Abstract
Exercise-induced muscular overload can trigger delayed onset muscle soreness (DOMS). DOMS is related to the indiscriminate use of analgesics and nonsteroidal anti-inflammatory drugs without proper guidance, decreased physical exercise adherence and degenerating sports performance, increased risk of injury, and reduced muscle strength and function. Dietary anthocyanins have been extensively studied as potential natural treatments for DOMS, but the indication, dosage, and form of use remain highly variable. Therefore, this review aims to synergize and present evidence relating to the effect of anthocyanins on DOMS in clinical studies. Notably, the results of anthocyanin supplementation for DOMS were found to be inconclusive. The use of protocols with lower anthocyanin doses yielded better results than those with high-dose supplements, suggesting that anthocyanin-rich foods are more accessible as therapeutic tools, leading to the conclusion that these foods could be used to prevent and treat DOMS. However, consumption protocols for this purpose are not yet well established, and the answer is dependent on the methodological quality of future studies.
Keywords: dietary supplements, exercise, myalgia, phytochemicals
INTRODUCTION
Physical exercise has numerous well-known health benefits that have been scientifically proven in clinical trials; it reduces premature mortality and contributes to the prevention of several diseases (Booth et al., 2012; Warburton and Bredin, 2017). According to the standard guidelines, to reap the benefits of exercise, individuals should engage in at least 150∼300 min of moderate physical exercise per week or 75∼150 min of vigorous physical exercise per week in sessions lasting at least 10 min (Haskell et al., 2007; Guthold et al., 2018).
During exercise, muscle overload is applied, which can vary depending on the exercise duration, modality, stimulus speed, frequency, and several other factors (Peake et al., 2017). This muscle overload can cause homeostatic alterations and local microtrauma (Appell et al., 1992; Luti et al., 2020). The occurrence of local microtrauma usually triggers delayed onset muscle soreness (DOMS) (Armstrong, 1984; Tokinoya et al., 2020). Although research is being conducted to determine the role of DOMS in the muscle adaptation process, such mechanisms are still in their beginning (Schoenfeld, 2012). Regarding the aspects related to athletic performance, the literature is vast in addressing harmful effects based on the magnitude and duration of DOMS, such as indiscriminate use of analgesics and nonsteroidal anti-inflammatory drugs without proper guidance, decreased physical exercise adherence and degenerating sports performance, increased risk of injury, and reduced muscle strength and function (Jalalvand et al., 2012; Pearcey et al., 2015; Lundberg and Howatson, 2018).
Lifestyle factors, such as hydration, adequate sleep, and proper eating habits, can optimize muscle recovery and minimize soreness (Evans, 1991; Quintero et al., 2018). In addition, dietary bioactive compounds have been extensively studied as important adjuvants to muscle recovery after exercise-induced muscle damage (EIMD) and may act to reduce DOMS owing to their potential antioxidant, anti-inflammatory, and regulatory activities (Ranchordas et al., 2017). These components are found in plant-based foods and comprise polyphenol groups, such as flavonoids, phytosterols, glucosinolates, and carotenoids (Angelo and Jorge, 2007).
Restoring homeostasis by consuming bioactive compound-rich foods can encourage the use of effective and safe strategies to control the adverse outcomes resulting from EIMD, such as muscle soreness. Among the several varieties of bioactive compounds, anthocyanin supplementation-one of the main classes of flavonoids-is widely studied for its supposed effects on muscle recovery (Bongiovanni et al., 2020), but the indication, dosage, and form of use remain highly variable, making it difficult to truly understand the role of these supplements and their indications based on the level of evidence. Therefore, this narrative review aims to gather and discuss available evidence about the effect of anthocyanins on DOMS in humans, as well as to identify the consistency of this information about the requirements for an effective and safe prescription.
MATERIALS AND METHODS
Despite being a narrative review, some search criteria were established to allow for the tracking of studies that would answer the main objective of this study. The research on the effect of anthocyanins on DOMS was compiled using the PubMed, Virtual Health Library, and Google Scholar databases and included works published between 2010 and 2021. However, the mechanisms and concepts involving DOMS have been described without time restrictions.
Various combinations of the following terms were performed: “muscle damage” OR “muscle damage induced by exercise” AND “supplementation” OR “nutrition” AND “antioxidants” OR “anthocyanins”. In all research platforms, the papers were selected based on the following criteria: 1) intervention studies conducted with humans; 2) mandatory assessment of muscle soreness; and 3) containing the dosage of anthocyanins from the supplements and food used for the intervention. The selected articles were presented and discussed in the topic “Anthocyanin-rich foods and supplements to prevent or treat Delayed Onset Muscle Soreness” of this review.
For the other topics of this review, the papers and information included were not obtained from a specific search criterion. However, all information was gathered from an extensive literature search and included in the present study to provide a broader and more complete view of the subject discussed.
RESULTS AND DISCUSSION
Mechanisms of DOMS
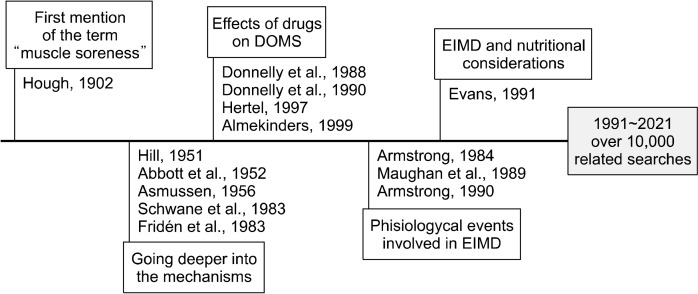
When searching for “delayed onset muscle soreness” on scientific websites, the oldest study that this term can track was conducted in 1983. However, performing a deeper search revealed that interest in this theme began in 1902 when the physician Dr. Hough (1902) published a work entitled “Ergographic studies in muscular soreness”. For the first time, soreness was suggested to be unrelated to fatigue intensity-which was already commonly studied at that time-but with muscle ruptures resulting from physical exercise. Since then, numerous authors have turned their attention to the mechanism of DOMS to clarify its etiology and several factors that permeate this mechanism.
Over the decades, the term has been increasingly used, and the nuances of this mechanism have been explored (Fig. 1) (Hough, 1902; Hill, 1951; Abbott et al., 1952; Asmussen, 1956; Fridén et al., 1983; Schwane et al., 1983; Armstrong, 1984; Donnelly et al., 1988; Maughan et al., 1989; Armstrong, 1990; Donnelly et al., 1990; Evans, 1991; Hertel, 1997; Almekinders, 1999). DOMS is now recognized as a phenomenon that can occur in situations of atypical exercise, resulting in ultra-structural muscle injury, for example, individuals who start an activity after a sedentary period and active people who change the intensity of training or participate in competitions that promote physical stimulation higher than usual (Hotfiel et al., 2018). Despite extensive research, the exact mechanism of DOMS remains unknown. The process of muscle damage and recovery associated with physical exercise involves complex structures and systems that require simplification. However, the most explored theory assumes that DOMS is caused by mechanical damage to skeletal muscle tissue after an exercise stimulus (Armstrong, 1990; Hotfiel et al., 2018).
Fig. 1.
Concepts and authors involved in the development of the first reported DOMS mechanisms. DOMS, delayed onset muscle soreness; EIMD, exercise-induced muscle damage.
Schwane et al.’s study (1983) is an important example of the mechanical damage theory of DOMS. They started from the whole base built on the concepts of DOMS at the time, investigating the hypothesis that exercises with a higher intensity of eccentric contraction (downhill run) triggered greater DOMS when compared with exercises with an equal volume of eccentric and concentric contractions (level run).
This reflection is crucial for developing study designs aimed at observing DOMS-related outcomes. The implementation of protocols with a predominance of eccentric contraction represents one of the ways of obtaining DOMS. Therefore, establishing adequate sample selection criteria to designate the ideal protocol with atypical exercises for these subjects is crucial. When the EIMD protocol causes sufficient overload in the skeletal musculature, the tissue repair response begins implicitly (Arnold et al., 2007).
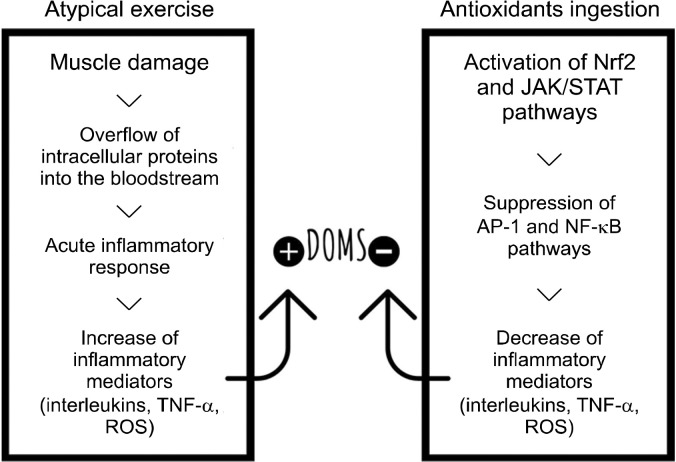
Following the rupture of the myofibrils involved in the contraction process, the tissues undergo structural and metabolic changes, increasing the flow of intracellular protein leakage into the bloodstream (Paulsen et al., 2012). These proteins function as trauma markers and favor the occurrence of an acute inflammatory response at the systemic level to promote tissue repair (Zaldivar et al., 2006).
Upon detecting muscle damage, macrophages are recruited to the muscle tissue, and in a quick sequence, the concentrations of inflammatory mediators and reactive oxygen species also increase (Paulsen et al., 2012). In addition to promoting the activation and feedback of oxidative and inflammatory pathways and favoring DOMS establishment, some mediators involved in this process, such as interleukins (ILs) and the tumor necrosis factor (TNF), are also capable of chemically sensitizing nociceptors-responsible structures for persistent pain manifestation (Rocha et al., 2007). DOMS can begin 8 h after an exercise and peak between 24 and 72 h, reducing gradually after this time (Armstrong, 1984; Zaldivar et al., 2006; Paulsen et al., 2012).
DOMS can be measured subjectively using a visual analog scale (VAS), which comprises a one-dimensional instrument, usually with a line from zero to 100 or 200 mm in which the extremes can represent “a few or no pain” and “intense, maximal pain” (Lau et al., 2015). DOMS may be clinically associated with touch sensitivity, muscle stiffness, local edema, and decreased muscle strength (Armstrong, 1990; Hotfiel et al., 2018). Fig. 2 depicts all possible DOMS mechanisms discussed in this topic.
Fig. 2.
Potential triggering mechanisms of DOMS and the role of dietary anthocyanins in this process. DOMS, delayed onset muscle soreness; TNF-α, tumor necrosis factor alpha; ROS, reactive oxygen species; Nrf2, nuclear factor-erythroid 2-related factor-2; JAK/STAT, Janus kinase/signal transducers and activators of transcription; AP1, activator protein-1; NF-κB, nuclear transcription factor kappa B.
Anthocyanins
Anthocyanins are widely distributed throughout the plant kingdom, being responsible for red, purple, and blue pigmentation in flowers, seeds, and fruits, such as red cabbage, eggplant, strawberry, blueberry, cherry, and acaí (Saigo et al., 2020). Anthocyanins, which are organic compounds that are soluble in water, are identified as secondary metabolites and are considered flavonoids (Khoddami et al., 2013), mainly due to their chemical structure. This structure is characterized by a 15-carbon skeleton based on a chrome ring with a second aromatic ring, by means of a single or additional sugar molecules, linked in several hydroxylated positions in the formation base (Pervaiz et al., 2017).
These sugar molecules are generally analogous to anthocyanidin (Sinopoli et al., 2019). For this reason, structural differences between anthocyanins are related to the number of hydroxyl groups, position, and type or number of sugars linked to the molecule (Peña-Sanhueza et al., 2017). In addition, anthocyanins can undergo structural changes according to environmental conditions, pH, temperature, light, solvent, and metal ions (Sinopoli et al., 2019). According to Chaves-Silva et al. (2018), over 650 different anthocyanin structures have been identified, all of which are derived from the six most common anthocyanidins (cyanidin, delphinidin, pelargonidin, malvidin, peonidin, and peturidine).
In the literature, more than 200 compounds of this class have been reported, and discussion about the possible beneficial effects of anthocyanin-rich foods on human health is linked to the high antioxidant activity of these substances and their potential role in modulating oxidative stress and proinflammatory processes (de Pascual-Teresa et al., 2010).
Anthocyanin-rich foods and supplements to prevent or treat DOMS
One of the theories that justify the use of anthocyanins for DOMS prevention and treatment starts from the idea that bioactive compound-rich foods help degrade the Kelch-like epichlorohydrin-associated protein 1 protein (Ma, 2013). This protein keeps the nuclear factor-erythroid 2-related factor-2 neutral, which, when active, migrates to the nucleus and interacts with the antioxidant response gene. This suppresses the pathways of nuclear transcription factor kappa B (NF-κB) and activator protein-1, increasing the expression of genes that regulate the inflammatory process (Nguyen et al., 2009).
Another possibility is that consuming anthocyanin-rich foods helps promote a direct increase in IL-10 concentrations (McAnulty et al., 2011; Tomé-Carneiro et al., 2012). IL-10 is cited as one of the main counter-regulating cytokines of the immune response. It primarily plays an anti-inflammatory role by significantly suppressing the synthesis of TNF-α, IL-6, IL-1, and other proinflammatory substances in monocytes and macrophages (Speretta et al., 2014). The production of proinflammatory mediators is interrupted by negative feedback when IL-10 binds to its receptor (IL-10R) and activates, in macrophages, the signaling pathway called Janus kinase/signal transducers and activators of transcription and activates the signal transducer and activator of transcription 3 (STAT3) by phosphorylation. STAT3, in turn, promotes the transcription of cytokine signaling suppressor 3, which minimizes NF-κB activity, as well as the release of stimulating substances from nociceptors (Fig. 2) (Riley et al., 1999; Murray, 2007).
Interventional studies with bioactive compounds at DOMS have grown significantly in recent years; however, the variety of foods and supplements evaluated is enormous, as well as the type of EIMD protocols implemented, and this can interfere with the conclusions and applicability of the results obtained. Bongiovanni et al. (2020) published a review that stratified the use of various supplements regarding muscle damage based on the level of evidence, including those rich in anthocyanins, such as beets and pomegranates. The authors used a broad search to select numerous supplements and foods in various preparations, with no detailed inclusion or exclusion criteria. Owing to the variability in dosages, administration form of supplements, and stated target audience, understanding their results and making accurate decisions are difficult; therefore, the focus of the current review was more specific, and all eight studies selected are summarized in Table 1.
Table 1.
Studies that investigated the effects of anthocyanin-rich supplements in the treatment and prevention of DOMS in the last 10 years (2010~2020)
| Reference | Supplement (placebo-controlled) | Study design | Subject (sample size calculation) | EIMD protocol | Result (treatment) |
|---|---|---|---|---|---|
| Trombold et al., 2011 | -Pomegranate juice: 500 mL a day, containing about 192 mg of anthocyanins -Placebo: the same amount of colored and artificially flavored juice |
-Double-blind, crossover experiment with 2 treatments, each lasting 15 days with a minimum 14-day washout period -EIMD protocol was performed on the 8th day of supplementation |
-17 healthy active men -No sample size calculation |
-3 sets of 20 unilateral eccentric elbow flexion+6 sets of 10 unilateral eccentric knee extension at 110% of unilateral 1RM -180 s of rest between sets |
-Decrease on elbow flexion strength -Increase on elbow flexion soreness -No differences in knee extensor muscles |
| Hutchison et al., 2016 | -Black currant nectar: 1,000 mL a day, containing about 630 mg of anthocyanins -Placebo: the same amount of black cherry powder juice |
-Double-blind, placebo-controlled study, lasting 8 days -EIMD protocol was performed on the 8th day of supplementation |
-24 healthy participants untrained, moderately active (6 men and 18 woman) -No sample size calculation |
-3 sets of 10 repetitions of eccentric knee extensions using a bar weighted with 115% of the respective 1RM weigh -180 s of rest between sets |
-No differences in soreness -Increase on CK activity -Increase on response of IL-6 -Higher ORAC levels |
| McCormick et al., 2016 | -Tart cherry juice: 600 mL a day, containing about 820 mg of anthocyanins -Placebo: the same amount of and artificial juice |
-Double-blind, crossover experiment with 2 treatments, each lasting 7 days with a 5-week washout period -EIMD protocol was performed on the 6th day of supplementation |
-9 highly trained men water polo players -No sample size calculation |
-The training spreadsheets were maintained throughout the intervention. On the 6th day, a water polo match was simulated | -No significant effect on the recovery (DOMS, CK, IL-6, among others) |
| Bell et al., 2016 | -Tart cherry juice: 200 mL a day, containing about 4.5 mg of anthocyanins -Placebo: the same amount of a synthetically derived fruit flavored |
-Double-blind, placebo-controlled study, lasting 8 days -EIMD protocol was performed on the 5th day of supplementation |
-16 semi-professional, male soccer players -No sample size calculation |
-An adaptation version of LIST | -Better performance indices -Decrease on DOMS -Minor response of IL-6 -No significant effect on CK |
| Brown et al., 2019 | -Tart cherry juice: 200 mL a day, containing about 4.5 mg of anthocyanins -Placebo: the same amount of a synthetically derived fruit flavored |
-Double-blind, placebo-controlled study, lasting 8 days -EIMD protocol was performed on the 5th day of supplementation |
-20 physically active woman -Sample size calculation by completing a power analysis of 80% |
-An adaptation version of LIST | -Less tendency to report soreness but no statistical evidence |
| Lynn et al., 2018 | -Bilberry juice: 400 mL a day, containing about 160 mg of anthocyanins -Placebo: the same amount prepared from a fruit-flavored maltodextrin powder |
-Single-blind, placebo-controlled study, lasting 8 days -EIMD protocol was performed on the 5th day of supplementation |
-21 recreational trained runners (16 men and 5 woman) -No sample size calculation |
-A real half marathon race | -Tendency to trigger harmful effect on DOMS and C reactive protein dosage, but no statistical evidence |
| Lima et al., 2019 | -Mixed juice: 480 mL a day, containing about 116 mg of anthocyanins -Placebo: the same amount of water mixed with maltodextrin |
-Double-blind, placebo-controlled study, lasting 9 days -EIMD protocol was performed on the 5th day of supplementation |
-30 physically active men -No sample size calculation |
-Downhill run (—15%) for 30 min at 70% of their VO2max speed | -Increase on DOMS values -Increase on CK concentrations |
| Lamb et al., 2019 | -Tart cherry juice and pomegranate juice: 500 mL a day, containing about 15 mg (TC) and 98 mg (POM) of anthocyanins -Placebo: the same amount of fruit-flavored drink |
-Double-blind, placebo-controlled study, lasting 9 days -EIMD protocol was performed on the 5th day of supplementation |
-36 nonresistance-trained men -No sample size calculation |
-5 sets of 10 maximal voluntary eccentric contractions of the nondominant elbow flexors on the isokinetic dynamometer -120 s of rest between sets |
-No significant effect on DOMS and CK |
DOMS, delayed onset muscle soreness; EIMD, exercise-induced muscle damage; RM, repetition maximum; CK, creatine kinase; IL, interleukin; ORAC, oxygen radical absorbance capacity; LIST, Loughborough intermittent shuttle test.
Trombold et al. (2011) conducted the oldest study found in our search. For this crossover, double-blind, placebo-controlled clinical trial, the authors selected 17 men with an average age of 21 years who had been active for at least three months. The intervention comprised ingesting 250 mL of pomegranate juice or placebo twice a day with a 12-h interval for 15 days and with a washout of at least 14 days between treatments. On the eighth day of supplementation, the volunteers performed the EIMD protocol (Table 1). Muscle pain was assessed before and at various times after exercise (2, 24, 48, 72, 96, and 168 h). For this, VAS associated with direct stimulation in the arm and leg muscles was used since the protocol included the upper and lower limbs. The results showed that both groups had increased pain after performing the protocol, as expected. The group that consumed pomegranate juice reported less muscle pain during the stimulation of the elbow flexor muscles (P=0.006) when compared with the placebo group. However, there was no significant difference between the groups when the perception of pain was assessed based on the stimulation of the knee extensor muscles.
From these results, the authors risked suggesting the possibility of using supplementation with pomegranate juice for athletes in competition periods as well as for individuals in the initial stages of training. However, the study’s methods and forms of presentation did not allow such inferences. The data collected with the VAS in the study by Trombold et al. (2011) were expressed only in their relative form using the P-value associated with the comparative graph. Although the descriptive analysis containing the raw data was not presented, the graphs allowed us to observe that the maximum pain intensity reported by the volunteers occurred between 24 and 48 h for both the upper and lower limbs, with the average value being <5 in the VAS. In this tool, this value represents moderate pain. Still, without the numerical presentation of measures of central tendency and variability, determining whether the response to the protocol was homogeneous among the volunteers was difficult, and this limited the ecological validity of the study. In addition, the external validity was compromised because the authors did not include a sample size calculation, limiting the extrapolation of the conclusions beyond the evaluated group (Gupta et al., 2016). Another crucial point was that the participants were instructed to maintain their usual diet, but no tool was used to assess eating habits. Dietary variations and measurement errors are thought to significantly interfere with the results because the components used in the intervention may also be present in other foods (Kirkpatrick et al., 2018). Therefore, analyzing and controlling these variables during the period stipulated for the intervention are extremely important.
Hutchison et al. (2016) conducted a double-blind, placebo-controlled study with 16 volunteers of both sexes with an average age of 20 years, all of whom were moderately active but were unaccustomed to strength training in the six months preceding the study. The volunteers were randomly assigned into two groups, in which they would receive approximately 1 L of blackcurrant nectar or the placebo drink divided twice a day for eight consecutive days. Although the dietary pattern was not assessed, the researchers listed anthocyanin-rich foods to avoid and instructed the participants to maintain their other eating habits. On the 5th day, the EIMD protocol was applied, and the pain perception variable was collected at various times (baseline, 24, 48, 72, and 96 h after exercise) using the VAS after performing a movement of full squat, without external weight, with an amplitude of 90°.
As expected, the two groups assessed by Hutchison et al. (2016) reported increased pain when compared with the baseline, presenting the most significant pain moment in 48 h (treatment group: 2.75±0.86; placebo group: 3.38±0.75) and returning to baseline values at 96 h post protocol of EIMD. However, when the two groups were compared, no statistically significant differences were found between the groups, although the treatment favored lower values of self-reported pain at all times. The lack of a sample size calculation was an important limitation because it may have reduced the power of detecting the effect, which was only demonstrated by trends (Gupta et al., 2016). In addition, the analysis of groups without gender stratification could be a limitation because studies have discussed the different forms of pain perception between men and women (de Araújo Palmeira et al., 2011). Such discussion mentions that the perception of pain comes from complex interactions between biological variables (e.g., gonadal hormones and genetics) and psychosocial variables (e.g., depression, anxiety, culture, and gender) and results indicate that women are more prone to increased pain sensitivity and chronicity (Bartley and Fillingim, 2013).
Two studies conducted in 2016 were tracked in the present study. The first was a crossover, randomized, double-blind, placebo-controlled study led by McCormick et al. (2016), with nine male water polo athletes averaging 18 years of age. A dose of 600-mL cherry juice or placebo was given for six consecutive days, with a five-day washout between treatments. The research included a convenience sample in which athletes from a specific institute were instructed to maintain the training sheet and, on the 6th day of the protocol, participated in a group simulation of a water polo match to replicate the demands of an official game. To assess DOMS, volunteers filled in a daily diary with VAS for pain during the six days of intervention, where 0 represented “no pain and no stiffness” and 10 represented “a lot of pain”. According to the results presented, the effect of time or treatment on volunteer-reported pain at any of the evaluated moments could not be determined. The fact that the study was conducted with well-conditioned individuals may have made it difficult to trigger muscle damage of greater magnitude and, consequently, minimized the effect of the protocol on achieving DOMS. The absence of an assessment of eating habits, sample size calculation, and the choice of the public versus the protocol may have limited the study (Gupta et al., 2016; Kirkpatrick et al., 2018).
Bell et al.’s study (2016) shares some similarities with that of McCormick et al.’s study (2016), previously presented. In addition to being performed with athletes, the randomized, placebo-controlled, double-blind clinical trial entailed offering 30 mL of cherry juice or placebo twice a day for eight consecutive days to the 16 semi-professional athletes of club soccer, with an average age of 25 years. The authors were concerned about possible dietary interference and provided a list of foods to avoid and instructed the participants to fill in the diet record diary, ensuring homogeneity of these variables at the baseline. The EIMD protocol was adapted from the Loughborough Intermittent Shuttle Test and was performed on the 5th day of treatment. Muscle pain was assessed under the stimulation of a squat movement using VAS in the following moments: baseline, 24, 48, and 72 h after following the protocol. Bell et al.’s (2016) results demonstrated that both groups showed a significant increase in DOMS 72 h after performing the proposed exercises, reinforcing that the protocol choice was adequate for the expected outcome. In addition to the effect of time on self-reported pain values, the group that received cherry juice supplementation was observed to have less pain perception when compared with the placebo group at 24 (P=0.044), 48 (P=0.018), and 72 h (P=0.007) after performing the EIMD protocol. Notably, the study omitted a crossover design or sample size calculation, which represents important characteristics for the ecological and external validity of the findings. However, the importance of the appropriate choice of EIMD protocol was revealed. Because all variables measured are dependent on muscle damage stimulation, this step of the design is essential, and if the protocol does not produce the expected outcome, all efforts of the other steps may be lost, invalidating the intervention.
Brown et al. (2019) conducted a randomized, placebo-controlled, double-blind study with 20 physically active women and an average age of 19 years, in which the effectiveness of cherry juice in DOMS and other variables related to EIMD was evaluated. The supplementation protocol was the same as in the study by Bell et al. (2016), which comprised 30 mL of treatment with cherry juice or the placebo twice a day for eight consecutive days. The inclusion and exclusion criteria were well detailed, and the participants were instructed to maintain their previous eating habits throughout the intervention period. The EIMD protocol was applied on the 5th day of supplementation, and the DOMS assessment was performed under the encouragement of a full squat, associated with the pain pressure threshold, using the VAS at the baseline, 24, 48, and 72 h after the battery of exercise. An increase in DOMS was observed in both groups, returning to baseline values in the 72-h period. This effect of time is expected given the behavior of the DOMS mechanism. However, when comparing the two groups (treatment and placebo), the authors reported that the group supplemented with cherry juice showed less tendency toward self-reported pain, but this has not been statistically proven. Brown et al.’s (2019) study was carefully designed and presented a sample size calculation in addition to well-defined criteria for selecting volunteers and information that allowed their replication. In addition, the participants were instructed to maintain their usual diet and complete a weighed food diary. The fact that no effect of supplementation on DOMS variables was found should be considered. The lack of anthocyanin dosages in plasma often occurs in studies of this nature, and this can be a limitation. This aspect interferes with the interpretation of the adequacy of supplementation and makes it difficult to make decisions regarding the amount of anthocyanins to be offered, which appears in a divergent way in the studies (Table 1).
Lynn et al. (2018) also performed a single-blind, randomized, placebo-controlled study with 21 individuals of both sexes and an average age of 30 years. The individuals were divided into two groups to receive 200 mL of bilberry juice or placebo twice a day for seven days. On the 5th day, the volunteers participated in a half marathon in the region (Sheffield Half Marathon) and provided data on DOMS with VAS after performing a full squat in the following moments: before the race, right after the race, and 24 and 48 h after the race. The results showed that the mean time of the test was similar between the groups, with no statistically significant differences. Both groups reported a significant increase in pain shortly after completing the half marathon, and surprisingly, the group that consumed supplementation with anthocyanin-rich juice showed a slight to moderate increase in pain perception after the half marathon. Lynn et al.’s study (2018) presented many positive points, such as sample size calculation, characterization of the supplement offered, and complete descriptions for its replication. However, the study also had some limitations. The authors reported that the travel time from the finish line to the laboratory differed among the volunteers, compromising the linearity of the data collection and, possibly, the results. In addition, because the researchers could not measure data on the participants’ eating habits, discussing the interference of other issues related to food choices with EIMD and DOMS responses is infeasible.
In a randomized, placebo-controlled, single-blind study conducted by Lima et al. (2019), 30 male physical education students with an average age of 22 years were selected and divided into two groups to receive 240 mL of antioxidant juice (made with a mix of fruits rich in anthocyanins-clarified apple juice with plum, blueberry, maqui berry, raspberry, and cranberry concentrates) or placebo twice a day for nine consecutive days. On the 5th day, both groups underwent a downhill run, under the conditions described in Table 1. For muscle pain assessment, participants were instructed to climb up and down from a 45-cm chair without external help and, after performing this movement, they should respond to VAS that varied between “no pain” and “very, very painful” in their extremities. The results revealed an increase in pain perception in both groups after performing the EIMD protocol. However, at all times reported (baseline, right after, 24, 48, 72, and 96 h after exercise), the treatment group had lower DOMS values than the placebo group (P< 0.05), indicating that supplementation had a hypothesized effect. Although Lima et al.’s (2019) results were very clear, the authors did not access food records or quantify the total consumption of phenolic compounds. This type of evaluation allowed us to observe whether the effects were solely due to the supplementation offered or if interferences existed in habits and stimuli external to the intervention. These results could be confirmed by measuring the volunteers’ total antioxidant capacity or plasma levels of anthocyanins, comparing the two groups and correlating with dietary records, to determine whether the volunteers had differences at baseline and during the intervention, eliminating confounding factors.
The last randomized, placebo-controlled, double-blind study screened in our search was that of Lamb et al. (2019) and had the participation of 36 nonresistance-trained men who were allocated to consume 250 mL of pomegranate juice, cherry juice, or placebo twice a day for nine days. On the 5th day, the EIMD protocol was applied, with a predominance of eccentric contraction, and muscle pain was assessed pre-exercise, immediately post-exercise, and at 24, 48, 72, and 96 h post-exercise. The results showed that supplementation tended to increase muscle pain values, but this difference was not statistically proven. The authors mentioned that the absence of a representative sample may have interfered with the identification of the effect. However, this was not the only limitation. In addition to not performing any biochemical quantification of markers related to oxidative stress and inflammation, the researchers reported that despite offering directions pertaining to eating habits, the volunteers consumed foods with a high content of bioactive compounds during the intervention period.
Dietary control through guidelines and dietary records has proved to be a critical point in intervention studies with supplements rich in bioactive compounds. Not all studies presented mentioned dietary guidelines, including indications to avoid the consumption of certain foods during the intervention period. Moreover, because they are interventions conducted in a free lifestyle, what the volunteer chooses to consume cannot be controlled. For this reason, the analysis of dietary records becomes an essential element for the data to be evaluated and treated correctly, allowing for the identification of confounding factors that can induce mistaken inferences in certain situations.
Based on the presented studies, the results of anthocyanin supplementation for DOMS are inconclusive. This is due to the diversity of intervention methods, including target audience selection, anthocyanin content in supplements, EIMD protocol, and study design issues (Kendall, 2003; Gupta et al., 2016; Kirkpatrick et al., 2018). We are unaware of previous reviews that have evaluated the use of anthocyanin-rich foods exclusively in DOMS. However, some studies have observed the use of these foods and supplements in various parameters of muscle recovery, which we can highlight-increased antioxidant capacity after exercise (Doma et al., 2021; Jones et al., 2021); performance (Doma et al., 2021); and creatine kinase and other biomarkers (Doma et al., 2021; Kimble et al., 2021).
Although doubts exist, research arouses interest in conducting interventions that assess the potential of anthocyanin-rich foods in DOMS treatment and prevention. Therefore, further studies are necessary given that they are dedicated to defining a design that allows the external and ecological validity of the results, prioritizing the following: representative sample selection by performing a sample size calculation; standardized dietary prescriptions and analysis of food records to correct possible confounding factors; quantification and standardization of anthocyanin amount in the supplements offered and bioavailability assessment; biochemical tests to verify the effect of supplementation on the individual’s total antioxidant capacity; and adequate choice of the EIMD protocol to the selected public because DOMS is dependent on muscle damage magnitude.
Despite these limitations, studies seem to agree with the supplementation period and EIMD protocol application given that the majority proposed a minimum supplementation of three days prior to performing strenuous exercise. Another common point among the papers presented is the process of selecting volunteers, in which those with pathological habits and conditions that directly interfere with the inflammatory and oxidative processes of the body, such as smoking, alcoholism, and chronic and autoimmune diseases, are excluded. In addition, based on the studies included in the review, supplements with higher dosages of anthocyanin in their composition (>600 mg) had a certain effect on inflammation and oxidative stress biomarkers, but as a direct effect on DOMS, the main effects were reported with the use of protocols with fewer supplements and lower concentrations of anthocyanins in their composition. The most recent studies seem to follow this trend and, probably, find a way to better understand this scenario.
From this, some important points about the main conclusions of this review can be discussed. First, based on the studies presented in this review, the sporadic consumption of anthocyanin-rich foods is insufficient to obtain these results. Consumption must occur in order to precede physical efforts in a programmed manner. Second, the best results were obtained from lower dosages, suggesting the effectiveness of more continuous and moderate consumption; future studies may start from this idea.
Therefore, given proven effects and properly established protocols, the use of anthocyanin-rich foods may be an accessible and important tool in DOMS prevention and treatment. Previous studies have already shown that the therapeutic effects of anthocyanins on human health are more frequent when the source foods are consumed in their entire form (Vizzotto, 2012). This can occur because, when isolating an active ingredient, the synergy between the components of the whole food is lost (Costa et al., 2015; Dias et al., 2015). This finding, combined with that of the present study, allows us to reflect on the benefits of an anthocyanin-rich diet over the use of isolated supplements, both in terms of consumption adherence and cost, in addition to supporting the idea that a balanced diet can meet all the needs of athletes and physical exercise practitioners without the need for supplements (Garthe and Maughan, 2018; Garthe and Ramsbottom, 2020).
The discovery of natural methods that minimize the deleterious effects of DOMS is of great interest to the scientific community and health professionals. Pain, touch sensitivity, muscle stiffness, local edema, reduced strength, and muscle contraction ability can decrease adherence to the practice of physical exercise and even stimulate the exacerbated consumption of drugs, representing an important health risk. Thus, the concern about the use of anthocyanin-rich supplements and foods as a resource to reduce the magnitude of DOMS remains, but the answer is dependent on the methodological quality of future studies.
Footnotes
FUNDING
None.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: TCMS, LRA. Analysis and interpre-tation: TCMS, LRA, JLG, HCMC. Data collection: TCMS. Writing the article: TCMS, ECM. Critical revision of the article: LRA, JLG, HCMC. Final approval of the article: all authors. Overall responsibility: LRA.
References
- Abbott BC, Bigland B, Ritchie JM. The physiological cost of negative work. J Physiol. 1952;117:380–390. doi: 10.1113/jphysiol.1952.sp004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almekinders LC. Anti-inflammatory treatment of muscular injuries in sport. An update of recent studies. Sports Med. 1999;28:383–388. doi: 10.2165/00007256-199928060-00001. [DOI] [PubMed] [Google Scholar]
- Angelo PM, Jorge N. Phenolic compounds in foods-A brief review. Rev Inst Adolfo Lutz. 2007;66:1–9. [Google Scholar]
- Appell HJ, Soares JM, Duarte JA. Exercise, muscle damage and fatigue. Sports Med. 1992;13:108–115. doi: 10.2165/00007256-199213020-00006. [DOI] [PubMed] [Google Scholar]
- Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990;22:429–435. doi: 10.1249/00005768-199008000-00002. [DOI] [PubMed] [Google Scholar]
- Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984;16:529–538. doi: 10.1249/00005768-198412000-00002. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen E. Observations on experimental muscular soreness. Acta Rheumatol Scand. 1956;2:109–116. doi: 10.3109/rhe1.1956.2.issue-1-4.12. [DOI] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell PG, Stevenson E, Davison GW, Howatson G. The effects of montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients. 2016;8:441. doi: 10.3390/nu8070441. https://doi.org/10.3390/nu8070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni T, Genovesi F, Nemmer M, Carling C, Alberti G, Howatson G. Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: current knowledge, practical application and future perspectives. Eur J Appl Physiol. 2020;120:1965–1996. doi: 10.1007/s00421-020-04432-3. [DOI] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Stevenson EJ, Howatson G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur J Sport Sci. 2019;19:95–102. doi: 10.1080/17461391.2018.1502360. [DOI] [PubMed] [Google Scholar]
- Chaves-Silva S, Santos ALD, Chalfun-Júnior A, Zhao J, Peres LEP, Benedito VA. Understanding the genetic regulation of anthocyanin biosynthesis in plants-Tools for breeding purple varieties of fruits and vegetables. Phytochemistry. 2018;153:11–27. doi: 10.1016/j.phytochem.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Costa ZG, de LOCe Silva C, Costa CML, Faria LJG. Estudo da estabilidade de antocianinas extraídas dos frutos de açaí (Euterpea oleracea Mart.); Proceedings of the Anais do XI Congresso Brasileiro de Engenharia Química em Iniciação Científica; 2015 Jul 19-22; Campinas, Brazil. pp. 2177–2182. [Google Scholar]
- de Araújo Palmeira CC, Ashmawi HA, de Paula Posso I. Sex and pain perception and analgesia. Rev Bras Anestesiol. 2011;61:814–828. doi: 10.1016/S0034-7094(11)70091-5. [DOI] [PubMed] [Google Scholar]
- de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MM, Martino HS, Noratto G, Roque-Andrade A, Stringheta PC, Talcott S, et al. Anti-inflammatory activity of polyphenolics from açai (Euterpe oleracea Martius) in intestinal myofibroblasts CCD-18Co cells. Food Funct. 2015;6:3249–3256. doi: 10.1039/C5FO00278H. [DOI] [PubMed] [Google Scholar]
- Doma K, Gahreman D, Connor J. Fruit supplementation reduces indices of exercise-induced muscle damage: a systematic review and meta-analysis. Eur J Sport Sci. 2021;21:562–579. doi: 10.1080/17461391.2020.1775895. [DOI] [PubMed] [Google Scholar]
- Donnelly AE, Maughan RJ, Whiting PH. Effects of ibuprofen on exercise-induced muscle soreness and indices of muscle damage. Br J Sports Med. 1990;24:191–195. doi: 10.1136/bjsm.24.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly AE, McCormick K, Maughan RJ, Whiting PH, Clarkson PM. Effects of a non-steroidal anti-inflammatory drug on delayed onset muscle soreness and indices of damage. Br J Sports Med. 1988;22:35–38. doi: 10.1136/bjsm.22.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ. Muscle damage: nutritional considerations. Int J Sport Nutr. 1991;1:214–224. doi: 10.1123/ijsn.1.3.214. [DOI] [PubMed] [Google Scholar]
- Fridén J, Sjöström M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Garthe I, Maughan RJ. Athletes and supplements: prevalence and perspectives. Int J Sport Nutr Exerc Metab. 2018;28:126–138. doi: 10.1123/ijsnem.2017-0429. [DOI] [PubMed] [Google Scholar]
- Garthe I, Ramsbottom R. Elite athletes, a rationale for the use of dietary supplements: A practical approach. PharmaNutr. 2020;14:100234. doi: 10.1016/j.phanu.2020.100234. https://doi.org/10.1016/j.phanu.2020.100234. [DOI] [Google Scholar]
- Gupta KK, Attri JP, Singh A, Kaur H, Kaur G. Basic concepts for sample size calculation: Critical step for any clinical trials! Saudi J Anaesth. 2016;10:328–331. doi: 10.4103/1658-354X.174918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Hertel J. The role of nonsteroidal anti-inflammatory drugs in the treatment of acute soft tissue injuries. J Athl Train. 1997;32:350–358. [PMC free article] [PubMed] [Google Scholar]
- Hill AV. The mechanics of voluntary muscle. The Lancet. 1951;258:947–951. doi: 10.1016/S0140-6736(51)91922-8. [DOI] [PubMed] [Google Scholar]
- Hotfiel T, Freiwald J, Hoppe MW, Lutter C, Forst R, Grim C, et al. Advances in delayed-onset muscle soreness (DOMS): Part I: Pathogenesis and diagnostics. Sportverletz Sportschaden. 2018;32:243–250. doi: 10.1055/a-0753-1884. [DOI] [PubMed] [Google Scholar]
- Hough T. Ergographic studies in muscular soreness. Am Phys Educ Rev. 1902;7:1–17. doi: 10.1080/23267224.1902.10649879. [DOI] [Google Scholar]
- Hutchison AT, Flieller EB, Dillon KJ, Leverett BD. Black currant nectar reduces muscle damage and inflammation following a bout of high-intensity eccentric contractions. J Diet Suppl. 2016;13:1–15. doi: 10.3109/19390211.2014.952864. [DOI] [PubMed] [Google Scholar]
- Jalalvand A, Anbarian M, Khorjahani A. The effects of a combination treatment (pre-exercise vitamin C & PNF stretching, post-exercise ultrasound treatment) on markers of exercise-induced muscle damage. Rev Bras Med Esporte. 2012;18:322–329. doi: 10.1590/S1517-86922012000500008. [DOI] [Google Scholar]
- Jones L, Bailey SJ, Rowland SN, Alsharif N, Shannon OM, Clifford T. The effect of nitrate-rich beetroot juice on markers of exercise-induced muscle damage: A systematic review and meta-analysis of human intervention trials. J Diet Suppl. 2021 doi: 10.1080/19390211.2021.1939472. https://doi.org/10.1080/19390211.2021.1939472. [DOI] [PubMed] [Google Scholar]
- Kendall JM. Designing a research project: randomised controlled trials and their principles. Emerg Med J. 2003;20:164–168. doi: 10.1136/emj.20.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble R, Jones K, Howatson G. The effect of dietary anthocyanins on biochemical, physiological, and subjective exercise recovery: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1963208. https://doi.org/10.1080/10408398.2021.1963208. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick SI, Collins CE, Keogh RH, Krebs-Smith SM, Neuhouser ML, Wallace A. Assessing dietary outcomes in intervention studies: pitfalls, strategies, and research needs. Nutrients. 2018;10:1001. doi: 10.3390/nu10081001. https://doi.org/10.3390/nu10081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb KL, Ranchordas MK, Johnson E, Denning J, Downing F, Lynn A. No effect of tart cherry juice or pomegranate juice on recovery from exercise-induced muscle damage in non-resistance trained men. Nutrients. 2019;11:1593. doi: 10.3390/nu11071593. https://doi.org/10.3390/nu11071593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WY, Blazevich AJ, Newton MJ, Wu SS, Nosaka K. Assessment of muscle pain induced by elbow-flexor eccentric exercise. J Athl Train. 2015;50:1140–1148. doi: 10.4085/1062-6050-50.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LCR, Barreto RV, Bassan NM, Greco CC, Denadai BS. Consumption of an anthocyanin-rich antioxidant juice accelerates recovery of running economy and indirect markers of exercise-induced muscle damage following downhill running. Nutrients. 2019;11:2274. doi: 10.3390/nu11102274. https://doi.org/10.3390/nu11102274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg TR, Howatson G. Analgesic and anti-inflammatory drugs in sports: Implications for exercise performance and training adaptations. Scand J Med Sci Sports. 2018;28:2252–2262. doi: 10.1111/sms.13275. [DOI] [PubMed] [Google Scholar]
- Luti S, Modesti A, Modesti PA. Inflammation, peripheral signals and redox homeostasis in athletes who practice different sports. Antioxidants. 2020;9:1065. doi: 10.3390/antiox9111065. https://doi.org/10.3390/antiox9111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn A, Garner S, Nelson N, Simper TN, Hall AC, Ranchordas MK. Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: a randomised, placebo-controlled trial. J Int Soc Sports Nutr. 2018;15:22. doi: 10.1186/s12970-018-0227-x. https://doi.org/10.1186/s12970-018-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan RJ, Donnelly AE, Gleeson M, Whiting PH, Walker KA, Clough PJ. Delayed-onset muscle damage and lipid peroxidation in man after a downhill run. Muscle Nerve. 1989;12:332–336. doi: 10.1002/mus.880120412. [DOI] [PubMed] [Google Scholar]
- McAnulty LS, Nieman DC, Dumke CL, Shooter LA, Henson DA, Utter AC, et al. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl Physiol Nutr Metab. 2011;36:976–984. doi: 10.1139/h11-120. [DOI] [PubMed] [Google Scholar]
- McCormick R, Peeling P, Binnie M, Dawson B, Sim M. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J Int Soc Sports Nutr. 2016;13:41. doi: 10.1186/s12970-016-0151-x. https://doi.org/10.1186/s12970-016-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- Peake JM, Neubauer O, Della Gatta PA, Nosaka K. Muscle damage and inflammation during recovery from exercise. J Appl Physiol. 2017;122:559–570. doi: 10.1152/japplphysiol.00971.2016. [DOI] [PubMed] [Google Scholar]
- Pearcey GE, Bradbury-Squires DJ, Kawamoto JE, Drinkwater EJ, Behm DG, Button DC. Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. J Athl Train. 2015;50:5–13. doi: 10.4085/1062-6050-50.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Sanhueza D, Inostroza-Blancheteau C, Ribera-Fonseca A, Reyes-Díaz M. Anthocyanins in berries and their potential use in human health. In: Shiomi N, Waisundara V, editors. Superfood and Functional Food-The Development of Superfoods and Their Roles as Medicine. IntechOpen; London, UK: 2017. pp. 155–172. [DOI] [Google Scholar]
- Pervaiz T, Songtao J, Faghihi F, Haider MS, Fang J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J Plant Biochem Physiol. 2017;5:2. doi: 10.4172/2329-9029.1000187. https://doi.org/10.4172/2329-9029.1000187. [DOI] [Google Scholar]
- Quintero KJ, Resende A, Leite GSF, Lancha AH., Jr An overview of nutritional strategies for recovery process in sports-related muscle injuries. Nutrire. 2018;43:27. doi: 10.1186/s41110-018-0084-z. https://doi.org/10.1186/s41110-018-0084-z. [DOI] [Google Scholar]
- Ranchordas MK, Rogerson D, Soltani H, Costello JT. Antioxidants for preventing and reducing muscle soreness after exercise. Cochrane Database Syst Rev. 2017;12:CD009789. doi: 10.1002/14651858.CD009789.pub2. https://doi.org/10.1002/14651858.CD009789.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–16521. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- Rocha APC, Kraychete DC, Lemonica L, de Carvalho LR, de Barros GAM, dos Santos Garcia JB, et al. Pain: current aspects on peripheral and central sensitization. Rev Bras Anestesiol. 2007;57:94–105. doi: 10.1590/S0034-70942007000100011. [DOI] [PubMed] [Google Scholar]
- Saigo T, Wang T, Watanabe M, Tohge T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr Opin Plant Biol. 2020;55:93–99. doi: 10.1016/j.pbi.2020.04.001. [DOI] [PubMed] [Google Scholar]
- Schoenfeld BJ. Does exercise-induced muscle damage play a role in skeletal muscle hypertrophy? J Strength Cond Res. 2012;26:1441–1453. doi: 10.1519/JSC.0b013e31824f207e. [DOI] [PubMed] [Google Scholar]
- Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc. 1983;15:51–56. doi: 10.1249/00005768-198315010-00010. [DOI] [PubMed] [Google Scholar]
- Sinopoli A, Calogero G, Bartolotta A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chem. 2019;297:124898. doi: 10.1016/j.foodchem.2019.05.172. https://doi.org/10.1016/j.foodchem.2019.05.172. [DOI] [PubMed] [Google Scholar]
- Speretta GF, Leite RD, de Oliveira Duarte AC. Obesity, inflammation and exercise: focus on TNF-alpha and IL-10. Rev Rev Hosp Univ Pedro Ernesto. 2014;13:61–69. doi: 10.12957/rhupe.2014.9807. [DOI] [Google Scholar]
- Tokinoya K, Ishikura K, Ra SG, Ebina K, Miyakawa S, Ohmori H. Relationship between early-onset muscle soreness and indirect muscle damage markers and their dynamics after a full marathon. J Exerc Sci Fit. 2020;18:115–121. doi: 10.1016/j.jesf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, et al. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012;110:356–363. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Trombold JR, Reinfeld AS, Casler JR, Coyle EF. The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. J Strength Cond Res. 2011;25:1782–1788. doi: 10.1519/JSC.0b013e318220d992. [DOI] [PubMed] [Google Scholar]
- Vizzotto M. Propriedades funcionais das pequenas frutas. Inf Agropecu. 2012;33:84–88. [Google Scholar]
- Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541–556. doi: 10.1097/HCO.0000000000000437. [DOI] [PubMed] [Google Scholar]
- Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ, et al. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100:1124–1133. doi: 10.1152/japplphysiol.00562.2005. [DOI] [PubMed] [Google Scholar]