Abstract
Mediterranean spotted fever-like illness (MSF-like illness) is a tick-borne disease caused by Rickettsia sibirica mongolitimonae first reported in France more than 25 years ago. Until today, more than 50 cases of MSF-like illness have been reported in different regions of Europe and Africa, highlighting variable clinical manifestation. Here we report a case of MSF-like illness following a bite from a Hyalomma tick in the Skopje region of North Macedonia.
Keywords: Rickettsia sibirica mongolitimonae, Hyalomma, North Macedonia, Mediterranean spotted fever-like illness
Mediterranean spotted fever (MSF)-like illness is tick-borne disease caused by Rickettsia sibirica mongolitimonae, an obligate intracellular bacterium, most probably transmitted by ticks of the genus Rhipicephalus and genus Hyalomma [1]. While cases of MSF, which are caused by Rickettsia conorii, are frequently detected in endemic areas of Europe around the Mediterranean Basin, MSF-like illness is reported sporadically in different European and African countries [1,2]. Here we present a case of MSF-like illness caused by R. sibirica mongolitimonae in a female patient after a bite from a Hyalomma tick in North Macedonia.
Case description
A female patient in her 60s visited an outpatient department of our clinic in Skopje, North Macedonia in June 2022. The patient reported a rash accompanied with myalgia, headache, fever (38.5 °C) and malaise. Symptoms had started 6 days earlier (on 15 June, Day 1) with high-grade fever, headache, myalgia and malaise. Three days later (Day 4), she noticed a tick surrounded with a circumscriptive erythematous patch under her left axilla (Figure 1). The patient visited the emergency department, where the tick was surgically removed, and antibiotic treatment (amoxicillin/clavulanic acid 1,000 mg twice a day) was administered. On Day 7 (the day she visited our clinic), a non-pruritic rash was noticed on both breasts and upper arms (Figure 2) and non-specific symptoms persisted despite antibiotic treatment.
Figure 1.
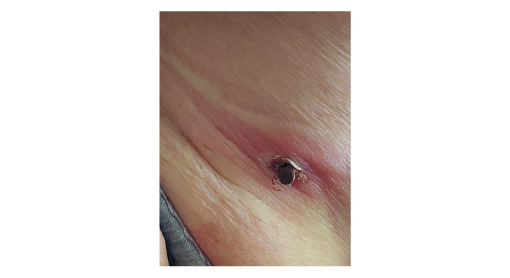
Hyalomma tick attached to the skin of left axillar region with circumscriptive erythematous patch around the infestation site, North Macedonia, June 2022
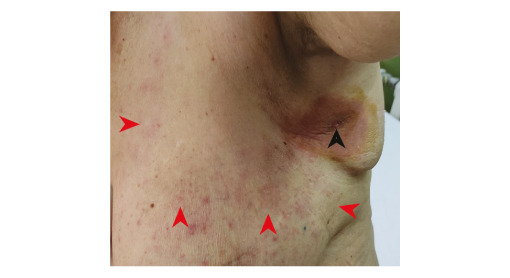
Figure 2.
Clinical presentation of Rickettsia sibirica mongolitimonae infection (6th day of illness), North Macedonia, June 2022
Black arrow: location of tick infestation. Red arrows: skin affected by maculopapular rash caused by infection.
The patient lived in a house in a rural area of the Skopje region in North Macedonia. She reported that she had not travelled outside that region in the previous 6 months. In the context of possible contact with animals, the patient reported that she only had a pet dog. She declined a household visit and blood sampling of the animal, therefore exposure of the dog to tick-borne pathogens could not be assessed.
During the consultation on Day 7, we also took a venous blood sample for biochemical analysis and complete blood count. A capillary blood sample from the tick infestation site and another venous blood sample were taken for direct detection of spotted fever group rickettsiosis (SFGR). A treatment regimen including doxycycline 100 mg twice daily for 14 days was started without awaiting the test results, and the patient was managed through the outpatient department. Control examination was conducted on Day 9, where the patient showed an excellent clinical response. The rash stopped spreading immediately after the antibiotic regimen was changed to tetracyclines, while the fever receded on the second day of the treatment. After 10 days of doxycycline treatment, another control examination was conducted. The patient reported no complaints, while hyperpigmentation was noticed at the previous skin lesions and tick infestation site.
In order to confirm the exposure to an SFGR member, a serum sample was taken 4 weeks after the symptom onset for examination of seroreactivity against R. conorii antigens.
Laboratory findings
Complete blood count and biochemical analysis
Complete blood count was performed on an automated hematology analyzer XS-1000i (Sysmex, Kobe, Japan). All biochemical analyses quantifying levels of creatinine, aspartate aminotransferase (AST), alanine transaminase (ALT) and C-reactive protein (CRP) were performed on a Cobas Integra 400 Plus II analyzer (Roche Diagnostics Corporation, Indianapolis, United States (US)). Among the initial laboratory findings, only C-reactive protein showed mild elevation (39 mg/L; reference: 0–10 mg/L). Complete blood count, AST, ALT and creatinine values were unremarkable (Table).
Table. Patient data, disease manifestation and laboratory findings, Rickettsia sibirica mongolitimonae infection, North Macedonia, June 2022.
| Case | ||
|---|---|---|
| General data | ||
| Patient age group (years)/sex | 60–70/female | |
| Date of illness onset | 16 June 2022 | |
| Tick bite reported | 18 June 2022 | |
| Reported location of tick encounter | Skopje region, North Macedonia | |
| Tick genus | Hyalomma | |
| Clinical manifestation (yes/no) | ||
| Fever | Yes | |
| Headache | Yes | |
| Myalgia | Yes | |
| Eschar | Yes | |
| Enlarged regional lymph nodes | No | |
| Local redness | Yes | |
| Systemic rash | Yes | |
| Outcome | Recovered | |
| Complications | Hyperpigmentation of skin affected by infection | |
| Laboratory findings | ||
| White blood cells | 8.1 × 103 µL | Reference: 4.0–11.0 × 103 µL |
| Platelets | 245 × 103 µL | Reference: 150–400 × 103 µL |
| AST | 34 U/L | Reference: 10–47 U/L |
| ALT | 35 U/L | Reference: 10–52 U/L |
| CRP | 39 mg/L | Reference: 0–10 mg/L |
| Creatinine | 83 µmol/L | Reference: 62–133 µmol/L |
| IgG against SFGR | + (1:512) | Negative (< 1:64) |
ALT: alanine transaminase; AST: aspartate transaminase; CRP: C-reactive protein; SFGR: spotted fever group rickettsiosis.
Morphological characterisation of the tick
Although the tick was not submitted for entomological examination, the patient provided a photography of the still-attached tick before removal at the emergency department (Figure 1). It was taxonomically identified as member of the genus Hyalomma according to taxonomic keys described previously [3].
Direct detection of the causative agent
We extracted total DNA from complete blood and the capillary blood sample using the NucleoSpin Tissue kit (Macherey Nagel, Düren, Germany). Initially, was assessed the presence of SFGR DNA with a probe-specific RealLine Rickettsia Species Fla-Format kit (Bioron Diagnostics, Römerberg, Germany; catalogue number VBD5392). The qPCR reaction was performed using the StepOne Real-Time PCR System (Applied Biosystems, San Francisco, US). From the two blood samples acquired from the patient, Rickettsia spp. DNA was detected only in capillary blood.
Subsequently, total DNA extracted from the capillary blood sample was analysed via high-throughput microfluidic real-time PCR amplification using 48.48 Dynamic Array IFC chips (Fluidigm, San Francisco, US) as described previously [4]. The capillary blood sample tested positive in the microfluidic PCR for Rickettsia spp. gltA gene. Sequencing of gltA, ompA and ompB gene fragments revealed the presence of R. sibirica mongolitimonae (GenBank accession numbers OP425002, OP515622 and OP605957, respectively). See the Supplement for details concerning validation of microfluidic real-time PCR and amplicon sequencing.
Indirect immunofluorescence analysis
Since antibodies against one SFGR member cross-react with antigens of all SFGR members [5], we examined exposure to tick-borne rickettsial pathogens via detection of IgG reactive with R. conorii using a commercial indirect immunofluorescence assay (Vircell, Granada, Spain; reference ‘PRICOG’). We examined twofold serial serum dilutions, starting from 1:32 up to 1:1,024. The serum sample acquired from the patient 4 weeks after the first medical examination tested positive for anti-SFGR IgG and showed seroreactivity up to dilution 1:512, suggesting recent infection with an SFGR member. The serum sample acquired during the first visit to our clinic was reactive only in dilution 1:32.
Discussion
MSF-like illness, caused by R. sibirica mongolitimonae infection, was previously described as an emerging disease in France, Portugal, Spain, Turkey and several African countries [1,2,6]. The illness probably occurs in a wider geographical area, since R. sibirica mongolitimonae was also detected in ticks from Israel and China [7,8]. On the Balkan peninsula, Turkey is the only country with previous reports of MSF-like illness [1], while MSF cases have been reported in Bulgaria, Croatia, Greece, Romania and Turkey [9-13]. Although MSF is not reported in Serbia, recent reports of tick-borne lymphadenopathy [14] and atypical clinical manifestations caused by Rickettsia helvetica [4] indicate circulation of SFGR in the local population.
Here, we report the first case of MSF-like illness detected in North Macedonia, caused by R. sibirica mongolitimonae and transmitted by a Hyalomma tick. According to the classification proposed for evaluation of MSF clinical manifestation [15], our case had a mild, typical and acute form of disease, given that the patient developed fever, headache, myalgia, eschar and a systemic rash, with elevated CRP levels as the only abnormal laboratory finding. Currently ticks of the genera Rhipicephalus and Hyalomma are proposed as the main vectors for R. sibirica mongolitimonae [1].
Earlier studies in North Macedonia noted the presence of Hyalomma and Rhipicephalus ticks in the Skopje region, the South-eastern region and the North-eastern region [16]. In addition, their presence was confirmed indirectly in Vardar region [16]. This report of human exposure to Hyalomma ticks are in accordance with previously published data, as the household of our patient is located within Skopje region and in near proximity to Vardar region.
Diagnostics of MSF-like illness require an experienced clinician who will consider R. sibirica mongolitimonae infection as a differential diagnosis and further require the existence of a highly specialised microbiological laboratory facility which is able to confirm the rickettsial aetiology of the disease. In addition, MSF-like illness presents a diagnostic challenge since it can appear during winter and can have variable clinical manifestations [1,17].
Conclusion
Considering that MSF or MSF-like illness are not nationally notifiable diseases in North Macedonia, there is a high possibility that MSF-like illness is neglected, especially in North Macedonian regions where presence of Hyalomma and Rhipicephalus ticks is reported. Since precise aetiological diagnosis of MSF-like illness requires a highly specialised microbiological laboratory facility, we are proposing cross-border cooperation of Balkan countries to standardise the diagnostic procedure and raise awareness among physicians, building diagnostic capacity and networks in which information about cases can be shared. This will allow to plan effective preventive measures and to assess the impact of this disease in the population of North Macedonia and as well as population of other Balkan countries possibly affected by R. sibirica mongolitimonae infection.
Ethical statement
Ethical approval was obtained by University Clinic for Infectious Diseases and Febrile Conditions, Skopje, North Macedonia, and the report was conducted in line with the Declaration of Helsinki, as revised in 2013.
Funding statement
This report received no funding.
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: Report design: DJ, SMa and PB; Data analysis: DM, IB, VS, SM, ACC, AEP, LMH, AFS. Bioinformatic analyses: AFS, ACC, SMo. Data interpretation: DJ, SM, PB, ACC, AEP. Critical revision of manuscript: ACC, PB, AEP, SMo, LMH, VS, DM.
References
- 1. Rajoelison P, Mediannikov O, Javelle E, Raoult D, Parola P, Aoun O. Rickettsia sibirica mongolitimonae human infection: A diagnostic challenge. Travel Med Infect Dis. 2018;26:72-3. 10.1016/j.tmaid.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 2. Nouchi A, Monsel G, Jaspard M, Jannic A, Angelakis E, Caumes E. Rickettsia sibirica mongolitimonae infection in a woman travelling from Cameroon: a case report and review of the literature. J Travel Med. 2018;25(1):tax074. 10.1093/jtm/tax074 [DOI] [PubMed] [Google Scholar]
- 3.Estrada-Peña A. Ticks of domestic animals in the Mediterranean region: a guide to identification of species. Zaragoza: University of Zaragoza; 2004. 131 p. [Google Scholar]
- 4. Banović P, Díaz-Sánchez AA, Simin V, Foucault-Simonin A, Galon C, Wu-Chuang A, et al. Clinical aspects and detection of emerging rickettsial pathogens: a "One Health" approach study in Serbia, 2020. Front Microbiol. 2022;12:797399. 10.3389/fmicb.2021.797399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson MT, Satjanadumrong J, Hughes T, Stenos J, Blacksell SD. Diagnosis of spotted fever group Rickettsia infections: the Asian perspective. Epidemiol Infect. 2019;147:e286. 10.1017/S0950268819001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibarra V, Portillo A, Palomar AM, Sanz MM, Metola L, Blanco JR, et al. Septic shock in a patient infected with Rickettsia sibirica mongolitimonae, Spain. Clin Microbiol Infect. 2012;18(8):E283-5. 10.1111/j.1469-0691.2012.03887.x [DOI] [PubMed] [Google Scholar]
- 7. Sun J, Lin J, Gong Z, Chang Y, Ye X, Gu S, et al. Detection of spotted fever group Rickettsiae in ticks from Zhejiang Province, China. Exp Appl Acarol. 2015;65(3):403-11. 10.1007/s10493-015-9880-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrus S, Perlman-Avrahami A, Mumcuoglu KY, Morick D, Baneth G. Molecular detection of Rickettsia massiliae, Rickettsia sibirica mongolitimonae and Rickettsia conorii israelensis in ticks from Israel. Clin Microbiol Infect. 2011;17(2):176-80. 10.1111/j.1469-0691.2010.03224.x [DOI] [PubMed] [Google Scholar]
- 9. Baltadzhiev I, Kevorkyan A, Popivanova N. Mediterranean spotted fever in child and adult patients: investigation from an endemic region in Bulgaria. Cent Eur J Public Health. 2020;28(3):187-92. 10.21101/cejph.a5608 [DOI] [PubMed] [Google Scholar]
- 10. Kuloglu F, Rolain JM, Akata F, Eroglu C, Celik AD, Parola P. Mediterranean spotted fever in the Trakya region of Turkey. Ticks Tick Borne Dis. 2012;3(5-6):298-304. 10.1016/j.ttbdis.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 11. Pitigoi D, Olaru ID, Badescu D, Rafila A, Arama V, Hristea A. Mediterranean spotted fever in southeastern Romania. BioMed Res Int. 2013;2013:395806. 10.1155/2013/395806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Efstratiou A, Karanis G, Karanis P. Tick-borne pathogens and diseases in Greece. Microorganisms. 2021;9(8):1732. 10.3390/microorganisms9081732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Punda-Polić V, Luksić B, Capkun V. Epidemiological features of Mediterranean spotted fever, murine typhus, and Q fever in Split-Dalmatia County (Croatia), 1982-2002. Epidemiol Infect. 2008;136(7):972-9. 10.1017/S0950268807009491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajinov Z, Roš T, Ivkov-Simić M, Gajić B, Prćić S, Matić M. Tick-borne lymphadenopathy acquired in Serbia: Report of two cases. Vojnosanitetski pregled. 2018;75(11):1134-7.
- 15. Baltadzhiev IG, Popivanova NI, Stoilova YM, Kevorkian AK. Mediterranean spotted fever--classification by disease course and criteria for determining the disease severity. Folia Med (Plovdiv). 2012;54(4):53-61. 10.2478/v10153-012-0006-4 [DOI] [PubMed] [Google Scholar]
- 16. Mertens M, Vatansever Z, Mrenoshki S, Krstevski K, Stefanovska J, Djadjovski I, et al. Circulation of Crimean-Congo Hemorrhagic Fever Virus in the former Yugoslav Republic of Macedonia revealed by screening of cattle sera using a novel enzyme-linked immunosorbent assay. PLoS Negl Trop Dis. 2015;9(3):e0003519. 10.1371/journal.pntd.0003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raoult D, Brouqui P, Roux V. A new spotted-fever-group rickettsiosis. Lancet. 1996;348(9024):412. 10.1016/S0140-6736(05)65037-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


