Abstract
Arma custos (Fabricius) (Hemiptera: Pentatomidae) is a natural predator that can control various agricultural and forestry pests. This study aimed to clarify the effects of temperature on the growth, reproduction, and population of the predator and to simulate its population growth. Using the age–stage, two-sex life table method, 18°C, 22°C, 26°C, 30°C, and 34°C were selected as the temperature conditions. A. custos can complete its life cycle at 18°C–30°C, and the developmental duration of each A. custos stage, adult pre-oviposition period, total pre-oviposition period, and the mean generation time (T) were shortened with the increase in temperature. The pre-adult mortality was significantly reduced at 26°C and 30°C. In addition, the fecundity of a single female and the gross reproductive rate were the highest at 30°C. Significant differences were observed in the intrinsic rate of increase (r) and the finite rate of increase (λ) under different temperature conditions, and both reached the maximum at 30°C. Results showed that adult A. custos raised at 26°C had a longer lifespan and the fecundity was higher at 30°C in comparison with the other temperatures. This study is the first to report the life cycle of A. custos at different temperatures, and the results can provide a scientific theoretical basis for the indoor artificial reproduction, outdoor release, and colonization of A. custos.
Keywords: age–stage two-sex life table, Arma custos, growth and development, population parameters, temperature
Arma custos (Fabricius) (Hemiptera: Pentatomidae) is widely distributed from Europe to Japan from approximately 30°N and northward (Xiao 1992; Rider and Zheng 2002; Gao et al. 2009; Zou et al. 2016; Zhao et al. 2018; Yin et al. 2021). As an excellent predatory natural enemy, A. custos can be artificially bred and utilized (Liao et al. 2019; Yang et al. 2019), and adults and nymphs of A. custos have good biological control effects on adults and larvae of more than 40 agricultural and forestry pests belonging to the orders Coleoptera, Lepidoptera, Hymenoptera, and Hemiptera (Zou et al. 2012, 2013; Guo et al. 2020; Yang et al. 2021). Previous studies have shown that A. custos prefers to feed on Ambrostoma quadriimopressum Motschulsky (Coleoptera: Chrysomelidae) and Dendrolimus spp. (Lepidoptera: Lasiocampidae) (Gao et al. 1993). In addition, A. custos can prey on quarantine pests such as Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) (Guo et al. 2011), Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) (Wang et al. 2012), and Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae) (Tang et al. 2019). Therefore, A. custos is a predatory natural enemy with great application value in agriculture and forestry.
The life activities of insects are affected by biotic and abiotic factors, with temperature being an important factor (Hoffmann 1984; Satar et al. 2005; Broufas et al. 2009; Shi et al. 2011). The effects of temperature on insects are mostly reflected in reproductive and metabolic behavior, such as growth and development, survival rate, adult emergence, mating, and oviposition (Pandey and Tripathi 2008; Forster 2011; Xu et al. 2019). In the temperature range required for the normal development of insects, the growth and development of insects show a sigmoid relationship with the increase in temperature, meaning that too high or low temperature affect the development of insects (Mathews et al. 1997; Du et al. 2007). Previous studies showed that the egg and nymph stages of A. custos were significantly shortened with increase in temperature from 18°C to 30°C (Xu et al. 1984); under different temperature treatments at 20°C, 25°C, and 30°C, the developmental duration and adult survival rate of A. custos were significantly different (Zhou et al. 2012); and the development period of the 3rd to 5th instar nymphs of the offspring was significantly shortened, and the adult lifespan of the offspring was also significantly decreased after A. custos was treated with high temperature at 35°C, while a low temperature of 18°C and a high temperature of 35°C significantly reduced the egg hatching rate (Li et al. 2021).
However, previous studies only focused on the effects of temperature on pre-adult growth, without considering the effects of temperature on A. custos reproduction. The adult stage of insects mainly completes the reproduction of the population. Whether insects can successfully develop into adults and the longevity of adult stage are of great significance to population growth. In our previous investigation and literature review, it was found that there were some problems in indoor rearing of A. custos (Zhu et al. 2022). For example, the feeding temperature during the propagation period and the germplasm preservation period was not clear, the mortality at each age was not understood, and the reproduction was unstable. Therefore, it is of great significance to explore the effects of different temperatures on the growth, development and reproduction of A. custos. By clarifying the regularity of the population growth regularity and decline of A. custos, the establishment of a dynamics model of A. custos population will help to predict its population dynamics, which is of great significance to ‘controlling insects with insects (Yan et al. 2021)’.
The temperature is one of the prominent ecological factors that affect insects, and the age–stage two-sex life table is a solid tool to evaluate its effects on the fitness and population growth of insects (Atlihan and Chi 2008; Régnière et al. 2012; Nitin et al. 2018). The conventional insect life table parameters are calculated based on the fact that the development of different instars is unified, and only the fecundity and longevity of female adults are recorded after adult emergence, ignoring the important role of male adults on population growth; this results in large errors in the calculated life table parameters (Huang and Chi 2012). Chi and Liu (1985) established the age–stage, two-sex life table to address the inadequacy of the conventional insect life table, and this technique has been widely used by many researchers in the study of the population dynamics of different insects (Chi et al. 2020; Chen et al. 2022).
In this study, based on Chi’s (1988) age–stage, two–sex life table theory, we collected development, survival, and reproduction data of A. custos at different temperatures (18°C, 22°C, 26°C, 30°C, and 34°C), and constructed the life table parameters, hence, we determined the suitable temperature range for the predator. Moreover, the change in population growth of the predator within 60 d was predicted to observe the influence of temperature on the population growth of A. custos, as well as, to provide new ideas for better indoor breeding and outdoor release application of A. custos.
Materials and Methods
Insect Rearing
Spodoptera frugiperda individuals were originally collected from corn fields on 19 June 2019 in Huashi village (25.909°N, 104.635°E, 1800 m above mean sea level), Jichangping Town, Panzhou, Guizhou Province, China. They were reared for three generations at the Institute of Entomology, Guizhou University, on fresh young maize leaves in a climate chamber (Jiangnan Instrument Factory, Ningbo, China) at 27 ± 1°C, 70% ± 5% relative humidity, and a photoperiod of 16:8 (L:D) h. In addition, 1st–3rd instar larvae were reared and concentrated in breeding boxes (60 × 60 × 55 cm). Given their cannibalistic behavior, the 4th–6th instar larvae were raised in breeding boxes (750 ml, depth 5.6 cm, bottom diameter 8.8 cm; top diameter 11.7 cm), with eight heads per box; the insects pupated in sterilized soil and cotton balls soaked in 10% honey water were placed in the boxes to provide nutrition to adults.
Arma custos adult males and females required for the experiments were provided by the natural enemies breeding center of Bijie Tobacco Company, Bijie, Guizhou Province, China, in June 2020. Subsequently, the cages (50 cm × 50 cm × 65 cm) with nymphs of A. custos were placed in an artificial climate chamber (Jiangnan Instrument Factory, Ningbo, China) set at 26 ± 1°C, 66% ± 5% relative humidity, and a photoperiod of 16:8 (L:D) h and continuously reared for more than three generations at the Institute of Entomology, Guizhou University. At 0800 and 2000 hours each day, 3–5 instar S. frugiperda larvae were provided to A. custos in the cage as food (Fig. 1). When adults emerged, they were paired and moved into plastic cups (300 ml); the mouth of the cup was sealed with a 100-mesh gauze, and a piece of paper (8 × 5 cm) was placed in the cup for oviposition. The same batch of eggs produced within 24 h was selected as the experimental insect source.
Fig. 1.
Arma custos eats the life history of Spodoptera frugiperda.
Experimental Methods
To evaluate the effect of different temperatures on the life cycle parameters of A. custos, we selected five temperatures, 18°C, 22°C, 26°C, 30°C, and 34°C, at a relative humidity of 66% ± 5% and a photoperiod of 16:8 (L:D) h. At the beginning of the experiment, 1,500 eggs produced by A. custos females (less than 24 h old) were collected; 150 eggs were randomly labeled under each temperature treatment group, and 150 eggs were placed in each temperature treatment group as the supplementary insect source.
Eggs were placed in 100 ml egg cups and sprayed with sterile distilled water regularly once a day to keep humid. The eggs were observed twice daily at 0800 and 2000 hours, until hatched. When the marked egg hatched, the time to hatching (unit: day) was recorded, and the number of eggs hatched without success or shriveled was recorded as death. After the egg hatched to the 1st instar nymph, it was removed from the egg cup using a soft brush and placed into a 300 ml plastic cup for rearing. Considering that the 1st instar nymphs do not need to feed on prey, the cotton balls soaked in 10% honey water were placed at the bottom of the cup to provide nutrition and the mouth of the cup was covered with a 100-mesh gauze. The 2nd instar nymphs were provided 3–5 instar S. frugiperda larvae for feeding. They were observed at 0800 and 2000 hours each day, and the excreta and prey corpses after feeding were cleaned at each observation time. Simultaneously, the developmental duration of each instar, the survival rate of nymphs, and the number of adults were recorded.
The number and time of emergence were recorded, and the newly emerged adults of A. custos were paired, with one male and one female, and placed into the paired cup with a piece of card for egg laying. If the ratio of male and female was different, then the corresponding heterosexual adult (female or male) was selected from the supplementary source for pairing. Furthermore, record fecundity and longevity of females and males, which are supplemented adult life parameters, were not measured. Using the KEYENCE VHX-1000 system to record each stage of A. custos.
Life Table Construction
The life table raw data for all individuals at different temperatures were organized and analyzed according to a previously reported age–stage, two–sex life table theory (Chi and Liu 1985; Chi 1988; Chi and Su 2006). The experiments were conducted based on the methods of relevant reports (Tuan et al. 2014; Hao et al. 2016; Liu et al. 2018). The computer program TWOSE–MSChart (Chi 2021a) was used to calculate the developmental duration of each stage of the population, adult pre-oviposition period (APOP), total pre-oviposition period (TPOP), fecundity, and other related population parameters. The standard errors of each parameter were estimated using the Bootstrap technology for 100,000 repeated assessments (Efron and Tibshirani 1993). The advantages of using bootstrap are well explained by Polat-Akköprü et al. (2015). The paired bootstrap test (TWOSEX–MSChart) was used to test the differences among the population parameters, development duration, and reproduction values of A. custos under different temperature treatments. Origin 2018 software was used to plot life cycle parameters, including lx, sxj, fxj, mx, exj, and vxj, of A. custos. The parameters were calculated as follows:
The age-specific survival rate (lx) refers to the survival rate from egg to age x, which was calculated as follows:
where sxj is the age-stage-specific survival rate curve (the probability that a newly laid egg will survive to age x at stage j), and m is the number of stages.
Age-specific fecundity (mx) refers to the average number of eggs produced by an individual at age x, which was calculated as follows:
where fxj is the age-specific fecundity of females (the daily number of eggs laid by a female adult at age x and stage j).
The age–stage life expectancy (exj), which represents the lifespan that an individual of age x and stage j is expected to survive, was calculated as follows:
where n is the last age in each population and s’iy is the probability that an individual of age x and stage y will survive to age i and stage j by assuming s’iy=1.
The age–stage-specific reproductive value (vxj) represents the contribution of an individual of age x and age j to the future population, which was calculated as follows:
The intrinsic rate of increase (r) of a population refers to the population growth rate when the population reaches a stable age–stage distribution. Based on the Euler–Lotka formula, age starts from 0, and it is calculated using the dichotomy iteration method (Goodman 1982).
The finite rate of increase (λ) was calculated as follows:.
The net reproductive rate (R0) represents the total number of offsprings that an individual produced during its lifetime, which was calculated as follows:
The mean generation time (T) was defined as the length of time that a population needs to increase to R0-fold when the stable increase rate r and λ are reached, that is, erT=R0 or λT=R0, which was calculated as.
The gross reproductive rate (GRR) was calculated as.
Population Projections
The TIMING–MSChart program (Chi 2021b) was used to simulate the population growth of A. custos at different temperature treatments. An initial population of 10 eggs was used for each temperature treatment.
Results
Developmental Duration, Adult Longevity, and Fecundity of A. custos at Different Temperatures
The 34°C treatment group could only develop to 2nd instar nymphs; however, other temperature groups could complete growth and development and produce offspring, and the developmental time gradually shortened with the increase in temperature. In addition, the differences among the treatments were significant. The longest developmental time of eggs (14.5 d) was recorded at 18°C, which was 3 times of that at 30°C and 3.4 times of that at 34°C (Table 1). Moreover, at 18°C, the developmental duration of each instar nymph was more than 10 d, the shortest adult duration at 30°C was only 55 d.
Table 1.
Duration of different developmental stages of Arma custos at different temperatures
| Developmental stage | Developmental Time (d) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | (18 ± 1)°C | N | (22 ± 1)°C | N | (26 ± 1)°C | N | (30 ± 1)°C | N | (34 ± 1)°C | |
| Egg | 150 | 14.450 ± 0.190a | 150 | 8.760 ± 0.040b | 150 | 6.390 ± 0.040c | 150 | 4.800 ± 0.030d | 150 | 4.240 ± 0.061e |
| 1st instar nymph | 123 | 12.850 ± 0.220a | 104 | 6.740 ± 0.180b | 125 | 4.120 ± 0.080c | 136 | 2.700 ± 0.060e | 65 | 3.170 ± 0.078d |
| 2nd instar nymph | 74 | 14.410 ± 0.530a | 79 | 6.190 ± 0.210b | 109 | 4.500 ± 0.090c | 113 | 4.130 ± 0.120d | 3 | 4.000 ± 0.000e |
| 3rd instar nymph | 66 | 10.050 ± 0.220a | 73 | 5.210 ± 0.150b | 103 | 3.840 ± 0.100c | 103 | 2.780 ± 0.040d | 0 | — |
| 4th instar nymph | 65 | 11.550 ± 0.260a | 71 | 6.280 ± 0.220b | 101 | 4.170 ± 0.100c | 102 | 3.170 ± 0.040d | 0 | — |
| 5th instar nymph | 64 | 17.330 ± 0.190a | 67 | 11.090 ± 0.190b | 97 | 7.080 ± 0.090c | 102 | 5.500 ± 0.060d | 0 | — |
| Pre-adult | 64 | 80.780 ± 0.850a | 67 | 43.790 ± 0.600b | 97 | 29.950 ± 0.220c | 102 | 22.930 ± 0.120d | 0 | — |
| Adult | 64 | 117.780 ± 6.870a | 67 | 112.940 ± 6.060a | 97 | 99.910 ± 4.520ab | 102 | 55.410 ± 2.110c | 0 | — |
The data in the table are presented as mean ± SE. Different lowercase letters in the same row indicate significant differences among developmental stages under different temperature treatments by the paired bootstrap test program (P<0.05); N represents the number of survived eggs and test insects alive.
Temperature significantly affected the adult longevity, APOP, TPOP, and fecundity of A. custos (Table 2). The longevity of the female and male adults significantly decreased as the temperature increased (P < 0.05). The longevity of female adults at 18°C and 30°C was 187 and 80 d, respectively, and that of male adults was 210 and 77 d, respectively. The APOP differed significantly among temperatures and progressively decreased with the increase in temperature. The longest APOP was 122 d at 18°C, and the shortest APOP was 13 d at 30°C. TPOP shows the same trend. On the contrary, the fecundity of A. custos gradually increased with the increase of temperature, and reached the maximum at 30°C with 474 eggs.
Table 2.
Adult longevity and reproductive parameters mean (± SE) of Arma custos at different temperatures
| Temperature (°C) | Adult Longevity/d | Adult pre-oviposition period (APOP)/d | Total pre-oviposition period (TPOP)/d | Oviposition days Od |
Fecundity (no. of eggs) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | N | Female | N | Female | N | Female | N | Female | ||
| 18 ± 1 | 33 | 209.760 ± 7.960a | 31 | 186.650 ± 11.60a | 14 | 121.930 ± 9.400a | 14 | 201.360 ± 9.830a | 1.790±0.190c | 14 | 2.930 ± 0.290c |
| 22 ± 1 | 30 | 174.630 ± 8.990b | 37 | 142.220 ± 7.630b | 30 | 61.870 ± 5.780b | 30 | 104.600 ± 5.840b | 9.000±1.220b | 30 | 242.700 ± 37.310b |
| 26 ± 1 | 47 | 139.960 ± 6.200c | 50 | 120.360 ± 6.390c | 44 | 40.980 ± 5.540c | 44 | 70.750 ± 5.560c | 16.270±1.380a | 44 | 399.950 ± 36.040a |
| 30 ± 1 | 55 | 77.250 ± 2.850d | 47 | 79.620 ± 3.230d | 45 | 13.330 ± 1.240d | 45 | 36.240 ± 1.290d | 20.290±1.650a | 45 | 474.240 ± 44.120a |
| 34 ± 1 | — | — | — | — | — | — | — | — | — | — | — |
The data in the table is mean ± SE. Different lowercase letters in the same column represent the difference of the parameter in the paired bootstrap test program under different temperature treatments (P<0.05). N represents the number of male and female adults in the first and second columns, respectively. The last three columns show the number of female adults that can lay eggs.
Mortality Rate at Different Temperatures
The mortality of A. custos reared at different temperatures is shown in Table 3. Temperatures significantly influenced the mortality rate of the 1st, 2nd, and 5th instar nymph of A. custos. However, no significant difference in mortality was observed between the 3rd and 4th instar nymph under different temperature treatments (P > 0.05). The mortality rate of pre-adult A. custos at 18°C and 22°C was significantly higher than that at 26°C and 30°C (Table 3).
Table 3.
Mortality of mean (±SE) at different developmental stages of Arma custos at different temperature treatments
| Developmental stage | Mortality Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| N | (18 ± 1)°C | N | (22 ± 1)°C | N | (26 ± 1)°C | N | (30 ± 1)°C | |
| Egg | 150 | 0 ± 0a | 150 | 0 ± 0a | 150 | 0 ± 0a | 150 | 0 ± 0a |
| 1st instar nymph | 123 | 0.180 ± 0.032a | 104 | 0.307 ± 0.038a | 125 | 0.167 ± 0.030ab | 136 | 0.093 ± 0.024b |
| 2nd instar nymph | 74 | 0.327 ± 0.038a | 79 | 0.167 ± 0.030b | 109 | 0.107 ± 0.025b | 113 | 0.153 ± 0.030b |
| 3rd instar nymph | 66 | 0.053 ± 0.018a | 73 | 0.040 ± 0.016a | 103 | 0.040 ± 0.016a | 103 | 0.067 ± 0.020a |
| 4th instar nymph | 65 | 0.007 ± 0.007a | 71 | 0.013 ± 0.009a | 101 | 0.013 ± 0.009a | 102 | 0.007 ± 0.007a |
| 5th instar nymph | 64 | 0.007 ± 0.007ab | 67 | 0.027 ± 0.013a | 97 | 0.027 ± 0.013a | 102 | 0 ± 0b |
| Pre-adult | 64 | 0.573 ± 0.040a | 67 | 0.553 ± 0.040a | 97 | 0.353 ± 0.039b | 102 | 0.320 ± 0.038b |
| Female | 31 | 0.207 ± 0.330b | 37 | 0.247 ± 0.035ab | 50 | 0.333 ± 0.038a | 47 | 0.313 ± 0.038a |
| Male | 33 | 0.220 ± 0.0338bc | 30 | 0.200 ± 0.032c | 47 | 0.313 ± 0.038ab | 55 | 0.367 ± 0.039a |
The lowercase letters in the same row indicate significant differences in mortality at different developmental stages of A. custos on different temperature (P<0.05). A paired bootstrap test was used to detect statistical differences in the mortality at different stages of A. custos on different the temperatures. Standard errors were estimated from 100,000 bootstrap resampling. N represents the number of viable eggs and test insects
The age–stage–specific survival curves (sxj) showed a similar trend (Fig. 2). The growth curves of A. custos under different temperature treatments have several overlaps because of the overlapping generation of A. custos and the complex and changeable growth and development relationship among individuals. In addition to the egg stage, the survival curve values of A. custos at the other developmental stage initially increased and then decreased with the increase of developmental time. In the present study, the survival rate of nymph treated at 30°C was the highest, and about 66.7% of the nymph could complete the entire developmental stage, followed by 64% at 26°C. By contrast, the survival rate of nymph at 22°C and 18°C was lower. Under 18°C, the male and female adults of A. custos had the longest development period, which could develop to the 306th day and 283rd day, respectively.
Fig. 2.
Age–stage-specific survival rate (sxj) of each developmental stage of Arma custos at different temperatures.
Population Survival Rate and Fecundity
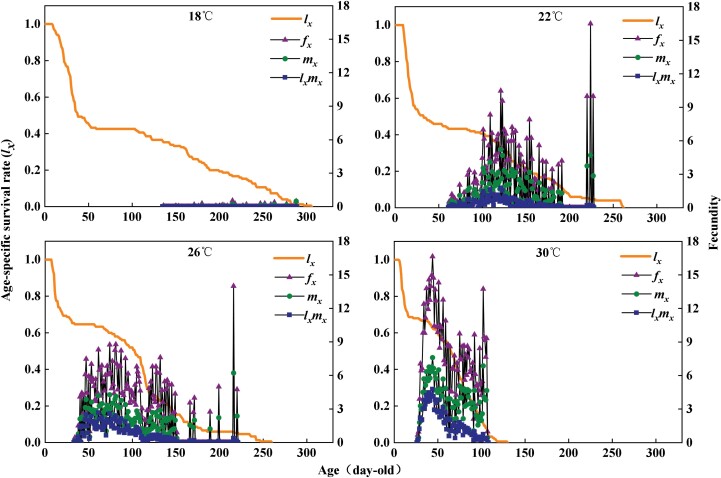
The age-specific survival rate curve (lx) of the population can reflect the change of A. custos from egg to death under different temperatures (Fig. 3). These groups showed decreased survival rates beginning at days 9, 10, 8, and 6. The age–stage fecundity (fxj), age-specific fecundity (mx), and age-specific net maternity (lxmx) are shown in Fig. 3. Under a treatment temperature of 18°C, the female adult can only produce a small number of eggs that are not hatched; thus, at this temperature, the fxj, mx, and lxmx curves of adult female A. custos only fluctuated slightly almost in a line. The values differed remarkably by temperatures. For example, the first peak of fxj occurred on day 225 with 16.5 offspring at 22°C, day 217 with 14 offspring at 26°C, and day 45 with 16.7 offspring at 30°C.
Fig. 3.
Age–stage-specific survival rate (lx), age–stage fecundity (fx), age–stage-specific fecundity (mx), and age–stage-specific maternity (lxmx) of Arma custos at different temperatures.
Life Expectancy and Reproduction Value
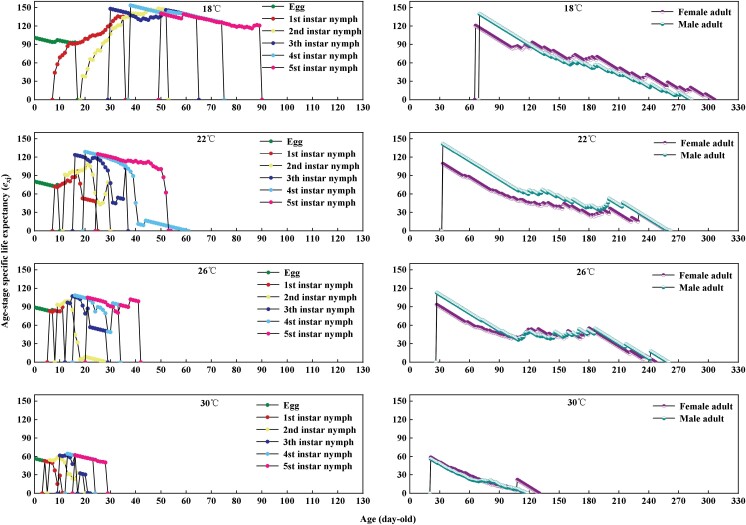
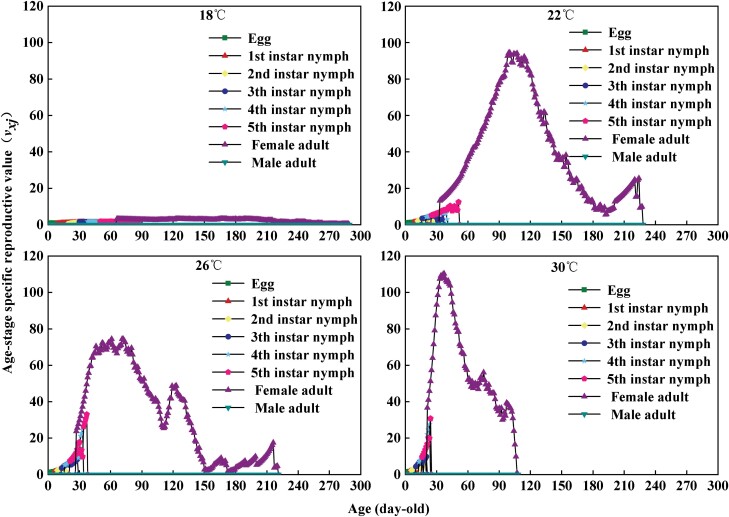
Temperature significantly affected the life expectancy exj of A. custos (Fig. 4). In general, exj decreased with the increase of age and temperature. The life expectancy at 18°C, 22°C, 26°C, and 30°C was 101, 89, 80, and 57, respectively. The reproductive value (vxj) is the contribution of an individual of age x and stage j to the future population of A. custos (Fig. 5). The vxj curve at each instar under different temperature treatments showed an upward trend, whereas it significantly increased when the female adults began to oviposit. Low fecundity was observed at 18°C, and the maximum value was only 3.3 on the 167th day; at 22°C treatment, it reached the highest peak of 94.6 on the 101st day; at 26°C treatment, it reached the maximum value of 74.5 on the 73rd day; the maximum value of 110.4 was reached on the 38th day at 30°C treatment.
Fig. 4.
Age–stage-specific life expectancy (exj) of each developmental stage of Arma custos at different temperatures.
Fig. 5.
Age–stage-specific reproductive value (vxj) of each developmental stage of Arma custos at different temperatures.
Life Table Parameters
Temperatures significantly affected the population parameters of A. custos (Table 4). The intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R0) of A. custos significantly increased with the increase in temperature. The highest r,λ, and R0 of A. custos were found at 30°C, which were 0.11 days−1, 1.12 days−1, and 142.27 offspring, respectively. On the contrary, the average generation period (T) of A. custos decreased with the increase in temperature. Furthermore, the GRR reached the maximum value at 30°C.
Table 4.
Population parameters (mean±SE) for Arma custos at different temperatures
| Temperature (°C) | Intrinsic rate of increase | Finite rate of increase | Net reproductive rate | Mean generation time | Gross reproductive rate |
|---|---|---|---|---|---|
| r (d-1) | λ (d-1) | R 0 (offspring) | T (d) | GRR (offspring) | |
| 18 ± 1 | -0.006 ± 0.001d | 0.994 ± 0.001d | 0.273 ± 0.074c | 217.338 ± 8.475a | 2.312 ± 0.575c |
| 22 ± 1 | 0.035 ± 0.002c | 1.036 ± 0.003c | 48.542 ± 10.816b | 111.239 ± 4.423b | 167.372 ± 37.396b |
| 26 ± 1 | 0.074 ± 0.004b | 1.077 ± 0.004b | 117.322 ± 18.180a | 64.305 ± 2.488c | 246.462 ± 38.150ab |
| 30 ± 1 | 0.111 ± 0.005a | 1.118 ± 0.005a | 142.273 ± 22.090a | 44.624 ± 1.180d | 313.242 ± 54.042a |
| 34 ± 1 | — | — | — | — | — |
The data in the table are the mean ± SE. Different lowercase letters in the same column indicate the significant difference analysis of this parameter in the paired bootstrap test program under different temperature treatments (P<0.05)
Population Projections
The projected population growth of A. custos for a 60-d period is shown in Fig. 6 for the four temperature treatments. At 18°C, the population had not begun to reproduce by 60 d. At 22°C, the adult emerged after 33 d. At 26°C, the second generation of eggs hatched after 35 d had developed to L1, L2, L3, L4, and L5 instars by 60 d. At 30°C, the second generation of eggs emerged after 28 d, and the second generation of adults emerged after 50 d. The estimates were generally higher for the 26°C and 30°C treatments than the other two treatments.
Fig. 6.
Population projections over a 60-d period for Arma custos at different temperatures and the overall population of each treatment.
Discussion
As an ectothermic organism, A. custos is sensitive to ambient temperature, which significantly affects its diffusion coefficient, distribution, and life cycle (Zhang 2017). A life table is an important tool of population ecology research. The use of natural enemies to control pests can help us understand the number, age structure, developmental rate, and reproductive capacity and predict the population dynamics of insects (Chi et al. 2019). To date, life table parameters of A. custos have been studied and obtained for different varieties of pests (Yang 2021). This study is the first to investigate how temperatures affect the development and reproduction of A. custos on S. frugiperda.
Life activities such as growth, development, reproduction and survival all have suitable temperature ranges. When the temperature exceeds the optimum range, the growth, development and reproduction of insects will be seriously affected (Hodek and Hodková 1988; Huang et al. 2008; Kang et al. 2022). For example, the developmental duration of each instar in Picromerus bidens (Hemiptera: Pentatomidae) and Picromerus lewisi (Hemiptera: Pentatomidae) (both predatory Pentatomidae) was shortened with the increase of temperature in the range of 18°C to 32°C, and the eggs could not hatch normally at 15°C and 35°C (Mahdian et al. 2008; Tang 2020). The nymph development rate of Podisus maculiventris (Hemiptera: Pentatomidae) increased with the increase of temperature from 18°C to 30°C, the eggs could hatch at 13.3°C to 32.7°C, and the nymphs could successfully develop into adults at 18.4°C to 32.7°C (Legaspi et al. 2005; Sunghoon et al. 2014). In this study, breeding temperatures were greater than 18°C and less than 34°C, which A. custos could only develop to second instar nymphs at 34°C, indicating that the maximum temperature for the large-scale reproduction of A. custos is must be well below 34°C. This is important information for the large-scale reproduction of A. custos.
Constructing the age-stage-specific survival rate (Sxj) and age-specific survival rate (lx) of A. custos based on the survival status of different instars can objectively describe the whole process of A. custos population from egg to adult death. It can better reflect the development rate and survival difference between individuals of A. custos. The results of this study showed that there was significant overlap in the different developmental stages of A. custos, with the increase of developmental time, the previous instar began to molt and transformed into the next instar or partially died. Therefore, the survival rate curve of each developmental stage showed a trend of increasing first and then decreasing. From the mortality rate, it can be seen that the mortality rate of the pre-adult and female adult A. custos raised at 26°C is higher than that of 30°C. At present, 26°C is often selected as the feeding temperature for indoor rearing A. custos (Zhang et al. 2017; Yang et al. 2021, 2022), according to the results of this study, raising the indoor rearing temperature to 30°C can reduce the mortality at different ages. Whether the insect population can reproduce to a certain number and continue depends on the successful development of the insect eggs into adults and the life span of adult stage (Hu et al. 2021).
For natural enemy insects, fecundity is more important in biological control than longevity. In this study, the life expectancy (exj) was the smallest at 30°C, but the fecundity (fx7, mx, and lxmx) of A. custos reached the maximum at this temperature, it indicated that the adult A. custos had a shorter lifespan at 30°C, but more eggs were laid. This condition was beneficial to the increase of the number of A. custos and pest management. Whether temperature is also an environmental factor affecting the energy distribution trade-off between A. custos reproduction and longevity requires further study.
Life table parameters (r,λ, GRR, R0, and T) as important indicators in insect population prediction, can reflect the population growth potential of insects in different environments (Hao et al. 2016). Our study showed that the 30°C treatment could prolong the oviposition period of A. custos, with the highest intrinsic of increase (r), the finite rate of increase (λ) and GRR, and the shortest average generation time (T) and adult lifespan under this treatment. Consistent with the conclusions obtained from studies on Hippodamia variegate (Goeze) (Coleoptera: Coccinellidae) and Orius minutus (Linnaeus) (Hemiptera: Anthocoridae) (Yue et al. 2009; Ding et al. 2016), within a certain range, increasing temperature is beneficial to the reproduction of insect populations. The results showed that, when a large number of A. custos need to be released in the field, compared with 26°C, 30°C was more conducive to the indoor propagation of A. custos.
This study compared the developmental duration, age-stage-specific survival rate (Sxj) and age-specific survival rate (lx), and life table parameters, etc. The results showed that 26°C–30°C is the suitable temperature range for the reproduction of A. custos, which is the first report of the suitable temperature range for artificial rearing in the large-scale reproduction of A. custos. The previous feeding studies were all carried out at 26°C (Zou et al. 2016; Yang 2021), without exploring the life table of gender age at different temperatures. In this study, at 26°C, A. custos can reproduce only 5–6 generations a year, which is consistent with the results of Zhang et al. (Zhang et al. 2017), while at 30°C, A. custos can reproduce 8–9 generations a year. Based on this, we suggest that the large-scale propagation temperature of A. custos can be adjusted from 26°C to 30°C for feeding, which not only shortens the reproduction cycle of A. custos, but also ensures that a large number of commercial A. custos can be obtained.
Managing pests by releasing a large number of natural enemy insects is a means of biological control. Practically, this can be achieved by releasing a large number of predators at one point in time or by sequentially releasing small numbers throughout a growing season to establish a more naturally distributed population of predators. However, the cost of submerged release of natural enemies to control pests is too high, especially like A. custos, the price of an A. custos is 0.44–0.74 USD in the Chinese market. According to the recommended dosage of 30 heads per 667 m2 (one mu) (Zou et al. 2016; Ren et al. 2022), the cost of biological control agent will reach 13.33–22.22 USD per 667 m2, which is very high compared with chemical prevention and control. Therefore, the field application of A. custos should be combined with the occurrence of field pests, the release age of A. custos, field temperature and other factors. In the field, we can reasonably use the developmental duration difference of each age according to meteorological data, and then release A. custos of lower ages in advance according to the prediction results of the pests, so that A. custos can better adapt to the environment in the field, develop to a more efficient pest control period (4-5 instar nymphs) (Tang et al. 2020; Sun et al. 2021), and finally form a population colonization. This can effectively increase the control effect and reduce the cost of control (Hu et al. 2021).
In summary, the most suitable breeding temperature for A. custos is 30°C, compared with the current breeding temperature of 26°C, we could get 8–9 generations A. custos per year feeding under 30°C, so we suggest that the rearing temperature should be increased 30°C, which can provide a sufficient source of insects for the continuous release of A. custos, and can also be used as a guarantee for the prevention and control of a large number of pests that suddenly break out in the field. Therefore, in the future, we need to do further research on the population growth dynamics of A. custos under the conditions of mimicking the changing temperature in nature and the actual conditions in the field.
Acknowledgments
We thank PhD student Rixin Jiang (Guizhou University) for help on photograph, and we also thank Yichai Chen (Guizhou University) for help on TWOSEX–MSChart. Finally, we would like to thank Dr. Phyllis G. Weintraub (retired from Agricultural Research Organization, Israel) for language editing of this manuscript. This research was supported by the Science-Technology Program of Guizhou Tobacco Company Zunyi Branch ([2018]11) and the Science-Technology Program of China Tobacco Head Company Guizhou Company ([2019]10).
Contributor Information
Jie Wang, Institute of Entomology, Guizhou University, Guiyang 550025, China; Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Moubtainous Region, Guiyang 550025, China.
Yinlin Mu, Institute of Entomology, Guizhou University, Guiyang 550025, China; Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Moubtainous Region, Guiyang 550025, China.
Can Yang, Institute of Entomology, Guizhou University, Guiyang 550025, China; Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Moubtainous Region, Guiyang 550025, China.
Lin Yang, Institute of Entomology, Guizhou University, Guiyang 550025, China; Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Moubtainous Region, Guiyang 550025, China.
Changhua Zhang, Guizhou Tobacco Company Zunyi Branch, Zunyi 563000, China.
Huiping Yu, Guizhou Tobacco Company Bijie Branch, Bijie 551700, China.
Zhimin Chang, Institute of Entomology, Guizhou University, Guiyang 550025, China; Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Moubtainous Region, Guiyang 550025, China.
Jiankun Long, Institute of Entomology, Guizhou University, Guiyang 550025, China; Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Moubtainous Region, Guiyang 550025, China.
Xiangsheng Chen, Institute of Entomology, Guizhou University, Guiyang 550025, China; Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Moubtainous Region, Guiyang 550025, China.
Author Contribution
Conceptualization: J.W. and X.-S.C.; methodology: J.W. and Y.-L.M.; software: Y.-L.M. and C.Y.; validation: L.Y., Z.-M.C. and J.-K.L.; formal analysis: J.W.; investigation: C.-H.Z.; resources: H.-P.Y.; data curation, J.W.; writing—original draft preparation: J.W.; writing—review and editing: J.W., Y.-L.M., C.Y., L.Y., Z.-M.C. and J.-K.L.; visualization: X.-S.C.; supervision: X.-S.C.; project administration: X.-S.C.; funding acquisition: X.-S.C. All authors have read and agreed to the published version of the manuscript.
References Cited
- Atlıhan, R., and Chi H.. . 2008. Temperature-dependent development and demography of Scymnus subvillosus (Coleoptera: Coccinellidae) reared on Hyalopterus pruni (Homoptera: Aphididae). J. Econ. Entomol. 101: 325–333. [DOI] [PubMed] [Google Scholar]
- Broufas, G. D., Pappas M. L., and Koveos D. S.. . 2009. Effect of relative humidity on longevity, ovarian maturation, and egg production in the olive Fruit Fly (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 102: 70–75. [Google Scholar]
- Chen, Y. C., Chen D. F., Yang M. F., and Liu J. F.. . 2022. The effect of temperatures and hosts on the life cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 13: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, H. 1988. Life–table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17: 26–34. [Google Scholar]
- Chi, H. 2021a. TWOSEX–MSChart: A Computer Program for the Age–Stage, Two–Sex Life Table Analysis. Accessed on November 25, 2021. Available online: http://140.120.197.173/Ecology/prod02.Htm.
- Chi, H. 2021b. TIMING–MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. Accessed on November 25, 2021. Available online: http://140.120.197.173/Ecology/prod02.Htm.
- Chi, H., Fu J. W., and You M. S.. . 2019. Age–stage, two–sex life table and its application in population ecology and integrated pest management. Acta Ecol. Sin. 62: 255–262. [Google Scholar]
- Chi, H., and Liu H.. . 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24: 225–240. [Google Scholar]
- Chi, H., You M. S., Atlihan R., Smith C. L., Kavousi A., Özgökçe M. S., Güncan A., Tuan S. J., Fu J. W., Xu Y. Y., . et al. 2020. Age-stage, two-sex life table: an introduction to theory, data analysis, and application. Entomol. Gen. 2: 103–124. [Google Scholar]
- Chi, H., and Su H. Y.. . 2006. Age–stage, Two–sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 35: 10–21. [Google Scholar]
- Ding, Y., Yang Q. F., Li Q., Jiang C. X., and Wang H. J.. . 2016. Effects of temperature on the development and reproduction of Orius minutus (Hemiptera: Anthocoridae). Acta Entomol. Sin. 59: 647–653. [Google Scholar]
- Du, Y., Ma C. S., Zhao Q. H., Ma G., and Yang H. P.. . 2007. Effects of heat stress on physiological and biochemical mechanisms of insects a literature review. Acta Ecol. Sin. 27: 1565–1572. [Google Scholar]
- Efron, B. and Tibshirani R. J.. . 1993. The bootstrap estimate of standard error. In: An introduction to the bootstrap. Chapman and Hall, New York, NY. 49–54. [Google Scholar]
- Forster, J., Hirst A. G., and Woodward G.. . 2011. Growth and development rates have different thermal responses. Am. Nat. 178: 668–678. [DOI] [PubMed] [Google Scholar]
- Gao, C. Q., Wang Z. M., and Yu E. Y.. . 1993. Study on artificial feeding technology of Arma chinensis (Fallou). J. Jilin Forest. Sci. Tech. 2: 16–18. [Google Scholar]
- Gao, Z., Zhang L. X., and Wang G. Q.. . 2009. Arma chinensisFallou Protection and utilization as a control agent of Sugarbeet insect-pests. Sugar Crops of China. (1): 70–72. [Google Scholar]
- Goodman, D. 1982. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 119: 803–823. [Google Scholar]
- Guo, W. C., Tuerxun, Y. M.Xu, J.Liu, J. J.Xu, P. L.Wang, J.He, Z. H.Xia, W. J.Fu, X. Y. Jing, and Zhang D. M.. 2011. Study and application on sustained and integrated control techniques of Colorado Potato Beetle. Xinjiang Agric. Sci. 48: 197–203. [Google Scholar]
- Guo, Y., Zhao C., Li J. Z., and Li D. S.. . 2020. Functional response ofArma chinensis(Fallou) to the first instar nymph of Tessaratoma papillosaDrury. Chin. J. Biol. Chin. 36: 826–831. [Google Scholar]
- Hao, Q., Huang Q., Liang W. B., Gong C. W., and Wang X. G.. . 2016. Age-stage two-sex life tables of Spodoptera litura (Lepidoptera: Noctuidae) at different temperatures. Acta Entomol. Sin. 59: 654–662. [Google Scholar]
- Hodek, I., and Hodková M.. . 1988. Multiple role of temperature during insect diapause: a review. . Entomol. Exp. Appl. 49: 153–165. [Google Scholar]
- Hoffmann, K.H. 1984. Metabolic and enzyme adaptation to temperature, pp. 1–32. In: Hoffmann, K.H. (eds), Environmental Physiology and Biochemistry of insects. Springer, Berlin, Heidelberg, Germany. [Google Scholar]
- Hu, C. X., Fan W., Zhang Q., Chen G. H., Yin H. H., Xu T. Y., Yang J. B., Yang H., Eu D. H., and Zhang X. M.. . 2021. Control effect of Orius similison Frankliniella occidentalisbased on the two-sex life table and the age-stage-specific predation rate. Scientia Agricultura Sinica. 54: 2769–2780. [Google Scholar]
- Huang, Y. B., and Chi H.. . 2012. Age–stage, two–sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 19: 263–273. [Google Scholar]
- Huang, Z., Ren S. X., and Musa P. D.. . 2008. Effects of temperature on development, survival, longevity, and fecundity of the Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) predator, Axinoscymnus cardilobus (Coleoptera: Coccinellidae). Biol. Control 46: 209–215. [Google Scholar]
- Kang, M. Y., Zou T. T., Zhan K. P., Chen Y. Y., Xiu D. Y., and Song L. W.. . 2022. Research progress on insect diapause induced by photoperiod and temperature. J. Jilin Forest. Sci. Technol. 51: 46–48. [Google Scholar]
- Legaspi, J. C., and Legaspi B. C.. . 2005. Life table analysis for Podisus maculiventris immatures and female adults under four constant temperatures. Environ. Entomol. 34: 990–998. [Google Scholar]
- Li, Z. Y., Du Q. X., Xie P. F., Yang Z. X., and Cai H. L.. . 2021. Effects of temperature on the biological characteristics of Arma chinensisand its offspring. China Plant Prot. 41: 17–22. [Google Scholar]
- Liao, P., Miao S. M., Xu R. N., Liu C. X., Chen G. K., Wang M. Q., Mao J. J., Zhang L. S., and Chen H. Y.. . 2019. Evaluation of a new liquid artificial diet of Arma chinensis Fallou (Hemiptera: Pentatomidae). Chin. J. Biol. Control 35: 9–14. [Google Scholar]
- Liu, Y. Y., Li G. Y., Yang L., Chi H., and Chen X. S.. . 2018. Demography and mass rearing of the medicinal blister beetle Epicauta impressicornis (Pic) (Coleoptera: Meloidae) at different temperatures. J. Econ. Entomol. 111: 2364–2374. [DOI] [PubMed] [Google Scholar]
- Mahdian, K., Tirry L., and Clercq P. D.. . 2008. Development of the predatory pentatomid Picromerus bidens (L.) at various constant temperatures. Belg. J. Zool. 138: 135–139. [Google Scholar]
- Mathews, M. C., Summers C. B., and Felton G. W.. . 1997. Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch. Insect. Biochem. Physiol. 34: 57–68. [Google Scholar]
- Nitin, K. S., Sridahr V., Onkar S. N., Chakravarthy A. K., and Atlihan R.. . 2018. Effect of temperature and CO2 on population growth of South American Tomato Moth, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on tomato. J. Econ. Entomol. 111: 1614–1624. [DOI] [PubMed] [Google Scholar]
- Pandey, A. K., and Tripathi C. P. M.. . 2008. Effect of temperature on the development, fecundity, progeny sex ratio and life-table of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. BioControl 53: 461–471. [Google Scholar]
- Polat-Akköprü, E., Atlihan R., Okut H., and Chi H.. . 2015. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 108: 378–387. [DOI] [PubMed] [Google Scholar]
- Ren, C. Y., Liu J., Luo M. H., Nie Z. Y., and Huang N.. . 2022. A review on Arma chinensis Fallou (Hemiptera: Pentatomidae): a natural enemy insect. Chin. Agric. Sci. Bull. 38: 100–109. [Google Scholar]
- Régnière, J., Powell J., Bentz B., and Nealis V.. . 2012. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 58: 634–647. [DOI] [PubMed] [Google Scholar]
- Rider, D. A., and Zheng L. Y.. . 2002. Checklist and nomenclatueal notes on the Chinese Pentatomidae (Heteroptera) I. Asopinae. Entomotaxonomia 24: 107–115. [Google Scholar]
- Satar, S., Kersting U., and Uygun N.. . 2005. Effect of temperature on development and fecundity of Aphis gossypii Glover (Homoptera: Aphididae) on cucumber. J. Pest Sci. 78: 133–137. [Google Scholar]
- Shi, P. J., Ikemoto T., and Ge F.. . 2011. Development and application of models for describing the effects of temperature on insects’ growth and development. Chin. J. Appl. Entomol. 48: 1149–1160. [Google Scholar]
- Sun, J. J., Wang M. Q., Tang Y. T., Li X. Y., Zhang L. S., and Li H.. . 2021. Predatory functional response of Arma custos (Hemiptera: Pentatomidae) to the larvae of Helicoverpa armigera (Lepidoptera: Noctuidae). J. Plant Prot. 48: 1081–1087. [Google Scholar]
- Sunghoon, B., Youngsoo S., and Yong-Lak P.. . 2014. Temperature-dependent development and survival of Podisus maculiventris (Hemiptera: Pentatomidae): implications for mass rearing and biological control. J. Pest Sci. 87: 331–340. [Google Scholar]
- Tang, Y.T. 2020. Study on potential of a novel natural enemy insect Picromerus lewisi Scott in biological control. Master’s Thesis, University of Chinese academy of agricultural sciences, Beijing, China. pp: 1–55. [Google Scholar]
- Tang, Y. T., Guo Y., Pan M. Z., Mao J. J., Chen H. Y., Zhang L. S., and Wang M. Q.. . 2020. Predation of Plutella xylostella larva by Arma chinensis. Plant Prot. 46: 155–160. [Google Scholar]
- Tang, Y. T., Li Y. Y., Liu C. X., Mao J. J., Chen H. Y., Zhang L. S., and Wang M. Q.. . 2019. Predation and behavior of Arma chinensis to Spodoptera frugiperda. Plant Prot. 45: 65–68. [Google Scholar]
- Tuan, S. J., Lee C. C., and Chi H.. . 2014. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age–stage, two-sex life table. Pest Manag. Sci. 70: 805–813. [DOI] [PubMed] [Google Scholar]
- Wang, W. L., Liu Q., Yan J. H., Kang Z., Lin Z. Q., Zhang X. H., Yang Q. M., Wang J., Ma J., and Sun Y. L.. . 2012. Preliminary observation of preyed ability ofArma chinensis(Fallou), a new natural enemy ofHyphantria cunea(Drury). J. Shandong Forest. Sci. Technol. 42: 11–14. [Google Scholar]
- Xiao, G.R. 1992. Heteroptera. In: Forest Insects of China. China Forestry Publishing House, Beijing, China. 342–344. [Google Scholar]
- Xu, C. H., Yan J. J., and Yao D. F.. . 1984. Relationship between temperature and development of Arma chinensis Fallou. Sci. Silvae Sin. 20: 96–99. [Google Scholar]
- Xu, L. Y., Liu S. Y., Yu J. F., Xu Y. Y., and Chen Z. Z.. . 2019. Effects of temperatures on the predation of Chrysoperla sinica (Neuroptera: Chrysopidae) on Sitobion avenae (Hemiptera: Aphididae). J. Environ. Entomol. 41: 605–611. [Google Scholar]
- Yan, J. J., Wang Y. L., Zhao R., Tian G. Q., and Li R.. . 2021. Analysis of population growth, development and fecundity dynamics of wolf spider Pardosa astrigera based on age-stage two-sex life table. J. Plant Prot. 48: 593–601. [Google Scholar]
- Yang, C. 2021. Predation, prey preference and life table of Arma chinensis (Fallou) on Spodoptera litura (Fabricius) and Helicoverpa asssulta (Guenée). Master’s Thesis, University of Guizhou, Guizhou, China. [Google Scholar]
- Yang, C., Mu Y. L., Wang J., Huang C. Y., Jia F. Z., Yu H. P., and Chen X. S.. . 2021. Starvation tolerance of Arma chinensis in different environments at suitable and low temperatures. J. Mount. Agric. Biol 40: 84–87. [Google Scholar]
- Yang, C., Mu Y. L., Wang J., Huang C. Y., Jia F. Z., Yu H. P., and Chen X. S.. . 2022. Predatory functional of Arma chinensis (Fallou) to the eggs and 3rd-instar larvae of two tobacco pests. Plant Prot. 48: 158–162. [Google Scholar]
- Yang, Z. H., Meng L., and Li B. P.. . 2019. Larval instar–dependent effects on predation behavior of stinkbug Arma chinensis attacking Spodooptera litura larvae. Chin. J. Ecol. 38: 3376–3381. [Google Scholar]
- Yin, Y. F., Zhu Y. J., Mao J. J., Gundersen-Rindal D. E., and Liu C. X.. . 2021. Identification and characterization of microRNAs in the immature stage of the beneficial predatory bug Arma chinensis Fallou (Hemiptera: Pentatomidae). Arch. Insect. Biochem. Physiol. 107: e21796. [DOI] [PubMed] [Google Scholar]
- Yue, J., He J., Zhang R., and He D. H.. . 2009. Life tables of laboratory population of Hippodamia variegate at different temperatures. Chin. Bull. Entomol. 46: 921–925. [Google Scholar]
- Zhang, H.P. 2017. Key biological and physiological factors affect the population establishment of Arma chinensis (Fallou) in greenhouses. Master’s Thesis, University of Chinese academy of agricultural sciences dissertation, Beijing, China. [Google Scholar]
- Zhang, H. P., Pan M. Z., Yi Z. J., Zhang C. H., Guo Y., Liu C. X., Zhang L. S., Wang M. Q., Jia F. Z., Yang Z. Y., . et al. 2017. Effects of short term starvation on longevity, fecundity and predation of Arma chinensis (Hemiptera: Pentatomidae). Chin. J. Biol. Control 33: 159–164. [Google Scholar]
- Zhao, Q., Wei J. F., Bu W. J., Liu G. Q., and Zhang H. F.. . 2018. Synonymize Arma chinensis as Arma custos based on morphological, molecular and geographical data. Zootaxa 4455: 161–176. [DOI] [PubMed] [Google Scholar]
- Zhou, S. L., Zhou C. Y., Ding H. L., Liu X. W., Gao J., Dong Z. Q., Xie Z. J., and Xu Q. S.. . 2012. Influence of different temperature on the growth and development ofArma chinensis. J. Jilin Forest. Sci. Technol. 41: 19–20, 32. [Google Scholar]
- Zhu, Y. J., Yin Y. F., Wang Z., Chen H. Y., and Liu C. X.. . 2022. A note of degeneration of Arma chinensis (Heteroptera: Pentatomidae) during the rearing process. Chin. J. Biol. Control 38: 159–165. [Google Scholar]
- Zou, D. Y., Wang M. Q., Zhang L. S., Zhang Y., Zhang X. J., and Chen H. Y.. . 2012. Taxonomic and bionomic notes on Arma chinensis (Fallou) (Hemiptera: Pentatomidae: Asopinae). Zootaxa 3382: 41–52. [Google Scholar]
- Zou, D. Y., Wu H. H., Coudron T. A., Zhang L. S., Wang M. Q., Liu C. X., and Chen H. Y.. . 2013. A meridic diet for continuous rearing of Arma chinensis (Hemiptera: Pentatomidae: Asopinae). Biol. Control 67: 491–497. [Google Scholar]
- Zou, D. Y., Xu W. H., Liu B. M., Bai Y. C., Liu X. L., Xu J. Y., Hu X., and Gu X. S.. . 2016. Research progress and prospects of Arma chinensis Fallou (Hemiptera: Pentatomidae). J. Environ. Entomol. 38: 857–865. [Google Scholar]