Abstract
The CD8+-T-cell response to human immunodeficiency virus type 1 (HIV-1) is considered to be important in host control of infection and prevention of AIDS. We have developed a single-cell enzyme immunoassay (enzyme-linked immunospot assay) specific for gamma interferon (IFN-γ) production stimulated by either autologous B-lymphoblastoid cell lines (B-LCL) infected with vaccinia virus vectors expressing HIV-1 proteins or synthetic peptides representing known HIV-1 CD8+ cytotoxic T-lymphocyte (CTL) epitopes. Single-cell IFN-γ production stimulated by HIV-1 Gag-, Pol-, and Env-expressing B-LCL was a reliable measure of HIV-1-specific T-cell immunity in peripheral blood CD8+ T cells from HIV-1 infected individuals. This method was more sensitive than stimulation of IFN-γ by direct infection of the cultures with HIV-1–vaccinia virus vectors. Comparable results were found for IFN-γ production in CD8+ T cells from HIV-1-negative, cytomegalovirus (CMV)-seropositive, healthy donors stimulated with B-LCL expressing the CMV pp65 lower matrix protein. HIV-1 peptides were immunodominant for both CD8+ single-cell IFN-γ production and CTL precursor frequencies. The number of cells producing IFN-γ decreased in individuals with late-stage HIV-1 infection and was temporally enhanced during combination antiretroviral therapy with two reverse transcriptase nucleoside inhibitors and a protease inhibitor.
Maintenance of memory CD8+ cytotoxic T-lymphocyte precursors (CTLp) specific for human immunodeficiency virus type 1 (HIV-1) is important in resistance to progression of disease during HIV-1 infection (2, 25, 33). We (23, 24) and others (9, 15, 32) have noted that higher levels of peripheral blood CTLp specific for various HIV-1 proteins are associated with slower progression of HIV-1 infection and lack of disease development in long-term nonprogressors. The anti-HIV-1 CTLp limiting dilution assay, however, is very labor-intensive and is dependent on the capacity of the CD8+ T cells to remain viable and proliferate in vitro over a 2-week culture period.
Consequently, we were interested in a simple, sensitive, and quantitative method to monitor the T-cell response against HIV-1 antigens. Recently, enumeration of gamma interferon (IFN-γ)-secreting cells specific for other viruses by the enzyme-linked immunospot (ELISPOT) assay has been reported to be a more sensitive measure of CD8+ T-cell reactivity than quantification of CTLp (3, 17, 19, 28). Cytokine release in this assay can be measured on the single-cell level, allowing direct calculation of antiviral T-cell frequencies (4, 10, 26, 27). It is not clear, however, whether this assessment of CD8+ T-cell function is a reliable indicator of anti-HIV-1 host response relative to disease progression.
We have therefore investigated anti-HIV-1 CD8+ T-cell responses measured as single-cell IFN-γ production by the ELISPOT assay. Our results show that detection of the number of IFN-γ-producing cells in response to vaccinia virus (VV) vectors encoding HIV-1 genes and to HIV-1 peptides is a reliable method for assessing anti-HIV-1 CD8+ T-cell reactivity.
MATERIALS AND METHODS
Study subjects.
The study population included 11 HIV-1-seropositive homosexual men from the Pittsburgh, Pa., portion of the Multicenter AIDS Cohort Study, a study of the natural history of HIV infection (14), and 10 HIV-1-seropositive adults enrolled at the Pittsburgh site of the Merck 035 trial (8) who received triple-combination antiretroviral therapy (zidovudine [ZDV], lamivudine [3TC], and indinavir [IDV]). Seventeen HIV-1-seronegative, heterosexual individuals, including six cytomegalovirus (CMV)-seropositive subjects, were used as controls. This study was approved by the University of Pittsburgh Institutional Review Board.
Effector cells and T-cell isolation.
Venous blood anticoagulated with heparin was separated on Ficoll-Hypaque gradients to obtain peripheral blood mononuclear cells (PBMC). The cells were frozen and stored at −135°C prior to thawing for use in the ELISPOT or CTLp assay. For some experiments, CD8+ or CD4+ T cells were enriched from PBMC by negative selection using immunomagnetic beads (DYNAL, Lake Success, N.Y.). Briefly, PBMC were incubated with a cocktail of beads containing anti-CD4 or anti-CD8 monoclonal antibodies and anti-CD19 and anti-CD56 antibodies to remove the CD4+ or CD8+ T cells, B cells, and NK cells. The levels of purity of CD8+ and CD4+ T cells were approximately 98 and 90%, respectively, as analyzed by flow cytometry.
Recombinant VV and synthetic peptides.
The VV constructs used in this study for expression of HIV-1 genes were vABT 141, which contains the gag coding sequence for p55 (VV-Gag); vABT 204, which contains the full-length pol gene, consisting of the reverse transcriptase (RT), protease, and integrase genes (VV-Pol); and vABT 408, which contains the combined gag-pol and env coding sequences (VV-GPE). These vectors contain coding sequences from the BH10 and HxB2 strains of HIV-1 (Therion Biologics, Cambridge, Mass.). The other HIV-1 vector was vPE 11, which contains the env coding region of the HIV-1 strain BH10 minus the signal sequence (VV-Env) (B. Moss, National Institutes of Health) (21). VV-CMV expressed the human CMV matrix tegument phosphoprotein pp65 (a gift of S. Riddell, Fred Hutchinson Cancer Research Center, Seattle, Wash.) (5). The NYCBH strain of VV (VV-Vac) was used as the control virus (Therion).
Synthetic peptides of HLA A2- and HLA B27-restricted, HIV-1 CTL epitopes were prepared by the University of Pittsburgh Cancer Institute Peptide Synthesis Facility (Table 1). A series of peptides with lengths of 20 amino acids (aa) that overlapped by 10 aa and that spanned aa 133 to 357 of HIV-1 Gag were provided by Washington Singer Labs, University of Exeter (Exeter, United Kingdom). HLA testing was done by sequence-specific oligonucleotide probe hybridization at the University of Pittsburgh Medical Center.
TABLE 1.
HLA class I-associated viral epitopes used in this study
| HLA | Epitope | Sequence (reference) |
|---|---|---|
| A2 | Gag p24151–159 | TLNAWVKVV (20) |
| A2 | Pol RT476–484 | ILKEPVHGV (31) |
| A2 | Env gp120192–199 | KLTSCNTSV (1) |
| A2 | Gag p1777–85 | SLYNTVATL (30) |
| A2 | Influenza virus M158–66 | GILGFVFTL (6) |
| A2 | HTLV-1a Tax11–19 | LLFGYPVYV (16) |
| B27 | Gag p24263–272 | KRWIILGLNK (7) |
HTLV-1, human T-cell leukemia virus type 1.
Preparation of stimulator cells.
The stimulator cells were autologous B-LCL immortalized by infection with Epstein-Barr virus (EBV) (filtered supernatant from the EBV-transformed B95-8 marmoset cell line; American Type Culture Collection, Manassas, Va.) (11). In some experiments, the stimulator cells were frozen and thawed autologous PBMC. The B-LCL or PBMC were counted, and 106 cells were transferred to a 15-ml conical centrifuge tube and spun at 450 × g for 10 min at room temperature. Supernatant was discarded, and the cells were resuspended in 1 ml of RPMI medium containing antibiotics (complete medium) with 5% fetal calf serum (FCS). VV constructs were added at an input virus-to-cell multiplicity of 4 to 1. The virus-cell mixture was then spun at 700 × g for 30 min at room temperature. Supernatant was discarded, and the cell pellet was dispersed and then washed with complete medium containing 5% FCS. The cells were resuspended in 1 ml of complete medium with 15% FCS and incubated for 16 h at 37°C in a 5% CO2 atmosphere. Expression of the VV-GPE vector is consistent in >70% of these infected B-LCL (11). The cell suspension was then transferred into a 60- by 15-mm petri dish and mixed with 3.5 ml of cold complete medium containing 5% FCS and 0.5 ml of psoralen (Sigma Chemical Co., St. Louis, Mo.). The mixture was exposed to long-wave UV light irradiation for 5 min to inactivate the VV and any residual, endogenous HIV-1. The cells were washed with warm complete medium containing 5% FCS and counted to determine the number and viability of irradiated-VV-infected cells. The irradiated-VV-infected cells were then resuspended in complete medium containing 15% FCS. One hundred microliters of this cell suspension was added to responder cells at an responder-to-stimulator cell ratio of 10:1 in a microwell with a nitrocellulose membrane of a 96-well plate (Milliliter; Millipore, Bedford, Mass.) for the ELISPOT assay or of a round-bottom 96-well cell culture plate for the CTLp assay.
ELISPOT assay for IFN-γ release from single, antigen-specific T cells.
Our single-cell assay was a modification of that described by Tanguay and Killion (29) and Lalvani et al. (17). Nitrocellulose membranes in microwells of 96-well plates (Millipore) were coated overnight at 4°C with 50 μl of anti-IFN-γ antibody (10 μg/ml; Mabtech, Stockholm, Sweden) per well. The antibody-coated plates were washed four times with phosphate-buffered saline (PBS; BioWhittaker, Walkersville, Md.) and blocked with 180 μl of RPMI medium (Life Technologies, Grand Island, N.Y.) containing 10% human serum (Sigma) per well over 1 h at 37°C. PBMC or CD8+ cells (CD4−, CD19−, and CD56−) (105 to 106) enriched by magnetic bead separation (DYNAL) and B-LCL or PBMC infected with VV vectors (104 to 105) were added to the wells in 200 μl of AIM V medium (Life Technologies) and incubated overnight at 37°C in 5% CO2. In certain experiments, PBMC or CD8+ cells were incubated overnight at 37°C in 5% CO2 with HLA-restricted HIV-1 peptides (10 μg/ml) in 96-well plates with nitrocellulose membranes. The plates were washed four times with PBS containing 0.05% Tween 20 (Sigma), and the secondary antibody (biotin-conjugated anti-IFN-γ monoclonal antibody; Mabtech) was added at 2 μl/ml in 100 μl/well. Plates were washed four times with PBS containing 0.05% Tween 20 after 2 h of incubation at 37°C in 5% CO2, and avidin-bound, biotinylated horseradish peroxidase H (Vectastain Elite kit; Vector Laboratories, Burlingame, Calif.) was added to the wells for 1 h at room temperature. The plates were washed three times with PBS containing 0.05% Tween 20 and three times with PBS, followed by 5 min of incubation with 100 μl of 3-amino-9-ethylcarbazole (Sigma) per well. The reaction was stopped with running tap water. The spots were counted with a dissecting microscope (Fostec microscope; Fryer, Pittsburgh, Pa.). Medium or phorbol 12-myristate 13-acetate (PMA) at 1 ng/ml (Sigma) and ionomycin at 1 μM/ml (Sigma) were used as negative and positive controls, respectively. In some experiments, VV-infected stimulator cells were incubated with anti-major histocompatibility complex (MHC) class I or anti-MHC class II antibody (1:100 dilution; Coulter, Miami, Fla.) for 45 min at 37°C in 5% CO2 before being added to the wells containing the responder cells. The results were expressed as either the numbers of spot-forming cells per 106 cells or the net numbers of spot-forming cells per 106 cells, i.e., the numbers of spots per 106 PBMC or CD8+ T cells specific for HIV-1 antigen minus the numbers of non-HIV-1-specific spots per 106 PBMC or CD8+ T cells.
Detection of memory CTL reactivity by limiting-dilution precursor frequency analysis.
The precursor frequencies of anti-HIV-1 CTLp were determined by limiting-dilution assay of freshly isolated and frozen and thawed PBMC (11). PBMC were seeded in complete medium containing 15% FCS at 0 (control), 250, 500, 1,000, 3,000, 6,000, 12,000, and 16,000 cells per well in 24 replicate wells of 96-well, round-bottom microtiter plates. To each well were added 2.5 × 104 gamma-irradiated allogeneic PBMC from one or two HIV-1-seronegative normal donors as feeder cells, 100 U of interleukin-2, and stimulator cells (1,600 stimulator cells per well) consisting of VV-GPE-infected, psoralen- and UV-inactivated B-LCL. The cells were cultured for 14 days at 37°C in a 5% CO2 atmosphere, with fresh complete medium containing 15% FCS and recombinant interleukin-2 being added every 5 days. On day 14, the cultured cells were divided, transferred to two new wells, and adjusted to 100 μl with complete medium containing 15% FCS.
For target cells, autologous B-LCL (3 × 106) were infected with VV–HIV-1 and VV-Vac control vectors at an input multiplicity of 4 to 1 and labeled with 150 μCi of Na251CrO4 in 1 ml of complete medium containing 15% FCS for 16 h at 37°C in a 5% CO2 atmosphere (11). The B-LCL were then washed three times with cold complete medium containing 5% FCS. The numbers and viability of the cells were monitored microscopically by trypan blue dye exclusion. Target cells were resuspended to a concentration of 105 cells/ml in complete medium containing 10% FCS. Each target cell solution (12.5 μl) was transferred into a LumaPlate-96 in duplicate wells, and cells were counted in a scintillation counter (Packard, Meriden, Conn.) as maximum counts per minute. The target cells were then added at 104 cells in 100 μl per well to 24 replicate wells of effector cells and incubated for 4 h at 37°C in a 5% CO2 atmosphere. Afterward, 25 μl of cell-free supernatant was transferred from each well into a LumaPlate-96 plate and radioactivity was assessed. The fraction of nonresponding wells was the number of wells in which the 51Cr release did not exceed the mean spontaneous release plus 10% of the 51Cr incorporation (total 51Cr release − spontaneous 51Cr release) divided by the number of wells assayed. The precursor frequency was estimated by the maximum-likelihood method with a statistical program provided by S. Kalams (Boston, Mass.). CTLp activity was expressed either as the precursor frequency and 95% confidence interval per 106 PBMC or as net precursor frequency per 106 PBMC, i.e., the number of CTLp per 106 PBMC specific for HIV-1 antigen minus the number of CTLp per 106 PBMC specific for non-HIV-1-expressing, VV-Vac-infected target cells.
Statistics.
Comparisons of the different cumulative parameters were done by Student's t test. Data among different groups were assessed by analysis of variance with the Scheffe multiple-comparison test and analyzed for associations between different parameters by the Pearson correlation coefficient test. A P value of >0.05 was considered not significant.
RESULTS
Optimal antigen stimulation conditions for inducing single-cell IFN-γ production with VV vectors.
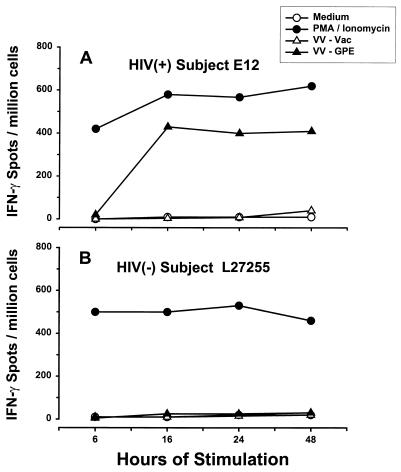
We initially determined the optimal conditions for antigen stimulation of IFN-γ-secreting, HIV-1-specific, CD8+ T lymphocytes in HIV-1-seropositive subjects. First, PBMC from HIV-1-infected subjects were cultured with graded doses of VV–HIV-1-infected stimulator cells, at responder cell-to-stimulator cell ratios of 2, 5, 10, 50, and 250 to 1, for various time periods (6, 16, 24, and 48 h). To account for possible immunomodulating effects of HIV-1 infection on this response, we also determined the optimal conditions for CMV antigen-specific, single-cell IFN-γ production induced by VV-CMV-infected stimulator cells in PBMC from HIV-1-seronegative–CMV-seropositive, healthy subjects. PMA-ionomycin was included as a positive control for stimulated IFN-γ production. We found that the optimal ratio of responder to stimulator cells for both HIV-1 and CMV antigens was 10 to 1 (data not shown) and that the optimal incubation time was 16 h, with higher specific ELISPOT frequencies and lower backgrounds than those of VV-Vac or medium controls (a representative example for the HIV-1 antigen is shown in Fig. 1A).
FIG. 1.
Effect of time of stimulation on the number of IFN-γ-producing cells. PBMC of HIV-1-infected subject E12 (CD4+ T-cell count, 1,017 cells/mm3; HIV-1 RNA load, <50 copies/ml; treated with 3TC, d4T [stavudine], and IDV) and HIV-1-negative donor L27255 were stimulated with medium alone (negative control), PMA-ionomycin (positive control), VV-Vac, or VV-GPE.
Seventeen HIV-1-negative persons had no HIV-1-specific IFN-γ responses (representative data are shown in Fig. 1B). The cumulative mean numbers (± standard errors [SE]) of spot-forming cells per 106 PBMC for the 17 persons were 9 (±4) with medium alone, 20 (±5) with VV-Vac stimulation, 28 (±4) with VV-Gag stimulation, 18 (±5) with VV-Pol stimulation, and 31 (±10) with VV-Env stimulation. CMV-specific IFN-γ was produced by PBMC from six of these HIV-1-negative individuals who were CMV seropositive and not by six who were CMV seronegative; i.e., numbers of spot-forming cells with VV-CMV stimulation were 230 (±80) for the six CMV seropositive donors and 15 (±7) for the six CMV seronegative donors (P < 0.01).
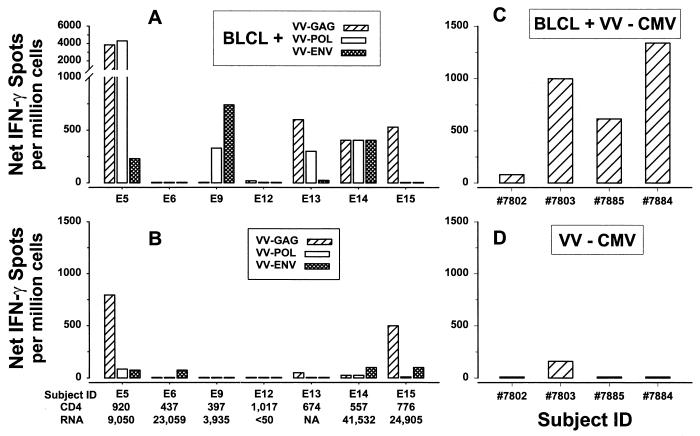
It has recently been reported that IFN-γ can be induced directly in PBMC by replicating VV vectors expressing HIV-1 genes (18). We therefore compared this antigen stimulation system to our VV-infected autologous B-LCL system and also with a system in which VV vector-infected PBMC are used as antigen-presenting cells (APC). PBMC stimulated with VV–HIV-1- or VV-CMV-infected, autologous B-LCL as APC had responses equal to or higher than those of PBMC stimulated directly with these VV vectors in most HIV-1-infected subjects (Fig. 2A and B) and HIV-1-negative, CMV-seropositive normal donors (Fig. 2C and D). These results were supported by the higher, cumulative results obtained with our technique (P < 0.01 for both anti-HIV-1 and anti-CMV CTL). VV–HIV-1- or VV-CMV-infected autologous B-LCL also had better APC function than did VV vector-infected, autologous PBMC (data not shown).
FIG. 2.
Comparison of results with inactivated, autologous B-LCL APC after direct infection with active, replication-competent VV–HIV-1 or VV-CMV vectors for stimulation of IFN-γ production in PBMC from seven HIV-1-infected subjects (A and B) and four HIV-1-negative, CMV-positive healthy donors (C and D). Results are shown as the net numbers of IFN-γ-producing cells after subtraction of the background reactivity to the VV-Vac-infected B-LCL (A and C) or to VV-Vac alone (B and D). CD4, CD4+ T-cell counts per mm3; RNA, copies of HIV-1 RNA per milliliter of plasma. None of the seven HIV-1-infected subjects except E12 were receiving antiretroviral therapy; E12 was receiving 3TC, d4T (stavudine), and IDV. NA, not available; ID, identification.
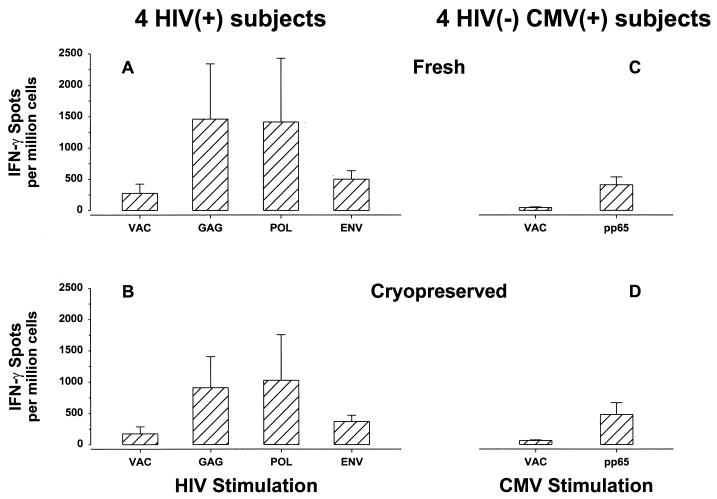
Single-cell IFN-γ production induced in cryopreserved PBMC.
Use of cryopreserved PBMC is essential for doing long-term, nonconcurrent prospective studies of T-cell immune function. The IFN-γ-producing cell frequency mediated by frozen and thawed PBMC was therefore compared to the frequency for fresh PBMC in HIV-1-infected subjects and in CMV-seropositive, HIV-1-seronegative normal donors. The frequency of IFN-γ-producing cells induced in frozen and thawed PBMC was comparable to that in freshly donated, matched PBMC from the same person tested prior to freezing for four HIV-1-infected subjects (Fig. 3A) and four HIV-1-negative, CMV-seropositive normal donors (Fig. 3B) (P value was not significant).
FIG. 3.
Comparison of levels of IFN-γ production in freshly donated PBMC from four HIV-1-infected subjects and four HIV-1-negative, CMV-positive, healthy donors tested prior to freezing of PBMC (A and C) and in matched, frozen and thawed PBMC (B and D). Results are expressed as means ± SE. The HIV-1-infected subjects were not receiving antiretroviral therapy and had CD4+ T cell counts of 663 ± 116/mm3 and HIV-1 RNA loads of 19,856 ± 8,493 copies/ml.
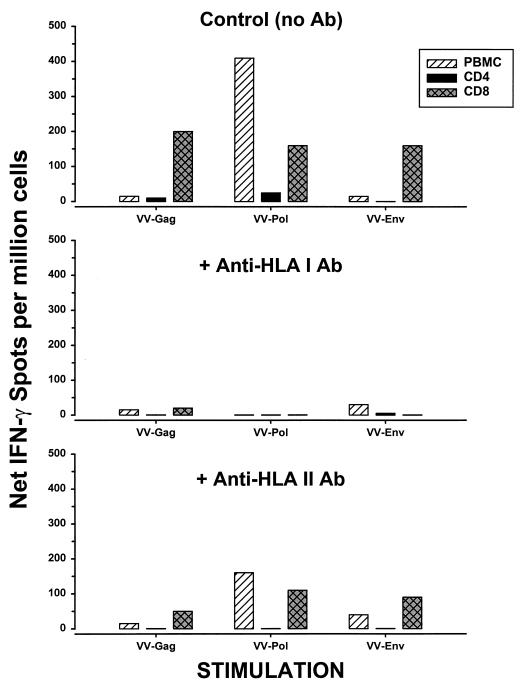
T-cell phenotype and HLA restriction patterns of the IFN-γ-producing cells.
PBMC were enriched for CD8+ and CD4+ T cells from HIV-1-infected subjects, stimulated by VV-GPE-infected autologous B-LCL, and tested by the ELISPOT assay for IFN-γ production. CD8+ T cells but not CD4+ T cells responded to all three HIV-1 proteins (Gag, Pol, and Env) (a representative example is shown in Fig. 4). This activity was blocked by anti-HLA class I antibody but not by anti-HLA class II antibody. The IFN-γ was therefore produced by HIV-1-specific, HLA class I-restricted, CD8+ T cells.
FIG. 4.
Comparison of numbers of HLA-associated, IFN-γ-producing cells in CD8+ T cells, CD4+ T cells, and PBMC. HIV-1-infected subject E5 (on no antiretroviral therapy) had a CD4+ T cell count of 920/mm3 and a plasma HIV-1 RNA load of 9,050 copies/ml. Ab, antibody.
Comparison of the frequencies of HIV-1-specific T cells by single-cell IFN-γ and CTLp assays.
The ELISPOT and limiting-dilution assays were done on 28 paired PBMC samples from 10 HIV-1-infected subjects on various types of antiretroviral therapy. The results showed that there was a significant correlation between the numbers of IFN-γ-producing cells and CTLp in response to Env (r = 0.64, P < 0.01) but not in response to Gag (r = 0.26, P was not significant) or Pol (r = 0.31, P was not significant). The mean net levels (± SE) of IFN-γ-producing cells and the net numbers of CTLp per 106 PBMC for these samples were 111 (±27) and 43 (±13), respectively, with Gag (P = 0.01), 146 (±45) and 227 (±94) with Pol (P was not significant), and 96 (±25) and 92 (±37) with Env (P was not significant). The background, mean numbers (±SE) of IFN-γ-producing cells from the HIV-1-seropositive subjects for the medium and VV-Vac controls were 23 (±9) and 87 (±18), respectively, and were 53 (±12) for the VV-Vac CTLp control.
Optimal antigen stimulation conditions for inducing single-cell IFN-γ production with synthetic peptides.
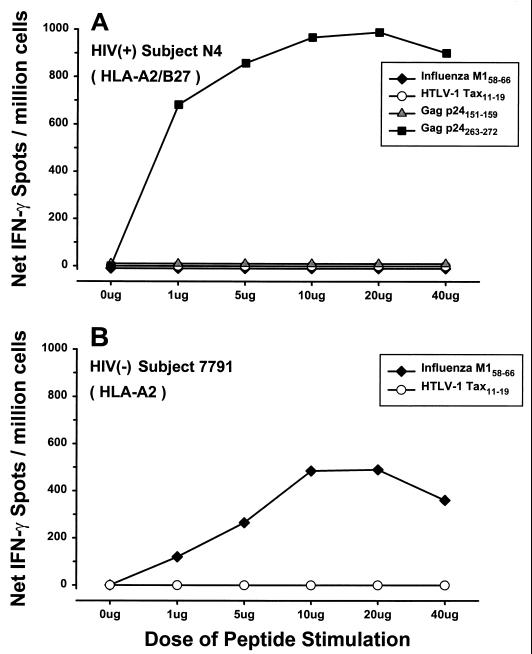
PBMC from HIV-1-infected subjects were tested for the optimal IFN-γ response to HIV-1 peptides by stimulation with various concentrations of peptides representing known CD8+ CTL epitopes (Table 1). Data in Fig. 5A show that 10 μg of HLA B∗27 Gag p24263–272 peptide per ml stimulated the highest IFN-γ responses in an HLA A∗02 B∗27-positive individual. PBMC from this study subject did not respond to another HIV-1 peptide, Gag p24151–159, specific for HLA A∗02. This concentration of peptide was also optimal for induction of IFN-γ for the HLA-A∗02 HIV-1 Gag p17, RT, and Env peptides listed in Table 1 in other HIV-1-positive subjects (data not shown). There was no IFN-γ response in PBMC from HIV-1-positive or -negative persons to a known human T-cell leukemia virus type 1 Tax HLA A∗02 peptide (negative control), whereas IFN-γ was produced in PBMC from many but not all of these persons in response to 10 μg of the influenza A virus matrix HLA A∗02 peptide (positive control) per ml (representative examples are shown in Fig. 5). Therefore, the 10-μg/ml concentration of peptide was used in all subsequent experiments.
FIG. 5.
IFN-γ responses of PBMC from an HIV-1-infected, HLA A∗02 B∗27 subject (N4; CD4+ T cell count, 828 cells/mm3; HIV-1 RNA load, not available; not receiving antiretroviral therapy) and an HIV-1-negative, healthy HLA A∗02 subject (7791) to various concentrations of viral peptides representing predetermined CD8+ CTL epitopes. HTLV-1, human T-cell leukemia virus type 1.
We next compared VV p55 Gag-infected B-LCL to an immunodominant HLA A∗02 peptide, Gag p1777–85, for stimulation of IFN-γ in 55 PBMC samples from eight HLA A∗02, HIV-1-infected subjects at different times during HIV-1 infection. The number of cells producing IFN-γ stimulated by the p17 peptide (1,088 ± 405/106 PBMC) was significantly higher than that stimulated by VV-Gag (163 ± 29/106 PBMC) (P = 0.002). The frequency of cells producing IFN-γ in response to the p17 peptide, however, correlated with that stimulated by VV-Gag (r = 0.51, P < 0.01). In total, four of the eight HLA A∗02, HIV-1-infected subjects tested had a positive response to the p17 peptide, using a cutoff of 39 spot-forming cells per million PBMC (defined as the mean ±2 SD of the value for the medium control). Thus, this Gag p17 peptide sequence appears to be a major CD8+ T-cell epitope for IFN-γ production in HIV-1 infection.
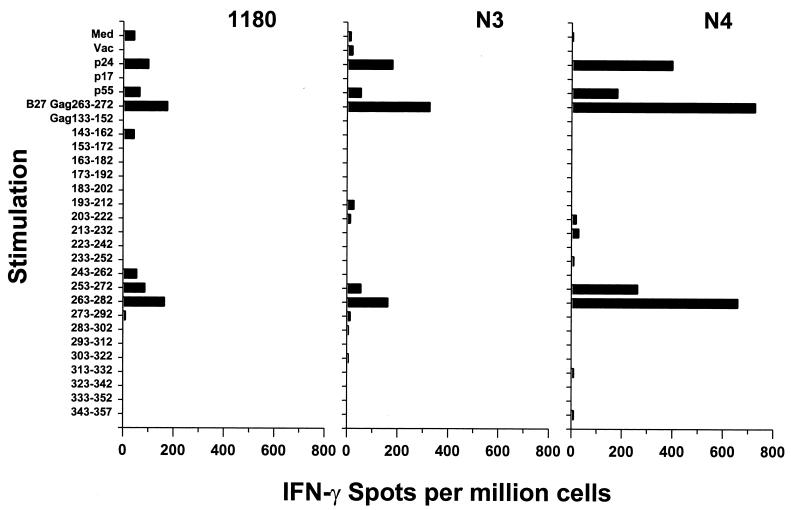
To further identify immunodominant epitopes for HIV-1-specific IFN-γ production, PBMC from three HIV-1-infected, HLA-A∗02 B∗27 subjects were stimulated with a series of 22 overlapping 20-aa peptides covering Gag residues 133 to 357. All three subjects had positive ELISPOT responses to stimulation with VV p55 Gag and p24 Gag but not with p17 Gag (Fig. 6). IFN-γ responses from these three subjects were induced by adjacent Gag p24253–272 (NPPIPVGEIY-KRWIILGLNK) and Gag p24263–282 (KRWIILGLNK-IVRMSPTSI) peptides (Fig. 6). We then showed that a known CTL epitope within this region, HLA B∗27 Gag p24263–272 (KRWIILGLNK) (7), was immunogenic in all three subjects by peptide concentration-dependent, IFN-γ reactivity (data not shown). The same immunodominant HLA B∗27 Gag p24263–272 epitope was also recognized in a 51Cr release CTL assay by PBMC from the three subjects and by T-cell clones generated from two of the subjects (N3 and 1180; clones were not generated from subject N4) (data not shown). Thus, the same HLA B∗27 Gag p24 epitope was dominant for both IFN-γ production and CTL reactivity in these three subjects.
FIG. 6.
Identification of an immunodominant Gag p24 HLA B∗27 epitope recognized by PBMC from three HLA-A∗02 B∗27-positive, HIV-1-infected subjects (subject N3: CD4+ T cell count, 497/mm3; plasma HIV-1 RNA load, 96,000 copies/ml; no concurrent antiretroviral therapy; subject N4: CD4+ T-cell count, 828/mm3; plasma HIV-1 RNA load, 50 copies/ml; no concurrent antiretroviral therapy; subject 1180: CD4+ T-cell count, 450/mm3; plasma HIV-1 RNA load, 2,379 copies/ml; concurrent treatment with IDV). Med, medium control.
Single-cell IFN-γ responses in individuals at different stages of HIV-1 infection and antiretroviral treatment.
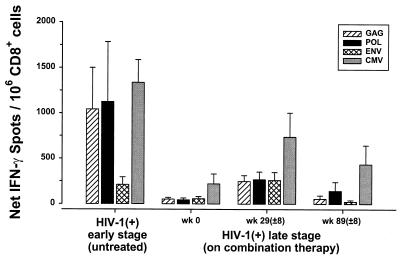
IFN-γ responses to HIV-1 were examined in 10 early-stage, HIV-1-infected subjects who were not receiving any antiretroviral therapy (mean CD4+ T-cell count, 609 ± 56/mm3; mean plasma HIV-1 RNA load, 32,886 ± 12,552 copies/ml). These responses were compared to those of 10 HIV-1-infected, late-stage subjects who had received ZDV alone for at least 6 months (termed week 0 in Fig. 7) (mean CD4+ T-cell count, 180 ± 33/mm3; mean plasma HIV-1 RNA load, 59,554 ± 11,842 copies/ml) after receiving triple-combination therapy for averages of 29 (±8) weeks (mean CD4+ T-cell count, 334 ± 31/mm3; mean plasma HIV-1 RNA load, 905 ± 519 copies/ml) and 89 (±8) weeks (mean CD4+ T-cell count, 430 ± 33/mm3; mean plasma HIV-1 RNA load, 837 ± 818 copies/ml). For this comparison, the ELISPOT data were calculated as the numbers of IFN-γ-producing cells per 106 CD8+ cells to normalize the results.
FIG. 7.
Single-cell IFN-γ responses of individuals with different stages of HIV-1 infection (means ± SE). IFN-γ responses against each of the HIV-1 proteins were examined for 10 HIV-1-infected, early-stage subjects who were not receiving any anti-HIV-1 therapy and for 10 HIV-1-infected, late-stage patients before (week 0) and after 29 (±8) weeks and 89 (±8) weeks of triple-combination antiretroviral therapy.
There was a significantly greater number of IFN-γ-producing, CD8+ cells detected in the untreated, early-stage HIV-1-infected subjects than in the late-stage subjects receiving ZDV monotherapy (with Gag, P < 0.04; with Pol, P < 0.10; with Env, P < 0.09; with CMV, P < 0.01) (Fig. 7). An enhancement in the number of IFN-γ-producing cells was found in the late-stage subjects after 29 weeks of combination antiretroviral therapy compared to the week 0 levels (with Gag, P < 0.01; with Pol, P < 0.02; with Env, P < 0.04; with CMV, P = 0.08). This enhancing effect was no longer evident in late-stage subjects after 89 weeks compared to numbers of IFN-γ-producing cells before initiation of combination therapy (with Gag, P = 0.92; with Pol, P = 0.32; with Env, P = 0.29; with CMV, P = 0.37).
DISCUSSION
This study shows that single-cell IFN-γ production as detected by the ELISPOT assay is a reliable measure of anti-HIV-1 CD8+ T-cell function. The data support the use of inactivated, autologous B-LCL that are infected overnight with VV vectors expressing Gag, Pol, and Env as APC for stimulation of HIV-1-specific, HLA class I-restricted IFN-γ production. The ELISPOT assay is similar to our method for stimulation of anti-HIV-1 CTLp (11), except that it requires only an overnight, 16-h incubation for detection of the IFN-γ-producing cells, rather than the 2 weeks of in vitro culture necessary for expansion of the CTLp. Like the CTLp assay, the ELISPOT assay can be performed using cryopreserved cells, allowing retrospective and batch testing. We also found comparable results for CMV antigen-stimulated IFN-γ production by CD8+ T cells from HIV-1-negative, CMV-seropositive, healthy persons. Thus, as has been noted by other investigators (3, 17, 19, 28), we believe that the single-cell IFN-γ assay has an advantage over the CTLp assay in not requiring replication of CD8+ T cells in vitro, with the potential variance of differential cell growth. The relatively short-term ELISPOT assay may also primarily measure direct, HIV-1 antigen-specific IFN-γ production, rather than secondary IFN-γ production due to nonspecific, paracrine effects of cytokines on the CD8+ cells.
The number of IFN-γ-producing CD8+ cells in response to the VV p55 Gag vector correlated with that induced by the HLA A∗02 p17 Gag77–85 peptide. We have also found that this IFN-γ reactivity correlated with the number of CD8+ cells expressing T-cell receptor for this peptide as determined by HLA class I tetramer staining (24a). Moreover, four of eight HLA A∗02 HIV-1-infected subjects had IFN-γ reactivity to the p17 Gag77–85 peptide. Use of VV–HIV-1 vectors for expression of antigens in the APC, however, obviates the necessity of matching HLA genotypes of the study subjects, as is required for T-cell tetramer staining or reactivity to peptides.
Our results confirm and extend those of Larsson et al. (18), who showed that VV–HIV-1 vectors can be used to stimulate single-cell IFN-γ production by CD8+ T cells, which can be detected by the ELISPOT assay. Our method of stimulating PBMC with VV–HIV-1- or VV-CMV-infected, inactivated B-LCL as APC, however, proved more sensitive than their method of stimulation of IFN-γ-producing cells directly in the cultures by replicating VV vectors (18) or by VV vector-infected, inactivated PBMC used as APC. This greater sensitivity may be due to a higher level of expression of viral antigen processed overnight through the HLA class I pathway in the infected B-LCL APC. It may also be related to the potential immunodysfunctional effects of replicating VV on the IFN-γ pathway (13) in the directly infected PBMC cultures. Another potential advantage of our system is that it controls for the number of APC and the level of viral antigen in the assay system.
In the present study, the number of cells producing IFN-γ in response to HIV-1 Env, but not Gag or Pol, correlated with the number of anti-HIV-1 CTLp. More extensive results from our laboratory show that there is a significant correlation between the number of IFN-γ-producing cells and CTLp responses for all three HIV-1 structural proteins (24a). Of further note is that our results suggest that Gag p24263–272 is a dominant immunogen for both IFN-γ production and CTL function in HIV-1-infected, HLA B∗27 persons. This finding extends the work of others who showed a similar correlation between human and murine PBMC stimulated by CTL peptides of influenza A virus (17), Epstein-Barr virus (28), and lymphocytic choriomeningitis virus (3, 19). We believe that the cumulative data suggest that although the same CD8+ cells appear to mediate these functions, they are not totally identical.
We have noted in longitudinal studies of patients receiving combination antiretroviral therapy that the number of IFN-γ-producing cells stimulated by HIV-1 peptides or APC expressing VV–HIV-1 vectors correlates with the number of CD8+ T cells staining with the HIV-1 peptide–HLA A2 tetramer complexes (24a). This result is similar to data in the murine lymphocytic choriomeningitis virus model (19) and suggests that these two assays assess similar CD8+ T cell populations. Enumeration of IFN-γ-producing cells has an advantage over that in the tetramer assay, however, in that the former assay is broadly applicable to all HLA types when VV–HIV-1 vector-expressing APC are used. It also does not require special, HLA tetrameric reagents when stimulation is done with HIV-1 peptides. These factors make the detection of single-cell production of IFN-γ by the ELISPOT assay an attractive method for quantifying HIV-1-specific, CD8+ T-cell reactivity.
An important finding in this study is that the number of anti-HIV-1 IFN-γ-producing, CD8+ T cells was associated with the stage of HIV-1 infection. Persons with relatively early infection, as determined by lower levels of HIV-1 RNA in plasma and higher numbers of CD4+ T cells, had greater numbers of HIV-1-specific, IFN-γ-producing cells than did later-stage subjects. The IFN-γ reactivity increased in these late-stage subjects after an average of 33 months on triple-combination therapy, although it did not reach levels found in the early-stage, untreated persons. These data support an early, immunoenhancing effect on numbers of circulating, anti-HIV-1 CD8+ CTLp in a subpopulation of late-stage patients on triple-combination therapy (12, 22, 24a). The enhanced, HIV-1-specific CTLp and IFN-γ responses in these patients were temporary, however, and decreased to baseline levels by 89 weeks of treatment. This drop in HIV-1 antigen-specific, CD8+ T-cell responses may be due to low HIV-1 antigenic burden in the patients on long-term combination drug therapy, which suggests that adjunct, immune-based approaches will be necessary to induce a sustained, HIV-1-specific T-cell reactivity in late-stage patients receiving combination antiretroviral therapy.
ACKNOWLEDGMENTS
This work was supported in part by grants U01 AI35041, U01 AI37984, R01 AI41388, and R01 AI41870 from the National Institutes of Health and a grant from Merck.
We thank Luann Borowski, Hongyi Li, Michelle Tseng, and Susan McQuiston for technical assistance, Walter Storkus, Theresa Whiteside, and Elaine Elder for advice on the ELISPOT assay, Adriana Zeevi for HLA typing, and William Buchanan and the Pitt Men's Study and Pitt Treatment Evaluation Unit staff for clinical assistance.
REFERENCES
- 1.Brander C, Pichler W J, Corradin G. Identification of HIV protein-derived cytotoxic T lymphocyte (CTL) epitopes for their possible use as synthetic vaccine. Clin Exp Immunol. 1995;101:107–113. doi: 10.1111/j.1365-2249.1995.tb02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brander C, Walker B D. T lymphocyte responses to HIV-1 infection: implications for vaccine development. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 3.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czerkinsky C, Andersson G, Ekre H P, Nilsson L A, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 5.Gavin M A, Gilbert M J, Riddell S R, Greenberg P D, Bevan M J. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993;151:3971–3980. [PubMed] [Google Scholar]
- 6.Gotch F, McMichael A, Rothbard J. Recognition of influenza A matrix protein by HLA-A2-restricted cytotoxic T lymphocytes. Use of analogues to orientate the matrix peptide in the HLA-A2 binding site. J Exp Med. 1988;168:2045–2057. doi: 10.1084/jem.168.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–216. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 8.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Jonas L, Meibohm A, Holder D, Schleif W A, Condra J H, Emini E A, Isaacs R, Chodakewitz J A, Richman D D. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. JAMA. 1998;280:35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 10.Herr W, Schneider J, Lohse A W, Meyer zum Buschenfelde K H, Wolfel T. Detection and quantification of blood-derived CD8+ T lymphocytes secreting tumor necrosis factor α in response to HLA-A2.1-binding melanoma and viral peptide antigens. J Immunol Methods. 1996;191:131–142. doi: 10.1016/0022-1759(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 11.Huang X L, Fan Z, Liebmann J, Rinaldo C. Detection of human immunodeficiency virus type 1-specific memory cytotoxic T lymphocytes in freshly donated and frozen-thawed peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 1995;2:678–684. doi: 10.1128/cdli.2.6.678-684.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalvakolanu D V. Virus interception of cytokine-regulated pathways. Trends Microbiol. 1999;7:166–171. doi: 10.1016/s0966-842x(99)01476-6. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow R A, Ostrow D G, Detels R, Phair J P, Polk B F, Rinaldo C R., Jr The Multicenter AIDS Cohort Study: rationale, organization and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 15.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenig S, Woods R M, Brewah Y A, Newell A J, Jones G M, Boone E, Adelsberger J W, Baseler M W, Robinson S M, Jacobson S. Characterization of MHC class I restricted cytotoxic T cell responses to tax in HTLV-1 infected patients with neurologic disease. J Immunol. 1993;151:3874–3883. [PubMed] [Google Scholar]
- 17.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson M, Jin X, Ramratnam B, Ogg G S, Engelmayer J, Demoitie M-A, McMichael A J, Cox W I, Steinman R M, Nixon D, Bhardwaj N. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1 positive individuals. AIDS. 1999;13:767–777. doi: 10.1097/00002030-199905070-00005. [DOI] [PubMed] [Google Scholar]
- 19.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 20.Parker K C, Bednarek M A, Hull L K, Utz U, Cunningham B, Zweerink H J, Biddison W E, Coligan J E. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J Immunol. 1992;149:3580–3587. [PubMed] [Google Scholar]
- 21.Polydefkis M, Koenig S, Flexner C, Obah E, Gebo K, Chakrabarti S, Earl P L, Moss B, Siliciano R F. Anchor sequence-dependent endogenous processing of human immunodeficiency virus 1 envelope glycoprotein gp160 for CD4+ T cell recognition. J Exp Med. 1990;171:875–887. doi: 10.1084/jem.171.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pontesilli O, Kerkhof-Garde S, Notermans D W, Foudraine N A, Roos M T L, Klein M R, Danner S A, Lange J M A, Miedema F. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldo C R, Jr, Gupta P, Huang X L, Fan Z, Mullins J I, Gange S, Farzadegan H, Shankarappa R, Munoz A, Margolick J B. Anti-HIV type 1 memory cytotoxic T lymphocyte responses associated with changes in CD4+ T cell numbers in progression of HIV type 1 infection. AIDS Res Hum Retrovir. 1998;14:1423–1433. doi: 10.1089/aid.1998.14.1423. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Rinaldo, C. R., Jr., X.-L. Huang, Z. Fan, J. B. Margolick, L. Borowski, A. Hoji, C. Kalinyak, D. K. McMahon, S. A. Riddler, W. H. Hildebrand, R. B. Day, and J. W. Mellors. Anti-human immunodeficiency virus type 1 (HIV-1) CD8+ T-lymphocyte reactivity during combination antiretroviral therapy in HIV-1-infected patients with advanced immunodeficiency. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 25.Rowland-Jones S, Tan R, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 26.Scheibenbogen C, Lee K H, Mayer S, Stevanovic S, Moebius U, Herr W, Rammensee H G, Keiholz U. A sensitive ELISPOT assay for detection of CD8+ T lymphocytes specific for HLA class I-binding peptide epitopes derived from influenza proteins in the blood of healthy donors and melanoma patients. Clin Cancer Res. 1997;3:221–226. [Google Scholar]
- 27.Scheibenbogen C, Lee K H, Stevanovic S, Witzens M, Willhauck M, Waldmann V, Naeher H, Rammensee H G, Keiholz U. Analysis of the T-cell response to tumor and viral peptide antigens by an IFNγ- ELISPOT assay. Int J Cancer. 1997;71:932–936. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O'Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A B, Callan M F C. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 29.Tanguay S, Killion J J. Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 1994;13:259–263. [PubMed] [Google Scholar]
- 30.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsomides T J, Walker B D, Eisen H N. An optimal viral peptide recognized by CD8+ T-cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolinsky S M, Korber B T M, Neumann A U, Daniels M, Kunstman K J, Whetsell A J, Furtado M R, Cao Y, Ho D D, Safrit J T, Koup R A. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 33.Yang O O, Walker B D. CD8+ cells in human immunodeficiency virus type 1 pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv Immunol. 1997;66:273–311. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]