Significance
Clarifying the recognition pathways of agonist and G protein to G protein–coupled receptor (GPCR) is essential to understand the signal transduction mechanism of GPCR. However, it is still challenging to simulate the full activation process of GPCR on a reasonable simulation timescale with conventional molecular dynamics (MD) methods. Here, we developed an MD simulation approach named supervised Gaussian accelerated MD (Su-GaMD) and revealed the full activation mechanism of adenosine (Ado) A1 receptor (A1R) (including adenosine Ado−A1R recognition, preactivation of A1R, and A1R−G protein recognition) in hundreds of nanoseconds simulations. The whole activation process and the metastable intermediate states revealed in this study could provide complementary structural characterizations to expand our perspectives on A1R drug discovery.
Keywords: G protein–coupled receptor, molecular dynamics simulations, ligand‒protein recognition pathway, protein‒protein recognition pathway, enhanced sampling method
Abstract
The full activation process of G protein–coupled receptor (GPCR) plays an important role in cellular signal transduction. However, it remains challenging to simulate the whole process in which the GPCR is recognized and activated by a ligand and then couples to the G protein on a reasonable simulation timescale. Here, we developed a molecular dynamics (MD) approach named supervised (Su) Gaussian accelerated MD (GaMD) by incorporating a tabu-like supervision algorithm into a standard GaMD simulation. By using this Su-GaMD method, from the active and inactive structure of adenosine A1 receptor (A1R), we successfully revealed the full activation mechanism of A1R, including adenosine (Ado)–A1R recognition, preactivation of A1R, and A1R–G protein recognition, in hundreds of nanoseconds of simulations. The binding of Ado to the extracellular side of A1R initiates conformational changes and the preactivation of A1R. In turn, the binding of Gi2 to the intracellular side of A1R causes a decrease in the volume of the extracellular orthosteric site and stabilizes the binding of Ado to A1R. Su-GaMD could be a useful tool to reconstruct or even predict ligand–protein and protein–protein recognition pathways on a short timescale. The intermediate states revealed in this study could provide more detailed complementary structural characterizations to facilitate the drug design of A1R in the future.
G protein–coupled receptors (GPCRs) are the largest family of receptors in the cell membrane (1, 2). GPCRs recognize a variety of external molecules and initiate various intracellular signaling cascades as responses that ultimately regulate body growth, development, and metabolism. They are widely distributed in the human body and participate in a variety of physiological roles (3). More than 30% of the drugs on the market target GPCRs (4).
The adenosine A1 receptor (A1R) is one of the four subtypes of the G protein–coupled adenosine receptor family that mediate the biological effects of endogenous adenosine (Ado) (5). Activation of the A1R is therapeutically desirable for ischemia-perfusion injury, atrial fibrillation, and neuropathic pain (6). Using regular A1R orthosteric agonists has failed in the development of analgesics because of a lack of sufficient on-target selectivity as well as off-tissue adverse effects (7). However, an allosteric modulator of A1R reported by Draper-Joyce et al. (8) exhibits analgesic efficacy. Moreover, an A1R-selective agonist has been discovered by Wall et al. (9) to elicit analgesia without respiratory depression through selectively activating Gob among the six Gi/o subtypes. A1R exists in a dynamic equilibrium between inactive and active states that can be selectively shifted by the binding of a ligand and through interaction with intracellular proteins such as Gi/o (10). The biased agonists with selectivity for the particular A1R conformational states are proposed as a better option for drug development by promoting Gi/o signaling without affecting other pathways mediated by A1R (11–14). Recently, the structural basis of A1R with agonists/antagonists, allosteric modulators, and G proteins has attracted great interest, and great breakthroughs have been made. With the use of X-ray crystallography and cryo-electron microscopy (cryo-EM) technology, a considerable number of A1Rs bound with agonists/antagonists, allosteric modulators, and G proteins have been resolved (8, 15–17). In 2017, Glukhova et al. (15) resolved the X-ray structure of A1R bound to the selective covalent antagonist DU172 (Protein Data Bank [PDB] code 5UEN). In the same year, Cheng et al. (16) reported the structure of A1R with a selective noncovalent antagonist PSB36 (PDB code 5N2S). These two structures, in which A1R is in its inactive state, provide a molecular basis for A1R subtype selectivity for antagonists. In 2018, Draper-Joyce et al. (17) revealed the cryo-EM structure of the A1R–Gi2 complex bound to its endogenous agonist Ado (Ado–A1R–Gi2 complex, PDB code 6D9H, 6D9H structure for short). Most recently, they resolved the cryo-EM structure of the A1R–Gi2 complex bound to its endogenous agonist Ado and a positive allosteric modulator MIPS521 (MIPS521–Ado–A1R–Gi2 complex, PDB code 7LD3) (8). In these two structures, A1R was fully activated with both Ado binding in the extracellular orthosteric pocket and Gi2 protein binding in the intracellular region. The detailed characterizations of these structures provide a solid structural foundation for the activation of A1R. However, the dynamic processes of ligand recognition, Gi2 protein recognition, and full activation of A1R have not been clarified. The experimentally observed ligand-bound states of A1R are chemically stable and can be utilized for the design of A1R-targeting drugs. In fact, the atomic-level description of the different metastable intermediate states characterized in the recognition process suggests complementary opportunities for the design of new A1R drugs. Hopefully, the future of drug design will involve atomic details of not only the experimentally observed ligand-bound state but also the whole ligand–protein network of recognition pathways, including all metastable intermediate states (18). A complete understanding of the full activation process of A1R (including the Ado recognition pathway, the G protein recognition pathway, and the full activation of A1R) will help to expand our perspectives on A1R drug discovery and development.
The dynamic process and recognition pathway of agonist–GPCR and GPCR–G protein are important to improve understanding of the signal transduction mechanism involved in the full activation process of GPCRs, while the full activation process occurs on a timescale of several milliseconds (19). The associated long timescale is difficult to access via conventional molecular dynamics (MD) simulations. Over the past decades, MD simulations have been applied to study the recognition and dissociation between ligands and GPCRs, including long-timescale conventional MD (cMD) (20, 21) and a variety of enhanced sampling MDs (21–28) including random acceleration MD (RAMD), steered MD (sMD), metadynamics (MTD), and accelerated MD (aMD). A detailed understanding of ligand-introduced GPCR activation has been developed in recent years, and GPCRs undergo significant conformational changes in extracellular and intracellular regions (2, 29, 30). Recently, Moro’s group (31) provided the supervised MD (SuMD) approach, which combined a tabu-like supervision algorithm on the ligand–receptor approaching distance with cMD simulations, to study the binding event and pathway between an antagonist and A2AR at dozens of nanoseconds. Moreover, with the emergence of active X-ray or cryo-EM structures of GPCRs, many cMD and enhanced sampling MD simulations have been shown to be successful in studying the activation mechanism of GPCRs (25, 32–36). However, MD studies focusing on the interaction between GPCRs and intracellular proteins are scarce, even though many GPCRs combined with intracellular proteins have been resolved experimentally. Notably, McCammon’s group (37) successfully simulated the binding of a G protein mimetic nanobody (Nb9-8) to the M2 muscarinic acetylcholine receptor (M2R) by using a Gaussian aMD (GaMD) method in a very long timescale simulation (4,500 ns). Due to the limitations of computational capacity, it is still very difficult to predict GPCR–G protein recognition pathways even with existing enhanced sampling MD methods.
Here, we provide a enhanced sampling technique (Su-GaMD) by incorporating a tabu-like supervision algorithm into a GaMD simulation. Su-GaMD can provide a more favorable way to discover the process by which GPCR interacts with ligand and intracellular protein at the nanosecond timescale. By using the Su-GaMD and GaMD methods, we simulated the Ado–A1R binding event and then the recognition process of Gi2 protein to A1R based on both the active and inactive A1R structures. The full activation mechanism of A1R (including the Ado–A1R recognition, the preactivation of A1R, and the A1R–G protein recognition) and the possible recognition pathways of Ado to A1R and Gi2 to A1R were revealed. The conformational changes occurring in both the intracellular and extracellular binding pockets of A1R were observed and the coupling between them was discussed. This study provides comprehensive insights into A1R characterization during its whole activation process and opens up avenues for the rational design of A1R drugs.
Results and Discussion
Design of the Su-GaMD Simulations.
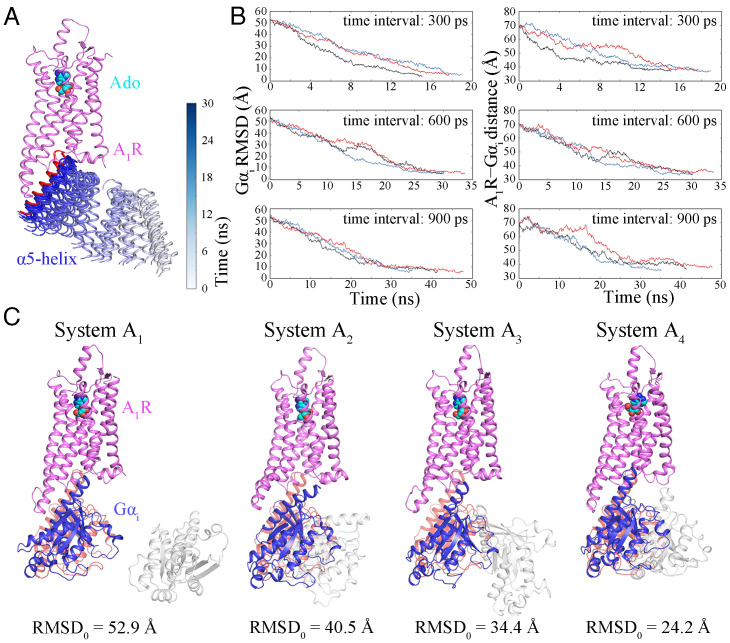
We developed the Su-GaMD method derived from SuMD and GaMD by exploiting a tabu-like supervision algorithm in a standard GaMD simulation. We used a simplified Ado–A1R–Gαi model (with the α subunit of the Gi2 protein [Gαi] to present the heterotrimeric Gi2 protein) to test the reliability of this Su-GaMD method. We placed Gαi >20 Å away from A1R (system A, SI Appendix, Fig. S1A) and performed Su-GaMD simulations to reconstruct the A1R–Gαi complex.
To select an appropriate time interval, we performed three independent Su-GaMD simulations for system A1 (Fig. 1C) with time intervals of 300, 600, and 900 ps. Each simulation was replicated three times. In all the simulations, Gαi was successfully observed to enter the intracellular binding site of A1R in less than 50 ns of the Su-GaMD simulation (Fig. 1A and Table 1 and SI Appendix, Table S1). During A1R–Gαi recognition, the Gαi rmsd (defined as the rmsd calculated on the heavy atoms in the main chain of Gαi relative to the 6D9H structure) fell to ∼4.7 Å and the A1R-Gαi distance (defined as the distance between the centers of mass [COMs] of the heavy atoms of the Gαi α5-helix [residues Lys331 to Phe355] and A1R) dropped to ∼35.9 Å (which was close to that of 32.8 Å in the 6D9H structure) (Fig. 1B and SI Appendix, Table S1). These results indicated that the A1R–Gαi complex close to the 6D9H structure was reconstructed through these Su-GaMD simulations. Considering a compromise of the sampling number and the simulation time, we chose the 600-ps interval (same as the previous SuMD works of Moro’s group (38, 39)) for the following Su-GaMD simulations.
Fig. 1.
(A) Recognition of Gαi (only the α5-helix is shown) to the intracellular binding site of A1R. Trajectories of the α5-helix of Gαi (ribbons) are colored by simulation time on a silver (0 ns) to blue (30 ns) scale. The α5-helix of Gαi in the 6D9H structure is shown as a red ribbon. (B) Time-dependent Gαi rmsds and A1R–Gαi distances using time intervals of 300, 600, and 900 ps. (C) Binding of Gαi to A1R was observed in the trajectories of replicates with different initial positions and orientations of Gαi. A1R is colored violet, Gαi in the 6D9H structure is colored pink, the initial position of Gαi is shown in silver, and Gαi in the final snapshot is colored blue.
Table 1.
Overview of the simulations in the present study
| System | Description | Method | Replicates | Time interval of Su-GaMD | Time* (ns) |
|---|---|---|---|---|---|
| A1 | A1R–Gαi binding event (rmsd0 = 52.9 Å) | Su-GaMD | 3 | 300 ps | 17.2 |
| A1R–Gαi binding event | Su-GaMD | 3 | 600 ps | 31.2 | |
| A1R–Gαi binding event | Su-GaMD | 3 | 900 ps | 41.4 | |
| A2 | A1R–Gαi binding event (rmsd0 = 40.5 Å) | Su-GaMD | 3 | 600 ps | 25.0 |
| A3 | A1R–Gαi binding event (rmsd0 = 34.4 Å) | Su-GaMD | 3 | 600 ps | 18.2 |
| A4 | A1R–Gαi binding event (rmsd0 = 24.2 Å) | Su-GaMD | 3 | 600 ps | 30.0 |
| B | Ado–A1R binding event (from active A1R) | Su-GaMD | 3 | 600 ps | 34.4 |
| A1R–Gi2 binding event | Su-GaMD | 3 | 600 ps | 40.2 | |
| C1 | Ado–A1R binding event (from inactive A1R) | Su-GaMD | 3 | 600 ps | 107.6 |
| Preactivation of A1R | GaMD | 3 | – | 150.0 | |
| C2 | A1R–Gi2 binding event | Su-GaMD | 3 | 600 ps | 61.6 |
*For each system, the Su-GaMD simulation time means the average value of three replicates.
To test the influence of the initial position and orientation of Gαi, we also performed Su-GaMD simulations for systems A2, A3, and A4 (Fig. 1C and Table 1 and SI Appendix, Table S2). We found that Gαi could enter its binding site in A1R and achieve an A1R–Gαi complex similar to the 6D9H structure in a reasonable Su-GaMD simulation time no matter where we placed it or what its orientation was in the beginning.
For comparison, a 1,000-ns unsupervised GaMD simulation was performed for system A1. We found that the stable A1R–Gαi complex could not be reached in this extremely long-time GaMD simulation (the minimum Gαi rmsd was 25.8 Å, see SI Appendix, Fig. S4B). In addition, we performed three parallel Su-MD simulations (without Gaussian acceleration) for system A1 and compared the results with those of Su-GaMD simulations. The Gαi rmsds and Gαi–A1R distances in the three replicates of Su-MD simulation are depicted in SI Appendix, Fig. S5. The mimimum Gαi rmsds and the minimum A1R–Gαi distances of the Su-MD simulations are depicted in SI Appendix, Table S3. We found that the Su-MD simulations could reconstruct the A1R–Gαi complex as well, but the simulation times were 45.0, 54.6, and 75.6 ns (SI Appendix, Fig. S5 and Table S3), which were longer than those of the Su-GaMD simulations (30.0, 30.0, and 33.6 ns; Fig. 1B and SI Appendix, Table S1). The mimimum Gαi rmsds of the Su-MD simulations were comparable to those of the Ga-SuMD simulations (4.6, 5.0, and 4.9 Å for Su-MD vs 4.9, 4.9, and 4.9 Å for Su-GaMD; SI Appendix, Tables S1 and S3), but the minimum A1R–Gαi distances of the Su-MD simulations were longer than those of the Ga-SuMD simulations (37.0, 37.2, and 38.8 Å for Su-MD vs 33.6, 33.6, and 35.2 Å for Su-GaMD; SI Appendix, Tables S1 and S3). In summary, we could reconstruct the A1R–Gαi complex in a binding mode similar to that of the 6D9H structure and observed the A1R–Gαi recognition process in less than 50 ns by using the Su-GaMD strategy, while this A1R−Gαi complex could not be reached even in long-time (e.g., 1,000 ns) unsupervised GaMD simulation. Further details are provided in SI Appendix. There was no overall conformational change of the receptor during the simulation of the A1R–Gαi recognition process.
After the verification of this Su-GaMD method, we employed it to investigate the full activation mechanism of A1R. The whole heterotrimeric Gi2 protein, including Gαi and Gβγ, was employed for the rest of the simulations.
Reconstruction of the Ado–A1R–Gi2 Complex from the Active A1R Structure.
Ado–A1R recognition pathway.
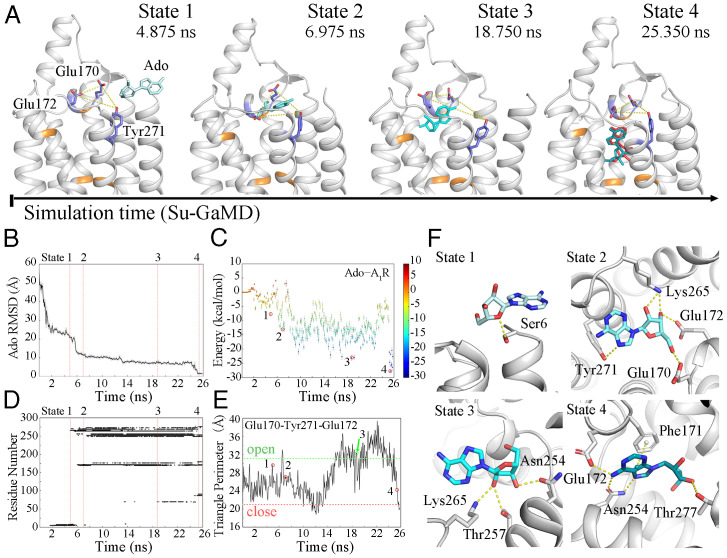
To investigate the Ado–A1R binding event, we performed Su-GaMD simulations for A1R with Ado >20 Å away from its orthosteric site (system B in Table 1, SI Appendix, Fig. S1B). Starting from free diffusion in the solvent, Ado gradually entered the extracellular binding site of A1R composed of Thr913.36, Phe171ECL2, Glu172ECL2, Leu2506.51, Asn2546.55, Thr2777.42, and His2787.43 in a 25.8-ns Su-GaMD simulation (Fig. 2, Movie S1). The Ado rmsd (defined as the rmsd calculated on all the heavy atoms of Ado relative to the 6D9H structure) fell from 57.2 Å to 1.4 Å (Fig. 2B) and the Ado–A1R distance (defined as the distance between the COMs of the heavy atoms of Ado and the residues Thr913.36, Phe171ECL2, Glu172ECL2, Leu2506.51, Asn2546.55, Thr2777.42, and His2787.43 that formed the Ado-binding pocket of A1R) decreased from 58.2 Å to 0.7 Å (which was comparable to that of 1.1 Å in the 6D9H structure) during the Su-GaMD simulation (black line in SI Appendix, Fig. S6B). This indicated that the final binding pose of Ado in A1R was close to that in the 6D9H structure at the end of the Su-GaMD simulation. During the simulation of the Ado–A1R binding event, Gi2 moved freely in the solvent and was >14.9 Å away from A1R. Thus, the possible pathway of Ado–A1R recognition was observed.
Fig. 2.
(A) The Ado–A1R recognition process. A1R is shown in silver; residues Thr913.36, Phe171ECL2, Leu2506.51, Asn2546.55, Thr2777.42, and His2787.43 are shown in orange; and Glu170ECL2, Glu172ECL2, and Tyr2717.36 are shown as blue sticks. Ado is shown as a cyan stick, and the pose of Ado in the 6D9H structure is colored red in state 4. Time-dependent (B) Ado rmsd, (C) binding free energy landscape for Ado–A1R, (D) Ado–A1R contact residues, and (E) the triangle perimeters of the Glu170ECL2–Tyr2717.36–Glu172ECL2 vestibular lid during the recognition process (the triangle perimeters of the open and closed states are depicted in green and red dashed lines). (F) The four metastable intermediate states in the Ado–A1R recognition pathway.
In the Ado–A1R binding free energy landscape we calculated (from the active A1R structure), the Ado–A1R recognition process was found to involve several metastable intermediate states (Fig. 2C). Four metastable intermediate states (states 1, 2, 3, and 4) were identified (Fig. 2 A and F), in which the binding free energies between Ado and A1R were −7.7, −13.1, −23.1, and −27.8 kcal·mol−1, respectively (Fig. 2C, points 1, 2, 3, and 4). A detailed analysis of contact residues during the Ado–A1R recognition process was also performed on the Su-GaMD trajectory, and all the residues of A1R within 4 Å of Ado in the Su-GaMD simulation were shown in the contact map (Fig. 2D).
States 1, 2, 3, and 4 depict the Ado–A1R recognition pathway along the Su-GaMD simulation time (Fig. 2 A and F). First (in state 1, at 4.875 ns), Ado interacted with A1R through residues in TM1 (Ser61.29-Gln91.32), ECL3 (Ser267ECL3), and TM7 (Tyr2717.36) (state 1 in Fig. 2D). A hydrogen bond was observed between the 5′-hydroxyl oxygen in the ribose moiety of Ado and the hydroxyl hydrogen of Ser61.29 (state 1 in Fig. 2F). Then (in state 2, at 6.975 ns), Ado entered the extracellular vestibule consisting of residues in ECL2 (Glu170ECL2-Lys173ECL2), ECL3 (Lys265ECL3), and TM7 (Pro2667.31-Tyr2717.36) (state 2 in Fig. 2D). The ribose moiety of Ado was accommodated by the side chains of Glu170ECL2, Glu172ECL2, and Lys265ECL3 through the hydrogen bonds between them, and the nitrogen in the 6-amino group of the purine ring in Ado formed a hydrogen bond with the hydrogen in the phenylhydroxyl group of Tyr2717.36 (state 2 in Fig. 2F). After that (in state 3, at 18.750 ns), Ado entered the site that approximate to the 6D9H binding conformation and formed stable contacts with ECL2 (residues Phe171ECL2 and Glu172ECL2) and TM5-TM7 (Met1775.35 and Ser2466.47-Thr2707.35) (state 3 in Fig. 2D). The ribose moiety of Ado formed hydrogen bonds with residues Asn2546.55, Thr2576.58, and Lys265ECL3 (state 3 in Fig. 2F). Finally (in state 4, at 25.350 ns), Ado reached the orthosteric binding site of A1R, making contact with TM3, ECL2, and TM5-TM7 (residues Val873.32, Leu883.33, Thr913.36, Phe171ECL2, Glu172ECL2, Met1775.35, Met1805.38, Leu2506.51, His2516.52, Asn2546.55, Ile2747.39, and Thr2777.42) (state 4 in Fig. 2D). Asn2546.55 located the purine ring of Ado through two hydrogen bonds, and notable interactions between Ado and the orthosteric site residues included π-π stacking with Phe171ECL2 and hydrogen bonds with Glu172ECL2 and Thr2777.42 (state 4 in Fig. 2F). Accordingly, the Su-GaMD simulation revealed the Ado–A1R binding event. We observed the possible recognition pathway and summarized all the amino acids involved in the binding event. ECL2 and ECL3 were important during recognition. The binding pathway of Ado and its metabolite inosine to A2AR has been explored successfully with the SuMD method by Moro’s group (40, 41). Most recently, the A1R recognition and dissociation of five endogenous, selective and nonselective agonists, namely, the binding and unbinding pathways to A1R, were simulated by Deganutti et al. (36) using SuMD. In our present study, most of the key residues involved in states 1, 2, 3, and 4 (i.e., Glu170ECL2, Phe171ECL2, Glu172ECL2, Asn2546.55, Thr2576.58, Lys265ECL3, Tyr2717.36, and Thr2777.42) were identified to compose the orthosteric or allosteric site in previous mutational and computational studies of ligand interactions in A1R (15, 36, 42–44).
Three independent Su-GaMD simulations were performed and produced similar results. The Ado rmsd and Ado–A1R distance in the three replicates of simulations are depicted in SI Appendix, Fig. S6 A and B. These simulations were performed on the model with truncation at residue Ser61.29. In addition, we performed another simulation on a model with the five N-terminal residues added (SI Appendix, Fig. S7), and this simulation showed a binding process similar to that discussed above.
“Open” and “closed” states of the orthosteric pocket.
Most noteworthy, we observed that the orthosteric pocket was open and closed in the antagonist-bound A1R (inactive state, PDB code 5N2S) and in the Ado–Gi2–bound A1R (active state, PDB code 6D9H), similar to M2R (37), in that the triangle perimeters of the Glu170ECL2–Tyr2717.36–Glu172ECL2 “vestibular lid” (defined as the sum of the length of all three sides of the triangle composed of the side chain Cδ atoms of Glu170ECL2 and Glu172ECL2 and the side chain oxygen atom of Tyr2717.36, shown in yellow dashed lines in Fig. 2A) were 31.1 Å (open) and 20.9 Å (closed), respectively. The triangle perimeter of the Glu170ECL2–Tyr2717.36–Glu172ECL2 vestibular lid was monitored along the Ado–A1R recognition process (Fig. 2E). When the Ado was removed from the orthosteric pocket and free in the solvent, the vestibular lid was closed. Following that, the vestibular lid gradually opened for Ado to enter the orthosteric binding site of A1R. At the end of the Su-GaMD simulation, Ado reached a position similar to that in the active Ado–A1R–Gi2 6D9H structure, and the vestibular lid was closed again. Thus, we observed the closed–open–closed conformational switch of the vestibular lid during the Ado–A1R recognition process. In addition, the Glu172ECL2–Lys265ECL3 salt bridge was regarded as a hindrance of the orthosteric site (36, 44, 45). We also monitored the Glu172ECL2–Lys265ECL3 salt bridge (calculated based on the minimum distance between the side chain nitrogen atom of Lys265ECL3 and the two carbonyl oxygens of Glu172ECL2) in our simulations (SI Appendix, Fig. S8). The Glu172ECL2–Lys265ECL3 salt bridge showed a similar closed–open–closed conformational switch to the vestibular lid during the Ado–A1R recognition process.
Recognition pathway of Gi2 to A1R.
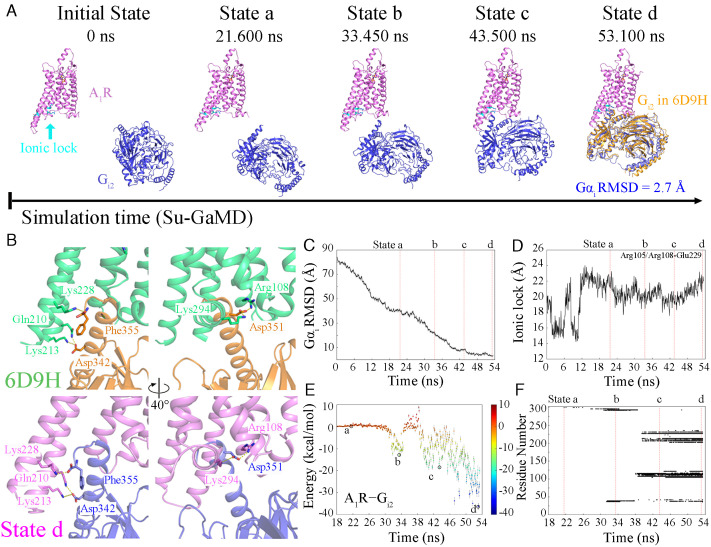
Immediately after the formation of the Ado–A1R complex, we investigated the recognition pathway of Gi2 to the active A1R. Starting from free diffusion in the solvent, in which the Gαi rmsd was 83.7 Å and the A1R–Gαi distance was 78.8 Å, Gi2 gradually entered the intracellular binding site of A1R in the 53.4-ns Su-GaMD simulation (Fig. 3A, Movie S2). During the Su-GaMD simulation of the A1R–Gi2 recognition process, the Gαi rmsd decreased to 2.7 Å (Fig. 3C) and the A1R–Gαi distance decreased to 32.7 Å (see the black line in Fig. S6D), suggesting that the Gi2 protein aligned well with that in the 6D9H structure at the end of the Su-GaMD simulation. The A1R maintained in the activated state, in which the “ionic lock” was broken (with an N–O distance of >8 Å; Fig. 3D).
Fig. 3.
(A) The landscape of the A1R−Gi2 recognition pathway. The relative position of Gi2 after global alignment of A1R (A1R is shown in violet, and Gi2 is shown in blue) to that of the 6D9H structure (Gi2 is shown in orange) is shown in state d. (B) The same key molecular interactions in the 6D9H structure (A1R and Gi2 are shown in green and orange, respectively) and state d (A1R and Gi2 are shown in violet and blue, respectively). Time-dependent (C) Gαi rmsd, (D) N–O distance between the guanidinium of Arg1053.50/Arg1083.53 and the carboxyl of Glu2296.30, (E) the binding free energy landscape for A1R−Gi2, and (F) A1R−Gi2 contact residues during the recognition process.
During the A1R–Gi2 recognition process, four metastable intermediate states (states a, b, c, and d) were identified (Fig. 3 A and E). In state a (at 21.600 ns), Gi2 made initial contacts with the H8 region of A1R (Lys3018.56 in H8) through its α5-helix (state a in Fig. 3 A and F). In state b (at 33.450 ns), Gi2 made further contacts with ICL1 in addition to H8 of A1R (i.e., Asn371.60, Ala39ICL1, Gln2938.48, and Lys2948.49, state b in Fig. 3 A and F), and the A1R–Gi2 binding free energy was −12.9 kcal/mol (state b in Fig. 3E). Then, Gi2 entered the cavity composed of TM3, TM4, ICL2, TM5, and TM6 by contacting Arg1083.53, Thr1124.38, Tyr115ICL2, Lys116ICL2, Gln2105.68, Lys2145.72, and Lys2286.29 of A1R at 43.500 ns (state c in Fig. 3 A and F). The A1R–Gi2 binding free energy decreased to −33.8 kcal/mol in state c (state c in Fig. 3E). Finally (state d, at 53.100 ns), Gi2 moved into the much deeper intracellular binding pocket of A1R and interacted with A1R through residues Gln38ICL1, Arg1053.50, Arg1083.53, Val1093.54, Thr1124.38, Leu113ICL2, Arg114ICL2, Tyr115ICL2, Lys116ICL2, Tyr2055.63, Arg2085.66, Gln2105.68, Lys2135.71, Lys2145.72, Lys2286.29, Glu2296.30, Lys2316.32, Leu2366.37, and Lys294H8 (state d in Fig. 3 A and F). The binding free energy decreased to −36.5 kcal/mol in state d (state d in Fig. 3E). Gi2 eventually entered the intracellular pocket and formed stable interactions with A1R at the end of the Su-GaMD simulation. The interaction interface was composed of TM3, ICL2, TM5-TM7, and H8 of A1R and the α5-helix, αN-helix, and αN-β1 loop of Gi2. All the key molecular interactions between A1R and Gi2 in the 6D9H structure and state d are included in SI Appendix, Table S5. Five of the seven interactions in the 6D9H structure were observed to maintained in state d. Specifically, Gln2105.68 and Lys2286.29 of A1R formed hydrogen bonds with Asp342 and Phe355 of the α5-helix of Gi2, and Arg1083.53, Lys294H8, and Lys2135.71 of A1R formed salt bridges with Asp351 and Asp342 of the α5-helix of Gi2 (SI Appendix, Table S5 and Fig. 3B). Thus, the structure of state d predicted by the Su-GaMD simulation revealed a similar mode of interaction compared with the A1R–Gi2 complex in the 6D9H structure. We can see from the contact residues in states a to d that the ICLs (especially ICL2) of A1R formed favorable contacts with Gi2, and they played an important role in the recognition and binding of the Gi2 protein. This important role of ICLs in A1R–Gi2 recognition is consistent with the previous long-timescale simulation of nanobody Nb9-8 to M2R (37).
To intuitively exhibit the evolution of interactions between A1R and Gi2 during the binding process, we performed protein residue network analyses. The networks between A1R and Gi2 for states a to d are shown in SI Appendix, Fig. S9. It was seen that the network strength between A1R and Gi2 increased gradually during the recognition process.
Three independent MD simulations showed similar results. The Gαi rmsd and A1R–Gαi distance during the A1R–Gi2 recognition of the three replicates are depicted in SI Appendix, Fig. S6 C and D. Similar to previous studies (8, 46), the helical domain of Gi2 that was not included in the cryo-EM structures was omitted in these simulations. This was based on the fact that the helical domain did not form direct contact with the atoms of A1R in the A1R–Gi2 ternary complex. In addition, we performed another simulation on a model with the helical domain rebuilt, and this simulation showed a similar A1R–Gi2 recognition process to that discussed above (SI Appendix, Fig. S10).
In summary, we reconstructed the Ado–A1R–Gi2 complex from A1R (in its active state) and free Ado and Gi2 using the Su-GaMD approach. The reconstruction process involved two stages, as follows: the Ado–A1R binding event (25.8 ns of supervision on Ado rmsd) and the A1R–Gi2 binding event (53.4 ns of supervision on Gαi rmsd). The Glu170ECL2–Tyr2717.36–Glu172ECL2 vestibular lid and the Glu172ECL2–Lys265ECL3 salt bridge showed a closed–open–closed conformational switch during the Ado–A1R binding event, and the ICLs played important roles in the A1R–Gi2 binding event.
Full Activation Mechanism of A1R: Reconstruction of the Ado–A1R–Gi2 Complex from the Inactive A1R Structure.
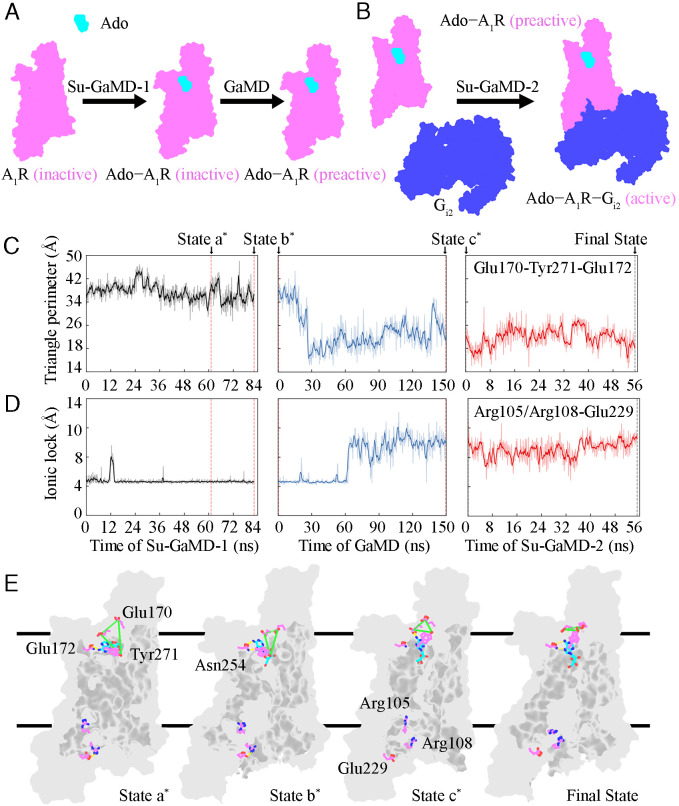
The full activation process of A1R from its inactive state was captured after its recognition with both Ado and Gi2. The whole reconstruction process of the Ado–A1R–Gi2 complex from the inactive A1R (i.e., the full activation mechanism of A1R) included three events, as follows: the Ado–A1R binding event, the A1R preactivation event just before Gi2 binds to A1R, and the A1R–Gi2 binding event. Consequently, three stages of simulations were performed to investigate the whole activation process (Fig. 4 A and B). The first stage was an 82.2-ns Su-GaMD simulation to investigate the Ado–A1R binding event (Su-GaMD-1, with the Ado rmsd supervised). The second stage was a 150-ns GaMD simulation to investigate the A1R conformational changes from the inactive state to the preactive state. The third stage was a 55.2-ns Su-GaMD simulation to investigate the A1R–Gi2 binding event from the preactive Ado–A1R complex (Su-GaMD-2, with Gαi rmsd supervised). A ternary Ado–A1R–Gi2 complex was achieved at the end of these three stages of simulations (SI Appendix, Fig. S11). The animations of the Ado–A1R binding event, the A1R preactivation event, and the A1R–Gi2 binding event are shown in Movies S3–S5.
Fig. 4.
Schematic diagram of the reconstruction process of the Ado−A1R−Gi2 complex from inactive A1R, including (A) the Ado−A1R binding event and the A1R preactivation event and (B) the A1R−Gi2 binding event. Ado (cyan outline), A1R (violet outline), and Gi2 (blue outline) are shown in surface model. Time dependent (C) triangle perimeter of the Glu170ECL2−Tyr2717.36−Glu172ECL2 vestibular lid and (D) N–O distance between the guanidinium of Arg1053.50/Arg1083.53 and the carboxyl of Glu2296.30 during the whole activation process. (E) Representative structures of A1R during the whole activation process. A1R is displayed as a gray surface model. Ado (cyan) and key residues in A1R (violet) are displayed in ball-and-stick. The Glu170ECL2−Tyr2717.36−Glu172ECL2 vestibular lid is depicted by green dashed lines, and hydrogen bonds are depicted by yellow dashed lines.
In the events involved in the A1R full activation process, we monitored the conformational changes of the vestibular lid of the orthosteric pocket and the ionic lock between Arg1053.50/Arg1083.53 and Glu2296.30 (Fig. 4 C and D). Four states of A1R were captured during the simulations, in which the characters involved in the full A1R activation process were clearly stated in the time sequence (states a*, b*, c* and final state in Fig. 4E).
Ado–A1R recognition.
Ado entered the orthosteric site of A1R, which made A1R reach state a* at 61.200 ns in Su-GaMD-1 (state a* in Fig. 4E). In state a*, the hydrogen in the 6-amino group of the purine ring of Ado formed a hydrogen bond with the oxygen atom of the amide group of Asn2546.55, which helped Ado locate the orthosteric site of A1R (state a* in Fig. 4E). The extracellular vestibular lid was fully open in state a* (with the Glu170ECL2–Tyr2717.36–Glu172ECL2 triangle perimeter of 40.2 Å, state a* in Fig. 4C). Afterward, at 82.200 ns in Su-GaMD-1, Ado adjusted its orientation in the orthosteric pocket and possessed a binding mode consistent with the 6D9H structure (state b* in Fig. 4E). After Ado entered the orthosteric pocket of A1R, the vestibular lid was still open (with a Glu170ECL2–Tyr2717.36–Glu172ECL2 triangle perimeter of 33.2 Å, state b* in Fig. 4C). The ionic lock between Arg1053.50/Arg1083.53 and Glu2296.30 was still closed (with an N–O distance of 4.0 Å, state b* in Fig. 4D) in state b*, which means that A1R remained in the inactive state.
Preactivation of A1R.
We performed three parallel 150-ns GaMD simulations and simulated the A1R from the inactive state to the preactive state. At the end of the 150-ns GaMD simulation, the Ado rmsd and the A1R rmsd (defined as the rmsd calculated on the heavy atoms of A1R (without including TM6) relative to the 6D9H structure) were 1.5 Å and 2.0 Å, the ionic lock between Arg1053.50/Arg1083.53 and Glu2296.30 was broken (with N–O distance of >8 Å, state c* in Fig. 4D), and the A1R achieved the preactive state (state c* in Fig. 4E), in which its ionic lock was broken but had not reached the full activation state of the Gi2-bound state. The vestibular lid changed to be closed (with the Glu170ECL2–Tyr2717.36–Glu172ECL2 triangle perimeter of 22.9 Å, state c* in Fig. 4C) in the preactive state. For comparison, we also performed three parallel 300-ns GaMD simulations for apo-A1R. The results in SI Appendix, Fig. S12 show that the ionic lock between Arg1053.50/Arg1083.53 and Glu2296.30 did not break during the three parallel simulations for the apo-A1R system. In contrast, the A1R achieved the preactive state (characterized by the breaking of the ionic lock) after the 150-ns GaMD simulations of the Ado-A1R system. These results indicated that the preactivation of A1R was the consequence of the Ado binding event. This preactivation of A1R is in agreement with the preactivated complex in the combined activation mechanism of a class B GPCR glucagon receptor revealed by Mattedi et al. (47) with MTD simulations.
Recognition between preactivated A1R and Gi2.
The landscape of the A1R–Gi2 recognition pathway from the preactive state of A1R is shown in SI Appendix, Fig. S11A. At 55.200 ns in Su-GaMD-2, the Ado–A1R–Gi2 complex was achieved (final state in Fig. 4E and SI Appendix, Fig. S11A), and this structure aligned well with the 6D9H structure (with an Ado rmsd of 2.0 Å, an A1R rmsd of 1.7 Å, and a Gαi rmsd of 2.9 Å; SI Appendix, Fig. S11 B–F). In the final state, the ionic lock broke (final state in Fig. 4E), the intracellular half of TM6 moved outward, and the bend angle (between the Cα atoms of Tyr2256.26, Leu2456.46, and Thr2576.58) of TM6 increased to 151.1°, which was comparable to the bend angle of 153.5° in the 6D9H structure. These results indicated that A1R was fully activated in the final state. When A1R was fully activated, the vestibular lid was closed (with the Glu170ECL2–Tyr2717.36–Glu172ECL2 triangle perimeter of 22.0 Å that was comparable to that of 20.9 Å in the 6D9H structure, see final state in Fig. 4C). Three independent MD simulations for each stage showed similar results. The Ado rmsds and Ado–A1R distances of the three replicates of Su-GaMD-1 trajectories as well as the Gαi rmsds and A1R–Gαi distances of the three replicates of Su-GaMD-2 trajectories are depicted in SI Appendix, Figs. S11 C and E, respectively. The A1R rmsd of the three replicates of the three stages of simulations are depicted in SI Appendix, Fig. S11F.
For more comparisons between Su-MD and Su-GaMD, we performed Su-MD simulations for the A1R–Gi2 recognition process from the preactive A1R and Gi2 (Su-MD-2, with Gαi rmsd supervised). The Gαi rmsds and Gαi–A1R distances in the three replicates of Su-MD-2 are depicted in SI Appendix, Fig. S13. The minimum Gαi rmsds and the minimum A1R–Gαi distances of Su-GaMD-2 and Su-MD-2 are depicted in SI Appendix, Table S4. It was seen that the Gαi rmsds of Su-GaMD-2 could reach the target value (<5 Å) in less than 75.0 ns, while the Gαi rmsds of Su-MD-2 could not reach the target value (<5 Å) in more than 100.2 ns. These findings indicated that Su-MD needs more computational cost than Su-GaMD in the simulation of the protein–protein recognition process.
Gi2-induced conformational changes feed back to the orthosteric pocket in A1R.
More interestingly, we observed coupling between the Ado–A1R and A1R–Gi2 binding events by calculating the Ado–A1R binding free energies and the volumes of the Ado-binding pocket in A1R for the four states during the whole activation process (Table 2). The volume change of the Ado-binding pocket and the Gi2-binding site during the full activation process is shown in SI Appendix, Fig. S14. On the intracellular side, the volumes of the Gi2-binding site in the inactive 5N2S and the active 6D9H structures were 797.9 Å3 and 1632.1 Å3, respectively. During the whole activation process, the volume of the Gi2-binding site dilated from 908.3 Å3 in state a* (comparable to that in the inactive 5N2S structure) to 1,768.0 Å3 in the final state (comparable to that in the active 6D9H structure). On the extracellular side, the volumes of the Ado–binding pocket in the inactive 5N2S and the active 6D9H structures were 424.0 Å3 and 318.9 Å3, respectively, which indicated shrinkage of the pocket after the full activation of A1R. During the reconstruction process of the Ado–A1R–Gi2 complex, the volume of the Ado–binding pocket in A1R shrank from 463.9 Å3 in state a* (comparable to that in the inactive 5N2S structure) to 321.4 Å3 in the final state (comparable to that in the active 6D9H structure). As a result of the volume decrease of the Ado-binding pocket in A1R, the Ado–A1R binding free energy decreased from −12.1 kcal/mol in state a* to −34.0 kcal/mol in the final state (comparable to the binding free energy of −34.2 kcal/mol in the fully active 6D9H structure). These results suggested that the intracellular binding of Gi2 to A1R showed a benefit in shrinking the extracellular orthosteric binding site and promoting the binding affinity of Ado in A1R. These results reflected the allosteric coupling between the intracellular Gi2 protein binding and the conformational changes in the extracellular orthosteric Ado-binding pocket of A1R. This observation was consistent with previous experimental studies for another class A GPCR, β1AR, in which the active-state structures of β1AR with the nanobody that exhibited G protein-like behavior binding in the intracellular site showed a 24 to 42% reduction in the volume of the extracellular orthosteric ligand-binding pocket compared with the inactive-state structures (48).
Table 2.
The volumes of the Ado−binding pocket and the Ado−A1R binding free energies for the 5N2S and 6D9H structures and states a*, b*, c* and the final state
| Entry | Ado−binding pocket volume (SE, Å3) | Ado−A1R binding free energy (SE, kcal/mol) |
|---|---|---|
| 5N2S structure | 424.0 | – |
| 6D9H structure | 318.9 | −34.2 (1.2)* |
| State a* | 463.9 (15.3)† | −12.1 (1.1)† |
| State b* | 433.0 (12.2)† | −11.8 (1.1)† |
| State c* | 331.5 (11.7)† | −24.5 (1.4)† |
| Final state | 321.4 (10.5)† | −34.0 (1.1)† |
*We performed 100 ns cMD simulations for the 6D9H structure embedded in POPC in a water box and extracted the last 1.2-ns trajectory to calculate the Ado−A1R binding free energy.
†We extracted the 1.2-ns trajectory prior to the frame of states a*, b*, c* and the final state to calculate the volume of the Ado-binding pocket and the Ado−A1R binding free energy, respectively.
Conclusions
In the present work, we developed a Su-GaMD approach by incorporating a tabu-like supervision algorithm into a standard GaMD simulation. The Su-GaMD simulations allowed us to identify the binding pathways and important intermediate states of the ligand and the G protein recognitions to GPCR. We successfully used this Su-GaMD method to investigate Ado–A1R recognition and the subsequent A1R–Gi2 recognition event within hundreds of nanoseconds of simulations. The possible recognition pathways and important intermediate states of the Ado–A1R and A1R–Gi2 binding events were identified, the Ado–A1R–Gi2 complex was reconstructed from both active and inactive A1R, and the full activation mechanism of A1R (i.e., the whole signaling process from the extracellular side to the intracellular side of A1R) was revealed. Starting from free diffusion in the solvent, Ado gradually entered the extracellular orthosteric site of A1R. After that, A1R achieved the preactive state that was characterized by the broken ionic lock between Arg1053.50/Arg1083.53 and Glu2296.30 on the intracellular side. Then, Gi2 recognized the intracellular binding site and bound to A1R, the Ado–A1R–Gi2 complex was reconstructed, and A1R was fully activated. The binding of Ado to the extracellular orthosteric site A1R initiates conformational changes and the preactivation of A1R. In turn, the binding of Gi2 to the intracellular side of A1R caused a decrease in the volume of the extracellular orthosteric pocket and stabilized the binding of Ado. These results reflect the allosteric coupling between the intracellular Gi2 protein binding and the conformational change in the extracellular orthosteric Ado-binding pocket of A1R. With this case study of A1R, we have proven the applicability of the Su-GaMD approach to reconstruct a ligand–GPCR–G protein complex in nanosecond-timescale simulations, and the ligand–GPCR and GPCR–G protein recognition pathways were identified.
Molecular biologists have recently focused on the key conformational states and molecular details provided by a significant number of experimentally resolved ligand–GPCR or GPCR–G protein structures. On the one hand, it is more urgent to understand how the binding complexes are formed and to define the transformation process between the key conformational states over time. Distinguished conformational states in the G protein signaling pathway have been previously resolved by excellent experimental scientists, and it is necessary to connect these states by computational approaches. On the other hand, the future of drug design will involve detailed characterization of experimentally resolved bound states as well as the metastable intermediate states (metabinding sites) predicted by computational techniques with more efficiency. Su-GaMD simulations for A1R provide a promising approach that can both predict the binding complexes and reveal the metastable intermediate states in the full activation process of A1R. Taken together, the computationally determined full activation mechanism provides comprehensive insights into the A1R activation process and contributes to the future design of small molecules that could bias the signaling of A1R.
Materials and Methods
All MD simulations were carried out using Amber 18 (49). The AMBER FF14SB force field (50) was used for proteins, the general AMBER force field (GAFF) (51) was used for ligands, and the AMBER lipid force field LIPID14 (52) was used for 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphorylcholines (POPCs). Ado and Gi2 were separately placed >20 Å away from A1R to reconstruct a ternary complex of Ado, Gi2, and A1R (Ado–A1R–Gi2) from both active and inactive A1R. The Ado–A1R recognition event and the A1R–Gi2 recognition event were investigated by using the Su-GaMD method with the rmsds of Ado or Gi2 protein supervised. For the inactive system, a 150-ns GaMD simulation was performed to obtain a preactive state of A1R before the A1R–Gi2 recognition event was simulated. Further details are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (Grant No. BSMS69004).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. Y.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203702119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information. The data have not been deposited in a publicly accessible database.
References
- 1.Sriram K., Insel P. A., G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Mol. Pharmacol. 93, 251–258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latorraca N. R., Venkatakrishnan A. J., Dror R. O., GPCR dynamics: Structures in motion. Chem. Rev. 117, 139–155 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Kenakin T., Theoretical aspects of GPCR-ligand complex pharmacology. Chem. Rev. 117, 4–20 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Congreve M., de Graaf C., Swain N. A., Tate C. G., Impact of GPCR structures on drug discovery. Cell 181, 81–91 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez-de-Terán H., Sallander J., Sotelo E., Structure-based rational design of adenosine receptor ligands. Curr. Top. Med. Chem. 17, 40–58 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Fredholm B. B., IJzerman A. P., Jacobson K. A., Linden J., Müller C. E., Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 63, 1–34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zylka M. J., Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol. Med. 17, 188–196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draper-Joyce C. J., et al. , Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 597, 571–576 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wall M. J., et al. , Selective activation of Gαob by an adenosine A1 receptor agonist elicits analgesia without cardiorespiratory depression. Nat. Commun. 13, 4150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo D., Heitman L. H., IJzerman A. P., Kinetic aspects of the interaction between ligand and G protein-coupled receptor: The case of the adenosine receptors. Chem. Rev. 117, 38–66 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Romagnoli R., Baraldi P. G., Moorman A. R., Borea P. A., Varani K., Current status of A1 adenosine receptor allosteric enhancers. Future Med. Chem. 7, 1247–1259 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Kashfi S., Ghaedi K., Baharvand H., Nasr-Esfahani M. H., Javan M., A1 adenosine receptor activation modulates central nervous system development and repair. Mol. Neurobiol. 54, 8128–8139 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Chen J. F., Eltzschig H. K., Fredholm B. B., Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug Discov. 12, 265–286 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson K. A., Gao Z. G., Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 5, 247–264 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glukhova A.et al., . Structure of the Adenosine A1 Receptor Reveals the Basis for Subtype Selectivity. Cell 168, 867–877.e13 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Cheng R. K. Y.et al., . Structures of Human A1 and A2A Adenosine Receptors with Xanthines Reveal Determinants of Selectivity. Structure 25, 1275–1285.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Draper-Joyce C. J., et al. , Structure of the adenosine-bound human adenosine A1 receptor-Gi complex. Nature 558, 559–563 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Fronik P., Gaiser B. I., Sejer Pedersen D., Bitopic ligands and metastable binding sites: Opportunities for G protein-coupled receptor (GPCR) medicinal chemistry. J. Med. Chem. 60, 4126–4134 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Du Y., et al. , Assembly of a GPCR-G protein complex. Cell 177, 1232–1242.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum D. M., et al. , Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 469, 236–240 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dror R. O., et al. , Pathway and mechanism of drug binding to G-protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. 108, 13118–13123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai Q., Shi D., Zhang Y., Liu H., Yao X., Exploration of the antagonist CP-376395 escape pathway for the corticotropin-releasing factor receptor 1 by random acceleration molecular dynamics simulations. Mol. Biosyst. 10, 1958–1967 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Ísberg V., Balle T., Sander T., Jørgensen F. S., Gloriam D. E., G protein- and agonist-bound serotonin 5-HT2A receptor model activated by steered molecular dynamics simulations. J. Chem. Inf. Model. 51, 315–325 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Saleh N., Ibrahim P., Clark T., Differences between G-protein-stabilized agonist-GPCR complexes and their nanobody-stabilized equivalents. Angew. Chem. Int. Ed. Engl. 56, 9008–9012 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Miao Y., Nichols S. E., Gasper P. M., Metzger V. T., McCammon J. A., Activation and dynamic network of the M2 muscarinic receptor. Proc. Natl. Acad. Sci. U.S.A. 110, 10982–10987 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamelberg D., Mongan J., McCammon J. A., Accelerated molecular dynamics: A promising and efficient simulation method for biomolecules. J. Chem. Phys. 120, 11919–11929 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Kruse A. C., et al. , Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482, 552–556 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provasi D., Bortolato A., Filizola M., Exploring molecular mechanisms of ligand recognition by opioid receptors with metadynamics. Biochemistry 48, 10020–10029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wingler L. M., McMahon C., Staus D. P., Lefkowitz R. J., Kruse A. C., . Distinctive Activation Mechanism for Angiotensin Receptor Revealed by a Synthetic Nanobody. Cell 176, 479–490.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada I., Ueda T., Kofuku Y., Eddy M. T., Wüthrich K., GPCR drug discovery: Integrating solution NMR data with crystal and cryo-EM structures. Nat. Rev. Drug Discov. 18, 59–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabbadin D., Moro S., Supervised molecular dynamics (SuMD) as a helpful tool to depict GPCR-ligand recognition pathway in a nanosecond time scale. J. Chem. Inf. Model. 54, 372–376 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya S., Vaidehi N., Mechanism of allosteric communication in GPCR activation from microsecond scale molecular dynamics simulations. Biophys. J. 112, 498a–499a (2017). [Google Scholar]
- 33.Yuan S., et al. , The molecular mechanism of P2Y1 receptor activation. Angew. Chem. Int. Ed. Engl. 55, 10331–10335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Sun J., Li D., Lin J., Activation and conformational dynamics of a class B G-protein-coupled glucagon receptor. Phys. Chem. Chem. Phys. 18, 12642–12650 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y., et al. , Molecular insights into ligand recognition and G protein coupling of the neuromodulatory orphan receptor GPR139. Cell Res. 32, 210–213 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deganutti G., et al. , Deciphering the agonist binding mechanism to the adenosine A1 receptor. ACS Pharmacol. Transl. Sci. 4, 314–326 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao Y., McCammon J. A., Mechanism of the G-protein mimetic nanobody binding to a muscarinic G-protein-coupled receptor. Proc. Natl. Acad. Sci. U.S.A. 115, 3036–3041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salmaso V., Sturlese M., Cuzzolin A., Moro S., . Exploring Protein-Peptide Recognition Pathways Using a Supervised Molecular Dynamics Approach. Structure 25, 655–662.e2 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Cuzzolin A., et al. , Deciphering the complexity of ligand-protein recognition pathways using supervised molecular dynamics (SuMD) simulations. J. Chem. Inf. Model. 56, 687–705 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Sabbadin D., et al. , Exploring the recognition pathway at the human A2A adenosine receptor of the endogenous agonist adenosine using supervised molecular dynamics simulations. MedChemComm 6, 1081–1085 (2015). [Google Scholar]
- 41.Deganutti G., Welihinda A., Moro S., Comparison of the human A2A adenosine receptor recognition by adenosine and inosine: New insight from supervised molecular dynamics simulations. ChemMedChem 12, 1319–1326 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Nguyen A. T. N., et al. , Extracellular loop 2 of the adenosine A1 receptor has a key role in orthosteric ligand affinity and agonist efficacy. Mol. Pharmacol. 90, 703–714 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Miao Y., Bhattarai A., Nguyen A. T. N., Christopoulos A., May L. T., Structural basis for binding of allosteric drug leads in the adenosine A1 receptor. Sci. Rep. 8, 16836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jespers W., et al. , Structural mapping of adenosine receptor mutations: Ligand binding and signaling mechanisms. Trends Pharmacol. Sci. 39, 75–89 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Mattedi G., Deflorian F., Mason J. S., de Graaf C., Gervasio F. L., Understanding ligand binding selectivity in a prototypical GPCR family. J. Chem. Inf. Model. 59, 2830–2836 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J., Miao Y., Mechanistic insights into specific G protein interactions with adenosine receptors. J. Phys. Chem. B 123, 6462–6473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattedi G., Acosta-Gutiérrez S., Clark T., Gervasio F. L., A combined activation mechanism for the glucagon receptor. Proc. Natl. Acad. Sci. U.S.A. 117, 15414–15422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warne T., Edwards P. C., Doré A. S., Leslie A. G. W., Tate C. G., Molecular basis for high-affinity agonist binding in GPCRs. Science 364, 775–778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Case D. A., et al. , AMBER 2018 (University of California, San Francisco, 2018). [Google Scholar]
- 50.Maier J. A., et al. , ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Wolf R. M., Caldwell J. W., Kollman P. A., Case D. A., Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Dickson C. J., et al. , Lipid14: The Amber lipid force field. J. Chem. Theory Comput. 10, 865–879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information. The data have not been deposited in a publicly accessible database.