Key Points
Question
What are the individual and total financial costs of head and neck cancer (HNC) survivorship care?
Findings
This retrospective review and economic evaluation found that the cost of HNC survivorship is higher than baseline costs for at least 5 years after diagnosis. Patients who were female, had hypopharyngeal tumors, or were treated with multimodal therapy had the highest risk for increased HNC survivorship costs.
Meaning
The findings of this review and economic evaluation suggest that practitioners should be cognizant of overall HNC survivorship costs and how these costs vary among subpopulations, especially when considering emerging treatments.
Abstract
Importance
Oncologic treatment is costly to the health care system and to individuals, but patients with head and neck cancer (HNC) also have long-term care needs after treatment. Survivors of HNC require specific consideration given their rapidly growing numbers. This subpopulation of cancer survivors often experiences long-term treatment-associated morbidity.
Objective
To describe the total and out-of-pocket (OOP) costs associated with HNC survivorship and the risk factors for financial toxicity among this population.
Design, Setting, and Participants
This was a retrospective review and economic evaluation of a cohort of US adults with a diagnosis of HNC from 2006 to 2018. The study used data the from IBM MarketScan Commercial Claims Database. Data were analyzed from November 2020 to June 2022.
Exposures
Treatment for HNC.
Main Outcomes and Measures
Total and OOP medical costs were assessed monthly and reported relative to the date of HNC diagnosis. The primary outcome was the difference between a patient’s mean monthly survivorship costs (13-60 months after diagnosis) and mean monthly baseline costs (7-12 months before diagnosis). Univariate and multivariable linear regression models were created for total and OOP costs to generate coefficient estimates with 95% CIs.
Results
The study cohort of this economic evaluation included 19 098 patients with HNC (median [range] age, 56 [18-64] years; 14 144 [74.1%] men and 4954 [25.9%] women; race and ethnicity were not considered). Throughout the survivorship period, median total and OOP costs were $372 per month and $31 per month higher than baseline costs, respectively, with variation in expenses by demographic information, health plan type, and oncologic variables. In the multivariable model, greater total and OOP excess survivorship costs were associated with female sex ($343/mo; 95% CI, $126 to $560 and $9/mo; 95% CI, $4 to $14). Highest and lowest total excess survivorship costs associated with cancer site were seen for hypopharyngeal ($1908/mo; 95% CI, $1102 to $2714) and oropharyngeal cancers (−$703/mo; 95% CI, −$967 to −$439) vs oral cavity cancers. Compared with surgery or radiation therapy alone, multimodal treatment was generally associated with excess OOP survivorship costs.
Conclusions and Relevance
The findings of this retrospective economic evaluation review suggest that the costs of HNC survivorship remain persistently elevated above baseline costs for at least 5 years after diagnosis. High survivorship costs were associated with female sex, hypopharyngeal tumors, and treatment with multimodal therapy. Practitioners should seek to minimize costs for these patients at higher-risk of financial toxicity after treatment and work to provide directed supportive services.
This retrospective economic review evaluates total and out-of-pocket cost associated with head and neck cancer survivorship and identifies risk factors for financial toxicity.
Introduction
The number of head and neck cancer (HNC) survivors has rapidly increased, currently comprising 436 060 individuals and 3% of all cancer survivors.1 This rapid increase is mainly associated with the epidemic increase in human papillomavirus (HPV)-associated oropharyngeal cancer incidence, compounded by an associated increase in survival.2,3,4,5 Despite evolving treatment paradigms, HNC treatment remains morbid, producing long-term medical needs for this growing population of survivors.6,7,8 Meanwhile, the individual cost burden of oncologic care is expanding,9,10 which contributes to the heavy financial burden on patients. This financial burden can create financial toxicity—the negative financial experience of patients affecting financial circumstances and resilience.11,12 Financial toxicity can interfere with clinical care, and negatively affect oncologic outcomes.4,13 Yet, there are limited data on the out-of-pocket (OOP) and total medical costs for the HNC population, especially longitudinal data that extends into the survivorship phase with appropriate comparison groups.11,14
In 2016, the American Cancer Society (ACS) published the HNC survivorship guidelines delineating the increased medical needs of HNC survivors.1 These guidelines include surveillance follow-up visits and screenings with a multidisciplinary group of practitioners and rehabilitation for long-term and late effects of treatment. The American Head & Neck Society’s Survivorship, Supportive Care, and Rehabilitation Service, built on the earlier ACS guidelines, published an updated set of recommended practices in HNC survivorship that emphasizes a multidisciplinary approach.15 Compared with other cancer populations, HNC populations are overrepresented by socioeconomically disadvantaged demographic groups, have higher levels of substance abuse, and have more comorbidities.16,17,18 Together, these factors likely form higher levels of baseline medical needs before the HNC diagnosis and a more substantial individual burden of medical expenses.16 However, these differences also complicate the study of HNC survivorship care because comparison to the general population or other oncologic groups may be obscured by baseline differences in comorbidities and medical utilization.
A recent study evaluating a national sample of patients with HNC demonstrated that this population constitutes a high-risk group for financial toxicity and has higher median and OOP annual expenses compared with patients who have other types of cancer.16 However, to our knowledge, no study to date has evaluated the longitudinal financial burden experienced by HNC survivors. Therefore, the aim of this study was to assess the overall and individual burden of medical expenses associated with HNC survivorship care through a longitudinal study of medical expenses before and after diagnosis. These findings are anticipated to inform the growing research and policy efforts around caring for HNC survivors and allow for targeting of groups at the highest risk for substantial costs.
Methods
The institutional review board of Washington University exempted this retrospective economic evaluation from review and waived informed consent because the study used deidentified data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Data Source
This study used data from the IBM MarketScan Commercial Claims Database, a large multipayer data set that is fully described elsewhere.19 Data from more than 150 million lives are collected primarily from large employers and some health plans to provide a source of adjudicated medical claims across encounter settings over time and linked by encrypted identifiers. The upper age limit of the database available at Washington University was 64 years. Clinical details, including staging and HPV status, were not available. The health plans captured were all based in the US.
Cohort Selection
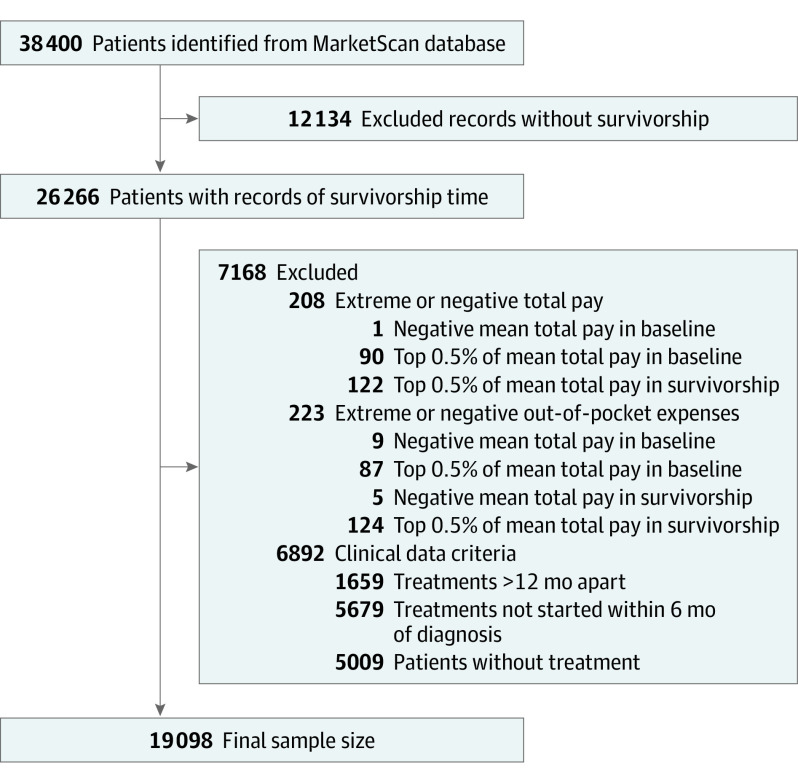
The MarketScan Database was queried for data from 2006 to 2018 for adults (18-64 years) with a diagnosis of HNC per the International Statistical Classification of Diseases and Related Health Problems, Ninth and Tenth Revisions (ICD-9 and -10; eTable 1 in the Supplement). To increase the specificity, we required either 1 inpatient diagnosis or an outpatient diagnosis followed by a confirmatory diagnosis within 30 to 90 days after the index encounter. Each individual’s first diagnosis that met these criteria was included in the cohort. Individuals with less than 12 months of continuous enrollment before the diagnosis were excluded (Figure 1). Patients receiving no treatments within 6 months of diagnosis were excluded. To minimize the inclusion of patients with recurrent or persistent disease, those receiving treatment 12 months after their initial treatment were excluded.
Figure 1. Cohort Selection Diagram.
Outcome Definitions
Total and OOP medical costs were assessed monthly and reported relative to the diagnosis date. Total medical costs referred to all medical costs captured by the patient’s payer, and therefore, included inpatient, outpatient, professional services, and pharmacy claims, plus individual OOP costs, but did not capture indirect costs (eg, travel and time off work). All costs were adjusted for inflation to 2018 US dollars. These costs differed from charges billed by the hospital and represented the actual costs incurred by the payer after applying any negotiated discounts. Time periods were defined in reference to the diagnosis date as follows: prediagnosis baseline (7-12 months before diagnosis), washout (1-6 months before diagnosis), diagnosis and treatment (0-12 months after diagnosis), and survivorship (13-60 months after diagnosis). The primary outcomes for total and OOP costs were the difference between individual patients’ mean monthly survivorship costs and mean monthly baseline costs, referred to as excess survivorship costs. Follow-up length was variable, but data from all complete months were used in calculating average survivorship period costs. The highest 0.5 percentile of average total and OOP costs from the baseline and survivorship period were removed to avoid extreme and highly influential outliers. Negative costs after averaging over baseline and survivorship periods were considered implausible and overly influential, and therefore, were excluded.
We hypothesized that median costs during the baseline and survivorship periods would be mostly stable over time, but there would be a meaningful difference between the survivorship and baseline period. We also hypothesized that this difference in survivorship vs baseline costs would vary among subgroups of HNC survivors. We further assumed a rise in costs during the washout period owing to symptoms and diagnostic procedures, followed by a substantial increase in cost during the diagnosis and treatment period associated with the cancer itself, and then a rapid decrease. These time periods were set a priori and were considered sufficiently wide to allow for individual variation in the timing of diagnosis, treatment start, and treatment completion. The appropriateness of these cutoffs was confirmed by visual inspection of the cost curves over time.
Covariate Definitions
Individuals were described at diagnosis by sex, age (by decade), employment status, relationship to the insurance plan member, plan type, geographic region of residence, and urban or rural residence. All patient variables were captured at the time of diagnosis. Oncologic variables included tumor site and treatment modalities per ICD-9 and -10 diagnosis codes and treatment procedure codes (eTables 1 and 2 in the Supplement), respectively. Elixhauser comorbidity index was calculated based on the ICD diagnosis codes.20 Each comorbidity category was included separately in the models as dichotomous variables.
Statistical Analysis
Descriptive statistics were used to report cohort characteristics. Median monthly costs for the cohort were reported graphically. Each categorical variable’s excess survivorship costs were summarized as the median and IQR. Univariate and multivariable linear regression models were created for total and OOP costs to generate coefficient estimates with 95% CIs. These coefficients represented the dollar difference in monthly excessive survivorship costs independently associated with the variable category compared with its reference. Normal distributions of the outcome variables were confirmed visually. All available variables were included in the models. Data analysis was conducted from November 2020 to June 2022.
Results
Cohort Description
The study cohort included a total of 19 098 individuals with HNC (median [range] age, 56 [18-64] years; 14 144 [74.1%] men and 4954 [25.9%] women; women; race and ethnicity data were not considered (Figure 1; Table 1). The most common HNC site was the oropharynx with 29.6% (5657 patients), followed by the oral cavity with 20.0% (3827 patients) and the larynx with 13.1% (2495 patients). Chemoradiotherapy was the plurality of treatment modalities, with 35.2% or 6725 patients. Most (84.4%; 15 678) patients resided in an urban area in the South region (42.4%; 8089), were active full-time employees (45.9%; 8740), and had a preferred provider organization (PPO) health plan (61.3%; 11 704 patients). The numbers of patients available at each follow-up interval are detailed in eTable 3 in the Supplement.
Table 1. Excess Survivorship Costs of Head and Neck Cancer, 2006 to 2018.
| Factors | No. (%) | Difference in survivorship vs baseline cost, median (IQR), $/mo | |
|---|---|---|---|
| Total cost | Out-of-pocket cost | ||
| Total survivors | 19 098 (100) | 371.64 (10.22 to 1411.33) | 31.49 (−4.79 to 110.48) |
| Male | 14 144 (74.1) | 381.18 (21.10 to 1412.87) | 33.53 (−2.86 to 112.49) |
| Female | 4954 (25.9) | 341.69 (−9.94 to 1407.62) | 26.04 (−12.24 to 105.52) |
| Age group, y | |||
| <40 | 1004 (5.3) | 258.79 (0 to 1052.61) | 22.51 (−7.23 to 101.79) |
| 40-44 | 1074 (5.6) | 308.93 (36.61 to 966.80) | 33.27 (0 to 105.37) |
| 45-49 | 2150 (11.3) | 364.78 (45.84 to 1284.14) | 39.43 (0 to 115.67) |
| 50-54 | 4041 (21.2) | 359.27 (22.02 to 1228.17) | 36.22 (−1.01 to 114.69) |
| 55-59 | 5663 (29.7) | 399.99 (14.21 to 1529.62) | 32.17 (−5.10 to 109.38) |
| ≥60 | 5166 (27.0) | 399.58 (−0.84 to 1732.82) | 27.05 (−12.72 to 109.69) |
| Employment status | |||
| Active full time | 8740 (45.9) | 326.59 (0 to 1228.45) | 29.78 (−6.50 to 108.16) |
| Other/unknown | 7676 (40.4) | 416.73 (30.08 to 1539.94) | 34.68 (−2.54 to 121.58) |
| Retiree | 2605 (13.7) | 407.70 (0 to 1708.17) | 29.72 (−6.17 to 92.78) |
| Health plan type | |||
| CDHP/HDHP | 1970 (10.3) | 246.96 (−11.57 to 1052.27) | 37.98 (−24.14 to 142.86) |
| Comprehensive | 868 (4.5) | 399.61 (5.11 to 1785.79) | 31.65 (−6.73 to 97.48) |
| EPO/missing | 573 (3.0) | 416.61 (0 to 1512.65) | 24.93 (−4.25 to 90.81) |
| HMO/POS | 3983 (20.9) | 372.20 (3.20 to 1398.23) | 19.45 (0 to 73.81) |
| PPO | 11 704 (61.3) | 385.72 (22.87 to 1455.50) | 37.71 (−6.45 to 120.68) |
| Geographic area | |||
| Northeast | 3182 (16.7) | 374.46 (0 to 1345.92) | 19.49 (−4.41 to 79.46) |
| North Central | 4650 (24.3) | 391.14 (19.14 to 1519.86) | 34.29 (−6.12 to 109.62) |
| South | 8089 (42.4) | 357.59 (4.49 to 1399.16) | 41.11 (−5.83 to 128.69) |
| West | 2935 (15.4) | 379.77 (24.11 to 1347.11) | 22.94 (−2.07 to 97.26) |
| Unknown | 242 (1.3) | 419.66 (33.79 to 1515.29) | 35.95 (−5.23 to 97.12) |
| Rural | 2904 (15.6) | 404.23 (31.64 to 1556.08) | 41.85 (−3.98 to 129.91) |
| Urban | 15 678 (84.4) | 374.35 (14.52 to 1408.23) | 31.19 (−4.14 to 109.17) |
| Cancer site | |||
| Hypopharynx | 265 (1.4) | 924.28 (190.49 to 3666.21) | 54.54 (0 to 152.67) |
| Larynx | 2495 (13.1) | 332.09 (11.16 to 1438.18) | 31.11 (−3.39 to 102.63) |
| Multiple sites | 1688 (8.8) | 420.89 (42.92 to 1559.71) | 34.90 (0 to 121.17) |
| Nasopharynx | 718 (3.8) | 692.09 (110.15 to 1875.20) | 36.57 (−0.97 to 129.24) |
| Oral cavity | 3827 (20.0) | 369.82 (10.43 to 1425.90) | 30.66 (−6.01 to 112.18) |
| Oropharynx | 5657 (29.6) | 331.20 (5.35 to 1148.37) | 31.91 (−4.49 to 106.90) |
| Other | 2177 (11.4) | 487.51 (45.88 to 1800.65) | 36.86 (−5.35 to 115.44) |
| Salivary | 1589 (8.3) | 189.13 (−59.70 to 874.58) | 16.66 (−13.86 to 79.12) |
| Sinonasal | 682 (3.6) | 896.89 (69.35 to 2914.04) | 42.47 (−6.85 to 149.68) |
| Treatment course | |||
| CT only | 398 (2.1) | 499.80 (0 to 4064.50) | 11.11 (−20.04 to 86.10) |
| CT and RT | 6725 (35.2) | 491.13 (79.57 to 1669.79) | 42.38 (−0.10 to 125.25) |
| RT only | 3313 (17.3) | 268.37 (0 to 936.72) | 27.28 (−5.84 to 90.71) |
| Surgery only | 3190 (16.7) | 96.74 (−78.21 to 543.14) | 13.14 (−15.29 to 67.82) |
| Surgery and CT | 115 (0.6) | 786.34 (0 to 5971.63) | 18.26 (−11.39 to 147.91) |
| Surgery and RT | 2660 (13.9) | 357.71 (4.87 to 1299.57) | 29.09 (−4.43 to 104.99) |
| Surgery, CT, and RT | 2697 (14.1) | 912.69 (182.52 to 4561.66) | 57.11 (0 to 165.48) |
| Elixhauser comorbidity score | |||
| None | 10 481 (54.9) | 388.25 (61.13 to 1305.16) | 40.83 (0 to 120.66) |
| 1 | 4949 (25.9) | 376.04 (−1.80 to 1439.92) | 28.06 (−10.73 to 102.32) |
| 2 | 2148 (11.2) | 313.42 (−108.02 to 1584.89) | 12.74 (−35.61 to 89.47) |
| ≥3 | 1520 (8.0) | 210.01 (−762.64 to 2306.91) | 0 (−96.05 to 79.55) |
Abbreviations: CDHP, consumer-driven health plan; CT, chemotherapy; EPO, exclusive provider organization; HDHP, high deductible health plan; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization; RT, radiation therapy.
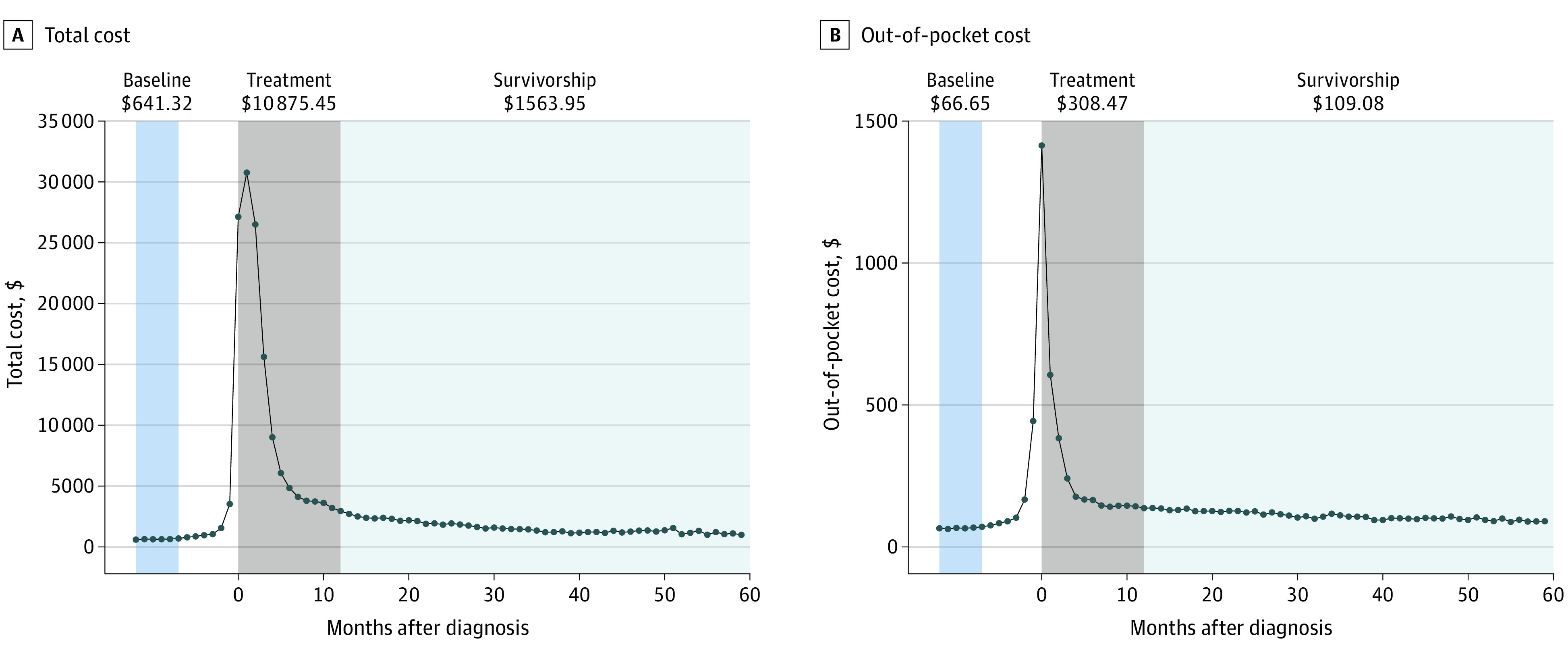
The total median monthly costs were $92 per month during the baseline period, including $17 per month of OOP costs (eTable 4 in the Supplement). On average, the median monthly total cost increased $22 per month. As expected, both the total and OOP costs began increasing in the months before diagnosis, then substantially increased during the diagnosis and treatment period (Figure 2). Within 12 months of diagnosis, both total and OOP costs decreased, then were largely stable in the survivorship period, decreasing by $28 per month (total) and $1 per month (OOP). During the survivorship period, median total and OOP costs were $372 per month and $31 per month higher than baseline (Table 1).
Figure 2. Median Monthly Head and Neck Cancer Treatment Expenses Over Time.
Factors Associated With Increased Survivorship Costs
Demographic, health plan, and oncologic variables observed substantial variation in total and OOP excess survivorship costs which persisted in the multivariable models (Table 2). In these models, total and OOP excess survivorship costs were higher in association with female sex ($318/mo; 95% CI, $99 to $538 and $9/mo; 95% CI, $4 to $15). Excess total costs were lowest in association with consumer-driven health plans and high deductible health plan (−$456/mo; 95% CI, $767 to $145) compared with PPO. However, compared with PPO plans, these plans were associated with the highest excess OOP costs ($18/mo; 95% CI, $10 to $26). The patient’s region was not associated with total excess survivorship costs, but excess OOP costs differences were observed and highest in the South ($37/mo; 95% CI, $30 to $43) compared with the Northeast. Patients in urban counties had lower total and OOP costs (−$113; 95% CI, −$388 to −$249 vs −9; 95% CI, −$15 to −$3, respectively).
Table 2. Factors Associated With Excess Survivorship Cost in Head and Neck Cancer, 2006 to 2018.
| Factors | Excess survivorship cost, estimate (95% CI), $/moa | |
|---|---|---|
| Total cost | Out-of-pocket cost | |
| Male sex | 0 [Reference] | 0 [Reference] |
| Female sex | 318.2 (98.9 to 537.5) | 9.6 (4.2 to 15.0) |
| Age group, y | ||
| ≥60 | 0 [Reference] | 0 [Reference] |
| <40 | 118.1 (−340.9 to 577.2) | −7.3 (−18.6 to 4.0) |
| 40-44 | −394.0 (−832.2 to 44.1) | −19.9 (−30.7 to −9.1) |
| 45-49 | −243.7 (−581.9 to 94.5) | −5.5 (−13.9 to 2.8) |
| 50-54 | −434.4 (−713.6 to −155.3) | −6.3 (−13.2 to 0.6) |
| 55-59 | −218.3 (−467.2 to 30.5) | −5.4 (−11.6 to 0.7) |
| Employment status | ||
| Active | 0 [Reference] | 0 [Reference] |
| Other/unknown | 21.8 (−181.9 to 225.5) | 11.7 (6.7 to 16.7) |
| Retiree | 37.4 (−275.7 to 350.5) | −8.0 (−15.8 to −0.3) |
| Health plan type | ||
| PPO | 0 [Reference] | 0 [Reference] |
| CDHP/HDHP | −456.3 (−767.2 to −145.5) | 18.1 (10.4 to 25.7) |
| Comprehensive | −63.9 (−537.5 to 409.6) | −14.6 (−26.3 to −2.9) |
| EPO/missing | 507.6 (−33.4 to 1048.7) | −27.7 (−41.1 to −14.4) |
| HMO/POS | −151.0 (−385 to 83.1) | −39.4 (−45.2 to −33.6) |
| Region | ||
| Northeast | 0 [Reference] | 0 [Reference] |
| North Central | 52.0 (−242.5 to 346.6) | 18.4 (11.1 to 25.7) |
| South | −149.6 (−415.8 to 116.7) | 36.9 (30.3 to 43.4) |
| West | −74.4 (−397.6 to 248.8) | 19.5 (11.6 to 27.5) |
| Unknown | −2244.3 (−10 892.5 to 6403.9) | −35.4 (−248.6 to 177.8) |
| Residence | ||
| Rural | 0 [Reference] | 0 [Reference] |
| Urban | −133.3 (−387.6 to 121.0) | −8.8 (−15.0 to −2.5) |
| Cancer site | ||
| Oral cavity | 0 [Reference] | 0 [Reference] |
| Hypopharynx | 1991.8 (1180.6 to 2802.9) | 21.7 (1.7 to 41.7) |
| Larynx | 227.7 (−118.5 to 573.8) | −3.2 (−11.7 to 5.3) |
| Multiple sites | −71.4 (−440.8 to 297.9) | 0.1 (−9.0 to 9.2) |
| Nasopharynx | 192.2 (−333.5 to 717.8) | 10.3 (−2.6 to 23.3) |
| Oropharynx | −718.0 (−984.7 to −451.4) | −13.6 (−20.2 to −7.1) |
| Other site | 0.4 (−348.6 to 349.5) | 3.4 (−5.2 to 12.0) |
| Salivary | −537.4 (−927.4 to −147.5) | −20.6 (−30.2 to −11.0) |
| Sinonasal | 1116.0 (586.0 to 1645.9) | 13.7 (0.7 to 26.8) |
| Treatment course | ||
| RT and CT | 0 [Reference] | 0 [Reference] |
| CT only | 796.3 (132.4 to 1460.3) | −11.7 (−28.0 to 4.7) |
| RT only | −1558.1 (−1841.5 to −1274.8) | −22.0 (−29 to −15.1) |
| Surgery only | −1954.5 (−2241.4 to −1667.5) | −38.1 (−45.1 to −31.0) |
| Surgery and CT | 2853.3 (1646.0 to 4060.6) | 44.7 (14.9 to 74.4) |
| Surgery and RT | −703.8 (−1010.5 to −397.1) | −8.4 (−15.9 to −0.8) |
| Surgery, CT, and RT | 2333.6 (2041.0 to 2626.2) | 25.5 (18.3 to 32.7) |
Abbreviations: CDHP, consumer-driven health plan; CT, chemotherapy; EPO, exclusive provider organization; HDHP, high deductible health plan; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization; RT, radiation therapy.
Models are adjusted for all variables listed and each Elixhauser comorbidity variable.
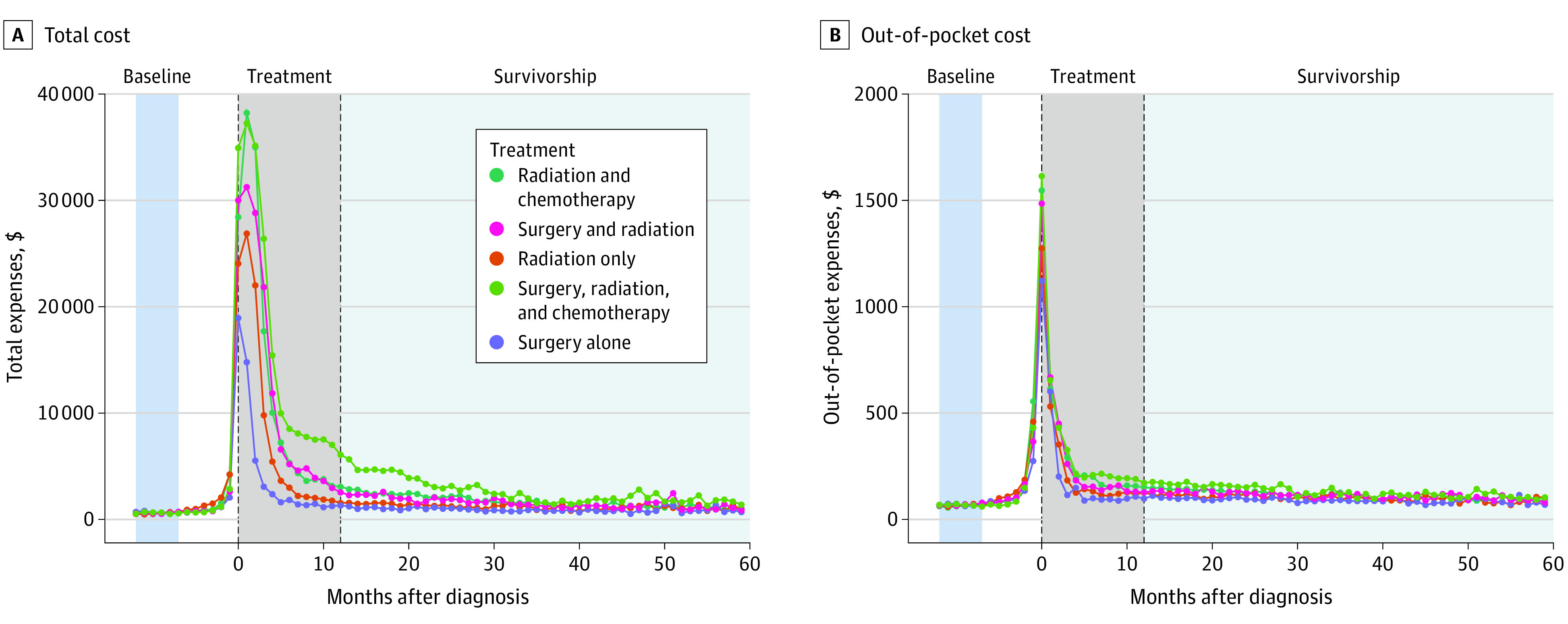
The highest total excess survivorship costs were seen for hypopharyngeal cancers ($1992/mo; 95% CI, $1181 to $2803) compared with oral cavity cancers (Figure 3). Compared with oral cavity cancers, the lowest total excess survivorship cost was associated with oropharyngeal cancers (−$718/mo; 95% CI, −$987 to −$451). Similar effects were seen in excess OOP expenses with lower costs associated with oropharyngeal and salivary sites (−$14/mo; 95% CI, −$20 to −$7; and −$21/mo; 95% CI, −$30 to −$11, respectively). Multimodal treatment was associated with higher excess total survivorship costs; specifically, in comparison to chemoradiotherapy, surgery plus chemoradiotherapy was associated with an additional $2334 per month of total expenses, whereas surgery alone was associated with $1954 per month less in total expenses. Similar effects were observed for excess OOP costs ($26/mo; 95% CI, $18 to $33; and −$38/mo; 95% CI, −$45 to −$31) for surgery plus chemoradiotherapy and surgery alone compared with radiation therapy and chemoradiotherapy (Figure 3).
Figure 3. Median Monthly Head and Neck Cancer Treatment Expenses by Treatment Type.
Discussion
The treatment period was anticipated to be the most costly for patients with cancer. However, oncologic patients also routinely have long-term expensive health care needs. The rapid increase in HNC survivors, their long-term treatment-associated morbidity, and their lower socioeconomic resources require special consideration of these survivorship costs. Although oncologic outcomes remain the primary focus of HNC research, health system resources and patients’ financial burdens are important to evaluate and target for improvement. To our knowledge, this study is the first to analyze long-term excess medical costs incurred by HNC survivors in a large national cohort. This study makes 3 important contributions. First, it established the time course for median medical expenses before and after treatment and demonstrates that costs remain elevated for 5 years after diagnosis. Second, it quantified the median excess ongoing monthly survivorship costs as $372 (total) and $31 (OOP). Third, it found that patients who were female, had hypopharyngeal tumors, or had been treated with multimodal therapy, were specific subgroups of HNC survivors with the highest risk for experiencing excess financial burden.
The existing literature on medical costs of HNC focuses primarily on the treatment period and has shown higher costs within 1 year of diagnosis associated with advanced stage, nasopharyngeal tumor site, multimodal therapy, region, and surgical complications.14,21,22 However, the treatment and survivorship phases of care present patients with unique challenges, such as the inability to work, duration of disability benefits, insurance coverage, and OOP limits. Prior studies that include the survivorship period have been restricted to the elderly population,23 fail to fully account for higher baseline spending among the HNC population,24 or focus on specific clinical comparisons. Additionally, some studies have relied on associated costs (those charges directly connected to an HNC diagnosis), which are likely to underestimate the total costs associated with the HNC diagnosis and its sequelae.11 To our knowledge, the present study analyzed the most extensive sample and represents the only study focusing on the survivorship period using individual patients’ baseline spending as the comparison group.
The financial burden of oncologic care on patients is increasingly recognized.25,26,27,28,29 Overall, cancer survivors have double the annual medical expenditure compared with patients with no cancer history.30,31 Although the costs during the survivorship period are lower than during treatment, the current study demonstrates that medical costs remain substantial for HNC patients and more than baseline at least 5 years after diagnosis. We have previously shown through an analysis of the Medical Expenditure Panel Survey16 that HNC survivors have median total medical expenses of $8384 per year, which is higher than other oncologic cohorts and comparable with the present study’s findings ($618/mo). The present study builds off this data with a much larger cohort (19 098 patients vs 489) and the addition of longitudinal health plan data, including cancer site and treatments received. In general, cancer costs decrease with increasing time from diagnosis, then escalate during end-of-life care.32 This decrease over time was observed in our data, but to a small degree as monthly total and OOP costs decreased.
Socioeconomically disadvantaged groups are disproportionately affected by HNC, and patients with poor general and mental health tend to incur higher costs related to their diagnoses.17 Given this population and the cost of HNC care, it is not surprising that 40.5% of HNC survivors report financial toxicity associated with cancer and its treatment.33 Patients with Medicaid insurance status, younger age, lower income, lower education, or unmarried status experience greater financial toxicity among cancer survivors, generally and specifically among those with head and neck cancer.13,33,34,35,36 Patients with HNC who experience greater financial toxicity complete treatment and cancer surveillance less often, require feeding tubes more often, and ultimately have inferior survival.4,13 The current study provides additional insights, demonstrating higher total and OOP costs for female sex, multimodal treatment, and hypopharyngeal tumors. Additionally, an individual exposure to OOP costs can differ by region and health plan. High deductible plans were associated with lower total costs, but a higher OOP burden, consistent with the cost- and risk-sharing design of these plans. This is in comparison with the most common plan type, PPOs, which typically have higher premiums but provide coverage for a wide array of health care specialties and services.
The unique demographics and biology of oropharyngeal cancer warrant particular attention.2,3,37 Oropharyngeal cancers account for the largest number of new HNC survivors, and this is anticipated to grow further with the continued increase in oropharyngeal cancer incidence.38 The long-term survival of HPV-associated oropharyngeal cancer is often excellent; however, it means a lengthy survivorship period and accrual excess costs.3 Although overall good quality of life is reported, especially for those with unimodal treatment, many continue to have long-term symptoms, especially related to swallowing and oral health.14,39,40,41 Compared with other HNC sites, swallowing dysfunction is worse on average after oropharyngeal cancer treatment, requiring short- or long-term gastrostomy tube use and weight loss.7 This subgroup demonstrated lower total and OOP excess survivorship costs, after accounting for demographic, regional, and treatment differences. This could be related to unmeasured differences in baseline health, severity of disease, subtle differences in treatment not captured by this analysis, or differences in health care utilization patterns. For example, women generally utilize more health care, including recommended preventive care, which results in higher costs,42,43 and may explain the higher HNC survivorship costs for women in the cohort. Regardless of cause, the excess survivorship costs for patients with oropharyngeal cancer remained $331 per month more than their baseline medical costs, a substantial amount compared with the median family income of $5186 per month in the US in 2012.44
Reducing the costs associated with HNC survivorship care will be challenging, and different strategies are needed to target total and patient OOP costs. First, reducing treatment when medically appropriate would directly decrease treatment costs,45 which would likely reduce survivorship costs because of improved morbidity and less disability. However, although reducing costs is important, it remains secondary to achieving oncologic outcomes, especially survival. Treatment de-escalation research is well underway for HPV-associated disease, and research towards more personalized approaches to other HNC sites holds promise for minimizing toxic therapies in patients unlikely to benefit.46,47 Avoiding treatments in excess of those recommended by evidence-based guidelines has been identified as a cost-control target in general oncology but is not well studied for HNC.48 Changing practice patterns may also reduce cost, such as shifting survivorship care toward primary care offices, which is recommended by the ACS guidelines.1,49 Unfortunately, few primary practitioners report sufficient training and capacity to provide this care.50 Actionable things at the practitioner and clinic level may include identifying patients at risk of financial toxicity and referring them to resources, such as financial counselors and social workers.
Meanwhile, society at large will need to reconsider how much cost sharing by HNC survivors is appropriate, and design insurance marketplace regulation in line with these values. Nonetheless, large cost reductions are likely possible. This is evident in the health systems of other Western nations where their patients with cancer have one-fourth of the OOP costs of their counterparts in the US and have achieved lower cancer mortality rates.51,52
Limitations
This study benefits from a large multipayer data source that captures all encounters during an individual’s enrollment period; however, it also has important limitations. The data source is a nonrandom sample, limited to those less than 65 years of age with US-based health insurance and is expected to underrepresent small health plans; therefore, it may not fully represent the overall HNC population. Data were unavailable after a change in health plan, which occurs frequently in the US and limits the available follow-up data. In addition, it is unknown how survivorship costs may have differed in patients lost to follow-up given any change in health plan, loss of coverage, or death. This study captured medical costs, which represent most oncologic costs31; however, other societal and personal costs were not captured but are estimated to be $3.4 billion annually in the US.53 The study analysis approximated average costs during the survivorship period to be stable, although we did note a small decrease in total and OOP costs over time. The study duration was truncated to 5 years after diagnosis, and as such, the findings may not persist beyond that time point. Additionally, several relevant clinical variables, including tumor stage, HPV status, recurrence, and mortality, were not available.
Conclusions
Overall, this retrospective economic evaluation review and economic evaluation establishes that the costs of HNC survivorship were persistently elevated above baseline for at least 5 years after diagnosis. Patient characteristics associated with risk for high costs of HNC survivorship were female sex, hypopharyngeal tumors, and treatment with multimodal therapy. Individuals and practitioners should be aware of these high-risk patients so as to seek ways to minimize costs when possible and to provide directed supportive services.
eTable 1. ICD-9 and -10 Diagnosis Codes for Head and Neck Cancer Sites
eTable 2. Current Procedural Terminology (CPT) and ICD-9-CM Procedure Codes for Treatment Categories
eTable 3. eTable 3. Number of Individuals at Each Time and Monthly Average Costs
eTable 4. Head and Neck Cancer Costs by Time Period
References
- 1.Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(3):203-239. doi: 10.3322/caac.21343 [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song JS, Vallance P, Biron V, Jeffery CC. Epidemiological trends of head and neck cancer survivors in Alberta: towards improved understanding of the burden of disease. J Otolaryngol Head Neck Surg. 2020;49(1):46. doi: 10.1186/s40463-020-00443-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen N, Fedewa S, Chen AY. Epidemiology and demographics of the head and neck cancer population. Oral Maxillofac Surg Clin North Am. 2018;30(4):381-395. doi: 10.1016/j.coms.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Ringash J, Bernstein LJ, Devins G, et al. Head and neck cancer survivorship: learning the needs, meeting the needs. Semin Radiat Oncol. 2018;28(1):64-74. doi: 10.1016/j.semradonc.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 7.Harris A, Lyu L, Wasserman-Winko T, George S, Johnson JT, Nilsen ML. Neck disability and swallowing function in posttreatment head and neck cancer patients. Otolaryngol Head Neck Surg. 2020;163(4):763-770. doi: 10.1177/0194599820923630 [DOI] [PubMed] [Google Scholar]
- 8.Cramer JD, Johnson JT, Nilsen ML. Pain in head and neck cancer survivors: prevalence, predictors, and quality-of-life impact. Otolaryngol Head Neck Surg. 2018;159(5):853-858. doi: 10.1177/0194599818783964 [DOI] [PubMed] [Google Scholar]
- 9.Kim P. Cost of cancer care: the patient perspective. J Clin Oncol. 2007;25(2):228-232. doi: 10.1200/JCO.2006.07.9111 [DOI] [PubMed] [Google Scholar]
- 10.Meropol NJ, Schrag D, Smith TJ, et al. ; American Society of Clinical Oncology . American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27(23):3868-3874. doi: 10.1200/JCO.2009.23.1183 [DOI] [PubMed] [Google Scholar]
- 11.Amonkar MM, Chastek B, Samant N, Teitelbaum A. Economic burden of resected squamous cell carcinoma of the head and neck in a US managed-care population. J Med Econ. 2011;14(4):421-432. doi: 10.3111/13696998.2011.584096 [DOI] [PubMed] [Google Scholar]
- 12.Witte J, Mehlis K, Surmann B, et al. Methods for measuring financial toxicity after cancer diagnosis and treatment: a systematic review and its implications. Ann Oncol. 2019;30(7):1061-1070. doi: 10.1093/annonc/mdz140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beeler WH, Bellile EL, Casper KA, et al. Patient-reported financial toxicity and adverse medical consequences in head and neck cancer. Oral Oncol. 2020;101:104521. doi: 10.1016/j.oraloncology.2019.104521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang J, Crawford K, Faraji F, Ramsey C, Kemp A, Califano JA III. An analysis of 1-year charges for head and neck cancer: targets for value-based interventions. Otolaryngol Head Neck Surg. 2020;163(3):546-553. doi: 10.1177/0194599820921401 [DOI] [PubMed] [Google Scholar]
- 15.Goyal N, Day A, Epstein J, et al. Head and neck cancer survivorship consensus statement from the American Head and Neck Society. Laryngoscope Investig Otolaryngol. 2021;7(1):70-92. doi: 10.1002/lio2.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massa ST, Osazuwa-Peters N, Adjei Boakye E, Walker RJ, Ward GM. Comparison of the financial burden of survivors of head and neck cancer with other cancer survivors. JAMA Otolaryngol Head Neck Surg. 2019;145(3):239-249. doi: 10.1001/jamaoto.2018.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massa ST, Rohde RL, Mckinstry C, et al. An assessment of patient burdens from head and neck cancer survivorship care. Oral Oncol. 2018;82:115-121. doi: 10.1016/j.oraloncology.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 18.Eytan DF, Blackford AL, Eisele DW, Fakhry C. Prevalence of Comorbidities among Older Head and Neck Cancer Survivors in the United States. Otolaryngol Head Neck Surg. 2019;160(1):85-92. doi: 10.1177/0194599818796163 [DOI] [PubMed] [Google Scholar]
- 19.IBM Watson Health . IBM MarketScan Research Databases for life sciences researchers. Accessed September 19, 2022. https://www.ibm.com/downloads/cas/0NKLE57Y
- 20.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 21.Tom MC, Ross RB, Koyfman SA, et al. Clinical factors associated with cost in head and neck cancer: implications for a bundled payment model. J Oncol Pract. 2019;15(6):e560-e567. doi: 10.1200/JOP.18.00665 [DOI] [PubMed] [Google Scholar]
- 22.Divi V, Tao L, Whittemore A, Oakley-Girvan I. Geographic variation in Medicare treatment costs and outcomes for advanced head and neck cancer. Oral Oncol. 2016;61:83-88. doi: 10.1016/j.oraloncology.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 23.Lang K, Menzin J, Earle CC, Jacobson J, Hsu MA. The economic cost of squamous cell cancer of the head and neck: findings from linked SEER-Medicare data. Arch Otolaryngol Head Neck Surg. 2004;130(11):1269-1275. doi: 10.1001/archotol.130.11.1269 [DOI] [PubMed] [Google Scholar]
- 24.Jacobson JJ, Epstein JB, Eichmiller FC, et al. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: commercial insurance, Medicare, and Medicaid. Head Neck Oncol. 2012;4:15. doi: 10.1186/1758-3284-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153-165. doi: 10.3322/caac.21443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekwueme DU, Zhao J, Rim SH, et al. Annual out-of-pocket expenditures and financial hardship among cancer survivors aged 18-64 years: United States, 2011-2016. MMWR Morb Mortal Wkly Rep. 2019;68(22):494-499. doi: 10.15585/mmwr.mm6822a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lentz R, Benson AB III, Kircher S. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. J Surg Oncol. 2019;120(1):85-92. doi: 10.1002/jso.25374 [DOI] [PubMed] [Google Scholar]
- 28.Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122(8):283-289. doi: 10.1002/cncr.29808 [DOI] [PubMed] [Google Scholar]
- 29.Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2016;109(2):djw205. doi: 10.1093/jnci/djw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekwueme DUYK, Yabroff KR, Guy GP Jr, et al. ; Centers for Disease Control and Prevention (CDC) . Medical costs and productivity losses of cancer survivors: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2014;63(23):505-510. [PMC free article] [PubMed] [Google Scholar]
- 31.Guy GP Jr, Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31(30):3749-3757. doi: 10.1200/JCO.2013.49.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1304-1312. doi: 10.1158/1055-9965.EPI-19-1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mady LJ, Lyu L, Owoc MS, et al. Understanding financial toxicity in head and neck cancer survivors. Oral Oncol. 2019;95:187-193. doi: 10.1016/j.oraloncology.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 34.Baddour K, Fadel M, Zhao M, et al. The cost of cure: Examining objective and subjective financial toxicity in head and neck cancer survivors. Head Neck. 2021;43(10):3062-3075. doi: 10.1002/hed.26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massa ST, Cass LM, Challapalli S, et al. Demographic predictors of head and neck cancer survival differ in the elderly. Laryngoscope. 2019;129(1):146-153. doi: 10.1002/lary.27289 [DOI] [PubMed] [Google Scholar]
- 36.Mols F, Tomalin B, Pearce A, Kaambwa B, Koczwara B. Financial toxicity and employment status in cancer survivors: a systematic literature review. Support Care Cancer. 2020;28(12):5693-5708. doi: 10.1007/s00520-020-05719-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahal BA, Catalano PJ, Haddad RI, et al. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1660-1667. doi: 10.1158/1055-9965.EPI-19-0038 [DOI] [PubMed] [Google Scholar]
- 38.American Cancer Society . Cancer Facts & Figures 2017. Accessed September 19, 2022. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html
- 39.Høxbroe Michaelsen S, Grønhøj C, Høxbroe Michaelsen J, Friborg J, von Buchwald C. Quality of life in survivors of oropharyngeal cancer: a systematic review and meta-analysis of 1366 patients. Eur J Cancer. 2017;78:91-102. doi: 10.1016/j.ejca.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 40.Scott SI, Kathrine Ø Madsen A, Rubek N, et al. Long-term quality of life & functional outcomes after treatment of oropharyngeal cancer. Cancer Med. 2021;10(2):483-495. doi: 10.1002/cam4.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Head MDA, Neck Cancer Symptom Working G; MD Anderson Head and Neck Cancer Symptom Working Group . Self-reported oral morbidities in long-term oropharyngeal cancer survivors: a cross-sectional survey of 906 survivors. Oral Oncol. 2018;84:88-94. doi: 10.1016/j.oraloncology.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owens GM. Gender differences in health care expenditures, resource utilization, and quality of care. J Manag Care Pharm. 2008;14(3)(suppl):2-6. doi: 10.18553/jmcp.2008.14.S3-A.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertakis KDAR, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49(2):147-152. [PubMed] [Google Scholar]
- 44.Federal Reserve Bank of St Louis.Median Family Income in the United States. Accessed September 19, 2022. https://fred.stlouisfed.org/series/MEFAINUSA646N.
- 45.Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32(9):865-882. doi: 10.1007/s40273-014-0169-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2017;8:4-11. doi: 10.1016/j.ctro.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaidar-Person O, Gil Z, Billan S. Precision medicine in head and neck cancer. Drug Resist Updat. 2018;40:13-16. doi: 10.1016/j.drup.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 48.Schleicher SM, Bach PB, Matsoukas K, Korenstein D. Medication overuse in oncology: current trends and future implications for patients and society. Lancet Oncol. 2018;19(4):e200-e208. doi: 10.1016/S1470-2045(18)30099-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vos JAM, Wieldraaijer T, van Weert HCPM, van Asselt KM. Survivorship care for cancer patients in primary versus secondary care: a systematic review. J Cancer Surviv. 2021;15(1):66-76. doi: 10.1007/s11764-020-00911-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bober SL, Recklitis CJ, Campbell EG, et al. Caring for cancer survivors: a survey of primary care physicians. Cancer. 2009;115(18)(suppl):4409-4418. doi: 10.1002/cncr.24590 [DOI] [PubMed] [Google Scholar]
- 51.Iragorri N, de Oliveira C, Fitzgerald N, Essue B. The out-of-pocket cost burden of cancer care: a systematic literature review. Curr Oncol. 2021;28(2):1216-1248. doi: 10.3390/curroncol28020117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow RD, Bradley EH, Gross CP. Comparison of cancer-related spending and mortality rates in the us vs 21 high-income countries. JAMA Health Forum. 2022;3(5):e221229. doi: 10.1001/jamahealthforum.2022.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000-2020. J Natl Cancer Inst. 2008;100(24):1763-1770. doi: 10.1093/jnci/djn384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-9 and -10 Diagnosis Codes for Head and Neck Cancer Sites
eTable 2. Current Procedural Terminology (CPT) and ICD-9-CM Procedure Codes for Treatment Categories
eTable 3. eTable 3. Number of Individuals at Each Time and Monthly Average Costs
eTable 4. Head and Neck Cancer Costs by Time Period