ABSTRACT
Background
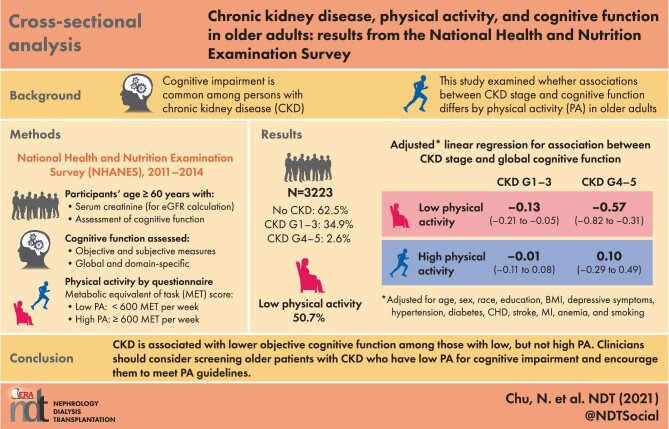
Cognitive impairment is common among persons with chronic kidney disease (CKD), due in part to reduced kidney function. Given that physical activity (PA) is known to mitigate cognitive decline, we examined whether associations between CKD stage and global/domain-specific cognitive function differ by PA.
Methods
We leveraged 3223 participants (≥60 years of age) enrolled in National Health and Nutrition Examination Survey (NHANES, 2011–2014), with at least one measure of objective cognitive function [immediate recall (CERAD-WL), delayed recall (CERAD-DR), verbal fluency (AF), executive function/processing speed (DSST), global (average of four tests) or self-perceived memory decline (SCD)]. We quantified the association between CKD stage {no CKD: estimated glomerular filtration rate [eGFR] ≥60 mL/min/1.73 m2 and albuminuria [albumin:creatinine ratio (ACR)] <30 mg/g; stages G1–G3: eGFR ≥60 mL/min/1.73 m2 and ACR ≥30 mg/g or eGFR 30–59 mL/min/1.73 m2; stages G4 and G5: eGFR <30 mL/min/1.73 m2} and cognitive function using linear regression (objective measures) and logistic regression (SCD), accounting for sampling weights for nationally representative estimates. We tested whether associations differed by PA [Global Physical Activity Questionnaire, high PA ≥600 metabolic equivalent of task (MET) · min/week versus low PA <600 MET · min/week] using a Wald test.
Results
Among NHANES participants, 34.9% had CKD stages G1–G3, 2.6% had stages G4 and G5 and 50.7% had low PA. CKD stages G4 and G5 were associated with lower global cognitive function {difference = −0.38 standard deviation [SD] [95% confidence interval (CI) −0.62 to −0.15]}. This association differed by PA (Pinteraction = 0.01). Specifically, among participants with low PA, those with CKD stages G4 and G5 had lower global cognitive function [difference = −0.57 SD (95% CI −0.82 to −0.31)] compared with those without CKD. Among those with high PA, no difference was found [difference = 0.10 SD (95% CI −0.29–0.49)]. Similarly, the CKD stage was only associated with immediate recall, verbal fluency, executive function and processing speed among those with low PA; no associations were observed for delayed recall or self-perceived memory decline.
Conclusions
CKD is associated with lower objective cognitive function among those with low but not high PA. Clinicians should consider screening older patients with CKD who have low PA for cognitive impairment and encourage them to meet PA guidelines.
Keywords: CKD, elderly, epidemiology, GFR, physical activity
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Cognitive impairment is common among persons with chronic kidney disease (CKD) due in part to reduced kidney function. Given that physical activity is known to mitigate cognitive decline, we examined whether associations between CKD stage and global/domain-specific cognitive function differ by physical activity.
What this study adds?
CKD is associated with worse global cognitive function, immediate recall, verbal fluency, executive functioning and processing speed among those with low, but not high physical activity. No associations were observed for delayed recall or self-perceived memory decline. Additionally, objective measures of cognitive function detected low cognitive performance more frequently than subjective measures.
What impact this may have on practice or policy?
Clinicians should consider objectively screening older patients with CKD who have low physical activity for cognitive impairment and encourage them to meet physical activity guidelines.
INTRODUCTION
Cognitive impairment is common among people of all ages with chronic kidney disease (CKD), with prevalence ranging from 13 to 58% [1–5]. The association between CKD and cognitive function has been extensively studied [2, 6–11], with collective evidence demonstrating that with each 10 mL/min/1.73 m2 decrease in estimated glomerular filtration rate (eGFR) among those with CKD, the odds of cognitive impairment increase by 10–12% [1, 11, 12]. Putative mechanisms underlying the association between CKD and cognitive decline include vascular dysfunction, lymphatic dysfunction, decreased clearance of uremic toxins and hemodynamic changes during dialysis [6, 7]. Nonetheless, prevention and treatment of cognitive impairment in CKD are limited [13]. A more thorough examination of the association between kidney function and cognitive function by specific cognitive domains can help discern potential differences in etiologies; for example, two of the most common types of dementia, Alzheimer's disease and vascular dementia, are often distinguished by disproportionate impairments in episodic memory and executive function, respectively [14].
Physical activity (PA) is likely protective against cognitive decline among the general population and persons with chronic health conditions [15–21]. After PA, older adults experience improved regulation of hippocampal function, neurogenesis, synaptic plasticity, cerebral blood flow and a reduction in cardiovascular risk and proinflammatory activity [15, 22, 23]. Given the high prevalence of cardiovascular disease in individuals with CKD, it is likely that the association between kidney function and cognitive function is modified by PA level [24, 25].
To test whether the association between CKD stage and cognitive function differs by PA level, we leveraged the National Health and Nutrition Examination Survey (NHANES, 2011–2014), a nationally representative cross-sectional study designed to assess the health and nutritional status of the US civilian noninstitutionalized resident population [26]. Our goals were to quantify the association between CKD stage and global and domain-specific cognitive function and test whether these associations differed between those with low and high PA levels.
MATERIALS AND METHODS
Study design
We conducted a cross-sectional study of 3223 participants ≥60 years of age from NHANES (2011–2014). Participants with measurements of serum creatinine for eGFR calculation and at least one assessment of cognitive function, as described below, were included in the study. Participants’ demographic information and health status were collected either through direct measurement or via self-report at each study cycle, including body mass index (BMI; weight divided by height squared), depressive symptoms [Patient Health Questionnaire (PHQ-9) ≥10] [27–29], hypertension [systolic blood pressure (SBP) ≥130 mmHg, diastolic blood pressure (DBP) ≥80 mmHg or reported current use of antihypertensive medication], diabetes (fasting blood glucose level ≥126 mg/dL, nonfasting glucose level ≥200 mg/dL, reported history of diabetes or current use of medications for diabetes or high blood sugar) and anemia (hemoglobin <12 g/dL in males, hemoglobin <11 g/dL in females or reported taking treatment for anemia in the past 3 months). History of coronary heart disease (CHD), myocardial infarction (MI), stroke and smoking (smoked at least 100 cigarettes in life) were collected by self-report using a questionnaire.
CKD stage
Serum creatinine and albuminuria [albumin:creratinine ratio (ACR)] were measured at each study cycle. Serum creatinine was used to calculate eGFR with the Chronic Kidney Diease Epidemiology Collaboration (CKD-EPI) equation [30]. We defined categories of CKD stage based on previously published guidelines: no CKD (eGFR ≥60 mL/min/1.73 m2 and ACR <30 mg/g), CKD stages G1–G3 (eGFR 30–59 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 and ACR ≥30 mg/g) and CKD stages G4 and G5 (eGFR < 30 mL/min/1.73 m2) [31].
Cognitive function
We defined global and domain-specific cognitive function using the objective and subjective measures available in NHANES at each cycle (2011–2014). Objective measures included four separate tests: word list learning trials from the Consortium to Establish a Registry for Alzheimer's Disease battery for immediate recall (CERAD-WL) and delayed recall (CERAD-DR) [32], the Animal Fluency (AF) test for verbal fluency [33, 34] and the Digit Symbol Substitution Test (DSST) for executive function and processing speed [35–37]. The word list learning task for immediate recall (CERAD-WL) and delayed recall (CERAD-DR) examines the ability to recall newly learned information and delayed memory and has been validated as a tool designed to discriminate those with potential cognitive impairment from those with healthy cognitive function [38, 39]. Specifically, 10 words were read aloud by the participant, immediately followed by three consecutive recalls [33, 40, 41]. The delayed recall of all 10 words occurred after the AF and DSST assessments, ∼8–10 min from the start of the word learning trials [33, 40, 41]. The AF is a verbal fluency test that assesses semantic memory, where participants are asked to name aloud as many animals as possible in 1 min [33, 34]. AF test scores have also been shown to discriminate those with normal cognitive function from those who have mild cognitive impairment and more severe forms of cognitive impairment, such as Alzheimer's disease and related dementia [42, 43]. The DSST is a highly sensitive, validated [44–46], paper-and-pencil cognitive test that primarily assesses attention and processing speed [35], but it is also linked to executive functioning [35–37]. The examination was conducted using a paper form that has a key at the top containing nine numbers paired with symbols. Participants copy the matching symbol in boxes that adjoin the numbers in 2 min. For each of the cognitive tests, 1 point was given for each correctly recalled, named, or matched response, with a higher score reflecting better cognitive function [33, 40, 41]. All objective tests were then standardized to a mean of 0 and standard deviation (SD) of 1 and averaged into a global cognitive composite score, as described in prior studies [47].
Subjective cognitive function, assessing self-perceived memory through at-home interviews, was also considered as a separate outcome. Specifically, participants reported whether or not they experienced worsening or more frequent confusion or memory loss during the past 12 months.
Physical activity
PA was collected via questionnaire based on the Global Physical Activity Questionnaire [48]. Participants reported their frequency of and time spent in vigorous and moderate work activity, walking or bicycling and vigorous or moderate recreational activities. Metabolic equivalent of task (MET) scores were assigned for each activity based on prior NHANES guidelines [49]: 8 for vigorous work-related activity, 4 for moderate work-related activity, 4 for walking or bicycling, 8 for vigorous leisure-time PA and 4 for moderate leisure-time PA. The total amount of PA was assessed by summing minutes of activity per week multiplied by the MET score of each activity. A high level of PA was defined as ≥600 MET·min/week and a low level of PA was defined as <600 MET·min/week based on the US PA guidelines [50].
Descriptive statistics
We summarized the distributions of characteristics in participants and presented the distribution in participants with no CKD, with CKD stages G1–G3 and CKD stages G4 and G5. We generated means and SDs for normally distributed continuous variables, medians and interquartile ranges (IQRs) for nonnormally distributed continuous variables and proportions for binary or categorical variables. Mobile examination center sample weights were accounted for in the analysis to produce estimates that are representative of the US non-institutionalized civilian resident population [51]. Descriptive statistics of the study population without imposed sampling weights are also available in the Supplementary data, Table S1.
PA, CKD and objective cognitive function
For the standardized objective cognitive tests, we used adjusted linear regression models to assess the difference in cognitive function by CKD stage to generate Cohen's d [52]; Cohen's d refers to the standardized difference between two means, where effect sizes of 0.2 SD are generally considered small, 0.5 SD medium and 0.8 SD large [53]. To assess whether associations differed between high and low PA levels, we tested the interaction between CKD stage and PA on cognitive function using a Wald test. Models were adjusted for potential confounders of CKD and cognitive decline, including age, sex, race, education, BMI, depressive symptoms, hypertension, diabetes, CHD, stroke, Myocardial Infarction (MI), anemia and smoking. The mobile examination center sample weights were accounted for to generate nationally representative estimates.
PA, CKD and subjective cognitive function
We used adjusted logistic regression models to quantify the association between CKD stage and subjective cognitive function [odds ratios (ORs)]. To test whether the association differed between high and low PA levels, we used an approach similar to that outlined above for objective measures.
Sensitivity analysis
To test whether inferences remained robust overall and by PA level, we first removed race from the CKD-EPI equation to adhere to the National Kidney Foundation and American Society of Nephrology's mission to move past race-based medicine [54]. Additionally, we excluded those with severe depressive symptoms, given that it is highly correlated with CKD, cognitive impairment and PA [7, 55–58]; the PHQ-9 has high validity and reliability against the gold standard criteria of a diagnosis of depression among long-term dialysis patients, as well as in primary care populations [28, 29]. We also tested whether inferences remained robust after more rigid control of anemia using continuous hemoglobin levels. Finally, given that smoking is a risk factor for CKD progression as well as cardiovascular morbidity [59, 60], we assessed whether smoking status (ever versus never smokers) significantly modifies associations between PA, CKD and cognitive function.
Statistical analysis
All statistical analysis was conducted using Stata version 16 (StataCorp, College Station, TX, USA) and we used a statistical significance cutoff of α < 0.05.
RESULTS
Participant characteristics
Among the study population, 16.8% were ≥80 years of age, 78.4% were non-Hispanic White and 54.2% were female (Table 1). Overall, 62.5% had no CKD, 34.9% had CKD stages G1–G3 and 2.6% had CKD stages G4 and G5. Additionally, 14.7% reported self-perceived memory impairment and median scores for objective cognitive tests were 20 (IQR 17–23) for immediate recall (CERAD-WL), 6 (IQR 5–8) for delayed recall (CERAD-DR), 18 (IQR 14–21) for verbal fluency (AF) and 53 (IQR 41–64) for executive function and processing speed (DSST). Additionally, 50.7% met criteria for low PA. Before adjustment, participants without CKD were more likely to meet PA guidelines compared with those with CKD stages G1–G3 (55.4% versus 38.6%) and CKD stages G4 and G5 (54.4% versus 21.5%) (Table 1).
Table 1.
Characteristics of participants ≥60 years of age from the NHANES (2011–2014) (n = 3223) with recorded cognitive function
| Characteristics | Overall (N = 3223) | No CKD (n = 2015) | CKD stages G1–G3 (n = 1124) | CKD stages G4 and G5 (n = 84) |
|---|---|---|---|---|
| Age (years) | ||||
| 60–69 | 53.7 | 64.2 | 33.6 | 32.1 |
| 70–79 | 29.5 | 26.6 | 34.9 | 36.3 |
| ≥80 | 16.8 | 9.2 | 31.5 | 31.6 |
| Race | ||||
| Mexican American | 3.8 | 3.9 | 3.4 | 5.9 |
| Other Hispanic | 3.8 | 3.9 | 3.5 | 2.7 |
| Non-Hispanic White | 78.4 | 78.6 | 78.7 | 66.8 |
| Non-Hispanic Black | 8.4 | 7.8 | 9.0 | 20.4 |
| Non-Hispanic Asian | 3.9 | 4.3 | 3.3 | 1.2 |
| Other | 1.7 | 1.4 | 2.1 | 3.0 |
| Female | 54.2 | 53.0 | 56.7 | 57.0 |
| BMI (kg/m2), mean (SE) | 29.0 (0.2) | 28.7 (0.3) | 29.5 (0.3) | 29.7 (0.9) |
| Education ≥12 years | 81.5 | 84.4 | 77.2 | 57.3 |
| Severe depressive symptoms | 7.6 | 6.8 | 8.5 | 17.7 |
| Hypertension | 75.5 | 70.0 | 86.0 | 88.8 |
| Treated hypertension | 93.7 | 92.6 | 95.2 | 93.5 |
| Diabetes | 23.3 | 17.8 | 32.2 | 59.7 |
| CHD | 10.0 | 7.1 | 15.4 | 19.0 |
| MI | 8.8 | 6.3 | 13.4 | 18.7 |
| Stroke | 7.7 | 5.0 | 12.6 | 16.8 |
| Anemia | 7.0 | 3.7 | 11.4 | 45.8 |
| Treated anemia | 4.3 | 2.8 | 6.6 | 16.5 |
| Smoking | 50.1 | 48.7 | 53.1 | 47.5 |
| Physical activity, median (IQR) | 540 (2000) | 840 (2400) | 180 (1440) | 0 (300) |
| Low physical activity | 50.7 | 44.6 | 61.4 | 78.5 |
Proportions (%) are presented unless stated otherwise, accounting for NHANES sampling weights. Serum creatinine was measured and used to calculate eGFR with the CKD-EPI equation. CKD stage was then defined by the following cut-points: no CKD: eGFR ≥60 mL/min/1.73 m2 and ACR <30 mg/g; CKD stages G1–G3: eGFR 30–59 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 and ACR ≥30 mg/g; CKD stages G4 and G5: eGFR <30 mL/min/1.73 m2. Severe depressive symptoms were defined as a Patient Health Questionnaire [PHQ-9] score ≥10. Physical activity was collected using the Global Physical Activity Questionnaire and converted to MET·min/week. High physical activity was defined as ≥600 MET·min/week. SE, standard error.
CKD and cognitive function
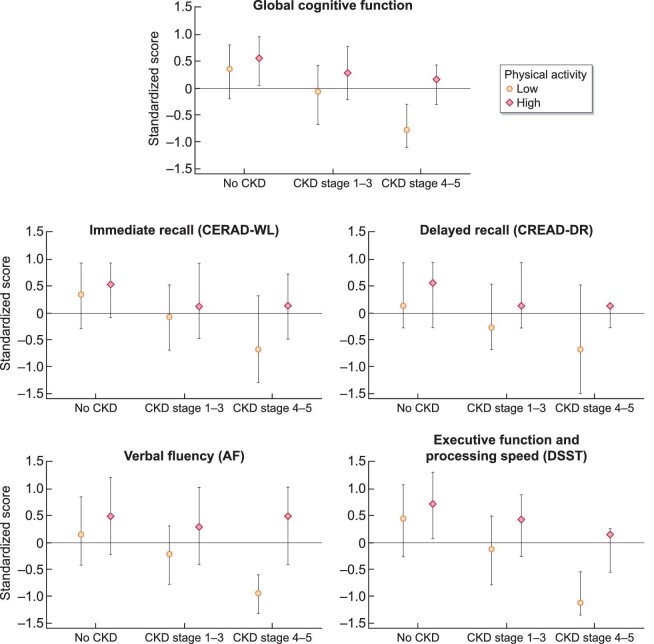
Before adjustment for risk factors, participants with CKD stages G1–G3 {Cohen's d = −0.32 SD [95% confidence interval (CI) −0.41 to −0.23]} and CKD stages G4 and G5 [Cohen's d = −0.81 SD (95% CI −1.00 to −0.62)] had significantly lower global cognitive function compared with those without CKD (P < 0.01) (Figure 1); similar results were found for domain-specific tests (Supplementary data, Table S2). After adjustment for risk factors, only participants with CKD stages G4 and G5 had lower global cognitive function compared with those without CKD [Cohen's d = −0.38 SD (95% CI −0.62 to −0.15)] (Table 2). This finding was consistent for domain-specific tests, particularly for executive function and processing speed [Cohen's d = −0.54 SD (95% CI −0.93 to −0.16)], immediate recall [Cohen's d = −0.38 SD (95% CI −0.56 to −0.21)] and verbal fluency [Cohen's d = −0.29 (95% CI −0.58 to −0.01)]. Those with CKD stages G1–G3 had poorer cognitive performance in executive function and processing speed [Cohen's d = −0.12 (95% CI −0.21 to −0.03)] compared with those without CKD. No associations were observed between CKD stage and delayed recall [CKD stages G1–G3 versus no CKD: Cohen's d = −0.03 (95% CI −0.14–0.08); CKD stages G4 and G5 versus no CKD: Cohen's d = −0.23 (95% CI −0.57–0.11)]. Additionally, CKD stages G1–G3 were associated with poorer self-perceived memory compared with those without CKD [OR 1.41 (95% CI 1.06–1.88)]; however, there was no association with self-perceived memory for those with CKD stages G4 and G5 compared with those without CKD [OR 1.16 (95% CI 0.51–2.62)] (Table 3).
FIGURE 1:
Objective cognitive test scores by CKD stage and physical activity level among participants ≥60 years of age from the NHANES (2011–2014) (N = 3223). Medians and IQRs are presented for cognitive test scores (standardized to a mean of 0 and an SD of 1). NHANES sampling weights were accounted for to obtain nationally representative estimates. Serum creatinine was measured and used to calculate eGFR with the CKD-EPI equation. CKD stage was then defined by the following cut-points: no CKD: eGFR ≥60 mL/min/1.73 m2 and ACR <30 mg/g; CKD stages G1–G3: eGFR 30–59 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 and ACR ≥30 mg/g; CKD stages G4 and G5: eGFR <30 mL/min/1.73 m2. Physical activity was collected using the Global Physical Activity Questionnaire and converted to MET·min/week, where ≥600 MET·min/week represents high physical activity.
Table 2.
CKD stage and objective assessments of cognitive function by levels of PA among participants ≥60 years of age from NHANES (2011–2014) (N = 3223).
| Overall, | Low physical activity, | High physical activity, | ||
|---|---|---|---|---|
| Stage and assessment | mean (95% CI) | mean (95% CI) | mean (95% CI) | P for interaction |
| Global cognitive function | ||||
| No CKD | 0 (ref) | 0 (ref) | 0 (ref) | |
| CKD stages G1–G3 | −0.07 (-0.15–0.00) | −0.13 (−0.21 to −0.05) | −0.01 (−0.11–0.08) | 0.03 |
| CKD stages G4 and G5 | −0.38 (−0.62 to −0.15) | −0.57 (−0.82 to −0.31) | 0.10 (−0.29–0.48) | 0.01 |
| Immediate recall (CERAD-WL) | ||||
| No CKD | 0 (ref) | 0 (ref) | 0 (ref) | |
| CKD stages G1–G3 | −0.10 (−0.19 to −0.00) | −0.17 (−0.31 to −0.02) | −0.03 (−0.19–0.14) | 0.24 |
| CKD stages G4 and G5 | −0.38 (−0.56 to −0.21) | −0.50 (−0.77 to −0.23) | −0.11 (−0.52–0.31) | 0.18 |
| Delayed recall (CERAD-DR) | ||||
| No CKD | 0 (ref) | 0 (ref) | 0 (ref) | |
| CKD stages G1–G3 | −0.03 (−0.14–0.08) | −0.10 (−0.25–0.04) | 0.05 (−0.11–0.21) | 0.13 |
| CKD stages G4 and G5 | −0.23 (−0.57–0.11) | −0.37 (−0.80–0.06) | 0.08 (−0.36–0.53) | 0.15 |
| Verbal fluency (AF) | ||||
| No CKD | 0 (ref) | 0 (ref) | 0 (ref) | |
| CKD stages G1–G3 | −0.09 (−0.19–0.01) | −0.13 (−0.25 to −0.00) | −0.05 (−0.18–0.07) | 0.34 |
| CKD stages G4 and G5 | −0.29 (−0.58 to −0.01) | −0.48 (−0.78 to −0.18) | 0.19 (−0.31–0.68) | 0.02 |
| Executive function and processing speed (DSST) | ||||
| No CKD | 0 (ref) | 0 (ref) | 0 (ref) | |
| CKD stages G1–G3 | −0.12 (−0.21 to −0.03) | −0.19 (−0.30 to −0.08) | −0.05 (−0.20–0.09) | 0.15 |
| CKD stages G4 and G5 | −0.54 (−0.93 to −0.16) | −0.75 (−1.18 to −0.32) | −0.01 (−0.51–0.50) | 0.02 |
Significant values (P < 0.05) are in bold.
Cognitive test scores are standardized to a mean of 0 and an SD of 1. Models are adjusted for age, sex, race, education, BMI, depressive symptoms, hypertension, diabetes, CHD, MI, stroke, anemia and smoking. NHANES sampling weights are accounted for in linear regression analyses to obtain nationally representative estimates. Serum creatinine was measured and used to calculate eGFR with the CKD-EPI equation. CKD stage was then defined by the following cut-points: no CKD: eGFR ≥60 mL/min/1.73 m2 and ACR <30 mg/g; CKD stages G1–G3: eGFR 30–59 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 and ACR ≥30 mg/g; CKD stages G4 and G5: eGFR <30 mL/min/1.73 m2. Physical activity was collected using the Global Physical Activity Questionnaire and converted to MET·min/week, where ≥600 MET·min/week represents high physical activity.
Table 3.
Association between renal function and self-perceived memory decline by physical activity (N = 3223)
| Overall, | Low physical activity, | High physical activity, | ||
|---|---|---|---|---|
| CKD stage | OR (95% CI) | OR (95% CI) | OR (95% CI) | P for interaction |
| No CKD | 1 (ref) | 1 (ref) | 1 (ref) | |
| CKD stages G1–G3 | 1.41 (1.06, 1.88) | 1.60 (1.09, 2.34) | 1.15 (0.76, 1.73) | 0.24 |
| CKD stages G4 and G5 | 1.16 (0.51, 2.62) | 1.19 (0.47, 3.03) | 1.24 (0.13, 11.47) | 0.97 |
Models are adjusted for age, sex, race, education, BMI, depressive symptoms, hypertension, diabetes, CHD, MI, stroke, anemia and smoking. NHANES sampling weights are accounted for in logistic regression analysis to obtain nationally representative estimates. Serum creatinine was measured and used to calculate eGFR with the CKD-EPI equation. CKD stage was then defined by the following cut-points: no CKD: eGFR ≥60 mL/min/1.73 m2 and ACR <30 mg/g; CKD stages G1–G3: eGFR 30–59 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 and ACR ≥30 mg/g; CKD stages G4 and G5: eGFR <30 mL/min/1.73 m2. Physical activity was collected using the Global Physical Activity Questionnaire and converted to MET·min/week, where ≥600 MET·min/week represents high physical activity.
PA, CKD stage and global cognitive function
The association between CKD and global cognitive function differed by PA level when comparing those with CKD stages G4 and G5 with those without CKD (P for interaction = 0.01). Before adjustment (Supplementary data, Table S2), among participants with low PA, those with CKD stages G4 and G5 had poorer global cognitive function compared with those without CKD [Cohen's d = −0.99 (95% CI −1.15 to −0.83)]; however, there was no association between CKD and global cognitive function among those with high PA [Cohen's d = −0.27 (95% CI −0.84–0.30)]. The association between CKD and global cognitive function did not differ by PA level when comparing those with CKD stages G1–G3 with those without CKD (P for interaction = 0.05).
After adjustment (Table 2), the association between CKD and global cognitive function differed by PA level for those with CKD stages G4 and G5 compared with those without CKD (P for interaction = 0.01). Among participants with low physical activity, those with CKD stages G4 and G5 had poorer global cognitive function compared with those without CKD [Cohen's d = −0.57 (95% CI −0.82 to −0.31)]; however, there was no association between CKD and global cognitive function among those with high PA [Cohen's d = 0.10 (95% CI −0.29–0.48)]. Similar results were observed by PA level when comparing those with CKD stages G1–G3 with those without CKD [low PA: Cohen's d = −0.13 (95% CI −0.21 to −0.05); high PA: Cohen's d = −0.01 (95% CI −0.11–0.08); P for interaction = 0.03].
PA, CKD stage and cognitive function by cognitive domain
Before adjustment (Supplementary data, Table S2) and after adjustment (Table 2), differences by PA level were consistent for verbal fluency (P for interaction = 0.02) and executive function and processing speed (P for interaction = 0.02). Specifically, among those with low PA, CKD stages G4 and G5 were associated with lower performance in verbal fluency compared with those without CKD [Cohen's d = −0.48 (95% CI −0.78 to −0.18)], but among those with high PA, there was no association [Cohen's d = 0.19 (95% CI −0.31–0.68)]. Similar findings were observed for executive function and processing speed when comparing CKD stages G4 and G5 with those without CKD [low PA: −0.75 (95% CI −1.18 to −0.32); high PA: −0.01 (95% CI −0.51–0.50)]. The association between CKD stages G1–G3 and domain-specific cognitive function did not differ by PA level for any of the cognitive domains (all P for interaction > 0.05) (Table 2). Similarly, the association between CKD and self-perceived memory impairment did not differ by PA (CKD stages G4 and G5 versus no CKD: P for interaction = 0.97; CKD stages G1–G3 versus no CKD: P for interaction = 0.24) (Table 3).
Sensitivity analyses
When we removed race from the CKD-EPI equation to classify CKD stage (Supplementary data, Tables S4 and S5), excluded participants with severe depressive symptoms (Supplementary data, Tables S6 and S7) or controlled more rigidly for anemia using continuous hemoglobin levels (Supplementary data, Tables S8 and S9), inferences remained robust across all global and domain-specific cognitive tests, overall and by PA level. Additionally, smoking status did not significantly modify associations between PA, CKD and cognitive function for all global and domain-specific cognitive tests (P > 0.05); nevertheless, subgroup analyses restricted to ever versus never smokers are presented in the Supplementary data, Tables S10 and S11.
DISCUSSION
In this nationally representative study of 3223 participants, we found that compared to participants without CKD in NHANES, only those with CKD stages G4 and G5 were associated with poorer global cognitive performance [CKD stages G1–G3: difference = −0.07 SD (95% CI −0.15–0.00); CKD stages G4 and G5: difference = −0.38 SD (95% CI −0.62 to −0.15)]. Similar findings were observed for domain-specific tests. Compared with those without CKD, CKD stages G4 and G5 were most strongly associated with poorer performance in executive function and processing speed [Cohen's d = −0.54 SD (95% CI −0.93 to −0.16)], immediate recall [Cohen's d = −0.38 SD (95% CI −0.56 to −0.21)] and verbal fluency [Cohen's d = −0.29 (95% CI −0.58 to −0.01)]. No associations were observed for delayed recall. Interestingly, self-perceived memory decline was only associated with CKD stages G1–G3 [OR 1.41 (95% CI 1.06–1.88)], but not with CKD stages G4 and G5 [OR 1.16 (95% CI 0.51–2.62)]. Nonetheless, associations with global cognitive performance differed by PA level for both CKD stages G4 and G5 (P for interaction = 0.01) and CKD stages G1–G3 (P for interaction = 0.03) compared with those without CKD, with greatest differences found among those in later CKD stages. Specifically, among participants with low PA, those with CKD stages G4 and G5 [Cohen's d = −0.57 (95% CI −0.82 to −0.31)] and CKD stages G1–G3 [(Cohen's d = –0.13 (95% CI −0.21 to −0.05)] had lower global cognitive function compared with those without CKD; however, among those with high PA, no differences were observed [CKD stages G1–G3 versus no CKD: Cohen's d = −0.01 (95% CI −0.11–0.08); CKD stages G4 and G5 versus no CKD: Cohen's d = 0.10 (95% CI −0.29–0.48)]. Consistent with findings for global cognitive performance, associations differed by PA for verbal fluency (P = 0.02) and executive function and processing speed (P = 0.02).
Previous studies of cognitive impairment and kidney disease report similar results [61–66]. Despite the different objective measures used to assess cognitive function, as well as the different algorithms used to assess kidney function, studies have consistently demonstrated that worse kidney function is associated with worse cognitive function. Our findings corroborate prior results in a diverse, nationally representative sample of adults ≥60 years of age. In this study, worse CKD was associated with moderately lower cognitive function [CKD stages G4 and G5 versus no CKD: difference = −0.38 SD (95% CI −0.62 to −0.15)] based on the Cohen's d scale. Interestingly, findings were not consistent when using a subjective measure of self-perceived memory function [CKD stages G1–G3: 1.41 (95% CI 1.06–1.88); CKD stages G4 and G5: 1.16 (95% CI 0.51–2.62)]. This is especially concerning given the lack of standardization in cognitive screening [67]; clinicians often rely on subjective, self-perceived screening rather than more thorough, objective testing [68]. Considering these findings in parallel with current clinical practice, nephrologists should consider using objective measures when available to detect lower cognitive performance related to reduced kidney function.
This study extends prior findings on the relationship between cognition and kidney function by investigating how physical activity modifies this association. Similar to the cognitive benefits observed among healthy older adults generally [15, 17–20], individuals with chronic health conditions [16] and among patients with end-stage kidney disease (ESKD) [21], our nationally representative study of persons ≥60 years of age demonstrates that the correlation between CKD and global cognitive performance was mitigated for those who met PA guidelines for both CKD stages G1–G3 [high PA: difference = −0.01 (95% CI −0.11–0.08); low PA: difference = −0.13 (95% CI −0.21 to −0.05)] and CKD stages G4 and G5 [high PA: difference = 0.10 SD (95% CI −0.29–0.48); low PA: difference = −0.57 SD (95% CI −0.82 to −0.31)] compared with those without CKD. Notably, these results are cross-sectional in nature; however, there is evidence from other studies that walking, pedaling and cycling can preserve cognition among patients with ESKD undergoing dialysis [69–73]. Additionally, other studies have found that impaired physical performance, such as slower gait speed, was an early marker of cognitive dysfunction [74]. It is likely that exercise and improvements in PA or physical performance can reduce cardiovascular risks [75, 76], improve cerebral blood flow and, in turn, mitigate the impact of cerebral infarcts on cognitive decline in the CKD population [77, 78].
Indeed, our results, though cross-sectional in nature, may reflect the potential vascular mechanisms underlying the relationship between kidney function, physical activity and cognitive function by investigating associations by specific cognitive domain. Findings were consistent for verbal fluency (CKD stages G4 and G5 versus no CKD: P for interaction = 0.02), as well as executive function and processing speed measured using the DSST, where the greatest differences were observed by activity level (CKD stages G4 and G5 versus no CKD: P for interaction = 0.02). Other objective tests linked more closely to memory, including the CERAD-WL (immediate recall), CERAD-DR (delayed recall) [32] and SCD (self-perceived memory decline), were less consistent. These findings support prior studies that have highlighted the implications of reduced kidney function on executive function, such as among those with ESKD [79, 80]. However, our findings extend those results to a novel, nationally representative population of older patients with CKD and observed that those with low PA are most vulnerable. Impairments in executive function in patients with CKD may suggest early signs of dementia, particularly vascular dementia [14, 81], and in many cases Alzheimer's disease [82, 83], likely as a result of highly prevalent traditional cardiovascular disease risk factors and inflammation that may predispose those with low PA to abnormal brain function [84]. Further prospective studies designed to assess whether programs to improve PA can mitigate a decline in cognitive function in patients with CKD are warranted given the lack of prevention and treatment strategies available for this vulnerable population [13].
There are several notable limitations that deserve comment. Most importantly, the cross-sectional nature of the data does not allow any causal inference; the relationship between CKD, cognitive function and physical activity should be studied prospectively to corroborate other studies in non-CKD populations that have found bidirectional associations between physical and cognitive function [15, 85–87]. Additionally, as an observational study, NHANES is subject to unmeasured confounding. Though we adjusted for many known correlates of kidney impairment, physical activity and cognitive function, for other unmeasured reasons, the same individuals who tend to have low activity and greater CKD severity may be at greater risk of reduced cognitive function. Finally, though we found no significant differences in the relationship between PA, CKD and cognitive function by smoking status (Supplementary data, Tables S10 and S11), further investigations designed to unpack the role of smoking behavior, CKD progression and PA on cognitive function are needed.
Despite these limitations, this study has many strengths. It extends prior findings to a large, novel, nationally representative sample of individuals ≥60 years of age. It additionally presents both subjective and objective measures of cognitive function, providing the opportunity to examine multiple cognitive domains. Finally, it includes a measure of PA that allows direct calculation of MET scores for comparison with current PA level standards.
In conclusion, while kidney function is associated with reduced global cognitive performance and executive function, meeting PA guidelines may mitigate those relationships. Additionally, objective measures of cognitive function detected low cognitive performance in those with CKD more frequently than subjective measures, particularly among those with low PA. Therefore clinicians should consider objectively screening older patients with CKD who have low PA for cognitive impairment and encourage them to meet PA guidelines.
Supplementary Material
Contributor Information
Nadia M Chu, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Jingyao Hong, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Oksana Harasemiw, Department of Internal Medicine, University of Manitoba, Winnipeg, MB, Canada.
Xiaomeng Chen, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Kevin J Fowler, The Voice of the Patient, Inc., Chicago, IL, USA.
Indranil Dasgupta, Heartlands Hospital Birmingham and Warwick Medical School, University of Warwick, West Midlands, UK.
Clara Bohm, Department of Internal Medicine, University of Manitoba, Winnipeg, MB, Canada.
Dorry L Segev, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Mara A McAdams-DeMarco, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
AUTHORS’ CONTRIBUTIONS
N.M.C. and M.M.D. participated in concept design, interpretation, drafting, critical revision and approval of the article. J.H. participated in data analysis, drafting and approval of the article. O.H., X.C., K.J.F., I.D. and C.B. participated in critical revision and approval of the article. D.L.S. participated in concept design, critical revision and approval of the article.
FUNDING
Study investigators were funded by the National Institute of Diabetes and Digestive and Kidney Disease, the National Institute of Allergy and Infectious Disease and the National Institute on Aging {grant numbers K01AG064040 [principal investigator (PI) N.M.C.], K24AI144954 [PI D.S.], R01AG055781 [PI M.A.M.-D.], R01DK120518 [PI M.A.M.-D.] and R01DK114074 [PI M.A.M.-D.], as well as by the Manitoba Medical Services Foundation (to C.B.). Funders had no role in the study design, data collection, analysis, reporting or decision to submit for publication.
CONFLICT OF INTEREST STATEMENT
C.B. has received research funding from Hope Pharmaceuticals and has ownership interest in Precision Advanced Digital Manufacturing. K.J.F. receives a stipend as the Patient Voice Editor of the Clinical Journal of the American Society of Nephrology, receives compensation as an advisor to Responsum CKD and the Kidney Research Institute and has ongoing consulting contracts with Bayer, Gilead, Natera, Hansa Biopharma, Veloxis, Talaris, Travere Therapeutics, Palladio Biosciences, Otsuka and eGenesis.
REFERENCES
- 1. Kurella Tamura M, Xie D, Yaffe Ket al. Vascular risk factors and cognitive impairment in chronic kidney disease: the Chronic Renal Insufficiency Cohort (CRIC) study. Clin J Am Soc Nephrol 2011; 6: 248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shea Y-F, Lee M-SC, Mok M-YMet al. Prevalence of cognitive impairment among peritoneal dialysis patients: a systematic review and meta-analysis. Clin Exp Nephrol 2019; 23: 1221–1234 [DOI] [PubMed] [Google Scholar]
- 3. Burns CM, Knopman DS, Tupper DEet al. Prevalence and risk of severe cognitive impairment in advanced chronic kidney disease. J Gerontol A Biol Sci Med Sci 2018; 73: 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarnak MJ, Tighiouart H, Scott TMet al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 2013; 80: 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta A, Mahnken JD, Johnson DKet al. Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC Nephrol 2017; 18: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viggiano D, Wagner CA, Martino Get al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol 2020; 16: 452–469 [DOI] [PubMed] [Google Scholar]
- 7. Drew DA, Weiner DE, Sarnak MJ. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis 2019; 74: 782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zammit A, Katz M, Bitzer Met al. Cognitive impairment and dementia in older adults with chronic kidney disease: a review. Alzheimer Dis Assoc Disord 2016; 30: 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Lone E, Connors M, Masson Pet al. Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2016; 67: 925–935 [DOI] [PubMed] [Google Scholar]
- 10. Kallenberg MH, Kleinveld HA, Dekker FWet al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD—a systematic review. Clin J Am Soc Nephrol 2016; 11: 1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Etgen T, Chonchol M, Förstl Het al. Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 2012; 35: 474–482 [DOI] [PubMed] [Google Scholar]
- 12. Kurella Tamura M, Wadley V, Yaffe Ket al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Kidney Dis 2008; 52: 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viggiano D, Wagner CA, Martino Get al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol 2020; 16: 452–469 [DOI] [PubMed] [Google Scholar]
- 14. Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci 2004; 226: 3–7 [DOI] [PubMed] [Google Scholar]
- 15. Sofi F, Valecchi D, Bacci Det al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med 2011; 269: 107–117 [DOI] [PubMed] [Google Scholar]
- 16. Cai H, Li G, Hua Set al. Effect of exercise on cognitive function in chronic disease patients: a meta-analysis and systematic review of randomized controlled trials. Clin Interv Aging 2017; 12: 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rathore A, Lom B. The effects of chronic and acute physical activity on working memory performance in healthy participants: a systematic review with meta-analysis of randomized controlled trials. Syst Rev 2017; 6: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly ME, Loughrey D, Lawlor BAet al. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev 2014; 16: 12–31 [DOI] [PubMed] [Google Scholar]
- 19. Busse AL, Gil G, Santarém JMet al. Physical activity and cognition in the elderly: a review. Dement Neuropsychol 2009; 3: 204–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dougherty RJ, Schultz SA, Kirby TKet al. Moderate physical activity is associated with cerebral glucose metabolism in adults at risk for Alzheimer's disease. J Alzheimers Dis 2017; 58: 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chu NM, McAdams-DeMarco MA. Exercise and cognitive function in patients with end-stage kidney disease. Semin Dial 2019; 32: 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol 2019; 15: 383–392 [DOI] [PubMed] [Google Scholar]
- 23. Silva MVF, Loures CdMG, Alves LCVet al. Alzheimer's disease: risk factors and potentially protective measures. J Biomed Sci 2019; 26: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jankowski J, Floege J, Fliser Det al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021; 143: 1157–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schrauben SJ, Hsu JY, Amaral Set al. Effect of kidney function on relationships between lifestyle behaviors and mortality or cardiovascular outcomes: a pooled cohort analysis. J Am Soc Nephrol 2021; 32: 663–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Center for Health Statistics . National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index.htm (August 2020, date last accessed) [Google Scholar]
- 27. Ricardo AC, Fischer MJ, Peck Aet al. Depressive symptoms and chronic kidney disease: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. Int Urol Nephrol 2010; 42: 1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watnick S, Wang PL, Demadura Tet al. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis 2005; 46: 919–924 [DOI] [PubMed] [Google Scholar]
- 30. Andrew SL, Lesley AS, Christopher HSet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eknoyan G, Lameire N, Eckardt Ket al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3: 5–14 [DOI] [PubMed] [Google Scholar]
- 32. Morris JC, Heyman A, Mohs RCet al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part 1. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39: 1159–1165 [DOI] [PubMed] [Google Scholar]
- 33. Brody DJ, Kramarow EA, Taylor CAet al. Cognitive performance in adults aged 60 and over: National Health and Nutrition Examination Survey, 2011–2014. Natl Health Stat Rep 2019; 126: 1–23 [PubMed] [Google Scholar]
- 34. Canning SJD, Leach L, Stuss Det al. Diagnostic utility of abbreviated fluency measures in Alzheimer's disease and vascular dementia. Neurology 2004; 62: 556–562 [DOI] [PubMed] [Google Scholar]
- 35. Jaeger J. Digit Symbol Substitution Test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 2018; 38: 513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thornton KE, Carmody DP. Symbol digit and the quantitative EEG. J Neurother 2012; 16: 210–222 [Google Scholar]
- 37. Amaresha AC, Danivas V, Shivakumar Vet al. Clinical correlates of parametric digit-symbol substitution test in schizophrenia. Asian J Psychiatr 2014; 10: 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sotaniemi M, Pulliainen V, Hokkanen Let al. CERAD-neuropsychological battery in screening mild Alzheimer's disease. Acta Neurol Scand 2012; 125: 16–23 [DOI] [PubMed] [Google Scholar]
- 39. Hailpern SM, Melamed ML, Cohen HWet al. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 2007; 18: 2205–2213 [DOI] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention, National Center for Health Statistics . National Health and Nutrition Examination Survey, 2013–2014 Data Documentation, Codebook, and Frequencies, Cognitive Functioning (CFQ_H). https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CFQ_H.htm (August 2020, date last accessed) [Google Scholar]
- 41. Centers for Disease Control and Prevention, National Center for Health Statistics . National Health and Nutrition Examination Survey 2011–2012, Data Documentation, Codebook, and Frequencies, Cognitive Functioning (CFQ_G). https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CFQ_G.htm (August 2020, date last accessed) [Google Scholar]
- 42. Clark LJ, Gatz M, Zheng Let al. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer's disease. Am J Alzheimers Dis Other Demen 2009; 24: 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta-analysis. Neuropsychologia 2004; 42: 1212–1222 [DOI] [PubMed] [Google Scholar]
- 44. Brody DJ, Kramarow EA, Taylor CAet al. Cognitive performance in adults aged 60 and over: National Health and Nutrition Examination Survey, 2011–2014. Natl Health Stat Rep 2019; 126: 1–23 [PubMed] [Google Scholar]
- 45. Bailey RL, Carmel R, Green Ret al. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr 2011; 94: 552–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bailey RL, Jun S, Murphy Let al. High folic acid or folate combined with low vitamin B-12 status: potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am J Clin Nutr 2020; 112: 1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson RS, Mendes De Leon CF, Barnes LLet al. Participation in cognitively stimulating activities and risk of incident Alzheimer's disease. JAMA 2002; 287: 742–748 [DOI] [PubMed] [Google Scholar]
- 48. Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health 2006; 14: 66–70 [Google Scholar]
- 49. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey 2011–2012 Data Documentation, Codebook, and Frequencies: Physical Acitivty (PAQ_G). https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PAQ_G.htm (August 2020, date last accessed) [Google Scholar]
- 50. US Department of Health and Human Services . Physical Activity Guidelines for Americans. https://health.gov/sites/default/files/2019-09/paguide.pdf (August 2020, date last accessed) [Google Scholar]
- 51. Chen T-C, Parker JD, Clark Jet al. National Health and Nutrition Examination Survey: estimation procedures, 2011–2014. Vital Health Stat 2 2018; 177: 1–26 [PubMed] [Google Scholar]
- 52. Cohen J. Statistical Power Analysis for Behavioral Sciences, rev. ed. New York: Academic Press, 1977 [Google Scholar]
- 53. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Cambridge, MA: Academic Press, 2013 [Google Scholar]
- 54. National Kidney Foundation, American Society of Nephrology . Establishing a Task Force to Reassess the Inclusion of Race in Diagnosing Kidney Diseases. https://www.kidney.org/news/establishing-task-force-to-reassess-inclusion-race-diagnosing-kidney-diseases (August 2020, date last accessed) [Google Scholar]
- 55. Bautovich A, Katz I, Smith Met al. Depression and chronic kidney disease: a review for clinicians. Aust N Z J Psychiatry 2014; 48: 530–541 [DOI] [PubMed] [Google Scholar]
- 56. Tsai Y-C, Chiu Y-W, Hung C-Cet al. Association of symptoms of depression with progression of CKD. Am J Kidney Dis 2012; 60: 54–61 [DOI] [PubMed] [Google Scholar]
- 57. Agganis BT, Weiner DE, Giang LMet al. Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis 2010; 56: 704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mafra D, Fouque D. Lower physical activity and depression are associated with hospitalization and shorter survival in CKD. Am Soc Nephrol 2014; 9: 1669–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 60. Hallan S, de Mutsert R, Carlsen Set al. Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? Am J Kidney Dis 2006; 47: 396–405 [DOI] [PubMed] [Google Scholar]
- 61. Hailpern SM, Melamed ML, Cohen HWet al. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 2007; 18: 2205–2213 [DOI] [PubMed] [Google Scholar]
- 62. Pereira AA, Weiner DE, Scott Tet al. Cognitive function in dialysis patients. Am J Kidney Dis 2005; 45: 448–462 [DOI] [PubMed] [Google Scholar]
- 63. Murray AM, Tupper DE, Knopman DSet al. Cognitive impairment in hemodialysis patients is common. Neurology 2006; 67: 216–223 [DOI] [PubMed] [Google Scholar]
- 64. Sehgal AR, Grey SF, DeOreo PBet al. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis 1997; 30: 41–49 [DOI] [PubMed] [Google Scholar]
- 65. Kurella M, Chertow GM, Fried LFet al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 2005; 16: 2127–2133 [DOI] [PubMed] [Google Scholar]
- 66. Yaffe K, Ackerson L, Kurella Tamura Met al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 2010; 58: 338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lin JS, O'Connor E, Rossom RCet al. Screening for cognitive impairment in older adults: a systematic review for the U.S. preventive services task force. Ann Intern Med 2013; 159: 601–612 [DOI] [PubMed] [Google Scholar]
- 68. Studart AN, Nitrini R. Subjective cognitive decline: the first clinical manifestation of Alzheimer's disease? Dement Neuropsychol 2016; 10: 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Baggetta R, D'Arrigo G, Torino Cet al. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: a secondary analysis of the EXCITE trial. BMC Geriatr 2018; 18: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Manfredini F, Mallamaci F, D'Arrigo Get al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol 2017; 28: 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McAdams-DeMarco MA, Konel J, Warsame Fet al. Intradialytic cognitive and exercise training may preserve cognitive function. Kidney Int Rep 2018; 3: 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stringuetta Belik F, Oliveira E Silva VR, Braga GPet al. Influence of intradialytic aerobic training in cerebral blood flow and cognitive function in patients with chronic kidney disease: a pilot randomized controlled trial. Nephron 2018; 140: 9–17 [DOI] [PubMed] [Google Scholar]
- 73. Graham-Brown MPM, March DS, Churchward DRet al. Design and methods of CYCLE-HD: improving cardiovascular health in patients with end stage renal disease using a structured programme of exercise: a randomised control trial. BMC Nephrol 2016; 17: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Koren MJ, Blumen HM, Ayers EIet al. Cognitive dysfunction and gait abnormalities in CKD. Clin J Am Soc Nephrol 2021; 16: 694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Penny JD, Salerno FR, Brar Ret al. Intradialytic exercise preconditioning: an exploratory study on the effect on myocardial stunning. Nephrol Dial Transplant 2019; 34: 1917–1923 [DOI] [PubMed] [Google Scholar]
- 76. Graham-Brown MPM, March DS, Young Ret al. A randomized controlled trial to investigate the effects of intra-dialytic cycling on left ventricular mass. Kidney Int 2021; 99: 1478–1486 [DOI] [PubMed] [Google Scholar]
- 77. Sachdev P, Kalaria R, O'Brien Jet al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014; 28: 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer's disease. Acta Neuropathol 2016; 131: 659–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kurella Tamura M, Larive B, Unruh MLet al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the frequent hemodialysis network trials. Clin J Am Soc Nephrol 2010; 5: 1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kurella Tamura M, Vittinghoff E, Hsu CYet al. Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int 2017; 91: 948–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hachinski V, Iadecola C, Petersen RCet al. National Institute of Neurological Disorders and Stroke—Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006; 37: 2220–2241 [DOI] [PubMed] [Google Scholar]
- 82. Albert MS, Moss MB, Tanzi Ret al. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 2001; 7: 631–639 [DOI] [PubMed] [Google Scholar]
- 83. Kray J, Lindenberger U. Adult age differences in task switching. Psychol Aging 2000; 15: 126–147 [DOI] [PubMed] [Google Scholar]
- 84. Drew DA, Weiner DE. Cognitive impairment in chronic kidney disease: keep vascular disease in mind. Kidney Int 2014; 85: 505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Erickson KI, Hillman C, Stillman CMet al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc 2019; 51: 1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Oberlin LE, Waiwood AM, Cumming TBet al. Effects of physical activity on poststroke cognitive function: a meta-analysis of randomized controlled trials. Stroke 2017; 48: 3093–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Daly M, McMinn D, Allan JL. A bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci 2015; 8. 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.