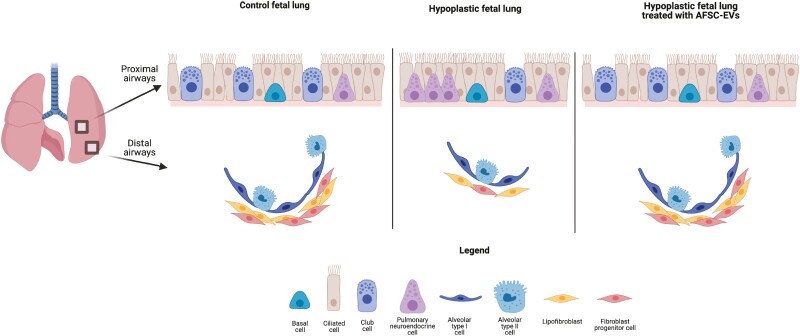
Abstract
Pulmonary hypoplasia secondary to congenital diaphragmatic hernia (CDH) is characterized by impaired branching morphogenesis and differentiation. We have previously demonstrated that administration of extracellular vesicles derived from rat amniotic fluid stem cells (AFSC-EVs) rescues development of hypoplastic lungs at the pseudoglandular and alveolar stages in rodent models of CDH. Herein, we tested whether AFSC-EVs exert their regenerative effects at the canalicular and saccular stages, as these are translationally relevant for clinical intervention. To induce fetal pulmonary hypoplasia, we gavaged rat dams with nitrofen at embryonic day 9.5 and demonstrated that nitrofen-exposed lungs had impaired branching morphogenesis, dysregulated signaling pathways relevant to lung development (FGF10/FGFR2, ROBO/SLIT, Ephrin, Neuropilin 1, β-catenin) and impaired epithelial and mesenchymal cell marker expression at both stages. AFSC-EVs administered to nitrofen-exposed lung explants rescued airspace density and increased the expression levels of key factors responsible for branching morphogenesis. Moreover, AFSC-EVs rescued the expression of alveolar type 1 and 2 cell markers at both canalicular and saccular stages and restored markers of club, ciliated epithelial, and pulmonary neuroendocrine cells at the saccular stage. AFSC-EV-treated lungs also had restored markers of lipofibroblasts and PDGFRA+ cells to control levels at both stages. EV tracking showed uptake of AFSC-EV RNA cargo throughout the fetal lung and an mRNA-miRNA network analysis identified that several miRNAs responsible for regulating lung development processes were contained in the AFSC-EV cargo. These findings suggest that AFSC-EV-based therapies hold potential for restoring fetal lung growth and maturation in babies with pulmonary hypoplasia secondary to CDH.
Keywords: exosome, extracellular vesicles, microRNA, congenital diaphragmatic hernia, pulmonary hypoplasia, lung development, fetal medicine, regenerative medicine
Graphical Abstract
Babies with congenital diaphragmatic hernia have hypoplastic lungs characterized by impaired branching morphogenesis and undifferentiated epithelium and mesenchyme. The authors demonstrate that amniotic fluid stem cell extracellular vesicles (AFSC-EVs) administered to rat hypoplastic fetal lungs restore branching and exert regenerative effects on epithelial and mesenchymal cells, partly through miRNA cargo transfer. AFSC-EV beneficial effects were obtained at translationally relevant time points.
Significance Statement.
This study describes a novel therapeutic approach to pulmonary hypoplasia, a devastating condition characterized by fetal lung underdevelopment and burdened by high mortality and morbidity rates. This therapy is based on the use of stem cell derivatives, called extracellular vesicles (EVs). The study shows that administering stem cell-derived EVs to an experimental model of the disease promotes fetal lung growth and maturation. The EVs enter the cells lining the diseased lungs and release microRNAs, genetic material that regulate biological processes such as lung development. This study shows that these beneficial effects are obtained at translationally relevant timepoints during gestation.
Introduction
Fetal lung underdevelopment, also known as pulmonary hypoplasia, is primarily characterized by decreased lung growth and maturation.1 The most common birth defect found in babies with pulmonary hypoplasia is congenital diaphragmatic hernia (CDH).1 CDH is characterized by incomplete closure of the fetal diaphragm and herniation of abdominal organs into the chest. At present, the mortality of CDH in high-income countries is 20%-30%,2,3 and more than 50% of survivors have long-term morbidity that may persist into adulthood.2,4 Pulmonary hypoplasia is a primary cause of these poor outcomes, and it persists even after surgical repair of the diaphragm and repositioning of the herniated organs back into the abdomen.2,5 The poor outcomes observed in infants and children with CDH have mobilized researchers to find an antenatal treatment that can promote normal lung development in fetuses with pulmonary hypoplasia secondary to CDH.6
We have recently reported that administration of extracellular vesicles derived from rat amniotic fluid stem cells (AFSC-EVs) rescues normal development in rodent fetal models of pulmonary hypoplasia.7 Specifically, we demonstrated that AFSC-EVs increase branching morphogenesis and promote epithelial cell differentiation in fetal rat lung explants harvested at the pseudoglandular stage.7 When we tested another source of EVs from bone marrow-derived mesenchymal stromal cells (MSC-EVs), we did not observe a similar rescue effect in lung branching morphogenesis and cell differentiation to that exerted by AFSC-EV treatment.7 Moreover, AFSC-EVs administered intratracheally in an in vivo rabbit fetal model of CDH increased the number of alveoli and promoted lipofibroblast differentiation at the alveolar stage of lung development.7 The effects of AFSC-EVs on rat fetal lung growth and maturation were mainly mediated by their RNA cargo, as enzymatically digesting AFSC-EV RNA ablated their beneficial effects in multiple models.7 Of the small RNA species contained in AFSC-EV cargo, miRNAs were the species most likely to mediate the regenerative effects observed on lung development.7
When we previously investigated the biological effects of AFSC-EVs on fetal hypoplastic lungs, we reported that fibroblast growth factor 10 (FGF10) expression was rescued to normal levels.7 In that study, we focused on the FGF10 pathway, as it plays a critical role in lung branching morphogenesis, epithelial proliferation, and lineage commitment.8 However, fetal lung development is a highly dynamic process that requires multiple pathways to coordinate branching morphogenesis and lung cell differentiation.9 Building on recent studies on the transcriptomic analysis and mapping of the rodent fetal lung,10,11 herein we investigated multiple signaling pathways that are known or unknown to be dysregulated in experimental CDH. The findings reported in the present study expand on our knowledge of pulmonary hypoplasia and further elaborate on the impact of AFSC-EVs on regulatory pathways that are key for lung development. Moreover, we further delineated the effects of AFSC-EV therapy on hypoplastic lungs at the canalicular and saccular stages. As CDH is typically diagnosed at the anatomy scan around 20 weeks of gestation, these stages are translationally relevant and provide an opportunity for designing clinical interventional studies. Lastly, the present study further characterized the AFSC-EV effects on epithelial and mesenchymal cell differentiation, specifically interrogating some of the most relevant cell types during the canalicular and saccular stages of lung development.
Materials and Methods
Extracellular Vesicle Isolation and Characterization
Extracellular vesicles were derived from c-kit+ rat AFSCs from a pregnant rat at embryonic day E12 using our established protocol.12 Briefly, AFSCs were grown to 70% confluence and the supernatant was collected and subjected to differential centrifugation (300g, 1200g, and 100 000g), as described.12 Extracellular vesicles were also isolated from bone marrow-derived mesenchymal stromal cells (Cell Biologics, catalog no RA-6043) as previously described.7 Both cultures were maintained below 6 passages and routinely monitored for appropriate morphology. Following the International Society for Extracellular Vesicles guidelines,13 EVs were characterized for size (nanoparticle tracking analysis), morphology (transmission electron microscopy), and expression of canonical EV-related protein markers (Western blot analysis), as described.7,12
Fluorescence Labeling of AFSC-EVs and In Vitro Tracking
AFSC-EVs were fluorescently labeled using ExoGlow-EV labeling kit (SBI System Biosciences), using the manufacturer’s protocol. The fluorescently labeled AFSC-EVs were added to E17.5 and E20.5 fetal rat explant cultures and co-incubated with 4ʹ,6-diamidino-2-phenylindole (DAPI) for 3 hours prior to imaging, using a 2-photon Leica SP8 confocal microscope (Leica).
Experimental Models of Pulmonary Hypoplasia
Ex Vivo
In fetal rats, CDH and pulmonary hypoplasia were induced via the administration of nitrofen to rat dams (100 mg) by oral gavage on embryonic day (E) 9.5. Dams were euthanized and fetal lungs were micro-dissected at the canalicular (E17.5) and saccular (E20.5) stages of lung development. The time points selected were chosen according to the developmental stages described previously.14,15 Lungs were grown as explants on nanopore membranes (Whatman, Thermo Fisher Scientific), and incubated for 72 h in culture medium alone, MSC-EVs (0.5% v/v), or rat AFSC-EVs (0.5% v/v). Lungs from fetuses of dams that received olive oil (no nitrofen) at E9.5 served as control. All explant specimens were snap frozen in liquid nitrogen and stored at −80°C for long-term storage or fixed and processed for histology using our established protocol.7
In Vitro
Primary epithelial cells and fibroblasts were derived from fetal rat lungs dissected at E17.5 and E18.5, respectively, using an established protocol.16 Briefly, single-cell suspensions were obtained from pooled lungs of control and nitrofen-exposed rat fetuses by trypsinization (0.25% trypsin; Gibco). Epithelial and fibroblast cells were isolated as described,7,16,17 grown for 7 days and then treated with culture medium alone, rat AFSC-EVs (0.5% v/v), or rat MSC-EVs for 24 h. Upon harvest, epithelial and fibroblast gene expression analysis was conducted.
Branching Morphogenesis Evaluation
To assess branching, rat fetal lung explants were processed for histology (hematoxylin/eosin staining) as described.7 Explants were evaluated for airspace density using the radial airspace count (RAC) and parenchymal to airspace ratio using the mean linear intercept (MLI), as recommended by the American Thoracic Society.18
mRNA Quantification with qRT-PCR
RNA extraction was conducted using Trizol and cDNA synthesis was performed using the VILO superscript RT III kit (Life Technologies), as described.7 Gene expression was conducted using the 2ΔΔCT method using ViiA qPCR system (ABI) following MIQE guidelines.19 Primers are listed in Table 1.
Table 1.
mRNAs assessed by real-time PCR.
| Gene name | Primer | Product size (bp) | GenBank accession number |
|---|---|---|---|
| Fgf10 | for: 5ʹ-AGCTGTTCTCCTTCACCAAGTA-3ʹ | 118 | NM_012951.1 |
| rev: 5ʹ-ACTCCGATTTCCACTGATGTTA-3ʹ | |||
| Fgfr2 | for: 5ʹ-TGCCTGGTGGAGAATGAATAC-3ʹ | 168 | NM_012712.1 |
| rev: 5ʹ-GGGCTGGGCATCACTATAA-3ʹ | |||
| Robo1 | for: 5ʹ-GCGAATCGGAGTGGTTAGTT-3ʹ | 101 | NM_022188.2 |
| rev: 5ʹ-GCGGGCCTTAATCTCGTAGT-3ʹ | |||
| Robo2 | for: 5ʹ-TGCAACCATTCGCTCTGTAGT-3ʹ | 223 | NM_032106.3 |
| rev: 5ʹ-ACCACCGATGCCAGCTATAA-3ʹ | |||
| Slit2 | for: 5ʹ-GAAGCAAGAGGCGGAAATACT-3ʹ | 163 | NM_022632.2 |
| rev: 5ʹ-TCCAACATGGTCAAGAAGTGTA-3ʹ | |||
| Ephb2 | for: 5ʹ-AACGGTGTGATCCTGGACTAT-3ʹ | 229 | DQ011670.1 |
| rev: 5ʹ-CCTTGATGCTGGTCTGGTACT-3ʹ | |||
| Efnb2 | for: 5ʹ-GTGGTGCTGCTGCTCAAGTA-3ʹ | 192 | NM_001107328.2 |
| rev: 5ʹ-CCCGCTGACCTTCTCGTAGT-3ʹ | |||
| Nrp1 | for: 5ʹ-TGGTGAGCCCTGTGGTCTA-3ʹ | 123 | NM_145098.2 |
| rev: 5ʹ-CATATTCCTCTGGCTTCTGGTAGT-3ʹ | |||
| Ctnnb1 | for: 5ʹ-CGCCAGTGGATTCCGTACT-3ʹ | 197 | NM_053357.2 |
| rev: 5ʹ-GCTTTCCTGATTGCCGTAAG-3ʹ | |||
| Wnt3a | for: 5ʹ-GTGCAAATGCCACGGACTAT-3ʹ | 198 | NM_001107005.2 |
| rev: 5ʹ-TCTGTGGGCACCTTGAAGTAT-3ʹ | |||
| Wnt5a | for: 5ʹ-AGCAGGTCGCAGGACAGTAT-3ʹ | 247 | NM_022631.3 |
| rev: 5ʹ-CGGACTTGGGTCGATGTAGA-3ʹ | |||
| Sema3a | for: 5ʹ-CGCACAAGACGACAGGATATAA-3ʹ | 142 | NM_017310.1 |
| rev: 5ʹ-TGCATTCCAAGAATGTGCTACT-3ʹ | |||
| Plxna | for: 5ʹ-TGAACACGCTGGCTCACTAT-3ʹ | 133 | XM_002729398.6 |
| rev: 5ʹ-CGCAGCATGCTCTCGTATC-3ʹ | |||
| Pdpn | for: 5ʹ-CCTCCACTTGCCAGCAGTA-3ʹ | 117 | NM_019358.1 |
| rev: 5ʹ-GCATGTGGTCCTCAATCATAAC-3ʹ | |||
| Sftpc | for: 5ʹ-AACGCCTTCTCATCGTGGTT-3ʹ | 247 | NM_017342.2 |
| rev: 5ʹ-GGCTTATAGGCGGTCAGGAG-3ʹ | |||
| Ager | for: 5ʹ-CCAACTACCGAGTCCGAGTCTA-3ʹ | 240 | NM_053336.2 |
| rev: 5ʹ-CTGACCGAAGCGTGAAGAGT-3ʹ | |||
| Abca3 | for: 5ʹ-GCAGTGCAACACGCTGTAGA-3ʹ | 163 | XM_006246039.4 |
| rev: 5ʹ-GCAGCAGAGGAAGCTGGTACT-3ʹ | |||
| Scgb1a1/Cc10 | for: 5ʹ-ATACCCTCCCACAGGAGACC-3ʹ | 153 | NM_013051.1 |
| rev: 5ʹ-AGGAGGGAGAGGGGAATGAC-3ʹ | |||
| Tp63 | for: 5ʹ-CATCAAAGCTCCATTGCTGTA-3ʹ | 107 | NM_001127343.1 |
| rev: 5ʹ-CTTTGCCCTGGTTTTGTAGAC-3ʹ | |||
| Foxj1 | for: 5ʹ-GTGAGCTGGAACCCCTCAAA-3ʹ | 238 | NM_053832.2 |
| rev: 5ʹ-GTGGCCAGGAAGGTCTCATC-3ʹ | |||
| Ascl1 | for: 5ʹ-GGCTCCAACAGCGTCTCTAC-3ʹ | 169 | NM_022384.2 |
| rev: 5ʹ-AGCGGTTGTCTGAGAATGAATA-3ʹ | |||
| Pdgfra | for: 5ʹ-TTCCTCCGGGCTATCGGATT-3ʹ | 270 | NM_012802.1 |
| rev: 5ʹ-ATTTCCACGTTGGGGTCCTC-3ʹ | |||
| Pparg | for: 5ʹ-GGTGAAACTCTGGGAGATCCT-3ʹ | 145 | NM_013124.3 |
| rev: 5ʹ-GGTCCACAGAGCTGATTCCG-3ʹ | |||
| Thy1 | for: 5ʹ-CAAGAGGAGGCTGCAAGCTA-3ʹ | 217 | NM_012673.2 |
| rev: 5ʹ-CAGGCTGAACTCATGCTGGA-3ʹ | |||
| Eln | for: 5ʹ-AGCAAGACCTGGGTTTGGAC-3ʹ | 162 | NM_012722.1 |
| rev: 5ʹ-CACTTTCTCTTCCGGCCACA-3ʹ | |||
| Acta2 | for: 5ʹ-GCTGCTCCAGCTATGTGTGA-3ʹ | 253 | NM_031004.2 |
| rev: 5ʹ-AGTTGGTGATGATGCCGTGT-3ʹ | |||
| Actb | for: 5ʹ-GTCCACCCGCGAGTACAAC-3ʹ | 260 | NM_031144.3 |
| rev: 5ʹ-GGATGCCTCTCTTGCTCTGG-3ʹ |
Protein Extraction and Western Blotting
Protein extraction and Western blotting were performed, as previously described.7 Briefly, cells or tissues were subjected to tissue extraction buffer (Thermo Fisher Scientific) containing protease and phosphatase inhibitor cocktail (Roche) and disrupted by sonication. The protein content was quantified using a bicinchoninic acid assay (Thermo Fisher Scientific), normalized to 10 µg, and loaded on 4%-12% NuPage MES mini-blot pre-cast gels (Thermo Fisher Scientific). Transfer was performed using the iBlot2 system (Thermo Fisher Scientific) and imaged using an Odyssey FC digital acquisition system (LI-COR Biosciences). Antibodies and concentrations are specified in Table 2.
Table 2.
Antibodies used for imaging and Western blotting.
| Antibody | Antibody host | Manufacturer/catalog no. | Application | Dilution factor |
|---|---|---|---|---|
| PDPN | Rabbit | Thermo Fisher/P5374 | WB, IF | 1:200 (IF), 1:1000 (WB) |
| EPCAM | Rabbit | Abcam/ab213500 | IF | 1:100 |
| SPC | Rabbit | Abcam/ab90716 | WB, IF | 1:400 (IF), 1:1000 (WB) |
| ECAD | Mouse | Abcam/ab231303 | IF | 1:100 |
| CC10 | Mouse | Santa Cruz/Sc-3659921 | WB, IF | 1:100 (IF), 1:1000 (WB) |
| FOXJ1 | Mouse | Santa Cruz/Sc-53139 | WB, IF | 1:50 (IF), 1:500 (WB) |
| P63 | Rabbit | Abcam/ab124762 | WB, IF | 1:50 (IF), 1:500 (WB) |
| ASCL1 | Rabbit | Invitrogen/14579482 | WB,IF | 1:250 (IF), 1:1000 (WB) |
| PPARG | Rabbit | Thermo Fisher/PA3 821A | WB, IF | 1:500 (IF), 1:1000 (WB) |
| PDGFRA | Goat | R&D Systems/AF1062 | WB, IF | 1:500 (IF), 1:1000 (WB) |
| IgG Alexa Fluor 488 | Rabbit | CST/4412S | IF | 1:1000 |
| IgG Alexa Fluor 555 | Mouse | CST/4409 | IF | 1:1000 |
| IgG Alexa Fluor 488 | Goat | Invitrogen/A11055 | IF | 1:1000 |
| IgG HRP | Mouse | CST/7076S | WB | 1:1000 |
| IgG HRP | Rabbit | CST/7074 | WB | 1:1000 |
| IgG HRP | Goat | Invitrogen/31402 | WB | 1:1000 |
Abbreviations: CST, cell signaling technology; IF, immunofluorescence; WB, Western blot.
Immunofluorescence Using Confocal Microscopy
Immunofluorescence was conducted using our established protocol.7 Paraffin-embedded sections were stained using the following antibodies: podoplanin (PDPN) for alveolar type 1 (AT1) cells, surfactant protein C (SPC) for alveolar type 2 (AT2) cells, both co-stained with epithelial adherens junction cadherin (ECAD); club-cell-specific 10 kD protein (CC10) for club cells; tumor protein 63 (P63) for basal cells; forkhead box protein J1 (FOXJ1) for ciliated epithelial cells; achaete-scute homolog 1 (ASCL1) for pulmonary neuroendocrine cells; platelet-derived growth factor receptor A (PDGFRA) and peroxisome proliferator-activated receptor gamma (PPARG) for mesenchymal cells. Antibodies and concentrations are provided in Table 2.
Results
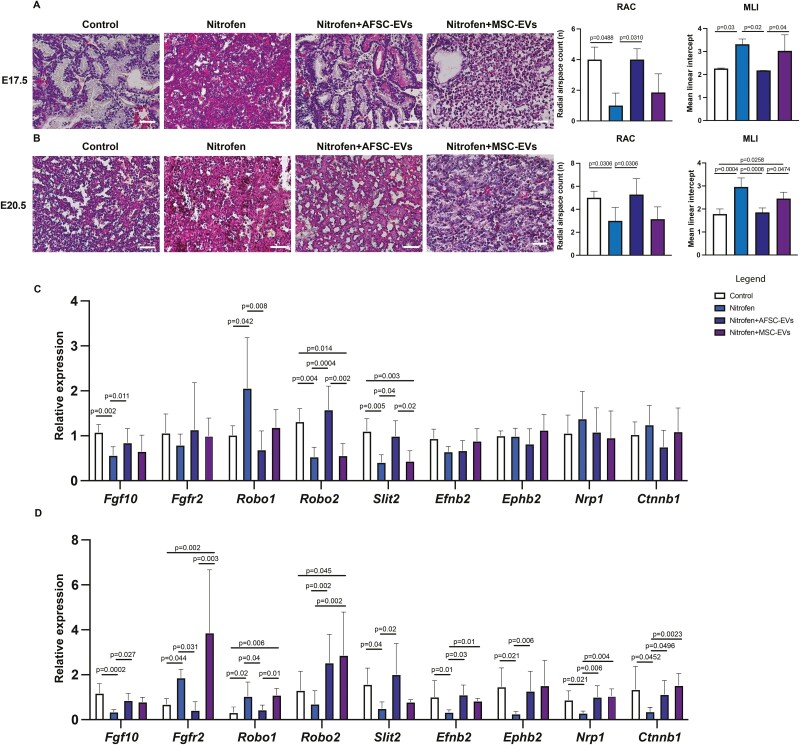
Administration of AFSC-EVs Rescues Branching Morphogenesis in Fetal Hypoplastic Lungs during the Canalicular and Saccular Stages of Lung Development
We first confirmed that nitrofen-exposed lungs harvested at both canalicular and saccular stages of lung development had impaired branching morphogenesis, as evidenced by fewer airspaces measured with RAC and a lower airspace to parenchyma ratio measured by MLI (Fig. 1A, 1B). In particular, nitrofen-exposed lungs had thickened septae with disorganized tissue surrounding the airspaces. Administration of AFSC-EVs to nitrofen-exposed lung explants rescued branching morphogenesis at both developmental stages, with increased density of airspaces and thin septae (Fig. 1A, 1B). When we investigated signaling pathways that are known to be responsible for branching morphogenesis,8 we found that nitrofen-exposed lungs harvested at either E17.5 or E20.5 had downregulated expression of Fgf10, roundabout guidance receptor 2 (Robo2), and slit guidance ligand 2 (Slit2) factors, as well as upregulated expression of Roundabout guidance receptor 1 (Robo1) (Fig. 1C, 1D). Moreover, fetal lungs harvested at E20.5 had higher levels of fibroblast growth factor receptor 2 (Fgfr2) and lower levels of Ephrin B2 (Efnb2), Ephrin type-B receptor 2 (Ephb2), Neuropilin 1 (Nrp1), and β-catenin 1 (Cttnb1) compared to control (Fig. 1D). Interestingly, nitrofen-exposed lungs did not have any dysregulation of other factors that are also involved in branching morphogenesis,8 such as Wnt family member 3a and 5a (Wnt3a and Wnt5a), Semaphorin 3a (Sema3a), and Plexin A (Plxna) at any of the time points (Supplementary Fig. 1). Following AFSC-EV administration, nitrofen-exposed lungs harvested at E17.5 or E20.5 had upregulated levels of Fgf10, Robo2, and Slit2 and downregulated levels of Fgfr2 and Robo1. Moreover, AFSC-EV treated hypoplastic lungs harvested at E20.5 had upregulated levels of Efnb2, Ephb2, Nrp1, and Ctnnb1 (Fig. 1C, 1D).
Figure 1.
Administration of AFSC-EVs rescues branching morphogenesis in fetal hypoplastic lungs during the canalicular and saccular stages of development. Representative images of hematoxylin and eosin stains and quantification of airspace density using the radial airspace count (RAC) and parenchymal to airspace ratio using the mean linear intercept (MLI), in A, canalicular and B, saccular stages of lung development. Scale bar = 50 µm. Gene expression levels of factors involved in branching morphogenesis in control and nitrofen-exposed untreated, AFSC-EV and MSC-EV treated lung explants at C, canalicular and D, saccular stages of lung development. Data were analyzed using Kruskal-Wallis test for E17.5 MLI and RAC and E20.5 RAC (A, B), as well as gene expression analysis (C; Robo2, D: Slit2, Efnb2, Ctnnb1, and D; Fgfr2) and one-way ANOVA using Tukey’s multiple comparison test for all other analyses (A-D). Data are presented as mean ± SD (n = at least 4 biological replicates per group for RAC, MLI and for gene expression analysis). Only significant differences (P < .05) are reported in the graphs.
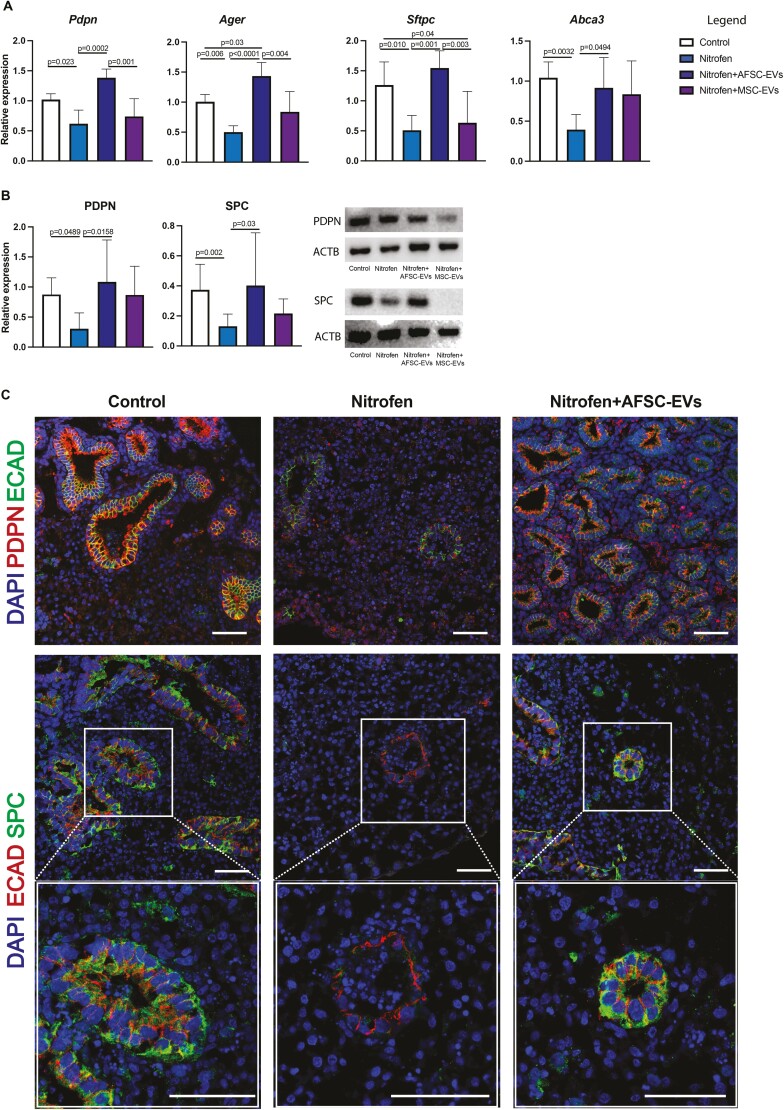
AFSC-EV Administration Promotes Differentiation of Epithelial Lung Cells at Canalicular and Saccular Stages of Lung Development
Nitrofen-exposed lungs have been reported to be immature, in part due to the altered differentiation of the proximal and distal airway epithelium. At the canalicular stage, we observed that nitrofen exposure decreased the expression of markers for AT1 (PDPN) and AT2 (SPC) cells but had no effect on markers of club cells (CC10), basal cells (P63), ciliated epithelial cells (FOXJ1), and pulmonary neuroendocrine cells (ASCL1) (Fig. 2A-2C; Supplementary Fig. 2). Administration of AFSC-EVs to nitrofen-exposed lungs harvested at the canalicular stage rescued the expression levels of AT1 and AT2 cell markers back to control. We confirmed these effects also on primary epithelial cells derived from nitrofen-exposed lungs during the canalicular stage (Supplementary Fig. 3).
Figure 2.
AFSC-EV administration promotes differentiation of alveolar lung epithelial cells at the canalicular stage of fetal lung development. Markers of AT1 (PDPN) and AT2 (SPC) were analyzed at A, gene (RT-qPCR), and B, protein (Western blot) expression levels. ACTB was used as a loading control. C, Representative immunofluorescence images of PDPN (red) and SPC (green) co-localization with the epithelial marker E-cadherin (ECAD). Bottom panels represent higher magnification images of this co-localization (C). DAPI was used as a nuclear stain. Scale bar = 50 µm. Data were analyzed using Kruskal-Wallis test for protein analysis (B) and using one-way ANOVA using Tukey’s multiple comparison test for all other analyses (A). Data are presented as mean ± SD (n = at least 4 biological replicates per group for gene and n = at least 7 for protein expression). Only significant differences (P < .05) are reported in the graphs.
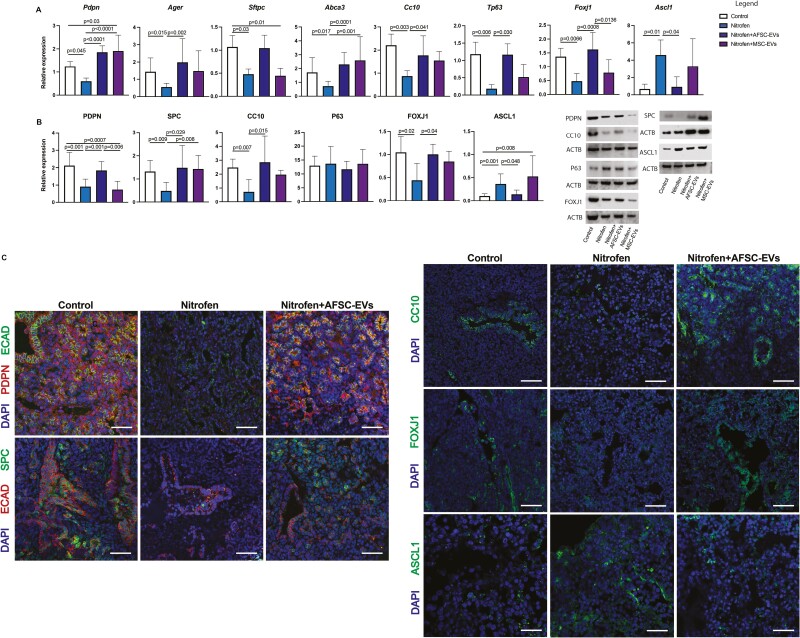
At the saccular stage, we observed a dysregulation of expression markers of proximal and distal epithelial cells (Fig. 3A-3C). Nitrofen-exposed lungs harvested at this stage and treated with AFSC-EVs had increased expression of AT1, AT2, club, and ciliated epithelial cells, and decreased expression of pulmonary neuroendocrine cells back to control, with only partial marker restoration with MSC-EV administration (Fig. 3A-3C; Supplementary Fig. 4).
Figure 3.
AFSC-EV administration promotes differentiation of alveolar and bronchial lung epithelial cells at the saccular stage of fetal lung development. Markers of AT1 (PDPN), AT2 (SPC), club (CC10), basal (P63), ciliated epithelial (FOXJ1) and pulmonary neuroendocrine (ASCL1) were analyzed at A, gene (RT-qPCR), and B, protein (Western blot) expression level. ACTB was used as a loading control. C, Representative immunofluorescence images of PDPN (red) and SPC (green) co-localization with the epithelial marker ECAD and ASCL1, CC10, FOXJ1 (green) immunolocalization in all fetal lung groups. DAPI was used as a nuclear stain. Scale bar = 50 µm. Data were analyzed using Kruskal-Wallis test for Ager, Sftpc, Tp63, Ascl1 (A), SPC, CC10, FOXJ1 protein (B), and using one-way ANOVA with Tukey’s multiple comparison test for all other analyses. Data are presented as mean ± SD (n = at least 4 biological replicates per group for gene and protein expression). Only significant differences (P < .05) are reported in the graphs.
AFSC-EV Administration Has Regenerative Effects on the Fetal Lung Mesenchyme
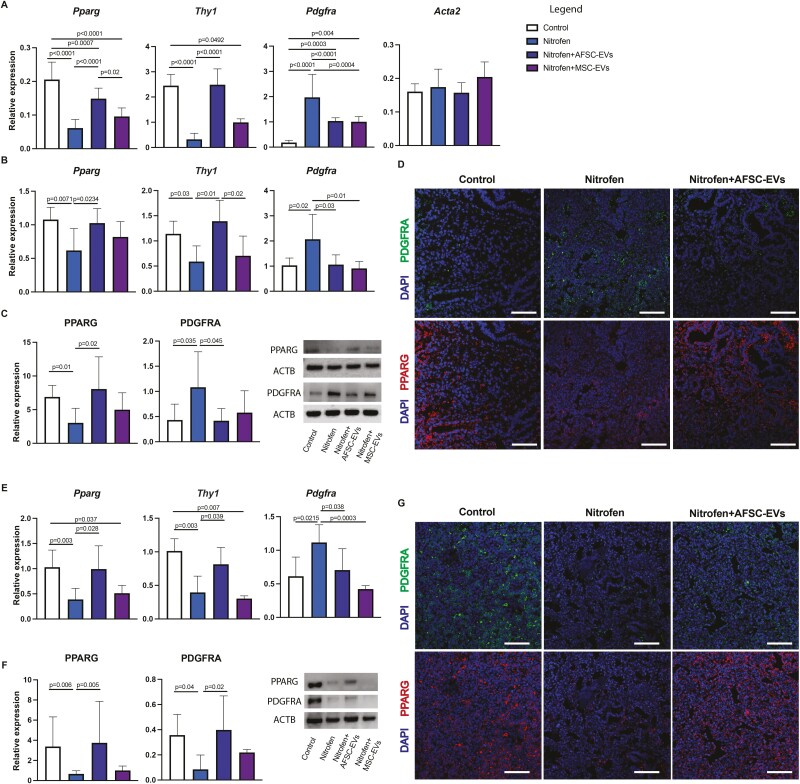
Several studies have reported that CDH hypoplastic lungs have undifferentiated mesenchyme,20,21 with altered expression of lipofibroblasts22-25 and PDGFRA+ cells.26,27 To study mesenchymal differentiation and lung fibroblast response to AFSC-EVs, we first derived primary fibroblasts from control and nitrofen-exposed lungs harvested at the canalicular stage. In primary cell culture, we observed a decreased expression of PPARG and Thy1, which are markers of lipofibroblasts.28 Moreover, we found an increase in PDGFRA expression, which is a marker of progenitor cells that mainly give rise to myofibroblasts,29 but no difference in expression of Actin alpha 2 (Acta2), a well-established marker of myofibroblasts.20,21 When we investigated the mesenchyme of fetal lung explants, we confirmed the decrease in lipofibroblast markers at both gene and protein expression levels in nitrofen-exposed fetal lungs harvested at canalicular (Fig. 4B-4D) and saccular stages (Fig. 4E-4G). The increase in PDGFRA+ cell expression noticed in primary fibroblasts derived from nitrofen-exposed lungs was also confirmed in the explant model at the canalicular stage (Fig. 4B-4D). Conversely, the protein expression of PDGFRA in fetal nitrofen-exposed lungs harvested at the saccular stage was decreased compared to control (Fig. 4F, 4G). Administration of AFSC-EVs to nitrofen-exposed primary fibroblasts or lung explants increased the expression of lipofibroblast markers (Fig. 4A-4G). Similarly, nitrofen-exposed primary fibroblasts and fetal lungs treated with AFSC-EVs had a decrease in PDGFRA expression levels compared to nitrofen-injured untreated fibroblasts or lung explants (Fig. 4B-4G).
Figure 4.
AFSC-EV administration has regenerative effects on the fetal lung mesenchyme. A, Mesenchymal markers for fibroblast (Pdgfra), lipofibroblast (Pparg, Thy1) and myofibroblast (Eln, Acta2) cells were analyzed in primary fibroblasts isolated from fetal lungs. Mesenchymal markers analyzed at B, (RT-qPCR), and B, protein (Western blot) expression levels at the canalicular stage. ACTB was used as a loading control. D, Representative immunofluorescence images of the increased PDGFRA+ fibroblasts and diminished lipofibroblast cells at the canalicular stage. DAPI was used as a nuclear stain. Scale bar = 50 µm. These same markers were also analyzed at E, gene and F, protein expression levels at the saccular stages. G, Representative immunofluorescence images of decreased PDGFRA+ fibroblast and lipofibroblast cells at the saccular stage. DAPI was used as a nuclear stain. Scale bar = 50 µm. Data were analyzed using Kruskal-Wallis test for Thy1 (A), Thy1 (B), PPARG (C), Pparg, Thy1, (E), PPARG, PDGFRA (F) and using one-way ANOVA using Tukey’s multiple comparison test for all other analyses. Data are presented as mean ± SD (n = at least 8 biological replicates per group for primary cell (A), n = at least 6 biological replicates for E17.5 gene and protein expression and n = at least 4 biological replicates for E20.5 gene and protein expression (E-F). Only significant differences (P < .05) are reported in the graphs.
AFSC-EV Mechanism of Action Is Partly Modulated by the Release of RNA Species
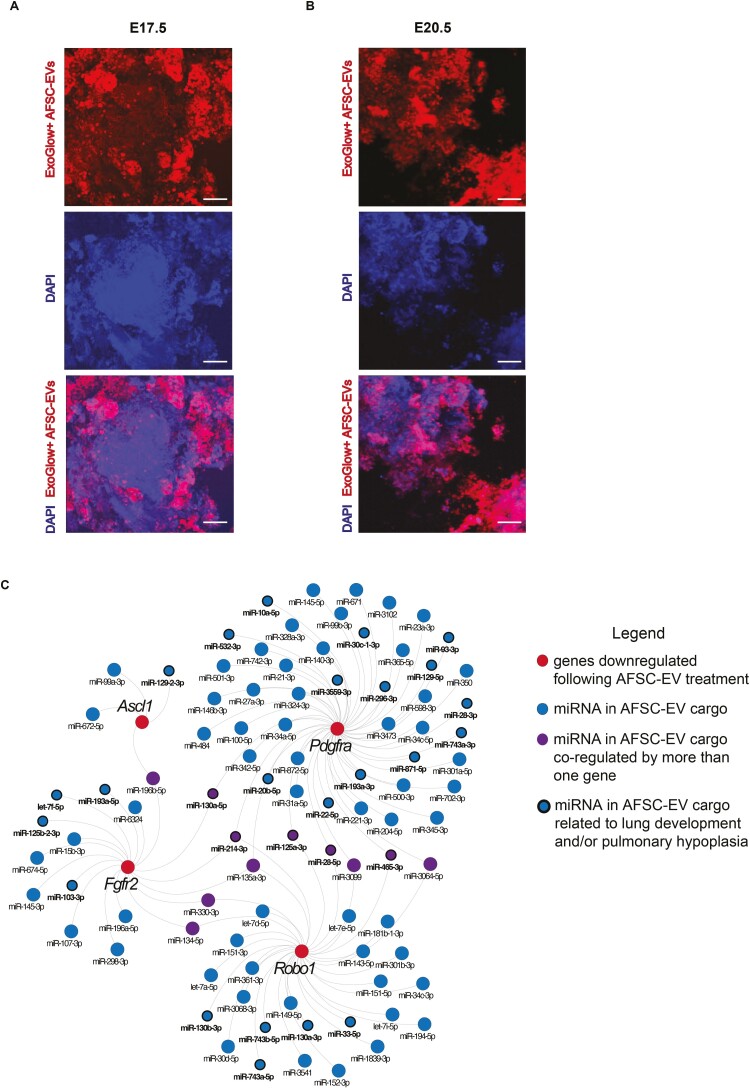
As we have previously demonstrated that AFSC-EV main mechanism of action toward fetal lung regeneration at E14.5 is through the release of the RNA cargo,7 in this study, we tracked the RNA cargo of AFSC-EVs that were administered to nitrofen-exposed lungs harvested at E17.5 and E20.5. At both stages of lung development, we observed that AFSC-EVs entered the lung tissue, as shown by the presence of their RNA cargo throughout the lung parenchyma (Fig. 5A, 5B; Supplementary online Video 1). In our previous small RNA sequencing studies,7 we identified some miRNAs that were in part responsible for the effects of AFSC-EVs on branching morphogenesis and fetal lung epithelial differentiation. In the present study, we linked the genes responsible for branching morphogenesis and cell differentiation that were downregulated following AFSC-EV treatment (Robo1, Fgfr2, Ascl1, and Pdgfra; Figs. 1, 3, 4) with miRNAs that were found to be in the AFSC-EV cargo by the small RNA sequencing analysis conducted in our previous study (Fig. 5C). Using miRWalk, we found 91 miRNAs that are predicted to downregulate the selected 4 genes, including 28 that are known to be involved in lung development (Fig. 5C). Of the 91 miRNAs, we identified 11 miRNAs that co-regulated more than one gene.
Figure 5.
AFSC-EVs modulate fetal lung development and maturation in part through their release of RNA species. Representative images of tracking studies using fluorescently labeled AFSC-EVs (red) in A, E17.5 and B, E20.5 fetal lung explants. DAPI was used as a nuclear stain. C, Network analysis of downregulated genes responsible for branching morphogenesis and cell differentiation following AFSC-EV treatment (Robo1, Fgfr2, Ascl1, and Pdgfra) with AFSC-EV cargo miRNA. Scale bar = 50 µm.
Discussion
In this study, we have shown that AFSC-EVs administered to rat fetal hypoplastic lungs harvested at canalicular and saccular stages of lung development exert a regenerative effect on the pulmonary epithelium and mesenchyme. These effects were through multiple pathways that are known to be involved in lung development8 and that we herein demonstrated to be targeted by AFSC-EVs. When we investigated the FGF pathway in the nitrofen model, we replicated the observation of other research groups that reported a consistent decrease in FGF10 expression at both canalicular and saccular stages,30-34 and an upregulation of FGFR2 expression at the saccular stage.35 As we had previously demonstrated in fetal lung explants harvested at the pseudoglandular stage,7 AFSC-EVs administered to explants harvested at both canalicular and saccular stages rescued the expression of both FGF10 and FGFR2 back to control levels. The ROBO/SLIT pathway is also essential for normal lung development, as shown in knockout studies,36,37 and is downregulated in nitrofen-exposed lungs of fetuses with CDH.38 In the present study, we confirmed the upregulation of ROBO1 and the corresponding downregulation of ROBO2 and SLIT2 expression in nitrofen-exposed lungs at both time points, which were rescued by AFSC-EV treatment to control levels. When we assessed factors involved in sending guidance cues for lung branching, we confirmed that the Ephrin pathway was downregulated at the saccular stage, as previously reported,39 and rescued by AFSC-EV treatment to control levels. Neuropilin is another guidance cue factor that has been reported to support the formation of alveolar ducts and alveoli in rodents40 and that, to the best of our knowledge, has not been investigated in nitrofen-exposed lungs. The present study shows for the first time that Neuropilin gene expression is downregulated in nitrofen-exposed lungs during the saccular stage and rescued by AFSC-EV administration. Similarly, β-catenin, which is important for the maintenance of lung epithelial progenitors,41 is herein reported for the first time to have downregulated expression in nitrofen-exposed lungs. Lastly, when we investigated other factors implicated in lung branching morphogenesis, such as Wnt family members WNT3A and WNT5A, as well as Semaphorin, and Plexin A,8 we found no differences between control and nitrofen-exposed lungs. These results support the notion that some but not all of the pathways involved in branching morphogenesis are dysregulated in human and experimental CDH.
In this study, we confirmed that fetal hypoplastic lungs have impaired epithelial cell differentiation in primary lung epithelial cells and lung explants, as we had reported at the pseudoglandular stage.7 In the distal airways, we found reduced expression of AT1 and AT2 markers at both canalicular and saccular stages of lung development, as previously reported by other groups.27,42-44 AT1 and AT2 downregulation was rescued by AFSC-EV administration to control levels, in line with our previous report in fetal lungs harvested at the pseudoglandular stage.7 When we investigated epithelial cell populations in the proximal airways, we observed no differences in the expression of club, basal, and ciliated epithelial cells at the canalicular stage, similar to reports from human and rodent models of pulmonary hypoplasia at the same time point.27,45 At the saccular stage, we observed downregulated expression of club and ciliated epithelial cell markers, as well as upregulated expression of pulmonary neuroendocrine cells in nitrofen-exposed lungs harvested. To the best of our knowledge, no study has investigated the expression of basal, club, and ciliated epithelial cells in nitrofen-exposed lungs at the saccular stage. Conversely, we confirmed the upregulation of pulmonary neuroendocrine cells at the saccular stage of lung development, as shown by several groups.46-48 The increased number of pulmonary neuroendocrine cells in CDH fetal lungs has previously been explained as an adaptive response to impaired lung development, whereby the amines and peptides secreted by these cells are implicated in lung development, repair, and regeneration.46,49 The dysregulated expression of epithelial cell populations lining the proximal airway was restored to normal levels by AFSC-EV administration at both canalicular and saccular stages.
The mesenchyme plays a critical role in lung development, as it provides support for the fetal lung epithelium.50-52 In this study, we confirmed that hypoplastic CDH lungs have decreased expression of lipofibroblasts, as previously shown in the nitrofen model21-23,25 and in the surgical rabbit model of CDH.7 Lipofibroblasts, a population of lipid-containing fibroblasts that develop during the canalicular stage, play a crucial role during fetal lung development, maintain tissue homeostasis, and respond to cellular injury.50,53 PDGFRA+ cells are a population of progenitors that mainly give rise to myofibroblasts.29 This cell population is key for lung development and its conditional inactivation during embryogenesis has been reported to result in alveolar simplification.29 In our study, we found increased levels of PDGFRA in vitro and ex vivo at the canalicular stage, as previously reported during the same developmental stage by Dingemann et al.26 The alteration in markers of lipofibroblasts and PDGFRA+ cells in nitrofen-exposed lungs compared to control could indicate that hypoplastic fetal lungs remain at an immature state, with decreased cell differentiation and increased levels of progenitor cells. This is in line with other studies reporting that the pulmonary mesenchyme is defective in the nitrofen model of pulmonary hypoplasia and may be due to the dysregulated epithelial-mesenchymal tissue interactions.20,44 The decrease in lipofibroblast markers observed in this study at the canalicular stage persisted also in the saccular stage of lung development. Conversely, we observed that PDGFRA protein expression was decreased in nitrofen-exposed lungs compared to control at the saccular stage. This could be due to simplification of the lung architecture, which is also observed in immature lungs affected by bronchopulmonary dysplasia, where PDGFRA expression has been reported to be downregulated.54-56 In both canalicular and saccular stages of lung development, AFSC-EV administration to nitrofen-exposed lungs restored markers of lipofibroblasts and PDGFRA+ cells back to control levels. These findings in the mesenchyme were similar to the improvements observed in lung epithelial differentiation. Given the close interaction between epithelium and mesenchyme compartments during lung development, it is difficult to delineate whether EV effects are directed toward one compartment and affect the other indirectly or are affecting both simultaneously.
The EV tracking experiments conducted in this and our previous study7 demonstrate that the AFSC-EV cargo uptake is not specific to an area or compartment of the lung. Studies investigating potential cell-type-specific transcriptomic changes are currently underway. Nonetheless, in the present study, we were able to find regulators of both lung epithelial and mesenchymal cells. In fact, our analysis of the AFSC-EV miRNA cargo and downregulated genes revealed 28 miRNAs that play a role in restored epithelial and mesenchymal cell differentiation, as well as branching morphogenesis. As an example, miR-20-5p, which belongs to the miR17~92 family, is of particular interest as it controls pulmonary surfactant gene expression in AT2 cells.57 Through antagomir studies, we demonstrated that miR-20-5p is responsible for AFSC-EV rescue effect of impaired autophagy, a key cellular mechanism that is dysregulated in CDH lungs.58 In addition to this cluster, miR-28-5p was also found in our network to regulate Robo1 and Pdgfra, and has been reported to be decreased in the nitrofen model of CDH.7,59 Interestingly, 11 miRNAs identified in our network regulate more than one of the target genes, indicating that target gene regulation can be coordinated by multiple combinations of miRNAs.60 Together, these findings support that AFSC-EV mechanism of action in restoring tissue homeostasis is partly ascribed to their miRNA cargo transfer, with predicted direct targets in Ascl1, Fgfr2, Pdgfra, and Robo1. Nonetheless, we recognize that AFSC-EV miRNAs may also cause indirect effects on the other target pathways and cells, as well as that other small RNA species contained in the AFSC-EV cargo may play a role in the observed lung regenerative effects.
This study provides further evidence that an AFSC-EV-based therapy holds potential for fetal lung regeneration in babies with pulmonary hypoplasia secondary to CDH. We have herein demonstrated that AFSC-EVs promote branching morphogenesis and rescue epithelial and mesenchymal cell homeostasis at both canalicular and saccular stages of lung development. Toward the translational advancement of this novel therapy, these time points are amenable for fetal intervention, especially in fetuses with CDH who are diagnosed prior to these stages. We specifically found that while the changes in differentiation of the distal airways occurred in both the canalicular and saccular stages, the proximal epithelium was rescued only in the saccular stage. However, it is difficult to translate these findings from a rodent model directly to human babies. Fetal interventions in human babies with CDH, such as fetal endoscopic tracheal occlusion, have been reported during both time points.61,62 Future studies in larger animal models will examine the optimal gestational age to administer the EV therapy and will address other aspects, such as route of delivery, dose, and frequency of administration. In fact, it is critical to carefully address these and other translational aspects before clinical application of EV-based therapies in human patients, as previously reported.63,64
We acknowledge that our study has some limitations. Our findings were obtained using the nitrofen model of CDH, which is based on the administration of an herbicide that targets retinoic acid synthesis.28,65 Nonetheless, although human pulmonary hypoplasia is not caused by maternal exposure to herbicides, nitrofen administration causes a degree of lung underdevelopment analogous to that of human fetuses with pulmonary hypoplasia.28,65 Moreover, retinoic acid is known to play a role in fetal lung development and CDH babies have low intracellular retinoic acid levels in the lungs and low retinol and retinol-binding protein levels in the cord blood.65,66 This model has been used for over three decades and the multiple pathways that are dysregulated in nitrofen-exposed hypoplastic lungs are similar to those reported in human studies.28 Nevertheless, findings from our study serve mainly as a proof-of-principle that AFSC-EVs rescue lung development in CDH hypoplastic lungs. Future studies will be designed to also address whether the rescue effects observed following AFSC-EV treatment are maintained in a model of lung compression that replicates what occurs clinically in fetuses with CDH. Moreover, we recognize that our study addressed the epithelial and mesenchymal compartments of the lung, for what is currently known. While there is a vast literature on epithelial differentiation in normal and hypoplastic lungs, relatively little is known about lung mesenchymal cells and their impairment in pulmonary hypoplasia. This is in line with a recent report by Tsukui et al, who highlighted that the diversity of lung fibroblasts has not been well characterized in normal or diseased lungs.52 Nonetheless, we are encouraged by the observation that AFSC-EVs are able to promote differentiation toward lipofibroblast in canalicular and saccular stages in rats, as well as during alveologenesis in fetal rabbits with CDH.7
Conclusion
In conclusion, this study confirms that administration of AFSC-EVs to hypoplastic fetal lungs rescues branching morphogenesis and promotes differentiation of epithelial and mesenchymal lung cells during the canalicular and saccular stages of lung development. Moreover, we provide further evidence that the RNA cargo could be responsible for the AFSC-EV mechanism of action toward lung regeneration. Taken together, these findings demonstrate that AFSC-EV treatment may represent an exciting new opportunity for antenatal intervention for CDH babies at clinically relevant time points.
Supplementary Material
Acknowledgments
The authors would like to thank Rachel Bercovitch for technical assistance and Paolo De Coppi for providing AFSCs in kind. We are indebted to the Imaging Facility and the Lab Animal Services at the Hospital for Sick Children, Toronto. The graphical abstract was designed using BioRender.com.
Contributor Information
Kasra Khalaj, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada.
Rebeca Lopes Figueira, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada.
Lina Antounians, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada.
Sree Gandhi, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada.
Matthew Wales, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada.
Louise Montalva, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada.
George Biouss, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada.
Augusto Zani, Developmental and Stem Cell Biology Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada; Division of General and Thoracic Surgery, The Hospital for Sick Children, Toronto, ON, Canada; Department of Surgery, University of Toronto, Toronto, ON, Canada.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR Project Grant 175300 to A.Z.), CIHR Fellowship (176535 to K.K.), American Thoracic Society (RP-2020-26 to R.L.F.), SickKids Restracomp Fellowship (to K.K.), SickKids Foundation (R00DH00000 to A.Z.), and University of Toronto Department of Surgery (to A.Z.).
Conflict of Interest
The authors declared no potential conflicts of interest.
Author Contributions
K.K.: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. R.L.F., L.A., L.M.: Collection and/or assembly of data, data analysis and interpretation, final approval of manuscript. S.G., M.W., G.B.: Collection and/or assembly of data, final approval of manuscript. A.Z.: Conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Cotten CM. Pulmonary hypoplasia. Semin Fetal Neonatal Med. 2017;22:250-255. 10.1016/j.siny.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 2. Zani A, Chung WK, Deprest J, et al. Congenital diaphragmatic hernia. Nat Rev Dis Primers. 2022;8:37. 10.1038/s41572-022-00362-w [DOI] [PubMed] [Google Scholar]
- 3. Gupta VS, Harting MT, Lally PA, et al. Mortality in congenital diaphragmatic hernia: a multicenter registry study of over 5000 patients over 25 years. Ann Surg. 2021. [DOI] [PubMed] [Google Scholar]
- 4. Spoel M, van der Cammen-van Zijp MHM, Hop WCJ, et al. Lung function in young adults with congenital diaphragmatic hernia; a longitudinal evaluation. Pediatr Pulmonol. 2013;48:130-137. 10.1002/ppul.22557 [DOI] [PubMed] [Google Scholar]
- 5. Hislop A, Reid L.. Persistent hypoplasia of the lung after repair of congenital diaphragmatic hernia. Thorax. 1976;31:450-455. 10.1136/thx.31.4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jeanty C, Kunisaki SM, MacKenzie TC.. Novel non-surgical prenatal approaches to treating congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19:349-356. 10.1016/j.siny.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 7. Antounians L, Catania VD, Montalva L, et al. Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci Transl Med. 2021;13:eaax5941. 10.1126/scitranslmed.aax5941 [DOI] [PubMed] [Google Scholar]
- 8. Horowitz A, Simons M.. Branching morphogenesis. Circ Res. 2008;103:784-795. 10.1161/CIRCRESAHA.108.181818 [DOI] [PubMed] [Google Scholar]
- 9. Herriges M, Morrisey EE.. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502-513. 10.1242/dev.098186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo M, Du Y, Gokey JJ, et al. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun. 2019;10:37. 10.1038/s41467-018-07770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ardini-Poleske ME, Clark RF, Ansong C, et al. LungMAP: the molecular atlas of lung development program. Am J Physiol Lung Cell Mol Physiol. 2017;313:L733-L740. 10.1152/ajplung.00139.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antounians L, Tzanetakis A, Pellerito O, et al. The regenerative potential of amniotic fluid stem cell extracellular vesicles: lessons learned by comparing different isolation techniques. Sci Rep. 2019;9:1837. 10.1038/s41598-018-38320-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antounians L, Figueira RL, Sbragia L, et al. Congenital diaphragmatic hernia: state of the art in translating experimental research to the bedside. Eur J Pediatr Surg. 2019;29:317-327. 10.1055/s-0039-1693993 [DOI] [PubMed] [Google Scholar]
- 15. Weng T, Zhongming C, Jin N, et al. Gene expression profiling identifies regulatory pathways involved in the late stage of rat fetal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1027-L1037. [DOI] [PubMed] [Google Scholar]
- 16. Caniggia I, Tseu I, Han RN, et al. Spatial and temporal differences in fibroblast behavior in fetal rat lung. Am J Physiol. 1991;261:L424-L433. 10.1152/ajplung.1991.261.6.L424 [DOI] [PubMed] [Google Scholar]
- 17. Tzanetakis A, Antounians L, Belfiore A, et al. Endoplasmic reticulum stress response is activated in pulmonary hypoplasia secondary to congenital diaphragmatic hernia, but is decreased by administration of amniotic fluid stem cells. Pediatr Surg Int. 2019;35:63-69. 10.1007/s00383-018-4376-4 [DOI] [PubMed] [Google Scholar]
- 18. Hsia CCW, Hyde DM, Ochs M, et al. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394-418. 10.1164/rccm.200809-1522ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611-622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 20. van Loenhout RB, Tseu I, Fox EK, et al. The pulmonary mesenchymal tissue layer is defective in an in vitro recombinant model of nitrofen-induced lung hypoplasia. Am J Pathol. 2012;180:48-60. 10.1016/j.ajpath.2011.09.032 [DOI] [PubMed] [Google Scholar]
- 21. Donahoe PK, Longoni M, High FA.. Polygenic causes of congenital diaphragmatic hernia produce common lung pathologies. Am J Pathol. 2016;186:2532-2543. 10.1016/j.ajpath.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedmacher F, Hofmann AD, Takahashi H, et al. Disruption of THY-1 signaling in alveolar lipofibroblasts in experimentally induced congenital diaphragmatic hernia. Pediatr Surg Int. 2014;30:129-135. 10.1007/s00383-013-3444-z [DOI] [PubMed] [Google Scholar]
- 23. Friedmacher F, Fujiwara N, Hofmann AD, et al. Evidence for decreased lipofibroblast expression in hypoplastic rat lungs with congenital diaphragmatic hernia. Pediatr Surg Int. 2014;30:1023-1029. 10.1007/s00383-014-3549-z [DOI] [PubMed] [Google Scholar]
- 24. Gosemann J-H, Doi T, Kutasy B, et al. Alterations of peroxisome proliferator-activated receptor γ and monocyte chemoattractant protein 1 gene expression in the nitrofen-induced hypoplastic lung. J Pediatr Surg. 2012;47:847-851. 10.1016/j.jpedsurg.2012.01.038 [DOI] [PubMed] [Google Scholar]
- 25. Friedmacher F, Fujiwara N, Hofmann AD, et al. Prenatal retinoic acid increases lipofibroblast expression in hypoplastic rat lungs with experimental congenital diaphragmatic hernia. J Pediatr Surg. 2014;49:876-81; discussion 881. 10.1016/j.jpedsurg.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 26. Dingemann J, Doi T, Ruttenstock E, et al. Abnormal platelet-derived growth factor signaling accounting for lung hypoplasia in experimental congenital diaphragmatic hernia. J Pediatr Surg. 2010;45:1989-1994. 10.1016/j.jpedsurg.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 27. Kunisaki SM, Jiang G, Biancotti JC, et al. Human induced pluripotent stem cell-derived lung organoids in an ex vivo model of the congenital diaphragmatic hernia fetal lung. Stem Cells Transl Med. 2021;10:98-114. 10.1002/sctm.20-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montalva L, Zani A.. Assessment of the nitrofen model of congenital diaphragmatic hernia and of the dysregulated factors involved in pulmonary hypoplasia. Pediatr Surg Int. 2019;35:41-61. 10.1007/s00383-018-4375-5 [DOI] [PubMed] [Google Scholar]
- 29. Li R, Bernau K, Sandbo N, et al. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. eLife. 2018;7:e36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teramoto H, Yoneda A, Puri P.. Gene expression of fibroblast growth factors 10 and 7 is downregulated in the lung of nitrofen-induced diaphragmatic hernia in rats. J Pediatr Surg. 2003;38:1021-1024. [DOI] [PubMed] [Google Scholar]
- 31. Acosta JM, Thébaud B, Castillo C, et al. Novel mechanisms in murine nitrofen-induced pulmonary hypoplasia: FGF-10 rescue in culture. Am J Physiol Lung Cell Mol Physiol. 2001;281:L250-L257. 10.1152/ajplung.2001.281.1.L250 [DOI] [PubMed] [Google Scholar]
- 32. Chalphin AV, Tracy SA, Lazow SP, et al. Congenital diaphragmatic hernia as a potential target for transamniotic stem cell therapy. J Pediatr Surg. 2020;55:249-252. [DOI] [PubMed] [Google Scholar]
- 33. Pederiva F, Ghionzoli M, Pierro A, et al. Amniotic fluid stem cells rescue both in vitro and in vivo growth, innervation, and motility in nitrofen-exposed hypoplastic rat lungs through paracrine effects. Cell Transplant. 2013;22:1683-1694. 10.3727/096368912X657756 [DOI] [PubMed] [Google Scholar]
- 34. Jesudason EC, Smith NP, Connell MG, et al. Peristalsis of airway smooth muscle is developmentally regulated and uncoupled from hypoplastic lung growth. Am J Physiol Lung Cell Mol Physiol. 2006;291:L559-L565. 10.1152/ajplung.00498.2005 [DOI] [PubMed] [Google Scholar]
- 35. Friedmacher F, Doi T, Gosemann J-H, et al. Upregulation of fibroblast growth factor receptor 2 and 3 in the late stages of fetal lung development in the nitrofen rat model. Pediatr Surg Int. 2012;28:195-199. 10.1007/s00383-011-2985-2 [DOI] [PubMed] [Google Scholar]
- 36. Xian J, Clark KJ, Fordham R, et al. Inadequate lung development and bronchial hyperplasia in mice with a targeted deletion in the Dutt1/Robo1 gene. Proc Natl Acad Sci USA. 2001;98:15062-15066. 10.1073/pnas.251407098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenberg JM, Thompson FY, Brooks SK, et al. Slit and robo expression in the developing mouse lung. Dev Dyn. 2004;230:350-360. 10.1002/dvdy.20045 [DOI] [PubMed] [Google Scholar]
- 38. Gonçalves AN, Correia-Pinto J, Nogueira-Silva C.. ROBO2 signaling in lung development regulates SOX2/SOX9 balance, branching morphogenesis and is dysregulated in nitrofen-induced congenital diaphragmatic hernia. Respir Res. 2020;21:302. 10.1186/s12931-020-01568-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takahashi T, Friedmacher F, Zimmer J, et al. Ephrin-B1, -B2, and -B4 expression is decreased in developing diaphragms and lungs of fetal rats with nitrofen-induced congenital diaphragmatic hernia. Eur J Pediatr Surg. 2019;29:113-119. 10.1055/s-0038-1675774 [DOI] [PubMed] [Google Scholar]
- 40. McGowan SE, McCoy DM.. Neuropilin-1 directs PDGFRα-entry into lung fibroblasts and signaling from very early endosomes. Am J Physiol Lung Cell Mol Physiol. 2021;320:L179-L192. 10.1152/ajplung.00149.2020 [DOI] [PubMed] [Google Scholar]
- 41. Ostrin EJ, Little DR, Gerner-Mauro KN, et al. β-Catenin maintains lung epithelial progenitors after lung specification. Development. 2018;145:dev160788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nguyen TM, Jimenez J, Rendin LE, et al. The proportion of alveolar type 1 cells decreases in murine hypoplastic congenital diaphragmatic hernia lungs. PLoS One. 2019;14:e0214793. 10.1371/journal.pone.0214793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chapin CJ, Ertsey R, Yoshizawa J, et al. Congenital diaphragmatic hernia, tracheal occlusion, thyroid transcription factor-1, and fetal pulmonary epithelial maturation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L44-L52. 10.1152/ajplung.00342.2004 [DOI] [PubMed] [Google Scholar]
- 44. Guilbert TW, Gebb SA, Shannon JM.. Lung hypoplasia in the nitrofen model of congenital diaphragmatic hernia occurs early in development. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1159-L1171. 10.1152/ajplung.2000.279.6.L1159 [DOI] [PubMed] [Google Scholar]
- 45. Serapiglia V, Stephens C, Joshi R, et al. Murine fetal tracheal occlusion increases lung basal cells via increased yap signaling. MedRxiv, doi: 10.1101/2021.09.17.21263741, September 22, 2021, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gosney JR, Okoye BO, Lloyd DA, et al. Pulmonary neuroendocrine cells in nitrofen-induced diaphragmatic hernia and the effect of prenatal glucocorticoids. Pediatr Surg Int. 1999;15:180-183. 10.1007/s003830050549 [DOI] [PubMed] [Google Scholar]
- 47. Santos M, Nogueira-Silva C, Baptista MJ, et al. Pulmonary epithelial cell differentiation in the nitrofen-induced congenital diaphragmatic hernia. J Pediatr Surg. 2007;42:1231-1237. 10.1016/j.jpedsurg.2007.02.014 [DOI] [PubMed] [Google Scholar]
- 48. IJsselstijn H, Perrin DG, de Jongste JC, et al. Pulmonary neuroendocrine cells in neonatal rats with congenital diaphragmatic hernia. J Pediatr Surg. 1995;30:413-415. 10.1016/0022-3468(95)90044-6 [DOI] [PubMed] [Google Scholar]
- 49. Song H, Yao E, Lin C, et al. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci USA. 2012;109:17531-17536. 10.1073/pnas.1207238109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Riccetti M, Gokey JJ, Aronow B, et al. The elephant in the lung: integrating lineage-tracing, molecular markers, and single cell sequencing data to identify distinct fibroblast populations during lung development and regeneration. Matrix Biol. 2020;91-92:51-74. 10.1016/j.matbio.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. El Agha E, Moiseenko A, Kheirollahi V, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20:261-273.e3. 10.1016/j.stem.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsukui T, Sun K-H, Wetter JB, et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 2020;11:1920. 10.1038/s41467-020-15647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rehan VK, Sugano S, Wang Y, et al. Evidence for the presence of lipofibroblasts in human lung. Exp Lung Res. 2006;32:379-393. 10.1080/01902140600880257 [DOI] [PubMed] [Google Scholar]
- 54. Popova AP, Bentley JK, Cui TX, et al. Reduced platelet-derived growth factor receptor expression is a primary feature of human bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L231-L239. 10.1152/ajplung.00342.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fulton CT, Cui TX, Goldsmith AM, et al. Gene expression signatures point to a male sex-specific lung mesenchymal cell PDGF receptor signaling defect in infants developing bronchopulmonary dysplasia. Sci Rep. 2018;8:17070. 10.1038/s41598-018-35256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li C, Lee MK, Gao F, et al. Secondary crest myofibroblast PDGFRα controls the elastogenesis pathway via a secondary tier of signaling networks during alveologenesis. Development. 2019;146:dev176354. 10.1242/dev.176354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gong Y, Xu W, Chen Y, et al. miR-20a-5p regulates pulmonary surfactant gene expression in alveolar type II cells. J Cell Mol Med. 2019;23:7664-7672. 10.1111/jcmm.14639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khalaj K, Antounians L, Figueira RL, et al. Autophagy is impaired in fetal hypoplastic lungs and rescued by administration of amniotic fluid stem cell extracellular vesicles. Am J Respir Crit Care Med. 206:476-487. 10.1164/rccm.202109-2168OC [DOI] [PubMed] [Google Scholar]
- 59. Zhu S, He Q, Zhang R, et al. Decreased expression of miR-33 in fetal lungs of nitrofen-induced congenital diaphragmatic hernia rat model. J Pediatr Surg. 2016;51:1096-1100. 10.1016/j.jpedsurg.2016.02.083 [DOI] [PubMed] [Google Scholar]
- 60. Mukherji S, Ebert MS, Zheng GXY, et al. MicroRNAs can generate thresholds in target gene expression. Nat Genet. 2011;43:854-859. 10.1038/ng.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Deprest JA, Nicolaides KH, Benachi A, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med. 2021;385:107-118. 10.1056/NEJMoa2027030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Deprest JA, Benachi A, Gratacos E, et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N Engl J Med. 2021;385:119-129. 10.1056/NEJMoa2026983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rohde E, Pachler K, Gimona M.. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21:581-592. 10.1016/j.jcyt.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 64. Khalaj K, Figueira RL, Antounians L, et al. Systematic review of extracellular vesicle-based treatments for lung injury: are EVs a potential therapy for COVID-19? J Extracell Vesicles. 2020;9:1795365. 10.1080/20013078.2020.1795365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coste K, Beurskens LWJE, Blanc P, et al. Metabolic disturbances of the vitamin A pathway in human diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 2015;308:L147-L157. 10.1152/ajplung.00108.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Loo CKC, Pearen MA, Pereira TN, et al. Lung and liver growth and retinoic acid status in human fetuses with congenital diaphragmatic hernia. Early Hum Dev. 2018;116:17-23. 10.1016/j.earlhumdev.2017.10.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.