Abstract
Complex iron nanoparticle-based drugs are one of the oldest and most frequently administered classes of nanomedicines. In the US, there are seven FDA-approved iron nanoparticle reference drug products, of which one also has an approved generic drug product (i.e., sodium ferric gluconate (SFG)). These products are indicated for the treatment of iron deficiency anemia and are administered intravenously. On the molecular level, iron nanomedicines are colloids composed of an iron oxide core with a carbohydrate coating. This formulation makes nanomedicines more complex than conventional small molecule drugs. As such, these products are often referred to as nonbiological complex drugs (e.g., by the nonbiological complex drugs (NBCD) working group) or complex drug products (e.g., by the FDA). Herein, we report a comprehensive study of the physiochemical properties of the iron nanoparticle product SFG. SFG is the single drug for which both an innovator (Ferrlecit) and generic product are available in the US, allowing for comparative studies to be performed. Measurements focused on the iron core of SFG included optical spectroscopy, inductively coupled plasma mass spectrometry (ICP-MS), X-ray powder diffraction (XRPD), 57Fe Mössbauer spectroscopy, and X-ray absorbance spectroscopy (XAS). The analysis revealed similar ferric-iron-oxide structures. Measurements focused on the carbohydrate shell comprised of the gluconate ligands included forced acid degradation, dynamic light scattering (DLS), analytical ultracentrifugation (AUC), and gel permeation chromatography (GPC). Such analysis revealed differences in composition for the innovator versus the generic SFG. These studies have the potential to contribute to future quality assessment of iron complex products and will inform on a pharmacokinetic study of two therapeutically equivalent iron gluconate products.
Keywords: nanomedicine, iron nanoparticle, physicochemical properties, colloidal iron carbohydrate drugs, non-biologic complex drugs, generic nanoparticles, sodium ferric gluconate
Graphical Abstract
INTRODUCTION
With advances in nanoengineering, the incorporation of nanotechnology into human drug products has aided in the development of new drugs with improved pharmacokinetics, efficacy, safety, and targeting properties.1-3 These products, often called nanomedicines, are products designed and engineered to have at least one dimension between 1 and 100 nm or to exhibit dimension-dependent properties or phenomena up to 1 μm.2,4 The use of nanotechnology in new medicines shows great promise, and in the United States, approximately 50 nanomedicines have been approved.5
One of the most frequently administered nanomedicines on the market is iron nanoparticles, which make up 14% of all FDA-approved nanodrugs. These products are iron-carbohydrate colloids/nanoparticles that are administered intravenously (IV) to treat iron deficiency anemia (IDA).1,6-10 IDA is commonly seen in patients with chronic kidney disease (CKD), which often accompanies type 2 diabetes; in cancer patients, for whom treatment with chemotherapeutic agents leads to anemia; and in some patients with autoimmune disorders.6,11-14
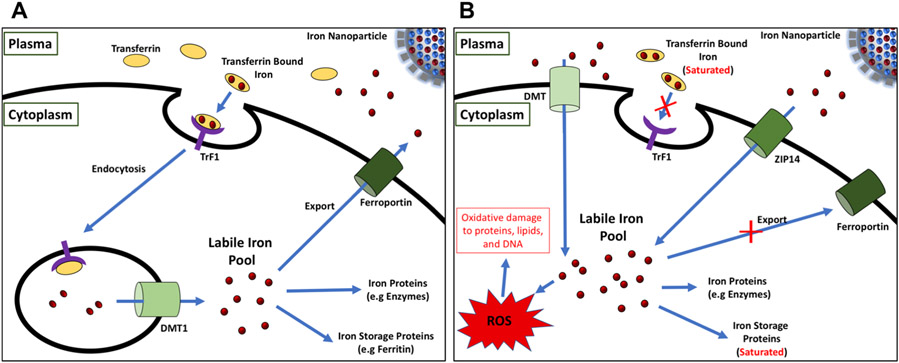
The overall goal of IV iron administration is to replenish the depleted iron cellular stores caused by IDA.15 Upon IV infusion, the iron drugs are processed via the reticuloendothelial system, after which the iron is transferred to transferrin in the plasma which delivers the iron to tissues to replenish iron deplete proteins and turn on erythrocyte production (red blood cell production).6,12,16-18 It is important that the release of the iron be controlled to avoid iron overload which results in saturation of transferrin and the presence of labile iron. Labile iron can be taken up by cells nonspecifically, leading to oxidative damage and ultimately toxicity (Figure 1).19,20 Iron nanoparticle drugs need to be designed such that iron release is controlled.
Figure 1.
Cartoon diagram of iron homeostasis under normal (A) and iron overload conditions (B). Under normal conditions, the iron released from the iron nanoparticle drug complex binds transferrin proteins in the plasma and is endocytosed into the cell via the transferrin receptor protein (TrF1). Endocytosed iron is then shuttled to the cytoplasm via the divalent metal transporter 1 (DMT1) and is either stored by iron storage proteins such as ferritin, used in iron dependent molecules/processes, or exported out of the cell via ferroportin. Under iron overload conditions, transferrin becomes completely saturated by iron resulting in the down regulation of the TrF1 pathway. The remaining nontransferrin bound iron is then transported into the cell via nonspecific metal transporters, DMT and ZIP14. The excess intracellular labile iron can react with cellular oxygen forming toxic reactive oxygen species (ROS), which may result in oxidative damage to proteins, lipids, and DNA.
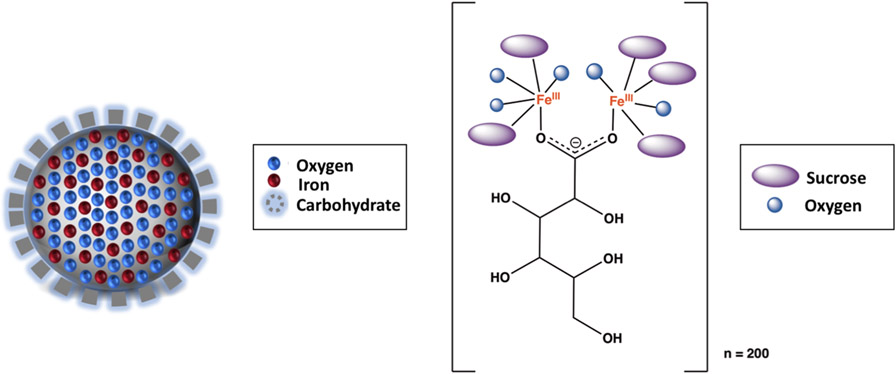
There are currently seven reference (innovator) and one generic IV iron nanoparticle drugs that are FDA approved: INFed (iron dextran), Dexferrum (iron dextran), Venofer (iron sucrose), Feraheme (ferumoxytol), Injectafer (ferric carboxymaltose), Monoferric (ferric derisomaltose), and Ferrlecit (sodium ferric gluconate complex), for which only the generic version of sodium ferric gluconate is available in the US.6,21 The general molecular features of each of these products are the same: they have a ferric iron oxyhydroxide core with a carbohydrate coating (Figure 2).11 The nature of the carbohydrate differs between products, and variability in physicochemical properties such as iron core size, hydrodynamic size, stability of iron nanoparticle complexes, and iron release between products have been reported.11,22-25
Figure 2.
A cartoon representation of an iron carbohydrate product (left) and the atomic connectivity of the iron-oxyhydroxide core with the gluconate ligand that forms the polynuclear iron nanoparticle (right). Each SFG nanoparticle complex is estimated to be composed of roughly ~200 sodium ferric gluconate units.
The single IV iron product for which a generic has been approved is Ferrlecit (Figure 3). This product, denoted here as ‘generic sodium ferric gluconate,’ was approved in 2011.26 There have been no additional generic iron nanoparticle type products approved in the subsequent eight years, despite an interest in approving additional products.1 In the intervening years, efforts to define how nanomedicines should be regulated have been made both by the FDA in the US and analogous regulatory agencies abroad.4,7,27 Generic nanomedicines in the US are subject to the same regulations as conventional small molecule drugs; however, nanoparticle-based drugs have unique properties.7,28 It has been suggested that new guidance for nanomedicines as a group or specific guidance for specific nanomedicines are needed.7,28,29 For the latter, there is product-specific guidance for SFG that was issued in 2013, in which qualitative (Q1) and quantitative (Q2) formulation sameness, in vivo pharmacokinetic bioequivalence studies, and in vitro characterization including physicochemical properties are recommended.6,27,29
Figure 3.
Brand (Ferrlecit) (left) and generic sodium ferric gluconate (right).
Our laboratory is currently conducting a study to compare brand SFG (Ferrlecit) and generic SFG in healthy volunteers.20 We have developed an approach that allows us to measure iron speciation in plasma, using liquid chromatography coupled to inductively coupled plasma mass spectrometry (LC-ICP-MS).20 The LC-ICP-MS technology allows us to simultaneously measure all iron species–colloidal iron, iron bound to transferrin, and labile iron and therefore provides a more comprehensive pharmacokinetic analysis of iron speciation in vivo. In previous studies, only some of these species (i.e., total iron and transferrin bound iron) could be directly measured.26,30-34 In conjunction with the in vivo pharmacokinetic data that we will obtain from the clinical trial, we sought to comprehensively evaluate the in vitro equivalence of the two drug products. By characterizing the in vitro properties of the two drugs and analyzing these data in light of the in vivo data to be obtained, the physicochemical properties that inform on the in vivo properties will be identified. These findings have the potential to inform on future in vitro/in vivo correlations and/or relationships.
Herein, we report a comparison of the physicochemical properties of brand SFG and generic SFG. The studies focused on the nature of the iron core and the nature of the carbohydrate complex and utilized a variety of analytical measures. We determined that the iron cores are equivalent but observed differences in the products’ particle size, molecular weight distribution, and iron lability.
MATERIALS AND METHODS
Materials.
Ferrlecit (NDC 0024-2792-10) (Sanofi U.S. LLC., Bridgewater, NJ, USA) and generic sodium ferric gluconate (SFG) (NDC 0591-0149-87) (Watson Pharma, Inc., Corona, CA, USA), containing 62.5 mg elemental iron per 5 mL (223.8 mM) sterile single-use vial were purchased. All drug stocks used throughout the investigation were from freshly opened vials. Trace metal grade nitric acid and hydrochloric acid were purchased from Fisher Scientific.
Data Presentation.
All data presented are presented as follows: average ± standard deviation.
Optical Spectra.
To determine the optical spectra of brand and generic SFG products, SFG samples were diluted to 0.050, 0.950, 0.136, 0.174, and 0.208 mM Fe concentrations in 0.9% NaCl solution. Full absorbance scans (220–800 nm) of each sample were taken in screw-capped quartz cuvettes (Starna Cells) using a Lambda 25 spectrometer (PerkinElmer). The molar extinction coefficients at 300 and 470 nm were calculated for both brand and generic SFG via linear fit to Beer’s law.
Total Iron Content.
The total iron content in both brand and generic SFG products were determined by inductively coupled plasma mass spectrometry (ICP-MS). Three different lots of both brand and generic SFG samples were diluted to a target elemental iron concentration of 0.4 mM Fe with deionized water. The diluted samples (200 μL) were then digested at 80 °C for 12 h in 67% nitric acid (500 μL) and deionized water (800 μL). A 6% nitric acid matrix was achieved by diluting hydrolyzed samples with deionized water (4 mL) prior to analysis by ICP-MS. Total elemental iron concentrations were determined by a 7700 Agilent ICP-MS (Agilent Technologies Inc., Santa Clara, CA, USA), employing an octopole reaction system cell (ORS) in He mode to remove interferences. ICP-MS run parameters were as follows: an argon carrier gas flow of 0.99 L/min, helium gas flow of 4.3 mL/min RF power of 1550 W, octopole RF of 200 V, and OctP bias of −18 V. Iron concentrations were derived from a calibration curve generated by a diluted series of iron atomic absorption standards (Millipore Sigma, Saint Louis, MO, USA) prepared in a 6% nitric acid matrix. Data analysis was performed using Agilent Mass Hunter software 4.4. Method validation was reported previously.20
Iron Core Measures.
X-ray Powder Diffraction (XRPD).
X-ray measurements of dried solutions were performed with an XRPD 3000TT (Seifert, Ahrensburg, Germany) using copper radiation (1.54178 Å, 40 kV, 30 mV) in Bragg Bretana configuration (automatic divergence slit, angular rate 0.18°/min). The samples were dried directly on the sample carrier (35 °C for 7 days in a drying chamber). Both pulverized and nonpulverized data were obtained. The nonpulverized data are shown here because the iron oxide iron hydroxide signal is less obscured by sucrose under these experimental conditions.
57Fe Mössbauer Spectroscopy.
Mössbauer spectroscopy was performed on both powder and liquid solution samples. Powdered samples were prepared by lyophilizing (223.8 mM Fe) brand and generic SFG (5 mL) in a speed vacuum concentrator (Thermo Fisher Scientific). Following lyophilization, samples were dried in an oven at 80 °C for 6 h. Once dried, a glass mortar and pestle were used to powder each sample prior to Mössbauer spectral analysis. For liquid measurements, undiluted (223.8 mM Fe) aqueous solution SFG samples were taken directly from their vials, immediately before Mössbauer spectral analysis.
The 57Fe Mössbauer spectra for both SFG products were recorded on a spectrometer from SEE Co. (Science Engineering & Education Co., Minneapolis, MN, USA) integrated with a Janis SVT-400-MOSS LHe/LN2 cryostat. The spectrometer was operated in the constant acceleration mode in transmission geometry. All experiments were carried out in the absence of an applied magnetic field. Powdered samples (ca. 40 mg) and aqueous solution samples (0.4 mL) of both SFG products (natural abundance Fe) were loaded under air into Delrin cups equipped with tight-fitting Delrin inserts designed to immobilize the contents of the cup. Aqueous solution samples were frozen in LN2 and stored under LN2 prior to analysis. Subsequently, data were collected at 80 K employing a velocity scale of ±10 mm s−1. The zero velocity of each Mössbauer spectrum refers to the centroid of a 25 μm metallic iron (α-Fe) foil collected at 295 K. The spectra were analyzed using the WMOSS program (SEE Co., formerly WEB Research Co., Edina, MN, USA) and fit to Lorentzian quadrupole doublets.
X-ray Absorption Spectroscopy (XAS).
X-ray absorption spectroscopy (XAS) was utilized to characterize the electronic and ligand metric parameters for the iron core within the SFG drugs. Samples were prepared in a nitrogen-purged glovebox in a water solution with 0.9% NaCl and 30% glycerol, at a final iron concentration of 1 mM. Samples were loaded into Lucite XAS cells wrapped with Kapton tape, flash-frozen in liquid nitrogen, removed from the glovebox, and stored in liquid nitrogen until data collection. XAS data were collected at the Stanford Synchrotron Radiation Lightsource (SSRL) on beamline 9–3. Beamline 9–3 is equipped with Si(220) double-crystal monochromator and utilizes a harmonic rejection mirror for focus and for reduction of harmonics. During data collection, samples were maintained at 10 K using an Oxford Instruments continuous-flow liquid helium cryostat. Protein fluorescence excitation spectra were collected using a 100-element germanium solid-state array detector. XAS spectra were measured using 5 eV steps in the pre-edge region (6900–7094), 0.25 eV steps in the edge region (7095–7135 eV), and 0.05 Å−1 increments in the extended X-ray absorption fine structure (EXAFS) region (to k = 13.0 Å−1), integrating from 1 to 30 s in a k3-weighted manner for a total scan length of approximately 40 min. X-ray energies were calibrated by recording an iron foil absorption spectrum simultaneously with the collection of drug data. The first inflection point for the iron foil edge was assigned at 7111.3 eV. Each fluorescence channel of each scan was examined for spectral anomalies prior to averaging. Data represent the average of four or five scans.
XAS data were processed using the Macintosh OS X version of the EXAFSPAK program suite integrated with Feff version 7.2 for theoretical model generation.35 Data reduction and processing of pre-edge transitions followed previously established protocols.36 Analysis of X-ray absorption near-edge spectroscopy (XANES) 1s → 3d transitions was completed using the EDG_FIT subroutine within EXAFSPAK. EXAFS fitting analysis was performed on raw/unfiltered data following our published protocol.36 EXAFS data were fit using both single- and multiple-scattering theoretical amplitude and phase functions for iron–oxygen/nitrogen, iron–sulfur, and iron–iron interactions calibrated utilizing published iron model data as controls. During spectral simulations, the scale factor was set to 0.95, and E0 values of −10, −12, and −15 were utilized for iron–oxygen/nitrogen, iron–sulfur, and iron–iron interactions, respectively. Metal–ligand coordination numbers were fixed at half-integer values, while only the absorber–scatterer bond length (R) and Debye–Waller factor (σ2) were allowed to freely vary during the fit.
Carbohydrate Shell/Overall Product Measures.
Forced Acid Degradation.
Three lots of both brand and generic SFG were diluted to a total iron concentration of 0.18 mM Fe with 0.9% NaCl, 0.24 M HCl solution, pH 0.3, in screw-capped quartz cuvettes (Starna Cells Inc., Atascadero, CA, USA). Full absorbance scans (220–820 nm) were recorded on a Lambda 25 spectrometer (PerkinElmer) every 2 h over a time period of 22 h, beginning immediately after sample dilution. Absorbances at 287 nm were plotted against time from which the first-order decay half-life was calculated using Prism software 5.01.
Dynamic Light Scattering (DLS).
To measure the particle size of both brand and generic SFG complexes, diluted (22.3 mM Fe in 0.9% NaCl (w/v) solution) and undiluted (223.8 mM Fe) SFG samples from three different lots of each product were analyzed in 1.5 mL disposable plastic cuvettes (BrandTech Scientific Inc., Essex, CT, USA) via DLS. In addition to 22.3 and 223.8 mM Fe SFG samples, 2.2, 3.0, 4.5, 9.0, 44.8, 56.0, 74.6, 111.9, and 149 mM Fe SFG brand SFG (lot A6123) and generic SFG (lot 1702105.1) samples in 0.9% NaCl solution were also run on DLS. DLS measurements were taken on a Zetasizer Nano ZSP (Malvern Instruments Ltd., Westborough, MA, USA) at 20 °C. Backscattering information was collected at a 173° angle using a 633 nm wavelength He–Ne laser. The hydrodynamic (Z-averaged) diameters of both diluted SFG complexes were calculated via Zetasizer software version 7.03. Due to the dark coloration and high concentration of the undiluted samples, the hydrodynamic diameters of each of the undiluted SFG samples were additionally calculated using corner scattering information obtained in quartz cuvettes (Starna Cells) via DLS. During the corner scattering experiments, the hydrodynamic diameters of both SFG complexes were measured on a photocorrelation spectrometer (Photocor Instruments, Moscow, Russia) at 20 °C. Corner scattering information was collected at a 90° angle using a 632 nm He–Ne laser. Autocorrelation functions were processed using DynaLS software (SoftScientific, Tirat Carmel, Israel). The hydrodynamic (Z-averaged) diameters Z–Dh of both undiluted SFG complexes were calculated using the following formula:
where di is the value of hydrodynamic diameters of the particles present in the particle size distribution and Ii is their corresponding intensities.
Analytical Ultracentrifugation (AUC).
The total loading sample average sedimentation coefficients of both brand and generic SFG products were measured by analytical ultracentrifugation (AUC). In order to determine lot to lot variation, three different lots of both brand SFG and generic SFG were used during this experiment. SFG samples were diluted to a 2.2 mM Fe total iron concentration in 0.9% NaCl solution prior to AUC measurement. AUC was performed using a Beckman Optima XL-A, An-60 Ti rotor, scanning absorbance optics, with 12 mm path length double sector graphite centerpieces and quartz lenses. All measurements were made at 470 nm at a speed of 42,000 rpm, 20 °C. Data collected over 100 scans were used as a representation of the whole run (radial step size of 0.003 cm). The total loading sample sedimentation coefficients of both brand and generic SFG samples for each concentration were calculated by integrating 100% of the loading concentration signal using an ls-g*(s) SEDFIT model where both RI noise and time-independent noise were accounted for. To ensure that the total loading sample sedimentation coefficients were not concentration-dependent, SFG samples were also diluted to four other total iron concentrations (0.9, 1.5, 1.8, and 2.6 mM Fe) in 0.9% NaCl solution and analyzed via AUC.
Gel Permeation Chromatography (GPC).
The molecular weight of both brand and generic SFG complexes was determined via gel permeation chromatography (GPC). SFG samples were diluted to a total iron concentration of 5.6 mM Fe with mobile phase solution (aqueous solution of sodium azide (0.02 w/v %) and 0.01% brand SFG at pH 7.0) prior to injection into the GPC. Commercial pullulan polysaccharide molecular weight standards (Sigma-Aldrich, St. Louis, MO, USA) ranging from 23–805 kDa were used to generate a molecular weight calibration curve. Both standards (40 μL) and samples (50 μL) were eluted through a 300 × 7.8 mm Toso Hass TSK Gel G40000SWxL column (spherical silica with a particle size of 8 μm and a pore size of 25 nm) at an isocratic flow rate of 0.5 mL/min on a Hewlett-Packard 1100 HPLC system coupled with an online refractive index detector (Optilab rEX, Wyatt Technology Corp., Santa Barbara, CA, USA). During analysis, the column temperature was maintained at 35 °C, while the autosampler temperature was maintained at 20 °C. The molecular weight of both brand and generic SFG complexes were calculated via multivariate curve (log molar mass (g/mol) vs volume (mL)) using Astra 6.1.1.17 software (Wyatt Technology Corp., Goleta, CA, USA).
Results.
A suite of physicochemical measurements were performed on brand and generic SFG to obtain fundamental characterization data and identify potential differences between the products. Because nanoparticle-based drugs are more complex than traditional small molecule drugs, their physicochemical properties are similarly more complex. The products are typically not singular, homomeric species; instead, they are complex, heterogeneous species.7,37 The synthesis of these products is perhaps predictive of this heterogeneity. For instance, the manufacturing process for sodium ferric gluconate consists of a series of controlled chemical reactions that include the mixing of ferric chloride and sodium carbonate to yield ferric hydroxide precursors, which then react with sodium gluconate and progressively react to form the complex, heterogeneous nanoparticle that is diluted with sucrose and water.38 The first set of experiments performed here focused on characterizing the iron core of the brand and generic SFG.27
Optical Spectra.
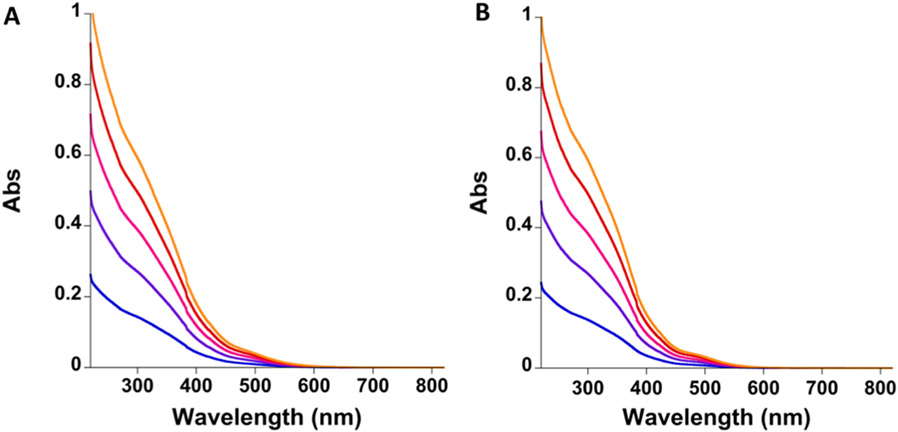
To measure and compare the optical spectra of both brand and generic SFG products, the products were diluted to a range of concentrations, and their optical spectra were recorded. Broad absorbance bands were observed between 300 and 500 nm for both products, indicative of iron-ligand peaks (Figure 4). The absorbance at 300 nm has previously been proposed to be an oxo-metal charge transfer band, while those between 470 and 500 nm have proposed to be as d-d bands arising from a six-coordinate, high-spin Fe(III) ground state (Figure 4).22 These spectra are consistent with those previously reported for SFG.22,39 The average brand SFG molar extinction coefficients at 300 and 470 nm were determined to be 2860 ± 25.3 and 274 ± 2.5 M−1 cm−1, whereas the average generic SFG molar extinction coefficients were determined to be 2830 ± 30.5 and 213 ± 2.10 M−1 cm−1 (Table 1). These values are similar to those previously reported (Table 1).22
Figure 4.
Overlay of the change in the averaged absorption spectra (n = 3), between 220 and 800 nm, of brand SFG (A) and generic SFG (B) at 0.050 (blue), 0.95 (purple), 0.136 (pink), 0.174 (red), and 0.208 mM (orange) Fe concentrations diluted in 0.9% NaCl solution.
Table 1.
Average Brand and Generic SFG Absorbance Spectra Molar Extinction Coefficients and Standard Deviation (n = 3)
| product | lot no. | diluent | cell path length (cm) | ε300 (M−1 cm−1) | ε470 (M−1 cm−1) | ref |
|---|---|---|---|---|---|---|
| brand SFG | A6122 | 0.9% NaCl | 1 | 2860 ± 25.3 | 274 ± 2.50 | data from this work |
| not recorded | water | 0.01 | 2670 | 250 | Kudasheva et al. 2004 | |
| generic SFG | 1602240.1 | 0.9% NaCl | 1 | 2830 ± 30.5 | 213 ± 2.10 | data from this work |
Total Iron Content.
Inductively coupled plasma mass spectrometry (ICP-MS) was used to measure the total iron content in three different lots of both brand and generic SFG formulations. To ensure that sample iron concentration was within a detectable range, both brand and generic SFG samples were diluted to 0.4 mM Fe in deionized water before being digested in trace metal grade nitric acid. The average total iron content, calculated from three lots of each product (n = 3 per lot), was measured to be 229.2 ± 10.3 mM Fe (12.8 mg/mL) for brand SFG and 234.8 ± 14.0 mM Fe (13.1 mg/mL) for generic SFG. No significant difference was determined between the brand and generic SFG total iron content by a two-tailed Student’s t test (p > 0.05). Similar to other studies, all measured brand and generic SFG total iron content were within 10% of the 223.8 mM Fe (12.5 mg/mL) total iron content listed on each drug label, indicating little difference in lot to lot variation (Table 2).6 Although ICP-MS has only recently begun to be employed to measure total iron in SFG products, it provides a robust measure of iron content.6
Table 2.
Average Brand and Generic SFG Product Total Elemental Iron Content and Standard Deviation (n = 3)
| product | lot no. | [Fe] (mM) | ref |
|---|---|---|---|
| brand SFG | A6104 | 233.8 ± 3.1 | data from this work |
| A6123 | 236.4 ± 7.6 | data from this work | |
| A6124 | 217.4 ± 2.6 | data from this work | |
| D2C283A | 225.7 ± 2.1 | Sun et al. 2018 | |
| D2C593A | 221.1 ± 3.2 | Sun et al. 2018 | |
| generic SFG | 1602192.1 | 219.1 ± 6.4 | data from this work |
| 1702105.1 | 239.5 ± 1.4 | data from this work | |
| 1702107.1 | 246.0 ± 10.1 | data from this work | |
| 132296.1 | 236.3 ± 4.1 | Sun et al. 2018 |
X-ray Powder Diffraction (XRPD).
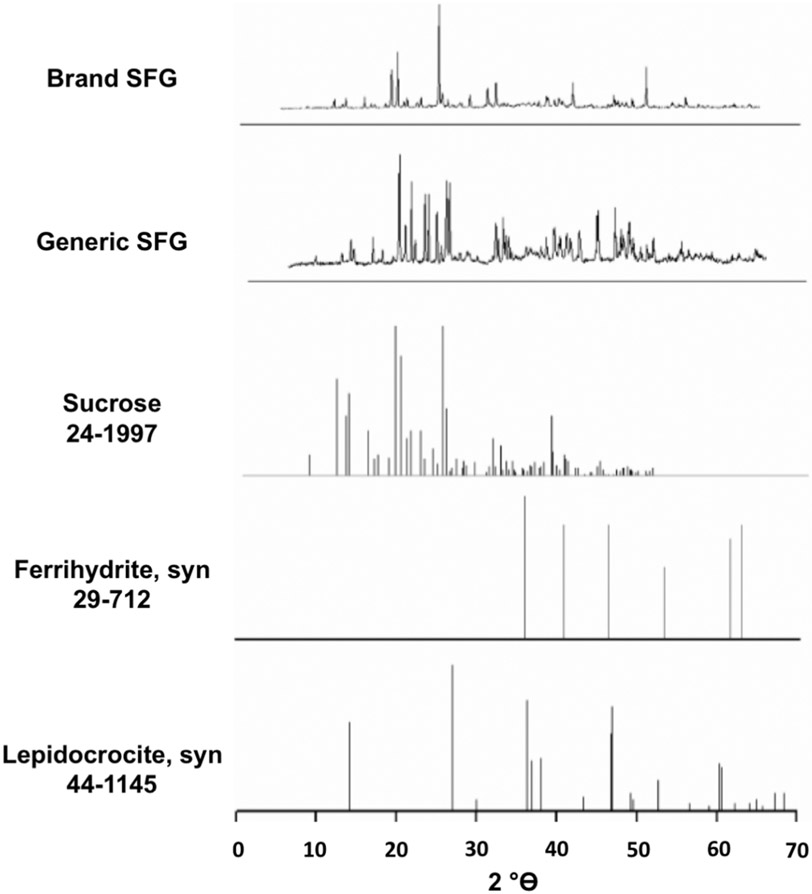
X-ray powder diffraction was employed to identify the crystalline structure of the sodium ferric gluconate nanoparticles. Samples dried for 7 days produced the X-ray powder patterns, shown in Figure 5. The spectra are dominated by the sucrose present in the product (peaks at 14° 2θ and 22° 2θ), as has been noted for XPRD of other iron nanoparticle products.22,40 Comparison of these data to the growing body of published XPRD data for iron nanoparticles revealed that the spectra are consistent with a ferrihydrite solid-state structure (observed peaks at 36° 2θ and 62° 2θ are characteristic of ferrihydrite). It must be noted that we cannot rule out the presence of some lepidocrocite character, as the strong carbohydrate (sucrose) signal makes it difficult to differentiate between ferrihydrite and lepidocrocite.22,40 The singular XRPD study of SFG – which utilized the brand product–similarly identified both ferrihydrite and lepidocrocite character.23 Both brand and generic products contain ferrihydrite like iron core characteristics, and generic SFG was observed to have a slightly more pronounced 36° 2θ ferrihydrite peak, which could indicate a slightly more uniform crystalline form in generic SFG than brand SFG.22 However, the current XRPD method is not a quantitative measure, and the XRPD data provide an indication of the overall crystalline form.
Figure 5.
X-ray powder diffraction pattern of brand and generic SFG nonpulverized powder samples dried for 7 days. X-ray measurements of nonpulverized dried solutions were performed with an XRPD 3000TT using copper radiation (1.54178 Å, 40 kV, 30 mV) in Bragg Bretana configuration (automatic divergence slit, angular rate 0.18°/min).
57Fe Mössbauer Spectroscopy.
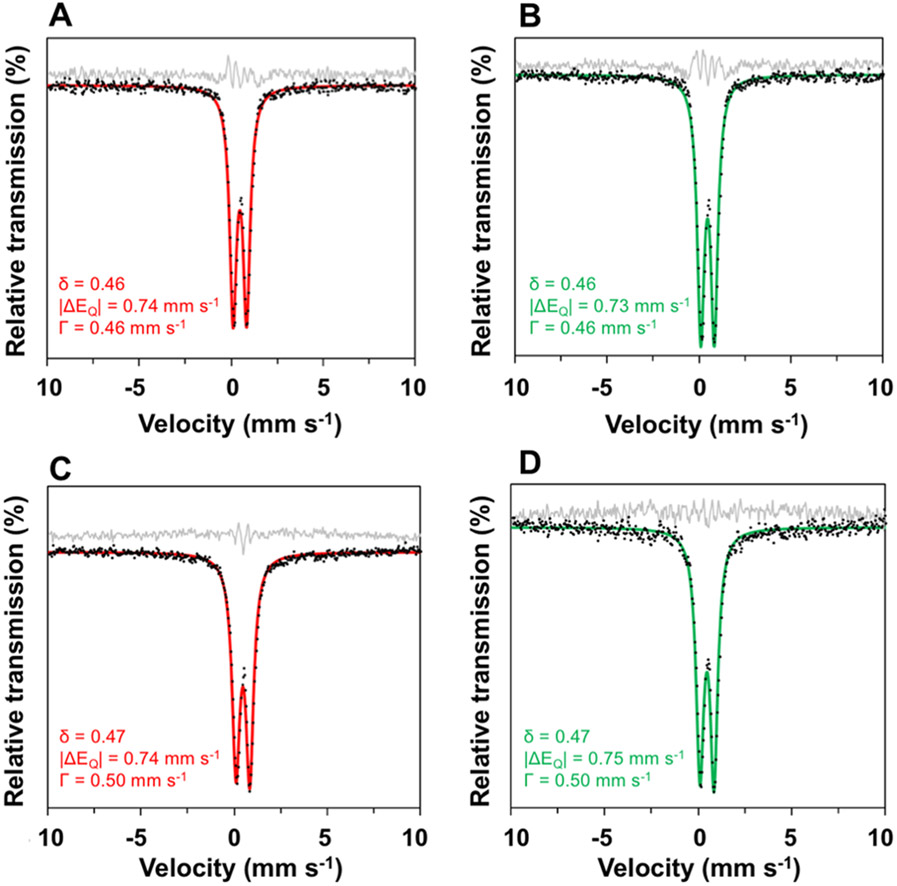
To further define the iron core, 57Fe Mössbauer spectra were obtained (Figure 6). The 80 K spectrum of the powdered brand complex exhibits a single quadrupole doublet with δ = 0.46 and ∣ΔEQ∣ = 0.74 mm s−1 (fwhm line width, Γ = 0.46 mm s−1), consistent with fast-relaxing ferric centers. Aqueous samples of the brand complex exhibit nearly identical parameters (δ = 0.46 and ∣ΔEQ∣ = 0.73 mm s−1), strongly suggesting that the Fe environments are unaffected by dissolution. Likewise, the 80 K spectrum of the powdered generic complex exhibits δ = 0.47 and ∣ΔEQ∣ = 0.74 mm s−1 (fwhm line width, Γ = 0.50 mm s−1), with solution parameters (δ = 0.47 and ∣ΔEQ∣ = 0.75 mm s−1) that closely match the solid-state values. The small differences in isomer shift (0.01 mm s−1) and quadrupole splitting (0.01–0.02 mm s−1) between brand and generic provide good evidence that these systems contain the same ferric gluconate core structure.
Figure 6.
57Fe Mössbauer spectra (80 K, zero applied field) of SFG as powder brand SFG (A), solution brand SFG (B), powder generic SFG (C), and solution generic SFG (D) samples at 80 K. Samples were not diluted for either powder or solution sample preparation. Experimental data: black dots. Best fits: solid red and green lines. Residuals: solid gray lines.
X-ray Absorption Spectroscopy (XAS).
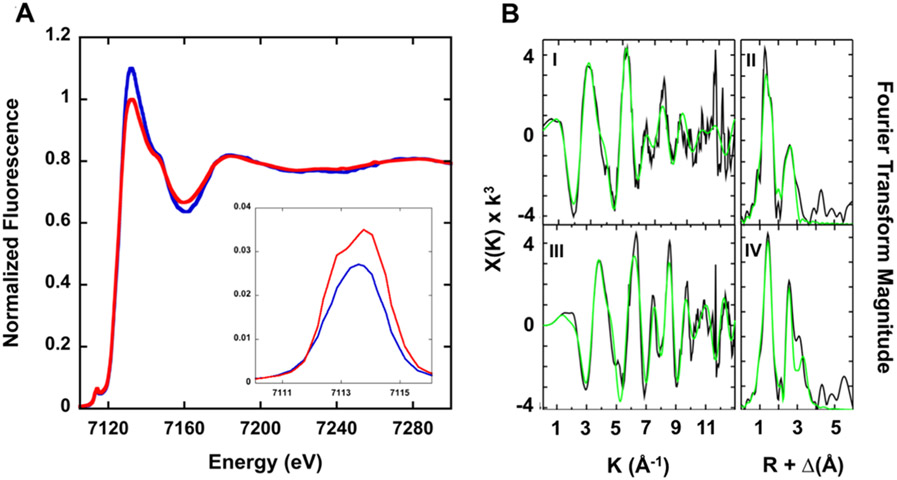
X-ray absorption spectroscopy (XAS) was used to characterize the electronic and ligand metric parameters for iron associated with both brand and generic SFG nanoparticle iron cores. The X-ray absorption near edge structure (XANES) spectra for both brand and generic SFG iron nanoparticles are shown in Figure 7A. The XANES pre-edge signals, centered at 7113.8 and 7114 eV for the brand and generic SRG samples, respectively, show signals consistent with Fe(III) models (Figure 7A, inset), while the area for the pre-edge peak feature for the brand sample (7.3 eV2) is slightly smaller than the area measured for the generic sample (9.5 eV2), suggesting an octahedral ligand coordination geometry that is slightly more symmetric for the brand sample.41-43 The first inflection point of the Fe edge in both samples occurs at 7126.5 eV (brand) and 7127 eV (generic), suggesting iron in both SFG samples is in the ferric oxidation state.44
Figure 7.
(A) Iron XANES for brand (blue) and generic (red) SFG products. (Inset) An expansion of the 1s–3d pre-edge transition peak for both brand (blue) and generic (red) SFG products. (B) Iron EXAFS and Fourier transform of EXAFS data for brand (I and II) and generic (III and IV) SFG products. (Left) Raw EXAFS data displayed in black and best fit in green. (Right) Corresponding Fourier transform plot of raw EXAFS data in black and best fit in green.
Simulations of the extended X-ray absorption fine structure (EXAFS) spectral region of the iron XAS spectra were used to provide the metrical parameters for iron bound to both samples. The asymmetric nature of the Fe EXAFS (Figure 7B left) and Fourier transforms of the EXAFS (Figure 7B right) indicate that iron in both samples is in a complex coordination geometry with substantial long-range order. Best fit parameters from simulations for the iron nearest neighbor ligand environment in the brand sample indicate that metal is coordinated with two distinct environments of only oxygen/nitrogen ligands centered at ca. 1.87 and 2.03 Å, while ligands for the generic sample occur at 1.91 and 2.06 Å (Figure 7B, Table 1). Coordination numbers for the Fe–O/N ligands obtained from the EXAFS simulations suggest ligands bound to the Fe(III) in the brand sample is six-coordinate and highly symmetric, while that is the generic sample is still six-coordinate (based on Fe–O/N bond lengths consistent with six-coordinate species in the Cambridge Structural Database) however the symmetry for this generic model is slightly distorted. Long-range scattering in both samples shows a distinct Fe⋯Fe vector at 3.05 Å (brand sample) and 3.01 Å for the generic sample.45 In addition, both samples show Fe⋯C scattering at 2.93 Å (brand) and at 3.76 Å (generic).
Slight differences between the pre-edge features and intensities, caused by differences in the immediate metal–ligand environments within the two SFG drug samples, were determined through XANES and EXAFS analysis. The XAS analysis indicates that the iron cores of brand and generic SFG nanoparticles are not identical but very similar. A further conclusion is that XAS has the potential to inform on any iron core differences between iron nanoparticle type drug products.
Forced Acid Degradation.
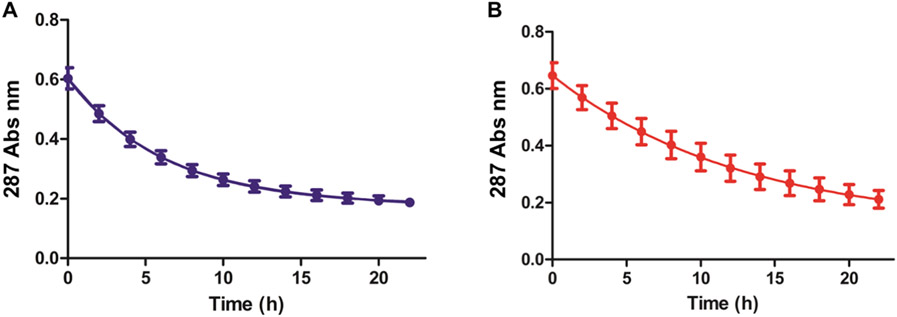
To compare the iron lability of both brand and generic SFG products, an in vitro forced acid degradation assay was performed. In this assay, the drug is subjected to a pH drop via the addition of HCl, and the drug’s optical spectrum is monitored. The SFG optical spectrum exhibits absorbance peaks between 250 and 500 nm, which have been attributed to the iron-oxy/iron-hydroxy structure of the core (Figure 4). As the product degrades, these absorbance peaks decrease. Full absorbance scans (220–820 nm) of 0.18 mM Fe diluted SFG products in 0.9% NaCl, 0.24 M HCl, at pH 0.3, were obtained every 2 h over the course of 22 h starting immediately after dilution (Figure 8). Decreases in the 287 nm absorbance were observed in both of the measured brand and generic SFG spectra (Figure 8). The half-lives of both products were then calculated by fitting these data to a first-order decay model (Table 3, Figure 8). The half-lives differed between the two products, with the brand exhibiting an average half-life of 4.3 h ± 0.3, and the generic exhibiting a longer average half-life of 9.4 h ± 2.4 (calculated from three lots of each product (n = 3 per lot)). This difference has the potential to inform on any differences in vivo data if observed. We note that a similar half-life of the brand SFG has been reported by Jan and co-workers (4.0 h ± 0.1) using this same assay.23 Of interest is the observed difference in half-life between the brand and generic. In Jahn and co-workers’ work, iron sucrose also had a half-life close to the brand SFG, while other iron products (dextran, isomaltoside 1000, carboxymaltose, and ferumoxytol) had considerably longer half-lives (<21 h).23
Figure 8.
Forced acid degradation of brand (A) and generic (B) SFG containing 0.18 mM elemental iron diluted in 0.9% NaCl, 0.24 M HCl solution. At t = 0 h, the drug is fully intact. Each point represents the average of three measurements taken at 287 nm on a UV–visible spectrometer. Data were fitted to a one phase exponential decay plot (R2 brand = 0.999 and R2 generic = 0.997).
Table 3.
Average Half-Lives and Standard Deviations (n = 3) of Brand and Generic SFG Products Diluted to a Total Elemental Iron Concentration of 0.18 mM Fe in 0.9% NaCl, 0.24 M HCl Solution
| product | lot no. | half-life (h) |
|---|---|---|
| brand SFG | A6104 | 4.3 ± 0.1 |
| A6123 | 4.5 ± 0.2 | |
| A6124 | 4.0 ± 0.4 | |
| Generic SFG | 1602192.1 | 7.1 ± 0.1 |
| 1702105.1 | 11.9 ± 3.5 | |
| 1702107.1 | 9.1 ± 0.8 |
Dynamic Light Scattering (DLS).
SFG complex products are administered intravenously, either diluted in 0.9% NaCl solution or undiluted. Dilution of nanoparticles can affect particle size; however, there are limited data on the particle size of the SFG products, and the data available were not performed at clinically relevant concentrations.6,23,38 We sought to determine the particle size of the SFG products for clinically relevant concentrations such that we could compare the brand and generic products and determine whether dilution affects particle size. These concentrations are “diluted”- 22.3 mM Fe in 0.9% NaCl and “undiluted” – 223.8 mM Fe.46 The complex particle sizes (Z-average hydrodynamic diameters) of both “dilute” and “undilute” brand and generic SFG complexes were measured by dynamic light scattering (DLS). The particle sizes of undiluted and diluted brand and generic SFG complexes were compared according to the draft guidance (Draft Guidance on Sodium Ferric Gluconate Complex) using a population bioequivalence statistical approach.27
DLS of Dilute Products.
The average complex particle sizes for the brand and generic SFG, diluted to 22.3 mM Fe in 0.9% NaCl, calculated from three lots of each product (n = 3 per lot), were measured to be 14.9 ± 0.1 nm and 10.1 ± 0.3 nm, respectively. A single peak was observed in all cases. The particle sizes of the diluted brand and generic SFG were found to differ from one another (p < 0.001) with a 95% confidence interval from −5.0 to −4.5 (mean difference = −4.7) (Table 4). When comparing lots, no differences in dilute SFG complex particle size were observed for brand SFG product lots (p > 0.05), whereas statistical differences were observed for the generic SFG product lots (p < 0.05) via one-way ANOVA tests (Table 4). Collectively, these data reveal that the brand and generic SFG have different particle sizes, with that of the brand being larger than generic SFG. All data were calculated using backscattering information.
Table 4.
Average Complex Particle Size (Z-Average Hydrodynamic Diameters) and Standard Deviation (n = 3) of Diluted (22.3 mM Fe in 0.9% NaCl Solution) and Undiluted (223.8 mM Fe) Brand and Generic SFG Product Samples Measured by Dynamic Light Scatteringa
| drug product | lot no. | Fe conc (mM) | pH | diluent | light scattering geometries | Z-average diameter (nm) | PDI |
|---|---|---|---|---|---|---|---|
| diluted brand SFG | A6122 | 22.3 | 6.8 | 0.9% NaCl | back | 14.8 ± 0.1 | 0.151 |
| A6123 | 22.3 | 6.8 | 0.9% NaCl | back | 15.0 ± 0.0 | 0.169 | |
| A6124 | 22.3 | 6.8 | 0.9% NaCl | back | 14.8 ± 0.4 | 0.178 | |
| undiluted brand SFG | A6122 | 223.8 | 8.5 | NA | back | 26.6 ± 1.1 | 0.218 |
| A6123 | 223.8 | 8.5 | NA | back | 26.0 ± 0.8 | 0.239 | |
| A6124 | 223.8 | 8.5 | NA | back | 25.9 ± 1.4 | 0.252 | |
| A6122 | 223.8 | 8.5 | NA | corner | 24.7 ± 1.0 | 0.044 | |
| diluted generic SFG | 162240.1 | 22.3 | 6.8 | 0.9% NaCl | back | 10.3 ± 0.1 | 0.144 |
| 1702105.1 | 22.3 | 6.8 | 0.9% NaCl | back | 9.8 ± 0.0 | 0.155 | |
| 1702107.1 | 22.3 | 6.8 | 0.9% NaCl | back | 10.3 ± 0.2 | 0.104 | |
| undiluted generic SFG | 162240.1 | 223.8 | 8.0 | NA | back | 17.8 ± 0.5 | 0.337 |
| 1702105.1 | 223.8 | 8.0 | NA | back | 16.7 ± 0.2 | 0.132 | |
| 1702107.1 | 223.8 | 8.0 | NA | back | 16.7 ± 0.1 | 0.16 | |
| 162240.1 | 223.8 | 8.0 | NA | corner | 14.0 ± 1.0 | 0.077 |
PDI: polydispersity index.
DLS of Undilute Products.
The average complex particle sizes for the brand and generic undilute SFG, calculated from three lots of each product (n = 3 per lot), were measured to be 26.2 ± 0.4 nm and 17.1 ± 0.6 nm. The particle sizes of the undiluted brand and generic SFG were found to differ from one another (p < 0.001) with a 95% confidence interval from −9.9 to −8.2 (mean difference = −9.1) (Table 4). When comparing lots, no differences in undilute SFG complex particle size were observed for brand SFG product lots (p > 0.05), whereas statistical differences were observed for the generic SFG product lots (p < 0.05) via one-way ANOVA tests. (Table 4). Similar to the “dilute” samples, these data reveal that the brand SFG particle size is larger than the generic particle size.
As it can be difficult to measure and calculate the particle sizes of darkly colored, nontransparent, and highly concentrated samples in solution, corner scattering light geometry DLS approaches, used to determine oil-in-water emulsion particle sizes and successfully employed to study viscous and nontransparent petroleum products, were used to validate the SFG complex particle sizes determined using backscattering (vide supra).47 The average undiluted complex particle sizes (n = 3), calculated using corner scattering information, of brand SFG (24.7 ± 1.0 nm) and generic SFG (14.0 ± 1.0 nm) were slightly smaller than those calculated via backscattering information but still significantly larger than those observed in diluted SFG samples (Table 4). Therefore, backscattering for undiluted SFG products appears to be valid.
Taken together, these data also show that dilution leads to a smaller measured particle size for both brand and generic products. This was confirmed by determining the statistical differences between the undilute and dilute products (brand and generic) using a two-tailed Student’s t test (p < 0.0001). (Table 4).
Concentration Dependent DLS Measurements.
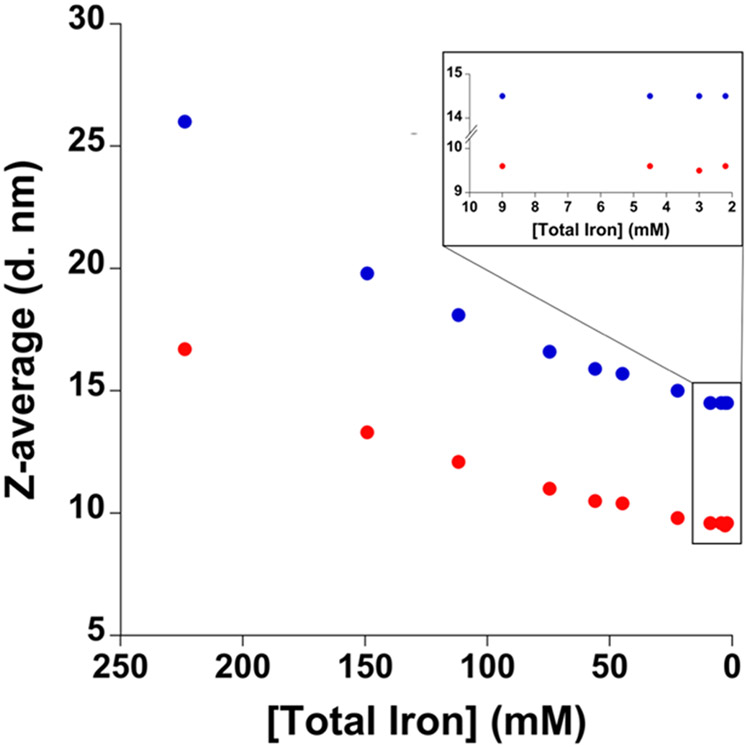
Given that statistically significant differences in complex particle size were observed between the diluted and undiluted SFG products, we expanded our studies to evaluate the particle size over a range of concentrations to understand the effects of dilution. Our approach involved measuring the particle size of the products [SFG (lot no. A6123), generic SFG (lot no. 1702105.1)] at the following concentrations: 2.2, 3.0, 4.5, 9.0, 22.3, 44.8, 56.0, 74.6, 111.9, 149.2, and 223.8 mM Fe in 0.9% NaCl (Figure 9). We observed a decrease in particle size as the product was diluted from 223.8 mM to 22.3 mM (Figure 9), after which no statistically significant changes were observed from 9.0 mM Fe to 2.2 mM Fe via one-way ANOVA (p > 0.05) (Figure 9). As our results indicate that dilution affects particle size for both brand and generic SFG, we compared our measurements to those previously reported with identical drug concentrations and diluents. Our measured brand SFG particle size (14.5 nm), at 2.2 mM Fe (100 times dilution) in 0.9% NaCl solution, was slightly larger than those previously reported (11.5, 11.9, and 12.1 nm), whereas our measured generic SFG particle size (9.6 nm) was slightly smaller than that previously reported (10.5 nm).6,48 One potential source of these differences between our measured particle sizes and those previously reported are product lot to lot differences. To the best of our knowledge, all previously reported SFG DLS particle size measurements have only measured samples with iron concentrations less than 4.5 mM Fe (50 times dilution); for example, in the comprehensive study by Sun and co-workers, a 100X dilution was utilized.6 These dilutions are lower than the concentrations used clinically, 22.3 mM Fe and 223.8 mM Fe; therefore, the studies reported herein expand the breadth of the understanding of the particle size as measured by DLS.6,23,38,48
Figure 9.
Particle size of brand SFG lot no. A6123 (blue) and generic SFG lot no. 1702105.1 (red) at 2.2, 3.0, 4.5, 9.0, 22.3, 44.8, 56.0, 74.6, 111.9, 149.2, and 223.8 mM Fe in 0.9% NaCl solution. Each point represents the average of three measurements taken on a Malvern Zetasizer Nano ZSP. The error bars represent the standard deviation from three different replicate samples. However, these error bars are often smaller than the symbol.
Analytical Ultracentrifugation (AUC).
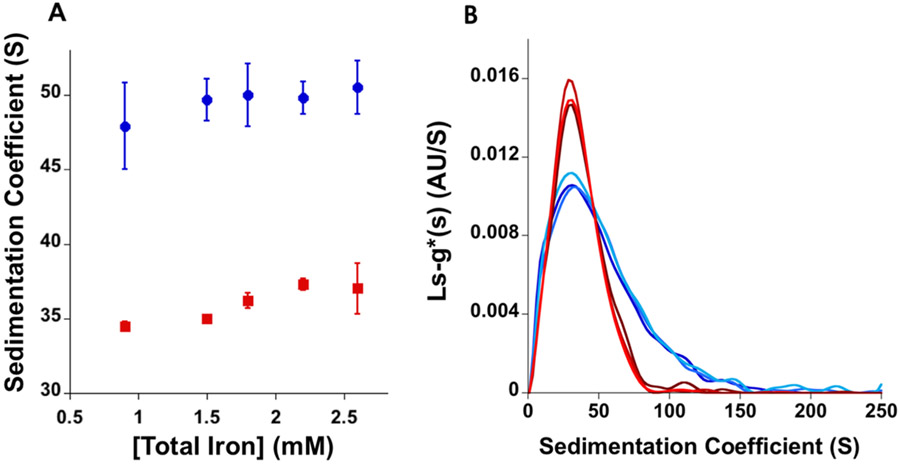
To identify potential differences between brand and generic SFG preparation solution heterogeneity, the total loading sample sedimentation coefficients were measured by analytical ultracentrifugation. The total loading sample sedimentation coefficients of both SFG products diluted to 2.2 mM Fe in 0.9% NaCl solution were determined by AUC. Following AUC, monomodal sedimentation coefficient distributions are observable for both brand and generic SFG products (Figure 10A). Similar to previously reported SFG product sedimentation coefficient distributions, brand SFG had a shorter and broader distribution than generic SFG (Figure 10B).6 The integration of the entire total loading sample sedimentation coefficient distribution shows the total loading sample sedimentation coefficient, averaged from three different product lots (n = 3 per lot), of brand SFG (49.8 ± 1.1 S) to be larger than that of generic SFG (37.3 ± 0.4 S). Interestingly, our measured total loading sample sedimentation coefficients are the opposite of those reported in the only other comparative AUC analysis of brand and generic SFG that showed brand SFG to have smaller sedimentation coefficient than generic SFG.6 This may be due to variability in the analytical method or intralab variability, suggesting that this approach may not be ideal for evaluating these types of products.6
Figure 10.
(A) The total loading sample averaged sedimentation coefficients of five different concentrations of total iron (0.9, 1.5, 1.8, 2.2, and 2.6 mM Fe) of brand (blue) and generic (red) SFG diluted in 0.9% NaCl solution (n = 3). (B) The average sedimentation coefficient distribution of brand (blue) and generic (red) SFG at 2.2 mM Fe diluted in 0.9% NaCl solution calculated from three lots of each product (n = 3 per lot).
Additionally, differences between brand and generic SFG sedimentation coefficient distributions were also found where brand SFG has a shorter and wider sedimentation coefficient distribution than generic SFG (Figure 10B). Little to no difference in total loading sample sedimentation coefficient or sedimentation distribution are seen between different SFG product concentrations (Figure 10A). As total sample loading sedimentation coefficient and distribution can be influenced by particle size, shape, molecular weight, and density, these measurements are collectively indicative of differences between the two products’ general properties.49,50
Gel Permeation Chromatography (GPC).
To compare the molecular weights of brand and generic SFG, gel permeation chromatography (GPC) using a refractive index detector was determined. Each product’s molecular weight was determined by comparing the retention time of the product to the retention time of known standards (pullulan polysaccharide molecular weight standards). Brand SFG was found to have an average molecular weight of 437.5 ± 3.0 kDa (calculated from three different product lots (n = 3 per lot), which falls within the molecular weight range previously reported (384.4–467.7 kDa) (Table 5).6 Generic SFG was found to have an average molecular weight of 342.0 ± 1.5 kDa (calculated from three different product lots (n = 3 per lot), which is slightly below the molecular weight range previously reported (363.7–387.4 kDa) (Table 5).6 Of the two products, brand SFG had the closest measured molecular weight to the predicted SFG complex formula ([NaFe2O3(C6H11O7)(C12H22O11)5]n=200) of 417.9 kDa. Evaluation of our data, in the context of previously reported data using this same GPC approach, shows that the molecular weight range for brand SFG is 384.4–467.7 kDa and generic is 342.0–387.4 kDa. The variability in the weight was higher for brand SFG (83.3 kDa versus 45.4 kDa).6 These differences may be caused by lot to lot variation. Together these data indicate that brand SFG has, on average, a larger molecular weight than generic SFG, although both fall within the expected product molecular weight range of 289–440 kDa.6,46
Table 5.
Gel Permeation Chromatography (GPC) Determined Weight-Averaged Molecular Weights (MW) of Brand and Generic SFG Samplesa
| product | lot no. | MW (kDa) |
standard deviation |
ref |
|---|---|---|---|---|
| Brand SFG | A6122 | 437.5 | 3.0 | data from this work |
| brand SFG | A5075 | 467.7 | 3.0 | Sun et al. 2018 |
| brand SFG | D2C283A | 384.4 | 1.2 | Sun et al. 2018 |
| brand SFG | D2C593A | 393.4 | 1.2 | Sun et al. 2018 |
| generic SFG | 1602240.1 | 342.0 | 1.5 | data from this work |
| generic SFG | 142290.1 | 363.7 | 1.9 | Sun et al. 2018 |
| generic SFG | 142241.1 | 365.9 | 5.4 | Sun et al. 2018 |
| generic SFG | 132996.1 | 387.4 | 2.1 | Sun et al. 2018 |
Diluted 40 times with mobile phase solution (aqueous solution of sodium azide (0.02 w/v %) and 0.01% brand SFG at pH 7.0). Both SFG samples and pullulan polysaccharide standards (21 kDa–805 kDa) were run on a 300 × 7.8 mm Toso Haas TSK Gel G40000SWXL column.
Discussion.
In this work, a comprehensive approach to measure the physicochemical properties of brand and generic sodium ferric gluconate products was taken. The goal was to determine if there are any differences between the two products that may inform regulatory decision making. In addition, we sought to evaluate the methods with regards to their viability of implementation and analysis. The current FDA draft guidance for sodium ferric gluconate products stipulates that the properties of both the iron core and the carbohydrate shell be determined to evaluate their sameness; however, the methods to be employed to obtain this characterization data are not specified. As there are multiple experimental approaches available, our experience with several different approaches may be informative for others seeking to compare products.
The methods we utilized can be divided into the two areas stipulated in the guidance: iron core and carbohydrate shell. The former provides information about the iron stoichiometry, oxidation state, and geometry. The latter provides information regarding particle size. In addition, we employed an acid degradation assay to measure the iron lability–which refers to the dissociation of ferric iron from the nanoparticles.
The measures of the iron core that were performed range from the routine, e.g., UV–visible spectroscopy, which requires a simple benchtop instrument, to the more specialized, e.g., XAS spectroscopy, which requires a synchrotron light source. Collectively, the measures gave an overall picture of the iron core by assessing various physicochemical properties. The ICP-MS data quantified the total iron in each product. ICP-MS requires a specialized instrument; however, if the instrument is available, the data obtained is highly accurate and straightforward to interpret. A second straightforward experiment performed was UV–visible spectroscopy. Here, spectra of the iron-oxy-hydroxide core were obtained and found to contain broad absorbance envelopes. UV–visible spectroscopy is typically accessible in research laboratories, and the spectra obtained can be viewed as a “fingerprint” of the iron core state. One limitation, however, is that in order to obtain accurate optical spectra for iron nanoparticle products, the sample must be diluted. Data that we obtained using DLS indicated that the particle size decreases as a result of dilution; thus, the UV visible spectra obtained may not be a true representation of the complex at the concentration utilized clinically. XAS spectroscopy provided clear information regarding oxidation state, ligands, and geometries; however, a drawback to this technique is the requirement of a synchrotron and expertise in this highly specialized measurement. Similarly, while Mössbauer provided information regarding the ferric core structure and oxidation state, it also requires specialized equipment and expertise. Of the methods employed, XRPD proved to be the most challenging and provided the least definitive data. The iron nanoparticle products have a sucrose excipient, which exhibits a very strong and broad XRPD signal. It is imperative that as much sucrose as possible be removed from the iron nanoparticle in order to obtain any signal for the iron-oxyhydroxide core. The methods utilized to remove the sucrose can include lyophilization, drying, and pulverization. Even with these methods, the XRPD signal for the iron core is often obscured by the sucrose signal making the data difficult to interpret.22,40 As such, while XRPD has historically been used to analyze iron centers in nanoparticles, it has limitations.
To measure the carbohydrate shell, which is a measure of the entire nanoparticle, we also used several approaches. One approach was DLS, which has been used previously for these types of products.6 DLS is relatively straightforward, and the instrumentation is common in many laboratories. A drawback to conventional DLS, however, is that it requires that the sample be diluted. We had access to corner scattering DLS, which allowed DLS measurement of undiluted nanoparticles, which is one form that is administered to patients. We observed that the undiluted nanoparticles were larger (~2-fold) than the diluted nanoparticles. We suggest that the addition of 0.9% NaCl to dilute the samples for DLS, to mimic how the products are diluted in the clinic, may affect the particle size because it increases ionic strength. Nonetheless, we note that regardless of whether the sample was diluted or not, the brand SFG was always measured to be larger than the generic products. As most laboratories only have access to conventional DLS instrumentation, we suggest that measurements of dilute samples may be useful as there are literature comparators available, but these measures should be taken with the consideration that dilution results in a smaller particle size. AUC was used to determine the sedimentation coefficient of brand and generic SFG. Due to a non-normal distribution of both brand and generic SFG observed in the AUC data, we could only determine the total loading sample average sedimentation coefficient. Our results are consistent with a larger total loading sample average sedimentation coefficient for brand SFG compared to generic. AUC is not a common instrument and is typically utilized for studies of proteins and other biological macromolecules. The data we obtained was limited because we could only determine the total loading sample average sedimentation coefficient, not a true sedimentation coefficient. The final measure of the carbohydrate cell was GPC using HPLC coupled to a refractive index detector, which provided a measure of the average molecular weights of the iron products. Here, we obtained data that were consistent with data that had been reported previously.6 This experimental approach is straightforward, and many laboratories have HPLC capability; however, it does require a refractive index detector, which is less common than a UV–visible or fluorescence detector.
A final measure that we performed was a “forced acid degradation” assay. In this experiment, the iron nanoparticle drug product is introduced into a solvent system that contains concentrated HCl, and the absorbance spectrum is monitored over time. The role of the acid is to hydrolyze the Fe-oxyhydroxide bonds causing the nanoparticle to dissociate and break down into small iron complexes and carbohydrate ligands. This breakdown is monitored by UV–visible spectroscopy over time. We observed that both brand and generic products break down under these conditions, with the generic exhibiting slower release (i.e., longer half-life) than the brand. We note that the generic particle size is smaller than the brand, and often rates of dissolution of simple, idealized drug particles are inversely related to particle size, leading to more rapid dissolution.7,25,51 Here, however, this trend was not followed, perhaps since sodium ferric gluconate complex in sucrose is a complex product, and since the comparison is across formulation (i.e., generic versus brand). A better future understanding of iron liberation via forced acid degradation testing may consider the architecture of iron-oxy-hydroxy nanoparticulate cores, their carbohydrate shell, and combined acid reactivity with diffusional boundary layer effects.
CONCLUSIONS
Nanoparticle-based drugs are more complex than their small molecule drug counterparts, and the goal of this work was to characterize the products and identify similar and different properties. Our focus was on two areas: comparison of the iron core properties and comparison of the entire product properties (iron core + carbohydrate shell). The carbohydrate shell, to which the iron atoms are coordinated via iron–oxygen bonds, serves as an integral component for drug release.27 As such, by comparing both the iron core and full products’ physicochemical properties, we were able to assess whether there are differences between the products and identify the source of the differences.
Overall, no differences in the iron cores were identified: both were determined to be ferric centers in an octahedral coordination environment, and each product contained an equivalent amount of total iron. We did, however, identify some differences in the physicochemical properties of the iron nanoparticles. These differences included differences in (i) iron lability measured via an acid release test (the iron release was more rapid (at least 2×) for the brand versus generic product), (ii) particle size measured via DLS (the particle size of the brand was larger than the generic, and the dilution of samples decreased the particle size), and (iii) molecular weight via GPC (the brand product had a larger molecular weight than the generic). These findings set the groundwork for future research aimed at determining which physicochemical properties inform on clinical activity, including application to a parallel crossover clinical study by our laboratory comparing the differences in labile iron content after iron gluconate administration in healthy volunteers. Moreover, these findings have the potential to contribute to the development of generic parental iron colloidal regulatory standards.
ACKNOWLEDGMENTS
S.L.J.M., M.A.K., and J.E.P. are grateful to the U.S. FDA for the support of this work (UO1FD005266). Additional support from the FDA for J.E.P. (1U01FD005946) is also gratefully acknowledged. Support was also provided by the University of Maryland School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014). J.E.P.B. is grateful for support from CBI (T32 GM066706) and AFPE. D.P.G. is grateful to the NIH (GM 119374) Corner scattering experiments were conducted at the Light Scattering Center, University of Maryland, College Park. We thank Heather M. Neu, Kiwon Ok, and Jordan D. Pritts for assistance with the ICP-MS analysis of SFG products. We also thank Karen Fleming, JHU Biophysics, for advice and assistance with AUC experiments and data workup.
Footnotes
The views expressed in this article are those of the authors and should not be interpreted as the official opinion or policy of the U.S. Food and Drug Administration, Department of Health and Human Services, or any other agency or component of the U.S. government. The mention of trade names, commercial products, or organizations is for clarification of the methods used and should not be interpreted as an endorsement of a product or manufacturer.
The authors declare no competing financial interest.
Contributor Information
Joel E. P. Brandis, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, Maryland 21201, United States
Kyle C. Kihn, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, Maryland 21201, United States
Marc B. Taraban, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, Maryland 21201, United States.
Julia Schnorr, Department of Pharmaceutical Technology and Biopharmaceutics, Johannes Gutenberg University Mainz, 55128 Mainz, Germany.
Alex M. Confer, Department of Chemistry, The Johns Hopkins University, Baltimore, Maryland 21218, United States.
Sharon Batelu, Department of Pharmaceutical Sciences, Wayne State University, Detroit, Michigan 48201, United States.
Dajun Sun, Office of Research and Standards, Office of Generic Drugs, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, Maryland 20993, United States.
Jason D. Rodriguez, Division of Pharmaceutical Analysis, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, St. Louis, MO 20903, United States
Wenlei Jiang, Office of Research and Standards, Office of Generic Drugs, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, Maryland 20993, United States.
David P. Goldberg, Department of Chemistry, The Johns Hopkins University, Baltimore, Maryland 21218, United States.
Peter Langguth, Department of Pharmaceutical Technology and Biopharmaceutics, Johannes Gutenberg University Mainz, 55128 Mainz, Germany.
Timothy L. Stemmler, Department of Pharmaceutical Sciences, Wayne State University, Detroit, Michigan 48201, United States.
Yihua Bruce Yu, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, Maryland 21201, United States.
Maureen A. Kane, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, Maryland 21201, United States.
James E. Polli, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, Maryland 21201, United States.
Sarah L. J. Michel, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, Maryland 21201, United States.
REFERENCES
- (1).Ventola CL Progress in nanomedicine: approved and investigational nanodrugs. P. and T 2017, 42 (12), 742. [PMC free article] [PubMed] [Google Scholar]
- (2).Faria M; Bjornmalm M; Thurecht KJ; Kent SJ; Parton RG; Kavallaris M; Johnston APR; Gooding JJ; Corrie SR; Boyd BJ; Thordarson P; Whittaker AK; Stevens MM; Prestidge CA; Porter CJH; Parak WJ; Davis TP; Crampin EJ; Caruso F Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol 2018, 13 (9), 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kargozar S; Mozafari M Nanotechnology and Nanomedicine: Start small, think big. Mater. Today: Proc 2018, 5 (7), 15492–15500. [Google Scholar]
- (4).FDA Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology; FDA, Office of the Commissioner, 2014. [Google Scholar]
- (5).Bobo D; Robinson KJ; Islam J; Thurecht KJ; Corrie SR Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm. Res 2016, 33 (10), 2373–2387. [DOI] [PubMed] [Google Scholar]
- (6).Sun D; Rouse R; Patel V; Wu Y; Zheng J; Karmakar A; Patri AK; Chitranshi P; Keire D; Ma J; Jiang W Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: Physicochemical Characterization. Nanomaterials 2018, 8 (1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Oner ZG; Michel SL; Polli JE Equivalence and regulatory approaches of nonbiological complex drug products across the United States, the European Union, and Turkey. Ann. N. Y. Acad. Sci 2017, 1407 (1), 26–38. [DOI] [PubMed] [Google Scholar]
- (8).Besarab A; Coyne DW , Iron supplementation to treat anemia in patients with chronic kidney disease. Nat. Rev. Nephrol 2010, 6 (12), 699. [DOI] [PubMed] [Google Scholar]
- (9).Pai AB Iron Oxide Nanoparticle Formulations for Supplementation. Met. Ions Life Sci 2019, 19. [DOI] [PubMed] [Google Scholar]
- (10).Pai AB Complexity of intravenous iron nanoparticle formulations: implications for bioequivalence evaluation. Ann. N. Y. Acad. Sci 2017, 1407 (1), 17–25. [DOI] [PubMed] [Google Scholar]
- (11).Zheng N; Sun DD; Zou P; Jiang W Scientific and regulatory considerations for generic complex drug products containing nanomaterials. AAPS J. 2017, 19, 619. [DOI] [PubMed] [Google Scholar]
- (12).Camaschella C Iron-deficiency anemia. N. Engl. J. Med 2015, 372 (19), 1832–1843. [DOI] [PubMed] [Google Scholar]
- (13).Weiss G; Goodnough LT Anemia of chronic disease. N. Engl. J. Med 2005, 352 (10), 1011–1023. [DOI] [PubMed] [Google Scholar]
- (14).CDC National Chronic Kidney Disease Fact Sheet, 2017; US Department of Health and Human Services, Centers for Disease Control and Prevention: https://www.cdc.gov/kidneydisease/pdf/kidney_factsheet.pdf, 2017. (accessed June 12, 2020). [Google Scholar]
- (15).Macdougall IC Strategies for iron supplementation: oral versus intravenous. Kidney Int. 1999, 55, S61–S66. [DOI] [PubMed] [Google Scholar]
- (16).Zhang D; Meyron-Holtz E; Rouault TA Renal iron metabolism: transferrin iron delivery and the role of iron regulatory proteins. J. Am. Soc. Nephrol 2007, 18 (2), 401–406. [DOI] [PubMed] [Google Scholar]
- (17).Geisser P; Burckhardt S The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011, 3 (1), 12–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Silverstein SB; Rodgers GM Parenteral iron therapy options. Am. J. Hematol 2004, 76 (1), 74–78. [DOI] [PubMed] [Google Scholar]
- (19).Brissot P; Ropert M; Le Lan C; Loréal O Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim. Biophys. Acta, Gen. Subj 2012, 1820 (3), 403–410. [DOI] [PubMed] [Google Scholar]
- (20).Neu HM; Alexishin SA; Brandis JEP; Williams AMC; Li W; Sun D; Zheng N; Jiang W; Zimrin A; Fink JC; Polli JE; Kane MA; Michel SLJ Snapshots of Iron Speciation: Tracking the Fate of Iron Nanoparticle Drugs via a Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometric Approach. Mol. Pharmaceutics 2019, 16, 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Zou P; Tyner K; Raw A; Lee S Physicochemical Characterization of Iron Carbohydrate Colloid Drug Products. AAPS J. 2017, 19 (5), 1359–1376. [DOI] [PubMed] [Google Scholar]
- (22).Kudasheva DS; Lai J; Ulman A; Cowman MK Structure of carbohydrate-bound polynuclear iron oxyhydroxide nanoparticles in parenteral formulations. J. Inorg. Biochem 2004, 98 (11), 1757–69. [DOI] [PubMed] [Google Scholar]
- (23).Jahn MR; Andreasen HB; Futterer S; Nawroth T; Schunemann V; Kolb U; Hofmeister W; Munoz M; Bock K; Meldal M; Langguth P A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm 2011, 78 (3), 480–491. [DOI] [PubMed] [Google Scholar]
- (24).Balakrishnan V; Rao M; Kausz A; Brenner L; Pereira B; Frigo T; Lewis J Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur. J. Clin. Invest 2009, 39 (6), 489–496. [DOI] [PubMed] [Google Scholar]
- (25).Neiser S; Rentsch D; Dippon U; Kappler A; Weidler PG; Gottlicher J; Steininger R; Wilhelm M; Braitsch M; Funk F; Philipp E; Burckhardt S Physico-chemical properties of the new generation IV iron preparations ferumoxytol, iron isomaltoside 1000 and ferric carboxymaltose. BioMetals 2015, 28 (4), 615–635. [DOI] [PubMed] [Google Scholar]
- (26).Baribeault D Sodium ferric gluconate (SFG) in complex with sucrose for IV infusion: bioequivalence of a new generic product with the branded product in healthy volunteers. Curr. Med. Res. Opin 2011, 27 (8), 1653–1657. [DOI] [PubMed] [Google Scholar]
- (27).FDA, Draft Guidance on Sodium Ferric Gluconate Complex. U.S. Department of Health and Human Services (HHS), F. a. D. A. F., Center for Drug Evaluation and Research (CDER). https://www.fda.gov/drugs/guidances-drugs/product-specific-guidances-generic-drug-development, 2013. (accessed Feb 12, 2019). [Google Scholar]
- (28).Borchard G; Flühmann B; Mühlebach S Nanoparticle iron medicinal products–Requirements for approval of intended copies of non-biological complex drugs (NBCD) and the importance of clinical comparative studies. Regul. Toxicol. Pharmacol 2012, 64 (2), 324–328. [DOI] [PubMed] [Google Scholar]
- (29).Emily M; Ioanna N; Scott B; Beat F Reflections on FDA Draft Guidance for Products Containing Nanomaterials: Is the Abbreviated New Drug Application (ANDA) a Suitable Pathway for Nanomedicines? AAPS J. 2018, 20 (5), 92. [DOI] [PubMed] [Google Scholar]
- (30).de Swart L; Hendriks JCM; van der Vorm LN; Cabantchik ZI; Evans PJ; Hod EA; Brittenham GM; Furman Y; Wojczyk B; Janssen MCH; Porter JB; Mattijssen VEJM; Biemond BJ; MacKenzie MA; Origa R; Galanello R; Hider RC; Swinkels DW Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica 2016, 101 (1), 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Gosriwatana I; Loreal O; Lu S; Brissot P; Porter J; Hider RC Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal. Biochem 1999, 273 (2), 212–220. [DOI] [PubMed] [Google Scholar]
- (32).Kolb A; Smit N; Lentz-Ljuboje R; Osanto S; Van Pelt J Non-transferrin bound iron measurement is influenced by chelator concentration. Anal. Biochem 2009, 385 (1), 13–19. [DOI] [PubMed] [Google Scholar]
- (33).Pai AB; Pai MP; Meyer DE; Bales BC; Cotero VE; Zheng N; Jiang W In vitro and in vivo DFO-chelatable labile iron release profiles among commercially available intravenous iron nanoparticle formulations. Regul. Toxicol. Pharmacol 2018, 97, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pai AB; Meyer DE; Bales BC; Cotero VE; Pai MP; Zheng N; Jiang W Performance of Redox Active and Chelatable Iron Assays to Determine Labile Iron Release From Intravenous Iron Formulations. Clin. Transl. Sci 2017, 10 (3), 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Rehr J; Ankudinov A Progress and challenges in the theory and interpretation of X-ray spectra. J. Synchrotron Radiat 2001, 8 (2), 61–65. [DOI] [PubMed] [Google Scholar]
- (36).Cook JD; Bencze KZ; Jankovic AD; Crater AK; Busch CN; Bradley PB; Stemmler AJ; Spaller MR; Stemmler TL Monomeric yeast frataxin is an iron-binding protein. Biochemistry 2006, 45 (25), 7767–7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Schellekens H; Stegemann S; Weinstein V; de Vlieger JS; Flühmann B; Mühlebach S; Gaspar R; Shah VP; Crommelin DJ How to regulate nonbiological complex drugs (NBCD) and their follow-on versions: points to consider. AAPS J. 2014, 16 (1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Yang Y; Shah RB; Faustino PJ; Raw A; Yu LX; Khan MA Thermodynamic stability assessment of a colloidal iron drug product: sodium ferric gluconate. J. Pharm. Sci 2010, 99 (1), 142–153. [DOI] [PubMed] [Google Scholar]
- (39).Geetha K; Raghavan MS; Kulshreshtha SK; Sasikala R; Rao CP Transition-metal saccharide chemistry: synthesis, spectroscopy, electrochemistry and magnetic susceptibility studies of iron (III) complexes of mono-and disaccharides. Carbohydr. Res 1995, 271 (2), 163–175. [Google Scholar]
- (40).Fütterer S; Andrusenko I; Kolb U; Hofmeister W; Langguth P Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD). J. Pharm. Biomed. Anal 2013, 86, 151–160. [DOI] [PubMed] [Google Scholar]
- (41).Dzul SP; Rocha AG; Rawat S; Kandegedara A; Kusowski A; Pain J; Murari A; Pain D; Dancis A; Stemmler TL In vitro characterization of a novel Isu homologue from Drosophila melanogaster for de novo FeS-cluster formation. Metallomics 2017, 9 (1), 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Westre TE; Kennepohl P; DeWitt JG; Hedman B; Hodgson KO; Solomon EI A multiplet analysis of Fe K-edge 1s→3d pre-edge features of iron complexes. J. Am. Chem. Soc 1997, 119 (27), 6297–6314. [Google Scholar]
- (43).Randall CR; Zang Y; True AE; Que L Jr; Charnock JM; Garner CD; Fujishima Y; Schofield CJ; Baldwin JE X-ray absorption studies of the ferrous active site of isopenicillin N synthase and related model complexes. Biochemistry 1993, 32 (26), 6664–6673. [DOI] [PubMed] [Google Scholar]
- (44).Rodrigues AV; Kandegedara A; Rotondo JA; Dancis A; Stemmler TL Iron loading site on the Fe–S cluster assembly scaffold protein is distinct from the active site. BioMetals 2015, 28 (3), 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Groom CR; Bruno IJ; Lightfoot MP; Ward SC The Cambridge structural database. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater 2016, 72 (2), 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ferrlecit. Sanofi Aventis U.S; LLC Bridgewater: NJ, 2011. [Google Scholar]
- (47).Burya YG; Yudin IK; Dechabo VA; Kosov VI; Anisimov MA Light-scattering study of petroleum asphaltene aggregation. Appl. Opt 2001, 40 (24), 4028–4035. [DOI] [PubMed] [Google Scholar]
- (48).Wu Y; Petrochenko P; Chen L; Wong SY; Absar M; Choi S; Zheng J Core size determination and structural characterization of intravenous iron complexes by cryogenic transmission electron microscopy. Int. J. Pharm 2016, 505 (1–2), 167–174. [DOI] [PubMed] [Google Scholar]
- (49).Wermeling J; Halsall H Sedimentation coefficient, frictional coefficient, and molecular weight: A preparative ultracentrifuge experiment for the advanced undergraduate laboratory. J. Chem. Educ 1982, 59 (12), 1076. [Google Scholar]
- (50).Diaz L; Peyrot C; Wilkinson KJ Characterization of polymeric nanomaterials using analytical ultracentrifugation. Environ. Sci. Technol 2015, 49 (12), 7302–7309. [DOI] [PubMed] [Google Scholar]
- (51).Serajuddin AT; Jarowski CI Effect of diffusion layer pH and solubility on the dissolution rate of pharmaceutical bases and their hydrochloride salts. I. Phernazopyridine. J. Pharm. Sci 1985, 74 (2), 142–7. [DOI] [PubMed] [Google Scholar]