Abstract
Objective
To evaluate the effect of myopia on retinal vascular bifurcation.
Methods
A cross-sectional study that retrospectively analyzed the fundus photographs and clinical data of 493 people who participated in routine physical examinations in Huadong Sanatorium. One eye of each subject was included in the analysis. Retinal vascular bifurcation measurements were extracted by using a validated computer program. One-way ANOVA and analysis of covariance were performed to compare the measurements across high myopia, low to moderate myopia, and non-myopia groups.
Results
The mean age was 41.83 ± 10.43 years and 63.49% were women. The mean spherical equivalent refraction (SER) was − 4.59 ± 3.07 D. Ninety-nine (20.08%) eyes met the definition of high myopia (SER ≤ -6.0 D), along with 234 (47.46%) low to moderate myopia (-6.0 D < SER <-0.5 D), and 160 (32.45%) non-myopia (SER ≥ -0.5 D). The differences in the arteriolar branching angle, venular branching coefficient, venular asymmetry ratio, venular angular asymmetry, and venular junctional exponent among the three groups remained significant (p < 0.05) after multivariate adjustment. Pairwise comparisons showed arteriolar branching angle and venular angular asymmetry in high myopia were significantly lower than low to moderate myopia (p < 0.001, p = 0.014 respectively) and non-myopia (p = 0.007, p = 0.048 respectively). Venular asymmetry ratio and venular branching coefficient in high myopia were significantly higher than low to moderate myopia (p = 0.029, p = 0.001 respectively) and non-myopia (p = 0.041, p = 0.043 respectively). There was a significant difference in venular junctional exponent between high myopia and low to moderate myopia (p = 0.031).
Conclusion
The vascular bifurcation differs in dependence on the myopic refractive error and a significant increase in the difference can be observed in high myopic eyes.
Introduction
A healthy vascular network is essential for efficient blood transport to various parts of the body, and damage to the network may cause ischemia, leakage, and other pathologic conditions which then lead to many diseases. The retinal vascular network can be directly observed in vivo and has therefore been studied widely in expectation of finding predictors of various diseases. Studies have shown that the retinal vascular network geometry changes are an important sign of vascular damage, which are associated with a variety of systemic and ocular diseases, such as hypertension [1], diabetes [2], cerebral small vessel disease [3], dementia [4], diabetic retinopathy [5], and glaucoma [6].
In myopic eyes, the expansion of sclera stretches and thins the underlying choroid and retina [7], which is responsible for the myopia-related retinal vascular alterations, for instance, narrower arterioles and venules [8–10], higher length to diameter ratios of retinal arterioles [11], straighter arterioles [9], and decreased vascular fractal dimension [12]. However, the relationship between myopia and vascular bifurcation remains controversial. Patton et al. [8] found no association of axial length (AL) with the angles at vessel bifurcations and junctional exponents in a small pseudophakic population. However, Lim et al. [9] demonstrated in a middle-aged and older Malay population that increased myopic refractive errors and longer AL were associated with more acute branching angles in arterioles, and increased branching coefficients (BC; quotient of the area of the branch and trunk vessels, Table 1) in both arterioles and venules. The discrepancy in the results may be due in part to potential or residual confounding. Aging, cardiovascular disease, cardio-active medications, smoking, etc. are known to be associated with the retinal vascular geometry, and are not possibly fully adjusted in a relatively older and heterogeneous study population, which in turn hinders our understanding of the relationship between myopia and retinal vascular geometry. In addition, if the bifurcation parameters change with the myopic elongation of the globe, a dose-response effect should be observed, which however has not been carefully studied yet.
Table 1.
Description of the retinal vascular bifurcation parameters measured for each retinal photograph
| Parameter | Abbreviation | Description |
|---|---|---|
| Branching angle | BA | Defined as the first angle subtended between two daughter vessels at each vascular bifurcation. |
| Branching coefficient | BC | Branching coefficient reflects the ratio between the diameters of the parent vessel and the diameters of its branches. Defined as the summed square of the mean vessel widths of each branch or daughter vessel divided by the square of the mean width of the parent vessel. |
| Asymmetry ratio | AF | Defined as the ratio of the square of the width of two branching vessel. |
| Angular asymmetry | AA | Defined as the difference between two daughter angles. |
| Junctional exponent | JE | Junctional exponent provides an index of caliber sizes of two daughter vessels relative to the parent vessel and is considered to represent an optimality state of microvascular networks [17]. It has been proposed that in an optimal state, the value of junctional exponent is three [17]. |
Therefore, we conducted a study investigating the differences in retinal vascular bifurcation parameters among high myopia, low to moderate myopia, and non-myopia in an otherwise healthy and relatively young population.
Methods
In this cross-sectional study, we retrospectively reviewed the fundus photographs and clinical data of 1100 people who participated in routine physical examinations in Huadong Sanatorium from June 2018 to December 2018. The present study was conducted in accordance with the ethical principles of the Declaration of Helsinki on medical research. The retrospective review of participants’ records and the waiver of consent were approved by the Medical Ethics Committee of Huadong Sanatorium. Subjects with the following conditions were excluded from this study: (1) History of cataract surgery, aphakic or pseudophakic, and self-reported refractive surgery in the selected eye (n = 0); (2) Diabetic retinopathy and other retinal vascular diseases in the selected eye (n = 2); (3) History of smoking, hypertension (medical history or meet the diagnostic criteria of hypertension[systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg] [13]), diabetes(medical history or glycosylated hemoglobin ≥ 6.5 mmol/l [14]) (n = 531); (4) Fundus images of poor quality, including those due to media opacities, small size of pupil or images out of focus (n = 74). Of the 1100 available people, 493 people were included in this study.
Each subject underwent a comprehensive ocular examination during physical examination, including pupillary examination, anterior segment examination, and complete retinal evaluation. Noncycloplegic autorefraction was performed using an auto-refractometer (Topcon KR-8900, Japan). Intraocular pressure was measured in mm Hg with a non-contact tonometer (Topcon CT-80 A, Japan). Digital fundus photography was taken by using a 45° digital retinal camera (Topcon-NW300; Japan) without mydriasis. Two retinal images of each eye were obtained, one centered at the optic disc and another centered at the fovea.
For each subject, we selected one optic disc-centered photograph from the eye with better photographic quality to perform measurement (when the photographic quality of both eyes was similar, the right eye was selected for analysis). The refractive state of this eye determined the refractive group that the subject was assigned to. Spherical equivalent refraction (SER) was calculated as the sum of the sphere power and half of the cylinder power, and the unit is diopter (D). Refractive errors were defined as high myopia (SER ≤-6.0 D) [15], low to moderate myopia ( -6.0 D < SER <-0.5 D) [15], and non-myopia (SER ≥ -0.5 D).
Measurements of retinal vascular bifurcation parameters
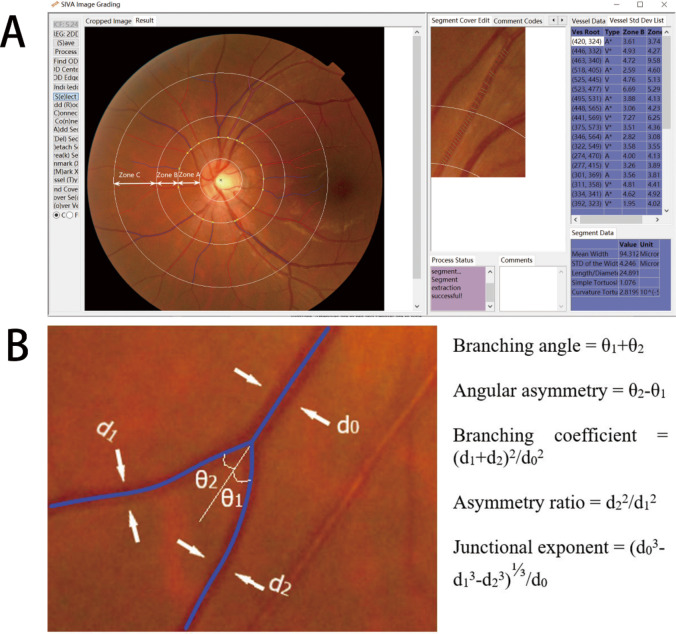
For each retinal photograph, a trained grader (SCX), masked to participants’ identities, used a semi-automated computer-assisted program (Singapore I Vessel Assessment [SIVA], version 4.0; National University of Singapore, Singapore) to quantitatively measure a series of retinal vascular bifurcation parameters. During image grading, SIVA divides the retina into three zones with reference to the optic disc location and size. Zone A, B, C are standardized and defined as the region from the disc margin to 1/2-disc diameter from the disc margin, 1/2-disc diameter to 1 disc diameter from the disc margin, and 1 disc diameter to 2-disc diameter from the disc margin, respectively (Fig. 1 A). Retinal vessel geometric parameters are measured and calculated based on the retinal vessels located in Zone B and C as measurements in these zones are more stable from curvature of the eye. A description of retinal bifurcation parameters is listed in Table 1 and depicted in Fig. 1B. Previous studies reported that SIVA produces repeatable measurements of the retinal vasculature in former preterm and term children and in adults; intra-observer and interobserver reproducibility were higher than 66% for vascular geometry [16].
Fig. 1.
(A) Retinal vascular geometric variables assessed quantitatively by the Singapore I Vessel Assessment software. Arterioles are in red and venules are in blue. (B) At each bifurcation, the width of the trunk vessel (d0) and its two branching vessels (d1 and d2, d1 > d2) are measured, as well as the branching angle (θ1 and θ2, θ1 < θ2)
Statistical analysis
Statistical analysis was performed using SPSS V.23.0 (SPSS Inc., Chicago, IL). The branching coefficient, branching angle, asymmetry ratio, and angular asymmetry were normally distributed among participants, as well as the logarithmically transformed junctional exponent. Descriptive statistics were presented as mean and standard deviation (SD) for normally distributed data.
Chi-square test (categorical variables) and one-way ANOVA (normally distributed continuous variables) were used to perform comparisons among different refractive error statuses. The multivariable-adjusted model was tested for each dependent variable with correction for confounding variables in analysis of covariance (ANCOVA). Age [18], glycosylated hemoglobin [19] (HbA1c), mean arterial pressure [1] (MAP, calculated as 1/3SBP + 2/3DBP [20]), body mass index [21] (BMI, calculated as weight in kilograms divided by the square of height in meters) and intraocular pressure [6] (IOP) were known to influence retinal vascular bifurcation parameters and included as covariates. In order to further identify which particular differences between pairs were significant, we used pairwise comparison to explore differences among three groups. Moreover, we used complete case analysis to deal with a small number of missing data on HbA1c (n = 19), BMI(n = 23), IOP(n = 7) and MAP (n = 4). The missing data was considered as missing completely at random. One-way ANOVA was performed after missing values were removed. Participants with missing data were excluded from analysis of covariance and pairwise comparisons.
Results
Retinal photographs of unilateral eyes from 493 people were included in this study. The mean age was 41.83 ± 10.43 year-old (ranged from 17 to 75 year-old ), with 180 men (36.51%) and 313 women (63.49%). The mean SER was − 4.59 ± 3.07 D. Ninety-nine (20.08%) eyes met the definition of high myopia (ranged from − 16.13 D to -6.00 D), along with 234 (47.46%) low to moderate myopia (ranged from − 5.88 D to -0.63 D), and 160 (32.45%) non-myopia (ranged from − 0.50 D to + 6.13 D). Systemic demographics and retinal vascular bifurcation parameters of the participants grouping by the refractive error status are summarized in Table 2. In general, myopic subjects were younger, and with lower HbA1c. Mean arteriolar branching angle, arteriolar branching coefficient, venular branching coefficient, venular asymmetry ratio, venular angular asymmetry, and arteriolar junctional exponent were significantly different among the three different refractive error status groups.
Table 2.
Clinical characteristics and retinal vascular bifurcation measurements, by refractive error status
| Parameter | High myopia | Low to moderate myopia | Non-myopia | p value |
|---|---|---|---|---|
| Number | 99 | 234 | 160 | |
| Age (years) | 38.67 (10.56) | 39.35 (8.95) | 45.48 (10.70) | < 0.001* |
| Female, n (%) | 62 (62.62%) | 142 (60.68%) | 109 (68.13%) | 0.486 |
| Spherical equivalent (D) | -8.62 (2.23) | -2.99 (1.44) | 0.26 (0.92) | < 0.001* |
| Intraocular pressure (mmHg) | 15.37 (2.76) | 15.40 (2.58) | 15.14 (2.46) | 0.750 |
| Body mass index (kg/m2) | 23.33 (3.78) | 22.48 (2.64) | 23.11 (2.84) | 0.208 |
| Glycosylated hemoglobin (%) | 5.30 (0.35) | 5.24 (0.28) | 5.41 (0.31) | < 0.001* |
| Total cholesterol (mmol/L) | 4.96 (0.68) | 4.95 (0.83) | 5.05 (0.81) | 0.299 |
| Systolic Blood Pressure (mmHg) | 115.01 (13.89) | 113.19 (12.35) | 115.19 (11.16) | 0.481 |
| Diastolic Blood Pressure (mmHg) | 71.04 (9.86) | 69.38 (8.36) | 69.67 (8.68) | 0.398 |
| Arteriolar branching angle (degree) | 70.11 (11.73) | 76.59 (11.41) | 75.30 (10.66) | < 0.001* |
| Venular branching angle (degree) | 74.51 (12.69) | 76.61 (9.94) | 75.97 (10.70) | 0.277 |
| Arteriolar branching coefficient | 1.48 (0.21) | 1.41 (0.18) | 1.47 (0.19) | 0.001* |
| Venular branching coefficient | 1.31 (0.18) | 1.26 (0.14) | 1.27 (0.15) | 0.014* |
| Arteriolar asymmetry ratio | 0.82 (0.07) | 0.82 (0.06) | 0.81 (0.07) | 0.112 |
| Venular asymmetry ratio | 0.74 (0.10) | 0.71 (0.10) | 0.72 (0.10) | 0.041* |
| Arteriolar angular asymmetry (degree) | 30.28 (13.52) | 33.76 (11.44) | 32.56 (11.25) | 0.051 |
| Venular angular asymmetry (degree) | 37.21 (13.55) | 41.39 (13.41) | 41.02 (12.46) | 0.026* |
| Log (arteriolar junctional exponent) | 0.41 (0.06) | 0.43 (0.05) | 0.41 (0.05) | 0.002* |
| Log (venular junctional exponent) | 0.45 (0.06) | 0.46 (0.05) | 0.46 (0.05) | 0.224 |
All data were expressed as mean (SD) or number (percentages), as appropriate. Chi-square test (categorical variables) and one-way ANOVA (normally distributed continuous variables) were used to perform comparisons. P-values were corrected for multiple comparisons using the Bonferroni method
*Statistical difference (p < 0.05) among participants with different refractive error status
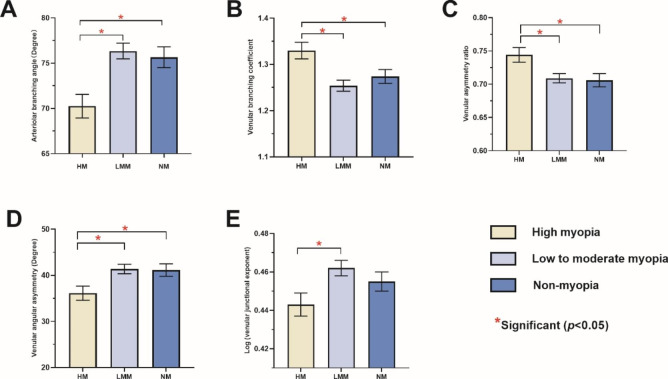
The differences in the arteriolar branching angle, venular branching coefficient, venular asymmetry ratio, venular angular asymmetry and venular junctional exponent among three groups remained significant after adjusting for age, HbA1c, MAP, BMI and IOP (Table 3). Figure 2 shows arteriolar branching angle and venular angular asymmetry in high myopia were significantly lower than low to moderate myopia (p < 0.001, p = 0.014 respectively) and non-myopia (p = 0.007, p = 0.048 respectively). Venular asymmetry ratio and venular branching coefficient in high myopia were significantly higher than moderate myopia (p = 0.029, p = 0.001 respectively) and non-myopia (p = 0.041, p = 0.043 respectively). There was a significant difference in venular junctional exponent between the high myopia group and the low to moderate myopia group (p = 0.031). However, the difference of venular junctional exponent in non-myopia and high myopia were not statistically significant (p = 0.504).
Table 3.
The comparison of the retinal vascular bifurcation measurements among participants with different refractive error statuses in multivariable-adjusted model
| Parameter | High myopia Adjusted mean (95% CI) |
Low to moderate myopia Adjusted mean (95% CI) |
Non-myopia Adjusted mean (95% CI) |
P |
|---|---|---|---|---|
| Arteriolar branching angle (degree) | 70.21 (67.64, 72.78) | 76.30 (74.60, 78.00) | 75.75 (73.49, 78.02) | < 0.001* |
| Venular branching angle (degree) | 74.47 (72.03, 76.92) | 76.80 (75.17, 78.42) | 76.13 (73.98, 78.28) | 0.299 |
| Arteriolar branching coefficient | 1.46 (1.42, 1.51) | 1.41 (1.39, 1.44) | 1.46 (1.42, 1.50) | 0.071 |
| Venular branching coefficient | 1.33 (1.30, 1.37) | 1.25 (1.23, 1.28) | 1.27 (1.24,1.30) | 0.001* |
| Arteriolar asymmetry ratio | 0.82 (0.81, 0.84) | 0.82 (0.81, 0.83) | 0.81 (0.80, 0.82) | 0.321 |
| Venular asymmetry ratio | 0.74 (0.72, 0.77) | 0.71 (0.69, 0.72) | 0.71 (0.69, 0.73) | 0.021* |
| Arteriolar angular asymmetry (degree) | 30.60 (27.89, 33.31) | 33.79 (31.99, 35.58) | 33.14 (30.75, 35.53) | 0.151 |
| Venular angular asymmetry (degree) | 36.10 (33.07, 39.13) | 41.35 (39.34, 43.37) | 41.19 (38.52, 43.85) | 0.014* |
| Log (arteriolar junctional exponent) | 0.41 (0.40, 0.43) | 0.42 (0.42, 0.43) | 0.42 (0.40, 0.43) | 0.251 |
| Log (venular junctional exponent) | 0.44 (0.43, 0.46) | 0.46 (0.45, 0.47) | 0.46 (0.44, 0.47) | 0.036* |
CI, confidence interval; Model adjusted for age, HbA1c, MAP, BMI, and IOP. P-values were corrected for multiple comparisons using the Bonferroni method
*Statistical difference (p < 0.05) among participants with different refractive error statuses
Fig. 2.
Bar charts showing the difference in the arteriolar branching angle (A), venular branching coefficient (B), venular asymmetry ratio (C), venular angular asymmetry (D), and log (venular junctional exponent deviation) (E) among high myopia, low to moderate myopia and non-myopia. Standard deviation bars are shown
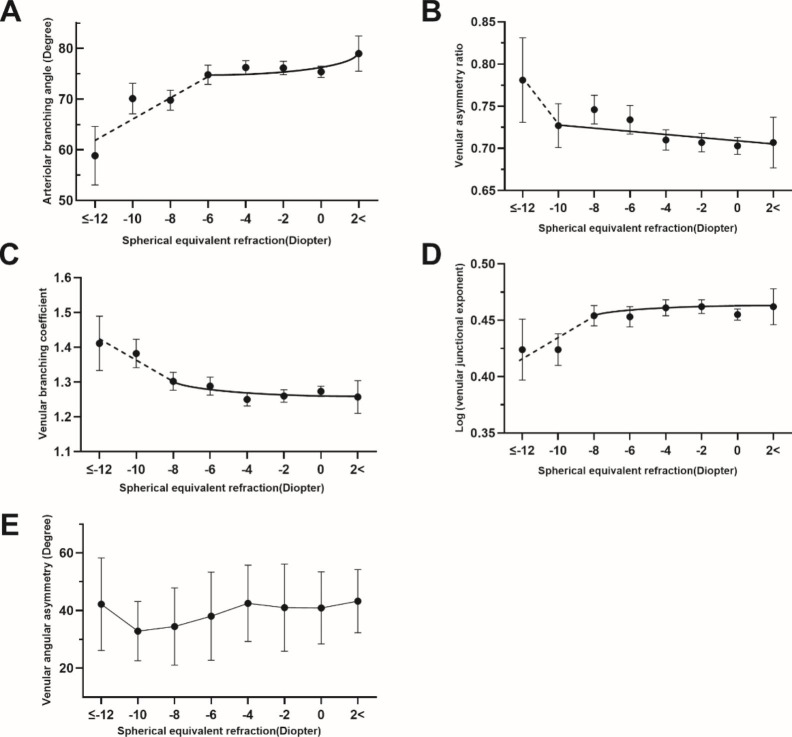
Figure 3 depicts the changes of arteriolar branching angle, venular asymmetry ratio, venular branching coefficient, venular junctional exponent, and venular asymmetry ratio with SER. It is observed that a sharp change of the slope occurred during the transition to higher myopia (roughly − 6D~-10D) in Fig. 3 (A ~ D) diagram. The slope for arteriolar branching angle by SER went steeper when SE ≤ -6D. For venular asymmetry ratio, venular branching coefficient, and venular junctional exponent, the cutoffs were − 10D, -8D, and − 8D, respectively. No clear cut-off point was observed for the chart of venular angular asymmetry (Fig. 3E).
Fig. 3.
Arteriolar branching angle (A), venular asymmetry ratio (B), venular branching coefficient (C), log (venular junctional exponent deviation) (D), and venular angular asymmetry(E) distribution by spherical equivalent refraction (SER) after multivariable adjustment. SER was evenly divided into seven groups from − 12D to 2D. Mean value with 95% confidence interval (95% CI) was plotted against each refraction group
A The slope for change in arteriolar branching angle by SER was steeper from − 6D. B The slope for change in venular asymmetry ratio by SER was steeper from − 10D. C The slope for change in venular branching coefficient by SER was steeper from − 8D. D The slope for change in log (venular junctional exponent deviation) by SER was steeper from − 8D.
Discussion
Retinal vascular bifurcation parameters are potential markers of the changes in microvascular structure and could be early indicators of ocular and systemic disease [1, 5]. In the present study on an otherwise healthy and relatively young population, we first validated the association between retinal vascular bifurcation parameters and myopia; then we found a much significant change of the parameters in high myopia. The deviation from a so-called optimal mode of vascular architecture [22, 23], could potentially compromise the blood oxygen transport to the peripheral inner retina.
We observed that arteriolar branching angle was significantly smaller in the high myopia group than in low to moderate myopia group and non-myopia group. Compared with the low to moderate myopia and non-myopia, high myopia had a more acute branching angle (70.11°±11.73° versus 76.59°±11.41° versus 75.30°±10.66°, p < 0.001). Evidence has shown a distortion of retinal branching network hampered microcirculatory transport, increased shear stress, reduced efficiency, and hence greater risk of vascular damage [2].
Increased venular branching coefficient was significantly associated with high myopia in the current study. Branching coefficient increases when the diameter of the branching venules increases disproportionately to that of the parent vessel. The increase in the diameter of branching vessels may be due to vasodilation and result in thinning of the vessel wall. The thinner walls of branching venules may be less resistant to wall shear stress than parent venules, resulting in endothelial inflammation. It also has been suggested that an increased venular branching coefficient may reflect inflammation and endothelial dysfunction triggered by hyperglycaemia in diabetes [24].
Our findings indicate a relationship between decreased venular angular asymmetry and high myopia, and between increased venular asymmetry ratio and high myopia. It is of interest to evaluate the possible pathophysiological basis of these results. Although we are not aware of any studies that have investigated how varying angular asymmetry and asymmetry ratio would affect stress in the vasculature, and what effect these changes will have on the retina of high myopia. Some studies have reported that venular asymmetry ratio changes may precede Alzheimer’s disease. Cabrera et al. observed that the venular asymmetry ratio was significantly higher in patients with cognitive impairment than in age-matched controls [25]. Frost et al. observed venular asymmetry ratio was higher in healthy individuals with high plaque burden, that change may be occurring early in Alzheimer’s disease pathogenesis [26]. It is possible that increased venular asymmetry ratio was associated with microvascular damage.
An interesting observation was that repeatedly, high myopia has been associated with a lower risk for the development and progression of diabetic retinopathy (DR) [27]. The possible mechanism of the protective effect has not been fully elucidated, but may include decrease in retinal blood flow perfusion, decrease in retinal / choroidal oxygen demand, posterior vitreous detachment (PVD) and changes in cytokine profile in high myopia [27]. Some of the factors are independent of the changes of myopic vasculature, such as liquefication of vitreous humor / PVD in myopia [28]. Some other factors, however, could be the sequelae of the compromising blood flow transportation. Chronicle sub-optimal blood supply, among others, may lead to the degeneration or adaptation of retinal ganglion cells (RGCs) and retinal pigment epithelium (RPE) or other retinal cells and, in turn, decrease the oxygen demand [29]. As for the changes of the vascular geometric parameters in myopia, some may either be the causes or indicators of the protective effect of high myopia for DR. The narrowing of vascular caliber and the decrease of vascular fractal dimension (the complexity of vascular branching) in high myopia are related to the decrease of retinal blood flow [30]. Studies consistently showed that increased retinal blood flow volume is a significant risk factor for DR development or progression [27]. High flow volume may increase the physical stress to the retinal vascular wall, as well as the biochemical damage of hyperglycemia in diabetes. On the other hand, larger arteriolar or venular branching angle have been observed in diabetic or DR patients [5, 19]. It was also reported that larger arteriolar branching angle was frequently a concomitant of other risk factors, such as poor glycemic control [31] and longer diabetes duration [17]. Therefore, a reduced blood flow in myopia, indicated by narrower retinal vessels and simpler fractal dimension, and the smaller branching angles in high myopia, may lessen the damage and be protective. However, there is a lack of direct evidence of the causality. Further studies are needed.
A strength of the present study in comparison with previous reports is that we selected a more homogenous otherwise healthy population to minimize potential or residual confounding. Second, by studying the dose-effect of myopia on retinal vascular bifurcation parameters, we demonstrated that compared to the lower myopia, it seems that high myopia compromises the integrity of retinal vasculature at an accelerated rate. The finding of the current study contributes to the body of evidence about the interaction between myopia and retinal vessels – myopia stretches the globe as well as the domestic vascular system, while the latter in turn depletes the blood supply to the eye, which may lead to the anatomic and functional damage of high myopia, and play a role in the pathogenesis of pathologic myopia.
We acknowledge that some limitations affect the present study: first, the cross-sectional design limits the ability to infer causality. Second, the axial length was not measured in the current study. However, in a healthy and relatively young population, SER can be a very good proxy for the axial length [32]. Third, the number of high myopia participants in our study was insufficient for subgroup modeling.
In summary, the vascular bifurcation differs in dependence on the myopic refractive error and a significant increase in the difference can be observed in high myopic eyes. We believe that our study is the first to show the markedly deleterious effect of high myopia on retinal bifurcation. And if the finding is validated in future longitudinal study, it will deepen our understanding of the pathophysiology of the progression of myopia.
Acknowledgements
Not applicable.
Authors’ information: 1Department of Ophthalmology, Zhongshan Hospital, Fudan University, Shanghai, China; 2Department of Ophthalmology, Huadong Sanatorium, Wuxi, Jiangsu Province, China; 3Department of Ophthalmology, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, Fujian Province, China; 4Center for Evidence-based Medicine, Fudan University, Shanghai, China.
Authors’ contributions
Caixia Sun, Yuanzhi Yuan, Jing Cong, and Xinyuan Wu wrote the main manuscript text and prepared figures and tables. Tingli Chen and Jing Wang collected clinical data and fundus photographs of participants. All authors reviewed the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due further analysis of data is in progress, but are available from the corresponding author on reasonable request.
Declarations
Ethic approval and consent to participate
All procedures adhered to the tenets of the Declaration of Helsinki, and local approval was received from the Medical Ethics Committee of Huadong Sanatorium. Medical Ethics Committee of Huadong Sanatorium waive the need for informed consent due to retrospective review of participants’ records.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Caixia Sun and Tingli Chen as equal contributors.
References
- 1.Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, Lau QP, Zhu AL, Klein R, Saw SM, et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J HYPERTENS. 2011;29(7):1380–91. doi: 10.1097/HJH.0b013e328347266c. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CY, Ikram MK, Klein R, Wong TY. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia. 2015;58(5):871–85. doi: 10.1007/s00125-015-3511-1. [DOI] [PubMed] [Google Scholar]
- 3.Doubal FN, de Haan R, MacGillivray TJ, Cohn-Hokke PE, Dhillon B, Dennis MS, Wardlaw JM. Retinal arteriolar geometry is associated with cerebral white matter hyperintensities on magnetic resonance imaging. INT J STROKE. 2010;5(6):434–9. doi: 10.1111/j.1747-4949.2010.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrory S, Taylor AM, Kirin M, Corley J, Pattie A, Cox SR, Dhillon B, Wardlaw JM, Doubal FN, Starr JM, et al. Retinal microvascular network geometry and cognitive abilities in community-dwelling older people: The Lothian Birth Cohort 1936 study. BRIT J OPHTHALMOL. 2017;101(7):993–8. doi: 10.1136/bjophthalmol-2016-309017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein R, Lee KE, Danforth L, Tsai MY, Gangnon RE, Meuer SE, Wong TY, Cheung CY, Klein B. The Relationship of Retinal Vessel Geometric Characteristics to the Incidence and Progression of Diabetic Retinopathy. Ophthalmology. 2018;125(11):1784–92. doi: 10.1016/j.ophtha.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu R, Cheung CY, Saw SM, Mitchell P, Aung T, Wong TY. Retinal vascular geometry and glaucoma: the Singapore Malay Eye Study. Ophthalmology. 2013;120(1):77–83. doi: 10.1016/j.ophtha.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 7.Ohsugi H, Ikuno Y, Oshima K, Tabuchi H. 3-D choroidal thickness maps from EDI-OCT in highly myopic eyes. Optom Vis Sci. 2013;90(6):599–606. doi: 10.1097/OPX.0b013e3182924017. [DOI] [PubMed] [Google Scholar]
- 8.Patton N, Maini R, MacGillivary T, Aslam TM, Deary IJ, Dhillon B. Effect of axial length on retinal vascular network geometry. AM J OPHTHALMOL. 2005;140(4):648–53. doi: 10.1016/j.ajo.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Lim LS, Cheung CY, Lin X, Mitchell P, Wong TY, Mei-Saw S. Influence of refractive error and axial length on retinal vessel geometric characteristics. Invest Ophthalmol Vis Sci. 2011;52(2):669–78. doi: 10.1167/iovs.10-6184. [DOI] [PubMed] [Google Scholar]
- 10.Cheung N, Tikellis G, Saw SM, Amirul IF, Mitchell P, Wang JJ, Wong TY. Relationship of axial length and retinal vascular caliber in children. AM J OPHTHALMOL. 2007;144(5):658–62. doi: 10.1016/j.ajo.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Quigley M, Cohen S. A new pressure attenuation index to evaluate retinal circulation. A link to protective factors in diabetic retinopathy. Arch Ophthalmol. 1999;117(1):84–9. doi: 10.1001/archopht.117.1.84. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Mitchell P, Liew G, Rochtchina E, Kifley A, Wong TY, Hsu W, Lee ML, Zhang YP, Wang JJ. Lens opacity and refractive influences on the measurement of retinal vascular fractal dimension. ACTA OPHTHALMOL. 2010;88(6):e234–40. doi: 10.1111/j.1755-3768.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J HYPERTENS. 2013;31(7):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 14.Fonville S, Zandbergen AA, Vermeer SE, Dippel DW, Koudstaal PJ, den Hertog HM. Prevalence of prediabetes and newly diagnosed diabetes in patients with a transient ischemic attack or stroke. CEREBROVASC DIS. 2013;36(4):283–9. doi: 10.1159/000353677. [DOI] [PubMed] [Google Scholar]
- 15.Haarman A, Enthoven CA, Tideman J, Tedja MS, Verhoeven V, Klaver C. The Complications of Myopia: A Review and Meta-Analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. doi: 10.1167/iovs.61.4.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang QF, Wei FF, Zhang ZY, Raaijmakers A, Asayama K, Thijs L, Yang WY, Mujaj B, Allegaert K, Verhamme P, et al. Reproducibility of Retinal Microvascular Traits Decoded by the Singapore I Vessel Assessment Software Across the Human Age Range. AM J HYPERTENS. 2018;31(4):438–49. doi: 10.1093/ajh/hpx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasongko MB, Wang JJ, Donaghue KC, Cheung N, Benitez-Aguirre P, Jenkins A, Hsu W, Lee ML, Wong TY. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 2010;33(6):1331–6. doi: 10.2337/dc10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes AD, Wong TY, Witt N, Evans R, Thom SA, Klein BE, Chaturvedi N, Klein R. Determinants of retinal microvascular architecture in normal subjects. Microcirculation. 2009;16(2):159–66. doi: 10.1080/10739680802353868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung CY, Sabanayagam C, Law AK, Kumari N, Ting DS, Tan G, Mitchell P, Cheng CY, Wong TY. Retinal vascular geometry and 6 year incidence and progression of diabetic retinopathy. Diabetologia. 2017;60(9):1770–81. doi: 10.1007/s00125-017-4333-0. [DOI] [PubMed] [Google Scholar]
- 20.Sainas G, Milia R, Palazzolo G, Ibba G, Marongiu E, Roberto S, Pinna V, Ghiani G, Tocco F, Crisafulli A. Mean Blood Pressure Assessment during Post-Exercise: Result from Two Different Methods of Calculation. J Sports Sci Med. 2016;15(3):424–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Tai E, Kueh YC, Wan HW, Wong TY, Shatriah I. Comparison of retinal vascular geometry in obese and non-obese children. PLoS ONE. 2018;13(2):e191434. doi: 10.1371/journal.pone.0191434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray CD. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc Natl Acad Sci U S A. 1926;12(3):207–14. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray CD. THE PHYSIOLOGICAL PRINCIPLE OF MINIMUM WORK APPLIED TO THE ANGLE OF BRANCHING OF ARTERIES. J GEN PHYSIOL. 1926;9(6):835–41. doi: 10.1085/jgp.9.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen ML, Broe R, Frydkjaer-Olsen U, Olsen BS, Mortensen HB, Peto T, Grauslund J. Retinal vascular geometry and its association to microvascular complications in patients with type 1 diabetes: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987) Graefes Arch Clin Exp Ophthalmol. 2017;255(2):293–9. doi: 10.1007/s00417-016-3454-3. [DOI] [PubMed] [Google Scholar]
- 25.Cabrera DD, Somfai GM, Arthur E, Kostic M, Oropesa S, Mendoza SC. Investigating Multimodal Diagnostic Eye Biomarkers of Cognitive Impairment by Measuring Vascular and Neurogenic Changes in the Retina. FRONT PHYSIOL. 2018;9:1721. doi: 10.3389/fphys.2018.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, Villemagne V, Rowe CC, Macaulay SL, Szoeke C, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry. 2013;3:e233. doi: 10.1038/tp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He M, Chen H, Wang W. Refractive Errors, Ocular Biometry and Diabetic Retinopathy: A Comprehensive Review. CURR EYE RES. 2021;46(2):151–8. doi: 10.1080/02713683.2020.1789175. [DOI] [PubMed] [Google Scholar]
- 28.Morita H, Funata M, Tokoro T. A clinical study of the development of posterior vitreous detachment in high myopia. Retina. 1995;15(2):117–24. doi: 10.1097/00006982-199515020-00005. [DOI] [PubMed] [Google Scholar]
- 29.Jonas JB, Ohno-Matsui K, Panda-Jonas S. Myopia: Anatomic Changes and Consequences for Its Etiology. Asia Pac J Ophthalmol (Phila) 2019;8(5):355–9. doi: 10.1097/01.APO.0000578944.25956.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. OPHTHALMIC RES. 2000;32(6):249–56. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 31.Li LJ, Lamoureux E, Wong TY, Lek N. Short-term poor glycemic control and retinal microvascular changes in pediatric Type 1 Diabetes patients in Singapore: a pilot study. BMC OPHTHALMOL. 2017;17(1):60. doi: 10.1186/s12886-017-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng L, Gwiazda J, Manny RE, Scheiman M, Weissberg E, Fern KD, Weise K. Limited change in anisometropia and aniso-axial length over 13 years in myopic children enrolled in the Correction of Myopia Evaluation Trial. Invest Ophthalmol Vis Sci. 2014;55(4):2097–105. doi: 10.1167/iovs.13-13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due further analysis of data is in progress, but are available from the corresponding author on reasonable request.