The acute respiratory distress syndrome (ARDS) is a highly complex syndrome that affects the microstructure of the lung in a variety of ways. The role of the alveolar epithelial barrier in the pathogenesis of and recovery from acute lung injury has gained interest recently.18 The mechanism for damage of the alveolar epithelial barrier may be specific and different for each type of injury. The additive effect of mechanical ventilation, including alveolar overdistention from positive-pressure ventilation, may contribute to the injury.
The traditional practice of using high-inflation pressures and high levels of positive end-expiratory pressure (PEEP) has been encouraged by the misperception that damage in acute lung injury is distributed uniformly. Recent work by Gattinoni4, 5 demonstrates that the nondependent lung often remains well aerated and has a normal compliance, whereas other areas fill with fluid and have altered gas-exchanging properties. These areas may not participate at all in gas exchange. Because of these physiologic changes, the lung has a smaller active surface area and the distribution of pressure is not uniform. One must, therefore, evaluate both tidal volume and PEEP on the basis of tidal transalveolar pressure and on changes in the inflection point of the static pressure-volume curve (Fig. 1) .14, 17
Figure 1.
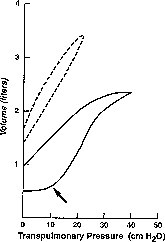
Typical static pressure-volume curves of a patient with normal lungs (dashed lines) and a patient with ARDS (solid lines). Note the minimal hysteresis and normal functional residual capacity in the patient without ARDS. The pressure-volume curve of the patient with ARDS demonstrates marked hysteresis and an inflection point on the inflation limb (arrow). The optimal PEEP will maximally recruit alveoli and obliterate this inflection point without overdistending alveoli.
Based on current evidence, it is justifiable to apply sufficient total PEEP (the sum of PEEP plus auto-PEEP) to maximize alveolar recruitment without overdistending alveolar units. This amount of PEEP obliterates any clear inflection point in the respiratory compliance curve. In the authors' experience, the level of PEEP required to reach the point at which oxygenation should be maximal is rarely greater than 15 cm H2O.
BAROTRAUMA
There are varied forms of pulmonary barotrauma. Pneumothorax, interstitial emphysema, pneumomediastinum, subcutaneous emphysema, and cyst formation are all prominent causes of iatrogenic increases in the degree of acute lung injury and, thus, of critical illness. Although less apparent, the microscopic damage caused by mechanical ventilation may be even more harmful. It is clear from animal studies that mechanical ventilation can induce acute lung injury similar to ARDS when overdistention of alveoli develops.3, 9, 26, 27 One of the first studies identifying the adverse effects of high peak-inspiratory pressure (PIP) on lung tissue and the ability of PEEP to modify the effect of PIP was performed by Webb and Tierney in 1974.27 Adult rats were ventilated for 60 minutes with five different ventilatory patterns (PIP/PEEP [cm H2O]: 14/0, 30/ 0, 45/0, 30/10, and 45/10). The histologic results were compared tocontrol animals not mechanically ventilated. Animals ventilated at 14/0 showed no difference from control animals, whereas animals ventilated at 45/0 demonstrated dramatic decreases in compliance and were cyanotic. All of these animals died with gross hemorrhagic pulmonary edema. Histology revealed marked perivascular and alveolar hemorrhage and edema. The addition of 10 cm H2O PEEP (45/10 group) limited the abnormalities to interstitial edema.
Detailed histopathologic changes induced by high PIP (40 cm H2O) in baby pigs have been described by Tsuno et al.26 After 22 hours of mechanical ventilation, pigs demonstrated severe hypoxemia on 40% oxygen, with 3 to 5 cm H2O PEEP. The lungs of these animals showed gross alveolar hemorrhage, alveolar neutrophil infiltration, alveolar macrophage and type-II pneumocyte proliferation, interstitial congestion and thickening, interstitial lymphocyte infiltration, emphysematous changes, and hyaline membrane formation. The changes were similar to those seen in early ARDS. Control animals ventilated at 18 cm H2O PIP showed no noticeable changes in lung function and histopathologic findings. What may be more clinically relevant, however, is that the effect of alveolar overdistension may be synergistic with previous lung injury. Dreyfuss et al3 compared the effects of different levels of lung distension during mechanical ventilation in intact anesthetized rats that were either exposed or not exposed to parenteral alpha-naphthylthiourea (ANTU), a toxin that damages the pulmonary endothelium. Mechanical ventilation with a tidal volume of 7 mL/kg body weight for 2 minutes was compared with that of 25, 33, and 45 mL/kg ventilation in rats exposed or not exposed to ANTU. The group ventilated with 45 mL/kg ventilation and treated with ANTU had significantly greater permeability pulmonary edema than would be predicted by adding together what the two injuries would cause separately. The microscopic complications detailed previously have important implications for patients with acute lung injury, as these complications may extend ventilatory dependency and increase rates of mortality and morbidity.
In humans, peak and plateau alveolar pressures frequently have been cited as important risk factors for ventilator-related barotrauma. One of the best-understood causes of barotrauma is alveolar overdistention. Slutsky and others25 believe that high plateau pressures (greater than 35 cm H2O) may be more harmful to patients than high concentrations of oxygen. The barotrauma of mechanical ventilation has been associated with a combination of high airway pressure and high levels of PEEP.22 This increased incidence of barotrauma with high levels of PEEP (greater than 15 cm H2O) appears related to increases in both mean and peak alveolar pressures. Some studies report a high incidence of pneumothorax and barotrauma with high levels of PEEP,21 whereas others observe no correlation between PEEP and barotrauma.23 This variable correlation between PEEP and barotrauma is not surprising in that the additive effect of inspiratory pressure plus PEEP may be more important than PEEP alone. It may be safer to add high levels of PEEP to small tidal volumes than to traditional tidal breaths (10 to 15mL/kg). The authors believe, as do others, that it is not PEEP but rather the peak alveolar pressure10 and the frequency of application of high pressure that contribute to barotrauma. Even traditional users of high levels of PEEP, such as Nelson's group,2 aim to decrease exposure to high alveolar pressures by reducing the intermittent mandatory ventilation rate to four breaths per minute. Nelson's patients, however, were exposed to high levels of airway pressure during these volume-controlled breaths secondary to decreased pulmonary compliance.
Ventilator strategies that expose the lung to the lowest peak alveolar pressure25 may be useful. Experimental evidence9, 26 shows that static transpulmonary pressures in excess of 30 cm H2O may aggravate or even cause barotrauma through epithelial and capillary injury. Based on these physiologic parameters, the authors have integrated the guidelines proposed by Kacmarek and Hickling8 into practice.
-
1
Maintain peak alveolar pressure of less than 35 cm H2O (may require tidal volume of 5 to 7 mL/kg).
-
2
Target PEEP 10 to 15 cm H2O.
-
3
Avoid air trapping and auto-PEEP (limits on inspiratory time and rate must be individualized).
This highly monitored and physiologically based strategy is described commonly as a "lung-protective strategy." Many of the new modes of ventilation address these concepts by being pressure targeted. These modes deliver gas in a decelerating flow pattern that results in lower peak-airway pressures and also may act to distribute pressure over a wider area.
Data suggest that ventilatory strategies that limit peak alveolar pressure and overdistention favorably affect outcome in ARDS. Gattinoni and coworkers6 have shown a marked decrease in mortality rates in patients with ARDS, using extracorporeal CO2 removal, low peak-inspiratory pressure (PIP less than 40 cm H2O), PEEP, and low-rate ventilation when compared with that of the multicentered adult extra-corporeal membrane oxygenation trial of the 1970s.29 The main difference in pulmonary management between the two series was the limitation of inflation pressure. Hickling and colleagues7 reported retrospective data on patients with ARDS that indicated an improvement in mortality rates as compared to Apache II prediction data, if an effort to maintain the maximum PIP at less than 40 cm H2O was specified. Fifty patients with ARDS and a predicted mortality rate of 40% had an actual mortality rate of only 16%. Lewandowski and coworkers12 reported a 16% mortality rate in a series of 38 patients with ARDS with a lung-injury score (Murray score)19 greater than 2.5, managed with pressure-controlled ventilation, permissive hypercapnia, and frequent body-position changes.
LUNG-PROTECTIVE VENTILATION MODES
Pressure-controlled or limited ventilation is being used widely now in an attempt to limit ventilator-associated lung injury. Pressure-controlledinverse ratio ventilation (PC-IRV) is one such mode. Because alveolar volume is a major determinant of PaO2, part of the rationale for using PC-IRV in patients with ARDS is to maintain relatively high alveolar volume but hold peak alveolar pressure below 35 cm H2O to minimize the risk of barotrauma.16, 20 PC-IRV may improve alveolar recruitment and therefore allow a rapid decrease in inspired oxygen concentration. The reversal of the inspiratory-to-expiratory wave pattern has been postulated to act as a splint to help open and maintain certain alveolar units against the tractive forces of diseased units that surround them. This splinting may remove the relatively high levels of PEEP and sustained pressures necessary to open them.15 Alveolar units that respond to this form of therapy may be more prevalent in the early phase of ARDS when atelectasis is widespread and the surface tension and viscosity of fluid lining the small airways are high. The authors advocate the early use of PC-IRV in ARDS20 to prevent the advancement of this collapsing tractive force.
Another highly successful mode to limit high-inflation pressure is pressure-regulated volume control (PRVC). This mode is currently available on the Servo Ventilator 300 (Siemens, Solna, Sweden) and is now available in the United States. PRVC is a union of volume and pressure ventilation controlled by the physiologic parameter of compliance. In this mode, inspiratory pressure is regulated to a value based on the volume/pressure calculation of the previous breath compared to a preset target volume. This mode, therefore, adds real-time ability to the monitoring of pressure and compliance, thereby adding computer control to a lung-protective strategy (Fig. 2) . The lung is opened and splinted throughout the entire ventilatory cycle by a pressure-volume pattern with variable pressure parameters. It is hoped that this computer-controlled mode will help limit ventilator-induced lung injury, thus yielding a decrease in time on the ventilator and a decrease in intensive care unit costs.
Figure 2.
The use of pressure-regulated volume control.
The use of lung-protective strategies may require ventilation of patients with smaller tidal volumes. This leads to reduced alveolar ventilation and thus, hypercapnia. The term permissive hypercapnia was coined by Hickling and co-workers in 1990.7 It is defined as a deliberate limitation of tidal volume to avoid barotrauma. The PaCO2 is allowed to increase to levels greater than normal (50 to 100 mm Hg). In certain settings, however, even mild levels of hypercapnia may be detrimental (i.e., increased intracranial pressure). To overcome these problems, a simple adjunct to remove CO2 in any form of pressure-controlled ventilation is tracheal gas insufflation. The technique, described by Ravenscraft et al,24 uses a thin (1.5 mm) catheter placed down a conventional endotracheal tube. Air or gas with higher Fio2 is delivered through the catheter at a flow rate of 2 to 6 L/minute. This procedure "flushes" CO2 from the proximal airway throughout the respiratory cycle. This is a useful adjunct to the treatment of severe ARDS by controlling the climb of CO2, which is observed secondary to the markedly increased proportion of dead-space ventilation.
EXTRAPULMONARY EFFECTS OF HIGH-INFLATION PRESSURE AND POSITIVE END-EXPIRATORY PRESSURE
High levels of PEEP and high peak pressures may have profound effects on cardiovascular function. The major cardiovascular effect of PEEP is a reduction of cardiac output related primarily to reduced ventricular preload. PEEP also can increase right ventricular afterload, leading to further reduction of left ventricular preload. Another reported complication in the use of high levels of PEEP has been the induction of supraventricular and ventricular dysrhythmias. In a recent study,2 researchers found that 33% of patients had episodes of supraventricular tachycardia. This group of researchers prophylactically placed patients who were on 20 cm H2O of PEEP on digoxin. Fluid retention with high peak pressures and PEEP has been described.11 Several mechanisms have been proposed, including depression of atrial natriuretic factor and stimulation of antidiuretic hormone. The physiologic purpose of this fluid retention may be to promote cardiac output in the face of high intrathoracic pressure, but the disadvantages of fluid overload must be anticipated and monitored.
In a recent report by Love et al,13 high levels of PEEP (greater than 15 cm H2O) were associated with decreased mesenteric blood flow despite normalization of cardiac output. Love concluded that high levels of PEEP may cause a redistribution of blood flow away from the mesenteric vascular bed. As splanchic ischemia has been implicated in themultiple organ failure syndrome, this may be an important finding related to the care of critically ill patients.
SUMMARY
There is a growing body of evidence suggesting that high levels of inflation pressure and high levels of PEEP may be injurious to lung tissue and other organ systems. Limiting peak alveolar pressures below 35 cm H2O may help in avoiding these injuries. The findings have led to the development of a lung-protective strategy that is based on physiologic parameters. This strategy, often using permissive hypercapnia and pressure-limited modes of ventilation, may gain widespread use in the near future. If this strategy reduces barotrauma, a reduction in the length of time on mechanical ventilation and mortality rates can be anticipated.
At our center we routinely initiate mechanical ventilation in patients with acute lung injury, using tidal volumes of approximately 6 mL/kg. This may be decreased further if peak alveolar pressures exceed 30 to 35 cm H2O. PEEP is added to maximize alveolar recruitment and oxygenation. Optimal PEEP is located at the inflection point of the respiratory compliance curve. Usually a PEEP of 8 to 12 cm H2O is sufficient. Although we usually initiate mechanical ventilation with a volume-cycled mode, we are not hesitant to switch rapidly to a pressure-limited mode if results are unsatisfactory. We believe that more attention to the potential harmful effects of pressure and volume on lung architecture may result in further improvement of survival in patients with acute respiratory failure.
Footnotes
Address reprint requests to Peter J. Papadakos, MD Division of Critical Care Medicine Department of Anesthesiology Box 604 University of Rochester Rochester, NY 14642
References
- 1.Cullen D.J., Caldena D.L. The incidence of ventilator-induced pulmonary barotrauma in critically ill patients. Anesthesiology. 1979;50:185–190. doi: 10.1097/00000542-197903000-00003. [DOI] [PubMed] [Google Scholar]
- 2.DeRusso S.M., Nelson L.D., Safcsak K., et al. Survival in patients with severe adult respiratory distress syndrome treated with high-level, positive end expiratory pressure. Crit Care Med. 1995;23:1485–1496. doi: 10.1097/00003246-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss D., Soler P., Saumon G. Mechanical ventilation-induced pulmonary edema: Interaction with previous lung alterations. Am J Respir Crit Care Med. 1995;151:1568–1575. doi: 10.1164/ajrccm.151.5.7735616. [DOI] [PubMed] [Google Scholar]
- 4.Gattinoni L., Presenti A. ARDS: The non-homogeneous lung: Facts and hypothesis. Critical Care Diagnosis. 1987;6:1–4. [Google Scholar]
- 5.Gattinoni L., Presenti A., Bombino M. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology. 1988;69:824–832. doi: 10.1097/00000542-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L., Presenti A., Mascheroni D., et al. Low-frequency positive-pressure ventilation with extra-corporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256:881–886. [PubMed] [Google Scholar]
- 7.Hickling K.G., Henderson S.J., Jackson R. Low mortality associated with low volume permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16:372–377. doi: 10.1007/BF01735174. [DOI] [PubMed] [Google Scholar]
- 8.Kacmarek R.M., Hickling K.C. Permissive hypercapnia. Resp Care. 1993;38:373–387. [Google Scholar]
- 9.Kolobow T., Moretti M.O., Fumagelli R., et al. Severe impairment of lung function induced by high peak airway pressure during mechanical ventilation. American Review of Respiratory Disease. 1987;135:312–315. doi: 10.1164/arrd.1987.135.2.312. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A., Pontoppidan H., Falke K.J., et al. Pulmonary barotrauma during mechanical ventilation. Crit Care Med. 1973;1:181–186. doi: 10.1097/00003246-197307000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Leithner C., Frass M., Pacher R., et al. Mechanical ventilation with positive end-expiratory pressure decreases release of alpha-atrial natriuretic peptide. Crit Care Med. 1987;15:484–488. doi: 10.1097/00003246-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowski K., Slama K., Falka K.J. In: Update of Intensive Care and Emergency Medicine. Vincent J.L., editor. Springer Verlag; Berlin: 1992. Approaches to improve survival in severe ARDS. [Google Scholar]
- 13.Love R., Choe E., Lippton H., et al. Positive end-expiratory pressure decreases mesenteric blood flow despite normalization of cardiac output. J Trauma. 1995;39:195–199. doi: 10.1097/00005373-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Marini J.J. New options for ventilatory management of acute lung injury. New Horizons. 1993;1:489–503. [PubMed] [Google Scholar]
- 15.Marini J.J., Kelsen S.G. Re-targeting ventilatory objectives in adult respiratory distress syndrome: New treatment prospects--persistent questions [editorial] American Review of Respiratory Diseases. 1992;146:2–3. doi: 10.1164/ajrccm/146.1.2. [DOI] [PubMed] [Google Scholar]
- 16.Marcy T.W., Marini J.J. Inverse ratio ventilation in ARDS rationale and implementation. Chest. 1991;100:494–504. doi: 10.1378/chest.100.2.494. [DOI] [PubMed] [Google Scholar]
- 17.Matamis D., Lemaire F., Harf A., et al. Total respiratory pressure volume curves in the adult respiratory distress syndrome. Chest. 1984;86:58–66. doi: 10.1378/chest.86.1.58. [DOI] [PubMed] [Google Scholar]
- 18.Mathay M.A., Folkesson H.G., Campagna A., et al. Alveolar epithelial barrier and acute lung injury. New Horizons. 1993;1:613–622. [PubMed] [Google Scholar]
- 19.Murray J.F., Matthay M.A., Luce J.M., et al. An expanded definition of the adult respiratory distress syndrome. American Review of Respiratory Disease. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 20.Papadakos P.J., Halloran W., Hessney J.I., et al. The use of pressure-controlled inverse ratio ventilation in the surgical intensive care unit. Trauma. 1991;31:1211–1214. doi: 10.1097/00005373-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Peterson G.N., Baier H. Incidence of pulmonary barotrauma in a medical ICU. Crit Care Med. 1983;11:67–69. doi: 10.1097/00003246-198302000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Pierson D.J. Alveolar rupture during mechanical ventilation: Role of PEEP, peak airway pressure, and distending volume. Respir Care. 1988;33:472–486. [Google Scholar]
- 23.Pingleton S.K. Complications of acute respiratory failure. American Review of Respiratory Disease. 1988;137:1463–1493. doi: 10.1164/ajrccm/137.6.1463. [DOI] [PubMed] [Google Scholar]
- 24.Ravenscraft S.A., Burke W.C., Nahum A., et al. Tracheal gas insufflation augments CO2 clearance during mechanical ventilation. American Review of Respiratory Diseases. 1993;48:345–351. doi: 10.1164/ajrccm/148.2.345. [DOI] [PubMed] [Google Scholar]
- 25.Slutsky A.S., et al. Mechanical ventilation ACCP consensus conference. Chest. 1993;104:1833–1859. doi: 10.1378/chest.104.6.1833. [DOI] [PubMed] [Google Scholar]
- 26.Tsuno K., Prato P., Kolobow T. Acute lung injury from mechanical ventilation at moderately high airway pressures. J App1 Physiol. 1990;69:956–961. doi: 10.1152/jappl.1990.69.3.956. [DOI] [PubMed] [Google Scholar]
- 27.Webb H.H., Tierney D.F. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: Protection by positive end-expiratory pressure. American Review of Respiratory Diseases. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 28.Woodring J.H. Pulmonary interstitial emphysema in the adult respiratory distress syndrome. Crit Care Med. 1984;13:786–791. doi: 10.1097/00003246-198510000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Zapol W.M., Snider M.T., Hill J.D., et al. Extra-corporeal membrane oxygenation in severe acute respiratory failure: A randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]


