Abstract
Increased expression of substance P (SP) and neurokinin-1 receptor (NK1R) has been noticed in patients with allergic rhinitis (AR) and allergic asthma (AA). However, little is known of the expression of SP and NK1R in monocytes and B cells of AR and AA. In the present study, the expression levels of SP and NK1R were determined by flow cytometry and mouse AR and AA models. The results showed that both percentages of SP+ monocytes and SP+ B cells, and mean fluorescence intensity (MFI) of SP in monocytes were elevated in the blood of AA and AR combined with AA (ARA) patients. Similarly, the percentages of NK1R+ monocytes were elevated in the blood of AR, AA, and ARA patients. Allergens Artemisia sieversiana wild allergen extract (ASWE), house dust mite extract (HDME), and Platanus pollen allergen extract (PPE) increased the expression density of SP molecules (determined by MFI) in an individual monocyte of AR patients. HDME and PPE appeared to enhance SP and NK1R expression in the B cells of ARA and AR patients. In the mouse AR and AA models, the percentages of NK1R+ monocytes and B cells were elevated in blood following OVA (ovalbumin) sensitization and challenge. Knocking out the FcεRI molecule completely abolished the OVA-induced upregulation of expression of NK1R in monocytes and B cells of AA mice. In conclusion, upregulated expressions of SP and NK1R may contribute to the pathogenesis of airway allergy.
Keywords: substance P, NK1R, monocyte, B cell, allergic rhinitis, allergic asthma
Upregulated expressions of SP and NK1R in monocytes and B cells may play a role in the pathogenesis of AA, ARA and AR. Aerosol allergens can enhance SP and NK1R expressions in monocytes and B cells, which contribute to the development of these allergic airway disorders. A FcεRI associated mechanism may be involved in the OVA-induced upregulation of expression of NK1R in the monocytes and B cells of AA mice.
Introduction
While allergic rhinitis (AR) is considered as an immunoglobulin (Ig) E-mediated inflammation of the upper airway [1], allergic asthma (AA) is recognized as an IgE-mediated inflammation of the lower airway [1]. AR combined AA (ARA) is characterized, nowadays, as a single disease related to upper and lower airway inflammation [2]. It is noticed that infiltration of inflammatory cells, particularly Th2 cells, eosinophils, and basophils, into nasal [3] and lung mucosal tissues [4] may specifically contribute to the pathophysiology of AR [5], AA [6], and ARA [7]. However, little is known about the involvement of SP/NK1R-expressing monocytes and B cells.
It was reported that monocytes have a central role in orchestrating local allergic inflammation [8]. And as a key immune cell in the bloodstream, monocytes have been used to characterize the severity of inflammation such as AR [9]. Moreover, monocytes comprise the largest population of cells within the airways of patients with AA [10]. It is found that the number of B cells is significantly higher in patients with allergic disease than in nonallergic controls [10], and that B cells are key players in AR [3] and AA [11] as they can produce allergen-specific IgE.
Substance P (SP) belongs to the family of tachykinin, which exhibits specific neuronal activity and pro-inflammatory activity [12]. It is produced by lymphocytes, macrophages [13], eosinophils, and dendritic cells [14], and has been implicated in the pathogenesis of inflammation [15]. Since SP is reported to be associated with AR [12] and AA [14], and monocytes can produce SP [16], we anticipate that SP expressing monocytes may be involved in the pathogenesis of AR and AA.
Neurokinin-1 receptor (NK1R) is a major receptor of SP [17], which expresses in inflammatory cells, such as mast cells and macrophages [18]. Activation of NK1R can mediate SP-induced migration of basophils [19] and consequently amplify the pro-inflammatory response [13]. Enhanced expression of NK1R has been observed in patients with AR [20], and NK1R-mediated neurogenic inflammation may be involved in AA [21]. Since SP/NK1R complex has been recognized as an integral part of the microenvironment of inflammation and is involved in the molecular bases of many human pathologies [22], it is likely that SP/NK1R-expressing monocytes and B cells are related to AR and AA.
It has been observed that the number of monocytes in nasal mucosa increases after continuous allergen stimulation, accompanied by the recruitment of Th2 cells and eosinophils in patients with AR [8]. Since allergens are the causative factors of allergic disease, and house dust mite (HDM) major allergens evoke upregulation of pulmonary SP, inflammation, and mucus hypersecretion [23], we examined the effect of allergens on the expressions of SP and NK1R on monocytes and B cells in the present study.
Immunoglobulin E (IgE) is recognized as the most important biological target for AR and AA treatment [24]. The interaction of FcεRI (high-affinity receptor of IgE)-bound IgE antibody with the allergen is an essential step for mast cell/basophilic activation and subsequent release of allergic mediators [25]. To further investigate the role of FcεRI in allergic airway disease, we examined the difference in NK1R expression on monocytes and B cells of wild-type (WT) and FcεRI knockout (KO) mice.
The aim of the present study is to investigate the expression of SP and NK1R in monocytes and B cells of the patients with AR, AA, and ARA, and the influence of allergens on SP and NK1R expression. We observed that the expression of SP and NK1R in monocytes and B cells was increased in the blood.
Materials and methods
Reagents
The following reagents were purchased from Biolegend (San Diego, CA, USA): Human TruStain FcX™ (Fc Receptor Blocking Solution), Zombie Aqua™ Fixable Viability kit, PE/Cy7-conjugated mouse anti-human CD14 antibody, APC/Cy7-conjugated mouse anti-human CD19 antibody, RBC Lysis Buffer, Zombie NIR™ Fixable Viability kit, TruStain FcX™ (anti-mouse CD16/32), Brilliant Violet (BV) 421-conjugated rat anti-mouse CD11b antibody, BV 510 rat anti-mouse Ly-6G/Ly-6C (Gr-1) antibody, APC-conjugated rat anti-mouse CD19 antibody. BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution kit was purchased from BD Pharmingen (San Jose, CA, USA). FITC-conjugated mouse anti-human SP antibody was purchased from Lifespan (Rochester, NY, USA), and its isotype control antibody FITC-conjugated mouse IgG was obtained from eBioscience (San Diego, CA, USA). APC-conjugated mouse anti-human NK1R and APC-conjugated mouse IgG3 isotype control antibodies were purchased from R&D Systems (Minneapolis, MN, USA). PE-conjugated rabbit anti-mouse NK1R antibody and its isotype control antibody PE-conjugated rabbit IgG were obtained from Novus (Colorado, USA). Artemisia sieversiana wild allergen extract (ASWE), house dust mite extract (HDME), or Platanus pollen allergen extract (PPE) were obtained from Macro Union Pharmaceutical Co. Ltd (Beijing, China). Most of the general chemicals, such as salts and buffer components were of analytical grade.
Patients and samples
A total of 25 AR, 26 AA, 19 ARA, and 17 HC (healthy control) subjects were recruited for the study. Their general characteristics were summarized in Table 1. The diagnosing criteria for AR in this experiment were in line with the 2015 AR Clinical Practice Guidelines issued by the American Academy of Otorhinolaryngology-Head and Neck Surgery [26]; the diagnosing criteria of AA conformed to the Global Initiative for Asthma [27]. Samples were obtained at the First Affiliated Hospital of Jinzhou Medical University, China. The informed consent from each volunteer, according to the declaration of Helsinki and agreement with the ethical committee of the First Affiliated Hospital of Jinzhou Medical University, has been obtained. The blood from each patient with AR, AA, ARA, and HC subjects was taken in outpatient clinics. From each subject, 10 ml of peripheral blood was taken into an EDTA-containing tube before centrifugation at 450g for 10 min. The cells were used for flow cytometric analysis and plasma was stored at −80℃ until use.
Table 1:
Characteristics of subjects
| Population | HC (n = 17) | AR (n = 25) | AA (n = 26) | ARA (n = 19) |
|---|---|---|---|---|
| Age (years) | 26 (21–35) | 33 (17–60) | 40 (23–60) | 35 (11–56) |
| Female/Male | 5/12 | 14/11 | 9/17 | 9/10 |
| Onset age (years) | na | 28 (4–46) | 40 (3–53) | 35 (8–48) |
| History (years) | na | 5 (1–30) | 6 (0.5–30) | 5.5 (2–35) |
| Artemisia (+) | 0 | 12 | 11 | 9 |
| House dust mite (+) | 0 | 10 | 7 | 7 |
| Platanu (+) | 0 | 9 | 8 | 5 |
Median values (range) are shown. Specific allergens were examined by skin prick test.
HC: healthy control, AR: allergic rhinitis, AA: allergic asthma, ARA: AR combined with AA.
Animals
BALB/c mice (6–8 weeks) were obtained from the Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). C57-WT mice and C57-FcεRI-KO mice (6–8 weeks) were obtained from GemPharmatech Co. Ltd (Jiangsu, China). The animals were bred and reared under strict ethical conditions according to international recommendations. They were housed in the Animal Experimental Center of the First Affiliated Hospital of Jinzhou Medical University in a specific pathogen-free environment with free access to standard rodent chow and water at a constant temperature of 23–28℃ and relative humidity of 60–75%. The animal experiment procedures were approved by the Animal Care Committee at Jinzhou Medical University.
Mouse sensitization and challenge
The OVA (ovalbumin)-induced mouse AR model was mainly adopted from a previous study by [28]. Briefly, OVA-sensitized and OVA-challenged (OVA-OVA) and OVA-sensitized and normal saline (NS) challenged (OVA-NS) mice were sensitized on days 0, 7, 14, and 21 with a subcutaneous multipoint injection of 50 µg of OVA and 1.5 mg of AL(OH)3 suspended in NS to a total volume of 0.5 ml. NS sensitized and NS challenged (NS-NS) mice received only an equal volume (0.5 ml) of NS on the same days. On day 28, OVA-OVA mice were challenged with nasal drops of either 20 µl of 1% OVA for 7 days, and OVA-NS mice and NS-NS mice were challenged with 20 µl of NS for 7 days, 10 µl on each side of the nasal cavity. On the last day, 3 h after the nasal drip was completed, the animals were sacrificed, and their blood was collected for analysis.
The OVA-induced mouse AA model was mainly adopted from a previous study [29]. Briefly, OVA-OVA mice and OVA-NS mice were sensitized on days 0 and 7 with intra-peritoneal injection of 10 µg of OVA and 1 mg of AL(OH)3 suspended in NS to a total volume of 0.5 ml. NS-NS mice received only an equal volume (0.5 ml) of NS on the same days. On days 14–21, OVA-OVA mice were exposed daily to aerosolized 10 mg/ml of OVA over a 30-min period, and OVA-NS mice and NS-NS mice were exposed daily to NS over a 30-min period. On the last day, 24 h after aerosol inhalation was completed, animals were sacrificed and their blood was collected for analysis.
Flow cytometric analysis of SP and NK1R expression in peripheral blood monocytes and B cells
The procedure for detecting the expression of SP and NK1R in human blood monocytes and B cells was performed as described previously [30]. Briefly, the cells were stimulated with or without ASWE, HDME, and PPE (all at a concentration of 0.1 and 1.0 µg/ml) for 1 h at 37°C, respectively, and 2 µg/ml of brefeldin A was added to the tube at the same time. To detect SP and NK1R expression in human blood monocytes (CD14+ cells) and B cells (CD19+ cells), the following antibodies were added to the test tubes: PE/Cy7-conjugated mouse anti-human CD14 and APC/Cy7-conjugated mouse anti-human CD19, before 100 µl of whole blood was added at room temperature for 15 min in the dark. Following the ligation of red blood cells, the white blood cells were fixed and permeabilized using Cytofix/Cytoperm™ Fixation/Permeabilization Kit according to the instructions from the manufacturer. APC-conjugated mouse anti-human NK1R antibody and FITC-conjugated mouse anti-human SP antibody were then added to the test tube, and APC-conjugated mouse IgG3 and FITC-conjugated rabbit IgG were used as isotype controls. The cells were incubated at 4°C for 30 min in the dark. Following washing with BD washing buffer, the cell pellets were resuspended in fluorescence-activated cell sorting (FACS)-flow solution and analyzed with FACS Verse flow cytometer (BD Biosciences, San Jose, CA). A total of 10 000 events were analyzed per population for each sample.
To detect SP and NK1R expression in mouse blood monocytes and B cells, BV421-conjugated rat anti-mouse CD11b antibody, BV 510™ anti-mouse Ly-6G/Ly-6C (Gr-1) antibody, and APC anti-mouse CD19 antibody were added to the test tubes before 100 µl of whole blood was added at room temperature for 15 min in the dark. Following the ligation of red blood cells, the white blood cells were fixed and permeabilized using Cytofix/Cytoperm kit according to the instructions from the manufacturer. PE-conjugated anti-mouse NK1R antibodies were then added to each tube for 30 min at 4°C. A total of 50 000 events were analyzed for each sample.
Statistical analysis
Statistical analyses were performed using SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). Data for the expression of SP and NK1R in monocytes and B cells of patients with AR, AA, and ARA are presented as scatter plots, in which a horizontal line indicates the median value. Data for the expression of NK1R on mouse blood monocytes and B cells are displayed as boxplots, which show the median, interquartile range, and the largest and smallest values for each experiment. The paired Mann–Whitney U test was employed in cases where the Kruskal–Wallis analysis revealed significant differences between groups, for the pre-planned comparisons of interest. For all analyses, P < 0.05 was taken as significant.
Results
Upregulated expressions of SP and NK1R in peripheral blood monocytes of patients with AR, AA, and ARA
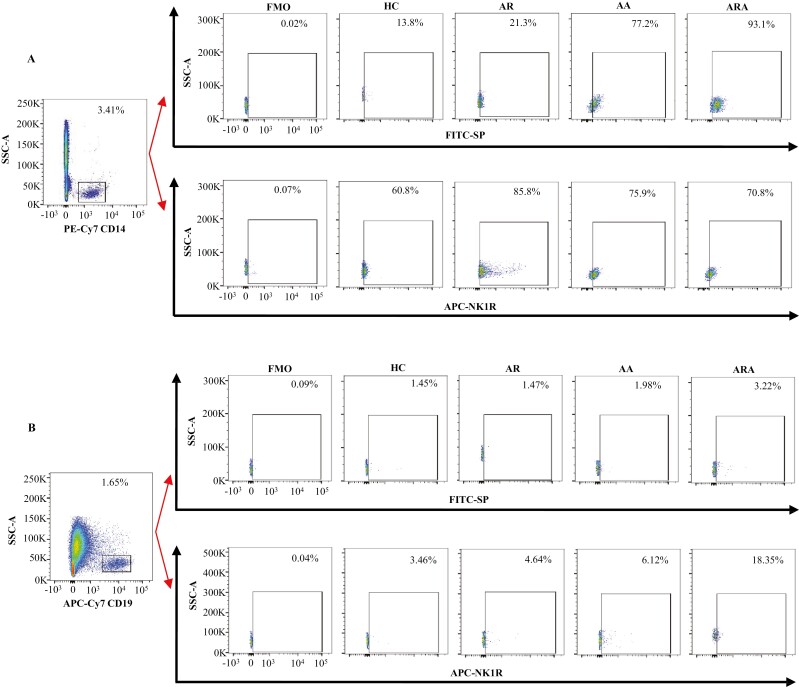
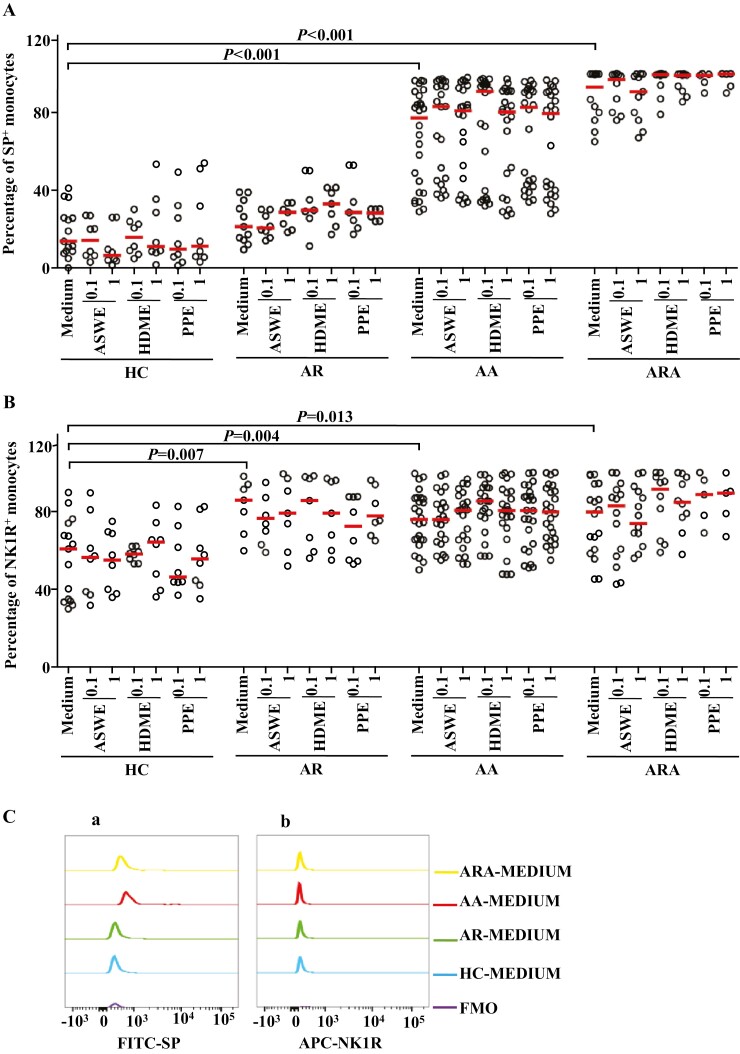
Monocytes have been reported to be involved in AR [9], AA [10], and ARA [31]. However, the expressions of SP and its major receptor NK1R in these airway allergic diseases have not been explored. In order to evaluate the involvement of SP and NK1R in airway allergic diseases, we investigated the expression of SP and NK1R in the peripheral blood monocytes of patients with AR, AA, and ARA. The results showed that the percentages of SP+ monocytes were increased by 4.6- and 5.7-fold in the blood of patients with AA and ARA compared with HC subjects, respectively (Figs 1A, 2A). Similarly, the percentages of NK1R+ monocytes were elevated by 41.1, 24.8, and 31.2% in the blood of patients with AR, AA, and ARA compared with HC subjects, respectively (Figs 1A, 2B).
Figure 1:
Flow cytometry analysis of the expressions of SP and NK1R in human peripheral blood monocytes and B cells from allergic rhinitis (AR), allergic asthma (AA), AR combined with AA (ARA), and healthy control (HC) subjects. (A) and (B) were the representative graphs of the gating strategies of SP+ and NK1R+ monocytes and B cells, respectively.
Figure 2:
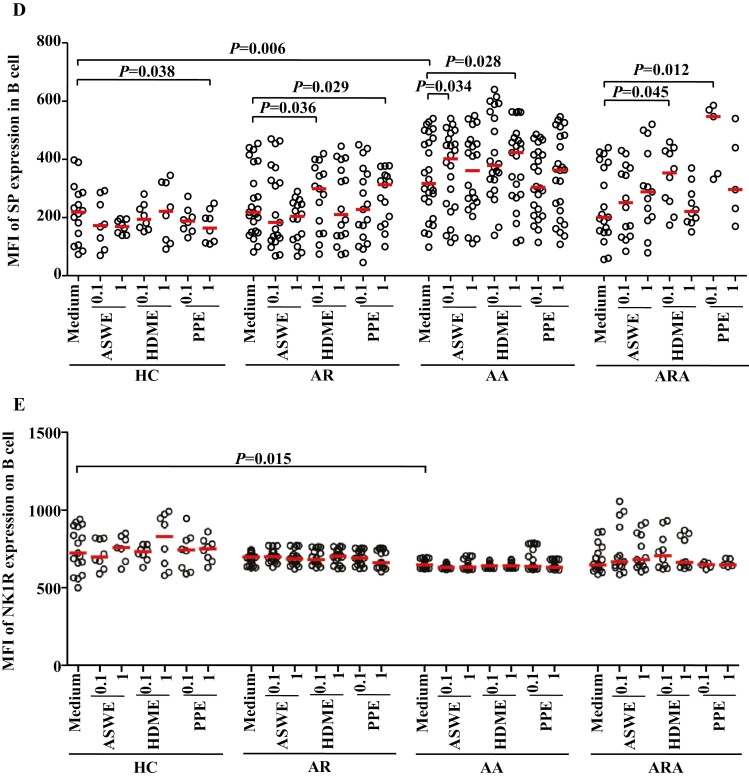
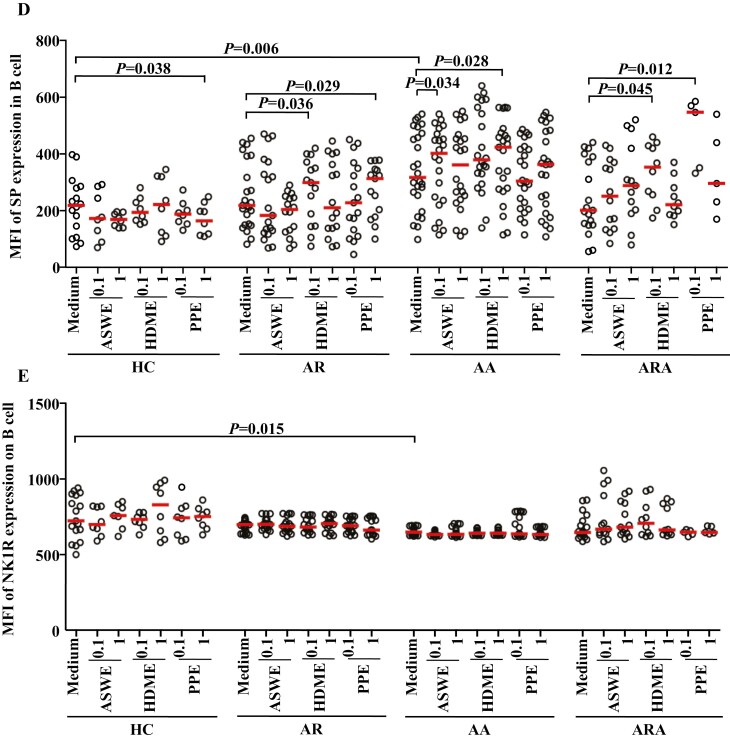
Flow cytometry analysis of expressions of SP and NK1R in human peripheral blood monocyte (CD14+ cells). (A) and (B) represented the percentages of SP+ and NK1R+ monocytes in the patients with allergic rhinitis (AR), allergic asthma (AA), AR combined with AA (ARA), and healthy control (HC) subjects, respectively. (Ca) and (Cb) showed representative flow cytometric figures of mean fluorescent intensity (MFI) of SP and NK1R expressions in the monocytes of AR, AA, ARA, and HC subjects. (D) and (E) represented the MFI of SP and NK1R in the monocytes of AR, AA, ARA, and HC subjects. Cells were stimulated with or without house dust mite extract (HDME), Artemisia sieversiana wild allergen extract (ASWE), or Platanus pollen allergen extract (PPE), all at the concentrations of 0.1 and 1 µg/ml for 1 h at 37°C. Each symbol represents the value of one subject. The median value is indicated by a horizontal line. P < 0.05 was taken as statistically significant. FMO: fluorescence minus one.
Mean fluorescence intensities (MFIs) of SP in monocytes were elevated by 1.6-fold and 73% in patients with AA and ARA (Fig. 2Ca and D), and NK1R on monocytes was increased by 15.7% in patients with AA (Fig. 2Cb and E).
Expression of SP and NK1R in peripheral blood B cells of the patients with AR, AA, and ARA
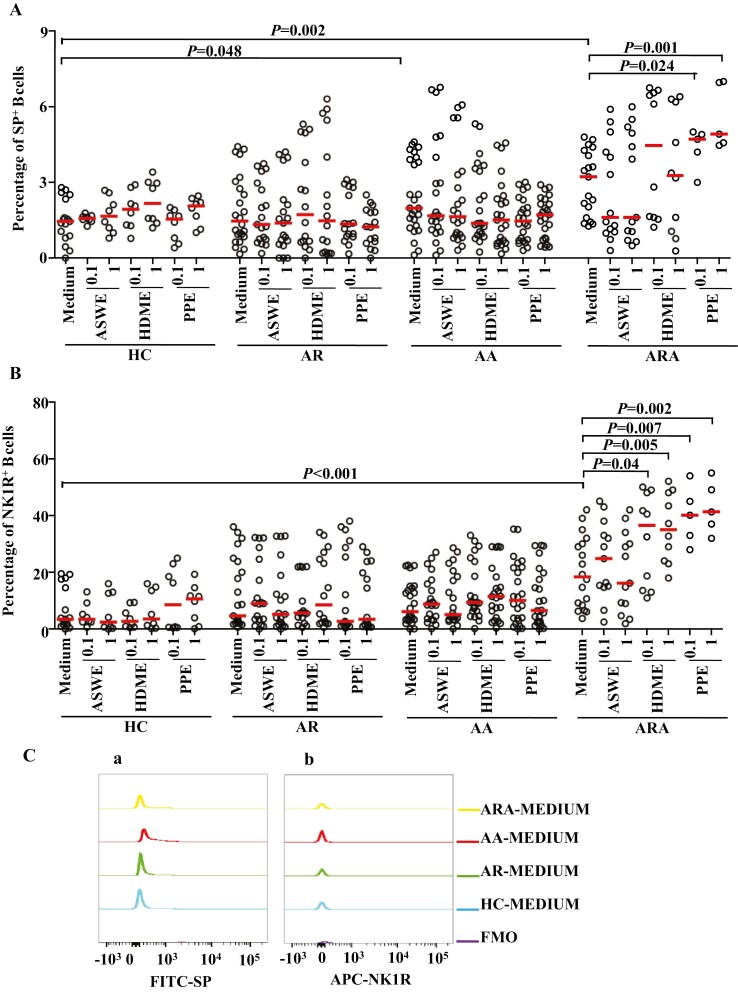
Close relationships between B cells and airway allergic diseases have long been recognized [10]. However, little is known about the expressions of SP and NK1R in B cells under allergic conditions. In order to further understand the involvement of SP and NK1R in airway allergic diseases, we investigated the expression of SP and NK1R in the peripheral blood B cells of patients with AR, AA, and ARA. The results showed that the percentages of SP+ B cells were increased by 36.2- and 1.2-fold in the blood of patients with AA and ARA compared with HC subjects (Figs 1B, 3A). The NK1R+ B cells were elevated by 4.3-fold in patients with ARA (Figs 1B, 3B).
Figure 3:
Flow cytometry analysis of expressions of SP and NK1R in human peripheral blood B cells (CD19+ cells). (A) and (B) represented the percentages of SP+ and NK1R+ B cells in the patients with allergic rhinitis (AR), allergic asthma (AA), AR combined with AA (ARA), and healthy control (HC) subjects, respectively. (Ca) and (Cb) showed representative flow cytometric figures of mean fluorescent intensity (MFI) of SP and NK1R in the B cells of AR, AA, ARA, and HC subjects. (D) and (E) represented the MFI of SP and NK1R in the B cells of AR, AA, ARA, and HC subjects. Cells were stimulated with or without house dust mite extract (HDME), Artemisia sieversiana wild allergen extract (ASWE), or Platanus pollen allergen extract (PPE), all at the concentrations of 0.1 and 1 µg/ml for 1 h at 37°C. Each symbol represents the value of one subject. The median value is indicated by a horizontal line. P < 0.05 was taken as statistically significant. FMO: fluorescence minus one.
Although MFI of SP in B cells increased by 45.1% (Fig. 3Ca and D), MFI of NK1R on B cells decreased by 10.3% in the blood of patients with AA (Fig. 3Cb and E).
Induction of altered expression of SP and NK1R in peripheral blood of patients with AR, AA, and ARA by allergens
In order to examine the influence of allergens on the expression of SP and NK1R in monocytes and B cells of airway allergic diseases, we investigated the effects of ASWE, HDME, and PPE allergens on SP and NK1R expression in the monocytes and B cells of patients with AR, AA, and ARA. The results showed that the allergens of ASWE, HDME, and PPE at the concentrations tested had little influence on the proportions of SP+ and NK1R+ monocytes from the patients with AR, AA, ARA, and HC subjects (Fig. 2). PPE at the concentrations of 0.1 and 1.0 µg/ml induced 46.3 and 52.5% increases in the proportion of SP+ B cells in the patients with ARA (Fig. 3A). HDME and PPE, both at the concentrations of 0.1 and 1.0 µg/ml, provoked 98.9 and 90.5%, and 40.1- and 1.3-fold greater proportions of NK1R+ B cells in the blood of patients with ARA (Fig. 3B).
In terms of MFI, ASWE at 1.0 µg/ml enhanced the expression of SP in monocytes by 27.8% in patients with AR. HDME at 0.1 and 1.0 µg/ml upregulated the expression of SP by 26.5 and 41.5%, and PPE at 0.1 and 1.0 µg/ml increased the expression of SP by 23.8 and 34.5% in the monocytes of patients with AR, respectively (Fig. 2D). HDME at 0.1 µg/ml also enhanced the expression of SP in monocytes by 65.7% in patients with AA (Fig. 2D). PPE at 0.1 and 1.0 µg/ml increased the expression of NK1R on monocytes by 34.4% in patients with ARA (Fig. 2E). Furthermore, HDME and PPE elevated the expression of SP in the B cells of AR patients, while HDME and ASWE augmented the expression of SP in the B cells of AA patients. Meanwhile, HDME and PPE at 0.1 µg/ml elevated the expression of SP by 75.6- and 1.7-fold in the B cells of patients with ARA, respectively (Fig. 3D).
Altered expressions of NK1R on blood monocytes and B cells in OVA-sensitized AR and AA mice
To further understand the expression of NK1R on monocytes and B cells in allergic conditions, we investigated the expression of NK1R on the monocytes and B cells of OVA-sensitized AR and AA mice by flow cytometry analysis.
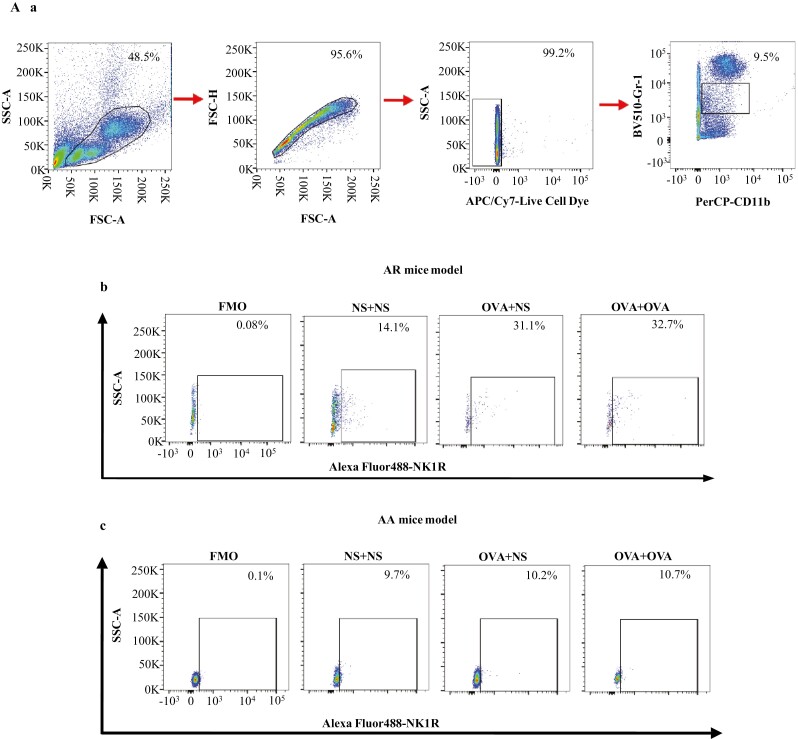
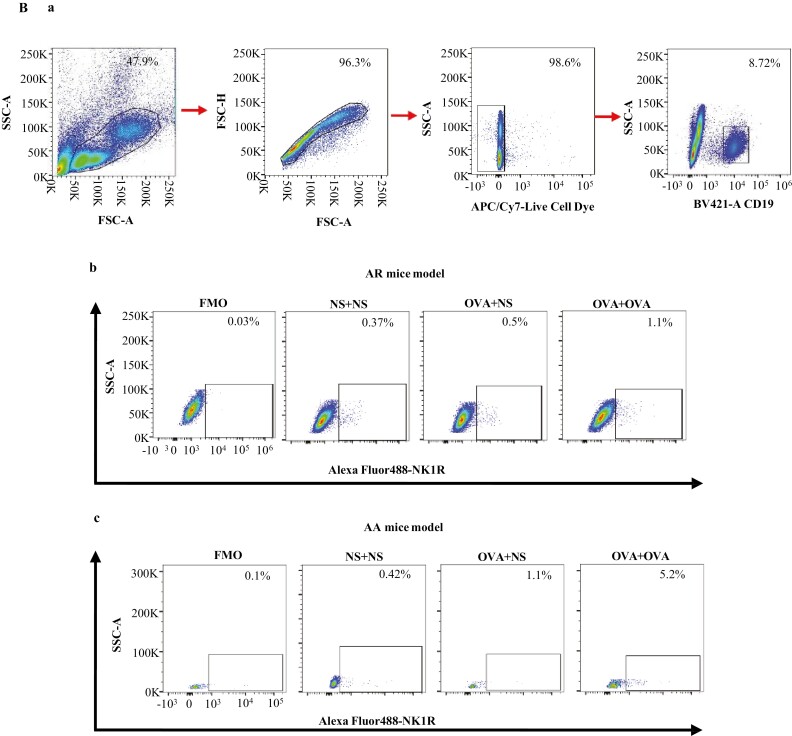
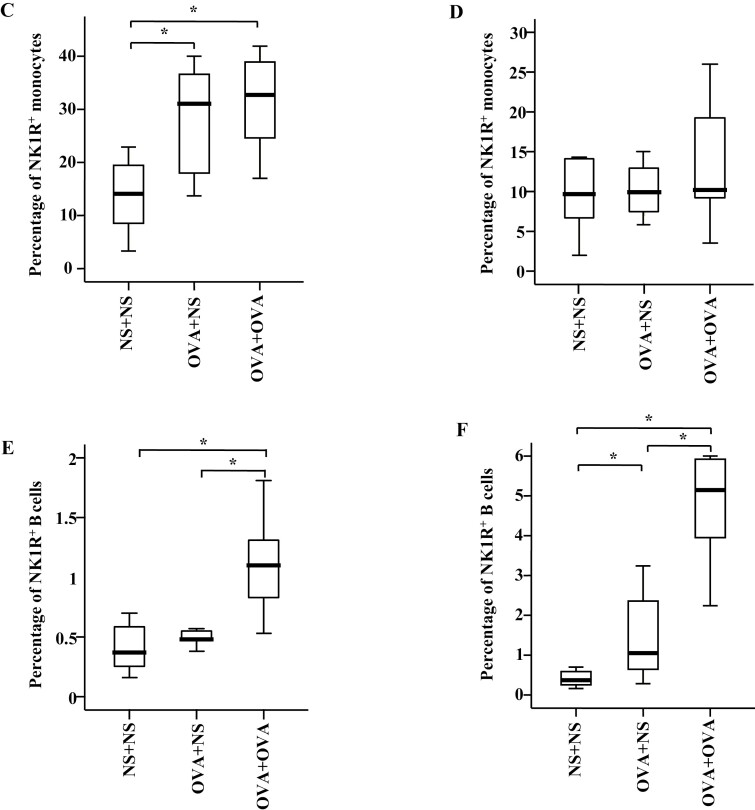
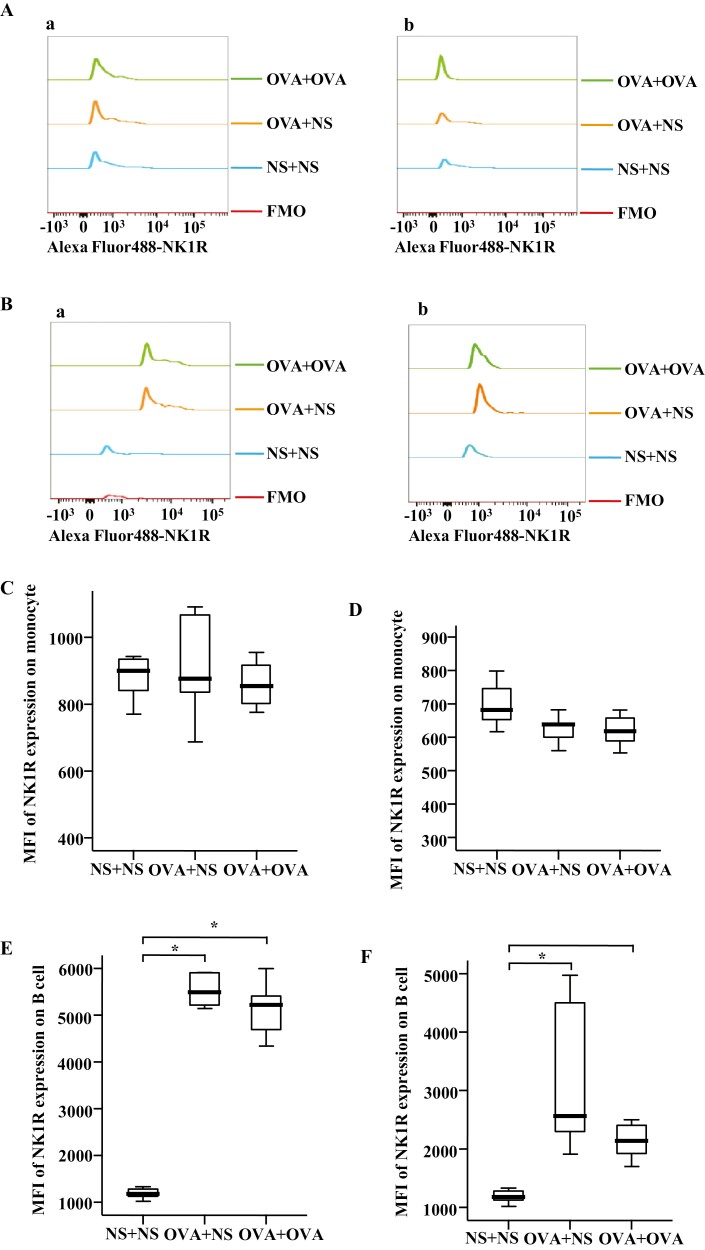
In the mouse AR model, the percentages of NK1R+ monocytes were elevated in the blood of OVA-OVA mice and OVA-NS mice compared with NS-NS mice (Fig. 4Ab and C). The percentages of NK1R+ B cells were enhanced in the blood of OVA-OVA mice compared with OVA-NS mice and NS-NS mice (Fig. 4Bb and E). MFI of NK1R expression on B cells was elevated in the blood of OVA-OVA mice of AR and OVA-NS mice of AR compared with NS-NS mice of AR (Fig. 5Ba and E).
Figure 4:
Flow cytometry analysis of the expression of NK1R on mouse monocytes and B cells. Monocytes were represented as CD11b+Ly-6C/G+ cells. B cells were represented as CD19+ cells. Mice were randomly selected into OVA-sensitized and OVA-challenged group (OVA + OVA), OVA-sensitized and normal saline (NS) challenged group (OVA + NS), and NS control group (NS + NS). (A) and (B) showed the gating strategies of monocytes and B cells in the blood of OVA-sensitized and non-sensitized mice, respectively. In both (A) and (B), (a) represented gating strategies of monocytes and B cells, while (b) and (c) represented the allergic rhinitis (AR) and allergic asthma (AA) models, respectively. (C) and (D) demonstrated changes in the percentages of NK1R+ monocytes of normal saline (NS) and OVA-sensitized AR and AA mice. (E) and (F) illustrated changes in the percentages of NK1R+ B cells of NS and OVA-sensitized AR and AA mice. A total of 7 animals were in each group. P < 0.05 was taken as statistically significant. FMO: fluorescence minus one.
Figure 5:
Mean fluorescence intensity (MFI) of NK1R expression on mouse monocytes and B cells. (A) and (B) showed the representative figures of MFI of NK1R expression on mouse monocytes and B cells, in which (a) and (b) represented the allergic rhinitis (AR) and the allergic asthma (AA) models, respectively. (C) and (D) demonstrated changes in MFI of NK1R on monocytes of normal saline (NS) and OVA-sensitized AR and AA mice. (E) and (F) illustrated changes in MFI of NK1R on B cells of NS and OVA-sensitized AR and AA mice. A total of 7 animals were in each group. P < 0.05 was taken as statistically significant.
In the mouse AA model, the percentages of NK1R+ B cells were enhanced in the blood of OVA-OVA mice and OVA-NS mice compared with NS-NS mice (Fig. 4Bc and F). The percentage of NK1R+ monocytes was also enhanced in the blood of OVA-OVA mice compared with OVA-NS mice (Fig. 4Ac and D). MFI of NK1R on B cells was elevated in the blood of OVA-OVA mice of AA and OVA-NS mice of AA compared with NS-NS mice of AA (Fig. 5Bb and F).
Expression of NK1R on monocytes and B cells of WT and FcεRI-KO AA mice
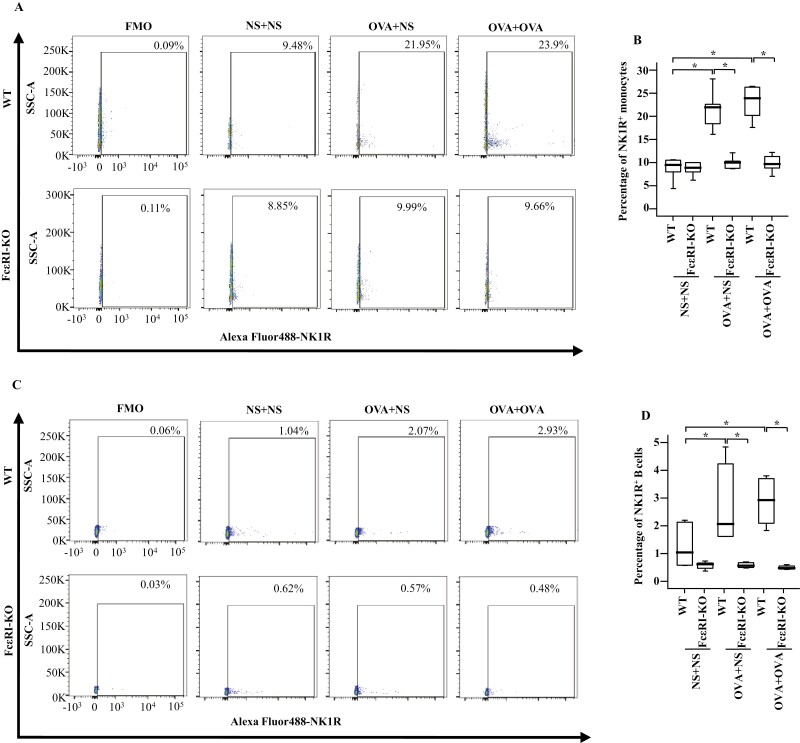
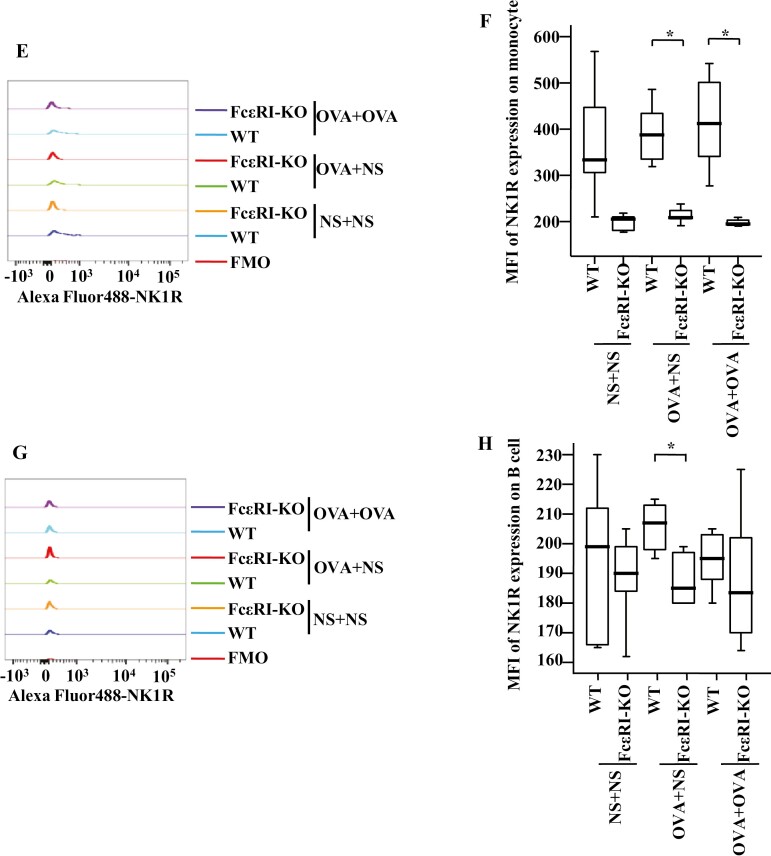
To examine the involvement of FcεRI in allergen-induced NK1R expression on monocytes and B cells in vivo, we studied the expression of NK1R on monocytes and B cells of OVA-induced mouse AA model using WT and FcεRI-KO mice. The results showed that OVA-induced enhancements of the proportions of NK1R+ monocytes (Fig. 6A and B) and B cells (Fig. 6C and D) in WT OVA-OVA mice and WT OVA-NS mice of AA were dramatically diminished in FcεRI-KO mice following the same treatment.
Figure 6:
Expressions of NK1R in monocytes and B cells in the blood of wild-type (WT) and FcεRI knockout (KO) AA mice. (A) and (C) showed representative graphs of the gating strategies of NK1R+ cells in monocytes and B cells. (B) and (D) demonstrated changes in the percentages of NK1R+ cells in the monocytes and B cells of WT and FcεRI KO mice. (E) and (G) showed the representative figures of MFI of NK1R expressions on mouse monocytes and B cells, respectively. (F) and (H) demonstrated changes in MFI of NK1R expressions on monocytes and B cells of WT and FcεRI-KO mice, respectively. A total of 7 animals were in each group. P < 0.05 was taken as statistically significant. FMO: fluorescence minus one.
Discussion
We clearly demonstrate that the expressions of SP and NK1R in the monocytes and B cells of patients with AA and ARA are increased in their peripheral blood, further suggesting that monocytes and B cells are involved in the pathogenesis of AA and ARA via a SP-NK1R-related mechanism. Since SP is a potent pro-inflammatory mediator, it is likely to contribute to the development of AA and ARA. Therefore, our study may be important for understanding the mechanism of AA and ARA. However, it appears that the increase of NK1R+ monocytes in the blood has only occurred in some AR patients, suggesting that AR has quite a different pathophysiological mechanism from the mechanism of AA and ARA in terms of expressions of SP and NK1R in monocytes and B cells. A report that plasma SP level is higher in patients with allergic airway diseases [19] may help clarify our current observations.
SP/NK1R complex has been recognized as an integral part of the microenvironment of inflammation and is involved in the molecular bases of many human pathologies [22]. For example, SP is reported to be associated with AR [12] and AA [14], monocytes can produce SP [16], enhanced expression of the NK1R has been observed in patients with AR [20], NK1R-mediated neurogenic inflammation may be involved in AA [21], activation of NK1R can mediate SP-induced migration of basophils [19], the number of B cells is significantly higher in patients with allergic disease than in nonallergic controls [10], and B cells are key players in AR [3] and AA [11] as they can produce allergen-specific IgE. Taking these findings and our current observation together, we anticipate that there must be enhanced SP/NK1R complexes in monocytes and B cells under allergic conditions in the airway, which are actively involved in the pathogenesis of AA, ARA, and, possibly, AR.
To the best of our knowledge, the current study is the first study that explores the effect of allergens on the expression of SP and NK1R in monocytes and B cells. Although the allergens of ASWE, HDME, and PPE had little influence on the proportions of SP+ and NK1R+ monocytes, ASWE, HDME, and PPE increased the expression density of SP molecules, as determined by MFI, in an individual monocyte of patients with AR. While HDME enhanced the expression of SP in the monocytes of patients with AA, PPE increased the expression of NK1R in the monocytes of patients with ARA. These results suggest that allergens can modulate SP and NK1R expression in monocytes in allergic airway disorders, particularly AR. HDME and PPE appear to enhance SP expression in the B cells of patients with ARA and AR, and NK1R expression in the B cells of the patients with ARA. HDME and ASWE augment the expression of SP in the B cells of AA patients. These observations implicate that aerosol allergens can alter SP and NK1R expression in the peripheral blood B cells of patients with allergic airway diseases. While direct evidence of allergens affecting the expression of SP and NK1R in monocytes and B cells is not available, the studies that show HDM [32] and pollen [33] are the most common allergens for allergic diseases such as AR and AA, and that the number of monocytes in nasal mucosa increases after continuous allergen stimulation in patients with AR [8] may help explain our current observations.
In mouse AR and AA models, the percentages of NK1R+ monocytes and B cells were elevated in blood following OVA sensitization and challenge, supporting the proposition that allergens can upregulate the expression of NK1R in monocytes and B cells of AR and AA. Therefore, it seems likely that monocytes and B cells constitutively express upregulated SP and NK1R in AA, the expression of SP and NK1R in AR will only upregulate after allergen induction. To our surprise, knocking out the FcεRI molecule, completely abolished OVA-induced upregulation of expression of NK1R in the monocytes and B cells of AA mice, suggesting that the action of OVA in AA mice is dependent on a FcεRI associated mechanism. A report demonstrating that the development of bronchial hyperresponsiveness was completely inhibited in OVA-sensitized mice treated with NK1R antagonist L-732138 [34] may help explain this observation.
In conclusion, upregulated expressions of SP and NK1R in monocytes and B cells may play a role in the pathogenesis of AA, ARA, and AR. Aerosol allergens can enhance SP and NK1R expressions in monocytes and B cells, which contribute to the development of these allergic airway disorders. A FcεRI associated mechanism may be involved in the OVA-induced upregulation of expression of NK1R in the monocytes and B cells of AA mice.
Glossary
Abbreviations
- AA
allergic asthma
- AR
allergic rhinitis
- ASWE
Allergens Artemisia sieversiana wild allergen extract
- HC
healthy control
- HDME
house dust mite extract
- IgE
immunoglobulin E
- KO
knockout
- MFI
mean fluorescence intensity
- NK1R
neurokinin-1 receptor
- OVA
ovalbumin
- PPE
Platanus pollen allergen extract
- SP
substance P
- WT
wild type
Contributor Information
Peixuan Han, Department of Pathophysiology, Translational Medicine Institute, Shenyang Medical College, Shenyang, Liaoning, China.
Liping Chen, Department of Respiratory Medicine, The Second Affiliated Hospital of Shenyang Medical College, Shenyang, Liaoning, China.
Dong Chen, Allergy and Clinical Immunology Research Centre, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China.
Ruiming Yang, Allergy and Clinical Immunology Research Centre, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China.
Wei Wang, Allergy and Clinical Immunology Research Centre, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China.
Jingyu Liu, Allergy and Clinical Immunology Research Centre, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China.
Shaoheng He, Department of Pathophysiology, Translational Medicine Institute, Shenyang Medical College, Shenyang, Liaoning, China; Allergy and Clinical Immunology Research Centre, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China.
Huiyun Zhang, Department of Pathophysiology, Translational Medicine Institute, Shenyang Medical College, Shenyang, Liaoning, China.
Funding
This project was sponsored by grants from the Shenyang Science and Technology Plan Project (19-109-4-17), Key Laboratory of Research on Pathogenesis of Allergen Provoked Allergic Disease, Liaoning Province (2018-30), the Climbing Scholar Project in Liaoning Province (2018-38), Innovation and Entrepreneurship Projects for High-Level Talent in Shenyang (2017CXCY-C-01), Science and Technology Innovation “double hundred project” in Shenyang (Z17-5-044), Leader Team Project in Jinzhou Medical University (2017–2020), and Liaoning Province Science and Technology Planning Project (2019JH8/10300053).
Conflict of interests
The authors declare that there are no conflicts of interest regarding the publication of this article.
Author contributions
P.H. carried out most of the experiments, generated the majority of the data, and wrote a large part of the first draft of the manuscript. R.Y. and W.W. performed the flow cytometry and challenge tests. L.C., D.C., and J.L. carried out the clinical study and participated in data analysis. S.H. and H.Z. designed and conducted the study, analyzed the data, and wrote the second and final drafts of the paper. All the authors contributed to the article, revised the manuscript, and approved the publication of this paper.
Ethical approval
The study has been approved by the Medical Ethical Committee of Shenyang Medical College and the Medical Ethical Committee of the First Affiliated Hospital of Jinzhou Medical University. Written informed consent was obtained from volunteers.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Kakli HA, Riley TD.. Allergic rhinitis. Prim Care 2016, 43, 465–75. doi: 10.1016/j.pop.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 2. Paiva Ferreira LKD, Aiva Ferreira LAM, Monteiro TM, Bezerra GC, Bernardo LR, Piuvezam MR.. Combined allergic rhinitis and asthma syndrome (CARAS). Int Immunopharmacol 2019, 74, 105718. doi: 10.1016/j.intimp.2019.105718. [DOI] [PubMed] [Google Scholar]
- 3. Eifan AO, Durham SR.. Pathogenesis of rhinitis. Clin Exp Allergy 2016, 46, 1139–51. doi: 10.1111/cea.12780. [DOI] [PubMed] [Google Scholar]
- 4. Koch S, Sopel N, Finotto S.. Th9 and other IL-9-producing cells in allergic asthma. Semin Immunopathol 2017, 39, 55–68. doi: 10.1007/s00281-016-0601-1. [DOI] [PubMed] [Google Scholar]
- 5. Lloyd CM, Saglani S.. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol 2013, 131, 1267–74; quiz 1275. doi: 10.1016/j.jaci.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 6. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012, 18, 673–83. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 7. Li HT, Chen ZG, Lin YS, Liu H, Ye J, Zou XL, et al. CpG-ODNs and budesonide act synergistically to improve allergic responses in combined allergic rhinitis and asthma syndrome induced by chronic exposure to ovalbumin by modulating the TSLP-DC-OX40L axis. Inflammation 2018, 41, 1304–20. doi: 10.1007/s10753-018-0779-6. [DOI] [PubMed] [Google Scholar]
- 8. Eguiluz-Gracia I, Bosco A, Dollner R, Melum GR, Lexberg MH, Jones AC, et al. Rapid recruitment of CD14+ monocytes in experimentally induced allergic rhinitis in human subjects. J Allergy Clin Immunol 2016, 137, 1872–1881.e12. doi: 10.1016/j.jaci.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 9. Moniuszko M, Kowal K, Jeznach M, Rusak M, Dabrowska M, Bodzenta-Lukaszyk A.. Phenotypic correlations between monocytes and CD4+ T cells in allergic patients. Int Arch Allergy Immunol 2013, 161, 131–41. doi: 10.1159/000343687. [DOI] [PubMed] [Google Scholar]
- 10. Heeringa JJ, Rijvers L, Arends NJ, Driessen GJ, Pasmans SG, van Dongen JJM, et al. IgE-expressing memory B cells and plasmablasts are increased in blood of children with asthma, food allergy, and atopic dermatitis. Allergy 2018, 73, 1331–6. doi: 10.1111/all.13421. [DOI] [PubMed] [Google Scholar]
- 11. De Vooght V, Carlier FC, Devos V, Haenen S, Verbeken E, Nemery B, et al. B-lymphocytes as key players in chemical-induced asthma. PLoS One 2013, 8, e83228. doi: 10.1371/journal.pone.0083228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chalastras T, Nicolopoulou-Stamati P, Patsouris E, Eleftheriadou A, Kandiloros D, Yiotakis I, et al. Expression of substance P, vasoactive intestinal peptide and heat shock protein 70 in nasal mucosal smears of patients with allergic rhinitis: investigation using a liquid-based method. J Laryngol Otol 2008, 122, 700–6. doi: 10.1017/S0022215107001454. [DOI] [PubMed] [Google Scholar]
- 13. Weinstock JV. Substance P and the regulation of inflammation in infections and inflammatory bowel disease. Acta Physiol 2015, 213, 453–61. doi: 10.1111/apha.12428. [DOI] [PubMed] [Google Scholar]
- 14. O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin F, Shanahan CP.. The role of substance P in inflammatory disease. J Cell Physiol 2004, 201, 167–80. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 15. Munoz M, Covenas R.. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–50. doi: 10.1007/s00726-014-1736-9. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Tian S, Douglas SD, Ho Z.. Morphine up-regulates expression of substance P and its receptor in human blood mononuclear phagocytes and lymphocytes. Cell Immunol 2000, 205, 120–7. doi: 10.1006/cimm.2000.1713. [DOI] [PubMed] [Google Scholar]
- 17. Lai JP, Cnaan A, Zhao H, Douglas SD.. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J Neurosci Methods 2008, 168, 127–33. doi: 10.1016/j.jneumeth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue J, Zhao H, An Y, Liang A, Zhao C.. [Expression of substance P receptor mRNA in nasal mucosa of rat in allergic rhinitis model]. Zhonghua Er Bi Yan Hou Ke Za Zhi 2000, 35, 247–50. [PubMed] [Google Scholar]
- 19. Cima K, Vogelsinger H, Kahler CM.. Sensory neuropeptides are potent chemoattractants for human basophils in vitro. Regul Pept 2010, 160, 42–8. doi: 10.1016/j.regpep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 20. Kaise T, Akamatsu Y, Ikemura T, Ohmori K, Ishii A, Karasawa A.. Involvement of neuropeptides in the allergic nasal obstruction in guinea pigs. Jpn J Pharmacol 2001, 86, 196–202. doi: 10.1254/jjp.86.196. [DOI] [PubMed] [Google Scholar]
- 21. Schuiling M, Zuidhof AB, Zaagsma J, Meurs H.. Involvement of tachykinin NK1 receptor in the development of allergen-induced airway hyperreactivity and airway inflammation in conscious, unrestrained guinea pigs. Am J Respir Crit Care Med 1999, 159, 423–30. doi: 10.1164/ajrccm.159.2.9804125. [DOI] [PubMed] [Google Scholar]
- 22. Rosso M, Munoz M, Berger M.. The role of neurokinin-1 receptor in the microenvironment of inflammation and cancer. ScientificWorldJournal 2012, 2012, 381434. doi: 10.1100/2012/381434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Z, Zhuang J, Zhao L, Gao X, Luo Z, Liu E, et al. Roles of bronchopulmonary C-fibers in airway hyperresponsiveness and airway remodeling induced by house dust mite. Respir Res 2017, 18, 199. doi: 10.1186/s12931-017-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abu-Rumeileh S, Halbgebauer S, Steinacker P, Anderl-Straub S, Polischi B, Ludolph AC, et al. CSF SerpinA1 in Creutzfeldt-Jakob disease and frontotemporal lobar degeneration. Ann Clin Transl Neurol 2020, 7, 191–9. doi: 10.1002/acn3.50980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dema B, Suzuki R, Rivera J.. Rethinking the role of immunoglobulin E and its high-affinity receptor: new insights into allergy and beyond. Int Arch Allergy Immunol 2014, 164, 271–9. doi: 10.1159/000365633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bousquet J, Schunemann HJ, Fonseca J, Samolinski B, Bachert C, Canonica GW, et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy 2015, 70, 1372–92. doi: 10.1111/all.12686. [DOI] [PubMed] [Google Scholar]
- 27. Von Mutius E. Presentation of new GINA guidelines for paediatrics. The Global Initiative on Asthma. Clin Exp Allergy 2000, 30, 6–10. doi: 10.1046/j.1365-2222.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 28. Mo JH, Kang EK, Quan SH, Rhee CS, Lee CH, Kim DY.. Anti-tumor necrosis factor-alpha treatment reduces allergic responses in an allergic rhinitis mouse model. Allergy 2011, 66, 279–86. doi: 10.1111/j.1398-9995.2010.02476.x. [DOI] [PubMed] [Google Scholar]
- 29. Pauwels RA, Brusselle GJ, Kips JC.. Cytokine manipulation in animal models of asthma. Am J Respir Crit Care Med 1997, 156, S78–81. doi: 10.1164/ajrccm.156.4.12-tac-1. [DOI] [PubMed] [Google Scholar]
- 30. Zheng W, Wang J, Zhu W, Xu C, He S.. Upregulated expression of substance P in basophils of the patients with chronic spontaneous urticaria: induction of histamine release and basophil accumulation by substance P. Cell Biol Toxicol 2016, 32, 217–28. doi: 10.1007/s10565-016-9330-4. [DOI] [PubMed] [Google Scholar]
- 31. Tang H, Li T, Han X, Sun J.. TLR4 antagonist ameliorates combined allergic rhinitis and asthma syndrome (CARAS) by reducing inflammatory monocytes infiltration in mice model. Int Immunopharmacol 2019, 73, 254–60. doi: 10.1016/j.intimp.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 32. Okamoto Y, Fujieda S, Okano M, Yoshida Y, Kakudo S, Masuyama K.. House dust mite sublingual tablet is effective and safe in patients with allergic rhinitis. Allergy 2017, 72, 435–43. doi: 10.1111/all.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mortuaire G, Michel J, Papon JF, Malard O, Ebbo D, Crampette L, et al. Specific immunotherapy in allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis 2017, 134, 253–8. doi: 10.1016/j.anorl.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 34. Garcia-Recio S, Gascon P.. Biological and pharmacological aspects of the NK1-receptor. Biomed Res Int 2015, 2015, 495704. doi: 10.1155/2015/495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.