Abstract

Increasing levels of air pollution are driving the need for the development of new processes that take “waste-to-chemicals”. Herein, we report the capture and conversion under ambient conditions of a major air pollutant, NO2, using a robust metal-organic framework (MOF) material, Zr-bptc (H4bptc = 3,3′,5,5′-biphenyltetracarboxylic acid), comprising {Zr6(μ3-O)4(μ3-OH)4(COO)12} clusters linked by 4-connected bptc4– ligands in an ftw topology. At 298 K, Zr-bptc shows exceptional stability and adsorption of NO2 at both low (4.9 mmol g–1 at 10 mbar) and high pressures (13.8 mmol g–1 at 1.0 bar), as measured by isotherm experiments. Dynamic breakthrough experiments have confirmed the selective retention of NO2 by Zr-bptc at low concentrations under both dry and wet conditions. The immobilized NO2 can be readily transformed into valuable nitro compounds relevant to construction, agrochemical, and pharmaceutical industries. In situ crystallographic and spectroscopic studies reveal strong binding interactions of NO2 to the {Zr6(μ3-O)4(μ3-OH)4(COO)12} cluster node. This study paves a circular pathway to enable the integration of nitrogen-based air pollutants into the production of fine chemicals.
Introduction
The growing emissions of nitrogen dioxide, NO2, from the combustion of fossil fuels contribute significantly to global warming, acid rain, and ozone depletion and have severe impacts on the environment and human health.1−4 State-of-the-art deNOx processes based upon selective catalytic reduction (SCR) incorporating precious metal catalysts, toxic chemicals, and significant energy consumption5 are struggling to meet increasingly stringent regulations.6 The transformation of waste into value-added chemicals is therefore becoming an important target in the development of “circular economy”, where products are made, used, and reused, rather than being disposed.7 Nitro compounds and their derivatives are important intermediates for a wide range of explosives, colorants, agrochemicals, and pharmaceuticals,8,9 but the state-of-the-art synthesis of nitro compounds relies heavily on the use of HNO3 and NH3 that are produced from the extremely energy-demanding Ostwald and Haber–Bosch processes, respectively.10 The capture and enrichment of pollutant NO2 and its conversion into nitro compounds are thus a promising route to achieve the circular utilization of reactive nitrogen resources, as well as reducing the carbon footprint for chemical industries.
Exploiting the high porosity and stability of porous materials for the reversible capture of target gases affords economically viable technologies for clean-up and mitigation of gaseous pollutants.11 Traditional porous materials, such as activated carbons,12 silica,13 and zeolites,14,15 have been tested for the adsorption of NO2. However, their limited structural stability and restricted design functionalization result in low and often irreversible adsorption. As emerging solid sorbents for a wide spectrum of gases and vapors,16,17 metal-organic framework (MOF) materials and their composites have been investigated for the adsorption of NO2.18 Although some systems have achieved high dynamic sorption (250–2138 ppm) in exceptional cases, the rapid structural degradation of the MOF host upon NO2 uptake has hampered their further applications (Table S1).19−24 To the best of our knowledge, only MFM-300(Al)25 and MFM-52026 have been reported to display fully reversible NO2 uptake for the pure gas over repeated cycles. However, MFM-300(Al) shows only a very low uptake of 1.4 mmol g–1 at low pressure (10 mbar and 298 K) owing to its inherently moderate binding sites (μ2-OH and aromatic C–H groups), while MFM-520 exhibits a low total uptake of 4.5 mmol g–1 (1.0 bar and 298 K) due to its limited porosity (surface area of 313 m2 g–1) (Figure S4). To enable the enrichment of NO2 within pores at low concentrations and the subsequent efficient conversion of NO2 to nitro compounds, the sorbent material must display high adsorption under both low and high pressures and afford sufficient stability upon regeneration. This represents an extremely challenging target.
Herein, we report the high adsorption of NO2 in a Zr-based MOF, Zr-bptc, which displays a fully reversible uptake of 4.9 and 13.8 mmol g–1 at 10 mbar and 1.0 bar, respectively, at 298 K. In addition, breakthrough experiments confirm that Zr-bptc exhibits highly selective retention of NO2 at low concentrations (2500 ppm) under both dry and wet conditions. Importantly, the immobilized NO2 molecules (4.9–13.8 mmol g–1) can be quantitatively converted to various nitro compounds under ambient conditions. The binding domains of NO2 (and also CO2 for comparison) in Zr-bptc have been determined by in situ synchrotron X-ray powder diffraction. The adsorbed NO2 molecules partially dimerize to N2O4 in the pore, and this has been studied by variable-temperature electron paramagnetic resonance (EPR) spectroscopy. The remaining NO2 monomers are stabilized by strong host–guest interactions with heats of adsorption (Qst) of 90 kJ mol–1, which have been visualized by in situ inelastic neutron scattering (INS) and EPR studies, coupled with density functional theory (DFT) calculations. More importantly, Zr-bptc can be fully regenerated upon the delivery of nitro compounds and reused, thus fulfilling the “waste-to-chemicals” target.
Experimental Methods
NO2 Adsorption Isotherms
Gravimetric sorption isotherms of NO2 were recorded at 298, 303, 308, and 313 K, maintained using a temperature-programmed water bath and furnace, on a Hiden Xemis system under ultrahigh vacuum (10–10 bar) using a turbo pumping system. Ultrapure research grade (99.999%) NO2 was purchased from BOC. In a typical gas adsorption experiment, acetone-exchanged Zr-bptc (50 mg) was loaded into the Xemis system and activated at 573 K under a dynamic high vacuum (10–10 bar) for 24 h to give fully desolvated Zr-bptc.
Breakthrough Experiments
The flow rate of the entering gas mixture was maintained at 40 mL min–1, and the gas concentration, C, of gases at the outlet was determined by mass spectrometry and compared with the corresponding inlet concentration C0, where C/C0 = 1 indicates a complete breakthrough. For breakthrough separation under wet conditions, a fixed bed was packed with Zr-bptc that had been treated and preadsorbed with water at 75% RH. A gas mixture of 0.25% NO2 (2500 ppm diluted in 22.25% He and 77.5% N2) was then passed through the fixed bed. Breakthrough separation of NO2/CO2 was conducted using a mixture of 0.25% NO2 (2500 ppm) and 6.25% CO2 diluted in 93.5% He. The concentrations of NO2 and CO2 were chosen to mimic a typical exhaust gas of combustion of diesels in marine transport (N2: 77.50%, O2: 13.75%, CO2: 6.25%, NO2: 0.212%, SO2: 0.17%, H2O: 0.025%, CO: 0.005%, hydrocarbons 0.005%).27
General Procedure for Conversion
Preactivated Zr-bptc (1.0 g) was packed in a fixed bed, and a gas flow of 2500 ppm NO2 (diluted in 77.5% N2 and 22.25% He) was passed through the column at 298 K until a complete breakthrough was achieved. The gas flow was switched off, and Zr-bptc loaded with 2500 ppm NO2 (denoted as NO2@Zr-bptc-N) was sealed for the study of conversion. The quantity of captured NO2 in NO2@Zr-bptc-N was determined by TGA, which shows a 19% weight loss between 25 and 330 °C, corresponding to an uptake of 5.1 mmol g–1 (Figure S23). This is consistent with that (4.9 mmol g–1) observed in the breakthrough experiment. Aromatic substrates (0.75 mmol) and CHCl3 (5.0 mL) were added to a 10 mL round-bottom flask and stirred for 5 min to obtain a clear solution. NO2@Zr-bptc-N containing 0.51 mmol NO2 was added to the solution under stirring at room temperature or 0 °C. Upon completion of the reaction, the mixture was centrifuged, the solid was recycled, and the supernatant was collected and reduced under vacuum for analysis. Nitration experiments were also conducted using Zr-bptc loaded with NO2 at 1 bar, 298 K (denoted as NO2@Zr-bptc-N*) (see SI 1.12). The quantity of captured NO2 in NO2@Zr-bptc-N* was determined by TGA, which shows a 40% weight loss between 25 and 330 °C, corresponding to an uptake of 14.3 mmol g–1 (Figure S23), consistent with that (13.8 mmol g–1) observed in the isotherm adsorption experiments.
Results and Discussion
The highly stable MOF Zr-bptc [Zr6O4(OH)4(bptc)3] (H4bptc = biphenyl-3,3′,5,5′-tetracarboxylic acid) incorporates 12-connected {Zr6(μ3-O)4(μ3-OH)4(COO)12} clusters linked by 4-connected bptc4– ligands to form an open and neutral framework in the ftw topology.28 Desolvated Zr-bptc exhibits cubic-shaped cages decorated with {Zr6} clusters and planar bptc4– linkers on the vertices and faces, respectively (denoted as Cage A; Figure 2a). Cage A has a diameter of ∼12 Å after considering the van der Waals’ radii, and they are interconnected through another type of smaller tetrahedral cages of ∼4.5 Å diameter (Cage B; Figure 2a) located at the 12 edges of cubic Cage A. The ratio of Cage A to B is 1:3. Desolvated Zr-bptc shows a Brunauer–Emmett–Teller (BET) surface area of 960 m2 g–1, a pore volume of 0.413 g cm–3, and high chemical, thermal, and water/moisture stability (Figures S1 and S2).
Figure 2.
Views of the crystal structures of Zr-bptc, [Zr6O4(OH)4(bptc)3·(NO2)7.5·(NO2)2.3·(N2O4)4.1], and [Zr6O4(OH)4(bptc)3·(CO2)2.8]. All structures were derived from Rietveld refinements of in situ synchrotron X-ray powder diffraction data collected at 298 K (C: gray; N: blue; O: red Zr: cyan; H: white). (a) Metal–ligand cage A (yellow) and B (rose) in Zr-bptc; (b) packing of adsorbed NO2 and N2O4 molecules in Zr-bptc; (c) enlarged view of binding sites of N2O4 in Zr-bptc; (d) enlarged view of the binding site of monomer NO2 in Zr-bptc; (e) packing of NO2–N2O4 in the pore of Zr-bptc (orange: N2O4; sea green: NO2 at site I; rose: NO2 at site II; key dipole–dipole interactions are labeled); and (f) enlarged view of the binding site of CO2 in Zr-bptc.
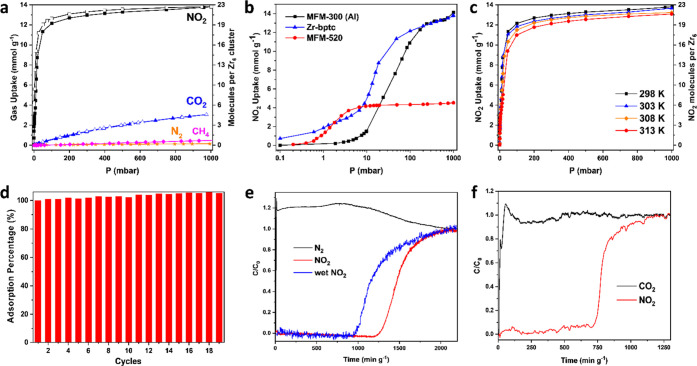
Isothermal adsorption of NO2 in Zr-bptc reveals exceptional uptakes of 1.8, 4.9, and 13.8 mmol g–1 at 0.001, 0.01, and 1.0 bar, respectively, at 298 K (Figures 1a–c and S5). The uptake of NO2 at very low pressure by Zr-bptc exceeds that of the state-of-the-art material, MFM-520 (1.3 and 4.2 mmol g–1 at 0.001 and 0.01 bar, respectively, at 298 K), thus representing a new benchmark for NO2 adsorption and confirming its potential for the efficient capture of NO2 at low concentrations. In addition, significant amounts of residual NO2 (30–50%) were observed upon pressure-swing desorption (Figures 1a and S5), indicating the strong binding of NO2 in Zr-bptc, which acts as an efficient NO2 store. Zr-bptc displays a comparable uptake capacity at 1.0 bar with the best-performing material, MFM-300(Al) (14.1 mmol g–1 at 298 K),25 and this high total uptake translates to high working capacities for the storage and conversion of NO2. Thus, the exceptional uptakes at both very low and ambient pressures endow Zr-bptc with great potential for the development of “waste-to-chemicals” processes. Although a number of metal-doped MOFs20,24,29 and MOF/graphite oxide composites23 show the dynamic adsorption of NO2 (ca. 500–1000 ppm) from gas mixtures under both dry and humid conditions, this is not comparable with the direct uptakes obtained via isotherm experiments with pure NO2 owing to the uncertainties in the former, which has been discussed in detail in our earlier report.25 Furthermore, these composite materials often display partial or complete structural degradation on NO2 adsorption–desorption cycles. In contrast, little change in structure or sorption capacity was observed for Zr-bptc after 19 cycles of adsorption and desorption of NO2 (Figures 1d, S1, and S2). Combined thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) determined the heats of adsorption for NO2 uptake in Zr-bptc to be 90 kJ mol–1 (Figure S9), substantially higher than for MFM-300(Al)25 and MFM-520(Zn)26 (61 and 58 kJ mol–1, respectively, determined by TGA–DSC; Figures S10 and S11), thus suggesting the presence of stronger interactions between Zr-bptc and adsorbed NO2 molecules. This is also consistent with the higher uptakes observed for Zr-bptc at both low and high pressures. Significantly, Zr-bptc shows low adsorption of CO2, CH4, and N2 at 298 K and 1.0 bar (3.05, 0.49, and 0.14 mmol g–1, respectively) (Figures 1a and S3). The value of Qst for CO2 uptake is 28 kJ mol–1 and shows little variation as a function of surface coverage, indicating weak host–guest interactions (Figures S12 and S13 and Table S2).
Figure 1.
Gas adsorption and dynamic separation data. (a) Adsorption isotherms for NO2, CO2, N2, and CH4 in desolvated Zr-bptc at 298 K (desorption of N2 and CH4 is omitted for clarity); (b) comparison of NO2 adsorption isotherms on a logarithmic scale for Zr-bptc, MFM-300(Al), and MFM-520 (desorption data are omitted for clarity); (c) sorption isotherms for NO2 in Zr-bptc (desorption data are omitted for clarity and shown in Figure S5); (d) cyclic adsorption–desorption of NO2 at 298 K between 0 and 200 mbar (a small gradual increase in the uptake was due to the minor amount of retained NO2 in Zr-bptc upon desorption under pressure-swing conditions); (e) breakthrough plots for N2/NO2 gas mixtures under dry and wet conditions (2500 ppm NO2 diluted in 77.5% N2 and 22.25% He); and (f) breakthrough plots for CO2/NO2 gas mixtures (2500 ppm NO2 and 6.25% CO2 diluted in 93.5% He).
Analysis of pure-component isotherms via ideal adsorbed solution theory (IAST)30 affords adsorption selectivities for various gas mixtures (N2, CO2, and NO2) at 298 K and 0–1.0 bar (Figure S14). The IAST selectivities for NO2/CO2 (1:99) and NO2/N2 (1:99) are ∼750 and >5000, respectively. It is worth noting that the very high IAST selectivities are subject to uncertainties owing to the extremely low adsorption of N2 or overlapping binding sites of NO2 and CO2 in Zr-bptc but confirm the potential of Zr-bptc in the selective adsorption of NO2 in the presence of CO2 and N2. Dynamic breakthrough experiments using a fixed bed packed with Zr-bptc were undertaken with gas mixtures of NO2/N2 (2500 ppm NO2 diluted in 77.5% N2 and 22.25% He) under both dry and wet (relative humidity: 75%) conditions and with NO2/CO2 (2500 ppm NO2 and 6.25% CO2 diluted in 93.5% He) at 298 K and 1.0 bar.27 Highly selective retention of NO2 by Zr-bptc was observed in all cases, showing a retention time of NO2 of 1200, 980, and 750 min g–1 for the mixture of NO2/N2 (dry), NO2/N2 (wet), and NO2/CO2, respectively (Figure 1e,f) (additional data are shown in Figures S6–S8). Compared with the dynamic adsorption capacity of NO2 (4.9 mmol g–1) under dry conditions, the small reduction (4.0 mmol g–1) under wet conditions is due to the competitive adsorption of water and reaction between adsorbed NO2 and H2O molecules in the pore. The dynamic selectivities of NO2/N2 and NO2/CO2 are estimated to be 151 and 24, respectively.
Rietveld refinement of the high-resolution synchrotron X-ray powder diffraction data of NO2-loaded Zr-bptc, [Zr6O4(OH)4(bptc)3·(NO2)7.5·(NO2)2.3·(N2O4)4.1], at 298 K revealed three independent binding sites, I, II, and III, which are assigned as NO2, NO2, and N2O4 molecules, respectively (Figures 2b, S15, and S20 and Table S3). The total crystallographic occupancy of NO2 molecules (18.0 NO2 per {Zr6} cluster) is slightly lower than that obtained from the isotherm (22.9 NO2 per {Zr6} cluster), which is likely due to the presence of a small amount of highly disordered NO2 molecules in the pores. The NO2 molecules at site I (7.5 NO2 per {Zr6} cluster) exhibit strong interaction with the carboxylate group [O2N···OOC = 2.64(1) and 2.62(4) Å] (Figure 2d). Furthermore, O centers from NO2 molecules form multiple supramolecular interactions with the aromatic C–H groups on benzene rings. The interaction [NO2···H–C = 2.00(7)], <C–H–O = 101.8(4)° and twofold hydrogen bond [NO2···H–C = 2.36(3), 2.91(8) Å, <C–H–O = 144.4(7), 156.5(7)°] (Table S4) stabilize the packing of NO2 molecules at site I. The multiple hydrogen bonds and dipole–dipole interactions between NO2 at site I and the framework suggest strong binding, consistent with the exceptionally high adsorption at low pressure. Three different dipole interactions including [O2N···benzene = 3.17(1) Å] and [NO2···NO2 = 2.01(4) and 2.71(8) Å] stabilize the NO2 molecules at site II (2.3 NO2 per {Zr6} cluster). Interestingly, NO2 molecules at site III (8.2 NO2 per {Zr6} cluster) are dimerized to N2O4 and stabilized by electrostatic and dipole interactions [N2O4···H–C = 2.03(6) and O2N···benzene = 3.27(8) Å] (Figure 2c). The packing of NO2–N2O4 molecules is sustained by multiple intermolecular dipole–dipole interactions based upon monomer-to-monomer, monomer-to-dimer, and dimer-to-dimer with distances ranging from 1.74(8) to 3.38(3) Å, offering an efficient storage environment (Figure 2e), consistent with the high Qst of Zr-bptc (90 kJ mol–1). Interestingly, the NO2 and N2O4 molecules immobilized in the pores of Zr-bptc on saturation show an unprecedented packing density of 1.83 g cm–3 at 298 K, higher than that observed in MFM-300(Al) (1.73 g cm–3 at 298 K), MFM-520 (1.42 g cm–3 at 298 K), liquid NO2 and N2O4 (1.45 and 1.44 g cm–3, respectively, at 294 K), and close to that of solid N2O4 (1.94 g cm–3 at 140 K).31 This result confirms a highly efficient packing of NO2/N2O4 molecules within the pores of Zr-bptc.
Two independent binding sites, I’ and II’, were located in cages B and A, respectively, in CO2-loaded Zr-bptc, [Zr6O4(OH)4(bptc)3·(CO2)2.8] (Figures S16–S19). The total crystallographic occupancy of 2.8 CO2 per {Zr6} cluster is lower than that obtained from the isotherm (5.2 CO2 per {Zr6} cluster), likely due to the presence of a large amount of highly disordered CO2 molecules in the pore owing to the weak host–guest interactions. CO2 molecules at site I’ (0.93 CO2 per {Zr6} cluster) display dipole interactions with the carboxylate group [O2C···OOC = 3.16(1) Å] (Figure 2f). In addition, multiple weak supramolecular interactions were observed between CO2I’ molecules and the benzene ring [O=C=O···H–C = 2.38(2)–3.47(5) Å] (Table S4). The CO2 molecules at site II’ (1.87 CO2 per {Zr6} cluster) are stabilized by the π···π interaction with the phenyl ring [O2C···benzene = 3.47(9) Å]. Overall, the host–guest binding interaction is notably weaker than that of NO2, consistent with low Qst (28 kJ mol–1) and the selective uptake of NO2 in the breakthrough experiment.
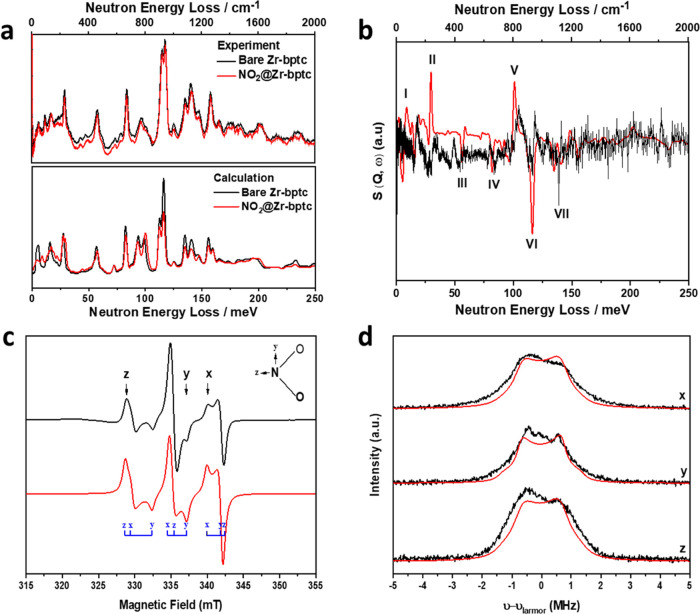
In situ INS, coupled with DFT calculations, enabled the visualization of the binding dynamics for NO2-loaded Zr-bptc with a focus on the −CH groups involved in the supramolecular contacts. Seven major changes in the INS spectra were observed on the adsorption of NO2 in Zr-bptc (Figure 3a,b). Peaks I–III at low energy transfer (<60 meV) and peaks IV–VII at high energy (80–150 meV) correspond to deformational modes of the phenyl ring and the twisting/wagging/scissoring modes of the aromatic −CH groups, respectively. These changes support the direct interactions between adsorbed NO2/N2O4 molecules and the soft −CH groups, consistent with the in situ crystallographic analysis.
Figure 3.
Spectroscopic data. (a) Comparison of the experimental (top) and DFT-simulated (bottom) INS spectra for bare and NO2-loaded Zr-bptc; (b) comparison of the difference plots for experimental and DFT-calculated INS spectra of bare and NO2-loaded Zr-bptc. No scale factor was used for the DFT calculations. S, dynamic structure factor; Q, difference between the incoming and outgoing wave vectors; ℏω, the energy change experienced by the sample; (c) continuous-wave X-band (9.72 GHz) EPR spectrum of NO2@Zr-bptc at 10 K (black) and simulation (red) with gx = 2.0055, gy =1.991, and gz =2.0028 and 14N nuclear hyperfine interactions (nuclear spin, I = 1) of Ax = 145, Ay = 127, and Az =185 MHz, where x, y, and z define the NO2 molecular axes (inset). NO2 has a C2v point symmetry with the z axis along the C2 rotation axis, y parallel to the O–O vector, and x normal to the NO2 plane; (d) X-band Davies ENDOR spectrum (black) at 5 K and the static magnetic fields indicated, shown by the arrows in panel (c), dominantly selecting the NO2x, y, and z axes (top to bottom), respectively. ENDOR gives pairs of transitions separated by the effective hyperfine coupling for the orientation selected, centered on the Larmor frequency of the nucleus being probed (14.9 MHz for 1H at 350 mT). The black lines are the experimental spectra, and the red lines are the calculated spectra.
The presence of adsorbed NO2 monomers in Zr-bptc is confirmed unambiguously by EPR spectroscopy on NO2@Zr-bptc at 10 K (Figure 3c), which shows signals for immobilized NO2 with a resolution of both the anisotropic electronic g-factor and 14N hyperfine interaction.32 The spectral line width is approximately double that of NO2-loaded MFM-300(Al)25 (Table S5), reflecting the higher concentration of monomeric NO2 in the pores of Zr-bptc, as found by the crystallographic study. The interaction of NO2 with the framework was probed by Davies ENDOR (electron–nuclear double resonance) spectroscopy at 5.7 K, which reveals weak 1H hyperfine interactions with frequencies around 2 MHz (Figure 3d).33 Based on a point-dipole model, these can be reproduced with a H···NO2 distance of 3.8 Å. The ENDOR spectra are rather broad and relatively insensitive to the magnetic field of measurement, i.e., with respect to the orientation of the NO2 molecule. This is presumably because of the multiple monomer NO2 binding sites within the pores of Zr-bptc; much stronger orientation selection is observed for NO2@MFM-300(Al) system, which has only one monomer binding site. This result is consistent with the enhanced packing density of NO2 in Zr-bptc. The H···NO2 distance from ENDOR at 10 K is longer than that found from the structural model determined at room temperature, indicating that the trapped NO2 molecules form stronger intermolecular interactions at low temperature. On warming the sample (200–360 K; Figure S21), the spectra broaden and the signal intensity increases as a function of the monomer–dimer equilibrium in the pores.26
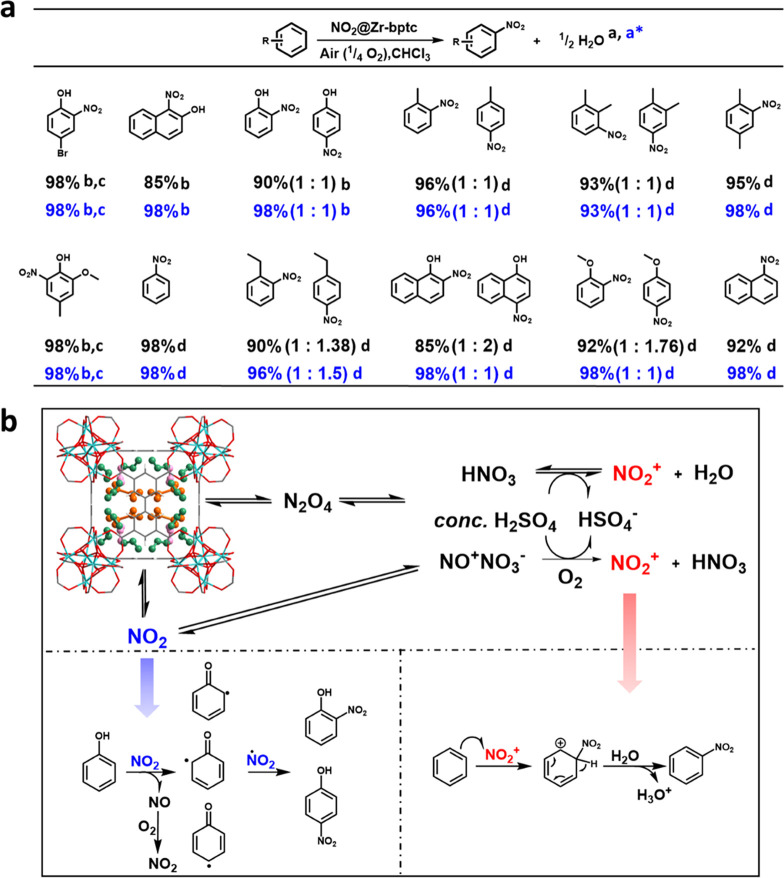
Nitration is widely used in the chemical industry to produce important nitro compounds (e.g., nitrobenzene), which are key intermediates for the synthesis of a wide range of explosives, colorants, agrochemicals, and pharmaceuticals.8,9,26,34 The global market for nitrobenzene is $9.8 billion in 2020 and is projected to reach $14.5 billion by 2027.35 The construction industry is the dominant end user of nitrobenzene and consumes approximately half of the world’s annual production. In addition, nitrobenzene is also used in the synthesis of paracetamol, which serves as a generic medicine globally. For other examples, nifedipine, entacapone, and niclosamide are nitro-group-containing medicines and are widely used to treat hypertension, Parkinson’s disease, and tapeworm infections, respectively.36 Nitration in the chemical industry is usually carried out using a mixture of concentrated acids (e.g., 20% nitric acid, 60% sulfuric acid, and 20% water for mononitration) at 80–120 °C.9 Because these processes are highly exothermic, enormous amounts of energy are consumed by cooling. Additionally, the industrial production of nitric acid via the oxidation of ammonia in the Ostwald process carries huge carbon footprints.10 Thus, nitration is widely considered one of the most energy-consuming and hazardous industrial processes. Although NO2 gas can be used as a nitration reagent, its highly corrosive and toxic nature renders the operation highly challenging.39 In addition, byproducts from overnitration and overoxidation are often obtained when using gaseous or liquid NO2 in nitration processes,40,41 and this is also confirmed by our tests on the nitration of a range of substrates with NO2 gas (Figures S27–S36). We sought to convert the captured NO2 by Zr-bptc to valuable nitro compounds at room temperature and without the use of mixtures of concentrated acids. Activated Zr-bptc was loaded with NO2 at 2500 ppm, which gives an uptake of 5.1 mmol g–1 (denoted as NO2@Zr-bptc-N; see the Experimental Methods and SI sections for details). Activated Zr-bptc was also loaded with NO2 at 1 bar, which gives an uptake of 14.3 mmol g–1 (denoted as NO2@Zr-bptc-N*; see the Experimental Methods and SI sections for details). A series of nitration experiments of aromatic compounds were then undertaken using NO2@Zr-bptc-N as the nitration source (Figures 4a and S37–S69). A fixed amount of NO2@Zr-bptc-N (containing 0.51 mmol NO2) was added to the mixture of aromatic substrates (0.75 mmol) and CHCl3 (5.0 mL) under stirring at room temperature or 0 °C, and the product was isolated by centrifugation and the supernatant was collected and analyzed by NMR spectroscopy. Importantly, the captured NO2 molecules can be quantitatively converted to nitro compounds in the absence or the presence of a catalytic amount (1%) of sulfuric acid, which is also confirmed by EPR analysis of the postreaction mixture that confirms the absence of residual NO2 (Fig. S24). Mononitration was achieved in a series of key aromatic compounds, including benzene, naphthalene, and p-xylene with yields of >85%. O- and p-substituted nitro compounds were obtained in nearly 1:1 ratio for phenol, toluene, and o-xylene and >1:1.4 ratio for ethylbenzene, anisole, and naphthalen-2-ol. Trace amounts of m-substituted nitro compound, which is a common product in the nitration process with gaseous NO2,40,41 were observed in these reactions. Control experiments using H4bptc or ZrOCl2·8H2O as the capture material were conducted, and little conversion was observed (Figure S26), demonstrating the key role of Zr-bptc in enriching NO2 from gas mixtures and releasing NO2 into the reaction medium (Figure S25).
Figure 4.
Schematic view of the utilization of NO2 pollutant for nitration processes. (a) Views of nitro compounds prepared using captured NO2. Yields are based on the captured NO2. aNO2@Zr-bptc-N was formed from Zr-bptc loaded with NO2 at 2500 ppm at 298 K to give a total capacity of NO2 of 5.1 mmol g–1. Conditions for nitration: a sample of NO2@Zr-bptc-N containing 0.51 mmol of NO2 was treated with 0.75 mmol of aromatic substrate in 5 mL of CHCl3 at room temperature. Yields and selectivity are in black. a*NO2@Zr-bptc-N* was formed from Zr-bptc loaded with NO2 at 1 bar at 298 to give a total capacity of NO2 of 14.3 mmol g–1. A sample of NO2@Zr-bptc-N* containing 1.43 mmol of NO2 was treated with 1.5 mmol of aromatic substrate in 5 mL of CH2Cl2 at room temperature. Yields and selectivity are in blue. bReaction conducted at 0 °C within 0.5 h; cthe reaction was completed within 1.0 h; da catalytic amount (1%) of conc. H2SO4 was added, and the reaction time was extended to 6.0 h; (b) proposed mechanism for the nitration reaction of phenol and benzene by NO2@Zr-bptc-N. The captured NO2 is released from the MOF into the reaction mixture as N2O4 or NO2. In the presence of conc. H2SO4, NO2+ is produced in the presence of O2, which drives the nitration process. For the nitration process in the absence of conc. H2SO4, the reaction occurs between NO2-derived radicals and substrate.37,38 The MOF plays a key role in the enrichment of NO2 from gas mixtures and facile release of captured NO2 into the reaction system.
In addition, EPR spectroscopy was employed to validate the proposed mechanism (Figure 4b), and the presence of signals of NO in the nitration of phenol demonstrated the presence of an alternative reaction pathway to nitronium ion-induced nitration, consistent with the short reaction time required (Figure 5a,b). The Zr-bptc recovered from these reactions can be regenerated fully via heating under dynamic vacuum. Uptake of NO2 and conversion efficiency in the synthesis of nitrobenzene were stable over three consecutive cycles (Figures S2 and S70 and Table S6). Thus, the integration of waste NO2 into the production of important nitro compounds not only holds the promise to reduce the carbon footprint of existing industrial nitration processes but also fulfills the requirement of “waste-to-chemicals” processes (Figure 6).
Figure 5.

EPR spectroscopy. (a) Continuous-wave X-band (9.72 GHz) EPR spectra of the nitration reaction mixture of phenol at 10 K (black) and simulation (red); and (b) continuous-wave X-band (9.72 GHz) EPR spectra of the nitration reaction mixture of benzene at 10 K (black) and simulation (red), confirming the absence of NO.
Figure 6.

Illustration of the nitrogen cycle for the synthesis of nitro compounds and regeneration of sorbent materials.
Conclusions
The robust porous MOF material, Zr-bptc, exhibits exceptional adsorption capacity of NO2 under both low- and high-pressure conditions and high heats of adsorption with an unprecedented packing density of NO2 in the pore. In situ synchrotron X-ray diffraction and INS studies, coupled with DFT calculations, unravel the molecular details on the host–guest binding that result in excellent adsorption performance. In situ EPR analysis demonstrates the existence of NO2–N2O4 equilibrium in this system. The successful synthesis of various fine chemicals from the key air pollutant demonstrates a promising “waste-to-chemicals” process for the recovery and circular utilization of reactive nitrogen-based wastes.
Acknowledgments
The authors thank EPSRC (EP/I011870), the Royal Society, and the University of Manchester for funding. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 742401, NANOCHEM). The authors are grateful to Diamond Light Source and Oak Ridge National Laboratory (ORNL) for access to Beamlines I11 and VISION (a DOE Office of Science User Facility), respectively. The computing resources were made available through the VirtuES and the ICE-MAN projects, funded by Laboratory Directed Research and Development program and Compute and Data Environment for Science (CADES) at ORNL. We acknowledge EPSRC for funding the UK National EPR Facility.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c07283.
Additional crystallographic information, gas adsorption data, thermogravimetric analysis, density functional theory (DFT) calculations, breakthrough data, stability test data, and conversion of captured NO2 are available (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lee M.; Shevliakova E.; Stock C.; Malyshev A. S.; Milly C. D. P. Prominence of the tropics in the recent rise of global nitrogen pollution. Nat. Commun. 2019, 10, 1437 10.1038/s41467-019-09468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial. Clean air for a sustainable world. Nat. Commun. 2021, 12, 5824. [DOI] [PMC free article] [PubMed]
- Wall D. H.; Nielsen U. N.; Six J. Soil biodiversity and human health. Nature 2015, 528, 69–76. 10.1038/nature15744. [DOI] [PubMed] [Google Scholar]
- Aravkin A. Y.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1223–1249. 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenović M.; Paprika M.; Marinković A. Denitrification techniques for biomass combustion. Renewable Sustainable Energy Rev. 2018, 82, 3350–3364. 10.1016/j.rser.2017.10.054. [DOI] [Google Scholar]
- WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. https://apps.who.int/iris/handle/10665/345329?locale-attribute=pt& World Health Organization (2021) (accessed July, 2022). [PubMed]
- Iaquaniello G.; Centi G.; Salladini A.; Palo E.; Perathoner S. Waste to chemicals for a circular economy. Chem. - Eur. J. 2018, 24, 11831–11839. 10.1002/chem.201802903. [DOI] [PubMed] [Google Scholar]
- Nepali K.; Lee H. Y.; Liou J. P. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. 10.1021/acs.jmedchem.8b00147. [DOI] [PubMed] [Google Scholar]
- Booth G.Nitro Compounds, Aromatic. In Ullmann’s Encyclopaedia of Industrial Chemistry; Wiley, 1988. [Google Scholar]
- Chen J. G.; Crooks M. R.; Seefeldt C. L.; Bren L. K.; Bullock M. R.; Darensbourg Y. M.; Holland L. P.; Hoffman B.; Janik J. M.; Jone K. A.; Kanatzidis G. M.; King P.; Lancaste M. K.; Lymar V. S.; Pfromm P.; Schneider F. W.; Schrok R. R. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, eaar6611 10.1126/science.aar6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Wang M.; Liang X.; Yuan J.; Yang H.; Wang S.; Ren Y.; Wu H.; Pan F.; Jiang Z. Organic molecular sieve membranes for chemical separations. Chem. Soc. Rev. 2021, 50, 5468–5516. 10.1039/D0CS01347A. [DOI] [PubMed] [Google Scholar]
- Abdulrasheed A. A.; Jalil A. A.; Triwahyono S.; Zaini A. A. M.; Gambo Y.; Ibrahim M. Surface modification of activated carbon for adsorption of SO2 and NOx: A review of existing and emerging technologies. Renewable Sustainable Energy Rev. 2018, 94, 1067–1085. 10.1016/j.rser.2018.07.011. [DOI] [Google Scholar]
- Ko G. J.; Ham D. S.; Kim K. J.; Zhu J.; Han B. W.; Chung J.; Yang M. S.; Cheng H.; Kim H. D.; Kang Y. C.; Hwang W. S. Biodegradable, flexible silicon nanomembrane-based NOx gas sensor system with record-high performance for transient environmental monitors and medical implants. NPG Asia Mater. 2020, 12, 71 10.1038/s41427-020-00253-0. [DOI] [Google Scholar]
- Wang X.; Hanson J. C.; Kwak J. H.; Szanyi J.; Peden C. H. Cation movements during dehydration and NO2 desorption in a Ba–Y, FAU zeolite: an in situ time-resolved X-ray diffraction study. J. Phys. Chem. C 2013, 117, 3915–3922. 10.1021/jp308307m. [DOI] [Google Scholar]
- Franus M.; Wdowin M.; Bandura L.; Franus W. Removal of environmental pollutions using zeolites from fly ash: A review. Fresenius Environ. Bull. 2015, 24, 854–866. [Google Scholar]
- Woellner M.; Hausdorf S.; Klein N.; Mueller P.; Smith W. M.; Kaskel S. Adsorption and detection of hazardous trace gases by metal–organic frameworks. Adv. Mater. 2018, 30, 1704679 10.1002/adma.201704679. [DOI] [PubMed] [Google Scholar]
- Liang Z.; Qu C.; Guo W.; Zou R.; Xu Q. Pristine metal–organic frameworks and their composites for energy storage and conversion. Adv. Mater. 2018, 30, 1702891 10.1002/adma.201702891. [DOI] [PubMed] [Google Scholar]
- Han X.; Yang S.; Schröder M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 2019, 3, 108–118. 10.1038/s41570-019-0073-7. [DOI] [Google Scholar]
- Peterson G. W.; Mahle J. J.; Decoste J. B.; Gordon W. O.; Rossin J. A. Extraordinary NO2 removal by the metal-organic framework UiO-66-NH2. Angew. Chem., Int. Ed. 2016, 55, 6235–6238. 10.1002/anie.201601782. [DOI] [PubMed] [Google Scholar]
- Shang S.; Yang C.; Wang C.; Qin J.; Li Y.; Gu Q.; Shang J. Transition metal inserted porphyrin metal-organic frameworks as π-backbonding adsorbents for NO2 removal. Angew. Chem., Int. Ed. 2020, 59, 19680–19683. 10.1002/anie.202007054. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Liu D.; Yin J.; Shang Y.; Du J.; Kang Z.; Wang R.; Chen Y.; Sun D.; Jiang J. An ultrafast responsive NO2 gas sensor based on a hydrogen-bonded organic framework material. Chem. Commun. 2020, 56, 703–706. 10.1039/C9CC09171H. [DOI] [PubMed] [Google Scholar]
- Ebrahim A. M.; Levasseur B.; Bandosz T. J. Interactions of NO2 with Zr-Based MOF: Effects of the size of organic linkers on NO2 adsorption at ambient conditions. Langmuir 2013, 29, 168–174. 10.1021/la302869m. [DOI] [PubMed] [Google Scholar]
- Levasseur B.; Petit C.; Bandosz T. J. Reactive adsorption of NO2 on copper-based metal-organic framework and graphite oxide/metal-organic framework composites. ACS Appl. Mater. Interfaces 2010, 2, 3606–3613. 10.1021/am100790v. [DOI] [PubMed] [Google Scholar]
- Ebrahim A. M.; Bandosz T. J. Ce(III) doped Zr-based MOFs as excellent NO2 adsorbents at ambient conditions. ACS Appl. Mater. Interfaces 2013, 5, 10565–10573. 10.1021/am402305u. [DOI] [PubMed] [Google Scholar]
- Han X.; Godfrey G. W. H.; Briggs L.; Davies J. A.; Cheng Y.; Daemen L. L.; Sheveleva M. A.; Tuna F.; McInnes J. L. E.; Sun J.; Drathen C.; George W. M.; Ramirez-Cuesta J. A.; Thomas M. K.; Yang S.; Schröder M. Reversible adsorption of nitrogen dioxide within a robust porous metal–organic framework. Nat. Mater. 2018, 17, 691–696. 10.1038/s41563-018-0104-7. [DOI] [PubMed] [Google Scholar]
- Li J.; Han X.; Zhang X.; Sheveleva M. A.; Cheng Y.; Tuna F.; McInnes J. L. E.; McPherson J. M. L.; Teat J. S.; Daemen L. L.; Ramirez-Cuesta J. A.; Schröder M.; Yang S. Capture of nitrogen dioxide and conversion to nitric acid in a porous metal–organic framework. Nat. Chem. 2019, 11, 1085–1090. 10.1038/s41557-019-0356-0. [DOI] [PubMed] [Google Scholar]
- Seddiek I. S.; Elgohary M. M. Eco-friendly selection of ship emissions reduction strategies with emphasis on SOx and NOx emissions. Int. J. Nav. Archit. Ocean Eng. 2014, 6, 737–748. 10.2478/IJNAOE-2013-0209. [DOI] [Google Scholar]
- Wang H.; Dong X.; Lin J.; Teat J. S.; Jensen S.; Cure J.; Alexandrov V. E.; Xia Q.; Tan K.; Wang Q.; Olson H. D.; Proserpio M. D.; Chabal J. Y.; Thonhauser T.; Sun J.; Han Y.; Li J. Topologically guided tuning of Zr-MOF pore structures for highly selective separation of C6 alkane isomers. Nat. Commun. 2018, 9, 1745 10.1038/s41467-018-04152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim A. M.; Bandosz T. J. Effect of amine modification on the properties of zirconium-carboxylic acid based materials and their applications as NO2 adsorbents at ambient conditions. Microporous Mesoporous Mater. 2014, 188, 149–162. 10.1016/j.micromeso.2014.01.009. [DOI] [Google Scholar]
- Myers A. L.; Prausnitz J. M. Thermodynamics of mixed-gas adsorption. AlChE J. 1965, 11, 121–127. 10.1002/aic.690110125. [DOI] [Google Scholar]
- Kvick Å.; McMullan R. K.; Newton M. D. The structure of dinitrogen tetroxide N2O4: neutron diffraction study at 100, 60, and 20 K and ab initio theoretical calculations. J. Chem. Phys. 1982, 76, 3754–3761. 10.1063/1.443414. [DOI] [Google Scholar]
- Shiotani M.; Moro G.; Freed J. H. ESR studies of NO2-adsorbed on Ti supported surfaces: Analysis of motional dynamics. J. Chem. Phys. 1981, 74, 2616–2640. 10.1063/1.441334. [DOI] [Google Scholar]
- Kulik L.; Lubitz W. Electron-nuclear double resonance. Photosynth. Res. 2009, 102, 391–401. 10.1007/s11120-009-9401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patai S.The Chemistry Of Amino, Nitroso And Nitro Compounds And Their Derivatives; The Hebrew University: Jerusalem, 1982. [Google Scholar]
- Nitrobenzene Market Size, Share & Trends Analysis Report By Application (Aniline Production, Synthetic Rubber Manufacturing), By End Use (Construction, Automotive), By Region, And Segment Forecasts, 2020 – 2027. https://www.grandviewresearch.com/industry-analysis/nitrobenzene-market (accessed July, 2022).
- Nepali K.; Lee Y. H.; Liou P. J. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. 10.1021/acs.jmedchem.8b00147. [DOI] [PubMed] [Google Scholar]
- Misiaszek R.; Crean C.; Geacintov E. N.; Shafirovich V. Combination of nitrogen dioxide radicals with 8-oxo-7, 8-dihydroguanine and guanine radical in DNA: oxidation and nitration end-products. J. Am. Chem. Soc. 2005, 127, 2191–2200. 10.1021/ja044390r. [DOI] [PubMed] [Google Scholar]
- Deng R.; You K.; Ni W.; Zhao F.; Liu P.; Luo H. Low-temperature and highly efficient liquid-phase catalytic nitration of chlorobenzene with NO2: remarkably improving the para-selectivity in O2-Ac2O-Hβ composite system. Appl. Catal., A 2020, 594, 117468 10.1016/j.apcata.2020.117468. [DOI] [Google Scholar]
- Bosch E.; Kochi J. K. Thermal and photochemical nitration of aromatic hydrocarbons with nitrogen dioxide. J. Org. Chem. 1994, 59, 3314–3325. 10.1021/jo00091a018. [DOI] [Google Scholar]
- Mori T.; Suzuki H. Ozone-mediated nitration of aromatic compounds with lower oxides of nitrogen (the Kyodai nitration). Synlett 1995, 1995, 383–392. 10.1055/s-1995-4979. [DOI] [Google Scholar]
- Qi X.; Cheng G.; Lu C.; Qian D. Nitration of simple aromatics with NO2 under air atmosphere in the presence of novel brønsted acidic ionic liquids. Synth. Commun. 2008, 38, 537–545. 10.1080/00397910701796758. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.