Abstract
The liver is a highly dynamic metabolic organ that plays critical roles in plasma protein synthesis, gluconeogenesis and glycogen storage, cholesterol metabolism and bile acid synthesis as well as drug/xenobiotic metabolism and detoxification. Research from the past decades indicate that autophagy, the cellular catabolic process mediated by lysosomes, plays an important role in maintaining cellular and metabolic homeostasis in the liver. Hepatic autophagy fluctuates with hormonal cues and the availability of nutrients that respond to fed and fasting states as well as circadian activities. Dysfunction of autophagy in liver parenchymal and non-parenchymal cells can lead to various liver diseases including non-alcoholic fatty liver diseases, alcohol associated liver disease, drug-induced liver injury, cholestasis, viral hepatitis and hepatocellular carcinoma. Therefore, targeting autophagy may be a potential strategy for treating these various liver diseases. In this review, we will discuss the current progress on the understanding of autophagy in liver physiology. We will also discuss several forms of selective autophagy in the liver and the molecular signaling pathways in regulating autophagy of different cell types and their implications in various liver diseases.
Keywords: AALD, DILI, HCC, Lipophagy, Mitophagy, NAFLD
1. Introduction
Autophagy is an evolutionarily conserved cellular degradation process that delivers cellular components (macromolecules, excess/or damaged organelles) to lysosomes (Klionsky and Emr 2000; Mizushima et al., 2008). Autophagy plays a critical role in cellular homeostasis through degradation of proteins, glycogen, lipids and organelles such as damaged mitochondria and excess endoplasmic reticulum (ER) to provide nutrients and biomolecular building blocks for cell survival (Mizushima and Komatsu 2011; Youle and Narendra 2011; Liu and Czaja 2013; Mizushima 2018; Chino and Mizushima 2020). There are three major types of autophagy: macroautophagy, microautophagy, and chaperon-mediated autophagy (CMA), which differ in how the cargos are delivered to lysosomes (Parzych and Klionsky 2014). Macro-autophagy involves the formation of a double-membrane autophagosome that envelops autophagic cargos, traffics them to a lysosome where the membranes fuse to form an autolysosome and the cargos are degraded by the lysosomal hydrolytic enzymes (Klionsky and Emr 2000). Microautophagy occurs when autophagic cargos are directly engulfed by an invaginated lysosome which bypasses the formation of autophagosomes (Schuck 2020). In CMA, cellular soluble proteins containing a recognition pentapeptide motif (KFERQ) are recognized by cytosolic chaperones, such as heat shock-cognate protein of 70 kDa (HSC70), which then binds to lysosome-associated membrane protein type 2A (LAMP-2A) to induce LAMP-2A multimerization to form a translocation complex resulting in the transport of CMA substrates cross of lysosomal membrane and degradation (Arias and Cuervo 2011).
The liver is one of the most dynamic organs in humans, and autophagy plays important roles in liver physiology and pathology (Yin et al., 2008; Ding 2010; Rautou et al., 2010). Autophagy was discovered from experiments performed using the liver in the 1960s (Deter et al., 1967). Dysfunction or dysregulation of autophagy is associated with multiple liver diseases, including alcohol associated liver disease (AALD) (Williams et al., 2014), non-alcoholic fatty liver disease (NAFLD) (Gonzalez-Rodriguez et al., 2014; Tanaka et al., 2016), non-alcoholic steatohepatitis (NASH) (Fallowfield 2011), drug-induced liver injury (DILI) (Ni et al., 2012), cholestasis, viral hepatitis (Sir et al., 2008b; Wang and Ou 2015), and hepatocellular carcinoma (HCC) (Chao et al., 2020). In this review, we briefly discuss the regulatory machinery of autophagy, selective autophagy including aggrephagy, mitophagy, ER-phagy and lipophagy as well as the role of autophagy in liver non-parenchymal cells in the context of liver pathophysiology and diseases. We also briefly discuss the emerging therapeutic approaches for targeting autophagy in treating liver diseases.
2. Regulation of autophagy
Autophagy is a multistep process involving a group of conserved genes known as autophagy-related genes (Atg). At present, over 40 Atgs have been identified, which are highly conserved from yeast to mammalian cells. These ATG proteins participate in different stages of autophagy including phagophore initiation, nucleation, expansion, autophagosome closure and fusion with lysosome, and the final steps of degradation and efflux of the breakdown products. The autophagy process has been described extensively in a series of other reviews (Klionsky and Emr 2000; Nakatogawa et al., 2009; Mizushima et al., 2011; Mizushima 2020) and will only be briefly mentioned here. In mammalian cells, the core ATG proteins can be roughly categorized into several functional groups: (i) The ULK kinase complex, consisting of ULK1 or ULK2 (Unc-51 like autophagy activating kinase 1 or 2, mammalian homologs of ATG1), ATG13, ATG101, and FIP200/RB1CC1 (focal adhesion kinase family interacting protein of 200 kDa or RB1-inducible coiled-coil 1, a putative yeast ATG17 homolog), phosphorylates downstream factors for initiation of phagophore formation (Mizushima 2010). The activity of the ULK complex is negatively regulated by mechanistic target of rapamycin complex 1 (mTORC1) and positively regulated by AMP-activated protein kinase (AMPK) (Egan et al., 2011; Egan et al., 2011; Kim and Guan 2011; Kim et al., 2011; Loffler et al., 2011). (ii) The Beclin1 (mammalian homologs of ATG6)-class III phosphoinositide 3-kinase (PI3K) complex, consisting of Beclin1/BECN1, ATG14L, p150 (the yeast ortholog is VPS15) and the class-III PI3 kinase VPS34 (Kihara et al., 2001), is responsible for the production of phosphatidylinositol-3-phosphate (PtdIns3P) directly from phosphatidylinositol (Burman and Ktistakis 2010). This PtdIns3P serves as a landmark on the isolation membrane to recruit other factors, such as ATG18, involved in the process of phagophore nucleation for autophagosome formation (Obara et al., 2008). The activity of this complex can be positively regulated by ULK1 (Russell et al., 2013). (iii) The only transmembrane ATG protein, ATG9, works together with ATG2 and WDR45/WIPI4 (mammalian homologs of ATG18) to function in the phagophore membrane elongation and retrieval process (Polson et al., 2010). Recent evidence suggests that ATG9 can facilitate phospholipid translocation across the autophagosome membrane for the expansion of the isolation membrane (Orii et al., 2021). When cells need to limit autophagy activity in normal conditions, ATG9 is ubiquitinated and degraded by the proteasome, a process mediated by the E3 ligase Met30 (Feng et al., 2021). This posttranslational regulation of ATG9 thus adds another layer of regulation on autophagy. (iv) The two ubiquitin-like conjugation systems (the ATG12-ATG5 system and the microtubule-associated protein 1A/1B light chain 3 (also known as LC3, mammalian homologs of ATG8), utilize ATG7 as the E1-like enzyme and ATG3 as the E2-like enzyme and ATG5-ATG12-ATG16L1 as the E3 ligase to facilitate the conjugation of LC3 with phosphatidylethanolamine (PE), which are important for the elongation of the isolation membrane and completion of the autophagosome (Ohsumi 2001). (v) A set of endosomal sorting complexes required for transport (ESCRT) including VPS37A, component charged MVB protein 2A (CHMP2A), and the AAA ATPase vacuolar protein sorting 4 (VPS4) regulate autophagosome closure in mammalian cells (Takahashi et al., 2018; Takahashi et al., 2019). In addition, ER-localized autophagy proteins EPG-3/vacuole membrane protein 1 (VMP1) and TMEM41B also play an essential role in controlling phagophore maturation and autophagosome closure (Moretti et al., 2018; Morita et al., 2018; Zhao and Zhang 2018). (vi) The mature autophagosome finally fuses with a lysosome to form an autolysosome in which the autophagic cargos are degraded by hydrolases and lipases. The fusion process is regulated by the fusion machinery soluble N-ethyl-maleimide-sensitive fusion protein (NSF) and attachment protein receptor (SNARE) family proteins, such as vesicle-associated membrane protein 8 (VAMP8), synaptosomal-associated protein 29 (SNAP29) and syntaxin 17 (STX17) (Fader et al., 2009; Furuta et al., 2010; Itakura et al., 2012). Additionally, LAMP-1 and LAMP-2, lysosomal membrane proteins, and RAB7 (a RAS-related GTP-binding protein), are required for the fusion of autophagosomes with lysosomes (Jager et al., 2004; Eskelinen 2006; Huynh et al., 2007; Hubert et al., 2016). The current understanding of the regulation of autophagy is summarized in Fig. 1.
Fig. 1. A simplified scheme of the autophagy process.
(1) mTORC1 negatively regulates ULK1/2 by phosphorylating Ser 317 and Ser 777, whereas AMPK positively regulates ULK1/2 by phosphorylating S637 and S757 of ULK1/2. Nucleation of the pre-autophagosome structure requires both the ULK complex and the PtdIns3K complex. Activated ULK1/2 phosphorylates Beclin-1, leading to VPS34 activation and phagophore formation. (2) Elongation and autophagosome formation are mediated by two “ubiquitin-like conjugation systems”. In general, the protease ATG4 first processes nascent LC3 to the cytosolic form LC3-I, and then ATG7 and ATG3 act as E1-, E2-like enzymes to further process LC3-I to the lipid-conjugated form LC3-II. Meanwhile, ATG7 (E1) and ATG10 (E2) promote the ATG12-ATG5 conjugation with ATG16L1 to form the ATG12-ATG5-ATG16L1 complex, which works as an E3 ligase to promote the formation of phosphatidylethanolamine (PE)-conjugated LC3-II. The phagophore transmembrane protein ATG9, with ATG2 and WIPI1/2 (ATG18) participates in the expanding phagophore by delivering membrane and lipids from donor sources such as ER and Golgi complex. The closure of autophagosome membranes requires ESCRT, including VPS37A, CHMP2A, and VPS4, as well as ER transmembrane proteins VMP1 and TMEM41B. (3) The autophagosome-lysosome fusion process is regulated by the fusion machinery NSF and SNARE family proteins, such as VAMP7, VAMP8, VAMP9, SNAP29 and STX17. STX17 interacts with SNAP29 and lysosomal VAMP8, which leads to autophagosome-lysosome fusion. In addition, LAMP-1 and LAMP-2, two lysosomal membrane proteins, and RAB7, a small GTPase, are required for the fusion of autophagosomes with lysosomes. Finally, the autophagosome content is degraded by acidic hydrolases within the lysosome.
In addition to the above-mentioned core ATG proteins, the completion of autophagy requires the involvement of other proteins. The recognition of cargo to the phagophore requires autophagy cargo receptors, such as sequestosome 1 (SQSTM1/p62, hereafter referred to as p62), to interact with LC3 on the concave side of the membrane (Komatsu and Ichimura 2010). Various receptors function in different types of selective autophagy such as aggrephagy, mitophagy, ER-phagy and lipophagy, connecting the cargo with the phagophore via binding to an LC3-family protein, and all of these types of selective autophagy have been implicated in various liver diseases as we will discuss in detail below.
3. Autophagy in liver physiology
The liver is a central organ in regulating metabolism of the body, which include the metabolism of carbohydrates, lipids and fatty acids, proteins and amino acids as well as drugs/xenobiotoics. Hepatocytes, the hepatic parenchyma cells that occupy approximately 80% of liver volume, carries out the majority of these physiological tasks. The remaining, “nonparenchymal” cells (NPCs) are intrahepatic cholangiocytes, epithelial biliary tree cells, liver sinusoidal endothelial cells that line the blood vessels, hepatic stellate cells (HSCs) that are involved in fibrosis and Kupffer cells that are hepatic resident macrophages. In addition to its metabolic functions, the liver is also a major secretory organ and its role has long been appreciated in regulating coagulation and hemostasis. Increasing evidence indicates that ~40% of the transcripts in the liver encode secreted proteins or hepatokins that also play a critical role in metabolism (Uhlen et al., 2015).
Under physiological conditions, autophagy plays an important role in maintaining cellular and metabolic homeostasis in the liver. The fluctuation of nutrients including amino acids, glucose and lipids in the liver is subjected to circadian regulation, and basal hepatic autophagy exhibits a rhythmic behavior in coordination with fluctuations of the body’s nutrient status. For example, hepatic autophagy activity and gene expression of autophagy-related genes peak just prior to the initiation of activity in animals exposed to a 12-h light/dark cycle (Pfeifer and Strauss 1981; Ma et al., 2011; Ryzhikov et al., 2019), indicating a role for autophagy in sensing the need for increased nutrient delivery to the brain, peripheral organs and tissues. In addition, autophagy also modulates recycling of key circadian regulators by selectively degrading circadian proteins such as cryptochrome circadian regulator 1 (CRY1) (Toledo et al., 2018). Under nutrient-deprivation, hepatic autophagy is activated leading to glycogenolysis, lipolysis and protein catabolism to supply the cell with glucose, fatty acids and amino acids as cellular fuel, and other organs with glucose (Schneider and Cuervo 2014; Ueno and Komatsu 2017). At the organismal level, hepatic autophagy fluctuates with hormonal cues including pancreatic hormones such as insulin and glucagon as well as gastric hormones such as Ghrelin and glucagon-like peptide-1 (GLP-1) analogue to either negatively or positively regulate autophagy in response to fed and fasting states (Yin et al., 2008; Zhang et al., 2015; He et al., 2016).
In addition, both sympathetic and parasympathetic branches of the central nervous system (CNS) regulate the physiological functions of the liver (Jensen et al., 2013). In response to starvation, epinephrine is secreted to promote autophagy in the liver (Mortimore and Poso 1987). Glial cell line-derived neurotrophic factor (GDNF) can also enhance autophagy in hepatocytes via inhibition of mTOR (Mwangi et al., 2019). Conversely, upon feeding, elevated circulating levels of inulin, glucose, adipokines, regulatory amino acids and bile acids inhibit hepatic autophagy to favor anabolic program (Mortimore and Poso 1984). In addition, thyroid hormones (THs) stimulate fatty acid β-oxidation, which is coupled with induction of hepatic autophagy to deliver fatty acids to the mitochondria (Sinha et al., 2012).
In addition to regulating energy and nutrient homeostasis in response to fasting and feeding, basal hepatic autophagy is critical for maintaining the homeostasis and quality control of proteins and organelles in hepatocytes. For instance, mice with liver-specific deletion of either Atg5 or Atg7 have accumulated ubiquitinated proteins, aberrant endoplasmic reticulum, excess peroxisomes, endoplasmic reticulum and damaged mitochondria (Komatsu et al., 2005; Ni et al., 2012; (Yang et al., 2016); Ni et al., 2019).
Together, the synergetic interplays of nervous, endocrine, and paracrine signals as well as circulating nutrient levels orchestrate autophagy in the liver, which plays an important role in physiological functions of the liver by maintaining cellular and metabolic homeostasis (Fig. 2). As a result, dysregulation of autophagy can lead to various liver pathogenesis and diseases as discussed in detail below.
Fig. 2. Physiological regulation of hepatic autophagy.
Hepatic autophagy fluctuates with hormonal cues that respond to feeding and fasting cycles. During fasting, neuronal and hormonal cues (eg. Glucagon, ghrelin and GLP-1), as well as decreased levels of circulating metabolites, such as glucose, and amino acids trigger autophagy. In contrast, feeding leads to elevated levels of circulating insulin, adipokines, glucose, amino acids and bile acid, all of which inhibit hepatic autophagy. Basal hepatic autophagy also exhibits a rhythmic behavior and is coordinated with fluctuations in nutrient status. Nutrient flux also initiates transcriptional regulation of hepatic autophagy. Farnesoid X Receptor (FXR); Forkhead box O (FOXO); Glucagon-Like Protein-1 (GLP1); G protein-coupled receptor (GPCR); Peroxisome proliferator-activated receptor (PPAR); transcription factor EB (TFEB); Zinc Finger With KRAB And SCAN Domains 3 (ZKSCAN3).
4. Dysregulation of hepatic autophagy in obesity and NAFLD
NAFLD, defined as hepatic steatosis that is not caused by alcohol, is the most common liver disease worldwide (Flegal et al., 2012), which can progress to the more severe non-alcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma (HCC) (Huang et al., 2020). Whereas NAFLD is estimated to occur in only 20–30% of the general adult population, it occurs in 70–80% of individuals with obesity and diabetes (Chalasani et al., 2012; Loomba and Sanyal 2013; Younossi et al., 2019). Conversely, NAFLD is thought to contribute to insulin resistance and type 2 diabetes to form a vicious cycle (Yki-Jarvinen 2014).
The mechanisms that contribute to the onset of NALFD are complicated often involving multiple “hits” related to: lifestyle, genetic susceptibility, adipose tissue dysfunction, insulin resistance, lipotoxicity, inflammation, oxidative stress, abnormal regulation of innate immunity, and disturbed gut microbiota (Tilg and Moschen 2010; Henao-Mejia et al., 2012; Birkenfeld and Shulman 2014; Haas et al., 2016). At the cellular level, dysregulation of hepatic lysosomal/autophagy has been linked to pathogenic steatosis in the context of NAFLD (Choi et al., 2013). Both genetic and diet-induced mouse models of obesity are associated with defective hepatic autophagy and CMA, which contribute to obesity-associated hepatic steatosis and insulin resistance (Singh et al., 2009; Yang et al., 2010; Schneider et al., 2014). Importantly, recent studies demonstrated that autophagic and lysosomal function are impaired in livers from patients with NAFLD (Fukuo et al., 2014; Gonzalez-Rodriguez et al., 2014). Restoration of autophagy by genetic (eg. through overexpression of Atg7 or TFEB) or chemical treatments (eg. the application of trehalose, rapamycin, carbamazepine or other pharmaceutical agents) can alleviate liver steatosis in mouse models of obesity (Singh et al., 2009; Yang et al., 2010; Sinha et al., 2014; Sun et al., 2015; DeBosch et al., 2016). Thus far, many small molecules (Rubinsztein et al., 2007; Fan et al., 2017) and peptides (Shoji-Kawata et al., 2013) as well as drug repurposing such as the calcium channel blocker verapamil (Park et al., 2014) have been developed to target autophagy or TFEB-mediated lysosomal biogenesis (Wang et al., 2017) and some also provide protection against NAFLD. Notably, many of the front-line therapies that improve NAFLD and NASH, including non-pharmacological caloric restriction (Marino et al., 2014) and exercise (He et al., 2012; Lira et al., 2013) have also been shown to activate autophagy.
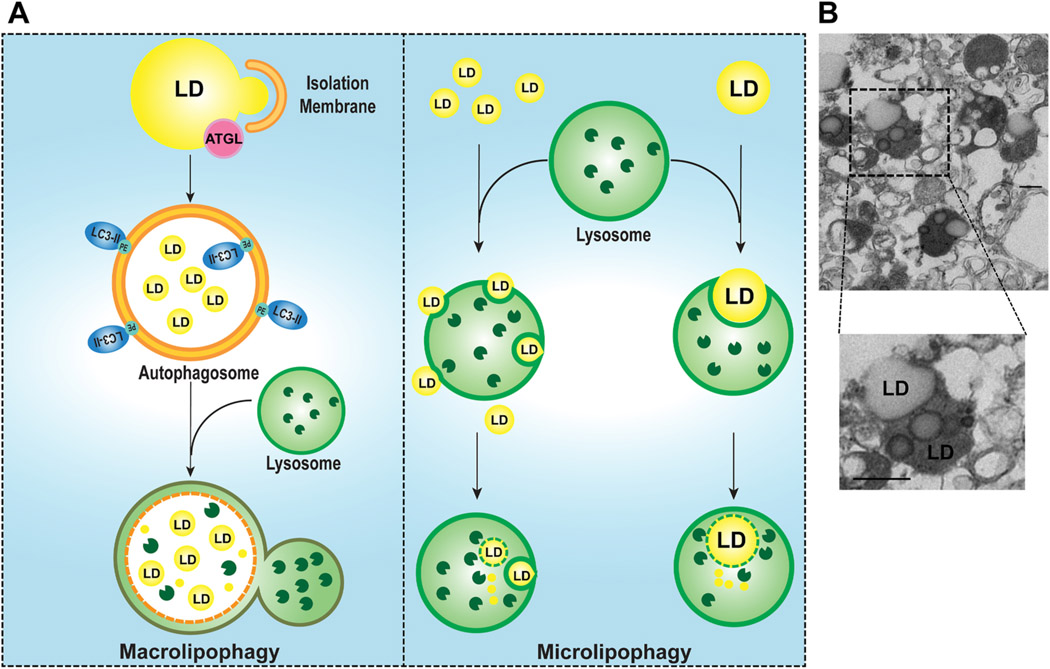
5. Lipid droplet, macrolipophagy and microlipophagy
NAFLD is characterized by the excess deposition of triglycerides and other neutral lipids in intracellular organelles called lipid droplets (LDs), which are derived from cytoplasmic leaflets of the ER membrane with a phospholipid monolayer (Olzmann and Carvalho 2019). In the liver, two major pathways regulate LD catabolism: lipolysis and lipophagy. Lipolysis is regulated by cytosolic neutral lipases such as hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL). In response to fasting, elevated catecholamines (e.g. epinephrine and norepinephrine) activate protein kinase A (PKA), which phosphorylates and activates HSL and the perilipins of the LD coating proteins. Phosphorylated perilipins then allow the exposure of the stored lipids to HSL and ATGL resulting in lipolysis. Genetic ablation or overexpression of ATGL in the liver exacerbates or alleviates steatosis in mouse livers, respectively (Wu et al., 2011). These findings suggest that cytosolic lipases are important regulators in LD turnover in hepatocytes (Mashek et al., 2015). In addition to lipase-mediated LD lipolysis, another emerging pathway to breakdown LD in hepatocytes involves an autophagy process known as “macrolipophagy” or “lipophagy” (Singh et al., 2009). During macrolipophagy, LDs are selectively taken up by autophagosomes likely via directly recruiting autophagy machinery proteins, and then autophagosome-enwrapped LD are further delivered to lysosomes where LDs are broken down by lysosomal acidic lipases. However, the selective receptor protein (s) for the recruitment of autophagosome to LDs are not yet identified. Nevertheless, increasing evidence supports a potential interplay between ATGL-mediated lipolysis and lipophagy, which may target different sized LDs to regulate hepatic lipid metabolism. It has been suggested that ATGL targets large LDs to break them down into smaller LDs for lipophagy (Schott et al., 2019). ATGL may also activate sirtuin 1 (SIRT1) to promote autophagy for further LD breakdown via macrolipophagy (Sathyanarayan et al., 2017).
In addition to autophagy, CMA directly degrades LD-associated proteins perilipin 2 (PLIN2) and perilipin 3 (PLIN3), which occurs concurrently with elevated levels of cytosolic ATGL and autophagy proteins on LD to promote LD lipolysis (Kaushik and Cuervo 2015). Moreover, recent evidence also suggests that lysosomes can directly target LDs resulting in LD breakdown independent autophagy, a process that has been termed “microlipophagy” (Schulze et al., 2020). In isolated lysosomes from fasted mouse liver, we found that most lysosomes contain small LDs. In some cases, a lysosome membrane invaginates and engulfs multiple small LDs. In other cases, lysosomes can directly engulf one relatively large LD (Fig. 3), supporting the existence of microlipophagy in hepatocytes. Trafficking and envelopment of LDs via autophagosomes to lysosomes is likely energy and time consuming, thus it is probable microlipophagy may be a more efficient “short-cut” pathway for small LDs catabolism rather than using autophagosomes.
Fig. 3. Macrolipophagy and microlipophagy regulate homeostasis of LDs in the liver.
Two major autophagy pathways are involved in the catabolism of LDs in hepatocytes: Type 1 macrolipophagy and Type 2 microlipophagy (A). In macrolipophagy, the isolation membrane and autophagosome are developed around the LDs and engulfed a portion of the LDs as a result of ATGL-mediated lipolysis in a piecemeal fashion. The autophagosome containing the LD then fuses with a lysosome to form an autolysosome where the LD is degraded by acidic lipases. In microlipophagy, multiple small LDs or a large LD can be directly taken up by a lysosome via lysosomal membrane invagination, and degraded by lysosomal acidic lipases generating free fatty acids. Whether a receptor protein is required for both lipophagy pathways remains unclear. (B) Representative images of electron microscopy analysis of purified lysosomal fractions from mouse livers that are fasted overnight. Lower panel is enlarged from the boxed area. Arrows: lysosomes with LD.
While the evidence supporting autophagy in regulating LD catabolism and protection against NAFLD seems compelling, it should be noted that autophagy has also been shown to promote the biogenesis of LDs. Upon starvation, intracellular membranes are degraded by autophagy to generate free fatty acids (FFAs), which promotes de nova LD biogenesis and an increased number of LDs (Nguyen et al., 2017). Since FFAs are toxic, increased LD formation likely serves as an adaptive protective mechanism against lipotoxicity (Nguyen et al., 2017). Moreover, mice with liver-specific deletion of Atg5, Atg7 or FIP200 are resistant to starvation or high fat diet-induced steatosis (Kim et al., 2013; Ma et al., 2013; Li et al., 2018; Takahashi et al., 2020). Mechanistically, it has been found that autophagy degrades nuclear receptor co-repressor 1 (NCoR1), which generally functions to inhibit the transactivation of nuclear receptors such as liver X receptor α (LXRα), a nuclear receptor responsible for fatty acid and triglyceride synthesis. Lack of autophagy leads to the accumulation of NcoR1 resulting in decreased LXRα-mediated fatty acid and triglyceride synthesis, and consequently decreased LD biogenesis (Takahashi et al., 2020). Notably, autophagy-deficient livers have increased liver injury and inflammation that may also alter the adaptive lipid metabolism pathways, including LXRα-mediated lipogenesis and peroxisome proliferator-activated receptor α (PPARα)-mediated lipid oxidation whereas increased p62-mediated nuclear factor erythroid 2-related factor 2 (Nrf2) activation (Ma et al., 2013; Li et al., 2018; Saito et al., 2019). Nrf2 activation contributes to the decreased fasting-induced hepatic steatosis as deletion of Nrf2 in L-Atg5 KO mice recovers hepatic steatosis after fasting (Li et al., 2018). Therefore, the role of autophagy in the development of NAFLD may be context-dependent, and more studies are needed to further elucidate the complex role and mechanisms.
6. Dysfunction of the ER and ER-phagy
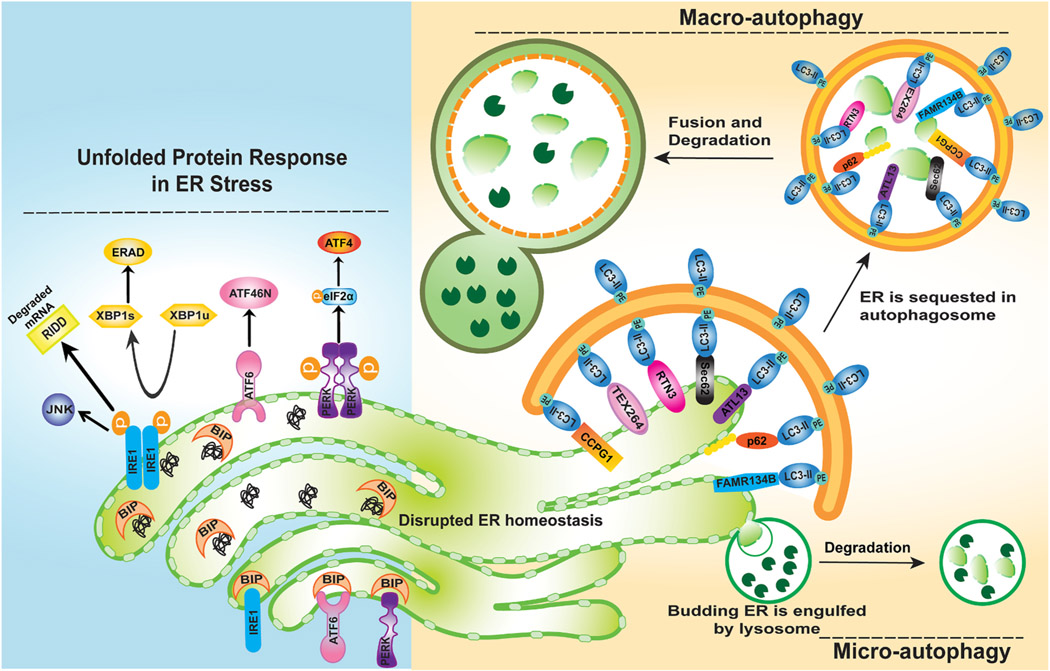
The endoplasmic reticulum (ER) is an essential cellular organelle and its key functions include but are not limited to protein processing, lipid metabolism, calcium storage and detoxification (Schwarz and Blower 2016; (Chino and Mizushima, 2020)). Any perturbations in ER homeostasis can induce an ER stress response to try and restore its homeostasis such as the unfolded protein response (UPR) that has three arms of components including PERK-ATF4, ATF6 and IRE1-XBP1, which activates signaling pathways that regulate protein production, protein folding, ER biogenesis, ER-associated degradation (ERAD), the regulated IRE1 dependent decay (RIDD) pathway to degrade mRNAs localize to the ER membranes and autophagy (Ding et al., 2007a; Ding et al., 2007b; Hetz 2012; Maurel et al., 2014). ER stress-induced autophagy may serve as a compensatory response to relief ER stress by degrading misfolded proteins (Ding and Yin 2008). In addition, ER-stress induced UPR can lead to ER expansion and subsequent ER-phagy to gain the ER homeostasis in yeast (Bernales et al., 2006). In the liver, the exposure of hepatocyte to xenobiotics can result in proliferation of smooth ER to produce cytochrome P-450 (CYPs) enzymes that mediate xenobiotic metabolization (Feldman et al., 1980; Masaki et al., 1987; Guevin et al., 2010). ER-phagy is thought to be important for degrading ER and restoring the ER content to normal levels. One early pioneer study conducted in yeast reveals that the unfolded protein response induced formation of autophagosomes that sequestered ER, suggesting the presence of ER-specific autophagy (Bernales et al., 2007). ER-phagy is also observed in plant and mammalian cells (Liu et al., 2012; Smith and Wilkinson 2017). Similar to lipophagy, ER-phagy also includes micro-ER-phagy and macro-ER-phagy. In micro-ER-phagy lysosome membrane invagination directly engulfs ER fragments (Schuck et al., 2014; Wilkinson 2019) whereas in macro-ER-phagy, the ER fragments are first packed into autophagosomes before fusing with lysosomes for degradation (De Leonibus, Cinque et al., 2019; Stolz and Grumati 2019). So far, a number of selective autophagy receptors for ER-phage have been identified including FAM134B (also known as Reticulophagy Regulator 1 (RETREG1) (Khaminets et al., 2015), RTN3L (the splice isoform of Reticulon 3, an RHD-containing ER-reshaping protein) (Grumati et al., 2017), SEC62 (Fumagalli et al., 2016), CCPG1 (cell-cycle progression gene 1) (Smith and Wilkinson 2018), atlastin3 (ATL3) (Chen et al., 2019), TEX264 (Chino et al., 2019), p62 (Yang et al., 2016), Epr1 (Zhao and Du 2020) and CALCOCO1 (calcium binding and coiled-coil domain 1) (Nthiga et al., 2020). These receptor proteins are either ER resident proteins or can interact with ER membrane proteins, but all receptor proteins have an LIR domain to directly interact with LC3 or GABARAP to recruit an autophagosome to the ER. These receptor proteins are critical for relieving ER stress and maintaining ER-homeostasis by promoting ER-phagy (Wilkinson 2019). In the liver, ER-phagy can remove excessive ER and CYP enzymes after xenobiotics are metabolized (Feldman et al., 1980; Masaki et al., 1987; Guevin et al., 2010; (Yang et al., 2016)) (Fig. 4). However, the exact role of these selective ER-phagy receptors in hepatic ER homeostasis have not been well characterized with the exception of p62 (Yang et al., 2016). More studies are needed to further investigate the roles of these ER-phagy receptor proteins in liver pathophysiology.
Fig. 4. ER-phagy during ER stress.
During ER stress, the stress sensors IRE1, ATF6, and PERK which are originally bound to BIP, are activated by dissociating from BIP. Activated IRE1 dimers trigger JNK activation and induce processing of un-spliced XBP1 (XBP1u) mRNA into its spliced form (XBP1s), which regulates protein production involved in the ERAD pathway. IRE1 also activates the RIDD pathway to degrade mRNAs localize to the ER membranes. Activated PERK phosphorylates eIF2α, which inhibits global translation and selectively increases mRNA translation of some stress-related proteins such as ATF4. Activated ATF6 is processed into active N-terminus cytosolic fragment (ATF6N) which translocates to the nucleus and regulates the expression of ER-stress related genes. In the liver, the exposure of hepatocytes to xenobiotics can result in proliferation of smooth ER to produce cytochrome P-450 (CYPs) enzymes that mediate xenobiotic metabolization. ER-phagy plays an important role in restoring ER homeostasis by removing damaged and excessive ER. Both micro-autophagy and macro-autophagy can be involved in removing ER. In micro-autophagy lysosome membrane invagination directly engulfs ER fragments, whereas in macro-autophagy, the ER fragments are first packed into autophagosomes through selective autophagy receptors before fusing with lysosomes for degradation. The selective ER-phagy receptors include but are not limited to TEX264, RTN3L, CCPG1, FAM134B, ATL3, p62 and SEC62. These receptor proteins are either ER resident proteins or can interact with ER membrane proteins, but all receptor proteins contain a LIR domain to directly interact with LC3 to recruit autophagosomes to the ER.
7. Autophagy in alcohol associated liver disease
Excessive alcohol consumption causes AALD, which is histologically similar to NAFLD with a spectrum of liver disorders and pathological changes, ranging from alcoholic steatosis to liver fibrosis, cirrhosis, alcoholic hepatitis (AH) and liver cancer (Gao and Bataller 2011; Williams et al., 2014; Nagy et al., 2016). The involvement of autophagy in AALD is still complex and controversial. Acute alcohol drinking activates autophagy as a protective mechanism (Ding et al., 2010), whereas chronic alcohol exposure impairs lysosome function, promotes hepatomegaly and hepatic protein accumulation resulting in steatosis and liver injury (Donohue and Thomes 2014; Chao et al., 2018c; Chao and Ding 2019).
Acute alcohol consumption activates autophagy through multiple mechanisms. Alcohol causes the generation of reactive oxygen species (ROS), ER stress, lipogenesis and mitochondrial damage (Ding et al., 2010; Nagy et al., 2016). Acute alcohol exposure significantly increased both mRNA and protein levels of various essential Atg genes/proteins via activating transcription factor FoxO3 and hypoxia-inducing factor-1 beta (HIF-1β) in mouse livers (Ni et al., 2013a; Ni et al., 2014a). Acute ethanol exposure also inhibits mTORC1 and increases the nuclear level of TFEB which increase lysosomal biogenesis and autophagy (Ding et al., 2010; Thomes et al., 2015). As a consequence, autophagy acts as a hepatoprotective mechanism to protect against acute alcohol-induced liver injury by decreasing hepatocyte apoptosis and steatosis through degradation of damaged mitochondria and LDs (Ding et al., 2011a; Ding et al., 2011b).
However, upon chronic alcohol exposure, various studies have revealed that hepatic autophagy is suppressed via multiple mechanisms (Kharbanda, McVicker et al. 1996, 1997; Schulze et al., 2017; (Chao et al., 2018c)). Studies using GFP-LC3 transgenic mice that were chronically fed with control and ethanol-containing liquid diets showed increased accumulation of GFP-LC3 puncta in hepatocytes fed with ethanol diet, suggesting increased biogenesis of autophagosomes (Thomes et al., 2012; Lin et al., 2013; Chao et al., 2018c). Nevertheless, subsequent studies revealed that the increased number of autophagosomes is due to decreased hepatic lysosome numbers and increased lysosomal pH and impaired trafficking of lysosomal enzymes by chronic ethanol exposure (Kharbanda, McVicker et al. 1996, 1997; Chao et al., 2018a; Chao et al., 2018c)). Decreased lysosome number in hepatocytes is associated with a decrease in nuclear TFEB accumulation and impairment of lysosomal functions resulting in “insufficient autophagy”, a term referring to increased autophagic flux that fails to reach its maximum capacity to meet cell degradation needs (Chao et al., 2018a; Chao et al., 2018c). Decreased hepatic TFEB is likely mediated by the activation of mTOR as pharmacological inhibition of mTOR partially rescued TFEB signaling and protected against alcohol-induced steatosis and liver injury (Chao et al., 2018c). In addition, chronic alcohol exposure can also lead to reduced lysosome mobility, impaired small guanosine triphosphate Rab7, Src kinase and dynamin 2 (a GTPase involved in scission of cell membrane and formation of microtubule bundles) activities resulting in the depletion of lysosomes and inhibition of hepatocyte lipophagy (Schroeder et al., 2015; Li and Ding 2017; Rasineni et al., 2017; Schulze et al., 2017). In addition to lipophagy, both acute and chronic ethanol exposure can induce parkin mitochondrial translocation and increase parkin-mediated mitophagy, which serves as another layer of protection against alcohol-induced liver injury (Williams et al., 2015a; Eid et al., 2016). Overall, current data seem support a temporal role of autophagy in AALD. Acuate alcohol exposure induces adaptive protective autophagy whereas this adaptive autophagy process becomes impaired in chronic alcohol exposure.
8. Aggrephagy, phase-phase separation and AALD
Misfolded proteins that fail to be rescued by chaperones or degraded through the ubiquitin-proteasome system (UPS) accumulate and form protein aggregates. Aggrephagy is a form of selective autophagy for degradation of these protein aggregates (Lamark and Johansen 2012; Feng et al., 2014). There are two major pathways for degrading misfolded proteins in eukaryotic cells. The UPS degrades short-lived and soluble misfolded proteins whereas aggrephagy is responsible for degradation of long-lived proteins and insoluble protein aggregates (Ding and Yin 2008; Lamark and Johansen 2012; Dikic 2017; Sun et al., 2020). Ubiquitinated proteins are recognized by ubiquitin binding autophagy receptors such as p62, neighbor of BRCA1 gene 1 (NBR1), optineurin (OPTN), nuclear dot protein 52 (NDP52, also called CALCOCO2), and TAX1BP1 (Tax1 binding protein 1) (Lamark and Johansen 2012; Lystad and Simonsen 2015; Sarraf et al., 2019).
These receptor proteins tether aberrant ubiquitinated proteins to the inner surface of the phagophore by conjugating with LC3/GABARAP family proteins (Lamark and Johansen 2012; Lystad and Simonsen 2015; Sun et al., 2020). p62 is the key factor that determines whether the ubiquitinated proteins are degrade by ubiquitin-proteasome system or aggrephagy (Danieli and Martens 2018). p62 is a common component of many human disease-related cellular inclusion bodies, such as Mallory-Denk bodies (MDBs), which are found in approximately 75% of patients with alcoholic hepatitis, and in nearly all patients with alcoholic cirrhosis (French et al., 1993). MDBs are also commonly found in livers of NASH and HCC patients, which mainly consist of cytoskeletal intermediate filament proteins keratin 8 (K8) and keratin 18 (K18), p62 and ubiquitin (Zatloukal et al., 2007). It has been shown that the UBA domain of p62 directly binds to ubiquitinated misfolded proteins and sequesters them into protein aggregates. These p62 positive aggregates then further recruit LC3-positive autophagosomes through p62’s LIR motif (Wild et al., 2014; Lee and Weihl 2017). Lahiri et al. found that p62 interacts with keratin aggregates that promote the enlargement and stabilization of MDBs as well as recruitment of other MDB-associated proteins such as NBR1 and Hsp25. p62 deficiency leads to the formation of smaller MDBs and prevents their maturation (Lahiri et al., 2016). These inclusion bodies have been widely used as morphological hallmarks of a variety of liver diseases including AALD and HCC, although their contributions to the disease pathogenesis remains elusive. It has been suggested that soluble misfolded proteins are more toxic, and thus the formation of insoluble inclusion bodies may be an adaptive protective mechanisms against proteotoxicity (Ding and Yin 2008; Manley et al., 2013).
Recent evidence indicates that intracellular p62 bodies and stress granules are not simple protein aggregates but rather form liquid-like protein condensates through liquid-liquid phase separation, and these condensates are cleared by selective autophagy (Noda et al., 2020; Yamasaki et al., 2020). Interestingly, the pre-autophagosomal structure formation is initiated by the Atg1 complex that undergoes phase separation to form condensates in yeast (Fujioka et al., 2020).
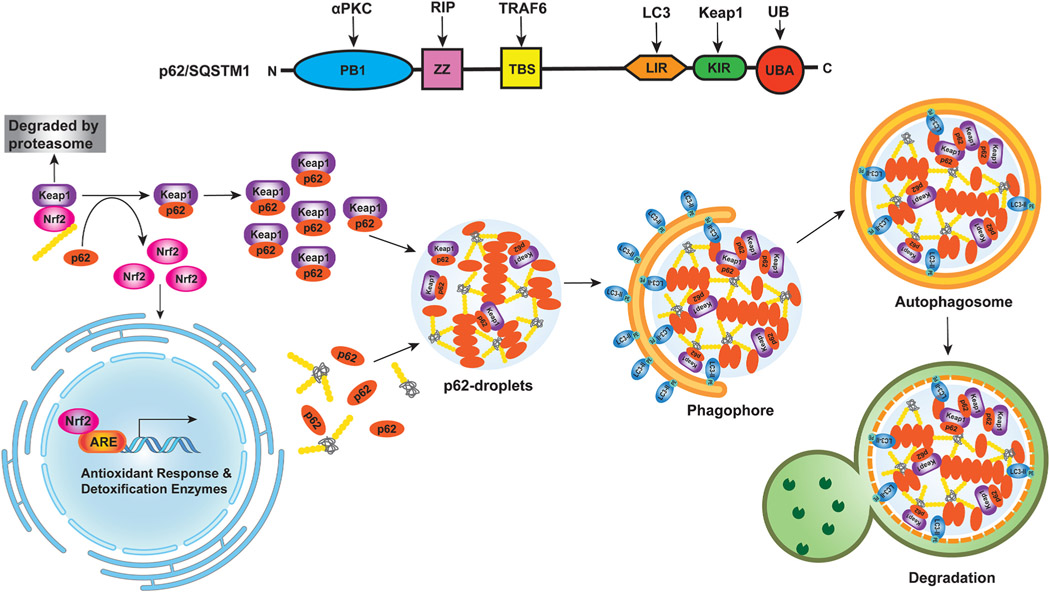
In mammalian cells, autophagosome-like vesicles form at the surface of protein-free droplets through partial wetting, a physics term referred to the ability of liquids to form interfaces with solid surfaces. The droplet surface tension supports the formation of membrane sheets and the bending sheets can either divide droplets for piecemeal sequestration or sequester entire droplets to trigger droplet autophagy or “fluidophagy” (Agudo-Canalejo et al., 2020). In hepatocytes, Kageyama et al. showed that p62 can form low-liquidity gel-like p62 bodies, which contain ubiquitin and core autophagy-related proteins such as ATG8 and direct autophagosome formation toward the p62-gel (Kageyama et al., 2021). Keap1, the negative regulator of Nrf2, reversibly sequesters in the p62-gels depending on its p62 binding activity resulting in the activation of Nrf2. They further generated a HyD-LIR-Venus mice that have defects in Atg8-interaction-dependent selective autophagy and showed that these mice have impaired turnover of p62-gels resulting in Nrf2 hyper-activation in mouse liver (Kageyama et al., 2021). These new findings indicate that p62-gels may serve as platforms for both autophagosome formation and Nrf2-mediated anti-oxidative stress response in the liver. More studies are needed to further elucidate the relevance of phase-phase separation-mediated selective autophagy in the context of liver diseases such as NAFLD and AALD (Fig. 5).
Fig. 5. p62-Nrf2 activation in liver cancer.
(1) Schematic illustration of p62 domain structure. Phox/Bemp1 (PB1) domain is involved in the binding of p62 to atypical PKC (aPKC) or ERK1. The ZZ zinc finger region and the TRAF6-binding domain, TBS, interact with RIP1 and TRAF6, respectively, to activate the p62-mediated nuclear factor-κB (NF-κB) pathway. LIR domain of p62 binds to LC3 protein to trigger the selective-autophagy pathway. KIR domain next to LIR binds to Keap1 and transports Keap1 into the autophagosome for degradation, which leads to the activation of the non-canonical Keap1-Nrf2 pathway. The ubiquitin-associated (UBA) domain on the C terminus binds to ubiquitinated proteins. Both the PB1 and UBA domains play an important role in p62-mediated aggregate formation. (2) Crosstalk between Nrf2 and p62 phase separation in selective autophagy. p62 regulates the Keap1-Nrf2 pathway through the KIR domain. In canonical Keap1-Nrf2 pathway, Keap1 mediates Nrf2 ubiquitination and leads to Nrf2 degradation via the proteasome. In non-canonical Keap1-Nrf2 pathway, p62 competitively binds to Keap1 leading to the stabilization of Nrf2 that can then translocate into the nucleus and activate the expression of antioxidant response-related detoxification enzymes, which protects against drug-induced hepatotoxicity and cancer cell survival. p62 is a multivalent protein and multivalent interactions between p62 and poly-ubiquitin or ubiquitinated misfolded proteins promotes p62 droplet formation, which can also recruit Keap1. The liquid-like properties of the p62 condensates are also thought to be crucial in the initiation of autophagosome formation for aggrephagy.
9. Autophagy in drug-induced liver injury
Drug-induced liver injury (DILI) is the most common cause of liver failure in the United States (Reuben et al., 2010). Most acute liver failure cases in the United States are caused by acetaminophen (APAP)-induced liver injury (AILI) (Larson et al., 2005). APAP is considered safe at therapeutic doses. However, APAP overdose can cause mitochondrial dysfunction, hepatocyte death, liver injury, and possible liver failure. Mitochondrial dysfunction, oxidative stress, and formation of APAP-adducts (APAP-AD) all contribute to AILI (Jaeschke et al., 2012; Ramachandran and Jaeschke 2019).
The role of autophagy in AILI was reviewed previously (Chao et al., 2018b) and will be briefly discussed here. Mice treated with rapamycin or Torin 1 to activate autophagy are protected against AILI whereas mice treated with chloroquine (CQ) to inhibit autophagy have increased AILI (Ni et al., 2012; Ni et al., 2016; Shan et al., 2019). Various other drugs that activate autophagy are also shown to protect against AILI in mice. For example, chlorpromazine (CPZ) protects against AILI by activating autophagy and decreasing activation of c-Jun-N-terminal kinase (JNK) (Li et al., 2019), a well-known mediator of AILI. IL-22 protects against AILI in mice by activating AMPK-dependent autophagy. Like CPZ, IL-22 also decreases JNK activation (Mo et al., 2018). While pharmacological activation of autophagy protects against AILI, liver-specific Atg5 knockout (KO) mice are paradoxically resistant to AILI due to the non-canonical activation of Nrf2 as a result of autophagy deficiency-induced p62 accumulation (Ni et al., 2012).
Mechanistically, autophagy protects against AILI by selectively removing damaged mitochondria and APAP-AD. In mitochondria/mitophagy, Parkin is translocated to mitochondrial outer membrane after APAP treatment in mice, and acute knockdown of Parkin in mouse livers inhibits mitophagy and increases AILI (Williams et al., 2015b). Furthermore, PINK1/Parkin double KO mice have increased liver injury and mortality after APAP treatment compared to WT mice, highlighting the importance of this pathway in APAP-induced mitophagy (Wang et al., 2019). Interestingly, mitophagy might help protect against AILI by inhibiting NLRP3 inflammasome activation (Shan et al., 2019). Autophagy also protects against AILI by selectively removing APAP-AD (Ni et al., 2016; Akakpo et al., 2019; Chen et al., 2020). APAP-AD formation is thought to be an important contributor to AILI (McGill et al., 2012). APAP-AD co-localized with GFP-LC3 positive autophagosomes and Lamp1 positive lysosomes in primary hepatocytes treated with APAP, and APAP-AD were found present in autophagosomes and lysosomes isolated from APAP-treated mouse livers (Ni et al., 2016). Autophagy inhibition by CQ or leupeptin increases serum APAP-AD levels and liver injury, and treatment with the autophagy activator Torin 1 decreased serum APAP-AD levels and liver injury in mice (Ni et al., 2016). Interestingly, serum levels of APAP-AD were increased in human APAP overdose patients with high alanine aminotransferase (ALT) values but not in overdose patients with low ALT values who lacked liver injury. However, serum LC3-II levels were increased in all APAP overdose patients regardless of ALT levels compared to healthy controls. Therefore, the combination of serum APAP-AD and LC3-II levels may be used as biomarkers for monitoring APAP-mediated liver injury (Ni et al., 2016). In addition to the early injury phase of AILI, future studies may be needed to determine the importance of selective APAP-AD and damaged mitochondria removal by autophagy in the liver recovery/regeneration phase from APAP overdose.
In addition to AILI, autophagy also protects against DILI caused by efavirenz (EFV), diclofenac, and cisplatin and may also help defend against DILI triggered by other drugs (Williams and Ding 2020). Overall, autophagy protects against DILI by upregulating selective autophagy pathways, such as mitophagy and selective degradation of reactive drug metabolites. Specific mechanisms involved in autophagy-mediated protection against DILI need clarification, but upregulation of selective autophagy pathways may be a promising target for future therapeutic options for DILI patients.
10. Autophagy in macrophages and inflammation
Chronic liver inflammation contributes to liver injury, fibrosis and cirrhosis. Inflammatory cells such as Kupffer cells (which are hepatic resident macrophages), neutrophils, and dendritic cells (DCs) all participate in liver inflammation (Koyama and Brenner 2017). Kupffer cells play critical roles during liver injury, and the hepatoprotective function of Kupffer cells includes inhibiting pathogen invasion and repairing tissue injury. However, elevated activities of Kupffer cells also aggravates liver inflammation in ALD, NAFLD, NASH, and liver ischemia-reperfusion injury (IRI) (Brenner et al., 2013; Dixon et al., 2013; Ding and Jaeschke 2016). Hepatic macrophage autophagy has recently been reported as a protective mechanism against liver steatosis, inflammation, and fibrogenesis (Mallat et al., 2014).
Mice fed with alcohol showed increased expression of pro-inflammatory markers and steatosis in myeloid-specific cannabinoid receptor 2 (CB2) KO mice compared to WT mice, and CB2 activation induces macrophage autophagy that protects against alcohol-induced steatosis and inflammation. Interestingly, the protection of CB2 agonist is lost in myeloid-specific Atg5 KO mice (Denaës et al., 2016), suggesting the beneficial role of CB2 activation is dependent on macrophage autophagy. Impaired autophagy in myeloid-specific Atg5 KO mice enhances pro-inflammatory cytokine production by activating pro-inflammatory M1 polarization (Liu et al., 2015), exacerbates liver inflammation and fibrosis after chronic CCl4 treatment (Lodder et al., 2015), and potentiates GalN/LPS induced liver injury (Ilyas et al., 2016). Mechanistically, it is likely that autophagy of macrophages can decrease mitochondrial ROS accumulation, inhibit the production of NLRP3 inflammasome-activated IL-1β, and promote the degradation of interferon regulatory factor 1 (IRF1) which silences chemokines, such as CCL5 and CXCL10 (Liang et al., 2019). Conversely, genetic overexpression of TFEB or pharmacological activation of TFEB by trehalose in macrophages induces lysosomal biogenesis and autophagy resulting in decreased production of pro-inflammatory IL-1β, leading to reduced atherosclerosis (Sergin et al., 2017). Therefore, targeting macrophage autophagy and lysosomal biogenesis may be a valued avenue for attenuating liver inflammation related diseases including NASH and alcoholic hepatitis and NAFLD associated atherosclerosis.
11. Autophagy in hepatic stellate cells and liver fibrosis
Chronic liver injury leads to liver scarring, termed fibrosis, and sustained fibrosis can lead to cirrhosis, and eventually progress to liver failure and HCC. Hepatic stellate cells (HSCs), which are sinusoidal pericytes, play critical roles in liver fibrogenesis (Friedman 2008; Weiskirchen et al., 2019). Upon activation, quiescent and LDs enriched HSCs transdifferentiate into collagen-secreting myofibroblasts that produce extracellular matrix. This activation process occurs in multiple chronic liver diseases such as cholestasis, NAFLD, viral hepatitis, NASH, AALD, and autoimmune disorders (Weiskirchen and Tacke 2016; Tsuchida and Friedman 2017). The key characteristic of HSC activation is the loss of retinoid-containing LDs. Autophagy contributes to HSC activation by providing the energy from LD breakdown in HSCs (Hernández–Gea et al., 2012; Mallat et al., 2014; Weiskirchen and Tacke 2019). In vitro studies have shown that autophagy activity is significantly increased during murine or human HSC activation, and pharmacologically inhibiting autophagy blocked the activation of HSCs (Thoen et al., 2011; Hernández–Gea et al., 2012). HSC-specific Atg7 KO mice have decreased HSC activation and liver fibrosis in response to either carbon tetrachloride (CCl4) or thioacetamide (TAA) (Hernández–Gea et al., 2012; Mallat et al., 2014). Atg2A is known to extend the phagophore by transferring lipids from the ER to the phagophore (Maeda et al., 2019). Atg2A is enriched in LDs of quiescent HSCs, and is critical in mediating cell activation and autophagy in HSCs. Downregulation of Atg2A by small interfering RNAs (siRNA) blocks autophagic flux and inhibits murine or human HSCs activation in vitro (Hong et al., 2018).
Various signaling pathways have been reported to activate autophagy in HSCs to promote fibrosis. Autophagy is activated by insulin-like growth factor binding protein-associated protein 1 (IGFBPrP1) or transforming growth factor β1 (TGF-β1) by inhibiting Akt/mTOR or activating ERK and JNK pathways in HSCs that leads to HSC activation and fibrosis (Huang et al., 2019; Zhang et al., 2021). Fibrotic livers are associated with hypoxia and increased release of high-mobility group box-1 (HMGB1). Either activation of hypoxia-inducible factor-1α or exogenous HMGB1 can activate autophagy in HSCs (Deng et al., 2014; Li et al., 2018), which may further worsen fibrosis. Furthermore, HSC autophagy is activated by chronic alcohol feeding due to increased ROS production and ER stress resulting in increased extracellular matrix (ECM) deposition (Hernández-Gea et al., 2013). Advanced glycation end products also induce autophagy during HSCs activation and subsequently contribute to fibrogenesis in patients with chronic hepatitis C (He et al., 2015).
To balance autophagy activation in HSCs, there are many signaling pathways that inhibit autophagy in HSCs. Guanine nucleotide-binding α-subunit 12 (Gα12) is highly expressed in HSCs and fibrotic liver. Gα12 activation promotes autophagy through ATG12–5 conjugation in HSCs, and HSC activation can be blocked by miR-16 which inhibits de novo synthesis of Gα12 (Kim et al., 2018). Interestingly, p62 directly binds to vitamin D receptor (VDR) and retinoid X receptor-alpha (RXRα) to promote their heterodimerization, which suppresses HSC activation and fibrosis (Duran et al., 2016). As autophagy deficiency leads to the accumulation of p62, it is therefore likely that autophagy inhibition and p62 accumulation may form a forward feedback loop to inhibit HSC activation and fibrosis. Altogether it is evident that autophagy is a central regulator in HSC activation, and reducing autophagy in HSCs may be a promising therapeutic strategy for treating liver fibrosis. However, one remaining challenge is to develop autophagy inhibitors to specifically target HSCs but not hepatocytes, as inhibition of autophagy in hepatocytes may lead to hepatocyte death accompanied with inflammation and fibrosis (Ni et al., 2014b; Ni et al., 2019).
12. Autophagy in liver endothelial cells
Liver sinusoidal endothelial cells (LSECs) are highly specialized endothelial cells that represent the first defense barrier of the liver between blood cells and hepatocytes or HSCs (DeLeve 2015; Ruart et al., 2019). LSECs are distinct from other endothelial cells since their basement membranes are characterized by fenestrae (DeLeve 2015). LSECs play a critical role in anti-inflammation, anti-fibrogenesis, and maintaining liver homeostasis. Loss of fenestration (capillarization) is observed in both acute liver injury and chronic liver diseases such as IRI (Boteon et al., 2017), liver cirrhosis (Ni et al., 2017), NAFLD (Hammoutene and Rautou 2019), NASH (Hammoutene et al., 2020), and HCC (Wilkinson et al., 2020). Recent studies have demonstrated that endothelial autophagy protects against various liver injuries. The characteristic of early liver IRI is LSEC injury and dysfunction (Miyashita et al., 2016). Autophagy activation induces angiogenesis during the recovery phase of ischemic injury, and endothelial autophagy activation participates in reducing liver IRI, which is mediated by the transcription factor Krüppel-like factor 2 (KLF2) that has an endothelial vasoprotection function. Overexpression of KLF2 activates autophagy in isolated LSECs, and KLF2 inducer, simvastatin, increases cell viability by stimulating KLF2-medaited autophagy in LSECs with biomechanical stimulation by shear stress, in an in vitro cell model that mimics liver IRI (Guixé-Muntet et al., 2017).
Autophagy is also upregulated during LSEC capillarization both in vitro and in vivo. The endothelial cell-specific Atg7 KO mice have aggravated liver fibrosis due to impaired antioxidant response after acute CCl4 treatment (Ruart et al., 2019). More recently, Hammoutene et al. showed that autophagy is impaired in LSECs of NASH patients. LSEC-specific Atg5 KO mice show an increase in LSEC apoptosis, inflammation and NASH development after high fat diet feeding (Hammoutene et al., 2020). In response to vascular injury, endothelial cells secrete various factors, such as von Willebrand factor (VWF), to promote platelet adhesion to the injured vessel wall which acts as one of the first lines of defense. VWF is generally stored in specific secretory granules known as Weibel-Palade bodies (WPBs) in endothelia cells, and it has been shown that autophagy is required for VWF secretion in cultured endothelial cells. Moreover, endothelial cell-specific Atg7 KO mice had a significant increase in measured bleeding time when compared with control mice but the role of autophagy-mediated VWF secretion from LSECs was not investigated (Torisu et al., 2013). However, some evidence suggests that VWF and platelet activation may impair liver regeneration in DILI and promote NASH development (Malehmir et al., 2019; Groeneveld et al., 2020). Therefore, whether the role of LSEC autophagy is beneficial or detrimental in liver diseases may be context dependent. For the secretory role of autophagy in LSECs, it may also be interesting to determine whether an autophagy receptor protein may be involved in selectively packing the VWF in autophagosomes.
13. Cholesterol and autophagy in fatty liver disease
The liver plays a key role in regulating whole body cholesterol and bile acid homeostasis. Hepatic cholesterol accumulation has been linked to non-alcoholic steatohepatitis (NASH) and is thought to cause organelle dysfunction, oxidative stress and hepatocyte injury (Mari et al., 2006; Puri et al., 2007; Wouters et al., 2008). Hepatic bile acid synthesis serves as the major cholesterol elimination mechanism (Li and Chiang 2014). Bile acids act as signaling molecules to regulate hepatic metabolic homeostasis. However, in cholestasis where hepatic bile acid accumulates, bile acids promote hepatocyte injury and inflammatory infiltration. More recently, bile acid hepatotoxicity has also been recognized as a potentially important contributor to liver injury in NASH (Arab et al., 2017; Zhou et al., 2017). Recent emerging evidence shows that pathological cholesterol and bile acid accumulation impairs hepatic autophagy activity and lysosomal function (Wang et al., 2018; Panzitt et al., 2020a).
Lysosomes play a highly significant role in regulating cholesterol homeostasis in hepatocytes (Soccio and Breslow 2004). Following receptor-mediated endocytosis, cholesterol-rich lipoproteins enter the endo-lysosomal pathway and cholesterol ester (CE) is hydrolyzed by lysosomal acidic lipase (LAL) to generate free cholesterol (FC) to be transported out of the lysosomes. In hepatocytes, FC predominantly resides in the plasma membrane while FC content is relatively low in other intracellular organelles such as the ER, Golgi, and mitochondria (Soccio and Breslow 2004; van Meer et al., 2008). While cells constantly generate FC to be used for membrane synthesis, sterol and bile acid synthesis, etc., accumulation of FC in intracellular organelles can be detrimental to their function. To prevent this, FC is delivered to the ER where acyl-CoA: cholesterol acyltransferase (ACAT) efficiently converts FC to CE that is subsequently stored in LDs. In addition to lysosomal CE hydrolysis, a number of neutral lipases can directly act on the LDs to hydrolyze CE to release free cholesterol (Ouimet and Marcel 2012). In macrophages, CE hydrolysis in the cytosol and lysosome appeared to be equally important in generating the intracellular FC pool (Ouimet et al., 2011). The same may be assumed in hepatocytes but could also depend on the cellular metabolic environment and lipid contents. Under conditions of a net increase of cellular cholesterol input, cholesterol esterification is predominant in limiting intracellular FC accumulation. In NASH, dysregulation of cholesterol homeostasis is associated with intrahepatic accumulation of FC that causes mitochondrial dysfunction, ER stress, lysosomal stress, and hepatocellular injury (Mari et al., 2006; Puri et al., 2007; Wouters et al., 2008).
Current evidence generally suggests that cholesterol-mediated lysosomal dysfunction impairs autophagic flux at the autophagosome-lysosome fusion step, possibly due to cholesterol accumulation in both autolysosomes and lysosomes. This is supported by studies of various genetic defects known to be associated with lysosomal cholesterol accumulation. Niemann-Pick disease, type C (NPC) is a progressive neurological disorder caused by mutations in NPC1 or NPC2 genes that result in defective lysosomal cholesterol export. NPC cells accumulate autophagy vacuoles due to delayed autophagosome clearance (Liao et al., 2007; Elrick et al., 2012). Niemann-Pick disease type A/B is another lysosomal storage disease due to defects in lysosomal acid sphingomyelinase (ASMase) (Schuchman and Desnick 2017). ASMase mediates the conversion of sphingomyelins to ceramides, and ASMase deficiency increases lysosomal membrane sphingomyelin content causing lysosomal membrane cholesterol retention (Ridgway 2000). Hepatocytes isolated from ASMase KO mice showed decreased autophagosome and lysosome fusion (Fucho et al., 2014). It has been shown that forced cholesterol loading in hepatocytes caused significant accumulation of autolysosomes, a condition associated with decreased lysosomal hydrolytic activity and increased autolysosomal membrane permeability (Wang et al., 2017). Such autophagy defects were further amplified when ACAT-mediated cholesterol esterification was inhibited (Wang et al., 2017), which supports the notion that FC accumulation in the lysosomes but not CE accumulation in the LDs is the underlying cause of autophagy impairment. Consistently, many cholesterol lowering therapeutics including statins, bile acid sequestrants and ezetimibe have been shown to promote autophagy activity in hepatocytes (Yamamura et al., 2014; Wang et al., 2015; Wang et al., 2017). Notably, hydroxypropyl-β-cyclodextrin, a cholesterol depleting agent proposed to treat NPC diseases, has been shown to promote autophagy (Cheng et al., 2006; Sarkar et al., 2013; Meske et al., 2015), suggesting that targeting lysosomal cholesterol accumulation is effective in restoring autophagy flux and attenuating autophagy vacuole accumulation in cells with lysosomal cholesterol accumulation. On the other hand, autophagy defects in hepatocytes are not known to cause CE accumulation in the LDs, which argues that lysosomes, but not autophagic flux per se, is important in regulating cellular cholesterol homeostasis.
Recent studies have revealed that FC accumulation was significantly higher in NASH livers than in normal livers and simple steatosis livers in humans (Puri et al., 2007). Cholesterol accumulation in hepatocytes causes mitochondrial dysfunction and lysosomal stress, which contributes significantly to injury and inflammation in NASH (Mari et al., 2006; Wouters et al., 2008). It was initially observed that forced cholesterol accumulation in hepatocytes resulted in significantly increased numbers of autolysosomes and lysosomes (Wang et al., 2017), suggesting a compensatory increase of autophagy and lysosomal biogenesis. More recently, TFEB was shown to be activated in response to cholesterol-induced lysosomal stress in hepatocytes (Wang et al., 2020), which provided a plausible molecular mechanism underlying such adaptive responses. This finding further suggests that in NASH livers impaired autophagic flux due to defective autolysosome clearance and compensatory induction of autophagy initiation and lysosomal biogenesis co-exist, which may underlie the difficulty of using traditional autophagy markers and autophagy flux assays in determining how hepatic autophagy activity changes in various experimental NAFLD and NASH models with different disease severity.
14. Bile acid and autophagy in cholestasis
Bile acid synthesis occurs exclusively in hepatocytes and plays a key role in whole body cholesterol elimination (Li and Chiang 2014). Cholesterol 7α-hydroxylase (CYP7A1) is an ER resident cytochrome P450 enzyme that catalyzes the first and rate-limiting step in the conversion of cholesterol into bile acids (Li and Chiang 2014). Bile acids are secreted from hepatocytes into the bile and subsequently released into the small intestine where bile acids act as physiological detergents to help emulsify dietary fat and facilitate intestine absorption of lipid and fat-soluble vitamins. Bile acids are efficiently re-absorbed at the terminal ileum and transported back to the liver. Bile acids circulate between the liver and intestine a few times a day via enterohepatic circulation of bile acids. Bile acid synthesis is regulated by bile acid-mediated feedback inhibition (Li and Chiang 2014). The nuclear receptor farnesoid x receptor (FXR) is highly expressed in the liver and intestine and is activated by bile acids (Kong et al., 2012). In hepatocytes, FXR induces small heterodimer partner (SHP) to repress CYP7A1 gene transcription (Goodwin et al., 2000). In the small intestine bile acid-activated FXR induces mouse fibroblast growth factor 15 (FGF15), which acts as an endocrine hormone to repress hepatic CYP7A1 gene transcription (Inagaki et al., 2005). Fibroblast growth factor 19 (FGF19) is the human ortholog of the mouse FGF15 (Song et al., 2009).
Recent studies have revealed that bile acid signaling inhibits autophagy activity via several transcriptional and signaling mechanisms. Bile acid-activated FXR was shown to transcriptional inhibit autophagy genes leading to reduced autophagy activity in hepatocytes (Lee et al., 2014; Manley et al., 2014; Seok et al., 2014). Furthermore, bile acid-induced intestine FGF15/19 has also been shown to inhibit TFEB and its target genes in autophagy and lysosome biogenesis (Byun et al., 2017; Wang et al., 2020). Given that FGF15/19 activates a number of nutrient signaling pathways including mTOR and ERK1/2 in hepatocytes, bile acid-FGF15/19 signaling has also been shown to inhibit autophagic flux via signaling mechanisms independent of its transcriptional control of autophagy genes (Manley et al., 2014; Byun et al., 2017). A more recent study reported that FXR induced Rubicon, a known inhibitor of autophagosome maturation (Panzitt et al., 2020b), which provided a molecular mechanism by which bile acids inhibited autophagic flux. Both bile acid concentration and FGF15/19 concentration increase in the portal blood during postprandial period where they can act as insulin-independent signaling molecules to regulate hepatic metabolic transition from fasting to feeding (Yamagata et al., 2004; Galman et al., 2005; Lundasen et al., 2006; Kir et al., 2011; Potthoff et al., 2011). From a physiological point of view, bile acid signaling may mediate postprandial inhibition of hepatic autophagy. During fasting and starvation, bile acid and other nutrient signaling pathways decrease and autophagy activity is increased.
One of the emerging areas of research is understanding the regulation and significance of hepatic autophagy in cholestasis, a liver disease associated with genetic and acquired defects of hepatic bile acid secretion and intrahepatic bile acid accumulation. Several studies have reported that autophagosome-lysosome fusion is inhibited in experimental models of cholestasis, which is consistent with the reported inhibitory effect of bile acids (Manley et al., 2014; Kim et al., 2018). Because super-physiological concentrations of bile acids found under cholestasis can activate various cellular stress kinases, it is likely that cellular signaling mechanisms mediate bile acid inhibition of autophagosome-lysosome fusion. Recently, it was found that mice with hepatic autophagy defect due to Atg5 or Atg7 deletion also exhibited characteristic features of cholestasis including elevated bile acids in the liver and blood, ductular reaction, impaired FXR activity, and gut microbiota remodeling (Khambu et al., 2019; Yan et al., 2020), suggesting that inhibited hepatic autophagy may in turn contribute to liver injury in cholestasis. In support of this, genetic mutations of FXR can cause cholestasis in humans (Gomez-Ospina et al., 2016). Currently, the molecular mechanisms and pathophysiological implications of the bile acid and autophagy crosstalk is far from clear, and further studies are still needed to fill this knowledge gap.
15. Autophagy and viral hepatitis
Hepatitis B virus (HBV) and hepatitis C virus (HCV) are the two main hepatitis viruses of chronic liver infection and are associated with the progression of liver fibrosis to HCC (Alavian et al., 2011; Abdoli et al., 2018). HBV is an enveloped DNA virus and infects approximately 250 million members of the global population chronically, and causes an estimated 800,000 deaths annually, mostly from liver cirrhosis and cancer (Daniel Lavanchy 2016; Polaris Observatory 2018; Razavi 2020). HCV is an enveloped RNA virus, which chronically infects approximately 70 million individuals worldwide, and causes an estimated 400, 000 death annually (Gower et al., 2014; Petruzziello et al., 2016; Razavi 2020). Increasing evidence indicates that both HBV and HCV infection affect the autophagy process resulting in impaired autophagic flux (Sir et al., 2008a; Sir et al., 2010a; Huang et al., 2013). Increased formation of autophagosomes can be induced by overexpression of the entire HBV genome or the hepatitis B virus X protein (HBx) alone. Mechanistically, the X protein directly binds with phosphatidylinositol 3-kinase to enhance its enzyme activity, resulting in the biogenesis of autophagosomes in hepatocytes (Tang et al., 2009; Sir et al., 2010b; Khan et al., 2018). Despite increased autophagosome formation, HBx dramatically impairs lysosomal acidification and autophagic degradation possibly through interaction with V-ATPase which decreases its lysosome targeting (Liu et al., 2014). In HBV related HCC patients, poor overall survival rate is associated with low expression of ATG5. Moreover, pharmacological activation of autophagy by amiodarone inhibits liver tumorigenesis in an orthotopic SD rat model (Lan et al., 2014). Altogether, autophagy not only promotes the replication of HBV, but it may also play a role in inhibiting HBV-related tumorigenesis.
Similar to HBV, HCV infection also increases the formation of autophagosomes but with delayed autophagosome maturation and autophagic flux (Ait-Goughoulte et al., 2008; Sir et al., 2008a; Sir et al., 2008b; Ke and Chen 2011; Huang et al., 2013). Sir et al. reported that HCV infection increased the formation of autophagosomes but impaired the fusion of autophagosomes with lysosomes resulting in defective autophagic flux (Sir et al., 2008a). Subsequent studies have revealed more complicated temporal changes of autophagic flux induced by HCV. HCV infection-induced autophagic flux is less efficient at the early stages but becomes more efficient at later stages (Huang et al., 2013; Wang and Ou 2015; Wang et al., 2015). This is likely because HCV enhances its replication by temporally regulating the autophagic flux through differentially inducing the expression of RUN domain and cysteine-rich domain containing Beclin 1-interacting protein (Rubicon) and ultraviolet radiation resistance-associated gene protein (UVRAG) (Wang et al., 2015). Increased expression of UVRAG by HCV infection stimulates the maturation of autophagosome, whereas increased expression of Rubicon in the early stage of HCV infection suppresses the maturation of autophagosomes resulting in the accumulation of autophagosomes to sustain HCV replication (Wang et al., 2015). HCV induces autophagy in hepatocytes by direct and indirect mechanisms. The expression of the HCV NS3-NS5B nonstructural polyprotein increases the formation of autophagosomes. Immunity-related GTPase family M protein (IRGM), a member of the small GTPase family, interacts with ATG5 and ATG10 to regulate autophagy. It has been shown that HCV NS3 interacts with IRGM and thus indirectly induces autophagy (Gregoire et al., 2011). In addition, NS5B, an RNA-dependent RNA polymerase and essential component of the HCV replication complex, interacts with ATG5 to promote autophagosome formation (Guevin et al., 2010; Sir et al., 2012). The HCV p7 ion channel protein directly binds to Beclin-1, but this binding does not increase the number of autophagosomes based on the changes of LC3-II levels. As an autophagic flux assay was not performed in this study, the effects of p7 on autophagic flux remains to be determined (Aweya et al., 2013). In addition to the direct effects on autophagy, HCV infection can also induce autophagy by indirect mechanisms such as increased ER stress and oxidative stress (Sir et al., 2008a; Medvedev et al., 2017).
In addition to general autophagy, both HBV and HCV can also induce mitophagy by increasing Pink1 and Parkin expression as well as increased Drp1-mediated mitochondrial fragmentation. Increased mitophagy is likely to promote cell survival and HBV or HCV-associated HCC development (Huang et al., 2018; Ma et al., 2020). It has been well documented that HCV infection increases LD formation in hepatocytes. These LDs are often associated with HCV-induced autophagosomes and autophagy levels are inversely correlated with steatosis in HCV patients (Vescovo et al., 2012). However, whether HCV-induced autophagy can also selectively target LDs (macrolipophagy) and its impact on HCV replication and liver pathogenesis need to be further studied. Nonetheless, it seems that both HBV and HCV can cause the accumulation of autophagosomes and allows the virus to use the autophagosome’s membrane to promote its own replication.
16. Autophagy-p62-Nrf2 axis and liver cancer
Hepatocellular carcinoma (HCC) is the most common liver cancer and the third leading cause of cancer related tumor death worldwide (Yang and Roberts 2010). Various factors including aflatoxin B1 exposure, infection with HBV, HCV, AALD, diabetes, obesity, NAFLD, and hemochromatosis (an iron overload disease), as well as genetic disorders such as mutations in tumor suppressors or oncogenes can contribute to HCC development (Farazi and DePinho 2006; Caruso, O’Brien et al., 2020). All the above HCC risk factors either inhibit autophagy (such as activation of oncogene Akt or loss of tumor suppressor genes Pten or Tsc1), have insufficient autophagy (NAFLD and AALD) or delayed autophagic flux (HBV and HCV) in the liver (Fig. 6). It is now well-documented that autophagy acts as a tumor suppressor and defective autophagy promotes liver tumorigenesis. Beclin 1 heterozygous mice and liver-specific Atg5 or Atg7 KO mice all developed spontaneous liver tumors when they are aged (Qu et al., 2003; Inami et al., 2011; Takamura et al., 2011; Ni et al., 2014b), providing genetic evidence to support the tumor suppressing role of autophagy. Autophagic removal of protein aggregates and dysfunctional organelles such as mitochondria and ER may help to ameliorate oxidative and metabolic stress as well as genome instability, and in turn prevent cell transformation. Liver tumor formation is associated with a spectrum of temporal changes characterized as increased hepatic oxidative DNA and mitochondrial damage, increased number of peroxisomes and ER content, cell death and compensatory hepatocyte proliferation, hepatomegaly, ductular reaction, inflammation, fibrosis, and eventual liver tumors. All of the above pathological features can be found in liver-specific Atg5 or Atg7 KO mice (Takamura et al., 2011; Ni et al., 2012; Ni et al., 2014b; Tian et al., 2015; Yang et al., 2016; Ni et al., 2019). Increased release of high mobility group box 1 (HMGB1) and hepatic activation of Yap (yes-associated protein) as well as mechanistic target of rapamycin (mTOR) and p62-Nrf2 activation have been implicated in the liver tumorigenesis of hepatic autophagy-deficiency, although each of these signaling pathway seems to have distinctive roles (Khambu et al., 2018; Lee et al., 2018; Ni et al., 2019; Yang et al., 2019b). Either deletion of HMGB1 or Yap inhibits liver tumor formation, but deletion of HMGB1 tends to reduce ductular reactions whereas deletion of Yap decreases hepatomegaly in liver-specific Atg7 KO mice (Khambu et al., 2018; Lee et al., 2018). In contrast, deletion of mTOR promotes the early development of liver tumors despite the improvement of hepatomegaly and live injury in liver-specific Atg5 KO mice (Ni et al., 2019).
Fig. 6. Various HCC risk factors impair hepatic autophagy.
Activation of oncogenes or loss of tumor suppressor genes (such as tsc1 or pten) causes mTORC1 activation. NAFLD and AALD impair TFEB-mediated lysosomal biogenesis and decrease autophagy proteins such as ATG7. Both HBV and HCV infection either impairs lysosomal function or inhibits fusion of autophagosomes with lysosomes, leading to autophagy inhibition or delayed autophagic flux. All these primary risk factors for chronic liver diseases thus lead to inhibition of hepatic autophagy. Impaired hepatic autophagy increased accumulation of damaged mitochondrial, oxidative stress, genomic instability and non-canonical p62-Keap1-Nrf2 activation, ultimately resulting in liver cancer.
Nrf2 is a transcription factor that regulates expression of several genes that are involved in antioxidant defense, drug and lipid metabolism as well as autophagy (Jain et al., 2010; Bartolini et al., 2018). In response to oxidative stress, Nrf2 is activated via the canonical Kelch-like ECH-associated protein 1 (Keap1)-Nrf2 pathway, in which Keap1 acts as an adaptor protein to assemble an E3 ubiquitin ligase complex to promote Nrf2 ubiquitination and proteasomal degradation (Jaramillo and Zhang 2013). Under autophagy defective conditions, Nrf2 is activated in a non-canonical Keap1-Nrf2 pathway through p62 as a result of decreased autophagic degradation of p62. Ectopic overexpressed p62 alone in vivo is sufficient to induce Nrf2 activation and Nrf2-dependent hepatomegaly, which requires Akt activation as inhibition of Akt attenuated hepatomegaly (He et al., 2020). Phosphorylated p62 competitively binds to Keap1 to increase stabilization and transcriptional activity of Nrf2 (Komatsu et al., 2010; Ichimura et al., 2013). In addition to phosphorylation, it is shown that p62 is ubiquitinated at lysine (K7) via K63-linkage by TRIM21 (Tripartite motif-containing protein 21), a ubiquitin E3 ligase. The ubiquitylated p62 inhibits p62 oligomerization and sequestration of Keap1, serving as a negative regulator of Nrf2. Indeed, TRIM21 KO mice have increased Nrf2 activation and protect against oxidative damages in the mouse liver induced by arsenic exposure (Pan et al., 2016). Increased Keap1-p62 positive aggregates and ameliorated liver dysfunction were observed in liver-specific Atg7 Nrf2-DKO mouse livers. In contrast, liver-specific Atg7 Keap1-DKO mouse livers exacerbated liver injury by constitutively activating Nrf2 (Komatsu et al., 2010). Notably, dysregulation of the p62-Keap1-Nrf2 axis has been observed in both human HCC patients and mouse liver cancer models (Takamura et al., 2011; Bartolini et al., 2018). Nrf2, as an oncogene, promotes tumorigenesis and contributes to the resistance of human tumors to chemotherapy, and increased Nrf2 expression is a prognostic factor for HCC (Sporn and Liby 2012; Zhang et al., 2015; Bartolini et al., 2018). Tumor cells often grow in harsh environments due to limited nutrient and oxygen supply. It is likely that enhanced Nrf2 activation may protect tumor cells against oxidative and metabolic stress during tumor development. Indeed, deletion of Nrf2 completely abrogated liver injury and tumorigenesis in liver-specific Atg5 or Atg7 KO mice (Komatsu et al., 2010; Ni et al., 2014b; Khambu et al., 2018). The dramatic attenuation of liver injury and complete elimination of liver tumorigenesis in liver-specific Atg5 KO mice by deletion of Nrf2 are very intriguing as these double knockout mice (Atg5 and Nrf2) are still lacking autophagy in their livers. These observations may suggest that persistent Nrf2 activation is more critical than the lack of autophagy for inducing liver injury. An alternative interpretation is that lack of autophagy is not sufficient to cause liver injury and tumorigenesis if Nrf2 is concurrently absent. As Nrf2 is a transcription factor that enhances gene expression of multiple genes and subsequent protein translation, it may lead to aberrant protein accumulation and proteotoxicity in the liver (Yang et al., 2019a). If this hypothesis is correct, then shutting down or decreasing protein translation may help to attenuate the detrimental effects of persistent Nrf2 activation in autophagy defective livers. Our recent findings that deletion of mTOR or Raptor improves hepatomegaly and liver injury in young liver-specific Atg5 KO mice seems to support this hypothesis (Ni et al., 2019; Yang et al., 2019a). However, these protections are transient as lack of mTOR leads to compensatory Akt activation and promotes early liver tumorigenesis in middle-aged liver-specific Atg5 KO mice, when the same age liver-specific Atg5 KO mice have not yet developed tumors (Ni et al., 2019). Liver-specific Raptor KO mice also have been shown to promote high fat diet and chemical carcinogen-induced liver tumorigenesis (Umemura et al., 2014). Thus, the mechanisms of liver tumorigenesis in autophagy defective livers appear to be very complex and may involve multiple distinctive temporal adaptive and secondary effects. Nonetheless, it seems among the known signaling pathways including HMGB1, Yap and mTOR, the p62-Nrf2 pathway seems to play a predominant role as deletion of either HMGB1, Yap or mTOR cannot completely eliminate the tumors or even promote early tumor development whereas deletion of Nrf2 completely eliminates tumor formation in autophagy defective livers. Notably, results from a reversible Atg5-shRNA knockdown mouse model using doxycycline (dox)-inducible system to temporarily inhibit Atg5-dependent autophagy in vivo showed that ATG5 knockdown (ATG5i) mice also developed hepatomegaly and liver injury (Cassidy et al., 2018). Interestingly, restoration of ATG5 in these ATG5i mice reversed hepatomegaly and liver injury but these mice still develop fibrosis. However, neither Nrf2 activation nor tumorigenesis was determined in these ATG5 restored mice. Nonetheless, these findings may imply that restoration autophagy is beneficial but may not eliminate all the liver pathogenesis in conditions with chronic impaired autophagy. In a chemical screen, a small compound called K67 that inhibits the interaction between phosphorylated p62-peptide and Keap1 was identified as an Nrf2 inhibitor. K67 inhibits HCC proliferation, tumor growth and increases sensitivity to the anticancer drugs (Saito et al., 2016; Yasuda et al., 2020). Therefore, inhibition of Nrf2 using small molecule inhibitors may be a promising approach for treating a subtype of HCC that have defective autophagy and persistent Nrf2 activation.
17. Conclusion and future perspectives
With the rapid progress of autophagy research in the past couple of decades, we have gained significant insights into the roles of autophagy in the regulation of cellular and metabolic homeostasis in a variety of cell types in the liver. Impaired autophagy in hepatocytes, macrophage/Kupfer cells and endothelial cells generally leads to detrimental effects in the liver whereas defective autophagy in HSCs may be beneficial against liver fibrosis and HCC. Both AALD and NAFLD, the two most common chronic liver diseases, are associated with defective hepatic autophagy. Moreover, defective autophagy also promotes liver tumorigenesis (see the summary of all genetic KO mouse models of either parenchymal or non-parenchymal cells with distinctive phenotypes in Table 1). Therefore, approaches to boost hepatic autophagy will be beneficial to improve these chronic liver diseases. Indeed, pharmacological activation of autophagy has been shown to protect against DILI, AALD and NAFLD in experimental animal models. As many high through-put screenings for autophagy inducers or Nrf2 inhibitors have been performed or ongoing, we anticipate more translational studies in the future to test these newly identified autophagy modulators for treating liver diseases.
Table 1.
Phenotypes of hepatic parenchymal and non-parenchymal knockout/knockdown mice.
| Cell Type | Genotype | Phenotype | Ref |
|---|---|---|---|
|
| |||
| Hepatocytes/cholangiocytes | Alb-Cre Atg7f/f Alb-Cre Atg5f/f Alb-Cre Fip200f/f Alb-Cre Lamp-2af/f Alb-Cre Atg5f/f,Nrf2f/f DKO Alb-Cre Atg5f/f, mTORf/fDKO Alb-Cre Atg5f/f, Raptorf/f DKO Alb-Cre Atg7f/f,Nrf2f/f DKO Alb-Cre Atg7f/f, Yapf/f DKO Alb-Cre Atg7f/f,p62−/− DKO Alb-Cre Tfebf/f |
insulin resistance, hepatocyte death, liver tumors impaired fasting-induced LD biogenesis inflammation, fibrosis; cholestasis hepatosteatosis reduced liver injury & tumorigenesis reduced liver injury & promote tumorigenesis reduced liver injury & promote tumorigenesis reduced liver injury reduced liver size & tumorigenesis reduced liver injury & tumorigenesis increased diet-induced steatosis |
Singh et al. (2009) Ma et al. (2013) Ni et al. (2014b, 2019) Komatsu et al. (2010) Lee et al. (2018) |
| Macrophage (Kupffer Cells) | Lys-Cre Atg5f/f | increased liver injury & inflammation | Lodder et al. 2015 |
| HSC | GFAP-Cre Atg7f/f | decreased HSCs activation & fibrosis | Hernández–Gea et al, (2012) |
| LSEC | VE-Cadherin-Cre Atg5f/f VE-Cadherin-Cre Atg5f/f |
Increased inflammation & fibrosis decreased VWF secretion |
Ruart et al. (2019) |
| Whole-body KO | ASMase KO Parkin KO Parkin, PINK1 DKO |
decreased diet-induced steatosis increased alcohol-induced liver injury increased AILI |
Fucho et al. (2014)
Wang et al. (2019) |
| Whole-Liver Knockdown | Atg5-RNAi (ATG5i) | increased liver injury & hepatomegaly | Cassidy et al., 2018 |
HSC: Hepatic stellate cell; LSECs: Liver sinusoidal endothelial cell.
However, some important issues remain to be resolved. As autophagy is highly dynamic in the liver, monitoring the dynamic spatiotemporal regulation of autophagy and autophagic flux is challenging. The liver has unique metabolic zonations, and zonular induction of autophagy and mitophagy has also been documented in mouse livers exposed to APAP or glucagon (Ni et al., 2013b; Springer et al., 2021). Previously developed autophagy reporter mice such as GFP-LC3 and Mito-QC may be helpful to monitor the dynamic spatiotemporal changes in autophagy and mitophagy in the liver (Mizushima 2009; McWilliams et al., 2016) and more reporter mice for other forms of selective autophagy such as lipophagy and ER-phagy are needed. In addition, the lessons learned from contrasting observations in mouse studies of the alteration of the same gene might be resolved by combining spatiotemporal regulation and level of gene alteration in vivo. Mice with genetic deletion of Atg (i.e. Atg5 or Atg7) quickly developed liver injury and inflammation as well as many other adaptive metabolic responses. These pre-existing conditions in the liver of autophagy-deficient mice may alter the response and complicate interpretation of the results when they are further challenged with stresses such as fasting, high fat diet or drug treatment. It may be helpful to consider using the acute KO approach such as inducible KO mice or AAV-TBG-cre delivery to dissect the role of autophagy deficiency in liver pathophysiology, as under the acute deletion phase many adaptive responses have not been developed yet. Finally, as there are various cell types that have distinctive functions in the liver, how to selectively target the autophagy process in a specific cell type without affecting the others remains to be an important issue.
Acknowledgements
This work was supported in part by the National Institutes of Health grants: R37 AA020518, R01 DK102142, U01 AA024733, R01 AG072895 and 1R21AG065720 (WXD); DK117965 (TL) and DK108835 (LY).
Abbreviations
- AALD
alcohol associated liver disease
- ACAT
cholesterol acyltransferase
- AH
alcoholic hepatitis
- AICAR
activator 5-Aminoimidazole-4-carboxamide ribonucleotide
- AILI
APAP-induced liver injury
- ALD
alcoholic liver disease
- AMPK
AMP-activated protein kinase
- APAP
acetaminophen
- APAP-AD
acetaminophen adducts
- ASMase
acid sphingomyelinase
- ATG
autophagy-related genes
- ATGL
adipose triglyceride lipase
- ATL3
atlastin-3
- CALCOCO1
calcium binding and coiled-coil domain 1
- CB2
cannabinoid receptor 2
- CCl4
carbon tetrachloride
- CCPG1
cell-cycle progression gene 1
- CE
cholesterol ester
- CHMP2A
charged multivesicular body protein 2A
- CMA
chaperon-mediated autophagy
- CNS
central nervous system
- CRY1
Cryptochrome Circadian Regulator 1
- CPZ
chlorpromazine
- CQ
chloroquine
- CYPs
cytochrome P-450
- CYP7A1
cholesterol 7α-hydroxylase
- DILI
drug-induced liver injury
- DCs
dendritic cells
- DRP1
dynamin-related protein 1
- EFV
efavirenz
- EPR1
effector cell peptidase receptor 1
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- ESCRT
endosomal sorting complexes required for transport
- FC
free cholesterol
- FFAs
free fatty acids
- FGF15
fibroblast growth factor 15
- FGF19
fibroblast growth factor 19
- FIP200
FAK family kinase-interacting protein of 200 kDa
- FoxO3
transcription factor forkhead box O-3
- FXR
farnesoid x receptor
- GDNF
glial cell line-derived neurotrophic factor
- GLP-1
glucagon-like peptide-1
- Gα12
Guanine nucleotide-binding α-subunit 12
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HMGB1
high-mobility group box-1
- HIF-1β
hypoxia-inducing factor-1 beta
- HSCs
hepatic stellate cells
- HSC70
heat shock-cognate protein of 70 kDa
- HSL
hormone sensitive lipase
- IGFBPrP1
insulin-like growth factor binding protein-associated protein 1
- IRGM
immunity related GTPase family M protein
- IRF1
interferon regulatory factor 1
- IRI
ischemia-reperfusion injury
- JNK
c-Jun-N-terminal kinase
- Keap1
Kelch-like ECH-associated protein 1
- KLF2
Krüppel-like factor 2
- KO
knockout
- K8
keratin 8
- LAL
lysosomal acidic lipase
- LAMP-1
lysosome-associated membrane protein 1
- LAMP-2A
lysosome-associated membrane protein type 2A
- LC3
microtubule-associated protein 1A/1B-light chain 3
- LDs
lipid droplets
- LSECs
liver sinusoidal endothelial cells
- LXRα
liver X receptor α
- NDP52
nuclear dot protein 52
- CALCOCO2
Calcium-binding and coiled-coil domain-containing protein 2
- MDB
Mallory-Denk bodies
- mTORC1
mechanistic target of rapamycin complex 1
- NAFLD
nonalcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NBR1
neighbor of BRCA1 gene 1
- NCoR1
nuclear receptor co-repressor 1
- NLRP3
NLR family pyrin domain containing 3
- NPC
Niemann-Pick disease, type C
- NPCs
nonparenchymal cells
- Nrf2
nuclear factor erythroid 2-related factor 2
- NSAIDs
non-steroidal anti-inflammatory drugs
- NSF
N-ethylmaleimide-sensitive fusion protein
- NS5B
nonstructural protein 5B
- OPTN
optineurin
- PE
phosphatidylethanolamine
- PI3K
phosphoinositide 3-kinase
- PINK1
PTEN-induced kinase 1
- PKA
protein kinase A
- PLIN2
perilipin 2
- PLIN3
perilipin 3
- PPARα
peroxisome proliferator-activated receptor α
- PtdIns3P
phosphatidylinositol-3-phosphate
- RAB7
RAS-related GTP-binding protein
- RB1CC1
RB1-inducible coiled-coil 1
- ROS
reactive oxygen species
- RTN3L
the spliced isoform of Reticulon 3
- Rubicon
RUN domain and cysteine-rich domain containing Beclin 1-interacting protein
- RXRα
retinoid X receptor-alpha
- SHP
small heterodimer partner
- siRNA
small interfering RNAs
- SIRT1
sirtuin 1
- SNAP29
synaptosomal-associated protein 29
- SNARE
Soluble NSF attachment protein receptor
- SQSTM1/p62
sequestosome 1
- STX17
syntaxin 17
- TAA
thioacetamide
- TFEB
transcription factor EB
- TEX264
testis expressed 264, an ER-phagy receptor
- TGF-β1
transforming growth factor β1
- THs
thyroid hormones
- TRIM21
tripartite motif-containing protein 21
- Tax1BP1
taxi-binding protein 1
- ULK1/2
unc-51 like autophagy activating kinase 1 or 2
- UPR
unfolded protein response
- UPS
ubiquitin-proteasome system
- UVRAG
ultraviolet radiation resistance-associated gene protein
- VAMP8
vesicle-associated membrane protein 8
- VDR
vitamin D receptor
- VMP1
vacuole membrane protein 1
- VPS4
vacuolar protein sorting 4
- VPS37A
vacuolar protein sorting 37 homolog A
- VWF
von Willebrand factor
- WPBs
Weibel-Palade bodies
- 4 MP
4-methylpyrazole
References
- Abdoli A, Alirezaei M, Mehrbod P, Forouzanfar F, 2018. Autophagy: the multipurpose bridge in viral infections and host cells. Rev. Med. Virol 28 (4), e1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudo-Canalejo J, Schultz SW, Chino H, Migliano SM, Saito C, Koyama-Honda I, Stenmark H, Brech A, May AI, Mizushima N, Knorr RL, 2020. Wetting regulates autophagy of phase-separated compartments and the cytosol. Nature 591 (7848), 142–146. 10.1038/s41586-020-2992-3. [DOI] [PubMed] [Google Scholar]
- Ait-Goughoulte M, Kanda T, Meyer K, Ryerse JS, Ray RB, Ray R, 2008. Hepatitis C virus genotype 1a growth and induction of autophagy. J. Virol 82 (5), 2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakpo JY, Ramachandran A, Duan L, Schaich MA, Jaeschke MW, Freudenthal BD, Ding WX, Rumack BH, Jaeschke H, 2019. Delayed treatment with 4-methylpyrazole protects against acetaminophen hepatotoxicity in mice by inhibition of c-jun n-terminal kinase. Toxicol. Sci 170 (1), 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian S, Ande S, Coombs K, Yeganeh B, Davoodpour P, Hashemi M, Los M, Ghavami S, 2011. Virus-triggered autophagy in viral hepatitis–possible novel strategies for drug development. J. Viral Hepat 18 (12), 821–830. [DOI] [PubMed] [Google Scholar]
- Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M, 2017. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 65 (1), 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E, Cuervo AM, 2011. Chaperone-mediated autophagy in protein quality control. Curr. Opin. Cell Biol 23 (2), 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aweya JJ, Mak TM, Lim SG, Tan YJ, 2013. The p7 protein of the hepatitis C virus induces cell death differently from the influenza A virus viroporin M2. Virus Res. 172 (1–2), 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini D, Dallaglio K, Torquato P, Piroddi M, Galli F, 2018. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res 193, 54–71. [DOI] [PubMed] [Google Scholar]
- Bernales S, McDonald KL, Walter P, 2006. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4 (12), e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, Schuck S, Walter P, 2007. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy 3 (3), 285–287. [DOI] [PubMed] [Google Scholar]
- Birkenfeld AL, Shulman GI, 2014. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 59 (2), 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boteon YL, Laing R, Mergental H, Reynolds GM, Mirza DF, Afford SC, Bhogal RH, 2017. Mechanisms of autophagy activation in endothelial cell and their targeting during normothermic machine liver perfusion. World J. Gastroenterol 23 (48), 8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Galluzzi L, Kepp O, Kroemer G, 2013. Decoding cell death signals in liver inflammation. J. Hepatol 59 (3), 583–594. [DOI] [PubMed] [Google Scholar]
- Burman C, Ktistakis NT, 2010. Regulation of autophagy by phosphatidylinositol 3-phosphate. FEBS Lett. 584 (7), 1302–1312. [DOI] [PubMed] [Google Scholar]
- Byun S, Kim YC, Zhang Y, Kong B, Guo G, Sadoshima J, Ma J, Kemper B, Kemper JK, 2017. A postprandial FGF19-SHP-LSD1 regulatory axis mediates epigenetic repression of hepatic autophagy. EMBO J. 36 (12), 1755–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso S, O’Brien DR, Cleary SP, Roberts LR, Zucman-Rossi J, 2020. Genetics of HCC: novel approaches to explore molecular diversity. Hepatology 73 (Suppl 1), 14–26. 10.1002/hep.31394. [DOI] [PubMed] [Google Scholar]
- Cassidy LD, Young AR, Pérez-Mancera PA, Nimmervoll B, Jaulim A, Chen H-C, McIntyre DJ, Brais R, Ricketts T, Pacey S, 2018. A novel Atg5-shRNA mouse model enables temporal control of autophagy in vivo. Autophagy 14 (7), 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ, 2012. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology 55 (6), 2005–2023. [DOI] [PubMed] [Google Scholar]
- Chao X, Ding WX, 2019. Role and mechanisms of autophagy in alcohol-induced liver injury. Adv. Pharmacol 85, 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X, Ni HM, Ding WX, 2018a. Insufficient autophagy: a novel autophagic flux scenario uncovered by impaired liver TFEB-mediated lysosomal biogenesis from chronic alcohol-drinking mice. Autophagy 14 (9), 1646–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X, Qian H, Wang S, Fulte S, Ding W-X, 2020. Autophagy and liver cancer. Clin. Mol. Hepatol 26 (4), 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X, Wang H, Jaeschke H, Ding WX, 2018b. Role and mechanisms of autophagy in acetaminophen-induced liver injury. Liver Int. 38 (8), 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X, Wang S, Zhao K, Li Y, Williams JA, Li T, Chavan H, Krishnamurthy P, He XC, Li L, Ballabio A, Ni HM, Ding WX, 2018c. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology 155 (3), 865–879 e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xiao Y, Chai P, Zheng P, Teng J, Chen J, 2019. ATL3 is a tubular ER-phagy receptor for GABARAP-mediated selective autophagy. Curr. Biol 29 (5), 846–855 e846. [DOI] [PubMed] [Google Scholar]
- Chen S, Li M, Jiang W, Zheng H, Qi LW, Jiang S, 2020. The role of Neu1 in the protective effect of dipsacoside B on acetaminophen-induced liver injury. Ann. Transl. Med 8 (13), 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Ohsaki Y, Tauchi-Sato K, Fujita A, Fujimoto T, 2006. Cholesterol depletion induces autophagy. Biochem. Biophys. Res. Commun 351 (1), 246–252. [DOI] [PubMed] [Google Scholar]
- Chino H, Hatta T, Natsume T, Mizushima N, 2019. Intrinsically disordered protein TEX264 mediates ER-phagy. Mol. Cell 74 (5), 909–921 e906. [DOI] [PubMed] [Google Scholar]
- Chino H, Mizushima N, 2020. ER-phagy: quality control and turnover of endoplasmic reticulum. Trends Cell Biol. 30 (5), 384–398. [DOI] [PubMed] [Google Scholar]
- Choi AM, Ryter SW, Levine B, 2013. Autophagy in human health and disease. N. Engl. J. Med 368 (19), 1845–1846. [DOI] [PubMed] [Google Scholar]
- Daniel Lavanchy MK, 2016. Global epidemiology of hepatitis B virus infection. Molecular and Translational Medicine 187–203. [Google Scholar]
- Danieli A, Martens S, 2018. p62-mediated phase separation at the intersection of the ubiquitin-proteasome system and autophagy. J. Cell Sci 131 (19), jcs214304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonibus C, Cinque L, Settembre C, 2019. Emerging lysosomal pathways for quality control at the endoplasmic reticulum. FEBS Lett. 593 (17), 2319–2329. [DOI] [PubMed] [Google Scholar]
- DeBosch BJ, Heitmeier MR, Mayer AL, Higgins CB, Crowley JR, Kraft TE, Chi M, Newberry EP, Chen Z, Finck BN, Davidson NO, Yarasheski KE, Hruz PW, Moley KH, 2016. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci. Signal 9 (416), ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeve LD, 2015. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 61 (5), 1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaës T, Lodder J, Chobert M-N, Ruiz I, Pawlotsky J-M, Lotersztajn S, Teixeira-Clerc F, 2016. The cannabinoid receptor 2 protects against alcoholic liver disease via a macrophage autophagy-dependent pathway. Sci. Rep 6 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Huang Q, Wang Y, Shen P, Guan F, Li J, Huang H, Shi C, 2014. Hypoxia-inducible factor-1alpha regulates autophagy to activate hepatic stellate cells. Biochem. Biophys. Res. Commun 454 (2), 328–334. [DOI] [PubMed] [Google Scholar]
- Deter RL, Baudhuin P, De Duve C, 1967. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J. Cell Biol 35 (2), C11–C16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, 2017. Proteasomal and autophagic degradation systems. In: Annual Review of Biochemistry, vol. 86, pp. 193–224. [DOI] [PubMed] [Google Scholar]
- Ding W-X, Jaeschke H, 2016. Autophagy in macrophages regulates the inflammasome and protects against liver injury. J. Hepatol 64 (1), 16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, 2010. Role of autophagy in liver physiology and pathophysiology. World J. Biol. Chem 1 (1), 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM, 2010. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139 (5), 1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Li M, Yin XM, 2011a. Selective taste of ethanol-induced autophagy for mitochondria and lipid droplets. Autophagy 7 (2), 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Manley S, Ni HM, 2011b. The emerging role of autophagy in alcoholic liver disease. Exp. Biol. Med 236 (5), 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM, 2007a. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem 282 (7), 4702–4710. [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM, 2007b. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am. J. Pathol 171 (2), 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Yin XM, 2008. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy 4 (2), 141–150. [DOI] [PubMed] [Google Scholar]
- Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE, 2013. Kupffer cells in the liver. Comprehensive Physiology 3 (2), 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue TM Jr., Thomes PG, 2014. Ethanol-induced oxidant stress modulates hepatic autophagy and proteasome activity. Redox Biol 3, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S, Roberts LR, Kisseleva T, Karin M, Diaz-Meco MT, 2016. p62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Canc. Cell 30 (4), 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan D, Kim J, Shaw RJ, Guan KL, 2011. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7 (6), 643–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ, 2011. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331 (6016), 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid N, Ito Y, Horibe A, Otsuki Y, 2016. Ethanol-induced mitophagy in liver is associated with activation of the PINK1-Parkin pathway triggered by oxidative DNA damage. Histol. Histopathol 31 (10), 1143–1159. [DOI] [PubMed] [Google Scholar]
- Elrick MJ, Yu T, Chung C, Lieberman AP, 2012. Impaired proteolysis underlies autophagic dysfunction in Niemann-Pick type C disease. Hum. Mol. Genet 21 (22), 4876–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen EL, 2006. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Aspect. Med 27 (5–6), 495–502. [DOI] [PubMed] [Google Scholar]
- Fader CM, Sanchez DG, Mestre MB, Colombo MI, 2009. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta 1793 (12), 1901–1916. [DOI] [PubMed] [Google Scholar]
- Fallowfield JA, 2011. Therapeutic targets in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol 300 (5), G709–G715. [DOI] [PubMed] [Google Scholar]
- Fan Y, Wang N, Rocchi A, Zhang W, Vassar R, Zhou Y, He C, 2017. Identification of natural products with neuronal and metabolic benefits through autophagy induction. Autophagy 13 (1), 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA, 2006. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat. Rev. Canc 6 (9), 674–687. [DOI] [PubMed] [Google Scholar]
- Feldman D, Swarm RL, Becker J, 1980. Elimination of excess smooth endoplasmic reticulum after phenobarbital administration. J. Histochem. Cytochem 28 (9), 997–1006. [DOI] [PubMed] [Google Scholar]
- Feng Y, Ariosa AR, Yang Y, Hu Z, Dengjel J, Klionsky DJ, 2021. Downregulation of autophagy by met30-mediated Atg9 ubiquitination. Proc. Natl. Acad. Sci. U. S. A 118 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ, 2014. The machinery of macroautophagy. Cell Res. 24 (1), 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL, 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. J. Am. Med. Assoc 307 (5), 491–497. [DOI] [PubMed] [Google Scholar]
- French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, Mendenhall CL, 1993. Pathology of Alcoholic Liver Disease. Seminars in Liver Disease, © 1993. Thieme Medical Publishers, Inc. [DOI] [PubMed] [Google Scholar]
- Friedman SL, 2008. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev 88 (1), 125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucho R, Martinez L, Baulies A, Torres S, Tarrats N, Fernandez A, Ribas V, Astudillo AM, Balsinde J, Garcia-Roves P, Elena M, Bergheim I, Lotersztajn S, Trautwein C, Appelqvist H, Paton AW, Paton JC, Czaja MJ, Kaplowitz N, Fernandez-Checa JC, Garcia-Ruiz C, 2014. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J. Hepatol 61 (5), 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Alam JM, Noshiro D, Mouri K, Ando T, Okada Y, May AI, Knorr RL, Suzuki K, Ohsumi Y, Noda NN, 2020. Phase separation organizes the site of autophagosome formation. Nature 578 (7794), 301–305. [DOI] [PubMed] [Google Scholar]
- Fukuo Y, Yamashina S, Sonoue H, Arakawa A, Nakadera E, Aoyama T, Uchiyama A, Kon K, Ikejima K, Watanabe S, 2014. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol. Res 44 (9), 1026–1036. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Noack J, Bergmann TJ, Cebollero E, Pisoni GB, Fasana E, Fregno I, Galli C, Loi M, Soldà T, 2016. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat. Cell Biol 18 (11), 1173–1184. [DOI] [PubMed] [Google Scholar]
- Furuta N, Fujita N, Noda T, Yoshimori T, Amano A, 2010. Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate fusion of antimicrobial and canonical autophagosomes with lysosomes. Mol. Biol. Cell 21 (6), 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galman C, Angelin B, Rudling M, 2005. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology 129 (5), 1445–1453. [DOI] [PubMed] [Google Scholar]
- Gao B, Bataller R, 2011. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141 (5), 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim MS, Kim KH, Shneider BL, Picarsic JL, Jacobson TA, Zhang J, He W, Liu P, Knisely AS, Finegold MJ, Muzny DM, Boerwinkle E, Lupski JR, Plon SE, Gibbs RA, Eng CM, Yang Y, Washington GC, Porteus MH, Berquist WE, Kambham N, Singh RJ, Xia F, Enns GM, Moore DD, 2016. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat. Commun 7, 10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillon J, Lo Iacono O, Corazzari M, Fimia GM, Piacentini M, Muntane J, Bosca L, Garcia-Monzon C, Martin-Sanz P, Valverde AM, 2014. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 5, e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA, 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6 (3), 517–526. [DOI] [PubMed] [Google Scholar]
- Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H, 2014. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol 61 (1 Suppl. l), S45–S57. [DOI] [PubMed] [Google Scholar]
- Gregoire IP, Richetta C, Meyniel-Schicklin L, Borel S, Pradezynski F, Diaz O, Deloire A, Azocar O, Baguet J, Le Breton M, Mangeot PE, Navratil V, Joubert PE, Flacher M, Vidalain PO, Andre P, Lotteau V, Biard-Piechaczyk M, Rabourdin-Combe C, Faure M, 2011. IRGM is a common target of RNA viruses that subvert the autophagy network. PLoS Pathog. 7 (12), e1002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld D, Cline-Fedewa H, Baker KS, Williams KJ, Roth RA, Mittermeier K, Lisman T, Palumbo JS, Luyendyk JP, 2020. Von Willebrand factor delays liver repair after acetaminophen-induced acute liver injury in mice. J. Hepatol 72 (1), 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumati P, Morozzi G, Hölper S, Mari M, Harwardt M-LI, Yan R, Müller S, Reggiori F, Heilemann M, Dikic I, 2017. Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. elife 6, e25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevin C, Manna D, Belanger C, Konan KV, Mak P, Labonte P, 2010. Autophagy protein ATG5 interacts transiently with the hepatitis C virus RNA polymerase (NS5B) early during infection. Virology 405 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guixé-Muntet S, de Mesquita FC, Vila S, Hernández-Gea V, Peralta C, García-Pagán JC, Bosch J, Gracia-Sancho J, 2017. Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury. J. Hepatol 66 (1), 86–94. [DOI] [PubMed] [Google Scholar]
- Haas JT, Francque S, Staels B, 2016. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu. Rev. Physiol 78, 181–205. [DOI] [PubMed] [Google Scholar]
- Hammoutene A, Biquard L, Lasselin J, Kheloufi M, Tanguy M, Vion A-C, Mérian J, Colnot N, Loyer X, Tedgui A, 2020. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J. Hepatol 72 (3), 528–538. [DOI] [PubMed] [Google Scholar]
- Hammoutene A, Rautou P-E, 2019. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol 70 (6), 1278–1291. [DOI] [PubMed] [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B, 2012. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481 (7382), 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Antonucci L, Yamachika S, Zhang Z, Taniguchi K, Umemura A, Hatzivassiliou G, Roose-Girma M, Reina-Campos M, Duran A, 2020. NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly. J. Hepatol 72 (6), 1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Sha S, Sun L, Zhang J, Dong M, 2016. GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem. Biophys. Res. Commun 476 (4), 196–203. [DOI] [PubMed] [Google Scholar]
- He Y, Zhu J, Huang Y, Gao H, Zhao Y, 2015. Advanced glycation end product (AGE)-induced hepatic stellate cell activation via autophagy contributes to hepatitis C-related fibrosis. Acta Diabetol. 52 (5), 959–969. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA, 2012. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482 (7384), 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Gea V, Hilscher M, Rozenfeld R, Lim MP, Nieto N, Werner S, Devi LA, Friedman SL, 2013. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J. Hepatol 59 (1), 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández–Gea V, Ghiassi–Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, Friedman SL, 2012. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142 (4), 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, 2012. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol 13 (2), 89–102. [DOI] [PubMed] [Google Scholar]
- Hong Y, Li S, Wang J, Li Y, 2018. In vitro inhibition of hepatic stellate cell activation by the autophagy-related lipid droplet protein ATG2A. Sci. Rep 8 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DQ, El-Serag HB, Loomba R, 2020. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol 18 (4), 223–238. 10.1038/s41575-020-00381-6, 1759–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kang R, Wang J, Luo G, Yang W, Zhao Z, 2013. Hepatitis C virus inhibits AKT-tuberous sclerosis complex (TSC), the mechanistic target of rapamycin (MTOR) pathway, through endoplasmic reticulum stress to induce autophagy. Autophagy 9 (2), 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T-J, Ren J-J, Zhang Q-Q, Kong Y-Y, Zhang H-Y, Guo X-H, Fan H-Q, Liu L-X, 2019. IGFBPrP1 accelerates autophagy and activation of hepatic stellate cells via mutual regulation between H19 and PI3K/AKT/mTOR pathway. Biomed. Pharmacother 116, 109034. [DOI] [PubMed] [Google Scholar]
- Huang XY, Li D, Chen ZX, Huang YH, Gao WY, Zheng BY, Wang XZ, 2018. Hepatitis B virus X protein elevates parkin-mediated mitophagy through lon peptidase in starvation. Exp. Cell Res 368 (1), 75–83. [DOI] [PubMed] [Google Scholar]
- Hubert V, Peschel A, Langer B, Groger M, Rees A, Kain R, 2016. LAMP-2 is required for incorporating syntaxin-17 into autophagosomes and for their fusion with lysosomes. Biology Open 5 (10), 1516–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S, 2007. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 26 (2), 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M, 2013. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell 51 (5), 618–631. [DOI] [PubMed] [Google Scholar]
- Ilyas G, Zhao E, Liu K, Lin Y, Tesfa L, Tanaka KE, Czaja MJ, 2016. Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1β. J. Hepatol 64 (1), 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA, 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabol. 2 (4), 217–225. [DOI] [PubMed] [Google Scholar]
- Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, Lee MS, Tanaka K, Komatsu M, 2011. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J. Cell Biol 193 (2), 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Kishi-Itakura C, Mizushima N, 2012. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151 (6), 1256–1269. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A, 2012. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev 44 (1), 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL, 2004. Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci 117 (Pt 20), 4837–4848. [DOI] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T, 2010. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem 285 (29), 22576–22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo MC, Zhang DD, 2013. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 27 (20), 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KJ, Alpini G, Glaser S, 2013. Hepatic nervous system and neurobiology of the liver. Comp. Physiol 3 (2), 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama S, Gudmundsson SR, Sou YS, Ichimura Y, Tamura N, Kazuno S, Ueno T, Miura Y, Noshiro D, Abe M, Mizushima T, Miura N, Okuda S, Motohashi H, Lee JA, Sakimura K, Ohe T, Noda NN, Waguri S, Eskelinen EL, Komatsu M, 2021. p62/SQSTM1-droplet serves as a platform for autophagosome formation and anti-oxidative stress response. Nat. Commun 12 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM, 2015. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol 17 (6), 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke PY, Chen SS, 2011. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest 121 (1), 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambu B, Huda N, Chen X, Antoine DJ, Li Y, Dai G, Kohler UA, Zong WX, Waguri S, Werner S, Oury TD, Dong Z, Yin XM, 2018. HMGB1 promotes ductular reaction and tumorigenesis in autophagy-deficient livers. J. Clin. Invest 128 (6), 2419–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambu B, Li T, Yan S, Yu C, Chen X, Goheen M, Li Y, Lin J, Cummings OW, Lee YA, Friedman S, Dong Z, Feng GS, Wu S, Yin XM, 2019. Hepatic autophagy deficiency compromises farnesoid X receptor functionality and causes cholestatic injury. Hepatology 69 (5), 2196–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, 2015. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522 (7556), 354–358. [DOI] [PubMed] [Google Scholar]
- Khan M, Imam H, Siddiqui A, 2018. Subversion of cellular autophagy during virus infection: insights from hepatitis B and hepatitis C viruses. Liver Res 2 (3), 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda KK, McVicker DL, Zetterman RK, Donohue TM Jr., 1996. Ethanol consumption alters trafficking of lysosomal enzymes and affects the processing of procathepsin L in rat liver. Biochim. Biophys. Acta 1291 (1), 45–52. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, McVicker DL, Zetterman RK, MacDonald RG, Donohue TM Jr., 1997. Flow cytometric analysis of vesicular pH in rat hepatocytes after ethanol administration. Hepatology 26 (4), 929–934. [DOI] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y, 2001. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol 152 (3), 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Guan KL, 2011. Regulation of the autophagy initiating kinase ULK1 by nutrients: roles of mTORC1 and AMPK. Cell Cycle 10 (9), 1337–1338. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL, 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol 13 (2), 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim DH, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, Lee MS, 2013. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med 19 (1), 83–92. [DOI] [PubMed] [Google Scholar]
- Kim KM, Han CY, Kim JY, Cho SS, Kim YS, Koo JH, Lee JM, Lim SC, Kang KW, Kim J-S, 2018. Gα12 overexpression induced by miR-16 dysregulation contributes to liver fibrosis by promoting autophagy in hepatic stellate cells. J. Hepatol 68 (3), 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Han SY, Yu KS, Han D, Ahn HJ, Jo JE, Kim JH, Shin J, Park HW, 2018. Impaired autophagy promotes bile acid-induced hepatic injury and accumulation of ubiquitinated proteins. Biochem. Biophys. Res. Commun 495 (1), 1541–1547. [DOI] [PubMed] [Google Scholar]
- Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ, 2011. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331 (6024), 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD, 2000. Autophagy as a regulated pathway of cellular degradation. Science 290 (5497), 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Ichimura Y, 2010. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 584 (7), 1374–1378. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou Y-S, Ueno I, Sakamoto A, Tong KI, 2010. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol 12 (3), 213–223. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T, 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol 169 (3), 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL, 2012. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 56 (3), 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Brenner DA, 2017. Liver inflammation and fibrosis. J. Clin. Invest 127 (1), 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri P, Schmidt V, Smole C, Kufferath I, Denk H, Strnad P, Rulicke T, Frohlich LF, Zatloukal K, 2016. p62/Sequestosome-1 is indispensable for maturation and stabilization of mallory-denk bodies. PloS One 11 (8), e0161083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamark T, Johansen T, 2012. Aggrephagy: selective disposal of protein aggregates by macroautophagy. International journal of cell biology 2012, 736905. 10.1155/2012/736905, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan SH, Wu SY, Zuchini R, Lin XZ, Su IJ, Tsai TF, Lin YJ, Wu CT, Liu HS, 2014. Autophagy suppresses tumorigenesis of hepatitis B virus-associated hepatocellular carcinoma through degradation of microRNA-224. Hepatology 59 (2), 505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM, Acute Liver Failure Study, G., 2005. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42 (6), 1364–1372. [DOI] [PubMed] [Google Scholar]
- Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD, 2014. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 516 (7529), 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Weihl CC, 2017. Regulation of SQSTM1/p62 via UBA domain ubiquitination and its role in disease. Autophagy 13 (9), 1615–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YA, Noon LA, Akat KM, Ybanez MD, Lee TF, Berres ML, Fujiwara N, Goossens N, Chou HI, Parvin-Nejad FP, Khambu B, Kramer EGM, Gordon R, Pfleger C, Germain D, John GR, Campbell KN, Yue Z, Yin XM, Cuervo AM, Czaja MJ, Fiel MI, Hoshida Y, Friedman SL, 2018. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nat. Commun 9 (1), 4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zeng C, Zheng B, Liu C, Tang M, Jiang Y, Chang Y, Song W, Wang Y, Yang C, 2018. HMGB1-induced autophagy facilitates hepatic stellate cells activation: a new pathway in liver fibrosis. Clin. Sci. (Lond.) 132 (15), 1645–1667. [DOI] [PubMed] [Google Scholar]
- Li T, Chiang JY, 2014. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev 66 (4), 948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chao X, Yang L, Lu Q, Li T, Ding WX, Ni HM, 2018. Impaired fasting-induced adaptive lipid droplet biogenesis in liver-specific Atg5-deficient mouse liver is mediated by persistent nuclear factor-like 2 activation. Am. J. Pathol 188 (8), 1833–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ding WX, 2017. Impaired Rab7 and Dynamin2 block fat turnover by autophagy in alcoholic fatty livers. Hepatol Commun 1 (6), 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ni HM, Jaeschke H, Ding WX, 2019. Chlorpromazine protects against acetaminophen-induced liver injury in mice by modulating autophagy and c-Jun N-terminal kinase activation. Liver Res 3 (1), 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Zhong Z, Kim SY, Uchiyama R, Roh YS, Matsushita H, Gottlieb RA, Seki E, 2019. Murine macrophage autophagy protects against alcohol-induced liver injury by degrading interferon regulatory factor 1 (IRF1) and removing damaged mitochondria. J. Biol. Chem 294 (33), 12359–12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Yao Y, Liu J, Yu Z, Cheung S, Xie A, Liang X, Bi X, 2007. Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1 −/− mouse brain. Am. J. Pathol 171 (3), 962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XC, Yin XM, 2013. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J. Hepatol 58 (5), 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z, 2013. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. Faseb. J 27 (10), 4184–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fang M, Hu Y, Huang B, Li N, Chang C, Huang R, Xu X, Yang Z, Chen Z, Liu W, 2014. Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation. Autophagy 10 (3), 416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Czaja MJ, 2013. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 20 (1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, Tanaka KE, Czaja MJ, 2015. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 11 (2), 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Burgos JS, Deng Y, Srivastava R, Howell SH, Bassham DC, 2012. Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 24 (11), 4635–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder J, Denaës T, Chobert M-N, Wan J, El-Benna J, Pawlotsky J-M, Lotersztajn S, Teixeira-Clerc F, 2015. Macrophage autophagy protects against liver fibrosis in mice. Autophagy 11 (8), 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler AS, Alers S, Dieterle AM, Keppeler H, Franz-Wachtel M, Kundu M, Campbell DG, Wesselborg S, Alessi DR, Stork B, 2011. Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 7 (7), 696–706. [DOI] [PubMed] [Google Scholar]
- Loomba R, Sanyal AJ, 2013. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol 10 (11), 686–690. [DOI] [PubMed] [Google Scholar]
- Lundasen T, Galman C, Angelin B, Rudling M, 2006. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med 260 (6), 530–536. [DOI] [PubMed] [Google Scholar]
- Lystad AH, Simonsen A, 2015. Assays to monitor aggrephagy. Methods 75, 112–119. [DOI] [PubMed] [Google Scholar]
- Ma D, Molusky MM, Song J, Hu CR, Fang F, Rui C, Mathew AV, Pennathur S, Liu F, Cheng JX, Guan JL, Lin JD, 2013. Autophagy deficiency by hepatic FIP200 deletion uncouples steatosis from liver injury in NAFLD. Mol. Endocrinol 27 (10), 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Panda S, Lin JD, 2011. Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J. 30 (22), 4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, McKeen T, Zhang J, Ding WX, 2020. Role and mechanisms of mitophagy in liver diseases. Cells 9 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Otomo C, Otomo T, 2019. The autophagic membrane tether ATG2A transfers lipids between membranes. Elife 8, e45777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, Leone V, Peiseler M, Surewaard BGJ, Rath D, Ali A, Wolf MJ, Drescher H, Healy ME, Dauch D, Kroy D, Krenkel O, Kohlhepp M, Engleitner T, Olkus A, Sijmonsma T, Volz J, Deppermann C, Stegner D, Helbling P, Nombela-Arrieta C, Rafiei A, Hinterleitner M, Rall M, Baku F, Borst O, Wilson CL, Leslie J, O’Connor T, Weston CJ, Adams DH, Sheriff L, Teijeiro A, Prinz M, Bogeska R, Anstee N, Bongers MN, Notohamiprodjo M, Geisler T, Withers DJ, Ware J, Mann DA, Augustin HG, Vegiopoulos A, Milsom MD, Rose AJ, Lalor PF, Llovet JM, Pinyol R, Tacke F, Rad R, Matter M, Djouder N, Kubes P, Knolle PA, Unger K, Zender L, Nieswandt B, Gawaz M, Weber A, Heikenwalder M, 2019. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med 25 (4), 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat A, Lodder J, Teixeira-Clerc F, Moreau R, Codogno P, Lotersztajn S, 2014. Autophagy: a multifaceted partner in liver fibrosis. BioMed Res. Int 2014 10.1155/2014/869390, 2014, 869390–869390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S, Ni HM, Kong B, Apte U, Guo G, Ding WX, 2014. Suppression of autophagic flux by bile acids in hepatocytes. Toxicol. Sci 137 (2), 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S, Williams JA, Ding W-X, 2013. Role of p62/SQSTM1 in liver physiology and pathogenesis. Exp. Biol. Med 238 (5), 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C, 2006. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metabol. 4 (3), 185–198. [DOI] [PubMed] [Google Scholar]
- Marino G, Pietrocola F, Madeo F, Kroemer G, 2014. Caloric restriction mimetics: natural/physiological pharmacological autophagy inducers. Autophagy 10 (11), 1879–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki R, Yamamoto A, Tashiro Y, 1987. Cytochrome P-450 and NADPH-cytochrome P-450 reductase are degraded in the autolysosomes in rat liver. J. Cell Biol 104 (5), 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashek DG, Khan SA, Sathyanarayan A, Ploeger JM, Franklin MP, 2015. Hepatic lipid droplet biology: getting to the root of fatty liver. Hepatology 62 (3), 964–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M, Chevet E, Tavernier J, Gerlo S, 2014. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci 39 (5), 245–254. [DOI] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H, 2012. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol 264 (3), 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams TG, Prescott AR, Allen GF, Tamjar J, Munson MJ, Thomson C, Muqit MM, Ganley IG, 2016. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol 214 (3), 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev R, Ploen D, Spengler C, Elgner F, Ren H, Bunten S, Hildt E, 2017. HCV-induced oxidative stress by inhibition of Nrf2 triggers autophagy and favors release of viral particles. Free Radic. Biol. Med 110, 300–315. [DOI] [PubMed] [Google Scholar]
- Meske V, Priesnitz T, Albert F, Ohm TG, 2015. How to reduce the accumulation of autophagic vacuoles in NPC1-deficient neurons: a comparison of two pharmacological strategies. Neuropharmacology 89, 282–289. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Nakanuma S, Ahmed A, Makino I, Hayashi H, Oyama K, Nakagawara H, Tajima H, Takamura H, Ninomiya I, 2016. Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eur. Surg 48 (2), 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, 2009. Methods for monitoring autophagy using GFP-LC3 transgenic mice. Methods Enzymol. 452, 13–23. [DOI] [PubMed] [Google Scholar]
- Mizushima N, 2010. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol 22 (2), 132–139. [DOI] [PubMed] [Google Scholar]
- Mizushima N, 2018. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol 20 (5), 521–527. [DOI] [PubMed] [Google Scholar]
- Mizushima N, 2020. The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol 63, 1–10. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M, 2011. Autophagy: renovation of cells and tissues. Cell 147 (4), 728–741. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ, 2008. Autophagy fights disease through cellular self-digestion. Nature 451 (7182), 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y, 2011. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol 27, 107–132. [DOI] [PubMed] [Google Scholar]
- Mo R, Lai R, Lu J, Zhuang Y, Zhou T, Jiang S, Ren P, Li Z, Cao Z, Liu Y, Chen L, Xiong L, Wang P, Wang H, Cai W, Xiang X, Bao S, Xie Q, 2018. Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics 8 (15), 4170–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti F, Bergman P, Dodgson S, Marcellin D, Claerr I, Goodwin JM, DeJesus R, Kang Z, Antczak C, Begue D, Bonenfant D, Graff A, Genoud C, Reece-Hoyes JS, Russ C, Yang Z, Hoffman GR, Mueller M, Murphy LO, Xavier RJ, Nyfeler B, 2018. TMEM41B is a novel regulator of autophagy and lipid mobilization. EMBO Rep. 19 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Hama Y, Izume T, Tamura N, Ueno T, Yamashita Y, Sakamaki Y, Mimura K, Morishita H, Shihoya W, Nureki O, Mano H, Mizushima N, 2018. Genome-wide CRISPR screen identifies TMEM41B as a gene required for autophagosome formation. JCB (J. Cell Biol.) 217 (11), 3817–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimore GE, Poso AR, 1984. Lysosomal pathways in hepatic protein degradation: regulatory role of amino acids. Fed. Proc 43 (5), 1289–1294. [PubMed] [Google Scholar]
- Mortimore GE, Poso AR, 1987. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu. Rev. Nutr 7, 539–564. [DOI] [PubMed] [Google Scholar]
- Mwangi SM, Li G, Ye L, Liu Y, Reichardt F, Yeligar SM, Hart CM, Czaja MJ, Srinivasan S, 2019. Glial cell line-derived neurotrophic factor enhances autophagic flux in mouse and rat hepatocytes and protects against palmitate lipotoxicity. Hepatology 69 (6), 2455–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LE, Ding WX, Cresci G, Saikia P, Shah VH, 2016. Linking pathogenic mechanisms of alcoholic liver disease with clinical phenotypes. Gastroenterology 150 (8). 10.1053/j.gastro.2016.02.035, 1756–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y, 2009. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol 10 (7), 458–467. [DOI] [PubMed] [Google Scholar]
- Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, Nomura DK, Olzmann JA, 2017. DGAT1-Dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev. Cell 42 (1), 9–21 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H-M, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding W-X, 2012. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol. Sci 127 (2), 438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Bhakta A, Wang S, Li Z, Manley S, Huang H, Copple B, Ding WX, 2014a. Role of hypoxia inducing factor-1beta in alcohol-induced autophagy, steatosis and liver injury in mice. PloS One 9 (12), e115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX, 2012. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 55 (1), 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Chao X, Yang H, Deng F, Wang S, Bai Q, Qian H, Cui Y, Cui W, Shi Y, Zong WX, Wang Z, Yang L, Ding WX, 2019. Dual roles of mammalian target of rapamycin in regulating liver injury and tumorigenesis in autophagy-defective mouse liver. Hepatology 70 (6), 2142–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Du K, You M, Ding WX, 2013a. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am. J. Pathol 183 (6), 1815–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX, 2016. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol 65 (2), 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Williams JA, Jaeschke H, Ding WX, 2013b. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol 1, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP, Jaeschke H, Ding WX, 2014b. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J. Hepatol 61 (3), 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Li J-M, Liu M-K, Zhang T-T, Wang D-P, Zhou W-H, Hu L-Z, Lv W-L, 2017. Pathological process of liver sinusoidal endothelial cells in liver diseases. World J. Gastroenterol 23 (43), 7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda NN, Wang Z, Zhang H, 2020. Liquid-liquid phase separation in autophagy. J. Cell Biol 219 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nthiga TM, Kumar Shrestha B, Sjottem E, Bruun JA, Bowitz Larsen K, Bhujabal Z, Lamark T, Johansen T, 2020. CALCOCO1 acts with VAMP-associated proteins to mediate ER-phagy. EMBO J. 39 (15), e103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Sekito T, Niimi K, Ohsumi Y, 2008. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J. Biol. Chem 283 (35), 23972–23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y, 2001. Molecular dissection of autophagy: two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol 2 (3), 211–216. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Carvalho P, 2019. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol 20 (3), 137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii M, Tsuji T, Ogasawara Y, Fujimoto T, 2021. Transmembrane phospholipid translocation mediated by Atg9 is involved in autophagosome formation. J. Cell Biol 220 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL, 2011. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metabol. 13 (6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Marcel YL, 2012. Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arterioscler. Thromb. Vasc. Biol 32 (3), 575–581. [DOI] [PubMed] [Google Scholar]
- Pan JA, Sun Y, Jiang YP, Bott AJ, Jaber N, Dou Z, Yang B, Chen JS, Catanzaro JM, Du C, Ding WX, Diaz-Meco MT, Moscat J, Ozato K, Lin RZ, Zong WX, 2016. TRIM21 ubiquitylates SQSTM1/p62 and suppresses protein sequestration to regulate redox homeostasis. Mol Cell 61 (5), 720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzitt K, Fickert P, Wagner M, 2020a. Regulation of autophagy by bile acids and in cholestasis - CholestoPHAGY or CholeSTOPagy. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis 1867 (2), 166017. [DOI] [PubMed] [Google Scholar]
- Panzitt K, Jungwirth E, Krones E, Lee JM, Pollheimer M, Thallinger GG, Kolb-Lenz D, Xiao R, Thorell A, Trauner M, Fickert P, Marschall HU, Moore DD, Wagner M, 2020b. FXR-dependent Rubicon induction impairs autophagy in models of human cholestasis. J. Hepatol 72 (6), 1122–1131. [DOI] [PubMed] [Google Scholar]
- Park HW, Park H, Semple IA, Jang I, Ro SH, Kim M, Cazares VA, Stuenkel EL, Kim JJ, Kim JS, Lee JH, 2014. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat. Commun 5, 4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parzych KR, Klionsky DJ, 2014. An overview of autophagy: morphology, mechanism, and regulation. Antioxidants Redox Signal. 20 (3), 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C, 2016. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol 22 (34), 7824–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer U, Strauss P, 1981. Autophagic vacuoles in heart muscle and liver. A comparative morphometric study including circadian variations in meal-fed rats. J. Mol. Cell. Cardiol 13 (1), 37–49. [DOI] [PubMed] [Google Scholar]
- Polaris Observatory C, 2018. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 3 (6), 383–403. [DOI] [PubMed] [Google Scholar]
- Polson HE, de Lartigue J, Rigden DJ, Reedijk M, Urbe S, Clague MJ, Tooze SA, 2010. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 6 (4), 506–522. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA, 2011. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metabol. 13 (6), 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ, 2007. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46 (4), 1081–1090. [DOI] [PubMed] [Google Scholar]
- Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B, 2003. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest 112 (12), 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2019. Acetaminophen hepatotoxicity. Semin. Liver Dis 39 (2), 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasineni K, Donohue TM Jr., Thomes PG, Yang L, Tuma DJ, McNiven MA, Casey CA, 2017. Ethanol-induced steatosis involves impairment of lipophagy, associated with reduced Dynamin2 activity. Hepatol Commun 1 (6), 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R, 2010. Autophagy in liver diseases. J. Hepatol 53 (6), 1123–1134. [DOI] [PubMed] [Google Scholar]
- Razavi H, 2020. Global epidemiology of viral hepatitis. Gastroenterol. Clin. N. Am 49 (2), 179–189. [DOI] [PubMed] [Google Scholar]
- Reuben A, Koch DG, Lee WM, G. Acute Liver Failure Study, 2010. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 52 (6), 2065–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway ND, 2000. Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochim. Biophys. Acta 1484 (2–3), 129–141. [DOI] [PubMed] [Google Scholar]
- Ruart M, Chavarria L, Campreciós G, Suárez-Herrera N, Montironi C, Guixé-Muntet S, Bosch J, Friedman SL, Garcia-Pagán JC, Hernández-Gea V, 2019. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J. Hepatol 70 (3), 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ, 2007. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov 6 (4), 304–312. [DOI] [PubMed] [Google Scholar]
- Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL, 2013. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol 15 (7), 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhikov M, Ehlers A, Steinberg D, Xie W, Oberlander E, Brown S, Gilmore PE, Townsend RR, Lane WS, Dolinay T, Nakahira K, Choi AMK, Haspel JA, 2019. Diurnal rhythms spatially and temporally organize autophagy. Cell Rep. 26 (7), 1880–1892 e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Ichimura Y, Taguchi K, Suzuki T, Mizushima T, Takagi K, Hirose Y, Nagahashi M, Iso T, Fukutomi T, 2016. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming. Nat. Commun 7 (1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, Okuda S, Lee HC, Ikeda K, Kanegae Y, Saito I, Auwerx J, Motohashi H, Suematsu M, Soga T, Yokomizo T, Waguri S, Mizushima N, Komatsu M, 2019. Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat. Commun 10 (1), 1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Carroll B, Buganim Y, Maetzel D, Ng AH, Cassady JP, Cohen MA, Chakraborty S, Wang H, Spooner E, Ploegh H, Gsponer J, Korolchuk VI, Jaenisch R, 2013. Impaired autophagy in the lipid-storage disorder Niemann-Pick type C1 disease. Cell Rep. 5 (5), 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf SA, Shah HV, Kanfer G, Ward ME, Youle RJ, 2019. Selective autophagic clearance of protein aggregates is mediated by the autophagy receptor, TAX1BP1. BioRxiv 558767. [Google Scholar]
- Sathyanarayan A, Mashek MT, Mashek DG, 2017. ATGL promotes autophagy/lipophagy via SIRT1 to control hepatic lipid droplet catabolism. Cell Rep. 19 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JL, Cuervo AM, 2014. Liver autophagy: much more than just taking out the trash. Nat. Rev. Gastroenterol. Hepatol 11 (3), 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JL, Suh Y, Cuervo AM, 2014. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metabol. 20 (3), 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott MB, Weller SG, Schulze RJ, Krueger EW, Drizyte-Miller K, Casey CA, McNiven MA, 2019. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J. Cell Biol 218 (10), 3320–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, McNiven MA, 2015. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology 61 (6), 1896–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman EH, Desnick RJ, 2017. Types A and B niemann-pick disease. Mol. Genet. Metabol 120 (1–2), 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, 2020. Microautophagy–distinct molecular mechanisms handle cargoes of many sizes. J. Cell Sci 133 (17). [DOI] [PubMed] [Google Scholar]
- Schuck S, Gallagher CM, Walter P, 2014. ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J. Cell Sci 127 (18), 4078–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze RJ, Krueger EW, Weller SG, Johnson KM, Casey CA, Schott MB, McNiven MA, 2020. Direct lysosome-based autophagy of lipid droplets in hepatocytes. Proc. Natl. Acad. Sci. U. S. A 117 (51), 32443–32452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze RJ, Rasineni K, Weller SG, Schott MB, Schroeder B, Casey CA, McNiven MA, 2017. Ethanol exposure inhibits hepatocyte lipophagy by inactivating the small guanosine triphosphatase Rab7. Hepatol Commun 1 (2), 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz DS, Blower MD, 2016. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell. Mol. Life Sci 73 (1), 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, Ma J, Kemper B, Kemper JK, 2014. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 516 (7529), 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, 2017. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun 8 (1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S, Shen Z, Zhang C, Kou R, Xie K, Song F, 2019. Mitophagy protects against acetaminophen-induced acute liver injury in mice through inhibiting NLRP3 inflammasome activation. Biochem. Pharmacol 169, 113643. [DOI] [PubMed] [Google Scholar]
- Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, Huerta C, Virgin HW, Helms JB, Eerland R, Tooze SA, Xavier R, Lenschow DJ, Yamamoto A, King D, Lichtarge O, Grishin NV, Spector SA, Kaloyanova DV, Levine B, 2013. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494 (7436), 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ, 2009. Autophagy regulates lipid metabolism. Nature 458 (7242), 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, Ilkayeva OR, Gooding J, Ching J, Zhou J, Martinez L, Xie S, Bay BH, Summers SA, Newgard CB, Yen PM, 2014. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology 59 (4), 1366–1380. [DOI] [PubMed] [Google Scholar]
- Sinha RA, You SH, Zhou J, Siddique MM, Bay BH, Zhu X, Privalsky ML, Cheng SY, Stevens RD, Summers SA, Newgard CB, Lazar MA, Yen PM, 2012. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Invest 122 (7), 2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Ann DK, Ou JH, 2010a. Autophagy by hepatitis B virus and for hepatitis B virus. Autophagy 6 (4), 548–549. [DOI] [PubMed] [Google Scholar]
- Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH, 2008a. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology 48 (4), 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Kuo CF, Tian Y, Liu HM, Huang EJ, Jung JU, Machida K, Ou JH, 2012. Replication of hepatitis C virus RNA on autophagosomal membranes. J. Biol. Chem 287 (22), 18036–18043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Liang C, Chen WL, Jung JU, Ou JH, 2008b. Perturbation of autophagic pathway by hepatitis C virus. Autophagy 4 (6), 830–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH, 2010b. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc. Natl. Acad. Sci. U. S. A 107 (9), 4383–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Wilkinson S, 2017. ER homeostasis and autophagy. Essays Biochem. 61 (6), 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Wilkinson S, 2018. CCPG1, a cargo receptor required for reticulophagy and endoplasmic reticulum proteostasis. Autophagy 14 (6), 1090–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccio RE, Breslow JL, 2004. Intracellular cholesterol transport. Arterioscler. Thromb. Vasc. Biol 24 (7), 1150–1160. [DOI] [PubMed] [Google Scholar]
- Song KH, Li T, Owsley E, Strom S, Chiang JY, 2009. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 49 (1), 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Liby KT, 2012. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Canc 12 (8), 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer MZ, Poole LP, Drake LE, Bock-Hughes A, Boland ML, Smith AG, Hart J, Chourasia AH, Liu I, Bozek G, Macleod KF, 2021. BNIP3-dependent mitophagy promotes cytosolic localization of LC3B and metabolic homeostasis in the liver. Autophagy 1–17. 10.1080/15548627.2021.1877469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A, Grumati P, 2019. The various shades of ER-phagy. FEBS J. 286 (23), 4642–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Wu R, Li P, Yu L, 2020. Phase separation in regulation of aggrephagy. J. Mol. Biol 432 (1), 160–169. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang S, Yu C, Pan Z, Liu Y, Zhao J, Wang X, Yun F, Zhao H, Yan S, Yuan Y, Wang D, Ding X, Liu G, Li W, Zhao X, Liu Z, Li Y, 2015. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am. J. Physiol. Endocrinol. Metab 309 (11), E925–E935. [DOI] [PubMed] [Google Scholar]
- Takahashi SS, Sou YS, Saito T, Kuma A, Yabe T, Sugiura Y, Lee HC, Suematsu M, Yokomizo T, Koike M, Terai S, Mizushima N, Waguri S, Komatsu M, 2020. Loss of autophagy impairs physiological steatosis by accumulation of NCoR1. Life Sci Alliance 3 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, He H, Tang Z, Hattori T, Liu Y, Young MM, Serfass JM, Chen L, Gebru M, Chen C, Wills CA, Atkinson JM, Chen H, Abraham T, Wang HG, 2018. An autophagy assay reveals the ESCRT-III component CHMP2A as a regulator of phagophore closure. Nat. Commun 9 (1), 2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Liang X, Hattori T, Tang Z, He H, Chen H, Liu X, Abraham T, Imamura-Kawasawa Y, Buchkovich NJ, Young MM, Wang HG, 2019. VPS37A directs ESCRT recruitment for phagophore closure. J. Cell Biol 218 (10), 3336–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N, 2011. Autophagy-deficient mice develop multiple liver tumors. Gene Dev. 25 (8), 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Hikita H, Tatsumi T, Sakamori R, Nozaki Y, Sakane S, Shiode Y, Nakabori T, Saito Y, Hiramatsu N, Tabata K, Kawabata T, Hamasaki M, Eguchi H, Nagano H, Yoshimori T, Takehara T, 2016. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 64 (6), 1994–2014. [DOI] [PubMed] [Google Scholar]
- Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li F, Wang Y, Tiollais P, Li T, Zhao M, 2009. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology 49 (1), 60–71. [DOI] [PubMed] [Google Scholar]
- Thoen LF, Guimarães EL, Dollé L, Mannaerts I, Najimi M, Sokal E, van Grunsven LA, 2011. A role for autophagy during hepatic stellate cell activation. J. Hepatol 55 (6), 1353–1360. [DOI] [PubMed] [Google Scholar]
- Thomes PG, Trambly CS, Fox HS, Tuma DJ, Donohue TM Jr., 2015. Acute and chronic ethanol administration differentially modulate hepatic autophagy and transcription factor EB. Alcohol Clin. Exp. Res 39 (12), 2354–2363. [DOI] [PubMed] [Google Scholar]
- Thomes PG, Trambly CS, Thiele GM, Duryee MJ, Fox HS, Haorah J, Donohue TM Jr., 2012. Proteasome activity and autophagosome content in liver are reciprocally regulated by ethanol treatment. Biochem. Biophys. Res. Commun 417 (1), 262–267. [DOI] [PubMed] [Google Scholar]
- Tian Y, Kuo CF, Sir D, Wang L, Govindarajan S, Petrovic LM, Ou JH, 2015. Autophagy inhibits oxidative stress and tumor suppressors to exert its dual effect on hepatocarcinogenesis. Cell Death Differ. 22 (6), 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR, 2010. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52 (5), 1836–1846. [DOI] [PubMed] [Google Scholar]
- Toledo M, Batista-Gonzalez A, Merheb E, Aoun ML, Tarabra E, Feng D, Sarparanta J, Merlo P, Botre F, Schwartz GJ, Pessin JE, Singh R, 2018. Autophagy regulates the liver clock and glucose metabolism by degrading CRY1. Cell Metabol. 28 (2), 268–281 e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisu T, Torisu K, Lee IH, Liu J, Malide D, Combs CA, Wu XS, Rovira II, Fergusson MM, Weigert R, Connelly PS, Daniels MP, Komatsu M, Cao L, Finkel T, 2013. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat. Med 19 (10), 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Friedman SL, 2017. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol 14 (7), 397. [DOI] [PubMed] [Google Scholar]
- Ueno T, Komatsu M, 2017. Autophagy in the liver: functions in health and disease. Nat. Rev. Gastroenterol. Hepatol 14 (3), 170–184. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, 2015. Proteomics. Tissue-based map of the human proteome. Science 347 (6220), 1260419. [DOI] [PubMed] [Google Scholar]
- Umemura A, Park EJ, Taniguchi K, Lee JH, Shalapour S, Valasek MA, Aghajan M, Nakagawa H, Seki E, Hall MN, Karin M, 2014. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metabol. 20 (1), 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW, 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol 9 (2), 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo T, Romagnoli A, Perdomo AB, Corazzari M, Ciccosanti F, Alonzi T, Nardacci R, Ippolito G, Tripodi M, Garcia-Monzon C, Lo Iacono O, Piacentini M, Fimia GM, 2012. Autophagy protects cells from HCV-induced defects in lipid metabolism. Gastroenterology 142 (3), 644–653 e643. [DOI] [PubMed] [Google Scholar]
- Wang C, Niederstrasser H, Douglas PM, Lin R, Jaramillo J, Li Y, Oswald NW, Zhou A, McMillan EA, Mendiratta S, Wang Z, Zhao T, Lin Z, Luo M, Huang G, Brekken RA, Posner BA, MacMillan JB, Gao J, White MA, 2017. Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nat. Commun 8 (1), 2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ni HM, Chao X, Ma X, Rodriguez YA, Chavan H, Wang S, Krishnamurthy P, Dobrowsky R, Xu DX, Jaeschke H, Ding WX, 2019. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol 22, 101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Park JY, Kwon O, Choe EY, Kim CH, Hur KY, Lee MS, Yun M, Cha BS, Kim YB, Lee H, Kang ES, 2015. Chronic HMGCR/HMG-CoA reductase inhibitor treatment contributes to dysglycemia by upregulating hepatic gluconeogenesis through autophagy induction. Autophagy 11 (11), 2089–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ou JH, 2015. Hepatitis C virus and autophagy. Biol. Chem 396 (11), 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tian Y, Ou JH, 2015. HCV induces the expression of Rubicon and UVRAG to temporally regulate the maturation of autophagosomes and viral replication. PLoS Pathog. 11 (3), e1004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ding WX, Li T, 2018. Cholesterol and bile acid-mediated regulation of autophagy in fatty liver diseases and atherosclerosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863 (7), 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ding Y, Li J, Chavan H, Matye D, Ni HM, Chiang JY, Krishnamurthy P, Ding WX, Li T, 2017. Targeting the enterohepatic bile acid signaling induces hepatic autophagy via a CYP7A1-AKT-mTOR Axis in mice. Cell Mol Gastroenterol Hepatol 3 (2), 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gunewardena S, Li F, Matye DJ, Chen C, Chao X, Jung T, Zhang Y, Czerwinski M, Ni HM, Ding WX, Li T, 2020. An FGF15/19-TFEB regulatory loop controls hepatic cholesterol and bile acid homeostasis. Nat. Commun 11 (1), 3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskirchen R, Tacke F, 2016. Liver fibrosis: which mechanisms matter? Clinical Liver Disease 8 (4), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskirchen R, Tacke F, 2019. Relevance of autophagy in parenchymal and non-parenchymal liver cells for health and disease. Cells 8 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskirchen R, Weiskirchen S, Tacke F, 2019. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol. Aspect. Med 65, 2–15. [DOI] [PubMed] [Google Scholar]
- Wild P, McEwan DG, Dikic I, 2014. The LC3 Interactome at a Glance. The Company of Biologists Ltd. [DOI] [PubMed] [Google Scholar]
- Wilkinson AL, Qurashi M, Shetty S, 2020. The role of sinusoidal endothelial cells in the axis of inflammation and cancer within the liver. Front. Physiol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, 2019. ER-phagy: shaping up and destressing the endoplasmic reticulum. FEBS J. 286 (14), 2645–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Ding WX, 2020. Role of autophagy in alcohol and drug-induced liver injury. Food Chem. Toxicol 136, 111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Manley S, Ding WX, 2014. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J. Gastroenterol 20 (36), 12908–12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Ni HM, Ding Y, Ding WX, 2015a. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol 309 (5), G324–G340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Ni HM, Haynes A, Manley S, Li Y, Jaeschke H, Ding WX, 2015b. Chronic deletion and acute knockdown of parkin have differential responses to acetaminophen-induced mitophagy and liver injury in mice. J. Biol. Chem 290 (17), 10934–10946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lutjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B, van Bilsen M, Shiri-Sverdlov R, Hofker MH, 2008. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of non-alcoholic steatohepatitis. Hepatology 48 (2), 474–486. [DOI] [PubMed] [Google Scholar]
- Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, Yang G, Mitchell GA, 2011. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54 (1), 122–132. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, Fukamizu A, 2004. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem 279 (22), 23158–23165. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Ohsaki Y, Suzuki M, Shinohara Y, Tatematsu T, Cheng J, Okada M, Ohmiya N, Hirooka Y, Goto H, Fujimoto T, 2014. Inhibition of Niemann-Pick-type C1-like1 by ezetimibe activates autophagy in human hepatocytes and reduces mutant alpha1-antitrypsin Z deposition. Hepatology 59 (4), 1591–1599. [DOI] [PubMed] [Google Scholar]
- Yamasaki A, Alam JM, Noshiro D, Hirata E, Fujioka Y, Suzuki K, Ohsumi Y, Noda NN, 2020. Liquidity is a critical determinant for selective autophagy of protein condensates. Mol Cell 77 (6), 1163–1175 e1169. [DOI] [PubMed] [Google Scholar]
- Yan S, Khambu B, Chen X, Dong Z, Guo G, Yin XM, 2020. Hepatic autophagy deficiency remodels gut microbiota for adaptive protection via FGF15-FGFR4 signaling. Cell Mol Gastroenterol Hepatol 11, 973–997. 10.1016/j.jcmgh.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ni HM, Ding WX, 2019a. The double-edged sword of MTOR in autophagy deficiency induced-liver injury and tumorigenesis. Autophagy 15 (9), 1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ni HM, Ding WX, 2019b. Emerging players in autophagy deficiency-induced liver injury and tumorigenesis. Gene Expr. 19 (3), 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ni HM, Guo F, Ding Y, Shi YH, Lahiri P, Frohlich LF, Rulicke T, Smole C, Schmidt VC, Zatloukal K, Cui Y, Komatsu M, Fan J, Ding WX, 2016b. Sequestosome 1/p62 protein is associated with autophagic removal of excess hepatic endoplasmic reticulum in mice. J. Biol. Chem 291 (36), 18663–18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JD, Roberts LR, 2010. Hepatocellular carcinoma: a global view. Nat. Rev. Gastroenterol. Hepatol 7 (8), 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li P, Fu S, Calay ES, Hotamisligil GS, 2010. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metabol. 11 (6), 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda D, Ohe T, Takahashi K, Imamura R, Kojima H, Okabe T, Ichimura Y, Komatsu M, Yamamoto M, Nagano T, 2020. Inhibitors of the protein–protein interaction between phosphorylated p62 and Keap1 attenuate chemoresistance in a human hepatocellular carcinoma cell line. Free Radic. Res 1–13. [DOI] [PubMed] [Google Scholar]
- Yin XM, Ding WX, Gao W, 2008. Autophagy in the liver. Hepatology 47 (5), 1773–1785. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H, 2014. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2 (11), 901–910. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP, 2011. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol 12 (1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F, 2019. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol 71 (4), 793–801. [DOI] [PubMed] [Google Scholar]
- Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB, 2007. From Mallory to Mallory-Denk bodies: what, how and why? Exp. Cell Res 313 (10), 2033–2049. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jiang N, Ping J, Xu L, 2021. TGFbeta1induced autophagy activates hepatic stellate cells via the ERK and JNK signaling pathways. Int. J. Mol. Med 47 (1), 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang C, Zhang L, Yang Q, Zhou S, Wen Q, Wang J, 2015. Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Canc. 15 (1), 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao TJ, 2015. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc. Natl. Acad. Sci. U. S. A 112 (4), 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Du LL, 2020. Epr1, a UPR-upregulated soluble autophagy receptor for reticulophagy. Autophagy 16 (11), 2112–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YG, Zhang H, 2018. The ER-localized autophagy protein EPG-3/VMP1 regulates ER contacts with other organelles by modulating ATP2A/SERCA activity. Autophagy 14 (2), 362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Learned RM, Rossi SJ, DePaoli AM, Tian H, Ling L, 2017. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun 1 (10), 1024–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]