
Results: Significant decreases in body weight, renal blood flow, and the number of white blood cells, coupled with significant increases in serum levels of blood urea nitrogen (BUN) and creatinine (Cre) were observed after CDDP administration. No significant changes in BP and HR were observed. Notably, the increases of serum BUN and Cre in KO mice were significantly higher than those in WT mice (BUN; 110.7 vs 279.4 mg/dL, Cre; 0.61 vs 1.82 mg/dL, for WT and KO, respectively). Furthermore, gene expression levels of AKI markers, such as KIM‐1 and NGAL, were significantly upregulated by CDDP administration in KO mice more than those in WT mice.
Conclusion(s): We clarified that ADAMTS13 deficiency exacerbates CDDP‐induced AKI in a mouse model. Modulation of plasma levels of ADAMTS13 (i.e. administration of recombinant ADAMTS13) may be a novel treatment option towards drug‐induced AKI.
PB0799
Multi‐scale modeling of SIPA showing VWF agglomeration and capture of platelets with high shear
D. Ku1; Z. Lu2; C. Bresette 3; C. Aidun4
1 Georgia Institute of Technology, Decatur, Georgia, United States; 2 Brown University, Providence, Rhode Island, United States; 3 Georgia Institute of Technology, Atlanta, Georgia, United States; 4 Georgia Tech, Atlanta, Georgia, United States
Background: Pathologically high shear rate conditions can allow platelets to aggregate on collagen or VWF surfaces. This process is difficult to visualize experimentally with concurrent molecular‐ and cellular‐resolutions.
Aims: Demonstrate physics of agglomeration and capture in first 10 ms.
Methods: We created a multi‐scale computational model of VWF protein folding, the non‐equilibrium molecular kinetics of A1‐GPIb, and high shear rate convection to delineate the flow‐mediated biophysics of VWF and platelets assembly into mural micro‐thrombi (Figure 1). The in silico predictions are compared to experimental observations of SIPA in an in vitro microfluidic chamber.
Results: We show that high shear initially creates VWF elongation. The VWF then wraps around platelets passing the strings to cause a local agglomeration in the flow. The soluble VWF entanglement occurs before mural capture of the agglomerate by immobilized VWF. Increasing soluble VWF concentration by ~20x in silico leads to a 2 ~ 3x increase in SIPA rates, matching the increase in occlusion rates found in vitro. The morphology of mural aggregates is primarily controlled by VWF molecular weight (length), where normal‐length VWF leads to cluster or elongated aggregates and ultra‐long VWF leads to loose aggregates seen by others' experiments. The entire SIPA process occurs on the order of 10 ms with the agglomerate traveling a lag distance of a few hundred microns before capture, matching in vitro results. Finally, we present phase diagrams of SIPA which provides biomechanistic rationales for a variety of thrombotic and hemostatic events in terms of platelet agglomeration and capture (Figure 2).
Conclusion(s): The model captures the early biophysics of SIPA under pathologically high shear rates to provide a mechanistic explanation for rapid platelet accumulation and VWF‐related thrombotic pathologies. sVWF tentacles reach out to collect platelets in the flow while iVWF tentacles capture the agglomerates in 10 ms.

PB0800
Quantitative von Willebrand factor multimer analysis in von Willebrand disease patients enrolled in the Zimmerman Program study
H. Clift 1; P. Christopherson2; V. Flood3; K. Friedman4; R. Montgomery5; S. Haberichter5
1 Versiti Wisconsin, Wauwatosa, Wisconsin, United States; 2 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 3 Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 4 Versiti/Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 5 Versiti Blood Center of Wisconsin, Milwaukee, Wisconsin, United States
Background: The diagnosis of von Willebrand disease (VWD) requires many assays including VWF multimer structure analysis. Accurate VWD subtype classification relies on a correct assessment of these multimers and their molecular weight distribution.
Aims: To assess VWF multimer structure in patients with VWD using a precise, quantitative multimer assay.
Methods: VWF multimers were analyzed with lithium dodecyl sulfate (LiDS) agarose gels and subsequent commercial VWF polyclonal antibody western blots for healthy controls and VWD subjects (index cases and family members with well‐defined genotypes and phenotypes) recruited through the Zimmerman Program. Densitometry was performed and area‐under‐the‐curve (AUC) calculated using Multi Gauge software. Percentage of low molecular weight (LMW, bands 1–5), intermediate molecular weight (IMW, bands 6–10) and high molecular weight (HWM) multimers (bands >10) was calculated for each sample.
Results: We defined multimer distribution ranges for healthy individuals and for each VWD subtype. Subjects with low VWF and type 2 N VWD displayed normal multimers. Although type 1 and 2 M VWD subjects had essentially normal multimers, there was often a subtle shift from HMW to LMW species (HMW AUC of 20%). Most type 1C subjects showed a more pronounced shift (HMW AUC of 15%). Type 2A and 2B VWD subjects demonstrated a loss of HMW multimers as expected (HMW AUC of 6–7%). Additionally, we correlated recurrent sequence variants (SVs), and their positions within VWF, with abnormal multimers in type 1 and 2 VWD (Table 1). Sixteen SVs spread across the D'D3, A1, and A2 domains coincided with abnormal multimers.
Conclusion(s): Using a detailed quantitative VWF multimer analysis method we were able to detect subtle distribution differences, as in type 1C VWD, as well as clear differences like those seen in type 2A/2B VWD. Additionally, we correlated causative SVs with abnormal multimers, paving the way for a more in‐depth analysis of these genetic variants.

PB0796
Characterization of 17 Novel Von Willebrand Factor Missense Mutations
M. Appiah 1; A. Shaida2; G. Mobayen2; T. McKinnon3
1 Imperial College London, london, England, United Kingdom; 2 Imperial College, London, England, United Kingdom; 3 Imperial College London, London, England, United Kingdom
Background: Von Willebrand factor (VWF) is a large multimeric glycoprotein that plays an important role in hemostasis. Qualitative or quantitative abnormalities in plasma derived VWF is resultant in Von Willebrand disease (VWD). Previous studies have established a strong association between missense mutations found in the heterogeneous protein and disease‐causing mechanisms. A recent cross‐sectional familial VWD study (the BRNO‐VWD study) identified 17 unrecognized missense mutations in suspected VWD patients in the South Moravian population.
Aims: To investigate the impact of 17 unrecognized missense mutations on VWF expression and function.
Methods: Site directed mutagenesis was used to create 17‐novel point mutations (R5K, G39K, D47V, E197K, V343M, A631R, C996S, C1031S, A1250D, E1292D, E1615D, D1691E, T1728S, R1830C, G1890E, T2023A, E2353K) in the pcDNA‐FL‐VWF expression vector that encodes for full length VWF. Recombinant VWF (rVWF) was expressed in HEK293T cells and VWF ELISA for quantification of protein levels in the media and cell lysate. The physiological function of rVWF was evaluated using static and shear‐based assays.
Results: The mutations G39R, D47V, A631T, C996S, G1890E all virtually abolished secretion of VWF, while mutations E1615D and E2353K reduced secretion. Interestingly co‐transfection with wtVWF rescued expression to varying extents and mutant monomers could be incorporated into multimers and secreted. Endo H and immunofluoresce staining demonstrated retention of mutant VWF within the ER. Significantly, none of the secreted mutations had abnormal function under shear stress, despite the T1728S and R1830C mutations being located in the A3 domain which contains the major collagen binding site. While the E1615D mutation in the A2 domain was cleaved faster by ADAMTS13 indicative of VWD type 2A.
Conclusion(s): The 17 novel mutations have varying effects on the VWF molecule and demonstrate the heterogenicity of VWD.
PB0351
Blood transfusion maintains endothelial cell quiescence ‐ implications in the prevention of stroke in pediatric sickle cell anemia
H. Fogarty 1; S. Ward2; E. Karampini2; S. Elliott3; A. Rehill2; A. Ahmad2; J. Velasquez2; R. Geoghegan4; H. Conroy4; M. Byrne5; U. Budde6; E. Tuohy7; C. McMahon4; J. O'Donnell2
1 Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 3 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Carlow, Ireland; 4 Department of Hematology, Children's Health Ireland (CHI) at Crumlin, Dublin, Ireland, Dublin, Dublin, Ireland; 5 National Coagulation Centre, St James's Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 6 Medilys Laborgesellschaft mbH, Department of Hämostaseology, Hamburg, Germany, Hamburg, Hamburg, Germany; 7 Department of Hematology, St James's Hospital, Dublin, Ireland, Dublin, Dublin, Ireland
Background: Sickle cell anemia (SCA) is characterized by chronic haemolysis, hypercoagulability and thrombosis, including stroke in 10% of children. The mainstays of treatment are hydroxyurea (HU) and chronic blood transfusion (BT). However, the biological mechanisms through which these therapies down‐regulate thrombotic risk remain poorly understood.
Aims: We hypothesized that HU and BT may attenuate SCA‐induced endothelial cell (EC) activation, coagulation activation and thereby mitigate thrombotic risk.
Methods: Following ethical approval and informed consent, 180 SCA children were recruited. Plasma samples were collected during steady‐state and tested for a range of Weibel Palade body (WPB) markers, including VWF:Ag, VWF multimers, VWF propeptide (VWFpp), FVIII:C, Angiopoeitin‐2 (Ang‐2) and Osteoprotegerin (OPG). In addition, plasma ADAMTS‐13 and thrombin generation were assessed.
Results: Plasma VWF:Ag, VWF:CB and VWFpp were all significantly reduced in the BT‐treated children (n = 84) compared to the HU‐treated cohort (n = 96) (Figure 1). In keeping with the concept of lower EC activation, plasma levels of other WPB biomarkers (Ang‐2 and OPG) were also significantly decreased in the BT group (Figure 1). While ADAMTS13 activity did not differ between the cohorts, abnormal circulating VWF multimers were observed in the HU‐treated but not in the BT children (Figure 1). The VWF/ADAMTS13 ratio was also significantly increased. Finally, markers of coagulation activation, including FVIII:C levels, endogenous thrombin potential (ETP) and peak thrombin were also significantly reduced in the BT cohort (Figure 1).
Conclusion(s): Although BT is recognized as the treatment of choice for children with severe SCA, the biological mechanisms underpinning its efficacy remain poorly understood. This is the first large study to directly address the effects of BT on SCA endotheliopathy and coagulopathy. Importantly, our novel data demonstrate that BT plays a critical role in maintaining normal EC quiescence and thus thrombin generation in SCA children.

VWF and von Willebrand Factor Disorders ‐ Clinical Conditions
VPB0844
Сompliance with Therapy in Patients with von Willebrand's Disease in Russia
N. Zozulya 1; N. Andreev1; O. Belyakova2; A. Korobkin3; K. Farizova3; E. Noskova4; N. Beksheneva5; E. Ursulenko6; J. Hammerschmidt7; J. Rozhdestvenskaya8; V. Sibirtseva9; E. Komartseva10; E. Oganesyan11; Y. Nered'ko12; L. Romanenko12; O. Leshina13; O. Serdyuk14; K. Elgakaeva15; Z. Yasakova15; O. Yastrubinetskaya16
1 National Research Center for Hematology, Moscow, Moskva, Russia; 2 Morozovskaya Children's Hospital of the Department of Health of Moscow, Moscow, Moskva, Russia; 3 State Budget‐Funded Health Care Institution Chelyabinsk Regional Clinical Hospital, Chelyabinsk, Sverdlovsk, Russia; 4 Kirov Regional Clinical Hospital, Kirov, Russia, Kirov, Kirov, Russia; 5 Children's Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, Kazan, Russia, Kazan, Tatarstan, Russia; 6 Irkutsk State Regional Children's Clinical Hospital, Irkutsk, Russia, Irkutsk, Irkutsk, Russia; 7 Tomsk Regional Clinical Hospital, Tomsk, Russia, Tomsk, Tomsk, Russia; 8 Kemerovo City Children's Cliinical Hospital №7, Kemerovo, Russia, Kemerovo, Kemerovo, Russia; 9 State Novosibirsk Regional Clinical Hospital, Novosibirsk, Russia, Novosibirsk, Novosibirsk, Russia, 10 Rostov Regional Clinical Hospital, Rostov‐on‐Don, Russia, Rostov, Rostov, Russia, 11 Stavropol Regional Clinical Oncological Dispensary, Stavropol, Russia, Stavropol’, Stavropol’, Russia, 12 Regional children clinical Hospital, Stavropol, Russia, Stavropol’, Stavropol’, Russia, 13 Volgograd Regional Clinical Hospital №1, Volgograd, Russia, Volgograd, Volgograd, Russia, 14 Clinical Oncological dispensary №1, Krasnodar, Russia, Krasnodar, Rostov, Russia, 15 Republican Children's Clinical Hospital named after E.P. Glinka, Grozny, Russia, Grozniy, Karachay‐Cherkess, Russia, 16 National Research Center for Hematology, Moscow, Russia, Moscow, Moskva, Russia
Background: von Willebrand's disease (VWD) is characterized by a significantly lower number of life‐threatening bleeding and a slight decrease in the quality of life. This imposes a number of features on compliance. To explore the situation in Russia, the Russian National Research Center for Hematology initiated this research in several regions.
Aims: To identify issues of VWD disease treatment that can be improved to preserve the quality of life and improve compliance.
Methods: Interviewing patients with VWD by phone or in person at the clinic. The questions included information on social status, anamnestic information on diagnosis, effectiveness and convenience of substitution therapy.
Results: 18 centers for hematology participated in the study of total. 277 patients took a part in it: 116 male, 158 female and 3 did not answer the question. The average age of respondents is 27.6 ± 18.0 y.o. Most respondents were diagnosed more than 5 years ago (58.8%), 3‐5y.a. was 17.3%, 1–3y.a. was 17.0%, less than 1 year ago 6.9%. 32.9% of respondents do not remember their type of VWD, 36.5% have 1 type, 18.8% have 2 type and 11.9% have the 3 type of disease. Most of the respondents work or study 69.0%. The 2/3 of responders (66.5%) were hospitalized due to bleeding in the last 3 years. Only half of them (52.2%) had therapy corrected after. 46.2% of respondents bleedings are reduces the quality of life. 15.9% follow only half of the doctor's recommendations and 3.4% do not follow them at all. Completely satisfied with the therapy are 53.1% of the respondents. However, 8.7% consider the therapy to be insufficiently effective.
Conclusion(s): The survey identified a need for work aimed at increasing patients' adherence to therapy, educate patients about intravenous injections. Communication between the patient and the doctor and timely correction of therapy could improve compliance as well.
VPB0841
A case of hematidrosis complicated with von Willebrand disease
M. Takeyama 1; S. Furukawa2; K. Ogiwara2; K. Nogami1
1 Nara Medical University, Kashihara, Nara, Japan; 2 Department of Pediatrics, Nara Medical University, Kashihara, Nara, Japan
Background: Hematidrosis is extremely rare disease characterized by bloody sweating from non‐traumatized skin and mucous membranes. We report 14‐year‐old girl of hematidrosis complicated with von Willebrand disease (VWD).
Aims: To diagnose whether bleeding symptoms are due to VWD or hematidrosis.
Methods: We showed her clinical course and analyzed her blood samples.
Results: She experienced recurrent episodes of redness of eyelids and oral bleedings for one year. Her laboratory data revealed low activity of von Willebrand factor (VWF:RCo) (29 IU/dL), and low VWF antigen (46 IU/dL). The VWF multimer analysis showed all multimers were present but all were in reduced concentration. She was, therefore, diagnosed with VWD type 1. However, she experienced recurrent history of bleedings from the intact skin over the palm, face, forearms, feet, nose and mouse with no evidence of trauma at the bleeding sites, resulting in a diagnosis of hematidrosis complicated with VWD. The episodes usually happed before going to junior high school in the morning and no bleeding symptoms were seen during the holidays. Each episode lasted several hours and was usually self‐limited. DDAVP (1‐deamino‐8‐D‐arginine vasopressin) was administered as a treatment for the bleedings, but no apparent effect was observed although VWF:RCo was elevated after administration of DDAVP. Comprehensive coagulation function analysis using rotational thromboelastometry (ROTEM), total thrombus‐formation analysis system (T‐TAS), and Multiplate were performed to evaluate her coagulation function and showed the almost normal range. Oral propranolol was commenced because the drug is considered effective for hematidrosis. No immediate effects were, however, observed after propranolol administration. She was referred to psychiatrist and her psychiatric disorders at school was revealed. The frequency of bleeding decreased remarkably after graduation form junior high school.
Conclusion(s): Her bleeding symptom was mainly because of not VWD but hematidrosis according to the data of comprehensive coagulation analysis and her clinical course.
VPB0839
Diagnostic Errors Of Von Willebrand Disease In A Developing Country: Analysis Of 38 Years Data
L. Hambardzumyan; N. Martirosyan1; A. Movsisyan2; M. Badikyan3; N. Sargsyan4; G. Tamamyan5; A. Ter‐Grigoryan6
1 Hematology Center after Prof. R. H. Yeolyan, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 2 Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Department of Pediatric Oncology and Hematology, Yerevan State Medical University, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 3 Hematology Center after Prof. R.H. Yeolyan, Yerevan, Yerevan, Armenia; 4 Hematology center after prof. R.H.Yeloyan Armenian Hemophilia and Thrombosis Center Yerevan State Medical University, Department of Pediatric Oncology and Hematology, Yerevan, Yerevan, Armenia; 5 Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R.H. Yeolyan, Yerevan, Armenia, Department of Pediatric Oncology and Hematology, Yerevan State Medical University, Yerevan, Armenia, Yerevan, Yerevan, Armenia; 6 Armenian Hemophilia and Thrombosis Center Yerevan State Medical University, Department of Pediatric Oncology and Hematology, Yerevan, Yerevan, Armenia
Background: Von Willebrand disease (VWD) is the most common inherited bleeding disorder. The incidence of symptomatic cases is 1 in 10,000 to 1 in 100,000.
Aims: The aim of our study is to evaluate the diagnostic workup and management of VWD in Armenia.
Methods: The medical records of all patients with VWD being managed in the only hematology center in Armenia (Hematology Center after Prof. R.H.Yeolyan), were retrospectively reviewed and analyzed.
Results: Since 1984 VWD was diagnosed in 32 patients (~1 in 100,000 of Armenia population) with the median age of 30 and mean age of 31.2 years. Four of them were falsely diagnosed with and managed as platelet‐type VWD by immunophenotyping. Sixteen patients were female (50%). Six (35.3%) of 17 available blood typing testes were O+. Two patients had no bleeding history, easy bruising and mucocutaneous bleeding occurred in 30 patients (93.8%), 10 (31.3%) of them had also joint, soft tissue bleeding, 2 had gastrointestinal and 7 (43.8% of females) had heavy menstrual bleeding. Anemia developed in 43.8% of cases. Fourteen patients (43.8%) had family history of bleeding. Von Willebrand factor antigen and functional assays (VWF:Ag and/or VWF:Act) were performed in 17 patients: 6 were < 30%, 1 was 30–50% and 10 were > 50% (3 of them had decreased FVIII/VWF:Ag), multimers were not assessed. FVIII activity was measured in 24 and was decreased in 14 (58.3%) cases. Automated analyzer revealed thrombocytopenia in 9 patients and blood film morphology in 3 of them. Nineteen (59,4%) patients had prolonged APTT. Antifibrinolytics were used in all bleeding episodes, after major bleeding 13 patients received plasma‐derived VWF concentrate, 3 patients FVIII, 1 patient desmopressin acetate.
Conclusion(s): VWD screening tests aren't constantly available and completely performed in Armenia so there is a high probability of misdiagnosis. Further efforts are needed for rediagnosis, subtype classifying and differential diagnosis.
PB0813
Performance Evaluation of a VWF Antigen Assay in a Multicenter Study
C. Gunnesch 1; M. Boehm‐Weigert1; R. Barten1; R. Biddle2; C. Eby3; R. Francis4; S. Pipe5; A. Siegemund6
1 Siemens Healthineers, Marburg, Hessen, Germany; 2 Siemens Healthineers, Glasgow, Delaware, United States; 3 Washington University, Saint Louis, Missouri, United States; 4 New York Presbyterian Hospital‐Columbia University, New York, New York, United States; 5 Departments of Pediatrics and Pathology, University of Michigan, Ann Arbor, Michigan, United States; 6 MVZ Limbach Magdeburg, Magdeburg, Sachsen‐Anhalt, Germany
Background: Initial evaluation of patients with suspected Von Willebrand Disease (VWD) usually includes the measurement of coagulation factor VIII, VWF antigen (Ag), and VWF activity. For the clinical laboratory it is beneficial if these assays can be performed on the same analyzer. In the U.S.A., a VWF Ag assay is not yet available on the Sysmex CS‐2500 System.
Aims: The aim of this study was to establish analytical and clinical performance data of the Siemens Healthineers VWF Ag assay on the SYSMEX CS‐2500 system.
Methods: Verification of the reportable range, linearity and the limit of quantification (LOQ) were performed. Reproducibility was tested at three US sites (five days with two runs per day and three replicates per run) using five samples spanning the reportable range. A method comparison to the STA‐LIATEST® VWF:Ag on Stago's STA R MAX system was performed with samples from the intended use population. Reference intervals were determined at three US sites with samples from healthy donors (n = 306).
Results: The verified assay reportable range is 4 to 300% of normal. The reproducibility studies demonstrate CVs between 2.03% and 5.96%. The method comparison study shows a good correlation. The reference intervals for blood group O (n = 147 subjects), for blood groups non‐O (n = 159 subjects) and for both groups combined are comparable to those published for other VWF Ag assays.
Conclusion(s): The Siemens Healthineers vWF Ag assay on the Sysmex CS‐2500 analyzer is very precise, has a clinically useful reportable range and shows good comparability to another commercial assay. The assay can thus be used to aid in the diagnosis of VWD. Disclaimer: Sysmex is a trademark of SYSMEX CORPORATION. The Siemens Healthineers vWF Ag assay is not available for sale in the U.S.A. Product availability may vary from country to country and is subject to varying regulatory requirements.
PB0822
A quantitative evaluation of high‐molecular forms of von Willebrand factor multimers in children's with different types of vWD
A. Poletaev1; E. Seregina 1; N. Karamian1; D. Fedorova1; A. Pshonkin1; S. Plyasunova1; P. Zharkov2
1 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 2 Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia
Background: High‐molecular forms of von Willebrand factor (vWF) multimers (HMWM) play a major role in primary hemostasis, however, the contribution of the amount of HMWM to the severity of bleeding in children is not fully understood.
Aims: To evaluate the relationship between the HMWM vWF count and the severity of clinical manifestations in children with different types of vWD.
Methods: There were 35 children under the age of 18 year with different types of vWD enrolled of this study. Out of 35 patients, 16 (45.7%) patients corresponded to type 1, 2 (5.7%) patients corresponded to type 3, and 12 (40%) patients corresponded to type 2 vWD. The severity of bleeding was assessed on the PBQ scale, with the calculation of the final bleeding score. The vWF:Ag, vWF:RCo, activity of blood coagulation factor VIII, vWF collagen binding and vWF multimers were evaluated in all samples. For a quantitative assessment of the photograph of the gel, the Imagej program was used, where the HMWM amount was estimated of brightness of the bands.
Results: The mean score PBQ was 4.05 points, median 4 points. The minimum PBQ was 1 point, the maximum was 12 points. There were no correlations between the amount of HMWM vWF and the degree of bleeding for all types of vWD (rs = 0,1, p = 0,7), for 1 type (rs = 0,02, p = 0,9) and for 2A; 2B types (rs = 0,13, p = 0,8). It is noteworthy that of the two functional tests evaluating the work of the HMWM vWF, a strong inverse relationship was found between the number of HIMF and vWF:CB (rs = −0,81, p = 0,015), while the dependence between the HMWM and vWF:RCo moderate and not significant (rs = −0,5, p = 0,21).
Conclusion(s): The evaluation of the amount of vWF IMMF does not provide additional information in terms of assessing the severity of clinical manifestations and predicting the severity of hemorrhagic syndrome in children's.


PB0832
Frequency of VWF single nucleotide variants in a single institution of Argentina
A. Woods 1; J. Paiva2; M. Casinelli2; M. Alberto3; A. Sanchez‐Luceros4
1 IMEX‐CONICET‐ANM, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 2 Academia Nacional de Medicina, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 3 National Academy of Medicine, Ciudad Autonoma de buenos aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 National Academy of Medicine, CABA, Ciudad Autonoma de Buenos Aires, Argentina
Background: As in other American countries, Argentineans are the result of the admixture between different migratory groups, mostly from Spain and Italy, and to a lesser extent from Sub‐Saharan Africa, and Southeast Asia. This genetic background can produce a high percentage of false positive/negative results due to differences in the genetic composition of our populations. Currently, there are no data on the frequency of VWF‐polymorphisms (SNVs) in Argentine population in EAHAD Coagulation Factor Variant Databases (VWF; Leiden Open Variation Database).
Aims: To analyze the frequency of common VWF‐SNVs in our region.
Methods: We analyzed genotypic data of subjects from our institution. Sixty‐seven SNVs within exons 17–28 including exon/intron boundaries were analyzed; 15 were intronic, 52 exonic; 34 were synonymous, 18 non‐synonymous. Allele frequencies were calculated for each SNV.
Results: Seven novel SNPs were observed: c.2281 + 16C > A, c.2442 + 38G > T, c.2581C > A (p.H861N) and c.2583 T > G (p.H861Q), c.3108 + 34G > A, c.3539‐49C > G and c.3539‐33G > C. The most common SNVs with major allele frequency (MAF) < 0.8 were (Table 1): • intron 17: c.2282‐42A > C • exon 18: c.2365A > G (p.T789A); c.2385 T > C (p.Y795=) • intron 19: c.2546 + 25 T > C • exon 28: c.4141A > G (p.T1381A); c.4414G > C (p.D1472H); c.4641 T > C (p.T1547=).
Conclusion(s): This is the first report of VWF‐SNVs frequency in a single institution of Argentina. In general, the allelic frequency of our population was quite similar to that described in European populations. However, some discrepancies were observed: •c.2282‐42A > C: our MAF was A, as described in Mancuso's paper (1989) and ISTH‐VWF database, but C in GRCh38.p13 Primary Assembly. •c.2546 + T > C: our MAF: T, as in ISTH‐VWF database, but C in Mancuso, and GRCh38.p13 Primary Assembly. •c.2555G > A (p.R852Q): our MAF was G, as in Mancuso and ISTH‐VWF database, but A in GRCh38.p13 Primary Assembly. •c.3951C > T (p.A1317=): our MAF was C, as in Mancuso and GRCh38.p13 Primary Assembly, but T in ISTH‐VWF database. •c.4141A > G (p.T1381A): our MAF was A, as in GRCh38.p13 Primary Assembly, but G in Mancuso and ISTH database.

PB0830
Sporadic Type 3 von Willebrand Disease due to homozygosity in a non‐consanguineous family
J. Cutler1; R. Wheeler 1; V. McDonald2; K. Forsyth3; Z. Yussuf3; R. Fenton3; S. Charrot3; D. Hart4; S. Sivapalaratnam5; M. Mitchell6
1 Viapath Analytics LLP, Guy's & St. Thomas' NHSFT, London, England, United Kingdom; 2 Barts Health NHS Trust, The Royal London Hospital, London, England, United Kingdom; 3 Barts Health NHS Trust, Royal London Hospital, London, England, United Kingdom; 4 Royal London Hospital Hemophilia Centre, Royal London Hospital, Barts Health NHS Trust, London, England, United Kingdom; 5 Barts Health NHS Trust, London, England, United Kingdom; 6 Viapath Analytics LLP, Guy's & St. Thomas' NHSFT, LONDON, England, United Kingdom
Background: ASH ISTH NHF WFH 2021 guidelines classifies type 3 VWD as a virtual absence of the VWF protein with associated very low FVIII levels, with a consequent severe bleeding phenotype. It has an autosomal recessive mode of inheritance and a prevalence of 0.55–3.2/million in Western countries. In consanguineous families, the index cases often present with homozygous pathogenic variants, whilst where parents are unrelated compound heterozygosity is by far the predominant presentation.
Aims: We report a case of sporadic type 3 VWD, presenting in a 9 month old female, the second child of two Caucasian, reportedly non‐consanguineous parents.
Methods: The patient presented with severe bleeding from the mouth following trauma which required haemostatic intervention. There was no prior bleeding history in the index case nor in the parents or elder sibling. Analysis of the coding region and flanking regions of VWF, was performed by PCR amplification and Sanger sequencing on DNA obtained via buccal swab.
Results: VWF antigen and GP1BM were both reported as <1 in the index case. A homozygous c.7483delC /p.(Leu2495Cysfs*8) variant was identified in exon 44 of VWF. Both parents and an older brother were subsequently shown to be heterozygous for this variant. VWF Levels are not available for the parents or elder sibling. The allele frequency for this variant is 1:141385 in Gnomad, and it has not been previously described in a homozygous presentation, or with a bleeding phenotype.
Conclusion(s): Reports of Type 3 VWD due to homozygous presentation of a VWF variant in non‐consanguineous families are very uncommon: the most common report being a second ‘as yet unidentified’ variant. This case is of interest as genuine homozygosity is confirmed via the detection of the heterozygous variant in each, asymptomatic, parent.
VPB0836
VWD Connect Foundation Severe Von Willebrand Disease Patient Registry
J. Cesta; A. Arapshian; C. Morgenthaler; C. Walsh; M. Gounder
VWD Connect Foundation, Wellington, Florida, United States
Background: Severe Von Willebrand Disease (sVWD) patients (estimated over 1500 U.S.) are an under‐studied population with severe bleeding not well‐characterized in literature. VWD Connect Foundation (VCF) has sponsored the design and launch of a sVWD online longitudinal natural history and outcomes patient registry.
Aims: The primary objective is to characterize the sVWD patient population, reporting on prevalence, genotypes, phenotypes, and management. The secondary objective is to provide a convenient online platform for participants (or caregivers) to self‐report clinical outcomes in real time. This critical endeavor also aims to provide researchers and other stakeholders with actionable data describing the sVWD population leading to better treatments, management, and ultimately a cure.
Methods: August 2021: VCF received IRB approval for protocol VWD‐001, The Severe Von Willebrand Disease Patient Registry: A Longitudinal Natural History and Patient Outcomes Study. December 2021: the Registry opened to a small cohort of participants. For the purposes of this Registry, sVWD patients may include Type 3 VWD and Severe Types 1; 1C; 2A; 2B; 2 M, and 2 N, excluding acquired VWD. Patients with unknown/other types may be considered severe if they have Von Willebrand Factor levels < 20%. Following informed consent and screening, participants complete modules, with continued participation as new modules are released. Future modules planned include: quality of life, family history, laboratory data, genetic data, reproductive bleeding, joints, treatments, prophylaxis and inhibitors. Participants will complete the Self‐Administered Bleeding Assessment Tool (Self‐BAT; Deforest et al, 2015) annually.
Results: As of January 2022, preliminary data has been collected on a small cohort, with enrollment and participation ongoing. Data includes patient demographics, medical history, prior and concomitant medication history, and Self‐BAT results.
Conclusion(s): Using the skill of its membership and dedicated medical professionals, VCF has successfully launched an online sVWD Patient Registry. Preliminary data from a larger cohort would be presented at the meeting.
VPB0838
When multiple genetic variants contribute to a bleeding phenotype
L. Fonseca; F. Martins Pereira; A. Brito; F. Lobo; M. Costa
Centro Hospitalar Tondela‐Viseu, Viseu, Viseu, Portugal
Background: Epistaxis is one of the most common complaints at emergency room and is recurrent among children. Most cases are of benign origin. Trauma, inflammation, vascular alterations, drugs or coagulopathies are the most frequent causes. In this latter case, it may be the first or even the only symptom.
Aims: To describe a clinical case.
Methods: A 9‐year‐old girl was referred to a coagulopathies specialist due to frequent and profuse epistaxis and gingivorrhagias. She had no bleeding complication during tooth extractions or vaccination. In family history, only her father and brother had recurrent episodes of epistaxis. No surgical history or medication were reported. The results of coagulation screening tests were: prolonged prothrombin time (PT) of 14.2 seconds, normal activated partial thromboplastin time (aPTT) of 34.2 seconds and low fibrinogen levels (1.8 g/L). The initial study also included a complete blood count and C‐reactive protein, which were normal. Coagulation factor levels were measured and FVIII and FVII were normal, but FV was slightly decreased (41%). The VWF:Ag was 80.5% and VWF:Rco was 79.3% (VWF:RCo/VWF:Ag ratio 0,98). Because of the reduced plasma levels of FV, genetic tests were required. A panel of 43 genes was made in Next‐Generation Sequencing (NGS).
Results: NGS showed 3 genetic alterations: functional polymorphism on F5 gene associated with decreased FV levels; mutation in heterozygosity on VWF gene (autosomal dominant variant) characterized by increased affinity of VWF for platelet GPIBa, suggestive of von Willebrand Disease (VWD) type 2B; mutation in heterozygosity on NBEAL2 gene associated with macrothrombocytopenia.
Conclusion(s): The study in this patient suggests that she has VWD type 2B. Although, the co‐inheritance of the other 2 variants may be contributing to the patient hemorrhagic phenotype. Additional studies are essential to elucidate the genotype–phenotype correlation.
PB0815
Evaluation of the diagnostic accuracy of VWF:Ac and VWF Ristocetin Cofactor Activity in the diagnosis of von Willebrand disease
B. Koc 1; S. Durmus2; E. Akkaya2; S. Genc3; B. Zulfikar4
1 Istanbul University, Oncology Institute, İstanbul, Istanbul, Turkey; 2 Istanbul University, Istanbul Faculty of Medicine, Istanbul, Istanbul, Turkey; 3 Acibadem Hospital, Maslak, Istanbul, Istanbul, Turkey; 4 Istanbul University Oncology Institute, Istanbul, Istanbul, Turkey
Background: VWF antigen (VWF:Ag) and Ristocetin Cofactor activity (VWF:RCo) are initial laboratory tests used in the diagnosis of von Willebrand disease (VWD). Due to the low reproducibility and sensitivity of the VWF:RCo test, the VWF:Ac test has emerged as an alternative diagnostic method, reflecting the binding of plateletGPIb receptors to VWF in the absence of ristocetin.
Aims: The aim of the study was to evaluate the performance of VWF:Ac Innovance (VWF:Ac) and VWF:RCo tests in measuring VWF activity, and to investigate the accuracy and the sensitivity in the diagnosis of VWD.
Methods: The study consisted 40 patients who have been diagnosed VWD previously and 20 healthy subjects. Plasma VWF:Ag, VWF:RCo, VWF:Ac, FVIII activity, platelet aggregation with 0.6 mL/1,2 mL ristosetin were measured using SysmexXN 6000 coagulation analyzer, and platelet count was measured by Beckman CoulterLH780 hematology analyzer.
Results: The comparison of VWF:RCo with VWF:Ac assay showed good agreement (y = 5.4544 + 0.996x, mean bias:0.6). VWFAg levels of type1, type2 and 3 patients were lower than those of control group(p = 0.000) being the lowest in type3 patients. VWF:RCo and VWF:Ac were also lower in all types of VWD compared with controls (p = 0.011 for type1 and p = 0.000 for type2 and 3), VWF:Ac and VWF:RCo in type3 patients were also different than type1 (p = 0.016, p0.034). FVIII levels were also significantly different than those of control, also, decreased FVIII was obtained in type3 VWD, compared to type1 and 2(p = 0.046, p = 0.006). Diagnostic value was evaluated by ROC curves; AUC was 0.662 for VWF:RCo/Ag and 0.548 for VWF:Ac/VWFAg for all group.
Conclusion(s): Our results indicate VWF:AC test showed good concordance and aggrement with VWF:RCo. However, we assumed that the VWF activity to antigen ratio had better diagnostic value for the classification of VWF deficiency.
Table 1: VWF:Ag, VWF:RCo, VWF:Ac, FVIII ve RIPA concentrations in type 1, type 2, and type 3 VWDs and healthy controls.


VPB0843
The ratio of von Willebrand factor activity to antigen in newly diagnosed essential thrombocythemia
K. Yokoyama
Tokai University Hachioji Hospital, Hachjoji, Tokyo, Japan
Background: Essential thrombocythemia (ET) is complicated with both thrombosis and hemorrhage. Hemorrhage often occurs ET patients with platelet count more than 100 e3/cmm, and one of the causes of hemorrhage in ET patients is thought to be acquired von Willebrand syndrome (AVWS). AVWS is characterized by abnormally von Willebrand factor ristocetin cofactor activity (VWF:Rco), and decreased VWF:Rco/VWF antigen (VWF:Ag) ratio less than 0.6.
Aims: To investigate the factors associated with decreased VWF:Rco/VWF:Ag less than 0.6 in newly diagnosed ET patients.
Methods: Records of patients who were newly diagnosed with ET according to the 2016 World Health Organization criteria between August 2018 and August 2021 at Tokai University Hachioji Hospital were reviewed. Data extracted included patients characteristics, laboratory data (e.g. complete blood cell counts, VWF:Ag, VWF:Rco, allele burden of JAL2V617F), history of thrombosis or hemorrhage, and use of antiplatelet drug at diagnosis. Retrospective analysis was performed to examine factors which effect on the VWF:Rco/VWF:Ag ratio. The ethics committee of the institutional review board approved the study.
Results: 32 patients were newly diagnosed with ET during study period. 10 of them were with VWF:Rco/VWF:Ag less than 0.6, and 22 of them were with VWF:Rco/VWF:Ag 0.6 or higher. Platelet levels of patients with VWF:Rco/VWF:Ag less than 0.6 was significantly higher than those of patients with VWF:Rco/VWF:Ag 0.6 or higher (1075+/−231 e3/cmm vs 764+/−187 e3/cmm, p = 0.03). Other factors (e.g. underlying diseases, white blood cell count, hematocrit, allele burden of JAL2V617F, use of antiplatelet drug at diagnosis were not different between two groups of patients (table). The ratio of patients with VWF:Rco/VWF:Ag less than 0.6 was significantly higher in ET patients with platelet count 100 e3/cmm or more (figure).
Conclusion(s): VWF:Rco/VWF:Ag ratios were significantly low in ET patients with higher platelet count.


PB0819
Hemostatic Response to Subcutaneous versus Intranasal Desmopressin in Patients with Von Willebrand Disease (VWD)
S. Saey 1; A. Sharathkumar2; W. Karla3; M. Krantz3; A. Currie3; U. Perepu3; M. Shinkle3
1 Carver College of Medicine, Iowa City, Iowa, United States; 2 Stead Family Department of Pediatrics, University of Iowa Children's Hospital, Iowa City, Iowa, United States; 3 University of Iowa Hospitals and Clinics, Iowa City, Iowa, United States
Background: The recent recall of intranasal desmopressin (DDAVP) has impacted management of patients with von Willebrand disease (VWD) in the United States, leaving one available alternative for home administration: subcutaneous DDAVP preparation at 6 mcg/1.5 mL. With weight‐based dosing at 0.3 mcg/kg/dose, individuals >20 Kg require multiple injections which decreases convenience and patient acceptance. Recognizing clinical need and limited alternatives, we adopted the practice of restricting DDAVP dosing to two subcutaneous injections (max dose = 12mcg) regardless of patient weight. Since the data to support this practice is sparce, we reviewed the response of DDAVP challenge tests for individuals who had both subcutaneous and intranasal DDAVP. We speculated that subcutaneous route would cause additional stress response due to injection site pain and different pharmacodynamics patterns from intranasal.
Aims: Compare intra‐patient DDAVP challenge response between subcutaneous and intranasal route.
Methods: This retrospective cohort study reviewed seven patients with VWD (type I and II) who underwent desmopressin challenge tests via intranasal and subcutaneous routes. Response variables included Von Willebrand Factor Antigen (VWF:Ag), VWF:Ristocetin activity (VWF:RCo), and factor VIII levels at baseline and 1 to 6 hours post‐DDAVP administration. Response was defined as peak increment in VWF:RCo >1.5X baseline.
Results: All seven patients responded to subcutaneous DDAVP challenge test (Table 1, Figure 1). While the intra‐patient responses via intranasal and subcutaneous route did not show significant difference in mean VWF:RCo peak to baseline ratio, p = 0.179, three patients (ID# 1,2,7) who did not respond to intranasal DDAVP responded to subcutaneous, p = 0.023. All five patients (ID#1,3,4,5,6) who received limited‐dose DDAVP (12 mg) responded as well. Study is limited by small sample size and reporting bias.
Conclusion(s): Our experience shows that subcutaneous DDAVP, maximum dosing 12 mg, is an effective alternative to intranasal DDAVP. Subcutaneous route may even be an option for patients previously unresponsive to intranasal administration. Larger studies are needed to clarify our observation.


PB0828
Evaluation of within‐subject variation of VWF multimers assay
M. Pikta1; T. Szanto 2; V. Banys3
1 Department of Laboratory Medicine, North Estonia Medical Centre, Tallinn, Estonia; Department of Health Technologies, Tallinn University of Technology, Tallinn, Estonia, Tallinn, Harjumaa, Estonia; 2 Coagulation Disorders Unit, Comprehensive Cancer Center, Helsinki University Hospital, Helsinki, Uusimaa, Finland; 3 Department of Physiology, Biochemistry, Microbiology and Laboratory Medicine, Institute of Biomedical Sciences, Faculty of Medicine, Vilnius University, Vilnius, Lithuania, Vilnius, Vilniaus Apskritis, Lithuania
Background: Von Willebrand factor (VWF) multimers (VWF:MM) play a major role in the phenotypic classification of von Willebrand disease (VWD). However, there is a high error rate, inconsistent interpretations of VWF:MM profiles and false classifications even in the experienced laboratories.
Aims: The aim of this study was to evaluate the within‐subject variation of VWF:MM assay using H5VW and H11VW gels (Sebia, France).
Methods: VWF:MM were subdivided as low molecular weight multimers (LMWM), intermediate molecular weight multimers (IMWM) and high molecular weight multimers (HMWM) according the manufacturer's recommendation. Laboratory profiles of 24 individuals referred for bleeding disorders evaluation were analyzed. For assessment of within‐subject variation, samples from non‐VWD individuals (n = 9), low VWF (n = 1), suspected VWD type 1 (n = 7), suspected VWD type 2A or 2 M (n = 5) and Hemophilia A carriers (n = 2) were collected over time and the VWF:MM evaluation was repeated twice (n = 20), three (n = 3) or four times (n = 1). The assessment criterion was an identical VWF:MM interpretation of results in each case (normal pattern/lack of IMWM and HMWM/relative decrease of HMWM).
Results: In the group with normal VWF:MM distribution, the within‐subject variations for LMWM, IMWM and HMWM were 7.3%, 7.0% and 4.3%, respectively. In the group with relative decrease of HMWM, the within‐subject variations for LMWM, IMWM and HMWM were 14.5%, 17.3% and 10.6%, respectively. In all cases repeated VWF:MM testing yielded identical interpretation.
Conclusion(s): Demonstrated within‐subject VWF:MM variability shows reasonable repeatability of results in both non‐VWD and VWD patient samples. Consistent interpretation of different follow‐up samples indicates the reliability of chosen VWF:MM assay.
VPB0840
Correlation study between VWF: RCO ristocetin cofactor activity and ISTH‐BAT in von Willebrand disease
R. Messaoudi 1; M. Amine2
1 University Hospital Center of Oran, Oran, Oran, Algeria; 2 UNIVERSITY HOSPITAL ORAN, ORAN, Oran, Algeria
Background: the Willebrand ristocetin cofactor (VWF: RCO) measures the ability of VWF to bind to platelets in the presence of ristocetin, it is a specific test that allows the diagnosis of von Willebrand disease, the ISTH hemorrhagic score ‐BAT allows clinical evaluation of bleeding symptomatology. The question is, does the clinical ISTH‐BAT score have the same diagnostic value as ristocetin cofactor activity?
Aims: To study the correlation between ristocetin cofactor (VWF: RCO) and the clinical ISTH‐BAT score.
Methods: In our series of 20 patients with von Willebrand disease, the study of the correlation between the cofactor activity of ristocetin VWF: RCO and the clinical haemorrhagic score ISTH‐BAT.
Results: The correlation between the cofactor activity of ristocetin VWF: RCO and the clinical haemorrhagic score ISTH‐BAT shows an inverse correlation, the more the VWF activity: RCO is lower the higher the ISTH‐BAT score is according to the following regression equation: ISTH‐BAT score = − 0.016 × (VWF: RCO) + 0.364. This correlation is medium (r = 0.42). The correlation study between the RCO: FVW activity and the clinical ISTH‐BAT score, shows that the clinical ISTH‐BAT score is partly explained by the RCO: FVW, despite the small number (only 20 patients). Therefore, the ISTH‐BAT clinical score may have a positive predictive value in the diagnosis of von Willebrand disease.
Conclusion(s): the ISTH‐BAT clinical score represents a very important clinical element for the diagnosis of von Willebrand disease. This score represents the first assessment of hemostasis in the face of any suspicion of the disease, this score can advantageously replace our failures in biology, especially in the most remote areas.
PB0834
Mixed Phenotype 1/2E Von Willebrand Disease in Two Slovak Patients: Case Report
J. Zolkova 1; Z. Kolkova2; D. Loderer2; J. Sokol3; T. Simurda4; A. Stryckova3; M. Dobrotova1; J. Ivankova5; I. Skornova1; Z. Lasabova6; P. Kubisz7; J. Stasko7
1 National Centre of Hemostasis and Thrombosis, Department of Hematology and Transfusiology, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin and University Hospital in Martin, Slovakia, Martin, Zilina, Slovakia; 2 Biomedical Center Martin, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Martin, Slovakia, Martin, Zilina, Slovakia; 3 National Centre of Thrombosis and Hemostasis, Department of Hematology and Transfusiology, Jessenius Faculty of Medicine, Comenius University in Bratislava, Martin University Hospital, Martin, Slovakia, Martin, Zilina, Slovakia; 4 Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, University Hospital Martin, Martin, Zilina, Slovakia; 5 Comenius University, The Jessenius Faculty of Medicine in Martin, Martin, Zilina, Slovakia; 6 Department of Molecular Biology and Genomics, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, BioMed Martin, Martin, Slovakia, Martin, Zilina, Slovakia; 7 National Centre of Thrombosis and Hemostasis, Department of Hematology and Transfusiology, Jessenius Faculty of Medicine, Comenius University in Bratislava, Martin University Hospital, Martin, Slovakia, Martin, Zilina, Slovakia
Background: Diagnostics of von Willebrand disease (VWD) is a complex process that aims to distinguish between qualitative and quantitative defects of von Willebrand factor (VWF) and reliably determine the subtype of VWD. In general, if all available VWF assays are used, genetic testing for screening mutations may only act to confirm a patient's phenotype. But the identification of causal mutations may be crucial for effective patient diagnosis and may also provide a more specific diagnosis to aid appropriate management.
Aims: Report the own experience with diagnostics of two patients with the mixed VWD phenotype.
Methods: The complete laboratory diagnostics of VWD was realized, routine coagulation testing, screening for VWD (VWF:Ac, VWF:Ag, FVIII:C, VWF:CBA) and discrimination tests (multimer analysis, genetic testing of the whole VWF gene).
Results: In the patients laboratory findings we observed proportionally reduced values of VWF:Ag and VWF:Ac, which does not indicate a dysfunctional VWF defect (V16: VWF:Ag = 0.25 IU/mL, VWF:Ac = 0.17 IU/mL, FVIII:C = 0,431 IU/mL, vWF:CBA = 0,11 IU/mL; V21: VWF:Ag = 0.13 IU/mL, VWF:Ac = 0.16 IU/mL, FVIII:C = 0,342 IU/mL, vWF:CBA = 0,034 IU/mL). However, multimer analysis revealed a deficiency of IMW and HMW multimers of VWF (V16: Low 12% (12–15%) Med 7% (22–35%) High 8% (47–70%); V21: Low 17,3% Med 1,1% High 5,8%). Genetic testing revealed a heterozygous missense mutation Trp1144Gly in exon 26 of VWF gene.
Conclusion(s): Mutations in exon 26 VWF in the D3 domain, such as Trp1144Gly, manifest as dominant type 1 VWD, but with abnormal multimers with typical 2A/IIE subtype features. The group of patients with similar mutations was designated as type 1/2E. Three studies demonstrated a significantly increased vWF:pp/vWF:Ag ratio in all 1/2E patients who carried Trp1144Gly mutation. This suggests a significant increase in the clearance of the vWF/FVIII complex. This work was supported by grant VEGA 1/0187/17.
PB0820
Von willebrand disease in older patients
C. Seaman
University of PIttsburgh, Pittsburgh, Pennsylvania, United States
Background: Bleeding symptoms in von Willebrand disease (VWD) may be minimal in the absence of hemostatic stressors, such as menstruation and childbirth; thus, patients seeking medical care for VWD are often younger. As a result, the natural course of VWD in older adults has not been well described.
Aims: To perform a descriptive analysis of older VWD patients receiving care at our Hemophilia Treatment Center (HTC).
Methods: We performed a retrospective electronic health record review of VWD patients with at least 1 HTC clinic visit between June 1, 2015, and May 31, 2021, and age 45 or older at the time of the visit. Data collected consisted of demographics; VWD‐related information; multimorbidity; and medications. A paired t‐test was performed to compare change in von Willebrand factor (VWF) levels and bleeding score, and McNemar's test was used to compare change in the proportion of patients with normal VWF levels and bleeding score. Multiple linear regression was performed to assess the influence of multimorbidity on change in VWF levels adjusted for VWD type.
Results: Seventy patients had 131 HTC clinic visits with an average of 1.9 (SD 1.3, range 1–9) visits per person. Patient characteristics are presented in Table 1. Among 62 invasive procedures in 36 patients (Table 1), nine were performed without VWD‐specific therapy. Bleeding occurred with two procedures, a colonoscopy with desmopressin and a spine surgery without VWD‐specific therapy. Mean baseline VWF levels and bleeding score were normal, and VWF levels increased with age in patients with type 1 VWD (Table 2). Multimorbidity did not predict change in VWF levels, beta = 0.044 (SE 0.047), p = 0.36.
Conclusion(s): Our findings underscore the importance of describing the natural course of VWD in older adults, especially the critical nature of determining bleeding risk in this population, to guide clinical decision‐making with the use of antihemostatic drugs and periprocedural VWD‐specific therapy.


VPB0835
Hemoglobin concentration is a determinate of plasma VWF and FVIII levels
M. Carter‐Febres; C. Tarango; E. Mullins
Cincinnati Children's Hospital, Cincinnati, Ohio, United States
Background: Von Willebrand Disease (VWD) is the most common bleeding disorder. Complicating the diagnosis, von Willebrand factor (VWF) and factor VIII (FVIII) are acute phase reactants, and levels fluctuate in response to many stimuli, including physical activity. A common scenario for evaluation of a bleeding disorder is in patients with bleeding symptoms leading to iron deficiency anemia. We therefore sought to determine if anemia leads to elevations in VWF and factor VIII levels.
Aims: Define the impact of hemoglobin levels on VWF and FVIII levels in persons without a known bleeding disorder.
Methods: This is a single‐site retrospective review of the electronic health record of patients that had von Willebrand profiles [VWF:Antigen (Ag), VWF:Activity (Act), and FVIII activity] obtained within 24 hours of a hemoglobin level. Data was retrieved from January 1, 2012, through June 1, 2021.
Results: We identified 4899 instances among 4552 patients of a VWF profile that had an associated hemoglobin level in a 24‐hour period in individuals that did not carry a diagnosis of VWD. 1134 (30%) of these instances were associated with a hemoglobin <12 g/dL. Stratifying by hemoglobin, we found that both VWF: Ag (p < 0.0001) and VWF:Act (p < 0.0001) were significantly elevated in patients with anemia (defined as hemoglobin <12 g/dL). This correlation was strongest in patients with a hemoglobin of <10 g/dL. FVIII levels also increased in strong association with anemia (p < 0.0001). Interestingly, we also observed that anemia was associated with a significant decrease in the VWF Act:Ag ratio (p < 0.01).
Conclusion(s): Anemia was found to be associated with elevations of VWF:Ag, VWF:Act, and FVIII. As many patients presenting for a bleeding disorder evaluation are anemic, the confounding effect of anemia should be considered during the diagnostic evaluation.


PB0807
Inhibitors in 3 patients with type 3 von Willebrand disease in a Portuguese Centre – management of bleeding and surgeries
C. Catarino 1; F. Rodrigues2; A. Pereira2; C. Peixoto3; L. Parusnikova3; J. Pestana4; S. Campaniço3; J. Lucas5; P. Afonso3
1 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisbon, Lisboa, Portugal; 2 Congenital Coagulopathies Reference Centre‐ Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisboa, Lisboa, Portugal; 3 Centro Hospitalar Universitário Lisboa Norte ‐ Hospital Santa Maria, Lisbon, Portugal, Lisboa, Lisboa, Portugal; 4 Hospital do Espirito Santo ‐ Évora, Évora, Evora, Portugal; 5 Hospital Nélio Mendonça, Funchal, Madeira, Portugal
Background: The development of alloantibodies against von Willebrand factor (VWF) represents a rare but serious complication of the treatment of VW disease (VWD), being associated with the lack of hemostatic response to VWF concentrates but, also, with the risk of anaphylactic reactions.
Aims: Demonstrade the efficacy and safety of recombinant activated factor VII (rFVIIa) in this group of patients.
Methods: We, retrospectively, evaluated data on bleeding episodes and surgical procedures in 3 patients with type 3 VWD with inhibitors (VWDi) followed at our center.
Results: Between 2006 and 2021, 3 patients with VWD and inhibitors (VWDi): 2 females and 1 male were diagnosed and treated at our center (ages 4–20). Inhibitors were suspected due to the absence of bleeding control while on therapy with FVIII/FVW concentrates; to laboratory results that did not match the administered therapy with FVIII/FVW concentrates; and to an allergic reaction related to the infusion of FVIII/FVW concentrate. During this period, more than 40 bleeding episodes were treated with rFVIIa. These episodes include mostly mucocutaneus bleeding (oral; gastrointestinal; gynecological; urological; epistaxis), large muscular hematomas; hemarthrosis and retroperitoneal hemorrhages. rFVIIa was also effective in several dental extractions and in 2 major surgeries (1 patient was submitted to an hysterectomy and another patient to an abdominal surgery due to a life‐threatening bleeding). Average dosage of rFVIIa was 100 micrograms and the schedule was decided according to the type and severity of the clinical situation. In severe bleeding, and in major surgeries rFVIIa was administered, in general, with 2 or 3 hours intervals. No adverse effects were observed related to hemostatic therapy.
Conclusion(s): Even though, in our patients, rFVIIa was clearly effective, we think it would be important to have a way to compile information on this group of patients, in order to determine the most appropriate approach.
PB0808
Hemostatic response to exercise training in patients with continuous‐flow left ventricular assist device
C. Chan 1; M. Passmore2; O. Tronstad3; H. Seale3; M. Bouquet2; A. Hogan4; N. Sowden4; D. Platts4; W. Chan4; A. Dashwood4; D. McGiffin5; A. Maiorana6; J. Suen7; J. Fraser2; B. Meyns8; L. Fresiello8; S. Jacobs8
1 Griffith University, Brisbane, Queensland, Australia; 2 Faculty of Medicine, University of Queensland, Brisbane, Queensland, Australia; 3 Physiotherapy Department, The Prince Charles Hospital, Brisbane, Queensland, Australia; 4 Department of Cardiology, The Prince Charles Hospital, Brisbane, Queensland, Australia; 5 Cardiothoracic Surgery & Transplantation, The Alfred Hospital, Melbourne, Victoria, Australia; 6 Allied Health Department, Fiona Stanley Hospital, Perth, Western Australia, Australia; 7 University of Queensland, Brisbane, Queensland, Australia; 8 Department of Clinical Cardiac Surgery, KU Leuven, Leuven, Vlaams‐Brabant, Belgium
Background: Acquired von Willebrand syndrome and platelet dysfunction are commonly diagnosed, which contributing to bleeding complications, in patients with a continuous‐flow left ventricular assist device (CF‐LVAD).
Aims: We aimed to investigate whether exercise training can stimulate endothelial release of von Willebrand factor (vWF) and organ‐reserved platelets into blood circulation by increasing the squeezing stress surrounding peripheral blood vessel walls and internal organs, potentially mitigating the bleeding risk in CF‐LVAD patients.
Methods: In a prospective, two‐centred comparative cohort study, 21 patients with an implanted CF‐LVAD (HeartMate 3, n = 12 and HeartWare Ventricular Assist Device, n = 9) either performed exercise at maximal intensity (n = 13) on an ergometer for approximately 10 minutes or sub‐maximal intensity (n = 8) on a treadmill for 30 minutes. Blood samples were taken pre‐ and post‐exercise for full blood examination, platelet aggregometry, vWF multimeric analysis, rotational thromboelastometry, coagulation, fibrinolytic and endothelial bio‐marker analysis.
Results: Exercise training resulted in an increased average platelet count (235 to 263 × 103/μL, p = 0.000009), with adenosine diphosphate (31 to 40 U, p = 0.000012), thrombin receptor activating peptide‐6 (55 to 65 U, p = 0.000002), and ristocetin (25 to 32 U, p = 0.0095) induced‐platelet aggregation also enhanced. Endothelial activation was demonstrated by increased overall vWF multimers (21.3% to 25.3% in high‐molecular‐weight, p = 0.00005; 20.4% to 25.1% in intermediate‐molecular‐weight, p = 0.00013; 27.9% to 31.8% in low‐molecular‐weight, p = 0.0021), collagen‐binding vWF activity (1.0 to 1.2 U/mL, p = 0.0008), ADAMTS13 (1.1 to 1.2 μg/mL, p = 0.0113), tissue plasminogen activator (2.7 to 3.0 ng/mL, p = 0.036) and D‐dimers (3.1 to 3.4 μg/mL, p = 0.0012). Additionally, exercise shortened clot formation times (66 to 63 seconds, p = 0.0002), with a corresponding increase in maximum clot firmness (65.8 to 67.2 mm, p = 0.00004).
Conclusion(s): Irrespective of device type and exercise regime, our results demonstrate that exercise training alters hemostatic parameters with increased endothelial release of vWF, platelet number and function, suggesting a potential non‐pharmacological role in mitigating bleeding risk in patients with CF‐LVAD.


PB0806
A screening assay for type 2B von Willebrand factor variants using an ELISA assay with ristocetin and a recombinant wild‐type glycoprotein Ib
P. Colpani1; L. Baronciani 1; G. Cozzi1; M. Pagliari2; A. Cairo3; E. Biguzzi4; F. Peyvandi5
1 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, Milano, Lombardia, Italy; 3 Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milano, Lombardia, Italy; 4 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 5 Fondazione IRCCS Ca′ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Type 2B patients are usually detected using the first level assays for von Willebrand disease (VWD), because of their loss of high molecular weight multimers (HMWM). Ristocetin‐induced platelet agglutination assay (RIPA) or genetic analysis of von Willebrand factor (VWF) exon 28 is then performed to confirm diagnosis. Type 2B with HMWM (e.g., p.Pro1266Leu) might be missed at the first screening or misdiagnosed as type 1/low‐VWF and RIPA or genetic analysis are rarely performed in these cases.
Aims: Due to the relative common presence of type 2B with HMWM variants in our type 2B population (25%), we developed a screening assay to identify the type 2B variants using patients' frozen plasma.
Methods: A wild‐type recombinant glycoprotein Ib (rGPIb) was immobilized on a 96‐wells ELISA plate using the 2D4 monoclonal antibody. A normal pooled plasma (NP), type 2B controls and patients' plasma were normalized to the lowest VWF antigen level. Each sample was seeded in presence of ristocetin at 2 different concentrations (0.06 and 0.12 mg/mL; Figure) and incubated for 2 hours. VWF was detected with an anti‐VWF HRP–labeled polyclonal antibody. Seventy patients with VWF levels borderline or reduced, at their first clinical evaluation due to bleeding diathesis, were assessed versus a group of 14 type 2B patients. Sanger sequencing of exon 28 was performed in patients with VWF increased binding to rGPIb.
Results: The mean values of the absorbance ratios between samples and NP, in comparison with those obtained with the type 2B patients are reported in the Table. Three patients had ratios ≥1.87 ± 0.70 (2B HMWM), two carried a type 2B variant.
Conclusion(s): This GPIb ELISA assay, that has the advantage to use frozen plasma, could help to identify type 2B variants and can be used to select patients to be investigated at molecular level.


PB0831
Using GP1bM Activity (VWF:GPIbM) versus Ristocetin Cofactor Activity (VWF:RCo) in von Willebrand Factor Panels
E. Wong 1; V. Kumar2; D. Goode2; A. Espinoza3; N. Martin3; L. Noh3; J. Baldwin4; T. Zeschmann4; F. Racke4; J. Dlott4; L. Worfolk4
1 Quest Diagnostics Nichols Institute, Chantilly, Virginia, United States; 2 Quest Diagnostics med fusion, Lewisville, Texas, United States; 3 Quest Diagnostics Nichols Institute, Secaucus, New Jersey, United States; 4 Quest Diagnostics, Secaucus, New Jersey, United States
Background: The 2021 ASH ISTH NHF WFH von Willebrand disease (VWD) guidelines recommend that platelet‐dependent von Willebrand factor (VWF) activity‐to‐antigen ratios below 0.7 be used to distinguish between various VWD types. The guidelines suggest using platelet binding activity assays of VWF over the ristocetin cofactor activity (VWF:RCo) assays for diagnosing VWD, a recommendation based on low certainty from diagnostic accuracy studies.
Aims: To determine how VWD diagnosis would change at various VWF activity‐to‐antigen ratios using the Siemens' GPIbM (VWF:GPIbM) Innovance and VWF:RCo assays.
Methods: Study of patient specimens submitted for comprehensive VWD analysis. VWF:GPIbM and VWF:RCo testing was performed per manufacturers guidelines using the Siemens BCS‐XP. VWD diagnosis was made using either VWF:RCo or VWF:GPIbM, at various VWF activity‐to‐antigen ratios.
Results: At all ratios, panels with GPIbM activity had definitive results in 83 of 83 (100%) specimens, while panels with VWF:RCo had definitive results in 74 of 83 (89%). For 9 specimens that did not have quantifiable VWF:RCo, diagnosis was probable Type 2A/2B VWD or aVWS (n = 3), type 2 M or Type 1 (n = 5), or Type 3 (n = 1) based on VWF:Ag of 6%. Of 5 the VWF panels that could not distinguish between Type 2 M or Type 1 disease by VWF:RCo, 4 (80%) could be discerned using VWF:GPIbM as either Type 1 or Type 2 M disease; 1 had a VWF activity‐to‐antigen ratio of 2.94 due to unknown interference.
Conclusion(s): VWF:GPIbM use in VWD comprehensive panels has greater sensitivity and specificity compared to panels incorporating VWF:RCo and should be used to preferentially replace VWF:RCo in the diagnosis of VWD. Disclaimer: BCS, INNOVANCE, and all associated marks are trademarks of Siemens Healthcare Diagnostics Products GmbH or its affiliates. Under FDA review (DE Novo classification request). Not available for sale in the USA. Product availability may vary from country to country and is subject to varying regulatory requirements.
PB0833
Usefulness of in‐silico prediction tools in the analysis of VWF genetic variants
A. Woods 1; J. Paiva2; D. Primrose3; M. Alberto4; A. Sanchez‐Luceros5
1 IMEX‐CONICET‐ANM, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 2 Academia Nacional de Medicina, CABA, Ciudad Autonoma de Buenos Aires, Argentina; 3 Universidad de Moron, Moron, Buenos Aires, Argentina; 4 National Academy of Medicine, Ciudad Autonoma de buenos aires, Ciudad Autonoma de Buenos Aires, Argentina; 5 National Academy of Medicine, CABA, Ciudad Autonoma de Buenos Aires, Argentina
Background: To determine if disease‐causing variants (DCV) observed in patients with von Willebrand disease (VWD) are related to their clinical and laboratory phenotypes, additional costly, labor‐intensive and time‐consuming experimental approaches are needed. In‐silico prediction tools were designed to predict the pathogenicity of DCV on the structure and/or function of the resulting protein. However, their performance can vary greatly. A reliable statistical ratefor evaluating in‐silico methods is to calculate the Matthews correlation coefficient (MCC) for each method, which considers true positives/negatives, false positives/negatives. Values≥0.7 indicate very strong agreement between prediction and observation.
Aims: • To predict pathogenicity of DCVs found in our VWD2 patients, using in‐silico methods. • To calculate MCC for each method in predicting both pathogenicity in DCVs and neutrality in benign single nucleotide variants (SNVs).
Methods: Thirty‐one DCVs, 35 synonymous and 17 non‐synonymousSNVs, all located within exons17‐28of VWF gene. Thirty in‐silico methods: I‐Mutant; PolyPhen; SIFT; SIFT4G; Mutation‐Taster; Provean; DANN; CADD; FunSeq2; Predict‐SNP2; GWAVA; Eigen; EigenPC; PhD‐SNP; BayesDel‐addAF; BayesDel‐noAF; LRT; M‐Cap; MVP; ListS2; MetaLR; MetaRNN; MetaSVM; MutPred; Revel; Deogen2; Mutation‐Assessor; FATHMM‐MKL; FATHMM‐XF; FATHMM.
Results: All DCVs were predicted as pathogenic: 25/31 (80.6%) by ≥70% of in‐silico methods; 4/31 (12.9%), by <40% of methods. Synonymous SNVs were predicted as benign: 34/35 (97.1%) by >70% of methods; 1/35 (2.8%) by 57.1% of methods. Non‐synonymous SNVs were predicted as benign: 12/17 (70.6%) by>70% of methods; 2/17 (11.7%) by <20 of methods; p.Pro1601Thr: pathogenic by all methods. DANN, CADD, and FunSeq2 showed MCC≥0.7; MVP and I‐mutant, the worst MCC (Figure 1).
Conclusion(s): DANN, CADD, FunSeq2 and Predict‐SNP2 showed the best performance. In‐silico methods might be excellent tools for supporting the classification of DCVs related to VWD. Synonymous SNVs showed higher predictive accuracy than non‐synonymous SNVs. p.Pro1601Thr as benign variant should be revised. Not all the in‐silico methods discriminate between DCVs and SNVs. This point needs further analysis to improve them.

PB0818
Response rate of different DDAVP dosing in Hemophilia A and von Willebrand disease
S. Sharma1; S. Pn'g2; D. Pepperell 2
1 Fiona Stanley Hospital, Hemophilia and Haemostatis Centre, Perth, Western Australia, Australia; 2 Fiona Stanley Hospital, Perth, Western Australia, Australia
Background: DDAVP is used in Hemophilia A and Von Willebrand disease (vWD) for hemostasis. Various DDAVP dosing regimens are used for the DDAVP challenge. The original dose suggested by Mannucci et al was 0.3 mcg/kg intravenously (IV), while others use 0.2mcg/kg IV dosing. Some national guidelines recommend a capped dose of 20 mcg IV or SC, while others have used a capped dose of 15 mcg SC.
Aims: The objective is to compare the response rate, and safety profile between 0.2mcg/kg and 0.3mcg/kg dosing during a DDAVP challenge.
Methods: This is a retrospective analysis using prospective data from the Australian Bleeding Disorders Registry (ABDR) from 1998 to 2017. Patients with Hemophilia A or vWD undergoing a DDAVP challenge were included. Response rate to DDAVP was defined as a greater than 50 IU/dL increase in FVIII (for hemophilia A), and greater than 50 IU/dL increase in FVIII and VWF:RCoF for patients with vWD. Changes in factor VIII, vWFAg and RiCoF were also obtained. Any adverse effects and changes in sodium levels were also recorded. These outcomes were then compared between individuals who received a < 0.25mcg/kg dosing of DDAVP compared with those receiving a greater dose (to accommodate rounding).
Results: 128 patients were included. 79 out of 85 patients (92.9%) who received a dose of less than 0.25mcg/kg were responsive, compared to 40 out of 43 patients (93.0%) receiving a dose greater than 0.25mcg/kg. Adverse events (predominantly facial flushing) were similar between groups; 10 out of 85 patients (11.7%) in the lower dosing group compared to 4 out of 43 (9.3%) in the higher dosing group. Data for incidence of hyponatraemia is still pending.
Conclusion(s): Response rates and adverse events were similar between the 0.2mcg/kg and 0.3mcg/kg DDAVP dosing groups, suggesting lower doses are equivalent in achieving the same response in patients with Hemophilia and vWD.
PB0826
A Systematic Review of Acquired von Willebrand Syndrome Associated With Cardiac Disorders: Epidemiology, Disease Burden, and Management
P. Du 1; R. Bharali2; S. Sun3
1 Takeda Development Center Americas, Inc., Hershey, Pennsylvania, United States; 2 HCD Economics, Daresbury, England, United Kingdom; 3 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States
Background: Acquired von Willebrand syndrome (aVWS) is a rare bleeding disorder, which can be caused by certain cardiac disorders. High blood shear rates specific to these disorders are associated with loss of high molecular weight multimers of von Willebrand factor (VWF), which impairs VWF function.
Aims: To describe the epidemiology, disease burden, and management of cardiac‐related aVWS.
Methods: A systematic review (PROSPERO 2021 CRD42021241527) of studies/reports published up to 31 January 2021 (no date limit) was performed using MEDLINE, Cochrane Central Register for Controlled Trials, EMBASE, and Web of Science databases utilizing keywords encompassing aVWS and cardiac conditions. Additional searches were performed using trial registries, gray literature (ethos.bl.uk, dart‐europe.eu, oatd.org), congresses, and Google Scholar. Identified studies were screened by 2 independent reviewers. Review articles and duplicates were removed, and data from identified publications extracted.
Results: Of 70 studies included, 37 were cohort studies, 24 case studies/series, 7 case–control studies, and 2 device experimental studies. Cumulative sample size was 2482 patients with cardiac conditions; median (range) age was 49 (1–89) years at aVWS diagnosis. aVWS occurred frequently in patients with cardiac failure on ventricular assist devices, mechanical circulatory support, or extracorporeal membrane oxygenation/life support (53–100% of patients; 34 cohort studies), or with aortic stenosis (8 cohort studies; patient % not available). Bleeding‐related events/complications (mostly gastrointestinal and intracranial bleeding, hemothorax, and epistaxis) were reported in 3–100% of patients (29 cohort studies). Deaths were reported in 16 cohort studies and 4 case reports (407/1378 patients); most common reasons were multiple organ failure (n = 24), cardiomyopathy (n = 10), and serious bleeding events (n = 8). Reported treatments primarily targeted underlying conditions and control of bleeding and related complications; VWF concentrates/factor VIII were rarely used (Table 1).
Conclusion(s): This systematic review highlights the limited data on aVWS epidemiology and disease burden and indicates the complexities of managing patients with cardiac‐related aVWS.

PB0805
Development of Personalized CRISPR/Cas9 Based Gene Correction Therapy for Von Willebrand Disease
I. Bär 1; P. Burgisser2; R. Bierings3; J. Eikenboom4; C. van Kwawegen1; F. Leebeek2; J. Voorberg5
1 Department of Hematology, Erasmus University Medical Centre, Rotterdam, the Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 2 Department of Hematology, Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 3 Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 4 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands; 5 Department of Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Von Willebrand Disease (VWD) is the most common inherited bleeding disorder. Current treatment options, including desmopressin/DDAVP administration or VWF‐concentrate therapy, provide no sufficient long‐term solutions. CRISPR/Cas9 gene targeting approaches present the opportunity to selectively target and correct causative mutations in VWF. Endothelial colony forming cells (ECFCs) from VWD patients are a powerful translational cell model of VWD and could potentially be used for demonstrating VWF gene correction ex vivo.
Aims: The aim of this study is to set a ground for personalized gene therapy in VWD by developing CRISPR/Cas9 gene correction methods in VWD patient‐derived ECFCs.
Methods: The Willebrand in the Netherlands (WiN) cohort study characterized >800 VWD patients. ECFCs were isolated from venous blood of selected VWD patients who enrolled in the BOEC‐MK study. Cellular phenotypes are determined using biochemical assays for VWF synthesis, secretion, and multimer composition and using confocal imaging of VWF storage in Weibel‐Palade‐bodies. VWD mutations will be corrected using a Cas9‐based cytosine base editor in conjunction with specific guide RNAs directed against the site of mutation. Single cell sorted ECFCs will be clonally expanded and will be evaluated for rescue of the disease phenotype. Correction of the mutation will be confirmed using DNA sequencing.
Results: A panel of causative VWF mutations has been selected for gene correction. Corresponding patient ECFCs have been isolated and their baseline disease phenotypes are being characterized using morphological and biochemical assays. ECFCs are currently being immortalized to overcome their limited proliferation ability.
Conclusion(s): Gene therapy is a promising treatment alternative for patients suffering from VWD. More studies are needed to evaluate which correction strategies are suitable for efficient and durable correction of VWF mutations in VWD. Funding was received from the Netherlands Organization for Scientific Research (NWO), Domain Applied and Engineering Sciences (TTW), ‘Connecting Innovators’ Open Technology Programme, Project#18712.
PB0823
The rare VWF non‐canonical splice‐site variant, c.8155 + 6 T > A, causes type 3 von Willebrand disease in homozygosity and results in perinuclear retention of VWF in endothelial cells
M. Sims 1; F. Burden1; J. Collins1; P. North2; L. Stefanucci1; A. Dinan1; S. Sivapalaratnam3; D. Hart4; M. Laffan5; W. Ouwehand6; S. Shapiro7; M. Frontini2
1 University of Cambridge, Cambridge, England, United Kingdom; 2 University of Exeter, Exeter, England, United Kingdom; 3 Barts NHS Trust, London, England, United Kingdom; 4 The Royal London Hospital Hemophilia Centre, Barts and The London School of Medicine and Dentistry, London, England, United Kingdom; 5 Centre for Hematology, Imperial College London, London, England, United Kingdom; 6 Department of Hematology, University of Cambridge and NHS Blood and Transplant, NIHR BioResource, Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, CB2 0PT, UK. Department of Hematology, University College London Hospitals, London, NW1 2BU, UK, Cambridge, England, United Kingdom; 7 Oxford University Hospitals National Health Service Foundation Trust, University of Oxford, and Oxford National Institute for Health Research Biomedical Research Centre, Oxford, England, United Kingdom
Background: A patient with type 3 von Willebrand disease (VWD3) was enrolled into a whole genome sequencing study as routine genetic analysis had not identified a causal genetic variant. The homozygous single nucleotide variant (SNV), c.8155 + 6 T > A, 4 nucleotides from the intron 51 donor splice site of VWF, was suspected to be aetiological.
Aims: To assess the functional consequences of c.8155 + 6 T > A.
Methods: The proband and controls were consented for studies approved by the East of England research ethics committee. Blood was taken from which platelet cDNA and endothelial colony forming cells (ECFCs) were derived. Subsequently, VWF‐directed CRISPR‐Cas9 RNAs were transduced into wild type (WT) human induced pluripotent stem cells (hiPSCs). Two clones were selected with different biallelic termination codons: at 120 nucleotides 3′ from the translation start site (knock out, KO); and 12 nucleotides 5′ of the position of the proband's SNV (Δexon50‐52). WT, KO, and Δexon50‐52 hiPSCs were differentiated to endothelial cells (iECs).
Results: c.8155 + 6 T > A resulted in the skipping of exon 50 (Figures 1A and 1B), and consequently a frameshift and termination codon 25 amino acids 3′ of exon 49 (Figure 1C). In the proband ECFCs, VWF was predominantly distributed in a perinuclear halo with minimal packaging into punctate structures (Figure 1D). These findings were replicated in iECs (Figure 2). Furthermore, 239 iECs were evaluated: 71% of Δexon50‐52 iECs did not contain VWF compared with 9% of WT (p = 2.1 x 10–14, Fisher Exact Test). Most of the remaining 29% of Δexon50‐52 iECs contained VWF in a perinuclear distribution.
Conclusion(s): Homozygous c.8155 + 6 T > A results in VWD3 due to a reduction in abundance and packaging of VWF in endothelial cells. This is likely to be due to truncation of VWF prior to the C‐terminal cysteine knot, which is crucial for VWF dimerisation and exit from the endoplasmic reticulum.


PB0825
Burden of Illness in Patients With von Willebrand Disease Receiving Prophylaxis Versus Potential Prophylaxis‐Eligible Patients: A Post‐Hoc Analysis of A European Socioeconomic Study
P. Du 1; G. Morgan2; S. Brighton2; F. Truong Berthoz3; N. Kemenyash3; S. Sun4
1 Takeda Development Center Americas, Inc., Hershey, Pennsylvania, United States; 2 HCD Economics, Daresbury, England, United Kingdom; 3 Takeda Pharmaceuticals International AG, Zürich, Zurich, Switzerland; 4 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States
Background: Limited real‐world evidence is available for the health benefits of prophylaxis in patients with von Willebrand disease (VWD).
Aims: To compare patient characteristics and disease burden in patients treated with prophylaxis with those identified as eligible for prophylaxis according to current VWD management guidelines but not currently receiving it.
Methods: Post‐hoc analysis of “Cost of VWD Across Europe, A Socioeconomic Study” (CVESS), a retrospective, descriptive, cross‐sectional study conducted in 2018 in France, Germany, Italy, Spain, and the UK. Patients aged >1 year, with a laboratory diagnosis of hereditary VWD and known VWD classification were eligible. Data were collected by clinicians using medical records and an optional patient questionnaire. The prophylaxis group had received intermittent or continuous prophylaxis to prevent bleeding in the previous 12 months. Prophylaxis‐eligible patients were defined in this analysis as those with severe (resulting in hospitalization or involving critical organs) and/or frequent (≥5 total, ≥3 joint, or ≥2 gastrointestinal) bleeds in the 12 months before the index date (ie, time of data collection), and were not prescribed prophylaxis. Ethical approval for CVESS and patient informed consent were obtained.
Results: Demographics and VWD severity/type for the prophylaxis (n = 229) and prophylaxis‐eligible (n = 102) groups are presented in Table 1. Lower levels of pain were reported in the prophylaxis versus prophylaxis‐eligible groups. Patients in the prophylaxis group were less likely to have ≥1 target joints (10.5% vs 23.5%; p = 0.002), and/or ≥1 chronically damaged joints (19.2% vs 35.3%, p = 0.002). Mental/behavioral disorders were the most common concomitant conditions; anxiety was reported in 12.2% of prophylaxis versus 27.5% of the prophylaxis‐eligible groups (p = 0.001). Rates of hospitalization were lower in the prophylaxis versus prophylaxis‐eligible groups (Table 2).
Conclusion(s): This European‐based real‐world study highlights the increased disease burden and unmet needs in patients with VWD and severe and/or frequent bleeds who had not received prophylaxis in the previous 12 months.


VPB0842
von Willebrand factor multimer analysis may predict the development of transplant‐associated thrombotic microangiopathy
S. Yamada 1; M. Kubo2; H. Okumura3; M. Matsumoto2
1 Nara Medical University, Kashihara, Nara, Japan; 2 Department of Blood Transfusion Medicine, Nara Medical University, Kashihara, Nara, Japan; 3 Toyama Prefectural Central Hospital, Toyama City, Toyama, Japan
Background: Transplant‐associated thrombotic microangiopathy (TA‐TMA) is a serious complication of allogenic hematopoietic stem cell transplantation (allo‐HSCT). It has been reported that von Willebrand factor (VWF) antigen (Ag) levels are higher in TA‐TMA patients; however, the relationship between VWF multimers and TA‐TMA has not been elucidated in detail.
Aims: To investigate the relationship between TA‐TMA and VWF multimers.
Methods: Plasma of patients was collected from day −7 to day 63 of allo‐HSCT to test for VWF antigen (Ag), VWF ristocetin cofactor (VWF:Rco), ADAMTS13 activity, VWF multimers, and VWF‐degradation product (VWF‐DP). VWF‐DP was measured using a monoclonal antibody that specifically recognizes Y1605 at the C‐terminal boundary, which is exposed following ADAMTS13‐mediated cleavage of the VWF A2 domain (Hayakawa et al. JTH. 2019).
Results: Fourteen patients were analyzed in this study. Six patients developed probable TA‐TMA (Cho, et al. Transplantation, 2010) and two patients developed definite TA‐TMA (Ho, et al. Biol Blood Marrow Trasplant, 2005). The results of VWF multimers and VWF parameters in the two definite TA‐TMA cases are shown in Figure 1. At the onset of TA‐TMA (Patient No.1, day 33 and No.2, day 20), unusually large VWF multimers (UL‐VWFMs) appeared in the plasma. These findings were not seen in probable TA‐TMA or others. Before the appearance of UL‐VWFMs, a defect of high molecular weight VWF multimers (HMW‐VWFMs) and increase in VWF‐DP were found in both patients with definite TA‐TMA. These results indicated that the defects of HMW‐VWFMs might be caused by excessive cleavage of VWFMs by ADAMTS13. In addition, the defects of HMW‐VWFMs were also found in patients with probable TA‐TMA or in non‐TA‐TMA patients; however, UL‐VWFMs did not appear after the defects of HMW‐VWFMs.
Conclusion(s): When UL‐VWFMs are found following the absence of HMWMs in VWF multimer analysis, the clinical state may shift from pre‐ to definite TA‐TMA.

PB0811
Retrospective Cohort Study of GPIbM and D1472H in Patients Referred for Von Willebrand Disease Testing
D. Dulak; N. Machin; F. Xavier; C. Seaman; M. Ragni
University of Pittsburgh, Pittsburgh, Pennsylvania, United States
Background: The 2021ASH ISTH NHF WFH Von Willebrand Disease (VWD) guidelines suggest newer assays measuring platelet‐binding activity, e.g. VWF:GPIbM assay over ristocetin‐based assays to reduce variability and diagnostic error. The VWF exon 28 D1472H polymorphism assay may also reduce false positive ristocetin‐based assay results. Few studies, however, compare GPIbM and D1472H by age, race, gender, and bleeding score.
Aims: This was a retrospective cohort study comparing GPIbM and D1472H data in patients undergoing VWF testing between June 2019 and May 2021.
Methods: Deidentified data were extracted from outpatient records under exempt protocol, PRO21060079, University of Pittsburgh Institutional Review Board, including VWF:RCo (platelet agglutination), GPIbM (latex immunoassay), and D1472H (next generation sequencing).
Results: Of 126 patients tested, 92 (73.0%) were female, including 34 PF (pregnant/hormone treated) and 58 NPF (nonPF); and 29 (27.0%) were male (Table). Overall, 77.0% were adults and 23.0% children; 77.8% Caucasian and 15.9% African American. Mean GPIbM was higher in females than males, 82 ± 5 IU/dL vs 71 ± 6; in PF than NPF, 86 ± 6 vs 71 ± 2 (p < 0.01); in adults than children, 80 ± 4 vs 77 ± 7, and in African Americans than Caucasians, 92 ± 9 vs 77 ± 4. GPIbM varied inversely with BAT, BS > =5 vs <5 in females, 73 ± 7 vs 89 ± 6; and BS > =3 vs <3 in males, 62 ± 10 vs 82 ± 7. GPIbM varied by VWF:RCo (<0.30, 0.30–0.50, > = 50), 61 ± 9, 76 ± 6, and 95 ± 6, respectively, with low GPIbM (< 52 IU/dL) in 38.1%, 26.0%, and 0%, respectively. Comparing VWF:RCo < 50 and > =50, GPIbM was low in 21/71 (29.6%) vs. 0/33 (0%), p = 0.001, mean GPIbM 72 ± 5 vs. 95 ± 6, p < 0.01, with correlation r = 0.472, p > 0.05, between GPIbM and VWF:RCo. Comparing D1472H+ and D1472H‐ groups, VWF:RCo, GPIbM, and correlation were not different.
Conclusion(s): Despite improved platelet‐binding, the GPIbM assay is elevated in the majority of those with VWF:RCo < 50, African Americans, females, with pregnancy and hormone use, limiting its diagnostic utility.

PB0812
Lack of Congruent VWF Levels, Genetics, and Bleeding Symptoms in Families with a Type 1 von Willebrand Disease Diagnosis
M. Schilthuis1; P. Christopherson2; S. Haberichter3; A. Paterson4; R. Montgomery3; V. Flood 1
1 Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 2 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 3 Versiti Blood Center of Wisconsin, Milwaukee, Wisconsin, United States; 4 The Hospital for Sick Children, Toronto, Ontario, Canada
Background: Type 1 von Willebrand Disease (VWD) is a common bleeding disorder defined by partial quantitative deficiency of von Willebrand factor (VWF). Inheritance is reported as autosomal dominant with variable penetrance.
Aims: To determine the percentage of families who show complete congruence of type 1 VWD diagnosis, VWF genotype, and bleeding phenotype.
Methods: Index cases (IC) and their family members were enrolled at 35 centers across the United States (310 families, 1402 individuals). Diagnosis of type 1 VWD was defined as VWF:Ag < 50 IU/dL or VWF:RCo < 54 IU/dL with normal activity/antigen ratio. Sanger sequencing of the VWF coding region (± comparative genomic hybridization) was performed for ICs with assessment of variant pathogenicity by ACMG criteria. Family members were analyzed for presence of variant (s) in IC for that family. Bleeding scores were calculated using the ISTH bleeding assessment tool.
Results: Familial congruence was determined across the three categories (presence/absence of genetic variant, low VWF levels, and abnormal bleeding score). Average family size was 4.5 individuals. Only 24% of families showed complete congruence of laboratory values, genotype, and bleeding phenotype. The proportion of congruent and incongruent families with or without a VWF variant did not differ (p = 0.24). Furthermore, the mean VWF:Ag was similar between those with and without a genetic variant (p = 0.15). The mean IC bleeding score was significantly higher in families with congruency of genetic variants, bleeding score, and VWF levels (p < 0.001).
Conclusion(s): Most families affected by type 1 VWD do not show complete congruence of VWF levels, genotype, and bleeding phenotype. Congruence was associated with higher bleeding scores in the IC affirming importance of symptoms in VWD diagnosis. Genetic changes outside the VWF coding region or locus or other explanations for bleeding phenotypes may be important in this population. Our study demonstrates the complicated nature of VWD inheritance.
PB0809
Performance of the ISTH‐BAT bleeding score in a population of newly diagnosed VWD in the U.S. Zimmerman Program
P. Christopherson 1; T. Abshire1; D. Doherty2; V. Flood3; S. Haberichter4; J. O'Donnell5; R. Montgomery4
1 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 2 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin 6, Dublin, Ireland; 3 Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 4 Versiti Blood Center of Wisconsin, Milwaukee, Wisconsin, United States; 5 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland
Background: Diagnosis of VWD can be challenging due to subjective bleeding assessment and variable laboratory testing.
Aims: We sought to evaluate newly diagnosed VWD subjects by analyzing VWF levels, bleeding scores and incorporating current Guidelines on the diagnosis of VWD.
Methods: Zimmerman Program Prospective Study patients referred to local hematologists and assessed for a bleeding disorder were enrolled and evaluated for VWD. 564 subjects with a new diagnosis of type 1/Low VWF were analyzed. Abnormal ISTH‐Bleeding Assessment Tool (BAT) scores were determined using published age and gender cutoffs. Central VWF:Ag and VWF:GPIbM were used to divide cohort by levels of <30, 30–50 and > 50 IU/dL (normal).
Results: 346 (61%) of newly diagnosed subjects had an abnormal BAT while 218 (39%) had a normal BAT with no significant difference in gender or VWF levels between those groups. The BAT did not correlate with VWF levels but did correlate with age. Both <30 and 30–50 groups had 35% of subjects with normal BATs, similar number of females, and no significant difference in age. When taking both levels and bleeding score into account, 201 subjects would be classified as type 1 VWD under new guidelines. Using a VWF level cutoff of 50, the sensitivity of the BAT to predict type 1 VWD was 65%, specificity 42% in all subjects. When adult women were evaluated separately, 107 (70%) had an abnormal BAT and 45 (30%) had a normal BAT with sensitivity of 73% and specificity of 33%.
Conclusion(s): While BATs are a useful tool to quantitate bleeding and assist with the diagnosis of VWD, the pediatric population, as well as patients who present with moderate to borderline VWF levels and bleeding, remain challenging. Further analysis of subject's subsequent samples and bleeding scores will provide insight into the diagnostic accuracy of VWD and utility of a BAT in this cohort.
PB0814
How do laboratories perform von Willebrand disease (VWD) diagnostics?
M. Hollestelle 1; J. Meijers2; J. Eikenboom3; P. Meijer1
1 ECAT Foundation (External quality Control for Assays and Tests), Voorschoten, The Netherlands, Voorschoten, Zuid‐Holland, Netherlands; 2 Department of Experimental Vascular Medicine, Amsterdam University Medical Centers, Amsterdam, The Netherlands and Department of Molecular Hematology, Sanquin Research, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Dysfunction or absence of von Willebrand factor (VWF) may lead to von Willebrand disease (VWD), which is a common inherited bleeding disorder. VWD is classified in 3 types: type 1 is a partial quantitative deficiency of VWF, type 3 is a complete quantitative deficiency of VWF and type 2 consist of qualitative abnormalities of VWF. To come to a correct VWD diagnosis multiple tests and a correct interpretation of these tests are needed.
Aims: The aim of the study was to gain insight into the approach of laboratories towards the VWD diagnosis with respect to the used algorithm, tests, cut‐off values and the applied guidelines.
Methods: Data from two samples of the external quality assessment (EQA) VWF surveys of the ECAT were evaluated. The classification of the samples by the participants were compared with the measurements found for various VWF parameters. Furthermore, results were analyzed of a questionnaire that was sent to hemostasis laboratories about the performance of VWD diagnostic approaches.
Results: The majority of participants indicated the correct classification for the two samples used in the EQA program. However, respectively 6 and 23% indicated another classification. For both samples significant differences were observed in the measurements of the VWF parameters divided by the classification of the participants, e.g. for VWF:RCo/VWF:Ag ratio (Figure 1). The questionnaire demonstrated that the testing approach varied between the laboratories, especially the parameters that were essential for the discrimination between VWD type 1 and healthy individuals and the cut‐off values used to discriminate between VWD type 1 and 2.
Conclusion(s): Diagnosis of VWD is heterogeneous in approach with significant differences in VWF measurements, used guidelines and as a consequence differences in applied cut‐off values. This will cause differences in the diagnosis of VWD and treatment of patients. Harmonization of approaches is needed.

VPB0837
Characterization of Cellular Pathogenic Mechanism of Type 1 von Willebrand Disease with A1500V mutation
Y. Chen 1; C. Chang2; S. Cheng3; S. Hu1; S. Lai1
1 Hemophilia care & research center, Division of Hematology/Oncology, Tri‐Service General Hospital, National Defense Medical Center, Taipei, Taipei, Taiwan (Republic of China); 2 School of Medicine, Graduate Institute of Clinical Medicine, Taipei Medical University, Taipei, Taiwan 3Hemophilia center, Division of Pediatric Hematology/Oncology, Taipei Medical University Hospital, Taipei, Taiwan, Taipei, Taipei, Taiwan (Republic of China); 3 Hemophilia and Rare Disease Treatment Center, Tungs' Taichung MetroHarbor Hospital, Taichung, Taiwan, Taichung, Taichung, Taiwan (Republic of China)
Background: We have found that 7 type 1 von Willebrand disease (VWD) patients harbored the same variant (c.4499C > T, p.Ala1500Val).
Aims: The aims of this study were to investigate the causative mutation of the variant and elucidate its pathogenic mechanism on cell expression.
Methods: Both wild type and homozygous variant VWF in the conditioned medium and cell lysates were analyzed by enzyme‐linked immunosorbent assay and VWF ristocetin cofactor activity assay. VWF multimers electrophoresis of the expressed mutated and wild type VWF in the HEK‐293 cells were performed. To evaluate the intracellular location of VWF, the cells were stained by immunofluorescence antibodies analyzed by Leica/Thunder microscope. The structure of VWF A2 domain was used to build up the A1500V mutant model using the protocol of Build Mutants (Bivia Discovery Studio 2019).
Results: Statistically significant decreases in VWF Ag in the cell lysate, medium and total amount in A1500V variant as compared to the wild type, as shown in Figure 1. There was no difference in the VWF secretion percentage in the A1500V mutation and wild type. The multimers analysis of heterozygous and homozygous A1500V‐VWF showed the presence of a full spectrum with slight reduction concentration. There was no increased retention of VWF in the endoplasmic reticulum for A1500V variant, identified by the co ‐localization of VWF and PDI, as shown in Figure 2. Structural comparison of wild type VWF and the A1500V VWF revealed significant alteration. The effect of the variant influenced on the protein stability in the VWF A2 domain.
Conclusion(s): We demonstrate the A1500V was a causative mutation for type 1 VWD. The mutation affected intracellular VWF biosynthesis significantly but did not influence secretion or multimer formation of VWF. In silico studies indicate that the mutation resulted in the structure change associated with the protein instability.
The VWF Ag level in cell lysate, medium and total amount were all higher in wild type compared to A1500V variant with statistical difference (619 ± 166 ng/mL vs 192 ± 456 ng/mL in cell lysate, 1062 ± 80 ng/mL vs 443 ± 69 ng/mL in medium and 1681 ± 226 ng/mL vs 635 ± 111 ng/mL in total amount). VWF Ag was expressed as mean ± standard error.
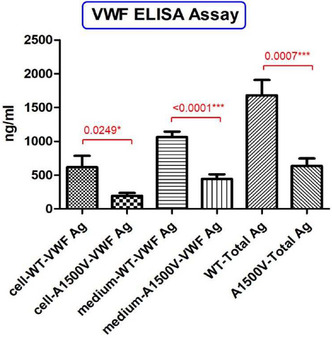
Cells were immunostained using rabbit‐anti‐VWF (green; Abcam) to visualize total VWF, and mouse anti‐PDI with DyLight ® ‐ 550 conjugated goat anti‐mouse IgG (red). Nucleus: DAPI (blue)。.

PB0816
In vitro investigation of emicizumab efficacy and mode of action in vWD type 2 and 3 samples
M. Locke 1; N. Receveur1; A. Kiialainen2; T. David2
1 F. Hoffmann‐La Roche, Ltd., Basel, Basel‐Stadt, Switzerland; 2 F. Hoffmann‐La Roche Ltd., Basel, Basel‐Stadt, Switzerland
Background: Von Willebrand factor (vWF) supports hemostasis by promoting platelet adhesion and activation, acting as a carrier for FVIII, and modulating fibrinolysis. Quantitative (type 1 or 3) or qualitative (type 2) defects in vWF lead to the bleeding disorder von Willebrand Disease (vWD). Emicizumab (Hemlibra®, Roche) has proven effective for prophylaxis in FVIII‐deficient Hemophilia A (HA) patients, providing a scientific rationale for its use in the management of type 3 vWD.
Aims: Investigate the efficacy and mechanisms by which emicizumab promotes hemostatic activity in HA and vWD plasma and whole blood.
Methods: Efficacy and Mode of Action (MoA) data was generated in multiple lots of HA and vWD patient plasmas and reconstituted blood using platelet aggregation/activation assays, thrombin generation (TG) assays and fibrinolysis assays.
Results: Emicizumab had no effect on platelet activation or aggregation, but promoted TG in HA and vWD type 3 plasmas (Table 1). Higher baseline TG was measured in reconstituted vWD type 2 N plasmas (5–40% residual FVIII) compared to type 3 plasma, which increased a further 2.4 to 3.3‐fold by the addition of 50 ug/ml emicizumab. In tissue plasminogen activator (tPA)‐induced fibrinolysis assays, emicizumab promoted surprisingly high anti‐fibrinolytic activity in vWD type 3 and HA samples (2.2 and 1.6‐fold extension to clot lysis times, respectively, Table 2) compared to the anti‐fibrinolytic activity of FVIII.
Conclusion(s): Emicizumab promotes hemostasis in HA and vWD plasma by supporting TG and delaying fibrinolysis, and is unlikely to directly affect platelet activation or aggregation. Our preliminary data suggest that emicizumab promotes higher TG in vWD type 2 N plasma when compared to HA and type 3 plasmas. The anti‐fibrinolytic mechanisms of emicizumab are under investigation. Overall, these data support the notion of emicizumab efficacy in vWD type 2 N and 3 patients and provide early insight into its MoA.


PB0821
Data mining for profiling the mutational landscape of von Willebrand disease
O. Seidizadeh 1; A. Cairo2; L. Baronciani3; M. Pagliari4; S. Siboni5; E. Biguzzi6; F. Peyvandi7
1 Fondazione IRCCS Ca'Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy 2. Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy, Milan, Lombardia, Italy; 2 Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milano, Lombardia, Italy; 3 Fondazione IRCCS Ca′ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milano, Lombardia, Italy; 4 Fondazione IRCCS Ca'Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, Milan, Lombardia, Italy; 5 Fondazione IRCCS Ca′ Granda, Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 6 Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 7 Fondazione IRCCS Ca′ Granda ‐ Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Von Willebrand disease (VWD) with a population prevalence of 1% is caused by mutations in the von Willebrand factor (VWF). However, its exact prevalence is not yet established. More than 900 distinct mutations have been found in the VWF so far.
Aims: To explore the VWF mutational burden and to estimate VWD prevalence in the general population using the genome Aggregation Database (gnomAD).
Methods: VWF variants were obtained from the gnomAD (v2.1.1; 141,456 subjects) and were compared to the Human‐Gene‐Mutation‐Database (HGMD) and Leiden‐Open‐Variation‐Database (LOVD v.2.0 and v.3.0). The following variants were considered pathogenic: nonsense and frame‐shift; splicing (+2/−2); splicing (+8/−8) and missense predicted deleterious by 3/3 and 7/7 tools respectively or being associated with VWD in HGMD and LOVD2/3. All identified pathogenic variants were used to evaluate the VWF mutational burden and VWD prevalence.
Results: Using gnomAD, we identified 505 distinct VWF pathogenic variants (244 unique): 287 not reported (novel) and 218 already reported. 138 of 218 gnomAD variants were not predicted as pathogenic by all in‐silico tools but were reported to be associated with VWD in HGMD and/or LOVD databases. Thirteen variants had a minor‐allele‐frequency (MAF) >0.01 in at least one population. In particular, 3 variants (p.Arg2185Gln, p.Met740Ile, p.His817Gln) were very frequent in Africans/Africans American (MAF: 0.11–0.18, Table 1). Due to these variants, the African population was excluded from the calculation of VWD prevalence in the general population and was analyzed standalone (Table 2). This analysis revealed a heterozygote frequency of 0.13 among all populations with a prevalence of 0.45 and 13.4 for recessively‐inherited and autosomal‐dominant, respectively among 100 individuals.
Conclusion(s): Through our systematic analysis of>140,000 individual exome/genome data from the gnomAD database, we found 287 novel VWF variants predicted as pathogenic. Our results demonstrated that the “true” worldwide prevalence of VWD is more than 10 times higher than that reported so far.


PB0829
In Vitro Field Study Assessing How Clinical Hemostasis Laboratories Analyze Recombinant and Plasma‐Derived von Willebrand Factor Products
P. Turecek 1; R. Ilk2; H. Gritsch1
1 Baxalta Innovations GmbH, a Takeda company, Vienna, Wien, Austria; 2 Takeda Manufacturing Austria AG, Vienna, Wien, Austria
Background: Several clinical laboratory methods have been established to measure plasma‐derived von Willebrand factor (pdVWF) in plasma samples, but few data are available on their use for analyzing recombinant VWF (rVWF).
Aims: Evaluate how clinical/diagnostic laboratories analyze pdVWF and rVWF in vitro.
Methods: Human VWF‐deficient plasma samples (HRF Inc., Raleigh, NC, USA) were spiked with rVWF or pdVWF (antihemophilic factor/VWF complex [human]), each at final concentrations of 1.0, 0.6, 0.2, 0.1 IU/mL von Willebrand factor:ristocetin cofactor activity [VWF:RCo] according to labeled VWF activity. The SSC/ISTH secondary coagulation standard lot #5 was used as a control. Participating laboratories received 3 sets of these blinded aliquots and assayed each set in an independent run. The mean results per assay were compared with the expected potency based on labeled activity of the drug. Microsoft Excel v16.0 and Minitab 20.4 were used for data arrangement, graphical visualization, and statistical analyses.
Results: Samples were analyzed by 22 laboratories from 11 countries (North America, n = 5; Europe, n = 16; India, n = 1). Depending on assay type, variability between laboratories was high, particularly for VWF:RCo activity. Geometric mean recovery of VWF:RCo for rVWF was acceptable (78–112% relative to labeled VWF:RCo target); measured pdVWF concentrations were significantly off‐target, with lower values (51–104%). For both products, high recovery of VWF antigen content with acceptable precision was achieved versus labeled VWF:RCo activity (109–122%) (Table 1). VWF platelet receptor glycoprotein Ib (GPIb) assays gave high recovery for rVWF (114–125%) and low recovery for pdVWF (62–74%). VWF collagen‐binding assays gave similar results to GPIb methods (Table 2).
Conclusion(s): This in vitro study indicates that the results of VWF assays used in clinical laboratories differ between rVWF and pdVWF, particularly for GPIb assays. These differences may arise from the higher multimeric structure of rVWF and should be considered when analyzing post‐infusion samples from patients treated with either product.

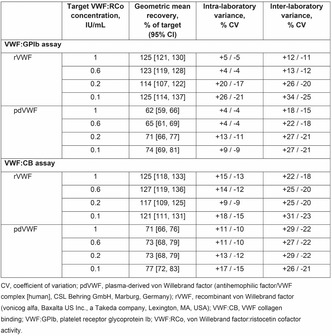
PB0810
A novel role for subtle enhanced VWF clearance in Low VWF pathogenesis highlights limitations of VWFpp/VWF:Ag ratio assessment of clearance
D. Doherty 1; M. Byrne2; M. Nolan3; J. O'Sullivan4; K. Ryan5; N. O'Connell6; S. Haberichter7; P. Christopherson8; J. Di Paola9; P. James10; M. Lavin11; J. O'Donnell12
1 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin 6, Dublin, Ireland; 2 National Coagulation Centre, St James's Hospital, Dublin, Ireland, Dublin, Dublin, Ireland; 3 St James's Hospital, Dublin, Dublin, Ireland; 4 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland; 5 National Coagulation Centre,St James's Hospital,Dublin8, Dublin, Dublin, Ireland; 6 St. James Hospital, Dublin, Ireland; 7 Versiti Blood Center of Wisconsin, Milwaukee, Wisconsin, United States; 8 Versiti Blood Research Institute, Milwaukee, Wisconsin, United States; 9 Washington University in St. Louis, St. Louis, Missouri, United States, 10 Department of Medicine, Queen's University, Kingston, Canada, Kingston, Ontario, Canada, 11 St James Hospital, Dublin, Dublin, Dublin, Ireland, 12 Irish Centre for Vascular Biology, School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland, Dublin, Dublin, Ireland
Background: Markedly enhanced VWF clearance represents an important pathogenic mechanism in Type 1C and Type 2 VWD. In contrast, previous studies suggest that reduced VWF biosynthesis is more important in patients with VWF levels in the 30‐50 IU/dL range.Aims: VWF clearance was investigated in the Low VWF Ireland cohort (LoVIC) using the recent 2021 ASH/ISTH/NHF/WFH guideline recommendation for increased clearance diagnosis (plasma VWF:Ag fall‐off >30% from peak at 4 hours following desmopressin).
Methods: Low VWF patients (n = 75) were recruited following ethical approval and informed consent. Following intravenous desmopressin (DDAVP), blood samples were drawn for VWF:Ag, VWF:RCo and FVIII:C at baseline (T0), 1 (T1) and 4 hours (T4).
Results: Based on ASH guideline criteria, 15/75 (20%) of Low VWF patients demonstrated enhanced VWF clearance (Figure 1). Low VWF patients with enhanced clearance demonstrated (i) significantly higher initial increases in plasma VWF:Ag levels at T1 (median 3.84 vs 2.89‐fold, p < 0.0001) and (ii) significantly greater fall‐offs in plasma VWF:Ag, VWF:RCo and FVIII:C levels between T1 and T4 (median 1.53 vs 1.20‐fold for VWF:Ag, p < 0.0001) (Figure 1). Thus, within the Low VWF cohort, there is a subgroup in whom desmopressin triggers enhanced endothelial release of VWF‐FVIII, which then undergoes enhanced plasma clearance. The steady‐state VWFpp/VWF:Ag ratio has been used to identify patients with Type 1C VWD. Interestingly, in the Low VWF cohort, we observed a marked discordance between VWFpp/VWF:Ag ratios and DDAVP fall‐off data. Even using the blood group O VWFpp/VWF:Ag 97.5th percentile cut‐off (>2.15), only 12% (9/75) of Low VWF patients were identified as suspected enhanced clearance.
Conclusion(s): Our findings define a novel role for subtle enhanced VWF clearance in Low VWF pathogenesis. Furthermore, our data highlight important limitations of the VWFpp/VWF:Ag ratio in identifying increased clearance in patients with mild to moderate reductions in plasma VWF levels.

PB0827
A Systematic Review of Acquired von Willebrand Syndrome Associated With Non‐Cardiac Diseases: Epidemiology, Disease Burden, and Management
P. Du 1; R. Bharali2; S. Sun3
1 Takeda Development Center Americas, Inc., Hershey, Pennsylvania, United States; 2 HCD Economics, Daresbury, England, United Kingdom; 3 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States
Background: Acquired von Willebrand syndrome (aVWS) is a rare bleeding disorder caused by certain underlying conditions, including neoplasms and autoimmune disorders. The pathogenic mechanism of aVWS varies by primary condition but can result in impaired von Willebrand factor (VWF) function, enhanced VWF clearance, or loss of high molecular weight VWF multimers.
Aims: To describe the epidemiology, disease burden, and management of non‐cardiac‐related aVWS.
Methods: A systematic review (PROSPERO 2021 CRD42021241527) of studies/reports published up to 31 January 2021 (no date limit) was performed using MEDLINE, Cochrane Central Register for Controlled Trials, EMBASE, and Web of Science databases utilizing keywords encompassing aVWS, excluding reports on cardiac conditions. Additional searches were performed using trial registries, gray literature (ethos.bl.uk, dart‐europe.eu, oatd.org), congresses, and Google Scholar. Identified studies were screened by 2 independent reviewers. Review articles and duplicates were removed, and data from identified publications were extracted.
Results: Of 300 studies included, 58 were cohort studies (total 13,822 patients) and 242 were case series/reports (327 patients); median (range) patient age at aVWS diagnosis was 55 (1–93) and 51 (1.5–90) years, respectively. Blood system‐related neoplasms were the most frequent underlying cause (Table 1). Bleeding‐related events were reported in 26 cohort studies (Table 2). VWF/factor VIII concentrate use (Table 2) was reported in only 103 patients in cohort studies. 103 deaths were reported in 6 cohort studies (0.7% of total 13,822 patients from 58 cohort studies), with the highest mortality (82/164 patients; 50%) reported in a study of patients with multiple myeloma and related plasma cell disorders.
Conclusion(s): Myeloproliferative and lymphoproliferative neoplasms were the most common non‐cardiac conditions associated with aVWS. This systematic review highlights the limited information available on epidemiology, burden of illness, and treatment of non‐cardiac‐related aVWS, and the need for further research to better understand this rare disease and improve the patients' unmet needs.


PB0817
Emicizumab ameliorates hemostasis in von Willebrand Disease type 3 but not type 2A murine models
G. Mc Cluskey 1; O. Christophe2; P. Lenting2; C. Denis3; C. Casari4
1 Inserm U1176 ‐ HITh, Le Kremlin‐Bicetre, Ile‐de‐France, France; 2 INSERM U1176, Le Kremlin Bicetre, Ile‐de‐France, France; 3 Inserm, Le Kremlin‐Bicêtre, Ile‐de‐France, France; 4 Inserm U1176 ‐ HITh, Le Kremlin Bicetre, Ile‐de‐France, France
Background: Emicizumab is a bi‐specific antibody used to treat hemophilia A patients. Von Willebrand Disease (VWD) is characterized by quantative/qualitative defects of von Willebrand factor (VWF) that can lead to secondary factor VIII (FVIII) deficiency. Successful off‐label use of emicizumab has been reported in VWD‐type 3 patients, re‐igniting the question of whether restoring FVIII‐like activity improves the bleeding phenotype in VWD patients.
Aims: To assess the effects of emicizumab on the bleeding profiles of VWD mouse models.
Methods: A tail‐vein‐transection (TVT) bleeding assay was applied to mouse models of VWD‐type 3 (Vwf−/−) and VWD‐type 2A(IIA) (VWFp.R1597W+/+/GP1BA+/+). Emicizumab (5 mg/kg), in conjunction with human factors IX and X, or FVIII (10%–100%), were injected.
Results: In VWD‐type 3 mice, one‐third of emicizumab‐receiving mice (5 out of 15) displayed a normal haemostatic response to the injury (blood lost <150 μL), whereas all buffer‐injected mice (n = 10) displayed severe bleeding phenotypes (p = 0.01). Consistently, 10/15 (66.7%) emicizumab‐treated mice spontaneously formed an occlusive clot, temporarily arresting the bleeding, compared to 2/10 (20%) buffer‐injected mice (p = 0.02). In contrast, emicizumab had no effect on the bleeding profiles of VWD‐type 2A mice (blood lost: 491+/−267 μL buffer‐injected, 503+/−279 μL emicizumab‐treated, p > 0.99). As control, we next compared emicizumab‐ and FVIII‐treatment in VWD‐type 2A mice subjected to TVT and, consistent with emicizumab treatment, replenishing 10‐ or 100% of FVIII activity was not sufficient to correct the bleeding phenotype of these mice (474+/−208 μL buffer‐injected, 382+/−170 μL 10% FVIII, p = 0.38; 306+/−241 μL 100% FVIII, p = 0.17).
Conclusion(s): As observed in patients, emicizumab improved the bleeding phenotypes in a mouse model of VWD‐type 3, suggesting that emicizumab assists in restoring primary clot formation in the absence of VWF. However, no amelioration was observed in VWD‐type 2A mice, suggesting that degraded VWF, even at low concentrations, has a negative effect on clot formation in a TVT bleeding model.
PB0824
Characteristics and Clinical Burden Among Patients With von Willebrand Disease Eligible for Prophylaxis: Results From a Retrospective US Cohort Study
P. Du 1; E. Swallow2; J. Marden2; E. Billmyer2; E. Yim2; S. Sun3
1 Takeda Development Center Americas, Inc., Hershey, Pennsylvania, United States; 2 Analysis Group, Inc., Boston, Massachusetts, United States; 3 Takeda Development Center Americas, Inc., Cambridge, Massachusetts, United States
Background: Although international guidelines recommend long‐term prophylaxis for patients with von Willebrand disease (VWD) who experience severe/frequent bleeding, it is not currently approved in the US.
Aims: Assess unmet needs by describing the real‐world characteristics and clinical burden among patients with VWD overall and those eligible for prophylaxis (i.e., with a history of severe/frequent bleeds).
Methods: This retrospective cohort study used de‐identified electronic medical records (EMR) data from an integrated US healthcare system. Two cohorts were analyzed: (1) patients with ≥2 diagnoses of VWD in the EMR (01/2004–12/2020), and (2) the subset of these patients who were eligible for prophylaxis (history of ≥3 joint bleeds, ≥2 gastrointestinal bleeds, ≥5 total bleeds in the last 12 months, or ≥1 major bleed). The index dates of these two cohorts were defined as the date of first VWD diagnosis and the date that a patient first met prophylaxis eligibility criteria, respectively. Patients were required to have ≥6 months of data before (baseline period) and after the index date (study period). Clinical characteristics and bleeding events were described.
Results: Overall, 396 patients with VWD, including a subset of 75 prophylaxis‐eligible patients, were included for analysis; none received long‐term prophylaxis. Patient demographics were similar between cohorts, except for age (Table 1). In both cohorts, the proportions of patients with comorbidities or bleeds increased between the baseline and study periods, and the proportions appeared higher in prophylaxis‐eligible patients. The most common comorbidities were chronic pulmonary disease, obesity, anemia, anxiety, and depression. Patients experienced more new bleeds than recurrent bleeds from the same anatomical location (Table 2).
Conclusion(s): In this retrospective US cohort study, patients with VWD experienced a high burden of bleeding‐related illness and other comorbidities. Nearly 20% of patients were eligible for prophylaxis owing to severe or frequent bleeds, and thus may have benefited from prophylactic treatment.


Platelets and Megakaryocytes
Megakaryocytes and Thrombopoiesis
VPB0366
Serial Thrombopoietin Levels in Immune Thrombocytopenia Patients on Treatment
P. Woolley 1; J. Westwood2; M. Scully3
1 University College London Hospitals NHS Foundation Trust, London, United Kingdom, London, England, United Kingdom; 2 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom; 3 University College London Hospitals NHS Foundation Trust, London, England, United Kingdom
Background: Measurement of serum thrombopoietin (TPO) levels may predict treatment response to TPO receptor agonist (TPO‐RA) therapy.
Aims: To evaluate the clinical relevance of TPO levels in ITP patients receiving TPO‐RAs.
Methods: Serum TPO concentration was measured by ELISA and using linear regression calculation, normal TPO range was 8.8 pg/mL ‐114 pg/mL from 50 normal controls. Written consent was obtained.
Results: 171 serial TPO samples from 105 patients were included in the analysis and patients divided into 3 groups; ITP patients not on treatment (n = 52), patients on Eltrombopag (n = 42) and patients on Romiplostim (n = 11). There was no correlation between TPO level and platelet count for patients not on treatment (p = 0.29). Patients on Eltrombopag had a median platelet count of 75x109/L(range 3x109/L‐333x109/L) a median TPO level of 181.45 pg/mL. Patients on Romiplostim had median platelet count of 61x109/L (range 2x109/L‐425x109/L) with a median TPO level of 61 pg/mL. TPO level was inversely proportional to platelet count for patients on Eltrombopag (p = 0.04) and Romiplostim (p = 0.02), however this relationship was lost for non‐responders (platelet count <30x109/L) on Eltrombopag (median TPO level 184.65 pg/mL (range 29.70 pg/mL‐1299.81 pg/mL) (p = 0.7) (Figure 1). Figure 2 demonstrates the difference between Eltrombopag and Romiplostim effect on TPO level. Adjustment for different drug dose removes this as a confounder. As drug dose increased so did platelet count (p = < 0.0001) and for both drugs an inverse relationship between TPO level and platelet count is noted (p = < 0.0001); median TPO level for Eltrombopag 187.81 pg/mL and Romiplostim 158.80 pg/mL. TPO‐RA responders (platelet count >30x109/L) had lower TPO levels than non‐responders.
Conclusion(s): TPO level and platelet count have an inverse relationship for patients on TPO‐RA therapy. Eltrombopag and Romiplostim affect TPO levels differently at any given platelet count for responsive patients with both drugs showing an inverse relationship to platelet count. This confirms TPO levels are a potentially clinically useful management tool in patients with ITP.


PB0355
Knockdown screening of microRNAs in thrombocytes and their role in thrombopoiesis
A. Al Qaryoute; W. Fallatah; S. Dhinoja; P. Jagadeeswaran
University of North Texas, Denton, Texas, United States
Background: Previous studies have shown that human platelets and megakaryocytes carry microRNAs suggesting their role in platelet function and megakaryocyte development, respectively. However, there is limited information on their role in megakaryocyte maturation. We hypothesize that zebrafish thrombocytes could be used as a model to study their role in megakaryocyte maturation because thrombocytes have megakaryocyte features. Recently, we have identified 15 microRNAs in thrombocytes using single‐cell RNA sequencing. Investigating their role in thrombocyte maturation should provide insight into thrombocyte development.
Aims: To knockdown microRNAs found in zebrafish thrombocytes by piggyback knockdown method and understand their role in thrombocyte maturation.
Methods: Piggyback knockdown ASO/VMO hybrids of each microRNA were injected into the adult fish. Blood was collected 48 hours post‐injection, and the thrombocytes were labeled by DiI‐C18 and quantified by flow cytometry. Likewise, we performed knockdowns in larvae and measured TTO using an arterial thrombosis assay.
Results: To evaluate the role of microRNAs in zebrafish thrombocyte maturation, we performed a knockdown of each of the 15 microRNAs and quantified the number of thrombocytes. We found that knockdown of mir‐223, let‐7b, and mir‐7148 increased total thrombocyte percentage. We then gated young, more mature, and fully mature thrombocyte populations according to the intensity of DiI and quantified these populations. The results showed that more mature and fully mature populations increased whereas the ratio of young to more mature populations decreased. We also confirmed the increase in total thrombocytes by knockdowns in larvae using TTOs.
Conclusion(s): We found knockdown of mir‐223, let‐7b, and mir‐7148 led to an increase in the percentage of the total, fully mature, and more mature thrombocytes, suggesting that mir‐223, let‐7b, and mir‐7148 are repressors for thrombocyte maturation. This information should be useful in understanding downstream targets for the microRNAs that ultimately could lead to novel therapeutic targets to treat thrombocytopenia.
PB0359
CRISPR genome editing coupled with optimized biomanufactured human platelets for molecular and functional investigation of platelet biogenesis
L. Mallo 1; A. Pongerard1; C. GACHET1; Y. KNAPP2; F. Lanza1; C. Strassel1
1 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 2 Université Avignon, LAPEC UPR4278, F‐84000 Avignon, France, Avignon, Provence‐Alpes‐Cote d'Azur, France
Background: Many rodent models represent powerful tools for our understanding of platelet disorders. However, the inherent differences between human and murine platelets limit their exploration. These drawbacks, combined with the difficulty in accessing patients and the desire to reduce animal experimentation, make CRISPR genome editing in CD34+ hematopoietic progenitors (HP) an innovative and powerful tool to better explore platelet pathologies and for future therapeutic applications.
Aims: Our goal was to develop an efficient method for large‐scale production of genetically modified platelets, proceeding from CRIPSR‐modified HP down to platelet production. As a proof of concept, we deleted the genes encoding GP1BA and TUBB1, known to play essential roles in platelet production and function.
Methods: Blood‐derived CD34+ HP were inactivated for GP1BA or TUBB1 using CRISPR/Cas9. MKs were then differentiated and subjected to a custom proprietary platelet release device (see accompanying abstract).
Results: CRISPR editing resulted in a 80–90% deletion efficiency for each targeted gene. This resulted in MK maturation defects previously identified in patients and mutant mice. These included for GP1BA, a less well‐developed demarcation membrane system and abnormal proplatelet morphology, characterized by fewer pseudopodial extensions, thicker shaft sections and increased diameter of the coiled elements. As expected for TUBB1, a profound defect in proplatelet extension was observed. For both deletions, fully mature MK when subjected to the platelet release device led to biomanufactured platelets exhibiting larger diameters and misarranged microtubule coils. Large production of these modified platelets is under way which will allow their functional evaluation.
Conclusion(s): These results predict that the CRIPSR/Cas9 technology combined with our platelet release device will represent a powerful tool for studying candidate genes involved in human platelet biogenesis and function.
PB0357
Megakaryocyte‐driven blood clot contraction and its structural mechanisms
O. Kim 1; A. Gagne2; R. Litvinov3; D. French2; L. Brass4; J. Weisel1
1 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 2 Department of Pathology and Laboratory Medicine, The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 3 University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania, United States; 4 Hematology‐Oncology Division, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, United States
Background: Contraction of blood clots and thrombi is driven by platelets. A growing body of evidence suggests a direct involvement of megakaryocytes (MKs) in local blood clotting and thrombosis, but it is unknown whether MKs can drive contraction of blood clots and, if so, what are the mechanisms of MK‐driven clot contraction.
Aims: To determine if MKs can induce contraction of clots and study dynamic structural alterations at the single‐cell levels.
Methods: CD41(+)CD42b(+) MKs were generated from human induced pluripotent stem cells and suspended in citrated platelet‐free human blood plasma at 4 million/mL. Clot formation and MK activation were induced by adding 2 U/mL thrombin and 3 mM CaCl2. Changes in clot size were followed for 60 min using an optical tracking system, and super‐resolution confocal microscopy of MK‐containing plasma clots was used to follow structural rearrangements of the fluorescently labeled cells and fibrin fibers.
Results: Thrombin‐activated MKs caused contraction of plasma clots with the average final extent of contraction of ~50% and two kinetic phases that differed in the mean kinetic rates and durations. The contractility of MKs was sensitive to inhibitors of the myosin II ATPase activity, actin polymerization, and αIIbβ3‐fibrin(ogen) binding, suggesting the same contractile machinery as in platelets. The biomechanical mechanism of MK‐driven clot contraction involves formation of filopodia and larger cellular protrusions that attach to fibrin fibers and undergo extension‐retraction cycles together with the cell body contraction. By pulling on the fibrin network, the contracting MKs actively remodel the clot, resulting in compaction of the entire MK‐fibrin meshwork.
Conclusion(s): Our results provide evidence for the ability of MKs to contract fibrin clots and elucidate the structural mechanisms of MK‐driven clot contraction, suggesting a novel mechanistic insight into the mechanobiology of MKs, which may play a direct role in modulating the permeability and obstructiveness of blood clots and thrombi.

PB0362
Molecular insights into the cause and treatment of congenital thrombocytopenia in mice lacking the co‐inhibitory receptor G6b‐B
A. MAZHARIAN1; B. Maître2; D. Hennequin3; M. Lourenco‐Rodrigues4; S. Heising5; H. De la salle6; S. Watson7; C. GACHET2; Y. Senis 8
1 Etablissement Français du Sang, University of Strasbourg, Strasbourg, France, Strasbourg, Alsace, France; 2 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 3 Inserm U1255, Etablissement Français du Sang Grand Est, Strasbourg, Alsace, France; 4 Inotrem, Nancy, Alsace, France; 5 Institute of Metabolism and Systems Research,University of Birmingham, UK, Birmingham, England, United Kingdom; 6 Inserm U1255, Etablissement Français du Sang, Université de Strasbourg, France, Strasbourg, Alsace, France; 7 University of Birmingham, Birmingham, UK, Birmingham, England, United Kingdom; 8 Inserm UMR‐S1255/Etablissement Français du Sang/University of Strasbourg, Strasbourg, Alsace, France
Background: Thrombocytopenia is a common platelet disorder with a variety of etiologies, including antibody‐mediated platelet activation and clearance, aberrant megakaryocyte (MK) development, infection and trauma. Clinical outcomes vary from mild purpura and bruising to severe, life‐threatening bleeding. Mice lacking the immunoreceptor tyrosine‐based inhibition motif‐containing co‐inhibitory receptor G6b‐B (Mpig6b, G6b knockout, KO) are born with a complex MK/platelet phenotype characterized by severe macrothrombocytopenia, expansion of the MK population and myelofibrosis. Platelets are almost completely devoid of the GPVI‐FcR gamma‐chain collagen receptor complex and a subset have increased surface immunoglobulins. A strikingly similar phenotype has been recently reported in patients with null and loss‐of‐function mutations in MPIG6B.
Aims: To better understand the cause and treatment of macrothrombocytopenia and GPVI down‐regulation in G6b KO mice.
Methods: G6b KO mice were either treated with standard therapies for macrothrombocytopenia, or crossed with mice Rag1 KO or Syk R41A loss‐of‐function mice to rescue the phenotype. Platelet count and volume were measured using a blood analyzer. Platelet surface receptor expression and activation were measured by flow cytometry. Platelet aggregation and secretion were measured by lumi‐aggregometry.
Results: Intravenous‐immunoglobulin resulted in a transient partial recovery of platelet counts in G6b KO mice, whereas crossing these mice with Rag1 KO mice, lacking B and T cells, had no effect. Treatment with the thrombopoietin mimetic Romiplostim rescued platelet count, GPVI expression and the response of G6b KO platelets to the GPVI‐specific agonist collagen‐related peptide. Loss‐of‐function of Syk tyrosine kinase (R41A) and treatment with the Syk kinase inhibitor BI1002494 rescued platelet counts and GPVI expression in G6b KO mice, whereas the Src family kinase inhibitor dasatinib did not.
Conclusion(s): These findings provide mechanistic insights into the cause of the disease and mode of action of Syk inhibitors in the treatment of congenital macrothrombocytopenia caused by mutations in G6b.
PB0358
In‐depth analysis of the effects of unsteady flows on platelet release from suspensions of proplatelet‐bearing megakaryocytes
O. Boiron1; F. Lanza2; C. Strassel2; Y. Knapp 3
1 CNRS, Université Aix‐Marseille, Ecole Centrale Marseille, IRPHE UMR7342, F‐13000 Marseille, France, Marseille, Provence‐Alpes‐Cote d'Azur, France; 2 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 3 Université Avignon, LAPEC UPR4278, F‐84000 Avignon, France, Avignon, Provence‐Alpes‐Cote d'Azur, France
Background: Recent research in the field of platelet production has shown that unsteady flow behavior favors the efficient generation of platelets from their precursors, the megakaryocytes (MK). We obtained yields of ~100 platelets/MK in a custom device presenting a succession of convergent‐divergent geometries in which continuous periodic flow conditions are generated. However, these yields still do not reach those obtained in vivo (≈1000–2000). Therefore, a better understanding of the mechanisms of platelet release from suspended MK should allow to promote the development of even more efficient systems.
Aims: The present study proposes to analyze spatio‐temporal influence between hydrodynamics and proplatelet‐bearing MK processed into our platelet release dev ice in order to identify parameters that influence platelet yield.
Methods: Flow conditions in the device were determined by computational fluid dynamics (CFD) simulations for various flow rates. Trajectories of small 1‐10 μm spherical inert particles dragged into the flow, mimicking either platelets or MK, were inferred from these simulations. Particle histories data through the device were statistically analyzed.
Results: We showed that, regardless of diameter, the particles underwent a complex history marked by alternating laminar and turbulent flow conditions. Turbulent regions at high Reynolds numbers (~2700) were observed downstream of the narrowest sections of the geometry where short applications of stresses have occurred. These short stresses, which dominate the hydrodynamic loading of the particles, were found to be essential for efficient platelet release. However, if the flow rate increased, the stresses on the particles increased but the residence time decreased resulting in a decreased platelet release. Detailed analysis of these opposing trends has identified the importance not of stress alone, but of “shear stress accumulation” in combination with turbulence as key elements of the mechanisms underlying platelet release.
Conclusion(s): This study opens new directions for the development of efficient large scale platelet release devices.
PB0363
Phosphoinositide dynamics in megakaryocyte/platelet granule integrity and platelet functions
S. Severin 1; M. Caux2; R. Mansour2; J. Xuereb2; G. Chicanne2; J. Viaud2; A. Vauclard2; F. Boal2; B. Payrastre2; H. Tronchere2
1 Institute of metabolic and cardiovascular diseases, Toulouse cedex 04, Midi‐Pyrenees, France; 2 Inserm UMR 1297, Institute of metabolic and cardiovascular diseases, Toulouse cedex 04, Midi‐Pyrenees, France
Background: Secretory granules are key elements for platelet functions. Granule biogenesis and integrity are regulated by fine‐tuned mechanisms that need to be further characterized. This impedes our understanding of the etiology of hemorrhagic syndromes associated to quantitative or qualitative abnormalities in platelet granules.
Aims: Here, we investigated the role of the phosphoinositide 5‐kinase PIKfyve and its lipid products, phosphatidylinositol 5 monophosphate (PtdIns5P) and phosphatidylinositol (3,5) bisphosphate (PtdIns (3,5)P2), in granule homeostasis in megakaryocytes and platelets.
Methods: For that, we invalidate PIKfyve by using a specific inhibitor STA 5326 or by silencing using shRNA in the megakaryocytic MEG‐01 cell line, which on many points resembles primary MKs and has been validated to study MK granule biogenesis, in imMKCLs and primary MKs and in freshly isolated human platelets.
Results: We show that PIKfyve expression and its lipid product levels increased with megakaryocytic maturation. In megakaryocytes, PtdIns5P and PtdIns(3,5)P2 were found in alpha and dense granule membranes with higher levels in dense granules. Pharmacological inhibition or knock‐down of PIKfyve in megakaryocytes decreased PtdIns5P and PtdIns(3,5)P2 synthesis and induced a vacuolar phenotype with a loss of alpha and dense granule identity. Consistently, permeant PtdIns5P and PtdIns(3,5)P2 were able to restore alpha and dense granule integrity following PIKfyve pharmacological inhibition. In platelets, we show that PIKfyve inhibition specifically altered the integrity of dense granules culminating in defects in their secretion, platelet aggregation and thrombus formation.
Conclusion(s): Our data demonstrate that PIKfyve and its products PtdIns(3,5)P2 and PtdIns5P control the maintenance of granule integrity both in megakaryocytes and platelets.
VPB0364
Defective megakaryopoiesis in ETV6‐variant carriers results from an aberrant megakaryocyte‐erythroid progenitor population
T. Bigot 1; E. Gabinaud1; L. Hannouche1; V. Sbarra1; D. Payet‐Bornet2; M. Loosveld3; D. Potier2; M. Alessi1; M. Poggi1
1 Aix Marseille Université, Marseille, Provence‐Alpes‐Cote d'Azur, France; 2 CNRS, Marseille Cedex 9, Provence‐Alpes‐Cote d'Azur, France; 3 Aix Marseille Université, Marseille Cedex 9, Provence‐Alpes‐Cote d'Azur, France
Background: Germline mutations in the ETV6 gene are responsible for a familial thrombocytopenia and leukemia predisposition. Although previous studies showed that ETV6 plays an important role in megakaryocyte (MK) maturation and platelet formation, the mechanisms by which ETV6 dysfunction promotes thrombocytopenia remain unclear.
Aims: To study the effect of ETV6 variations on the differentiation of hematopoietic‐stem/progenitor‐cells (HSPC) at a single cell level and discover changes mediating the differentiation defect.
Methods: Peripheral CD34 + ‐cells were isolated from controls and patients carrying two different ETV6 variants (P214L localized in the central domain and F417LfsTer4 in the ETS domain) and differentiated in MK. F417LfsTer4 has never been reported. Transcriptomic profiles were analyzed by scRNA sequencing at day 6 and 11 of culture.
Results: The transcriptomic profile of the F417LfsTer4 and P214L ETV6 are similar, indicating that the same dysregulation is observed with both variations. The patients' cells strongly differ from those of controls at each timepoint (Figure 1). ETV6 deficient condition is characterized by a higher proportion of HSPC (18 ± 8.2 vs 3.9 ± 0.6%) and megakaryocyte‐erythroid progenitor (MEP, (50 ± 6.7 vs 39 ± 2.0%), a reduced proportion of megakaryocyte‐progenitor/megakaryocyte (MKP/MK, 13 ± 2.3 vs 38 ± 1.8%) and a lack of platelets. Genes differentially expressed between genotypes were identified for each hematopoietic cell stage. The transcriptomic profile of HSPCs and GMPs overlapped between control and patient cells. Pathway analysis using gene ontology identified the onset of divergence at the CMP stage, particularly for genes involved in the “regulation of protein localization in the nucleus”. Divergence intensifies at MEP and MkP/Mk (Figure 2) stages. Mitochondrial cellular metabolism (i.e. oxidative phosphorylation, respiratory electron transport chain, ATP synthesis) is the most significantly downregulated pathway in patients.
Conclusion(s): ETV6 mutations affect early hematopoiesis, at the level of the CMP/MEP transition, resulting in an aberrant MEP population and a defect in megakaryopoiesis. Studying mitochondrial cellular metabolism may represent an interesting target to investigate.


PB0352
Secretion of transforming growth factor β1 from megakaryocytes is mediated by autophagy signaling pathways
I. Becker 1; J. Lykins2; M. Barrachina3; H. Roweth4; B. Nieswandt5; S. Whiteheart6; K. Machlus7; J. Italiano8
1 Boston Children's Hospital, Boston, Massachusetts, United States; 2 University of Kentucky College of Medicine, Lexington, Kentucky, United States; 3 Harvard Medical School, Boston Children's Hospital, Boston, Massachusetts, United States; 4 Harvard Medical School, Brigham and Women's Hospital, BOSTON, Massachusetts, United States; 5 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 6 Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, Lexington, KY, USA, Lexington, Kentucky, United States; 7 Vascular Biology Program, Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts, United States; 8 Harvard Medical School, Boston, Massachusetts, United States
Background: Recent advances in single cell omics have identified that megakaryocytes (MKs) can function beyond platelet production by contributing to the maintenance of bone marrow homeostasis and immunity. Clonal hematopoiesis causes an accumulation of immature MKs in the bone marrow, which in turn instigates myeloproliferative disorders such as myelofibrosis. Myelofibrosis progression has been associated with an increased release of profibrotic cytokines such as transforming growth factor β1 (TGFβ1) from MKs into the bone marrow. While the underlying mechanisms remain elusive, unconventional trafficking mechanisms involving the cytoskeletal regulator RhoA and the endosomal GTPase Arf6 have been implicated in other cell types.
Aims: The aim of the study was to identify whether TGFβ1 release from MKs is autophagy‐dependent, and whether underlying signaling pathways can be targeted to alter cytokine secretion in vivo.
Methods: MK secretion was analyzed in the presence of autophagy and cytoskeletal inhibitors using cytokine arrays and immunosorbent assays. Immunofluorescence staining for TGFβ1 and secretory regulators was performed on native and in vitro‐matured MKs. Alterations in hematopoietic progenitor populations in mouse models with platelet secretion defects were assessed in cryosections by immunofluorescence and by flow cytometry.
Results: Treatment of MKs with autophagy or RhoA pathway inhibitors markedly reduced TGFβ1 secretion in vitro without affecting secretion of other soluble factors and was associated with an intracellular accumulation of TGFβ1 and autophagosomes. Colocalization of TGFβ1 with autophagosomes in native MKs correlated with Arf6 expression patterns. Moreover, myelofibrosis was identified in mice selectively lacking Arf6 in platelets and MKs, while other mouse models with platelet secretory defects did not exhibit altered marrow morphology.
Conclusion(s): Our data suggest that TGFβ1 trafficking is uncoupled from conventional granule secretion, but dependent on the RhoA and Arf6 signaling pathways that regulate secretory autophagy. Future studies will evaluate MK autophagy as a possible therapeutic target in myelofibrosis.
PB0356
The emerging role of Kupffer cells in thrombopoietin generation
D. Karakas 1; J. Li2; H. Ni3
1 University of Toronto, Toronto, Ontario, Canada; 2 Department of Laboratory Medicine, University of Toronto| Canadian Blood Services Centre for Innovation, Toronto, ON, Canada, Toronto, Ontario, Canada; 3 St. Michael's Hospital, Keenan Research Centre, Toronto, Ontario, Canada
Background: Thrombopoietin (TPO) is the physiological regulator of the hematopoietic stem cell niche (HSC), megakaryopoiesis and platelet production. We reported that platelet GPIbα is required for platelet‐mediated TPO generation (Blood, 2018). However, the mechanism of the platelet‐hepatocyte interaction is unknown. Platelets in the vessel are separated from hepatocytes by a fenestrated endothelium. Potential intermediaries for TPO generation include hepatocyte luminal protrusions, and Kupffer cells, which are essential for desialylated platelet (dPLT) clearance.
Aims: Determine whether Kupffer cells contribute to TPO generation independently or in concert with hepatocellular protrusions.
Methods: Murine Kupffer cells were depleted with Clodronate Liposomes. Murine liver endothelial fenestrations were reduced with treatment of 250 ppb arsenite in their drinking water. Kupffer cell depleted and arsenite‐treated mice were transfused 3x108 dPLTs. Sera and platelet counts were recorded, and TPO quantified by ELISA. Primary murine Kupffer cells were isolated and cultured alone or with dPLTs, and media supernatant was added to hepatocytes for TPO qPCR analysis.
Results: Kupffer cell depleted mice showed a TPO decrease of 43.6%(±16) 2 days post depletion, which could not be rescued with dPLT transfusion. Arsenite‐treated mice had a nadir of 61.3%(±4) baseline TPO levels, and were also non‐responsive to dPLT transfusions. These findings demonstrates that Kupffer cells facilitate platelet‐hepatocyte luminal protrusion contact for TPO generation. Surprisingly, Kupffer cell depletion in GPIbα‐deficient mice, which lack platelet‐mediated TPO generation, had a TPO decrease of 22.5%(±5) and in vitro Kupffer cell supernatant increased hepatocellular TPO expression by 2.43 fold. Interestingly, these data suggest that Kupffer cells promote baseline hepatocellular TPO production via secretory factor release.
Conclusion(s): Our data demonstrates that Kupffer cells are essential for platelet‐mediated TPO generation and promote constitutive hepatocellular TPO production. These findings have broad impacts for the HSC niche, megakaryopoiesis and platelet production, as well as therapeutic discovery for thrombocytopenia patients.
PB0353
RhoB drives megakaryocyte transendothelial migration in RhoA‐deficient mice
M. Englert 1; K. Aurbach1; T. Heib2; I. Becker3; L. Wackerbarth4; C. Kusch5; U. Knaus6; C. Stigloher7; I. Pleines1; B. Nieswandt1; Z. Nagy8
1 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 2 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Ulm, Bayern, Germany; 3 Boston Children's Hospital, Boston, Massachusetts, United States; 4 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, München, Bayern, Germany, 5 University Hospital Würzburg, Germany, Würzburg, Bayern, Germany; 6 Conway Institute, School of Medicine, University College Dublin, Dublin, Ireland, Belfield, Dublin, Ireland; 7 Imaging Core Facility, Theodor‐Boveri Institute of Biosciences, Biocenter, Julius‐Maximilians‐Universität Würzburg, Würzburg, Bayern, Germany; 8 Institute of Experimental Biomedicine, Chair I, University Hospital Wuerzburg, Wuerzburg, Germany, Würzburg, Bayern, Germany
Background: Platelet‐producing megakaryocytes (MKs) are large cells in the bone marrow (BM), localized in close vicinity to vascular sinusoids. The penetration of the endothelial barrier and subsequent intravascular protrusion formation by MKs are key steps in platelet biogenesis, however the molecular mechanisms regulating these processes are unclear. It was previously reported that entire MKs transmigrate into the BM sinusoids in mice lacking RhoA conditionally in the MK lineage (RhoAfl/fl;Pf4‐Cre mice; here referred to as RhoA‐deficient for simplicity). RhoB is closely related to RhoA and regulates cell migration and invasion in other cell types, although its role in MKs remains unknown.
Aims: To dissect the mechanisms underlying transendothelial migration of RhoA‐deficient MKs in the BM.
Methods: RhoB‐deficient (constitutive), RhoA‐deficient (conditional), RhoA/RhoB double‐deficient and RhoA‐deficient/RhoB‐heterozygous mice were analyzed for platelet production. MK number, localization and transmigration were assessed in stained cryosections using immunofluorescence microscopy. RhoB protein levels in MKs and platelets were determined by Western blotting.
Results: We found that RhoB is expressed in wild‐type (WT) MKs and platelets and becomes highly overexpressed in RhoA‐deficient MKs. We hypothesized that increased RhoB levels might induce the observed MK transmigration phenotype in RhoA‐deficient mice. While RhoB‐deficient mice displayed microthrombocytopenia, but no evidence of increased MK transmigration, double‐deficient mice exhibited severe macrothrombocytopenia, but reconstituted MK distribution at BM sinusoids to normal, compared to RhoA‐deficient mice. Interestingly, we found that RhoA‐deficient/RhoB‐heterozygous mice, in which MKs express WT levels of RhoB, also showed a reverted phenotype with normal MK localization.
Conclusion(s): We conclude that the increased RhoB levels contribute to MK transmigration in RhoA‐deficient MKs and that RhoA and RhoB have opposing roles in this lineage. These findings suggest that MK localization and proplatelet formation are critically regulated by a balanced expression of RhoA and RhoB.
VPB0365
Differential Autoregulation of RUNX1 by Isoforms RUNX1 B and RUNX1 C in Megakaryocytic Cells
L. Guan 1; A. Rao2
1 Sol Sherry Thrombosis Research Center, Lewis Katz School of Mediciine, Temple University, Philadelphia, Pennsylvania, United States; 2 Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania, United States
Background: Haplodeficiency of RUNX1, a key hematopoietic transcription factor, causes impaired megakaryocyte (MK) differentiation, thrombocytopenia, platelet dysfunction, and predisposition to AML. Two major RUNX1 isoforms RUNX1C and RUNX1B differ by 14 AA and are expressed from distinct promoters, P1 and P2, respectively. Little is known about the differential effects of RUNX1 isoforms.
Aims: We studied the effects of RUNX1B and RUNX1C on RUNX1 autoregulation.
Methods: Studies were performed on RUNX1 regulation in PMA‐treated megakaryocytic HEL cells and in HeLa cells, which express negligible RUNX1.
Results: ChIP studies using HEL cells and PCR amplification showed RUNX1 binds to 5 consensus sites in P1 and one site in P2 promoters (1–1.5 Kb). In luciferase reporter promoter studies, mutating RUNX1 binding sites 1 and 2 in P1 promoter reduced activity; mutating sites 3 and 4 increased activity, indicating their functional effects. Mutating binding site in P2 promoter increased activity. In co‐transfection studies in HeLa cells (with negligible RUNX1), increasing RUNX1C concentration increased P1 promoter activity and RUNX1B reduced promoter activity. With constant expression of RUNX1C, increasing RUNX1B reduced P1 promoter activity. Moreover, RUNX1B expression reduced P2 promoter activity, while RUNX1C increased this activity. Thus, RUNX1B and RUNX1C have differential autoregulatory effects on RUNX1 promoters. We then examined RUNX1 protein levels in HEL cells by immunoblotting. RUNX1B overexpression decreased (~50%) RUNX1C; RUNX1C overexpression increased RUNX1B (2‐fold). In studies in HEL cells using RUNX1C‐specific antibody, and expression plasmids for hemagglutinin‐tagged‐RUNX1B and c‐Myc‐tagged RUNX1C, RUNX1B overexpression decreased RUNX1C; RUNX1C overexpression increased RUNX1B. Preliminary studies suggest that the isoforms regulate MYL9 (RUNX1 target) expression differentially.
Conclusion(s): RUNX1B and RUNX1C autoregulate RUNX1 expression differentially in an isoform‐specific manner in megakaryocytic cells. This may be important in understanding altered downstream gene expression and in modulating RUNX1 for therapeutic purposes in inherited RUNX1 haplodeficiency states. (Supported by NIH grants R01HL109568 and R01137376).
PB0354
Production of platelets in vitro in functionalised 3‐dimensional scaffolds mimicking the bone marrow niche
H. Foster 1; M. Colzani1; G. Bouet Chalon1; D. Howard1; C. Di Buduo2; N. Muller‐Sienerth3; A. Waller1; Y. Sun4; J. Shepherd5; A. Evans1; P. Soprano6; M. Parsons7; Y. Ying Sims8; E. Turro9; P. Maguire10; S. Best5; R. Cameron5; A. Balduini11; G. Wright3; C. Ghevaert12
1 Wellcome‐MRC Cambridge Stem Cell Institute, University of Cambridge, Cambridge, England, United Kingdom; 2 Department of Molecular Medicine, University of Pavia, Pavia, Italy, Pavia, Lombardia, Italy; 3 Cell Surface Signaling Laboratory, Wellcome Trust Sanger Institute, Cambridge, England, United Kingdom; 4 University of Birmingham, Birmingham, England, United Kingdom; 5 Cambridge Centre for Medical Materials, University of Cambridge, Cambridge, England, United Kingdom; 6 Department of Molecular Medicine, University of Pavia, Pavia, Italy, Pavía, Lombardia, Italy; 7 University College Dublin, Dublin, Dublin, Ireland; 8 Wellcome Trust Sanger Institute, Cambridge, England, United Kingdom; 9 Icahn School of Medicine at Mount Sinai, New York, New York, United States, 10 University College Dublin, Ireland, Dublin, Dublin, Ireland, 11 University of Pavia, Pavia, Italy, Pavía, Lombardia, Italy, 12 University of Cambridge, Cambridge, England, United Kingdom
Background: The safety, quality and supply of donor‐derived platelet units intended for transfusion have improved over the past decades but significant problems still remain. In vitro‐derived platelets offer a possible alternative but up‐scaling production is hindered by our limited understanding of thrombopoiesis (the release of platelets by their mother cell, the megakaryocyte).
Aims: Here, we have developed an integrated strategy aiming to mimic ex vivo the bone marrow physiological niche that promotes thrombopoiesis by mature megakaryocytes in order to identify key elements that promote platelet production.
Methods: Proteomic and genomic analysis was employed to analyze differential expression levels of transmembrane proteins in cells that were shown to promote platelet production through direct contact with megakaryocytes. A panel of 259 recombinant biotinylated‐transmembrane proteins were immobilized on streptavidin coated surfaces to screen in order to identify proteins that promoted platelet production.
Results: ACVR1B, CRTAM, MUCEN and BTN1A1 were identified to improve platelet production from either cord blood‐ (ACVR1B) or pluripotent stem cells‐derived (CRTAM, MUCEN and BTN1A1) megakaryocytes. Using two different methodologies, we functionalise either collagen‐ or silk‐based 3‐dimensional scaffolds and confirm increased functional platelet production by up to two‐fold.
Conclusion(s): This unbiased approach has allowed us to identify novel proteins whose role in platelet formation was previously unknown and highlights the potential gain of recreating the MK niche to allow in vitro platelets to become a viable alternative for transfusion.
PB0361
Coxsackievirus B3 infection of CD34+ cells impairs megakaryocyte and platelet production through activation of Toll‐like receptors 7 and 8
M. Schattner 1; C. Rodriguez2; N. Charo3; S. Tatti4; R. Gomez5; L. D'Atri6
1 Institute of Experimental Medicine, caba, Buenos Aires, Argentina; 2 Institute of Experimental Medicine, National Academy of Medicine‐CONICET, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Institute of Experimental Medicine, National Academy of Medicine‐CONICET, CABA, Buenos Aires, Argentina; 4 Clinical Hospital, Ciudad Autónoma de Buenos Aires, Buenos Aires, Argentina; 5 Laboratory of Animal Virus, Institute of Biotechnology and Molecular Biology, UNLP‐CONICET, La Plata, Buenos Aires, Argentina; 6 Institute of Experimental Medicine, National Academy of Medicine‐CONICET, Ciudad Autónoma de Buenos Aires, Buenos Aires, Argentina
Background: Increasing evidence indicates that hematopoietic progenitor cells (CD34+ cells), megakaryocytes (MKs), and platelets (PLT) express toll‐like receptors (TLRs) allowing the contribution of these cells to the immune response and inflammation. However, whether TLRs' activation on CD34+ modulates megakaryo/thrombopoiesis during viral infections is still unclear.
Aims: We evaluated whether the single‐strand RNA Coxsackievirus B3 (CVB3) infection modulates human MK development and PLT production through TLR7/8 pathway.
Methods: CD34+ cells derived from human umbilical cord blood were exposed to CVB3 or UV‐irradiated CVB3 and then stimulated with thrombopoietin in the presence or absence of TLR7/8 antagonists. TLRs expression, proliferation rate, CD34* differentiation, MK maturation, and PLT biogenesis were determined by flow cytometry and mRNA of Fli‐1, RUNX‐1, NF‐E2, and IFN‐beta by qPCR.
Results: CD34+ cells express both TLR7 and TLR8 (Figure 1A‐B). Exposure of cells to CVB3 resulted in productive infection as determined by the presence of viral infectious particles in culture supernatants after 24 h and 11 dpi (Figure 1C). Total cell number, differentiation towards MKs (CD41 + cells), MK maturation (CD42b and CD42a + cells), and platelet biogenesis, were markedly reduced (n = 6, p < 0.05) in infected cultures (Figure 1 D‐H). The mRNA expression of transcription factors involved in these processes including Fli‐1, RUNX‐1, and NF‐E2, was also downregulated in infected cells (n = 5, p < 0.05) (Figure 1I‐K). The reduction in MK growth was not due to alteration of cell proliferation but was associated with an increase in cell apoptosis and the RNA levels of IFN‐beta (Figure 2 A‐C). Decreased cell number, MK maturity, PLT production, and apoptosis were significantly reversed by TLR7/8 antagonists (Figure 2 D‐G) and partially mimicked by Imiquimod, an agonist of TLR7/TLR8 (n = 4, p < 0.05).
Conclusion(s): These data suggest a new role for TLR7/8 in megakaryo/thrombopoiesis during viral infections that might contribute to a better understanding of the molecular bases underlying thrombocytopenia during infectious processes.


PB0360
SHP099 inhibits megakaryopoiesis, thrombopoiesis and Mpl signaling
L. Zimmermann1; D. Hennequin2; C. Di Buduo3; A. Balduini4; Y. Senis5; A. Mazharian 6
1 Etablissement Français du Sang, University of Strasbourg, Strasbourg, France, Strasbourg, Alsace, France; 2 Inserm U1255, Etablissement Français du Sang Grand Est, Strasbourg, Alsace, France; 3 Department of Molecular Medicine, University of Pavia, Pavia, Italy, Pavia, Lombardia, Italy; 4 University of Pavia, Pavia, Italy, Pavía, Lombardia, Italy; 5 Inserm UMR‐S1255/Etablissement Français du Sang/University of Strasbourg, Strasbourg, Alsace, France, 6 Etablissement Français du Sang, University of Strasbourg, Strasbourg, France, Strasbourg, Alsace, France
Background: The non‐transmembrane protein‐tyrosine phosphatase (PTP) Src homology (SH2) domain‐containing PTP 2 (Shp2), encoded by the proto‐oncogene PTPN11, has been implicated in many cellular processes, including survival, proliferation and differentiation of multiple cell types, including megakaryocytes (MKs). Shp2 has been demonstrated to regulate signalling from a variety of tyrosine kinase‐linked receptors, including cytokine and growth factor receptors and is a well‐known positive regulator of the Ras‐MAPK pathway. Targeting Shp2 pharmacologically, therefore represents a therapeutic strategy for many Ras‐driven cancers.Aims: Investigate the role of Shp2 in mouse and human megakaryocytopoiesis and thrombopoietin (Tpo) receptor Mpl signaling, through the use of a specific allosteric Shp2 inhibitor, SHP099.
Methods: MK maturation, Mpl signaling, proplatelet formation and platelet release were analyzed in primary mouse and human hematopoietic progenitors treated with SHP099, by flow cytometer, capillary‐based immunoassays and microscopy assays respectively. SHP099 was also tested on human MK cultured ex vivo in an artificial three‐dimensional (3D) miniature marrow bioreactor system.
Results: Treatment of primary bone marrow (BM)‐derived MKs with 10 microM SHP099 affect slightly cell viability, however it resulted in an inhibition of proliferation and maturation, with decreased number of mature MKs and reduced ploidy, as well as a decrease number of proplatelet‐forming MKs. Similar results were observed in human CD34 + ‐derived MKs. SHP099 also inhibited the number of platelet released from CD34 + ‐derived MKs, showing that Shp2 activation is required for platelet formation. Moreover, Tpo‐induced Erk1/2 and Akt phosphorylation was significantly decreased in CD34 + ‐derived MKs, demonstrating that Shp2 is a positive regulator of Mpl signaling. Using a 3D ex vivo BM niche model made of silk fibroin, confocal microscopy analysis also revealed a reduction in human proplatelet formation and branching, and a decreased number of platelets released in the presence of SHP099.
Conclusion(s): Shp2 activation is essential for mouse and human megakaryocytopoiesis and in mediating Mpl signal transduction.
Platelet Function and Interactions
PB0888
Anaphylactic Shock To A Hematological Disorder Patient During Transfusion Of Platelet Aphaeresis Unit Stored In Platelet Additive Solution: A Rare Case Report
B. poluru mranikrinda
Sparsh Hospital, BANGALORE, Karnataka, India
Background: Adverse transfusion reactions from apheresis platelets (AP) stored in Platelet additive solution (PAS) are uncommon, as PAS is a balanced electrolyte solution developed to replace plasma from platelet concentrates as the storage medium.
Aims: To evaluate the cause of anaphylactic reaction that occurred in a patient after transfusion of AP stored in PAS.
Methods: An AP product was donated by a blood group A male, processed using PAS. The patient developed severe Hypotension with Tachycardia immediately after 10 minutes of transfusion. Pre‐transfusion and Post transfusion vitals showed a marked decrease in Blood Pressure from 110/70 mmHg to 70/50 mm Hg with Pulse increased from 88 bpm to 118 bpm. Oxygen saturations were normal. Serological testing included an antibody screen (gel technology), a direct antiglobulin test (DAT), and Sterility testing of the transfused product.
Results: A 61‐year‐old blood group O patient with Aplastic anemia developed severe hypotension within the first 10 minutes of the start of AP in PAS transfusion despite premedication with Inj.Avil and Inj.Hydrocort. The post‐transfusion reaction evaluation was non‐significant showing negative antibody screen and also negative DAT, both for immunoglobulin G and C3d; No bacterial growth was seen in the transfused product and patient blood culture was sterile. Ruling out hemolytic and septic transfusion reactions. The patient was resuscitated with Inj. Adrenaline and got shifted to ICU for further follow‐up. The patient vitals got stabilized after 4 hours and got discharged in hemodynamically stable condition. Notably, a group Of patient at a different hospital received a split of the same apheresis unit, with no reaction.
Conclusion(s): To our knowledge, this is the first case with anaphylactic shock reported from AP transfusion stored in PAS. The fact that one transfusion recipient had anaphylactic shock whereas another did not have any reported reaction highlights the potential importance of recipient variables in adverse transfusion reactions.
VPB0897
Platelet indices at the titanium rod implantation in rabbit femur
E. Volokitina; I. Antropova; S. Kutepov; D. Chelchushev; M. Dobrinskaia
Urals State Medical University, Ekaterinburg, Sverdlovsk, Russia
Background: Blood loss, venous thromboembolism, aseptic fragility of prosthesis components and periprosthetic infection are the most severe complications encountered in major joint replacements (MJR). Platelets are the first cells which contact injured tissues and implant surface, they catalyze stable clot formation, stimulate regeneration and take part in antimicrobial protection.
Aims: To study the features of platelet parameters dynamics depending on its initial state during titanium rod implantation in rabbit femur.
Methods: The experiment included 20 Chinchilla rabbits. Parameters: platelet count, MPV, PDW, PCT, P‐LCR (Sysmex XN‐1000), ADP aggregation (Chrono‐Log 700) were determined before surgery, 1 day and 8 weeks after surgery. Animals were divided into 2 groups according to the score number calculated using preoperative platelet parameters (Table 1). HPI group (high platelet index) included 8 rabbits with 5–8 scores. In LPI group (low platelet index) group ‐ 8 rabbits with 9–11 scores. Titanium rod (0.2x0.6 cm) was implantated according to press fit principle in rabbit femur. Animals were withdrawn from the experiment 8 weeks after surgery. The study was approved by Local Ethics Committee of the Ural State Medical University in accordance with national and EU regulations. Statistical analysis was performed using “Statistica” software package.
Results: Platelet parameters before orthopedic surgery, 1 day and 8 weeks after titanium rod implantation are shown in Figure 1. Before surgery, the HPI group had significantly higher values of platelet count, MPV, and ADP‐induced aggregation than the LPI group. In postoperative period, the values of these indicators were also higher in the HPI group in comparison to the LPI group.
Conclusion(s): The number of platelets, cell volume, aggregation activity in postoperative period depend on its initial parameters. In this regard the possibility of predicting the functional activity of platelets and the success in repair process during major orthopedic operations would be helpful.


VPB0898
Utilizing a Quartz Crystal Microbalance with dissipation (QCM‐D) to Study Platelet NO‐Signaling Under Flow Conditions
D. Castaneda‐Zaragoza 1; M. Saito2; P. Jurasz3
1 Department of Pharmacology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada; 2 Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, Canada; 3 Department of Pharmacology, Faculty of Medicine and Dentistry/Cardiovascular Research Centre/Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Alberta, Canada
Background: Platelet function testing has been a subject of research due to its potential role in predicting platelet responses and acute coronary syndromes risk. Human platelets produce nitric oxide (NO), an important factor that negatively regulates platelet adhesion and aggregation, and previous studies have shown that platelets of patients with acute coronary syndromes have reduced NO production. While previous studies have used Quartz Crystal Microbalance with dissipation (QCM‐D) under flowing conditions to assess platelet aggregation and stiffness of the resulting aggregate, QCM‐D has not been used to study platelet NO‐signaling.
Aims: To assess the effects of the nitric oxide synthase substrate L‐arg and inhibitor L‐NAME on the platelet aggregation profile to collagen‐coated sensors using QCM‐D.
Methods: Platelets were isolated from healthy donors and resuspended to a concentration of 2.0 x 108/ml. QCM‐D sensors were coated with collagen (10ug/ml) for 1 hour. Platelets were pre‐incubated for 15 minutes with either vehicle, L‐Arg (100uM), or L‐NAME (1 mM) at 37°C. The platelets were then flowed through the QCM at 1.410 mL/minute and the negative change in frequency (ΔF) and dissipation (D) was measured over 5 minutes.
Results: L‐arg significantly reduced the ‐ΔF to 70.3 ± 8.4% of control (Figure 1), demonstrating a reduction in mass on the sensor consistent with enhanced NO production and inhibition of platelet adhesion/aggregation. Conversely, L‐NAME caused a ‐ΔF of 115.5 ± 12.3% of control. Similarly, L‐arg caused a significant reduction in D, consistent with a reduced stiffness of the platelet layer, an effect not observed with L‐NAME (Figure 2).
Conclusion(s): L‐arg reduces platelet aggregation on collagen‐coated sensors as measured by QCM under flow conditions, consistent with an enhanced NO inhibitory effect. Further, the QCM assay may be a novel and highly sensitive technique to assess platelet NO‐signaling and the function of platelets of patients at increased risk of acute coronary syndromes.


PB0879
Inhibition of apoptosis during cold storage of platelet concentrates better maintains both platelet functionality and survival
I. Marini 1; Y. Tamamushi1; C. Maettler1; L. Pelzl2; K. Althaus2; S. Nowak‐Harnau3; T. Backchoul4
1 Institute for Clinical and Experimental Transfusion Medicine (IKET), University Hospital of Tuebingen, Germany, stuttgart, Baden‐Wurttemberg, Germany; 2 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tuebingen, Baden‐Wurttemberg, Germany; 3 Center for Clinical Transfusion Medicine, Tuebingen, Baden‐Wurttemberg, Germany; 4 Institute for Clinical and Experimental Transfusion Medicine, Medical Faculty of Tuebingen, University Hospital of Tuebingen, Tuebingen, Germany, Tübingen, Baden‐Wurttemberg, Germany
Background: Apheresis‐derived platelet concentrates (APCs) is an essential medical treatment. However, the storage at room temperature enhances the risk of bacterial contamination. Recently, we showed that cold storage of APCs better conserves platelet functions but reduced cell half‐life because of apoptosis activation.
Aims: To investigated the impact of apoptosis inhibition on platelet functions and half‐life during cold storage.
Methods: APCs were stored for 7 days at 4°C with or without the apoptosis inhibitor G04 (RhoA‐GTPase inhibitor). The functionality of cold‐stored platelets was assessed by flow cytometer (CD63, CD62 and PAC expression, upon TRAP), aggregometry assay and thrombin generation. The platelet responsiveness to extracellular matrix proteins was determined using the platelet adhesion assay. Platelet survival was investigated using the NOD/SCID mouse model.
Results: The presence of the inhibitor better maintains CD63 and PAC1 expression during cold storage (CD63, p = 0.035; PAC1, p = 0.005). No differences in the expression of CD62 were observed with or without inhibitor (p = 0.728). Furthermore, the capability to generate thrombin was not impaired in the presence of G04 (p = 0.956). Interestingly, the aggregation ability was better conserves in platelet incubated with G04 (TRAP, p = 0.019). Next, comparable adhesion ability was detected with or without inhibitor (p = 0.447). More importantly, the inhibition of apoptosis induced a significant higher percentage of circulating cold‐stored platelets in vivo compared to untreated cells (5 h post injection, p = 0.046).
Conclusion(s): Our results show that the inhibition of the apoptotic pathway significantly reduces the clearance of cold‐stored platelets without affecting cell functionality. Therefore, the use of apoptosis inhibitors may be a promising strategy to prolong the storage time and reduce the risk of bacterial infection post‐transfusion.
PB0855
Platelet dysfunction in HFpEF patients: plasma and whole blood measurements
G. D'Italia 1; D. Coenen2; T. Lemmens3; S. Wielders3; A. Al‐Abadi3; S. Toutouh3; J. Franssen4; S. Mourmans4; B. Tullemans3; J. Weerts4; A. Barandiaran Aizpurua4; V. van Empel4; B. Schroen4; J. Cosemans5
1 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, maastricht, Limburg, Netherlands; 2 University of Kentucky College of Medicine, Lexington, KY, USA, Lexington, Kentucky, United States; 3 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 4 Department of Cardiology, Maastricht University Medical Centre (MUMC+), Maastricht, Netherlands, Maastricht, Limburg, Netherlands, 5 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands
Background: Heart failure with preserved ejection fraction (HFpEF) results from a complex interplay of systemic syndromes, that include diabetes and hypertension and are characterized by chronic low‐grade inflammation and microvascular dysfunction (MVD). In recent years, the importance of platelets in vascular inflammation and endothelial dysfunction emerged, suggesting an unexplored role for platelets in MVD. However, the role of platelets in HFpEF is still poorly examined.
Aims: To investigate whether HFpEF patients present alterations in platelet functions.
Methods: Endothelial and platelet activation markers were measured in plasma from HFpEF patients with and without type 2 diabetes mellitus (T2DM) and age‐ and sex‐matched hypertensive controls. Moreover, platelet integrin αIIbβ3 activation and platelet α‐granule secretion were measured by flow cytometry using freshly isolated platelets from HFpEF patients (N = 80) and hypertensive controls (N = 25, recruitment in progress), stimulated with different agonists. Microfluidics assays with whole blood from HFpEF patients and controls were performed to measure adhesion, platelet activation markers (CD62P, fibrin(ogen), annexin A5) and thrombus growth under arterial flow conditions.
Results: Markers for endothelial cell activation, ICAM‐1, VCAM‐1, are increased in HFpEF patients compared to hypertensive controls, reflecting the inflammatory state in HFpEF. On the other hand, platelet activation markers, β‐TG, PF4, TSP‐1, are decreased, especially in HFpEF patients with type 2 diabetes mellitus. Moreover, platelets from HFpEF patients show decreased integrin αIIbβ3 activation and α‐granule secretion upon stimulation with collagen‐related peptide and TRAP‐6, and a tendency for an overall reduction in platelet surface coverage, activation of platelets and thrombus formation under flow.
Conclusion(s): In sum, our preliminary results show endothelial cell activation and platelet dysfunction in HFpEF patients compared to hypertensive controls, suggesting a possible unrecognized role of platelets in HFpEF.
PB0863
The influence of hemolysis on platelet reactivity in patients undergoing aortic valve replacement surgery
S. Sánchez1; A. García1; F. García1; N. Marín2; J. García‐Estañ2; J. Moraleda1; D. Iyu 2
1 Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Murcia, Spain; 2 University of Murcia, Murcia, Murcia, Spain
Background: Cardiopulmonary bypass (CPB) with extracorporeal circulation is the procedure that is performed in patients undergoing aortic valve replacement surgery. CPB is a relatively safe technique although may have some complications such as tissue damage via decreased organ perfusion. In this setting hemolysis seems to be an important contributor to these complications since the increase of free hemoglobin in plasma decreases Nitric Oxide (NO)‐bioavailability and may lead to platelet aggregation/activation and vasoconstriction, resulting in thrombosis and reduced organ perfusion.
Aims: To investigate the influence of hemolysis on thrombin‐receptor activating peptide (TRAP)‐induced platelet reactivity in patients undergoing aortic valve replacement.
Methods: Platelet aggregation (by platelet counting) and activation (by P‐selectin expression) were measured in seventeen patients by flow cytometry in whole blood in response to thrombin‐receptor activating peptide (TRAP), in the absence or presence of a range of different concentrations of NONOate (nitric oxide donor). Vasodilator‐stimulated phosphoprotein (VASP) phosphorylation was determined using a cytometric bead (VASPFix) assay as a measure of cAMP/cGMP. All the determinations were measured in three different moments: Before (M1), During (M2) and After (M3) the CPB procedure.
Results: NONOate (nitric oxide donor) inhibited TRAP‐induced platelet aggregation and activation in a dose‐dependent manner in the three experimental groups (M1, M2 and M3) with no significance difference among them (Table 1). The effects of NONOate were directly related to changes in cAMP/cGMP, assessed by VASP phosphorylation (Table 2).
Conclusion(s): The overall effects of NONOate (nitric oxide donor) on platelet reactivity and VASP phosphorylation are the consequence of an adequate bioavailability of nitric oxide in these patients under our experimental conditions. These results indicate that the level of hemolysis brought about by CPB in these patients might not be sufficient to interfere with the physiological regulation of nitric oxide on platelet function in vivo.


PB0868
Detection of hyperfunctioning platelets and novel phenotypic platelet subtypes
M. Jongen; L. Schmied; S. Meinke; R. Fiskesund; P. Höglund.
Center for Hematology and Regenerative Medicine, Karolinska Institutet, Huddinge, Stockholms Lan, Sweden
Background: Platelets are important mediators in maintaining homeostasis and in pathology of bleeding and thrombosis. In addition, they are now known to be highly heterogeneous in phenotype markers such as size, density and receptor content as well as their function with both highly reactive platelets as well as hard to activate platelet subtypes. However, while many clinical methods exist to detect hypofunctioning platelets, methodology for the detection of hyperfunctioning platelets is lacking and limited to high‐on‐treatment response detection when treated with anti‐platelet agents.
Aims: We aim to design a highly polychromatic flow cytometry panel to detect platelet subtypes in hyperfunctioning platelets.
Methods: We designed a novel multi‐color flow cytometry panel that utilizes modern innovations in flow cytometers and new dyes to map distinct platelet phenotypes in hyperfunctioning platelets. Our ultimate goal is to identify clinically relevant subtypes of platelets that predict thrombosis risk more accurately in individuals with a higher risk due to smoking, obesity and diabetes so that anti‐platelet treatment can be tailored to those patients with a clinically relevant high risk. Furthermore, results were analyzed using both traditional subsequent gating strategies as well as new unsupervised clustering algorithms such as Principal Component Analysis, t‐distributed stochastic neighbor embedding (t‐SNE) and Uniform manifold approximation and projection (UMAP).
Results: We designed a 14 color panel with antibodies against CD9, CD29, CD31, CD32, CD36, CD41, CD42a, CD61, CD62P, CD63, CD107a, CD154, MHC class 1 and PAC‐1. This combination of markers has the potential to detect known and novel subtypes of platelets in healthy volunteers and can be used in patient samples in the future.
Conclusion(s): Detecting multiple subtypes of platelets brings clinically relevant hyperfunction of platelets a step closer and can lead us to find specific subtypes to target with anti‐platelet treatment in the future.
PB0871
Fusogenic liposomes label human platelets without impacting normal platelet function
C. Kempster 1; J. Cull2; J. Gibbins3; A. Pollitt2
1 University of Reading, Reading, England, United Kingdom; 2 University of Reading, READING, England, United Kingdom; 3 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: Platelets play a critical role at sites of vascular injury to arrest bleeding, yet hyper‐responsive platelets can be causative to heart attacks and stroke. Understanding platelet molecular mechanisms is therefore important to identify novel drug targets and improved therapeutics. However, since platelets lack a cell nucleus, conventional methods used to identify processes in nucleated cells cannot directly be applied.
Aims: This project aims to use biocompatible liposomes with a unique fusogenic nature to deliver cargo directly into the cytoplasm of platelets. Enabling biological processes and molecular mechanisms to be studied directly in human platelets. The first step towards this goal is to determine if fusogenic liposomes can fuse with human platelets without impacting function.
Methods: Granule secretion was measured using P‐selectin as a marker of platelet activation. Annexin V binding was used as a measure of apoptotic platelets. Platelet adhesion and spreading on Fibrinogen was quantified using a convolutional neural network (CNN). Confocal microscopy assessed the extent of cell labelling and cargo delivery by fluorescently labeled fusogenic liposomes.
Results: Fluorescently labeled fusogenic liposomes efficiently fuse with the membrane of human platelets. Spontaneous fusion with platelets does not lead to platelet activation in the presence of PGI2, with no significant increase in P‐selectin surface expression, or the induction of phosphatidylserine translocation to the outer platelet membrane as measured by Annexin V binding. Normal platelet behavior is unaffected by liposome fusion as measured by agonist induced P‐selectin exposure and platelet spreading.
Conclusion(s): These results demonstrate that fusogenic liposomes can label human platelets without impacting normal cell behavior, revealing a method to deliver cargo directly into the cytoplasm of human platelets to interrogate both known and novel molecular mechanisms in vitro, and in real time.
PB0883
Platelet reactivity and thrombogenicity in Framingham Heart Study participants with hypertriglyceridemia
B. Nkambule 1; M. Chan2; A. Lachapelle2; F. Thibord3; J. Cunha4; R. Pashek5; M. Chen2; A. Johnson2
1 NHLBI, Boston, Massachusetts, United States; 2 NHLBI, Framingham, Massachusetts, United States; 3 Population Sciences Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, Boston, Massachusetts, United States; 4 NHLBI and Boston University's Framingham Heart Study, Framingham, Massachusetts, United States; 5 NHLBI, Allston, Massachusetts, United States
Background: Hypertriglyceridemia is associated with hypercoagulability, but the association between triglyceride levels and altered platelet function remains unclear.
Aims: The aim of this study was to assess the association between platelet function and triglyceride levels.
Methods: We included participants from the Framingham Heart Study (FHS) Third Generation cohort, New Offspring Spouse (NOS) and OMNI cohort who attended the third examination cycle (2016–2019). Eligible participants were categorized into four triglyceride (TG) subgroups. We assessed platelet responses to adenosine diphosphate using flow cytometry and light transmission aggregometry. In addition, the levels of thrombogenicity were determined using the novel Total Thrombus formation Analysis System (TTAS).
Results: The study included 1897 (55.53%) FHS participants with normal TG levels (TG levels<100 mg/dL); 883 (25.85%) participants with high‐normal TGs (TG levels 100–150 mg/dL); 378 (11.06%) with borderline high TGs (TG levels>150–199 mg/dL); and 258 (13.60%) participants with HTG (TG levels>200 mg/dL) (Table 1). In our linear mixed effects model adjusted for age, sex, alcohol consumption, aspirin use, and statin use, TG levels were associated with decreased platelet P‐selectin exocytosis (CD62P) (b = 0.006, p = 0.022). Similarly in the subgroup analysis %CD62P levels were inversely associated with borderline‐high TG (b = −1.155, p = 0.0095), and high TG levels b = −1.188, p = 0.0071). Lastly, high‐normal, and borderline‐high TG levels were associated with increased levels of collagen‐dependent thrombogenicity (b = 0.236, p = 0.0006) measured with TTAS.
Conclusion(s): TG levels are associated with altered platelet activation and aggregation even at TG levels considered as normal. Furthermore, increased platelet‐driven thrombogenicity is directly associated with TG levels.

VPB0904
Flow cytometry test with ADP stimulation for platelet functional activity assessment
E. Ponomarenko 1; A. Ignatova1; A. Filkova2; A. Pisaryuk3; M. Teterina3; I. Meray3; Z. Kobalava3; A. Sveshnikova1; G. Novichkova2; M. Panteleev4
1 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 2 National Medical Research Center of Pediatric Hematology, Oncology and Immunology Named after Dmitry Rogachev, Russian Ministry of Healthcare, Moscow, Moskva, Russia; 3 City Clinical Hospital named after V.V. Vinogradov, Russian Ministry of Healthcare, Moscow, Moskva, Russia; 4 Center for Theoretical Problems of Physicochemical Pharmacology, Moscow, Moskva, Russia
Background: Adenosine diphosphate (ADP) potentiates platelet activation caused by other agonists as well as stimulates platelets on its own. There are studies on platelet functional activity after activation with ADP using flow cytometry, however, no standard protocol is developed. It's important in monitoring antiplatelet therapy in cardio patients with the acute coronary syndrome, which includes ADP receptors' inhibitors.
Aims: The aim of our study was to develop a flow cytometry protocol for platelet functional activity assessment after stimulation with ADP and to test it on cardio patients on dual antiplatelet therapy.
Methods: Venous blood was collected into vacuum plastic tubes with sodium citrate, final concentration 3.8%. 20 μL of blood was diluted 1:20 in HEPES‐buffered Tyrode buffer. For platelet activation, a mixture of ADP (5 μM) and 2.5 mM CaCl2 was used. We determined forward scatter, side scatter, CD42bPE, CD61‐PE, CD62P‐Alexa Fluor 647, PAC‐1‐FITC, annexin V‐Alexa Fluor 647 binding and mepacrine release levels.
Results: We found that blood should not be transported, while time after blood collection and inter‐operator variability did not influence the results. Platelets should be activated and dyed simultaneously for 15 minutes with the addition of CaCl2. We used our protocol on children aged 2 months‐3 years old (n = 5) and healthy adult donors aged 18–45 years old (n = 22) and found that after stimulation both groups similarly had 5‐fold increase in CD62p and PAC‐1 binding levels, while no dense granules release was observed. We then used our protocol on cardio patients on dual antiplatelet therapy (acetylsalicylic acid + ticagrelor or prasugrel, n = 9) and found that after a month of therapy stimulated platelets of cardio patients had 2.7‐fold lower CD62p and CD61 and 1.2‐fold lower PAC‐1 binding levels compared to healthy donors.
Conclusion(s): We developed a flow cytometry protocol for comprehensive platelet functional activity assessment after stimulation with ADP, which is sensitive to dual antiplatelet therapy.
PB0847
Management of adult idiopathic thrombocytopenic purpura (ITP) patients with antinuclear antibody (ANA)
M. Borhany 1; S. Zafar1; M. Abid1; T. Shamsi2
1 National Institute of Blood Diseases and Bone marrow Transplantation, Karachi, Sindh, Pakistan; 2 National Institute of Blood Diseases and BMT, KARACHI, Sindh, Pakistan
Background: Idiopathic thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by persistent thrombocytopenia due to autoantibody binding to platelet antigens causing their premature destruction by the reticuloendothelial system.
Aims: Following study was conducted to determine the likelihood of positive ANA markers in adult ITP patients and other autoimmune conditions along with their management.
Methods: This prospective observational study was conducted from January to December 2021 after taking informed consent. Patient's medical history, clinical data and outcome was collected using patients performa and analyzed using SPSS version 23.
Results: A total of 143 patients were screened for ANA. This includes 38 males (26.6%), 105 females (73.4%) with a median age of 38 years (IQR‐25), 94 acute ITP (64.7%) and 49 chronic ITP (34.3%). ANA markers were positive in 41 (28.7%), AMA and ASMA in 28 (19.6%) patients. The frequency of positive ANA was higher in females 32 (78%) as compared to males 8(22%) and in acute 25 (61.0%) as compared to chronic 16 (39%) ITP patients. Among 41 (27.3%) positive ANA patients, 6(15.38%) had systemic lupus erythematosus (SLE) and 1(2.4%) patient had hypothyroidism. Lab parameters showed significant difference with median platelet (24 IQR‐49 vs 81 IQR‐178 vs 82.5 IQR‐150) x109/L and hemoglobin (12.05 g/dL IQR‐3 vs 12.06 IQR‐2 vs 12.40 IQR 2) from baseline to 3 months and follow‐up with (p value = ≤0.001). Frequency of bleeding symptoms is presented in Figure 1. Skin related complications observed includes alopecia in 3(2.09%) and skin rashes in 4(2.79%), whereas 11 (7.6%) patients had history of abortions. Overall treatment response is presented in Table 1.
Conclusion(s): Overall treatment response with first and second line therapy in acute (90.4%) and chronic (94.9%) ITP patients was observed to be effective with decreased bleeding symptoms. However prevalence of 28.7% of ANA indicates concerns for developing other autoimmune conditions such as SLE.


PB0864
TRAP‐Induced platelet reactivity is inhibited by omega‐3 fatty acid‐derived prostaglandin E3 (PGE3)
J. Osete‐Lobato1; F. García2; N. Marín1; J. García‐Estañ1; D. Iyu 1
1 University of Murcia, Murcia, Murcia, Spain; 2 Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Murcia, Spain
Background: Thrombin is one of the most important factors that regulates platelet reactivity and coagulation. Clinical trials have consistently shown that omega‐3 fatty acid supplementation lowers the risk for cardiovascular mortality and morbidity [7]. Since omega‐3 fatty acids are the main precursors of PGE3 in vivo, [13,14,15] it would be relevant to investigate the effects of PGE3 on Thrombin Receptor Activating Peptide (TRAP)‐induced platelet reactivity to determine the receptors and possible mechanisms of action of these compounds.
Aims: To investigate whether omega‐3 fatty acid‐derived PGE3 might inhibit TRAP‐induced platelet reactivity, especially in the presence of an Gi‐coupled EP3 receptor antagonist, where the effects of PGE3 at the Gs‐coupled EP4 receptor would be potentiated.
Methods: Platelet aggregation (by platelet counting) and activation (by P‐selectin expression) were measured by flow cytometry in whole blood in response to thrombin‐receptor activating peptide (TRAP) and PGE3, in the absence or presence of the EP3 and EP4 receptor antagonists: DG‐041 and ONO‐AE3‐208. Vasodilator‐stimulated phosphoprotein (VASP) phosphorylation was determined using a cytometric bead (VASPFix) assay as a measure of cAMP.
Results: PGE3 inhibited TRAP‐induced platelet aggregation and activation. This inhibition was enhanced in the presence of an Gi‐coupled EP3 receptor antagonist and abolished in the presence of an Gs‐coupled EP4 receptor antagonist (Table 1). The effects of PGE3 were directly related to changes in cAMP, assessed by VASP phosphorylation (Table 2).
Conclusion(s): The general effects of PGE3 on human platelet reactivity are the consequence of a balance between promotory and inhibitory effects at receptors that have contrary effects on adenylate cyclase. When the balance is shifted towards a major interaction of PGE3 with EP4 receptor its ability to inhibit platelet function increases. These results indicate a potential mechanism by which omega‐3 fatty acids underlie cardioprotective effects.

PB0882
Rab GTPase Protein localization, regulation and function in platelets
N. Nguyen 1; A. Melrose2; I. Parra‐Izquierdo2; J. Pang2; T. Zheng2; O. McCarty3; J. Aslan2
1 Oregn Health & Science University, Portland, Oregon, United States; 2 Oregon Health & Science University, Portland, Oregon, United States; 3 Oregon Health and Science University, Portland, Oregon, United States
Background: Rab GTPases serve as master regulators of vesicle biogenesis, trafficking, and fusion in eukaryotic cells. Platelets express over 40 different Rab proteins with potential roles in granule secretion, receptor trafficking and other cell physiological process critical to platelet function.In previous work, we examined Rab localization and distribution in platelets using fluorescent microscopy. However, the specific localization and distribution of Rab and the mechanisms by which Rabs and their effectors regulate complex vesicular trafficking in platelets remain to be elucidated.
Aims: Study the regulation and function of Rab GTPases in platelets.
Methods: Through a combination of immunofluorescence microscopy, time‐lapse microscopy, Western blot analysis, and pharmacological inhibition, we quantified the regulation of >10 different Rab GTPases in both resting and platelets stimulated with the collagen receptor GPVI/ITAM agonist crosslinked collagen‐related peptide (CRP‐XL).
Results: Through conventional fluorescent microscopy, we observed that Rab proteins dynamically localized to distinct vesicle populations upon activation of platelets. Phos‐tag gel electrophoresis and Western blot analysis revealed that Rab7, Rab8, and other Rab associated proteins were phosphorylated following platelet activation by CRP‐XL; phosphorylation of Rab proteins was reduced by pharmacological inhibitors of protein kinase C (PKC), as well as kinases known to target Rabs in other cell types including TAK1 and TBK1. Moreover, our preliminary experiments suggest that select platelet functions were inhibited by novel pharmacological inhibitors of Rab family members including the Rab7 GTPase inhibitor CID 1067700 and the Rab8‐binding stapled peptide StRIP16.
Conclusion(s): Platelets express a variety of Rab GTPase family members that localize to distinct vesicular compartments in quiescent and activating platelets. Future work will determine how different Rabs orchestrate intracellular vesicular trafficking activities associated with platelet function.
PB0887
Platelet function in MS‐NASH mice: a newly developed obesity model
C. Peng 1; S. Whiteheart2
1 Department of Pharmacology, University of Kentucky College of Medicine, Lexington, Kentucky, United States; 2 Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, Lexington, KY, USA, Lexington, Kentucky, United States
Background: Obesity is a multifactorial disease with many co‐morbidities leading to multi‐organ dysfunction. It is also associated with recurrent atherothrombotic events and inadequate responses to antiplatelet therapy. Current studies point towards the dysregulation of platelet signaling in both receptor and second messenger pathways as being key drivers for increased risk of atherosclerosis and cardiovascular disease in obesity. However, data from these studies are conflicting and warrant more experimentation to understand platelet function in metabolic disease. Recently, a new mouse model, MS‐NASH, was developed to study obesity. This inbred, polygenic, mouse model is clinically translatable given its ability to respond to anti‐diabetic drugs, its intact leptin pathway, and its mimicry of the multifaceted aspects of the human metabolic syndrome, disease pathophysiology, and associated comorbidities.
Aims: This study aims to evaluate platelet phenotypes in the MS‐NASH, obese mouse model.
Methods: Twelve‐week‐old MS‐NASH mice (Strain #030888, Jackson Laboratory) and C57BL/6J mice were fed a Purina 5008 chow. To validate the model, food intake and weight were recorded. EchoMRI was used to determine fat, lean, free water, and total water mass. Tail‐bleeding assays were used to characterize the hemostasis phenotype.
Results: MS‐NASH mice had higher overall weight, fat percentage, and total water mass, corresponding with an increased food intake. Lean and free water mass were unaltered. Bleeding times were increased in the MS‐NASH mice compared to the C57BL/6J mice (p < 0.0001). Interestingly, bleeding time and body weight positively correlated in the MS‐NASH mice.
Conclusion(s): Based on this preliminary study, we validated MS‐NASH mice as a model for obesity. Furthermore, we demonstrated that the MS‐NASH mice have increased bleeding times which are positively correlated with their body mass, suggesting dysfunctional hemostasis in these obese mice. This work is supported by the NIH/NHLBI (HL150818), VA, and the CCTS TL1 fellowship (TR 1997–5 A1).
PB0892
The effect of PCSK9 on platelet aggregation and on the inflammatory activation of endothelial cells
A. Tsouka1; A. Tselepis 2
1 Atherothrombosis Research Centre, Department of Chemistry, University of Ioannina, 45,110 Ioannina, Greece, Ioannina, Ioannina, Greece; 2 Atherothrombosis Research Centre, Department of Chemistry, University of Ioannina, Ioannina, Ioannina, Greece
Background: PCSK9 is a serine protease that induces endosomal and lysosomal degradation of the LDL receptor, which leads to increased plasma LDL‐cholesterol levels. Recent studies have shown that PCSK9 is involved in the pathophysiology of inflammation and has effects on other cell types.
Aims: Aim of the present study was to investigate the effect of mature recombinant PCSK9 (recPCSK9) on platelet aggregation and on the expression of inflammatory markers on endothelial cells.
Methods: Washed platelets (WP) were isolated from healthy volunteers and adjusted at 250.000 platelets/μl. WP were incubated with various concentrations of PCSK9 and then activated with (0.01–0.025 units/mL) thrombin or (0.25 mm) Aracidonic Acid (AA) that induces platelet aggregation 20 and 28%, respectively which was monitored by Light Transmittance Aggregometry. Human Umbilical Vein Endothelial Cells (HUVECs) were cultured to reach confluency and then pre‐incubated with recPCSK9 for 5 min followed by activation with (0.05 ng/mL) Tumor Necrosis Factor‐α (TNF‐α) for 6 h. Cell activation was studied by the determination of the membrane expression of ICAM‐1 (Intercellular Adhesion Molecule‐1) using flow cytometry and the fluorescently labeled antibody, CD54‐PE.
Results: recPCSK9 at concentrations of 300‐1600 ng/mL does not induce platelet aggregation, however at concentrations ≥800 ng/mL it increases by 2.5 times the thrombin‐induced platelet aggregation (from 20 to 48%) and doubles the platelet aggregation induced by A.A (from 28 to 56%). recPCSK9 at concentrations of 500–1500 ng/mL does not induce the expression of ICAM‐1 in HUVECs (MFI values), while it enhances the action of TNF‐α by 1.5 times at a concentration of 1.000 ng/mL (from 53 to 79.5) and by 2.5 times at a concentration of 1.500 ng/mL (from 53 to 132.5).
Conclusion(s): recPCSK9 potentiates the thrombin‐ or AA‐induced platelet activation as well as the inflammatory activation of the endothelial cells. The underlying mechanisms, as well as the significance of this action at the clinical level, are under investigation.
PB0895
Changes in immature platelet fraction after major trauma: a prospective cohort study
A. Rossetto1; P. Armstrong2; L. Green3; K. Brohi4; T. Warner2; P. Vulliamy 5
1 Centre for Trauma Science, Blizard Institute, Queen Mary University, London, United Kingdom, London, England, United Kingdom; 2 Queen Mary, University of London, London, England, United Kingdom; 3 Queen Mary Univeristy of London, UK ‐ Barts Health NHS Trust, London, UK, London, England, United Kingdom; 4 Queen Mary University of London, London, England, United Kingdom; 5 Queen Mary University of London, london, England, United Kingdom
Background: Alterations in platelet behavior after severe injury are an important contributor to trauma‐induced coagulopathy, but little is known about how hemorrhage and tissue injury influence platelet turnover.
Aims: To determine whether the immature platelet fraction (IPF) is associated with injury severity and clinical outcome after trauma.
Methods: We conducted a prospective observational cohort study of injured patients at a single major trauma centre. Using a Sysmex XE automated analyzer, we measured the IPF at 2 h, 24 h and 72 h post injury. We defined patients with an injury severity score (ISS) < 15 as mild–moderate and those with an ISS of >15 as severely injured.
Results: A total of 194 patients were included, with a median ISS of 13 (IQR 4–25) and an overall mortality of 12% (23/194). At 2 h, the IPF in severely injured patients (5.0 [3.5–7.8]%) was significantly (p = 0.001) higher than that in patients with mild–moderate injuries (3.9 [2.5–5.7]%). A similar trend was evident at 24 h (6.4 (5.1–8.5)% vs 3.6 (2.8–7.8)%; p = 0.03) and 72 h (7.1 (5.0–10.2)% vs 4.8 (3.6–7.5)%; p = 0.04), with a progressive increase in IPF between 2 h and 72 h in severely injured patients (Figure 1A). Platelet count showed the inverse pattern, with lower counts in severely injured patients at each timepoint and a progressive decline between 2 h and 72 h (Figure 1B). The mortality rate in patients with both IPF and platelet count below the cohort medians (11/35, 31.4%) was four‐fold higher (p < 0.001) than that in the remainder of the cohort (12/159, 7.5%).
Conclusion(s): These results demonstrate that the IPF increases in the circulation in the acute phase after major trauma, and that low IPF values in the context of a low platelet count are associated with higher mortality. More detailed investigations of the origins and beneficial effects of circulating immature platelets after injury are warranted.

VPB0900
Platelets and von Willebrand factor after exposure to turbulent, but low shear flow with implications in mechanical circulatory support
A. Liu; D. Bark
Washington University in St. Louis, St. Louis, Missouri, United States
Background: Heart failure is a major cause of death with the gold standard of treatment being heart transplantation. However, there are various limitations to this solution including patient eligibility and donor supply. Despite its many benefits, VADs exhibit significant limitations in hemocompatibility. Many ventricular assist devices (VADs) introduce turbulent flow characteristics, leading to detrimental effects on various blood components including platelets and proteins, such as von Willebrand factor (VWF).
Aims: Aim 1 (Evaluate effects of turbulent flow on VWF cleavage): We hypothesize that the increase in rotational speed and presence of turbulent flow will increase VWF cleavage. Aim 2 (Evaluate effects of turbulent flow on platelet activation): We hypothesize that the increase in rotational speed and turbulent flow will increase platelet activation.
Methods: We will draw peripheral whole blood and separate out the red blood cells to isolate plasma proteins and platelets. After isolation, we will expose platelet poor plasma to three different flow conditions calculated by preliminary results, followed by a multimer blot to assess VWF cleavage. To evaluate platelet activation after samples are exposed to vortexing, we will use flow cytometry to assess integrin (alpha)IIb(beta)3 conformational change CD41 expression and p‐selectin release by CD62 expression.
Results: Our results demonstrate the power of a simple vortexer assay in studying biological samples in a complex flow environment without requiring large sample. Although we are not the first to use this assay, we are the first to our knowledge to fully investigate the role of turbulence that can be created through this assay.
Conclusion(s): Our findings provide the field with a better understanding of the flow conditions that lead to VWF extension and cleavage in addition to platelet activation found in turbulence. This lays the groundwork for improving designs of blood‐contacting mechanical devices resulting in a lowered risk of bleeding and clotting complications after device implantation.


PB0848
Characterization of the uric acid transporter URAT1 (SLC22A12) in platelets and megakaryocytes
I. Boukhatem 1; J. Bélanger2; O. Ghafoud2; S. Fleury3; M. Welman3; M. Lordkipanidzé3
1 Montreal Heart Institute & University of Montreal, Montréal, Quebec, Canada; 2 Montreal Heart Institute & University of Montreal, Montreal, Quebec, Canada; 3 Montreal Heart Institute, Montréal, Quebec, Canada
Background: URAT1, encoded by the SLC22A12 gene in humans, acts as an organic anion transporter that plays a key role in uric acid and oxidative homeostasis. High levels of uric acid lead to urate crystals formation in the joints or in urine resulting in gouty arthritis or kidney stones, respectively. Less well‐known, the epidemiological association of hyperuricemia with adverse cardiovascular events poses the question of the contribution of high uric acid circulating levels to platelet reactivity and thrombosis.
Aims: To characterize URAT1 expression and function in human platelets and megakaryocytes.
Methods: URAT1 expression was verified by immunoblotting and by flow cytometry using independent antibodies in platelets and the megakaryocytic cell line MEG‐01, with HEK293 as positive controls. Platelet aggregation in response to classical platelet agonists (collagen, arachidonic acid, ADP and thrombin receptor activating peptide (TRAP)), in the presence or absence of pharmacological URAT1 inhibitors (lesinurad and verinurad) was verified by light transmission aggregometry in washed platelets prepared from whole blood of healthy male and female volunteers.
Results: URAT1 immunoreactivity was detected on the surface of and within platelets and MEG‐01 cells, at the expected molecular weight of 65 kD. Cell fractionation experiments were consistent with flow cytometry results. Incubation of washed platelets with uric acid (50–100 μg/mL) did not induce spontaneous platelet aggregation, nor did it induce synergistic effects in the presence of low concentrations of classical platelet agonists. However, collagen‐, arachidonic acid‐, ADP‐, and to a lesser extent TRAP‐induced platelet aggregation was inhibited by pre‐incubation of washed platelets with either lesinurad (IC50 125 nM ‐ 1.6 μM) or verinurad (IC50 145 nM ‐ 14 μM).
Conclusion(s): Human platelets and megakaryocytes express the URAT1 transporter. Whether platelets and megakaryocytes can sense and respond to uric acid fluctuations in their environment remains uncertain and merits further investigation.
PB0886
Alcohol intake including wine drinking is associated with decreased platelet reactivity in a large population sample
R. Pashek 1; B. Nkambule2; M. Chan3; F. Thibord4; A. Lachapelle3; J. Cunha5; M. Chen3; A. Johnson3
1 NHLBI, Allston, Massachusetts, United States; 2 NHLBI, Boston, Massachusetts, United States; 3 NHLBI, Framingham, Massachusetts, United States; 4 Population Sciences Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, Boston, Massachusetts, United States; 5 NHLBI and Boston University's Framingham Heart Study, Framingham, Massachusetts, United States
Background: Alcohol consumption has been linked to decreased platelet function. Whether this link is dependent on sex or type of alcoholic beverage consumed (wine/liquor/beer) remains unclear.
Aims: We aimed to assess associations between weekly alcohol consumption, platelet reactivity, and beverage type in men and women in a large‐scale population cohort.
Methods: Cross‐sectional data was obtained from participants enrolled in the Framingham Heart Study (N = 3427). Alcohol consumption was assessed by standardized medical history questionnaire, including quantity, frequency, and beverage type. White versus red wine consumption was measured using the Harvard semi‐quantitative food frequency questionnaire (FFQ). Five bioassays measured platelet reactivity traits across several agonists in whole‐blood and platelet‐rich plasma (PRP) samples. Linear mixed effects models adjusted for age, sex, and aspirin use, were used to evaluate associations between platelet reactivity and alcohol consumption.
Results: Total alcohol consumption was associated with decreased platelet reactivity, with stronger associations among wine and liquor compared to beer (Figure 1). Though the majority of reactivity traits (88%, at p < 0.01) had larger effect sizes among women, sex‐interaction tests were mostly insignificant. Among individuals who only consumed either red or white wine, decreased platelet reactivity traits (29%, at p < 0.01) were found to be moderately associated with total white wine consumption; however, red wine appeared to have no significant relationship with platelet reactivity.
Conclusion(s): We confirm associations between alcohol consumption and decreased platelet reactivity. Effects may be stronger for liquor and wine drinking, and in women; however, different alcohol preferences between sexes are noted that may contribute to these differences (Table 1), not including non‐drinkers (n = 809). Our results show that red wine consumption is not strongly associated with lower platelet function, contrasting some prior studies. Since polyphenol and chemical content varies widely among wines, our study suggests wine type cannot simply be generalized in its effects on platelet reactivity.


PB0853
The potential of pre‐activated platelets to contribute to thrombus formation
I. De Simone 1; C. Baaten2; J. Gibbins3; H. ten Cate4; J. Heemskerk5; C. Jones3; P. van der meijden6
1 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, MAASTRICHT, Limburg, Netherlands; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Institute for Molecular Cardiovascular Research, University Hospital Aachen, RWTH Aachen University, Aachen, Germany, Maastricht, Limburg, Netherlands; 3 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom; 4 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 5 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 6 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Thrombosis Expertise Center, Heart + Vascular Center, Maastricht University Medical Center, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands
Background: Platelet activation results in a conformational change of integrin αIIbβ3, which regulates the adhesiveness of platelets to fibrinogen and VWF and is necessary for thrombus formation. Prior integrin activation can be reversed. We hypothesize that platelet activation with different agonists results in diverse integrin (in)activating effects over time, thereby affecting the participation of pre‐activated platelets in thrombus formation.
Aims: To study short‐and long‐term platelet responses upon GPVI and PAR stimulation and to investigate whether pre‐activated platelets contribute to thrombus formation.
Methods: Short‐and long‐term effects of GPVI and PAR stimulation were evaluated via flow cytometry (PAC‐1 and fibrinogen binding) and plate‐based aggregation. Pre‐activated washed platelets were added to washed red blood cells and fibrinogen and were perfused over collagen type I or fibrinogen surfaces, to assess the role of pre‐activated platelets in thrombus formation.
Results: After 30 minutes of PAR stimulation, with thrombin or TRAP6, platelets showed 30–50% less PAC‐1 (p < 0.01) and fibrinogen binding (p < 0.05) than after 10 minutes. The ability of platelets to aggregate also decreased after 30 minutes of PAR stimulation compared to immediately after agonist addition (p < 0.05). Contrarily, neither PAC‐1 and fibrinogen binding nor aggregation were diminished after 30 minutes of GPVI stimulation with CRP‐XL. Compared to unstimulated platelets, short‐ or long‐term pre‐activation of platelets (0 or 30 minutes, respectively) with CRP‐XL resulted in the formation of larger heterogeneous thrombi on collagen and fibrinogen surfaces. Large heterogeneous thrombi were also formed when platelets were pre‐activated short‐term with TRAP6. However, after longer incubation with TRAP6, the formed homogeneous thrombi on collagen and fibrinogen resembled those of control, unstimulated platelets.
Conclusion(s): The long‐term integrin αIIbβ3 activation by prior platelet triggering with GPVI agonists is accompanied by a more prolonged response and enhanced thrombus formation in comparison to PAR triggering. When integrin αIIbβ3 reverses, platelets retain their normal aggregation pattern under flow.
PB0854
Evaluation of the hemostatic ability of the new device Total Thrombus Formation Analysis System’ (T‐TAS) for thrombocytopenic patients. In vitro effect of Thrombosomes®
S. Samanbar1; A. Moreno‐Castaño2; J. Piñeyroa3; M. Pino4; S. Torramade‐Moix5; J. Martinez‐Sanchez6; K. Moskowitz7; G. Escolar5; M. Diaz‐Ricart 8
1 Hematopathology, Pathology Department, CDB, Hospital Clinic, IDIBAPS, University of Barcelona, Spain, Barcelona, Catalonia, Spain; 2 Hematopathology, Pathology Department, Centre de Diagnòstic Biomèdic. Hospital Clinic Barcelona, Barcelona, Catalonia, Spain; 3 Hematology Department, Hospital Clinic, Barcelona, Spain, Barcelona, Catalonia, Spain; 4 Hospital Clinic Barcelona, Barcelona, Catalonia, Spain; 5 Hematopathology, Department of Pathology, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain, Barcelona, Catalonia, Spain; 6 Josep Carreras Leukemia Research Institute, Hospital Clínic de Barcelona, Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 7 Cellphire Inc, Cellphire Therapeutics, Inc., Rockville, MD, US, Rockville, Maryland, United States; 8 Hospital Clinic, IDIBAPS, University of Barcelona, Spain, Barcelona, Catalonia, Spain
Background: Bleeding complications in thrombocytopenic patients constitute a significant problem in clinical practice. Current laboratory approaches fail to evaluate hemostasis in thrombocytopenia and also the efficacy of platelet transfusion. The Total Thrombus Formation Analysis System (T‐TAS) is a new approach proposed to explore hemostasis in samples with low platelet counts. T‐TAS may become an alternative tool to explore the hemostatic effect of hemoderivatives.
Aims: To validate T‐TAS as a suitable device to evaluate hemostasis in samples from thrombocytopenic patients before and after platelet transfusion. To explore the hemostatic efficiency of in vitro addition of Thrombosomes®, a human platelet‐derived lyophilized hemostatic agent proposed for the treatment of bleeding in thrombocytopenia.
Methods: Whole blood from hematological thrombocytopenic patients (n = 28) (platelet count <30x10^3 platelets/μl) was collected before and after platelet transfusion. Hemostasis was evaluated through T‐TAS, with specific chips (HD) containing microcapillary channels (50‐μm‐deep) coated with collagen and tissue factor. Area under the curve (AUC) and occlusion times (OT, min) were registered for both samples before and after addition of Thrombosomes® (50x10^3/μl).
Results: Values (Mean ± SEM) of platelet counts were of 10 ± 1.2 and 24 ± 2 (x10^3 platelets/μl) and hematocrits (HCT) were of 24 ± 0.6% and 27 ± 1%, before and after platelet transfusion, respectively. T‐TAS demonstrated significant improvements after platelet transfusion (see Table 1). Addition of Thrombosomes® increased AUC and shortened OT in 60% and 100% of samples from non‐transfused (p˂0.05) and transfused patients, respectively.
Conclusion(s): The newly designed system T‐TAS, using a modified microchip‐based flow chamber, was able to measure hemostasis in samples with thrombocytopenia and low hematocrit. T‐TAS was useful to evaluate the hemostatic capacity of transfused platelets. Moreover, T‐TAS offers a platform to assess the hemostatic effect of hemoderivatives, such as Thrombosomes®, which enabled hemostasis even in those samples in which it was severely impaired.

PB0856
Chronic inflammation of the liver and bile ducts contributes to platelet hypoactivity and platelet‐leukocyte aggregate formation in Mdr2−/− mice
H. Englert 1; J. Gerwers1; N. Wolska1; J. Krause1; D. Schwinge1; C. Schramm2; T. Renné3
1 University Medical Center Hamburg Eppendorf, Hamburg, Hamburg, Germany; 2 University Medical Centre Hamburg Eppendorf, Hamburg, Hamburg, Germany; 3 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany
Background: Platelets and leukocytes are known to be key players in inflammation. Increased thrombotic risk has been linked to liver disease due to a dynamic disequilibrium between pro‐ and anticoagulation. Formation of platelet‐leukocyte aggregates (PLAs) is a recently observed phenomenon, associated with a wide range of cardiovascular diseases, stroke, and inflammatory diseases, but has not been described previously in liver diseases. Cholangitis is a chronic liver disease, characterized by inflammation, fibrosis, and biliary strictures, eventually resulting in cirrhosis, liver failure and cancer. Intrahepatic cholestasis and cholangitis can be mimicked using Mdr2 (Abcb4) KO mice, which fail to secrete phospholipid from the liver into the biliary system.
Aims: In this study we aimed to investigate the role of platelets and the formation of PLAs in a mouse model of cholangitis.
Methods: Anti‐coagulated blood from 4‐, 8‐, 12‐, and 20‐week‐old Mdr2−/− and control (wild‐type, WT) mice was activated with ADP, thrombin, or collagen‐related peptide (CRP‐xl) and analyzed for activation markers (p‐selectin expression and integrin GPIIb/IIIa activation) using flow cytometry. PLAs were measured by flow cytometry in a resting state, without the addition of any activators. Liver lysates were analyzed for inflammatory markers using a multiplex cytokine assay.
Results: Mdr2−/− platelets were slightly preactivated under resting conditions but appear to be hypoactive when stimulated. An increase in PLA formation in Mdr2−/− compared to WT animals was noted. Liver lysates from Mdr2−/− mice showed a pro‐inflammatory cytokine profile in comparison to WT mice.
Conclusion(s): Taken together, our data suggest that chronic liver and biliary inflammation in Mdr2−/− mice promote platelet hypoactivity and PLA formation.
PB0860
Smartphone‐based detection of the coagulation cascade and/or platelet activation in ‘dip‐stick’ microcapillary strips
D. Hodge 1; T. Vujic2; R. Sariyer3; A. Edwards3; C. Jones4
1 Reading School of Biological Sciences, University of Reading, Whiteknights, Reading, United Kingdom, Reading, England, United Kingdom; 2 University of Reading, Reading, England, United Kingdom; 3 Reading School of Pharmacy, University of Reading, Whiteknights, Reading, United Kingdom, Reading, England, United Kingdom; 4 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: The lack of platelet function testing suitable for primary care prevents the use of platelets as biomarkers and/or for monitoring anti‐platelet therapy. To enable rapid haemostatic analysis in wider settings, we have adapted microfluidic technology with smart‐phone imaging to develop a ‘dip‐stick’ haemostatic function analyzer.
Aims: To develop a proof‐of‐principle, inexpensive, simple ‘dip‐stick’ device to detect coagulation/platelet activation.
Methods: Microcapillary Film (MCF) is manufactured from melt‐extruded Teflon® FEP. It contains 10 parallel capillaries with a mean 200 μm diameter. Capillaries were coated internally with a hydrophilic polymer (PVOH) and then thrombin. Coated capillaries were dipped into whole citrated blood from healthy volunteers (n = 6) for 30 seconds. Capillary rise was imaged with a smart‐phone camera. Height of capillary rise was analyzed in ImageJ.
Results: Capillaries coated with thrombin showed a concentration dependant reduction in capillary rise, with an average intra‐strip coefficient of variation (CV) < 6%, and an average inter‐strip CV <3%. In strips loaded with 50 units/mL thrombin, mean capillary rise was 24.1% lower than control (p < 0.0005). Pre‐treatment of blood with hirudin to inhibit thrombin completely reversed this effect; capillary rise reduced by 0.37% compared to control (p > 0.05). Pre‐treatment of blood with the peptide GPRP, which allowed platelet activation but inhibited clot formation partially reversed thrombin's effect on capillary rise, reducing by 6.1% relative to control (p < 0.05), but 18.0% higher than thrombin alone (p < 0.0005). We also showed that hematocrit correlated with rise in agonist‐free capillaries yet reduction in rise due to thrombin was independent of blood cells counts.
Conclusion(s): We have developed a reproducible proof‐of‐principle ‘dip‐stick’ haemostatic function analyzer with the capability of measuring coagulation/platelet function, independently or in combination. This demonstrates the potential utility of this device as an assay that could be used point‐of‐care and in large scale platelet function studies, meeting important medical and research needs.
PB0861
Smartphone‐based detection of platelet response to ADP in microcapillary strips
D. Hodge 1; O. Godfrey2; B. Khatri2; A. Edwards3; C. Jones4
1 Reading School of Biological Sciences, University of Reading, Whiteknights, Reading, United Kingdom, Reading, England, United Kingdom; 2 University of Reading, Reading, England, United Kingdom; 3 Reading School of Pharmacy, University of Reading, Whiteknights, Reading, United Kingdom, Reading, England, United Kingdom; 4 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: The lack of platelet function testing for primary care prevents effective monitoring of patients on anti‐platelet therapy and the ability to personalize therapy. To enable rapid platelet function analysis in a wider setting we have developed a microfluidic ‘dip‐stick’ coupled with smart‐phone imaging that allows rapid assessment of platelet activation by ADP, a pathway regularly targeted by anti‐platelet drugs.
Aims: To develop a proof‐of‐principle, inexpensive, simple ‘dip‐stick’ device to detect platelet activation via ADP at point of care.
Methods: Microcapillary Film (MCF) is manufactured from melt‐extruded Teflon® FEP. It contains 10 parallel capillaries with a 200 μm diameter. Capillaries were coated internally with hydrophilic polymer (PVOH) or modified coating solution ‘Coating X' and then ADP. Coated capillaries were dipped into whole citrated blood from healthy volunteers (n = 5) for 30 seconds. Capillary rise was imaged with a smart‐phone camera. Height of capillary rise was analyzed in ImageJ.
Results: Capillaries coated with PVOH did not demonstrate an ADP concentration dependant reduction in capillary rise and showed high variability with an average intra‐strip coefficient of variation (CV) of 24%, and an average inter‐strip CV of 14%. Treatment of blood with cangrelor did not significantly impact capillary rise. However, capillaries coated with ‘Coating X' demonstrated an ADP concentration dependant reduction in capillary rise and were reproducible; with an average intra‐strip CV value below 7%, and an average inter‐strip CV value below 4%. In strips loaded with ADP at 100uM, mean reduction of capillary rise was −10.8%, upon treatment of blood with cangrelor this decreased to 0.78% (p < 0.0005).
Conclusion(s): We have developed a rapid, reproducible, proof‐of‐concept ‘dip‐stick’ platelet function analyzer capable of measuring platelet response to ADP and the inhibitory action of cangrelor. This demonstrates the potential utility of this device as an assay that could be widely used at point‐of‐care, meeting the unmet need of on‐treatment testing.
PB0869
Single platelet function in thrombocytopenia patients measured with droplet microfluidics
M. Jongen; P. Höglund
Center for Hematology and Regenerative Medicine, Karolinska Institutet, Huddinge, Stockholms Lan, Sweden
Background: Platelet functional heterogeneity has been a topic of interest in several studies. However, only a droplet‐based method can study pure intrinsic heterogeneity without the influence of adjacent platelets. This method has previously shown to detect novel subtypes of differently responsive platelets that contribute to the total response of platelets as a collective.
Aims: To adapt and apply the single platelet droplet microfluidic assay to detect subtypes of platelets in different patient groups suffering from thrombocytopenia.
Methods: The innovative droplet microfluidic protocol involves compartmentalizing platelets in monodisperse droplets with agonists while excluding paracrine signaling. High frequency encapsulation (250 Hz) is coupled with flow cytometry for high throughput quantification of platelet responses to multiple agonists. The ideal intake of platelets is in platelet rich plasma at a concentration of 5x109/L and consumption is 60 μL/hr, so for mildly thrombocytopenic patients no adaptation is required and for severely thrombocytopenic patients a slow centrifugation of the platelet rich plasma can increase the concentration as required.
Results: Platelets were individually encapsulated in water‐in‐oil droplets with a mean diameter of 25 μm. In healthy individuals, it is shown that there exist hyperresponsive platelet subpopulations visible after addition of low concentration agonist that are not detectable outside droplets and drive the collective response. Furthermore, at high concentrations of agonists we can detect hyposensitive platelets that do not respond with submaximal agonist activation. We hypothesize that these are increased in leukemia patients where there is a functional defect in addition to the low counts and that the hyperresponsive population is enhanced in immune thrombocytopenic patients where a compensatory mechanism prevents bleeding even at very low counts.
Conclusion(s): This study demonstrates the value of a high throughput droplet microfluidics and flow cytometry workflow for measuring platelet heterogeneity and its application to thrombocytopenic patients of different origins.

PB0874
Assessing Circulation Persistence of Human Platelet Products in a NOD‐SCID Mouse Model
B. Kuhn 1; K. Moskowitz2
1 Cellphire Therapeutics, Inc., Rockville, Maryland, United States; 2 Cellphire Inc, Cellphire Therapeutics, Inc., Rockville, MD, US, Rockville, Maryland, United States
Background: Measuring survival of human platelets following their circulation in the blood allows for investigations of function, clearance, and the impact of their treatment prior to infusion. The Nonobese diabetic/severe combined immunodeficiency (NOD‐SCID) mouse is an ideal animal model given its compatibility with xeno‐transfusion. Herein, we use carboxyfluorescein diacetate succinimidyl ester (CFDA‐SE) to fluorescently label apheresis platelets, cold‐stored platelets (CSP), 6% Dimethylsulfoxide cryopreserved platelets (CPP), and Thrombosomes®, a human platelet derived lyophilized hemostatic (LHP), to measure their circulation persistence in NOD–SCID mice.
Aims: Assess the circulation persistence of apheresis platelets, cold‐stored platelets, cryopreserved platelets, and Thrombosomes®.
Methods: Human apheresis platelets were incubated with 50 μM CFDA‐SE at room temperature for 60 minutes. Labeled platelets were used as a test article or further processed into CSP (stored at 4°C unagitated), CPP (stored at −80°C), or LHP (stored at room temperature). NOD‐SCID mice (n = 6 per group) were infused by tail vein injection and whole blood samples were taken by submandibular venipuncture. The study infusion dates were staggered to accommodate processing of each product. Apheresis platelets were infused 48 hours post‐collection, CSP and CPP were infused 8 days after collection, and LHP were infused 9 days after platelet collection. Labeled platelet counts were measured by flow cytometry.
Results: Apheresis platelets had the highest initial recovery (2 minutes post infusion) at 69%, followed by CSP at 38%, CPP at 21%, and finally LHP at 9% (Figure 1). These differences in platelet recovery persisted throughout the other timepoints.
Conclusion(s): The NOD‐SCID mouse model of platelet circulation persistence successfully differentiated between four platelet products. It demonstrated that LHP are cleared more rapidly than liquid stored platelets and that CSP and CPP are intermediate in clearance. These differences in circulation persistence are seemingly correlated to platelet activation and cell damage induced by the different platelet treatment conditions.

PB0889
Endogenous nitroxyl (HNO) production in a normal biological system and its role in platelet function
R. Pozner 1; Y. Doctorovich1; M. Maccaferro1; S. Suarez2; M. Marti3; F. Doctorovich2; P. Ivani1; M. Schattner1
1 Experimental Medicine Institute. National Academy of Medicine. CONICET., CABA, Ciudad Autonoma de Buenos Aires, Argentina; 2 INQUIMAE, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, C.A.B.A. Argentina., CABA, Ciudad Autonoma de Buenos Aires, Argentina; 3 Depto. de Química Biológica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, C.A.B.A. Argentina., CABA, Ciudad Autonoma de Buenos Aires, Argentina
Background: Platelet inhibition is the focus of numerous investigations. Although several inhibitors are widely used, HNO (a reduced form of NO) is raising a great clinical interest. HNO shares vasodilatory properties with NO, but also showed increased muscle contractility, acceleration of ventricular relaxation and decrease in cardiac load. These data come from the use of HNO‐releasing drugs, however, it is unknown if this gasomediator can be synthesized endogenously by platelets.
Aims: In this study, we evaluated the generation of HNO by platelets (PRP) and its impact on functional responses.
Methods: HNO production was quantified in real‐time by two experimental strategies: detection of spectral changes of a porphyrin of Mn(III), and current changes using a high sensitivity selective electrochemical sensor ‐based on a porphyrin of Co(II)‐. The concentration of HNO was estimated from a calibration curve made with Angeli's salt (Figure 1A). Fibrinogen binding and P‐selectin expression were evaluated by cytofluorimetry.
Results: Stimulation of platelets with classical agonist TRAP, ADP, or arachidonic acid (AA) promoted the generation of HNO (Figure 1B, n = 5, p < 0.05). These results were corroborated by spectrophotometry. In order to modulate HNO levels, and considering that platelets synthesize NO, we use reducing agents such as ascorbic acid (Asc) or 2‐mercaptoethanol. The production of HNO by ADP, TRAP, or AA was significantly higher in the presence of both drugs (Figure 1c). In addition, it was observed that all the platelet activation parameters evaluated (aggregation, fibrinogen binding, and P‐selectin externalization) were significantly inhibited by both HNO‐elevating agents, in a concentration‐dependent manner. On the contrary, in the presence of L‐Cysteine or Glutation, the TRAP‐ or ADP‐induced HNO production were reduced and platelet function improved.
Conclusion(s): These data constitute the first evidence of HNO production by a biological system under normal conditions and suggest a negative feedback loop for this endogenous gasomediator in platelets.

VPB0901
Effects of platelets activated by different agonists on fibrin formation and thrombin generation
I. Muravlev1; A. Dobrovolsky2; O. Antonova1; S. Khaspekova1; A. Mazurov 1
1 National Medical Research Center for Cardiology, Russian Ministry of Health, Moscow, Moskva, Russia; 2 NATIONAL MEDICAL RESEARCH CENTER OF CARDIOLOGY Ministry of Health of the Russian Federation, DEPARTMENT OF CLINICAL PROBLEMS OF ATHEROTHROMBOSIS, Moscow, Moskva, Russia
Background: Platelet procoagulant function is usually assessed by exposure of phosphatidylserine (PS), which serves as a substrate for assembling coagulation complexes, and/or by the activity of these complexes. However, the direct effects of platelets on fibrin formation have not been thoroughly studied so far.
Aims: We investigated the effects of platelets activated by different agonists on fibrin formation and thrombin generation and compared these effects with PS expression.
Methods: A modified version of a recalcification assay was developed for studying the effects of platelets on fibrin formation. Washed human platelets were not activated or activated by ionophore A23187, collagen, arachidonic acid, ADP, and TRAP (Thrombin Receptor Activating Peptide), and spin down on the bottom of 96‐well plates. Plasma was added to platelets, recalcified, and fibrin formation was followed by changes in light absorption. Platelets prepared in the same way were tested in the thrombin generation test. PS expression was evaluated by annexin V staining using flow cytometry.
Results: Platelets significantly accelerated both fibrin formation and thrombin generation. They shortened the lag phase and increased the maximal rate of plasma clotting, increased the peak and the maximal rate of thrombin generation. In both tests, platelets were presumably activated by endogenous thrombin formed in plasma after triggering coagulation reactions. However, pretreatment of platelets with exogenous agonists additionally increased their procoagulant activity. Maximal activity was observed after pretreatment with A23187, lesser with collagen and arachidonic acid, and minimal with ADP and TRAP (the latter might be ineffective due to the competition with endogenous thrombin). Effects of differently activated platelets on fibrin formation and thrombin generation in general corresponded with the levels of PS expression.
Conclusion(s): The ability of platelets activated by different agonists to accelerate fibrin formation correlates with their action on thrombin generation and is determined by the level of PS expression. Support RFBR # 20–015‐00106.
PB0877
Discriminating platelet subpopulations on the expression level of HLA‐I highlights the high reactivity of young versus old platelets
C. Angénieux1; A. Couvidou1; N. Brouard1; A. Eckly2; P. Mangin1; B. Maître 1
1 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 2 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strassbourg, Alsace, France
Background: High proportion of young platelets has been associated with cardiovascular diseases and impaired responsiveness to thienopyridines. So far, the lack of reliable tools to precisely discriminate various platelet subpopulations according to their age has prevented detailed analysis of platelet reactivity.
Aims: To assess the platelet reactivity according to their age based on HLA‐I expression level that we recently showed to be over‐represented on young platelets.
Methods: Platelet activation of hirudinated human blood was assessed by flow cytometry (FC) for different platelet subsets based on their HLA‐I expression. These populations were further cell sorted and their intrinsic properties were determined by FC and electron microscopy (EM).
Results: HLA I expression level allows the identification of three platelet subpopulations regarding to their age (HLA low, dim and high). In response to different soluble agonists, levels of fibrinogen binding, phosphatidylserine exposure showed a two‐ to four‐ fold increase in the reactivity of young HLA‐I high platelets as compared to HLA‐I dim ones whereas old HLA‐I low platelets were the least reactive. Moreover, the highest capacity of HLA‐I high platelets to simultaneously express annexin V and α‐granule factor V or activated αIIbβ3 after coactivation with TRAP and CRP indicated that the procoagulant feature of platelets is age‐related. In parallel, we demonstrated that HLA‐I is reliable to guide platelet cell sorting without any platelet activation as shown by EM and FC; stimulation with TRAP and CRP confirmed an intrinsic ability of HLA‐I high platelets to become procoagulant.
Conclusion(s): Using HLA‐I expression as a way to finely discriminate and isolate platelet subpopulations according to their age, we showed that the young HLA‐I high population is the most reactive and prone to become procoagulant. These results open up new perspectives to investigate deeply the physio/pathological role of young platelets.
PB0872
Fully automated platelet Differential Interference Contrast image analysis via deep learning
C. Kempster 1; G. Butler2; E. Kuznecova1; K. Taylor1; N. Kriek3; G. Little3; M. Sowa3; T. Sage3; L. Johnson3; J. Gibbins4; A. Pollitt3
1 University of Reading, Reading, England, United Kingdom; 2 Johns Hopkins University, Johns Hopkins School of Medicine, Maryland, United States; 3 University of Reading, READING, England, United Kingdom; 4 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: Platelets mediate arterial thrombosis, a leading cause of myocardial infarction and stroke. During injury, platelets adhere and spread over exposed subendothelial matrix substrates of the damaged blood vessel wall. The mechanisms which govern platelet activation and their interaction with a range of substrates are therefore regularly assessed and investigated using platelet spreading assays. These assays often use differential interference contrast (DIC) microscopy to assess platelet morphology whereby analysis is performed by manually annotating the perimeter of each platelet.
Aims: To implement a convolutional neural network (CNN) to automate platelet spreading analyses and abrogate time consuming and biased manual analysis.
Methods: The CNN was trained using 120 generalized training images. Increasing the number of training images increased the mean average precision (mAP) of the CNN. Platelets were spread in the presence and absence of inhibitors (dasatinib, ibrutinib, and PRT‐060318) or agonist (thrombin) known to impact platelet morphology, and over a range of different substrates (CRP‐XL, vWF and fibrinogen). The CNN performance was compared to a trainer, and five manual annotators.
Results: A convolutional neural network (CNN) allowed fully automated analysis of platelet spreading assays captured by DIC microscopy when platelets were spread over a range of substrates, and when in the presence or absence of inhibitors or agonist. Significant variation was observed between individual annotators, highlighting biases associated with manual analyses. Results were consistent in quantifying the spread area of platelets, which were comparable to published literature.
Conclusion(s): The application of a CNN enables, for the first time, automated analysis of unlabelled platelet spreading assays captured by DIC microscopy which has the potential to standardize analyses across laboratories.
PB0876
Platelet function testing and timing of bypass surgery ‐ A platelet function sub‐study from the RAPID CABG randomized trial
M. Lordkipanidzé 1; G. Wells2; M. Le May2; F. Rubens2; L. Chen2; M. Ruel2; A. Chong2; M. Welman1; L. Perrault3; V. Chan2; J. Tanguay3; D. So2
1 Montreal Heart Institute, Montréal, Quebec, Canada; 2 University of Ottawa Heart Institute, Ottawa, Ontario, Canada; 3 Montreal Heart Institute, Montreal, Quebec, Canada
Background: The RAPID CABG (NCT02668562) randomized trial demonstrated early coronary artery bypass surgery (CABG), 2–3 days after ticagrelor cessation, was non‐inferior in incurring perioperative bleeding compared to waiting 5–7 days. Platelet function testing has been proposed as a tool to guide timing to surgery. In this pre‐specified platelet function sub‐study, we evaluate perioperative platelet function as a determinant of bleeding.
Aims: To assess whether pharmacodyamic measurement of platelet function following ticagrelor cessation is a predictor of bleeding and ischemic outcomes.
Methods: Patients with acute coronary syndromes and requiring CABG were randomly assigned to early surgery, 2–3 days after ticagrelor cessation, vs standard delay of 5–7 days. Platelet function was measured using the VerifyNow P2Y12 assay before, immediately after and at 24, 48 and 72 hours post‐CABG. The primary outcome was bleeding, defined as class 3 or 4 (severe and massive) universal definition of peri‐operative bleeding (UDPB). Secondary outcomes included: other bleeding outcomes (BARC type 4, TIMI CABG‐related bleeding, cardiac tamponade) and ischemic outcomes (CV death, stroke, myocardial infarction, refractory ischemia, urgent unplanned revascularization).
Results: In the per‐protocol population, platelet function measures were available in 60/65 patients in the early group and 54/58 in the delayed group. At all perioperative time points, the delayed group had significantly higher platelet reactivity (Figure A). Proportion of patients with low platelet reactivity (LPR ‐ defined by PRU < 85, a correlate to increased bleeding risk), was similar in the early (5%) vs. delayed (1.9%) groups (p = 0.62). A higher proportion had high platelet reactivity (HPR, defined as PRU > 208) in the delayed (89%) vs. the early (45%) group at time of surgery (p < 0.001). Preoperative platelet reactivity was not associated with blood loss (Figure B). Correlation to other bleeding and ischemic outcomes will be presented.
Conclusion(s): Platelet function recovery after ticagrelor cessation followed expected pharmacokinetics, but did not predict perioperative bleeding.

PB0878
The Impact of Dual Anti‐platelet, Anti Coagulation Therapy on Hemostatic Plug Structure and Function
C. Mansi 1; J. Severa2; T. Marar2; T. Stalker2
1 Thomas Jefferson University, Norwood, Pennsylvania, United States; 2 Thomas Jefferson University, Philadelphia, Pennsylvania, United States
Background: Background: Anti‐thrombotic medications carry an inherent risk of bleeding, which may be exacerbated when anti‐coagulant and anti‐platelet therapeutics are combined. Our lab has previously shown differences in the effects of anti‐platelet versus anti‐coagulant drugs on the structure and function of hemostatic plugs.
Aims: Aims: We examined whether dual anti‐thrombotic treatment consisting of combined anti‐platelet and anti‐coagulant therapeutics is different than either therapy alone on hemostatic plug structure and function.
Methods: Methods: Mice were treated with the P2Y12 antagonist clopidogrel and the Factor Xa inhibitor rivaroxaban across a range of doses, either alone or in combination. The hemostatic response was assessed using a mouse jugular vein puncture injury model. Platelet accumulation, activation, and fibrin deposition were evaluated with quantitative multiphoton fluorescence microscopy.
Results: Results: Mice treated with clopidogrel alone had significantly impaired platelet accumulation at the site of injury along with prolonged bleeding times and failure to achieve hemostasis at the highest doses of clopidogrel used (10–25 mg/Kg, p < 0.05). Mice given rivaroxaban alone instead showed a dose‐dependent reduction in fibrin deposition (p < 0.05 at 1 mg/Kg) with no impact on bleeding. Mice treated with both clopidogrel and rivaroxaban had platelet and fibrin accumulation that was similar to either drug given alone, however, dual anti‐thrombotic therapy resulted in impaired hemostasis at doses that had no impact on bleeding when given in isolation.
Conclusion(s): Conclusion: Combined administration of anti‐platelet and anti‐coagulant therapeutics has a greater impact on hemostatic plug function than either drug alone. Our findings highlight how platelet and fibrin deposition are differentially impacted by the use of either anti‐thrombotic therapy in isolation, resulting in increased bleeding in the setting of dual therapy. We speculate that this enhanced bleeding stems from the combined loss of both ADP‐ and thrombin‐mediated platelet activation. Our findings may help clinicians better understand bleeding risk associated with dual anti‐thrombotic therapy.
PB0845
P2Y receptor antagonists and tyrosine kinase inhibitors reduce oxLDL‐mediated procoagulant platelet activity
T. Zheng1; T. Kohs1; J. Pang1; S. Reitsma1; I. Parra‐Izquierdo1; A. Melrose 1; M. Larson2; C. Williams1; M. Hinds1; O. McCarty3; J. Aslan1
1 Oregon Health & Science University, Portland, Oregon, United States; 2 Augustana University, Sioux Falls, South Dakota, United States; 3 Oregon Health and Science University, Portland, Oregon, United States
Background: Dyslipidemia and lipoprotein accumulation in the vasculature drive atherogenesis and cardiovascular disease and are risk factors for clinically significant atherothrombotic events, such as myocardial infarction, stroke, and sudden death. In these contexts, oxidized low density lipoprotein (oxLDL) contributes to atherosclerosis through interactions with peripheral blood cells, in particular platelets. However, the mechanisms by which oxLDL affects platelet activation and function, and how to best therapeutically target and safely prevent such responses remains to be elucidated.
Aims: The aim of this study is to investigate how oxLDL upregulates glycoprotein VI (GPVI) mediated platelet hemostatic and procoagulant responses, and how traditional and emerging antiplatelet therapies affect oxLDL‐enhanced platelet activity ex vivo.
Methods: Human platelets were isolated and treated with oxLDL alone and in combination with GPVI specific agonist, crosslinked collage‐related peptide (CRP‐XL) and assayed for hemostatic and procoagulant responses in the presence of inhibitors of purinergic receptors (P2Y; ticagrelor, AR‐C 66096, MRS2179), cyclooxygenase (COX; aspirin, indomethacin), Bruton's tyrosine kinase (BTK; ibrutinib), and spleen tyrosine kinase (Syk; fostamatinib/R406).
Results: Ex vivo, oxLDL enhanced GPVI‐mediated platelet dense granule secretion, alpha granule secretion, integrin activation, thromboxane generation, and platelet aggregation, as well as platelet procoagulant phosphatidylserine exposure and fibrin generation. Our study further demonstrates that CD36 signaling from oxLDL activation overlaps with the GPVI signaling pathway, in such that the phosphorylation of proteins along the Syk‐BTK‐PI3K/Akt axis is enhanced. P2Y antagonists (e.g., ticagrelor), BTK inhibitors (e.g., ibrutinib), and Syk inhibitors (e.g. fostamatinib) reduced oxLDL‐mediated platelet responses and procoagulant activity, whereas COX inhibitors (e.g., aspirin) had no significant effect.
Conclusion(s): Altogether, our results demonstrate that oxLDL enhances ex vivo platelet responses and procoagulant activity downstream of GPVI signaling in a manner that may be reduced by P2Y antagonists (ticagrelor) and tyrosine kinase inhibitors (ibrutinib and fostamatinib), but not significantly affected by COX inhibitors (aspirin).
PB0870
Platelet functional phenomics in the post‐acute stage of pulmonary embolism
S. von Ungern‐Sternberg1; V. ten Cate2; M. Nagler3; M. Panova‐Noeva4; B. Dahlen5; J. Prochaska2; S. Heitmeier6; C. Gerdes7; S. Konstantinides8; T. Münzel9; C. Espinola‐Klein10; K. Lackner11; H. Spronk12; H. ten Cate13; P. van der meijden14; K. Leineweber7; P. Wild2; K. Jurk 15
1 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 2 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 3 Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Rheinland‐Pfalz, Germany; 4 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, mainz, Rheinland‐Pfalz, Germany; 5 Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 6 Bayer AG, Wuppertal, Germany, Wuppertal, Nordrhein‐Westfalen, Germany; 7 Bayer AG, Wuppertal, Nordrhein‐Westfalen, Germany; 8 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 9 Center for Cardiology ‐ Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 10 Center for Cardiology, Cardiology III ‐ Angiology; Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, mainz, Rheinland‐Pfalz, Germany, 11 Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, mainz, Rheinland‐Pfalz, Germany, 12 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart + Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands, 13 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands, 14 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Thrombosis Expertise Center, Heart + Vascular Center, Maastricht University Medical Center, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands, 15 University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: Enhanced platelet aggregation is associated with increased risk of venous thromboembolism and higher platelet activity has been demonstrated in acute pulmonary embolism (PE) compared to deep vein thrombosis. However, robust longitudinal studies investigating platelet function in the post‐acute phase of PE are lacking.
Aims: Elucidate platelet functionality and the effect of antithrombotic therapies in post‐acute PE.
Methods: A subgroup of 206 confirmed patients with PE from the Genotyping and Molecular Phenotyping of Venous ThromboEmbolism (GMP‐VTE) project, a multicenter prospective cohort study of 693 confirmed VTE cases, was analyzed. This study was approved by the local Ethics Committee. All patients signed the informed consent. Platelet fibrinogen binding, degranulation and pro‐coagulant activity in whole blood (flow cytometry), aggregability (light transmission aggregometry) and thrombin generation (calibrated automated thrombography) in platelet‐rich plasma were assessed in the acute PE event (BL) and during follow‐up examinations at 3/6, 12 and 24 months. Repeated measures ANOVA was used to test change over time, and pairwise t‐tests for comparisons between time points (e.g. Δt1‐t0).
Results: A significant cessation of anticoagulants was observed after 12 (BL:84.2%; 12 m:72.8%) and 24 (35.9%) months follow‐up, whereas antiplatelet therapy was directly stopped after PE confirmation for most cases (BL:37%; 3/6 m:11.1%). Ex vivo platelet fibrinogen‐binding (Δ12m‐BL = ‐11%, p < 0.0001), CD63 (Δ12m‐BL = ‐3%, p < 0.0001) and tissue factor surface expression (Δ12m‐BL = ‐12%, p = 0.028) were stably downregulated at 12 months follow‐up independent of antithrombotic therapy. Impaired epinephrine‐induced platelet aggregation (Δ3/6 m‐BL = +15%, p = 0.0032) and tissue factor‐triggered endogenous thrombin potential (ETP) (Δ3/6 m‐BL = +696 nM*min, p = 0.015) in the acute phase were normalized at 3/6 months follow‐up of non‐antiplatelet drug users and for ETP additionally increased for non‐anticoagulant users (Δ3/6 m‐BL = +2741 nM*min, p < 0.0001).
Conclusion(s): Platelet function is altered in acute PE, and recovers over the span of 3 to 12 months in the post‐acute phase. Continued antiplatelet and anticoagulant therapy maintain platelet hyporeactivity in the post‐acute stage of PE.
PB0873
Histology of Shear‐Induced Platelet Aggregated (SIPA) Thrombus Formed in vitro
D. Ku 1; D. Kim2
1 Georgia Institute of Technology, Decatur, Georgia, United States; 2 FDA, Silver Spring, Maryland, United States
Background: Shear‐Induced Platelet Aggregation (SIPA) can form quickly and grow to occlusive thrombi to stop blood flow under arterial hemodynamic conditions. The structure of SIPA clot may contain valuable information on mechanism of formation and a way to treat arterial occlusions. However, it is hard to collect a structurally intact SIPA clot clinically.
Aims: Create large SIPA clot to study its structure via histology.
Methods: Macroscopic thrombi are made from porcine whole blood in a collagen‐coated, large‐scale, glass stenosis with high shear flow conditions of an atherosclerotic artery. The millimeter‐sized thrombi are harvested for histology and scanning electron microscopy.
Results: Histological images showed 3 distinctive regions of thrombus architecture. 1) String‐like platelet aggregates extend from the wall in stripes alternating with free RBCs upstream of the throat. The strings were > 10x as long as they were wide and reached out to join the strings from the opposite wall. B) Near the apex, the thrombus was a dense mass with microchannels that effectively occludes the lumen. C) In the downstream expansion region, the thrombus occupied the centerline as a cone with an annulus of free blood in the flow separation zone. A thin shell of platelets at the wall of the expansion, separate from the core. The total clot is estimated to contain 1.23 billion platelets with pores on the order of 10–100 microns.
Conclusion(s): The histology reveals a complex structure of arterial thrombi that grow from their tips under high shear stress with VWF‐platelet strings. The occluding thrombus is dominant at the apex and contains many microchannels that allow some mass transport through the bulk of the thrombus. This architecture can create occlusion or hemostasis rapidly with minimal platelet material, yet remain porous for potential delivery of lytic agents to the core of the thrombus.


PB0849
High‐Throughput Screening to Identify Novel Small‐Molecule Inhibitors of Clot Retraction
C. Buitrago 1; M. Menezes2; F. Glickman3; B. Coller2
1 The Rockefeller University, new york, New York, United States; 2 The Rockefeller University, New York, New York, United States; 3 The Rockefeller University, new York, New York, United States
Background: Clot retraction, the ultimate phase of platelet thrombus formation, is critical for clot stabilization. It requires functional αIIbβ3 receptors (Glanzmann thrombasthenia patients have diminished or absent clot retraction), fibrin, and the integrated actions of the platelet actin‐myosin contractile and cytoskeletal systems. Disturbances in the mechanical properties of a clot have clinical implications as they are associated with both bleeding and thrombosis.
Aims: We recently demonstrated that platelets treated with the αIIbβ3 antagonist peptide RGDW, which eliminates fibrinogen‐mediated platelet aggregation, are still able to retract clots. We have exploited this observation to develop a robust high‐throughput screening assay to identify chemical compounds that inhibit clot retraction.
Methods: We miniaturized and standardized a clot retraction assay in a 384 well microtiter plate format. 1. Wash fresh platelets and treat with RGDW to prevent platelet‐fibrinogen interaction. 2. Add test compounds to the wells of polystyrene 384‐well microtiter plates using a pin tool. 3. Add CaCl2 and thrombin to the wells. 4. Add platelet/RGDW mixture, 5. After 60 min, image the plate using Image Xpress (Figure). Negative controls consist of wells containing platelets without compounds. Positive controls are wells containing platelets that are not stimulated with thrombin or platelet treated with mAb 7E3, an anti‐αIIbβ3 mAb known to inhibit clot retraction.
Results: To date, we have screened more than 400,000 compounds with a ‘hit’ rate of 0.34%. The Z' factor was 0.5–1.0, indicating that the assay has a high degree of reproducibility. Confirmation and curation of the hits is underway, and several classes of compounds have been identified, including kinase inhibitors with known antiplatelet effects as well as many compounds that have not previously been reported to have antiplatelet activity.
Conclusion(s): Our novel high‐throughput screen has identified compounds that inhibit clot retraction. Ongoing studies will identify their mechanism(s) of action.

PB0852
Platelet reactivity is associated with arterial tonometry, vessel diameters and calcium burden
J. Cunha 1; M. Chan2; B. Nkambule3; F. Thibord4; A. Lachapelle2; R. Pashek5; R. Vasan6; J. Rong7; E. Benjamin6; N. Hamburg6; M. Chen2; G. Mitchell8; A. Johnson2
1 NHLBI and Boston University's Framingham Heart Study, Framingham, Massachusetts, United States; 2 NHLBI, Framingham, Massachusetts, United States; 3 NHLBI, Boston, Massachusetts, United States; 4 Population Sciences Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, Boston, Massachusetts, United States; 5 NHLBI, Allston, Massachusetts, United States; 6 Boston University, Boston, Massachusetts, United States; 7 Boston University, Framingham, Massachusetts, United States; 8 Cardiovascular Engineering Inc, Framingham, Massachusetts, United States
Background: Arterial tonometry and aortic and coronary calcium measures are useful indicators of cardiovascular disease (CVD) risk. Prior studies found positive associations between serum vascular growth factors (angiopoietin‐2), tonometry measures (carotid‐femoral pulse wave velocity, mean arterial pressure) and CVD risk. Activated platelets release angiopoietin‐1, P‐selectin and microvesicles, which may provide a link between vascular structure and platelet function.
Aims: We analyzed platelet function, arterial tonometry, aortic and coronary calcium, and aortic diameters measured in the Framingham Heart Study's Gen3/NOS/Omni‐2 cohorts. We hypothesized that higher platelet reactivity would be associated with subclinical CVD measures, including arterial stiffness and higher calcium (Agatston) scores.
Methods: Platelet reactivity data in whole blood or platelet rich plasma was collected by 5 assay platforms in up to 7 agonists in n = 3429 individuals as previously described (Grech et al. PMID 34939182). We used linear mixed effects models with platelet reactivity phenotypes as outcomes adjusting for CVD risk factors (age, sex, aspirin‐use, body mass index, height, heart rate, hypertension medication, lipid medication, total cholesterol:HDL, diabetes, smoking) and accounting for family structure. Calcium values were assessed as ln‐transformed (Agatston+1).
Results: Higher vessel calcium displayed a pattern of association with higher platelet reactivity (Figure 1), whereas larger vessel diameters were generally associated with lower platelet reactivity. The arterial tonometry measures (niCFPWV, CPP, AI, GRC, Pf, and Zc) had a combination of positive and negative associations with platelet aggregation measures, including regions with opposite relations between niCFPWV and Zc, which are likely mediated by diameter effects.
Conclusion(s):. Vessel diameter associations are consistent with lower shear environments invoking less platelet reactivity, as platelets can be provoked by shear forces alone, and are potentiated by higher shear. The associations with vessel calcium suggest subclinical atherosclerosis may provoke platelet activation through multiple pathways of activation, thereby contributing to increased thrombosis risk.


PB0865
Surfing the PHOME for Novel Anti‐Platelet Agents: Empirical Evaluation of a Bioinformatic Drug Re‐Purposing Algorithm
G. Jarvis 1; H. Newman2; O. Elhakeem3; M. Alshahrani4; G. Gkoutos5; R. Hoehndorf6; P. Schofield3
1 University of Sunderland, Sunderland, England, United Kingdom; 2 University of Warwick, Coventry, England, United Kingdom; 3 University of Cambridge, Cambridge, England, United Kingdom; 4 National Center for Artificial Intelligence, Riyadh, Ar Riyad, Saudi Arabia; 5 University of Birmingham, Birmingham, England, United Kingdom; 6 King Abdullah University of Science and Technology, Thuwal, Makkah, Saudi Arabia
Background: The PHOME (PHarmacology + proteOME) is a drug re‐purposing algorithm that exploits large publicly available datasets to identify novel drug/target interactions in platelets. The PHOME integrates pharmacological, proteomic, tissue expression and gene function data into interaction networks.
Aims: (1) To use the PHOME to rank known compounds according to their ability to modulate platelet function. (2) To use in vitro platelet testing to evaluate the predictive effectiveness of the PHOME in identifying compounds with platelet modulatory actions.
Methods: 1. Drug Selection using the PHOME: A platelet protein–protein interaction (PPI) network was constructed using the Gene Expression Atlas (https://www.ebi.ac.uk/gxa/home) and STRING (https://string-db.org/) databases, with drug/target annotations from STITCH (http://stitch.embl.de/). We scored drug effects in the Platelet‐PPI‐Network generating outputs for 553 well‐known and/or clinically used compounds. Lower p values (Wilcoxon rank‐sum test) predict effects on collagen binding (GO:0005518), platelet activation (GO:0030168) and platelet aggregation (GO:0070527). Ten compounds scored p = 1 in each GO category and were selected for testing as controls. Ten compounds were selected randomly from 37 that scored p = 0 in each category. 2. Drug Evaluation: These 20 drugs were assigned ID codes to remove operator bias. Human washed platelets were pre‐incubated with the 20 drugs at 100 μM or 4 vehicle controls. Collagen (1 μg/mL)‐induced aggregation and static adhesion to collagen, collagen‐related peptide (CRP) and fibrinogen were measured.
Results: 5/10 p = 1 drugs substantially inhibited aggregation and 5/10 had no discernible effect. 6/10 p = 0 drugs inhibited aggregation and 4/10 did not. 3/10 p = 1 drugs reduced adhesion to collagen, 1/10 to CRP and 2/10 to fibrinogen. For the p = 0 drugs, the equivalent results were 1/10, 0/10 and 1/10.
Conclusion(s): These data suggest that the PHOME does not effectively predict drugs that modify platelet function, although 11 compounds displayed inhibitory actions. Scrutiny of database entries may suggest novel hypotheses about platelet function and enable algorithm optimisation.
PB0867
Development of an endothelialized in vitro thrombosis model with a disease relevant extracellular matrix
A. Drysdale1; A. Unsworth2; S. White1; S. Jones 1
1 Manchester Metropolitan University, Manchester, England, United Kingdom; 2 Manchester Metropolitan University, Manchester, England, United Kingdom
Background: Acute coronary syndrome (ACS) occurs following the rupture or erosion of an atherosclerotic plaque. The exposed extracellular matrix (ECM), a dynamic, complex network of glycoproteins, collagens and growth factors, activates circulating platelets, initiating thrombosis. Antiplatelet drugs represent the cornerstone treatment for ACS, however there is a need for safer more efficacious therapies, the development of which are dependent on disease relevant models.
Aims: The aim of this study was to develop an in vitro thrombosis model with an endothelial phenotype and ECM relevant to ruptured or eroded plaques, to enable the development of a more stratified antithrombotic approach.
Methods: The composition of the ECM produced by endothelial cells (ECs) and smooth muscle cells (SMCs) under healthy conditions and following disease related stimuli was identified using mass spectrometry. Thrombus formation under arterial flow conditions was measured using fluorescent microscopy on the cell‐derived matrices, and on recombinant ‘erosion’ and ‘rupture’ composites designed from ECM characterization in the literature.
Results: Methods were successfully developed to remove ECs and SMCs, leaving an intact ECM for thrombus formation, and to retrieve the cell‐derived ECM in sufficient quantities for mass spectrometry. The extracellular matrix generated by ECs and SMCs varied considerably between the different cell treatments and this was reflected in the thrombogenicity of the different cell‐derived matrices, with significant differences in the size of the thrombi produced (p < 0.05). Differences were also observed in the thrombogenicity of the composite matrices, with larger thrombi formed under ‘rupture’ conditions.
Conclusion(s): Developing disease‐relevant in vitro thrombosis models will facilitate the stratification of antithrombotic treatment based on plaque phenotype and will contribute towards reducing the use of animals in cardiovascular research.
PB0893
Storage of apheresis platelets in lipaemic plasma affects aspects of platelet function and surface receptor expression
D. van der Wal 1; C. Linnane2; H. Aung3; R. Webb3; D. Marks3
1 Australian Red Cross Lifeblood, Sydney, New South Wales, Australia; 2 Australian Red Cross Lifeblood, Alexandria, New South Wales, Australia; 3 Australian Red Cross Lifeblood, Cronulla, New South Wales, Australia
Background: Lipaemia in blood donations is typically caused by consuming a high fat meal prior to donating, metabolic disorders, or medication. The visual appearance of lipaemic platelet concentrates often raises questions concerning safety and quality, hence they are often discarded.
Aims: As there is little data regarding the quality of platelets stored in lipaemic plasma, this was investigated.
Methods: Double apheresis platelet components were pooled, centrifuged and resuspended in 70% SSP+/30% plasma, either control (non‐lipaemic) and moderately lipaemic plasma (n = 5), or non‐lipaemic and severely lipaemic plasma (n = 6). Plasma was graded for extent of lipaemia based on opacity. Platelet concentrates were stored for 7 days at 20–24°C with agitation. Platelet metabolism, mitochondrial membrane polarization (TMRE), activation and function were measured. Data were analyzed using a two‐way repeated measures ANOVA.
Results: Storage of platelets in moderately and severely lipaemic plasma had no effect on platelet concentration, mean platelet volume, pH, glucose consumption or lactate production. Mitochondrial membrane depolarisation was significantly higher in platelets stored in moderately (p = 0.001) and severely lipaemic plasma (p = 0.037). Storage in lipaemic plasma had no effect on phosphatidylserine exposure, cell surface CD62P and soluble CD62P release. However, CD62P expression following TRAP‐6 was blunted in platelets stored in moderately and severely lipaemic plasma (p = 0.009 and p = 0.002) respectively. Storage in moderately and severely lipaemic plasma reduced surface GPIbα (p = 0.015 and p = 0.008), GPVI (p = 0.027 and p = 0.008) and CD61 (p = 0.007). Most viscoelastic parameters (thromboelastography) were not significantly different. Release of PF4 was increased (p = 0.0058) in platelets stored in severely lipaemic plasma (p = 0.007).
Conclusion(s): Storage of platelets in lipaemic plasma does not affect platelet metabolism, but differentially affects platelet receptor expression and their ability to respond to platelet agonists. These changes may be reduce their efficacy upon transfusion, warranting further investigation.
PB0884
Platelet hyperaggregability in Framingham Heart Study participants living with obesity
B. Nkambule 1; M. Chan2; A. Lachapelle2; F. Thibord3; J. Cunha4; R. Pashek5; M. Chen2; A. Johnson2
1 NHLBI, Boston, Massachusetts, United States; 2 NHLBI, Framingham, Massachusetts, United States; 3 Population Sciences Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, Boston, Massachusetts, United States; 4 NHLBI and Boston University's Framingham Heart Study, Framingham, Massachusetts, United States; 5 NHLBI, Allston, Massachusetts, United States
Background: Obesity is an independent risk factor for non‐communicable disease and the most prevalent comorbidity associated with poor prognosis in thrombotic disorders. Persistent platelet activation and chronic immune activation contribute to a hypercoagulable state in obesity.
Aims: To assess platelet function in Framingham Heart Study (FHS) participants living with obesity.
Methods: We included 3429 participants from the Framingham Heart Study (FHS) Third Generation cohort, New Offspring Spouse (NOS) and OMNI cohort. We excluded participants (n = 24) who were classified as underweight (BMI < 18.5) and (n = 2) who had missing data. A total of 3403 Eligible participants were categorized into four subgroups based on the WHO body mass index (BMI) classification. We assessed the levels of adenosine diphosphate (ADP)‐induced platelet degranulation (%CD62P expression) and integrin activation (%PAC‐1 binding) using flow cytometry. In addition, we assessed agonist‐induced aggregation (3.19 μM ADP, 0.061 mg/mL, collagen, 4.48 μM TRAP‐6 amide) using whole blood impedance aggregometry.
Results: The included FHS participants comprised of 28.8% lean individuals (BMI < 25; n = 979); 36.9% participants classified as overweight (BMI = 25.0–29.9; n = 1255); 29.4% participants living with Class 1 obesity (BMI > 30.0; n = 1001), and 4.9% Class 2 or 3 obesity (BMI≥35.0, n = 168). In participants with class 1 obesity, the levels of ADP‐induced %PAC‐1 binding were decreased (ß = ‐0.11, SE = 0.05, p = 3.23E‐02) when compared to lean participants. In addition, participants who were classified as overweight, had hyper‐aggregable platelets in response ADP (ß = 0.16, SE = 0.16, p = 1.74E‐04); collagen (ß = 0.11, SE = 0.04, p = 5.46E‐03); TRAP‐6 (ß = 0.17, SE = 0.04, p = 8.73E‐05) compared to lean individuals. Lastly, in participants with class 1 obesity, platelets were hyperreactive to ADP (ß = 0.17, SE = 0.047, p = 2.8E‐04) and TRAP‐6 (ß = 0.29, SE = 0.05, p = 1.33E‐09).
Conclusion(s): In individuals with obesity, platelets are hypo‐responsive to ADP‐induced integrin activation and hyper‐responsive to ADP, and TRAP‐6 induced aggregation.

PB0857
Increased Platelet Activation and Platelet‐Leukocyte Aggregate Formation in Primary Sclerosing Cholangitis
J. Gerwers 1; H. Englert1; N. Wolska2; F. Glaser2; J. Hartl2; I. Schregel2; M. Sebode2; S. Steinmann2; J. Weltzsch2; C. Weiler‐Normann2; C. Schramm3; T. Renné4; C. Deppermann5
1 University Medical Center Hamburg Eppendorf, Hamburg, Hamburg, Germany; 2 University Medical Centre Hamburg‐Eppendorf, Hamburg, Hamburg, Germany; 3 University Medical Centre Hamburg Eppendorf, Hamburg, Hamburg, Germany; 4 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany; 5 University Medical Centre Mainz, Mainz, Rheinland‐Pfalz, Germany
Background: Primary sclerosing cholangitis (PSC) is an inflammatory disease of the intra‐ and extrahepatic bile ducts resulting in cholestatic liver disease, liver failure and potentially cholangiocarcinoma. The pathophysiology of PSC is still largely unknown. Platelets and leukocytes contribute to inflammatory processes and regulate immune reactions in liver disease. A previous study showed an increased expression of glycoprotein Ibɑ (GPIbɑ) on the platelet surface in patients with PSC compared to a control cohort indicating a hypercoagulable state in PSC patients.
Aims: We aimed to investigate platelet functions in PSC to explore if platelets play a role in PSC pathogenesis.
Methods: Blood was collected from PSC patients and healthy controls. We used platelet aggregometry and flow chambers assays (vWF and collagen) to analyze thrombus formation under static conditions and flow. Flow cytometry was used to quantify the surface expression of major platelet receptors, platelet reactivity upon stimulation with ADP, CRP‐XL and TRAP‐6 and formation of platelet‐leukocyte‐aggregates (PLAs) in blood samples from PSC patients. We further used ELISAs to detect platelet surface receptor shedding.
Results: Platelets from PSC patients formed larger and denser clots compared to healthy controls, implicating a hyper‐activated state. GPIbɑ and P‐Selectin expression on the platelet surface in the resting state was lower compared to controls. We observed increased soluble glycoprotein VI (sGPVI) levels in plasma. Threshold for platelet activation – assessed by P‐Selectin exposure and aIIbb3‐integrin activation ‐ was higher. We also found an increased tendency of PLA formation both in resting and activated state in blood from PSC patients.
Conclusion(s): Higher platelet activation threshold and an increased tendency to form PLAs suggest that platelets have an exhausted phenotype in PSC and that they contribute to thromboinflammatory processes in PSC.
PB0862
Platelet activation via glycoprotein VI initiates thrombin generation: a potential role for platelet‐derived factor IX?
L. Li1; M. Roest2; J. Meijers3; B. de Laat4; R. Urbanus5; P. de Groot6; D. Huskens 7
1 Department of Platelet pathophysiology, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 2 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 3 Department of Molecular Hematology, Sanquin Research, Amsterdam, the Netherlands, Department of Experimental Vascular Medicine, Amsterdam UMC, University of Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 5 Center for Benign Hematology, Thrombosis and Hemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 6 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands, 7 Synapse Research Institute, Maastricht, Limburg, Netherlands
Background: In a platelet‐rich environment, coagulation can be activated by collagen via a FXIIa dependent pathway and via a platelet activation dependent pathway.
Aims: To delineate the platelet‐derived trigger that initiates coagulation after platelet activation by collagen.
Methods: Thrombin generation (TG) initiated with collagen, with Glycoprotein VI (GPVI) specific agonists (collagen‐related peptide, CRP‐XL, or convulxin) or an α2β1 specific agonist (GFOGER) was measured in PRP and PPP. Inhibitors of FXIIa (corn trypsin inhibitor, CTI) and active‐site inactivated FVIIa or anti‐FVII antibodies were used to establish the mechanism of initiation. To determine the platelet‐derived trigger of coagulation, the activation of isolated coagulation factors by activated washed platelets, and the inhibition of this activation by specific antibodies was studied. Also, TG in factor‐depleted plasmas reconstituted with activated washed platelets was investigated.
Results: Stimulation of PRP with collagen initiated TG, even in the presence of FXIIa‐ and FVIIa‐ inhibitors. GPVI agonists, but not α2β1 agonists, initiated TG in PRP but not in PPP (Figure 1). GPVI agonists could also initiate fibrin formation in PRP. Stimulation of platelets via GPVI, initiated TG in FVII‐ and FXII‐depleted plasma but failed to initiate TG in FVIII or FIX depleted plasma. Addition of CRP‐XL to washed platelets in buffer with FX and FVIII resulted in FX activation. FX activation was abolished in the presence of an inhibiting anti‐FIX antibody, suggesting CRP‐XL stimulated platelets release FIXa. Furthermore, addition of FVIII to FIX‐deficient plasma restored TG induced by GPVI activated platelets, and was inhibited by an anti‐FIX Ab.
Conclusion(s): Stimulation of platelets via GPVI initiated TG independent of the conventional FXII activation or tissue factor pathways. Our study provides new evidence that platelet activation by GPVI can initiate TG via platelet‐derived FIX(a) activity.

PB0885
Pregnancy‐specific glycoprotein 9 potentiates platelet function mediated by immunoreceptor tyrosine‐based activation motif pathways
I. Parra‐Izquierdo 1; A. Melrose1; J. Pang1; J. Lo1; M. Hinds1; A. Maloyan1; G. Dveskler2; O. McCarty3; J. Aslan1
1 Oregon Health & Science University, Portland, Oregon, United States; 2 Uniformed Services University of the Health Sciences, Bethesda, Maryland, United States; 3 Oregon Health and Science University, Portland, Oregon, United States
Background: Pregnancy‐specific glycoproteins (PSGs) are immunoglobulin superfamily members secreted by the syncytiotrophoblast layer of the placenta into the maternal circulation during pregnancy. PSGs exert immunoregulatory functions by triggering anti‐inflammatory responses in circulating leukocytes. However, the effect of PSGs on platelet functional responses remains largely unknown.
Aims: To determine whether PSGs regulate human platelet function ex vivo.
Methods: Recombinant PSGs were produced in HEK293 and CHO cells. Platelets were isolated from healthy volunteers and incubated with PSGs in the presence or absence of classical platelet agonists such as collagen‐related peptide (CRP‐XL). Platelet function was analyzed by Western Blot, static adhesion assays, flow cytometry, aggregation, and secretion assays.
Results: PSG1, PSG4, PSG7 and PSG9 did not induce platelet granule secretion or integrin activation ex vivo. However, PSG9 concentration‐dependently potentiated granule secretion and integrin activation induced by suboptimal concentrations of the glycoprotein (GP)VI agonist CRP‐XL. PSG9 also potentiated platelet aggregation following stimulation with CRP‐XL. Conversely, incubation with PSG9 completely abrogated platelet function induced by the Toll‐like receptor (TLR)2 ligands Pam2CSK4 and Pam3CSK4 and had no effects on thrombin‐induced responses. Studies using Western Blot demonstrated that PSG9 primed CRP‐XL‐mediated tyrosine phosphorylation as well as phosphorylation of substrates downstream of the protein kinase C (PKC) and protein kinase B (Akt) pathways. Mechanistic studies with select pharmacologic inhibitors showed that PSG9‐mediated platelet priming is dependent on Akt and on nuclear factor (NF)‐κB and Bruton's‐tyrosine kinase (BTK) pathways. Finally, stimulation of whole blood with PSG9 also potentiated granule secretion and integrin activation upon incubation with CRP‐XL, but not with thrombin receptor activator peptide 6 (TRAP6).
Conclusion(s): Our results suggest that PSG9 modulates platelet function in an agonist‐specific manner and therefore it may play an important role in regulating hemostasis during pregnancy.
PB0890
The heparan sulfate proteoglycan perlecan induces conflicting patterns of thrombus formation upon activation of GPVI: a novel role in thrombus stability?
I. Provenzale 1; J. Gibbins2; J. Heemskerk3; P. van der meijden4; C. Jones2
1 Dept of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands and Institute for Cardiovascular and Metabolic Research (ICMR), School of Biological Sciences, University of Reading, Reading, United Kingdom, Reading, England, United Kingdom; 2 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom; 3 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 4 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Thrombosis Expertise Center, Heart + Vascular Center, Maastricht University Medical Center, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands
Background: Proteoglycans are a heterogeneous family of proteins with covalently bound sulfated glycosaminoglycans. Perlecan, a matrix heparan sulfate proteoglycan secreted by endothelial cells and smooth muscle cells, has been proved to bind to the platelet and megakaryocyte‐specific ITIM receptor G6bB, regulating glycoprotein VI (GPVI) signaling. Contrastingly its C‐term fragment can enhance collagen induced platelet adhesion via the integrin α2β1
Aims: We investigated the potential dual role of perlecan to induce platelet adhesion and to regulate GPVI mediated thrombus formation.
Methods: Platelet degranulation and integrin αIIbβ3 activation was measured by flow cytometry following pre‐incubation with proteoglycans and dose‐dependent activation with suboptimal concentrations of collagen‐related peptide (CRP‐XL). In spreading assays, platelets were allowed to interact with collagen and perlecan for 45 minutes. Whole blood thrombus formation under flow was measured on CRP‐XL surfaces with(out) perlecan. After perfusion the formed thrombi were stained for P‐Selectin.
Results: Perlecan enhanced CRP‐XL‐induced platelet degranulation and integrin αIIbβ3 activation in a time and concentration‐dependent manner. Immobilized perlecan supported platelet adhesion and spreading. However, in combination with collagen, perlecan reduced the platelet spreading. In whole blood perfusion over a combination of CRP‐XL and von Willebrand factor (VWF), co‐immobilized perlecan reduced the buildup of thrombi, but simultaneously changed the thrombus structure. Morphological analyses indicated smaller, denser and more regularly shaped thrombi, with increased P‐selectin exposure when perlecan was present. Perfusion onto perlecan alone resulted in a monolayer of platelets without multilayered thrombus formation.
Conclusion(s): Perlecan enhanced adhesion and GPVI‐mediated platelet activation. However, when co‐immobilized, it suppressed GPVI‐mediated spreading and thrombus formation, while still enhancing local platelet activation. Whether this is due to a slower kinetic of platelet deposition or to an enhanced thrombus contractility remains unclear. These findings reflect the intrinsic structural features of perlecan, acting as a ligand for platelets and simultaneously competing with GPVI ligands.
VPB0902
Deletion of TGF‐β1 on platelets attenuates pulmonary arterial hypertension by enhancing aerobic glycolysis of pulmonary arterial smooth muscle cell
Z. Ming; Y. Zhu; X. Gong; M. Lu; X. Zeng; J. Gao; Y. Guo; L. Liu; R. Ma
Department of Pharmacology, Tongji Medical College Of Huazhong University Of Science And Technology, Wuhan, Hubei, China (People's Republic)
Background: Metabolic reprogramming is a hallmark of pulmonary arterial hypertension. Platelet activation have been implicated in Pulmonary Arterial Hypertension (PAH). Whereas the role of platelet in the pathogenesis of PAH remains unclear.
Aims: To explore the effect of platelet derived TGF‐β1 on pulmonary arterial smooth muscle cells proliferation, migration, and aerobic glycolysis.
Methods: We explored the platelet function of SU5416/hypoxia mice and MCT injected rats PAH model. Then we investigated pulmonary arterial smooth muscle cells aerobic glycolysis after treated with platelet supernatant, TGF β RI, PKM2 and other antagonists were applied to identify the underlying mechanism. In addition, platelet specific deletion TGFβ1 mice were exposed to chronic hypoxia and SU5416. Cardiopulmonary hemodynamics, vascular remodeling and aerobic glycolysis of PASMC was determined.
Results: We demonstrate that platelet released TGFβ1 enhances the aerobic glycolysis of pulmonary arterial smooth muscle cells after platelet activation via increasing PKM2 expression. Mechanistically, platelet derived TGFβ1 regulates PKM2 expression through mTOR/c‐Myc/PTBP1‐hnRNPA1 pathway. Platelet TGFβ1 deficiency mice are significantly protected from SU5416 plus chronic hypoxia induced PAH, including attenuated increases in right ventricular systolic pressure and less pulmonary vascular remodeling. Also, in Pf4 cre + Tgfb1 fl/fl mice PAMSCs showed lower glycolysis capacity and their PKM2 expression decreased.
Conclusion(s): Our data demonstrate that TGFβ1 released by platelet contributes to the pathogenesis of PAH and further highlights the role of platelet in PAH.
PB0859
The uptake and utilization of fatty acids by platelets
M. Hindle; K. Naseem
University of Leeds, Leeds, England, United Kingdom
Background: As platelets circulate in the blood, they require the mitochondrial oxidation of glucose and fatty acids (FA) to meet the energy demands of survival and vascular surveillance. There is a basic understanding of glucose utilization in platelets, where on activation they adopt a glycolytic phenotype. However, little is currently known about the uptake and metabolic processing of FA.
Aims: We sought to understand exogenous FA uptake, trafficking and utilization in blood platelets, and to study downstream effects on platelet metabolism and biology.
Methods: We used a combination of fluorescent flow cytometry (FFC), confocal laser scanning microscopy (CLSM) and bioenergetic measurements to profile the platelets in response to FA.
Results: Measuring platelet uptake of FA by FFC with the FA analogue BODIPY™ 558/568 C12 led to a concentration‐dependent increase in uptake, which was reduced in response to platelet activation. Using CLSM it was observed FA accumulated within the cells and were co‐localized with mitochondrial markers TOMM22 or MitoTracker™ Deep Red. Having established that FA were internalized and trafficked under quiescent conditions we used the C16 FA palmitate to profile metabolic function. Treatment of platelets with palmitate in the presence of tetramethylrhodamine ethyl ester, a marker of mitochondrial membrane potential (ΔmΨ), led to an increase in ΔmΨ measured by FFC. Bioenergetic studies using a Seahorse XFp metabolic analyzer indicated that palmitate increased both basal oxygen consumption rate (OCR) and maximal respiration. This was coupled with a decrease in extracellular acidification rate (ECAR), suggesting reduced glycolysis. Interestingly uptake of a fluorescent glucose analogue, 2‐NBDG, demonstrated that FA supply also drove an increase in glucose uptake.
Conclusion(s): The import of FA into mitochondria led to increased ΔmΨ, OCR and glucose uptake. However, ECAR was reduced, indicative of a rewiring of glucose metabolism under lipidaemic stress. Together these data suggest that FA accumulation in platelets leads to metabolic reprogramming.
PB0891
Evolution of the Platelet Plug and Leukocyte Recruitment After Initial Hemostasis In Vivo
J. Severa 1; C. Mansi2; T. Stalker3
1 Thomas Jefferson University, Cardeza Hemotology, Philadelphia, Pennsylvania, United States; 2 Thomas Jefferson University, Norwood, Pennsylvania, United States; 3 Thomas Jefferson University, Philadelphia, Pennsylvania, United States
Background: Platelets express the leukocyte recruiting adhesion molecule P‐selectin upon activation and subsequent alpha‐granule release. Leukocyte migration through the endothelium and interactions with platelets via platelet‐leukocyte aggregates are well described; however, the ability of leukocytes to infiltrate and migrate through a hemostatic plug via platelet interaction after vascular injury remains poorly understood.
Aims: This study aims to elucidate changes in platelet plug architecture and the ability of leukocytes to migrate through the plug for up to 60 minutes after hemostatic injury.
Methods: A mouse jugular puncture injury model was used in conjunction with quantitative multi‐photon microscopy to measure platelet accumulation, alpha granule secretion, fibrin deposition, and leukocyte recruitment over one hour post‐injury.
Results: Bleeding cessation was achieved within 47 ± 2.22 seconds following puncture injury of the mouse jugular vein. We found that platelet recruitment continued beyond initial hemostasis, as total platelet accumulation was significantly increased 30 minutes post‐injury as compared to 5 min post‐injury (p < 0.05). Fibrin deposition on the extravascular side of the injury site also continued to increase following initial hemostasis (p < 0.05, 5 vs. 30 min post‐injury). In association with these changes in plug architecture, we observed a time dependent increase in leukocyte recruitment to the platelet plug within the first hour post‐injury, particularly on the intraluminal side of the vessel (p < 0.0001, 5 vs. 60 min post‐injury).
Conclusion(s): Taken together, our results describe changes in hemostatic plug structure and function that occur after hemostasis is achieved. Continued fibrin deposition suggests ongoing localized thrombin activity over an extended period at the injury site. Concurrently, leukocytes are recruited and infiltrate the interior of the platelet plug. These findings suggest continued evolution of hemostatic plug structure and function after bleeding cessation occurs to facilitate plug stabilization and wound healing.
PB0851
Platelet secretion in wound healing: the essence of appropriate granule biogenesis
D. Coenen 1; S. Whiteheart2
1 University of Kentucky College of Medicine, Lexington, KY, USA, Lexington, Kentucky, United States; 2 Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, Lexington, KY, USA, Lexington, Kentucky, United States
Background: Non‐healing wounds cause a substantial global burden, both clinical and economic. Wound healing comprises multiple processes, e.g. hemostasis, inflammation, angiogenesis, and remodeling, in which different cell types are involved, including platelets, leukocytes, and endothelial cells. In various clinical and veterinary settings, platelet‐rich plasma or growth factors are applied to the wound to accelerate wound repair processes. However, detailed functional knowledge of these applications is lacking, partially due to insufficient mechanistic insights about platelet activity in different wound healing phases.
Aims: We aim to investigate the role of the platelet's secretory machinery in wound healing.
Methods: A 4‐mm biopsy punch was used to make two symmetrical full‐thickness circular excisions on the back of mice with defects in platelet granule biogenesis (Nbeal2−/− and Serglycin−/− mice) and wildtype mice (C57BL/6). Wounds were photographed and measured daily using calipers to determine percentage wound healing compared to baseline (day 0). Mice were sacrificed on day 3 and day 7, when blood was drawn and (partially healed) wounds were harvested with a 6‐mm biopsy punch for further analyses.
Results: Nbeal2−/− mice showed severely impaired wound healing with distinctive wound morphology when compared to wildtype mice. Interestingly, and in contrast with Nbeal2 deficient mice, serglycin deficiency did not lead to altered wound healing, despite both Nbeal2 and serglycin playing important roles in platelet granule biogenesis and cargo packaging.
Conclusion(s): Until now, the role of platelets and their releasate in wound healing has not been investigated sufficiently. We are the first to specifically study platelet secretion in wound healing and ultimately provide a broader understanding of platelets in the complete wound healing process. Preliminary results suggest appropriate platelet granule biogenesis with sufficient granule cargo levels to be essential in skin wound healing. Additional analyses will have to elucidate more specific mechanisms. This work is supported by the NIH/NHLBI (HL150818) and the VA.
PB0875
Mechanosensitive Ion Channels Piezo1 in Red Blood Cells are Involved in Platelet‐Driven Blood Clot Contraction
R. Litvinov 1; N. Evtugina2; R. Giniatullin3; J. Weisel4
1 University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania, United States; 2 Institute of Fundamental Medicine and Biology, Kazan (Volga region) Federal University, Kazan, Russian Federation, Kazan, Tatarstan, Russia; 3 A. I. Virtanen Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland, Kuopio, Pohjois‐Savo, Finland; 4 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States
Background: Piezo1 is a mechanosensitive cationic channel expressed in many cell types, including blood cells. Piezo1 opens in response to mechanical perturbation of the plasma membrane, resulting in a boost of intracellular [Ca2+]i. Since platelet‐driven contraction of blood clots is followed by compression of red blood cells (RBCs), it is conceivable that mechanical activation of Piezo1 in RBCs followed by Ca2+ influx can change cell deformability and/or platelet contractility, thus modulating clot contraction.
Aims: To reveal if Piezo1 channels in RBCs and platelets play a role in the contraction of blood clots.
Methods: Effects of a Piezo1 agonist, Yoda1 (5 μM), and antagonist, GsMTx‐4 (1 μM), on clot contraction in vitro were studied in fresh human blood anticoagulated with a corn trypsin inhibitor to keep the physiological [Ca2+]. Clot formation and contraction were induced by thrombin (1 U/mL) either in whole blood or in platelet‐rich plasma, and the clot shrinkage was followed using an optical tracking system.
Results: Adding the Piezo1 agonist Yoda1 to whole blood before clotting increased the rate and extent of clot contraction significantly compared to control (p = 0.025). In platelet‐rich plasma Yoda1 caused the opposite effect, with a moderate decrease in the rate and extent of clot contraction (p = 0.06), perhaps by competing with the natural Piezo1 activation in distorted platelets. Adding the Piezo1 antagonist, GsMTx‐4, caused a marked decrease in the rate and extent of clot contraction relative to control both in whole blood and in platelet‐rich plasma (p = 0.0006 and p = 0.01, respectively).
Conclusion(s): Activation of Piezo1 channels in RBCs enhances contraction of blood clots, likely mimicking the mechanical activation of Piezo1 in compressed RBCs during platelet‐induced clot shrinkage. The subsequent Ca2+ influx in RBCs could affect their rigidity and promote platelet contractility via biomechanical feedback, ATP/ADP release or cause phosphatidylserine exposure followed by enhanced thrombin generation.
PB0866
Unidentified pre‐analytical factors influence measures of platelet reactivity in the Framingham Heart Study
M. Chan1; M. Chen1; F. Thibord2; B. Nkambule3; A. Lachapelle1; C. Wallace de Melendez1; Z. Schneider1; J. Cunha4; R. Pashek5; J. Grech1; P. Armstrong6; T. Warner6; A. Johnson 1
1 NHLBI, Framingham, Massachusetts, United States; 2 Population Sciences Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, Boston, Massachusetts, United States; 3 NHLBI, Boston, Massachusetts, United States; 4 NHLBI and Boston University's Framingham Heart Study, Framingham, Massachusetts, United States; 5 NHLBI, Allston, Massachusetts, United States; 6 Queen Mary, University of London, London, England, United Kingdom
Background: The assessment of platelet reactivity is key in diagnosing bleeding disorders and the evaluation of anti‐platelet drug efficacy. However, there is a prevailing “one‐size fits all” approach in the interpretation of measures of platelet reactivity, with arbitrary cut‐offs based on healthy volunteer responses.
Aims: Using the large platelet reactivity dataset obtained in the Framingham Heart Study (FHS), we assessed whether any pre‐analytical or technical factors contribute to these platelet reactivity measures.
Methods: Blood and platelet‐rich plasma were obtained from the FHS (N = 3429) and details such as time and date of draw, anti‐platelet medication use, any deviations from a standard blood draw were noted. Platelet reactivity was measured in response to a range of agonists in five platelet assays: light transmission aggregometry (LTA), Optimul aggregometry, Multiplate aggregometry, Total Thrombus‐formation Analysis System (T‐TAS) and flow cytometry. Lipemic and hemolyzed samples were excluded from analysis, and linear mixed‐effect models were used to determine factors which modulated platelet reactivity traits.
Results: As expected, aspirin strongly attenuated platelet responses, most notably to arachidonic acid (final aggregation LTA, β = −1.724, p < 9.48e‐657). P2Y12 antagonists attenuated ADP responses in all assays. Furthermore, sex and age robustly modulated agonist responses (Figure). Fasting status did not alter platelet reactivity measures. Interestingly, draw times later in the day and longer time from draw to test attenuated platelet responses. Finally, agonist batch, phlebotomist, and assay technician (more so for “hands on” assays such as flow cytometry) had a moderate‐large effect on platelet reactivity.
Conclusion(s): This study highlights the importance of considering pre‐analytical variables and technical factors in the interpretation of platelet reactivity measures. Caution must be exercised in the use of standard ranges, unless stratified by age and sex. In large studies agonist batch and technician variation should be reduced and steps should be taken to standardize time of blood draw.
PB0850
Lowe syndrome in platelets: OCRL controls the reorganization of actin and tubulin filaments during platelet activation
A. Bura 1; M. Bender2; A. Jurak Begonja1
1 Department of Biotechnology, University of Rijeka, Croatia, Rijeka, Primorsko‐Goranska, Croatia; 2 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: Lowe syndrome (LS) is a rare X‐linked disorder characterized by a triade of symptoms affecting the brain, the eyes, and the kidneys. It is caused by mutations in the oculocerebrorenal syndrome of Lowe protein (OCRL) which is a 5‐phosphatase that dephosphorylates phosphatidylinositol‐4,5‐bisphosphate [PI(4,5)P2] to phosphatidylinositol‐4‐monophosphate (PI4P). PI(4,5)P2 regulates actin nucleation and reorganization which is a crucial step during platelet (PLT) activation. Moreover, it has been shown that LS patients have occasional bleeding problems that led us to investigate the role of OCRL in PLTs.
Aims: Our aim is to determine the molecular mechanism by which OCRL regulates PLT cytoskeletal rearrangements.
Methods: We pharmacologically inhibited OCRL and used confocal and electron microscopy to visualize PLTs, Western blot for the analysis of PLT signaling pathways, and flow cytometry to investigate PLT activation markers and F‐actin levels.
Results: We show that OCRL inhibition increases PI(4,5)P2 levels in PLTs and impairs PLT spreading on fibrinogen, resulting in the extensive formation of actin nodules which colocalize with proteins implicated in actin dynamics (ARP2/3, Vinculin, SNX9). These nodules also colocalize with pTyr showing they are sites of active signaling. Furthermore, OCRL inhibition decreases the levels of MLC phosphorylation upon stimulation with thrombin and TRAP‐6, although without change in net F‐actin. Interestingly, OCRL inhibition also impairs the disassembly of the microtubular coil during PLT spreading and it increases the levels of acetylated tubulin. Altered cytoskeletal changes did not change P‐selectin surface expression or αIIbβIII activation upon platelet activation. Finally, we show that the kinetics of GPIbα surface downregulation is impaired upon OCRL inhibition.
Conclusion(s): OCRL controls actin and tubulin reorganization as well as GPIbα downregulation in PLTs but has no effect on PLT degranulation or integrin activation.
PB0881
Calcium‐and integrin‐binding protein 2 negatively regulates in vivo thrombosis and platelet functions by attenuating S1P production
M. Naik; K. Golla; N. Shaik; P. Patel; U. Naik.
Thomas Jefferson University, Philadelphia, Pennsylvania, United States
Background: CIB family consists of four members, CIB1, CIB2, CIB3, and CIB4. CIB1 positively regulates platelet function. The expression and function of other members in platelets is not known.
Aims: To evaluate the function of CIB2 in platelets.
Methods: We generated Cib2flox/Pf4cre + mice. Cib2flox/Pf4cre‐ mice were used as controls. In‐vivo tail bleeding, microfluidic assays, and tMCAO model of stroke as well as aggregation, Ca2+ levels, western blotting, and RT‐PCR were performed.
Results: We found that CIB2 is expressed in platelets. MK/platelet specific loss of Cib2 enhances hemostatic and thrombotic function of platelets which is opposite to Cib1 ablation thus ruling out the proposed compensatory role for Cib2. The absence of Cib2 results in increase in platelet aggregation (p < 0.05) and intracellular calcium rise when stimulated with low concentrations of agonists which was overcome by increased agonist concentration. Additionally, the in‐vitro thrombus formation was significantly (p < 0.05) enhanced in Cib2‐null platelets. The observed gain of function is due to enhanced platelet signaling and not due to increased expression of surface receptors. Furthermore, deletion of Cib2 results in enhanced cerebral infract size, which is opposite to that observed in Cib1 KO mice. CIB1 interacts and translocate sphingosine kinase 1 (SK1), a key enzyme involved in S1P synthesis. CIB2 on the other hand inhibits SK1 translocation since it lacks the calcium‐myristoyl switch. It is therefore possible that in the absence of Cib2 increased S1P is generated causing enhanced platelet function. Inhibition of S1PR2, a major S1P receptor using JTE‐013, we found that the agonist‐induced hyper‐aggregation and increase in calcium level in Cib2flox/Pf4cre + platelets were reduced to control levels. Furthermore, treatment of Cib2flox/Pf4cre + mice with JT3‐013 reduced the infract size to control level.
Conclusion(s): These in‐vivo and in‐vitro results suggest that Cib2, an endogenous suppressor of SK1, negatively regulates thrombosis and stroke by attenuating S1P production.
PB0894
Population‐based reference ranges of platelet function tests and their discriminative ability for cardiovascular disease
S. von Ungern‐Sternberg 1; M. Panova‐Noeva2; V. ten Cate3; B. Dahlen4; G. Buch5; S. Rapp6; J. Prochaska3; K. Strauch7; M. Beutel8; A. Schuster9; K. Lackner10; T. Münzel11; P. Wild3; K. Jurk12
1 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 2 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, mainz, Rheinland‐Pfalz, Germany; 3 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 52 Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; 3 German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany; 4 Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), University Medical Center of the Johannes Gutenberg University Mainz, Obere Zahlbacher Str. 69, 55,131, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 62 Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 74 Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), University Medical Center of the Johannes Gutenberg University Mainz, Obere Zahlbacher Str. 69, 55,131, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 85 Department of Psychosomatic Medicine and Psychotherapy, University Medical Center of the University of Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 96 Department of Ophthalmology, University Medical Center Mainz, Langenbeckstr. 1, 55,131 Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 10 Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, mainz, Rheinland‐Pfalz, Germany, 11 Center for Cardiology ‐ Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 12 University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: Hyperactive platelets contribute to different cardiovascular diseases. However, platelet function testing is preferentially applied for the diagnosis of platelet‐related bleeding disorders.
Aims: This study aimed to 1) generate age‐ and sex‐specific reference values for platelet (re)activity markers from population‐based “cardiovascular healthy” individuals and 2) evaluate the discriminative potential of values outside reference ranges for cardiovascular disease.
Methods: A “cardiovascular healthy” subgroup (n = 341; 51% female) was defined in the Gutenberg Health Study after exclusion of cardiovascular‐related diseases and medications. This study was approved by the local Ethics Committee including signed participant informed consent. The 5th/95th percentiles defined platelet function reference values. Age−/sex‐dependency and cardiovascular risk determinants of platelet function parameters (flow cytometry, light transmission aggregometry, PFA‐200, calibrated automated thrombography) were assessed by multivariable linear regression. Age−/sex‐adjusted logistic regression in the total sample (n = 789) estimated the probability of having coronary artery disease (CAD), myocardial infarction (MI), stroke, atrial fibrillation (AF), chronic heart failure (CHF) and venous thromboembolism (VTE) for values outside the reference range.
Results: Men (median:2.60%,IQR:1.20/5.31) showed higher percentage of CD63+ platelets than women (1.90%,0.70/3.68), which both decreased with age. Spontaneous and collagen‐induced platelet aggregability increased (β [log‐transformed dependent variable, DV] = 0.096, p = 0.002; β = 1.59, p = 0.036) and collagen/ADP‐induced closure time decreased (β [log‐transformed DV] = −0.03, p = 0.038) with age. Tissue factor‐induced endogenous thrombin potential (ETP) decreased (β = −33.8, p = 0.018) and lag time increased (β = 0.327, p = 0.0078) with age. Arterial hypertension, obesity and active smoking were age, sex and other risk factor‐independent determinants of platelet aggregability, thrombin generation and ex vivo activation. Values outside the reference range for collagen‐induced platelet aggregability and thrombin generation were related to increased prevalence of CAD, MI, AF, CHF and VTE. History of stroke and VTE were more prevalent with values exceeding the reference range for CD63+ platelets (OR:5.77;95%CI:1.42;23.41; p = 0.014) and spontaneous aggregation (OR:2.75;95%CI:1.14;6.66; p = 0.025), respectively.
Conclusion(s): Platelet function test values outside of population‐based reference ranges may be useful markers of cardiovascular disease.
PB0858
Effect of Agonist Stimulation and Antiplatelet Drugs on Platelet Contractility
D. Hiebner 1; M. Kenny1; S. Lickert2; I. Schoen1
1 Royal College of Surgeons in Ireland, Dublin, Dublin, Ireland; 2 ETH Zürich, Zurich, Zurich, Switzerland
Background: Within the developing thrombus, the dense packing of platelets and fibrin is believed to hinder the diffusion of soluble agonists, ultimately directing each platelets' activation response and giving rise to the core‐shell thrombus architecture. Defects in platelet contractility result in decreased overall thrombus stability and increased bleeding. How soluble agonists and anti‐platelet drugs might direct the development of platelet traction forces is incompletely understood.
Aims: To evaluate the potency of platelet agonists to induce contractility in adherent platelets and the modulation of platelet contractility by antiplatelet agents.
Methods: Blood was obtained from healthy consenting volunteers as approved by the RCSI Research Ethics Committee (REC1405). Washed platelets were seeded onto fibrinogen‐coated micropost arrays or glass coverslips, in the presence of various agonists or antiplatelet agents. Traction force microscopy and fluorescence image analysis were used to assess the mechanical (total force per cell, force per area) and morphological (spreading area, f‐actin organization) characteristics of single platelets.
Results: Agonist treatment resulted in contrasting fractions of contractile versus non‐contractile cells, as well as in a variable coupling between platelet spreading and contractility. PAR agonists thrombin and PAR‐4 activating peptide yielded the highest total forces per cell, followed by TRAP‐6, CRP‐XL and then ADP. Thrombin‐activated platelets showed the least spreading and the highest force per area. Traction forces and spreading area correlated closely for CRP‐XL and ADP but were largely decoupled for all PAR agonists. Morphological analysis of agonist‐treated platelets revealed modified f‐actin arrangements which potentially could aid the identification of specific contractile signatures. Antiplatelet agents blocking GPVI, thromboxane A2 and P2Yx signaling pathways influenced platelet contractility to varying degrees.
Conclusion(s): Platelet contractility is controlled by integrating different extracellular signals, including the identity of soluble agonists and antiplatelet agents, as present at different locations in the developing thrombus.
PB0880
Freeze Dried Platelet Derivatives (Thrombosomes®) Retain Hemostatic Properties During Heparin Complexation with Protamine
K. Moskowitz; S. Xu.
Cellphire Therapeutics, Inc, Rockville, Maryland, United States
Background: A human lyophilized platelet derived hemostatic, Thrombosomes®, are currently in phase 2 trials in thrombocytopenic bleeding patients. A prerequisite to the assessment of Thrombosomes® as a potential hemostatic agent to quell bleeding associated with CABG surgery is compatibility with heparin:protamine. Herein we investigated heparin:protamine treatment's impact on Thrombosomes® hemostatic potential, in vitro.
Aims: Investigate Thrombosomes®’ ability to generate thrombin in the presence of pharmacologic concentrations of heparin:protamine.
Methods: A prophylactic level of heparin, 0.1 U/mL plasma, was combined with 5 and 50 K/μL Thrombosomes® and assayed using a Thrombin Generation Assay (TGA). A therapeutic level of heparin, 0.8 U/mL plasma, was tested without reversal and with 0.5‐, 1‐, and 1.5‐times the recommended reversal dose of protamine (4, 8, or 12 μg/mL, respectively) along with 10, 25, or 50 K/μL Thrombosomes® using a TGA and activated clotting time (ACT) measurements.
Results: Thrombosomes® at 5 and 50 K/μL returned peak thrombin production to normal in 0.1 U/mL heparin (Figure 1A). Thrombosomes® at 10 to 50 K/μL was unable to return peak thrombin generation when 0.8 U/mL heparin and 4 ug/mL protamine (1/2 P). (Figure 1B, C). However, reversal of heparin with recommended (P) and excess (3/2 P) doses of protamine resulted in thrombin generation potential of Thrombosomes® similar to when no heparin was added (Figure 1C). There is a trend towards lower ACT with higher concentrations of Thrombosomes® in the sample groups containing excess heparin (Figure 2).
Conclusion(s): Thrombosomes® restored hemostasis at prophylactic heparin dose. Thrombosomes® retain thrombin generation potential at therapeutic dose of heparin:protamine, and accelerated clotting in a dose dependent manner. These results support the assessment of Thrombosomes® for their potential use in CABG surgery and other clinical applications wherein heparin has been given and reversed by protamine. Future work to confirm Thrombosomes® usage in animal models where heparin‐protamine complexation is planned.
PB0896
Under increased platelet turnover large and small platelets are produced simultaneously
M. Wolff 1; S. Handtke2; S. Schwarz2; A. Greinacher3; T. Thiele2
1 University Medicine Greifswald, Greifswald, Mecklenburg‐Vorpommern, Germany; 2 Department of Transfusion Medicine, University Medicine Greifswald, Greifswald, Mecklenburg‐Vorpommern, Germany; 3 Universitätsmedizin Greifswald, Greifswald, Mecklenburg‐Vorpommern, Germany
Background: Human platelets vary in size, function, and age. These platelet features change when platelet turnover increases (e.g. by major surgery, sepsis). We established a platelet apheresis model to deplete platelets from healthy volunteers with subsequent production of young platelets and minimal interfering factors or comorbidities.
Aims: We aim to further characterize large and small human platelets during normal and increased platelet generation regarding age and function.
Methods: Blood was obtained from healthy plateletpheresis donors before and on days 3, 7, 10 after apheresis. Platelets were isolated and separated into large and small platelet fractions by differential centrifugation. We analyzed reticulated platelets labeled with Cy5‐conjugated oligo‐dT and oligo‐dA, HLA‐I expression by binding of anti‐HLA‐A, B, C‐APC antibodies and desialylation of platelet glycoproteins by binding of Fluorescein‐labeled lectins (RCA, ECL), using flow cytometry.
Results: The overall proportion of RNA‐rich young platelets increased after plateletpheresis (p = 0.0059, Figure 1A) and was more pronounced in small platelets on day 7 and 10 compared to large ones (Figure 2A). Likewise, HLA‐I expression, as a marker for young platelets, was significantly increased after plateletpheresis in unseparated (day 3: p = 0.0062, Figure1B), large (day 3: p = 0.0039) and small platelets (day 7: p = 0.0156, Figure 2B). Interestingly, platelet desialylation, which is increased during aging of platelets, was not significantly changed after plateletpheresis. Large platelets were more desialylated compared to small platelets at all time points (Figure 2C).
Conclusion(s): Platelet loss and consecutive platelet production induced by apheresis, increased the proportion of reticulated platelets with higher HLA‐I expression in small and large platelets. This indicates simultaneous synthesis of young large and small platelets. The assumption that large platelets always represent younger platelets has to be revised when platelet turnover increases.


PB0846
ZO‐2 Enriched Tight Junction‐like Structures at Sites of Platelet–Platelet Contact
M. Nagy1; M. Bender2; N. Poulter3; A. Sickmann4; X. Stéphenne5; S. Brouns6; R. Koenen7; H. ten Cate8; J. Heemskerk9; C. Baaten 10
1 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 2 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 3 Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, United Kingdom; Centre of Membrane Proteins and Receptors (COMPARE), Universities of Birmingham and Nottingham, Midlands, United Kingdom, Birmingham, England, United Kingdom; 4 Leibniz‐Institut für Analytische Wissenschaften – ISAS – e.V. Dortmund, Germany, Dortmund, Nordrhein‐Westfalen, Germany; 5 Laboratoire d'Hépatologie Pédiatrique et Thérapie Cellulaire, Unité PEDI, Institut de Recherche Expérimentale et Clinique, Université catholique de Louvain (UCLouvain), Brussels, Belgium, Woluwe‐Saint‐Lambert, Brussels Hoofdstedelijk Gewest, Belgium; 6 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands, 7 Maastricht University, Maastricht, Limburg, Netherlands; 8 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 9 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands, 10 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Institute for Molecular Cardiovascular Research, University Hospital Aachen, RWTH Aachen University, Aachen, Germany, Maastricht, Limburg, Netherlands
Background: Platelets express junction proteins that are known to regulate cell–cell adhesion, barrier formation and communication in tissue forming cells. Previous research has shown that the gap junction proteins expressed by platelets regulate platelet activation in an αIIbβ3‐dependent manner. Also, platelet activation processes are negatively regulated by endothelial cell‐selective adhesion molecule (ESAM) and junctional adhesion molecule A (JAM‐A), proteins that are part of the tight junctions (TJ) of endothelial cells. However, the role of zonula occludens 2 (ZO‐2) ‐an intracellular component of the endothelial TJs‐ in the formation of inter‐platelet contacts has not yet been described.
Aims: To unravel the molecular composition, in particular ZO‐2, of tight platelet–platelet contacts.
Methods: Using phosphoproteomics databases, ZO‐2 phosphorylation in activated platelets was investigated. Isolated platelets were allowed to fully spread on a fibrinogen surface. Fixed samples were stained to assess F‐actin polymerization in relation to ZO‐2 and ESAM distribution using confocal and/or super‐resolution fluorescence microscopy. Platinum replica electron microscopy was applied for ultrastructural visualization of the cytoskeleton at sites of platelet–platelet interaction.
Results: ZO‐2 undergoes extensive phosphorylation changes upon agonist‐induced platelet activation as well as cAMP‐mediated platelet inhibition. Confocal and super‐resolution fluorescence microscopy indicated a marked redistribution and clustering of ZO‐2 molecules at sites of platelet–platelet contacts, in which ZO‐2 colocalized with ESAM. Furthermore, platinum replica electron microscopy revealed that inter‐platelet contacts resulted in the merging of the circumferential actin bundles between interacting platelets. These phenomena were antagonized by cAMP elevation.
Conclusion(s): Jointly, these data point to the assembly of tight junction‐like structures, where intracellular components of tight junctions accumulate at sites of close platelet–platelet contact. This work was supported by the Dutch Heart Foundation (2020 T020 to C.B.)
VPB0899
Mechanisms of HLA‐KO iPSC‐platelets immune escape from NK cells
C. Flahou 1; M. Iwasaki2; S. Nakamura2; N. Sugimoto2; K. Eto2
1 Center for iPS cell Research and Applications, Kyoto University, Kyoto, Kyoto, Japan; 2 Kyoto University, Kyoto, Kyoto, Japan
Background: In some cases, patients can become refractory to platelet transfusion due to qllo‐immune responses. Therefore, to make iPS‐derived platelets suitable for the widest majority, we chose to edit out the expression of HLA class‐I on the surface of induced pluripotent stem cell‐derived platelets (iPSC‐platelets). While we observed that their HLA‐KO precursor cells elicited an NK cell response, the HLA‐KO platelets did not.
Aims: We propose to identify factors which influence the difference in sensitivity to NK cell cytotoxicity between HLA‐KO iPSC‐platelets and their precursors, HLA‐KO iPSC‐derived immortalized megakaryocytes progenirot cell line (imMKCLs).
Methods: We used both discovery and hypothesis‐driven methodologies. In the discovery approach, we used improved mass spectrometry analysis to compare the proteome of both cell types, then a LIMMA‐based statistical analysis extracts differentially represented pathways. In vitro assays subsequently validated relevant candidates. We also used live‐imaging to characterize contact and killing events, as well as visualize the formation of immunological synapses between NK and target cells.
Results: Proteins involved in repression of immune processes were up‐regulated in HLA‐KO platelets compared to HLA‐KO imMKCLs. This includes integrin pathways and known NK cells inhibitors. Moreover, platelet precursor cells formed immunological synapses with NK cells, ending in either tolerance or killing.
Conclusion(s): For iPSC‐derived therapies to move towards clinical trials, considerations about their HLA‐compatibility, as well as their deep functional characterization are fundamental. HLA‐KO iPSC‐platelets display a stronger immuno‐regulatory proteome than their precursor cells and this work has identified crucial pathways governing NK cell toxicity and tolerance to both platelets and their precursor cells. These findings represent important insights for future clinical trials of universal, HLA knockout platelets.

VPB0903
Matrix metalloproteinase‐13: a novel player of platelet activation and in vivo thrombus formation
S. Momi 1; M. Sebastiano2; E. Falcinelli3; P. Gresele3
1 University of Perugia, Italy, Perugia, Umbria, Italy; 2 University of Perugia, Perugia, Umbria, Italy; 3 University of Perugia, Department of Medicine and Surgery, Section of Internal and Cardiovascular Medicine, Perugia, Umbria, Italy
Background: Some MMPs are regulators of platelet function. Platelets express and release MMP‐2 that enhances platelet aggregation by cleaving PAR1 at a non conventional site. Atherosclerotic plaques are rich in MMP‐13 that is also known to cleave PAR1 of cardiac cells. No studies however have explored whether MMP‐13 can activate platelets.
Aims: Our aim was to explore the effect of MMP‐13 on platelet PAR1, in vitro platelet activation and signal transduction, and to assess whether it modulates in vivo platelet‐mediated thrombosis.
Methods: The cleavage site of PAR1 by rhMMP‐13 was characterized using CHO cells transfected with pcDEF3/PAR1 T7‐ tagged human PAR‐1; platelet function was assessed by flow‐cytometry; in vivo hemostasis in mice by the tail tip‐transection bleeding time; in vivo arterial thrombosis by a photochemical‐injury femoral artery thrombosis model.
Results: MMP‐13 cleave PAR1 on CHO cells, a reaction blocked by the antibody SPAN12 directed against the PAR1 N‐terminal residues 35–46. Active MMP‐13 potentiated ADP‐induced human platelet P‐selectin expression and platelet/leukocyte aggregate formation and triggered platelet G protein activation, effects abolished by WAY170523c (MMP‐13 inhibitor) and by the blockade of platelet PAR1 by SPAN12. The ex vivo formation of platelet/monocyte aggregates, and, to a lesser extent platelet/neutrophil aggregates were reduced in MMP‐13−/−mice as compared with WT mice suggesting that endogenous MMP‐13 plays a role in platelet activation. Femoral artery thrombosis was significantly delayed (27 ± 1.4 vs 11.5 ± 1.7 min, respectively, p < 0.01) and the tail tip transection bleeding time was mildly, but significantly prolonged (390 ± 76 vs 120 ± 11 sec, p < 0.0001) in MMP‐13−/− mice as compared to WT mice.
Conclusion(s): Our data data show that active MMP‐13 activates platelets by cleaving PAR1 at a non canonical site. MMP‐13 plays a role in in vivo platelet activation and thrombus formation at a vessel wall‐injury site. MMP‐13 represents a novel modulator of platelet function and a potential target of antithrombotic therapy.
Platelet Proteomics and Genomics
PB0369
Exploring Platelet Proteins Altered in Severe COVID‐19 Using Gene Ontology (GO) Pathways
L. Goudswaard 1; K. Burley2; C. Williams2; K. Heesom2; S. Mundell2; A. Poole2; I. Hers2
1 University Of Bristol, Bristol, England, United Kingdom; 2 University of Bristol, Bristol, England, United Kingdom
Background: SARS‐CoV‐2 infection causing COVID‐19 is associated with a high incidence of thrombotic complications, a phenomenon in which platelets play an important contributory role. COVID‐19 is associated with alterations in platelet function, including increased platelet–neutrophil aggregates and impaired integrin αIIbβ3 activation. The mechanisms underlying such effects remain unclear.
Aims: In this study, we aimed to identify changes in the platelet proteome in patients with COVID‐19 and explore implicated biological pathways.
Methods: Patients hospitalized with COVID‐19 (N = 7) and healthy controls (N = 6) were recruited between October 2020 and February 2021. Proteomics analysis was performed on washed platelets using Tandem Mass Tag Mass Spectrometry (TMT‐MS). Alterations in platelet proteins were analyzed using Gene Ontology (GO) pathways.
Results: A total of 5773 proteins were quantified in COVID‐19 patients and controls. There was differential expression of 858 (15%) proteins between patients with severe COVID‐19 infection and controls (false discovery rate p < 0.05). Pathway analysis revealed expression changes in gene products associated with regulation of platelet activation (GO:0010543), with reduced expression of platelet kinases (PRKCA, PRKCD, PRKCQ, LYN, SYK and JAK2), glycoproteins (GP5 and GP9) and PLEK, known positive regulators of integrin activation. There was increased expression of alpha‐ and dense‐granule lumen gene products (GO:0031093 and GO:0031089), including protease inhibitors SERPINA3, SERPING1 and SERPINF2
Conclusion(s): Our results demonstrate diverse changes in signaling and secretion pathways important to platelet activity in patients hospitalized with COVID‐19. Increased abundance of platelet granule proteins is in keeping with published plasma proteome descriptions, suggesting platelet uptake of plasma proteins. Platelet granule proteins involved in the regulation of hemostasis may contribute to the increased risk of thrombosis seen in this patient cohort and point towards potential therapeutic targets for COVID‐19 related thrombotic complications.
VPB0374
The role of miRNAs in regulation of platelet activity ‐ a bioinformatic analysis
Z. Wicik 1; C. Eyileten1; D. Jakubik1; J. Jarosz‐Popek1; M. Wolska2; A. Nowak1; D. Keshwani1; D. Von Lewinski3; H. Sourij3; J. Siller‐Matula4; M. Postula1
1 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 2 Medical University of Warsaw, warsaw, Mazowieckie, Poland; 3 Medical University of Graz, Graz, Steiermark, Austria, 4 medical university of vienna, vienna, Wien, Austria
Background: Circulating miRNAs can be determined from plasma, serum, or whole blood, and they can be used as diagnostic and prognostic biomarkers as well as therapeutic targets including cardiovascular diseases (CVDs).
Aims: Herein, we present original results from bioinformatic analyses, which identified top 22 platelet‐related miRNAs including hsa‐miR‐320a, hsa‐miR‐16‐5p, hsa‐miR‐106a‐5p, hsa‐miR‐320b, hsa‐miR‐15a‐5p, hsa‐miR‐15b‐5p, hsa‐miR‐195‐5p, hsa‐miR‐92a‐3p as widely involved in platelet reactivity and associated diseases, including CVDs, Alzheimer's and cerebrovascular diseases, cancer, hypertension.
Methods: Bioinformatics analysis miRNA targets prediction, data filtering, and visualization as interaction networks, Identification of the top miRNAs regulating genes associated with platelet‐related processes and expressed in relevant tissues, Identification of the most promising genes targeted by platelet‐related miRNAs, Enrichment analysis by in silico analysis.
Results: Analysis focused on the identification of the highly regulatory targets shared between those miRNAs identified 43 of them. Best ranked genes associated with overall platelet activity and most susceptible for noncoding regulation were PTEN, PIK3R1, CREB1, APP and MAPK1. Top targets also strongly associated with CVD were VEGFA, IGF1, ESR1, BDNF and PPARG. Top targets associated also with other platelet‐related diseases including cancer identified in our study were TP53, KRAS (highest number of hits overall) and CCND1. The most affected pathways by top miRNAs and top targets included diseases associated with GFRs, platelet activation, signaling and aggregation, Signaling by VEGF, MAPK family signaling cascades and signaling by Interleukins. Terms specific only for miRNAs included coronary artery disease, platelet degranulation and neutrophil degranulation, while for targeted genes it was ESR1 mediated signaling, extra‐nuclear estrogen signaling and endometriosis.
Conclusion(s): Our results show the novel features of platelet physiology and may provide a basis for further clinical studies focused on platelet reactivity. They also show in which aspects miRNAs can be promising biomarkers of platelet related pathological processes.


PB0368
Proteomics demonstrate differences in protein lifespan regulation in platelets from adult and pediatric donors
A. Garzon Dasgupta 1; A. Ignatova2; V. Zgoda3; A. Martyanov4; A. Sveshnikova2
1 Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology Ministry of Healthcare of Russian Federation, Moscow, Moskva, Russia; 2 Dmitry Rogachev National Medical Research Center Of Pediatric Hematology, Oncology and Immunology, Moscow, Moskva, Russia; 3 Orekhovich Institute of Biomedical Chemistry, Moscow, Moskva, Russia; 4 Center for Theoretical Problems of Physicochemical Pharmacology, Moscow, Moskva, Russia
Background: Differences between platelet functioning in adults and children have been reported previously. Such differences include hyper‐responsiveness to stimulation for children platelets or different pattern for granule secretion. However, the possible origin of these differences was not determined.
Aims: Here we aim to determine differences in functionality in platelets from adult and pediatric donors by means of proteomics and continuous flow cytometry.
Methods: Platelet proteomics and analysis of functional responses were performed for two cohorts of healthy donors: 4 adults (23.5 ± 2.6 years) and 4 children (13.0 ± 3.6 years). Conventional platelet responses to stimulation with ADP, PAR1AP or collagen related peptide (CRP) were measured by continues and end‐point flow cytometry. For proteomics analysis washed platelets were used. Platelet protein lysates were analyzed using LC‐Orbitrap MS. The analysis of raw data was performed by MaxQuant software. The resulting relative abundances analysis was performed in Python 3.7. In addition, a UniProt‐based and Gene Ontology (GO) – based study was conducted.
Results: Differences in functional responses between adult and pediatric platelets were shown both for resting state and after stimulation. We performed label‐free quantitative proteomics to detect protein abundances. We selected age‐specific proteins that have significant >2‐fold difference in copy number between adult and pediatric groups. It appears that selected proteins are more abundant in children donors, than adult. GO‐based analysis showed high levels of ribosome‐related proteins (P08865, P06730, P22626) along with proteosome subunits (P60900, P49720, P25788, P25789) and proteins related to the intracellular vesicle transport (Q9NRW1, Q9H0U4, O75396, etc.).
Conclusion(s): Preliminary results suggest, that the vesicle trafficking and proteosome proteins differ the most between the groups. This may indicate specific regulation of protein content by platelets due to total high initial concentration of proteins. All parts of this study were supported by the Russian Science Foundation grant 21–74‐20,087.
PB0370
Characterization of the human platelet glycome upon storage using tandem mass spectrometry
M. Lee‐Sundlov1; R. Grozovsky2; A. Hanneman3; K. Hoffmeister 1
1 Versiti Translational Glycomics Center, Milwaukee, Wisconsin, United States; 2 University of Miami, Miami, Florida, United States; 3 The Glycomics Center, Division of Molecular, Cellular and Biomedical Sciences, University of New Hampshire, Durham, NH; 4Charles River Laboratories, Shrewsbury MA, Shrewsbury, Massachusetts, United States
Background: Changes in surface glycan determinants, specifically sialic acid loss, determine platelet life span. The gradual loss of stored platelet quality, known as platelet storage lesion, is a complex process that fundamentally involves carbohydrate structures.
Aims: To determine the platelet Glycome of fresh and room temperature stored platelets.
Methods: We applied lipophilic extraction and glycan release protocols to sequentially profile glycosphingolipids, N‐ and O‐linked glycans using freshly isolated and seven‐day stored platelets. Analytical methods including matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS), tandem mass spectrometry (MSn), and liquid chromatography (LC) were used to obtain structural details of selected glycans and terminal epitopes.
Results: The fresh platelet repertoire of surface structures observed diverse N‐glycans, including high mannose and multiple polylactosamine repeats, and blood group glycans. O‐glycans consisted of sialylated and fucosylated core‐1 and core‐2 structures. Lactosyl‐ and globo‐ series structures and sialylated gangliosides dominated glycosphingolipids. We observed a loss of sialylated O‐glycans and a reciprocal increase in neutral structures following platelet storage at room temperature.
Conclusion(s): The data indicate that loss of sialylated O‐glycans is associated with platelet storage lesion and untimely removal of platelets following storage.
VPB0373
Alteration of circulating platelet‐related and diabetes‐related microRNAs in individuals with type 2 diabetes: Results from a stepwise hypoglycaemic clamp study
C. Eyileten 1; Z. Wicik1; D. Keshwani1; A. Nowak1; F. Aziz2; F. Aberer2; P. Pferschy2; N. Tripolt2; C. Sourij2; B. Prietl2; F. Prüller2; D. Von Lewinski2; J. Siller‐Matula3; M. Postula1; H. Sourij2
1 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 2 Medical University of Graz, Graz, Steiermark, Austria, 3 medical university of vienna, vienna, Wien, Austria
Background: Platelet function impairs in T2DM patients and hypoglycemia could increase the risk of cardiovascular complications. No study aimed to analyze the miRNA expression related to platelets in response to acute hypoglycemia in T2DM.
Aims: We aimed to investigate the influence of hypoglycemia on several selected miRNAs (based on bioinformatic analysis‐hsa‐miR‐16‐1, hsa‐miR‐34a, hsa‐miR‐129‐2, hsa‐miR‐15a, hsa‐miR‐15b, hsa‐miR‐106a) in subjects at an early stage of T2DM on metformin monotherapy, without established CVD and antiplatelet therapy during a stepwise hypoglycemic clamp experiment and a follow‐up seven days after the clamp event.
Methods: 14 patients with T2DM were included, all participants were on metformin monotherapy and they underwent a hyperinsulinemic‐euglycemic and subsequently a hyperinsulinaemic‐hypoglycaemia clamp experiment. Platelet activity, coagulation, endothelial function and inflammatory markers were determine and miRNAs expressions were analyzed.
Results: MiR‐106a‐5p, miR‐15b, miR‐15a, miR‐16‐5p, miR‐223 and miR‐126 showed significant increasing trend after euglycaemic clamp followed by hypoglycaemic clamp. MiR‐16‐5p was negatively correlated with interleukin (IL)‐6, intercellular adheasion molecule (ICAM) and vascular cell adheasion molecule (VCAM) (p = 0.002, p < 0.001, p = 0.016, respectively), whereas miR‐126 was positively correlated with VCAM (p < 0.001). There were negative correlations between miR‐16‐5p, miR‐126 and coagulation factors, including factor VIII and vWF. MiR‐126, miR‐129‐2‐3p and miR‐15b showed correlation with platelet function. Disease enrichment analysis showed that targets present in platelets of all analyzed miRNAs are associated with cancer related phenotypes.
Conclusion(s): Our study found that acute hypoglycemia could modulate miRNAs expression during an hyperinsulinaemic‐hypoglycaemic clamp experiment. It is worth noticing that up to this day, only a few studies have directly investigated the impact of hypoglycemia on thrombotic parameters, platelet function, and inflammatory biomarkers. In the first stage, applying bioinformatics analysis tools, we associated crucial biological function and signaling pathways related to the miRNAs with the most prominent differential expression pattern in T2DM associated with glucose metabolism, inflammation, thrombosis, and platelet function.


VPB0372
Subtype‐specific plasma signatures of platelet‐related protein releasate in acute pulmonary embolism
G. Baidildinova 1; V. ten Cate2; M. Nagler3; M. Panova‐Noeva4; S. Rapp5; T. Koeck6; J. Prochaska2; S. Heitmeier7; C. Gerdes8; S. Schwers8; S. Konstantinides9; T. Münzel10; C. Espinola‐Klein11; K. Lackner12; H. Spronk13; H. ten Cate14; P. van der meijden15; K. Leineweber8; P. Wild2; K. Jurk16
1 Maastricht University, Mainz, Rheinland‐Pfalz, Germany; 2 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 3 Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Rheinland‐Pfalz, Germany; 4 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, mainz, Rheinland‐Pfalz, Germany, 52 Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 6 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 7 Bayer AG, Wuppertal, Germany, Wuppertal, Nordrhein‐Westfalen, Germany; 8 Bayer AG, Wuppertal, Nordrhein‐Westfalen, Germany; 9 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 10 Center for Cardiology ‐ Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 11 Center for Cardiology, Cardiology III ‐ Angiology; Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, mainz, Rheinland‐Pfalz, Germany, 12 Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, mainz, Rheinland‐Pfalz, Germany, 13 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart + Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands, 14 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands, 15 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Thrombosis Expertise Center, Heart + Vascular Center, Maastricht University Medical Center, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands, 16 University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: Recent evidence suggests that the two subtypes of pulmonary embolism (PE), isolated PE (iPE) and deep vein thrombosis (DVT)‐associated PE (DVT‐PE) differ in terms of plasma protein profiles in the acute phase.
Aims: This study aimed to determine specific plasma signatures for proteins related to platelets in acute iPE and DVT‐PE compared to isolated DVT (iDVT).
Methods: Within the Genotyping and Molecular Phenotyping of Venous ThromboEmbolism (GMP‐VTE) Project, a multicenter prospective cohort study of 693 confirmed VTE cases, a highly sensitive targeted proteomics approach based on dual‐antibody proximity extension assay was applied. Whole leg ultrasonography was performed to exclude DVT in individuals with iPE. This study was approved by the local Ethics committee including signed patient informed consent. A total of 135 platelet‐related candidate proteins were selected from 444 proteins according to mass spectrometry‐based platelet proteomics databases and a systematic literature screen. LASSO‐regularized logistic regression models were utilized for analysis of regulated proteins.
Results: LASSO‐analysis selected 33 and 30 of 135 platelet‐related proteins in iPE and DVT‐PE vs. iDVT, respectively. The majority of the selected proteins did not overlap between PE‐subtypes. While iPE‐specific proteins were assigned to be predominantly released via shedding mechanisms and extracellular vesicles, degranulation was identified as a major release mechanism assigned to DVT‐associated PE‐specific proteins. Network analysis demonstrated three interconnected clusters of specifically regulated proteins in iPE linked to immunoreceptor signaling, pathogen clearance and chemotaxis, whereas for DVT‐associated PE one cluster was linked to tissue remodeling and leukocyte trafficking.
Conclusion(s): Machine learning‐based analysis revealed specific plasma signatures and differential release mechanisms of proteins related to platelets in iPE and DVT‐ PE. These data suggest that the platelet protein releasate contributes to the differential regulation of plasma proteins in acute PE compared to iDVT, which may be associated with different platelet activation patterns. GB is supported by EU‐TICARDIO No. 813409.
PB0371
New experimental evidence for a role of platelets in cholesterol‐induced vascular and valvular calcification
Z. Jiang 1; C. Oury2
1 University of Liege, Liege, Liege, Belgium; 2 Laboratory of Cardiology, GIGA Institute, University of Liège, Liège, Belgium, Liege, Liege, Belgium
Background: Hypercholesterolemia promotes calcification of arteries and of the aortic valve. The extent of calcification predicts poor cardiovascular outcome. Several studies indicate that platelets could play an active role in the calcification process. However, the link between hypercholesterolemia, platelets and vascular or valvular calcification remains unclear.
Aims: Our study aimed at assessing the role of platelets in arterial and aortic valve calcification under conditions of hypercholesterolemia.
Methods: We used a well‐established rabbit model of aorta and aortic valve calcification induced by 16 weeks of a cholesterol‐rich diet (0.3% cholesterol) supplemented with vitaminD2 (25.000 U/day) for 15 days. Rabbits were treated with the antiplatelet drug ticagrelor administered in food. Vascular and valvular calcification were detected by computed tomography (CT) and alizarin red staining of heart sections. The presence of platelets on aortic valve surface was analyzed by scanning electron microscopy. We then conducted an unbiased proteomic analysis of releasates of platelets isolated from rabbit blood to assess cholesterol‐induced changes in platelet function and identify potential soluble mediators of calcification.
Results: Aortic wall and valvular calcification developed in all rabbits fed a cholesterol rich‐diet (n = 13). Platelets were detected near calcification nodules on aortic valves. Ticagrelor treatment (n = 6) inhibited ex vivo platelet reactivity to ADP, and it reduced by half the number of rabbits with detectable aortic macrocalcification on CT scans, while fully preventing aortic valve calcification. Among 543 detected proteins, the 16‐week cholesterol‐rich diet modified the levels of 79 proteins from platelet releasates as compared to baseline. Remarkably, 40 of these proteins are known regulators of vascular or valvular calcification, including paraoxonase‐1, heparanase, periostin, fetuins, serpin proteases, thrombospondins, fibulins, CTGF, HGF, CD44, VCAM‐1, vitronectin, fibronectin, and the complement system.
Conclusion(s): Platelet inhibition with ticagrelor limits vascular and valvular calcification induced under hypercholesterolemia. Platelets may represent sources of soluble mediators driving the calcification process.
PB0367
Temporal labelling of aging platelets in mice indicates differential regulation and retention of granule protein encoding mRNAs
P. Armstrong 1; N. Kirkby2; C. Gutmann3; H. Allan4; A. Joshi3; M. Crescente5; J. Mitchell2; M. Mayr3; T. Warner1
1 Queen Mary, University of London, London, England, United Kingdom; 2 Imperial College London, London, England, United Kingdom; 3 King's College London, London, England, United Kingdom; 4 Blizard Institute, Queen Mary University of London, London, England, United Kingdom; 5 Manchester Metropolitan University, Manchester, England, United Kingdom
Background: Platelets inherit mRNA from their parent megakaryocyte. Young newly formed platelets are characterized as those with the highest levels of inherited mRNA, as this is rapidly lost once platelets are released, and age, within the circulation. Previous studies have identified the general decay half‐life of platelet mRNA to be approximately 6 hours, but it is unclear if this is decay is similar for all mRNAs.
Aims: To use in vivo temporal labelling to investigate platelet mRNA dynamics.
Methods: Fluorescent anti‐CD42c antibodies (Emfret) were injected (i.v) at various timepoints to C57Bl6 mice (12‐14wk) to identify age‐specific platelet populations: younger (< 24 h), older (>1 days old) and global. Following isolation by cell sorting, RNA was extracted (2.5million per sample) and qRT‐PCR performed for a panel of highly expressed platelet mRNAs. Unbiased clustering analysis was performed to compare age and abundance of individual RNAs.
Results: Principal component analysis demonstrated each analyzed population clustered individually but with differential directionality for individual mRNAs; indicating potential for individual patterns of decreased abundance. Focused comparison and grouping of mRNAs revealed the amount of loss varied by specific mRNA (median fold change ranging from −5.3 to −66.4) but was not contingent on starting abundance. In particular, those mRNAs classified as associated with signaling proteins (Gnas, Atp2a3, Alox12, Rgs18, Sh3bgrl3) demonstrated the greatest reduction in abundance, whilst those associated with granule proteins (Srgn, Nrgn, B2m, Pf4, Pbpp) demonstrated the least (mean ± s.e.m fold change: −35.6 ± 6.8 versus −9.2 ± 1.9 respectively, p < 0.05, n = 4).
Conclusion(s): The variable rates of loss observed in this dataset strongly suggest that platelet mRNA is under more refined control than previously appreciated. Further research is required to determine the mechanisms and purpose of such regulation, particularly if retention of granule protein encoding mRNAs correlates with the phenomenon of downstream de novo protein production previously reported by some groups.
Platelet Receptors
PB0380
Platelet glycoprotein analysis by flow cytometry, could further diagnostic information be gained from the mean fluorescence intensity values?
A. McCormick; O. Galvez; S. Nasim; M. Panetta; Y. Daniel.
Viapath Analytics, London, England, United Kingdom
Background: Platelet receptor glycoprotein expression is assessed in our laboratory by flow cytometry. The statistical components collected for analysis are percentage of gated cells and mean fluorescent intensity (MFI). An arbitrary cut off of >80% is considered to demonstrate full expression but is limited by lack of standardization and the subjective nature of interpretation. The Cytoflex™ flow cytometer (Beckman coulter) has been recently validated. Among other features, the Cytoflex™ offers the capability to detect 13 colors and exhibits major sensitivity for small particles. Use of DURAClone ™ technology has improved the turn around time, offered antibody standardization from batch to batch and allowed for a range of glycoproteins antibodies to be expressed in a single tube.
Aims: Our study aims to assess if MFI data obtained during platelet glycoprotein analysis could be used to support diagnosis of platelet receptor dysfunction.
Methods: EDTA whole blood from patients with suspected glycoprotein deficiency is collected. Following incubation, 20,000 platelet events are counted on the Cytoflex™. The gate strategy is applied and the platelet population subsequently interrogated for each different antibody on the panel. The results are collected by quantifying the % of positive antibody expression and the MFI.
Results: We have collated MFI values from 20 control values to assess if a reference cut‐off may be viable. Results from genetically confirmed patients with Glanzmann's Thrombasthenia (GT) and Bernard Soulier syndrome (BSS) were compared (Table 1). The MFI data obtained from control samples (with exception of the platelet activation marker CD62p) were shown to display normal distribution by Shapiro–Wilk analysis. A potential threshold was set at Median – 2xS.D.
Conclusion(s): The presented data suggests that MFI may be considered as an additional reporting parameter in the diagnosis of altered platelet glycoprotein expression. A combination of MFI and % positive antibody expression may prove a more robust diagnostic tool.

PB0391
Human platelets do not express a functional FcERI receptor on their surface
C. Tacquard1; F. Tupin 2; S. Magnenat3; C. Metz‐Favre4; P. Mertes5; C. Gachet3; B. Hechler3
1 EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, 2) Department of Anesthesia and Intensive Care, Strasbourg University Hospital, Strasbourg, France, Strasbourg, Alsace, France; 2 INSERM, EFS Grand‐Est, Strasbourg University, Strasbourg, Alsace, France; 3 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France; 4 Chest Disease Department, Strasbourg University Hospital, Strasbourg, France, Strasbourg, Alsace, France; 5 Department of Anesthesia and Intensive Care, Strasbourg University Hospital, Strasbourg, France, Strasbourg, Alsace, France
Background: Anaphylaxis, the most severe and potentially life‐threatening form of immediate hypersensitivity reaction, involves mainly a classical pathway mediated by antigen‐specific immunoglobulins (IgE) and their high‐affinity Fc receptor (FcERI) present on mast cells and basophils. It has been suggested that platelets contribute to IgE‐mediated anaphylaxis, particularly the most severe reactions, but the mechanism of their activation is not clearly established.
Aims: We assessed the effective presence and functionality of the FcERI receptor on human platelets.
Methods: The presence of FcERI on human platelets from healthy donors was evaluated by flow cytometry and Western blotting using two mouse monoclonal antibodies (mAbs) (CRA‐1 and 9E1) against the human FcERI alpha‐chain. The functionality of FcERI was investigated on platelets from healthy donors and allergic patients.
Results: FcERI was undetectable on platelets from healthy donors by Western blot and flow cytometry analyses using the mAbs CRA‐1 and 9E1, while these antibodies detected FcERI on human basophils. Incubation of washed platelets from healthy donors with anti‐TNP (trinitro‐phenyl) IgE for 1 h, followed by washing and challenge with TNP‐BSA did not induce platelet aggregation. In addition, exposure of citrated whole blood from allergic patients to their specific allergen or to an anti‐human IgE mAb (G7‐18), a specific FcERI activator, did not induce P‐selectin exposure or activation of integrin alphaIIbbeta3 (PAC‐1) on platelets, whereas activation of basophils was evidenced by their significant increase in surface CD63 exposure. Finally, stimulation of citrated platelet‐rich plasma from allergic patients with their specific allergen or with the mAb G7‐18 did not result in platelet aggregation.
Conclusion(s): Human platelets do not have a functional FcERI receptor on their surface. These results do not however exclude a contribution of platelets to IgE‐mediated anaphylaxis, which could be indirect, potentially involving other effectors.
VPB0394
iPSC‐derived megakaryocytes as a model to study human platelet integrin αIIbβ3 function
K. Fong 1; O. Kim2; A. Gagne3; C. Jobaliya3; J. Maguire3; M. Poncz4; D. French3; R. Litvinov5; J. Weisel6; W. Degrado7; L. Brass1
1 Hematology‐Oncology Division, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, United States; 2 Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 3 Department of Pathology and Laboratory Medicine, The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 4 Department of Pediatrics, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, United States; 5 University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania, United States; 6 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 7 Department of Pharmaceutical Chemistry, University of California, San Francisco, San Francisco, California, United States
Background: Integrin αIIbβ3, is the major fibrin(ogen) receptor on activated human platelets, supporting platelet aggregation and clot contraction during the hemostatic response to injury. αIIbβ3 is expressed on megakaryocytes (MKs) as well as platelets. Historically, methods to study αIIbβ3 structure/function relationships have been limited to humans with naturally occurring sequence variants, transgenic mouse models, and expression studies in cultured cell lines.
Aims: To determine whether the αIIbβ3 receptor expressed on human induced pluripotent stem cell (iPSC)‐derived CD41 + CD42+ iMKs is functional, and whether CRISPR/Cas9 gene editing can be used to generate isogenic lines to support integrin structure/function studies.
Methods: Hematopoietic progenitor cells (HPC) were generated from iPSCs using a 2D directed differentiation protocol. iMKs were generated from HPCs cultured in previously established cytokine mixtures. αIIbβ3 activation was analyzed by flow cytometry using the activation‐dependent monoclonal antibody, PAC‐1. Clot contraction was measured using a Thromboimager Analyzer with iMKs resuspended in platelet‐poor plasma in the presence of 2 U/mL thrombin and 3 mM calcium.
Results: The mean fluorescence intensity (MFI) for PAC‐1 binding was 6128 ± 203 (n = 4) for thrombin‐stimulated, 241 ± 17 (n = 4) for unstimulated and 185 ± 70 (n = 4) for EDTA‐treated cells. Previous work in CHO cells showed the αIIb calf domain variants V760A and H787A to be constitutively and non‐constitutively active, respectively. The V760A constitutively active variant created in an established control human iPSC line showed a two‐fold increase in PAC‐1 binding to iMKs (432 ± 144, n = 3) compared to the non‐constitutively active H787A variant (223 ± 91, n = 4, p = 0.06) and control iMKs (241 ± 17, n = 3, p = 0.07). All of the control and variant iMKs resuspended in platelet poor plasma were able to support clot contraction.
Conclusion(s): The ability to generate and study specific variants in the platelet integrin αIIbβ3 receptor using iPSCs and the CRISPR/Cas9 technology demonstrates the use of iMKs in studying platelet integrin‐structure function relationships.
PB0376
Inhibition of the p75 pan neurotrophic receptor partly mitigates BDNF‐induced platelet aggregation
S. Fleury 1; I. Boukhatem2; M. Welman1; É. Maurand3; B. Allen1; H. Saragovi4; M. Lordkipanidzé1
1 Montreal Heart Institute, Montréal, Quebec, Canada; 2 Montreal Heart Institute & University of Montreal, Montréal, Quebec, Canada; 3 Université Paris‐Descartes, Paris, Ile‐de‐France, France; 4 Lady Davis Institute, Montréal, Quebec, Canada
Background: The Brain‐Derived Neurotrophic Factor (BDNF) promotes neuronal growth and survival, acting through alternatively spliced isoforms of the full length TrkB receptor and the 75 kDa pan‐neurotrophic receptor (p75NTR). TrkB and p75NTR receptors cross‐regulate each other's signal and ligand bindings. BDNF is present in brain and serum, and platelets can have 1000‐fold greater BDNF than neurons. We have shown that washed platelets aggregate in response to BDNF via the activation of a truncated TrkB receptor. Whether p75NTR is present in platelets and regulates BDNF‐induced platelet aggregation remains unknown.
Aims: To characterize p75NTR expression and function in platelets.
Methods: Washed platelets were prepared from whole blood of healthy male and female volunteers. p75NTR expression was assessed by immunoblotting, flow cytometry and confocal microscopy, and quantified by ELISA (n = 20). Platelets were activated using classical agonists (ADP, collagen, thrombin receptor activating peptide [TRAP]) and BDNF, in the presence of p75NTR inhibitor THX‐B or negative control inactive analog, THX. Aggregation was measured by light transmission aggregometry. Signaling pathways downstream of BDNF activation were assessed by anti‐phosphoprotein immunoblot.
Results: p75NTR immunoreactivity was detected on the surface of (28.3 ± 19.4%) and within (56.4 ± 18.4%) platelets. p75NTR levels quantified by ELISA were highly variable, with a 10‐fold difference between highest (382 pg/mL) and lowest values (38 pg/mL), and did not change following platelet activation. Collagen‐, ADP‐, and TRAP‐induced aggregation were not affected by the p75NTR inhibitor, THX‐B. Inhibition of BDNF‐induced platelet aggregation showed high interindividual variation (32.4 ± 36.8% inhibition): THX‐B‐induced responses ranged from no effect in 50%, partial effect in 30% to a complete abrogation of platelet aggregation in 20% of participants.
Conclusion(s): Pharmacological inhibition of p75NTR abrogated BDNF‐induced platelet aggregation in some but not all healthy volunteers. Whether this variability in aggregation is related to the high interindividual variation in platelet p75NTR levels remains to be established.
PB0375
Deciphering the TLT‐1 Fibrinogen ligand interaction
s. Branfield 1; X. Vales2; A. Washington3; C. wu4; P. Akamine2; J. González2
1 oakland university, auburn hills, Michigan, United States; 2 UNIVERSITY OF PUERTO RICO, SAN JUAN, Puerto Rico, United States; 3 Oakland University, Rochester, Michigan, United States; 4 OAKLAND UNIVERSITY, ROCHESTER HILLS, Michigan, United States
Background: The platelet integrin αIIbβ3’s interaction with the plasma clotting fibrinogen protein plays a central role in thrombosis and hemostasis. Upon activation, platelets release TREM‐Like Transcript‐1 (TLT‐1) from their a‐granules onto their surface. Early studies have demonstrated that fibrinogen is a ligand for TLT‐1. TLT‐1 binding fibrinogen was a surprising discovery since αIIbβ3, the most abundant platelet receptor, also binds fibrinogen and facilitates platelet aggregation. It is difficult to understand why platelets have two fibrinogen binding receptors. Very little is known about the TLT‐1‐ffibrinogen interaction.
Aims: Delineate the TLT‐1 ‐ Fibrinogen molecular interaction.
Methods: To develop a binding assay using an Octet Qke Bio‐layer Interferometry (BLI) to delineate the molecular interaction between TLT‐1 and fibrinogen and use mass spectrometry to identify specific regions of fibrinogen that interact with TLT‐1.
Results: We first used BLI to demonstrate the TLT‐1 Fibrinogen interaction. Figure 1 illustrates a strong association with no dissociation, suggesting a strong interaction between the TLT‐1 and fibrinogen. To localize the exact binding sites for this molecular interaction, we digested fibrinogen using trypsin and carried out an immunoprecipitation (IP) followed by Liquid Chromatography‐Mass Spectrometry (LC–MS/MS). We isolated and identified four potential peptides and are currently using the BLI to identify which of these regions are critical for binding TLT‐1. We are also identifying the regions of TLT‐1 that interact with fibrinogen. This poster will detail the current state of these studies.
Conclusion(s): The TLT‐1/fibrinogen interaction has an equilibrium dissociation constant (KD) of 3.02 ± 0.20 nM. This interaction may play a larger role in hemostasis and inflammation that previously thought.

PB0381
Impact of Coagulation Factor XI (FXI) on the Binding of Platelet Glycoprotein Ibα to the von Willebrand Factor A1 Domain
M. Nakayama 1; S. Goto2; S. Takemoto3; H. Oka2; H. Yokota3; S. Takagi4
1 Tokai University, Isehara, Kanagawa, Japan; 2 Department of Medicine (Cardiology), Tokai University School of Medicine, Isehara, Kanagawa, Japan; 3 Image Processing Research Team, Center for Advanced Photonics, RIKEN, Wako, Saitama, Japan; 4 Graduate School of Engineering, The University of Tokyo, Bunkyō, Tokyo, Japan
Background: Platelet glycoprotein (GP)Ibα have specific binding sites for multiple proteins such as von Willebrand factor (VWF) and coagulation factor XI (FXI). The structural basis for different biological functions mediated by FXI and VWF binding with GPIbα is still to be elucidated.
Aims: To clarify the potential impacts of FXI binding to GPIbα‐VWF complex using MD simulations.
Methods: The binding site(s) of FXI on GPIbα is not fully known. Since K252 and K253 in FXI are positively charged amino acids, all negatively charged amino acids (GLU and ASP) in targeted N‐terminus GPIbα were assumed to be potential binding sites for FXI in this study (Figure 1). The position and velocity vector of each atom and water molecule were calculated in each 2 × 10–15 second with NAnoMolecular Dynamics (NAMD) software using Chemistry at Harvard Macromolecular Mechanics (CHARMM)‐36 force field. All models were calculated 450 ns.
Results: The molecular dynamic simulation revealed approximately 4.2 to 27.3 times greater positional fluctuations in atoms constructing GPIbα bound with FXI as compared to its binding with VWF. Non‐covalent binding energy generated by GPIbα binding with FXI is approximately 5% to 45% as compared to those generated by its binding with VWF. The structure of GPIbα binding with VWF is stable with minimal influence by its additional binding with FXI (Figure 2), which was confirmed by biological experiments in exogenously added FXI condition (final concentration of 80 μg/mL) showing no significant difference in platelet adhesion on VWF under wall shear rate of 1500 s‐1.
Conclusion(s): FXI binding with GPIbα minimally influenced the structure and biological function of GPIbα binding with VWF.


PB0382
The antibody JAQ1 binds to both human and mouse GPVI but differently affect their function and in vivo depletion
S. Navarro 1; H. Brown2; J. Heemskerk3; M. Kuijpers4; D. Stegner5; B. Nieswandt6
1 University Hospital Würzburg, Würzburg, Bayern, Germany; 2 University hospital Würzburg, Würzburg, Bayern, Germany; 3 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 4 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 5 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 6 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: Platelet glycoprotein (GP) VI is the major platelet collagen receptor and a promising antithrombotic target. This was first demonstrated in mice using the monoclonal antibody JAQ1, which inhibits completely collagen‐related peptide (CRP) and collagen‐induced platelet activation, aggregation and thrombus formation. Injection of JAQ1‐IgG induces transient thrombocytopenia and depletion of GPVI from platelets, whereas its F(ab´)‐fragment only blocks GPVI without affecting platelet count or GPVI expression in vivo.
Aims: The aim of this study was testing the effects of JAQ1 on human GPVI function in vitro and the immunodepletion of the receptor in newly generated hGPVI‐knockin (hGPVItg/tg) mice in vivo.
Methods: We used flow cytometry, aggregometry, spreading assays and ex vivo flow adhesion studies to characterize the effect of JAQ1 on human platelets and hGPVItg/tg mouse platelets. The effects of JAQ1 treatment on hGPVI expression in vivo was analyzed in hGPVItg/tg mice.
Results: Flow cytometry revealed binding of JAQ1 to human GPVI, but not to other species, including rat, hamster, rabbit, swine, cat, dog, horse, and cow. In hGPVItg/tg mouse platelets, JAQ1 binding did not cause detectable activation unless cross‐linked, comparable to the effect on wild‐type platelets. Remarkably, in contrast to mouse GPVI, JAQ1 did not inhibit CRP or collagen binding to human GPVI. In vivo, JAQ1‐treatment induce complete depletion of mGPVI in wild‐type mice, but only partially downregulate the receptor in hGPVItg/tg mice, which was in both groups accompanied by a comparable transient thrombocytopenia.
Conclusion(s): The binding site of JAQ1 on GPVI is conserved between mouse and human, but differs in its functional significance. In vivo targeting of this site with JAQ1 IgG results in different immuno‐depletion efficacies, indicating that epitope‐specific effects could be involved in this process.
PB0379
Functional expression of GFP‐tagged integrin αIIb in a transgenic (knockin) mouse line
C. Kusch 1; D. Johnson2; P. Burkard3; P. Öftering3; D. Stegner4; S. Beck5; B. Nieswandt6
1 University Hospital Würzburg, Germany, Würzburg, Bayern, Germany; 2 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 3 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Germany, Würzburg, Bayern, Germany; 4 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany, 5 University Hospital and Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 6 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany
Background: As heterodimeric adhesion molecules, integrins facilitate cell–cell and cell‐matrix interactions. The dominant platelet integrin αIIbβ3 is a paradigmatic model to study integrin biology and is central in thrombosis, hemostasis, and inflammation. Dynamic visualization of αIIbβ3 in vivo as well as in vitro is difficult as its ligation, e.g. by antibodies, rapidly alters its localisation (clustering and capping) and function.
Aims: To generate and analyze a mouse line expressing eGFP‐tagged integrin αIIbβ3.
Methods: Generation and characterization of a mouse line expressing eGFP‐tagged integrin αIIb (Itga2bGFP) allowing the dynamic visualization of αIIbβ3 in the absence of antibodies.
Results: It has previously been shown in heterologous systems (CHO cells) that tagging of the integrin αIIb‐subunit with GFP is possible without impairing the integrin's functionality (Buensuceso et al., JBC 2003). Following this strategy, we generated a mutant mouse line expressing eGFP fused to the intracellular tail of αIIb (Itga2bGFP). Flow cytometric analyses of Itga2bGFP platelets confirmed the expression of the αIIb‐eGFP fusion protein. Notably, αIIbβ3 surface abundance was slightly reduced in platelets from homozygous mutant mice but unaltered in heterozygous platelets compared to wild‐type controls. Surface expression of major platelet glycoproteins, as well as αIIbβ3 activation and P‐selectin exposure in response to classical platelet agonists were indistinguishable between Itga2bGFP and control platelets. This was confirmed in aggregometry studies and by normal tail bleeding times of Itga2bGFP mice. Fluorescence microscopy confirmed that the eGFP signal allows to monitor αIIbβ3 in live platelets during spreading on fibrinogen in vitro and in intravital microscopy‐based disease models.
Conclusion(s): Itga2bGFP mice express functional eGFP‐tagged integrin αIIbβ3 and now enable studies on αIIbβ3 dynamics in platelets and megakaryocytes in vitro and in vivo. This work was supported by TR240 grant with project number 374031971 and BE5084/5–1.
PB0389
The C‐Type Lectin CD93 Regulates Platelet Activation Induced by PAR4 Stimulation
S. Trivigno 1; M. Vismara2; M. Zarà3; L. Galgano1; S. Carnevali Carlino2; I. Canobbio2; F. Galvagni4; M. Orlandini4; M. Torti5; G. Guidetti2.
1 University School for Advanced Studies IUSS, Pavia, Pavia, Lombardia, Italy; 2 University of Pavia, Pavia, Pavia, Lombardia, Italy; 3 Centro Cardiologico Monzino IRCCS, Milano, Milano, Lombardia, Italy; 4 University of Siena, Siena, Siena, Toscana, Italy; 5 University of Pavia, Pavia, Lombardia, Italy
Background: CD93 is a member of group XIV in the C‐type lectin superfamily and plays a prominent role in inflammation, vascular diseases and cancer. In endothelial cells, CD93 is an adhesion molecule regulating angiogenesis through the interaction with the extracellular matrix protein Multimerin‐2. CD93 also regulates leukocyte recruitment and migration, as well as efferocytosis. The extracellular domain of CD93 can be released as a soluble factor by proteolytic shedding and its plasma levels are increased in different disorders. Despite its emerging importance in several vascular contexts, the contribution of CD93 to platelet biology is still unknown.
Aims: In this study we investigated the role of CD93 in platelet activation induced by physiological platelet agonists.
Methods: Platelet aggregation, secretion, protein phosphorylation and platelet–neutrophil aggregate formation induced by several agonists were investigated in whole blood and purified human and murine platelets by lumiaggregometry, immunoblotting, and flow cytometry.
Results: CD93 is expressed in human and murine platelets, as demonstrated by immunoblotting and immunofluorescence microscopy analyses. Stimulation of human platelets with thrombin or collagen induced CD93 tyrosine phosphorylation and shedding, promoting its release as a soluble extracellular domain. Platelets from CD93‐knockout (KO) mice aggregated normally when stimulated with convulxin, thrombin or U46619, but displayed a defective aggregation upon selective stimulation of PAR4. Moreover, activation of integrin αIIbβ3 and α‐granule release induced by PAR4‐activating peptide were significantly reduced in CD93KO platelets, and formation of platelet–neutrophil aggregates was also impaired. These functional defects were mirrored by a reduction of protein tyrosine phosphorylation and protein kinase C activation. Interestingly, analysis of platelet response upon multiple stimulations over time with low doses of PAR4‐activating peptide revealed a more pronounced desensitization of CD93‐deficient platelets compared to wild type cells.
Conclusion(s): This study reveals a novel role for CD93 in platelet function as a selective regulator of PAR4‐mediated signaling.
PB0393
Use of spectral flow cytometry to immunophenotype platelets
A. Vadgama 1; J. Boot1; H. Allan1; C. Mein1; P. Armstrong2; T. Warner2
1 Blizard Institute, Queen Mary University of London, London, England, United Kingdom; 2 Queen Mary, University of London, London, England, United Kingdom
Background: Platelet function is mediated through expression of specialized surface markers and dedicated signaling pathways. Changes in expression levels of these surface markers following platelet activation have been shown to underlie haemostatic response and thrombus formation.
Aims: To develop an assay to immunophenotype platelets using spectral flow cytometry.
Methods: Blood was taken via venepuncture from healthy volunteers (n = 23) and centrifuged to produce platelet‐rich plasma. Spectral flow cytometry (Cytek Aurora) was used to simultaneously determine the expression levels of 14 markers in resting platelets and platelets activated with ADP (adenosine diphosphate), TRAP‐6 amide (thrombin receptor‐activating peptide ligand), U46619 (thromboxane A2 receptor ligand), PAR‐4 (thrombin receptor PAR‐4 ligand), and CRP‐XL (collagen‐related peptide). Median fluorescence intensity (MFI) was recorded, analyzed with NovoExpress software and GraphPad Prism software using an ANOVA or mixed‐effects analysis, and presented as mean ± s.e.m.
Results: Following platelet activation, changes in surface marker expression varied according to agonist stimulation. In general terms, expression of 9 markers (CD9, CD36, CD63, CD107a, CD62P, CD29, CD41, CD61, PAC‐1) increased following activation, whilst the expression of 4 markers (CD31, CD42a, CD42b, GPVI) decreased, and that of CLEC‐2 remained unchanged. The largest increase in expression of CD62P was in response to TRAP‐6 (9818 ± 2140 to 53,186 ± 2165, p < 0.0001), and that of PAC‐1 to PAR‐4 (633 ± 90 to 4866 ± 494, p < 0.0001). The largest decrease in CD42b was in response to PAR‐4 (14,014 ± 1171 to 6447 ± 530, p < 0.0001).
Conclusion(s): Using spectral flow cytometry, we have developed an assay that can concurrently characterize expression of 14 platelet surface markers in a low‐volume sample. It reliably detects changes in response to activation that are consistent with existing literature. Such an assay will be a powerful tool not only in investigating basal and activation‐dependent platelet heterogeneity, but also in detecting abnormalities in surface marker expression associated with conditions such as inherited bleeding disorders or immune thrombocytopaenia.
PB0384
GPVI is a binding site for factor VIII on platelets
R. Sekar 1; A. Mimoun2; M. Bou Jaoudeh3; S. Loyau4; S. Delignat5; V. Daventure5; J. Rayes6; Y. Boulaftali7; M. Jandrot‐Perrus8; V. Proulle9; S. Lacroix‐Desmazes10
1 INSERM UMRS 1138, Sorbonne University, Paris, Ile‐de‐France, France; 2 INSERM UMRS 1138, Paris, Ile‐de‐France, France; 3 INSERM UMRS1138 ‐ Sorbonne University, Paris, Ile‐de‐France, France; 4 INSERM U1148, Laboratory for Vascular Translational Science, Paris, Ile‐de‐France, France; 5 Inserm, Paris, Ile‐de‐France, France; 6 University of Birmingham, Birmingham, England, United Kingdom; 7 Inserm U1148, Paris, Ile‐de‐France, France; 8 LVTS, UMR_S1148 INSERM, Université de Paris, France, PARIS, Ile‐de‐France, France; 9 INSERM UMRS1138, Paris, Ile‐de‐France, France, 10 INSERM, Paris, Ile‐de‐France, France
Background: Factor VIII (FVIII) is an essential actor to form the tenase complex at the surface of activated platelets. FVIII binding partners on platelets include phosphatidylserine (PS) and fibrin‐bound‐αIIbβ3. Screening for FVIII interactions with platelet‐expressed glycoproteins (GP) indicated FVIII binding to GPVI, the cross‐linking of which by collagen, activates platelets.
Aims: To characterize the FVIII/GPVI interaction and decipher its influence on platelet functions.
Methods: Binding of full‐length FVIII (FL‐FVIII), B domain‐deleted FVIII (BDD‐FVIII) and BDD‐FVIII mutated in the C1 and C2 domains (C1R2090A/K2092A/F2093A, C2R2215A/R2220A) was investigated using ELISA on immobilized human monomeric recombinant GPVI (rGPVI) or Fc‐fused dimeric GPVI (GPVI‐Fc), and by flow cytometry. FVIII was pre‐incubated alone or with varying amounts of von Willebrand factor (VWF), or human monovalent monoclonal IgG anti‐A2, anti‐C1, anti‐C2 FVIII domains. The effect of FVIII on collagen‐induced platelet aggregation and GPVI‐dependent downstream phosphorylation was further studied on washed platelets.
Results: FL‐FVIII and BDD‐FVIII, but not plasma‐derived FVIII, bound in a dose‐dependent manner to GPVI‐Fc and rGPVI. VWF inhibited FVIII binding to GPVI. Interestingly, C1‐ and C2‐specific, but not A2‐specific, monovalent IgG also inhibited the interaction. Accordingly, FVIII variants mutated in the C1 and C2 target epitopes lost binding capacity to GPVI. VWF‐free FVIII binding to resting and thrombin‐activated platelets was confirmed using flow cytometry, albeit at high concentration. In these conditions, FVIII inhibited by more than 50% collagen‐induced aggregation, while FIX had no effect; and by 52 ± 19% global GPVI‐dependent downstream phosphorylation, as compared to a 72 ± 7% inhibition induced by the anti‐GPVI Fab 9O12.
Conclusion(s): Our results identify GPVI as a novel non‐PS binding partner for FVIII on platelets, in adjunct to fibrin‐bound‐ αIIbβ3. We also observed that elevated FVIII concentrations inhibit collagen‐mediated platelet activation. Whether the FVIII‐GPVI interaction plays a modulatory role at the surface of activated platelets during thrombus formation, remains to be determined.
PB0387
GPR56 is the platelet collagen receptor activator of G protein 13
L. Pan1; T. Bernadyn2; R. Adili3; M. Holinstat4; G. Tall 2
1 University of Michigan Medical School, Ann Arbor, Michigan, United States; 2 University of Michigan, Ann Arbor, Michigan, United States, 3 University of Michigan, ANN ARBOR, Michigan, United States; 4 Department of Pharmacology, University of Michigan, Ann Arbor, Michigan, United States
Background: We recently identified the adhesion GPCR, GPR56/ADGRG1 as a platelet cell surface receptor that utilizes fibrillar collagen and blood flow‐induced shear force to activate G protein 13. GPR56 is activated by a unique tethered‐peptide‐agonist mechanism. We propose that activation of GPR56 serves to initiate the actin cytoskeletal rearrangements that elicit platelet shape change and support primary platelet activation during hemostasis.
Aims: The goals of our current study are to: 1. Quantify GPR56 mRNA and cell surface receptor levels in human and mouse platelets. 2. Characterize the robust activation of G13 by GPR56 in the context of weaker G protein i signaling that GPR56 may also provide. 3. Use new transgenic mouse models to further understand the role of GPR56 in the regulation of hemostasis.
Methods: GPR56 expression and cell surface protein abundance will be measured by a combination of techniques including qPCR (directed and Taqman GPCR array), RNAseq, immunoblotting and flow cytometry. Ex vivo platelet signaling assays (Rho‐GTP production, platelet spreading, and adenylyl cyclase activity) will be performed to understand the interplay of GPR56 mediated G13 and Gi signaling. In vivo hemostasis assays will be performed using new transgenic mouse strains.
Results: GPR56 is a highly abundant platelet GPCR. Preliminary results indicate that its abundance is similar to protease activated receptors (PAR) and purinergic receptors (P2Y). Human GPR56 activates G13 and weakly stimulates Gi, although mouse GPR56 seems to only activate G13. Global Gpr56 knockout mice exhibit hemostasis defects. New data may be presented to examine the effects of a platelet‐specific Gpr56 deletion on these processes.
Conclusion(s): We identified GPR56 as a platelet collagen receptor that appears to work in concert with the repertoire of known platelet collagen receptors. We propose that GPR56 operates as transient collagen receptor to evoke platelet signaling and does not directly mediate stable platelet adhesion to collagen.
PB0390
Identification of novel inhibitors of the platelet collagen receptor GPVI by a phenotypic screening assay
S. Troitiño 1; I. Izquierdo2; N. Gómez‐Romero2; F. Rodríguez Del Río2; G. Prieto Da Cuña2; L. Hermida‐Nogueira1; E. Domínguez3; M. Loza4; Á. García1
1 Platelet Proteomics Group, Center for Research in Molecular Medicine and Chronic Diseases (CiMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain, Santiago de Compostela, Galicia, Spain; 2 Platelet Proteomics Group, Center for Research in Molecular Medicine and Chronic Diseases (CiMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain, Santiago De Compostela, Galicia, Spain; 3 Innopharma Screening Platform, BioFarma Research group, Center for Research in Molecular Medicine and Chronic Diseases (CIMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain, Santiago De Compostela, Galicia, Spain; 4 Head of Innopharma Screening Platform, BioFarma Research group, Center for Research in Molecular Medicine and Chronic Diseases (CIMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain, Santiago De Compostela, Galicia, Spain
Background: Current antiplatelet therapies are often associated with significant risks of bleeding and hemorrhage by seriously compromising platelet function. Finding antiplatelet drugs modulating thrombus formation without significantly altering hemostasis is a need. The collagen receptor glycoprotein VI (GPVI) is a promising candidate, since not only is uniquely expressed in platelets and megakaryocytes, but also its blockade inhibits thrombosis.
Aims: To identify GPVI potential inhibitors by phenotypic screening that can progress into preclinical trials as novels antithrombotic candidates.
Methods: Platelet‐based intracellular calcium mobilization (FLIPR Calcium 4) phenotypic screen assay was used to identify inhibitors. Label‐free dynamic mass redistribution assay was carried out to confirm ligand binding. Series of derivatives of the hits obtained were synthesized by using a medicinal chemistry strategy. Functional studies were based on platelet aggregation and spreading assays. Viability Calcein‐AM assay by flow cytometry was used to test the potential toxicity of ligands.
Results: Two molecules showing efficacy at inhibiting intracellular calcium release and platelet aggregation through GPVI modulation, SIL‐ENA and SEDN2, were identified as ligands. SEDN2 was the most potent aggregation inhibitor and was used as a scaffold to obtain 19 chemical derivatives. Two of those derivatives showed a potent antiplatelet activity and were prioritized, together with SEDN2, as candidates for preclinical thrombosis studies. The candidates have negligible effects in platelet viability at micromolar doses. We are performing studies to further characterize their antithrombotic potential in vivo by evaluating their selectivity and bioavailability.
Conclusion(s): Novel antiplatelet agents targeting GPVI were identified and prioritized by combining relevant phenotypic screens and functional studies in human platelets.
PB0386
The WASH‐complex subunit Strumpellin regulates integrin αIIbβ3 trafficking in murine platelets
M. Spindler 1; Y. Schurr2; L. Reil2; B. Nieswandt2; L. Machesky3; M. Bender2
1 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 2 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 3 Cancer Research UK Beatson Institute, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, Scotland, United Kingdom
Background: The platelet specific integrin αIIbβ3 mediates platelet adhesion, aggregation and plays a central role in thrombosis and hemostasis. In resting platelets, αIIbβ3 is expressed on the membrane surface and in intracellular compartments. Upon platelet activation, the number of surface‐expressed αIIbβ3 is increased by the translocation of internal granule pools to the plasma membrane. The five‐subunit Wiskott‐Aldrich syndrome protein and Scar homolog (WASH) complex is the major endosomal actin polymerization‐promoting complex and has been implicated in the generation of actin networks involved in endocytic trafficking of integrins in other cell types. Strumpellin is a subunit of the WASH complex but its role in platelet function is unknown.
Aims: The aim of this study was to investigate the role of Strumpellin in platelet function.
Methods: We took advantage of the megakaryocyte/platelet‐specific Strumpellin‐deficient mice and performed flow cytometry, qPCR, quantitative mass spectrometry and different microscopic techniques.
Results: Strumpellin‐deficient mice displayed a normal platelet count. In contrast to other surface membrane glycoproteins (e.g. α2, β1, GPV, GPIb) the surface expression of integrin αIIbβ3 was approximately 20% decreased on Strumpellin‐deficient platelets. While the release of the internal αIIbβ3 pool after platelet activation was unaffected, fibrinogen uptake was delayed. The number of platelet α‐granules was slightly but significantly increased in Strumpellin‐deficient platelets. Interestingly, reduced integrin αIIbβ3 expression was also observed in primary Strumpellin‐deficient megakaryocytes, however their ploidy levels were unaltered. Quantitative PCR revealed normal mRNA level of Itga2b and Itgb3 in mutant megakaryocytes, suggesting unaltered transcription of these genes. Quantitative proteome analysis of isolated αIIbβ3‐positive vesicular structures from Strumpellin‐deficient platelets revealed an enrichment of protein markers, which are associated with the endoplasmatic reticulum, Golgi complex and early endosomes.
Conclusion(s): These results point to a so far unidentified role of the WASH complex subunit Strumpellin in integrin αIIbβ3 trafficking in murine platelets.
PB0392
Pim kinase: A novel regulator of platelet and megakaryocyte thromboxane A2 and C‐X‐C‐R motif receptors
S. Nock1; L. Hutchinson2; K. Naseem3; S. Jones1; S. Mundell2; A. Unsworth 4; M. blanco‐Lopez1
1 Manchester Metropolitan University, Manchester, England, United Kingdom; 2 University of Bristol, Bristol, England, United Kingdom; 3 University of Leeds, Leeds, England, United Kingdom, 4 Manchester Metropolitan University, Manchester, England, United Kingdom
Background: Pim kinases have recently been identified to play a role in the regulation of platelet function and thrombosis. Deletion or inhibition of Pim‐1 results in reduced thrombus formation without altered hemostasis, suggesting it is a desirable antiplatelet target. In other cell types, Pim kinases have been shown to regulate CXCR4 and we have previously described that Pim‐1 regulates platelet function via regulation of TPαR signaling.
Aims: We set out to investigate the mechanism by which Pim‐1 regulates TPαR and CXCR4 signaling in platelets and megakaryocytes.
Methods: HEK293 cells transfected with Flag or GFP‐tagged TPαR and MEG01 cells (expressing CXCR4) were treated with Pim kinase inhibitors (AZD1208, LGH447) for 10 minutes. Receptor levels (surface and total) were then assessed using both flow cytometry and microscopy. TxB2 generation was measured by ELISA and calcium mobilization was measured to determine effects on downstream signaling.
Results: No alteration in TxB2 generation indicates Pim kinase regulates platelet TxA2 receptor signaling independently of COX1 regulation. Pim kinase inhibition causes internalization of both the CXCR4 and TPαR when assessed using flow cytometry and fluorescence microscopy. Consistent with the receptors being internalized, a reduction in TxA2 and SDF1a mediated calcium mobilization, and phospho‐PKC signaling was observed following treatment of platelets, HEK293 and MEG01 cells with Pim kinase inhibitors.
Conclusion(s): Inhibition of Pim kinase attenuates TPαR and CXCR4 signaling via receptor internalization. CXCR4 and TPαR share similar consensus sequences, containing homologous serine residues within their intracellular loops suggesting that Pim could phosphorylate TPαR in a similar mechanism to CXCR4, regulating receptor expression at the platelet and megakaryocyte surface. Reduction of platelet and megakaryocyte TPαR and CXCR4 receptor levels and signaling offers a novel platelet targeting strategy, especially in inflammatory conditions, such as rheumatoid arthritis and SLE that are associated with increased circulating plasma levels of thromboxane A2 and SDF‐1a.
PB0378
Ticagrelor enhances platelet TP receptor surface expression in patients due to P2Y12 / TP dimerization
M. Keith 1; J. Hutchinson2; J. Khalil1; M. Aungraheeta3; S. Mundell1
1 University of Bristol, Bristol, England, United Kingdom; 2 University of Bristol, BRISTOL, England, United Kingdom; 3 North Bristol NHS Trust, Bristol, England, United Kingdom
Background: Dual antiplatelet therapy (DAPT) targeting both TP receptor ligand production and P2Y12 receptor activation is standard following percutaneous coronary intervention (PCI) in coronary artery disease patients. Trial data remain inconclusive but suggest redundancy of aspirin in the presence of strong P2Y12 inhibitors such as ticagrelor or prasugrel.
Aims: To explore potential interplay between the Gαq‐coupled TP and Gαi‐coupled P2Y12 receptor pathways at the levels of surface expression, molecular interaction and platelet function.
Methods: Platelets were obtained with informed consent from acute coronary syndrome (ACS) patients undertaking DAPT, or from healthy volunteers. Platelet activation was assessed by ratiometric monitoring of ligand‐induced calcium flux. Receptor expression in platelets and HEK293T cells transiently overexpressing tagged P2Y12 and TP receptors was assessed by flow cytometry. Receptor interaction was assessed by immunoprecipitation and Western blotting from lysed cells.
Results: Longitudinal comparisons of platelet responses from patients at the end of DAPT revealed significant reduction in U46619‐induced, TP‐mediated calcium flux in the presence of ticagrelor plus aspirin, compared to aspirin alone. However, patient ticagrelor treatment was associated with a significant increase in both P2Y12 and, unexpectedly, TP platelet surface expression as assessed by flow cytometry. This was recapitulated ex vivo using healthy donor platelets, and further explored through co‐expression of tagged P2Y12 and TPα in HEK293T cells where enhanced surface expression of each receptor was observed, compared to single receptor transfectants. This appeared specific, since no such enhancement was seen upon substitution of P2Y12 with the similarly Gαi‐coupled μOR. Finally, tagged, overexpressed P2Y12 and TP receptors were demonstrated to interact in reciprocal co‐immunoprecipitation experiments indicating dimerization.
Conclusion(s): These data suggest co‐trafficking of P2Y12 and TP receptors, that can be modulated through inhibition of P2Y12 by ticagrelor. They highlight the complex relationship between P2Y12 and TP receptor biology helping to inform the debate on DAPT versus monotherapy.
PB0388
Platelet glycoprotein Ibα carries ABO blood group antigens
K. Tiemeyer 1; M. Hollenhorst1; K. Aoki2; M. Ishihara2; C. Bertozzi3
1 Stanford University, Palo Alto, California, United States; 2 University of Georgia; 3 Stanford University, Stanford, California, United States
Background: ABO blood group is associated with risk of thrombosis and bleeding. Research into this association has focused largely on the impact of ABO antigens on von Willebrand factor (VWF). Platelet ABO antigens may also play an important role in hemostasis and thrombosis. Few studies have investigated the expression of ABO antigens on the platelet binding partner of VWF, GPIbα.
Aims: To confirm that platelet GPIbα carries ABO antigens, to determine if these antigens are present on N‐ vs O‐glycans, and to estimate the number of ABO antigens carried by a single molecule of GPIbα.
Methods: The ectodomain of GPIbα was purified from human platelets of known blood type. The purified protein was treated with recombinant α‐N‐acetylgalactosaminidase (cleaves terminal α‐N‐acetylgalactose found in the A antigen, Figure 1A), α‐galactosidase (cleaves terminal α‐galactose found in the B antigen, Figure 1B), or Peptide N‐glycosidase F (PNGaseF, cleaves N‐glycans) and subjected to immunoblotting using monoclonal anti‐A, anti‐B, or Ulex europeaus (UEA) lectin (binds H antigen). The monosaccharide composition of the purified glycoprotein was determined by high‐performance anion‐exchange chromatography.
Results: GPIbα purified from donors of blood type A and B showed reactivity with antibodies against blood group A and B antigens, respectively (Figure 1C). GPIbα purified from donors of blood type O, A, and B showed reactivity with UEA lectin (Figure 1D). Treatment of type A GPIbα with α‐N‐acetylgalactosaminidase but not PNGase F abolished anti‐A reactivity (Figure 1E). Treatment of type B GPIbα with α‐galactosidase eliminated anti‐B antigen reactivity (Figure 1C). Monosaccharide analysis showed a mean molar fucose‐to‐GPIbα ratio of 5.83 (Table 1).
Conclusion(s): Human platelet GPIbα carries ABO blood group antigens, most likely on O‐glycans. On average, a single molecule of GPIbα carries less than 6 ABO antigens. These data confirm and extend prior research that has suggested a role for GPIbα ABO antigen expression in hemostasis and thrombosis.


PB0377
Heparin and heparin proteoglycan‐mimetics activate platelets via PEAR1 and PI3Kbeta
C. Kardeby 1; A. Evans1; J. Campos1; C. Smith1; A. Slater1; E. Martin1; S. Severin2; A. Brill1; G. Pejler3; Y. Sun1; S. Watson4
1 University of Birmingham, Birmingham, England, United Kingdom; 2 Institute of metabolic and cardiovascular diseases, Toulouse cedex 04, Midi‐Pyrenees, France; 3 Uppsala University, Uppsala, Uppsala Lan, Sweden; 4 University of Birmingham, Birmingham, UK, Birmingham, England, United Kingdom
Background: Platelet Endothelial Aggregation Receptor 1 (PEAR1) is a single‐transmembrane orphan receptor primarily expressed on platelet and endothelial cells. Genetic variants of PEAR1 have repeatedly and independently been identified as top hits associated with cardiovascular diseases. We have reported that the sulfated polysaccharide fucoidan and synthetic fucoidan‐mimetics activate PEAR1 by binding to the 13th EGF‐like repeat containing a BXXBB sequence (B is a positively charged amino acid), a sequence similar to known heparin‐binding consensus sequences. This led us to speculate that PEAR1 may be a receptor for a heparin‐based proteoglycans.
Aims: The present study aims to investigate if PEAR1 is activated by heparin proteoglycans.
Methods: A proteoglycan‐mimetic was created by conjugating heparin to an albumin protein core. Nanobodies were raised against the 12‐14th EGF‐like repeats in PEAR1 through VIB Nanobody Core Technology, Brussels.
Results: We show that a heparin proteoglycan‐mimetic causes platelet aggregation and phosphorylation of PEAR1 and Akt in washed platelets, and aggregation in platelet‐rich plasma. The response to the heparin proteoglycan‐mimetic was abolished by a novel anti‐PEAR1 nanobody and by a selective inhibitor of PI3Kbeta, TGX 221. Unfractionated heparin stimulated full aggregation in washed platelets in 4 out of 7 donors after a delay of more than 30 minutes but not in platelet‐rich plasma.
Conclusion(s): We have shown that PEAR1 is a receptor for heparin and heparin‐based proteoglycan‐mimetics. Suggesting that the endogenous ligand for PEAR1 may be the only heparin‐containing proteoglycan expressed by mast cells, serglycin. Heparin and heparin proteoglycans, together with Fc‐epsilon RI‐alpha, represent three separate ligands for PEAR1; all of which are expressed by the mast cell. Further studies are required to address the significance of the mast cell–PEAR1 signaling axis in health and disease.

PB0383
Increased platelet procoagulant activity driven by fibrin‐GPVI interaction alters clot structure
J. Sandrin Gauer 1; C. Duval2; R. Xu3; F. Macrae2; C. Tiede2; D. Tomlinson2; S. Watson4; R. Ariëns5
1 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds., Leeds, England, United Kingdom; 2 University of Leeds, Leeds, England, United Kingdom; 3 Discovery and Translational Science Department, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds., Leeds, England, United Kingdom; 4 University of Birmingham, Birmingham, UK, Birmingham, England, United Kingdom; 5 Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, United Kingdom, Leeds, England, United Kingdom
Background: The GPVI‐pathway has previously been reported to be involved in procoagulant platelet activity via GPVI‐collagen binding. More recently, newly discovered fibrin‐GPVI interactions have been reported to play a role in thrombosis. Nevertheless, the impact of GPVI‐fibrin interaction on the development of procoagulant platelets, and how interfering with this interaction alters clot structure, remains to be established.
Aims: Our study aims were to investigate the role of GPVI‐fibrin interaction on the development of procoagulant platelet and, subsequently, to characterize the effects of modulating GPVI‐fibrin interaction on clot structure.
Methods: Procoagulant platelets were analyzed in platelet‐rich plasma (PRP) clots by SEM (wild‐type and GPVI‐deficient samples) and confocal microscopy. Procoagulant platelets were determined, and clot structure analysis assessing clot density, porosity, and retraction was performed in PRP or whole‐blood clots from healthy volunteers in the presence of tyrosine kinase inhibitors (PRT‐060318, ibrutinib and dasatinib), Affimer proteins inhibiting GPVI‐fibrin(ogen) interaction, and eptifibatide control.
Results: In the presence of fibrin (and absence of collagen), GPVI‐deficient clots, and clots where GPVI‐fibrin(ogen) interaction or GPVI‐signaling pathway were inhibited (by Affimer and tyrosine kinase inhibitors, respectively) showed fewer procoagulant platelets and decreased fibrin fiber density. Clot porosity was increased in the presence of all inhibitors, however, final clot weight following whole‐blood clot retraction was altered only by ibrutinib. The effects of tyrosine kinase inhibitors on procoagulant platelet number were exacerbated by eptifibatide, which also significantly impaired whole‐blood clot retraction.
Conclusion(s): Our results show that interaction of GPVI with fibrin plays a role in the development of procoagulant platelets and indicate that modulating this interaction has downstream effects on clot structure. Our findings suggest that targeting GPVI‐fibrin interaction may alleviate structural characteristics commonly associated with a pro‐thrombotic phenotype and, thereby, have important implications in development of novel anti‐thrombotic interventions.
PB0385
GPIbα shedding is restricted to the inner platelet membrane
K. Six 1; M. Van den Hauwe2; R. De Rycke3; I. Sagi4; E. Gardiner5; V. Compernolle6; H. Feys7
1 Belgian Red Cross Flanders, Ghent, Oost‐Vlaanderen, Belgium, 21. Transfusion Research Center, Belgian Red Cross Flanders, Ghent, Oost‐Vlaanderen, Belgium, 32. Department of Biomedical Molecular Biology and Expertise Centre for Transmission Electron Microscopy, Ghent University ‐ 3. Center for Inflammation Research and BioImaging Core, VIB, Ghent, Oost‐Vlaanderen, Belgium, 44. Department of Biological Regulation, Weizmann Institute of Science, Rehovot, HaMerkaz, Israel; 5 John Curtin School of Medical Research, The Australian National University, Canberra, Australian Capital Territory, Australia, 61. Transfusion Research Center, Belgian Red Cross Flanders ‐ 6. Blood services, Belgian Red Cross Flanders ‐ 7. Faculty of Medicine and Health Sciences, Ghent University, Ghent, Oost‐Vlaanderen, Belgium, 71. Transfusion Research Center, Belgian Red Cross Flanders ‐ 7. Faculty of Medicine and Health Sciences, Ghent University, Ghent, Oost‐Vlaanderen, Belgium
Background: GPIbα is an abundant transmembrane receptor on the platelet cytoplasmic membrane. Following strong and sustained platelet activation, ADAM17 catalyzes GPIbα proteolysis thereby releasing the soluble glycocalicin ectodomain.
Aims: We aimed to understand the enzyme‐substrate relationship in space and time.
Methods: ADAM17 and GPIbα were detected using labeled monoclonal antibodies in human resting platelets or after activation via GPVI and PARs, using immunofluorescence and western blot analysis.
Results: To chase glycocalicin, resting platelets were preincubated with anti‐GPIbα monoclonal antibody before activation. After activation, the majority of antibody was detected in the platelet pellet with only 11.2 ± 4.0% released in supernatant despite 82.1 ± 4.9% of GPIbα effectively shed. Furthermore, preincubation of resting platelets with anti‐GPIbα antibody 5G6, targeting the ADAM17 cleavage site, did not inhibit glycocalicin release (93 ± 6% relative to control without 5G6). When 5G6 was present during activation, glycocalicin release was partially inhibited (59 ± 11% relative to control, p = 0.02; n = 5). Finally, pretreatment of platelets with the membrane impermeable O‐sialoglycoprotein endopeptidase removed a 45 kDa N‐terminal fragment of surface‐exposed GPIbα but had no effect on the molecular weight or amount of glycocalicin released in platelet supernatant following activation‐dependent shedding. Statistically, >80% of GPIbα was an ADAM17 substrate, while <2% of released GPIbα was surface‐exposed. This agrees with our transmission electron microscopy findings using anti‐ADAM17 immunogold labeling, showing ADAM17 resides almost exclusively (96 ± 2% of label; n ≥ 25 platelets) within the inner membranes of both resting and activated platelets. This intracellular detection was confirmed by immunofluorescence microscopy and flow cytometry where successful ADAM17‐antigen detection required permeabilization with saponin. In addition, only membrane‐permeable sheddase inhibitors marimastat, GM6001 and KP‐457 significantly inhibited GPIbα shedding (88 ± 5%, 88 ± 4% and 95 ± 1%, respectively). In contrast, membrane‐impermeable ADAM17 inhibitors D1A12 (11 ± 11%) and recombinant prodomain (7 ± 10%; ≥6) could not inhibit metalloproteolysis of GPIbα.
Conclusion(s): The majority of GPIbα is a substrate for ADAM17, but surface‐exposed GPIbα is not.
Platelet Signaling
PB0396
Characterization of thrombogenic effects of platelet tyrosine phosphatase SHP2 inhibition using the allosteric inhibitor SHP099
D. Fernández de la Fuente 1; L. Hermida‐Nogueira2; J. Huang3; S. Veiras4; M. Kuijpers5; J. Heemskerk6; Á. García2
1 School for Cardiovascular Diseases (CARIM), Maastricht University, 6200 MD Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 2 Platelet Proteomics Group, Center for Research in Molecular Medicine and Chronic Diseases (CiMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain, Santiago de Compostela, Galicia, Spain; 3 Department of Biochemistry, CARIM, Maastricht University, 6200 MD Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 4 Department of Anesthesiology and Intensive Care Medicine, Clinical University Hospital of Santiago, Santiago de Compostela, Spain, Santiago de Compostela, Galicia, Spain; 5 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 6 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands
Background: Protein tyrosine phosphatase SHP2 is a known regulator of platelet signal transduction potentially suppressing GPVI‐induced activation. Currently, there are clinical trials with derivatives of the allosteric drug SHP099, which inhibits SHP2, as therapy for solid cancers. Human mutations of SHP2 gene, PTPN11, have been described to be linked to a variety of diseases.
Aims: Assessment of the potential thrombogenic effect of drug‐induced SHP2 inhibition.
Methods: Washed human platelets were incubated with SHP099 and stimulated with the GPVI agonists collagen‐related peptide (CRP) or collagen for aggregation and flow cytometric measurements. Drug effects of shear‐dependent thrombus and fibrin formation were determined using whole‐blood microfluidics over low and high collagen concentrations. In all assays, effects of co‐inhibition of P2Y12 ADP receptor blocker were examined. Thromboelastography was used to evaluate the effect on clot formation.
Results: Pharmacological inhibition of SHP2 in washed platelets did not alter GPVI‐dependent platelet aggregation regardless of the GPVI agonist used and P2Y12 inhibition. However, inhibition of SHP2 triggered faster integrin GPIIbIIIa opening, when assesed after GPVI activation. Moreover, in whole blood microfluidic assays, the drug enhanced the buildup of thrombus volume on collagen surfaces. When limiting the thrombus buildup by P2Y12 inhibition, this enhancement was abolished. In the presence of tissue factor and coagulation, the drug significantly enhanced thrombus volume, size, and contraction as well as fibrin formation. In thromboelastography, tissue factor‐induced blood clotting profiles were enhanced with tranexamic acid, preventing fibrinolysis.
Conclusion(s): The thrombogenic effect of SHP2 inhibition is not confined to early GPVI activation and requires the presence of shear and/or coagulation.
PB0400
Rac1 inhibition causes impaired GPVI signaling in human platelets through GPVI shedding and loss of PLCγ2 phosphorylation
R. Neagoe 1; D. Stegner2; B. Nieswandt3; S. Watson4; N. Poulter5
1 University of Würzburg, Wuerzburg, Bayern, Germany; 2 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 3 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 4 University of Birmingham, Birmingham, UK, Birmingham, England, United Kingdom; 5 Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, United Kingdom; Centre of Membrane Proteins and Receptors (COMPARE), Universities of Birmingham and Nottingham, Midlands, United Kingdom, Birmingham, England, United Kingdom
Background: Platelets are anucleated blood cells with a main role in primary hemostasis and thrombosis. Rac1 is a small Rho GTPase that is activated in platelets upon stimulation with various ligands, including collagen and thrombin, which are ligands for the glycoprotein VI (GPVI) receptor. Rac1‐deficient murine platelets present impaired lamellipodia formation, aggregation and reduced PLCγ2 activation, which is not dependent on the phosphorylation status of the protein. The effect of Rac1 inhibition on human platelets is less‐well studied.
Aims: To investigate the role of Rac1 in GPVI‐dependent human platelet activation.
Methods: Washed human platelets were stimulated by GPVI agonists (collagen and collagen‐related peptide) in the presence of the Rac1‐specific inhibitor EHT1864 and analyzed for platelet activation, aggregation, spreading, protein phosphorylation and GPVI shedding. The effect of Rac1 inhibition on GPVI receptor clustering was studied using single‐molecule super‐resolution microscopy.
Results: In human platelets, the inhibition of Rac1 by EHT1864 had no significant effect on GPVI clustering on collagen fibers but decreased the ability of platelets to spread, aggregate or becoming activated in response to GPVI agonists. Additionally, in contrast to what is observed in murine Rac1‐deficient platelets, EHT1864 enhanced GPVI shedding in both resting and activated platelets and reduced the phosphorylation levels of PLCγ2.
Conclusion(s): Rac1 activity is required for both human and murine platelet activation in response to GPVI‐ligands, but Rac1’s mode of action differs between both species.
VPB0408
Molecular mechanisms of apoptosis in platelets and megakaryocytes in pediatric immune thrombocytopenia
F. Franzoso 1; M. Schmuge2; C. Gowin2; T. Stein2
1 University Children's Hospital Zürich, Zürich, Zurich, Switzerland; 2 University Children's Hospital Zürich, Zurich, Zurich, Switzerland
Background: Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder characterized by low platelets counts and a mostly mild‐ and in rare occasion life threatening bleeding symptoms. Previous studies, also from our group, have demonstrated a role of platelet apoptosis in the pathogenesis of childhood ITP. A mechanistic understanding of the ITP pathogenesis is still lacking, and most treatments increase the platelet counts and prevent the severe clinical manifestations a limited number of the patients only.
Aims: In our present study, we aimed to investigate apoptosis molecular mechanisms in ITP platelets and their precursors, the megakaryocytes (MKs).
Methods: We isolated platelets in vitro as described before also by our group (1,2). To differentiate MKs from PBMCs peripheral blood from acute and chronic ITP patients and controls, we used an adapted protocol from Salunke et al (3). As control cells we used the human megakaryoblastic cell line MEG‐01, treated for 4 h with plasma from acute and chronic ITP patients and healthy controls. We performed silencing experiments in control and ITP platelets and MKs, MEG‐01 by using siRNA caspase‐3 or suppressing apoptosis by treating with pan‐caspase inhibitors (Z‐VAD‐FMK), and, in comparison, with the apoptosis inducer ABT‐737. We determined the expression at mRNA levels of apoptosis pathway regulatory genes (Bax, Bcl‐2, Bid, Bak, Apaf‐1, cytochrome‐c, caspase‐3, −8, −9 and clusterin [CLU]) by qRT‐PCR.
Results: We could demonstrate increased expression levels of some apoptotic genes such as CLU, cytochrome‐c, Bax and Bak in ITP platelets and MKs compared to healthy controls from 5 ITP patients as well as in MEG‐01 treated cells that could be silenced by using siRNA caspase‐3.
Conclusion(s): Our results indicate a possible impairment of apoptosis pathway via upregulation of Clusterin and Bax in platelets and in their producers MKs that can lead to platelet destruction in ITP disease.
PB0395
Avidity‐dependent differential signaling by CLEC‐2 receptors
S. Kunapuli1; C. Dangelmaier 1; J. Kostyak2
1 Temple University School of Medicine, Philadelphia, Pennsylvania, United States; 2 Temple University School of Medicine, Philladelphia, Pennsylvania, United States
Background: C type lectin receptor family members play a role in many cells including platelets where they are crucial in the separation of lymphatic and blood vessels during development. The CLEC‐2 receptor contains the canonical intracellular hemITAM motif through which it signals to activate Syk. One proposed hypothesis for the signaling cascade is that Syk bridges two receptors through phosphorylated hemITAM motifs. We demonstrated that the phosphorylated hemITAM activates PI3 kinase/Tec kinase pathways to activate Syk.
Aims: To address this controversy, we used a CLEC‐2 selective agonist and studied the role of Tec kinases in platelet activation.
Methods: We activated platelets with different concentrations of CLEC‐2 monoclonal antibodies in the presence and absence of different inhibitors.
Results: Platelet activation and downstream signaling were abolished in murine and human platelets in the presence of the Tec kinase inhibitor Ibrutinib when a low concentration of a CLEC‐2 antibody was used to crosslink CLEC‐2 receptors. This inhibition was overcome by increasing concentrations of the CLEC‐2 antibody. Similar results were obtained in Lyn null platelets or X‐linked immunodeficient (Xid) mouse platelets, with an inactivating mutation in Btk.
Conclusion(s): We conclude that at low crosslinking (avidity) conditions of CLEC‐2, Tec kinases play an important role in the activation of Syk but, at higher crosslinking (avidity) conditions, their role becomes less important and other mechanisms take over to activate Syk.


PB0407
Establishing the signaling pathways regulating reversible platelet integrin activation
J. Zou 1; S. Sun1; I. De Simone2; H. ten Cate3; P. de Groot4; J. Heemskerk5; M. Roest6; B. de Laat7
1 Maastricht University, MAASTRICHT, Limburg, Netherlands; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, MAASTRICHT, Limburg, Netherlands; 3 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 4 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands; 5 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 6 Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 7 Department of Functional Coagulation, Synapse Research Institute, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands
Background: For long it is known that collagen‐ and thrombin‐induced platelet signaling pathways are intrinsically reversible, and coupled to these the conformation change leading to integrin αIIbβ3 activation required for platelet aggregation. However, which signaling pathways contribute to a more or less persistent platelet aggregation in hemostasis and thrombosis is still not fully elucidated.
Aims: To establish the platelet signaling pathways induced by collagen or thrombin receptors that regulate early and late integrin αIIbβ3 activation.
Methods: Platelets were activated dose‐dependently with the glycoprotein VI (GPVI) agonist CRP‐XL or any of the PAR agonists thrombin, SFLLRN and AYPGKF. Flow cytometry was used to measure integrin αIIbβ3 activation (FITC‐PAC1 mAb) and P‐selectin expression over time. A panel of pharmacological inhibitors was used to block specific signaling pathways before or after the agonist addition.
Results: When pre‐treated with blocking compounds, agonist‐induced integrin αIIbβ3 activation decreased in the order: protein kinase C (PKC) > glycogen synthase kinase 3 (GSK3) > β‐arrestin > phosphatidylinositol‐3‐kinase (PI3K) > [Ca2+]i elevation. Blocking compound application at 10–20 min after agonist CRP‐XL or TRAP6 resulted in the same potency order of integrin closure. Expression of P‐selectin was not antagonized by late application of compounds. After selective stimulation by PKC agonist PMA, integrin activation was prevented, and integrin closure was enhanced, again in the order of compounds blocking PKC > GSK3 > β‐arrestin > PI3K > [Ca2+]i elevation. Induction of [Ca2+]i elevation by thapsigargin resulted in limited integrin activation that was only enhanced combined with weaker agonists such as TRAP6 or PMA.
Conclusion(s): Similarly, for GPVI and PAR agonists, PKC isoforms provided the strongest mechanism of short‐ and long‐term integrin αIIbβ3 activation. This role is only enhanced by additional [Ca2+]i elevation with weak platelet agonists.
PB0402
Elucidation of Specific Platelet Signaling Pathways Perturbed in SLFN14‐Mutated Patients with Inherited Macrothrombocytopenia and Bleeding
R. Stapley; L. Garcia‐Quintanilla; C. Smith; N. Morgan
University of Birmingham, Birmingham, England, United Kingdom
Background: SLFN14 is a novel hematopoietic‐specific endoribonuclease with an unidentified role. SLFN14 missense mutations have previously been found in five unrelated families with inherited thrombocytopenia and platelet function defects. However, it remains unclear how SLFN14 mutations contribute to platelet function defects in humans.
Aims: To investigate platelet activation and signaling pathways in SLFN14‐mutated patients with platelet function defects, macrothrombocytopenia and bleeding.
Methods: Whole blood counting and platelet function assays including platelet aggregation and flow cytometry were used to assess overall platelet function in patients from three families with missense mutations in SLFN14. Signaling downstream of the major platelet receptors GPVI, PAR‐1 and ADP receptors was also assessed by western blot.
Results: Patients with mutations in SLFN14 (K219N, V220D and K218E) all presented with macrothrombocytopenia and reduced aggregation responses to collagen, ADP and PAR‐1‐receptor activating peptide. Transmission electron microscopy of platelets also showed reduced numbers of dense granules compared to controls, correlating with the reduced ATP secretion observed by lumiaggregometry (n = 1–5 patients per family). K219N and V220D patients (n = 2 patients per mutation) show reduced phosphorylation of Syk‐Y525/526, LAT‐Y200 and PLCg2‐Y1217 in response to collagen and collagen‐related peptide.
Conclusion(s): SLFN14‐mutated patients have macrothrombocytopenia and significant platelet function defects in response to collagen, ADP and PAR‐1‐peptide. Reduced GPVI downstream signaling in K219N and V220D patients suggest SLFN14 mutations may mediate ITAM receptor signaling upon activation. These defects in important hemostatic pathways explain the patients' mild to moderate bleeding. Further investigation is however still required to determine how SLFN14 mediates effects on these pathways.
PB0401
Endothelial‐induced regulation of platelet signaling under flow: establishing the endothelial‐controlled platelet phosphoproteome
I. Provenzale 1; F. Solari2; C. Schönichen3; D. Fernández de la Fuente4; S. Brouns5; M. Kuijpers6; P. van der meijden7; J. Gibbins8; A. Sickmann9; C. Jones8; J. Heemskerk10
1 Dept of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands and Institute for Cardiovascular and Metabolic Research (ICMR), School of Biological Sciences, University of Reading, Reading, United Kingdom, Reading, England, United Kingdom; 2 Leibniz‐Institut für Analytische Wissenschaften – ISAS – e.V. Dortmund, Germany, Dortmund, Nordrhein‐Westfalen, Germany, 3 Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Center for Thrombosis and Hemostasis, University Medical Center of the Johannes Gutenberg‐University of Mainz, Germany, Maastricht, Limburg, Netherlands; 4 School for Cardiovascular Diseases (CARIM), Maastricht University, 6200 MD Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 5 Dept of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands; 6 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 7 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands; Thrombosis Expertise Center, Heart + Vascular Center, Maastricht University Medical Center, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 8 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom; 9 Department of Protein Dynamics, Leibniz Institute for Analytical Sciences ‐ ISAS‐e.V., Dortmund, Germany; Medizinische Fakultät, Medizinisches Proteom‐Center, Ruhr‐Universität Bochum, Germany; Department of Chemistry, College of Physical Sciences, University of Aberdeen, United Kingdom., Dortmund, Nordrhein‐Westfalen, Germany, 10 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands
Background: Whole blood based microfluidic assays represent new tools to model thrombosis and hemostasis in vitro. Yet these assays often lack key vascular elements that regulate platelet activation and control thrombus formation.
Aims: Development of an endothelialised microfluidic system to reveal mechanisms of endothelial control of platelet and coagulant processes. Use of a phosphoproteomic approach to delineate endothelial effects on platelets.
Methods: Human umbilical vein endothelial cells (HUVEC), cultured in microfluidic channels coated with collagen and tissue factor, were used for whole blood perfusion (shear rate 1000/s). Fluo4‐loaded platelets and labeled fibrinogen were added to measure platelet activation and fibrin formation. Calcium responses were also measured in isolated platelets exposed to HUVEC, and post‐activated with thrombin or collagen‐related peptide (CRP‐XL). Guided by functional assays, (un)stimulated platelets exposed to HUVEC (n = 3 donors, 11 conditions) were used for label‐free phosphoproteomic analysis. Peptides were generated with a bottom‐up proteomic approach, phopsphopeptides were enriched using Fe(III)‐IMAC based workflow and analyzed via LC–MS/MS.
Results: Whole blood perfusion over sub‐confluent HUVEC resulted in reduced platelet adhesion with surface area coverage (SAC) of 4.6 ± 3.4%, when compared to absence of HUVEC (40.4 ± 6.9 %SAC, p < 0.001). Residual thrombi were restricted to areas between HUVEC, and the platelets had low Ca2+ responses. Under coagulant condition, HUVEC strongly delayed the fibrin formation (p < 0.01). Under stasis, pre‐incubation with HUVEC suppressed the platelet Ca2+ responses to thrombin or CRP‐XL. In mass‐spectrometric analyses, 5463 phospho‐peptides were identified (77.5% Ser, 15.1% Thr, 7.4% Tyr) with good correlation between biological replicates (Pearson 0.86). Multiple regulated proteins were identified with consensus PKA and PKG phosphorylation motives, pointing to activation control via prostacyclin, nitric oxide and other endothelial‐derived biomolecules, being antagonized by platelet post‐stimulation.
Conclusion(s): Endothelial cells negatively regulate platelet responses (under flow) through phosphorylation changes affecting collagen and thrombin receptor‐induced activation involving multiple pathways.
PB0406
Differential regulation of GPVI‐stimulated Y/S phosphorylation of Syk and Btk by both PKC and PKA in human platelets
P. Zhang 1; F. Solari2; U. Walter3; J. Heemskerk4; A. Sickmann5; K. Jurk6
11Department of Protein Dynamics, Leibniz Institute for Analytical Sciences ‐ ISAS‐e.V., Dortmund, Germany; 2 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Germany; 3Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands, Dortmund, Nordrhein‐Westfalen, Germany; 2 Leibniz‐Institut für Analytische Wissenschaften – ISAS – e.V. Dortmund, Germany, Dortmund, Nordrhein‐Westfalen, Germany, 31 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Germany;, Mainz, Rheinland‐Pfalz, Germany; 4 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 5 Department of Protein Dynamics, Leibniz Institute for Analytical Sciences ‐ ISAS‐e.V., Dortmund, Germany; Medizinische Fakultät, Medizinisches Proteom‐Center, Ruhr‐Universität Bochum, Germany; Department of Chemistry, College of Physical Sciences, University of Aberdeen, United Kingdom., Dortmund, Nordrhein‐Westfalen, Germany; 6 University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: Platelet glycoproteins (GP), including GPIbα, GPVI, αIIbβ3 integrin and Clec‐2, activate Src family kinases resulting in the membrane‐recruitment of both spleen tyrosine kinase (Syk) and Bruton tyrosine kinase (Btk), followed by their Y‐ phosphorylation (Y352, Y525/526 and Y551, respectively) and activation. Activated Syk and Btk stimulate phospholipase Cγ2, elevate IP3, Ca2+, 1,2‐diacylglycerol and increase protein kinase C (PKC) activity. Syk and Btk are crucial for platelet activation, but their regulation by other major signaling pathways is not well understood.
Aims: Here we investigated the regulation of Syk/Btk Y/S phosphorylation by PKC and protein kinase A (PKA) in GPVI‐stimulated platelets.
Methods: Aggregation of washed human platelets was stimulated by convulxin and monitored by light transmission aggregometry. Platelet PKC was activated with phorbol dibutyrate (PDBu) or inhibited with GF109203X (GFX), while PKA was activated with iloprost. Phospho‐antibodies against Syk/Btk phospho‐sites were used to measure their time‐dependent (10–300 s) regulation in convulxin‐stimulated platelets by western blotting.
Results: Convulxin induced strong platelet aggregation and a rapid, transient up‐regulation of Syk at pY525/526, pY352, pS297 and of Btk at pY551, pY223, pS180. PDBu alone induced platelet aggregation, major increases of Syk pS297 and Btk pS180, but only minor changes in Btk pY551. GFX partially inhibited convulxin‐induced aggregation, abolished convulxin‐stimulated pS‐sites, but increased convulxin‐induced Syk/Btk pY‐sites. Effects of PKA activation resembled those of PKC inhibition. Currently, additional proteins of the GPVI/Syk/Btk signalosome are analyzed.
Conclusion(s): In human platelets, GPVI‐stimulated PKC isoforms mediate Syk S297 and Btk S180 phosphorylation and negatively affect their Y‐phosphorylation. PKA stimulation suppresses the PKC‐mediated phosphorylation events. This implies that the GPVI/Syk/Btk signalosome is regulated by serine/threonine protein kinases, thus strongly modulating the Syk/Btk activation signal in human platelets. This study was approved by our local Ethics committee and followed the Declaration of Helsinki. P.Z. is supported by EU TICARDIO No. 813409.
PB0399
Evidence for a PI3‐kinase independent pathway in the regulation of Rap1b activation downstream of the P2Y12 receptor in platelets
S. Kunapuli; C. Dangelmaier
Temple University School of Medicine, Philadelphia, Pennsylvania, United States
Background: Platelet activation by adenosine diphosphate (ADP) is mediated through two G‐protein‐coupled receptors, P2Y1 and P2Y12, which signal through Gq and Gi, respectively. P2Y1 stimulation leads to phospholipase C activation and an increase in cytosolic calcium necessary for CalDAG‐GEF1 activation. Engagement of P2Y12 inhibits adenylate cyclase, which reduces cAMP, and activation of PI3‐kinase, which inhibits RASA3 resulting in sustained activated Rap1b.
Aims: We evaluated the role of PI3 kinase in the activation of Rap1B downstream of ADP receptors.
Methods: In this study, we activated human platelets with 2‐MeSADP in the presence of LY294002, a PI3‐kinase inhibitor, AR‐C69931MX, a P2Y12 antagonist, or MRS2179, a P2Y1 antagonist. We measured Rap1B activation by a pulldown assay with a kit from cell signaling. We measured the phosphorylation of Akt on Ser473 as an indicator of PI3‐kinase activity.
Results: LY294002 and ARC69931MX abolished 2MeSADP‐induced Akt phosphorylation. MRS2179 reduced 2MeSADP‐induced Akt phosphorylation but did not abolish it. Rap1b activity, however, was only reduced, but not ablated, using LY294002 and was completely inhibited by ARC69931MX or MRS2179. Similarly; 2MeSADP‐induced Akt phosphorylation was abolished in the P2Y12 knock out mice platelets without much effect on Rap1B. Conversely, Rap1B activation was abolished, while Akt phosphorylation was unaffected, in the P2Y1 knock out mouse platelets
Conclusion(s): These data suggest that ADP‐induced Rap1b activation requires both P2Y1 and P2Y12. In addition, although stimulation of P2Y12 results in PI3‐kinase activation leading to Akt phosphorylation and Rap1b activation, Rap1b activation can occur independently of PI3‐kinase downstream of P2Y12. Thus we propose that the P2Y12 receptor can regulate Rap1 through RASA3 in a pathway independent of PI3‐kinase.


PB0405
Loss of zinc transporters ZIP1 and ZIP3 augments platelet reactivity in response to G protein‐coupled receptor agonists and accelerates thrombus formation in vivo
A. Elgheznawy1; P. Öftering2; M. Englert3; F. Kaiser4; C. Kusch3; U. Gbureck5; M. Bösl6; B. Nieswandt7; T. Vögtle 8; H. Hermanns1
1 Medical Clinic II, Division of Hepatology, University Hospital Würzburg, Würzburg, Bayern, Germany; 2 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Germany, Würzburg, Bayern, Germany; 3 Institute of Experimental Biomedicine I, University Hospital and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 4 Department for Functional Materials in Medicine and Dentistry, University Hospital Würzburg, Würzburg, Bayern, Germany; 5 Department for Functional Materials in Medicine and Dentistry, University Hospital Würzburg, Wür, Bayern, Germany; 6 Institute of Experimental Biomedicine I, University Hospital and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, 97,080 Würzburg, Germany, Würzburg, Bayern, Germany; 7 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 8 University Hospital Würzburg and University of Würzburg, Würzburg, Bayern, Germany
Background: Zinc (Zn2+) is an essential micronutrient and also considered as an important mediator for thrombosis and hemostasis. Zn2+ transporters ‐ ZIPs and ZnTs ‐ are widely expressed in eukaryotic cells, including platelets. However, our understanding of the transport mechanisms that regulate Zn2+ homeostasis in platelets is limited.
Aims: To explore the potential role of the well‐known Zn2+ transporters ZIP1 and ZIP3 in maintaining platelet Zn2+ homeostasis and in the regulation of platelet function.
Methods: Using mice globally lacking ZIP1 and ZIP3 (ZIP1/3 DKO), we investigated the role of the Zn2+ importers in various in vitro experiments and in vivo experiments of platelet function.
Results: While inductively coupled plasma ‐ mass spectrometry (ICP‐MS) measurements indicated unaltered overall Zn2+ concentrations in platelets of ZIP1/3 DKO mice, we observed a significantly delayed and less efficient Zn2+ release upon thrombin‐stimulated platelet activation. This resulted in a hyperactive platelet response not only in response to thrombin, but also towards other G protein‐coupled receptor (GPCR) agonists. Immunoreceptor tyrosine‐based activation (ITAM)‐coupled receptor agonist signaling, however, was unaffected. Augmented GPCR responses were accompanied by enhanced Ca2+ signaling and PKC activation. Further functional analysis of ZIP1/3 double deficient mice revealed enhanced platelet aggregation, bigger thrombus volume under flow ex vivo and faster in vivo thrombus formation.
Conclusion(s): The current study identifies ZIP1 and ZIP3 as important regulators for the maintenance of platelet Zn2+ homeostasis and function. Funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (project number 374031971‐TRR 240).
PB0397
PPAR‐alpha inhibition of platelet activation and thrombosis
L. Stanger1; A. Yamaguchi1; R. Adili2; M. Holinstat 3
1 Department of Pharmacology, University of Michigan, Ann Arbor, Michigan, United States, 2 University of Michigan, ANN ARBOR, Michigan, United States; 3 University of Michigan, Ann Arbor, Michigan, United States
Background: Eicosanoids were recently shown to regulate platelets through activation of peroxisome proliferator‐activated receptors (PPARs). There exists a significant gap in our knowledge of how a transcription regulator such as PPAR can regulate activity in the platelet. Importantly, determining the underlying mechanism by which PPAR limits platelet activation in a non‐genomic manner represents a significant gap in our understanding of how fatty acids modulate platelet function to decrease the risk of a thrombotic event.
Aims: To delineate the mechanisms by which eicosanoid activation of PPAR‐alpha attenuates platelet activation and clot formation.
Methods: co‐IP experiments were conducted to determine the protein complex interactions of PPAR with other proteins and signaling pathways in the platelet. Additionally, we have assessed PKC activation and calcium mobilization and investigated the signaling correlates in platelet activity including small G protein Rap1, integrin GPIIb/IIIa, and platelet aggregation. Finally, we evaluated the role of PPAR in regulating thrombosis in vivo in the mouse.
Results: PKC activation and calcium mobilization were found to be dependent on PPAR activation. Rap1‐GTP binding was decreased following PPAR activation and aggregation, granule secretion, and integrin activation was all attenuated in PPAR activated condition, but were rescued when PPAR activity was blocked. Finally, the extent of clot formation was significantly decreased with PPAR activation and was reversed in animals lacking PPAR.
Conclusion(s): this study defined PPAR as an important signaling protein regulating the extent of platelet activity ex vivo as well as the ability to form a clot in vivo. These studies help to determine that PPAR‐alpha activation represents a novel method for limiting platelet activation and thrombosis and this PPAR may represent a new target for directed antiplatelet therapy.
PB0398
Fatty acids negatively regulate platelet function through formation of noncanonical 15‐lipoxygenase‐derived eicosanoids
A. Yamaguchi1; B. Tourdot2; S. Perry3; G. Lee4; N. Rhoads1; C. van Hoorebeke3; J. Sorrentino3; J. Yeung2; C. Li5; C. Freedman3; T. Holman6; M. Holinstat 5
1 Department of Pharmacology, University of Michigan, Ann Arbor, Michigan, United States; 2 Cincinnati Children's Hospital, Ann Arbor, Michigan, United States; 3 University of California Santa Cruz, Santa Cruz, California, United States; 4 Vanderbilt University, Ann Arbor, Michigan, United States; 5 University of Michigan, Ann Arbor, Michigan, United States; 6 University of California Santa Cruz, Santa Cruz, Michigan, United States
Background: The antiplatelet effect of polyunsaturated fatty acids is primarily attributed to its metabolism to bioactive eicosanoids by oxygenases such as lipoxygenases (LOX). Platelets have demonstrated the ability to generate 15‐LOX‐derived eicosanoids; however, whether 15‐LOX is in the platelet and further required to the formation of its eicosanoids remain unclear.
Aims: We sought to elucidate whether 15‐LOX is required for the formation of 15‐LOX eicosanoids in the platelet and determine the mechanistic effects of 15(S)‐hydroxyeicosatrienoic acid (15‐HETrE) and 15(S)‐hydroxyeicosatetraenoic (15‐HETE) on platelet reactivity.
Methods: Platelet aggregation and intracellular signaling were assessed in human platelets treated with the 15‐LOX eicosanoids. Leukocyte‐depleted platelets were treated with inhibitors for 15‐LOX‐1 (ML351), 15‐LOX‐2 (1A1U) or 12‐LOX (ML355) prior to agonist‐induced aggregation. To assess 15‐LOX expression in platelets, 15‐LOX‐1, 15‐LOX‐2 and 12‐LOX enzymes were used as controls.
Results: Treatment with 15‐HETrE or 15‐HETE was shown to attenuate collagen‐induced platelet aggregation (Figure 1A) due to inhibition of PKC activation (Figure 1B), calcium mobilization (Figure 1C), ⍺IIbβ3 activation (Figure 1D), and granule secretion (Figure 1E‐F). While 15‐HETrE was demonstrated to inhibit platelets through activation of PPARβ (Figure 2A), 15‐HETE was shown to activate PPAR⍺ (Figure 2B). Although platelets treated with DGLA were shown to form 15‐HETrE, treatment with 15‐LOX‐1 or 15‐LOX‐2 inhibitor did not rescue collagen‐induced platelet aggregation (Figure 2C) or ATP secretion (Figure 2D). Additionally, expression of 15‐LOX‐1 (Figure 2E), but not 15‐LOX‐2 (Figure 2F), was decreased in leukocyte‐depleted platelets compared to non‐depleted platelets.
Conclusion(s): These findings suggest that 15‐LOX eicosanoids regulate platelet activity, but are formed through a 15‐LOX‐independent pathway in platelets. Furthermore, since 15‐LOX is highly expressed in leukocytes, it is reasonable to suggest that its regulation of platelet reactivity may involve a transcellular mechanism between platelets and leukocytes through the formation of the eicosanoids. This study aids to better understand role of 15‐LOX on regulation of platelet activity.


PB0403
Real‐time assessment of inter‐platelet communication and coordination of calcium signals by connexin gap junctions
K. Taylor 1; A. Bye2; J. Mitchell3; S. Ray1; J. Gibbins4
1 University of Reading, Reading, England, United Kingdom; 2 St Georges University London, London, England, United Kingdom; 3 University of Birmingham, Birmingham, England, United Kingdom; 4 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: Following vascular injury, platelets rapidly aggregate to prevent blood loss and promote wound repair. Mechanisms regulating inter‐platelet communication and coordination of these responses are incompletely understood. Platelets express several members of the connexin gap junction family that form intercellular channels. Selective inhibitory peptides of these channels reduce thrombus formation in vivo, but inter‐cellular signals conveyed by platelet connexins remain unclear.
Aims: To evaluate the role of connexins in coordination of agonist‐evoked platelet calcium signaling.
Methods: Calcein diffusion between platelets was employed as a marker of connexin activation during thrombus formation assays. Briefly, 10% of platelets were co‐stained with calcein and CD61 antibodies, allowing monitoring of calcein diffusion beyond these ‘donor’ platelets. For calcium imaging, platelets were spread on fibrinogen and TRAP6‐evoked calcium transients were monitored by confocal microscopy.
Results: Under arteriolar shear we formed thrombi and tracked movement of calcein dye from donor platelets into the cytosol of adjacent cells. To evaluate the role for connexins in this process, we used the pan‐connexin inhibitor carbenoxolone or the connexin 37 (Cx37)‐specific peptide inhibitor 37,43Gap27. In the presence of carbenoxolone calcein diffusion was reduced by 60%, whilst 37,43Gap27 led to a 30% reduction. We therefore investigated whether multiple connexin isoforms contributed to inter‐cellular communication using a peptide cocktail to target the major platelet connexins (Cx37, Cx40 and Cx62). This approach reduced dye transfer to the same extent as carbenoxolone. Finally, we studied TRAP6‐evoked calcium signals in immobilized platelets and calculated the time interval between calcium spikes in adjacent cells (lag time). Incubating of platelets with carbenoxolone reduced coordination of calcium signals and increased lag time.
Conclusion(s): Connexins facilitate coordination of inter‐platelet calcium signals, which may involve diffusion of cytosolic calcium or IP3. It is interesting to speculate that connexins may relay signals between the core and shell to regulate thrombus formation.
VPB0409
GRK2 regulates ADP signaling in platelets
X. Zhao 1; M. Cooper1; Y. Yarman1; J. Michael1; K. Chuprun2; W. Koch2; S. McKenzie1; M. Tomaiuolo1; T. Stalker1; L. Zhu3; P. Ma1
1 Thomas Jefferson University, Philadelphia, Pennsylvania, United States; 2 Temple University, Philadelphia, Pennsylvania, United States; 3 Soochow University, Soochow, Jiangsu, China (People's Republic)
Background: The critical role of GRK2 in regulating cardiac function has been well documented for over three decades. Therefore, targeting GRK2 has been extensively studied as a novel approach to treat cardiovascular disease. However, little is known about its role in hemostasis and thrombosis.
Aims: To investigate the function of GRK2 during platelet activation and how GRK2 regulates the hemostatic response to injury.
Methods: We took advantage of using a megakaryocyte/platelet‐specific GRK2−/− mouse model. We examined the impact of GRK2 deficiency on platelet activation and platelet accumulation at the site of vascular injury.
Results: Deletion of GRK2 in mouse platelets causes increased platelet accumulation following laser‐induced injury in cremaster muscle arterioles, particularly in the shell region of thrombi. GRK2−/− platelets have increased platelet aggregation in response to ADP, but not to PAR4 receptor agonist, TxA2, or convulxin. Furthermore, GRK2−/− platelets retain the ability to aggregate in response to ADP re‐stimulation, indicating that GRK2 contributes to ADP receptor desensitization. Underlying these changes in GRK2−/− platelets is an increase in Ca2+ mobilization, RAP1 activation, and Akt phosphorylation in response to ADP, and an attenuated rise of cAMP levels in response to ADP in the presence of prostaglandin I2. Cangrelor treatment eliminates the phenotypic difference in platelet accumulation between WT and GRK2−/− mice at the site of injury. In addition, absence of GRK2 increases ADP‐induced pulmonary thromboembolism. Pharmacologic inhibition of GRK2 activity in human platelets increases platelet activation in response to ADP. Finally, we show that GRK2 binds to endogenous Gβγ subunits during platelet activation.
Conclusion(s): Taken together, we have demonstrated for the first time that 1) GRK2 regulates ADP signaling via P2Y1 and P2Y12, 2) GRK2 interacts with Gβγ and functions as a signaling hub in platelets for fine‐tuning GPCR signaling, and 3) maintaining GRK2 activity in platelets may be beneficial for prevention of thrombotic diseases.
PB0404
Connexins are post‐translationally modified in activated platelets; potential role of calpain
A. Elgheznawy1; K. Taylor 2; S. Parkes2; J. Gibbins3
1 Medical Clinic II, Division of Hepatology, University Hospital Würzburg, Würzburg, Bayern, Germany; 2 University of Reading, Reading, England, United Kingdom; 3 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom
Background: Platelets express several connexins that assemble as hemichannels at the plasma membrane or dock to form gap junctions with adjacent cells facilitating the release or transfer of bioactive molecules. Selective inhibition of connexin 37 (Cx37), Cx40 or Cx62 reduces thrombus formation in vivo and limits platelet activation in vitro. To date, several mechanisms of post‐translational modification (PTM) are known to regulate the activity of connexins. The regulation of platelet connexins by PTMs warrants further investigation.
Aims: We aimed to investigate the role of proteolysis‐mediated PTM of Cx62 by calpain.
Methods: Proteolytic cleavage of Cx62 was assessed by immunoblot and platelet function was studied by aggregometry and calcium flux assays. We tracked efflux and transfer of calcein dye to evaluate Cx62 activation in single platelets by flow cytometry or within thrombi formed under arteriolar shear rates.
Results: We performed in silico analysis of Cx62 to scan for post‐translational modification sites and identified a potential calpain cleavage site within its extracellular face. Immunoblot analysis revealed a cleavage product of Cx62 that appeared following platelet activation, which was blocked by calpeptin. To understand whether Cx62 PTM has functional consequences on hemichannel activities, we designed a mimetic peptide for the predicted cleaved Cx62 N‐terminal sequence (62Pept‐NT) as a decoy substrate to diminish Cx62 PTM in platelets. Treatment of platelets with 62Pept‐NT delayed calcein dye release from activated platelets with no effect on platelet aggregation. Finally, we studied the functional consequence of calpain cleavage of platelet Cx62 within thrombi. Under control conditions we observed real‐time transfer of calcein dye between platelets. Indicative of a role for calpain, addition of 62Pept‐NT reduced intercellular communication by 30%.
Conclusion(s): Our data demonstrate the presence of a calpain cleavage site on the extracellular face of Cx62 that regulates the opening of hemichannels and gap junctions.
Vascular Biology
Blood Cells and Vessel Wall
PB1266
Maphosphamide, a cyclophosphamide analog, increases proinflammatory and permeability responses on endothelial cells in vitro
J. Martinez‐Sanchez 1; R. Pascual‐Diaz2; M. Palomo1; A. Moreno‐Castaño3; H. Ventosa2; M. Rovira4; G. Escolar2; E. Carreras1; M. Diaz‐Ricart5
1 Josep Carreras Leukemia Research Institute, Hospital Clínic de Barcelona, Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 2 Hematopathology, Department of Pathology, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 3 HEMATOPATHOLOGY, PATHOLOGY DEPARTMENT, CENTRE DE DIAGNÒSTIC BIOMÈDIC. HOSPITAL CLINIC BARCELONA, BARCELONA, Catalonia, Spain; 4 Stem Cell Transplantation Unit, Hospital Clínic de Barcelona, IDIBAPS, Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 5 Hospital Clinic, IDIBAPS, University of Barcelona, Spain, Barcelona, Catalonia, Spain
Background: Graft‐versus‐host disease (GVHD) remains a major complication of allogeneic hematopoietic cell transplantation (allo‐HCT). Allo‐HCT causes endothelial activation and damage. In addition, endothelial dysfunction plays a key role in the GVHD pathophysiology. The immunosuppressive drugs administered during allo‐HCT exhibit a detrimental impact on the endothelium. Administration of post‐transplant cyclophosphamide has significantly decreased GVHD incidence.
Aims: To evaluate the effect of maphosphamide (MPH), a cyclosphosphamide analog with direct activity, on endothelial cells in comparison to cyclosporine A (CSA), a standard immunosuppressant with known damaging effect on the endothelium.
Methods: Human microvascular endothelial cells (ECs) in culture were exposed (48 h) to MPH (2 μg/mL) and CSA (200 ng/mL), to explore changes in markers of endothelial damage, such as: i) the adhesion receptor VCAM‐1, ii) production of VWF, iii) the cell permeability protein VE‐cadherin, and iv) activation of the inflammation‐related protein p38 MAPK.
Results: Both MPH and CSA induced statistically significant increments in VCAM‐1 expression in comparison to control cells (fold increases: 2.8 ± 0.4 and 2.6 ± 0.3, respectively, p < 0.01). While the production of VWF was enhanced in response to CSA (fold increase: 1.4 ± 0.2, p < 0.05), it was reduced with MPH (0.8 ± 0.1, p < 0.05). VE‐cadherin expression decreased significantly in response to MPH (fold increase: 0.7 ± 0.1, p < 0.01), whereas no effect was observed with CSA. Activation of p38 MAPK occurred in response to both compounds but it was higher and faster for CSA.
Conclusion(s): Our results demonstrate that maphosphamide exerted a proinflammatory effect on endothelial cells similar to the observed with cyclosporine A. The decreased expression of VE‐cadherin with maphosphamide could indicate an increased endothelial permeability. Therefore, the cyclophosphamide analog is not exempt of an activating action on the endothelium. Further studies are needed to explore the mechanisms of cyclophosphamide in this setting.
PB1272
CRISPR‐Based Knockout Red Blood Cells as Tools to Study Red Blood Cell Surface Protein Involvement in Thrombosis
N. Ouazzani Chahdi 1; J. Meijers2; R. van Bruggen3
1 Sanquin Research, University of Amsterdam, Utrecht, Utrecht, Netherlands; 2 Department of Molecular Hematology, Sanquin Research, Amsterdam, the Netherlands, Department of Experimental Vascular Medicine, Amsterdam UMC, University of Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Molecular Hematology, Sanquin Research, University of Amsterdam, Amsterdam, The Netherlands, Uithoorn, Noord‐Holland, Netherlands
Background: Thrombosis is a vascular disease that involves unwanted clotting of blood within any vessel, resulting in an obstruction. This obstruction is the result of an imbalance in blood coagulation. Venous thrombosis is characterized by a high content of red blood cells (RBCs) in the clot. RBCs contribute largely to the size and stability of a venous clot. However, it remains unclear how RBCs affect clotting. CRISPR/Cas9‐ mediated genome editing of RBC proteins enables the study of specific proteins and their effect on clot formation in more detail.
Aims: The aim of this study was to develop a new approach to investigate clot formation and fibrinolysis by using CRISPR‐based knockout (KO) RBCs.
Methods: Several membrane proteins suspected to play a role in clot formation were targeted and KO cells were generated using CRISPR/Cas9 in hematopoietic stem cells. Growth and differentiation of cultured RBCs was analyzed by flow cytometry. These cultured RBCs were used to form 5 μL clots by incubating the RBCs and human recalcified citrated plasma with tissue factor. Contracting clots were visualized and the fibrin content quantified by confocal microscopy. Moreover, lysis of these clots was measured using the HALO assay.
Results: Our CRISPR approach resulted in KO efficiencies varying from 80–95% in differentiated RBCs. Flow cytometry data showed no differences in RBC maturation and enucleation between the wildtype and KO cells. Small blood clots were made using these cultured RBCs and fibrin was successfully visualized and quantified. In addition, lysis of these clots occurred in the presence of tPA.
Conclusion(s): Highly efficient KO RBCs were obtained using CRISPR/Cas9. No differences were observed in the growth and differentiation between wildtype and KO RBCs, suggesting that Cas9‐mediated genome editing does not affect the RBC differentiation process. Our data show that these cultured RBCs can be used in assays to study clotting and fibrinolysis.
PB1261
Delayed CCL26 trafficking in the secretory pathway of STX5 depleted Endothelial Cells
S. Hordijk 1; M. Kat2; R. Bierings3
1 Erasmus University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 2 Molecular Hematology, Sanquin Research and Landsteiner Laboratory, Amsterdam UMC, University of Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 3 Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands
Background: The SNARE protein Syntaxin 5 (STX5) is involved in trafficking secretory cargo between the endoplasmic reticulum (ER) and the Golgi. However, the kinetic details behind this remain undefined. The Retention Using Selective Hooks (RUSH) system allows for the coordinated retention and release of a protein of interest. Using this system with a fluorescently‐tagged secretory protein probe such as the chemokine CCL26 allows us to investigate the dynamics of the secretory pathway in absence of STX5, its interactors and regulators. Hereby we can characterize their roles in secretion and further unravel the secretory pathway of endothelial cells (ECs).
Aims: To investigate the effect of STX5 depletion on trafficking of secretory proteins in ECs.
Methods: We performed short hairpin (sh)RNA‐based STX5 knockdown in human umbilical vein endothelial cells (HUVECs) followed by transient transfection with a streptavidin‐KDEL_SBP‐GFP‐CCL26 construct. The time required for translocation from the ER to the Golgi following addition of biotin was used to quantify and compare progression of CCL26 through the secretory pathway.
Results: A delay of CCL26 trafficking was often observed in STX5 depleted cells (Figure 1). Quantification of reporter translocation time to the Golgi showed a significant difference between shCTRL and shSTX5 transduced HUVECs. In some cells, the absence of STX5 caused (prolonged) ER retention of the probe as Golgi translocation did not occur within 60 minutes (Figure 2).
Conclusion(s): STX5 plays an essential role in the expedited delivery of secretory proteins from ER to the Golgi.

PB1267
Endothelial damage biomarkers panel to predict response to extracorporeal photopheresis in steroid refractory acute GVHD patients
J. Martinez‐Sanchez 1; M. Palomo1; O. Penack2; A. Moreno‐Castaño3; S. Torramade‐Moix4; A. Ramos1; H. Ventosa4; M. Rovira5; G. Escolar4; E. Carreras1; J. Cid6; M. Diaz‐Ricart7
1 Josep Carreras Leukemia Research Institute, Hospital Clínic de Barcelona, Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 2 Hematology, Oncology and Tumor Immunology Department, Charité Universitätsmedizin Berlin, Berlin, Germany., Berlin, Berlin, Germany; 3 HEMATOPATHOLOGY, PATHOLOGY DEPARTMENT, CENTRE DE DIAGNÒSTIC BIOMÈDIC. HOSPITAL CLINIC BARCELONA, BARCELONA, Catalonia, Spain; 4 Hematopathology, Department of Pathology, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 5 Stem Cell Transplantation Unit, Hospital Clínic de Barcelona, IDIBAPS, Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 6 Apheresis & Cellular Therapy Unit, Department of Hemotherapy and Hemostasis, ICMHO, Hospital Clínic de Barcelona, IDIBAPS, Universitat de Barcelona, Barcelona, Spain., Barcelona, Catalonia, Spain; 7 Hospital Clinic, IDIBAPS, University of Barcelona, Spain, Barcelona, Catalonia, Spain
Background: Extracorporeal photopheresis (ECP) is an effective and safe procedure in treating patients with symptomatic graft‐versus‐host disease (GVHD). ECP is used as a second line treatment for those patients with acute GVHD (aGVHD) who are steroid‐refractory (SR‐aGVHD). Clinical and experimental evidence demonstrates that endothelial damage is involved in aGVHD. Therefore, sustained endothelial dysfunction may be a pathophysiological substrate for SR‐aGVHD.
Aims: To measure circulating endothelial damage biomarkers to: i) analyze the degree of endothelial damage in SR‐aGVHD patients before initiating ECP; ii) investigate the effect of ECP on the endothelial activation state, and iii) explore differences depending on the response to ECP.
Methods: SR‐aGVHD patients treated with ECP (n = 35) were classified into good (GR) and bad (BR) responders depending on their clinical improvement after ECP. Plasma samples were collected at initiation (PRE) and at the end (POST) of ECP treatment. We analyzed endothelial activation biomarkers, such as soluble Vascular Cell Adhesion Molecule‐1 (sVCAM‐1), von Willebrand Factor (VWF), thrombomodulin (TM), soluble TNF receptor 1 (sTNFR1), and angiopoietin 2 (ANG2); GVHD markers, such as suppression of tumorigenicity 2 (ST2), regenerating islet‐derived 3‐alpha (REG3alpha), and T‐cell immunoglobulinmucin‐3 (TIM3); soluble C5b9 (sC5b9) for complement activation; and circulating dsDNA for neutrophil extracellular traps (NETs). Results were compared in each group and between both groups.
Results: Decreased levels of sTNFR1, TIM3, sC5b9, sVCAM‐1, TM, ST2 and ANG2 were observed in PRE samples of GR vs. BR (Table 1). Levels of these markers did not change substantially in the GR POST samples, whereas most of them kept increasing in the BR group. Association of those biomarkers with AUC >0.60 (ROC curves) resulted in PPV of 94.44% and NPV of 54.55% to negatively respond to EPC (p < 0.009).
Conclusion(s): We propose a panel of endothelial damage biomarkers in patients with SR‐aGVHD that could predict the clinical response to ECP.

VPB1278
Influence of titanium dioxide particles on redox processes in human erythrocytes in vitro
A. Skarabahatava 1; M. Sadaunichuk1; E. Venskaya1; N. Aliakhnovich2
1 Institute of Biophysics and Cell Engineering of NAS of Belarus, Minsk, Minskaya Voblasts', Belarus, 2 2Vitebsk State Order of Peoples' Friendship Medical University, Vitebsk, Vitsyebskaya Voblasts', Belarus.
Background: During the last decades, it was believed that titanium and its compounds are biologically inert, which led to its widespread use in medical practice. However, the growing number of published studies on the effects of TiO2 confirms the importance of studying the issue of its biosafety.
Aims: The aim of this work is to evaluate the possibility of titanium dioxide particles of different sizes (nano‐ and microparticles, food titanium) influencing the redox balance of human erythrocytes in vitro.
Methods: Erythrocytes were separated by centrifugation of blood at 2000 g during 5 min in PBS buffer pH 7.4. After they were diluted to 2% hematocrit in the same buffer and loaded with titanium dioxide particles at a final concentration of 0.01 mg/mL and incubated for 3 hours at 37°C with constant stirring. ROS level was assessed by fluorescent of 2′,7′‐dichlorodihydrofluorescein diacetate (DCFDA). Catalase glutathione and peroxidase activity were assessed by the standard methods. Statistical analysis was carried out using Wilcoxon test (n = 7).
Results: It was shown that during short‐term exposure (30 min), all studied particles were able to stimulate the formation of ROS in erythrocytes. Also, under the conditions of modeling oxidative stress using tert‐butyl hydroperoxide all the particles increased the level of DCFDA fluorescent. But food titanium dioxide mostly contributes to the observed effect. It was found that all types of the particles after a 3‐h incubation reduce the activity of antioxidant defense enzymes (glutathione peroxidase and catalase) by an average of 20% (p < 0.05), but the difference between the effects of different types of particles could not be identified.
Conclusion(s): Thus, it has been shown that titanium dioxide particles are capable of stimulating the oxidative processes development in the cell, and, also inhibiting the work of its defense system, which may contribute to their toxic effect.
VPB1279
Oxidative Stress Biomarkers and Their relevance to fatty Acid‐Binding Protein in Stage‐V Chronic Kidney Disease Patients on Hemodialysis
D. Sridharan 1; V. Bansal1; F. Siddiqui1; P. Karumanchi2; A. Odeh1; O. Iqbal1; B. Kantarcioglu3; J. Fareed4
1 Loyola University Medical Center, Maywood, Illinois, United States; 2 Loyola University Medical Center, Palatine, Illinois, United States; 3 Loyola University Chicago, Oak Park, Illinois, United States; 4 Loyola University Chicago, Maywood, Illinois, United States
Background: Oxidative stress plays a major role in the progression of kidney disease. Nitric Oxide (NO), malondialdehyde (MDA), nitrotyrosine, annexin V, myeloperoxidase (MPO), non‐esterified fatty acids (NEFA), fatty acid binding protein (FABP) and inflammatory cytokines are commonly used biomarkers of oxidative stress in various clinical conditions.
Aims: The purpose of this investigation is to compare the levels of circulating oxidative stress biomarkers including L‐FABP in stage V chronic kidney disease (CKD‐V) patients on hemodialysis (HD) compared to normal healthy controls.
Methods: Blood samples were collected in sodium citrate tubes from stable patients with CKD‐V who are treated at Loyola University Medical Center Outpatient Dialysis Unit. Normal human plasma (NHP) samples was commercially obtained. Sandwich enzyme‐linked immunosorbent assay (ELISA) methods were utilized to evaluate plasma levels of NO, MDA, nitrotyrosine, annexin V, MPO, troponin, VEGF, NEFA, plasminogen activator inhibitor 1 (PAI‐1),inflammatory cytokines and L‐FABP.
Results: The CKD‐V cohort showed a statistically significant elevation in the levels of annexin V (7.57 ± 5.24 ng/mL) NO (34.74 ± 19.74 pg/mL), and L‐FABP (106,599 ± 176,729 pg/mL) compared to the NHP group. Lower levels of MPO (100.2 ± 19.28 mU/mL) and NEFA (0.56 ± 0.34 mEq/L) was observed in comparison to the NHP group (p < 0.05). L‐FABP levels were significantly elevated, almost 20‐fold higher in CKD‐V patients compared to the NHP group. Spearman correlation revealed a negative correlation of L‐FABP with NEFA (r = −0.22; p < 0.05) and a positive correlation of L‐FABP with nitrotyrosine (r = 0.36; p < 0.05) in the CKDV cohort.
Conclusion(s): These results suggest that as oxidative stress increases in chronic kidney disease patients, the rate of lipid peroxidation also increases, thereby converting NEFAs into lipoperoxides. As levels of these lipoperoxides increase, L‐FABP levels also increase as a protective measure to excrete the toxic byproducts out of the body.
PB1270
Erythrocyte Components Affect Vascular Calcification By Modulating Nitric Oxide Signaling At Sites Of Intramural Microhemorrhage
M. PAVLAKI1; K. Moiko 2; A. Thomaidis3; A. Kourkoulis3; A. Markakis3; G. Chalikias3; S. Konstantinides3; D. Tziakas3
1 Dpt. of Cardiology, Democritus University of Thrace, Alexandroupolis, Greece, Alexandroupolis, Evros, Greece; 2 Democritus University of Thrace, Alexandroupolis, Evros, Greece; 3 Cardiology Dpt. University of Thrace, Alexandroupolis, Evros, Greece
Background: We have previously shown that red blood cells (RBCs) enhance vascular smooth muscle cell (SMC) osteogenesis via mechanisms partly dependent on nitric oxide (NO) generation. Interestingly, osteoinductive effects were exerted by RBC membranes, but not by intact or lysed RBCs.
Aims: To dissect the mechanisms underlying the osteoinductive properties of RBC membranes as opposed to intact or lysed RBCs, with particular focus on arginase activity and NO scavenging by hemoglobin.
Methods: Lysed RBCs and RBC membranes (ghosts), isolated from human blood, were examined in vitro for enhancement of osteogenic differentiation and calcification of aortic SMCs in culture as assessed by Alizarin staining. Oxidative neutralization of RBC cytoplasm served to evaluate the NO‐scavenging activity of oxyhemoglobin. Arginase activity and content were assessed in the calcification assay and by sample immunoblotting followed by band densitometry analysis, respectively.
Results: The arginase content of the RBC cytoplasm exceeded that of RBC ghosts by approximately one order of magnitude. Using the calcification assay of aortic SMCs, we could show that the osteoinductive effect of RBC ghosts was enhanced by physical contact with the aortic SMCs and varied among different donors. Lysed RBC showed almost no effect when added alone to SMCs, and inhibited RBC ghost‐mediated calcification. However, the inhibitory effect of the RBC cytosol could be reversed following oxidation of oxyhemoglobin and after inhibition of arginase by L‐norvaline.
Conclusion(s): Incomplete clearance of lysed RBC at sites of intraplaque microhemorrhage may promote NO dependent vascular calcification. Our results suggest that, in addition to RBC membrane effects, the RBC cytosol may also exert NO dependent osteoinductive activity under oxidative conditions in association with neutralization of hemoglobin's NO scavenging acitivity. Such an oxidative milieu has been reported to exist in human atherosclerotic lesions. Interindividual variability in the balance between NO synthase and arginase activity may determine the presence and extent of calcification.
PB1274
Trauma Induces Intravascular Hemolysis, which is Associated with Adverse Clinical Outcomes: Potential Role of Released Arginase‐1, Depletion of L‐arginine, and Reduced Production of Nitric Oxide
T. Schaid 1; A. D'Alessandro2; E. Moore3; C. Silliman4; A. Sauaia4; M. DeBot1; C. Erickson4; I. Lacroix4; A. Cralley4; A. Banerjee5; K. Jones5; A. Ghasabyan6; S. Mitra5; M. Cohen4; K. Hansen4
1 University of Colorado, Denver, Colorado, United States; 2 Department of Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States; 3 Ernest E Moore Shock Trauma Center atDenver Health, Denver, Colorado, United States; 4 University of Colorado, Aurora, Colorado, United States; 5 University of Colorado, Department of Surgery, Aurora, Colorado, United States; 6 University of Colorado, Department of Surgery, Denver, Colorado, United States
Background: Severe injury may induce hemolysis, releasing intracellular RBC proteins, such as arginase‐1. Transfused RBC's can lyse in the trauma‐associated, thromboinflammatory milieu, which may have serious clinical implications given the frequency of blood transfusion after trauma. There are currently no studies examining the relationship between trauma‐induced hemolysis and clinical outcomes.
Aims: Use comprehensive proteomics to link trauma‐induced hemolysis to outcomes and unmask potential mechanisms of post‐injury organ dysfunction.
Methods: Blood was collected from injured patients at a Level I Trauma Center. Proteomics were performed using targeted liquid chromatography coupled with mass spectrometry. Intracellular RBC proteins as well as haptoglobin, L‐arginine, and ornithine were measured at multiple timepoints. Unpaired t‐tests and multiple linear regression were used to analyze association between injury, hemolysis, and outcomes.
Results: Intracellular RBC proteins were elevated on emergency department (ED) arrival through 24 hr versus healthy controls. Haptoglobin decreased over 24 hr in those severely injured with shock (NISS >25, base excess <−6 mEq/L). Severe injury and shock were associated with elevated arginase‐1 on ED arrival that decreased over 24 hr but remained elevated, reduced L‐arginine on ED arrival that continued decreasing over 24 hr, and elevated ornithine on ED arrival that decreased over 24 hr (Figure 1). Over 24 hr, RBC transfusions were negatively correlated with haptoglobin concentrations. Additionally, elevated hemolysis proteins and increased arginine conversion to ornithine were associated with mortality and adverse outcomes (Table1).
Conclusion(s): Trauma and shock result in intravascular hemolysis, which is exacerbated by RBC transfusions and associated with worse outcomes. Arginase‐1 release with depletion of L‐arginine necessary for nitric oxide production may contribute to organ dysfunction. This study raises important questions about how the trauma milieu causes hemolysis, which may be exacerbated by exogenous RBC's given during resuscitation. Additional investigation is needed on endogenous versus transfused RBC lysis in trauma, which may unveil critical implications for resuscitation. Research was supported by NIH 1RM1GM131968–01.


PB1253
What defines Platelet Responsiveness? “Aggregation and secretion are independently regulated”
E. Alfatani.
School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland,123 St Stephens Green, Dublin 2; ebtihalalfatani@rcsi.com, Sandyford, Dublin, Ireland
Background: Platelet activation plays an important role in arterial thrombosis in coronary heart disease, stroke, and peripheral arterial disease.
Aims: Therefore this study aims to determine if platelet aggregation is a true measure of global platelet responsiveness; or whether ATP secretion may reveal a different dynamic. .
Methods: 108 normal healthy volunteers were assessed for indices of platelet function, in response to 3 different agonists over a physiological dose range as follows: thrombin receptor activating peptide (TRAP; 2.9‐33uM), collagen‐related peptide (CRP‐XL; 0.1–2 ug/ml) and thromboxane A2‐mimetic (U46619; 0.1–15 uM). Specifically, we examined the dose–response nature of each individual's responsiveness to platelet agonists in parallel assays of platelet aggregation (assessed by light transmission in PRP) and platelet ATP secretion (assessed by luminometry).
Results: The results show significant inter‐individual differences in aggregation and secretion responses to all 3 agonists in the donor population. For example, the maximal extent of platelet aggregation varies in response to TRAP from a minimum 31.50% to maximum 120%). Similarly, the maximal extent of ATP secreted in response to TRAP from minimum 0.11 to maximum 4.67 pmoles / 106 platelets, demonstrating a 40 to 50 fold range in secretion‐capacity for this dense‐granule component. A greater than 10 fold difference in secretion of ATP was also observed in response to CRP and U46619 in this donor cohort. There is a strong positive correlation between responses to all 3 agonists in the aggregation assays and, separately, in the secretion assays. However, there is no correlation between platelet secretion and aggregation responses.
Conclusion(s): Substantial inter‐individual variations in platelet responses are observed in normal healthy donors using 3 different agonists (TRAP; CRP and U46619). Aggregation and secretion are independently regulated, suggesting that more studies are merited to investigate if ATP secretion might provide better insights into clinical risks in patient populations.
PB1255
Disclosing the role of endothelial dysfunction in the pathogenesis of unprovoked venous thromboembolism by using patient‐specific endothelial colony‐forming cells
A. Cancellara 1; M. Bacci2; L. Bertolani3; E. Romualdi4; V. Pessi5; C. Lodigiani2; M. Donadini6; S. Della Bella1; F. Calcaterra1; D. Mavilio1
1 Unit of Clinical and Experimental Immunology (UCEI), Department of Medical Biotechnologies and Translational Medicine, University of Milan; IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy, Rozzano, Lombardia, Italy; 2 Center for Thrombosis and Hemorrhagic Diseases, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy, Rozzano, Lombardia, Italy; 3 Unit of Clinical and Experimental Immunology (UCEI); IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy, Rozzano, Lombardia, Italy; 4 UO Medicina 2, Ospedale di Circolo e Fondazione Macchi, ASST Sette Laghi, Varese, Italy, Varese, Lombardia, Italy; 5 Università dell'Insubria, Dipartimento di Medicina e Chirurgia, Varese, Italy, Varese, Lombardia, Italy; 6 Università dell'Insubria, Dipartimento di Medicina e Chirurgia, Varese; Centro Trombosi ed Emostasi, Ospedale di Circolo e Fondazione Macchi, ASST Sette Laghi, Varese, Italy, Varese, Lombardia, Italy
Background: Venous thromboembolism (VTE) is a multifactorial disease that often occurs in the presence of provoking factors; major risk factors induced provoked VTE (pVTE) whereas weakly provoked (wpVTE) is associated with minor ones. Nevertheless, up to 50% of VTE events are defined as unprovoked (uVTE) as their pathogenesis is still unknown. In this regard, the presence of endothelial alterations deserves to be explored as endothelial dysfunction (ED) is known to trigger thrombus formation. Endothelial colony‐forming cells (ECFCs) are the true endothelial progenitors proposed as cell therapy product or liquid biopsy in vascular disorders.
Aims: To investigate whether ECFCs in VTE patients show alterations that may reflect a perturbation of the endothelial compartment involved in uVTE pathogenesis.
Methods: ECFCs were isolated and expanded from peripheral blood mononuclear cells obtained from 40 VTE patients (13 uVTE, 16 wpVTE, 11 pVTE) and 10 matched healthy donors (HDs). ECFCs were analyzed for efficiency of isolation, cell viability and growth. ED was assessed by in vitro functional assays.
Results: ECFCs were isolated from the three subgroups of VTE patients with a similar efficiency as HDs in term of frequency and number of colonies. In pVTE, a trend in the reduction of the frequency of subjects who gave origin to ECFCs was observed. Time of appearance was longer in VTE with the difference being significant only in wpVTE patients. VTE ECFCs showed a high rate of early senescence (passage≤2), with wpVTE group being characterized by the highest frequency.
Conclusion(s): Our results indicate alterations in the growth of VTE ECFCs thus confirming their use to investigate ED role in uVTE pathogenesis. A deeper characterization of ECFCs, still ongoing, will allow the identification of the molecular mechanisms involved in ED in uVTE and to disclose whether they are associated with uVTE only are shared with secondary VTE.
PB1262
The Role of Endothelial Glycocalyx Degradation in COVID‐19
H. Jerndal 1; C. Ahlm2; A. Fors Connoly2; J. Normark3
1 Umeå University Hospital, Umeå, Vasterbottens Lan, Sweden; 2 Department of Clinical Microbiology, Umeå University, SWE, Umea, Vasterbottens Lan, Sweden; 3 Department of Clinical Microbiology, Umeå University Hospital, Umeå, Vasterbottens Lan, Sweden
Background: The endothelial glycocalyx (eGLX), a proteoglycan‐ and glycoprotein‐rich layer covering the endothelial cells, has been shown to play an important role during infectious diseases. It regulates vascular permeability, prevents thrombosis, and modulates leukocyte adhesion and inflammatory response.
Aims: The aim of this study was to investigate eGLX degradation during COVID‐19.
Methods: GlycoCheck is a non‐invasive test where a video microscope is placed under the tongue. From the measurements, eGLX thickness can be quantified. The eGLX damage is measured through the Perfused Boundary Region, (PBR), which is the zone where erythrocytes penetrate the protective eGLX. 40 patients with COVID‐19 were included at the Umeå University Hospital after written informed consent. GlycoCheck measurements were performed during the acute phase of disease and were compared to follow up. Statistical analysis with related‐samples Wilcoxon signed rank test was performed.
Results: 23 out of 40 patients have to this date completed 1 follow up GlycoCheck measurement, 1–2 months after the acute phase of disease. Among these 23 patients, the PBR was significantly increased during the acute phase of disease compared to follow up 1–2 months later (p < 0.01). Mean PBR among first visits during the acute phase was 2.32 μm (SEM 0.05), compared to the mean PBR of follow up visits, which was 2.12 μm (SEM 0.04).
Conclusion(s): Our preliminary results show significant differences between the thickness in eGLX between patients during the acute phase of COVID‐19 compared to follow up measurements of the same patients 1–2 months after, thus indicating a degradation of eGLX during the acute phase of COVID‐19.

PB1263
Evidence for mitochondrial dysfunction in blood‐derived endothelial colony forming cells isolated from patients with antiphospholipid syndrome
L. Kabir 1; R. Maughan1; K. Paschalaki1; A. Randi2; D. Carling3; D. Arachchillage4; J. Mason1; C. Pericleous1
1 National Heart and Lung Institute, Imperial College London, London, England, United Kingdom; 2 Imperial College London, London, England, United Kingdom; 3 MRC London Institute of Medical Sciences, Imperial College London, London, England, United Kingdom; 4 Department of Immunology and Inflammation, Imperial College London, London, England, United Kingdom
Background: Endothelial dysfunction is a critical event in antiphospholipid syndrome (APS), a systemic autoimmune disease characterized by the persistent presence of pathogenic antiphospholipid antibodies (aPL), thrombotic and obstetric complications. aPL activate endothelial and immune cells, tipping the haemostatic balance to an inflammatory and pro‐coagulant state. Altered metabolism and mitochondrial function in patient‐derived immune cells may contribute to APS pathogenesis but these mechanisms have yet to be explored in the endothelium.
Aims: To examine how aPL impact endothelial metabolism and mitochondrial function using ex vivo blood‐derived endothelial colony forming cells (ECFCs) from patients with APS.
Methods: ECFCs were isolated from peripheral blood mononuclear cells (PBMCs) obtained from patients with APS (n = 11; 5 with arterial thrombosis, 3 venous, 3 with both) and healthy controls (HC, n = 11). Experiments were performed at passage 4–5. Protein expression was assessed by immunoblot, respiratory and glycolytic measurements using the Seahorse XFe96 Flux Analyzer, and mitochondrial network analysis by cytochrome c immunofluorescence. Non‐parametric Mann–Whitney and Spearman's data analysis was performed.
Results: Colony number per 10^7 PBMC seeded, time in culture to colony appearance and time to passage were similar between patient and control ECFC. Diminished basal mitochondrial respiration and OXPHOS‐linked ATP production (oxygen consumption rate, p < 0.05) in APS ECFCs provides evidence for mitochondrial dysfunction; a tendency towards a glycolytic phenotype was also evident. OXPHOS immunoblot analysis correlated with basal respiration measurements (r = 0.7, p = 0.04). Concomitantly, we observed suppressed mitochondrial MnSOD expression along with reduced mitochondrial footprint and increased fragmentation of the endothelial mitochondrial network in APS versus HC ECFCs, indicating mitochondrial stress.
Conclusion(s): Perturbed mitochondrial energy homeostasis and increased glycolysis are pronounced in APS patient‐derived ECFCs, signifying pathological metabolic reprogramming. Given the importance of mitochondria in endothelial homeostasis, dysfunctional mitochondria may contribute to the pro‐coagulant and inflammatory features of endothelial dysfunction in APS.
PB1256
The role of FSAP in regulating endothelial permeability
X. Cui 1; B. Stavik2; P. Sandset3; S. Kanse4
1 Oslo University Hospital, OSLO, Oslo, Norway; 2 Oslo university hospital, Oslo, Oslo, Norway; 3 University of Oslo, Oslo, Oslo, Norway, 4 university of Oslo, Oslo, Oslo, Norway
Background: Factor VII activating protease (FSAP) is a circulating serine protease that is involved in regulating hemostasis and inflammation. FSAP‐deficient mice subjected to thromboembolic stroke exhibit a larger infarct volume, which indicates the protective role of FSAP in the stroke. Our recent research showed that exogenous FSAP is protective in stroke. FSAP has been shown to modulate hyaluronic acid‐mediated alterations in endothelial permeability. Therefore, we have investigated the influence of FSAP on the regulation of endothelial permeability by other factors.
Aims: To characterize the role of FSAP in vascular permeability.
Methods: Human umbilical vein endothelial cells (HUVEC) and aorta endothelial cells (HAoEC) were seeded into transwells before they were treated with histones, recombinant human thrombin and human VEGF‐A165. The passage of streptavidin‐horseradish peroxidase (HRP) through the endothelial monolayer was measured. Immunofluorescence staining of VE‐Cadherin and ZO‐1 was used to determine the patency of the endothelial layer. Taqman was used to detect the mRNA expression of TLR2. The serine protease domain (SPD) of FSAP was compared to the inactive Marburg‐I of FSAP (MI‐FSAP).
Results: Histones, thrombin and VEGF significantly increased permeability of HUVEC as well as HAoEC. FSAP‐SPD abolished the permeability increases by extracellular histones, but not by thrombin and VEGF. FSAP‐SPD alone had no effect on the endothelial permeability. The disturbance of endothelial junctions by the extracellular histones were reversed by FSAP‐SPD. FSAP‐SPD repressed the overexpression of TLR2 induced by extracellular histones. Inactive MI‐FSAP had no such effect.
Conclusion(s): Tissue injury, inflammation and NETosis leads to the release of extracellular histones. Histones activate Pro‐FSAP and this, in turn, degrades histones and reverses the increased endothelial permeability by histones. This could contribute to the protective effects of FSAP observed in stroke.


PB1273
Mechanical Injury of a tissue‐engineered human arterial construct replicates the thrombotic and pharmacological properties of the native artery
J. Ranjbar 1; w. Njoroge2; J. Roe3; P. Roach4; J. Gibbins5; Y. Yang2; A. Harper6
1 Keele University, Stoke‐on‐Trent, England, United Kingdom; 2 Keele University, STOKE‐ON‐TRENT, England, United Kingdom; 3 Loughborough University, loughborough, England, United Kingdom; 4 Loughborough University, Loughborough, England, United Kingdom; 5 Institute for Cardiovascular and Metabolic Research, School of Biological sciences, University of Reading, Reading, UK, Reading, England, United Kingdom; 6 Keele University, Stoke on Trent, England, United Kingdom
Background: Intravital microscopy in mice is widely used to study in vivo thrombus formation – however the relevance of these studies to human physiology is unclear. Previously we have developed a 3D, tissue‐engineered human arterial construct (TEAC) that replicates the primary [1] and secondary haemostatic properties of the native artery, which could be used as an alternative to current in vivo experiments.Aims: The aim of this study was to identify whether our model was able to replicate thrombus formation in response to mechanical and FeCl3 injury, as well as its sensitivity to clinically‐relevant anti‐thrombotic drugs.Methods: Our TEAC was incorporated into a novel 3D printed microfluidic chamber and perfused recalcified citrated blood under arterial shear conditions. Platelets were fluorescently‐labelled with CSFE. Thrombus formation was monitored by fluorescence microscopy for up to 13 minutes after perfusion onset in either intact channels, or those subjected to mechanical or FeCl3 injury.Results: Both mechanical and FeCl3 injury models induced a reproducible thrombotic response, whilst there was limited thrombus formation seen in the uninjured channels. Under physiological flow with human blood, thrombus formation was faster and more extensive in TEACs subject to mechanical injury (average fluorescence = 11.7 ± 2.4 arbitrary units) compared to those injured with FeCl3 Injury (2.7 ± 0.4, p < 0.05, n=10). Mechanically‐injured arterial constructs also had enhanced tissue factor activity and shorter prothrombin times compared to those subjected to FeCl3 injury in static assays. Pre‐treatment of whole blood with ticagrelor or rivaroxaban before perfusion over mechanically‐injured TEACs significantly reduced the thrombus formation when compared to the untreated control (p < 0.05, n = 8 and 6 respectively).Conclusion(s): Our study demonstrates that tissue‐engineered human arterial constructs are a viable replacement to current murine thrombosis models, as they can replicate the haemostatic processes of the native artery, and are sensitive to clinically‐relevant anti‐platelet and anti‐coagulant therapies.
VPB1277
Selective angiostatin neutralization promotes endothelial migration an early stage of angiogenesis
M. Saito 1; P. Jurasz2
1 Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB, Canada, Edmonton, Alberta, Canada; 2 Department of Pharmacology, Faculty of Medicine and Dentistry/Cardiovascular Research Centre/Mazankowski Alberta Heart Institute, University of Alberta, Edmonton, Alberta, Canada
Background: Cardiovascular diseases are the primary cause of morbidity and mortality worldwide, and many coronary artery disease and peripheral artery disease patients are not eligible for standard therapies. Hence, therapeutic angiogenesis has been a sought‐after experimental treatment strategy. Its limitation has been the sole focus on the delivery of pro‐angiogenic factors and/or endothelial progenitor/stem cells, and not on concomitant suppression of counteracting endogenous negative angiogenesis regulators such as angiostatin. As such, our lab recently synthetized neutralizing peptides derived from ATP synthase, a known angiostatin target, to counteract the effects of angiostatin in an effort to promote therapeutic angiogenesis.Aims: To investigate whether angiostatin neutralization promotes angiogenesis.Methods: In vitro experiments were performed using Human Cardiac Microvascular Endothelial Cells (HMVEC‐C). HMVEC‐C were incubated with angiostatin (30 μg/mL) and neutralizing peptides (Alfa1‐3 or non‐neutralizing delta; 3uM) under hypoxia conditions (95% N2/5% CO2) for 48 hours. HMVEC‐C endothelial nitric oxide synthase (eNOS), matrix metalloproteinase‐14 (MMP‐14) and MMP‐2 levels were measured using immunoblot, and zymography as molecular markers of angiogenesis. HMVEC‐C nitric oxide (NO) production was measured using DAF‐FM‐based flow cytometry and migration was assayed using a MMP‐dependent modified Boyden chamber model.Results: Angiostatin decreased HMVEC‐C eNOS, MT1‐MMP, and MMP‐2 proteins levels, while only the combined incubation of alfa peptides 1‐3 reversed angiostatin's effects (eNOS: 91.5 ± 7.5%; MMP‐14 104.5 ± 5.3%; MMP‐2: 83.7± 17.1% of control, respectively). Similarly, angiostatin decreased HMVEC‐C NO production and HMVEC‐C migration, and these effects of angiostatin were reversed by the combined effects of alfa peptides 1‐3 (NO production: 93.5 ± 15.8% and migration 93.3 ± 17.5% of control, respectively).Conclusion(s): Combined the Alfa 1‐3 peptides counteracted the effects of angiostatin, increasing eNOS, MMP‐14 and, MMP2 levels, and NO production leading to increased HMVEC‐C migration; thus, demonstrating their potential for their further study in in vivo ischemic models.
PB1271
Multiparameter analysis of the time‐dependent roles of Platelet and Coagulation Pathways in Collagen‐ and Tissue Factor‐Induced Thrombus Formation
S. Navarro 1; D. Stegner2; B. Nieswandt3; M. Kuijpers4; J. Heemskerk5
1 University Hospital Würzburg, Würzburg, Bayern, Germany; 2 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 3 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 4 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University; Thrombosis Expertise Centre, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, The Netherlands, Maastricht, Limburg, Netherlands; 5 Department of Biochemistry, CARIM, Maastricht University, Maastricht, the Netherlands; Synapse Research Institute Maastricht, The Netherlands, Maastricht, Limburg, Netherlands
Background: In hemostasis and thrombosis, the complex process of thrombus formation involves different receptors and molecular pathways of platelet and coagulation activation. These pathways are considered to operate continuously together at the same time, but this has not been fully investigated.
Aims: To elucidate the time‐dependency of key pathways of thrombus and clot formation, initiated by collagen and tissue factor surfaces, where coagulation is triggered via the extrinsic route.
Methods: We adapted a microfluidics whole‐blood assay with the Maastricht flow chamber to acutely block molecular pathways involved in thrombus formation by pharmacological intervention at desired time points.
Results: The microfluidic system adapted ad hoc for this study revealed crucial roles of glycoprotein VI (GPVI)‐induced platelet signaling via Syk kinase as well as factor VIIa‐induced thrombin generation, both of which were confined to the first minutes of thrombus formation. In contrast, platelet activation via the protease‐activating receptors 1/4 (PAR1/4) and integrin αIIbβ3 activity appeared to be prolongedly active and extended to later stages of thrombus and clot formation. Finally, the data also pointed to a pivotal role of GPVI and the ITAM signaling in the generation of a procoagulant surface on platelets and therefore the enhancement of thrombin generation and consequently fibrin deposition at level of the thrombus surface.
Conclusion(s): This study presents a new efficient adaptation of the Maastricht microfluidic system, allowing disclosure of the time‐dependent roles of key pathways of platelet activation and coagulation in the thrombus development. This work thereby revealed a more persistent contribution of thrombin receptor‐induced platelet activation than of collagen receptor‐induced platelet activation to the thrombotic process.
PB1275
Hemostasis after vessel injury occurs under high shear conditions
A. Yakusheva 1; K. Butov2; G. Bykov3; G. Závodszky4; F. Ataullakhanov3; M. Panteleev3; P. Mangin5
1 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065, Strasbourg, Alsace, France; 2 Dmitry Rogachev Pediatric Hematology and Immunology Hospital, Moscow, Moskva, Russia; 3 Center for Theoretical Problems of Physicochemical Pharmacology, Moscow, Moskva, Russia; 4 Computational Science Lab, Faculty of Science, Institute for Informatics, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 5 Université de Strasbourg, INSERM, EFS Grand‐Est, BPPS UMR‐S1255, FMTS, F‐67065 Strasbourg, France, Strasbourg, Alsace, France
Background: Blood flow is a major regulator of hemostasis and thrombosis. The current view is that intermediate flows occur in healthy vessels, while high shear levels are reached notably during thrombosis. Targeting high shear has been proposed as an innovative strategy to selectively block thrombosis with a minor impact on hemostasis. The shear rates occurring at the edge of the lesion on a healthy vessel are unknown.
Aims: To measure experimentally the shear rates occurring in wounds in a context relevant to hemostasis.
Methods: Two models of vessel puncture in human and mice were developed and characterized. Doppler probes were used to measure blood flow in the vessels throughout the hemostatic process, and ComSol software allowed to recalculate shear rates and elongational flows in the wound. We used a model with a catheter in which the shear rate was calculated by applying Poiseuille's equation with volumetric rates of blood loss.
Results: Shear rates at the edge of the wound reached high values the medians of which were 22,000 s−1, 25,000 s−1 and 7000 s−1 after puncture of the carotid artery, aorta or saphenous vein, respectively. These results were confirmed in a human venous puncture model where shear rates in an implanted catheter in the cubital vein reached 2000–27,000 s−1. In all models, elevated levels of elongational flows exceeding 1000/s were found. The shear rates steeply decreased with the increase in injury size which was explained by the low hydrodynamic resistance of the injuries compared to the resistance of the downstream vessel network.
Conclusion(s): The relative vessel‐wound resistance determines the decrease of shear rate with increase in injury size. Various types of lesions in small and large mouse and human vessels result in high levels of shear rates and elongational flows, which are therefore not specific to arterial thrombosis, but also relevant to hemostasis.
PB1264
Phenotypic heterogeneity within healthy donor endothelial colony forming cells explored by morphological analysis and RNA expression profiling
S. Laan 1; R. Dirven2; I. van Moort3; T. Kuipers4; H. Mei4; J. Eikenboom2; R. Bierings5; S. Boer6
1 Leiden University Medical Center, Leiden, The Netherlands., Leiderdorp, Zuid‐Holland, Netherlands; 2 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, The Netherlands., Leiden, Zuid‐Holland, Netherlands; 3 Erasmus MC, University Medical Center Rotterdam, Rotterdam, Zuid‐Holland, Netherlands; 4 Sequencing Analysis Support Core, Department of Biomedical Data Sciences, Leiden University Medical Centre, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 5 Erasmus MC, University Medical Center Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 6 Department of Internal medicine, division of Thrombosis and Hemostasis, Leiden University Medical Centre, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Endothelial colony forming cells (ECFCs) derived from patients can be used to investigate pathogenic mechanisms of vascular diseases like von Willebrand disease in their native environment. Previously, considerable phenotypic heterogeneity has been observed between ECFC clones derived from healthy donors. This heterogeneity needs to be well understood in order to use the ECFCs as endothelial models for vascular disease.
Aims: Determine phenotypic and gene expression differences between ECFCs derived from healthy controls.
Methods: A total of 33 ECFC clones derived from 16 healthy controls were analyzed. Gene expression of a panel of Weibel‐Palade body (WPB) content proteins, Von Willebrand Factor (VWF) transcription factors and endothelial cell markers was measured in all ECFC clones by qPCR. The transcriptome of a selection of ECFC clones (n = 15) is currently being analyzed using bulk RNA sequencing as an unbiased method to examine the gene expression heterogeneity. Phenotypic profiling of the ECFCs was done using confocal microscopy, followed by automated WPB segmentation and morphometric analysis using CellProfiler. WPB quantity per cell, length and eccentricity were measured.
Results: Through hierarchical clustering of RNA expression profiles we could distinguish two major clusters within the ECFCs, which were mainly driven by differences in expression of endothelin‐1, α‐SMA, tPA, OPG, IL‐8, VWF, GATA6, P‐selectin and CD34 (Figure 1). Phenotypic profiling of these groups showed significantly more WPBs in cluster one compared to cluster two (Figure 2). Furthermore, the WPBs in cluster two were significantly shorter and rounder.
Conclusion(s): We observed a range of different RNA expression patterns between ECFC clones and clear differences in WPB count and structure, which can be used to more accurately classify ECFCs. The observed differences between the ECFC controls further emphasize the need to compare ECFC clones with similar characteristics when studying patient derived ECFCs. Funding by SYMPHONY: NWO‐NWA.1160.18.038 and Landsteiner Foundation for Blood Transfusion Research, Grant Number:1852.


PB1265
Role of a cellular antiprotease named Protease‐Nexin‐1 in the development of atherosclerosis
C. Madjene 1; Y. Boulaftali2; V. Arocas2; S. Loyau3; B. Ho‐Tin‐Noé4; M. Bouton2
1 INSERM U1148 ‐ Laboratory for vascular and translational Science ‐, Paris, Ile‐de‐France, France; 2 Inserm U1148, Paris, Ile‐de‐France, France; 3 INSERM U1148, Laboratory for Vascular Translational Science, Paris, Ile‐de‐France, France; 4 INSERM, PARIS, Ile‐de‐France, France
Background: The Protease/Antiprotease balance is involved in the development of atherosclerotic plaque and can particulary influence its stability. We have previously shown, that Protease Nexin‐1 (PN‐1), a serpin (Serine Protease Inhibitor) expressed by smooth muscle cells is significantly increased in human fatty streaks and fibrolipidic lesions.We have demonstrated that both platelets and intimal foam cells, including vascular smooth muscle cells (vSMCs), are the main sites of PN‐1 presence in early atheroma. We have also observed that treatment of human vSMCs with modified LDL induce overexpression of PN‐1 .
Aims: First, we have studied the impact of PN‐1 in the development of atherosclerosis in vivo on LDL R−/− mice.Then, we studied the role of PN‐1 in the responses of vSMCs during atherosclerosis: such as their ability to internalize oxidized LDLs, their proliferation potential, and their death.
Methods: We analyzed the development of atherosclerosis plaques on PN‐1 atherosclerotic deficient mice and WT atherosclerotic mice using oil red staining in en‐face preparation of the aorta.We studied overexpression or underexpression of PN‐1 by lentiviral transduction of SMCs incubated with increasing concentrations of oxidized LDLs. We analyzed the internalization of ox LDLs by Bodipy labeling, the PDGF‐induced proliferation of SMCs by MTT proliferation test and the cell death induced by IL‐1β by lactate deshydrogenase assay.
Results: Our in vitro results suggest that PN‐1i) limits oxLDL internalization capacity by SMCs,ii) promotes SMC proliferation and iii) limits their death. In vivo data show that PN‐1 atherosclerotic deficient mice develop,a higher number of atherosclerotic lesions than the controls. Lesion size percentage is indeed 5 times higher in PN‐1deficient mice compared to their relative controls.
Conclusion(s): The results obtained are promising and suggests a protective role of PN‐1 in atherosclerosis.
PB1268
Anti‐thrombotic effects of the proteasome inhibitor carfilzomib on the endothelium
A. Hjazi1; C. Gonzalez‐Maroto1; M. Rodriguez Gutierrez1; T. Page1; T. McKinnon 2
1 Imperial College, London, England, United Kingdom; 2 Imperial College London, London, England, United Kingdom
Background: Carfilzomib (CFZ) is a second‐generation proteasome inhibitor used to treat multiple myeloma. Potent inhibition of the proteasome results in chronic proteotoxic endoplasmic reticulum (ER) stress, leading to apoptosis. While CFZ has improved survival rates in multiple myeloma, it is associated with an increased risk of cardiovascular adverse effects. While this has been putatively linked to cardiotoxicity, CFZ could potentially also exhibit adverse effects on the endothelium.
Aims: To investigate the effects of CFZ on the endothelium.
Methods: HUVECS were treated with CFZ and expression of relevant markers of ER stress, inflammation and thrombosis measured. In some experiments cells were stimulated with TNFα following CFZ treatment. Immunofluorescent staining was used to determine VWF string formation following drug treatment and turbidity and Factor X activation assays used to assess clot formation on endothelial cells.
Results: CFZ treatment of sub‐confluent HUVECS induced BIP and ubiquitin expression indicating induction of ER stress, however CFZ treatment of confluent HUVECS did not induce ER stress indicating CFZ cannot induce ER stress in resting HUVECS. Interestingly, CFZ treatment induced the expression of eNOS, tissue plasminogen activator and thrombomodulin and was able to reduce TNFα induced ICAM‐1 and tissue factor expression, suggesting a potential protective effect on the endothelium. Consistent with these observations, CFZ reduced factor Xa generation and fibrin clot formation on the endothelium following TNF treatment and prevented the formation of VWF‐platelet strings and leukocyte adhesion under shear stress.
Conclusion(s): This data demonstrates that CFZ is unable to induce ER stress in confluent resting endothelial cells and can conversely attenuate the pro‐thrombotic effects of TNF on the endothelium. This study suggests that CFZ does not negatively alter HUVECS, and proteasome inhibition of the endothelium may offer a potential way to prevent thrombosis. Further studies are now required to establish the mechanisms behind these effects.
PB1257
Custodiol solution improves hemocompatibility of decellularized liver scaffold
M. Dias 1; B. Paranhos1; J. Ferreira2; R. Fonseca3; C. Batista4; R. Martins‐Santos1; C. Andrade5; L. Faccioli6; A. Silva7; F. Nogueira8; G. Domont8; R. Goldenberg1
1 Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil. Instituto Nacional de Ciência e Tecnologia em Medicina Regenerativa, INCT‐REGENERA, Universidade Federal do Rio de Janeiro, UFRJ, Rio de Janeiro, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil; 2 Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil.Instituto de Bioquímica Médica Leopoldo de Meis, Universidade Federal do Rio de Janeiro, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil; 3 Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil. Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil; 4 Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Brazil., Rio de Janeiro, Rio de Janeiro, Brazil; 5 Departamento de Histologia e Embriologia, Universidade do Estado do Rio de Janeiro, UERJ, Rio de Janeiro, RJ, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil; 6 Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA, Pittsburgh, Pennsylvania, United States; 7 Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil; 8 Laboratório de Proteômica /LADETEC, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brasil, Rio de Janeiro, Rio de Janeiro, Brazil
Background: Organ decellularization is one of the most promising approaches of tissue engineering to overcome the shortage of organs available for transplantation. However, there are key hurdles that still hinder its clinical application, and the lack of hemocompatibility of decellularized materials is a central one.
Aims: In this work, we aimed to evaluate whether Custodiol (HTK solution), a common solution used in organ transplantation, can reduce the thrombogenicity of decellularized liver scaffolds (DLS).
Methods: To reach this aim Wistar rat livers were transferred to be perfused through the portal vein using an infusion pump at 3 mL/min with water for 2 hours followed by 1% Triton X‐100 for 2 hours and SDS 1% for 18 h. Then, we performed ex vivo DLS blood perfusion (n = 6), coagulation assays (n = 3), platelets aggregation assays (n = 3), recellularization (n = 3), proteomic analysis (n = 6), and in vivo transplantation (n = 6). All data were described as means ± standard deviations (SD). The comparison between groups was performed using a paired Student's t‐test or one‐way ANOVA with Tukey's multiple comparisons test.
Results: In this work, we demonstrate that Custodiol increased the hemocompatibility of DLS obtained from rat livers. We showed that Custodiol inhibited ex vivo, in vitro, and in vivo blood coagulation to such extent that allowed successful transplantation of whole‐liver scaffolds into recipient animals. DLS previously perfused with Custodiol showed no signs of platelet aggregation and maintained in vitro and in vivo cellular compatibility. Proteomic analysis revealed that proteins related to platelet aggregation were reduced in Custodiol samples while control samples were enriched with thrombogenicity‐related proteins. We also identified distinct components that could potentially be involved with this anti‐thrombogenic effect and thus require further investigation.
Conclusion(s): Therefore, Custodiol perfusion emerge as a promising strategy to reduce the thrombogenicity of decellularized biomaterials and could benefit several applications of whole‐organ tissue engineering.


PB1252
Role of matrix Gla protein in vascular calcification: hard chemistry for soft vessels
S. Agten 1; D. Suylen2; A. Gentier3; L. Schurgers2; T. Hackeng2
1 Department of BIochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 3 Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands
Background: Vascular calcification is implicated in pathologies such as atherosclerosis and chronic kidney disease. However, much is unclear regarding initiation and inhibition of calcification while detection can only be achieved at a late stage, hampering intervention. A key player in this process is matrix Gla protein (MGP), a heavily post‐translationally modified inhibitor of calcification. While MGP is recognized as one of the strongest inhibitors of ectopic calcification, its mechanism of action is unknown. Further research into MGP's function is hampered by the inability in obtaining homogenously modified variants of MGP.
Aims: To study intra‐ and extracellular structure–function relationships of different variants of MGP containing combinations of serine‐phosphorylation and glutamate‐γ‐carboxylation.
Methods: We have employed a 3‐fragment, one‐pot, chemical protein synthesis approach to synthesize all possible combinations of post‐translationally modified variants of MGP (unmodified, phosphorylated, carboxylated, phosphorylated‐carboxylated). These variants were tested for their ability to inhibit in vitro formation of calcium phosphate deposits. Cellular effects involved with calcification were evaluated in a vascular calcification assay where MGP variants were added to human vascular smooth muscle cells (hVSMC) cultured under calcifying conditions.
Results: Our chemical protein synthesis approach has provided access to diverse homogenous post‐translationally modified variants of MGP offering unique tools for research of MGP's structure–function relationships. First results of biological evaluation showed that unmodified MGP is unable to interfere with calcium phosphate precipitation whereas all post‐translationally modified variants showed a dose‐dependent inhibitory effect. Moreover, we could show a dose‐dependent inhibitory effect of post‐translationally modified MGP on calcifying hVSMCs.
Conclusion(s): Results of both the non‐cellular and cellular vascular calcification model assays confirm importance of glutamate‐γ‐carboxylation but also show a clear effect of serine‐phosphorylation on inhibition of calcium crystal formation. Currently, we investigate effects of addition of different MGP variants on hVSMC differentiation and phenotype switching. Project is funded by the Dutch Heart Foundation (2019 T013) to SMA.
PB1258
Modulation of monocytes inflammatory and hemostatic phenotype after transcatheter aortic valve replacement and consequences on post‐procedural complications
F. Lassalle1; F. Vincent1; M. Rosa1; A. Rauch1; F. Dumezy2; C. Roumier2; D. Dombrowicz1; C. Gheeraert1; P. Lefebvre1; B. Staels1; A. Vincentelli1; E. Van Belle1; S. Susen3; A. Dupont 1
1 Univ. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐ EGID, F‐59000 Lille, France, Lille, Nord‐Pas‐de‐Calais, France; 2 Department of Hematology, CHU Lille, Lille, Nord‐Pas‐de‐Calais, France; 3 CRC‐MHC, Lille University Hospital, Lille, France, Lille, Nord‐Pas‐de‐Calais, France
Background: Aortic valve stenosis, the most common valvular disorder in the elderly, leads to a very disturbed blood flow (low arterial pulsatility, high shear stress). Its treatment consists in a transcatheter aortic valve replacement (TAVR) allowing an immediate reopening of the valve with sudden pulsatility and shear stress correction. However, TAVR remains an inflammatory procedure, whose main post‐procedural complications are bleeding and thrombosis involving mechanisms poorly understood. Recently, a close link between blood coagulation and innate immune system has been described, termed immunothrombosis, including monocytes as major actor.
Aims: To study the modulation of circulating monocytes phenotype due to acute changes of blood flow conditions in order to better understand the mechanisms involved in post‐procedural complications of TAVR.
Methods: Patients who underwent TAVR were included in the study (NCT02628509). We studied the circulating monocytes subpopulations repartition by flow cytometry before (T0) and one day after TAVR (D1) for 36 patients and performed transcriptomic analysis of classical monocytes at T0 and D1 for 12 patients after cell sorting.
Results: We observed an increase of monocytes levels at D1 versus T0 (p < 0.0001), linked to an increase of classical (CD14++CD16‐) and intermediate (CD14++CD16+) monocytes (p < 0.0001 for both). High preprocedural rates of intermediate monocytes were associated with one‐year mortality whereas high postprocedural levels of non‐classical monocytes (CD14 + CD16++) were associated with bleeding or thrombotic complications at one month. Transcriptomic analysis revealed 267 up‐ and 28 down‐regulated genes at D1 versus T0. The most upregulated genes encoded for leukocyte activation and inflammatory response and among genes implicated in coagulation, THBD (thrombomodulin) and F5 (factor V) were the 2 most upregulated genes.
Conclusion(s): TAVR modulates monocytes inflammatory and hemostatic phenotype in link with TAVR post‐procedural complications suggesting that immunothrombosis processes play a role in the occurrence of TAVR post‐procedural complications.
PB1269
Inflammatory stimuli reveal a differential glycocalyx response between venous and arterial endothelial cells
A. Milusev 1; R. Rieben2; N. Sorvillo2
1 Faculty of Medicine, University of Bern, Switzerland, Bern, Bern, Switzerland; 2 Department for biomedical research (DBMR), university of Bern, Switzerland, Bern, Bern, Switzerland
Background: The glycocalyx is a layer of sugars on endothelial cells (EC) enriched in heparan sulfate (HS). During inflammation, alteration in sulfation and shedding of HS occurs, activating both pro‐inflammatory and pro‐coagulant pathways. Arterial and venous EC respond differently to inflammatory cues, but whether this is due to distinct glycocalyx/HS dynamics is not known.
Aims: Here we investigate differences in sulfation and/or shedding of HS between arterial and venous cells in response to inflammatory stimuli.
Methods: Isolated porcine arterial and venous EC were cultured under static, high (typical for arteries) or low (typical for veins) shear stress using a 3D microfluidic system. HS expression and ‐shedding were detected using immunofluorescence. Sulfation was indirectly determined by quantitation of Ndst1 transcripts, encoding a HS‐sulfotransferase. Next, shedding of HS was quantified after EC activation with human serum, tumor necrosis factor alpha (TNFα) or lipopolysaccharide (LPS).
Results: HS was detected on arterial cells exclusively under flow, while it was observed on venous cells in both static and flow conditions, indicating that glycocalyx dynamics differ between these cells. Assessment of shear stress revealed that non‐physiological shear stress impacts Ndst1 expression, causing an increase of sulfation in arterial cells (low shear) and a decrease in venous cells (high shear), without impact on the overall HS expression. Next, glycocalyx was detected upon EC activation. Surprisingly, no HS shedding from venous cells was observed when activated with human serum, TNFα or LPS whereas HS was shed from arterial EC.
Conclusion(s): The different glycocalyx response upon shear stress and activation suggests that physiological shear is essential to maintain normal HS sulfation levels and indicates that the glycocalyx on venous but not arterial cells is resistant to shedding upon inflammation. This could account for the diverse behavior of venous and arterial cells during inflammatory events.
PB1254
Functional alterations involved in increased bleeding in hereditary hemorrhagic telangiectasia mouse models
C. Egido‐Turrion1; E. Rossi2; C. Ollauri‐Ibáñez1; M. Pérez‐García3; M. Sevilla1; J. Bastida 4; J. González‐Porras5; A. Rodríguez‐Barbero1; C. Bernabeu6; J. Lopez‐Novoa1; M. Pericacho7
1 Universidad de Salamanca. Institute for Biomedical Research of Salamanca (IBSAL), Salamanca, Castilla y Leon, Spain; 2 Université Paris Cité ‐ Faculty of Pharmacy, Paris, Ile‐de‐France, France; 3 Department of Internal Medicine, Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain, Salamanca, Castilla y Leon, Spain; 4 Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain; On behalf of “Grupo Español de Alteraciones Plaquetarias Congénitas (GEAPC)”, Sociedad Española de Trombosis y Hemostasia (SETH), Salamanca, Castilla y Leon, Spain; 5 Department of Hematology, Complejo Asistencial Universitario de Salamanca (CAUSA), Instituto de Investigación Biomédica de Salamanca (IBSAL), Universidad de Salamanca (USAL), Spain, Salamanca, Castilla y Leon, Spain; 6 Centro de Investigaciones Biológicas Margarita Salas, Consejo Superior de Investigaciones Científicas (CSIC), Madrid, Madrid, Spain; 7 Universidad de Salamanca. Instituto de Investigaciones Biomédicas de Salamanca (IBSAL), SALAMANCA ‐ 37,007, Castilla y Leon, Spain
Background: Hereditary Hemorrhagic Telangiectasia (HHT) is an autosomal‐dominant genetic disorder involving defects in two predominant genes known as endoglin (ENG; HHT‐1) and activin receptor‐like kinase 1 (ACVRL1/ALK1; HHT‐2). It is characterized by mucocutaneous telangiectases that, due to their fragility, frequently break causing recurrent epistaxis and gastrointestinal bleeding. Because of the severity of hemorrhages, the study of the hemostasis involved in these vascular ruptures is critical to find therapies for this disease.
Aims: The aim of this study is to analyze the different processes involved in hemostasis in the most common genetic models of HHT, namely Eng+/− for HHT‐1 and Alk1+/− for HHT‐2.
Methods: The analysis of the general process of hemostasis was performed by studying the bleeding time of the tail. Platelet activation and aggregation analyses were performed for the study of primary hemostasis, together with in vitro or in vivo platelet‐endothelium interaction studies. Fibrinolysis was studied by studying serum levels of fibrinogen, tPA, PAI‐1, and D‐dimer.
Results: In Eng+/− mice, the results of in vivo and in vitro assays suggest deficient platelet‐endothelium interactions that impair a robust and stable thrombus formation. Consequently, the thrombus could be torn off and dragged by the mechanical force exerted by the bloodstream, leading to the reappearance of hemorrhages (Figure 1A). In Alk1+/− mice, overactivation of the fibrinolysis system was observed (Figure 1B).
Conclusion(s): These results support the idea that endoglin and Alk1 haploinsufficiency leads to a common phenotype of impaired hemostasis, but through different mechanisms. This contribution opens new therapeutic approaches to HHT patients' epistaxis.
PB1259
Platelet α‐granule content induces endothelial damage and blood brain barrier breakdown in experimental stroke
V. Göb 1; L. Zimmermann2; K. Hemmen3; G. Stoll4; B. Nieswandt5; K. Heinze6; M. Schuhmann4; D. Stegner7
1 University Hospital and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 2 Department of Neurology, University Hospital Würzburg, Würzburg, Bayern, Germany; 3 Rudolf Virchow Center, University of Würzburg, Würzburg, Bayern, Germany; 4 University Hospital Würzburg, Würzburg, Bayern, Germany; 5 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 6 Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany; 7 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Biomaging University of Würzburg, Würzburg, Bayern, Germany
Background: Ischemic stroke is a major cause of death and disability worldwide. Even if a rapid recanalization is achieved, ongoing lesion development can be observed (referred to as reperfusion injury). Activation of platelets and the thereby induced secretion of granules are critically involved in stroke pathology since absence of granule secretion protects mice in experimental stroke. At the same time, loss of blood brain barrier (BBB) integrity is commonly observed especially in the early hours after stroke.
Aims: In the current study, we aimed to elucidate a potential role of platelet secretion on BBB integrity.
Methods: We subjected mice to transient middle cerebral artery occlusion (tMCAO), a model of ischemic stroke. Vascular integrity was monitored by 2‐photon intravital microscopy, and the degree of albumin extravasation was assessed in brain cryosections and by western blot analysis. To investigate the influence of platelet granule content on endothelial cells we measured the trans endothelial electrical resistance (TEER) of murine brain microvascular endothelial cell (MBMEC) monolayers and assessed cell morphology by immunohistochemistry.
Results: We confirmed BBB breakdown as an early event following ischemic stroke using intravital microscopy. The degree of albumin extravasation into the brain parenchyma increased with progressive reperfusion times. Absence of platelet α‐granule secretion (Nbeal2−/− mice) protected mice from this increased vascular permeability and reduced endothelial cell damage whereas this was not the case upon absent dense granule secretion (Unc13d−/− mice). Immunohistochemistry and subsequent image analysis revealed severely impaired MBMEC monolayer integrity upon incubation with α‐granule content explaining the loss of barrier function. This was confirmed by TEER measurements.
Conclusion(s): Our results revealed an unexpected effect of platelet secretion on BBB integrity. Promising α‐granule components could already be identified and will be validated until the ISTH conference. Targeting these α‐granule components could offer an anti‐platelet therapy that does not increase the risk of intracranial hemorrhages.
PB1260
Importance of erythrocyte Arginase‐1 for vascular smooth muscle cell no signaling and calcification
R. Gogiraju 1; L. Renner2; M. Bochenek3; K. Zifkos4; M. Molitor2; P. Wenzel4; S. Danckwardt3; T. Münzel5; K. Schäfer4
1 University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 2 Department of Cardiology, University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 3 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 5 Center for Cardiology ‐ Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: Erythrocytes (red blood cells, RBCs) actively participate in the control of vascular nitric oxide (NO) bioavailability. Increased expression of arginase‐1 (ARG1), an important regulator of NO production by competing with endothelial NO synthase (eNOS) for L‐arginine, is seen in erythrocytes of patients at risk for vascular dysfunction. Our group has previously shown that atherosclerotic plaque bleeding and erythrocyte extravasation promote vascular calcification by mechanisms involving NO.
Aims: To determine the significance of ARG1 in murine erythrocytes for vascular smooth muscle cell (VSMC) NO signaling, osteoblastic differentiation and calcification.
Methods: Atherosclerosis‐prone mice lacking ARG1 in RBCs (apoE−/− RBC.ARG1 knockout, KO) were generated by crossing erythropoietin Cre recombinase transgenic mice into the apolipoprotein E‐deficient background followed by intercrosses with ARG1flox/flox transgenic mice and feeding Western type diet.
Results: Conditional deletion of ARG1 increased the erythrocyte NO content in an eNOS‐dependent manner, without affecting erythrocyte numbers, morphology or removal in the spleen. Vascular calcification was significantly enhanced in apoE−/− RBC.ARG1 knockout mice, as shown by molecular imaging of osteogenic activity using OsteoSense™ and verified by Alizarin staining of calcium phosphate deposits on cross‐sections through the aortic root. Incubation of VSMCs with lysed RBC membranes from apoE−/− RBC.ARG1‐KO mice also accelerated osteoblastic differentiation in vitro, and calcium deposits and osteogenic marker mRNA levels decreased in the presence of a NO scavenger or inhibition of NO signaling. Increased expression of the protein denitrosylase GSNOR (S‐nitrosoglutathione reductase), degradation of S‐nitrosoglutathione to glutathione and reduced protein S‐nitrosation of ARG1 were identified as mechanisms leading to VSMC calcification in mice lacking ARG1 in erythrocytes.
Conclusion(s): Our findings suggest an important role of erythrocyte ARG1 in the control of vascular L‐arginine metabolism, NO bioactivity and signaling and identify GSNOR and protein S‐denitrosation of ARG1 as potential target to prevent vascular smooth muscle cell calcification.
PB1276
Identification of a red cell hemostatic mechanism causing COVID‐19 microvascular obstruction
Y. Yuan 1; M. Wu2; I. Alwis3; R. Smythe3; J. Maclean3; t. Woodruff4; T. Barrett5; a. rapkiewicz6; J. Shaun7
1 Heart Research Institute, University of Sydney, Melbourne, Victoria, Australia; 2 Heart Research Institute, Charles Perkins Centre, The University of Sydney, Sydney, New South Wales, Australia; 3 Heart Research Institute, The University of Sydney, Sydney, New South Wales, Australia; 4 School of Biomedical Sciences, University of Queensland, Brisbane, Queensland, Australia; 5 NYU Grossman School of Medicine, New York, New York, United States; 6 Department of Pathology, NYU Long Island School of Medicine, New York, New York, United States; 7 Heart Research Institute, University of Sydney, Sydney, New South Wales, Australia
Background: Severe COVID‐19 infection is associated with a wide spectrum of clinical manifestations, leading to systemic thromboinflammation and multiorgan dysfunction. The primary cause of multiorgan damage is widespread endothelial injury, leading to microangiopathy and organ ischemia. The molecular mechanisms by which ischemic endothelial cells causes microvascular obstruction remains ill defined.
Aims: Identification of distinct microvascular occlusion mechanisms in COVID‐19.
Methods: The microvasculature of multiple organs from patients dying from COVID‐19, myocardial infarction or stroke were analyzed by H&E, immunohistochemistry, SEM and CLEM. Animal models of gut ischemia and stroke were also examined. Intravital confocal microscopy examined endothelial injury and microvascular obstruction mechanisms mediated by platelets, red cells and fibrin.
Results: We demonstrate the existence of a distinct microvascular hemostatic mechanism mediated by hemolyzed red blood cells (RBC), independent of platelets and fibrin. Extensive RBC hemolysis was apparent in the microvasculature of COVID‐19 patients and in humans with major organ ischemia, leading to widespread microvascular obstruction. This RBC hemostatic mechanism was triggered by organ ischemia and associated with localized accumulation of hemolyzed RBCs at sites of endothelial necroptosis. RBC hemolysis was impaired in animals lacking the necroptosis mediator, MLKL or the C9 component of complement, indicating the involvement of cell intrinsic and extrinsic membrane lytic processes. Intravital microscopy revealed that the RBC hemostatic mechanism was triggered by the fragmentation of lyzed RBCs and the deposition of RBC membranes on the surface of dying endothelial cells, forming an endovascular sealant that prevents interstitial bleeding.
Conclusion(s): Our studies demonstrate the existence of a previously unidentified microvascular hemostatic mechanism mediated by hemolyzed RBCs. Dysregulation of this hemostatic mechanism is linked to microvascular obstruction and bleeding in COVID‐19 and ischemic diseases.
Endothelial Cell Signaling.
VPB1285
The role of endothelial colony‐forming cells in acute deep vein thrombosis
L. Silva 1; S. Montalvão2; S. Huber3; S. Soares3; A. Lemos Junior3; O. Camargo‐Junior4; C. Simões4; M. Abreu4; E. de Paula5; J. Annichino‐Bizzacchi6
1 University of Campinas, Campinas, Sao Paulo, Brazil, 22Hemostasis and Thrombosis Laboratory, Hematology and Hemotherapy Center, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil; 3 UNICAMP, Campinas, Sao Paulo, Brazil; 4 PUCC, Campinas, Sao Paulo, Brazil; 5 School of Medical Sciences of the University of Campinas, Campinas, Sao Paulo, Brazil; 6 Hemostasis and Thrombosis Laboratory, Hematology and Hemotherapy Center, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil
Background: Deep Vein Thrombosis (DVT) is characterized by the formation of a thrombus within deep veins, and it is described as a multifactorial disease whereas the interaction of risk factors can trigger the thrombotic process. Thirty percent of DVT are classified as non‐provoked DVT, and the search for new factors involved in the process is a challenge. Endothelial investigation can be an interesting point in this situation but has limitations about the anatomical access.
Aims: To evaluate the role of endothelial cells in patients with acute DVT when compared to healthy individuals.
Methods: The study was approved by a recognized medical ethics committee and after the volunteer's consent, the blood was collected to isolate the Endothelial Colony‐Forming Cells (ECFCs). Once these cells were obtained and properly immunophenotyped by flow cytometry, it was assessed the endothelial function through proliferation, apoptosis, and migration assays.
Results: Twenty‐five patients were selected, however, only 5 had their ECFCs successfully isolated – a group of study. Interestingly, all ECFCs patients were non‐provoked DVT (n = 3) or associated with minor risk factors (n = 2). The median age of the DVT group was 55 years against 33 years of the control group, the other clinical and descriptive data are shown in table 1. Regarding the ECFS, the morphology was similar in both groups (table 1), however, the ECFCs isolation was a challenge in DVT patients, with 20% of success vs. 50% in controls. The endothelial data through automated live cell analysis (Incucyte®) evidenced increased endothelial cell death in DVT compared to the controls, significantly at 24 hours of experiment, table 2. In proliferation and wound healing assay, despite variations between the medians, it was not found statistical significance among the comparations, probably due to the small sample size of DVT patients.
Conclusion(s): Our data suggest an alteration of endothelial cells in acute DVT.


VPB1284
Biomarker profiling to investigate the molecular and cellular pathogenesis of endothelium in atrial fibrillation
G. Dungan 1; A. Odeh1; F. Siddiqui1; D. Hoppensteadt2; O. Iqbal1; A. Darki2; J. Fareed2; m. syed1
1 Loyola University Medical Center, Maywood, Illinois, United States; 2 Loyola University Chicago, Maywood, Illinois, United States
Background: Atrial Fibrillation (AF) is the most prevalent cardiac arrythmia and is one of the main causes of cardiovascular morbidity.
Aims: This study seeks to examine the potential relationship between various endothelial, thrombotic, and inflammatory biomarkers in AF patient plasma.
Methods: Citrated blood samples from 111 AF patients with confirmed clinical diagnosis of atrial fibrillation were enrolled in this study. Control plasma samples represented 50 healthy individuals. All samples were analyzed using commercially available ELISA methods for the endothelial biomarkers ICAM‐1, VEGF, the thrombotic biomarkers tPA, PAI‐1, Annexin V, the inflammatory biomarkers IL‐6, TNFα, CRP, and Ang‐II. All results were tabulated and correlation analysis between each respective biomarker was carried out using GraphPad prism software.
Results: All of the biomarker studies showed varying levels of increase in patient plasma as compared to normal controls. The AF patients showed a statistically significant Spearman correlation between the following biomarker pairings: ICAM‐1 vs TNFα (r = 0.527, p = 0.012, n = 22), CRP vs IL‐6 (r = 0.489, p = 0.004, n = 33), TNFα vs IL‐6 (r = 0.654, p < 0.001, n = 41), Annexin V vs VEGF (r = 0.628, p < 0.001, n = 40), VEGF vs CRP (r = 0.518, p = 0.023, n = 19), tPA vs PAI‐1 (r = 0.672, p < 0.001, n = 28), tPA vs Ang‐II (r = −0.532, p = 0.004, n = 28).
Conclusion(s): These studies suggest the integrated expression of endothelial and inflammatory biomarkers as well as the coordinated expression of endothelial and thrombotic biomarkers in the pathophysiology of atrial fibrillation. Profiling patient levels of these markers may be helpful in screening AF disease progression as well as designing optimal therapeutic approaches for AF.
PB1282
Models for identification of endothelial glycocalyx degradation mechanisms during Puumala virus infection
C. Jacquet 1; M. Bally2; C. Ahlm3; A. Fors Connoly3
1 Umeå Hospital University, 90,185 Umeå, Sweden, Umeå, Vasterbottens Lan, Sweden; 2 Department of Clinical Microbiology, Umeå University, 90,185 Umeå, Sweden, Umeå, Vasterbottens Lan, Sweden; 3 Department of Clinical Microbiology, Umeå University, SWE, Umea, Vasterbottens Lan, Sweden
Background: The endothelial glycocalyx (eGLX) cell layer is composed of glycosaminoglycans (e.g., heparan sulfate (HS)) and glycoproteins (e.g., syndecan‐1 [SDC1], thrombomodulin). Degradation of vascular eGLX has been implicated in diabetes, coronary atherosclerosis, and kidney failure pathogenesis. Previously, we found an association between plasma levels of SDC‐1 and disease severity of hemorrhagic fever with renal syndrome (HFRS) caused by Puumala orthohantavirus.
Aims: Investigate eGLX shedding during orthohantavirus infection; and identify potential mechanisms of eGLX degradation through implementation of in vitro models.
Methods: In total 44 HFRS patients with serologically verified HFRS at the Umeå University hospital were included. Using commercial ELISA assays the plasma concentrations of SDC1, MMP9, and HS were quantified. Using Quartz Crystal Microbalance with Dissipation (QCM‐D) monitoring (AWSensors), effects of Heparinase (2 units/mL) or MMP‐9 (500 ng/mL) were followed on biotinylated HS (b‐HS) or SDC1 protein linked to supported lipid bilayers formed on SiO2 sensors. Enzyme immunoassays were performed on confluent GEnC cells on 96‐wells. After Heparinases incubation, cells were fixed and stained with anti‐HS antibody and HRP‐coupled secondary antibody. TBM was added before reading the absorbance at 450 nm.
Results: In HFRS cohort, HS and SDC1 plasmatic levels were significantly increased during disease compared to follow up (p < 0.01). MMP‐9 was also detected as higher concentration in plasma during disease than at follow up (p < 0.001) (Figure.1). QCM‐D experiments showed that Heparinase can specifically degrade b‐HS (∆f = −28% ±8; ∆D = −41% ±11). MMP‐9 sheddase, at plasmatic concentration during acute phase of HFRS, was also effective against SDC1 (∆f = −57% ±12; ∆D = −68% ±18) (Figure.2). In vitro results on GEnC cells showed HS degradation by Heparinase at concentrations ranging from 0.125 units/mL to 2 units/mL.
Conclusion(s): We identified two potential candidates that could contribute to cleavage of the eGLX during HFRS and could potentially contribute to disease pathogenesis and severity.


PB1283
Endothelial P2X7 promotes venous thromboembolism
E. Ollivier 1; V. Gourdou‐Latyszenok2; F. Couturaud3; C. Lemarie4
1 INSERM, UMR 1304, GETBO, BREST, Bretagne, France; 2 INSERM, UMR 1304, BREST, Bretagne, France; 3 Inserm, Univ Brest, CHRU Brest, UMR 1304, GETBO, Departement de Medecine Interne et Pneumologie, Brest, France, Brest, Bretagne, France; 4 INSERM, UMR 1304, brest, Bretagne, France
Background: Venous thromboembolism (VTE) affects 117 people per 100,000 each year and is an important cause of morbidity and mortality. VTE can lead to 1) death through pulmonary embolism, 2) the post‐thrombotic syndrome, or 3) chronic pulmonary hypertension resulting in significant chronic respiratory compromise. Inflammatory pathways are intricately involved in thrombus formation and therefore in VTE mechanisms. In pathological conditions, adenosine triphosphate (ATP) is released in the extracellular compartment and is recognized as a danger signal by several cell types including endothelial cells. Recent data indicated that the CD39/CD73 system involved in the metabolism of ATP into AMP might protects against thrombosis by downregulating the pro‐inflammatory pathway of the inflammasome. ATP can also interact with the P2X7 receptor involved in inflammation causing a wide range of responses.
Aims: To determine how the endothelial P2X7 receptor contributes to venous thromboembolism.
Methods: HUVECs were incubated with BzATP, thrombin or both. HUVECs were primed with TNF‐alpha prior to stimulation. We used immunofluorescence, western blot and real‐time quantitative PCR analyses to assess P2X7 expression by endothelial cells, activation of p38 and NFkB activation and expression of pro‐inflammatory and pro‐coagulant genes.
Results: We confirmed that endothelial cells expressed P2X7 in vitro in HUVECs and in vivo after induction of venous thrombosis by ligation of the inferior vena cava. Treatment of endothelial cells with BzATP and thrombin induced the activation of p38 and NFkB signaling pathways. This appears to be associated with an increased expression of IL‐1b and downregulation of thrombomodulin expression. The expression of adhesion molecules, ICAM‐1 and VCAM‐1, tends to increase.
Conclusion(s): Our data suggest that ATP released in the extracellular space following cell damage or activation induced a pro‐inflammatory response in endothelial cells through P2X7 activation. P2X7 might have a pro‐thrombotic role exacerbating venous thromboembolism.
PB1281
Non‐parenchymal origin of alternatively spliced tissue factor in chronic liver disease
C. Lewis1; K. Stone1; D. Kuhnell2; S. Langevin2; A. Soto‐Gutierrez3; P. Van Dreden4; J. Luyendyk5; V. Bogdanov 6
1 Division of Hematology/Oncology, Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States; 2 Department of Environmental & Public Health Sciences, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States; 3 Department of Pathology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States; 4 Diagnostica Stago, Gennevilliers, France., Gennevilliers, Ile‐de‐France, France; 5 Department of Pathobiology and Diagnostic Investigation, Michigan State University, East Lansing, Michigan, United States; 6 College of Medicine, University of Cincinnati, Cincinnati, Ohio, United States
Background: Expression of alternatively spliced Tissue Factor (asTF), an integrin activator, is low in the healthy liver. Recently, we reported that plasma asTF is elevated in patients with chronic liver disease (CLD).
Aims: To explore the potential cellular sources and mechanistic underpinnings of asTF's upregulation in CLD.
Methods: Extracellular microvesicles (MVs) and asTF protein were measured in CLD plasma. MVs were concentrated by ultracentrifugation and characterized using antibody selection and CountBright™ Plus Absolute Counting Beads. Immunohistochemistry was used to detect asTF protein in livers of mice with experimental hepatic fibrosis (carbon tetrachloride (CCl4) or high trans‐fat diet (HTFD)), and in human liver (healthy and cirrhotic). asTF mRNA and protein expression were studied in human cell lines HepG2 and Huh7 (hepatocytes), LX‐2 (stellate cells), and TMNK1 (liver sinusoidal endothelium).
Results: Plasma asTF levels associated strongly with those of CD31+/CD41‐ MVs; significance rose with increasing CLD severity. Compared to control mice, asTF staining was increased along the endothelium lining of central veins of both CCl4‐challenged and HTFD‐fed mice (Figure 1). asTF co‐localized with CD31 in the human liver with minimal expression in healthy tissue, high expression in low‐stage fibrosis, and moderate expression in late‐stage fibrosis (Figure 2). Constitutive asTF expression was extremely low in HepG2 and Huh7 cells compared to that observed in LX‐2 and TMNK1 cells. Treatment of TMNK1 cells with TNFα (20 ng/mL) induced asTF protein, which persisted beyond 6 hrs post‐induction. Notably, TNFα treatment of TMNK1 cells increased the release of both asTF protein and MVs. Interestingly, treatment of LX‐2 cells with angiotensin II (10 nM) transiently induced asTF expression.
Conclusion(s): Non‐parenchymal cells, particularly endothelium, display inducible asTF expression that may contribute to increased asTF in CLD. The precise role of asTF in CLD progression requires further study because asTF's impact on integrin signaling may comprise a therapeutic target.


PB1280
Investigation of podoplanin signaling complexes in primary lymphatic endothelial cells
T. Al Thunayan 1; M. Tomlinson2
1 Student, Reading, England, United Kingdom; 2 University of Birmingham, Birmingham, England, United Kingdom
Background: The lymphatic system is an extensive network of vessels which plays an important role in numerous physiological and pathological conditions such as the removal of excess fluid from tissues, immune surveillance and cancer metastasis. Despite this importance little is known about its formation. However, blood platelets have been found to play a vital role in the separation of the lymphatic vasculature from blood vessels throughout embryonic development and into adulthood. This role is independent of the classical function of platelets in thrombosis and hemostasis and is mediated by the interaction of the platelet C‐type lectin‐like receptor, CLEC‐2, with Podoplanin on lymphatic endothelial cells (LECs). The mechanism by which Podoplanin relays signals from platelets to the LECs is unclear.
Aims: This study aims to identify membrane associated Podoplanin containing signaling complexes in primary LECs and to understand the spatial regulation of these complexes.
Methods: LEC Podoplanin complexes were preserved by cell lysis in a Brij 97 buffer to preserve tetraspanin domains. Podoplanin containing complexes were immunoprecipitated, proteins separated by SDS‐PAGE and the presence of complex members determined by Western blotting. Super resolution microscopy (dSTORM) was used to study the spatial distribution of these complexes on the LEC surface.
Results: Podoplanin forms membrane associated complexes with multiple proteins, including the tetraspanin CD9. The composition of Podoplanin complexes changes following stimulation of the LEC signaling receptor VEGFR3, a receptor essential for lymphangiogenesis.
Conclusion(s): Podoplanin resides in tetraspanin domains, the composition of which changes upon the initiation of VEGFR3 signaling. The functional consequence of these dynamic changes is yet to be elucidated.
Inflammation and Sepsis.
VPB1293
Thromboinflammatory biomarkers of cardiorenal syndrome in patients undergoing maintenance hemodialysis in end stage renal disease
P. Karumanchi 1; V. Bansal2; F. Siddiqui2; D. Hoppensteadt3; J. Fareed3
1 Loyola University Medical Center, Palatine, Illinois, United States; 2 Loyola University Medical Center, Maywood, Illinois, United States; 3 Loyola University Chicago, Maywood, Illinois, United States
Background: Cardiovascular disease is a highly common complication in patients with end stage renal disease (ESRD) on maintenance hemodialysis. Current literature reveals that inflammation and thrombosis are integral to CRS development and key cardiac and renal biomarkers are elevated in CRS patients.
Aims: This study aims to demonstrate that thrombo‐inflammatory biomarkers and laboratory parameters give a telling narrative of ESRD progression to CRS.
Methods: Plasma samples were collected from 95 ESRD patients which were recruited with an approved IRB. Normal human plasma (NHP) was obtained from a commercial. Thrombo‐inflammatory biomarkers, including Annexin V, MPO, Troponin, L‐FABP, VEGF, D‐dimer, TNF‐alpha, IL‐6, CRP, eNOS, Nitrotyrosine, MDA, NEFA, NO, vWF and MCP‐1 were measured using commercially available ELISA methods. Results were then statistically analyzed using GraphPad Prism v.9; the results were compiled into mean ± SEM, percent change comparison to NHP, analyzed for significance.
Results: Most of the thrombo‐inflammatory CRS biomarkers were elevated in ESRD patients. Biomarkers including Annexin V (ESRD 7.57 ± 5.24, 23.64% vs. 6.125 ± 8.29, p < 0.001), L‐FABP (106599.43 ± 176728.97, 1983% vs. 5116.33 ± 1767.88, p < 0.0001), D‐dimer (ESRD 1447.01 ± 2103, 650.53% vs. 192.8 ± 212.9, p < 0.0001), TNF‐alpha (ESRD 2.52 ± 1.06, 35.87% vs. 1.85 ± 1.20, p < 0.0001), IL‐6 (ESRD 5.211 ± 10.36, 317.21% vs. 1.25 ± 1.24, p < 0.0001), CRP (ESRD 14.53 ± 14.11, 2214.43% vs. 0.63 ± 0.99, p < 0.0001), NO (ESRD 34.74 ± 19.74, 151.74% vs. 13.8 ± 5.60, p < 0.0001), vWF (ESRD 200.7 ± 25.89, 183.03% vs. 70.91 ± 23.13 p < 0.0001) and MCP‐1 (148.4 ± 56.09, 67.49% vs. 88.6 ± 27.25, p < 0.0001) showed significant increase when compared to NHP.
Conclusion(s): This study suggests that thrombo‐inflammatory biomarkers may be helpful in the understanding of the molecular pathogenesis of CRS in ESRD patients.

VPB1292
Contribution of isoproterenol in hemostatic alterations in vivo
Y. Prado 1; F. Marchant2; V. Alejandro3; F. Simon3
1 Millennium Institute in Immunology and Immunotherapy, Santiago, Region Metropolitana, Chile; 2 Millennium Institute in Immunology and Immunotherapy, santiago, Region Metropolitana, Chile; 3 Universidad Andrés Bello, Santiago, Region Metropolitana, Chile
Background: Chronic sympathetic (over)activity is a known feature of many inflammatory diseases including sepsis and COVID‐19 and is presented with high levels of circulant catecholamines that are associated with poor outcomes including thrombotic episodes derived from hemostatic alterations, multi organic failure and death. The β‐adrenergic receptor (β‐AR) subtype is known for regulate cardiac function and interestingly, emerging evidence has shown that β‐AR overactivity exerts deleterious effects besides of cardiac dysfunction, for instance, boosting inflammation through the enhanced expression of pro‐inflammatory cytokines as TNF‐α, IL‐1β, IL‐6 and IL‐18.
Aims: Considering the known crosstalk between inflammation and coagulation/thrombosis, we aimed to explore whether β‐AR stimulation promote hemostatic alterations conducing to thrombotic episodes.
Methods: Male Sprague–Dawley rats aged 8 weeks were anesthetized with isoflurane and subcutaneously injected with a single dose of the β‐AR agonist, isoproterenol (ISO, 5 mg/kg of body weight) in 2 mL (4 bolus of 500 μL) or the same volume of saline (n = 3). At 3 hours blood were collected, centrifugated at 1500 g for 15 minutes and plasma was undergone to hematological and coagulation analysis. Kidneys were harvested for histopathological analysis. The results are presented as ±SD. Significant differences were assessed by the t‐student test (Mann–Whitney). The experimental protocols were approved by the Bioethics and Biosafety Committee of the Andrés Bello University.
Results: ISO treated rats exhibited decreased lymphocytes, red blood cells, hemoglobin and hematocrit. Conversely, granulocytes and d‐dimer levels were increased in ISO rats regarding to controls. Histopathological analysis of ISO treated rats showed high vascular presence of red blood cells.
Conclusion(s): ISO stimulation promotes in vivo alterations in hemostasia that could enhance procoagulant states. Results suggest that β‐AR could be considered as target to improve the treatment of patients with inflammatory diseases, to reduce hemostatic alterations and procoagulant states.
PB1291
Biomarkers of endothelial cell dysfunction in HIV‐infected patients
E. Mayne 1; T. Abdool‐Carim2; M. Lederman3; N. Funderburg4; S. Louw5
1 Department of Pathology, Faculty of Health Sciences, University of Cape Town and National Health Laboratory Service, Cape Town, Western Cape, South Africa; 2 University of Witwatersrand, Johannesburg, Gauteng, South Africa; 3 Case Western Reserve University, Cleveland, Ohio, United States; 4 Ohio State University, Columbus, Ohio, United States; 5 National Health Laboratory Service (NHLS) / University of the Witwatersrand (Wits), Johannesburg, Gauteng, South Africa
Background: HIV‐infected patients are at increased risk of vascular disease and arterial thrombosis, linked to endothelial dysfunction and chronic inflammation.
Aims: To evaluate the presence of biomarkers of endothelial dysfunction in plasma the coagulation status of leukocytes in HIV‐infected patients.
Methods: Venous blood was collected with approval from the Wits Human Research Ethics Committee from healthy controls (n = 13) and compared with samples collected from HIV‐infected patients (n = 22). Biomarker levels in plasma were evaluated using the MILLIPLEX MAP Human Cardiovascular Disease (CVD) Magnetic Bead Panel 2 (Merck KGaA, Darmstadt, Germany). Flow cytometry was performed on an LSR II flow cytometer and analyzed on FlowJo (both Becton‐Dickinson, Franklin Lakes, USA). Monocytes and neutrophils were identified by light scatter characteristics and expression of HLA‐DR, CD33, CD14, CD16 and CD69 (monocytes) and CD33, CD16 and CD69 (neutrophils). Tissue factor was expressed as a percentage of positive cells. Descriptive statistics were computed and values were compared with a Mann–Whitney U test on Graphpad Prism (Graphpad, California, United States). A p‐value of <0.05 was considered significant.
Results: HIV‐infected patients showed elevated humoral markers of inflammation and endothelial dysfunction (GDF‐15, p‐selectin and sICAM‐1) when compared with uninfected controls. The leukocytes in these patients showed upregulation of the procoagulant tissue factor expression (Table 1).
Conclusion(s): HIV infection predisposes to endothelial dysfunction. In this study, we investigated the contribution of both humoral and cellular factors to increased cardiovascular risk. GDF‐15, an independent biomarker of cardiovascular disease and sICAM‐1 and p‐selectin, markers associated with leukocyte trafficking across the endothelium were significantly elevated in HIV‐infected patients in the absence of clinical cardiovascular disease. Tissue factor expression by leukocytes was also increased and which contributed to a general procoagulant state. Future directions will examine these factors in HIV‐infected patients with established vascular disease.

PB1288
Impact of periodontal inflammation on the cardiac tissues in a murine model
H. El Itawi 1; F. Batool2; C. Stutz2; F. Zobairi‐El Ghazouani3; O. Huck2; F. Toti2
1 INSERM U1260, Strasbourg, Alsace, France; 2 University of Strasbourg, Strasbourg, Alsace, France; 3 University of Strasbourg, 67,084, Alsace, France
Background: Periodontitis is a highly prevalent, oral inflammatory disease of infectious origin associated with cardiovascular worsened outcome, Porphyromonas gingivalis being the main causative pathogen.
Aims: To compare the effects of mechanical and/or P.gingivalis gum injury on the heart tissue in a murine experimental model of periondontitis.
Methods: In C57BL‐6 mice, a 2 or 4‐weeks gum injury was performed by ligatures with silk thread (LIG) soaked or not with P.gingivalis (LP and LP4w, thrice/week). Data were compared to 2‐weeks P.gingivalis oral gavage (gvg), intraperitoneal injection (IP). Other IP‐treated mice were simultaneously injected with Cl‐amidin (4 mg/mL), a PAD4 inhibitor (IP + Inh). After sacrifice, cardiac proteins were extracted from the left heart, pro‐atherothrombotic and pro‐inflammatory markers assessed by western blot.
Results: Compared to untreated mice (CTL) or P.gingivalis gavage, IP induced elevation of all inflammatory markers in heart tissue: VCAM (IP:0.8 ± 0.1,CTL:0.2 ± 0.05, p < 0.0001), Gasdermin (IP:1.2 ± 0.2,CTL0.2 ± 0.06 p = 0.0009),TNF‐alpha (IP:1.6 ± 0.2,CTL:2 ± 0.03 p < 0.0001), IL‐6 (IP:1.1 ± 0.6,CTL 0.2 ± 0.05 p < 0.001), IL‐10 (IP: 1.2 ± 0.2 CTL 0.3 ± 0.09, p = 0.04). PAD‐4 only down‐regulated IL‐10 (IP + INH:0.3 ± 0.06, IP:1.2 ± 0.2), suggesting the impact of netosis on anti‐inflammatory responses. After 2 weeks‐ligature, P.gingivalis‐soaked threads significantly upregulated VCAM and Gasdermin (LP:0.5 ± 0.1, p < 0.0001;LP:0.6 ± 0.1, p < 0.001 respectively; CTL:0.2 ± 0.055), while LIG had no effect. A 4‐weeks sustained treatment raised the expression of VCAM(LP4W: 0.4 ± 0.03, CTL 0.2 ± 0.03 p < 0.0001), TNF‐alpha (LP4W: 0.7 ± 0.1,CTL 0.2 ± 0.03 p < 0.0001) and IL‐6 (LP4w: 0.8 ± 0.1, CTL 0.2 ± 0.05 p < 0.001). Strikingly, only the IL‐10 expression reached the peak values induced by IP (LP4w: 1.1 ± 0.2,IP: 1.2 ± 0.2 vs CTL0,3 ± 0,09, p = 0.04), strongly suggesting an anti‐inflammatory effect prompted by P.gingivalis. Indeed, gavage led to elevated IL‐10 already after 2 weeks (0.9 ± 0.03).
Conclusion(s): Data confirm the model as suitable for the study of remote cardiac effects by P.gingivalis local infection. Moreover, low but sustained pathogen‐induced periodontitis alter the balance of heart inflammatory mediators. The control of netosis appears valuable to preserve or delay heart tissue inflammation.
PB1289
Gasdermin D is dispensable for NET formation in vivo
H. Englert 1; C. Rangaswamy1; M. Divivier1; J. Göbel1; I. Hermans‐Borgmeyer1; K. Mowen2; T. Fuchs1; T. Renné3
1 University Medical Center Hamburg Eppendorf, Hamburg, Hamburg, Germany; 2 Scripps Research, San Diego, California, United States; 3 Institut für Klinische Chemie und Laboratoriumsmedizin, Zentrum für Diagnostik, UKE, Hamburg, Germany, Hamburg, Hamburg, Germany
Background: Neutrophil Extracellular Traps (NETs) are scaffolds of extracellular fibers, primarily composed of unfolded double‐stranded DNA and granular enzymes, that are released from activated neutrophils. NETs have a role in host defense; however, excessive NET formation contributes to inflammatory and thrombotic diseases. The histone citrullinating protein peptidyl arginine deiminase 4 (PAD4) and the pore‐forming protein gasdermin d (GSDMD) have been proposed to play important roles for NET formation.
Aims: In this study, we aimed to study the role of GSDMD and PAD4 in NET formation in experimental models of septicemia and sterile neutrophilia.
Methods: We generated Gsdmd−/− and Pad4−/− knock‐out mice on a Dnase1−/‐Dnase1l3−/− deficient background. We induced sterile neutrophilia and septicemia in Dnase1−/‐Dnase1l3−/−, Dnase1−/‐Dnase1l3−/−Gsdmd−/− and Dnase1−/‐Dnase1l3−/‐Pad4−/− mice using a hepatic expression vector encoding the gene for G‐CSF and by injecting LPS and heat‐killed E. coli, respectively. The formation of NET clots in vitro from isolated murine neutrophils was tested using different activators and pharmacological inhibitors.
Results: Dnase1−/‐Dnase1l3−/−Gsdmd−/− and Dnase1−/‐Dnase1l3−/‐Pad4−/− mice were not protected from death during sterile neutrophilia or septicemia. Histological analysis of lung sections showed that NET‐derived clots occluded blood vessels in both mouse strains. Furthermore, we found that the genetic or pharmacological inhibition of GSDMD blocks in vitro NET formation in PMA‐activated murine neutrophils.
Conclusion(s): Taken together, we unexpectedly found that GSDMD deficiency blocked PMA‐induced NET formation in vitro, however it did not inhibit NET formation in our two independent in vivo models of sterile neutrophilia and septicemia.
PB1290
The contribution of peptidylarginine deiminase 4 and NETs to a dysregulated VWF/ADAMTS13 axis in Staphylococcus aureus bacteremia in patients
C. Martens 1; M. Peetermans2; M. Jacquemin3; P. Verhamme4; K. Martinod1
1 KU Leuven, Leuven, Vlaams‐Brabant, Belgium; 2 Medical Intensive Care Unit, Department of General Internal Medicine, University Hospitals Leuven, Leuven, Belgium; Laboratory for Clinical Infectious and Inflammatory Diseases, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 3 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium; Department of Vascular Medicine and Hemostasis, University Hospitals Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium; 4 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium
Background: Neutrophils are key players in sepsis pathogenesis, with neutrophil extracellular traps (NETs) providing a thrombus scaffold that also binds von Willebrand factor (VWF). The enzyme peptidylarginine deiminase 4 (PAD4) is not only important for NET formation but also inactivates ADAMTS13. We hypothesized that inactivation of ADAMTS13 by PAD4 might occur in vivo in Gram‐positive sepsis and contribute to clinical outcome.
Aims: To obtain clinical evidence of the balance between NETs/PAD4 and VWF/ADAMTS13 in systemic infection.
Methods: With appropriate ethical approval and informed consent, we collected plasma samples from hospitalized patients with Staphylococcus aureus bacteremia within 36 hours after bacteremia onset (n = 66, baseline), and 1 day (n = 58), 4 days (n = 49) and 7 days (n = 32) after enrollment. Samples were analyzed for PAD4, NET biomarkers, VWF antigen, and ADAMTS13 activity. Correlation analyses were performed using Spearman correlation with a two‐sided significance level of 0.05.
Results: Despite low prevalence of severe sepsis, we found elevated levels of H3Cit (2.60 [1.56–4.57] ng/ml), MPO‐DNA (8.32 [4.71–12.2] ng/ml), H3Cit‐DNA (58.2 [40.4–91.4] ng/ml), PAD4 (1.61 (0.79–4.41 ng/mL), and VWF:Ag (3.07 (2.16–3.84 IU/mL), and decreased ADAMTS13 activity (50 [37.0–65.8] %) (median (IQR), values at baseline). Whereas C‐reactive protein (CRP) decreased during longitudinal follow‐up, PAD4, VWF, MPO‐DNA, and H3Cit remained high. PAD4 antigen was negatively correlated with ADAMTS13 activity (r = −0.382, p = 0.002), and H3Cit levels were positively correlated with VWF:Ag throughout the disease course. Baseline PAD4 levels were positively correlated with APACHE‐II score, a predictor of mortality (r = 0.322, p = 0.009), and VWF was correlated with CRP (r = 0.304, p = 0.014 at baseline) and fibrinogen (r = 0.344, p = 0.015 at day 1).
Conclusion(s): We report elevated NETs and PAD4 in Gram‐positive bacteremia, together with reduced ADAMTS13 activity. This supports the hypothesis that inactivation of ADAMTS13 by PAD4 occurs in systemic S. aureus infection, and may contribute to sepsis pathogenesis.
PB1286
GPVI drives pulmonary inflammation by mediating platelet–neutrophil complex formation in a murine model of ARDS
P. Burkard 1; C. Schonhart2; D. Köhler3; P. Rosenberger3; B. Nieswandt2
1 University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Germany, Würzburg, Bayern, Germany; 2 Institute of Experimental Biomedicine, University Hospital Würzburg and Rudolf Virchow Center for Integrative and Translational Bioimaging, University of Würzburg, Würzburg, Bayern, Germany; 3 University Department of Anesthesiology and Intensive Care Medicine, University Hospital Tübingen, Tübingen, Baden‐Wurttemberg, Germany
Background: The Acute Respiratory Distress Syndrome (ARDS) is a detrimental inflammatory disease state associated with high mortality. Especially, the infiltration of neutrophils into the pulmonary airspace is causative for the acute inflammation and lung injury. A role of platelet glycoprotein (GP)VI to host defense in a model of pneumonia‐derived sepsis was suggested, but the underlying mechanisms remained elusive.
Aims: We aimed to mechanistically dissect the contribution of GPVI to thrombo‐inflammation in the acute phase of experimental ARDS in mice.
Methods: GPVI was depleted in wild‐type mice by injecting JAQ1 antibody. Acute alveolar inflammation was induced by intranasal instillation of lipopolysaccharide (LPS). A bronchoalveolar lavage (BAL) was performed after 4 hours and infiltrated cells, myeloperoxidase (MPO) activity, hemoglobin content and a set of cytokines were determined. MPO activity and Evans blue dye extravasation in the processed lung were analyzed. Furthermore, high resolution, multi‐color confocal microscopy of cryosections and the ventilated lung (intravital confocal microscopy) was performed to assess cell–cell interactions during acute inflammation.
Results: Control mice developed a profound inflammatory response to LPS characterized by pulmonary and blood neutrophilia, hypothermia, and increased blood lactate levels. In contrast, GPVI depleted mice were clearly protected from pulmonary and systemic compromises as evidenced by reduced hypothermia and blood lactate levels, while inflammatory bleeding was not increased. This was accompanied by a markedly mitigated pulmonary neutrophilic infiltration and reduced platelet–neutrophil complex (PNC) formation in lung tissue as detectable in cryosections and by intravital microscopy. Remarkably, however, the extent of neutrophil extracellular trap (NET) formation was not different between control and GPVI‐depleted animals. BAL analyses revealed diminished inflammatory cytokine levels and neutrophilic infiltrates in GPVI‐depleted mice.
Conclusion(s): GPVI drives alveolar inflammation by promoting neutrophil recruitment and PNC formation, but not NETosis. GPVI inhibition might be a promising strategy to reduce the acute pulmonary inflammation causing ARDS.
PB1287
The role of extracellular vimentin in fibrin formation
M. Cruz 1; M. Martinez‐Vargas1; L. Brubaker2; A. Cebula2; A. Yee3; R. Rumbaut2
1 Baylor College of Medicne, Houston, Texas, United States; 2 Baylor College of Medicine, Houston, Texas, United States; 3 Texas Children's Hospital/Baylor College of Medicine, Houston, Texas, United States
Background: Thrombosis is frequently manifested in critically ill patients with systemic inflammation, including sepsis. The coagulopathy in systemic inflammation is often associated with increased levels of fibrinogen and D‐dimer. There are studies that have reported elevated levels of vimentin in serum/plasma of septic patients.
Aims: We sought to investigate the relationship between vimentin and fibrinogen in fibrin polymerization.
Methods: We measured vimentin levels in plasma from healthy subjects and critically ill patients with systemic inflammation. We used recombinant vimentin protein to conduct biochemical studies and fibrin formation assays. Anti‐vimentin antibodies were used to analyze fibrin polymerization and fibrinolysis assays, to analyze fibrin clot structure using confocal microscopy, and for co‐immunoprecipitation studies. Size exclusion chromatography was utilized to isolate extracellular vimentin from plasma.
Results: The levels of vimentin in plasma derived from critically ill subjects with sepsis were in average three‐fold higher value compared to that of healthy subjects. We determined that vimentin interacts directly with fibrinogen and enhances fibrin formation. Anti‐vimentin antibody effectively blocked fibrin formation and caused changes in the fibrin structure in plasma. Additionally, confocal imaging demonstrated plasma vimentin enmeshed in the fibrin fibrils. Co‐immunoprecipitation assays demonstrated a direct interaction between extracellular vimentin and fibrinogen in plasma from critically ill patients but not in healthy plasma. Lastly, in contrast to healthy plasma, vimentin in plasma from critically ill patients co‐eluted with fibrinogen from the size exclusion column chromatography.
Conclusion(s): This study describes a novel role for extracellular vimentin in regulating fibrin formation and clot structure. The data suggest that elevated levels of an aberrant extracellular vimentin in critically ill patients with systemic inflammation potentiates fibrin clot formation; being consistent with the notion that plasma vimentin may contribute to the pathogenesis of thrombosis.
Innate and Adaptive Immunity
PB1296
Elevated NET‐formation and citrullinated‐fibrinogen are found in a porcine model of acute limb ischemia reperfusion injury
V. Zollet 1; I. Arenas2; S. Hirsiger3; B. Brahim2; M. Petrucci2; J. Mikulic2; D. Casoni2; J. Wang2; O. Beslac4; K. Nettelbeck2; L. Garcia2; l. Fuest5; E. Vögelin6; M. Constantinescu5; R. Rieben7; N. Sorvillo7
1 University of Bern, Switzerland, fribourg, Fribourg, Switzerland; 2 DBMR, bern, Bern, Switzerland; 3 Inselspital, Bern, Bern, Switzerland, 4 dbmr, bern, Bern, Switzerland; 5 Inselspital, bern, Bern, Switzerland; 6 Inselpspital, bern, Bern, Switzerland; 7 Department for biomedical research (DBMR), university of Bern, Switzerland, Bern, Bern, Switzerland
Background: Ischemia/reperfusion injury (IRI) is a pathological inflammatory process in which activated infiltrating neutrophils release neutrophil extracellular traps (NETs), a decondensed chromatin meshwork. During NET‐formation, the intracellular enzyme peptidyl‐arginine deiminase 4 (PAD4) is discharged to the extracellular milieu, where it citrullinates proteins leading to loss of protein function. While NET‐derived PAD4 was found to citrullinate plasma proteins in several diseases, its role in IRI is unknown.
Aims: Here, we aim to determine the effect of NETs and PAD4‐mediated protein citrullination in a model of acute limb IRI.
Methods: Amputated pig forelimbs were exposed to 1 h or 9 h of ischemia and then reperfused in vivo for 12 h. Limb weight and compartmental pressure were measured, and skeletal muscle was analyzed by immunofluorescence (TUNEL assay and dystrophin staining) to evaluate tissue damage. Myeloperoxidase and citrullinated histone 4 were used to detect neutrophils and NETs, respectively. Candidate citrullinated proteins were immuno‐precipitated from plasma samples using an anti‐citrulline probe and identified by Western blot.
Results: Limb weight and compartmental pressure were increased (0.3‐ and 3.4‐fold, respectively) in limbs that underwent 9 h ischemia compared to 1 h. Reperfused limbs after 9 h ischemia also displayed higher infiltration of neutrophils and NETs. Interestingly, immunohistochemical analysis revealed that the extent of disruption of skeletal muscle architecture correlated with the presence of NETs, suggesting a role for NETs in tissue damage. Next, we looked for citrullinated plasma fibrinogen and antithrombin, which could play a critical role in IRI. We found a 0.5‐fold increase of citrullinated fibrinogen in plasma of pigs which were grafted a limb after 9 h ischemia. Citrullinated antithrombin did not rise during IRI.
Conclusion(s): Our data reveal that NETs and levels of citrullinated fibrinogen correlated with tissue damage in IRI. Given its established role in the formation of plasmin‐resistant thrombi, this suggests that citrullinated fibrinogen contributes to the thrombo‐inflammatory events in IRI.
PB1294
Pulmonary thrombosis promotes sever flu in mice exposed to cigarette smoke
T. Kaminski; T. Brzoska; K. Robinson; T. Nyunoya; P. Sundd
University of Pittsburgh, Pittsburgh, Pennsylvania, United States
Background: Epidemiological evidence suggests that prior exposure to cigarette smoke (CS) or habitual smoking increases the risk of influenza A virus (IAV)‐triggered respiratory failure (severe flu). Although emerging evidence supports the role of thrombo‐inflammation in the development of CS and IAV‐triggered lung injury, the innate immune mechanism that contributes to this morbidity remains poorly understood.
Aims: To determine whether neutrophil‐platelet aggregation and NETs generation in the pulmonary microcirculation promotes CS and flu‐induced severe lung injury.
Methods: We used mouse model of 4‐week long exposure to CS with subsequent infection with IAV A/PR/8/34 (H1N1) strain. The body weight changes and oxygen saturation were measured every day for 7 days post IAV infection followed by assessment of lung injury. Lungs were harvested for histological assessment and estimation of viral titer using RT‐PCR. Quantitative fluorescence intravital lung microscopy (qFILM) was conducted 72–96 h post IAV‐infection to visualize the lung microcirculation of mice intravenously administered with fluorescent dextran, anti‐Ly6G Ab and anti‐CD49Ab to visualize pulmonary microcirculation, neutrophils and platelets, respectively.
Results: Mice exposed to CS+IAV manifested significantly more weight loss, drop in oxygen saturation and lung injury compared to mice exposed to room‐air+IAV. qFILM revealed that severity of lung injury in CS+IAV exposed mice was associated with significantly larger regions with impaired blood flow and vascular leakage in the lung, which was secondary to significantly more pulmonary vascular occlusion by platelet‐rich neutrophil‐platelets aggregates in mice exposed to CS+IAV than room‐air+IAV.
Conclusion(s): These initial results suggest that CS primes pro‐thrombo‐inflammatory signaling in neutrophils and platelets to promote severe lung injury following flu.
PB1295
Age related differences in monocyte subsets, cytokine patterns and the production of anti‐SARS‐CoV‐2 antibodies during acute COVID‐19 – a prospective observational longitudinal study
A. Pirabe 1; S. Heber2; W. Schrottmaier3; A. Schmuckenschlager4; S. Treiber3; D. Pereyra5; J. Santol1; E. Pawelka6; M. Traugott6; C. Schörgenhofer7; T. Seitz3; M. Karolyi8; B. Jilma7; U. Resch1; A. Zoufaly9; A. Assinger3
1 Institute of Vascular Biology and Thrombosis Research, Medical University of Vienna, Vienna, Wien, Austria; 2 Institute of Physiology, Medical University of Vienna, Vienna, Wien, Austria; 3 Medical University of Vienna, Vienna, Wien, Austria; 4 Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Wien, Austria; 5 Division of Visceral Surgery, Medical University of Vienna, Vienna, Wien, Austria; 6 Fourth Medical Department with Infectious Diseases and Tropical Medicine, Klinik Favoriten/Kaiser‐Franz‐Josef Hospital, Vienna, Wien, Austria; 7 Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Wien, Austria; 8 Department of Medicine IV, Clinic Favoriten, Vienna, Wien, Austria; 9 Department of Medicine IV, Clinic Favoriten; Faculty of Medicine, Sigmund Freud University, Vienna, Wien, Austria
Background: The COVID‐19 pandemic highlighted the vulnerability of the elderly population towards infectious threats, illustrating that aging is accompanied by dysregulated immune responses summarised in terms like inflammaging, immunoparalysis and immunothrombosis.
Aims: To gain a better understanding on the underlying mechanisms of the age‐associated risk of adverse outcome in individuals experiencing a SARS‐CoV‐2 infection, we analysed the impact of age on circulating monocyte phenotypes, activation markers, inflammatory cytokines, haemostatic parameters and the onset of anti‐SARS‐CoV‐2 antibody production in the context of COVID‐19 disease progression and outcome.
Methods: Blood samples were collected at the day of hospital admission followed by repeated blood draws (EK1315/2020). Blood parameters were quantified by bead‐based immunoassays and flow cytometry. Mixed linear models were applied to explore if the cytokine levels, monocyte subset ratios and antibody titers develop differently over the disease course in younger and elderly COVID‐19 patients and whether this development in turn differs between the outcome groups.
Results: Our data indicate no age‐associated differences in monocyte subset composition. However, age and outcome are associated with differences in monocyte activation status. Moreover, a distinct cytokine pattern of IL‐6, IL‐8 and TNF in elderly survivors versus non‐survivors suggests that older patients with adverse outcome experience an inappropriate immune response. Anti‐ spike S1 IgGs are reduced in elderly. However, deceased patients had higher anti‐spike IgG levels than patients requiring intensive care indicating that antibodies are not necessarily protective against adverse outcome. In addition, strong correlations were found between anti‐spike S1‐, anti‐nucleocapsid and anti‐RBD IgGs and platelet counts implying that platelets may play an underestimated role in the production of anti‐SARS‐CoV‐2 antibodies.
Conclusion(s): Our study underscores the importance of longitudinal monitoring in elderly COVID‐19 patients, as dynamic changes after symptom onset can be observed, which allow for a differentiated insight into confounding factors that impact the complex pathogenesis following an infection with SARS‐CoV‐2.
Non‐coding RNAs
VPB1297
Fingerprint of novel circulating microRNAs identify patients with stroke‐embolic stroke of undetermined source
C. Eyileten1; Z. Wicik1; J. Jarosz‐Popek1; A. Nowak1; A. Shahzadi2; D. Keshwani1; T. Adem1; D. Mirowska‐Guzel1; G. Pare3; M. Postula1; M. Wolska 4
1 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 2 Istanbul Cerrahpasa University, Istanbul, Istanbul, Turkey; 3 McMaster University, Hamilton, Ontario, Canada; 4 Centre for Preclinical Research and Technology (CePT), Medical University of Warsaw, Warsaw, Mazowieckie, Poland
Background: Circulating levels of selected miRNAs open up new avenues for the identification of more effective and specific biomarkers to identify and risk‐stratify stroke patients.
Aims: Aim is to identify and select specific miRNAs to be used as disease biomarkers to improve both prognosis and prediction.
Methods: 48 patients with embolic stroke of undetermined source (ESUS) were involved in the study. We divided the patient groups based on patients who had a second stroke or TIA and did not have. Total RNA was extracted from plasma and quality was assessed with fluorometric electrophoresis. Microarray analysis by Affymetrix. Statistical analysis: in TAC software and R using Signal information obtained from the TAC output, FDR correction, logistic regression, calculated AUC using ROCp R package. Coexpression analysis to identify genes authentically expressed was performed using Spearman correlation (cutoff = 0.9, Rpval = 0.05).
Results: MiR‐4786, miR‐1205, miR‐548ar‐3p, miR‐518e‐3p were found the most differentially expressed miRNAs between the groups (patients with one stroke vs multiple strokes). miR‐4786 was studied only in patients with acute leukemia. Several studies showed the importance of miR‐1205 in cell carcinoma and ovarian cancer progression. Moreover, so far only one study showed the regulation of miR‐548ar‐3p in breast cancer. Finally only one study showed the alteration of miR‐518e‐3p in Parkinson's disease patients. Our enrichment analysis showed IL‐2 signaling pathway, lipid and lipoprotein metabolism, BDNF signaling pathway, MAPK signaling pathway, Intellectual Disability, Alzheimer's Disease are significantly related to ESUS‐ patients.
Conclusion(s): Our results identified several novel circulating miRNAs that are down‐ or up‐regulated in ESUS‐stroke patients, as showing the predictive significance for the assessment of risk of the second stroke. Among those with the most relevant differential expression, several miRNAs were already identified to play a role in the pathophysiology of neurovascular diseases, paving the way to a new class of smart pathophysiology‐based biomarkers in stroke.


VPB1298
Platelet‐derived miRNAs and platelet‐derived extracellular vesicles as emerging diagnostic and prognostic biomarkers in acute ischemic stroke patients
C. Eyileten 1; D. Jakubik1; A. Shahzadi2; J. Jarosz‐Popek1; D. Keshwani1; A. Gasecka1; M. Wolska3; A. Nowak1; S. De Rosa4; E. van der Pol5; D. Mirowska‐Guzel1; I. Kurkowska‐Jastrzebska6; A. Czlonkowska6
1 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 2 Istanbul Cerrahpasa University, Istanbul, Istanbul, Turkey; 3 Medical University of Warsaw, Warsaw, Mazowieckie, Poland; 4 Magna Graecie University, Catanzaro, Calabria, Italy; 5 University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 6 Institute of Psychiatry and Neurology, Warsaw, Mazowieckie, Poland
Background: Ischemic stroke (IS) is one of the most frequent causes of death.
Aims: We aimed to analyze the circulating platelet‐derived miR‐19a‐3p, miR‐186‐5p, Let‐7f, platelet‐extracellular vesicles (EVs), leukocyte‐EVs, and endothelial‐EVs levels 24‐h and 7‐days after IS as novel diagnostic and prognostic/predictive biomarkers.
Methods: Blood samples of 28 patients with acute IS were collected 24‐h and 7‐days after stroke and 35 age‐ and gender‐matched individuals free of stroke. Platelet reactivity by AA‐, TRAP‐, ADP‐induced platelet aggregometry. PlasmaRNA was extracted; quality RNA was assessed: fluorometric assay; RT‐PCR for miRNAs expression and flow‐cytometry for EVs determination.
Results: Patients with IS on day‐1 had significantly higher platelet reactivity assessed by AA‐induced platelet aggregometry compared to controls (p = 0.001). Patients with normal platelet activation had significantly higher miR‐186‐5p expression levels compared to patients with HPR at day‐1 acute‐stroke (p = 0.034). 7‐days after stroke, expression levels of miR‐186‐5p decreased in the same patients with normal platelet reactivity (p = 0.036). Patients with HPR had significantly elevated platelet‐EVs (CD62) concentration compared to patients with normal platelet reactivity at the day of 1 acute‐stroke (p = 0.012). Patients with HPR had significantly higher leukocyte‐EVs (CD45) concentration compared to patients with normal platelet function at day‐1 stroke (p = 0.002). Diagnostic values of baseline miRNAs and EVs were evaluated with receiver operating characteristic curve analysis. ROC curve showed that pooling the miR‐19a‐3p expressions, platelet‐EVs, and leukocyte‐EVs concentration yielded a higher AUC than the value of each individual biomarker as AUC was 0.893 (95% CI, 0.79–0.99). Patients with moderate stroke had significantly elevated miR‐19a‐3p expression compared to patients with minor stroke at the day of acute IS. ROC curve was 0.867, (95% CI, 0.74–0.10) p = 0.001.
Conclusion(s): Our analysis showed alteration of circulating miRNAs and EVs after IS. Combination of miR‐19a‐3p, Let‐7f, platelet‐EVs and leukocyte‐EVs might have a diagnostic value for acute stroke and miR‐19a‐3p can predict the severity of stroke in IS patients.


Protease Activated Receptors
VPB1301
Mechanism of hepatocyte growth factor‐induced decreased expression of protein C inhibitor in HepG2 cells
N. Akita 1; T. Okamoto2; J. Nishioka1; K. Suzuki1; T. Hayashi3
1 Suzuka University of Medical Science, Suzuka, Mie, Japan; 2 Shimane University, Izumo, Shimane, Japan; 3 Mie Prefectural College of Nursing, Tsu, Mie, Japan
Background: Protein C inhibitor (PCI), a member of the SERPIN family, inhibits an anticoagulant activated protein C (APC), which inactivates blood coagulation cofactors, factors Va and VIIIa in plasma. We have so far shown that PCI is expressed in various human tissues, including liver and kidneys, suppresses tumor infiltration by inhibiting urinary plasminogen activator (uPA) and tumor metastasis by inhibiting angiogenesis, and regulates liver regeneration by inhibiting hepatocyte growth factor activator (HGFA), which is an activator of hepatocyte growth factor precursor to hepatocyte growth factor (HGF). Recently, we showed that HGF decreases PCI expression in HepG2 cells, although the detailed mechanism is still unclear.
Aims: In the present study, we investigated the detailed signal transduction mechanism of HGF‐induced decreased expression of PCI in HepG2 cells.
Methods: HepG2 cells were cultured using RPMI1640 containing 10% fetal bovine serum (FBS). Confluent HepG2 cells were treated with various signal transduction inhibitors such as c‐Met inhibitor, MEK inhibitor, PI3K inhibitor, JUN kinase inhibitor and ERK inhibitor, in the absence of FBS for 24 h, and the culture supernatants were recovered. PCI concentration in the culture supernatants was measured using specific ELISA.
Results: PCI expression in HepG2 cells was decreased by HGF treatment, and this HGF‐induced decreased expression of PCI in HepG2 cells was recovered by c‐Met inhibitor, MEK inhibitor and PI3K inhibitor treatments, but JUN kinase inhibitor and ERK inhibitor had no effect on it.
Conclusion(s): Our results indicated that HGF decreases PCI expression in hepatocytes via c‐Met, MEK and PI3K.
PB1300
Intestinal epithelial protease‐activated receptor‐1 affects gastrointestinal motility and the enteric nervous system
G. Pontarollo; O. Dremova; V. Nguyen; K. Endres; C. Reinhardt
Johannes Gutenberg University Mainz, Mainz, Rheinland‐Pfalz, Germany
Background: Protease‐activated receptor‐1 (PAR1), traditionally called the thrombin receptor (F2r), is expressed in several cell types in the gastrointestinal (GI) tract, where it mediates (patho)physiological functions. The GI tract presents its own intrinsic nervous system, the enteric nervous system (ENS), controlling GI transit and composed by the submucosal and myenteric plexuses. PAR1 modulates GI motility by affecting smooth muscle cell contraction/relaxation and sensory neurons. However, the underlying molecular pathways and the cell‐specific contribution of PAR1 on the ENS remain elusive.
Aims: To analyze the cell type‐specific role of PAR1 on GI motility and ENS morphology.
Methods: Two conditional PAR1‐knockout mouse models were generated: flF2rxVil‐Cre strain (F2rΔIEC) and flF2rxVE‐Cdh‐Cre strain (F2rΔEC), in which PAR1 is deleted in the intestinal epithelial or endothelial cells. GI motility was analyzed by monitoring the ex vivo fecal transit time in the colon (cm/min). The ENS was studied on the myenteric plexus isolated from the mid‐ and distal‐small intestine. Longitudinal muscle‐myenteric plexus (LMMP) layers were dissected and stained for β3‐tubulin (Tuj1), a pan‐marker for axons and dendrites. The status of the ENS was assessed by quantifying the Tuj1+ area on a region of interest. For each strain, knockout mice were compared to wild type (WT) littermates. Results were presented as mean ± SEM and evaluated by independent samples Student’s t test.
Results: GI motility assay revealed that F2rΔIEC mice present an increased fecal speed compared to WT controls. Quantification of β3‐tubulin highlighted a lower Tuj1+ area in the mid‐small intestine of F2rΔIEC mice, while F2rΔEC mice showed an opposite, albeit not significant, trend. In both mouse models, distal‐small intestine was unaffected.
Conclusion(s): Intestinal PAR1 mediates different effects on GI motility and ENS, depending on the cell type. In particular, PAR1‐deletion in the epithelium leads to an impaired neuronal network in the jejunum, but increased GI motility in the colon.


PB1299
EPCR−PAR1 biased signaling regulates perfusion recovery and neovascularization in peripheral ischemia via mechanism involving nitric oxide
M. Bochenek 1; R. Gogiraju2; S. Großmann3; J. Krug3; J. Orth3; S. Reyda1; G. Georgiadis4; S. Konstantinides1; T. Münzel5; J. Griffin6; P. Wild7; C. Espinola‐Klein3; W. Ruf8; K. Schäfer9
1 Center for Thrombosis and Hemostasis, University Medical Center, Mainz, Rheinland‐Pfalz, Germany, 2 University Medical Center Mainz, Mainz, Rheinland‐Pfalz, Germany; 3 Department of Cardiology, University Medical Center, Mainz, Rheinland‐Pfalz, Germany; 4 Department of Cardiology, Democritus University of Thrace, Alexandroupolis, Evros, Greece; 5 Center for Cardiology – Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Rheinland‐Pfalz, Germany; 6 Department of Molecular Medicine, The Scripps Research Institute, La Jolla, California, United States; 7 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Rheinland‐Pfalz, Germany; 8 Center for Thrombosis and Hemostasis, Johannes Gutenberg University Medical Center, Mainz, Rheinland‐Pfalz, Germany; 9 University Medical Center Mainz, Mainz, Rheinland‐Pfalz, Germany
Background: Blood clot formation initiates ischemic events, but the role of coagulation during postischemic tissue repair is poorly understood. The endothelial protein C receptor (EPCR) regulates coagulation and vascular signaling by protease activated receptors (PARs).
Aims: To study the effect of coagulation receptors in ischemia‐induced new vessel formation.
Methods: The mouse model of unilateral hindlimb ischemia (HLI) was used to induce new vessel formation, and reperfusion of the distal hindlimbs was monitored over four weeks using Laser Doppler perfusion imaging (LDPI). Gastrocnemius (GC) muscles of the ischemic and contralateral control hindlimb were analyzed. Human microvascular endothelial cells were subjected to hypoxia (1% oxygen) and the role of EPCR was examined.
Results: Here, we show in an unilateral hindlimb ischemia model in mice that endothelial EPCR−PAR1 signaling supports reperfusion and neovascularization. Whereas deletion of PAR2 or PAR4 did not impair angiogenesis, EPCR and PAR1 deficiency or PAR1 resistance to cleavage by activated protein C in PAR1 R46Q mice caused markedly reduced postischemic reperfusion in vivo and angiogenesis in vitro. These findings were corroborated in primary human endothelial cells stimulated with biased PAR1 agonist peptides. Loss of EPCR−PAR1 signaling upregulated hemoglobin expression and reduced endothelial nitric oxide (NO) bioavailability. Defective angiogenic sprouting was rescued by the NO donor DETA‐NO, and NO scavenging or inhibition of endothelial NO signaling increased hemoglobin and mesenchymal marker expression in primary endothelial cells. Endarterectomy specimens from patients with ischemic peripheral artery disease also showed reduced endothelial EPCR expression associated with increased hemoglobin expression and NO bioavailability.
Conclusion(s): Our data implicate endothelial EPCR−PAR1 signaling in neovascularization after ischemia and identify endothelial hemoglobin expression as an unexpected link between coagulation signaling and the preservation of endothelial cell NO bioavailability and prevention of fibrosis.
Stem Cells and Vascular Cell Growth
PB1304
Characterising endothelial colony‐forming cells in the context of HIV: People living with HIV have elevated endothelial colony‐forming cell frequency
A. Khawaja 1; R. Maughan2; K. Paschalaki2; C. Pericleous2; J. Mason2; M. Nelson3; G. Taylor4; A. Randi1; M. Boffito3; M. Emerson2
1 Imperial College London, London, England, United Kingdom; 2 National Heart and Lung Institute, Imperial College London, London, England, United Kingdom; 3 Chelsea and Westminster Hospital NHS Foundation Trust, London, England, United Kingdom; 4 Department of Infectious Disease, Imperial College London, London, England, United Kingdom
Background: Improvements in HIV management have transformed a once fatal infection into a manageable chronic condition, resulting in an ageing population of people living with HIV (PLHIV). PLHIV are less likely to die from AIDS‐related illness, but are twice as likely to develop cardiovascular disease (CVD). Patient‐derived endothelial colony‐forming cells (ECFCs) are being increasingly used to study endotheliopathy of specific diseases, but have not been exploited within the context of HIV where the pathophysiology of CVD remains unclear.
Aims: To assess whether ECFC can be successfully isolated from PLHIV.
Methods: Whole blood was obtained from PLHIV on effective antiretroviral therapy (ART) (n = 10) or HIV‐negative people accessing Pre‐Exposure Prophylaxis (PrEP, n = 10). Peripheral blood mononuclear cells (PBMCs) were isolated and monitored for ECFC growth. Day of colony emergence, time to first split (P0 split), and ECFC frequency were used to define isolation kinetics. A previously isolated HIV‐negative, ART‐naïve control group was used as a reference control group. Statistical significance was determined by one‐way ANOVA with Tukey's multiple comparison test.
Results: ECFCs emerged in 10/10 PLHIV, 8/10 PrEP users and 12/13 controls. From the first colony emerging, PLHIV‐ECFCs took 22.5 ± 1.5 days (mean ±SEM) for colonies to establish for first split, which was significantly longer than both PrEP‐ECFCs (15.4 ± 2.1 days, p < 0.05) and reference ECFCs (14.9 ± 1.1 days, p < 0.01). PLHIV had higher ECFC frequencies (0.52 ± 0.15 colonies/1 × 106 PBCMs) compared to both the PrEP group (0.21 ± 0.06 colonies/1 × 106 PBCMs, p < 0.05) and reference control group (0.13 ± 0.03 colonies/1 × 106 PBCMs, p < 0.01).
Conclusion(s): ECFCs from PLHIV on effective therapy can be isolated and will be used to explore the molecular effects of ART and HIV infection upon endothelial thrombo‐inflammatory properties. The significance of higher ECFC frequency and the longer time for colony establishment in PLHIV is currently unknown, but may provide a route to defining, preventing and treating endothelial dysfunction in this population.
VPB1305
Сarrying out stem cell autotransplantation without cryo‐freezing
I. Berger1; A. Achilova2; A. Kayumov2; G. Makhamadalieva 2; Z. Rajabova2
1 Head of the Scientific Laboratory of Hemostasis Pathology, Tashkent, Toshkent, Uzbekistan; 2 Republican Scientific and Practical Medical Center of Hematology (RSPMTSG) of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Toshkent, Uzbekistan
Background: In the Center of Hematology (Uzbekistan) in 2021, 31 autologous transplantations of hematopoietic blood stem cells (HSCC) were performed. In 11 patients (Group 1), transplantation was performed without the use of cryofreezing of the obtained HSCC and 20 auto‐HSCCs (Group 2) with freezing according to standard protocols.
Aims: Elimination of toxicity from DMSO during autotransplantation in patients with multiple myeloma.
Methods: The stem cells obtained by apheresis on the Teruma "Spectra Optia" apparatus were stored for 48 h at a temperature of +4 – +8 C in the refrigerator. Group 2 underwent HSC autotransplantation with DMSO freezing at −90C, which were stored for 1–2 months. The number of CD 34+ cells was determined by flow cytometry on a BD FACS Calibur apparatus using Beckman Coulter reagents (USA)
Results: When collecting HSCs in both groups, CD 34+ averaged 5.3 × 106 cells/ml, stem cell viability was 100%, and 48 h later, stem cell viability before transfusion in patients of group 1 was 95%, in group 2, after defrosting after 2 months, it was 99%. During autotransplantation of the 1st group of patients, there were no undesirable manifestations from the entry of DMSO into the patient's blood (signs of cardiorespiratory toxicity and allergic reactions). The absence of toxic complications contributed to the reduction of bed‐days in the sterile ward, and averaged 18 days. In group 2, during auto‐HSC, an increase in blood pressure was recorded – in 85% of cases, a decrease in SPO2 – in 55%, nausea in 100% of patients, vomiting – in 20% of cases. Bed‐days amounted to 24 days.
Conclusion(s): Conclusion: The use of HSC without freezing improves the results of clinical tolerability, while the viability of stem cells enters the target normative values (90–99%), and also reduces the number of bed‐days spent in the hospital, which determines its economic efficiency.
PB1303
Generation of iPSC‐derived vascular cells deficient in tissue factor pathway inhibitor
V. Bröker 1; A. Akbulut2; L. Schurgers3; T. Hackeng3
1 Maastricht University, Maastricht, Limburg, Netherlands; 2 Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 3 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands
Background: Tissue factor pathway inhibitor (TFPI) is a Kunitz‐type inhibitor that acts as a constitutive inhibitor of the extrinsic coagulation pathway. Previous studies have shown that TFPI‐deficiency leads to the accelerated formation of atherosclerotic plaques in animal models.
Aims: The aim of this study is to generate iPSC‐derived vascular smooth muscle cells (SMC) and endothelial cells (EC) deficient in TFPI. These cells will serve as a robust platform for studying the impact of TFPI on cardiovascular pathologies.
Methods: Human induced pluripotent stem cells (iPSC) were modified by using a CRISPR/Cas9‐mediated approach targeting the FXa‐binding Kunitz(K)2‐domain of TFPI. Genetically modified cells were selected, characterized by pluripotency marker staining, and further differentiated into SMC and EC using small molecule‐based protocols. Expression of TFPI was assessed using Western blot and an in house‐developed ELISA.
Results: CRISPR/Cas9‐mediated modification of iPSCs resulted in a 12‐bp in‐frame deletion at the K2‐domain. Modified cells exhibited a slightly altered iPSC morphology but showed positive expression of pluripotency markers. Furthermore, cells were successfully differentiated into SMC and EC as confirmed by positive expression of respective markers. Western blot analysis revealed that TFPI was still expressed in modified cells pointing to the presence of a 4 amino acid truncated (∆~605 Da) and possibly unfunctional protein.
Conclusion(s): The generation of TFPI‐deficient cell lines will give further insights into the initiation and progression of cardiovascular diseases. Whether the expressed protein is active still remains to be elucidated. However, the disruption of the crucial K2‐domain suggests improper protein function. iPSC‐based methods allow incorporation of engineered cells in autologous microfluidic systems and co‐culture models improving physiologic and pathologic representation in vitro and therefore will lead to more reliable in vitro research outcomes.
PB1302
Lineage specific vascular smooth muscle cell differentiation from pluripotent stem cells: A developmental bias towards aortic calcification
A. Akbulut 1; N. Rapp2; H. Davaapil3; S. Sinha3; L. Schurgers4
1 Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands; 2 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, The Netherlands, Maastricht, Limburg, Netherlands; 3 Department of Medicine and Wellcome ‐MRC Cambridge Stem Cell Institute, University of Cambridge, Cambridge, England, United Kingdom; 4 Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Limburg, Netherlands
Background: Vascular calcification is an active process that contributes to cardiovascular disease wherein the vascular smooth muscle cell (SMC) is the protagonist to disease progression. The developmental heterogeneity of SMC may attribute to the site‐specific occurrence of calcification.
Aims: We aimed to generate and characterise various lineage SMCs from human induced pluripotent stem cells (iSMCs). Next, we aimed to determine whether the various lineage iSMCs would be capable to calcify and might there be differences in calcification between the various iSMCs.
Methods: For this study we performed stepwise lineage specification to generate iSMCs. Lateral mesoderm (LM), paraxial mesoderm (PM) and neural crest (NC) are induced before iSMC specification. Then iSMCs were subjected to calcification assay on various concentrations of calcium and serum. Next, we compared the calcification potential of the various lineages under optimised conditions against each other. Thereafter, we performed bulk RNA‐sequencing on various iSMCs in calcification vs control experiments.
Results: Differentiation to various lineages, characterisation of iSMC subpopulations demonstrates that cells ubiquitously express canonical SMC markers. Next, we checked functionality with regards to calcification deposition. Either lowering serum concentration or increasing total calcium levels resulted in increased calcification in all cell types, confirming both functionality and dose response. On comparing calcification of the three different sub‐populations, we found that LM iSMCs calcify more and faster than the two other lineages. No difference was found between calcification of NC or PM iSMCs. Upon performing RNA‐sequencing DEGs are present between lineages. Further within the PC2 there is a drift for NC and PM populations that is absent in the LM‐iSMCs.
Conclusion(s): Lateral mesoderm iSMCs calcify more and faster than both paraxial mesoderm and neural crest iSMCs in our disease‐in‐a‐dish calcification model. This confers to aortic calcification that canonically starts at the aortic root. We are currently investigating mechanism and translation of our findings.
Venous Thromboembolism
Antiphospholipid Syndrome
VPB0418
Clinical and immunological features of antiphospholipid syndrome
A. Baya Chatti 1; S. Skhiri2; A. Guiga2; W. Ben Yahia2; A. Atig2; N. Ghannouchi2
1 Farhat Hached Hospital, Tunis, Sousse, Tunisia; 2 Farhat Hached Hospital, Sousse, Sousse, Tunisia
Background: Antiphospholipid syndrome (APS) is a common cause of acquired hypercoagulability. Its major presentations are thrombotic (arterial, venous, or microvascular) and pregnancy morbidity.
Aims: This study aimed to describe clinical and serological features of APS, in a cohort of Tunisian patients.
Methods: We conducted a retrospective study including 19 patients meeting the international Sidney consensus criteria, over an 11‐year period [2009 to 2020]. Clinical, biological and immunological data were analysed.
Results: The mean age at diagnosis was 31.4 [20–59]. The gender ratio (F/M) was 8.5. APS was secondary (63.2%) to systemic lupus erythematosus (91.7%) and to immune thrombocytopenic purpura (8.3%). It was inaugurated by thrombosis (68.4%) followed by obstetric events (31.6%). Thrombosis occurred in 15 patients. Ten patients developed Venous thrombosis with 16 episodes during follow up, mainly unprovoked (68.8%), involving lower extremities (n = 13), portal vein, cerebral vein thrombosis and Budd Chiari syndrom in one case respectively. Nine arterial thrombosis occurred in seven patients: Limb ischemia, cerebrovascular accident, myocardial infarction in two cases respectively, and ischemic optic neuropathy, mesenteric and appendicular ischemia, in one case respectively. One patient had intracardiac thrombus. Skin manifestations (n = 5) were acrocyanosis, vasculitis and necrotic ulcers in two cases respectively, livedo reticularis, purpura and Raynaud syndrome in one case respectively. Eleven females developed obstetric complications: Foetal losses (n = 13) with 17 intrauterine foetal deaths (n = 8) and eight miscarriage (n = 5), prematurity (n = 5), preeclampsia (n = 3) and intrauterine growth restriction (n = 1). Biology revealed prolonged activated partial thromboplastin time (n = 13), hrombocytopenia (n = 6) (two patients with primary APS), and autoimmune hemolytic anemia in five patients with APS secondary to SLE. Immunological profiles showed isolated LA positivity (n = 10), triple positivity (n = 4), Anti‐ACL/LA (n = 3), Anti‐β2GPI/LA and isolated anti‐ACL in one patient respectively.
Conclusion(s): APS is a heterogeneous disorder in terms of clinical and immunological features. It must be considered in women with gestational complications or in case of recurrent thrombosis.
PB0410
DRVVT in APLA‐ As a single test and in combination with anti B2‐glycoprotein and anti cardiolipin
P. Balasubramanian 1; T. Geevar2; R. Dave2; J. Jude prakash3; J. Mathew3; A. Abraham4; J. L3; S. Nair2
1 All India Institute of Medical Sciences, AIIMS, Rishikesh, Rishikesh, Uttarakhand, India; 2 Christian Medical College and Hospital, Vellore, Vellore, Tamil Nadu, India; 3 Christian Medical College, Vellore, Vellore, Tamil Nadu, India; 4 Christian Medical College, Vellore, vellore, Tamil Nadu, India
Background: APLA is one of the most common acquired thrombophilias.
Aims: To study the significance of dilute russel viper venom test (DRVVT) towards the diagnosis of antiphospholipid antibody syndrome (APLA) – As a stand alone test and in combination with antibodies to β2‐glycoprotein I (anti‐β2‐ GPI) and cardiolipin (aCL).
Methods: This was a six month retrospective study. The electronic medical records of 451 patients referred for lupus anticoagulant (LAC) test were reviewed. LAC was done by DRVVT. Anti IgG, IgM β2‐GPI and IgG aCL were done by enzyme linked immunosorbent assay (ELISA).
Results: 451 patients were referred for LAC test in view of arterial thrombosis (12.4%), venous thrombosis (10.1%), thrombocytopenia (7%), pregnancy morbidity (8.6 %), systemic lupus erythematosus (16%), other autoimmune causes (6.8%). DRVVT positivity was seen in 49 cases (23%). Mild positivity in 29, moderate in 14 and marked in 6 cases. In DRVVT positive patients, IgG anti‐β2‐GPI was positive in 8 cases, IgM anti‐β2‐GPI in 9 and IgG aCL in 15 cases. Repeat LAC testing was sent only for 12 cases. Triple positivity was seen in 10 out of 29 cases. In DRVVT negative patients (89%), IgG anti‐β2‐GPI was negative in all cases, IgM anti‐β2‐GPI was positive in 21 cases and IgG aCL in 5. In other indications (38.5%) referred for LAC, only two cases were mild positive for DRVVT. Secondary causes were excluded for analysis of sensitivity, specificity of different test modalities (Table 1). DRVVT had highest sensitivity and specificity in cases presenting with thrombosis. IgG aCL was more specific in arterial thrombosis and anti IgG β2 GPI was more specific in venous thrombosis, thrombocytopenia and abortions.
Conclusion(s): APLA is diagnosed based on clinical and laboratory criteria. DRVVT as a single test has higher sensitivity and specificity in thrombosis but simultaneous testing for other antiphospholipid antibodies increases the specificity.

PB0417
Single centre experience of lupus anticoagulant testing
K. Mordue; C. Lentaigne
University Hospitals Plymouth NHS Trust, Plymouth, England, United Kingdom
Background: Lupus Anticoagulant (LA) is one of three laboratory criteria defined by evidence‐based, international guidelines to identify antiphospholipid syndrome (APS). LA testing focuses on patients likely to have APS – those with unprovoked VTE, arterial thrombosis and pregnancy morbidity. These guidelines further detail appropriate testing following an initial positive LA to diagnose APS, including confirmation of persistent LA, anti‐cardiolipin (ACL) or anti‐β2‐glycoprotein‐1 (B2GP1) antibodies.
Aims: To audit the use and interpretation of LA at our institution against ISTH guidance (Devreese et al., 2020).
Methods: Retrospective analysis was performed on LA requests received within the Coagulation laboratory at University Hospitals Plymouth, during August 2021. The requests and results were interrogated against an audit questionnaire addressing ISTH guideline standards.
Results: A total of 65 LA requests were received; 66% had appropriate clinical indications. The majority (56.9%) were from Neurology – stroke was the most common clinical indication. LA was detected in 18.6% of requests; 2/11 by DRVVT and PTT‐LA, 8/11 by PTT‐LA, and 1/11 by DRVVT; 5 patients had equivocal LA results by DRVVT. All four anticoagulated patients tested using Taipan Snake Venom Time were negative. Of the 16 positive or equivocal LA results, 62.5% were repeated at >12 weeks to demonstrate persistence. ACL was included with 92.3% of LA requests, whilst B2GP1 was only requested with 53.8%; 50% of positive ACL and B2GP1 were appropriately repeated.
Conclusion(s): This small, single‐institution snapshot of LA testing has provided reassurance that most requests were for appropriate clinical indications and were appropriately followed up with a second test >12 weeks; however, it was concerning that many were not accompanied by B2GP1 request. Incomplete investigation of a positive LA may lead to underdiagnosis of APS. As Neurology are the main contributor of LAS requests, their involvement in Haemostasis MDTs will help reinforce appropriate and complete testing.
PB0411
Hydroxychloroquine as an adjunct treatment for patients with triple positive thrombotic antiphospholipid syndrome‐ a single centre experience
C. Crossette‐Thambiah 1; M. Laffan2; D. Arachchillage3
1 Imperial College London, London, United Kingdom; 2 Centre for Haematology, Imperial College London, London, United Kingdom; 3 Department of Immunology and Inflammation, Imperial College London, London, United Kingdom
Background: Hydroxychloroquine (HCQ) is an antimalarial drug that has been used for many years for the treatment of inflammatory rheumatic diseases, especially systemic lupus erythematous (SLE). Evidence suggests that HCQ has anticoagulant and antiplatelet effects in addition to anti‐inflammatory effects. Therefore, it would be an attractive option for patients with antiphospholipid syndrome (APS) especially those with triple positive APS carrying the highest risk of recurrence despite adequate anticoagulation. However, use of HCQ is the not the standard treatment for patients with APS.
Aims: To determine the benefit of HCQ in patients with triple positive APS as adjunct treatment with warfarin.
Methods: We performed a 3‐year follow‐up 23 triple positive thrombotic APS treated with HCQ 200mg once or twice daily in combination with warfarin in a single UK centre.
Results: Median age was 47 (range 22–74) years and 67% were female. Baseline characteristics of are summarised in Table 1. Prior to commencing HCQ 23 patients, 43.5% (10/23) had had ischaemic stroke, one catastrophic APS and the remainder venous thromboembolism (VTE). Of the 10 patients with stroke, 80% (8/10) were treated with warfarin to target INR of 2.5 (2.0–3.0) and two (20%) to INR of 3.5 (3.0–4.0) due to recurrent thrombosis. All stroke patients had an annual MRI brain to detect new white matter lesions or evidence of new infarction or silent bleeding events. All demonstrated only the expected interval maturation with no progression of lesions or bleeding. None of the 23 patients developed recurrent thrombosis or bleeding over 3‐year follow‐up. All patients had ophthalmology review but none developed retinal toxicity or other side effects.
Conclusion(s): These data support the use of HCQ in patients with triple positive APS on standard intensity anticoagulation with warfarin to prevent thrombosis recurrence without significant increase in bleeding or other complications.

PB0415
The effect of a gut microbiome intervention on antiphospholipid syndrome: the ROMAS study
V. Jansen 1; D. van Mourik1; K. van Bergen en Henegouwen1; T. Noordermeer2; M. Limper3; R. Urbanus2; S. Middeldorp4; T. van Mens1
1 Amsterdam UMC, Amsterdam, Noord‐Holland, Netherlands; 2 Center for Benign Haematology, Thrombosis and Haemostasis, Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Utrecht, Utrecht, Netherlands; 3 Department of Rheumatology and Clinical Immunology, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands; 4 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands
Background: The etiology of the autoantibodies in patients with antiphospholipid syndrome (APS) is unknown. Consequently, there is no curative treatment and management of this autoimmune disease is based on anticoagulation, which has suboptimal safety and efficacy. There is a clinical need for novel therapies acting in a more ‘upstream’ way, thus directly influencing the pathogenic APS‐antibodies. The gut microbiome contributes to auto‐immunity and contains peptide homologues to the main auto‐antigen, beta‐2‐glycoprotein‐I, which may affect disease activity. Alteration of the gut microbiota with vancomycin diminishes disease activity in mice but patient data is lacking.
Aims: To evaluate whether alteration of the gut microbiome affects disease activity in patients with APS.
Methods: In this intervention study, APS patients with stable disease, i.e. no recent thrombotic events or pregnancy complications, were included. They received oral vancomycin, 500 mg 4 times daily for 7 days, previously shown to alter gut microbiota composition without systemic effects. The main study outcome was the composite of a panel of clinical endpoint‐related biomarkers, measured at multiple time points [Figure 1]. This panel consisted of criteria and non‐criteria APS antibodies, complement and inflammation markers and coagulation parameters. Effect of the intervention was measured by a comparison of the biomarker composite, expressed as the first principle component of the panel, in a pretest‐posttest design.
Results: 15 patients with antiphospholipid syndrome were included. Baseline characteristics are displayed in Table 1.
Conclusion(s): The primary outcome will be presented at the conference in July 2022.


PB0412
Laboratory detection of Lupus Anticoagulant using Dilute Russell's Viper Venom test (DRVVT) and/or Activated Partial Thromboplastin time (APTT): Does it matter?
T. Geevar 1; R. Dave1; P. Elizabeth2; A. Samuel3; J. Thamburaj3; J. Mammen3; S. Nair1
1 Christian Medical College and Hospital, Vellore, Vellore, Tamil Nadu, India; 2 Christian Medical College, Vellore, Tamil Nadu, India; 3 Christian Medical College, Vellore, Vellore, Tamil Nadu, India
Background: Lupus anticoagulant (LA) is one of the three laboratory criteria for the identification of the antiphospholipid syndrome (APS). Primary criteria for LA detection include prolonged clotting time of a phospholipid dependent test, the failure of correction on mixing study, shortening of the clotting time in the presence of high phospholipid concentrations, and absence of other inhibitors. Dilute Russel Viper Venom Test (DRVVT) is the preferred test, and second test should be a sensitive Activated Partial Thromboplastin time (APTT). LA should be considered positive if one of the two test systems gives a positive result.
Aims: To describe the pattern of LA testing in a tertiary centre in South India and correlate with other antiphospholipid antibodies (APLAs) and clinical features.
Methods: This was a retrospective study wherein all consecutive LA requests from 1st January 2021 to 30th June 2021 were reviewed. LA testing was performed using DRVVT screen and confirm reagents (STA‐Staclot DRVV Screen and STA‐Staclot DRVV Confirm) and APTT reagent (STA‐PTT Automate) using three steps, Screen‐mix‐confirm. Clinical details and other APLA results were obtained from the electronic medical records.
Results: There were a total of 1471 LA requests during the study period. 41 cases were excluded in view of interferences due to anticoagulants like Heparin, low molecular weight heparin and Dabigatran. A total of 137 LA positive cases were identified, of which 73 were positive by both DRVVT and APTT, 54 positive only by DRVVT and 10 positive only by APTT. There was maximum correlation with other APLAs when LA was positive by both APTT and DRVVT as shown in Figure 1.
Conclusion(s): Positive LA by APTT alone did not show any correlation, while LA positive by both DRVVT and APTT showed maximum correlation with the presence of other APLAs.

PB0413
Insights on antiphospholipid antibody syndrome metabolomics by NMR
L. Martins1; M. Honorato 2; S. Montalvão3; E. Braga1; L. Silva2; E. de Paula4; F. Orsi2; L. Tasic1; J. Annichino‐Bizzacchi2
1 Biological Chemistry Laboratory, Department of Organic Chemistry, Institute of Chemistry, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil; 2 Hemostasis and Thrombosis Laboratory, Hematology and Hemotherapy Center, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil, 3 Hemostasis and Thrombosis Laboratory, Hematology and Hemotherapy Center, University of Campinas (UNICAMP), Campinas, Sao Paulo, Brazil; 4 School of Medical Sciences of the University of Campinas, Campinas, Sao Paulo, Brazil
Background: Antiphospholipid antibody syndrome (APS) is an acquired autoimmune thrombophilia that causes arterial, venous or microvascular thrombosis, and pregnancy morbidity, such as recurrent abortion, late fetal death, and severe preeclampsia. APS is diagnosed based on clinical and antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin, and anti‐β2 glycoprotein I.
Aims: This study aimed to map serum signatures by NMR, and investigate if there is a connection among variation in metabolites and APS.
Methods: Individuals with APS (n = 32, Table 1) were analyzed, matched by age and gender with control individuals (HC, n =32, Table 1) to ensure homogeneity in the basal metabolic rates. The NMR data sets were recorded on Bruker AVANCE III 600 MHz spectrometer using the inverse triple‐core probe at 25°C. 1H‐NMR data were processed, normalized by the sum, centered on the mean, and analyzed in MetaboAnalyst 5.0 software platform.
Results: The Partial Least Squares – Discriminant Analysis (PLS‐DA, Figure 1A) and ortho‐PLS‐DA (Figure 1B) performed onto CPMG data enabled us to distinguish between the APS and HC groups. The main metabolites related to APS were identified based on multi and univariate analysis results (Figure 1C). APS‐individuals showed lower serum amounts of lipids, glutamine (Gln), lysine (Lys), and valine (Val), while the amounts of isoleucine (Ile), and threonine (Thr) were slightly higher compared to HC‐individuals. Recently, in an animal model study, it was shown that Val and Ile metabolic pathways showed the major roles in regulating platelet activation in thrombosis.
Conclusion(s): The modified levels of those branched chain amino acids might be associated with the risk of APS, and it is still to verify if amino acids – Gln, Lys, Val, Ile, and Thr are potential targets for illuminating previously unknown pathways in APS, which open up possibilities for further studies. The Ethics Research Committee approved the experimental procedures.


VPB0419
Renal function at enrollment but not change over time is associated with arterial thrombotic events in patients positive for lupus anticoagulant
M. Colling1; F. Posch2; C. Ay3; S. Koder4; I. Pabinger3; J. Gebhart 5
1 Medical University of Vienna, Vienna, Wien, Austria; 2 Medical University of Graz, Graz, Steiermark, Austria; 3 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 4 Medical University of Vienna, Wien, Wien, Austria; 5 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria
Background: Patients with lupus anticoagulant (LA) are at risk for arterial and venous thromboembolic events (ATE, VTE). Patients who experience ATE may constitute a distinct subgroup and benefit from specialized therapeutic approaches. Further work is needed to better characterize this subgroup.
Aims: Identify patient characteristics and laboratory parameters associated with the development of ATE in LA positive patients.
Methods: Patients with at least 2 positive LA tests were monitored for ATE within the prospective Vienna Lupus Anticoagulant and Thrombosis Study (EC. 068/2001 and 1268/2014)
Results: This analysis included 187 patients, 156 female (83%) with a median age of 40.5 years (IQR 31.0–55.0) and median follow‐up of 11.4 years. Twenty‐seven prospective ATEs occurred and the 10‐year prospective ATE incidence was 13.9% [95% CI: 8.3–19.6]. Prospective ATE risk was associated with age, body mass index (BMI), history of ATE, active smoking, diabetes, VKA use at baseline, aCL IgM positivity, aβ2GPI IgM positivity, mean platelet volume, creatinine, and estimate glomerular filtration rate (CKD‐EPI) (Table 1). After adjusting for traditional arterial thrombotic risk factors (age, sex, BMI, active smoking, diabetes), history of ATE, prior history of both ATE and VTE, creatinine, and eGFR remained independently associated with prospective risk of ATE (Table 1). There was a significant difference in 10‐year incidence of ATE when patients were stratified by baseline eGFR (Gray’s test, p = 0.019, Figure 1). However, in the patients who had a follow‐up creatinine measured within two years of enrollment (n = 88), change in eGFR (neither any change nor decrease) did not associate with prospective ATE risk (p = 0.17).
Conclusion(s): After adjusting for traditional arterial risk factors, renal function at baseline, but not change in eGFR over two years, was associated with prospective risk of ATE. This suggests that the duration of exposure to impaired renal function may play a role in the increased risk of ATE.

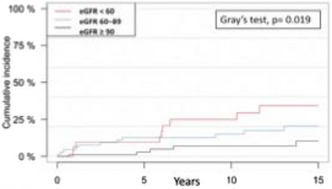
PB0414
Antiphospholipid syndrome‐related venous thromboembolism: A nationwide study
H. Hwang 1; S. Bang2; J. Lee3; S. Kim3; Y. Kim4; M. Kim5; J. Hong6; H. Yhim7
1 Soonchunhang University Gumi Hospital, Gumi, Kyongsang‐bukto, Republic of Korea; 2 Seoul National Bundang Hospital, Bandang, Kyonggi‐do, Republic of Korea; 3 Seoul National Bundang Hospital, Bundang, Kyonggi‐do, Republic of Korea; 4 Soonchunhyang University Seoul Hospital, Seoul, Seoul‐t'ukpyolsi, Republic of Korea; 5 Soonchunhyang University Gumi Hospital, Gumi, Kyongsang‐bukto, Republic of Korea; 6 Seoul National Hospital, Seoul, Seoul‐t'ukpyolsi, Republic of Korea; 7 Chonbuk, Jeonju, Cholla‐bukto, Republic of Korea
Background: Direct oral anticoagulants (DOAC) are widely used for prevention of arterial thrombosis and the treatment and secondary prevention of venous thromboembolism (VTE), but the efficacy and safety of DOACs for the treatment in patients with antiphospholipid syndrome‐related VTE (APS‐VTE) are uncertain.
Aims: This nationwide population‐based epidemiologic study examined the efficacy and safety of DOAC versus warfarin for patients with APS‐VTE between 2014 and 2018.
Methods: APS‐VTE is defined as both VTE code and APS code (D686) being detected twice at least 12 weeks apart within 180 days of index initial VTE event.
Results: APS‐VTE patients (n = 462) accounted for 0.54% of all VTE cases (n = 84,916). Among 410 individuals with APS‐VTE (210 female, 51.2%), 209 patients received DOACs, and 201 patients received warfarin (n = 201) for anticoagulation. The recurrent VTE occurred in 8 of 209 patients (3.8%) who received DOACs and in 7 of 201 (3.5%) who received warfarin (relative risk [RR], 1.099; 95% CI, 0.41–2.98; p = 1). The arterial thrombosis occurred in 8 of 209 patients (3.8%) who received DOAC and in 20 of 201 (10%) who received warfarin (RR, 0.385; 95% CI, 0.17–0.85; p = 0.024). The composite outcome was significantly lower in patients (9.1%) on DOAC than patients (16.3%) on warfarin (RR, 0.537; 95% CI, 0.32–0.91; p = 0.028). The safety outcome (all bleeding) occurred in 7 of 209 (3.4%) in DOAC and 7 of 201 (3.5%) in warfarin (RR, 0.96; 95% CI, 0.34–2.69; p = 0.84).
Conclusion(s): The VTE recurrence and bleeding on DOAC are comparable, but the arterial thrombosis and composite outcomes patients treated with DOAC are significantly lower than those treated with warfarin among APS‐VTE.

PB0416
Falsely elevated PT‐INR results measured with Quick point‐of‐care methods and Quick laboratory method in patients with antiphospholipid syndrome
A. Kristoffersen 1; T. Lindahl2; H. Alvheim3; A. Stavelin3; M. Hellum4; U. Ørvim Sølvik5; T. Geir5; E. Brodin6; S. Sandberg7; C. Henriksson4
1 Haraldsplass Deaconess Hospital and Haukeland University Hospital, Bergen, Hordaland, Norway; 2 Linköping University, Linköping, Ostergotlands Lan, Sweden; 3 Noklus, Haraldsplass Deaconess Hospital, Bergen, Hordaland, Norway; 4 Institute of Clinical Medicine, Faculty of Medicine, University of Oslo and Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway; 5 Department of Global Public Health and Primary Care, University of Bergen, Bergen, Hordaland, Norway; 6 Akershus University Hospital, Lørenskog, Akershus, Norway; 7 Noklus, Haraldsplass Deaconess Hospital, Department of Global Public Health and Primary Care, University of Bergen and Norwegian Porphyria Centre, Department of Medical Biochemistry and Pharmacology, Haukeland University Hospital, Bergen, Norway, Bergen, Hordaland, Norway
Background: Falsely elevated PT‐INR results are reported in some patients with antiphospholipid syndrome (APS), but it is not clear which methods and patients that are affected.
Aims: To study which PT‐INR methods are most prone to interference, and if antibody profiles in APS‐patients may explain such interference.
Methods: Blood was drawn from 29 controls and 21 APS‐patients for 1 – 4 times per participant. PT‐INR was analysed with five different point‐of‐care (POC) instruments (three Quick methods (capillary blood, no dilution) and two Owren methods (1:21 and 1:6 dilution)); one Quick laboratory method (1:3 dilution), and one Owren laboratory method (1:21 dilution, used as the reference PT‐INR method). Chromogenic factor X was analysed as an alternative monitoring method. To establish antibody profiles, lupus anticoagulant, anti‐cardiolipin, anti‐beta‐2‐glycoprotein I, anti‐phosphatidylserine/prothrombin (anti‐PS/PT) and anti‐beta‐2‐glycoprotein I domain I (anti‐B2GPI‐DI) antibodies were analysed.
Results: Falsely elevated PT‐INR results (>20% PT‐INR difference compared to the reference method) were seen in up to five of 21 APS‐patients with the Quick methods (both POC instruments and laboratory method), but not with POC Owren methods (Figure 1). The five APS‐patients were tetra‐positive (including anti‐PS/PT antibodies) with an anti‐B2GPI‐DI antibody titer above 500 U/mL. Falsely elevated PT‐INR and high titer antiphospholipid antibodies were seen consistently over time. Tetra‐positivity was also seen in six of 21 APS‐patients without falsely elevated PT‐INR, but their anti‐B2GPI‐DI antibody titer was either normal (< 30 U/mL, n = 2) or less than 180 U/mL (n = 4).
Conclusion(s): PT‐INR POC Quick methods with no dilution of patient samples have the highest risk of falsely elevated PT‐INR in APS‐patients, but PT‐INR Quick laboratory methods may also give falsely elevated results in some of these patients. Very high anti‐B2GPI‐DI antibody titers were only seen in the five APS‐patients with falsely elevated PT‐INR, and in such patients, Owren methods should be used.

VPB0420
Increased expression of protein disulfide isomerase A1 on platelet surface in lupus anticoagulant‐associated thrombosis
A. Romer1; N. Samadi 2; L. Hell1; C. Ay3; J. Gebhart4; I. Pabinger3
1 Division of Haematology and Haemostaseology, Department of Medicine I, Medical University of Vienna, Vienna, Wien, Austria; 2 Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 3 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 4 Medical University of Vienna, Vienna, Wien, Austria
Background: Antiphospholipid antibody syndrome (APS) is an autoimmune disorder defined by the presence of antiphospholipid antibodies (aPLAs) in combination with the occurrence of venous and/or arterial thromboembolism (TE). Among aPLA positives, the ones who have lupus anticoagulant (LA) are at highest risk of developing thromboembolism (TE). So far, the role of platelets in the pathogenesis of LA+TE+ patients is not fully investigated. In previous platelet proteomic analysis, we discovered that protein disulphide isomerase A1 (PDI A1), a member of the PDI family, was significantly upregulated in platelets and plasma of LA+TE+ patients (Hell et al, Exp Mol Med, 2020, 52, 66–78). Several studies indicated the association of PDIs and thrombosis formation, however, the exact function of these proteins is not clear yet.
Aims: To investigate the levels of PDI A1 on platelet surface in LA+TE+ patients in comparison to control subjects.
Methods: Platelets from LA positive patients with a history of TE and platelets from age and sex matched healthy controls were analyzed for differences in PDI A1 surface expression in vivo by flow cytometry.
Results: We investigated 21 LA+TE+ patients (67% females, median age: 48 years), all of whom were triple positive (in addition anticardiolipin and anti‐ß2‐glycoprotein I antibodies) and 21 healthy controls (67% female, median age: 48 years) (Table 1). We found significantly higher levels of PDI A1 on platelet surface in LA+TE+ patients in comparison to healthy individuals (median IQR: LA+TE+ 14.03 (12.41–16.78), controls 11.9 (8.7–17.01), p = 0.021) (Figure 1).
Conclusion(s): The meaningful upregulation of PDI A1 on the surface of platelets in LA+TE+ patients together with our previous findings about the prothrombotic platelet proteome in APS patients and enhanced levels of PDI A1 in plasma indicate a role for PDI and a contribution of platelets in thrombotic events in LA positive patients.


Platelet surface PDI A1 expression of LA+TE+ patients (n = 21) compared to healthy controls (n = 21).
Artificial Valves
PB0422
Polymeric prosthetic heart valves with low thrombogenicity
S. Melo 1; A. Aqil2; B. Ditkowski3; C. Jérôme2; P. Lancellotti4; C. Oury3
1 University of Liège – GIGA, Liège, Liege, Belgium; 2 Center for Education and Research on Macromolecules (CERM), CESAM‐UR, University of Liège, B‐4000 Liège, Belgium, Liège, Liege, Belgium; 3 Laboratory of Cardiology, GIGA‐Cardiovascular Sciences, University of Liège, Liège, Belgium, Liège, Liege, Belgium; 4 Laboratory of Cardiology, GIGA Institute, University of Liège, Liège, Belgium; Gruppo Villa Maria Care and Research, Maria Cecilia Hospital, Cotignola, and Anthea Hospital, Bari, Italy, Liege, Liege, Belgium
Background: Valvular heart diseases (VHD) affect about 13% of the elder population (>75), which represents more than 100 million people worldwide. The only available treatment is heart valve replacement, either with mechanical or biological prostheses. Mechanical prostheses provide long‐term durability, but inevitably bring along a high risk of thromboembolism. On the other hand, biological prostheses provide better hemo/biocompatibility, but they are prone to degeneration. Alternatively, polymeric (particularly polyurethane (PU)‐derived) materials have been suggested and investigated as potential heart valve biomaterials.
Aims: To develop and test innovative non‐isocyanate polyhydroxyurethanes (PHUs) for prosthetic heart valves, with improved mechanical and anti‐thrombogenic properties, resulting in enhanced hemocompatibility.
Methods: PHUs were obtained by polyaddition between different bis‐cyclic‐carbonates and amines. The ultimate tensile strength (MPa), strain (%) and elastic modulus (MPa) of synthesized PHUs were measured under dry and wet conditions and compared to native valves. Hemolysis tests were performed in the presence of washed red blood cells from human blood. The activation of the complement and coagulation cascade was evaluated after incubation of the PHU samples with citrate‐anticoagulated platelet‐poor plasma. Platelet adhesion was quantified upon incubation of the samples with platelet‐rich plasma.
Results: A Dacron reinforcement of the selected PHU rendered mechanical properties that were comparable to the ones of a native valve. This PHU did not cause hemolysis or complement activation, it induced less coagulation activation than medical grade PU, and caused extremely low levels of platelet adhesion. A tri‐leaflet prosthetic heart valve with a precisely defined design was produced by injection molding.
Conclusion(s): PHU materials displayed overall improved hemocompatibility. A cost effective, environmentally friendly and mechanically competent prosthetic heart valve was developed and could be further explored as an alternative for valve replacement in VHD patients.
PB0421
Prevalence of guideline discordant aspirin use and associated adverse events in patients on warfarin for mechanical valve replacement
B. Haymart1; X. Kong1; G. Barnes1; J. Errickson1; E. Kline‐Rogers2; M. Ali3; V. Shah4; J. Froehlich2; S. Kaatz 4
1 University of Michigan, Ann Arbor, Michigan, United States; 2 University of Michigan Health, Ann Arbor, Michigan, United States; 3 Beaumont Hospital, Royal Oak, Michigan, United States; 4 Henry Ford Hospital, Detroit, Michigan, United States
Background: For patients on vitamin K antagonists for mechanical aortic or mitral valve replacements, the 2020 American College of Cardiology and American Heart Association Valvular Heart Disease Guidelines recommend only adding aspirin in patients with a specific indication for antiplatelet therapy. This is in contrast to prior guidelines which recommended concomitant aspirin therapy.
Aims: To assess the prevalence of guideline discordant aspirin use among patients with mechanical heart valves on warfarin and to compare adverse event rates among patients with and without concomitant aspirin.
Methods: Patients on warfarin for mechanical heart valve replacement were identified from the Michigan Anticoagulation Quality Improvement Initiative (MAQII) registry. Patients with an indication for antiplatelet therapy, including myocardial infarction, percutaneous coronary intervention, or coronary artery bypass within the past 12 months, were excluded. Aspirin and non‐aspirin cohorts were formed based on aspirin use at enrollment. Demographics and co‐morbidities were compared using t‐test and chi‐square test while bleeding and thrombotic rates were compared using Poisson regression model. HAS‐BLED (bleeding risk) and CHA2DS2‐VASc (ischemic stroke risk) scores were used to adjust event rates.
Results: A total of 182 patients met inclusion criteria, with 121 (66.5%) using aspirin (Table 1). The cohorts had similar demographics, time in therapeutic INR range, and HAS‐BLED and CHA₂DS₂‐VASc scores. The aspirin cohort had a significantly higher rate of bleeding (25 vs 12 per 100 patient‐years, p < 0.001) and emergency department visits for bleeding (14.1 vs 3.6 per 100 patient‐years, p < 0.001) compared to the non‐aspirin cohort. There was no observed difference in rates of ischemic stroke between the cohorts (0.21 vs 0.40 per 100 patient‐years, p = 0.69).
Conclusion(s): A significant proportion of patients on warfarin for mechanical valve replacement are on guideline discordant aspirin therapy. Aspirin de‐prescribing may safely reduce bleeding events and emergency department utilization.

Cancer Associated Thrombosis
PB0908
Bleeding versus thrombosis: Case series on dilemma of VTE management in cancer patients and role of radiotherapy
A. Bolarinwa; O. Popoola; M. Elebute
Marcelle Ruth Cancer Centre and Specialist Hospital, Lagos, Lagos, Nigeria
Background: Oncology patients with major bleeding who later develop Venous Thromboembolism present a difficult therapeutic dilemma because of risk of recurrent fatal pulmonary embolism if untreated and life threatened hemorrhage if treated with anticoagulant.
Aims: To highlight challenges of managing coexisting bleeding and thrombosis in cancer as well as role of local hemostatic radiotherapy.
Methods: This is a case series of 4 patients with major bleeding and Cancer Associated Thrombosis.
Results: The first two patients are middle aged woman with advanced cervical cancer. They both presented with massive pulmonary embolism and bleeding per vagina requiring transfusion support. The patients were commenced on low molecular weight heparin (LMWH) for the treatment of PE and a local hemostatic mechanism like packing the vagina with adrenaline‐soaked gauze was put in place. Despite this, the bleeding persisted. Although intravenous tranexamic acid reduced the bleeding but there are concerns on its procoagulant effect. The dose of anticoagulant was reduced in one and was completely withdrawn in another due to risk of exsanguination. They both had hemostatic radiotherapy to the cervix which significantly control the bleeding, and this allowed recommencement of anticoagulation. They both made significant improvement in their symptoms. The other two patients were managed for prostate CA and anaplastic large cell lymphoma. They both had clinically significant bleeding and DVT. The later developed PE. In these two, anticoagulation was only possible after resolution of bleeding with hemostatic radiotherapy. One of them have recurrence of symptoms while the other eventually died of pulmonary embolism.
Conclusion(s): The cases reported above highlights the therapeutic dilemma of managing patient with VTE and major bleeding particularly in cancer settings. Challenges include deciding when to hold on with anticoagulation, appropriate choice of hemostatic treatment. Achieving hemostasis with radiotherapy in these patients allows use of appropriate anticoagulant in managing their VTE.
VPB0942
Association between RET and BRAF(V600) oncogenic status and risk of venous and pulmonary thromboembolism in patients with papillary thyroid cancer
D. Katalinic 1; I. Aleric2; A. Vcev2; M. Solter3; L. Toetome4
1 Faculty of Dental Medicine and Health and Faculty of Medicine, J. J. Strossmayer University of Osijek, Osijek, Osjecko‐Baranjska, Croatia; 2 Faculty of Dental Medicine and Health and Faculty of Medicine, J. J. Strossmayer University of Osijek, Osijek, Osjecko‐Baranjska, Croatia; 3 School of Medicine, University of Zagreb, Zagreb, Grad Zagreb, Croatia; 4 Center for Cancer Medicine, Oslo, Oslo, Norway
Background: Papillary thyroid cancer (PTC) and its variants (classical (CV), tall cell (TCV) and follicular variant (FV)) accounts for approximately 80% of all thyroid cancers. Mutations of the RET and BRAF(V600) genes are found in nearly 70% of PTC cases. They are able to trigger the activation of mitogen‐activated protein kinase pathway and to promote neoplastic cell proliferation and possible hypercoagulability. The number of studies on venous thromboembolism (VTE) and pulmonary embolism (PE) coexist with PTC is limited and medical data are contradictory.
Aims: The aim of the study was to perform the genomyc analysis of the RET and BRAF(V600) mutations in patients with PTC and to determine the prevalence and risk factors associated with VTE and PE taking into consideration the status of RET and BRAF(V600) genotype.
Methods: The study included 112 patients, aged 49–74 years. Mutations of the RET and BRAF(V600) genes were detected using Real‐time polymerase chain reaction. Diagnosis of the PTC and its variants were confirmed with cyto/histopathological examination. Diagnostic strategies were evaluated for VTE and PE.
Results: The most important genomic, cyto/histopathological and prognostic elements regarding all three variants of the PTC: CV‐PTC (n = 78): RET and BRAF(V600) mutations are common findings, with BRAF(V600) confirming a worse prognosis. The 65% of these patients have shown stabile disease course, with 25% with metastatic nodes; TCV‐PTC (n = 8): Aggressive behavior has been attributed to BRAF(V600) mutation. FV‐PTC (n = 26): Absence of BRAF(V600) mutation was the most important genetic element. Although some studies have shown a possible increase in VTE and PE occurrence in PTC patients harbouring RET and BRAF(V600) mutation, our studie could not confirm these data.
Conclusion(s): The presence of RET and BRAF(V600) mutation status and increased risk of VTE and PE in patients with PTC could not be confirmed
PB0922
The prevalence of thrombophilia in patients with cancer associated thromboembolism
J. Hirmerová 1; I. Šubrt2; Z. Hajšmanová3
1 University Hospital, Faculty of Medicine in Pilsen, Charles University, Czech Republic, Pilsen, Plzensky kraj, Czech Republic; 2 Institute of Medical Genetics, University Hospital, Faculty of Medicine in Pilsen, Charles University, Czech Republic, Pilsen, Plzensky kraj, Czech Republic; 3 Institute of Clinical Biochemistry and Haematology, University Hospital, Pilsen, Czech Republic, Pilsen, Plzensky kraj, Czech Republic
Background: Cancer is a significant risk factor for venous thromboembolism (VTE) which applies not only to the risk of the first VTE event but also to the risk of VTE recurrence. The presence of thrombophilia may further increase the risks. However, thrombophilia testing is not usually performed in cancer patients.
Aims: The aim of our study was to assess the prevalence of thrombophilia in patients with cancer associated thromboembolism (CAT).
Methods: We retrospectively evaluated prospectively collected data of patients with active cancer and objectively confirmed VTE. The study was performed in a tertiary care hospital. We included the patients who had visited thrombosis clinic since 2006 to 2020. We obtained complete history of the patients and performed thrombophilia testing. All patients provided a written informed consent. For statistical evaluation, Student´s T‐test and chi‐square test were used.
Results: Of 87 patients with CAT (mean age 68.5 ± 9.4; 36.8% females), 24.1% had a positive family history and 29.9% had a positive personal history of VTE. The prevalence of thrombophilia was 28.7% (FVL 17.2%, prothrombin gene mutation G20210A 2.3%, antithrombin deficiency 6.9% and antiphospholipid syndrome 3.4%). Comparing the CAT groups with and without thrombophilia, the age and gender distribution was similar (mean age 68.6 vs 68.5, p = 0.939; females 44.0% vs 33.9%, p = 0.375). In the group with CAT and thrombophilia, more patients had a positive family history of VTE (36.0% vs 19.4%, p = 0.101) and a positive personal history of VTE (44.0% vs 24.2%, p = 0.068) but the differences did not reach statistical significance.
Conclusion(s): The prevalence of thrombophilia in the patients with CAT is quite high, especially in those who already have a positive history of VTE. Larger and prospective studies should address the question whether selected patients with CAT might benefit from thrombophilia testing in assessing their risk of recurrence and tailoring secondary thromboprophylaxis.
PB0934
Khorana score as a predictor of thrombotic risk in outpatients with malignant neoplasms
Y. Vivas1; E. Medina2; E. Abreu2; S. Rezende 3; D. RIbeiro4
1 Fundação Hemominas, Belo Horizonte, Minas Gerais, Brazil; 2 University Federal de Minas Gerais, Brazil, Belo horizonte, Minas Gerais, Brazil; 3 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 4 University Hospital, Universidade Federal de Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil
Background: Armand Trousseau suggested the possible association between neoplasia and venous thromboembolism (VTE) in 1985. Numerous studies have been published as to elucidate this link. Cancer patients have a 4 to 7 times greater risk of developing VTE, which represents the second major cause of death.
Aims: To evaluate the incidence of VTE, VTE‐related death and the role of the Khorana score to predict VTE in patients with solid cancer or multiple myeloma who will initiate chemotherapy.
Methods: This is an ongoing, prospective cohort study. Inclusion criteria was adult patients, >18 years with a first and recent diagnosis of solid neoplasia or multiple myeloma, attended at the Outpatient Oncology Clinic of University Hospital, University Federal de Minas Gerais, Brazil. Patients were consecutively included from July 2021 to January 2022. The follow‐up time is six months. The Khorana score was calculated based on the patient's first interview. The outcome was VTE and any death.
Results: Ninety‐five patients have been included to date. Mostly are women (57.9%), aged less than 60 years old (52.6%). The main primary sites of the tumor are breast (23.2%), lymph nodes (14.7%), colon (12.6%), and pancreas (7.4%). The Khorana score stratification was 0 (12.6%), 1 (34.7%), 2 (20.0%), 3 (6.3%), 4 (1.1%), and 5 (1.1%). To date, there were 5 deaths (5.3%) and 5 VTE events (5.3%), being 3 pulmonary thromboembolism and 2 VTE. Patients with Khorana score 0 did not present VTE, while 5.9% and 25% of Khorana score 1–2 and ≥3, respectively, had VTE (p = 0.04), with an increased incidence of VTE of patients with Khorana 3 greater than or equal to 3 (p 0.06, RR 5.33, CI 1,04–27,25).
Conclusion(s): Patients with Khorana score ≥ 3 had a higher incidence of VTE in comparison with Khorana < 3, similar to the data found in the literature.
PB0932
Survey on the management of cancer associated thrombosis (CAT) in haemato‐oncology patients with thrombocytopenia
H. Okoye 1; K. Korubo2; H. Omunakwe3; C. Efobi4; N. Onodingene5; N. Ugwu6
1 College of Medicine University of Nigeria Ituku Ozalla, Enugu, Enugu, Nigeria; 2 University of Port Harcourt Teaching Hospital, Port Harcourt, Rivers, Nigeria; 3 Department of Haematology, Rivers State University Teaching Hospital Rivers state, Port Harcourt, Rivers, Nigeria; 4 Department of Haematology and Blood Transfusion, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus Anambra state, Awka, Anambra, Nigeria; 5 Pamo University of Medical Sciences, Iriebe Rivers State, Port Harcourt, Rivers, Nigeria; 6 Department of Haematology and Immunology, Faculty of Basic Clinical Sciences, Abakaliki Ebonyi state, Abakaliki, Ebonyi, Nigeria
Background: Arterial or venous thrombosis can complicate cancer. Up to 20% of oncology patients develop venous thromboembolism (VTE), and VTE is not uncommon in haemato‐oncology. However, haemoto‐oncology is complicated by thrombocytopenia.
Aims: To access the knowledge and practice of haematologists in Nigeria as regards the management of thrombocytopenia and cancer‐associated thrombosis (CAT).
Methods: This was a survey that was shared electronically with haematologists in Nigeria over a period of 6 months using a pretested questionnaire. The questionnaire tested participants’ knowledge and management practices of thrombocytopenia in CAT in haemato‐oncology. Data were managed with Microsoft Excel and analysed using statistical package for social sciences version 21.
Results: We had 106 respondents, 70 (66%) were Consultant Haematologists, about 30.2% of the respondents saw 6–10 haemato‐oncology patients in a month. Fifty‐Seven (53.8%) of the respondents risk assessed their patients for CAT, 63 (59.4%) of the respondents saw 1–2 haemato‐oncology patients with thrombosis in 3 months. The most common mode of treatment was pharmacological – 94 (88%) of respondents used low molecular weight heparin. The most common haemato‐oncology associated with thrombocytopenia was Acute leukemias 69 (67%). The most common decision taken by respondents was to stop anticoagulants and transfuse platelets because the most frequent concern was the risk of bleeding in this group of patients.
Conclusion(s): Many haematologists have a high level of awareness, knowledge, and good practice regarding thrombocytopenia in CAT in Haemato‐oncology patients, however, there is a need for improved knowledge and unified protocols for treatment in line with newer treatment guidelines.

PB0920
Performance of the Thrombogyn and Khorana scores for prediction of venous thromboembolism in ovarian cancer patients after major surgery
N. Guman 1; H. Wiegers2; M. Schaafsma3; F. Mulder1; S. Mom2; N. van Es2
11. Amsterdam University Medical Centers, University of Amsterdam; 2. Tergooi Medical Center, Amsterdam, Noord‐Holland, Netherlands; 2 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 3 Antoni van Leeuwenhoek, Netherlands Cancer Institute, Amsterdam, Noord‐Holland, Netherlands
Background: Guidelines recommend extended thromboprophylaxis for prevention of venous thromboembolism (VTE) in ovarian cancer patients undergoing major surgery. Individual VTE risk, however, may vary widely. The Thrombogyn and Khorana scores may be helpful in identifying low‐risk patients for whom shorter duration thromboprophylaxis could be beneficial.
Aims: To evaluate the predictive performance of the Thrombogyn and Khorana scores for VTE in ovarian cancer patients after major surgery.
Methods: A retrospective cohort study was performed in women with ovarian cancer who underwent major surgery between January 2017 and December 2020. Those with ongoing therapeutic anticoagulation or recent minor surgery were excluded. The primary outcome was pulmonary embolism, deep‐vein thrombosis, and cerebral vein thrombosis during 90‐day follow‐up. Discriminatory performance of continuous scores was evaluated by the area under the receiver operating curve (AUROC) and dichotomous scores by the cumulative VTE incidence in high‐ and low‐risk groups with corresponding hazard ratio (HR). Subgroup analyses were performed in patients receiving standard (< 10 days) and extended thromboprophylaxis (>26 days).
Results: 253 women were included (see Table for baseline characteristics). Scores could be calculated for 245 (97%) patients, of whom 18 developed VTE (7.5%, 95% CI: 4.6–11.3%) during 90‐day follow‐up. The AUROC at 90 days for the continuous Thrombogyn and Khorana scores were 0.50 (95% CI: 0.39–0.61) and 0.57 (95% CI: 0.44–0.70), respectively. Thrombogyn score ≥2 and Khorana score ≥3 were not significantly associated with VTE risk (HR 0.7, 95% CI: 0.4–1.9 and 2.3, 95% 0.5–7.0, respectively; Figure). Analyses in 119 (49%) patients with standard and 100 (41%) with extended prophylaxis showed similar results.
Conclusion(s): The Thrombogyn did not discriminate between ovarian cancer patients at high and low VTE risk after major surgery. The Khorana score showed better discrimination, although not significant. These results do not support their use in guiding the duration of thromboprophylaxis in this setting.


PB0910
Comparison of standard vs low dose of direct oral anticoagulants for the secondary prophylaxis of venous thromboembolism in patients affected by hematologic malignancies
A. Serrao1; M. Chavez Orellana2; G. Assanto3; R. Mormile3; E. Baldacci3; C. Santoro4; A. Chistolini 2
1 Hematology, Sapienza University, Rome, Lazio, Italy; 2 Hematology, Sapienza University of Rome, Rome, Lazio, Italy; 3 Sapienza University of Rome, Rome, Lazio, Italy; 4 Hematology, University Hospital Policlinico Umberto I, Rome, Rome, Lazio, Italy
Background: Venous thromboembolism (VTE) is one serious complication in patients with hematologic malignancies due to the release of procoagulant factors by neoplastic cells, central venous catheter and chemotherapy. Considering that hematologic malignancies represent a non‐transient VTE risk factor, the treatment and secondary prophylaxis of VTE are an important step in the therapeutic approach of hematologic patients. Although direct oral anticoagulants (DOACs) demonstrated their efficacy and safety in the treatment of acute phase of VTE cancer related, there are few data in the setting of the secondary prophylaxis.
Aims: The aim of our study was to evaluate the efficacy and safety of DOACs in the VTE secondary prophylaxis in hematologic patients, comparing full and low dose of DOACs.
Methods: This retrospective monocentric study collected data of patients affected by hematologic malignancies with VTE (pulmonary embolism and deep vein thrombosis of typical sites) treated with DOACs. We divided the patients in 2 groups according to DOACs dose performed after the first 6 months of VTE treatment: one group continue with standard dose and the other group shifted to apixaban 2.5 BID or rivaroxaban 10 QD.
Results: We studied 55 patients (Table 1). After a median time of 6 months from a first VTE, 29 patients continued with DOAC at full dose, 26 patients shifted to DOACs at reduced dose. After a median time of 24 months (range 19–32) no thrombotic recurrences were observed in the two groups. We reported 4 minor bleeding events: 3 in the group treated with full dose and 1 in the other group. We did not report any statistically significant differences in terms of DOACs efficacy and safety between the two groups.
Conclusion(s): Our experience supports the use of DOACs also at reduced dosage in the VTE secondary prophylaxis in patients affected by hematologic malignancies.

VPB0943
Laboratory assessment of prothrombotic status in patients with primary brain tumors
O. Melniсhnikova 1; J. Zhilenkova2; E. Zolotova2; O. Sirotkina3; K. Pishchulov4; T. Vavilova4; O. Moiseeva4; M. Simakova4
1 Almazov National Medical Research Centre, Saint‐Peterburg, Saint Petersburg City, Russia; 2 Almazov National Medical Research Centre, Saint Petersburg, Saint Petersburg City, Russia; 3 PNPI named by BP Konstantinov of NRC Kurchatov Institute, St. Petersburg, Saint Petersburg City, Russia; 4 Almazov National Medical Research Centre, St. Petersburg, Saint Petersburg City, Russia
Background: In patients with brain tumors, including gliomas (GM), venous thromboembolism (VTE) is a common complication (20–30%). A rise the platelet – derived circulating extracellular vesicles (EVs) level and activation system coagulation in plasma may contribute to VTE in patients GM.
Aims: To evaluate the use of routine laboratory tests (D‐dimer, platelet count) in combination with thrombin generation test and relative abundance platelet‐derived EVs in improving VTE risk factor stratification in patients with primary brain tumors.
Methods: In the present study, 11 patients with newly diagnosed GM and 10 healthy volunteers were analyzed. Platelet‐derived EVs isolation and identification were performed using Exo‐FACS kit (HansaBioMed Life Sciences, Estonia) and monoclonal antibodies CD41‐PE/Cy7 as platelet specific markers, determination of the relative abundance by CytoFlex B4‐R2‐V2 (Beckman Coulter, USA). Thrombin generation test (TGT) were performed by the calibrated automated thrombography method (Thrombinoscope BV, Netherlands).
Results: In patients with GM, the relative abundance of the CD41+ EVs and D‐dimer level were significantly higher than in controls (44.3 [40.5; 52.4]% vs 27.2 [22.9; 31]%, p = 0.002; 0.46 [0.38; 1.85] μg/ml FEU vs 0.36 [0.27; 0.40] μg/ml FEU, p = 0.03, respectively). There was a trend towards an increase in peak and rate thrombin formation (p = 0.06) in the GM group. Significant correlation between circulating CD41+ EVs and activation trombin (TGT parameter’s) in the GM group was shown (p < 0.05). During short follow‐up period, 3 patients (27%) developed thrombosis, had tumour size more than 5 cm, thrombocytopenia < 150*109/L, increased VI (> 95 nmol/min) and D‐dimer (> 5 μg/ml FEU).
Conclusion(s): The present pilot study showed that TGT and platelet EVs analysis, in combination with D‐dimer and platelet count, can be used to assess the risk of VTE in GM patients. The results of the study need to be confirmed in a larger prospective study.
PB0912
Evaluation of activated protein C resistance in women undergoing tamoxifen therapy: Pilot study
M. Didembourg 1; J. Douxfils2; S. Reda3; H. Rühl4; L. Morimont5
1 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), Namur, Namur, Belgium, 21 Qualiblood s.a., 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS)., 5000, Namur, Belgium; 3 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 4 Institute of Experimental Haematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany, 51. Qualiblood s.a., 2. University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), 5000, Namur, Belgium
Background: Tamoxifen, a selective estrogen receptor modulator (SERM), is known to reduce hormono‐dependent breast cancer recurrence but is associated with an increased risk of venous thromboembolism. As already demonstrated with oral contraceptives, compounds interacting with estrogen receptors can impact hemostasis and a resistance towards the activated protein C (APC) is now well described for combined oral contraceptive (COC). Nevertheless, the underlying mechanism of tamoxifen‐induced procoagulable state remains unclear.
Aims: To assess APC resistance in women and men with hormono‐dependent breast cancer undergoing tamoxifen therapy, using the validated ETP‐based APC resistance assay.
Methods: Overall, 21 women and 2 men (mean age = 57) under active tamoxifen therapy were included. The ETP‐based APC resistance assay was assessed on the CAT system, using ThromboScreen ‐TM (Diagnostica Stago, France) as triggering reagent in absence and in presence of exogenous APC. As several patients were on anticoagulant therapy, all samples were treated with the DP filter device to remove the impact of anticoagulation on thrombin generation. To estimate the influence of tamoxifen on the normalized APC sensitivity ratio (nAPCsr), results were compared to a historical reference cohort of 47 healthy individuals (19 women and 28 men; mean age = 24). The nAPCsr results stood between 0 to 10, with higher values representing a higher resistance towards APC.
Results: The mean nAPCsr ± SD of patients under tamoxifen therapy was 3.23 ± 0.93 (range: 1.31 to 4.9) which was significantly higher from the mean nAPCsr of the control group (1.04 ± 0.59 (range: 0.00 to 2.10)) (p‐value < 0.001). This correspond to nAPCsr observed with second generation COC or factor V Leiden.
Conclusion(s): The nAPCsr values of patients undergoing tamoxifen therapy were significantly higher compared to healthy individuals, supporting APC resistance. Further analyses are needed to confirm the results of this pilot study.

PB0916
The role of anticoagulation in the management of tumour thrombosis: A single centre retrospective study
U. Faruqi 1; R. Darroch2; H. Verma2; G. Bahra3; M. Flynn2; A. Danaee2
1 Guys and St Thomas NHS Foundation Trust, London, England, United Kingdom; 2 Guys and St Thomas' NHS Foundation Trust, London, England, United Kingdom; 3 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom
Background: There are numerous guidelines addressing anticoagulation in cancer associated thrombosis and the role of direct oral anticoagulants (DOAC), yet there is no consensus regarding the role of anticoagulation in tumour thrombosis and whether or not it has any impact on survival.
Aims: We conducted a single centre retrospective observational study reviewing the diagnosis, management and outcomes of tumour thrombus and the role of anticoagulation.
Methods: CT reports with the term “tumour thrombus” between 2019 and 2021 were identified. Electronic patient records were reviewed to identify patient demographics, site of tumour thrombus, management and outcome.
Results: 68 patients were identified. The baseline characteristics are documented in (table 1). The most common malignancy was renal (32% of patients), correlating with the most common thrombus sites identified as the inferior vena cava and renal vein (table 2). 46/68 (68%) received anticoagulation and 22/68 (32%) were never anticoagulated. Resolution of tumour thrombus occurred in 14% of those therapeutically anticoagulated, 10% prophylactically anticoagulated and 9% not anticoagulated. 2/36 therapeutically anticoagulated patients and 2/22 of those patients not anticoagulated developed progressive tumour thrombus in association with disease progression. There were 5 major bleeding events as defined by ISTH criteria requiring intervention or admission. Four of these occurred on therapeutic anticoagulation and one on prophylaxis. 39% of those therapeutically anticoagulated and 41% of patients not anticoagulated died by 6 months.
Conclusion(s): This small observational cohort did not demonstrate a positive impact of anticoagulation on six month survival. Treatment of the underlying malignancy is likely the main predictor of resolution or progression of tumour thrombus. The distinction between tumour thrombus and bland thrombus radiologically is critical and can be challenging but important and would guide management with regard to the need for anticoagulation. Further larger studies are required to corroborate our findings and potentially identify those patients who might benefit from anticoagulation.


PB0923
The incidence and risk factors of thrombotic complications in patients with diffuse large B cell lymphoma at Trang Hospital
S. Jutiamornlerd
Trang Hospital, Trang, Trang, Thailand
Background: Thrombotic complications are a serious event in patients with diffuse large B cell lymphoma (DLBCL). The incidence and risk factors for developing arterial thromboembolism (ATE) events is still unclear and therefore needs to be studied further.
Aims: To identify the incidence and risk factors of venous thromboembolism (VTE) and arterial thromboembolism (ATE) events in DLBCL patients.
Methods: This observational study was conducted as a retrospective and prospective cohort study from January 2010 to December 2021 in Trang Hospital.
Results: 189 patients newly diagnosed with DLBCL were included in this study. The average age was 62.07 ± 15.99years and the average body mass index (BMI) was 22.61 ± 4.63kg/m2. Baseline characteristics were 53.4% males, 24.3% BMI ≥25kg/m2, 41.8% performance status >1, 51.9% stage >2, 63.5% lactate dehydrogenase >247U/L, 26.5% hemoglobin < 10 g/dL, 14.3% white blood cell ≥11,000/μL, 9.5% platelet count ≥450,000/μL and 15.3% mass size ≥7.5cm. The most common underlying illnesses in DLBCL patients were hypertension (12.8%), hyperlipidemia (11.1%), diabetes mellitus (10.6%) and other underlying illnesses were atrial fibrillation (1.1%), sequelae of CVA (4.8%), Old myocardial infarction (1.1%) and no previous VTE. The incidence of VTE was 1.6% deep vein thrombosis (DVT) and 1.1% pulmonary embolism (PE). The incidence of ATE was 1.1% myocardial infarction and 0.5% ischemic stroke. Only mass size ≥7.5cm. was significantly associated with PE in DLBCL patients (p‐value 0.0258). Risk factor of ATE events in DLBCL patients found only BMI ≥25kg/m2 was nearly significantly associated with ischemic stroke (p‐value 0.058).
Conclusion(s): The incidence of PE in DLBCL patients was similar compared to Western country studies but low incidence of ATE events. DLBCL patients with bulky disease seem to increase the risk for developing PE. A large prospective cohort study with longer duration follow‐up is required for further confirmation.
VPB0944
Anticoagulant associated bleeding risk assessment model for cancer associated thrombosis patients: A systematic review
G. Poenou 1; E. Toledano2; H. Helfer3; L. Plaisance2; E. Versini2; N. Diab4; S. Djennaoui2; I. Mahe5
1 Assistance publique Hopitaux de paris, Colombes, Ile‐de‐France, France; 2 Assistance Publique Hôpitaux de Paris, Colombes, Ile‐de‐France, France, 3 assistance publique hôpitaux de Parus, Colombes, Ile‐de‐France, France; 4 Assistance Publique Hôpitaux de Paris, colombes, Ile‐de‐France, France; 5 Assistance Publique Hôpitaux de Paris, Université de Paris, 3. Unité Inserm UMR_S1140 Innovation thérapeutique en hémostase, 4. INNOVTE‐FCRIN, Colombes, Ile‐de‐France, France
Background: Patients with venous thromboembolism event (VTE) in the context of cancer should be receiving anticoagulant as long that the cancer is active. Therefore, a tailor‐made anticoagulation strategy should rely on an individualized assessment of the risks of recurrent VTE and anticoagulant associated bleeding. Yet, no existing risk assessment model (RAM) for anticoagulant associated bleeding risk has been validated in cancer associated thrombosis (CAT).
Aims: The aim of this review is to investigate the currently available RAM for anticoagulant associated bleeding after VTE in their applicability in the CAT population, and to provide new insights on how we can succeed in developing a new anticoagulant associated bleeding RAM in current medical care of CAT patients.
Methods: A systematic search for peer‐reviewed publications up to September 20th, 2021 was performed in PubMed. Studies were eligible if they comprised patients with VTE, and used a design for developing a prediction model, score, or other prognostic tools for anticoagulant associated bleeding during anticoagulant treatment, including systematic reviews. Studies focusing on predicting the risk of anticoagulation associated bleeding in a non‐VTE based population were excluded.
Results: 15 RAMs were found in the literature. (Table 1) Just the CAT‐BLEED was developed for CAT patients and none of the presented RAMs developed on VTE general population were externally validated in a population of CAT patients. By focusing on methodology and applicability for CAT patients, the current review illustrates the limitations of the available RAMs for anticoagulant associated bleeding in CAT patients. To obtain a better RAM to assess the anticoagulant‐associated bleeding risk in CAT patients, we deemed it necessary to answer the questions in WHOM, HOW, WHAT, and WHEN do we assess the anticoagulant‐associated bleeding risk.
Conclusion(s): The development of RAM for bleeding risk assessment in patients with CAT is warranted.

PB0929
Comparison between full dose and reduced dose enoxaparin in the management of cancer‐associated venous thromboembolism
J. Ong1; E. Leitinger 2; S. Chunilal3
1 Monash Health, North Melbourne, Victoria, Australia; 2 Monash Health, Clayton, Victoria, Australia; 3 Monash Health, Melbourne, Victoria, Australia
Background: Treatment of cancer‐associated venous thromboembolism (VTE) is challenging due to high rates of both recurrent thrombosis and major bleeding. Low molecular weight heparin remains the preferred anticoagulant in select patients. Trials evaluating dalteparin have reduced the dose after one month. The optimal enoxaparin dosing regimen for cancer‐associated VTE remains uncertain.
Aims: To compare the efficacy and safety of full dose enoxaparin (1 mg/kg twice daily or 1.5 mg/kg daily) versus reduced dose enoxaparin (≤1 mg/kg daily) in the management of cancer‐associated VTE.
Methods: A single‐centre, retrospective audit was performed on patients with active cancer and acute VTE between January 2014 and December 2018. All patients received full dose enoxaparin for at least the first 28 days. Following this, patients either remained on full dose enoxaparin or were changed to reduced dose enoxaparin, at their treating clinician’s discretion. The primary outcome was recurrent VTE at six months. All suspected recurrences were blindly adjudicated by two reviewers. Secondary outcomes were rates of ISTH‐defined major bleeding and mortality. The study received institutional ethics committee approval.
Results: 257 patients (median age 64, 53% female) were identified with active cancer and acute VTE. 141 patients (55%) remained on full dose enoxaparin and 116 patients (45%) received reduced dose enoxaparin. Patient characteristics and outcomes are shown in Table 1 and Table 2. There was no difference in rates of recurrent VTE at six months between reduced dose enoxaparin and full dose enoxaparin (5.2% versus 5.7%, p = 0.86). There was also no difference in major bleeding events (9.5% versus 7.1% p = 0.49). Mortality was lower in patients who received reduced dose enoxaparin (16% versus 39%, p < 0.01). 97% of deaths were due to cancer, with no deaths attributable to VTE or bleeding.
Conclusion(s): The efficacy of reduced dose enoxaparin appears comparable to full dose enoxaparin in the management of cancer‐associated VTE, though without less major bleeding.


PB0921
Safety and efficacy of direct oral anticoagulant in elderly patients with cancer: real‐world challenges
F. Haque 1; N. Mnyama2; O. Adeniyi‐zaccheus2; R. Wells2; V. Brown2; A. Maraveyas2
1 Hull University Teaching Hospital NHS Trust, UK, COTTINGHAM, England, United Kingdom; 2 Hull University Teaching Hospital NHS Trust, UK, Hull, England, United Kingdom
Background: Anti‐Xa DOAC (direct oral anticoagulants) is an accepted treatment option for patients with cancer‐associated thrombosis (CAT) and is recommended in current guidelines. Their use, however, comes with some safety caveats. Several consensus statements suggest consideration of relative risks for increased bleeding before prescribing an oral anticoagulant vs. low molecular weight heparin.
Aims: Evaluate the safety and efficacy of DOAC prescribing habits in patients with cancer treated in an acute oncology service.
Methods: Retrospectively collected data from the electronic medical record system of a cohort of 449 patients with cancer receiving DOAC, apixaban or rivaroxaban, from September 2019 to August 2020 in HUTH NHS trust. Median follow‐up period was 6.6 months. The outcomes studied were recurrent venous thromboembolism (rVTE), major bleeding (MB), and clinically relevant non‐major bleeding (CRNMB). We considered the incidence of bleeding events in the presence vs. absence of relative contraindications to DOAC prescription. Analyses were performed with SPSS v27, IBM Corp TM and STATA SE17.
Results: Median age was 75 years, 254 (56.6%) male, 209 (46.5%) had metastatic disease. 197 (43.9%) were on DOAC for CAT, 194 (43.2%) for atrial fibrillation (43.2%). 5/449 (1.1%) had rVTE, all in the CAT cohort. 21 (4.7%) had bleeding with 8 (1.8%) MB. 164 (36.5%) patients had the presence of relative contraindications for DOAC prescription; among them, 10 (6.1%) had bleeding with 3 (1.6%) MB. The odds of bleeding in patients with relative contra‐indication was 1.6 (95% CI 0.7, 3.9; p = 0.284). Luminal involvement in all tumour types increased the risk of bleeding (OR 2.6; 95% CI 0.9, 7.4, p = 0.075).
Conclusion(s): Despite the more geriatric profile of these patients, overall rVTE and bleeding events were in keeping with rates seen in the registration studies. However, we found a trend for more frequent bleeding events in patients that had DOACs prescribed in the presence of relative contraindications.


PB0914
Antithrombin activity and association with risk of thrombosis and mortality in patients with cancer
C. Englisch 1; O. Königsbrügge2; F. Moik3; P. Quehenberger4; M. Preusser5; I. Pabinger6; C. Ay6
1 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 3 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Austria; Division of Oncology, Department of Internal Medicine, Medical University of Graz, Graz, Austria, Vienna, Steiermark, Austria; 4 Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 5 Department of Medicine I, Clinical Division of Oncology, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 6 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Venous thromboembolism (VTE) and arterial thromboembolism (ATE) are common complications in cancer patients. Antithrombin is a potent plasma anticoagulant and its deficiency is an established risk factor for VTE. In patients with cancer the association of antithrombin and risk of thrombosis is unclear.
Aims: To investigate the influence of antithrombin‐activity‐levels on risk of cancer‐associated VTE, ATE and all‐cause mortality, we utilized the dataset of the Vienna Cancer and Thrombosis Study (CATS).
Methods: CATS is an observational cohort study including patients with newly diagnosed or recurrent cancer. Patients were followed for objectively diagnosed VTE and ATE for up to 2 years. VTE and ATE were quantified in competing risk analysis, with all‐cause mortality as competing outcome. Overall survival was compared with a log‐rank test using a Kaplan Meier analysis.
Results: 1,127 patients were included (45% female, median age: 62 years [interquartile range, IQR: 52–68]). Over a median follow‐up of 18 months (IQR: 6–24), 110 patients were diagnosed with VTE (cumulative 2‐year incidence: 9.8%, 95% confidence interval [CI]: 8.2–11.5), 32 with ATE (cumulative 2‐year incidence: 2.8%, 95% CI: 1.9–3.9) and 563 patients had died (2‐year mortality: 50%). In the whole patient population antithrombin was not associated with risk of VTE (SHR: 1.00; 95% CI: 0.99–1.01), ATE (SHR: 1.00; 95% CI: 0.98–1.03) or mortality (HR: 1.00; 95% CI: 0.99–1.00) (table 1). In patients with brain tumors antithrombin was not associated with VTE (SHR: 0.99; 95% CI: 0.96–1.03), but, unexpectedly, higher levels showed association with ATE (SHR: 1.02; 95% CI: 1.00–1.04), and mortality (HR: 1.01; 95% CI: 1.00–1.02; >75th versus < 75th percentile: log‐rank 0.002; table 1, figure 1).
Conclusion(s): In patients with cancer, antithrombin was not associated with risk of VTE, ATE or all‐cause mortality. However, in the subgroup of brain cancer patients, higher levels were associated with ATE and poor overall survival, which needs confirmation and further investigation.

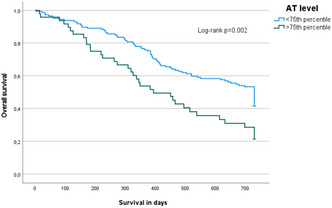
PB0928
Direct oral anticoagulants for treatment of venous thromboembolism in patients with hematological malignancies
R. Robinson1; G. Spectre2; M. Lishner3; O. Sharabi4; E. Robinson5; O. Hamburger Avnery6; A. Gafter‐Gvili7; P. Raanani2; A. Leader 8
1 Institute of Hematology, Davidoff Cancer Centre, Rabin Medical Centre, Kfar Saba, HaMerkaz, Israel; 2 Institute of Hematology, Davidoff Cancer Centre, Rabin Medical Centre, Petah Tikva, HaMerkaz, Israel; 3 Meir Research Institute, Meir Medical Center, Ra'anana, HaMerkaz, Israel; 4 Sackler School of Medicine, Tel Aviv University, Petah Tikva, HaMerkaz, Israel; 5 Intensive Care Unit, Rabin Medical Centre, Kfar Saba, HaMerkaz, Israel; 6 Institute of Hematology, Meir Medical Center, Kfar Saba, HaMerkaz, Israel, 7 internal medicine A, Rabin Medical Centre, Hod Hasharon, HaMerkaz, Israel; 8 Rabin Medical Center, Petah Tikva, HaMerkaz, Israel
Background: Data is needed on direct oral anticoagulants (DOACs) for treatment of venous thromboembolism (VTE) in hematological malignancies (HM). Retrospective studies to date lacked a control group and included patients with atrial fibrillation and/or VTE, but not VTE alone.
Aims: Assess incidence of VTE recurrence and bleeding in HM patients treated with low molecular weight heparin (LMWH) or DOACs for acute VTE.
Methods: Retrospective cohort study including patients with active HM and newly‐diagnosed VTE treated at Rabin or Meir Medical Centers (1/2015‐7/2021). Patients indexed on the first day of anticoagulation for VTE and followed for 12 months. The primary outcome was a composite of recurrent VTE, clot progression, major bleeding or clinically relevant non‐major bleeding (CRNMB). Cumulative incidence [95% confidence interval (CI)] was calculated for each anticoagulation group (LMWH, DOAC) and hazard ratios (HR) were calculated using cox‐proportional hazards model, with death as a competing risk.
Results: 143 HM patients treated with LMWH (96) or DOACs (47) for acute VTE were included. Table 1 shows patient characteristics. HM was lymphoma in 83 (58%). DOAC‐treated patients were older, had more pulmonary embolism and less splanchnic vein thrombosis. The 12‐month cumulative incidence of the primary outcome (Figure 1) was 24.2% (95% CI 15.9–33.5%; n = 22) in the LMWH group and 18.5% (8.5–31.5%; n = 8) in the DOAC group (HR 1.51 [0.695–3.297]). Two recurrent VTE occurred (both in the DOAC group while off‐treatment). Nine (9.4%) LMWH‐treated patients had major bleeding compared to 1 (2.1%) DOAC‐treated patient (HR 4.85 [0.64–36.56]). There were 13 (13.5%) CRNMB cases in the LMWH group and 5 (10.6%) in the DOAC group (HR 1.44 [0.52–3.98]).
Conclusion(s): This study generates the hypothesis that DOACs may be a safe and effective alternative to LMWH for VTE in patients with lymphoma or plasma cell dyscrasia. Larger prospective studies are needed to confirm these findings.


PB0931
Mechanisms of cancer associated thrombosis in gynaecological cancer patients‐tumour or treatment effect?
M. Ward1; S. O'Toole2; F. Abu Saadeh3; Z. Marchocki4; E. Ibrahim5; F. Martin6; N. Gleeson7; J. O'Leary8; L. Norris 9
1 Dept Histopathology, Trinity St. James's Cancer Institute, St. James's Hospital, Dublin 8 Ireland, Dublin 8, Dublin, Ireland; 2 Dept of Histopathology & Dept of Obstetrics and Gynaecology, Trinity St. James's Cancer Institute, St. James's Hospital, Dublin 8, Dublin, Dublin, Ireland; 3 Dept of Gynae‐Oncology, Trinity St. James's Cancer Institute, Dublin 8, Ireland, Dublin, Dublin, Ireland; 4 Dept of Gynae‐Oncology, Trinity St. James's Cancer Institute, St. James's Hospital, Dublin 8, Ireland, Dublin, Dublin, Ireland; 5 Dept of Gynae‐Oncology, Trinity St. James's Cancer Institute, St. James's Hospital, Dublin 8, Dublin, Dublin, Ireland; 6 Dept of Obstetrics and Gynaecology, Trinity St. James's Cancer Institute, Dublin 8, Ireland, Dublin, Dublin, Ireland; 7 Dept of Gynae‐oncology, Trinity St. James's Cancer Institute, St. James's Hospital, Dublin 8, Dublin, Dublin, Ireland; 8 Dept of Histopathology, Trinity St. James Cancer Institute, St. James's Hospital, Dublin 8, Dublin, Dublin, Ireland; 9 Trinity St. James's Institute, Dublin 8, Dublin, Ireland
Background: Gynaecological cancers patients are at high risk of venous thromboembolism(VTE) however the mechanism by which VTE occurs is not understood and could be tumour or treatment related.
Aims: The aim of this study is to determine the mechanism of hypercoagulability in gynaecological cancer patients and to assess the role of tumour and treatment factors in the enhanced thrombin generation observed in patients who subsequently develop VTE.
Methods: mRNA expression of Prothrombin(F2), Tissue Factor(TF), Tissue Factor Pathway Inhibitor‐1 (TFPI‐1), TFPI‐2, endothelial protein C receptor(EPCR), protein S(PS), protein C (PC), thrombomodulin (TM), Factor V (FV) and VIII (FVIII) was measured in tumour biopsies from 26 treatment naïve gynaecological cancer patients who developed VTE compared with matched controls. Plasma levels of FV, F8, TFPI, TM, PS, EPCR were determined by ELISA and were correlated with thrombin generation(ETP) in patients who subsequently developed VTE (VTE group). Ethical approval and informed consent was obtained.
Results: No significant differences in tumour mRNA expression was observed in patients who developed VTE compared with those who remained thrombosis free. In treatment naïve patients, pre‐operative ETP and Factor VIII were significantly increased in patients who developed VTE (VTE group) compared with matched controls (Non VTE group) (p < 0.05; p < 0.03). FVIII correlated with peak thrombin levels in the VTE group (r = 0.560). In patients treated with neoadjuvant chemotherapy before surgery, TM was reduced in the VTE group compared with the non VTE group (p < 0.03). TM correlated negatively with ETP in the VTE group (r = −4558).
Conclusion(s): Tumour expression of coagulation proteases does not appear to be implicated in VTE in gynaecological cancer patients. FVIII levels contribute to the pro‐coagulant activity observed in treatment naive gynaecological cancer patients who develop VTE. Reduced levels of TM may be linked to chemotherapy associated endothelial damage and result in impaired activation of protein C at the endothelial surface resulting in chemotherapy associated VTE.
VPB0939
Impact of age‐adjusted international prognostic index for predicting venous thromboembolism in thai patients with diffuse large B‐cell lymphoma
N. Hantrakun 1; P. Phinyo2; A. Tantiworawit1; E. Rattaritamrong1; C. Chai‐Adisaksopha1; T. Rattanathammethee1; S. Hantrakool1; P. Piriyakhuntorn1; T. Punnachet1; P. Niprapan1; L. Norasetthada1
1 Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand, Sri Phum, Muang, Chiang Mai, Thailand; 2 Center for Clinical Epidemiology and Clinical Statistic, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand, Sri Phum, Muang, Chiang Mai, Thailand
Background: Lymphoma is among the malignant diseases at high risk for venous thromboembolism (VTE). The international prognostic index (IPI) and age‐adjusted IPI (aaIPI) have been used to predict survival outcome for patients with diffuse large B‐cell lymphoma (DLBCL).
Aims: This study aimed to determine the VTE incidence and the impact of aaIPI for predicting VTE among Thai patients with DLBCL compared with the Khorana risk score (KRS).
Methods: This was a retrospective cohort study including all adult patients with newly diagnosed pathologically confirmed DLBCL. Patients with VTE were objectively identified by radiologists. Kaplan‐Meier method and log‐rank test were used to examine the differences in the rate of VTE occurrence in each aaIPI group. We used the area under the receiver operating characteristic (ROC) to quantify the ability of aaIPI to predict VTE.
Results: From 591 newly diagnosed DLBCL patients with median age 58 (range 16–93) years, 32 patients had VTE within the first year of DLBCL diagnosis, given the incidence of VTE of 5.4% (95% confidence interval [CI], 3.7–7.6). VTE patients had more advanced stage (75% vs. 56.3%; p = 0.043) and higher LDH (78.1% vs. 54.9%; p = 0.010) than non‐VTE patients. The 1‐year cumulative incidence of VTE was 1.47%, 3.87%, 7.65%, and 8.46% for aaIPI 0 to 3, respectively (p = 0.008) (Figure 1A). Patients with aaIPI ≥ 2 had significantly higher 1‐year cumulative incidence of VTE than patients with aaIPI < 2 (8.0% vs. 2.8%; hazard ratio, 3.51; 95% CI, 1.57–7.83; p = 0.001) (Figure 1B). The area under the ROC for VTE prediction was 0.61, 0.64, 0.65 for KRS, IPI, and aaIPI, respectively.
Conclusion(s): The incidence of VTE among Thai patients with DLBCL was comparable to Caucasians. The aaIPI might be helpful to identify DLBCL patients with a higher risk of VTE especially in patients with aaIPI ≥ 2.

VPB0945
Poor performance status and elevated factor VIII levels increase thrombotic risk in lymphoma and multiple myeloma patients
I. Sánchez Prieto; I. Gutiérrez Jomarrón; C. Martínez Vázquez; J. García Suárez
Hospital Universitario Príncipe de Asturias, Madrid, Madrid, Spain
Background: Venous thromboembolism (VTE) is an important cause of morbimortality in cancer, resulting from a combination of genetic and acquired conditions. Thrombosis incidence in lymphoma is variable (1,5–59,5%), being higher in non‐Hodgkin lymphoma (NHL) than in Hodgkin disease (6,5 vs.4,7%), and in aggressive compared to indolent NHL (16,3 vs.3,8%). VTE incidence in multiple myeloma (MM) is 15,2%. Despite the demonstrated thrombotic risk in these patients, thromboprophylaxis is largely underutilized. Recently, new thrombotic risk assessment models (RAMs) have been developed.
Aims: To identify risk factors for thrombosis and explore thrombotic RAMs in lymphoma and MM patients.
Methods: This prospective, observational study includes 63 patients diagnosed with lymphoma/MM between February 2020 and June 2021 at our centre. The variables recorded were: thrombosis characteristics; clinical, treatment and laboratory variables (including acquired and genetic‐Thrombo inCode‐ thrombophilia testing at cancer diagnosis). Approval by the local medical ethics committee and patients’ informed consent were obtained.
Results: Median age at cancer diagnosis was 64 (IQR:51–72). MM was diagnosed in 16 (25,4%) patients and lymphoma in 47 (74,6%), the majority being NHL (87,2%). At a median follow‐up of 9,1 months (IQR:5,1–12), 6 (9,5%) patients developed VTE (2 deep venous thrombosis,2 pulmonary embolisms,1 catheter‐related,1 internal jugular vein thrombosis); median time to VTE was 36 days (IQR:4–92). Thrombosis was more common in patients with ECOG2 or more (28,6% vs.4,1%; p = 0,019). Baseline median factor VIII was higher in VTE (393%; IQR 246–344) than in non‐VTE group (211%; IQR 157–241) (p = 0,005). Other risk factors appear in Table 1. Table 2 shows thrombotic risk according to different RAMs.
Conclusion(s): Thrombosis incidence in our population is similar to previously described, with impaired ECOG and elevated factor VIII increasing VTE risk. Existing thromboprophylaxis didn't significantly reduce thrombosis, and RAMs stratification showed no association with thrombosis; this may be due to the small population analyzed. Improved RAMs combining clinical, laboratory and genetic variables are needed to prevent thrombosis in lymphoma and MM.
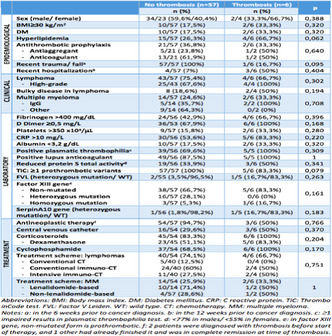

PB0936
Pre‐surgical ovarian vein thrombosis in women with ovarian malignancies as predictor of pulmonary embolism
H. Wiegers 1; L. Beenen2; M. Schaafsma3; S. Middeldorp4; B. Hutten5; S. Mom6; L. Scheres7
1 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 2 Department of Radiology, Amsterdam University Medical Centres, Amsterdam, Noord‐Holland, Netherlands; 3 Antoni van Leeuwenhoek, Netherlands Cancer Institute, Amsterdam, Noord‐Holland, Netherlands; 4 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands; 5 Department of Epidemiology and Data Science, Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 6 Department of Obstetrics and Gynaecology, Amsterdam UMC, University of Amsterdam, Amsterdam Reproduction & Development research institute, Amsterdam, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 7 Radboud University Medical Center & Leiden University Medical Center, Nijmegen, Gelderland, Netherlands
Background: Ovarian vein thrombosis (OVT) is a rare disorder. Few data are available on the risk factors for OVT. Current evidence suggests a role for local factors, as the risk of OVT is increased during pregnancy, in the postpartum period and in patients with gynaecological malignancies. Furthermore, several case reports have described the occurrence of pulmonary embolism (PE) after OVT.
Aims: To assess the association between preoperative OVT and postoperative PE in women undergoing surgery for an ovarian malignancy.
Methods: In this case‐control study women surgically treated for ovarian cancer FIGO stages I‐IV were included. Cases were consecutive women with postoperative PE within 90 days after surgery between January 2015 and July 2020, controls were women without postoperative PE within 90 days. Cases were matched (1:2 ratio) by age, malignancy disease stage and type of surgery with controls. Without knowledge of the occurrence of postoperative PE, one experienced radiologist assessed presence of OVT on pre‐operative imaging (computed tomography with intravenous contrast).
Results: A total of 57 patients (19 cases, 38 controls) were included, with a median age of 70 years (interquartile range 62–76) and mean BMI of 26 kg/m2 (standard deviation 5.2). Surgeries concerned: interval debulking (54.4%), primary debulking (40.4%) or staging surgery (5.2%) of which 95% were open procedures, and 5% laparoscopic. Of the 19 cases with postoperative PE, two (10.5%, 95% confidence interval [CI] 2.9–31.4) were found to have preoperative OVT, whereas none of the 38 controls (0%) had OVT on pre‐operative imaging. In absence of an OVT in the control group, a conservative estimate of the Odds Ratio (assuming 0.5 additional OVT in both cases and controls) would yield: 11.0 (95% CI 0.5 – 241.4).
Conclusion(s): OVT on preoperative imaging might be a predictor of postoperative PE in women with ovarian malignancy. Confirmation from larger studies is needed.
PB0905
D‐dimer level measured at first incident cancer‐related venous thromboembolism is a predictor for recurrence: A retrospective cohort study
H. Almajed 1; F. Aleidan2
1 King Saud bin Abdulaziz University for Health Sciences, Riyadh, Ar Riyad, Saudi Arabia; 2 King Abdulaziz Medical City, Ryiadh, Ar Riyad, Saudi Arabia
Background: Cancer is considered as a major risk factor of venous thromboembolism (VTE). Whether D‐dimer level at first incident cancer‐related VTE can predict recurrence is not fully elucidated.
Aims: To assess the association between D‐dimer level measured at first incident cancer‐related VTE and risk of recurrence.
Methods: In this study, all patients with first incident cancer‐related VTE were retrospectively retrieved and followed‐up for a period of 36 months. Measured levels of D‐dimer and clinical predictors for each patient prior intervention were collected. Univariate and multivariant cox regression were used to estimate hazard ratio (HR) and 95% confidence interval (CI).
Results: A total of 73 (34.3%) of 213 cancer‐related VTE patients had recurrent VTE. The crude recurrence rate was 11.4 per 100 person‐years (95% CI, 9.2–14.2). The mean value of D‐dimer was significantly higher seen in recurrent VTE patients than those without recurrence (14.8 ±7.5 mg/L vs 4.8 ±5.7 mg/L, p < 0.001). The highest positive likelihood ratio using Area under the receiver operating characteristic (ROC) curve occurred when a D‐dimer threshold of 8.67 mg/L was chosen. At this threshold the D‐dimer was 92% sensitive and 80% specific in predicting recurrent VTE. The area under the ROC curve was 0.924 (95% CI 0.887 to 0.960, p < 0.001, Figure 1). Patients with a D‐dimer ≥8.67 mg/L at first VTE in our study were 3.1‐fold more likely to have a recurrence than those with lower D‐dimer results (Table 1).
Conclusion(s): A D‐dimer ≥8.67 mg/L measured at first incident cancer‐related VTE was associated with a 3.1‐fold increased hazard of recurrence events. Our finding suggests that a Low D‐dimer level at time of first cancer‐related VTE is suitable in the clinical practice to avoid extended‐duration anticoagulation.


PB0926
Low concentrations of rFVIIa bypass changes in ROTEM coagulation parameters induced by abelacimab in vitro
Y. Khder 1; S. Cote2; P. Hoffmann3; D. Bloomfield4
1 MYRA Life Science Services, Rosenau, Alsace, France; 2 Independent Consultant, Saint Louis, Alsace, France; 3 N/A, Lady’s Island, South Carolina, United States; 4 Anthos Therapeutics, Inc., Cambridge, Massachusetts, United States
Background: Low doses of recombinant activated factor VII (rFVIIa) have been used to prevent or stop bleeding in patients with severe factor XI (FXI) deficiency. A similar strategy was proposed to bypass the pharmacodynamic effects of abelacimab.
Aims: To test whether low concentrations of rFVIIa could correct the changes in abelacimab‐induced coagulation parameters as measured by rotational thromboelastometry (ROTEM) in a whole blood in vitro assay.
Methods: Blood specimens were obtained in citrate tubes (6 healthy donors). Specimens were incubated with 15 and 30 μg/mL of abelacimab or vehicle for 10 min at 37°C. Specimens were subsequently spiked with rFVIIa at 0.5 and 1 μg/mL or vehicle. Clot formation was monitored using ROTEM®delta analyzers and the non‐activated thromboelastometry (NATEM) test, using recalcification reagent. CT (clotting time; sec), CFT (clot formation time; sec), alpha angle, etc. were measured and were compared with reference ranges provided by the manufacturer.
Results: Abelacimab at 15 and 30 μg/mL concentrations increased CT by 61% and 64%, CFT by 37% and 32%, and decreased alpha angle by 10% and 14% vs baseline, respectively. Adding rFVIIa at 0.5 and 1.0 μg/mL to abelacimab‐spiked samples shortened CT by 21% and 38%, CFT by 33% and 49%, and increased alpha angle by 29% and 47%, respectively (Figure 1). NATEM parameters remained within normal reference ranges when rFVIIa was added (Figure 1).
Conclusion(s): Low concentrations of rFVIIa (0.5 to 1 μg/mL) were able to correct the effects of abelacimab as assessed by rotational thromboelastometry. Normalizing without over‐correcting ROTEM coagulation parameters suggests that tested concentrations of rFVIIa are unlikely to result in a hypercoagulable state. Our data support using low doses of rFVIIa for bleeding management in patients treated with abelacimab.

PB0935
Plasma MiRNAs and the risk of venous thromboembolism in patients with glioblastoma: A nested case‐control study
J. Riedl 1; M. Hackl2; A. Diendorfer2; M. Preusser3; G. Widhalm4; K. Roessler4; I. Pabinger5; C. Ay5; F. Erhart4
1 Medical University of Vienna, Wien, Wien, Austria; 2 TAmiRNA GmbH, Vienna, Austria., Vienna, Wien, Austria; 3 Medical University of Vienna, Department of Medicine I, Division of Oncology, Vienna, Wien, Austria; 4 Medical University of Vienna, Department of Neurosurgery, Vienna, Wien, Austria; 5 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Patients with high‐grade gliomas are at high risk of venous thromboembolism (VTE). MicroRNAs (miRNAs) are small non‐coding RNAs with multiple roles in tumor biology as well as in hemostasis.
Aims: We aimed to explore the association between miRNAs and risk of VTE in high‐grade glioma.
Methods: We conducted a nested‐case control study within 152 glioblastoma patients that had been included in the Vienna Cancer and Thrombosis Study (CATS), a prospective cohort study with the aim to identify risk factors for VTE in patients with newly diagnosed or recurrent cancer. At study inclusion a single blood draw is taken and patients are thereafter followed for a maximum of two years. The primary endpoint was symptomatic VTE. In total, 24 patients (16%) developed VTE. Out of the 128 patients who did not develop VTE during follow‐up we randomly selected 24 age‐ and sex‐matched controls. Four patients had to be excluded due to missing plasma samples, leading to a final group size of 21 patients with VTE (=cases) and 23 without VTE (=controls). RNA‐Seq was performed in plasma by using the miND® NGS workflow and analysis pipeline (TAmiRNA, Vienna, Austria).
Results: In an exploratory analysis adjusted for platelet count, we found several miRNAs differentially expressed in controls vs. cases. miRNAs that showed the highest magnitude of downregulation in controls vs. cases were miR‐224‐5p, miR‐4433b‐3p, and miR‐139‐3p, while miR‐454‐3p, miR‐183‐5p, miR‐122‐5p, miR‐122‐3p, miR483‐3p and miR‐885‐3p showed the highest magnitude of upregulation (Figure 1). However, after adjustment for multiple testing none of the results achieved statistical significance.
Conclusion(s): Several miRNAs showed a trend towards differential expression in glioblastoma patients who later developed VTE compared to patients without VTE. Larger studies are needed to clarify whether miRNAs might identify patients at high risk of VTE.


PB0938
Treatment of splanchnic vein thrombosis with anticoagulant therapy in cancer patients: A systematic review and meta‐analysis
A. Zahrai 1; M. Carrier2; A. Delluc3
1 University of Ottawa and The Ottawa Hospital, Ottawa, Ontario, Canada; 2 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 3 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada
Background: Nowadays, SVTs occur more commonly in patients with cancer, who have a higher thrombotic risk than non‐cancer patients. Clinical guidelines on treatment of SVTs come mostly from small observational studies and expert clinician recommendations. Anticoagulants are often recommended to treat SVTs in cancer patients.
Aims: To determine the anticoagulation type, duration, dosing, effectiveness and safety in adult patients with cancer‐associated SVTs.
Methods: We searched EMBASE, MEDLINE and CENTRAL electronic databases for clinical studies that evaluate anticoagulants given for cancer‐associated SVTs in adult patients using an expert‐developed specific search strategy from inception to April 30, 2021, without any restrictions. Clinicaltrials.gov was also investigated for additional studies. We evaluated SVT recurrence, recanalization and extension, major and clinically relevant non‐major bleeding and all‐cause mortality, between patients receiving anticoagulants and those not receiving anticoagulants for cancer‐associated SVT. We screened studies in duplicates for inclusion, will quantitatively pool the results of included studies and perform subgroup analyses of involved subpopulations.
Results: 3332 studies were retrieved for screening and 18 studies (12 full text publications and 6 abstracts) have been included for data extraction to date. A preliminary review of each included study appears to favor using anticoagulants to treat SVTs in cancer patients with favorable outcomes such as recurrence, extension, recanalization and all‐cause mortality. Bleeding outcomes appear to be more variable across studies. Meta‐analysis is ongoing.
Conclusion(s): Physicians need to balance the risk of thrombosis with that of bleeding when treating patients with cancer‐associated SVTs using anticoagulants. While meta‐analysis is pending, a preliminary overview of included studies demonstrated that anticoagulants can be useful in reducing the thrombotic and mortality risks in these patients, while risk of bleeding varied across clinical studies. Our study may lead to improvements in the health and economic burden of cancer‐associated SVTs.
VPB0941
The role of selectins in development of acute pulmonary embolism in cancer patients
I. Darwish1; B. Kantarcioglu 2; A. Darki3; F. Siddiqui4; O. Iqbal4; D. Hoppensteadt3; J. Fareed3
1 Loyola University Chicago, Stritch School of Medicine, Maywood, Illinois, United States; 2 Loyola University Chicago, Oak Park, Illinois, United States; 3 Loyola University Chicago, Maywood, Illinois, United States; 4 Loyola University Medical Center, Maywood, Illinois, United States
Background: Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third leading cause of cardiovascular death. Cancer represents and added risk of adverse outcomes in PE patients. Adhesion molecules such as selectins have been investigated in several studies of VTE patients. However, their causative role in thrombosis and cancer remains unclear.
Aims: We sought to quantify the levels of P‐Selectin, E‐Selectin and L‐Selectin in a patient cohort, comprised of acute PE, to determine their relevance on pathophysiology of VTE.
Methods: Whole blood samples were drawn from patients within 24 h of confirmed diagnosis of acute PE under an Institutional Review Board approved protocol. The samples were tested for P‐Selectin, E‐Selectin and L‐Selectin utilizing commercially available ELISA assays. The distribution of co‐morbidities in PE patients, including demographic information, collected through the review of patient electronic medical records. Circulating levels of each biomarker were compared according to cancer diagnosis. p < 0.05 was considered statistically significant.
Results: Table 1 depicts the demographic information and distribution of co‐morbidities in acute PE patients. We did not observe any difference in the levels of P‐Selectin and E‐Selectin in comparison to cancer vs. non cancer status (p > 0.05). The levels of L‐Selectin were significantly lower in cancer patients compared to non‐cancer patients (1313.78 ± 661.537 vs 1529.06 ±539.72, p: 0.01425).
Conclusion(s): Our results have shown that L‐selectin levels in acute PE patients significantly differ in patients with cancer vs. non cancer. The L‐Selectin and E‐selectin levels in cancer subgroups while not significantly different showed a trend towards decreased values. Additional data including larger number of patients may provide definitive data on these biomarkers and their role in cancer.


PB0906
Incidences and predictors of venous thromboembolism and mortality in patients diagnosed with lung cancer among Saudi population: Retrospective two‐centre cohort study
R. Almesfir 1; N. Alqudaibi2; F. Aleidan3
1 King Saud bin Abdulaziz University for Health Sciences (KSAU‐HS), Saudi Arabia, Ar Riyad, Saudi Arabia, 2 King Saud bin Abdulaziz University for Health Sciences (KSAU‐HS), Ryiadh, Ar Riyad, Saudi Arabia; 3 King Abdulaziz Medical City, Ryiadh, Ar Riyad, Saudi Arabia
Background: Venous Thromboembolism a well‐known complication in patents diagnosed with cancer. Lung cancer considered among the group of malignancies with the highest incidence rates of VTE.
Aims: To determine the incidence rates and assess the predictors associated with VTE and mortality in patients diagnosed with lung cancer.
Methods: In this two‐centres study we retrospectively followed up 497 patients aged ≥18 years with a lung cancer. In this Saudi cohort study, the incidence of VTE and mortality were assessed. The association between characteristics of patients, VTE and mortality were explored by estimating hazard ratio (HR) and 95% confidence interval (CI) using univariate and multivariate cox regression.
Results: Of the 497 patients with lung cancer, 98 patients developed VTE and 280 patients died with an incidence rate of 9.8 per 100 person‐year (PY,95% CI, 8.0–11.8) and 26.5 per 100 PY (95% CI, 23.9–29.3), respectively. Presence of NSCLC, low albumin level, and high grade of Eastern Cooperative Oncology Group (ECOG) performance status were found independent predictors associated with the development of VTE; Table 1. VTE carries a high mortality rate (HR, 3.10; 95% CI, 2.53–3.91; p < 0.001; Table 2). Using Kaplan‐Meier, the estimate VTE median time (months) was significantly lower in NSCLC (28.7 months) than SCLC (33.7 months, p = 0.002 by the log‐rank test). The estimate survival median time was significantly lower in NSCLC (23.8 months) than in SCLC (29.7 months, p < 0.001 by the log‐rank test).
Conclusion(s): The incidence rate for VTE in lung cancer patients was 9.8 per 100 YP and the mortality was 26.5 per 100 PY. Presence of NSCLC, low albumin level, and high grade of ECOG performance status were significant predictors for VTE. Patients who developed VTE had 3.10‐fold higher mortality rate than patients with no VTE.
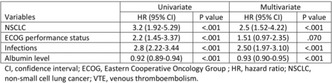

PB0925
Abelacimab has no effect on platelet aggregation induced by TRAP‐6 and collagen
Y. Khder 1; S. Cote2; P. Hoffmann3; D. Bloomfield4
1 MYRA Life Science Services, Rosenau, Alsace, France; 2 Independent Consultant, Saint Louis, Alsace, France; 3 N/A, Lady’s Island, South Carolina, United States; 4 Anthos Therapeutics, Inc., Cambridge, Massachusetts, United States
Background: Factor XI (FXI) has been identified as a ligand to ApoER2 and GPIbα on the surface of stimulated platelets. The impact of FXI inhibition by abelacimab on platelet aggregation remains to be determined.
Aims: To test the effects of increasing concentrations of abelacimab on platelet aggregation following stimulation with collagen or thrombin receptor activating peptide‐6 (TRAP‐6) compared with vehicle and active control (abciximab, anti‐GP2b3a) and to determine the effects of both agents on thrombin generation.
Methods: Whole blood was obtained in EDTA and citrate tubes (6 healthy donors). Specimens were spiked with vehicle, abelacimab (250, 500, or 1000 nM), or abciximab (50 nM). Platelet aggregation was recorded following induction using collagen (1 μg/mL) or TRAP‐6 (8 μM using a Multiplate® impedance aggregometer (Roche Diagnostics International Ltd, Switzerland). The area under the curve (AUC) for platelet aggregation was determined in arbitrary aggregation*time (AU*min). Thrombin generation was also measured at the above abelacimab and abciximab concentrations in platelet‐rich plasma using Thrombinoscope CAT (Calibrated Automated Thrombogram; Stago CH S.A.) and tissue factor reagent (1 pM); fluorescence was measured by an automated plate reader fluorometer (Fluoroskan Ascent, Thermolabsystems, Helsinki, Finland).
Results: Abelacimab showed no inhibitory or stimulatory effects on platelet aggregation induced by collagen or TRAP‐6 (Figure 1). In contrast, significant reductions in collagen‐induced platelet aggregation were observed with abciximab. Abelacimab resulted in significant delays in lag time and the time to peak concentration of thrombin generation; abciximab had no effect on thrombin generation.
Conclusion(s): Targeting FXI/FXIa by abelacimab does not affect platelet aggregation. However, abelacimab had an inhibitory effect on thrombin generation. As expected, abciximab inhibited collagen‐induced platelet aggregation but did not affect thrombin generation.

PB0937
Venous thromboembolism in bladder cancer: Scope of the Problem and Patients’ Perspectives (VTE‐BC)
O. Abdullah1; A. Young 2; D. Parashar3; A. Ignatowicz4
1 Haematology Department/Royal London Hospital, Coventry, England, United Kingdom; 2 Warwick Medical School/University of Warwick, Coventry, England, United Kingdom; 3 Health sciences /University of Warwick, Gibbet hill street, Coventry, England, United Kingdom; 4 Institute of Applied Health Research/ College of Medical and Dental Sciences /University of Birmingham, Birmingham, England, United Kingdom
Background: Venous thromboembolism (VTE) presents a challenge in the management of cancer patients and is a common cause of morbidity and mortality. Bladder cancer (BC) patients are at high risk of VTE; however, the problem is not well quantified. Patients’ awareness of cancer‐associated thrombosis (CAT) early in their cancer pathway has hitherto been neglected.
Aims: The aims of this study were to investigate the scope of VTE in patients with BC in the UK and explore patients’ understanding of CAT from their experience of having BC and treatment.
Methods: A sequential mixed‐methods study was applied: i) quantitative to explore the incidence and risk factors for VTE in BC, by cohort and case‐control analysis, followed by ii) qualitative through semi‐structured interviews among patients with BC and healthcare professionals (HCPs), utilising thematic analysis.
Results: VTE incidence in patients with BC was around 6.0 per 1000 from national datasets, lower than previously published. Cystectomy and stage IV disease significantly increased the risk of VTE in BC patients; adjusted odds ratio (OR); OR 2.88 (95% CI 1.63–5.07), p < 0.001 and OR 4.41 (95% CI 1.85–10.50), p = 0.002, respectively, while chemotherapy was found to have borderline significance as a risk factor; OR 2.56 (95% CI 1.03–6.32), p = 0.041. Patients had a lack of awareness of CAT; they received limited education on VTE. Three major themes emerged from the interviews: ‘it’s all about the cancer’ (CAT was not a priority for patients with BC or HCPs), ‘a labyrinthine process’ (information about VTE was bewildering and compartmentalised along the cancer continuum of care) and ‘improving the poor deal’ (patients’ and HCPs’ offered suggestions to improve communication around CAT).
Conclusion(s): VTE is an important clinical problem in patients with BC. HCPs can build on the findings of VTE‐BC to improve individualised CAT care through multidisciplinary inclusion of CAT discussion during patient encounters.


PB0917
The Ottawa Score did not predict recurrent venous thromboembolism in the prospective TROPIQUE cohort study
C. Frere 1; P. Debourdeau2; A. lamblin3; F. Cajfinger4; N. Falvo5; Y. Benhamou6; M. Sevestre7; D. Farge8
1 Sorbonne Université, Paris, Ile‐de‐France, France; 2 Institut Sainte‐Catherine, Avignon, Provence‐Alpes‐Cote d'Azur, France; 3 LEO Pharma, Voisins‐le‐Bretonneux, Ile‐de‐France, France; 4 Assistance Publique Hôpitaux de Paris, Paris, Ile‐de‐France, France; 5 CHU de Dijon‐Bourgogne, Dijon, Bourgogne, France; 6 CHU Charles‐Nicolle, Rouen, Nord‐Pas‐de‐Calais, France; 7 CHU Picardie, Amiens, Nord‐Pas‐de‐Calais, France; 8 Université de Paris, Paris, Ile‐de‐France, France
Background: Recurrent venous thromboembolism (VTE) occurs frequently in cancer patients despite curative anticoagulation. The Ottawa score was designed to stratify patients according to their risk of recurrent VTE within 6 months after starting anticoagulation.
Aims: To analyze the predictive value of the Ottawa risk score among cancer patients from the TROPIQUE study cohort.
Methods: TROPIQUE was a prospective multicenter observational cohort of adult cancer patients with newly diagnosed VTE treated with low molecular weight heparin (LMWH) for 6 months. For all patients included in the study, we retrospectively calculated the modified Ottawa score as follows: female sex, lung cancer, and prior history of VTE, each accounting for 1 point; breast cancer and cancer stage I + II each accounting for −1 point. Patients with an Ottawa score ≥ 1 were classified at high‐risk for recurrent VTE. The primary endpoint was recurrent VTE within the first 6 months of anticoagulation. All clinical events were centrally adjudicated. The Fine‐Gray competing risk model was used to take into account the competing risk of death.
Results: A total of 409 patients were analyzed on an intention‐to‐treat basis. At study entry, patients were aged 65.0 ± 12.1 years. The majority of index VTE events were deep vein thrombosis (47.2%) or pulmonary embolism (35.5%). The main primary cancer site was gastrointestinal (24.4%), followed by lung (17.4%) and breast (15.9%), and 249 (60.9%) patients had metastatic disease. The modified Ottawa score was high (≥1) in 163 (39.9%) patients. The overall 6‐month cumulative incidence of recurrent VTE was 6.2 % (95% CI: 4.0–9.5), and 6.6% (95% CI: 4.0–10.7) versus 5.7% (95% CI: 2.6–12.5) in the Ottawa low‐intermediate and high‐risk groups, respectively. The C‐statistic value was 0.52 (95% CI: 0.46–0.58).
Conclusion(s): In this prospective cohort of cancer patients receiving LMWH for VTE, the Ottawa score failed to accurately predict the risk of recurrent VTE.

PB0930
Cancer associated thrombosis on bevacizumab: Risk of recurrence and bleeding if bevacizumab is stopped or continued
M. Mayenga1; N. Falvo2; I. MAHÉ3; A. Jannot4; B. Gazeau5; G. Meyer6; O. Sanchez7; B. Planquette 4
1 Hôpital Foch, Suresnes, Ile‐de‐France, France; 2 CHU de Dijon‐Bourgogne, Dijon, Bourgogne, France; 3 Department of Internal Medicine, APHP, Paris, Ile‐de‐France, France; 4 APHP, Paris, Ile‐de‐France, France; 5 APHP, paris, Ile‐de‐France, France; 6 Hôpital Européen Georges Pompidou, Paris, Ile‐de‐France, France; 7 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France
Background: Cancer associated thrombosis (CAT) is a common complication of cancer and its management is complicated by an increased risk of recurrence and bleeding in these patients. Bevacizumab is an effective anti‐angiogenic therapy but increases the risk of bleeding and potentially the risk of CAT.
Aims: The objective of this study was to evaluate the efficacy and tolerance of anticoagulant therapy in the treatment of CAT occurring on bevacizumab, according to the continuation or discontinuation of bevacizumab.
Methods: This was a retrospective multicenter study. Patients with anticoagulated deep vein thrombosis or pulmonary embolism occurring on bevacizumab between June 2000 and December 2020 were included. The primary end point combined the occurrence of thromboembolic recurrence and the occurrence of clinically significant major or non‐major bleeding during the entire follow‐up period. Secondary end points were recurrence of thromboembolic events, occurrence of bleeding, occurrence of major bleeding, and overall survival.
Results: Of the 162 patients included, bevacizumab was stopped in 70 patients (stop group) and continued in 92 patients (continuation group). During follow‐up, 30% of patients experienced CAT recurrence or major or non‐major bleeding in the stop group and 29% in the continuation group. Analysis of survival to first event showed no significant difference between the two groups (p = 0.19). The same result was found in the multivariate analysis and taking into account the time of exposure to bevacizumab. There was no difference between the groups in recurrence, bleeding, major bleeding, or overall survival.
Conclusion(s): This study suggests the efficacy and safety of anticoagulant therapy with bevacizumab. However, a larger non‐inferiority study would be needed to confirm this result.

PB0933
A randomized clinical trial to evaluate the effect of rosuvastatin with enoxaparin on circulating tissue factor extracellular vesicles following ovarian cancer surgery
A. Osataphan1; R. Patell 2; R. Rosovsky3; P. Elavalakanar1; A. Bregar4; A. Ramos4; L. Garrett1; S. Ren5; D. Neuberg5; M. Shea1; J. Zwicker2
1 BIDMC, Boston, Massachusetts, United States; 2 Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States; 3 Massachusetts General Hospital, Boston, Massachusetts, United States; 4 MGH, Boston, Massachusetts, United States; 5 DFCI, Boston, Massachusetts, United States
Background: Ovarian cancer is among the most prothrombotic tumor diagnoses, and the majority of venous thromboembolism (VTE) occur within 60 days of surgery. Tissue factor bearing extracellular vesicles (TF‐EV) are prothrombotic and have been linked to thrombosis in cancer and hypercoagulability of surgery. Rosuvastatin has been shown to reduce the generation of EV and decrease VTE risk in non‐cancer populations. Whether rosuvastatin reduces TF‐EV and prevents VTE following ovarian cancer surgery is unknown.
Aims: To evaluate effect of combining rosuvastatin with enoxaparin on circulating TF‐EV following ovarian cancer surgery.
Methods: In a pilot trial for women undergoing ovarian cancer, women were randomized to enoxaparin 40 mg daily for 30 days or enoxaparin 40 mg for 30 days with rosuvastatin 40 mg daily from days 15 to 60. Women who did not want randomization received enoxaparin 40 mg for 30 days per standard of care. Total EV and TF‐EV were assessed by flow cytometry. Bilateral lower extremity ultrasound was performed on day 30 and 60.
Results: Sixteen women received enoxaparin alone and seven women were randomized to enoxaparin with rosuvastatin. Circulating TF‐EV were markedly elevated after surgery and decreased with time (Figure 1). There was no difference in number of circulating TF‐EV with enoxaparin versus enoxaparin with rosuvastatin on day 30 (7.7 ± 10.9 × 103 TF‐EV/μL versus 5.7 ± 5.1 × 103 TF‐EV/μL, p = 0.65) or day 60 (5.6 ± 6.2 TF‐EV/μL vs. 3.8 ± 3.6 × 103 TF‐EV/μL, p = 0.53 respectively). Similarly, there were no differences in CRP or D‐dimer between groups. No one experienced lower extremity deep vein thrombosis although portal vein thrombosis was diagnosed in enoxaparin arm. No major hemorrhages were observed.
Conclusion(s): The addition of rosuvastatin to enoxaparin following ovarian cancer surgery does not appear to impact number of circulating EV‐TF nor alter markers of coagulation. Asymptomatic DVT was not observed in this cohort following ovarian cancer surgery.

VPB0940
Biomarkers of coagulopathy in patients with different types of acute leukemia
Y. Hisada 1; S. Archibald1; K. Bansal2; S. Dwarampudi2; A. Mullen2; S. Bhatia2; R. Bhatia2; N. Mackman1; R. Gangaraju2
1 University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States; 2 University of Alabama at Birmingham, Birmingham, Alabama, United States
Background: Acute leukemia is associated with coagulopathy. Acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL) have a similar incidence of disseminated intravascular coagulation (DIC, 9–22%) and venous thromboembolism (VTE, 2–13%). Interestingly, acute promyelocytic leukemia (APL), a subtype of AML, has an increased incidence of DIC (60–85%) compared with other types of leukemia. This suggests that there are different mechanisms of coagulopathy among different types of acute leukemia.
Aims: To compare levels of various biomarkers, including extracellular vesicle (EV) tissue factor (TF) activity, phosphatidylserine (PS)+ EVs, citrullinated histone H3 (H3Cit)‐DNA complex, active plasminogen activator inhibitor 1 (PAI‐1) and tissue plasminogen activator (tPA), in patients with different types of acute leukemia.
Methods: Plasma from 30 APL, 281 non‐APL AML, and 78 ALL patients were collected. Levels of D‐dimer, prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, and complete blood count were obtained from medical records. We measured levels of EVTF activity and H3Cit‐DNA complex using in‐house assays and PS+ EVs, active PAI‐1 and tPA using commercially available assays.
Results: APL patients had significantly higher levels of EVTF activity compared to non‐APL AML and ALL patients. In contrast, non‐APL AML and ALL patients had higher levels of PS+ EVs compared to APL patients. There was no difference in levels of H3Cit‐DNA between the three groups. Non‐APL AML patients had the lowest levels of active PAI‐1 followed by ALL and then APL patients. Levels of tPA were not different between the three groups.
Conclusion(s): Our results suggest that TF+ EVs contribute to the activation of coagulation cascade in APL. In non‐APL AML and ALL patients PS+ EVs may contribute to the hypercoagulable state. Low levels of active PAI‐1 in non‐APL AML patients suggest that fibrinolysis may be enhanced in these patients.
VPB0946
Clinical impact of thromboprophylaxis in pancreatic cancer patients: Data from ACT4CAT study
N. Tsoukalas; A. Christopoulou; E. Timotheadou; I. Athanasiadis; A. Koumarianou; S. Peroukidis; G. Samelis; A. Psyrri; N. Kapodistrias; A. Nikolakopoulos; C. Andreadis; A. Ardavanis; E. Samantas; C. Papandreou; D. Mavroudis; A. Bokas; V. Barbounis; A. Athanasiadis; P. Papakotoulas; I. Boukovinas
On behalf of the Hellenic Society of Medical Oncology (HeSMO, http://www.hesmo.gr/en), Athens, Greece, Athens, Attiki, Greece
Background: Pancreatic adenocarcinoma (PaC) carries the highest risk of Venus ThromboEmbolism (VTE) among all cancers, with VTE rates from 5%–41% in retrospective cohorts and up to 67% in postmortem series. The development of VTE in 1 out of 5 patients with PaC, was often associated with significant decreases in PFS and OS.
Aims: Record the clinical practice regarding thromboprophylaxis in patients with active pancreatic cancer.
Methods: ACT4CAT is prospective observational study conducted by HeSMO, recording the clinical practice of thromboprophylaxis in ambulatory ca patient (pts) with solid tumors. This report focuses on pancreatic cancers. Patients enrolled after signed informed consent.
Results: 170 PaC patients enrolled from 18 oncology departments; 118 completed the study (69.4%). Age was 65.5 ± 10.7 years, BMI 25.2 ± 4.1 g/m2, males 59.4%. Khorana score 2 had 63.5%, 28.85%:3, 5.3%:4 and 2.4%:5. Locally advanced disease had 9%, while 76.2% metastatic. Neoadjuvant treatment received 2% of patients and 1% adjuvant while 71% & 22% 1st line and 2nd line respectively. 79.1% of patients treated with Highly Thrombogenic Agents (HTAs) including: 34.2% platinum and 75.9% antimetabolites. Detailed high thrombotic risk factors presented in figure. Thromboprophylaxis duration lasted 5.6 ± 3.5 months (min:1, max:24 months). Anticoagulants administered: tinzaparin 95.8%, fondaparinux 1.8%, bemiparin 0.6% and enoxaparin 1.8%. Intermediate dose administered to 78.8% of patients irrelevant of Khorana score, metastasis, or treatment line. Regarding efficacy only 2 pulmonary embolisms reported (1.2%, 95% CI: 0.1–4.2%). One minor bleeding reported (0.6%, 95% CI: 0.0–3.20, all events occurred in patients with metastatic disease.
Conclusion(s): Administration of thromboprophylaxis in patients with pancreatic cancer was safe and effective even at intermediate dose and for long duration. Oncologists seem that consider thromboprophylaxis in patients with pancreatic cancer in daily clinical practice.

PB0907
Three‐month outcomes in cancer patients with superficial‐ or deep vein thrombosis in the lower limbs. Results from the RIETE Registry
L. Bertoletti 1; P. Debourdeau2; C. Font3; J. López‐Núñez4; C. Gómez‐Cuervo5; I. MAHÉ6; R. Otero‐Candelera7; M. Adarraga8; P. López‐Miguel9; M. Monreal10
1 CHU Saint Etienne, Saint‐Etienne, Rhone‐Alpes, France; 2 Department of Supportive Care Oncology. Institut Sainte Catherine, Avignon, Provence‐Alpes‐Cote d'Azur, France; 3 Hospital Clínic de Barcelona, Barcelona, Catalonia, Spain; 4 Department of Internal Medicine. Hospital Germans Trias i Pujol. Badalona, Barcelona. Department of Medicine. Universitat Autònoma de Barcelona. Institut de Recerca Germans Trias i Pujol, Badalona, Barcelona. Spain, Barcelona, Catalonia, Spain; 5 Department of Internal Medicine. Hospital Universitario 12 de Octubre., Madrid, Castilla y Leon, Spain; 6 Department of Internal Medicine, APHP, Paris, Ile‐de‐France, France; 7 Medical Surgical Unit of Respiratory Diseases. Hospital Universitario Virgen del Rocio, Sevilla, Spain. Instituto de Biomedicina de Sevilla (IbiS), Sevilla, Spain. Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Sevilla, Andalucia, Spain; 8 Department of Internal Medicine. Hospital Universitario Reina Sofía., Córdoba, Andalucia, Spain; 9 Department of Pneumonology. Hospital General Universitario de Albacete, Albacete, Andalucia, Spain, 10 Department of Internal Medicine, Hospital Universitari Germans Trias i Pujol. Badalona, Barcelona, Spain Chair for the Study of Thromboembolic Disease, Faculty of Health Sciences, UCAM – Universidad Católica San Antonio de Murcia, Spain, Barcelona, Andalucia, Spain
Background: Little is known on superficial vein thrombosis (SVT) management in patients with active cancer.
Aims: Describe SVT characteristics and evolution in patients with active cancer.
Methods: Data from the RIETE registry were analyzed to compare the clinical characteristics and 90‐day outcomes in patients with active cancer and lower‐limb SVT; and compared with two groups: active cancer and lower‐limb deep vein thrombosis (DVT); lower‐limb SVT without cancer. The primary outcome was subsequent VTE (including symptomatic SVT, DVT or pulmonary embolism (PE)). Secondary outcomes were major bleeding and mortality.
Results: From March 2015 to April 2021, 110 patients with cancer and SVT, 1,695 with cancer and DVT, 1,030 with SVT but no cancer were included in the RIETE registry. Most patients in all subgroups (93%, 99% and 96%, respectively) were anticoagulated, with lower daily doses of low‐molecular‐weight heparin (114 ± 58, 163 ± 44, and 106 ± 50 IU/kg, respectively) in those with SVT. At 3 months, 101 patients (3.6%) developed subsequent VTE (SVT 13, DVT 41, PE 47), 72 (2.5%) had major bleeding and 282 (9.9%) died. Evolution according to groups are presented in the table. On multivariable analysis, only cancer was found to be an independent predictor for subsequent VTE (HR = 2.04; 1.15–3.62).
Conclusion(s): Risk of subsequent VTE (including symptomatic SVT, DVT or PE) are similar in cancer patients with isolated SVT than in those with isolated DVT.

PB0909
Microparticles and D‐Dimers improve prediction of chemotherapy‐ associated thrombosis in cancer patients
M. Chekkal 1; N. Yafour2; M. Elhorri3; A. Saadi Ouslim4; A. Adda5; N. Zmouli5; M. Bennaoum5
1 Medicine faculty. Oran1 university. Oran university establishment (EHU). Oran. Algeria., Oran, Oran, Algeria; 2 Department of hematology. EHU Oran. Medicine faculty. Oran1 University, Oran, Oran, Algeria; 3 HMRUO, Oran, Oran, Algeria; 4 Department of Biochemistry. EHU Oran. Medicine faculty. Oran1 University, Oran, Oran, Algeria; 5 Department of Hemobiology and Blood Transfusion. EHU Oran. Medicine Faculty, Oran University. Oran. Algeria, Oran, Oran, Algeria
Background: The cancer is associated to a state of hypercoagulability which can be the cause of venous thromboembolism (VTE) representing an undeniable cause of morbidity and mortality. Nevertheless, the pathophysiology of thrombi formation has not yet been fully elucidated and large variations of the risk have been demonstrated related to cancer characteristics, to the patient itself or to treatment.
Aims: In order to predict the risk of VTE in our patients, we conducted a prospective cohort study in cancer patients who will receive chemotherapy.
Methods: First, we evaluated the risk of cancer‐related VTE by hypercoagulability parameters (D‐dimers, microparticles, V Leiden mutation). In a second step, we tested the predictive value of the Khorana risk score (KRS) of cancer‐ related VTE. Then, we developed and tested the predictive value of an expanded score based on the addition of predictive biomarkers to the KRS parameters.
Results: 165 patients were included in our study. After an observation period of 2 years, 10 patients (6.0%) developed a VTE. Among the criteria studied, only the D‐dimers and the microparticles were predictive of VTE in cancer. We validated the Khorana risk score in our study population. The positive predictive value (VPP) of this score was 13,6% and the negative predictive value (VPN) was 97,9%. After adding 2 predictive biomarkers (D‐dimers and microparticles), the expanded score had a PPV of 23,5% and a VPN of 98,6%. An increased score ≥to the cut‐off value of 4 set by a ROC curve was associated with a higher risk of VTE (HR = 8.753, 95% CI [2.348–32.629]).
Conclusion(s): The addition of biomarkers to the routine clinical and biological parameters of the Khorana score enhances the predictive potential of VTE risk in cancer. This will allow a better stratification of patients according to the risk of VTE with the aim of optimizing thromboprophylaxis.
PB0924
Prevalence, treatment and prognosis of tumor thrombi in renal cell carcinoma
F. Kaptein 1; T. van der Hulle1; S. Braken1; E. van Gennep1; J. Buijs1; M. Burgmans1; S. Cannegieter1; E. Du Chatinier1; M. Huisman2; E. van Persijn van Meerten1; H. Versteeg1; R. Pelger1; F. Klok3
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 3 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Renal cell carcinoma (RCC) can be complicated by a venous tumor thrombus (TT), of which the optimal management is unknown.
Aims: Our aim was to assess the prevalence of TT in RCC, its current management and its association with venous thromboembolism (VTE), arterial thromboembolism (ATE), major bleeding (MB) and mortality.
Methods: 647 patients diagnosed with RCC between 2010–2019 in our hospital were included and followed from RCC diagnosis until two years after, or until an outcome of interest (VTE, ATE and MB) or death occurred, depending on the analysis. The study was approved by the local Institutional Review Board and oral informed consent was obtained. Cumulative incidences were estimated with death as a competing risk. Cox regression was used to identify predictors and the prognostic impact.
Results: At RCC diagnosis 86 patients had a TT (13%), of which 34 were limited to the renal vein (‘RV’), 37 to the inferior vena cava below the diaphragm (‘IVC’), and 15 extended above the diaphragm (‘AD’); 20 patients (23%) started therapeutic anticoagulation and 45 (52%) underwent thrombectomy with/without anticoagulant therapy. During a median follow‐up of 24 months (IQR 7.0–24), 36 patients were diagnosed with VTE (5.6%), 11 with ATE (1.7%) and 45 with MB (7.0%). Patients with TT were more often diagnosed with VTE (aHR 6.1, 95% CI 2.5–15; Figure 1), with increasing VTE risks in more proximal TT levels (adjusted 2‐year cumulative incidence RV 7.4%, IVC 22% and AD 45%, respectively; Figure 2). TT patients receiving anticoagulation were less often diagnosed with VTE (HR 0.56, 95% CI 0.13–2.5), with a tendency to have more MB events (HR 3.4, 95% CI 0.95–12) compared to those without anticoagulation.
Conclusion(s): Patients with RCC‐associated TT were at high risk of developing VTE. Future studies should establish which of these patients benefit from anticoagulation therapy.


PB0911
Comparison of effectiveness and safety of direct oral anticoagulant versus low molecular weight heparin treatment for venous thromboembolism in patients with active cancer – the OSCAR UK Study
A. Cohen 1; C. Wallenhorst2; C. Becattini3; B. Schaefer4; K. Abdelgawwad4; G. Psaroudakis4; G. Brobert5; C. Coleman6; A. Ekbom7; C. Ay8; A. Lee9; A. Khorana10; M. Carrier11; M. Rivera12; C. Marinez2
1 Department of Haematology Medicine, Guy's and St Thomas' NHS Foundation Trust, London, England, United Kingdom; 2 Institute for Epidemiology, Statistics and Informatics GmbH, Frankfurt, Hessen, Germany; 3 University of Perugia, Perugia, Umbria, Italy; 4 Bayer AG Medical Affairs & Pharmacovigilance, Pharmaceuticals, Berlin, Berlin, Germany; 5 Integrated Evidence Generation & Business Innovation, Bayer AB, stockholm, Stockholms Lan, Sweden; 6 School of Pharmacy, University of Connecticut, Hartford, Connecticut, United States; 7 Karolinska Institutet, Stockholm, Stockholms Lan, Sweden; 8 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria; 9 Division of Hematology, Department of Medicine, University of British Columbia, Vancouver, British Columbia, Canada, 10 Cleveland Clinic, Taussig Cancer Institute and Case Comprehensive Cancer Center, Cleaveland, Ohio, United States, 11 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada, 12 Integrated Evidence Generation & Business Innovation, Bayer AG, Berlin, Berlin, Germany
Background: The risk and benefit for direct oral anticoagulants (DOACs) in patients with venous thromboembolism (VTE) associated with active cancer varies with cancer type and the associated bleeding risk. Current guidelines from the International Society on Thrombosis and Haemostasis (ISTH) recommend caution in patients with some cancer types.
Aims: Estimate the incidence of VTE recurrence, significant bleeding (major or requiring hospitalisation) and all‐cause mortality in patients with active cancer and incident VTE, comparing low molecular weight heparin (LMWH) with DOAC.
Methods: OSCAR UK’s source population consists of all patients in the UK Clinical Practice Research Datalink between 01/2013 and 10/2020, with linked information on hospitalisation and cause of death. Adults diagnosed with active cancer (with a record of diagnosis or treatment in the last 6 months), who experienced hospitalisation, emergency department admission or primary care visit with a VTE, and were eligible to receive DOACs according to the ISTH recommendations, were included. Exclusions: therapeutic use of anticoagulants prior to cancer‐associated thrombosis (CAT), a history of atrial fibrillation, cardiac valve replacement, VTE other than deep vein thrombosis or pulmonary embolism, end‐stage renal failure, thrombocytopenia, initiation of palliative care or current pregnancy. Propensity scores for DOAC initiation were used to identify comparable groups of CAT patients with DOAC and LMWH treatment. Both exposure cohorts were observed for up to one year. Risks of VTE recurrence and significant bleeding events were estimated with sub‐distribution hazard ratios accounting for the competing risk of mortality. All‐cause mortality was estimated from hazard ratios.
Results: The CAT cohort comprised > 2,500 patients; 23.5% received DOACs and 76.5% LMWH. The commonest cancer types were in descending order: breast (21.0%), lung (12.6%), prostate (12.1%), and unresected cancers of the lower gastrointestinal tract (9.9%).
Conclusion(s): Benefit–risk of all study outcomes comparing anticoagulation with DOAC vs. LMWH will be presented.
PB0913
Epidemiology of asymptomatic venous thrombotic events in pediatric cancer patients: A population‐based study from Canada
B. Ells 1; K. Kulkarni2; Z. Forbrigger3; A. Sharathkumar4
1 Dalhousie University, IWK Health Centre, Bridgewater, Nova Scotia, Canada; 2 IWK Health Centre and Dalhousie University, Halifax, Nova Scotia, Canada; 3 Dalhousie University, IWK Health Centre, Halifax, Nova Scotia, Canada; 4 Stead Family Department of Pediatrics, University of Iowa Children’s Hospital, Iowa City, Iowa, United States
Background: Venous thrombotic events (VTE) are a well‐recognized complication in pediatric cancer patients with extremely variable incidence (1–73%). However, majority of the reports focus on symptomatic VTE. In contrast to adult literature, population‐based studies on exact incidence and characteristics of asymptomatic VTE in pediatric cancer patients are lacking.
Aims: To determine the incidence and characteristics of asymptomatic VTEs in contrast to symptomatic VTEs in pediatric cancer patients.
Methods: After ethics approval, a population‐based database of all pediatric oncology patients treated at the Izaak Walton Killam (IWK) Health Centre since January 2000 to December 2019, was created by amalgamating data from (i) Pediatric oncology hospital database (ii) Cancer in Young People in Canada registry (iii) pharmacy database and (iv) hospital health records. Data on patients with symptomatic and asymptomatic VTE was extracted. All radiology reports of all patients were reviewed for VTE according to a standardized protocol. Asymptomatic VTE was defined as “Intraluminal filling defect detected within venous system (including right side of the heart and pulmonary arteries)” during imaging studies (Chest X‐ray, computed tomography [CT], CT venography, magnetic resonance imaging [MRI], MR venography, ultrasound doppler [U/S], echocardiography [ECHO], venogram and linogram) in patients with no clinical history of VTEs.
Results: A total of 18,390 reports were reviewed for 748 subjects. VTE incidence was: symptomatic 36 (4.81%) in 26 patients; asymptomatic/clinically unsuspected 79 (10.56%) in 56 patients. Symptomatic VTEs were significantly associated with CVL. Table 1 shows the incidence and characteristics of VTE. Most VTE were detected on ultrasonography (Table 2).
Conclusion(s): The current large population‐based study establishes a best estimate of incidence and characteristics of asymptomatic VTEs in pediatric oncology patients after exhaustive review of 18,390 radiological studies. With a greater understanding of asymptomatic VTE incidence, further investigation is required to explore risk factors, clinical impact and outcomes associated with asymptomatic VTE.


PB0927
Abelacimab does not influence the effects of two commonly used antiplatelet agents in vitro
Y. Khder 1; S. Cote2; P. Hoffmann3; D. Bloomfield4
1 MYRA Life Science Services, Rosenau, Alsace, France; 2 Independent Consultant, Saint Louis, Alsace, France; 3 N/A, Lady’s Island, South Carolina, United States; 4 Anthos Therapeutics, Inc., Cambridge, Massachusetts, United States
Background: In previous studies, we demonstrated that abelacimab does not affect platelet function; concomitant use of abelacimab with antiplatelet drugs was not previously evaluated.
Aims: To assess the effects of increasing concentrations of abelacimab on the inhibition of platelet aggregation by aspirin or ticagrelor.
Methods: We concurrently conducted 2 studies. Blood specimens were obtained in EDTA and citrate tubes (6 healthy donors for each study). In the first study, whole blood was spiked with vehicle, aspirin (1 mg/mL), or aspirin (1 mg/mL) + abelacimab (125, 250, and 500 nM). Platelet aggregation was measured by impedance platelet aggregometry following induction with collagen (3.2 μg/mL) or arachidonic acid (0.5 mM) (Multiplate® analyzer, COLtest and ASPItest reagents; Roche Diagnostics International Ltd, Rotkreuz, Switzerland). In the second study, whole blood was spiked with vehicle, ticagrelor (3 μM), or ticagrelor (3 μM) + abelacimab (125, 250, and 500 nM). Platelet aggregation was measured with the same methodology following induction by ADP (6.4 μM) or thrombin receptor activating peptide‐6 (TRAP‐6; 32 μM).
Results: In the presence of aspirin, platelet aggregation induced with collagen or arachidonic acid was inhibited by 60% and 71%, respectively (p < 0.05). Abelacimab at 125, 250, and 500 ng/mL did not attenuate or increase the level of inhibition achieved with aspirin alone (Figure 1). In presence of ticagrelor, platelet aggregation induced with ADP or TRAP‐6 was inhibited by 54% and 31%, respectively (p < 0.05). Abelacimab at 125, 250, and 500 ng/mL did not alter platelet inhibition achieved with ticagrelor alone (Figure 2).
Conclusion(s): Abelacimab at clinically relevant concentrations did not alter the inhibitory effects of aspirin or ticagrelor. These results suggest that pharmacodynamic interactions between abelacimab and two commonly used antiplatelet agents are unlikely.


PB0915
Risk factors of venous thromboembolism among elderly patients with acute myeloid leukemia by race in the United States
A. Faiz; C. Philipp
Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey, United States
Background: There are limited data for risk factors of venous thromboembolism (VTE) in patients with acute myeloid leukemia (AML) by race.
Aims: To determine risk factors of VTE among elderly patients with AML by race in the United States.
Methods: For this retrospective cohort study, the United States SEER‐Medicare database (2000 to 2015) was used for patients ≥ 65 years diagnosed with AML. Race was defined as White, Black or Asian. VTE was defined as a diagnosis of deep vein thrombosis or pulmonary embolism during the study period. Logistic regression was used to examine the risk factors of VTE by race and Cox proportional regression was used to evaluate the effect of VTE on mortality.
Results: Among 21,520 of AML patients, VTE was diagnosed in 10.6% of 18,731 Whites, 13.4% of 1,362 Blacks and 5.6% of 1,310 Asians. The risk factors of VTE in Whites were age < 75 years, female sex and comorbidities including anemia (OR = 1.6, 95% CI, 1.4–1.8), hypertension (OR = 1.9, 95% CI, 1.7–2.2), chronic lung (OR = 1.2, 95% CI, 1.1–1.4) and kidney (OR = 1.3, 95% CI, 1.2–1.5) disease, hyperlipidemia (OR = 1.3, 95% CI, 1.1–1.4), heart failure (OR = 1.3, 95% CI, 1.2–1.4), obesity (OR = 1.2, 95% CI, 1.1–1.4), chemotherapy (OR = 1.3, 95% CI, 1.2–1.5) and presence of a catheter (OR = 2.0, 95% CI, 1.8–2.2). For Blacks, hyperlipidemia (OR = 2.4, 95% CI, 1.7–3.4), heart failure (OR = 1.6, 95% CI, 1.1–2.2) and presence of a catheter (OR = 2.3, 95% CI, 1.6–3.4) and for Asians, age < 75 years, female sex, hypertension (OR = 2.8, 95% CI, 1.3–6.0) and presence of a catheter (OR = 2.1, 95% CI, 1.2–3.7) and chemotherapy (OR = 1.9, 95% CI, 1.1–2.2) were risk factors for VTE. A diagnosis of VTE was not associated with reduced survival in AML patients irrespective of race.
Conclusion(s): Our findings may help to establish a risk profile for VTE in elderly patients diagnosed with AML.
PB0918
Comparison of the Khorana, PROTECHT and ONKOTEV risk assessment models to predict venous thromboembolism in patients with pancreatic cancer
C. Frere 1; L. Gauthier2; C. Canivet3; S. Gourgou2; L. Buscail3; B. Bournet3; D. Farge4
1 Sorbonne Université, Paris, Ile‐de‐France, France; 2 Institut du Cancer de Montpellier, Montpellier, Languedoc‐Roussillon, France; 3 Université de Toulouse, Toulouse, Midi‐Pyrenees, France; 4 Université de Paris, Paris, Ile‐de‐France, France
Background: Several risk assessment models (RAMs) have been developed to identify cancer patients at high‐risk of venous thromboembolism (VTE) when undergoing chemotherapy who may benefit from thromboprophylaxis. The clinical usefulness of these RAMs remains debated in pancreatic cancer (PC) patients.
Aims: To analyze the discriminatory performance of the Khorana, PROTECHT, and ONKOTEV RAMs for VTE in patients with newly diagnosed PC undergoing ambulatory chemotherapy enrolled in the BACAP prospective multicenter cohort study (2014–2020).
Methods: The 6‐month cumulative risk of VTE was estimated using death as a competing event. Sub‐distribution hazard ratios (SHR) for dichotomized RAMs were estimated using the Fine and Gray competing risks regression model to assess VTE risk difference between low/intermediate‐ and high‐risk patients. The time‐dependent concordance index (C‐index) was calculated to evaluate the RAMs discriminatory performance, with confidence intervals (CI) calculated by repeating analyses in 250 bootstrap samples.
Results: 762 PC patients were enrolled prior to chemotherapy, of whom 125 (16.4%) developed VTE during a 6‐month follow‐up period. The rate of PC patients predicted at “high‐risk of VTE” was 28.1% using the Khorana score, 99.0% using the PROTECHT score and 34.1% using the ONKOTEV score. Whichever score was used, the 6‐month cumulative incidence of VTE did not differ between patients predicted at “high‐risk of VTE” and those predicted at “low/intermediate risk of VTE” (Table 1). The RAM C‐index value was 0.54 (95% CI 0.49–0.59) for the Khorana score, 0.53 (95% CI 0.49–0.59) for the PROTECHT score and 0.56 (95% CI 0.50–0.61) for the ONKOTEV score.
Conclusion(s): In the BACAP cohort, the Khorana, PROTECHT and ONKOTEV RAMs failed to accurately predict VTE in patients with newly diagnosed PC receiving chemotherapy. Effective RAM are needed to predict VTE risk in PC patients, meanwhile primary pharmacological prophylaxis of VTE in patients with locally advanced or metastatic pancreatic cancer receiving chemotherapy and having a low bleeding should be implemented.

PB0919
Polygenic risk scores for prediction of cancer‐associated venous thromboembolism: A UK Biobank analysis
N. Guman 1; F. Mulder1; B. Ferwerda2; A. Zwinderman2; H. Buller3; N. van Es2; P. Kamphuisen4
11. Amsterdam University Medical Centers, University of Amsterdam; 2. Tergooi Medical Center, Amsterdam, Noord‐Holland, Netherlands; 2 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 3 Amsterdam University Medical Centers, University of Amsterdam, amsterdam, Noord‐Holland, Netherlands, 41. Tergooi Medical Center; 2. Amsterdam University Medical Centers, University Medical Center, Amsterdam, Noord‐Holland, Netherlands
Background: Guidelines recommend primary thromboprophylaxis for selected cancer patients at high risk of venous thromboembolism (VTE). Improvement of traditional clinical risk assessment models for VTE in cancer patients is needed.
Aims: To assess the predictive performance of three polygenic VTE risk scores derived in the general population (De Haan et al. 2012 [5‐SNP score], Klarin et al. 2019 [297‐SNP score], and Lindström et al. 2019 [37‐SNP score]) in cancer patients enrolled in the UK Biobank, a large‐scale prospective, observational cohort.
Methods: Participants with an incident cancer diagnosis after UK Biobank enrollment were included, using data from cancer registries or inpatient diagnosis data. Those with an anticoagulant therapy prescription at enrollment or history of VTE were excluded. The primary outcome was pulmonary embolism or deep‐vein thrombosis. Patients were followed for 12 months after cancer diagnosis. Performance of the continuous scores was assessed by time‐dependent c‐indices and subdistribution hazard ratios (SHR) in univariable and multivariable analyses adjusted for sex and age. The risk in patients in the upper quartile of the polygenic scores was compared to that in the lower three quartiles.
Results: 36,479 cancer patients (median age 66; 49% females) were included of whom 874 (2.4%) developed VTE during 12‐month follow‐up. All continuous scores were significantly associated with VTE in univariable and multivariable analyses (SHRs 1.3 to 1.5). The 297‐SNP score showed the best discrimination (c‐index 0.59, 95%‐CI: 0.58–0.61). For this score, the cumulative VTE incidence was 3.6% (95% CI: 3.2–4.0) in the upper quartile versus 2.0% (95% CI: 1.8–2.2) in the lower quartiles (SHR 1.8, 95% CI: 1.6–2.1). Performance of all scores is summarized in the Table and Figure.
Conclusion(s): Polygenic VTE risk scores developed for the general population can identify cancer patients with about a 2‐fold increased VTE risk, and may potentially improve VTE risk assessment on top of clinical risk scores.


Genetic Risk Factors of Thrombosis
VPB1314
ABO blood group as an additional risk factor for venous thromboembolism
E. Ristovska 1; T. Makarovska Bojadjieva1; S. Useini1; E. Velkova1; E. Petkovic1; V. Dejanova Ilijevska1; J. Trajkova1; L. Ismaili2; M. Tashkovska3
1 Institute for transfusion medicine, Skopje, Skopje, Skopje, Macedonia; 2 Institute for transfusion medicine, Tetovo, Skopje, Tetovo, Macedonia; 3 City General Hospital 8th September, Skopje, Skopje, Skopje, Macedonia
Background: Higher thrombotic risk associated with non‐O blood group individuals is observed especially when in combination with thrombophilia.
Aims: To estimate the association of ABO blood type with venous thromboembolism (VTE).
Methods: In this retrospective case control study we enrolled 40 patients with confirmed VTE. In all of them ABO blood group was determined using serologic methods such as column agglutination technique which included forward and reverse typing. Thrombophilia testing was performed in 12 patients with unprovoked VTE event.
Results: ABO distribution among VTE patients is as follows: A in 19 (47.5%), O in 5 (12.5%), B in 10 (25.0%) and AB in 6 (15.0%) patients. The prevalence of O blood group in VTE patients was significantly lower (12.5%) in comparison to non‐O blood types (87.5%). Blood group A is more prevalent and group O is less prevalent in VTE patients in comparison to our general population in which the frequency of A and O blood group is 41% and 32% respectively. In 6 VTE patients Covid 19 was confirmed few weeks prior to the thrombotic event (4‐A, 1‐O and 1‐B blood group). In 12 patients tested for thrombophilia, 36 mutations were discovered with the following frequency: FII G20210A‐5.5%, FVL G1691A‐13.8%, FXIII V34L‐8.3%, FGB G455A‐11.1%, ITGA2 C807T‐5.5%, ITG B3 T1565C‐2.7%, PAI‐1 4G/5G675‐27.7%, MTHFR C677T‐25%, from which 24 were heterozygous and 12 were homozygous (2‐FVL, 2‐FXIII, 1‐FGB, 6‐PAI‐1 and 1 case with MTHFR mutation). All of the patients have more than one mutation in different combinations and their ABO distribution was similar as in the rest of the VTE patients.
Conclusion(s): According to the results, individuals with non‐O blood group are more prone to VTE. ABO phenotype and especially ABO genotype may be additional diagnostic tool for assessment of the VTE risk together with thrombophilia markers.
PB1309
Study the role of endothelial protein C receptor gene polymorphisms in Indian DVT patients
A. Sharma 1; K. Kishor1; A. Biswas2; R. Ranjan1; R. Kumar1; S. Tyagi1; R. Saxena3; J. Oldenburg4; M. Mahapatra5
1 All India Institute of Medical Sciences, New Delhi, Delhi, India; 2 Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Bonn, Nordrhein‐Westfalen, Germany; 3 The Medicity Hospital Gurgaon, gurgaon, Haryana, India; 4 Institute of Experimental Hematology and Transfusion Medicine and Centre for Rare Diseases, Universitätsklinikum Bonn, Bonn, Nordrhein‐Westfalen, Germany; 5 All India Institute of Medical Sciences, Delhi, Delhi, India
Background: Endothelial Protein C Receptor (EPCR) is an emerging factor and plays a vital role in the pathogenesis of DVT. It augments PC activation via binding with the thrombin‐thrombomodulin complex. EPCR polymorphisms have been reported to influence the risk of DVT whereas their association with Indian DVT patients is not yet studied
Aims: To identify the prevalence of EPCR gene variants (4678G>C, 6936A>G, and 23bp insertion) and their role in Indian DVT patients
Methods: 75 DVT patients (M:37, F:38), median age 45 years range (20–60 years) and similar age and sex‐matched healthy controls were studied. EPCR gene variants were detected by allele‐specific PCR and sequencing
Results: DVT was seen in the lower limb in 65 (86%) patients and upper limb in 10 (14%) patients. The variant allele C for 4678G>C was significantly higher in controls than patients (Patients: C = 6%, Control: C = 12.6%, p = 0.047) but the frequency of variant genotypes (GC, CC) was not significantly increased. (Patients: GC = 20%, CC = 2.6%, Controls: GC = 9.3%, CC = 1.3%. p = 0.143, χ 2 = 3.89). The allele frequency of variant allele G for 6936A>G polymorphism was significantly higher in patients than controls (Patient: G = 16%, Control: G = 6%, p = 0.011, χ2 = 6.50). Genotypic frequency of AG and GG genotype was also significantly increased (Patients: AG = 24%, GG = 4%, Controls: AG = 10.6%, GG = 1.3%, p = 0.040, χ 2 = 6.04). Subjects carrying AG and GG genotypes had an increased risk of DVT (AG: OR: 2.55, CI: (1.11–6.62), GG: OR: 3.26, (CI: 0.680–32.46). A 23 bp insertion was absent in all the study subjects
Conclusion(s): The protective role of 4678G>C polymorphism was identified which is similar to previous results obtained from the west, whereas 6936G>A polymorphism has a causative role in DVT. Therefore, 6936G>A polymorphism detection may support in identifying Indians having a high risk of DVT
VPB1311
Klinefelter sindrome and thromboembolic disease
A. Calonge Arribas 1; J. Dos santos2; A. Álvarez Aramburu2; L. Valderas Monge2
1 Hospital Universitario de Navarra, Pamplona, Navarra, Spain; 2 University Hospital of Navarra, Pamplona, Navarra, Spain
Background: Klinefelter's Syndrome (KS) is the most common sex chromosome disorder. The genetic background is the extra X chromosome. Venous thromboembolism (VTE) has been observed among KS patients.
Aims: The aim of the study was to present a case series of VTE in patients with KS
Methods: Descriptive study of three clinical cases with KS and review of the literature on this entity
Results: Case 1 29‐year‐old male with a history of KS on testosterone treatment who was admitted for extensive deep vein thrombosis (DVT) in the left lower limb up to the iliac vein and bilateral massive pulmonary embolism (PE). Anticoagulation is started with enoxaparin at therapeutic doses and later bridging therapy with acenocoumarol. Since the cause of the venous thromboembolic disease was attributed to KS and testosterone treatment, the patient was left on anticoagulation indefinitely without further thromboembolic episodes. Case 2 35‐year‐old male with a history of KS, smoker and obese without testosterone treatment. He was admitted in 2015 for idiopathic DVT in the right popliteal region. He received heparin at therapeutic doses for 6 months. In 2021 he presented new DVT in left popliteal region also idiopathic. However, it was impossible to switch him to acenocoumarol treatment despite a total weekly dose of almost 50 mg. After requesting authorisation from the hospital inspectorate, rivaroxaban treatment was started indefinitely. Case 3 22‐year‐old male with a history of unprovoked DVT in 2016 anticoagulated with acenocoumarol who was admitted for pulmonary thromboembolism despite anticoagulation. During admission we found the patient's phenotype characteristic, so we requested a hormonal study that showed us primary hypogonadism and the karyotype confirmed our diagnostic suspicion of KS.
Conclusion(s): According to the literature, patients with KS appear to be at increased risk of thromboembolic events with a high prevalence of recurrence of DVT or PE as reflected in our case series.
VPB1313
VKORC1‐1639AA genotype as a possible risk factor for venous thromboembolism in elderly patients with FV Leiden mutation
S. Kapustin 1; A. Chechulova2; J. Drijun1; Z. Sidorova1; V. Burakov1; V. Soroka3; V. Soldatenkov1; L. Papayan4
1 Russian Research Institute of Hematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 2 Dzhanelidze Research Institute of Emergency Medicine, St. Petersburg, Saint Petersburg City, Russia; 3 Dzhanelidze Research Institute of Emergency Medicine, Saint Petersburg, Saint Petersburg City, Russia; 4 Russian Research Institute of Haematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia
Background: Vitamin K plays a crucial role in hemostasis by activating both procoagulant (FII, VII, IX, X) and anticoagulant (proteins C, S, Z) factors. Vitamin K‐epoxide reductase 1 (VKORC1) G‐1639A gene polymorphism affects enzyme activity and bioavailability of vitamin K. Little is known about the role of the VKORC1 G‐1639A variation in venous thromboembolism (VTE) development, in particular, in patients with inherited thrombophilia.
Aims: To study the role of the VKORC1 G‐1639A gene polymorphism in VTE development in patients from North‐Western Russia.
Methods: We included 600 VTE patients (294 men and 306 women, mean age 43.6 ± 15.3 years) originated from the North‐Western region of Russia in the study. The control group (CG) consisted of 200 healthy individuals of the same origin. Genotyping for the VKORC1 G‐1639A, FII G20210A and FV G1691A variations was performed by PCR‐RFLP. Intergroup differences in genotype frequencies were assessed by Fisher’s exact test. The study was approved by the local ethical committee.
Results: Distribution of the VKORC1 G‐1639A gene variants was similar in both VTE patients and controls. In the group of patients without FII and FV gene mutations, the frequency of the VKORC1‐1639AA genotype was almost equal to that in CG (17.1% vs. 16.5%, respectively). VKORC1‐1639AA genotype frequency in patients with the FV Leiden was 4‐fold higher than in those having the FII G20210A mutation (19.6% vs. 4.4%, respectively; OR = 5.2; p = 0.021). Notably, among patients having FV Leiden variant, the VKORC1‐1639AA genotype frequency was much higher in the group with late‐onset disease (after 45 years) compared to young VTE patients (31.6% vs. 11.9%; OR = 3,4; p = 0,02). Thus, in elderly VTE patients with FV Leiden, the VKORC1‐1639AA variant was almost 2‐fold overrepresented when compared to CG (31.6% vs. 16.5%; OR = 2,4; p = 0,033).
Conclusion(s): We suggest that the VKORC1‐1639AA genotype could increase VTE risk in elderly patients with FV Leiden mutation.
VPB1312
Polymorphism of genes associated with fibrin clot structure or/and lysis rate could influence the risk of pulmonary embolism in young patients with venous thromboembolism
A. Chechulova 1; S. Kapustin2; Z. Sidorova2; J. Drijun2; V. Soldatenkov2; L. Papayan3; V. Soroka4; A. Tomchenko4
1 Dzhanelidze Research Institute of Emergency Medicine, St. Petersburg, Saint Petersburg City, Russia; 2 Russian Research Institute of Hematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 3 Russian Research Institute of Haematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 4 Dzhanelidze Research Institute of Emergency Medicine, Saint Petersburg, Saint Petersburg City, Russia
Background: Fibrin clot structure and rate of its lysis could influence the risk of deep‐vein thrombosis (DVT) or/and pulmonary thromboembolism (PE). Association between genetic factors regulating these phenotypes and risk of thromboembolic complications remains debatable.
Aims: To study association between DNA polymorphism of the alpha‐fibrinogen (FI‐A), factor XIII (FXIII‐A), TPA, PAI‐1 genes and risk of PE development in young patients with venous thromboembolism (VTE).
Methods: We analyzed 243 VTE patients under 45 years, which were divided into three clinical groups: isolated DVT (“DVT only”) (n = 154; 63.4%), DVT complicated by PE (“DVT+PE”) (n = 53; 21.8%) or isolated PE (“PE only”) (n = 36; 14.8%). Genotyping for the FI‐A Thr312Ala, FXIII‐A Val34Leu and PAI‐1‐675 4G/5G polymorphisms was performed by PCR‐RFLP. TPA 311 bp Ins/Del gene polymorphism was resolved by PCR and subsequent gel electrophoresis. Intergroup differences in genotype distributions were assessed by Fisher’s exact test and characterized by odds ratio (OR) and p‐value.
Results: Comparison of genotype distributions revealed several differences between the clinical VTE groups (see table 1). Frequency of the FI‐A 312 Ala/Ala variant was 2‐fold higher in the group “PE only” than in patients with isolated DVT (22.2% vs. 11.0%, OR = 2.3; p = 0.098). In the group “DVT only”, heterozygous genotype of the FXIII‐A gene was more prevalent than in patients with DVT complicated by PE (48.7% vs. 30.2% OR = 2.2; p = 0.024). On the contrary, the FXIII‐A 34 Leu/Leu variant was more frequently seen in patients with the signs of PE (11.2% vs. 6.5% in “DVT only” group; OR = 1.8; p = 0.23). Finally, the frequency of the TPA Del/Del genotype was 2‐fold higher in patients with isolated PE than in the group “DVT only” (38.9% vs. 18.8%; OR = 2.7; p = 0.014).
Conclusion(s): FI‐A 312 Ala/Ala, TPA Del/Del and possibly FXIII‐A 34 Leu/Leu variants could increase the risk of PE development in young VTE patients.

PB1306
Evaluation of a LAMP assay for detection of Prothrombin Allele and Factor V Leiden
K. Hickey 1; T. Pitchford2; S. Kitchen3
1 Department of Coagulation, Sheffield Teaching Hospitals NHSFT, sheffield, England, United Kingdom; 2 Sheffield Teaching Hospital NHS Foundation Trust, Sheffield, England, United Kingdom, 3 , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, England, United Kingdom
Background: Prothrombin allele G20210A (PTA) and Factor V Leiden (FVL) are both inherited risk factors for venous thromboembolism and constitute part of a standard thrombophilia screen. Our laboratory evaluated the performance of reagents for PTA genotyping and FVL genotyping (La Car MDx, Belgium), tested using a Magnetic Induction Cycler (Bio Molecular System, Australia). These methods employ Loop Mediated Isothermal Amplification (LAMP). The main advantage of these LAMP methods is the ability to perform analysis on whole blood bypassing the need for DNA extraction typical required in PCR.
Aims: To compare the performance of LAMP methods against the current routine methods for genotyping PTA and FVL.
Methods: A mixture of citrate and EDTA samples stored at either 4 or −20 degrees and previously tested for PTA and FVL were included in this study. The predicate method was PTA and FVL reagents (Roche, UK) analysed on the LightCycler 2.0 (Roche, UK).
Results: Previously tested PTA whole blood samples comprising 117 wild‐type, 61 heterozygous and 9 homozygous samples were evaluated. LAMP PTA genotyping demonstrated complete agreement with our current method. Previously tested FVL whole blood samples comprising 95 wild‐type, 96 heterozygous and 21 homozygous samples were evaluated. LAMP FVL genotyping demonstrated complete agreement with our current method.
Conclusion(s): The LAMP method for PTA and FVL provides a more straightforward testing process by eliminating the need for time‐consuming DNA extraction, thus shortening the preparation and with a reduced amplification/analysis time compared to LightCycler method. Overall, we had 100% agreement between PCR and LAMP methods for FVL and PTA genotyping.

PB1307
Characterization of a novel genetic variant in family with protein S deficiency
V. Votin1; J. Moon1; J. Lim Conley1; J. Kain1; A. Gavini2; I. Tomas3; B. Lewis 1; M. Ero1
1 Machaon Diagnostics, Inc., Berkeley, California, United States; 2 Kaiser Permanente, Vacaville, California, United States; 3 Unidade de Saúde Familiar da Barrinha, Esmoriz, Aveiro, Portugal
Background: Congenital Protein S deficiency is associated with thrombophilia and an increased risk of thromboembolism. It is caused by pathogenic variants in the PROS1 gene. Data about the role of the PLG gene in thrombosis is conflicting.
Aims: Finding two genetic variants in these genes in a 46‐year‐old patient, we aimed to determine their role in the protein S deficiency in his large affected family.
Methods: Next‐generation sequencing and Sanger sequencing were used to detect two genetic variants in the family members: p.Cys228Gly in the PROS1 gene and p.Lys38Glu in the PLG gene. Previous results for protein S activity and related tests were analyzed, as well as family history for deep vein thrombosis (DVT).
Results: The variants (one novel, one pathogenic for type 1 plasminogen deficiency) segregate with disease within the family. All five family members presenting with protein S deficiency have both variants in the heterozygous state, while the two family members who are unaffected have neither variant.
Conclusion(s): Thus, the variants may be individually or together contributing to the deep vein thrombosis in the affected family members.
PB1308
Differential proteomic profile of patients with Pulmonary Embolism (PE) related to events during follow‐up: chronic thromboembolic pulmonary hypertension (CTEPH) or occult cancer
V. Sánchez López1; E. Mendoza Zambrano2; J. Oto 3; E. Arellano Orden1; O. Tura Ceide4; C. FONT5; M. Saez6; P. Medina7; I. Blanco8; J. Barberà9; R. Otero Candelera1
1 Institute of Biomedicine of Seville (IBiS), Virgen del Rocio University Hospital, Sevilla, Andalucia, Spain; 2 Virgen del Rocio University Hospital, Sevilla, Andalucia, Spain; 3 Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 4 Hospital Clínic‐Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS); University of Barcelona, Barcelona, Catalonia, Spain; 5 Hospital Clínic de Barcelona, Barcelona, Catalonia, Spain; 6 Centro Andaluz de Estudios Bioinformáticos (CAEBI), Sevilla, Andalucia, Spain; 7 Haemostasis, Thrombosis, Arteriosclerosis and Vascular Biology Research Group, Medical Research Institute Hospital La Fe, Valencia, Comunidad Valenciana, Spain; 8 Hospital Clínic‐Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Catalonia, Spain; 9 Hospital Clínic‐Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS); University of Barcelona, Barcelona, Catalonia, Spain
Background: In the follow‐up of patients diagnosed with PE, either CTEPH or cancer can be identified. We hypothesized that cancer and CTEPH might share pathogenic pathways such as inflammation, cell proliferation, or apoptosis.
Aims: Analyze the proteomic profile of patients with CTEPH, uncomplicated PE and PE patients with occult cancer to identify proteins dysregulated in PE group compared to those with occult cancer and CTEPH.
Methods: Fourteen patients with CTEPH, 6 with venous thrombosis disease (VTD) who were subsequently diagnosed with cancer, and 7 with PE who did not present neither CTEPH nor occult cancer after 2 years follow‐up were evaluated. Citrated plasma was obtained from all of them and used for protein quantification in a mass spectrometer by iTRAQ© labeling. After quality control and normalization, differential expression analysis was performed using the Kruskal‐Wallis test with the Benjamini‐Hochberg (BH) correction for multiple testing.
Results: A total of 382 proteins were determined in all groups, 28 were found to be differentially expressed in the 3 groups of patients. We selected five of them based on their under‐ or overexpression in the PE group, compared to the groups that developed cancer or CTEPH (Table 1).
Conclusion(s): The circulating proteins identified could help to differentiate patients with PE who are at risk for presenting subsequent cancer or CTEPH.

PB1310
Decreased mitochondrial DNA copy number in patients with history of recurrent venous thromboembolism
R. Vostatek 1; C. Englisch2; P. Hohensinner3; S. Nopp4; I. Pabinger5; C. Ay5
1 Medical University of Vienna, Austria, Vienna, Wien, Austria; 2 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 3 Center for Biomedical Research, Medical University of Vienna, Vienna, Wien, Austria; 4 Medical University of Vienna, Vienna, Wien, Austria; 5 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Venous thromboembolism (VTE) is the third most common cardiovascular disease (CVD) and occurs in all age groups, albeit the risk increases sharply with age. There is evidence indicating a role for mitochondria in cardiovascular aging. However, in the context of VTE this has not been investigated in detail.
Aims: We aimed to explore a biomarker reflecting biological aging (i.e. human mitochondrial DNA copy number (mtDNA)) and its association with risk of VTE.
Methods: We measured the average mtDNA from sampled isolated blood via a qPCR‐based assay kit and analyzed their association with risk of VTE in a case‐control study of patients with confirmed recurrent VTE and healthy individuals. Patients had at least one unprovoked VTE event and were included at least 3 months after the most recent VTE event.
Results: We included 98 patients with recurrent VTE (median [25th–75th percentile] age: 58 [48–64] years, 43.9% women), and 91 unrelated healthy controls (median age: 52 [45–59] years, 52.7% women) from the same ethnic background and geographic region. The analysis of mtDNA showed a high variation and differed significantly between patients and healthy controls (figure 1). Patients showed a lower average mtDNA value compared to healthy controls: 663 (78.75–2204.5) vs. 2832 (724–4350) per diploid cell; p < 0.001). After adjustment for age, sex, BMI, and smoking, mtDNA was independently associated with VTE risk (odds ratio per increase in 100 mtDNA per diploid cell: 0.97, 95% CI 0.955–0.986).
Conclusion(s): We have observed decreased mtDNA levels in patients with VTE, indicating an association of biological aging with risk of VTE. The association between biological aging and risk of VTE needs further investigation to understand whether changes of biomarkers of biological aging are the cause or consequence of VTE.

Post‐thrombotic Syndrome
PB0426
Patients reporting of post‐traumatic stress disorder after experiencing venous thromboembolic event
R. Rosovsky 1; W. Robertson2; F. Klok3
1 Massachusetts General Hospital and Harvard Medical School, Newton, Massachusetts, United States; 2 National Blood Clot Alliance, Philadelphia, Pennsylvania, United States; 3 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: The main focus of care for patients with venous thromboembolism (VTE) is to prevent recurrent VTE, major bleeding and death. While these are highly relevant, they do not capture patient‐relevant outcomes such as quality of life (QOL) and functional abilities.
Aims: To evaluate degree of PTSD in VTE patients and how that impacts QOL and functional abilities.
Methods: The Post Traumatic Stress Disorder (PTSD) Checklist – Civilian version (PCL‐C) was one of twelve validated instruments included in an anonymous survey aimed at exploring functional limitations, pain, dyspnea, depression and PTSD in VTE patients. The survey was placed on the website of National Blood Clot Alliance and online via Facebook, Instagram, LinkedIn and Twitter™ to better understand the degree and impact of PTSD in adults (≥18 years) diagnosed with pulmonary embolism (PE), deep vein thrombosis (DVT) or both.
Results: 3372 people took the survey between August 2021 and January 2022 of which 86% were women with mean age of 43 years (range 18–70). 36% and 22% of respondents had PE or DVT, respectively, and 42% had both. Over 50% had their VTE >1 year ago and 79% were on anticoagulation. Of the 2714 who completed the PTSD portion of the survey, 18.2% reported little or no PTSD symtpoms, 4.6% reported some PTSD symptoms, 33.7% reported moderate to moderately high PTSD symptoms and 43.6% reported high severity of PTSD symptoms (Figure 1). Analyses correlating PTSD with QOL, pain, anxiety, depression and functional abilities are underway and will be available by the conference.
Conclusion(s): This is the largest survey aimed at investigating the long‐term emotional effects in VTE survivors. PTSD was frequent among this highly self‐selected group of responders. Information gleaned from this survey is the first step in gaining a broader understanding of this important outcome and can help guide future research.

PB0427
Patients reporting on relevant functional limitations following an episode of venous thromboembolism: the post venous thromboembolism functional scale
R. Rosovsky 1; W. Robertson2; F. Klok3
1 Massachusetts General Hospital and Harvard Medical School, Newton, Massachusetts, United States; 2 National Blood Clot Alliance, Philadelphia, Pennsylvania, United States; 3 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Beyond recurrent venous thromboembolism (VTE), major bleeding and death, quality of life (QOL) and functional ability are highly relevant clinical outcomes of VTE survivors. However, these features and other important determinants of well‐being have not been well explored.
Aims: To investigate the long term functional limitations in VTE patients.
Methods: The Post Venous Thromboembolism Functional Status (PVFS) Scale was one of twelve validated instruments included in an anonymous survey aimed at exploring functional limitations, pain, dyspnea, depression, post‐traumatic stress disorder (PTSD) and post thrombotic panic syndrome in VTE patients. The survey was circulated online (website of National Blood Clot Alliance), and via social media (Facebook, Instagram, LinkedIn and Twitter™) to better understand the long‐term impact of pulmonary embolism (PE) and deep vein thrombosis (DVT).
Results: 3372 people took the survey between August 2021 and January 2022 (Table 1). Of the 2924 who completed the PVFS portion of the survey, during their everyday life, 13% report no limitations and no symptoms, pain, depression, or anxiety (SPDA), 38% report negligible limitations although still have persistent SPDA, 31% suffer from limitations, occasionally needing to reduce usual activities due to SPDA, 16% are not able to perform all usual activities due to SPDA and 2% are dependent on nursing care due to SPDA. Figure 1a and 1b correlate PVFS with anxiety or depression and pain or discomfort, respectively. Analyses correlating PVFS with QOL, dyspnea and functional abilities are underway and will be presented at the conference.
Conclusion(s): This is the largest survey aimed at investigating the long‐term functional limitations that may be present after a VTE. Half of the respondees reported moderate to severe functional limitations, which was associated by a higher prevalence of depression, anxiety and pain. Understanding how these health determinants affect outcomes is crucial to improve the care for these patients.


VPB0430
Erythrocyte‐derived microvesicles and microRNA‐144/451 cluster in patients with post‐thromboembolic syndrome
O. Sirotkina 1; A. Ulitina2; J. Zhilenkova3; E. Zolotova4; M. Simakova5; T. Vavilova5
1 PNPI named by BP Konstantinov of NRC Kurchatov Institute, St. Petersburg, Saint Petersburg City, Russia; 2 Pavlov First Saint‐Petersburg State Medical University, St/Petersburg, Saint Petersburg City, Russia; 3 Almazov National Medical Research Centre, Saint Petersburg, Saint Petersburg City, Russia; 4 Almazov National Medical Research Centre, St. Peterburg, Saint Petersburg City, Russia; 5 Almazov National Medical Research Centre, St. Petersburg, Saint Petersburg City, Russia
Background: There is a shortage of information about the contribution of microvesicles (MVs) to the development of hypercoagulation and thrombosis. At the same time, microRNAs are known to be widely represented in MVs and transmitted signals between cells. Therefore, MVs and microRNAs are hot topics in thrombosis research. Also, the microRNA‐144/451 cluster is involved in regulating the erythrocyte life cycle.
Aims: Our study aimed to assess the number of erythrocyte‐derived MVs and the abundance of microRNAs in both erythrocytes and MVs in patients with post‐thromboembolic syndrome and in healthy controls.
Methods: The study included 18 patients with pulmonary embolism (PE) complicated by chronic thromboembolic pulmonary hypertension (CTEPH) or chronic thromboembolic disease (CTED) and 12 controls without a history of thromboembolic episodes. The number of MVs was measured by flow cytometry with the Exo‐FACS Kit (HansaBioMed) and fluorescently labeled anti‐erythrocyte antibodies (CD235a+). The expression levels of miR‐144‐3p, miR‐451a, miR‐451b were detected by RT‐PCR with miRCURY LNA Probe PCR Kit (QIAGEN).
Results: The total number of MVs and the number of CD235a+MVs were higher in PE compared with controls (82.9 [69.9–93.7] vs 52.8 [42.7–72.7] and 1.3 [0.6–1.5] vs 0.3 [0.2–0.5], respectively, p < 0.050). The levels of both miR‐144 and miR‐451a were several orders of magnitude higher in erythrocytes than in MVs. However, miR‐451b was not detected in MVs, either in PE or controls. The level of miR‐144‐3p was lower in PE than in controls both in MVs and erythrocytes (0.13 [0.06–0.32] vs 0.47 [0.27–0.55] and 15,207 [13,594–116,707] vs 218,315 [89,613–308,311], respectively, p < 0.050), whereas the level of miR‐451a was lower only in MVs (937 [421–1956] vs 1875 [1687–2406], p < 0.050). Moreover, in CTEPH, levels of both miR‐144‐3p and miR‐451a were lower than in CTED.
Conclusion(s): Our results are consistent with the hypothesis that both erythrocyte‐derived MVs and the microRNA‐144/451 cluster contribute significantly to the risk of post‐thromboembolic syndrome development. The study is supported by RFBR №20‐04‐00257.
PB0428
Post‐thrombotic syndrome in patients with venous thromboembolism treated with rivaroxaban – Results from the FIRST Registry
V. Speed; L. Roberts; J. Patel; R. Patel; R. Arya
King's College Hospital, London, England, United Kingdom
Background: Post‐thrombotic syndrome (PTS) is a common complication of deep vein thrombosis (DVT) and affects 20–50% of patients. Recent studies have reported that rivaroxaban may reduce the risk of PTS compared with warfarin. Data reporting the incidence and severity of PTS after treatment with rivaroxaban in routine practice are limited.
Aims: To report the incidence and severity of PTS in patients managed with rivaroxaban for DVT enrolled in the FIRST registry assessed using the patient‐reported Villalta scale (PRV).
Methods: The FIRST registry was a UK only, non‐interventional prospective multicentre study, investigating the incidence of long‐term complications of VTE in patients treated with rivaroxaban. (Clinicaltrials.gov NCT02248610). Participants with a diagnosis of DVT were sent the PRV by mail. The PRV is a validated adaptation of the Villalta scale. Questionnaire data were entered locally onto the web‐based registry database. The presence of PTS was reported as a PRV score >4 and categorised according to severity. Data were analysed with descriptive statistics. Comparison between subgroups was made using a Mann‐Whitney test for continuous data or the chi square or Fisher’s exact test for categorical data.
Results: 934 (74%) of participants were enrolled in the FIRST registry with a lower limb DVT. 628/934 remained in follow‐up on the 7/1/20 when the questionnaires were sent to local sites. In total, 215 (34%) were returned. 44.7% of participants were reported to have PTS. Participants with proximal index events were more likely to report PTS (p < 0.05) (Table 1.) Post thrombotic syndrome was severe in 9.8% of cases (Table 2).
Conclusion(s): In this cross‐sectional sub study from the FIRST registry, PTS was reported at a higher frequency in participants treated with rivaroxaban in routine clinical practice to that reported in post hoc phase III analyses, but at a comparable frequency to emerging real world data.


PB0423
Finding the gap in pediatric post‐thrombotic syndrome
D. Bastas1; L. Brandão 2; J. Vincelli3; N. Amiri3; K. Abdul‐Samad3; S. Stephens3; L. Avila1
1 The Hospital for Sick Children, TORONTO, Ontario, Canada; 2 The Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada, Toronto, Ontario, Canada; 3 The Hospital for Sick Children, Toronto, Ontario, Canada
Background: Children with deep vein thrombosis (DVT) are at risk of developing post‐thrombotic syndrome (PTS), a long‐term complication that includes poor endurance. It is critical to understand the extent to which movement is affected in these patients, to improve their management.
Aims: To assess movement ability, physical functioning, and activity level in children with/without PTS.
Methods: Patients aged 8–21 years with prior limb DVT were prospectively recruited in this cross‐sectional study. Patients completed the Movement Ability Measure (MAM‐CAT), a standardized questionnaire that assesses self‐perceived current (“now”) and preferred (“would like”) movement ability, and the gap between them. MAM‐CAT evaluates 6 areas of movement: strength, accuracy, endurance, flexibility, speed, and adaptability. Other measures included activity level (Godin Exercise Questionnaire), physical functioning (PROMIS‐PF), and PTS severity (CAPTSure). The association between PTS (as a continuous measure and classified as no/mild/moderate‐severe PTS) and movement and activity were analyzed using generalized least squares for correlated data. Ethics board approval and informed consent were obtained.
Results: Sixty‐six patients were recruited between October 2020 and January 2022; characteristics are shown in Table 1. Ninety‐two DVT‐affected limbs were assessed for PTS. Increasing PTS severity was associated with lower current movement ability and a larger gap between current vs. preferred movement ability. Nearly all aspects of movement were affected (Table 2). Adjusted for sex, age, and affected limb, increasing PTS severity was associated with a wider gap between current vs. preferred movement ability, and lower physical functioning: for every point increase in CAPTSure‐PTS score, there was a 0.16‐point increase in movement ability gap (p < 0.001) and a 0.20‐point decrease in physical functioning scores (p = 0.002).
Conclusion(s): In children, PTS severity is associated with greater movement restrictions and impaired physical functioning. Furthermore, there is a gap between the patient’s current vs. desired movement ability. Reducing this gap is a critical aspect of PTS management in children.


PB0429
Impact of venous thromboembolism on physical functioning: Results from a prospective cohort study
D. Steiner 1; S. Nopp2; B. Weber3; O. Schlager4; K. Janata‐Schwatczek5; O. Königsbrügge1; F. Klok6; I. Pabinger7; C. Ay7
1 Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 2 Medical University of Vienna, Vienna, Wien, Austria; 3 Department of Dermatology, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 4 Department of Medicine II, Division of Angiology, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 5 Department of Emergency Medicine, Medical University of Vienna, Vienna, Austria, Vienna, Wien, Austria; 6 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 7 Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Vienna, Wien, Austria
Background: Venous thromboembolism (VTE) is a common disease with various long‐term sequelae, including chronic functional limitations. The clinical course, degree, risk factors and mechanisms of this are incompletely understood.
Aims: We aimed to investigate the impact of acute VTE on functional status at diagnosis and follow‐up, and its determinants.
Methods: Patients with acute VTE were included in a prospective cohort study and followed up for at least three months. Functional status was assessed at baseline (within 3 weeks of diagnosis) and after 3 to 6 months using the Post‐VTE Functional Status (PVFS) scale and the Patient Reported Outcomes Measurement Information System (PROMIS) physical function short form 10a. To investigate potential risk factors for functional limitations after VTE, we evaluated the influence of age, sex, pulmonary embolism (PE) diagnosis, PVFS one month before diagnosis (assessed retrospectively at study inclusion), smoking status, and comorbidities on the PROMIS physical function score at three‐month follow‐up in a multivariable linear regression model.
Results: The study population consisted of 109 patients (64.2% men) with a median (25th to 75th percentile) age of 53.1 (41.0–62.2) years. 59 (54.1%) patients had deep vein thrombosis (DVT) and 50 (45.9%) PE, of whom 21 had concurrent DVT. At diagnosis and three‐month follow‐up, 99 (90.1%) and 60 (55.0%) patients, respectively, had at least some degree of functional limitation (PVFS ≥ 1; Figure 1). In a multivariable linear regression model, PE diagnosis (p = 0.001), higher age (p = 0.023), and lower physical functioning before (p = 0.017) and at diagnosis (measured with PROMIS, p < 0.001) were significantly associated with decreased physical functioning at follow‐up.
Conclusion(s): Most patients experienced a decrease in physical functioning after being diagnosed with VTE and every second patient still reported functional limitations at three months despite adequate treatment. Age, PE as index event, and functional status before and at diagnosis predicted functional limitations at follow‐up.

PB0424
Post thrombotic syndrome: Quality of life and incidence in patients under treatment with direct oral anticoagulants
A. Poretto 1; L. Spiezia2; A. Codognola3; E. Campello4; D. Tormene3; F. Dalla Valle3; P. Simioni5
1 Padova university hospital, SALGAREDA, Veneto, Italy; 2 Padova University Hospital, Padova, Italy, Padova, Veneto, Italy; 3 Padova university hospital, Padova, Veneto, Italy; 4 University of Padova, Padova, Veneto, Italy; 5 Padua University Hospital, Padua, Veneto, Italy
Background: Post‐thrombotic syndrome (PTS) is a disabling complication of venous thrombotic disease. It is still unclear wheter treatment with direct oral anticoagulants (DOACs) reduces the incidence of PTS compared to treatmen with vitamin K antagonist (vKA). There is no effective treatment strategy, but the use of compression stockings is recommended, with conflicting results.
Aims: The primary aim was to compare the incidence of PTS in DOACs vs warfarin, according also with the presence of thrombophilia. The second aim was to assess the quality of life of affected patients by investigating the role of compression stockings.
Methods: 538 patients with deep vein thrombosis of the lower limbs, evaluated at the Thrombotic and Hemorrhagic Diseases Unit of the University Hospital of Padua, between 2014 and 2020, were enrolled, treated with DOACs for at least 3 months and compared with a historical sample of 538 patients, referred to our centre, treated with vKA. The possible onset of PTS (Villalta criteria) was recorded in the following months. The quality of life was investigated through the VEINES questionnaire, recording also the use of elastic stockings.
Results: The Hazard ratio (HRa) was statistically significant for DOACs than vKA (HRa 2.49; 95% CI; 1.94–3.20), especially for rivaroxaban (HRa 2.68; 95% CI; 1.92–3.74). Thrombophilia was not statistically associated with the risk of PTS regardless of the type of anticoagulant used (p < 0.05). Symptoms most suffered were heaviness in the lower limbs (60%), severe limitation of social activities (25.7%). 46 (65.7%) patients reported a limited use of elastic stockings. Even with low compliance, more people reported an improvement of the symptoms (18.6%) than those who reported a worsening (5.7%).
Conclusion(s): DOACs reduce the incidence of PTS more than vKA, thrombophilia does not influence the risk. The graduated elastic compression improves the quality of life of patients with PTS, that is strongly affected by symptoms.
PB0425
The CLUES study: A critical look at understanding the emotional suffering of blood clot survivors
R. Rosovsky 1; W. Robertson2; F. Klok3
1 Massachusetts General Hospital and Harvard Medical School, Newton, Massachusetts, United States; 2 National Blood Clot Alliance, Philadelphia, Pennsylvania, United States; 3 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Important aspects of post‐venous thromboembolic (VTE) syndrome are dyspnea, pain, anxiety, depression and post‐traumatic stress disorder (PTSD). The correlations between all of these features and their impact on functional outcomes and quality of life (QOL) are not well studied and thus poorly understood.
Aims: To evaluate features of post‐VTE syndromes and their impact on functional outcomes and QOL.
Methods: Twelve validated instruments focusing on several determinants of well‐being that play important roles in recovery after VTE were included in an anonymous survey aimed at exploring patient relevant outcomes after VTE (Table 1). The survey was placed on the website of National Blood Clot Alliance, a patient advocacy organization, and online via Facebook, Instagram, LinkedIn and Twitter™ to better understand the degree and impact of PTSD in adults (≥18 years of age) diagnosed with pulmonary embolism, deep vein thrombosis, or both.
Results: 3372 people took the survey between August 2021 and January 2022. Respondent characteristics are described in Table 2. 75% of participants report feeling moderately anxious or depressed, 60% report having moderate or extreme pain, 53% have some or major problems with performing their usual activities and 40% get sudden feelings of panic quite or very often. Because the survey only recently closed, additional analyses are currently being conducted to learn about the prevalence of each determinant as well as how they impact and correlate with one another. Results will be shared at the conference.
Conclusion(s): This is the largest collection of surveys aimed at investigating the frequency and degree of dyspnea, pain, anxiety, depression, PTSD and post thrombotic panic syndrome in patients diagnosed with VTE and their impact on functional outcomes and health‐related QOL. The information collected and analyses generated from these surveys will provide a greater understanding of these important issues and will help guide future patient care, education and research.


Thrombophilia
VPB0963
Clinico‐laboratory findings in patients with hereditary thrombophilia in the north‐western region of Russia
V. Soldatenkov 1; O. Soldatenkova2; L. Papayan3; N. Silina2; K. Komissarov2; S. Kapustin1; V. Burakov2; N. Saltykova2; O. Matvienko4; O. Smirnova4
1 Russian Research Institute of Hematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 2 Russian Scientific Research Institute of Hematology and Transfusiology, Saint‐Petersburg, Saint Petersburg City, Russia; 3 Russian Research Institute of Haematology and Transfusiology, Saint Petersburg, Saint Petersburg City, Russia; 4 Russian Research Institute of Hematology and Transfusiology, Saint‐Petersburg, Saint Petersburg City, Russia
Background: Nowadays the role of hereditary thrombophilias in thrombosis pathogenesis is obvious and it’s necessary to reveal its impact on clinical course.
Aims: To study clinico‐laboratory features of patients with hereditary thrombophilia.
Methods: 131 case‐records of patients, who underwent treatment at our department in 2017–2022, were studied. Inclusion criteria: verified arterial or venous thrombosis and confirmed hereditary thrombophilia. Exclusion criteria: JAK2, CALR, MPL mutations. The following groups of patients were formed on the base of the molecular genetics and coagulation tests: I – isolated FV Leiden mutation (n = 20), II – isolated mutation in Prothrombine G20210A (n = 8), III – isolated antithrombine deficiency (n = 5), IV – isolated Protein C deficiency (n = 1), V – isolated FVIII elevation (n = 19), VI – isolated hyperhomocysteinemia (MTHFR, MTRR mutations, confirmed phenotypically) (n = 10), VII – isolated primary antiphospholipid syndrome (n = 3), VIII – combination of three and more thrombophilia markers (n = 9), IX – combination of two strong thrombophilia markers (n = 6), X – strong and moderate thrombophilia markers combination (n = 50). Clinical and laboratory data of those groups were analyzed.
Results: 1. 208 strong or moderate thrombophilia markers were found. The most common was FV Leiden mutation (23.08%) and hyperhomocysteinemia (27.88%). 2. Combined thrombophilias were found in 49.62%. Combination of Leiden mutation with hyperhomocysteinemia was the most common combined thrombophilia (26.15%). 3. 508 thrombotic events were found: 15.75% in arterial vessels, 84.25% in venous vessels. The most common arterial localization – popliteal artery (26.25%), and venous localization – popliteal and superficial femoral veins (21.5% and 20.1%). 4. In 29.77% of patients the first thrombotic event was life‐threatening (69.23% – pulmonary embolism). 5. Some initial thromboses had atypical localization (renal vein, transverse and sigmoid brain sinuses) 6. The median age of first thrombosis was 40.5 years.
Conclusion(s): Young patients with atypical or life‐threatening thrombosis should be tested for hereditary thrombophilia.
PB0950
Clinical correlation between lupus anticoagulant (LA) and thrombosis – Ampang Hospital EXPERIENCE
S. Nasution1; T. Kunju 2; V. Selvaratnam3; L. Kian Boon4
1 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Kuala Lumpur, Kuala Lumpur, Malaysia; 2 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Selangor, Malaysia; 3 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Ampang Jaya, Selangor, Malaysia; 4 National Institutes of Health, Ministry of Health Malaysia, Shah Alam, Selangor, Malaysia
Background: Lupus Anticoagulant (LA) positivity is associated with thrombosis including venous and arterial thrombosis and recurrent pregnancy loss. In Ampang Hospital, a national haematology referral centre, dilute Russell Viper Venom time (dRVVT), PTT‐LA and Staclot‐LA are used to confirm the presence of LA antibody as per ISTH guideline.
Aims: To determine the prevalence of LA and its correlation with thrombosis.
Methods: All LA requests were collected and clinical data of each patient was reviewed retrospectively from the electronic Hospital Information System (eHIS) between 2010 and 2018. Rejected requests and insufficient clinical data were excluded from the analysis.
Results: A total of 460 LA tests were requested for various thrombotic episodes; 351 (86.7%) females and 54 (13.3%) males. Out of all LA tests performed, 26.3% (n = 121) were positive and 69.3% (n = 319) were negative. 20 (4.5%) cases with no clear indication was rejected. Of those with positive results, VT has the highest correlation with LA positivity (23%), followed by AT (19%), recurrent miscarriage (14.9%), others (28.9%). Although LA is associated with recurrent miscarriage, there is only a very small number of patients who were tested positive. Out of 341 samples sent for pregnancy losses, only 5.3% (n = 18) had a positive LA test. Of 121 patients with recurrent miscarriages, majority of them were tested negative LA (only 14.9% were tested LA positive), which indicates not all recurrent miscarriages are due to LA (p‐value 0.0015). Further analysis will be performed as we would like to see the correlation between positive LA and recurrent thrombotic events.
Conclusion(s): Our analysis showed only a small cohort of patients with unexplained thrombosis had a positive LA test. We suspect that most LA test are requested as part of a routine battery of investigation at diagnosis without critical clinical evaluation. Clinicians should be aware that not all unexplained thrombosis is due to APLS.
PB0957
Co‐inheritance of protein C deficiency and homozygous factor V Leiden mutation: 10‐year follow‐up on a case with severe presentation
A. Sarmento 1; C. Almeida1; M. Mendes2; L. Borges2
1 Centro Hospitalar Baixo Vouga, Aveiro, Aveiro, Aveiro, Portugal; 2 Centro Hospitalar do Baixo Vouga, Aveiro, Aveiro, Aveiro, Portugal
Background: Factor V Leiden (FVL) mutation and protein C (PC) deficiency are inherited thrombophilias that lead to an increased risk of thrombosis and their individual effect on thrombotic risk has been described before. However, very few cases of concurrence have been described.
Aims: We describe the follow‐up of a now 10‐year‐old Caucasian female diagnosed with protein C deficiency and homozygous factor V mutation with a severe presentation at birth.
Methods: Collected data was obtained from patient record.
Results: In 2011, our newborn patient underwent right hand catheterization at birth, following a tonic‐clonic seizure. Soon after, she developed persistent upper limb cyanosis, which prompted the diagnosis of either severe arterial spasm or arterial thrombosis. Cyanosis later improved, but not completely, which ultimately led to necrosis and amputation of the 2nd to 4th fingertips. Brain magnetic resonance imaging also showed multiple hypoxic‐ischemic lesions, while cerebral ultrasound was suggestive of bilateral infarction. There was no family history of thrombophilic defects. Thrombophilia testing revealed type I PC deficiency (PC:activity 21%; PC:antigen 26%) and homozygous FVL mutation. PROC gene sequence analysis detected a heterozygous mutation. Due to the severity of this episode and the presence of two major inherited thrombotic risk factors, indefinite anticoagulation was proposed. Low‐molecular‐weight‐heparin (LMWH) was given initially, with anti‐Xa monitoring. At 21 months old, LMWH was switched to oral anticoagulation with vitamin K antagonist. Neurodevelopment of our now 10‐year‐old patient has been normal, with no recorded sequelae of hypoxic‐ischemic encephalopathy. No new thrombotic events were registered or suspected, while also not presenting any major bleeds, partially due to the fact of having a time in therapeutic range (TTR) above 90%.
Conclusion(s): Co‐inherited thrombophilias may present a challenge at presentation, especially in the absence of family history. The coexistence of multiple thrombophilic disorders and its possible supra‐additive effect on overall thrombotic risk is not well clarified.
VPB0964
Homocysteine as an independent thrombosis risk factor
G. Sottilotta 1; G. Nicolò2
1 Hemophilia Centre – Thrombosis and Hemostasis Service. Great Metropolitan Hospital of Reggio Calabria (Italy), REGGIO CALABRIA, Calabria, Italy; 2 Hemophilia Centre – Thrombosis and Hemostasis Service. Great Metropolitan Hospital of Reggio Calabria (Italy), Reggio Calabria, Calabria, Italy
Background: Elevated levels of homocysteine (HCY) are associated with arterial and venous thrombosis via several mechanisms such as increased tissue factor expression, enhanced platelet reactivity, increased thrombin generation, and endothelial dysfunction
Aims: To evaluate if hyper HCY (hHCY) may be considered as an independent thrombosis risk factor and its incidence in different sites of thrombosis
Methods: We retrospectively analysed clinical data of 319 patients, 184 with hHCY and 135 with normal HCY (nHCY), followed by our centre during the last 5 years. We excluded other conditions of thrombophilia: Factor V Leiden and Prothrombin G20210, Protein C and S deficiency, presence of anticardiolipin or anti‐beta2 glycoprotein‐I antibodies and lupus anticoagulant
Results: 29/184 with hHCY were found with thrombosis. Of these: 18/29 (62.1%) had venous thrombosis (DVT), 11/29 (37.9%) had arterial thrombosis. We considered three hHCY severity levels: mild 15–29 μmol/L, moderate 30–100 μmol/L and severe>100 μmol/L. 22/135 (16.3%) nHCY patients were diagnosed with thrombosis. Of these: 17/135 (12.6%) had DVT and 5/135 arterial thrombosis (3.7%). Even in this case, we observed more DVT (17/22,77.3%) than arterial thrombosis (5/22,22.7%). The comparison about incidence of thrombosis between hHCY and nHCY groups evidenced similar percentages (15.8% and 16.3% respectively). Results are resumed in table 1. We found different thrombosis percentages comparing the subjects with normal and mild hHCY to those with moderate hHCY: people with moderate hHCY had a much higher percentage of arterial thrombosis (61.5%) than those with nHCY (22.7%) and patients with mild hHCY (15.8%) (see table 2), even if these data were not sufficient to hypothesize a correlation between levels of HCY and sites of thrombosis
Conclusion(s): Our analysis did not demonstrate that hHCY can be considered an independent risk factor for thrombosis. In our opinion, as reported by other authors, hHCY could be only considered as a prothrombotic cofactor


PB0947
Thrombophilia screening in patients with ocular venous thrombosis
M. Alrifai 1; U. Sachs2; C. Kelm1
1 University Hospitals Giessen, Germany, Giessen, Hessen, Germany; 2 Giessen University Hospital, Giessen, Hessen, Germany
Background: Ocular venous thrombosis (OVT) is a common retinal vascular disease, which may result in significant visual loss. The influence of thrombophilia in OVT patients is still controversial. The therapeutic consequences of OVT are not well studied. Here, we report upon a thrombophilia screening in 177 consecutive patients with OVT.
Aims: We aimed to assess the role of thrombophilia as a causative or additive risk factor in the development of ocular thrombosis.
Methods: 177 patients with ocular venous thrombosis were examined. Four to six weeks after the acute episode, patients were assessed for thrombophilia in the Department of Thrombosis and Hemostasis.
Results: 46 patients (26 %) had a positive history of thromboembolic events. Laboratory tests revealed antiphospholipid antibodies in 29, lipoprotein (a) elevation in 35, factor VIII:c elevation in 37, antithrombin deficiency in 5, prothrombin (G20210A) polymorphism in 7 and factor V Leiden mutation in 17 patients.
Conclusion(s): Antiphospholipid antibodies and elevated levels of lipoprotein (a) appear to be enriched in patients with OVT. Since both factors have an impact on the arterial system, we consider OVT a consequence of arterial pathology, which could support the hypothesis that atherosclerosis contributes to the etiology of OVT and recommend the evaluation of atherosclerosis status.
PB0953
Free protein S antigen represents more reliable assay for protein S deficiency testing compared with protein S activity and should be used as the first and main test in the diagnostic protocol
S. Margetić 1; F. Tomić2; I. Vuga2
1 Department of Clinical Chemistry, Sestre milosrdnice University Hospital Center, Vinogradska 29, 10 000 Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia; 2 University Hospital Center Sestre milosrdnice, Zagreb, Croatia, Zagreb, Grad Zagreb, Croatia
Background: Heterozygous protein S (PS) deficiency is a well known risk factor for thrombophilia with plasma levels of PS of 35–60%. However, diagnostics of PS deficiency using the most common PS activity (PS:Ac) assays is difficult due to interfering preanalytical and analytical factors leading to false positive results.
Aims: To investigate whether free PS antigen (fPS:Ag) assay could be used as an initial assay to determine hereditary PS deficiency in order to improve current diagnostic protocol with PS:Ac as first test
Methods: PS:Ac (functional clot‐based test) and fPS:Ag (immunological test with monoclonal antibody to unbound PS) were measured with commercial assays (Protein S Ac and Innovance Free PS Ag, Siemens Healthineers, Germany) in 133 consecutive patients referred for testing to our laboratory.
Results: Results of protein PS:Ac and fPS:Ag testing are presented in Table 1. In 88 (66.2%) patients, both fPS:Ag and PS:Ac were within reference ranges. In 12 (9%) patients both fPS:Ag and PS:Ac were decreased, among which in 11/133 (8.3%) due to pregnancy or warfarin therapy. PS deficiency was confirmed in 1 (0.8%) patient with decreased both PS:Ac and fPS:Ag at two separate occasions. FPS:Ag assay correctly classified all 133 samples as normal and abnormal. In contrast, in 31/133 (24.1%) samples, PS:Ac levels were slightly decreased and confirmed as false positive in retesting, with fPS:Ag levels within reference range.
Conclusion(s): This study confirmed that fPS:Ag should be used as first diagnostic step in hereditary protein S testing due to its much lower falsely decreased values and unclear results and less susceptibility to interferences compared to PS:Ac. In the vast majority of patients, in case of normal fPS:Ag result, no further testing is needed. Based on the results obtained in our laboratory we changed our previous practice with PS:Ac as first diagnostic step and applied fPS:Ag as initial and main diagnostic assay (Figure 1).


PB0960
Adherence to thrombophilia testing guidelines and accuracy of thrombophilia work‐up: A single‐center cross sectional study
K. Vrotniakaite‐Bajerciene 1; F. Schmidli2; K. Jalowiec2; H. Broughton2; J. Schneider2; A. Haynes3; J. Brodard2; A. Rovo2; J. Kremer Hovinga4; A. Angelillo‐Scherrer2
1 Bern University Hospital, Bern, Bern, Switzerland; 2 Bern University Hospital, Department of Hematology and Central Hematology Laboratory, Bern, Bern, Switzerland; 3 Bern University, CTU Bern, Bern, Bern, Switzerland; 4 Department of Hematology and Central Hematology Laboratory, Bern University Hospital, University of Bern, Bern, Bern, Switzerland
Background: Selective thrombophilia testing has been introduced to clinical practice in 2010 considering low clinical utility of systematic thrombophilia testing. Although guidance adherence and accuracy of thrombophilia testing has been proven to be poor in acute or general hospital care, it has not been assessed in an outpatient specialized center yet.
Aims: To examine the adherence to thrombophilia testing guidance and accuracy of work‐up at a tertiary thrombophilia center.
Methods: We conducted a single‐center cross sectional study of 3686 patients referred to the thrombophilia consultation, at the Bern University Hospital (Switzerland), from 01/2010 to 10/2020. We studied the adherence to indication of thrombophilia testing according to international and internal guidelines at a specialized thrombophilia center and evaluated the accuracy of thrombophilia work‐up.
Results: Thrombophilia testing was performed in 3550 patients (94%) and 1258 patients (28.9%) displayed at least one thrombophilia. Most of the included patients (2407, 65%) were referred because of venous thromboembolism (VTE) and arterial thromboembolism (591, 16%), followed by asymptomatic patients (567, 15%) and pregnancy morbidity (121 patients, 3%). Only 1208 work‐ups (33%) were adherent to the guidance of the thrombophilia center, mostly in asymptomatic patients (Table 1). Significantly more positive results for heterozygous and homozygous factor V Leiden mutation and protein S deficiency were found in patients tested not according to internal and international guidance (Table 2). Three thousand three hundred nineteen thrombophilia work‐ups (3319, 95.6%) were accurate, whereas 79 (2.3%) were performed inappropriately on anticoagulation and 59 (1.7%) on contraceptive treatment or in pregnancy.
Conclusion(s): Our study shows very good accuracy of thrombophilia work‐up but poor adherence to thrombophilia testing guidelines at a specialized thrombophilia center in Switzerland. A more standardized and simpler approach to thrombophilia work‐up is needed.

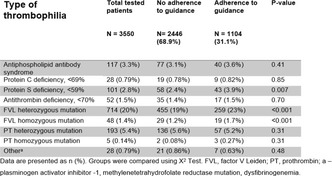
PB0958
Assessment of 3 new functional antithrombin, protein C and protein S assays
P. Suchon 1; E. Maccary2; P. Morange3
1 Aix Marseille Univ, INSERM, INRAE, C2VN, Marseille, France; Laboratory of Haematology, La Timone Hospital, Marseille, France, marseille, Provence‐Alpes‐Cote d'Azur, France; 2 HORIBA Medical International – Montpellier France, Montpellier, Languedoc‐Roussillon, France; 3 C2VN, INRAE, INSERM Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France
Background: Yumizen G1550 is a recently launched fully automated hemostasis analyser. Horiba has developed reagents for thrombophilia screening.
Aims: The aim of the study was to compare the performances of the couple Horiba/Yumizen G1550 with the local configuration (Stago or Hyphen BioMed/STA‐R Max) for the measurement of antithrombin, protein C (PC) and protein S (PS).
Methods: All 3 Horiba reagents were functional assays measuring antithrombin, PC and PS activities. Local instrument was the Stago STA‐R Max. The following reagents were used for measuring antithrombin and PC activities: STA Stachrom AT III (Stago), HEMOCLOT PC (Hyphen BioMed). Repeatability was assessed using 2 levels of controls (30 replicates). Reproducibility was estimated after 25 runs of 2 levels of controls. Values of repeatability and reproducibility were previously calculated for the local configuration (repeatability: 10 replicates of plasma sample; reproducibility: 30 runs of control samples). Passing Bablok and Bland Altman methods were used for the comparison. Of note the local configuration did not allow a comparison for PS measurement (antigenic free PS measured with STA Liatest Free PS‐Stago). Agreement between the 2 configurations was assessed using an 80% cut‐off for antithrombin deficiency and 70% for PC deficiency.
Results: As compared with the local configuration the Horiba/Yumizen configuration displayed good performances for AT and PC activities (table). Of note PC activity CVs were lower with the Yumizen configuration for both repeatability (4.0 and 1.6% vs 4.2 and 4.0%) and reproducibility (3.9 and 4.6% vs 7.4 and 8.7%). As expected CVs were higher for PS activity than antigenic free PS. We obtained the following values for correlation: – Antithrombin: r = 0.96; bias = 11%; 1/22 conflicting sample. – PC: r = 0.85; bias = 16%; 10/67 (15%) conflicting samples.
Conclusion(s): Horiba reagents showed good performances as compared with local configuration. Although the couple Horiba/Yumizen led to a misclassification in 10/67 samples incorrectly classified as PC deficiencies.

PB0961
Continued suspected direct oral anticoagulant interference on thrombophilia testing: Experience of a reference laboratory
E. Wong 1; L. Worfolk2; L. Noh3; J. Dlott2
1 Quest Diagnostics Nichols Institute, Chantilly, Virginia, United States; 2 Quest Diagnostics, Secaucus, New Jersey, United States; 3 Quest Diagnostics Nichols Institute, Secaucus, New Jersey, United States
Background: Since approval of direct oral anticoagulants (DOACs) for anticoagulation, the diagnosis of thrombophilia risk factors has become challenging, especially when clot‐based assays are utilized. Falsely elevated protein S and C activities and false‐positive lupus anticoagulant results may occur with DOAC use. Little is known of the interference seen in subsequent years when educational efforts were undertaken to make licensed care providers aware of these interferences.
Aims: Examination of DOAC interference before and after DOACs were introduced to the US market.
Methods: Retrospective review of venous thrombophilia testing from January 2008 to December 2020 at a coagulation reference referral laboratory. To identify potential DOAC interference, we reviewed specimens submitted for both a protein C activity test and a dilute Russell viper venom test (dRVVT), which is a lupus anticoagulant test. DOAC interference was suspected when protein C activity was elevated (>200%) and dRVVT testing did not demonstrate an inhibitor pattern. DOAC interference was strongly suspected when protein C activity was elevated (>200%) and dRVVT testing demonstrated an inhibitor pattern. Annual percentages of thrombophilia tests with suspected and strongly suspected DOAC interference were compared.
Results: Percentage of suspected DOAC interference increased annually from 2008 to 2016, which corresponds to the introduction of DOACs to the US market. Strongly suspected DOAC interference increased from 0.21% in 2008, peaking at 5.07% (24.1‐fold increase) in 2015; test results of elevated protein C activity without a dRVVT inhibitor pattern increased from 0.48% in 2008, peaking at 15.12% (31.5‐fold increase) in 2014. Suspected interference of 7–9% has continued since 2016.
Conclusion(s): While strongly suspected DOAC interference has markedly decreased since the peak in 2015, suspected DOAC interference still affects a substantial percentage of thrombophilia test orders despite educational efforts by our laboratory and other investigators as evidenced by publications in the literature and choosing wisely recommendations by professional societies
https://www.abstractscorecard.com/shared/uploads/scorecard/Tasks/upload/16333/MFNLWBDK‐1204177‐1‐ANY(2).jpg

PB0955
Current knowledge on factor V Leiden mutation as a risk factor for recurrent venous thromboembolism: A systematic review and meta‐analysis
D. Eppenberger1; H. Nilius2; B. Anagnostelis1; C. Huber3; M. Nagler 4
1 University of Bern, Bern, Bern, Switzerland; 2 Inselspital, Bern University Hospital, Bern, Bern, Switzerland; 3 Helsana health insurance, Zurich, Zurich, Switzerland; 4 Inselspital, Bern University Hospital, and University of Bern, Bern, Bern, Switzerland
Background: Thrombophilia screening is widely done in clinical practice, and it is claimed that the extent of venous thromboembolism (VTE) recurrence risk in patients with common defects is still not fully understood.
Aims: We aimed to summarize all high‐quality data of epidemiological studies prospectively observing VTE recurrence in patients with and without heterozygous factor V Leiden (FVL) mutation in various subgroups.
Methods: We searched MEDLINE and EMBASE databases for cohort studies prospectively assessing VTE recurrence in patients with and without FVL (PROSPERO: CRD42021182800). Data were extracted on cohort and study‐level. The methodological quality was assessed using the Newcastle‐Ottawa Scale (NOS). Relative risks (RR) were calculated overall and in subgroups using a fixed‐effects model.
Results: From 30 cohorts, 22 studies were finally included summarizing 13’244 patients. Heterozygous FVL was identified in 2779 individuals (21%). The methodological quality was estimated to be high in 18 studies (82%). The overall RR was 1.4 (95% CI 1.28, 1.55), consistent across subgroups.
Conclusion(s): Pooling all high‐quality epidemiological data, the risk of recurrent VTE was increased by 40% in patients with heterozygous FVL mutation. Against the background of established risk factors, the FVL mutation plays only a marginal role in the risk assessment for recurrent VTE.
PB0949
Variability in thrombophilia testing in large international physician survey
M. Ellis 1; O. Avnery1; B. Hunt2
1 Meir Medical Center, Kfar Saba, HaMerkaz, Israel; 2 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom
Background: Thrombophilia testing is indicated when the presence of hypercoagulability influences management. Evidence‐based recommendations for testing are available for certain clinical situations however, guidance is absent or lacks support from robust clinical study data in numerous clinical situations.
Aims: To determine worldwide thrombophilia testing practices of thrombosis physicians across a range of clinical situations for which clinical guidelines are lacking or are based on low grade clinical evidence.
Methods: A SurveyMonkey® questionnaire was distributed to 60 international thrombosis physicians, posted on the ISTH website and on the Twitter feed of one of the authors (BH). Survey questions included respondents' characteristics and geographic location. Clinical scenarios covered unusual site venous thrombosis, arterial thrombosis, obstetric complications and family screening. Respondents indicated whether no testing, hereditary thrombophilia testing, antiphospholipid antibody testing or both should be performed. Consensus levels were predefined.
Results: Of 227 respondents, 155 consultant physicians whose main clinical interest is thrombosis and hemostasis were included in this analysis. 97% of respondents do not perform any testing after venous thromboembolism (VTE) with major provocation while 73.6% test after an unprovoked event, establishing reasonable guideline adherence. No consensus was found regarding contraceptive‐related VTE, unusual site thrombosis, intra uterine growth restriction, preeclampsia or preceding contraceptive use with a familial thrombosis history. Moderate consensus existed for ischemic stroke, recurrent pregnancy loss, placental abruption and implantation failure. Consensus existed regarding no role for screening prior to oral contraception in patients with migraine and transdermal hormone replacement following cancer‐associated thrombosis. Physicians in the Middle East were more likely to perform testing than those in the United States, Europe, Canada and the United Kingdom.
Conclusion(s): Marked variability in thrombophilia testing exists among thrombosis experts across a broad range of clinical entities, despite the availability of published guidelines. Clinical studies should be encouraged in areas where knowledge gaps exist.
PB0951
Shortened prothrombin time and activated partial thromboplastin time tests as a risk factor for venous or arterial thrombosis in Mexico
Y. Lee‐Tsai 1; L. Rosales‐Badillo2; H. Leyton‐Rivera2; A. Majluf Cruz3
1 Hospital General San Juan de Dios, Guatemala, Guatemala, Guatemala; 2 Unidad de Investigación Medica de Trombosis, Hemostasia y Aterogénesis. Instituto Mexicano del Seguro Social, Ciudad de Mexico, Distrito Federal, Mexico; 3 Unidad de Investigación Medica de Trombosis, Hemostasia y Aterogénesis. Instituto Mexicano del Seguro Social, Mexico City, Distrito Federal, Mexico
Background: Abnormally prolonged Prothrombin Time (PT) and activated Partial Thromboplastin Time (APTT) tests have been always considered as markers of a hemophilic state. However, there is growing evidence suggesting that they also may be associated with a thrombophilia status when they are shortened.
Aims: To compare the results of the PT and APTT tests in a cohort of patients with thrombosis vs. healthy controls in Mexico.
Methods: In this prospective, cohort, case control study, male and female patients and a group control were included. The study period was January 2014 to December 2021. Several variables were analyzed including type and number of thrombosis, comorbidities, and the results of PT and APTT tests, blood cell counts, and liver and renal function tests. Women under hormonal treatment or pregnant were excluded. Blood samples were always obtained after the anticoagulant effect was washed‐out. Thrombosis was classified in three subgroups: venous, arterial, or both.
Results: We included 4,649 cases (1,769 males (38%) and 2,880 women (62%)), and 1,000 controls. The statistical analysis was performed considering three cut‐off values regarding the shortness of the PT and APTT tests: (0.2, 0.3, and 0.5 or more sec). As compared with the control group, there was a significant shortness of the tests in all subgroups of patients as compared with controls (p < 0.001). These differences were more pronounced in the APTT results (Table 1). Also, there was a significant relationship between the degree of shortness of the APTT test and the number of thrombotic episodes.
Conclusion(s): The results of this large cohort analysis strongly suggest that shortness of the PT and APTT tests are significantly associated with a history of thrombotic events in the Mexican population.

PB0954
Thrombotic profile according to antithombin deficiency and SERPINC1 gene analysis: A French retrospective cohort study
L. Khider1; M. DELRUE2; T. Mirault1; N. Gendron3; O. Sanchez4; B. Planquette5; L. Darnige6; S. Gandrille6; A. STEPANIAN7; D. Smadja8; E. Messas9; V. SIGURET10; L. Mauge 11
1 HEGP, Paris, Ile‐de‐France, France; 2 AP‐HP.Nord / University of Paris, 75010, Ile‐de‐France, France; 3 Hôpital européen Georges Pompidou, Inserm UMRS_1140, Paris, Ile‐de‐France, France; 4 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 5 APHP, Paris, Ile‐de‐France, France; 6 Hematology, HEGP, Paris, Paris, Ile‐de‐France, France; 7 Lariboisière Hospital/University of Paris, Paris, Ile‐de‐France, France; 8 HEGP, Inserm UMRS_1140, Paris, Ile‐de‐France, France; 9 Vascular Medicine, HEGP, Paris, Paris, Languedoc‐Roussillon, France, 10 INSERM UMR‐S‐1140/University of Paris, Paris, Ile‐de‐France, France, 11 APHP, HEGP, Paris, Paris, Ile‐de‐France, France
Background: Inherited AT deficiency (ATD) is associated with a 16‐fold risk of venous thromboembolism (VTE) and arterial thrombotic events (ATE). In ATD, the association of clinical data (thrombotic history, anticoagulant treatment, recurrence risk) with genetic characterization of SERPINC1 and ATD type are scarce. These data would be useful to optimize the antithrombotic management in ATD.
Aims: To describe thrombotic events, anticoagulant treatment and outcomes of patients with ATD, according to the deficiency type and SERPINC1 sequence gene variations.
Methods: Retrospective cohort study with inherited ATD genetically characterized (SERPINC1 gene analysis) with clinical and laboratory data reported in a standardized form. Quantitative variables were expressed as medians [25th‐75th percentiles] and categorical variables as percentages on available data.
Results: From 2000 to 2021, we included 63 patients (31 [24–52] years; 53% men; 20.9 % with BMI >30 kg/m2), of whom 26.7% had a first‐degree family history of thrombosis. Fifty‐three (80.3 %) patients had a personal history of thrombosis. Type I ATD was the most frequent (59.0%) and all had a thrombosis history (Figure 1). First thrombotic event occurred at 30 yo [21–42] comprising VTE (88.7%) and ATE (11.3%). Median follow‐up since the first thrombotic event was 53.7 months [25.4–144.8]. Initial antithrombotic therapy (median 6.1 months [3.1–7.6]) was stopped after the first event in 44.7% patients. Patients with a long‐term treatment were notably on DOAC (36.0%) or vitamin K antagonist (44.0%). Thrombotic recurrence occurred in 23 patients (43.4%) mainly type 1 ATD (Figure 1), 17.4% were still on aspirin, 8.7% on DOAC, 4.3% on LMWH and 69.6% had no antithrombotic therapy.
Conclusion(s): We report high thrombotic recurrence risk in ATD patients, particularly in type I. Our study suggests that ATD type and SERPINC1 gene analysis is useful to predict thrombotic risk in ATD patients and could guide antithrombotic course duration to prevent thrombotic recurrence in selected patients.

PB0952
The von Willebrand Factor/ADAMTS13 ratio is a good indicator of venous thromboembolism risk. Results from the RETROVE project
D. Llobet 1; C. Vallvé2; S. Mojal2; M. Carrasco2; N. Vilalta2; J. Mateo2; J. Millón2; J. Souto3
1 Hospital de la Santa Creu i Sant Pau, Barcelona, Catalonia, Spain; 2 Hospital de la Santa Creu i Sant Pau, Barcelona, Catalonia, Spain; 3 Genomics of Complex Diseases Group, Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau, Barcelona, Catalonia, Spain
Background: High von Willebrand factor (vWf) levels are a known risk factor of venous thrombosis (VT). ADAMTS13 is the regulator of vWf. Recently, our group has described that low ADAMTS13 levels are associated with VT risk in women. Also, Pagliari, et al. reported that ADAMTS13 activity levels < 86% are associated with a moderate risk of VT. Lastly, it has been recently described in several studies about ADAMTS 13 levels and COVID, they found that a high vWf/ADAMTS13 ratio is associated with a higher severity of COVID and also that low ADAMTS13 is associated with lower survival.
Aims: To evaluate if the vWf/ADAMTS13 ratio increases VT risk.
Methods: Patients consisted of 400 individuals with VT recruited by the RETROVE Project (Riesgo de Enfermedad TROmboembólica VEnosa). Also, 400 healthy controls were included. Controls were not matched for sex or age due to the design of the project. We determined antigen ADAMTS13 and vWf by ELISA and their cut‐off points were set at P10 (≤60% ADAMTS13) and P90 (>183% fvW) respectively. The VT risk was estimated with binary logistic regression. We estimated the crude and age‐adjusted odds ratio.
Results: vWf levels above 183% were associated with higher VT risk (adj‐OR: 8.48 [5.48–13.12 95% CI], p < 0.001). Also, high ORs were found with vWf > 183% regardless of ADAMTS13 levels, suggesting that vWf is the main factor responsible for VT risk. A synergetic effect of high vWf levels plus low ADAMTS13 levels were found, the adj‐OR increases to 13.47 ([5.10–35.53], p = 0.0001) when low ADAMTS13 levels were included in the calculations. Regarding the behavior of vWf/ADAMTS13 ratio, we found the following results (Table 1).
Conclusion(s): In the RETROVE project, the von Willebrand Factor/ADAMTS13 ratio is a good indicator of venous thromboembolism risk. Spanish grants: FIS PI12/00612, FIS PI 15/00269, and FIS PI 18/00434

PB0959
Predictive factors for thrombophilia diagnosis: A single‐center cross sectional study
K. Vrotniakaite‐Bajerciene 1; F. Schmidli2; K. Jalowiec2; H. Broughton2; J. Schneider2; A. Haynes3; J. Brodard2; A. Rovo2; J. Kremer Hovinga4; A. Angelillo‐Scherrer2
1 Bern University Hospital, Bern, Bern, Switzerland; 2 Bern University Hospital, Department of Hematology and Central Hematology Laboratory, Bern, Bern, Switzerland; 3 Bern University, CTU Bern, Bern, Bern, Switzerland; 4 Department of Hematology and Central Hematology Laboratory, Bern University Hospital, University of Bern, Bern, Bern, Switzerland
Background: Clinical utility of thrombophilia testing has been proven to be limited. As only high‐risk thrombophilia seems to influence a further treatment decision, more clear and homogenous selection criteria for high‐risk thrombophilia are needed.
Aims: The aim of this study was to investigate the strongest predictive factors for hereditary low‐ and high‐risk thrombophilia, and for antiphospholipid syndrome (APS).
Methods: We conducted a single‐center cross sectional study of 3686 patients referred to the thrombophilia consultation, at the Bern University Hospital (Switzerland), from 01/2010 to 10/2020. We investigated predictive factors of getting a positive result for hereditary and acquired thrombophilia in a cohort of patients selected for thrombophilia work‐up.
Results: In 3550 patients (94%), a partial or full thrombophilia testing was performed and 1258 patients (28.9%) displayed at least one thrombophilia. Most of the included patients (2407, 65%) were referred because of venous thromboembolism (VTE) and arterial thromboembolism (591, 16%), followed by asymptomatic patients (567, 15%) and pregnancy morbidity (121 patients, 3%) (Table 1). Younger age (< 50 years) and positive personal and first‐ and second‐degree family history for VTE were associated with hereditary low‐risk thrombophilia (defined in Table 2), whereas the presence of risk factors and co‐morbidities reduced the likelihood of a hereditary low‐risk thrombophilia diagnosis. Hereditary high‐risk thrombophilia was more likely to be diagnosed in young female patients (< 50 years) without co‐morbidities, with a positive first‐degree family history for VTE and with high D‐dimer level (>500 μg/L) at time of the work‐up, while the presence/absence of risk factors did not affect a positive hereditary high‐risk thrombophilia finding. Meanwhile, APS was more likely to be diagnosed in patients with ≥2 co‐morbidities (Table 2).
Conclusion(s): Several clinical characteristics were associated with hereditary and acquired thrombophilia. A more personalized approach to improve hereditary high‐risk thrombophilia diagnosis yield, including when risk factors are present, should be prospectively assessed.
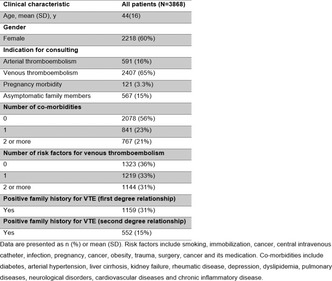

VPB0962
Fracture mechanics of human blood clots: Fibrinogen concentration is a critical determinant of clot toughness
R. Kadaba Ramanujam 1; K. Garyfallogiannis2; T. Yu3; C. Nagaswami4; R. Litvinov5; J. Weisel6; J. L. Bassani2; P. K. Purohit2; V. Tutwiler7
1 Rutgers‐State University of New Jersey, New Brunswick, New Jersey, United States, 2 university of Pennsylvania, Philadelphia, Pennsylvania, United States; 3 Rutgers‐State University of New Jersey, Philadelphia, Pennsylvania, United States; 4 Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 5 University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania, United States; 6 Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States; 7 Rutgers University, New Brunswick, New Jersey, United States
Background: Embolization, or rupture of a thrombus, is a complication of thrombosis that increases mortality risk by up to 30%. Fibrin provides mechanical and structural stability to blood clots. Increased fibrin(ogen) concentration, which is a thrombophilia, is associated with (pro)‐thrombotic conditions and embolization risk.
Aims: To characterize the dependence of mechanical toughness (resistance to rupture), on fibrin(ogen) concentration.
Methods: We performed single edge notch fracture tests to examine fibrin rupture under a constant strain rate (Figure 1A). We utilized confocal and scanning electron microscopy to quantify fibrin network structure. A finite element model using fibrin constitutive laws was used to independently probe the relationship between fibrin(ogen) concentration and resistance to rupture.
Results: Our results revealed that increasing fibrin(ogen) concentration (1–10mg/ml) results in an increase in toughness (Gc) (3–15 N/m p < 0.0001) (Figure 1B) and the maximum force prior to rupture (Fmax) (0.08–0.43N p < 0.0001). Correlation analysis revealed no dependence of Gc on initial crack lengths across all fibrinogen concentrations studied, demonstrating that toughness is a well‐defined material parameter. Increased fibrinogen concentration resulted in fewer (16–10 number of fibers p < 0.0001), thicker (0.15–0.35 μm p < 0.0001) fibrin fibers with a reduction in pore size (2.3–2 μm p < 0.0001). Finite element modeling was required to capture the dissipative effects of fluid flow on the resistance to rupture; these effects cannot be easily measured experimentally. Our modeling suggests a power law relationship of the toughness to fibrinogen concentration (Figure 1C), suggesting a critical strain drives scission of the fibrin fibers.
Conclusion(s): These findings reveal a significant contribution of fibrin(ogen) concentration to the resistance of fibrin clots to rupture, suggesting a role in propensity for embolization.

PB0956
Venous thrombosis associated with combined oral contraceptives: Role of intrinsic thrombin generation potential
A. Rauch 1; A. van Hylckama Vlieg2; F. Rosendaal3
1 University of Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011‐EGID, France, Lille, Nord‐Pas‐de‐Calais, France; 2 Leiden University, Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Ideally, women at high risk of venous thromboembolism (VT) when using combined oral contraceptives (COC), should be identified prior to starting.
Aims: To assess if TG‐potential (estimated prior starting COC) can identify women at high risk of VT.
Methods: TG‐potential was evaluated in 1072 premenopausal women (444 cases; 628 controls from the MEGA study) with the assumption that measurements performed in cases (who all discontinuated COC use) late after thrombosis was mimicking their intrinsic TG‐potential. In controls using COC at the time of the blood draw, we estimated their intrinsic TG‐potential in the absence of use COC by substracting the mean COC effect from their measured TG‐potential. To assess the risk of VT, odds ratios (ORs) were calculated for TG‐potential (peak‐height or activated protein C sensitivity ratio [APCsr]) after stratification in quartiles. Using the 75th‐percentile of TG‐potential in controls as cut‐off, the effect of COC use, elevated TG‐potential, and combination on VT risk was assessed with adjustment on age, body mass index and family history of VT (using women with neither risk factor as reference ). Analyses were also stratified on progestogen type.
Results: The ORs of VT for TG‐potential in the top quartile (compared with the lowest quartile) were 11.6 (95% confidence interval [CI]: 6.3–21) for peak‐height, 2.3 (95% CI: 1.6–3.4) for APCsr. The combination of elevated TG‐potential and subsequent COC use (OR (peak‐height) = 16.7 [95% CI: 10.5–26], OR (APCsr) = 13.3 [95% CI: 8.4–21]) was higher than expected from individual risks. After exclusion of FV‐Leiden and prothrombin‐G20210A carriers, women with elevated peak‐height levels were still the most vulnerable to COC (Table 1). This synergistic effect on VT risk was higher in women using third‐generation COC than levonorgestrel‐ones, even after exclusion of of FV‐Leiden and prothrombin‐G20210A mutations.
Conclusion(s): High TG‐potential is associated with an increased risk for VT and COC use potentiates such association.

PB0948
ATHNdataset Hereditary Antithrombin Deficiency (HAD) Pilot Project (ATHN 12) Registry from the American Thrombosis and Hemostasis Network (ATHN)
M. DeSancho 1; E. Suvar1; J. Santos2; M. Recht3
1 Weill Cornell Medicine, New York, New York, United States; 2 American Thrombosis Hemostasis Network, Iowa City, Iowa, United States; 3 American Thrombosis and Hemostasis Network, Rochester, New York, United States
Background: Hereditary antithrombin deficiency (HAD) is an uncommon high‐risk thrombophilia. Heterozygotes carriers usually present with a first thrombotic event before age 30 mainly venous thrombosis (VT).
Aims: We designed a national HAD registry to collect clinical, laboratory, and treatment data of patients with HAD with the goal of optimizing their management.
Methods: Ongoing prospective HAD registry (ATHN12) created in 2020 to identify a cohort of 100 adults and pediatric patients with HAD followed at thrombosis centers affiliated with the American Thrombosis Hemostasis Network. We collected demographics, laboratory data, thrombosis risk factors, thrombotic events, pregnancies and procedures, antithrombotic therapy, and use of antithrombin concentrate.
Results: 52 patients were identified: 67% females; mean age 35.5 years; 54% had type 1 HAD. There were 67 VT and 3 arterial thrombosis. 65% patients had at least 1 VT and 53% recurrent events. The most common thromboses were lower extremity DVT (52%) and pulmonary embolism (31%) (Table 1). Anticoagulation was prescribed in 97% of patients with VT: 30% direct oral anticoagulants (DOACs), 31% warfarin, and 39% LMWH or UFH (Table 2). There were 69 pregnancies in 27 women; of these, 9 had first trimester spontaneous abortions and one third trimester fetal loss. Overall there were 46.4% pregnancy complications (Table 2). LMWH was prescribed in 49% pregnancies. There were 6 pre‐term deliveries (< 37 weeks’ gestation). Antithrombin concentrate was administered in 29% pregnancies before delivery. Anticoagulation was continued in 45% of women post‐delivery and in 14% anticoagulation was switched to warfarin or DOAC. 79% patients had a total of 108 procedures. Overall, 40% patients received AT concentrate without any adverse events.
Conclusion(s): Recurrent VT was the most common presentation. DOACs and warfarin were equally prescribed in these patients. LWMH was only prescribed in half of the pregnancies and AT concentrates in less than one third of deliveries without adverse events.


Visceral Vein Thrombosis
VPB0438
Thrombotic events of the spleno‐portal axis in pre and post liver transplantation
A. Ortiz Lopez 1; M. Hernández Aínsa2; I. Rivas Estabén3; S. Angós Vázquez4; N. Gemperle Ortiz4; M. Moles Guerrero4; V. Murillo Cortés4; s. Lorente Pérez4; m. Dobón Rebollo4; L. Palomera Bernal4; O. Gavín Sebastian4
1 HEMATOLOGY RESIDENT, Zaragoza, Aragon, Spain; 2 Hospital Lozano Blesa, Zaragoza, Aragon, Spain; 3 Hospital Lozano Blesa, Andorra (Teruel), Aragon, Spain; 4 Hospital Lozano Blesa, Zaragoza, Aragon, Spain
Background: The alteration in the complex physiological balance between pro and anticoagulant factors existing in cirrhotic patients leads to an increased risk of deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) and, notably, thrombosis of the splenoportal axis (PT).
Aims: To determine the incidence of PT in cirrhotic patients before and after liver transplantation and to analyze the management of anticoagulation in this area.
Methods: Descriptive and single‐center observational study, in which a retrospective cohort of 78 patients undergoing liver transplant from March 2017 to August 2020 was reviewed.
Results: Of 78 patients, 80.8% (63/78) were men and 19.2% (15/78) women, with a median age of 60 years at the liver transplant. The main causes of liver transplant were alcohol (62%) and viral (16%). Before transplant, there was 18% (14/78) of PT, being diagnosed with a median of 8 months before transplant. The 86% (12/14) received anticoagulation, 75% (9/12) with low molecular weight heparin (LMWH) and the rest with anti‐vitamin K (AVK). Because of severe thrombocytopenia the 14% (2/14) couldn´t received anticoagulation. We found two episodes of DVT before liver transplant, one of them also diagnosed with PT. After transplant, we found 5.1% (4/78) of PT, detected with a median of 3 days after the transplant. The 50% (2/4) were treated with LMWH. There was 7.7% (6/78) of DVT, diagnosed with a median of 7 months after transplant, 66.7% (4/6) of them treated with LMWH. The percentage in both types of events that did not receive anticoagulation was due to severe thrombocytopenia.
Conclusion(s): The incidence of PT found is similar to the one published in the reviewed literature. The high incidence of thrombosis after liver transplant forces us to implement improvements actions in the management of possible factors that favors the thrombotic event.

PB0433
Ovarian vein thrombosis: A retrospective cohort study
F. De Pascali 1; N. Foti2; W. Ageno3; M. Donadini4
1 University of Insubria, Como, Lombardia, Italy; 2 School of Medicine, University of Insubria, Varese, Varese, Lombardia, Italy; 3 Department of Medicine and Surgery, Research Center on Thromboembolic Disorders and Antithrombotic Therapies, University of Insubria, Varese, Italy., VARESE, Lombardia, Italy; 4 Research Center on Thromboembolic Disorders and Antithrombotic Therapies, University of Insubria, Varese, VARESE, Lombardia, Italy
Background: Ovarian vein thrombosis (OVT) is an unusual manifestation of venous thromboembolism (VTE), with a lack of evidence on its management.
Aims: To collect data about OVT characteristics, anticoagulant therapy (AC) and outcomes.
Methods: Observational, monocentric, retrospective study including consecutive adult patients diagnosed with OVT from 2007 to 2021 and referred to the Thrombosis center of Varese hospital. Data were collected on demographics characteristics, clinical presentation, OVT extension, AC, VTE recurrence and bleeding events.
Results: 38 women were included, with a mean age of 57.6 ± 19.8 years. 32 patients (84%) had at least one major VTE risk factor: cancer 23 (60.5%), chemotherapy 13 (34,2%), surgery or major trauma in the last 3 months 8 (21%), infection 2 (5.2%), pregnancy/post‐partum 3 (8%), hormonal therapy 3 (7.9%), previous VTE 2 (5%). Most of the patients (31/38) were asymptomatic. Left ovarian vein was involved in 32 patients (84%). Pulmonary embolism (PE) was diagnosed in 2 (5%) cases. 30 patients (78.9%) received AC with low molecular weight heparin (LMWH) as initial treatment, mainly at full dose (28/30); 2 (6,6%) received lower doses due to brain metastasis or acute renal insufficiency. 20 patients (66,6%) received extended anticoagulation (>3 months), either with LMWH (16; 8%) or warfarin (3; 15%) or apixaban (1; 5%). 2 patients were subsequently switched from LMWH to apixaban. Over a mean follow up of 32.7 months, recurrent VTE occurred in 1 patient (2.6%) who had been previously treated with AC; there were 8 bleeding events (21%), including 3 major (7.9%).
Conclusion(s): OVT was often incidentally diagnosed, and it was rarely associated with concomitant PE. Cancer and surgery were frequent risk factors. Most of the patients received AT. During follow‐up major bleedings were not negligible, whereas recurrent VTE was low.
VPB0435
Vascular involvement in Behçet's disease
A. Baya Chatti 1; S. Skhiri2; A. Guiga2; W. Ben Yahia2; A. Atig2; N. Ghannouchi2
1 Farhat Hached Hospital, Tunis, Sousse, Tunisia; 2 Farhat Hached Hospital, Sousse, Sousse, Tunisia
Background: Behçet’s disease (BD) is a systemic inflammatory vasculitis with a wide range of manifestations including vascular involvement.
Aims: Our study aimed to describe clinical, therapeutic and evolutionary features of vascular involvement in BD.
Methods: We conducted a retrospective study, including 77 patients meeting the international study group criteria for BD, followed for a period of 18 years [2002–2020]. Patient’s data were collected and analysed.
Results: The mean age at diagnosis was 34.4 years [20–60]. The gender ratio (M/F) was 1.85. Vascular involvement concerned 28 patients (36.4%) and initiated the disease in 17.9% of cases. Vascular manifestations were dominated by venous thrombosis (92.9%) with 45 thrombotic events during follow‐up. Deep venous thrombosis involved most frequently the lower limbs (53.3%), followed by iliac veins (13.3%), inferior vena cava and cerebral veins (8.9%, respectively), jugular veins and pulmonary embolism (6.7%, respectively), renal and hepatic veins (4.4%, respectively), upper limb, superior vena cava, gastrointestinal, central retinal, and ovarian veins in one case each. Superficial venous thrombosis involved upper limbs (n = 3) and lower limbs (n = 1). Four patients (12.9%) developed arterial thrombosis: Mesenteric infarction, myocardial infarction, ophthalmic artery occlusion and intracardiac thrombus. Arterial aneurysm were observed in two patients: of the pulmonary artery revealed by recurrent haemoptysis and of the renal artery revealed by lumbar pain. Therapeutically, anticoagulants were initiated in 88.9%, corticosteroids in 74% and immunosuppressive treatment in 29.7% of cases (cyclophosphamide in 6 patients and azathioprine in 2 patients). In terms of evolution, after an average follow‐up of 59 months, 48% patients presented recurrences. Sequelae occurred in 59.3% of patients: Chronic venous insufficiency in 14 patients and chronic Budd Chiari syndrome with hepatic cirrhosis in two patients, one of whom died of hepatic encephalopathy.
Conclusion(s): Vascular involvement in BD is heterogeneous in term of presentations and severity. Early diagnosis and management are essential to reduce complications.
VPB0436
Clinical and etiological features of vena cava thrombosis: 69 cases study
A. Mabrouk 1; I. Naceur2; T. Ben Achour2; M. Jridi2; M. Khanfir2; I. Ben Ghorbel2; M. Lamloum2; F. Saïd2; H. Houman2
1 Faculty of Medicine of Tunis, Manar University, Ben Arous, Ben Arous, Tunisia; 2 Faculty of Medicine of Tunis, Manar University, Tunis, Tunis, Tunisia
Background: Vena cava thromboses (VCT) are rare and remains under‐recognized.
Aims: The aim of this study was to describe clinical and etiological features and outcomes of vena cava thromboses.
Methods: We conducted a retrospective study of patients with vena cava thromboses diagnosed in an internal medicine department over a period time of 20 years.
Results: Sixty nine patients with vena cava thrombosis were enrolled. The mean age was 44 years. Sex‐ratio M/F was 2.45. Forty five patients had inferior vena cava thrombosis (IVCT) and 26 patients had superior vena cava thrombosis (SVCT); 2 patients had both superior and inferior vena cava thrombosis. Eighteen patients had personal history of deep venous thrombosis. IVCT was discovered incidentally in 67% of cases, otherwise it was revealed by bilateral lower limbs edema. Patients presented with lower limbs edema (57%), and collateral venous circulation (17%). Concomitant thromboses were found in 75% of patients: lower extremities (n = 32), atypical locations (n = 4), pulmonary embolism (n = 7) and superficial thrombophlebitis (n = 2). Six patients had Behcet’s disease and 7 had malignancy. Inherited thrombophilia was noted in 2 cases. SVCT was discovered incidentally in 5 cases and revealed by a superior vena cava syndrome in 58% of cases. Collateral circulation (62%), bilateral upper extremities edema (30%), and dyspnea (23%) were other signs of SVCT. SVCT was associated to other locations in 84.6% of cases: upper extremities (n = 12), cerebral (n = 2), lower extremities (n = 2), sub clavicular (n = 2) and retinal (n = 1). Pulmonary embolism was diagnosed in 3 cases. Behcet’s disease (n = 13) and malignancy (n = 6) were the main causes of SVCT. Association of IVCT and SVCT were noted in patient with Behçet’s disease (n = 1) and hyperhomocysteinemia (n = 1).
Conclusion(s): In our series Vena cava thrombosis seems to be often associated with other concomitant deep vein thrombosis. Behcet’s disease and malignancies are the major causes of vena cava thrombosis.
VPB0437
Identifying clinical predictors for thrombus progression in cirrhotic patients with untreated splanchnic vein thrombosis
H. McMurry 1; J. Sabile1; B. Elstrott1; B. Chobrutskiy1; A. Mohinani2; S. Patel1; S. Gowda1; J. Shatzel2
1 Oregon Health and Science University, Portland, Oregon, United States; 2 Oregon Health & Science University, Portland, Oregon, United States
Background: Splanchnic vein thrombosis (SVT) occurs in a heterogenous group of patients secondary to a variety of risk factors including liver disease. There is equipoise regarding the utility of anticoagulation in cirrhotic patients with SVT.
Aims: We sought to identify clinical factors predictive of new or progressive thrombosis in a cohort of patients with untreated SVT, with the goal of identifying patients most likely to benefit from therapeutic anticoagulation.
Methods: This was a retrospective cohort study of cirrhotic patients over 18 years of age diagnosed with an SVT at the Oregon Health & Science University from 2015 –2020, including only patients who were not initially treated with anticoagulation. The primary study endpoint was a composite of the following: imaging‐confirmed progression of SVT, development of cavernous transformation, intestinal ischemia, portal cholangiopathy or new venous or arterial thrombosis.
Results: 261 patients were included in the analysis. Forty percent of all patients experienced the primary composite endpoint. Multivariable logistic regression found that only the presence of pancreatitis or abdominal infection at diagnosis was associated with increased likelihood of experiencing the primary composite endpoint (OR 3.61, p = 0.02). Attempts to derive an integer‐based risk model found an AUC that was only marginally higher than that of chance alone (0.57). However, there was a statistically significant difference of overall survival between patients that did and did not experience the primary composite endpoint.
Conclusion(s): Overall, only the presence of pancreatitis or active intra‐abdominal infection was found to be predictive of thrombotic progression in patients with cirrhosis and SVT. Predictive modeling implies that the risk factors for thrombus progression are heterogenous. Thrombotic progression was associated with increased mortality.


PB0434
Safety and efficacy of anticoagulant treatment in patients with ovarian vein thrombosis: A systematic review and meta‐analysis
C. Vassallo 1; L. Muscat Baron2; N. Riva1; A. Gatt3; J. Calleja‐Agius4
1 Faculty of Medicine and Surgery, University of Malta, Msida, Malta; 2 Faculty of Medicine and Surgery, University of Malta, Msida, Not Applicable, Malta; 3 Department of Pathology, Faculty of Medicine and Surgery, University of Malta, Msida, Not Applicable, Malta; 4 Department of Anatomy, Faculty of Medicine and Surgery, University of Malta, Msida, Not Applicable, Malta
Background: Ovarian vein thrombosis (OVT) is a rare category of venous thromboembolism (VTE). It is usually reported during pregnancy/puerperium, but risk factors also include malignancies, pelvic surgery and local infections. The treatment typically consists of antibiotics and anticoagulants; however, it is debated whether anticoagulation is appropriate for all aetiologies of OVT.
Aims: To evaluate safety and efficacy of anticoagulant treatment for OVT by performing a systematic review of the literature and meta‐analysis.
Methods: The protocol was registered a priori in PROSPERO (CRD42021270883). We performed a bibliographic search within the electronic databases MEDLINE, EMBASE and CENTRAL from inception to July 2021. We included randomized control trials and observational studies/case‐series enrolling at least 10 adult patients diagnosed with OVT and treated with any anticoagulant drugs. The following outcomes were extracted: mortality, major bleeding and recurrent VTE during anticoagulant treatment, and vessel recanalization. We calculated weighted mean proportions using a random‐effects model.
Results: We retrieved 11 observational studies enrolling OVT patients treated with anticoagulants (n = 361); 4 studies evaluated also OVT patients who were not anticoagulated (n = 286). Six studies included only post‐partum OVT, while the remaining included mixed aetiologies. The most common anticoagulants were warfarin and heparin; average treatment duration ranged from 1 to 9 months. In treated patients, mortality rate was 2.18% (95% CI, 0–10.03%; I 2 = 63.0%; 4/134 patients, 5 studies); major bleeding 0.95% (95% CI, 0–3.60%; I 2 = 0%; 1/129 patients, 2 studies); recurrent VTE 2.10% (95% CI, 0.04–6.16%; I 2 = 4.2%; 2/101, 5 studies); and vessel recanalization 92.8% (95% CI, 74.6–100%; I 2 = 83.2%; 134/149 patients, 6 studies). The rate of recurrent VTE in untreated patients was 9.88% (95% CI, 5.95–14.65%; I2 = 0%; 18/184 patients, 2 studies).
Conclusion(s): There is a scarcity of literature available on the use of anticoagulation for OVT. Our results suggest that anticoagulant treatment is associated with reduced VTE recurrence and low major bleeding events.
PB0432
The ADAMTS‐13/von Willebrand factor (VWF) ratio as a prognostic biomarker for the development of portal vein thrombosis (PVT) in patients with compensated cirrhosis
R. De Cristofaro 1; S. Lancellotti2; M. Sacco1; M. Tardugno1; A. De Magistris3; M. Basso4; M. Pompili5; F. Ponziani5; F. Santopaolo5; A. Grieco5
1 Catholic University of Sacred Heart, Translational Medicine and Surgery Department, Rome, Lazio, Italy; 2 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Rome, Lazio, Italy; 3 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Lazio, Italy; 4 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Center for Hemorrhagic and Thrombotic Diseases and Haemophilia Center, Rome, Rome, Lazio, Italy; 5 Fondazione Policlinico Universitario A. Gemelli, IRCCS, Department of Digestive, Endocrine and Metabolic System, Institute of Internal Medicine and Geriatrics, Rome, Rome, Lazio, Italy
Background: Cirrhosis is associated with an increased risk of PVT in which the decrease of the portal vein flow velocity < 15 cm/s, leads an unbalance between the decreased zinc‐protease ADAMTS‐13, due to the pro‐fibrogenic transformation of stellate cells, and the inflammation‐driven heightened VWF and FVIII levels, predisposing to platelet/VWF‐rich thrombi formation in micro‐/macro‐liver‐ circulation. Liver cirrhosis is characterized by the alteration of both pro and anticoagulant proteins. Thus, a significant reduction of the protein C/FVIII ratio was reported in patients with NAFLD. Other studies indicated a prevalence of congenital thrombophilia conditions, as risk factors of PVT development.
Aims: This observational prospective clinical trial on compensated cirrhotic patients (ClinicalTrial.gov: NCT03322696) could identify ADAMTS‐13/VWF:GpIbR ratio as a key predictive factor of PVT development in cirrhotic patients. All participants signed informed consent.
Methods: The final 80/118 enrolled subjects were followed for 48 months and underwent Doppler‐ultrasound liver examination and clinical (Child‐Pugh and MELD‐score) and hematochemical (PT%, APTT%, AT%, PC%, PS%, LAC, fibrinogen, FVIII:C, D‐dimer) assessment. VWF antigen and VWF:GpIbR were measured by chemiluminescence assays; ADAMTS‐13:activity by a FRET‐based‐assay; both FV Leiden G1691A and the prothrombin G20210A polymorphisms by using the RT‐PCR; statistical analyses by the SPSS‐software.
Results: Five patients presented PVT (cumulative incidence = 6.3%) (characteristics showed in Table 1), occurred between 4 and 36 months after the enrollment. The ADAMTS‐13/VWF:GpIbR ratio sensitivity and specificity as PVT predictor was assessed by a ROC curve (Figure 1), highlighting a cut‐off‐value = 0.40, below which the risk to develop a PVT complication increases (OR 14.6, 95% C.I.:1.36–157.2, p = 0.027) as also confirmed by a Cox‐regression‐analysis, where the risk to develop a PVT rises exponentially after 30th month‐follow‐up.
Conclusion(s): As a function of disease progression, the ADAMTS‐13/VWF unbalance in the sinusoidal vessel predisposes to thrombotic diathesis. Thus, the ADAMTS‐13/VWF:GpIbR ratio, significantly associated with liver failure, could be a predictive biomarker for PVT development.


PB0431
Anticoagulant therapy for splanchnic vein thrombosis: an individual patient data meta‐analysis
M. Candeloro 1; E. Valeriani2; M. Monreal3; W. Ageno4; N. Riva5; R. Lopez Reyes6; M. Peris7; J. Beyer Westendorf8; S. Schulman9; V. Rosa10; J. LÓPEZ‐NÚÑEZ11; J. Garcia‐Pagan12; M. Magaz12; M. Senzolo13; A. De Gottardi14; M. Di Nisio15
1 "G. D'Annunzio" University, Chieti, Italy, Chieti, Abruzzi, Italy; 2 Diagnostic and Therapeutic Medicine Department, University Campus Bio‐Medico of Rome, Italy, Rome, Lazio, Italy; 3 Department of Internal Medicine, Hospital Universitari Germans Trias i Pujol, Badalona, Barcelona, Spain Chair for the Study of Thromboembolic Disease, Faculty of Health Sciences, UCAM – Universidad Católica San Antonio de Murcia, Spain, Barcelona, Andalucia, Spain; 4 Department of Medicine and Surgery, Research Center on Thromboembolic Disorders and Antithrombotic Therapies, University of Insubria, Varese, Italy, VARESE, Lombardia, Italy; 5 Department of Pathology, Faculty of Medicine and Surgery, University of Malta, Msida, Malta, Msida, Not Applicable, Malta; 6 Department of Pneumonology, Hospital Universitari i Politècnic La Fe, Valencia, Spain, Valencia, Comunidad Valenciana, Spain; 7 Department of Pneumonology, Hospital Universitari i Politècnic La Fe, Valencia, Spain, Castellón, Comunidad Valenciana, Spain; 8 Department of Medicine, Division Hematology, Dresden University Hospital, Dresden, Sachsen, Germany; 9 McMaster University, Hamilton, Ontario, Canada, 10 Department of Internal Medicine, Hospital Universitario Virgen de Arrixaca, Murcia, Spain, Murcia, Murcia, Spain, 11 Department of Internal Medicine, Hospital Germans Trias i Pujol, Badalona, Barcelona, Department of Medicine, Universitat Autònoma de Barcelona, Institut de Recerca Germans Trias i Pujol, Badalona, Barcelona, Spain, Barcelona, Catalonia, Spain, 12 Hepatic Hemodynamic Laboratory, Liver Unit, Hospital Clínic, IDIBAPS and CIBEREHD, University of Barcelona, Barcelona, Spain, Barcelona, Catalonia, Spain, 13 Multivisceral Transplant Unit, University Hospital of Padua, Padua, Italy, Padova, Veneto, Italy, 14 Gastroenterology and Hepatology, Ente Ospedaliero Cantonale, Università della Svizzera Italiana, Lugano, Switzerland, Lugano, Ticino, Switzerland, 15 Department of Medicine and Ageing Sciences, University “G D’Annunzio”, Chieti‐Pescara, Italy, Chieti, Abruzzi, Italy
Background: Robust evidence on the optimal management of splanchnic vein thrombosis (SVT) is lacking and guideline recommendations are heterogeneous.
Aims: To evaluate the effectiveness and safety of anticoagulation for SVT.
Methods: This individual patient data meta‐analysis was performed according to the PRISMA guideline. We searched MEDLINE and EMBASE up to June 2021 and clincaltrial.gov. Data from individual datasets were verified, merged, and any discrepancy with published data was resolved by contacting study authors. Main effectiveness outcomes were recurrent venous thromboembolism (VTE) and mortality; the main safety outcome was major bleeding. Incidence rates were expressed as number of events per 100 patient‐years (/100 p‐y) with relative 95% confidence intervals (95% CI). Hazard ratios (HRs) were estimated with Cox proportional models considering anticoagulant treatment as time‐varying.
Results: A total of 1635 patients were included from 3 studies. Eighty‐five percent of patients received anticoagulation for a median duration of 316 days (range 1 to 730 days). Overall, incidence rates for recurrent VTE, major bleeding, and mortality were 5.3/100 p‐y (95% CI, 5.1 to 5.5), 4.4/100 p‐y (95% CI, 4.2 to 4.6), and 13.0/100 p‐y (95% CI, 12.4 to 13.6), respectively. The incidence rates of all outcomes were lower during anticoagulation and higher after treatment discontinuation or when anticoagulation was not administered. In multivariable analysis, anticoagulant treatment appeared to be associated with a lower risk of recurrent VTE (HR 0.42; 95% CI, 0.27 to 0.64), major bleeding (HR 0.48; 95% CI, 0.30 to 0.75), and mortality (HR 0.23; 95% CI, 0.18 to 0.31). Results were consistent in patients with cirrhosis, solid cancer, myeloproliferative neoplasms, unprovoked SVT, and SVT associated with transient or persistent non‐malignant risk factors.
Conclusion(s): In patients with SVT the risk of recurrent VTE and major bleeding is substantial. Anticoagulant treatment is associated with reduced risk of both outcomes.


VTE Diagnosis
VPB0978
Characteristics of the deep vein thrombosis of the upper limb
R. Ben Salah 1; O. Frikha2; F. Mkaouar2; S. Marzouk3; Z. Ahloul4
1 Hedi Chaker Hospital, Sfax, Sfax, Tunisia; 2 Departement of Internal Medicine, Hédi Chaker Hospital, Sfax, Sfax, Tunisia; 3 Departement of Internal Medicine, Hédi Chaker Hospital, Sfax, Sfax, Tunisia; 4 University of Sfax, SFAX, Sfax, Tunisia
Background: The incidence of deep vein thrombosis (DVT) of the upper limb is increasing due to the frequent use of intravenous devices for various indications.
Aims: We report in our work the epidemiological characteristics and the etiological profile of DVT of the upper limb.
Methods: This is a retrospective study carried out in an internal medicine department collecting the files of patients with DVT of upper limb over a period of 24 years from 1996 to 2020.
Results: Twenty‐three patients presented with upper limb DVT. They were 16 men (69.56%) and 7 women (30.43%). The average age was 46 years. DVT involved the subclavian vein (16 cases ), the axillary vein (10 cases), the internal jugular vein (13 cases), the brachiocephalic venous trunk (TVBC) (6 cases) and the basilic vein in one case. It was associated with superior vena cava thrombosis in two patients and venous thrombosis of the lower limb complicated by pulmonary embolism in a single patient. It was bilateral in one case. The etiological investigation revealed an anti‐phospholipid syndrome in 6 cases (26.08%). Constitutional thrombophilia was observed in 5 patients (21.73%). Four patients had Behçet's disease (17.39%). DVT revealed neoplasia in 3 cases (13%): 2 men had bronchopulmonary cancer and one woman, followed for endometrial neoplasia, had metastasis in the thymic compartment. The survey was negative in 5 patients (21.73%). The evolution under anticoagulants was favorable in 21 patients. Elsewhere, DVT was fatal in one case and complicated by pulmonary embolism in one patient.
Conclusion(s): DVT of the upper limb is associated with a higher mortality rate. This is due to the underlying disease. Hence, it is important to carry out an etiological investigation in search of neoplasia or autoimmune diseases.
PB0968
Right heart thrombus and pulmonary embolism: Early diagnosis and therapeutic management
N. Diaconu; T. Cuzor
Institut of Cardiology, Chisinau, Chisinau, Moldova
Background: Prompt diagnosis of pulmonary embolism (PE) remains challenging, which often results in a delayed or inappropriate treatment of this life‐threatening condition. Mobile thrombus in the right cardiac chambers is a neglected cause of PE. It poses an immediate risk to life and is associated with an unfavorable outcome and high mortality.
Aims: The aim of this study was to analyze echocardiographic findings and to assess therapeutic management in 110 patients with proven PE, in 7 (6,3%) cases with floating RA thrombus diagnosed with transthoracic and transesophageal echocardiography in the first 24 h after hospitalization.
Methods: Echocardiography displayed a mobile ovoid, polycyclic or worm‐like right atrial mass, always associated with signs of PH. This study is part of national research project 20.80009.8007.28.
Results: All patients with atrial thrombosis showed signs of high PH, with maximum systolic pressure in PA 64,2 ± 3,42 mmHg and the average 37,65 ± 2,41 mmHG. The echocardiographic results were revealed a more dilation of RA (with area 27,53 ± 3,13 cm2), VD (39,5 ± 3,11 cm), PA trunk (28,1 ± 2,41 cm), ETT confirmed MCConnell sign in 4 patients, with the relationship of end‐diastolic diameter VD/VS > 1; more obvious dilation of the IVC diameter (23.11 ± 2.11 cm); increase in the tricuspidal regurgitation, which averaged 3.8 ± 1.77 m/sec. All these patients had significantly lower index TAPSE (15.54 ± 4.21 mm) and the tissue index S ´m RV (8.6 ± 1.21 cm/sec), the signs that confirmed the presence of systolic dysfunction of RV. Also, in this group was observed a significant reduction in the relation TAPSE/Pulmonary Artery Systolic Pressure, the average being 0.29 ± 1.11 (0.18–0.37). We observed two cases of floating migratory thrombus from the RA cavity into the LA through the patent oval foramen, with the signs of mechanical obstruction of the mitral valve orifice.
Conclusion(s): Our data suggest that echocardiographic examination is necessary in all suspected PE and has to be done quickly for emergency treatment in patients with floating RA thrombus.
PB0970
Measuring Factor VII Activating Protease (FSAP) in venous thrombosis using a newly developed FSAP generation assay
M. Etscheid 1; K. Hanschmann2; P. Sandset3; S. Kanse4
1 Paul Ehrlich Institute Federal Institute for Vaccines and Biomedicines, Langen, Rheinland‐Pfalz, Germany; 2 Paul Ehrlich Institute, Langen, Hessen, Germany; 3 University of Oslo, Oslo, Oslo, Norway, 4 university of Oslo, Oslo, Oslo, Norway
Background: FSAP is a plasma protease involved in thrombosis, haemostasis and inflammation. A single nucleotide polymorphism (SNP) in the FSAP gene (“Marburg I”), with a strongly reduced proteolytic activity, has been associated with thrombotic events, but a role in venous thrombosis (VT) is uncertain.
Aims: We developed a kinetic assay to measure FSAP generation directly in plasma and demonstrate its suitability in plasma samples of patients with VT.
Methods: FSAP generation was measured with a specific fluorogenic substrate (Ac‐Pro‐DTyr‐Lys‐Arg‐AMC), using histones as activation trigger. Two‐chain FSAP served as calibrator in the FSAP generation assay (FGA), pooled plasma was used as internal control and for normalization. The raw fluorescence curves were transferred into a TGA software package (Technoclone, Vienna) to determine lag phase, time to peak (TtPeak), velocity, peak FSAP and endogenous FSAP potential (EFP).
Results: The histone‐triggered FGA is highly FSAP‐specific with no interference by other proteases like clotting factors or fibrinolytic enzymes. A good linearity was seen for TtPeak, peak FSAP and EFP. The assay was found suitable for heparinized plasma, when treated with polybrene, and clearly identified Marburg I carrier. Plasma of postmenopausal women with or without a history of VT (each group n = 50) showed highly variable results, with only weak, non‐significant differences between groups. Marburg I carrier were equally present in both groups and when excluding these from statistical evaluation, a delayed FSAP generation became significantly different between groups.
Conclusion(s): The FGA is robust and reproducible, measuring various aspects of FSAP activity in plasma. In a one‐step assay design, the contribution of FSAP antigen, sequence variations and inhibition/inactivation are captured. The Marburg I‐SNP did not correlate with history of VT. Focussing on this SNP may hamper risk stratification regarding FSAP activity and thrombosis, and may explain the difficulty in finding a clear consensus on FSAP and VT in the past.
VPB0979
Use of D‐dimer for the exclusion of new pulmonary embolism in anticoagulated patients: A multicenter retrospective study
E. Beaudoin1; S. Kaka2; E. Gagnon1; A. Durivage1; i. boulais1; G. Le Templier1; D. Toupin1; G. Le Gal3; B. Gouin 1
1 Centre Hospitalier Universitaire de Sherbrooke/Université de Sherbrooke, Sherbrooke, Quebec, Canada; 2 University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada; 3 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada
Background: The use of plasma D‐dimer level to exclude pulmonary embolism (PE) in low‐intermediate risk non‐anticoagulated patients has been well studied. However, its use for patients on chronic direct‐acting oral anticoagulant (DOAC) therapy is unclear.
Aims: We aimed to evaluate D‐dimer performance for the exclusion of PE among patients on DOACs who presented to the emergency department (ED) and underwent lung imaging for clinically suspected PE.
Methods: Patients aged ≥ 18 years on DOAC therapy who presented to the ED between January 2010 and June 2019 and had, in one visit (or in two visits within two days), a plasma D‐dimer measurement and an imaging test to assess PE (CT pulmonary angiography or ventilation‐perfusion scintigraphy) were included. The primary outcome was to evaluate the diagnostic accuracy of a negative D‐dimer in the exclusion of a new PE.
Results: Our study included 109 patients. Only four patients (3.7%) had a new diagnosis of PE, of which two had active cancer. The sensitivity of abnormal conventional or age adjusted D‐dimer thresholds to detect PE in this population was 100.0% (95% CI 39.8–100.0) for both thresholds, whilst the negative predictive value to exclude PE in this population was 100.0% (95% CI 91.8–100.0 and 93.8–100.0 respectively for each D‐dimer threshold).
Conclusion(s): In patients on chronic DOAC therapy, plasma D‐dimer level appears to have a high sensitivity and a high negative predictive value. This study suggests a possible role of plasma D‐dimer level in the exclusion of PE among patients on DOAC therapy with a clinical suspicion of PE. However, these findings are hypothesis‐generating only, and further studies are required.


B0977
Endogenous thrombin generation potential in patients with pulmonary embolism and its relevance to thrombo‐inflammatory biomarkers
F. Siddiqui 1; A. Darki1; D. Hoppensteadt1; B. Kantarcioglu1; M. Monreal2; J. Fareed1
1 Loyola University Chicago, Maywood, Illinois, United States; 2 Department of Internal Medicine, Hospital Universitari Germans Trias i Pujol. Badalona, Barcelona, Spain Chair for the Study of Thromboembolic Disease, Faculty of Health Sciences, UCAM – Universidad Católica San Antonio de Murcia, Spain, Barcelona, Andalucia, Spain
Background: Pulmonary embolism (PE) is a catastrophic outcome in patients with venous thrombosis and third leading cardiovascular cause of death after myocardial infraction and stroke, estimates around 100,000 deaths annually. Inflammation, hemostatic dysregulation including fibrinolytic imbalance, endothelial compromise, and generation of thrombotic mediators, along with cellular defects and hemodynamic derangement contribute to the pathogenesis of PE. Endogenous thrombin generation contributes to the overall pathology of PE.
Aims: The purpose of this study is to investigate endogenous thrombin potential (ETP) and the circulating levels of biomarkers of thrombo‐inflammation in PE samples.
Methods: Citrated blood samples from 400 patients with confirmed PE were collected at Loyola University Medical Center and Gottlieb Memorial Hospital under an IRB approved protocol. Normal human plasma (NHP) comprised of 25 male and 25 female samples were obtained from a commercial source. Thrombin generation studies were carried out using a commercially available a kinetic system. Endogenous GAG’s contents were measured by fluorogenic quenching method. Thrombin generation parameters such as peak thrombin, lag time and area under the curve (AUC) were compiled. Plasma samples were retrospectively analyzed for biomarkers, including D‐Dimer, PAI‐1, tPA, TAFIa, vWF, CRP, IL6, TFPI and VEGF using commercially available sandwich ELISA methods. Results were compiled as mean ± SD and analyzed for significance and correlation by using GraphPad Prism software.
Results: On a cumulative basis, PE patients showed a wide variation in thrombin generation parameters (Table 1). Biomarker analysis showed elevated levels of D‐Dimer (37.05‐fold), CRP (35.34‐folds), IL6 (21.62‐folds), tPA (4.69‐folds), PAI‐1a (3.95‐folds), VEGF (2.38‐folds), TFPI (2.17‐folds), GAG’s (1.92‐folds), vWF (1.89‐folds), and TAFIa (1.21‐folds). Peak thrombin levels exhibited varying degree of positive and negative correlations with various biomarkers (Figure 1).
Conclusion(s): PE patients exhibit a wide variation in endogenous thrombin generation parameters. However, biomarkers of hemostatic activation, vascular dysfunction and inflammation were increased.
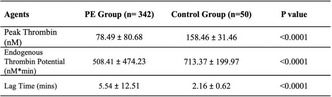

PB0966
Potential utility of a multi‐component coagulation factor panel to detect portal vein thrombosis and assess hemostasis in chronic liver disease
C. Lewis1; K. Bari2; C. Xie3; K. Sherman2; M. Vasse4; P. Van Dreden5; V. Bogdanov 6
1 Division of Hematology/Oncology, Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States; 2 Division of Digestive Diseases, Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States; 3 Department of Environmental & Public Health Sciences, University of Cincinnati College of Medicine, Cincinnati, Ohio, United States; 4 Foch Hospital, Suresnes, Ile‐de‐France, France; 5 Diagnostica Stago, Gennevilliers, France., Gennevilliers, Ile‐de‐France, France; 6 College of Medicine, University of Cincinnati, Cincinnati, Ohio, United States
Background: Portal vein thrombosis (PVT) prior to liver transplant (LT) is a major contributor to morbidity, but the means of detecting and/or predicting PVT are limited.
Aims: To explore whether plasma coagulation factor activity levels can help detect and/or evaluate risk for PVT in subjects with chronic liver disease (CLD) awaiting LT.
Methods: Informed consent was obtained from each subject; study was approved by institutional review boards. Circulating levels of Factor V (FV), Factor VIII (FVIII), Protein C (PC), and Protein S (PS) activity levels and the concentrations of D‐dimer, sP‐selectin, and alternatively spliced tissue factor (asTF) were assessed in two CLD cohorts with severity scored using the Child‐Turcotte‐Pugh (CTP) formula [ambulatory, n = 42 (CTP‐A, n = 12; CTP‐B, n = 19; CTP‐C, n = 11) and LT, n = 43 (CTP‐A, n = 12; CTP‐B, n = 28; CTP‐C, n = 3)]. There were no cases of PVT in the ambulatory cohort, and 4 cases of confirmed PVT in the LT cohort.
Results: A strong inverse correlation between FV and FVIII activity levels and the presence of PVT was found in the LT cohort (p = 0.030 and p = 0.016, respectively). This enabled us to develop a logistic regression‐based compensation score (CS) to identify LT patients at risk of PVT. CS = −7.17 × ln(FV%) – 3.73 × ln(FVIII%) + 47 When FV and FVIII values were subject‐matched and graphed as a correlation plot, the four subjects with PVT clustered in the extreme bottom left quadrant (Figure); 2 subjects with PVT had a CS > 4.0 and the other two had CS in the 0–4 range. CS values in all subjects without PVT were below 0.
Conclusion(s): Conclusion: We demonstrate for the first time the feasibility of using a combination of FV and FVIII plasma activity levels to identify CLD coagulopathy associated with high risk of PVT. Larger studies are warranted to validate our findings.

PB0974
Diagnostic efficacy of ECG‐derived ventricular gradient for the detection of chronic thromboembolic pulmonary hypertension in patients with acute pulmonary embolism
D. Luijten 1; F. Meijer2; G. Boon3; Y. Ende‐Verhaar3; R. Bavalia4; L. El Bouazzaoui5; M. Delcroix6; A. Mairuhu7; M. Huisman8; S. Middeldorp9; P. Pruszcyk10; D. Ruigrok11; P. Verhamme12; A. Vonk Noordegraafonk Noordegraaf11; J. Vriend13; H. Vliegen2; F. Klok14
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Cardiology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 4 Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 5 Department of Pulmonology, Haga Teaching Hospital, Den Haag, Zuid‐Holland, Netherlands; 6 Department of Pneumology, KU Leuven University Hospitals Leuven, Leuven, Vlaams‐Brabant, Belgium; 7 Department of Internal Medicine, Haga Teaching Hospital, Den Haag, Zuid‐Holland, Netherlands; 8 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 9 Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands, 10 Department of Internal Medicine and Cardiology, Medical University of Warsaw, Warszawa, Mazowieckie, Poland, 11 Department of Pulmonology, Amsterdam UMC, VU University Medical Centre, Amsterdam, Noord‐Holland, Netherlands, 12 Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium, Leuven, Vlaams‐Brabant, Belgium, 13 Department of Cardiology, Haga Teaching Hospital, Den Haag, Zuid‐Holland, Netherlands, 14 Leiden University Medical Center, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands
Background: Application of the chronic thromboembolic pulmonary hypertension (CTEPH)‐rule out criteria (manual electrocardiogram [ECG] reading and N‐terminal pro‐brain natriuretic peptide [NTproBNP] blood test) can rule out CTEPH in patients with pulmonary embolism (PE) and persistent dyspnea without echocardiography (InShape II algorithm). Increased pulmonary pressure in CTEPH may also be identified using automated ECG‐derived ventricular gradient optimized for right ventricular pressure overload (VG‐RVPO).
Aims: The diagnostic performance of the VG‐RVPO for the detection of CTEPH and the incremental diagnostic value of the VG‐RVPO as new rule‐out criteria in the Inshape II algorithm were evaluated.
Methods: A predefined analysis of the Inshape II study was performed, which included all patients in whom a baseline ECG and a follow‐up ECG 3–6 months after PE diagnosis were available.
Results: 60 PE patients were included of which 5 (8.3%) were ultimately diagnosed with CTEPH. The mean baseline VG‐RVPO was −18.12 mV · ms for CTEPH patients and −21.57 mV · ms for non‐CTEPH patients (mean difference 3.46 mV · ms [95% CI −29.03 to 35.94]). The VG‐RVPO normalized in patients with and without CTEPH, without a clear between‐group difference (mean Δ VG‐RVPO of −8.68 and −8.42 mV · ms respectively; mean group difference of −0.25 mV · ms, [95% CI −12.94 to 12.44]). The overall predictive accuracy of baseline VG‐RVPO, follow‐up RVPO and Δ VG‐RVPO for detection of CTEPH was moderate to poor (ROC AUC 0.611, 0.514 and 0.539, respectively). Up to 76% of the needed echocardiograms could have been avoided with VG‐RVPO as new rule‐out criteria in the Inshape II algorithm, however at cost of missing up to 80% of the CTEPH diagnosis. Only one scenario decreased the need for echocardiograms with 9.5% and detected all CTEPH cases
Conclusion(s): We could not demonstrate additional diagnostic value of VG‐RVPO as standalone test or as on top of the InShape II algorithm.


PB0967
Pulmonary hemodynamics at rest and during exercise in patients with chronic thromboembolic pulmonary disease
A. Dhayyat 1; J. Hilde2; Ø. Jervan3; K. Stavem4; W. Ghanima5; K. Steine6
1 Ostfold Hospital Trust, Oslo, Oslo, Norway; 2 Akershus University Hospital, Oslo, Oslo, Norway; 3 Ostfold Hospital Trust, oslo, Oslo, Norway; 4 Department of pulmonology, Akershus University Hospital, oslo, Oslo, Norway; 5 Clinic of Internal Medicine, Østfold Hospital, Norway, Department of Hematology, Oslo University Hospital and Institute for Clinical Medicine, University of Oslo, Norway, Sarpsborg, Ostfold, Norway; 6 Department of cardiology, Akershus University Hospital, Lørenskog, Akershus, Norway
Background: Patients with chronic thromboembolic pulmonary disease without pulmonary hypertension at rest (CTEPD) may suffer from exercise intolerance. An important research question is therefore if this is due to abnormal hemodynamic responses in the pulmonary circulation during exercise.
Aims: To characterize pulmonary hemodynamics by exercise in patients with CTEPD by right heart catheterization and contribute to the definition of CTEPD helping to identify the main cause of exercise limitation.
Methods: 23 symptomatic patients with persistent perfusion defects on V/Q scan all diagnosed after pulmonary embolism (PE) underwent exercise right heart catheterization. The diagnosis of chronic thromboembolic pulmonary disease (CTEPD) was defined by the abscence of resting PH (mPAP >20 mmHg) proposed at the 6th World Symposium on Pulmonary Hypertension. Exercise induced pulmonary hypertension (EIPH) was defined by combining mPAP >30 mmHg and TPR >3 WU
Results: Six patients was diagnosed with PH. 17 with CTEPD. Four patients with CTEPD met the criteria for EIPH, and three CTEPD patients had borderline EIPH (mPAP ranging from 27–31 mmHg, TPR ranging from 2,5–3,0 WU). One CTEPD patient was unable to perform exercise right heart catheterization due to leg cramps.
Conclusion(s): Up till now, four out of seventeen patients with CTEPD have met the criteria for EIPH, demonstrating latent abnormal pulmonary vascular responses in nearly a quarter of patients with CTEPD. However, it is too early to draw any conclusion about the incidence of EIPH among CTED patients in this ongoing study.

PB0971
Clinical and echocardiographic variables associated with residual perfusion defects after pulmonary embolism
Ø. Jervan 1; J. Gleditsch2; S. Parker3; D. Rashid2; W. Ghanima4; K. Steine5
1 Hospital of Ostfold Norway, Fredrikstad, Ostfold, Norway; 2 Department of Radiology, Hospital of Ostfold, Kalnes, Sarpsborg, Ostfold, Norway; 3 Department of physical Medicine and Rehabilitation, Hospital of Ostfold, Kalnes, Moss, Ostfold, Norway; 4 Østfold Hospital Foundation, Sarpsborg, Ostfold, Norway; 5 Department of Cardiology, Akershus University Hospital, Lørenskog, Akershus, Norway
Background: Residual perfusion defects (RPD) after pulmonary embolism (PE) are common, and the detection of RPD is clinically relevant even in the absence of pulmonary hypertension.
Aims: The aim of this study was to explore baseline characteristics and echocardiographic findings at follow‐up associated with RPD after PE.
Methods: The study cohort is a part of the PE REHAB‐trial (NCT03405480), an ongoing randomized controlled trial to assess the effect of pulmonary rehabilitation in patients with persistent dyspnea after PE. Patients aged 18–75 years with a history of PE, confirmed by computed tomography pulmonary angiography 6–72 months earlier and persistent dyspnea corresponding to modified Medical Research Council dyspnea scale ≥ 1 were included. Participants (n = 169) completed a diagnostic work‐up consisting of standard transthoracic echocardiography and ventilation/perfusion (V/Q) scintigraphy. V/Q scintigraphy were deemed either positive or negative, according to the European Association of Nuclear Medicine criteria. Clinical data at time of PE‐diagnosis was obtained. Thrombotic burden at diagnosis was assessed using the Mean Bilateral Proximal Extension of the Clot (MBPEC)‐score, where higher number indicates more proximal emboli. Clinical and echocardiographic characteristics were explored in univariate analyses using t‐test or Mann‐Whitney U‐test. Multiple logistic regression was performed to assess the association between selected variables and the presence of residual perfusion defects.
Results: RPD were detected in 52/169 (31%) of PE‐survivors with persistent dyspnea. Higher MBPEC‐score at diagnosis and reduced pulmonary acceleration time at follow‐up were associated with RPD; adjusted odds ratios 1.76 (95% CI: 1.16–2.67, p‐value < 0.01) and 0.67 (95% CI: 0.55–0.82, p‐value < 0.001) per 10 milliseconds, respectively. Pulmonary acceleration time had greater feasibility compared to other echocardiographic measurements of right ventricular function and pulmonary hypertension.
Conclusion(s): RPD are common after pulmonary embolism. Greater thrombotic burden at diagnosis and shortened pulmonary acceleration time at echocardiographic follow‐up may help identify patients with RPD.


PB0965
The use of transthoracic echocardiography in adults hospitalized with acute pulmonary embolism
B. Azad 1; K. Steckham2; T. Azad3; C. Ainsworth2; L. Lau4; S. Mithoowani2; D. Siegal5
1 Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada, Ottawa, Ontario, Canada; 2 Department of Medicine, McMaster University, Hamilton, ON, Canada, Hamilton, Ontario, Canada; 3 Ottawa Hospital Research Institute, Ottawa, ON, Canada, Ottawa, Ontario, Canada; 4 University of Ottawa Heart Institute, Ottawa, ON, Canada, Ottawa, Ontario, Canada; 5 University of Ottawa and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada
Background: Right ventricular dysfunction (RVD) predicts adverse outcomes in patients with acute pulmonary embolism (PE). However, the use of inpatient transthoracic echocardiography (TTE) to evaluate RVD is variable and its impact on management and outcomes is uncertain, especially in the presence of other markers of RV strain (RV enlargement on CT, elevated troponin).
Aims: Our aims were to (1) characterize the use of TTE in adults hospitalized with PE, (2) explore the relationship between different markers of RVD, and (3) compare outcomes of patients managed with or without TTE.
Methods: We present preliminary data of a multicentre retrospective cohort study of consecutive adults hospitalized with acute PE at four academic hospitals in Hamilton (2) and Ottawa (2) from 2017–2018.
Results: We identified 489 patients (mean age 65 ± 15 years, 47% male). Most patients had a PE involving segmental or larger vessels (88%). TTE was conducted during hospitalization in 184 patients (38%; 44% Hamilton and 21% Ottawa). A higher proportion of patients with elevated troponin (56% vs. 30% p < 0.001) and RV strain on CT (58% vs. 29% p < 0.001) underwent TTE than those without these findings. TTE was more frequent among patients who required intensive care compared to those who did not (61% vs. 33% p < 0.001). A higher proportion of patients with elevated troponin or RV strain on CT had TTE findings of RV enlargement (50% vs. 21%, p < 0.001; 55% vs. 19% p < 0.001) and reduced RV function (50% vs. 25%, p = 0.003; 60% vs. 22% p < 0.001).
Conclusion(s): Hospitalized patients with acute PE frequently undergo TTE but utilization varies across centres. Patients with elevated troponin and RV strain on CT were more likely to have RV enlargement and impaired function on TTE. Clarification on the role of inpatient TTE in this population is required to direct appropriate use.
PB0972
Adjusted D‐dimer cutoff levels to rule out pulmonary embolism in patients hospitalized for COPD exacerbation: results from the SLICE trial
D. Jimenez 1; C. Rodriguez1; L. Jara‐Palomares2; E. Tabernero3; A. Tenes1; S. Gonzalez1; W. Briceño1; J. Lobo4; R. Morillo1; B. Bikdeli5
1 Hospital Ramon y Cajal, Madrid, Madrid, Spain; 2 Medical Surgical Unit of Respiratory Diseases. Hospital Universitario Virgen del Rocio, Sevilla, Spain. Instituto de Biomedicina de Sevilla (IbiS), Sevilla, Spain. Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Sevilla, Andalucia, Spain; 3 Hospital Cruces, Bilbao, Pais Vasco, Spain; 4 Hospital Araba, Araba, Pais Vasco, Spain; 5 Brigham and Women’s Hospital, Boston, Massachusetts, United States
Background: For patients with suspected pulmonary embolism (PE), age‐ or clinically‐adjusted D‐dimer threshold level can be used to define a negative test that safely exclude PE and reduce the use of chest imaging. However, the utility of this approach in patients hospitalized for chronic obstructive pulmonary disease (COPD) exacerbation is undefined.
Aims: To assess the clinical usefulness and diagnostic accuracy of the age‐ and clinical probability‐adjusted strategies of D‐dimer interpretation, compared with the conventional fixed threshold, in patients hospitalized for exacerbations of COPD.
Methods: We run a post hoc analysis of the patients who required hospitalization for exacerbation of COPD and were randomized to the intervention arm in the SLICE trial. Using the conventional fixed strategy as the reference, we compared the proportion of patients with a negative D‐dimer result, and the negative predictive value and sensitivity of three D‐dimer threshold strategies for initial PE or subsequent diagnosis of venous thromboembolism (VTE): the age‐adjusted strategy, the Wells‐adjusted strategy, and the YEARS‐adjusted strategy.
Results: A total of 368 patients were included. Using a conventional threshold, 182 (49.5%) patients had negative D‐dimer values, of whom 1 (0.6%) had PE diagnosed during the initial work‐up (sensitivity, 94.1%). The use of an age‐adjusted threshold increased the number of patients in whom PE could be excluded from 182 to 233 patients (63.3%), and the proportion of false‐negative findings increased to 1.7% (sensitivity, 76.5%). With the use of a D‐dimer threshold based on the Wells score or YEARS criteria, 64.4% and 71.5% had negative values, and the proportion of false‐negative findings was 2.5% (sensitivity, 64.7%) and 2.7% (sensitivity, 58.8%), respectively.
Conclusion(s): In patients hospitalized for COPD exacerbation, compared with the conventional fixed strategy, age‐ or clinically‐adjusted strategies of D‐dimer interpretation were associated with a larger proportion of patients in whom PE could be considered ruled out with a higher failure rate.

PB0973
Residual pulmonary vascular obstruction and recurrence after acute pulmonary embolism: a systematic review and meta‐analysis of individual participant data
P. Robin1; R. Le Pennec1; M. Eddy2; L. Sikora3; P. Le Roux1; M. Carrier4; F. Couturaud5; C. Tromeur6; B. Planquette7; O. Sanchez8; R. Pesavento9; M. Rodger10; M. Kovacs11; R. Mallick12; P. Salaun1; G. Le Gal 13
1 Inserm, Univ Brest, CHRU Brest, UMR 1304, GETBO, Nuclear Medicine Department, Brest, France, Brest, Bretagne, France; 2 Department of Medicine, University of Ottawa, Ottawa Hospital Research Institute, Thrombosis Research Group, Ottawa, Canada, Ottawa, Ontario, Canada; 3 Health Sciences Library, University of Ottawa, Ottawa, Ontario, Canada, Ottawa, Ontario, Canada; 4 University of Ottawa at The Ottawa Hospital and Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 5 Inserm, Univ Brest, CHRU Brest, UMR 1304, GETBO, Departement de Medecine Interne et Pneumologie, Brest, France, Brest, Bretagne, France; 6 Department of Internal Medicine and Chest Diseases, CHU de Brest, Brest, Bretagne, France; 7 APHP, Paris, Ile‐de‐France, France; 8 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 9 Department of Internal Medicine, University of Padua, Padua, Italy, Padua, Veneto, Italy, 10 Department of Medicine, McGill University, Montreal, QC, Canada, Montreal, Quebec, Canada, 11 Division of Hematology, Department of Medicine, University of Western Ontario, London, Ontario, Canada, London, Ontario, Canada, 12 Ottawa Hospital Research Institute, Ottawa, Ontario, Canada, 13 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada
Background: The optimal duration of anticoagulant therapy after a first unprovoked venous thromboembolism (VTE) is controversial due to tightly balanced risks and benefits of indefinite anticoagulation for secondary prevention of recurrence. A better assessment of the risk of recurrence after a first VTE event following discontinuation of anticoagulation is necessary to determine the optimal, individualized management strategy.
Aims: To assess the association between residual pulmonary vascular obstruction (RPVO) on planar lung scan and recurrent VTE at one year in patients with a first acute pulmonary embolism (PE) who discontinued anticoagulant therapy following an initial treatment of ≥ 3 months.
Methods: Study selection: Prospective cohort studies and randomised controlled trials in patients who experienced a first episode of objectively confirmed acute PE, completed at least 3 months of anticoagulant therapy and did not have any recurrence during this period. Datasources: MEDLINE, EMBASE, and the Cochrane Central Registry of Controlled Trials.
Results: Four studies were identified, individual data obtained for all 809 patients. RPVO (i.e. obstruction > 0%) was found in 407 patients (50.3%) after a median of 6.6 months of treatment. Recurrent VTE or death due to PE occurred in 114 patients (14.1%) for an annual risk of 6.4% (95% CI 4.7%–8.6%). Out of the 114 recurrences, 63 occurred within one year after discontinuation of anticoagulant therapy corresponding to a risk of 8.1% (6.4%, 9.8%) at 1 year. Recurrent VTE occurred in 50 of 483 (10.4%, 95% CI 6.7%–15.7%) participants with RPVO < 5%, and in 64 of 326 (19.6%, 95% CI 13.5%–27.6%) participants with RPVO ≥ 5%: hazard ratio 2.08 (95% CI 1.82–2.38).
Conclusion(s): In this individual participant data meta‐analysis, RPVO is a significant predictor of the risk of recurrent events. However, the risk of recurrent events remains too high in patients without residual perfusion defect for it to be used as a stand alone test to decide on anticoagulation discontinuation.

PB0976
CTPA in pregnant women with suspected pulmonary embolism: effect on neonatal thyroid function
M. Righini 1; A. Elias2; O. Sanchez3; J. Schmidt4; D. Aujesky5; P. Roy6; G. Le Gal7
1 Division of Angiology and Hemostasis, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland, Geneva, Geneve, Switzerland; 2 Nice University Hospital, Nice, Provence‐Alpes‐Cote d'Azur, France; 3 Innovative Therapies in Haemostasis, INSERM, Université de Paris, Paris, France, Paris, Ile‐de‐France, France; 4 Clermont‐Ferrand University Hospital, Clermont‐ferrand, Auvergne, France; 5 Insel University Hospital, Bern, Bern, Switzerland; 6 Angers University Hospital, Angers, Centre, France; 7 Ottawa University Hospital, Ottawa, Ontario, Canada
Background: Neonatal hypothyroidism is often raised as a potential concern for the use of Computed Tomography Pulmonary Angiography (CTPA) in pregnant women with suspected PE.
Aims: To assess the incidence of hypothyroidism among newborns from mothers exposed to CTPA.
Methods: Pregnant women with clinically suspected PE, included in a multicentre multinational prospective diagnostic management outcome study, based on pretest clinical probability assessment, high‐sensitivity D‐dimer testing, bilateral lower limb venous compression ultrasonography, and computed tomography pulmonary angiography (CTPA). Results of the Guthrie tests were systematically collected for newborns of all women who required CTPA as part of the diagnostic strategy. A TSH level above 15 U/ml was used to define hypothyroidism.
Results: Out of the 166 women included in the Swiss participating centers, 149 underwent a CTPA including 14 with twin pregnancies. Eight women suffered a pregnancy loss and results of the Guthrie could not be retrieved for 4 newborns. All Guthrie levels were reported as being below 15 U/ml. The incidence of neonatal hypothyroidism was 0/151 (0.0%, 95% CI: 0.0–2.5%).
Conclusion(s): We did not identify any case of neonatal hypothyroidism in our cohort of 149 pregnant women investigated for suspected PE using a CTPA. Along with previous literature data, this provides further reassuring data regarding the use of CTPA in this indication.
PB0975
The effect of rivaroxaban on the diagnostic value of D‐dimer
H. Mohamad 1; S. Vikum2; C. Jørgensen3; M. Tavoly4; L. Garabet5; W. Ghanima6
1 University of Oslo, Norway and Østfold Hospital, Norway, Oslo, Oslo, Norway; 2 Clinic of Internal Medicine, Østfold Hospital, Norway and Institute for Clinical Medicine, University of Oslo, Norway, Oslo, Oslo, Norway; 3 Ostfold hospital, Sarpsborg, Ostfold, Norway; 4 Sahlgrenska Hospital, Gothenburg, Vastra Gotaland, Sweden; 5 Department of Multidisciplinary Laboratory Medicine and Medical Biochemistry, Akershus University Hospital, Norway, Lørenskog, Akershus, Norway; 6 Clinic of Internal Medicine, Østfold Hospital, Norway, Department of Hematology, Oslo University Hospital and Institute for Clinical Medicine, University of Oslo, Norway, Sarpsborg, Ostfold, Norway
Background: Preemptive anticoagulation is widely used in the pre‐diagnostic phase of deep vein thrombosis (DVT). In some parts of the world anticoagulation is administered prior to D‐dimer testing. Direct oral anticoagulants (DOACs) have been shown to be a safe alternative empiric treatment to low‐molecular weight heparin. However, the impact of DOACs on D‐dimer in patients with suspected DVT is not well established.
Aims: This study aimed to determine the effect of empiric rivaroxaban on the diagnostic performance of D‐dimer.
Methods: D‐dimer was measured before and after the administration of one to two doses (15 mg) of rivaroxaban in 418 outpatients, referred with suspected DVT to the emergency department at Østfold hospital, Norway. To determine whether the effect of rivaroxaban significantly impacted D‐dimer results, we calculated the diagnostic performance of the test before and after the administration of rivaroxaban.
Results: Median D‐dimer values decreased from 1.0 mg/L (IQR, 0.7–2.2) to 0.9 mg/L (IQR, 0.6–2.0) and 286 (68.4%) of the patients had a decrease in D‐dimer after administration of rivaroxaban. For the patients with decreased D‐dimer, D‐dimer decreased by 18.0% and 25.3% on average for the patients who took one and two tablets of rivaroxaban, respectively. Thirty‐six patients (8.9%) with positive D‐dimer (≥ 0.5 mg/L) before taking rivaroxaban had negative D‐dimer values (<0.5 mg/L) after rivaroxaban intake (Table 1). Of these, two had DVT diagnosed by CUS and had Wells scores of 2 and −2, respectively. The sensitivity of D‐dimer decreased from 99.0% (95% CI, 94.6–99.8) to 97.0% (95% CI, 91.6–99.0) (Table 1).
Conclusion(s): Rivaroxaban administered before measuring D‐dimer may reduce D‐dimer levels leading to false negative results and reduced sensitivity. As this study lacked a control group, caution is advised in interpretation of D‐dimer results after the administration of rivaroxaban. Whether these results apply to other anticoagulant drugs should be examined in future trials.

PB0969
PEBSI – Pulmonary Embolism Bleeding Score Index
B. Dzudovic 1; B. Subotic2; A. Neskovic3; J. Matijasevic4; S. Salinger5; J. Dzudovic6; S. Obradovic2
1 Clinic of Emergency Internal Medicine, Military Medical Academy, Belgrade, Not Applicable, Serbia; 2 Clinic of Cardiology, Military Medical Academy, Belgrade, Vojvodina, Serbia; 3 Department of Cardiology, Clinical Hospital Centre Zemun, Belgrade, Vojvodina, Serbia; 4 Clinic of Intensive Pulmonology, Institute of Pulmonary Diseases Vojvodina, Sremska Kamenica, Novi Sad, Vojvodina, Serbia; 5 Clinic of Cardiology, Clinical Centre Nis, Nis, Vojvodina, Serbia; 6 National Poison Control Center, Military Medical Academy, Belgrade, Vojvodina, Serbia
Background: Estimation of bleeding risk is an unmet need for individualized therapy in acute pulmonary embolism (PE) patients with increased mortality risk.
Aims: To form a new score that is easy to use and can detect PE patients at low risk for major bleeding (MB) who may benefit from thrombolysis.
Methods: This is a retrospective cross‐sectional study that included consecutive PE patients who received thrombolysis using a tissue‐plasminogen activator (tPA). Patients’ characteristics on admission to the hospital were analyzed with regard to the occurrence of major bleeding during the first 7 days. Major bleeding was defined according to the modified International Society of Thrombosis and Haemostasis (ISTH) criteria (“overt” bleeding is the only modification from the original criteria). Pulmonary embolism bleeding score index (PEBSI) was created using multivariate regression analyses, and finely, the dichotomous index was used for the discrimination of patients at low risk for MB from those at high risk.
Results: Overall 367 PE patients were treated with tPA during the 6‐year period (2015–2021). Among them, 29 (7.9%) fulfilled the criteria for MB. Five factors were identified as significantly associated with MB and were used to build the PEBSI score: previous bleeding, recent surgery, diabetes, the use of drugs that could be associated with bleeding, and anemia. PEBSI score showed significant prediction power for 7‐day MB revealing a c‐index value of 0.794 (95CI% 0.698–0.889). Patients with PEBSI scores of 0 or 1 had a low risk for MB (2.8%) and those with scores > 1 had a high risk for MB (18.6%) (p < 0.001). Internal validation of the PEBSI score using a randomly, equally split method confirmed the discriminative value of the PEBSI score.
Conclusion(s): Novel PEBSI score has significant power to discriminate patients at low risk for MB on thrombolytic therapy from those at high risk.


VTE Epidemiology
PB1330
Characteristics and trends of venous thromboembolism – Local experience
D. Kigitovica; V. Gibietis; E. Rusa; S. Strautmane; A. Zaicenko; K. Dzirnieks; J. Birzulis; A. Skride
Riga Stradins University, Riga, Riga, Latvia
Background: Venous thromboembolism (VTE) is an increasingly recognised condition due to improved radiological visualisation and clinical recognition; however, real‐life experience differs between countries. VTE is still associated with significant mortality.
Aims: We aimed to assess VTE‐related temporal trends in a tertiary hospital patient cohort.
Methods: The prospective cohort study, conducted in a single university hospital from 2014 till 2021, included patients ≥18 years presenting with symptomatic acute VTE. Statistical analyses were conducted using SPSS 23.0.
Results: Between 2014 and 2021 a total of 648 patients were admitted to the hospital with VTE. In 2014–2017 in comparison to 2018–2021, body mass index, patient age did not differ in patients with VTE, and had no impact on mortality (p > 0.05). In total, 19.4% of patients were diagnosed with cancer, with trend to increase in last years among patients with VTE (p < 0.05). During follow‐up 52.3% (n = 66) of patients with cancer died, 30‐day mortality was 37.9% (n = 25), 1‐year mortality was 89.4%. Out of 648 patients 172 died, 30‐day mortality was 40.1%, impact of cancer on mortality (p < 0.05). Episode of VTE after surgical operation was observed in 11% of patients that were admitted to the hospital with no significant decrease in incidence in last years and no impact on mortality (p > 0.05). Thromboprophylaxis after surgery was not prescribed to 70.4% of patients. Immobility before episode of PE was noted in 15.6% of patients. Pulmonary artery systolic pressure >50 mmHg on echocardiography was noticed in 21.3% or 66 patients, only 2% of patients revealed CTEPH confirmed by right heart catheterisation after episode of VTE.
Conclusion(s): Mortality of VTE might be high due to advanced stage of concomitant diseases. Long‐term complications of VTE are underevaluated. Funding: Support for involving doctoral students in scientific research and studies. Project/agreement No.8.2.2.0/20/I/004
PB1321
Focused on cerebral venous thrombosis
S. Herrero Martin 1; J. Hernández Cristobal2; M. Mora Argumanez3
1 Universitary Guadalajara Hospital, Guadalajara, Castilla‐La Mancha, Spain; 2 Universitary Hospital Guadalajara, Guadalajara, Castilla‐La Mancha, Spain; 3 Universitary Hospital Guadalajra, Guadalajara, Castilla‐La Mancha, Spain
Background: Cerebral venous thrombosis (CVT) is a major cause of stroke in young adults. Is more frequent in women, it may appear related to pregnancy or the use of hormonal contraceptives. Its symptoms are nonspecific, often with a normal neurological examination. Its diagnosis is based on imaging tests and in most cases, if treatment is started early, the prognosis may be favorable.
Aims: Describe the characteristics of CVT in patients that we have had in our clinic.
Methods: We compiled data from patients with CVT followed up in our hemostasis consultation in the last 6 years. Patient data were collected through hospital medical records.
Results: We have a series of 15 cases, most of them women (73.3%). The average age of the series is 38.6 years (range from newborn to 64 years). The most frequent symptoms were epileptic seizures in 40% and headache in 33.3%. In 45.5% of women, CVT was related to pregnancy or the puerperium, and in 27.3% of the women it was associated with taking oral contraceptives. Thrombophilia (genetic or acquired) has been found to be involved in 33.3% of cases. The remaining cases were associated with breast cancer (1), trauma (1), severe anemia (1), and SARS‐CoV vaccines (1). In two cases (13.3%) decompressive neurosurgery was required. In two cases (13,3%), direct oral anticoagulant (apixaban and dabigatran) were used. In 60% of cases anticonvulsant treatment was associated. In 40% of cases, the evolution was very favorable without sequelae, recurrent headache was found in 53,3% of cases.
Conclusion(s): CVT is a rare but important cause of stroke in young adults. CVT it is not easy to diagnose, partly due to its relative rarity, its multiple and various clinical manifestations and interpret correct brain imaging. We must keep it in mind to avoid delay in diagnosis and treatment.


VPB1342
Aetiological profile of inferior veina cava thrombosis
R. Ben Salah 1; F. Mkaouar2; O. Frikha2; F. Frikha2; Z. Bahloul3
1 Hedi Chaker Hospital, Sfax, Sfax, Tunisia; 2 Departement of Internal Medicine, Hédi Chaker Hospital, Sfax, Sfax, Tunisia; 3 University of Sfax, SFAX, Sfax, Tunisia
Background: Venous thromboses of the inferior vena cava (ITC) is an infrequent localization and to date there are few series published on the subject.
Aims: The aim of our study concerning TCI is to describe the etiological profile of the patients ITC, the and the treatments used.
Methods: In this retrospective study, we reviewed all the records of patients hospitalized in the internal medicine department for TCI over a period from January 1, 1996 to February 2021.
Results: They were 12 men and 10 women with an average age of 37.7 years (range: 23–80 years). The clinical symptoms included: edema of the lower limbs (11 cases), ascites (2 cases) and abdominal collateral circulation (8 cases). The diagnosis was confirmed by abdominal venous Doppler echo in 11 patients, by abdominal CT angiography (7 cases) and by conventional venography in one case. An association with a pelvic manifestation in 3 cases: an ovaritis 1 case, a pyelitis 1 case and an epididymitis 1 case. The favoring factors retained were the post partum in 2 cases and a traumatic fracture in the lower limb in one patient. An etiological investigation was carried out in all cases because of the unusual character. Behçet's disease was retained in 6 cases, APS in 5 cases, resistance to activated protein C in 2 cases and leiomyosarcoma‐like neoplasia of the inferior vena cava in 1 case. Anticoagulant treatment was administered in all cases for a period of 3 months to 8 years discussed on a case‐by‐case basis with operative management and placement of a prosthesis in the case of leiomyosarcoma.
Conclusion(s): The pathology of the inferior vena cava is very representative of venous pathology. Early diagnosis is necessary and anticoagulant treatment is essential to improve the vital prognosis.
VPB1351
The Manipal deep venous thrombosis registry: Clinical characterization, treatment patterns and 2‐year outcomes of DVT patients in India
A. Singh 1; D. Shivakumar2; M. Virdiana2; A. Srikanth2; T. Devasia3
1 Department of Medicine, Kasturba Medical College & Hospital, Manipal Academy of Higher Education, Manipal, India, Manipal, Karnataka, India; 2 Kasturba Medical College & Hospital, Manipal Academy of Higher Education, Manipal, India, Manipal, Karnataka, India; 3 Department of Cardiology, Kasturba Medical College & Hospital, Manipal Academy of Higher Education, Manipal, India, Manipal, Karnataka, India
Background: Deep vein thrombosis (DVT) is associated with significant morbidity and mortality due to its recurrence and complications. The recent substantial data on DVT and its complications and long‐term outcomes are limited in India.
Aims: This Manipal DVT registry is describing the clinical characteristics, treatment patterns, and outcomes of DVT patients.
Methods: This is a retrospective observational registry study carried out on all patients with a diagnosis of DVT confirmed by Doppler ultrasonography between 2014 and 2018 in a tertiary care center in South India. We have followed up the patients for 2‐years for the DVT‐related outcomes and correlated with the risk factors.
Results: A total of 612 eligible patients of DVT were included with the mean age of 49.5 ± 15.3 years. Among all, 420 (68.6%) were males and 192 (31.4%) were females. One hundred eleven (18%) patients who have had a previous history of DVT and hypertension, malignancy, trauma/surgery, and varicose veins were presented in 154 (25.2%), 44 (7.2%), 96 (15.7%), and 143 (23.4%) patients respectively. Four hundred forty‐six (72.9%) and 96 (11.6%) patients received warfarin and newer oral anticoagulants (NOAC) respectively. In composite major adverse outcomes, pulmonary embolism 66 (10.8%) was major followed by DVT recurrence 33 (5.4%) and death 16 (2.6%). A total of 123 (20.1%) patients were readmitted with DVT‐related complications. The previous history of DVT and malignancy and smoking were the significant risk factors associated with 2‐year composite outcomes with a p‐value < 0.05.
Conclusion(s): Manipal DVT registry provides information about risk factors, clinical presentation, and treatment of DVT. The previous history of DVT, smoking, and presence of varicose veins, discoloration of the limb is associated with increased risk of readmission, major adverse cardiovascular events (MACE), or death. Patients with DVT associated with malignancy have reduced survival.


VPB1345
Assessment of venous thromboembolism risk factors in hospitalized patients at the University of Nigeria Teaching Hospital, Enugu Nigeria
E. Ezigbo 1; T. Nwagha2; V. Nwuzor3
1 University of Nigeria, Enugu Campus, ENUGU, Enugu, Nigeria; 2 Department of Haematology and Immunology, University of Nigeria Teaching Hospital, Enugu, Nigeria, Enugu, Enugu, Nigeria; 3 Department of Medical Laboratory Sciences, University of Nigeria Enugu Campus, Enugu, Nigeria, Enugu, Enugu, Nigeria
Background: Venous thromboembolism (VTE) is a common, lethal disease that recurs frequently with serious long‐term complications. To improve survival and prevent complications, risk factors need to be evaluated in hospital patients. This will help to modify exposures and target prophylaxis to the appropriate individual. There is a paucity of data for VTE risk assessment in Nigeria hospitals.
Aims: This study, assessed VTE risk factors in hospitalized patients at the University of Nigeria Teaching Hospital, Enugu.
Methods: This was an observational, case‐control study on the prevalence of VTE risk factors in hospital patients. Three hundred and fifty (350) patients were recruited for the study: This comprised 150 medical patients, 140 surgical patients and a population of 60 healthy subjects served as the control group. Subjects were evaluated once using the Caprini risk assessment model (RAM).
Results: Over 50% of all hospitalized patients, were at risk for VTE. Surgical patients were at a higher risk than medical patients were. Hemoglobin concentration was associated with the risk of VTE in surgical patients, while d‐dimer was associated with VTE risk in medical patients.
Conclusion(s): This study shows a high prevalence of VTE risk factors among hospitalized patients at the University of Nigeria Teaching Hospital.

PB1338
Impact of COVID‐19 pandemic on VTE investigation and diagnosis rate in Belfast Health and Social Care Trust, Northern Ireland
N. Sotiropoulou 1; S. Hannan2; G. Benson3; R. Gooding4; C. Neill3; C. Corrigan4
1 Queens University Belfast, Enniskillen, Northern Ireland, United Kingdom; 2 Queens University Belfast, Belfast, Northern Ireland, United Kingdom; 3 Belfast HSCT, Belfast, Northern Ireland, United Kingdom; 4 Belfast Health and Social Care Trust, Belfast, Northern Ireland, United Kingdom
Background: COVID‐19 infection is associated with an increased risk of thrombosis including venous thromboembolism (VTE). We investigated the impact of the COVID‐19 pandemic on the number of scans performed to investigate suspected VTE and the positivity rate in a large health trust.
Aims: Compare number of scans performed to investigate suspected VTE in 2020 and 2021 with the number performed in 2019 prior to the COVID‐19 pandemic. We then looked at the rate of positive results to determine if our local experience reflects the global picture. We also calculated the rate of hospital acquired thrombosis during the same time frame to determine if this rate was impacted by the pandemic.
Methods: We searched all radiology systems for number of CT Pulmonary Angiogram (CTPA), ventilation perfusion (V/Q) scan, ultrasound (USS) Doppler (lower and upper limbs) performed and number positive for VTE. We then cross referenced with hospital admission in preceding 90 days to determine number of hospital acquired thrombosis (HAT).
Results: Despite the onset of COVID‐19 pandemic there was a 15% reduction in number of scans performed in 2020 reflecting initial public health campaign and down turn of all routine services. However by 2021 there was a 25% increase in scans compared to pre‐pandemic levels. Positivity rate was similar between years (11–14%). As was the proportion of hospital acquired thrombosis (40–43%). Incidence of VTE events rose by 31% – with 30.4% of all PEs diagnosed in 2021 associated with COVID (Table 1).
Conclusion(s): Incidence of confirmed VTE has risen markedly compared to pre‐pandemic levels reflecting global experience. Our study highlights this rise in potentially avoidable morbidity and significant resource implications for our local healthcare infrastructure.

PB1335
Prevalence of venous thromboembolism and its associations with demographics, disease spectrum and hydroxyurea use in a large racially homogenous population of sickle cell disease patients in Nigeria
H. Okoye 1; C. Ezekekwu2; T. Nwagha3; K. Korubo4; H. Omunakwe5; O. Nnachi6; A. Madu2; B. Nwogoh7; C. Efobi8; E. Muoghalu9; C. Nonyelu10; A. Okoye6; O. Obodo11; C. Ugwu9; M. Egolum12; o. nnachi6; I. Okpala2
1 College of Medicine University of Nigeria Ituku Ozalla, Enugu, Enugu, Nigeria; 2 College of Medicine, University of Nigeria, Ituku Ozalla campus, Enugu, Enugu, Enugu, Nigeria; 3 College of Medicine University of Nigeria/University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria; 4 University of Port Harcourt Teaching Hospital, Port Harcourt, Rivers, Nigeria; 5 Department of Haematology, Rivers State University Teaching Hospital Rivers state, Port Harcourt, Rivers, Nigeria; 6 Alex Ekwueme Federal University Teachinh Hospital Abakaliki, Abakaliki, Ebonyi, Nigeria; 7 University of Benin, Benin, Edo, Nigeria; 8 Department of Haematology and Blood Transfusion, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus Anambra state, Awka, Anambra, Nigeria; 9 UNTH, Enugu, Enugu, Nigeria, 10 College of Medicine, University of Nigeria, Ituku Ozalla campus, Enugu, Enugu, Ekiti, Nigeria, 11 University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria, 12 UNTH, ENUGU, Enugu, Nigeria
Background: Sickle cell disease (SCD) is associated with altered haemostatic balance. There are reports of high prevalence of venous thromboembolism (VTE) in SCD. There are no previous studies reporting the prevalence of VTE in SCD in Nigeria, a country which bears the greatest burden of SCD. We hypothesize that there is no significant association between patient demographics, disease phenotype, clinical spectrum, organ damage and use of hydroxyurea and VTE.
Aims: To determine the prevalence of VTE in SCD in Nigeria and its associations.
Methods: This is a multicentre retrospective study where case notes of adult (age ≥18 years) SCD patients receiving care at the centres were reviewed. Information on demographics, steady state hemogram parameters, clinical phenotypes, duration of follow‐up, history of VTE including risk factors and management were collected.
Results: Out of the 509 SCD patients with a median (IQR) duration of follow‐up of 2 (1–5) years., 10 (2.0%) had VTE (9 DVT and PE). Their median (IQR) age was 27 (22.8–30.3) years, out of which 4 were below 25 years, 5 were between 25 and 39 years and only 1 was ≥40 years. Identifiable risk factors for VTE include positive family history (2, 20%) surgery, splenectomy, paraplegia, and cancer associated thrombosis (1, 10% each). No identifiable risk in 4. There was no significant association between VTE and age, gender and haemoglobin phenotype. VTE was significantly associated with viscosity/vaso‐occlusive phenotype {vaso‐occlusive crisis – p‐value 0.035; acute chest syndrome – p‐value 0.002, odd ratio 8 95% CI 2.2–28.9; osteonecrosis‐ p‐value 0.012 OR 5.24 95% CI1.45–18.91 Pulmonary hypertension and stroke also showed significant association with VTE – p‐value 0.001 OR 23.3 95% CI 5.18–105.06 and p‐value 0.032 OR 9.35 95% CI 0.87–53.25. Hydroxyurea use had no significant association with VTE.
Conclusion(s): prevalence of VTE in Nigeria is low and it is significantly associated with viscosity/vaso‐occlusive phenotype. pulmonary hypertension and stroke.


PB1317
Clinical series of patients with non‐bacterial thrombotic endocarditis not associated with cancer or lupus anticoagulant/antiphospholipid antibodies
A. Casanegra 1; P. Patrzalek2; D. Houghton1; R. Kurmann1; W. Kuczmik3; D. Hodge4; A. Azza5; K. Klarich1; W. Wysokinski1
1 Mayo Clinic, Rochester, Minnesota, United States; 2 Wroclaw Medical University, Wroclaw, Dolnoslaskie, Poland; 3 Medical University of Silesia, Katowice, Slaskie, Poland; 4 Mayo Clinic, Jacksonville, Florida, United States; 5 Mayo Clinic, Rochester, Minnesota, United States
Background: Non‐bacterial thrombotic endocarditis (NBTE) is either related to cancer or lupus anticoagulant/antiphospholipid antibodies (LA/aPLa) with or without connective tissue disease.
Aims: Describe NBTE not associated with cancer or LA/aPLa.
Methods: An electronic search of medical records of patients from the Mayo Clinic enterprise was performed to identify all patients with NBTE, without underlying cancer or LA/aPLa. All patients had negative evaluation for connective tissue disease, negative testing for LA by RVVVT method, for anticardiolipin and anti‐beta 2 glycoprotein 1 both IgG and IgM antibodies. All patients had negative blood cultures and an infectious disease consult to rule out infectious endocarditis.
Results: Sixteen patients (12 females, mean age 53.8 ± 14.9, range: 29–88 years) had NBTE diagnosed by transesophageal echocardiogram between June 2011 and December 2021. Twelve patients had mitral valves (MV) affected, 10 with diffuse valve thickening; thrombi were attached to the mitral in 9 and to the ventricular side in 4 cases; 8 were attached to the tip and 2 involving commissure; 6 thrombi were mobile. The NBTE size ranged from 0.27 to 1.31cm. Two patients had moderate MV regurgitation, and one moderate/severe stenosis. Eight patients had aortic valve (AV) affected: 6 with global and 2 partial valve thickening; 4 thrombi were attached to the ventricular and 3 aortic side; 3 were attached to the tip and 3 involving commissure; 3 were mobile masses. AV NBTE size ranged from 0.46 to 1.58cm. Two patients had moderate AV regurgitation. NBTE was associated with embolic complications to the brain (n = 11), kidney and coronaries (n = 2 each), and spleen (n = 1). All patients but one were treated with anticoagulation (11 warfarin, 4 apixaban).
Conclusion(s): This first clinical series of patients with cancer negative and LA/aPLa negative NBTE shows that majority of patients are women, both mitral and aortic valves are affected with predominately central nervous system embolic destination.
PB1334
The (T) thrombosis (I) in patients with (L) lower (L) limb (I) injuries (R) requiring (I) immobilisation (TILLIRI) study: A prospective observational multicentre study – Early Results
T. O Halloran 1; M. Watts1; S. Egan2; M. O’Donoghue3; B. Bleach4; T. Breslin5; M. Alshafie5; J. O'Driscoll5; B. Nemeth6; S. Cannegieter6; S. O'Rourke7; M. Barlow7; N. Salter8; A. Wakai9; N. O'Connell10; M. Nolan11; M. Crowley12; A. Burke13; M. Maxwell14; D. O Keeffe1
1 University Hospital Limerick, Limerick, Limerick, Ireland; 2 University Hospital Limerick, University of Limerick, limerick, Limerick, Ireland; 3 St Johns Hospital, University of Limerick Hospital Group, limerick, Limerick, Ireland; 4 Ennis Hospital, University of Limerick Hospital Group, Ennis, Clare, Ireland; 5 Mater Misericordiae University Hospital, Dublin, Dublin, Ireland; 6 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 7 Midland Regional Hospital Tullamore, Tullamore, Offaly, Ireland; 8 St Vincents University Hospital, Dublin, Dublin, Ireland; 9 Beaumont Hospital, Dublin, Dublin, Ireland, 10 St. James Hospital, Dublin, Ireland, 11 St James's Hospital, Dublin, Dublin, Ireland, 12 Cork University Hospital, Cork, Cork, Ireland, 13 Sligo University Hospital, Sligo, Sligo, Ireland, 14 Sligo University Hospital, Dublin, Dublin, Ireland
Background: Patients requiring temporary lower limb immobilisation after an injury have an increased venous thromboembolism (VTE) risk. The magnitude of this reported risk varies between studies, likely due to heterogeneity in study design. Contextualizing risks within populations is therefore challenging. Moreover, contributing risk factors remain incompletely characterized.
Aims: We aim to determine the 90‐day incidence of symptomatic VTE after temporary lower limb immobilisation and to externally validate the “Thrombosis Risk Prediction for patients with cast immobilisation (TRiP (cast))” risk assessment model (RAM), which was developed using data from the MEGA case‐control study (Nemeth B (Cannegieter S) et al. DOI: 10.1016/j.eclinm.2020.100270).
Methods: TILLIRI is a prospective, multicentre observational study including 12 participating sites within the Irish Network for VTE Research (INViTE). Consecutive patients aged 18 years and older with an immobilised injured lower limb are eligible for inclusion. The primary outome is the incidence of symptomatic VTE at 90 days. We also aim to externally validate the TRiP (cast) RAM within the TILLIRI cohort using clinical variables collected at the index visit. In contrast to earlier studies, this is a highly unselected population. Our target sample size is 3900 patients.
Results: 912 patients have been included to date from 12 sites. Follow up is complete for 770. 691/770 90% did not have pharmacological thromboprophylaxis prescribed. In line with our study protocol, an interim analysis of 90‐day VTE rate will be performed after 1000 patients have completed follow‐up and all data recorded centrally. To date, the mean (SD) TRiP (cast) score is 5.4 ± 2.4.
Conclusion(s): Predicted and actual recruitment are similar in this large prospective observational study within INViTE, demonstrating feasibility. The TILLIRI study is recruiting a highly unselected population, an important consideration for generalizability of outcomes. We anticipate that our planned interim analysis will be conducted in Q1 2022 and that recruitment will complete on target.
PB1319
Management of superficial venous thrombosis based on individual risk profiles: protocol for the development and validation of three prognostic prediction models in large primary care cohorts
F. van Royen1; M. van Smeden2; C. Moons3; F. Rutten2; G. Geersing 2
1 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands, Utrecht, Utrecht, Netherlands; 2 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 3 UMC Utrecht/Utrecht University, Utrecht, Utrecht, Netherlands
Background: Superficial venous thrombosis (SVT) is considered a benign thrombotic condition in most patients. However, it can also cause serious complications such as clot progression to deep venous thrombosis (DVT) and pulmonary embolism (PE). Although most SVT patients are encountered in primary healthcare, studies on SVT nearly all were focused on patients seen in the hospital setting.
Aims: To describe the protocol for the development and external validation of three prognostic prediction models for relevant clinical outcomes in SVT patients seen in primary care: (i) prolonged (painful) symptoms within 14 days since SVT diagnosis, (ii) for clot progression to DVT or PE within 45 days, and (iii) for clot recurrence within 12 months.
Methods: Data will be used from four primary care routine healthcare registries from both the Netherlands and the UK; one will be used for the development of the prediction models and the remaining three will be used as external validation cohorts. The study population will consist of patients ≥18 years with a diagnosis of SVT. Selection of SVT cases will be based on a combination of ICPC/READ/Snowmed coding and free text clinical symptoms.
Results: Predictors considered are sex, age, BMI, clinical SVT characteristics, and co‐morbidities including: (history of any) cardiovascular disease, diabetes, autoimmune disease, malignancy, thrombophilia, pregnancy and presence of varicose veins. The prediction models will be developed using multivariable logistic regression analysis techniques for model i and ii and for model iii a Cox proportional hazards model will be used. They will be validated by internal‐external cross‐validation as well as external validation.
Conclusion(s): There are currently no prediction models available for predicting the risk of serious complications for SVT patients presenting in primary care settings. The described prediction models should help identify patients at highest risk for complications and support clinical decision‐making for this understudied disorder.

PB1332
Cerebral venous thrombosis: Clinical, radiological, biological and etiological characteristics of a French Prospective Cohort: Comparison with ISCVT cohort
A. Triquenot Bagan1; I. Crassard2; L. Drouet3; P. Morange4; V. Wolff5; G. Godenèche6; V. Le Cam Duchez 7
1 Neurology Department, ROUEN, Haute‐Normandie, France; 2 Neurology Department, PARIS, Ile‐de‐France, France; 3 Cardiology, Lariboisière, APHP, Paris, Ile‐de‐France, France; 4 C2VN, INRAE, INSERM Aix‐Marseille University, Marseille, Provence‐Alpes‐Cote d'Azur, France; 5 Neurology departement, STRASBOURG, Alsace, France; 6 Neurology departement, LA ROCHELLE, Bretagne, France; 7 INSERM U1096 EnVI, Vascular Hemostasis Unit, Rouen University Hospital, Rouen, Haute‐Normandie, France
Background: Cerebral venous thrombosis (CVT) is a rare disease with highly variable clinical presentation and outcome. Etiological assessment may be negative. T. The mechanisms involved in this variability remain unknown.
Aims: The aim of this multicenter French study registered on ClinicalTrials.gov (NCT02013635) was therefore to prospectively recruit a cohort of patients with cerebral venous thrombosis (FPCCVT) in order to study thrombin generation and clot degradation and to evaluate their influence on clinical radiological characteristics.
Methods: This prospective, multicenter, French study was conducted from July 2011 to September 2016. Consecutive patients (aged >15 years) referred to the stroke units of 21 French centers and who had a diagnosis of symptomatic CVT were included. The diagnosis of CVT had to be confirmed by imaging. Clinical, radiological, biological and etiological characteristics were recorded at baseline, at acute phase, at 3 months and at last follow‐up visit. Thrombophilia screening and the choice of treatment were performed by the attending physician. All data were compared with data from the International Study on CVT.
Results: Two‐hundred‐and‐thirty‐one patients were included: 117 (50.6%) had isolated intracranial hypertension, 96 (41.5%) had focal syndrome. During hospitalization, 229 (99.1%) patients received anticoagulant treatment. Median length of hospital stay was 10 days. Five patients died during hospitalization (2.2%). At 3 months, 216 patients (97.0%) had follow‐up with neurological data based on an outpatient visit. The mean duration of antithrombotic treatment was 9 months and the mean time to last follow‐up was 10.5 months. At the end of follow‐up, 8 patients had died and 26 patients were lost to follow‐up. At least one risk factor was identified in 200 patients.
Conclusion(s): We demonstrated that the FPCCVT cohort had characteristics similar to the historical ISCVT cohort. Nevertheless, the initial clinical presentation was less severe in our study probably due to an improvement in diagnostic methods between the two studies.
PB1340
Quality of life after a first venous thrombosis in elderly
H. Wang 1; M. Cushman2; F. Rosendaal3; A. van Hylckama Vlieg4
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Larner College of Medicine at the University of Vermont, Colchester, Vermont, United States; 3 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, the Netherlands, Leiden, Zuid‐Holland, Netherlands; 4 Leiden University, Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: Limited information exists regarding short and long‐term effects of venous thrombosis on quality of life in the elderly.
Aims: To assess the impact of a first venous thrombosis on quality of life in older patients with venous thrombosis.
Methods: Analyses were performed in the AT‐AGE study, a multi‐center case‐control study performed in Vermont, USA and Leiden, NL, comprising of 401 cases with a first VT and 431 control subjects, all aged ≥70 years. We measured health‐related quality of life using the generic SF‐36 questionnaire. All patients filled in the questionnaire twice, i.e., soon after the venous thrombosis (visit 1) and one year after the venous thrombosis (visit 2) while controls filled in the questionnaire once. We used a difference of ≥5 points in any subscale of the SF36 as clinically or socially relevant. The study was approved by the Medical Ethical Committee of the Leiden University Medical Center and by the Committee of Human Research of the University of Vermont.
Results: Except for mental health, the quality of life of patients was decreased after their VT compared with that of controls (visit 1: mean difference for physical score: −15.9 (−19.3 to −12.4)) and for mental score: −12.5 (−15.7 to −9.3)) (Table). Apart from general health, the quality of life of patients was improved in the year after venous thrombosis, i.e., the physical score was 49.5 (46.9–52) at visit 1 and 55.2 (52.4–58) at visit 2 (Figure).
Conclusion(s): Quality of life of patients soon after the venous thrombosis was impaired in all domains (except for mental health) compared with the controls in the elderly. Quality of life of patients was improved one year after the venous thrombosis (except for general health). More detailed analysis regarding disease specific quality of life will be available at ISTH conference.


VPB1346
Frequency and risk factors associated with thromboembolic events in a Sickle Cell Disease cohort
D. Werneck Rodrigues1; L. Fonseca 2; S. Souza3; T. Espósito2; N. Magalhães3; A. Santos2; R. Almeida4; J. Almeida1; M. Thomaz1
1 Fundação Hemominas, Juiz de Fora, Minas Gerais, Brazil; 2 Centro Universitário Presidente Antonio Carlos – UNIPAC, Juiz de Fora, Minas Gerais, Brazil; 3 Faculdade de Ciências Médicas e da Saúde de Juiz de Fora ‐SUPREMA, Juiz de Fora, Minas Gerais, Brazil; 4 Faculdade de Ciências Médicas e da Saúde de Juiz de Fora – SUPREMA, Juiz de Fora, Minas Gerais, Brazil
Background: Sickle cell disease (SCD) has high risk factors for venous stasis, endothelium activation and blood hypercoagulability, making the patients particularly vulnerable to thromboembolic events (TE) which is one of the main causes of hospitalization in SCD.
Aims: Identify the frequency of TE in a cohort of patients with SCD, correlating epidemiological and nosological data.
Methods: This is a longitudinal study cohort type carried out at Fundação Hemominas Juiz de Fora (JF) from March, 2013 to 2017. Figure 1 shows a flowchart of selection, inclusion and exclusion of research subjects. Adults and children of both genders were included. The variables analyzed were: type of SCD, gender, age, smoking, pregnancy, oxygen saturation (SpO2), arterial hypertension (AH), ACS, TE and pulmonary hypertension (PH). The research was approved by local REC. The ID grant was HHSN2682011000071 – National Institute of Health.
Results: Patients were evaluated for epidemiological and SCD complications data. The results were described in Table 1. Most patients were genotype HbSS/HbSβ0 (68.4%), and self‐reported as mixed or black race (~80.0%). Smoking was more present within men (35.4%). 59.2% of women had already been pregnant. Pulmonary embolism (PE) was exclusive in adults (1.6%), AH and PH were more common in this age group too. High rates of ACS were obtained, occurring in 64.2% of children and 58.9% of adults. Even though, infarctive stroke was more present in children (4.0%).
Conclusion(s): The occurrence of TE was associated with the presence of severe SCD genotypes, pregnancy and smoking. Two of these factors are not supposed to occur in children, what may justify the absence of PE events in this age group of our cohort. Also, high rates of ACS can be underestimating TE events count, since their clinical manifestations are similar.


PB1339
Plasma levels of P‐selectin and future risk of venous thromboembolism
S. Swamy 1; S. Brækkan2; T. Ueland3; J. Hansen4; O. Snir5
1 Thrombosis Research and Expertise Center (TREC), Department of Clinical Medicine, University of Tromsø – The Arctic University of Norway, Tromsø, Norway, Tromsø, Troms, Norway; 2 UiT – The Arctic University of Norway, Tromsø, Troms, Norway; 3 Research Institute of Internal Medicine, Oslo University Hospital, Rikshospitalet, Oslo, Norway, Oslo, Oslo, Norway; 4 Thrombosis Research Center, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway; 5 UiT – The Arctic University of Norway, Tromso, Troms, Norway
Background: Elevated P‐selectin levels have been observed following acute venous thromboembolism (VTE) and are reported to predict recurrent VTE and cancer‐associated VTE. Whether plasma P‐selectin levels are associated with risk of future incident VTE in the general population remains unclear.
Aims: To investigate the association between plasma P‐selectin levels and risk of future incident VTE.
Methods: We performed a nested case‐control study in 415 VTE patients and 843 age‐ and sex‐matched controls derived from the general population (Tromsø IV cohort 1994–2007). The levels of soluble P‐selectin were measured in plasma samples collected at baseline (1994/95) using ELISA. Samples were categorized according to quartile cut‐offs in the control population. Logistic regression models were used to estimate odds ratios (OR) for VTE across quartiles of plasma P‐selectin in overall‐ and sex‐stratified analysis after adjustment for age, sex, body mass index and C‐reactive protein levels.
Results: We found no association between P‐selectin levels in plasma and risk of VTE in the overall analyses. However, sex‐stratified analyses revealed that women with plasma P‐selectin levels in the highest quartile (>44.3 ng/mL) had higher risk of VTE (OR 1.63, 95% CI: 1.01–2.64) compared with women with plasma P‐selectin levels in the lowest quartile (≤29.9 ng/mL) (Table 1). In contrast, higher levels of P‐selectin were apparently associated with lower risk of VTE in men (OR for highest vs. lowest quartile of P‐selectin: 0.69, 95% CI: 0.42–1.15). The observed associations were stronger when the time between blood sampling and VTE was shorter (Figure 1).
Conclusion(s): Our findings suggest that elevated levels of plasma P‐selectin are associated with an increased risk of future VTE in women but not in men.


VPB1341
Mortality associated with venous thrombosis in Brazil: Evidence of inequalities between low/middle‐ and high‐income countries
I. Batista 1; S. Rezende2; M. Cherchiglia3; E. de Paula4; F. de Andrade Orsi5
1 School of Medical Sciences of the University of Campinas, Campinas, Brazil, Recife, Pernambuco, Brazil; 2 Department of Internal Medicine, Faculty of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 3 Department of Epidemiology of the Federal University of Minas Gerais, Belo Horizonte, Brazil, Belo Horizonte, Minas Gerais, Brazil; 4 School of Medical Sciences of the University of Campinas, Campinas, Sao Paulo, Brazil; 5 School of Medical Sciences of the University of Campinas, Campinas, Brazil, Campinas, Sao Paulo, Brazil
Background: Despite evidence of reducing venous thrombosis (VT) mortality rates in high‐income countries, scarce information is available on VT‐related mortality in Brazil or other low/middle income countries.
Aims: To describe VT‐related deaths in Brazil and to evaluate its impact on overall mortality over the years.
Methods: By accessing the Brazilian database of death certificates, we determined the proportion of deaths related to pulmonary embolism (PE) and other VT and the main underlying associated causes of death between 2000 to 2018. The mortality trends were analysed in the entire population as well as divided by region, sex and age.
Results: VT was reported in 349.298 death registries, 271.159 of which were PE‐related, revealing that VT‐related deaths accounted for 1.6% of all deaths. These rates remained stable during the study period as opposed to the declining VT‐related deaths rates reported in high‐income countries (Figure 1). Cancer (40.6%) and injuries (36.5%) were the most common underlying causes associated with VT‐related deaths in Brazil. The magnitude of the association between injury and VT‐related death increased as the income of the Brazilian region decreased, outgrowing the impact of cancer on VT‐related death in some poor areas (Table 1). Trends on VT‐related death were similar between sex and age groups, except for infants. In this age group, congenital and infectious diseases were the most important underlying causes associated with death.
Conclusion(s): In Brazil, the impact of VT on overall mortality is low and the main VT‐associated underlying causes of death are cancer and injury. These results reveal the inequalities in VT mortality across countries and underscore the importance of carrying out multinational studies, not only for diversity documentation but for delineating health policies.


PB1337
Changing pattern of venous thrombosis events in 2021 associated with COVID in a teaching hospital
H. Rowswell 1; T. Nokes2
1 University Hospitals Plymouth NHS Trust, Exeter, England, United Kingdom; 2 University Hospital Plymouth NHS Trust, Plymouth, England, United Kingdom
Background: University Hospitals Plymouth, NHS trust, is a teaching hospital which has been a venous thromboembolism (VTE) exemplar centre since 2010. Data on VTE events, whether hospital acquired (HAT) and radiology scans performed, has been collected since 2010.
Aims: To review whether COVID had an impact on number of VTE events diagnosed, number of HAT events and radiological investigations performed.
Methods: Collect VTE outcome data from computed tomography pulmonary angiography (CTPA), ventilation perfusion scans (V/Q) and Doppler ultrasounds of upper and lower limbs. Review positive VTE events as to whether they meet criteria for HAT and compare outcome data for 2021 with an average from 2010 – 2020.
Results: In 2021 number of VTE events was 944, an increase of 153 (19%) over previous 11 years. HAT events were 206, increased by 17 (9%) though as a percentage of total VTE fell from 24% to 22%. Total pulmonary embolism (PE) was 590, increase of 136 (30%), although deep vein thrombosis (DVT) events were 354, representing an increase of 18 (5%). CTPA scans rose by 891 (41%) to 7021 though DUS remained similar with 2714 scans against an average of 2704 and VQ scans reduced to 339 compared with 545. Of the positive VTE events, 72 (8%) were associated with a COVID diagnosis and a significant number 67 (93%) being PE compared to 5 (7%) DVT.
Conclusion(s): There is likely to be an association between VTE increase and shielding as well as positive COVID events causing a decrease in usual physical activity. It is not entirely clear why PE increased significantly but as COVID is a respiratory virus and causes significant inflammation within the lungs, this may have impacted on these figures. Initial review of data confirms no significant changes to VTE risk factors. We will continue to collect and monitor this data in 2022.

VPB1344
ABO blood group and factor viii level as risk factors for idiopathic venous thromboembolism
A. Diaz 1; M. Vazquez2; M. Ordoñez2; F. Neria2; J. Cachá2; M. Gomez2
1 SEHH, Madrid, Madrid, Spain; 2 Mostoles Hospital, Madrid, Madrid, Spain
Background: Non‐O group (A, B, AB) individuals have consistently been found to demonstrate increased incidence of both arterial and venous thromboembolism (VTE) compared to O group individuals. On the other hand, several studies have demonstrated that high factor VIII (FVIII) level constitute a prevalent, dose‐dependent risk factor for VTE, both first episode and recurrence. The ABO locus is a major determinant of plasma levels of von Willebrand factor (VWF)/FVIII levels. Non‐O individuals have 25% higher level of VWF/FVIII than O individuals.
Aims: To study ABO group, FVIII level and their possible association as risk factors in a selected population of patients with a first episode of idiopathic VTE.
Methods: We studied retrospectively our series of 178 patients with a first episode of idiopathic VTE objectively diagnosed in our hospital from 2014 to 2019. We analyzed clinical data as well as thrombophilia studies. Informed consent was obtained. FVIII level was determined 6 months after the acute episode. Acute phase response was excluded. ABO phenotyping was performed at the Blood Bank. Statistical analysis included the Chi‐squared test, p values less than 0.05 being considered significant.
Results: Non‐O group was more frequent in patients (78%) than in the reference population of blood donors (56%), A1 phenotype being also more frequent (94% vs 80%). Inversely, O group was less frequent (22% vs 44%) (table 1) FVIII level was high (>190%) in most patients (79%) and very high (≥ 230%) in 51% of them. There were significant differences in FVIII level depending on the ABO blood group both considering each possible group or non‐O group vs O group (table 2).
Conclusion(s): Non‐O blood group and elevated FVIII level are the most important risk factors in this population and appear to be associated. They should be taken into account in the long‐term management of these patients.
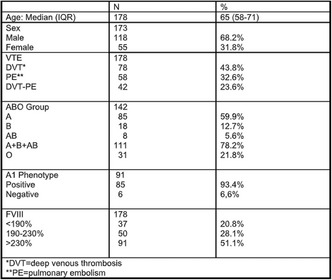

VPB1347
Higher in‐hospital mortality of cancer‐associated pulmonary embolism: Findings from the CURES registry
J. Liu 1; Z. Zhang2; P. Yang3; Z. Zhai2
1 China‐Japan Friendship Hospital, Beijing, Beijing, China (People's Republic); 2 Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China‐Japan Friendship Hospital, Beijing, Beijing, China (People's Republic); 3 Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing, Beijing, China (People's Republic)
Background: Venous thromboembolism (VTE), especially pulmonary embolism (PE), has been the second leading cause of death in patients with cancer. Characterization of patients with cancer‐associated PE and their risk factors may help improve survival and prognosis.
Aims: This study aimed to analyze the characteristics of cancer‐associated PE and evaluate the clinical predictors of in‐hospital death of patients with cancer and acute PE.
Methods: Adult patients with acute symptomatic PE were enrolled between 2009 and 2015. Patients with cancer were selected for the evaluation of risk factors using univariate and multivariate logistic regression models. Primary outcome measures were all‐cause in‐hospital mortality and bleeding.
Results: Among the 7438 patients with acute PE, 12.1% were diagnosed with cancer. The most common type of cancer was lung cancer (38.2%), while 2.9% patients had pancreatic cancer and 10.3% patients received chemotherapy. The rates of all‐cause death and bleeding events were higher in patients with cancer than in those without cancer (9.6% vs 2.6%, p < 0.001; 5.8% vs 4.3%, p < 0.001, respectively). Fatal PE was the most prevalent cause of death in patients with and without cancer. Pancreatic cancer was the strongest risk factor for mortality. Age >80 years, pulmonary infection, Borg scale ≥4, pulse ≥110 beats/min, thrombocytopenia, PaO2 < 60 mm Hg, and major bleeding were independent risk factors. Initial anticoagulation and surgery within 1 month were independent protective factors for mortality. The rate of bleeding events and major bleeding in the included patients with pulmonary infection was 11.2% and 3.2%, respectively, which were higher than those in the cancer population.
Conclusion(s): PE patients with cancer had higher in‐hospital mortality and bleeding rates compared with patients without cancer. During hospitalization, more appropriate management and care may be required for symptomatic PE patients with risk factors.


VPB1348
Serial platelet mapping can be used to define platelet hyperactivity throughout pregnancy
G. Mosca; L. Skeith; L. Yamaura; J. Baxter; P. Skeith
University of Calgary, Calgary, Alberta, Canada
Background: Hypercoagulability occurs in pregnancy due to increased procoagulant factors and decreased anticoagulant factors. However, less is known about platelet contribution to hypercoagulability and venous thromboembolism (VTE). Platelet mapping (PLM) using thrombelastography (TEG) analysis can be used to activate platelets at the adenosine diphosphate (ADP) receptor or the thromboxane A2 (AA) receptor, to evaluate clot strength. By using PLM to identify the platelet contribution to hypercoagulability in pregnancy, we can better understand the mechanism of pregnancy‐related VTE.
Aims: To evaluate platelet contribution to hypercoagulability during healthy pregnancy and to use PLM analysis to assess aspirin use.
Methods: Following ethics approval, eligible pregnant women who provided informed consent were enrolled in a single centre, prospective cohort and were followed serially with TEG whole‐blood analysis monthly during the first and second trimester, and every two weeks during the third trimester. Platelet hyperactivity was defined as an ADP and AA maximal amplitude value (MA; a measure of clot strength) ≥55 mm. Mean MA values were calculated at each timepoint to measure the duration of hypercoagulability. Descriptive statistics were used.
Results: Seven consecutive participants were enrolled (mean age = 32 ± 2.7 years). TEG‐defined hypercoagulability increased throughout pregnancy (Figure 1). Platelet hyperactivity was identified in both the ADP and AA pathways, and increased throughout pregnancy (Figure 1). The AA‐MA values for a single participant taking aspirin (162 mg) daily were above 55 mm for the majority of her pregnancy, suggesting platelet hyperactivity (Figure 2).
Conclusion(s): This study identified that platelet‐mediated hypercoagulability begins early in pregnancy and increases as pregnancy progresses. The data suggests that further analysis is needed to better understand the role of aspirin and platelet contribution in hypercoagulability. Further investigation is needed to determine if point‐of‐care TEG‐analysis can identify platelet hyperactivity in pregnant women at risk for VTE.
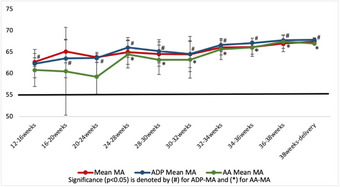

VPB1349
Thrombotic events as a manifestation of vasculitis/vasculopathy induced by cocaine adulterated with levamisole in a Colombian cohort
M. Plaza 1; T. Urrego2; A. Restrepo3; C. Muñoz1
1 Hospital San Vicente Fundación, Medellin, Antioquia, Colombia; 2 Clínica IPS Universitaria, Medellin, Antioquia, Colombia; 3 Universidad de Antioquia, Envigado, Antioquia, Colombia
Background: The illegal consumption of cocaine adulterated with levamisole is increasing worldwide. Vasculitis and agranulocytosis generated by this component have been widely studied, however, both micro and macrovascular thrombotic vasculopathy continues to be an area of study due to its impact on the prognosis and additional management measures.
Aims: To determine the frequency of patients with thrombotic vasculopathy as a manifestation and to describe the clinical and main laboratory findings.
Methods: A descriptive, retrospective study was performed. Patients with levamisole‐induced vasculopathy (LIVEN) evaluated from December 2010 to December 2021 were included. Descriptive statistics were calculated through Jamovi, ver 1.6.7.0.
Results: A total of 60 patients with a diagnosis of LIVEN were included in the follow‐up cohort: 16 presented thrombotic vasculopathy as a manifestation (26.6%). The mean age was 34 years, 81.2% of the patients were men. Thrombotic microangiopathy was documented in 13 patients by skin biopsy (21.6%) and only 3 presented macrovascular venous thromboembolic events (5%). The events of macrovascular thrombosis were deep vein thrombosis, 1 in the upper limb and 2 in the lower limbs. 75% of patients with thrombotic vascular disease had at least one antiphospholipid antibody present. None of the patients included in the follow‐up presented arterial thrombosis as a manifestation (Tables 1–2)
Conclusion(s): Thrombotic manifestations are infrequent in patients with LIVEN, microvascular involvement by biopsy being more frequent than macrovascular venous thromboembolic events


PB1322
A ten‐year Australian experience of splanchnic vein thrombosis
J. Oktaviana1; B. Lui1; P. Ho 2; H. Lim1
1 Northern Health, Epping, Victoria, Australia; 2 Northern Health, Victoria, Australia, Epping, Victoria, Australia
Background: There is lack of consensus in the management of splanchnic vein thrombosis (SVT), in part due to the rarity and heterogeneity of these cases.
Aims: To evaluate the risk factors, diagnosis, management and complications of SVT.
Methods: A ten‐year retrospective evaluation of consecutive SVT presentations at Northern Health, Victoria, Australia, from January 2011 to December 2020 was conducted. The mean follow‐up was 19 months. The results were compared to our DVT/PE database of the same period.
Results: 98 patients (64 males; mean age 64 years) presented with 99 episodes of SVT. 94% were symptomatic with the main portal vein most commonly involved (n = 61). SVT patients were more likely male and have active malignancy compared to those with DVT/PE (Table 1). 41% had concurrent intra‐abdominal infection, 14% had recent intra‐abdominal surgery and 33% had active malignancy. Thirty‐four patients (35 cases) had underlying cirrhosis. Thirty‐two patients were screened for JAK2 V617F mutation of which three patients (including 1 cirrhotic patient) were subsequently diagnosed with myeloproliferative neoplasm. Patients with cirrhosis were more likely to not be anticoagulated compared to non‐cirrhotic patients and those with DVT/PE. Eighteen patients (including 5 cirrhotic patients) with SVT received direct oral anticoagulant without known bleeding or thrombotic complications. Cirrhotic patients reported more recurrent thrombotic events (3 SVT and 2 DVT/PE) or clot progression (n = 1) compared to non‐cirrhotic patients with two recurrent thrombotic events (1 SVT and 1 DVT) and one clot progression (15.6 vs 3.3 events/100‐person‐years; HR 7.0 (95% CI 1.3–36.6), p = 0.022). The rate of clinically significant bleeding on anticoagulation was comparable across groups.
Conclusion(s): Splanchnic vein thrombosis is more likely to be provoked compared to DVT/PE, with malignancy and abdominal infections as common causes. Cirrhotic patients have a higher rate of thrombotic complications compared to non‐cirrhotic patients (HR 7.0, 95% CI 1.3–36.6) suggesting a careful assessment of individualised anticoagulation decision is needed.

PB1329
The predictive value of pulmonary infarction in patients with acute pulmonary embolism on persistent symptoms and adverse events
F. Kaptein 1; L. Kroft2; L. van Dam3; J. Stöger1; M. Ninaber1; M. Huisman4; F. Klok5
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Radiology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 3 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 4 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 5 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: Pulmonary infarction (PI) is relatively common in acute pulmonary embolism (PE) with a reported incidence of nearly 30%. The impact on mortality and short‐term outcomes seems limited, but the effect on persistent symptoms and functional impairment is largely unknown.
Aims: To evaluate the predictive value of suspected pulmonary infarction at acute PE diagnosis on long‐term clinical outcomes.
Methods: We studied an existing cohort of 99 patients with computed tomography pulmonary angiography (CTPA) confirmed acute PE in whom 3‐month follow‐up data were available. The CTPAs were re‐evaluated by an expert thoracic radiologist, who was blinded for clinical outcomes, for signs of PI (i.e. consistent with a peripheral wedge‐shaped consolidation in a region of pulmonary embolism, with decreased contrast enhancement; Figure). We investigated the association between signs of PI at PE diagnosis and clinical symptoms at initial presentation, adverse events (recurrent venous thromboembolism, PE‐related readmission and mortality) and self‐reported persistent symptoms (dyspnea, pain and post‐PE functional impairment, the latter defined as ‘new/progressive dyspnea, exercise intolerance and/or diminished functional status following adequately treated PE, without an apparent alternative explanation’) at 3‐month follow‐up using univariate Cox regression analysis.
Results: At PE diagnosis, 57 patients (58%) had suspected PI, comprising a median of 1% (IQR 1–3) of the total lung parenchyma. Patients with suspected PI presented more often with hemoptysis and pleural pain, and with more proximal PE on CTPA than patients without suspected PI (Table). There was no association with adverse events, persistent dyspnea or pain at 3‐month follow‐up, but signs of PI predicted more functional impairment (OR 3.0, 95% CI 1.0–9.1).
Conclusion(s): Patients with signs of PI had a different clinical presentation at acute PE diagnosis than patients without those signs, but no association with adverse events was found. Additionally, signs of PI predicted post‐PE functional impairment.

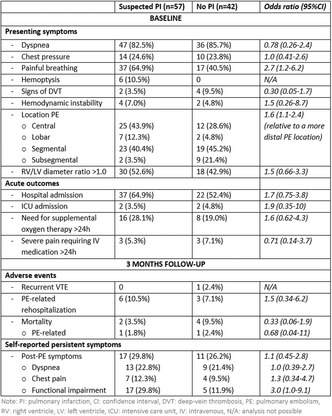
PB1331
Using natural language processing to diagnose venous thromboembolism: A systematic review
B. Lam 1; T. Chiasakul2; D. Karagkouni1; W. Robertson3; R. Rosovsky4; L. Lake3; I. Vlachos1; J. Zwicker1; R. Patell1
1 Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States; 2 King Chulalongkorn Memorial Hospital, Arlington, Virginia, United States; 3 National Blood Clot Alliance, Philadelphia, Pennsylvania, United States; 4 Massachusetts General Hospital and Harvard Medical School, Newton, Massachusetts, United States
Background: Venous thromboembolism (VTE) is the leading cause of preventable in‐hospital mortality, and identifying these events is a core metric of hospital quality measures. Understanding the current incidence of VTE can be challenging because organizations often rely on humans to review charts and diagnoses codes. Natural language processing (NLP) offers a promising alternative, allowing for rapid extraction of relevant data from extensive medical records.
Aims: To conduct a systematic review of studies that use NLP to identify VTE events in the electronic health record.
Methods: We conducted a literature search of PubMed, MEDLINE, EMBASE, and Web of Science using Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. Search criteria included original studies with the terms “artificial intelligence” and “venous thromboembolism” published from inception through April 20, 2021. Only studies that employed NLP to assist in the diagnosis or detection of VTE were included. Two reviewers assessed each study for eligibility and adherence to reporting standards using the Transport Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) tool. The pooled sensitivity, specificity, and 95% CI were calculated using a bivariate random effects model.
Results: 556 studies were identified. Sixteen studies with a total of 28 NLP models were included. There was wide variability in adherence to TRIPOD (Figure 1), and some TRIPOD items were deemed not applicable. Sixteen NLP models from 10 studies included sensitivity and specificity as a performance measure. The overall sensitivity of these models was 0.86 (95% CI, 0.73–0.94) and the overall specificity was 0.99 (95% CI, 0.98–0.99) (Figure 2).
Conclusion(s): Sensitivity of NLP to identify VTE events varied greatly between different algorithms, but overall specificity was high. Further studies using standardized performance measures are needed.


VPB1343
Prolonged hypercoagulability occurs following pelvic and acetabular fractures, as defined by serial thrombelastography
A. Clarke; L. Skeith; R. Korley; A. Dodd; P. Duffy; R. Martin; P. Skeith
University of Calgary, Calgary, Alberta, Canada
Background: Despite standard thromboprophylaxis, patients who sustain pelvic or acetabular (PA) fractures have a historically high venous thromboembolism (VTE) rate (12%). Thrombelastography (TEG) is a point‐of‐care analysis tool from which maximal amplitude (MA) values ≥65mm can quantify hypercoagulability and increased VTE risk in trauma patients.
Aims: (1) To utilize serial TEG analysis to define hypercoagulability duration following surgically treated PA fractures. (2) To compare MA values between patients with and without VTE.
Methods: After obtaining informed consent, consecutive adult patients with surgically treated PA fractures were enrolled from a single centre into this prospective cohort study with research ethics board approval. Serial whole‐blood analysis was performed using a TEG6s hemostasis analyzer at timepoints determined a priori. Exclusion criteria: therapeutic anticoagulation, bleeding disorders, active malignancy, and pregnancy. Patients received standardized thromboprophylaxis with prophylactic‐dose low‐molecular‐weight heparin (LMWH) for 28 days post‐operatively. VTE was defined as symptomatic pulmonary embolism (PE) or deep vein thrombosis (DVT), or asymptomatic proximal DVT from screening doppler ultrasound performed three days post‐operatively. One‐sided t‐tests were used to compare the MA threshold (≥65 mm) and serial MA measures, and MA values between patients with and without VTE (α = 0.05).
Results: Thirty‐three patients with a mean age of 47 ± 19 years were included (10 females, 30%). The majority (96%) of patients were hypercoagulable at two weeks. 50% of patients remained hypercoagulable at the time of LMWH discontinuation, and 14% remained hypercoagulable at 3‐months post‐operatively. There were seven VTE events (21.2%) (Figure 1), including six symptomatic PEs. Mean MA for all VTE events over all timepoints closest to diagnosis was 68.2 mm ± 4.8 mm, while the overall mean for non‐VTE was 64.0 mm ± 7.1 mm (p = 0.06) (Table 1).
Conclusion(s): Prolonged hypercoagulability occurs in half of PA patients beyond discontinuation of standardized thromboprophylaxis. Serial TEG analysis warrants further study to help predict VTE risk and inform clinical recommendations for thromboprophylaxis duration following PA fractures.


PB1316
Differences in coagulation parameters associated with glucocorticoid treatment in patients with a first venous thrombosis: Data from the MEGA study
E. Camilleri 1; N. van Rein1; N. Biermasz2; O. Dekkers3; R. Feelders4; M. Huisman5; M. Kaya6; F. Klok7; O. Meijer8; A. Pereira8; F. Rosendaal9; F. Shokri10; B. van Vlijmen11; S. Cannegieter1
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Medicine, Division of Endocrinology and Center for Endocrine Tumors Leiden, Leiden, Zuid‐Holland, Netherlands; 3 Department of Clinical Epidemiology and Department of Medicine, Division of Endocrinology and Center for Endocrine Tumors Leiden, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 4 Department of Internal Medicine, Division of Endocrinology, Erasmus MC University Medical Center, Rotterdam, The Netherlands, Rotterdam, Zuid‐Holland, Netherlands; 5 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 6 Department of Clinical Oncology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 7 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 8 Department of Medicine, Division of Endocrinology and Center for Endocrine Tumors Leiden, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 9 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden University, Leiden, Zuid‐Holland, Netherlands, 10 Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands, 11 Department of Internal Medicine, Division of Thrombosis and Hemostasis, Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: Glucocorticoid (GC) treatment increases the risk of venous thromboembolism (VTE) through unclear mechanisms. Particularly, it is unclear to what extent GCs influence coagulation and fibrinolysis.
Aims: To assess whether GC treatment is associated with changes in coagulation and fibrinolysis in outpatients with a first VTE, by comparing patients treated with GC with untreated patients.
Methods: The MEGA‐study is a case‐control study into causes of VTE, where clinical data were collected on 4956 patients and blood was sampled in a random 50%. Data on 2547 patients could be linked to the Dutch Pharmacies Register to obtain information on GC‐prescriptions, out of whom a blood sample was available in 1155 patients. Patients were classified as treated with GC if the date of blood sampling was within a period of GC‐use, and untreated if they never had a GC prescription (i.e. excluding later GC‐users). Fibrinogen, factor(F)V, FVIII, FX, von Willebrand factor, protein(P)C, PS, thrombin activatable fibrinolysis inhibitor, plasminogen activator inhibitor‐1, clot lysis time (CLT) and endogenous thrombin potential (ETP) were measured in each group. Mean differences and 95% confidence intervals (CI) were estimated with linear regression adjusted for possible confounders.
Results: Of 1155 patients, 31 were using GC at the time of blood sampling, whereas 890 never were. Table 1 shows general characteristics of the two groups. Mean levels of all pro‐ and anti‐coagulant parameters, except for FX, were higher in the patients treated with GC compared to the untreated (Table 2). ETP was not different between the two groups (mean difference −24.7 [95% CI −94.2–44.7]) whereas CLT was prolonged in the patients using GC.
Conclusion(s): Mean levels of all anti‐ and pro‐coagulant proteins were higher in patients treated with GC compared to the untreated, with a similar ETP. CLT was prolonged in users of a GC. These findings are in line with the literature on patients with Cushing’s disease.


PB1324
Evaluating routine data sources and efficient methods to determine the incidence of venous thromboembolism and major bleeding in unselected medical and surgical inpatients
D. Horner 1; S. Rex2; M. Bursnall2; K. de Wit3; S. Goodacre2; B. Hunt4
1 Salford Royal NHS Foundation Trust, Lymm, England, United Kingdom; 2 University of Sheffield, Sheffield, England, United Kingdom; 3 Queen's University, Kingston, Ontario, Canada; 4 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom
Background: The accuracy of clinical outcome data is key to any future study of tailored thromboprophylaxis in hospitalised inpatients. However, any such study will require a large sample size.
Aims: We sought to evaluate the use of efficient methods to determine the feasibility of a data enabled implementation study of VTE risk assessment models. Our aim was to compare the accuracy of available coding (ICD10) and local registry data to determine key VTE and bleeding outcomes, against case note review by trained research assistants.
Methods: A multi‐centre observational, cohort study with prespecified feasibility criteria conducted at four NHS sites. We identified a consecutive cohort of general medical and surgical patients requiring hospitalisation during the calendar year 2019, then scrambled patient episodes into random batches to mitigate known and unknown confounders. Research assistants subsequently undertook retrospective case note review for each patient episode through electronic health records, in sequential batches. We extracted data on the subsequent diagnosis of hospital acquired thrombosis (HAT) and major bleeding events as per internationally agreed definitions, up to 90 days following discharge. We then collated multiple routine outcome data sources for each patient episode and compared them to a reference standard diagnosis of case note review.
Results: 2115 patients were analysed with an original hospital admission occurring in the calendar year 2019. Of these, 107 patient episodes were ineligible, leaving 2008 episodes for analysis. The sensitivity was 62% (95% CI 54% to 69%) for the use of coding data to detect HAT events and 81% (95% CI 75% to 87%) for local registry entries. The sensitivity of coding to detect major bleeding events was 38% (95% CI 27% to 50%).
Conclusion(s): In this multicentre cohort study, efficient data methods used to identify HAT and major bleeding events performed poorly when compared to direct case note review by trained research staff.

PB1328
The Venous Thrombosis Registry in Østfold Hospital (The TROLL registry): Design and cohort description
C. Jørgensen 1; M. Tavoly2; H. Pettersen3; E. Førsund3; C. Roaldsnes3; M. Olsen3; E. Tjønnfjord3; J. Gleditsch4; A. Galovic3; S. Vikum5; S. Brækkan6; W. Ghanima7
1 Ostfold hospital, Sarpsborg, Ostfold, Norway; 2 Sahlgrenska Hospital, Gothenburg, Vastra Gotaland, Sweden; 3 Østfold Hospital, Sarpsborg, Ostfold, Norway; 4 Department of Radiology, Hospital of Ostfold, Kalnes, Sarpsborg, Ostfold, Norway; 5 Clinic of Internal Medicine, Østfold Hospital, Norway and Institute for Clinical Medicine, University of Oslo, Norway, Oslo, Oslo, Norway; 6 Thrombosis Research Center, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway; 7 Østfold Hospital Foundation, Sarpsborg, Ostfold, Norway
Background: The incidence of venous thromboembolism (VTE) is expected to increase over the next decades, further increasing its substantial impact on patients and health care resources. Registries have the benefit of reporting real‐world data without excluding clinically important subgroups.
Aims: To describe a Norwegian VTE registry and to characterize the registered population.
Methods: The Venous Thrombosis Registry in ØstfOLd HospitaL (TROLL) is an ongoing registry of consecutive patients diagnosed with, treated and/or followed up for VTE at Østfold Hospital, Norway, since 2005. Baseline and follow‐up data, including demography, clinical features, risk factors, diagnostic procedures, types of VTE and treatment were collected during hospitalization and scheduled outpatient visits.
Results: From 2005 to 2021, 5037 patients had consented for research in TROLL. Median age was 67 years (IQR 55–77) and 2622 (52.1%) were males. 2736 (54.3%) had pulmonary embolism (PE), 2034 (40.4%) had deep vein thrombosis (DVT), and 265 (5.3%) had upper extremity DVT, splanchnic or cerebral sinus vein thrombosis. 2330 (46.3%) were classified as unprovoked VTE whereas 1131 (22.5%) had cancer associated thrombosis. (Table) Direct oral anticoagulants were the most frequent treatment (39.3%) followed by low‐molecular weight heparins (30.4%) and vitamin K antagonists (30.3%). Outpatient treatment of DVT increased from 71% in 2005 to 77% in 2019 whereas, outpatient treatment for PE increased from 4% in 2005 to 23% in 2019.
Conclusion(s): TROLL is a relatively large ongoing population‐based registry of unselected VTE patients, with high‐quality data that represent a valuable source of real‐world data on the management and outcomes of VTE. The findings to date correspond well with those reported from other registries of unselected VTE patients. The long‐term follow‐up of patients in TROLL and linkage to other local and national registries, and local biobank provide unique opportunities for research on the management, biomarkers, risk factors and short‐ and long‐term outcomes of VTE.

PB1336
Clustering of coagulation factors in acute venous thromboembolism identifies clusters of thromboinflammatory and endothelial activation markers as independent predictors of recurrence and mortality
A. Pallares Robles 1; V. ten Cate2; A. Schulz3; J. Prochaska2; T. Koeck4; S. Heitmeier5; R. Ewert6; M. Halank7; C. Espinola‐Klein8; K. Lackner9; T. Münzel10; H. ten Cate11; A. ten Cate‐Hoek12; S. Konstantinides13; P. Wild2
1 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands, Mainz, Rheinland‐Pfalz, Germany; 2 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; Preventive Cardiology and Preventive Medicine, Department of Cardiology, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 3 Preventive Cardiology and Preventive Medicine, Center for Cardiology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, 55131, Germany, Mainz, Rheinland‐Pfalz, Germany; 4 Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 5 Bayer AG, Wuppertal, Germany, Wuppertal, Nordrhein‐Westfalen, Germany; 6 Department of Internal Medicine, Pulmonary Diseases, University Medicine Greifswald, Greifswald, Germany, Greifswald, Mecklenburg‐Vorpommern, Germany; 7 Department of Internal Medicine I and Pulmonology, Carl Gustav Carus Hospital, University of Dresden, Germany, Dresden, Sachsen, Germany; 8 Department of Cardiology, University Medical Center Mainz, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany; 9 Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center of the Johannes Gutenberg‐University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 10 Center for Cardiology – Cardiology I, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine‐Main, Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany, 11 Departments of Biochemistry and Internal Medicine, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands;, Maastricht, Limburg, Netherlands, 12 Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, the Netherlands; Thrombosis Expertise Center, Heart+ Vascular Center, Maastricht University Medical Center, Maastricht, the Netherlands, Maastricht, Limburg, Netherlands, 13 Center for Thrombosis and Hemostasis, University Medical Center Mainz, Germany, Mainz, Rheinland‐Pfalz, Germany
Background: Blood coagulation is a complex and highly coordinated process, modulated by procoagulant and anticoagulant factors. Genetic or environmental defects in their hemostatic balance contribute to venous thromboembolism (VTE). We hypothesized that patterns in coagulation system activity observed in acute VTE may also predict recurrent events.
Aims: To identify patterns of coagulation factors and their relation with risk factors and clinical outcomes of VTE patients.
Methods: Acute‐phase plasma samples from 612 subjects from the GMP‐VTE project were analyzed. This study was approved by the local Ethics Committee. All patients signed the informed consent. FII, FVII, FVIII, FIX, FX, FXI, FXIII, activated Protein C (aPC), Protein S (PS), D‐dimer, fibrinogen, tissue factor protein inhibitor (TFPI), thrombomodulin and plasminogen activator inhibitor‐1 levels were quantified, and principal component (PC) analysis was used to derive coagulation system patterns. Cox regression and elastic net‐regularized linear regression were used to identify associations of PC scores with clinical outcome and risk factors.
Results: Four PCs were retained, accounting for 51.3% of the total variance. PC1 was mainly driven by vitamin K dependent factors (FII, FVII, FX, and aPC); PC2 by FVIII, FIX, FXI, and fibrinogen; PC3 by D‐dimer and FXIII, and TFPI and PS contributed to PC4. PC3 and PC4 significantly predicted recurrent VTE risk (HR: 1.27; 95 %CI: [1.05–1.53]; p = 0.014, and 1.31 [1.06–1.62]; p = 0.013, respectively), independently of age, sex, body mass, VTE risk factors, thrombus site, and medication intake. Additionally, PC2 and PC4 exhibited good prognostic ability for all‐cause death (HR: 1.36 [1.04–1.77]; p = 0.024, and 1.76 [1.42–2.20]; p < 0.001). PC4 scores were elevated in subjects with comorbidities reflecting endothelial dysfunction (diabetes, chronic kidney disease, coronary artery disease).
Conclusion(s): Principal component analysis of the coagulation system characterized clusters of thrombo‐inflammation and endothelial activation markers related to recurrent VTE and mortality. APR is supported by EU‐TICARDIO No. 813409.
PB1315
Predictors of pregnancy associated venous thromboembolism: A case‐control study
M. AlSheef 1; a. abu‐shaheen1; A. Zia Zaidi2; O. Alarfaj3
1 King Fahad Medical City, Riyadh, Ar Riyad, Saudi Arabia; 2 King Fahad Medical City, Riydadh, Ar Riyad, Saudi Arabia; 3 King Fahad Medical City, riyadh, Ar Riyad, Saudi Arabia
Background: Venous thromboembolism (VTE) manifesting as pulmonary embolism (PE) or deep vein thrombosis (DVT), is an important cause of morbidity and the most common cause of maternal death during pregnancy and the postpartum period.
Aims: We conducted this study to describe the predictors of pregnancy‐induced VTE (DVT and PE), among pregnant women.
Methods: A case‐control study was conducted at a tertiary care center in Riyadh. A total of 180 patients diagnosed with pregnancy‐associated thrombosis were included in this study as cases and 200 subjects who did not develop VTE were included as controls. Demographic data were collected by medical chart review. Risk factors of VTE were collected using the risk assessment tool of the Royal College of Obstetricians and Gynecologists. The main outcome measures were VTE, manifesting as PE or DVT.
Results: The results of the multivariate analysis identifying the predictors of having VTE showed the following factors: family history (OR = 50.47, 95% CI: 6.78–375.64, p‐value <0.0001), thrombophilia (OR = 21.99, 95% CI: 2.83–170.63, p‐Value = 0.003), and gross varicose veins (OR = 17.15, 95% CI: 3.93–74.87, p‐value <0.0001).
Conclusion(s): The findings of this study show that family history, thrombophilia, and gross varicose vein were the provoking factors for VTE, exceeding other transient risk factors. Prophylaxis is recommended for women at the highest risk of pregnancy‐related VTE, such as those with inherited thrombophilia, a strong family or personal history of VTE, and with gross varicose veins.
PB1320
Predictive factors of clot propagation towards deep venous thrombosis and/or pulmonary embolism in superficial venous thrombosis patients: A systematic review
F. van Royen1; M. van Smeden2; S. van Doorn3; F. Rutten2; G. Geersing 2
1 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands, Utrecht, Utrecht, Netherlands; 2 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands; 3 University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, Utrecht, Utrecht, Netherlands
Background: Superficial venous thrombosis (SVT) can be left untreated in most patients. However, a subset of patients will develop clot propagation towards deep venous thrombosis (DVT) and/or pulmonary embolism (PE). Clinical characteristics predictive of clot propagation will help identify those patients at highest risk and thus those benefitting from early intervention.
Aims: To identify all clinically relevant predictive factors and their predictive value for clot progression towards DVT and/or PE in patients with SVT.
Methods: The protocol of this systematic review is registered at the International Prospective Register of Systematic Reviews (PROSPERO) with protocol number CRD42021262819. Pubmed/MEDLINE and Embase were systematically searched until July 22 2021 for studies describing SVT patients, DVT and/or PE as the outcome and presenting predictive factors. The CHARMS checklist for Prognostic Factor studies was used for systematic extraction of study characteristics and the QUIPS tool for risk of bias assessment. Per identified predictive factor, relevant effect estimates were extracted such as odds ratio’s (OR) and hazard ratio’s (HR) and if not presented, OR’s were calculated. Effect estimates of the most reported predictors were summarised in forest plots.
Results: Twenty‐two studies were included. The most reported predictive factors were age > 75 years, male sex, history of venous thromboembolism (VTE), absence of varicose veins and cancer. Clinical SVT characteristics, such as length or location of the clot, were often insufficiently reported. Effect estimates showed wide variation as exemplified by the univariable OR’s for history of VTE (range 0.52–6.92). Due to the heterogeneity in study design, population, outcome assessment and predictor assessment, formal meta‐analysis by pooling of results was deemed inappropriate.
Conclusion(s): Age > 75, sex, history of VTE, absence of varicose veins and cancer were the most reported predictive factors for clot propagation in SVT patients. Identifying patient‐factors related to increased risk of SVT complications will help support clinical decision‐making.
PB1325
Chronobiology of acute deep vein thrombosis: A 28 year study of lower extremity duplex ultrasounds
D. Houghton; D. Vlazny; R. Meverden; P. Daniels; M. Bartlett; G. Hesley; A. Lekah; A. Casanegra; W. Wysokinski
Mayo Clinic, Rochester, Minnesota, United States
Background: Day light savings time (DLST) changes have been shown to increase the presentations of myocardial infarctions (MI).
Aims: Evaluate the influence of DLST on diagnosis of deep vein thrombosis (DVT).
Methods: Lower extremity venous Duplex ultrasound (DUS) radiology reports from the inception of electronic archiving through December 31, 2020 were evaluated across the Mayo Clinic Enterprise. A natural language processing algorithm was used to identify acute DVT from radiology text reports. DUS reports from the state of Arizona were a “control” population as they not participate in DLST.
Results: A total of 357,703 lower extremity venous DUS reports in 237,052 patients from January 1, 1992 to December 30, 2020 were identified. In the overall sample including both spring and fall DLST changes, there were a similar number of DUS performed (and probability of acute DVT) before and after time changes. Multivariable nominal logistic regression models were used to adjust for differences in age, sex, and race in the sample of DUS reports before and after DLST. The results remained negative overall (OR 1.01, 95% CI 0.97–1.04), for both the spring and fall time changes, with both 7 and 30‐day windows, and within both the control (Arizona) and other geographic regions (Table 1). No difference in the diagnosis of acute DVTs was found in December vs January, n = 2863 (9.55%) and n = 2641 (9.34%) respectively (p = 0.39). However, the probably of acute DVT was higher on the weekends (Figure 1) compared to weekdays, 9.90% vs 8.99%, respectively (p < 0.001). The probability of acute DVT also varied by month of the year (Figure 2), with a statistically significant lower probability in the 2nd Quarter of the year compared to the rest of the year (8.61% vs 9.27%).
Conclusion(s): Daylight savings time changes do not influence the incidence of acute DVT.


PB1326
Incidence of pregnancy‐associated venous thromboembolism: Second nationwide study
H. Hwang 1; S. Bang2; J. Lee3
1 Soonchunhang University Gumi Hospital, Gumi, Kyongsang‐bukto, Republic of Korea; 2 Seoul National Bundang Hospital, Bandang, Kyonggi‐do, Republic of Korea; 3 Seoul National Bundang Hospital, Bundang, Kyonggi‐do, Republic of Korea
Background: Pregnancy is a well‐known transient risk factor for venous thromboembolism (VTE).
Aims: This was the second nationwide study to examine the relationship between the increased proportion of patients of advanced maternal age and the incidence rate of pregnancy‐associated VTE (PA‐VTE) during the study period (2014–2018) compared with those in a previous study (2006–2010).
Methods: Using the Korean Health Insurance Review and Assessment Service database, we retrospectively identified all PA‐VTE events using both diagnostic and medication codes.
Results: Out of 124,228 VTE events, 510 (0.4%) cases of PA‐VTE were identified in 499 women (median 34, range 20–49). The incidence rate of PA‐VTE per 10,000 (PA‐VTE/104d) in this second study (2.62) was 3.2 times higher than the rate in the first study (0.82). In the second study, the PA‐VTE/104d of women in their 40s (5.46) was three times higher than that of women in their 20s (1.80) (relative risk [RR], 3.03; 95% CI, 2.04–4.51; p < 0.01). The incidence rate in their 40s in second study was 2.3 times higher than that in their 40s in first study. Cases of PA‐VTE/104d occurred more frequently in cases of multiparity than in those with nulliparity, in cesarean section compared with vaginal delivery, and in multiple compared with single pregnancies. Most cases of PA‐VTE occurred in postpartum period (321/510, 62.9%). The PE was the frequent type of VTE in postpartum (231/321, 72%)
Conclusion(s): Advanced maternal age increases the risk of PA‐VTE. Obstetricians need to be cautious about the risk of VTE, particularly during postpartum in women in their 40s.


PB1318
Patient characteristics associated with all‐cause mortality of incident hospitalized pulmonary embolism patients: A nationwide cohort study
Q. Chen; N. van Rein; S. Cannegieter
Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: The prognosis after a pulmonary embolism (PE) varies from no symptoms to death. However, large‐scale studies on patient characteristics associated with a worse prognosis are rare.
Aims: To explore patient characteristics associated with all‐cause mortality of incident hospitalized PE patients.
Methods: Incident PE patients (ICD‐10 code: I26) admitted to hospitals in The Netherlands between 2013 and 2018 were identified using nationwide data from Statistics Netherlands. Age, sex, socioeconomic status, prior antithrombotic agents use, and comorbidities were studied as patient characteristics. All patients were followed from incident PE diagnosis for 1 year or until date of death. To study the association of patient characteristics with mortality, we estimated cumulative incidences and hazard ratios (HRs) of 30‐day or 1‐year all‐cause mortality by Kaplan‐Meier estimator and Cox regression respectively.
Results: 61,322 incident PE patients were included with a mean age of 64.8 ± 15.6 years. The overall 30‐day and 1‐year cumulative incidence of all‐cause mortality was 10.05% (95% confidence interval (CI) 9.82–10.29%) and 24.22% (95% CI 23.88–24.56%), respectively. For 30‐day all‐cause mortality, older age was a significant risk factor (HR = 1.04 per 1‐year increase, 95% CI 1.03–1.04), while sex was not. Immigration background, not married or in partnership, low household income, and prior use of antithrombotic agents (within 6 months before the incident PE) were associated with 30‐day mortality. Amongst comorbidities, intracerebral hemorrhage, malignant tumor, abnormal liver function, heart failure, and stroke showed the strongest associations (i.e., HR > 2). The association pattern for 1‐year all‐cause mortality was similar, where the association was strongest for cancer (HR = 5.08, 95% CI 4.91–5.25). Detailed results are presented in the Tables.
Conclusion(s): This study showed that increasing age, an immigration background, being single, a low household income, prior use of antithrombotic agents and most comorbidities were associated with all cause‐mortality after a PE. This information may help to predict prognosis of PE.


VPB1350
Non‐O blood type is a risk factor for venous thromboembolism in acutely injured patients requiring orthopaedic surgery
P. Skeith; S. Yee; J. Petrowitsch; A. Clarke; L. Yamaura; K. Rondeau; L. Skeith
University of Calgary, Calgary, Alberta, Canada
Background: Patients with a non‐O blood type are at increased risk for venous thromboembolism (VTE) based on data from large studies. The association between non‐O blood type and major provoked VTE following major orthopaedic surgery is less well defined, especially in trauma patients with different injury types.
Aims: To compare blood group and VTE incidence in trauma patients who require surgical treatment.
Methods: Consecutive adult patients undergoing surgery for acute acetabular, pelvic, hip, femur, or humerus fractures were included in this prospective cohort. Exclusion criteria were prior VTE, pre‐injury therapeutic anticoagulation, or pregnancy. Following informed or surrogate consent, patients underwent blood work that included blood typing and were subsequently followed for 3‐months. All patients received low‐molecular weight‐heparin thromboprophylaxis for 14 days post‐operatively for upper extremity injuries, or 28 days for pelvic or lower extremity injuries. VTE was defined as symptomatic PE or DVT, or asymptomatic DVT found on screening Doppler ultrasound on postoperative day three. T‐tests, chi square and Fisher's Exact tests were used to compare between those with and without VTE and logistic regression was used to evaluate potential risk factors for VTE.
Results: A total of 287 patients were included, with 17 VTE event (5.9%). There were no differences in age and sex between those with and without VTE (Table 1). Compared with type‐O, type‐A blood carried a 7‐fold increased risk for VTE and type‐B carried a 6.3‐fold increased risk for VTE (Table 2). Compared with a hip fracture, sustaining an acetabular fracture was associated with the highest VTE incidence for fracture type (Table 2).
Conclusion(s): This study supports that non‐O blood type is associated with a higher risk of provoked VTE following orthopaedic fracture surgery in trauma patients. Further investigation, including von Willebrand factor and factor VIII levels, is warranted to better understand this relationship.


PB1323
Venous thromboembolism in obese patients: A ten‐year experience
B. Wee1; B. Lui1; P. Ho 2; H. Lim1
1 Northern Pathology Victoria, Department of Haematology, Northern Health, Epping, VIC, Epping, Victoria, Australia; 2 Northern Health, Victoria, Australia, Epping, Victoria, Australia
Background: Obesity is an increasing global threat with known associations to venous thromboembolism (VTE). There is, however, a lack of consensus in the VTE management of obese patients, particularly in extremes of weight (>120 kg).
Aims: To review the impact of obesity on VTE treatment and outcomes.
Methods: A retrospective analysis was conducted of VTE presentations (DVT ± PE) to Northern Health, Victoria, Australia, from January 2011 to December 2020. Morbidly obese patients (defined as >120 kg) were compared to those ≤120 kg. Patients with malignancy and those without documented weight were excluded. The median follow‐up was 55 months.
Results: 2362 VTE cases, including 194 patients with weight >120kg were identified (see Table 1). The morbidly obese patients were younger (median age 47 years) and more likely to have previous history of VTE (n = 78, 40%). They were more likely to present with unprovoked VTE (59% vs 45%, p < 0.001) and major VTE (75% vs 67%, p = 0.028) compared to those ≤120kg. Warfarin was the most common choice of anticoagulant in patients >120 kg. The morbidly obese patients had the highest rates of VTE recurrence off anticoagulation (7.8/100‐patient years; HR 2.0 (95% CI 1.3–3.0)) when comparing to patients ≤120 kg. There were no differences in clinically significant bleeding rates. A subanalysis of enoxaparin anti‐Xa levels performed on patients >120 kg (see Table 2) demonstrated no differences in the rate of therapeutic levels achieved using uncapped (1 mg/kg twice daily) or reduced (<1 mg/kg twice daily) strategies, with no differences in thrombosis or bleeding rates across strategies.
Conclusion(s): Morbid obesity is associated with increased clot burden and higher rate of VTE recurrence off anticoagulation without increased clinically significant bleeding. No differences were seen between uncapped or reduced enoxaparin dosing strategies in these patients. As the prevalence of global obesity rises, it is important to optimise the anticoagulation strategies in this population.


PB1333
Hand grip strength and risk of incident venous thromboembolism: The Tromsø Study
O. Leknessund 1; V. Morelli2; B. Strand3; J. Hansen4; S. Brækkan4
1 UiT – The Artic University of Norway, Tromsø, Troms, Norway; 2 UiT – The Arctic University of Norway, Tromsø, Norway, Tromsø, Troms, Norway; 3 Norwegian Institute of Public Health, Oslo, Oslo, Norway; 4 Thrombosis Research Center, Department of Clinical Medicine, UiT – The Arctic University of Norway, Tromsø, Norway, Tromso, Troms, Norway
Background: Hand grip strength (HGS), commonly used as a proxy of whole‐body muscular strength, is associated with a wide range of adverse health outcomes and mortality. However, there is limited data on the association between HGS and risk of venous thromboembolism (VTE).
Aims: To investigate the association between HGS and risk of incident VTE in a population‐based cohort.
Methods: Participants (n = 13,648) from the 4–7th surveys of the Tromsø Study (T4‐T7, enrolment: 1994–2016) were followed throughout 2020, and all incident VTEs were recorded. HGS of the non‐dominant hand was measured using a Martin vigorimeter (T4‐T6) and a Jamar Digital Dynamometer (T7). Hazard ratios (HR) for VTE according to low HGS (< 25th percentile) were estimated using Cox regression models, and adjusted for age, sex, body height, body mass index, physical activity, and comorbidities (cardiovascular disease and cancer).
Results: During a median of 6.9 years of follow‐up, 545 incident VTEs occurred. The crude incidence rate per 1000 person‐years was 4.5 (95% CI: 3.9–5.2) in those with low HGS and 2.7 (95% CI: 2.4–3.0) in those with normal/high HGS (≥25th percentile). Participants with low HGS had a 27% higher risk of VTE (HR 1.27, 95% CI: 1.02–1.56) compared to those with normal/high HGS (Table 1). Subgroup analyses revealed that the risk estimates were higher for unprovoked VTE (HR 1.37, 95% CI: 0.96–1.91) and deep vein thrombosis (DVT, HR 1.50, 95% CI: 1.13–1.99). Similar results were found in analyses restricted to men, women, and elderly (>75 years), respectively. Furthermore, the association between low HGS and overall VTE was stronger in analysis restricted to the first 5 years of follow‐up (HR 1.48, 95% CI: 1.01–2.17).
Conclusion(s): A weak HGS was associated with increased risk of VTE, and particularly unprovoked VTE and isolated DVT. Our findings suggest that poor muscle strength is a risk factor for VTE.

PB1327
Health‐Related Quality of Life and physical capacity in patients with residual perfusion defects after pulmonary embolism
Ø. Jervan 1; S. Parker2; J. Gleditsch3; D. Rashid3; W. Ghanima4
1 Hospital of Ostfold Norway, Fredrikstad, Ostfold, Norway; 2 Department of physical medicine and rehabilitation, Hospital of Ostfold, Kalnes, Moss, Ostfold, Norway; 3 Department of Radiology, Hospital of Ostfold, Kalnes, Sarpsborg, Ostfold, Norway; 4 Østfold Hospital Foundation, Sarpsborg, Ostfold, Norway
Background: Residual perfusion defects (RPD) are common after pulmonary embolism (PE), but their impact on Health‐Related Quality of Life (HRQoL) and physical capacity is not well known.
Aims: The aim of this study was to explore differences in HRQoL and physical capacity between PE‐survivors with and without RPD.
Methods: The study cohort is a part of the PE REHAB‐trial (NCT03405480), an ongoing randomised controlled trial investigating the effect of pulmonary rehabilitation in patients with persistent dyspnea after PE. Patients aged 18–75 years with a history of PE within 6–72 months, confirmed by computed tomography pulmonary angiography, were included in the present study. Patients with significant cardiopulmonary comorbidities or diagnosed chronic thromboembolic pulmonary hypertension were excluded. Participants (n = 274) performed ventilation/perfusion (V/Q) scintigraphy and the Incremental Shuttle Walk Test (ISWT) to assess physical capacity. V/Q scintigraphy were deemed either positive or negative, according to the European Association of Nuclear Medicine criteria. In addition, participants completed the Euroqol 5‐level (EQ5D5L) and Pulmonary Embolism Quality of Life‐questionnaires. Data are presented as median and interquartile range, and analyses were performed using Mann‐Whitney U‐test.
Results: RPD were present in 71/274 (26%). Hypothyroidism was more prevalent in those with RPD, but otherwise there were no significant differences regarding demographic data or comorbidities. Patients with RPD had significantly reduced exercise capacity compared to those without, median ISWT distance 660 vs 805 meters, p‐value 0.01. Apart from reduced EQ5D visual analogue scale (71 vs 65 points, p‐value 0.02) in patients with RPD, there were no differences in other measurements of HRQoL.
Conclusion(s): RPD are common after PE and are associated with impaired physical capacity. EQ5D visual analogue scale was the only measurement of HRQoL being slightly reduced in this cohort.

VTE Prophylaxis
PB1369
Challenges of anticoagulation management service and need of establishing pharmacist‐led anticoagulation clinic in tertiary care teaching hospital, Ethiopia: A qualitative study
T. Tadesse
Tikur Anbessa Specialized Hospital, College of Health Sciences, Addis Ababa University, Addis Ababa, Adis Abeba, Ethiopia
Background: The complexity of anticoagulation management has led to the advancement of a variety of care models comprising patient self‐management, anticoagulation clinics, and pharmacist‐managed services in many countries.
Aims: To explore the challenges of AMS, and assess the need for establishing pharmacist‐led anticoagulation clinic (PLAC) at Tikur Anbessa Specialized Hospital (TASH), Addis Ababa, Ethiopia
Methods: A qualitative study was conducted at TASH. A semi‐structured interview guide was used by interviewing 15 physicians from different specialties, heads of pharmacy and laboratory departments, and 20 patients to explore their overall ideas, perception, experience about AMS and the challenges of AMS, and the need of establishing PLAC in the hospital.
Results: Only 3 physicians responded they had warfarin initiation and maintenance dosing protocols. Out of 15 respondents, three, seven, six, and four of them stated that they had protocols for venous thromboembolism (VTE) risk assessment; VTE prophylaxis and treatment; and bleeding risk assessment, respectively. Out of the total, 80% of respondents stated that inadequately trained healthcare professionals (HCPs), absence of separate anticoagulation clinic for anticoagulation management and patient education, irregular availability of anticoagulants and INR testing, and long appointment time, were the main challenges of existing AMS. All patients mentioned they have bought the anticoagulant and tested their INR out of the hospital on more than one occasion. Fourteen (70%) described that there was a long waiting time that affects their satisfaction towards the AMS provided to them. The interviewees suggested that it is necessary to establish PLAC with the well‐adopted standard operating procedure (SOP), skilled manpower, sufficient training for assigned staff, and sustainable supply of anticoagulants and INR testing.
Conclusion(s): There were many limitations in the anticoagulation management service of the studied hospital. Based on these recommendations and literature consultation and experience elsewhere PLAC was established in TASH.
PB1371
Translation and validation of the Caprini risk assessment score for Urdu speakers
K. Lalani1; J. Bishop‐Royse2; J. Tariman2; A. Tafur 3
1 NorthShore University HealthSystem, Skokie, Illinois, United States; 2 DePaul University Chicago, Chicago, Illinois, United States; 3 Northshore University Hospital, Evanston, Illinois, United States
Background: Venous Thromboembolism (VTE) is a potentially fatal condition and determination of risk level and identification of risk factors for VTE should be performed for the post‐operative period. The Caprini Risk Assessment Score (CRS), is a validated risk assessment tool used widely to predict post‐operative VTE in the Western world, but it has limited utility among resource‐poor and developing countries due to limited English language proficiency of potential instrument users.
Aims: We aimed to translate the CRS tool to Urdu and validate its use in the Urdu speaking population through correlation between self‐administered and healthcare professional‐administered scores.
Methods: The CRS was translated into Urdu by a certified translation service. Focus group interviews were conducted to identify comprehension challenges with the translation. For validation, self‐administered CRS scores were compared to the advanced practice nurse (APN)‐administered scores. The study was approved by the DePaul University, Chicago IRB under a waiver of informed consent.
Results: A 6‐member focus group (average age 51.5y (range 38–68), 33% male, average education level grade 14 (range 8–16)) provided feedback for the final revisions of the translated tool. 30 volunteers (average age 50.1y (range 28–65), 50% male, average education level grade 15.4 (range 12–18) completed the final tool and were interviewed by the APN to obtain comparative scores. All participants were proficient in both English and Urdu. Using SPSS v.23, Pearson’s correlation coefficient between patient‐ and APN‐administered scores was excellent (1.000).
Conclusion(s): The first Urdu version of the patient‐completed risk assessment model is another step forward in addressing the huge economic and health burden related to VTE. One study limitation was the lack of volunteers with below high school education level.


VPB1381
A case series in pregnant women receiving therapeutic LMWH, is monitoring anti‐Xa levels necessary?
S. Chong1; P. Liew2; V. Selvaratnam 3; S. Tan2
1 Dr, Telok Panglima Garang, Selangor, Malaysia; 2 Hospital Ampang, Ampang Jaya, Selangor, Malaysia; 3 Department of Haematology, Ampang Hospital, Ministry of Health Malaysia, Ampang Jaya, Selangor, Malaysia
Background: Pregnancy increases the risk of thrombosis. It's a constant issue for treating doctors since it raises the risk of venous thromboembolism (VTE) and valve thrombosis in women who have mechanical heart valves (MHV). The dosage of low molecular weight heparin (LMWH) is usually determined by weight, but the management of LWMH dosage during pregnancy is debatable. Some research imply that dose adjustments should be monitored with anti‐Xa levels, while others argue that weight‐based dosing eliminates the need for adjustments.
Aims: The anti‐Xa level in MHV and VTE patients was the main focus of our investigation. Secondly, we compared their actual LMWH dosing and dosage adjustments in both groups based on anti‐Xa levels.
Methods: This is a retrospective cohort between 2020 and 2021 of all pregnant women who were therapeutically anticoagulated with LMWH and had anti‐Xa levels of at least 1 peak (± trough) antenatally.
Results: During the study period, 4 (80.0%) of the women with MHV (n = 5) were kept on the same LMWH dosage throughout the antenatally, whereas 1 (20.0%) required dose modifications based on trough and peak anti‐Xa levels. 4 (57.1%) of the women with VTE (n = 7) showed subtherapeutic levels of peak anti‐Xa in the VTE group. However, the dosage was not changed throughout pregnancy. LMWH therapy did not cause any bleeding or clotting issues. No reports of maternal thrombotic complications were identified.
Conclusion(s): In pregnant women with high risk such as MHV and VTE, monitoring by trough and peak anti‐Xa levels maybe be use for dosage guidance on a case‐by‐case basis. However a well‐designed prospective trial evaluating the effect of anti‐Xa monitoring strategies on clinically important outcomes is needed.
VPB1379
Multi‐dimensional instrument assessment of adherence to warfarin therapy: methodological study
M. Sousa Vianna1; M. Praxedes2; I. Afonso Reis1; A. Silvina Pagano1; C. Barbosa Ferreira1; R. Costa Viana1; D. Vieira Nascimento1; M. Parreiras Martins 1
1 Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 2 Universidade Federal do Recôncavo da Bahia, Salvador, Bahia, Brazil
Background: Adherence to warfarin therapy is relevant in clinical practice due to the great variability in dose‐response and the risk of complications. To date, there are no instruments for multi‐dimensional assessments of warfarin therapy validated in Brazilian Portuguese.
Aims: To describe the process of development the instrument focused on a multi‐dimensional assessment of adherence to warfarin therapy.
Methods: The elaboration of the instrument entitled “Questionnaire for the evaluation of multi‐dimensional adherence to warfarin therapy” followed these stages: (i) conceptual map; (ii) preliminary version of the instrument; (iii) analysis performed by judges; (iv) semantic analysis of the items through a pre‐test with 30 patients. The quantitative analysis was performed by calculating the Content Validity Coefficient (CVC) for the instruction and for each item.
Results: Six judges assessed the content validity and a sample of 30 patients participated in the pre‐test. Of the initial 35 items, 14 were removed and 19 were modified, resulting in 21 items in the final version of questionnaire. The CVC calculation was 0.7, demonstrating that the items are relevant. The domains and their number of related items were: self‐care related to warfarin therapy (7); health monitoring (4) and administration of pills (10). Approximately 77% of the patients completely understood all the items. The semantic analysis indicated the need to modify three of the 21 items of the scale. The changes involved inclusion and modification of alternatives and inclusion of terms in the wording.
Conclusion(s): The development process of the multi‐dimensional instrument to assess adherence to warfarin followed the internationally recommended steps and was successfully carried out. The results obtained showed that the instrument has adequate content validity, with good acceptance and understanding of the items by the patients. It is recommended that the psychometric properties of the developed instrument be evaluated to ensure its validity and reliability to achieve the proposed objectives.

PB1354
Venous thromboembolism prophylaxis use by pediatric orthopedic surgeons in Canada
J. Curwin 1; W. MacNevin1; R. El‐Hawary2; K. Kulkarni2
1 Dalhousie University, Halifax, Nova Scotia, Canada; 2 IWK Health Centre and Dalhousie University, Halifax, Nova Scotia, Canada
Background: A novel pediatric venous thromboembolism (VTE) screening tool was developed and implemented in 2016 at the IWK Health Centre in Halifax, Canada, which safely reduced use of thromboprophylaxis by 47.9% with no increase in VTE in the pediatric orthopedic surgical population (POSP). There is currently no data on the current practices or protocols for VTE prophylaxis for POSP in Canada.
Aims: The current survey was designed to assess current practices regarding VTE prophylaxis in POSP in Canada.
Methods: After ethics approval, a 22 question survey was administered electronically to all Canadian Pediatric Orthopaedic Group (CPOG) members. The survey contained questions on respondent demographics and background, current VTE prophylaxis practices and experiences including indications for prophylaxis, existence of VTE protocols and details regarding further potential collaboration. Descriptive statistical analyses were completed on the survey responses.
Results: Of the 100 CPOG members, 49 (49%) responded. Table 1 shows the demographic characteristics and practices of respondents. Table 2 highlights VTE prophylaxis practices and experiences in Canada. 57% (n = 28) do not have a defined protocol for VTE prophylaxis and 18% were uncertain if they do. 85% (n = 26) were open to utilizing a VTE prophylaxis screening tool and 12% are uncertain if they would be.
Conclusion(s): This study has demonstrated that a uniform protocol for VTE prophylaxis does not exist in most Canadian centres, despite its need. There was a nation‐wide interest in adopting a perioperative VTE prophylaxis screening tool to optimize pharmacologic thromboprophylaxis use in POSP. The goal of future research is national implementation and standardization of such a screening tool through collaboration with other institutions in a multi‐centre study.


PB1359
Prevention of VTE in hospitalized patients with acute infectious disease: Online CME improves physician competence and confidence
M. Harris 1; C. Padbury2; M. LaCouture3
1 Medscape, Atlanta, Georgia, United States; 2 Medscape, New York, New York, United States; 3 Medscape, New York, New York, United States
Background: Clinicians need updates on VTE prevention strategies for hospitalized medically ill patients.
Aims: This study examined whether interactive, case‐based, online CME could improve competence and confidence of cardiologists related to prevention of VTE in patients hospitalized with acute infectious disease.
Methods: Educational design included a “test, then teach” approach to elicit cognitive dissonance, with evidence‐based feedback provided following each learner response. Educational effect was assessed using a repeated‐pair design with pre‐/post‐assessment. Three multiple choice questions assessed knowledge/competence, and 1, rated on a Likert‐type scale, assessed confidence. A paired samples t‐test was conducted on overall average number of correct responses and for confidence rating, and a McNemar’s test was conducted at the question level (significance level, p < 0.05).
Results: 186 cardiologists were included in the analysis. 83% of cardiologists improved their competence related to use of evidence‐based antithrombotic strategies for VTE prevention in patients hospitalized with acute infectious disease (p < 0.001), showing a 75% relative change in responses correct from pre‐ to post. On a question level, further results indicate: 54% improvement 41% reinforcement (p < 0.001) in competence in use of evidence‐based antithrombotic strategies for VTE prevention in patients hospitalized with COVID‐19; 60% improvement and 25% reinforcement (p < 0.001) in competence of the use of evidence‐based risk assessment tools to determine need for thromboprophylaxis in patient hospitalized with COVID‐19; 49% measurable increase in confidence in ability to prevent VTE in patients hospitalized with COVID‐19, resulting in 41% who were mostly or very confident after education (p < 0.001).
Conclusion(s): This study demonstrates the success of interactive, case‐based, online CME at improving competence and confidence of cardiologists related to prevention of VTE in hospitalized patients with acute infectious disease. Case‐based education with interactive questions and detailed explanations of best practices should be employed more often to help clinicians apply knowledge and evidence into practice to improve patient management.
PB1358
Effectiveness of an electronic decision support system for management of venous thromboembolism in hospitalized patients
Y. Guo 1; Y. Yang2; Y. Yao3
1 Sixth Medical Center, Chinese PLA General Hospital, Beijing, Beijing, China (People's Republic); 2 Sixth Medical Center, Chinese PLA General Hospital, 100853, Beijing, China (People's Republic); 3 Chinese Pla General Hospital, 100853, Beijing, China (People's Republic)
Background: Different scores have been recommended for symptomatic venous thromboembolism (VTE) risk assessment, however, there is a heterogeneity in the handling of these risk scores for the clinician.
Aims: To evaluate the effectiveness of an electronic decision support system for management of VTE in hospitalised patients.
Methods: An electronic decision support system for VTE risk assessment and integrated care (DeVTEare) based on hospital information system has been developed, which consisting of VTE, PE, and bleeding risk assessment (Padua for medical patients, Caprini for surgical patients, Geneva, sPESI for PE, HAS‐BLED), together with the guideline‐recommended treatments. The DeVTEare was input in hospital information system in Sixth Medical Center, Chinese PLA General Hospital since August 1, 2021. The VTE events were included into the study between January 1, 2021 to January 1, 2022.
Results: There were 36,005 adult patients (mean age, SD, 53,19, female, 47.1%) admitted to the hospital, and 169 (0.5%) inhospitalised DVT and 22 (0.1%) PE, respectively. Using DeVTEare, the rate of VTE risk assessment was 98.5% for all in‐hospital patients, the rate of PE risk assessment was 90.4% for patients at high‐risk of VTE, the rate of preoperative PE risk assessment (Geneva score) was 94.4%, and the assessment rate of PE severity (sPESI) was 91.7% in patients admitted with PE (n = 203, mean, SD, 66,15, female 42.4%), while bleeding risk assessment was 92.1%. There were 34.8% patients receiving surgery and 8.1% inhospitalised medical patients at high risk of VTE. After adjusting age, sex, CAD, hypertension, diabetes, cancer, etc., the odd ratio (OR, 95% confidential interval, CI) of DeVTEare use was 0.58 (0.39–0.86) (p = 0.007) for inhospitalised VTE, 0.64 (0.49–0.84) (p = 0.001) for the death of hospitalized VTE, and 0.58 (0.45–0.74) (p < 0.001) for the death of inhospitalised PE.
Conclusion(s): The electronic decision support system for VTE risk assessment and integrated care, DeVTEare, facilitated VTE prevention and reduced hospitalized VTE events.
VPB1376
the use of aspirin thromboprophylaxis compared to alternative anticoagulants in elective total hip replacement: A retrospective cohort study
R. Norman 1; J. Kielly2; K. Osei Bonsu3; R. Chitsike4; S. Young5
1 Eastern Region Health Authority, St. John’s, Newfoundland and Labrador, Canada; 2 Memorial University School of Pharmacy / Eastern Region Health Authority, St. John’s, Newfoundland and Labrador, Canada; 3 Memorial University School of Pharmacy, St. John’s, Newfoundland and Labrador, Canada; 4 Eastern Region Health Authority / Memorial University Faculty of Medicine, St. John’s, Newfoundland and Labrador, Canada; 5 Memorial University / Eastern Region Health Authority, St. John’s, Newfoundland and Labrador, Canada
Background: The role of Aspirin (ASA) thromboprophylaxis following elective total hip replacement (THR) remains unclear given the lack of high‐quality evidence and differing guideline recommendations. There is limited data on current prescribing patterns of ASA versus other anticoagulants in THR.
Aims: To identify prescribing patterns of ASA compared to alternative anticoagulants in patients who undergo THR within a Canadian health region.
Methods: We conducted a retrospective cohort study of patients who had elective THR between January–June 2019. Data collected included drug and patient‐specific characteristics. Continuous variables were presented as means with standard deviation and frequencies and percentages for categorical data. Chi‐square and t‐tests were used to compare proportions and means between treatment groups for categorical and continuous variables respectively. Multivariable logistic and Poisson regressions were used to determine predictors of treatment choices (ASA versus rivaroxaban) and estimate the association of treatment with healthcare utilization.
Results: A total of 180 patients who underwent THR were included, with 153/180 (85%) receiving ASA and 27/180 (15%) receiving rivaroxaban. The most common ASA regimen prescribed was 325mg daily (152/153, 99.3%) for a mean of 43 ± 6.9 days. Patient characteristics that differed for ASA versus rivaroxaban respectively included a history of malignancy (6.5% vs 51.9%, p < 0.001), previous VTE (0% vs 11.1%, p = 0.001), thrombophilia (0% vs 3.7%, p = 0.02) and receipt of chemotherapy (0% vs 11.1%, p < 0.001). Multivariate regression showed patients with a history of malignancy were more likely to receive rivaroxaban compared to ASA (odds ratio 31.65, 95% CI 18.22–2.4 × 104, p < 0.001). No differences were seen between the groups for health care utilization.
Conclusion(s): This study showed ASA utilized as thromboprophylaxis following elective THR in a substantial percentage of patients within the health region. Significant differences were seen between characteristics of patients who received ASA versus rivaroxaban. The clinical relevance of the observed differences should be further explored.
VPB1377
The use of warfarin in patients with heart diseases in Brazil and factors associated with non‐adherence to treatment
W. Freitas Nunes De Sousa1; N. Sernizon Guimarães1; R. Pedra De Souza1; M. Da Costa Rocha1; A. De Oliveira Magalhães Mourão1; K. Braga Gomes Borges1; C. Margotto Bartollo1; M. Praxedes2; M. Parreiras Martins 1
1 Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 2 Universidade Federal do Recôncavo da Bahia, Salvador, Bahia, Brazil
Background: Oral anticoagulants (OAC) are indicated for primary and secondary thromboprophylaxis and warfarin has been extensively used in clinical practice. Non‐adherence to treatment can lead to therapeutic failure, increased risk of thromboembolic events and death.
Aims: The aim of this study was to assess factors associated with non‐adherence to warfarin therapy in patients with heart disease.
Methods: This is a retrospective cohort study developed in an anticoagulation clinic at the Southeastern Brazil. Data were collected by patient interview and review of medical records. Variables included sociodemographic, clinical and behavioral data. Self‐reported non‐adherence was the outcome of interest. Univariate analysis was performed to identify variables with p‐value < 0.20. A multivariate model was obtained by calculating the relative risk with exponentiation of regression coefficients and the results were described by incidence risk ratios (IRR).
Results: A total of 312 patients were included, with a mean age of 60.4 ± 13.5 years, with predominance of women (n = 187; 59.9%) and atrial fibrillation (AF)/flutter was the main indication for warfarin use (n = 232; 74.4%). Overall, 55.5% (n = 173) of study participants were considered non‐adherent. The risk factors associated with non‐adherence to warfarin therapy were the presence of systemic arterial hypertension (IRR = 1.36), AF/flutter (IRR = 1.50), INR 2.5–3.5 (IRR = 1.56), absenteeism (IRR = 1.15) and non‐severe bleeding reports (IRR = 1.14). The protective factors associated to non‐adherence were higher time in therapeutic range (TTR) (IRR = 0.97), use of amiodarone (IRR = 0.54) and use of diuretics (IRR = 0.70).
Conclusion(s): The knowledge of factors associated with non‐adherence is relevant to clinical practice as it may substantiate educational interventions to improve self‐care and consequently adherence to warfarin therapy.
PB1357
Pharmacological thromboprophylaxis to prevent venous thromboembolism in ambulatory patients with cancer undergoing antineoplastic drugs in real world clinical practice: A cohort study
M. Donadini 1; A. Squizzato2; P. Ferrazzi3; S. Maggioni4; C. Sacco3; C. Lodigiani5
1 Department of Medicine and Surgery, University of Insubria and ASST Sette Laghi, Varese, Italy, Varese, Lombardia, Italy; 2 University of Insubria, Varese and Como, Italy, Como, Lombardia, Italy; 3 IRCCS Humanitas Research Hospital, Rozzano, Italy, Rozzano, Lombardia, Italy; 4 School of Medicine, University of Insubria, Varese, Italy, Milano, Lombardia, Italy; 5 Center for Thrombosis and Hemorrhagic Diseases, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy, Rozzano, Lombardia, Italy
Background: Venous thromboembolism (VTE) is the second leading cause of death in cancer patients. Clinical prediction models can identify ambulatory patients undergoing antineoplastic drugs who may benefit from primary pharmacological thromboprophylaxis. Evidence is scant on determinants of thromboprophylaxis and outcomes in clinical practice.
Aims: To estimate the incidence of VTE in a cohort of cancer ambulatory patients undergoing antineoplastic drugs, who were systematically assessed for VTE pharmacological thromboprophylaxis.
Methods: In a retrospective, observational cohort study, based on a standardized protocol being active at the Humanitas Research Hospital from 2012, consecutive adult ambulatory patients starting antineoplastic drugs were evaluated for VTE thromboprophylaxis (low molecular weight heparin 80 UI/Kg) based on Khorana score and clinical judgment. The primary outcome was symptomatic VTE and asymptomatic deep vein thrombosis at ultrasound performed at 6 months. The study was approved by the local institutional review board.
Results: 443 patients were enrolled up to November 2019. Baseline characteristics are described in Table 1. Among 206 patients (46.5%) who were given thromboprophylaxis, 112 (54.4%) had Khorana score ≥ 2, as compared to 30/227 (13.2%) who did not receive thromboprophylaxis (p < 0.01). Indications for thromboprophylaxis in the remaining 94 patients who had Khorana score < 2 were: previous VTE (6 patients), other VTE risk factors (31), cancer‐related risk factors (34), thrombophilia (5), multiple indications (18). Patients receiving thromboprophylaxis had a worse performance status (p 0.03). At 6 months, VTE occurred in 32/443 (7.2%) patients, of whom 12/206 (5.9%) on thromboprophylaxis and 20/227 (8.9%) not receiving thromboprophylaxis (p 0.27). The same comparison for major bleeding was 3.9% and 2.6% (p 0.45) and for death 4.4% and 0.4% (p 0.01).
Conclusion(s): In real‐world clinical practice setting, pharmacological thromboprophylaxis choice was based on Khorana score or other clinical determinants. This strategy was associated with similar VTE incidence as compared to clinical trials.

VPB1375
Efficacy and safety of extended thromboprophylaxis with Apixaban after endovenous ablation for varicose veins in patients with a history of thrombophlebitis and venous thromboembolism
S. Machuskyi 1; O. Voloshyn2; O. Suzdalenko1; V. Gubka1; V. Pavlychenko1
1 Angiolife Vascular Center, Zaporizhzhia, Zaporiz'ka Oblast', Ukraine; 2 Angiolife Vascular Center, Zaporizhzhua, Zaporiz'ka Oblast', Ukraine
Background: Venous thromboembolism is extremely serious complication after endovenous ablation, occurring more frequently in patients with superficial and deep vein thrombosis in past medical history.
Aims: Evaluation of efficacy and safety of prolonged thromboprophylaxis after endovenous procedures in patients at high thrombotic risk.
Methods: 62 patients (28 males and 36 females with a mean age of 42.3 ± 8.7 years) were included. 54 patients had a history of superficial vein thrombosis, which was conservatively treated (there were no clinical signs of phlebitis and complete or partial recanalization of the superficial veins was achieved). The average time from the onset of the phlebitis to the moment of ablation was 85 ± 12.2 days. 8 patients had history of deep vein thrombosis provoked by a major risk factor (complete recanalization of deep veins with reflux less than 1.5 s was detected in all patients). In all patients, thromboprophylaxis with Apixaban 2.5 mg BID for 30 days after endovenous ablation was used. The detection of thromboembolism was based on serial ultrasound examination of the venous system at 1, 7, 30 and 90 days after ablation.
Results: None of the patients had life‐threatening complications during the follow‐up period (pulmonary embolism or major bleeding). In 1 patient (1,61%), distal deep vein thrombosis in the sural vein was detected 7 days after treatment, which was successfully treated with therapeutic doses of Apixaban for 3 months. In 2 patients (3,27%), EHIT 2 was noted, which was resolved spontaneously during 1‐st month of observation. 2 patients (3,27%) had minor bleeding (1 nasal and 1 gynecological due to intrauterine polyp) during the observation period of 3 months.
Conclusion(s): Extended thromboprophylaxis with Apixaban 2.5 mg BID is a safe and effective approach in reducing the risk of thromboembolism in patients requiring endovenous ablation, but further studies are needed to test the hypothesis in a larger patient population.
VPB1382
Prolonged post‐operative hypercoagulability can be defined using serial thrombelastography in patients with metastatic bone disease
L. Yamaura; L. Skeith; M. Monument; P. Skeith
University of Calgary, Calgary, Alberta, Canada
Background: Presence of malignancy places patients with metastatic bone disease (MBD) undergoing orthopaedic surgery at 7‐fold higher risk for post‐operative venous thromboembolism (VTE) compared to non‐cancer orthopaedic patients. Thromboprophylaxis guidelines are undefined for this vulnerable group, as the extent and duration of post‐operative hypercoagulability (and concomitant VTE risk) remain unknown. Thrombelastography (TEG) is a point‐of‐care blood test that can evaluate coagulation, from clot formation to lysis. Maximal amplitude (MA) measures clot strength and values ≥65 mm can define hypercoagulability and predict VTE events in orthopaedic surgery patients.
Aims: Quantify the extent and duration of post‐operative hypercoagulability and evaluate coagulation profiles in those with and without VTE events.
Methods: Consecutive adults with MBD undergoing orthopaedic surgery for fracture treatment were enrolled into this single‐centre, prospective cohort study (HREBA.CC‐20‐0147). Patients provided informed consent to collect blood samples (pre‐operatively; on post‐operative day (POD) 1, 3, and 5; and 2‐, 6‐, and 12‐weeks post‐operatively) and undergo a bilateral lower extremity screening Doppler ultrasound on POD3. TEG analysis was performed using TEG®6s haemostasis analyzers (Haemonetics Corporation). Image‐confirmed VTE was captured throughout the study and defined as symptomatic or asymptomatic. Thromboprophylaxis prescription and compliance were recorded. Descriptive statistics and one‐sample t‐tests were performed to compare MA values at VTE diagnosis with mean MA of patients without VTE complications.
Results: Nineteen participants (68 ± 12 years) were enrolled. VTE incidence rate was 21.1% (Table1). At the time of VTE diagnosis, mean MA was 68.3 ± 5.3 mm and all VTE events occurred by POD5. Within this timeframe, regardless of VTE incidence, most patients were hypercoagulable (POD3: 82.4%; 2‐weeks: 91.7%). Hypercoagulability persisted at 6‐weeks (mean MA: 65.0 ± 4.7 mm) and normalized at 12‐weeks (mean MA: 63.1 ± 5.1 mm) post‐operatively (Figure 1).
Conclusion(s): Patients with MBD are at highest risk for VTE complications within 2‐weeks post‐operatively. Duration of hypercoagulability may extend beyond 6‐weeks post‐operatively, warranting further investigation into disease‐specific hypercoagulability thresholds.


VPB1384
Pilot study of effect on thromboprophylaxis using system‐wide multifaceted quality improvement intervention based on clinical decision support system in hospitalized patients
K. Zhen 1; Q. Gao1; Z. Zhai2
1 China‐Japan Friendship Hospital, Beijing, Beijing, China (People's Republic); 2 Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China‐Japan Friendship Hospital, Beijing, Beijing, China (People's Republic)
Background: The VTE prophylaxis rate in hospitalized patients is low. System‐wide multifaceted quality improvement intervention may be an efficient strategy to optimize the VTE prophylaxis.
Aims: To explore the effectiveness of multifaceted quality improvement intervention in VTE prophylaxis in hospitalized patients.
Methods: Hospitalized patients admitted in the department of respiratory and critical care medicine, orthopedic, and general surgery wards were allocated into two groups based on their department admitted. The intervention group received regular care combined with multifaceted quality improvement intervention. The control group implemented the hospital’s current VTE prophylaxis measures. We analyzed the improvement of VTE risk assessment rate, the VTE prophylaxis rate, and the occurrence of hospital‐related VTE events.
Results: A total of 3,644 eligible patients were enrolled in the study. The VTE prophylaxis rate of the intervention group increased from 22.93% to 34.56% (p < 0.001), and the incidence of HA‐VTE events increased from 0.49% to 1.00% (p = 0.366). In the control group, the VTE prophylaxis rate increased from 21.65% to 27.16% (p = 0.091), and the incidence of HA‐VTE events increased from 0.47% to 2.02% (p = 0.001).
Conclusion(s): Multifaceted quality improvement intervention strategy is expected to facilitate implementation of recommended VTE prophylaxis and reducing the incidence of HA‐VTE incidents in hospital. However, it is necessary to conduct more multi‐center clinical trials in the future to provide more reliable real‐world evidence.

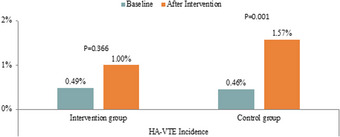
VPB1378
A systematic review of factors associated with non‐adherence to the use of coumarin derivatives or direct oral anticoagulants
W. Freitas Nunes De Sousa1; N. Sernizon Guimarães1; C. Costa Viana1; P. Machado1; A. Fonseca Medeiros1; M. Sousa Vianna1; R. Pedra De Souza1; C. Margotto Bartollo1; M. Praxedes2; M. Parreiras Martins 1
1 Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil; 2 Universidade Federal do Recôncavo da Bahia, Salvador, Bahia, Brazil
Background: Non‐adherence to thromboprophylaxis treatment with oral anticoagulants (OAC) is a public health problem and may be associated with high mortality rates.
Aims: We sought to synthesize the factors associated with non‐adherence to therapy with coumarin derivatives or direct oral anticoagulants.
Methods: A systematic review was performed at electronic databases Medline, Embase, CINAHL, Lilacs and grey literature (Google Scholar, MedNar, OpenGray, ProQuest Dissertations and Theses and hand search). This study was conducted according to Cochrane's method and PRISMA. The registration on PROSPERO is CRD42020223555.
Results: Overall, 1,270 studies were identified, and nine studies were selected for this review. In hand search, 77 studies were found, but none included. The associated factors with non‐adherence were heterogeneous, and some factors were described as both risk and protection for non‐adherence, with few variables showing consistent results among the studies. The factors associated only as risk were male sex, hospitalization, bleeding and Charlson score. The variables white race, number of medications in use and polypharmacy, duration of OAC therapy and CHADS2 or CHA2DS2VASc with scores ranging from 2 to ≥5 were cited just as protective factors.
Conclusion(s): Our systematic review showed risk and protective factors associated with non‐adherence. In clinical practice, the knowledge on the factors associated with non‐adherence is helpful to identifing patients at higher risk of complications that would benefit from individualized interventions.
PB1365
A tool integrated in the electronic health record to guide proper decision‐making regarding peri‐endoscopic anticoagulant use
A. Plender 1; L. Faber2; S. Graumans1
1 Rode Kruis Ziekenhuis, Beverwijk, The Netherlands, Amsterdam, Noord‐Holland, Netherlands; 2 Rode Kruis Ziekenhuis, Beverwijk, The Netherlands, Bloemendaal, Noord‐Holland, Netherlands
Background: Anticoagulants, like vitamin‐K‐antagonists (VKA) and direct oral anticoagulants (DOAC), are widely used, also among patients who undergo endoscopic procedures. To balance between bleeding and thrombotic risks careful decisions have to be made about whether and for how long anticoagulants have to be stopped peri‐endoscopically and if bridging is necessary (VKA). In our hospital, we created a tool in the electronic health record (EHR) to aid this decision making.
Aims: Firstly, this study aims to assess whether the tool is used properly. Secondly, how many bleeding and thrombotic events have occurred since the implementation of the tool.
Methods: Orders placed for endoscopies by medical specialists between 2018 and 2021 were included. By filling in the bleeding and thrombotic risks, the tool automatically generates an advice for the peri‐procedural anticoagulation. It was checked whether the decision‐making tool was filled in correctly. Correct use was defined as following the national protocol as shown in pop‐ups in the tool. Data about complications after endoscopies were collected retrospectively. A complication was defined as any thrombotic or bleeding event occurring within 30 days after endoscopy.
Results: In total 986 endoscopies were included for the analysis. The cumulative incidence for thrombotic or bleeding events was 2%. 14 bleeding events occurred, of which 1 needed intervention. There were 3 thrombotic events. In 89% the tool was used correctly; the main error was incorrect bleeding risk (8%).
Conclusion(s): This study evaluates the use of an EHR‐integrated decision‐making tool to aid proper anticoagulant use peri‐endoscopically. By analyzing the incorrect usage of the tool, we formulated several suggestions to improve the tool. As this study is not a comparative one, it does not provide evidence on whether the tool is able to reduce complications. Future research will examine whether this tool leads to fewer peri‐endoscopic complications.


PB1432
Predicting DVT Incidence based on risk factors by using Artificial Neural Networks
Y. Yang 1; Z. Zhongbin1; Y. Guo2
1 the 6th Medical Centre of Chinese PLA General Hospital, Beijing, Beijing, China (People's Republic); 2 Sixth Medical Center, Chinese PLA General Hospital, Beijing, Beijing, China (People's Republic)
Background: Deep vein thrombosis (DVT) is a common multifactorial disease. If the disease is not prevented as soon as possible, it may prolong the cure time and even lead to death.
Aims: To develop a simple, cost‐effective, accurate and non‐invasive prediction model by artificial intelligence machine‐learning model for early detection of high‐risk DVT patients.
Methods: 1295 patients were selected including all 729 patients with DVT and 566 patients without from January 2011 to December 2020 in the 6th Medical Centre of Chinese PLA General Hospital. An artificial neural network (ANN) predictive procedure divided three‐quarters of the patients (993 cases) randomly as training sample data to construct the predictive model while one quarter of the patients (302 cases) used to test the effectiveness of the constructed model. ANN inputs were gender, age, fibrinogen, D‐Dimer, hypertension, coronary heart disease, tumor, diabetes, chemotherapy, surgery etc.
Results: When the number of hidden‐nodes was set to 8 and the number of epochs was set to 800 in the artificial neural network model, the accuracy reached the highest with an average rate of 81.47% and variance of 0.0001. The prediction accuracy of ANN model was higher than that of linear Logistic regression model with an average rate of 79.80%.
Conclusion(s): With the help of computer‐based ANN model, the high‐risk DVT patients could be detected as early as possible, conducive to disease prevention and the DVT incidence reduction.
PB1361
Comparing the proportion of medical and surgical hospital inpatients requiring pharmacological thromboprophylaxis using different risk assessment models: an observational cohort study
D. Horner 1; R. Doonan2; G. Mike3; S. Goodacre3; B. Hunt4; K. de Wit5
1 Salford Royal NHS Foundation Trust, Lymm, England, United Kingdom; 2 Northern Care Alliance NHS Foundation Trust, Salford, England, United Kingdom; 3 University of Sheffield, Sheffield, England, United Kingdom; 4 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 5 Queen's University, Kingston, Ontario, Canada
Background: Risk Assessment Models (RAMs) are used at the point of hospital admission to quantify the individual risk of venous thromboembolism and guide prescribing of pharmacological prophylaxis. We recently conducted a systematic review describing 24 unique RAMs, with little evidence of comparative impact.
Aims: We sought to compare proportional recommendations for pharmacological thromboprophylaxis using six commonly applied RAMs alongside the UK Department of Health VTE risk assessment tool. Our main aim was to objectively characterise potential variation in prescribing.
Methods: A single centre observational cohort study. We identified a consecutive cohort of adult medical and surgical patients requiring hospitalisation during the calendar year 2019. We excluded patients requiring critical care admission, anticoagulation and pregnant/post‐partum patients. We extracted initial clinical data using hospital admission records and VTE risk assessments completed prospectively by clinical teams using a structured note within the electronic healthcare record. We identified additional variables apriori to enable further completion of 6 prespecified RAMs; research assistants then undertook case note review and extracted data points necessary to facilitate retrospective completion. We considered a sample of 250 patients to be sufficient to provide estimates of prescribing with a confidence interval range approaching 10%.
Results: We analysed 543 hospital episodes with VTE risk assessment performed. 289 episodes met exclusion criteria, leaving 254 episodes suitable for data extraction and comparative analysis. Overall recommendations for prescribing varied substantially between RAMs, ranging from 10% for the IMPROVE associative score to 91% for the Department of Health VTE risk assessment tool. Variation persisted throughout medical (11 to 92%) and surgical subgroups (9 to 88%).
Conclusion(s): Existing VTE RAMs produce highly variable rates of recommended pharmacological prophylaxis, when applied to a cohort of medical and surgical inpatients. The national UK Department of Health VTE RAM conferred significantly higher rates of prescribing than any other method.


VPB1373
Pre‐operative thromboprophylaxis does not cause increased bleeding events in patients with surgically‐treated hip fractures
H. Krzyzaniak; D. You; L. Skeith; P. Skeith
University of Calgary, Calgary, Alberta, Canada
Background: Current guidelines recommend pre‐operative initiation of thromboprophylaxis if surgery for hip fracture is delayed for more than 12 h to prevent post‐operative VTE. Yet, thromboprophylaxis is often not administered due to concerns regarding increased peri‐operative bleeding. Recent studies have found conflicting evidence on this topic.
Aims: The purpose of this study was to determine usage rates of pre‐operative thromboprophylaxis in this patient population and to compare the complication rate between patients who received and did not receive pre‐operative thromboprophylaxis following hip fracture surgery.
Methods: A prospective cohort (REB16‐2240) of patients over 50 years of age with hip fractures amenable to surgical treatment were recruited at a Level I trauma centre. Pre‐operative thromboprophylaxis was administered at the discretion of the surgical team. All patients received 28 days of thromboprophylaxis post‐operatively with low‐molecular‐weight‐heparin. Major and clinically relevant non‐major bleeding was based on International Society on Thrombosis and Haemostasis definitions. Standardized z‐scores and two‐sample t‐tests were used to compare outcomes.
Results: Of the 121 patients included in this study, 31 (25.6%) were given pre‐operative thromboprophylaxis. No significant differences were found between patients who received and did not receive pre‐operative thromboprophylaxis in terms of demographics, comorbidities, fracture type, or surgical procedure. Patients who were given pre‐operative thromboprophylaxis did not experience increased peri‐operative bleeding, but did have significantly longer time to surgery (p = 0.02), longer surgical duration (p = 0.03), and increased 3‐month mortality (p = 0.02) (Table 1).
Conclusion(s): Patients who were given pre‐operative thromboprophylaxis did not experience increased peri‐operative bleeding or VTE events; however, further research is needed to confirm these findings. The increased 3‐month mortality experienced in these patients may be attributed to pre‐fracture comorbidities, prolonged time to surgery, and duration of the operation.

PB1352
Risk factors of venous thromboembolism in patients with varicose disease of the lower extremities
O. Atamanyuk; V. Atamaniuk
Ivano‐Frankivsk National Medical University, Ivano‐Frankivsk, Ivano‐Frankivs'ka Oblast', Ukraine
Background: The true frequency of venous thromboembolism (VTE) after surgical treatment of lower extremity varicose vein disease is still uncertain, there is no evidence of the need for routine thromboprophylaxis.
Aims: To stratify the risk factors for VTE in patients with varicose vein disease.
Methods: A prospective study was conducted from 2019–2021 on 260 consecutive patients who underwent surgical treatment. Prior to surgery, risk factors for VTE were assessed with the Caprini score and ultrasound of the venous system of the lower extremities was performed. Sonographic monitoring in the post‐surgical period was performed in 72 h, after 7 days and 1 month. Postoperative VTEs were defined as EHIT (classes II–IV), DVT, and PE.
Results: Methods of treatment and clinical characteristics of patients are shown in Table 1. Analyzing the risk factors of VTE on the Caprini score: low risk was in 55 (21.1%) patients, moderate risk – 159 (61.2%), high – 46 (17.7%). All patients with low and moderate risk of VTE for thromboprophylaxis underwent elastic compression for 1 month, with a high risk factor additionally used pharmacoprophylaxis by LMWH or rivaroxaban 10 mg for one week. 5 (1.9%) patients in the postoperative period developed thrombotic complications. EHIT II was diagnosed in 1 (0.4%) patient with a moderate risk of VTE on the second day after treatment. Distal DVT was detected in 4 (1.5%) with a high risk of VTE. Clinically, no manifestations of PE were observed.
Conclusion(s): The main risk factors for VTE are previous episodes of deep or superficial vein thrombosis (p = 0.001), age of patients (p = 0.014), duration and volume of surgery (p = 0.001). Patients with high risk of VTE should be prescribed pharmacoprophylaxis for 7 days, and patients with a history of thrombotic events should have at least one month of that.

PB1363
Red‐light exposure reduces venous thrombosis in mice
E. Andraska1; C. Kaltenmeier1; A. arivudainambi1; E. Mihalko1; M. Dyer1; S. Shea2; R. Steinman1; M. Rosengart1; M. Neal 3
1 University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 2 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States; 3 Trauma and Transfusion Medicine Research Center, Department of Surgery, University of Pittsburgh, Pittsburgh, PA, Pittsburgh, Pennsylvania, United States
Background: Recent evidence suggests a link between venous thromboembolism (VTE) development and activation of the innate immune system. Studies suggest that light therapy, or photobiomodulation, augments immunity.
Aims: Develop a novel VTE prophylactic strategy utilizing photobiomodulation.
Methods: WT C57BL/6J mice were exposed to ambient (n = 13, fluorescent white light, 300 lux), red (n = 13, 617 nm, 1,700 lux), and blue (n = 13, 421 nm, 1,700 lux) light with 12:12h light:dark cycle. After 72h light exposure, mice underwent a model of VTE that includes ligation of the inferior vena cava. Thrombus and blood samples were harvested for analysis. Platelet aggregation and activation (CD41+/CD62P+) were evaluated. Platelet releasate was isolated and neutrophil extracellular trap formation (NETosis) was measured. Groups were compared with parametric (mean ± SD, t‐test) or non‐parametric tests (median[interquartile range], Mann‐Whitney test) depending on distribution of data. Patients undergoing cataract surgery with implantation of blue‐light filtering intraocular lenses (IOL) versus Natural IOL were evaluated for risk of VTE using multivariable logistic regression clustered by hospital.
Results: Red‐light exposure markedly reduced clot weight compared to ambient‐ and blue‐light exposed mice (ambient 21 [13–23]mg, red 2 [0–7.75]mg, blue 21 [18–29]mg; p < 0.01, Figure 1). Red‐light exposed mice had reduced levels of platelet aggregation (ambient 17.5 ± 3.4 ohm vs. red 5.0 ± 1.5 ohm; p = 0.02) and activation (CD62P+: ambient 77.6 ± 5.0% vs. red 61.8 ± 1.8%; p = 0.02). NETs were reduced in clots from red‐light exposed animals compared to blue and white. Platelet releasate from these animals resulted in reduced NETosis (fold‐increase from baseline: ambient 1.9 ± 0.1 vs. red 1.1 ± 0.1, p = 0.03). In a risk adjusted analysis, blue‐light filtering IOL were associated with reduced risk of VTE (OR 0.89; 95% CI 0.80–0.99; p = 0.04).
Conclusion(s): Exposure to red‐light reduced thrombus burden in a murine model of VTE. Reduced platelet activation and stimulation of neutrophils may be integral to reduce thrombosis. Human cataract patients display reduced VTE after unmasking the benefit of red‐light. Photobiomodulation is potentially a promising alternative for VTE prophylaxis.

PB1355
Thromboprophylaxis during lower limb immobilisation after injury – a review of 2 years’ practice at King’s College Hospital, London
E. Marrinan1; V. Speed2; G. Giron3; L. Georgiou4; R. Harris1; M. Al‐Agil1; L. Roberts1; R. Patel2; R. Arya2; J. Czuprynska 1
1 King's College Hospital, London, England, United Kingdom; 2 Kings College Hospital, London, England, United Kingdom; 3 King's College Hospital NHS Foundation Trust, London, England, United Kingdom; 4 Princess Royal University Hospital, London, England, United Kingdom
Background: The optimal thromboprophylaxis strategy for lower limb immobilisation is undetermined. The King’s College Hospital (KCH) risk assessment, for conservatively‐managed outpatients, offers weight‐based prophylactic‐dose low‐molecular‐weight heparin for the duration of immobilisation with 1 or more of: personal venous thromboembolism (VTE) history, active cancer or cancer treatment, pregnancy or < 6 weeks post‐partum, or Achilles tendon rupture.
Aims: To report the incidence of VTE in lower limb injury managed with immobilisation, VTE risk assessment rate, and the proportion of patients who received thromboprophylaxis.
Methods: Electronic medical records were searched using CogStack and Emergency department software to identify patients 18 years or over with lower limb injury conservatively managed with immobilisation between 1/1/2019–31/12/2020. Demographic, prescribing and the nature of injury and immobilisation data were collected. Objectively diagnosed VTE events were included if they occurred within 90 days of lower limb injury presentation. Data are described using descriptive statistics. Subgroup data are compared using the Mann‐Whitney test for continuous data or Chi‐squared for categorical data.
Results: 3482 patients were identified. Characteristics, VTE risk assessment rate and risk factors are summarised in table 1 and site of injury in Figure 1. There were 19/3482 (0.55%) episodes of VTE reported, occurring at median 18 (IQR 12–45) days from injury of which most were distal deep vein thrombosis (68.4%). 3236 patients (92.9%) had a documented VTE risk assessment. 352 patients (10.1%) received thromboprophylaxis or were already prescribed anticoagulation. Approximately half of VTE events occurred despite thromboprophylaxis.
Conclusion(s): Our results confirm a low incidence of VTE in patients with lower limb immobilisation using a simplified risk assessment tool. The risk assessment rate was high and a low proportion of patients received thromboprophylaxis.


PB1356
Efficacy and safety of prophylactic anticoagulation in adult and children patients with primary nephrotic syndrome: a systematic review and meta‐analysis
F. De Pascali 1; F. Brunini2; G. Rombolà2; A. Squizzato3
1 University of Insubria, Como, Lombardia, Italy; 2 Nephrology Unit, ‘Ospedale di Circolo’, ASST Settelaghi, Varese, Italy, Varese, Lombardia, Italy; 3 University of Insubria, Varese and Como, Italy, Como, Lombardia, Italy
Background: Nephrotic Syndrome (NS) in a disease associated with an increased incidence of venous thromboembolic events (VTE), approximately 10%. There are no solid recommendations about the best prevention strategy for VTE in NS.
Aims: To evaluate the efficacy and safety of prophylactic anticoagulation in patients with NS.
Methods: Studies evaluating prophylactic anticoagulation in NS were identified by electronic search of MEDLINE and EMBASE database until December 2021. Weighted mean proportion and 95% confidence intervals (CIs) of thromboembolic and hemorrhagic events were calculated using a fixed‐effects and a random‐effects model. Statistical heterogeneity was evaluated using the I2 statistic. The difference in the thrombotic events among groups were estimated as pooled odds ratio (OR) and corresponding 95% confidence interval (CI).
Results: Five cohort studies, for a total of 414 adult patients, were included. All the studies were at high risk of bias. Only two study had a control group. Weighted mean incidence of pulmonary embolism and deep vein thrombosis in patients who received VTE prophylaxis was 1.8% (95% CI 0.6–3.5%; I2 4.4%) and 0.9% (95% CI 0.2–2.2%; I2 43.4%%) at fixed‐effect model, respectively; no patient developed renal vein thrombosis. Weighted mean incidence of major bleeding in patients who received VTE prophylaxis was 2.3% (95% CI 1–4.2%; I2 25.4%) at random‐effect model, respectively. Patients who received VTE prophylaxis had a non‐statistically significant reduced risk of pulmonary embolism [OR 0.59 (CI 95% 0.13–2.65; I2 64.4%)], and an increased risk of major bleedings [OR 2.08 (CI 95% 0.41–10.45; I2 0%)] compared to whom did not received it.
Conclusion(s): Our findings suggest that prophylactic anticoagulation in adult patients with primary NS may reduce the risk of VTE, even if it may be associated with a not negligible bleeding risk.

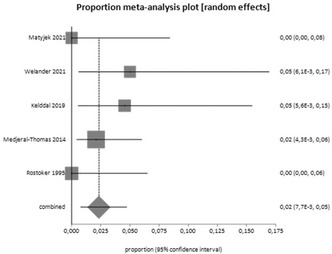
PB1360
Inter‐rater reliability of the UK Department of Health VTE risk assessment tool for medical and surgical hospital admissions
D. Horner 1; B. Daniels2; M. Holland3; S. Goodacre4; B. Hunt5; K. de Wit6
1 Salford Royal NHS Foundation Trust, Lymm, England, United Kingdom; 2 University of Manchester, Salford, England, United Kingdom; 3 University of Bolton, Bolton, England, United Kingdom; 4 University of Sheffield, Sheffield, England, United Kingdom; 5 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 6 Queen's University, Kingston, Ontario, Canada
Background: Half of all venous thromboembolic events relate to recent hospitalisation. These events are potentially preventable, through risk assessment and thromboprophylaxis. The majority of UK NHS hospitals use the Risk Assessment Model (RAM) proposed by the Department of Health, derived through expert consensus. It is unclear whether this tool has good inter‐rater reliability or is completed accurately within a pragmatic setting.
Aims: We sought to evaluate the inter‐rater reliability of the Department of Health RAM within a cohort of acute medical and surgical patients requiring emergency hospitalisation.
Methods: A single centre prospective observational cohort study, conducted during 2020 within a convenience sample of general medical and surgical patients requiring emergency hospitalisation. All patients underwent VTE risk assessment conducted by clinical teams during routine care. Risk assessment was subsequently repeated by trained research assistants within 24h of admission, blinded to the original evaluation. All research assistants underwent standardised VTE risk assessment training. A minimum sample size of 194 patients was predetermined. We used Cohen’s Kappa as a measure of inter‐rater reliability.
Results: During the study period, our observer team repeated 274 risk assessments. We excluded 38 cases classed as medical patients with no mobility restriction. 236 patient episodes with a completed clinical and research assistant risk assessment profile were analysed. 137 (58.1%) patients were hospitalised for medical reasons, and the remaining 99 (41.9%) for surgical reasons. Our results show substantial inter‐observer agreement for the Department of Health tool when used in acute medical admissions (Cohen’s Kappa 0.75 [95% CI 0.64 to 0.86]) but only moderate agreement (0.56 [95% CI 0.36 to 0.75]) for surgical admissions. The reliability of individual risk components was highly variable, with inter‐rater agreement ranging from 0.49 to 1.00
Conclusion(s): These findings suggest decision making on pharmacological prophylaxis may vary between clinicians for the same patient, despite the use of a national RAM.


PB1368
Risk of bleeding and venous thromboembolism in patients using therapeutic dosage of Direct Oral Anticoagulants undergoing total hip or total knee arthroplasty
M. Smeets 1; E. Kristiansen2; B. Nemeth1; M. Huisman3; S. Cannegieter1; A. Pedersen2
1 Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands; 2 Department of Clinical Epidemiology and Department of Clinical Medicine, Aarhus University, Aarhus, Midtjylland, Denmark; 3 Department of Medicine – Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, Zuid‐Holland, Netherlands
Background: About 1.0%‐1.5% of patients undergoing total hip (THA) or total knee arthroplasty (TKA) still develop postoperative VTE, indicating that current clinical practice of thromboprophylaxis is not sufficient in high‐risk individuals. Results from dose‐ranging studies with direct oral anticoagulants (DOACs) suggest a higher efficacy of therapeutic dosages of DOACs for VTE prevention, albeit at an increased bleeding risk.
Aims: To estimate the feasibility of providing DOACs in therapeutic dose as thromboprophylaxis for high‐risk VTE patients, we aimed to determine risks of bleeding and VTE in patients who underwent THA or TKA and who are also treated with long‐term DOAC in therapeutic dose, e.g., for atrial fibrillation (AF).
Methods: We conducted a registry‐based cohort study on Danish data from 2000 through 2018. The primary cohort of interest consisted of AF patients on therapeutic dosages of DOACs who underwent THA or TKA. Two control cohorts were formed: (1) patients undergoing THA/TKA using a prophylactic dose and (2) AF patients on therapeutic dose of DOACs not undergoing THA/TKA. 6‐ and 12‐week cumulative incidences of bleeding and VTE with death as competing risk were assessed.
Results: A total of 1,595 patients were included in the therapeutic dose THA/TKA cohort (Table 1). Of these patients 15 developed bleeding and 14 developed VTE. The 6‐ and 12‐week cumulative incidences for bleeding were 0.3% (95% CI 0.1 to 0.7) and 0.9% (95% CI 0.6 to 1.5), respectively (Table 2). For VTE these numbers were 0.8% (95% CI 0.4 to 1.3) and 0.9% (95% CI 0.5 to 1.4). Bleeding complications were lower in both control cohorts while VTE incidence was slightly higher in the THA/TKA control cohort on regular thromboprophylaxis.
Conclusion(s): These results suggest that a therapeutic dosage of DOACs might be a feasible option to prevent VTE after THA/TKA in patients at high risk of VTE which can form a basis for further studies.


PB1370
Anticoagulation control, outcomes and associated factors in patients receiving warfarin in long‐term care in Africa: A systematic review
T. Tadesse 1; G. Temesgen2; D. Yadeta3; L. Chelkaba2; T. Gedif2
1 Tikur Anbessa Specialized Hospital, College of Health Sciences, Addis Ababa University, Addis Ababa, Adis Abeba, Ethiopia; 2 School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Adis Abeba, Ethiopia; 3 School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Adis Abeba, Ethiopia
Background: Oral anticoagulation therapy with warfarin requires frequent monitoring level of anticoagulation by the international normalized ratio (INR).
Aims: Therefore, this systematic review aimed to systematically summarize anticoagulation control, treatment outcomes, and associated factors in long‐term patients receiving warfarin in Africa.
Methods: The literature search was conducted in PubMed, Cochrane Library, African Journal of Online databases, Google Scholar, and Google. An advanced search strategy was computed to retrieve relevant studies related to anticoagulation control and outcomes. Duplication, title and abstract screening, and full‐text assessment were conducted in Covidence software. Study quality was assessed using the Joanna Briggs Institute Critical appraisal quality assessment tool. The systematic review is registered in PROSPERO (CRD42021260772) and performed based on the PRISMA guideline.
Results: Out of 298 identified articles, 18 articles were eligible for the final review and analysis. The mean of 39.4 ± 8.4% time in therapeutic range (TTR) (29.4% to 57.3%), 36.7 ± 11.5% TTR (range 25.2–49.7%) and 46% TTR (43.5–48.5 %) was computed from studies that determined TTR by Rosendaal, direct and cross‐section‐of‐the‐files methods, respectively. The lowest percentage of TTR was 13.7%, while the highest was 57.3% was observed in this review. The highest percentage of patients (32.25%) who had TTR ≥ 65% was reported in Tunisia, but the lowest percentages were in Namibia (10%, TTR ≥ 65%) and Kenya (10.4%, TTR ≥ 70%). Generally, 10.4–32.3% of study participants achieved desired optimal anticoagulation level. Regarding secondary outcomes, 1.6–7.5% and 0.006–59% of patients experienced thromboembolic complications and bleeding events, respectively. The presence of chronic comorbidities, taking more than two drugs, and presence of medications that potentially interact with warfarin was the frequently reported predictors of poor anticoagulation therapy.
Conclusion(s): Oral anticoagulation control was suboptimal in patients taking warfarin as evidenced by low TTR in Africa. Therefore, there is an urgent need for further improving oral anticoagulation management service.
PB1372
Rivaroxaban in patients with intestinal failure on parenteral nutrition (TINCRBEL study)
T. van Haaps 1; R. Bavalia2; R. Mathôt3; M. Serlie4; S. Middeldorp5; M. Coppens6
1 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, the Netherlands., Amsterdam, Noord‐Holland, Netherlands; 2 Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 3 Department of Hospital Pharmacy, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 4 Department of Endocrinology and Metabolism, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands; 5 Radboud University Medical Center, Nijmegen, Gelderland, Netherlands; 6 Amsterdam University Medical Centers, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands
Background: Patients with intestinal failure (due to short bowel syndrome [SBS], other malabsorption or pseudo‐obstruction) on parenteral nutrition require central venous access which puts them at high risk for thrombosis. As INR control on vitamin K antagonist is often poor in these patients, many are on subcutaneous heparin for secondary prevention. The direct oral anticoagulant rivaroxaban is resorbed proximally in the gastro‐intestinal tract and might therefore be sufficiently resorbed.
Aims: To evaluate pharmacokinetics (PK) of rivaroxaban in patients on parenteral nutrition and to evaluate clinical outcome.
Methods: In this ongoing prospective cohort study, patients undergo a 6‐point PK analysis within 2 weeks after starting rivaroxaban 20 mg. Patients with adequate resorption compared to reference ranges taken from patients without intestinal failure. Those within range were allowed to continue rivaroxaban and were followed clinically for at least six months. Those below range were extensively counselled and allowed to continue as a shared decision. Clinical outcomes include: thrombosis, major bleeding and death.
Results: Seventeen patients, median age 61 yrs (IQR: 44–70 yrs) are thus far included (10 with SBS). Anticoagulation was prescribed for VTE (n = 16) or AF (n = 1). After PK analysis, rivaroxaban was continued in 14 (82%) patients. PK data are presented in Table 1. Mean Cmin was 37 ng/mL (90% CI 24–50), Cmax was 203 ng/mL (90% CI 155–251), and median Tmax was 3.0 h. During a cumulative follow‐up duration of 22.5 yrs thus far, no patients experienced thrombosis, major bleeding. One patient died at an elderly age, which was not related to the study drug.
Conclusion(s): These preliminary results suggest an adequate resorption of rivaroxaban in patients requiring parental nutrition due to SBS or intestinal malabsorption. Despite a high inter‐individual variability, this patient population can be treated with rivaroxaban. However, future results of this study should confirm the current clinical outcomes.


VPB1380
Prolonged platelet‐mediated hypercoagulability occurs following surgically treated hip fractures
P. Skeith; A. Dodd; P. Duffy; R. Martin; R. Korley; L. Skeith
University of Calgary, Calgary, Alberta, Canada
Background: Hip fractures are an epidemic and despite thromboprophylaxis, venous thromboembolism (VTE) rates remain high (2.5–5%). Serial thrombelastography (TEG) analysis can quantify hypercoagulable state and increased VTE risk. Platelet mapping (PLM) can be used to activate platelets at the adenosine diphosphate (ADP) receptor or the Thromboxane A2 (AA) receptor, to evaluate clot strength when platelets are activated through those specific receptors.
Aims: (1) To evaluate platelet contribution to hypercoagulability and (2) to compare hypercoagulability between arthroplasty and fracture fixation surgical treatments.
Methods: Consecutive adult patients undergoing acute hip fracture surgery were enrolled in this prospective cohort study. Exclusion criteria were prior VTE, active malignancy, or pre‐injury therapeutic anticoagulation. Following informed or surrogate consent, serial TEG and PLM analyses were performed at admission, post‐operative day (POD) 1, 3, 5, 7 and at 2‐, 4‐, 6‐ and 12‐weeks post‐operatively, using the TEG6s hemostasis analyzer (Haemonetics Corporation). All patients received low molecular weight heparin (LMWH) for 28 days post‐operatively. Hypercoagulability was defined as maximal amplitude (MA; a measure of clot strength) over 65mm based on TEG analysis. T‐tests were used to compare MA values with the hypercoagulability threshold and between arthroplasty and fracture fixation.
Results: Fifty patients were enrolled with a mean age of 78.0 (SD = 11.0) years and 56% being female. Twenty‐nine (58%) were treated with arthroplasty. TEG analysis demonstrated hypercoagulability until 12‐weeks for over 50% of patients and PLM identified platelet‐mediated hypercoagulability based on elevated ADP‐MA and AA‐MA, with more pronounced platelet contribution demonstrated via the AA pathway (Figure 1). Patients treated with arthroplasty demonstrated a trend towards more pronounced hypercoagulability (Figure 2).
Conclusion(s): This study supports further investigation into the safety and efficacy of prolonged thromboprophylaxis for some patients and the investigation of anti‐platelet thromboprophylaxis following hip fracture surgery.
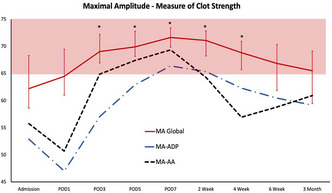

PB1364
Weight‐adjusted dosing of tinzaparin for thromboprophylaxis in obese patients
C. Pfrepper 1; E. Koch2; M. Weise2; M. Metze3; R. Siegemund4; A. Siegemund2; S. Petros2
1 University Hospital Leipzig, Leipzig, Germany, Leipzig, Sachsen, Germany; 2 Division of Hemostaseology, University Hospital Leipzig, Leipzig, Germany, Leipzig, Sachsen, Germany; 3 Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, Leipzig, Sachsen‐Anhalt, Germany; 4 Medical ICU, University Hospital Leipzig, Leipzig, Germany, Leipzig, Sachsen, Germany
Background: Knowledge about weight‐adjusted dosing of tinzaparin for prophylaxis in obese medical patients is limited and it remains unclear whether this approach leads to an accumulation of the drug.
Aims: To evaluate the anti‐Xa activity in obese patients on tinzaparin thromboprophylaxis adjusted for actual body weight.
Methods: This prospective study included patients with a body mass index (BMI) ≥ 30 kg/m2 treated with tinzaparin once daily adjusted for actual body weight (table 1). Anti‐Xa‐, anti‐IIa activity, von Willebrand factor (VWF) antigen, VWF activity, factor VIII, D‐dimer, prothrombin fragments and thrombin generation were measured four h after subcutaneous injection between day 1 and 14 after the initiation of tinzaparin.
Results: We included 121 plasma samples from 66 patients (48.5% female), with a median age of 60 (range 23–85) years, a median weight of 125 (range 82–300) kg and a median BMI of 41.9 (range 30.1–88.6) kg/m2. The target anti‐Xa activity of 0.2–0.4 IU/mL was achieved in 80 plasma samples (66.1%), 39 (32.2%) were below and two (1.7%) above the target range. The median anti‐Xa activity was 0.26 (IQR 0.20–0.31) IU/mL, 0.23 (IQR 0.16–0.28) IU/mL and 0.21 (IQR 0.17–0.26) IU/mL on the days 1–3, 4–6 and 7–14, respectively. The anti‐Xa activity was not different between the weight groups (p = 0.188), figure 1. Subcutaneous injection into the upper arm compared to the abdomen resulted in a lower endogenous thrombin potential (455 vs. 1533 nmol/L*min, p = 0.008), a lower peak thrombin (32 vs. 149 nmol/L, p = 0.008) and a trend to a higher anti‐Xa activity (0.023 vs 0.020 IU/L, p = 0.075).
Conclusion(s): Dosing of tinzaparin adjusted for actual body weight in obese patients seems not to lead to accumulation or overdosing. The majority of patients achieve anti‐Xa activity in the target range. In addition, there is a significant difference in thrombin generation depending on the injection site.


PB1366
Safety and efficacy of apixaban as thromboprophylaxis in multiple myeloma patients receiving chemotherapy: A prospective cohort study
Z. Sayar 1; C. Gates2; S. Bristogiannis3; A. Patel2; M. Ogunbiyi4; A. Tailor2; K. Yong2; M. Thomas5
1 University College London Hospitals NHS Foundation Trust, London, UK; Whittington Hospital, London, UK, London, England, United Kingdom; 2 University College Hospital, London, England, United Kingdom; 3 Evaggelismos General Hospital, Athens, Greece; 4 University College, London, London, England, United Kingdom; 5 University College Hospitals London NHS Foundation Trust, London, England, United Kingdom
Background: Apixaban 2.5mg twice‐daily replaced low‐molecular‐weight‐heparin as thromboprophylaxis (TP) for multiple myeloma (MM) patients receiving outpatient‐based chemotherapy considered at high‐risk of venous thromboembolism (VTE) on 1st November 2019 in our regional centre (Figure 1).
Aims: This prospective cohort study aimed to assess the safety and efficacy of apixaban as thromboprophylaxis in high‐thrombotic risk patients with MM.
Methods: Data was systematically collected from the electronic noting system for service evaluation, retrospectively for the historic cohort (1st Nov 2018 – 1st Nov 2019) prior to introduction of the novel thromboprophylactic strategy, and prospectively (1st Nov 2019– 1st Nov 2020) following the change of local guidelines. Exclusion criteria included antithrombotic treatment other than thromboprophylaxis or contraindication to thromboprophylaxis e.g. thrombocytopenia. Data collected included previous VTE history, thromboprophylactic agent, thrombosis and bleeding events.
Results: There were 102 MM patients in the historic and 147 in the prospective cohort (Table 1). VTE prophylaxis was prescribed in 80% historic and 78% prospective cohort. After the introduction of the amended thromboprophylactic strategy, prescriptions of apixaban increased from 27% to 53%, whilst aspirin prescriptions fell from 62% to 41%. After the introduction of apixaban, thrombotic events reduced from 3% (3/102) to 1.4% (2/147). All thrombotic events in the historic cohort occurred despite aspirin as thromboprophylaxis and on a pomalidomide‐containing regimen. No thrombotic events occurred on prophylactic apixaban in either cohort. Five bleeding events occurred in the historic cohort: one major bleed (on apixaban TP) and two clinically‐relevant non‐major bleeding (CRNMB). In the prospective cohort, there was one major bleed (aspirin as TP) and one CRNMB. Across both cohorts, major bleeding occurred in 1.2% (1/82) and CRNMB in 1.2% (1/82) patients on prophylactic apixaban.
Conclusion(s): This data supports the use of apixaban as thromboprophylaxis in MM patients at high‐thrombotic risk, with low thrombotic rates and acceptably low rates of major bleeding and CRNMB.


PB1362
Decision‐analysis modelling of effectiveness and cost‐effectiveness of thromboprophylaxis for medical inpatients
D. Horner 1; S. Davis2; S. Goodacre2; A. Pandor2; M. Holland3; K. de Wit4; B. Hunt5; X. Griffin6
1 Salford Royal NHS Foundation Trust, Lymm, England, United Kingdom; 2 University of Sheffield, Sheffield, England, United Kingdom; 3 University of Bolton, Bolton, England, United Kingdom; 4 Queen's University, Kingston, Ontario, Canada; 5 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 6 Queen Mary University of London, London, England, United Kingdom
Background: Medical inpatients are at increased risk of venous thromboembolism (VTE) which can be life‐threatening or result in chronic complications. Thromboprophylaxis reduces the risk of VTE but incurs costs and potentially increases the risk of bleeding. Risk assessment models (RAMs) are currently used to target thromboprophylaxis at higher risk medical inpatients. It is currently uncertain whether this individualised approach is cost effective.
Aims: To determine the balance of costs, risks, and benefits for different thromboprophylaxis strategies in hospitalised medical inpatients.
Methods: Decision‐analytic modelling to estimate the following outcomes for alternative thromboprophylaxis strategies: thromboprophylaxis usage, VTE incidence and treatment, major bleeds including intracranial haemorrhage, chronic thromboembolic complications, and overall survival. We used model parameters from the published literature and evaluated costs over a life time horizon. We included adult medical inpatients other than those requiring critical care admission. Key interventions evaluated included no thromboprophylaxis, thromboprophylaxis for all and and thromboprophylaxis given according to RAMs. Key outcome measures included costs and quality‐adjusted‐life‐years (QALYs).
Results: Our base case results showed that thromboprophylaxis for all has a high probability (>99%) of being the most cost‐effective strategy (£20,000 per QALY threshold) when applying RAM performance data typical of that observed across several RAMs in a cohort of medical inpatients. A sensitivity analysis showed that targeting thromboprophylaxis using a Padua score ≥3 (84% of cohort) has a 76.6% probability of being the most cost‐effective strategy when assuming higher RAM performance (sensitivity 99.9%; specificity 23.7%).
Conclusion(s): Thromboprophylaxis for all appears to be the most cost‐effective strategy for medical inpatients. A cost‐effective RAM would need to have high sensitivity and identify low risk patients who could forego thromboprophylaxis.


VPB1374
Impact of implementation of primary thromboprophylaxis protocol in hospitalized hematologic malignancies patients
A. López Sacerio 1; M. Tejeda Ramón1; M. Suárez Rodríguez1; J. Cruz Rodríguez1; A. Morales Helguera2
1 Arnaldo Milián University Hospital, Santa Clara, Villa Clara, Cuba; 2 “Martha Abreu” de Las Villas Central University, Santa Cllara, Villa Clara, Cuba
Background: Venous thromboembolism (VTE) is a frequent complication in hematologic malignancies (HM). Thromboprophylaxis is still inconsistent and not risk adjusted in our hospital.
Aims: To evaluate the efficacy and safety of a risk stratified VTE primary thromboprophylaxis in hospitalized HM patients.
Methods: An observational prospective cohort study was conducted in 384 HM patients between July 2018 to January 2022, at Arnaldo Milián Hospital, Santa Clara, Cuba. According to the application of a protocol based on a logistic regression VTE predictive risk model, the cohort was divided into a pre‐protocol cohort (211 patients between July 2018 to December 2020) and a post‐protocol cohort (173 patients between January 2021 to January 2022). The internally and externally validated previous score, included 5 predictive factors: hypercholesterolemia, tumoral activity, use of thrombogenic drugs, diabetes mellitus and immobilization. Patients were divided into low and high risk. Informed consent was obtained, and the protocol was approved by a medical ethics committee complying with the Declaration of Helsinki.
Results: During the implementation of the protocol, 119 patients were high risk and 54 low risks. Adherence to thromboprophylaxis strategy was 95.8%. In high‐risk patients, daily thromboprophylaxis with low‐molecular weight heparin was the most frequently used (71,43%) followed by unfractionated heparin (28,57%) every 12 h. No bleeding or adverse reactions associated with anticoagulants were reported. The cut‐off value for discontinuation of thromboprophylaxis in thrombocytopenic patients was < 30 × 109/L. VTE incidence had a significant decrease before (27,96%) and after (2,89%) the implementation of the protocol (p < 0.001). Overall survival at one year was 77,3% in the pre‐protocol cohort and increased to 91,8 % in the post‐protocol set
Conclusion(s): The implementation of a primary thromboprophylaxis protocol based on the VTE predictive score is a tool that allows the use of efficacy and safe personalized thromboprophylaxis in hematologic malignancies.
PB1353
Predicting post‐discharge venous thromboembolism and major bleeding among medical patients: External validation of a risk score derived from readily available laboratory tests
S. Hyder1; G. Barnes 2; H. Han1; S. Ash2; S. Woller3; B. Horne4; S. Stevens5
1 University of Michigan Health System, Ann Arbor, Michigan, United States; 2 University of Michigan, Ann Arbor, Michigan, United States; 3 Intermountain Medical Center, Intermountain Healthcare, MURRAY, Utah, United States; 4 Intermountain Medical Center Heart Institute, Salt Lake City, Utah, United States; 5 Department of Medicine, Intermountain Medical Center, Murray, Utah, United States
Background: Medical patients experience increased risk of venous thromboembolism following hospital discharge. Extended duration thromboprophylaxis (EDT) can reduce VTE risk but may carry bleeding risks. Evidence for risks and benefits of EDT remains limited. A recent study used readily available blood count and chemistry labs to derive 90‐day risk scores for VTE and bleed outcomes.
Aims: External validation of the Intermountain Risk Score for hospital‐associated venous thromboembolism (HA‐VTE IMRS) and major bleeding (HA‐MB IMRS).
Methods: Retrospective cohort study of adult patients discharged alive from medical services at an academic medical center between 2015–2019. Patients were excluded for known VTE, preexisting anticoagulant use, or recent surgical procedure. HA‐VTE IMRS and HA‐MB IMRS were calculated and dichotomized as high‐ or low‐risk as described in the derivation manuscript. 90‐day post‐discharge VTE and bleeding outcomes were assessed using ICD‐10 codes and blood bank transfusion records.
Results: 113,578 patients remained in the final analysis. For VTE prediction, 66,340 (58.4%) had a low‐risk HA‐VTE IMRS < 7, versus 47,238 (41.6%) high‐risk ≥7. For bleed prediction, 71,576 (63%) had a low‐risk HA‐MB IMRS < 8, versus 42,002 (37%) high‐risk ≥ 8. VTE incidence was 1.1% in high‐risk versus 0.6% in low‐risk cohort. Major bleeding incidence was 1.3% in high‐risk versus 0.1% in low‐risk cohort. AUCs for VTE and bleed outcome discrimination were 0.585 and 0.78, respectively. Patients with a combined high‐risk VTE score and low‐risk bleeding score comprised 14.5% of the population.
Conclusion(s): In this external validation study, the HA‐VTE IMRS did not discriminate well for VTE but the HA‐MB IMRS had high discriminatory ability for major bleeding events. A sizable minority of patients were categorized as high VTE risk with low bleed risk, a population which may benefit from EDT. Further prospective research on these risk scores may better define efficacious and safe EDT among medical patients.


VTE Treatment
VPB0469
Pulmonary cement embolism after kyphoplasty: Clinical and Laboratory aspects of pro‐coagulant effects
V. Muster 1; P. Jud2; K. Gütl2; F. prüller2; L. Schweiger2; T. Gary2; M. Brodmann2; R. raggam2
1 Medical University of Graz, Graz, Steiermark, Austria; 2 Medical University Graz, Graz, Steiermark, Austria
Background: PMMA is most commonly used in kyphoplasty. During injection, migration of cement‐particles and hence embolization in the venous circulation system can occur. A cement PE is therefore a potential complication.
Aims: We present a case of a 65‐yo female with cement PE after kyphoplasty. Ex‐vivo plasmatic coagulation studies using PMMA with hydroxylapatit were performed.
Methods: A solution with sterile water and PMMA in a concentration of 0.5 g/ml was made and 0, 25, 100 and 250 μl were added to the citrated kaolin test and measured with a thrombelastogram.
Results: A 65‐yo woman presented three days after kyphoplasty with dyspnea, chest pain and swelling in the right calf. CTPA was performed revealing cement PE. Compression ultra sound confirmed a deep vein thrombosis. Enoxaparin 80 mg b.i.d. was started and was switched to edoxaban 60mg once daily after 5 days. The citrated kaolin test analyzed with the thrombelastogram showed a R‐time of 330 s, 295 s, 250 s, 215 s for 0, 25, 100 and 250 μl of the solution added, respectively. With increasing quantity of the cement powder solution, a faster clot formation is present. The reduction of the R time measured between no cement powder solution and 250yl of cement powder solution added to the citrated kaolin test is 34.9%.
Conclusion(s): In our study we found a reduction of the R time in the thrombelastogram with increasing quantity of bone cement showing that a coagulation activation is happening and a clot is formatted in a shorter time. Previous studies could not show this effect. With the results of our study we support that anticoagulation should be started in case of cement PE.


PB0457
Retrospective study of COVID patients diagnosed with VTE
B. Packham
Thrombosis & Anticoagulation, Luton, England, United Kingdom
Background: We found that the number of patients diagnosed with PE following COVID began to increase so we wanted to study the trend and triggers In a recent review of our COVID‐19 admissions, the incidence of VTE in patients with SARSCoV‐2 was 34.5% in imaged patients (119 patients had CT pulmonary angiograms, and 41patients had identified pulmonary thromboembolism (Barnet Hospital, unpublished data, 2020 Kumar el at 2020).
Aims: To review the number of patients referred to the clinic before and present, make a comparison of number of VTEs before and during COVID pandemic
Methods: Retrospective cohort study was conducted from Jan until June 2020. All patients who have confirmed as COVID with VTE were included, suspected COVID were excluded from the study The anticoagulation team all uploaded information to excel and this was analysed and report produced
Results: A total of 657 patients were referred to the anticoagulation clinic between Jan to June 2020 Out of them 184 had VTE out of that number 32 were identified as COVID 19. DOAC drug prescribed Total number 32 Apixaban 5 mg BD 20 Rivaroxaban 20 mg 8 Dabigatran 150 mg 0 Edoxaban 60 mg OD 0 LMWH 3
Conclusion(s): Conclusions: Most patients did not have any issues related to the VTE but they were still recovering as a result of COVID. They reported feeling tired, short of breath and weak. These are not the usual symptoms reported by VTE patients apart from PE patients We treated them for duration of three months but if these patients were not fully mobile or had on going symptoms we plan to continue the therapy for a total of six months Keywords: COVID, DOAC
PB0459
Retrieval rate and complications of inferior vena cava filters placed during acute venous thromboembolism
D. Santagata 1; W. Ageno2; M. Venturini3; M. Donadini4; F. Fontana3; F. Piacentino3
1 University of Insubria, Como, Lombardia, Italy; 2 Department of Medicine and Surgery, Research Center on Thromboembolic Disorders and Antithrombotic Therapies, University of Insubria, VARESE, Lombardia, Italy; 3 Department of Diagnostic and Interventional Radiology, University of Insubria, Ospedale di Circolo e Fondazione Macchi, Varese, Lombardia, Italy; 4 Research Center on Thromboembolic Disorders and Antithrombotic Therapies, University of Insubria, Varese, Lombardia, Italy
Background: Over the last two decades, inferior vena cava filters (IVFC) placement has increased, especially with the advent of retrievable IVCF. Despite an increase of IVCF retrieval after Food and Drug Administration warnings, retrieval rates remain poor. In addition, current indications for filter removal do not include a formal evaluation of patients’ comorbidities.
Aims: To evaluate the retrieval rate of IVCF and its determinants and to describe the long‐term clinical course of patients with IVCF placement, including VTE (Venous Thromboembolism) events and filter complications.
Methods: A single‐center retrospective cohort study was performed enrolling all patients diagnosed with acute deep vein thrombosis (DVTs) and/or pulmonary embolism (PE), who underwent IVCF placement from 2015 to 2019. The primary outcome was retrieval rates at 3, 6 and 12 months.
Results: 139 patients underwent IVCF placement due to temporary contraindications to full dose anticoagulation for acute VTE, 31 of whom remained without any anticoagulation even at discharge. We found a removal rate of 2,1%, 13,5% and 29,2% at 3, 9 and 12 months, respectively. Active cancer was significantly associated with the decision to not remove IVCF (OR 0,16; CI 95% 0,05 to 0,52), whereas surgical procedures up to 1 month before VTE event were significantly associated with filter removal (OR 3,43; CI 95% 1,19 to 9,88). During follow‐up, 11 patients (7,9%) had lower limb DVT (4 during anticoagulation) and 7 patients (5,0%) were diagnosed with device‐related complications (3 IVCF‐tilting, 2 IVCF‐occlusions and 1 caval‐penetration). Overall, the mortality rate at 1 year was 42,6%.
Conclusion(s): Despite the 2014 FDA warning, the retrieval rate of IVCF is low. However, the results may be in part explained by the high mortality rate, that reflects a highly comorbid population. Indeed, active cancer was associated with the choice to not remove filters, whereas transient factors such as surgery were associated with filter retrieval.
PB0448
Median serum trough concentration of apixaban in pulmonary embolism patients after one month of therapy
J. Dzudovic1; B. Dzudovic 2; M. Crevar Sakac3; S. Obradovic4
1 National Poison Control Center, Military Medical Academy, Belgrade, Vojvodina, Serbia; 2 Clinic of Emergency Internal Medicine, Military Medical Academy, Belgrade, Not Applicable, Serbia; 3 Faculty of Pharmacy/University of Belgrade, Belgrade, Vojvodina, Serbia; 4 Clinic of Cardiology, Military Medical Academy, Belgrade, Vojvodina, Serbia
Background: Apixaban is one of recommended direct oral anticoagulants in the treatment of pulmonary embolism (PE), and this therapy is considered to be efficacious and safe. Although no therapeutic drug monitoring is recommended, there are no studies that measured apixaban concentrations in PE patients.
Aims: To measure and evaluate concentration variability of apixaban in serum during the trough phase after one month of stable therapy with recommended drug dosage.
Methods: This is a single‐center retrospective cross‐sectional study of PE patients who were discharged with apixaban in the therapy. After one month of stable therapy with 5mg of apixaban bid, during the expected trough phase of the drug concentration (15 min before the next dose of the drug) a blood sample from the cubital vein was taken for laboratory analysis. Trough apixaban plasma concentrations were measured by liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Basic patients’ characteristics were collected on the hospital admission for statistical analysis. Apixaban concentrations were presented in ng/ml and as median with interquartile range. Creatinine clearance was calculated using the Cockcroft‐Gault equation. Recurrent PE or bleeding were one‐month events of interest.
Results: Overall 22 patients in the period from 2019 till 2021 were included in the analysis (11 men and 11 women). The mean age and creatinine clearance were 61 years (SD = 14) and 84 ml/min (SD = 38), respectively. The median apixaban concentration in the trough phase with interquartile range (25th‐75th percentile) was 220.78 (121.67 – 246.92). Minimal concentration was 57.67, and maximal was 2327.57 which was an outlier because the 90th percentile was at 358.25. No one‐month event was noticed.
Conclusion(s): Apixaban concentrations measured in the trough phase in PE patients after one month of therapy were within a wide range of values. However, no recurrent PE or bleeding was noticed among this small group of PE patients.
PB0462
Watchfull waiting: A conservative management of rivaroxaban overdose
I. Machado1; S. Teixeira 2; L. Gonçalves3; D. Cibele1; M. Carvalho3; C. Neves4; C. Koch3
1 Centro Hospitalar e Universitário São João, Porto, Porto, Portugal; 2 Centro Hospitalar e Universitário São João, Maia, Porto, Portugal; 3 Center of Thombosis and Haemostasis, Department of Immunohemotherapy, Centro Hospitalar e Universitário São João, Porto, Porto, Portugal; 4 Center of Thombosis and Haemostasis, Department of Immunohemotherapy, Centro Hospitalar e Universitário São João, Porto, Porto, Portugal
Background: Rivaroxaban is an active direct factor Xa inhibitor mainly used to treat venous thromboembolism, as well as for the prevention of embolic stroke in atrial fibrillation, with a standard dose of 20 mg once daily. According to literature, the maximum factor Xa inhibition by rivaroxaban occurs after 2–4 h and the terminal half‐life is 8–12 h.
Aims: The aim of this study was to review a clinical case with an overdose of rivaroxaban.
Methods: A 78‐year‐old female patient presented to the hospital 3 h after a deliberate self‐intoxication with 1400 mg of rivaroxaban (70 tablets of 20 mg). At the admission time, she was conscious (Glasgow Coma Scale 15/15), with no alterations in vital signs or any symptoms or signs of hemorrhage.
Results: In Portugal, where there are no established pharmacological tools to fully reverse the anticoagulant effect of rivaroxaban, prothrombin complex concentrate (PPC) can be used to improve hemostasis. The first approach was to give 50 g of oral activated charcoal, diuresis stimulation and close monitoring of arterial blood pressure in bed rest. At the same time, rivaroxaban concentrations, Prothrombin Time and Activated Partial Thromboplastin Time were monitored (Figures 1 and 2). Initial hemogram and biochemistry parameters were normal, in particular, normal renal function. The overdose was asymptomatic in a patient with low risk of bleeding. The rivaroxaban concentration in plasma was in the trough level 18 h post‐ingestion and the patient recovered uneventfully. She was discharged to the psychiatric ward on day 3.
Conclusion(s): We report a case of rivaroxaban overdose with favorable outcome with conservative management – it did not require administration of PPC, fresh frozen plasma or any blood products. A close clinical surveillance and specific assays to monitoring rivaroxaban concentrations can help the management of this unusual situations.


PB0454
Venous thromboembolism reoccurrence during factor Xa inhibitors in young patients associates with thoracic outlet syndrome and development of chronic thromboembolic pulmonary hypertension
M. Kaksonen 1; M. Pentikäinen2; P. Simonen2; R. Lassila3
1 Helsinki University Hospital and University of Helsinki, Vantaa, Uusimaa, Finland; 2 Helsinki University Hospital, Heart and Lung Center and University of Helsinki, Helsinki, Uusimaa, Finland; 3 Helsinki University Hospital, Helsinki, Uusimaa, Finland
Background: Direct oral anticoagulants (DOAC) are the drug of choice in treating venous thromboembolism (VTE). During DOAC trials a 2–3% treatment failure rate has been observed. The predisposing factors for this treatment failure have not been addressed before in the literature.
Aims: Our aim was to elucidate associated factors of recurrent VTE under coagulation factor Xa inhibitor (FXaI) treatment.
Methods: Ten consecutive consultation cases of FXaI failure were included. Caprini, Padua, chronic thromboembolic pulmonary hypertension (CTEPH), and local VTE (Helsinki University Hospital) risk factor scores were assessed in all patients. Thrombophilia screen and coagulation activity follow‐up were performed, and the anticoagulation strategy was individually tailored.
Results: The affected patients were young (mean age 37.5, range 22–55), and 6 were women. Although, initially many patients appeared to have unprovoked VTE, all were found with major VTE risk factors. Seven patients had chronic venous obstruction: 5 subclavian (thoracic outlet syndrome, TOS), one common iliac, and one with chronic paraplegia. Five patients had multiple VTE risk factors and 4 had thrombophilia. The VTE risk scores varied from the lowest (TOS patients) to the highest risk (multiple risk factors/thrombophilia). The FXaI failure occurred on average at 99 days of therapy (range 15–279) without evident noncompliance. D‐dimer levels were low upon FXaI failure, and re‐thrombosis was resistant to further anticoagulation. Initial VTE was pulmonary embolism (PE) in 8 patients, and upon recurrent PE, 6 of them developed CTEPH after the FXaI failure. All the CTEPH and majority (8/10) of all patients required mechanical/surgical interventions. All patients are alive with individually tailored anticoagulation.
Conclusion(s): Our series of these young patients illustrates the importance of the suspicion of TOS and thorough assessment of risk factors upon VTE and its reoccurrence. Impaired fibrinolysis deserves further studies. The tailoring of effective management strategies and follow‐up are needed.
PB0450
Study of the thrombin generation in anticoagulated patients with hemorrhage complications
R. Gauthier 1; A. Desvoilhes2; L. Talon2; T. Sinegre2; J. Schmidt3; F. Moustafa3; D. Teissandier4; A. Lebreton5
1 CHU Clermont‐Ferrand, Notre‐Dame‐de‐l'Île‐Perrot, Quebec, Canada; 2 Hematology Department, CHU Clermont‐Ferrand, Clermont‐Ferrand, Auvergne, France; 3 Emergency Unit, CHU Clermont Ferrand, Clermont‐Ferrand, Auvergne, France, 4 CHU Clermont Ferrand, Clermont‐Ferrand, Auvergne, France; 5 Service d’hématologie biologique, CHU Clermont‐Ferrand, Clermont‐Ferrand, France, Clermont‐Ferrand, Rhone‐Alpes, France
Background: While prothrombin complex concentrates (PCC) have been proven to be effective to reverse vitamin K antagonists (VKA), PCC is also used to reverse direct oral anticoagulants (DOACs) in a non‐specific manner. We previously measured thrombin generation (TG) following the reversion of bleeding patients treated with OAC with CAT method and showed a hypercoagulable state after reversion for patients treated with DOACs (ISTH 2019 Poster PB1659).
Aims: Our study, supported by Stago, aims to evaluate the new thrombin generation system (ST‐Genesia®) using leftover plasma of our study.
Methods: Plasma of patients treated with either VKA, rivaroxaban(R) or apixaban(A) were collected at the patients’ arrival (V1), 30mins(V2); 6H(V3) and 24H(V4) post‐reversion. We compared with Bland‐Altman plots peaks and endogenous thrombin potentials (ETP) obtained with both instruments.
Results: Hypercoagulability was detected for DOACs at V2 (mean ± SD ETP A: 2302 ± 693.3; R: 2628 ± 1111 nM.min) (ETP healthy controls intervals: 1017 to 1667 nM.min as 5th and 95th percentiles) and was maintained to V4 (mean ± SD ETP A: 2348 ± 321,2; R: 2495 ± 418,5 nM.min). No hypercoagulability was detected for VKAs after reversion (mean ± SD ETP V2: 982.0 ± 480.5; V3: 1137 ± 326.8; V4: 1061 ± 365.6 nM.min). As for the peak (healthy controls intervals: 178,2 to 359,6 nM as 5th and 95th percentiles), only patients treated with rivaroxaban became hypercoagulable at V4 (mean ± SD R: 427.7 ± 38.78 nM).
Conclusion(s): Patients treated with VKA and reversed with PCC have regained a normal coagulation, while patients treated with DOACs and reversed with PCC had hypercoagulability, as observed with CAT. Agreements between the two methods were satisfactory for both TG parameters.

VPB0472
Development of a consensus‐based cross‐domain protocol for the management of elastic compression stocking therapy: a modified Delphi study
R. Schreurs; M. Joore; H. ten Cate; A. ten Cate
Maastricht University Medical Center, Maastricht, Limburg, Netherlands
Background: Elastic compression stocking (ECS) therapy is commonly used in patients with deep venous thrombosis (DVT) and chronic venous disease (CVD). The provision of ECS therapy is complex, and studies indicate a lack of practical guidance and suboptimal collaboration among health care professionals.
Aims: We aimed to reach consensus on critical issues of ECS therapy among the involved health care professionals and patients.
Methods: A three‐round modified Delphi analysis was performed in the Netherlands in which 56 health care professionals (internists, dermatologists, general practitioners, emergency room nurses, home care nurses, medical stocking suppliers, and occupational therapists) and seven patients were invited. The 21 statements included in this analysis were based on information collected from a previously conducted Functional Resonance Analysis Method and Realist Evaluation. We used 7‐point Likert scale questions and a 75% threshold for consensus. This study was supported by ZonMW, the Netherlands Organisation for Health Research and Development.
Results: Of the 63 persons invited for this study, 59 (94%) agreed to participate and responded in the first questionnaire round; of whom 52 were health care professionals and seven were patients (five DVT and two CVD). The overall response rate for the three questionnaire rounds was 91%. After completion of the rounds, full consensus was achieved on 19 out of 21 statements. No consensus was reached on the need for a follow‐up appointment for CVD patients and who should be responsible to determine the ECS type (custom‐made or standard).
Conclusion(s): We identified 19 consensus‐driven recommendations on treatment decisions and collaboration in ECS therapy among an interdisciplinary panel of health care professionals and patients. These recommendations form a basis for consensus‐driven optimization of ECS therapy and should ideally be incorporated in a general cross‐domain protocol for ECS therapy in patients with DVT and CVD.
PB0442
Two stage intravital imaging mouse model to assess venous thromboembolism in sickle cell disease
T. Brzoska; T. Kaminski; P. Sundd
University of Pittsburgh, Pittsburgh, Pennsylvania, United States
Background: Sickle cell disease (SCD) patients have an increased risk of venous thromboembolism (VTE). Population‐based studies demonstrated that VTE has a cumulative incidence rate of approximately 25% in adult SCD patients and is associated with higher risk of mortality. VTE in SCD is most commonly manifested as deep vein thrombosis (DVT) with associated pulmonary embolism (PE). Although autopsy studies have regularly discovered pulmonary thromboembolic lesions in SCD patients, the pathophysiology of VTE in SCD remains largely unknown due to the lack of relevant animal VTE model. Understanding the mechanisms that promote VTE in SCD is imperative to identify its prevention and treatment measures.
Aims: To establish an intravital microscopy approach to probe VTE in SCD.
Methods: DVT in SCD mice pre‐treated with hemin was induced by surgical ligation of femoral vein. Venous thrombus formation was visualized using intravital multi‐photon‐excitation (MPE) microscopy. To trigger acute PE, femoral vein ligation was removed and the venous thrombus was observed to spontaneously detach and travel to the SCD mouse lung. This method allowed real time visualization of acute PE in vivo using MPE microscopy of intact lung in live breathing mice.
Results: Venous thrombus took the form of a large mass of elliptical shape which extended in the long‐axis direction of the femoral vein. It was composed of fibrin, erythrocytes and sparse platelets. Over time, thrombus was infiltrated by migrating neutrophils. Acute PE involved embolization of the pulmonary arterioles and the arteriolar bottle‐necks located at the junction of pulmonary arterioles and capillaries. The embolization of arteriolar circulation led to loss of blood flow in the arterioles and the down‐stream capillaries.
Conclusion(s): Herein we introduce an intravital microscopy approach to probe VTE in SCD live mouse. Our model has potential application in investigating the molecular determinants of VTE associated with SCD as well as evaluating efficacy of new antithrombotic drugs.
PB0456
Inferior vena cava filter in a tertiary Hospital of Barcelona: a descriptive study of 185 cases
M. Montoya 1; C. Gabara2; N. López2; C. Zamora2; A. Morancho2; J. Moisés2; C. FONT2; S. Jiménez2; F. Zarco2; M. Burrel2; P. Bermúdez2; E. Serrano2; J. Aibar2
1 Hospital Clínic de Barcelona, Spain; 2 Hospital Clínic de Barcelona, Barcelona, Catalonia, Spain; 3 Hospital Clínc de Barcelona, Barcelona, Catalonia, Spain
Background: Inferior vena cava filters (IVCF) have become an important part of venous thromboembolic disease’s (VTD) therapy. IVCF are used to prevent embolization of a lower extremity deep vein thrombosis (DVT) when the risk of pulmonary embolism (PE) is high. Nevertheless, guidelines differ on the recommended indications for IVCF placement due to lacking evidence of their benefit.
Aims: To evaluate the adherence to different guidelines recommendations regarding to the indication for IVCF placement and to assess retrieval rates and their complications in a tertiary hospital.
Methods: A retrospective observational study was performed including 185 patients in which an IVCF was placed between 2015 and 2020 in a tertiary hospital of Barcelona. We considered absolute indication if it was included in all clinical guidelines revised and relative indication if it was included in some (Table 1).
Results: The main epidemiologic and clinical data of the patients are shown in Table 2. The mean age was 63.4 years (standard derivation 15), and 58.4% were men. 76.6% had an age‐adjusted Charlson Comorbidity Index score equal or higher than 4. 45% of the patients had cancer. 32.4% had previous history of VTD (80% PE, 20% DVT). Regarding to filter placement indication, 70 (38%) patients had no indication, 87 (47%) had an absolute indication and 28 (15%) had a relative indication. There was an important charge of complications associated with the filter (12.4%), being the most frequent one’s instrumental failure (34.8%) and thrombosis (30.4%). IVCF was not removed in 38.9% of the patients and 17.8% of them had a VTD recurrence. The mortality rate was 55.7%.
Conclusion(s): There is an important proportion of patients in which a IVCF is placed without a strong indication, and this becomes remarkable because there is an important charge of complications associated. More than a half of the patients died during the follow‐up.


PB0464
Peri‐operative management of anticoagulation and bleeding in patients undergoing CDT for acute iliofemoral DVT
K. White 1; K. Creeper2; S. Black3; N. Thulasidasan3; K. Breen4
1 Barts Health NHS Trust, London, England, United Kingdom; 2 Guy's and St Thomas's NHS Foundation Trust, Kings College London, London, England, United Kingdom; 3 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 4 Guy's & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Catheter directed thrombolysis (CDT) is an evolving modality used as an adjunct to anticoagulation in selected patients with acute iliofemoral deep vein thrombosis (DVT) (1–3). To date, outcomes have primarily focused on vascular patency but there is a paucity of data pertaining to anticoagulation management and bleeding outcomes (4).
Aims: To review the anticoagulation, management and incidence of bleeding associated with vascular intervention for acute iliofemoral DVT.
Methods: A single centre, retrospective audit of chart and laboratory databases for all patients that presented with an acute iliofemoral DVT between December 2019 and November 2021. Data pertaining to patient demographics, vascular intervention, anticoagulation and incidence of bleeding were collated and analysed.
Results: A total of 75 patients (51% female) with a median age 43 years (IQR 32, 55) were identified. The median duration of catheter directed thrombolysis with alteplase 0.01 mg/kg/hr was 48 h. 47 (63%) also underwent venous stenting. 51 (68%) developed hypofibrinogenemia (fibrinogen <1.0 g/L) and 44 (59%) received supplemental cryoprecipitate. Major bleeding was observed in 7 patients (9%) and was significantly associated with a fibrinogen ≤ 0.5g/L (Χ 2 6.5, df = 1, p < 0.05). 8 patients underwent repeat intervention with the majority (n = 5, 62.5%) performed within one month of initial intervention. None of those who underwent repeat intervention had bleeding complications. 69 (92%) were treated with single agent, therapeutic dalteparin pre‐procedure and for 6 weeks post intervention. 57 (76%) patients were switched to a direct oral anticoagulant at 6 weeks.
Conclusion(s): The risk of major bleeding with CDT is low and not increased by re‐intervention. Hypofibrinogenemia ≤ 0.5 g is strongly associated with increased risk of bleeding and is a common complication of CDT; therefore, justifying the recommendation for regular monitoring and consideration of replacement.
PB0461
Patient outcomes of catheter directed thrombolysis for upper extremity deep vein thrombosis
Y. Tan 1; N. Thulasidasan2; S. Black2; K. Breen3
1 Guy's and St Thomas' NHS Foundation Trust, Croydon, England, United Kingdom; 2 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 3 Guy's & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Upper extremity deep vein thrombosis (UEDVT) accounts for < 10% of deep vein thrombosis (DVT). UEDVT can be idiopathic or can be due to thoracic outlet syndrome, thrombophilia, central venous catheter (CVC), oral contraceptives and cancer. Treatment recommendations are largely based on indirect evidence on the treatment of lower extremity DVT, with anticoagulation being the mainstay of treatment. Catheter directed thrombolysis (CDT) can potentially be considered in a minority of cases.
Aims: To evaluate immediate and medium term outcomes in UEDVT including recurrent venous thromboembolism (VTE) and clinically significant bleeding in patients receiving CDT.
Methods: Review of records of patients receiving CDT for UEDVT at St Thomas’ Hospital, London between December 2019 and November 2021 to extract information on risk factors, post procedure VTE recurrence and complications.
Results: 10 patients (median age 41.5 years) received CDT for UEDVT. 6 (60%) patients had thoracic outlet syndrome, 1 (10%) was idiopathic and 3 (30%) had provoked UEDVT due to oral contraceptives, CVC and lupus nephritis. 6 (60%) patients received concurrent vascular intervention such as venoplasty or thrombectomy with CDT. 4 (60%) proceeded with cervical rib excision based on MRI findings. All patients commenced treatment dose low molecular weight heparin post CDT. VTE recurred in 3 (30%) patients. No bleeding was reported with CDT monotherapy, however one patient (10%) developed haematoma and haemothorax after combined CDT, venoplasty and rib excision. Median admission length was 6 days, with median follow up of 18 months. 2 (20%) patients were lost to follow up.
Conclusion(s): CDT is associated with low rate of VTE recurrence and bleeding. Our study has highlighted that this is a complex group of patients with a need for more robust data comparing the efficacy and safety of CDT with anticoagulation and if it has any long term benefit on reduction of post thrombotic syndrome.
PB0465
Feasibility study of patient self‐management of Warfarin using the Fearon algorithm
A. Wilson 1; J. Saunders1; S. Vazquez1; A. Jones1; M. Fearon2; P. Wegener3; D. Witt1
1 University of Utah College of Pharmacy, Salt Lake City, Utah, United States; 2 Epitome Pharmaceuticals Ltd., Halifax, Nova Scotia, Canada; 3 Epitome Pharmaceuticals Ltd., San Diego, California, United States
Background: The Fearon algorithm (FA), a patient‐specific warfarin dosing protocol, improves time in therapeutic range (TTR) when used by an anticoagulation management service (AMS). However, there is lack of evidence demonstrating the feasibility of FA use under patient self‐management (PSM) protocols. This before‐and‐after study compared measures of INR control between 25 patients using FA guidance for warfarin management under AMS versus PSM protocols.
Aims: To assess the feasibility of using the FA to support PSM protocols.
Methods: Eligible patients taking warfarin under AMS use of the FA for a 6‐month period were included in this study and followed for an additional 6 months after implementing PSM protocols. Patients were instructed to follow the FA using individualized nomograms developed to match patient‐specific dose‐effect curves (Figure 1). TTR was compared between AMS and PSM phases using a one‐tailed Wilcoxon Signed‐Rank Test.
Results: A total of 30 patients were initially enrolled. Of these, 26 (86.7%) completed 6 months of AMS using the FA and 25 (83.3%) successfully transitioned to PSM using the FA. The 5 patient removals were due to significant bleeding (2, 6.67%), poor protocol adherence (2, 6.67%), and hospitalization resulting in death unrelated to anticoagulation (1, 3.33%). After accounting for periprocedural holds of warfarin, median TTR following the AMS phase using the FA was 65% (IQR 57%–77%); a 9% increase from baseline (p < 0.01). For patients completing the PSM phase, median TTR was 64% (IQR 53%–77%) (p = 0.16), demonstrating the feasibility of FA use to support PSM protocols. PSM maintained TTR improvement from baseline (p < 0.01).
Conclusion(s): Implementation of PSM using the FA resulted in no significant negative impacts on patient outcomes. TTR was not negatively affected by introducing PSM to patients with prior history using the FA. This study demonstrated that PSM can be implemented without deterioration of INR control in patients using the FA.

PB0444
Endoscopic evaluation in patients with gastrointestinal bleeding receiving anticoagulation for venous thromboembolism
N. Brunton 1; W. Wysokinski2; D. Vlazny2; D. Hodge3; H. Siddiqui3; D. Houghton2; A. Casanegra2
1 Danbury Hospital, Danbury, Connecticut, United States; 2 Mayo Clinic, Rochester, Minnesota, United States; 3 Mayo Clinic, Jacksonville, Florida, United States
Background: Gastrointestinal (GI) bleeding is a well described complication in patients anticoagulated for venous thromboembolism (VTE) with and without malignancy. Little data exists on endoscopic evaluation, management, and outcomes in this population.
Aims: To describe the endoscopic evaluation strategies, findings, and management in patients with GI bleeding receiving anticoagulation therapy for VTE.
Methods: Review of medical records of 3287 consecutive, anticoagulated patients enrolled in the Mayo Clinic VTE Registry between March 2013 through October 2021 that experienced GI major (MB) or clinically relevant non major bleeding (CRNMB).
Results: Three percent (109 patients, mean age 67, range 26–100, 52.3% female) had GI bleeding while on anticoagulation, 62 (56.9%) MB and 47 (43.1%) CRNMB. Cancer was present in 76 (69.7%), 22 (20.2%) from the GI tract (Table 1). In 18 patients bleeding was managed outpatient. Transfusion was needed in 62 patients (56.9%). Endoscopic evaluation was completed in 72 (66.1%): upper endoscopy in 51, lower in 40, and capsule in 2 patients. A bleeding source was identified with endoscopy in 49 (44.5%) patients and an endoscopic procedure to address active bleeding was performed in 19 (26.4%) (Table 2). Therapeutic anticoagulation was held in 90 patients (82.6%) for a mean of 3 days (range 0–116). For 21 patients, anticoagulation was reduced to a prophylactic dose temporarily and in 4 permanently. Anticoagulation was indefinitely discontinued in 30 cases (27.5%). During follow up 56 (51.4%) of the patients died, and 20 (18.3%) had a recurrent MB or CRNMB, 16 of them from the GI tract.
Conclusion(s): Two thirds of patients with GI bleeding undergo endoscopic evaluation but a bleeding source is identified in only half of those cases, and only a fifth are amenable for treatment. GI bleeding is a significant cause of anticoagulation discontinuation


PB0447
Adherence and persistence to Direct Oral Anticoagulants (DOACs) in the community following newly diagnosed Venous Thromboembolism (VTE) – A pharmacy‐linkage study
C. Dix 1; H. Bortz2; M. da Gama3; M. Treloar3; M. Reynolds3; T. Day4; H. Tran5
1 The Alfred Hospital, Melbourne, Victoria, Australia; 2 Pharmacy Department, The Alfred Hospital, Melbourne, Victoria, Australia; 3 NostraData, Melbourne, Victoria, Australia; 4 Department of Haematology, Flinders Medical Centre, Adelaide, South Australia, Australia; 5 Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia AND Department of Medicine, Central Clinical School, Monash University, Melbourne, Australia, Melbourne, Victoria, Australia
Background: Adherence to medications is a clinical concern across many chronic illnesses. Adherence and persistence to DOACs for VTE is critical to ensure adequate treatment of the thrombosis and, if used for an extended duration, to prevent new VTE episodes.
Aims: Assess the adherence and persistence to DOACs in patients in the community following admission to hospital with a new VTE.
Methods: We retrospectively reviewed community pharmacy dispensing data on all patients with newly diagnosed VTE and prescribed a DOAC (apixaban or rivaroxaban) between January 2018 and December 2019 at our institution. Proportion of days covered (PDC) was used to assess adherence at 90 days, and 6‐ and 12‐months. Persistence was measured by participants having a dispensed supply of DOAC at the end of the intended treatment period, as long as there were no significant gaps in supply (>60 days). Ethics approval was granted
Results: We identified 225 patients who met inclusion criteria. Overall PDC at 90 days, 6months and 12 months were 84.6%, 86.2% and 86.1% respectively. Apixaban had a higher mean PDC than rivaroxaban, at 86.2% and 80.6% respectively (p < 0.01). Females had a higher PDC than males, 87.3% and 81.2% respectively (p < 0.05). 133 (64%) were persistent with therapy and there were no clinical predictors of improved persistence.
Conclusion(s): In patients with newly diagnosed VTE treated with a DOAC, adherence rates were high at over 80%, with females and those prescribed apixaban having higher adherence. Only two‐thirds were persistent with therapy for the intended treatment duration. These findings may assist clinicians in potentially identifying those at risk of poor adherence.


PB0453
Real‐world use of Andexanet Alfa for anti‐Xa inhibitor reversal: A case series
A. Jones 1; A. Brewster2; M. Jolley3; J. Saunders1; A. Wilson1; S. Vazquez1; M. Crowther4; D. Witt1
1 University of Utah College of Pharmacy, Salt Lake City, Utah, United States; 2 Idaho State University, Pocatello, Idaho, United States; 3 Jolley's Corner Variety Inc., Salt Lake City, Utah, United States; 4 McMaster University, Hamilton, Ontario, Canada
Background: While andexanet has been approved for reversal of the anti‐Xa inhibitors apixaban and rivaroxaban, few studies have described important aspects of real‐world clinical use such as dosing, timing in relationship to last dose of oral anticoagulant, and outcomes related to safety and effectiveness.
Aims: Describe use and 30‐day patient outcomes of andexanet for anti‐Xa reversal at an academic medical center.
Methods: This was a case series of adult patients who were administered andexanet for anti‐Xa inhibitor reversal.
Results: We identified 18 orders for andexanet between 2018–2022; 15 were administered. Median patient age was 68 (interquartile range: 58–75), 60% were female, 80% were white, and 66% were receiving apixaban. The most common indication for andexanet was intracranial bleeding (73.3%). Last dose of anti‐Xa inhibitor was < 8 h in 2 patients (13.3%), unknown in 5 (33.3%), and >8 h in 8 (53.3%). Per dosing guidelines, three patients on rivaroxaban were administered incorrect doses (too low, too high, and incorrect infusion rate, respectively). Infusion times ranged from 39 min to 12 h, with a median time of 2 h. Seven patients received ≥1 additional reversal/hemostatic agent: prothrombin complex concentrate (5, 33.3%), desmopressin (2, 13.3%), fresh frozen plasma (1, 6.7%), and phytonadione (1, 6.7%). Three patients received an agent at an outside hospital. Five patients experienced adverse events within 30 days: 4 deaths (26.7%) and 1 stroke (6.7%). All adverse events occurred within 8 days of andexanet administration.
Conclusion(s): Many patients received inappropriate administration of andexanet and received additional reversal/hemostatic agents. The last dose of anti‐Xa inhibitor was administered greater than 8 h prior to factor Xa reversal with andexanet in over half of patients. These findings suggest that development of, and adherence to, administration guidelines are required to ensure safe and appropriate administration of andexanet alfa.
PB0460
Evaluation of edoxaban plasma concentration data from clinical practice
V. Speed 1; O. McNally2; R. Byrne3; R. Patel1; R. Arya1; J. Patel1
1 Kings College Hospital, London, England, United Kingdom; 2 King's College London, London, England, United Kingdom; 3 King's College Hospital, London, England, United Kingdom
Background: Edoxaban prescribing is increasing in popularity in the United Kingdom. However, the latest guidance from the International Society of Thrombosis and Haemostasis suggests avoiding in patients >120 kg or with a BMI >40 kg/m2 for the treatment of venous thromboembolism. Data describing edoxaban exposure in this population are lacking and complicated by reported reduced efficacy in those with a high creatinine clearance in the atrial fibrillation population.
Aims: To report an analysis of edoxaban plasma concentration data from clinical practice and describe edoxaban exposure in those with high bodyweight (>120 kg) or high creatinine clearance (90mL/min).
Methods: Between 2017 and 2020, edoxaban plasma concentrations drawn from patients attending the anticoagulation clinic at King’s College Hospital, London as part of routine care were retrospectively reviewed, with patient characteristics collated from the electronic patient record. Edoxaban concentrations were determined using an anti‐Xa chromogenic assay (Diagnostica Stago), which has a lower limit of quantification (LLOQ) of 20ng/mL. A trough concentration was defined as a level drawn after 20 h post dose.
Results: 279 edoxaban plasma concentrations were drawn from 257 patients. The majority of patients were anticoagulated for stroke prevention in AF (91.8%) – Table 1. 104/279 trough concentrations were reported, 43/104 (41%) of which were LLOQ (Figure 1). 5/8 (63%) of patients >120kg and 18/37 (49%) of those with a creatinine clearance >90mL/min had a reported trough less than 20ng/mL (LLOQ).
Conclusion(s): A high proportion of edoxaban trough concentrations were reported as LLOQ from clinical practice data. How this relates to outcomes requires further investigation and further work is required on the influence of body weight and renal function on edoxaban pharmacokinetics.


PB0466
Physician practice behaviors surrounding dose‐reduction of direct oral anticoagulants for the extended phase treatment of venous thromboembolism
S. Woller 1; D. Groat2; R. Rosovsky3; K. Sanfilippo4; L. Baumann Kreuziger5; M. Gaddh6; E. Eyster7; K. Martin8
1 Intermountain Medical Center, Intermountain Healthcare, MURRAY, Utah, United States; 2 Intermountain Medical Center, Murray, Utah, United States; 3 Massachusetts General Hospital, Boston, Massachusetts, United States; 4 Washington University School of Medicine St. Louis, St. Louis, Missouri, United States; 5 Medical College of Wisconsin, Milwaukee, Wisconsin, United States; 6 Emory University School of Medicine, Atlanta, Georgia, United States; 7 Penn State University School of Medicine, State College, Pennsylvania, United States; 8 Northwestern Memorial Hospital, Chicago, Illinois, United States
Background: Two direct oral anticoagulants (DOACs), apixaban and rivaroxaban, have been studied in reduced doses for extended‐phase treatment of venous thromboembolism (VTE). However, scant evidence exists surrounding clinician practice and decision‐making regarding dose reduction for extended‐phase treatment of VTE.
Aims: Report clinician practice and characteristics surrounding dose‐reduction of DOACs for extended‐phase VTE treatment.
Methods: We conducted a 16‐question REDCap survey between 7/14/2021 and 9/13/2021 among ISTH 2021 Congress attendees, and online via Twitter™. We performed logistic regression to explore factors associated with dose‐reduction. Considering dosing behaviors, we used k‐means clustering and minimized the silhouette score while maintaining sufficient cluster size. We assessed for clustering of clinicians by characteristics around dose reduction decision‐making. Descriptive statistics were used to describe the clusters.
Results: 171 clinicians responded, of whom most were attending academic physicians that practiced in North America (Table 1). Clinicians who treated larger volumes of patients had higher odds of electing dose‐reduction. We identified 5 clusters that showed distinct patterns of behavior regarding dose‐reduction. Cluster 1) Rarely dose reduces and is the cluster most likely to prescribe rivaroxaban; Cluster 2) Dose reduces with considerable frequency, does not consider age when dose‐reducing, is least likely to temporarily re‐escalate dosing, and prescribes apixaban and rivaroxaban equally; Cluster 3) Dose reduces < 50% of the time, but temporarily re‐escalates dosing during increased VTE risk; Cluster 4) Dose reduces with considerable frequency, temporarily re‐escalates dosing during increased VTE risk, and is most likely to prescribe apixaban; Cluster 5) Dose reduces with highest frequency, and takes the fewest risk factors into consideration to not dose reduce (Figure 1).
Conclusion(s): Most clinicians elect to dose‐reduce DOACs for extended‐phase anticoagulation. The likelihood of a clinician to dose‐reduce increased with volume of patients treated. Clinician prescribing patterns clustered around VTE risk factors as well as re‐escalation during high‐risk periods.


VPB0468
Levels of heparin induced anti‐PF4 antibodies and endogenous glycosaminoglycans and their relationship with inflammatory biomarkers in pulmonary embolism patients
B. Kantarcioglu 1; A. Darki2; F. Siddiqui2; D. Hoppensteadt2; J. Lewis3; R. Krämer4; J. Fareed2
1 Loyola University Chicago, Oak Park, Illinois, United States; 2 Loyola University Chicago, Maywood, Illinois, United States; 3 Loyola University Chicago, Northfield, Illinois, United States; 4 Heidelberg University, Heidelberg, Baden‐Wurttemberg, Germany
Background: Increased levels of inflammatory cytokines have been shown in venous thrombosis. Endothelial damage and shedding of endothelial glycocalyx have also been shown in certain thrombotic disease states, conferring that they have important functions in circulatory homeostasis.
Aims: We sought to quantify the levels of anti‐PF4 antibody isotypes together with endogenous glycosaminoglycans (GAGs) in a patient cohort, comprised of PE, to determine their impact on pathophysiology of VTE and HIT and observe whether there is a relationship in between and inflammatory marker subtypes.
Methods: Whole blood samples were drawn from patients within 24 h of confirmed diagnosis of acute PE under an Institutional Review Board approved protocol. The samples were tested for anti‐PF4 isotypes of IgA, IgG, and IgM ELISA assay, endogenous GAGs with heparin red assay and inflammatory biomarkers with biochip assay. Circulating levels of each biomarker were compared to control plasma. p < 0.05 was considered statistically significant.
Results: The levels of anti‐PF4 Ig A, Ig G and endogenous GAGs were elevated in acute PE patients compared to normal controls (p < 0.05). The increase in anti‐PF4 Ig M was not significant (p: 0.78). Anti PF4 Ig A were correlated with IL‐2, IL‐6 and TNFA. Anti PF4 Ig G were correlated with IL‐6 and d‐dimer. Anti PF4 Ig M were correlated with IL‐2 and MCP‐1. The levels of endogenous GAGs were correlated with TNF‐α, IL‐10, IL‐8, IL‐1β, IL‐1α and VEGF. We observed significant correlations in between anti‐PF4 antibody isotypes. The correlation between the levels of anti‐PF4 isotypes and endogenous GAGs were insignificant.
Conclusion(s): Our findings supports that the development of thrombosis is likely due to releasing of PF4 antigen from platelets and shedding of endogenous GAGS from the endothelial cells causing activation of endothelial cells, platelets, and leukocytes, resulting in inflammatory cytokine release and triggering of the coagulation system.


PB0439
Efficacy and safety of apixaban versus warfarin in Morbid obese patients with venous thromboembolism
H. Almajed 1; F. Aleidan2
1 King Saud bin Abdulaziz University for Health Sciences, Riyadh, Ar Riyad, Saudi Arabia; 2 King Abdulaziz Medical City, Ryiadh, Ar Riyad, Saudi Arabia
Background: Venous thromboembolism (VTE) includes deep venous thrombosis (DVT) and pulmonary embolism (PE) and affects approximately 25% of extremely high obese individuals annually. Obesity is a known risk factor for new onset and recurrent VTE.
Aims: To compare the risk of recurrent VTE and Major bleeding (MB) among VTE patients with morbid obesity who initiated apixaban or warfarin.
Methods: This retrospective two center cohort study included morbidly obese patients with BMI ≥40 kg/m2 who were prescribed apixaban or warfarin for VTE between January 1st, 2018, to December 31st, 2020. Data was extracted from the electronic record system at King Abdulaziz Medical City and King Fahad Medical City in Riyadh city, Saudi Arabia. The primary outcome was incidence of bleeding and recurrent VTE. Secondary outcomes included all‐cause mortality and readmission.
Results: The study included 198 patients in warfarin group and 114 in apixaban group. Baseline characteristics were mostly well matched except for the follow up duration which was significantly longer in the warfarin group as compared to apixaban (p = 0.006). The annual incidence rate of primary outcome was similar between warfarin and apixaban (2.98% vs.1.57%; RR:2.51; 95% CI 0.95–7.64; p = 0.206).
Conclusion(s): Our finding suggests that apixaban use is safe and effective as warfarin in morbidly obese patients. Further prospective studies are necessary to confirm this finding.
PB0452
Changing management of superficial vein thrombosis: A 5 year single site observational study
R. Gooding 1; R. Lavery2; G. Benson2; C. Corrigan2; W. Hamilton2; T. McKernan2; C. Neill2; T. Srikanth2; P. Steele2
1 Belfast Trust (HSCNI/NHS), Belfast, Northern Ireland, United Kingdom; 2 Belfast HSCT, Belfast, Northern Ireland, United Kingdom
Background: Optimal management of superficial vein thrombosis (SVT) remains uncertain, requiring careful balancing of thrombotic and bleeding risks, symptoms and health economic considerations. There are conservative (without anticoagulation) or anticoagulation options: established parenteral anticoagulation [fondaparinux] brings high treatment burden and financial cost. Direct Oral Anticoagulants (DOACs) may be a safe and effective option, with potential to reduce treatment burden and cost, whereas Low Molecular Weight Heparin is commonly used for acute venous thromboembolism.
Aims: This retrospective study assessed the management of SVT at a single site over 5 years to better understand the changing management of SVT for patients scanned to exclude DVT, as part of a quality improvement project.
Methods: A search of the venous thrombosis service database (Belfast HSCT) was conducted, where a lower limb doppler ultrasound scan found SVT as the sole positive finding. Scans associated with DVT or PE were excluded. Demographic data including age and gender were collected for 2017–2021, along with management in terms of anticoagulation type or no anticoagulation.
Results: Of 662 positive scans, the mean number per half‐year was 66, with 54% female. Approximately 20% of patients were in the 50–59, 60–69 and 70–79 year brackets each. Management patterns are shown in Graph 2: approximately 6% were managed with no anticoagulation through the study period. Fondaparinux use dropped from 66% of cases in year 1 to 13% in year 5. DOAC use increased from 1% to 24%. LMWH use increased from 27% to 59% over study period, and was the most frequently used anticoagulant from year 2. The majority of patients were treated with anticoagulation for 6 weeks.
Conclusion(s): A host of anticoagulants appear effective and safe for management of SVT: our study shows increasing use of LMWH and DOACs, likely reflecting a combination of newer publications, drug cost, and increasing confidence in DOACs management of SVT.

VPB0467
Factors influencing the decision making on thrombolytic therapy in normotensive patients with pulmonary embolism: Data from SIRENA registry
N. Cherepanova 1; I. Mullova1; T. Pavlova2; A. Erlikh3; O. Barbarash4; S. Berns5; E. Schmidt5; D. Duplyakov6
1 Samara Regional Cardiology Dispensary, Samara State Medical University, Samara, Samara, Russia; 2 Samara State Medical University, Samara, Samara, Russia; 3 Pirogov Russian National Research Medical University, City clinical hospital №29, Moscow, Moskva, Russia; 4 Russian Academy of Sciences; Scientific Institution Research Institute for Complex Issues of Cardiovascular Diseases, Kemerovo, Kemerovo, Russia; 5 Scientific Institution Research Institute for Complex Issues of Cardiovascular Diseases, Kemerovo, Kemerovo, Russia; 6 Research and Development Institute, Samara State Medical University, Samara Regional Cardiology Dispensary, Samara, Samara, Russia
Background: The place of thrombolytic therapy (TLT) in not high risk pulmonary embolism (PE) is not clearly defined.
Aims: To study the features of the use of TLT in normotensive patients with PE in real clinical practice.
Methods: Analysis data from patients with a diagnosis of PE were included in the Russian multicenter observational prospective register "SIRENA" (RusSIan REgistry of pulmoNAry embolism), admitted from 15.04.2018 to 15.04.2019. The features of management of normotensive patients with PE who received TLT (group 1) was compared to that of randomly selected gender‐, and year‐matched normotensive patients who did not receive TLT (group 2) (ratio, 1:1).
Results: 660 patients was enrolled in the registry, 609 of them with a lifetime confirmed diagnosis of PE. TLT was performed in 152 patients with PE (25%), of which 101 (66.2%) was normotensive. In the TLT group hospital mortality was 4 (4%) patients and 6 (5.9%) patients in group 2 (р = 0,748). Floating blood clot in the veins of the lower extremities (odds ratio (ОR) 11,6, 95% confidence interval (CI) 2,22–60,60), syncope in the debut of PE (ОR 1,67, 95% CI 0,73–3,78), respiratory rate over 22 per minute (ОR 1,12, 95% CI 1,02–1,23), systolic pressure in pulmonary artery by echocardiography over 40 mm Hg (ОR 6,7; 95% CI 2,44–18,74) were independent clinical factors that significantly influence the doctor's decision to perform thrombolysis. The probability of performing TLT decreased in the presence of history of chronic kidney disease (OR 0.1, 95% CI 0.02–0.53), surgery in the previous 12 months (OR 0.19, 95% CI 0.05–0.71), an increase of the right atrium over 45 mm Hg (OR 0.96, 95% CI 0.09–0.99). Bleeding during hospitalization was registered in 10 (9.9%) patients of group 1, including BARC 3 in 2 patients.
Conclusion(s): In real clinical practice, there is an unreasonably high frequency of TLT in normotensive patients with PE.
VPB0470
Long‐term results of tinzaparin for the treatment of superficial vein thrombosis of the lower limbs
C. Papageorgopoulou; K. Nikolakopoulos; S. Papadoulas; S. Kakkos
Department of Vascular Surgery, University Hospital of Patras, Patras, Greece, Patras, Akhaia, Greece
Background: Superficial vein thrombosis (SVT) of the lower limbs is associated with an increased risk of recurrent venous thromboembolism (VTE), but long‐term risksbeyond three years are largely unknown.
Aims: To identify the frequency of recurrent VTE in patients with lower limb SVT during a follow‐up through five years following initial presentation.
Methods: Consecutive patients with SVT were treated with subcutaneous tinzaparin (Innohep™, LEOPharma, Denmark). Patients were stratified into three groups by the duration of treatment: group 1 (≤30 days) and group 2 (31–60 days), which run in parallel and patients received mostly an intermediate or therapeutic dose and also a subsequent group 3 where patients received an intermediate dose (131iu/Kg) for 90 days. The composite primary endpoint of this prospective cohort study was recurrent VTE, defined as occurrence of clinically evident SVT recurrence, deep‐vein thrombosis or pulmonary embolism.
Results: A total of147patients with a median age of 58.2 years were treated (group 1, n = 60, group 2, n = 38 and group 3, n = 49). Four patients died, two were lost to follow‐up andthe remaining 141 patients were followed‐up for five years. Recurrent VTE occurring in 27/147 patients (five‐year rate 18.5%, Figure 1), including 18 events of recurrent SVT, 7 events of deep‐vein thrombosis and 2 events of pulmonary embolism. Fifteen events occurred early (during the first three months) and the remaining 12 late. Five‐year recurrent VTE‐freerates were significantly better with prolonged anticoagulation for the initialSVT event. These rates were71.5% in group 1, 84.1%in group 2 and 91.7% in group 3 (Figure 2, p for trend = 0.005).
Conclusion(s): Long‐term recurrent VTE rates in patients presenting with SVT are not negligible, supporting the notion that SVT is not a benign disease. Anticoagulation for three months, a duration similar with VTE, may improve patient outcomes.


VPB0471
Renal function impact in clinical and biologial results from patients treated with doacs in real world
M. Plaza Seijas 1; M. Corrochano2; S. Mojal3; C. Moret Puig4; R. Acosta‐Isaac5; J. Souto6
1 Research Institute, Hospital de la Santa Creu i Sant Pau, Barcelona., Barcelona, Catalonia, Spain; 2 Thrombosis and Haemostasis Unit, Madrid, Madrid, Spain; 3 Hospital de la Santa Creu i Sant Pau, Barcelona, Catalonia, Spain; 4 Hospital de la Santa Creu i Sant Pau, Barcelona, BARCELONA, Catalonia, Spain; 5 Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau., Barcelona, Catalonia, Spain; 6 Genomics of Complex Diseases Group, Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau, Barcelona, Catalonia, Spain
Background: The clinical and anticoagulant effect of direct oral anticoagulants (DOACs) is influenced by renal function. The EMA and FDA warned about the use of Edoxaban in patients with a glomerular filtration rate (GFR) > 95 mL/min, after the ENGAGE trial. Edoxaban could have less effectiveness in supranormal GFR. This was also observed in the ROCKET‐AF trial with rivaroxaban and in the ARISTOTLE trial with apixaban, but not with dabigatran.
Aims: To compare the characteristics of patients with a GFR ≥95 mL/min with those who have normal GFR, including biological parameters (Anti‐FXa activity), and the thrombotic complications incidence.
Methods: Prospective observational, unicentric study of anticoagulated patients with DOACs (MACACOD project, NCT 04042155). Anti‐Xa pre‐dose (trough) and post ‐dose (peak, 2h after intake) activity (HemosIL, Liquid Anti‐Xa, Werfen) were measured in all patients receiving edoxaban, apixaban and rivaroxaban. Edoxaban plasmatic concentrations were also measured (HemosIL, Liquid Anti‐Xa calibrated for edoxaban) in a subgroup of patients (n = 387) treated with edoxaban. Statistical analysis: Bivariate analysis of basal variables. For categorical variables Chi‐squared or Fisher test were used, as well as Mann‐Whitney U test for continued variables.
Results: We have analyzed 1030 patients anticoagulated with DOACs. 1000 were anticoagulated due to atrial fibrillation (AF) and 30 due to VTE. 132 patients had a GFR ≥90mL/min; 80 edoxaban, 24 apixaban, 23 dabigatran and 5 rivaroxaban. Total follow‐up was 1202 patients‐year with a median follow‐up of 12.9 months. Basal population characteristics are shown in Table 1, and the clinical outcomes in Table 2. There were no significant differences in Anti‐Xa activity, neither at trough nor at peak; and the same for edoxaban plasmatic concentrations.
Conclusion(s): In our registry, DOACs in general and edoxaban in particular, the Anti‐Xa activity and edoxaban plasmatic concentration do not seem to be influenced by GFR. Furthermore, we did not observe relevant differences in the incidence of major complications.


PB0458
Rivaroxaban for management of Superficial Vein Thrombosis
M. Rees 1; R. Cloudsdale2; S. Bladen2; R. Alikhan2; D. Gosrani2
1 Cardiff & Vale University Health Board, Cardiff, Wales, United Kingdom; 2 Cardiff & Vale UHB, Cardiff, Wales, United Kingdom
Background: Superficial venous thrombosis (SVT) is associated with increased risk of DVT/PE. In 2015 we introduced an SVT treatment pathway in line with BSH guidelines (LMWH or fondaparinux) and Oct 2019 amended this pathway including rivaroxaban 10mg daily
Aims: To assess the efficacy and safety of introducing rivaroxaban for the management of SVT.
Methods: Retrospective case‐notes review of UHW DVT Clinic, Oct 2019 – Jan 2022, patients with SVT >3 cm from the SFJ who received anticoagulation. Outcomes: SVT recurrence, DVT, PE within 90 days of anticoagulation and safety outcomes major or clinically relevant non‐major bleeding while on anticoagulation.
Results: 4905 scans, 17% (757/4905) positive for venous thrombosis, 21% SVT (157/757): age 26 – 92 years; 56% (88) female and 44% (69) male. 129 (82%) SVT patients received rivaroxaban with no recurrences and 28 (18%) received LMWH (6%), fondaparinux 16 (10%) or serial imaging 3 (2%) due to malignancy (4), pregnancy/post‐partum (11), patient‐choice (7), drug‐drug interaction (2), allergy (1), contra‐indication (1). Risk factors for SVT: 107 (68.2%) varicose veins, 56 (35.7%) BMI > 30, 54 (34.4%) Age > 65, 37 (23.6%) previous VTE/SVT, 17 (10.8%) family history VTE, 10 (6.4%) COCP/HRT.
Conclusion(s): 82% (129) patients with SVT >3cm from the SFJ were treated with rivaroxaban 10mg OD, 101 (78%) received treatment for 6 weeks and then stopped with no recurrent event up to 90 days and 2 (1.6%) CRNMBs. These results suggest that rivaroxaban is a safe, effective treatment for the management of superficial vein thrombosis

PB0441
No pharmacological effect of the P‐glycoprotein inhibitor tamoxifen on edoxaban plasma levels in patients with breast cancer: The PHIX‐IT study
F. Bosch 1; F. Mulder2; A. Willemsen3; M. Rentinck3; N. van Es4; R. Mathôt5; P. Kamphuisen6
1 Tergooi Medical Center, Amsterdam, Noord‐Holland, Netherlands; 2 Amsterdam UMC, location AMC, Amsterdam, Noord‐Holland, Netherlands; 3 Tergooi Medical Center, Hilversum, Noord‐Holland, Netherlands; 4 Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Noord‐Holland, Netherlands; 5 Department of Hospital Pharmacy, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, the Netherlands, Amsterdam, Noord‐Holland, Netherlands, 61. Tergooi Medical Center; 2. Amsterdam University Medical Centers, University Medical Center, Amsterdam, Noord‐Holland, Netherlands
Background: Edoxaban is an oral factor Xa inhibitor frequently used for treatment of cancer‐associated thrombosis. Concomitant treatment with the strong P‐glycoprotein inhibitor tamoxifen, a widely used drug for breast cancer, can increase edoxaban plasma levels, which can result in increased bleeding risk. According to guidelines, caution is required in patients who are treated with tamoxifen and edoxaban simultaneously, although there are no pharmacological data available.
Aims: To assess the in‐vivo effect of the P‐glycoprotein inhibitor tamoxifen on edoxaban plasma levels in patients with breast cancer.
Methods: This was a prospective, open‐label, single‐sequence crossover study in breast cancer patients with an indication for tamoxifen and no indication for anticoagulation. Edoxaban was given in a dose of 60 mg once daily for 4 days, first without tamoxifen and later with concomitant tamoxifen in steady‐state (Figure 1). Edoxaban plasma levels (liquid chromatography‐mass spectrometry (LC‐MS)) and anti‐factor Xa levels were measured on day 4 of edoxaban use just before intake (T = 0; trough) and hourly in the 4 h after intake to identify Cmax and Tmax.
Results: Twenty‐four women with breast cancer scheduled for tamoxifen were included. The median age was 56 years (IQR 51–63) and in 18 patients (75%) the tumor was resected. Median edoxaban plasma levels were not different without and with concomitant tamoxifen use at T = 0 (14.3 μg/ml vs 12.5 μg/ml, p = 0.83) and at Tmax (289.0 μg/ml vs 293.5 μg/ml, p = 0.51). Similary, anti‐Xa levels were not different at T = 0 (12.8 ng/ml vs. 14.0 ng/ml, p = 0.98) and Tmax (215.5 ng/ml vs. 214.6 ng/ml, p = 0.98) (Figure 2).
Conclusion(s): These data show there is no pharmacological effect of tamoxifen on edoxaban plasma levels and anti‐Xa activity in patients with breast cancer and therefore the fear of a clinically important drug‐drug interaction is not justified.


PB0443
Direct oral anticoagulants compared with warfarin for the treatment of left ventricular thrombosis post myocardial infarction
R. Byrne 1; J. Czuprynska1; V. Speed1; J. Byrne1; P. Scott1; R. Clapham1; R. Patel2; L. Roberts1; R. Arya2; J. Patel3
1 King's College Hospital, London, England, United Kingdom; 2 Kings College Hospital, London, England, United Kingdom; 3 King's College Hospital NHS Foundation Trust, London, England, United Kingdom
Background: Left ventricular thrombus (LVT) is a major complication of acute myocardial infarction (AMI). Until recently anticoagulation with a vitamin K antagonist has been the mainstay of treatment for LVT. DOACs provide a more acceptable form of anticoagulation however, outcome data on their use in LVT post AMI is limited.
Aims: To compare efficacy and safety of DOACs versus warfarin for patients diagnosed with LVT post AMI at King’s College Hospital, London.
Methods: Patients diagnosed with LVT post AMI between 1st January 2018 and 31st December 2020 were identified using the CogStack search tool. A retrospective case note review was conducted and each patient followed for 1 year after the index event. The primary endpoint was resolution of LV thrombus on repeat imaging, with secondary endpoints of bleeding (major bleed [MB] and clinically relevant non‐major bleed [CRNMB]) or stroke or systemic embolism (SSE).
Results: Fifty‐seven patients were diagnosed with an LVT post AMI (warfarin 72% and DOAC 28% – rivaroxaban, apixaban or edoxaban). Baseline characteristics were mostly well matched (Table 1). 89% of patients received concurrent dual antiplatelet therapy. The overall rate of thrombus resolution was 75% at first follow up imaging (Table 1). Thrombus resolution in the warfarin group was 71% compared with 85% in the DOAC group (p = 0.47). There were two SSE events, 1 in the warfarin and 1 in the DOAC group. There were 4 episodes of MB or CRNMB in the warfarin group, and none in the DOAC group (Table 2). There were two minor bleeding events, 1 in the warfarin group and 1 in the DOAC group. The overall difference in documented bleeding was not statistically significant (p = 0.66).
Conclusion(s): Our data suggests that DOACs are as safe and effective as warfarin for the treatment of LVT associated with AMI and supports further widespread use for this indication.


PB0445
Calibration of the International Normalized Ratio dedicated to Rivaroxaban according to WHO guidelines
M. Chekkal 1; N. Yafour2; A. Adda3; A. Boulenouar3; N. Zmouli3; M. Elhorri4; A. Saadi Ouslim5; M. Bennaoum3
1 Medicine Faculty, Oran1 University, Oran University Establishment (EHU), Oran, Algeria; 2 Department of Hematology, EHU Oran, Medicine Faculty, Oran1 University, Oran, Oran, Algeria; 3 Department of Hemobiology and Blood Transfusion, EHU Oran, Medicine Faculty, Oran1 University, Oran, Algeria; 4 HMRUO, Oran, Oran, Algeria; 5 Department of biochemestery, EHU Oran, Medicine faculty, Oran1 University, Oran, Oran, Algeria
Background: The arrival of Non‐ VKA Oral Anticoagulants (NOACs) has upset the landscape of antithrombotics. One of the most used is Rivaroxaban that has an anti Xa activity with predictable pharmacokinetics and pharmacodynamics and does not require routine monitoring except in certain situations such as surgery, drug interactions, hemorrhagic or thrombotic manifestations. In these situations, what would be the routine test to use for monitoring: PT, PT Ratio, PT % activity, INRVKA, INRRivaroxaban or other tests?
Aims: Our objective was to establish a reliable and routinely available means for monitoring Rivaroxaban therapy by comparing inter‐thromboplastin variations on different hemostasis tests: PT, PT Ratio, PT % activity, INRVKA and INR Rivaroxaban.
Methods: In a first step, the inter‐reagent variability of routine tests (PT, PT Ratio, PT % activity, INRVKA) results using various thromboplastins (Recombiplastine®, Neoplastine plus®, PT fibrinogen®, PT fibrinogen HS Plus®) was evaluated by the average coefficient of variation tested in 60 patients under Rivaroxaban. In a second step, we carried out a secondary calibration according to the “WHO 1999” guidelines to deduce the ISI of each thromboplastin tested and we calculated a specific INR for rivaroxaban (INR Rivaroxaban). We then evaluated the impact on the average CV in 60 patients under Rivaroxaban.
Results: The average CVs of PT and PT % activity were 15.96% and 12.37% respectively. Those of PT Ratio and INRVKA were 9.09% and 9.96%, respectively, while that of INR Rivaroxaban was 3.87% below the threshold value of 5%.
Conclusion(s): Calibration of INR with patient plasmas treated with Rivaroxaban allowed harmonizing results and to reduce the variations between different thromboplastins. INR Rivaroxaban is an inexpensive and routinely feasible test that can be a reliable test for monitoring patients treated with Rivaroxaban.
PB0446
Factors associated with anticoagulant treatment initiation among venous thromboembolism patients with cancer
S. Shah1; A. Dhamane 2; M. Ferri2; R. Bruette1; V. Noxon1; X. Liu2; J. Jiang2; X. Luo3
1 STATinMED Research, Plano, Texas, United States; 2 Bristol‐Myers Squibb Company, Lawrenceville, New Jersey, United States; 3 Pfizer, Inc., Groton, Connecticut, United States
Background: Cancer is associated with increased risk of venous thromboembolism (VTE) and associated mortality. Clinical guidelines recommend anticoagulant treatment among VTE patients with cancer. Understanding the factors associated with anticoagulation treatment initiation in a real‐world setting may help address treatment gaps in this high‐risk patient population.
Aims: To evaluate factors associated with anticoagulant treatment initiation among VTE patients with cancer.
Methods: VTE patients with cancer aged ≥65 were identified from 1/1/2014–12/31/2019 in the SEER‐Medicare database. Patients were required to be enrolled for ≥6‐months prior to and ≥ 30‐days after their first VTE (index date). Cancer status was identified from SEER or Medicare database in the 6‐months prior through 30‐days post index date. Patients were identified as treated or untreated depending on whether they initiated anticoagulant treatment within 30‐days after index date. Demographics and VTE‐related variables (Table) were measured on index date. Comorbidity and cancer related variables (Table) were measured in the 6‐months prior to index date. Logistic regression was used to assess the demographic and clinical characteristic factors associated with anticoagulant treatment initiation.
Results: A total of 28,468 VTE patients with cancer met eligibility criteria: approximately 46% (n = 12,971) were classified as treated and 54% (n = 15,497) as untreated. Mean age was 75 for treated and 76 for untreated and majority were female (>50% for both). A number of demographic, VTE‐related, cancer‐related and comorbidity‐related factors were significantly associated with anticoagulant treatment initiation (p < 0.05) (Table). For example, pulmonary embolism (PE) with deep vein thrombosis (DVT) diagnosis (OR [CI] = 3.6 [3.3–3.8]), PE without DVT diagnosis (OR [CI] = 2.1 [2.0–2.3]), VTE in inpatient setting (OR [CI] = 2.0 [1.9–2.2]) and having pancreatic cancer (OR [CI] = 1.6 [1.4–1.8]) were associated with higher likelihood of receiving anticoagulant treatment.
Conclusion(s): The decision to initiate anticoagulant treatment among VTE patients with cancer is associated with a range of demographic, cancer‐related, VTE‐related, and comorbidity‐related factors.

PB0449
Direct oral anticoagulants in the management of cerebral sinus vein thrombosis: A single centre experience
U. Faruqi 1; V. Grilo2; P. Holmes3; S. Wong3; K. Breen4
1 Guys and St Thomas NHS Foundation Trust, London, England, United Kingdom; 2 Garcia De Orta's Hospital, Almada, Lisboa, Portugal; 3 Guy's and St Thomas' NHS Foundation Trust, London, England, United Kingdom; 4 Guy's & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Cerebral sinus vein thrombosis accounts for up to 1% of strokes. Anticoagulation is largely adopted as part of management and whilst direct oral anticoagulants (DOACs) are now first line in venous thromboembolism (VTE), there is limited data on efficacy in CSVT.
Aims: We conducted a single centre retrospective observational study reviewing the use of DOACs in patients with CSVT.
Methods: We identified patients with CSVT managed at Guys and St. Thomas’ NHS Foundation Trust between May 2016 and April 2021 by screening CT and MRI reports for keywords associated with a diagnosis of CSVT. Electronic patient records were reviewed to identify patient demographics and management.
Results: 30 adult patients with a diagnosis of CSVT were identified (Table 1). Four patients were transferred to other trusts for neurosurgical management or were out of area and are therefore excluded from the review of subsequent anticoagulation management. 23/26 (88%) patients received anticoagulation. Three patients were not anticoagulated (two chronic thrombosis at diagnosis and one thrombotic thrombocytopenic purpura). 78% received initial anticoagulation with a DOAC or were later converted to a DOAC from heparin or warfarin. 80% (16/20) continued on longterm anticoagulation and in 15/16 this was with a DOAC (2/15 dabigatran, 5/15 apixaban, 5/15 rivaroxaban, 3/15 edoxaban). Two patients had clot extension: one on warfarin, one on a DOAC with an uncontrolled myeloproliferative neoplasm. One patient had a major bleed (subdural requiring intervention once on warfarin then on rivaroxaban 20 mg therefore changed to apixaban 2.5 mg BD longterm); two patients had minor bleeding with menorrhagia on a DOAC.
Conclusion(s): This small study supports the efficacy and safety of DOACs in the management of CSVT and provides the option for secondary prophylaxis for those requiring longterm anticoagulation. Future directions would include larger studies to assess outcomes for those anticoagulated with DOACS.

PB0455
Low molecular weight heparin beyond 12 months in patients with cancer associated thrombosis. CAT‐LONG study
S. Lopez‐Ruz 1; M. Barca‐Hernando2; S. Marin‐Romero3; M. Elias‐Hernandez4; R. Otero‐Candelera4; L. Jara‐Palomares4
1 Hospital Universitario Virgen del Rocio, Sevilla, Spain, Sevilla, Andalucia, Spain; 2 Medical Surgical Unit of Respiratory Diseases, Hospital Universitario Virgen del Rocio, Sevilla, Spain, Sevilla, Andalucia, Spain; 3 Medical Surgical Unit of Respiratory Diseases, Hospital Universitario Virgen del Rocio, Sevilla, Spain, Instituto de Biomedicina de Sevilla (IbiS), Sevilla, Spain, Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Córdoba, Andalucia, Spain; 4 Medical Surgical Unit of Respiratory Diseases, Hospital Universitario Virgen del Rocio, Sevilla, Spain, Instituto de Biomedicina de Sevilla (IbiS), Sevilla, Spain, Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III, Madrid, Sevilla, Andalucia, Spain
Background: The clinical guidelines suggest that, in patients with cancer associated thrombosis (CAT), anticoagulant treatment should be continued beyond 6 months as long as the cancer is active. But data beyond 12 months is scarce, and we know that risk of bleeding is not fixed over time, as showed those studies that analyse anticoagulant treatment up 12 months.
Aims: Analyse safety of low molecular weight heparin (LMWH) beyond 12 months in patients with CAT.
Methods: We performed a post‐hoc analysis of consecutive CAT patients evaluated in several prospective studies from Oct‐2008 to Dec‐2019 (Qca, TiCAT, endothelial dysfunction). Primary outcome was clinically relevant bleeding (CRB) according to ISTH criteria. We compare two periods (1–12 vs. 12–24 months). For the survival analysis (time to event), the curves were compared using the Log Rank test (Mantel‐Cox) or the Generalized Wilcoxon test. Cox regression was performed with non‐parametric proportional hazards adjustment. We performed a sensitivity analysis to investigate the potential heterogeneity of treatment effects. For the competing risks analysis, the Fine‐Gray test was used.
Results: 588 patients were included, of which 33.8% (n = 199) received LMWH beyond 12 months. During the 2 years of follow‐up, there were 85 CRB (14%), 71 in the period 1–12 months and 14 in the period 12–24 months. Comparing 12–24 vs. 1–12 months, hazard ratio (HR) of CRB was 0.2 (95% CI: 0.1–0.37; p < 0.001). The competing risk analysis of CRB of both periods (1–12 vs. 12–24 months) showed a lower subdistribution hazard ratio (SHR) during the period 12–24 months (SHR: 0.3, 95% CI: 0.16–0.55, p < 0.001). In the period 12–24 months, subgroup analyses (metastasis, age, incidental thrombosis, ECOG performance status and sex) revealed no differences in CRB.
Conclusion(s): In cancer associated thrombosis, clinically relevant bleedings during LMWH treatment were significantly lower beyond 12 months.
PB0463
Outcomes in clinical studies of interventions for venous thromboembolism in non‐pregnant adults: A scoping review
T. Tritschler 1; E. Cusano2; M. Mathieu3; B. Hutton4; B. Shea4; R. Shorr4; L. Skeith2; L. Duffett4; N. Langlois5; L. Cowley4; S. Ng6; S. Dubois7; C. West7; P. Tugwell4; G. Le Gal8
1 Inselspital, Bern University Hospital, Bern, Bern, Switzerland; 2 University of Calgary, Calgary, Alberta, Canada; 3 University of Ottawa, Ottawa, Ontario, Canada; 4 Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario, Canada; 5 Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 6 Faculty of Medicine & Health, University of New South Wales, Sydney, New South Wales, Australia; 7 CanVECTOR, Ottawa, Ontario, Canada; 8 Department of Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Canada, Ottawa, Ontario, Canada
Background: The development of a core outcome set (COS), defined as an agreed minimum set of outcomes that should be measured and reported in trials of a specific disease, aims to increase the relevance of study findings to key stakeholder groups and improve standardization.
Aims: As the first step in developing a COS for venous thromboembolism (VTE) treatment studies, we aimed to generate an inclusive list of unique outcomes reported in previous VTE treatment studies and classify them in domains.
Methods: MEDLINE, Embase and CENTRAL were searched for prospective studies reporting on interventions for VTE in non‐pregnant adults. Study selection and data extraction were performed in blocks based on publication date, starting with 2015–2020 and subsequent 1‐year periods, until no new outcome was identified. Outcomes were classified in domains, which are groups of closely related outcomes, and domains were classified in four core areas including death, pathophysiological manifestations/abnormalities life impact and resource use.
Results: Of 7100 records identified, we included 240 publications, representing 165 (69%) distinct studies. We identified 205 unique outcomes that were grouped into 48 domains. A total of 30 (13%) studies covered ≥3 of the 4 core areas; death was included in 102 (43%), pathophysiological manifestations/abnormalities in 218 (91%), life impact in 41 (17%), and resource use in 25 (10%) studies.
Conclusion(s): Most VTE treatment studies evaluated pathophysiological features of VTE, but few studies reported outcomes that measure life impact or resource use. Our findings will inform next steps in the development of a COS for VTE treatment studies. (Funded by the Canadian Institutes of Health Research and supported by the Canadian Venous Thromboembolism Research Network.)

PB0440
Experience of therapeutic drug monitoring in patients prescribed direct oral anticoagulants (DOACs) of low body weight (<55 kg) at Guy’s & St Thomas’ NHS foundation trust
J. Bartoli‐ Abdou 1; V. Collings1; S. Acar2; G. Bahra1; N. McCutcheon1; Z. Mahir1; K. Breen3
1 Guy's & St Thomas' NHS Foundation Trust, London, England, United Kingdom; 2 King's College London, London, England, United Kingdom; 3 Guy's & St Thomas NHS Foundation Trust, Kings College London, London, England, United Kingdom
Background: Patients of low body weight (LBW) were under‐represented in phase‐III clinical trials for DOACs and experience using these agents in this population is lacking. Guidance recommends LBW patients have therapeutic drug monitoring (TDM) with anti‐Xa levels.
Aims: Review anti‐Xa levels and clinical management of LBW (<55 kg) patients prescribed DOACs at GSTT between 01/01/2018 and 31/12/2019.
Methods: Anti‐Xa levels conducted between 01/01/2018–31/12/2019 were extracted from laboratory records and records reviewed to identify patients weighing <55 kg, date and time of previous dose, DOAC regime, past medical history, renal function, dose changes as a result of level, bleeding or thrombotic event within 3‐months of level and current anticoagulation regime of patient was recorded. Descriptive statistics were used and non‐parametric tests were used to compare categorical variables.
Results: 83 anti‐Xa levels from 45 unique LBW patients were available for analysis. Median weight was 51.2 kg (IQR: 44.5–53.1), Table 1. Of the anti‐Xa levels available, 27 were peak levels (32.5%) and 20 were troughs levels (24.1%). The remaining 36 (43.4%) were neither peak or trough with 15 (18.1%) having no documented time of last dose or sample. Levels were within reference range for 33 (70.2%) samples (Table 2). Dose changes were made following high anti‐Xa levels in 5 (50%) cases. Patients prescribed the licensed dose in this cohort were more likely to have a peak or trough result outside of the reference range (Chi sq = 4.889, p = 0.027). In the 3‐months following each assay, 5 (11%) patients experienced a bleeding event, 1 (20%) of which was major (Table 2). 1 (2.2%) patient experienced a line associated thrombus.
Conclusion(s): DOACs are potentially suitable for use in LBW. Whilst dosing decisions should not be based on anti‐Xa levels in the general population, it may be beneficial in LBW. Anti‐Xa levels above the reference range was not associated with bleeding.
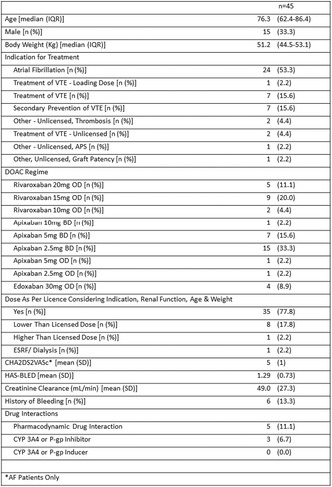

Women's Health
Estrogens and Progestinics
VPB1389
Descriptive analysis of venous thromboembolic disease in patients under contraceptive treatment in sector III of Aragón
I. Rivas Estabén 1; A. Ortiz López2; S. Angós Vázquez3; N. Gemperle Ortiz3; M. Moles Guerrero3; V. Murillo Cortés3; O. Gavín Sebastian3; L. Palomera Bernal3
1 Hospital Lozano Blesa, Andorra (Teruel), Aragon, Spain; 2 Hospital Clínico Lozano Blesa, Zaragoza, Aragon, Spain; 3 Hospital Lozano Blesa, zaragoza, Aragon, Spain
Background: In the present study, we analysed the profile of women, users of contraceptives, who have suffered a thrombotic event, to identify predisposing factors in order to minimise the risk and to be able to prescribe these drugs more safely.
Aims: To describe the type of thrombotic event in women taking contraceptives and to identify the environmental factors that precipitate and contribute to the magnitude of the event.
Methods: Observational, descriptive, cross‐sectional and retrospective study of a cohort of patients diagnosed with thrombosis while taking hormonal contraceptives from 2010 to 2020. Epidemiological, clinical and biological data were obtained from computerised medical records, the Emergency Department and the GOTA programme of patients seen at the Hospital Clínico Lozano Blesa in Zaragoza. The bibliography was obtained from electronic databases.
Results: The study population consisted of 190 women, of whom 52 met the inclusion criteria. 9.6% had a family history of VTE. The CVRFs present were: smoking (34.6%), obesity (29%), dyslipidaemia (17%) and arterial hypertension (4%); medical comorbidities: autoimmune disease (15.5%), mental illness (15.5%), respiratory pathology (11.5%), chronic venous insufficiency (9.6%), neoplastic disease (4%); environmental risk factors (30.8%); type of contraceptive: combined (79%), gestagen only (2%), no information (19%); gestagen generation: second (9.8%), third (34.2%) and fourth (56%); clinical manifestations of VTE: DVT (69.2%), PE (40.4%), DVT + PE (17.3%), SVT (5.8%), DVT + SVT (4%) and others locations (2%); positive thrombophilia (34.6%); recurrence of VTE (2%).
Conclusion(s): 1. The most common clinical manifestation of VTE in women who experience thrombosis in the context of contraceptive use is proximal DVT of the lower extremities. 2. The risk of VTE from contraceptives is determined by both the dose of oestrogen and the type of progestogen. The safest option is an oral contraceptive containing levonorgestrel combined with a low dose of estrogen (20 mg).


PB1388
Thromboelastography assessing coagulation profile in young women using hormonal contraception
Y. Tera 1; Y. Deng2; M. Othman1
1 Queen's University, Kingston, Ontario, Canada; 2 Queen’s University, Kingston, Ontario, Canada
Background: Hormonal contraception (HC) is used by 9% of women worldwide. Although rare, thrombosis represents a serious side effect of HC. The risk of thrombosis is influenced by the estrogen component, dose, type, use duration, and family history. The risk increases two‐folds (10–20/10,000) in women using HC compared to healthy, non‐pregnant women, not using HC. Conventional haemostasis testing may not detect hypercoagulability or predict thrombosis risk.
Aims: To assess the coagulation profile in association with hormonal contraception in young female using thromboelastography (TEG) and evaluate the potential value of TEG in monitoring the possible risk of thrombosis and guiding individualized approach hormonal contraception.
Methods: 300 young females were recruited from two post‐secondary institutions for this study. Data on age, type, duration and whether first or recurrent use, were collected. Citrated whole blood was drawn within the first 14 day of menstrual cycle. TEG was performed using a TEG® 5000 Hemostasis System. The following major parameters were evaluated: time to clot formation (R), rate of clot formation (K), speed of clot propagation (α), strength of clot (MA), Clot lysis after 30 min. (LY30) and clotting index (CI). Student t test was used to assess the difference in TEG parameters between HC users and non‐users.
Results: 27 females (17 users and 10 non‐users), aged 19‐ 29 were tested. The Average reported duration of use was 5 years. Comparative analysis of TEG parameters of the two age‐matched groups showed no significant difference in any of the parameters. However, there is a trend towards hypercoagulability observed in α (p = 0.09) and the trend towards fibrinolysis observed in LY30 (0.09), in HC users compared to the non‐users (Table 1).
Conclusion(s): Based on this small sample, there seems to be no change in coagulation profile with HC use. Additional assessments are needed before a final conclusion can be made.

VPB1390
Common risk factors for arterial and venous thrombosis and the recurrence rate in young non‐pregnant women
N. Vereina 1; T. Movchan2; V. Chulkov2
1 South Ural State Medical Universiry, Chelyabinsk, Sverdlovsk, Russia; 2 South Ural Medical University, Chelyabinsk, Sverdlovsk, Russia
Background: The identification of common thrombotic risk factors seems important for evaluation the overall hypercoagulation status and prognosis.
Aims: To determine factors associated with arterial and/or venous thrombosis and the recurrence rate of thrombosis in non‐pregnant women aged 18–44 years.
Methods: 319 women referred to the antithrombotic centre from 2010 to 2020 were enrolled in case‐control study: 134 patients with first verified arterial and/or venous thrombosis and 185 women without thrombosis. Exclusion criteria: pregnancy and 6 weeks after delivery at the time of the first thrombosis; mental disorders. The demographic, clinical, laboratory and treatment characteristics were recorded. The prospective observation with median time 52 months (range from 1 to 10 years) was carried out for cases.
Results: In cases 100 women (74.6%) had venous (83 – VTE; 17 – other localizations) and 33 (24.6%) – arterial thrombosis (30 – ischemic stroke, 3 – myocardial infarction). One patient with antiphospholipid syndrome had pulmonary embolism with renal artery thrombosis. In multiple regression analysis significant common determinants for the first thrombosis were: gallbladder diseases (OR – 12; 1 95% CI 2.5 – 67.5), cardiovascular diseases (OR = 10.2; 95% CI 3.6–29.1); total cholesterol level (OR = 7.7; 95% CI 4.4 – 13.5); respiratory diseases (OR = 3.7; 95% CI 1.0–11.5); the use of combined hormonal contraceptives (OR = 3.4; 95% CI 1.7–6.8); varicose veins of the lower extremities (OR = 2.5; 95% CI 1.0–4.9). At follow‐up, 12 women (8, 95 %) had a recurrence of thrombosis: 8 – VTE; 3 – ischemic strokes, 1 – cerebral vein thrombosis).
Conclusion(s): Independent common factors, associated with arterial and venous thrombosis in young non‐pregnant women were gallbladder diseases, cardiovascular diseases; total cholesterol level, respiratory diseases, the use of combined hormonal contraceptives and varicose veins of the lower extremities. The recurrence rate was 8, 95% during follow‐up on average for 4.3 years.
PB1386
Comparison of the ETP‐based thrombomodulin assay versus the ETP‐based APC resistance assay on the ST Genesia system
L. Morimont 1; M. Didembourg2; A. Carlo3; J. Dogné4; J. Douxfils5
11. Qualiblood s.a., 2.University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), 5000, Namur, Belgium; 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), Namur, Namur, Belgium; 3 Diagnostica Stago, Asnières Sur Seine, Ile‐de‐France, France; 4 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS), 5000, Namur, Belgium, 51 Qualiblood s.a., 2 University of Namur, Department of Pharmacy, Namur Thrombosis and Hemostasis Center (NTHC), Namur Research Institute for Life Sciences (NARILIS)., 5000, Namur, Belgium
Background: Resistance towards activated protein C(APC) in women treated with combined hormonal contraceptive (CHC) can be measured using an endogenous thrombin potential (ETP)‐based assay, ideally targeting 90% ETP inhibition in healthy individuals. The only commercially available kit for APC resistance assessment on an automated thrombin generation platform, i.e., the ST Genesia, is the STG‐ThromboScreen kit which uses thrombomodulin (TM) and targets 50% ETP inhibition. Nevertheless, previous assays based on the addition of exogenous APC instead of TM are better known to assess CHC‐induced APC resistance.
Aims: To compare the sensitivity of the 50% ETP‐based TM resistance assay and the 90% ETP‐based APC resistance assay (recently implemented) on the ST Genesia system, in women using CHCs.
Methods: The TM‐based assay consisted of using STG‐ThromboScreen TS‐TM and STG‐ThromboScreen TS+TM while the APC‐based assay consisted of using the STG‐ThromboScreen TS‐TM in absence and presence of exogenous APC. This study involved 96 individuals which were stratified into several subgroups: healthy individuals (men and women not using CHC) (n = 56), women using 2nd generation CHCs (n = 18), 3rd generation CHCs (n = 13) and the so‐called “other” CHCs (n = 9).
Results: The mean ETP inhibition % (±SD) of healthy individuals equaled 89.5% ± 8.1% in presence of APC, and 64.2% ± 12.7% in presence of TM. Differences between healthy individuals and women using CHCs were significant with both assays. No significant difference was observed among women using different CHCs generation with the TM‐based assay while significant differences were observed between the “other” CHCs user group versus 2nd and 3rd generation CHCs user groups with the APC‐based assay.
Conclusion(s): APC resistance test based on the addition of exogenous APC achieving 90% ETP inhibition is preferable over commercial TM‐based assay targeting 50% ETP inhibition for the assessment of CHC‐induced APC resistance. Further investigations are required to confirm our results and consider the use of the ETP‐based APC resistance assay in clinics.
PB1385
Concurrent use of Tranexamic acid and hormonal therapy for the management of heavy menstrual bleeding in women
A. Epstein 1; O. Turan2; R. Abdul‐Kadir3
1 Royal Free London NHS Foundation Trust, LONDON, England, United Kingdom; 2 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free London NHS Foundation Trust, London, England, United Kingdom; 3 Royal Free London NHS Foundation Trust, London, England, United Kingdom
Background: Heavy menstrual bleeding (HMB) is the most common gynaecological symptom associated with impaired physical and social wellbeing. Both hormonal therapies and the anti‐fibrinolytic Tranexamic acid (TXA) are used as a first line therapy for reducing menstrual blood loss. There are concerns that their concurrent use may increase the risk of venous thromboembolism (VTE).
Aims: The aim of this study is to review the effectiveness and safety of this combination therapy for management of HMB in women.
Methods: Retrospective data collection from electronic patient records of 175 women (100 (57%) with an inherited bleeding disorder and 75 (43%) without) with HMB who received combination therapy.
Results: The median age of the women was 20–29 years, 98 (56%) were overweight or obese. None had a personal or family history of VTE. Von Willebrand disease was the commonest bleeding disorders affecting 38 (38%) of the women. Median pictorial blood assessment chart score was 137. 28 (16%) had iron deficiency anaemia and 36 (20%) had uterine fibroids. The median duration of therapy was 3 years with 88 (50%) of women using the treatments for more than 4 years. Hormonal therapies used were combined oral contraceptives in 78 (44%) women, Levo‐norgestrel IUS in 28 (16%), cyclical progesterone in 54 (31%), Progesterone only pill (desogestrel 75 mcg) in 14 (8%). 168 (96%) of women used TXA only during the period; 71 (41%) of whom required TXA more than 7 days a month. 12 (7%) also used TXA for other indications; bruising and epistaxis. Median PBAC score 6 months after treatment was 71 and 150 (86%) women rated the combined therapy as effective. 18 (10%) women required an additional therapy; 6 (3%) endometrial ablation and 12 (7%) hysterectomy.
Conclusion(s): Concurrent use of TXA and hormonal therapy is safe, effective and well tolerated in women with HMB.
PB1387
Heavy menstrual bleeding from anticoagulation therapy significantly impacts on women’s quality of life – findings from the PERIOD programme of work
J. Patel 1; O. Nzelu2; L. Roberts3; J. Johns2; J. Ross2; R. Arya4
1 King's College Hospital Foundation NHS Trust and King's College London, Surrey, England, United Kingdom; 2 King's College Hospital Foundation NHS Trust, London, England, United Kingdom; 3 King's College Hospital, London, England, United Kingdom; 4 Kings College Hospital, London, England, United Kingdom
Background: There is increasingly recognition that women prescribed anticoagulant therapy experience heavier bleeding during their menstrual cycles, potentially impacting on their quality of life (QoL). Little research exists which reports this, from the women’s perspective.
Aims: To quantify menstrual blood loss and quality of life in women newly prescribed anticoagulant therapy following initiation of anticoagulation therapy, compared to a control group.
Methods: Women between the ages of 18–50, newly commenced on anticoagulation therapy were recruited from the anticoagulation clinics at King’s College Hospital, UK. Subjects were asked to complete two online validated instruments: the menstrual bleeding questionnaire and a menstrual pictogram, during two consecutive menstrual cycles. In parallel, a control group was recruited. Descriptive statistics was used to analyse the data, with non‐parametric tests applied to compare differences between groups. Ethical approval for the study has been granted (REC reference: 19/SW/0211).
Results: 102 women returned completed questionnaires; 38 in the anticoagulated group and 64 in the control group. Table 1 outlines the demographic information. Table 2 compares the menstrual pictogram blood loss and menstrual questionnaire quality of life scores during cycle 1 and cycle 2 for both groups. Menstrual pictogram blood loss significantly correlated with menstrual QoL scores, Spearman’s rho 0.673 (cycle 1) and 0.693 (cycle 2), p < 0.001. Women in the anticoagulated group were more likely to miss days off work, avoid social activities, experience episodes of bleeding that soaked through to outer clothing and report the end date of their cycle being unpredictable, compared to the control group.
Conclusion(s): Our findings confirm that women newly commencing oral anticoagulation suffer with heavy menstrual bleeding (HMB) which significantly impacts on their quality of life. Further research which helps to identify those women more likely to suffer with HMB and circumventing this, along with determining the optimal anticoagulant treatment strategy is required.


Pregnancy and Pregnancy Complications
PB1401
Severe Covid‐19 infection in pregnancy complicated by heparin induced thrombocytopenia
S. Kazi 1; M. Smith2; K. Talks3
1 Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, England, United Kingdom; 2 Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, England, United Kingdom; 3 Newcastle Haemophilia Comprehensive Care Centre, Royal Victoria Infirmary, Newcastle upon Tyne, England, United Kingdom
Background: As the pandemic has progressed with emergence of new variants, data on pregnancies with COVID‐19 infection has evolved. Pregnant women represent a high‐risk population with increasing incidence of maternal morbidity and mortality
Aims: We present a case report of a 31‐year‐old primipara presenting with severe COVID‐19 infection and thrombosis antepartum complicated by the development of heparin induced thrombocytopenia (HIT) post partum.
Methods: A 31 year not previously vaccinated for SARS‐CoV‐2, presented at 28 weeks gestational age with severe COVID‐19 infection requiring Non‐invasive respiratory support. A CT pulmonary angiogram revealed COVID pneumonitis with distal pulmonary emboli. Blood tests‐ platelet count of 280 X 109/L, Prothrombin time (PT) of 12 s, Partial thromboplastin time (APTT) of 28 s and a derived Fibrinogen of 6.9 g/L. She received Tocilizumab/Sarilumab, steroids and treatment dose of low molecular weight heparin (LMWH). With ongoing concerns of fetal distress and deteriorating maternal condition requiring invasive respiratory support, an emergency Cesarean section (CS) was performed on day 3 of admission. Intravenous heparin infusion was restarted post‐operatively and LMWH recommenced on day 1 after the CS. On day 13 post CS HIT screen was requested with positive anti‐PF4/heparin antibodies (Acustar®) (optical density 7.2). LMWH was stopped; argatroban initiated due to need for procedures and the platelet count improved. There was no evidence of further extension or recurrence of thrombo embolic disease.
Results: Thrombocytopenia is frequent in critically ill patients. The occurrence of HIT in critically ill COVID‐19 patients has been described.
Conclusion(s): Critical illness and complications such as COVID‐19 associated coagulopathy and HIT occurring during pregnancy require multidisciplinary plan with close collaboration between various specialties. Adverse outcomes of COVID‐19 infection in pregnancy may be uncommon and therefore international collaboration such as ISTH COV‐PREG‐Coag Registry is critical improve the global understanding of the infection in pregnancy.

VPB1421
Protein C and antithrombin III of pregnant women visiting antenatal clinic in University of Calabar Teaching Hospital, Calabar, Nigeria
D. Okpokam 1; A. Okoli1; E. Akwiwu1; J. Akpotuzor1; A. Udo2
1 University of Calabar, Calabar, Cross River, Nigeria; 2 University of Calabar Teaching Hospital, Calabar, Cross River, Nigeria
Background: Haemostatic changes in pregnancy are significant, essential and have the potential to cause adverse pregnancy outcomes.
Aims: To assess and provide information on some coagulation parameters, fibrinogen, Protein C, and Antithrombin III of pregnant women attending University of Calabar Teaching Hospital.
Methods: 120 pregnant women, aged 18–45 years were recruited, sixty (60) age‐matched non‐pregnant women. Ethical clearance with the Reg. number (UCTH/HREC/33/553) and informed consent (well‐structured questionnaire) from all subjects was obtained. Citrated and serum samples were used. PT, APTT and TT were assayed using the One‐Stage Quick method respectively, FIB was assayed using Clauss method, while PC and ATIII were assayed using ELISA technique.
Results: 52.5% of pregnant women were 19–28 years, 30.8% were 29–38 year and 16.7% were 39–48 years. Pregnant women with tertiary have the highest (58.3%), secondary (29.2%) and primary education (12.5%). It was observed that FIB (4.40 g/l), PC (3715.83 pg/ml) and AT III (1346.65 ng/ml) showed significant increase when compared with non‐pregnant women (p < 0.001). Also APTT and TT showed significant differences (p < 0.05) in age ranges. TT and PC were significantly decreased (p < 0.05) in Multiparous 4 and Multiparous 3 when compared with Primiparous and Nulliparous among pregnant women based on parity respectively. PT and FIB (13.88 s, 4.71 g/L) were significantly increased in 3rd trimester when compared with the 1st and 2nd trimester (13.12 s, 13.19 s) and (4.18 g/L, 4.29 g/L) respectively. Relationships were mild positive between PT Versus AT III (r = 0.176, p < 0.05) and mild negative between PC Versus APTT (r = 0.137, p < 0.05).
Conclusion(s): FIB and ATIII are significantly higher, PC activity was significantly low, while multigravidity has an influence on protein C, among pregnant women. Health care givers should be aware of the pregnancy induced changes to aid the proper management and monitoring of pregnant women with bleeding or other thromboemoblic disorders in this locality.


PB1403
What is the extent of coagulation abnormalities in the development of pregnancy complications?
A. Khosravi 1; M. Paridar2; A. Kordian3; O. Kiani Ghalehsardi4; V. Takhviji5; M. Karimzadeh6
1 *Transfusion Research center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 2 Ministry of health and medical edjucation, Tehran, Iran, Tehran, Tehran, Iran; 3 Ahvaz University of Medical Sciences, Ahvaz, Iran, Tehran, Tehran, Iran; 4 Iran University of Medical Sciences, Tehran, Iran, Tehran, Tehran, Iran; 5 Transfusion Research center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tehran, Tehran, Iran; 6 Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tehran, Tehran, Iran
Background: Pregnancy is an acquired hypercoagulable state. Moreover, many studies show that the acquired or inherited thrombophilia can be associated with recurrent pregnancy loss.
Aims: We have aimed to evaluate the coagulation status of patients with pregnancy complications in a large scale design.
Methods: In this retrospective observational study, we evaluate 1345 patients with pregnancy complications that referred to the Iranian Blood Transfusion Organization. We use prothrombin time (PT) and activated partial prothrombin time (aPTT) to screen these patients and evaluate the clinical history, the prevalence of activated protein C resistance (APCR), protein C(PrC), protein S(PrS), and antithrombin III deficiencies, Lupus Anticoagulant (LA) and factor deficiency
Results: The most frequent complaint was thrombophilia (97.3%), followed by Bleeding and thrombosis (1.9%) and bleeding tendency (0.5%). We observed 39.4%, 11.9% and 1.3% abortion in first, Second and third‐trimester, respectively. Out of the 1219 thrombophilic patients, 94.1% had a negative thrombophilia screen, while 5.7% had at least one thrombophilic defects. The most frequent defect was APCR (3%), followed by pregnancy‐induced PrS deficiency (1.6%), LA (0.3%), AT (0.2%), PrC (0.2%), PrS (0.2%). In a close observation of 19 patients with Bleeding and thrombosis and 6 with a bleeding tendency, 79.2% and 85.7% of both groups were normal in coagulation screening.
Conclusion(s): Our findings indicate that thrombophilia can be a common presentation in women with pregnancy loss, but the majority of these patients have a negative thrombophilia screen. Recurrent miscarriage and thrombophilia are merely associated, and the evidence for possible benefits of thrombophilia screening is lacking.
VPB1418
Factor V Leiden polymorphism, prothrombin G20210A mutation, and methylenetetrahydrofolate reductase C677T polymorphism in recurrent pregnancy loss
F. Megdich 1; I. Krichen1; L. Mbarek2; I. Ben Ayed2; K. Chaabene3; C. Kallel4
1 Department of Hematology/University of Monastir, Sfax/Tunisia, Sfax, Tunisia; 2 Department of Molecular Biology/University of Sfax, Sfax, Sfax, Tunisia; 3 Gynecology Obstetrics and Reproductive Medicine/University of Sfax, Sfax, Sfax, Tunisia; 4 Department of Hematology/University of Monastir, Sfax, Sfax, Tunisia
Background: Recurrent pregnancy loss (RPL) is a major public health problem. Although the prominent effect of acquired thrombophilia is well established, studies of the involvement of the hereditary thrombophilia remain controversial.
Aims: Therefore, our objective was aimed to explore the incidence and the role of genetic prothrombotic markers as risk factors for RPL in the south of Tunisia.
Methods: 67 women who presented RPL were enrolled in this study. The detection of factor V Leiden (FVL) G1691A polymorphism and the prothrombin G20210A mutation were performed by PCR‐AS (polymerase chain reaction‐specific allele) while the MTHFR C677T polymorphism was established by PCR‐RFLP (polymerase chain reaction ‐Restriction fragment length polymorphism). Statistics were based on chi‐square tests (chi2).
Results: The mean age of our patients was 31 ± 6 years. Our study revealed polymorphisms as follows: FVL G1691A (21% heterozygous and 3% homozygous variants), MTHFR C677T (50.7% heterozygous and 10.5% homozygous variants). However, results showed a total absence of prothrombin G20210A mutation in both patients and the control group. Added to that, a significant incidence of FVL heterozygosity was found in our patients with a 2.597 times increase of the risk of RPL (Odds Ratio (OR): 2.597; 95% CI (1.005–6.709)). Furthermore, the MTHFR heterozygosity presented a significant incidence in our series with a 1.515 times rise of the RPL’s risk (OR 1.515; 95% CI (0.993–2.311)). The statistical association of both FVL and MTHFR polymorphisms in the heterozygous form increased the risk of RPL over 2.749 times (OR: 2.749; 95% CI (1.331–5.679)).
Conclusion(s): Our study confirms the involvement of the heterozygosity of both FVL and MTHFR C677T polymorphism in the RPL's occurrence. Also, homozygous variants have been found only in patients. Therefore, rising the number of analyzed samples would be necessary to have a better molecular profile of women and to ensure an better monitoring.
PB1399
Semiological assessment and use of scales in patients with excessive menstrual bleeding in primary care: A pending task
B. Diaz Jordan
Hospital General de Valdepenas, Valdepenas, Castilla‐La Mancha, Spain
Background: Excessive menstrual bleeding (EMB) is a common consultation in primary care. There are various scales to quantify losses and promptly refer to specialized care, however, their use remains limited in routine clinical practice.
Aims: The objective is to describe the semiological tools for referral to Hematology for excessive menstrual bleeding, and their knowledge about quantification scales.
Methods: Prospective, analytical and multicentre study in which 20 family doctors belonging to the Integrated Care Management of Valdepenas (Spain) participated through telephone consultation. Demographic and statistical data were collected.
Results: The median age was 50 years (range: 38–61 years) with parity in both sexes. The median distance between the health center and the hospital was 25 km (range: 1–51 km). The semiological sign that had the highest score among family doctors to refer a patient with EMB was secondary anemia (100%), followed by blood volume loss (90%) and dysmenorrhea (85%). 100% of the sample reported requesting a complete blood count, iron profile and basic hemostasis study within the first consultation, although only 15% would request an initial thyroid profile. Only 5% of the participants use any of the quantification scales described (Highman, PBAC, Philipp) in their usual practice, with the days of bleeding being the most common way of quantifying the EMB (97%).
Conclusion(s): It is essential to increase training on the etiological factors and consequences of EMB, as well as the implementation of validated quantification scales, in order to optimize its management and early referral for study/treatment.
VPB1422
Revision of the diagnostic methods used for the detection of pulmonary embolism in pregnant women at the Centre Hospitalier Universitaire de Sherbrooke
M. Patterson; G. Le Templier; L. Savoie
CHUS, Sherbrooke, Quebec, Canada
Background: The diagnosis of pulmonary embolism in pregnant women poses a particular challenge, given that several symptoms of pulmonary embolism are often expected findings in pregnant women, and that patients and some clinicians may be reluctant to use imaging for fear of radiation.
Aims: We aim to describe the incidence of pulmonary embolism in pregnant patients in which this diagnosis was suspected in our hospital and to compare it with the 5% incidence found in medical literature. We also want to describe the use of serum D‐dimer assay in our hospital and assess whether there has been an increase in its use since the appearance of the ARTEMIS study in march 2019.
Methods: We collected data from our local computerized clinical record for all pregnant patients aged 18 to 45 who underwent an investigation for pulmonary embolism between January 2015 and November 2019.
Results: Of the 186 of these patients, 4 (2.2%; 95% CI [0.84% – 5.40%]) were diagnosed with a confirmed pulmonary embolism. There was no statistically significant difference with the value of 5% found in the literature (p value at 0.07). Before March 2019, serum D‐dimer was measured in 57.7% of pregnant patients in whom there was suspicion of pulmonary embolism. After March 2019, D‐dimer was measured in 82.6% of these patients, representing a statistically significant increase in their use before and after March 2019, with a p‐value of 0.02.
Conclusion(s): These results demonstrate that there is no statistically significant difference between the incidence of pulmonary embolism in pregnant women in which this diagnosis is suspected in our hospital and that found in medical literature. Secondary, we have found an increase in the use of D‐dimer in the diagnostic approach for pulmonary embolism in pregnant patients in our hospital after March 2019.
VPB1427
The use of rotational thromboelastometry in the management of thromboprophylaxis in pregnancy
L. Stanciakova 1; M. Dobrotova2; P. Holly3; J. Zolkova2; L. Vadelova4; I. Skornova2; J. Ivankova3; M. Brunclikova2; M. Grendar5; J. Danko6; P. Kubisz2; J. Stasko2
1 National Centre of Haemostasis and Thrombosis, Department of Haematology and Transfusiology, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Martin University Hospital, Martin, Slovak Republic, Martin, Zilina, Slovakia; 2 National Centre of Hemostasis and Thrombosis, Department of Hematology and Transfusiology, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin and University Hospital in Martin, Slovakia, Martin, Zilina, Slovakia; 3 Comenius University, The Jessenius Faculty of Medicine in Martin, Martin, Zilina, Slovakia; 4 Centre of Immunology in Martin, s.r.o., Martin, Slovak Republic, Martin, Zilina, Slovakia; 5 Biomedical Center Martin, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Slovakia, Martin, Zilina, Slovakia; 6 Department of Gynaecology and Obstetrics, Jessenius Faculty of Medicine in Martin, Martin University Hospital, Martin, Slovak Republic, Martin, Zilina, Slovakia
Background: Anticoagulant thromboprophylaxis with low‐molecular‐weight heparin (LMWH) for patients with a previous venous thromboembolism (VTE) is recommended. There are only limited cases of its use in the prevention of thrombotic complications in pregnancy.
Aims: The authors present the results of a prospective and longitudinal study of haemostasis in at‐risk pregnant patients.
Methods: Blood samples were taken at these time points: T1 during the 10th–12th week of pregnancy (WP), T2 in the 16th–18th WP, T3 between the 26th–28th WP, T4 in the 35th–36th WP and T5 between the 6th–8th week following the delivery. Results of clotting time (CT), clot formation time (CFT), alpha angle (AA) and maximum clot firmness (MCF) were compared between T1–T4 and T5 when selected parameters are expected to be normalized. The authors also compared the results with the data of the control group. Written informed consent of included patients was obtained and the study was approved by the Ethics committee of the Jessenius Faculty of Medicine. Study complies with the Declaration of Helsinki. The authors do not have any conflict of interest.
Results: The authors observed shortening of CFT in EXTEM (p 0.0007 from the 26th WP vs. control group) and INTEM (p 0.002 from the 35th WP), increase in AA in EXTEM, INTEM, and HEPTEM, and the persistent increase in MCF in all three mentioned tests. According to the results, LMWH dose was modified in particular patients.
Conclusion(s): Even after the postpartum period, AA in EXTEM was steeper when comparing with the control group (p 0.0007). This may indicate that haemostasis is not completely normalized after 6–8 weeks after delivery. ROTEM seems to be a helpful tool for the individual assessment of the termination of secondary thromboprophylaxis. Acknowledgement: The authors thank the support of projects of the Agency for the Support of Research and Development APVV‐16‐0020 and Scientific Grant Agency 1/0549/19.
VPB1428
High prevalence of non RhD antibodies among antenatal women attending tertiary care Centre Hyderabad Pakistan
A. Sultana 1; I. Ujjan2
1 Lumhs Jamshoro Pakistan, Hyderabad, Sindh, Pakistan; 2 Lumhs, Hyderabad, Sindh, Pakistan
Background: The presence of red cell alloantibodies during pregnancy can result in severe obstetrical complications. Antibodies of the Rh and Kell systems had proven frequently reported to be associated with obstetrical complications. With the introduction of anti‐D prophylaxis, the incidence of RhD alloimmunization in pregnancy has been significantly reduced. Next to anti‐D, non‐D antibodies like anti‐K and anti‐c have been implicated in severe HDFN and are a serious concern for Obstetrition. This highlights the need for tertiary care hospitals to establish a well‐defined protocol for the management of non RhD alloimmunization in antenatal females.
Aims: To determine RBC Allo‐Immunization and specificities among antenatal women attending Tertiary care Centre Hyderabad Pakistan.
Methods: A total of 56 samples were studied and it was a non probability convenience sampling. Pregnant women of any age, parity and irrespective of the gestational age were included in the study and those having known autoimmune disease were excluded. The plasma/serum was used for antibody screening and identification using antihuman globulin gel cards (ID‐card LISS/Coombs). Data was analyzed by using SPSS version 21. p‐value less then < 0.05 was considered significant.
Results: The overall prevalence of alloantibodies in our study was 12.5% (6/56). The most prevalent significant alloantibodies were anti‐D and anti‐K respectively The non RhD antibodies specificity were included anti‐k (n = 2/33.33%), anti‐ E(n = 1/16.66%), anti‐c(n = 1/16.66%). There was significant association between RBC alloimmunisation and Rh phenotype, parity.
Conclusion(s): The occurrence alloimmunization in Rh negative was 33.33% whereas in Rh positive it was 66.66% which is greater than that reported in comparable studies. Rh D antibodies and Kell antibodies were common antibodies observed in pregnant women. However a significant fraction was non‐RhD alloimmunization among Rh (D) positive pregnant women.

VPB1417
Venous thromboprophylaxis in 108 hospitalized pregnant women with COVID‐19: Experience of a Public Hospital in Argentina from April 2020‐July 2021
B. Grand 1; M. Gonzalez Alcantara2; L. Voto2
1 Department of Maternal and Fetal Medicine. Hematology. Hospital Juan A Fernández, School of Medicine. University of Buenos Aires, CABA, Buenos Aires, Argentina; 2 Department of Maternal and Fetal Medicine. Hospital Juan A Fernández, School of Medicine. University of Buenos Aires, Buenos Aires, Buenos Aires, Argentina
Background: Coronavirus (COVID‐19) infection is a pandemic caused by SARS‐CoV‐2, appeared in Wuhan, in November 2019 and in March 2020 in Argentina. Initially it predisposes patients to thrombotic disease. Pregnancy is a hypecoagulable state and the optimal thromboprophylaxis in pregnancy is unknown. We work with the available information adapted to our hospital requirements.
Aims: To describe 1. Venous thromboprophylaxis in hospitalized pregnant women with COVID‐19 infection. 2. Maternal outcome.
Methods: 108 pregnant women were hospitalized between April 2020‐july 2021 (6 in the Intensive Care Unit (ICU), 4 required mechanical ventilation, 6 in Medical Clinic Unit and the rest in the Obstetrics Unit, patients were not vaccinated). Median age 29 (range 16–41). Trimester 1st 3.7%(4); 2nd 15.7%(17); 3rd 79.7%(86) (>37 weeks: 47); postpartum 0.9%(1). Treatment: We elaborated local guides and low molecular weight heparin (LMWH) enoxaparin 40 mg/d was prescribed to all pregnant women with COVID‐19 when hospitalized, 8 received intermediated doses and those more than 37 week received unfractionated heparin (UFH). Outpatient prolongation of thromboprophylaxis depends on risk stratification, those admitted to ICU continue with enoxaparin until delivery.
Results: 41 patients were discharged pregnant and 64 postpartum, 1 ectopic pregnancy and 2 abortions. There were neither maternal deaths nor venous thromboembolism (VTE) during the acute period of infection or during hospitalization. One women that was admitted to de ICU and require ARM with a BMI >40 and with LMWH developed a deep vein thrombosis 2 months later at 34 week of gestation and was anticoagulated with full doses of LMWH.
Conclusion(s): During the first period of COVID 19 infection in our country, hospitalized women received either LMWH enoxaparin at fixed doses of 40 mg/day or UFH if near delivery. No VTE or maternal death were observed during hospitalization. We suggest to continue VTE prophylaxis during the antenatal period in those patients admitted to ICU until delivery.
VPB1419
Markers of endothelial dysfunction as predictors of recurrent severe preeclampsia
M. Nikolaeva 1; A. Momot2; V. Terekhina3; A. Kudinov3
1 Department of Obstetrics and Gynaecology, Altai State Medical University, Barnaul, Russian Federation, Barnaul, Altaisky krai, Russia; 2 Altai Branch of FSBI «National Research Center for Hematology» Russian Ministry of Healthcare, Barnaul, Altaisky krai, Russia, 3 ФГБОУ ВО АГМУ МЗ РФ, Barnaul, Altaisky krai, Russia
Background: Women with a history of severe preeclampsia (PE) are at high risk of disease recurrence, which is realized in 10–25% of women. Taking into account the theory of pathogenesis of PE, the study of circulating biomarkers of endothelial dysfunction, such as endothelin‐1 (ET‐1) and endothelial microvesicles (EVs), looks promising.
Aims: To study the role of endothelial dysfunction markers in predicting the recurrence of early preeclampsia.
Methods: A prospective randomized observational study was conducted including 127 women with a history of severe early PE. The inclusion point is the preconception period (PC). The endpoint is delivery, the surrogate points – the level of ET‐1 (pmol/ml) and endothelial EVs at the PC period and at 11–12, 19–21 and 27–28 weeks of gestation. Before the end of the study, 30 patients were excluded from the study according to the inclusion/exclusion criteria. The remaining 97 patients were randomized into 2 groups according to the observed pregnancy outcomes: one with a beneficial gestation course and delivery at term (control group, n = 59) and another one with recurrent severe PE in the present pregnancy (main group, n = 38).
Results: The study at the PC period showed that the median (Me) of ET‐1 level was Me = 0.55 [95% CI:0.39–0.90] in the main group versus Me = 0.40 [95% CI:0.27–0.65] in the control group (p = 0.0382). The same data were obtained in the study of endothelial EVs, the level of which was significantly higher in women with realized PE, in relation to the control group: Me = 21.8 [95% CI:20.2–32.6] and Me = 14.5 [95% CI:13.4–17.3] (p = 0.0021), respectively. The ROC analysis showed that the level of endothelin > 0.514 (AUC‐0.609; 95% CI:0.559–0.745), as well as the level of endothelial EVs > 1.71 (AUC‐0.648; 95% CI:0.453–0.813) had a predictive ability of moderate strength.
Conclusion(s): The findings seem to be a background for the use of biomarkers of endothelial dysfunction as a prognostic predictor of recurrent PE starting from the PC period.
VPB1423
Easy clot characteristics of the placenta in a case of congenital hypofibrinogenemia
Y. Seki 1; T. Ushiki2; M. Masuko3; J. Takizawa3; H. Sone3; N. Okumura4
1 Niigata University Medical and Dental Hospital, Minami‐uonuma, Niigata, Japan; 2 Department of Blood Transfusion, Niigata University Medical and Dental Hospital, Niigata, Niigata, Japan; 3 Division of Hematology, Endocrinology and Metabolism, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Niigata, Japan; 4 Department of Health Sciences, Shinshu University School of Medicine, Matsumoto, Nagano, Japan
Background: We report a case of congenital hypofibrinogenemia (Fbg) in which three embryo transfers were performed in a case of artificial insemination failure, pregnancy was achieved, and the child was born with regular replacement therapy.
Aims: To investigate the thrombogenicity of the new gene mutation, we examined it in pathological specimens.
Methods: The patient was 38‐year‐old female. She was referred to us for embryo transfer after multiple failed artificial insemination attempts, and successfully conceived, delivered, and gave birth twice with replacement therapy with a dried human Fbg preparation as reported in this conference 2021. Genetic analysis of the DNA sequence of the PCR products in the Aα, Bβ, and γ chains revealed that the nucleotides in the γ chain exon8 were heterozygous for TGT (normal and wild type) and CGT (mutant type); Cys (cysteine; normal and wild type) was replaced by Arg (arginine; mutant type). Cys (cysteine; normal and wild type) was replaced by Arg (arginine; mutant), and no existing report was found [γp.C352R(Niigata II)]. After two deliveries, we examined the placental pathology in detail, and found that there were many thrombi in the placental blood vessels, which was clearly different from normal deliveries.
Results: In the Human fibrinogen database‐Groupe d'etude sur l'hemostase et la thrombose (geht.org), previously reported mutations and the presence or absence of thrombotic tendency are described in detail, but there are many mutations with unknown thrombotic tendency. There are many mutations with unknown thrombogenicity. The new γp.C352R (Niigata II) is a mutation that may cause easy thrombosis during replacement therapy with dried human Fbg.
Conclusion(s): In this case, placenta with Fbg >150mg/dL at implantation and >100mg/dL during pregnancy under regular supplementation of dried human fibrinogen showed a tendency to thrombus. Further study will be necessary to study the optimal target Fbg concentration during pregnancy and delivery in cases with similar mutations.
VPB1425
Platelets and neutrophils co‐drive procoagulant potential in antiphospholipid syndrome during pregnancy
E. Agbani1; E. Mahe2; S. Chaturvedi3; L. Yamaura4; P. Skeith4; M. Barber4; M. Choi4; A. Lee5; L. Skeith 4
1 University of Calgary, CALGARY, Alberta, Canada; 2 University of Calgary, University of Calgary, Alberta, Canada; 3 Johns Hopkins University, Baltimore, Maryland, United States; 4 University of Calgary, Calgary, Alberta, Canada; 5 University of British Columbia, Calgary, Alberta, Canada
Background: Antiphospholipid syndrome (APS) may occur as a primary condition or secondary to an underlying disease, most often Systemic Lupus Erythematosus (SLE). Platelets are activated in APS, but their pathogenic role, particularly in pregnancy‐associated thrombosis or placental complications, is unclear. Also, while it is known that platelet‐leucocyte aggregates (PLAs) are increased in APS, their role in pregnancy and SLE/APS is unknown.
Aims: To demonstrate the procoagulant potential of platelet and leukocytes in the prothrombotic state of SLE/APS.
Methods: We utilised a high‐resolution dynamic imaging approach to examine platelet and neutrophil membranes, and their interactions in platelet‐rich‐plasma plus from two pregnant patients with SLE and secondary APS (week 20–22) and gestational age‐matched healthy pregnant controls (PC). Both SLE/APS patients were maintained on therapeutic doses/regimen of low‐molecular‐weight heparin (LMWH) and acetylsalicylic acid (ASA) (162 mg/day), hydroxychloroquine and prednisone; and one received azathioprine and rituximab in addition. Platelet‐rich‐plasma plus (PRP+) was reconstituted to contain leucocytes by centrifuging whole blood at 180 g for 17 min, followed by careful extraction of the whole supernatant, which included the upper plasma fraction (PRP) and the buffy coat.
Results: Pregnancy in SLE patients with APS was associated with platelet and neutrophil activation that has functional procoagulant activity evidenced by surface thrombin generation, despite standard of care therapy with LMWH and ASA, and treatment of their underlying SLE with immunosuppression.
Conclusion(s): Further research will focus on identifying the mechanisms underlying platelet activation and platelet‐leukocyte interactions and their prothrombotic effects in SLE/APS.
PB1392
Ovarian vein thrombosis case series at King Fahad Medical City in Riyadh, Saudi Arabia
M. AlSheef 1; A. Abu‐shaheen1; A. Zia Zaidi2
1 King Fahad Medical City, Riyadh, Ar Riyad, Saudi Arabia; 2 King Fahad Medical City, Riydadh, Ar Riyad, Saudi Arabia
Background: Ovarian vein thrombosis (OVT) is uncommon, occurring with a variable frequency of 1/600 to 1/2000 pregnancies. It is associated with different conditions, including thrombophilia, malignancy, sepsis, intraabdominal and pelvic inflammatory conditions, pregnancy, postnatal period, and specific surgical interventions, particularly gynecological surgeries.
Aims: To identify the potential risk factors for ovarian venous thrombosis and elaborate on the standard treatment strategies in its management.
Methods: Our study consists of 18 patients diagnosed with ovarian vein thrombosis (OVT) between 2005 and 2016; the data were collected from the Health Information Management system at King Fahad Medical City (KFMC) in Riyadh, Saudi Arabia using a standard format. The diagnosis was made via one of the following clinical tools: Doppler ultrasonography, computed tomography (CT) scan, or magnetic resonance imaging (MRI).
Results: Our study found that OVT involved the right ovarian vein more often than the left and mainly occurs in women during their postnatal period. These patients' other risk factors included hypertension, diabetes, and a high body mass index (BMI). The most common presenting complaints were abdominal pain and fever. The most common treatment was the administration of enoxaparin (low molecular weight heparin) for an average duration of one to three months, which was associated with a low recurrence rate of OVT.
Conclusion(s): Physicians should be vigilant and have a low threshold for suspicion of OVT in female patients presenting with lower abdominal pain and fever in their postnatal period. Additionally, it is suggested to use low molecular weight heparin as initial therapy for OVT for one to three months, resulting in a high remission rate.
PB1393
Clinical timing of uterotonic medications, tranexamic acid (TXA), and blood transfusions in postpartum hemorrhage (PPH) management
G. Lee1; A. Serpas1; C. Bereuter 1; J. Chu2; H. Ahmadzia1
1 George Washington University School of Medicine and Health Sciences, Washington, District of Columbia, United States; 2 University of Maryland, Baltimore, Maryland, United States
Background: PPH is the cause of 11% of maternal deaths in the United States. Studies examining optimum timing of medications for hemorrhage management/prevention and period of highest risk after delivery are limited.
Aims: To describe the patterns of clinical timing for use of uterotonics (misoprostol, methergine, carboprost), TXA, and transfusions in the management of postpartum hemorrhage in the obstetric population.
Methods: Patients who delivered at the George Washington University Hospital from July 2019 to May 2021 with a quantitative blood loss (QBL) greater than or equal to 1000 mL were identified. Delivery time, QBL, methods of interventions were analyzed, including administration of uterotonics (misoprostol, methergine, carboprost), TXA, transfusions, and other surgical interventions.
Results: We included n = 463 PPH patients of which 102 (22%) received carboprost, 170 (37%) received methergine, 143 (31%) received misoprostol, 141 (30%) received TXA, and 111 (24%) received transfusions. 2 patients underwent a hysterectomy (<1%), and 7 (1%) patients required uterine artery embolization. 411 (89%) patients were given the PPH diagnosis within an hour of delivery with 170 (37%) patients sub‐typed as intraoperative/intrapartum PPH, defined as ≥1000 mL QBL within the immediate delivery period, and the remaining 241 (52%) sub‐typed as ≤1 h postpartum. The remaining 52 (11%) patients were diagnosed after 1 hr with the latest diagnosis at 22.3 h postpartum. Figure 2 demonstrates that there was no correlation between QBL and time to PPH diagnosis (r‐squared = 0.011).
Conclusion(s): The majority of PPH patients were diagnosed within 1 h postpartum, however, QBL was not correlated with the time elapsed to PPH diagnosis. Additional analyses plan to examine time to administration of the first agent, time to transfusion and any racial/ethnicity interactions and correlation of degree of pre‐existing anemia and PPH diagnosis.


PB1394
Evaluating the haemostatic changes in pregnancy via global coagulation assays: A series of 27 pregnant females in the first trimester
M. Cheong 1; W. Wong2; C. Tan2; H. Kaur1; H. Ng2; L. Lee1
1 Singapore General Hospital, Singapore, Not Applicable, Singapore; 2 Department of Haematology, Singapore General Hospital, Singapore, Not Applicable, Singapore
Background: Pregnancy is a hypercoagulable state and is associated with thrombotic complications. However, traditional screening coagulation assays are not sensitive to detecting such haemostatic changes. Global coagulation assays such as clot waveform analysis (CWA) and thrombin generation assays (TGA) may be able to detect changes in the coagulation status of pregnant patients. While TGA changes have been studied in the pregnant population, aPTT‐CWA has yet to be explored.
Aims: We aim to study the changes in aPTT‐CWA and TG in females who are pregnant in their first trimester compared with a normal control population.
Methods: Plasma samples were collected from patients during the first trimester of pregnancy, defined as before 12 weeks of gestation. Standard coagulation assays were performed. aPTT‐CWA parameters were generated using CS2100i coagulometer (Sysmex). TGA was performed using the Calibrated Automated Thrombogram (CAT). Results were compared to normal controls.
Results: 27 females in the first trimester of pregnancy were included in this study (Table 1). PT and aPTT parameters were within the normal reference ranges. D‐Dimer was elevated with a mean of 0.59 mg/L, and Protein S was on the lower limit of normal with a mean of 57%. Compared with controls, aPTT‐CWA parameters were significantly higher in pregnant patients, suggesting hypercoagulability (min1: 6.41%/s vs 4.69%/s, p < 0.001; min2: 1.01%/s2 vs 0.74%/s2, p < 0.001, max2: 0.85%/s2 vs 0.67%/s2, p < 0.001). This trend was also demonstrated in thrombin generation assay with CAT parameters of mean Estimated Thrombin Potential (ETP) and Peak Thrombin significantly higher (ETP: 2233.47 nM/min vs 1370.78 nM/min, p < 0.001, Peak Thrombin 388.05 nM vs 274.58 nM, p < 0.001) (Table 2).
Conclusion(s): Changes in haemostasis indicative of hypercoagulability happens early in pregnancy in the first trimester, and these changes are demonstrated via global coagulation assays. Such assays like the aPPT‐CWA may serve as potential assays to monitor coagulation status in pregnancy.


PB1396
Validation of clinical reference ranges for viscoelastometric assessment of haemostasis (TEG® 6S) and standard laboratory tests in obstetric patients
E. Crossland; D. White; J. Bamber; R. Tassell; M. Besser; E. Symington; M. Robinson; W. Thomas; S. MacDonald
Cambridge University Hospitals NHS Foundation Trust, Cambridge, England, United Kingdom
Background: To prevent excessive bleeding during delivery, pregnancy causes a hypercoagulable state by reducing natural anticoagulants and increasing procoagulant factors. Such changes are reflected in standard laboratory tests (SLTs). During emergent situations, SLTs often do not reflect the current haemostatic profile due to excessive turnaround time. The Thromboelastograph (TEG® 6S) is capable of assessing coagulation and fibrinolysis rapidly, within 20 min, favouring its use in urgent clinical situations including massive haemorrhage during delivery. Manufacturer, reference ranges are provided for all parameters.
Aims: Are reference ranges to represent haemostasis in late pregnancy, both for viscoelastometry and Standard Laboratory tests required and can they be applied to represent the haemostatic profile in a more clinically represntative manner?
Methods: This study was a prospective observational study involving the enrolment of 69 pregnant women near full term attending the elective C‐Section clinic at The Rosie Hospital, Cambridge, UK between 8th of February 2021 and 28th of April 2021. All participants were recruited, with full informed consent. The aim of this study was to confirm coagulation changes in late pregnancy and determine pregnancy specific reference ranges for viscoelastic and SLTs using CLSI EP28‐A3c.
Results: Although reference ranges of late pregnancy patients were within the reference ranges stated by the manufacturer, the distribution of results differed. All parameters demonstrated a hypercoagulable profile, close to the hypercoagulable extreme of the manufacturer ranges. This reduces the sensitivity of the manufacturer ranges for detecting deviations from baseline. Locally validated reference ranges improve sensitivity to detect risk of bleeding, reflected in abnormal viscoelastic parameters
Conclusion(s): The results observed confirmed the hypercoagulable state experienced during late pregnancy. These results provide additional evidence for the need for establishment of obstetric specific reference ranges on the TEG® 6s point of care analyser.


PB1413
Evaluation of the qLabs FIB system, a novel rapid and easy‐to‐use point‐of‐care system to quantify functional fibrinogen level using a single drop of citrated whole blood sample
S. Sanfilippo1; L. Buisson2; H. Rouabehi2; F. Depasse3; E. Peynaud‐Debayle4; B. Dumont4; T. Donnet 2
1 Diagnostica Stago R&D, Gennevilliers, France., GENNEVILLIERS, Ile‐de‐France, France; 2 Diagnostica Stago R&D, Gennevilliers, France., Gennevilliers, Ile‐de‐France, France; 3 Diagnostica Stago, Asnières Sur Seine, Ile‐de‐France, France; 4 Department of Biological Hematology and Transfusion, Louis‐Mourier Hospital, Colombes, France., Colombes, Ile‐de‐France, France
Background: Fibrinogen is an early predictor of obstetric postpartum hemorrhage (PPH) severity, and current guidelines recommend maintaining the plasma fibrinogen level above 2.0 g/L (Kozek‐Langenecker et al., 2016 and Shaylor et al., 2017). However, laboratory assessment of fibrinogen concentration from plasma, involves time‐consuming preanalytical steps that could impact the time to diagnosis and management of massive bleeding. The electrometer and the test strip are components of the qLabs FIB system (Micropoint Bioscience) (Figure). After 15 μL citrated blood drop deposition on the strip, the electrometer measures blood flow rate along microfluidic thrombin‐coated channels and displays fibrinogen plasma concentration taking into account the sample hematocrit measured simultaneously. This point‐of‐care device allows a functional fibrinogen measurement from 1.0 to 4.0 g/L of plasma in less than 10 min and is insensitive to platelet count (until 800 000/μL) as well as heparin therapy (until 2 UI/mL).
Aims: Evaluate analytical performances of the qLabs FIB system.
Methods: The qLabs FIB was evaluated through a comparative study with the STA®‐ Liquid Fib assay on STAR Max (Stago). A three‐laboratory comparison study was conducted to assess reproducibility and repeatability of the qLabs FIB using whole blood samples and plasma Quality Controls.
Results: Comparison analysis showed a robust correlation (Passing Bablok regression, R2>0,92) between the qLabs FIB and the reference method. The mean bias was ± 0.4 g/L for a fibrinogen level below 1.5 g/L, and inferior to 15% (95%, CI) (± 0.2 g/L) for a fibrinogen level within the [1,5–4.0 g/L] range. Between‐run/between‐site and within‐run reproducibility were satisfactory with CVs ≤10% and ≤8% respectively.
Conclusion(s): The qLabs FIB system enables a rapid and reliable measurement of fibrinogen levels from citrated whole blood. Further studies should investigate the performance of qLabs FIB device in critical care settings and confirm its ability to guide management of critical bleeding, especially for PPH.

PB1404
Impact of Tissue factor promoter gene polymorphism with Recurrent pregnancy loss in India
K. Kishor 1; A. Sharma1; S. Maharana2; C. Laha Roy2; R. Ranjan1; R. Kumar1; M. Mahapatra3; R. Saxena4
1 All India Institute of Medical Sciences, New Delhi, Delhi, India; 2 Blood and Vascular Biology Research Lab, Department of Life Sciences, School of Life Sciences, Central University of Tamil Nadu, Thiruvarur, Tamil Nadu, India; 3 All India Institute of Medical Sciences, Delhi, Delhi, India; 4 The Medicity Hospital Gurgaon, gurgaon, Haryana, India
Background: Recurrent pregnancy loss (RPL) is a common clinical anomaly resulting in repeated fetal loss and occurs with a frequency of 0.5–3% in all couples trying to conceive. It is complex, multifactorial disease, and its etiology remains unexplained in 40–50% of all RPL. Elevated level of tissue factor (TF) has been associated with DVT whereas, its association with Indian RPL patients is not yet studied.
Aims: To look for the association of TF with RPL in India and to elucidate the role of TF promoter gene polymorphism with TF plasma levels.
Methods: RPL patients with at least 3 consecutive pregnancy losses before 20 weeks of gestational age and equal number of healthy women with, at least one naturally conceived pregnancy were recruited for this study. Total 10 ml of whole blood was collected from each participant after obtaining proper informed consent. Plasma TF levels were determined by ELISA. TF promoter polymorphism 603A>G and prothrombotic mutations (Factor V Leiden and MTHFR 677C>T) were detected by PCR‐RFLP.
Results: 70 RPL patients, median age 33 years range (21–44 years) were recruited. Mean plasma TF levels were significantly higher in patients than controls (74.85 ± 12.30pg/ml and 69.95 ± 12.15 pg/ml, respectively p = 0.019). FV Leiden was significantly higher in patients 5 (7.1%) and absent in controls (p < 0.001). The distribution of MTHFR 677 C>T polymorphism was similar 12 (17.1%) in patients and 8 (11.4%) controls (p = 0.46). AG and GG genotypes of 603 A>G polymorphism was significantly higher (31.4%) in patient than (15.7%) controls (p = 0.046). Relation between the TF levels and their genotypes and relative risk of RPL are shown in table.
Conclusion(s): High TF level is significantly associated with Indian RPL patients. 603A>G polymorphism associated with high plasma TF level and 3.4‐fold increases the risk of RPL. Thus, TF 603 A>G polymorphism detection may help in identifying high risk of DVT.

PB1409
Evaluating the haemostatic effects of tranexamic acid in women with preeclampsia: An experimental ex vivo study
H. Okoye 1; M. Othman2; T. Nwagha3; D. Onwusulu4; R. Onoh5; C. Chigbu6
1 College of Medicine University of Nigeria Ituku Ozalla, Enugu, Enugu, Nigeria; 2 Queen's University, Kingston, Ontario, Canada; 3 College of Medicine University of Nigeria / University of Nigeria Teaching Hospital, Enugu, Enugu, Nigeria; 4 Faculty of Medicine, Nnamdi Azikiwe University, Awka, Anambra, Nigeria; 5 Alex Ekwueme Federal University Teachinh Hospital Abakaliki, Abakaliki, Ebonyi, Nigeria; 6 College of Medicine, University of Nigeria, Ituku Ozalla campus, Enugu, Enugu, Enugu, Nigeria
Background: Tranexamic acid (TXA) use has gone beyond common indications like post‐partum haemorrhage, and post‐surgical bleeding. However, concerns remain around a potential increased risk of peripartum venous thromboembolism, more so in preeclampsia.
Aims: To evaluate the haemostatic effects of TXA ex‐vivo in women with preeclampsia.
Methods: This was an ex‐vivo study involving 45 normal pregnant women and 45 women with preeclampsia (9 mild and 36 with severe features) matched for age, gestational age and body mass index. The blood samples were collected, with informed consent, at the time of delivery and divided into 2 parts. The 1st served as the pre ‐TXA sample while the 2nd part was spiked with TXA and served as post‐TXA sample. Plasma levels of D‐Dimer and plasmin–antiplasmin complex (PAP) were determined using ELISA kits from Elabscience® Biotechnology Inc Texas USA. Ethical approval was obtained from Research Ethics Committee of University of Nigeria Teaching Hospital, Enugu. Statistical analysis was done using SPSS version 21.
Results: The mean D‐dimer and PAP values in the pre‐TXA samples differ significantly between groups. Following spiking with TXA, the mean D‐dimer and PAP levels did not significantly differ in the pre‐TXA vs post‐TXA samples (p 0.560 and 0.500 respectively) in preeclampsia cohort. In normal pregnancy, the mean D‐dimer levels in the post‐TXA samples did not differ significantly (p = 0.071) from the pre‐TXA samples. However, the mean value of PAP in post‐TXA samples was significantly higher (p = 0.048) than that of pre‐TXA samples following TXA spiking.
Conclusion(s): Tranexamic acid did not have any significant effect on D‐dimer and PAP levels in preeclampsia suggesting that it may not increase the thrombotic risks in preeclampsia patients. However, further large‐scale studies are recommended to explore the in‐vivo effects of TXA and the mechanism behind the differential effects in pre‐eclampsia and normal pregnancy.
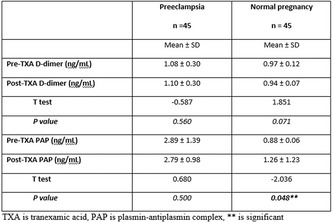

PB1410
The prenatal assessment of congenital bleeding disorder in carrier women with menorrhagia
K. Perveen 1; M. Borhany1; M. Abid1; T. Shamsi2
1 National Institute of Blood Diseases and Bone Marrow Transplantation, Karachi, Sindh, Pakistan; 2 National Institute of Blood Diseases and BMT, KARACHI, Sindh, Pakistan
Background: Heavy menstrual bleeding in the absence of any pelvic pathology raises the possibility of an acquired or inherited bleeding disorder. The prevention of these bleeding disorders is possible through prenatal diagnosis of fetal genetic disease.
Aims: The aim is to evaluate the early diagnosis and management of menorrhagia and to assess the prenatal status of congenital bleeding disorder in carrier mother.
Methods: This study was conducted at National Institute of Blood Diseases from 2019 to 2021. Medical history was collected by using patient’s performa and prenatal testing was performed through Chorionic villus sampling (CVS) for the detection of congenital bleeding disorder in the fetus. CVS samples were collected through an ultrasound guided procedure and gene sequencing was run on extracted genomic DNA for respective factor defect (SPSS‐version 23).
Results: A total of 98 females with frequent complaints of Menorrhagia were recruited in the study; out of which 42 (42.8%) were having one of the factor deficiency as FVIII; 8 (18.6%), FVII & GT 6 (14%) in each, and VWD 5 (11.6%), with median age 23 (IQR = 10) years. Consanguinity in 31 (73.8%) and positive family history was reported in 30 (71.4%) patients, history of complications includes; 20 (47.6%) anemia, 10 (23.8%) thrombocytopenia, 6 (14.2%), PCOs, 4 (9.5%) fibroid and 2 (4.7%) hemorrhagic ovarian cyst. Significant association between frequency of menorrhagia with congenital bleeding disorder were observed (p‐value = 0.034 as showed in Figure 1). Management with pack red cell, fresh frozen plasma, Novo‐seven and plasma concentrates were given to reduce frequent bleeding events in females. Apart from that 30 (71.4%) carrier mothers had pregnancies who were screened at 12 week for prenatal testing; 5 (16%) had affected fetus while 25 (83.3%) fetuses were either carrier or normal.
Conclusion(s): Prenatal testing through CVS is safe and effective way of prevention, early diagnosis and appropriate management may furnish better life style in future.

PB1411
Management of antithrombin deficiency in pregnancy – 2 cases report
F. Pires 1; A. Freixo2; C. Peixoto3; C. Câmara1; M. Galvão4; Á. Beleza4
1 CHULN, Santa Maria's Hospital – Portugal, Lisbon, Lisboa, Lisboa, Portugal; 2 Centro Hospitalar Lisboa Norte, Hospital de Santa Maria, Lisboa, Lisboa, Portugal; 3 Centro Hospitalar Universitário Lisboa Norte – Hospital Santa Maria, Lisbon, Portugal, Lisboa, Lisboa, Portugal; 4 Centro Hospitalar Universitário Lisboa Norte – Hospital Santa Maria, Lisboa, Lisboa, Portugal
Background: Inherited AntiThrombin Deficiency (ATD) is a rare thrombophilia with a high risk of thrombosis (1). As pregnancy is a maintained state of hipercoagulability, the presence of ATD significantly increases the risk of maternal venous thromboembolism (VTE) (2). Some serious gestational complications are associated with ATD (3). Despite prophylaxis with low molecular weight heparin (LMWH), pregnant women could have VTE and may need Antithrombin replacement therapy (ATRT) for anti‐Xa peak level > 0.5 IU/mL and throught level > 0.1IU/mL (4,5).
Aims: Presentation of two clinical cases of ATD management in pregnancy.
Methods: Data from clinical cases followed at Centro Hospitalar Lisboa Norte were collected using their medical records.
Results: Case 1 27 years old woman, 13 weeks gestation, with inherited ATD and a personal history of deep vein thrombosis (DVT) and pulmonary embolism (PE) at the age of 14 (2 weeks after starting the oral contraceptive pill), presented a new episode of DVT while on enoxaparin 40mg/day plus AAS. She was started on enoxaparin at therapeutic dose (60mg/bid) and 15 weeks later she had a suspected PE, started on 100 mg enoxaparin and was referred to our department. She started anti‐Xa and Antithrombin levels monitoring at peak. She needed higher doses enoxaparin and ATRT (graph 1), with no bleeding episodes. Case 2 21 week pregnant woman diagnosed with a DVT and PE, was admitted at her local hospital and started on therapeutic dose enoxaparin 80 mg/bid. 3 days after she entered the ER with a new DVT episode. A thrombophilia study was performed, which revealed ATD The patient continued therapy with LMWH and started ATRT. The patient is now 33 weeks pregnant on ATRT with no bleeding episodes.
Conclusion(s): ATD is a rare condition with a strong impact in pregnancy. An adequate individualized management, higher anticoagulation doses and ATRT are safe and efficient in preventing VTE.

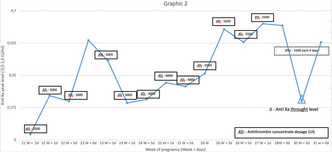
VPB1416
Postpartum thromboprophylaxis following caesarean delivery
M. Bastrash; C. Antinora; M. Plourde; M. Trahan; C. Simard; M. Koolian; E. Suarthana; K. wou; I. Malhamé
McGill University, Montreal, Quebec, Canada
Background: Caesarean delivery (CD) increases the risk of venous thromboembolism (VTE) and bleeding events. Despite best practice recommendations for postpartum thromboprophylaxis following CD, heterogeneity of practice remains.
Aims: In the absence of a standardized institutional prescription algorithm, among women with CD, we aimed (1) To describe the current practice for postpartum thromboprophylaxis, and (2) To compare the incidence of postpartum VTE and bleeding events between those with and without postpartum thromboprophylaxis.
Methods: We performed a retrospective cohort study. We reviewed medical records of a simple random sample of all CD between January and December 2020. We identified factors associated with postpartum thromboprophylaxis and assessed adherence to the Society for Obstetricians and Gynaecologists of Canada (SOGC) best practice guidelines. VTE, primary and secondary major bleeding (MB), and clinically relevant nonmajor bleeding (CRNMB) events were compared among women with and without thromboprophylaxis using a two‐way Chi‐square or Fisher’s exact tests, as appropriate.
Results: Of 322 included patients with CD, 81 (25.2%) received postpartum anticoagulation. Body‐mass index, pre‐existing diabetes, hypertensive disorders, fetal growth restriction, gestational age at delivery, preterm premature rupture of membranes, prolonged antepartum hospitalization, strict bedrest, unplanned CD, peripartum infection, prior anticoagulation use (including in the antepartum period), length of stay, and hospital readmission were associated with postpartum thromboprophylaxis. In total, 68/199 (34.2%) of patients requiring postpartum thromboprophylaxis as per SOGC guidelines received anticoagulation. Low‐molecular‐weight heparin was chosen in 80/81 (98.8%) of participants, with a first dose given at 12.0 (interquartile range [IQR] 8.0–14.0) h after delivery for a total of 2.0 (IQR 2.0–3) days. One patient developed a pulmonary embolism following hospital discharge. Participants receiving thromboprophylaxis had more primary MB and CNRMB events (Table 1).
Conclusion(s): Only one‐third of women qualifying for postpartum thromboprophylaxis received anticoagulation. Participants who received thromboprophylaxis were also at increased risk of primary bleeding events.

VPB1429
Association between a first‐trimester retrochorionic hematoma and plasminogen activity level
N. Tikhonova 1; N. Kuznetsova2; A. Milovanov1; E. Goufman1; T. Fokina1; M. Boltovskaya1; N. Nizyaeva1
1 A.P. Avtsyn Research Institute of Human Morphology, Moscow, Moskva, Russia; 2 the Rostov State Medical University, Rostov‐on‐Don, Rostov, Russia
Background: Retrochorionic hematomas are the most common cause of vaginal bleeding in the first trimester of pregnancy. Disturbances in the blood coagulation system could probably lead to the appearance of retrochorionic hematomas
Aims: To assess the association of a first‐trimester retrochorionic hematoma with plasminogen activity (%PLG) and level of IgG and IgM binding to plasminogen in system circulation.
Methods: The study included 73 consecutive consenting pregnant women (18–40 years old, 5–13 weeks of gestation):16 patients had retrochorionic hematoma, and 57 women were with uncomplicated pregnancy. Tests were performed in thrombocyte free plasma. We analyzed such characteristics as prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), fibrinogen (FB) and %PLG. The concentration of soluble fibrin‐monomer complexes (SFMCs) was determined by ortho‐phenanthroline test. The content of D‐dimer, plasminogen, levels of IgG and IgM binding plasminogen were revealed by ELISA. Statistical analysis was performed using Spearman's rank correlations(r), ROC analysis, the nonparametric Mann‐Whitney test with a critical value of p < 0.05. Data are presented as median with interquartile range (Me; Q1‐Q3).
Results: We revealed a weak positive correlation (r = 0.29) in healthy women between aPTT (30.6s; 29.1–33.3) and IgM‐Plg (89.4 u/ml; 67.2–119.6), but patients with hematomas had a stronger correlation (r = 0.51) between aPTT (32.8s; 30.6–36.0) and IgM‐Plg (100.6u/ml; 76.5–140.3). Patients with hematomas was a positive correlation (r = 0.55) between TT (19.0s; 18.1–20.8) and IgM‐Plg. In the control group showed a weak negative correlation (r = −0.27) between gestational age and PT (11.5 s; 11.2–12.0). There was a weak positive correlation with FB (3.3 g/L;2.8–3.9) (r = 0.33) and %PLG (116.5%;91.8–143.4) (r = 0.38). Patients with hematomas had higher PT (12.5 s; 12.1–12.9) (p = 0.0006), SFMCs (4.0 mg/L; 3.0–5.3) and lower %PLG (82.5%; 66.7–91.0) (p = 0.0007), than healthy women SFMCs (3.0 mg/L; 3.0–4.0) (p = 0.004). ROC analysis was applied to estimate the sensitivity (SN) and specificity (SP) of %PLG to differ women with retrochorionic hematoma from healthy pregnant women (SN = 100%; Sp = 68.4%, AUC = 0.85). The ratio of %PLG/IgM‐Plg had a high significance (SN = 75%; Sp = 88%; AUC = 0.82).
Conclusion(s): Plasminogen activity and %PLG/IgM‐Plg ratio could be used as potential markers to revealed hematoma, what is important, especially in the early pregnancy.

PB1395
Pregnancy as a protective factor for bleeding in immune thrombocytopenia regardless of platelet count
F. Chuliber 1; A. Muñoz2; L. Llera2; V. Privitera3; D. Mezzarobba2; M. Villagra Iturre2; E. Viñuales2; J. Arbelbide2; D. Penchasky2
1 Hospital ItaIiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Hospital Italiano de Buenos Aires, Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital Italiano de Buenos Aires, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina
Background: Immune thrombocytopenia (ITP) has distinct features in pregnancy, accounting for 1–2/1000 of them.
Aims: To compare the proportion of bleeding between pregnant and non‐pregnant ITP patients and describe the clinical characteristics and treatment in pregnant ITP patients.
Methods: We retrospectively included a cohort of ITP pregnant women who attended a university hospital between 2007–2021. Patients with a diagnosis of ITP in pregnancy or with a relapse in pregnancy were considered. A comparison was performed with a group of consecutive IT non‐pregnant women (1:1) of the same age range. A multiple logistic regression model was constructed to evaluate the pregnancy/bleeding relationship.
Results: Thirty‐four ITP pregnant patients (38 pregnancies) were analyzed and, 38 non‐pregnant women with ITP were as a comparator group. The proportion of patients who bled was significantly lower in the pregnant group. A small proportion presented major bleeding in both groups (Table 1). Pregnant patients had higher platelet counts (at diagnosis and nadir). Fewer pregnant women required treatment for ITP, but the rate of complete response (>100 × 109/L) was higher in non‐pregnant women (table 1). In the bivariate analysis, a platelet count (nadir) lower than 30 x109/L was associated with a higher risk of bleeding [ORc 18.56 (95% CI 5.36‐ 64.18), p < 0.0001]. In contrast, pregnancy was a protective factor of bleeding [ORc 0.03 (95% CI 0.007–0.12), p < 0,001]. In the multivariate analysis, pregnancy persisted as a protective factor regardless of platelet count [ORa 0.04 (95% CI 0.01–0.20), p < 0.0001]. In the pregnant group, 47% had a history of ITP. In 21 pregnancies, it was necessary to initiate treatment for ITP. The main indication was a low platelet count with a high risk of bleeding. Corticosteroids were the most common therapy. Thirty‐eight percent of the therapies were indicated in preparation for delivery (Table 2).
Conclusion(s): We observed that ITP pregnant patients had significantly lower hemorrhagic manifestations than non‐pregnant patients regardless of platelet count.
PB1405
Features of changes in platelet aggregation activity in pregnant women with preeclampsia
B. Kurbanov
Tashkent Pediatric Medical Institute, Tashkent, Toshkent, Uzbekistan
Background: The most important indicators of the plasma and platelet components of the hemostasis system in pregnant women with preeclampsia are of great importance in assessing the development of the disease severity.
Aims: to determine the character of changes in platelet aggregation activity in pregnant women with preeclampsia.
Methods: We studied the platelet aggregation activity in 112 pregnant women with preeclampsia and in 50 women with a physiological course of pregnancy in the third trimester. An assessment was made of the total aggregation activity of platelets according to the types of aggregation curves.
Results: The increase in the intensity of aggregation in pregnant women with preeclampsia with ADP stimulation of 1·10‐3 m was 16.1% and 7.9%, respectively; ADP 1·10‐5 m – 15.1% and 10.6%; ADP 1·10‐7 m – 62.3% and 62.2%; adrenaline – 27.4 and 30.3% (p < 0.01). The intensity of secondary platelet aggregation during stimulation with ADP 1·10‐5 m and adrenaline was also significantly higher than in conditionally healthy women (p < 0.001). At the same time, the slope of the aggregation curve at the stage of secondary aggregation exceeded that in healthy pregnant women (by 5.8%, respectively) (p > 0.05). These changes were combined with an increase in the adhesiveness index by 11% in pregnant women. When evaluating the aggregation curves in healthe pregnants, a slight decrease in the frequency of two‐phase curves of platelet aggregation was revealed due to an increase in irreversible aggregation during stimulation with ADP 1·10‐5 m and adrenaline. An increase in platelet aggregation activity, along with hypoaggregation, took place during stimulation with ADP 1·10‐7 m, while the number of two‐phase irreversible aggregation increased.
Conclusion(s): To sum up, pregnant women with preeclampsia have signs of thrombocytopathy and coagulopathy of in combination with a changes in platelet aggregation activity of the blood.

VPB1426
How can changes in secondary haemostasis be useful for the management of pregnancy?
L. Stanciakova 1; M. Dobrotova2; P. Holly3; J. Zolkova2; L. Vadelova4; I. Skornova2; J. Ivankova3; M. Brunclikova2; M. Samos5; T. Bolek5; M. Grendar6; P. Kubisz2; J. Stasko2
1 National Centre of Haemostasis and Thrombosis, Department of Haematology and Transfusiology, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Martin University Hospital, Martin, Slovak Republic, Martin, Zilina, Slovakia; 2 National Centre of Hemostasis and Thrombosis, Department of Hematology and Transfusiology, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin and University Hospital in Martin, Slovakia, Martin, Zilina, Slovakia; 3 Comenius University, The Jessenius Faculty of Medicine in Martin, Martin, Zilina, Slovakia; 4 Centre of Immunology in Martin, s.r.o., Martin, Slovak Republic, Martin, Zilina, Slovakia; 5 Department of Internal Medicine I, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Martin University Hospital, Martin, Slovak Republic, Martin, Zilina, Slovakia; 6 Biomedical Center Martin, Comenius University in Bratislava, Jessenius Faculty of Medicine in Martin, Slovakia, Martin, Zilina, Slovakia
Background: In the course of physiological pregnancy the haemostatic balance changes towards hypercoagulability. Low‐molecular‐weight heparin (LMWH) is recommended for secondary thromboprophylaxis in pregnant women with previous venous thromboembolism (VTE). However, there are only several studies focused on the effect of LMWH on haemostatic parameters during pregnancy of patients with prior VTE and thromboprophylaxis.
Aims: The authors present results of prospective and longitudinal evaluation of changes in haemostasis in high‐risk pregnant women.
Methods: Blood samplings were performed during pregnancy (T1‐T4) and once after the postpartum period (T5). Results of coagulation factor VIII (FVIII) activity, protein S (PS) function, ProC Global ratio and anti‐Xa activity were assessed and compared with the control group. Written informed consent of included patients was obtained and the study was approved by the Ethics committee of the Jessenius Faculty of Medicine. Study complies with the Declaration of Helsinki. The authors do not have any conflict of interest.
Results: Despite secondary thromboprophylaxis, there was a significant increase in FVIII activity between T1 and T5 (p 0.0003), T2 and T4 (p 0.0144), T3 and T5 (p 0.0007) and between T4 and T5 (p < 0.0001). We observed decrease in PS function (T2‐T5 vs. the controls, p value < 0.0001) and ProC Global ratio did not normalize after the postpartum period – p < 0.0001 between control group and T5 for both PS and ProC Global).
Conclusion(s): Regular testing of LMWH effectiveness in terms of routine monitoring of anti‐Xa activity to tailor the dose of LMWH is not suggested. Our results show that haemostasis may not be restored even 6–8 weeks following delivery. Therefore, it is questionable when is it safe to withdraw secondary thromboprophylaxis in patients with previous VTE. Acknowledgement: The authors thank the support of projects of the Agency for the Support of Research and Development APVV‐16‐0020 and Scientific Grant Agency (Vega) 1/0549/19.
VPB1430
Evaluation of hemostasis disorders and the level of neutrophil extracellular traps in pregnant women with thrombophilia
I. Vasilenko 1; S. Gasparyan2; I. Orfanova2; A. Fomicheva1; V. Metelin3
1 M.F. Vladimirsky Moscow Regional Clinical and Research Institute (MONIKI), A.N. Kosygin Russian State University, Moscow, Moskva, Russia; 2 Stavropol State Medical University, Stavropol, Stavropol', Russia; 3 MONIKI, RSU named A.N. Kosygin, Moscow, Moskva, Russia
Background: Three main factors have been found to play the leading role in the occurrence of thromboembolic complications in pregnant women: genetic thrombophilia, hyperhomocysteinemia and antiphospholipid syndrome. However, the detailed mechanisms responsible for adverse pregnancy outcomes in the presence of thrombophilia remain incompletely understood.
Aims: The purpose of this study was to identify the relationship between hemostasis disorders and trapped DNA levels in pregnant women with a history of obstetric complications.
Methods: 337 women with thrombophilia and placental insufficiency (age 19 to 36 years, median age 29 years) in the second trimester of pregnancy were examined. A control group consisted of 34 nonpregnant women aged 21 to 35 years (median age 28 years). Hemostasis studies were performed using routine tests and the laboratory diagnostic system "Thrombodynamics Registrator T‐2" ("HemaCore", Russia). The level of neutrophil extracellular traps (NETs) in whole blood smears was counted using the automatic microscopy system.
Results: Analysis of platelet count, TV, ACTV, PV, AT III did not reveal statistically significant differences in the comparison groups. Fibrinogen levels in pregnant women were 1.3 times higher than in the control group (p < 0.05). The baseline clot growth rate, clot size, and clot density were found to be statistically significantly higher in the examined pregnant women with thrombophilia than in the control group (p < 0.05). Clot growth rate V (μm/min) and fibrin clot size at 30 min of follow‐up (CS, μm) were the most informative. Non‐pregnant women's %NETs averaged 5.7 ± 2.7% (1.8–11.9%), while in complicated pregnancy %NETs was statistically significantly (p < 0.0001) increased at 12.6 ± 4.1% (2.9–18.6%).
Conclusion(s): The findings expand our knowledge of the relationship between the immune system and hemostasis, whose disorders are associated with the risk of thromboembolic complications in pregnancy, which may contribute to the search for more effective personalized pregnancy management protocols, prediction of thrombosis threat and adequate and justified pregravid prevention.
PB1398
Loss of opportunities in the diagnosis and treatment of Primary obstetric antiphospholipid syndrome (POAPS). What is hidden behind the daily clinical practice?
G. de Larrañaga 1; S. Udry2; S. Perés3; F. Aranda3; S. Morales Perez4; C. Belizna5; E. Esteve‐Valverde6; D. Fernández Romero7; J. Alijotas‐Reig8; O. Latino7
1 Hospital Of Infectious Diseases "Dr. Francisco J. Muñiz", Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 2 Acute Hospital "Dr. Carlos G. Durand and Research center “Fundación Respirar”, Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 3 Hospital of Infectious Diseases "Dr. Francisco J. Muñiz", Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 4 Internal Medicine Althaia Healthcare Network of Manresa, Barcelona, Catalonia, Spain; 5 University Hospital Angers, Angers, Pays de la Loire, France; 6 Fundación Althaia de Manresa, Barcelona, Cantabria, Spain; 7 Acute Hospital "Dr. Carlos G. Durand", Ciudad Autónoma de Buenos Aires, Ciudad Autonoma de Buenos Aires, Argentina; 8 Valld'Hebron University Hospital, Barcelona, Cantabria, Spain
Background: Patients who meet the clinical criteria for POAPS sometimes are not adequately studied, due to failure on the request of serological studies to make the proper diagnosis. This leads to underdiagnosis of this pathology, exposing patients to new thrombotic and/or obstetric events that would be preventable with a timely diagnosis and a proper treatment.
Aims: To identify and quantify the loss of opportunities, in terms of time, in the diagnosis and treatment of POAPS.
Methods: Ninety‐nine POAPS patients [median age: 32 years; (28–37)], strictly according to the international criteria, were studied. Preventable clinical events associated with POAPS during a GAP time were identified through a retrospective analysis. The GAP time was defined as the time between the date when the patient meets the clinical criteria and the date when the diagnosis was finally made. The number of preventable obstetric/thrombotic events and the GAP time (years) were recorded. Patients were grouped and compared whether they were attended at a Specialized or General Health Center.
Results: During the GAP time 134 pregnancies (56 live births and 78 pregnancy losses) and 87 preventable events were registered (46 miscarriages, 32 fetal losses and 9 premature deliveries before the 34th weeks due to preeclampsia or placental insufficiency and 1 thrombosis). The mean GAP time (± SD) was 4.27 years ± 3.58 (range: 0–21 years). A number of 20.37 preventable events per year /100 patients was estimated. Remarkably, all patients that presented with preventable events were not attended at specialized health centers.
Conclusion(s): The underdiagnosis of POAPS leads to the occurrence of preventable obstetric events, a loss of time resulting in a loss of lives. We consider that it mainly because of a lack of knowledge on the diagnostic criteria for POAPS from the medical staff and the lack of diagnostic support in General health centers.


PB1402
Management of inherited antithrombin deficiency in pregnancy: The western Australian experience
M. Alexander1; D. Pepperell1; A. Keegan 2
1 Fiona Stanley Hospital, Perth, Western Australia, Australia; 2 King Edward Memorial Hospital, Perth, Western Australia, Australia
Background: Inherited antithrombin deficiency is a rare, high‐risk thrombophilia associated with obstetric and neonatal morbidity and mortality. The use of low molecular weight heparin (LMWH) and antithrombin concentrate (AT3) appears to improve maternal and fetal outcomes; however, there is a lack evidence‐based recommendations to guide clinicians in the management of these complex pregnancies.
Aims: To provide a descriptive analysis of the management of all pregnancies (>20 weeks gestation) in women with inherited antithrombin deficiency across Western Australia from January 2015 – January 2022.
Methods: We undertook a multicentre, retrospective cohort analysis based on predefined maternal characteristics, LMWH dosing (weight‐based vs anti‐Xa guided), AT3 use and maternal and neonatal outcomes.
Results: Seven women with inherited antithrombin deficiency delivered eight live infants. The average maternal age was 33 years and 50% of pregnancies were conceived on LMWH. LMWH was initially weight‐based (1 mg/kg BD) then up‐titrated to achieve an anti‐Xa level between 0.6–1.0 IU/mL. The average dose of LMWH was 1.39 mg/kg BD, however wide variation in LMWH dosing was noted (1.03–1.90 mg/kg BD). Two women required non‐elective caesarean sections for obstetric indications, but all deliveries received AT3 at induction followed by an additional dose 24 h later. There was marked variation in dosing (amount and duration) of AT3 based on clinicians’ prescribing preferences and clinical need. One woman had a large volume postpartum haemorrhage, two women developed wound haematomas and one woman developed a sagittal sinus thrombosis on day 19 postpartum. Four pregnancies were affected by significant placental insufficiency including severe preeclampsia and intrauterine growth restriction. Two neonates required admission to Neonatal Intensive Care.
Conclusion(s): The Western Australian experience of inherited antithrombin deficiency in pregnancy mirrors international literature, with significant maternal and fetal morbidity despite variable use of LMWH and AT3. There is a need for development of evidence‐based guidelines to improve outcomes in these high‐risk pregnancies.
PB1412
Inherited thrombophilia and non‐O blood type associated with recurrent spontaneous abortion
L. Spiezia1; A. Poretto 2; E. Borella3; G. Turatti3; M. Marobin3; C. Simion3; E. Campello4; D. Tormene5; P. Simioni6
1 Padova University Hospital, Padova, Italy, Padova, Veneto, Italy; 2 Padova university hospital, SALGAREDA, Veneto, Italy; 3 Padova University Hospital, Padova, Veneto, Italy; 4 University of Padova, Padova, Veneto, Italy; 5 Padova University Hospital, Padova, Veneto, Italy; 6 Padua University Hospital, Padua, Veneto, Italy
Background: Several studies evaluated the association between inherited thrombophilias (IT) and recurrent spontaneous abortion (RSA) without reaching definitive conclusions.
Aims: We assessed the prevalence of IT (antithrombin (AT), protein C (PC) and protein S (PS) deficiencies; factor V G1691A mutation (FV Leiden), prothrombin G20210A mutation and non‐O blood type in a group of women with a history of RSA.
Methods: N. 255 unselected women with a history of RSA (i.e. ≥ 3 consecutively) were considered for enrollment between December 2011 and December 2019. A group of age‐matched women, with at least one normal pregnancy and no history of adverse pregnancy outcomes acted as controls. All participants underwent screenings for IT and ABO genotyping.
Results: N. 223 (mean age 37 yrs, range 25–42 yrs) women were enrolled. The prevalence of obesity and non‐O blood was significantly higher in cases than controls (p 0.01 and <0.05, respectively). The odds ratio (OR) of RSA in women with vs. without IT was 2.10 (95% CI, 1.26–3.50). The OR of RSA in women with non‐O vs. O blood type was 1.46 (95% CI, 1.01–2.13). We found concomitant IT and non‐O in n. 29 (13%) cases and n. 16 (7%) controls, OR 2.62 (95% CI, 1.32–5.17).
Conclusion(s): IT and non‐O blood type appear to be individually associated with a significantly higher risk of RSA, which is further increased in subjects carrying both. Larger studies are warranted to validate our findings.
PB1414
COVID‐19‐associated hemostasis alterations in pregnancy: a prospective, case‐control study
Z. Bagoly1; E. Tóth 2; R. Orbán‐Kálmándi3; T. Deli4; O. Török4; L. Lóczi5; J. Tóth6; S. Molnár4; Z. Krasznai4
1 University of Debrecen, Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary; 2 University of Debrecen, Faculty of Medicine, Department of Gynecology and Obstetrics, Debrecen, Hajdu‐Bihar, Hungary; 3 University of Debrecen; Faculty of Medicine, Department of Laboratory Medicine, Division of Clinical Laboratory Sciences, Debrecen, Hajdu‐Bihar, Hungary; 4 University of Debrecen; Faculty of Medicine, Department of Gynecology and Obstetrics, Debrecen, Hajdu‐Bihar, Hungary; 5 University of Debrecen; Faculty of Medicine, Debrecen, Hajdu‐Bihar, Hungary; 6 University of Debrecen; Faculty of Medicine, Department of Laboratory Medicine, Debrecen, Hajdu‐Bihar, Hungary
Background: Coronavirus disease‐19 (COVID‐19) is associated with disturbed hemostasis balance. Little is known about COVID‐19‐associated hemostasis alterations in pregnancy and their associations with the clinical course.
Aims: We aimed to test hemostasis alterations in COVID‐19‐positive pregnant women as compared to non‐infected pregnancies and to correlate results with maternal and perinatal outcomes.
Methods: In this single‐center observational case‐control study, 80 women with acute COVID‐19 infection at 24–40 gestational weeks (COVID‐19+ group) and 80 healthy age‐ and gestational week‐matched pregnant women (COVID‐19‐ group) were enrolled. All women were outpatients with mild/no symptoms at admission. Acute infection was confirmed/ruled out using SARS‐CoV‐2 RT‐PCR and/or antigen test. Blood taken on admission was analyzed for markers of inflammation (CRP, ferritin, IL‐6), D‐dimer, fibrinogen, von Willebrand factor antigen, factor VIII (FVIII) and factor XIII (FXIII) activity, clot‐lysis assay, thrombin generation, ACE1, ACE2 activity, and anti‐SARS‐CoV‐2 antibody levels. Detailed clinical parameters of pregnancy, labor and post‐partum period were registered. Pregnancies were followed for 6 weeks after childbirth.
Results: In the COVID‐19+ group, APTT was significantly prolonged, while PT, fibrinogen, and D‐dimer levels did not significantly differ from the non‐infected group. FVIII activity was significantly lower in the COVID‐19+ group as compared to COVID‐19‐ group (183.7 ± 55.9% vs. 201.7 ± 49.2%, p = 0.03). Similarly, FXIII activity was significantly reduced in COVID‐19+ pregnant women (80.6 ± 23.5% vs. COVID‐19‐ group: 91.6 ± 22.5%, p = 0.04). Pregnancy‐associated complications were observed in 5 COVID‐19+ cases, with marked alterations of coagulation screening tests, clot‐lysis assay, and increased D‐dimer. Perinatal complications were observed in 6 cases and one newborn tested positive for SARS‐CoV‐2. Two cases of severe post‐partum hemorrhage were observed in the COVID‐19+ group. No post‐partum thrombotic events occurred.
Conclusion(s): In this cohort, third‐trimester COVID‐19+ pregnancies were associated with reduced levels of FVIII and FXIII activity. In a few cases, marked alterations of hemostasis and fibrinolysis balance occurred, which were accompanied by pregnancy complications.
PB1406
A tertiary centre experience of late amniocentesis in obstetric management of women with inherited bleeding disorders
B. Madan 1; J. Cutler2; G. Gray3; S. Sankaran4; M. Mitchell5
1 Guy's & St. Thomas' NHS Foundation Trust, London, England, United Kingdom; 2 Viapath Analytics LLP, LONDON, England, United Kingdom; 3 Guys and St Thomas' Hospitals NHS Foundation Trust, London, England, United Kingdom; 4 Guys' and St Thomas' NHS Foundation Trust, London, England, United Kingdom; 5 Viapath Analytics LLP, London, England, United Kingdom
Background: The significant risk of birth‐trauma to affected foetuses of women with inherited bleeding disorders (IBD) is well‐recognised. In absence of the knowledge whether the foetus is affected or not, a restrictive approach of avoiding standard obstetric‐interventions that increase foetal bleeding‐risk is adopted. Consequently, women with unaffected foetuses may be unnecessarily subjected to restrictive birth‐plans. Knowledge of foetal mutation status is key in avoiding this. Pre‐natal diagnosis is not always acceptable because of associated risk of miscarriage. Ultrasound sexing can reduce the risk by 50% in haemophilia carriers but pregnancies with unaffected male foetus are unnecessarily subject to restrictive plans. It cannot be used in autosomal recessive conditions.
Aims: To present the experience of late amniocentesis at > 34 weeks gestation at our Tertiary Obstetric Bleeding Disorders Clinic.
Methods: Late amniocentesis was offered to women carrying a potentially affected foetus. Foetal diagnosis was confirmed by presence or absence of familial mutation. 17 late amniocenteses were performed in women with IBD who had an identifiable mutation: 15 in haemophilia A carriers, and 2 in Type 2 vWD. 9 women had a single pregnancy, while 4 women had 2 pregnancies.
Results: The result informed birth‐plans in 15/17 (88%) cases. The foetus was found to be affected in 4/17 cases (23.5%). A total of 11/17 foetuses (64.7%) were found to be unaffected, facilitating normal management of delivery in local‐hospitals. The result could not be obtained in 2 cases due to poor DNA quality. In one case the amniocentesis was technically challenging due to foetal‐position and was re‐attempted successfully at a later date.
Conclusion(s): Late amniocentesis is a valuable technique in obstetric management of selected women with IBD. It has a significantly positive impact in clinical decision‐making and in guiding management of labour and delivery in women who decline early prenatal diagnosis or in those who present late.
PB1397
Psychological and cognitive factors involved in decision‐making process of haemophilia carriers in reproductive choices
I. Cutica 1; M. Mortarino2; I. Garagiola3; G. Pravettoni4; F. Peyvandi5
1 University of Milan, Italy, Milan, Lombardia, Italy; 2 Fondazione IRCCS Ca’Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy, Milan, Lombardia, Italy; 3 Angelo Bianchi Bonomi Haemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, and Luigi Villa Foundation, Milan, Italy, Milan, Lombardia, Italy; 4 Department of Oncology and Hemato‐Oncology, University of Milan, and Director of Applied Research Unit for Cognitive and Psychological Science, European Institute of Oncology, Milan, Italy, Milano, Lombardia, Italy; 5 Fondazione IRCCS Ca’ Granda – Ospedale Maggiore Policlinico, UOC Medicina Generale, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Lombardia, Italy
Background: Carriers of haemophilia (HCs), as an X‐linked recessive trait, face a multitude of challenging reproductive decisions linked to the possibility of having an affected child, such as opting for pre‐implantation genetic testing (PGT), or choosing prenatal diagnosis (DPN) and then whether to continue or interrupt pregnancy in case of affected male fetus. Notwithstanding, the emotional and cognitive aspects of such decision‐making processes were not explored in literature yet.
Aims: The aim of this study was to investigate whether cognitive and emotive factors, such as decision style1, risk perception 2, coping strategies used in response to stress3 and emotions about haemophilia, might modulate HCs’ reproductive decisions.
Methods: Forty HCs were interviewed through questionnaires comprising standardized scales for psychological variables and ad‐hoc open questions.
Results: Descriptive statistics about sociodemographic data, hemophilia‐linked variables and pregnancy‐related variables are reported in Table 1. Results for psychological tests are reported in Table 2. A series of ANOVAs show that HCs who have a rational decision‐making style tend to chose DPN or PGT, whereas those with an avoidant decision‐making style tend to choose routine pregnancy analysis (RPA) (F = 6.7, p = 0.002). Moreover, the score to COPE is lower in women who chose DPN or PGT with respect to those who chose RPA (F = 4.94, p = 0.025). Although familial haemophilia does not impact, per se, on pregnancy decisions, women who chose DPN or PGT have deep negative emotions linked to haemophilia family history more frequently than those who chose RPA (F = 13.42, p = 0.001). As concerning pregnancy outcomes, the low number of haemophilic male foetuses (who might require a decision) do not allow to perform statistical analyses.
Conclusion(s): HCs who have a rational decision‐making style, a low ability to cope with stressors, and who experienced discomfort, in childhood, for familial haemophilia, tend to choose PGT or DPN over RPA.


PB1408
Pregnancy‐induced change in platelet transcription, metabolism, and aggregation: A longitudinal study from pre‐pregnancy to postpartum
E. Montenont 1; H. Campbell1; J. Rowley2; N. Tolley1; A. Blair1; C. Stubben1; J. Cox1; R. Souvenir3; D. Abel4; A. Weyrich5; M. Rondina1; R. Campbell1
1 University of Utah, Salt Lake City, Utah, United States; 2 University Of Utah, Salt Lake City, Utah, United States; 3 University of Iowa, Iowa City, Iowa, United States; 4 UCLA, Los Angeles, California, United States; 5 Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, United States
Background: Pregnancy is associated with increased risk for venous thromboembolism. Although previous studies have characterized how pregnancy alters the hemostatic and fibrinolytic system, the effect of pregnancy on platelet function remains relatively unknown.
Aims: We examined platelet activation and aggregation, the transcriptome and metabolome longitudinally beginning prior to pregnancy and continuing through the postpartum period.
Methods: We collected blood from 20 healthy women before pregnancy, during each trimester of pregnancy and 2–6 weeks postpartum. At each visit, we isolated leukocyte‐depleted platelets for RNA‐sequencing and metabolic studies. Platelet activation basally and in response to agonists was measured by flow cytometry. Washed platelet aggregation was also performed.
Results: The platelet transcriptome and metabolome was significantly altered during pregnancy with the most significant changes occurring in the 3rd trimester. Pathway analysis between pre‐pregnancy and the 3rd trimester revealed significant changes to mitochondrial pathways, integrin signaling, and estrogen receptor signaling. In addition, we observed significant changes to platelet‐specific genes including FCGR2A, ITGA2B, GP6, and genes regulating procoagulant platelet formation (p < 0.05). Basal platelet activation and in response to agonists did not change significantly over the course of pregnancy or postpartum. In contrast, washed platelet aggregation declined in response to ADP (p < 0.006) and collagen (p < 0.0001) over the course of pregnancy with the lowest response postpartum. Thrombin‐induced platelet aggregation significantly increased compared to pre‐pregnancy levels during the 2nd (p = 0.03) and 3rd (p = 0.01) trimester, again with marked decline postpartum (p = 0.006 compared to 3rd trimester). Decreased platelet aggregation in the 3rd trimester correlated with increased oxidized glutathione levels and with genes involved with glutathione metabolism, which are known to reduce collagen‐ and ADP‐induced aggregation (r = −0.508, p = 0.06).
Conclusion(s): Pregnancy is associated with unique changes in platelet transcription and metabolism. Although not associated with traditional markers of platelet activation, these changes were associated with reduced collagen‐ and ADP‐induced aggregation and increased thrombin‐induced aggregation.
PB1415
Use of recombinant von Willebrand factor concentrate for peri‐partum haemostatic management of women with type 2 VWD; a case series
O. Turan 1; M. Musarara2; R. Abdul‐Kadir3
1 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free London NHS Foundation Trust, London, England, United Kingdom; 2 Katharine Dormandy Haemophilia and Thrombosis Centre, Royal Free Hospital NHS Trust London, London, England, United Kingdom; 3 Royal Free London NHS Foundation Trust, London, England, United Kingdom
Background: Recombinant VWF concentrate (rVWF) ensures adequate replacement of VWF activity without accumulation of FVIII.
Aims: We describe a case series of three women who were treated with recombinant VWF.
Methods: Pharmacokinetic studies were performed at 32–34 weeks gestation. VWF:RiCo, VWF:Ag, FVIII were assessed pretreatment, 30 min, 3, 6, and 24 h post‐infusion.
Results: Two women had type 2M and one had 2B VWD. All women received 60iu/kg rVWF. Mean pre‐treatment levels of VWF:Rico, VWF:Ag, FVIII were 14, 90, 50, 137 IU/dL respectively. Pharmacokinetic studies showed 736% increase in VWF:RiCo (mean 103 IU/dl) at 30 mins and dropped by 2,14,32% (mean 101, 87, 59 IU/dL) at 3,6,24 h, respectively. VWF:Ag increased by 92% (mean 173 IU/dL) at 30 mins, followed by 2, 1 and 11% reduction at 3, 6 and 24 h post‐treatment (mean 170, 168 and 158 IU/dL). Factor VIII did not change at 30 mins and rose by 17, 1 and 25% at 3, 6 and 24 h (mean 160, 162 and 203 IU/dL) (Figure 1). Two women had planned a Caesarean section with spinal anaesthesia without any complications. One woman with type 2B VWD developed thrombocytopenia; platelet count was 32 × 109/L when she attended in labour at 38 weeks. Due to platelet clumping and difficulty obtaining an accurate level, emergency Caesarean section was performed with general anaesthesia. She developed uterine atony and postpartum haemorrhage (1500 ml). rVWF was stopped by 2 weeks post‐partum. All women were also given tranexamic acid for 6 weeks.
Conclusion(s): Recombinant VWF concentrate has provided adequate peripartum haemostasis while preventing rise of FVIII in the first 24 h after delivery when VTE risk is highest.

PB1407
The increased risk of venous thromboembolism (VTE) in obese pregnant women is due to more prolonged exposure to a procoagulant state
S. McNeill 1; F. Jordan2; V. Gibson3; D. Freeman2; C. Bagot4
1 NHS Greater Glasgow and Clyde, GLASGOW, Scotland, United Kingdom; 2 University of Glasgow, Glasgow, Scotland, United Kingdom; 3 NHS Greater Glasgow and Clyde, Glasgow, Scotland, United Kingdom; 4 Department of Haematology, Glasgow Royal Infirmary, Glasgow, England, United Kingdom
Background: VTE remains the leading direct cause of maternal death, with obese women over‐represented within the data. A better understanding of the pathophysiology underlying the increased risk of VTE in obese pregnant women is needed to develop more effective preventative strategies.
Aims: To characterise the prothrombotic phenotype of obese pregnant women.
Methods: 262 women, grouped by BMI class, were recruited to a British Heart Foundation funded prospective longitudinal study, approved by the West of Scotland Research Ethics Committee. Following written informed consent, blood samples were obtained at the antenatal booking appointment and repeated at 28 weeks’ gestation. Thrombin generation was performed using the Calibrated Automated Thrombogram method. Protein S (PS), and fibrinogen were assayed by the ACL TOP analyser. Tissue factor pathway inhibitor (TFPI), and plasminogen activator inhibitor‐1 (PAI‐1) were quantified by ELISA.
Results: At booking, endogenous thrombin potential (ETP) was significantly higher in obese (p = 0.0003) compared to lean women (Figure 1). By 28 weeks, ETP had increased and there was no longer any difference between BMI groups. The increase in ETP with advancing pregnancy was significantly greater in lean (19.92%) compared to obese class‐II women (−3.37%), (p = 0.025). A similar pattern was observed for peak thrombin, velocity index and PAI‐1. Fibrinogen was significantly higher in obese compared to lean women at booking (p < 0.001) and remained higher, whilst increasing, with advancing gestation (p < 0.001) (Figure 2). TFPI and PS were significantly higher at booking in obese compared to lean women (p < 0.001 and p = 0.03, respectively), with a greater percentage decrease by 28 weeks (TFPI: lean −6.9%, obese class‐III −24%; PS: lean −3.8%, obese class‐III −15.2%).
Conclusion(s): Obese women are exposed to the prothrombotic state earlier in pregnancy than lean women which, accompanied by the greater percentage reduction in TFPI and PS by 28 weeks’ gestation, may explain why this group is at increased risk of death from VTE.
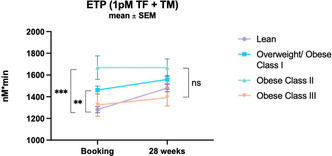

PB1400
Neurologically asymptomatic patients with pure obstetric antiphospholipid syndrome cumulate progressive microstructural brain damages, mild functional abnormalities, and subtle cognitive impairments
J. Gris 1; S. Sandiramourty2; T. Hai Nguyen2; J. Beregi2; F. Pereira3
1 University of Montpellier and University Hospital of Nîmes, Nîmes, Languedoc‐Roussillon, France; 2 Department of Radiology/Medical Imaging, University Hospital of Nîmes, Nîmes, Languedoc‐Roussillon, France; 3 University of Nîmes, Nîmes, Languedoc‐Roussillon, France
Background: Despite being asymptomatic, women with pure obstetric antiphospholipid syndrome (oAPS) hold microstructural brain damage not observed in conventional neuroradiological exams (PMID: 26201292). It is unclear whether the subtle cerebral injuries are progressive or affecting brain function.
Aims: To examine the evolution of the microstructure cerebral injuries, the extension of the functional brain states, and the respective cognitive performance in pure oAPS with a low‐dose aspirin (LDA) primary thromboprophylaxis.
Methods: 6‐year noninvasive neuroimaging‐informed follow‐up in 66 women with oAPS and 17 controls sharing the same medical history. oAPS were categorized as positive for lupus anticoagulant (LA) and/or aβ2GPI‐IgG (LA/aβ2GPI‐G‐positive) or negative (LA/aβ2GPI‐G‐negative). White‐matter (WM) integrity along three time‐points using longitudinal diffusion‐tensor imaging (DTI) and gray‐matter (GM) damages (cortical thickness and subcortical volume) were assessed. Brain network analyzes were conducted using functional MRI. The mini‐mental state examination (MMSE) before the first and second MRI sessions and a comprehensive 1‐h battery of neuropsychological tests were performed. Correlations were built between scores related to the verbal fluency and complex attention and abnormal neurobiological markers in neuroimaging.
Results: DTI indices changed significantly over time in both APS groups, but not in controls, LA/aβ2GPI‐G‐positive women demonstrating stronger changes, with progressive WM impairments. Some focused decrease in cortical thickness and subcortical volume, i.e. on GM, were detected. No clinical neurologic nor mental health manifestations were observed. Evidences of progressive modifications in brain networks, and finally in functional connectivity, were demonstrated.
Conclusion(s): oAPS women taking LDA studied over a 6 years period showed evidence of subtle progressive microstructural brain damage despite absence of any perceptible clinical symptoms and of detectable lesions using conventional imaging. LA or aβ2GPI‐G positive women developed more significant alterations than the other oAPS women. The long‐term clinical significance deserves to be investigated.
VPB1420
Systemic and local changes in the tissue factor activity and its inhibitor in early severe preeclampsia
M. Nikolaeva 1; A. Momot2; K. Shchekleina3; A. Kudinov3
1 Department of Obstetrics and Gynaecology, Altai State Medical University, Barnaul, Russian Federation, Barnaul, Altaisky krai, Russia; 2 Altai Branch of FSBI «National Research Center for Hematology» Russian Ministry of Healthcare, Barnaul, Altaisky krai, Russia, 3 ФГБОУ ВО АГМУ МЗ РФ, Barnaul, Altaisky krai, Russia
Background: The contribution of disorders in the hemostasis system determines 5–15% of gestational complications including preeclampsia (PE). In this case, tissue factor (TF) plays a key role in the initiation of the pathological process, enhancing the processes of inflammation, angiogenesis, and apoptosis as key mechanisms for the development of PE.
Aims: To study the expression of tissue factor and tissue factor pathway inhibitor in the systemic circulation and by placental tissues during the development of early severe preeclampsia
Methods: a prospective observational cohort study was performed. The main group included 40 women at low risk of developing PE (according to the results of calculations in the Astraia program in the first trimester), but with realized early severe PE. The control group included 40 pregnant women with a physiological gestation course and delivery at term. The endpoint is delivery, surrogate points are levels of TF and tissue factor pathway inhibitor (TFPI) at 19–21 and 27–28 weeks of gestation. After delivery, the expression of TF and TFPI by placental tissues was studied by immunohistochemistry.
Results: At all control points, the patients of the main group had a significant increase in the level of TF in the blood plasma (Figure 1), with a simultaneous decrease of TFPI (Figure 2). TF expression in placenta in the case of realized PE was lower: Me = 3.99% (95% CI: 3.27–4.85) versus control values – Me = 2.76% (95% CI: 2.14–3.86) (p = 0.0113). At the same time, TFPI expression in placenta in the compared groups did not differ from each other.
Conclusion(s): Early severe PE is characterized by increased expression of TF in the systemic circulation and a decrease in TF expression at the local level.


VPB1424
Hypercoagulability and inflammatory markers in a case of congenital TTP complicated by fetal demise
L. Skeith 1; K. Hurd1; L. Almeida1; A. Dufour1; A. Lee2; D. Goodyear1; L. Girard1; J. Soucie1; J. Nicholas1; P. Skeith1; E. Agbani3
1 University of Calgary, Calgary, Alberta, Canada; 2 University of Victoria, Victoria, British Columbia, Canada; 3 University of Calgary, CALGARY, Alberta, Canada
Background: Patients who are diagnosed with thrombotic thrombocytopenic purpura (TTP) during pregnancy are at an increased risk of complications including fetal death. We describe a case of a 32‐year‐old G3P0 who presented with microangiopathic hemolytic anemia, thrombocytopenia (platelet count 13), labile blood pressure, proteinuria and fetal demise at 20 weeks’ gestation. She was diagnosed with congenital TTP (cTTP) based on an ADAMTS13 level of 0.69%, inhibitor level < 12 Units/mL, and two gene abnormalities of ADAMTS13.
Aims: To better understand the pathophysiology of pregnancy complications in cTTP through platelet and proteomic studies.
Methods: We performed plasma quantitative shotgun proteomics analysis, which we coupled with detailed fluorescence imaging, and the systematic quantification of procoagulation and inflammation markers in platelet‐rich‐plasma re‐constituted to contain neutrophils. We compared experimental outcomes in cTTP to 4 pregnant healthy controls (PC), 4 gestational hypertension (GH), and 4 preeclampsia or HELLP syndrome (PE) patients (Research Ethics Board Approval #REB18‐1545).
Results: We identified 15 proteins upregulated in cTTP, and a sub‐analysis revealed that S100A8 and S100A9 were distinctly overexpressed (6–7‐fold increase) in our cTTP patient, but not in PC, GH or PE patients. High‐resolution platelet imaging from our cTTP patient showed acquired platelet and neutrophil activation, and classic structures of platelet‐neutrophil aggregates. Compared to all other participant groups, our cTTP patient had increased P‐selectin, tissue factor expression, annexin‐V binding on platelets and neutrophils, and localized thrombin generation, which is suggestive of hypercoagulability. We visualised in cTTP, but not PC, GH or PE, loose thrombus consisting of activated and procoagulant platelets and neutrophils trapped within mesh‐like structures resembling a fibrin‐network.
Conclusion(s): Evidence of platelet‐neutrophil activation and interaction, platelet hypercoagulability, and proinflammation as detected by S100A8 and S100A9 proteins were identified in our case of congenital TTP with fetal demise.
PB1391
Iron deficiency in pregnancy is highly prevalent despite suboptimal screening: A population‐based cohort study
A. Alam; V. Jain; P. Kaul; S. Lapner; C. Wu; H. Sun
University of Alberta, Edmonton, Alberta, Canada
Background: Iron deficiency anemia (IDA) is highly prevalent among pregnant women worldwide. Despite its association with adverse pregnancy outcomes, suboptimal testing results in under recognition.
Aims: To describe (1) the frequency of iron deficiency (ID) screening and (2) the prevalence and risk factors of ID and IDA among all pregnancies associated with hospitalized live births in Alberta, Canada.
Methods: This is a retrospective population‐based cohort study using the Alberta Pregnancy Birth Cohort which was created by linked administrative databases and previously described. Research ethics approval was obtained. Hemoglobin and ferritin tests ordered between January 1, 2014 and December 31, 2017 were retrieved from laboratory database. Anemia was defined as hemoglobin < 110 g/l in first and third trimester and < 105 g/l in second trimester. ID was defined as ferritin < 30 mcg/l in any trimester. IDA was defined as at least one record of concurrent anemia and low ferritin within the same trimester. Logistic regression was used to identify risk factors of IDA.
Results: Among the 207,355 pregnancies with available hemoglobin results, 36,500 (18%) had anemia at least once during pregnancy (Figure 1). Only 1 in 3 pregnancies with anemia had a concurrent ferritin test and over 80% of them demonstrated ID. Ferritin screening occurred at least once in only 60% of our cohort and a high prevalence of ID (61%) was identified among those tested. Ferritin testing was more frequent in first trimester (42%) than subsequent trimesters (21–24%), and infrequently tested postpartum (8%). 85% of the women were in the younger age group (Table 1). Advanced maternal age, multiparity and African and Asian ethnicity were identified as significant predictors of IDA.
Conclusion(s): Concordant with prior studies, testing of IDA is suboptimal among pregnant women, with racialized groups at highest risk for IDA. Further investigation is ongoing to identify gaps in care.