Abstract
Exploiting the pluripotent properties of embryonic stem cells (ESCs) holds great promise for regenerative medicine. Nevertheless, directing ESC differentiation into specialized cell lineages requires intricate control governed by both intrinsic and extrinsic factors along with the actions of specific signaling networks. Here, we reveal the involvement of the p21-activated kinase 4 (Pak4), a serine/threonine kinase, in sustaining murine ESC (mESC) pluripotency. Pak4 is highly expressed in R1 ESC cells compared with embryonic fibroblast cells and its expression is progressively decreased during differentiation. Manipulations using knockdown and overexpression demonstrated a positive relationship between Pak4 expression and the clonogenic potential of mESCs. Moreover, ectopic Pak4 expression increases reprogramming efficiency of Oct4-Klf4-Sox2-Myc-induced pluripotent stem cells (iPSCs) whereas Pak4-knockdown iPSCs were largely incapable of generating teratomas containing mesodermal, ectodermal and endodermal tissues, indicative of a failure in differentiation. We further establish that Pak4 expression in mESCs is transcriptionally driven by the core pluripotency factor Nanog which recognizes specific binding motifs in the Pak4 proximal promoter region. In turn, the increased levels of Pak4 in mESCs fundamentally act as an upstream activator of the Akt pathway. Pak4 directly binds to and phosphorylates Akt at Ser473 with the resulting Akt activation shown to attenuate downstream GSK3β signaling. Thus, our findings indicate that the Nanog-Pak4-Akt signaling axis is essential for maintaining mESC self-renewal potential with further importance shown during somatic cell reprogramming where Pak4 appears indispensable for multi-lineage specification.
Keywords: Pak4, Nanog, Akt, pluripotency, induced pluripotent stem cells (iPSCs), murine embryonic stem cells (mESCs)
Graphical Abstract
Graphical Abstract.
Significance Statement.
PI3K/Akt signaling plays an integral role in balancing pluripotency versus differentiation in ESCs although the upstream signals involved are not well understood. Here we show that Pak4, a serine-threonine kinase best known for signaling contributions in different cancers, functions as a direct upstream activator of Akt to elicit control over the pluripotent state of mESCs. This finding can likely be exploited for iPSCs technology since manipulating Pak4 expression in somatic cells can modify their reprogramming efficiency. These findings provide new insights into the regulatory control of pluripotency maintenance together with offering practical opportunities to optimize experimental manipulations of ESCs.
Introduction
Embryonic stem cells (ESCs) are derived from the inner cell mass (ICM) of pre-implantation blastocysts and possess two characterizing features, namely their self-renewal ability as well as their capacity for multi-lineage specification and differentiation.1-4 These features enable ESCs to form each of the 3 germ layers (pluripotency) and they are consequently employed in studies of mammalian development and in cell-based therapies. Even though ESCs have remarkable developmental plasticity, a major challenge involves how to efficiently control their generation into the desired lineages. Therefore, to harness the powerful self-developing properties of ESCs, it is first essential to understand the cellular mechanisms governing pluripotency and to dissect the pathways regulating lineage commitment.
Previous studies have indicated that mouse ESCs can be maintained in a state of pluripotency in the presence of soluble factors or small-molecule inhibitors. For example, the cytokine leukemia inhibitory factor (LIF) drives self-renewal of mouse ESCs by activating the transcription factor STAT35-8 while the MAP kinase/ERK kinase (MEK) and glycogen synthase kinase 3 (GSK3) inhibitors (2i, PD0325901 and CHIR99021) can increase the expression of pluripotency factors in ESCs.9 Furthermore, BIO (6-bromoindirubin-3ʹ-oxime), a GSK3-specific pharmacological inhibitor, activates the Wnt/β-catenin pathway and constrains the differentiation of ESCs via maintaining the expression of various transcription factors including Oct4, Sox2, Nanog, Klf4, Stat3, and Rex1.10,11 Nonetheless, while a broad complement of factors can sustain pluripotency, the trio of Oct4, Sox2, and Nanog are considered to represent the core elements.12
Oct4 belongs to the Pit-Oct-Unc (POU) family of homeodomain proteins and plays a critical role in the establishment and maintenance of ESC pluripotency, for example, pluripotency is lost in the inner cell mass (ICM) of Oct4-null embryos which only differentiates to the trophoblast lineage.13,14 Similarly, mESCs deficient in Sox2 differentiate into trophectoderm15 and Sox2-null blastocysts fail to form a pluripotent ICM.16 Nanog was discovered via a screen for pluripotency factors that could sustain mESC self-renewal in the absence of LIF,17,18 and intriguingly, Nanog-null mESCs still sustain pluripotency although with the tendency to differentiate.18,19 Thus, it is proposed that Nanog is essential for the acquisition of pluripotency, but is dispensable once pluripotency is achieved.19 Such reports reflect the general consensus that Oct4, Sox2 and Nanog occupy the top hierarchical position among signaling networks acting to maintain the pluripotency characteristics of ESCs. However, many important details of the underlying mechanisms largely remain unclear, particularly the details of how different circuitries are interconnected.
A range of studies has established the essential role of PI3K/Akt signaling in maintaining the undifferentiated state of ESCs as well as regulating somatic cell reprogramming. For example, several reports involving murine ESCs have shown that Akt functions upstream to phosphorylate GSK3α and GSK3β at Ser21 and Ser9 residues, respectively, with the resulting GSK3α/β inactivation serving to upregulate the pluripotency factors TBX3 and Nanog.20,21 In contrast, PI3K/Akt inhibition achieved with PI3K or p110β-specific inhibitors along with deficiencies in PI3K-related genes results in the downregulation of pluripotency markers and loss of ESC self-renewal potential.22-24 Moreover, ectopic expression of constitutively active Akt was sufficient to maintain pluripotency in mouse and primate ESCs in the absence of feeder cells.25 Furthermore, several reports have demonstrated that PI3K/Akt signaling promotes induced pluripotent stem cell (iPSC) generation via inhibiting GSK3β and FOXO1, while concurrently enhancing glycolysis, a metabolic characteristic of pluripotent cells.26-28 Consistently, other evidence shows that inhibition of Akt hinders somatic cell reprogramming, which is mitigated by the inhibition of GSK3.29 Moreover, another study reported that PI3K/Akt signaling was indispensable for the generation and maintenance of iPSCs, at least in the end-stage of reprogramming.30 Together these reports provide strong evidence that PI3K/Akt signaling is required for the modulation of ESC self-renewal and pluripotency, as well as the generation of iPSCs.
In this study, we performed comparative proteomics to discover differences between mouse embryonic fibroblast cells (MEF) and mESC-R1 (R1) cells which reflect differentiated and pluripotent cell states, respectively. Following on from this analysis, functional screening led to the identification of p21-activated kinase 4 (Pak4) as a novel pluripotency maintaining factor. Pak4 or p21-activated kinase 4 is a member of the small Pak gene family of serine/threonine kinases. The six members of the family can be broadly categorized into two groups depending on structural and functional differences. Group A (also termed group I) includes Pak1, −2 and −3 whereas group B/II includes Pak4, −5 and −6.31 All Pak members contain an N-terminal p21-binding domain (PBD) and C-terminal kinase domain while Pak4 also possesses a pseudosubstrate domain (PSD). Pak4 has been associated with neurological conditions such as Parkinson’s disease31 but has been most intensively studied from the perspective of malignancy which promotes the progression of different cancer types. For example, Pak4 signaling has been variously linked to the control of cell proliferation,32 survival,33 invasion and metastasis,34 the epithelial-mesenchymal transition, drug resistance35,36, and the maintenance of cancer stem cell-like phenotypes.37 However, few studies to date have considered the role of Pak4 in regulating ESC pluripotency and somatic cell reprogramming.
Herein, we reveal that Pak4 expression in mESCs is indispensable for maintaining their stemness characteristics while also being strongly induced during somatic cell reprogramming. We further establish that Pak4 acts upstream of the PI3K/Akt pathway to facilitate mESC pluripotency through direct activation of Akt. Moreover, we reveal that Pak4 transcription in mESCs is driven by Nanog. These results identify Pak4 as a novel pluripotency regulator together while providing a previously unrecognized coordinating element acting between Nanog and its downstream transcriptional program.
Materials and Methods
Cell Culture
Mouse embryonic fibroblast cells (MEF) and HEK-293T were cultured in high glucose (25mM) DMEM (Thermo Fisher Scientific) with 10% v/v fetal bovine serum (BI, Biological Industries), 4 mM L- glutamine, 1% v/v penicillin-streptomycin (Gibco) and 1mM pyruvate (Gibco) and maintained at 37 °C in a humidified 5% CO2-containing atmosphere. Mouse embryonic stem cells (mESC-R1 and mESC-AB2.2) were maintained in DMEM (Thermo Fisher Scientific) with 10% v/v ES cell fetal bovine serum (Gibco), 10% Knockout Serum Replacement (Gibco), 2mM Glutamax, 100 μM non-essential amino acids (Gibco), 0.1 mM β-mercaptoethanol (Sigma), 1% v/v penicillin-streptomycin (Gibco), 1 mM sodium pyruvate (Invitrogen) and 1000 U ml−1 LIF (Millipore) on gelatin (0.1% v/v)-coated plates. Fifty percent DMEM/F12 (Gibco), 50% Neurobasal (Gibco), 1% N-2 supplement (100×) (Gibco), 2% B27 supplement (50×) (Gibco), 2 mM Glutamax, 0.05% BSA (Gibco), 100 μM non-essential amino acids, 0.1 mM β-mercaptoethanol (Sigma), 1% v/v penicillin-streptomycin (Gibco), 1mM sodium pyruvate (Invitrogen) N2B27 medium for serum-free culture. For the 2i+LIF cultures, N2B27 medium was supplemented with CHIR99021 (MCE 3 μM), PD0325901 (MCE 1 μM) and 1000 U ml−1 LIF. Cell line authenticity was verified by STR analysis.
Proteomic and Bioinformatic Analyses
The detection of differentially expressed proteins between immortalized MEF and mESC-R1 cells was conducted commercially by PTM Biolabs Inc. using an integrated approach involving a label-free strategy, HPLC fractionation, and LC–MS/MS analyses.
The resulting MS/MS data were processed using the MaxQuant search engine (v1.5.2.8). Tandem mass spectra were searched against mouse database concatenated with a reverse decoy database. Trypsin/P was specified as a cleavage enzyme allowing up to 2 missing cleavages. The first search range was set to 20 ppm for precursor ions, main search range set to 5 ppm, and fragment ions set to 0.02 Da. Carbamidomethyl on Cys was specified as fixed modification and oxidation on Met, acetylation on protein N-terminal, deamidation on Asn, and Gln was specified as variable modifications. Label-free quantification method was LFQ, FDR was adjusted to <1% and min. score for modified peptides score was set >40. Student’s t-test was used to evaluate the significant differences and proteins with a fold change of above 2 or below 1/2 and P-value < .05 were considered as differentially expressed proteins. The mass spectrometry data have been deposited to the ProteomeXchange Consortium with the dataset identifier PXD034181. For further functional screening, we selected 10 candidate proteins within the top 100 down-regulated proteins (2.7<R1/MEF ratio < 5.5).
RNA Inference, Transduction, and Transfection
Experiments involving gene knockdown and overexpression were conducted using lentiviral-mediated transduction with short hairpin RNAs (shRNAs) (Supplementary Table S1), pSin-based constructs (Supplementary Table S2) and the STEMCCA plasmids-expressing Oct4, Sox2, Klf4, and c-Myc. Lentiviral particles were generated by transfection of 293T cells with PLKO.1 vectors containing specific shRNAs, pSin-based constructs, or STEMCCA plasmids along with pREV, pGag, pVSVG at the ratio of 2:2:2:1 or PSPAX2 and Pmd2.g at the ratio of 2:2:1 in Opti-MEM medium (Gibco) for 48 h. Supernatants were filtered with a 0.45 μm PVDF filter before infecting target cells for 24 h and subsequent selection with 5 μg/mL puromycin. Alternatively, transfections were performed with the indicated plasmids (Supplementary Table S2) using the lipofectamine-2000 reagent (Invitrogen) according to the manufacturer’s instructions and analyzed after 48 h.
In Vitro Differentiation of Mouse Embryonic Stem Cells
Mouse ESCs were cultured in LIF deprivation ESC medium or both LIF deprivation and treated with 10−7 M RA (Sigma) on gelatin (0.1% v/v)-coated plates. Embryoid body formation was induced by incubating the ESCs in an ESC medium without LIF under non-adherent conditions. The hanging drops method was used to obtain EBs. Briefly, ES cells were dissociated into single cells and diluted into 200-300 cells/μL before culturing in hanging drops (20 μL/drop) for 3 days, followed by reseeding on gelatin-coated 6-well plates for another 10 days.
Alkaline Phosphatase Staining
Single cell suspensions of mouse embryonic stem cells were plated at 200 cells per cm2 in GMEMβ/10% FCS with the indicated LIF concentrations or 2i+LIF medium or serum and LIF free N2B27 medium for 3-5 days. After incubation, the resulting colonies were fixed in 4% formaldehyde for 15 min at room temperature before rinsing 3 times with PBS. Thereafter, the colonies were stained using the Alkaline phosphatase Detection Kit (Millipore/STEMGENT) according to the manufacturer’s instruction and the numbers of AP-positive colonies counted. Where indicated, colonies were scored into 3 morphology categories, “domed,” “mixed,” and “flattened” representing undifferentiated, mixed, and differentiated colonies, respectively.
Induction of Pluripotent Stem Cells
Primary mouse embryonic fibroblast cells (MEF) were isolated from E13.5 embryos and induction of pluripotent stem cells was carried out according to a previous study.38 MEF cells (passage 1) were infected with two sequential rounds of lentiviral supernatants containing STEMCCA plasmids-expressing Oct4, Sox2, Klf4, and c-Myc followed by selection with 5 μg/mL puromycin. The cells were then harvested and plated onto mitomycin-C-treated (10 μg/mL for 2.5 h) MEF feeders and cultured with mouse ESC medium. After 14 days infection, the colonies were stained for alkaline phosphatase and the numbers of AP-positive clones were counted or picked and cultured onto new feeder coated dishes as needed. For Pak4 silencing and overexpression, MEF cells (passage 1) were infected with two sequential rounds of lentiviral supernatants containing OKSM (Oct4, Klf4, Sox2, and c-Myc) and Pak4 shRNAs or pSin-based Pak4 followed by induction as the description above. The efficiency of knockdown and overexpression was detected by Western blot after 4 days post-infection prior to plating the cells onto feeders.
Western Blotting and Immunoprecipitation
Cell lysates were prepared with RIPA buffer containing protease inhibitors (Beyotime) and clarified by centrifugation at 10 000×g for 15 min at 4 °C. Equal protein amounts as determined using the Bio-Rad RC/DC protein assay were electrophoresed by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 4% skim milk and incubated with primary antibodies overnight at 4 °C, decorated with horseradish peroxidase-conjugated secondary antibodies with detection using chemiluminescence (Advansta). Alternatively, for immunoprecipitations, cell lysates prepared with IP buffer (0.5% NP-40, 20 mM Tris pH 7.4, 150 mM NaCl, 1.5 mM MgCl2) and protease inhibitor cocktail (Solarbio) were incubated with primary antibodies adsorbed to protein A/G-Sepharose (Invitrogen) beads for 4 h. After washing the beads 5 times with IP buffer the samples were eluted and subjected to Western blotting. Antibody sources/dilutions are shown in Supplementary Table S3.
Immunofluorescence Staining
The iPS cells (passage 3) were cultured on mitomycin-C-treated MEF feeders for 4 days before fixation with 4% formaldehyde. Thereafter, the cells were permeabilized with 0.2% Triton X-100, blocked with 1% BSA in PBS, and washed using PBS with 0.05% Tween-20 (PBST). Primary antibodies in PBST were then added overnight at 4 °C before washing and the addition of appropriate fluorochrome-conjugated secondary antibodies for 1 h at room temperature. Samples were counterstained with DAPI, mounted in anti-fade mounting medium, and images collected using a Zeiss LSM 700 confocal microscope.
qRT-PCR
Total RNA was extracted using the TRIzol Reagent (Invitrogen) as previously described.39 cDNA was prepared from total RNA using the PrimeScriptTM RT reagent kit (TaKaRa) according to the manufacturer’s instructions. Quantitative RT-PCR analyses were performed using the specified primers (Supplementary Table S4) with the One-Step PrimeScript RT-PCR kit (TaKaRa). Relative expression values were calculated using the comparative Ct method normalized against the beta-actin housekeeping gene.
Dual-Luciferase Reporter Assay
HEK-293T cells seeded in 2 cm2 plates were co-transfected with the indicated pGL3-based reporter plasmids (Supplementary Table S2) along with Renilla luciferase. After 24 h the results were assessed using the Dual-Luciferase Reporter Assay System (Promega) with firefly luciferase values corrected against Renilla measurements according to the manufacturer’s instructions.
ChIP Assays
The Millipore ChIP kit was used according to the manufacturer’s instructions. Briefly, cells were first cross-linked with 1% formaldehyde for 10 min at room temperature before quenching the reaction by adding 0.125M glycine. Nuclei were then isolated and resuspended in lysis buffer (50 mM Tris–HCl (pH 8.1), 10 mM EDTA, 1% SDS, and protease inhibitors). Cell lysates were sonicated and incubated with protein A/G-Sepharose beads conjugated with either anti-Nanog antibodies or control IgG. Bound DNA fragments were subjected to semi-quantitative RT-PCR using the specified primers (Supplementary Table S4).
CRISPR/Cas9
CRISPR/Cas9-mediated Pak4 gene-editing vectors were constructed by annealing gRNA oligonucleotide pairs (Supplementary Table S1) and subcloning into lentiCRISPRv2 (one vector system) according to the Zhang laboratory protocol. Lentiviral particles produced as described above using a 1:2:2 mixture of plasmids (Pmd2.g, PSPAX2, and lentiCRISPRv2) were used to transduce target mESC cells. After selection with 5 μg/mL puromycin, stably infected cells were plated in 96 well plates and single-cell clones screened by Western blot and gDNA sequencing to identify Pak4 knockout cells.
In Vitro Kinase Assays
Recombinant Pak4, Akt, and mutant proteins were isolated from transfected HEK-293T cells. Briefly, cells were individually transfected with pSin-Flag-based plasmids encoding Flag-Pak4, Flag-Akt WT, Flag-Akt (T308A), Flag-Akt (S473A), and Flag-Akt (T308A&S473A) using Lipofectamine-2000 reagent (Invitrogen) according to the manufacturer’s instructions. Cell lysates prepared using IP lysis buffer (0.5% NP-40, 150 mM NaCl, 20 mM HEPES, pH 7.5, 2 mM EDTA, and 1.5 mM MgCl2) supplemented with protease inhibitor cocktail were incubated with anti-Flag (M2) antibody-conjugated beads (Sigma). After overnight incubation at 4 °C, the beads were washed 5 times with IP buffer before competitive elution of the recombinant proteins with 150 ng/μL 3× Flag peptides (APE × Bio) for 1 h at 4 °C. The eluates were then transferred to 30 kDa protein concentration columns (Millipore) to concentrate the recombinant proteins and dialyze out the 3× Flag peptides. In vitro kinase assays were performed as 60 μL reactions containing 1× kinase buffer (CST), 7 μg Flag-Pak4 as kinase, and 3 μg WT or mutant Flag-Akt as substrates, supplemented with 30 μM ATP at 30 °C for 30 min. After the reaction, the samples were subjected to Western blot analysis.
Teratoma Formation
BALB/c nude mice (5 weeks old, male) were obtained from Shanghai SLAC Laboratory Animal Co. Ltd. After 3 days of acclimatization, mice were injected subcutaneously (s.c.) with 1.0 × 106 iPS cells (passage 5) in the posterior flanks. Mice were maintained for 4 weeks before the animals were humanely sacrificed and the teratomas excised, fixed, and subjected to histopathological analysis using H&E staining (Servicebio). Studies were conducted with approval from the Animal Research Ethics Committee of the University of Science and Technology of China.
Statistical Analysis
Data were assumed to be normally distributed and continuous variables were expressed as mean ± SD. All analyses were performed by two-tailed Student’s t-test using GraphPad Prism 8 with significance defined as P ≤ .05. Reproducibility and the number of replicates used are defined in the corresponding Figure legends.
Results
Identification of Pak4 as a Pluripotency Factor in mES Cells
Previous studies have established a general paradigm where pluripotency factors undergo inactivation and/or downregulation following differentiation. Based on this principle, we sought to uncover novel pluripotency factors by conducting comparative proteomic analyses between mouse embryonic fibroblast cells (MEF) and mESC-R1 cells (R1). For further screening, we selected 10 of the top 100 proteins that were downregulated in MEF cells (refer Methods) as these proteins were potentially involved in maintaining ESC pluripotency (Fig. 1a). Consistent with the screening data, all 10 proteins were upregulated to some degree, but of these candidates, Pak4, Pex3, Gart, Pgam5, Rcc2, and Got1 were most noticeably increased in R1 cells (Fig. 1b). Moreover, we found by qPCR analysis that the levels of all 6 genes were likewise increased in R1 compared to MEF cells (Supplementary Fig. S1a).
Figure 1.
Identification of Pak4 as a pluripotency relevant factor in mES cells. (a) Heat map comparisons of 10 candidate proteins (Pak4, Pex3, Gart, Pgam5, Rcc2, Got1, Acsl4, Fh, Aco2, and Sdha) showing significantly higher differential expression levels in mouse embryonic fibroblast cells (MEF) versus mES-R1 cells. (b) Western blotting comparing expression of the 10 proteins from (a) in MEF versus mES-R1 cells. Sox2 and actin served as positive and loading controls, respectively. (c) Western blot analysis of Pak4 and Sox2 levels in mES-R1 cells induced to differentiate after treatment of 10 nM RA for 0–4 days. (d) Expression of Nanog and the Yamanaka factors (Oct4, Sox2, Klf4, and c-Myc) measured by Western blot in mES-R1 cells transduced with control (pLKO.1) or 3 independent shRNAs targeting Pak4. (e–h) mES-R1 cells from (d) were cultured at 200 cells per cm2 in serum/LIF medium (e, f) or 2i+LIF medium (g, h) for 4 days. After alkaline phosphatase (AP) staining (e, g), the differentiation states of the resulting colonies were classified as “domed” (black arrow; undifferentiated), “mixed” (blue arrow) or “flattened” (green arrow; differentiated) (f, h). (i) R1 cells transfected were transfected with an empty Flag vector (control) or Flag-Pak4 and the expression of Nanog and the Yamanaka factors were measured by Western blot. (j, k) R1 cells from (i) were cultured to establish colonies under chemically defined conditions (serum and LIF free N2B27 medium) for 4 days (j). Thereafter, alkaline phosphatase staining was performed and total numbers of AP-positive colonies were counted (k). (b-k) Data represent three independent experiments. (f, h, k) Data are mean ± SD, n = 3, *P < .05; **P < .01; ***P < .001; ns, not significant, two-tailed paired Student’s t-test. (e, g, j) Scale bar represents 200 μm.
Next, to interrogate the functional role of these proteins in the regulation of ESC pluripotency, we individually knocked down each gene in R1 cells and examined the effects on the expression of the pluripotency factors Oct4, Nanog, Klf4, and Sox2. Notably, among the 6 genes, we found that only knockdown of Pak4 led to the decreased expression of Oct4, Nanog, Klf4, and Sox2 (Supplementary Fig. S1b), which strongly suggested the loss of pluripotency. In addition, the expression of Pak4 was downregulated in response to LIF deprivation (Supplementary Fig. S1c, S1d), retinoic acid (RA)-induced ES cell differentiation, and embryoid body formation (Fig. 1c and Supplementary Fig. S1e). Together these results proposed that Pak4 may exert a role in maintaining mESC pluripotency and we consequently focused our studies on Pak4.
First, to ensure the effects of Pak4 knockdown in mESCs were specific, we employed 3 independent shRNAs targeting Pak4. Indeed, knockdown with all 3 shRNAs in independent ES clones (R1 and AB2.2) resulted in remarkably decreased expression of pluripotency markers (Fig. 1d and Supplementary Fig. S1f), ruling out the possibility of off-target effects and clonal differences. Moreover, Pak4 knockdown reduced and increased the percentage of “domed/mixed” and “flattened” colonies, respectively, indicative of more differentiated colonies (Fig. 1e, 1f). Similarly, after the assays were conducted in 2i+LIF medium, Pak4 silencing also decreased the percentage of “domed” morphology colonies, and to a greater degree compared with LIF/serum culture conditions (Fig. 1g, 1h). Likewise, Pak4 knockdown in AB2.2 ESCs also significantly decreased the percentage of undifferentiated colonies (Supplementary Fig. S1g, S1h). Conversely, ectopic expression of Pak4 increased the expression of the pluripotency markers (Fig. 1i) with concomitant increases in the number of AP-positive colonies (Fig. 1j, 1k). Together, these data demonstrate an important role of Pak4 in sustaining mESC pluripotency and protecting mESCs from differentiation.
Pak4 Modulates the Efficiency of Somatic Cell Reprogramming
Considering the positive effects of Pak4 on maintaining mESC pluripotency, we hypothesized that Pak4 would also facilitate somatic cell reprogramming. To test this notion, we first examined the expression levels of Pak4 in primary MEF cells and induced pluripotent stem cells (iPSCs). Instructively, we found that both Pak4 mRNA and protein levels were progressively increased during OKSM (Oct4, Klf4, Sox2, and c-Myc)-induced MEF cell reprogramming (Fig. 2a). Moreover, the high comparative levels of Pak4 in R1 cells compared to primary MEF were also reflected in iPSCs (passage 3) (Fig. 2b), with correspondingly high expression of the core pluripotency markers (Fig. 2c).
Figure 2.
Pak4 dictates somatic cell reprogramming efficiency. (a) Primary MEF cells were transfected with OKSM (Oct4, Klf4, Sox2, c-Myc) to promote reprogramming to iPSCs. The relative mRNA levels of Pak4 were determined by qPCR (upper panels) and Western blot analyses of Pak4 (lower panels) at 0-, 4-, and 8-days post-transfection. The relative levels of Pak4 were determined by densitometry and normalization against the actin loading control. (b) The mRNA (upper panels) and protein (lower panels) levels of Pak4 as determined by qPCR and Western blot, respectively, were used to compare Pak4 expression levels of Pak4 in primary MEF, OKSM-derived iPS cells (passage 3), and mES-R1 cells. (c) Western blot analyses of Oct4, Nanog, Klf4, and Sox2 in the cells from (b). (d) Primary MEF cells were transfected with OKSM to induce iPSCs in conjunction with transduction of control (pLKO.1) or Pak4 targeting shRNAs followed by selection with 5 μg/mL puromycin. Four days after infection, Western blot was used to confirm Pak4 knockdown efficiency with reprogramming efficacy assessed after 14 days by counting total AP-positive colonies. (e) The iPSC assay in (d) was repeated on primary MEF cells transfected with Flag control or a Flag-tagged Pak4 construct. (f) Comparison of the mRNA levels of Oct4, Sox2, Nanog, Rex1, Fbx15, and Esg1 determined by semi-quantitative PCR in MEF, mES-R1, and control and Pak4-shRNA OKSM-derived iPSC colonies. (g) Comparison of protein levels of Pak4, Oct4, Sox2, Nanog, and Klf4 in control and Pak4-shRNA OKSM-derived iPSC colonies. (h) Control and Pak4-shRNA OKSM-derived iPSCs were injected s.c. into the right posterior flanks of nude mice. Teratomas derived from all control iPSC colonies exhibited mesodermal, ectodermal, and endodermal tissues but only 1 of the 6 OKSM+shPak4 derived colonies tested gave rise to a multi-lineage teratoma. (a–e) Data represent three independent experiments. (h)n = 5 nude mice. (a–e) Data are mean ± SD, n = 3, *P < .05; **P < .01; ***P < .001; ns, not significant, two-tailed paired Student’s t-test. (d–e, h) Scale bar represents 100 μm, 50 μm, respectively.
Next, to functionally verify that Pak4 contributes to MEF reprogramming, we silenced Pak4 expression during OKSM-mediated reprogramming. Indeed, knockdown of Pak4 markedly inhibited iPSC reprogramming efficiency as evident through the significantly decreased number of AP-positive colonies (Fig. 2d). Conversely, ectopic Pak4 expression resulted in increased numbers of AP-positive colonies (Fig. 2e). These results suggest that Pak4 was indispensable for somatic cell reprogramming. Intriguingly, semi-quantitative reverse transcription PCR (RT-PCR) and immunofluorescence analyses revealed that ES cell marker genes including Oct4, Sox2, Nanog, SSEA1, Rex1, Fbx15, and Esg1 remained highly expressed in OKSM+shPak4 derived colonies and at similar levels to the control OKSM-derived iPSCs (Fig. 2f and Supplementary Fig. S2). Notably, analysis of individual iPSC colonies after Pak4 silencing revealed variable protein levels of the core transcription factors although each different colony showed reductions in at least two of the four factors compared to the shRNA control (Fig. 2g). Furthermore, evaluation of teratoma formation ability in the iPSCs derived from these experiments showed that just 1 of 6 OKSM+shPak4-derived colonies gave rise to teratoma containing mesodermal, ectodermal, and endodermal tissues (Fig. 2h). Collectively, these findings indicate that Pak4 is required for efficient reprogramming of MEF cells.
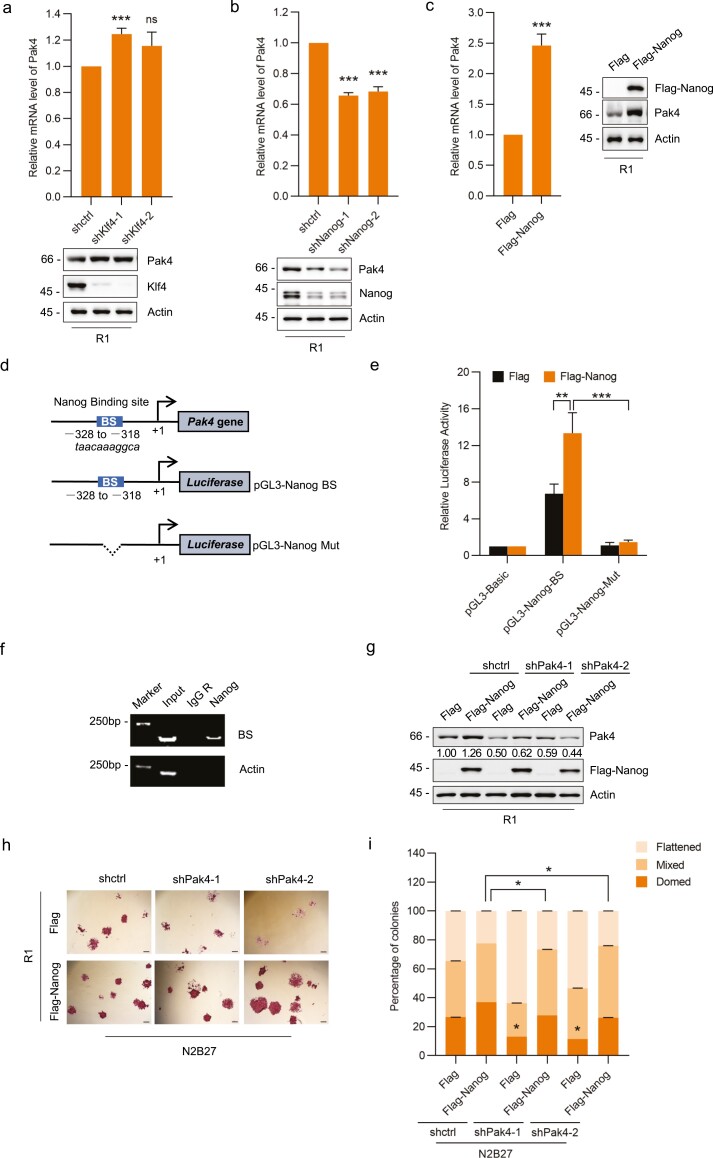
Pak4 Is Transcriptionally Regulated by Nanog
Given the elevated Pak4 transcript levels associated with pluripotent mESCs and iPSCs, it was reasonable to assume that Pak4 may be regulated by one or more of the major pluripotency-associated transcription factors. Analysis of the proximal promoter region of the Pak4 gene using the JASPAR database for potential Oct4, Sox2, Klf4, or Nanog binding motifs revealed that only high scoring binding sites (BSs) for Klf4 and Nanog Supplementary (Table S5). Thereafter, we knocked down either Klf4 and Nanog in R1 cells but only knockdown of Nanog led to markedly decreased Pak4 mRNA and protein levels (Fig. 3a, 3b). Consistently, ectopic expression of Nanog resulted in strong increases in both Pak4 mRNA and protein levels (Fig. 3c), implying that Nanog may be involved in the transcriptional regulation of Pak4.
Figure 3.
Pak4 is transcriptionally regulated by Nanog (a, b) mES-R1 cells were transduced with control (pLKO.1) or shRNAs targeting Klf4 (a) or Nanog (b) and relative Pak4 mRNA levels measured by qPCR (upper panels). Western blot analyses (lower panels) were used to determine the protein levels of Pak4 and the efficiency of knockdown. (c) mES-R1 cells were transfected with either Flag control or Flag-Nanog and relative Pak4 mRNA and protein levels were measured by qPCR (upper panels) and Western blot, respectively. Nanog overexpression was confirmed by blotting against Flag. (d) Schematic illustration of the putative Nanog binding site present in the proximal promoter region of Pak4 and the design of pGL3-based luciferase reporter constructs (BS, BS-deletion mutant). (e) HEK-293T cells were transfected with pGL3 (control) or the individual Pak4 promoter reporters in combination with Renilla and/or expression vectors encoding Flag (empty) or Flag-Nanog. After 24 h, dual luciferase measurements were performed and firefly luciferase activities for the pGL3-Nanog and -Nanog-Mut constructs normalized to the pGL3-basic levels. (f) Chromatin immunoprecipitation (ChIP) assays were performed in mES-R1 cells against negative control IgG or anti-Nanog antibodies. Semi-quantitative PCR was performed using ChIP primers targeting the region encompassing the Nanog BS in the Pak4 promoter or alternatively actin control primers. (g) mES-R1 cells were transduced with control (pLKO.1) or Pak4 targeting shRNAs in combination with transfected with either Flag or Flag-Nanog. Western blot analyses were conducted to confirm Nanog overexpression and Pak4 knockdown, respectively. (h, i) Cells from (g) were cultured for 3 days under chemically defined conditions and after alkaline phosphatase (AP) staining (h), the differentiation state of the resulting colonies was classified as “domed” (undifferentiated), “mixed” or “flattened” (differentiated) (i). (a–i) Data represent 3 independent experiments. (a–c, e, i) Data are mean ± SD, n = 3, *P < .05; **P < .01; ***P < .001; ns, not significant, two-tailed paired Student’s t-test. (h) Scale bar represents 200 μm.
To further test this notion, we constructed luciferase reporter plasmids based on the Pak4 promoter that contained either the intact or truncated Nanog BS (Fig. 3d). Indeed, luciferase reporter activity in the intact Nanog BS construct was responsive to ectopic expression of Flag-Nanog, while decisively, all reporter activity was abrogated when this binding sequence was deleted (Fig. 3e). Moreover, chromatin immunoprecipitation (ChIP) assays against Nanog showed that chromatin fragments of the Pak4 gene promoter containing the Nanog BS were recovered from R1 cells (Fig. 3f). As anticipated, ectopic expression of Nanog increased the percentage of undifferentiated colonies formed by R1 mESCs. But remarkably, Pak4 knockdown significantly attenuated the proportion of undifferentiation colonies formed in the presence of ectopically expressed Nanog (Fig. 3g–3i). Thus, Pak4 is a direct transcriptional target of Nanog in mESCs with further evidence showing that Pak4 expression is necessary, albeit partially, for driving the downstream pluripotency program of Nanog.
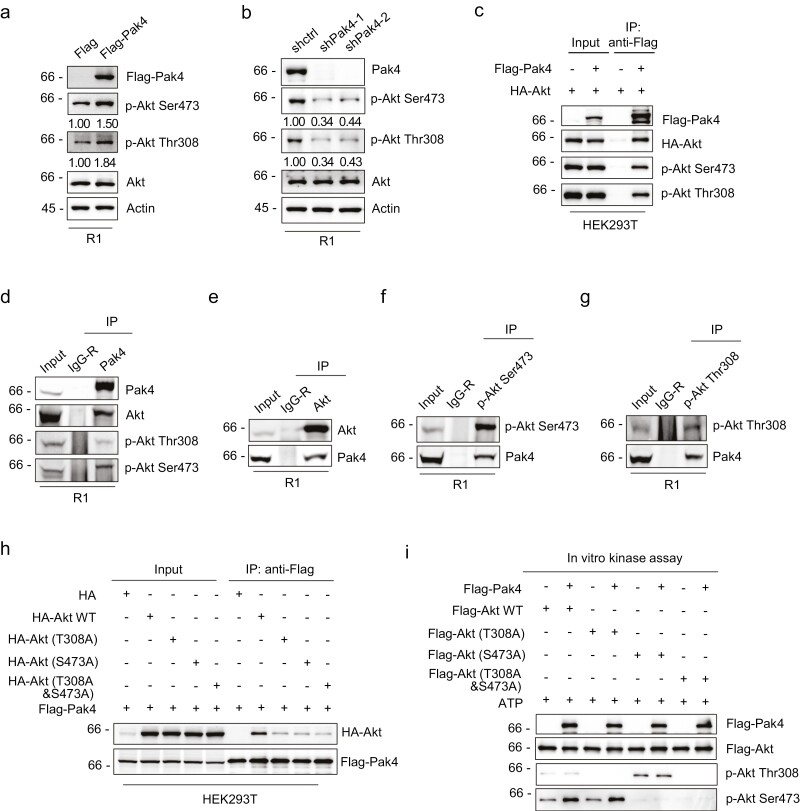
Pak4 Phosphorylates Akt at Serine 473
After deciphering how Pak4 was transcriptionally regulated in the pluripotent condition, we next considered its potential downstream targets. Prior cues have linked Pak1 to the regulation of Akt, either in a kinase-independent manner by serving as a scaffold to facilitate Akt stimulation by PDK140 or in a kinase-dependent manner by facilitating Ser473 phosphorylation in Akt.41 As expounded in the Introduction, strong evidence links PI3K/Akt signaling to pluripotency maintenance although few studies have associated Pak4 with the regulation of this pathway. Thus, to investigate the possibility that Pak4 was linked to Akt signaling, we examined the expression levels and activation of Akt after manipulating Pak4 expression in R1 cells. The latter was measured by assessing the relative phosphorylation levels of the Thr308 and Ser473 residues in Akt, generally considered requirements for Akt activity.42 Notably, ectopic expression of Flag-Pak4 resulted in markedly enhanced Akt phosphorylation at both Thr308 and Ser473 residues, whereas Pak4 knockdown led to markedly decreased phosphorylation (Fig. 4a, 4b). Manipulating Pak4 did not impact Akt expression levels, proposing that Pak4 was involved in Akt activation.
Figure 4.
Pak4 phosphorylates Akt at Ser473 (a) mES-R1 cells were transfected with either Flag or Flag-Pak4 constructs, and Western blotting was conducted against total, Ser473 (p-Akt Ser473) and Thr308-phosphorylated (p-Akt Thr308) forms of Akt. The relative levels of phosphorylated Akt were determined by densitometry with normalization against total Akt levels. Actin served as a loading control throughout. (b) mES-R1 cells transduced with control (pLKO.1) or Pak4 targeting shRNAs were subjected to Akt phosphorylation analysis as per (a). (c) HEK-293T cells were transfected with the indicated combinations of Flag-Pak4 (+) and HA-Akt (+) or empty control vectors (−) before conducting immunoprecipitations with anti-Flag (M2) antibodies. The samples were then subjected to Western blotting against Flag and HA along with antibodies against the Ser473 and Thr308-phosphorylated forms of Akt. (d–g) Immunoprecipitations were performed using mES-R1 cells against control IgG (IgG-R) in combination with antibodies against Pak4 (d), Akt (e), p-Akt Ser473 (f), and p-Akt Thr308 (g). The precipitated samples were subjected to immunoblotting as shown against total Akt, p-Akt Ser473, and p-Akt Thr308 and Pak4. Input control samples were used to verify all immunoprecipitation experiments. (h) HEK-293T cells were transfected with Flag-Pak4 in the indicated combination with an empty HA control vector or the indicated Akt constructs (WT, single T308A, and S473A substitution mutants or dual T308A&S473A mutant) before conducting immunoprecipitations with anti-Flag (M2) antibodies. The immunoprecipitated samples were then subjected to Western blotting against HA and Flag. (i) In vitro kinase assays performed with recombinant forms of Flag-Akt WT, Flag-Akt (T308A), Flag-Akt (S473A), Flag-Akt (T308A&S473A) in combination with Flag-Pak4 in the presence of ATP. The reactions were subjected to Western blotting against Pak4 and Akt to reveal Flag-Akt and Flag-Pak4, respectively, with Akt phosphorylation assessed with p-Akt Thr308 and p-Akt Ser473 antibodies. (a–i) Data represent 3 independent experiments.
Many modulators of Akt, both positive and negative, regulate the activation status of Akt through direct binding, for example, as occurs with Ras43 and the PH domain leucine-rich repeat protein phosphatase (PHLPP).44 Notably, co-immunoprecipitation (co-IP) analyses conducted between ectopically expressed Flag-Pak4 and HA-Akt indicated that Flag-Pak4 interacted with HA-Akt including its Thr308 and Ser473 phosphorylated forms (Fig. 4c). Importantly, associations between the endogenous forms of Pak4 and the Thr308/Ser473 phosphorylated forms of Akt could be readily reproduced in experiments conducted with R1 cells in a fully reciprocal manner (Fig. 4d–g). To further determine whether the interaction between Pak4 and Akt was dependent on Akt phosphorylation status, we assessed the potential of single and double substitution mutants of Akt (T308A, S473A, and T308A&S473A) to associate with Pak4. Indeed, the amount of HA-Akt co-precipitated with Flag-Pak4 was greatly diminished when either phosphorylated site was mutationally inactivated, with the levels of associated Akt similar for the single and double mutants (Fig. 4h). Collectively, these results indicate that Pak4 preferentially interacts with phosphorylated Akt although whether Pak4 kinase activity is directly responsible for Akt phosphorylation remained to be defined.
To address this question, we turned to in vitro kinase assays utilizing purified recombinant forms of Pak4, wildtype (WT) Akt, and the single and double substitution Akt mutants. Here the inclusion of Pak4 in these assays resulted in remarkably increased Ser473 phosphorylation levels in WT and T308A Akt mutant while the presence of Pak4 had no impact on the levels of T308 phosphorylation (Fig. 4i). As expected, no significant phosphorylation signals were observed in the T308A&S473A double Akt mutant. These findings indicate that Pak4 can directly and selectively promote the phosphorylation of Akt at Ser473.
Pak4 Maintains mESC Pluripotency via Activation of Akt
Given that Pak4 promotes phosphorylation of Akt and Akt signaling is sufficient to maintain pluripotency in ESCs,25 we sought to determine whether Pak4 could regulate mESC pluripotency via Akt activation. Notably, we observed that knockdown of Akt in Pak4-overexpressing R1 cells inhibited the expression increases in Oct4, Nanog, Klf4, and Sox2 (Fig. 5a). Moreover, along with diminished Akt activation represented by Ser473 phosphorylation levels, Pak4 overexpression also resulted in increases in GSK3β Ser9 phosphorylation levels, and this was attenuated following Akt knockdown, fully consistent with previous reports linking GSK3α/β inactivation with Akt activation.20,21 Consistently, similar findings were observed when R1 cells were treated with the Akt inhibitor, MK2206 (Fig. 5b). Conversely, expression of the constitutively active S473D form of Akt served to significantly restore the expression levels of Oct4, Nanog, Klf4, and Sox2 that were diminished upon Pak4 silencing (Fig. 5c). Lastly, to ensure the veracity of these data we repeated the key regulatory experiments in R1 cells which had alternatively undergone CRISPR-CAS9 mediated deletion of Pak4 (Supplementary Fig. S3a, S3b). Consistent with the preceding data, the reintroduction of the constitutively active S473D Akt mutant in Pak4-null ES cells strongly rescued the expression levels of Oct4, Nanog, Klf4, and Sox2 (Fig. 5j), while an inactive Akt mutant (S473A) failed to restore the depressed expression levels of Oct4, Nanog, Klf4 and Sox2 (Fig. 5k). Thus, Akt activation is necessary and sufficient for the actions of Pak4 in mediating the maintenance of pluripotency factors in mESCs.
Figure 5.
Pak4 maintains mES cells pluripotency via activating Akt1. (a) mES-R1 cells were transduced with control (pLKO.1) or Akt1 targeting shRNAs in combination with transfection of either Flag or Flag-Pak4 as indicated. Western blot analyses were conducted with antibodies against Flag, Akt, p-Akt Ser473, Ser9 phosphorylated GSK3β, Oct4, Nanog, Klf4, and Sox2. Where indicated, the relative levels of each protein were determined by densitometry with normalization against the actin loading control. (b) mES-R1 cells transfected with either Flag or Flag-Pak4 were treated with or without 2μM Akt1 inhibitor MK2206 for 24h. Western blot analyses were conducted as per panel (a). (c) mES-R1 cells were transduced with control (pLKO.1) or Pak4 targeting shRNAs in combination with transfection of either empty (Flag) vector or the constitutively active Flag-Akt S473D mutant. Western blot analyses were conducted as per panel (a). (d, f, h) The AP-positive colonies number were counted respectively from (a), (b), (c) mES-R1 cells cultured for 3 days with indicated medium and treatments. (e, g, i) Alkaline phosphatase staining was performed respectively from (a), (b), (c) mES-R1 cells cultured for 3 days with indicated medium and treatments. (j, k) CRISPR-CAS9-directed gene knockout of Pak4 was performed in mES-R1 cells to derive Pak4−/− cells. WT and Pak4−/− cells mES-R1 cells were then transfected with either an empty (Flag) vector or the constitutively active Flag-Akt S473D (j) or constructively inactive Flag-Akt S473A (k) mutants. Thereafter, Western blot analyses were conducted against Flag and Pak4 along with Oct4, Nanog, Klf4, Sox2, and actin. (a–k) Data represent 3 independent experiments. (d, f, h) Data are mean ± SD, n = 3, *P < .05; **P < .01; ***P < .001; ns, not significant, two-tailed paired Student’s t-test. (e, g, i) Scale bar represents 200 μm.
In parallel with the aforementioned assays, we assessed the functional impact of manipulating Akt and/or Pak4 on clonogenicity. As anticipated from the observed levels of pluripotency factors, knockdown of Akt inhibited the clonogenicity of R1 cells and particularly reversed the increases promoted by ectopic Flag-Pak4 expression (Fig. 5d, 5e) while chemical inhibition of Akt profoundly inhibited the increased AP-positive colony numbers resulting from ectopic Flag-Pak4 expression (Fig. 5f, 5g). In addition, expression of the S473D form of Akt was sufficient to rescue the inhibition of clonogenicity induced by Pak4 knockdown (Fig. 5h, 5i).
Lastly, we sought to verify that Akt activation was essential for the effects of Pak4 on sustaining ES cell pluripotency. Importantly, we found that ectopic expression of the constitutively active S473D Akt mutant significantly restored the expression levels of Oct4, Nanog, Klf4, Sox2 in Pak4 knockdown mESCs, together with the number of AP-positive colonies (Fig. 5c, 5h, and 5i). Consistently, the S473D Akt mutant also strongly rescued the expression levels of Oct4, Nanog, Klf4, and Sox2 in Pak4-null ES cells (Fig. 5j), while the equivalent experiment with the inactive S473A Akt mutant failed to affect Oct4, Nanog, Klf4, and Sox2 expression levels (Fig. 5k). Collectively, these data suggest that activation of Akt is indispensable for Pak4 to promote mESC pluripotency.
Discussion
Deciphering ESC transcriptional and signaling networks are essential for understanding the cellular mechanisms that govern pluripotency. Prior developmental studies have shown that homozygous deletion of Pak4 in mice results in embryonic lethality on day 11.5 with profound neuronal defects associated with the migration and differentiation of neurons and interneurons.45 Other defects in vessel formation are associated with impaired heart function and placental insufficiency.45,46 Interestingly, inhibiting Pak4 expression in zebrafish with morpholinos is also developmentally lethal47but this finding was later contradicted using an alternative gene knockout strategy.48 Nevertheless, the findings in mice appear to predict that Pak4 was involved in the regulation of pluripotency and differentiation events although the precise mechanisms remain undefined.49 Consistent with this thesis, our data showed that knockdown of Pak4 was remarkably associated with loss of ESC pluripotency while enforced Pak4 expression in mESCs overcame differentiation signals to restore pluripotency. And in teratoma formation in nude mice, considered the most stringent of pluripotency assays, we showed that iPSCs without Pak4 expression largely failed to form teratomas displaying multi-lineage commitment. Furthermore, assessment of somatic cell reprogramming revealed that Pak4 was highly expressed in OKSM-induced iPSCs with knockdown of Pak4 inhibiting the formation of OKSM-induced iPSC colonies. Collectively these findings identify Pak4 as a novel mESC pluripotency maintenance factor. In addition, our study further elucidated how Pak4 was regulated along with defining its downstream connection to Akt signaling.
Although Pak4 has been intensively studied in the context of cancer and neurological disease, most literature focuses on signaling events downstream of Pak4. Thus, there is a general need to better elucidate how Pak4 transcription is regulated in different physiological and pathological contexts. One prior study in pancreatic cancer cells showed that Pak4 expression was associated with enhanced levels of stemness-associated transcription factors (Oct4/Nanog/Sox2 and Klf4).37 Indeed, we definitively identified Pak4 as a direct transcriptional target of Nanog, but not other members Oct4, Klf4, or Sox2. Instructively, we also found that Pak4 knockdown strongly impaired mESC pluripotency even with ectopic expression of Nanog, indicating Nanog exerts its function to maintain stemness of mESCs through Pak4 expression. Interestingly, we found knockdown or deletion of Pak4 in mESCs resulted in general attenuation of OKSM expression, raising the possibility of a positive feedback loop involving Pak4. While this notion remains to be established it is pertinent to note the precedents linking Akt signaling effects on GSK3α/β to the upregulation of Nanog.20,21 It has been previously reported that MEK/ERK inhibitor PD0325901 combined with GSK3 inhibitor CHIR99021 can prevent mESC differentiation20,50 and consistently we found that Pak4 overexpression inhibited GSK3β through effects on Akt signaling. Moreover, we found that knockdown or chemical inhibition of Akt with MK2206 impaired the actions of Pak4 in sustaining mESC stemness, while conversely, expression of a constitutively activated Akt mutant restored the ability of Pak4 to maintain pluripotency.
Numerous studies have elaborated that the PI3K/Akt signaling is essential for maintaining pluripotency of stem cells, promoting somatic cell reprogramming, and regulating cell fate determination.51 In addition, Akt signaling sufficiently maintains pluripotency in mouse and primate ESCs,25 with Pak1 shown to activate Akt via Ser473 phosphorylation.41 According to our data, Pak4 overexpression strongly increased Akt phosphorylation levels, proposing a tangible association between Pak4 and Akt. Substantiating this link, we identified direct binding between Pak4 and Akt, and showed that Pak4 preferentially Akt interacted with the Thr308 and Ser473 phosphorylated forms of Akt. Phosphorylation at either or both of these residues is equated with Akt kinase activation and most famously, PDK1 facilitates phosphorylation Thr308 while Ser473 is phosphorylated by mTORC2.52 Nevertheless, many other kinases and phosphatases have been described as regulators or downstream effectors of Akt. Further in vitro kinase assays verified that Pak4 phosphorylated Akt at Ser473 but not Thr308 and moreover, Ser473 phosphorylation was independent of Thr308 phosphorylation, at least in vitro. Some studies have reported that Thr308 phosphorylation is a prerequisite for Ser473 phosphorylation53,54 and we observed that manipulating Pak4 in mESCs resulted in co-regulation of Thr308 and Ser473 phosphorylation. Together this proposes that like Pak1,41 Pak4 acts as an Akt Ser473 kinase which acts cooperatively with a presently unidentified Thr308 Akt kinase to promote full activation of Akt in mESCs. Also, with respect to Akt phosphorylation, a more recent role involving protein stability55 does not appear to be fully relevant here since Pak4 manipulations primarily affected Akt phosphorylation, not total Akt levels.
On the basis of our data, we propose a working model depicting an indispensable role of Pak4 in maintaining mESC identity (Fig. 6). As a transcriptional target of Nanog, Pak4 interacts with and phosphorylates Akt at Ser473, thereafter leading to the activation of Akt signaling and promoting the stemness of mESCs.
Figure 6.
Working model for the role of Nanog-Pak4-Akt pathway in maintaining mESC pluripotency. As a transcriptional target of Nanog, Pak4 interacts with and phosphorylates Akt at Ser473, thereafter leading to the activation of Akt signaling and promoting the stemness of mESCs.
Conclusion
In this article, we identify the serine/threonine kinase Pak4 as an indispensable pluripotency regulator, acting as a direct upstream activator of Akt signaling. Driven by Nanog, Pak4 functions to maintain the pluripotent state of murine embryonic stem cells and moreover, manipulating Pak4 expression can be practically exploited to increase the reprogramming efficiency of OKSM-induced iPSCs.
Supplementary Material
Acknowledgments
We thank Dr Qidong Li for the valuable discussions. We also thank Prof. Xiaoyuan Song (Division of Life Sciences and Medicine, University of Science & Technology of China) for providing STEMCCA plasmid.
Contributor Information
Fangyuan Cheng, Division of Life Sciences and Medicine, The First Affiliated Hospital of University of Science & Technology of China, and CAS Center for Excellence in Molecular Cell Science, Innovation Center for Cell Signaling Network. Hefei, Anhui, People’s Republic of China.
Mingyue Li, Division of Life Sciences and Medicine, The First Affiliated Hospital of University of Science & Technology of China, and CAS Center for Excellence in Molecular Cell Science, Innovation Center for Cell Signaling Network. Hefei, Anhui, People’s Republic of China.
Rick Francis Thorne, Translational Research Institute, Henan Provincial People’s Hospital, Academy of Medical Science, Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; Henan key Laboratory of Stem cell Differentiation and Modification, Henan Provincial People’s Hospital, Henan University, Zhengzhou, Henan, People’s Republic of China.
Guangzhi Liu, Henan key Laboratory of Stem cell Differentiation and Modification, Henan Provincial People’s Hospital, Henan University, Zhengzhou, Henan, People’s Republic of China.
Yuwei Zhang, Henan key Laboratory of Stem cell Differentiation and Modification, Henan Provincial People’s Hospital, Henan University, Zhengzhou, Henan, People’s Republic of China.
Mian Wu, Division of Life Sciences and Medicine, The First Affiliated Hospital of University of Science & Technology of China, and CAS Center for Excellence in Molecular Cell Science, Innovation Center for Cell Signaling Network. Hefei, Anhui, People’s Republic of China; Translational Research Institute, Henan Provincial People’s Hospital, Academy of Medical Science, Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; Henan key Laboratory of Stem cell Differentiation and Modification, Henan Provincial People’s Hospital, Henan University, Zhengzhou, Henan, People’s Republic of China.
Lianxin Liu, Division of Life Sciences and Medicine, The First Affiliated Hospital of University of Science & Technology of China, and CAS Center for Excellence in Molecular Cell Science, Innovation Center for Cell Signaling Network. Hefei, Anhui, People’s Republic of China.
Funding
This work was supported by Grant (2018YFA0107100) from the National Key R&D Program of China and Grants (81820108021, 31871437) from the National Natural Science Foundation of China.
Conflict of Interest
The authors declare that they have no competing interests.
Author Contributions
F.Y.C.: conception and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript; M.Y.L.: collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript; R.F.T: manuscript writing, and final approval of manuscript; G.Z.L., Y.W.Z: data analysis and interpretation; W.M., L.X.L.: conception and design, data analysis and interpretation, final approval of manuscript.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. All raw data are available from the corresponding author upon request.
References
- 1. Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634-7638. 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagy A, et al. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424-8428. 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reubinoff BE, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399-404. 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 5. Smith AG, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688-690. 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 6. Williams RL, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684-687. 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 7. Matsuda T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261-4269. 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ying QL, Nichols J, Chambers I, Smith A.. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281-292. 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 9. Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519-523. 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sato N, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55-63. 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 11. Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947-956. 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940-954. 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosner MH, et al. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686-692. 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 14. Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379-391. 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 15. Masui S, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625-635. 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 16. Avilion AA, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126-140. 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643-655. 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 18. Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631-642. 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 19. Chambers I, et al. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230-1234. 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 20. Wray J, et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol. 2011;13:838-845. 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niwa H, et al. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118-122. 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 22. Paling NRD, et al. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063-48070. 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 23. Kingham E, Welham M.. Distinct roles for isoforms of the catalytic subunit of class-IA PI3K in the regulation of behaviour of murine embryonic stem cells. J Cell Sci. 2009;122:2311-2321. 10.1242/jcs.046557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh AM, et al. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10:312-326. 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe S, et al. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697-2707. 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- 26. Silva J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu Y, et al. Stimulation of somatic cell reprogramming by ERas-Akt-FoxO1 signaling axis. Stem Cells. 2014;32:349-363. 10.1002/stem.1447. [DOI] [PubMed] [Google Scholar]
- 28. Zhu H, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81-94. 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang Y, et al. Differential effects of Akt isoforms on somatic cell reprogramming. J Cell Sci. 2014;127:3998-4008. 10.1242/jcs.150029. [DOI] [PubMed] [Google Scholar]
- 30. Zunder ER, et al. A continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell. 2015;16:323-337. 10.1016/j.stem.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Won SY, et al. PAK4 signaling in health and disease: defining the PAK4–CREB axis. Exp Mol Med. 2019;51:1-9. 10.1038/s12276-018-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tabusa H, Brooks T, Massey AJ.. Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol. Cancer Res. 2013;11:109-121. 10.1158/1541-7786.MCR-12-0466. [DOI] [PubMed] [Google Scholar]
- 33. Park MH, et al. p21-Activated kinase 4 promotes prostate cancer progression through CREB. Oncogene. 2013;32:2475-2482. 10.1038/onc.2012.255. [DOI] [PubMed] [Google Scholar]
- 34. Cai S, et al. Overexpression of P21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion. J Exp Clin Cancer Res. 2015;34:1-10. 10.1186/s13046-015-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yeo D, et al. The role of p21-activated kinases in pancreatic cancer. Pancreas. 2015;44:363-369. 10.1097/MPA.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 36. Park JJ, et al. The p21-activated kinase 4-Slug transcription factor axis promotes epithelial- mesenchymal transition and worsens prognosis in prostate cancer. Oncogene. 2018;37:5147-5159. 10.1038/s41388-018-0327-8. [DOI] [PubMed] [Google Scholar]
- 37. Tyagi N, et al. p-21 activated kinase 4 (PAK4) maintains stem cell-like phenotypes in pancreatic cancer cells through activation of STAT3 signaling. Cancer Lett. 2016;370:260-267. 10.1016/j.canlet.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, et al. p53-facilitated miR-199a-3p regulates somatic cell reprogramming. Stem Cells. 2012;30:1405-1413. 10.1002/stem.1121. [DOI] [PubMed] [Google Scholar]
- 39. Han C, et al. Regulation of l-threonine dehydrogenase in somatic cell reprogramming. Stem Cells. 2013;31:953-965. 10.1002/stem.1335. [DOI] [PubMed] [Google Scholar]
- 40. Higuchi M, et al. Scaffolding function of PAK in the PDK1–Akt pathway. Nat Cell Biol. 2008;10:1356-1364. 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 41. Mao K, et al. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J Mol Cell Cardiol. 2008;44:429-434. 10.1016/j.yjmcc.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balasuriya N, et al. Genetic code expansion and live cell imaging reveal that Thr-308 phosphorylation is irreplaceable and sufficient for Akt1 activity. J Biol Chem. 2018;293:10744-10756. 10.1074/jbc.RA118.002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yue Y, et al. Ras GTPase-activating protein binds to Akt and is required for its activation. J Biol Chem. 2004;279:12883-12889. 10.1074/jbc.M312308200. [DOI] [PubMed] [Google Scholar]
- 44. Gao T, Furnari F, Newton AC.. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13-24. 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 45. Qu J, et al. PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol Cell Biol. 2003;23:7122-7133. 10.1128/MCB.23.20.7122-7133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tian Y, et al. Essential role for the Pak4 protein kinase in extraembryonic tissue development and vessel formation. Mech Dev. 2009;126:710-720. 10.1016/j.mod.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 47. Law SHW, Sargent TD.. Maternal pak4 expression is required for primitive myelopoiesis in zebrafish. Mech Dev. 2013;130:181-194. 10.1016/j.mod.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Law SHW, Sargent TD.. The serine-threonine protein kinase PAK4 is dispensable in zebrafish: identification of a morpholino-generated pseudophenotype. PLoS One. 2014;9:e100268. 10.1371/journal.pone.0100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tian Y, Lei L, Minden A.. A key role for Pak4 in proliferation and differentiation of neural progenitor cells. Dev Biol. 2011;353:206-216. 10.1016/j.ydbio.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 50. Gu H, et al. Mitochondrial E3 ligase March5 maintains stemness of mouse ES cells via suppression of ERK signalling. Nat Commun. 2015;6:1-12. 10.1038/ncomms8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu JSL, Cui W.. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050-3060. 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 52. Bozulic L, Hemmings BA.. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol. 2009;21:256-261. 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 53. Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098-1101. 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 54. Scheid MP, Marignani PA, Woodgett JR.. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247-6260. 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liao Y, Hung MC.. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19-42. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. All raw data are available from the corresponding author upon request.