Abstract
Newly-diagnosed or relapses of immunoglobulin A nephropathy (IgAN) have been associated with COVID-19 vaccination in the literature. Most reported cases were mild clinical diseases characterized by microscopic haematuria and do not require dialysis treatment. This current case report describes a 55-year-old male patient that presented to the emergency department with acute kidney injury after receiving the first dose of the mRNA-1273 COVID-19 vaccine. After admission, his renal function deteriorated rapidly, and then he developed uraemic encephalopathy. He underwent emergency haemodialysis with a rapid improvement in his mental status. Renal biopsy showed newly-diagnosed IgA nephropathy along with markedly elevated plasma level of galactose-deficient-IgA1 (Gd-IgA1) antibody. The patient did not receive immunosuppressive treatment and is now dialysis-free. Immune activation is considered an essential factor in developing or exacerbating IgAN following COVID-19 vaccination. This current case report demonstrates that elevated Gd-IgA1 antibody may be the potential mechanistic link between COVID-19 vaccination and IgAN.
Keywords: Acute kidney injury, immunoglobulin A nephropathy, COVID-19 vaccination, galactose-deficient IgA1, case report
Introduction
Immunoglobulin A nephropathy (IgAN) following vaccination for the coronavirus disease 2019 (COVID-19) has been reported after the worldwide vaccination programme. For example, most reported patients suffering from de novo or relapsed IgAN had received an mRNA vaccine, either BNT-162b2 (Pfizer) or mRNA-1273 (Moderna),1,2 while a few cases of IgAN were reported after the ChAdOx1-S (AstraZeneca) vaccination.3 Most cases reported in the literature had mild disease and the IgAN resolved spontaneously after conservative treatments.4 This current case report describes a patient with newly-diagnosed IgAN that presented with acute kidney failure 2 weeks after receiving the first dose of the mRNA-1273 vaccine. To the best of our knowledge, this is the first report of newly-diagnosed IgAN associated with the COVID mRNA vaccination presenting as nephrotic syndrome, acute kidney injury progressing to uraemia and concomitant elevation of plasma galactose-deficient IgA1 (Gd-IgA1) antibody.
Case report
On 26 August 2021, a 55-year-old male patient presented to the Emergency Department, Far Eastern Memorial Hospital, New Taipei City with acute kidney injury (AKI) and abnormal liver function having experienced nausea, vomiting and general malaise for 2 weeks after receiving the first dose of the mRNA-1273 vaccine on 12 August 2021. He had no major systemic disease, no genetic or psychosocial history, except chronic hepatitis B infection. Nine months before admission, a comprehensive health check-up report showed normal renal function without microscopic haematuria or proteinuria. Laboratory tests revealed the following levels: serum blood urea nitrogen 54 mg/dl (reference range 7–25 mg/dl); creatinine 1.94 mg/dl (reference range 0.7–1.3 mg/dl); total bilirubin 0.85 mg/dl (reference range 0.3–1.0 mg/dl); aspartate aminotransferase 86 U/l (reference range 13–39 U/l); and alanine aminotransferase 114 U/l (reference range 0–41 U/l). Gross haematuria was not noted in this patient. Complete blood cell count showed the following: white cell count 10 540 cells/μl (reference range 3800–10 400 cells/μl); haemoglobin 19.9 g/dl (reference range 13.0–17.0 g/dl); and platelet count 130 000/μl (reference range 140 000–400 000/μl).
On admission on 19 September 2021, his body temperature was 36.2°C, pulse rate 80 beats per minute, respiratory rate 20 times per minute and blood pressure 110/90 mmHg. Urinalysis revealed proteinuria, haematuria and pyuria (4+ urine protein, 35.6 red blood cells per high-power field and 21.7 white blood cells per high-power field). Granular casts and free epithelial cells were found in the urine sediment. Abdominal ultrasonography was unremarkable. His serum albumin and globulin were 2.0 g/dl (reference range 3.5–5.7 g/dl) and 3.5 g/dl (reference range 2.0–3.5 g/dl), respectively. His 24-h urine protein excretion was 11.57 g (reference range <0.2 g). Unfortunately, his renal function deteriorated rapidly after admission. Uraemic encephalopathy with generalized seizures ensued. Magnetic resonance imaging was undertaken and reported no intracranial space-occupying lesion. He was treated with emergency haemodialysis and his consciousness quickly improved.
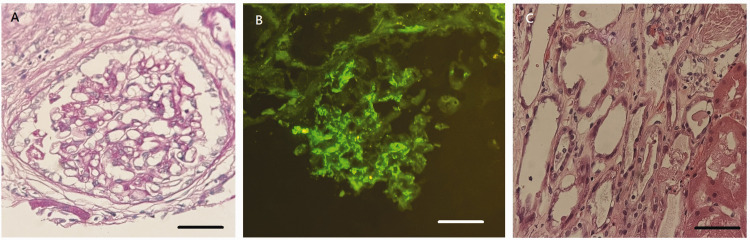
Elevated serum IgA (505.1 mg/dl; reference range 66–433 mg/dl) and decreased complement C3 (78.2 mg/dl; reference range 87.0–200.0 mg/dl) and complement C4 (12.2 mg/dl; reference range 19.0–52.0 mg/dl) were noted. Renal biopsy was then performed on 1 October 2021 (Figure 1). Microscopically, the glomeruli showed focal mild increase of mesangial cellularity and matrix in more than 50% of glomeruli (M1). Many tubules showed flattened epithelium with loss of nuclei, compatible with acute tubular necrosis (ATN). There was no endocapillary hypercellularity (E0), absence of segmental glomerulosclerosis (S0) and absence of crescent formation (C0). An estimated 20% of the cortical area showed tubular atrophy (T0) and interstitial fibrosis with focal mononuclear cell infiltration was noted. Periodic acid Schiff stain revealed focal mild mesangial proliferation. The immunofluorescence study showed granular deposition of IgA and C3 (++). The pathological diagnoses were IgAN and ATN. The Oxford Classification of IgA nephropathy (MEST-C) was M1E0S0T0C0.5
Figure 1.
Representative photomicrographs of tissue sections from a renal biopsy undertaken from a 55-year-old male patient that presented with acute kidney injury and abnormal liver function having experienced nausea, vomiting and general malaise for 2 weeks after receiving the first dose of the mRNA-1273 COVID-19 vaccine. (a) The glomeruli showed mild increase of mesangial cellularity and matrix (scale bar 20 µm) along with (b) significant immunoglobulin A deposition (++) (scale bar 20 µm) and (c) Many tubules showed flattened epithelium with loss of nuclei, compatible with acute tubular necrosis (scale bar 20 µm). The colour version of this figure is available at: http://imr.sagepub.com.
Based on the pathological findings, the patient’s plasma level of Gd-IgA1 antibody was measured using an enzyme-linked immunosorbent assay (Immuno-Biological Laboratories (IBL America), Minneapolis, MN, USA). The result was 21 603 ng/ml. The patient did not receive immunosuppressive treatment. The total serum IgA level decreased over time and follow-up serum IgA before hospital discharge was within reference range (382.2 mg/dl). Three months after admission, follow-up creatinine decreased to 4.0 mg/dl. The patient recovered from renal failure and is dialysis-free, but proteinuria persists. The above case report was written according to the CARE guidelines.6
Discussion
Immunoglobulin A nephropathy, or Berger’s disease, is the most common primary glomerulonephritis globally, with the highest incidence in eastern Asia.7 IgAN is thought to be an autoimmune disease with genetic predispositions.8 Notably, bacterial infections, various viral infections, including the COVID-19,9 may induce IgAN and IgA vasculitis.8
Recently published case reports have raised concerns about de novo or relapsed IgAN following COVID-19 vaccination.4 According to the literature, most post-vaccination glomerulonephritis is minimal change disease or IgAN.2 Typically, post-vaccination IgAN presents as gross haematuria and is self-limiting.10,11 In a large case series reported recently,2 among a total of 13 cases of IgAN (eight new and five relapse cases, only three patients received immunosuppression. Notably, most newly-diagnosed and relapse cases improved under conservative treatments within 2 weeks and no case required dialysis.2 In the recent case series,12 there were two cases diagnosed as IgAN undergoing dialysis. One patient was a 66-year-old female with diabetic nephropathy and had already been receiving dialysis before biopsy.12 The other patient was a 57-year-old male with chronic kidney disease and high creatinine levels (6.2 mg/dl).12 He received systemic treatment with steroid and cyclophosphamide.12 At 4 week follow-up, his renal function had not recovered and he continued to require dialysis.12 The current patient exhibited a unique clinical presentation as IgAN associated with nephrotic range proteinuria, ATN and AKI, leading to the need for dialysis. The patient did not receive immunosuppressive therapy due to his critical illness but he still recovered from end-stage renal disease.
Levels of Gd-IgA1 antibody have never been investigated in previous case reports of IgAN associated with the COVID-19 vaccination. The induction of Gd-IgA1 and subsequent formation of immune complexes with anti-glycan autoantibodies is the pivotal mechanism of IgAN.13 In the current patient, the substantial elevation of Gd-IgA1 level to more than 20 000 ng/ml was consistent with the pathogenesis of IgAN. Based on our recent study of 98 IgAN patients,14 the medium level of Gd-IgA1 in 98 patients with IgAN was 9005 ng/ml (25–75 percentile: 5705–12313 ng/ml), compared with 605 ng/ml (25–75 percentile: 14.6–2703 ng/ml) in normal subjects. Strong activation of the innate immune system by mRNA vaccine may lead to the non-specific activation of T and B lymphocytes and exacerbation of underlying autoimmunity.4 While the vaccine-related glomerular disease does not obliterate the benefits of mass COVID-19 vaccination, it raises the question that if the second dose of mRNA vaccine is an appropriate recommendation for patients that suffer from such severe illness post-vaccination.
In conclusion, this current case highlights the possibility of acute renal failure and IgAN after COVID-19 vaccination. Elevated plasma levels of Gd-IgA1 antibody demonstrates that non-specific immune activation may be the potential mechanistic link between COVID-19 vaccination and IgAN.
Acknowledgement
The authors thank Mrs Ruo-Wei Hung for her assistance in measuring the plasma level of Gd-IgA1.
Footnotes
Author contributions: Y.S.C. and Y.L.C. took care of the patient. Y.S.C., C.W.Y., C.C.T., M.D.A., S.F.C. and Y.L.C. wrote the manuscript. C.C.T. performed the pathology analysis. M.D.A. and W.C.C. analysed the case and edited the manuscript.
The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Far Eastern Memorial Hospital, Taiwan (no. FEMH-2019-C-023) and the Ministry of Science and Technology, Taiwan (no. MOST 109-2314-B-418-011-MY3).
ORCID iD: Yen-Ling Chiu https://orcid.org/0000-0003-4504-5571
References
- 1.Abramson M, Mon-Wei Yu S, Campbell KN, et al. IgA Nephropathy After SARS-CoV-2 Vaccination. Kidney Med 2021; 3: 860–863. 2021/07/20. DOI: 10.1016/j.xkme.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klomjit N, Alexander MP, Fervenza FC, et al. COVID-19 Vaccination and Glomerulonephritis. Kidney Int Rep 2021; 6: 2969–2978. 2021/10/12. DOI: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenoglio R, Lalloni S, Marchisio M, et al. New Onset Biopsy-Proven Nephropathies after COVID Vaccination. Am J Nephrol 2022; 53: 325–330. 2022/03/31. DOI: 10.1159/000523962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bomback AS, Kudose S, D'Agati VD. De Novo and Relapsing Glomerular Diseases After COVID-19 Vaccination: What Do We Know So Far? Am J Kidney Dis 2021; 78: 477–480. 2021/06/29. DOI: 10.1053/j.ajkd.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimarchi H, Barratt J, Cattran DC, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91:1014–1021. DOI:10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Gagnier JJ, Riley D, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Dtsch Arztebl Int 2013; 110: 603–608. 2013/10/01. DOI: 10.3238/arztebl.2013.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo SC, Goh SM, Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology (Carlton) 2019; 24: 885–895. DOI:10.1111/nep.13592. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med 2013; 368: 2402–2414. DOI:10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 9.Pérez A, Torregrosa I, D’Marco L, et al. IgA-Dominant Infection-Associated Glomerulonephritis Following SARS-CoV-2 Infection. Viruses 2021; 13: 587. DOI: 10.3390/v13040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita Y, Yoshida K, Ichikawa D, et al. Abrupt worsening of occult IgA nephropathy after the first dose of SARS-CoV-2 vaccination. CEN Case Rep 2022; 11: 302–308. 2022/01/07. DOI: 10.1007/s13730-021-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nihei Y, Kishi M, Suzuki H, et al. IgA Nephropathy with Gross Hematuria Following COVID-19 mRNA Vaccination. Intern Med 2022; 61: 1033–1037. 2022/02/04. DOI: 10.2169/internalmedicine.8787-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caza TN, Cassol CA, Messias N, et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney360 2021; 2: 1770–1780. 2022/04/05. DOI: 10.34067/KID.0005372021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak J, Julian BA, Tomana M, et al. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 2008; 28: 78–87. 2008/01/29. DOI: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu YL, Lin WC, Shu KH, et al. Alternative Complement Pathway Is Activated and Associated with Galactose-Deficient IgA(1) Antibody in IgA Nephropathy Patients. Front Immunol 2021; 12: 638309. 2021/06/29. DOI: 10.3389/fimmu.2021.638309. [DOI] [PMC free article] [PubMed] [Google Scholar]