Abstract
The antiphospholipid syndrome (APS) is usually defined by the association of clinical manifestations that comprise venous and/or arterial thrombosis, recurrent fetal losses, and thrombocytopenia, along with the presence of anticardiolipin (aCL) antibodies and/or lupus anticoagulant. Various infectious diseases can induce aCL; however, these antibodies are not usually associated with thrombotic events, as happens with autoimmune diseases, in which these antibodies need the presence of β2-glycoprotein I. Levels of immunoglobulin G (IgG) and IgM aCL antibodies were determined by enzyme-linked immunosorbent assay for 243 patients with chronic hepatitis C virus (HCV) infection and 100 healthy controls. Clinical events of APS, the level of β2-glycoprotein dependence of aCL, the presence of cryoglobulins and other autoantibodies, and cross-reactivity between purified aCL and HCV were evaluated. Positive results for aCL antibodies were found more frequently (3.3%) for the patients with HCV infection than for healthy controls (0%). All positive aCL antibodies were β2-glycoprotein I independent. No significant association was found between aCL antibodies and clinical manifestations of APS, neither was one found between the presence of other autoantibodies or cryoglobulins and that of aCL. Finally, no cross-reactivity between aCL antibodies and HCV antigens was observed. As previously reported, aCL antibodies seem to be an epiphenomenon, and they do not have clinical or laboratory significance in HCV patients.
Infection with hepatitis C virus (HCV) may lead to an autoantibody response. It has been reported that chronically infected HCV patients have anti-smooth muscle antibodies, rheumatoid factor, anti-liver-kidney-microsomal (aLKM) antibodies, anticardiolipin (aCL) antibodies, and low titers of antinuclear antibodies (ANA) (1, 6, 8, 31).
Antiphospholipid (aPL) antibodies, such as aCL and lupus anticoagulant (LA), are a group of antibodies with an apparent affinity for anionic phospholipids. New data indicate that the antigenic targets of aPL detected in conventional aCL and LA assays are phospholipid-binding plasma proteins, most notably β2-glycoprotein I (β2GPI) and prothrombin, or complexes of these proteins with phospholipids (28). aPL antibodies are detected in patients with autoimmune and infectious diseases and other conditions. In patients with autoimmune diseases, they have been associated with thrombosis, thrombocytopenia, fetal loss, and a variety of other clinical manifestations (livedo reticularis, valvular heart disease, etc.). This clinical association has been defined as antiphospholipid syndrome (APS) (2, 11). In this syndrome, aCL antibodies require β2GPI to bind cardiolipin, but aCL antibodies induced by infections do not usually require this cofactor to bind the anionic phospholipid and are considered nonpathogenic (21, 23).
In previous studies, members of our group tested aCL antibodies and their cofactor dependence in sera from patients with infectious diseases such as syphilis, leprosy, human immunodeficiency virus infection, and rickettsiosis. The aCL antibodies showed cofactor independence as well as a nonsignificant association with APS (24, 25). However, similarities in antigenic specificity and cofactor dependence of aCL in the sera of patients with human parvovirus B19 infection and of patients with systemic lupus erythematosus have been recently reported (19); furthermore, one case of APS associated with cytomegalovirus infection has also been linked to the presence of anti-β2GPI (16).
Recently, a study conducted with patients with chronic HCV infection showed a high prevalence of immunoglobulin G (IgG) and/or IgM aCL associated with clinical manifestations of APS (26). However, neither nonspecific binding in enzyme-linked immunosorbent assay (ELISA) nor the cofactor dependence of aCL was determined in these patients. We studied the aCL levels in patients with HCV infection, their clinical significance, and their cofactor dependence. We also studied the cross-reactivity of purified aCL with HCV proteins.
MATERIALS AND METHODS
Patients.
Levels of aCL antibodies were determined for 243 patients (123 women and 120 men; age range, 14 to 74 years; mean = 50 years) who were positive for anti-HCV antibodies by the second-generation ELISA (HCV 3.0 ELISA; Ortho, Neckargemünd, Germany) and positive for HCV RNA by a reverse transcriptase PCR (Amplicor HCV; Roche, Branchburg, N.J.). We also included 100 healthy controls. Patients were randomly selected among persons who were diagnosed with HCV infection in our hospital during the last 10 years and who had been followed up in the hepatology clinic throughout that period. Liver biopsies were performed on 228 out of the 243 patients, and cirrhosis was detected in only 25. Twenty-seven percent of the patients with HCV had been treated with alpha interferon (IFN-α); however, most of them (69%) had stopped receiving the treatment at least 6 months before the study.
Detection of aCL and cofactor dependence by ELISA.
aCL antibodies (IgG and IgM isotypes) were measured by a previously described ELISA method (15). Of note, specific binding was calculated by subtracting the optical density obtained in the wells that did not contain the antigen (nonspecific binding) from that of the ones that were coated with it.
IgG and IgM aCL-positive standards were obtained from an international reference laboratory (that of E. N. Harris in Louisville, Ky.) (12). The concentration of aCL was measured in international units (a unit being equivalent to the binding activity of 1 mg of aCL/ml) (13). The samples were considered positive when the value was higher than 10 GPL or 10 MPL.
A modified ELISA, previously described by members of our laboratory (24), was used to evaluate the specific binding to β2GPI (aCL's cofactor). Basically, this ELISA was similar to the aCL ELISA except that we used 1% bovine serum albumin (BSA) instead of fetal calf serum as a blocking and diluted solution, due to the fact that BSA does not contain the serum cofactor.
Detection of LA activity.
All patients' plasma samples with aCL antibodies were evaluated for the presence of LA activity according to the criteria defined by the SSC Subcommittee for the Standardization of Lupus Anticoagulants (4). An activated partial thromboplastin time test (APTT) was performed with PTT-LA (Diagnostica Stago, Asmières, France); kaolin clotting time was measured by the method followed by Exner et al. (9), using kaolin from Sigma, and the diluted Russell's viper venom time was determined using Russell's viper venom from Diagnostica Stago, with the cephalin being diluted 1:8 (31). Mixing studies with normal plasma were performed for both the APTT and the diluted Russell's viper venom time in a 1:1 ratio (patient plasma to normal plasma). An APTT-based test containing hexagonal-phase phospholipid (Staclot-LA; Diagnostica Stago) was used as a confirmatory test.
aCL purification by chromatography.
Before performing the purification of the specific aCL antibodies, we chose to include a step consisting of the isolation of total serum immunoglobulins. The immunoglobulin fraction of serum was isolated using quaternary aminoethyl Sephadex A-50 affinity column chromatography.
These immunoglobulins were used to purify the specific aCL antibodies using a cardiolipin affinity column prepared as previously described (22). Fractions containing anticardiolipin activity were measured by using the ELISA for aCL antibodies described above. Their degree of purity was checked out by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (17).
Cross-reactivity of aCL with HCV proteins.
Once the IgG aCL antibodies were purified from the serum of a patient with high titers, we studied their reactivity with some of the HCV proteins using the RIBA-3 assay (Chiron, Emeryville, Calif.) by following the manufacturer's instructions.
Other antibody studies.
ANA, aLKM antibodies, antimitochondrial antibodies (AMA), and anti-gastric parietal cell antibodies (PCA) were detected by indirect immunofluorescence using air-dried cryostat sections from rat liver, kidney, and stomach. The tests were considered positive when the titer was equal to or higher than 1/80.
For the detection of cryoglobulins, 10 ml of blood was drawn from fasting patients and allowed to clot, and the serum was then separated at 37°C. The samples were stored at 4°C for 2 weeks, and the cryoprecipitate was evaluated. Positive samples were then rewarmed to 37°C to determine if the cryoprecipitate redissolved. Cryocrit quantification was done by centrifugation at 3,000 × g for 30 min in a Wintrobe tube. Cryoglobulin characterization at 37°C was done by high-resolution protein electrophoresis, which allows discrimination of small protein bands (10). Samples exceeding trace amounts were evaluated by immunofixation.
Clinical protocol.
The clinical protocol included the epidemiological analysis of the HCV infection source, a physical examination, a liver function test, portal vein ecographic-Doppler study, testing for antibodies to human immunodeficiency virus, an evaluation of the presence of alpha-fetoprotein by radioimmunoassay, and previous clinical findings of thrombosis detected by ecography or as a clinical manifestation. All the patients had been diagnosed with chronic HCV infection and had been monitored as outpatients once or twice yearly for the last 10 years.
Statistical analysis.
A chi-square test was used to ascertain differences between the control population and the HCV-infected individuals regarding the presence of aCL. Among patients with aCL, we used the same statistical approach to determine the association of aCL with other autoantibodies (ANA, aLKM antibodies, PCA, and AMA). Probability values of less than 0.05 were considered statistically significant.
RESULTS
aCL antibodies and LA activity.
The prevalence of aCL antibodies was 0% (0 of 100) in the control group, whereas 6.6% (16 of 243) of the patients with HCV infection were positive for aCL; nevertheless, after the nonspecific binding was subtracted, this prevalence rate dropped to 3.3% (8 of 243). In this case, the difference between the groups was not statistically significant (P = 0.07). This phenomenon was observed in all the patients who were positive for aCL; remarkably, the majority of patients that became negative for aCL when nonspecific binding was considered were those that had tested positive with low aCL titers (less than 24 GPL or MPL). None of the cirrhotic patients were positive for aCL antibodies.
The isotype distribution for aCL was 62.5% (5 of 8) for IgG and 25% (2 of 8) for IgM, and 12.5% (1 of 8) of the patients tested positive for both.
Similar optical density values were observed when BSA was used in the ELISA instead of fetal calf serum. Therefore, these aCL antibodies were not β2GPI dependent.
None of the patients with aCL antibodies was positive for LA.
Cross-reactivity of purified aCL antibodies.
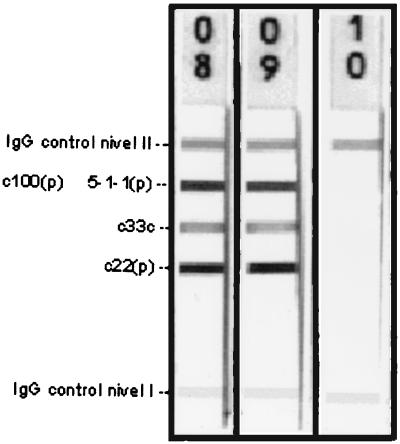
aCL antibodies were purified by affinity chromatography and probed in a RIBA-3 assay to determine their cross-reaction with HCV proteins. The concentration of purified aCL antibodies that we obtained after using the two columns was 100 GPL (approximately two-thirds of the initial sample). The absence of reactivity with any of the bands is shown in Fig. 1.
FIG. 1.
RIBA-3 performed using purified aCL antibodies. Reactivities with c100(p), c33c, and c22(p) bands (specific to HCV) with the isolated serum immunoglobulin fraction before (lane 8) and after (lane 9) removal of aCL antibodies are demonstrated. No reactivity was demonstrated with any HCV antigens using purified aCL antibodies (lane 10).
Other antibodies.
Three aCL-positive patients and 45 aCL-negative patients were positive for ANA. All the patients with aCL were negative for AMA, aLKM antibodies, and PCA. Among the aCL-negative patients, 2 were positive for aLKM antibodies and 8 were positive for PCA. The results are summarized in Table 1.
TABLE 1.
Distribution of aCL-positive and aCL-negative patients according to clinical and serological findings
| Parameter | No. of patients/no. tested (%)
|
|
|---|---|---|
| aCL positive | aCL negative | |
| Thrombocytopenia (<150 × 103) | 1/8 (12.5) | 41/222 (18.5) |
| Thrombosis | 0/8 (0) | 2/219 (0.9) |
| ANA (>1/80) | 3/8 (37.5) | 45/219 (20.5) |
| AMA (>1/80) | 0/8 (0) | 0/219 (0) |
| aLKM antibodies (>1/80) | 0/8 (0) | 2/219 (0.9) |
| PCA (>1/80) | 0/8 (0) | 8/219 (3.6) |
No significant differences were found regarding the frequency of cryoglobulinemia in patients with aCL and in patients without these antibodies. The percentages were 12.5 and 12.3, respectively.
Clinical study.
None of the patients with aCL antibodies had clinical or ecographic thrombosis, and only one had thrombocytopenia. No significant differences were seen in comparison with patients without aCL antibodies.
Among the patients treated with IFN-α, none was aCL positive. On the other hand, 4.5% (8 of 179) of the nontreated patients were aCL positive.
DISCUSSION
Only a few studies of aCL in connection with chronic HCV infection have been conducted (8, 26), and many questions remain after reviewing them. The first is related to the prevalence of aCL among patients with chronic HCV. In our study, 3% of the patients with chronic HCV infection had aCL (IgG and/or IgM), and none of them had LA. Other reports have described a frequency of aCL ranging from 3 to 22% (5, 8, 26, 30). These differences may be due to the methods used; thus, it matters whether a commercial ELISA kit or a homemade standardized ELISA method that is internationally accepted is used to determine the level of aCL antibodies (27). In a multicenter study, an evaluation of nine commercial kits used to quantify aCL antibodies showed variations of 31 to 60% and 6 to 50% for the IgG and IgM aCL isotypes, respectively (27). An evaluation of aCL antibody laboratory methods by the United Kingdom External Quality Survey showed that the variance coefficient was very high for both homemade and commercial kits. The discrepancy of results is more common with samples from patients with low levels of aCL antibodies (30). Another variable that can alter the aCL assay results has to do with considering or ignoring the nonspecific binding of the antibodies in the ELISA plate (7, 30)—that is, the bindings of the antibody on the well that has not been coated with the antigen. This phenomenon is seen more frequently with the IgM and IgA isotypes. None of the studies of aCL antibodies in chronic HCV infection considered the nonspecific binding in the ELISA plate, which is in fact a very important issue because the frequency of nonspecific binding is high in the sera of some patients (7, 30). In our study, the frequency of aCL was 6.6%, and it dropped to 3.3% when the nonspecific binding was subtracted. Matsuda et al. (20) used an international standard ELISA method similar to the one used in our study, but they did not take into consideration the nonspecific binding of the antibodies; thus, the positive low levels of aCL in HCV patients reported in previous studies may have been false positives.
We did not find aCL in patients with liver cirrhosis. Biron et al. (3) found a high frequency of aCL in cirrhotic patients when the cirrhosis was caused by either HCV or alcohol abuse. This discrepancy may be due to the fact that these patients usually have hypergammaglobulinemia, which leads us to hypothesize that perhaps these results are false positives (7).
Many HCV patients are treated with IFN-α. Matsuda et al. (20) and Leroy et al. (18) studied the influence of this treatment on aCL and reported that this therapeutic approach did not change the frequency of aCL antibodies. In our study we also demonstrated that IFN-α did not affect the aCL reactivity of samples from patients with HCV.
Some authors have tried to correlate aCL and mixed cryoglobulinemia in patients with HCV and found no significant association (5, 30). In our study, we did not find any, either.
There are no data concerning the prevalence of LA in patients with chronic HCV. In this study, all the patients with aCL were LA negative. The correlation between aCL and LA is very high in autoimmune diseases (14, 32). Nevertheless, studies of aCL in patients with some infectious diseases (e.g., syphilis) have never showed the presence of LA despite the fact that these patients were positive for aCL (15, 29). However, in patients with some infectious diseases it is possible to detect LA antibodies. For example, members of our group found LA in patients with Q fever (25). Therefore, it seems that the relation between chronic HCV and LA is similar to the one between LA and syphilis.
Another subject of interest is whether aCL antibodies are pathogenic in patients with chronic HCV infection. aCL antibodies are found in APS patients as well as in patients with autoimmune diseases such as systemic lupus erythematosus (secondary APS) (2, 11). Typically, these aCL antibodies are cofactor dependent (β2GPI dependent). In infectious diseases, aCL antibodies are cofactor independent and are not associated with clinical manifestations (25). Only one patient with cytomegalovirus infection who had thrombosis and cofactor-dependent aCL has been reported (16). In this study, we found aCL cofactor independence in all the HCV patients studied. Recently, Cacoub et al. (5) did not find anti-β2GPI antibodies in patients with HCV and aCL.
Prieto et al. (26) found a relationship between aCL and thrombosis or thrombocytopenia in patients with chronic HCV infection. In our study, we found no clinical manifestations of APS among the aCL-positive patients. Other authors have also reported the absence of thrombotic events in patients with HCV and aCL (5, 20).
Here we demonstrated (both by ELISA and by affinity-purified chromatographic methods) that aCL antibodies associated with HCV infection are cofactor independent. These aCL antibodies do not require β2GPI for binding to cardiolipin that is either fixed on the ELISA plate or immobilized in the column with acrylamide-bisacrylamide. Furthermore, we and others have found no correlation between these aCL cofactor-independent antibodies and the clinical manifestation of APS.
Finally, it remains unknown whether aCL antibodies are an epiphenomenon of HCV infection or whether a cross-reactivity exists between aCL and any HCV antigen. In this study, we found no cross-reactivity with purified aCL antibodies using the RIBA technique.
In conclusion, the prevalence of aCL antibodies in HCV patients was higher than it was in the control group, but these antibodies had no clinical significance. As is the case for other autoantibodies described in conjunction with HCV infection, aCL antibodies seem to be an epiphenomenon, since they do not present cross-reactivity with any of the HCV proteins.
ACKNOWLEDGMENTS
This work was supported by National Institute of Health (Spain) grants FIS (98/0194) and BEFI (90/9035).
REFERENCES
- 1.Abuaf N, Lunel F, Giral P, Borotto E, Laperche S, Poupon R, Opolon P, Huraux J M, Homberg J C. Non-organ specific autoantibodies associated with chronic C virus hepatitis. J Hepatol. 1993;18:359–364. doi: 10.1016/s0168-8278(05)80281-8. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon-Segovia D, Sanchez-Guerrero J. Primary antiphospholipid syndrome. J Rheumatol. 1989;16:482–488. [PubMed] [Google Scholar]
- 3.Biron C, Andreani H, Blanc P, Ramos J, Ducos J, Guigue N, Michel H, Larrey D, Schved J F. Prevalence of antiphospholipid antibodies in patients with chronic liver disease related to alcohol or hepatitis C virus: correlation with liver injury. J Lab Clin Med. 1998;131:243–250. doi: 10.1016/s0022-2143(98)90096-8. [DOI] [PubMed] [Google Scholar]
- 4.Brandt J T, Triplett D A, Alving B, Scharrer I. Criteria for the diagnosis of the lupus anticoagulants: an update. Thromb Haemostasis. 1995;74:1185–1190. [PubMed] [Google Scholar]
- 5.Cacoub P, Musset L, Amoura Z, Guilani P, Chabre H, Lunel F, Poynard T, Opolon P, Piette J C. Anticardiolipin, anti-β2-glycoprotein I, and antinucleosome antibodies in hepatitis C virus infection and mixed cryoglobulinemia. J Rheumatol. 1997;24:2139–2144. [PubMed] [Google Scholar]
- 6.Clifford B D, Donahue D, Smith L, Cable E, Luttig B, Manns M, Bonkovsky H L. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology. 1995;21:613–619. [PubMed] [Google Scholar]
- 7.Cowchock S, Fort J, Muñoz S, Norberg R, Maddrey W. False positive ELISA tests for anticardiolipin antibodies in sera from patients with repeated abortion, rheumatologic disorders and primary biliary cirrhosis: correlation with elevated polyclonal IgM and implications for patients with repeated abortion. Clin Exp Immunol. 1988;73:289–294. [PMC free article] [PubMed] [Google Scholar]
- 8.De Larrañaga G, Harris E N, Pierangeli S S, Alonso B, Schroder M T, Muzzio E, Fainboim H A. Low prevalence of autoimmune antiphospholipid antibodies in infectious diseases of the liver. Lupus. 1996;5:521. [Google Scholar]
- 9.Exner T, Rickard K A, Kronenberg H. A sensitive test anticoagulant and its behavioral patterns. Br J Haematol. 1978;40:143–151. doi: 10.1111/j.1365-2141.1978.tb03648.x. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Bragado F, Acebedo G, Vilardell M. Lymphocyte subpopulations in essential mixed cryoglobulinemia. Ann Rheum Dis. 1986;45:159–164. doi: 10.1136/ard.45.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris E N, Baguley E, Asherson R, Hughes G R V. Clinical and serological features of the antiphospholipid syndrome. Br J Rheumatol. 1987;26(Suppl. 2):19. [Google Scholar]
- 12.Harris E N, Gharavi A E, Patel S P, Hughes G R V. Evaluation of the anticardiolipin antibody test: report of an international workshop held 4 April 1986. Clin Exp Immunol. 1987;68:215–222. [PMC free article] [PubMed] [Google Scholar]
- 13.Harris E N. Special report. The Second International Anti-Cardiolipin Standardization Workshop/the Kingston Anti-Phospholipid Antibody Study (KAPS) group. Am J Clin Pathol. 1990;94:476–484. doi: 10.1093/ajcp/94.4.476. [DOI] [PubMed] [Google Scholar]
- 14.Harris E N, Boey M L, Mackworth-Young C G, Gharavi A E, Patel B M, Loizou S, Hughes G R V. Anticardiolipin antibodies: detection by radioimmunoassay and association with thrombosis in systemic lupus erythematosus. Lancet. 1983;ii:1211–1214. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 15.Hunt J E, McNeil H P, Morgan G J, Crameri R M, Krillis S A. Antiphospholipid β2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992;1:75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 16.Labarca J A, Rabaggliati M, Radrigan F J, Rojas P P, Perez C P, Ferrés M V, Acuña G G, Bertin P A. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24:197–200. doi: 10.1093/clinids/24.2.197. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Leroy V, Arvieux J, Jacob M C, Maynard-Muet M, Baud M, Zarski J P. Prevalence and significance of anticardiolipin, anti-beta2 glycoprotein I and anti-prothrombin antibodies in chronic hepatitis C. Br J Haematol. 1998;101:468–474. doi: 10.1046/j.1365-2141.1998.00722.x. [DOI] [PubMed] [Google Scholar]
- 19.Loizou S, Cazabon J K, Walport M J, Tait D, So A K. Similarities of specificity and cofactor dependence in serum antiphospholipid antibodies from patients with human parvovirus B19 infection and from those with systemic lupus erythematosus. Arthritis Rheum. 1997;40:103–108. doi: 10.1002/art.1780400115. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda J, Saitoh N, Gotoh M, Gohchi K, Tsukamoto M, Syoji S, Miyake K, Yamanaka M. High prevalence of aPL antibodies and anti-thyroglobulin antibodies in patients with hepatitis C virus infection treated with interferon-α. Am J Gastroenterol. 1995;90:1138–1141. [PubMed] [Google Scholar]
- 21.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Koike T. Anticardiolipin cofactor(s) and differential diagnosis of autoimmune disease. Lancet. 1990;336:177–178. doi: 10.1016/0140-6736(90)91697-9. [DOI] [PubMed] [Google Scholar]
- 22.McNeil H P, Krillis S A, Chesterman C N. Purification of antiphospholipid antibodies using a new affinity method. Thromb Res. 1988;52:641–648. doi: 10.1016/0049-3848(88)90136-3. [DOI] [PubMed] [Google Scholar]
- 23.McNeil H P, Simpson R J, Chesterman R J, Krillis S A. Antiphospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: β2 glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ordi J, Selva A, Monegal F, Porcel J M, Martinez-Costa X, Vilardell M. Anticardiolipin antibodies and dependence of a serum cofactor. A mechanism of thrombosis. J Rheumatol. 1993;20:1321–1324. [PubMed] [Google Scholar]
- 25.Ordi-Ros J, Selva-O'Callaghan A, Monegal-Ferran F, Monasterio-Aspiri Y, Vilardell-Tarrés M. Prevalence, significance and specificity of antiphospholipid antibodies in Q fever. Clinic Infect Dis. 1994;18:213–217. doi: 10.1093/clinids/18.2.213. [DOI] [PubMed] [Google Scholar]
- 26.Prieto J, Yuste J R, Beloqui O, Cirera M P, Riezu J I, Aguirre B, Sangro B. Anticardiolipin antibodies in chronic hepatitis C. Implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology. 1996;23:199–204. doi: 10.1002/hep.510230201. [DOI] [PubMed] [Google Scholar]
- 27.Reber G, Arvieux J, Comby E, Degenne E, de Moerloose P, Sanmarco M, Potron G. Multicenter evaluation of nine commercial kits for quantification of anticardiolipin antibodies. The Working Group on Methodologies in Haemostasis from the GEHT (Group d'Etudes sur l'Hemostase et la Thrombose) Thromb Haemostasis. 1995;73:444–452. [PubMed] [Google Scholar]
- 28.Roubey R A. Immunology of the antiphospholipid antibody syndrome. Arthritis Rheum. 1996;39:1444–1454. doi: 10.1002/art.1780390903. [DOI] [PubMed] [Google Scholar]
- 29.Schultz D R. Antiphospholipid antibodies. Basic immunology and assays. Semin Arthritis Rheum. 1997;26:724–729. doi: 10.1016/s0049-0172(97)80041-8. [DOI] [PubMed] [Google Scholar]
- 30.Selva-O'Callaghan A, Ordi-Ros J, Monegal-Ferran F, Martinez N, Cortés-Hernandez F, Vilardell-Tarres M. IgA anticardiolipin antibodies. Relation with other antiphospholipid antibodies and clinical significance. Thromb Haemostasis. 1998;79:282–285. [PubMed] [Google Scholar]
- 31.Thiagarajan P, Pengo V, Shapiro S S. The use of the diluted Russell viper venom time for the diagnosis of the lupus anticoagulants. Blood. 1986;68:869–874. [PubMed] [Google Scholar]
- 32.Triplett D A, Brand J T, Musgrave K A, Orr C A. The relationship between lupus anticoagulants and antibodies to phospholipid. JAMA. 1988;259:550–554. [PubMed] [Google Scholar]