Abstract
Background
Anaesthetists have traditionally ventilated patients’ lungs with tidal volumes (TVs) between 10 and 15 ml kg−1 of ideal body weight (IBW), without the use of PEEP. Over the past decade, influenced by the results of the Acute Respiratory Distress Syndrome Network trial, many anaesthetists have begun using lower TVs during surgery. It is unclear whether the benefits of low TV ventilation can be extended into the perioperative period.
Methods
We reviewed the records of 29 343 patients who underwent general anaesthesia with mechanical ventilation between January 1, 2008 and December 31, 2011. We calculated TV kg−1 IBW, PEEP, peak inspiratory pressure (PIP), and dynamic compliance. Cox regression analysis with propensity score matching was performed to examine the association between TV and 30-day mortality.
Results
Median TV was 8.6 [7.7–9.6] ml kg−1 IBW with minimal PEEP [4.0 (2.2–5.0) cm H2O]. A significant reduction in TV occurred over the study period, from 9 ml kg−1 IBW in 2008 to 8.3 ml kg−1 IBW in 2011 (P=0.01). Low TV 6–8 ml kg−1 IBW was associated with a significant increase in 30-day mortality vs TV 8–10 ml kg−1 IBW: hazard ratio (HR) 1.6 [95% confidence interval (CI) [1.25–2.08], P=0.0002]. The association remained significant after matching: HR 1.63 [95% CI (1.22–2.18), P<0.001]. There was only a weak correlation between TV kg−1 IBW and dynamic compliance (r=−0.006, P=0.31) and a weak-to-moderate correlation between TV kg−1 IBW and PIP (r=0.32 P<0.0001).
Conclusions
Use of low intraoperative TV with minimal PEEP is associated with an increased risk of 30-day mortality.
Keywords: intraoperative care, pulmonary ventilation, tidal volume
Editor’s key points.
-
•
Low tidal volume (TV) ventilation (5–8 ml kg−1) with PEEP is of established benefit as part of ‘lung protection’ in ICU patients with acute lung injury.
-
•
However, the advantages in patients undergoing surgery are not established.
-
•
In this large retrospective study, ventilation using low TVs and minimal PEEP was associated with increases in 30-day mortality and length of hospital stay.
-
•
This suggests that ventilation using low TV alone might be harmful, and low TVs are beneficial only when used with PEEP.
-
•
More prospective data are required to confirm these findings.
Approximately 20 million general anaesthetics are administered annually in the USA alone. Traditionally, with the intent of ameliorating atelectasis induced hypoxia, anaesthetists have ventilated patients with tidal volumes (TVs) between 10 and 15 ml kg−1 of body weight without the use of PEEP.1 Over the past decade, influenced by the results of the Acute Respiratory Distress Syndrome Network (ARDSNet) trial, many care providers have strayed from this traditional teaching and begun using lower TV ventilation intraoperatively.2 The ARDSNet trial showed that critically ill patients with ARDS profited from a 9% survival benefit when TVs of 6 ml kg−1 predicted body weight (with PEEP) were chosen as opposed to the traditional 12 ml kg−1. Further investigations have shown that mechanical ventilation with traditional TVs initiates or aggravates an inflammatory response, when compared with low TV ventilation with PEEP, not only in injured lungs but in healthy lungs as well.3 4 Consequently, clinicians have started utilizing the ‘low TV’ concept with the assumption that healthy patients also need ‘lung protection’ from the detrimental effects of high TVs.5 While there is clear evidence of survival benefit in the intensive care unit (ICU) with ARDS patients, it remains unclear whether these benefits can be extended into the operating theatre and applied to patients without acute lung injury (ALI)/ARDS. We investigated the hypothesis that the use of low intraoperative TVs would be associated with a decrease in perioperative morbidity and mortality.
Methods
After obtaining Institutional Review Board approval and waiver of informed consent, we queried our anaesthesia data warehouse for all adult (age >18) in-patient and day of admission (DAS) surgical cases performed at our institution under general anaesthesia (GA) with tracheal intubation (TT) and mechanical ventilation between January 1, 2008 and December 31, 2011, excluding cardiac, thoracic, liver transplant, and palliative (e.g. percutaneous feeding tube placement, and tracheostomy) procedures. Patients who underwent more than one anaesthetic during their admission were excluded. Our institutional data warehouse was then queried to obtain administrative and outcome data. Patients missing administrative, outcome data, or both were excluded (Fig. 1 ).
Fig 1.
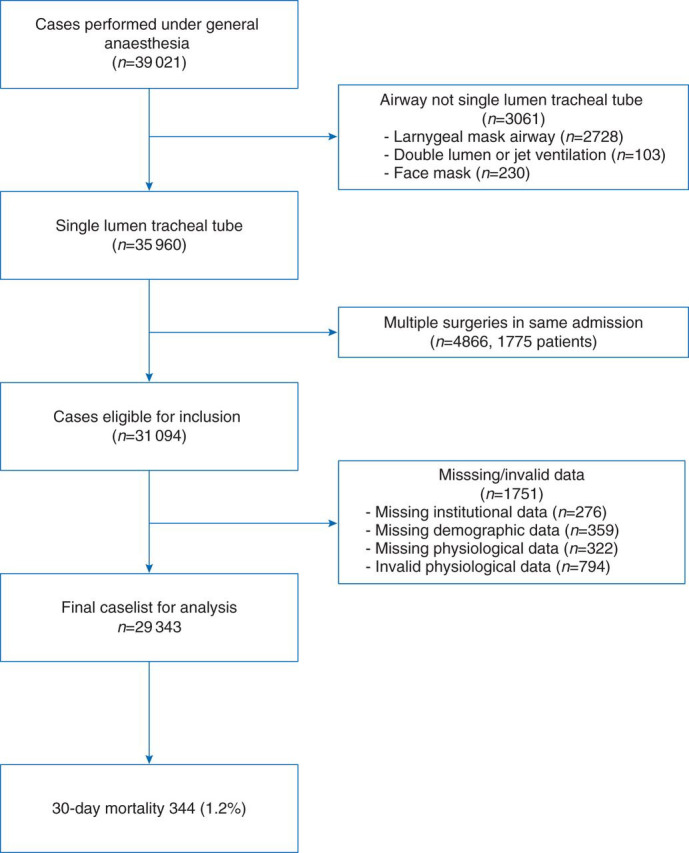
The CONSORT style flow diagram showing exclusion criteria.
Ventilator management and calculation of respiratory variables
Patients were mechanically ventilated using a variety of General Electric Healthcare (Madison, WI, USA) anaesthesia machines. During the study period, the following models were in use at our institution: Aisys Carestation, Datex-Ohmeda ADU Carestation, Aestiva/5, and Aespire. Expired TV and other physiologic data were recorded every 15 s by our anaesthesia information management system (AIMS, CompuRecord, Philips, Andover, MA, USA). Ventilator mode, plateau pressure, and set TV were not recorded because of limitations of our AIMS. Anaesthetic agent (inhaled vs i.v.), ventilation mode (volume control vs pressure control), ventilator settings, and fraction of inspired oxygen ( ) were chosen at the discretion of the attending anaesthetist.
For each mechanically ventilated patient, we then calculated the median value of the following variables over the length of intubation: expired TV, respiratory rate, peak inspiratory pressure (PIP), PEEP, and . Median dynamic compliance was calculated as median TV divided by (PIP–PEEP).6 We then calculated the median TV per kg, and the median TV kg−1 ideal body weight (IBW). IBW was determined using the Devine formula for men and the Robinson formula for women.7
All cases with TVs <250 ml and/or TV <3 or >20 ml kg−1 and PIP <3 cm H2O were excluded, because these data points were more than four standard deviations (sds) from the mean and likely represented artifacts. Excluding cases with low or negative PIP also filtered out most spontaneously breathing patients, since PIP is negative during spontaneous ventilation. Cases with PEEP >16 cm H2O were excluded as being invalid because our ventilators cannot apply PEEP values >16 cm H2O. Patients with IBW <40 kg or height <140 cm (more than 4 sds from the mean) were excluded, since the formulae for calculating IBW become inaccurate at extremes of size.7
Assessment of preoperative risk
To assess general preoperative risk, we used the ASA physical status score, age, gender, race, BMI, presence of obstructive lung disease (asthma, COPD, or both), and use of preoperative inhaled steroids, as recorded in the AIMS. Patients were stratified by BMI according to the World Health Organization definitions, and the BMI group was included as a covariate.8 In addition, we included a category for severely obese patients (BMI >35 kg m−2).9
As a measure of co-existing illness we used the All Patient Refined—Diagnosis Related Group (APR-DRG) severity of illness (SOI) and risk of mortality (ROM) scores (extreme, major, moderate, and minor, for both scores).10 The APR-DRG is a classification scheme developed by 3M based on nationwide samples and used by Medicare to determine the estimated resource consumption of a patient. The scores are calculated based on primary and secondary discharge diagnoses and thus capture all co-morbidities present during an admission. Although SOI and ROM are highly correlated for many conditions, they often differ because they relate to distinct patient attributes.11 The APR-DRG ROM has been shown to correlate well with mortality among ICU patients and to be a suitable tool for risk-adjusting 30-day mortality among acute myocardial infarction patients.12, 13, 14
Outcomes
The primary outcome examined was 30-day mortality. Mortality data were obtained either from the Social Security Administration Death Master File (National Technical Information Service, Alexandria, VA, USA) or from our institutional data (discharge disposition of ‘expired’). As a secondary outcome we examined the difference in observed vs expected length of stay (O-E LOS). Observed LOS was based on admission and discharge dates. Expected LOS was obtained from the University Health System Consortium.15 As the distribution of the difference in O-E LOS was not normal, we decided a priori to make a binary cut-off where a difference of >2 days’ excess stay was considered a negative outcome.
Statistical methods
Descriptive data are presented as mean (sd), median [inter-quartile range (IQR)], or n (%). For two group comparisons, χ 2 tests were used for categorical variables and either the Student t-test or the Wilcoxon-Mann–Whitney test for continuous variables, as appropriate. Spearman correlation or Kendall’s τ-b correlation was used to describe the bivariate association between two continuous variables. Ordinary least square regression, allowing both linear and quadratic terms, was fitted to assess the trend of TV used over the study period and to assess the relationship between median and TV. To investigate a possible non-linear relationship between TV and the log of the hazard ratio (HR)/odds ratio (OR), we decided a priori to create five TV groups. Quartiles were also created for age, BMI, and length of intubation. The largest group was always chosen as the reference group.
To verify that exclusions were random and not clustered, excluded cases were compared with the study population with respect to TV IBW and 30-day mortality. Because of the fact that ∼20% of the excluded observations were emergency cases (see the Results section), we repeated the same comparisons for only those excluded observations that were non-emergent. The Breslow–Day test for homogeneity of the ORs was used to check that the 30-day mortality of emergency cases was not different between the study cohort and the excluded cohort.
Multivariable modelling
Cox regression analyses were performed to assess the associations of TV kg−1 IBW with 30-day mortality. Weighted Schoenfeld Residuals were used to check the proportional hazard assumption for the TV groups. The effect of TV on O-E LOS >2 days among patients alive at discharge was assessed using logistic regression. For both the Cox and logistic regressions, stepwise selection was used to identify additional predictors of outcomes. Initial covariates considered included ASA physical status, age, gender, race, BMI group, laparoscopic vs open surgery, type of surgery, service year, presence of TT in situ, and length of intubation, PIP and dynamic compliance.
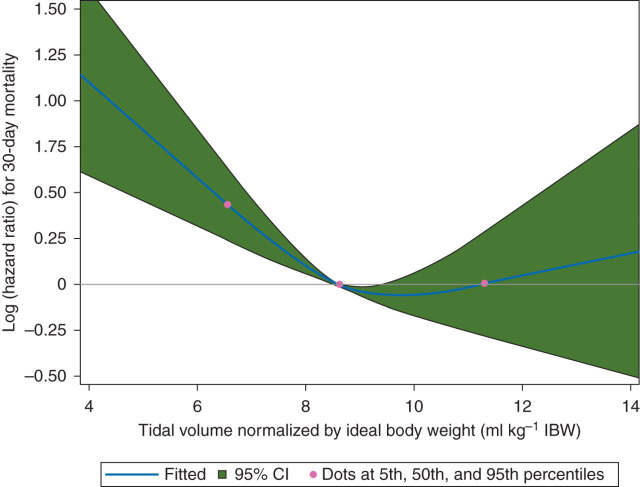
Restricted cubic spline (RCS) analysis was used to characterize the dose–response relationship between median TV ml kg−1 IBW and 30-day mortality, while adjusting for the same set of covariates as used in the final Cox model. The 5th, 50th, and 95th percentiles of TV ml kg−1 IBW were chosen as the default knots for model fitting.16
Propensity score matching
To confirm the independent effect of low TV on 30-day mortality, we undertook a propensity score-matched analysis. The TV 3–6, 6–8, 10–12, and 12–20 ml kg−1 IBW groups were each compared with the TV 8–10 ml kg−1 IBW group. Hereafter, we refer to them as the treated and the untreated groups, respectively. First, a forward stepwise logistic regression model was fitted to predict the propensity of receiving TV 6–8 ml kg−1 IBW. The following preoperative factors were used in the propensity score: age, ASA physical status, IBW, BMI, gender, race, emergency status, presence of obstructive disease, use of inhaled steroids, surgical specialty, use of laparoscopy, service year, the APR-DRG SOI and ROM scores, and an indicator representing attending anaesthetist. We then performed the propensity score matching and outcome analysis using the MatchIt, coxph, and Zelig packages in R 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria).17, 18, 19 We paired the treated and untreated subjects with a 1:1 ratio based on the logit of the propensity scores using the nearest neighbour matching without replacement. A caliper width of 0.2 units was used. We then carefully checked the balance of each preoperative factor between treated and untreated groups by examining the mean difference between the groups, standardized by the sd of the treated group. When the balance goal was achieved, we ran the Cox regression to estimate the HR of 30-day mortality for the treated group. Among those who were discharged, we also compared the LOS (using a Poisson regression) and the odds of having O-E LOS >2 days (using a logistic regression) between the two groups. All analyses were further adjusted for the following intraoperative variables: dynamic compliance, length of intubation, and presence of TT before arrival in the operating theatre.
Calculation of respiratory variables variable was done using Perl 5.10. Statistical analysis and plot generation were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA) and R versions 2.15.3 and 3.0.1.
Results
A total of 29 343 cases met the inclusion criteria and had complete data sets. There were 1751 (5.6% of eligible) cases excluded because of missing institutional data (n=276), baseline patient characteristics (n=359), physiological data (n=322), or invalid physiological data (n=794). (Figure 1) The mortality rate for these excluded subjects tended to be higher; however, after careful investigation, we found that nearly 20% (n=293) of the excluded cases were marked as emergencies, vs 9.6% of included cases. The ORs of 30-day mortality for emergency cases were not significantly different between the study cohort and the excluded cohort (Breslow–Day test for homogeneity: P=0.12). Further, the difference in TV ml kg−1 IBW between emergent and non-emergent cases was only 0.22 ml kg−1 IBW [95% CI: 0.14∼0.30]. Among the excluded cases that were non-emergent, the excluded subjects were not statistically different from the study sample with respect to the TV used and 30-day mortality (results not shown).
Baseline characteristics broken down by TV ml kg−1 IBW are given in Table 1 . For the entire cohort, the median TV was 525.8 [472–601] ml and the median TV ml kg−1 IBW was 8.6 [7.7–9.6]. A higher proportion of women were ventilated artificially using high TV ml kg−1 IBW (Table 1). This is likely to be the result of two factors. First, our ventilators have a default set TV of 500 ml. Secondly, on average, females are shorter than males and since IBW is calculated based on height, which in turn results in an increase in the TV ml kg−1 IBW seen in females.
Table 1.
Patient characteristics. *Sub-specialty surgery includes: gynaecologic surgery, neurosurgery, orthopaedics, plastic surgery, spinal surgery, renal transplant urologic surgery, vascular surgery, and other
| Variable | Entire cohort n (%), mean(sd) median [IQR] |
TV IBW group, ml kg−1n (%), mean (sd), median [IQR] |
P-value |
||||
|---|---|---|---|---|---|---|---|
| 29 343 | [3–6], n=488 | [6–8], n=8846 | [8–10], n=14 877 | [10–12], n=4412 | [12–20], n=720 | ||
| Age | 54.9 (16.1) | 60.4 (16.9) | 56 (16.8) | 54.5 (15.9) | 53.9 (15.3) | 53.3 (14.7) | <0.00001 |
| Female gender | 16 001 (54.5) | 110 (22.5%) | 3289 (37.2%) | 8912 (59.9%) | 3130 (70.9%) | 560 (77.8%) | <0.00001 |
| ASA physical status | |||||||
| I, II | 14 708 (50.1) | 163 (33.4%) | 4221 (47.7%) | 7984 (53.7%) | 2047 (46.4%) | 293 (40.7%) | <0.00001 |
| III | 12 101 (41.2) | 238 (48.8%) | 3678 (41.6%) | 5826 (39.2%) | 2000 (45.3%) | 359 (49.9%) | <0.00001 |
| IV, V | 2534 (8.6) | 87 (17.8%) | 947 (10.7%) | 1067 (7.2%) | 365 (8.3%) | 68 (9.4%) | <0.00001 |
| Weight (kg) | 75 [63.5–88.6] | 75.5 [63.5–86.8] | 74.1 [63–86.4] | 74 [62–87.3] | 78.6 [67–93.2] | 84.1 [72.4–100] | <0.00001 |
| IBW (kg) | 60.9 [54.2–70.6] | 75.1 [64.4–80] | 66.2 [59.3–75.1] | 59.3 [54.2–68.5] | 55.9 [52.2–61.6] | 52.5 [49.1–57.6] | <0.00001 |
| BMI (kg m−2) | 26.3 [23–30.3] | 23.6 [20.8–27.1] | 24.5 [21.7–27.9] | 26.4 [23.2–30.2] | 29.3 [25.9–34.2] | 33.6 [28.6–39.5] | <0.00001 |
| BMI group | |||||||
| 10–18.5 | 876 (3%) | 67 (13.7%) | 502 (5.7%) | 288 (1.9%) | 18 (0.4%) | 1 (0.1%) | <0.00001 |
| 18.5–25 | 10 883 (37.1%) | 235 (48.2%) | 4201 (47.5%) | 5566 (37.4%) | 831 (18.8%) | 50 (6.9%) | <0.00001 |
| 25–30 | 9793 (33.4%) | 127 (26%) | 2828 (32%) | 5134 (34.5%) | 1528 (34.6%) | 176 (24.4%) | <0.00001 |
| 30–35 | 4654 (15.9%) | 42 (8.6%) | 945 (10.7%) | 2453 (16.5%) | 1028 (23.3%) | 186 (25.8%) | <0.00001 |
| 35– | 3137 (10.7%) | 17 (3.5%) | 370 (4.2%) | 1436 (9.7%) | 1007 (22.8%) | 307 (42.6%) | <0.00001 |
| Obstructive disease (asthma/COPD) | 3821 (13%) | 71 (14.5%) | 1052 (11.9%) | 1863 (12.5%) | 695 (15.8%) | 140 (19.4%) | <0.00001 |
| Inhaled steroid use | |||||||
| Obstructive disease and steroids | 548 (1.9%) | 8 (1.6%) | 131 (1.5%) | 281 (1.9%) | 110 (2.5%) | 18 (2.5%) | 0.001 |
| Obstructive disease and no steroids | 3273 (11.2%) | 63 (12.9%) | 921 (10.4%) | 1582 (10.6%) | 585 (13.3%) | 122 (16.9%) | <0.00001 |
| No obstructive disease, no steroids | 25 522 (87%) | 417 (85.5%) | 7794 (88.1%) | 13 014 (87.5%) | 3717 (84.2%) | 580 (80.6%) | <0.00001 |
| TV, median (ml) | 525.8 [472–601] | 419 [339–460] | 481 [443–536] | 528 [478.7–605.5] | 603 [554–669] | 682 [634.1–754] | <0.00001 |
| TV, mean (ml) | 505.8 [452.2–574.1] | 408.8 [347.6–450.6] | 465.1 [423–517.3] | 507.8 [458.8–577.3] | 574.9 [525.4–636.5] | 649.7 [600.9–721.9] | <0.00001 |
| TV, median (ml kg−1 IBW) | 8.6 [7.7–9.6] | 5.7 [5.4–5.9] | 7.4 [6.9–7.7] | 8.9 [8.4–9.4] | 10.6 [10.3–11.1] | 12.7 [12.3–13.2] | <0.00001 |
| TV (ml kg−1 IBW) | 1.7 [1.4–2.1] | 1.5 [1.2–1.9] | 1.5 [1.2–1.8] | 1.7 [1.4–2.1] | 2 [1.6–2.5] | 2.5 [2–3.1] | <0.00001 |
| PEEP (cm H2O) | 4 [2.2–5] | 3.2 [2.1–4.9] | 4 [2.2–5] | 4 [2.2–5] | 4 [2.3–5] | 4 [2.4–5] | <0.00001 |
| PIP (cm H2O) | 21 [18–25] | 17 [14–21.2] | 19 [16.2–23] | 21.7 [18.1–25] | 24 [21–27.7] | 25.3 [22.5–29] | <0.00001 |
| Dynamic compliance (ml cm−1 H2O) | 30.5 [25–37] | 30.7 [22.4–39.6] | 31.1 [25–37.6] | 30.2 [24.9–36.6] | 30.2 [25.4–36.5] | 31.9 [27–38.5] | <0.00001 |
| Dynamic compliance group (ml cm−1 H2O) | |||||||
| 0–25 | 7317 (24.9) | 158 (32.4%) | 2207 (24.9%) | 3824 (25.7%) | 1019 (23.1%) | 109 (15.1%) | <0.00001 |
| 25–30 | 6721 (22.9) | 77 (15.8%) | 1827 (20.7%) | 3488 (23.4%) | 1149 (26%) | 180 (25%) | <0.00001 |
| 30–37 | 8031 (27.4) | 106 (21.7%) | 2443 (27.6%) | 4053 (27.2%) | 1216 (27.6%) | 213 (29.6%) | 0.039 |
| 37–150 | 7274 (24.8) | 147 (30.1%) | 2369 (26.8%) | 3512 (23.6%) | 1028 (23.3%) | 218 (30.3%) | <0.00001 |
| Ventilatory frequency (bpm) | 10 [9–12] | 12 [10–13] | 11 [10–12] | 10 [9–12] | 10 [8–11] | 9.5 [8–10] | <0.00001 |
| Fraction of inspired oxygen | 0.75 [0.68–0.8] | 0.76 [0.70–0.88] | 0.75 [0.69–0.81] | 0.74 [0.67–0.8] | 0.74 [0.66–0.8] | 0.73 [0.57–0.8] | <0.00001 |
| Length of intubation (min) | 182 [129–264] | 146.5 [98–222.2] | 174 [124–253] | 183 [129–265] | 195 [137–283] | 209.5 [145–298.5] | <0.00001 |
| Tracheal tube placement | |||||||
| By anaesthesia provider in OR | 29 116 (99.2%) | 482 (98.8%) | 8758 (99%) | 14 771 (99.3%) | 4389 (99.5%) | 716 (99.4%) | 0.019 |
| Before OR (in situ) | 227 (0.8%) | 6 (1.2%) | 88 (1%) | 106 (0.7%) | 23 (0.5%) | 4 (0.6%) | 0.019 |
| APR-DRG SOI | |||||||
| Minor | 12 784 (43.6%) | 141 (28.9%) | 3523 (39.8%) | 6805 (45.7%) | 1999 (45.3%) | 316 (43.9%) | <0.00001 |
| Moderate | 10 873 (37.1%) | 174 (35.7%) | 3254 (36.8%) | 5505 (37%) | 1670 (37.9%) | 270 (37.5%) | 0.742 |
| Severe | 4212 (14.4%) | 108 (22.1%) | 1444 (16.3%) | 1960 (13.2%) | 590 (13.4%) | 110 (15.3%) | <0.00001 |
| Extreme | 1474 (5%) | 65 (13.3%) | 625 (7.1%) | 607 (4.1%) | 153 (3.5%) | 24 (3.3%) | <0.00001 |
| APR-DRG ROM | |||||||
| Minor | 20 665 (70.4%) | 251 (51.4%) | 5835 (66%) | 10 795 (72.6%) | 3247 (73.6%) | 537 (74.6%) | <0.00001 |
| Moderate | 5491 (18.7%) | 132 (27%) | 1782 (20.1%) | 2663 (17.9%) | 791 (17.9%) | 123 (17.1%) | <0.00001 |
| Severe | 2201 (7.5%) | 65 (13.3%) | 826 (9.3%) | 1013 (6.8%) | 257 (5.8%) | 40 (5.6%) | <0.00001 |
| Extreme | 986 (3.4%) | 40 (8.2%) | 403 (4.6%) | 406 (2.7%) | 117 (2.7%) | 20 (2.8%) | <0.00001 |
| Race | |||||||
| Asian | 1608 (5.5%) | 17 (3.5%) | 469 (5.3%) | 882 (5.9%) | 212 (4.8%) | 28 (3.9%) | 0.002 |
| Black | 3853 (13.1%) | 76 (15.6%) | 1222 (13.8%) | 1874 (12.6%) | 566 (12.8%) | 115 (16%) | 0.004 |
| Hispanic | 3224 (11%) | 46 (9.4%) | 775 (8.8%) | 1657 (11.1%) | 633 (14.3%) | 113 (15.7%) | < 0.00001 |
| Other | 3803 (13%) | 52 (10.7%) | 1167 (13.2%) | 1904 (12.8%) | 600 (13.6%) | 80 (11.1%) | 0.144 |
| White | 16 855 (57.4%) | 297 (60.9%) | 5213 (58.9%) | 8560 (57.5%) | 2401 (54.4%) | 384 (53.3%) | < 0.00001 |
| Surgery type | |||||||
| General surgery | 11 244 (38.3%) | 193 (39.5%) | 3631 (41%) | 5670 (38.1%) | 1533 (34.7%) | 217 (30.1%) | <0.00001 |
| Sub-specialty surgery* | 18 099 (61.7%) | 295 (60.5%) | 5215 (59.0%) | 9207 (61.9%) | 2879 (65.3%) | 503 (69.9%) | <0.00001 |
| Laparoscopic surgery | 8574 (29.2%) | 111 (22.7%) | 2686 (30.4%) | 4550 (30.6%) | 1084 (24.6%) | 143 (19.9%) | <0.00001 |
| Emergency | 2817 (9.6%) | 63 (12.9%) | 988 (11.2%) | 1331 (8.9%) | 373 (8.5%) | 62 (8.6%) | <0.00001 |
Characteristics of ventilation
Median TV kg−1 IBW decreased from 9.0 ml kg−1 in 2008 to 8.3 ml kg−1 in 2011 (P<0.01 for both the linear and quadratic terms). The decrease was non-linear with a more pronounced decline earlier in the study period. Thirty-seven per cent (37.7%) of patients in 2011 were ventilated with TV 6–8 ml kg−1 IBW vs only 22.7% in 2008 (P<0.0001). Figure 2 illustrates that there were only weak correlations between dynamic compliance (Fig. 2 a) and PEEP (Fig. 2 c) and TV ml kg−1 IBW (r=−0.006, P=0.31; r=0.03, P<0.001, respectively). There was a weak-to-moderate correlation between PIP (Fig. 2 b) and TV ml kg−1 IBW (r=0.32, P<0.0001). Median in the TV 3–6 ml kg−1 IBW group was 0.76 (76%) vs 0.73 (73%) in the TV 12–20 ml kg−1 IBW group (P<0.001). However, linear regression between median and TV ml kg−1 IBW as a continuous variable showed only a very weak correlation (r=−0.07, P<0.0001).
Fig 2.
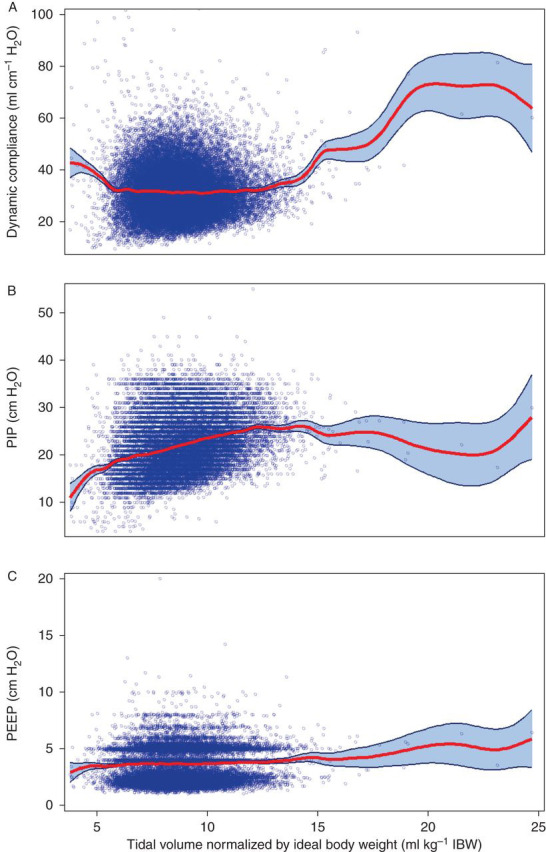
The relationship between intraoperative airway pressures and tidal volume. Scatterplots with smoothed regression line and 95% CI showing the relationship between several intraoperative ventilator variables and TV normalized by IBW. (a) Dynamic compliance, r=−0.006, P=0.31; (b) PIP, r=0.32, P<0.0001; (c) PEEP, r=0.033, P<0.0001.
Effect of intraoperative TV on outcomes
The results of the stepwise Cox regression are given in Table 2 . Preliminary analysis showed a high correlation between PIP and dynamic compliance (r=−0.76, P<0.001); therefore, only dynamic compliance was included in the final model. After adjusting for all retained co-factors, the risk of 30-day mortality significantly increased with TVs of 6–8 ml kg−1 IBW, compared with the reference (largest) group of TV 8–10 ml kg−1 IBW. The HR for TV 6–8 ml kg−1 IBW was 1.61 (95% CI 1.25–2.08, P=0.0002). TVs >8–10 ml kg−1 IBW were not associated with an increased ROM. The dose–response curve generated by the RCS analysis indicated a threshold TV of 9.7 ml kg−1 IBW (P<0.0006, Fig. 3 ). Low dynamic compliance was found to be significantly associated with an increased risk of 30-day mortality, while the length of intubation was not (Table 2). There was no significant interaction between TV ml kg−1 IBW and length of intubation (P=0.38). If PIP was included in the model instead of dynamic compliance, the HR for TV 6–8 ml kg−1 IBW was 1.72 (95% CI 1.35–2.91, P<0.001) and the HR for PIP >28 cm H2O was 1.68 (95% CI 1.24–2.29, P<0.001). We also found a significant association between TV 6–8 ml kg−1 IBW and an O-E LOS of >2 days (Table 2).
Table 2.
Multivariate models for 30-day mortality and O-E LOS >2 days. *The P-values for the overall test for TV (ml) per kg IBW for 30-day mortality and LOS O-E >2 days are P<0.015 and P<0.010. NS, not significant
| Variable | 30-day mortality |
O-E LOS >2 days |
||||
|---|---|---|---|---|---|---|
| HR | 95% Confidence limits | P-value | OR | 95% Confidence limits | P-value | |
| TV IBW group (ml kg−1)* | ||||||
| 3–6 | 1.372 | 0.816, 2.307 | 0.2335 | 1.365 | 1.065, 1.748 | 0.0139 |
| 6–8 | 1.611 | 1.249, 2.077 | 0.0002 | 1.108 | 1.024, 1.199 | 0.0109 |
| 8–10 (ref.) | – | – | – | – | – | – |
| 10–12 | 1.056 | 0.694, 1.608 | 0.799 | 0.914 | 0.827, 1.009 | 0.0735 |
| 12–20 | 2.081 | 0.941, 4.602 | 0.0704 | 0.714 | 0.57, 0.893 | 0.0032 |
| Dynamic compliance (ml cm−1 H2O) | ||||||
| 0–25 | 1.403 | 1.041, 1.89 | 0.0263 | 0.716 | 0.648, 0.791 | <0.0001 |
| 25–30 | 0.967 | 0.695, 1.346 | 0.8427 | 0.906 | 0.826, 0.994 | 0.0369 |
| 30–37 (ref.) | – | – | – | – | – | – |
| 37–150 | 0.994 | 0.712, 1.387 | 0.9704 | 1.029 | 0.941, 1.125 | 0.5351 |
| Obstructive disease | ||||||
| with steroid use | 0.527 | 0.194, 1.429 | 0.2078 | NS | NS | NS |
| without steroid use | 1.273 | 0.981, 1.651 | 0.0693 | NS | NS | NS |
| Length of intubation (h) | ||||||
| 0–2 | 1.219 | 0.932, 1.595 | 0.1477 | 0.618 | 0.558, 0.686 | <0.0001 |
| 2–4 (ref.) | – | – | – | – | – | – |
| 4–6 | 0.94 | 0.684, 1.293 | 0.7047 | 1.614 | 1.487, 1.752 | <0.0001 |
| >6 | 1.065 | 0.752, 1.507 | 0.7223 | 3.002 | 2.724 | <0.0001 |
| TT before operating theatre | 2.218 | 1.64, 3 | <0.0001 | NS | NS | NS |
| Age (yr) | ||||||
| <45 (ref.) | – | – | – | – | – | – |
| 45–55 | 1.442 | 0.885, 2.349 | 0.1418 | 1.138 | 1.032, 1.254 | 0.0096 |
| 56–65 | 1.502 | 0.957, 2.36 | 0.0771 | 1.21 | 1.099, 1.332 | 0.0001 |
| >65 | 1.917 | 1.275, 2.882 | 0.0018 | 1.197 | 1.086, 1.319 | 0.0003 |
| Female gender | 1.024 | 0.8, 1.311 | 0.8524 | 1.213 | 1.127, 1.307 | <0.0001 |
| Race | ||||||
| White (ref.) | – | – | – | – | – | – |
| Asian | 0.553 | 0.29, 1.057 | 0.0731 | NS | NS | NS |
| Black | 0.911 | 0.671, 1.236 | 0.5492 | NS | NS | NS |
| Hispanic | 1.064 | 0.771, 1.468 | 0.7075 | NS | NS | NS |
| Other | 0.908 | 0.653, 1.262 | 0.564 | NS | NS | NS |
| BMI group (kg m−2) | ||||||
| 10–18.5 | 1.273 | 0.822, 1.972 | 0.2797 | 0.885 | 0.728, 1.075 | 0.2192 |
| 18.6–25 (ref.) | – | – | – | – | – | – |
| 25–30 | 0.869 | 0.672, 1.123 | 0.2832 | 1.001 | 0.923, 1.087 | 0.9733 |
| 30–35 | 0.757 | 0.535, 1.069 | 0.1141 | 1.186 | 1.071, 1.315 | 0.0011 |
| >35 | 0.573 | 0.35, 0.937 | 0.0266 | 1.202 | 1.061, 1.361 | 0.0037 |
| ASA physical status | ||||||
| I or II | 0.332 | 0.165, 0.669 | 0.002 | 0.889 | 0.821, 0.962 | 0.0035 |
| III (ref.) | – | – | – | – | – | – |
| IV or V | 1.967 | 1.511, 2.562 | <0.0001 | 1.066 | 0.945, 1.202 | 0.3004 |
| APR-DRG SOI | ||||||
| Minor | – | – | – | – | – | – |
| Moderate | 1.282 | 0.551, 2.982 | 0.5643 | 1.237 | 1.142, 1.341 | <0.0001 |
| Severe | 1.665 | 0.652, 4.252 | 0.2862 | 1.125 | 0.99, 1.278 | 0.0711 |
| Extreme | 3.615 | 1.368, 9.554 | 0.0096 | 1.616 | 1.298, 2.011 | <0.0001 |
| APR-DRG ROM | ||||||
| Minor | – | – | – | – | – | – |
| Moderate | 1.793 | 0.832, 3.865 | 0.136 | 1.092 | 0.992, 1.202 | 0.0722 |
| Severe | 8.142 | 3.626, 18.284 | <0.0001 | 1.361 | 1.166, 1.589 | <0.0001 |
| Extreme | 30.193 | 12.908, 70.624 | <0.0001 | 1.653 | 1.289, 2.121 | <0.0001 |
| Laparoscopic surgery | 0.933 | 0.701, 1.241 | 0.6336 | 0.633 | 0.579, 0.692 | <0.0001 |
| General surgery | 1.173 | 0.898, 1.532 | 0.242 | 1.526 | 1.416, 1.644 | <0.0001 |
| Emergency | 1.154 | 0.896, 1.486 | 0.2663 | 1.193 | 1.058, 1.346 | 0.0039 |
| Year of service | ||||||
| 2008 | 1.064 | 0.782, 1.447 | 0.6932 | 0.837 | 0.764, 0.918 | 0.0002 |
| 2009 | 1.001 | 0.736, 1.362 | 0.9958 | 0.642 | 0.585, 0.704 | <0.0001 |
| 2010 | 0.833 | 0.609, 1.139 | 0.2517 | 0.654 | 0.597, 0.716 | <0.0001 |
| 2011 (ref.) | – | – | – | – | – | – |
Fig 3.
The effect of intraoperative tidal volume on 30-day mortality. RCS curve showing the dose–response relationship between median TV per kg IBW and 30-day mortality. Dots show the 5th, 50th, and 95th percentiles of TV per kg IBW. The shaded area shows the 95% CI. The CI >0.00 indicates a significant association between TV per kg IBW <9.7 ml kg−1 and 30-day mortality.
Results of propensity score matching
Propensity score matching resulted in 13 108 successfully matched pairs out of 14 466 patients in the four ‘treated’ TV groups (Table 3 ). The IQR of the standardized mean difference for all preoperative factors was within 4% of the treated group after matching. The largest standardized mean difference was 13% for IBW in the TV 3–6 ml kg−1 IBW group, corresponding to an absolute difference in IBW between the TV 3–6 ml kg−1 IBW and the untreated group of <1 kg IBW. The unadjusted HR for death after propensity score matching among patients ventilated with TVs of 6–8 vs 8–10 ml kg−1 IBW was 1.63 (95% CI 1.22–2.18, P<0.001) (Table 3). When further adjusted for intraoperative variables, the HR was 1.61 (95% CI 1.20–2.15, P=0.001). Patients in the 6–8 ml kg−1 IBW group also had longer stays (relative LOS 1.08, 95% CI 1.06–1.09, P<0.001), and were more likely to have an O-E LOS of >2 [OR 1.15 (95% CI 1.05–1.26, P=0.005)]. In contrast, patients in the TV 10–12 ml kg−1 IBW group had shorter stays (relative LOS for the TV 10–12 ml kg−1 IBW group 0.95, 95% CI 0.93–0.97, P<0.001).
Table 3.
Results of propensity score matching. *Insufficient mortality (n=10) in this group to calculate HR
| TV IBW group | Matched/treated | 30-day mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted HR |
Adjusted HR |
||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||||
| 3–6 | 463/488 | 1.27 | 0.65 | 2.50 | 0.487 | 1.39 | 0.66 | 2.90 | 0.384 |
| 6–8 | 7534/8846 | 1.63 | 1.22 | 2.18 | <0.001 | 1.61 | 1.20 | 2.15 | 0.001 |
| 8–10 (untreated) | – | – | – | – | – | – | – | – | – |
| 10–12 | 4407/4412 | 1.00 | 0.60 | 1.67 | 0.995 | 1.11 | 0.65 | 1.91 | 0.694 |
| 12–20 | 704/720 | 2.34 | 0.61 | 9.06 | 0.217 | N/A* | |||
|
LOS, relative ratio (RR) |
O-E LOS >2 days |
||||||||
| RR | 95% CI | P-value | OR | 95% CI | P-value | ||||
| 3–6 | 463/488 | 1.04 | 0.98 | 1.10 | 0.153 | 1.20 | 0.84 | 1.72 | 0.320 |
| 6–8 | 7534/8846 | 1.08 | 1.06 | 1.09 | <0.001 | 1.15 | 1.05 | 1.26 | 0.002 |
| 8–10 (untreated) | – | – | – | – | – | – | – | – | – |
| 10–12 | 4407/4412 | 0.95 | 0.93 | 0.97 | <0.001 | 0.92 | 0.82 | 1.04 | 0.179 |
| 12–20 | 704/720 | 0.87 | 0.82 | 0.92 | <0.001 | 0.64 | 0.46 | 0.89 | 0.009 |
Discussion
The results of the present study suggest that mechanical ventilation utilizing low TVs with minimal PEEP is associated with an increase in 30-day mortality and hospital LOS. The association was seen in both the standard and propensity score-matched analyses. Our data also show that a significant reduction in intraoperative TV used at our institution occurred over the 5-year study period, suggesting that the results of the ARDSNet trial did indeed have an impact on anaesthetic practice at our institution.
It must be emphasized that the ARDSNet trial investigated a subpopulation of critically ill patients—those with severe acute respiratory illness. We believe that such patients cannot be compared with a general surgical population undergoing GA. Critically ill patients with ALI/ARDS have decreased compliance and are often treated with PEEP in order to increase functional residual capacity, reduce shunt fraction, and avoid atelectrauma.20 In the ARDSNet trial, the low TV group had a mean PEEP of 8–9 cm H2O throughout the first 3 days. Anaesthetists, on the other hand, rarely utilize PEEP in their daily practice. The median PEEP seen in our population was 4 cm H2O, similar to that found in another recent large study.5 Why anaesthetists are reluctant to use PEEP remains speculative, but is most likely linked to the shorter duration of mechanical ventilation, limitations of the ‘bag in the bellow’ ventilator and circle system, and most importantly, the fact that most patients come to the operating theatre with healthy lungs and so demonstrate normal oxygenation without the use of PEEP. While there is recent evidence that the use of low intraoperative TV plus PEEP of 10 cm H2O does attenuate the release of some inflammatory markers and may improve postoperative lung function, another study found that even among critically ill patients with preexisting ALI, intraoperative low TV ventilation was not associated with improved oxygenation or decreased in-hospital mortality.4 21 22
A recently published multi-centre randomized controlled trial explicitly exploring the intraoperative differences between non-protective ventilation (TV 10–12 ml kg−1 IBW, no PEEP, no recruitment manoeuvres) and protective ventilation (TV 6–8 ml kg−1 IBW, 6–8 cm H2O PEEP, recruitment manoeuvres every 30 min) in patients with an intermediate-to-high risk of postoperative pulmonary complications found a significant reduction in postoperative pulmonary complications in the protective ventilation group.23 We believe that the result of our study complement the findings of this trial, since no study to date has ever proved that low TVs in isolation are superior to standard ventilation strategies, but are beneficial only in conjunction with the application of PEEP and recurrent recruitment manoeuvres. In fact, a pilot study exploring the feasibility of using automated alerts to recommend low intraoperative TV in patients with ARDS/ALI found that while the clinicians were very amenable to reducing median intraoperative TV, they did not concomitantly increase the PEEP setting. The study found no outcome differences between the two groups, again suggesting the critical role of PEEP and recruitment.24 This supports our belief that applying only one component of the ARDSNet protocol (low TV) in isolation is a misapplication of evidence-based medicine.
Additionally, the ARDSNet protocol also limited the plateau airway pressure in the low TV group to ≤30 cm H2O vs ≤50 cm H2O in the conventionally ventilated group. Tobin asserted in an editorial in response to the ARDSNet results that the larger difference in plateau pressures may have been the reason why the ARDSNet trial found a benefit to lower TV whereas previous studies had not been able to identify the same effect.25 More recently, Fernández-Pérez and colleagues26 demonstrated that intraoperative peak airway pressure is associated with postoperative ALI. Consequently, we were concerned that poor compliance and high airway pressures might have been the reason why anaesthetists chose lower TVs in our cohort, raising the question as to whether the harm associated with low TV was actually the result of increased PIP in the low TV groups. While there was a weak-to-moderate, but significant correlation, between PIP and TV (Fig. 2 b), the correlation was actually positive—higher PIP was correlated with higher TV. This indicates that clinicians in most cases were not setting low TVs solely in response to high airway pressures. Further, both the multivariate and the propensity score-matched analyses showed that low TV remained significant even after adjusting for either dynamic compliance or PIP. This bolsters our finding that low TV with minimal PEEP is in and of itself independently associated with poorer outcomes.
As to the reason why patients whose lungs were ventilated with lower TVs and minimal PEEP had poorer outcomes, we speculate that atelectasis plays a central role. The induction of GA has long been known to be a promoter for the development of dependent zone atelectasis. To reduce the right-left shunting, anaesthetists have historically applied large TV to recruit these zones and improve systemic oxygenation.1 High has also been linked to the development of atelectasis during the perioperative period.27 In contrast to the recent findings of Blum and colleagues,28 the median in our study population was high across all TV groups (Table 1). Thus, the effects of absorption atelectasis were likely similar in the high TV vs low TV groups. This reassured us that any increased atelectasis was a result of the low TV itself and not attributable to ventilation with a higher in the low TV vs the high TV groups.
Investigations have shown that impaired oxygenation caused by perioperative atelectasis persists for days after the surgical procedure.29, 30, 31 Experimental work has linked atelectatic regions of the lung to translocation and increased bacterial growth providing an optimal nidus for the development of lower airway tract infections.32 33 There is also some clinical evidence in humans linking atelectasis to pneumonia.34 Regardless of the exact aetiology, perioperative respiratory complications increase 30-day mortality. Canet and colleagues35 studied 2464 patients and found a 30-day mortality of 19.5% among those who developed a postoperative pulmonary complication vs only 0.5% among those who did not.
Limitations
This was a retrospective single-centre study and as such we can report only associations and not causation. Clinical or administrative data on postoperative respiratory complications (e.g. pneumonia) and cause of death were unavailable, so it is very possible that the increased 30-day mortality seen among patients with low TVs was not attributable to the low TV per se but to other factors not captured in our analysis. However, the use of the APR-DRG ROM and SOI scores provided a robust risk-adjustment that has been well validated by other investigators.12, 13, 14 Another limitation is that we were unable to collect intraoperative plateau pressure and mode of ventilation utilized. However, mode of ventilation is simply a means to an end (decreased PIP) so lack of this data point is likely not significant. We also were unable to quantify oxygenation status and shunt fraction either intraoperatively or after operation because arterial blood gases were not collected for most patients in the cohort. This limited our ability to stratify patients based on intrinsic lung disease. We also acknowledge that even though the propensity score-matching analysis supported the results found in the Cox regression model, the magnitude of the biases in distributions between the TV groups for certain covariates was considerable (Table 1) and unlikely to be fully offset by the propensity score. Thus, the definitive answer to our question can be obtained only through a randomized controlled trial. Finally, the mortality rate may have been underestimated because of missing or unreported data in the death master file. Overall though, we do believe that the large number of patients, diversity of patient characteristics and procedures, and use of well-validated measures of coexisting disease and SOI provide reassurance that our conclusions are not the result of statistical error but do represent real phenomena.
In conclusion, while other studies have demonstrated the beneficial effects of low intraoperative TV with PEEP in patients at increased risk for pulmonary complications, we have shown that in a mixed general surgical population, ventilation with low TV and minimal PEEP has a detrimental effect on both 30-day mortality and hospital LOS.
Authors’ contributions
M.A.L.: study design, data extraction and analysis, and manuscript preparation; P.J.M.: data extraction and analysis; H.M.L.: statistical analysis; L.H.: manuscript review and revision; G.W.F.: study design, manuscript preparation, and final approval of manuscript.
Declaration of interest
None declared.
Handling editor: J. P. Thompson
Post Publication Comments
References
- 1.Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. N Engl J Med. 1963;269:991–996. doi: 10.1056/NEJM196311072691901. [DOI] [PubMed] [Google Scholar]
- 2.Brower RG, Matthay BMA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Choi G, Wolthuis EK, Bresser P, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology. 2006;105:689–695. doi: 10.1097/00000542-200610000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Wolthuis EK, Choi G, Dessing MC, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2007;108:46–54. doi: 10.1097/01.anes.0000296068.80921.10. [DOI] [PubMed] [Google Scholar]
- 5.Blum JM, Fetterman DM, Park PK, Morris M, Rosenberg AL. A description of intraoperative ventilator management and ventilation strategies in hypoxic patients. Anesth Analg. 2010;110:1616–1622. doi: 10.1213/ANE.0b013e3181da82e1. [DOI] [PubMed] [Google Scholar]
- 6.Harris RS. Pressure-volume curves of the respiratory system. Respiratory Care. 2005;50:78–98. [PubMed] [Google Scholar]
- 7.Shah B, Sucher K, Hollenbeck C. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract. 2006;21:312. doi: 10.1177/0115426506021003312. [DOI] [PubMed] [Google Scholar]
- 8.Obesity: Preventing and Managing the Global Epidemic . Report of a WHO consultation. World Health Organ Tech Rep Ser. World Health Organization; Geneva: 2000. pp. 1–253. Report No. 894. [PubMed] [Google Scholar]
- 9.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Averill RF, Goldfield NI, Muldoon J, Steinbeck BA, Grant TM. A closer look at all-patient refined DRGs. J AHIMA. 2002;73:46–50. [PubMed] [Google Scholar]
- 11.Hughes J. Mortality Measurement: Development of the 3M™ All Patient Refined Diagnosis Related Groups. Agency for Healthcare Research and Quality; Rockville, MD: 2009. http://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/mortality/Hughessumm.html March 2009 [Internet] www.ahrq.gov [cited 2013 August 6] Available from. (accessed 13 August 2013) [Google Scholar]
- 12.Goldfield N, Averill R. On ‘risk-adjusting acute myocardial infarction mortality: are APR-DRGs the right tool? Health Serv Res. 2000;34:1491–1495. Discussion 1495–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Baram D, Daroowalla F, Garcia R, et al. Use of the All Patient Refined-Diagnosis Related Group (APR-DRG) risk of mortality score as a severity adjustor in the medical ICU. Clin Med Circ Respir Pulm Med. 2008;2:19–25. doi: 10.4137/ccrpm.s544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano PS, Chan BK. Risk-adjusting acute myocardial infarction mortality: are APR-DRGs the right tool? Health Serv Res. 2000;34:1469–1489. [PMC free article] [PubMed] [Google Scholar]
- 15.Meurer S. Mortality risk adjustment methodology for University Health System’s Clinical Data Base [Internet] Mortal Meas. 2009 http://www.ahrq.gov/qual/mortality/Meurer.html [cited 2012 November 5]. Available from. (accessed 13 August 2013) [Google Scholar]
- 16.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 17.Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Analysis. 2007;15:199–236. [Google Scholar]
- 18.Imai K, King G, Lau O. Toward a common framework for statistical analysis and development. J Comput Graph Stat. 2008;17:892–913. [Google Scholar]
- 19.Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2013. http://projects.iq.harvard.edu/zelig [Internet]. [cited October 4]. Available from. (accessed 13 August 2013) [Google Scholar]
- 20.Mols G, Priebe HJ, Guttmann J. Alveolar recruitment in acute lung injury. Br J Anaesth. 2006;96:156–166. doi: 10.1093/bja/aei299. [DOI] [PubMed] [Google Scholar]
- 21.Chaiwat O, Vavilala MS, Philip S, et al. Intraoperative adherence to a low tidal volume ventilation strategy in critically ill patients with preexisting acute lung injury. J Crit Care. 2011;26:144–151. doi: 10.1016/j.jcrc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–1321. doi: 10.1097/ALN.0b013e31829102de. [DOI] [PubMed] [Google Scholar]
- 23.Futier E, Constantin J-M, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 24.Blum JM, Stentz MJ, Maile MD, et al. Automated alerting and recommendations for the management of patients with preexisting hypoxia and potential acute lung injury: a pilot study. Anesthesiology. 2013;119:295–302. doi: 10.1097/ALN.0b013e3182987af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobin MJ. Culmination of an era in research on the acute respiratory distress syndrome. N Engl J Med. 2000;342:1360–1361. doi: 10.1056/NEJM200005043421808. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Pérez E, Sprung J, Afessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax. 2009;64:121. doi: 10.1136/thx.2008.102228. [DOI] [PubMed] [Google Scholar]
- 27.Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology. 2003;98:28–33. doi: 10.1097/00000542-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Blum JM, Maile M, Park PK, et al. A description of intraoperative ventilator management in patients with acute lung injury and the use of lung protective ventilation strategies. Anesthesiology. 2011;115:75–82. doi: 10.1097/ALN.0b013e31821a8d63. [DOI] [PubMed] [Google Scholar]
- 29.Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L. Pulmonary densities during anesthesia with muscular relaxation—a proposal of atelectasis. Anesthesiology. 1985;62:422–428. doi: 10.1097/00000542-198504000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Lindberg P, Gunnarsson L, Tokics L, et al. Atelectasis and lung function in the postoperative period. Acta Anaesthesiol Scand. 1992;36:546–553. doi: 10.1111/j.1399-6576.1992.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 31.Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102:838–854. doi: 10.1097/00000542-200504000-00021. [DOI] [PubMed] [Google Scholar]
- 32.van Kaam AHLC, Lutter R, Lachmann RA, et al. Effect of ventilation strategy and surfactant on inflammation in experimental pneumonia. Eur Respir J. 2005;26:112–117. doi: 10.1183/09031936.05.00144504. [DOI] [PubMed] [Google Scholar]
- 33.van Kaam AH, Lachmann RA, Herting E, et al. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med. 2004;169:1046–1053. doi: 10.1164/rccm.200312-1779OC. [DOI] [PubMed] [Google Scholar]
- 34.Fujita T, Sakurai K. Multivariate analysis of risk factors for postoperative pneumonia. Am J Surg. 1995;169:304–307. doi: 10.1016/S0002-9610(99)80163-9. [DOI] [PubMed] [Google Scholar]
- 35.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.