Abstract
A captive male Linnaeus’s two-toed sloth died without any obvious clinical signs. At necropsy, multifocal ulceration at the lumbar and perianal skin, mitral valve vegetation, and multifocal hemorrhage in the leptomeninges were observed. Histopathologically, suppurative meningo-ventriculitis, dermatitis, and endocarditis characterized by severe neutrophilic infiltration were observed. Gram-positive cocci arranged in pairs or chains were present in these inflammatory lesions. Streptococcus agalactiae gene was detected in the skin, heart, and brain tissues by PCR and sequence analysis. These findings may indicate that S. agalactiae primarily infected the skin and then caused septicemia resulting in endocarditis and meningo-ventriculitis. The present case suggests that S. agalactiae infection can cause severe meningo-ventriculitis in two-toed sloth without any specific clinical signs.
Keywords: bacterial infection, meningitis, sloth, streptococcus agalactiae, ventriculitis
Streptococcal infections in humans, domestic animals, and aquatic animals are caused by Streptococcus agalactiae, dysagalactiae, canis, equi, suis, bovis, halichoeri, and iniae [3, 5, 10, 12, 17,18,19]. These streptococcal species exist in the upper respiratory tract, urogenital tract, mammary gland, and skin, and cause opportunistic infection [19]. Moreover, Streptococcus spp. may infect skin wounds and cause bacteremia, resulting in systemic infection [19]. Typical lesions of streptococcal infections are dermatitis, pneumonia, endocarditis, and meningitis [12, 17, 19]. Endocarditis is typically observed at the mitral valve in dogs and pigs [10, 12]. Meningitis can complicate choroiditis and ventriculitis [4, 12, 17, 18]. Microscopically, suppurative inflammation with Gram-positive cocci in pairs or chains can be detected [4, 12, 17, 18].
Linnaeus’s two-toed sloth (Choloepus didactylus) belongs to the genus Choloepus [16]. Sloths are slow moving, arboreal herbivorous, that show quadrupedal suspensory locomotion [16]. Some arboviruses and protozoans are known to infect sloths without causing apparent signs of illness [8]. Bacterial infection of the nervous system has not been reported in the sloth. To our knowledge, streptococcal infection has not been reported in the sloth. The present paper describes pathological findings of a case of meningo-ventriculitis caused by S. agalactiae infection in a sloth.
A male two-toed sloth of unknown age was imported from the republic of Guyana to Japan in 2009 and kept at a zoological facility. In 2021, the animal was found dead holding on to a tree. No obvious clinical sign was noted by the keepers, and further clinical examination was not performed. At necropsy, multifocal hair loss and ulcers covered with pus and scab were observed in the skin of the lumbar and perianal regions (Figs. 1 and 2). A 2-mm-sized vegetation at the mitral valve was observed (Fig. 3). There were multiple hemorrhagic foci in the leptomeninges at the left temporal lobe and cerebellum (Fig. 4). On the cut surface of the formalin-fixed brain, the lateral ventricles were occupied by exudates.
Fig. 1.

Overview of the sloth at necropsy. Hair loss in the lumbar region.
Fig. 2.
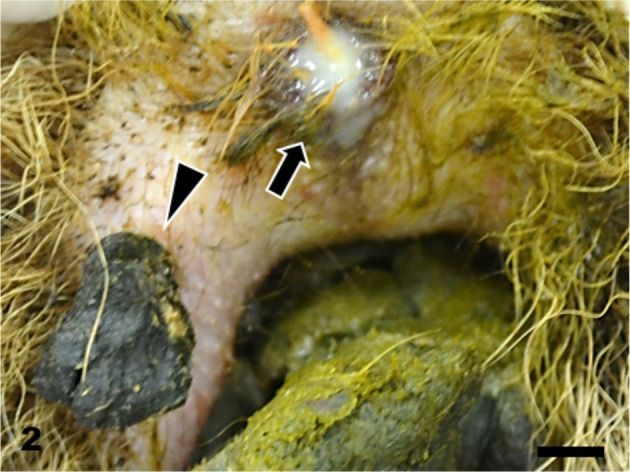
Skin. An ulcer covered with pus (arrow) and scab (arrowhead) at the perianal region. Bar=5 mm.
Fig. 3.
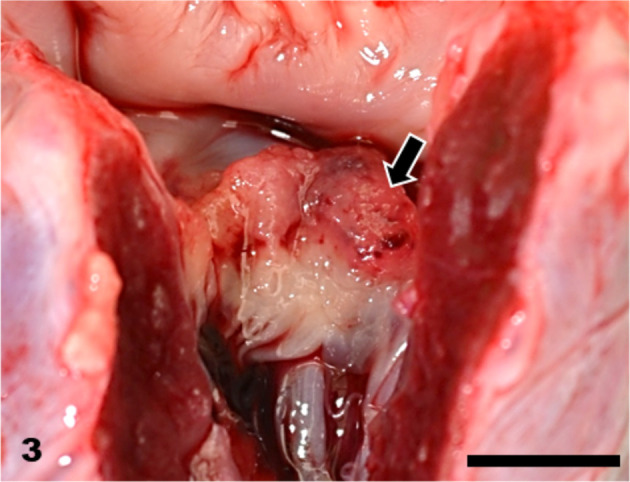
Heart. Vegetation at the mitral valve (arrow). Bar=5 mm.
Fig. 4.
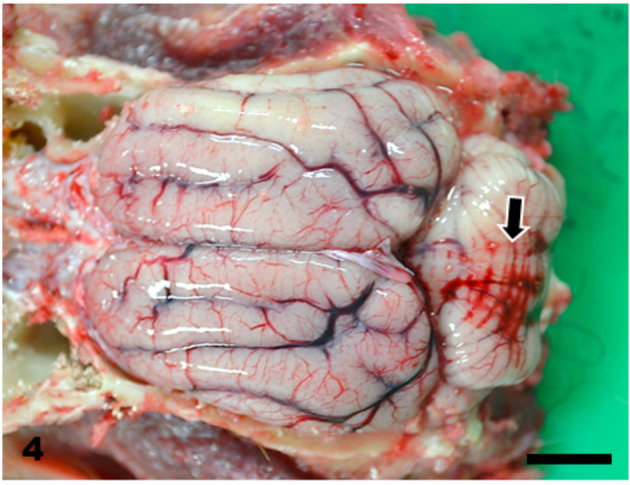
Brain. Marked hemorrhage in the leptomeninges at the cerebellum (arrow). Bar=10 mm.
The brain and tissues of the visceral organs were collected and fixed in 10% neutral buffered formalin and routinely embedded in paraffin. Sections were cut at 4 µm and stained with hematoxylin and eosin. Some sections of the skin, heart, and brain were also stained with Gram stain (Brown Hopps method). Histopathologically, the skin lesions at the lumbar and perianal areas were characterized by severe ulceration and serocellular crust formation mainly consisting of degenerated neutrophils. In the dermis, neutrophil infiltration and clusters of cocci were observed, accompanied by fibrosis and vascularization. In the heart, fibrin and numerous cocci were attached to the mitral valve and numerous neutrophils infiltrated the valve (Fig. 5). Neutrophil infiltration, fibrosis and clusters of cocci were observed in the endocardium of the left ventricle (Fig. 6). In the brain, numerous neutrophils and fewer macrophages infiltrated the lateral ventricles and choroid plexus epithelial cells were sloughed off (Fig. 7). Neutrophils also infiltrated the ependymal layer and the surrounding cerebral parenchyma (Fig. 8). Hemorrhage and mild infiltration of neutrophils and macrophages were observed in the cerebral, cerebellar, and brain stem leptomeninges. A few cocci were observed in the lateral ventricles and brain vascular. The cocci in the skin, heart, and brain were stained Gram-positive and were often arranged in pairs or chains (Fig. 9). Some of the cocci were phagocytosed by neutrophils. A few small foci of neutrophils and bacterial embolus were observed in the liver and the kidney glomeruli.
Fig. 5.
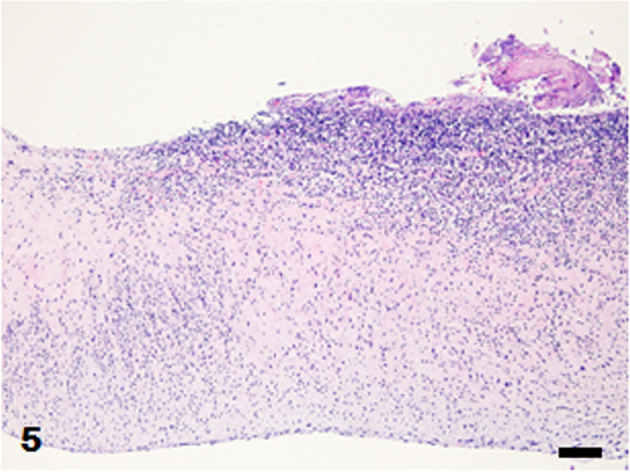
Mitral valve. Numerous inflammatory cells infiltrate the valve wall. Fibrinous exudates with cocci are attached to the valve. Hematoxylin and eosin (HE) stain. Bar=100 µm.
Fig. 6.
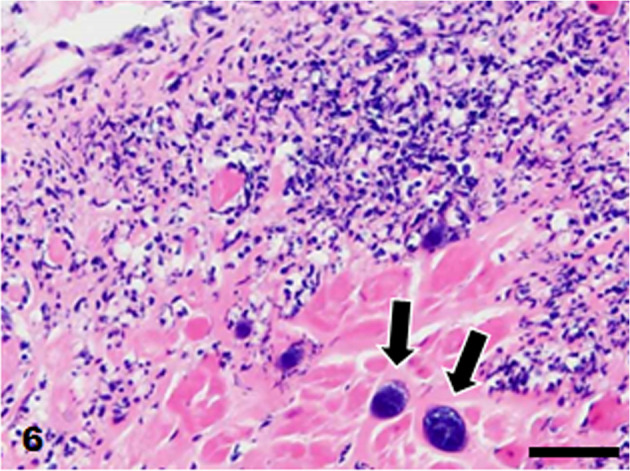
Heart. Neutrophil infiltration and clusters of cocci (arrows) in the endocardium. HE stain. Bar=50 µm.
Fig. 7.
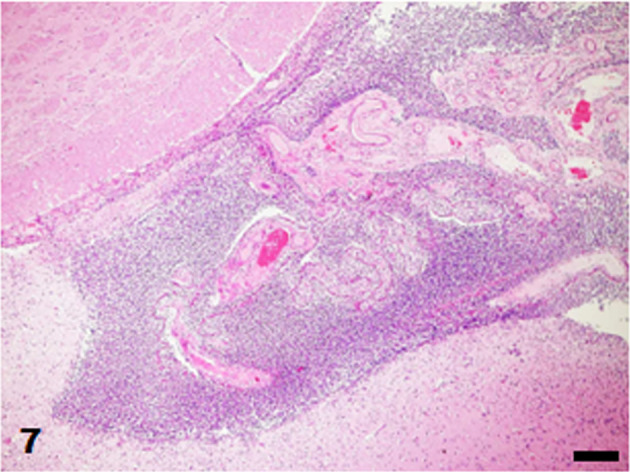
Lateral ventricle. Severe infiltration of inflammatory cells in the ventricle. HE stain. Bar=50 µm.
Fig. 8.
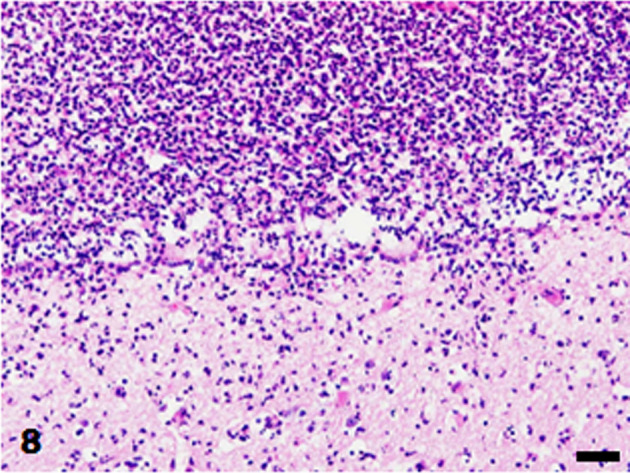
Lateral ventricle. Neutrophils infiltrate the ependymal layer and the surrounding cerebral parenchyma. HE stain. Bar=50 µm.
Fig. 9.
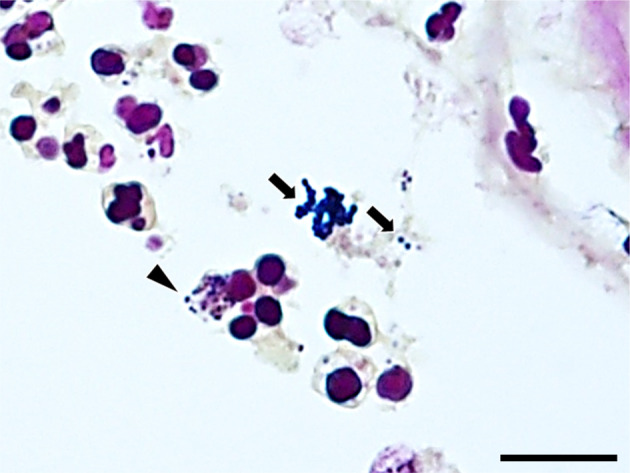
Choroid plexus. Gram-positive cocci are arranged in pairs or chains (arrows). Some of the cocci are phagocytosed by neutrophils (arrowhead). Gram stain (Brown Hopps method). Bar=10 µm.
Based on the histopathological findings, systemic streptococcal infection was suspected, and PCR examination was conducted by using formalin-fixed paraffin-embedded (FFPE) tissues of the skin, heart, and brain (frontal lobe and thalamus). Total DNA was extracted using the QIAmp® DNA FFPE Tissue Kit (QIAGEN, Venlo, Netherlands). Specific primer pair for Streptococcus spp. (5′ Sag 1 and 3′ B) [2] and a PCR master mix (KOD One® PCR Master Mix -Blue-; TOYOBO, Osaka, Japan) were used. Electrophoresis of the PCR products revealed a 150 bp band in all samples (Fig. 10). These PCR products were purified with a High Pure PCR Product Purification Kit (Roche, Basel, Switzerland) and sequenced by the Sanger method. Sequence data were queried using NCBI (National Center for Biotechnology Information) BLAST (Basic Local Alignment Search Tool; https://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequence of the PCR products showed 98% homology to S. agalactiae in all samples (Table 1). Bacterial culture was not available.
Fig. 10.
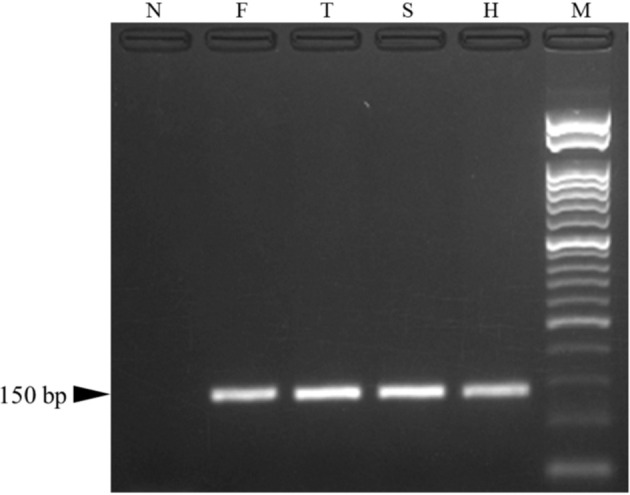
Gel electrophoresis, Streptococcus spp. gene PCR. Bands of 150 bp are observed in samples of the frontal lobe (F), thalamus (T), skin (S), and heart (H). bp, base pairs; N, negative control; M, 50 bp ladder.
Table 1. Results of NCBI (National Center for Biotechnology Information) BLAST (Basic Local Alignment Search Tool) query (top 3).
| Ranking | Description | Scientific name | Query cover (%) | E-value | Percent identity (%) |
|---|---|---|---|---|---|
| 1 | Streptococcus agalactiae strain 01173 chromosome, complete genome | Streptococcus agalactiae | 98 | 1.00E-43 | 98.15 |
| 2 | Streptococcus agalactiae 515 chromosome, complete genome | Streptococcus agalactiae 515 | 98 | 1.00E-43 | 98.15 |
| 3 | Streptococcus agalactiae strain Sag 153 chromosome, complete genome | Streptococcus agalactiae | 98 | 1.00E-43 | 98.15 |
In the present case, suppurative dermatitis, endocarditis, and meningo-ventriculitis, with bacterial infection were observed. Bacterial emboli were found in multiple visceral organs. Streptococcus agalactiae gene was detected in the skin, heart, and brain tissues by PCR examination. The distribution and histopathological findings were comparable to systemic streptococcal infections in domestic animals [4, 10, 12, 17,18,19].
Streptococcus agalactiae is a group B Streptococcus (GBS), which is one of the most important pathogens of infectious diseases in human neonates [9, 14]. Also, the number of cases of invasive GBS infection in adults has been increasing in the past two decades [6, 9]. Adults suffering from GBS disease often have underlying conditions such as diabetes, obesity, pregnancy, and ageing, which may compromise the immune system [6, 9]. In such cases, common clinical presentations are skin and soft tissue infection, bacteremia, osteomyelitis, pneumonia, and arthritis [6, 9]. However, a few cases of endocarditis and meningitis associated with GBS infection have been reported in adults without any underlying conditions [1, 7]. In the present case, a sloth without any underlying conditions was infected by S. agalactiae. The skin lesion was thought of as a responsible lesion for infection in the present case, which has also been described in human cases [9, 19].
The ependymal layer lacks tight junction, and thus is vulnerable to bacterial entry via the cerebrospinal fluid [4, 11]. Also, choroiditis often accompanies ventriculitis [4]. Streptococcal meningo-ventriculitis has been reported in humans, dogs, cows, and pigs [9, 12, 17, 18]. Human and animal patients with meningitis often present acute clinical signs such as fever, hyperesthesia, photophobia, confusion, vomiting, depression, and nuchal rigidity [14, 15]. The ventricular lesion of the present case was in an acute stage with severe neutrophil infiltration. However, the animal did not show apparent clinical signs. On the other hand, the meninges were spared from severe inflammation, which may explain the lack of clinical signs in the present case. Similarly, human patients of primary bacterial ventriculitis often lack clinical signs [13].
This is the first report of S. agalactiae infection in the CNS of a sloth. The pathological findings were comparable to those of human and domestic animal cases. The present case did not show any obvious clinical signs, which may be associated with the affected area of the brain. The sloth is a slow-moving animal, which can make it difficult for zoo keepers and veterinarians to notice behavioral changes and neurological signs.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this manuscript.
REFERENCES
- 1.Barile AJ, Kallen AJ, Wallace MR. 1999. Fatal group B streptococcal meningitis in a previously healthy young adult. Clin Infect Dis 28: 151. doi: 10.1086/517185 [DOI] [PubMed] [Google Scholar]
- 2.Berridge BR, Bercovier H, Frelier PF. 2001. Streptococcus agalactiae and Streptococcus difficile 16S-23S intergenic rDNA: genetic homogeneity and species-specific PCR. Vet Microbiol 78: 165–173. doi: 10.1016/S0378-1135(00)00285-6 [DOI] [PubMed] [Google Scholar]
- 3.Bowater RO, Dennis MM, Blyde D, Stone B, Barnes AC, Delamare-Deboutteville J, Horton MA, White M, Condon K, Jones R. 2018. Epizootics of Streptococcus agalactiae infection in captive rays from Queensland, Australia. J Fish Dis 41: 223–232. doi: 10.1111/jfd.12701 [DOI] [PubMed] [Google Scholar]
- 4.Cantile C, Youssef S. 2015. Nerve system. pp. 250–406. In: Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, Vol. 1, 6th ed. (Maxie MG ed.), Elsevier, Amsterdam. [Google Scholar]
- 5.Elliott JA, Facklam RR, Richter CB. 1990. Whole-cell protein patterns of nonhemolytic group B, type Ib, streptococci isolated from humans, mice, cattle, frogs, and fish. J Clin Microbiol 28: 628–630. doi: 10.1128/jcm.28.3.628-630.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francois Watkins LK, McGee L, Schrag SJ, Beall B, Jain JH, Pondo T, Farley MM, Harrison LH, Zansky SM, Baumbach J, Lynfield R, Snippes Vagnone P, Miller LA, Schaffner W, Thomas AR, Watt JP, Petit S, Langley GE. 2019. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA Intern Med 179: 479–488. doi: 10.1001/jamainternmed.2018.7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita H, Nakamura I, Tsukimori A, Sato A, Ohkusu K, Matsumoto T. 2015. Severe infective endocarditis in a healthy adult due to Streptococcus agalactiae. Int J Infect Dis 38: 43–45. doi: 10.1016/j.ijid.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 8.Gilmore DP, Da Costa CP, Duarte DP. 2001. Sloth biology: an update on their physiological ecology, behavior and role as vectors of arthropods and arboviruses. Braz J Med Biol Res 34: 9–25. doi: 10.1590/S0100-879X2001000100002 [DOI] [PubMed] [Google Scholar]
- 9.Hanna M, Noor A. 2022. Streptococcus group B. https://www.ncbi.nlm.nih.gov/books/NBK553143/ In: StatPearls [Internet], StatPearls Publishing, Treasure Island [accessed on May 26, 2022].
- 10.Jensen HE, Gyllensten J, Hofman C, Leifsson PS, Agerholm JS, Boye M, Aalbæk B. 2010. Histologic and bacteriologic findings in valvular endocarditis of slaughter-age pigs. J Vet Diagn Invest 22: 921–927. doi: 10.1177/104063871002200611 [DOI] [PubMed] [Google Scholar]
- 11.Jiménez AJ, Domínguez-Pinos MD, Guerra MM, Fernández-Llebrez P, Pérez-Fígares JM. 2014. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2: e28426. doi: 10.4161/tisb.28426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamm CG, Ferguson AC, Lehenbauer TW, Love BC. 2010. Streptococcal infection in dogs: a retrospective study of 393 cases. Vet Pathol 47: 387–395. doi: 10.1177/0300985809359601 [DOI] [PubMed] [Google Scholar]
- 13.Lesourd A, Magne N, Soares A, Lemaitre C, Taha MK, Gueit I, Wolff M, Caron F. 2018. Primary bacterial ventriculitis in adults, an emergent diagnosis challenge: report of a meningoccal case and review of the literature. BMC Infect Dis 18: 226. doi: 10.1186/s12879-018-3119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mańdziuk J, Kuchar EP. 2021. Streptococcal meningitis. https://www.ncbi.nlm.nih.gov/books/NBK554448/ In: StatPearls, StatPearls Publishing, Treasure Island [accessed on May 26, 2022]. [PubMed]
- 15.Muñana KR. 1996. Encephalitis and meningitis. Vet Clin North Am Small Anim Pract 26: 857–874. doi: 10.1016/S0195-5616(96)50109-9 [DOI] [PubMed] [Google Scholar]
- 16.Padberg J. 2017. Xenarthran nervous systems. pp. 383–412. In: Evolution of Nervous Systems, 2th ed. (Kaas JH ed.), Elsevier, Amsterdam. [Google Scholar]
- 17.Reams RY, Glickman LT, Harrington DD, Thacker HL, Bowersock TL. 1994. Streptococcus suis infection in swine: a retrospective study of 256 cases. Part II. Clinical signs, gross and microscopic lesions, and coexisting microorganisms. J Vet Diagn Invest 6: 326–334. doi: 10.1177/104063879400600308 [DOI] [PubMed] [Google Scholar]
- 18.Seimiya Y, Ohshima K, Itoh H, Ogasawara N, Okutomo M, Tanaka S. 1992. Clinicopathology of meningoventriculitis due to Streptococcus bovis infection in neonatal calves. J Vet Med Sci 54: 871–874. doi: 10.1292/jvms.54.871 [DOI] [PubMed] [Google Scholar]
- 19.The Center for Food Security and Public Health2020. Zoonotic streptococcosis. https://www.cfsph.iastate.edu/Factsheets/pdfs/streptococcosis.pdf [accessed on May 26, 2022].


