Abstract
Background
Corticosteroids are medications with anti‐inflammatory and immunosuppressant properties. Systemic corticosteroids administered through the oral, intravenous, or intramuscular routes have been used to treat various types of low back pain, including radicular back pain (not due to spinal stenosis), non‐radicular back pain, and spinal stenosis. However, there is uncertainty about the benefits and harms of systemic corticosteroids for low back pain.
Objectives
To evaluate the benefits and harms of systemic corticosteroids versus placebo or no corticosteroid for radicular low back pain, non‐radicular low back pain, and symptomatic spinal stenosis in adults.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was September 2021.
Selection criteria
We included randomized and quasi‐randomized trials in adults of systematic corticosteroids versus placebo or no corticosteroid.
Data collection and analysis
We used standard Cochrane methods. The major outcomes were pain, function, need for surgery, serious adverse effect, and presence of hyperglycemia. The minor outcomes were quality of life, successful outcomes, non‐serious adverse events, and withdrawal due to adverse events. We used GRADE to assess the certainty of evidence for each outcome.
Main results
Thirteen trials (1047 participants) met the inclusion criteria. Nine trials included participants with radicular low back pain, two trial with low back pain, and two trials with spinal stenosis. All trials blinded participants to receipt of systemic corticosteroids. Seven trials were at low risk of bias, five at unclear risk, and one at high risk of selection bias. Two trials were at high risk of attrition bias. Doses and duration of systemic corticosteroid therapy varied.
Radicular low back pain
For radicular low back pain, moderate‐certainty evidence indicated that systemic corticosteroids probably slightly decrease pain versus placebo at short‐term follow‐up (mean difference (MD) 0.56 points better, 95% confidence interval (CI) 1.08 to 0.04 on a 0 to 10 scale) and may slightly increase the likelihood of experiencing improvement in pain at short‐term follow‐up (risk ratio (RR) 1.21, 95% CI 0.88 to 1.66; absolute effect 5% better (95% CI 5% worse to 15% better).
Systemic corticosteroids may not improve function at short‐term follow‐up (standardized mean difference (SMD) 0.14 better; range 0.49 better to 0.21 worse) and probably increase the likelihood of improvement in function at short‐term follow‐up (RR 1.52, 95% CI 1.22 to 1.91; absolute effect 19% better, 95% CI 8% better to 30% better). Systemic corticosteroids were associated with greater improvement in function versus placebo at long‐term follow‐up (MD −7.40, 95% CI −12.55 to −2.25 on the 0 to 100 Oswestry Disability Index) and greater likelihood of functional improvement (RR 1.29, 95% CI 1.06 to 1.56), based on a single trial. There was no difference in likelihood of surgery (RR 1.00, 95% CI 0.68 to 1.47).
Evidence indicated that systemic corticosteroids (administered as a single dose or as a short course of therapy) are not associated with increased risk of any adverse event, serious adverse events, withdrawal due to adverse events, or hyperglycemia, but estimates were imprecise as some trials did not report harms, and harms reporting was suboptimal in trials that did provide data. Limitations included variability across trials in interventions (e.g. corticosteroid used, dose and duration of treatment), clinical settings, and participants (e.g. duration of symptoms, methods for diagnosis); limited utility of subgroup analyses due to small numbers of trials; methodologic limitations or suboptimal reporting of methods by some trials; and too few trials to formally assess for publication bias using graphical or statistical tests for small sample effects.
Non‐radicular low back pain
Evidence on systemic corticosteroids versus placebo for non‐radicular pain was limited and suggested that systemic corticosteroids may be associated with slightly worse short‐term pain but slightly better function.
Spinal stenosis
For spinal stenosis, limited evidence indicated that systemic corticosteroids are probably no more effective than placebo for short‐term pain or function.
Authors' conclusions
Systemic corticosteroids appear to be slightly effective at improving short‐term pain and function in people with radicular low back pain not due to spinal stenosis, and might slightly improve long‐term function. The effects of systemic corticosteroids in people with non‐radicular low back pain are unclear and systemic corticosteroids are probably ineffective for spinal stenosis. A single dose or short course of systemic corticosteroids for low back pain does not appear to cause serious harms, but evidence is limited.
Plain language summary
Systemic corticosteroids for radicular and non‐radicular low back pain
Key messages
For radicular low back pain:
– systemic corticosteroids may have small benefits on pain and function soon after treatment (short‐term follow‐up), and might improve function for a while after treatment (long‐term follow‐up);
– benefits appear small, but systemic corticosteroids may be useful as they have few side effects when used for a short period of time, are widely available, and have low costs; systemic corticosteroids probably do not help avoid surgery;
– it is unclear whether effectiveness of systemic corticosteroids varies according to the duration of symptoms as optimal dose and duration of treatment for radicular low back pain is unknown; avoiding higher doses may reduce risk of harms.
For non‐radicular back pain and spinal stenosis:
– benefits of systemic corticosteroids are unclear.
What is low back pain and how is it treated?
Systemic corticosteroids are anti‐inflammatory medicines that work throughout the body. In people with radicular low back pain (sciatica, or back pain with leg pain related to a pinched nerve in the back, usually from a slipped or bulging disc), corticosteroids may relieve pain by decreasing swelling and related compression from slipped or bulging discs, thereby reducing pressure on the affected nerves. Other types of back pain (such as 'non‐radicular' [or without nerve involvement] or back and leg pain due to spinal stenosis [narrowing of the spine]) may also have an inflammatory component that could respond to systemic corticosteroids.
What did we do?
We searched medical databases for clinical trials comparing corticosteroids versus placebo (dummy treatment) or no corticosteroids in adults with low back pain. We compared and summarized their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We included 13 studies with 1047 people. Six studies were conducted in the US, four in Europe, one in Australia, one in Brazil, and one in Iran. Nine studies evaluated people with radicular low back pain, two studies evaluated people with non‐radicular low back pain, and two studies evaluated people with spinal stenosis. In five studies, back pain was of short duration and in the other studies the duration of back pain was longer or mixed. The average age of people in the studies ranged from 36 to 47 years, except for the studies of spinal stenosis, where the average age was about 58 years. Pain scores before treatment were about 6.6 to 9.0 points on a scale of 0 to 10. The dose and length of treatment with systemic corticosteroids or placebo varied across studies.
Main results
For radicular low back pain, systemic corticosteroids appear to slightly reduce pain in the short term and allow resumption of normal activities, and might slightly improve people's ability to perform normal activities at long‐term. Systemic corticosteroids probably do not reduce the likelihood of undergoing surgery to remove a slipped or bulging disc and had no impact on quality of life.
For other types of low back pain (non‐radicular low back pain or spinal stenosis), the effects of systemic corticosteroids were unclear or suggested no benefit.
In the doses given in the studies, systemic corticosteroids for low back pain did not appear to cause serious harms, but evidence was limited.
What are the limitations of the evidence?
For radicular low back pain, we are moderately confident about effects on short‐term pain, short‐term function, long‐term function (but based on only one study), likelihood of surgery, and short‐term quality of life. For spinal stenosis, we are moderately confident only about effects on short‐term pain and function. For other time points and outcomes and for non‐radicular low back pain, we have little confidence about the evidence. Factors that decreased confidence in findings included flaws in how the studies were designed (patients were not assigned to treatments randomly, a lot of patients dropped out of the study, or there were large differences between patients assigned to different treatments), not having enough studies or participants to be certain about the results, and variations between studies in results and methods.
For people with radicular low back pain, studies are needed to determine the optimal dose and duration of systemic corticosteroid therapy, how effectiveness varies according to symptom duration, and whether imaging is needed to confirm the diagnosis of a bulging or slipped disc before using systemic corticosteroids. Studies are also needed to clarify effects of systemic corticosteroids on quality of life, long‐term outcomes, and harms.
For people with non‐radicular low back pain or spinal stenosis, research showing that some people have an inflammatory component would be helpful to determine whether well‐designed clinical trials should be conducted, and which people should be included in them.
How up to date is this evidence?
The evidence is current to September 2021.
Summary of findings
Background
Low back pain is highly prevalent and can result in major adverse impacts on quality of life and function (Deyo 2006; Hoy 2012). Low back pain is associated with high direct costs and is a common reason for missed work or reduced productivity while at work, therefore also resulting in high indirect costs (Luo 2004; Martin 2008). Several treatments are available for low back pain, including various pharmacologic treatments, non‐invasive non‐pharmacologic treatments, non‐surgical injection and interventional therapies, and surgical treatments.
Corticosteroids are a class of medications that are structurally similar to the human adrenal hormone cortisol, with potent anti‐inflammatory and immunosuppressant effects. For low back pain, corticosteroids can be administered directly into spinal structures with injections, as well as systemically, most commonly via the oral, intravenous, or intramuscular routes.
Systemic corticosteroids have long been used for treatment of radicular low back pain. One of the earliest studies on systemic corticosteroids for low back pain reported on 100 consecutive patients with radicular pain due to a herniated intervertebral disc who were treated with a tapering course of dexamethasone (Green 1975). It found that all patients reported relief of pain within 24 to 48 hours, and 80% did not undergo surgery after 15 months of follow‐up. There was no non‐corticosteroid control group. Subsequently, randomized placebo‐controlled trials evaluated systemic corticosteroids for radicular low back pain. In the trials, a diagnosis of radicular low back pain was based on presence of symptoms or signs of suggesting nerve root impingement at the L4, L5, or S1 level (often non‐specifically described as 'sciatica'); in most studies, imaging correlation was not performed to confirm presence of a herniated disc or nerve root impingement. Randomized trials have also evaluated the effects of systemic corticosteroids for non‐radicular low back pain and symptomatic spinal stenosis (narrowing of the spinal column). Although spinal stenosis can also cause radicular symptoms, it is often associated with neurogenic claudication, which usually manifests as leg pain that is exacerbated with standing and certain postures. Results of randomized trials of systemic corticosteroids for low back pain conditions have been inconsistent, with most individual trials reporting no or small benefits, often with imprecise estimates.
A 2017 guideline from the American College of Physicians did not recommend systemic corticosteroids, due to low‐to‐moderate quality of evidence no to small benefit versus placebo (Qaseem 2017). Prior guidelines from 2007 recommended against use of systemic corticosteroids (Chou 2007). Nonetheless, 14.5% of people with chronic low back pain who had seen a provider in the last year reported use of corticosteroids in the past 30 days (Carey 2009), and 5% of people evaluated in an emergency room for low back pain received corticosteroids (Friedman 2010). Given the ongoing use of systemic corticosteroids for people with low back pain and uncertainty regarding benefits and harms, our aim was to perform the first Cochrane Review on this topic, in order to provide an up‐to‐date synthesis of the evidence on systemic corticosteroids versus placebo for radicular low back pain (not due to spinal stenosis), non‐radicular low back pain, or symptomatic spinal stenosis.
Description of the condition
Low back pain is defined as pain or discomfort occurring between the lower posterior margin of the rib cage and the horizontal gluteal fold (Deyo 2014). The presence of radiating symptoms to the lower extremities characterizes radicular low back pain; in this condition the leg symptoms are often more severe than the back pain (Chou 2007). Symptoms include pain or paresthesias that occur in a dermatomal distribution corresponding to the affected nerve root, with or without motor deficits in the corresponding myotomal distribution. Radicular low back pain is most commonly due to a herniated disc, which occurs in about 4% to 5% of people with low back pain and often presents acutely. Other causes of radicular low back pain include spinal stenosis and foraminal stenosis, often due to degenerative conditions within the spine, which tend to present more chronically. Symptoms of spinal stenosis often occur in the bilateral lower extremities and may be associated with neurogenic claudication (development of lower extremity symptoms when the person assumes a standing position, which accentuates narrowing in the spinal canal). In this review, we use the term 'radicular pain' to refer to radicular pain not attributed to spinal stenosis; people with symptomatic spinal stenosis are addressed separately.
Non‐radicular low back pain refers to low back pain without radicular symptoms. Some people with non‐radicular low back pain have a clear pathoanatomic cause for their low back pain. However, non‐radicular back pain is most commonly non‐specific in the sense that it cannot be attributed to a specific spinal pathology or condition (Jarvik 2002). Although degenerative findings such as degenerated discs, facet joint arthropathy, and bulging discs are common in people with non‐radicular low back pain, such findings are only weakly associated with the presence and severity of low back symptoms (Chou 2011). Uncommon causes of low back pain include serious conditions such as cancer, fracture, inflammatory arthritis, and infection.
Low back pain is also often classified according to duration as acute (less than four weeks), subacute (four to 12 weeks), or chronic (longer than 12 weeks) (Chou 2007). The natural history in most people with acute radicular or non‐radicular low back pain is for there to be marked improvement in symptoms over days to weeks, whereas there is often little improvement over time in people with chronic symptoms (da C Menezes Costa 2012); subacute back pain represents an intermediate state between acute and chronic low back pain.
Description of the intervention
The term 'corticosteroids' generally refers to hormones that resemble cortisol and are associated with stronger glucocorticoid (anti‐inflammatory) effects relative to mineralocorticoid (sodium retention) effects (van der Laan 2008). Several corticosteroids are available, including prednisone, prednisolone, methylprednisolone, dexamethasone, betamethasone, and hydrocortisone. Corticosteroids vary in their relative potency, duration of action, and glucocorticoid relative to mineralocorticoid effects. Corticosteroids can be administered orally as well as parenterally (e.g. intravenously or intramuscularly). Doses and duration of corticosteroid therapy for low back pain are not well standardized and vary widely across studies; the duration ranges from a single dose to various tapering regimens in which the dose is reduced over days to weeks. Generic forms are available for all the corticosteroids listed above. Hyperglycemia is a common adverse event associated with short‐term corticosteroid use. Corticosteroids are also associated with several other adverse events that are either less common or usually only occur with longer‐term use, including infection, osteoporosis, psychiatric effects, and adrenal suppression (McDonough 2008).
How the intervention might work
The most common cause of radicular low back pain is a herniated intervertebral disc (Ropper 2015). The pathophysiology of radiculopathy from a herniated disc is related to compression of the nerve, as well as the local release of inflammatory cytokines. The effects of corticosteroids are presumed to be related to their anti‐inflammatory effects, which may help reduce swelling and related compression on the affected nerve. For non‐radicular low back pain and spinal stenosis, the rationale for use of systemic corticosteroids is less clear (Eskin 2014), although it is possible that some people may have an inflammatory component to their symptoms.
Why it is important to do this review
The use of systemic corticosteroids for low back pain remains relatively common even though it is not recommended in the most recent guideline (Qaseem 2017). Prior systematic reviews of systemic corticosteroids for radicular back pain have been performed (Roncoroni 2011; Abdel Shaheed 2019), but one review is outdated (Roncoroni 2011). In addition, the reviews focused on systemic corticosteroids for radicular pain, and did not address non‐radicular low back pain or spinal stenosis. Therefore, an up‐to‐date systemic review that addresses benefits and harms of systemic corticosteroids for radicular as well as non‐radicular low back pain and spinal stenosis is needed to better inform clinicians, patients, and policymakers making decisions regarding their use.
Objectives
To evaluate the benefits and harms of systemic corticosteroids versus placebo or no corticosteroid for radicular low back pain, non‐radicular low back pain, and symptomatic spinal stenosis in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs (e.g. alternating allocation or allocation by day of week). We excluded observational studies due to the increased potential for bias and confounding.
Types of participants
Inclusion criteria
Trials that enrolled adults aged 18 years or older with acute, subacute, or chronic radicular low back pain, non‐radicular low back pain, or spinal stenosis.
Trials that recruited people from primary care settings, specialty settings, or emergency room settings.
Exclusion criteria
Trials that focused on people with serious spinal pathology (e.g. cancer, fracture, cauda equina syndrome, inflammatory diseases) or in which such people accounted for more than 10% of participants.
Trials that focused on treatment of pregnant women or in which pregnant women accounted for more than 10% of participants.
Trials in which participants received systemic corticosteroids for the purpose of reducing postoperative pain following lumbar spine surgery. We excluded trials in which more than 10% of participants underwent spinal surgery within three months prior to enrolment.
Types of interventions
We included trials that administered any dose or regimen of systemic corticosteroids (prednisone, prednisolone, methylprednisolone, dexamethasone, betamethasone, hydrocortisone) through the oral, intravenous, or intramuscular routes with a comparison against placebo or no corticosteroid. We excluded studies that evaluated epidural, intra‐articular, or local (e.g. trigger point) administration of corticosteroids. We excluded head‐to‐head trials of steroids versus another active intervention.
Types of outcome measures
We focused on participant‐centered clinical outcomes. We did not evaluate physiologic, biomechanical (e.g. range of motion, measures of muscular strength), and laboratory parameters.
Major outcomes
Pain, measured as mean improvement from baseline.
Pain, measured as the percentage of participants with pain relief, based on improvements recorded using a visual analogue scale (VAS) or numerical rating scale (NRS).
Function, measured as mean improvement from baseline.
Function, measured as the percentage of participants with a successful functional outcome on the Oswestry Disability Index (ODI), the Roland‐Morris Disability Questionnaire (RDQ), or another scale for function/back‐specific disability.
Percentage of participants who underwent surgery.
Percentage of participants who experienced a serious adverse event.
Percentage of participants with hyperglycemia.
Minor outcomes
Quality of life, as measured by mean improvement in the 36‐item Short‐Form or another validated quality of life measure.
Percentage of participants with a successful composite outcome (e.g. improvement in pain and function).
Percentage of participants with a successful global measure of improvement.
Percentage of participants who experienced any adverse event.
Percentage of participants who withdrew because of an adverse event.
Search methods for identification of studies
Electronic searches
We searched the following databases with no language restrictions from the inception of the database:
the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, which includes the Cochrane Back and Neck Group Specialized Register);
MEDLINE OvidSP;
MEDLINE In‐Process & Other Non‐Indexed Citations via OvidSP;
Embase (OvidSP);
International Pharmaceutical Abstracts (IPA) (OvidSP);
Web of Science Core Collection.
We conducted the initial search on 14 March 2016 and update searches on MEDLINE, CENTRAL, and Embase on 7 September 2021. We searched Embase from 2015 forward to capture studies not then in CENTRAL as Embase is routinely searched for reports of trials to populate CENTRAL. We used the methods of Cochrane Back and Neck (Furlan 2015), as well as the Cochrane Handbook for Systematic Reviews of Interventions to develop our search strategies (Higgins 2021), which can be found in Appendix 1.
Searching other resources
We checked the reference lists of relevant articles for additional citations. We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch), and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) for unpublished or ongoing studies on 14 March 2016, with an update search on 7 September 2021.
Data collection and analysis
Selection of studies
Two review authors (RC and TD) independently screened citations identified by the literature search after all members of the review team pilot tested a sample of 100 citations to determine the inter‐rater reliability. Pilot testing indicated high inter‐rater reliability and the inclusion/exclusion were not modified. We resolved disagreements regarding final inclusion/exclusion by discussion and consensus or through the arbitration of a third review author (NH).
Data extraction and management
Two review authors (RC and TD) independently extracted data using a standardized data extraction form. We resolved discrepancies by discussion or through the arbitration of a third review author (NH). We extracted the following information:
study characteristics (author, year of publication, language, country, clinical setting, funding source, number randomized and analyzed, loss to follow‐up);
participant characteristics (age, sex, race, duration and severity of symptoms, use of imaging for participant selection);
intervention characteristics for the treatment (corticosteroid used, dose, duration, and co‐interventions) and placebo;
timing of follow‐up assessments, analyzed as immediate (less than two weeks since initiation of treatment), short‐term (two weeks to three months since initiation of treatment), intermediate term (greater than three months to less than 12 month), and long‐term (two months or greater); and
study results for each primary and secondary outcome at the specified time frames.
When there were multiple time points reported within a category, we extracted results closest to one week for immediate, eight weeks for short‐term, and six months for intermediate‐term follow‐up. For long‐term follow‐up, we used the longest duration of follow‐up, as well as results closest to one year. We converted daily and total corticosteroid doses to prednisone equivalents, using the calculator available at www.mdcalc.com/steroid-conversion-calculator, which is based on the following conversion ratios (Chrousos 2014; Schimmer 2011).
| Corticosteroid | Approximate equivalent dose (mg) |
| Hydrocortisone | 20 |
| Methylprednisolone | 4 |
| Prednisolone | 5 |
| Prednisone | 5 |
| Betamethasone | 0.80 |
| Dexamethasone | 0.80 |
Assessment of risk of bias in included studies
We assessed the risk of bias for each included study using the risk of bias assessment tool developed by Cochrane Back and Neck (Table 4; Table 5) (Furlan 2015). Two review authors (RC and TD) independently performed the risk of bias assessment and resolved disagreements through discussion or arbitration by a third review author (NH) when consensus could not be reached. We graded each potential source of bias as high, low, or unclear risk, and provided a quote from the study report together with a justification for our judgment in the risk of bias table. We summarized the risk of bias judgments across different studies for each of the domains listed. We presented the figures generated by the risk of bias tool to provide summary assessments of the risk of bias.
1. Sources of risk of bias.
| Bias domain | Source of bias | Possible answers |
| Selection | (1) Was the method of randomization adequate? | Yes/no/unsure |
| (2) Was the treatment allocation concealed? | Yes/no/unsure | |
| Performance | (3) Was the participant blinded to the intervention? | Yes/no/unsure |
| (4) Was the care provider blinded to the intervention? | Yes/no/unsure | |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/no/unsure |
| Attrition | (6) Was the dropout rate described and acceptable? | Yes/no/unsure |
| (7) Were all randomized participants analyzed in the group to which they were allocated? | Yes/no/unsure | |
| Reporting | (8) Were reports of the study free of suggestion of selective outcome reporting? | Yes/no/unsure |
| Selection | (9) Were the groups similar at baseline regarding the most important prognostic indicators? | Yes/no/unsure |
| Performance | (10) Were co‐interventions avoided or similar? | Yes/no/unsure |
| (11) Was the compliance acceptable in all groups? | Yes/no/unsure | |
| Detection | (12) Was the timing of the outcome assessment similar in all groups? | Yes/no/unsure |
| Other | (13) Were other sources of potential bias unlikely? | Yes/no/unsure |
2. Criteria for a judgment of 'yes' for the sources of risk of bias.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for trials with 2 groups), rolling a dice (for trials with ≥ 2 groups), drawing of balls of different colors, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, preordered sealed envelopes, sequentially ordered vials, telephone call to a central office, and preordered list of treatment assignments. Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the participants included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. |
| 3 | Index and control groups are indistinguishable for the participants or if the success of blinding was tested among the participants and it was successful. |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested among the care providers and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored 'yes' if the success of blinding was tested among the outcome assessors and it was successful or:
|
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias a 'yes' is scored (note: these percentages are arbitrary, not supported by literature). |
| 7 | All randomized participants are reported/analyzed in the group they were allocated to by randomization for the most important moments of effect measurement (minus missing values) irrespective of non‐compliance and co‐interventions. |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of the protocol, assessing that the published report includes enough information to make this judgment. |
| 9 | Groups had to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of participants with neurologic symptoms, and value of main outcome measure(s). |
| 10 | If there were no co‐interventions or they were similar between the index and control groups. |
| 11 | The review author determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number, and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered for several sessions; therefore it is necessary to assess how many sessions each participant attended. For single‐session interventions (e.g. surgery), this item is irrelevant. |
| 12 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures. |
| 13 | Other types of biases. For example:
|
Measures of treatment effect
For dichotomous outcomes, we quantified treatment effects with the risk ratio (RR) and 95% confidence interval (CI). Dichotomous outcomes for pain relief and successful functional (back pain‐specific disability), composite, or global outcomes were variably defined and reported across trials. We defined 'success' on dichotomous outcomes as any one of the following: 1. improvement of greater than 50% from baseline; 2. improvement of greater than 30% from baseline; 3. 'good' or 'excellent' results on a categorical pain rating scale, or similar; or 4. another definition for a successful outcome. For trials that reported more than one dichotomous result for an outcome, we selected one result to analyze, based on the prioritized order presented in this list. For people with radiculopathy, we analyzed improvement in leg pain; if leg pain was not reported, we analyzed back pain or overall pain. We analyzed dichotomous outcomes using Review Manager 5 (Review Manager 2020). For dichotomous outcomes, we classified the magnitude of effects based on the RR estimate as small (RR less than 1.25 or greater than 0.8), moderate (RR 1.25 to 2.00 or 0.5 to 0.8), or large (RR greater than 2.0 or less than 0.5) (Furlan 2015; Ostelo 2008).
For continuous outcomes, we quantified treatment effects by the difference in change from baseline (mean and 95% CI). We analyzed results adjusted for baseline differences (e.g. analysis of covariance or multiple regression) if available. If mean difference (MD) in change from baseline was not available, we analyzed MD in follow‐up scores. Because analyses using adjusted estimates on continuous outcomes (e.g. analysis of covariance or using estimates from multiple regression) could not be performed using Review Manager 5, we used Stata/SE 14.1 (Stata). For pain, we converted various pain intensity scales to a common 0 to 10 scale. Rescaling is accepted and common in the back pain field (Kopec 2000), and facilitates comparisons of results across trials. In addition, research has established agreement between NRS and VAS for pain intensity, enabling meta‐analysis of trials using different measures of pain intensity (Shafshak 2021). For function, given the variability in available scales, for the primary analysis we reported the standardized mean difference (SMD). Evidence indicates that common measures of function such as the RDQ and the ODI are correlated and similarly responsive to pool in meta‐analysis (Chiarotto 2016). For continuous outcomes, we defined the magnitude of effects as small (less than 10% difference on the scale or SMD of 0.2 to less than 0.5), medium (10% to 20% or 0.5 to 0.8), and large (greater than 20% or greater than 0.8), as used in other Cochrane Review from the Back and Neck group (Rubinstein 2011). We considered differences below the threshold for small to indicate no difference. We analyzed outcomes at predefined time points (immediate, short‐term, intermediate‐term, and long‐term).
Unit of analysis issues
If trials included multiple corticosteroid treatment arms (e.g. treatment arms using different corticosteroids or different doses of the same corticosteroid), we planned to combine the arms if results were similar. If results differed, we planned to analyze the treatment arms separately, dividing the placebo comparison group by the number of treatment arms to avoid double‐counting of data. However, no trial included multiple corticosteroid treatment arms.
Dealing with missing data
We contacted study authors for missing or incompletely reported data. In cases where the data were reported as the median and interquartile range and we were unable to obtain the mean and 95% CIs from the study authors, we assumed that the median was the mean and that the width of the interquartile range was equivalent to 1.35 times the 95% CI (Higgins 2021). We estimated data from graphs and figures when the information was not presented in tables or text. If information regarding standard deviations was missing and could not be obtained from study authors, we imputed the standard deviation using the mean coefficient of variation from the other trials in the same meta‐analysis. In situations in which the standard deviation for follow‐up score was not reported but standard deviations for baseline and change scores were reported, the standard deviation for follow‐up score was back calculated based on the standard deviations for the baseline and change scores, assuming the correlation between baseline and follow‐up score to be 0.5.
Assessment of heterogeneity
We assessed statistical heterogeneity based on visual inspection of the forest plot (e.g. for overlapping CIs and the direction of effect) and the I² statistic. We considered an I² greater than 75% to represent substantial heterogeneity, 40% to 75% to represent moderate heterogeneity, and less than 40% to represent low heterogeneity (Higgins 2021).
Assessment of reporting biases
We performed comprehensive literature searches, including searches of multiple electronic databases and review of reference lists, to reduce the possibility of reporting bias, and did not restrict inclusion based on publication status or language. We planned to generate funnel plots and performed the Egger test to evaluate for small‐sample effects, for meta‐analyses with at least 10 trials (Sterne 2011). However, no analysis had at least 10 trials.
Data synthesis
We combined the results from individual trials through meta‐analysis. We pooled results for each primary and secondary outcome at prespecified follow‐up durations (immediate, short, intermediate, or long‐term; see Data extraction and management). The premise for pooling was that based on application of the inclusion criteria, the trials were similar in terms of the intervention evaluated (a corticosteroid) and use of a placebo comparator. We separately pooled trials of participants with radicular pain not due to spinal stenosis, non‐radicular pain, and spinal stenosis, due to assumed variability in the underlying pathophysiology, which could impact responsiveness to corticosteroids. For radiculopathy not due to spinal stenosis, we analyzed trials that did not require imaging confirmation of herniated disc together with trials of confirmed herniated disc, because the diagnosis of radiculopathy prior to administration of systemic corticosteroids is often made without imaging confirmation in clinical practice. Presence of spinal stenosis was based on clinical factors that suggested spinal stenosis (e.g. primarily bilateral symptoms, chronic presentation in older adults, and presence of neurogenic claudication); in addition, all trials of spinal stenosis required imaging correlation. Within each low back pain category, we anticipated at least some heterogeneity because of likely differences between trials in terms of participant characteristics, settings, and interventions (e.g. specific corticosteroid used, dose, and duration); therefore, we used a random‐effects model. If statistical heterogeneity is low, results of the random‐effects model are similar to a fixed‐effect model. For trials too dissimilar to pool, we summarized the results narratively.
Subgroup analysis and investigation of heterogeneity
When there were at least three trials in a meta‐analysis, we performed subgroup analysis based on the following factors.
Risk of bias: high risk of bias versus low risk of bias; we also stratified trials according to use of a blinded versus non‐blinded design.
Dose of corticosteroid: high or low total dose based on whether it was above or below the median total dose administered for the trials in the analysis in prednisone equivalents.
Number of corticosteroid doses: single dose, multiple corticosteroid doses over less than one week, multiple corticosteroid doses over one week or longer.
Route of administration: intravenous or intramuscular versus oral.
Clinical setting: emergency room versus non‐emergency room.
Duration of symptoms: acute (less than four weeks) versus non‐acute (four weeks or longer).
For trials of radiculopathy or spinal stenosis: required imaging confirmation of herniated disc (radiculopathy) or spinal stenosis versus no imaging confirmation required.
We stratified all analyses according to whether participants had radicular pain, non‐radicular pain, or spinal stenosis, and we analyzed outcomes according to the prespecified categories for duration of follow‐up. For effects on function using a continuous measure, the primary analysis reported the SMD, and we performed subgroup analyses on the actual improvement in the RDQ, and the ODI using the original scales.
We planned to perform meta‐regression on the factors described above for meta‐analyses with at least 10 trials, but no meta‐analysis met this threshold and meta‐regression was not performed.
Sensitivity analysis
For continuous outcomes for pain and function, we based the primary analysis on MDs in improvement from baseline, adjusted for baseline differences when available. We performed a sensitivity analysis based on MDs at follow‐up, and used MDs at follow‐up if MDs from baseline were not available. For long‐term outcomes, we based the primary analysis on the longest duration of follow‐up available. We performed a sensitivity analysis based on outcomes recorded closest to one year of follow‐up. We planned to examine the impact of outlier trials on findings by performing sensitivity analyses excluding outliers, to help assess the robustness of findings. However, there were no outlier trials, in part due to the small number of trials in all analyses.
Summary of findings and assessment of the certainty of the evidence
We constructed summary of findings tables for systemic corticosteroid versus placebo or no systemic corticosteroid for the major outcomes. We created separate summary of findings tables for participants with radicular low back pain, non‐radicular low back pain, and spinal stenosis. We focused on outcomes reported at short‐term follow‐up (two weeks to three months), because this was the predefined time period with the most data available. We analyzed dichotomous and continuous outcomes as described in the Measures of treatment effect section.
Two review authors (RC and TD) independently assessed the certainty of the evidence using the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), and adapted in the updated Cochrane Back and Neck methods guidelines (Furlan 2015) (Appendix 2). For comparisons and outcomes with data, we graded the overall evidence as high, moderate, low, or very low certainty. Because the review was restricted to RCTs and quasi‐RCTs, we initially assumed the overall certainty of evidence to be high. We downgraded the evidence for factors that decreased the overall certainty, such high or unclear risk of bias, imprecision, inconsistency, indirectness, and publication bias, and gave explanations in the footnotes of the tables (Appendix 2).
The interpretation of the overall evidence grade categories (high, moderate, low, very low, or no evidence) is shown in Appendix 2.
Results
Description of studies
Results of the search
The searches retrieved 4210 unique (after deduplication) citations. After screening titles and abstracts, we retrieved the full text of 15 articles potentially meeting inclusion criteria for further review. Of these, we included 13 trials that met the inclusion criteria and excluded two studies. No studies are awaiting classification. We identified one ongoing study (Liu 2020). The flow of trials is presented in the PRISMA chart in Figure 1.
1.

Study flow diagram.
Included studies
We included 13 RCTs. Sample sizes ranged from 29 to 269 (1047 participants).
Six trials were conducted in the US (Eskin 2014; Friedman 2006; Friedman 2008; Goldberg 2015; Haimovic 1986; Holve 2008), four trials in Europe (Finckh 2006; Hedeboe 1982; Hofferberth 1982; Porsman 1979), one trial in Australia (Balakrishnamoorthy 2015), one trial in Brazil (Rodrigues 2014), and one trial in Iran (Akbari Aghdam 2020). Four trials recruited participants from emergency rooms (Balakrishnamoorthy 2015; Eskin 2014; Friedman 2006; Friedman 2008), one trial from primary care clinics (Goldberg 2015), one trial from emergency rooms or primary care clinics (Holve 2008), one trial from specialty or primary care clinics (Akbari Aghdam 2020), two trials enrolled hospital inpatients (Finckh 2006; Porsman 1979), and four trials did not report the clinical setting (Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Rodrigues 2014). Trials enrolled participants from one or two centers, with the exception of one trial that enrolled participants from three centers (Goldberg 2015), and three trials did not report the number of centers (Akbari Aghdam 2020; Haimovic 1986; Hofferberth 1982).
The duration of follow‐up from initiation of systemic corticosteroids ranged from five to seven days (Eskin 2014), to one to four years (Haimovic 1986). Seven trials followed participants for six weeks or less (Balakrishnamoorthy 2015; Eskin 2014; Finckh 2006; Friedman 2006; Friedman 2008; Hofferberth 1982; Porsman 1979), one trial followed participants for two months (Akbari Aghdam 2020), and five trials followed participants for 12 weeks or longer (Goldberg 2015; Haimovic 1986; Hedeboe 1982; Holve 2008; Rodrigues 2014). Two trials were conducted in or before 1980 (Hedeboe 1982; Porsman 1979), and five trials in or after 2000 (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2006; Friedman 2008; Goldberg 2015). Six trials did not clearly report when they were conducted; two of these were published in the 1980s (Haimovic 1986; Hofferberth 1982) and four were published in or after 2008 (Akbari Aghdam 2020; Eskin 2014; Holve 2008; Rodrigues 2014). Three trials reported funding from a government agency or research foundation (Eskin 2014; Goldberg 2015; Holve 2008), four trials reported no funding (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2008; Rodrigues 2014), and six trials did not report funding sources (Akbari Aghdam 2020; Friedman 2006; Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979). No trial reported pharmaceutical company or other industry funding. Of the trials that did not report funding sources, all except for two (Akbari Aghdam 2020; Friedman 2006) were published prior to 1990. One trial was published in German (Hofferberth 1982), and the others were published in English. Details for each included trial are presented in the Characteristics of included studies table.
Participants
Nine trials evaluated participants with radicular low back pain (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2008; Goldberg 2015; Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Holve 2008; Porsman 1979), two trials participants with non‐radicular low back pain (Eskin 2014; Friedman 2006), and two trials participants with spinal stenosis (Akbari Aghdam 2020; Rodrigues 2014). Imaging correlation was required in two trials of participants with radicular low back pain (Finckh 2006; Goldberg 2015), and in both trials of participants with spinal stenosis (Akbari Aghdam 2020; Rodrigues 2014). The other trials based diagnosis of radicular or non‐radicular low back pain on history and physical examination findings without imaging correlation.
In five trials, the duration of symptoms was acute (less than four weeks) (Eskin 2014; Finckh 2006; Friedman 2006; Friedman 2008; Holve 2008). Two trials enrolled mixed populations of participants with acute and non‐acute symptoms; in one of these trials, participants had symptoms for up to three months (mean 31 days) (Goldberg 2015), and in the other, the duration of symptoms ranged from a few days to six months (mean 8.2 weeks) (Porsman 1979). Two trials enrolled participants with chronic symptoms (mean 36 months [Hofferberth 1982] or greater than four months [Akbari Aghdam 2020]). Four trials, including one trial of participants with spinal stenosis (Rodrigues 2014), did not report the duration of symptoms (Balakrishnamoorthy 2015; Haimovic 1986; Hedeboe 1982; Rodrigues 2014).
The mean age of participants was 58 to 59 years in the trials of spinal stenosis (Akbari Aghdam 2020; Rodrigues 2014); in the other trials, the mean age ranged from 36 to 47 years. One trial did not report participant age (Haimovic 1986). In 10 trials that reported sex, the proportion of women ranged from 31% to 62% (Akbari Aghdam 2020; Balakrishnamoorthy 2015; Eskin 2014; Finckh 2006; Friedman 2006; Friedman 2008; Goldberg 2015; Hedeboe 1982; Hofferberth 1982; Holve 2008; Porsman 1979); women comprised less than 40% of the population in five trials (Eskin 2014; Hedeboe 1982; Hofferberth 1982; Holve 2008; Porsman 1979). Four trials reported race/ethnicity; in two trials, most participants were White (Eskin 2014; Goldberg 2015) and in two trials most participants were Hispanic or Latino (Friedman 2006; Friedman 2008).
Nine trials reported baseline pain (Akbari Aghdam 2020; Balakrishnamoorthy 2015; Eskin 2014; Finckh 2006; Friedman 2006; Friedman 2008; Goldberg 2015; Holve 2008; Rodrigues 2014). Standardized to a 0 to 10 scale, baseline pain scores ranged from 6.6 to 9.0. Six trials reported baseline function. Four trials reported baseline scores on the ODI of 27 to 63 (0 to 100 scale) (Akbari Aghdam 2020; Balakrishnamoorthy 2015; Finckh 2006; Goldberg 2015), and two trials reported baseline scores on the RDQ of 16 (0 to 24 scale) (Holve 2008; Rodrigues 2014).
Interventions
The systemic corticosteroid was prednisone in four trials (Eskin 2014; Goldberg 2015; Holve 2008; Rodrigues 2014), prednisolone in one trial (Akbari Aghdam 2020), methylprednisolone in three trials (Finckh 2006; Friedman 2006; Friedman 2008), and dexamethasone in five trials (Balakrishnamoorthy 2015; Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979). The route of administration was intravenous in two trials (Balakrishnamoorthy 2015; Finckh 2006), intramuscular in five trials (Friedman 2006; Friedman 2008; Hedeboe 1982; Hofferberth 1982; Porsman 1979), and oral in six trials (Akbari Aghdam 2020; Eskin 2014; Goldberg 2015; Haimovic 1986; Holve 2008; Rodrigues 2014). Systemic corticosteroids were administered as a single dose in four trials (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2006; Friedman 2008), as a five‐ to seven‐day course in five trials (Akbari Aghdam 2020; Eskin 2014; Haimovic 1986; Hedeboe 1982; Porsman 1979), and as a nine‐ to 21‐day course in four trials (Goldberg 2015; Hofferberth 1982; Holve 2008; Rodrigues 2014). The corticosteroid dose on the first day ranged from 10 mg to 400 mg prednisone equivalents, with the total corticosteroid dose ranging from 50 mg to 1050 mg prednisone equivalents. One trial used weight‐based dosing (prednisone 1 mg/kg/day for one week, decreasing by one third each week for three weeks) (Rodrigues 2014).
Outcomes
Major outcomes
Nine trials reported pain as a continuous outcome (Akbari Aghdam 2020; Balakrishnamoorthy 2015; Eskin 2014; Finckh 2006; Friedman 2006; Friedman 2008; Goldberg 2015; Holve 2008; Rodrigues 2014). Pain scales were a 0 to 10 VAS (Balakrishnamoorthy 2015; Rodrigues 2014) or NRS (Akbari Aghdam 2020; Friedman 2006; Friedman 2008; Goldberg 2015), 0 to 100 VAS (Finckh 2006), 0 to 5 verbal rating scale (RDQ Pain) (Holve 2008), or 0 to 3 verbal rating scale (Eskin 2014).
Six trials reported pain as a dichotomous outcome: proportion with no or mild pain (Eskin 2014), pain score decreased 20 or greater on a 0 to 100 scale (Finckh 2006), no back pain in previous 24 hours (Friedman 2006; Friedman 2008), improvement of 5 points or greater on a 0 to 10 scale (Goldberg 2015), or improvement in pain (Haimovic 1986).
Eight trials reported function as a continuous outcome (Akbari Aghdam 2020; Balakrishnamoorthy 2015; Finckh 2006; Friedman 2006; Friedman 2008; Goldberg 2015; Holve 2008; Rodrigues 2014). Function was measured using the ODI (scored 0 to 100) in four trials (Akbari Aghdam 2020; Balakrishnamoorthy 2015; Finckh 2006; Goldberg 2015), the RDQ (scored 0 to 24) in two trials (Holve 2008; Rodrigues 2014), and a modified (18 item) Roland Morris Disability Questionnaire (RDQ‐18, scored 0 to 24) in two trials (Friedman 2006; Friedman 2008). For methods of analyzing continuous outcomes, two trials reported adjusted differences in change from baseline (Balakrishnamoorthy 2015; Goldberg 2015) and four trials reported or enables calculation of unadjusted differences in change from baseline (Akbari Aghdam 2020; Finckh 2006; Friedman 2006; Friedman 2008). Three trials only reported differences in means at follow‐up (Eskin 2014; Holve 2008; Rodrigues 2014).
Four trials reported function as a dichotomous outcome (able to resume normal activities [Balakrishnamoorthy 2015; Eskin 2014], RDQ‐18 score of 0 [Friedman 2006; Friedman 2008], or improvement greater than 50% on the ODI [Goldberg 2015]). Seven trials reported one or more outcomes at immediate‐term follow‐up (Balakrishnamoorthy 2015; Eskin 2014; Finckh 2006; Friedman 2006; Friedman 2008; Haimovic 1986; Holve 2008), eight at short‐term follow‐up (Akbari Aghdam 2020; Balakrishnamoorthy 2015; Finckh 2006; Friedman 2006; Friedman 2008; Goldberg 2015; Holve 2008; Rodrigues 2014), one at intermediate‐term follow‐up (Holve 2008), and two at long‐term follow‐up (Goldberg 2015; Haimovic 1986).
Two trials reported risk of serious adverse events (Balakrishnamoorthy 2015; Goldberg 2015), and two trials reported no serious adverse events in any group (Friedman 2006; Friedman 2008).
Two trials reported risk of hyperglycemia (transient hyperglycemia [Finckh 2006] or blood sugar increase 50% or greater [Hofferberth 1982]) and two trials reported no hyperglycemia in any group (Friedman 2006; Friedman 2008).
Minor outcomes
Three trials reported global improvement ("clear improvement [Hedeboe 1982]," "full symptom relief or greatly improved" [Hofferberth 1982], or positive "effect" [not otherwise defined] [Porsman 1979]). Three trials reported risk of subsequent surgery within 30 days (Finckh 2006), within 52 weeks (Goldberg 2015), or within an unspecified duration (Porsman 1979).
Five trials reported risk of any adverse event (Balakrishnamoorthy 2015; Friedman 2006; Friedman 2008; Goldberg 2015; Hedeboe 1982), and one trial reported no "significant" adverse effects in any group (Eskin 2014).
Three trials reported risk of withdrawal due to adverse events (Goldberg 2015; Hedeboe 1982; Porsman 1979).
Excluded studies
We excluded two RCTs because they compared systemic corticosteroids against pregabalin or gabapentin, with no placebo arm (Ko 2016; Oros 2019). See Characteristics of excluded studies table.
Ongoing studies
We identified one ongoing study (Liu 2020). See Characteristics of ongoing studies table.
Risk of bias in included studies
The risk of bias assessments are summarized in Figure 2 and Figure 3 and described in more detail below. Five trials met criteria for overall low risk of bias: four trials had low risk of bias in all domains (Eskin 2014; Friedman 2006; Friedman 2008; Goldberg 2015) and one trial had low risk of bias in all domains except for allocation concealment and attrition bias (Akbari Aghdam 2020). Three trials were at high risk of bias overall: one trial had high attrition (38%) and between‐group baseline differences (Balakrishnamoorthy 2015), one trial had very high attrition (66%) at 30 days (Finckh 2006), and one trial was quasi‐randomized (sequential allocation) (Holve 2008). Five trials were at unclear risk of bias overall because they reported inadequate information to assess multiple risk of bias domains (Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979; Rodrigues 2014).
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
Seven trials had low risk of selection bias based on randomization and allocation concealment methods (Balakrishnamoorthy 2015; Eskin 2014; Finckh 2006; Friedman 2006; Friedman 2008; Goldberg 2015; Rodrigues 2014). One trial had high risk of selection bias due to inadequate randomization and allocation concealment methods (sequential allocation) (Holve 2008). Three trials had unclear randomization methods (Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979) and five trials had unclear allocation concealment methods (Akbari Aghdam 2020; Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979).
Blinding
Participants were blinded in all trials and care providers were blinded in all trials except for one (Rodrigues 2014), in which blinding of care providers was unclear. Hence, there is low risk of performance bias and low risk of detection bias for self‐reported outcomes.
Two trials had unclear risk of detection bias for assessor‐reported outcomes as it was unclear whether outcome assessors were blinded or not (Hedeboe 1982; Porsman 1979). The remaining trials were at low risk of detection bias.
Incomplete outcome data
Two trials reported high attrition at short‐term follow‐up (38% lost to follow‐up at six weeks [Balakrishnamoorthy 2015] and 66% lost to follow‐up at 30 days [Finckh 2006]) and were at high risk of attrition bias. One trial was at unclear risk of attrition bias (Hedeboe 1982). All other trials had low risk of attrition bias.
Selective reporting
Six trials had unclear risk of selective reporting because primary outcomes were not clearly specified (Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Holve 2008; Porsman 1979; Rodrigues 2014). The other trials had low risk of reporting bias.
Other potential sources of bias
One trial reported a difference in baseline pain scores (8.1 [95% CI 7.4 to 8.8] for corticosteroid versus 7.0 [95% CI 6.2 to 7.8] for placebo on a 0 to 10 scale) (Balakrishnamoorthy 2015) and four trials reported insufficient data to assess baseline differences (Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979).
In four trials, it was unclear if co‐interventions were avoided or similar (Haimovic 1986; Hofferberth 1982; Holve 2008; Rodrigues 2014).
In four trials, compliance was unclear (Hedeboe 1982; Hofferberth 1982; Porsman 1979; Rodrigues 2014). As previously noted, no trial reported industry funding, though six trials did not report funding sources (Akbari Aghdam 2020; Friedman 2006; Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979).
One trial excluded participants who discontinued treatment or had protocol violations (Porsman 1979), the method of analysis was unclear for one trial (Haimovic 1986), and one trial excluded participants who had drug complications (Akbari Aghdam 2020), but we did not downgrade for failure to perform intention‐to‐treat analysis because this impacted only 3/52 participants in Porsman 1979 and 7/93 participants in Akbari Aghdam 2020. All other trials conducted intention‐to‐treat analysis.
In one trial, timing of long‐term follow‐up was unclear and may have varied between groups (Haimovic 1986). In all other trials, timing of outcome assessment was similar between groups.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Systemic corticosteroids compared with placebo for radicular low back pain (not due to spinal stenosis).
| Systemic corticosteroids compared with placebo for radicular low back pain (not due to spinal stenosis) | ||||||
|
Patient or population: people with radicular low back pain (not due to spinal stenosis) Settings: primary care, emergency room, or inpatient Intervention: systemic corticosteroid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no systemic corticosteroid | Systemic corticosteroid | |||||
|
Pain (continuous) Standardized to 0–10 scale Follow‐up: short‐term (2 weeks to < 3 months) |
The mean pain score in the placebo groups ranged from 2.0 to 4.32a | The mean pain score in the intervention groups was 0.56 points better (1.08 better to 0.04 better) | Not applicable | 430 (5) | ⊕⊕⊕⊝ Moderateb | Systemic corticosteroids probably slightly decrease pain at short‐term follow‐up. |
|
Pain (dichotomous) Pain improved (definitions varied)c Follow‐up: short‐term (2 weeks to < 3 months) |
302/1000 | 365/1000 (266 to 501) | RR 1.21 (0.88 to 1.66) | 345 (2) | ⊕⊕⊕⊝ Moderated | Systemic corticosteroids may slightly increase the likelihood of experiencing improvement in pain at short‐term follow‐up. Absolute effect 5% better (5% worse to 15% better); relative effect 21% better (12% worse to 66% better). |
|
Function (continuous) SMD (scales varied) Follow‐up: short‐term (2 weeks to < 3 months) |
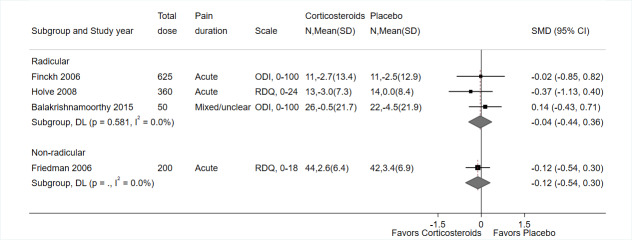
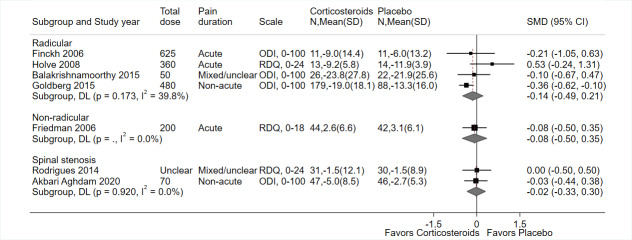
In the placebo groups, the mean score on the ODI (scale 0 to 100) ranged from 20.7 to 41.1 (3 trials) and the mean score on the RDQ was 4.1 (1 trial) | The SMD for function in the intervention groups was 0.14 better (range 0.49 better to 0.21 worse); the MD ranged from 2.90 better to 6.40 better on the ODI (3 trials) and 2.70 points worse on the RDQ (1 trial) | Not applicable | 364 (4) | ⊕⊕⊝⊝ Lowd,e | Systemic corticosteroids may not improve function at short‐term follow‐up. |
|
Function (dichotomous) Function improved (definitions varied)f Follow‐up: short‐term (2 weeks to < 3 months) |
342/1000 | 520/1000 (417 to 653) | RR 1.52 (1.22 to 1.91) | 403 (3) | ⊕⊕⊕⊝ Moderateg | Systemic corticosteroids probably increase the likelihood of experiencing improvement in function at short‐term follow‐up. Absolute effect 19% better (8% better to 30% better); relative effect 52% better (22% better to 91% better). |
|
Participants who underwent surgery Underwent surgery Follow‐up: varied (within 30 days, within 90 days, within 6 months, within 52 weeks, or within an unspecified time frame) |
171/1000 | 171/1000 (116 to 251) | RR 1.00 (0.68 to 1.47) | 442 (5) | ⊕⊕⊕⊝ Moderated | Systemic corticosteroids probably have no effect on the likelihood of subsequent surgery. |
|
Participants who experienced a serious adverse event Adverse event requiring hospitalization or resulting in death Follow‐up: long‐term (≥ 12 months) |
23/1000 | 17/1000 (3 to 100) | RR 0.74 (0.13 to 4.33) | 267 (2) | ⊕⊝⊝⊝ Very lowd,h,i,j | We are uncertain whether systemic corticosteroids have an effect on the likelihood of experiencing serious adverse events. |
|
Participants with hyperglycemia "Transient hyperglycemia" or blood sugar increase ≥ 50 mg/dL Follow‐up: immediate‐term (< 2 months), based on duration of treatment |
232/1000 | 248/1000 (109 to 573) | RR 1.07 (0.47 to 2.47) | 151 (2) | ⊕⊕⊝⊝ Lowd,k | Systemic corticosteroids (administered as a single large dose or as a short course) may not increase the likelihood of experiencing hyperglycemia. Absolute effect 5% worse (14% worse to 4% better); relative effect 7% worse (53% better to 147% worse). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LBP: low back pain; MD: mean difference; ODI: Oswestry Disability Index; RDQ: Roland Morris Disability Questionnaire; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aBased on the mean pain score reported at follow‐up in the placebo groups in the trials. bDowngraded one level for risk of bias (three of five trials were at high risk of bias). cDefinitions were no back pain in previous 24 hours (Friedman 2008), or improvement in pain score of 5 points or greater on a 0 to 10 scale (Goldberg 2015). dDowngraded one level due to imprecision (confidence interval for pooled estimate crossed zero for continuous outcomes or crossed one for dichotomous outcomes). eDowngraded one level for risk of bias (three of four trials were at high risk of bias). fDefinitions were able to resume normal activity (Balakrishnamoorthy 2015), RDQ‐18 = 0 (Friedman 2008), or improvement in pain score of 5 points or greater on a 0 to 10 scale (Goldberg 2015). gDowngraded one level due to indirectness (variability in definitions of functional improvement). hDowngraded one level due to risk of bias (all trials had unclear risk of bias). iDowngraded one level for inconsistency (I² = 43%). jDowngraded one level for indirectness (poorly defined and variable definitions). kDowngraded one level due to risk of bias (one trial had unclear risk of bias and the other trial had high risk of bias).
Summary of findings 2. Systemic corticosteroids compared with placebo for non‐radicular low back pain.
| Systemic corticosteroids compared with placebo for non‐radicular low back pain | ||||||
|
Patient or population: people with non‐radicular low back pain Settings: primary care, emergency room, or inpatient Intervention: systemic corticosteroid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no systemic corticosteroid | Systemic corticosteroid | |||||
|
Pain (continuous) Standardized to 0–10 scale Follow‐up: short‐term (2 weeks to < 3 months) |
The mean pain score in the placebo group was 2.3a | The mean pain score in the intervention group was 0.60 point worse (2.20 worse to 1.00 better) | Not applicable | 86 (1) | ⊕⊕⊝⊝ Lowb,c | Systemic corticosteroids may slightly increase pain at short‐term follow‐up. |
|
Pain (dichotomous) No pain in last 24 hours Follow‐up: short‐term (2 weeks to < 3 months) |
571/1000 | 542/1000 (377 to 794) | RR 0.95 (0.66 to 1.39) | 86 (1) | ⊕⊕⊝⊝ Lowb,c | Systemic corticosteroids may have no effect on the likelihood of experiencing improvement in pain at short‐term follow‐up. Absolute effect 3% worse (24% worse to 18% better); relative effect 5% worse (34% worse to 39% better). |
|
Function (continuous) RDQ‐18 (scale 0–18) Follow‐up: short‐term (2 weeks to < 3 months) |
The mean RDQ‐18 score in the placebo group was 3.1a | The mean RDQ‐18 score in the intervention group was −0.50 point better (−3.19 better to 2.19 worse) | Not applicable | 86 (1) | ⊕⊕⊝⊝ Lowb,c | Systemic corticosteroids may slightly improve function at short‐term follow‐up. |
|
Function (dichotomous) RDQ‐18 = 0 Follow‐up: short‐term (2 weeks to < 3 months) |
738/1000 | 775/1000 (605 to 982) | RR 1.05 (0.82 to 1.33) | 86 (1) | ⊕⊕⊝⊝ Lowb,c | Systemic corticosteroids may have no effect on the likelihood of experiencing improvement in function at short‐term follow‐up. Absolute effect 3% better (15% worse to 22% better); relative effect 5% better (18% worse to 33% better). |
| Participants who underwent surgery | — | — | — | — | — | Not reported. |
| Participants who experienced a serious adverse event | — | — | — | — | — | Serious adverse events and hyperglycemia were not reported. |
|
Participants who experienced any adverse event Adverse event regardless of severity Follow‐up: short term (2 weeks to < 3 months) |
452/1000 | 203/1000 (104 to 398) | RR 0.45 (0.23 to 0.88) | 86 (1) | ⊕⊕⊝⊝ Lowd | Systemic corticosteroids may reduce the likelihood of experiencing any adverse event. Absolute effect 25% better (6% to 44% better); relative effect 55% better (12% to 77% better) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RDQ‐18: 18‐item Roland‐Morris Disability Questionnaire; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aBased on the mean pain score reported at follow‐up in the placebo groups in the trials. bDowngraded one level due to imprecision. cDowngraded one level due to inconsistency (unable to assess inconsistency due to only one trial). dDefinitions were able to resume normal activities or RDQ‐18 = 0.
Summary of findings 3. Systemic corticosteroids compared with placebo for spinal stenosis.
| Systemic corticosteroids compared with placebo for spinal stenosis | ||||||
|
Patient or population: people with spinal stenosis Settings: primary care, emergency room, or inpatient Intervention: systemic corticosteroid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no systemic corticosteroid | Systemic corticosteroid | |||||
|
Pain (continuous) Standardized to 0–10 scale Follow‐up: short‐term (2 weeks to < 3 months) |
The mean pain scores in the placebo groups (2 trials) were 5.87 and 5.97a | The mean pain score in the intervention group was 0.20 point better (1.91 better to 1.50 worse) | Not applicable | 154 (2) | ⊕⊕⊕⊝
Moderateb |
Systemic corticosteroids probably have no effect on pain at short‐term follow‐up. |
|
Function (continuous) RDQ‐18 (scale 0–18) Follow‐up: short‐term (2 weeks to < 3 months) |
In the placebo groups, the mean score on the ODI (scale 0 to 100) was 42.9 (1 trial) and the mean score on the RDQ (scale 0–24) was 13.80 (1 trial) a | The SMD for function in the intervention groups was −0.02 better (range 0.33 better to 0.30 worse); the MD ranged was 2.28 points better on the ODI (1 trial) and 0.02 worse on the RDQ (1 trial) | Not applicable | 154 (2) | ⊕⊕⊕⊝ Moderateb | Systemic corticosteroids probably have no effect on function at short‐term follow‐up. |
| Participants who experienced any adverse event | — | — | — | — | — | No trials |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; ODI: Oswestry Disability Index; RDQ: Roland‐Morris Disability Questionnaire; RDQ‐18: 18‐item Roland‐Morris Disability Questionnaire; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aBased on the mean pain score reported at follow‐up in the placebo groups in the trials. bDowngraded one level due to imprecision.
We compared systemic corticosteroids to placebo. Results were analyzed separately for radicular low back pain, non‐radicular low back pain, and spinal stenosis. We found no trials comparing corticosteroid to no corticosteroid.
Radicular low back pain (not due to spinal stenosis)
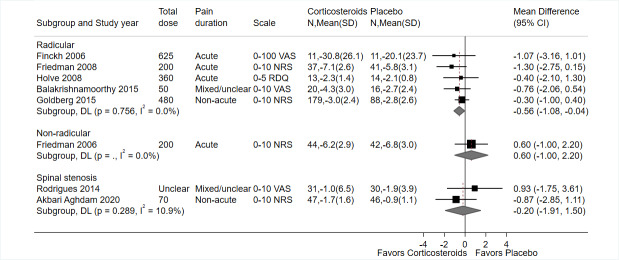
Nine trials evaluated 727 participants with radicular low back pain (not due to spinal stenosis) (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2008; Goldberg 2015; Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Holve 2008; Porsman 1979). Two trials were at low risk of bias overall (both had low risk of bias in all domains) (Friedman 2008; Goldberg 2015), three trials were at high risk of bias overall (Balakrishnamoorthy 2015; Finckh 2006; Holve 2008), and four trials had unclear risk of bias due to insufficient information in multiple domains (Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979). See Table 1.
Three trials evaluated participants with acute symptoms (Finckh 2006; Friedman 2008; Holve 2008), two trials evaluated participants with mixed acute and non‐acute symptoms (Goldberg 2015; Porsman 1979), one trial evaluated participants with non‐acute symptoms (Hofferberth 1982), and in three trials the duration of symptoms was unclear (Balakrishnamoorthy 2015; Haimovic 1986; Hedeboe 1982).
Imaging correlation of herniated disc and symptoms was required in two trials (Finckh 2006; Goldberg 2015).
Three trials administered systemic corticosteroids as a single dose (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2008), three trials as a seven‐day course (Haimovic 1986; Hedeboe 1982; Porsman 1979), and three trials as a nine‐ to 15‐day course (Goldberg 2015; Hofferberth 1982; Holve 2008). The total corticosteroid dose in prednisone equivalents ranged from 50 mg (Balakrishnamoorthy 2015) to 1050 mg (Hofferberth 1982); the dose on the first day ranged from 50 mg (Balakrishnamoorthy 2015) to 625 mg (Finckh 2006).
Major outcomes
Pain (continuous)
Five trials reported pain as a continuous outcome at one or more of the prespecified time points (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2008; Goldberg 2015; Holve 2008). Across time points, data were available from one to five trials. Evidence was strongest for short‐term pain, which was based on data from five trials, two of which had low risk of bias in all domains (Friedman 2008; Goldberg 2015).
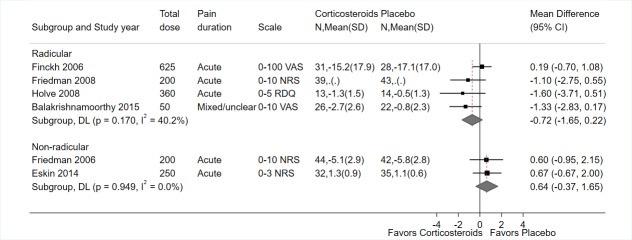
Systemic corticosteroids were associated with similar, small decreases in pain intensity versus placebo at immediate‐term (MD −0.72 on a 0 to 10 scale, 95% CI −1.65 to 0.22; I² = 40%; 4 trials, 212 participants; Figure 4; Balakrishnamoorthy 2015; Finckh 2006; Friedman 2008; Holve 2008), short‐term (MD −0.56, 95% CI −1.08 to −0.04 on a 0 to 10 scale; I² = 0%; 5 trials, 430 participants; Figure 5; Balakrishnamoorthy 2015; Friedman 2008; Goldberg 2015; Finckh 2006; Holve 2008), and long‐term (one‐year) follow‐up (MD −0.60, 95% CI −1.35 to 0.15 on a 0 to 10 scale; 1 trial, 234 participants; Goldberg 2015), but estimates at immediate‐ and long‐term were imprecise (CIs crossed 0). At intermediate‐term, one trial (27 participants) found systemic corticosteroids associated with greater change from baseline than placebo (−3.4 with corticosteroids versus −1.5 with placebo on the 0 to 5 RDQ Pain score), but CIs or statistical significance were not reported and could not be calculated (Holve 2008).
4.
Corticosteroid versus placebo, immediate‐term (less than two weeks) pain (continuous).
5.
Corticosteroid versus placebo, short‐term (two weeks to three months) pain (continuous).
Statistical heterogeneity was moderate for immediate‐term pain. There were no subgroup differences in effects on immediate‐term pain based on study setting, total or initial corticosteroid dose, route of corticosteroid administration, corticosteroid dosing regimen, overall risk of bias, duration of pain, or whether imaging correlation was required. However, stratified analyses were limited by the small number of trials. There was no statistical heterogeneity in the pooled estimate for short‐term pain.
Pain (dichotomous)
Four trials reported pain as a dichotomous outcome at one or more of the prespecified time points (Finckh 2006; Friedman 2008; Goldberg 2015; Haimovic 1986). The trials varied in their definitions of positive pain outcomes as improvement in pain score of 20 mm or greater on a 0 to 100 scale (Finckh 2006), no back pain in the last 24 hours (Friedman 2008), improvement in pain score of 5 points or greater on a 0 to 10 scale (Goldberg 2015), and improvement in pain (not otherwise defined) (Haimovic 1986).
Estimates were imprecise for systemic corticosteroids versus placebo and the likelihood of a positive pain outcome at any follow‐up, though the estimate favored systemic corticosteroids at immediate‐, short‐, and intermediate term (immediate‐term: RR 1.46, 95% CI 0.83 to 2.59; I² = 0%; 2 trials, 93 participants; Analysis 1.1; Figure 6; Finckh 2006; Haimovic 1986; short‐term: RR 1.21, 95% CI 0.88 to 1.66; I² = 0%; 2 trials, 345 participants; Analysis 1.2; Figure 7; Friedman 2008; Goldberg 2015); intermediate‐term: RR 0.86, 95% CI 0.30 to 2.44; 1 trial, 33 participants; Analysis 1.3; Figure 8; Haimovic 1986; long‐term follow‐up: RR 0.97, 95% CI 0.56 to 1.68; I² = 57%; 2 trials, 300 participants; Analysis 1.4; Figure 9; Goldberg 2015; Haimovic 1986). At each time point, data were available from only one or two trials. Evidence was strongest at short‐term, as the estimate was based on two trials with low risk of bias in all domains (Friedman 2008; Goldberg 2015). Statistical heterogeneity was moderate at long‐term follow‐up, based on two trials.
1.1. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 1: Pain (dichotomous), immediate (< 2 weeks) – by type of pain
6.

Corticosteroid versus placebo, immediate‐term (less than two weeks) pain (dichotomous).
1.2. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 2: Pain (dichotomous), short‐term (2 weeks to 3 months) – by type of pain
7.

Corticosteroid versus placebo, short‐term (two weeks to three months) pain (dichotomous).
1.3. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 3: Pain (dichotomous), intermediate (> 3 to < 12 months)
8.

Corticosteroid versus placebo, intermediate‐term (greater than three to less than 12 months) pain (dichotomous).
1.4. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 4: Pain (dichotomous), long‐term (≥ 12 months)
9.

Corticosteroid versus placebo, long‐term (12 months or greater) pain (dichotomous).
Function (continuous)
Five trials reported function as a continuous outcome at one or more of the prespecified time points (Balakrishnamoorthy 2015; Finckh 2006; Friedman 2008; Goldberg 2015; Holve 2008). Three trials reported function using the ODI (0 to 100 scale), one trial the RDQ (0 to 24 scale), and one trial the RDQ‐18 (0 to 18 scale). Two trials had low risk of bias in all domains (Friedman 2008; Goldberg 2015).
There was no evidence of a difference between systemic corticosteroids and placebo in function at immediate‐term follow‐up (SMD −0.04, 95% CI −0.44 to 0.36; I² = 0%; 3 trials, 97 participants; Figure 10; Balakrishnamoorthy 2015; Finckh 2006; Holve 2008). Two trials measured function with the ODI (differences 3.00 and −0.26 on a 0 to 100 scale) (Balakrishnamoorthy 2015; Finckh 2006), and one trial used the RDQ (difference −3.00, 95% CI −8.95 to 2.95 on a 0 to 24 scale) (Holve 2008).
10.
Corticosteroid versus placebo, immediate‐term (less than two weeks) function (continuous).
There was no evidence of a difference between systemic corticosteroids and placebo at short‐term follow‐up (SMD −0.14, 95% CI −0.49 to 0.21; 4 trials, 364 participants; Figure 11; Balakrishnamoorthy 2015; Finckh 2006; Goldberg 2015; Holve 2008). There was statistical heterogeneity (I² = 40%). In three trials that evaluated function using the ODI, including one trial with low risk of bias in all domains that required imaging correlation (Goldberg 2015), the difference favored corticosteroids (SMD −5.77, 95% CI −9.83 to −1.70, on a 0 to 100 scale; I² = 0%; Balakrishnamoorthy 2015; Finckh 2006; Goldberg 2015) and in one trial that used the RDQ, there was no evidence of a difference (SMD 2.70, 95% CI −1.05 to 6.45 on a 0 to 24 scale; Holve 2008). One other trial that could not be pooled found no difference between systemic corticosteroids and placebo in median RDQ‐18 scores at short‐term follow‐up (0 in both groups; Friedman 2008).
11.
Corticosteroid versus placebo, short‐term (two weeks to three months) function (continuous).
At intermediate‐term, one trial found systemic corticosteroids and placebo associated with similar change from baseline on the RDQ (mean: 15 with corticosteroids versus 14 with placebo, on a 0 to 24 scale); the statistical significance of the findings were not reported and could not be calculated (Holve 2008).
At long‐term (one year) follow‐up, one trial with low risk of bias in all domains found systemic corticosteroids were associated with greater improvement in the ODI than placebo (difference −7.40, 95% CI −12.55 to −2.25, on a 0 to 100 scale; Goldberg 2015). The magnitude of the difference was small.
Function (dichotomous)
Three trials evaluated function as a dichotomous outcome (Balakrishnamoorthy 2015; Friedman 2008; Goldberg 2015). The trials varied in their definitions of improvement in function as return to normal activities (Balakrishnamoorthy 2015), RDQ‐18 score=0 (indicating no disability) (Friedman 2008), and improvement in ODI of greater than 50% (Goldberg 2015). Two of the trials had low risk of bias in all domains (Friedman 2008; Goldberg 2015).
At immediate‐term follow‐up, there was a greater likelihood of having no disability with corticosteroids versus placebo based on one trial with low risk of bias in all domains, though the estimate was imprecise (defined as an RDQ‐18 score of 0; RR 1.49, 95% CI 0.95 to 2.35; 82 participants; Analysis 1.5; Figure 12; Friedman 2008). At short‐term follow‐up, the estimate for likelihood of a positive functional outcome was similar, but more precise (CIs did not cross 0), based on three trials (RR 1.52, 95% CI 1.22 to 1.91; I² = 0%; Analysis 1.6; Figure 13; Balakrishnamoorthy 2015; Friedman 2008; Goldberg 2015). One trial with low risk of bias in all domains found systemic corticosteroids associated with greater likelihood of experiencing greater than 50% reduction in the ODI at one year than placebo (RR 1.29, 95% CI 1.06 to 1.56; 267 participants; Analysis 1.14; Figure 14; Goldberg 2015).
1.5. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 5: Function (dichotomous), immediate (< 2 weeks) – by type of pain
12.

Corticosteroid versus placebo, immediate‐term (less than two weeks) function (dichotomous).
1.6. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 6: Function (dichotomous), short‐term (2 weeks to 3 months) – by type of pain
13.

Corticosteroid versus placebo, short‐term (two weeks to three months) function (dichotomous).
1.14. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 14: Function (dichotomous), long‐term (≥12 months)
14.

Corticosteroid versus placebo, long‐term (12 months or greater) function (dichotomous).
No trial evaluated the likelihood of a positive functional outcome at intermediate‐term follow‐up.
Participants who underwent surgery
Five trials evaluated the likelihood for participants to undergo subsequent surgery within 30 days (Finckh 2006), within 90 days (Hedeboe 1982), within six months (Holve 2008), within 52 weeks (Goldberg 2015), or within an unspecified time frame (Porsman 1979). There was no evidence of a difference between systemic corticosteroids and placebo in the likelihood of subsequent surgery (RR 1.00, 95% CI 0.68 to 1.47; I² = 0%; 5 trials, 442 participants; Analysis 1.15; Figure 15; Finckh 2006; Goldberg 2015; Hedeboe 1982; Holve 2008; Porsman 1979).
1.15. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 15: Surgery (radicular)
15.

Corticosteroid versus placebo, subsequent surgery.
Participants who experienced a serious adverse event
One trial reported few serious adverse events, with no difference between systemic corticosteroids and placebo (RR 0.74, 95% CI 0.13 to 4.33; 267 participants; Goldberg 2015), and one other trial reported no serious adverse events in either group (58 participants; Balakrishnamoorthy 2015).
Participants with hyperglycemia
Two trials found no evidence of a difference between systemic corticosteroids and placebo in risk of hyperglycemia, defined as "transient hyperglycemia" (Finckh 2006) or blood sugar increase 50 mg/dL or greater (Hofferberth 1982) (RR 1.07, 95% CI 0.47 to 2.47; I² = 8%; 151 participants; Analysis 1.22; Figure 16). One other trial reported no cases of hyperglycemia in either group (78 participants; Friedman 2008). For all harms, the number of trials was too small and estimates too imprecise to assess the effects of corticosteroid dose, duration of treatment, or route of administration on risk of adverse events.
1.22. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 22: Hyperglycemia – by type of pain
16.

Corticosteroid versus placebo, hyperglycemia.
Minor outcomes
Quality of life
One study with low risk of bias in all domains found systemic corticosteroids were associated with slightly improved SF‐36 Physical and Mental Component scores versus placebo at short‐term (three‐week) and long‐term (one‐year) follow‐up (MD 2.2 to 3.6 points on a 0 to 100 scale; 267 participants), but differences were below the threshold for small magnitude and imprecise except for the Physical Component score at three weeks and the Mental Component score at one year (Goldberg 2015).
Participants with a successful composite outcome
None of the trials reported percentage of participants with radicular pain with a successful composite outcome.
Participants with a successful global measure of improvement
Three trials evaluated the likelihood for participants to experience global improvement. This was defined as "clear improvement," "full symptom relief or greatly improved," or "effect" (not otherwise defined). At immediate‐term, there was no evidence of a difference between systemic corticosteroids and placebo in likelihood of experiencing global improvement (RR 1.26, 95% CI 0.88 to 1.81; I² = 43%; 179 participants; Analysis 1.25; Figure 17; Hedeboe 1982; Hofferberth 1982; Porsman 1979). One of these trials also found no difference in likelihood of clear improvement at three months (RR 1.26, 95% CI 0.46 to 3.46; Hedeboe 1982). All the trials reporting global improvement had unclear risk of bias in multiple domains.
1.25. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 25: Global improvement, immediate (< 2 weeks)
17.

Corticosteroid versus placebo, immediate‐term (less than two weeks) global improvement.
Participants who experienced any adverse event
There was no evidence of a difference between systemic corticosteroids and placebo in risk of any adverse event (RR 1.19, 95% CI 0.89 to 1.59; I² = 13%; 4 trials, 442 participants; Analysis 1.28; Figure 18; Balakrishnamoorthy 2015; Friedman 2008; Goldberg 2015; Hedeboe 1982).
1.28. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 28: Any adverse event – by type of pain
18.

Corticosteroid versus placebo, any adverse event.
Participants who withdrew because of an adverse event
Two trials reported no evidence of a difference between systemic corticosteroids and placebo in risk of withdrawal due to adverse events, but the estimate was extremely imprecise (RR 0.69, 95% CI 0.10 to 4.79; I² = 0%; 316 participants; Analysis 1.42; Figure 19; Goldberg 2015; Porsman 1979). One other trial reported no withdrawals due to adverse events in either group (39 participants; Hedeboe 1982).
1.42. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 42: Withdrawal due to adverse events
19.

Corticosteroid versus placebo, withdrawal due to adverse events.
Non‐radicular low back pain
Two trials evaluated 153 participants with acute non‐radicular low back pain (Eskin 2014; Friedman 2006). Both were at low risk of bias overall. One trial administered prednisone 50 mg per day for five days orally (total dose 250 mg) (Eskin 2014), and the other trial administered one dose of methylprednisolone 160 mg intramuscularly (prednisone equivalents 200 mg) (Friedman 2006). Both trials evaluated outcomes at immediate‐ and short‐term. See Table 2.
Major outcomes
Pain (continuous)
There was no evidence of a difference between systemic corticosteroids and placebo at immediate‐term or short‐term follow‐up (immediate term: MD 0.64, 95% CI −0.37 to 1.65, on a 0 to 10 scale; I² = 0%; 2 trials, 153 participants; Figure 4; Eskin 2014; Friedman 2006; short‐term: MD 0.60, 95% −1.00 to 2.20, on a 0 to 10 scale; 1 trial, 86 participants; Figure 5; Friedman 2006).
Pain (dichotomous)
There was no evidence of a difference between systemic corticosteroids and placebo in likelihood of a positive pain outcome (defined as having no or mild pain or having no back pain in the last 24 hours) at immediate‐term or short‐term follow‐up (immediate‐term: RR 0.82, 95% CI 0.61 to 1.09; I² = 0%; 2 trials, 165 participants; Analysis 1.1; Figure 6 Eskin 2014; Friedman 2006; short‐term: RR 0.95, 95% CI 0.66 to 1.39; 1 trial, 86 participants; Analysis 1.2; Figure 7; Friedman 2006).
Function (continuous)
One trial (86 participants) found no evidence of a difference between systemic corticosteroids and placebo in function at immediate‐term or short‐term follow‐up as measured by the RDQ‐18 (scale 0 to 18) (immediate‐term: MD −0.12, 95% CI −0.54 to 0.30; 86 participants; Figure 10; short‐term: MD −0.08, 95% CI −0.50 to 0.35; 86 participants; Figure 11; Friedman 2006).
Function (dichotomous)
There was no evidence of a difference between systemic corticosteroids and placebo in likelihood of a positive functional outcome at immediate‐term and short‐term (immediate‐term: RR 0.98, 91% CI 0.81 to 1.20; I² = 0%; 2 trials, 165 participants; Analysis 1.5; Figure 12; Eskin 2014; Friedman 2006; short‐term: RR 1.05, 95% CI 0.82 to 1.33; 1 trial, 86 participants; Analysis 1.6; Figure 13; Friedman 2006). The definition of functional improvement was "able to resume normal activities" (Eskin 2014) and an RDQ‐18 score of 0 (Friedman 2006).
Participants who underwent surgery
Neither trial reported participants with non‐radicular pain who underwent surgery.
Participants who experienced a serious adverse event
Neither trial reported participants with non‐radicular pain who experienced a serious adverse event.
Participants with hyperglycemia
There were no episodes of hyperglycemia requiring medical attention in participants with non‐radicular pain.
Minor outcomes
Quality of life
Neither trial reported quality of life in participants with non‐radicular pain.
Participants with a successful composite outcome
Neither trial reported percentage of participants with non‐radicular pain with a successful composite outcome.
Participants with a successful global measure of improvement
Neither trial reported percentage of participants with non‐radicular pain with a successful global measure of improvement.
Participants who experienced any adverse event
One trial (86 participants) found systemic corticosteroids were associated with a lower likelihood of any adverse event versus placebo at one week (21% with corticosteroids versus 45% with placebo; RR 0.45, 95% CI 0.23 to 0.88; Analysis 1.28; Friedman 2006). The most commonly reported adverse event was upper gastrointestinal complaints (9% with corticosteroids versus 21% with placebo; RR 0.42, 95% CI 0.14 to 1.27; Analysis 1.36). There was no evidence of a difference in likelihood of an adverse event at one month (9% with corticosteroids versus 10% with placebo; RR 0.95, 95% CI 0.26 to 3.57).
1.36. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 36: Gastrointestinal adverse events – by type of pain
One trial of 67 participants with non‐radicular low back pain reported "no significant side effects" in either arm; harms were otherwise not reported (Eskin 2014).
Participants who withdrew because of an adverse event
Neither trial reported percentage of participants with non‐radicular pain who withdrew because of an adverse event.
Spinal stenosis
Two trials evaluated 154 participants with spinal stenosis based on presence of symptoms (e.g. neurogenic claudication) and correlated imaging findings (Akbari Aghdam 2020; Rodrigues 2014). In one trial, participants had to have refractory symptoms, defined as no improvement with analgesic pain medications and physical therapy for at least four months (Akbari Aghdam 2020). The other trial did not report the duration of symptoms (Rodrigues 2014). Both trials were at low risk of bias; however, one trial had an unclear risk of allocation concealment and attrition bias (Akbari Aghdam 2020), and the other trial had unclear risk of bias in several domains (Rodrigues 2014). In one trial, dosing of systemic corticosteroids was weight based, starting at prednisone 1 mg/kg/day orally with a one third reduction per week, for three weeks (Rodrigues 2014). In the other trial, participants received prednisolone 10 mg daily for seven days (Akbari Aghdam 2020). In both trials, outcomes were assessed at short‐term. See Table 3.
Major outcomes
Pain (continuous)
There was no evidence of a difference between systemic corticosteroids and placebo in short‐term pain (MD on a 0 to 10 scale, −0.20, 95% CI −1.91 to 1.50; I² = 11%; Figure 5; Akbari Aghdam 2020; Rodrigues 2014).
Function (continuous)
There was no evidence of a difference between systemic corticosteroids and placebo in short‐term function (SMD −0.02, 95% CI −0.33 to 0.30; I² = 0%; Figure 11) (Akbari Aghdam 2020; Rodrigues 2014).
Participants who underwent surgery
Neither trial reported percentage of participants with spinal stenosis who underwent surgery.
Participants who experienced a serious adverse event
Neither trial reported percentage of participants with spinal stenosis who experienced a serious adverse event.
Participants with hyperglycemia
Neither trial reported percentage of participants with spinal stenosis with hyperglycemia.
Minor outcomes
Quality of life
One trial found no difference between systemic corticosteroids and placebo on any SF‐36 domain at short‐term follow‐up (Rodrigues 2014).
Participants with a successful composite outcome
Neither trial reported percentage of participants with spinal stenosis with a successful composite outcome
Participants with a successful global measure of improvement
Neither trial reported percentage of participants with spinal stenosis with a successful global measure of improvement.
Participants who experienced any adverse event
Neither trial reported percentage of participants with spinal stenosis who experienced any adverse event.
Participants who withdrew because of an adverse event
Neither trial reported percentage of participants with spinal stenosis who withdrew because of an adverse event.
Discussion
Summary of main results
This Cochrane Review is based on 13 placebo‐controlled RCTs of systemic corticosteroids for the management of low back pain. Results are summarized for radicular low back pain (not related to spinal stenosis) (Table 1), non‐radicular low back pain (Table 2), and spinal stenosis (Table 3).
For radicular low back pain (not due to spinal stenosis), five trials provided moderate‐certainty evidence that systemic corticosteroids are probably more effective than placebo for pain measured as a continuous outcome at short‐term (less than two weeks) follow‐up (MD −0.56, 95% CI −1.08 to −0.04). The magnitude of this effect was small, based on prespecified criteria. Effects were similar but imprecise (CIs crossed 0) for pain measured as a continuous outcome at immediate‐ or long‐term (low‐ or very low‐certainty evidence). Systemic corticosteroids were also associated with small improvement in function at long‐term follow‐up, based on one large, low risk of bias trial (MD −7.40, 95% CI −12.55 to −2.25, on a 0 to 100 scale; moderate‐certainty evidence) (Goldberg 2015). However, this finding is difficult to interpret because there were no differences at earlier follow‐up periods for this outcome in analyses based on multiple (three or four) trials, though evidence was very low or low certainty at earlier time points due to risk of bias, inconsistency, and imprecision. A mechanism for such a delayed effect of systemic corticosteroids is unclear. Systemic corticosteroids were associated with increased likelihood of experiencing improvement in pain (dichotomous) at immediate‐term follow‐up (RR 1.46, 95% CI 0.83 to 2.59; I² = 0%; low‐certainty evidence), but suggested smaller or no differences at longer follow‐up (moderate‐certainty evidence at short‐term and low‐ or very low‐certainty evidence at intermediate‐ or long‐term). For function measured as a dichotomous outcome, estimates and short‐term follow‐up favored systemic corticosteroids but were imprecise and not significant. Systemic corticosteroids were associated with increased likelihood of experiencing improvement in function at short‐term and long‐term follow‐up (short‐term: RR 1.52, 95% CI 1.22 to 1.91; I² = 0%; long‐term: RR 1.29, 95% CI 1.06 to 1.56). The certainty of evidence was moderate. Factors that complicate interpretation of effects of systemic corticosteroids on likelihood of pain or functional improvement are variability in how 'improvement' was defined, and failure of most trials to report these outcomes (most estimates were based one or two trials). Five trials provided low‐certainty evidence of no effect of systemic corticosteroids on likelihood of undergoing surgery (RR 1.00, 95% CI 0.68 to 1.47; I² = 0%; moderate‐certainty evidence), though the time frame for assessing whether participants had undergone surgery varied. Systemic corticosteroids had no effect on quality of life assessed using the SF‐36 Physical Component or Mental Component score, with differences below the predefined threshold for small.
Evidence was limited for effects of systemic corticosteroids versus placebo on non‐radicular low back pain (two trials) and spinal stenosis (two trials). For non‐radicular pain, systemic corticosteroids may slightly worsen pain and slightly improve function versus placebo at immediate‐ or short‐term follow‐up (low‐ or moderate‐certainty evidence). For spinal stenosis, there is probably no difference between systemic corticosteroids and placebo at short‐term follow‐up.
Regarding harms, there was low‐certainty evidence that systemic corticosteroids are not associated with increased risk of any adverse event, serious adverse events, or hyperglycemia in trials of participants with radiculopathy. Estimates were imprecise and reporting of harms was suboptimal, with some trials not reporting harms.
Overall completeness and applicability of evidence
We utilized methods to assure completeness in identification of relevant trials for this review. We conducted searches of multiple electronic databases, and supplemented searches with review of reference lists of relevant articles and searches on clinical trials registries. We did not apply language or date restrictions. The search identified no ongoing or unpublished trials.
This review was restricted to placebo‐controlled RCTs of systemic corticosteroids. We did not evaluate the effects of systemic corticosteroids compared with other medications or non‐pharmacologic treatments. We also did not evaluate the effects of non‐systemic use of corticosteroids, such as administration as an epidural, intradiscal, facet joint, or trigger point injection or administration perioperatively, and did not evaluate effects of systemic corticosteroids for cervical or thoracic back pain.
To increase applicability of our findings to clinical practice, we included trials of participants with different types of back pain (radicular, non‐radicular, or spinal stenosis) and did not apply restrictions based on duration of back pain or clinical setting (e.g. emergency room, primary care clinic, or hospital). Reflecting variability in clinical practice, some trials required back pain diagnoses to be confirmed with imaging findings and others did not. The duration of treatment with systemic corticosteroids, dose of corticosteroids, and type of corticosteroid used varied across trials, reflecting the lack of standardization in dosing regimens for systemic corticosteroids for low back pain. Although sensitivity and stratified analyses on effects of participant, setting, and intervention factors were limited by the small number of trials, there were no clear signals that these factors impacted estimated effects and there were no significant subgroup differences.
Trials varied with regard to whether they reported major outcomes (pain and function) as continuous or dichotomous measures. Effect estimates based on continuous measures indicate mean effects, whereas effect estimates based on dichotomous measures indicated the likelihood of achieving a clinically relevant outcome. Therefore, these methods provide complementary information. As noted above, trials reporting pain or function as dichotomous measures varied in how they defined improvement, potentially introducing heterogeneity into analyses on these outcomes, and continuous outcomes were reported more frequently than dichotomous outcomes. Few trials reported quality of life, function, or global improvement. Trials did not consistently report harms, methods for assessing and classifying severity of harms were not well reported and definitions for some harms (e.g. hyperglycemia) were varied. The trials were not designed to assess risk of harms associated with longer‐term or recurrent exposure to systemic corticosteroids, such as decreased bone density or fracture, or serious but uncommon harms such as psychosis, myopathy, or infection.
Quality of the evidence
Five trials were at overall low risk of bias (Akbari Aghdam 2020; Eskin 2014; Friedman 2006; Friedman 2008; Goldberg 2015), based on no risk of bias domain assessed as being at high risk of bias and adequate information to sufficiently judge risk of bias. All trials blinded participants and care providers to receipt of systemic corticosteroids except for one (Rodrigues 2014), in which it was unclear if care providers were blinded. Of the trials at overall low risk of bias, four were assessed as being at low risk of bias in all domains (Eskin 2014; Friedman 2006; Friedman 2008; Goldberg 2015) and one had unclear allocation concealment and attrition but otherwise was low risk of bias for all domains (Akbari Aghdam 2020). Three trials were assessed as being at high risk of bias, primarily due to high attrition or use of quasi‐randomization (sequential allocation) (Balakrishnamoorthy 2015; Finckh 2006; Holve 2008). Five trials reported inadequate information to assess multiple risk of bias domains and were, therefore, at unclear risk of bias (Haimovic 1986; Hedeboe 1982; Hofferberth 1982; Porsman 1979; Rodrigues 2014). All these trials except for one were published in or before 1986, when standards for conducting trials and reporting methods were not well standardized. Methods for assessing and reporting harms were also suboptimal. Our results were similar when we excluded trials at high risk of bias in a sensitivity analyses (Balakrishnamoorthy 2015; Finckh 2006; Holve 2008), though stratified analyses were limited by the small number of trials.
Therefore, the certainty of evidence was generally low, with some moderate ratings. In addition to risk of bias, evidence was most commonly downgraded for imprecision.
Potential biases in the review process
Our review had limitations. We pooled trials that used different corticosteroids that varied in dosing and duration of treatment, utilized different methods for diagnosing participants, and enrolled participants with different duration of symptoms. Although stratified analyses were conducted on these factors, estimates were imprecise and the number of trials was small, limiting their usefulness. However, statistical heterogeneity was low in pooled analyses on major outcomes (pain and function), with the exception of short‐term function.
We analyzed continuous outcomes in the following order of preference: adjusted difference in change from baseline, unadjusted difference in change from baseline, and follow‐up scores (not accounting for baseline differences). The impact and interpretation of our prioritization of continuous outcomes measures depends on the magnitude of baseline differences and whether the differences reflect 'true' variability in responsiveness to treatment or regression to the mean (Fu 2016). However, there were only baseline differences in one trial in our review and a sensitivity analysis based on the analysis of follow‐up scores provided results similar to the primary analysis, suggesting that our prioritization approach for continuous outcome measures had little impact on findings. In addition, the statistical methods we used to analyze continuous outcomes were based on standard parametric assumptions regarding the distribution of outcomes. Meta‐analysis based on parametric assumptions is widely performed and accepted in the low back pain field. Some data suggest that outcomes in pain trials may be skewed, which could result in inaccurate pooled estimates. However, evidence suggests that change scores, which we used to analyze continuous outcomes, tend towards normal distribution even when baseline or follow‐up scores are skewed, and that no statistical test on non‐parametric VAS pain data increased the likelihood of reporting between‐group differences (Dexter 1995; Vickers 2005). Furthermore, we analyzed pain and function as dichotomous and continuous outcomes. Analysis of dichotomous outcomes does not involve parametric assumptions and results of analyses of dichotomous outcomes were generally consistent with analyses of continuous outcomes.
We could not formally assess for publication bias due to the small number of trials, which limits the usefulness and reliability of statistical and graphical methods for assessing for small‐sample effects (a potential marker of publication bias) (Sterne 2011). However, we did not identify any potentially eligible trials in searches on clinical trials registries and did not identify potentially eligible trials published only as conference abstracts; therefore, we did not suspect publication bias. We were also limited in our ability to detect reporting bias since few trials had a published protocol. However, no trial reported industry funding, which has been associated with increased likelihood of publication bias.
Agreements and disagreements with other studies or reviews
Our findings are similar to a prior systematic review that found no difference between systemic corticosteroids and placebo in people with radicular low back pain for pain assessed as a dichotomous outcome or in likelihood of subsequent surgery (Roncoroni 2011). A difference with our review is that the prior review found corticosteroids associated with increased risk of any adverse event. However, it included any adverse event data from three trials that we did not use because they appeared to report selected adverse events or incomplete data on adverse events (Finckh 2006; Hofferberth 1982; Porsman 1979). Another difference is that the prior review found no effect on pain measured as a continuous outcome, and did not evaluate function. In addition, the review is outdated (searches conducted in February 2010) and our review includes additional trials published after the prior review was conducted. A more recent systematic review (searches conducted in October 2018) found potential small improvement in function (but not pain) with oral systemic corticosteroids or with a single intramuscular administration of methylprednisolone, but noted uncertainty in findings (Abdel Shaheed 2019). It was restricted to people with radiculopathy not due to spinal stenosis (sciatica) and stratified trials by route of administration. We did not analyze systemic corticosteroids separately by route of administration because they all result in systemic effects. This enabled us to conduct meta‐analysis, including analyses stratified according to route of administration (we found no subgroup differences based on the route of administration). Our review also expands upon prior systematic reviews by evaluating non‐radicular low back pain and spinal stenosis, evaluating additional outcomes (including surgery, quality of life, global improvement, and hyperglycemia and other adverse events), evaluating pain and function as both dichotomous and continuous outcomes, and evaluating outcomes at multiple prespecified time points.
Authors' conclusions
Implications for practice.
Our review has potential implications for practice. Evidence showing the effectiveness of pharmacologic and non‐invasive, non‐pharmacologic therapies for the treatment of radicular low back pain is limited. Although surgery may be an option for some people with radicular low back pain and epidural corticosteroid injections may provide short‐term benefit, some people may prefer to avoid these procedures or may not be appropriate candidates, and costs may pose a barrier. Our review suggests that for radicular low back pain, systemic corticosteroids may be associated with small benefits on pain and function at short‐term follow‐up. Although additional research is needed to verify small beneficial effects on long‐term function (findings based on a single trial), systemic corticosteroids may be an option for some people for short‐term relief of pain and improvement in function. The benefits of systemic corticosteroids were classified as small using our predefined criteria and below proposed minimum clinically important differences (Ostelo 2008). The magnitude of benefits of systemic corticosteroids on pain appears similar to that observed with other medications recommended in low back pain guidelines (Qaseem 2017), such as non‐steroidal anti‐inflammatory drugs (Machado 2017) and antidepressants (Ferreira 2021); however, comparing interventions is based on indirect (across‐study) comparisons and should be interpreted with caution. In people with radicular low back pain, benefits of systemic corticosteroids appear similar to those observed with epidural corticosteroids (Chou 2015; Oliveira 2020). In addition, the value that patients place on interventions associated with small benefits may vary according to contextual factors, including the availability of other effective treatments, potential harms, costs, and preferences around avoiding more‐invasive treatments (Salas Apaza 2021; Dekker 2019; Sedaghat 2019). Therefore, despite the small mean benefits, some people with radicular low back pain may wish to utilize systemic corticosteroids due to the lack of more effective, proven pharmacologic therapies for radicular pain; the potential for immediate symptom relief; low cost and wide availability; or because of a preference to avoid epidural corticosteroid injection. Estimates of effectiveness based on dichotomous outcomes (the likelihood of experiencing improvement beyond a specified threshold) complement findings based on continuous outcomes (mean improvement) and can be useful for informing decisions regarding systemic corticosteroids. For example, even when the mean benefit of pain is small, an increased likelihood of experiencing a greater than 30% improvement in pain might inform the decision to use corticosteroids. To be consistent with the randomized trials, use of systemic corticosteroids should be reserved for people with clear signs and symptoms of radicular pain (e.g. pain, weakness, and sensory changes in an L4, L5, or S1 nerve root distribution and positive straight leg or crossed straight leg raise test). Currently, it is unclear whether effectiveness of systemic corticosteroids varies according to the duration of symptoms or if imaging correlation is necessary for benefit. Prior to receiving systemic corticosteroids, patients should be counseled regarding the short‐term and small mean benefit on pain and function, the uncertain long‐term benefit, and the lack of impact on likelihood of subsequent surgery. Patients should also be counseled that although single‐dose and short courses of systemic corticosteroids do not appear to be associated with serious harms, evidence on harms is limited. The optimal dose and duration of systemic corticosteroids for radicular low back pain is unknown, but avoiding higher doses may reduce the risk of harms. The largest trial (and the only one to demonstrate long‐term benefit) used a 15‐day tapering course of oral prednisone starting at 60 mg daily.
For non‐radicular back pain and spinal stenosis, limited evidence indicates no short‐term benefits of systemic corticosteroids, with longer‐benefits unclear. A variety of pharmacologic and non‐pharmacologic therapies are available for treatment of non‐radicular back pain, though evidence on effective therapies for spinal stenosis is currently limited.
Implications for research.
For people with radicular low back pain (not due to spinal stenosis), trials are needed to determine the optimal dose and duration of systemic corticosteroid therapy, how effectiveness varies according to duration of symptoms, and whether limiting this therapy to patients with imaging‐confirmed herniated disc with nerve impingement is associated with improved outcomes. Trials are also needed to clarify effects of systemic corticosteroids on quality of life, long‐term outcomes, and harms. We identified one in‐progress randomized placebo‐controlled trial of oral corticosteroids in people with acute radiculopathy (Liu 2020).
For people with non‐radicular low back pain and spinal stenosis, prior to conducting additional randomized trials, research to determine whether some people have an inflammatory component that may be responsive to systemic corticosteroids would be helpful to define appropriate populations to study.
History
Protocol first published: Issue 12, 2016
Acknowledgements
We thank the peer reviewers, Bruno T Saragiotto, Masters and Doctoral Programs in Physical Therapy, Universidade Cidade de São Paulo, Brazil; Martin Underwood, Warwick Clinical Trials Unit and copy‐editor Anne Lawson, Central Production Service, Cochrane. The authors would also like to thank Shireen Harbin of Cochrane Back and Neck for helping to conduct the initial searches.
Appendices
Appendix 1. Search strategies for main databases
CENTRAL, MEDLINE, and Embase
1. Randomized Controlled Trial/
2. Controlled clinical trial/
3. Controlled Study/
4. Double Blind Procedure/
5. Single Blind Procedure/
6. randomization/
7. placebo/
8. (allocat$ or assign$).ab.
9. (compare or compared or comparing or comparison or comparative).ti,ab.
10. (controlled adj7 (study or design or trial)).ti,ab.
11. (cross‐over or crossover).ti,ab.
12. placebo$.ti,ab.
13. random$.ti,ab.
14. ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).ti,ab.
15. or/1‐14
16. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
17. human/ or normal human/ or human cell/
18. 16 and 17
19. 16 not 18
20. 15 not 19
21. exp BACKACHE/
22. ischialgia/
23. exp sciatic neuropathy/
24. sciatica/
25. (backache or back‐ache).ti,ab,kw.
26. ((back or lumb$) adj3 (pain or radicul$ or polyradicul$)).ti,ab,kw.
27. coccydynia.ti,ab,kw.
28. lumbago.ti,ab,kw.
29. dorsalgia.ti,ab,kw.
30. sciatica.ti,ab,kw.
31. coccyx.ti,ab,kw.
32. back disorder$.ti,ab,kw.
33. spondylosis.ti,ab,kw.
34. exp vertebral canal stenosis/ [includes lumbar spine stenosis]
35. exp intervertebral disk disease/ [includes intervertebral disk degeneration]
36. exp intervertebral disk hernia/ [includes lumbar disk hernia]
37. intervertebral disk/
38. ((spine or spinal or lumb$) adj3 stenosis).ti,ab,kw.
39. ((disc$ or disk$) adj degenerat$).ti,ab,kw.
40. ((disc$ or disk$) adj prolapse$).ti,ab,kw.
41. ((disc$ or disk$) adj herniat$).ti,ab,kw.
42. or/21‐41
43. exp steroid/
44. exp corticosteroid/
45. exp glucocorticoid/ [includes methylprednisone (meprednisone)]
46. exp steroid therapy/ [includes corticosteroid therapy]
47. prednisone/
48. dexamethasone/
49. betamethasone/
50. cortisone/
51. hydrocortisone/
52. triamcinolone/
53. (corticosteroid$ or glucocortic$ or adrenal cortex hormone$ or steroid$ or corticoid$ or prednis$ or methylprednis$ or dexamet$ or betamet$ or hydrocort$ or cortisone$ or triamcin$).ti,ab,kw.
54. or/43‐53
55. 20 and 42 and 54
56. limit 55 to yr=2015‐2016
57. limit 55 to em=201501‐201611
58. 56 or 57
IPA
1. ((back or lumb$) adj3 (pain or radicul$ or polyradicul$)).mp.
2. (backache or back‐ache).mp.
3. dorsalgia.mp.
4. lumbago.mp.
5. sciatica.mp.
6. coccydynia.mp.
7. coccyx.mp.
8. back disorders.mp.
9. ((spine or spinal or lumb$) adj3 stenosis).mp.
10. ((disc$ or disk$) adj degenerat$).mp.
11. ((disc$ or disk$) adj prolapse$).mp.
12. ((disc$ or disk$) adj herniat$).mp.
13. or/1‐12
14. (corticosteroid$ or glucocortic$ or adrenal cortex hormone$ or steroid$ or corticoid$ or prednis$ or methylprednis$ or dexamet$ or betamet$ or hydrocort$ or cortisone$ or triamcin$).mp.
15. 13 and 14
Web of Science
# 4 #3 AND #2 AND #1
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years
# 3 TOPIC: (random* OR placebo OR blind OR trial OR study OR compare OR compared OR comparison OR comparative OR controlled)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years
# 2 TS=(Corticosteroid$ OR glucocortic* OR “adrenal cortex hormone$” OR steroid$ OR corticoid$ OR prednis* OR methylprednis* OR dexamet*OR betamet* OR hydrocort* OR cortisone* OR triamcin*)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years
# 1 TS=(((back OR lumb*) NEAR/3 (pain or radicul* or polyradicul*)) OR backache OR back‐ache OR coccyx OR coccydynia OR sciatica OR lumbago OR dorsalgia OR ((spine OR spinal OR lumb*) NEAR/3 stenosis) OR ((disc$ OR disk$) NEAR/2 (degenerat* OR herniat* OR prolapse$)))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=All years
Indexes:
Science Citation Index Expanded (SCI‐EXPANDED) – 1900‐present·
Social Sciences Citation Index (SSCI) – 1956‐present·
Arts & Humanities Citation Index (A&HCI) – 1975‐present·
Conference Proceedings Citation Index‐ Science (CPCI‐S) – 1990‐present·
Conference Proceedings Citation Index‐ Social Science & Humanities (CPCI‐SSH) – 1990‐present·
Emerging Sources Citation Index (ESCI) – 2015‐present
Appendix 2. The GRADE approach to evidence synthesis
We categorized the certainty of the evidence as follows.
High (⊕⊕⊕⊕): further research is very unlikely to change our confidence in the estimate of effect.
Moderate (⊕⊕⊕⊝): further research is likely to have an important impact in our confidence in the estimate of effect.
Low (⊕⊕⊝⊝): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low (⊕⊝⊝⊝): any estimate of effect is very uncertain.
We graded the evidence available to answer each subquestion on the domains in the following manner.
Study design: we restricted the review to randomized trials, so we initially assumed the certainty of evidence was high.
Risk of bias: we downgraded by one level if greater than 25% but less than 50% of the participants in an analysis were enrolled in trials assessed at high risk of bias, and we downgraded by two levels if greater than 50% of the participants in an analysis were enrolled in trials assessed at high risk of bias.
Inconsistency: we downgraded by one level if the I² statistic was greater than 75% or if there was only one trial in an analysis (since it is not possible to assess inconsistency).
Indirectness: we downgraded by one level if greater than 50% of the participants were assessed as being outside the target group. As all outcomes were clinical outcomes and we did not perform indirect comparisons, this type of indirectness was not relevant for this review.
Imprecision: we downgraded by one level if the confidence interval for a risk ratio estimate crossed one and the upper limit of the confidence interval was greater than 1.20 or less than 0.80, or if the mean difference estimate crossed 0 and the upper or lower limit of the confidence interval was greater than 1 point above or below 0 on a 0 to 10 scale; and we downgraded by two levels if the confidence interval for a risk ratio estimate crosses 1 and the upper or lower limit of the confidence interval was greater than 2.00 or less than 0.50, or if the mean difference estimate crossed 0 and the upper or lower limit of the confidence interval was greater than 2 points above or below 0. We based the confidence interval thresholds on the predefined categories for small or large magnitude of benefits.
Publication bias: we downgraded by one level when we detected reporting bias or publication bias, based on identification of unpublished trials, presence of funnel plot asymmetry, or discrepancies between study protocols and the completed trials.
Data and analyses
Comparison 1. Corticosteroid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain (dichotomous), immediate (< 2 weeks) – by type of pain | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Radicular | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.83, 2.59] |
| 1.1.2 Non‐radicular | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.09] |
| 1.2 Pain (dichotomous), short‐term (2 weeks to 3 months) – by type of pain | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Radicular | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.88, 1.66] |
| 1.2.2 Non‐radicular | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.66, 1.39] |
| 1.3 Pain (dichotomous), intermediate (> 3 to < 12 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Radicular | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.44] |
| 1.4 Pain (dichotomous), long‐term (≥ 12 months) | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.56, 1.68] |
| 1.4.1 Radicular | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.56, 1.68] |
| 1.5 Function (dichotomous), immediate (< 2 weeks) – by type of pain | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Radicular | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.95, 2.35] |
| 1.5.2 Non‐radicular | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.20] |
| 1.6 Function (dichotomous), short‐term (2 weeks to 3 months) – by type of pain | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Radicular | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.22, 1.91] |
| 1.6.2 Non‐radicular | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.82, 1.33] |
| 1.7 Function (dichotomous), short‐term (radicular) – by corticosteroid dose | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.96, 1.72] |
| 1.7.1 Dose below the median | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.96, 1.38] |
| 1.7.2 Dose at or above the median | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.06, 2.74] |
| 1.8 Function (dichotomous), short‐term (radicular) – by dosing regimen | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.96, 1.72] |
| 1.8.1 Single dose | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.96, 1.38] |
| 1.8.2 For > 7 days | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.06, 2.74] |
| 1.9 Function (dichotomous), short‐term (radicular) – by route of administration | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.96, 1.72] |
| 1.9.1 Oral | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.06, 2.74] |
| 1.9.2 Intravenous | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.90, 1.87] |
| 1.9.3 Intramuscular | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.36] |
| 1.10 Function (dichotomous), short‐term (radicular) – by clinical setting | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.96, 1.72] |
| 1.10.1 Emergency | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.96, 1.38] |
| 1.10.2 Non‐emergency | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.06, 2.74] |
| 1.11 Function (dichotomous), short‐term (radicular) – by duration of symptoms | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.96, 1.72] |
| 1.11.1 Acute | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.36] |
| 1.11.2 Non‐acute | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.06, 2.74] |
| 1.11.3 Unclear/mixed | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.90, 1.87] |
| 1.12 Function (dichotomous), short‐term (radicular) – by imaging correlation | 4 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.98, 1.44] |
| 1.12.1 Imaging correlation required | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.06, 2.74] |
| 1.12.2 Imaging correlation not required/unclear | 3 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.97, 1.27] |
| 1.13 Function (dichotomous), short‐term (radicular) – by risk of bias | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.22, 1.91] |
| 1.13.1 Not high risk of bias | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.27, 2.22] |
| 1.13.2 High risk of bias | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.90, 1.87] |
| 1.14 Function (dichotomous), long‐term (≥12 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.15 Surgery (radicular) | 5 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.68, 1.47] |
| 1.15.1 Radicular | 5 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.68, 1.47] |
| 1.16 Surgery (radicular) – by risk of bias | 5 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.68, 1.47] |
| 1.16.1 Not high risk of bias | 4 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.69, 1.49] |
| 1.16.2 High risk of bias | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.06] |
| 1.17 Surgery (radicular) – by route of administration | 5 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.68, 1.47] |
| 1.17.1 Oral | 3 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.63, 2.03] |
| 1.17.2 Intravenous | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 2.83] |
| 1.17.3 Intramuscular | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.57, 1.63] |
| 1.18 Surgery (radicular) – by duration of symptoms | 5 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.68, 1.47] |
| 1.18.1 Acute | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.05, 1.97] |
| 1.18.2 Non‐acute | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.50, 2.44] |
| 1.18.3 Unclear/mixed | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.66, 1.63] |
| 1.19 Surgery (radicular) – by imaging correlation | 5 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.68, 1.47] |
| 1.19.1 Imaging correlation required | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.37, 2.24] |
| 1.19.2 Imaging correlation not required/unclear | 3 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.65, 1.59] |
| 1.20 Serious adverse events | 2 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.33] |
| 1.20.1 Radicular | 2 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.33] |
| 1.21 Hyperglycemia – by route of administration | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.47, 2.47] |
| 1.21.1 Intravenous | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.23, 93.70] |
| 1.21.2 Intramuscular | 3 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.69] |
| 1.22 Hyperglycemia – by type of pain | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.47, 2.47] |
| 1.22.1 Radicular | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.47, 2.47] |
| 1.22.2 Non‐radicular | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.23 Hyperglycemia – by clinical setting | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.47, 2.47] |
| 1.23.1 Emergency | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.23.2 Non‐emergency | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.23, 93.70] |
| 1.23.3 Mixed/unclear | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.69] |
| 1.24 Hyperglycemia – by imaging correlation | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.47, 2.47] |
| 1.24.1 Imaging correlation required | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.23, 93.70] |
| 1.24.2 Imaging correlation not required/unclear | 3 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.69] |
| 1.25 Global improvement, immediate (< 2 weeks) | 3 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.88, 1.81] |
| 1.25.1 Radicular | 3 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.88, 1.81] |
| 1.26 Global improvement, immediate (radicular) – by route of administration | 3 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.88, 1.81] |
| 1.26.1 Oral | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.48] |
| 1.26.2 Intramuscular | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.01, 2.03] |
| 1.27 Any adverse event – by duration of symptoms | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 1.27.1 Acute | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.28, 2.19] |
| 1.27.2 Non‐acute | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.96, 1.30] |
| 1.27.3 Unclear/mixed | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.48, 11.10] |
| 1.28 Any adverse event – by type of pain | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.28.1 Radicular | 4 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.89, 1.59] |
| 1.28.2 Non‐radicular | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.23, 0.88] |
| 1.29 Any adverse event – by clinical setting | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 1.29.1 Emergency | 3 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.85] |
| 1.29.2 Non‐emergency | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.96, 1.30] |
| 1.29.3 Unclear/mixed | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 6.32 [0.84, 47.69] |
| 1.30 Any adverse event – by risk of bias | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 1.30.1 Low risk of bias | 4 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.58, 1.85] |
| 1.30.2 High risk of bias | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.37, 4.19] |
| 1.31 Any adverse event – by corticosteroid dose | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 1.31.1 Dose below the median | 3 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.85] |
| 1.31.2 Dose at or above the median | 2 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.37, 10.98] |
| 1.32 Any adverse event – by imaging correlation | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 1.32.1 Imaging correlation required | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.96, 1.30] |
| 1.32.2 Imaging correlation not required/unclear | 4 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.48, 2.65] |
| 1.33 Any adverse event – by dosing regimen | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 1.33.1 Single dose | 3 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.41, 1.85] |
| 1.33.2 For 1–7 days | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 6.32 [0.84, 47.69] |
| 1.33.3 For > 7 days | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.96, 1.30] |
| 1.34 Any adverse event – by route of administration | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 1.34.1 Oral | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.96, 1.30] |
| 1.34.2 Intravenous | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.37, 4.19] |
| 1.34.3 Intramuscular | 3 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.37, 3.61] |
| 1.35 Gastrointestinal adverse events – by duration of symptoms | 3 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.78] |
| 1.35.1 Acute | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.31] |
| 1.35.2 Non‐acute | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.68, 3.93] |
| 1.35.3 Mixed/unclear | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 3.49 [0.71, 17.03] |
| 1.36 Gastrointestinal adverse events – by type of pain | 3 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.40, 3.81] |
| 1.36.1 Radicular | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.91, 4.21] |
| 1.36.2 Non‐radicular | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.14, 1.27] |
| 1.37 Gastrointestinal adverse events – by clinical setting | 3 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.78] |
| 1.37.1 Emergency | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.31] |
| 1.37.2 Non‐emergency | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.68, 3.93] |
| 1.37.3 Mixed/unclear | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 3.49 [0.71, 17.03] |
| 1.38 Gastrointestinal adverse events – by corticosteroid dose | 3 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.78] |
| 1.38.1 Dose below the median | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.15, 8.72] |
| 1.38.2 Dose at or above the median | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.68, 3.93] |
| 1.39 Gastrointestinal adverse events – by imaging correlation | 3 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.78] |
| 1.39.1 Imaging correlation required | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.91, 4.21] |
| 1.39.2 Imaging correlation not required/unclear | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.31] |
| 1.40 Gastrointestinal adverse events – by dosing regimen | 3 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.78] |
| 1.40.1 Single dose | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.31] |
| 1.40.2 For > 7 days | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.91, 4.21] |
| 1.41 Gastrointestinal adverse events – by route of administration | 3 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.78] |
| 1.41.1 Oral | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.68, 3.93] |
| 1.41.2 Intramuscular | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.15, 8.72] |
| 1.42 Withdrawal due to adverse events | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.42.1 Radicular | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.43 Withdrawal due to adverse events (radicular) – by duration of symptoms | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.43.1 Non‐acute | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.03, 7.77] |
| 1.43.2 Unclear/mixed | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.06, 14.50] |
| 1.44 Withdrawal due to adverse events (radicular) – by route of administration | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.44.1 Oral | 2 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.44.2 Intramuscular | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.45 Withdrawal due to adverse events (radicular) – by clinical setting | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.45.1 Non‐emergency | 2 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.45.2 Unclear/mixed | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.46 Withdrawal due to adverse events (radicular) – by dosing regimen | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.46.1 For 1–7 days | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.06, 14.50] |
| 1.46.2 For > 7 days | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.03, 7.77] |
| 1.47 Withdrawal due to adverse events (radicular) – by imaging correlation | 3 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.79] |
| 1.47.1 Imaging correlation required | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.03, 7.77] |
| 1.47.2 Imaging correlation not required/unclear | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.06, 14.50] |
1.7. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 7: Function (dichotomous), short‐term (radicular) – by corticosteroid dose
1.8. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 8: Function (dichotomous), short‐term (radicular) – by dosing regimen
1.9. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 9: Function (dichotomous), short‐term (radicular) – by route of administration
1.10. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 10: Function (dichotomous), short‐term (radicular) – by clinical setting
1.11. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 11: Function (dichotomous), short‐term (radicular) – by duration of symptoms
1.12. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 12: Function (dichotomous), short‐term (radicular) – by imaging correlation
1.13. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 13: Function (dichotomous), short‐term (radicular) – by risk of bias
1.16. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 16: Surgery (radicular) – by risk of bias
1.17. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 17: Surgery (radicular) – by route of administration
1.18. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 18: Surgery (radicular) – by duration of symptoms
1.19. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 19: Surgery (radicular) – by imaging correlation
1.20. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 20: Serious adverse events
1.21. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 21: Hyperglycemia – by route of administration
1.23. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 23: Hyperglycemia – by clinical setting
1.24. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 24: Hyperglycemia – by imaging correlation
1.26. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 26: Global improvement, immediate (radicular) – by route of administration
1.27. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 27: Any adverse event – by duration of symptoms
1.29. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 29: Any adverse event – by clinical setting
1.30. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 30: Any adverse event – by risk of bias
1.31. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 31: Any adverse event – by corticosteroid dose
1.32. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 32: Any adverse event – by imaging correlation
1.33. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 33: Any adverse event – by dosing regimen
1.34. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 34: Any adverse event – by route of administration
1.35. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 35: Gastrointestinal adverse events – by duration of symptoms
1.37. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 37: Gastrointestinal adverse events – by clinical setting
1.38. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 38: Gastrointestinal adverse events – by corticosteroid dose
1.39. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 39: Gastrointestinal adverse events – by imaging correlation
1.40. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 40: Gastrointestinal adverse events – by dosing regimen
1.41. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 41: Gastrointestinal adverse events – by route of administration
1.43. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 43: Withdrawal due to adverse events (radicular) – by duration of symptoms
1.44. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 44: Withdrawal due to adverse events (radicular) – by route of administration
1.45. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 45: Withdrawal due to adverse events (radicular) – by clinical setting
1.46. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 46: Withdrawal due to adverse events (radicular) – by dosing regimen
1.47. Analysis.

Comparison 1: Corticosteroid versus placebo, Outcome 47: Withdrawal due to adverse events (radicular) – by imaging correlation
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akbari Aghdam 2020.
| Study characteristics | ||
| Methods | Clinical setting: outpatient clinics (primary care or specialty) Number randomized: 100 Number analyzed: 93 Attrition: 7% Funding source: not reported Country: Iran Publication language: English |
|
| Participants | Mean age: 59 years with corticosteroid vs 60 years with placebo Women: 68% with corticosteroid vs 57% with placebo Race: not reported Type of back pain: spinal stenosis Duration of symptoms: > 4 months (mean or median not reported) Baseline pain (NRS, scale 0–10): 6.85 with corticosteroid vs 6.73 with placebo Baseline function (ODI, 0–100): 46.6 with corticosteroid vs 45.6 with placebo Imaging correlation required |
|
| Interventions | Prednisolone 10 mg (10 mg prednisone equivalents) PO (47 participants) Placebo (46 participants) Duration of treatment: 7 days Duration of follow‐up: 2 months |
|
| Outcomes | Pain: NRS (scale 0–10) Function: ODI (scale 0–100) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Unclear risk | By pharmacist, unclear if blinded to participant status. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded to intervention; placebo similar to the prednisolone tablet. |
| Blinding of care provider | Low risk | Nurse blinded to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurse blinded to study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7% (7/100) of participants excluded due to drug complications. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes specified and reported. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | 7% (7/100) of participants excluded due to drug complications; not downgraded due to small number of participants. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Balakrishnamoorthy 2015.
| Study characteristics | ||
| Methods | Clinical setting: emergency room Number randomized: 58 Number analyzed: 36 Attrition: 38% Funding source: none reported Country: Australia Publication language: English |
|
| Participants | Mean age: 39 years with corticosteroid vs 37 years with placebo Women: 59% with corticosteroid vs 45% with placebo Race: not reported Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: not reported Baseline pain (VAS, scale 0–10): 8.1 with corticosteroid vs 7.0 with placebo Baseline function (ODI, 0–100): 62 with corticosteroid vs 63 with placebo No imaging correlation required |
|
| Interventions | Dexamethasone 8 mg (50 mg prednisone equivalents) IV (29 participants) Placebo (29 participants) Duration of treatment: single dose Duration of follow‐up: 6 weeks |
|
| Outcomes | Pain: VAS (scale 0–10) Disability: ODI (scale 0–100) Disability (dichotomous): ability to resume normal activities Any adverse event Serious adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant filled data sheets placed in locked box. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition 38% (22/58) at 6 weeks. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes described and reported. |
| Baseline group similarity | Unclear risk | Groups not similar at baseline. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing of outcome assessment similar. |
Eskin 2014.
| Study characteristics | ||
| Methods | Clinical setting: emergency room Number randomized: 79 Number analyzed: 67 Attrition: 15% Funding source: Emergency Medical Associates Research Foundation Country: US Publication language: English |
|
| Participants | Mean age: 39 years with corticosteroid vs 41 years with placebo Women: 33% with corticosteroid vs 27% with placebo Race: 56% with corticosteroid vs 60% with placebo White; 18% with corticosteroid vs 20% with placebo Hispanic; 8% with corticosteroid vs 10% with placebo Black Type of back pain: non‐radicular Duration of symptoms: acute, median: 23 hours with corticosteroid vs 15 hours with placebo Baseline pain (VAS, scale 0–10): 8.0 with corticosteroid vs 8.0 with placebo Baseline function: not reported No imaging correlation required |
|
| Interventions | Prednisone 50 mg/day PO (32 participants) Placebo (35 participants) Duration of treatment: 5 days Duration of follow‐up: 5–7 days |
|
| Outcomes | Pain: VRS (scale 0–3) Pain (dichotomous): no or mild pain Function (dichotomous): ability to resume normal activities |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 15% (12/79). |
| Selective reporting (reporting bias) | Low risk | Primary outcomes described and reported. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Finckh 2006.
| Study characteristics | ||
| Methods | Clinical setting: hospital inpatient Number randomized: 65 Number analyzed: 59 at 3 days, 22 at 30 days Attrition: 9% at 3 days, 66% at 30 days Funding source: none reported Country: Switzerland Publication language: English |
|
| Participants | Mean age: 49 years with corticosteroid vs 45 years with placebo Women: 45% with corticosteroid vs 59% with placebo Race: not reported Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: acute, median: 15 days Baseline leg pain (VAS, scale 0–100): 67 with corticosteroid vs 63 with placebo Baseline back pain (VAS, scale 0–100): 47 with corticosteroid vs 55 with placebo Baseline global pain (VAS, scale 0–100): 65 with corticosteroid vs 61 with placebo Baseline function (ODI, 0–100): 26.7 with corticosteroid vs 26.7 with placebo Neurologic deficits: 52% with corticosteroid vs 34% with placebo Imaging correlation required |
|
| Interventions | Methylprednisolone 500 mg (625 mg prednisone equivalents) IV bolus (31 participants) Placebo IV (29 participants) Duration of treatment: single dose Duration of follow‐up: 30 days |
|
| Outcomes | Sciatic pain: VAS (scale 0–100) Low back pain: VAS (scale 0–100) Global pain: VAS (scale 0–100) and McGill Pain Questionnaire Function: ODI (scale 0–100) Pain (dichotomous): VAS decrease ≥ 20 Underwent spine surgery Harms: transient hyperglycemia, facial flush |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator. |
| Allocation concealment (selection bias) | Low risk | Sequential opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessment reported as blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9% (9/65) at 3 days, 66% (43/65) at 30 days. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes described and reported. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Friedman 2006.
| Study characteristics | ||
| Methods | Clinical setting: emergency room Number randomized: 87 Number analyzed: 86 Attrition: 1% Funding source: not reported Country: US Publication language: English |
|
| Participants | Mean age: 36 years with corticosteroid vs 36 years with placebo Women: 64% with corticosteroid vs 54% with placebo Race: 63% with corticosteroid vs 42% with placebo Latino; 23% with corticosteroid vs 44% with placebo Black; 12% with corticosteroid vs 7% with placebo White Type of back pain: non‐radicular Duration of symptoms: acute, median: 44 hours with corticosteroid vs 63 hours with placebo Baseline back pain (VAS, scale 0–10): 8.6 with corticosteroid vs 9.1 with placebo Baseline function: not reported No imaging correlation required |
|
| Interventions | Methylprednisolone 160 mg (200 mg prednisone equivalents) IM (44 participants) Placebo IM (43 participants) Duration of treatment: single dose Duration of follow‐up: 1 month |
|
| Outcomes | Pain: NRS (scale 0–10) Pain (dichotomous): no back pain in previous 24 hours Function (dichotomous): RDQ‐18 = 0 (scale 0–18); able to resume normal activities Function: RDQ‐18 (scale 0–18) Any adverse medication effects Upper gastrointestinal adverse effects Hyperglycemia requiring medical attention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered research bags; assignment not known to research assistant distributing bags. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | By blinded research assistants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 1% (1/87). |
| Selective reporting (reporting bias) | Low risk | Primary outcomes described and reported. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Friedman 2008.
| Study characteristics | ||
| Methods | Clinical setting: emergency room Number randomized: 82 Number analyzed: 78 Attrition: 5% Funding source: none Country: US Publication language: English |
|
| Participants | Mean age: 39 years with corticosteroid vs 37 years with placebo Women: 54% with corticosteroid vs 51% with placebo Race: 69% with corticosteroid vs 67% with placebo Hispanic/Latino; 22% with corticosteroid vs 21% with placebo African‐American/Black; 8% with corticosteroid vs 7% with placebo White Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: acute, median: 48 hours Baseline pain (VAS, scale 0–10): 8.9 with corticosteroid vs 9.1 with placebo Baseline function: not reported No imaging correlation required |
|
| Interventions | Methylprednisolone: 160 mg (200 mg prednisone equivalents) IM (37 participants) Placebo (41 participants) Duration of treatment: single dose Duration of follow‐up: 1 month |
|
| Outcomes | Pain: NRS (scale 0–10) Pain (dichotomous): no back pain in prior 24 hours Function: RDQ‐18 (scale 0‐18) Function (dichotomous): RDQ‐18 = 0; resumed usual activities Any adverse event |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator. |
| Allocation concealment (selection bias) | Low risk | Sequential unmarked syringes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 5% (4/82). |
| Selective reporting (reporting bias) | Low risk | Primary outcomes described and reported. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | No issues noted. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Goldberg 2015.
| Study characteristics | ||
| Methods | Clinical setting: primary care clinics Number randomized: 269 Number analyzed: 234 Attrition: 13% Funding source: National Institute of Arthritis and Musculoskeletal and Skin Diseases Country: US Publication language: English |
|
| Participants | Mean age: 46 years with corticosteroid vs 47 years with placebo Women: 46% with corticosteroid vs 42% with placebo Race: 71% with corticosteroid vs 57% with placebo White; 24% with corticosteroid vs 8% with placebo Asian; 5% with corticosteroid vs 10% with placebo multiracial; 2% with corticosteroid vs 3% with placebo African‐American; 0.6% with corticosteroid vs 5% with placebo Native American; 1% with corticosteroid vs 0% with placebo Pacific Islander; 7% with corticosteroid vs 16% with placebo unknown; 19% with corticosteroid vs 32% Hispanic with placebo Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: acute and non‐acute, mean: 30 days Baseline pain (NRS, scale 0–10): 6.6 with corticosteroid vs 6.9 with placebo Baseline function (ODI, 0–100): 51.2 with corticosteroid vs 51.1 with placebo Imaging correlation required |
|
| Interventions | Prednisone 20 mg PO 3 times/day for 5 days; 20 mg twice/day for 5 days; 20 mg once/day for 5 days (181 participants) Placebo (88 participants) Duration of treatment: 15 days (each prednisone dose given for 5 days) Duration of follow‐up: 1 year |
|
| Outcomes | Pain: NRS (scale 0–10) Pain (dichotomous): pain improved ≥ 5 points Function: ODI (scale 0–100) Function (dichotomous): ODI improved > 50% Quality of life: SF‐36 Physical Component and Mental Component Summary score (scale 0–100) Underwent surgery Any adverse event Serious adverse events Withdrawal due to adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization list. |
| Allocation concealment (selection bias) | Low risk | Centralized allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All staff and investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 13% (35/269). |
| Selective reporting (reporting bias) | Low risk | Primary outcomes described and reported. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Haimovic 1986.
| Study characteristics | ||
| Methods | Clinical setting: unclear Number randomized: 33 Number analyzed: 27 Attrition: 18% Funding source: not reported Country: US Publication language: English |
|
| Participants | Mean age: not reported Women: not reported Race: not reported Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: not reported Baseline pain: not reported Baseline function: not reported No imaging correlation required |
|
| Interventions | Dexamethasone 64 mg (400 mg prednisone equivalents) PO day 1; 32 mg (200 mg prednisone equivalents) PO day 2; 16 mg (100 mg prednisone equivalents) PO day 3; 12 mg (75 mg prednisone equivalents) PO day 4; and 8 mg (50 mg prednisone equivalents) PO days 5–7 (21 participants) Placebo (12 participants) Duration of treatment: 7 days Duration of follow‐up: 1–4 years |
|
| Outcomes | Pain (dichotomous): early improvement: resting low back pain or radicular pain on straight leg raising reported as 'definitely less' than before treatment Pain (dichotomous): late or sustained improvement: pain score ≤ 3 (scale 0–6) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 18% (6/33). |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes unclear. |
| Baseline group similarity | Unclear risk | Baseline data not provided. |
| Co‐interventions avoided or similar | Unclear risk | Information not provided. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Unclear risk | Analysis methods unclear. |
| Timing of outcome assessment similar | Unclear risk | Timing appeared to vary. |
Hedeboe 1982.
| Study characteristics | ||
| Methods | Clinical setting: unclear Number randomized: 39 Number analyzed: 39 Attrition: not reported Funding source: not reported Country: Denmark Publication language: English |
|
| Participants | Mean age: 44 years with corticosteroid vs 40 years with placebo Women: 47% with corticosteroid vs 25% with placebo Race: not reported Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: not reported Baseline pain: not reported Baseline function: not reported No imaging correlation required |
|
| Interventions | Dexamethasone 4 mg/mL, 16 mg (100 mg prednisone equivalents) IM 4 times/day for 1 day; 8 mg (50 mg prednisone equivalents) IM 4 times/day for 1 day; 8 mg (50 mg prednisone equivalents) IM 3 times/day for 1 day; 4 mg (25 mg prednisone equivalents) IM 3 times/day for 1 day; 4 mg (25 mg prednisone equivalents) IM twice/day for 3 days (19 participants) Placebo (20 participants) Duration of treatment: 7 days Duration of follow‐up: 3 months |
|
| Outcomes | Pain (dichotomous): clear improvement, not defined Underwent surgery Any adverse event Withdrawal due to adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcomes assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes not defined. |
| Baseline group similarity | Unclear risk | Baseline data not provided. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Unclear risk | Compliance not reported. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Hofferberth 1982.
| Study characteristics | ||
| Methods | Clinical setting: not reported Number randomized: 100 Number analyzed: 91 Attrition: 8% Funding source: not reported Country: Germany Publication language: German |
|
| Participants | Not reported according by intervention group, data for entire sample Mean age: 47 years Women: 31% Race: not reported Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: non‐acute, mean: 36 months Baseline pain: not reported Baseline function: not reported No imaging correlation required |
|
| Interventions | Dexamethasone phosphate 8 mg (50 mg prednisone equivalents) IM 3 times/day for 5 days; 4 mg (25 mg prednisone equivalents) IM times/day for 3 days; 4 mg (25 mg prednisone equivalents) IM twice/day for 1 day; 4 mg (25 mg prednisone equivalents) IM once/day for 1 day (38 participants) Placebo (53 participants) Duration of treatment: 10 days Duration of follow‐up: 11 days |
|
| Outcomes | Pain (dichotomous): full symptom relief or greatly improved symptoms Hyperglycemia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 9% (9/100). |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome not described and only reported pain improvement; function not reported. |
| Baseline group similarity | Unclear risk | Baseline data not provided. |
| Co‐interventions avoided or similar | Unclear risk | Use of co‐interventions unclear. |
| Acceptable compliance | Unclear risk | Compliance unclear. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Holve 2008.
| Study characteristics | ||
| Methods | Clinical setting: emergency room or primary care Number randomized: 29 Number analyzed: 27 Attrition: 7% Funding source: Kaiser Foundation Research Institute Country: US Publication language: English |
|
| Participants | Mean age: 39 years with corticosteroid vs 46 years with placebo Women: 37% (overall) Race: not reported Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: acute, ≤ 1 week Baseline pain (RDQ Pain, 0–5 VRS): 3.8 with corticosteroid vs 3.1 with placebo Baseline function (RDQ function, scale 0–24): 16 with corticosteroid vs 16 with placebo No imaging correlation required |
|
| Interventions | Prednisone: 60 mg PO once/day for 3 days; 40 mg PO once/day for 3 days; 20 mg PO once/day for 3 days (13 participants) Placebo (14 participants) Duration of treatment: 9 days Duration of follow‐up: 6 months |
|
| Outcomes | Pain: RDQ Pain (scale 0–5) Disability: RDQ (scale 0–24) Quality of life: SF‐12 Mental Health Component (scale 0–100) Underwent surgery |
|
| Notes | We imputed the standard deviation using the mean coefficient of variation from the trials of the same meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequential allocation. |
| Allocation concealment (selection bias) | High risk | Sequential allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research nurse blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 6.9% (2/29). |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes not reported. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Unclear risk | Use of co‐interventions not reported. |
| Acceptable compliance | Low risk | No issues noted. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Porsman 1979.
| Study characteristics | ||
| Methods | Clinical setting: hospital inpatient Number randomized: 52 Number analyzed: 49 Attrition: 6% Funding source: not reported Country: Denmark Publication language: English |
|
| Participants | Mean age: 47 years with corticosteroid vs 42 years with placebo Women: 32% with corticosteroid vs 33% with placebo Race: not reported Type of back pain: radicular (not due to spinal stenosis) Duration of symptoms: acute and non‐acute, mean: 8.2 with corticosteroid vs 8.3 weeks with placebo No imaging correlation required |
|
| Interventions | Dexamethasone 64 mg (400 mg prednisone equivalents) PO day 1; 32 mg (200 mg prednisone equivalents) PO day 2; 24 mg (150 mg prednisone equivalents) PO day 3; 12 mg (75 mg prednisone equivalents) PO day 4; 8 mg (50 mg prednisone equivalents) PO days 5–7 (25 participants) Placebo (24 participants) Duration of treatment: 7 days Duration of follow‐up: 9 days |
|
| Outcomes | Pain (dichotomous): treatment effect (undefined) Underwent surgery Withdrawal due to adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Low risk | Placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcomes assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 6% (3/52). |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes not described. |
| Baseline group similarity | Unclear risk | Baseline data not provided. |
| Co‐interventions avoided or similar | Low risk | No issues noted. |
| Acceptable compliance | Unclear risk | Compliance not reported. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
Rodrigues 2014.
| Study characteristics | ||
| Methods | Clinical setting: unclear Number randomized: 61 Number analyzed: 61 Attrition: 0% Funding source: none Country: Brazil Publication language: English |
|
| Participants | Mean age: 58 years with corticosteroid vs 58 years with placebo Women: not reported (reported no difference between groups) Race: not reported Type of back pain: spinal stenosis Duration of symptoms: not reported Baseline pain (VAS scale 0–10): 7.68 with corticosteroid vs 7.07 with placebo Baseline function (RDQ, scale 0–24): 16.16 with corticosteroid vs 15.27 with placebo Imaging correlation required |
|
| Interventions | Corticoid (prednisone) 1 mg/kg/day PO with 1/3 dose reduction/week (31 participants) Placebo (30 participants) Duration of treatment: 21 days Duration of follow‐up: 12 weeks |
|
| Outcomes | Pain: VAS (scale 0–10) Function: Roland Morris Disability Questionnaire (RDQ, scale 0–24) Quality of life: SF‐36 Physical Component and Mental Health Components (scale 0–100) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo. |
| Blinding of care provider | Unclear risk | Unclear if provider blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 0% (0/61). |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes not described. |
| Baseline group similarity | Low risk | Groups similar at baseline. |
| Co‐interventions avoided or similar | Unclear risk | Co‐interventions not reported |
| Acceptable compliance | Unclear risk | Adherence not reported. |
| Intention to treat | Low risk | Intention‐to‐treat analysis. |
| Timing of outcome assessment similar | Low risk | Timing similar. |
IM: intramuscular; IV: intravenous; NRS: numerical rating scale; ODI: Oswestry Disability Index; PO: oral; RDQ: Roland‐Morris Disability Questionnaire; RDQ‐18: 18‐item Roland‐Morris Disability Questionnaire; SF‐12: 12‐item Short Form; VAS: visual analogue scale; VRS: verbal rating score.
Characteristics of excluded studies [ordered by study ID]
Characteristics of ongoing studies [ordered by study ID]
Liu 2020.
| Study name | OASIS |
| Methods | Randomized controlled trial |
| Participants | 200 |
| Interventions | Prednisolone 50 mg orally once/day up to 3 days then tapering over 10 days, for maximum of 13 days vs placebo |
| Outcomes | Leg pain intensity (primary), back pain intensity, disability, time to recovery, quality of life, treatment success, adverse events, cost‐effectiveness |
| Starting date | Not reported |
| Contact information | Chang Liu: chang.liu1@sydney.edu.au |
| Notes |
Differences between protocol and review
Following publication of the protocol (Chou 2016), we made the following changes.
We modified the inclusion criteria to include quasi‐randomized as well as randomized controlled trials.
We changed the following outcomes from minor outcomes (in the protocol) to major outcomes: percentage of participants who underwent surgery, percentage of participants who experienced a serious adverse event, and percentage of participants with hyperglycemia.
The definition for overall low risk of bias originally was for trials to be low risk of bias on at least six of 12 risk of bias domains. We modified the definition for low risk of bias to also require participants to not have serious risk of bias (e.g. attrition greater than 35%, or quasi‐randomized design) on any risk of bias domain.
We removed the assessment of clinical relevance using the criteria in the 2009 Cochrane Back and Neck guidance, which was removed from the updated 2015 Cochrane Back and Neck guidance (definitions for classifying the magnitude of effect were retained).
Contributions of authors
RC drafted the protocol and the review and made revisions.
TD, NH, RZP, RL, and JHM reviewed the draft and made revisions.
RF reviewed the draft, made revisions, and conducted meta‐analyses.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
RC has conducted systematic reviews funded by the Agency for Healthcare Research and Quality and the American Pain Society that included systemic corticosteroids, and he led a guideline from the American College of Physicians and the American Pain Society that addressed systemic corticosteroids.
RZP: none.
RF: Oregon Health & Science University (employment).
RL owns stocks in companies that make systemic corticosteroids (Pfizer Inc, Amgen, Eli Lilly and Company, Merck, Johnson & Johnson Health Care Systems Inc, Gilead Sciences). His declaration was referred to the Cochrane Funding Arbiter, and it was determined that there was no breach of Cochrane policy.
NH: none.
JHM: none
TD: none.
New
References
References to studies included in this review
Akbari Aghdam 2020 {published data only}
- Akbari Aghdam H, Andalib A, Adadiyan Ardakani H, Telloo M, Sheikhbahaei E. A short-term oral corticosteroid for refractory lumbar spinal stenosis: a double-blinded randomized placebo-controlled trials. International Journal of Rehabilitation Research 2020;43:342-6. [DOI] [PubMed] [Google Scholar]
Balakrishnamoorthy 2015 {published data only}
- Balakrishnamoorthy R, Horgan I, Perez S, Keijzers G, Steele M. Steroids for emergency patients with low back pain and radiculopathy (SEBRA): the SEBRA trial. EMA – Emergency Medicine Australasia 2013;25(S1):17. [Google Scholar]
- Balakrishnamoorthy R, Horgan I, Perez S, Steele MC, Keijzers GB. Does a single dose of intravenous dexamethasone reduce Symptoms in Emergency department patients with low Back pain and RAdiculopathy (SEBRA)? A double-blind randomised controlled trial. Emergency Medicine Journal 2015;32:525-30. [DOI] [PubMed] [Google Scholar]
Eskin 2014 {published data only}
- Eskin B, Shih RD, Allegra JR, Fiesseler FW, Silverman M. Steroids for back pain: a randomized double-blind placebo-controlled trial. Academic Emergency Medicine 2010;17:s154. [Google Scholar]
- Eskin B, Shih RD, Fiesseler FW, Walsh BW, Allegra JR, Silverman ME, et al. Prednisone for emergency department low back pain: a randomized controlled trial. Journal of Emergency Medicine 2014;47(1):65-70. [DOI] [PubMed] [Google Scholar]
Finckh 2006 {published and unpublished data}
- Finckh A, Zufferey P, Schurch MA, Balague F, Waldburger M, So AK. Short-term efficacy of intravenous pulse glucocorticoids in acute discogenic sciatica. Spine 2006;31:377-81. [DOI] [PubMed] [Google Scholar]
Friedman 2006 {published data only}
- Friedman BW, Holden L, Esses D, Bijur PE, Choi HK, Solorzano C, et al. Parenteral corticosteroids for emergency department patients with non-radicular low back pain. Journal of Emergency Medicine 2006;31:365-70. [DOI] [PubMed] [Google Scholar]
Friedman 2008 {published data only}
- Friedman BW, Esses D, Solorzano C, Choi HK, Cole M, Davitt M, et al. A randomized placebo-controlled trial of single-dose IM corticosteroid for radicular low back pain. Spine 2008;33(18):E624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2015 {published data only}
- Goldberg H, Firtch W, Tyburski M, Pressman A, Ackerson L, Hamilton L, et al. Oral steroids for acute radiculopathy due to a herniated lumbar disk. JAMA 2015;313:1915-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haimovic 1986 {published data only}
- Haimovic IC, Beresford HR. Dexamethasone is not superior to placebo for treating lumbosacral radicular pain. Neurology 1986;36:1593-4. [DOI] [PubMed] [Google Scholar]
- Haimovic IC, Beresford HR. Treatment of lumbosacral radicular pain with dexamethasone: a controlled study. Neurology 1985;35(Suppl 1):252. [Google Scholar]
Hedeboe 1982 {published data only}
- Hedeboe J, Buhl M, Ramsing P. Effects of using dexamethasone and placebo in the treatment of prolapsed lumbar disc. Acta Neurologica Scandinavica 1982;65:6-10. [DOI] [PubMed] [Google Scholar]
Hofferberth 1982 {published data only}
- Hofferberth B, Gottschaldt M, Grass H, Bűttner K. The usefulness of dexamethasonephosphate in the conservative treatment of lumbar pain – a double-blind study [Űber den wert der anwendung von dexamethasonphosphat in der konservativen therapie der lumboischialgie]. Archiv fűr Psychiatrie und Nervenkrankheiten 1982;231:359-67. [DOI] [PubMed] [Google Scholar]
Holve 2008 {published data only}
- Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. Journal of the American Board of Family Medicine 2008;21:469-74. [DOI] [PubMed] [Google Scholar]
Porsman 1979 {published data only}
- Porsman O, Friis H. Prolapsed lumbar disc treated with intramuscularly administered dexamethasone phosphate. Scandinavian Journal of Rheumatology 1979;8:142-4. [DOI] [PubMed] [Google Scholar]
Rodrigues 2014 {published data only}
- Rodrigues LC, Natour J. A double-blind, randomized controlled, prospective trial assessing the effectiveness of oral corticoids in the treatment of symptomatic lumbar canal stenosis. Journal of Negative Results in BioMedicine 2014;13:13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues LC, Natour J. Double-blind randomized controlled prospective trial assessing the effectiveness of oral corticoids in the treatment of lumbar-canal stenosis in symptomatic patients. Annals of the Rheumatic Diseases 2013;72:720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ko 2016 {published data only}
- Ko S, Kim S, Kim J, Oh T. The effectiveness of oral corticosteroids for management of lumbar radiating pain: randomized, controlled trial study. Clinics in Orthopedic Surgery 2016;8:262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oros 2019 {published data only}
- Oroa M, Oros Jar M, Grabar V. Steroids and l-lysine aescinate for acute radiculopathy due to a herniated lumbar disk. Medicina (Kaunas) 2019;55(11):736. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Liu 2020 {published data only}
- Liu C, Abdel Shaheed C, McLachlan A, Latimer J, Li Q, Buchbinder R, et al. OASIS – a randomised, placebo-controlled trial of oral glucocorticoids for leg pain in patients with acute sciatica: trial protocol. BMJ Open 2020;10:e040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdel Shaheed 2019
- Abdel Shaheed C, Maher CG, Buchbinder R, Ng B, Enke O, Guzowski R, et al. Efficacy and harms of orally, intramuscularly, or intravenously administered glucocorticoids for sciatica: a systematic review and meta-analysis. European Journal of Pain 2019;24:518-35. [DOI] [PubMed] [Google Scholar]
Carey 2009
- Carey TS, Freburger JK, Holmes GM, Castel L, Darter J, Agans R, et al. A long way to go: practice patterns and evidence in chronic low back pain care. Spine 2009;34(7):718-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiarotto 2016
- Chiarotto A, Maxwell LJ, Terwwee CB, Wells GA, Tugwell P, Ostelo RW. Roland-Morris Disability Questionnaire and Oswestry Disability Index: which has better measurement properties for measuring physical functioning in nonspecific low back pain? Systematic review and meta-analysis. Physical Therapy 2016;96(10):1620-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chou 2007
- Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annals of Internal Medicine 2007;147(7):478-91. [DOI] [PubMed] [Google Scholar]
Chou 2011
- Chou R, Qaseem A, Owens DK, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Diagnostic imaging for low back pain: advice for high-value health care from the American College of Physicians. Annals of Internal Medicine 2011;154(3):181-9. [DOI] [PubMed] [Google Scholar]
Chou 2015
- Chou R, Hashimoto R, Friedly J, Fu R, Bougatsos C, Dana T, et al. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Annals of Internal Medicine 2015;163(5):373-81. [DOI] [PubMed] [Google Scholar]
Chrousos 2014
- Chrousos GP. Chapter 39. Adrenal corticosteroids & adrenocortical antagonists. In: Katzung BG, Masters SB, Trevor AJ, editors(s). Basic & Clinical Pharmacology. 12th edition. New York (NY): McGraw-Hill Companies, Inc, 2012:697-714. [Google Scholar]
da C Menezes Costa 2012
- da C Menezes Costa L, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ 2012;184:E613-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dekker 2019
- Dekker J. The minimal clinically important difference re-considered. Osteoarthritis and Cartilage 2019;27(10):1403-4. [DOI] [PubMed] [Google Scholar]
Dexter 1995
- Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology 1995;82:896-902. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deyo 2006
- Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine 2006;31(23):2724-7. [DOI] [PubMed] [Google Scholar]
Deyo 2014
- Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. Report of the NIH Task Force on research standards for chronic low back pain. Journal of Pain 2014;15(6):569-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2021
- Ferreira GE, McLachlan AJ, Lin CC, Zadro JR, Abdel-Shaheed C, O'Keeffe M, et al. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. BMJ 2021;372:m4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2010
- Friedman BW, Chilstrom M, Bijur PE, Gallagher EJ. Diagnostic testing and treatment of low back pain in United States emergency departments: a national perspective. Spine 2010;35(24):E1406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 2016
- Fu R, Holmer H. Change score or followup score? Choice of mean difference estimates could impact meta-analysis conclusions. Journal of Clinical Epidemiology 2016;76:108-17. [DOI] [PubMed] [Google Scholar]
Furlan 2015
- Furlan AD, Pennick V, Bombardier C, Tulder M. 2015 updated method guidelines for systematic reviews in the Cochrane Back and Neck Group. Spine 2015;40(21):1660-73. [DOI] [PubMed] [Google Scholar]
Green 1975
- Green LN. Dexamethasone in the management of symptoms due to herniated lumbar disc. Journal of Neurology, Neurosurgery, and Psychiatry 1975;38(12):1211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.traintraining.cochrane.org/handbook/archive/v6.2.
Hoy 2012
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis & Rheumatism 2012;64(6):2028-37. [DOI] [PubMed] [Google Scholar]
Jarvik 2002
- Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Annals of Internal Medicine 2002;137(7):586-97. [DOI] [PubMed] [Google Scholar]
Kopec 2000
- Kopec JA. Measuring functional outcomes in persons with back pain: a review of back-specific questionnaires. Spine 2000;25(24):3110-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Luo 2004
- Luo X, Pietroban R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 2004;29(1):79-86. [DOI] [PubMed] [Google Scholar]
Machado 2017
- Machado GC, Maher CG, Ferreira PH, O Day R, Pinheiro MB, Ferreira ML. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Annals of the Rheumatic Diseases 2017;76:1269-78. [DOI] [PubMed] [Google Scholar]
Martin 2008
- Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008;299(6):656-64. [DOI] [PubMed] [Google Scholar]
McDonough 2008
- McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Current Opinion in Rheumatology 2008;20(2):131-7. [DOI] [PubMed] [Google Scholar]
Oliveira 2020
- Oliveira CB, Maher CG, Ferreira ML, Hancock MJ, Oliveira VC, McLachlan AJ, et al. Epidural corticosteroid injections for sciatica: an abridged Cochrane systematic review and meta-analysis. Spine 2020;45(21):E1405-15. [DOI] [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33(1):90-4. [DOI] [PubMed] [Google Scholar]
Qaseem 2017
- Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2017;166:514-30. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roncoroni 2011
- Roncoroni C, Baillet A, Durand M, Gaudin P, Juvin R. Efficacy and tolerance of systemic steroids in sciatica: a systematic review and meta-analysis. Rheumatology 2011;50(9):1603-11. [DOI] [PubMed] [Google Scholar]
Ropper 2015
- Ropper AH, Zafonte RD. Sciatica. New England Journal of Medicine 2015;372(13):1240-8. [DOI] [PubMed] [Google Scholar]
Rubinstein 2011
- Rubinstein SM, Middelkoop M, Assendelft WJ, Boer MR, Tulder MW. Spinal manipulative therapy for chronic low-back pain. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD008112. [DOI: 10.1002/14651858.CD008112.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salas Apaza 2021
- Salas Apaza JA, Franco JV, Meza N, Madrid E, Loézar C, Garegnani L. Minimal clinically important difference: the basics. Medwave 2021;21(3):e8149. [DOI] [PubMed] [Google Scholar]
Schimmer 2011
- Schimmer BP, Funder JW. Chapter 42. ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In: Brunton LL, Chabner BA, Knollmann BC, editors(s). Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th edition. New York (NY): McGraw-Hill Companies, Inc, 2011:1209-36. [Google Scholar]
Sedaghat 2019
- Sedaghat AR. Understanding the minimal clinically important difference (MCID) of patient-reported outcome measures. Otolaryngology – Head and Neck Surgery 2019;161(4):551-60. [DOI] [PubMed] [Google Scholar]
Shafshak 2021
- Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. Journal of Clinical Rheumatology 2021;27(7):282-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stata [Computer program]
- Stata. Version 14. College Station, TX, USA: StataCorp, 2015. Available at www.stata.com.
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
van der Laan 2008
- Laan S, Meijer OC. Pharmacology of glucocorticoids: beyond receptors. European Journal of Pharmacology 2008;585(2-3):483-91. [DOI] [PubMed] [Google Scholar]
Vickers 2005
- Vickers AJ. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Medical Research Methodology 2005;5:35. [DOI: 10.1186/1471-2288-5-35] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chou 2016
- Chou R, Pinto RZ, Fu R, Lowe RA, Henschke N, Dana T. Systemic corticosteroids for radicular and non-radicular low back pain. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD012450. [DOI: 10.1002/14651858.CD012450] [DOI] [PMC free article] [PubMed] [Google Scholar]