Abstract
Background:
Streptococcus pyogenes, or Group A Streptococcus (GAS), causes acute pharyngitis and necrotizing fasciitis. Seasonal variations in GAS infections are not robustly characterized. We assessed seasonal variations and risk factors of GAS pharyngitis and ICD-10-diagnosed necrotizing fasciitis.
Methods:
From the period 2010–2019, we conducted a case–control study using laboratory-confirmed cases of GAS pharyngitis and a descriptive observational study of necrotizing fasciitis using ICD-10 codes. Data were collected from TriNetX, a federated research network. We extracted seasonal (quarterly) incidence rates. We used an autoregressive integrated moving average (ARIMA) model to assess seasonal variations. Demographic characteristics and 1-month outcomes were compared among adults with or without GAS pharyngitis.
Results:
We identified 224,471 adults with GAS pharyngitis (test-positive) and 546,142 adults without it (test-negative). GAS pharyngitis adults were younger (25.3 versus 30.2 years of age, p < 0.0001), more likely to be Hispanic individuals (10% versus 8%, p < 0.0001) and slightly more likely to be Black or African American individuals (14% versus 13%, p < 0.0001). Propensity score matching found that adults with test-positive cases of GAS pharyngitis had a higher risk of acute rheumatic fever while having no significant differences in risk of intensive care unit admission and mortality compared with test-negative cases. GAS pharyngitis average incidence peaked in the winter while dipping in the summer (0.32 versus 0.18 and 4.07 versus 1.78 per 1000 adults and pediatric patients, respectively). Necrotizing fasciitis diagnoses were highest during summer (0.032 per 1000 adults). There was a significant ARIMA seasonal variation in the time series analysis for adult and pediatric GAS pharyngitis (p < 0.0001 and p = 0.014, respectively). Necrotizing fasciitis diagnosis was not associated with seasonal variation (p = 0.861).
Conclusion:
Peaks in GAS pharyngitis occur in the winter months. ICD code–based necrotizing fasciitis did not show a quarterly seasonal variation.
Keywords: group A streptococcus, necrotizing fasciitis, pharyngitis, seasonal variation, Streptococcus pyogenes
Background
Streptococcus pyogenes, or Group A Streptococcus (GAS), causes suppurative and non-suppurative manifestations. Pharyngitis and necrotizing fasciitis (NF) are two clinically relevant suppurative manifestations, while the streptococcal toxic shock syndrome (STSS) represents a condition with streptococcal exotoxin pathophysiology. Unlike other upper respiratory infections, such as influenza,1 the seasonal variations in the incidence of GAS infections are not as robustly characterized, perhaps due to the heterogeneity in the various presentations of GAS infection. Understanding the seasonality of GAS infections provides an opportunity for early clinical identification and implementation of preventive interventions to reduce the burden of GAS disease during high-transmissions seasons.
Previous studies investigated seasonal variations in specific clinical manifestations of GAS, for example, soft tissue infections. The US Centers for Disease Control and Prevention highlighted seasonal trends in GAS isolation from patients with NF or STSS, illustrating that these severe GAS infections are consistently more frequent in the northern hemisphere winter and early spring, with a nadir in the late summer and fall.2 Lamagni et al.3 corroborated these findings in Europe, reporting peaking rates of severe GAS in the winter and spring using cultures in patients with clinical signs of STSS.
An analogous seasonal cadence seems evident in less severe GAS infections such as pharyngitis but with greater variation. An Australian study using microbiologic and serologic evidence reported a bimodal seasonal pattern of GAS pharyngitis, with peaks in the winter/spring and autumn. Interestingly, GAS carriage rates were highest for children in the winter and spring but showed no seasonal differences in the adult population.4 A 4-year surveillance effort in Sweden identified a trend toward a lower likelihood of GAS pharyngotonsillitis during the summer months of August and September.5
Further investigation is warranted to characterize the cyclic dynamics of GAS pharyngitis in a larger US-based population. Examining GAS pharyngitis is critical at this time, as COVID-19 has altered the differential diagnosis for patients with pharyngitis. Viral infections, predominantly rhinovirus, influenza, adenovirus, and parainfluenza, are the etiological cause of 50–80% of all sore throat cases.6 In comparison, COVID-19 has now been found to present with sore throat in up to 17.4% of patients.7 GAS is the most common bacterial etiologic pathogen in pharyngitis.8 To aid the clinician in diagnosing and managing acute pharyngitis, we aim to illustrate the seasonal incident frequencies of test-positive GAS pharyngitis and underscore the clinical features and mortality differences in the adult population. We also aim to describe the seasonal variation of GAS pharyngitis in the pediatric population and that of the International Classification of Diseases, 10th Revision (ICD-10) code-based NF.
Methods
Study design and population
We collected laboratory-identified cases of GAS pharyngitis using Logical Observation Identifiers Names and Codes (LOINC) codes (Supplemental eTable 1) and cases of NF using the ICD-10 code M72.6. All records were collected within TriNetX, a de-identified database containing more than 85 million distinct patient records from 58 healthcare organizations (Supplemental eMethods). Selection bias in our data collection was limited, as the patient datasets were previously procured by TriNetX. In this procurement process, patients without diagnoses or codes were excluded from the dataset. We collected seasonal incident cases of GAS pharyngitis and NF from winter 2010 to fall 2019 using a 3-month lookback period, which prevented multiple positive cases for the same patient within a 3-month window from being duplicated into the incident population. Winter was defined as December 21–March 20, spring as March 21–June 20, summer as June 21–September 20, and fall as September 21–December 20. Incidence rates were gathered using a denominator of patients within the TriNetX database receiving any ICD-10 diagnosis or lab test within the corresponding season and then calculated per 1000 patients. The denominator included patients 17 years of age and younger for the incidence calculation of pediatric GAS pharyngitis, while the denominator included patients 18 years of age and older for the incidence calculation of adult GAS pharyngitis and NF. However, 95% confidence intervals were constructed using a Poisson distribution. We employed weighted averages using case counts in the corresponding periods to determine the average incidence per season. For adult and pediatric GAS pharyngitis patients, we calculated the proportion of tests returning positive during each quarter. We used weighted methods to determine the mean test positivity ratio per season. Additional clinical variables included demographic characteristics, diagnoses, and selected laboratory test results (Supplemental eTables 2 and 3). The patient population was divided into two cohorts based on the presence or absence of positive or negative testing for GAS pharyngitis (Supplemental eMethods).
Outcome measures
The primary outcome was the quarterly incidence of positive GAS pharyngitis tests. Incidence rates were reported separately for adult and pediatric populations. The incidence rate was also reported for adult patients with an ICD-10 diagnosis of NF. Secondary outcomes included matched differences in 1-month risk of acute rheumatic fever (ARF), intensive care unit (ICU) admission, and death (Supplemental eTable 4) among adult patients with or without positive GAS pharyngitis tests.
Statistical analysis
Using Stata as statistical software, an autoregressive integrated moving average (ARIMA) regression model computed seasonal changes in incidence. We initially tested the seasonal variation for a stationary or non-stationary pattern using a trend graph analysis, a correlogram, and the formal Dickey–Fuller and Phillips–Perron tests. We then determined the autoregressive order (p), moving average order (q), and integrated difference order (d) values for the ARIMA and autoregressive moving average (ARMA) models. The autocorrelation (AC) and partial AC functions were used to determine the q and p values using the number of quarterly lags exceeding the 95% confidence bands. We created a line plot of the differencing operator or first-order difference of the seasonal time series variable to determine d. We generated residual errors, plotted the values around the mean, and performed the formal Portmanteau test to determine whether residuals were white noise. We performed a post-estimation analysis using Akaike’s information criterion (AIC) and Bayesian information criterion (BIC). We used the autoregressive (AR) and moving average (MA) roots to verify that the estimated ARMA process was stationary and invertible. Finally, we forecasted log-transformed incidence rates to assess the degree of estimation.
Baseline characteristics, recorded within 1 month or less before receiving a diagnostic evaluation for GAS, were compared using t tests and chi-square tests. We used TriNetX propensity score matching to compare the group of adults testing positive and testing negative for GAS pharyngitis. Both groups were matched by age at diagnosis, sex, presence of influenza or pneumonia, and presence of COVID-19. These covariates were selected based on the degree of difference between the two cohorts and the consequential impact of these parameters on outcomes of interest. Cohort matching was performed on the TriNetX software, using 1:1 nearest neighbor matching with propensity score differences (calipers) of less than or equal to 0.1. However, 1-month outcomes of ARF, ICU admission, and mortality were used to calculate risk and odds ratios. The significance of this study was set at p < 0.05.
Results
Clinical features of patients testing positive for GAS pharyngitis
We identified 224,471 adults with a positive test for GAS pharyngitis and 546,142 adults with a negative test. Patients testing positive for GAS pharyngitis were more likely to be younger, being on average nearly 5 years younger to those testing negative. The cohort testing positive for GAS pharyngitis had a higher fraction of men. Demographic comparisons of race and ethnicity showed that test-positive cases of GAS pharyngitis had a larger constituency of Black or African Americans and Hispanic or Latino individuals. Patients testing negative for GAS pharyngitis had a significantly higher prevalence of acute or subacute illnesses, such as influenza, pneumonia, and COVID-19. Adults with test-positive cases of GAS pharyngitis had less prevalence of chronic comorbidities such as HIV-1 infection, type 2 diabetes, chronic obstructive pulmonary disease (COPD), heart failure, and neoplasms (Table 1). The two cohorts did not have significantly different levels of inflammatory laboratory values, such as leukocyte counts, C-reactive protein, and ferritin. However, those testing positive for GAS pharyngitis showed considerably lower markers of cell injury, represented by lower levels of lactate dehydrogenase and alanine aminotransferase.
Table 1.
Baseline characteristics of adults testing positive for GAS and adults testing negative for GAS (values recorded within 1 month prior to laboratory evaluation for GAS).
| Cohort demographics | |||
|---|---|---|---|
| Subject characteristics | Adults testing positive for GAS pharyngitis | Adults testing negative for GAS pharyngitis | p |
| N = 224,471 | N = 546,152 | ||
| Age at diagnosis (years), M (±SD) | 25.3 (14.9) | 30.2 (17.5) | <0.0001 |
| % of cohort male sex (n) | 38% (84,504) | 37% (202,118) | <0.0001 |
| Race (n) | |||
| White | 70% (157,764) | 74% (401,841) | <0.0001 |
| Black or African American | 14% (30,777) | 13% (72,819) | <0.0001 |
| Asian | 2% (3415) | 2% (8565) | 0.3579 |
| Ethnicity (n) | |||
| Hispanic or Latino | 10% (22,704) | 8% (41,435) | <0.0001 |
| Comorbidities (n) | |||
| COVID-19 | 0.073% (164) | 0.226% (1236) | <0.0001 |
| Influenza and pneumonia | 0.389% (874) | 0.663% (3623) | <0.0001 |
| HIV | 0.111% (250) | 0.135% (736) | 0.0091 |
| Type 2 diabetes mellitus | 0.846% (1899) | 1.309% (7147) | <0.0001 |
| Rheumatoid arthritis | 0.016% (37) | 0.028% (153) | 0.0034 |
| Systemic lupus erythematosus | 0.039% (87) | 0.064% (352) | <0.0001 |
| Transplanted organs or tissues | 0.053% (118) | 0.139% (759) | <0.0001 |
| COPD | 0.114% (255) | 0.308% (1682) | <0.0001 |
| Heart failure | 0.116% (261) | 0.284% (1552) | <0.0001 |
| Fibrosis and cirrhosis of liver | 0.032% (72) | 0.084% (457) | <0.0001 |
| Acute kidney failure and CKD | 0.204% (458) | 0.644% (3519) | <0.0001 |
| Neoplasms | 0.736% (1651) | 1.330% (7266) | <0.0001 |
| Laboratory values, M (±SD) | |||
| Hemoglobin A1C | 6.66 (1.89) | 6.78 (2.03) | 0.0191 |
| Leukocytes (/µl) | 10.2 (76.2) | 10.6 (79.4) | 0.7005 |
| Lymphocytes (/µl) | 3.98 (25.4) | 2.1 (11.2) | <0.0001 |
| T4 helper cells (/µl) | 605 (317) | 428 (372) | 0.0003 |
| C-reactive protein (mg/L) | 19.4 (43.6) | 16.7 (32.8) | 0.0664 |
| Ferritin (ng/ml) | 309 (1133) | 780 (7154) | 0.1390 |
| Creatinine (mg/L) | 0.878 (0.695) | 1.1 (1.71) | <0.0001 |
| Albumin (g/dl) | 4.08 (0.559) | 3.81 (0.745) | <0.0001 |
| Lactate dehydrogenase (IU/ml) | 269 (220) | 391 (867) | 0.0344 |
| Aspartate aminotransferase (U/liter) | 30.7 (114) | 48.3 (306) | <0.0001 |
| Alanine aminotransferase (U/L) | 33.0 (69.1) | 41.2 (162) | 0.0005 |
| Alkaline phosphatase (U/L) | 92.5 (65.2) | 102 (91.9) | <0.0001 |
GAS, Group A Streptococcus; CKD, chronic kidney failure; COPD, chronic obstructive pulmonary disease.
Morbidity and mortality outcomes
In an unmatched comparison, patients testing positive for GAS pharyngitis had a significantly higher 1-month risk of ARF while a considerably lower 1-month risk of ICU admission and mortality compared with those with a negative GAS test. When matching for age at diagnosis, sex, influenza, pneumonia, and COVID-19, the two groups were not significantly different in terms of 1-month outcomes of mortality or ICU admission. However, the risk of ARF remained significantly higher in those testing positive for GAS (Table 2).
Table 2.
Differences in 1-month outcomes between adults testing positive for GAS pharyngitis and adults testing negative for GAS pharyngitis.
| Before matching | After matching for age, sex, COVID-19, and influenza and pneumonia | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Adult testing positive for GAS pharyngitis N = 224,471 |
Adult testing negative for GAS pharyngitis N = 546,152 |
OR (95% CI) | p | Adult testing positive for GAS pharyngitis N = 224,471 |
Adult testing negative for GAS pharyngitis N = 224,471 |
OR (95% CI) | p |
| ARF | 49 (0.022%) | 74 (0.014%) | 1.611 (1.123, 2.312) | 0.0089 | 49 (0.022%) | 24 (0.011%) | 2.0242 (1.253, 3.328) | 0.0034 |
| ICU admission | 185 (0.082%) | 611 (0.112%) | 0.736 (0.625, 0.868) | 0.0005 | 185 (0.082%) | 215 (0.096%) | 0.860 (0.707, 1.047) | 0.1334 |
| Deceased | 72 (0.032%) | 535 (0.098%) | 0.327 (0.256, 0.419) | <0.0001 | 72 (0.032%) | 91 (0.041%) | 0.791 (0.581, 1.078) | 0.1366 |
ARF, acute rheumatic fever; CI, confidence interval; GAS, Group A Streptococcus; OR, odds ratio.
Seasonal analysis of GAS pharyngitis in adults
From winter 2010 to fall 2019, we found a recurring seasonal pattern in the incidence rates of GAS pharyngitis among adults. The weighted average incidence rates consistently peaked in the winter (0.32 per 1000 patients) and spring (0.28 per 1000 patients) while reaching a trough in the summer (0.18 per 1000 patients) and rising again in the fall (0.25 per 1000 patients) (Figure 1). The weighted average proportion of tests returning positive for GAS displayed highs in the spring and winter, 16% and 15%, respectively, while reaching slightly lower values in the summer and fall, at 14%. For the GAS rate in adults, the outcome of the Dickey–Fuller and Phillips–Perron tests and the correlogram showed no decay, implying a stationary pattern of incidence (Supplemental eFigure 2). The AC and partial AC of the differential of adult GAS rate showed three and four lags outside of the 95% confidence bands, determining a q (MA) value of 3 and a p (AR) value of 4 (Supplemental eFigures 3 and 4). The rate showed a significant correlation with a coefficient of −1.38 (p < 0.0001), with an AR coefficient also highly significant of 0.88 (p < 0.0001). The MA coefficient of 0.26 did not reach statistical significance (p = 0.269). Post-estimation AIC and BIC values were −41.9 and −35.6, respectively. Portmanteau test for white noise was 20.5 (p = 0.2), and the AR and MA roots lying inside the circle estimated stability (Supplemental eFigures 5 and 6). Forecasted rates corresponded closely to the logarithmic calculated rates (Supplemental eFigure 7).
Figure 1.
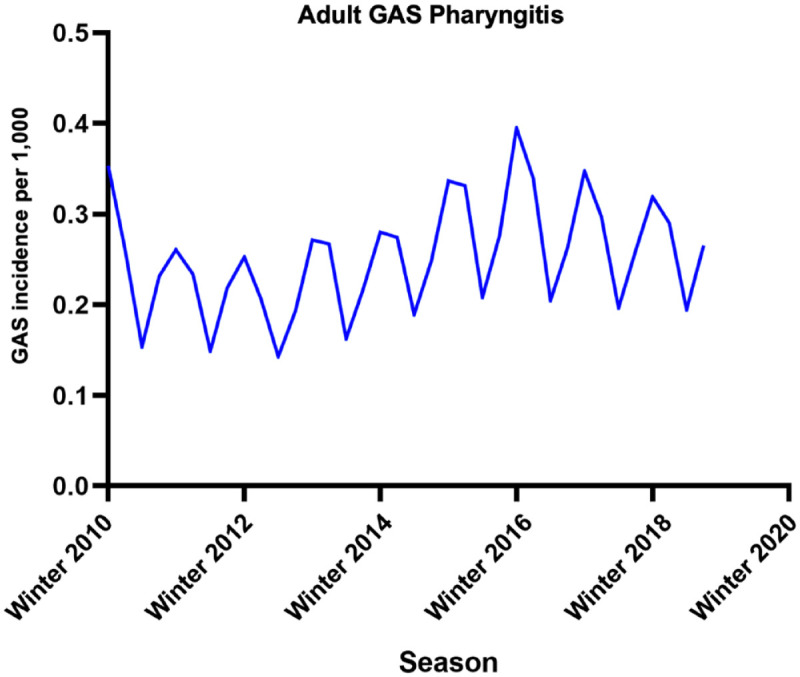
Seasonal variation in adults testing positive for GAS from winter 2010 to fall 2019. Incidences are displayed as cases per 1000 patients.
Seasonal analysis of GAS pharyngitis in pediatrics
We found much higher incidence overall while following the same seasonal cycle, with weighted average incidence most elevated in the winter (4.07 per 1000 patients) and spring (3.37 per 1000 patients), lowest in the summer (1.78 per 1000 patients), and climbing once more in the fall (3.27 per 1000 patients) (Figure 2). The weighted average proportion of tests returning positive for GAS displayed peaks in the winter and spring at 38%, decreased to a midpoint of 34% in the fall, and reached a nadir in the summer at 30%. Dickey–Fuller test, Phillips–Perron test, and the correlogram for the GAS pharyngitis pediatric rate showed a stationary effect (Supplemental eFigure 9). The AC and partial AC of the differential of GAS pediatric rate also showed three and four lags outside of the 95% confidence bands, determining a q (MA) value of 3 and a p (AR) value of 4 (Supplemental eFigures 10 and 11). The rate showed a significant correlation with a coefficient of 0.74 (p = 0.014), with an AR (0.91, p < 0.0001) and an MA coefficient (0.44, p = 0.034) also significant. Post-estimation AIC and BIC values were −41.9 and −35.6, respectively. Portmanteau test for white noise was 48.03 (p < 0.0001). However, the AR and MA roots resided inside the circle suggesting stability (Supplemental eFigures 12 and 13). Forecasted rates overlayed well with the observed logarithmic calculated pediatric rates (Supplemental eFigure 14).
Figure 2.
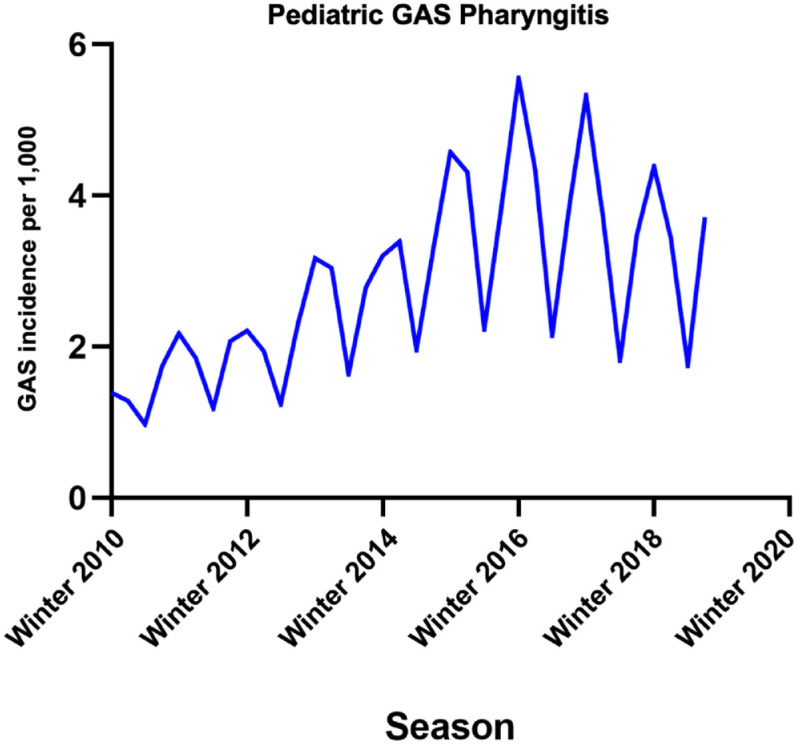
Seasonal variation in pediatrics testing positive for GAS from winter 2010 to fall 2019. Incidences are displayed as cases per 1000 patients.
Seasonal analysis of adult ICD-code-based NF
NF showed the highest weighted average incidence in the summer (0.032 per 1000 patients), while the incidence in the winter, spring, and fall was 0.027, 0.029, and 0.029 per 1000 patients, respectively (Supplemental eFigure 15) (Figure 3). Dickey–Fuller test, Phillips–Perron test, and the correlogram for the NF rate showed a non-stationary effect (Supplemental eFigure 16). The AC and partial AC of the differential of adult NF rate also showed four and zero lags outside of the 95% confidence bands, determining a q (MA) value of 4 and a p (AR) value of 0 (Supplemental eFigures 17 and 18). The rate did not show a significant correlation with a coefficient of 0.0008 (p = 0.861) but with an AR significant at L1–L4 (L1: −1.4, p < 0.0001). Post-estimation AIC and BIC values were −30.5 and −21.6, respectively. Portmanteau test for white noise was 6.6 (p = 0.96), and the AR and MA roots lying inside the circle estimated stability (Supplemental eFigures 19 and 20). Forecasted rates overlaid well with the logarithmic calculated NF rates (Supplemental eFigure 21).
Figure 3.
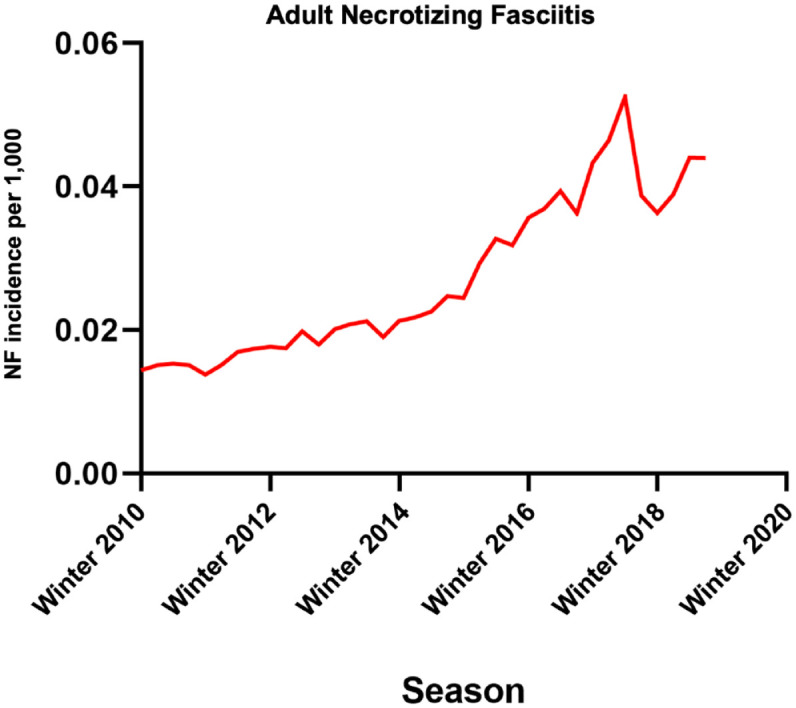
Seasonal variation in adult ICD-10-diagnosed necrotizing fasciitis from winter 2010 to fall 2019. Incidences are displayed as cases per 1000 patients.
Discussion
Based on our findings GAS pharyngitis for adult and pediatric populations shows a statistically significant quarterly pattern of incidence that are highest in the winter and lowest in the summer. This study did not show a statistically significant seasonal variation in ICD-10-diagnosed NF incidence rates. These findings aid in further characterizing the seasonality of GAS pharyngitis, corroborating findings of studies of GAS pharyngitis in populations outside of the United States and reported patterns of GAS soft tissue infections. As beta-hemolytic streptococci are transmitted via respiratory droplets, it is possible to conclude that incidence rates of GAS would be highest in colder months, when individuals are more likely to congregate indoors.9 Elucidation of GAS pharyngitis seasonality in tropical climates may allow for further understanding of how these social and environmental factors interact.
Another factor to consider is the intrafamilial spread of GAS from pediatrics to adults, especially in schooling. Children are the principal vectors of GAS transmission, and incidence rates of GAS pharyngitis are much higher in the pediatric population than among adults due to crowding in school or home environments.10 Since both populations show the highest infection rates during the winter, the seasonal variation in adult GAS pharyngitis is likely heavily influenced by children’s social factors. It has been reported that primary cases of GAS pharyngitis can cause secondary infections within the same household in 43% of cases.4 Thus, the absence of children in the highly transmissive environment of schools during the summer months likely accounts for the attendant low incidence of adult GAS pharyngitis during this season.
A potential confounder within our case–control baseline comparisons between subjects testing positive or negative for GAS pharyngitis is that a physician’s utilization of a GAS throat swab may not always correlate to a clinician’s suspicion of GAS pharyngitis. This discrepancy may be most apparent within older populations with underlying comorbidities, wherein a GAS test may be used within a broader diagnostic workup. One may also presume that the seasonal results of this study could be impacted by seasonal variations in physician usage of GAS throat swabs. However, our findings indicate that changes in GAS screening frequencies stem from seasonal changes in the likelihood of an individual being infected. This is illustrated by the fact that seasonal changes in the use of GAS testing parallel seasonal fluctuations in the proportion of tests returning positive. For example, when the use of tests is highest in the winter and spring months, we see that the positive test ratio is also highest in both adult and pediatric populations. Correspondingly, when the use of tests is lowest in the summer, we observe that the proportion of positive tests is also at a nadir. Past studies have demonstrated this phenomenon, with Andersson et al.5 reporting that the proportion of positive GAS rapid antigen tests is highest in April and lowest in August. Thus, the dynamics of GAS screenings likely originate from the dynamics of GAS infections.
A remaining limitation to quantifying the incidence of GAS pharyngitis is that colonization does not necessarily equate with bacterial infection and disease. Adults with throat pain have a significantly higher prevalence of GAS than healthy controls.11 The increased presence of GAS signals an increased likelihood of confirmed infection. Still, the LOINC employed in this study are structured to identify the presence or absence of GAS rather than the quantity of GAS present. We could not correlate sore throat symptoms and the clinical diagnosis of pharyngitis with the test results due to the ICD-code and laboratory-based nature of the study.
Another limitation is the study’s inability to separate all causative organisms of NF from those specifically due to GAS. Past seasonal analyses of GAS soft tissue infections have used case criteria based on the isolation of GAS from a normally sterile site or wound in combination with clinical signs of NF.2,3 With the database used in this study, we could not replicate this case definition as we were unable to link microbiological evidence of GAS to clinical signs of NF in a temporal manner. The database used captured a large population of patients experiencing clinical signs of NF, but multiple reports quantify GAS as only causing about 10% of these total NF cases.12,13 Thus, irrespective of our seasonal findings in ICD-10-diagnosed NF, it remains possible that NF due to GAS may seasonally mirror the patterns we have found in GAS pharyngitis.
Although the retrospective study design has limitations, it included the largest US-based seasonal analysis of GAS pharyngitis and spanned the most years studied. For the clinician managing a patient presenting with the signs of GAS pharyngitis, symptoms such as sore throat, fever, and tonsillitis will continue to be the most reliable indicator of the disease, regardless of the season. Characterization of the seasonality of GAS pharyngitis serves to supplement understanding of who should be screened for GAS pharyngitis and at what time of year is most useful. Thus, when building a diagnostic approach to sore throat, our results inform clinicians of an increased likelihood of GAS pharyngitis in younger adults and children in the winter and spring. The demographic metrics of our study also illustrate an increased likelihood of a positive GAS test in Black or African American and Hispanic or Latino individuals, calling for additional exploration into how social determinants of health may be influencing GAS colonization. In the future management of GAS, specifically regarding the reemerged development of GAS vaccines,14 comprehension of these seasonal and socially influenced factors may provide insight into vaccine administration.
A fundamental goal of antibiotic therapy for GAS pharyngitis is to prevent rare but severe complications caused by ARF. This study illustrates significantly higher rates of ARF in test-positive GAS patients with pharyngitis compared with those who test negative for GAS, with the caveat that a 1-month outcome window will not catch all possible cases of ARF. Prevention of ARF relies on prompt antibiotic treatment, as it eradicates GAS before an autoimmune response can develop.15 Identifying the seasonal frequencies may aid in assessing persons with increased ARF risk, potentially supporting more targeted and effective treatment.
Our data show that the seasonality of GAS pharyngitis follows the same seasonal cadence as influenza.1 Initial studies have also identified this seasonal pattern in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),16 and it remains to be seen how SARS-CoV-2 may impact the future seasonality of GAS pharyngitis. Although there is a great overlap between GAS pharyngitis and viral respiratory diseases in both seasonality and symptomatology, the unmatched adult comparisons show that distinguishing between these etiologies is important due to the differences in morbidity and mortality, in addition to aiding antibiotic stewardship efforts. Influenza and other viral respiratory infections are also tied to the development of invasive GAS (iGAS) diseases, such as NF, STSS, and bacteremia.17 The seasonal overlap described herein with GAS pharyngitis warrants subsequent investigation of whether viral–bacterial mechanisms may also contribute to the pathogenesis of streptococcal pharyngitis.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361221132101 for Seasonal variations and risk factors of Streptococcus pyogenes infection: a multicenter research network study by Matthew Kennis, Alex Tagawa, Vanessa M. Kung, Gabrielle Montalbano, Isabella Narvaez, Carlos Franco-Paredes, Lilian Vargas Barahona, Nancy Madinger, Leland Shapiro, Daniel B. Chastain and Andrés F. Henao-Martínez in Therapeutic Advances in Infectious Disease
Acknowledgments
None.
Footnotes
ORCID iDs: Lilian Vargas Barahona  https://orcid.org/0000-0003-3330-808X
https://orcid.org/0000-0003-3330-808X
Daniel B. Chastain  https://orcid.org/0000-0002-4018-0195
https://orcid.org/0000-0002-4018-0195
Andrés F. Henao-Martínez  https://orcid.org/0000-0001-7363-8652
https://orcid.org/0000-0001-7363-8652
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Matthew Kennis, Division of Infectious Diseases, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA.
Alex Tagawa, Center for Gait and Movement Analysis (CGMA), Children’s Hospital Colorado, Aurora, CO, USA.
Vanessa M. Kung, Division of Infectious Diseases, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA
Gabrielle Montalbano, Division of Infectious Diseases, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA.
Isabella Narvaez, Division of Infectious Diseases, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA.
Carlos Franco-Paredes, Hospital Infantil de México, Federico Gómez, México City, México.
Lilian Vargas Barahona, Division of Infectious Diseases, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA.
Nancy Madinger, Division of Infectious Diseases, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA.
Leland Shapiro, Division of Infectious Diseases, School of Medicine, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA; Division of Infectious Diseases, Rocky Mountain Regional Veterans Affairs Medical Center, Aurora, CO, USA.
Daniel B. Chastain, Department of Clinical and Administrative Pharmacy, University of Georgia College of Pharmacy, Albany, GA, USA
Andrés F. Henao-Martínez, Division of Infectious Diseases, School of Medicine, University of Colorado Anschutz Medical Campus, 12700 E. 19th Avenue, Mail Stop B168, Aurora, CO 80045, USA.
Declarations
Ethics approval and consent to participate: Research using TriNetX does not require ethical approval because patient-identifiable information is not accessible to users. As such, informed consent was not required.
Consent for publication: Non applicable.
Author contributions: Matthew Kennis: Conceptualization; Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Alex Tagawa: Formal analysis; Writing – review & editing.
Vanessa M. Kung: Writing – review & editing.
Gabrielle Montalbano: Writing – review & editing.
Isabella Narvaez: Writing – review & editing.
Carlos Franco-Paredes: Writing – review & editing.
Lilian Vargas Barahona: Writing – review & editing.
Nancy Madinger: Writing – review & editing.
Leland Shapiro: Writing – review & editing.
Daniel B. Chastain: Writing – review & editing.
Andrés F. Henao-Martínez: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Shapiro reports receiving grants from the Emily Foundation during the conduct of the study. Dr Henao-Martínez reported receiving a K12-clinical trial award as a co-principal investigator for the Expanded Access IND Program (EAP) to provide the Yellow Fever vaccine (Stamaril) to persons in the United States outside the submitted work. No other disclosures were reported. The Editor-in-Chief of Therapeutic Advances in Infectious Disease is an author of this article; therefore, the peer review process was managed by alternative members of the Board and the submitting Editor had no involvement in the decision-making process.
Availability of data and materials: The corresponding author had full access to data in the study and had final responsibility for the decision to submit the manuscript for publication. The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.
References
- 1. Tamerius J, Nelson MI, Zhou SZ, et al. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect 2011; 119: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson GE, Pondo T, Toews K-A, et al. Epidemiology of Invasive Group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis 2016; 63: 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamagni TL, Darenberg J, Luca-Harari B, et al. Epidemiology of severe streptococcus pyogenes disease in Europe. J Clin Microbiol 2008; 46: 2359–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danchin MH, Rogers S, Kelpie L, et al. Burden of acute sore throat and group a streptococcal pharyngitis in school-aged children and their families in Australia. Pediatrics 2007; 120: 950–957. [DOI] [PubMed] [Google Scholar]
- 5. Andersson M, Pallon J, Cronberg O, et al. Seasonal variations in use and outcome of rapid antigen detection tests and cultures in pharyngotonsillitis: a register study in Primary Care. BMC Infect Dis 2021; 21: 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolford RW, Goyal A, Belgam Syed SY, et al. pharyngitis. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2022, https://www.ncbi.nlm.nih.gov/books/NBK519550/ [PubMed] [Google Scholar]
- 7. Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on ‘Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis’. J Med Virol 2020; 92: 714–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen JF, Pauchard J-Y, Hjelm N, et al. Efficacy and safety of rapid tests to guide antibiotic prescriptions for Sore Throat. Cochrane Database Syst Rev 2020; 6: CD012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamburger M, Puck TT, Hamburger VG, et al. Studies on the transmission of hemolytic streptococcus infections: III. Hemolytic streptococci in the air, floor dust, and bedclothing of hospital wards and their relation to cross infection. J Infect Dis 1944; 75: 71–78. [Google Scholar]
- 10. Coffey PM, Ralph AP, Krause VL. The role of Social Determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis 2018; 12: e0006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gunnarsson RK, Holm SE, Söderström M. The prevalence of beta-haemolytic streptococci in throat specimens from healthy children and adults: implications for the clinical value of Throat cultures. Scand J Prim Health Care 1997; 15: 149–155. [DOI] [PubMed] [Google Scholar]
- 12. Wong C-h, Chang H-C, Pasupathy S, et al. Necrotizing fasciitis. J Bone Joint Surg 2003; 85: 1454–1460. [PubMed] [Google Scholar]
- 13. Salcido R, Ahn C. Necrotizing fasciitis. Adv Skin Wound Care 2007; 20: 288–293. [DOI] [PubMed] [Google Scholar]
- 14. Dale JB, Walker MJ. Update on group A streptococcal vaccine development. Curr Opin Infect Dis 2020; 33: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karthikeyan G, Guilherme L. Acute rheumatic fever. Lancet 2018; 392: 161–174. [DOI] [PubMed] [Google Scholar]
- 16. D’Amico F, Marmiere M, et al. Covid-19 seasonality in temperate countries. Environ Res 2022; 206: 112614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrera AL, Huber VC, Chaussee MS. The association between invasive group A streptococcal diseases and viral respiratory tract infections. Front Microbiol 2016; 7: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361221132101 for Seasonal variations and risk factors of Streptococcus pyogenes infection: a multicenter research network study by Matthew Kennis, Alex Tagawa, Vanessa M. Kung, Gabrielle Montalbano, Isabella Narvaez, Carlos Franco-Paredes, Lilian Vargas Barahona, Nancy Madinger, Leland Shapiro, Daniel B. Chastain and Andrés F. Henao-Martínez in Therapeutic Advances in Infectious Disease


