Abstract
The ability of the leishmanial parasite UR6 to act as an immunoprophylactic and immunotherapeutic agent against Leishmania donovani infection in BALB/c mice was investigated. Unlike the virulent L. donovani AG83 (MOHOM/IN/1983/AG83), UR6 given through intracardiac route failed to induce visceral infection, but when it was injected subcutaneously, UR6 induced a short-lived and localized self-healing skin lesion. Priming of peritoneal macrophages with UR6 in vitro induced superoxide (O2−) generation, whereas similar experiments with virulent AG83 inhibited O2− generation. It was observed that priming of mice with either live or sonicated UR6 in the absence of any adjuvant provided strong protection against subsequent virulent challenge. Further, UR6-primed infected mice not only displayed a strong antileishmanial delayed-type hypersensitivity (DTH) response but also showed an elevated level of the serum antileishmanial immunoglobulin G2a (IgG2a) isotype, whereas infected mice failed to mount any antileishmanial DTH response and showed an elevated level of IgG1. This indicates that UR6 priming and subsequent L. donovani infection allowed the expansion of Th1 cells. Our studies indicate that UR6 has potential to be used as an immunoprophylactic and immunotherapeutic agent against experimental visceral leishmaniasis.
The protozoan parasite Leishmania causes at least three major forms of human diseases, including cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis, and visceral leishmaniasis (VL) (12). VL is the most severe systemic disease among the three main categories of leishmaniasis (12). In India, almost 44 million people in 28 districts of Bihar and 5.5 million people in 8 districts of West Bengal are at risk for VL (47).
Chemotherapy of leishmaniasis has been restricted mainly to the use of antimonials (5), despite cardiac and renal toxicity. The emergence of drug resistance and nonavailability of prophylactic agents are major obstacles to their control. However, the development of protective immunity following spontaneous resolution of both CL (15) and VL (22) suggests that perhaps the induction of protection by vaccination could be feasible in the control of leishmaniasis.
Studies on mice and humans suggest that T-cell-mediated immune responses play a central role in the outcome of leishmaniasis (8). Preferential expansion of functionally distinct CD4+ T-cell subsets that are highly polarized to either the Th1 or Th2 pattern determines the outcome of the disease. Knowledge about defense mechanisms in leishmaniasis can be expected to aid in establishing a strategy for developing such a vaccine.
Development of a vaccine for CL has been the focus of much attention. Certain subcellular fractions (or pure antigens) and live, attenuated parasites have been implicated as potential vaccine candidates (17). Early studies in animal models immunized with killed parasites plus adjuvant have demonstrated that reductions in the live parasite burden can also be achieved in VL (17). Reports that many leishmanial antigens are cross-reactive (27) motivated the use of a first-generation vaccine for CL against VL (27, 28). The use of live Leishmania vaccine has been abandoned because of problems associated with the virulence of available vaccine lines. These problems led to the efforts to develop a safe, live Leishmania vaccine line by gene replacement (45). Heterologous carriers like the Salmonella system (23) that carried the gp63 gene of Leishmania major and recombinant gp63 cloned in a plasmid have been used as modern tools of genetic vaccination. But in all of these cases adjuvant was required to achieve protection. In absence of adjuvant, little protection was observed. In a recent report, it has been shown that Leishmania donovani promastigotes entrapped in neutral liposomes conferred around 73% protection in experimental VL (2).
Development of a safe and effective vaccine line is thus besieged with problems. While peptide vaccines suffer from poor immunogenicity and genetic restriction in the T-cell compartment, genetic vaccines are not likely to reach 100% protection because of their restrictiveness and lack of the full repertoire of antigens involved in a protective immune response (34, 46). For this reason there were various attempts at vaccinations using attenuated or avirulent forms of Leishmania. Gamma-irradiated Leishmania (38) or attenuated Leishmania derived from long-term culture in vitro (17) has been shown to yield substantial protection in mice against subsequent challenge with L. major.
Recently we have reported that the leishmanial parasite UR6 (MMOM/IN/1978/UR6) is highly effective as an immunoprophylactic and immunotherapeutic agent against L. donovani infection in a hamster model (29). The leishmanial parasite UR6 was found to stimulate superoxide generation in hamster macrophages (Mφs), to display abundant message for KMP II, and to lack LPG (29). Interestingly, unlike the case for other systems, UR6-mediated protection is observed in absence of any adjuvant. This observation prompted us to study the efficacy of UR6 as an immunoprophylactic and immunotherapeutic agent against L. donovani infection in the murine model.
MATERIALS AND METHODS
Animals, parasites, animal infection, and parasite burden.
Four- to 6-week-old BALB/c mice, reared in the Institute facility (originally brought from Jackson Laboratory, Bar Harbor, Maine were used irrespective of sex. Leishmania strain UR6 (MHOM/IN/1978/UR6) was originally isolated from the bone marrow aspirate of a kala-azar patient and has been maintained in Ray's modified medium (37) for more than 20 years in our laboratory. L. donovani strain AG83 (MHOM/IN/1983/AG83) and L. major strain NIH Friedlin were maintained in vitro in M-199 containing 10% fetal calf serum (FCS) as described before (29). Amastigotes were prepared from the spleens of AG83-infected mice on a discontinuous Percoll gradient as described by Hart et al., (18). For infection, mice were inoculated with 2 × 107 AG83 amastigotes or 2 × 107 promastigotes in 0.5 ml of saline through the tail vein (31).
Splenic and hepatic parasite burdens in mice were determined as described by Stauber (43), and results were expressed as mean parasite number ± standard deviation (SD).
Transformation of amastigotes to promastigotes.
Infected spleens were cultured at 22°C for 5 to 7 days in Schneider's Drosophila medium, supplemented with 20% FCS for synchronized transformation of amastigotes to promastigotes. Promastigotes were seen after 5 days, and thereafter the parasites were routinely subcultured (40).
MAb and polyclonal antibodies.
L. major-specific monoclonal antibodies (MAb) 5E6-G11 and 2G11-H2 (isotype immunoglobulin G1 [IgG1]; ascities fluid from mouse was the kind gift of E. Handman, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). Mouse IgG1 and goat anti-mouse IgG1 (heavy chain specific) were purchased from Sigma, St. Louis, Mo.
Iodination of anti-mouse IgG1 and CS-RIA with promastigotes.
Ten micrograms of goat anti-mouse IgG1 was iodinated by the chloramine-T method (14). The specific activity of the 125I-labeled anti-IgG1 was 107,141 to 121,374 cpm/μg of protein. The cell surface radioimmunoassay (CS-RIA) was carried out as described elsewhere (16). The extent of binding of MAb 5E6-G11-H2 to leishmanial parasites (promastigotes) was assessed by use of 125I-labeled anti-IgG1. Nonspecific IgG1 was used as a control. Optimal dilution of MAb and secondary Ab to perform CS-RIA was done as described elsewhere (29).
Preparation of heat-killed, sonicated, and formaldehyde-fixed UR6.
Heat-killed, formaldehyde-fixed, or sonicated forms of UR6 were prepared as described previously (29).
Priming of mice with various forms of UR6.
Mice were primed subcutaneously (s.c.) as well as through the intravenous (i.v.) or intraperitoneal (i.p.) route either with a graded number of live UR6 organisms or with its equivalent in the form of heat-killed, sonicated, or formaldehyde-fixed parasites in 0.5 ml of saline.
Preparation of parasite antigen.
Parasite antigen was prepared as described elsewhere (29). Briefly, stationary-phase promastigotes were harvested, washed three times in phosphate-buffered saline (PBS), and then resuspended in PBS at a concentration of 5 × 108 promastigotes per ml. The material was frozen and thawed five times in liquid nitrogen and then sonicated three times (for 30 s at maximum output with a 1-min interval between each sonication) on a Labsonic-2000 sonicator (Labonic L; B. Braun Melsungen AG, Melsungen, Germany). The sonicated material was then centrifuged at 2,000 rpm for 10 min, and the resulting supernatant was designated crude soluble antigen (CSA).
Assay of superoxide (O2−) in Mφs.
Superoxide (O2−) generation in Mφs was determined by nitroblue tetrazolium (NBT) reduction assay (30). Briefly, thioglycolate-induced peritoneal Mφs were adjusted to 4 × 106 per ml in RPMI 1640 medium (GIBCO) containing 10% FCS. One hundred microliters of sonicated AG83 or UR6 (equivalent to 6 × 107 parasites/ml) was mixed with 200 μl of cell suspension (equivalent to 8 × 106 Mφs) and allowed to adhere to 22-mm-diameter glass coverslips. Mφs stimulated with lipopolysaccharide (LPS) (500 ng/ml; Sigma) were used as a positive control. After 3, 6, and 12 h of incubation at 37°C in 5% CO2 in moist air, NBT (0.5 mg/ml) was added and the coverslips were kept at 37°C for 1 h. The reaction was terminated with 200 μl of cold PBS, and cells were fixed with ice-cold methanol and finally stained with 0.5% safranin. Mφs showing blue color were scored, and results were expressed as percent NBT-positive cells.
Assay of antileishmanial DTH response.
The delayed-type hypersensitivity (DTH) response in the infected and protected groups of BALB/c mice was studied using CSA as an antigen as described elsewhere (9).
Infection of Mφ culture.
Splenic mononuclear cells isolated from BALB/c mice (40) were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 12 mM HEPES, and 50 U of gentamicin per ml (complete medium) at a concentration of 106 cells/ml. The cells (4 ml) were allowed to adhere to 60-mm-diameter petri dishes at 37°C in 5% CO2 in moist air. Three hours after plating, the petri dishes were washed with warm RPMI 1640 medium to remove nonadherent cells and were reincubated in complete medium. L. donovani AG83 or UR6 promastigotes (5 × 107) were added to the culture at a parasite/Mφ ratio of 20:1. The petri dishes were kept at 37°C for 4 h to allow for internalization of parasites and infected cultures were then thoroughly washed with warm RPMI solution to remove free parasites and then incubated in complete medium at 37°C for different time periods (12, 18, and 24 h). Culture supernatant was collected and assayed for nitrite and tumor necrosis factor alpha (TNF-α). Cultures without added parasites were run in parallel.
Measurement of nitrite production.
Nitrite production in Mφ culture supernatant was assayed by the Griess reaction (13) using the nitric oxide assay kit of Boehringer Mannheim. In brief, 80 μl of RPMI 1640 medium was incubated with 50 μl of 1% sulfanilide and 50 μl of 1% n-1-naphthylethylenediamine dihydrochloride in 2.5% H3PO4 in the presence of nitrate reductase (10 μl of a 1-U/ml solution) at room temperature for 15 min. Nitrite was quantitated by measuring the optical density at 550 nm against a standard solution of sodium nitrite. Nitrite production in LPS-stimulated cultured Mφs was taken as the positive control.
Estimation of released TNF-α.
TNF-α production in Mφ culture supernatant was measured with the Factor-Test-X mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit in a solid-phase ELISA using the multiple-antibody sandwich principle (32). Released TNF-α was quantitated at 450 nm against a murine TNF-α standard. TNF-α production in Staphylococcus aureus Cowan 1-stimulated cultured Mφs was taken as the positive control.
Collection of sera and assay for antileishmanial IgG1 and IgG2a.
Sera were prepared from the clotted blood of five or six mice for analysis by enzyme immunoassay. The status of IgG1 and IgG2a in the sera of normal and infected mice was studied by enzyme immunoassay as described previously (10). MAb R616.7 and K123 were used as positive controls for mouse anti-IgG1 and anti-IgG2a, respectively.
Statistical analysis.
Results were expressed as means ± SDs for individual sets of experiments. In each experiment, about 5 to 10 animals were used in each group. Each experiment was performed three to six times, and the representative data from one set of these experiments are presented. The extent of variation between experiments was within 10%. A one- or two-tailed Student t test for analyzing significance was performed.
RESULTS
i.v. inoculation of UR6 and AG83 in BALB/c mice.
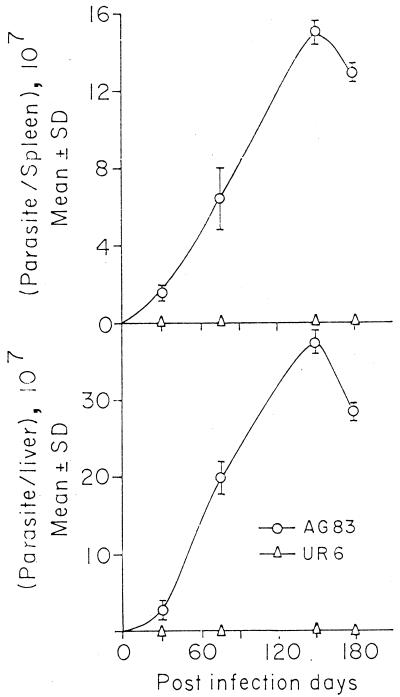
BALB/c mice (groups of six) were inoculated i.v. with 2 × 107 AG83 amastigotes or UR6 promastigotes, and at 5 months after infection hepatic and splenic parasite burdens were determined. It was observed that the parasite burden was very high in the case of AG83-infected BALB/c mice, whereas UR6-infected BALB/c mice failed to show any detectable parasites in the spleen and liver (Fig. 1).
FIG. 1.
Parasite burdens in spleens and livers of BALB/c mice in a progressive infection after inoculation with AG83 amastigotes and UR6 promastigotes through the tail vein.
UR6-induced cutaneous lesions in BALB/c mice.
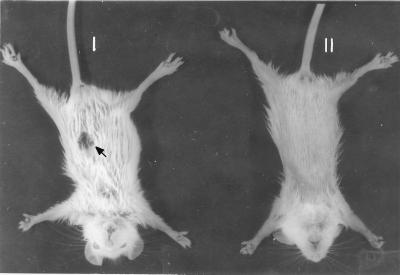
Seven BALB/c mice were inoculated with graded doses of UR6 (107, 108, and 109) s.c. It was observed that UR6 induced a very short-lived, self-healing skin lesion (Fig. 2), and the size of the lesion was directly proportional to the size of the UR6 inoculum. On increasing the number of UR6 parasites inoculated, the size of the lesion also increased. The lesion lasted for about 3 to 4 days and then tended to heal. No detectable parasites could be observed in the visceral organs of these mice throughout the period of investigation.
FIG. 2.
Skin lesions in BALB/c mice in response to s.c. challenge with live UR6. I, lesion at site of injection; II, healing response.
Ability of L. major-specific MAb (5E6-G11 and 2G11-H2) to bind to leishmanial parasites.
The ability of the L. major-specific MAb 5E6-G11 and 2G11-H2 to bind to leishmanial parasites was studied. It was observed that MAb 5E6-G11 showed maximum binding to L. major and minimum binding to AG83, with an intermediate level of binding to UR6. On the other hand, MAb 2G11-H2 showed maximum binding to L. major and essentially comparable levels of binding to UR6 and AG83 (Table 1).
TABLE 1.
Binding of L. major-specific MAb 5E6-G11 and 2G11-H2 to leishmanial parasites
| Ab | Relative binding (mean cpm ± SD) to:
|
||
|---|---|---|---|
| L. major Friedlin | Leishmania strain UR6 | L. donovani AG83 | |
| 5E6-G11 | 17,400 ± 823.07 | 14,327 ± 3,672 | 9,360 ± 246.00 |
| 2G11-H2 | 20,286 ± 5,532.40 | 14,441 ± 2,442 | 14,232 ± 420.00 |
| Nonspecific IgG1 | 3,831 ± 156.90 | 4,201 ± 637.80 | 4,007 ± 134.35 |
Priming of mice with live UR6 s.c. before virulent challenge and determination of splenic parasite burden.
The ability of UR6 to protect against virulent L. donovani (AG83) challenge was studied. BALB/c mice were primed with graded doses of UR6 as indicated in Table 2. The mice were divided into five groups (A through E; eight mice in each group). In groups B through E, each mouse was injected with UR6 twice at a 15-day interval, and 15 days after the second injection, animals were infected with live AG83 amastigotes. At 83 days postinfection, animals were sacrificed and the splenic parasite burden was determined. Group A mice received saline instead of UR6, whereas group E mice received only UR6. The mice receiving 107 (group B), 108 (group C), and 109 (group D) UR6 organisms showed 36, 67.6, and 95.4% reductions in the splenic parasite burden, respectively, compared to the infected controls (group A). As expected, mice receiving only UR6 (group E), failed to show any detectable parasites in the spleen.
TABLE 2.
Comparison of promastigote recovery in in vitro culture and parasite burdens in spleens of mice after s.c. priming with graded doses of live UR6 before infection with L. donovani AG83
| Group | Priming with live UR6
|
Infection with AG83 on day 0 | Splenic parasite burden (mean ± SD) | % Reduction in splenic parasite burden on day 83 with respect to group A | No. of parasites transformed in vitro culture (mean ± SD) | % Reduction in transformed promastigotes with respect to group A in in vitro culture | |
|---|---|---|---|---|---|---|---|
| Day 30 | Day 15 | ||||||
| A | + | (1.5 ± 0.2) × 107 | (4.8 ± 0.12) × 107 | ||||
| B | 107 | 107 | + | (9.6 ± 0.21) × 106 | 36.0a | (3.1 ± 0.1) × 107 | 35.41c |
| C | 108 | 108 | + | (4.86 ± 0.09) × 106 | 67.6b | (1.0 ± 0.09) × 107 | 79.16c |
| D | 109 | 109 | + | (6.8 ± 0.08) × 105 | 95.4c | (3.5 ± 0.04) × 106 | 92.70c |
| E | 109 | 109 | − | NDd | ND | ||
P < 0.01.
P < 0.005.
P < 0.0005.
ND, parasite not detected.
The number of amastigotes in the spleen in relation to transformed promastigotes recovered from the infected spleen (groups A through E) cultured for 96 h in the medium was calculated as described elsewhere (40). It was observed that the percent reductions in the splenic parasite burdens for amastigotes (as evident from the stamp smear) and promastigotes (as evident from the cultured splenic sections) went essentially hand in hand (Table 2).
Priming with live, sonicated, heat-killed, and formaldehyde-fixed UR6 s.c. before virulent challenge.
Ten mice in each group were immunized s.c. with either 109 live UR6 organisms (group B), 109 sonicated UR6 organisms (group C), 109 heat-killed UR6 organisms (group D), or 109 formaldehyde-fixed UR6 organisms (group E). The animals were primed three times at 15-day intervals, and 30 days after the last priming, animals were infected with virulent AG83 parasites. Mice (groups A through E) were sacrificed on day 90 postinfection, and the liver and splenic parasite burdens were determined (Table 3). It was evident that although all three forms of UR6 tested provided essentially comparable levels of protection, the sonicated UR6 was marginally more effective (the reductions in the splenic and hepatic parasite burdens were 99.5 and 93.68%, respectively). For the rest of the experiments, 109 sonicated UR6 organisms were used.
TABLE 3.
Parasite burdens in spleens and livers of BALB/c mice after priming with various forms of UR6 before infection with L. donovani AG83a
| Group | Priming with 109 UR6 organisms prior to infection with AG83 | Parasite count (mean ± SD) in:
|
% Reduction in parasite burden with respect to group A in:
|
||
|---|---|---|---|---|---|
| Spleen | Liver | Spleen | Liver | ||
| A | (2.70 ± 0.966) × 107 | (5.13 ± 0.66) × 107 | |||
| B | Live UR6 | (2.58 ± 1.06) × 105 | (6.00 ± 2.70) × 106 | 99.04b | 88.30e |
| C | Heat-killed UR6 | (4.80 ± 2.30) × 105 | (1.33 ± 0.834) × 107 | 98.22b | 74.03d |
| D | Formaldehyde-fixed UR6 | (2.28 ± 0.98) × 106 | (3.20 ± 1.14) × 106 | 91.55c | 93.76e |
| E | Sonicated UR6 | (1.35 ± 0.35) × 105 | (1.35 ± 0.77) × 105 | 99.50b | 93.68e |
All groups received AG83 on day 0, and splenic and liver parasite burdens were counted on day 90. Mice were primed with UR6 in days 60, 45, and 30 before infection.
P < 0.01.
P < 0.025.
P < 0.005.
P < 0.0005.
Priming of mice with sonicated UR6 through intracardiac i.v., i.p., and s.c. routes.
Eight mice in each group were primed through intracardiac i.v. (group B), i.p. (group C), and s.c. (group D) routes, and the mice were infected with amastigotes after the last injection and sacrificed 180 days after infection. Injection of UR6 promastigotes s.c. induced 100% immunity, in terms of both liver and splenic parasite burdens, against the high-dose challenge with virulent AG83. Although this immunity was not seen in the case of i.v. immunization with UR6 promastigotes, the i.p. route afforded an intermediate degree of protection (62.69% for spleen and 95.50% for liver) (Table 4).
TABLE 4.
Parasite burdens in spleens and livers of BALB/c mice after priming through various routes before infection with L. donovani AG83a
| Group | Route used for priming | Parasite count (mean ± SD) in:
|
% Reduction in parasite burden with respect to group A in:
|
||
|---|---|---|---|---|---|
| Spleen | Liver | Spleen | Liver | ||
| A | (1.30 ± 0.22) × 108 | (2.87 ± 0.69) × 108 | |||
| B | i.v. | (1.44 ± 0.95) × 108 | (6.27 ± 3.25) × 108 | 0b | 0c |
| C | i.p. | (4.85 ± 0.55) × 107 | (1.28 ± 0.19) × 107 | 62.69d | 95.50d |
| D | s.c. | (2.53 ± 0.98) × 105 | 0 | 99.80d | 100d |
All groups received AG83 on day 0, and splenic and liver parasite burdens were counted on day 180. Mice were primed with UR6 on days 90, 60, and 30 before infection.
P < 0.025.
P < 0.05.
P < 0.005.
Use of live or sonicated UR6 as an immunoprophylactic agent.
Thirty-day-infected mice were divided into three groups (nine mice in each group). Group B mice were injected with 109 live UR6 organisms, group C mice were injected with 109 sonicated UR6 organisms, and group A infected mice received only saline. All three groups were sacrificed on day 165 after infection, and splenic and liver parasite burdens were determined. Mice receiving live UR6 (group B) showed reductions in the splenic and liver parasite burdens to 97.46 and 82.7%, respectively, with respect to saline-treated infected control group (group A). Similar results were obtained for mice receiving sonicated UR6 (group C) (Table 5).
TABLE 5.
Parasite burdens in spleens and livers of BALB/c mice given live or sonicated UR6 after infection with L. donovani AG83a
| Group | Priming of infected mice with 109 UR6 organisms | Parasite count (mean ± SD) in:
|
% Reduction in parasite burden with respect to group A in:
|
||
|---|---|---|---|---|---|
| Spleen | Liver | Spleen | Liver | ||
| A | (1.50 ± 0.10) × 108 | (3.70 ± 0.26) × 108 | |||
| B | Live UR6 | (3.80 ± 1.25) × 106 | (6.40 ± 0.36) × 107 | 97.46b | 82.70b |
| C | Sonicated UR6 | (6.20 ± 0.37) × 106 | (5.71 ± 2.81) × 107 | 95.66b | 84.56b |
All groups received AG83 on day 0, and splenic and liver parasite burdens were counted on day 165. Mice were primed with UR6 on days 30, 60, and 90 after infection.
P < 0.005.
Superoxide generation in vitro in Mφs in response to L. donovani UR6 or AG83 promastigotes.
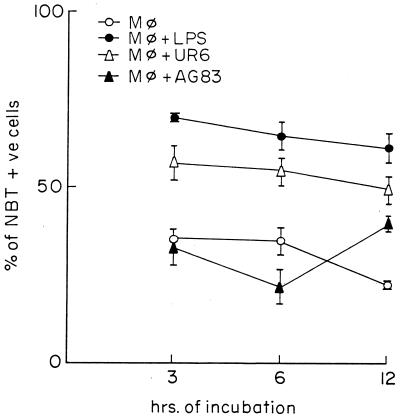
Peritoneal Mφs of BALB/c mice were incubated for 3 h, 6 h, and 12 h with sonicated UR6 or AG83 promastigotes, and the extent of superoxide generation was assayed by NBT reduction assay. LPS was used as a nonspecific stimulator of Mφs, and it was observed that LPS stimulated the maximum level of O2− generation in Mφs as evident from the percentage of NBT-positive cells. Incubation with sonicated UR6 promastigotes caused a significantly higher number of NBT-positive cells than incubation with AG83 promastigotes. When Mφs were incubated with medium alone, a basal level of NBT-positive cells was observed (Fig. 3).
FIG. 3.
Superoxide (O2−) generation (in terms of NBT reduction) in peritoneal Mφs of BALB/c mice. Mφs were stimulated or not with LPS or incubated in presence of UR6 or AG83. + ve, positive. Results are means and SDs.
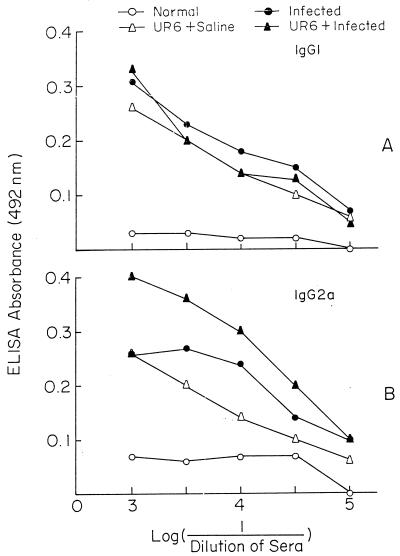
Antileishmanial DTH response and status of antileishmanial antibody in sera of infected and UR6-immunized BALB/c mice.
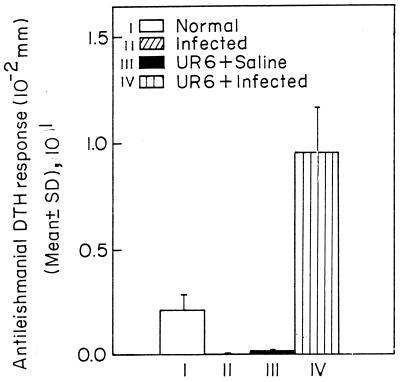
Normal BALB/c mice were divided into four groups (groups I through IV; 7 mice in each group). Group III and IV mice were injected thrice at 15-day intervals with 109 sonicated UR6 organisms, while group I and II mice were injected thrice at 15-day intervals with normal saline. Fifteen days after the last injection, group II and IV mice were challenged with live AG83 promastigotes, while group I and III mice received saline. After 120 days of infection, using L. donovani CSA, DTH was measured with a dial calliper. DTH was expressed as the absolute footpad thickness increase, in 10−2 mm (Fig. 4). In each experiment, group I mice were immunized with the test antigen. The increase in footpad thickness at 24 h in these mice was taken as the background footpad swelling caused by the eliciting antigen alone. All DTH data (specific DTH) are corrected for the background reading. The DTH responses in groups II, III, and IV were 0.21 ± 0.06, 0, 0.02 ± 0, and 0.95 ± 0.22, respectively (Fig. 4).
FIG. 4.
Antileishmanial DTH response in the BALB/c mice. I, normal mice; II saline-treated infected mice; III, normal mice primed with 108 UR6 organisms; IV, infected mice primed with 108 UR6 organisms. Sonicated UR6 was given to the primed animals as described in the text. Animals were injected with UR6 on days 0, 15, and 30, and 15 days after the last injection, animals were injected with L. donovani amstigotes.
The antileishmanial antibody isotype IgG2a and IgG1 status in the sera of all of the above four groups was studied. It was observed that antileishmanial IgG1 levels were comparable in groups II, III, and IV (Fig. 5A). On the other hand, antileishmanial IgG2a was found to be much elevated (Fig. 5B) in the UR6-primed infected group (i.e., the protected group [group IV]) compared to groups II and III.
FIG. 5.
Antileishmanial isotypes present in the sera of BALB/c mice.
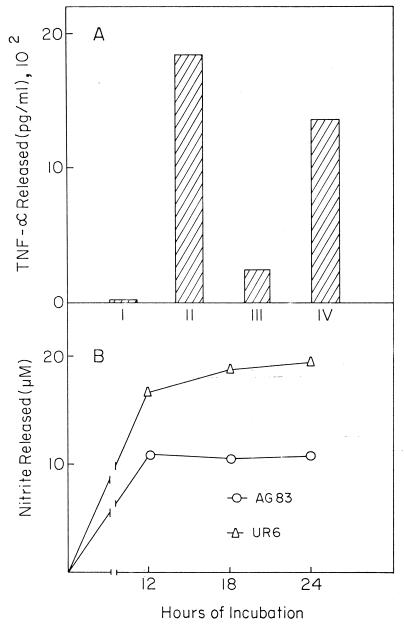
Release of TNF-α.
UR6 promastigotes were able to induce a 68-fold increase in TNF-α production in cultured Mφs. On the other hand, AG83 induced only a 12.5-fold increase in TNF-α formation. In the control experiments, S. aureus Cowan 1 caused a 92-fold increase in nitrite formation (Fig. 6A).
FIG. 6.
TNF-α (A) and nitric oxide (B) induction from BALB/c Mφs stimulated with UR6 or AG83. Released TNF-α and nitric oxide were measured as indicated in the text. (A) TNF-α released by BALB/c Mφs alone (I) or stimulated with UR6 (III) or AG83 (IV) was compared to that released by Mφs stimulated with S. aureus Cowan 1 (1,850 pg/ml) (II) (B) Nitric oxide released by BALB/c Mφs stimulated with AG83 or UR6 was corrected for the background stimulation with Mφs alone. For comparison, the nitric oxide released by LPS-stimulated Mφs was 23.8, 22.0, and 23.7 μM at 12, 18, and 24 h, of incubation, respectively. The data shown represent one experiment of five.
Nitrite production in cultured Mφ supernatant.
Incubation of murine Mφs with UR6 promastigotes induced a 2.5-fold increase in nitrite, compared to the 1.8-fold increase when Mφs were challenged with AG83 promastigotes (Fig. 6B). LPS caused a 3.2- to 3.8-fold increase in nitrite formation.
DISCUSSION
The present study was under taken to assess the protective ability of the atypical leishmanial parasite UR6 against virulent challenge with L. donovani AG83 in BALB/c mice. To date prophylactic immunization against VL has required either the use of an adjuvant (20) or the establishment of prior infection with a low dose of the parasite (26). Without the use of adjuvants like CFA, glucan, BCG, or liposomes, the efficacy was found to be reduced quite dramatically. Although substantial protective immunity could be induced by prophylactic immunization with gamma-irradiated Leishmania tropica promastigotes (21), irradiated promastigotes are not suitable as the basis of a vaccine. The importance of the present study is in establishing the fact that mice immunized with an atypical promastigote (UR6) without adjuvant can acquire long-lasting resistance against relatively large infecting challenges that are otherwise lethal due to systemic disease.
The leishmanial parasite UR6, unlike AG83, was unable to visceralize (Fig. 1) but was able to induce a short-lived, self-healing skin lesion in the BALB/c mice (Fig. 2) and showed cross-reactivity with the L. major-specific MAb 5E6-G11 and 2G11-H2 (Table 1). Previously Sacks et al. (39) have shown that L. tropica, a species historically associated with CL, caused a mild VL. Earlier, we have demonstrated that UR6 priming in hamsters offered protection against virulent L. donovani challenge (29). Hence, we became interested in studying the efficacy of UR6 as an immunoprophylactic immunotherapeutic agent in mice.
The UR6-mediated protection was found to be dependent on the immunization regimen, i.e., (i) the size of the challenge, (ii) the number of immunizations, and (iii) the form of the parasite. It was observed that priming with increasing numbers (107 to 109) of UR6 parasites resulted in a 36 to 95% reduction in the splenic or liver parasite burden (Table 2). When BALB/c mice were primed with different forms of UR6 (live, sonicated, heat-killed, or formaldehyde-fixed UR6) before infection, the reductions in splenic and liver parasitemias were in the range of 91 to 99% and 88 to 93%, respectively. It was observed that the priming with the sonicated form of UR6 resulted in the maximum reduction in both splenic (99%) and hepatic (93%) parasite burdens (Table 3). The best route of priming was seen to be multiple s.c. immunization with equivalent to 109 UR6 organisms (Table 4). The most effective regimen was delineated as multiple s.c. immunization with 109 UR6 organisms in either the live or the sonicated form. This is in agreement with the report that s.c. inoculation of L. major conferred a high degree of protection in CBA mice (38), although Liew et al. have reported opposite results (24). This discrepancy in the results may be due to the difference in the mouse strains used in the experiments. The percent reduction in the splenic parasite burden was further validated by comparison with the recovered promastigotes from the in vitro culture of the spleens from the same group of mice (Table 2). It was further observed that UR6 priming was also effective in established infection models (Table 5).
Precisely how parasites are killed within activated Mφs is presently unclear. Mφ activation by lymphokines results in an array of physiological and metabolic changes in the host cell, some of which might theoretically contribute to the antileishmanial effects (1). Evidence has been provided that the enhanced capacity of the activated Mφs to exert antileishmanial effects is closely correlated with the ability of these cells to secrete high levels of reactive oxygen intermediates (8). Since UR6 induces O2− generation in Mφs, it is plausible that UR6 priming induced O2− generation and that this resulted in the destruction of the intracellular parasites.
It has been shown that reactive nitrogen intermediates are major effector molecules in the inhibition of intracellular proliferation of L. major (8). The innate resistance of mice to infection with L. major can be well correlated with the induction of Mφ nitrate synthase for synthesis of nitric oxide (NO) by gamma interferon and TNF-α (6). Studies on Mφs from Lshr and Lshs congenic mice infected with L. donovani amastigotes prior to priming and activation with recombinant gamma interferon and LPS revealed a direct correlation between TNF-α release and nitrate production, indicating a definite autocrine role for TNF-α in production of reactive nitrogen intermediates and leishmanicidal activity (6). We have observed that nitrite and TNF-α production was higher in the cultures of UR6-activated Mφs than in those activated by AG83 promastigotes. Moreover, although only a basal level of nitrite was produced on Mφ activation by AG83 over a period of time, nitrite production slowly increased with time in the cultures of UR6-activated Mφs. There was a direct correlation between TNF-α release and nitrite production by UR6-activated Mφs.
Patients with CL and VL recover after drug treatment and gain resistance against reinfection as the antibody titer decreases and cell-mediated immunity is expressed, as assessed by skin reactivity to parasite antigens (7, 35). The preferential expansion of functionally distinct CD4+ T-cell subsets that are highly polarized to either the Th1 or Th2 pattern determines the outcome of the disease. In order to assess the T-cell clones implicated in UR6-mediated protection, we assayed for Th1 and Th2 cells indirectly in terms of the immunoglobulin isotype compartment. Th1 and Th2 cells help B cells to produce IgG2a and IgG1, respectively (33, 44). Susceptible mice infected with L. major mount a Th2 response and produce IgG1 antibodies, whereas the resistant mice suppress this activity and enhance IgG2a responses (7). Some of our previous studies with L. donovani indicated that both Th1 and Th2 cells coexist in the spleens of infected BALB/c mice or the relatively susceptible C57BL/6 mice, whereas the antileishmanial T-cell response was biased towards a Th1 response in the resistant B6C-H-2Bm12 strain (4, 42). IgG1 titers increased in uncontrolled infections and remained lower in association with regression (Fig. 5). On the other hand, IgG2a titers were higher in the case of UR6-protected animals. The effective stimulation of the IgG2a isotype has thus been associated with the presence of antileishmanial Th1 cells in the repertoire. Here, UR6 priming in infected mice allowed the preferential expansion of Th1 cells.
A positive DTH response towards leishmanial antigens is an indication of cell-mediated immunity, and there are reports of an association between the DTH response and protection in the L. major system (11, 19, 41). Development of DTH in UR6-immunized infected mice, in contrast with its suppression in nonimmunized controls, coincided with control or progression of disease in the respective groups. Thus, generation of suppression of DTH in response to L. donovani infection in BALB/c mice is abrogated by effective prophylactic immunization.
Our method of priming was quite distinct and novel because it did not require any adjuvant, unlike the case for other systems. In other systems, Corynebacterium parvum (17) or glucan (20) was used as an adjuvant with leishmanial parasites. Ali and Afrin have even demonstrated the use of neutral (3) and positively charged (2) liposomes as adjuvants in experimental visceral leishmaniasis in both hamster and mouse models. Recently, Rivier et al. (38) obtained 39 to 53% protection conferred by gp63 in the absence of adjuvant, which again increased (64 to 68%) in the presence of an adjuvant like C. parvum or BCG37. In experimental L. donovani infection the extent of protection of reported by others (2, 3, 20, 25, 36) was up to 60 to 80%, and in our case the maximum reduction in parasite burden that could be achieved in the murine model was 95 to 99% in the spleen and 80 to 90% in the liver. Previously we have shown that priming of hamsters with either live or sonicated UR6 in the absence of any adjuvant provided strong protection against virulent challenge. UR6-mediated protection was also observed in hamsters with established infection. Further, UR6-primed infected hamsters displayed a greatly extended life span compared to infected hamsters (29). To our knowledge, this is the first report showing that an attenuated parasite can be used for immunotherapy in the murine model, in the absence of an adjuvant, against experimental VL. Studies are under way to prime mice in combination with immunostimulatory agents such as BCG together with UR6 to eliminate the residual parasites.
ACKNOWLEDGMENT
We thank Vineeta Bal, NII, New Delhi, India, for conducting ELISAs.
REFERENCES
- 1.Adams D O, Hamilton T A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 2.Afrin F, Ali N. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect Immun. 1997;65:2371–2377. doi: 10.1128/iai.65.6.2371-2377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali N, Afrin F. Protection of mice against visceral leishmaniasis by immunisation with promastigote antigen incorporated in liposomes. J Parasitol. 1997;83:70–75. [PubMed] [Google Scholar]
- 4.Basak S K, Saha B, Bhattacharya A, Roy S. Immunobiological studies on experimental visceral leishmaniasis. II. Adherent cell-mediated down-regulation of delayed-type hypersensitivity response and up-regulation of B-cell activation. Eur J Parasitol. 1992;22:2041–2045. doi: 10.1002/eji.1830220813. [DOI] [PubMed] [Google Scholar]
- 5.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell J M. Genetic susceptibility to leishmanial infections: studies in mice and man. Parasitology. 1996;112(Suppl.):S67–S74. [PubMed] [Google Scholar]
- 7.Blackwell J M, Roberts B, Alexander J. Response of BALB/c mice to leishmanial infection. Curr Top Microbiol Immunol. 1985;122:97–106. doi: 10.1007/978-3-642-70740-7_15. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan C, Rollinghoff M. The immune response to Leishmania: mechanisms of parasite control and evasion. Int J Parasitol. 1998;28:121–134. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 9.Dhaliwal J S, Liew F Y, Cox F E G. Specific suppressor T cells for delayed-type hypersensitivity in susceptible mice immunized against cutaneous leishmaniasis. Infect Immun. 1985;49:417–423. doi: 10.1128/iai.49.2.417-423.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frinkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P, Park L S, Schooley K A, Coffman R L, Monsmann T R, Paul W E. Lymphokine control of in vivo immonuglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 11.Frommel D, Lagrange P M. BCG: a modifier of immune responses to parasites. Parasitol Today. 1989;5:188–190. doi: 10.1016/0169-4758(89)90143-9. [DOI] [PubMed] [Google Scholar]
- 12.Graham P C C. Introduction. In: Peters W, Killick-Kendrick R, editors. The leishmaniasis in biology and medicine. New York, N.Y: Academic Press; 1987. [Google Scholar]
- 13.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Grob P M, David E, Warren T C, DeLeon R P, Farina P R, Homon C A. Characterization of a receptor for human monocyte-derived neutrophil chemotactic factor/interleukin-8. J Biol Chem. 1990;265:8311–8316. [PubMed] [Google Scholar]
- 15.Grogl M, Thompson T N, Franke E D. Drug resistance in leishmaniasis. I. Its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992;47:117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- 16.Grunow R, D'Apuzzoo M, Wyss-Coray T, Frutig K, Pichler W J. A cell surface ELISA for the screening of monoclonal antibodies to antigens on viable cells in suspension. J Immunol Methods. 1994;171:93–102. doi: 10.1016/0022-1759(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 17.Handman E. Leishmania vaccines: old and new. Parasitol Today. 1997;13:236–238. doi: 10.1016/s0169-4758(97)01060-0. [DOI] [PubMed] [Google Scholar]
- 18.Hart D T, Vickerman K, Coombs G H. A quick, simple method for purifying Leishmania mexicana amastigotes in large numbers. Parasitology. 1981;82:345–355. doi: 10.1017/s0031182000066889. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel F P, Sadick M D, Holday B J, Coffman R L, Locksley R M. Reciprocal expression of interferonγ or interleukin 4 during the resolution of progression of murine leishmaniasis. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holbrook T W, Cook J A, Parker B W. Immunisation against Leishmania donovani: glucan as an adjuvant with killed promastigotes. Am J Trop Med Hyg. 1981;30:762–768. doi: 10.4269/ajtmh.1981.30.762. [DOI] [PubMed] [Google Scholar]
- 21.Howard J G, Liew F Y, Hale C, Nicklin S. Prophylactic immunisation against experimental leishmaniasis. II. Further characterisation of protective immunity against fatal Leishmania tropica infection induced by irradiated promastigotes. J Immunol. 1984;132:450–455. [PubMed] [Google Scholar]
- 22.Jackson J E, Tally J D, Ellis W Y, Mebrahtu Y B, Lawyer P G, Were J B, Reed S G, Panisko D M, Iimmer B L. Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania spp. from patients unresponsive to pentavalent antimony therapy. Am J Trop Med Hyg. 1990;39:464–480. doi: 10.4269/ajtmh.1990.43.464. [DOI] [PubMed] [Google Scholar]
- 23.Liew F Y. Role of cytokines un killing of intracellular pathogens. Immunol Lett. 1991;30:193–197. doi: 10.1016/0165-2478(91)90024-5. [DOI] [PubMed] [Google Scholar]
- 24.Liew F V, Hale C, Howard J G. Prophylactic immunisation against experimental leishmaniasis. IV. Subcutaneous immunisation prevents the induction of protective immunity against fatal Leishmania major infection. J Immunol. 1985;135:2095–2101. [PubMed] [Google Scholar]
- 25.Mansohn-Bahr P E C. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell G F, Curtis J M, Handman E. Resistance to cutaneous leishmaniasis in genetically susceptible BALB/c mice. Aust J Exp Biol Med Sci. 1981;59:555–565. doi: 10.1038/icb.1981.48. [DOI] [PubMed] [Google Scholar]
- 27.Modabber F. TDR Twelfth Programme Report. Geneva, Switzerland: World Health Organization; 1995. Leishmaniasis; p. 195. [Google Scholar]
- 28.Modabber F. TDR Ninth Programme Report. Geneva, Switzerland: World Health Organization; 1992. Leishmaniasis; pp. 77–87. [Google Scholar]
- 29.Mukhopadhyay S, Sen P, Bhattacharya S, Mazumdar S, Roy S. Immunoprophylaxis and immunotherapy against experimental visceral leishmaniasis. Vaccine. 1998;17:289–296. doi: 10.1016/s0264-410x(98)90017-2. [DOI] [PubMed] [Google Scholar]
- 30.Murray H W. Susceptibility of Leishmania to oxygen intermediates and killing of normal macrophages. J Exp Med. 1981;153:1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray H W, Berman J D, Wright S D. Immunochemotherapy for intracellular Leishmania donovani infection: gamma interferon plus pentavalent antimony. J Infect Dis. 1988;157:973–978. doi: 10.1093/infdis/157.5.973. [DOI] [PubMed] [Google Scholar]
- 32.Neta R, Oppenheim J J, Douches S D. Interdependence of the radio protective effects of human recombinant interleukin-1 alpha, tumor necrosis factor alpha, granulocyte colony stimulating factor, and murine recombinant granulocyte-macrophage colony stimulating factor. J Immunol. 1988;140:108–111. [PubMed] [Google Scholar]
- 33.Noben T N, Kroof P, Muller I. Susceptibility to Leishmania major infection in Interleukin 4 deficient mice. Science. 1996;271:987–999. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 34.Nossal G J V. Triumphs and trials of immunology in 1980s. Immunol Today. 1988;9:286–291. doi: 10.1016/0167-5699(88)91316-3. [DOI] [PubMed] [Google Scholar]
- 35.Pearson R D, Wheeler D A, Harrison L H, Kay H D. The immonubiology of leishmaniasis. Rev Infect Dis. 1983;5:907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- 36.Rachamin N, Jaffe C L. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J Immunol. 1993;150:2322–2331. [PubMed] [Google Scholar]
- 37.Ray J C. Cultivation of various leishmania parasites on solid medium. Ind J Med Res. 1932;20:355–357. [Google Scholar]
- 38.Rivier D, Shah R, Bovay P, Mauel J. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasite Immunol. 1993;15:75–84. doi: 10.1111/j.1365-3024.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 39.Sacks D L, Kenney R T, Kreutzer R D, Jaffe C L, Gupta A K, Sharma M C, Sinha S P, Neva F A, Saran R. Indian kala-azar caused by Leishmania tropica. Lancet. 1995;345:959–961. doi: 10.1016/s0140-6736(95)90703-3. [DOI] [PubMed] [Google Scholar]
- 40.Saha B, Roy H, Pakrashi A, Chakraborty R N, Roy S. Immunological studies on experimental visceral leishmaniasis. I. Changes in lymphoid organs and their possible role in pathogenesis. Eur J Immunol. 1991;21:577–581. doi: 10.1002/eji.1830210307. [DOI] [PubMed] [Google Scholar]
- 41.Scott P, Natoviz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T-cell lines that transfer immunity or exacerteation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:675–684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen E, Roy S. Immunobiological studies on experimental visceral leishmaniasis. V. The IABm12 mutation specifies resistance to infection. Scand J Immunol. 1998;47:431–435. doi: 10.1046/j.1365-3083.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- 43.Stauber L A. Host resistance to the Khartoum strain of Leishmania donovani. Rice Inst Pam. 1958;45:80–96. [Google Scholar]
- 44.Stevens T, Bossie A, Sandersv V. Regulation of antibody isotype secretion by subsets of Ag specific helper T-cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 45.Titus R G, Gueiros-Filho F I, de Frietas L A, Beverley S M. Development of a safe, live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci USA. 1995;92:10267–101271. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Regenmortel M H V. Structural and functional approaches to the study of protein antigenicity. Immunol Today. 1989;10:266–272. doi: 10.1016/0167-5699(89)90140-0. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. Health Situation, South East Asia Region, New Delhi. Geneva, Switzerland: World Health Organization; 1993. Implementation of the global strategys for health for all by the year 2000: second evaluation. [Google Scholar]