ABSTRACT
Background
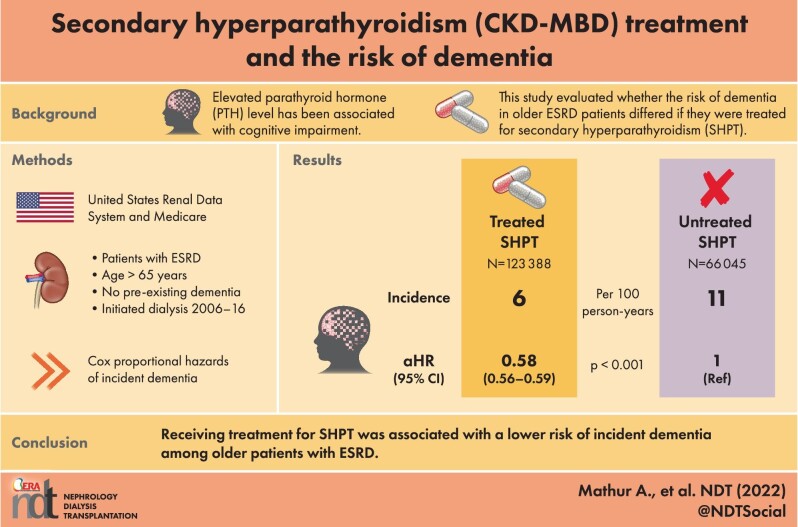
Elevated parathyroid hormone (PTH) levels have been reported as a potential risk factor for cognitive impairment. Compared with the general population, older adults with end-stage renal disease (ESRD) who are frequently affected by secondary hyperparathyroidism (SHPT) are at increased risk of developing dementia. The main objective of our study was to evaluate if the risk of dementia in older (age ≥66 years) ESRD patients differed if they were treated for SHPT.
Methods
Using the United States Renal Data System and Medicare claims, we identified 189 433 older adults without a diagnosis of dementia, who initiated dialysis between 2006 and 2016. SHPT treatment was defined as the use of vitamin D analogs, phosphate binders, calcimimetics or parathyroidectomy. We quantified the association between treated SHPT and incident dementia during dialysis using a multivariable Cox proportional hazards model with inverse probability weighting, considering SHPT treatment as a time-varying exposure.
Results
Of 189 433 older ESRD adults, 92% had a claims diagnosis code of SHPT and 123 388 (65%) were treated for SHPT. The rate of incident dementia was 6 cases per 100 person-years among SHPT treated patients compared with 11 cases per 100 person-years among untreated patients. Compared with untreated SHPT patients, the risk of dementia was 42% lower [adjusted hazard ratio (aHR) = 0.58, 95% confidence interval (CI): 0.56–0.59] among SHPT treated patients. The magnitude of the beneficial effect of SHPT treatment differed by sex (Pinteraction = .02) and race (Pinteraction ≤ .01), with females (aHR = 0.56, 95% CI: 0.54–0.58) and those of Asian (aHR = 0.51, 95% CI: 0.46–0.57) or Black race (aHR = 0.51, 95% CI: 0.48–0.53) having a greatest reduction in dementia risk.
Conclusion
Receiving treatment for SHPT was associated with a lower risk of incident dementia among older patients with ESRD. This work provides additional support for the treatment of SHPT in older ESRD patients.
Keywords: dialysis, elderly, ESRD, hyperparathyroidism, mineral metabolism
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Secondary hyperparathyroidism (SHPT), characterized by elevated parathyroid hormone levels (PTH), is prevalent among dialysis patients.
What this study adds?
Elevated PTH has been associated with cognitive impairment.
What impact this may have on practice or policy?
Our study demonstrates that treatment of SHPT lowers dementia risk, highlighting the additional burden of SHPT, and provides additional support for treatment of SHPT.
INTRODUCTION
Up to 70% of older (age >65 years) patients with end-stage renal disease (ESRD) suffer from cognitive impairment [2–4]. Furthermore, the incidence of dementia and Alzheimer's disease, the most commonly diagnosed types of dementia, begins to rise soon after dialysis initiation and is 10 times higher than the incidence in community-dwelling older adults [5]. Older ESRD patients who develop dementia are at a 1.5-fold higher risk of disability, 2-fold increased risk of hospitalization and a 2-fold higher risk of subsequent mortality [5]. Age, race, sex, diabetes, hemodialysis vintage, prior history of stroke and race have been identified as risk factors for cognitive impairment and dementia among ESRD patients, however, these are difficult to modify [6, 7]. Therefore, the identification of modifiable risk factors for cognitive decline and dementia in older ESRD patients is critical.
Secondary hyperparathyroidism (SHPT), characterized by severely elevated parathyroid hormone levels (PTH), arises from the metabolic abnormalities of ESRD affecting the majority of ESRD patients and represents one such potentially modifiable dementia risk factor. PTH can cross the blood–brain barrier binding to receptors in the brain thereby affecting cognition [8–10]. In patients without ESRD, elevated PTH levels are associated with impaired cognitive function and dementia [11–15].
Further, several single-institution studies have demonstrated improvement in specific cognitive domains after treatment of SHPT [16–18]; such treatments for SHPT include medications like phosphate binders, vitamin D analogs and calcimimetics or surgical parathyroidectomy. Yet, despite the existence of SHPT treatment guidelines, it remains untreated in up to 30%–60% of patients, representing a potential missed opportunity to address one of the central complications of ESRD: cognitive decline and dementia [1, 19, 20]. Furthermore, SHPT disease severity, management and outcomes vary by race [21–23].
Given that PTH impacts cognitive function, we hypothesized that the incidence of dementia would differ between older ESRD patients who were and were not treated for SHPT. Therefore, we sought to: (i) investigate whether SHPT treatment was associated with dementia risk and specifically Alzheimer's disease risk; and (ii) identify subgroups of SHPT patients who would benefit the most from SHPT treatment.
MATERIALS AND METHODS
Study population
Using the United States Renal Data System (USRDS), we identified 597 003 older (aged ≥66 years) ESRD patients who initiated dialysis between 2006 and 2016. We included patients on either hemodialysis or peritoneal dialysis. We restricted the study population to those with Medicare as a primary payer during 1-year pre-ESRD and Medicare Part A, B and D at the time of dialysis initiation (n = 217 444). Among older ESRD patients with Medicare, we further excluded those with a claim for dementia prior to dialysis (n = 23 459) and those without data from CMS-2728 (n = 4552). The final study population included 189 433 patients, assuming all dialysis patients had SHPT because PTH levels begin to rise in stage 3 chronic kidney disease [24–26] (Fig. 1). Although adynamic bone disease (ABD) is increasing in prevalence and unlike SHPT is associated with suppressed PTH, it may represent a small percentage of our population. We followed the study population until the date of transplantation, end of Medicare coverage, death or administrative censoring (31 December 2016), whichever came first.
FIGURE 1:
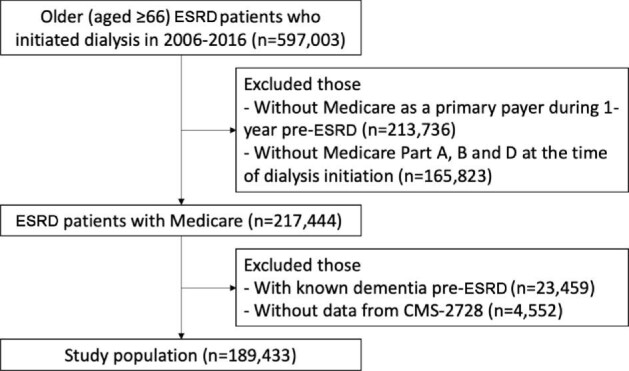
Study population was defined to examine the association of secondary hyperparathyroidism treatment during dialysis with incident dementia among older end-stage renal disease patients.
SHPT treatment
Treated SHPT was defined as receiving medications including vitamin D analogs (calcitriol, doxercalciferol, paricalcitol, ergocalciferol), phosphate binders (calcium acetate, calcium carbonate sevelamer, lanthanum carbonate, aluminum hydroxide) or cinacalcet, or undergoing surgical parathyroidectomy. These were chosen as treatments because low vitamin D levels and elevated phosphate levels both independently stimulate PTH and can independently lead to hyperparathyroidism even in non-dialysis patients [27, 28]. We did not include additional chronic kidney disease-mineral and bone disorder (CKD-MBD) treatments such as treatment of bone abnormalities. Medication use was obtained using Medicare Part D. Parathyroidectomy was ascertained using International Classification of Diseases 9th Revision (ICD-9), International Classification of Diseases 10th Revision (ICD-10) and Current Procedural Terminology (CPT) codes (Supplementary data, Table S1). We allowed a 30-day gap between the last day of a previous prescription and the first day of a current prescription. For example, a patient who had been prescribed cinacalcet for 30 days on 1 April 2015 and again for 30 days on 30 May 2015 was considered as treated between 1 April 2015 and 29 June 2016. Patients who underwent parathyroidectomy were considered treated since the operation day.
Dementia and Alzheimer's disease diagnoses
Incident dementia and Alzheimer's disease, the most common types of dementia, were defined as diagnosed via claims. Diagnoses were ascertained using a validated ICD-9 and ICD-10 codes using the previously published definitions (Supplementary data, Table S1) [5, 29–32]. Incident dementia and Alzheimer's disease were defined as having at least one inpatient or two outpatient claims during a 1-year period and the earliest date was used as the date of first diagnosis of dementia or Alzheimer's disease [29, 31].
Risk of dementia comparing treated with untreated SHPT patients
To quantify the association between SHPT treatment and incident dementia after dialysis initiation, we used a Cox proportional hazards model which treated the SHPT treatments as time-varying. We assessed the proportional-hazard assumption by using a log-log plot. We adjusted confounding using a propensity score method, designed to deal with confounding by indication in many pharmacoepidemiological studies [33]. First, we used a logistic model to estimate the propensity scores, probabilities of being treated for SHPT conditional on age as a categorical variable (66–69, 70–79, 80+ years), sex, race/ethnicity, employment status, geographic regions, the primary cause of ESRD, dialysis modality, body mass index (BMI) and comorbidities (hypertension, diabetes mellitus, cerebrovascular disease, atherosclerotic heart disease, heart failure, peripheral vascular disease, cancer, functional impairment, alcohol dependence and smoking). Also, a time-varying covariate defined as days of previous medication prescriptions was included in the model to account for the severity of SHPT. Then, in the Cox model, we used weights defined as the inverse of the probability being treated for SHPT for the treated patients and the inverse of the probability of being untreated for SHPT for the untreated patients.
Risk of dementia by type of treatment and in subgroups
We used Cox proportional hazards model to compare the association of incident dementia by type of treatment (medication, parathyroidectomy and no treatment). To estimate the propensity scores, we used a multinomial logistic model including the same covariates included in the model above. Additionally, we performed stratified analyses by age (66–69, 70–79, and 80+ years), sex (female versus male), race (White, Black, Asian and other) and diabetes. We chose to perform these analyses among patients at high risk for cognitive impairment or more severe hyperparathyroidism.
Sensitivity analysis
As the PTH level is not available in the data, we assessed a minimum strength of association of unmeasured confounding by the severity of SHPT. The associations with both the SHPT treatment and incident dementia were calculated on the risk ratio scale conditional on the measured covariates to cancel out the observed association by estimating E-value as follows: 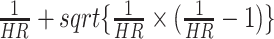 [34, 35]. We additionally performed two sensitivity analyses. We limited our study population to patients who had a diagnosis code of SHPT. We performed another analysis in which we were limited to patients only treated with calcimimetics or parathyroidectomy.
[34, 35]. We additionally performed two sensitivity analyses. We limited our study population to patients who had a diagnosis code of SHPT. We performed another analysis in which we were limited to patients only treated with calcimimetics or parathyroidectomy.
Statistical analysis
A P-value ˂.05 was considered statistically significant. All analyses were performed using Stata 16.0/MP for Linux (College Station, TX, USA). Missing data were handled using the missing-indicator method.
RESULTS
Study population
Of 189 433 older (age ≥66 years) ESRD adults, the majority of patients were 70–79 years of age, of White race, unemployed and had diabetes as the primary cause of ESRD (Table 1). Approximately half were females. The most common co-morbidities were hypertension followed by diabetes mellitus. The majority of patients lived in the Southern and Mid-Western regions of the USA. Of this entire cohort, 123 388 (65%) were treated for SHPT. Of this cohort, 93.5% were on hemodialysis and 92% had a claims diagnosis code of SHPT. Among SHPT treated patients, 25.4% were aged ≥80 years whereas among SHPT untreated patients, 35.2% were aged ≥80 years. Of the treated group, 16.2% had functional impairment whereas 25.8% of the untreated group had functional impairment. Otherwise, the treated and untreated groups were similar (Table 1).
Table 1.
Characteristics of older (aged ≥66 years) end-stage renal disease patients by secondary hyperparathyroidism treatment status (n = 189 433)
| Patients without SHPT treatment | Patients with SHPT treatment | |||
|---|---|---|---|---|
| Overall | (n = 66 045) | (n = 123 388) | P-value | |
| Age at diagnosis (years) | <.001 | |||
| 66–69, n (%) | 22.4 | 18.3 | 24.5 | |
| 70–79, n (%) | 48.8 | 46.5 | 50.1 | |
| 80 or older, n (%) | 28.8 | 35.2 | 25.4 | |
| Female, n (%) | 51.0 | 48.9 | 52.1 | <.001 |
| Race | <.001 | |||
| White, n (%) | 75.2 | 77.9 | 73.7 | |
| Black, n (%) | 18.8 | 17.3 | 19.5 | |
| Asian, n (%) | 4.4 | 3.4 | 5.0 | |
| Other, n (%) | 1.6 | 1.3 | 1.8 | |
| Currently employed, n (%) | 2.0 | 1.6 | 2.3 | <.001 |
| Geographic regions | <.001 | |||
| Northeast, n (%) | 18.6 | 18.1 | 18.9 | |
| South, n (%) | 37.4 | 37.2 | 37.6 | |
| Midwest, n (%) | 25.7 | 27.5 | 24.7 | |
| West, n (%) | 18.3 | 17.2 | 18.8 | |
| BMI (kg/m2) | <.001 | |||
| <25, n (%) | 36.0 | 38.9 | 34.5 | |
| 25–29.9, n (%) | 29.8 | 29.2 | 30.1 | |
| ≥30, n (%) | 34.2 | 31.9 | 35.4 | |
| Modality—hemodialysis, n (%) | 94.2 | 95.7 | 93.4 | <.001 |
| Primary cause of ESRD | <.001 | |||
| Diabetes mellitus, n (%) | 43.9 | 40.1 | 46.0 | |
| Hypertension, n (%) | 35.2 | 35.5 | 35.0 | |
| Glomerulonephritis, n (%) | 5.0 | 4.3 | 5.3 | |
| Others, n (%) | 15.9 | 20.1 | 13.7 | |
| Comorbidity | ||||
| Hypertension, n (%) | 87.6 | 85.4 | 88.8 | <.001 |
| Diabetes mellitus, n (%) | 55.5 | 53.1 | 56.7 | <.001 |
| Cerebrovascular disease, n (%) | 10.9 | 12.2 | 10.2 | <.001 |
| Atherosclerotic heart disease, n (%) | 25.3 | 26.1 | 24.8 | <.001 |
| Heart failure, n (%) | 39.9 | 43.5 | 37.9 | <.001 |
| Peripheral vascular disease, n (%) | 15.6 | 16.9 | 15.0 | <.001 |
| Cancer, n (%) | 10.5 | 12.0 | 9.8 | <.001 |
| Functional impairment, n (%) | 19.6 | 25.8 | 16.2 | <.001 |
| Alcohol dependence, n (%) | 0.7 | 1.0 | 0.6 | <.001 |
| Current smoker, n (%) | 3.8 | 3.7 | 3.9 | .13 |
SHPT, secondary hyperparathyroidism; BMI, body mass index; ESRD, end-stage renal disease.
Risk of dementia by SHPT treatment
The incident rate of dementia was 6 cases per 100 person-years among SHPT treated patients compared with 11 cases per 100 person-years in untreated patients (Table 2). The median time to dementia diagnosis was 8.6 years [95% confidence interval (CI): 8.5–8.9 years]. Over the study period, 16.5% (n = 136 858) of untreated SHPT patients were diagnosed with dementia, compared with 17.4% (n = 52 575) among those with treated SHPT. Compared with untreated SHPT patients, the risk of dementia was 42% lower [adjusted hazard ratio (aHR) = 0.58, 95% CI: 0.56–0.59] in SHPT treated patients after adjusting for age, sex, race/ethnicity, employment status, geographic region, BMI, dialysis modality type, the primary cause of ESRD, days of previous medication prescription and comorbidities (hypertension, diabetes mellitus, cerebrovascular disease, atherosclerotic heart disease, heart failure, peripheral vascular disease, cancer, functional impairment, alcohol dependence and smoking) (Table 2A).
Table 2.
Risk of incident dementia in older ESRD patients with secondary hyperparathyroidism (SHPT) comparing those who received treatment during dialysis in our study cohort (A) or only in patients with a diagnosis code of SHPT (B) with those who did not receive treatment, and also comparing treatment by parathyroidectomy compared with cinacalcet (C). Adjusted hazard ratio (aHR) and 95% confidence interval (CI) is reported
| SHPT treatment | Incident rate (cases/100 person-years) | Risk of incident dementia [aHR (95% CI)]a |
|---|---|---|
| A. Entire cohort | ||
| No | 11 | Reference |
| Yes | 6 | 0.58 (0.56–0.59) |
| B. Restricted only to patients with claims diagnosis of SHPT | ||
| No | 10 | Reference |
| Yes | 6 | 0.63 (0.62–0.65) |
| C. Restricted only to patients receiving either treatment | ||
| Cinacalcet | 7.3 | Reference |
| Parathyroidectomy | 6.2 | 0.91 (0.66–1.26) |
Adjusted for age at diagnosis sex, race/ethnicity, employment, geographic region, body mass index, dialysis modality type, primary cause of end-stage renal disease, comorbidities (hypertension, diabetes mellitus, cerebrovascular disease, atherosclerotic heart disease, heart failure, peripheral vascular disease, cancer, functional impairment, alcohol dependence and smoking) and days of previous medication prescription.
Risk of dementia by treatment in subgroups
The magnitude of the beneficial effect of SHPT treatment differed by sex (Pinteraction = .01) and race (Pinteraction ≤.01), but not by age or diabetic status (Table 3). Among female patients, SHPT treatment was associated with a 44% reduction in incident dementia risk (aHR = 0.56, 95% CI: 0.54–0.58) compared with a 40% (aHR = 0.60, 95% CI: 0.57–0.62) reduction in males. Among patients of Asian race, SHPT treatment was associated with a 49% reduction (aHR = 0.51, 95% CI: 0.46–0.57) and among those of Black race treatment was associated with a 50% reduction (aHR = 0.50, 95% CI: 0.48–0.53) in dementia risk. Among patients of White race, SHPT treatment was associated with a 39% reduction in dementia risk (aHR = 0.61, 95% CI: 0.59–0.62).
Table 3.
The impact of secondary hyperparathyroidism treatment on incident dementia stratified by age, sex, race and diabetes status compared with no treatment. Hazard ratios were adjusted for age at diagnosis, sex, race/ethnicity, employment, geographic region, body mass index, dialysis modality type, primary cause of end-stage renal disease, comorbidities (hypertension, diabetes mellitus, cerebrovascular disease, atherosclerotic heart disease, heart failure, peripheral vascular disease, cancer, functional impairment, alcohol dependence and smoking) and days of previous medication prescriptions
| Effect modifier | Incident dementia [aHR (95% CI)] | |||
|---|---|---|---|---|
| Age | 66–69 | 70–79 | ≥80 | |
| No treatment | Ref | Ref | Ref | |
| Treatment | 0.58 (0.54–0.61) | 0.58 (0.56–0.60) | 0.60 (0.57–0.62) | |
| Pinteraction | .93 | .45 | ||
| Sex | Male | Female | ||
| No treatment | Ref | |||
| Treatment | 0.60 (0.57–0.62) | 0.56 (0.54–0.58) | ||
| Pinteraction | .01 | |||
| Race | White | Black | Asian | Other |
| No treatment | Ref | Ref | Ref | Ref |
| Treatment | 0.61 (0.59–0.62) | 0.50 (0.48–0.53) | 0.51 (0.46–0.57) | 0.62 (0.50–0.75) |
| Pinteraction | <.001 | .01 | .86 | |
| Diabetes | No | Yes | ||
| No treatment | Ref | Ref | ||
| Treatment | 0.59 (0.56–0.61) | 0.57 (0.55–0.59) | ||
| Pinteraction | .34 | |||
Risk of dementia by type of treatment
The incident rate of dementia was 6 cases per 100 person-years in medically treated patients and 5 cases per 100 person-years in surgically treated patients who underwent parathyroidectomy. When comparing parathyroidectomy with medical treatment, there was no difference in the risk of dementia (aHR = 1.12, 95% CI: 0.75–1.67).
Risk of Alzheimer's disease by treatment
The incident rate of Alzheimer's disease was 1 case per 100 person-years in SHPT treated patients compared with 2 cases per 100 person-years in SHPT untreated patients. After adjusting for confounders, the risk of Alzheimer's disease was 33% lower (aHR = 0.67, 95% CI: 0.64–0.71) in SHPT treated patients compared with SHPT untreated patients (Table 4).
Table 4.
Risk of Alzheimer's disease in older end-stage renal patients with secondary hyperparathyroidism (SHPT) comparing those who received treatment during dialysis with those who did not receive treatment. Adjusted hazard ratio (aHR) and 95% confidence interval (CI) is reported
| SHPT treatment | Incident rate (cases/100 person-year) | Risk of Alzheimer's disease [aHR (95% CI)] |
|---|---|---|
| No | 2 | Reference |
| Yes | 1 | 0.67 (0.63–0.70) |
Adjusted for age at diagnosis sex, race/ethnicity, employment, geographic region, body mass index, dialysis modality type, primary cause of end-stage renal disease, comorbidities (hypertension, diabetes mellitus, cerebrovascular disease, atherosclerotic heart disease, heart failure, peripheral vascular disease, cancer, functional impairment, alcohol dependence and smoking) and days of previous medication prescription.
Sensitivity analysis
The risk of incident dementia in treated patients was 0.58 (95% CI: 0.56–0.59) times lower than in untreated patients and E-value was 2.84 for the estimate and 2.78 for the CI, which seems moderately robust. To explain away the observed association, a risk ratio of 2.84 between an unmeasured confounder and each of SHPT treatment and dementia conditional on the measured covariates is needed. An association between an unmeasured confounder and each of SHPT treatment and dementia would require a risk ratio of 2.78 each to make the CI cross 1. We performed two additional sensitivity analyses. If we limited our study population to those with a diagnosis code of secondary hyperparathyroidism only (n = 162 861), the incident rate of dementia was 10 cases per 100 person-years in untreated SHPT patients and 6 cases per 100 person-years in treated patients (Table 2B). Compared with untreated SHPT patients, the risk of dementia was 36% lower (aHR = 0.63, 95% CI: 0.62–0.65) in treated patients. If we restricted the study population to patients who received cinacalcet or parathyroidectomy after dialysis initiation (n = 25 018), there was no difference in risk of incident dementia between the two treatments (Table 2C).
DISCUSSION
In this national study of 189 433 dementia-free Medicare beneficiaries on dialysis for at least 1 year, patients who were treated for SHPT had a 42% lower risk of incident dementia and a 37% lower risk of Alzheimer's disease compared with those who were not treated. Among SHPT treated patients, females, patients of Black or Asian race, had the lowest risk of dementia with treatment. There was no difference in dementia risk in patients who were treated medically compared with surgically.
Prior studies have demonstrated that in the general adult population, the prevalence of dementia is ∼5%, primarily occurring in older adults [36]. In contrast, in patients with ESRD, 10% of younger patients develop dementia and up to 16% of older patients develop dementia [2, 5]. A total of 12 modifiable risk factors have been identified, accounting for 40% of all dementias [37]. However, in older patients, with ESRD, a state of end-organ damage, majority of these either cannot be modified or even if addressed, will not impact dementia risk. For example, medications to control blood pressure do not mitigate vascular dementia among ESRD patients [38]. However, high PTH which occurs in SHPT affects nearly all ESRD patients, has been associated with cognitive impairment, is treatable, and may represent a modifiable risk factor for incident dementia.
The existing literature demonstrates a mixed relationship between SHPT and cognitive impairment. Previous cross-sectional studies in SHPT patients demonstrate impairments inconsistently across various cognitive domains of executive function, memory, attention or global cognitive function [17, 39–43]. Increasing SHPT disease severity, characterized by higher PTH levels, has also been associated with greater gray matter volume on magnetic resonance imaging and worse scores on the Montreal Cognitive Assessment (MoCA), indicative of global cognitive function [8]. Two previous studies noted an association between PTH and Alzheimer's disease using two different definitions of the disease [11, 12]. In contrast, another study did not find an association between PTH and Alzheimer's disease but rather with developing vascular dementia [14]. A study by Chou and colleagues demonstrated improved global cognitive function and dementia rating scores following surgical treatment of SHPT [16]. However, thus far, no study has evaluated whether the risk of incident dementia differs by treated or untreated SHPT. Our study demonstrated a significant reduction in both incident dementia and Alzheimer's disease with SHPT treatment, suggesting a link between high PTH levels and cognitive impairment.
Prior studies have also identified risk factors for incident dementia specifically in ESRD patients including older age at hemodialysis initiation, underweight BMI, patients with diabetes, those with a history of transient ischemic attack or stroke, or those who were institutionalized [5]. Interestingly, we did not find that the benefit of SHPT treatment differed by age or diabetic status.
Our study also found that up to 35% of older ESRD patients with SHPT are not treated. While this may reflect disease severity or temporal shifts in the treatment landscape or practice patterns, it may also reflect a lack of consensus exists regarding optimal SHPT treatment regimens resulting in significant treatment variation. Despite recommended treatment targets by organizations such as the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Kidney Disease: Improving Global Outcomes (KDIGO), studies have demonstrated that PTH levels in the United States are rising [1, 19, 44]. The percentage of dialysis facilities reporting PTH levels <300 pg/mL declined from 82% in 2010 to 22% in 2014, whereas the percentage of dialysis facilities reporting PTH upper target of ≥600 pg/mL increased from 7% in 2010 to 62% in 2014 [44]. Surgical parathyroidectomy is ultimately required in 15% of dialysis patients at 10 years and in 38% at 20 years [45]. In our study, both medical and surgical treatments had a lower risk of incident dementia. Furthermore, we noted that the risk of incident dementia was comparable between medically treated and surgically treated patients. If we restricted our study population to patients who only received cinacalcet or parathyroidectomy after dialysis initiation, there was no significant difference in risk of incident dementia between the two treatments as shown by the hazard ratio crossing the reference line.
Our study has several limitations including those associated with large retrospective databases such as missing values, coding errors, confounding by indication and selection bias. The retrospective design of the study is a major limitation. Additionally, Medicare is not necessarily the primary payor for all older patients on hemodialysis in the USA, and therefore our results may not be entirely generalizable to all older dialysis patients. However, Medicare is the single largest insurer for older ESRD patients, and therefore this inclusion criterion is frequently used to study this population of patients [46]. Another potential limitation is that some patients may have received over-the-counter medications or medications that were bundled with a dialysis payment that were not captured. A major limitation of our study is the lack of PTH levels, serum calcium, phosphate and alkaline phosphatase, which were not available due to limitations of the datasets and could render uncertainty about the diagnosis. However, because PTH levels begin to rise in stage 3 CKD, nearly all dialysis patients have elevated PTH levels [24–26]. In our study population, 92% had a diagnosis code for SHPT. It was also difficult to measure and factor into the analyses SHPT disease severity, appropriateness of no treatment, adherence to medications or determining if surgical parathyroidectomy was performed appropriately (addressing all four parathyroid glands). It was also difficult to determine who would be overtreated for SHPT and have low PTH levels, a risk factor for ABD. Although we wanted to focus our study on patients with elevated PTH which is typical of SHPT, we did examine risk factors for ABD including dialysis modality, drug-induced osteoporosis and those that were prescribed bisphosphonates. Of the study population, 3.2% had at least one inpatient or outpatient claim of drug-induced osteoporosis within 1 year since ESRD diagnosis and 2.0% were ever prescribed bisphosphonates during the study follow-up (1.3% within 1 year since ESRD diagnosis). Based on this, the minority of our study population may have had ABD, but without a histopathologic diagnosis, we are unable to know the exact number. However, our findings have built upon existing literature and demonstrate an association between SHPT and incident dementia, identify subgroups who have the greatest beneficial effects of treatment, and emphasize the need for SHPT treatment in older patients with ESRD.
As the prevalence of older patients on dialysis grows and the risk of incident dementia rises, it is critical to understand potentially modifiable risk factors. Our work demonstrates that patients who are treated for SHPT have a significantly lower risk of developing dementia. These findings should be incorporated into shared treatment decision-making and encourage treatment for SHPT to reduce the risk of incident dementia.
Supplementary Material
Contributor Information
Aarti Mathur, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
JiYoon B Ahn, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Whitney Sutton, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Nadia M Chu, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Alden L Gross, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Dorry L Segev, Department of Surgery, New York University Langone Health, NY, NY, USA.
Mara McAdams-DeMarco, Department of Surgery, New York University Langone Health, NY, NY, USA.
CONFLICT OF INTEREST STATEMENT
No authors report a conflict of interest relevant to this research. D.L.S. reports personal fees from Sanofi-Aventis and Novartis outside the submitted work. The results presented in this article have not been published previously in whole or part, except in abstract format. No authors have any disclosures or conflicts to report.
FUNDING
Funding for this study was provided in part by the National Cancer Institute, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and the National Institute on Aging (NIA); grant numbers T32CA126607 (W.S.), K23AG053429 (PI: A.M.), R01DK120518 (PI: M.McA-DeM.), R01AG055781 (PI: M.McA-DeM.), K01AG064040 (PI: N.M.C.), K01AG050699 (PI: A.L.G.) and K24AI144954 (PI: D.L.S.).
REFERENCES
- 1. Ketteler M, Block GA, Evenepoel Pet al. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what's changed and why it matters. Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 2. Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 2011; 79: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis 2008; 15: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vanderlinden JA, Ross-White A, Holden Ret al. Quantifying cognitive dysfunction across the spectrum of end-stage kidney disease: a systematic review and meta-analysis. Nephrology (Carlton) 2019; 24: 5–16 [DOI] [PubMed] [Google Scholar]
- 5. McAdams-DeMarco MA, Daubresse M, Bae Set al. Dementia, Alzheimer's disease, and mortality after hemodialysis initiation. Clin J Am Soc Nephrol 2018; 13: 1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu NM, Shi Z, Haugen CEet al. Cognitive function, access to kidney transplantation, and waitlist mortality among kidney transplant candidates with or without diabetes. Am J Kidney Dis 2020; 76: 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo Y, Murray AM, Guo YDet al. Cognitive impairment and associated risk factors in older adult hemodialysis patients: a cross-sectional survey. Sci Rep 2020; 10: 12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gong X, Zou L, Wu Het al. Altered brain structural and cognitive impairment in end-stage renal disease patients with secondary hyperparathyroidism. Acta Radiol 2020; 61: 796–803 [DOI] [PubMed] [Google Scholar]
- 9. Macdonald RL, Zhang ZD, Ono Set al. Up-regulation of parathyroid hormone receptor in cerebral arteries after subarachnoid hemorrhage in monkeys. Neurosurgery 2002; 50: 1083–1091 [DOI] [PubMed] [Google Scholar]
- 10. Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem 1995; 270: 15455–15458 [DOI] [PubMed] [Google Scholar]
- 11. Kipen E, Helme RD, Wark JDet al. Bone density, vitamin d nutrition, and parathyroid hormone levels in women with dementia. J Am Geriatr Soc 1995; 43: 1088–1091 [DOI] [PubMed] [Google Scholar]
- 12. Ogihara T, Miya K, Morimoto S. Possible participation of calcium-regulating factors in senile dementia in elderly female subjects. Gerontology 1990; 36: 25–30 [DOI] [PubMed] [Google Scholar]
- 13. Bjorkman MP, Sorva AJ, Tilvis RS. Does elevated parathyroid hormone concentration predict cognitive decline in older people? Aging Clin Exp Res 2010; 22: 164–169 [DOI] [PubMed] [Google Scholar]
- 14. Hagstrom E, Kilander L, Nylander Ret al. Plasma parathyroid hormone is associated with vascular dementia and cerebral hyperintensities in two community-based cohorts. J Clin Endocrinol Metab 2014; 99: 4181–4189 [DOI] [PubMed] [Google Scholar]
- 15. Kellett KA, Williams J, Vardy ERet al. Plasma alkaline phosphatase is elevated in Alzheimer's disease and inversely correlates with cognitive function. Int J Mol Epidemiol Genet 2011; 2: 114–121 [PMC free article] [PubMed] [Google Scholar]
- 16. Chou FF, Chen JB, Hsieh KCet al. Cognitive changes after parathyroidectomy in patients with secondary hyperparathyroidism. Surgery 2008; 143: 526–532 [DOI] [PubMed] [Google Scholar]
- 17. Lourida I, Thompson-Coon J, Dickens CMet al. Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS One 2015; 10: e0127574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallace HJ, Wallace IR, McCaffrey P. Cognitive decline reversed by cinacalcet. QJM 2015; 108: 59–61 [DOI] [PubMed] [Google Scholar]
- 19. Tentori F, Wang M, Bieber BAet al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015; 10: 98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dombrowsky A, Borg B, Xie Ret al. Why is hyperparathyroidism underdiagnosed and undertreated in older adults? Clin Med Insights Endocrinol Diabetes 2018; 11: 1179551418815916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greene B, Kim SJ, McCarthy EPet al. Effects of social disparities on management and surgical outcomes for patients with secondary hyperparathyroidism. World J Surg 2020; 44: 537–543 [DOI] [PubMed] [Google Scholar]
- 22. Omije D, Norris K, Wang Jet al. Race is a major determinant of secondary hyperparathyroidism in uremic patients: comparative study of Blacks and Hispanics. Clin Nephrol 2008; 70: 312–318 [DOI] [PubMed] [Google Scholar]
- 23. Owda A, Elhwairis H, Narra Set al. Secondary hyperparathyroidism in chronic hemodialysis patients: prevalence and race. Ren Fail 2003; 25: 595–602 [DOI] [PubMed] [Google Scholar]
- 24. Andress DL, Coyne DW, Kalantar-Zadeh Ket al. Management of secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Endocr Pract 2008; 14: 18–27 [DOI] [PubMed] [Google Scholar]
- 25. Levin A, Bakris GL, Molitch Met al. Prevalence of abnormal serum vitamin d, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71: 31–38 [DOI] [PubMed] [Google Scholar]
- 26. Sprague SM, Crawford PW, Melnick JZet al. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol 2016; 44: 316–325 [DOI] [PubMed] [Google Scholar]
- 27. Lau WL, Obi Y, Kalantar-Zadeh K. Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol 2018; 13: 952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001; 22: 477–501 [DOI] [PubMed] [Google Scholar]
- 29. Taylor DH Jr., Ostbye T, Langa KMet al. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis 2009; 17: 807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolfgram DF, Szabo A, Murray AMet al. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int 2015; 35: 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McAdams-DeMarco MA, Bae S, Chu Net al. Dementia and Alzheimer's disease among older kidney transplant recipients. J Am Soc Nephrol 2017; 28: 1575–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guerra C, Linde-Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care 2012; 16: R233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jackson JW, Schmid I, Stuart EA. Propensity scores in pharmacoepidemiology: beyond the horizon. Curr Epidemiol Rep 2017; 4: 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mathur MB, Ding P, Riddell CAet al. Web site and R package for computing E-values. Epidemiology 2018; 29: e45–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med 2017; 167: 268–274 [DOI] [PubMed] [Google Scholar]
- 36. Ponjoan A, Garre-Olmo J, Blanch Jet al. Epidemiology of dementia: prevalence and incidence estimates using validated electronic health records from primary care. Clin Epidemiol 2019; 11: 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Livingston G, Huntley J, Sommerlad Aet al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viggiano D, Wagner CA, Martino Get al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol 2020; 16: 452–469 [DOI] [PubMed] [Google Scholar]
- 39. Driessen M, Wetterling T, Wedel Tet al. Secondary hyperparathyroidism and depression in chronic renal failure. Nephron 1995; 70: 334–339 [DOI] [PubMed] [Google Scholar]
- 40. Gilli P, De Bastiani P. Cognitive function and regular dialysis treatment. Clin Nephrol 1983; 19: 188–192 [PubMed] [Google Scholar]
- 41. Leinau L, Murphy TE, Bradley Eet al. Relationship between conditions addressed by hemodialysis guidelines and non-ESRD-specific conditions affecting quality of life. Clin J Am Soc Nephrol 2009; 4: 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puy L, Bugnicourt JM, Liabeuf Set al. Cognitive impairments and dysexecutive behavioral disorders in chronic kidney disease. J Neuropsychiatry Clin Neurosci 2018; 30: 310–317 [DOI] [PubMed] [Google Scholar]
- 43. Kalaitzidis RG, Karasavvidou D, Tatsioni Aet al. Risk factors for cognitive dysfunction in CKD and hypertensive subjects. Int Urol Nephrol 2013; 45: 1637–1646 [DOI] [PubMed] [Google Scholar]
- 44. National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42: S1–S201 [PubMed] [Google Scholar]
- 45. Schneider R, Slater EP, Karakas Eet al. Initial parathyroid surgery in 606 patients with renal hyperparathyroidism. World J Surg 2012; 36: 318–326 [DOI] [PubMed] [Google Scholar]
- 46. Muzaale AD, Massie AB, Wang MCet al. Risk of end-stage renal disease following live kidney donation. JAMA 2014; 311: 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.