Abstract
Objectives:
BioNTech (Pfizer) and CoronaVac (Sinovac) vaccines are two of the most administered coronavirus disease-2019 (COVID-19) vaccines worldwide. Vaccination against severe acute respiratory syndrome-coronavirus-2 has caused a diagnostic challenge in oncological 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) studies. The aim of our study was to evaluate the 18F-FDG PET/CT findings of the two most commonly administered vaccines worldwide.
Methods:
Patients over 18 years old who underwent 18F-FDG PET/CT for oncological purposes in our institution between January 13, 2021 and January 31, 2022, who received a single or second dose of the BioNTech or CoronaVac vaccines in the last two months, were included in the study. Descriptive analyses were presented as mean, standard deviation, frequency and ratio. Additionally, chi-square test was used to analyze categorical variables.
Results:
Ipsilateral deltoid muscle hypermetabolism was observed in 6.9% (n=15) and 14.3% (n=22) patients who received CoronaVac and BioNTech vaccines, respectively. Ipsilateral axillary lymph node hypermetabolism was observed in 11% (n=24) and 41.6% (n=64) patients who received CoronaVac and BioNTech vaccines, respectively. Synchronous deltoid muscle and axillary lymph node hypermetabolism was observed in 4.14% (n=9) and 12.33% (n=19) patients who received CoronaVac and BioNTech vaccines, respectively. Significant differences were detected between CoronaVac and BioNTech vaccines in terms of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and synchronous deltoid muscle and axillary lymph node hypermetabolism (p<0.05).
Conclusion:
COVID-19 vaccination may result in ipsilateral axillary lymph node hypermetabolism, ipsilateral deltoid muscle hypermetabolism, or synchronous deltoid muscle and axillary lymph node hypermetabolism with different frequencies depending on the type of vaccination. Although synchronous deltoid muscle and axillary lymph node hypermetabolism can reduce misinterpretation of 18F-FDG PET/CT, to avoid misinterpretation, it is important to question the vaccination history during ongoing COVID-19 vaccination process.
Keywords: 18F-FDG PET/CT, CoronaVac, BNT162b2, hypermetabolism, axillary lymph node
Abstract
Amaç:
BioNTech (Pfizer) ve CoronaVac (Sinovac) aşıları, dünya çapında en çok uygulanan koronavirüs hastalığı-2019 (COVID-19) aşılarından ikisi olmuştur. Şiddetli akut solunum yetmezliği sendromu-koronavirüs-2’ye karşı aşılama, onkolojik 18F-florodeoksiglukoz (FDG) pozitron emisyon tomografisi/bilgisayarlı tomografi (PET/BT) çalışmalarında tanısal zorluklara neden olmaktadır. Çalışmamızın amacı, dünya çapında en sık uygulanan iki aşının 18F-FDG PET/BT bulgularını değerlendirmektir.
Yöntem:
13 Ocak 2021 ile 31 Ocak 2022 tarihleri arasında kurumumuzda onkolojik amaçlı 18F-FDG PET/BT yapılan ve görüntüleme öncesi son iki ayda tek veya ikinci doz BioNTech veya CoronaVac aşısı yapılmış 18 yaş üstü hastalar çalışmaya dahil edildi. Tanımlayıcı analizler ortalama, standart sapma, frekans ve oran olarak sunuldu. Ayrıca kategorik değişkenlerin analizinde ki-kare testi kullanıldı.
Bulgular:
CoronaVac ve BioNTech aşısı olan hastaların sırasıyla %6,9’unda (n=15) ve %14,3’ünde (n=22) ipsilateral deltoid kas hipermetabolizması gözlendi. CoronaVac ve BioNTech aşısı olan hastaların sırasıyla %11’inde (n=24) ve %41,6’sında (n=64) ipsilateral aksiller lenf nodu hipermetabolizması gözlendi. Senkron deltoid kas ve aksiller lenf nodu hipermetabolizması, CoronaVac ve BioNTech aşıları yapılan hastaların sırasıyla; %4,14’ünde (n=9) ve %12,33’ünde (n=19) gözlendi. CoronaVac ve BioNTech aşıları arasında ipsilateral deltoid kas hipermetabolizması, ipsilateral aksiller lenf nodu hipermetabolizması ve senkron deltoid kas ve aksiller lenf nodu hipermetabolizması açısından anlamlı fark saptandı (p<0,05).
Sonuç:
COVID-19 aşısı, aşı tipine bağlı olarak farklı sıklıkta ipsilateral aksiller lenf nodu hipermetabolizması, ipsilateral deltoid kas hipermetabolizması veya senkron deltoid kas ve aksiller lenf nodu hipermetabolizması ile sonuçlanabilir. Senkron deltoid kas ve aksiller lenf nodu hipermetabolizması 18F-FDG PET/BT’nin yanlış yorumlanmasını azaltabilse de, yanlış yorumlamayı önlemek için devam eden COVID-19 aşılama sürecinde aşı geçmişini sorgulamak önemlidir.
Introduction
In late 2019, a new respiratory coronavirus disease-2019 (COVID-19) was detected in Wuhan, China’s Hubei Province which was caused by a novel coronavirus named severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) (1,2,3). A few months later, the World Health Organization (WHO) announced SARS-CoV-2 outbreak as a Public Health Emergency of International Concern on January 30, 2020 and subsequently reported COVID-19 as a pandemic on March 11, 2020 (1,3,4,5). With the aim of stopping the spread of the virus, many countries worldwide have reorganized the public order with strict restrictions that impact social life, businesses school, travel, economy and etc. (1,3).
To protect human health and reduce the unfavorable effects of COVID-19 on daily life, the development of vaccines and spread of vaccinations against SARS-CoV-2 has been accepted as the foremost prevention worldwide (6). As of January 12, 2022, WHO has evaluated that the AstraZeneca/Oxford, Johnson and Johnson, Moderna, BioNTech/Pfizer, Sinopharm, Sinovac, Covaxin, Covovax, Nuvaxovid vaccines against COVID-19 have met the necessary criteria for safety and efficacy (7). To date, BNT162b2 (BioNTech/Pfizer) and CoronaVac (Sinovac) have been the two of the most administered vaccines worldwide (8).
In a multicenter study by Dai et al. (9), it was revealed that cancer patients with COVID-19 have an almost threefold higher mortality ratio than COVID-19 patients without cancer. Prior vaccination of patients receiving cancer treatment, patients with advanced stage cancer and patients with hematologic malignancies and lung cancer have been recommended (10). Thus, vaccination prioritization of cancer and chronic diseases has been implemented in many countries.
Injection site pain and swelling, fatigue, headache, myalgia, chills, arthralgia, and lymphadenopathy are the adverse events after BNT162b2 and Coronavac vaccination (11,12). In addition to adverse events, vaccination against SARS-CoV-2 has caused a diagnostic challenge in oncologic 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT). Various studies have shown that 18F-FDG avid ipsilateral lymphadenopathy on 18F-FDG PET/CT was detected in approximately 50% and 10% of patients who received the BNT162b2 and CoronaVac vaccines, respectively (13,14). The aim of our study was to evaluate the 18F-FDG PET/CT findings of the two most commonly administered vaccines and possible factors affecting these findings as age, sex, systemic treatment, hematological malignancy, and days between vaccination and 18F-FDG PET/CT study. To the best of our knowledge, this study includes the largest number of participants comparing 18F-FDG PET/CT findings of both vaccines in the literature.
Materials and Methods
This retrospective study was conducted with the approval of the University of Health Sciences Turkey, Gulhane Scientific Research Ethics Committee of our institution (decision no: 2021/297). Patients over 18 years old who underwent 18F-FDG PET/CT for oncological purposes in our institution between January 13, 2021 and January 31, 2022 were reviewed. Patients who received a single or booster dose of the BNT162b2 or CoronaVac vaccines in the last two months before 18F-FDG PET/CT were included in the study. Patients who have not been vaccinated against COVID-19, patients who have been vaccinated after 18F-FDG PET/CT imaging, patients who have been vaccinated against other infections, 18F-FDG PET/CT imaging other than oncological purpose, patients who have missing data of vaccination and patient history, patients with known or congruent axillary lymph node metastasis, patients who had undergone axillary lymph node dissection before and patients with more than two months between vaccination and 18F-FDG PET/CT imaging were excluded from the study (Figure 1).
Figure 1.
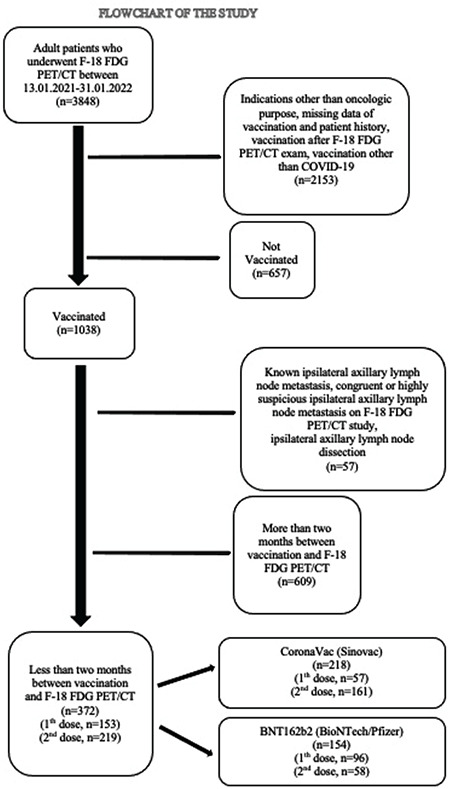
Flow chart of the study
FDG: Fluorodeoxyglucose, PET/CT: Positron emission tomography/computed tomography
CoronaVac vaccine contained SARS-CoV-2 antigen (600SU) and aluminum hydroxide (0.45 mg/mL) as an adjuvant (11). BNT162b2 vaccine contained 30 micrograms of tozinameran, a single-stranded, 5’-capped messenger RNA (mRNA) (12). The demographic data, medical diagnosis, and treatment history of patients, information about the date of vaccination, vaccination site and vaccine brand were obtained from the patient anamnesis form filled before 18F-FDG PET/CT imaging.
A 64-slice CT-integrated PET scanner (Discovery 690-GE Healthcare, Milwaukee, Wisconsin, USA) was used for PET/CT imaging. After fasting for at least 6 h, patients with blood glucose levels below 150 mg/dL were intravenously injected with 3.7 MBq/kg (0.1 mCi/kg) 18F-FDG. Approximately one hour later, CT (120 kV, 10-90 mA) and PET (3 min per bed) images of the area from the vertex to mid-thigh were acquired. Sagittal, coronal, transverse sections and maximum intensity projection images were obtained by iterative reconstruction (12 subset and four iteration) and attenuation correction. The images were analyzed using Advantage Workstation 4.6 (GE Healthcare). All PET/CT images were analyzed by two nuclear medicine physicians with 6 and 13 years of 18F-FDG PET/CT experience. Considering previous studies, maximum standard uptake value (SUVmax) values were measured by placing a region of interest at the injection site and at the ipsilateral axillary lymph node with the highest 18F-FDG uptake and compared with the SUVmax values of the contralateral side. 18F-FDG uptake in the deltoid muscle and axillary lymph node was accepted as positive if the ipsilateral-to-contralateral SUVmax value ratio was at least 1.5 (13,15).
Statistical Analysis
Statistical analyzes were performed using version 26.0 SPSS (IBM Corp., Armonk, New York, USA) and Stata/MP 16 (Stata Corporation, College Station, Texas, USA) softwares. Descriptive analyzes were presented as mean, standard deviation (SD) and frequency. The Kolmogorov-Smirnov test was used to determine whether the obtained parameters confirmed a normal distribution. Chi-square test was used to analyze categorical variables. A tetrachoric correlation test was used to analyze the correlations between the binary variables. Student’s t-test was used for comparing the normally distributed continuous variables. While investigating the associations between continuous, ordinal and dichotomous variables, the correlation coefficients (r) and their significance (p) were calculated using the Point Bi-serial and Pearson correlation tests.
Logistic regression models were fit with a binary dependent variable [ipsilateral deltoid muscle hypermetabolism (yes/no), ipsilateral axillary lymph node uptake (yes/no)] and with the following independent variables: Age, gender, systemic therapy (yes/no), hematologic malignancy (yes/no), vaccine brand, days after the single dose of vaccination, days after the booster dose of vaccination. The test of Hosmer and Lemeshow goodness of fit statistics were used to assess model fit. A 5% type-1 error level was used to infer statistical significance.
Results
General Demographics
Of 372 adults analyzed in this study, 161 (43.27%) were female and 211 (56.72%) were male. Their mean age was 57.24 (SD: 12.73). The number of patients who received CoronaVac and BNT162b2 vaccines was 218 (58.60%) and 154 (41.39%), respectively. The number of patients who received the single dose and booster doses were 153 (41.12%) and 219 (58.87%), respectively. The number of patients who received the single dose and booster doses of CoronaVac vaccine was 57 (15.32%) and 161 (43.27%), respectively. The number of patients who received the single dose and booster doses of BNT162b2 vaccine was 96 (25.80%) and 58 (15.59%), respectively. Table 1 summarizes the demographic characteristics of the study population.
Table 1. Demographic characteristics of study population.
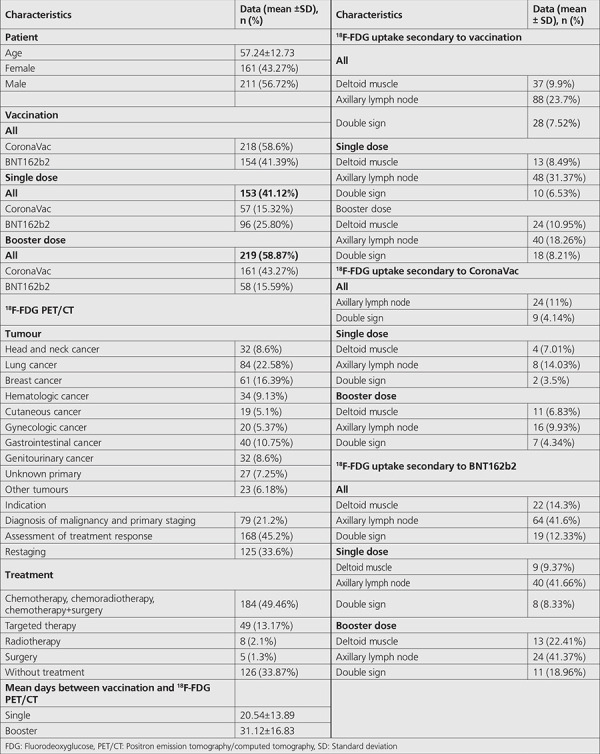
The distribution of the 18F-FDG PET/CT indications in numbers were; as head and neck cancer: 32 (8.6%), lung cancer: 84 (22.58%), breast cancer: 61 (16.39%), hematologic malignancy: 34 (9.13%), cutaneous cancer: 19 (5.1%), gynecologic cancer: 20 (5.37%), gastrointestinal cancer: 40 (10.75%), genitourinary cancer: 32 (8.6%), unknown primary: 27 (7.25%) and other tumors: 23 (6.18%).
The mean interval between the single dose of vaccination and 18F-FDG PET/CT imaging was 20.54 (SD: 13.89) (range, 1-59). The mean interval between the booster dose of vaccination and 18F-FDG PET/CT imaging was 31.12 (SD: 16.83) (range, 1-60).
In terms of positive ipsilateral axillary lymph node hypermetabolism, the median days after the single dose of CoronaVac and BNT162b2 vaccinations were 8 and 11, respectively. Additionally, in terms of positive ipsilateral axillary lymph node hypermetabolism, the median days after the booster dose of CoronaVac and BNT162b2 vaccination were 16 and 17, respectively.
The number of patients who received chemotherapy-chemoradiotherapy- surgery+chemotherapy, targeted therapy, radiotherapy, surgery and no treatment were 184 (49.46%), 49 (13.17%), 8 (2.1%) and 5 (1.3%) and 126 (33.87%) respectively. The number of patients who received systemic therapy (chemotherapy, chemoradiotherapy, surgery+chemotherapy and targeted therapy) was 233 (62.6%).
18F-FDG PET/CT study indications were diagnosis of malignancy and primary staging (n=79, 21.2%), assessment of treatment response (n=168, 45.2%) and restaging (n=125, 33.6%).
Vaccination caused 18F-FDG uptake in the ipsilateral deltoid muscle and axillary lymph nodes was detected in 9.9% (n=37) and 23.7% (n=88) of patients, respectively. Additionally, synchronous ipsilateral deltoid muscle and axillary lymph node hypermetabolism [Double sign (“DS”)] (16) were detected in 28 patients (7.52%) (Figures 2, 3). Additionally, supraclavicular lymph node hypermetabolism was detected secondary to vaccination in 8 (2.15%) patients, 7 of whom (4.54%) were secondary to the BNT162b2 vaccine (Figure 4).
Figure 2.
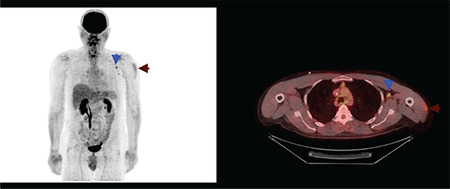
Maximum intensity projection and axial fusion image of 18F-FDG PET/CT. Synchronous hypermetabolism localised to the left deltoid muscle and left level 2 axillary lymph node secondary to BNT162b2 (BioNTech/Pfizer) vaccination (blue and claret arrows)
FDG: Fluorodeoxyglucose, PET/CT: Positron emission tomography/computed tomography
Figure 3.
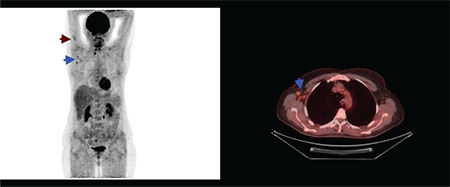
Maximum intensity projection and axial fusion image of 18F-FDG PET/CT. Synchronous hypermetabolism localised to the right deltoid muscle and right level 1 axillary lymph nodes secondary to BNT162b2 (BioNTech/Pfizer) vaccination (blue and claret arrows)
FDG: Fluorodeoxyglucose, PET/CT: Positron emission tomography/computed tomography
Figure 4.
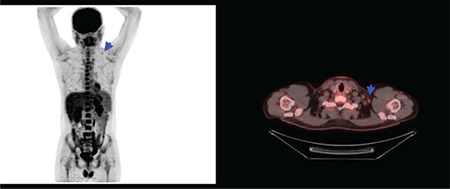
Maximum intensity projection and axial fusion image of 18F-FDG PET/CT. Left hypermetabolic supraclavicular lymph node secondary to BNT162b2 (BioNTech/Pfizer) vaccination (blue arrows)
FDG: Fluorodeoxyglucose, PET/CT: Positron emission tomography/computed tomography
Ipsilateral Deltoid Muscle Hypermetabolism Based Analysis
Ipsilateral deltoid muscle hypermetabolism was detected in 6.9% (n=15) and 14.3% (n=22) patients who received CoronaVac and BNT162b2 vaccines, respectively. A significant difference was detected between CoronaVac and BNT162b2 vaccines in terms of ipsilateral deltoid muscle hypermetabolism (p<0.05). Ipsilateral deltoid muscle hypermetabolism was detected in 7.01% (n=4) and 9.37% (n=9) patients who received a single dose of CoronaVac and BNT162b2 vaccines, respectively. It was not detected any significant difference between only a single dose of CoronaVac and BNT162b2 vaccines in terms of ipsilateral deltoid muscle hypermetabolism (p>0.05). Ipsilateral deltoid muscle hypermetabolism was detected 6.83% (n=11) and 22.41% (n=13) patients who received booster doses of CoronaVac and BNT162b2 vaccines, respectively. A significant difference was detected between the booster doses of CoronaVac and BNT162b2 vaccines in terms of ipsilateral deltoid muscle hypermetabolism (p<0.05).
There was a significant correlation between BNT162b2 vaccination and ipsilateral deltoid muscle hypermetabolism (p<0.05, rtet: 0.2562).
There was a significant association between ipsilateral deltoid muscle hypermetabolism and days passed after the booster dose of vaccination [Odds ratio (OR): 0.877; 95% confidence interval (CI), 0.829-0.928; p<0.05], days passed after the single dose of vaccination (OR, 0.748; 95% CI, 0.636-0.880; p<0.05) and age (OR, 0.965; 95% CI, 0.942-0.990; p<0.05).
Ipsilateral Axillary Lymph Node Hypermetabolism Based Analysis
Ipsilateral axillary lymph node hypermetabolism was detected in 11% (n=24) and 41.6% (n=64) patients who received CoronaVac and BNT162b2 vaccines, respectively. A significant difference was detected between CoronaVac and BNT162b2 vaccines in terms of ipsilateral axillary lymph node hypermetabolism (p<0.05). Ipsilateral axillary lymph node hypermetabolism was detected in 14.03% (n=8) and 41.66% (n=40) patients who received a single dose of CoronaVac and BNT162b2 vaccines, respectively. A significant difference was detected between the single dose of CoronaVac and BNT162b2 vaccines in terms of ipsilateral axillary lymph node hypermetabolism (p<0.05). Ipsilateral axillary lymph node hypermetabolism was detected in 9.93% (n=16) and 41.37% (n=24) patients who received booster doses of CoronaVac and BNT162b2 vaccines, respectively. A significant difference was detected between the booster doses of CoronaVac and BNT162b2 vaccines in terms of ipsilateral axillary lymph node hypermetabolism (p<0.05).
There was a significant correlation between BNT162b2 vaccination and ipsilateral axillary lymph node hypermetabolism (p<0.05, rtet: 0.5707).
There was a significant correlation between ipsilateral deltoid muscle hypermetabolism and ipsilateral axillary lymph node hypermetabolism (p<0.05, rtet: 0.7112).
There was a significant association between ipsilateral axillary lymph node hypermetabolism and days passed after the booster dose of vaccination (OR, 0.927; 95% CI, 0.898-0.958; p<0.05), days passed after the single dose of vaccination (OR, 0.927; 95% CI, 0.893-0.962; p<0.05) age (OR, 0.957; 95% CI, 0.936-0.976; p<0.05) and booster dose (OR, 2.04; 95% CI, 1.261-3.319; p<0.05).
“DS” Based Analysis
The percentage of “DS” in patients receiving CoronaVac and BNT162b2 vaccines were 4.14% (n=9) and 12.33% (n=19), respectively. A significant difference was detected between CoronaVac and BNT162b2 vaccines in terms of “DS” (p<0.05). The percentage of “DS” in patients receiving the single dose of CoronaVac and BNT162b2 vaccines were 3.5% (n=2) and 8.33% (n=8), respectively. It was not detected any significant difference between the single dose of CoronaVac and BNT162b2 vaccines in terms of “DS” (p>0.05). The percentage of “DS” in patients receiving booster doses of CoronaVac and BNT162b2 vaccines were 4.34% (n=7) and 18.96% (n=11), respectively. A significant difference was detected between the booster doses of CoronaVac and BNT162b2 vaccines in terms of “DS” (p<0.05).
75.67% and 31.81% patients with ipsilateral deltoid muscle and axillary lymph node hypermetabolism had “DS”, respectively.
There was a significant correlation between BNT162b2 vaccination and “DS” (p<0.05, rtet: 0.3472).
There was a significant association between “DS” and days passed after the second dose of vaccination (OR, 0.878; 95% CI, 0.824-0.936; p<0.05), days passed after the single dose of vaccination (OR, 0.768; 95% CI, 0.649-0.908; p<0.05) and age (OR, 0.961; 95% CI, 0.934-0.988; p<0.05).
Age, Gender, Therapy, Hematologic Malignancy Based Analysis
In terms of gender, it was not detected any significant difference between the positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p>0.05).
In terms of age, a significant difference was detected between the positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p<0.05).
In terms of systemic therapy, it was not detected any significant difference between positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p>0.05).
In terms of hematologic malignancy, it was not detected any significant difference between positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p>0.05).
Time Based Analysis
In terms of days passed after the single and booster dose of vaccination, a significant difference was detected between positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p<0.05).
In terms of days passed after the single dose of BNT162b2 vaccination, a significant difference was detected between positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p<0.05). There was a significant and negative correlation between days passed after the single dose of BNT162b2 vaccination and ipsilateral deltoid muscle hypermetabolism (p<0.05, rpb: -0.401), ipsilateral axillary lymph node hypermetabolism (p<0.05, rpb: -0.539) and “DS” (p<0.05, rpb: -0.371).
In terms of days passed after the booster dose of BNT162b2 vaccination, a significant difference was detected between positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p<0.05). There was a significant and negative correlation between days passed after the booster dose of BNT162b2 vaccination and ipsilateral deltoid muscle hypermetabolism (p<0.05, rpb: -0.651), ipsilateral axillary lymph node hypermetabolism (p<0.05, rpb: -0.554) and “DS” (p<0.05, rpb: -0.570).
In terms of days passed after the single and booster dose of CoronaVac vaccination, it was not detected any significant difference between positive and negative groups of ipsilateral deltoid muscle hypermetabolism, ipsilateral axillary lymph node hypermetabolism and “DS” (p>0.05).
In terms of single dose, ipsilateral axillary lymph node hypermetabolism was detected in 35.41%, 27.08%, 20.83%, 8.33%, 4.16%, 2.08%, and 2.08% patients at 1st-7th weeks after vaccination, respectively.
In terms of booster dose, ipsilateral axillary lymph node hypermetabolism was detected in 32.5%, 12.5%, 17.5%, 12.5%, 15%, 7.5%, and 2.5% of patients 1st-6th and 9th weeks after vaccination, respectively.
Discussion
Immunization against SARS-CoV-2 reduces the risk of life-threatening disease, therefore vaccination has been used as a necessary instrument against COVID-19 (4). Mass vaccination campaigns have been in progress worldwide by implementing additional booster dose. To date, BNT162b2 (BioNTech/Pfizer) and CoronaVac (Sinovac) vaccines have been the two of the most administered vaccines globally (8). BNT162b2 consist of “nucleoside-modified mRNA” encoding the “viral spike glycoprotein” of SARS-CoV-2 (12,17,18,19). CoronaVac is inactivated virus vaccine that contains inactivated SARS-CoV-2 as antigen (11). Although, BNT162b2 stimulates remarkably higher levels of SARS-CoV-2-specific binding and neutralizing antibody responses, CoronaVac elicits higher CD4+ and CD8+ T-cell responses than BNT162b2 (20). After vaccination, two main responses generated are the formation of cytotoxic T-lymphocytes in cellular response and formation of mature B-cells in the germinal center (GC) of the lymph node in humoral response (21,22). mRNA COVID-19 vaccines generate significant GC responses and the GC response substantially participates in the humoral response (22,23,24). In a study by Turner et al. (25), after examining the fine needle aspirates of draining axillary lymph nodes, it was detected that BNT162b2 vaccine stimulate GC B-cell responses eliciting the strong humoral immunity. In another study by Ellebedy et al. (24) similar results was founded that BNT162b2 vaccine has the capacity to elicit powerful GC reactions that are essential for durable humoral immunity.
The vulnerability of cancer patients to the influenza virus is a well-known and influenza infection increases the risk of death tenfold compared to patients without malignancy (4,26).
Based on this experience, the potential consequences of COVID-19 in cancer patients have been worrisome since the beginning of the pandemic. Patients with cancer have higher rates of severe disease and fatality after COVID-19 than the general population. In a recent meta-analysis (26 studies, 23,736 patients), Venkatesulu et al. (27) found nearly threefold higher odds of death in cancer patients affected by COVID-19 than in those without cancer (28). According to the Global Cancer Observatory Database, nearly 19.3 million new cancer cases have been declared in 2020 globally (29). Moreover, the incidence of cancer may increase, causing serious challenges for healthcare providers in protecting cancer patients at risk of COVID-19 (4). Prior vaccination of patients receiving cancer treatment, patients with advanced stage cancer and patients with hematologic malignancies and lung cancer have been recommended (10). Therefore, considering the possible high risk of severe disease, cancer patients have been prioritized in vaccination campaigns in most countries (30,31).
In addition to adverse events, vaccination against SARS-CoV-2 has caused a diagnostic challenge in cancer patients. Solitary 18F-FDG avid axillary lymphadenopathy secondary to COVID-19 vaccination may challenge nuclear medicine physician and oncologist in patients diagnosed with lymphoma, breast cancer, malignant melanoma and other cutaneous malignancies (16). In the studies by Cohen et al. (32), Orevi et al. (16) and Eifer et al. (13) have shown that vaccination with BNT162b2 resulted in 18F-FDG-positive axillary (45.6%, 66%, and 45%, respectively) lymph nodes ipsilateral to the injection site, especially after the booster dose. In another study by Sahin (14), the ipsilateral hypermetabolic axillary lymph nodes were detected in 9.9% (18/182) patients vaccinated with CoronaVac and 37.5% (9/24) patients vaccinated with BNT162b2. In our study, findings followed previous studies as ipsilateral axillary lymph node hypermetabolism was detected in 11% and 41.6% of patients who received CoronaVac and BNT162b2 vaccines, respectively. It has been suggested that 18F-FDG avid lymph nodes are related to the activation of the immune response (13,16). Mok et al. (20) detected that BNT162b2 elicited significantly higher PRNT50, PRNT90, sVNT, spike receptor binding, spike N-terminal domain binding, spike S2 domain binding, spike FcR binding and antibody avidity levels than CoronaVac. The stronger humoral responses of BNT162b2 than the CoronaVac vaccine may explain the detection of ipsilateral hypermetabolic axillary lymph nodes in approximately 4 times more patients in favor of BNT162b2 vaccine in our study.
After the proximal arm injection, 18F-FDG uptake secondary to immune reaction is substantially limited to regionally draining axillary lymph nodes. Additionally, the subsequent draining to supraclavicular lymph nodes may also be uncommonly observed (16). In the studies by Orevi et al. (16) and Cohen et al. (32), 18F-FDG avid supraclavicular lymph nodes were detected in 5.7% and 7.9% of the BNT162b2 vaccination, respectively. In our study, supraclavicular lymph node hypermetabolism was detected secondary to vaccination in 8 (2.15%) patients, 7 of whom (4.54%) were secondary to BNT162b2 vaccine.
In a study by Eifer et al. (13), it was detected a strong inverse association between hypermetabolic ipsilateral lymph nodes secondary to BNT162b2 vaccination and patient age. In another study by Sahin (14), a statistically significant difference was detected between patients with and without hypermetabolic axillary lymph nodes in terms of age. In their study, Cohen et al. (32) stated that patients younger than 62 and 64 years of age show a higher incidence of vaccine-associated hypermetabolic lymphadenopathy (VAHL) after the single and booster dose of BNT162b2 vaccine, respectively. In our study, there was a significant and inverse association between age and ipsilateral axillary lymph node hypermetabolism. Brockman et al. (33) detected that responses to mRNA-based COVID-19 vaccination in older adults are quantitatively and functionally impaired. Additionally, age was found as the most crucial factor in the antibody response to COVID-19 mRNA vaccination (33). Impaired primary and secondary antibody responses to vaccination in the elderly are considered responsible for this situation (13,34).
Eifer et al. (13) detected that a strong inverse association between hypermetabolic ipsilateral lymph nodes secondary to BNT162b2 vaccination and immunosuppressive treatment and the presence of hematologic disease. In their study, Cohen et al. (32) stated that the detection of VAHL was not different in patients receiving chemotherapy, radiotherapy, biological treatment, or immunotherapy. In our study, it was not detected any significant association between ipsilateral axillary lymph node hypermetabolism and systemic treatment and presence of hematologic disease. Various chemotherapeutics and targeted therapies in the treatment plan, days passed after the last treatment, hematologic disease burden, bone-marrow reserves, and other differences in the study population may be responsible for the discordant results.
In a study by Orevi et al. (16), “DS” was detected in the 20% of the vaccinated patients. Additionally, it was determined that 95% of patients with 18F-FDG accumulation at the injection site had “DS” and both the specificity and positive predictive value of “DS” for vaccination-caused lymphadenopathy were 100%. Additionally, “DS” was considered a marker to prevent misinterpretation of 18F-FDG PET/CT studies and further unnecessary procedures (16). In our study 75.67% patients with ipsilateral deltoid muscle hypermetabolism had “DS” (higher for BNT162b2). Additionally, 31.81% patients with ipsilateral axillary lymph node hypermetabolism had “DS” (higher for CoronaVac). Although “DS” can reduce false positive results in cancer patients, in cases with only axillary lymph node hypermetabolism, vaccination history based follow-up and correlation with other imaging modalities would be useful to avoid misinterpretation.
In the study by Eifer et al. (13), the number of days from the last vaccine dose and the number of vaccine dose was significantly associated with increased odds of hypermetabolic lymph nodes. In our study, the number of days from the last vaccine dose was significantly associated with decreased odds of ipsilateral axillary lymph node hypermetabolism. Additionally, the number of vaccine doses was significantly associated with increased odds of ipsilateral axillary lymph node hypermetabolism. In a comparison of two studies, the direction of association between hypermetabolic axillary lymph node and the number of days from the last vaccine dose was incongruent. As the number of days after the last dose of vaccination increases, the detection of axillary lymph node hypermetabolism is expected to decrease. However, hypermetabolic axillary lymph nodes may not be detected if the interval is too short. In the study by Cohen et al. (32), the first five days after the first vaccine, the third week after the first vaccine and at least three weeks after the booster dose administration was defined as appropriate time window for 18F-FDG PET/CT imaging (the incidence of VAHL is lower).
In the study by Eifer et al. (13), no association was found between ipsilateral deltoid muscle hypermetabolism and patient age. The number of vaccine doses was associated with increased odds of ipsilateral deltoid muscle hypermetabolism. Additionally, the number of days from the last vaccination was associated with decreased odds of ipsilateral deltoid muscle hypermetabolism. In our study, the number of days from the last vaccine dose and age was associated with decreased odds of ipsilateral deltoid muscle hypermetabolism. Impaired inflammatory responses and wound healing in the elderly and resolution of inflammation with time after vaccination are considered responsible (35). Furthermore, in our study there was not any significant association between ipsilateral deltoid muscle hypermetabolism and the number of vaccine dose. Hypermetabolism at the injection site is induced by the traumatic and inflammatory effect of the injection (13,16). In contrast to the complicated immune response that causes axillary lymph node hypermetabolism, trauma and inflammation caused by hypermetabolism at the injection site may not depend on the number of vaccination.
In the study by Sahin (14), the rates of hypermetabolic axillary lymph node after 1st dose of CoronaVac and BNT162b2 vaccination were 9.6% and 35%, respectively. Additionally, the rates of hypermetabolic axillary lymph node after 2nd dose of CoronaVac and BNT162b2 vaccinations were 10.2% and 50%, respectively (14). In the study by Cohen et al. (32), VAHL secondary to BNT162b2 vaccination was detected in 36.4% of the patients after the first dose and in 53.9% of patients after the booster dose. In our study, ipsilateral axillary lymph node hypermetabolism secondary to CoronaVac vaccination was detected in 14.03% (n=8) and 9.93% (n=16) patients after the single dose and booster dose, respectively. Additionally, ipsilateral axillary lymph node hypermetabolism secondary to BNT162b2 vaccination was detected in 41.66% (n=40) and 41.37% (n=24) patients after only a single and booster dose, respectively. It was determined that the results of our study were partially close to those of the aforementioned studies.
In the study by Sahin (14), the ipsilateral hypermetabolic axillary lymph nodes were detected in 9.9% patients vaccinated with CoronaVac and 37.5% (9/24) patients vaccinated with BNT162b2. However, the number of patients vaccinated with BNT162b2 was low (n=24) and statistical comparison could not be made between the two patient groups (14). In our study, ipsilateral axillary lymph node hypermetabolism was detected in 11% and 41.6% of patients who received CoronaVac and BNT162b2 vaccines, respectively. Additionally, a significant difference was detected between the CoronaVac and BNT162b2 vaccines with respect to ipsilateral axillary lymph node hypermetabolism. Furthermore, there was a significant correlation between BNT162b2 vaccination and ipsilateral axillary lymph node hypermetabolism. In their study, Mok et al. (20) found that 91.8% and 100% of the participants vaccinated with CoronaVac and BNT162b2, respectively, reached the 50% protection threshold 1 month after the second dose of vaccination. Further, 6 months after the second dose of vaccination, those ratios decreased to 16.3% and 79.6%, respectively. BNT162b2 was found to be more immunogenic and durable than the CoronaVac vaccine in terms of peak and waning antibody titers (20). In a study by Cohen et al. (22), correlation between VAHL secondary to BNT162b2 and humoral immunity in hematologic malignancy was researched. VAHL ratios were 10% in patients with negative serology, 31.3% in patients with low and 72.2% in patients with high anti-spike titers. Additionally, a positive statistically significant correlation was found between VAHL and serology ranks (rs=0.530, Pv <0.001) and it was suggested that the detection of VAHL indicates a higher likelihood of antibody production and an effective humoral response (22). We thought that the results of our study reflect the different immunogenic properties and effects of CoronaVac and BNT162b2 vaccines via 18F-FDG hypermetabolism. Although, BNT162b2 can cause a more hypermetabolic axillary lymph node than CoronaVac vaccine in parallel to its higher immunogenicity, to comment on serological antibody titer-related vaccine efficacy for COVID-19 infection is beyond the scope of the results of our study.
In a study by Eshet et al. (36), avid axillary lymphadenopathy was observed up to 7-10 weeks after the booster dose of BNT162b2 vaccination. In our study, ipsilateral axillary lymph node hypermetabolism was observed up to 7th and 9th week after the single and booster doses, respectively. Additionally, after the single and booster doses, ipsilateral axillary lymph node hypermetabolism was most frequently seen within the first month after vaccination and detected in 91.65% and 75% of patients, respectively. Further, after the single and booster dose, ipsilateral axillary lymph node hypermetabolism was most frequently seen within the first week after vaccination and detected in 35.41% and 32.5% of patients, respectively. Although postponing the 18F-FDG PET/CT study for a few weeks after COVID-19 vaccination reduces the possibility of ipsilateral hypermetabolic axillary lymph nodes, it cannot be completely prevented according to the results. Additionally, it should also be considered that long-term delay of 18F-FDG PET/CT study may adversely affect patient management.
Study Limitations
The main limitations of the study are the retrospective design, the inaccessibility to the vaccination records and patient histories of all patients who underwent 18F-FDG PET/CT. Axillary lymph nodes were not examined histopathologically due to invasive procedure. These studies were not repeated to follow-up the hypermetabolic axillary lymph nodes. Additionally, the association between vaccine-specific immune response and axillary lymph node hypermetabolism could not be investigated because to the lack of serological tests.
Conclusion
In our study population, 18F-FDG PET/CT detected ipsilateral axillary lymph node hypermetabolism in 11% and 41.6% of cancer patients receiving the inactivated SARS-CoV-2 vaccine and the novel mRNA-based COVID-19 vaccine, respectively. Although, the number of days after vaccination and age were negatively associated with ipsilateral axillary lymph node hypermetabolism, vaccination of booster dose was positively associated with ipsilateral axillary lymph node hypermetabolism. Additionally, BNT162b2 vaccination showed a positive relationship with ipsilateral axillary lymph node hypermetabolism. To avoid misinterpretation and unnecessary invasive procedures, it is necessary to enquire the COVID-19 vaccination history, particularly in young adults who received the booster dose of BNT162b2 vaccination recently. Although, misinterpretation of 18F-FDG PET/CT and further unnecessary invasive procedures could be reduced when “DS” was observed, in cases with only ipsilateral axillary lymph node hypermetabolism, vaccination history based follow-up and correlation with other imaging modalities would be useful to avoid misinterpretation. In cancer patients who have tumors with a predilection for axillary lymph node involvement, it would be useful to perform vaccination in the contralateral arm, which is away from the tumoral nodal drainage zone. Ipsilateral axillary lymph node hypermetabolism was most frequently observed within the first month after vaccination. Postponing the 18F-FDG PET/CT study for a few weeks after COVID-19 vaccination reduces the possibility of ipsilateral hypermetabolic axillary lymph nodes. According to our results, the visualization of hypermetabolic axillary lymph nodes cannot be completely prevented as it can be seen up to 7th and 9th week after the single and booster dose. It should also be considered that long-term delays in 18F-FDG PET/CT study may adversely affect patient management. The results of our study reflect the different immunogenic properties and effects of CoronaVac and BNT162b2 vaccines via 18F-FDG hypermetabolism. BNT162b2 can cause more hypermetabolic axillary lymph nodes than CoronaVac vaccine in parallel to its higher immunogenicity. Prospective 18F-FDG PET/CT studies with specific serological antibody titers must evaluate the efficacy of vaccines against COVID-19 infection via 18F-FDG hypermetabolism.
Acknowledgments
We thank Nur Aydınbelge-Dizdar MD, Semra İnce MD, Kürşat Okuyucu MD, Aslı Ayan MD, Alev Noyaner Çınar MD, Alper Özgür Karaçalıoğlu MD, Bengül Günalp MD, Aylin Çomak MD for their contribution in routine 18F-FDG PET/CT reporting. In addition, we thank to Beyza Bedi MD and Serhan Mahmudov MD especially and nuclear medicine residency students for filling in the routine medical history forms.
Footnotes
Ethics
Ethics Committee Approval: This retrospective study was conducted with the approval of the University of Health Sciences Turkey, Gulhane Scientific Research Ethics Committee of our institution (decision no: 2021/297).
Informed Consent: Informed consent for using medical images was provided from the patients.
Peer-review: Internally peer-reviewed.
Authorship Contributions
Surgical and Medical Practices: H.Ş., E.A., Concept: H.Ş., E.A., Design: H.Ş., E.A., Data Collection or Processing: H.Ş., E.A., Analysis or Interpretation: H.Ş., E.A., Literature Search: H.Ş., E.A., Writing: H.Ş., E.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Uddin M, Mustafa F, Rizvi TA, Loney T, Suwaidi HA, Al-Marzouqi AHH, Eldin AK, Alsabeeha N, Adrian TE, Stefanini C, Nowotny N, Alsheikh-Ali A, Senok AC. SARS-CoV-2/COVID-19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses. 2020;12:526. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 Novel Coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, WHO. WHO Director-General‘s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. (accessed on 15.03.2022). Available from: [Internet] https://www.who.int/dg/speeches/detail/who-director-generals-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 4.Mandal A, Singh P, Samaddar A, Singh D, Verma M, Rakesh A, Ranjan R. Vaccination of cancer patients against COVID-19: towards the end of a dilemma. Med Oncol. 2021;38:92. doi: 10.1007/s12032-021-01540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum. (accessed on 15.03.2022). Available from: [Internet] https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum#:~:text=On%2030%20January%202020%20following,of%20International%20Concern%20(PHEIC)
- 6.Klimek L, Agache I, Cooke E, Jutel M, Akdis CA, O’Hehir R. COVID-19 vaccines-The way forward. Allergy. 2022;77:15–16. doi: 10.1111/all.14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19 advice for the public: Getting vaccinated. (accessed on 15.03.2022). Available from: [Internet] https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice.
- 8.Coronavirus Vaccine Tracker, By Carl Zimmer, Jonathan Corum, Sui-Lee Wee and Matthew Kristoffersen Updated March 11. (accessed on 15.03.2022). Available from: [Internet] https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 9.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Li Z, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, Jaffee EM, Wherry EJ, Soria JC, D’Souza G; AACR COVID-19 and Cancer Task Force. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11:233–236. doi: 10.1158/2159-8290.CD-20-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CoronaVac(Sinovac) package leaflet. (accessed on 15.03 2022). Available from: [Internet] https://www.covidvaccine.gov.hk/pdf/CoronaVac_ENG_PI_brief.pdf.
- 12.Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eifer M, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Shams J, Keret N, Gorfine M, Eshet Y. COVID-19 mRNA vaccination: age and immune status and its association with axillary lymph node PET/CT uptake. J Nucl Med. 2022;63:134–139. doi: 10.2967/jnumed.121.262194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin O. Hypermetabolic axillary lymphadenopathy on FDG PET/CT Due to COVID-19 vaccination. Selcuk Med J. 2021;37:269–275. [Google Scholar]
- 15.Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–898. doi: 10.1007/s00259-011-1729-9. [DOI] [PubMed] [Google Scholar]
- 16.Orevi M, Chicheportiche A, Ben Haim S. Lessons learned from post-COVID-19 vaccination PET/CT studies. J Nucl Med. 2022;63:453–460. doi: 10.2967/jnumed.121.262348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency. Covid-19 mRNA vaccine (Comirnaty): EU summary of product characteristics. 2020. (accessed 15.03.2022). Available from: [Internet] https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty.
- 18.European Medicines Agency. Comirnaty: CHMP public assessment report. 2020. (accessed 15.03.2022). Available from: [Internet] http://www.ema.europa.eu.
- 19.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, Quandt J, Bidmon N, Ulges A, Baum A, Pascal K, Maurus D, Brachtendorf S, Lörks V, Sikorski J, Koch P, Hilker R, Becker D, Eller AK, Grützner J, Tonigold M, Boesler C, Rosenbaum C, Heesen L, Kühnle MC, Poran A, Dong JZ, Luxemburger U, Kemmer-Brück A, Langer D, Bexon M, Bolte S, Palanche T, Schultz A, Baumann S, Mahiny AJ, Boros G, Reinholz J, Szabó GT, Karikó K, Shi PY, Fontes-Garfias C, Perez JL, Cutler M, Cooper D, Kyratsous CA, Dormitzer PR, Jansen KU, Türeci Ö. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. doi: [Internet] https://doi.org/10.1101/2020.12.09.20245175.
- 20.Mok CKP, Cohen CA, Cheng SMS, Chen C, Kwok KO, Yiu K, Chan TO, Bull M, Ling KC, Dai Z, Ng SS, Lui GC, Wu C, Amarasinghe GK, Leung DW, Wong SYS, Valkenburg SA, Peiris M, Hui DS. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2022;27:301–310. doi: 10.1111/resp.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettini E, Locci M. SARS-CoV-2 mRNA Vaccines: immunological mechanism and beyond. Vaccines (Basel) 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen D, Hazut Krauthammer S, Cohen YC, Perry C, Avivi I, Herishanu Y, Even-Sapir E. Correlation between BNT162b2 mRNA Covid-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur J Nucl Med Mol Imaging. 2021;48:3540–3549. doi: 10.1007/s00259-021-05389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lederer K, Castaño D, Gómez Atria D, Oguin TH, Wang S, Manzoni TB, Muramatsu H, Hogan MJ, Amanat F, Cherubin P, Lundgreen KA, Tam YK, Fan SHY, Eisenlohr LC, Maillard I, Weissman D, Bates P, Krammer F, Sempowski GD, Pardi N, Locci M. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295.e5. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellebedy A, Turner J, O’Halloran J, Kalaidina E, Kim W, Schmitz A, Lei T, Thapa M, Case J, Amanat F, Rauseo A, Haile A, Klebert M, Suessen T, Middleton W, Krammer F, Teefey S, Diamond M, Presti R, Xie X, Shi PY. SARS-CoV-2 mRNA vaccines induce a robust germinal centre reaction in humans. Available from: [Internet] https://assets.researchsquare.com/files/rs-310773/v1_covered.pdf?c=1637595722.
- 25.Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, Lei T, Thapa M, Chen RE, Case JB, Amanat F, Rauseo AM, Haile A, Xie X, Klebert MK, Suessen T, Middleton WD, Shi PY, Krammer F, Teefey SA, Diamond MS, Presti RM, Ellebedy AH. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitterman R, Eliakim-Raz N, Vinograd I, Zalmanovici Trestioreanu A, Leibovici L, Paul M. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev. 2018;2:CD008983. doi: 10.1002/14651858.CD008983.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatesulu BP, Chandrasekar VT, Girdhar P, Advani P, Sharma A, Elumalai T, Hsieh CE, Elghazawy HI, Verma V, Krishnan S. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr. 2021;5:pkaa102. doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengar M, Chinnaswamy G, Ranganathan P, Ashok A, Bhosale S, Biswas S, Chaturvedi P, Dhamne C, Divatia J, D’Sa K, Jain H, Laskar S, Moulik NR, Mummudi N, Nair S, Nayak L, Nayak P, Patkar S, Pawaskar P, Ramaswamy A, Shetty O, Singh A, Sridhar E, Thorat J, Badwe R, Pramesh CS; TMH COVID-19 action group. Outcomes of COVID-19 and risk factors in patients with cancer. Nat Cancer. 2022;3:547–551. doi: 10.1038/s43018-022-00363-4. [DOI] [PubMed] [Google Scholar]
- 29.GLOBOCAN 2020: New Global Cancer Data | UICC. (accessed 15.03.2022). Available from: [Internet] https://www.uicc.org/news/globocan-2020-new-global-cancer-data.
- 30.Giesen N, Sprute R, Rüthrich M, Khodamoradi Y, Mellinghoff SC, Beutel G, Lueck C, Koldehoff M, Hentrich M, Sandherr M, von Bergwelt-Baildon M, Wolf HH, Hirsch HH, Wörmann B, Cornely OA, Köhler P, Schalk E, von Lilienfeld-Toal M; COVID-19 guideline panel of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO) 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination and therapy. Eur J Cancer. 2021;147:154–160. doi: 10.1016/j.ejca.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fendler A, de Vries EGE, GeurtsvanKessel CH, Haanen JB, Wörmann B, Turajlic S, von Lilienfeld-Toal M. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022;19:385–401. doi: 10.1038/s41571-022-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48:1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brockman MA, Mwimanzi F, Lapointe HR, Sang Y, Agafitei O, Cheung PK, Ennis S, Ng K, Basra S, Lim LY, Yaseen F, Young L, Umviligihozo G, Omondi FH, Kalikawe R, Burns L, Brumme CJ, Leung V, Montaner JSG, Holmes D, DeMarco ML, Simons J, Pantophlet R, Niikura M, Romney MG, Brumme ZL. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J Infect Dis. 2022;225:1129–1140. doi: 10.1093/infdis/jiab592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 35.Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: a mini-review. Gerontology. 2013;59:159–164. doi: 10.1159/000342344. [DOI] [PubMed] [Google Scholar]
- 36.Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology. 2021;300:E345–E347. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]


