Abstract
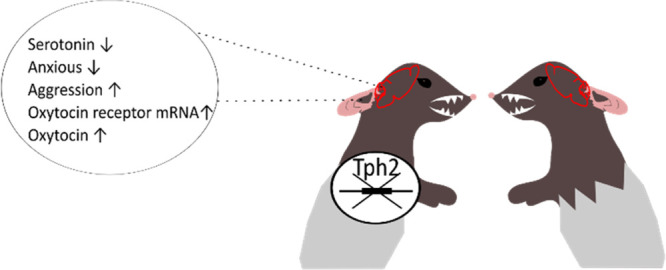
The central serotoninergic system is critical for stress responsivity and social behavior, and its dysregulations have been centrally implicated in virtually all neuropsychiatric disorders. Genetic serotonin depletion animal models could provide a tool to elucidate the causes and mechanisms of diseases and to develop new treatment approaches. Previously, mice lacking tryptophan hydroxylase 2 (Tph2) have been developed, showing altered behaviors and neurotransmission. However, the effect of congenital serotonin deficiency on emotional and social behavior in rats is still largely unknown, as are the underlying mechanisms. In this study, we used a Tph2 knockout (Tph2–/–) male rat model to study how the lack of serotonin in the rat brain affects anxiety-like and social behaviors. Since oxytocin is centrally implicated in these behaviors, we furthermore explored whether the effects of Tph2 knockout on behavior would relate to changes in the oxytocin system. We show that Tph2–/– rats display reduced anxiety-like behavior and a high level of aggression in social interactions. In addition, oxytocin receptor expression was increased in the infralimbic and prelimbic cortices, paraventricular nucleus, dorsal raphe nucleus, and some subregions of the hippocampus, which was paralleled by increased levels of oxytocin in the medial frontal cortex and paraventricular nucleus but not the dorsal raphe nucleus, central amygdala, and hippocampus. In conclusion, our study demonstrated reduced anxiety but exaggerated aggression in Tph2–/– male rats and reveals for the first time a potential involvement of altered oxytocin system function. Meanwhile, the research of oxytocin could be distinguished in almost any psychiatric disorder including anxiety and mental disorders. This research potentially proposes a new target for the treatment of such disorders, from a genetic serotonin deficiency aspect.
Keywords: serotonin, social behavior, affective behavior
Introduction
Serotonin (5-HT) has long been recognized to modulate the stress response and social behavior, and its dysfunction has been implicated in numerous psychiatric disorders. 5-HT synthesis is dependent on the rate-limiting enzyme tryptophan hydroxylase (Tph). There are two Tph isoforms, of which Tph2 is predominantly expressed in the brain.1 Indeed, Tph2 mRNA has been detected in multiple brain regions including the frontal cortex, thalamus, hippocampus, hypothalamus, and amygdala.2 The discovery of Tph2 opened up a new area of research. Human studies reported an association between functional Tph2 variants and personality traits3 as well as various neuropsychiatric disorders.4
Animals with targeted deletion of genes encoding mediators of the serotonergic transmission have been proven to be a powerful tool for detailed understanding of the contributions of the genetic basis of traits related to mood disorders. To model human Tph2 gene variance, Tph2 knockout (Tph2–/–) mice have been generated, which could sufficiently mimic human Tph2 polymorphisms.
Tph2–/– rats were introduced in 2016.5 Studies employing Tph2–/– rats showed increased aggressive behavior,6 increased levels of the neuroplasticity marker brain-derived neurotrophic factor in the prefrontal cortex under basal conditions,7 and an impaired response to acute stress exposure.7,8 However, at the behavioral level, the study of Tph2–/– rats is still inadequate. As to whether the rat model also demonstrates anxiety-like phenotypes and further social disturbances like in Tph2–/– mice remains to be established, as well as the potential underlying neurobiological mechanisms.
Taking human and mouse Tph2 data together, the changes in the expression of enzymes appear to particularly affect the domains of affective and social behavior. One molecule that is centrally implicated in both these behavioral domains is oxytocin.
Because 5-HT and oxytocin both have effects on anxiety and social processes, the attention for interactions between 5-HT and oxytocin is increasing. Based on the above, we hypothesized that the behavioral characteristics of Tph2–/– rats are related to altered oxytocin signaling. To test this hypothesis, we checked the alteration of the oxytocin system in different brain regions.
Results and Discussion
Results
Reduced Anxiety in Tph2 Knockout Rats
The elevated plus maze is a classic assay to assess anxiety levels. Tph2–/– rats spent more time in the open arms relative to Tph2+/+ rats (Figure 1A, ANOVA: F2,23 = 4.29, p = 0.03, permutation test: gTph2+/+<Tph2–/– = 1.05 [0.17; 2.05], p = 0.04), indicating a lower anxiety level in Tph2–/– rats. Consistently, Tph2–/– rats entered closed arms less often compared to Tph2+/+ rats (Figure 1B, ANOVA: F2,23 = 3.29, p = 0.06, permutation test: gTph2+/+<Tph2–/– = 1.1 [−2.35; 0.08], p = 0.03). Notably, there is also a medium, albeit non-significant, effect between Tph2+/+ and Tph2+/– groups (Figure 1B, gTph2+/+<Tph2+/– = 0.56 [−1.43; 0.40], p = 0.22). In other words, the fewer the Tph2 gene copies, the less frequent the rats enter closed arms.
Figure 1.

Elevated plus maze test. (A) Time spent in open arms, (B) closed-arm entries, (C) latency to enter open arms, (D) total distance moved on the elevated plus maze. nTph2+/+ = 10, nTph2–/– = 7, nTph2+/– = 9. The Hedges’ g for two comparisons against the shared control Tph2+/+ are shown in the Cumming estimation plot. The raw data are plotted on the upper axes. On the lower axes, mean differences are plotted as bootstrap sampling distributions. Each mean difference is depicted as a dot. Each 95% confidence interval is indicated by the ends of the vertical error bars.
The latency of the first entry into the open arms is a less conventional anxiety-related parameter but is of interest as it reflects the approach–avoidance conflict concerning aversive open arms. In our experiment, we did not find any noticeable effect between Tph2+/+ and Tph2+/– groups (Figure 1C, ANOVA: F2,23 = 1.13, p = 0.34). However, a trending effect was found between Tph2+/+ and Tph2–/– groups (gTph2+/+<Tph2–/– = 0.78 [−0.32; 1.87], p = 0. 12), which suggests that Tph2–/– rats have a higher latency of entering into aversive arms.
Finally, locomotor activity was evaluated by checking total distance rats traveled on the elevated plus maze. We could not establish a difference between Tph2+/+ and Tph2+/– or between Tph2+/+ and Tph2–/– groups (Figure 1D, ANOVA: F2,23 = 0.27, p = 0.76, permutation test: gTph2+/+<Tph2–/– = −0.32 [−1.37; 0.75], p = 0. 51). In conclusion, there is no discernible difference in the locomotor activity among three groups. We therefore conclude that differences in the elevated plus maze assay reflect reduced anxiety levels in the Tph2–/– rats, which is not due to a change in locomotor activity.
Elevated Aggressiveness in Tph2 Knockout Rats
Following the elevated plus maze test (24 h later), two unfamiliar rats from the same genotype were exposed to each other in a novel context for 20 min after being isolated for 3.5 h in a separate housing room (Figure 2A). We found a large genotype effect on total no contact behavior (Figure 2B, ANOVA: F2,23 = 42.28, p < 0.01, permutation test: gTph2+/+<Tph2–/– = −6.38 [−7.98; −4.5], p < 0.01), indicating that Tph2–/– rats have a higher level of active social interaction compared with Tph2+/+ rats. However, the prolonged social interaction of Tph2–/– rats manifested as increased mounting behaviors. Indeed, the Tph2+/– groups showed a trend toward more mounting behaviors than the Tph2+/+ group (Figure 2C, ANOVA: F2,23 = 7.21, p < 0.01, permutation test: gTph2+/+<Tph2+/– = 0.64 [−0.42; 1.36], p = 0.16). Meanwhile, mounting behavior was significantly increased in Tph2–/– in comparison with Tph2+/+ rats (gTph2+/+<Tph2–/– = 1.47 [0.80; 3.83], p = 0.01). In other words, the disruption of the Tph2 gene leads to more mounting behavior.
Figure 2.
Social behavior test. (A) Behavioral categories, (B) total no contact (%), (C) total mounting (%), and (D) total aggressiveness (combined time mounting, fighting, defending, and chasing, %). nTph2+/+ = 10, nTph2–/– = 7, nTph2+/– = 9. The Hedges’ g for two comparisons against the shared control Tph2+/+ are shown in the Cumming estimation plot. The raw data are plotted on the upper axes. On the lower axes, mean differences are plotted as bootstrap sampling distributions. Each mean difference is depicted as a dot. Each 95% confidence interval is indicated by the ends of the vertical error bars.
Inter-male mounting may be a marker for dominance or aggressiveness. Finally, we assessed the total time spent on aggressiveness, which included aggressive behaviors, mounting, and chasing behaviors all together. We found that Tph2–/– and Tph2+/– male rats spent more time on aggressive behaviors compared to wild-type controls (Figure 2D, ANOVA: F2,23 = 13.81, p < 0.01, permutation test: gTph2+/+<Tph2–/– = 2.13 [1.09; 7.78], p < 0.01, gTph2+/+<Tph2+/– = 0.77 [0.184; 1.56], p = 0.10). The fighting was also observed and are shown in Supplementary Figure S1 (ANOVA: F2,23 = 9.06, p < 0.01, permutation test: gTph2+/+<Tph2–/– = 1.74 [1.18; 4.53], p < 0.01). We concluded that Tph2 gene knockout is sufficient to increase aggressiveness in male rats.
Altered Oxytocin Receptor mRNA Expression in Tph2 Knockout Rats
We found that homozygous and heterozygous Tph2 knockout was sufficient to alter both anxiety and aggressive behaviors in male rats relative to wild-type controls. Due to its role in intensive interactions with 5-HT, we proposed that oxytocin may be a relevant mediator. To test this, we first examined oxytocin receptor gene expression (mRNA levels) in areas previously associated with anxiety and aggression (Figure 3A). We presented four subregions to parallel the receptor and oxytocin levels in Figure 3, while some other data was as presented in Table 1.
Figure 3.
Oxytocin receptor mRNA expression and oxytocin levels (left side: oxytocin receptor mRNA expression; right side: oxytocin level). (A) Brain punching site diagram, (B) prelimbic cortex, (C) ventral CA1 region, (D) ventral CA3 region, (E) dorsal raphe nucleus, (F) medial frontal cortex, (G) hippocampus, (H) paraventricular thalamic nucleus, and (I) central nucleus of the amygdala. For oxytocin ELISA results, n = WT (6), Tph2–/– (6), for oxytocin receptor PCR results, Tph2+/+ = 10, nTph2–/– = 10. The Hedges’ g between Tph2+/+ and Tph2–/– is shown in the above the Gardner–Altman estimation plot. Both groups are plotted on the left axes; the mean difference is plotted on floating axes on the right as a bootstrap sampling distribution. The mean difference is depicted as a dot, and the 95% confidence interval is indicated by the ends of the vertical error bar. Abbreviations: PL, prelimbic cortex; MPFC, medial prefrontal cortex; IL, infralimbic cortex; CA1, field CA1 of the hippocampus; CA3, field CA3 of the hippocampus; DG, granular layer of the dentate gyrus; PVN, paraventricular thalamic nucleus; Amg, central amygdala nucleus; CeA, central amygdala; Hipp, hippocampus; DR, dorsal raphe nucleus.
Table 1. The Oxytocin Receptor mRNA Expression Levels in Different Brain Regions.
| location | Hedge’s g [95% CI] Tph2+/+ < Tph2–/– | P value |
|---|---|---|
| infralimbic cortex | 1.14 [0.07; 2.18] | 0.02 |
| dorsal dentate gyrus | 0.93 [−0.02; 1.64] | 0.05 |
| ventral dentate gyrus | 1.22 [0.32; 2.05] | 0.02 |
| paraventricular thalamic nucleus | 1.49 [0.52; 2.66] | <0.01 |
| central nucleus of the amygdala | 0.44 [−0.52; 1.31] | 0.32 |
Oxytocin receptor mRNA expression levels were found to be increased in the infralimbic cortex (Table 1, gTph2+/+<Tph2–/– = 1.14 [0.07; 2.18], p = 0.02), paraventricular nucleus (Table 1, gTph2+/+<Tph2–/– = 1.49 [0.52; 2.66], p < 0.01), prelimbic cortex (Figure 3B, gTph2+/+<Tph2–/– = 1.54 [0.44; 2.33], p < 0.01), and dorsal raphe nucleus (Figure 3E, gTph2+/+<Tph2–/– = 2.35 [1.34; 3.37], p < 0.01). In this study, the hippocampus was functionally segmented into dorsal and ventral compartments, and three regions were tested, namely, CA1, CA3, and the granular layer of the dentate gyrus. In the dorsal hippocampal compartment, the expression of oxytocin receptors was largely increased in the dentate gyrus (Table 1, gTph2+/+<Tph2–/– = 0.93 [−0.02; 1.64], p = 0.05). In the CA3 region, a small change was found, and no change was found in the CA1 region. However, in the ventral hippocampal compartment, the expression in CA1 (Figure 3C, gTph2+/+<Tph2–/– = 1.74 [0.99; 2.39], p < 0.01), CA3 (Figure 3D, gTph2+/+<Tph2–/– = 1.05 [0.13; 1.91], p = 0.03), and dentate gyrus (Table 1, gTph2+/+<Tph2–/– = 1.22 [0.32; 2.05], p = 0.02) were all largely increased. We conclude that oxytocin receptor expression was elevated consistently throughout the brain in Tph2–/– relative to Tph2+/+ rats.
Altered Oxytocin Levels in Tph2 Knockout Rats
In addition to examining receptor expression levels, we also determined oxytocin concentration (Figure 3A). Because of the sensitivity of the assay, several areas were merged to achieve sufficient peptide levels (e.g., prelimbic and infralimbic cortex). This is justified because of the indiscriminate receptor mRNA elevation in the pooled regions. Our oxytocin ELISA results indicated that the oxytocin level was largely increased in the medial prefrontal cortex (Figure 3F, gTph2+/+<Tph2–/– = 1.55 [0.80; 2.3], p = 0.02), hippocampus (Figure 3G, gTph2+/+<Tph2–/– = 0.86 [−0.64; 1.94], p = 0.14), paraventricular thalamic nucleus (Figure 3H, gTph2+/+<Tph2–/– = 2.5 [1.69; 3.63], p < 0.01), and central nucleus of the amygdala (Figure 3I, gTph2+/+<Tph2–/– = 2.13 [0.9; 3.57], p < 0.01). The oxytocin expression in the dorsal raphe nucleus is shown in Supplementary Figure S2. We conclude that, similar to the oxytocin receptor, the ligand is found more abundantly in the areas sampled of Thp2–/– male rats, relative to wild-type controls.
Discussion
The results from this study reveal that the knockout of Tph2 significantly affects rats’ behavior and influences oxytocin levels and the expression of its receptors. Tph2–/– rats are less anxious and show more social interaction. However, social interaction is dominated by high levels of aggression and mounting.
Tph2–/– rats exhibited less anxiety-like behaviors in the elevated plus maze as supported by a longer duration in open-arm and a reduction in closed-arm entries. However, the data collected showed a trend toward the opposite. Contrary to Tph2–/– rats, 5-HT transporter knockout rats, which harbor a high brain 5-HT concentration, showed high sensitivity to environmental stimuli.8,9 Hence, it is possible that the reduced anxiety level of Tph2–/– rats relates to an attenuated environmental sensitivity, reducing the awareness of the difference between the open and closed arms. At the same time, the decreased anxiety level is independent of activity, as total distance traveled does not differ between genotypes. Interestingly, an 82% serotonergic neurotoxin-induced depletion of 5-HT in the rat medial prefrontal cortex increased anxiety-like behavior on the elevated plus maze.10 Given the fact that the depletion of 5-HT ab origine probably leads to compensatory responses as often seen in conventional knockout animal models,11 the finding that Tph2–/– rats were less anxious may also be due to 5-HT-mediated developmental or compensatory changes that contribute to the anxiolytic profile.
As 5-HT regulates the aggression in both sexes, enhanced serotonergic activity could inhibit intermale aggression, while hindering 5-HT signaling will stimulate aggression.12,13 5-HT transporter knockout rats exhibit less aggression, more prosocial behaviors with a high sensitivity to social stimuli.9 In our case, Tph2–/– rats had outburst aggressive behaviors almost immediately when housed together with another rat in a novel environment (data not shown), as reported Tph2–/– rats have more dense social networks, a more unstable hierarchy, and normal social memory.14 Therefore, we propose that Tph2–/– rats have a deficit in updating environmental information, leading to disrupted transmission of social information like hierarchy and social network. At the same time, we noticed that Tph2–/– rats spent more time on social contact with their assigned partner, but in an ‘antisocial’ manner with increased mounting behavior. As the animals were tested in male–male social interactions, the mounting behavior might be an act of showing social dominance, which is in line with our previous finding in the resident intruder test.6
The reduced anxiety in Tph2–/– rats may relate to altered oxytocin signaling. Oxytocin infusion into the prelimbic cortex decreased anxiety-like behavior, and pharmacological blockade of the oxytocin receptor prevented this anxiolytic effect, indicating that the anxiolytic effects of oxytocin are mediated, at least in part, through oxytocin receptors in the prelimbic cortex.15 Although we did not measure oxytocin levels and oxytocin receptor mRNA expression levels in the same animals, it is well possible that the anxiolytic phenotype of Tph2–/– rats related to elevated oxytocin levels in the medial frontal cortex and enhanced oxytocin receptor expression in the prelimbic cortex. Besides, amygdala plays a key role in emotional processing16 including anxiety, fear learning, and memory17,18 with γ-aminobutyric acid-ergic (GABAergic) interneurons serving critically for some inhibitory circuits.19 Presumably, 5-HT could alter the GABAergic tone via 5-HT2A receptors.20−22 Meanwhile, oxytocin also serves as a potent modulator of inhibitory GABA transmission in the central amygdala. For instance, oxytocin infusion into the central amygdala increased GABA activity in this region.23 In line with a previous report that oxytocin infusion into the central amygdala could decrease anxiety,24 in our experiment, Tph2–/– rats exhibit a lower anxiety level with the oxytocin levels being largely increased in the central nucleus of the amygdala. We therefore suspect that increased oxytocin in this nucleus lowers anxiety levels in Tph2–/– rats by enhancing GABA transmission. The hippocampus can be functionally segmented into dorsal, intermediate, and ventral compartments, with the dorsal part mediating cognitive functions and the ventral part implicated in stress, emotion, and affect.25,26 Previously, it has been reported that a serotoninergic lesion of the ventral hippocampus leads to increased anxiety-like behaviors in the elevated plus maze, showing that 5-HT has an anxiety dampening role in the ventral hippocampus.27 Surprisingly, in our Tph2–/– rat model, under conditions of life-long deficiency of brain 5-HT, rats expressed reduced anxiety. At the same time, we noticed that oxytocin receptor mRNA expression levels were mostly increased in the ventral but not dorsal compartment of Tph2–/– rats. As intracerebroventricular infusion of oxytocin into the lateral ventricle has anxiolytic effects,28,29 the decreased anxiety as observed in Tph2–/– rats may relate in part to increased oxytocin signaling in the hippocampus. Further investigation is needed to delineate the specific role of oxytocin in the hippocampal subregions and their contribution to Tph2–/– behavior.
Also, the altered social behaviors in Tph2–/– rats may relate to altered oxytocin signaling. More specifically, Tph2–/– males show more aggression.30,31 Even female Tph2–/– mice showed more aggression in an environment-enriched terrarium test,32 and further supported by Kästner and colleagues,32 even weanlings (3–4 weeks old) of both sexes showed elevated aggression in a modified resident–intruder test.30 Furthermore, increased obsessive–compulsive-like behavior was observed in Tph2–/– mice in the marble burying test.30,33 Tph2–/– mice show no difference in total locomotor activity or exploratory behaviors in the open-field test, but they spent less time in the central field, indicative for elevated anxiety-like traits.33 In some studies, it is also reported that Tph2–/– mice displayed marginally reduced anxiety-like behavior.34 In animal studies, oxytocin was first indicated to be involved in depressive behaviors originating from the finding that intracerebroventricular oxytocin administration diminished the immobility time in mice in the forced swimming test.35 After that, it has been shown that intraperitoneal oxytocin administration reduced the immobility in this test.36 Central administration of selective 5-HT agonists increased the expression of oxytocin mRNA in hypothalamic nuclei,37 which is consistent with reports that 5-HT and 5-HT fibers influence brain regions rich in oxytocin.38−40 Central injection of oxytocin reduces anxiety in the rat social interaction test, which is fully blocked by an antagonist of 5-HT2A/2C receptors.41
The prelimbic cortex participates in the regulation of social interaction,42 and oxytocin regulates social approach and preference behaviors.43 Therefore, together with social interaction data from our experiment, we propose that oxytocin in the prelimbic cortex promotes social interaction in Tph2–/– rats. Selective deletion of oxytocin receptors on serotonergic dorsal raphe neurons reduced resident–intruder aggression in males.44 In line with this finding, the oxytocin receptor mRNA expression level in the dorsal raphe nucleus is greatly increased in Tph2–/– rats, which may explain their increased aggressiveness during social interaction. As the change of oxytocin in the dorsal raphe nucleus is slightly decreased in Tph2–/– rats, the increased oxytocin receptor mRNA expression levels could reflect a compensation for reduced oxytocin levels in this region. At the same time, altered GABA transmission in the amygdala also results in exaggerated fear, which may explain the high aggressiveness level of Tph2–/– rats during social interaction.
It is worth mentioning that whether increased oxytocin protein at different brain regions is due to increased production, storage, or release of oxytocin still needs further investigation. A quantitative analysis of the amount of precursor and intermediate forms of oxytocin could be done to provide information about the production status of oxytocin. Oxytocin is largely stored in large dense-core vesicles and dendrites45 and released through Ca2+-dependent exocytosis.46 Intracerebral microdialysis is proper for release monitoring as oxytocin could only biologically function in extracellular space and its fluctuations could sufficiently reflect local oxytocin-releasing situations.47
Although in human beings Tph2 complete dysfunction is a very rare situation, there is an association between Tph2 polymorphisms and neuropsychiatric disorders.48,49 Tph2 knockout rats magnify the phenotype and provide information in the context of 5-HT and transdiagnostic behavior. At the same time, some limitations should be taken into account. We only tested male animals, while sex difference could impact the development of oxytocin system50 and oxytocin-dependent behaviors.51 Besides, due to the small brain-punching sample volume, samples used to assess oxytocin levels in the hippocampus and medial prefrontal cortex involve a mixture of subregions. Besides, it should also be considered that housing conditions, dominance, and social hierarchies could impact the results.52,53 In this study, animals were separately housed with the same genotypes from weaning, and the behaviors in each cage were monitored to ensure that no fighting would occur between cage mates. Because the animals were exposed to mixed-genotype peers in the litters and co-housed with a same-genotype partner from weaning, the animals were very familiar with their cage mates. Nonetheless, potential confounds due to the cage environment cannot be completely eliminated. Therefore, it would be worth trying to conduct validation experiments under single-housing conditions. In addition, an upward trend of oxytocin receptor expression has been reported in female Tph2–/– mice, which was the opposite of our male rat results.54 As to whether this phenomenon is due to sex or species differences still needs to be investigated. Thus, repeating the studies in females would be beneficial. Besides, research about whether the manipulation of oxytocin could generate behavioral changes in Tph2–/– rats would have a great impact, also demonstrating the correlation between the expression of oxytocin receptor mRNA and the expression of oxytocin receptor protein.
In conclusion, we demonstrated that rats lacking Tph2 display a series of behavioral changes, which gives us more insights into the effects of long-term 5-HT deficiency. Meanwhile, the behavioral changes originating from congenital brain 5-HT deficiency are sometimes different from acquired short-term 5-HT deficiency due to medical intervention, which suggests that compensatory pathways developed in Tph2–/– rats, with participation of the oxytocin system. The overall increase in oxytocin levels and receptor expression suggests that interventions decreasing oxytocin signaling may have the potential to normalize the anxiolytic and anti-social behavior in those suffering from low Thp2 availability.
Methods
Animals
Tph2 knockout (Tph2–/–) rats were generated by a truncation mutation.55 Tph2–/–, wild-type (Tph2+/+), and heterozygous (Tph2+/–) rats were derived by crossing heterozygous rats (dark agouti) that were out crossed with wild-type rats (DA/OlaHsd) (Jacob Human and Molecular Genetics Center, Medical College of Wisconsin, Milwaukee, USA). For behavioral testing, 26 male rats (nTph2+/+ = 10, nTph2–/– = 7, nTph2+/– = 9) with the same genotype were housed two to three per cage (25 × 25 × 35 cm3, length × width × height) starting from weaning with 2 cm sawdust bedding in a 12 h light–dark cycle from 8 a.m. to 8 p.m. at a temperature of 21 ± 1 °C under controlled environmental conditions (humidity 45–60%), with food and water provided ad libitum. Rats between 70 ± 14 days old were used for all experiments, exclusively during the light period. For molecular testing, another cohort of 20 rats (nTph2+/+ = 10, nTph2–/– = 10) was housed under same conditions, that is, two to three per cage with the same genotype. All efforts to retain animals as humane as possible were made according to the three Rs for all animals used.56
All procedures were executed in accordance with the Dutch legal ethical guidelines of animal experiments, as approved by the Central Committee Animal Experiments, the Hague, the Netherlands.
Elevated Plus Maze
Anxiety-like behavior was measured using the elevated plus maze. The maze, elevated 50 cm from the floor, consisted of two open arms (50 × 10 cm, 10 lux) and two closed arms (50 × 10 cm) that were enclosed by a side wall. Rats were placed in the center of the maze, facing the open arm and given freedom to explore the apparatus for 5 min,57 while being recorded by a camera suspended above the center of the maze. Total open and closed arm entries, duration, and latency as well as total distance traveled on all arms were quantified. Results were collected using Observer EthoVision version (Noldus, Wageningen, the Netherlands) by a researcher blind to treatment conditions.
Social Behavior
Two unfamiliar animals with the same genotype were exposed to each other in a novel context for 20 min after being isolated for 3.5 h in a separate housing room. The novel context consisted of a PhenoTyper cage (45 × 45 × 45 cm3) with standard sawdust bedding (2 cm). Rats had no access to food or water during the experiment. Each 20 min session was recorded, and videos were scored using JWatcher version 1.0 (Dan Blumstein’s Lab, University of California, Los Angeles; The Animal Behavior Lab, Macquarie University, Sydney, Australia). Social interaction and aggressive interaction parameters for each individual rat were scored by the same experimenter as shown in Table 2. The data from two Tph2–/– rats were removed from the analysis because of a fierce fight between the two animals, which ended with one of the rats hiding in a corner and not moving anymore.
Table 2. Social Interaction and Aggressive Interaction Behaviors Measured during the Social Interaction Test.
| social interaction | non-social interaction | |
|---|---|---|
| social exploration | no contact | |
| grooming each other | rearing | |
| self-grooming | ||
| total aggressiveness | fighting | |
| mounting | ||
| chasing | ||
| defensing |
Analysis of Oxytocin Receptor mRNA Expression Levels
To eliminate the effects from behavioral testing on gene expression, another independent group of rats was used for a molecular study for which we used Tph2+/+ and Tph2–/– rats. The rats were sacrificed through decapitation and immediately frozen at −80 °C. The left hemisphere was used for qPCR. Brain regions were dissected according to The Rat Brain in Stereotaxic Coordinates 6th Edition(58) by brain punching using a Cryostat machine. We punched out the prelimbic cortex (Bregma 4.20–2.52 mm), infralimbic cortex (Bregma 3.72–2.52 mm), paraventricular thalamic nucleus (Bregma −1.20 to −3.96 mm), central amygdaloid nucleus (Bregma −1.44 to −3.24 mm), granular layer of the dentate gyrus (dorsal) (Bregma −2.16 to −3.00 mm), granular layer of the dentate gyrus (ventral) (Bregma −4.36 to −5.04 mm), field CA1 of the hippocampus (dorsal) (Bregma −2.52 to −3.00 mm), field CA1 of the hippocampus (ventral) (Bregma −4.36 to −5.04 mm), field CA3 of the hippocampus (dorsal) (Bregma −2.52 to −3.00 mm), field CA3 of the hippocampus (ventral) (Bregma −4.36 to −5.04 mm), and the dorsal raphe nucleus (Bregma −6.96 to −8.40 mm). The location of the brain punches is shown in Figure 3. Total RNA was isolated by a single step of guanidinium isothiocyanate/phenol extraction by using a PureZOL RNA Isolation Reagent (Bio-Rad Laboratories, Segrate, Italy) according to the manufacturer’s instructions and quantified by spectrophotometric analysis. The samples were then processed for real-time polymerase chain reaction (RT-PCR) to assess the expression of the oxytocin receptor (primers and probe assay ID: Rn00564446_g1, purchased from Life Technologies). In particular, an aliquot of each sample was treated with DNAse (Thermo Scientific, Rodano, Italy) to avoid DNA contamination. Purified RNA was analyzed by the TaqMan qRT-PCR One-Step RT-PCR kit for probes (Bio-Rad Laboratories, Italy) with a TaqMan RT-PCR instrument (CFX384 real-time system, Bio-Rad Laboratories). After the initial retrotranscription step, 39 cycles of PCR were performed. Samples were run in 384-well formats in triplicate as multiplexed reactions with a normalizing internal control (36b4; forward primer: TTCCCACTGGCTGAAAAGGT; reverse primer: CGCAGCCGCAAATGC; probe: AAGGCCTTCCTGGCCGATCCATC, purchased from Eurofins MWG Operon, Germany). A comparative cycle threshold (Ct) method was used to calculate the relative target gene expression.
Analysis of Oxytocin Levels
The right hemisphere was used to measure oxytocin levels. We focused on the medial frontal cortex, paraventricular thalamic nucleus, dorsal raphe nucleus, central nucleus of the amygdala, and hippocampus. Due to the detection range limit, we pooled the CA1, CA3, and dentate gyrus regions from the ventral and dorsal parts of the hippocampus. Brain regions were punched using the same method as described above. Then, the brain punching samples were homogenated in RIPA buffer (Sigma, lot. R0278) with a proteinase inhibitor (Thermo Scientific Halt Protease Inhibitor Cocktail, Lot. WF327612). The location of the brain punches is shown in Figure 3. After centrifugation at 4 °C at 10,000 rcf for 10 min, the supernatant was collected and diluted by PBS. The protein concentration was measured using Micro BCA Protein Assay Kit (Thermo Fisher, lot. WF325481). Finally, the supernatant calibrated into the same protein concentration was used for the measurement of oxytocin levels using an ELISA kit (Abcam, lot. 133050), according to the manufacturer’s instructions.
Statistical Analysis
Statistical inference was chiefly based on effect size (Hedges’ g) and confidence intervals. P-values were estimated using non-parametric permutation tests. Confidence intervals and p-values were estimated by shuffling the group labels over 5000 permutations. The results are represented as Gardner–Altman plots and reported in the text as effect size [lower bound; upper bound of 95% confidence interval], p value. Effect size interpretations follow Cohen’s 1998 guidelines.57 Small effect: g > 0.2; medium effect: g > 0.4; large effect: g > 0.8. The code and the table to reproduce this analysis are provided freely: https://gitlab.socsci.ru.nl/preclinical-neuroimaging/tph2. Figure assets with a CC-BY license were obtained from https://scidraw.io/.59−63
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.2c00448.
Result of fighting during the social interaction test and result of oxytocin in the dorsal raphe nucleus (PDF)
Author Contributions
J.H. and F.C. designed the experiments, analyzed the data, and edited the paper. X.M. performed the experiments, analyzed the data, and drafted the manuscript. J.G. assisted with data analysis and edited the paper. G.S., P.S., N.H., and J.S. performed the experiments and interpreted the data.
The study was supported by the EU H2020 MSCA ITN project “Serotonin and Beyond” (N 953327).
The authors declare no competing financial interest.
Supplementary Material
References
- Walther D. J.; Peter J.-U.; Bashammakh S.; Hörtnagl H.; Voits M.; Fink H.; et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. 10.1126/science.107819. [DOI] [PubMed] [Google Scholar]
- Zill P.; Büttner A.; Eisenmenger W.; Bondy B.; Ackenheil M. Regional mRNA expression of a second tryptophan hydroxylase isoform in postmortem tissue samples of two human brains. Eur. Neuropsychopharmacol. 2004, 14, 282–284. 10.1016/j.euroneuro.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gutknecht L.; Jacob C.; Strobel A.; Kriegebaum C.; Müller J.; Zeng Y.; et al. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int. J. Neuropsychopharmacol. 2007, 10, 309–320. [DOI] [PubMed] [Google Scholar]
- Waider J.; Araragi N.; Gutknecht L.; Lesch K.-P. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: A perspective. Psychoneuroendocrinology 2011, 36, 393–405. 10.1016/j.psyneuen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Kaplan K.; Echert A. E.; Massat B.; Puissant M. M.; Palygin O.; Geurts A. M.; et al. Chronic central serotonin depletion attenuates ventilation and body temperature in young but not adult Tph2 knockout rats. J. Appl. Physiol. 2016, 120, 1070–1081. 10.1152/japplphysiol.01015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters D. G. A.; de Boer S. F.; Terneusen A.; Newman-Tancredi A.; Varney M. A.; Verkes R.-J.; et al. Enhanced aggressive phenotype of Tph2 knockout rats is associated with diminished 5-HT1A receptor sensitivity. Neuropharmacology 2019, 153, 134–141. 10.1016/j.neuropharm.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Brivio P.; Sbrini G.; Peeva P.; Todiras M.; Bader M.; Alenina N.; et al. TPH2 Deficiency Influences Neuroplastic Mechanisms and Alters the Response to an Acute Stress in a Sex Specific Manner. Front. Mol. Neurosci. 2018, 11, 389. 10.3389/fnmol.2018.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrini G.; Brivio P.; Bosch K.; Homberg J. R.; Calabrese F. Enrichment Environment Positively Influences Depression- and Anxiety-Like Behavior in Serotonin Transporter Knockout Rats through the Modulation of Neuroplasticity, Spine, and GABAergic Markers. Genes 2020, 11, 1248. 10.3390/genes11111248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg J. R.; Lesch K.-P. Looking on the Bright Side of Serotonin Transporter Gene Variation. Biol. Psychiatry 2011, 69, 513–519. 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Pum M. E.; Huston J. P.; Müller C. P. The role of cortical serotonin in anxiety and locomotor activity in Wistar rats. Behav. Neurosci. 2009, 123, 449. 10.1037/a0014478. [DOI] [PubMed] [Google Scholar]
- Knobelman D. A.; Hen R.; Blendy J. A.; Lucki I. Regional patterns of compensation following genetic deletion of either 5-hydroxytryptamine1A or 5-hydroxytryptamine1B receptor in the mouse. J. Pharmacol. Exp. Ther. 2001, 298, 1092–1100. [PubMed] [Google Scholar]
- Carrillo M.; Ricci L. A.; Coppersmith G. A.; Melloni R. H. Jr. The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology 2009, 205, 349–368. 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- Yanowitch R.; Coccaro E. F. The neurochemistry of human aggression. Adv. gene. 2011, 75, 151–169. 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- Alonso L.; Peeva P.; Stasko S.; Bader M.; Alenina N.; Winter Y.; et al. Constitutive depletion of brain serotonin differentially affects rats’ social and cognitive abilities. bioRxiv 2021, 10.1101/2021.09.23.461469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S.; Dong S. M.; Maurer S. D.; Post C.; Leuner B. Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action. Neuropharmacology 2017, 125, 1–12. 10.1016/j.neuropharm.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. E.Emotion circuits in the brain .2000, 23 ( (1), ), 155–184. [DOI] [PubMed]
- Duvarci S.; Pare D. Amygdala microcircuits controlling learned fear. Neuron 2014, 82, 966–980. 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P. H.; Tye K. M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanits H.; Milenkovic I.; Mahr N.; Pataraia E.; Hainfellner J. A.; Kovacs G. G.; et al. GABAA receptor subunits in the human amygdala and hippocampus. Immunohistochemical distribution of 7 subunits. J. Comp. Neurol. 2018, 526, 324–348. 10.1002/cne.24337. [DOI] [PubMed] [Google Scholar]
- Jiang X.; Xing G.; Yang C.; Verma A.; Zhang L.; Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology 2009, 34, 410–423. 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- McDonald A. J.; Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience 2007, 146, 306–320. 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie D. G. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J. Neurophysiol. 1999, 82, 69–85. 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Huber D.; Veinante P.; Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005, 308, 245–248. 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Bale T. L.; Davis A. M.; Auger A. P.; Dorsa D. M.; McCarthy M. M. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J. Neurosci. 2001, 21, 2546–2552. 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale E.; Pehrson A. L.; Jeyarajah T.; Li Y.; Leiser S. C.; Smagin G.; et al. Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS spectrums 2016, 21, 143–161. 10.1017/S1092852915000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M. S.; Dong H.-W. Are the dorsal and ventral hippocampus functionally distinct structures?. Neuron 2010, 65, 7–19. 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W.; Cook A.; Scholl J. L.; Mears M.; Watt M. J.; Renner K. J.; et al. Serotonin in the ventral hippocampus modulates anxiety-like behavior during amphetamine withdrawal. Neuroscience 2014, 281, 35–43. 10.1016/j.neuroscience.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S.; Slattery D. A.; Uschold-Schmidt N.; Reber S. O.; Neumann I. D. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 2014, 42, 225–236. 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Windle R. J.; Shanks N.; Lightman S. L.; Ingram C. D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997, 138, 2829–2834. 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M.; Kane M. J.; Briggs D. I.; Sykes C. E.; Shah M. M.; Francescutti D. M.; et al. Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J. Neurochem. 2012, 121, 974–984. 10.1111/j.1471-4159.2012.07739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosienko V.; Bert B.; Beis D.; Matthes S.; Fink H.; Bader M.; et al. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Translational. Transl. Psychiatry 2012, 2, e122–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kästner N.; Richter S. H.; Urbanik S.; Kunert J.; Waider J.; Lesch K.-P.; et al. Brain serotonin deficiency affects female aggression. Sci. Rep. 2019, 9, 1366. 10.1038/s41598-018-37613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelieva K. V.; Zhao S.; Pogorelov V. M.; Rajan I.; Yang Q.; Cullinan E.; et al. Genetic Disruption of Both Tryptophan Hydroxylase Genes Dramatically Reduces Serotonin and Affects Behavior in Models Sensitive to Antidepressants. PLoS One 2008, 3, e3301 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L.; Popp S.; Waider J.; Sommerlandt F. M. J.; Göppner C.; Post A.; et al. Interaction of brain 5-HT synthesis deficiency, chronic stress and sex differentially impact emotional behavior in Tph2 knockout mice. Psychopharmacology 2015, 232, 2429–2441. 10.1007/s00213-015-3879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenberg G. Short-term behavioral effects of neurohypophyseal peptides in mice. Peptides 1981, 2, 1–8. 10.1016/S0196-9781(81)80003-4. [DOI] [PubMed] [Google Scholar]
- Arletti R.; Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987, 41, 1725–1730. 10.1016/0024-3205(87)90600-X. [DOI] [PubMed] [Google Scholar]
- Jørgensen H.; Kjær A.; Knigge U.; Møller M.; Warberg J. Serotonin stimulates hypothalamic mRNA expression and local release of neurohypophysial peptides. J. Neuroendocrinol. 2003, 15, 564–571. 10.1046/j.1365-2826.2003.01032.x. [DOI] [PubMed] [Google Scholar]
- Emiliano A. B. F.; Cruz T.; Pannoni V.; Fudge J. L. The interface of oxytocin-labeled cells and serotonin transporter-containing fibers in the primate hypothalamus: a substrate for SSRIs therapeutic effects?. Neuropsychopharmacology 2007, 32, 977–988. 10.1038/sj.npp.1301206. [DOI] [PubMed] [Google Scholar]
- Ho S. S.; Chow B. K.; Yung W. H. Serotonin increases the excitability of the hypothalamic paraventricular nucleus magnocellular neurons. Eur. J. Neurosci. 2007, 25, 2991–3000. 10.1111/j.1460-9568.2007.05547.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E.; Swanson L. W.; Steinbusch H. W. M.; Verhofstad A. A. J. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983, 277, 355–360. 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Yoshida M.; Takayanagi Y.; Inoue K.; Kimura T.; Young L. J.; Onaka T.; et al. Evidence That Oxytocin Exerts Anxiolytic Effects via Oxytocin Receptor Expressed in Serotonergic Neurons in Mice. J. Neurosci. 2009, 29, 2259. 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez L. E.; Rujano M.; Tucci S.; Paredes D.; Silva E.; Alba G.; et al. Medial prefrontal transection enhances social interaction. I: behavioral studies. Brain Res. 2000, 887, 7–15. 10.1016/S0006-8993(00)02931-0. [DOI] [PubMed] [Google Scholar]
- Lukas M.; Toth I.; Reber S. O.; Slattery D. A.; Veenema A. H.; Neumann I. D. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 2011, 36, 2159–2168. 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani J. H.; Williams Avram S. K.; Cui Z.; Song J.; Mezey É.; Senerth J. M.; et al. Raphe serotonin neuron-specific oxytocin receptor knockout reduces aggression without affecting anxiety-like behavior in male mice only. Genes, Brain Behav. 2015, 14, 167–176. 10.1111/gbb.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by Oxytocin and Vasopressin. Neuron 2012, 76, 142–159. 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Knobloch H. S.; Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 2014, 8, 31. 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K.; Bosch O. J.; Krömer S. A.; Singewald N.; Neumann I. D. Release of Oxytocin in the Rat Central Amygdala Modulates Stress-Coping Behavior and the Release of Excitatory Amino Acids. Neuropsychopharmacology 2005, 30, 223–230. 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Gainetdinov R. R.; Beaulieu J.-M.; Sotnikova T. D.; Burch L. H.; Williams R. B.; et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 2005, 45, 11–16. 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Beaulieu J. M.; Gainetdinov R. R.; Caron M. G. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell. Mol. Life Sci. 2005, 63, 6. 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborski S., Mintz E. M., Caldwell H. K.. Sex Differences in the Embryonic Development of the Central Oxytocin System in Mice. J. Neuroendocrinol. 2016, 28 (), 10.1111/jne.12364. [DOI] [PubMed] [Google Scholar]
- Dumais K. M.; Bredewold R.; Mayer T. E.; Veenema A. H. Sex differences in oxytocin receptor binding in forebrain regions: Correlations with social interest in brain region- and sex- specific ways. Horm. Behav. 2013, 64, 693–701. 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Perkins A. E.; Varlinskaya E. I.; Deak T. Impact of housing conditions on social behavior, neuroimmune markers, and oxytocin receptor expression in aged male and female Fischer 344 rats. Exp. Gerontol. 2019, 123, 24–33. 10.1016/j.exger.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.; Hiura L. C.; Yang E.; Broekman K. A.; Ophir A. G.; Curley J. P. Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Horm. Behav. 2019, 114, 104551. 10.1016/j.yhbeh.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Huo Y.; Zhang Y.; Guo H.; Liu Y.; Fang Q.; Zhang J. Tph2–/– female mice restore socio-sexual recognition through upregulating ERα and OTR genes in the amygdala. PLoS One 2018, 13, e0193395 10.1371/journal.pone.0193395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M.; Kaplan K.; Echert A.; Puissant M.; Geurts A. Genomic Tryptophan Hydroxylase 2 (Tph2) Mutation in Rats Increases Mortality and Disrupts Eupneic Breathing During Postnatal Development. FASEB J. 2015, 29, 861.2. 10.1096/fasebj.29.1_supplement.861.2. [DOI] [Google Scholar]
- Russell W. M. S., Burch R. L.. The principles of humane experimental technique. Methuen: 1959. [Google Scholar]
- Pellow S.; Chopin P.; File S. E.; Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C.. The rat brain in stereotaxic coordinates: hard cover edition: Elsevier; 2006. [Google Scholar]
- Asiminas A.Adult_hooded_rat_sitting. Zenodo2020.
- Asiminas A.Adult_Hooded_Rat_Grooming. Zenodo 2020.
- Asiminas A.Adult_Hooded_Rat_Hunching. Zenodo 2020.
- Asiminas A.Adult_Hooded_Rat_Standing. Zenodo. 2020.
- Asiminas A.Adult_Hooded_Rats_Fight. Zenodo. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




