Abstract
Mechanical homeostasis emerges following normal development of the arterial wall and requires thereafter a slow balanced degradation and deposition of extracellular matrix constituents within an unchanging mechanical state. Recent findings suggest that homeostasis is compromised in arterial aging, which contributes to the structural stiffening that is characteristic of aged central arteries. Matrix metalloproteinases (MMPs) have strong proteolytic activity and play fundamental roles in matrix turnover. Here, we use Mmp12−/− mice to examine effects of a potent metalloelastase, MMP-12, on the biomechanical phenotype of the thoracic and abdominal aorta in young and naturally aged mice. A key finding is that germline deletion of the gene (Mmp12) that encodes MMP-12 alters biomechanical properties from normal more in young adult than in older adult mice. Consequently, percent changes in biomechanical properties during aortic aging are greater in wild-type than in MMP-12 deficient mice, though with similar overall decreases in elastic energy storage and distensibility and increases in calculated pulse wave velocity. Reduced elastic energy storage compromises the ability of the aorta to augment antegrade and retrograde blood flow while an increased pulse wave velocity can adversely affect end organs, both conditions being characteristic of aortic aging in humans. In summary, MMP-12 is fundamental for establishing homeostatic values of biomechanical metrics in the aorta and its absence leads to a pre-aged aortic phenotype in young mice.
Keywords: aging, homeostasis, stiffness, distensibility, pulse wave velocity
Introduction
The extracellular matrix endows large (elastic) arteries with much of their biomechanical functionality. In particular, elastic fibers endow these arteries with the capacity to distend (compliance) and store strain energy (resilience). The energy that is stored upon distension in systole is used during diastole to work on the blood and augment flow. Fibrillar collagens, on the other hand, endow the wall with stiffness and strength, the former via a strain stiffening due to the straightening of undulated fibers. Adventitial collagen, in particular, stress-shields the elastin- and smooth muscle cell-rich media and protects against transmural rupture at high blood pressures. Collagen, but not elastin, turns over continually in maturity. As (human) arteries age, elastin is gradually fatigue-damaged and functionally degraded, causing a shift in pressure load bearing from the compliant elastin to the stiffer collagen (O’Rourke and Hashimoto, 2007), the half-life of which can increase in aging via non-enzymatic cross-linking. This shift leads to a structural stiffening of large arteries in aging and the associated reduction in distensibility increases central pulse pressures — the primary cause of isolated systolic hypertension in aging.
This arterial extracellular matrix is established, maintained, and remodeled by multiple cell types, including the endothelial cells, smooth muscle cells, and fibroblasts as well as resident and recruited macrophages. It is largely the balance or imbalance in matrix degradation and deposition by these cells that dictates fundamental tissue level properties (Valentin and Humphrey, 2009). Among other proteolytic enzymes, matrix metalloproteinases (MMPs) play particularly important roles in matrix turnover within the arterial wall. There are over 20 MMPs, at least 15 of which are found in the vasculature (Newby, 2006; Raffetto and Khalil, 2008). MMPs are secreted in latent form (proMMP), sequestered within the matrix, and subsequently activated by various biomolecules (including other MMPs) as well as by mechanical stress. Indeed, mechanical stress can also both stimulate MMP gene expression (Asanuma et al., 2003; Ruddy et al., 2009) and affect the susceptibility of the matrix to proteolytic cleavage (Chang et al., 2012). MMPs are thus highly mechano-regulated, consistent with their important role in the turnover of the matrix that governs the mechanical properties in development, homeostasis, and disease.
Among the many MMPs, a metalloelastase (MMP-12) produced mainly by macrophages has garnered increasing attention recently. It is implicated in arterial aging, aortic aneurysms and dissections, and atherosclerosis, among other vascular conditions (Brankovic et al., 2019; Luttun et al., 2004; Proietta et al., 2014; Wang et al., 2010). In this paper, we test the hypothesis that MMP-12 is also an important contributor to the emergence of mechanical homeostasis in the aorta of young adult mice. Toward this end, we use validated biaxial testing protocols and methods of quantification to biomechanically phenotype and compare segments of the thoracic and abdominal aorta in young and naturally aged wild-type (WT) and MMP-12 null (Mmp12−/−) mice.
Methods
Animals
All live animal procedures were approved by the Institutional Animal Care and Use Committees of Yale University and the Veteran Affairs Connecticut Healthcare System. Minimum sample size was determined as follows. One of our main outcome measures is pulse wave velocity (PWV), for which we wanted to detect a 25% increase at α=0.05 and power 1-β=0.80 using a two-sided test. Previously, we observed in WT mice a relative standard deviation (SD)/mean of 18.4% for distensibility (Spronck et al., 2021). Since PWV is inversely related to the square root of distensibility, this relative error scales by ½, yielding a relative error in PWV of 9.2%. For a typical PWV of 4 m/s, this corresponds to a SD of 0.368 m/s. A 25% change in PWV, hence, corresponds to a Cohen’s effect size of 25%∙4/0.368=2.72 (Cohen, 1988). This gives a required minimum sample size of n=4 per group as calculated using G*Power 3.1.9.7 (Faul et al., 2009; Faul et al., 2007). For some groups, a higher number of mice was included as these mice were simultaneously used for other experiments. Young (13–14 weeks, n = 8) and older (52–53 weeks, n = 5) male C57BL/6J (WT) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA); young (13–14 weeks, n = 4) and older (52–53 weeks, n = 4) male Mmp12−/− mice were obtained by breeding B6.129X-Mmp12tm1Sds/J mice, originally purchased from The Jackson Laboratory. Following euthanasia, using either an intraperitoneal injection of pentobarbital sodium and phenytoin sodium (Beuthanasia-D; 150 mg/kg) or inhaled isoflurane (3%) followed by gentle cervical dislocation, the descending thoracic aorta (DTA) and suprarenal abdominal aorta (SAA) were excised carefully and side branches were ligated using 9–0 sutures.
Biomechanical testing
The biomechanical testing protocol and constitutive modeling have been described previously (Ferruzzi et al., 2013). Briefly, excised arteries (mean unloaded lengths of 5.31 and 5.75 mm for the DTA and SAA, respectively) were mounted on paired glass micropipets using 6–0 sutures, submerged in Hanks’ Balanced Salt Solution (HBSS) at room temperature to ensure passive behavior, and their in vivo values of axial stretch (λz,iv) were inferred from the recorded axial force (f) during pressurization cycles from 60 to 140 mmHg; λz,iv corresponds to that axial stretch at which the variation in f is minimal during pressurization. Arteries were then maintained at λz,iv during equilibration for 15 minutes under a cyclic pressurization from 80 to 140 mmHg. Subsequent preconditioning consisted of four pressurization cycles from 10 to 140 mmHg, again at λz,iv.
Biaxial data (pressure, outer diameter, axial force, and axial stretch) were collected on-line during seven sequential testing protocols consisting of, in-duplicate, distension-deflation cycles from 10 to 140 mmHg at three fixed values of axial stretch (0.95λz,iv, 1.00λz,iv, and 1.05λz,iv) and, in-duplicate, extension-shortening cycles from f = 0 to f = fmax at four fixed pressures (10, 60, 100, and 140 mmHg), where fmax corresponds to the maximum specimen-specific force reached during the pressurization protocol at axial stretch 1.05λz,iv. For each (duplicate) protocol step, the second cycle was used for analysis, from which the unloading data were extracted since they enable energy storage (i.e., the non-dissipated part) to be computed, from which stress and stiffness can be computed using standard formulae (Ferruzzi et al., 2013).
Constitutive modeling
Passive biomechanical data were fit with a four-fiber family constitutive relation of the form (Ferruzzi et al., 2013)
| (1) |
with c, , and model parameters and C = FTF the right Cauchy-Green deformation tensor, with F the deformation gradient tensor. denotes the orientation of fiber family i, with the angle of orientation with respect to the axial direction in the axial-circumferential plane in the traction-free configuration. IC = C: I = CAA (trace) and (fiber stretch squared) are coordinate invariant measures of deformation, with I the second-order identity tensor. The four fiber families were assumed to be oriented axially , circumferentially , and symmetric diagonally . The Cauchy stress tensor t can then be obtained, in physical components, from
| (2) |
where p is a Lagrange multiplier enforcing incompressibility and δij are components of the identity tensor I. Best-fit values of the eight free constitutive parameters were determined via a constrained (to be non-negative) nonlinear least-squares regression (Trust Region Reflective algorithm). Combined data sets from the seven biaxial protocols were used in this regression to minimize the sum-of-squared differences between measured and modeled luminal pressure and total axial force, each normalized by mean experimental measures.
The parameterized constitutive relation was then used to compute stored energy, biaxial Cauchy stress, biaxial material stiffness (Cijkl, specifically in circumferential and axial directions), circumferential structural stiffness (ℎ ∙ CƟƟƟƟ ; with ℎ the loaded wall thickness), a distensibility coefficient (D), and pulse wave velocity (PWV) at any configuration. In particular, the material stiffness in a current state, linearized about a physiologically relevant in vivo state, was calculated using (Baek et al., 2007)
| (3) |
with Fo the deformation gradient tensor between the chosen reference configuration and a finitely deformed configuration, and Co the right Cauchy-Green deformation tensor corresponding to Fo. The distensibility coefficient was calculated as (Spronck and Humphrey, 2019)
| (4) |
with ds and dd the systolic and diastolic diameters, and Ps and Pd the systolic and diastolic pressures. Finally, the pulse wave velocity was calculated as
| (5) |
with ρ = 1050 kg/m3 the assumed mass density of blood. Preliminary telemetry recordings by our group revealed comparable blood pressures in n=5 young mice without and with loss of MMP-12, though on an Apoe−/− background (Ps/Pd = 133/88 mmHg in Apoe−/−; Ps/Pd = 126/93 mmHg in Apoe−/−∙Mmp12−/−). Although Apoe−/− mice develop arterial hypertension with age, control mice do not (Yang et al. (1999), the latter being confirmed in another study showing that blood pressure does not change after one year of aging in C57BL/6J mice (De Moudt et al., 2020). Taken together, it appears that neither knock-out of Mmp12 nor biological age influence arterial blood pressure appreciably over the conditions studied herein. Therefore, and for consistency, the various geometric and biomechanical metrics were calculated and compared predominantly at Ps/Pd = 120/80 mmHg for all groups.
Quantitative histology
Consistent with previous work (Spronck et al., 2021), for each of the experimental groups (DTA and SAA for both young and old WT and young and old Mmp12−/− mice), we fixed 3–4 arteries in 10% neutral buffered formalin for 24 hours, followed by storage in ethanol. Subsequently, the vessels were embedded in paraffin and sectioned into 5-μm thick, 10-μm spaced, transverse sections that were mounted on a glass slide. Two slides per specimen were prepared and stained using a Movat pentachrome or Verhoeff-Van Gieson (VVG) stain, respectively. These sections were then imaged using an Olympus BX/51 microscope equipped with a DP70 digital camera (effective sensor resolution of 4080 × 3072 pixels, corresponding to a pixel size of 2.1 μm at a 2/3” (8.8 × 6.6 mm2 sensor size) and using a 20x objective (UPlanFl 20x, NA 0.50, optical resolution at λ = 400 nm of ~0.49 μm) and 0.5x tube lens, resulting in a total magnification of 10x and hence an image resolution of 0.21 μm (fulfilling the Nyquist criterion). When arterial sections exceeded the microscope’s field of view (0.88 × 0.66 mm2), multiple images were acquired and stitched together using Image Composite Editor software (Microsoft Research). Custom MATLAB (R2020a, MathWorks, Natick, MA) scripts based on hue-saturation-lightness (HSL) thresholding were used to extract cross-sectional area fractions for elastin (φe), collagen (φc), smooth muscle (φm) and ground substance / glycosaminoglycans (φg). φe was averaged from VVG and Movat images; φm and φg were extracted from Movat images; φc was calculated such that φe + φc + φm + φg = 1.
Because MMP-12 is a macrophage metalloelastase, we stained another three slides per specimen for cluster of differentiation 68 and 163 (CD68 using AA312–326 at 1:100, antibodies-online, Aachen, Germany and CD163 using ab34164 at 1:400, Abcam, Cambridge, United Kingdom), as well as F4/80 (using MA5–16363 at 1:75, Thermo Fisher Scientific, Waltham, MA), all with 3,3′-diaminobenzidine (DAB) on a horseradish peroxidase conjugated secondary antibody. Macrophage-stained slides were imaged using an Aperio AT2 Dx system (Leica Biosystems, Deer Park, IL, USA) with a 20x/0.75 NA Plan Apo objective (optical resolution at λ = 400 nm of ~0.33 μm) and an image resolution (effective pixel size) of 0.25 µm. Custom HSL thresholding-based MATLAB scripts were again used to extract cross-sectional area fractions positive for the respective macrophage stains.
Statistics
Mechanical and structural (histological) metrics were compared using a two-way (genotype and age) analysis of variance (ANOVA) followed by Bonferroni-corrected post-hoc tests. Using this full 2×2 design (2 genotypes x 2 ages), we can distinguish between genotype, age, and genotype*age interaction effects. p < 0.05 was considered statistically significant. All statistical analyses were performed using MATLAB.
Results
Standard histological sections revealed differences (detailed below) in cross-sectional geometry and composition across the four basic study groups, young and old WT and young and old Mmp12−/− aortas. Results are shown separately for the thoracic (Figure 1) and abdominal (Supplemental Figure S1) segments. Given that the microstructural differences are visually subtle, we used consistent, quantitative histology to detect and compare changes between the groups. Associated quantification of medial : adventitial areas as well as medial and adventitial elastin, fibrillar collagen, glycosaminoglycans (GAGs), and smooth muscle (cytoplasm) is given in Supplemental Table S1, and illustrated in Figure 2 in terms of cross-sectional area and area percentage. Wall thickness and thus cross-sectional area increased significantly with natural aging to 1 year, but more so in WT than in mutant aortas and more so in the thoracic than in the abdominal segment (Table S1a, DTA: loaded total cross-sectional area increased 68% from 0.179±0.001 to 0.300±0.005 mm2 (p<0.001) in WT but only 39% from 0.162±0.002 to 0.225±0.010 mm2 (p<0.001) in mutant aortas, p<0.001 for ANOVA interaction term; SAA: increased 40% from 0.163±0.001 to 0.228±0.007 mm2 (p<0.001) in WT and 37% from 0.138±0.002 to 0.189±0.006 mm2 (p<0.001) in mutant aortas; p=0.091 for interaction). Thus, absence of MMP-12 appeared to attenuate age-related vessel wall thickening in the DTA and (borderline significant) in the SAA. Importantly, these changes in thickness were achieved differently across groups, with the thoracic aortic medial : adventitial ratio remaining constant with aging in WT (change in media percentage from 65.9±1.0 % to 68.3±1.0 %, p=1.000) but decreasing with aging in the mutant (from 72.1±1.0 % to 63.7±1.7 %, p<0.001) mice while the abdominal aortic medial : adventitial ratio remained constant with aging in both genotypes (both p=1.000). The increase in loaded medial cross-sectional area in the thoracic aorta of the WT mice resulted from increases in all four primary constituents considered, namely elastin (+57%, from 0.033±0.001 to 0.052±0.005 mm2, p=0.007, perhaps frayed, giving the appearance of an increase), collagen (+93%, from 0.033±0.001 to 0.064±0.004 mm2, p<0.001), GAGs (+203%, from 0.003±0.000 to 0.009±0.000 mm2, p<0.001), and smooth muscle (+64%, from 0.049±0.000 to 0.080±0.001 mm2, p<0.001). In mutant mice, the increase in medial area resulted from significant increases in collagen (+28%, from 0.046±0.001 to 0.058±0.003 mm2, p=0.004) and smooth muscle (+50%, from 0.031±0.000 to 0.046±0.001 mm2, p<0.001), but not elastin (+9%, from 0.035±0.001 to 0.038±0.005 mm2, p=1.000). GAGs decreased significantly (−48%, from 0.006±0.000 to 0.003±0.000 mm2, p=0.017). The increase in adventitial thickness was due to increased collagen in both genotypes (wild-type: +57%, from 0.060±0.002 to 0.094±0.001 mm2, p<0.001; mutant: +79%, from 0.044±0.001 to 0.079±0.001 mm2, p<0.001). Such compositional and structural changes are expected to alter the biomechanical properties.
Figure 1.
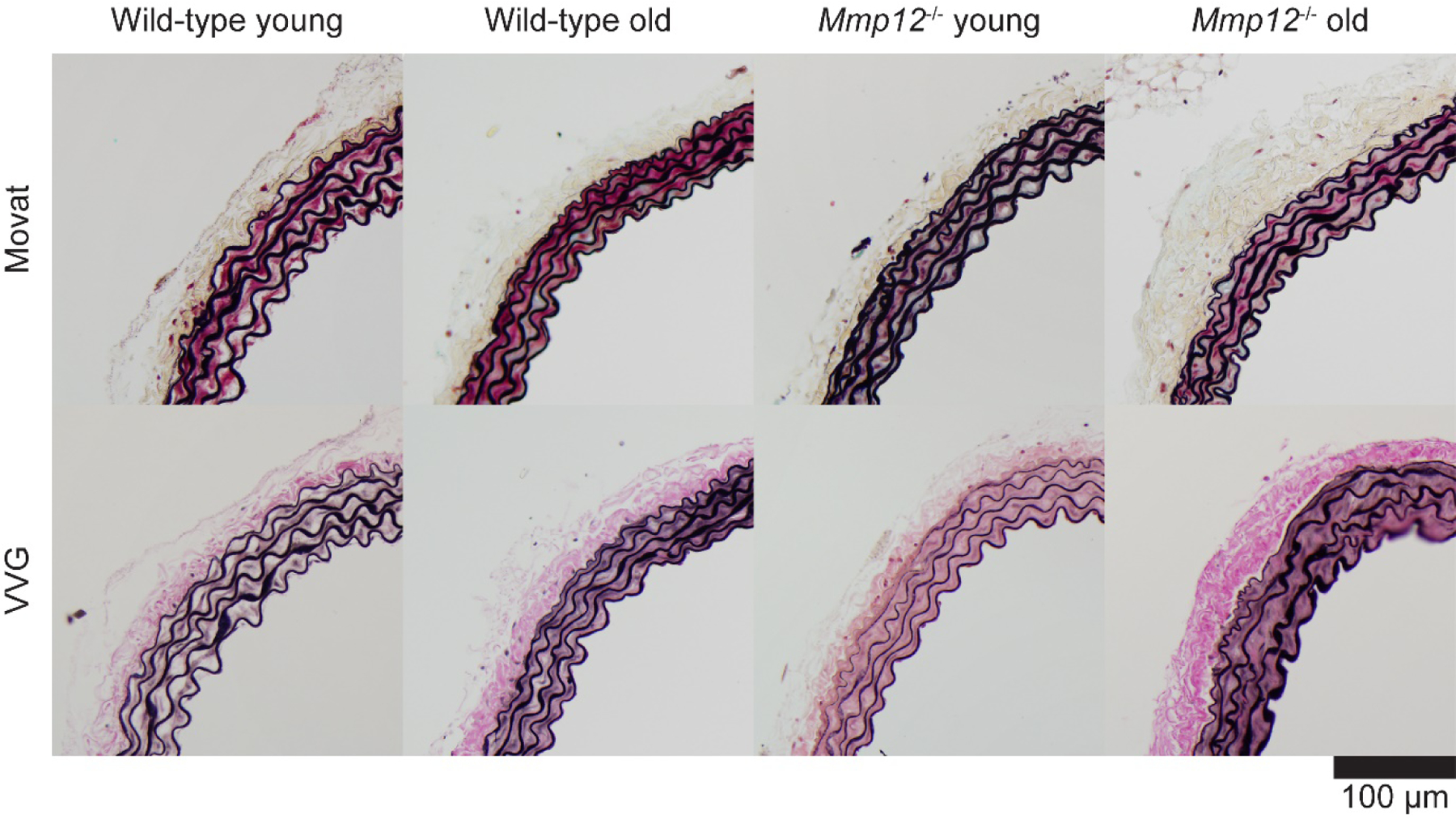
Representative transverse histological sections of the descending thoracic aorta (DTA) stained using Movat’s pentachrome, which shows elastin and nuclei black, collagen fibers yellowish, ground substance or glycosaminoglycans blue, cell cytoplasm pink, and (if present) fibrin red, or Verhoeff-van Gieson (VVG), which shows elastin and nuclei black and collagen pink. Mmp12−/−, matrix metalloproteinase 12 null. See Supplemental Figure S1 for similar results for the suprarenal abdominal aorta (SAA).
Figure 2.

Histology-based quantification of structural wall components for the descending thoracic aorta (DTA, A and C) and the suprarenal abdominal aorta (SAA, B and D). A and B represent absolute, loaded cross-sectional areas at systolic blood pressure (P=120 mmHg); C and D represent area percentages normalized to the total wall cross-sectional area. Loaded cross-sectional constituent areas were obtained by multiplying the specimen-specific loaded cross-sectional areas as obtained from biomechanical analyses with the area percentages as obtained from quantitative histology. Mmp12−/−, matrix metalloproteinase 12 null.
Our biaxial testing protocols enable measurement and calculation of multiple geometric and mechanical metrics that collectively help to define the biomechanical phenotype. Overall results were qualitatively similar between the thoracic (DTA) and abdominal (SAA) segments. Noting that the values of many of these metrics depend strongly on both the distending pressure and degree of in vivo axial stretch, select metrics are plotted versus these applied loads / boundary conditions in the first two rows of Figures 3 and S2 and as a function of the biaxial state of stretch in the last two rows; specific values at physiological values of pressure and axial stretch are given in Tables S2a and S2b for both segments (DTA and SAA) and all four study groups, with key metrics compared graphically in Figure 4. Of note, we have found that changes in the in vivo axial stretch, biaxial material stiffness, and elastically stored energy are particularly revealing metrics (Ferruzzi et al., 2015; Ferruzzi et al., 2018; Spronck et al., 2020). In the DTA, the in vivo value of axial stretch decreased modestly with aging (Table S2a; −6% in WT (p=0.211), −5% in mutant (p=0.824), p=0.017 for ANOVA aging effect) while systolic elastic energy storage decreased dramatically (−44% in WT (p<0.001), −32% in mutant (p=0.005), p<0.001 for ANOVA aging effect). Systolic circumferential material stiffness remained nearly constant with aging in both genotypes (+3% in WT (p=1.000), +1% in mutant (p=1.000), p=0.845 for ANOVA aging effect) while axial stiffness decreased dramatically in both (−35% in WT (p<0.001), −29% in mutant (p=0.023), p<0.001 for ANOVA aging effect). These differential circumferential and axial findings emphasize the importance of biaxial data. Note that circumferential structural stiffness increased with age (+52% in WT (p=0.205), +42% in mutant (p=0.483), p=0.010 for ANOVA aging effect) due to the concomitant increase in wall thickness (+47% in WT (p<0.001), +37% in mutant (p<0.001), p<0.001 for ANOVA aging effect).
Figure 3.

Detailed descending thoracic aorta (DTA) mechanics. A–D: Metrics during pressure distension at the in vivo value of axial stretch. F–J: Metrics during axial stretching at a pressure of 120 mmHg. K,O: circumferential and axial Cauchy stress-stretch curves plotted at in vivo axial stretch (K) and a distending pressure of 120 mmHg (O) for all four study groups. Symbols indicate means, error bars indicate standard errors. Enlarged symbols represent values at systolic blood pressure (A–E,K) and the in vivo axial stretch (F–J,O). L–N: group-averaged contour plots of elastically stored energy (L), circumferential material stiffness (M), and axial material stiffness (N) as a function of circumferential and axial stretch for wild-type young (blue lines) and wild-type older (orange lines) aortas. Enlarged symbols correspond to systolic blood pressure and in vivo axial stretch. Panels P–R are the same as L–N, except for Mmp12−/− aortas. Circ., circumferential; Mmp12−/−, matrix metalloproteinase 12 null. See Figure S2 for similar results for the suprarenal abdominal aorta.
Figure 4.

Key geometric and biomechanical metrics, evaluated at a common distending pressure of 120 mmHg and individual values of the in vivo axial stretch for all four experimental groups and both the descending thoracic aorta (DTA) and the suprarenal abdominal aorta (SAA). Key findings: 1) In vivo axial stretch (E) decreased with age in both the DTA and SAA (ANOVA p=0.017 and p=0.001; Supplemental Table 2). In the SAA, this effect was significantly larger in wild-type than in Mmp12−/− mice (ANOVA interaction term p=0.023). 2) Elastic energy storage (I) decreased with age in both the DTA and SAA (ANOVA both p<0.001) but did not differ between genotypes. 3) Circumferential material stiffness (G) remained nearly constant with aging, but differed between genotypes (ANOVA p=0.016 and p=0.001 for DTA and SAA). 4) Axial material stiffness (J) decreased significantly with aging in both genotypes (ANOVA p<0.001 for both DTA and SAA). In the DTA, axial material stiffness was significantly lower in the Mmp12−/− than in the wild-type groups (ANOVA p=0.008). 5) Local pulse wave velocity (L) as calculated from distensibility (K) using the Bramwell-Hill equation increases with age and is higher in Mmp12−/− than wild-type groups (ANOVA p<0.023). In the SAA, the age-associated pulse wave velocity increase was larger in wild-type mice than in Mmp12−/− mice (ANOVA interaction term p=0.006). Further figure panels: A: Outer diameter; B: Circumferential stretch; C,F: Circumferential and axial Cauchy stress; D: Wall thickness; H: Circumferential structural stiffness. Distensibility and pulse wave velocity were calculated using (systolic/diastolic) pressures of 120/80 mmHg. Overbars indicate p<0.05 for Bonferroni post-hoc tests after two-way analysis of variance (ANOVA). Circ., circumferential; Mmp12−/−, matrix metalloproteinase 12 null.
Percent increases or decreases with aging were, in general, greater for WT than for Mmp12−/− aortas. Importantly, in the DTA (Table S2a), values of elastically stored energy and distensibility were lower in young Mmp12−/− mice than in young WT mice (systolic stored energy: 63±1.6 vs. 73±3.5 kPa, p=0.216 (borderline significant); distensibility: 41.2±2.5 vs. 63.2±3.6 MPa−1, p=0.005), while values of PWV were (borderline) higher (4.83±0.15 vs. 3.92±0.13 m/s, p=0.125). When comparing older Mmp12−/− and WT mice, these differences were essentially absent (systolic stored energy: 43±2.8 vs. 41±1.4 kPa, p=1.000; distensibility: 33.3±6.3 vs. 35.4±0.9 MPa−1, p=1.000; PWV: 5.60±0.61 vs. 5.19±0.07 m/s, p=1.000). This pattern was also seen in the SAA for distensibility and PWV (Table S2b), with significant differences between young groups (distensibility: 39.6±1.1 vs. 58.0±2.8 MPa−1, p<0.001; PWV: 4.91±0.07 vs. 4.07±0.10 m/s, p<0.001) but not between old groups (distensibility: 29.6±1.3 vs. 31.9±1.0 MPa−1, p=1.000; PWV: 5.68±0.13 vs. 5.47±0.08 m/s, p=0.866). Taken together, absence of MMP-12 pre-aged the aorta in young mice in many ways.
Histological quantification of macrophage-related markers is presented in Figure S3a and Tables S3a and S3b. Representative sections are shown in Figures S3b and S3c. In the DTA (Table S3a), percentages of stained area were significantly higher in the mutant aortas for all three stains (ANOVA genotype term p<0.001 for CD68, p=0.014 for CD163, and p=0.045 for F4/80). Observations were similar for the SAA (Table S3b; ANOVA genotype term p=0.004 for CD68, p=0.019 for CD163, and p=0.011 for F4/80). Aging-related differences were less uniform: in the DTA (Table S3a), percentages of CD68 and CD163 stained areas were higher in the older than in the young aortas (ANOVA p=0.007 and p=0.038, respectively) while in the SAA (Table S3b), the area percentage was higher in the older than in the young aortas for only F4/80 (ANOVA p=0.049). When comparing the DTA and SAA, in the young WT group, the percentage of CD68 stained area was significantly higher in the SAA than in the DTA (0.697±0.154 vs. 0.110±0.045 %, p=0.019); the percentage of F4/80+ stained area was significantly lower in the SAA than in the DTA (0.330±0.062 vs. 0.557±0.030 %, p=0.028). The latter was also true for the young mutant group (0.213±0.082 vs. 0.706±0.128 %, p=0.017). In the older mutant group, the percentage of CD68+ stained area was significantly lower in the SAA than in the DTA (0.888±0.020 vs. 2.163±0.288 %, p=0.009). Taken together, these results show that the number of macrophages was markedly higher in the mutant aortas.
Discussion
It is increasingly recognized that macrophages, both resident and recruited, play key roles in soft tissue development, homeostasis, and adaptation (Okabe and Medzhitov, 2016; Wynn et al., 2013), not just disease progression. Among the many factors secreted by macrophages, MMP-12 is a potent elastase that can compromise elastic fiber integrity. Progressive damage to or degradation of elastic fibers is a characteristic of arterial aging in humans (Kielty, 2006; Yanagisawa and Wagenseil, 2020), though much less so in mice because of the normally long half-life of vascular elastin and the short life-span of mice (Ferruzzi et al., 2018; Spronck et al., 2020). Nevertheless, given the aforementioned increased attention to the role of MMP-12 in vascular aging and disease, in humans as well as in murine models, there was strong motivation to collect and compare the first detailed histo-mechanical data on aortic phenotype as a function of aging in Mmp12−/− mice.
A primary finding herein was that, relative to age-matched WT controls, differences in aortic phenotype due to the absence of MMP-12 were typically greater in younger than in older mice, especially in terms of decreased elastic energy storage, decreased distensibility, and increased PWV — all markers of aortic aging. Given the hemodynamic importance of PWV with regard to arterial (structural) stiffening in aging (McEniery et al., 2005), the calculated increases in PWV to values approaching 5.0 m/s in both aortic segments in young Mmp12−/− mice is particularly revealing. These findings are reminiscent, in part, of reported effects of fibrillin-1 deficiency in Marfan syndrome wherein mutation-associated increases in aortic stiffness are most apparent in younger individuals and less so in older individuals (de Wit et al., 2013), suggesting that effects of natural aging can eventually catch-up and dominate during later periods. Indeed, we previously showed in young mice on a mixed background that effects of hypertension on the aortic phenotype are less severe when superimposed on a fibulin-5 null mouse model of accelerated aortic aging (Spronck et al., 2020) due to the already marked effects of compromised elastic fibers in normotension in maturity (Ferruzzi et al., 2015). Another primary finding herein is a corollary to the first: percent biomechanical changes in the murine aorta due to aging to one year were generally lower in the absence of MMP-12. This finding could be interpreted as attenuated aging in the mutants or, as we suggest, a consequence of significant pre-aging prior to 14 weeks of age due to the absence of MMP-12, which implies a compromised homeostasis. Regardless, such aortic aging occurred despite the apparent near preservation of the elastic lamellae. In this regard, note that the histological suggestion of an increase in elastin in aortic aging in WT mice (Figure 1; Table S1) may have reflected slight fraying of the elastin within the lamellar structures, which would have increased VVG staining. Such fraying, in turn, could expose matricryptic sites (Davis et al., 2000) that could stimulate the observed increased collagen deposition in the media. There is a need to consider this possibility further, which was beyond the present scope.
There are few biomechanical findings to which the present data can be compared directly. Liu et al. (2015) reported that absence of MMP-12 in mice attenuates arterial stiffening, though measured using atomic force microscopy in both a femoral injury model and aortic aging. Such measurements do not address the in-plane tensile stiffness that is functionally important in aging-related arterial stiffening. Brankovic et al. (2019) performed biaxial tests on common carotid arteries excised from ~30- and 102-week old C57BL/6 wild-type and Mmp12−/− mutant mice and similarly reported that absence of MMP-12 attenuated biomechanical effects of aging. They noted further that biaxial stiffening in aging tended to associate with an increased thickness of the wall and decreased in vivo axial stretch, the latter two being consistent with our findings for the aorta. They suggested, however, that absence of MMP-12 tended to reduce age-related stiffening more in the axial than in the circumferential direction. Our results differ from theirs in that we found that the axial material stiffness decreased in aging, but less so in the absence of MMP-12. Reasons for this difference in interpretation potentially stem from differences in the way stiffness was calculated. Brankovic and colleagues reported stiffness as dσ/dλ for an undisclosed stress-stretch relation , which from the associated citation appeared to be a uniaxial exponential relation. We submit that given the multiaxial state of stress and strain, stiffness should be calculated using an appropriate tensorial relation, most easily accomplished in a referential form followed by a push-forward operation to obtain a spatial form. We used a small deformation superimposed on large approach (Baek et al., 2007) to accomplish this while obtaining a value that is appropriate over a cardiac cycle at the in vivo value of axial stretch. As discussed elsewhere, different metrics have different meanings (Spronck and Humphrey, 2019), thus suggesting a need for caution when comparing across studies. Using our same method for computing material stiffness, however, we previously found that the axial component of material stiffness similarly decreased in the DTA, SAA, and common carotid artery in both the fibulin-5 null model of accelerated aging (Ferruzzi et al., 2015) and in mice naturally aged to 100 weeks (Ferruzzi et al., 2018), with both mouse models on a mixed genetic background. These calculated reductions in axial stiffness appeared to mirror the measured reductions in the in vivo value of axial stretch, hence consistent with findings herein and suggestive of possible compensatory reductions in axial stiffness in cases when circumferential stiffness may increase, as in aging (Humphrey et al., 2009). Finally, we previously reported preliminary results (Spronck et al., 2018) related to the present study using WT mice on a mixed genetic background rather than a C57BL/6J background. Differences between that preliminary report and the present work may be attributable to this difference in genetic background, particularly given our recent findings of marked influences of background on aortic remodeling (Spronck et al., 2021).
Although our findings for the thoracic (DTA) and abdominal (SAA) aorta were similar qualitatively, they differed markedly in degree, with more severe changes and differences in the DTA. These regional differences are similar to those found previously in both angiotensin II induced and high salt + eNOS inhibition induced hypertension (Bersi et al., 2017; Spronck et al., 2020), with hypertensive remodeling tending to be more adaptive in the infrarenal abdominal aorta than in the DTA. Simply put, neither we nor others know the specific reason for these regional differences. That is, it is well known that many regional differences exist along the aorta, including those in embryonic lineage of the smooth muscle cells, cell receptor densities (e.g., AT1R), elastin : collagen ratios, values of intramural and wall shear stress, degree of perivascular tissue support, and so on, but there has not yet been a definitive link between these known regional differences and those in aortic remodeling in hypertension or aging.
Another primary finding herein was the significant numerical increase in CD68+ macrophages in the Mmp12−/− aortas, especially in young mice. Given the global knockout, no increase in number could compensate for the absence of macrophage-derived MMP-12, but this finding suggests that the cells sensed this absence and attempted nonetheless to compensate via proliferation. This is an important observation for these mice were otherwise normal, with normal blood pressure and no overt disease that usually associates with increased numbers of macrophages, as, for example, in angiotensin II-induced hypertension (Moore et al., 2015). That is, it appears that there was an attempt by the cells to rectify the absence of MMP-12 during development and into maturity, again suggesting an important role for this MMP-12 outside of disease. Of particular importance here, a prior study of the mouse aorta classified differential gene expression into four groups, including one for genes that were expressed during both development (from embryogenesis) and maturity, and MMP-12 was the only MMP included in this group (McLean et al., 2005). Their data thus support the present finding that MMP-12 plays important roles in both development, during which homeostatic biomechanical metrics emerge, and maturity, during which homeostatic responses can occur.
Notwithstanding the many important findings, this study was not without limitations. We only studied male mice given the sole availability of male Mmp12−/− mice from another laboratory here at Yale. Yet, sex differences in MMP-12 activity have been reported. For example, attenuated arterial stiffening in female Ldlr−/− mice was linked to reduced MMP-12 production by macrophages within the atherosclerotic lesions; deletion of Mmp12 attenuated this sex bias (Liu et al., 2019). This example reinforces the need for future studies on the biomechanical effects of MMP-12 to include both male and female mice. We also focused on but one MMP — MMP-12, a potent metalloelastase produced primarily by macrophages. There is, therefore, a pressing need for systematic study of the effects of other MMPs on arterial composition and properties in aging as well as other conditions, including hypertension. Wang et al. (2012) showed that chronic administration of a broad spectrum MMP inhibitor (PD166739) reduced elastic fiber fragmentation and collagen deposition in aortic aging in rats, noting the particular importance of reducing MMP-2 (a gelatinase, affecting elastin and degraded collagen) and MMP-13 (a collagenase, affecting collagens I and III, among others) activity. Studies in humans have similarly implicated other MMPs, including MMP-9 (a gelatinase), with a linear correlation emerging between increased stiffness and the level of MMP-9 (Yasmin et al., 2005). Many other examples exist, thus reinforcing the need for further understanding, with the reminder that proper MMP-driven degradation is needed to balance extracellular matrix deposition even in mechanical homeostasis and homeostatic adaptations, both in the vasculature and in other structural soft tissues (Rodriguez-Feo et al., 2005). Given the apparent “pre-aging” of the aortas in young Mmp12−/− mice herein, and noting that MMP-12 degrades multiple extracellular matrix constituents beyond elastin (Newby, 2006; Raffetto and Khalil, 2008), we submit that MMP-12 is critical in normal aortic development and maturation because of need for rapid degradation, not just deposition, of many extracellular matrix constituents within evolving biomechanical states during these periods of increasing hemodynamic loading. Evidence from studies contrasting effects of BAPN (β-aminopropionitrile) in young versus older mice confirms the rapid deposition of matrix components in developing mice that depend on lysyl oxidase activity for cross-linking (Zheng et al., 2020), but such deposition must be accompanied by degradation to promote optimal properties (e.g., orientations and undulations) as the aorta continues to grow. Multiple MMPs participate in such degradation, even late prenatally and early postnatally (McLean et al., 2005).
In summary, our histological and biaxial biomechanical data suggest that MMP-12 is essential for establishing the normal homeostatic state in the thoracic and abdominal aorta and its absence results in a pre-aged aortic phenotype in young mice. Although it may appear that effects of aging are attenuated in the absence of MMP-12, given the marked pre-aging, it is expected that this non-homeostatic pre-aging could increase the severity of early onset diseases, thus suggesting that further study is warranted on roles of MMPs in aortic development and homeostasis.
Supplementary Material
Acknowledgments
This work was supported, in part, by grants from the United States NIH (U01 HL142518, R01 HL155105), the Department of Veterans Affairs (I0-BX004038), the Netherlands Organisation for Scientific Research (Rubicon 452172006), and the European Union’s Horizon 2020 research and innovation program (No 793805).
References
- Asanuma K, Magid R, Johnson C, Nerem RM, Galis ZS, 2003. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol 284, H1778–1784. [DOI] [PubMed] [Google Scholar]
- Baek S, Gleason RL, Rajagopal K, Humphrey J, 2007. Theory of small on large: potential utility in computations of fluid–solid interactions in arteries. Comput. Methods Appl. Mech. Eng 196, 3070–3078. [Google Scholar]
- Bersi MR, Khosravi R, Wujciak AJ, Harrison DG, Humphrey JD, 2017. Differential cell-matrix mechanoadaptations and inflammation drive regional propensities to aortic fibrosis, aneurysm or dissection in hypertension. J. R. Soc. Interface 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankovic S, Hawthorne EA, Yu X, Zhang Y, Assoian RK, 2019. MMP12 preferentially attenuates axial stiffening of aging arteries. J. Biomech. Eng 141, 081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Flynn BP, Ruberti JW, Buehler MJ, 2012. Molecular mechanism of force induced stabilization of collagen against enzymatic breakdown. Biomaterials 33, 3852–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical power analysis for the behavioral sciences, 2nd ed. L. Erlbaum Associates, Hillsdale, N.J. [Google Scholar]
- Davis GE, Bayless KJ, Davis MJ, Meininger GA, 2000. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol 156, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moudt S, Hendrickx JO, De Munck DG, Leloup AJ, Martinet W, De Meyer GR, Fransen P, 2020. YI 2.2 Spontaneous Cardiovascular Ageing of C57bl6 Mice Results in the Development of Aortic Stiffness Prior to Periphral Blood Pressure Alterations. Artery Res 26, S13–S14. [Google Scholar]
- de Wit A, Vis K, Jeremy RW, 2013. Aortic stiffness in heritable aortopathies: relationship to aneurysm growth rate. Heart Lung Circ 22, 3–11. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG, 2009. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A, 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Ferruzzi J, Bersi MR, Humphrey JD, 2013. Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann. Biomed. Eng 41, 1311–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruzzi J, Bersi MR, Uman S, Yanagisawa H, Humphrey JD, 2015. Decreased elastic energy storage, not increased material stiffness, characterizes central artery dysfunction in fibulin-5 deficiency independent of sex. J. Biomech. Eng 137, 031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruzzi J, Madziva D, Caulk AW, Tellides G, Humphrey JD, 2018. Compromised mechanical homeostasis in arterial aging and associated cardiovascular consequences. Biomech. Model. Mechanobiol 17, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Eberth JF, Dye WW, Gleason RL, 2009. Fundamental role of axial stress in compensatory adaptations by arteries. J. Biomech 42, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, 2006. Elastic fibres in health and disease. Expert Rev. Mol. Med 8, 1–23. [DOI] [PubMed] [Google Scholar]
- Liu SL, Bae YH, Yu C, Monslow J, Hawthorne EA, Castagnino P, Branchetti E, Ferrari G, Damrauer SM, Pure E, Assoian RK, 2015. Matrix metalloproteinase-12 is an essential mediator of acute and chronic arterial stiffening. Sci. Rep 5, 17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Bajpai A, Hawthorne EA, Bae Y, Castagnino P, Monslow J, Pure E, Spiller KL, Assoian RK, 2019. Cardiovascular protection in females linked to estrogen-dependent inhibition of arterial stiffening and macrophage MMP12. JCI Insight 4, e122742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L, 2004. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation 109, 1408–1414. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Yasmin Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, Investigators, A., 2005. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J. Am. Coll. Cardiol 46, 1753–1760. [DOI] [PubMed] [Google Scholar]
- McLean SE, Mecham BH, Kelleher CM, Mariani TJ, Mecham RP, 2005. Extracellular matrix gene expression in the developing mouse aorta, in: Miner JH (Ed.), Extracellular Matrix in Development and Disease Elsevier, Amsterdam; Boston, pp. 81–128. [Google Scholar]
- Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT, Lieu M, Samuel CS, Diep H, Kemp-Harper BK, Tare M, Ricardo SD, Guzik TJ, Sobey CG, Drummond GR, 2015. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol 309, H906–917. [DOI] [PubMed] [Google Scholar]
- Newby AC, 2006. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res 69, 614–624. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Hashimoto J, 2007. Mechanical factors in arterial aging: a clinical perspective. J. Am. Coll. Cardiol 50, 1–13. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R, 2016. Tissue biology perspective on macrophages. Nat. Immunol 17, 9–17. [DOI] [PubMed] [Google Scholar]
- Proietta M, Tritapepe L, Cifani N, Ferri L, Taurino M, Del Porto F, 2014. MMP-12 as a new marker of Stanford-A acute aortic dissection. Ann. Med 46, 44–48. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA, 2008. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol 75, 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Feo JA, Sluijter JP, de Kleijn DP, Pasterkamp G, 2005. Modulation of collagen turnover in cardiovascular disease. Curr. Pharm. Des 11, 2501–2514. [DOI] [PubMed] [Google Scholar]
- Ruddy JM, Jones JA, Stroud RE, Mukherjee R, Spinale FG, Ikonomidis JS, 2009. Differential effects of mechanical and biological stimuli on matrix metalloproteinase promoter activation in the thoracic aorta. Circulation 120, S262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronck B, Ferruzzi J, Bellini C, Caulk AW, Murtada SI, Humphrey JD, 2020. Aortic remodeling is modest and sex-independent in mice when hypertension is superimposed on aging. J. Hypertens 38, 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronck B, Humphrey J, 2019. Arterial Stiffness: Different Metrics, Different Meanings. J. Biomech. Eng 141, 091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronck B, Latorre M, Wang M, Mehta S, Caulk AW, Ren P, Ramachandra AB, Murtada SI, Rojas A, He CS, Jiang B, Bersi MR, Tellides G, Humphrey JD, 2021. Excessive adventitial stress drives inflammation-mediated fibrosis in hypertensive aortic remodelling in mice. J. R. Soc. Interface 18, 20210336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronck B, Ramachandra AB, Toczek J, Han J, Sadeghi M, Humphrey JD, 2018. Knock-out of matrix metalloproteinase-12 exacerbates compromised mechanical homeostasis in arterial aging. Artery Res 24, 69. [Google Scholar]
- Valentin A, Humphrey J, 2009. Evaluation of fundamental hypotheses underlying constrained mixture models of arterial growth and remodelling. Philos. Trans. A Math. Phys. Eng. Sci 367, 3585–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, Kapoor K, Talan M, Lakatta EG, 2012. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension 60, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z, 2010. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest 120, 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW, 2013. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Wagenseil J, 2020. Elastic fibers and biomechanics of the aorta: Insights from mouse studies. Matrix Biol 85–86, 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Powell-Braxton L, Ogaoawara AK, Dybdal N, Bunting S, Ohneda O, Jin H, 1999. Hypertension and endothelial dysfunction in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol 19, 2762–2768. [DOI] [PubMed] [Google Scholar]
- Yasmin, McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB, 2005. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler. Thromb. Vasc. Biol 25, 372–378. [DOI] [PubMed] [Google Scholar]
- Zheng HQ, Rong JB, Ye FM, Xu YC, Lu HS, Wang JA, 2020. Induction of thoracic aortic dissection: a mini-review of beta-aminopropionitrile-related mouse models. J. Zhejiang Univ. Sci. B 21, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


