Abstract
Psoriasis—an immune-mediated skin disease—implicates in its pathophysiology by circulating pro-inflammatory cell populations, cytokines, and their interactions with the epidermis. The direct effect of approved anti-interleukin- (IL-)17A and anti-IL-17R biologic therapy on immunophenotyping of peripheral blood mononuclear lymphocytes’ (PBMCs) relative sub-population frequencies in psoriasis patients has not yet been described. Using multiparameter flow cytometry we examined T-cell subpopulations characterized by CCR6, CCR4, and CXCR3 chemokine receptor surface expression at baseline and after initiation of biologic therapy in PBMCs collected from 30 psoriasis patients. Increased CD3+CD4+CXCR3+, CD3+CD4+CCR6+CCR4+CXCR3+(CXCR3+-Th17), and CD3+CD4+CCR6+CCR4-CXCR3+(Th17.1) cell populations were observed in patients with psoriasis in comparison to healthy individuals (n = 10). IL-17 therapeutic blockade decreased CD3+CD4+CCR6+, CD3+CD4+CXCR3+, CD3+CD4+CCR6-CXCR3+(Th1), CD3+CD4+CCR6+CCR4+(Th17), CD3+CD4+CCR6+CCR4+CXCR3+(CXCR3+-Th17), and CD3+CD4+CCR6+CCR4-CXCR3+(Th17.1) cell populations in responding psoriasis patients. Moreover, CD3+CD4-CCR6+, CD3+CD4-CXCR3+, CD3+CD4-CCR6+CCR4+(Tc17), and CD3+CD4-CCR6-CXCR3+(Tc1) percentages were also inhibited. Modulation of the same cell sub-populations was also assessed in patients treated with methotrexate (n = 4), apremilast (n = 4), and anti-IL-23 biologic treatment (n = 4). In our study, the levels and functional capacity of peripheral pro-inflammatory Th1, Th17, and additional CCR6+T cell sub-gated populations from psoriasis patients that were treated with anti-IL-17 or anti-IL-17R targeted biologic therapy were explored for the first time. Our data clearly demonstrate that early anti-IL-17 mediated clinical remission is accompanied by a significant decrease of Th1, Th17, CXCR3+-Th17, and Th17.1 cells.
Keywords: anti-IL-17, IL-17, inflammation, psoriasis, T cells
Psoriasis is induced by circulating pro-inflammatory cell populations and the IL-23/Th17 pathway has been arguably recognized as a crucial component for the development of the disease. In psoriasis patients receiving biologic IL-17 therapeutic blockade circulating Th1, Th17 and sub-populations as CXCR3+-Th17 and Th17.1 were decreased as early as 3 months after treatment initiation. Early anti-IL17 mediated clinical remission is accompanied by a significant decrease of specific circulating pro-inflammatory cell populations in therapy-responding patients.
Graphical Abstract
Graphical Abstract.
Introduction
Psoriasis is an immune-mediated skin disease. Psoriasis vulgaris is identified by plaque-type clinical manifestations of the skin [1]. The pathogenesis of the disorder implicates various circulating pro-inflammatory cell populations, cytokines, and their interactions with epidermis, which in term promote keratinocyte hyperproliferation and dysfunctional differentiation [2, 3]. Although, the exact mechanism underlying chronic inflammation is not completely understood, immune dysregulation involving the predominance of effector cell populations has been considered of paramount importance for the pathological processes that eventually contribute to the pathogenesis of the disease [4].
In the early stages, immuno-mediated destruction is promoted by IFNa producing plasmacytoid dendritic cells, which favor skewing of myeloid dendritic cells (mDCs) towards a pro-inflammatory DC phenotype, which expresses both tumor necrosis factor (TNF) and interleukin (IL)-23. Such skewing induces T-cell differentiation toward T helper (Th) 17 and T cytotoxic (Tc) 17 subsets. Moreover, T-cell production of IFNγ has been demonstrated to be involved in the pathogenic cascade of the disease. Furthermore, the IL-23/IL-17 inflammation pathway promotes keratinocyte activation and recruitment of T effector subsets. For example, keratinocyte-produced C–C motif chemokine ligand (CCL−) 20 induces migration of C–C motif chemokine receptor (CCR−) 6 expressing, IL-17 producing T cells in the epidermis, while expression of C–X–C motif chemokine ligand (CXCL-) 9 and CXCL10 among other chemokines by keratinocytes, promotes C–X–C motif chemokine receptor (CXCR-) 3 producing T-cell accumulation. The chemokine-attracted T populations in the skin further produce IL-17, establishing a loop that enhances IL-17 signaling. Activation of dendritic cells, pro-inflammatory cytokine production, T cell subset differentiation, and accumulation in epidermis and chemokine production by triggered keratinocytes concomitantly induce the development of psoriatic skin lesion.
While it may be well established that Th17 cells are of paramount importance for the elicitation of psoriasis, a variety of studies demonstrates how not all Th17 cells are participating equally in inflicting damage [5, 6]. The combined production of both CCR6 and CCR4 surface markers characterizes IL-17 producing CD4+ T cells selectively (Th17 cells), whereas co-expression of both chemokine receptors CCR6 and CXCR3 identifies a heterogenous population of cells that produce IL-17 and IFNγ [7]. This IFNγ producing CCR6+CXCR3+CCR4− (Th17.1 or exTh17) population has been reported to be elevated in bronchoalveolar lavage in sarcoidosis patients [8], to contribute to disease activity in patients with multiple sclerosis [9] and finally predict the response of abatacept treatment in rheumatoid arthritis patients [10]. Other CD4+CCR6+ subpopulations include double positive CCR4+CXCR3+ cells (CXCR3+-Th17), and double-negative CCR4−CXCR3− cells (DN) [11]. Differences in cell surface phenotype characterization within the CD3+CD4+CCR6+ compartment, as shaped by discrete expression of CCR4 or/and CXCR3 markers identify various sub-populations with different immunological behaviors. A meticulous analysis of such cell populations, including their behavior following IL-17 targeted biologic treatment approved for the management of psoriatic patients is needed but to our knowledge has not yet been conducted.
While it is established that various alterations both in the number and the function of T-cell populations that differ between different disease severity phenotypes promote the pathogenesis of psoriasis, only a few studies explore the effect of approved biologic disease-modifying anti-rheumatic drugs (bDMARDs) on immunophenotyping of peripheral blood lymphocytes. On that basis, using flow cytometry we aimed to compare psoriasis-characteristic phenotypes of peripheral T helper and cytotoxic cell populations between 30 patients with psoriasis and 10 healthy controls. To witness not only the alterations of such populations but also the relevance of cell-specific reshaping to treatment’s response, we studied psoriasis patients before and after secukinumab (a human IgG1k monoclonal antibody (mAb) that selectively binds to IL-17A) or brodalumab (a human IgG2 anti-IL-17 receptor A mAb) treatment.
Patients and methods
Patients and healthy controls
Participants for this study were patients diagnosed with moderate to severe [Psoriasis Area and Severity Index (PASI) > 7] psoriasis vulgaris (n = 30) attending the outpatient clinic of the Department of Dermatology, University General Hospital of Larissa. Our study sample exploring anti-IL-17 therapeutic blockade was composed of 17 males and 13 females with a median age of 52.5 years (interquartile range of 46–63 years). Patients reported onset of the disease at a mean age of 40 years (SD = 12 years). Clinical parameters and patient characteristics are summarized in Table 1. All patients received either secukinumab (n = 18) or brodalumab (n = 12). Ten age- and sex-matched healthy controls (6 males, 4 females), with a mean age of 49 years were also included in the study. Another cohort of 12 psoriasis patients (8 males and 4 females with a mean age of 49 years) treated with methotrexate (n = 4), apremilast (n = 4), and anti-IL-23 (n = 4) treatment was studied to assess their effect in cell subset changes. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University General Hospital of Larissa, University of Thessaly (ECA #19/14-11-2019). Written informed consent was obtained from all subjects involved in the study. Psoriasis disease activity or response to biologic therapy was assessed by PASI score and PASI score improvement, respectively, with a ≥75% improvement accounting for response and a <75% improvement for no response.
Table 1:
Major demographic and clinical parameters of 30 patients with psoriasis
| Patient | Gender | Age | Age of disease’s Onset | Previous Treatment* | Washout Period (months) | Current Treatment | Response to treatment | |
|---|---|---|---|---|---|---|---|---|
| Baseline PASI | 3 months PASI |
|||||||
| 1 | M | 51 | 48 | cDMARDS, apremilast | 6 | Secukinumab | 21.6 | 0 |
| 2 | M | 66 | 46 | Naïve | n/a | Secukinumab | 21 | 0.5 |
| 3 | M | 53 | 29 | Cyclosporine, retinoids | n/a | Secukinumab | 20 | 0 |
| 4 | M | 53 | 41 | Naïve | n/a | Secukinumab | 11.9 | 0 |
| 5 | M | 65 | 55 | Cyclosporine, retinoids | n/a | Brodalumab | 18.5 | 1.4 |
| 6 | F | 38 | 31 | Naïve | n/a | Brodalumab | 10 | 0 |
| 7 | M | 52 | 48 | Etanercept | 34 | Brodalumab | 12.8 | 1.7 |
| 8 | M | 70 | 60 | Ustekinumab, secukinumab, apremilast | 1 | Brodalumab | 10 | 20 |
| 9 | F | 37 | 26 | cDMARDS | n/a | Brodalumab | 8.1 | 0.5 |
| 10 | M | 49 | 43 | Retinoids, cDMARDS | n/a | Secukinumab | 11.6 | 1.5 |
| 11 | M | 64 | 25 | Naïve | n/a | Brodalumab | 25.5 | 2.3 |
| 12 | M | 67 | 60 | Apremilast | 0 | Brodalumab | 30 | 2.8 |
| 13 | M | 67 | 53 | Adalimumab, cDMARDS, etanercept, cyclosporine, apremilast | 1 | Secukinumab | 10.4 | 0 |
| 14 | M | 44 | 37 | cDMARDS, cyclosporine, etanercept, ustekinumab | 1 | Secukinumab | 14.1 | 1.2 |
| 15 | M | 45 | 42 | cDMARDS, adalimumab | 3 | Secukinumab | 13.2 | 0 |
| 16 | F | 63 | 19 | cDMARDS, cyclosporine, etanercept, ustekinumab | 0 | Secukinumab | 11.9 | 0.5 |
| 17 | F | 57 | 51 | cDMARDS, cyclosporine, retinoids, ustekinumab | 0 | Secukinumab | 21.2 | 1.8 |
| 18 | F | 27 | 18 | Naïve | n/a | Secukinumab | 17 | 1.2 |
| 19 | M | 61 | 32 | cDMARDS, cyclosporine | n/a | Secukinumab | 8.5 | 0 |
| 20 | M | 41 | 27 | ustekinumab, adalimumab | 13 | Brodalumab | 7.8 | 0 |
| 21 | M | 50 | 34 | cDMARDS | n/a | Secukinumab | 10.9 | 0.4 |
| 22 | F | 49 | 36 | cDMARDS, etanercept, ustekinumab | 0 | Brodalumab | 8.5 | 2.8 |
| 23 | F | 52 | 43 | Retinoids, cDMARDs, ustekinumab, secukinumab | 1 | Brodalumab | 13.5 | 4.6 |
| 24 | F | 39 | 26 | cDMARDS | n/a | Brodalumab | 18 | 4.1 |
| 25 | F | 62 | 29 | cDMARDS | n/a | Secukinumab | 8 | 1.8 |
| 26 | F | 54 | 48 | Naïve | n/a | Secukinumab | 26.6 | 2.2 |
| 27 | F | 48 | 38 | cDMARDS, etanercept | 1 | Secukinumab | 13.5 | 1.2 |
| 28 | F | 57 | 35 | ustekinumab | 5 | Brodalumab | 7.2 | 0 |
| 29 | F | 66 | 46 | cDMARDS, adalimumab | 0 | Secukinumab | 20.5 | 16.2 |
| 30 | M | 46 | 60 | cDMARDS, etanercept, ustekinumab | 1 | Secukinumab | 11 | 10.5 |
Treatment in chronological order.
Abbreviation: PASI, Psoriasis Area and Severity Index; F, female; M, male; naive patient naïve to biologic treatment; cDMARDs conventional disease-modifying anti-rheumatic drugs; washout period months since last biologic or apremilast therapy; n/a patient has not received prior biologic or apremilast treatment.s
Peripheral blood samples (up to 30 ml) were collected from registered participants and layered on the surface of a LymphoPrep gradient (Axis-Shield, Oslo, Norway). Peripheral blood mononuclear cells (PBMCs) were isolated following a protocol including centrifugation and were then washed twice with serum free RPMI 1640 (Pan Biotech). Cell counting was accomplished with a Neubauer hemocytometer and cell viability was determined by trypan blue exclusion, consistently exceeding 95%. PBMCs were then resuspended in cell cryoprotective solution containing 10% dimethyl sulfoxide (DMSO) and 90% fetal calf serum (FCS), aliquoted into cryotubes (Corning, Thermo Fisher Scientific), placed at −80°C for one day, and then stored in liquid nitrogen containers until used.
Phenotypic analysis of PBMCs by multiparameter flow cytometry
Phenotypic assessment of T cells was performed as previously described [12] using the following anti-human mAbs: fluorescein isothiocyanate (FITC)-conjugated anti-CXCR3 (clone G025H7), phycoerythrin (PE)-conjugated anti-CCR6 (clone G034E3), peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (clone SK7), allophycocyanin (APC)-conjugated anti-CCR4 (clone L291H4), and PE-Cy7 conjugated anti-CD4 (clone RPA-T4) obtained by BioLegend, San Diego, USA. Isolated PBMCs (0.5–1 × 106 cells) after being washed in phosphate-buffered saline (PBS) were resuspended in staining buffer (containing PBS and 2% FCS), subsequently incubated with labeled mAbs for 30 min on ice, and then fixed with 2% paraformaldehyde solution. Flow cytometry analysis was performed using a Guava EasyCyte 8 (Merck-Millipore, Burlington, USA) cytometer with logarithmic amplification and a forward side light scatter-based gate for total lymphocyte population was applied. To ensure accurate measurement of infrequent T-cell subsets, at least 3 × 105 events were collected within the lymphocyte gate for each sample. Based on isotype control mAbs false-positive and high background readouts were excluded. Results reproducibility was assessed by performing experimental testing at least in duplicate. Data acquisition was achieved using BD Bioscience CellQuest software and data were analyzed with FlowJo, version 10.0.7 (BD Biosciences).
Detection of intracellular IFNγ and IL-17 expression by T cells
To measure intracellular IFNγ and IL-17A production by T-cell subsets, cells were either left untreated or stimulated with PMA (50 ng/ml) plus ionomycin (1 μg/ml) in the presence of Brefeldin A (10 μg/ml) in RPMI medium containing 10% FCS for 6 h, as previously described [13]. Activated PBMCs were surface stained using FITC-conjugated anti-CXCR3 (clone G025H7), PE-conjugated anti-CCR6 (clone G034E3), APC-conjugated anti-CCR4 (clone L291H4) and PE-Cy7-conjugated anti-CD4 (clone RPA-T4) mAbs, fixed and then permeabilized using commercially available Perm Wash Buffer (BioLegend, San Diego, USA). IFNγ and IL-17A were detected using APC-Cy7-conjugated anti-IFNγ (clone 4S.B3) and PerCP-conjugated anti-IL-17A (clone BL168) mAbs, respectively (obtained by BioLegend, San Diego, USA).
Magnetic cell isolation
Peripheral blood CD4+ T lymphocytes were purified by either positive or negative magnetic cell selection using CD4 mAb coated microbeads and a CD4-T cell negative isolation kit, respectively, as previously described. Every cell sorting procedure was performed according to manufacturer’s instructions and yielded >90% pure CD4+ T lymphocytes, as consistently evaluated by fluorescence-activated cell sorter (FACS) staining.
Statistical analysis
Descriptive analysis of our data (counts, means, or medians whenever appropriate) was used to describe the characteristics of the study sample. Percentages of cells expressing cell surface epitopes and MFI values were presented as mean ± SD or median and interquartile range (IQR) whenever appropriate for each group. Differences between the control and patient group, and between different time points in the patient group were assessed, once data were checked for normality by Shapiro–Wilk test, performing the indicated test. Differences between groups are reported by mean values and 95% CI or median values of difference, depending on the type of comparison (parametric or non-parametric test). Simple linear regression was used to assess the effect of previous treatment received on cell inhibition. P ≤ 0.05 was considered significant. Statistical calculations were performed using GraphPad Prism, version 9 software.
Results
In total, 25 of the 30 psoriasis patients achieved a ≥75% improvement in PASI score at 3 months after secukinumab or brodalumab initiation. Of the five non-responding patients, four achieved a therapy-mediated decrease (<75%) in PASI score. In one patient PASI score was further increased 3 months after therapy initiation. Significant differences in routine laboratory lymphocyte analysis at baseline and at 3 months were not observed (Supplementary Table S1).
Increased percentages of CD3+CD4+CXCR3+ T cells, and CXCR3 expression among CD3+CD4+CCR6+CCR4− and CD3+CD4+CCR6+CCR4+ lymphocyte subsets in psoriasis patients compared to healthy controls
The gating strategy of T lymphocyte subsets used for T-cell phenotyping is shown in Supplementary Fig. S1. Supplementary Fig. S1A shows representative flow cytometry plots revealing subdividing strategy for lymphocytes, CD4+ T cells, CD4+CCR6+ cells, CD4+CCR4+ cells, CD4+CXCR3+ cells, CD3+CD4+CCR6+CCR4+(Th17), CD3+CD4+CCR6+CCR4+CXCR3+(CXCR3+-Th17) and CD3+CD4+CCR6+CCR4+CXCR3− (CXCR3−-Th17) cells, CD3+CD4+CCR6+CCR4-CXCR3+(Th17.1) and CD3+CD4+CCR6+CCR4-CXCR3− (DN) cells. CD3+CD4-CCR6+CCR4+(Tc17) cells were sub-gated accordingly. Supplementary Table S2 summarizes the phenotyping characterization of cell subsets and their matching names.
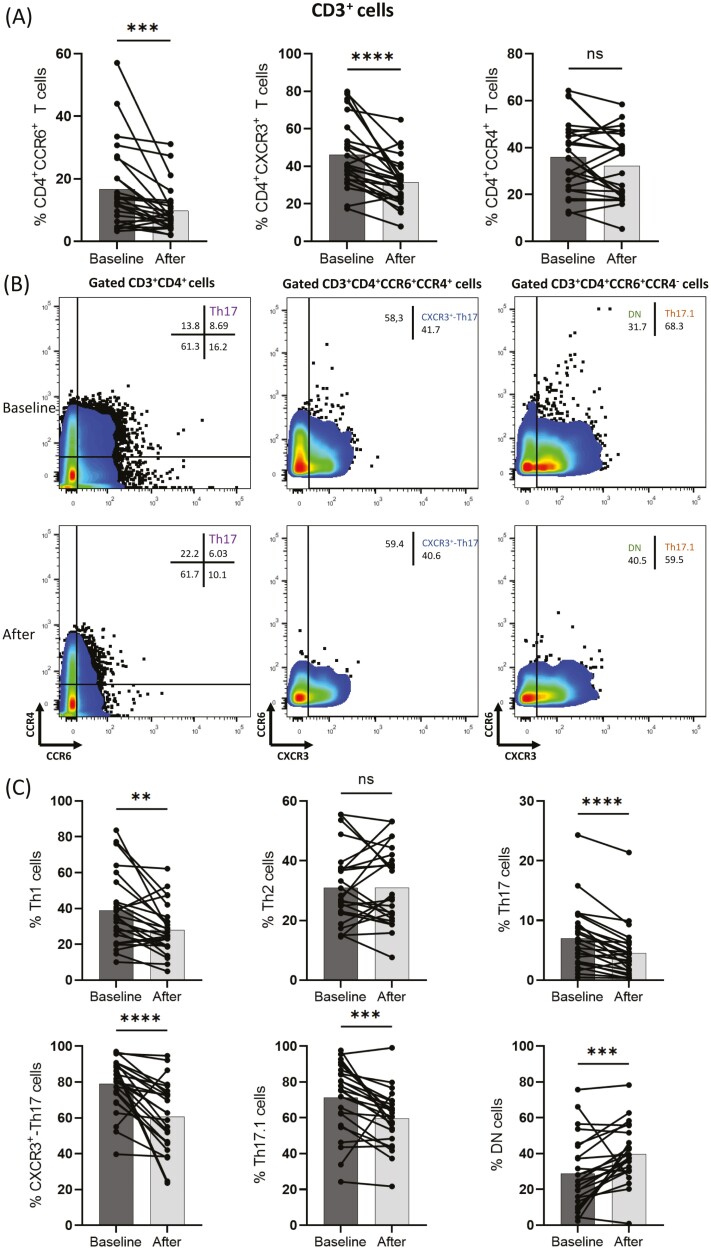
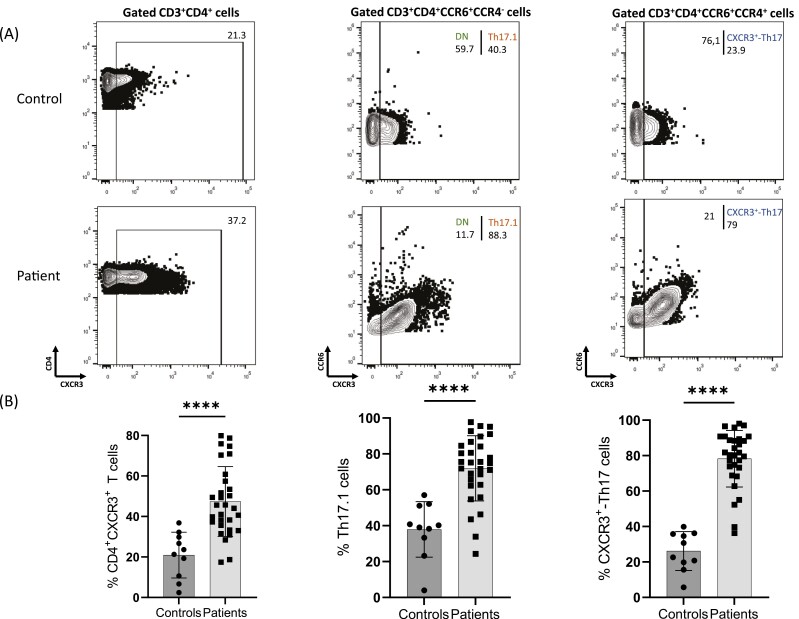
Figure 1A shows representative flow cytometry plots of CD4+CXCR3+ T population, Th17.1 and CXCR3+-Th17 subsets from a healthy control and a psoriatic patient. Analysis of PBMC flow cytometry at baseline shows an increase (mean = 26.5, 95% CI 14.6–38.3) in the frequency of CD4+CXCR3+ T cell subset in patients (mean = 47.4, SD = 17.2) in comparison to healthy controls (mean = 20.9, SD = 11.3), P < 0.0001. Th17.1 cell percentage is increased (mean = 34.1, 95% CI 21.1–47) in patients (mean = 71.9, SD = 18.2) compared to healthy individuals (mean = 37.9, SD = 15.4), P < 0.0001 and CXCR3+-Th17 cell percentage is also increased (difference between medians 54.4) in psoriasis patients (mean = 78.3, SD = 16) compared to healthy controls (mean = 26.2, SD = 11), P < 0.0001 (Fig. 1B).
Figure 1:
Analysis based on flow cytometry plots from peripheral blood mononuclear cells (PBMCs) from healthy controls (n = 10), and psoriasis patients (n = 30) was performed. Individual cell subsets were sub-gated according to the expression of CD3, CD4, CCR6, CCR4, and CXCR3 surface markers. (A) Representative flow cytometric plots show increased frequencies of Th17.1 and CXCR3+-Th17 populations in psoriatic patients compared to healthy controls. (B) Box graphical representation showing different frequencies of CD3+CD4+CXCR3+, Th17.1, and CXCR3+-Th17 between patients with psoriasis and healthy controls. Percentages out of origin population from which each population is further sub-gated, as described in Supplementary Fig. S1. Bar graphs showing the mean ± SD. ****P ≤ 0.0001 by Mann–Whitney test or unpaired t-test.
IL-17A therapeutic blockade decreased CD3+CD4+CCR6+ and CD3+CD4+CXCR3+ T-cell sub-populations in psoriasis patients
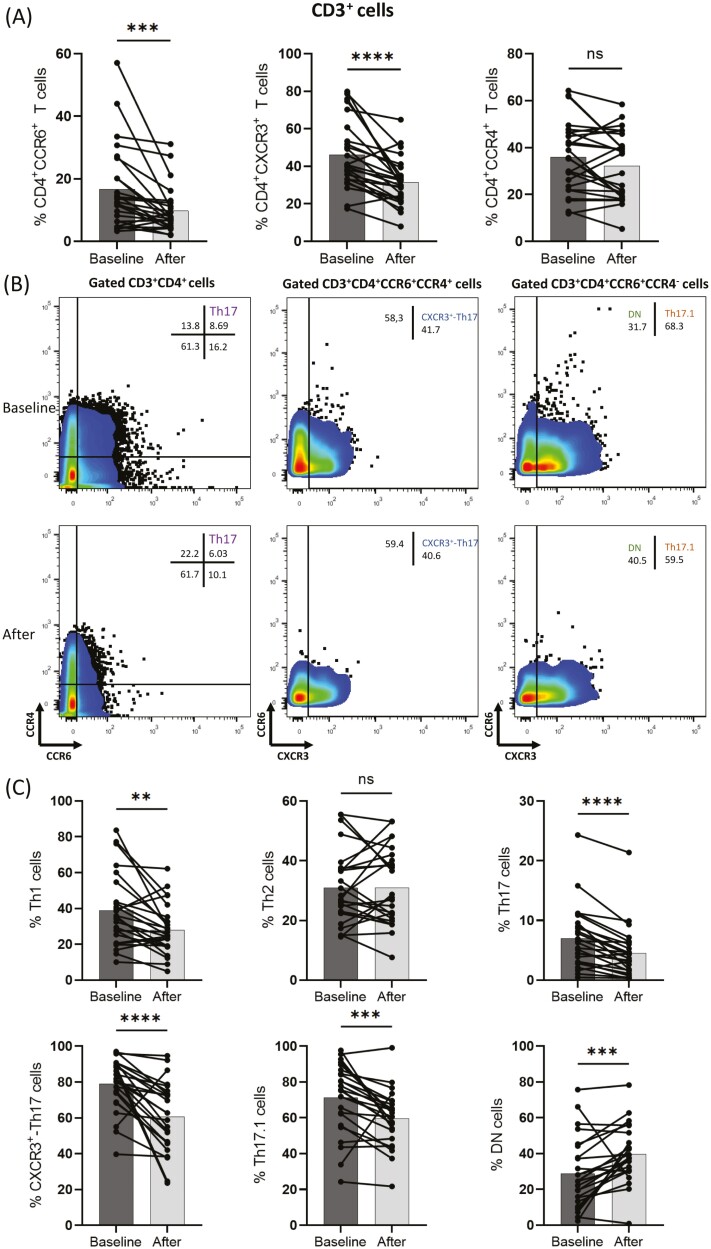
As shown in Fig. 2A, in responding patients secukinumab and brodalumab treatment decreased (median of difference −3.3) the percentage of CD3+CD4+CCR6+ population after treatment (mean = 9.8, SD = 7.3) compared to baseline (mean = 16.7, SD = 13.4), P = 0.0001. The percentage of CD3+CD4+CXCR3+ T cells was also significantly decreased (median of differences −10.20) in responding patients at 3 months of intervention (mean = 31.6, SD = 12.6) compared to baseline (mean = 46.2, SD = 18.1), P < 0.0001. CD3+CD4+CCR4+cells did not differ significantly between before and after treatment.
Figure 2:
Analysis based on flow cytometry plots from peripheral blood mononuclear cells (PBMCs) from responding to therapy psoriasis patients (n = 25) was performed. Individual cell subsets were sub-gated based on the expression of CD3, CD4, CCR6, CCR4, and CXCR3 surface markers. (A) Changes in frequency of CD3+CD4+CCR6+T cells, CD3+CD4+CXCR3+T cells, and CD3+CD4+CCR4+T cells in peripheral blood at baseline and after anti-IL-17 biologic therapy. The percentage out of CD4 population is displayed. (B) Representative flow cytometric plots showing changes in T-cell phenotypes in a psoriasis patient at baseline and after anti-IL17 biologic therapy. (C) Box graphical representation showing a significant decrease in Th1, Th17, CXCR3+-Th17, and Th17.1 cell subsets after anti-IL17 biologic therapy and an increase in DN cells in peripheral blood from psoriasis patients. Percentages out of origin population from which each population is further sub-gated, as described in Fig. 1. Bar graphs showing the mean ± SD. ns P > 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 by Wilcoxon signed rank test or paired t-test.
IL-17 therapeutic blockade decreased Th1, Th17 cell populations and expression of CXCR3 among CD4+CCR6+CCR4+ and CD4+CCR6+CCR4− cell subsets in psoriasis patients
Supplementary Fig. S1B shows representative flow cytometry plots revealing subdividing strategy for CD3+CD4+CCR6−CXCR3+(Th1) and CD3+CD4+CCR6-CXCR3−CCR4+(Th2). CD3+CD4−CCR6−CXCR3+(Tc1) and CD3+CD4-CCR6-CXCR3−CCR4+(Tc2) cells were gated accordingly.
Figure 2B shows representative flow cytometry plots of Th17, CXCR3+-Th17, and Th17.1 cells from a psoriasis patient at baseline and after 3 months of biologic treatment. In general, the percentage of Th17, CXCR3+-Th17, Th17.1, and Th1 was significantly decreased in responding to therapy psoriasis patients following treatment compared to baseline. More specifically, the initial Th17 cell percentage mean = 7 (SD = 5.2) decreased (mean = −2.4, 95% CI −3.3 to −1.5) after therapy to mean = 4.6 (SD = 4.4), P < 0.0001, the initial CXCR3+-Th17 cell percentage mean = 78.9 (SD = 14.6) also decreased (median of difference −17.5) to mean = 60.6 (SD = 19.6), P < 0.001 and the initial Th17.1 cell percentage mean = 71.2 (SD = 19.4) decreased (median of difference −11.3) to mean = 59.7 (SD = 15.5), P = 0.002 after intervention, whereas the percentage of Th2 cells remained largely unchanged (Fig. 2C).
IL-17 therapeutic blockade decreased CD3+CD4−CCR6+, CD3+CD4−CXCR3+, Tc1, and Tc17 cell populations in psoriasis patients
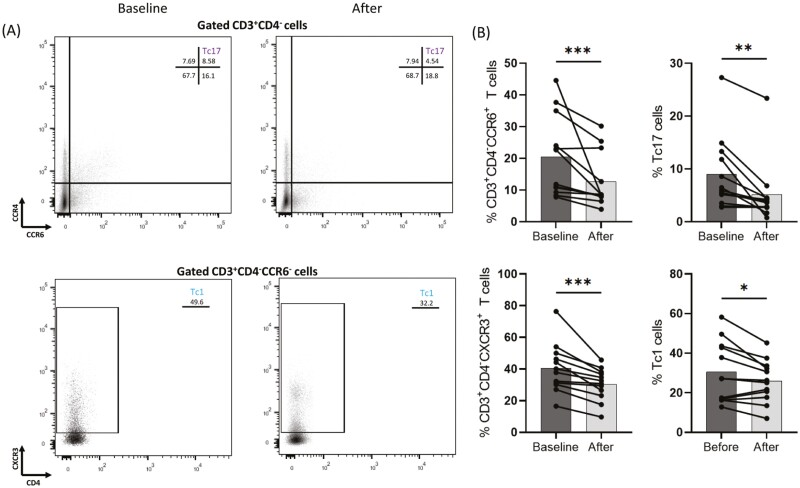
Figure 3A shows representative flow cytometry plots of Tc17 and Tc1 cells from a psoriasis patient at baseline and after 3 months of anti-IL-17 biologic treatment. As shown in Fig. 3B anti-IL17A and anti-IL17R biologics decreased (median of difference −3.9) the percentage of CD3+CD4-CCR6+T cells after therapy (mean = 12.7, SD = 8.6) compared to baseline (mean = 20.5, SD = 12.8), P = 0.001. The percentage of CD3+CD4−CXCR3+T cells was also significantly decreased (median of difference −8.1) in patients after biologic intervention (mean = 30.5, SD = 10.1) compared to baseline (mean = 40.5, SD = 15.5), P = 0.0005. Moreover, biologic therapy decreased (mean = −4.6, 95% CI −8.8 to −0.4) the percentage of Tc1 subset from mean = 30.5 (SD = 15.4) to mean = 25.9 (SD = 10.7), P = 0.0341 and the percentage of Tc17 subset was also inhibited (mean = −3.9, 95% CI −6.2 to −1.5) from mean = 9 (SD = 7) before therapy to mean = 5.1 (SD = 6) at 3 months after treatment initiation, P = 0.0038.
Figure 3:
Analysis of CD3+CD4− sub-population based on flow cytometry plots from peripheral blood mononuclear cells (PBMCs) from responding psoriasis patients was conducted (n = 12). Individual cell subsets were sub-gated based on the expression of CD3, CD4, CCR6, CCR4, and CXCR3 surface markers. (A) Representative flow cytometric plots showing changes in T-cell phenotypes in a psoriasis patient at baseline and after anti-IL17 biologic therapy. (B) Box graphical representation showing a significant decrease in CD3+CD4−CCR6+T, CD3+CD4-CCR6+CCR4+(Tc17), CD3+CD4−CXCR3+T, and CD3+CD4−CCR6-CXCR3+(Tc1) cell subsets after anti-IL17 biologic therapy in peripheral blood from psoriasis patients. Percentages out of origin population from which each population is further sub-gated. Bar graphs showing the mean ± SD. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 by Wilcoxon signed rank test or paired t-test.
Effect of previous treatments on cell-subset changes compared to anti-IL17A initiation
We also assessed whether any treatment initiated before anti-IL17A or anti-IL-17R biologic treatment was associated with changes in the percentages of cell subset populations. The analysis included baseline and 3-month results of patients (n = 25) subdivided into five groups according to previous treatment: naïve (n = 6), on csDMARDs (n = 8), apremilast (n = 3), etanercept, or adalimumab (anti-TNF) (n = 4), and patients who were treated with ustekinumab (n = 4) before secukinumab or brodalumab initiation. For Th17 and each cell sub-population (CXCR3+-Th17, and Th17.1) a separate univariate linear regression was performed to evaluate the effect of prior therapy (independent variable) on cell-subsets at baseline and at 3 months (dependent variable). The naïve group was used as a reference group for each analysis. As shown in Supplementary Table S3, prior treatment with apremilast affected changes of CXCR3+-Th17 percentages. No other statistically significant differences were found.
Methotrexate, apremilast, and anti-IL-23 treatment also modulate pro-inflammatory cell populations
We also evaluated, in a limited number of samples, whether the effect the of anti-IL17 therapeutic blockade on cell is specific for anti-IL-17 biologics or a similar effect is mediated after treatment of psoriasis patients with other agents. PBMC samples from 12 responders, including 5 naïve to previous therapy, 4 previously treated with csDMARDs, and 3 treated with biologic therapy were assessed at (treatment initiation) and at 3 months of their respective therapy. We assessed the effect of methotrexate (n = 4), of apremilast (n = 4) and of anti-IL-23 biologic treatment (n = 4; guselkumab or risankizumab). As shown in Supplementary Fig. S2 methotrexate treatment inhibited CD4+CXCR3+(mean = -13, 95% CI -25.12 to -0.88), Th1 (mean = −6.85, 95% CI −10.99 to −2.71) and CXCR3+-Th17 (mean = −10.25, 95% CI −16.79 to −3.7) cell frequencies. Apremilast treatment inhibited the percentage of CD4+CXCR3+(mean = −6.525, 95% CI −8.26 to −4.79) and Th1 cells (mean = −8.6, 95% CI −17.14 to −0.061) but did not affect CXCR3+-Th17, while anti-IL-23 biologic treatment decreased the percentage of CD4+CCR6+(mean = −6.81, 95% CI −13.46 to −0.167), Th17 (mean = −4.84, 95% CI –9.24 to −0.447) and CXCR3+-Th17 (mean = −28.76, 95% CI −51.49 to −6.037) cells.
Discussion
In this study, we explored for the first time the levels and functional capacity of peripheral pro-inflammatory phenotypically characterized as Th17 population (CCR6+ and CCR4+), Th1 subset (CXCR3+ CCR6− and CCR4−), and sub-populations of Th17 that have switched into IFNγ producing (Th1 like cells) (CCR6+ CCR4− CXCR3+) [14–16] in psoriasis patients at baseline and at 3 months after initiation of treatment with anti-IL-17 or anti-IL-17R targeted biologic therapy. Our data clearly demonstrate that early anti-IL17 mediated clinical remission is accompanied by a significant decrease of Th17, CXCR3+-Th17, Th17.1, and Th1 cells.
In our study, we found that CD4+CCR6+T cells had the most significant decrease after anti-IL17 or anti-IL17R biologic therapy. Recent studies suggest that CD4+CCR6+ population is heterogeneous [17, 18]. Secukinumab or brodalumab therapy did not affect proportions of CD3+CD4+ and CD3+ CD4- populations (Supplementary Fig. S3), whereas treatment significantly decreased Th17, Th17.1 CXCR3+-Th17 cells and altered their relative distribution in origin population CD4+CCR6+ after therapy (Supplementary Fig. S4). Such changes were not found in non-responders (Supplementary Fig. S5). Interestingly, IL-17 targeted therapy affected to a various extent the percentages of these T-cell populations, but intracellular IL-17 production also differed between these cell subsets in psoriasis patients as shown in Supplementary Fig. S6, further indicating that the immunophenotypic characteristics of those cells and their ability to be affected by treatment-induced IL-17 inhibition is diverse and must be characterized further at the functional level.
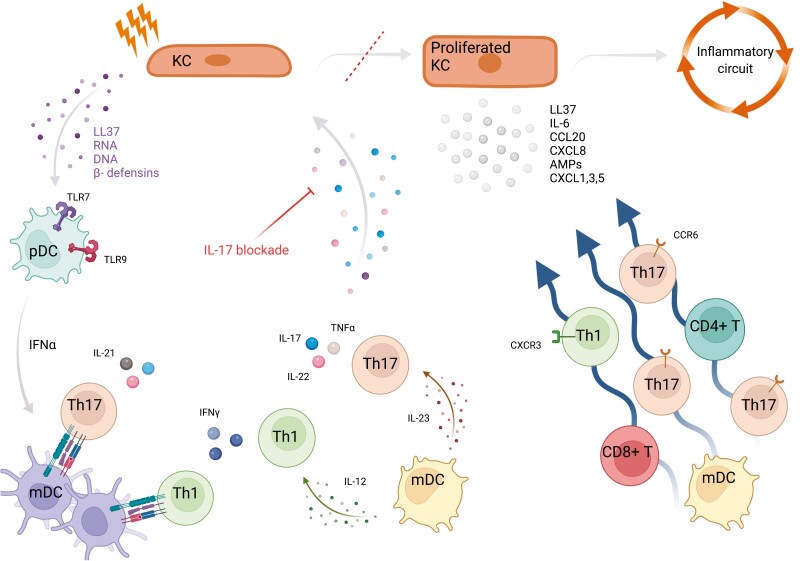
Our study cannot provide a mechanistic explanation for the observed changes in cell subset percentages other than that which is logical to assume i.e. that directly or indirectly those changes are mediated by IL-17 inhibition. Previous studies have shown that the pathophysiology of psoriasis involves the establishment of a pro-inflammatory loop that starts with keratinocyte damage induced by trauma, infection, or drugs [19]. As a result, complexes of self-nucleic acids with AMPs, such as LL37 (cathelicidin), β-defensins, and S100 or with IL-26 derived from Th17 cells have been reported to activate pDCs, through TLR7, TLR8, and/or TLR9 stimulation [20]. Activated pDCs produce type I interferons, which promote mDC maturation and Th differentiation [21, 22]. The two populations of psoriasis-associated mDCS, which are suggested to originate from precursors in circulation, upon activation produce IL-23, TNFα, IL-6, and IL-12 driving differentiation of T cells toward a Th17 and/or Th1 phenotype, and also present antigens to T cells establishing structures in the dermis that resemble lymphoid tissue [23, 24]. Interestingly, the formation of such dermal clumps is heavily relied on the CCL20/CCR6 chemokine system [25]. Skewing of T cells towards a Th17, a Th1, or a Th1-like Th17 phenotype results in IL-17, IL-21, IL-22, and IFNγ production [24, 26]. Keratinocytes respond to such signals by chemokine (including CCL20 and CXCL8-11), AMP, and cytokine (including IL-6, TNF-a) production [27, 28]. Subsequently, further CCR6+ dendritic and T-cell subset recruitment and Th17 differentiation are established, which amplify IL-17-mediated proinflammatory signals [29]. Such a feed-forward inflammatory loop has been proposed to be facilitated in psoriasis [20, 30]. Though we cannot speculate with confidence, it could be possible the therapeutic blockage of IL-17A or IL-17R may exert an inhibitory effect on keratinocyte proliferation (Fig. 4). Blocking IL-17, a cytokine that appears to be crucial for the maintenance of the pro-psoriatic circuit, decreases among others CCL20 and CXCL9-10 levels. That inhibits inflammatory dendritic and T cell dermal accumulation, hinders pro-Th17 differentiation signals, and attenuates loop-amplifying AMP and cytokine (IL-23, IL-12, and IL-6) production.
Figure 4:
A schematic representation of pro-inflammatory events facilitated during the establishment of interleukin- (IL-) 17 mediated psoriasis feed-forward circuit. Skin damage induces the release of self-nucleotides. Complexed self-nucleotides with antimicrobial peptides (AMPs) are recognized by Toll-like receptors (TLRs) of plasmacytoid dendritic cells (pDCs). Interferon (IFN) α produced, stimulates myeloid dendritic cells (mDCs), which express T helper (Th) 17 and Th1 differentiating cytokines. Furthermore, cell to cell interactions boost differentiating signals. Th17 produces IL-17, IL-21, IL22, tumor necrosis factor (TNF) α, among various cytokines that promote keratinocyte proliferation. Keratinocyte proliferation amplifies IL-23-producing signals, resulting in release of pro-inflammatory cell-attracting chemokines, thus inducing the inflammatory circuit established in psoriasis. Treatment with either anti-IL17A or anti-IL17RA biologics interrupts the IL-17-dependent inflammatory loop. KC, keratinocyte. Created with BioRender.com, under license to DPB.
While consensus regarding Th17 differentiation has not yet been achieved, IL-23, IL-1, and IL-6 have been considered instrumental for such a process to take place. TNFα has also been reported to possess indirect Th17 activating capacities [31]. Those cytokines have been reported to upregulate RORγt, thus inducing a Th17 phenotype and IL-17 production [16, 32–34]. Although, the model of STAT3/RORγt mirrors the traits of Th17 differentiation, it fails to reflect its complexity, since Th17 has been recognized as a population with high plasticity [35]. Specifically, exposure to IL-12 induces Th17 cells to IFNγ production [36], as it inhibits RORγt and upregulates T-bet [37]. Moreover, differentiation of Th17 into Th1-like populations has been reported to be IL-23-induced [38]. Inhibition of IL-17 mediated amplification of psoriasis inflammatory loop may translate in lower levels of IL-21 and IL-23, affecting the skewing of Th17 populations towards Th1-like cell subsets.
Recently developed biologic therapies that target not only the IL-23-Th17-IL-17 axis but the TNF-α-signaling as well, exhibit high efficacy and safety in patients with psoriasis. Biologically targeted therapies exert their immunosuppressive action by modulating immune responses. Such therapies may revert the established pro-inflammatory milieu through their direct effect on cytokine-producing cell populations. In recent studies, methotrexate has been found to decrease Th17, Th1 cell percentages in blood and restore Treg populations and their immunosuppressive function in patients with psoriasis [39, 40]. Also, small molecule and biologic therapies have been shown to increase Th2, Treg cell populations and decrease Th1, Th9, and Th22 cells in blood of patients after treatment [12, 41]. Previously, our group has demonstrated that apremilast, a phosphodiesterase 4 inhibitor successfully used for the treatment of the disorder, increases IL-10 producing regulatory B cells and decreases IFNγ and IL-17 producing pro inflammatory T subsets in psoriasis and psoriatic arthritis patients [12]. Etanercept has been found to induce apoptosis in dermal dendritic cells [42], but another study failed to illustrate a possible correlation between clinical response and T-cell compartmentalization in peripheral blood or epidermis [43]. Moreover, infliximab also decreased frequencies of circulating Th1 and Th17 cells in a small number of patients [44] in PBMCs but failed to inhibit IL-22 production by epidermal CD4+ T cells retained in restored skin six years after treatment initiation [45]. A single study has demonstrated that adalimumab decreases Th1, Th17, and Th22 populations and their associated transcription factors [46], contrasting another study that showed that adalimumab indeed increased Th17 cells in circulation in psoriatic arthritis patients [47]. Of relevance to the present study, recent data indicated that in patients with psoriasis IL-17 and IL-22 producing cells are in a substantial proportion CD3- innate lymphoid cells (ILC), and adalimumab treatment decreased the percentages of circulating NKp44+ILC3 [48]. Another study demonstrated that guselkumab and secukinumab decrease inflammatory monocyte-like and dendritic-like cells in lesioned skin [49]. A report examining a PsA patient’s invariant T, Th17, and Th17.1 cells in blood showed no significant difference between populations at baseline compared to after ixekizumab treatment [50].
In a recent report, researchers allocated psoriatic arthritis patients in a standard bDMARD treatment group and a strategic bDMARD treatment group, in which specific a biologic agent was chosen based on phenotypical characteristics of T helper cells as shown by flow cytometry analysis of patients’ blood. Patients allocated in the strategic bDMARD group were accordingly categorized in four types of phenotypical profiles with a matching biologic therapy: dominant Th1 phenotype/ustekinumab, dominant Th17 phenotype/ secukinumab, high Th1, and Th17 phenotype/secukinumab or anti-TNF and low Th1 and Th17 phenotype/adalimumab or infliximab. Patients receiving strategically chosen biologic treatment achieved at 6 months of treatment higher rates of low disease activity (SDAI, DAS28, ACR20) compared to the standard bDMARD treatment group [51]. We herein provide evidence that secukinumab and/or brodalumab most likely reform circulating Th17 cell phenotype profile of psoriasis patients and suggest that clinical remission is associated with cell subset re-shaping. Psoriasis patients with a dominant Th17 and Th1-like Th17 subset profile may be benefited from anti-IL-17 and/or anti-IL-17RA treatment. Moreover, early assessment during treatment by flow cytometry indicative of Th17 cell and sub-populations inhibition may operate as a surrogate marker of subsequent clinical remission.
To assess whether the effect of anti-IL-17 targeted therapy is drug-specific, we explored in a limited number of patients with available PBMCs, the influence of other treatment regimens on cell-subset changes. Methotrexate-treated patients in remission showed a decrease in CD4+CXCR3+, Th1, and CXCR3+-Th17 cell frequencies. Apremilast treatment inhibited the percentage of CD4+CXCR3+and Th1 cells but did not affect CXCR3+-Th17, while anti-IL-23 biologic treatment decreased the percentage of CD4+CCR6+, Th17, and CXCR3+-Th17 cells. Those data imply an effect on various cell subsets, non-specific to anti-IL-17 targeted therapy but more data are needed to shed light on the extent of their influence.
Also, when an analysis was made to assess the effect of previous treatments on cell-subset changes in patients who consequently received anti-IL17A initiation it was found that only apremilast exerted significant changes and this was noted only for CXCR3+-Th17 percentages. However, the observed data are not sufficient enough as the number of patients analyzed was relatively small and the interpretation of data must be treated with caution.
Our study demonstrates for the first that T-cell population characterization merely by surface chemokine receptor phenotyping is sufficient to provide a vital information concerning the likely outcome of patients that were treated with IL-17 targeted treatment, irrespectively whether that treatment relates to the inhibition of IL-17A or IL17-RA. Our data should be treated with caution since larger independent studies must be conducted to elucidate whether these cell subsets could be considered as potential patient classifying markers that could be implemented towards more personalized treatment options in the future.
Supplementary Material
Acknowledgments
We are grateful to Professor Eirini I. Rigopoulou for her critical comments.
Glossary
Abbreviations
- bDMARDs
biologic disease-modifying anti-rheumatic drugs
- CCL
C-C motif chemokine ligand
- CCR
C-C motif chemokine receptor
- csDMARDs
conventional synthetic disease-modifying anti-rheumatic drugs
- CXCL
C-X-C motif chemokine ligand
- CXCR
C-X-C motif chemokine receptor
- IFN
interferon
- IL
interleukin
- mAb
monoclonal antibody
- mDCs
myeloid dendritic cells
- PASI
Psoriasis Area and Severity Index
- PBMCs
peripheral blood mononuclear lymphocytes
- Tc
T cytotoxic
- Th
T helper.
Contributor Information
Sotirios G Tsiogkas, Department of Rheumatology and Clinical Immunology, Faculty of Medicine, University of Thessaly, Larissa, Greece.
Athanasios Mavropoulos, Department of Rheumatology and Clinical Immunology, Faculty of Medicine, University of Thessaly, Larissa, Greece.
Efthimios Dardiotis, Department of Neurology, Faculty of Medicine, University of Thessaly, Larissa, Greece.
Efterpi Zafiriou, Department of Dermatology, Faculty of Medicine, University of Thessaly, Larissa, Greece.
Dimitrios P Bogdanos, Department of Rheumatology and Clinical Immunology, Faculty of Medicine, University of Thessaly, Larissa, Greece.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University General Hospital of Larissa, University of Thessaly (ECA #19/14-11-2019). Informed consent was obtained from all subjects involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research was financed by both Greece and the EU (European Social Fund-ESF) through the Operational Program “Human Resources Development, Education and Lifelong Learning 2014-2020” in the context of the project “Development of a predictive response algorithm in psoriatic patients based on clinical, serological, immunological, and genetic biomarkers” (MIS 5048946).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Conceptualization, D.P.B., E.D., and E.Z.; methodology, S.G.T., A.M., and D.P.B.; validation, S.G.T. and A.M.; clinical assessment, S.G.T., D.P.B., and E.Z.; data curation, S.G.T., A.M., and E.Z.; original draft preparation, D.P.B. and S.G.T.; writing, review and editing, S.G.T., A.M., E.D., E.Z., and D.P.B.; supervision, D.P.B. and E.Z.; project administration, D.P.B. All authors have read and approved the finalized published version of the manuscript.
References
- 1. Armstrong AW, Read C.. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020, 323, 1945–60. [DOI] [PubMed] [Google Scholar]
- 2. Rendon A, Schäkel K.. Psoriasis pathogenesis and treatment. Int J Mol Sci 2019, 20, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harden JL, Krueger JG, Bowcock AM.. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun 2015, 64, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowes MA, Suárez-Fariñas M, Krueger JG.. Immunology of psoriasis. Annu Rev Immunol 2014, 32, 227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peters A, Lee Y, Kuchroo VK.. The many faces of Th17 cells. Curr Opin Immunol 2011, 23, 702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu X, Tian J, Wang S.. Insight into non-pathogenic Th17 cells in autoimmune diseases. Front Immunol 2018, 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007, 8, 639–46. [DOI] [PubMed] [Google Scholar]
- 8. Ramstein J, Broos CE, Simpson LJ, et al. IFN-γ producing t-helper 17.1 cells are increased in sarcoidosis and are more prevalent than t-helper type 1 cells. Am J Respir Crit Care Med 2016, 193, 1281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Langelaar J, Van Der Vuurst De Vries RM, Janssen Met al. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain 2018;141:1334–49. [DOI] [PubMed] [Google Scholar]
- 10. Maeda S, Osaga S, Maeda T, et al. Circulating Th17.1 cells as candidate for the prediction of therapeutic response to abatacept in patients with rheumatoid arthritis: an exploratory research. PLoS One 2019, 14, e02151921–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol 2015, 11, 415–29. [DOI] [PubMed] [Google Scholar]
- 12. Mavropoulos A, Zafiriou E, Simopoulou T, et al. Apremilast increases IL-10-producing regulatory B cells and decreases proinflammatory T cells and innate cells in psoriatic arthritis and psoriasis. Rheumatol 2019, 58, 2240–50. [DOI] [PubMed] [Google Scholar]
- 13. Tsiogkas SG, Mavropoulos Α, Skyvalidas DN, et al. Delphinidin diminishes in vitro interferon-γ and interleukin-17 producing cells in patients with psoriatic disease. Immunol Res 2022, 70, 161–73. [DOI] [PubMed] [Google Scholar]
- 14. Basdeo SA, Cluxton D, Sulaimani J, et al. Ex-Th17 (Nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J Immunol 2017, 198, 2249–59. [DOI] [PubMed] [Google Scholar]
- 15. Sallusto F, Zielinski CE, Lanzavecchia A.. Human Th17 subsets. Eur J Immunol 2012, 42, 2215–20. [DOI] [PubMed] [Google Scholar]
- 16. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, et al. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007, 8, 942–9. [DOI] [PubMed] [Google Scholar]
- 17. Wacleche VS, Goulet JP, Gosselin A, et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology 2016, 13, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dankers W, den Braanker H, Paulissen SMJ, et al. The heterogeneous human memory CCR6+ T helper-17 populations differ in T-bet and cytokine expression but all activate synovial fibroblasts in an IFNγ-independent manner. Arthritis Res Ther 2021, 23, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Meglio P, Villanova F, Nestle FO.. Psoriasis. Cold Spring Harb Perspect Med 2014, 4. doi: 10.1101/cshperspect.a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiricozzi A, Romanelli P, Volpe E, et al. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci 2018, 19. doi: 10.3390/ijms19010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med 2005, 202, 135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Wang LL, Yang HY, et al. Interleukin-21 is associated with the severity of psoriasis vulgaris through promoting CD4+ T cells to differentiate into Th17 cells. Am J Transl Res 2016, 8, 3188–96. [PMC free article] [PubMed] [Google Scholar]
- 23. Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol 2009, 129, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marinoni B, Ceribelli A, Massarotti MS, et al. The Th17 axis in psoriatic disease: pathogenetic and therapeutic implications. AutoImmun Highlights 2014, 5, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim T-G, Jee H, Fuentes-Duculan J, et al. Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J Invest Dermatol 2014, 134, 1462–5. [DOI] [PubMed] [Google Scholar]
- 26. Shi Y, Chen Z, Zhao Z, et al. IL-21 induces an imbalance of Th17/treg cells in moderate-to-severe plaque psoriasis patients. Front Immunol 2019, 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 2008, 159, 1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M, et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 2011, 131, 677–87. [DOI] [PubMed] [Google Scholar]
- 29. Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 2009, 129, 2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li B, Huang L, Lv P, et al. The role of Th17 cells in psoriasis Th17 cells. Immunol Res 2020, 68, 296–309. [DOI] [PubMed] [Google Scholar]
- 31. Chiu HY, Cheng YP, Tsai TF.. T helper type 17 in psoriasis: from basic immunology to clinical practice. Dermatologica Sin 2012, 30, 136–41. [Google Scholar]
- 32. Chen Z, Tato CM, Muul L, et al. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum 2007, 56, 2936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007, 8, 950–7. [DOI] [PubMed] [Google Scholar]
- 34. McGeachy MJ, Cua DJ.. Th17 cell differentiation: the long and winding road. Immunity 2008, 28, 445–53. [DOI] [PubMed] [Google Scholar]
- 35. Muranski P, Restifo NP.. Essentials of Th17 cell commitment and plasticity. Blood 2013, 121, 2402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity 2009, 30, 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007, 204, 1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhaumik S, Basu R.. Cellular and molecular dynamics of Th17 differentiation and its developmental plasticity in the intestinal immune response. Front Immunol 2017, 8, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan K, Xu W, Huang Y, et al. Methotrexate restores the function of peripheral blood regulatory T cells in psoriasis vulgaris via the CD73/AMPK/mTOR pathway. Br J Dermatol 2018, 179, 896–905. [DOI] [PubMed] [Google Scholar]
- 40. Priyadarssini M, Chandrashekar L, Rajappa M.. Effect of methotrexate monotherapy on T-cell subsets in the peripheral circulation in psoriasis. Clin Exp Dermatol 2019, 44, 491–7. [DOI] [PubMed] [Google Scholar]
- 41. Solberg SM, Aarebrot AK, Sarkar I, et al. Mass cytometry analysis of blood immune cells from psoriasis patients on biological therapy. Eur J Immunol 2021, 51, 694–702. [DOI] [PubMed] [Google Scholar]
- 42. Malaviya R, Sun Y, Tan JK, et al. Etanercept induces apoptosis of dermal dendritic cells in psoriatic plaques of responding patients. J Am Acad Dermatol 2006, 55, 590–7. [DOI] [PubMed] [Google Scholar]
- 43. van Lingen RG, Körver JEM, van de Kerkhof PCM, et al. Relevance of compartmentalization of T-cell subsets for clinical improvement in psoriasis: effect of immune-targeted antipsoriatic therapies. Br J Dermatol 2008, 159, 91–6. [DOI] [PubMed] [Google Scholar]
- 44. Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010, 130, 1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheuk S, Wikén M, Blomqvist L, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 2014, 192, 3111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luan L, Han S, Wang H, et al. Down-regulation of the Th1, Th17, and Th22 pathways due to anti-TNF-α treatment in psoriasis. Int Immunopharmacol 2015, 29, 278–84. [DOI] [PubMed] [Google Scholar]
- 47. Hull DN, Williams RO, Pathan E, et al. Anti-tumour necrosis factor treatment increases circulating T helper type 17 cells similarly in different types of inflammatory arthritis. Clin Exp Immunol 2015, 181, 401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Villanova F, Flutter B, Tosi I, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol 2014, 134, 984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mehta H, Mashiko S, Angsana J, et al. Differential changes in inflammatory mononuclear phagocyte and T-cell profiles within psoriatic skin during treatment with guselkumab vs. secukinumab. J Invest Dermatol 2021. doi: 10.1016/j.jid.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 50. Koga T, Matoba M, Sato T, et al. Evaluation of circulating invariant T cells before and after IL-17 inhibitor treatment in a patient with psoriatic arthritis. Clin Immunol 2018, 197, 107–9. [DOI] [PubMed] [Google Scholar]
- 51. Miyagawa I, Nakayamada S, Nakano K, et al. Precision medicine using different biological DMARDs based on characteristic phenotypes of peripheral T helper cells in psoriatic arthritis. Rheumatol 2019, 58, 336–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.