Abstract
Background/Objectives:
Sleep measures, such as duration and onset timing, are associated with adiposity outcomes among children. Recent research among adults has considered variability in sleep and wake onset times, with the Sleep Regularity Index (SRI) as a comprehensive metric to measure shifts in sleep and wake onset times between days. However, little research has examined regularity and adiposity outcomes among children. This study examined the associations of three sleep measures (i.e., sleep duration, sleep onset time, and SRI) with three measures of adiposity (i.e., body mass index [BMI], waist circumference, and waist-to-height ratio [WHtR]) in a pediatric sample.
Subjects/Methods:
Children (ages 4 – 13 years) who were part of the U.S. Newborn Epigenetic STudy (NEST) participated. Children (N = 144) wore an ActiGraph for one week. Sleep measures were estimated from actigraphy data. Weight, height, and waist circumference were measured by trained researchers. BMI and WHtR was calculated with the objectively measured waist and height values. Multiple linear regression models examined associations between child sleep and adiposity outcomes, controlling for race/ethnicity, child sex, age, mothers’ BMI and sleep duration.
Results:
When considering sleep onset timing and duration, along with demographic covariates, sleep onset timing was not significantly associated with any of the three adiposity measures, but a longer duration was significantly associated with a lower BMI Z-score (β=−.29, p< .001), waist circumference (β=−.31, p<.001), and WHtR (β=−.38, p< .001). When considering SRI and duration, duration remained significantly associated with the adiposity measures. The SRI and adiposity associations were in the expected direction, but were non-significant, except the SRI and WHtR association (β=−0.16, p= .077) was marginally non-significant.
Conclusions:
Sleep duration was consistently associated with adiposity measures in children 4-13 years of age. Pediatric sleep interventions should focus first on elongating nighttime sleep duration, and examine if this improves child adiposity outcomes.
INTRODUCTION
Consistent evidence has shown that shorter sleep duration is a prominent risk factor for childhood obesity (1,2). Both cross-sectional and longitudinal studies, using subjective and objective measures of sleep, across different stages of child development have demonstrated these relationships (3). Two recent meta-analyses with data totaling over 75,000 children demonstrated that shorter sleep duration was associated with twice the risk for having overweight or obesity (4), and for every one hour longer in sleep duration, children’s risk of overweight/obesity decreased by 21% (5). Shorter sleep duration during childhood has also been linked with adverse markers of metabolic health, including greater abdominal adiposity and waist circumference (4).
Aside from sleep duration, there is increasing consideration as to how other sleep measures - such as timing and its variability - relate to child obesity outcomes (6–8). Data on sleep timing suggests that later bed times and greater variability in sleep onset timing predict higher weight status and adiposity (6–11). Data on sleep variability remain limited, with inconsistencies in measurement methods across investigations, such as how sleep variability is defined (e.g., variability in sleep duration, sleep or wake onset, or sleep habits) and measured (e.g., sleep diaries, actigraphy) (1).
There are several measures of sleep regularity that have been developed to measure variability in sleep, which include interdaily stability, standard deviation, and social jetlag (12,13). More recent measures of sleep regularity include the composite phase deviation (14) and the Sleep Regularity Index (SRI) (15). The SRI was developed as a metric for quantifying the shifts in both sleep/wake onset times from day to day (15). Compared to other variability measures (15,16), it accounts for multiple sleep periods across the 24-hour period, such as naps, rather than a single nighttime sleep period (12), and measures variability between consecutive days, which have a particularly harmful impact on circadian alignment. Research using the SRI has examined associations between variability of sleep timings and adiposity outcomes in older adults (16), as well as cognitive and mental health outcomes in adolescents (17,18).
However, the relationship between sleep regularity and adiposity has rarely been examined in younger children, which is critical because sleep habits are established and long-term health behaviors are formed during this timeframe (19–21). Furthermore, since sleep regularity has been shown to be associated with adiposity outcomes in adults (16), it may also be associated with adiposity outcomes among children. As such, research examining how sleep regularity is associated with child adiposity outcomes is warranted. Specifically, investigating how sleep regularity is independently associated with child adiposity outcomes, in comparison to more-common measures of sleep (e.g., duration and onset timing), is needed in order to elucidate how various sleep factors are related to obesity among children. A more comprehensive understanding of child sleep characteristics, beyond sleep duration and onset, may be critical for optimizing sleep interventions for childhood obesity prevention. For example, this could provide researchers and intervention scientists with a clearer understanding of mechanisms driving the sleep-obesity relationship, elucidate additional targets of intervention and offer more comprehensive sleep measures to examine relations with adiposity outcomes.
In addition, multiple measures of child adiposity should be examined in relation to multiple sleep measures in order to obtain a more comprehensive evaluation on child health risk. While body mass index (BMI) is the most common measure of adiposity, waist circumference has also been shown to provide additional variance, above and beyond BMI, when predicting coronary artery disease risk factors among children and adolescents (22). When stratifying by BMI levels, children with overweight and obesity and a larger waist-to-height ratio (WHtR) were more likely to have an increased cardiovascular risk compared to children with overweight and obesity, but smaller WHtR (23). A meta-analysis among children found that WHtR, waist circumference, and BMI were equally predictive of cardio-metabolic risk factors (24), but WHtR was a better predictor of adiposity compared to BMI and waist circumference (25). Given the evidence that WHtR is a greater predictor of adiposity (25), further sleep research should examine WHtR, while also considering BMI and waist circumference in order to gain a more comprehensive understanding.
This study used data from a large, prospective observational study to examine associations between sleep duration, sleep timing (sleep onset timing), and the regularity of sleep/wake patterns (using the SRI), on child weight and adiposity outcomes. The first aim was to examine associations between two sleep measures used in prior research (i.e., sleep onset timing and sleep duration) with a more comprehensive assessment of child adiposity (i.e., including multiple adiposity measures: BMI, waist circumference, and WHtR) than has been previously investigated. The second aim was to determine whether there is a unique association between sleep regularity and adiposity outcomes after accounting for sleep duration.
MATERIALS AND METHODS
Study design and participant sample
This study included follow-up data from children enrolled in the prospective Newborn Epigenetic STudy (NEST). The primary aim of the NEST study was to identify early-life environmental and nutritional exposures associated with epigenetic alterations during infancy that could alter chronic disease susceptibility. Mother-infant dyads were enrolled in NEST during pregnancy and a subset participated in a follow-up study, where data on children’s sleep were collected at ages ranging from 4 to 13 years.
Recruitment and enrollment procedures have been described elsewhere (26,27). Briefly, women were recruited during pregnancy from prenatal clinics serving Duke University Hospital and Durham Regional Hospital Obstetrics facilities between April 2005 to June 2011. Women were eligible to participate if they were >18 years of age, currently pregnant, English or Spanish-speaking, and intended to use one of two local obstetrics facilities for their index pregnancy to enable access to labor and birth outcome data. Women who met these eligibility criteria and who provided written informed consent were enrolled.
Approximately 3 years later mothers and their children were recruited to participate in the follow-up study to NEST to examine prenatal and childhood factors related to obesity. Eligibility for this follow-up study were singleton birth, English-speaking, and the child being at least 3-years-old. During the follow-up period, all adult participants provided written informed consent. Youth provided verbal assent; written assent was obtained for children aged 12 or older (n=304). Children were also instructed on how to wear an ActiGraph sensor and asked to wear the device for one week. The NEST study and the follow-up study were both approved by the Duke Health Institutional Review Board.
Actigraphy data
Primary caregivers (predominately mothers) and children in the NEST follow-up study were asked to wear an ActiGraph GT3x+ accelerometer to wear for a week on their non-dominant hip. Additionally, participants were reminded to wear the accelerometers via daily text messages on the digital platform developed for the purpose of the study (28). Data collection was carried out throughout the entire calendar year, with children participating at different times during the year. Therefore, children could have participated during a school term or during a vacation. Also, the study was completed in Durham, North Carolina, where some public schools follow a year-round academic calendar. Every child included in the final analyses had at least one weekend day of data, along with data for several weekdays.
Among 304 mother-child dyads that were enrolled, accelerometer data were available from n=297 children. Children were instructed to wear the accelerometers continuously day and night, but were told that if they felt uncomfortable wearing them to bed, they could remove them for the night. Data were downloaded at a 30-second epoch using the ActiLife software. To ensure reliable estimation of sleep measures, we included in these analyses children who had worn the sensor continuously for at least five days (n=182) and excluded children who had continuous non-wearing time longer than 120 minutes (represented as 120 consecutive zeros). To do so, we first excluded subjects with actigraphy of less than 7200 minutes. Then among the rest of subjects, we detected the longest continuous wearing time, and included only those who had at least 7200 minutes of wearing time. The final sample size for these analyses was N = 144. Figure 1 includes a flow diagram to show how the final sample of children was determined for the analyses.
Figure 1.
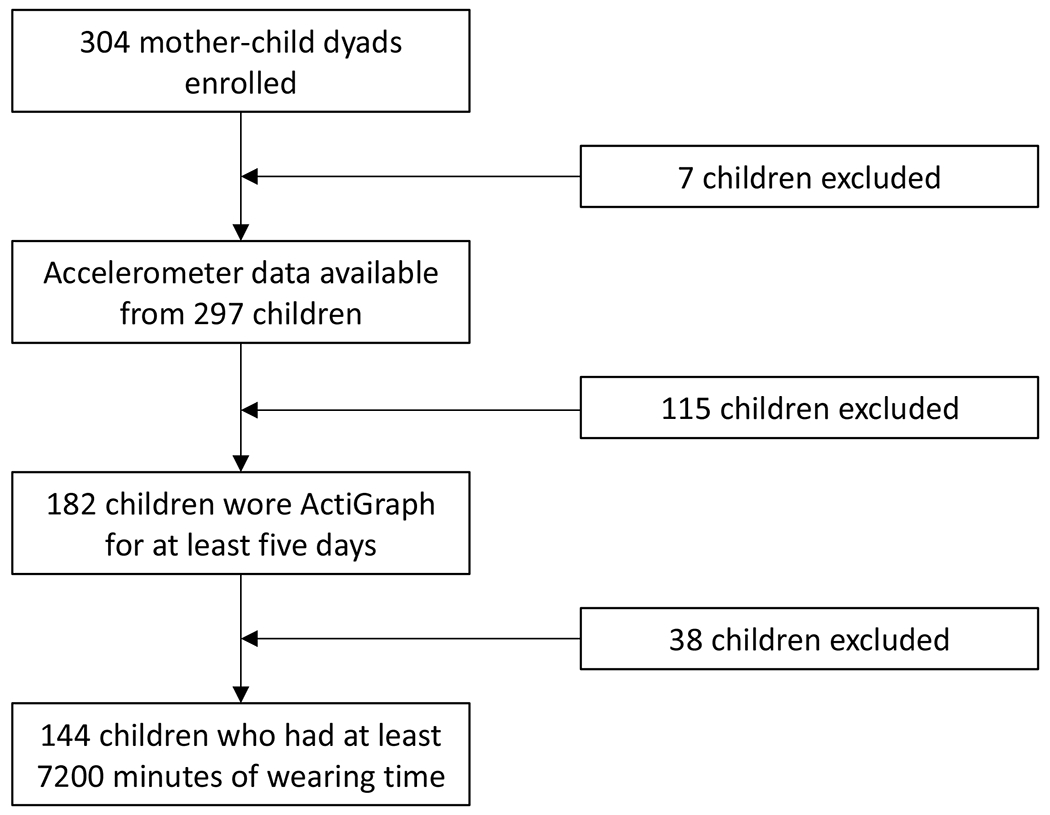
Flow diagram describing the cohort for analyses
Additionally, we examined any group differences between children not included in the analyses (n=160) vs. those included (n=144). There were no group differences found with respect to gender, race, and adiposity measures. However, children who were included in the analyses were older (mean age = 7.81) than children who were not included (mean age = 7.10).
Sleep measures
The sleep-related metrics (sleep duration, sleep onset time, and SRI) were extrapolated after detecting sleep-wake cycles using our previously devised algorithm (29). A robust sigmoid-transformed cosine model was fitted (30), and a change point between two consecutive sleep and wake times was estimated to detect the precise sleep and wake onset timings. These critical timepoints are expressed as minutes of the day since midnight (i.e., 00:00) in order to derive other metrics and statistical analyses. Nocturnal sleep duration (denoted sleep duration throughout the manuscript) was defined as the minutes between a sleep onset time and its successive sleep wake time and averaged across days for each child. Sleep Regularity Index (SRI) was derived from the sleep onset and wake onset times using a previously developed method (16), with larger SRI values being associated with more sleep regularity.
Weight and adiposity measures
Body mass index
Both mother and children’s weight and height were measured by trained researchers during the follow-up study visit. Following the National Health and Nutrition Examination Survey (NHANES) protocol (31), the mother and child were taken into the examination room to collect height (cm and ft) and weight (lbs) using research-grade equipment. Participants removed their shoes prior to measurement. For height, participants stood with their back against the wall, with heels pressed against the wall and with their back straight. For weight, participants removed heavy jackets that could significantly impact weight and the scale was recalibrated prior to measurement. BMI was calculated as (weight [kg] / height[m]2). BMI Z-score was calculated for each child and included in the analyses.
Waist circumference
Children’s waist circumference was also measured during the follow-up visit by trained researchers. Participants stood with their arms spread out in a “T”. Measurements were taken around the waist, right above the hips. Values were quantified in centimeters (cm).
Waist-to-height ratio
Children’s waist-to-height ratio was calculated by dividing waist measurements by height measurements. Values were quantified in cm.
Additional measures
Maternal race was obtained from a maternal self-report survey that was completed at enrollment during pregnancy. Mothers completed additional surveys at follow-up, where they reported total household income and their highest educational degree obtained.
Statistical analyses
Data were first examined for potential outliers. Two cases were removed where children’s BMI and waist circumference values indicated possible measurement error. These values were deemed implausible for children’s age and the cases were not included in any of the reported analyses.
Multiple linear regression models were fit separately for sleep onset and SRI. All models included control variables (race/ethnicity, child sex, age, mothers’ current BMI) and sleep duration. We also included the season children were in the study by categorizing participants by spring (March 21st to June 21st), summer (June 22nd to September 22nd), and fall (September 23rd to December 21st). Winter (December 22nd to March 20th) was the reference group. Race/ethnicity, child sex, and age were dummy coded. For race/ethnicity, two dummy coded variables were created: Black and Other (i.e., Hispanic children and all other races/ethnicities), with children who were White as the reference group. For age, two dummy coded variables were created as there were few children in the older ages: middle aged children (i.e., 7 to 9) and older aged children (i.e., 10 to 13), with children 4 to 6 as the reference group. By categorizing age, this allowed for a more equal distribution across age groups, compared to if age was treated as continuous. Child sex was coded as one dummy variable, with boys coded as 0 and girls coded as 1. Models were fit for each of the three outcome measures of adiposity, leading to a total of six separate models. To account for multiple testing, the Benjamini-Hochberg procedure was used to adjust the p-values of the sleep measures included in the regression analyses (32). All analyses were computed in R Version 4.0.5. Statistical significance was defined a priori as p < .05.
RESULTS
Descriptive Statistics
A total of N=144 children’s actigraphy data were selected for analysis. Of the 144 children, 70 were female, with a mean age of 7.81 years (SD = 1.93 years, range = 4 to 13 years). The sample was predominantly Black (62.5%), followed by White (32.6%), and Hispanic/Other (4.9%). Mean mother BMI was 32.70 kg/m2 (SD = 9.70, range = 16.42 to 64.30). Mean age of mother at follow-up was 35.70 years (SD = 6.13, range = 24 to 51). About 36% of mothers had a high school diploma, 10% had an associate’s degree, 20% had a bachelor’s degree, and 20% had a master’s or doctoral degree. The remaining 14% of mothers did not have a degree. Mean total household income was $58,369/year (SD = 79,014.18, range = 0 to 500,000). Continuous variables (i.e., sleep measures and adiposity measures) were summarized by their mean and standard deviations in Table 1. Table 2 provides the inter-correlations between the variables included in the analyses.
Table 1.
Summary of Demographic Variables, Season of Participation, Adiposity Outcomes and Sleep Measures Included in Regression Models
| Variable | M or percentage | SD | Range (min, max) |
|---|---|---|---|
| Demographic Variables | |||
| Child age | 7.81 | 1.93 | (4, 13) |
| White children | 32.6% | ||
| Black children | 62.5% | ||
| Hispanic/other children | 4.9% | ||
| Mother’s BMI (kg/m2) | 32.70 | 9.70 | (16.42, 64.30) |
| Season | |||
| Winter | 20.8% | ||
| Spring | 23.6% | ||
| Summer | 20.1% | ||
| Fall | 35.4% | ||
| Adiposity Outcomes | |||
| Child BMI Z-score | 0.56 | 1.18 | (−4.94, 2.63) |
| Child waist circumference (cm) | 67.18 | 12.03 | (45.90, 104.00) |
| Child waist-to-height ratio (cm) | .51 | .06 | (.39, .72) |
| Sleep Measures | |||
| Sleep Onset Time | 10:11PM | 1h 15min | (7:46PM, 3:08AM) |
| Sleep duration | 9h 20min | 50min 23sec | (7h 2min, 11h 58min) |
| SRI | .90 | .05 | (.71, .98) |
Table 2.
Inter-correlations of the Variables Included in the Regression Models
| BMI Z-score | Waist | WHtR | Black | Other | Child sex | Age | Mom BMI | Sleep Onset | Sleep Duration | SRI | Spring | Summer | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Waist | .62*** | ||||||||||||
| WHtR | .71*** | 0.84*** | |||||||||||
| Black | .23** | .20* | .17* | ||||||||||
| Other | −.03 | −.09 | .02 | −.29*** | |||||||||
| Child Sex | .19 | .03 | −.01 | .12 | −.03 | ||||||||
| Age | .10 | .61*** | .17* | .09 | −.06 | .10 | |||||||
| Mom BMI | .23** | .29*** | .26** | .37*** | −.02 | −.05 | .22** | ||||||
| Sleep Onset | .20** | .23** | .22** | .32*** | .04 | .04 | .21* | .37*** | |||||
| Sleep Duration | −.35*** | −.44*** | −.45*** | −.23** | .02 | −.05 | −.20* | −.27** | −.25 ** | ||||
| SRI | −.19* | −.25** | −.19* | −.35*** | .04 | .01 | −.23** | −.24** | −.37*** | .00 | |||
| Spring | −.11 | −.20* | −.21* | −.08 | .03 | .02 | −.04 | −.04 | .11 | .07 | −.01 | ||
| Summer | .07 | .06 | .09 | .03 | −.03 | .03 | .02 | .11 | .10 | .18* | −.10 | −.28*** | |
| Fall | −.16 | −.06 | −.12 | .03 | −.03 | −.05 | .04 | −.01 | −.11 | −.05 | .09 | −.41*** | −.37*** |
Note:
p < .05,
p < .01,
p < .001.
WHtR = waist-to-height ratio; SRI = Sleep Regularity Index
When examining the bivariate associations between the three sleep measures and adiposity outcomes, SRI and sleep duration were negatively associated with all three adiposity outcomes (rs range from −.19 to −.45) while sleep onset timing was positively associated with all three adiposity outcomes (rs range from .20 to .23). While SRI and sleep onset timing (r = −.37, p < .001) and sleep onset timing and sleep duration (r = −.25, p < .01) were significantly correlated with each other, SRI and sleep duration were not correlated (r = .00, p = ns).
Multiple Linear Regression Model Results
Table 3 shows the results from regression models for three adiposity outcomes, using sleep duration and sleep onset times as explanatory variables, adjusting for ethnicity/race, child sex, age, mothers’ BMI, and season. Sleep onset was not significantly associated with any of the three adiposity measures, but a longer sleep duration was significantly associated with a lower BMI Z-score (β = −.29, p < .001), waist circumference (β = −.31, p < .001), and WHtR (β = −.38, p < .001). Of the covariates included in the models, children aged 7 to 9 and 10 to 13 had a higher BMI and waist circumference.
Table 3.
Multiple Linear Regression Models – Sleep Duration and Sleep Onset as Explanatory Variables
| BMI Z-score | Waist circumference | WHtR | ||||
|---|---|---|---|---|---|---|
| Variable | Beta | p | Beta | p | Beta | p |
| Black | .055 | .548 | .026 | .730 | −0.009 | .920 |
| Other | −.009 | .915 | −.056 | .393 | 0.021 | .783 |
| Child sex | .084 | .278 | −.007 | .905 | −0.022 | .760 |
| Middle age child | .151 | .100 | .400** | .000 | 0.114 | .189 |
| Old age child | −.083 | .354 | .524** | .000 | 0.024 | .776 |
| Mother BMI | .111 | .209 | .063 | .386 | 0.105 | .213 |
| Spring | −.195 | .053 | −.287** | .001 | −0.313** | .001 |
| Summer | −.058 | .562 | −.093 | .258 | −0.054 | .568 |
| Fall | −.290** | .004 | −.228** | .006 | −0.287** | .003 |
| aSleep Onset | .034 | .702 | .029 | .702 | 0.075 | .558 |
| aSleep Duration | −.293*** | .001 | −.309*** | .000 | −0.384*** | .000 |
Note:
p < .05,
p < .01,
p < .001.
Betas are standardized betas. WHtR = waist-to-height ratio; SRI = Sleep Regularity Index
Multiple testing was controlled for using the Benjamini-Hochberg Procedure
Table 4 shows the results from regression models for three adiposity outcomes, using sleep duration and SRI as explanatory variables, adjusting for ethnicity/race, child sex, age, mothers’ BMI Z-score, and season. The associations between SRI and the adiposity measures were in the expected direction but non-significant: BMI (β=−.14, p=.114), waist circumference (β=−.11, p=.115), and WHtR (β=−.16, p= .077). Sleep duration was significantly associated with all three outcomes (ps < .001). Of the covariates included in the models, children’s age was associated with a higher BMI Z-score and waist circumference in the expected direction - older children had a higher BMI Z-score and wider waist.
Table 4.
Multiple Linear Regression Models – Sleep Duration and SRI as Explanatory Variables
| BMI Z-score | Waist circumference | WHtR | ||||
|---|---|---|---|---|---|---|
| Variable | Beta | p | Beta | p | Beta | p |
| Black | .013 | .891 | −.007 | .928 | −.048 | .586 |
| Other | −.015 | .851 | −.061 | .349 | .018 | .806 |
| Child sex | .092 | .230 | −.001 | .989 | −.012 | .865 |
| Middle age child | .131 | .146 | .385*** | .000 | .099 | .246 |
| Old age child | −.114 | .210 | .500*** | .000 | −.008 | .930 |
| Mother BMI | .106 | .219 | .059 | .402 | .106 | .192 |
| Spring | −.188 | .054 | −.282** | .001 | −.298** | .001 |
| Summer | −.055 | .577 | −.090 | .265 | −.044 | .633 |
| Fall | −.278** | .006 | −.219** | .008 | −.273** | .005 |
| aSRI | −.138 | .114 | −.109 | .115 | −.156 | .077 |
| aSleep Duration | −.318*** | .000 | −.328*** | .000 | −.418*** | .000 |
Note:
p < .05,
p < .01,
p < .001.
Betas are standardized betas. WHtR = waist-to-height ratio; SRI = Sleep Regularity Index
Multiple testing was controlled for using the Benjamini-Hochberg Procodure
As a sensitivity analysis, we conducted two sets of ANOVAs (results not reported here) to assess the associations between seasonality and sleep duration and sleep onset as these two sleep measures could change with season. We found that there was a significant association with sleep duration and seasonality, with children sleeping more during the summer than in the winter. There was no association between sleep onset and seasonality. Additionally, we conducted three full models (i.e., BMI Z-score, waist circumference, and WHtR) that included all three sleep metrics simultaneously. The results did not differ between the full models and separate models: the SRI/adiposity associations were in the expected direction but non-significant while sleep duration was significantly associated with all outcomes. The three full models are included in the Supplementary Materials.
DISCUSSION
Summary of the Findings
This study examined the associations between three measures of sleep (i.e., onset timing, duration, and regularity) and three measures of adiposity (i.e., BMI, waist circumference, and WHtR) among children. For our first aim, we examined the association between two commonly used sleep measures in child research (i.e., sleep duration and sleep onset timing) and the three adiposity outcomes. While sleep onset timing was not associated with any of the adiposity measures, sleep duration was negatively associated with all three outcomes.
Sleep duration has been shown to be associated with BMI and waist circumference in a plethora of research among children (33–35) and our study corroborated those prior associations. Importantly, children in our study who slept longer also had lower WHtR. Although WHtR is not examined as often as the two other outcomes, Nam and colleagues (36) similarly found a negative association between sleep duration and WHtR among South Korean adolescents. The link between sleep duration and weight in children could reflect a number of factors and the directionality is not completely understood. A review of sleep duration and dietary intake studies suggested that those who do not get enough sleep may compensate for drowsiness and fatigue throughout the day by eating high fat foods and diets lacking in healthy nutrients that boost energy if only temporarily (37).
Although some researchers have found associations between sleep onset timing and adiposity outcomes (6,8), not all made similar discoveries (38). In the current study, the correlation coefficients between sleep onset timing and the three adiposity measures were all significant and in the expected direction. This suggests when considered alone, sleep onset timing is associated with different weight measures among children, but once sleep duration is accounted for, the association is no longer significant. Thus, a later sleep onset may only relate to adiposity outcomes when it leads to a shorter sleep duration. Additionally, most prior research has focused on adults and adolescents, as research interest in sleep onset timing and obesity among young children is relatively recent (8). More research may be needed to better understand how sleep onset timing, sleep duration and child adiposity are interrelated.
We also examined the association between a relatively novel measure of sleep, sleep regularity as measured by SRI, and the three adiposity outcomes. While children with a higher SRI had lower adiposity measures, the associations were not significant. Although SRI has not been studied in children, prior research has found associations between SRI in BMI in adult populations (16,39). While a clear mechanism has not been established, Roane and colleagues argue lower sleep regularity can lead to poorer planning of health behaviors, such as physical activity and healthy eating, which are associated with weight outcomes (40). Furthermore, Bei and colleagues found sleep regularity to be associated with cortisol and allostatic load (an indicator of metabolic health) in a sample of adults (41), and could also be possible mechanisms linking sleep regularity to adiposity. While our study did not find a significant association between SRI and the adiposity outcomes, the direction of the associations corroborates prior research examining other child weight outcomes in relation to sleep variability measures (42,43).
Given that the associations were in the expected direction, the modest sample size of our study (N=144) may warrant additional investigation of the SRI/adiposity relationship.
Strengths, Limitations, and Implications/Future Directions
The strength of our study lies in objective sleep measures (i.e., actigraphy) and multiple child adiposity outcomes. Given that the majority of literature examining child sleep and obesity relies on parent- or self-report surveys (44,45), the use of objective measures in this study helps to advance the field by improving measurement accuracy and minimizing recall and social desirability biases. This is important given the possibility of slight overestimation or underestimation of weight and height when measured by self-report (46) and inaccuracies of sleep when measured with subjective surveys (47). Further, the use of continuously worn objective assessment of sleep and wake allows for assessing SRI, which has not been previously examined in relation to child adiposity. In addition, our novel sleep-wake cycle detection algorithm (29) provided accurate detection of sleep-wake cycles, which in turn provides an accurate estimation of sleep/wake onset times, sleep duration, and SRI, allowing us to adopt measures that are only possible to reliably collect from actigraphy; hence expanding the finite limitations of self-report surveys of sleep.
This study results herein should be interpreted in the context of some limitations. First, a sleep diary to keep a daily record of the children’s sleep was not included in our study. Because of this, we were not able to assess how self-report sleep quality may have added to our interpretation of the results with respect to SRI and/or adiposity outcomes. A second limiting factor is that without a heart rate monitor or electroencephalogram sensor, sleep onset times extrapolated from actigraphy data are based on activity counts, which could lead to an overestimation of the sleep duration. Third, given that the study was cross-sectional, we were not able to examine any longitudinal associations, which may exist between the adiposity and sleep measures. Although SRI has been validated in adults, it has not been validated among children. As a construct, SRI may manifest differently in children. We did not collect data on if children were in school while they wore the Actigraph watches. Given that schools in North Carolina have both year-round schools and schools with summer breaks, we were not able to determine if students were in school or not, although we were able to control for the seasonal period. The sleep duration measure did not include daytime napping, which could underestimate the duration of sleep among young children. We also did not include subjective psychological variables (e.g., depression or anxiety) which may be associated with both sleep and adiposity outcomes; however, this may be a direction for future research.
There are several implications and future directions of our study. Sleep duration is clearly important for child health, even after including measures of sleep variability and timing. If children are not getting enough sleep, even if they go to bed early or consistently, they are more likely to have greater adiposity that may negatively affect other aspects of health behaviors. For example, children with obesity may be less likely to initiate physical activity compared to their peers without obesity (48). Higher weight gain during late childhood and adolescence has also been associated with psychological health problems, such as depression and body dissatisfaction (49). These psychological health problems may further exacerbate sleep problems (50), leading to additional weight gain.
Given that sleep duration was associated with the adiposity outcomes and SRI was marginally associated with the adiposity outcomes, both of these sleep factors may have an influence on child health. This finding supports the sleep health framework (51,52); a theoretical model that posits sleep health is multidimensional and includes components beyond sleep duration, such as variability and timing. Although sleep duration is important for child health, including multiple measures of sleep may be more predictive of health than just including one measure. Intervention scientists targeting the weight of children should consider not only sleep duration, but also sleep regularity, as targeting both duration and consistency of a child’s sleep and wake cycles is likely to lead to improved outcomes, rather than focusing on just one aspect of sleep.
There are several future directions to consider. First, researchers may wish to replicate these findings in other samples. Since SRI is not well studied among children, future research should begin to integrate this measure more often within obesity-related studies. This would require researchers to collect actigraphy measures continuously over a longer period of time (e.g., 5 or more days). To provide support for directionality (e.g., Do sleep problems lead to higher WHtR or vice versa?) longitudinal studies would be warranted. Other measures of adiposity and additional sleep parameters could be considered in follow-up research. For example, Arora et al assessed objective measures of sleep duration and adiposity in a similar aged cohort as the one in the current study, but included measures of body fat percentage and fat mass (53). To further approach the multi-dimensional nature of sleep, future research could include sleep fragmentation or efficiency, and daytime napping. Although previous research has found that additional sleep over the weekend to compensate for short sleep during the weekdays is associated with lower childhood obesity risk (54), given that additional sleep during the weekend would likely lead to less sleep regularity (e.g., a lower SRI), future research could consider how weekend sleep compensation among children interacts with sleep regularity measures. Finally, to support causality, experimental studies that attempt to manipulate sleep duration and/or regularity and examine what effect this has on weight or weight-related behaviors would be needed.
Conclusions
The purpose of this study was to consider which sleep measure, out of the two common measures (sleep onset timing and sleep duration) and one less common measure (sleep regularity), is the most associated with child weight and adiposity outcomes. When considering sleep duration and sleep onset simultaneously, sleep duration was associated with all three adiposity measures. When considering sleep regularity and sleep duration simultaneously, the sleep regularity/adiposity associations were in the expected direction, but non-significant, while sleep duration was significantly associated with all three adiposity outcomes. The results of the current study suggest that sleep duration, even when factoring in sleep onset timing, significantly influences adiposity outcomes among children. While sleep regularity does not influence adiposity to the same extent as sleep duration, the results suggest sleep irregularity could be associated with adiposity in children. Future research examining child adiposity and sleep associations should include SRI and interventions targeting the sleep of children should not only consider increasing sleep duration, but also reducing variability in child sleep/wake patterns.
Supplementary Material
Acknowledgements
This research was supported by the National Institute of Environmental Health Sciences (R01ES016772, P30ES011961 pilot project, R21ES014947,P01ES022831, and R24ES028531), the US Environmental Protection Agency (RD-83543701), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD084487), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK085173), and the Duke Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. In addition, this research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Competing Interests
The authors declare no competing interests.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Morrissey B, Taveras E, Allender S, Strugnell C. Sleep and obesity among children: a systematic review of multiple sleep dimensions. Pediatr Obes. 2020;15(4):e12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller MA, Bates S, Ji C, Cappuccio FP. Systematic review and meta-analyses of the relationship between short sleep and incidence of obesity and effectiveness of sleep interventions on weight gain in preschool children. Obes Rev. 2021;22(2):e13113. [DOI] [PubMed] [Google Scholar]
- 3.Ash T, Taveras EM. Associations of short sleep duration with childhood obesity and weight gain: summary of a presentation to the National Academy of Science’s Roundtable on Obesity Solutions. Sleep Health. 2017. Oct;3(5):389–92. [DOI] [PubMed] [Google Scholar]
- 4.Fatima Y, Doi S a. R, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16(2):137–49. [DOI] [PubMed] [Google Scholar]
- 5.Ruan H, Xun P, Cai W, He K, Tang Q. Habitual sleep duration and risk of childhood obesity: systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015. Dec;5(1):16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golley RK, Maher CA, Matricciani L, Olds TS. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes. 2013. Apr;37(4):546–51. [DOI] [PubMed] [Google Scholar]
- 7.Jarrin DC, McGrath JJ, Drake CL. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Int J Obes. 2013. Apr;37(4):552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AL, Lumeng JC, LeBourgeois MK. Sleep patterns and obesity in childhood. Curr Opin Endocrinol Diabetes Obes. 2015. Feb;22(1):41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scharf RJ, DeBoer MD. Sleep timing and longitudinal weight gain in 4- and 5-year-old children. Pediatr Obes. 2015;10(2):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011. Feb 1;127(2):e345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung Ka-Fai, Katherine Ka-Ki Kan Wing-Fai Yeung. Sleep duration, sleep–wake schedule regularity, and body weight in Hong Kong Chinese adolescents. Biol Rhythm Res. 2012;44(2):169–79. [Google Scholar]
- 12.Fischer D, Klerman EB, Phillips AJK. Measuring sleep regularity: theoretical properties and practical usage of existing metrics. Sleep [Internet]. 2021. Apr 17 [cited 2021 Jul 9];(zsab103). Available from: 10.1093/sleep/zsab103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006. Jan;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
- 14.Fischer D, Vetter C, Roenneberg T. A novel method to visualise and quantify circadian misalignment. Sci Rep. 2016. Dec 8;6:38601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017. Dec;7(1):3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the Sleep Regularity Index in older adults and associations with cardiometabolic risk. Sci Rep. 2018. Dec;8(1):14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustamante CMV, Rodman AM, Dennison MJ, Flournoy JC, Mair P, McLaughlin KA. Within-person fluctuations in stressful life events, sleep, and anxiety and depression symptoms during adolescence: a multiwave prospective study. J Child Psychol Psychiatry. 2020;61(10):1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunsford-Avery JR, Damme KSF, Engelhard MM, Kollins SH, Mittal VA. Sleep/wake regularity associated with default mode network structure among healthy adolescents and young adults. Sci Rep. 2020. Dec;10(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westenhoefer J. Establishing dietary habits during childhood for long-term weight control. Ann Nutr Metab. 2002;46(1):18–23. [DOI] [PubMed] [Google Scholar]
- 20.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002. Dec;31(6):175–84. [DOI] [PubMed] [Google Scholar]
- 21.Dennison BA, Russo TJ, Burdick PA, Jenkins PL. An intervention to reduce television viewing by preschool children. Arch Pediatr Adolesc Med. 2004. Feb 1;158(2):170. [DOI] [PubMed] [Google Scholar]
- 22.Janssen I. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics. 2005. Jun 1;115(6):1623–30. [DOI] [PubMed] [Google Scholar]
- 23.Khoury M, Manlhiot C, McCrindle BW. Role of the Waist/Height Ratio in the Cardiometabolic Risk Assessment of Children Classified by Body Mass Index. J Am Coll Cardiol. 2013. Aug;62(8):742–51. [DOI] [PubMed] [Google Scholar]
- 24.Lo K, Wong M, Khalechelvam P, Tam W. Waist-to-height ratio, body mass index and waist circumference for screening paediatric cardio-metabolic risk factors: a meta-analysis. Obes Rev. 2016;17(12):1258–75. [DOI] [PubMed] [Google Scholar]
- 25.Brambilla P, Bedogni G, Heo M, Pietrobelli A. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. Int J Obes. 2013. Jul;37(7):943–6. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012. Jul 1;7(7):735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, Demark-Wahnefried W, et al. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health. 2011. Dec;11(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry CM, Sabhlok A, Saba VC, Majors AD, Schechter JC, Levine EL, et al. An Automated Text-Messaging Platform for Enhanced Retention and Data Collection in a Longitudinal Birth Cohort: Cohort Management Platform Analysis. JMIR Public Health Surveill. 2019. Apr 2;5(2):e11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Perera R, Engelhard M, Lunsford-Avery J, Kollins S, Fuemmeler B. A generic algorithm for sleep-wake cycle detection using unlabeled actigraphy data. In: 2019 IEEE EMBS International Conference on Biomedical & Health Informativs (BHI). 2019. p. 1–4. [Google Scholar]
- 30.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–904. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Anthropometry procedures manual [Internet]. [cited 2021 Aug 13]. Available from: http://www.cdc.gov/nchs/data/nhanes/bm.pdf
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 33.Eisenmann JC, Ekkekakis P, Holmes M. Sleep duration and overweight among Australian children and adolescents. Acta Paediatr. 2006;95(8):956–63. [DOI] [PubMed] [Google Scholar]
- 34.Carson V, Tremblay MS, Chaput JP, Chastin SFM. Associations between sleep duration, sedentary time, physical activity, and health indicators among Canadian children and youth using compositional analyses. Appl Physiol Nutr Metab. 2016. Jun;41(6 (Suppl. 3)):S294–302. [DOI] [PubMed] [Google Scholar]
- 35.Gong QH, Li SX, Li H, Cui J, Xu GZ. Insufficient sleep duration and overweight/obesity among adolescents in a Chinese population. Int J Environ Res Public Health. 2018. May 15;15(5):997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam GE, Han K, Kim DH, Lee JH, Seo WH. Sleep duration is associated with body fat and muscle mass and waist-to-height ratio beyond conventional obesity parameters in Korean adolescent boys. J Sleep Res. 2017;26(4):444–52. [DOI] [PubMed] [Google Scholar]
- 37.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015. Nov 1;6(6):648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pattinson CL, Smith SS, Staton SL, Trost SG, Thorpe KJ. Investigating the association between sleep parameters and the weight status of children: night sleep duration matters. Sleep Health. 2018. Apr 1;4(2):147–53. [DOI] [PubMed] [Google Scholar]
- 39.Lo K, Keung V, Cheung C, Tam W, Lee A. Associations between sleep pattern and quality and cardiovascular risk factors among Macao school students. Child Obes. 2019. Sep 1;15(6):387–96. [DOI] [PubMed] [Google Scholar]
- 40.Roane B, Seifer R, Sharkey K, Van Reen E, Bond T, Raffray T, et al. What role does sleep play in weight gain in the first semester of university? Behav Sleep Med. 2015;13(6):491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bei B, Seeman TE, Carroll JE, Wiley JF. Sleep and physiological dysregulation: a closer look at sleep intraindividual variability. Sleep [Internet]. 2017. Sep 1 [cited 2021 Jul 9];40(9). Available from: 10.1093/sleep/zsx109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong L, Martinez AJ, Buysse DJ, Harvey AG. A composite measure of sleep health predicts concurrent mental and physical health outcomes in adolescents prone to eveningness. Sleep Health. 2019. Apr;5(2):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou M, Lalani C, Banda JA, Robinson TN. Sleep duration, timing, variability and measures of adiposity among 8- to 12-year-old children with obesity. Obes Sci Pract. 2018. Oct 18;4(6):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guidolin M, Gradisar M. Is shortened sleep duration a risk factor for overweight and obesity during adolescence? A review of the empirical literature. Sleep Med. 2012. Aug;13(7):779–86. [DOI] [PubMed] [Google Scholar]
- 45.Quist JS, Sjödin A, Chaput JP, Hjorth MF. Sleep and cardiometabolic risk in children and adolescents. Sleep Med Rev. 2016. Oct;29:76–100. [DOI] [PubMed] [Google Scholar]
- 46.Poston WSC, Jitnarin N, Haddock CK, Jahnke SA, Day RS. Accuracy of self-reported weight, height and BMI in US firefighters. Occup Med. 2014. Jun 1;64(4):246–54. [DOI] [PubMed] [Google Scholar]
- 47.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zabinski MF, Saelens BE, Stein RI, Hayden-Wade HA, Wilfley DE. Overweight children’s barriers to and support for physical activity. Obes Res. 2003;11(2):238–46. [DOI] [PubMed] [Google Scholar]
- 49.Goldfield GS, Moore C, Henderson K, Buchholz A, Obeid N, Flament MF. Body dissatisfaction, dietary restraint, depression, and weight status in adolescents. J Sch Health. 2010;80(4):186–92. [DOI] [PubMed] [Google Scholar]
- 50.Stein MB, Belik SL, Jacobi F, Sareen J. Impairment associated with sleep problems in the community: relationship to physical and mental health comorbidity. Psychosom Med. 2008. Oct;70(8):913–9. [DOI] [PubMed] [Google Scholar]
- 51.Buysse DJ. Sleep health: can we define it? Does It matter? Sleep. 2014. Jan 1;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meltzer LJ, Williamson AA, Mindell JA. Pediatric sleep health: it matters, and so does how we define it. Sleep Med Rev. 2021. Jun 1;57:101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arora T, Gad H, Omar OM, Choudhury S, Chagoury O, Sheikh J, et al. The associations among objectively estimated sleep and obesity indicators in elementary schoolchildren. Sleep Med. 2018. Jul 1;47:25–31. [DOI] [PubMed] [Google Scholar]
- 54.Wing YK, Li SX, Li AM, Zhang J, Kong APS. The effect of weekend and holiday sleep compensation on childhood overweight and obesity. Pediatrics. 2009. Nov 1;124(5):e994–1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


