Abstract
Background
Early treatment of coronavirus disease 2019 (COVID-19) with remdesivir in high-risk patients, including those with immunosuppression of different causes, has not been evaluated. The objective of this study was to assess the clinical effectiveness of early remdesivir treatment among patients with mild to moderate COVID-19 at high risk of progression.
Methods
This prospective cohort comparative study was conducted in a tertiary referral center in Mexico City. Patients with mild to moderate COVID-19 at high risk for progression were treated with an ambulatory 3-day course of remdesivir. The primary efficacy composite outcome was hospitalization or death at 28 days after symptom onset. A Cox proportional hazards regression model was used to identify associations with the primary outcome.
Results
From December 1, 2021, to April 30, 2022, a total of 196 high-risk patients were diagnosed with COVID-19, of whom 126 were included in this study (43%, 54/126, received remdesivir; 57%, 72/126, did not receive remdesivir). Baseline clinical characteristics were similar between groups; autoimmune diseases (39/126), solid organ transplant (31/126), and malignant neoplasms (24/126) were the most common immunocompromising conditions. Diabetes mellitus was strongly associated with the primary outcome in both groups. Prior severe acute respiratory syndrome coronavirus 2 infection or vaccination was not independently associated with COVID-19 progression. Treatment with remdesivir significantly reduced the odds of hospitalization or death (adjusted hazard ratio, 0.16; 95% CI, 0.06–0.44; P < .01).
Conclusions
Early outpatient treatment with remdesivir significantly reduces hospitalization or death by 84% in high-risk, majority immunosuppressed patients with Omicron variant COVID-19.
Keywords: COVID-19, OPAT, immunosuppression, remdesivir
In this prospective cohort of predominantly immunosuppressed high-risk patients with mild to moderate COVID-19, outpatient treatment with 3 days of intravenous remdesivir was effective in reducing the odds of hospitalization and/or death from all causes at 28 days post-symptom onset.
Disease severity and mortality from coronavirus disease 2019 (COVID-19) have decreased in the general population, primarily due to widespread vaccination and possibly to evolution to less severe variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus [1, 2]. However, immunosuppressed patients experience diminished vaccine efficacy and higher rates of severe COVID-19 [3, 4]. Therefore, they are still at higher risk of hospitalization or death from COVID-19 than the general population [5–7], and close clinical management is advised [8].
Early treatment with remdesivir in patients with an increased risk of progression avoids hospitalization and death [9]. These findings have been reproduced in a small cohort of solid organ transplant (SOT) recipients [10], but not in larger cohorts or in patients with different kinds of immunosuppression. In addition, evidence from large randomized controlled trials supports the early administration of antiviral agents in treating high-risk patients with COVID-19 [11, 12]. Consequently, the World Health Organization’s latest treatment recommendations for patients with COVID-19 at high risk of hospitalization include the antivirals nirmatrelvir/ritonavir, remdesivir, and molnupiravir [13]. However, access to these treatments in low- and middle-income countries (LMICs) is limited [14], and plans for equitable distribution are yet to be implemented.
There is a limited supply of COVID-19 treatments in Mexico. Anti-SARS-CoV-2 monoclonal antibodies are not available, and nirmatrelvir/ritonavir just became available in August 2022. On January 17, 2022, based on the results of the PINETREE clinical trial by Gottlieb et al. [9], local guidelines at our institution included remdesivir as the standard of care for high-risk patients with early COVID-19. We report the clinical outcomes of high-risk patients treated with early ambulatory remdesivir, compared with those who did not receive any treatment. We aimed to compare the proportion of high-risk patients with mild to moderate COVID-19 who progressed to hospitalization or death between those who received early remdesivir and those who did not.
METHODS
Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” is a national referral center for rheumatologic, oncologic, hematologic, and renal diseases and is a specialized center for SOT and hematopoietic stem cell transplant (HSCT). From the beginning of the COVID-19 pandemic, our institution became an only-COVID-19 center and has gradually resumed attention for non-COVID-19 patients. This study is part of the COVID-19 prospective cohort that started at our institution on March 16, 2020. We included patients aged ≥18 years with high-risk conditions with confirmed mild to moderate COVID-19 who attended the emergency department at our institution from December 1, 2021, to April 30, 2022.
High-risk conditions for COVID-19 progression were defined as having >1 of the following: age ≥60 years, body mass index (BMI) >35 kg/m2, uncontrolled diabetes [15], uncontrolled arterial hypertension [16], cerebrovascular disease, ischemic heart disease, chronic renal disease on renal replacement therapy, liver cirrhosis, pregnancy, no vaccine history against SARS-CoV-2 or incomplete vaccine schedule (1 dose from a 2-dose primary series), and immunosuppression. In addition, immunosuppression was defined as ≥1 of the following: primary immunodeficiency, active cancer receiving chemotherapy and/or immunotherapy, HIV with CD4+ T-cell count <200 cells/mL, chronic steroid therapy (>2 weeks of prednisone >15 mg/d or its equivalent), SOT, HSCT, active autoimmune disease on immunosuppressive treatment, and recent use of anti-CD20 or antimetabolite drugs.
Per local guidelines, starting on January 17, 2021, patients were treated with a 3-day outpatient course of remdesivir when the following criteria were met: ≤7 days from symptom onset, confirmed COVID-19 (positive polymerase chain reaction [PCR] or antigen test), oxygen saturation at room air ≥90%, and >1 high-risk condition for COVID-19 progression. The threshold for oxygen saturation on room air was selected based on the normal gasometric values reported for Mexico City (altitude 2240 above sea level) [17]. Patients who met these criteria received an intravenous infusion of 200 mg remdesivir on day 1 and 100 mg on days 2 and 3. The comparison group also met the inclusion criteria. Patients who did not receive remdesivir were diagnosed with COVID-19 before the remdesivir outpatient policy was implemented (no specific early COVID-19 treatments were available at the time).
The primary outcome was a composite of hospitalization (>24-hour in-hospital stay) or death from any cause at day 28 after symptom onset. Considering a prevalence of 21% for hospitalization or death for immunosuppressed patients during the Omicron variant period [6], a prevalence of 0.07% for hospitalization or death in a remdesivir-treated high-risk population [9], a probability of type 1 error of 0.05%, and a statistical power of 90%, we calculated a sample size of at least 94 patients (47 per group).
A nonprobabilistic consecutive sampling of all patients who met the inclusion criteria was implemented. Clinical data were collected prospectively. Categorical variables were reported as frequencies and proportions. Continuous variables were reported using mean and standard deviation or median and interquartile range (IQR) values. Comparisons between the remdesivir and nonremdesivir groups were made using the chi-square test or Fisher exact test, as appropriate, and 2 independent-sample Wilcoxon tests. A bivariate analysis was used to identify factors associated with the primary outcome. Hazard ratios (HRs) and 95% CIs were calculated. To find an independent association between remdesivir treatment and the primary outcome, a multivariate Cox proportional hazards regression model that included variables of clinical and biological importance and those with P < .2 in bivariate analysis was constructed. The assumption of proportional hazards was met for the modeling. Adjusted HRs (aHRs) and 95% CIs were calculated. A 2-sided P value <.05 was considered statistically significant. Missing data were not replaced. Statistical analysis was done using STATA, version 15.1 (StataCorp, College Station, TX, USA).
RESULTS
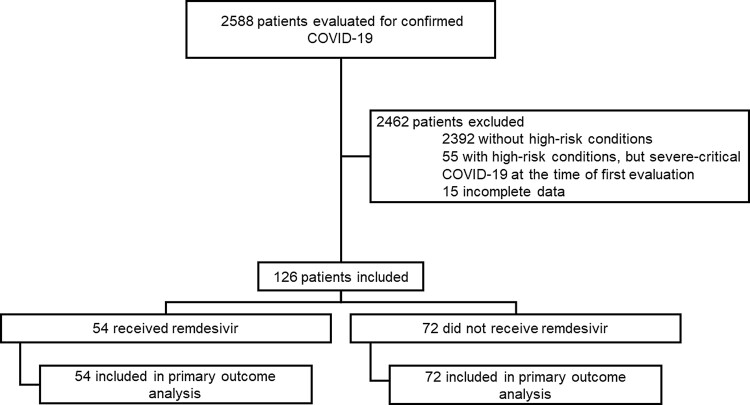
During the study period, 2588 patients were evaluated at our institution for COVID-19. One hundred ninety-six were considered high-risk per our definition, 55 were initially classified as severe/critical COVID-19, and 141 were initially classified as mild or moderate COVID-19. Fifteen patients were excluded because they were referred to other institutions for follow-up; thus, no information to evaluate the outcomes was available. For this study, we included 126 patients with mild to moderate COVID-19: 54/126 (42.9%) received remdesivir, and 72/126 (57.1%) did not receive remdesivir (Figure 1).
Figure 1.
Enrollment and inclusion. Abbreviation: COVID-19, coronavirus disease 2019.
The median age (IQR) was 49 (35–63) years, and 57.1% (72/126) were female. Comorbidities were present in 99.2% (125/126), immunosuppression was present in 93.7% (118/126), 88.1% (111/126) had 2 or more high-risk conditions for COVID-19 progression (48/54 in the remdesivir-treated group and 63/72 in the non-remdesivir-treated group), vaccination status was complete for 79.4% (100/126), and previous COVID-19 was self-reported by 9.3% (19/126). One patient did not report any comorbidities; however, he was considered high risk because he had not received any dose of the SARS-CoV-2 vaccine, and his age was >65. Diagnosis was made by PCR in 92.8% (117/126), and the median cycle threshold (ct) value (IQR) was 23 (21–27). Omicron was the predominant strain in 94.8% of the samples monitored by SGTF (110/116; 10 patients diagnosed with rapid antigen testing). The rest of the baseline clinical characteristics are described in Table 1.
Table 1.
Clinical Characteristics of the Study Population
| Characteristic | All n = 126 (100%) | Received Remdesivir n = 54 (42.9%) | Did Not Receive Remdesivir n = 72 (57.1%) | P |
|---|---|---|---|---|
| Sex—female, No. (%) | 72 (57.1) | 26 (48.1) | 46 (63.9) | .344 |
| Age, median (IQR), y | 49 (35–63) | 43 (31–61) | 51.5 (41.4–64.5) | <.001 |
| Obesity, No. (%) | 24 (19.1) | 10 (18.5) | 14 (19.4) | .896 |
| Type 2 diabetes mellitus, No. (%) | 27 (21.4) | 10 (18.5) | 17 (23.6) | .491 |
| Arterial hypertension, No. (%) | 40 (31.8) | 14 (25.9) | 26 (36.1) | .224 |
| Cerebrovascular disease, No. (%) | 4 (3.2) | 1 (1.9) | 3 (4.2) | .635 |
| Ischemic heart disease, No. (%) | 5 (4.0) | 2 (3.7) | 3 (4.2) | 1.000 |
| Chronic lung disease, No. (%) | 6 (4.8) | 3 (5.6) | 3 (4.2) | 1.000 |
| Advanced chronic kidney disease,a No. (%) | 9 (7.1) | 4 (7.4) | 5 (6.9) | .920 |
| Liver cirrhosis,a No. (%) | 6 (4.8) | 1 (1.9) | 5 (6.9) | .237 |
| Solid organ malignant neoplasm,a No. (%) | 9 (7.1) | 5 (9.3) | 4 (5.6) | .424 |
| Malignant hematologic disorders,a No. (%) | 15 (11.9) | 5 (9.3) | 10 (13.9) | .427 |
| Uncontrolled HIV infection,a No. (%) | 2 (1.6) | 0 (0) | 2 (2.8) | .506 |
| Autoimmune disorders,a No. (%) | 39 (31.0) | 16 (29.6) | 23 (31.9) | .781 |
| Solid organ transplant recipient,a No. (%) | 31 (24.6) | 13 (24.1) | 18 (25.0) | .905 |
| Hematopoietic stem cell transplant recipients,a No. (%) | 7 (5.6) | 4 (7.4) | 3 (4.2) | .461 |
| RT-PCR ct value, median (IQR), units (n = 117) | 23 (21–27) | 21 (23–26) n = 45 | 23.5 (20.5–28) n = 72 | .702 |
| Previous vaccination against SARS-CoV-2, No. (%) | 100 (79.4) | 45 (83.3) | 55 (76.4) | .340 |
| Previous SARS-CoV-2 infection, No. (%) | 19 (9.3) | 5 (9.3) | 14 (19.4) | .114 |
| Time from symptom onset to hospital presentation, median (range), d | 4 (0–7) | 3 (0–7) | 4 (1–7) | .82 |
| Time from symptom onset to remdesivir administration, median (range), d | … | 3 (0–7) | … |
Abbreviations: ct, cycle threshold; IQR, interquartile range; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Indicates comorbidities considered to be immunocompromising.
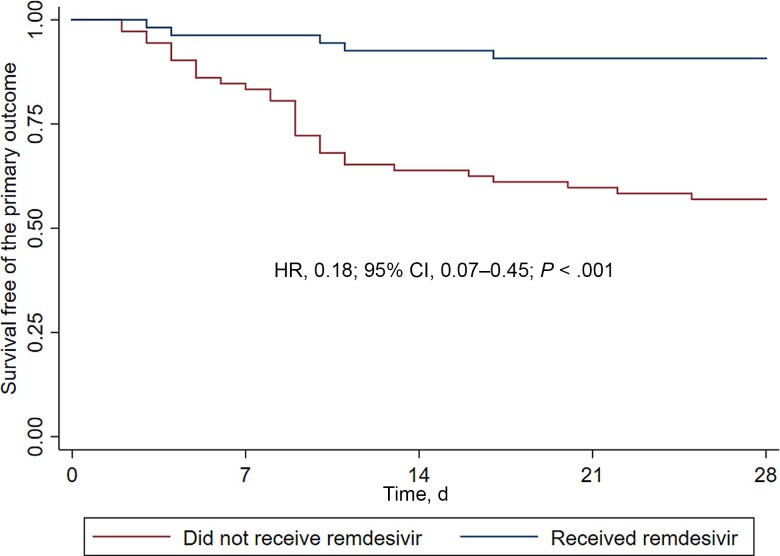
The primary outcome was directly assessed at our institution and occurred in 5/54 (9.3%) remdesivir-treated patients vs 31/72 (43.1%) non-remdesivir-treated patients (P < .01) (Table 2). On bivariate analysis, age ≥60 years (HR, 2.57; 95% CI, 1.33–4.94), diabetes mellitus (HR, 3.40; 95% CI, 1.76–6.59), and cirrhosis (HR, 3.38; 95% CI, 1.19–9.58) were associated with increased frequency of death or hospitalization. Previous SARS-CoV-2 vaccination was inversely associated with the primary outcome (HR, 0.48; 95% CI, 0.24–0.96). In multivariate analysis, diabetes mellitus (adjusted HR, 3.35; 95% CI, 1.58–7.07) was independently associated with the primary outcome (Table 3). Treatment with remdesivir was independently associated with lower risk of hospitalization or death in bivariate (HR, 0.18; 95% CI, 0.07–0.45) and multivariate analyses (aHR, 0.16; 95% CI, 0.06–0.44) (Figure 2 and Table 3).
Table 2.
Hospitalization and/or Death
| Outcome | All n = 126 (100%) | Received Remdesivir n = 54 (42.9%) | Did Not Receive Remdesivir n = 72 (57.1%) | P |
|---|---|---|---|---|
| Primary outcome, No. (%) | 36 (28.5) | 5 (9.3) | 31 (43.1) | <.001 |
| All-cause hospitalization, No. (%) | 27/36 | 5/5 | 22/31 | .004 |
| COVID-19-related hospitalization, No. (%) | 20/27 | 0/5 | 20/22 | <.001 |
| All-cause death, No. (%) | 9/36 | 0/5 | 9/31 | .007 |
Italic formatting indicates subsections of the primary outcome. Patients who died were previously hospitalized.
Abbreviation: COVID-19, coronavirus disease 2019.
Table 3.
Association of Clinical Variables With Hospitalization and/or Death
| Characteristic | HR (95% CI), P | aHR (95% CI), P |
|---|---|---|
| Male sexa | 1.37 (0.71–2.64), .432 | 0.95 (0.43–2.10), .908 |
| Age ≥60 ya | 2.57 (1.31–3.80), .003 | 1.52 (0.69–3.35), .295 |
| BMI >35 kg/m2 | 1.04 (0.45–2.36), .934 | |
| Diabetes mellitusa | 3.40 (1.76–6.59), <.001 | 3.35 (1.58–7.07), .002 |
| Arterial hypertension | 1.25 (0.63–2.46), .527 | |
| Cerebrovascular disease | 2.17 (0.52–9.05), .287 | |
| Ischemic heart disease | 1.67 (0.40–6.96), .480 | |
| Chronic lung disease | 1.05 (0.25–4.39), .943 | |
| Chronic kidney disease | 1.79 (0.63–5.07), .271 | |
| Cirrhosisa | 3.38 (1.19–9.58), .022 | 1.28 (0.37–4.87), .716 |
| Solid organ malignant neoplasm | 1.35 (0.41–4.41), .617 | |
| Malignant hematologic disordersa | 2.23 (0.98–5.11), .057 | 1.70 (0.61–4.75), .312 |
| Autoimmune disordersa | 0.62 (0.28–1.37), .239 | 0.77 (0.26–2.31), .640 |
| Solid organ transplant recipienta | 0.44 (0.17–1.13), .088 | 0.33 (0.10–1.08), .067 |
| Hematopoietic stem cell transplant recipient | 0.47 (0.06–3.43), .457 | |
| Previous SARS-CoV-2 vaccinationa | 0.48 (0.24–0.96), .037 | 0.65 (0.27–1.56), .340 |
| Previous SARS-CoV-2 infectiona | 0.87 (0.34–2.23), .767 | 0.58 (0.21–1.57), .281 |
| Treatment with remdesivira | 0.18 (0.07–0.45), <.001 | 0.16 (0.06–0.44), <.001 |
Abbreviations: aHR, adjusted hazard ratio; BMI, body mass index; HR, hazard ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Indicates variables included in the multivariable regression model.
Figure 2.
Kaplan-Meier estimates for hospitalization and/or death. Reported HR corresponds to bivariate analysis according to treatment with remdesivir. Abbreviation: HR, hazard ratio.
DISCUSSION
This study shows real-world evidence on the effectiveness of remdesivir for the early treatment of COVID-19 in high-risk patients. All baseline characteristics were similar between groups, except for age. Increasing age is a well-known risk factor for severe COVID-19 [18]. In our study, patients from the remdesivir-treated group were a median of 7 years younger than those in the non-remdesivir-treated group. Although this might have contributed to the higher hospitalization or death rate seen in the non-remdesivir-treated group, age was not independently associated with the primary outcome.
Hospitalization or death was high in this population (28.5%). Possible reasons for this include the variety of immunocompromising conditions present in the patients evaluated, inclusion of patients with 2 or more high-risk conditions (possibly adding to the odds of a worse outcome), diabetes and hypertension were considered for inclusion only if uncontrolled, inclusion criteria were wider compared with those used in the PINETREE study, and the primary outcome also included hospitalizations or death not directly related to COVID-19. The patients seen at our institution have multiple and complex medical disorders; thus, hospitalizations for underlying diseases in the 28-day follow-up are expected. As seen in Table 2, the events directly related to progression of COVID-19 were fewer, but still significantly different in both groups.
COVID-19 severity has changed throughout the pandemic [1]. To control for this effect, we included patients in similar pandemic time frames. Both groups were assessed during the time when the Omicron BA.1/BA.2 variant was the dominant circulating variant in Mexico [19]; therefore, no impact of the different variants of the virus is expected. There might be concern for including patients in the last part of the Delta wave, but for Mexico City the entry of Omicron BA.1/BA.2 was earlier than in other Mexican states [20]. Furthermore, by this time, our institute had implemented a PCR test (Thermo Fisher TaqPath) where 1 of the 3 target genes is not detected (called S gene dropout or S gene target failure [SGTF]). This test was used as a marker for the Omicron variant, pending sequence confirmation, and 94.8% of the samples in this study were positive for the SGTF marker.
Interestingly, in our study diabetes mellitus was associated with risk of hospitalization or death from COVID-19, despite the lower severity of disease reported for the Omicron variant. While the lower severity of Omicron is still controversial, the observation of diabetes mellitus as a risk factor for progression has been reported by other researchers [21] and warrants future investigations on the intricate relationship between SARS-CoV-2 and dysglycemia.
The findings on SOT as a variable with potentially lower odds of hospitalization or death are interesting. Although this was not replicated in the multivariate analysis, it certainly highlights observations from other investigators suggesting that immunosuppressive drugs for solid organ transplant might play an beneficial anti-inflammatory role and that the higher risk of COVID-19 progression in these patients is possibly driven by their other comorbidities, especially diabetes mellitus and cardiovascular disease [22, 23]. The recently published retrospective cohort [10] that evaluated early remdesivir treatment in 24 SOT recipients found results similar to ours regarding lower probability of hospitalization and/or death. However, the small sample size, the retrospective nature of the study, and the inclusion of only SOT recipients may limit the generalizability of their observations, especially to patients with other immunocompromising diseases. In contrast, our study showed a positive effect of early remdesivir treatment for SOT recipients as well as for patients with other immunocompromising conditions.
Some studies have suggested that previous SARS-CoV-2 natural infection as protection against severe reinfections is extremely effective [24]. Reinfection in our study group was seen in 9.3% of the population, without difference among treatment groups. However, this proportion of reinfections is low, which might influence the lack of association with protection. Also, immunocompromised patients are expected to present an impaired immune response against natural SARS-CoV-2 infection [25], and thus might not display this so-called protection against reinfection.
The SARS-CoV-2 vaccines used in Mexico are varied. BNT162b2 (Pfizer BioNTech) and AD122 Covishield (Astra Zeneca) account for >70% of the total national adult vaccination program [26]. Vaccine efficacy, although not assessed, is expected to be low among the patients included in this study. The proportion of vaccinated patients in both groups was similar, eliminating potential confounders for the effect of remdesivir. The negative association with the primary outcome observed on bivariate analysis was not observed on multivariate analysis, probably reflecting the low unvaccinated proportion of the population and that vaccine efficacy is expected to be low in immunocompromised patients, leaving them at high risk for COVID-19 progression. Of note, an overall vaccine coverage <80% should raise awareness for emphasizing this public health intervention among these high-risk groups.
The burden of COVID-19 in developing countries is higher, probably reflecting limited access to appropriate health care, limited access to antiviral treatments, and previously overwhelmed health care systems [14, 27]. The need for early treatments that could potentially alleviate the burden of COVID-19 has been highlighted since the beginning of the pandemic [28]. Effectively treating high-risk patients helps prevent an important number of COVID-19-related hospitalizations, thereby lowering costs at the health care system level, as well as costs and hospital-acquired complications at the patient level.
Our study serves as proof of concept regarding the feasibility of an outpatient remdesivir treatment clinic for early COVID-19 in the setting of a developing country and in the absence of other efficacious treatments. Patients received the intravenous infusion on day 1, were instructed on the appropriate care for an intravenous peripheral access, and were sent home. They returned on days 2 and 3 to receive the infusion using the same peripheral access. There were no adverse events related to the peripheral access, including catheter-related infections during treatment or follow-up. This reflects the expertise of the outpatient parenteral antimicrobial therapy (OPAT) nursing team at our institution and reinforces the feasibility of these kinds of programs for COVID-19.
The strengths of our study include the clear and structured definition of high-risk conditions, inclusion of predominantly immunosuppressed patients whose vaccine response is expected to be low, different types of immunosuppression, successful implementation of an OPAT-like clinic for COVID-19 in a developing country, inclusion of patients during the same time point of the pandemic, and prospective collection of data. Also, while inclusion criteria were based on the PINETREE study, the wider range of values allows for the criteria in our study to be used in other centers as they were adapted for pragmatism.
The limitations of our findings include that the observations were done in 1 center, the median age was 7 years younger in the remdesivir group (a known risk factor for adverse outcomes), treatment was not randomized, there were no available data on post-COVID-19 syndrome for these patients due to lack of long-term follow-up, and the time from vaccination or prior COVID-19 episode to current COVID-19 could not be evaluated due to the self-reported nature of these variables.
CONCLUSIONS
Early outpatient treatment with remdesivir significantly reduces hospitalization or death by 84% in high-risk, majority immunosuppressed patients with Omicron variant COVID-19.
Acknowledgments
The authors thank and recognize the hard work of the emergency staff department, the molecular virology and clinical microbiology laboratories, and the administrative staff who facilitated the work for this study.
Financial support. This study did not receive private funding. Remdesivir was donated to our institution by Mexico City’s Health Department. All other resources and expenses were covered by the Infectious Diseases Department, the Emergency Department, and the Virology Laboratory of Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán.”
Author contributions. S.R.L., D.K.S., J.S.O., G.M.R.P., A.P.L.: conceived the idea. S.R.L. J.Z.S.: enrollment. S.R.L., B.A.M.G., J.Z.S., C.M.R.M., K.T.T.: data management. B.A.M.G.: statistical analysis. S.R.L., T.H.G., M.F.G.L., D.K.S., J.S.O., G.M.R.P., A.P.L.: protocol implementation. S.R.L., B.A.M.G.: led the writing of the manuscript. All authors contributed equally to the revision of the manuscript.
Patient consent. This study is part of the prospective COVID-19 cohort at Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán.” It has been approved by the local Investigation and Ethics Committees under the approval reference INF-3333. All patients gave written informed consent.
Contributor Information
Sandra Rajme-López, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
Bernardo A Martinez-Guerra, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
Jessica Zalapa-Soto, Internal Medicine Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
Carla M Román-Montes, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
Karla M Tamez-Torres, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
María F González-Lara, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
Thierry Hernandez-Gilosul, Emergency Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
David Kershenobich-Stalnikowitz, General Direction, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
José Sifuentes-Osornio, General Direction, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
Alfredo Ponce-de-León, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
Guillermo M Ruíz-Palacios, Infectious Diseases Department, Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán,’ Mexico City, Mexico.
References
- 1. Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global Omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis 2022; 116:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohammed I, Nauman A, Paul P, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother 2022; 18:2027160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 2022; 376:e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Global Health 2022; 10:e326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grainger R, Kim AHJ, Conway R, Yazdany J, Robinson PC. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol 2022; 18:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malahe SRK, Hoek RAS, Dalm VASH, et al. Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: a prospective observational study. Clin Infect Dis 2022:ciac571. doi: 10.1093/cid/ciac571. [DOI] [PMC free article] [PubMed]
- 7. Lee M, Quinn R, Pradhan K, et al. Impact of COVID-19 on case fatality rate of patients with cancer during the Omicron wave. Cancer Cell 2022; 40:343–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li P, De Vries AC, Kamar N, Peppelenbosch MP, Pan Q. Monitoring and managing SARS-CoV-2 evolution in immunocompromised populations. Lancet Microbe 2022; 3:e325–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colaneri M, Amarasinghe N, Rezzonico L, et al. Early remdesivir to prevent severe COVID-19 in recipients of solid organ transplant: a real-life study from Northern Italy. Int J Infect Dis 2022; 121:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernal A J, Da Silva MM G, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk. Nonhospitalized adults with COVID-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Therapeutics and COVID-19: living guideline. 2022. Available at:https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3. Accessed June 20, 2022. [PubMed]
- 14. Usher AD. The global COVID-19 treatment divide. Lancet 2022; 399:779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 6. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care 2022; 45:S83–96. [DOI] [PubMed] [Google Scholar]
- 16. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension 2020; 75:1334–57. [DOI] [PubMed] [Google Scholar]
- 17. Pérez-Padilla JR. Adaptation to moderate altitude hypoxemia: the example of the valley of Mexico. Rev Invest Clin 2022; 74:4–15. [DOI] [PubMed] [Google Scholar]
- 18. Starke KR, Reissig D, Petereit-Haack G, Schmauder S, Nienhaus A, Seidler A. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Global Health 2021; 6:e006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cedro-Tanda A, Gómez-Romero L, De Anda-Jauregui G, et al. Early genomic, epidemiological, and clinical description of the SARS-CoV-2 Omicron variant in Mexico City. Viruses 2022; 14:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dirección General de Epidemiología . Reporte de vigilancia genómica del virus SARS-CoV-2 en México Distribución nacional y estatal. 2022. Available at: https://www.gob.mx/salud/acciones-y-programas/direccion-general-de-epidemiologia. Accessed June 25, 2022.
- 21. Skarbinski J, Wood MS, Chervo TC, et al. Risk of severe clinical outcomes among persons with SARS-CoV;2, infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: a retrospective cohort study. Lancet Reg Health Am 2022; 12:100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belli LS, Fondevila C, Cortesi PA, et al. Protective role of tacrolimus. Deleterious role of age and comorbidities in liver transplant recipients with COVID-19, results from the ELITA/ELTR multi-center European study. Gastroenterology 2021; 160:1151–63.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gatti M, Rinaldi M, Bussini L, et al. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect 2022; 28:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med 2021; 385:2487–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phadke VK, Scanlon N, Jordan SC, Rouphael NG. Immune responses to SARS-CoV-2 in solid organ transplant recipients. Curr Transpl Rep 2021; 8:127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Secretaria de Salud de México . Información de la vacuna. 2022. Available at: https://vacunacovid.gob.mx/informacion-de-la-vacuna/. Accessed June 20, 2022.
- 27. Levin AT, Owusu-Boaitey N, Pugh S, et al. Assessing the burden of COVID-19 in developing countries: systematic review, meta-analysis and public policy implications. BMJ Global Health 2022; 7:e008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim PS, Read SW, Fauci AS. Therapy for early COVID-19. JAMA 2020; 324:2149. [DOI] [PubMed] [Google Scholar]