Summary
Chimeric antigen receptor (CAR)-T cell therapy represents a breakthrough in the immunotherapy field and has achieved great success following its approval in 2017 for the treatment of B cell malignancies. While CAR-T cells are mostly applied as anti-tumor therapy in the present, their initial concept was aimed at a more general purpose of targeting membrane antigens, thus translating in many potential applications. Since then, several studies have assessed the use of CAR-T cells toward non-malignant pathologies such as autoimmune diseases, infectious diseases and, more recently, cardiac fibrosis, and cellular senescence. In this review, we present the main findings and implications of CAR-based therapies for non-malignant conditions.
Keywords: chimeric antigen receptor, autoimmune diseases, infectious diseases, fibrosis, CAR-T cell, adoptive cell transfer
Introduction
Immunotherapy has been a paradigm change in cancer treatment. The first documented attempts to use an immune-based therapy to treat cancer date back to William Coley in the 1890s, who used bacteria extracts injections directly into his patients’ tumors [1]. Although the data generated in those experiments are hard to interpret, the main idea of immunostimulation as cancer therapy has been carried on.
Bone marrow transplantation, the oldest form of cell-based therapy, was pioneered by the work of E. Donnall Thomas in the 1950s. He established the basic concepts that allowed for the first successful bone marrow transplantation in humans, a procedure that remains, up to now, the only curative option for a variety of hematological malignancies.
Since the establishment of bone marrow transplants, much has been achieved regarding immune-based therapy, whether humoral or cellular.
The first monoclonal antibody was approved for B cell lymphoma in 1997 [2], and subsequently many others have been licensed for different malignancies. Later, antibodies were conjugated to chemotherapy agents in order to enhance potency and target specificity [3]. Checkpoint inhibitors [4] and bi-specific antibodies [5], which can play different roles such as engaging the host immunity against a tumor target, have also reached clinical use more recently. However, the most striking progress has been the approval in 2017 of chimeric antigen receptor (CAR)-T cells to treat B cell malignancies [6]. Considered the first ‘living drug’ to reach the market, CAR-T cells comprise T lymphocytes genetically engineered to express a CAR. The most common CAR configuration consists of an extracellular single-chain variable fragment (scFv) domain for major histocompatibility complex (MHC)-unrestricted target recognition, coupled to the transmembrane and intracellular signaling regions (Fig. 1). First-generation CARs were initially proposed in 1993 [7] and had a single CD3 zeta chain intracellular domain, which is able to replicate the T cell receptor (TCR)-derived signal 1 required for T cell activation. Second-generation CARs incorporated a costimulatory domain, usually CD28 or 41BB, while third-generation CARs have two costimulatory domains in tandem [8]. Additional developments, comprising the so-called fourth-generation CARs, included the expression or secretion of additional molecules like cytokines, to improve efficiency [9]. Even though CAR-T cells have reached their epitome in the treatment of B cells malignancies, the technology was not initially designed solely for this purpose and took advantage of many concepts developed during the study of other disease entities, such as human immunodeficiency virus (HIV) [10]. Notably, CAR-T cells clinical success in the cancer setting has opened a new horizon of possibilities, not only for cancer treatment, but also for a variety of diseases in which specific membrane antigens become expressed or up-regulated in pathological cells. These cells can be immunologically targeted and as so, could benefit from CAR-T cell therapy, especially chronic diseases for which immunological memory may provide long-term gain. A landscape covering different approaches of CAR-T development for non-malignant pathologies will be further described here.
Figure 1.
CAR generations. First-generation CARs comprised a single CD3zeta chain, while second-generation CARs included a costimulatory signaling domain. Later, third-generation CARs emerged combining two different costimulatory molecules, the most common being 4-1BB and CD28. Further developments, comprising fourth-generation CARs, included the expression or secretion of additional molecules to improve CAR-T cell performance.
CAR-T cells in infectious diseases
CAR-T cell development for HIV: past and present
HIV and the associated acute immunodeficiency syndrome were described in the beginning of the 1980s and rapidly spread throughout the world. The significant human and economic burden associated with the disease-generated massive research efforts toward an efficacious treatment. Many drugs were developed, and even though the combination antiretroviral therapy (cART) is highly effective in controlling viral replication, it fails to eliminate the virus, and viremia recrudescence from the latent reservoir is observed upon treatment interruption [11]. The first reports of CAR-T cells clinical use date back to the 1990s, as an attempt to cure HIV. Those first-generation CARs comprised a CD4 extracellular domain associated to the CD3ζ (CD4ζCAR) signaling domain, which were transduced into CD8+ T cells (Fig. 2A). Clinical studies showed that while the strategy was feasible and safe, it failed to consistently control HIV replication [10, 12–15]. Nonetheless, one of the pioneering trials provided valuable information on CAR-T cell persistence, with decay half-lives exceeding 16 years [16]. Since then, CAR-T cell design evolved and key factors, such as effector function, persistence, and susceptibility to exhaustion, have been greatly optimized (reviewed in Chicaybam et al. [17]). Although second-generation CARs show better in vitro activity against HIV and in vivo persistence, this has never translated into clinical benefit [18]. Several challenges remain, such as the optimal extracellular target, long-term persistence, and the ability to target the latent viral reservoir.
Figure 2.
Past and present approaches for anti-HIV CAR-T cells. (A) The first-generation CAR construct used CD4 as the extracellular domain, coupled with a CD8 transmembrane region and a CD3zeta chain intracellular signaling. This structure rendered the CAR-T cell susceptible to HIV infection and showed limited viral control. To improve HIV infection resistance and persistence, subsequent designs have been optimized. (B) A third-generation CAR with the scFv fraction based on a broadly neutralizing antibody (bNAb) against gp120 and two costimulatory domains (CD28 and 4-1BB) is currently undergoing clinical investigation. (C) A second-generation CAR also comprising a scFv based on anti-gp120 bNAb with a single CD28 costimulatory domain, is expressed after CCR5 disruption using megaTAL nucleases or zinc-finger nucleases, and incorporation of the donor template in its site. This strategy protects against insertional mutagenesis and has been proven safe in a clinical trial. (D) Co-expression of the CAR and inhibitory molecules has also been described. On (D), a dual-CAR-T cell co-expresses two CAR constructs independently along with the CXCR4-CD34 fusion inhibitor. The CD4 extracellular is common to both receptors, which differ only in the costimulatory domain (4-1BB or CD28). In a humanized BLT mouse model, the dual CAR has shown better viremia control compared to the separate constructs or a third-generation CAR with both co-stimulatory domains.
HIV is a highly mutating virus and a pivotal point on cellular invasion is the recognition of CD4, mediated by the viral envelope protein (Env) gp120 binding. Because this domain is a key target, CD4 poses an attractive extracellular CAR domain, as the binding site of gp120 tends to remain conserved. However, CD4 expression also renders the CAR-T cells susceptible to lytic infection and destruction. In fact, the clinical studies did not show any beneficial role of these first-generation CARs with CD4 and the zeta chain. CARs with scFv derived from broadly neutralizing antibodies (bNAbs) that target conserved sites in the Env protein have also been developed [19–21]. One of them, a third-generation CAR with the scFv derived from a Nab directed against gp120, VRC01 [21], is undergoing clinical trial (NCT03240328) (Fig. 2B).
Protection of CAR-T cells from HIV infection is also paramount for therapy success and several approaches have tried to prevent HIV entrance in the cells [18]. CAR-T cells with targeted disruption of the HIV co-receptor CCR5 using zinc finger nucleases have been developed [22, 23] and are currently undergoing clinical investigation (NCT03617198). Recently, a promising new CAR engineering strategy enabling high rates of homology-directed repair (HDR) has been described. In this study, CCR5 was disrupted using megaTAL nucleases, and HDR allowed for simultaneous incorporation of the donor template into the CCR5 site (Fig. 2C). The resulting CAR-T cells showed better inhibition of HIV in vitro compared to non-CCR5 disrupted cells, and also added a potential new safety tool against insertional mutagenesis [20, 24]. This strategy was proved safe in a clinical trial [25]. Co-expression of CAR and inhibitory molecules, such as C34-CXCR4, an HIV fusion inhibitor, has also been described [26]. In a recent approach, Maldini et al. developed a dual CD4-based CAR-T cell that expresses both 41BB- and CD28- second-generation CARs independently, along with the C34-CXCR4 fusion inhibitor [27] (Fig. 2D). This construct resulted in superior proliferative capacity and persistence in a humanized BLT (bone marrow, liver, and thymus) mouse model when compared with the separate second-generation CARs or with a third-generation construct comprising both 41BB and CD28 costimulatory domains. The dual CAR expressing the fusion inhibitor led to reduction in acute viremia in vivo and enhanced suppression of viral load in a combined treatment with cART compared with cART alone.
Yet another way of targeting poor persistence of CAR-T cells in vivo was the generation of CD34+ hematopoietic stem and progenitor cells (HSPC)-derived CAR-T. In an immunodeficient murine model, CD4ζCAR was transduced into human CD34+ HSPC, generating T and B lymphocytes, NK cells, and myeloid cells in the animals. The authors showed that CAR-expressing cells were present in the thymus, spleen, blood, and bone marrow. Upon HIV challenge, CAR-T cells proliferated and were seemingly better at suppressing viral load than controls [28, 29]. However, a subsequent study with non-human primates failed to show a significant effect on viremia in the absence of cART, even though the cells persisted for as long as 2 years [30].
HIV-targeted CAR-T cells must also overcome the low antigen burden, which poses a challenge for long-term persistence. Preceding CAR-T infusion with TCR-specific viral antigen immunization is a way to address this issue and has already been included in clinical trials for B cell malignancies and glioblastoma (NCT01430390, NCT00709033, NCT01109095, and NCT03186118). Double-specific CAR-T cells are manufactured using cytomegalovirus (CMV), Epstein–Barr virus (EBV), or adenovirus TCR-specific cells [31–33] and after infusion, vaccination with viral epitopes is able to elicit CAR-T proliferation and enhance antitumor activity. However, it may prove counterproductive, as repetitive TCR activation leads to terminal differentiation and diminished proliferative capacity. The fine-tuning of stimulation frequency and intensity is yet another challenge for this approach.
Despite the high efficacy of cART, HIV elimination has not been achieved due to the presence of a latent virus reservoir [34]. The ‘shock and kill’ strategy with latency-reversing agents (LRAs) aimed at reversing quiescence to reestablish immune surveillance, also failed to destroy the reservoir [35–38]. The main sites of HIV persistence are CD4 T follicular helper cells (TFH) in B cell follicles [39]. Cytotoxic T lymphocytes (CTLs) are incapable of reaching these follicles because they lack CX chemokine receptor 5 (CXCR5), which guides cell trafficking in the B cell zone through a CXC chemokine ligand 13 (CXCL13) gradient [39]. Co-expression of CAR and CXCR5 is a reasonable approach that has been described in a simian model. As a proof of principle, the SIV-targeted CAR-CXCR5 cells showed in vitro migration through a CXCL13 gradient, and ex vivo homing to B follicles [40]. The combination of a CAR-T and LRAs is also being investigated in a clinical trial (NCT03980691).
CAR T cells in other infectious diseases
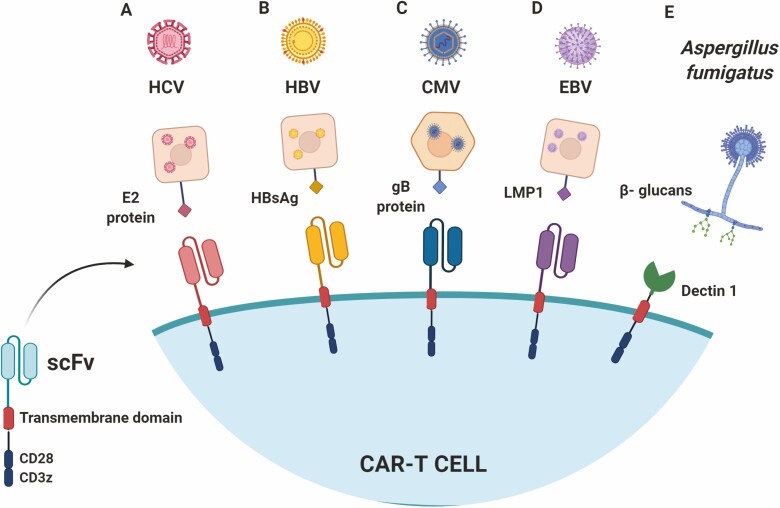
Beyond HIV, CARs specific for hepatitis C virus (HCV) have recently been developed based on an scFv targeting a highly conserved epitope of the E2 glycoprotein present in the HCV viral capsid. Sautto et al. [41, 42] evaluated the activity of anti-HCV CAR-T cells in vitro and were able to induce lysis of target cells transfected with HCV E2 (Fig. 3A). However, this anti-HCV CAR-T has not yet been tested in vivo. Because the structural properties of the E2 glycoprotein have not been well established, further studies are necessary to expand this approach.
Figure 3.
Current development of CAR-T cells for infectious diseases beyond HIV. (A). Second-generation CARs were designed with scFv domains anti-HCV (A) and HBV(B) antigens. Other CARs were designed to recognize epitopes of proteins found on the membrane of the target cells which are essential for virus entrance, as the gB protein in CMV infection (C) and LMP1 in EBV infection (D). (E) Second-generation CAR using the Dectin-1 protein extracellular portion, a natural receptor of B-glucans which are present in Aspergillus sp. cell wall.
In other models, such as for hepatitis B infections (HBV), some CARs have also been developed to prevent hepatocellular carcinoma progression and to approach chronic diseases. In this last example, the sequences of proteins S and L, both present on the surface of HBV infected cells, are used as targets for scFvs (Fig. 3B). These CARs are engineered to be expressed in lymphocytes from healthy donors. In this study, Bohne et al. [43] observed that anti-HBV CAR-T cells were able to recognize hepatocytes infected by HBV in vitro producing IL-2 and IFN-g following targeted recognition. It was also possible to observe that the production of these cytokines occurred at earlier time points in S-specific CAR-T cells than in L-specific cells, probably due to the high expression of protein S on the surface of infected cells.
Kruse et al. [44] also created CARs to recognize other HBV antigens by using different sizes of spacers in the structure of CARs. The authors observed that CARs that contained larger spacers were more promptly activated, producing higher levels of pro-inflammatory cytokines such as TNF-a, IL-2, and IFN-g when co-cultured with different cell lines infected by HBV in vitro. Subsequently, it was observed in vivo that after HBV infection and subsequent anti-HBV CAR-T treatment, there was a reduction in plasmatic HBV antigens and DNA, although it was not possible to observe complete elimination of the virus. However, the use of anti-HBV CAR-T cells is still more promising than the use of the anti-HBV therapies already known and currently used, like the nucleoside analogues mentioned previously. This aspect is particularly interesting because these analogs block the synthesis of viral DNA but do not affect the production of antigens. Thus, hepatocytes infected by HBV, even under treatment with nucleoside analogs, can still be targeted by anti-HBV CAR-T cells [45].
The use of CARs has also been evaluated in models of CMV infection. Anti-gb CAR-T cells, which target a protein expressed in the viral capsid, were successfully activated after co-culture with CMV-infected cells, in addition to showing satisfactory production of TNF-a and IFN-g [46] (Fig. 3C). However, in 2016, Proff et al. [47] noted that CMV-infected cells were resistant to death by anti-CMV CAR-T cells. Subsequently, it was possible to verify that some anti-apoptotic viral factors, which can inhibit the death of infected host cells, contribute to dampening the cytotoxicity of T cells. The same was observed when anti-CMV CAR-T cells were co-cultured with fibroblasts not infected by CMV, but with overexpression of the UL37x1 and UL36 proteins, showing that in addition to the deficient antigen presentation, these viral proteins protect the infected cells, blocking the cytotoxic effector functions of anti-CMV CAR-T cells. These observations do not exclude the potential for the use of antiviral CAR-T cells, since T lymphocytes remain capable of exerting non-cytotoxic functions through the secretion of cytokines and granzymes, which are important for cleavage of essential proteins related to CMV replication [48].
CARs have also been developed to recognize EBV proteins. In one of these approaches, Tang et al. [49] used a CAR specific for EBV latent membrane protein 1 (LMP1) (Fig. 3D). The CAR-T cells were co-cultured with nasopharyngeal carcinoma cells overexpressing LMP1, resulting in activation and production of cytokines such as IFN-g and IL-2. The study also evaluated the activity of these CAR-T cells in vivo following the formation of tumors overexpressing LMP1 in mice. Anti-EBV CAR-T cells were injected intra-tumorally and led to a reduction in tumor growth. However, it is known that a great amount of LMP1 is located in endosomes, which decreases its accessibility on the surface of EBV-associated tumor cells. Consequently, CAR T cells targeting LMP1 act in a level below their potential [50], although Tang et al. [51] also showed that neural progenitor cells overexpressing LMP1 were killed by the anti-EBV lymphocytes. It is still a challenge to reproduce the same outcomes considering the physiological levels of LMP1 and the respective cytotoxicity potential of T cells carrying the CARs. Nonetheless, these data show that this approach is promising and deserves further development for EBV-associated malignancies.
Another recent attempt of bringing CAR-T technology into the infectious diseases setting was the development of a CAR-T cell to fight opportunistic Aspergillus fungal infections, which poses a real hazard to immunocompromised patients. Kumaresan et al. [52] designed a CAR based on the pattern recognition receptor Dectin-1 that recognizes β-glucans present in the fungi cell wall. The so-called ‘D-CAR’ (Fig. 3E) comprising the extracellular portion of Dectin-1, the CD28 costimulatory domain, and the CD3z chain was able to reduce hyphal growth in vitro and lead to a decrease in fungal burden on in vivo NOD SCID-γ (NSG) mice models of lung and cutaneous Aspergillus infection. Despite being an interesting proof-of-concept, it has been shown that Dectin-1 can bind ligands other than β-glucans and may even serve as a co-stimulus when binding to CD4 and CD8 T cells [53, 54]. This lack of specificity is daunting as regards to translating this approach to the clinic.
Despite the great potential of using CAR-T cells in infectious diseases, it must be considered that vital organs are involved, such as the liver in different types of Hepatitis and therefore T cells can both induce remission of the disease or damage as in the case of cytokine release syndrome. Thus, the use of CAR-T cells should be carefully assessed for each type of disease.
One of the alternatives to solve this issue would be to use suicide genes to eliminate the CAR-T cells, or even to bet on the transient expression of these CARs using, for example, the electroporation of CAR mRNA. By doing so, it would be possible to monitor and transfer in a staggered manner the ideal number of T cells to patients and monitor their toxic effects [45].
CAR-T cells in autoimmune diseases
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multiple organ systems involvement, where autoantibodies against DNA and nuclear proteins form immune complex deposits consistently linked to disease pathogenesis [55]. Rituximab, an anti-CD20 monoclonal antibody, has been tried clinically in lupus nephritis, but failed to induce durable remissions [56, 57]. It has been hypothesized that immune complexes could block macrophage phagocytosis, and therefore, Rituximab doses need to be higher [58, 59]. Furthermore, incomplete B cell depletion and the dosing schedule may have contributed to the lack of success. Alternatively, CD19-targeted CAR-T cells are capable of inducing long lasting and profound B cell depletion through direct cytotoxicity, posing an attractive option for lupus treatment [60].
Indeed, Kansal et al. showed that CD19-targeted CD8+CAR-T cells were capable of reversing or deferring lupus manifestations in two murine models (NBZ/W and MRL-lpr), with considerable extension of the animals’ lifespan [60]. Because auto reactive CD4+ T cells might enhance disease, CD8+ T cells were purified and used to express a second-generation CAR, with the extracellular single-chain Fv domain of an anti-CD19 antibody, linked to CD28 and a variant CD3z terminus. Jin et al. further corroborated these findings using the MRL-lpr lupus model and a similar CAR-T configuration. However, in this study, a second construct was added for comparison, which used 41BB instead of CD28 costimulatory domain (Fig. 4A). Profound and sustained B cell depletion was achieved and correlated with amelioration of lupus symptoms [61]. The 41BB construct had better therapeutic effects compared to the one with CD28, which is consistent with previous findings that show longer persistence and lower exhaustion levels with 41BB signaling domain [62, 63]. The results displayed are encouraging for further research in lupus, especially since anti-CD19 CAR-T cells are already licensed for clinical use. Indeed, CD19 CAR-T cells for SLE have recently moved into a phase I clinical study (NCT03030976). Following a similar rationale, anti-B cell maturation antigen (BCMA) CAR-T cells initially developed against multiple myeloma [64, 65] are currently in a phase I/II clinical study (NCT04146051) using mRNA-transfected T cells to treat myasthenia gravis, an autoimmune disease in which B cells target the acetylcholine receptor in neuromuscular junctions.
Figure 4.
CAR-T cells for autoimmune diseases. (A) Second-generation CAR with scFv domain targeting CD19 for B cell depletion in SLE. (B) CAAR expressing extracellular cadherin domains of Dsg3 recognizes BCRs anti-Dsg3, leading to autoreactive memory B cells elimination in PV. (C) Tregs transduced with anti-TNP CAR suppressed inflammation and improved survival in a murine model of TNP-induced colitis. (D) Similarly, Tregs expressing anti-carcinoembryonic antigen CAR reduced the severity of colitis and subsequent colorectal tumor burden in two types of induced colitis in mice. (E) Intranasally delivered anti-myelin oligodendrocyte glycoprotein CAR Tregs reached various brain areas and suppressed inflammation, leading to clinical improvement in a MS murine model.
A novel approach to target autoimmunity was used for pemphigus vulgaris (PV), a life-threatening autoimmune disorder characterized by disseminated skin and mucosal blistering that can lead to fatal infections [66]. PV is caused by autoantibodies against Dsg3 (Desmoglein-3) and although CD20-targeted therapy induces remission in 95% of patients, most of them will relapse [67]. During active disease and relapse, the same anti-Dsg3 B cell clones are observed. Therefore, elimination of those memory cells could lead to PV cure. Ellebrecht et al. created a chimeric auto-antibody receptor (CAAR) [66], which comprised the cadherin domains 1–4 of Dsg3 as extracellular domains, coupled to the CD8 transmembrane domain linked to 41BB costimulatory and CD3z signaling domains (Fig. 4B). The authors were also able to observe, in a murine model of PV, that these CAAR-T cells were capable of selectively eliminating anti-Dsg3 cells without off-target related toxicity [66]. There is a phase I clinical trial (NCT04422912) ongoing for this disease aiming to evaluate the maximum tolerated dose and optimal infusion schedule of Dsg3-CAART cells in mucosal-dominant PV.
Yet another alternative in the context of autoimmune diseases is the introduction of CARs into regulatory T cells (Treg), generating antigen specific Treg cells. Infusion of polyclonal Treg cells has been used in phase I trials to halter type I diabetes and graft-versus-host-disease, with limited success and good safety profile [39]. Designing an antigen-specific Treg would allow for lower doses necessary to induce immunosuppression, and the possibility to redirect Treg cells to specific tissues, minimizing the off-target effects. A few models have been described in this scenario. In one of the first descriptions, by Elinav et al. [68], using a murine 2,4,6-Trinitrophenol (TNP)-induced colitis model, Treg cells were transduced with a second-generation CAR comprising a scFv specific for TNP (Fig. 4C). After retroviral transduction, Tregs maintained high Foxp3 expression and showed antigen-specific suppression activity in vitro, while significantly alleviating symptoms of TNP-induced colitis in vivo. A subsequent report by Blat et al. [69] using two different models of murine colitis (T-cell- and chemically induced) also suggests good potential for clinical use of CAR Treg for ulcerative colitis amelioration and colon cancer hindering. The designed CAR model targeted the membrane proximal A3 domain of the carcinoembryonic antigen molecule (Fig. 4D), which is overexpressed in colitis and colon cancer, leading to significantly reduced inflammation and lower tumor burden.
Multiple sclerosis (MS), an autoimmune demyelinating disease that affects the central nervous system (CNS) and leads to significant morbidity, has also been subject of interest in CAR-T therapy development. In a murine experimental autoimmune encephalomyelitis (EAE) model of MS, CNS-targeted CAR-Treg cells were effective in suppressing inflammation and reducing symptoms [70]. Interestingly, the CAR construct CARαMOG-FoxP3, which included a scFv anti-rat myelin oligodendrocyte glycoprotein (Fig. 4E), was also coupled in trans to the murine FoxP3 gene, separated by a 2A peptide. Forcing FoxP3 in this strategy aims to lock T cells in the regulatory phenotype, avoiding plasticity-derived phenotypes that could drive the cells toward a proinflammatory profile. A lentiviral vector was used to transduce murine naive CD4 cells, which were later delivered intranasally to treat EAE. CAR-Treg cells could reach various brain areas and suppress inflammation, as demonstrated by reduced levels of IFN-g mRNA in the brain and decreased demyelination as assessed by immunohistochemical markers. The benefits persisted even after rechallenging with a second EAE-inducing inoculum, showing the persistence of cells. Very importantly, the Treg cells retained their suppressive phenotype even after challenged with lipopolysaccharides (LPS)-stimulated macrophages, thought to reverse the Treg phenotype into an effector one. These findings show a promising new treatment strategy for MS and warrant further studies [70].
The main concern regarding the Treg strategy is the possibility that the inflammatory milieu could convert the Treg cells into T effector cells, with catastrophic consequences [71]. A few strategies have been proposed to overcome this issue, such as good manufacturing practices quality control using Treg cell-specific demethylated region as a marker for effector T cell conversion potential [72]. Suicide switches linked to Foxp3 inactivation or inflammatory cytokines expression are also envisioned [73]. Another strategy regards modulation of signal strength, since Tregs demand lower TCR affinity for activation [74]. Although promising, further investigation of this aspect is paramount for safe clinical use of CAR Treg cells.
CAR-T cells in fibrotic diseases: emerging applications
Cardiac diseases remain one of the leading mortality causes in the world [75]. Most myocardial diseases lead to cardiac fibrosis, which diminishes organ function and contributes to progression of heart failure [76]. Fibrosis reversing therapies are extremely limited and novel treatment strategies are needed. Recently, Aghajanian et al. identified an endogenous target for cardiac fibroblasts – the fibroblast activation protein (FAP) – through gene signature analysis of healthy and diseased human hearts [76]. In a murine model of cardiac injury induced by angiotensin II and phenylephrine, a FAP-targeted CD8 CAR-T cell (Fig. 5A) was capable of significantly reducing fibrosis and restoring cardiac function post-injury. Interestingly, CAR-T cells targeting FAP were previously described in 2013 by Tran et al. [77] to target solid tumors. As stromal fibroblasts are highly present in the tumor microenvironment and contribute to progression and metastasis, FAP-expressing stromal cells were an attractive target for CAR-based therapy. However, third-generation FAP-targeting CAR-T cells in this study showed limited antitumor activity and induction of cachexia and other toxicities, which were attributed to the expression of FAP on bone marrow stromal cells that could also be recognized in an on-target/off-tumor fashion [77]. Nonetheless, shortly after this first study, three other reports using FAP-targeting CAR-T cells were published that contrasted the previous results. Kakarla et al. [78] used an anti-FAP CAR specific for both murine and human FAP with a CD28 costimulatory domain against murine lung cancer models and saw a decrease in tumor growth that could be further enhanced by co-treatment with tumor-targeting cells. Accordingly, Schubert et al. [79] also reported in vitro and in vivo functionality of anti-FAP CAR-T cells in a mesothelioma xenograft model. In an additional example, Wang et al. [80] developed a second-generation CAR with the 41BB activation domain specific for murine FAP and tested it against mesothelioma and lung cancer mouse models. The authors reported that the CAR-T cells showed specific cytotoxicity against FAP+ cells that resulted in inhibited tumor growth. Importantly, both Kakarla et al. and Wang et al. reported no clinical toxicity. The reasons behind the different toxic effects seen in these studies are still not clear.
Figure 5.
CAR-T cells for cardiac fibrosis and senescence-associated liver fibrosis. (A) Anti-FAP second-generation CAR-T cells were recently proposed as a treatment strategy for cardiac fibrosis, as FAP is highly expressed in cardiac fibroblasts. (B) A novel approach using a uPAR-targeting CAR brings to light the possibility of driving T cells against senescent cells present in several diseases, specifically liver fibrosis, in which senescent hepatic stellate cells play an important role.
In the study by Aghajanian et al. targeting cardiac fibrosis, no off-target effect was observed in non-cardiac organs, neither delay in wound healing. Notably, perivascular fibrosis persisted, consistent with the absence of FAP expression in perivascular fibroblasts. Overall, it seems that the anti-FAP CAR approach has good potential for clinical application both for malignant and non-malignant conditions. Considering the prevalence of cardiac disease, notably the ischemic type, in the population, the development of such an approach is of great need.
Recently, great attention has been brought by the possibility of using CAR-T cells to target cellular senescence and reversing senescence-associated pathologies, as shown by Amor et al. [81]. Cellular senescence is a well-characterized state consisting of stable cell cycle arrest and a secretory program that modulates the tissue microenvironment [82]. Senescence is a physiological process that prevents pre-malignant cells proliferation and promotes wound healing. However, pathological accumulation of senescent cells associated with secreted pro-inflammatory factors can lead to tissue inflammation and damage, which empowers progression of degenerative diseases, such as atherosclerosis, diabetes, osteoarthritis, lung, and liver fibrosis. Through gene expression analysis of three distinct senescence models, urokinase-type plasminogen activator (uPAR) was identified as a cell-surface protein consistently and specifically upregulated in senescent cells. A second-generation uPAR-specific CAR was constructed (Fig. 5B), using an anti-mouse uPAR single-chain variable fragment linked to the CD28 costimulatory molecule and the CD3z signaling domain [81]. Those CAR-T cells were effective in ablating senescent cells in vitro and in vivo using murine models of lung adenocarcinoma treated with senescence inducing drugs and two models of liver fibrosis (chemical- and diet-induced), in which senescent hepatic stellate cells contribute to disease progression. Elimination of senescent cells translated into improved survival in a lung cancer model, and restored liver function, without notable toxicities in the proposed doses. Even though it is quite early for clinical application and further studies to address safety are needed, this model shows that senolytic CAR-T cells may be a feasible treatment for several senescence-associated pathologies.
Conclusion and perspectives
In the last decade, enormous advances have been achieved with anti-tumor CAR-T cell therapy, most notably in fighting hematological malignancies. However, CAR-T technology has great potential for broad clinical use in the non-malignant setting as well, especially for infectious and immune-mediated diseases. As discussed in this review, even though CAR-T cell therapy for HIV cure so far has been unsuccessful, several new concepts have been developed which deserve further exploration. Besides HIV, CAR-T cells targeted against HBV, HCV, CMV, EBV, and Aspergillus have been designed but are still in an early preclinical stage, with limited efficacy demonstrated so far. CAR-T development for other infectious diseases like tuberculosis has been proposed [83], but its feasibility is yet to be determined. For autoimmune diseases, CAR-T cell therapy brings a palpable opportunity for ameliorating or even curing a variety of chronic and degenerative diseases, with ongoing clinical trials. Furthermore, with the recently highlighted ability to target pathological fibrosis and cellular senescence, two processes deeply connected to chronic inflammation and cancer, the horizon of possibilities gets even wider.
Acknowledgements
We would like to thank Dr. Leonardo Chicaybam and Dr. Adriana Bonomo for the critical reading and suggestions made to this manuscript. The Editor-in-Chief and editorial team would like to thank the handling editor, Tao Dong, and the following reviewers, Jessica Borger and Eliane Piaggio, for their contribution to the publication of this article.
Glossary
Abbreviations
- BLT
Bone marrow, liver, and thymus
- BCMA
B cell maturation antigen
- bNAbs
Broadly neutralizing antibodies
- CAR
Chimeric antigen receptor
- CAAR
Chimeric auto-antibody receptor
- cART
Combination antiretroviral therapy
- CMV
Cytomegalovirus
- CNS
Central nervous system
- CTLs
Cytotoxic T lymphocytes
- CXCR5
CX chemokine receptor 5
- CXCL13
CXC chemokine ligand 13
- Dsg3
Desmoglein 3
- EAE
Encephalomyelitis
- EBV
Epstein-Barr virus
- Env
Envelope protein
- FAP
Fibroblast activation protein
- HDR
Homology-directed repair
- HIV
Human immunodeficiency virus
- HSPC
Hematopoietic stem and progenitor cells
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- LMP1
EBV latent membrane protein 1
- LRA
Latency reversing agent
- MS
Multiple sclerosis
- NSG
NOD Scid-γ
- PV
Pemphigus vulgaris
- scFv
Single-chain variable fragment
- SLE
Systemic lupus erythematosus
- Tfh
Follicular helper T cells
- TNP
2,4,6-Trinitrophenol
- Treg
Regulatory T cell
- uPAR
Urokinase-type plasminogen activator
Contributor Information
Mariana Torres Mazzi, Immunology and Tumor Biology Program - Research Coordination, Brazilian National Cancer Institute (INCA), Rio de Janeiro, Brazil.
Karina Lôbo Hajdu, Immunology and Tumor Biology Program - Research Coordination, Brazilian National Cancer Institute (INCA), Rio de Janeiro, Brazil.
Priscila Rafaela Ribeiro, Immunology and Tumor Biology Program - Research Coordination, Brazilian National Cancer Institute (INCA), Rio de Janeiro, Brazil.
Martín Hernán Bonamino, Immunology and Tumor Biology Program - Research Coordination, Brazilian National Cancer Institute (INCA), Rio de Janeiro, Brazil; Vice - Presidency of Research and Biological Collections (VPPCB), Oswaldo Cruz Foundation (FIOCRUZ), Rio de Janeiro, Brazil.
Funding
K.L.H. receives a scholarship from the Brazilian Ministry of Health. M.H.B receives scholarships from the Brazilian National Council for Scientific and Technological Development (CNPq), and Rio de Janeiro Foundation for Research Support (FAPERJ). P.R.R. received a scholarship from the Coordination of Superior Level Staff Improvement (CAPES).
Author contributions
M.T.M., K.L.H., and P.R.R. wrote the original draft. M.T.M., K.L.H., P.R.R., and M.H.B. edited the manuscript and wrote the final version.
Conflict of interest
The authors declare no conflict of interest.
Data availability
No new data were generated or analyzed in support of this research.
References
- 1. Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl 1953;276:1–103. [PubMed] [Google Scholar]
- 2. Grillo-López AJ, White CA, Dallaire BK, Varns CL, Shen CD, Wei A, Leonard JE, McClure A, Weaver R, Cairelli S, Rosenberg J. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr Pharm Biotechnol. 2000. Jul;1(1):1–9. 10.2174/1389201003379059 [DOI] [PubMed] [Google Scholar]
- 3. Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH, Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012. Jun 20;30(18):2183–9. 10.1200/JCO.2011.38.0410. Epub 2012 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cameron F, Whiteside G, Perry C. Ipilimumab: first global approval. Drugs. 2011. May 28;71(8):1093–104. 10.2165/11594010-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 5. Jen EY, Xu Q, Schetter A, Przepiorka D, Shen YL, Roscoe D, Sridhara R, Deisseroth A, Philip R, Farrell AT, Pazdur R. FDA Approval: Blinatumomab for Patients with B-cell Precursor Acute Lymphoblastic Leukemia in Morphologic Remission with Minimal Residual Disease. Clin Cancer Res. 2019. Jan 15;25(2):473–477. 10.1158/1078-0432.CCR-18-2337. Epub 2018 Sep 25. [DOI] [PubMed] [Google Scholar]
- 6. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014. Oct 16;371(16):1507–17. 10.1056/NEJMoa1407222. Erratum in: N Engl J Med. 2016. Mar 10;374(10): 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993. Jan 15;90(2):720–4. 10.1073/pnas.90.2.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Sun J, Liu H et al. Immunotherapy deriving from CAR-T Cell treatment in autoimmune diseases. J Immunol Res 2019;2019:5727516. 10.1155/2019/5727516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–54. 10.1517/14712598.2015.1046430. Epub 2015 May 18. [DOI] [PubMed] [Google Scholar]
- 10. Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000. Aug 1;96(3):785–93. [PubMed] [Google Scholar]
- 11. Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C; ANRS VISCONTI Study Group . Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013. Mar;9(3):e1003211. 10.1371/journal.ppat.1003211. Epub 2013 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991. Mar 8;64(5):1037–46. 10.1016/0092-8674(91)90327-u [DOI] [PubMed] [Google Scholar]
- 13. Roberts MR, Qin L, Zhang D, Smith DH, Tran AC, Dull TJ, Groopman JE, Capon DJ, Byrn RA, Finer MH. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994. Nov 1;84(9):2878–89. [PubMed] [Google Scholar]
- 14. Walker RE, Bechtel CM, Natarajan V, Baseler M, Hege KM, Metcalf JA, Stevens R, Hazen A, Blaese RM, Chen CC, Leitman SF, Palensky J, Wittes J, Davey RT Jr, Falloon J, Polis MA, Kovacs JA, Broad DF, Levine BL, Roberts MR, Masur H, Lane HC. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000. Jul 15;96(2):467–74. [PubMed] [Google Scholar]
- 15. Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, Macken C, Richman DD, Christopherson C, June CH, Lazar R, Broad DF, Jalali S, Hege KM. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002. Jun;5(6):788–97. 10.1006/mthe.2002.0611 [DOI] [PubMed] [Google Scholar]
- 16. Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012. May 2;4(132):132ra53. 10.1126/scitranslmed.3003761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chicaybam L, Bonamino MH, Luckow Invitti A et al. Overhauling CAR T cells to improve efficacy, safety and cost. Cancers (Basel). 2020;12(9):2360. 10.3390/cancers12092360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhlmann AS, Peterson CW, Kiem HP. Chimeric antigen receptor T-cell approaches to HIV cure. Curr Opin HIV AIDS. 2018. Sep;13(5):446–453. 10.1097/COH.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ali A, Kitchen SG, Chen ISY, Ng HL, Zack JA, Yang OO. HIV-1-Specific Chimeric Antigen Receptors Based on Broadly Neutralizing Antibodies. J Virol. 2016. Jul 11;90(15):6999–7006. 10.1128/JVI.00805-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hale M, Mesojednik T, Romano Ibarra GS, Sahni J, Bernard A, Sommer K, Scharenberg AM, Rawlings DJ, Wagner TA. Engineering HIV-Resistant, Anti-HIV Chimeric Antigen Receptor T Cells. Mol Ther. 2017. Mar 1;25(3):570–579. 10.1016/j.ymthe.2016.12.023. Epub 2017 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu B, Zou F, Lu L, Chen C, He D, Zhang X, Tang X, Liu C, Li L, Zhang H. Chimeric Antigen Receptor T Cells Guided by the Single-Chain Fv of a Broadly Neutralizing Antibody Specifically and Effectively Eradicate Virus Reactivated from Latency in CD4+ T Lymphocytes Isolated from HIV-1-Infected Individuals Receiving Suppressive Combined Antiretroviral Therapy. J Virol. 2016. Oct 14;90(21):9712–9724. 10.1128/JVI.00852-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maier DA, Brennan AL, Jiang S, Binder-Scholl GK, Lee G, Plesa G, Zheng Z, Cotte J, Carpenito C, Wood T, Spratt SK, Ando D, Gregory P, Holmes MC, Perez EE, Riley JL, Carroll RG, June CH, Levine BL. Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5. Hum Gene Ther. 2013. Mar;24(3):245–58. 10.1089/hum.2012.172. Epub 2013 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Didigu CA, Wilen CB, Wang J, Duong J, Secreto AJ, Danet-Desnoyers GA, Riley JL, Gregory PD, June CH, Holmes MC, Doms RW. Simultaneous zinc-finger nuclease editing of the HIV coreceptors ccr5 and cxcr4 protects CD4+ T cells from HIV-1 infection. Blood. 2014. Jan 2;123(1):61–9. 10.1182/blood-2013-08-521229. Epub 2013 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008. Jul;26(7):808–16. 10.1038/nbt1410. Epub 2008 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang WT, Levine BL, June CH. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014. Mar 6;370(10):901–10. 10.1056/NEJMoa1300662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leslie GJ, Wang J, Richardson MW, Haggarty BS, Hua KL, Duong J, Secreto AJ, Jordon AP, Romano J, Kumar KE, DeClercq JJ, Gregory PD, June CH, Root MJ, Riley JL, Holmes MC, Hoxie JA. Potent and Broad Inhibition of HIV-1 by a Peptide from the gp41 Heptad Repeat-2 Domain Conjugated to the CXCR4 Amino Terminus. PLoS Pathog. 2016. Nov 17;12(11):e1005983. 10.1371/journal.ppat.1005983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maldini CR, Claiborne DT, Okawa K, Chen T, Dopkin DL, Shan X, Power KA, Trifonova RT, Krupp K, Phelps M, Vrbanac VD, Tanno S, Bateson T, Leslie GJ, Hoxie JA, Boutwell CL, Riley JL, Allen TM. Dual CD4-based CAR T cells with distinct costimulatory domains mitigate HIV pathogenesis in vivo. Nat Med. 2020. Nov;26(11):1776–1787. 10.1038/s41591-020-1039-5. Epub 2020 Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhen A, Rezek V, Youn C et al. Stem-cell based engineered immunity against HIV infection in the humanized mouse model. J Vis Exp 2016;(113):54048. 10.3791/54048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhen A, Kamata M, Rezek V, Rick J, Levin B, Kasparian S, Chen IS, Yang OO, Zack JA, Kitchen SG. HIV-specific Immunity Derived From Chimeric Antigen Receptor-engineered Stem Cells. Mol Ther. 2015. Aug;23(8):1358–1367. 10.1038/mt.2015.102. Epub 2015 Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhen A, Peterson CW, Carrillo MA, Reddy SS, Youn CS, Lam BB, Chang NY, Martin HA, Rick JW, Kim J, Neel NC, Rezek VK, Kamata M, Chen ISY, Zack JA, Kiem HP, Kitchen SG. Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. PLoS Pathog. 2017. Dec 28;13(12):e1006753. 10.1371/journal.ppat.1006753. Erratum in: PLoS Pathog. 2018. Mar 12;14 (3): e1006891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang X, Wong CW, Urak R, Mardiros A, Budde LE, Chang WC, Thomas SH, Brown CE, La Rosa C, Diamond DJ, Jensen MC, Nakamura R, Zaia JA, Forman SJ. CMVpp65 Vaccine Enhances the Antitumor Efficacy of Adoptively Transferred CD19-Redirected CMV-Specific T Cells. Clin Cancer Res. 2015. Jul 1;21(13):2993–3002. 10.1158/1078-0432.CCR-14-2920. Epub 2015 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun J, Huye LE, Lapteva N, Mamonkin M, Hiregange M, Ballard B, Dakhova O, Raghavan D, Durett AG, Perna SK, Omer B, Rollins LA, Leen AM, Vera JF, Dotti G, Gee AP, Brenner MK, Myers DG, Rooney CM. Early transduction produces highly functional chimeric antigen receptor-modified virus-specific T-cells with central memory markers: a Production Assistant for Cell Therapy (PACT) translational application. J Immunother Cancer. 2015. Feb 18;3:5. 10.1186/s40425-015-0049-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakazawa Y, Huye LE, Salsman VS, Leen AM, Ahmed N, Rollins L, Dotti G, Gottschalk SM, Wilson MH, Rooney CM. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol Ther. 2011. Dec;19(12):2133–43. 10.1038/mt.2011.131. Epub 2011 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang H, Wallace Z, Dorrell L. Therapeutic Targeting of HIV Reservoirs: How to Give T Cells a New Direction. Front Immunol. 2018. Dec 4;9:2861. 10.3389/fimmu.2018.02861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014. Oct;1(1):e13–21. 10.1016/S2352-3018(14)70014-1. Epub 2014 Sep 15. [DOI] [PubMed] [Google Scholar]
- 36. Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog. 2015. Sep 17;11(9):e1005142. 10.1371/journal.ppat.1005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, Emad F, Buckheit R 3rd, McCance-Katz EF, Lai J, Kennedy M, Chander G, Siliciano RF, Siliciano JD, Deeks SG. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014. Mar;58(6):883–90. 10.1093/cid/cit813. Epub 2013 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012. Mar 23;36(3):491–501. 10.1016/j.immuni.2012.01.014. Epub 2012 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maldini CR, Ellis GI, Riley JL. CAR T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol. 2018. Oct;18(10):605–616. 10.1038/s41577-018-0042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haran KP, Hajduczki A, Pampusch MS, Mwakalundwa G, Vargas-Inchaustegui DA, Rakasz EG, Connick E, Berger EA, Skinner PJ. Simian Immunodeficiency Virus (SIV)-Specific Chimeric Antigen Receptor-T Cells Engineered to Target B Cell Follicles and Suppress SIV Replication. Front Immunol. 2018. Mar 20;9:492. 10.3389/fimmu.2018.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sautto GA, Wisskirchen K, Clementi N, Castelli M, Diotti RA, Graf J, Clementi M, Burioni R, Protzer U, Mancini N. Chimeric antigen receptor (CAR)-engineered T cells redirected against hepatitis C virus (HCV) E2 glycoprotein. Gut. 2016. Mar;65(3):512–23. 10.1136/gutjnl-2014-308316. Epub 2015 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sautto G, Tarr AW, Mancini N, Clementi M. Structural and antigenic definition of hepatitis C virus E2 glycoprotein epitopes targeted by monoclonal antibodies. Clin Dev Immunol. 2013;2013:450963. 10.1155/2013/450963. Epub 2013 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bohne F, Chmielewski M, Ebert G, Wiegmann K, Kürschner T, Schulze A, Urban S, Krönke M, Abken H, Protzer U. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology. 2008. Jan;134(1):239–47. 10.1053/j.gastro.2007.11.002. Epub 2007 Nov 4. [DOI] [PubMed] [Google Scholar]
- 44. Kruse RL, Shum T, Tashiro H, Barzi M, Yi Z, Whitten-Bauer C, Legras X, Bissig-Choisat B, Garaigorta U, Gottschalk S, Bissig KD. HBsAg-redirected T cells exhibit antiviral activity in HBV-infected human liver chimeric mice. Cytotherapy. 2018. May;20(5):697–705. 10.1016/j.jcyt.2018.02.002. Epub 2018 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bertoletti A, Tan AT. Challenges of CAR- and TCR-T cell-based therapy for chronic infections. J Exp Med 2020;217(5):e20191663. 10.1084/jem.20191663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Full F, Lehner M, Thonn V, Goetz G, Scholz B, Kaufmann KB, Mach M, Abken H, Holter W, Ensser A. T cells engineered with a cytomegalovirus-specific chimeric immunoreceptor. J Virol. 2010. Apr;84(8):4083–8. 10.1128/JVI.02117-09. Epub 2010 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Proff J, Walterskirchen C, Brey C, Geyeregger R, Full F, Ensser A, Lehner M, Holter W. Cytomegalovirus-Infected Cells Resist T Cell Mediated Killing in an HLA-Recognition Independent Manner. Front Microbiol. 2016. Jun 9;7:844. 10.3389/fmicb.2016.00844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Domselaar R, de Poot SA, Remmerswaal EB, Lai KW, ten Berge IJ, Bovenschen N. Granzyme M targets host cell hnRNP K that is essential for human cytomegalovirus replication. Cell Death Differ. 2013. Mar;20(3):419–29. 10.1038/cdd.2012.132. Epub 2012 Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang X, Zhou Y, Li W, Tang Q, Chen R, Zhu J, Feng Z. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J Biomed Res. 2014. Nov;28(6):468–75. 10.7555/JBR.28.20140066. Epub 2014 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Verweij FJ, van Eijndhoven MA, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, Kieff E, Geerts D, van der Kant R, Neefjes J, Middeldorp JM, Pegtel DM. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 2011. Jun 1;30(11):2115–29. 10.1038/emboj.2011.123. Epub 2011 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang X, Tang Q, Mao Y, Huang X, Jia L, Zhu J, Feng Z. CD137 Co-Stimulation Improves The Antitumor Effect Of LMP1-Specific Chimeric Antigen Receptor T Cells In Vitro And In Vivo. Onco Targets Ther. 2019. Nov 7;12:9341–9350. 10.2147/OTT.S221040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, Roszik J, Rabinovich B, Olivares S, Krishnamurthy J, Zhang L, Najjar AM, Huls MH, Lee DA, Champlin RE, Kontoyiannis DP, Cooper LJ. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci U S A. 2014. Jul 22;111(29):10660–5. 10.1073/pnas.1312789111. Epub 2014 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006. Jan;6(1):33–43. 10.1038/nri1745 [DOI] [PubMed] [Google Scholar]
- 54. Bode K, Bujupi F, Link C, Hein T, Zimmermann S, Peiris D, Jaquet V, Lepenies B, Weyd H, Krammer PH. Dectin-1 Binding to Annexins on Apoptotic Cells Induces Peripheral Immune Tolerance via NADPH Oxidase-2. Cell Rep. 2019. Dec 24;29(13):4435–4446.e9. 10.1016/j.celrep.2019.11.086 [DOI] [PubMed] [Google Scholar]
- 55. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008. Feb 28;358(9):929–39. 10.1056/NEJMra071297 [DOI] [PubMed] [Google Scholar]
- 56. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, Zhang D, Brunetta PG. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010. Jan;62(1):222–33. 10.1002/art.27233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P, Dall’Era M, Looney RJ, Rovin B, Dragone L, Brunetta P. Peripheral Blood B Cell Depletion after Rituximab and Complete Response in Lupus Nephritis. Clin J Am Soc Nephrol. 2018. Oct 8;13(10):1502–1509. 10.2215/CJN.01070118. Epub 2018 Aug 8. Erratum in: Clin J Am Soc Nephrol. 2019. Jan 7;14(1): 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007. Sep 1;179(5):3351–61. 10.4049/jimmunol.179.5.3351 [DOI] [PubMed] [Google Scholar]
- 59. Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007. Sep 1;179(5):3351–61. 10.4049/jimmunol.179.5.3351 [DOI] [PubMed] [Google Scholar]
- 60. Kansal R, Richardson N, Neeli I et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019; 11(482):eaav1648. 10.1126/scitranslmed.aav1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jin X, Xu Q, Pu C et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2020. 10.1038/s41423-020-0472-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kawalekar OU, O’ Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J, Keith B, Snyder NW, Blair IA, Milone MC, June CH. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016. Mar 15;44(3):712. 10.1016/j.immuni.2016.02.023. Epub 2016 Mar 15. Erratum for: Immunity. 2016. Feb 16;44(2): 380–90. [DOI] [PubMed] [Google Scholar]
- 63. Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015. Oct 12;28(4):415–428. 10.1016/j.ccell.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin L, Xing L, Acharya CM et al. CD8+ Anti-BCMA mRNA CAR T-cells effectively kill human multiple myeloma cells in vitro and in vivo. Blood 2017;130(Supplement 1):3067. 10.1182/blood.V130.Suppl_1.3067.3067 [DOI] [Google Scholar]
- 65. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, Lam LP, Morgan RA, Friedman K, Massaro M, Wang J, Russotti G, Yang Z, Campbell T, Hege K, Petrocca F, Quigley MT, Munshi N, Kochenderfer JN. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019. May 2;380(18):1726–1737. 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, Milone MC, Payne AS. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016. Jul 8;353(6295):179–84. 10.1126/science.aaf6756. Epub 2016 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Colliou N, Picard D, Caillot F, Calbo S, Le Corre S, Lim A, Lemercier B, Le Mauff B, Maho-Vaillant M, Jacquot S, Bedane C, Bernard P, Caux F, Prost C, Delaporte E, Doutre MS, Dreno B, Franck N, Ingen-Housz-Oro S, Chosidow O, Pauwels C, Picard C, Roujeau JC, Sigal M, Tancrede-Bohin E, Templier I, Eming R, Hertl M, D’Incan M, Joly P, Musette P. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013. Mar 6;5(175):175ra30. 10.1126/scitranslmed.3005166 [DOI] [PubMed] [Google Scholar]
- 68. Elinav E, Adam N, Waks T, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009. May;136(5):1721–31. 10.1053/j.gastro.2009.01.049. Epub 2009 Jan 27. [DOI] [PubMed] [Google Scholar]
- 69. Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther. 2014. May;22(5):1018–28. 10.1038/mt.2014.41. Epub 2014 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fransson M, Piras E, Burman J. et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation. 9, 112 (2012). https://doi.org/ 10.1186/1742-2094-9-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009. Sep;10(9):1000–7. 10.1038/ni.1774. Epub 2009 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008. Jun;38(6):1654–63. 10.1002/eji.200838105 [DOI] [PubMed] [Google Scholar]
- 73. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016. Apr 20;3:16011. 10.1038/mto.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Plesa G, Zheng L, Medvec A, Wilson CB, Robles-Oteiza C, Liddy N, Bennett AD, Gavarret J, Vuidepot A, Zhao Y, Blazar BR, Jakobsen BK, Riley JL. TCR affinity and specificity requirements for human regulatory T-cell function. Blood. 2012. Apr 12;119(15):3420–30. 10.1182/blood-2011-09-377051. Epub 2012 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva; World Health Organization; 2018. [Google Scholar]
- 76. Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA. Targeting cardiac fibrosis with engineered T cells. Nature. 2019. Sep;573(7774):430–433. 10.1038/s41586-019-1546-z. Epub 2019 Sep 11. Erratum in: Nature. 2019. Dec;576(7785): E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tran E, Chinnasamy D, Yu Z, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210(6):1125–1135. doi: 10.1084/jem.20130110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kakarla S, Chow KK, Mata M, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21(8):1611–1620. 10.1038/mt.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schuberth PC, Hagedorn C, Jensen SM, Gulati P, van den Broek M, Mischo A, Soltermann A, Jüngel A, Marroquin Belaunzaran O, Stahel R, Renner C, Petrausch U. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med. 2013. Aug 12;11:187. 10.1186/1479-5876-11-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, Antzis M, Cotner CE, Johnson LA, Durham AC, Solomides CC, June CH, Puré E, Albelda SM. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014. Feb;2(2):154–66. 10.1158/2326-6066.CIR-13-0027. Epub 2013 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, Kulick A, Houlihan S, Peerschke E, Friedman SL, Ponomarev V, Piersigilli A, Sadelain M, Lowe SW. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020. Jul;583(7814):127–132. 10.1038/s41586-020-2403-9. Epub 2020 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017. Jun 1;169(6):1000–1011. 10.1016/j.cell.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Parida SK, Poiret T, Zhenjiang L, Meng Q, Heyckendorf J, Lange C, Ambati AS, Rao MV, Valentini D, Ferrara G, Rangelova E, Dodoo E, Zumla A, Maeurer M. T-Cell Therapy: Options for Infectious Diseases. Clin Infect Dis. 2015. Oct 15;61(Suppl 3):S217–24. 10.1093/cid/civ615 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.