Abstract
Background:
Guidance is lacking for how to combine urinary biomarker data across studies that use different measures of urinary dilution, i.e., creatinine or specific gravity.
Methods:
Among 741 pregnant participants from four sites of The Infant Development and Environment Study (TIDES) cohort, we assessed the relation of maternal urinary di-2-ethylhexyl phthalate (DEHP) concentrations with preterm birth. We compared scenarios in which all sites measured either urinary creatinine or specific gravity, or where measure of dilution differed by site. In addition to a scenario with no dilution adjustment, we applied and compared three dilution-adjustment approaches: a standard regression-based approach for creatinine, a standard approach for specific gravity (Boeniger method), and a more recently developed approach that has been applied to both (covariate-adjusted standardization method). For each scenario and dilution-adjustment method, we estimated the association between a doubling in the molar sum of DEHP (∑DEHP) and odds of preterm birth using logistic regression.
Results:
All dilution-adjustment approaches yielded comparable associations (odds ratio [OR]) that were larger in magnitude than when we did not perform dilution-adjustment. A doubling of ∑DEHP was associated with 9% greater odds of preterm birth (OR: 1.09, 95% CI: 0.91, 1.30) when applying no dilution adjustment method, whereas dilution-adjusted point estimates were higher, and similar across all scenarios and methods: 1.13–1.20 (regression-based), 1.15–1.18 (Boeniger), and 1.14–1.21 (covariate-adjusted standardization).
Conclusions:
In our applied example, we demonstrate that it is possible and straightforward to combine urinary biomarker data across studies when measures of dilution differ.
Keywords: Biomarkers, creatinine, dilution, specific gravity, standardization, urine
Introduction
Combining data across multiple studies to assess associations of environmental chemical exposures and health outcomes1–12 is becoming common practice, particularly when individual studies typically have small sample sizes.2 However, combining urinary biomarkers can be challenging, in part, due to how different studies account for urinary dilution.
The concentration of a biomarker in urine is directly related to an individual’s hydration status.13 As such, failure to account for hydration can induce measurement error in the exposure of interest when using exposure biomarkers measured in urine. Given the same true exposure biomarker level, the level measured in a dilute urine sample is lower than the level measured in a concentrated urine sample. To address this issue, urinary creatinine or specific gravity are often measured as proxies for an individual’s hydration status at the time of urine sample collection.13
When hydration is assessed using specific gravity, urinary biomarker concentrations are usually adjusted for using the so-called Boeniger method,14–16 which yields dilution-adjusted concentrations in the original units of the biomarker. In contrast, when hydration is using creatinine, one of the more commonly used adjustment approaches is to divide the biomarker concentration by the creatinine concentration, yielding dilution-adjusted concentrations measured in weight of biomarker per weight of creatinine.13 As such, it is not straightforward to combine urinary biomarker data when different measures of dilution are used because common methods do not yield the same units of exposure biomarker concentration. A previous study addressed this issue by using raw (not dilution-adjusted) biomarker concentrations, thereby maintaining the same units of concentration across studies. The authors accounted for urinary dilution by including the study-specific rank of each sample’s creatinine or specific gravity value as a covariate in subsequent regression models.3 While this approach circumvents the issue of non-compatible units of exposure, it does not directly address a second issue that these measures of dilution are often affected by multiple exogenous or endogenous factors, independent of underlying hydration status.13,16,17
Several studies have shown that urinary creatinine (a waste product from skeletal muscle that is excreted by the kidneys) levels can be influenced by an individual’s age,13,16,17 height and body mass index (BMI),13,16 gender or sex,13,16 race and ethnicity,13,16 certain health conditions (e.g., hypertension),16 and time of day of sample collection.16,18 Similarly, urinary specific gravity (a measure of the relative density of urine to water) can be influenced by the same factors as creatinine,16,18–23 though perhaps to a lesser extent.16 Given this, Barr et al. proposed an approach (which we will refer to as the regression-based method),13,24 which aims to account for urinary dilution as well as the factors that may influence the given measure of dilution,13 via inclusion of both the uncorrected (raw) biomarker concentration and urinary dilution measure in a regression model, along with covariates believed to influence the measure of dilution. While this approach was developed for use with creatinine, it has been extended for use with specific gravity as well.24
Compared to these two approaches, it has previously been shown that accounting for factors that influence measures of dilution using a covariate-adjusted standardization method minimizes bias resulting from differential measurement error in subsequent estimates from regression models, when estimating effects of dilution-adjusted biomarker concentrations on health outcomes.17 Further, we demonstrated that this method could be applied when using either creatinine or specific gravity.16 Therefore, this method may offer a comparable means of adjusting urinary biomarkers for hydration, even when dilution measures differ.
We explored the potential implications for combining data with different urinary dilution measures using data from The Infant Development and Environment Study (TIDES) cohort, a multi-center prospective pregnancy cohort with four demographically and geographically diverse study sites in the United States, all of which had measures of both specific gravity and creatinine for the same maternal urine samples. We compared estimates of the association between urinary DEHP metabolite concentration and odds of preterm birth using three dilution-adjustment approaches: the regression-based method,13,17 the Boeniger method,14 and the covariate-adjusted standardization method,17 and compared these estimates to that when no urinary dilution-adjustment approach was applied. We further compared estimates across scenarios in which the dilution measures varied across study sites. We chose this as our applied example given that a previous study observed an association between DEHP metabolite concentration and preterm birth, in the TIDES cohort.24
Methods
Study population
TIDES recruited pregnant women from four sites: University of California, San Francisco (UCSF); University of Minnesota (UMN); University of Rochester Medical Center (URMC); and University of Washington, Seattle Children’s Hospital (UW).25 Urine samples were collected at three prenatal study visits; however, we only included samples from the third trimester study visit (median 32 weeks’ gestation) in the present analysis, as only these samples had measures of both urinary creatinine and specific gravity. We restricted this analysis to 741 women with urine samples collected prior to delivery; quantified DEHP metabolites; estimated gestational age at delivery; and measures of both specific gravity and creatinine, with values within three standard deviations (SDs) of the site-specific mean. Of these 741 women, 196 were recruited from UCSF, 204 from UMN, 209 from URMC, and 132 from UW. Prior to participation in TIDES, all women provided signed informed consent, and all study procedures were approved by site-specific institutional review boards (IRBs) as well as the Icahn School of Medicine at Mount Sinai (the TIDES Coordinating Center).
Urinary biomarkers of hydration and phthalate exposure
As previously described,24 concentrations of four DEHP metabolites (mono-2-ethylhexyl phthalate [MEHP], mono-2-ethyl-5-hydroxyhexyl phthalate [MEHHP], mono-2-ethyl-5-oxohexyl phthalate [MEOHP], and mono-2-ethyl-5-carboxypentyl phthalate [MECPP]) were quantified by the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, and machine-read values were used for concentrations below the limit of detection (LOD).24 We calculated the molar sum of DEHP (∑DEHP) by dividing the concentration of each DEHP metabolite by its respective molecular weight, summing these values, and multiplying by the molecular weight of MECPP (to retain original units of metabolite concentrations26). Specific gravity was measured within 30 minutes of sample collection using a hand-held refractometer (National Instrument Company, Inc., USA) calibrated with deionized water before each measurement was taken.27 At the time of phthalate metabolite assays, urinary creatinine levels (mg/dL) were measured using a commercially available colorimetric assay kit (Product ID: EIACUN; Enzo Life Sciences, Farmingdale, NY, USA) that is based on the Jaffe reaction. This chemistry is the same as that utilized by clinical laboratories for the quantification of urinary creatinine.
Preterm birth
Gestational age at delivery was based on first available ultrasound or self-reported last menstrual period, and we considered a birth preterm if delivery occurred prior to 37 completed weeks of gestation.24
Statistical analyses
Dilution assignment scenarios:
To emulate a study in which urinary biomarkers from multiple studies are combined for data analysis, we estimated the association of urinary log2-∑DEHP concentration with odds of preterm birth under four scenarios, basing regression models on a previous study of urinary phthalate biomarker concentrations and preterm birth in TIDES.24 The first two scenarios used biomarker concentrations adjusted for dilution using either (1) specific gravity or (2) creatinine for all participants. The third scenario adjusted for dilution using specific gravity for two sites (UCSF and UMN) and creatinine for two sites (URMC and UW), and the fourth scenario switched these assignments. We chose these groupings such that combined sites were not from the same broad geographic region (e.g., western United States) and yielded the most comparable grouping sample sizes (grouping A = 400 women and grouping B = 341 women).
Dilution-adjustment approaches:
We compared three different approaches that account for urinary dilution: the regression-based method, Boeniger method, and covariate-adjusted standardization method. Importantly, all three methods generate dilution-adjusted biomarker concentrations that retain their original units (e.g., ng/mL), regardless of whether specific gravity or creatinine is used as the measure of urinary dilution.
The first approach, the regression-based method, uses the raw (i.e., reported) biomarker concentrations in regression models, along with the measure of urinary dilution as a covariate.13,24 To ensure consistency across all four dilution assignment scenarios, each observation was assigned either their site-specific z-score (continuous) of specific gravity or creatinine (log2-transformed prior to calculation of z-scores), depending on the scenario.
The second approach, the Boeniger method, has traditionally been used for specific gravity but recently extended for use with creatinine as well.16 This method uses the following formulae to directly adjust biomarker concentrations for urinary dilution. For specific gravity, , where Esg is the specific gravity-standardized exposure biomarker concentration, Eo is the observed exposure biomarker concentration, SGmedian is the median of specific gravity values in the study sample, and SGo is the observed specific gravity value. For creatinine, , where Ecr is the creatinine-standardized exposure biomarker concentration, Eo is the observed exposure biomarker concentration, Crmedian is the median of creatinine concentrations in the study sample, and Cro is the observed urinary creatinine concentration. Importantly, we used the site-specific medians in our application of this approach.
The third approach, covariate-adjusted standardization, was originally developed for use with creatinine,17 and has a similar formula to the Boeniger method above. For creatinine, , where Ecr is the creatinine-standardized exposure biomarker concentration, Eo is the observed exposure biomarker concentration, Crp is the predicted creatinine concentration for each observation based on a prediction model of urinary creatinine, and Cro is the observed urinary creatinine concentration for each observation. For specific gravity , where Esg is the specific gravity-standardized exposure biomarker concentration, Eo is the observed exposure biomarker concentration, SGp is the predicted specific gravity for each observation, and SGo is the observed specific gravity for each observation. To estimate the predicted creatinine or specific gravity values for each observation, we used a series of site-specific linear regression models where the outcome was either creatinine (log2-transformed due to non-normality) or specific gravity. As shown in the directed acyclic graph (DAG, eFigure 1), this method aims to explicitly adjust for factors known (or hypothesized) to influence measures of dilution (e.g., creatinine, specific gravity), such that the only residual variability in the dilution measure results from underlying hydration status.13,17 Covariates for these models included maternal age (years), gestational age at sample collection (weeks), maternal height (cm), pre-pregnancy BMI (kg/m2), maternal Hispanic or Latina ethnicity, parity (nulliparous or parous), pre-eclampsia/gestational hypertension (based on International Classification of Diseases [ICD]-9 or 10 codes in medical chart), and maternal race (White, Black, Asian, or Other/multiple race [UCSF, UMN, and UW sites]; or, White, Black, or Other/multiple race [URMC site]). We did not include Asian as a separate race category for the URMC model as there were few women who identified as this race.
Descriptive statistics:
We calculated several descriptive statistics of maternal and urine sample collection characteristics, including the mean (SD) of continuous variables and sample size (%) for categorical variables, both in the overall study sample and by study site. We also calculated the median (interquartile range, IQR) of raw ∑DEHP and dilution-adjusted ∑DEHP (using the Boeniger or covariate-adjusted standardization method), for the overall study sample and by study site. We visualized the distributions of ∑DEHP concentrations using box-plots. Finally, we calculated the Spearman’s correlations (ρ) for all pairwise combinations of raw and dilution-adjusted concentrations (using the Boeniger or covariate-adjusted standardization method). We did not include the regression-based method in these descriptive analyses since that approach does not directly standardize the biomarker concentrations for urinary dilution, and instead does so indirectly via inclusion of the measure of dilution as a covariate in subsequent regression models.
Preterm birth regression models:
Prior to fitting regression models, we log2-transformed ∑DEHP given its non-normality. We modeled the relation of a doubling of ∑DEHP with odds of preterm birth via logistic regression models, estimated using a robust Huber-White sandwich estimation of variance. We included as covariates maternal age (years), pre-pregnancy BMI, maternal education level (highest achieved: high school or less, any college/technical school, any graduate work), and maternal race (White, Black, Other/multiple race). We also included study site to account for systematic differences across sites and inherent correlation of observations within sites. For models applying the regression-based or covariate-adjusted standardization method, we additionally included z-scores of the measure(s) of dilution as an additional covariate. However, as a sensitivity analysis, we fitted models where we applied the covariate-adjusted standardization method but excluded the z-scores of the measure(s) of dilution, as a previously described variation of this general approach.17
In regression analyses, we addressed missing covariate data (7% of sample) using multiple imputation by chained equations (50 imputed data sets). We performed all analyses using Stata v15.1 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
Descriptive statistics of study sample
The average (SD) age was 30.5 years (5.5) in the overall study sample (n = 741), with women being older (on average) at the UCSF site (34.2 years) and younger at the URMC site (26.4 years) (Table 1). Sixty-seven percent of women identified as White, overall, with 80% of women at UMN identifying as White and only 47% at URMC (where 35% identified as Black) (Table 1). Nearly 58% of women at UCSF attained some Graduate (i.e., post-Baccalaureate) education, while 14% of women at URMC attained this level of education (Table 1). Preterm delivery occurred in 8% of the overall sample, with URMC having the highest proportion (11%) and UW having the lowest (4%) (Table 1). The average (SD) gestational age at sample collection was ~33 weeks (3), with UW having the highest average gestational age at collection (~37 weeks) (Table 1). Average specific gravity and creatinine were lowest at UCSF (1.013 and 76 mg/dL, respectively) and highest at URMC (1.018 and 123 mg/dL, respectively) (Table 1).
Table 1.
Maternal and urine sample characteristics in the overall TIDES study sample and by study site.
| Characteristic | Overall n = 741 |
UCSF n = 196 |
UMN n = 204 |
URMC n = 209 |
UW N = 132 |
|---|---|---|---|---|---|
| Maternal age (years), mean (SD) | 30.5 (5.5) | 34.2 (4.1) | 30.7 (4.5) | 26.4 (5.5) | 31.4 (4.6) |
| Missing | 1 | 0 | 1 | 0 | 0 |
| Maternal race, n (%) | |||||
| White | 499 (67) | 137 (70) | 161 (79) | 99 (47) | 102 (77) |
| Black | 100 (14) | 6 (3) | 14 (7) | 74 (35) | 6 (6) |
| Asian | 52 (7) | 31 (16) | 9 (4) | 2 (1) | 10 (10) |
| Other, unknown, or multiple race(s) | 89 (12) | 22 (11) | 19 (9) | 24 (11) | 14 (14) |
| Missing | 1 | 0 | 1 | 0 | 0 |
| Maternal Hispanic or Latina ethnicity, n (%) | 67 (9) | 24 (12) | 6 (3) | 28 (13) | 9 (7) |
| Missing | 9 | 0 | 6 | 1 | 2 |
| Maternal highest educational attainment, n (%) | |||||
| High school or less | 105 (14) | 6 (3) | 9 (5) | 86 (41) | 4 (3) |
| Any college/technical schoola | 318 (43) | 76 (39) | 90 (45) | 93 (44) | 59 (45) |
| Any Graduate work | 312 (42) | 114 (58) | 101 (51) | 30 (14) | 67 (52) |
| Missing | 6 | 0 | 4 | 0 | 2 |
| Maternal height (cm), mean (SD) | 164.8 (7.3) | 164.9 (6.5) | 166.2 (7.6) | 162.6 (6.6) | 166.2 (8.0) |
| Missing | 3 | 0 | 1 | 0 | 2 |
| Maternal weight (kg), mean (SD) | 69.6 (17.6) | 65.3 (13.4) | 68.9 (15.3) | 75.3 (22.1) | 68.1 (15.8) |
| Missing | 8 | 0 | 4 | 1 | 3 |
| Pre-pregnancy body mass index (kg/m2), mean (SD) | 25.6 (6.3) | 24.0 (4.7) | 24.9 (5.1) | 28.5 (7.9) | 24.7 (5.6) |
| Missing | 8 | 0 | 4 | 1 | 3 |
| Parity, n (%) | |||||
| Primiparous or multiparous | 316 (45) | 79 (43) | 85 (43) | 102 (53) | 50 (42) |
| Missing | 43 | 11 | 4 | 15 | 13 |
| Pre-eclampsia/gestational hypertension, n (%)b | 102 (14) | 14 (8) | 30 (15) | 42 (20) | 16 (12) |
| Missing | 27 | 25 | 1 | 1 | 0 |
| Preterm deliveryc, n (%) | 59 (8) | 12 (6) | 19 (9) | 23 (11) | 5 (4) |
| Gestational age at sample collection (weeks), mean (SD) | 32.6 (2.9) | 32.4 (2.2) | 31.8 (2.1) | 31.2 (2.6) | 36.5 (2.2) |
| Urinary creatinine concentration (mg/dL), mean (SD) | 92.8 (66.4) | 76.0 (58.1) | 87.5 (65.1) | 123.1 (72.6) | 78.1 (52.5) |
| Urinary specific gravity, mean (SD) | 1.015 (0.007) | 1.013 (0.007) | 1.014 (0.007) | 1.018 (0.007) | 1.014 (0.007) |
Abbreviations: cm = centimeter; dL = deciliter; kg = kilogram; m = meter; mg = milligram; UCSF = University of California, San Francisco; UMN = University of Minnesota; URMC = University of Rochester Medical Center; UW = University of Washington
Up to Bachelor’s degree
Based on medical chart review and International Classification of Diseases 9th or 10th edition codes
< 37 weeks completed gestation
Descriptive statistics of ∑DEHP concentrations
While median concentrations of ∑DEHP (ng/mL of MECPP) were comparable for raw (i.e., not dilution-adjusted) and dilution-adjusted concentrations (standardized using either specific gravity or creatinine), the interquartile range (IQR) was widest for the raw concentrations (Figure 1, eTable 1). Median (IQR) concentrations were consistently highest for URMC samples and lowest for UCSF, regardless of adjustment for urinary dilution (eTable 1). Pairwise Spearman’s correlations between raw and dilution-adjusted concentrations were lowest for those that included raw concentrations, particularly when creatinine was used as the measure of dilution (Figure 2). For example, the correlation between raw ∑DEHP and creatinine-standardized ∑DEHP using the Boeniger method was ρ = 0.63, whereas between raw ∑DEHP and specific gravity-standardized ∑DEHP using the Boeniger method was ρ = 0.80 (Figure 2). While correlations between dilution-adjusted concentrations were all high (ρ ≥ 0.84), they were higher when the same measure of dilution was used (Figure 2). When measures of dilution differed, correlations were comparable when applying either the Boeniger (ρ = 0.87) or covariate-adjusted standardization method (ρ = 0.88) (Figure 2).
Figure 1.
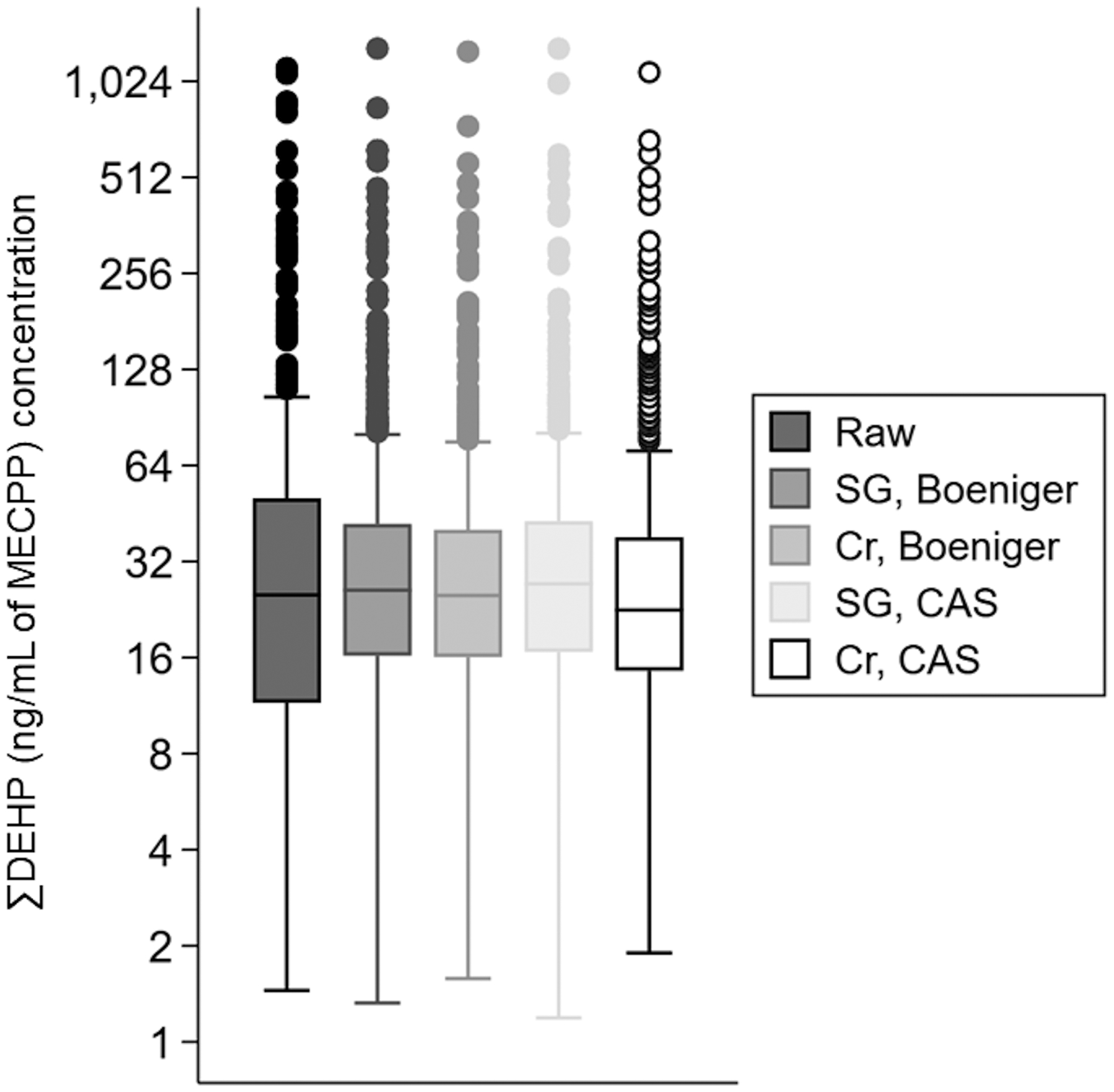
Box-plots of ∑DEHP (ng/mL of MECPP) by urine dilution-adjustment method and measure of dilution. X-axis legend key: CAS = covariate-adjusted standardization; Cr = creatinine; SG = specific gravity. Abbreviations: DEHP = di-2-ethylhexyl phthalate; MECPP = mono(2-ethyl-5-carboxypentyl) phthalate; mL = milliliter; ng = nanogram.
Figure 2.
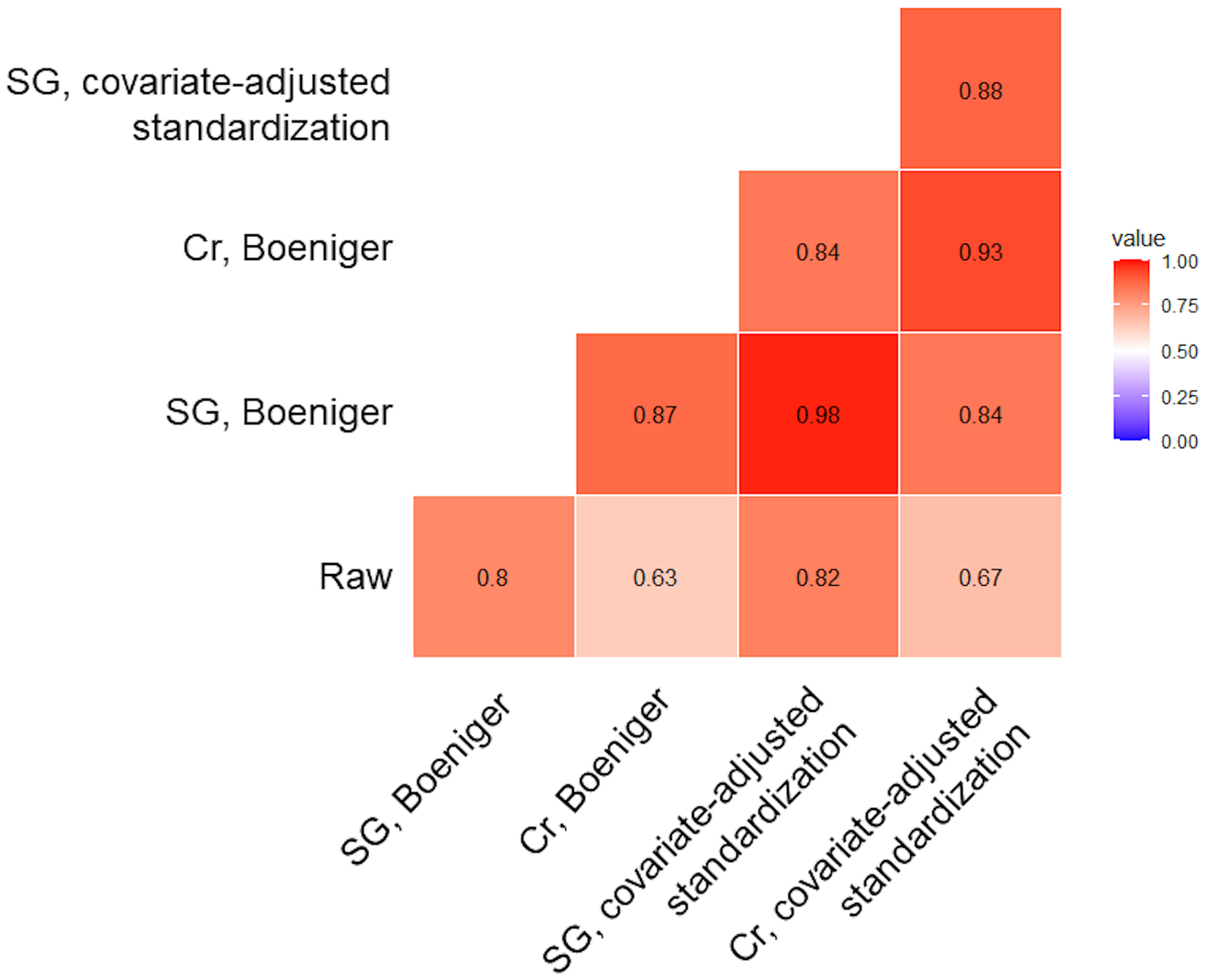
Spearman’s correlation heat-map for all pairwise comparisons of raw (not dilution adjusted) and dilution-adjusted ∑DEHP (ng/mL of MECPP) concentrations, among the overall TIDES study sample (n = 741). Abbreviations: Cr = creatinine; SG = specific gravity; DEHP = di-2-ethylhexyl phthalate; MECPP = mono(2-ethyl-5-carboxypentyl) phthalate; mL = milliliter; ng = nanogram.
Preterm birth regression models
When urinary dilution was ignored, a doubling in ∑DEHP was associated with greater odds (OR: 1.09, 95% CI: 0.91, 1.30) of preterm birth (Figure 3, eTable 2). For all dilution-adjustment methods under all four scenarios, the OR was greater in magnitude compared to the estimate using raw concentrations (OR range: 1.13–1.20, regression-based; 1.15–1.18, Boeniger; 1.14–1.21, covariate-adjusted standardization) (Table 1). Within dilution assignment scenario, all dilution-adjustment methods yielded comparable effects estimates with similar precision (Figure 2 and eTable 2). For example, in scenario 3 (where UCSF and UMN measured specific gravity while URMC and UW measured creatinine), ORs were 1.13 (95% CI: 0.89, 1.43) for the regression-based method, 1.15 (95% CI: 0.91, 1.44) for Boeringer, and 1.14 (95% CI: 0.91, 1.44) for covariate-adjusted standardization (eTable 2).
Figure 3.
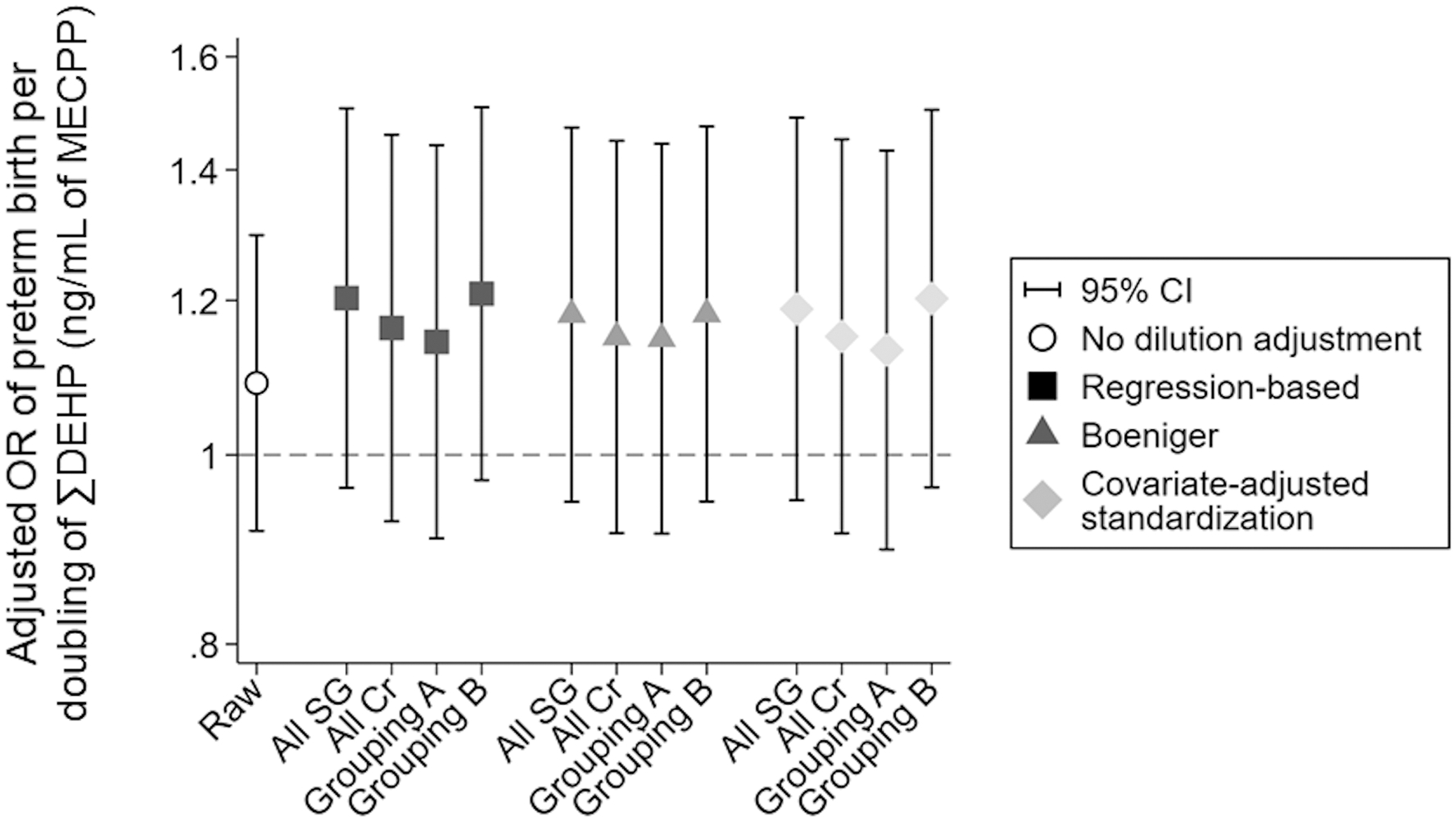
Associations of raw (not dilution-adjusted) and dilution-adjusted log2-∑DEHP (ng/mL of MECPP) concentrations with odds of preterm birth. All models adjusted for maternal age, pre-pregnancy BMI, maternal education level (highest achieved), maternal race, and study site. Regression-based and covariate-adjusted standardization models also adjusted for measure of dilution (z-standardized). X-axis key: Cr = creatinine; grouping A = UCSF and UMN adjusted using specific gravity; UW and URMC adjusted using creatinine; grouping B = UCSF and UMN adjusted using creatinine; UW and URMC adjusted using specific gravity; raw = no dilution adjustment; SG = specific gravity. Other abbreviations: CI = confidence interval; DEHP = di-2-ethylhexyl phthalate; MECPP = mono(2-ethyl-5-carboxypentyl) phthalate; mL = milliliter; ng = nanogram; OR = odds ratio.
In our sensitivity analysis, effect estimates were comparable between models in which the covariate-adjusted standardization method was applied, but dilution measure z-scores were either included or excluded as a covariate (eTable 3).
Discussion
Using biospecimen and participant data collected from four different sites in the TIDES cohort, we described three methods by which researchers can effectively harmonize urinary biomarker data, even when different measures of dilution are used across biospecimens. We separately applied the regression-based method,13,24 Boeniger method,14–16 and covariate-adjusted standardization method16,17 to third trimester ∑DEHP concentrations, and estimated the associations between a doubling of ∑DEHP and odds of preterm birth. With each dilution-adjustment method, we observed generally comparable estimates of associations across various scenarios (i.e., assigning some TIDES sites to creatinine and others to specific gravity), all of which were greater in magnitude than the association for raw (not dilution-adjusted) ∑DEHP. There are several reasons why the three methods yielded similar estimates of association, regardless of scenario.
First, specific gravity and creatinine were highly correlated in our study sample (Spearman’s ρ = 0.90), implying that they are relatively exchangeable for one another, at least in this study sample. In general, given that creatinine and specific gravity are both related to hydration status, and creatinine is one of the major dissolved solids in urine (thereby directly affecting its density), they would be expected to be highly correlated with one another in most situations; this, for example, has been observed in a previous study of adults who participated in the 2007–2008 cycle of National Health and Nutrition Examination Survey (NHANES) (ρ = 0.8).19 However, the degree to which they are correlated is likely dependent on the homogeneity of the population in which they are measured. Given that measures of creatinine may be more influenced by factors such as age,13,16,17 body size (e.g., BMI and height),13,16 gender or sex,13,16 race and ethnicity,13,16 and certain health conditions (e.g., hypertension, diabetes, kidney disease),16 a more diverse study population (with respect to these factors) would be expected to have a lower correlation between these two measures of dilution, generally. Additionally, different specimen collection and storage protocols across sites or studies may be another source of variability, even for the same measure of dilution. For example, specific gravity measured from thawed (i.e., previously frozen) as opposed to fresh samples (i.e., prior to freezing), may systematically differ since precipitation of urinary sediments often occurs in thawed urine.28 As such, the choice of dilution-adjustment method may have a greater impact on subsequent analyses in even more heterogenous populations and/or with differing specimen collection/storage protocols, wherein explicitly accounting for these factors during dilution-adjustment (as with the covariate-adjusted standardization method) may be advantageous.
Second, several of the aforementioned factors known to influence creatinine and specific gravity were included as covariates in our regression models of preterm birth, making the three dilution-adjustment methods more comparable. The covariate-adjusted standardization method, however, has the advantage of 1) allowing for different covariate adjustment sets to be used for the adjustment for urinary dilution, across cohorts and/or measures of dilution, and 2) allows for these covariate adjustment sets to differ from subsequent health outcome regression models.
Last, all dilution-adjustment methods accounted for the variability in the measures of dilution, either by directly standardizing the biomarker concentrations (i.e., Boeniger and covariate-adjusted standardization methods) or indirectly by including z-scores (specific to each measure of dilution) as a covariate in regression models (i.e., regression-based method). Importantly, while the covariate-adjusted standardization method used in this study includes z-scores of the measure(s) of dilution as a covariate, we still consider this method a “direct” standardization approach. As described in O’Brien et al., the measure of dilution is included as a covariate to block backdoor paths between covariates that influence the dilution measure and the outcome of interest.17 While our sensitivity analysis showed that associations were robust to excluding dilution z-scores when using the covariate-adjusted standardization method, this may not always occur, particularly when there are important unmeasured covariates related to both the measure of dilution and the outcome of interest.17
While using site- or study-specific rank (or z-scores) of creatinine or specific gravity has the advantage of accounting for systematic differences in their measurement at the site or study level, we previously showed that the rank-ordering between creatinine and specific gravity can be inconsistent for the same biospecimen.16 As such, unless all major individual-level sources of variability in the measurement of creatinine or specific gravity are adjusted for (either as covariates in subsequent health outcome regression models or via covariate-adjusted standardization), use of rank-order or dilution z-scores alone is likely insufficient to mitigate bias in resulting associations of the biomarker with the health outcome of interest. This is highlighted by the fact that while associations with preterm birth were largely comparable across the three dilution-adjustment methods as well as across the dilution assignment scenarios tested in our study, we observed somewhat larger differences in estimates with respect to the assignment scenarios. This is likely due to residual confounding of associations by some unmeasured factor (or factors) that influenced creatinine and/or specific gravity, such as additional sample collection characteristics (e.g., time of day of sample collection14,16,21) and/or diet.29 Additionally, an important consideration in the use of z-scores is the fact that a one SD unit may equate to very different changes in creatinine or specific gravity on their original scales, depending on the variability of these measures in the included studies. While one could generate z-scores by indexing to a target population instead of internally standardizing via site- or study-specific z-score, the latter approach has the advantage of directly adjusting for systematic differences in measurement of creatinine or specific gravity across sites or studies, which may otherwise not be straightforward or possible to explicitly account for in analysis.
While we have not compared our findings in this study with independent simulations, a previous simulation study demonstrated that the covariate-adjusted standardization method was robust in its ability to minimize the differential measurement error of urinary biomarker data, when compared to other dilution-adjustment methods (including the regression-based method),17 in scenarios similar to the one described in our study. Further, whereas other approaches had increasing bias with increasing effect sizes, covariate-adjusted standardization was minimally biased across the range of effect sizes tested.17 As such, it may be the preferred method, generally, when evaluating urinary biomarker data, but especially when pooling biomarker data across studies that used different measures of urinary dilution.
Conclusions
Researchers can combine urinary biomarker data from multiple studies when creatinine, specific gravity, or both are used as measures of urinary dilution. Results were similar for regression-based, Boeniger, and covariate-adjusted standardization approaches, but approaches based upon covariate-adjusted standardization may be more appropriate for broader applications with different sources of variability.
Supplementary Material
Funding
JRK and JPB were supported by NIH OD U24 OD023382 and NIEHS R01 ES030078. BMW, KMO, and KKF were supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health. ESB was supported by NIEHS P30 ES005022. RHNN, GLM, and SS were supported by NIEHS R01ES016863 and R01ES025169.
Footnotes
Conflict of interest
The authors report no perceived or actual conflicts of interest.
Data availability
The data utilized for this study are not publicly available but de-identified data may be available on request with review of a detailed data analysis plan by the TIDES Investigator Team. Access to data will also require approval by your institution’s IRB and a formal data use agreement. Contact the corresponding author for more information.
References
- 1.Padula AM, Monk C, Brennan PA, et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors-implications for research on perinatal outcomes in the ECHO program. J Perinatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley JP, Barrett ES, Beamer PI, et al. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) Program. J Expo Sci Environ Epidemiol 2020;30(3):397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendiola J, Meeker JD, Jørgensen N, et al. Urinary concentrations of di(2-ethylhexyl) phthalate metabolites and serum reproductive hormones: pooled analysis of fertile and infertile men. J Androl 2012;33(3):488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley JP, Engel SM, Braun JM, et al. Prenatal Phthalate Exposures and Body Mass Index Among 4- to 7-Year-old Children: A Pooled Analysis. Epidemiology 2016;27(3):449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel SM, Bradman A, Wolff MS, et al. Prenatal Organophosphorus Pesticide Exposure and Child Neurodevelopment at 24 Months: An Analysis of Four Birth Cohorts. Environ Health Perspect 2016;124(6):822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adgent MA, Carroll KN, Hazlehurst MF, et al. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ Int 2020;143:105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadopoulou E, Haug LS, Sakhi AK, et al. Diet as a Source of Exposure to Environmental Contaminants for Pregnant Women and Children from Six European Countries. Environ Health Perspect 2019;127(10):107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balalian AA, Liu X, Herbstman JB, et al. Prenatal exposure to organophosphate and pyrethroid insecticides and the herbicide 2,4-dichlorophenoxyacetic acid and size at birth in urban pregnant women. Environ Res 2021;201:111539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harley KG, Engel SM, Vedar MG, et al. Prenatal Exposure to Organophosphorous Pesticides and Fetal Growth: Pooled Results from Four Longitudinal Birth Cohort Studies. Environ Health Perspect 2016;124(7):1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Q, Cornelis MC, Townsend MK, et al. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect 2014;122(6):616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philippat C, Mortamais M, Chevrier C, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect 2012;120(3):464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starling AP, Engel LS, Calafat AM, et al. Predictors and long-term reproducibility of urinary phthalate metabolites in middle-aged men and women living in urban Shanghai. Environ Int 2015;84:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005;113(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 1993;54(10):615–27. [DOI] [PubMed] [Google Scholar]
- 15.Levine L Evaluation of urinary lead determinations. I. The significance of the specific gravity. J Ind Hyg Toxicol. 1945;27:217–223. [Google Scholar]
- 16.Kuiper JR, O’Brien KM, Ferguson KK, Buckley JP. Urinary specific gravity measures in the U.S. population: Implications for the adjustment of non-persistent chemical urinary biomarker data. Environ Int 2021;156:106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ Health Perspect 2016;124(2):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaines LG, Fent KW, Flack SL, et al. Effect of creatinine and specific gravity normalization on urinary biomarker 1,6-hexamethylene diamine. J Environ Monit 2010;12(3):591–9. [DOI] [PubMed] [Google Scholar]
- 19.Xia Y, Wong LY, Bunker BC, Bernert JT. Comparison of creatinine and specific gravity for hydration corrections on measurement of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in urine. J Clin Lab Anal 2014;28(5):353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkins HB, Pagnotto LD, Smith HL. Concentration adjustments in urinalysis. Am Ind Hyg Assoc J 1974;35(9):559–65. [DOI] [PubMed] [Google Scholar]
- 21.MacPherson S, Arbuckle TE, Fisher M. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol 2018;28(5):481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health 2001;74(1):63–7. [DOI] [PubMed] [Google Scholar]
- 23.Nermell B, Lindberg AL, Rahman M, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res 2008;106(2):212–8. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson KK, Rosen EM, Barrett ES, et al. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environment International 2019;133:105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett ES, Sathyanarayana S, Janssen S, et al. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol 2014;176:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun JM, Bellinger DC, Hauser R, et al. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 2017;58:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swan SH, Sathyanarayana S, Barrett ES, et al. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod 2015;30(4):963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saetun P, Semangoen T, Thongboonkerd V. Characterizations of urinary sediments precipitated after freezing and their effects on urinary protein and chemical analyses. Am J Physiol Renal Physiol 2009;296(6):F1346–54. [DOI] [PubMed] [Google Scholar]
- 29.Lykken GI, Jacob RA, Munoz JM, Sandstead HH. A mathematical model of creatine metabolism in normal males--comparison between theory and experiment. Am J Clin Nutr 1980;33(12):2674–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized for this study are not publicly available but de-identified data may be available on request with review of a detailed data analysis plan by the TIDES Investigator Team. Access to data will also require approval by your institution’s IRB and a formal data use agreement. Contact the corresponding author for more information.


