Abstract
The function of antioxidant polyphenols has been demonstrated for their ability to protect against a variety of diseases. However, some antioxidants have been shown to be pro-oxidant. Some of the important antioxidant enzymes are glutathione transferases (GST), which are involved in maintaining redox homeostasis. GST class Pi (GSTP1-1) hyper-activation is a feature that is found in cancer. This work aims to demonstrate the relationship between the phytochemicals of 18 plants and their ability to act as antioxidant/pro-oxidant agents, as well as their effects on the activity of GSTP1-1 and their cellular toxicity. Tamarindus indica, Cinnamomum verum, and Alpinia galanga extracts had high phytochemical contents, moderate heavy metal levels, and antioxidant/pro-oxidant activities. Among the main plant components identified using high-performance liquid chromatography, only chlorogenic acid, catechin, and quercetin can function as antioxidants and pro-oxidants. Hibiscus sabdariffa, C. verum, A. galanga, T. indica, Gossypium arboreum, and Punica granatum were among the plant extracts examined that inhibited the activity of the purified recombinant GSTP1-1, with the inhibition constant values ranging from 0.48 to 1.67 mg of gallic acid equivalent/g. The level of cytotoxicity was also studied to determine the effects of these extracts on human Caucasian breast cancer. The findings revealed that plants with high phenol content had an antioxidant/pro-oxidant capacity as well as inhibition of the activity of GST. However, the cytotoxic effect was not associated with all of the extracts, which indicates that polyphenols interact with other components that may influence their observed behavior.
Keywords: antioxidant capacity, cytotoxicity, glutathione transferase inhibition, polyphenol compounds, pro-oxidant activity
INTRODUCTION
Natural substances used in the treatment of human disease are increasingly becoming necessary worldwide. Several pharmaceutical companies are investing botanical resources in scientific facilities that have a significant possibility for treating humans to explore both their active components and their potential pharmacological uses (Dutta et al., 2014; Šantić et al., 2017). Antioxidant molecules serve an important role in maintaining the oxidative-antioxidant balance, and they enhance the antioxidant defenses, both of which are required for controlling many important physiological functions (Tan et al., 2018).
Phenolic compounds are natural antioxidant compound with beneficial effects that can be widely used. These include anti-inflammatory, antimutagenic, antiallergic, and anticarcinogenic effects (León-González et al., 2015; Hussain et al., 2016; Stefanello et al., 2018). The beneficial effects are mostly due to their redox properties, which allow them to function as reducing agents, hydrogen donors, singlet oxygen quenchers, and metal ion chelates (Hussain et al., 2016; Dróżdż et al., 2017). Phenolic compounds are categorized by their origin, biological function, and chemical structure. These compounds can be divided into two major groups of flavonoids and nonflavonoids (e.g., phenolic acids, phenolic alcohols, and tannins) based on the number of aromatic rings they have and their binding affinity to other various compounds (Lipiński et al., 2017). Flavonoids are classified into subclasses based on the degree of oxidation of the heterocyclic chain, such as anthocyanins, flavanols, flavans, flavones, and isoflavones (Maqsood et al., 2013).
The cell’s enzymatic and nonenzymatic antioxidant defense systems prevent oxidant mediated damage from occurring in biomolecules like lipids, proteins, and deoxyribonucleic acid (DNA) by neutralizing free radicals.
By neutralizing free radicals, the cell defense systems using enzymatic and nonenzymatic antioxidants prevent oxidant mediated damage from affecting diverse biomolecules like lipids, proteins, and DNA. However, by scavenging free radicals, antioxidants may prevent apoptosis from occurring in malignant cells that are induced via oxidative stress during chemotherapy. Antioxidants can also have antiapoptotic and cancer-promoting effects in cancer patients.
Dietary phenolic activity may be considered as a prooxidative and cytotoxic at high doses under certain conditions. The pro-oxidant properties of these compounds depend on various variables like the phenolic class, the presence of oxygen or transition metal ions, alkaline pH, their concentration levels, and characteristics of solubility (Olas, 2018). Phenolic autoxidation provides phenol radicals that react with oxygen, producing a reactive oxygen species (ROS) and a complex combination of semiquinones and quinones. The antioxidants exhibited pro-oxidant activity, especially when Cu2+ and Fe2+ were present. Phenolic pro-oxidant activity induces cellular lipid peroxidation, damage to DNA, and cellular apoptosis (Eghbaliferiz and Iranshahi, 2016). A more precise advantage for certain cellular targets and biological processes exist for the pro-oxidant properties of antioxidant compounds, as they tend to play a significant role in the prevention of certain forms of cancer and disorders stemming from oxidative stress (Yordi et al., 2012; León-González et al., 2015; Eghbaliferiz and Iranshahi, 2016).
A multifunctional xenobiotic metabolic enzyme called glutathione transferase (GST) catalyzes the conjugation of glutathione (GSH) with anticancer drugs and restricts their penetration and targeting abilities in cells and cellular DNA, respectively (Zhang et al., 2019). This appears to be mediated primarily by the GST class Pi (GSTP1-1). GSTP1-1 is directly involved in reducing quinones to hydroquinones, eliminating the redox cycle-induced ROS production, and thereby mediating oxidative stress (Lei et al., 2020). GSTP1-1 dimerizes into larger aggregates, inhibiting the process by which it binds to the transcription and apoptosis signal protein C-Jun N-terminal kinase and preventing it from activating when ROS is present (Lei et al., 2020).
Human tumors can display high levels of GSTP1-1 expression, inhibiting oxidative stress and preventing the death of tumor cells. New drugs that target GSTs are being studied in clinical trials as adjuvant therapies with anticancer agents that will be used for improving the efficacy of chemotherapy, enhancing cellular signaling pathways in cancer cells, and cellular apoptosis (Louis et al., 2016; Bartolini et al., 2019; Lei et al., 2020). Thus, the pro-oxidant capacity of polyphenols and the inhibition of GSTP1-1 may present a potential combination that can be used for cancer therapy.
The purpose of this study is to investigate the relationship between the type of phenolic content in 18 commonly used plants for use as antioxidants, some metal ion contents and their ability to act as oxidizing agents (H2O2 producers), and inhibitors of GSTP1-1 enzyme activity, as well as to test their level of toxicity on breast cancer cells (MCF-7). This may explain why, in addition to their natural antioxidant action, some plants can potentially act as pro-oxidants to inhibit the proliferation of breast cancer cells and improve treatment efficacy.
MATERIALS AND METHODS
Chemicals
Folin-Ciocateu’s reagent was purchased from Merck Company (Kenilworth, NJ, USA). 1,1-Diphenyl-2-picryl-hydrazyl (DPPH), ascorbic acid, xylenol orange, and phenolic compounds including apigenin-7-glucoside, catechin, cinnamic acid, caffeic acid, curcumin, chlorogenic acid, epigallocatechin gallate, ferulic acid, gallic acid, gentisic acid, 18-α glycrrytinic acid, hesperidin, quercetin, p-coumaric acid, syringic acid, protocatechuic acid, pyrogallol, and vanillic acid were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). All materials and culture media used for in vitro culture, lysozymes, isopropyl-β-D-thiogalactoside (IPTG), Ni-immobilized metal affinity chromatography matrix, and Epoxy-activated Sepharose 6B were also purchased from Sigma-Aldrich Company.
Plant materials
The plants used in the study are well-known in the local market for their traditional uses. Eighteen kinds of plants (Table 1) were purchased from Haraz Company for Food Industries and Natural Products (Cairo, Egypt). The plants were identified and validated by the Herbarium Plants Department of the National Research Center (NRC-Plant Drug Discovery Herbarium, Cairo, Egypt).
Table 1.
List of scientific and common names of the plants used in the study
| Plant name | Latin name | Used part | Family |
|---|---|---|---|
| Turmeric | Curcuma longa | Rhizome | Zingiberaceae |
| Ginger | Zingiber officinale | Rhizome | |
| Galangal | Alpinia galanga | Rhizome | |
| Tamarind | Tamarindus indica | Seed | Fabaceae |
| Cinnamon | Cinnamomum verum | Bark | Lauraceae |
| Clove | Syzygium aromaticum | Seed | Myrtaceae |
| Celery | Apium graveolens | Seed | Apiaceae |
| Pomegranate | Punica granatum | Seed | Lythraceae |
| Cress | Lepidium sativum | Seed | Brassicaceae |
| Radish | Raphanus sativus | Seed | |
| Rocket salad | Eruca sativa | Seed | |
| Turnip | Brassica rapa var. rapa | Seed | |
| Cabbage | Brassica oleracea | Seed | |
| White cauliflower | Brassica oleracea var. botrytis | Seed | |
| Kenaf | Hibiscus cannabinus | Seed | Malvaceae |
| Roselle | Hibiscus sabdariffa | Seed | |
| Cotton | Gossypium arboreum | Seed |
Preparation of plant extracts
Using a domestic blender, one gram of dried plants was ground up and mixed with (1:10 w/v) 70% of a solvent (ethanol or acetone) and stored at 55°C for 2 h. The extracted material was centrifuged at 1,000 g for 10 min, filtered through Whatman no. 1 filter paper (GE Healthcare Life Science, Buckinghamshire, UK), and stored at −20°C for future analysis.
Phytochemical analysis
Total phenolic contents (TPC): The concentration of TPC in the extracts was calculated using a series of standard gallic acid solutions (2.5∼20.0 μg/mL), as stated in Singleton and Rossi (1965).
Total flavonoid contents (TFC): The TFC in extracts was determined using a series of standard rutin solutions (2.5∼50.0 μg/mL), as described in the aluminum chloride colorimetric method (Dewanto et al., 2002).
Total anthocyanin contents (TAC): TAC was measured according to the pH differential method (Fuleki and Francis, 1968).
Total condensed tannin (TCT) contents: Determination of TCT content using the vanillin reaction was based on the procedure reported by Broadhurst and Jones (1978). Investigated samples were expressed as mg of catechin equivalent (CE) per g of the dry plant.
Total hydrolysable tannin (THT) contents: Concentration of THT after a reaction with potassium iodate (KIO3) to produce a colored product was determined according to the method of Çam and Hıșıl (2010). The results were expressed as mg tannic acid equivalent (TAE) per g dry plant (mg TAE/g dry plant).
Heavy metal contents: A concentration of copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) ions from the 18 plant extracts were each measured using the Agilent 5100 Synchronous Vertical Dual View Inductively (Agilent Technologies Inc., Santa Clara, CA, USA) coupled plasma-optical emission spectroscopy, with the Agilent Vapor Generation Accessory VGA 77 (Agilent Technologies Inc.). Table 2 explains how the experiment was prepared according to the operating conditions including wavelength, intensity, torches power, nebulizer flow, viewing mode, background correction, correlation coefficient, and calibration error (American Public Health Association et al., 2017). For each series of the measurements, the intensity calibration curve was constructed from a blank and three or more standards from Merck Company. A standard reference material and quality control sample from the National Institute of Standards and Technology confirmed the readings from the instruments.
Table 2.
Operating conditions for the determination of copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) ions using inductively coupled plasma-optical emission spectroscopy
| Operating condition | Cu | Fe | Mn | Zn |
|---|---|---|---|---|
| Wavelength (nm) | 327.395 | 238.204 | 257.61 | 213.857 |
| Intensity | 53,785 | 41,015 | 222,170 | 42,501 |
| Torches power (kW) | 1.2 | 1.2 | 1.2 | 1.2 |
| Nebulizer flow (L/min) | 0.7 | 0.7 | 0.7 | 0.7 |
| Plasma flow (L/min) | 12 | 12 | 12 | 12 |
| Viewing mode | Axial | Axial | Axial | Axial |
| Background correction | Fitted | Fitted | Fitted | Fitted |
| Correlation coefficient | 0.98 | 0.98 | 0.98 | 0.98 |
| Calibration error (%) | 10 | 10 | 10 | 10 |
| Calibration fit | Rational | Rational | Rational | Rational |
High performance liquid chromatography (HPLC) of phenolic compounds: Extracts (70% ethanol or acetone) of Tamarindus indica, Cinnamomum verum, and Alpinia galanga were subjected to the HPLC analysis at the National Research Centre (Cairo, Egypt). The HPLC analysis was conducted using an Agilent Technologies 1100 series liquid chromatograph equipped with an auto sampler and a diode-array detector (Agilent Technologies, Inc.). The detection limit was defined as the concentration at which the signal to noise ratio was equal to or greater than three (Kim et al., 2006).
Antioxidant capacity using the free radical scavenging activity method
Blois (1958) and Leong and Shui (2002) described the method used in this study to determine the activity of DPPH free radical scavenging for each sample. The EC50 value is the concentration of the sample which is required to scavenge 50% of DPPH free radicals. EC50 concentrations were calculated after constructing the percent of activity of DPPH scavenging versus the log extract concentrations curve using vitamin C as a standard.
Pro-oxidant capacity by following hydrogen peroxide producing activity
Plant extracts and phenolic compounds were examined to investigate their ability to produce hydrogen peroxide (H2O2) using the ferrous ion oxidation-xylenol orange assay (Long et al., 1999; Banerjee et al., 2003).
Biological materials
Expression and purification of recombinant hGSTP1-1: Prof. Bengt Mannervik (Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden) kindly gifted the wild-type human GSTP1-1 for use in this study. The hGSTP1-1 was expressed in the Escherichia coli strain XL1-Blue from the plasmid pKK-D in the presence of 0.2 mM IPTG (Kolm et al., 1995). The cells were harvested at 3,000 g for 10 min and were disrupted via ultrasonication. The hGSTP1-1 was purified using the Ni-immobilized metal affinity chromatography described by Hegazy et al. (2004).
Preparation of human erythrocyte homogenate: Venous blood (20 mL) was collected for the preparation of cytosolic hemolysate (Hamed et al., 2011).
Preparation of human placenta homogenate: Human placenta (38∼40 weeks gestation) (n=3) was obtained from healthy mothers who had no known history of any physiological or pathological problems. The placenta was collected immediately after each female’s delivery from Imbaba General Hospital (participants gave informed consent) for use in this study. Placental tissue preparation was performed according to the method described by Collier et al. (2002).
Purification of GSTs by affinity chromatography
The cytosolic fractions of erythrocyte and the placenta were purified via an affinity chromatography using a GSH-Sepharose 6B column. The reduced GSH was coupled with epoxy-activated Sepharose 6B, as described by Simons and Vander Jagt (1977). The homogeneity of the purified fractions was analyzed with native polyacrylamide gel electrophoresis (PAGE) (7%), as described by Davis (1964).
GST activity determination
By measuring the increase in the concentration of the conjugation product of GSH and 1-chloro-2,4-dinitrobenzene at 340 nm, the activity of GST was determined (Habig et al., 1974). Protein was estimated using the Coomassie brilliant blue G-250 and bovine serum albumin as a standard (Bradford, 1976).
GST inhibition studies
The different plant extracts were evaluated for their ability to inhibit the GSH-conjugating activity of GST under standard assay conditions. By measuring the activity of the enzyme in the presence of varying concentrations of the inhibitor and plotting the percentage activity values versus inhibitor concentrations, the inhibition constant (IC50) values were determined.
Cytotoxicity study
Cell line authentication and culture: The human breast cancer cell line (human Caucasian breast adenocarcinoma, MCF-7) and normal skin fibroblasts (BJ1) were identified and used for this study. All of the following procedures were carried out in a sterile area with a Laminar flow cabinet biosafety class II level (SG403INT, Baker, Sanford, ME, USA). The cells were suspended in Dulbecco’s modified essential medium HCT116 with a mixture of 1% antibiotic-antimycotic (10,000 U/mL of potassium penicillin, 10,000 μg/mL of streptomycin sulfate, and 25 mg/mL Amphotericin B) and 1% L-glutamine at 37°C under 5% CO2. The cells were batch cultured for 10 days and seeded at a concentration of 10×103 cells/well in a fresh complete growth medium. 96-well microtiter plastic plates were used at 37°C for 24 h under 5% CO2 in a water jacketed carbon dioxide incubator (TC2323, Sheldon Manufacturing Inc., Cornelius, OR, USA). The media was aspirated, fresh medium (without serum) was added and cells were incubated either alone (negative control) or with different concentrations of sample (extract) to produce a final concentration (100, 50, 25, 12.5, 6.25, 3.125, 1.56, and 0.78 μg/mL).
Cell viability assay: Cell viability was assessed via the mitochondrial dependent reduction of yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to purple formazan (Mosmann, 1983). After 48 h of incubation, the media was aspirated, and 40 μL of MTT salt (2.5 μg/mL) was added to each well, and four more hours of incubation occurred at 37°C under 5% CO2. To stop the reaction and the formed crystals from dissolving, 200 μL of 10% sodium dodecyl sulfate in deionized water was added to each well to be incubated overnight at 37°C (Thabrew et al., 1997). The level of absorbance was measured using a microplate multi-well reader (model 3350, Bio-Rad Laboratories Inc., Hercules, CA, USA) at 595 nm with a reference wavelength of 620 nm. Dimethyl sulfoxide (DMSO) was used as the vehicle for the dissolution of plant extracts, and the final concentration that was recorded for the cells was less than 0.2%. The percentage of change in viability was calculated according to the following formula:
Ethics approval
Volunteers provided blood and placenta samples, with an informed verbal agreement always being acquired prior to the interview by local requirements. All experiments were performed under Egyptian laws and National Research Center regulations approved by the Medical Research Ethics Committee (MREC) number 16195. The human breast cancer cell line (MCF-7) and normal skin fibroblasts (BJ1) were performed and identified by the Drug Bioassay-Cell Culture Laboratory, National Research Center, Cairo, Egypt.
Statistical analysis
All data are reported as mean±standard deviation for n=3 to 4 independent experiments. Data were statistically analyzed using one-way analysis of variance option in SAS 9.3 (IBM Corp., Armonk, NY, USA). Significant differences among means were separated using Duncan’s test. The P-values of less than 0.05 were considered to be significant. Principal component analysis (PCA) was used to graphically display groupings of different plants relative to the measurements taken (SIMICA-P11.5 software, Umetrics, Umeå, Sweden). Pearson correlations were used to test the relationships between different measurements that were taken. For the cell viability assay, the statistical significance was assessed between samples and the negative control (vehicle cells) using the SPSS 11 (SPSS Inc., Chicago, IL, USA) independent t-test. A probit analysis for IC50 and IC90 was conducted using the SPSS 11 system.
RESULTS
TPC
Different plant samples (1 g of each sample) were extracted in 10 mL (1:10 w/v) of different solvents (water, 30% ethanol, 70% ethanol, 30% methanol, 70% methanol, 30% acetone, and 70% acetone) in our preliminary study (data not shown). For the process of plant extraction, 70% ethanol was used, while the greatest phenolic yield was obtained using 70% acetone; for the extraction of three plants from the Zingiberaceae family. The TPC of 70% of solvent plant extracts are summarized in Table 3. The extracts from C. verum, T. indica, and A. galanga had the greatest phenolic values [35.5±0.5, 33.2±0.8, and 26.8±3.4 gallic acid equivalents (GAE)/g, respectively]. There was a considerable amount of phenolic content from the Curcuma longa extract (18.1±2.3 GAE/g). The remaining plants had phenolic levels ranging from 1.1 to 13.3 GAE/g.
Table 3.
Phenolic compound contents, antioxidant, and pro-oxidant activities of different plant extracts
| Plant name | TPC (mg GAE/g dry plant) | TFC (mg RE/g plant) | TAC (mg CGE/g plant) | TCT (mg CE/g dry plant) | THT (mg TAE/g dry plant) | DPPH EC50 (mg) | H2O2 production (μM) |
|---|---|---|---|---|---|---|---|
| Curcuma longa | 18.14±2.30c | 50.00±5.80a | 0 | 114.70±4.20c | 35.90±6.00a | 3.33±0.39hi | 7.53±0.51d |
| Zingiber officinale | 8.95±0.63e | 2.65±0.30fg | 0 | 24.30±3.36e | 25.40±1.90c | 29.60±0.41c | 0.58±0.08e |
| Alpinia galanga | 26.83±3.40b | 17.00±0.85c | 27.04±2.40e | 61.30±0.67d | 21.80±0.32d | 0.52±0.09i | 28.40±1.50c |
| Tamarindus indica | 33.20±0.78a | 4.66±1.00e | 117.00±2.60b | 445.00±3.45a | 21.50±0.22d | 0.35±0.00i | 48.31±3.70a |
| Cinnamomum verum | 35.50±0.50a | 1.73±0.30fgh | 250.00±15.30a | 243.00±3.35b | 30.10±1.40b | 0.81±0.07i | 39.24±0.63b |
| Syzygium aromaticum | 2.95±0.46gh | 2.80±0.20fg | 0 | 10.20±0.36h | 0.59±0.09j | 69.20±10.50b | ND |
| Apium graveolens | 13.26±1.25d | 24.30±2.00b | 1.09±0.20g | 5.66±0.22ij | 14.30±0.23e | 4.51±0.39h | ND |
| Punica granatum | 9.66±0.74e | 4.80±1.11e | 66.90±5.90d | 18.70±0.33f | 19.90±0.04d | 5.50±0.03gh | 1.09±0.07e |
| Lepidium sativum | 9.53±3.15e | 3.60±0.72ef | 0 | 1.97±0.36k | 2.33±0.05ij | 4.40±0.40h | ND |
| Raphanus sativus | 12.89±0.45d | 0.42±0.07h | 0 | 2.49±0.08jk | 8.26±0.26fg | 8.50±1.48fg | ND |
| Eruca sativa | 7.59±2.10ef | 2.80±0.00fg | 0 | 5.10±0.30ijk | 4.00±0.10hi | 20.10±3.14d | ND |
| Brassica rapa var. rapa | 7.50±1.30ef | 2.26±0.25fgh | 3.10±0.90g | 3.96±0.20ijk | 9.90±0.15f | 0.43±0.02i | ND |
| Brassica oleracea | 12.64±0.38d | 1.75±0.25fgh | 0 | 5.80±0.04i | 10.10±0.60f | 13.43±1.53e | ND |
| Brassica oleracea var. botrytis | 7.82±0.65ef | 1.11±0.20gh | 0 | 3.10±0.07ijk | 9.70±1.10f | 10.80±0.77ef | ND |
| Hibiscus cannabinus | 5.28±0.13fg | 9.66±0.61d | 0 | 1.74±0.12k | 5.70±0.23gh | 19.29±0.66d | ND |
| Hibiscus sabdariffa | 1.14±0.19h | 2.90±0.17fg | 92.14±10.10c | 2.39±0.16jk | 3.30±0.24hij | 123.20±9.78a | ND |
| Gossypium arboreum | 1.70±0.34h | 4.73±0.70e | 12.00±2.40f | 13.74±0.39g | 1.60±0.19ij | 8.69±0.04fg | ND |
Values are presented as mean±SD.
Means with different letters (a-k) within the same column are significantly different at P<0.05.
TPC, total phenol content; TFC, total flavonoid content; TAC, total anthocyanin content; TCT, total condensed tannin; THT, total hydrolyzed tannin; DPPH, 1,1-diphenyl-2-picryl-hydrazyl radical; EC50, the concentration of the extract which is required to scavenge 50% of DPPH free radicals; GAE, gallic acid equivalents; RE, rutin equivalents; CGE, cyanidin-3-glucoside equivalents; CE, catechin equivalents; TAE, tannic acid equivalents; ND, not detected.
TFC
The greatest flavonoid concentration was found in the C. longa extracts [50.0±5.8 rutin equivalents (RE)/g] compared with all of the other plant extracts that were measured. The highest flavonoid concentration was found in Apium graveolens (24.3±2.0 RE/g), followed by A. galanga (17.0±0.9 RE/g) and Hibiscus cannabinus (9.7±0.6 RE/g). The flavonoid content of the remaining plants was lower (<5.0 RE/g), as shown in Table 3.
TAC
The highest anthocyanin concentration was found in the C. verum extracts [250.0±15.3 mg cyanidine-3-glucoside equivalents (CGE)/g], followed by T. indica (117.0±2.6 mg CGE/g), Hibiscus sabdariffa (92.14±10.1 mg CGE/g), and Punica granatum (66.9±5.9 mg CGE/g). In the extracts of A. galangal, G. arboreum, Brassica rapa var. rapa, and A. graveolens, the content of anthocyanin was low (Table 3).
Total condensed and hydrolysable tannin contents
The values of TCT and THT from the 18 plants were represented as mg CE/g plant and mg TAE/g plant, respectively (Table 3). The highest value for condensed tannins (445.0±3.4) was found in the T. indica extracts, followed by the C. verum extracts. Condensed tannins for C. longa were 114.7±4.2 mg CE/g and 61.3±0.7 CE/g for A. galanga. The highest hydrolysable tannin concentrations (35.9±6.0 TAE/g and 30.1±1.4 TAE/g, respectively) belonged to the C. longa and C. verum extracts. A. galanga, T. indica, and P. granatum also contained significant hydrolyzable tannin concentrations (in the range of 20∼25 TAE/g).
Antioxidant capacity using DPPH scavenging activity
The EC50 values for the scavenging activity of DPPH revealed that the T. indica seed extract had a high antioxidant capacity (0.35±0.00 mg/g plant) compared with the other plant extracts. Furthermore, B. rapa var. rapa, A. galanga, and C. verum had excellent antioxidant capabilities, with EC50 values of less than 0.81±0.07 mg. However, the antioxidant capabilities of the other investigated plants range from moderate (EC50≥3.33 mg) to weak (EC50≥69.2 mg) (Table 3).
Pro-oxidant activity (H2O2 producing activity) for the examined plant extracts and their heavy metal contents
After being incubated for 24 h at a concentration of 250 mg per extract, the production of H2O2 was measured in the 18 plant extracts, as tabulated in Table 2. To assess their levels and production ability of H2O2, concentrations of Cu2+, Fe2+, Mn2+, and Zn2+ were evaluated in the tested plants (Table 4). The T. indica and C. verum extracts had the highest generation capacity of H2O2 (48.3±3.7 and 39.2±0.63 μmol/mg plant, respectively), followed by A. galanga (28.4±1.5 μmol/mg plant). The T. indica extract had a high level of Cu2+ (10.02 mg/kg plant), the C. verum extract had a high level of Mn2+ (245.37 mg/kg plant), and the A. galanga extract had a high level of Fe2+ (714.71 mg/kg plant). The extracts of C. longa, P. granatum, and Zingiber officinale generate low amounts of H2O2 (7.53±0.51 μM, 1.09±0.07 μM, and 0.58±0.08 μM) and contain high levels of Mn2+ and Fe2+. The other plants that were tested released H2O2 at concentrations less than 3.0 μM. Although Z. officinale had high levels of Fe2+ (395.58 mg/kg) and Mn2+ (291.09 mg/kg), their ability to produce H2O2 was relatively low (0.58±0.08 μmol/mg plant). The A. graveolens seeds also had the greatest quantities of Cu2+ and Fe2+ (11.31 mg/kg and 614.50 mg/kg, respectively). However, under the experimental conditions, their extracts are unable to generate H2O2. The same is true for the extracts of Syzygium aromaticum (which also includes a high amount of zinc), B. rapa var. rapa, Brassica oleracea, and Eruca sativa which have significant iron quantities (Table 4).
Table 4.
Heavy metal concentrations of the examined plants (unit: mg/kg plant)
| Plant name | Some heavy metal concentration | |||
|---|---|---|---|---|
|
| ||||
| Copper | Iron | Manganese | Zinc | |
| Curcuma longa | 2.32±0.20i | 175.58±11.30gh | 113.19±10.40c | 10.16±2.00f |
| Zingiber officinale | 5.23±0.75g | 395.58±48.80c | 291.09±19.90a | 20.15±1.80e |
| Alpinia galanga | 8.97±0.60bc | 714.71±45.50a | 98.68±13.30c | 21.83±2.10de |
| Tamarindus indica | 10.02±1.20b | 39.21±5.13j | 4.87±0.49g | 14.02±1.16f |
| Cinnamomum verum | 6.82±0.86ef | 81.79±8.50ig | 245.37±26.40b | 11.59±0.97f |
| Syzygium aromaticum | 6.97±0.60ef | 90.55±11.90i | 30.70±4.30e | 43.54±3.60a |
| Apium graveolens | 11.31±0.46a | 614.50±15.50b | 52.03±5.56d | 28.65±2.90c |
| Punica granatum | 8.49±0.73cd | 220.00±43.60fg | 11.58±2.40fg | 15.06±1.87f |
| Lepidium sativum | 3.08±0.40hi | 144.86±9.78h | 18.49±2.60efg | 25.27±3.00cde |
| Raphanus sativus | 2.40±0.50i | 68.59±7.56ij | 11.38±1.50fg | 25.46±2.40cde |
| Eruca sativa | 2.27±0.56i | 229.64±11.30ef | 15.88±2.80efg | 28.35±4.20c |
| Brassica rapa var. rapa | 5.97±0.60fg | 329.90±43.00d | 22.62±3.00efg | 41.47±9.70a |
| Brassica oleracea | 3.12±0.27hi | 249.45±32.00ef | 24.01±3.40ef | 27.13±3.50cd |
| Brassica oleracea var. botrytis | 3.87±0.67h | 272.50±33.60e | 25.80±3.30ef | 30.31±2.70bc |
| Hibiscus cannabinus | 12.39±0.88a | 177.03±20.80gh | 13.67±1.46efg | 34.96±3.60b |
| Hibiscus sabdariffa | 7.45±1.00de | 81.07±9.15ig | 10.34±1.00fg | 29.37±3.30c |
| Gossypium arboreum | 7.22±1.00ef | 97.44±13.60i | 9.38±1.68fg | 24.90±2.10cde |
Values are presented as mean±SD.
Means with different letters (a-i) within the same column are significantly different at P<0.05.
Correlation between different phenolic compounds, antioxidant/pro-oxidant activities, and metal contents of the plants investigated
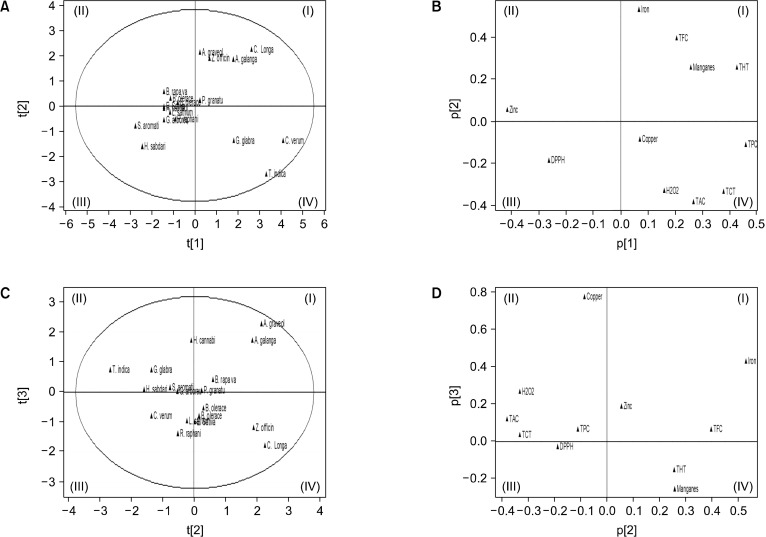
To categorize the polyphenol concentrations, antioxidant capacities, pro-oxidant activity, and metal ion contents of the 18 plants that were studied, the PCA was used. The score plots (Fig. 1A and 1C) were used to compare the 18 plant extracts’ similarities and differences. A substantially positive correlation between TPC, TCT, and THT (0.81 and 0.70, respectively) was revealed from the statistics using the Pearson correlation matrix on plant samples with various polyphenol concentrations, DPPH radical scavenging activities, and metal levels. TPC, TAC, and THT similarly had a strong negative correlation with the level of zinc metal (0.66, 0.59, and 0.68, respectively). The THT showed a highly positively correlation with the Mn2+ contents (0.68). The data also showed a moderately positively correlation between TPC and TAC (0.50), TFC and THT (0.53), and TCT and THT (0.54). A moderately negatively correlation was shown between TPC and the antioxidant activity of DPPH (0.50), as well as the contents of TCT and zinc (0.53). The loading score graphs (Fig. 1B and 1D) were used to overview the differential relevance of the eight variable parameters. According to the results of the analysis, the extracts of T. indica and C. verum were found in quadrant I, which has the highest TFC, THT, and Mn2+ levels as revealed in the loading score plots (Fig. 1B and 1D). A. galangal, C. longa, Z. officinale, and A. graveolens were grouped in quadrant IV, which has the highest levels of TPC, TAC, TCT, Cu2+, and pro-oxidant activity.
Fig. 1.
Scores and Loadings plot of principle components analysis. (A, C) Scores plot for the 18 examined plant extracts. (B, D) Loadings plot for results of 9 different variables. The t[1], t[2], and t[3] indicate matrix of scores that summarizes X variables. The p[1], p[2], and p[3] indicate matrix of loading showing the influence of variables. TPC, total phenol contents; TFC, total flavonoid contents; TAC, total anthocyanin contents; THT, total hydrolyzed tannin; TCT, total condensed tannin; DPPH, 1,1-diphenyl-2-picryl-hydrazyl-scavenging activity; H2O2, pro-oxidant activity; different metal ions contents (copper, manganese, iron, and zinc). PC1, first principal components; PC2, second principal components; PC3, third principal components.
HPLC identification and quantification of phenolic compounds extracted from the three plants exhibiting dual antioxidant/pro-oxidant activity (T. indica, C. verum, and A. galanga)
The phenolic components in T. indica, C. verum, and A. galanga extracts were identified by HPLC, as shown in Table 5. The T. indica extract contained 0.09 mg of protocatechuic acid, representing 25% of the total phenolic components (0.336 mg/g dry plant). Significant levels of quercetin (0.07 mg/g), catechin, rutin, and apigenin-7-glucoside (0.04 mg/g) were found, with values of 19.7%, 11.3%, 10.4%, and 10.5%, respectively. Less than 10% of the total phenolic compounds (caffeic acid, syringic acid, and cinnamic acid) were accounted for by the other identified compounds. The C. verum extract had cinnamic acid (5.4 mg/g) and p-coumaric acid (3.81 mg/g), representing 56.3% and 39.7%, respectively, of the total phenolic compounds (9.6 mg/g plant). The total content of phenolic compounds from the other identified compounds ranged from 1.55% (apigenin of 0.15 mg/g plant) to 0.15% (chrysin=0.01 mg/g plant). Cinnamic acid maintained the highest content in the A. galanga extract (1.02 mg/g), accounting for 45% of the identified phenolics (2.26 mg/g) (Table 5). There were also significant amounts of chrysin (0.23 mg/g), catechin (0.21 mg/g), p-coumaric acid (0.19 mg/g), and rosmarinic (0.18 mg/g), with 10%, 9.3%, 8.3%, and 7.8%, respectively.
Table 5.
Identified phenolic compounds of Tamarindus indica, Cinnamomum verum, and Alpinia galanga extracts using HPLC analysis
| Phenolic group | Compound name | Plant | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| T. indica | C. verum | A. galanga | |||||||
|
|
|
|
|||||||
| Rt (min) | mg/g dry plant | Rt (min) | mg/g dry tissue | Rt (min) | mg/g dry tissue | ||||
| Benzoic acid | Vanillic | 24.8 | 0.016 | 25.6 | 0.027 | 26.3 | 0.014 | ||
| Protocatechuic acid | 9.9 | 0.085 | 10.3 | 0.032 | 10.1 | ND | |||
| p-Hydroxybenzoic acid | 15.1 | ND | 15.7 | ND | 16.0 | 0.009 | |||
| Syringic acid | 23.0 | 0.022 | 24.0 | ND | 24.8 | 0.019 | |||
| Cinnamic acid | Cinnamic | 42.8 | 0.00017 | 43.4 | 5.40 | 45.7 | 1.018 | ||
| p-Coumaric acid | 37.2 | ND | 37.8 | 3.81 | 37.2 | 0.187 | |||
| Chlorogenic acid | 20.6 | ND | 21.4 | ND | 22.5 | 0.092 | |||
| Caffeic acid | 21.4 | 0.0079 | 22.2 | ND | 23.2 | ND | |||
| Ferulic acid | 32.4 | ND | 33.4 | ND | 35.5 | 0.074 | |||
| Sinapinic acid | 33.8 | ND | 34.7 | 0.022 | 36.3 | 0.003 | |||
| Rosmarinic | 40.0 | 0.0304 | 40.0 | ND | 42.6 | 0.177 | |||
| Flavonoid | Rutin | 36.2 | 0.0350 | 36.7 | 0.045 | 38.4 | 0.018 | ||
| Quercetin | 43.5 | 0.0663 | 46.7 | 0.014 | 46.7 | 0.014 | |||
| Naringin | 37.8 | ND | 45.7 | ND | 40.6 | ND | |||
| Kaempferol | 46.5 | ND | 47.0 | 0.077 | 49.6 | 0.11 | |||
| Myricetin | 39.5 | ND | 42.0 | ND | 42.0 | ND | |||
| Apigenin-7-glucoside | 38.8 | 0.0354 | 39.4 | ND | 40.6 | 0.093 | |||
| Apigenin | 46.0 | ND | 46.5 | 0.149 | 48.9 | ND | |||
| Chrysin | 52.0 | ND | 52.4 | 0.014 | 54.7 | 0.226 | |||
| Hesperidin | 38.5 | ND | 39.0 | ND | 41.0 | ND | |||
| Catechin | 18.6 | 0.038 | 19.4 | ND | 20.2 | 0.21 | |||
HPLC, high performance liquid chromatography; Rt, retention time; ND, not detected.
Antioxidant and pro-oxidant activities of the HPLC identified phenolic compounds
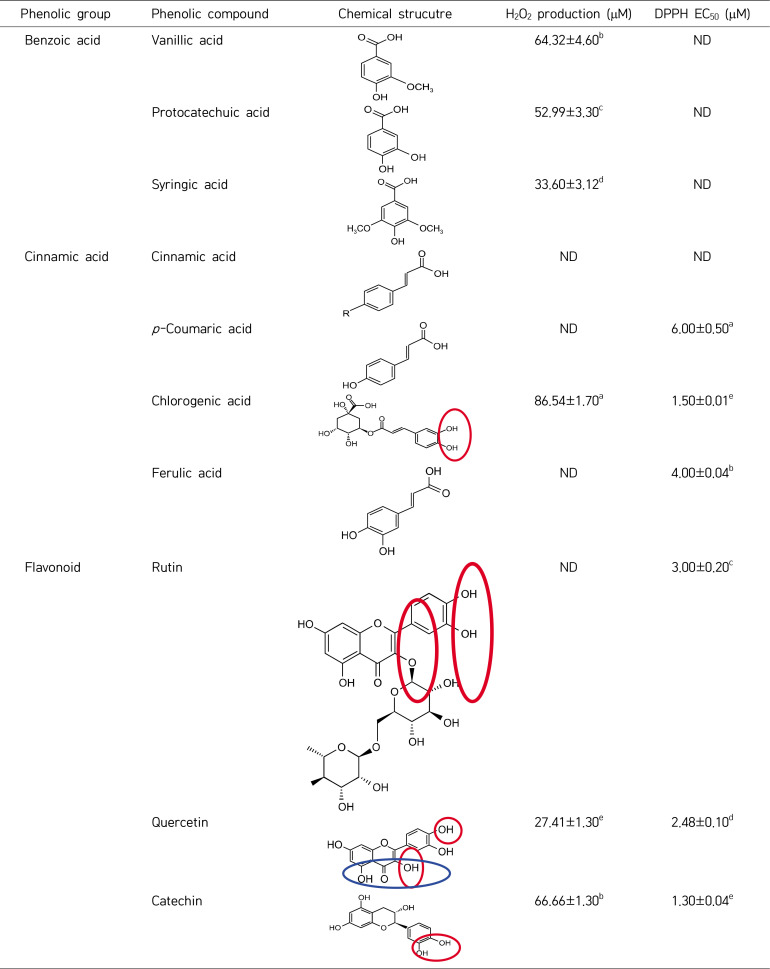
The antioxidant/pro-oxidant activity of the identified phenolic compounds were described in the HPLC analysis, as well as the chemical structure of the potential plant extracts of T. indica, C. verum, and A. galgana (Table 6). Each phenolic compound (250 μM) was incubated in a 50 mm sodium phosphate buffer with a pH of 7.4 at 37°C for 24 h. Both chlorogenic acid and flavonoids, such as quercetin and catechin, have a dual antioxidant and pro-oxidant action with significant values. The amounts of H2O2 that were produced by vanillic acid, protochatoic acid, and syringic acid represented 64.3, 52.9, and 33.6 mM, respectively. Cinnamic acid did not exhibit any antioxidant or pro-oxidant activity among the cinnamic acid derivative chemicals that were studied. Hydroxy-cinnamic acid derivatives, such as p-coumaric and ferulic acids, were found to have antioxidant action.
Table 6.
Important phenolic compounds with antioxidant and/or pro-oxidant properties identified in Tamarindus indica, Cinnamomum verum, and Alpinia galanga plant extracts
Values are presented as mean±SD.
The chemical structures were drawn using ACD/ChemSketch 2018.2.1 program (ACD/Labs, Toronto, ON, Canada).
Means with different superscript letters within the same column are significantly different at P<0.05.
DPPH, 1,1-diphenyl-2-picryl-hydrazyl; EC50, the concentration of the extract which is required to scavenge 50% of DPPH free radicals; ND, not detected.
Inhibitory effect of plant extracts on GST activity
The 18 plant extracts were tested for their ability to inhibit the predominant GST activity affinity purified from recombinant hGSTP1-1, human erythrocytes, and the GSTs from the placenta (Table 7). The IC50 is the inhibitory concentration required to inhibit GST activity by about 50%, was calculated by graphing the relative values of residual enzyme activity in the presence of an inhibitor versus the extract concentrations (0.004∼10 mg/g plant). The IC50 values were calculated as mg GAE/g plant (Table 7) indicated that the extracts of H. sabdariffa, C. verum, and A. galanga have the strongest inhibitory activity on the hGSTP1-1 enzyme, with IC50 values of 0.48±0.06, 0.66±0.08, and 0.82±0.07 mg GAE/g, respectively. The T. indica (IC50=1.12±0.26 mg GAE/g) and G. arboreum (IC50=1.59±0.18 mg GAE/g) extracts inhibit hGSTP1-1 activity identically. The T. indica and Z. officinale extracts have IC50 values on the erythrocyte GST of 1.80±0.18 and 3.60±0.49 mg GAE/g, respectively. The G. arboreum and Lepidium sativum extracts show nearly identical inhibitory actions on the erythrocyte GST (IC50=6.80±0.74 and 6.84±0.55 mg GAE/g, respectively). The C. longa extract inhibited the activity of erythrocyte GST (IC50=7.30±0.90 mg GAE/g). The other plants investigated had little to no inhibitory effects on the activity of erythrocyte GST (IC50 values greater than 10 mg/g dry plant). With an IC50 value of 0.75±0.06 mg GAE/g, the extract of T. indica was affected the activity of the purified placenta GST the most. The G. arboreum, H. sabdariffa, and P. granatum extracts all had confirmed effects on the placenta GST, with IC50 values of 2.70±0.42, 4.32±0.76, and 4.74±0.52 mg GAE/g, respectively. The IC50 values for the other plants examined on the placenta GST ranged from 7.20±0.60 to 34.80±1.70 mg GAE/g, as shown in Table 7.
Table 7.
IC50 values of the plant extracts examined on affinity purified GSTs (unit: mg GAE/g dry plant)
| Examined plant | hGSTP1-1 | Erythrocyte GST | Placenta GST |
|---|---|---|---|
| Curcuma longa | 4.54±0.67d | 7.30±0.90d | 8.80±0.92f |
| Zingiber officinale | 5.30±0.55d | 3.60±0.49e | 11.30±0.85e |
| Alpinia galanga | 0.82±0.07efg | 11.80±0.96b | 10.80±1.00e |
| Tamarindus indica | 1.12±0.26ef | 1.80±0.18f | 0.75±0.06i |
| Cinnamomum verum | 0.66±0.08efg | 12.78±0.85b | 12.18±0.40e |
| Syzygium aromaticum | 4.52±0.68d | NI | 7.20±0.60f |
| Apium graveolens | 4.80±0.63d | 10.40±0.96c | 7.40±0.45f |
| Punica granatum | 1.67±0.35e | 10.50±0.85c | 4.74±0.52g |
| Lepidium sativum | 5.36±0.50d | 6.84±0.55d | 7.23±0.88f |
| Raphanus sativus | 14.60±0.88a | NI | 19.30±0.95c |
| Eruca sativa | 12.60±1.00b | NI | 30.40±1.90b |
| Brassica rapa var. rapa | NI | 12.45±0.83b | 16.30±1.20d |
| Brassica oleracea | 11.80±1.10b | 16.00±1.00a | 34.80±1.70a |
| Brassica oleracea var. botrytis | NI | 9.90±0.70c | 16.75±0.96d |
| Hibiscus cannabinus | 6.63±0.84c | NI | NI |
| Hibiscus sabdariffa | 0.48±0.06fg | NI | 4.32±0.76g |
| Gossypium arboreum | 1.59±0.18e | 6.80±0.74d | 2.70±0.42h |
Values are presented as mean±SD.
Means with different letters (a-i) within the same column are significantly different at P<0.05.
IC50, the quantity of phenolic extract in GAE per gram of dry plant that inhibits enzyme activity by 50%; GAE, gallic acid equivalent; GST, glutathione transferase; NI, no inhibition (inhibition of GST activity could not be detected under our experiment conditions).
The cytotoxic effect of some plant extracts on MCF-7 cancer cell lines
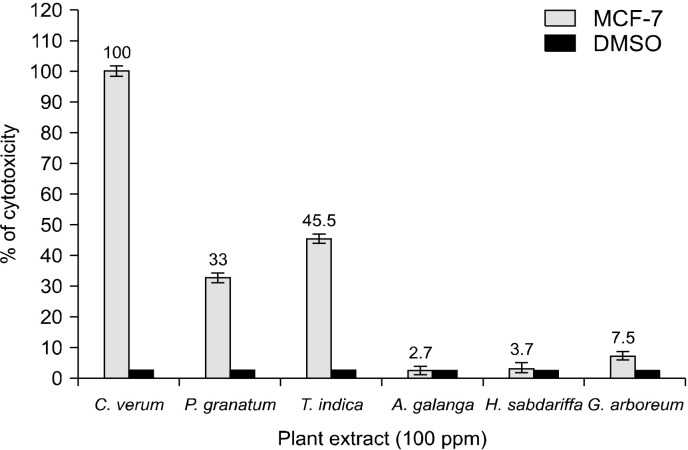
Cytotoxicity was examined for the most effective plants with dual antioxidant/pro-oxidant activities or the inhibitory activity of GST on human Caucasian breast cancer (MCF-7). The cytotoxicity of the extracts of C. verum, P. granatum, T. indica, A. galangal, H. sabdariffa, and G. arboreum at 100 ppm on MCF-7 resulted in the following cellular viability inhibition: 100%, 0%, 33.0%, 45.5%, 2.7%, 3.7%, and 7.5%, respectively (Fig. 2).
Fig. 2.
The cytotoxic effect of the examined plant extracts on breast cancer cell line (human Caucasian breast adenocarci-noma, MCF-7) at a concentration of 100 ppm compared to cells treated with dimethyl sulfoxide (DMSO) as a negative control. Error bars represent standard deviation from the mean.
DISCUSSION
The natural plant extracts that are used in traditional medicines in the field of ethnopharmacology are also productive for use in pharmacological models and are regarded as alternative therapies for various diseases (Majouli et al., 2017). Plant-derived antioxidants are a wide group of natural compounds with reduced or radical scavenging capabilities, particularly for dietary fruits and vegetables. These compounds attract a great deal of attention for scientists, pharmacologists, and physicians due to their effective preventive and therapeutic actions. Antioxidants demonstrate their benefits by directly reducing oxidative stress. Oxidative stress is the outcome of imbalances between ROS production and antioxidants. Oxidative stress in living cells can contribute to the breakdown of lipids, proteins, and nucleic acids, leading to the over-expression of oncogenes, the development of mutagens, and inflammation (Szymanska et al., 2016).
There is a wide variety of Mediterranean flora that includes a considerable number of native medicinal and aromatic plants. Several of these have many potential pharmacological and antioxidant uses (Grigorakis and Makris, 2018). Broad distribution and biological activity were correlated with the following plant families, which are widely used in food and traditional medicine: Zingiberaceae, Fabaceae, Lauraceae, Myrtaceae, Apiaceae, Lythraceae, Brassicaceae, and Malvaceae (Sharma and Kapoor, 2015). Chemical diversity among the members of these families may contribute to their activities. Isolated phenolic compounds, such as flavonoids, phenolic acids, and polysaccharides from certain family members, are thought to be responsible for such activities (Vadivel et al., 2016).
A 70% solvent extraction yielded the highest overall phenolic component concentration for all of the plants in this investigation. The extraction of phenolic compounds from plant materials depends mostly on the nature of the sample matrix, the solvent and extraction methods, time and storage conditions, the presence of interfering substances, and the chemical properties of the phenolics. This includes the molecular structure, polarity, concentration, and the number of aromatic rings and hydroxyl groups (Koffi et al., 2010).
Phenolic compounds are a chemically diverse group of secondary metabolites found in plants, and they contain antioxidant capabilities with a range of health benefits (Csepregi and Hideg, 2018). The antioxidant activities of phenolic compounds are mostly due to their redox characteristics which allow them to operate as reducing agents, hydrogen donors, and oxygen quenchers. The hydroxyl (OH−) groups of phenolics are effective H-donating antioxidants that scavenge the ROS. Thus, they block the subsequent radical production process. Typically, an increase in the OH group increases in the antioxidant potential in vitro (Quideau et al., 2011).
Our results indicated the relationship between the content of phenolic compounds in plant extracts and their antioxidant ability. The T. indica extracts containing the highest number of phenolic compounds also have the highest antioxidant ability. This is valid for the extracts of C. verum and A. galanga, as well the high phenolic content that is correlated with a strong antioxidant capacity. Previous research has found that the antioxidant potential corresponds to the content of phenolic compounds and flavonoids, agreeing with the findings of this study (Wong et al., 2006; Martins et al., 2015).
The observed antioxidant effect of these extracts could be attributed to plant extracts’ electron-donating activity, which results in the formation of a stable product and the termination of a free radical chain reaction. Strong scavenging abilities of the T. indica, C. verum, and A. galanga extracts can be related to the structural conformation of the phenolic compound. Phenolic compounds can provide the required component as a radical scavenger, even though phenolic compounds containing high concentrations of hydroxyl groups can reduce DPPH particles very quickly (Vadivel et al., 2011).
However, the concentration of the phenol and antioxidant power cannot always be associated. This is true for the B. rapa var. rapa extracts with a low phenolic content and a high antioxidant ability. A high level of antioxidant activity in the extract of B. rapa var. rapa can be attributed to the combined action of vitamin C and a number of biologically active substances found in the extract itself. Vitamin C and phenolic compounds account for 80% of the antioxidant production in Brassica plants (Sharma and Kapoor, 2015).
Similarly, when the total flavonoid and anthocyanin contents obtained in this study were compared with the antioxidant properties, there was no association between the concentration of flavonoids and antioxidant capacity. For example, the highest content of flavonoids in the C. longa extracts (50.0±5.8 RE/g) was associated with a low antioxidant capacity. However, a high concentration of anthocyanin in the C. verum and T. indica extracts was associated with their strong antioxidant capacity. This relationship was not valid in the case of the A. galanga and B. rapa var. rapa extracts, as concentrations of flavonoids and anthocyanins were low, and their antioxidant capacities were high.
The relationship between the concentration and antioxidant capacity relationship was valid for some plant extracts and invalid for others as far as condensed tannins are concerned. For example, the highest value was observed in the T. indica extracts, followed by the C. verum extracts, were associated with powerful antioxidants. However, in the case of the C. longa, A. galanga, and B. rapa var. rapa extracts, no relationship existed between the concentration of tannins and their antioxidant capacity.
Broad nutritional effects have been attributed to antioxidant activity, free radical scavenging mechanisms, and transition metal ion chelating activity of flavonoids (Lipiński et al., 2017). The structural differences of flavonoids based on the degree and pattern of hydroxylation, methylation, and glycosylation are typically associated with an antioxidant action. They may be due to 1) the catechol group on the B-ring which causes high stability for the radical formed after the capture of the free radical, 2) the two and three double bond in conjugation with the 4-ox function of the carbonyl group on the C ring, and 3) the presence of the hydroxyl group at the three and five positions (Maqsood et al., 2013).
It is not always possible to correlate the concentration of phenolic compounds with the antioxidant capacity according to the results of this study. This can be explained in particular by the structural factors of the antioxidant properties, including the phenolic group, their nature, the alternatives, and the changes in glycosylation (Wang et al., 2018). Other factors, including the presence of different active compounds in the plant, may alter the antioxidant capacity, synergistic effects, experimental conditions, and different antioxidant identification mechanisms. Some compounds interact strongly with DPPH and others that interact more slowly (de Brum et al., 2013).
In some cases, phenolic compounds have been considered to be toxic and mutagenic compounds. The toxicity of phenolic compounds has not been completely identified and has been ignored for several years (Olas, 2018). Under certain conditions, phenolic compounds can have pro-oxidant activity, as they often oxidize at high concentrations of acidic pH and in the presence of transition metals (particularly Fe2+ and Cu2+). The pro-oxidant activity of a number of phenolic compounds that cause oxidative stress, either through the generation of ROS or through the inhibition of antioxidant systems such as GST, result in oxidative damage to cells and biological macromolecules. Unlike its antioxidant properties, this pro-oxidant activity is not necessarily detrimental to biological systems and human health and can be used therapeutically to treat oxidative stress. In addition, it is a potential anticancer mechanism (Yordi et al., 2012; Eghbaliferiz and Iranshahi, 2016).
According to the present results, the large amounts of metal ions in the extracts are not the main reason for the high ability to generate H2O2, but may act as a catalyst. Addition of 500 μM of the metal chelating agent (ethylenediaminetetraacetic acid) lowers the concentration of the generated H2O2; except for the T. indica extracts (data not shown). This may suggest that the presence of transition metal ions, including Fe2+ and Cu2+, may be related to the pro-oxidant potential of some plants (e.g., A. galanga). However, the composition of some phenolic compounds in other plants may be related to the development of the production of H2O2 (e.g., C. longa). In the case of the A. graveolens seeds, this was not true. A. graveolens contain maximum levels of Fe and Cu, and B. rapa var. rapa seeds contain a high level of Zn.
The presence of the transition metal ions, specifically in Fe2+ and Cu2+, had a profound influence on the pro-oxidant activity of phenolic compounds. The Fe2+ is the most common transition metal ion in many processes that are related to oxidative stress in biological systems. Extra amounts of heavy metal ions in a living cell will catalyze the generation of ROS through a Fenton reaction. H2O2 is quickly converted to the hydroxyl radical (OH・) in a Fenton reaction. The hydroxyl radical is known as a strong oxidant that can lead to oxidative cell damage. In addition, the presence of a higher concentration of copper in the cells makes them more sensitive to the pro-oxidant activity of phenols. Cu2+ is a potent catalyst for oxidative reactions in most of the studies conducted on phenolic pro-oxidant behavior. Manganese is an essential trace element that has protective or toxic effects depending on the concentration level, and it can have different pro-oxidant and antioxidant properties. An overdose of Mn2+ results in pro-oxidant activity. The Zn is an antioxidant element that exercises its activity indirectly and plays several biological roles in the human body. It is an antagonist to redox-active transition metals including Cu2+ and Fe2+. In addition to its antioxidant activity, an overdose of Zn results in pro-oxidant activity. Phenolic antioxidants have been shown to function as pro-oxidants under situations such as high concentrations of transition metal ions, alkali pH, and the presence of oxygen molecules (Eghbaliferiz and Iranshahi, 2016).
The extracts of T. indica, C. verum, and A. galanga have a strong ability to act as antioxidants and pro-oxidants in our experimental conditions. Therefore, the HPLC analysis was performed to identify and measure the phenolic compounds extracted from the three plant extracts. Cinnamic acid had the highest concentrations of the C. verum and A. galanga extracts according to our results. The presence of p-coumaric and protocatechuic acids has been indicated among the phenolic acids. Also among the flavonoids are rutin, quercetin and catechin. These three plants showed significant antioxidant activity. The oldest traditional medicines have many therapeutic activities including anticancer, antiinflammatory, antiallergic, antidiabetes, and immunosuppressant. The specific phenolic compounds detected in these plant extracts agree with the compounds identified in literature, such as catechin and cinnamic acid (Guneidy et al., 2017; Uchenna et al., 2018).
The double nature of the main phenolic compounds identified in the plant extracts that were tested for their antioxidant/pro-oxidant capacities has been investigated. The findings of the current investigation show that antioxidant capacity compounds do have pro-oxidant activity and comprise phenolic acids like protochatuic acid. The flavonoid compounds quercetin and catechin also exhibited and antioxidant/pro-oxidant activity. Our results contradict those of Eghbaliferiz and Iranshahi (2016), which reported that high molecular weight phenols, such as hydrolysable and condensed tannins, have little to no properties. However, the pro-oxidant potential of tannin compounds such as catechins, epigallocatechins, and epicatechins was detected by Akagawa et al. (2003). When the typical antioxidant citric acid compound was subjected to the same conditions, its H2O2 generation (0.548 mM) was smaller than that of the phenolic compounds investigated in this study (data not shown).
The chemical structures of the substances under consideration are models of the primary structural aspects associated with their antioxidant and oxidative capabilities, which are as follows: 1) the presence of a 3’,4’-dihydroxy on the B ring (for example, catechin and quercetin); 2) the presence of a double bond between the second and third carbons in the C ring, paired with a keto group at the fourth position, allowing the electron to be located between the A and B rings; and 3) the presence of hydroxyl group substitutions at position three of the C ring and position five of ring A (Michalak, 2006; León-González et al., 2015).
Compounds with similar structures demonstrated identical patterns in dual activities. The antioxidant and pro-oxidant activity of chlorogenic acid may be due to the presence of three adjacent OH groups. In addition, the flavonoid compound quercetin (2, 3 double bond in ring C beside 4-oxo arrangement) may be considered as a potent pro-oxidant, as explained by León-González et al. (2015). Flavonoids typically exist in foods such as O-glycosides with sugars bound to C3. Glycosylated flavonoids lose their pro-oxidant function relative to aglycones (Rahal et al., 2014).
The total number of hydroxyl groups in the phenol ring is linked to the antioxidant properties, but the pro-oxidant activity of phenol is linked to the configuration of the total number of hydroxyl groups 2, 3-double bond in the C ring and 4-oxo in the molecule (Procházková et al., 2011; Eghbaliferiz and Iranshahi, 2016). The number of OH group substitutes (especially in ortho position) has the greatest pro-oxidant ability (Eghbaliferiz and Iranshahi, 2016).
Inconclusive and conflicting results exist from previous research on the antioxidant and pro-oxidant functions of polyphenols. For example, León-González et al. (2015) suggested that heavy metals mediated the pro-oxidant behavior of polyphenols by reducing metal ions involved in redox-cycling and producing hydroxyl radicals via the Fenton reaction. However, the antioxidant activity of quercetin complexes with metal ions is greater than pure quercetin according to study by Xu et al. (2019). In the case of cellular oxidation, polyphenols are “double edged weapons.” The type, physiological or nutritional dosages, presence of metal ions, and management of compounds or other drugs may all be critical factors that affect the balance between the harmful and beneficial effects of these compounds in relation to the maintenance of healthy biological systems (Yordi, 2012; Castañeda-Arriaga et al., 2018). The pro-oxidant activity of dietary polyphenols and their ability to induce DNA damage, along with cellular apoptosis, have been proposed as a potential anticancer therapy to be used either on its own or as an adjuvant therapy combined with classical chemotherapy (Yordi, 2012; León-González et al., 2015).
Cells possess their own antioxidant defense systems which include antioxidants, such as GSH, and antioxidant enzymes, including glutathione peroxidase, glutathione reductase, and GST. Cells enhance the antioxidant defense system when they are exposed to oxidative stress conditions resulting from the pro-oxidant capacity of certain phenolics and flavonoids. For example, the flavonoid antioxidant compound epigallocatechin gallate potentiates hepatotoxicity by suppressing the nuclear factor Nrf2, the key protein in the regulation of antioxidant enzymes such as GST (Eghbaliferiz and Iranshahi, 2016). The ability of some phenolic acids and flavonoids to influence GST activity has been demonstrated in other studies (Muller et al., 2000; Pifferi and Restani, 2003).
GST proteins are overexpressed in a variety of human cancers, contributing to poor outcomes and decreased patient survival. GSTP1 plays a role in cancer initiation, progression, spread, and resistance to anticancer treatments. GSTP1 has emerged as a potential therapeutic target for cancer treatment because of studies conducted on antioxidant and redox biology. These inhibitors have the potential to improve the efficacy of chemotherapy and reduce drug resistance. More research is needed to understand the function of these inhibitors, as well as their long-term effects, before they can be used safely in cancer treatment (Singh and Reindl, 2021).
GSTP1-1, the only enzyme of the Pi class, is the most prominent erythrocyte isoenzyme, accounting for 95% of the total GST pool (Bocedi et al., 2019). Another isoform of GST Pi is found in normal placental tissue and accounts for 67% of the total GST concentration in this tissue. The concentration of GST in the placenta decreases during its development and is absent in adult tissues. However, it was found in adult tissues throughout the carcinogenesis process, suggesting that it could be a cancer biomarker (Noguti et al., 2012).
A simple and reproducible GST purification technique for recombinant hGSTP1-1, erythrocyte, and placenta homogenates was conducted using an affinity chromatography column. The electrophoresis pattern in our results employed the presence of one major GST form bound to the affinity column as one band that could be detected on PAGE stained for protein and GST activity (data not shown). The affinity chromatography matrix has been used extensively to purify cytosolic GST enzymes as they greatly facilitated to display the excellent specificity and yield of these enzymes (Aliya et al., 2003).
Eighteen plant extracts were tested for their ability to inhibit the activity of purified major GSTs in this study. According to our findings, the T. indica, C. verum, and A. galangal extracts had the greatest influence on GST activity in erythrocytes, placenta, and hGSTP1-1. In addition to these three strong extracts, the extracts of H. sabdariffa, G. arboreum, and P. granatum have potent inhibitory actions on the hGSTP1-1 enzyme. The high polyphenol content of plant extracts could be linked to the strong inhibitory activity of GST in these extracts. These results are consistent with the T. indica, C. verum, and A. galangal extracts. Conversely, the G. arboreum and H. sabdariffa extracts do not fall into this category. There are various interpretations of the confounding relationship between the inhibitory potency and phenol. The overall phenol content does not include all probable inhibitors. Inhibition can be determined by considering the synergy between inhibitors in the mixture. This was determined by the concentration of the individual inhibitors, their structure, and their interaction.
On MCF7 cell lines, the cytotoxic effect of five extracts with a high ability to inhibit GST activity was investigated. The cinnamon (C. verum) extracts were the most cytotoxic (100% then zero), followed by the T. indica (45.5% inhibition) and A. galangal (33.3% cell viability) extracts. The plants with high phenol content have an antioxidant/pro-oxidant capacity, as well as an inhibitor of GST activity, implying that more research into its potential use in cancer treatment and drug resistance is required.
The plant-derived chemicals, whether delivered as whole foods or isolated molecules, have an undeniable influence on all stages of cancer, including breast cancer, proven by the majority of preclinical oncology research (Kubatka et al., 2020). Kubatka et al. (2020) reported on the anticancer effect of Cinnamomum zeylanicum L. essential oil on two types of human breast adenocarcinoma cells (MCF-7 and MDA-MB-231), validating cinnamon’s protective and therapeutic potential in animal breast cancer models. A methanolic extract of T. indica (100∼1,000 g/mL) has considerable anticancer action that is dependent on the treatment concentration and period (24∼72 h) (Hussein et al., 2017). Furthermore, the cytotoxicity examination of silver nanoparticles from the fruit shell of the T. indica fruit, indicating that silver nanoparticles induce apoptosis in MCF-7 cells (Gomathi et al., 2020). Examination of the oxidative activity and generation of free radicals by the T. indica extract’s silver nanoparticles in such a study revealed the involvement of the generation of ROS and the oxidative stress state that causes MCF-7 cell death. Polyphenols from P. granatum have powerful anticancer activity in a variety of ways, including antiestrogenic, antiproliferative, antiangiogenic, antiinflammatory, and antimetastatic activity, according to Moga et al. (2021). Pomegranate extracts were found to be cytotoxic in a dose- and time-dependent manner. Findings from Cao et al. (2021) also suggest that cotton seed (G. arboreum) ethanol extracts have cytotoxic activity.
The simultaneous presence of beta-carotene and antioxidants improves cell protection against oxidative stress. However, the carcinogenic activity of β-carotene has been observed when administered in high-dose supplementation, which contains only synthetic beta-carotene, and in individuals with chronic oxidative stress observed in heavy smokers (Palozza et al., 2003; Eghbaliferiz and Iranshahi, 2016). Polyphenols have pro-oxidant activity in normal tissues at regular daily dosages. All concerns about natural antioxidants’ pro-oxidant action are connected to large concentrations. The cellular antioxidant defense cannot protect cells from the huge levels of ROS produced by pro-oxidant substances at high dosages (Eghbaliferiz and Iranshahi, 2016). These indicate that the concentration and potency of the extracts, exposure time, and the type of cells evaluated all play an essential biological effect.
In the end we must mention that,
• Some of the plants described in this study have an excellent combination of antioxidant and pro-oxidant activities, in addition to cytotoxic effects on cancer cells. This study could help with the long-term prospective use of some plant extracts (e.g., C. verum and T. indica) as adjuvant medicines.
• Moreover, further studies and experiments should be conducted at the biological level, rather than the laboratory, to research and understand the action and efficacy of these plant extracts or one of their phenolic compounds that are active in the biological system.
• Pro-oxidant activity of natural polyphenols may be considered as a modern oxidation therapy technique for cancer.
Studies on antioxidants or pro-oxidants of polyphenols should concentrate on bioavailability (absorption, metabolism, and distribution of cells and tissues) to suggest whether it is in vitro acceptable in vivo. It should be noted that the involvement of polyphenol with other components can affect their behavior. In addition, in vivo assays can be more relevant to their metabolites than the molecule itself in terms of biological activity.
Footnotes
FUNDING
The research was funded by the National Research Center, Cairo, Egypt (grant no. 11010342).
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
This work has been carried out in cooperation with all authors. RAG: designed the manuscript diagram, searched the literature, analyzed the data, wrote the manuscript and took on the task of publishing it. ERZ and AAMG: participate in designing experiments, analyzing data, performing experiments, and reviewing manuscripts. NSEDS: participate in designing experiments, analyzing data, performing experiments. AS: participate in the design of experiments; participate in experiments, statistical study of data and involvement in the review of the manuscript. All authors have read and approved the manuscript.
REFERENCES
- Akagawa M, Shigemitsu T, Suyama K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci Biotechnol Biochem. 2003;67:2632–2640. doi: 10.1271/bbb.67.2632. [DOI] [PubMed] [Google Scholar]
- Aliya S, Reddanna P, Thyagaraju K. Does glutathione S-transferase Pi (GST-Pi) a marker protein for cancer? Mol Cell Biochem. 2003;253:319–327. doi: 10.1023/A:1026036521852. [DOI] [PubMed] [Google Scholar]
- American Public Health Association, author; American Water Works Association, author; Water Environment Federation, author. Standard Methods for the Examination of Water and Wastewater. 23rd ed. American Public Health Association; Washington, DC, USA: 2017. p. 1545. [Google Scholar]
- Banerjee D, Madhusoodanan UK, Sharanabasappa M, Ghosh S, Jacob J. Measurement of plasma hydroperoxide concentration by FOX-1 assay in conjunction with triphenylphosphine. Clin Chim Acta. 2003;337:147–152. doi: 10.1016/j.cccn.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Bartolini D, Torquato P, Piroddi M, Galli F. Targeting glutathione S-transferase P and its interactome with selenium compounds in cancer therapy. Biochim Biophys Acta Gen Subj. 2019;1863:130–143. doi: 10.1016/j.bbagen.2018.09.023. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bocedi A, Noce A, Marrone G, Noce G, Cattani G, Gambardella G, et al. Glutathione transferase P1-1 an enzyme useful in biomedicine and as biomarker in clinical practice and in environmental pollution. Nutrients. 2019;11:1741. doi: 10.3390/nu11081741. https://doi.org/10.3390/nu11081741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Sci Food Agric. 1978;29:788–794. doi: 10.1002/jsfa.2740290908. [DOI] [Google Scholar]
- Çam M, Hışıl Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010;123:878–885. doi: 10.1016/j.foodchem.2010.05.011. [DOI] [Google Scholar]
- Cao H, Sethumadhavan K, Cao F, Wang TTY. Gossypol decreased cell viability and down-regulated the expression of a number of genes in human colon cancer cells. Sci Rep. 2021;11:5922. doi: 10.1038/s41598-021-84970-8. https://doi.org/10.1038/s41598-021-84970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda-Arriaga R, Pérez-González A, Reina M, Alvarez-Idaboy JR, Galano A. Comprehensive investigation of the antioxidant and pro-oxidant effects of phenolic compounds: A double-edged sword in the context of oxidative stress? J Phys Chem B. 2018;122:6198–6214. doi: 10.1021/acs.jpcb.8b03500. [DOI] [PubMed] [Google Scholar]
- Collier AC, Tingle MD, Paxton JW, Mitchell MD, Keelan JA. Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum Reprod. 2002;17:2564–2572. doi: 10.1093/humrep/17.10.2564. [DOI] [PubMed] [Google Scholar]
- Csepregi K, Hideg É. Phenolic compound diversity explored in the context of photo-oxidative stress protection. Phytochem Anal. 2018;29:129–136. doi: 10.1002/pca.2720. [DOI] [PubMed] [Google Scholar]
- Davis BJ. Disc electrophoresis. II. Method and application to human serum proteins. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- de Brum TF, Zadra M, Piana M, Boligon AA, Fröhlich JK, de Freitas RB, et al. HPLC analysis of phenolics compounds and antioxidant capacity of leaves of Vitex megapotamica (Sprengel) Moldenke. Molecules. 2013;18:8342–8357. doi: 10.3390/molecules18078342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dróżdż P, Šėžienė V, Pyrzynska K. Phytochemical properties and antioxidant activities of extracts from wild blueberries and lingonberries. Plant Foods Hum Nutr. 2017;72:360–364. doi: 10.1007/s11130-017-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta KN, Chetia P, Lahkar S, Das S. Herbal plants used as diuretics: A comprehensive review. JPCBS. 2014;2:27–32. [Google Scholar]
- Eghbaliferiz S, Iranshahi M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother Res. 2016;30:1379–1391. doi: 10.1002/ptr.5643. [DOI] [PubMed] [Google Scholar]
- Fuleki T, Francis FJ. Quantitative methods for anthocyanins. 2. Determination of total anthocyanin and degradation index for cranberry juice. J Food Sci. 1968;33:78–83. doi: 10.1111/j.1365-2621.1968.tb00888.x. [DOI] [Google Scholar]
- Gomathi AC, Rajarathinam SRX, Sadiq AM, Rajeshkumar S. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J Drug Deliv Sci Technol. 2020;55:101376. doi: 10.1016/j.jddst.2019.101376. https://doi.org/10.1016/j.jddst.2019.101376. [DOI] [Google Scholar]
- Grigorakis S, Makris DP. Characterisation of polyphenol-containing extracts from Stachys mucronata and evaluation of their antiradical activity. Medicines. 2018;5:14. doi: 10.3390/medicines5010014. https://doi.org/10.3390/medicines5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneidy RA, Gad AM, Zaki ER, Ibrahim FM, Shokeer A. Antioxidant or pro-oxidant and glutathione transferase P1-1 inhibiting activities for Tamarindus indica seeds and their cytotoxic effect on MCF-7 cancer cell line. J Genet Eng Biotechnol. 2020;18:74. doi: 10.1186/s43141-020-00077-z. https://doi.org/10.1186/s43141-020-00077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneidy RA, Meguid NA, Abdel-Ghany SS, Saleh NSM, Zaki ER, Hamed RR. Inter-individual variation of normal and Down syndrome glutathione transferase in response to different phenolic compounds. RJPBCS. 2017;8:184–201. [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- Hamed RR, Maharem TM, Abdel-Meguid N, Sabry GM, Abdalla AM, Guneidy RA. Purification and biochemical characterization of glutathione S-transferase from Down syndrome and normal children erythrocytes: A comparative study. Res Dev Disabil. 2011;32:1470–1482. doi: 10.1016/j.ridd.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Hegazy UM, Mannervik B, Stenberg G. Functional role of the lock and key motif at the subunit interface of glutathione transferase p1-1. J Biol Chem. 2004;279:9586–9596. doi: 10.1074/jbc.M312320200. [DOI] [PubMed] [Google Scholar]
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. https://doi.org/10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein SI, Yaseen NY, Jawad SQ, Abd ST. Seeds of Tamarindus indica as anti-cancer in some cell line. IJABR. 2017;7:360–362. [Google Scholar]
- Kim KH, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–473. doi: 10.1016/j.foodchem.2005.01.032. [DOI] [Google Scholar]
- Koffi E, Sea T, Dodehe Y, Soro S. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. JAPS. 2010;5:550–558. [Google Scholar]
- Kolm RH, Stenberg G, Widersten M, Mannervik B. High-level bacterial expression of human glutathione transferase P1-1 encoded by semisynthetic DNA. Protein Expr Purif. 1995;6:265–271. doi: 10.1006/prep.1995.1034. [DOI] [PubMed] [Google Scholar]
- Kubatka P, Kello M, Kajo K, Samec M, Jasek K, Vybohova D, et al. Chemopreventive and therapeutic efficacy of Cinnamomum zeylanicum L. Bark in experimental breast carcinoma: Mechanistic in vivo and in vitro analyses. Molecules. 2020;25:1399. doi: 10.3390/molecules25061399. https://doi.org/10.3390/molecules25061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Gu X, Alvarado AG, Du Y, Luo S, Ahn EH, et al. Discovery of a dual inhibitor of NQO1 and GSTP1 for treating glioblastoma. J Hematol Oncol. 2020;13:141. doi: 10.1186/s13045-020-00979-y. https://doi.org/10.1186/s13045-020-00979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. doi: 10.1016/S0308-8146(01)00251-5. [DOI] [Google Scholar]
- León-González AJ, Auger C, Schini-Kerth VB. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem Pharmacol. 2015;98:371–380. doi: 10.1016/j.bcp.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Lipiński K, Mazur M, Antoszkiewicz Z, Purwin C. Polyphenols in monogastric nutrition-A review. Ann Anim Sci. 2017;17:41–58. doi: 10.1515/aoas-2016-0042. [DOI] [Google Scholar]
- Long LH, Lan AN, Hsuan FT, Halliwell B. Generation of hydrogen peroxide by "antioxidant" beverages and the effect of milk addition. Is cocoa the best beverage? Free Radic Res. 1999;31:67–71. doi: 10.1080/10715769900300611. [DOI] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Majouli K, Hamdi A, Hlila MB. Phytochemical analysis and biological activities of Hertia cheirifolia L. roots extracts. Asian Pac J Trop Med. 2017;10:1134–1139. doi: 10.1016/j.apjtm.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Maqsood S, Benjakul S, Shahidi F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit Rev Food Sci Nutr. 2013;53:162–179. doi: 10.1080/10408398.2010.518775. [DOI] [PubMed] [Google Scholar]
- Martins N, Ferreira ICFR, Barros L, Carvalho AM, Henriques M, Silva S. Plants used in folk medicine: The potential of their hydromethanolic extracts against Candida species. Ind Crops Prod. 2015;66:62–67. doi: 10.1016/j.indcrop.2014.12.033. [DOI] [Google Scholar]
- Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 2006;15:523–530. [Google Scholar]
- Moga MA, Dimienescu OG, Bălan A, Dima L, Toma SI, Bîgiu NF, et al. Pharmacological and therapeutic properties of Punica granatum phytochemicals: Possible roles in breast cancer. Molecules. 2021;26:1054. doi: 10.3390/molecules. https://doi.org/10.3390/molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Noguti J, Barbisan LF, Cesar A, Dias Seabra C, Choueri RB, Ribeiro DA. Review: In vivo models for measuring placental glutatione-S-transferase (GST-P 7-7) levels: a suitable biomarker for understanding cancer pathogenesis. In Vivo. 2012;26:647–650. [PubMed] [Google Scholar]
- Olas B. Berry phenolic antioxidants-Implications for human health? Front Pharmacol. 2018;9:78. doi: 10.3389/fphar.2018.00078. https://doi.org/10.3389/fphar.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palozza P, Serini S, Di Nicuolo F, Piccioni E, Calviello G. Prooxidant effects of b-carotene in cultured cells. Mol Aspects Med. 2003;24:353–362. doi: 10.1016/S0098-2997(03)00031-1. [DOI] [PubMed] [Google Scholar]
- Pifferi G, Restani P. The safety of pharmaceutical excipients. Farmaco. 2003;58:541–550. doi: 10.1016/S0014-827X(03)00079-X. [DOI] [PubMed] [Google Scholar]
- Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. https://doi.org/10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šantić Ž, Pravdić N, Bevanda M, Galić K. The historical use of medicinal plants in traditional and scientific medicine. Psychiatr Danub. 2017;29:787–792. [PubMed] [Google Scholar]
- Sharma P, Kapoor S. Biopharmaceutical aspects of Brassica vegetables. J Pharmacogn Phytochem. 2015;4:140–147. [Google Scholar]
- Simons PC, Vander Jagt DL. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977;82:334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Singh RR, Reindl KM. Glutathione S-transferases in cancer. Antioxidants. 2021;10:701. doi: 10.3390/antiox10050701. https://doi.org/10.3390/antiox1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Stefanello FS, dos Santos CO, Bochi VC, Fruet APB, Soquetta MB, Dörr AC, et al. Analysis of polyphenols in brewer's spent grain and its comparison with corn silage and cereal brans commonly used for animal nutrition. Food Chem. 2018;239:385–401. doi: 10.1016/j.foodchem.2017.06.130. [DOI] [PubMed] [Google Scholar]
- Szymanska R, Pospisil P, Kruk J. Plant-derived antioxidants in disease prevention. Oxid Med Cell Longev. 2016;2016:1920208. doi: 10.1155/2016/1920208. https://doi.org/10.1155/2016/1920208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WJ, Yang YC, Zhou Y, Huang LP, Xu L, Chen QF, et al. Diacylglycerol acyltransferase and diacylglycerol kinase modulate triacylglycerol and phosphatidic acid production in the plant response to freezing stress. Plant Physiol. 2018;177:1303–1318. doi: 10.1104/pp.18.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabrew MI, Hughes RD, McFarlane IG. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J Pharm Pharmacol. 1997;49:1132–1135. doi: 10.1111/j.2042-7158.1997.tb06055.x. [DOI] [PubMed] [Google Scholar]
- Uchenna UE, Shori AB, Baba AS. Tamarindus indica seeds improve carbohydrate and lipid metabolism: An in vivo study. J Ayurveda Integr Med. 2018;9:258–265. doi: 10.1016/j.jaim.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivel V, Nandety A, Biesalski HK. Antioxidant, free radical scavenging and type II diabetes-related enzyme inhibition properties of traditionally processed Jequirity bean (Abrus precatorius L.) Int J Food Sci Technol. 2011;46:2505–2512. doi: 10.1111/j.1365-2621.2011.02774.x. [DOI] [Google Scholar]
- Vadivel V, Sriram S, Brindha P. Distribution of flavonoids among Malvaceae family members-A review. Int J Green Pharm. 2016;10:S33–S45. [Google Scholar]
- Wang TY, Li Q, Bi KS. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J Pharm Sci. 2018;13:12–23. doi: 10.1016/j.ajps.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Li HB, Cheng KW, Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. doi: 10.1016/j.foodchem.2005.05.049. [DOI] [Google Scholar]
- Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24:1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordi EG, Pérez EM, Matos MJ, Villares EU. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. In: Bouayed J, Bohn T, editors. Nutrition, Well-Being and Health. IntechOpen; London, UK: 2012. pp. 23–48. [Google Scholar]
- Zhang J, Gu Y, Chen B. Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther. 2019;12:1937–1945. doi: 10.2147/OTT.S191621. [DOI] [PMC free article] [PubMed] [Google Scholar]