Abstract
IGlutamatergic transmission is widely implicated in neuropsychiatric disorders, and the discovery that ketamine elicits rapid-acting antidepressant effects by modulating α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) signaling has spurred a resurgence of interest in the field. This review explores agents in various stages of development for neuropsychiatric disorders that positively modulate AMPARs, both directly and indirectly. Despite promising preclinical research, few direct and indirect AMPAR positive modulators have progressed past early clinical development. Challenges such as low potency have created barriers to effective implementation. Nevertheless, the functional complexity of AMPARs sets them apart from other drug targets and allows for specificity in drug discovery. Additional effective treatments for neuropsychiatric disorders that work through positive AMPAR modulation may eventually be developed.
Keywords: Glutamatergic modulators, AMPA, Ampakines, mGluR modulators, Ketamine, Depression
Over the past two decades, the direct modulation of the glutamatergic system has received considerable attention as a novel approach for treating major depressive disorder (MDD).1–3 This attention was catalyzed by emerging clinical evidence showing that subanesthetic doses of the noncompetitive allosteric N-methyl-D-aspartate receptor (NMDAR) antagonist ketamine have rapid and long-lasting antidepressant effects in patients with treatment-resistant depression.4
Ketamine is a synthetic phencyclidine derivative with one chiral center made up of two enantiomers ((R)-ketamine and (S)-ketamine). It has been used as a dissociative anesthetic and analgesic drug for over 60 years. The (S)-enantiomer of ketamine has three- to four-fold higher affinity binding to the NMDAR than its (R)-counterpart, is a more potent anesthetic and analgesic, and was also selected for development as an antidepressant. However, recent evidence suggests that (R)-ketamine may elicit sustained antidepressant effects without the side effects associated with (S)-ketamine.5,6 (S)-ketamine (esketamine) nasal spray was approved by the United States Food and Drug Administration (FDA) in March 2019 as an adjunctive treatment for patients with treatment-resistant depression and in 2020 for adults with major depression with acute suicidal ideation or behavior7; it was also approved by the European Commission in December 2019 for the same indications. Esketamine currently needs to be administered in conjunction with Risk Evaluation and Mitigation Strategy (REMS) precautions due to its transient dissociative and psychotomimetic side effects and potential abuse liability, which has also been reported at antidepressant doses.7
This recent approval of esketamine for treatment-resistant depression represents a major breakthrough in the field of psychiatry, where no mechanistically novel drugs had been developed for over four decades. The discovery also led to direct and indirect glutamatergic modulators being considered as an entirely new class of antidepressant. As a result, clinical studies have assessed the antidepressant potential of several alternative voltage-dependent NMDAR agents, including GluN2B-selective allosteric modulators (CP-101,606, CERC-301, and Ro 25–6981)8 and glycine binding site modulators (AV-101 and rapastinel).9,10 Despite promising preliminary results at either the preclinical and/or early clinical stages, these molecules failed to duplicate the rapid, robust, and clinically meaningful antidepressant effects of ketamine,11 suggesting that mechanisms other than NMDAR inhibition may be involved in reproducing ketamine’s therapeutic effects.12,13 A subsequent surge of preclinical studies attempted to back-translate clinical findings in order to identify the cellular and molecular mechanisms of action underlying ketamine’s unique antidepressant effects and to develop other glutamatergic modulators that lacked the undesirable side effects induced by NMDAR antagonism (including psychotomimetic or dissociative symptoms, possible abuse liability, and evidence of brain lesions associated with chronic abuse).8,14–16
Much is still unknown about the cellular actions of ketamine, but growing evidence suggests that its antidepressant effects are associated with rapid improvements in synaptic plasticity in executive areas of the brain.17–19 For instance, one of the striking effects of acute ketamine administration in rodents is its ability to rapidly restore (within 24 hours of administration) dendritic arborization and the density of synaptic spines reduced by chronic stress, an effect observed only after several weeks of treatment with traditional antidepressants20,21; it should be noted, however, that some of these effects appear to be sex-dependent.22,23 This effect coincides with the peak of ketamine’s antidepressant effects and is considered essential for its therapeutic actions. The molecular mechanisms thought to be involved in these processes include enhanced α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor (AMPAR) throughput relative to the NMDAR, which leads to increased release of brain-derived neurotrophic factor (BDNF) at the synapses and to rapid and transient activation of mammalian target of rapamycin complex (mTORC) signaling.24 This increase in mTORC signaling was shown to increase the local expression of synaptic proteins, consistent with the rapid formation of new dendritic spines observed after ketamine treatment.20,25 In addition, the antidepressant-like effects of ketamine in rodents were abolished in the presence of NBQX (2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f] quinoxaline), an AMPAR antagonist.14,15,26
Further confirming the key role of AMPAR activation in inducing rapid antidepressant effects, preclinical studies demonstrated that the ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) exerts AMPAR-dependent sustained antidepressant effects without displaying significant binding affinity for NMDARs.15 Furthermore, the antidepressant actions of (2R,6R)-HNK appear to be related to acute increases in excitatory neurotransmission resulting by AMPAR activation followed by long-term adaptation characterized by increased levels of GluA1 and GluA2 AMPAR synaptic subunits.15,27 Interestingly, the antidepressant properties of (2R,6R)-HNK were not associated with psychotomimetic effects, suggesting that direct modulation of AMPARs, excluding NMDAR antagonism, might preserve antidepressant efficacy while limiting dissociative side effects. Researchers examining the potential involvement of (2R,6R)-HNK in NMDAR function further observed off-site effects at supraphysiologic doses but not at antidepressant-relevant concentrations.28–30
Taken together, these data support the notion that direct positive modulation of AMPARs could be a promising approach for developing rapid-acting, efficacious, and safer glutamatergic antidepressants. Presently, however, most research in this field has been performed exclusively in preclinical settings, and few selective AMPAR agents have been clinically evaluated for neuropsychiatric indications. Despite the notable challenges in developing AMPAR-modulating therapies, including low potency and poor metabolic stability, a clear impetus exists to explore the therapeutic potential of this receptor.
This review specifically explores the manner in which targeting positive AMPAR modulation may help expand the armamentarium of novel neuropsychiatric drugs. We discuss the structural and functional properties of AMPARs, with an emphasis on AMPAR positive allosteric modulators (PAMs) and other related compounds; describe attempts to develop translational biomarkers by modulating AMPA throughput; provide an in-depth review of direct AMPAR modulators; introduce promising research in the field of indirect AMPAR modulators; and discuss challenges and unresolved problems associated with this line of inquiry. It should be noted that a full exploration of AMPAR antagonist modulation is outside the scope of this review.
Structural and functional properties of AMPARs
AMPARs are ionotropic, homo- or hetero-tetrameric complexes composed of four subunits (GluA1–4) with a unique base architecture. Four distinct domains comprise the receptor: extracellular N-terminal and ligand-binding domains, the transmembrane domain that forms the ion channel, and a C-terminal domain located in the cytoplasm. The extracellular and transmembrane domains are connected through peptide linkers that give the receptor unique flexibility and allow for complex gating behaviors.31,32 Particularly important in drug discovery are the upper (D1) lobes of the ligand-binding domain,33 where adjacent subunits dimerize back-to-back to form acceptor sites for PAMs. Notably, a number of AMPA-modulating drugs, including ampakines, bind to allosteric sites that lie in proximity to the agonist site, at an interface between GluA subunits. This D1 interface is also targeted by mRNA-processing events involving the alternative splicing (flip/flop) of exons 14 and 15, which can change the functional selectivity of these binding sites as well as glutamate sensitivity (reviewed in34). The flip isoform, which confers hyperexcitability, has already been targeted in disorders such as epilepsy,35 although no specific therapeutics have been developed or tested.
Adding to this complexity, each subunit confers distinct channel ion selectivity and kinetic properties, as well as functional and trafficking properties. The state of one subunit can also influence the binding properties of another, with research showing that the affinity of the ligand-binding domain increases when other ligand-binding domains are occupied.36 Moreover, the N-terminal domain of GluA3 (which is primarily responsible for anchoring the receptor at the synapse and restricting mobility) is distinctively flexible and has unconstrained lower lobes that underlie the important role of GluA3 in allosteric gating regulation.37 The ability of a drug to influence this increased motility may be important when considering pharmacotherapy for depression, as individuals with MDD appear to have significantly decreased levels of GluA3.38 Receptors that contain the GluA2 subunit restrict Ca2+ permeability, decreasing channel conductance. These reductions in conductance have been shown to play an important role in synaptic plasticity because they consolidate long-term potentiation after initial NMDAR-independent facilitation through Ca2+-permeable AMPARs.39 Improving the throughput of both Ca2+ permeable and Ca2+ impermeable AMPARs could be key to developing new therapeutics for depression, given that researchers have observed drastic changes in synaptic connectivity associated with depression (reviewed in17).
Aside from the main pore-forming subunits, the expression of auxiliary subunits can further differentiate the functionality of AMPARs, altering aspects of channel conductance and gating as well as AMPAR expression at the synapse.40,41 Although relatively little is known about these mechanisms of action, they should be kept in mind when discussing the advantages and pit-falls associated with different positive AMPAR modulators in the treatment of depression and other neuropsychiatric disorders. The structural and functional disparities seen among AMPARs underscore the diversity of their possible roles in the central nervous system.
AMPA throughput and gamma oscillations: Converging evidence of a cross-species translational biomarker
Gamma oscillations in the brain are intricately regulated by AMPAR-mediated depolarization and gamma aminobutyric acid (GABA)A modulation, which induce an altered excitatory/inhibitory balance that results in pathological brain states.42,43 Moreover, several lines of evidence indicate a direct relationship between gamma oscillation and depression.44 Notably, acute subanesthetic-dose ketamine infusion is associated with robust increases in gamma power, as demonstrated by clinical research.45,46 Multiple forms of receptor-mediated modulation are involved in ketamine’s mechanism of action at the synapse, and recent data indicate that ketamine both silences GABAergic inhibitory synapses and increases glutamate release, thus enhancing AMPAR throughput.8,47 Research suggests that the mechanism underlying the increased gamma oscillations48 and gamma power observed in response to ketamine administration may be associated with decreased activity in GABAergic interneurons and with the disinhibition of excitatory pyramidal neurons.49,50 Studies also found that 0.5 mg/kg of ketamine, administered intravenously to healthy volunteers, resulted in a robust (greater than four-fold) increase in resting state gamma power with strong effects in the prefrontal cortex; this increase peaked at the end of the infusion and persisted for at least three hours.51 The mechanism of antidepressant response to ketamine is, however, likely to be more complex, given that NMDAR blockade of interneurons and the subsequent disinhibition of pyramidal neurons does not consistently produce antidepressant effects.52 Acute administration of the ketamine metabolite (2R,6R)-HNK also increases gamma oscillations, even though this metabolite does not inhibit NMDARs at concentrations that increase gamma power.15 Because AMPAR blockade can attenuate HNK-induced gamma oscillations, enhanced AMPAR activity is likely to be the mechanism by which HNK increases gamma power, although the precise mechanism underlying this increased AMPAR activity remains unknown.53 Taken together, these findings underscore the close relationship between gamma oscillations and inhibition/excitation balance.42,43 One theory posits that ketamine’s rapid antidepressant effects may be initiated by its antagonistic effects on NMDARs, and that these effects regulate glutamate/GABA and/or AMPAR/NMDAR activity. The relatively sustained effects seen in individuals treated with ketamine suggest that long-term changes in neural plasticity are also likely to play a role.
In addition, quantitative electroencephalography (qEEG) performed pre- and post- ketamine challenge in both rodents and humans found substantial increases in gamma-band power that have been implicated in the activation of fast ionotropic excitatory receptors, specifically AMPARs.54,55 This effect was also found to be associated with the (2R,6R)-HNK metabolite.15
Together, the findings to date suggest that sustained and rapid antidepressant effects are associated with AMPAR activation, encouraging continued investigation into therapies that target AMPARs for treating depression.
AMPAR positive modulators in drug discovery
AMPARs and their modulators were first considered as important targets for drug discovery in depression for two major reasons First, because there is evidence that the glutamatergic system may be dysregulated in neuropsychiatric disorders and, second, because these components play an essential role in the synaptic plasticity that underlies learning and memory (reviewed in56,57). Direct agonist-induced activation of AMPARs was initially explored, but this avenue was tempered by the realization that global activation of the central nervous system did not allow for sufficient processing of the generated signals. In contrast, positive modulation allows for greater sensitivity and specificity and has been more promising in treating neuropsychiatric disorders (reviewed in58). AMPAR PAMs can increase throughput in multiple ways, including slowing the rate of receptor desensitization by preventing conformational transitions or prolonging glutamatergic synaptic currents.59 Unlike the direct global approach, this process does not corrupt spatial or temporal information, allowing AMPARs to communicate effectively. Below, we review the different AMPAR PAMs, including nootropic racetams, AMPAkines, and other modulators that have been studied over the years for their putative neuropsychiatric effects. Additional study details are provided in Table 1, and the chemical structure of each AMPAR PAM can be found in Fig. 1.
TABLE 1.
AMPAR positive allosteric modulators (PAMs).
| Drug name | Reference | Diagnostic groups | Dosing and treatment groups | Primary outcome measure | Region of interest | Phase of development | Significance and findings |
|---|---|---|---|---|---|---|---|
| AMPAKINES | |||||||
| CX516 | 71 | Cognitive Enhancement | CX516 (35 mg/kg) | DNMS paradigm | N/A | Preclinical | CX516 improved performance on the DNMS paradigm, even on days when the drug was not administered. |
| 72 | Depression | CX516 (5 mg/kg) | Reducing submissive behavior | N/A | Preclinical | CX516 significantly reduced submissive behavior in Sprague-Dawley rats. | |
| 70 | Scn2a Haplo-insufficient mice (ADHD model) | CX516 (10–40 mg/kg) | OFT, EPM, LDB, 3-chamber social interaction, social memory test, SDTT, resident-intruder, fear conditioning and extinction | N/A | Preclinical | CX516 improved measures of hyperactivity but did not rescue measures of anxiety-like behaviors in mice. | |
| 73 | Age-related decline | CX516 (30 mg/kg) | BDNF and NGF mRNA expression | Hippocampus, piriform cortex, amygdala, dentate gyrus | Preclinical | CX516 administration to Sprague-Dawley rats significantly increased BDNF and NGF mRNA expression in the dentate gyrus, amygdala, and piriform cortex. | |
| 69 | Tm4sf2−/y mice (model of intellectual disability) | CX516 (5 mg/kg for 5 days) | LDB, emergence and fear conditioning, MWM, electrophysiological measures | Hippocampus | Preclinical | All behavioral measures were improved after CX516 administration, excitatory/inhibitory balance was restored, and synaptic potentiation was increased. | |
| 67 | Schizophrenia | CX516 (300–900 mg over 4 weeks) | Cognitive Battery and Neuropsychological Assessments (not otherwise described) | N/A | Case study | As monotherapy, CX516 did not improve measures of psychosis or cognition in schizophrenia patients. | |
| 66 | Schizophrenia | CX516 (900 mg, TID) | Cognitive battery, PANSS-Positive and General Subscales | N/A | Clinical trial | CX516 had no statistically significant clinical effect or benefit. | |
| NCT00040443 | Mild cognitive impairment | CX516 (900 mg) | 15-Item Word List Delayed Recall | N/A | Clinical trial | CX516 modestly improved scores on the 15-Item Word List Delayed Recall, a measure of short-term episodic memory. | |
| 68 | Fragile X | CX516 (300 mg) | Vineland Adaptive Behavior Scale | N/A | Clinical trial | Inconclusive with regard to antidepressant effects in patients with Fragile X syndrome. | |
| CX546 | 75 | mGluR5-deficient mice | CX546 (15 mg/kg) | LI, PPI | N/A | Preclinical | CX546 significantly improved LI and PPI from disruptions caused by mGluR5 knockout. |
| 74 | PCP-induced cognitive deficits | CX546 (10, 40, or 80 mg/kg) | NOR task | N/A | Preclinical | CX546 reversed object memory after administration of PCP to Lister-hooded rats. | |
| 79 | Neurogenesis | CX546 (50 μm) | BrdU, NeuN, TUNEL (cell viability assay), Tau | Subventricular zone | Preclinical | Increased neurogenesis and dendritogenesis were observed after CX546 treatment of stem and progenitor cell cultures, with no increase in cell death. | |
| 77 | Shank3 mutant mice (ASD model) | CX546 (5–20 mg/kg) | Three chamber test, social interaction, electrophysiological measures | Hippocampus | Preclinical | At 10 mg/kg and above, CX546 enhanced hippocampal EPSCs, the AMPA/NMDA ratio, and all behavioral measures. | |
| 76 | ASD mice models (Shank3 mutant and BTBR) | CX546 (15 mg/kg) | Three chamber test, male/female reciprocal interaction, OFT, grooming behavior | N/A | Preclinical | No changes in all measures of social interaction or exploratory behavior, but CX546 did increase self-grooming behavior. | |
| 78 | Synaptic plasticity | CX546 (250 μm daily for 2 weeks) | Electrophysiological recordings, synaptophysin, cell viability assay | Hippocampus | Preclinical | Prolonged AMPAkine exposure in hippocampal slice cultures pruned dendritic spines and increased efficiency in synaptic communication in the hippocampus. It did not cause cell death. | |
| CX614 | 85 | Behavior | CX614 (1 mg/kg, 4 mg/kg) | FST, photoresistor actimeter (locomotor activity) | N/A | Preclinical | CX614 decreased time spent immobile in the FST but did not affect general locomotor activity in either Albino Swiss mice or Wistar rats. The effects were abolished by administration of NBQX, an AMPAR antagonist. |
| 86 | Spatial navigation | CX614 (2.5 mg/kg) | Y-maze, gamma oscillation frequency and power | Hippocampus | Preclinical | AMPAR modulation with CX614 differentially affected slow and fast gamma of Long Evans rats of different ages. The rate of slow and fast gamma events decreased in older animals administered CX614, but the peak power of fast gamma events increased in young animals administered CX614. | |
| 83 | Synaptic plasticity | CX614 (10 μm, 50 μm) | BDNF release and phosphorylation of TrkB, mTOR, p-70S6K, and 4EBP1 | Hippocampal slices, neuronal cultures | Preclinical | CX614 rapidly stimulated local dendritic protein synthesis mediated by increased BDNF release. | |
| 81 | Structural plasticity, dendritogenesis | CX614 (0.5–10 μm) vs. ketamine (0.001–10 μm) | Tyrosine hydroxylase, GluA1, GluA2, TrkB, BDNF, p-70S6K | Mesencephalic dopaminergic neurons | Preclinical | CX614 mimicked the actions of ketamine in promoting dendritogenesis and structural synaptic plasticity through a BDNF-mediated mechanism. | |
| 80 | MPP(+) induced toxicity | CX614 (50 μm) | BDNF expression, lactate dehydrogenase release | Hippocampal and mesencephalic cultured slices | Preclinical | Pre-treatment with CX614 significantly reduced MPP(+)-induced toxicity mediated by an increase in BDNF. | |
| 87 | R6/2 mice (Huntington’s disease model) | CX614 (20–200 mM) | Electrophysiological recordings | Medium striatal spiny neurons | Preclinical | CX614 significantly increased the synaptic activity of both wild-type and R6/2 mice. | |
| 84 | Depression | CX614 (50 μm, incubated for 5 durations from 3 h to 48 h) | AMPAR (GluA1–3) mRNA and protein expression, BDNF expression, TrkB phosphorylation | Hippocampal slices | Preclinical | Prolonged (12–24 h) treatment with CX614 reduced GluA1–3 mRNA, increased BDNF levels, and increased synaptic TrkB activation. | |
| 82 | Depression | CX614 (1 mg/kg) in addition to imipramine, reboxetine, or escitalopram | FST | N/A | Preclinical | When used adjunctively with imipramine and reboxetine in albino Swiss mice, CX614 enhanced the medication’s antidepressant effects. There was no enhancement with escitalopram. | |
| CX691 | 88 | Behavior | CX691 (0.1 mg/kg, 0.3 mg/kg, 1 mg/kg) | BDNF mRNA expression, scopolamine-induced deficits in cued fear conditioning, a temporal (24 h)-induced deficit in NOR, attentional set shifting | Whole brain | Preclinical | In Lister hooded rats, CX691 attenuated scopolamine-induced impairments of cued fear conditioning and, at sub-chronic doses, improved attentional set-shifting and increased BDNF mRNA expression. |
| 89 | Alzheimer’s disease | CX691 (0.03–0.3 mg/kg for 10 days) | BDNF protein levels, MWM | Hippocampus | Preclinical | CX691 rescued hippocampal BDNF levels and performance on the spatial memory task after amyloid-beta injection to Wistar rats. | |
| 73 | Age-related decline | CX691 (3 mg/kg) | BDNF and NGF mRNA expression | Hippocampus, piriform cortex, amygdala | Preclinical | CX691 significantly increased BDNF and NGF mRNA expression in the somatosensory cortex, amygdala, and piriform cortex of Sprague-Dawley rats. | |
| 90 | Healthy elderly volunteers | CX691 (500 mg) | Wordlist learning, picture memory, N-back, symbol recall, maze task, pursuit rotor, symbol digit substitution test, continuous trail making test | N/A | Clinical trial | CX691 improved measures of short-term memory but impaired episodic memory. | |
| CX717 | 92 | Cognitive enhancer | CX717 (0.3–1.5 mg/kg) | [18F]FDG PET imaging, DMS | Dorsolateral prefrontal cortex, medial temporal lobe, dorsal striatum | Preclinical | CX717 reversed reductions in cognitive performance and brain activation that were induced by sleep deprivation, potentially by increasing the firing of task-specific hippocampal cells. |
| 94 | Alzheimer’s disease | CX717 (20 mg/kg) | 5CSRTT, NOR task | N/A | Preclinical | CX717 reduced the number of incorrect responses in the 5CSRTT in both control animals and in a model of cognitive impairment induced by bilateral vestibular differentiation of Wistar rats. However, this dose had detrimental effects in the NOR task. | |
| 91 | Depression | CX717 (20 mg/kg) | FST, BDNF, noradrenaline, dopamine, serotonin, glutamate | Prefrontal cortex | Preclinical | Within 30 minutes, CX717 transiently improved performance on the FST and increased all biological measures in Sprague-Dawley rats. | |
| 95 | Cognitive enhancer | CX717 (100–1000 mg | Polysomnography, EEG, cognitive battery | N/A | Clinical (phase unknown) | CX717 administered after sleep deprivation did not affect cognitive measures, but the highest dose (1000 mg) modestly helped recovery sleep. | |
| NCT03375021 | ADHD | CX717 (200–800 mg) | ADHD-Rating Scale, CGI, HAM-D, ADHD-Self Rating Scale, HAM-A, Pittsburgh Sleep Quality Index, Stroop Color and Word Test, Trail Making Test, Continuous Performance Task, Forward and Backward Digit Span | N/A | Clinical – Phase 2 | Results not yet posted. | |
| Org 26576 | 96 | Depression | Org 26576 (1–10 mg/kg) | BrdU | Hippocampus and prelimbic cortex | Preclinical | Chronic administration to Sprague-Dawley rats increased cell proliferation. Cells born after Org 26576 treatment had increased survival rates. Acute administration had no effect. |
| 97 | Acute stress | Org 26576 (10 mg/kg) | BDNF mRNA | Hippocampus, frontal and prefrontal cortex | Preclinical | BDNF mRNA was increased after Org 26576 treatment in the hippocampus, but not in the frontal or prefrontal cortex, of Sprague-Dawley rats. | |
| 100 | Schizophrenia | Org 26576 (3–30 mg/kg) plus the antipsychotics risperidone, olanzapine, and haloperidol | Conditioned avoidance response, catalepsy test, electrophysiological measures | Medial prefrontal cortex | Preclinical | Administered adjunctively with risperidone and olanzapine, but not haloperidol, Org 26576 improved behavioral measures in Wistar rats. EPSPs in the prefrontal cortex were augmented when Org 26576 was added to risperidone treatment, but not the other antipsychotics. | |
| 99 | ADHD | Org 26576 (100–300 mg) | Adult ADHD Investigator Symptom Rating Scale | N/A | Clinical – Phase 1 | No statistically significant treatment effects were found. | |
| 98 | Depression | Org 26576 (100 mg BID, 400 mg BID, 600 mg BID, placebo) | MADRS, cognitive tests, perception of emotion, hGh, cortisol | Hippocampus | Clinical – Phase 2 | Symptomatic improvement was greater in the treatment group than in the placebo group. There was also an association with growth hormone increases and cortisol decreases at the end of treatment. Improvements in executive functioning and speed of processing were seen in the group treated with 400 mg BID. | |
| S47445(CX1632, Tulrampator) | 103 | Depression and anxiety | Four doses, S47445 (0.3–10 mg/kg) vs fluoxetine (18 mg/kg) | EPM, OFT, Splash test, FST, TST, fur coat test, NSF | N/A | Preclinical | Chronic administration of S47445 had antidepressant- or anxiolytic-like effects in an anxio-depressive phenotypic mouse model (C57BL6/NTac mice) and in a Wistar rat model of anhedonia after chronic mild stress. |
| Depression and anxiety | S47445 (1–10 mg/kg) | OFT, BDNF, mTOR, phosphor-mTOR, 4EBP1, phosphor-4EBP1 | Prefrontal cortex and hippocampus | Preclinical | S47445 had antidepressant-like and anxiolytic-like effects in bulbectomized C57BL/6J mice. Related measures of BDNF and the mTOR pathway were rescued in the hippocampus, but not in the cortex. | ||
| 107 | Stress-related disorders | S47445 (1–10 mg/kg) | EPM, LDB, splash test, depolarization-evoked glutamate release, synaptic vesicle-associated proteins, mGluR5s, glucocorticoid receptors, oxytocin receptors | Hippocampus | Preclinical | S47445 corrected all behavioral and biological measures after chronic perinatal stress to Sprague-Dawley rats, including the balance between excitatory and inhibitory neurotransmission that predicts stress-based behavioral alterations. | |
| 105 | Age-related decline | S47445 (10 mg/kg) | LTP, VGlut1, spinophilin | Hippocampus, cortex | Preclinical | S47445 significantly counteracted deficits in LTP that resulted from old age and rescued post-synaptic dendritic spines in OFA rats and C57Bl/6 mice, as well as in primary cortical cell cultures. | |
| 102 | Age-related decline | S47445 (1–10 mg/kg) | mRNA and protein levels of BDNF, NT-3, NGF | Prefrontal cortex and hippocampus | Preclinical | Two weeks of chronic treatment with S47445 corrected the altered expression of all neurotrophins measured in aged Wistar Han rats. | |
| 106 | Age-related decline | S47445 (1–10 mg/kg) vs. S 38093 (0.3–3 mg/kg) | NOR | N/A | Preclinical | Both S47445 and S38093 effectively enhanced the performance of Swiss mice on the NOR task. | |
| 108 | Alzheimer’s disease | S47445 (5, 10, 50 mg) | Mini-Mental State Examination, Cornell Scale for Depression in Dementia, Alzheimer’s Disease Assessment Scale | N/A | Clinical – Phase 1 | S47445 was safe and well-tolerated but did not improve any measures of cognition or mood over placebo. | |
| 105 | Cognitive enhancer | S47445 (0.03–100 μm) | Novel object recognition test, spontaneous alteration in T maze, spontaneous locomotor, functional observation | Frontal cortex, hippocampus | Clinical – Phase 2 | S47445 enhanced cognition and had potential neurotrophic and neuroprotective effects. | |
| S18986 | 117 | Neonatal excitotoxic and inflammatory damage | S18986 (10 mg/kg) | Cell death (Fluorojade-B), anti-apoptosis inducible factor, anti-cleaved caspase-3, activated microglia, BDNF mRNA expression | Whole brain | Preclinical | S18986 caused dose-dependent and long-lasting protection of both white matter and cortical grey matter against excitotoxic and inflammatory insult in Swiss mice. |
| 119 | Cognitive enhancement | S18986 (0.3–100 mg/kg acute, or 0.3–30 mg/kg chronically) | One-trial object recognition test | N/A | Preclinical | Both acute and subchronic oral pretreatment in Wistar rats increased recognition of the familiar object, facilitating a form of episodic memory. | |
| 115 | Cognitive enhancement | S18986 (0.03–1 mg/kg) vs. donepezil (0.03 mg/kg) | Contextual Serial Discrimination task | N/A | Preclinical | Both donepezil and S18986 reversed memory deficits in older C57Bl/6 mice by increasing contextually correct responses. | |
| 120 | Cognitive enhancement | S18986 (0.03–0.3 mg/kg) vs. memantine (0.1–10 mg/kg) | Sequential Alternation task | N/A | Preclinical | Both memantine and S18986 reversed memory deficits in aged C57Bl/6 mice but did not modify results in younger mice. | |
| 121 | Cognitive enhancement | S18986 (0.1–1 mg/kg) | Radial Arm Maze with declarative and working memory paradigms | N/A | Preclinical | At 0.1 mg/kg, S189886 selectively improved performance in both declarative and working memory tasks in older C57Bl/6 mice. | |
| 118 | Cognitive enhancement | S18986 (0.1–1 mg/kg for 4 months) | Operant-delayed alternation task, Reinforcer Devaluation task, oxidative stress (4-hydroxy-nonenal and malondialdehyde) | Pre-limbic cortex, hippocampus | Preclinical | S18986 dose-dependently improved results in the reinforcer devaluation task, although there was no effect in the operant-delayed alternation task. In aged Sprague-Dawley rats, levels of oxidative stress were increased, but this was reversed by S18986. | |
| 122 | Cognitive enhancement | S18986 (0.3–1 mg/kg for 3 days) | NOR task | N/A | Preclinical | All dosages of S18986 significantly improved object recognition in Sprague-Dawley rats. | |
| 116 | Age-related decline | S18986 (0.1–1 mg/kg for 16 weeks) | Locomotor activity, spatial memory, forebrain cholinergic neurons, midbrain dopaminergic neurons, microglia in hippocampus | Whole brain | Preclinical | S18986 attenuated all measures of age-related decline in Sprague-Dawley rats. | |
| 123 | BDNF levels | S18986 (300 μm) with and without [S]-AMPA | BDNF mRNA and protein expression | Primary cortical neurons | Preclinical | S18986 failed to rescue levels of BDNF when applied alone but maximally enhanced BDNF mRNA levels when applied in conjunction with [S]-AMPA. | |
| OTHER AMPAR THROUGHPUT POSITIVE ALLOSTERIC MODULATORS | |||||||
| LY392098 | 126 | Depression | LY392098 (0.1–10 mg/kg) | FST, TST | N/A | Preclinical | LY392098 reduced immobility in the FST and TST, with a minimum effective dose of 0.5 mg/kg in both Sprague-Dawley rats and NIH-Swiss mice. |
| 124 | Depression | LY392098 (5 mg/kg) vs fluoxetine (20 mg/kg) | FST, TST, MB, SPT | N/A | Preclinical | LY392098 functioned like a traditional antidepressant and reduced immobility in the TST conducted with BALB/c mice, but it did not rescue marble burying or sucrose preference behavior. | |
| 127 | Neuro-psychiatric disorders | LY392098 (1, 5, and 10 mM) | BDNF protein and mRNA expression | Primary cortical neuronal cultures | Preclinical | LY392098 had a time- and concentration-dependent effect, significantly increasing levels of both BDNF protein and mRNA. | |
| 125 | TARP γ-8 null mice | LY392098 | FST | N/A | Preclinical | LY392098 had an antidepressant-like effect on the FST in wild-type mice, but this effect was abolished in the knockout mice. | |
| 128 | Synaptic plasticity | LY392098 (0.001–10 mg/kg) | Extracellular electrophysiological recordings | Prefrontal cortex | Preclinical | LY392098 increased the probability of action potential discharge in Sprague-Dawley rats, suggesting that it is a powerful modulator of AMPARs and primarily impacts the prefrontal cortex. | |
| LY404187 and LY451646 | 140 | Healthy controls | LY451646 (0.025–0.5 mg/kg for 21 days, or acutely) | BrdU labeling | Hippocampus | Preclinical | Both chronic and acute doses of LY451646 increased progenitor cell proliferation in Sprague-Dawley rats; larger increases were seen with chronic doses. |
| 131 | Healthy controls | LY404187 (0.1–1 mg/kg for 7 days or acutely) vs. LY451646 (0.1–0.5 mg/kg for 7 days or acutely) | BDNF protein and mRNA expression, TrkB phosphorylation | Hippocampus | Preclinical | In Sprague Dawley rats, acute-dose LY451646 increased BDNF mRNA, but chronic low-dose treatment decreased BDNF and TrkB mRNA, suggesting that AMPAR potentiators may modulate BDNF and TrkB in a dose- and time-dependent manner. | |
| 137 | Cognitive enhancement | LY451646 (0.1–1 mg/kg) administered with ketamine | Spatial delayed response task | N/A | Preclinical | After ketamine-induced impairments in working memory, LY451646 rescued all measures in rhesus monkeys. | |
| 129 | Parkinson’s disease | LY404187 (0.5 mg/kg/day, 28 days) | Rotometer score, dopamine and metabolite levels, tyrosine hydroxylase, GAP-43 levels | Striatum, substantia nigra | Preclinical | LY404187 provided functional and neurochemical protection from lesions to the striatum and substantia nigra (in Sprague-Dawley rats and C57B16J mice), even when treatment was delayed until after cell death occurred. | |
| 136 | BDNF+/− null mice | LY451646 (5 mg/kg) vs. ketamine (50 mg/kg) | FST, OFT, BDNF levels, TrkB phosphorylation | Hippocampus | Preclinical | Both ketamine and LY451646 produced rapid antidepressant effects, but neither significantly impacted BDNF levels or TrkB phosphorylation. | |
| 133 | Synaptic plasticity | LY404187 (0.1–10mM) | Neurite length, neurofilament, BDNF, and TrkB expression | SH-SY5Y cell line | Preclinical | LY404187 increased neurite length, neurofilament expression, and TrkB expression through a BDNF-mediated mechanism. | |
| 135 | Depression and anxiety | LY451646 (0–3 mg/kg) vs. + citalopram (0–10 mg/kg) | FST, EZM | N/A | Preclinical | LY451646 had antidepressant effects in the FST and enhanced the effects of citalopram in NMRI mice. LY451646 blocked the anxiolytic effects of citalopram in the other behavioral tasks but had no anxiogenic effects alone. | |
| 140 | Depression | LY451646 (0.25–2.5 mg/kg) | FST, TST | N/A | Preclinical | There was a dose-dependent decrease in immobility in the FST and TST following LY451646 administration to Sprague-Dawley rats. | |
| 134 | Depression and anxiety | LY451646 (3 mg/kg) | FST, OFT, EZM, MB | N/A | Preclinical | LY451646 rescued all measures of antidepressant efficacy but had no anxiolytic effects in NMRI mice. | |
| 138 | Depression | LY451646 (0.125 mg/kg) | Novelty-induced feeding suppression, Y-maze, GluA1 and GluA2, corticosterone | Hippocampus | Preclinical | LY451646 normalized anxiety and corticosterone levels in CD1 mice after exposure to chronic social stress, in contrast to previous studies that found no impact on anxiety-like behaviors. | |
| 139 | Ethanol intoxication | LY451646 (2 ml/kg) | Motor coordination, operant task disruptions, glucose utilization, BOLD signal | N/A | Preclinical | LY451646 attenuated all effects of ethanol-induced intoxication in Sprague-Dawley rats. | |
| LY503430 | 141 | Parkinson’s disease | LY503430 (0.1–0.5 mg/kg) | Rotameters, dopamine, tyrosine hydroxylase, GAP-43, BDNF | Substantia nigra, whole brain | Preclinical | LY503430 significantly protected all neurochemistry and behavioral measures when administered before substantia nigra lesion (in Sprague-Dawley rats) or MPTP administration (in C57Bl6J mice). It also rescued these measures when administered after injury. |
| 142 | Parkinson’s disease | LY503430 (1 mg/kg) | Rotameters, BDNF, GAP-43, general histology | Substantia nigra and striatum | Preclinical | LY503430 improved all behavioral and biological measures without impacting spontaneous movement in both Sprague-Dawley rats and C57B16J mice. | |
| LY451395 (mibampator) | 144 | Agitation or aggression in Alzheimer’s disease | LY451395 (3 mg) | NPI-D, CMAI-C, CSDD, FrSBe, CGI-S-AA | N/A | Clinical | Mibampator did not improve scale ratings compared to placebo, except on the FrSBe. |
| 143 | Alzheimer’s disease | LY451395 (0.2 mg for 28 days, 1.0 mg thereafter) | ADAS-Cog | N/A | Clinical | No changes from baseline on the ADAS-Cog after treatment. | |
| BIIB-104 (PF-04958242) | 149 | Proof of concept | PF-04958242 (up to 32 μm) | Functional potency, functional efficacy, secretory apparent permeability | Mouse embryonic stem (mES) cell-derived neurons | Preclinical | BIIB-104 displayed low oral pharmacokinetic variability as well as a promising therapeutic index that suggests a low chance of adverse events in humans at the doses measured. |
| NCT01749098 | Cognitive enhancement after ketamine-induced deficits | PF-04958242 (0.25–0.35 mg daily for 5 days) | Hopkins Verbal Learning Test, CogState battery, PANSS, Weschler Digit Span Test | N/A | Clinical – Phase 1 | Results not yet posted. | |
| NCT01518894 | Schizophrenia | PF-04958242 (0.1–0.2 mg for 14 days) | Composite of pharmacokinetics, CogState battery, Matrics Consensus Cognition Battery, Drug Effect Questionnaire, C-SSRS | N/A | Clinical – Phase 1 | Results not yet posted. | |
| NCT02332798 | Schizophrenia | PF-04958242 (0.2–0.35 mg for 14 days) | Observed plasma concentration, Cmax, Tmax, AUCT, oral clearance, terminal half-life, accumulation ratio, abnormal clinical laboratory measures, ECG, vital signs, neurological examination, C-SSRS | N/A | Clinical – Phase 1 | PF-04958242 was well-tolerated, even at the higher doses, in stable schizophrenia patients. | |
| NCT01511510, NCT01159483, NCT02228395, NCT01238679, NCT04079101 | Healthy volunteers | PF-04958242 (0.01–0.8 mg once or daily) | Composite of pharmacokinetics, Drug Effect Questionnaire, Digit Symbol Substitution Test, C-SSRS, Cmax, Tmax, AUCT, oral clearance, terminal half-life, adverse events, abnormal clinical laboratory measures, ECG, vital signs, neurological examination, renal clearance, urine elimination | N/A | Clinical - Phase 1 | PF-04958242 was well-tolerated in healthy volunteers, with only minor adverse events such as nausea, muscular weakness, and fatigue. | |
| NCT01365338 | Working memory | PF-04958242 (0.075–0.15 mg single dose) | fMRI, arterial spin labeling data | N/A | Clinical – Phase 1 | No results posted. | |
| 148 | Cognitive enhancement | BIIB-104 (0.35 mg on day 1, 0.25 mg for days 2 – 5) | Hopkins Verbal Learning Test, CogState battery, PANSS, CADSS | N/A | Clinical – Phase 1 | BIIB-104 significantly reduced ketamine-induced impairments on memory tasks without worsening psychotomimetic symptomology. | |
| NCT02855411 | Schizophrenia | PF-04958242 (0.15–0.5 mg daily for 12 weeks) | Matrics Consensus Cognitive Battery, adverse events, Schizophrenia Cognition Rating Scale, PANSS, CGI, Ataxia, C-SSRS | N/A | Clinical – Phase 2 | Trial was terminated (not due to safety concerns). | |
| NCT03745820 | Schizophrenia | BIIB-104 (0.15–0.5 mg daily for 12 weeks) | Matrics Consensus Cognitive Battery, adverse events, performance-based skills assessment, Schizophrenia Cognition Rating Scale, PANSS, CGI, ataxia, C-SSRS | N/A | Clinical - Phase 2 | Recruitment stage. | |
| TAK-137 | 154 | Neurological disorders | TAK-137 (0.03–1 mg/kg) vs. LY451646 (0.03–1 mg/kg) | Ca2+ influx, whole-cell patch clamp recordings, BDNF production, BrdU staining, novel object recognition test, DMS | Primary neurons, hippocampus | Preclinical | Compared to LY451646, TAK-137 induced higher BDNF production, significantly improved performance on cognitive tasks in Sprague-Dawley rats, and lowered risks of seizures. |
| 150 | Depression | TAK-137 (0.03 and 0.3 mg/kg for 3 days) | mTOR and p70S6K phosphorylation, BDNF production, NSF test, locomotor activity, PPI of acoustic startle | Primary cortical neurons | Preclinical | TAK-137 increased phosphorylation along the mTOR pathway. It also shortened feeding latency but not prepulse inhibition in Sprague-Dawley and Wistar-Kyoto rats. | |
| 152 | Schizophrenia | TAK-137 (3–10 mg/kg) | Locomotion, social interaction, 5-CSRTT, radial arm maze, DMS tasks, Reversal Learning Test | N/A | Preclinical | TAK-137 significantly enhanced performance on all measures of social interaction, attention, working memory, and cognitive flexibility in various strains of rats. | |
| HBT1 | 154 | Neuro-psychiatric and neurological disorders | HBT1 | BDNF production | Primary cortical neurons | Preclinical | HBT1 caused a bell-shaped response curve for BDNF production in primary neurons with no agonistic properties. |
Abbreviations: 4EBP1, Eukaryotic translation initiation factor 4E-binding protein 1; 5CSRTT, five-choice serial reaction time task; ADAS-Cog, Alzheimer’s Disease Assessment Scale – Cognitive Subscale; ADHD, attention deficit hyperactivity disorder; AMPA, α–amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; AMPAR, AMPA receptor; ASD, autism spectrum disorder; AUCT, area under the curve across time; BDNF, brain-derived neurotrophic factor; BID, twice per day; BOLD, blood-oxygen level dependent; BrdU, bromodeoxyuridine; CADSS, Clinician-Administered Dissociative States Scale; CGI, Clinical Global Impressions scale; CGI-S-AA, Clinical Global Impressions – Severity Agitation/Aggression scale; CMAI-C, Cohen-Mansfield Agitation Inventory – Community Version; Cmax, maximum plasma concentration; CSDD, Cornell Scale for Depression in Dementia; C-SSRS, Columbia-Suicide Severity Rating Scale; DMS, delayed match-to-sample task; DNMS paradigm, delayed nonmatch-to-sample paradigm; ECG, electrocardiogram; EEG, electroencephalography; [18F] FDG PET, 2-deoxy-2-[fluorine-18]fluoro-D-glucose positron emission tomography; EPM, elevated plus maze; EPSC, excitatory postsynaptic current; EPSP, excitatory postsynaptic potential; EZM, elevated zero max; fMRI, functional magnetic resonance imaging; FrSBe, Frontal Systems Behavior Scale; FST, forced swim test; GAP-43, growth-associated protein 43; GluA1, glutamate receptor A1; HAM-D, Hamilton Depression Rating Scale; hGh, human growth hormone; LDB, light/dark box; LI, latent inhibition; LTP, long-term potentiation; MADRS, Montgomery-Asberg Depression Rating Scale; MB, marble burying; mGluR5, metabotropic glutamate receptor 5; MPP, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; mTOR, mammalian target of rapamycin; MWM, Morris water maze; NBQX, 2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline; NeuN, Hexaribonucleotide Binding Protein-3; NGF, nerve growth factor; NMDA, N-methyl-D-aspartate; NOR task, novel object recognition task; NPI-D, Neuropsychiatric Inventory – Distress scale; NSF, novelty suppressed feeding; NT-3, neurotrophin-3; OFA, Oncins France Strain A; OFT, open field test; PANSS, Positive and Negative Syndrome Scale; PCP, phencyclidine; PPI, prepulse inhibition; p-70S6K, Ribosomal protein S6 kinase beta-1; SDTT, social dominance tube test; SPT, sucrose preference test; TID, three times per day; TARP γ − 8, transmembrane AMPAR regulatory protein γ − 8; Tmax, time to arrive at maximum plasma concentration; TrkB, Tropomyosin receptor kinase B; TST, tail suspension test; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; vGlut1, Vesicular glutamate transporter 1.
FIGURE 1.
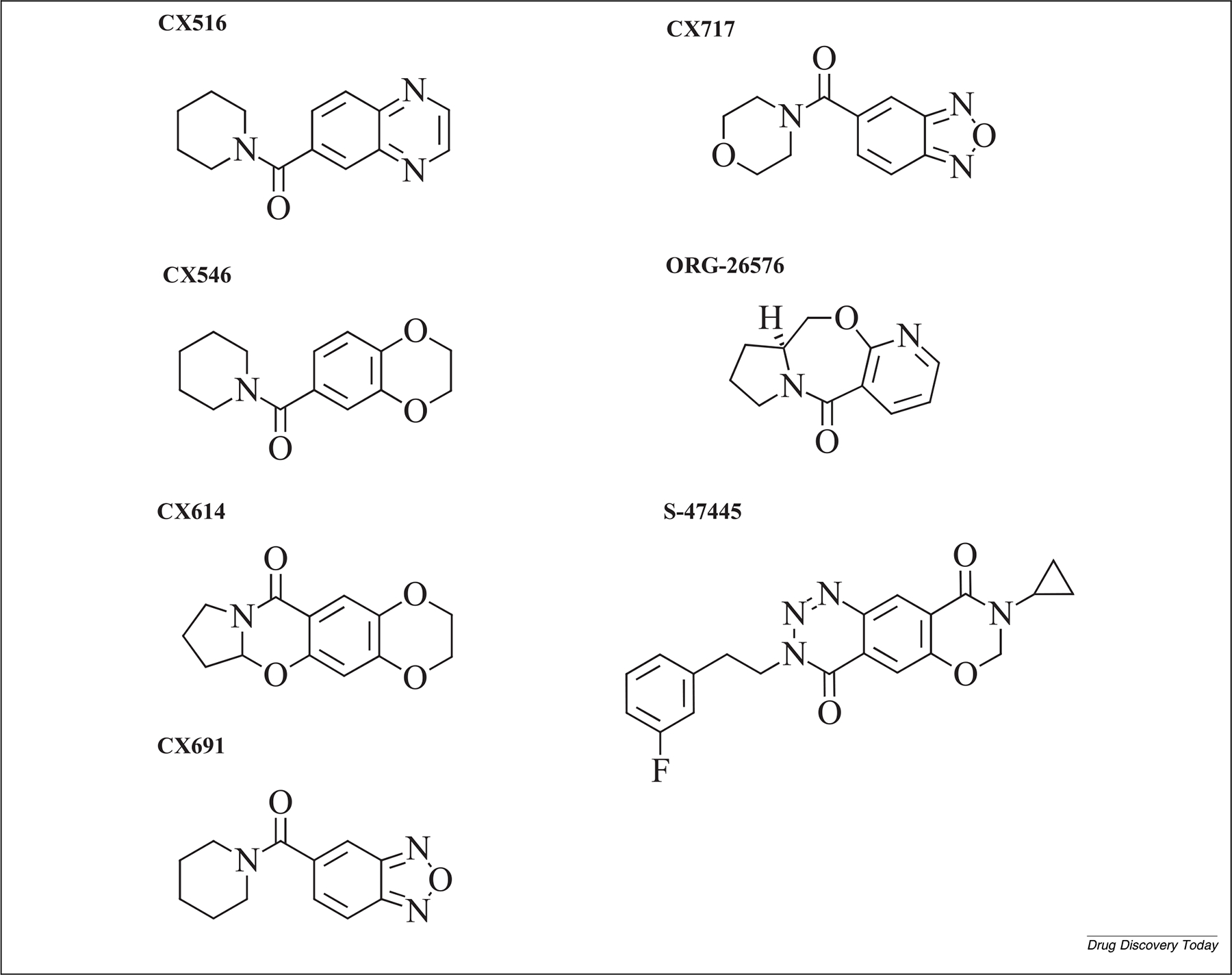
Molecular structure of AMPAkines and other α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) positive allosteric modulators (PAMs).
Nootropic racetams
Nootropic racetams (piracetam, oxiracetam, pramiracetam, aniracetam, phenylpiracetam) are AMPAR potentiators first developed in the late 1960s to limit cognitive impairment and memory decline associated with normal aging and neurodegenerative diseases.60 Racetams increase glutamatergic transmission by increasing the maximal density of AMPA-binding sites in the synaptic membrane, thereby enhancing the overall efficacy of AMPA-stimulated Ca2+ influx.61 Despite mixed clinical evidence, both piracetam and pramiracetam were approved in Europe and the United States as cognitive enhancers, and aniracetam was approved in Europe. Unfortunately, racetams are limited by their low potency and/or poor brain bioavailability and also induce rare, mild, and transitory side effects.60,62
Ampakines
AMPAkines, also known as ‘CX compounds’, are a series of AMPAR PAMs developed in the late 1990s to early 2000s. They are typically benzamides and have a closely related chemical structure in common.63 Originally designed to enlarge the therapeutic armamentarium, AMPAkines are low-potency drugs selected to avoid the risk of AMPA agonism-dependent serious side effects such as seizures. AMPAkines have been investigated for the treatment of neuropsychiatric, neurological, and neurodegenerative disorders64 and, most recently, have been under clinical development for drug-induced respiratory depression and sleep apnea.
AMPAkines exert their effects by stabilizing AMPARs in a channel-open state after glutamatergic release, which increases the amplitude and duration of glutamate-induced AMPA currents.63 Two distinct subfamilies of AMPAkine compounds exist. Type I compounds (such as CX546) prolong synaptic response, whereas type II compounds (such as CX516 and CX614) increase amplitude.64 By shifting current dynamics, both subgroups of AMPAkines lower the induction threshold and increase the magnitude of long-term potentiation,65 thereby improving memory and learning. Although other AMPAkines exist, they have not yet been classified. Relevant AMPAkines with potentially promising neurotrophic properties are described in-depth below, in approximate order of development. It should be noted that, to date, most of these compounds have been investigated for neuropsychiatric indications other than depression.
CX516
CX516 was one of the first AMPAkines to be developed, and has been clinically tested as a cognitive enhancer in mild cognitive impairment (MCI) (NCT00040443), Alzheimer’s Disease (NCT00001662), schizophrenia,66,67 and Fragile X syndrome.68 In clinical trials, however, the results were largely negative because of CX516’s low potency, short half-life, and extensive metabolism. Nevertheless, promising results have recently been obtained in animal models of intellectual disability,69 attention deficit hyperactivity disorder (ADHD),70 and other behavioral measures.71,72 Evidence also suggests that CX516 increases nerve growth factor (NGF) and BDNF in areas linked to neurodegenerative disease.73
CX546
The pharmacological properties of CX546 have specifically been explored in animal models of schizophrenia, where it showed promising results.74,75 More recently, CX546 was also explored in animal models related to autism spectrum disorder, where mixed results have been observed.76,77 Biologically, CX546 appears to be an effective mediator of neurogenesis, dendritogenesis, and increased structural plasticity.78,79 However, its low oral bioavailability limits the clinical development of this agent.
CX614
CX614 is a prototypical benzamide compound classified as an allosteric modulator. It was initially investigated as a putative new treatment for neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases.80 In preclinical comparisons, CX614 enhanced structural plasticity and dendritic outgrowth at the same rate as ketamine,81 and also enhanced the effects of the conventional antidepressants imipramine and reboxetine.82 Other preclinical evidence demonstrated its ability to rapidly upregulate BDNF protein levels83,84 and behavioral antidepressant-like effects82,85 as well as influence the rate, frequency, and power of gamma effects in an age–drug interaction associated with spatial memory.86 In preclinical models of Huntington’s disease, CX614 prevented AMPAR desensitization, slowed deactivation, and facilitated glutamate release, significantly increasing synaptic activity by augmenting the frequency and amplitude of spontaneous and miniature excitatory postsynaptic currents (mEPSCs) in transgenic mouse models of Huntington’s chorea compared to control mice.87 Despite some initial promise in preclinical models, development of these compounds appears to have been halted.
CX691
CX691 has been studied for its pro-cognitive effects in animal models of Alzheimer’s disease, where it was found to increase BDNF protein expression in the hippocampus and improve spatial learning and memory.88,89 These data are supported by research showing upregulation of BDNF and NGF mRNA by CX691 in the dentate gyrus, amygdala, and piriform cortex.73 CX691 was also clinically tested in a pilot study of healthy elderly volunteers, where it was found to significantly ameliorate short-term memory.90
CX717
The ampakine CX717 has positive allosteric modulatory properties at the AMPAR. It is believed to facilitate transmission at cortical synapses that use glutamate as a neurotransmitter. In preclinical models, CX717 enhanced cognitive properties and demonstrated antidepressant-like effects.91–94 Although initial investigations of its ability to improve cognition in sleep-deprived adults were not promising,95 this agent entered clinical development for the treatment of ADHD (NCT03375021); no results have yet been listed. It was also investigated for use in Alzheimer’s disease, but the trial was halted.
ORG 26576
Promising preclinical studies demonstrated that ORG-26576, which was developed as a potential treatment for depression and ADHD, increased hippocampal neurogenesis and BDNF synthesis and improved spatial memory.96,97 The compound did not, however, meet trial endpoints in subsequent Phase 2 clinical trials for MDD,98 ADHD,99 or schizophrenia,100 and its development has been discontinued.
S47445 (CX1632)
Chronic treatment with the recently developed AMPAkine S47445 (CX1632, tulrampator) was found to rescue deficits in synaptic plasticity and cytoarchitecture in the hippocampus of aged mice101 and to increase levels of neurotrophins in both the hippocampus and the prefrontal cortex.102 Antidepressant and anxiolytic-like effects were also reported in three animal models of depression in young-adult animals: mice exposed to chronic corticosterone treatment, rats subjected to chronic mild stress,103 and bulbectomized mice.104 The behavioral effects were again accompanied by increased levels of hippocampal neurogenesis and increased BDNF levels.103,104 S47445 also had pro-cognitive effects in animal models.105–107
Building on this work, S47445 is being investigated as a potential treatment for MDD, Alzheimer’s disease, and MCI (NCT02626572, NCT02805439). However, the results of a first double-blind, placebo-controlled clinical trial of chronic S47445 treatment for individuals with mild to moderate Alzheimer’s disease and comorbid depression found that S47445, although well tolerated, did not significantly improve cognitive or depressive symptoms.108
Other AMPAR-throughput PAMs
Since the early 2000s, Eli Lilly has developed several AMPAR PAMs, which have been investigated specifically for their putative antidepressant properties and possible applications in schizophrenia, Parkinson’s disease, and ADHD.109 PAMs of the biarylpropylsulfonamide class (LY392098, LY404187, LY451395, LY503430, and BIIB-104) are thought to enhance AMPA neurotransmission by reducing desensitization of the ion channel.110–113 The agents are listed below in approximate order of development.
S18986
Servier developed S18986 as a putative cognitive enhancer for normal aging. This agent increases excitatory activity by enhancing the amplitude of extracellular excitatory field potentials.114 A number of positive preclinical studies found that S18986 improved performance in memory tasks and increased BDNF protein levels in the hippocampus of aged rodents,115–122 although it did not increase BDNF mRNA levels.123 Nevertheless, a recent clinical trial found that S18986 exerted no clinically relevant effects in controlling cognitive symptoms in individuals with MCI (NCT00202540).
LY392098
In preclinical studies, LY392098 demonstrated antidepressant effects such as reducing weight loss and immobility time in the tail suspension test in mice,124 an effect that was blocked by knocking out the transmembrane AMPAR regulatory protein (TARP) γ − 8.125 In another preclinical study, LY392098 reduced immobility in both the forced swim and tail suspension tests in a dose-dependent manner.126 It was also found to upregulate BDNF levels in cortical neuronal cultures.127 Using electrophysiological approaches, Baumbarger and colleagues128 found that the effects of LY392098 were primarily located in prefrontal cortical neurons. To date, however, no clinical trials have been conducted with LY392098.
LY404187 and LY451646
LY404187 has been explored preclinically for its efficacy in improving cognition and in animal models of depression, schizophrenia, Parkinson’s disease, and ADHD.129 LY404187 is a stable racemic mixture of R and S stereoisomers (LY451646 was identified as the active isomer) that activates all AMPARs, but it appears to be more potent at GluA2- and GluA4-subunit-containing receptors and has preference for the flip splice variant.110,130 Chronic treatment with both LY404187 and LY451646 increased BDNF mRNA and protein expression in the hippocampus of rats131,132 and also increased neurite length and neurofilament expression.133 In animal models, LY451646 displayed antidepressant-like properties in the forced swim and tail suspension tests,134,136 significantly improved measures of working memory137 and chronic social stress,138 and prevented the effects of ethanol-induced intoxication on cognition.139 LY451646 also increased cell proliferation in the dentate gyrus, suggesting an ability to positively modulate hippocampal neurogenesis.140 To date, however, no clinical trials have been conducted with these compounds.
LY503430
LY503430 has been evaluated for its potential neuroprotective effects in animal models of Parkinson’s disease, where it significantly improved biological measures such as BDNF levels and decreased indicators of neurotoxicity.141 Other studies found that LY503430 protected against neurotoxicity induced by 6-hydroxydopamine or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyri dine (MPTP) infusion into the substantia nigra or striatum of rats.142 However, to date, no clinical trials have been conducted with this compound.
LY451395
LY451395 (mibampator) has been clinically evaluated for Alzheimer’s disease. However, clinical trials found that LY451395 did not affect dementia symptoms in participants with mild to moderate Alzheimer’s Disease,143 limit agitation and aggression,144 or reduce apathy.145
BIIB-104
Pfizer recently developed the nootropic agent BIIB-104 (PF-04958242).113 Preclinical evidence found that that BIIB-104 improved synaptic connections, working memory, and cognitive performance in animal models of schizophrenia.113,146–149 This agent, which appears to be well-tolerated, is now in Phase 2 clinical trials for cognitive impairment in individuals with schizophrenia (NCT03745820, NCT2855411).
TAK-137
TAK-137, developed by Takeda, appears to affect glutamatergic transmission by increasing the potency and binding affinity of AMPA for AMPARs.150,151 It is now under preclinical evaluation for the treatment of cognitive impairment in schizophrenia and as a potential new antidepressant. In several different animal models of schizophrenia, TAK-137 showed only limited effective-ness in controlling positive symptoms but significantly improved social interaction, working memory, and other cognitive functions.152 Another preclinical study recently compared the antidepressant properties of TAK-137 to those of ketamine and found that, in rats, three days of treatment with TAK-137 produced similar behavioral effects as acute ketamine without inducing any psychotomimetic side effects.153 Finally, in preliminary Phase 1 studies with both healthy volunteers and ADHD participants, TAK-137 was found to be safe and well-tolerated and to have a wide therapeutic window (NCT02334982, NCT02163915).
HBT1 and OXP1
HBT1 and OXP1 were identified as promising candidates in a recent high-throughput screening study conducted to discover new AMPAR potentiators specifically selected for their low agonistic effect in Ca2+ influx assays in primary neurons and low risk of bell-shaped response and seizure.154 HBT1 exhibited neurotrophic properties and increased BDNF protein levels in primary neurons.154 Given that this is preliminary research, the exact mechanism of action of these agents remains unknown.
Indirect AMPAR modulators
Other glutamatergic agents have also been investigated for their ability to indirectly modulate AMPAR activity and function. Indeed, recent evidence suggests that the rapid antidepressant effects associated with a variety of glutamatergic drugs under investigation both involves and requires AMPAR modulation, as demonstrated previously for ketamine and (2R,6R)-HNK. This area deserves attention when considering the impact of AMPARs in neuropsychiatric disorders, although further studies are needed to provide a more thorough understanding of indirect AMPAR modulation. Rather than provide a comprehensive review of these agents, we discuss a few promising indirect AMPAR modulators currently under development for the pharmacological treatment of neuropsychiatric disorders.
Metabotropic glutamate receptor (mGluR)2/3 antagonists
mGluRs are G-protein-coupled receptors that mediate secondary messenger pathways. They are traditionally subclassified into three groups based on sequence homology, G-protein coupling, and ligand selectivity. Given their ability to modulate excitatory transmission driven by ionotropic glutamate receptors and to fine-tune cellular response to glutamate signaling in the brain, the selective pharmacological modulation of mGluRs has been investigated as a novel strategy for developing glutamatergic-based antidepressants.
Preclinical evidence suggests that group II (mGluR2 and mGluR3) antagonists are presently the most promising of these compounds for the development of new, rapid-acting antidepressants.155–157 Preclinical studies found that antagonizing mGluR2/3s elicits an antidepressant-like effect that is blocked by the administration of an AMPAR antagonist.157,158 Other preclinical studies found that the antidepressant effects of both racemic ketamine and (2R,6R)-HNK were entirely abolished after administering an mGluR2/3 agonist.159,160 These promising preclinical results have spurred interest in some of these agents as drug candidates.157,158,161,162 One such agent is TS-161, a prodrug of TP0178894 and an orthosteric mGluR2/3 antagonist. A Phase 1, randomized, placebo-controlled, double-blind study evaluated the safety profile, tolerability, and pharmacokinetics of TS-161 in 70 healthy volunteers (NCT03919409), but results have not yet been posted. Phase 2 proof-of-concept trials of this agent in individuals with treatment-resistant depression are anticipated in 2022.
Reelin
Reelin is an extracellular matrix protein involved in regulating neural migration during brain development and in synaptic plasticity in the adult brain163 that appears to parallel some of ketamine’s molecular mechanisms. Reelin levels were found to be downregulated in the CA4 hippocampal region in postmortem brain samples from individuals with MDD.164
Preclinical studies found that chronic stress both causes depressive-like behaviors and decreases the number of Reelin + cells in the dentate gyrus subgranular zone (SGZ).165 These deficits have been speculated to underlie the alterations that chronic stress exerts on depressive-like behaviors, hippocampal neurogenesis, and synaptic plasticity.166 Preclinical studies also found that, as with ketamine, a single intrahippocampal infusion of Reelin rescued both the depressive-like behaviors and the neurochemical alterations associated with animal models of depression within 24 hours.167 Furthermore, these rapid antidepressant-like effects were blocked by administration of the AMPAR antagonist CNQX, suggesting that AMPAR currents are required for Reelin’s pharmacological effects.167 Reelin has also been shown to enhance AMPAR neurotransmission by increasing the number of AMPARs at postsynaptic sites.168 Chronic stress has also been found to result in deficits in hippocampal AMPAR expression and in altered synaptic mTORC levels, both of which were rescued by Reelin in a manner similar to ketamine.167,169 Finally, Reelin also appears to mediate some of ketamine’s rapid-acting antidepressant effects.170 Taken together, these data appear to indicate that the actions of Reelin on AMPARs may be one important molecular mechanism through which Reelin exerts rapid-acting antidepressant-like effects, underscoring the possibility that AMPAR modulators may be a novel avenue for developing new, rapid-acting antidepressants.169 Although Reelin has shown promise in preclinical studies, clinical research has yet to be conducted on this protein.
AMPA modulators in the treatment of neuropsychiatric disorders: Unsolved challenges
Despite the encouraging preclinical results described above, most AMPAR modulators developed to treat neurological and neuropsychiatric disorders have, to date, failed to demonstrate any relevant clinical effects. This may be due to several factors, including the lack of a proper in vitro or in vivo model, lack of target engagement, and the fact that early positive results are often overshadowed by larger failures in the clinical setting, which prevents agents from entering Phase 3 trials.
CX516 provides a good example of this process. The agent had promising preclinical results and positive preliminary findings in elderly participants with mild cognitive impairment (NCT00040443). When investigated for its ability to improve cognition in schizophrenia, however, the interaction of CX516 with common antipsychotics negated any initial positive impact.66 This challenge of translating basic science and preclinical findings into clinical results has been well-documented. Models to measure clinical efficacy in neurological and neuropsychiatric disorders often fall short due to their inability to capture the complexity and individuality present in human patients. Thus, optimizing the design and use of in vitro and in vivo models to analyze disorders is critical to future drug development efforts. In the context of AMPA modulators, current in vitro models have many challenges, given that the heterogeneity of AMPARs and their many splice variants, modulatory proteins, and post-translational modifications create a complex environment that cannot be replicated in cell cultures used for basic research. In vivo research is also associated with unique challenges, such as the variety of behavioral tests that cannot adequately mimic human behavior and cognition. As an example, the forced swim test in rodents, which is commonly used to measure despair in animal models of depression, has recently been criticized for its lack of real-world applicability.171
In addition to broader issues in the field regarding the modeling of neurological and neuropsychiatric disorders, the pharmacodynamics and pharmacokinetics of AMPAR positive modulators have presented specific barriers to success. For instance, CX516 and other AMPAkines have low potency and a short half-life, both of which may have contributed to their clinical failure. It should be noted, however, that more potent AMPAkines have also been unsuccessful in clinical trials,172 perhaps because of low solubility or poor metabolic stability, given that higher potency often comes at the cost of these two important factors.173 Another potential issue is the limited understanding of the specificity of many of these AMPAR modulators, particularly because subunit interactions are extremely important for determining receptor function. Another major technological barrier to discovering effective molecules is the lack of biomarkers showing how these modulators influence AMPARs in vivo. Developing tools to delineate the deactivation or desensitization of receptors post-treatment would be particularly valuable for the future discovery of novel AMPAkines.
Conclusions
Given the enormous potential of novel positive AMPAR modulators, there is considerable reason for optimism regarding their future development as cognitive enhancers and novel therapeutics for a variety of neuropsychiatric disorders, despite the difficulties discussed above. AMPAR PAMs target the synapse in a unique way, improving neuronal communication deficits. In addition, the structural and functional complexity of AMPARs suggests that developing chemically diverse AMPAR PAMs is necessary for determining which agents will be most successful for which disorder. The diverse expression patterns of receptor subunits and isoforms may allow the targeting of specific regions, an opportunity that sets AMPARs apart from other drug targets. As an example, Xia and colleagues174 showed that certain AMPAkines had a greater effect in the hippocampus than in the thalamus. Other positive modulators have shown specificity in targeting the flip or flop isoform of GluA2 (reviewed in175). Given the functional complexity of AMPARs, many opportunities exist to develop highly specific therapeutics for multiple disorders where glutamatergic transmission is impacted. As a result, identifying novel chemotypes has now become a priority in drug development, with companies such as Eli Lilly, GlaxoSmithKline, and others developing high-throughput screens to determine high-potency AMPAR modulators that are effective for different conditions. With improved tools and measurements, AMPAR PAMs remain some of the most promising treatment avenues for neurological and neuropsychiatric disorders.
Acknowledgements
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Funding and role of funding sources
Funding for this work was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002927), as part of the authors’ official duties as Government employees. Additional funding was provided by a NARSAD Independent Investigator Award to Dr Zarate, by a Brain and Behavior Mood Disorders Research Award to Dr Zarate, and by a NARSAD Young Investigator Award to Dr Kadriu. The views expressed do not necessarily reflect the views of the NIH, the Department of Health and Human Services, or the United States Government.
Abbreviations:
- ADHD
attention deficit hyperactivity disorder
- AMPA
a-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- AMPAR
AMPA receptor
- BDNF
brain-derived neurotrophic factor
- GABA
gamma aminobutyric acid
- HNK
hydroxynorketamine
- MCI
mild cognitive impairment
- MDD
major depressive disorder
- mTORC
mammalian target of rapamycin complex
- NGF
nerve growth factor
- NMDAR
noncompetitive allosteric N-methyl-D-aspartate receptor
- PAM
positive allosteric modulator
Biographies

Laura Musazzi, PhD is an Associate Professor at the School of Medicine and Surgery, University of Milano Bicocca. Her research interests include behavioral stress at the synaptic level, regulation of synaptic function, neuroplasticity, gene expression with regard to acute or chronic stress, and the pathogenesis of neuropsychiatric and neurodegenerative disorders with a focus on discovering novel mechanisms of action for psychotropic drugs.

Jenessa Johnston is a PhD student at the University of Victoria and a Pre-Doctoral Fellow at the National Institute of Mental Health (NIMH). Her research focuses on the molecular mechanisms underlying novel antidepressants, and on the discovery of diagnostic and therapeutic biomarkers for various neuropsychiatric disorders.

Bashkim Kadriu, MD is a board-certified psychiatrist. He is currently Director and Clinical Leader, Neuroscience Experimental Medicine, Janssen Research and Development, Janssen Pharmaceutical Companies of Johnson & Johnson. He previously worked as a neuroscientist at the Experimental Therapeutics and Pathophysiology Branch (ETPB), NIMH. His research interests include early drug development, as well as the neurobiological correlates of mood disorders, with a particular emphasis on discovering biosignatures that guide novel therapies and their mechanisms of action.
Footnotes
Conflicts of interest
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the United States Government but will share a percentage of any royalties that may be received by the Government. Dr Kadriu is now a full-time employee at Janssen Research & Development, LLC and owns stock and stock options in Johnson & Johnson; this work was completed while he was a full-time employee of the NIMH. Dr Musazzi is a consultant and has received speaker fees from Janssen Italy. Dr Popoli has received research contracts from Rodin Therapeutics (now Alkermes) in the last 36 months and received research contracts from Merck, GlaxoSmithKline, Servier, Sigma-Tau, Fidia, and Abbott. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- 1.Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem Pharmacol 2006; 71: 1273–88. [DOI] [PubMed] [Google Scholar]
- 2.Nisenbaum ES, Witkin JM, Positive allosteric modulation of AMPA receptors: a novel potential antidepressant therapy, in: Skolnick P (Ed.), Glutamate-based therapies for psychiatric disorders – Milestones in drug therapy, Birkäuser, Basel, 2010, pp. 39–56. [Google Scholar]
- 3.Skolnick P Glutamate-based therapies for psychiatric disorders – Milestones in drug therapy, Basel: Birkäuser; 2010. [Google Scholar]
- 4.Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU, Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories, Psychol Med 46 (2016) 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 2015; 5: e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 2014; 116: 137–41. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed July 27, 2021.
- 8.Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol 2019; 22: 119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park LT, Kadriu B, Gould TD, Zanos P, Greenstein D, Evans JW, et al. A randomized trial of the N-methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int J Neuropsychopharmacol 2020; 23: 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent, J Psychiatr Pract 21 (2015) 140–149. [DOI] [PubMed] [Google Scholar]
- 11.Kadriu B, Deng ZD, Kraus C, Henter ID, Lisanby SH, Zarate CA, Not so fast: recent successes and failures in treating depression, J Clin Psychiatry 81 (2020) 19ac13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duman RS. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Res 2018; 7: F1000 Faculty Rev-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 2018; 70: 621–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould TD, Zanos P, Zarate CA Jr. Ketamine mechanism of action: separating the wheat from the chaff. Neuropsychopharmacology 2017; 42: 368–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Zheng D, Xu J, Lam W, Yew DT, Brain damages in ketamine addicts as revealed by magnetic resonance imaging, Front Neuroanat 7 (2013) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22: 238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ionescu DF, Felicione JM, Gosai A, Cusin C, Shin P, Shapero BG, Deckersbach T. Ketamine-associated brain changes: a review of the neuroimaging literature. Harv Rev Psychiatry 2018; 26: 320–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rantamaki T, Kohtala S. Encoding, consolidation, and renormalization in depression: synaptic homeostasis, plasticity, and sleep integrate rapid antidepressant effects. Pharmacol Rev 2020; 72: 439–65. [DOI] [PubMed] [Google Scholar]
- 20.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. , Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation, Science 364 (2019) eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar A, Kabbaj M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry 2016; 80: 448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, Pitychoutis P. Sex differences in the temporal neuromolecular and synaptogenic effects of the rapid-acting antidepressant drug ketamine in the mouse brain. Neuroscience 2019; 398: 182–92. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Wang N, Yang C, Li X-M, Zhou Z-Q, Yang J-J, Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex, Eur Psychiatry 29 (7) (2014) 419–423. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, Duman RS, mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists, Science 329 (5994) (2010) 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G, Manji HK, Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors, Biol Psychiatry 63 (2008) 349–352. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer CL, Dutra JK, Tseng WC, Weber ML, Bogart LJ, Hales K, Pang J, Volfson D, am Ende CW, Green ME, Buhl DL, Pharmacological evaluation of clinically relevant concentrations of (2R,6R)-hydroxynorketamine, Neuropharmacology 153 (2019) 73–81. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM, Effects of a ketamine metabolite on synaptic NMDAR function, Nature 546 (7659) (2017) E1–E3. [DOI] [PubMed] [Google Scholar]
- 29.Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, Lovett J, Kim S, Wang F-H, Schmidt S, Jenne CE, Yuan P, Morris PJ, Thomas CJ, Zarate CA, Moaddel R, Traynelis SF, Pereira EFR, Thompson SM, Albuquerque EX, Gould TD, Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function, Proc Natl Acad Sci U S A 116 (11) (2019) 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. Zanos et al. reply. Nature 2017; 546: E4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert A, Howe JR, How AMPA receptor desensitization depends on receptor occupancy, J Neurosci 23 (3) (2003) 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Nafría J, Herguedas B, Watson JF, Greger IH, The dynamic AMPA receptor extracellular region: a platform for synaptic protein interactions, J Physiol 594 (19) (2016) 5449–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partin KM, AMPA receptor potentiators: from drug design to cognitive enhancement, Curr Opin Pharmacol 20 (2015) 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer B, Keinanen K, Verdoorn T, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg P, Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS, Science 249 (4976) (1990) 1580–1585. [DOI] [PubMed] [Google Scholar]
- 35.Lykens NM, Coughlin DJ, Reddi JM, Lutz GJ, Tallent MK. AMPA GluA1-flip targeted oligonucleotide therapy reduces neonatal seizures and hyperexcitability. PLoS One 2017; 12: e0171538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prieto ML, Wollmuth LP, Gating modes in AMPA receptors, J Neurosci 30 (12) (2010) 4449–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sukumaran M, Rossmann M, Shrivastava I, Dutta A, Bahar I, Greger IH, Dynamics and allosteric potential of the AMPA receptor N-terminal domain, EMBO J 30 (2011) 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 2013; 16: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu SJ, Zukin RS, Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death, Trends Neurosci 30 (3) (2007) 126–134. [DOI] [PubMed] [Google Scholar]
- 40.Greger IH, Mayer ML, Structural biology of glutamate receptor ion channels: towards an understanding of mechanism, Curr Opin Struct Biol 57 (2019) 185–195. [DOI] [PubMed] [Google Scholar]
- 41.Greger IH, Watson JF, Cull-Candy SG, Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins, Neuron 94 (4) (2017) 713–730. [DOI] [PubMed] [Google Scholar]
- 42.Gandal MJ, Sisti J, Klook K, Leitman V, Liang Y, Thieu T, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry 2012; 2: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kehrer C, Maziashvili N, Dugladze T, Gloveli T, Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia, Front Mol Neurosci 1 (2008) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bi K, Chattun MR, Liu X, Wang Q, Tian S, Zhang S, Lu Q, Yao Z, Abnormal early dynamic individual patterns of functional networks in low gamma band for depression recognition, J Affect Disord 238 (2018) 366–374. [DOI] [PubMed] [Google Scholar]
- 45.Rivolta D, Heidegger T, Scheller B, Sauer A, Schaum M, Birkner K, Singer W, Wibral M, Uhlhaas PJ, Ketamine dysregulates the amplitude and connectivity of high-frequency oscillations in cortical-subcortical networks in humans: evidence from resting-state magnetoencephalography-recordings, Schizophr Bull 41 (5) (2015) 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw AD, Saxena N, Jackson LE, Hall JE, Singh KD, Muthukumaraswamy SD, Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex, Eur Neuropsychopharmacol 25 (8) (2015) 1136–1146. [DOI] [PubMed] [Google Scholar]
- 47.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, Luscher B, Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment, Biol Psychiatry 80 (6) (2016) 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Rühlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai L-H, A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior, Mol Psychiatry 17 (5) (2012) 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Homayoun H, Moghaddam B, NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons, J Neurosci 27 (43) (2007) 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widman AJ, McMahon LL, Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy, Proc Natl Acad Sci U S A 115 (13) (2018) E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanacora G, Smith MA, Pathak S, Su H-L, Boeijinga PH, McCarthy DJ, Quirk MC, Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects, Mol Psychiatry 19 (9) (2014) 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller OH, Moran JT, Hall BJ, Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition, Neuropharmacology 100 (2016) 17–26. [DOI] [PubMed] [Google Scholar]
- 53.Bartos M, Vida I, Jonas P, Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks, Nat Rev Neurosci 8 (1) (2007) 45–56. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Jing L, Toledo-Salas J-C, Xu L, Rapid-onset antidepressant efficacy of glutamatergic system modulators: the neural plasticity hypothesis of depression, Neurosci Bull 31 (1) (2015) 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA, Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects, Mol Psychiatry 24 (7) (2019) 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arai AC, Xia Y-F, Suzuki E, Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus, Neuroscience 123 (4) (2004) 1011–1024. [DOI] [PubMed] [Google Scholar]
- 57.Javitt DC, Glutamate as a therapeutic target in psychiatric disorders, Mol Psychiatry 9 (11) (2004) 984–997. [DOI] [PubMed] [Google Scholar]
- 58.Ward SE, Bax BD, Harries M, Challenges for and current status of research into positive modulators of AMPA receptors, Br J Pharmacol 160 (2010) 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM, Mechanism of positive allosteric modulators acting on AMPA receptors, J Neurosci 25 (2005) 9027–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malykh AG, Sadaie MR, Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders, Drugs 70 (3) (2010) 287–312. [DOI] [PubMed] [Google Scholar]
- 61.Copani A, Genazzani A, Aleppo G, Casabona G, Canonico PL, Scapagnini U, Nicoletti F, Nootropic drugs positively modulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-sensitive glutamate receptors in neuronal cultures, J Neurochem 58 (1992) 1199–1204. [DOI] [PubMed] [Google Scholar]
- 62.Waegemans T, Wilsher CR, Danniau A, Ferris SH, Kurz A, Winblad B, Clinical efficacy of piracetam in cognitive impairment: a meta-analysis, Dement Geriatr Cogn Disord 13 (4) (2002) 217–224. [DOI] [PubMed] [Google Scholar]
- 63.Staubli U, Rogers G, Lynch G, Facilitation of glutamate receptors enhances memory, Proc Natl Acad Sci U S A 91 (2) (1994) 777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arai A, Kessler M, Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior, Curr Drug Targets 8 (2007) 583–602. [DOI] [PubMed] [Google Scholar]
- 65.Lynch G, Memory enhancement: the search for mechanism-based drugs, Nat Neurosci 5 (S11) (2002) 1035–1038. [DOI] [PubMed] [Google Scholar]
- 66.Goff DC, Lamberti JS, Leon AC, Green MF, Miller AL, Patel J, Manschreck T, Freudenreich O, Johnson SA, A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia, Neuropsychopharmacology 33 (3) (2008) 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marenco S, Egan MF, Goldberg TE, Knable MB, McClure RK, Winterer G, Weinberger DR, Preliminary experience with an ampakine (CX516) as a single agent for the treatment of schizophrenia: a case series, Schizophr Res 57 (2–3) (2002) 221–226. [DOI] [PubMed] [Google Scholar]
- 68.Berry-Kravis E, Krause SE, Block SS, Guter S, Wuu J, Leurgans S, Decle P, Potanos K, Cook E, Salt J, Maino D, Weinberg D, Lara R, Jardini T, Cogswell J, Johnson SA, Hagerman R, Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial, J Child Adolesc Psychopharmacol 16 (5) (2006) 525–540. [DOI] [PubMed] [Google Scholar]
- 69.Murru L, Vezzoli E, Longatti A, Ponzoni L, Falqui A, Folci A, et al. Pharmacological modulation of AMPAR rescues intellectual disability-like phenotype in Tm4sf2-/y mice. Cereb Cortex 2017; 27: 5369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tatsukawa T, Raveau M, Ogiwara I, Hattori S, Miyamoto H, Mazaki E, et al. Scn2a haploinsufficient mice display a spectrum of phenotypes affecting anxiety, sociability, memory flexibility and ampakine CX516 rescues their hyperactivity. Mol Autism 2019; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hampson RE, Rogers G, Lynch G, Deadwyler SA, Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance, J Neurosci 18 (7) (1998) 2740–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, Crites G, Malatynska E, Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model, Eur J Pharmacol 440 (1) (2002) 27–35. [DOI] [PubMed] [Google Scholar]
- 73.Radin DP, Johnson S, Purcell R, Lippa AS, Effects of chronic systemic low-impact ampakine treatment on neurotrophin expression in rat brain, Biomed Pharmacother 105 (2018) 540–544. [DOI] [PubMed] [Google Scholar]
- 74.Damgaard T, Larsen DB, Hansen SL, Grayson B, Neill JC, Plath N, Positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors reverses sub-chronic PCP-induced deficits in the novel object recognition task in rats, Behav Brain Res 207 (1) (2010) 144–150. [DOI] [PubMed] [Google Scholar]
- 75.Lipina T, Weiss K, Roder J, The ampakine CX546 restores the prepulse inhibition and latent inhibition deficits in mGluR5-deficient mice, Neuropsychopharmacology 32 (4) (2007) 745–756. [DOI] [PubMed] [Google Scholar]
- 76.Rhine MA, Parrott JM, Schultz MN, Kazdoba TM, Crawley JN, Hypothesis-driven investigations of diverse pharmacological targets in two mouse models of autism, Autism Res 12 (3) (2019) 401–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, Yao H, Yang J, Liu H, Liu Y, Jia F, Qi C, Lynn-Jones T, Hu H, Fu Z, Feng G, Wang W, Wu S, Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice, Nat Neurosci 22 (8) (2019) 1223–1234. [DOI] [PubMed] [Google Scholar]
- 78.Chang P-Y, Prenosil GA, Verbich D, Gill R, McKinney RA, Prolonged ampakine exposure prunes dendritic spines and increases presynaptic release probability for enhanced long-term potentiation in the hippocampus, Eur J Neurosci 40 (5) (2014) 2766–2776. [DOI] [PubMed] [Google Scholar]
- 79.Schitine C, Xapelli S, Agasse F, Sardà-Arroyo L, Silva AP, De Melo Reis RA, et al. Ampakine CX546 increases proliferation and neuronal differentiation in subventricular zone stem/progenitor cell cultures. Eur J Neurosci 2012; 35: 1672–83. [DOI] [PubMed] [Google Scholar]
- 80.Jourdi H, Hsu Y-T, Zhou M, Qin Q, Bi X, Baudry M, Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation, J Neurosci 29 (27) (2009) 8688–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G, Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling, Mol Psychiatry 23 (4) (2018) 812–823. [DOI] [PubMed] [Google Scholar]
- 82.Wolak M, Siwek A, Szewczyk B, Poleszak E, Pilc A, Popik P, Nowak G, Involvement of NMDA and AMPA receptors in the antidepressant-like activity of antidepressant drugs in the forced swim test, Pharmacol Rep 65 (4) (2013) 991–997. [DOI] [PubMed] [Google Scholar]
- 83.Jourdi H, Hamo L, Oka T, Seegan A, Baudry M, BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices, Neuropharmacology 56 (5) (2009) 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM, Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression, Neuroscience 159 (1) (2009) 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szewczyk B, Poleszak E, Sowa-Kućma M, Wróbel A, Słotwiński S, Listos J, Wlaź P, Cichy A, Siwek A, Dybała M, Gołembiowska K, Pilc A, Nowak G, The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test, Amino Acids 39 (1) (2010) 205–217. [DOI] [PubMed] [Google Scholar]
- 86.McHail DG, Dumas TC, Hippocampal gamma rhythms during Y-maze navigation in the juvenile rat, Hippocampus 30 (5) (2020) 505–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cepeda C, Cummings DM, Hickey MA, Kleiman-Weiner M, Chen JY, Watson JB, Levine MS, Rescuing the corticostriatal synaptic disconnection in the R6/2 mouse model of Huntington’s disease: exercise, adenosine receptors and ampakines, PLoS Curr 2 (2010) RRN1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woolley ML, Waters KA, Gartlon JE, Lacroix LP, Jennings C, Shaughnessy F, Ong A, Pemberton DJ, Harries MH, Southam E, Jones DNC, Dawson LA, Evaluation of the pro-cognitive effects of the AMPA receptor positive modulator, 5-(1-piperidinylcarbonyl)-2,1,3-benzoxadiazole (CX691), in the rat, Psychopharmacology (Berl) 202 (1–3) (2009) 343–354. [DOI] [PubMed] [Google Scholar]
- 89.Mozafari N, Shamsizadeh A, Fatemi I, Allahtavakoli M, Moghadam-Ahmadi A, Kaviani E, Kaeidi A, CX691, as an AMPA receptor positive modulator, improves the learning and memory in a rat model of Alzheimer’s disease, Iran J Basic Med Sci 21 (2018) 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wezenberg E, Jan Verkes R, Ruigt GSF, Hulstijn W, Sabbe BGC, Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers, Neuropsychopharmacology 32 (6) (2007) 1272–1283. [DOI] [PubMed] [Google Scholar]
- 91.Gordillo-Salas M, Pascual-Antón R, Ren J, Greer J, Adell A, Antidepressant-like effects of CX717, a positive allosteric modulator of AMPA receptors, Mol Neurobiol 57 (8) (2020) 3498–3507. [DOI] [PubMed] [Google Scholar]
- 92.Hampson RE, España RA, Rogers GA, Porrino LJ, Deadwyler SA, Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717, Psychopharmacology 202 (1–3) (2009) 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kara NZ, Flaisher-Grinberg S, Einat H, Partial effects of the AMPAkine CX717 in a strain specific battery of tests for manic-like behavior in black Swiss mice, Pharmacol Rep 67 (5) (2015) 928–933. [DOI] [PubMed] [Google Scholar]
- 94.Zheng Y, Balabhadrapatruni S, Masumura C, Darlington CL, Smith PF, Effects of the putative cognitive-enhancing ampakine, CX717, on attention and object recognition memory, Curr Alzheimer Res 8 (2011) 876–882. [DOI] [PubMed] [Google Scholar]
- 95.Boyle J, Stanley N, James LM, Wright N, Johnsen S, Arbon EL, Dijk D-J, Acute sleep deprivation: the effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep, J Psychopharmacol 26 (8) (2012) 1047–1057. [DOI] [PubMed] [Google Scholar]
- 96.Su XW, Li XY, Banasr M, Koo JW, Shahid M, Henry B, Duman RS, Chronic treatment with AMPA receptor potentiator Org 26576 increases neuronal cell proliferation and survival in adult rodent hippocampus, Psychopharmacology (Berl) 206 (2009) 215–222. [DOI] [PubMed] [Google Scholar]
- 97.Fumagalli F, Calabrese F, Luoni A, Shahid M, Racagni G, Riva MA, The AMPA receptor potentiator Org 26576 modulates stress-induced transcription of BDNF isoforms in rat hippocampus, Pharmacol Res 65 (2012) 176–181. [DOI] [PubMed] [Google Scholar]
- 98.Nations KR, Dogterom P, Bursi R, Schipper J, Greenwald S, Zraket D, et al. , Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: an exploratory, randomized, double-blind, placebo-controlled trial, J Psychopharmacol 26 (2012) 1525–1539. [DOI] [PubMed] [Google Scholar]
- 99.Adler LA, Kroon RA, Stein M, Shahid M, Tarazi FI, Szegedi A, et al. , A translational approach to evaluate the efficacy and safety of the novel AMPA receptor positive allosteric modulator org 26576 in adult attention-deficit/hyperactivity disorder, Biol Psychiatry 72 (2012) 971–977. [DOI] [PubMed] [Google Scholar]
- 100.Jardemark K, Marcus MM, Malmerfelt A, Shahid M, Svensson TH, Differential effects of AMPA receptor potentiators and glycine reuptake inhibitors on antipsychotic efficacy and prefrontal glutamatergic transmission, Psychopharmacology (Berl) 221 (2012) 115–131. [DOI] [PubMed] [Google Scholar]
- 101.Giralt A, Gómez-Climent M.à., Alcalá R, Bretin S, Bertrand D, Delgado-García JM, et al. , The AMPA receptor positive allosteric modulator S 47445 rescues in vivo CA3-CA1 long-term potentiation and structural synaptic changes in old mice, Neuropharmacology 123 (2017) 395–409. [DOI] [PubMed] [Google Scholar]
- 102.Calabrese F, Savino E, Mocaer E, Bretin S, Racagni G, Riva MA, Upregulation of neurotrophins by S 47445, a novel positive allosteric modulator of AMPA receptors in aged rats, Pharmacol Res 121 (2017) 59–69. [DOI] [PubMed] [Google Scholar]
- 103.Mendez-David I, Guilloux JP, Papp M, Tritschler L, Mocaer E, Gardier AM, et al. , S 47445 produces antidepressant- and anxiolytic-like effects through neurogenesis dependent and independent mechanisms, Front Pharmacol 8 (2017) 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pilar-Cuellar F, Castro E, Bretin S, Mocaer E, Pazos à., Díaz à., S 47445 counteracts the behavioral manifestations and hippocampal neuroplasticity changes in bulbectomized mice, Prog Neuropsychopharmacol Biol Psychiatry 93 (2019) 205–213. [DOI] [PubMed] [Google Scholar]
- 105.Bretin S, Louis C, Seguin L, Wagner S, Thomas JY, Challal S, et al. , Pharmacological characterisation of S 47445, a novel positive allosteric modulator of AMPA receptors, PLoS One 12 (2017) e0184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Louis C, Llopis K, Danober L, Panayi F, Lestage P, Beracochea D, New procognitive enhancers acting at the histamine H3 and AMPA receptors reverse natural forgetting in mice: comparisons with donepezil and memantine in the object recognition task, Behav Pharmacol 30 (2019) 351–357. [DOI] [PubMed] [Google Scholar]
- 107.Morley-Fletcher S, Zuena A, Mairesse J, Gatta E, Van Camp G, Bouwalerh H, et al. , The reduction in glutamate release is predictive of cognitive and emotional alterations that are corrected by the positive modulator of AMPA receptors S 47445 in perinatal stressed rats, Neuropharmacology 135 (2018) 284–296. [DOI] [PubMed] [Google Scholar]
- 108.Bernard K, Gouttefangeas S, Bretin S, Galtier S, Robert P, Holthoff-Detto V, et al. , A 24-week double-blind placebo-controlled study of the efficacy and safety of the AMPA modulator S47445 in patients with mild to moderate Alzheimer’s disease and depressive symptoms, Alzheimers Dement (N Y) 5 (2019) 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Neill MJ, Witkin JM, AMPA receptor potentiators: application for depression and Parkinson’s disease, Curr Drug Targets 8 (2007) 603–620. [DOI] [PubMed] [Google Scholar]
- 110.Miu P, Jarvie K, Radhakrishnan V, Gates MR, Ogden A, Ornstein PL, et al. , Novel AMPA receptor potentiators LY392098 and LY404187: effects on recombinant human AMPA receptors in vitro, Neuropharmacology 40 (2001) 976–983. [DOI] [PubMed] [Google Scholar]
- 111.Ornstein PL, Zimmerman DM, Arnold MB, Bleisch TJ, Cantrell B, Simon R, et al. , Biarylpropylsulfonamides as novel, potent potentiators of 2-amino-3-(5-methyl-3-hydroxyisoxazol-4-yl)-propanoic acid (AMPA) receptors, J Med Chem 43 (2000) 4354–4358. [DOI] [PubMed] [Google Scholar]
- 112.Ryder JW, Falcone JF, Manro JR, Svensson KA, Merchant KM, Pharmacological characterization of cGMP regulation by the biarylpropylsulfonamide class of positive, allosteric modulators of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, J Pharmacol Exp Ther 319 (2006) 293–298. [DOI] [PubMed] [Google Scholar]
- 113.Shaffer CL, Patel NC, Schwarz J, Scialis RJ, Wei Y, Hou XJ, et al. , The discovery and characterization of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor potentiator N-{(3S,4S)-4–4-(5-cyano-2-thienyl)phenoxytetrahydrofuran-3-yl}propane-2-sulfonamide (PF-04958242), J Med Chem 58 (2015) 4291–4308. [DOI] [PubMed] [Google Scholar]
- 114.Bernard K, Danober L, Thomas JY, Lebrun C, Muñoz C, Cordi A, et al. , DRUG FOCUS: S 18986: a positive allosteric modulator of AMPA-type glutamate receptors pharmacological profile of a novel cognitive enhancer, CNS Neurosci Ther 16 (2010) e193–e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Béracochéa D, Philippin JN, Meunier S, Morain P, Bernard K, Improvement of episodic contextual memory by S 18986 in middle-aged mice: comparison with donepezil, Psychopharmacology 193 (2007) 63–73. [DOI] [PubMed] [Google Scholar]
- 116.Bloss EB, Hunter RG, Waters EM, Munoz C, Bernard K, McEwen BS, Behavioral and biological effects of chronic S18986, a positive AMPA receptor modulator, during aging, Exp Neurol 210 (2008) 109–117. [DOI] [PubMed] [Google Scholar]
- 117.Destot-Wong K-D, Liang K, Gupta SK, Favrais G, Schwendimann L, Pansiot J, et al. , The AMPA receptor positive allosteric modulator, S18986, is neuroprotective against neonatal excitotoxic and inflammatory brain damage through BDNF synthesis, Neuropharmacology 57 (2009) 277–286. [DOI] [PubMed] [Google Scholar]
- 118.Kelly SJ, Bernard K, Muñoz C, Lawrence RC, Thacker J, Grillo CA, et al. , Effects of the AMPA receptor modulator S 18986 on measures of cognition and oxidative stress in aged rats, Psychopharmacology 202 (2009) 225–235. [DOI] [PubMed] [Google Scholar]
- 119.Lebrun C, Pillière E, Lestage P, Effects of S 18986–1, a novel cognitive enhancer, on memory performances in an object recognition task in rats, Eur J Pharmacol 401 (2000) 205–212. [DOI] [PubMed] [Google Scholar]
- 120.Vandesquille M, Krazem A, Louis C, Lestage P, Béracochéa D, S 18986 reverses spatial working memory impairments in aged mice: comparison with memantine, Psychopharmacology (Berl) 215 (2011) 709–720. [DOI] [PubMed] [Google Scholar]
- 121.Marighetto A, Valerio S, Jaffard R, Mormede C, Muñoz C, Bernard K, Morain P, The AMPA modulator S 18986 improves declarative and working memory performances in aged mice, Behav Pharmacol 19 (2008) 235–244. [DOI] [PubMed] [Google Scholar]
- 122.Bertaina-Anglade V, la Rochelle CD, Muñoz C, Morain P, Bernard K, Comparison of single vs. multiple administrations of the AMPA receptors modulator S 18986 in the object recognition task in rats, Fundam Clin Pharmacol 21 (2007) 349–354. [DOI] [PubMed] [Google Scholar]
- 123.Lockhart BP, Rodriguez M, Mourlevat S, Peron P, Catesson S, Villain N, et al. , S18986: a positive modulator of AMPA-receptors enhances (S)-AMPA-mediated BDNF mRNA and protein expression in rat primary cortical neuronal cultures, Eur J Pharmacol 561 (2007) 23–31. [DOI] [PubMed] [Google Scholar]
- 124.Farley S, Apazoglou K, Witkin JM, Giros B, Tzavara ET, Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm, Int J Neuropsychopharmacol 13 (2010) 1207–1218. [DOI] [PubMed] [Google Scholar]
- 125.Gleason SD, Kato A, Bui HH, Thompson LK, Valli SN, Stutz PV, et al. , Inquiries into the biological significance of transmembrane AMPA receptor regulatory protein (TARP) γ-8 through investigations of TARP γ-8 null mice, CNS Neurol Disord Drug Targets 14 (2015) 612–626. [DOI] [PubMed] [Google Scholar]
- 126.Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P, Antidepressant-like actions of an AMPA receptor potentiator (LY392098), Neuropharmacology 40 (2001) 1028–1033. [DOI] [PubMed] [Google Scholar]
- 127.Legutko B, Li X, Skolnick P, Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator, Neuropharmacology 40 (2001) 1019–1027. [DOI] [PubMed] [Google Scholar]
- 128.Baumbarger P, Muhlhauser M, Yang CR, Nisenbaum ES, LY392098, a novel AMPA receptor potentiator: electrophysiological studies in prefrontal cortical neurons, Neuropharmacology 40 (2001) 992–1002. [DOI] [PubMed] [Google Scholar]
- 129.O’Neill MJ, Murray TK, Whalley K, Ward MA, Hicks CA, Woodhouse S, et al. , Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson’s disease, Eur J Pharmacol 486 (2004) 163–174. [DOI] [PubMed] [Google Scholar]
- 130.Quirk JC, Nisenbaum ES, LY404187: a novel positive allosteric modulator of AMPA receptors, CNS Drug Rev 8 (2002) 255–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mackowiak M, O’Neill MJ, Hicks CA, Bleakman D, Skolnick P, An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study, Neuropharmacology 43 (2002) 1–10. [DOI] [PubMed] [Google Scholar]
- 132.O’Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES, AMPA receptor potentiators for the treatment of CNS disorders, Curr Drug Targets CNS Neurol Disord 3 (2004) 181–194. [DOI] [PubMed] [Google Scholar]
- 133.Voss OP, Milne S, Sharkey J, O’Neill MJ, McCulloch J, Molecular mechanisms of neurite growth with AMPA receptor potentiation, Neuropharmacology 52 (2007) 590–597. [DOI] [PubMed] [Google Scholar]
- 134.Andreasen JT, Fitzpatrick CM, Larsen M, Skovgaard L, Nielsen SD, Clausen RP, et al. , Differential role of AMPA receptors in mouse tests of antidepressant and anxiolytic action, Brain Res 1601 (2015) 117–126. [DOI] [PubMed] [Google Scholar]
- 135.Fitzpatrick CM, Larsen M, Madsen LH, Caballero-Puntiverio M, Pickering DS, Clausen RP, Andreasen JT, Positive allosteric modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptors differentially modulates the behavioural effects of citalopram in mouse models of antidepressant and anxiolytic action, Behav Pharmacol 27 (2016) 549–555. [DOI] [PubMed] [Google Scholar]
- 136.Lindholm JS, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC, et al. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf+/− heterozygous null mice. Neuropharmacology 2012; 62: 391–7. [DOI] [PubMed] [Google Scholar]
- 137.Roberts BM, Holden DE, Shaffer CL, Seymour PA, Menniti FS, Schmidt CJ, et al. , Prevention of ketamine-induced working memory impairments by AMPA potentiators in a nonhuman primate model of cognitive dysfunction, Behav Brain Res 212 (2010) 41–48. [DOI] [PubMed] [Google Scholar]
- 138.Schmidt MV, Trümbach D, Weber P, Wagner K, Scharf SH, Liebl C, et al. , Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus, J Neurosci 30 (2010) 16949–16958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones N, Messenger MJ, O’Neill MJ, Oldershaw A, Gilmour G, Simmons RMA, et al. , AMPA receptor potentiation can prevent ethanol-induced intoxication, Neuropsychopharmacology 33 (2008) 1713–1723. [DOI] [PubMed] [Google Scholar]
- 140.Bai F, Bergeron M, Nelson DL, Chronic AMPA receptor potentiator (LY451646) treatment increases cell proliferation in adult rat hippocampus, Neuropharmacology 44 (2003) 1013–1021. [DOI] [PubMed] [Google Scholar]
- 141.Murray TK, Whalley K, Robinson CS, Ward MA, Hicks CA, Lodge D, et al. , LY503430, a novel α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson’s disease, J Pharmacol Exp Ther 306 (2003) 752–762. [DOI] [PubMed] [Google Scholar]
- 142.O’Neill MJ, Murray TK, Clay MP, Lindstrom T, Yang CR, Nisenbaum ES, LY503430: pharmacology, pharmacokinetics, and effects in rodent models of Parkinson’s disease, CNS Drug Rev 11 (2005) 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, Sperling R, AMPA potentiator treatment of cognitive deficits in Alzheimer disease, Neurology 68 (2007) 1008–1012. [DOI] [PubMed] [Google Scholar]
- 144.Trzepacz PT, Cummings J, Konechnik T, Forrester TD, Chang C, Dennehy EB, et al. , Mibampator (LY451395) randomized clinical trial for agitation/aggression in Alzheimer’s disease, Int Psychogeriatr 25 (2013) 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ruthirakuhan MT, Herrmann N, Abraham EH, Chan S, Lanctôt KL, Pharmacological interventions for apathy in Alzheimer’s disease, Cochrane Database Syst Rev 5 (2018) CD012197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bednar MM, DeMartinis N, Banerjee A, Bowditch S, Gaudreault F, Zumpano L, Lin FR, The safety and efficacy of PF-04958242 in age-related sensorineural hearing loss: a randomized clinical trial, JAMA Otolaryngol Head Neck Surg 141 (2015) 607–613. [DOI] [PubMed] [Google Scholar]
- 147.Ishii T, Stolz JR, Swanson GT, Auxiliary proteins are the predominant determinants of differential efficacy of clinical candidates acting as AMPA receptor positive allosteric modulators, Mol Pharmacol 97 (2020) 336–350. [DOI] [PubMed] [Google Scholar]
- 148.Ranganathan M, DeMartinis N, Huguenel B, Gaudreault F, Bednar MM, Shaffer CL, et al. , Attenuation of ketamine-induced impairment in verbal learning and memory in healthy volunteers by the AMPA receptor potentiator PF-04958242, Mol Psychiatry 22 (2017) 1633–1640. [DOI] [PubMed] [Google Scholar]
- 149.Shaffer CL, Reverse and forward translational neuropharmacology in psychiatric drug discovery, Clin Pharmacol Ther 103 (2018) 193–195. [DOI] [PubMed] [Google Scholar]
- 150.Suzuki A, Murakami K, Tajima Y, Hara H, Kunugi A, Kimura H, TAK-137, an AMPA receptor potentiator with little agonistic effect, produces antidepressant-like effect without causing psychotomimetic effects in rats, Pharmacol Biochem Behav 183 (2019) 80–86. [DOI] [PubMed] [Google Scholar]
- 151.Witkin JM, Martin AE, Golani LK, Xu NZ, Smith JL, Rapid-acting antidepressants, Adv Pharmacol 86 (2019) 47–96. [DOI] [PubMed] [Google Scholar]
- 152.Tanaka M, Kunugi A, Suzuki A, Suzuki N, Suzuki M, Kimura H, Preclinical characterization of AMPA receptor potentiator TAK-137 as a therapeutic drug for schizophrenia, Pharmacol Res Perspect 7 (2019) e00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suzuki A, Tajima Y, Kunugi A, Kimura H, Electrophysiological characterization of a novel AMPA receptor potentiator, TAK-137, in rat hippocampal neurons, Neurosci Lett 712 (2019) 134488. [DOI] [PubMed] [Google Scholar]
- 154.Kunugi A, Tajima Y, Kuno H, Sogabe S, Kimura H, HBT1, a novel AMPA receptor potentiator with lower agonistic efect, avoided bell-shaped response in in vitro BDNF production, J Pharmacol Exp Ther 364 (2018) 377–389. [DOI] [PubMed] [Google Scholar]
- 155.Chaki S, Koike H, Fukumoto K. Targeting of metabotropic glutamate receptors for the development of novel antidepressants. Chronic Stress (Thousand Oaks) 2019; 3: 2470547019837712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Musazzi L, Targeting metabotropic glutamate receptors for rapid-acting antidepressant drug discovery, Expert Opin Drug Discov 16 (2021) 147–157. [DOI] [PubMed] [Google Scholar]
- 157.Witkin JM, mGlu2/3 receptor antagonism: a mechanism to induce rapid antidepressant effects without ketamine-associated side-effects, Pharmacol Biochem Behav 190 (2020) 172854. [DOI] [PubMed] [Google Scholar]
- 158.Koike H, Chaki S, Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats, Behav Brain Res 271 (2014) 111–115. [DOI] [PubMed] [Google Scholar]
- 159.Dong C, Zhang J-C, Yao W, Ren Q, Ma M, Yang C, et al. , Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine, Int J Neuropsychopharmacol 20 (2017) 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, et al. , (2R, 6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions, Proc Natl Acad Sci U S A 116 (2019) 6441–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koike H, Iijima M, Chaki S, Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats, Pharmacol Biochem Behav 107 (2013) 20–23. [DOI] [PubMed] [Google Scholar]
- 162.Chaki S, mGlu2/3 receptor antagonists as novel antidepressants, Trends Pharmacol Sci 38 (2017) 569–580. [DOI] [PubMed] [Google Scholar]
- 163.Lee GH, D’Arcangelo G, New insights into reelin-mediated signaling pathways, Front Cell Neurosci 10 (2016) 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fatemi SH, Earle JA, McMenomy T, Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression, Mol Psychiatry 5 (654–63) (2000) 571. [DOI] [PubMed] [Google Scholar]
- 165.Lussier AL, Lebedeva K, Fenton EY, Guskjolen A, Caruncho HJ, Kalynchuk LE, The progressive development of depression-like behavior in corticosterone-treated rats is paralleled by slowed granule cell maturation and decreased reelin expression in the adult dentate gyrus, Neuropharmacology 71 (2013) 174–183. [DOI] [PubMed] [Google Scholar]
- 166.Caruncho HJ, Brymer K, Romay-Tallón R, Mitchell MA, Rivera-Baltanás T, Botterill J, et al. , Reelin-related disturbances in depression: implications for translational studies, Front Cell Neurosci 10 (2016) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brymer KJ, Johnston J, Botterill JJ, Romay-Tallon R, Mitchell MA, Allen J, et al. , Fast-acting antidepressant-like effects of Reelin evaluated in the repeated-corticosterone chronic stress paradigm, Neuropsychopharmacology 45 (2020) 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qiu S, Zhao LF, Korwek KM, Weeber EJ, Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus, J Neurosci 26 (2006) 12943–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Johnston JN, Thacker JS, Desjardins C, Kulyk BD, Romay-Tallon R, Kalynchuk LE, Caruncho HJ, Ketamine rescues hippocampal reelin expression and synaptic markers in the repeated-corticosterone chronic stress paradigm, Front Pharmacol 11 (2020) 559627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim J-W, Herz J, Kavalali ET, Monteggia LM, A key requirement for synaptic Reelin signaling in ketamine-mediated behavioral and synaptic action, Proc Natl Acad Sci USA 118 (2021). e2103079118. 10.1073/pnas.2103079118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Kloet ER, Molendijk ML, Coping with the forced swim stressor: towards understanding an adaptive mechanism, Neural Plast 2016 (2016) 6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wesensten NJ, Reichardt RM, Balkin TJ, Ampakine (CX717) effects on performance and alertness during simulated night shift work, Aviat Space Environ Med 78 (2007) 937–943. [DOI] [PubMed] [Google Scholar]
- 173.Jamieson C, Basten S, Campbell RA, Cumming IA, Gillen KJ, Gillespie J, et al. , A novel series of positive modulators of the AMPA receptor: discovery and structure based hit-to-lead studies, Bioorg Med Chem Lett 20 (2010) 5753–5756. [DOI] [PubMed] [Google Scholar]
- 174.Xia Y-F, Kessler M, Arai AC, Positive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor modulators have different impact on synaptic transmission in the thalamus and hippocampus, J Pharmacol Exp Ther 313 (2005) 277–285. [DOI] [PubMed] [Google Scholar]
- 175.Pøhlsgaard J, Frydenvang K, Madsen U, Kastrup JS, Lessons from more than 80 structures of the GluA2 ligand-binding domain in complex with agonists, antagonists and allosteric modulators, Neuropharmacology 60 (2011) 135–150. [DOI] [PubMed] [Google Scholar]


