Abstract
There are still no definite treatment modalities for interstitial cystitis (IC). Meanwhile, stem cell therapy is rising as potential alternative for various chronic diseases. This study aimed to investigate the safety of the clinical-grade mesenchymal stem cells (MSCs) derived from human embryonic stem cells (hESCs), code name MR-MC-01 (SNU42-MMSCs), in IC patients. Three female IC patients with (1) symptom duration >6 months, (2) visual pain analog scale (VAS) ≥4, and (3) one or two Hunner lesions <2 cm in-office cystoscopy within 1 month were included. Under general anesthesia, participants received cystoscopic submucosal injection of SNU42-MMSCs (2.0 × 107/5 mL) at the center or margin of Hunner lesions and other parts of the bladder wall except trigone with each injection volume of 1 mL. Follow-up was 1, 3, 6, 9, and 12 months postoperatively. Patients underwent scheduled follow-ups, and symptoms were evaluated with validated questionnaires at each visit. No SNU42-MMSCs-related adverse events including immune reaction and abnormalities on laboratory tests and image examinations were reported up to 12-month follow-up. VAS pain was temporarily improved in all subjects. No de novo Hunner lesions were observed and one lesion of the first subject was not identifiable on 12-month cystoscopy. This study reports the first clinical application of transurethral hESC-derived MSC injection in three patients with IC. hESC-based therapeutics was safe and proved to have potential therapeutic efficacy in IC patients. Stem cell therapy could be a potential therapeutic option for treating IC.
Keywords: interstitial cystitis, embryonic stem cell, mesenchymal stem cell, clinical trial, Hunner lesion
Graphical Abstract
The current manuscript describes the clinical trials of human embryonic stem cell (hESC)-derived multipotent mesenchymal stem cells (MMSCs) for patients with interstitial cystitis (IC). We provide the clinical importance and advance that cystoscopic submucosal injection of the hESC-derived MMSC was safe and exhibited potential therapeutic efficacy in human subjects, without no adverse events.
Lessons Learned.
This phase 1 clinical trial used mesenchymal stem cells derived from human embryonic stem cells to treat interstitial cystitis patients with Hunner leisons.
Transurethral application of the human embryonic stem cell derivative is safe and feasible, improving the prospects of stem cell therapy for patients with interstitial cystitis.
The dosage of stem cells needs to be refined.
Significance Statement.
This study reports the first clinical application of transurethral human embryonic stem cell (hESC)-derived mesenchymal stem cell injection in three patients with interstitial cystitis (IC). This hESC-based therapeutics was safe and proved to have potential therapeutic efficacy in IC patients. Stem cell therapy could be a potential therapeutic option for treating IC.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic inflammatory disease that is characterized by chronic bladder or pelvic pain associated with bladder filling and usually accompanies lower urinary tract symptoms, such as frequency, nocturia, urgency, and urgency urinary continence.1,2 IC/BPS can be briefly categorized into two subtypes—those with positive Hunner lesions—“a circumscript, redden mucosal area with abnormal vessels radiating towards central scar with or without coagulum” on cystoscopy (Hunner-type IC) and those with negative Hunner lesions on cystoscopy (non-Hunner-type IC).3 If Hunner lesion is present, transurethral fulguration of the lesion is recommended.4 The procedure is effective for rapid alleviation of pain; however, symptom recurrence is common. According to American Urological Association (AUA) guidelines, individualized and multidisciplinary treatment approaches for IC/BPS are recommended, based on the unique characteristics of each patient, but only the behavioral/non-pharmacologic treatments, including behavior modification, stress and pain management, are recommended to all patients.1,2,5,6 Other treatment modalities have limited grade of recommendation and evidence strength, in other words, there is no definite cure for IC/BPS.
Stem cell therapy is emerging as a potent treatment option in regenerative medicine as stem cells are capable of self-renewal, differentiation into damaged cells, and paracrine effect.7-9 Wide range of chronic diseases including voiding dysfunction is a possible candidate. Mesenchymal stem cells (MSCs) are one of the reliable sources for stem cell therapy.10-12 MSCs can be obtained from adult or fetal tissues, or pluripotent stem cells (PSCs), such as human embryonic stem cells (hESCs) and induced PSCs (iPSCs). Favorable outcomes using different types of MSCs have been reported in clinical trials of various urological diseases, including erectile dysfunction, stress urinary incontinence, and detrusor underactivity, but all of the studies used autologous MSCs derived from muscle or adipose tissue.
Previously, we reported the benefits and non-tumorigenicity of multipotent MSCs (MMSCs) derived from hESCs in various preclinical voiding dysfunction models.13-19 MMSCs ameliorated the bladder voiding function and histological injuries, including urothelium denudation, mast cell infiltration, tissue fibrosis, and apoptosis. Recently, a combination of two-photon intravital microscopic imaging and single-cell and genome-wide transcriptome analyses demonstrated that the engrafted MMSCs were progressively integrated as perivascular cells, which could support the regenerative capacity of the damaged urothelium and stimulate the anti-inflammatory micro-environment via secreting WNT family member 5A (WNT5A).16 Notably, hESC-derived MMSCs exhibited a superior therapeutic efficacy compared to their adult tissue-derived counterpart. Limited evidence of adverse outcomes, including abnormal growth, tumorigenesis, or immune-mediated transplant rejection over 12-month post-injection, were apparent. Furthermore, MMSCs could overcome the frequently observed limitations in the adult tissue-derived MSCs, including functional loss and heterogeneity, following ex vivo expansion. Based on these findings, in this study, we developed clinical-grade MSCs derived from hESCs into the cell-type product with code name MR-MC-01 (SNU42-MMSCs) and investigated its potential in treating patients with IC. The primary outcome was the safety and tolerability of SNU42-MMSCs, and the secondary outcome was the efficacy of SNU42-MMSCs in clinical symptoms including pain, voiding profiles, and characteristics of Hunner lesions on cystoscopy.
Materials and Methods
Ethics Statement
All animal experiments (two independent sets with five animals per group) were approved and performed in compliance with the guidelines of the Institutional Animal Care and Use Committee of the University of Ulsan College of Medicine (IACUC-2019-12-290). The clinical trial was approved by the Institutional Review Board of the center (2019-1451), and the use of SNU42-MMSCs for the protocol was approved by the Korean Ministry of Food and Drug Safety. This protocol was registered on ClinicalTrials.gov (NCT04610359). Informed consent was obtained from all participants.
Manufacturing Clinical-Grade MMSCs (SNU42-MMSC)
Clinical-grade SNU42-MMSCs were manufactured in a fully validated good manufacturing practices facility under strict environmental control monitoring systems and routine microbial testing regimens at Mirae Cell Bio Co., Ltd. (Seoul, Republic of Korea). Master and working cell banks were established using hESC line SNUhES42 (Seoul National University), registered with the Korea Centers for Disease Control and Prevention.
The multipotent mesenchymal stem cell (MMSCs) used in this study were generated by differentiation of hESCs through embryoid body (EB) formation for 2 days. The mesenchymal cells (those migrating to the lower compartment of a porous Transwell membrane (8 µm) over 5 days) were isolated as previously described.13,20 Clinical and research-grade MMSCs were established using the same differentiation procedure, except for using different hESC lines, SNUhES42 and H9, respectively. Detail procedures for in vitro characterizing MMSCs were described in the Supplementary Methods.
Determining the Dose Ranges and Final Formulation
SNU42-MMSCs were administered at approximately 2.0 × 107 cells/60 kg person, calculated by converting an animal dose to the human equivalent dose (HED) according to body weight.21 The dose of the clinical investigational drug was determined based on the results of efficacy and toxicity tests. A non-toxic dose of SNU42-MMSCs, based on the no-observed-adverse-effect level (NOAEL) derived from the toxicity study, was determined as 2.0 × 106 cells/head in 20 g Balb/c-nu/nu mice. The cell concentrations and doses tested were 1.0 × 105 cells, 2.5 × 105 cells, 5.0 × 105 cells, and 1.0 × 106 cells in preclinical models of IC. Considering safety, the lowest dose with an observable efficacy (1.0 × 105 cells/300 g rat) was selected to calculate the human dose using the aforementioned method.
SNU42-MMSCs were cryopreserved using Cryostor CS10 cryopreservation solution (#210102, BioLifeSolutions, Bothell, WA, USA). On the day of the injection, vials of cryopreserved SNU42-MMSCs were thawed and resuspended in normal saline at a concentration of 2.2 × 107 cells/5.5 mL. Formulated SNU42-MMSCs were stored at 2-8°C up to 3 hours prior to administration.
Therapeutic Efficacy of SNU42-MMSCs in the Preclinical Model
Chronic IC was induced in female 8-week-old Sprague-Dawley rats (OrientBio, Gapyong, Gyeonggi-do, Korea) by administering protamine sulfate (PS, 10 mg/rat; Sigma-Aldrich, St. Louis, MO, USA), followed by instillation of lipopolysaccharide (LPS, 750 µg/rat, Sigma-Aldrich) for 30 minutes, as previously described.10,15,22 One week after the final instillation of PS/LPS, 1 × 105 MMSCs were directly injected into the outer layer of the bladder (anterior wall). To simulate the same procedures in the clinical study, both the clinical-grade (SNU42-MMSC) and research-grade (H9-MMSC) cells were cryopreserved, and on the day of injection, vials of cryopreserved cells were thawed and washed twice with 10 mL normal saline to minimize the effects of the cryopreservation medium (10% DMSO). The usage of the cryopreserved cells and lower cell dosage was different from previous preclinical studies, wherein freshly cultured cells were trypsinized, washed, and suspended in phosphate-buffered saline before injection (2 × 105 cells).13,14,16 We compared the therapeutic efficacy of the SNU42-MMSCs with that of the research-grade H9-MMSCs which were used in the previous preclinical studies.10,13,15,17 As a control treatment, dimethyl sulfoxide (DMSO, 67457-178-50; Mylan Pharmaceuticals Inc. Canonsburg, PA, USA) suspended in PBS was instilled for 30 minutes.23 The bladder voiding function and tissue injuries were assessed by awake cystometry and histological analysis, as previously described.10,13,15,17,22
Clinical Trial: Patient Selection
Adult female IC patients with symptoms lasting for more than 6 months, scoring ≥4 on the visual pain analog scale (VAS), and one or two Hunner lesions of <2 cm as observed in-office cystoscopy within 1 month were eligible for inclusion. Patients who were not suitable for participating in the clinical study based on the exclusion criteria and clinical investigators’ consideration were excluded. The main contraindications were pregnancy, uncontrolled systemic diseases, active or recurrent infection, immunosuppression or immunodeficiency, and malignancies. Detailed inclusion and exclusion criteria are provided in Supplementary Table S1.
Stem Cell Injection in Human Subjects
Under general anesthesia, patients were aseptically draped in lithotomy position. Before the injection, the locations and sizes of Hunner lesions were documented according to the bladder map which divided the bladder wall into 22 areas (Supplementary Fig. 1). SNU42-MMSCs were injected using a 70-cm-long 22-gauge needle, which is commonly used for intravesical Botox injection. Patients received submucosal injections of SNU42-MMSCs at the centers or margins of Hunner lesions and other parts of the bladder wall (except trigone), and the injection volume at each of the 5 sites was 1 mL.
Follow-up Protocol
Patients were scheduled for follow-up visits at 1, 3, 6, 9, and 12 months postoperatively. At each visit, patients underwent thorough vital sign monitoring, physical examination, and scheduled workups, including chest x-ray, laboratory tests, and abdominopelvic computed tomography (CT) to rule out any adverse events such as malignancy. The immunological response was monitored via HLA (human leukocyte antigen) and PRA (panel reactive antibody) testing. Patient symptoms were evaluated using the VAS pain scale, O’Leary-Sant IC symptom index/problem index (ICSI/ICPI), pelvic pain and urgency/frequency (PUF), global response assessment (GRA), and a 3-day voiding diary. Locations and extent of Hunner lesions were evaluated with flexible cystoscopy. The overall follow-up schedule is listed in Supplementary Table S2.
Statistical Analysis of the Clinical Study
The sample size was not based on statistical power calculation because the size of the study population was not sufficient to draw a conclusive statistical analysis. Summary data of each patient were used for the assessment of adverse events and clinical symptoms.
Results
Generation and Characterization of SNU42-MMSCs
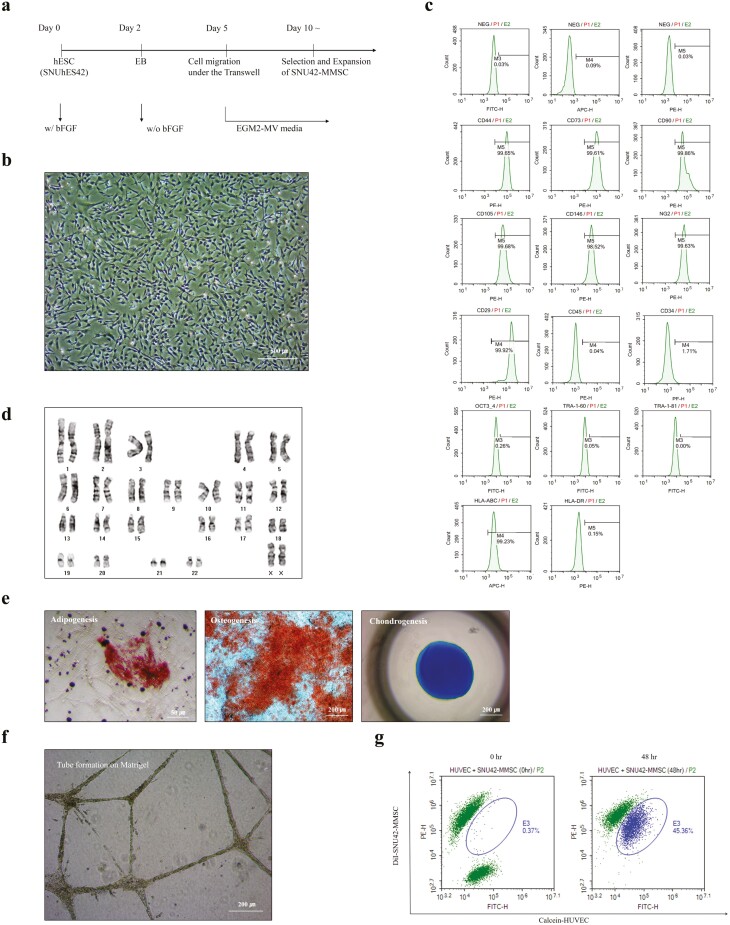
The SNU42-MMSCs were established as described in a previous study where research-grade MMSCs (H9-MMSC) were established using a porous membrane-mediated isolation method.20 Briefly, SNUhES42 colonies were allowed to form EBs for 2 days during initial differentiation. After plating the EBs on a Transwell for 5 days, the cells migrated to the lower compartment of the Transwell were gently scarped, and were enzymatically dissociated for subculture (Fig. 1a). The cells were replated, and they exhibited >99% purity within 7 days of differentiation. Like H9-MMSCs (Supplementary Fig. 2), SNU42-MMSCs exhibited spindle-shaped fibroblast-like morphology (Fig. 1b) and expressed MSC (CD90, CD105, CD73, CD44, and CD29) and pericyte (CD146 and NG2)-specific surface antigens. However, the expression of hematopoietic stem cell markers (CD45 and CD34), pluripotency markers (OCT-3/4, TRA-1-60, and TRA-1-81), and MHC class II (HLA-DR) was almost negligible (Fig. 1c). Established SNU42-MMSCs were expanded for tens of passages without chromosomal abnormalities (Fig. 1d), and their multipotency was examined by differentiation into cells of osteogenic, chondrogenic, and adipogenic lineages (Fig. 1e, Supplementary Fig. 3). Moreover, SNU42-MMSCs exhibited a tube-forming ability in Matrigel (Fig. 1f), and potent angiogenic effects were observed following dye transfer assays in the presence or absence of carbenoxolone, a gap junction intercellular communication inhibitor (Fig. 1g, Supplementary Fig. 4).
Figure 1.
Generation and characterization of clinical-grade SNU42-MMSCs. (a) Experimental scheme for the generation of clinical-grade MMSCs (SNU42-MMSC) using the Transwell-based differentiation of a hESC line, SNUhES42. (b) SNU42-MMSCs exhibited unique fibroblastic cell morphology. Scale bar = 500 µm. (c) SNU42-MMSCs were analyzed for specific surface antigen marker expression of MSCs (CD90, CD105, CD73, CD44, and CD29), pericytes (CD146 and NG2), hematopoietic stem cells (CD45 and CD34), pluripotent stem cells (OCT-3/4, TRA-1-60, and TRA-1-81), and MHC class I (HLA-ABC) and class II (HLA-DR). (d) Karyotypic analysis of SNU42-MMSCs. The isolated cells showed stable proliferation without chromosomal changes. (e) Differentiation potential of SNU42-MMSCs was shown by adipogenesis (left, Oil Red staining, scale bar = 50 µm), osteogenesis (middle, Alizarin Red S staining, scale bar = 200 µm), and chondrogenesis (right, Alcian blue staining, scale bar = 200 µm). (f) In vitro tube formation assay. Scale bar = 200 µm. (g) Dye transfer (circle in the plot) was detected in a co-culture of DiI-labeled SNU42-MMSCs (MMSC-DiI, red) and calcein-labeled human umbilical vein endothelial cells (HUVEC-Calcein, green). The results for the characterization of the research-grade (H9-MMSC) cells are presented in Supplementary Fig. 2.
Therapeutic Efficacy of SNU42-MMSCs in Preclinical Model
Next, the therapeutic efficacy of SNU42-MMSCs was compared with that of H9-MMSCs and one current treatment (DMSO) using a chronic IC animal model with the instillation of LPS. Awake cystometric analysis showed that SNU42-MMSCs had superior therapeutic efficacy compared to H9-MMSCs and DMSO instillation in terms of restoring bladder voiding function defects, such as increased non-voiding contraction, basal and micturition pressure, decreased micturition interval, bladder capacity, micturition volume, and residual volume (Fig. 2a, 2b). SNU42-MMSCs were the most effective in restoring the histological injuries commonly observed in IC patients, including denuded urothelium, increased mast cell infiltration, and apoptosis (Fig. 2c, Supplementary Fig. 5).
Figure 2.
Therapeutic efficacy of SNU42-MMSCs in a preclinical study. (a) Representative awake cystometry results, (b) quantitative bladder voiding parameters, and histological examinations, and (c) at 1 week after the injection of the indicated MMSCs (1 × 105 cells) or a single instillation of 50% DMSO. The clinical-grade SNU42-MMSC and research-grade H9-MMSC cells were established using the same differentiation procedure (porous membrane-mediated isolation of MSCs from different hESC lines).20 Data are presented as the mean ± SEM from two independent sets with five animals in each group. Statistical analyses were performed using one-way ANOVA with Bonferroni post hoc tests. *P < .05, **P < .01, ***P < .001 compared to the LPS-IC group; #P < .05, ##P < .001, ###P < .001 compared to the DMSO group, $$$P < .001. IAP, intra-abdominal pressure; IVP, intravesical pressure; Sham, sham-operated. Representative histological results are presented in Supplementary Fig. 5.
Clinical Trial: Baseline Demographics of Patients
Five patients were screened, but two patients were excluded due to the following reasons, respectively; positive syphilis on serology tests and Hunner lesions larger than 2 cm (Supplementary Fig. 6). A total of three patients were enrolled and all patients completed the study. The median age was 71 years (range: 68-77), and symptom duration was more than 1 year, respectively. All patients had previous refractory symptoms to oral medication such as pentosan polysulfate. Two patients had experienced recurrence after intravesical instillation therapy and endoscopic operation. All of them had negative urine cytology or bladder biopsy to rule out combined malignancy before screening (Table 1).
Table 1.
Baseline demographics of patients.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age at enrollment (years) | 77 | 68 | 71 |
| Age at symptom onset (years) | 69 | 67 | 60 |
| Body mass index (kg/m2) | 25.9 | 27.5 | 26.2 |
| Previous IC treatment | |||
| Medication | Yes | Yes | Yes |
| Intravesical treatment | Yes | None | Yes |
| TURC | Twice | None | Four times |
| Baseline voiding profile | |||
| Frequency (/day) | 9 | 9.3 | 25 |
| Presence of urgency | Yes | Yes | Yes |
| Functional bladder capacity (cc) | 300 | 200 | 200 |
| Baseline Hunner lesions | |||
| Location(s) | P7, P9 | R1-P1, P5 | D5-D6, D1-D2 |
| Size | 1.5 cm, 0.6 cm | 1.9 cm, 1 cm | 0.8 cm, 0.6 cm |
Locations of Hunner lesions are based on the bladder map (Supplementary Fig. 1).
Abbreviation: TURC, transurethral resection and cauterization.
Safety of SNU42-MMSCs in Human Subjects
No SNU42-MMSCs-related adverse events (including immune reaction or abnormalities on clinical tests) were reported. Patients underwent pre- and postoperative HLA class I and II antibody screening. Patient 1 showed weak positive HLA class I antibody on preoperative PRA screening, but single antigen assay—Luminex technology with mean fluorescence intensity (MFI) showed negative results. The subject reported temporary right-side suprapubic pain after stem cell injection, but it was tolerable and did not require opioid analgesics, additional intervention, or admission.
Efficacy of SNU42-MMSCs in Human Subjects
Pain significantly improved 1 month after the procedure in all patients, but subsequent changes differed. However, none of the patients required gabapentin or opioid analgesics that unexpectedly aggravated transient pain and was well controlled with non-steroidal anti-inflammatory drugs during the study period. Patient 2, who presented the most drastic improvement in post-procedural pain presented the same degree of recurred symptoms at 12-month follow-up. Transurethral resection and cauterization (TURC) was performed for the Hunner lesions and there was no evidence of malignancy in resected bladder tissue. In other participants, no significant aggravation was determined based on the symptom questionnaires (Table 2). Moreover, none of the patients presented de novo Hunner lesions or any abnormalities in the bladder. The extent of Hunner lesions remained comparable or slightly decreased; one Hunner lesion of patient 1 was not identifiable on 12-month cystoscopy (Fig. 3).
Table 2.
Changes in clinical symptoms after stem cell injection.
| VAS | ICI total | ICSI | ICPI | PUF total | PUF-S | PUF-B | GRA | |
|---|---|---|---|---|---|---|---|---|
| A. Patient 1 | ||||||||
| Pre | 8 | 20 | 9 | 11 | 14 | 9 | 5 | — |
| Post 1 mo | 3 | 17 | 7 | 10 | 16 | 9 | 7 | 5 |
| Post 3 mo | 4 | 17 | 9 | 8 | 16 | 11 | 5 | 5 |
| Post 6 mo | 5 | 19 | 9 | 10 | 14 | 9 | 5 | 5 |
| Post 12 mo | 5 | 21 | 10 | 11 | 15 | 10 | 5 | 5 |
| B. Patient 2 | ||||||||
| Pre | 10 | 24 | 11 | 13 | 17 | 11 | 6 | — |
| Post 1 mo | 2 | 12 | 7 | 5 | 4 | 4 | 0 | 7 |
| Post 3 mo | 4 | 15 | 7 | 8 | 12 | 8 | 4 | 6 |
| Post 6 mo | 6 | 30 | 16 | 14 | 23 | 14 | 9 | 4 |
| Post 12 mo | 10 | 29 | 14 | 15 | 26 | 17 | 9 | 2 |
| C. Patient 3 | ||||||||
| Pre | 4 | 34 | 18 | 16 | 27 | 18 | 9 | — |
| Post 1 mo | 2 | 32 | 17 | 15 | 24 | 16 | 8 | 4 |
| Post 3 mo | 1 | 34 | 18 | 16 | 25 | 16 | 9 | 5 |
| Post 6 mo | 9 | 36 | 20 | 16 | 26 | 17 | 9 | 5 |
| Post 12 mo | 4 | 36 | 20 | 16 | 27 | 18 | 9 | 6 |
Abbreviations: GRA, global response assessment; ICSI/ICPI, O’Leary-Sant IC symptom index/problem index; PUF, pelvic pain, and urgency/frequency; VAS, visual pain analog scale.
Figure 3.
Serial cystoscopic images of Hunner lesions in each patient. (a) Patient 1, (b) Patient 2, and (c) Patient 3. Hunner lesions in each patient at pre-, 1-month, 3-month, 6-month, and 12-month post-stem cell injection.
Discussion
The present study reports the first clinical application of SNU42-MMSCs, the hESCs-derived clinical-grade MMSCs, for the treatment of Hunner-type IC. The SNU42-MMSCs were manufactured using strict quality control and were revealed to be non-tumorigenic in good laboratory practice (GLP) preclinical studies required by the Korea Ministry of Food and Drug Safety (KFDA) prior to this phase I study. A single injection of SNU42-MMSCs into the submucosal layer of the urinary bladder was safe and well tolerated and showed therapeutic efficacy in subjective symptom relief.
The predominant problem with IC is recurrence due to the lack of definite treatment modalities. In a retrospective study involving 91 patients who underwent TURC for Hunner lesions from October 2011 to December 2017, the recurrence rates after TURC were 12.7%, 40%, and 55.2% at 6, 12, and 18 months, respectively. Recurrence patterns of Hunner lesions were unpredictable.24 IC is a loco-extensive disease affecting the entire bladder; bladder tissues are affected by Hunner-type IC presented pan cystitis with varying degrees of severe inflammation and urothelial denudation in the entire bladder.25,26 If Hunner lesions affect the most vulnerable part of the bladder, local control of the lesion is merely a temporary option. Stem cells undergo self-renewal and simultaneously give rise to various progenitors to replace the damaged cells,8,9 so the concept that transplanted cells would regenerate in an abnormal, damaged bladder is a lucrative basis of a possible alternative treatment option. Furthermore, MSCs could secrete a wide range of tropic factors, stimulating the micro-environments favorable to tissue regeneration and immunomodulation response.27,28 In our preclinical studies, MMSC administration significantly activated sonic hedgehog and WNT family genes, resulting in the upregulation of their downstream growth factors in bladder tissues. Notably, the therapeutic potency of MMSCs was abrogated by the interference of WNT or insulin-like growth factor signaling.1,6 Consistent with these results, the silencing of WNT5A in MMSCs significantly impaired core functions of MSCs, including migration and self-renewal potency.7 In this context, identifying the molecular nature of secretomes from MMSCs could be required to precisely elucidate their mode of action for treating IC.
For successful clinical application of stem cells, the type and dosage of stem cells and the route of administration must be clearly defined. Our study used SNU42-MMSCs, the drug substance of MMSCs, which have shown therapeutic efficacy, as examined by various assays (histology, functional studies, and gene expression analysis), in various rat models of bladder dysfunction.10,13,17-19 The MMSC treatment had superior therapeutic efficacy (both short-term and long-term) when compared to the counterparts from adult tissues such as bone marrow and umbilical cord in different rat models of bladder dysfunction and also had long-term safety in the rat model of HCl-induced IC.10,13,16 The optimal therapeutic dosage of MMSCs in our rat model was 1 ~ 2.5 × 105/mL, which was converted to HEDs. Our route of stem cell transplantation was transurethral bladder injection via cystoscopy, which is the most familiar route for most urologists.
Various clinical trials have investigated the efficacy of autologous muscle- or adipose tissue-derived MSCs in urological diseases including stress urinary incontinence and detrusor underactivity.9,29 Using autologous MSCs might be safer than using allogenic MSCs, but the challenges of hampered engraftment and survival of transplanted cells remain. Large-scale production of MSCs derived from adult tissues has limits in maintaining primitive function and proliferative capacities,11,12 especially if the cells are obtained from tissues of an aged donor. Moreover, MSCs that undergo higher passage numbers are more likely to provoke an innate immune attack upon transplantation.30 The SNU42-MMSCs used in the present study are derived from the hESCs, which is a cost-effective alternative because of the unlimited expansion potential and pluripotency of these cells.20,31 Moreover, the therapeutic efficacy of SNU42-MMSCs is superior to that of adult tissue-derived MSCs reported in our previous studies.10,13,17 Moreover, acquiring a sufficient amount of autologous tissue harbors biopsy-related adverse events, including pain, bleeding, and chances of infection. SNU42-MMSC is a product-type stem cell that does not require tissue sampling from subjects in a clinical setting. Furthermore, isolating autologous stem cells from recipients’ tissue requires a certain amount of time after tissue harvest so that there is a gap between stem cell preparation and transplantation. Finally, there is always a possibility of variations in the total amounts of injected cells in autologous stem cells therapy. Several clinical trials, which used autologous stem cells in urological diseases, have injected different amounts of stem cells.9
The possibility of adverse events, especially carcinogenesis, is considered one of the major concerns of clinical uses of hESC-derived cells. Several clinical trials in the fields of ophthalmology32-35 and cardiology36 have used hESC-derived cells. In a study with subretinal transplantation of hESC-derived retinal pigment epithelial (hESC-RPE) cells in patients with Stargardt’s macular dystrophy and atrophic age-related macular degeneration, no evidence of adverse proliferation, rejection, or serious ocular or systemic safety issues related to the procedure was observed within a median follow-up of 22 months.34 Adverse events were associated with vitreoretinal surgery and immunosuppression. A recent publication on 5-year biosafety of hESC-RPE cells in seven subjects with Stargardt’s macular degeneration reported that none of the patients experienced systemic or local adverse reactions, except for two patients who presented transiently elevated post-procedural intraocular pressure.32 In six patients with ischemic left ventricular dysfunction, hESC-derived cardiovascular progenitors embedded in a fibrin patch were delivered epicardially during a coronary artery bypass procedure. No tumor was detected during the median 18-month follow-up, and none of the patients presented arrhythmias.36 In our preclinical study with MMSC transplantation in the HCl-induced IC rat model, no tumors were observed up to 12-month follow-up when monitored with nanoScan positron emission tomography/MRI imaging.13 To summarize, hESC-derived cells have potential risk for tumorigenesis, but local injection of the cells in the target organ may be safe as the efficacy, non-invasiveness, time-saving potential, and consistency overcome the disadvantages.
The main difference between the previous clinical trials using hESC-derived cells and our study is the use of immunosuppressants. In clinical trials using hESC-RPE cells or patches, patients were prescribed a temporary systemic immunosuppression regimen, such as oral tacrolimus, mycophenolate mofetil, or oral prednisolone to prevent rejection. These regimens led to a wide range of side effects, including fatigue, diarrhea, stomachache, uncontrolled diabetes, infection, and shortness of breath. We did not use a systemic immunosuppressant for the following reasons: First, the injection site was the submucosa of the bladder, and the transplanted cells were highly likely to be localized between the submucosa and the detrusor, which was expected to result in a limited systemic response. Second, the IC bladder presents changes in immune responses like B-cell abnormalities; cyclosporine A, which modulates immunity, is recommended as a fifth-line treatment for IC, according to the AUA guidelines. Concurrent prescription of an immunosuppressant is most likely to overestimate the therapeutic efficacy of SNU42-MMSCs in future studies. Third, the actual injected number of cells might be less than expected as needle injection results in a tiny defect of the urothelium, which could be a route for cell spillage. In addition, humans urinate regularly, so spilled cells into the urinary bladder are immediately diluted by urine and get washed out during voiding.
The therapeutic efficacy of SNU42-MMSCs varied among study participants, but VAS pain improved significantly (VAS ≤4) at 1-month post-procedure in all subjects. The size of Hunner lesions is not always proportional to the degree of pain, but initially large (nearly 2 cm) Hunner lesions were more likely to persist throughout the follow-up. Meanwhile, the extent of small Hunner lesions (<1 cm) tended to decrease; one Hunner lesion of patient 1 was not identifiable at 12-month follow-up. This trend was not applicable to patient 3, who initially had the smallest Hunner lesions, but the longest symptom duration (11 years), previous history of TURC (four times), and relatively decreased functional bladder capacity compared to the other subjects. Although this is a phase I study designed to explore the safety of clinical-grade MMSCs at minimum therapeutic dosage, the disease duration of IC and extent of previous therapy may play a key role in the variation of therapeutic efficacy.
This study presents the preliminary investigation on the safety of treatment with SNU42-MMSCs in IC patients but is limited by its small number of patients. Long-term safety monitoring is scheduled for up to 5 years for these patients. A follow-up clinical trial that investigates the safety of a higher dose and modified injection method of SNU42-MMSCs to decide the optimal therapeutic dosage and protocol for a greater number of patients is about to be launched. Further investigations should focus on an optimal dosage and injection schedule of SNU42-MMSCs to maximize the efficacy with continuous safety monitoring, to suggest stem cell injection as a possible therapeutic modality for treating IC patients.
Supplementary Material
Contributor Information
Jung Hyun Shin, Department of Urology, Ewha Womans University, Mokdong Hospital, Seoul, Korea.
Chae-Min Ryu, Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea; Department of Biomedical Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Hwan Yeul Yu, Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea; Department of Biomedical Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Juhyun Park, Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Ah Reum Kang, Mirae Cell Bio Co., Ltd., Seoul, Korea.
Jeong Min Shin, Mirae Cell Bio Co., Ltd., Seoul, Korea.
Ki-Sung Hong, Mirae Cell Bio Co., Ltd., Seoul, Korea; Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea.
Eun Young Kim, Mirae Cell Bio Co., Ltd., Seoul, Korea.
Hyung-Min Chung, Mirae Cell Bio Co., Ltd., Seoul, Korea; Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea.
Dong-Myung Shin, Department of Biomedical Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Myung-Soo Choo, Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Funding
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF-2020R1A2C1007789, NRF-2022R1C1C102368); by an NRF MRC grant funded by the Korean government (MSIP) (NRF-2018R1A5A2020732); and by the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare, Nos. 21D0804L1 and RS-2022-00040242).
Conflict of Interest
This clinical study was funded by the research funding/contracted research (#2019-1451) between M.-S.C. (Asan Medical Center) and E.Y.K. (Mirae Cel Bio Co., Ltd). The other authors declared no potential conflicts of interest.
Author Contributions
J.H.S.: conception and design, administrative support, collection and assembly of data, data analysis and interpretation, manuscript writing. C.-M.R., H.Y.Y.: collection and/or assembly of data, data analysis and interpretation. J.P.: final approval of manuscript. A.R.K.: provision of study material, administrative support. J.M.S.: provision of study material, collection and/or assembly of data. K.-S.H.: collection and/or assembly of data, mnuscript writing. E.Y.K., H.-M.C.: provision of study material, final approval of manuscript. D.-M.S.: manuscript writing, final approval of manuscript. M.-S.C.: conception and design, final approval of manuscript.
Data Availability
All data are incorporated into the article and its online supplementary material.
References
- 1. Clemens JQ, Erickson DR, Varela NP, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2022;208:34-42. 10.1097/JU.0000000000002756 [DOI] [PubMed] [Google Scholar]
- 2. Homma Y, Akiyama Y, Tomoe H, et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int J Urol. 2020;27:578-589. 10.1111/iju.14234 [DOI] [PubMed] [Google Scholar]
- 3. Akiyama Y, Hanno P.. Phenotyping of interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26(Suppl 1):17-19. 10.1111/iju.13969 [DOI] [PubMed] [Google Scholar]
- 4. Ronstrom C, Lai HH.. Presenting an atlas of Hunner lesions in interstitial cystitis which can be identified with office cystoscopy. Neurourol Urodyn. 2020;39:2394-2400. 10.1002/nau.24500 [DOI] [PubMed] [Google Scholar]
- 5. Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545-1553. 10.1016/j.juro.2015.01.086 [DOI] [PubMed] [Google Scholar]
- 6. Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185:2162-2170. 10.1016/j.juro.2011.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang JM, Chung S, Yun K, et al. Long-term effects of human induced pluripotent stem cell-derived retinal cell transplantation in Pde6b knockout rats. Exp Mol Med. 2021;53:631-642. 10.1038/s12276-021-00588-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim A, Shin DM, Choo MS.. Stem cell therapy for interstitial cystitis/bladder pain syndrome. Curr Urol Rep. 2016;17:1. 10.1007/s11934-015-0563-1 [DOI] [PubMed] [Google Scholar]
- 9. Shin JH, Ryu CM, Yu HY, et al. Current and future directions of stem cell therapy for bladder dysfunction. Stem Cell Rev Rep. 2020;16:82-93. 10.1007/s12015-019-09922-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim Y, Jin HJ, Heo J, et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018;32:2672-2684. 10.1038/s41375-018-0151-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim J, Heo J, Ju H, et al. Glutathione dynamics determine the therapeutic efficacy of mesenchymal stem cells for graft-versus-host disease via CREB1-NRF2 pathway. Sci Adv. 2020;6:eaba1334. 10.1126/sciadv.aba1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim J, Heo J, Yu HY, et al. Small-sized mesenchymal stem cells with high glutathione dynamics show improved therapeutic potency in graft-versus-host disease. Clin Transl Med. 2021;11:e476. 10.1002/ctm2.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim A, Yu HY, Lim J, et al. Improved efficacy and in vivo cellular properties of human embryonic stem cell derivative in a preclinical model of bladder pain syndrome. Sci Rep. 2017;7:8872. 10.1038/s41598-017-09330-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryu CM, Yu HY, Lee HY, et al. Longitudinal intravital imaging of transplanted mesenchymal stem cells elucidates their functional integration and therapeutic potency in an animal model of interstitial cystitis/bladder pain syndrome. Theranostics. 2018;8:5610-5624. 10.7150/thno.27559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin JH, Ryu C-M, Ju H, et al. Synergistic effects of N-acetylcysteine and mesenchymal stem cell in a lipopolysaccharide-induced interstitial cystitis rat model. Cells. 2019;9:86. 10.3390/cells9010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu HY, Lee S, Ju H, et al. Intravital imaging and single cell transcriptomic analysis for engraftment of mesenchymal stem cells in an animal model of interstitial cystitis/bladder pain syndrome. Biomaterials. 2022;280:121277. 10.1016/j.biomaterials.2021.121277 [DOI] [PubMed] [Google Scholar]
- 17. Lee SW, Ryu CM, Shin JH, et al. The therapeutic effect of human embryonic stem cell-derived multipotent mesenchymal stem cells on chemical-induced cystitis in rats. Int Neurourol J. 2018;22:S34-S45. 10.5213/inj.1836014.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shin JH, Ryu C-M, Ju H, et al. Therapeutic efficacy of human embryonic stem cell-derived multipotent stem/stromal cells in diabetic detrusor underactivity: a preclinical study. J Clin Med. 2020;9:2853. 10.3390/jcm9092853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu HY, Shin JH, Yun H, et al. A preclinical study of human embryonic stem cell-derived mesenchymal stem cells for treating detrusor underactivity by chronic bladder ischemia. Stem Cell Rev Rep. 2021;17(6):2139-2152. 10.1007/s12015-021-10204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong KS, Bae D, Choi Y, et al. A porous membrane-mediated isolation of mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part C Methods. 2015;21:322-329. 10.1089/ten.TEC.2014.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Food and Drug Administration. Guidance for Industry on Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Availability. 70 FR 42346. 2005. https://purl.fdlp.gov/GPO/LPS119812. Accessed January 15, 2019.
- 22. Ryu CM, Shin JH, Yu HY, et al. N-acetylcysteine prevents bladder tissue fibrosis in a lipopolysaccharide-induced cystitis rat model. Sci Rep. 2019;9:8134. 10.1038/s41598-019-44631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tyagi P, Hsieh VC, Yoshimura N, et al. Instillation of liposomes vs dimethyl sulphoxide or pentosan polysulphate for reducing bladder hyperactivity. BJU Int. 2009;104:1689-1692. 10.1111/j.1464-410X.2009.08673.x [DOI] [PubMed] [Google Scholar]
- 24. Han JY, Shin JH, Choo MS.. Patterns and predictors of Hunner lesion recurrence in patients with interstitial cystitis. Neurourol Urodyn. 2019;38:1392-1398. 10.1002/nau.23998 [DOI] [PubMed] [Google Scholar]
- 25. Akiyama Y, Niimi A, Nomiya A, et al. Extent of Hunner lesions: the relationships with symptom severity and clinical parameters in Hunner type interstitial cystitis patients. Neurourol Urodyn. 2018;37:1441-1447. 10.1002/nau.23467 [DOI] [PubMed] [Google Scholar]
- 26. Kim A, Han JY, Ryu CM, et al. Histopathological characteristics of interstitial cystitis/bladder pain syndrome without Hunner lesion. Histopathology. 2017;71:415-424. 10.1111/his.13235 [DOI] [PubMed] [Google Scholar]
- 27. Chen Z, Liu L, Chen Y, et al. Periostin attenuates cyclophosphamideinduced bladder injury by promoting urothelial stem cell proliferation and macrophage polarization. Stem Cells Transl Med. 2022;11(6):659-673. 10.1093/stcltm/szac025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiaie N, Ghanavati SPM, Miremadi SS, et al. Mesenchymal stem cell-derived exosomes for COVID-19 therapy, preclinical and clinical evidence. Int J Stem Cells. 2021;14:252-261. 10.15283/ijsc20182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilleran J, Diokno AC, Ward E, et al. Improved global response outcome after intradetrusor injection of adult muscle-derived cells for the treatment of underactive bladder. Int Urol Nephrol. 2021;53(7):1331-1338. 10.1007/s11255-021-02847-1 [DOI] [PubMed] [Google Scholar]
- 30. Moll G, Rasmusson-Duprez I, von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565-1574. 10.1002/stem.1111 [DOI] [PubMed] [Google Scholar]
- 31. Li X, Kim CY, Shin JM, et al. Mesenchymal stem cell-driven activatable photosensitizers for precision photodynamic oncotherapy. Biomaterials. 2018;187:18-26. 10.1016/j.biomaterials.2018.09.041 [DOI] [PubMed] [Google Scholar]
- 32. Li SY, Liu Y, Wang L, et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up. Cell Prolif. 2021;54:e13100. 10.1111/cpr.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sung Y, Lee MJ, Choi J, et al. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients. Br J Ophthalmol. 2021;105:829-837. 10.1136/bjophthalmol-2020-316225 [DOI] [PubMed] [Google Scholar]
- 34. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516. 10.1016/S0140-6736(14)61376-3 [DOI] [PubMed] [Google Scholar]
- 35. Mehat MS, Sundaram V, Ripamonti C, et al. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology. 2018;125:1765-1775. 10.1016/j.ophtha.2018.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Menasché P, Vanneaux V, Hagège A, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71:429-438. 10.1016/j.jacc.2017.11.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.