Significance
Childhood exposure to social disadvantage and related stressors is a major risk factor for altered brain development. The extent that these associations begin prenatally, when white matter is rapidly developing, remains unclear. In a sample of 289 neonates enriched for social disadvantage experienced prenatally, we use prospective data beginning early in gestation to show that prenatal exposure to social disadvantage is related to aberrant fronto-limbic white matter microstructure in the first weeks of life. The association between maternal psychosocial stress and cingulum bundle (CB) microstructure was observed in the setting of less-severe socioeconomic hardship. Associations between early life adversity and white matter development begins in utero, and fronto-limbic pathways are particularly vulnerable during this critical period of white matter development.
Keywords: prenatal, social disadvantage, depression, stress, diffusion MRI
Abstract
Early life adversity (social disadvantage and psychosocial stressors) is associated with altered microstructure in fronto-limbic pathways important for socioemotional development. Understanding when these associations begin to emerge may inform the timing and design of preventative interventions. In this longitudinal study, 399 mothers were oversampled for low income and completed social background measures during pregnancy. Measures were analyzed with structural equation analysis resulting in two latent factors: social disadvantage (education, insurance status, income-to-needs ratio [INR], neighborhood deprivation, and nutrition) and psychosocial stress (depression, stress, life events, and racial discrimination). At birth, 289 healthy term-born neonates underwent a diffusion MRI (dMRI) scan. Mean diffusivity (MD) and fractional anisotropy (FA) were measured for the dorsal and inferior cingulum bundle (CB), uncinate, and fornix using probabilistic tractography in FSL. Social disadvantage and psychosocial stress were fitted to dMRI parameters using regression models adjusted for infant postmenstrual age at scan and sex. Social disadvantage, but not psychosocial stress, was independently associated with lower MD in the bilateral inferior CB and left uncinate, right fornix, and lower MD and higher FA in the right dorsal CB. Results persisted after accounting for maternal medical morbidities and prenatal drug exposure. In moderation analysis, psychosocial stress was associated with lower MD in the left inferior CB among the lower-to-higher socioeconomic status (SES) (INR ≥ 200%) group, but not the extremely low SES (INR < 200%) group. Increasing access to social welfare programs that reduce the burden of social disadvantage and related psychosocial stressors may be an important target to protect fetal brain development in fronto-limbic pathways.
Exposure to early life adversity (social disadvantage and related psychosocial stressors) during sensitive periods of brain development in early childhood is a major risk factor for aberrant brain development (1). Understanding when associations between exposure to early life adversity and differences in white matter development begin to emerge may inform the timing and design of preventative interventions. Various forms of social disadvantage, such as low parental education, reduced family income, and neighborhood deprivation, have been associated with lasting reductions in prefrontal, amygdala, and hippocampal volumes (2). However, less is known about these effects on white matter development more specifically, including their regional specificity and timing. The cingulum bundle (CB), uncinate, and fornix are key white matter tracts that connect fronto-limbic brain regions and are similarly altered by social disadvantage (3, 4). Studies using diffusion MRI (dMRI) have shown that childhood social disadvantage is related to lower fractional anisotropy (FA) in the uncinate, fornix, and CB in children age 8–10 y (4) and adults (3). To date, just one study has focused on perinatal social disadvantage in preterm and term-born neonates, linking social disadvantage with lower FA in the uncinate at term-equivalent age (5). This study, however, focused on family-level factors, including caregiver education, employment status, occupation, primary language spoken, age at child delivery, and family structure. In addition to family-level factors, aspects of the broader social environment, including neighborhood disadvantage, may also be related to brain development in childhood (2).
Exploring prenatal and early postnatal effects may be particularly important due to the rapid proliferation of immature oligodendrocyte cells starting from 28 wk gestation, followed by myelination of white matter fibers in the first weeks of life, which progresses in an orderly, regionally specific, anterior-posterior manner (6, 7). Thus, prenatal white matter development is a critical period of heightened neuronal plasticity that may be disrupted by early life adversity in utero. Yet little is known about associations between social disadvantage and prenatal white matter development in humans. Preclinical work in rodents suggests that resource deprivation and exposure to other related stressors produces proinflammatory cytokines and inhibits the expression of proteins responsible for axonal differentiation and myelination, resulting in fewer mature, myelin-producing oligodendrocytes in developing white matter (8–10). Exposure to social disadvantage and related stressors may also exacerbate reactive oxygen species, causing both direct injury to and resulting apoptosis of oligodendrocyte precursors (11) and arrest of oligodendrocyte differentiation without cell death (12). Taken together, these findings suggest that in utero exposure to social disadvantage may impair the microstructural maturation of white matter in developing humans during a period of high brain plasticity.
While childhood exposure to social disadvantage is associated with altered white matter connectivity (3, 4), social disadvantage co-occurs with other forms of early life adversity. One pathway by which social disadvantage may shape offspring brain development includes maternal psychosocial stress (13). To date, only a handful of studies have examined maternal psychosocial stress in pregnancy and neonatal white matter connectivity (14–17). Maternal anxiety in the third trimester has been shown to predict lower FA in the CB, fornix, and uncinate in neonates (15, 18) and higher mean diffusivity (MD) in prefrontal white matter at age 1 mo (16); white matter regions also associated with social disadvantage (3, 4). Maternal perinatal depression has been related to higher MD in frontal white matter (16) and fewer white-matter fibers projecting from the amygdala to the prefrontal cortex in neonates (17). While these findings highlight the role of maternal psychosocial stress, the extent to which social disadvantage may have a dissociable relationship with white matter connectivity at birth is unclear. Indeed, social disadvantage may contribute unique variance in what may be a common pathway translating macro- and micronutrient deficiencies and direct and indirect neuroinflammatory effects of household, outdoor, and water pollutants to the developing brain. Prior studies of maternal psychosocial stress have typically examined social disadvantage as a confound in adjusted analyses (14, 15, 17). Treating social disadvantage as a confound, rather than as an independent variable of interest, limits the understanding of its direct contribution to early brain development. The extent that experiencing severe socioeconomic hardship may exacerbate associations between maternal psychological stress and aberrant fetal white matter development is also unclear.
This study focuses on 289 healthy, term-born neonates with brain neuroimaging acquired at birth. Mothers were identified in the first trimester of pregnancy and oversampled for low income. As described in Luby et al. (19), observed variables collected during pregnancy were used to define two latent factors: social disadvantage (education, health insurance, income-to-needs ratio [INR], neighborhood disadvantage, and nutrition) and psychosocial stress (depression, stress, life events, and racial discrimination; see also SI Appendix). The overall objective of this study was to examine prenatal exposure to social disadvantage and psychosocial stress in relation to white matter connectivity at birth. The CB, uncinate, and fornix were selected as key fronto-limbic tracts of interest because they connect the amygdala and hippocampus with the frontal cortex (20–22), are vulnerable to the adverse effects of early life adversity (5, 14–18), and have frequently been implicated in the development of socioemotional impairments, highlighting their relevance as a biomarker for psychopathology (23, 24). The corpus callosum (CC) and corticospinal tract (CST) were also included as negative control tracts. The CC is a large anterior–posterior tract in proximity to the CB with branching fibers, but it does not connect fronto-limbic brain regions. The CST forms the primary motor pathway, with a highly directional, inferior–superior orientation.
Because links between early life adversity and brain development are complex and likely multifactorial, we sought to test the multiple, nonexclusive ways in which social disadvantage and psychosocial stress may have shared, unique, and/or moderating relationships on white matter development. The first objective of this study was to examine social disadvantage and psychosocial stress in relation to CB, uncinate, and fornix microstructure to understand the relative contribution of each factor accounting for the other factor. We hypothesized that both social disadvantage and psychosocial stress would be independently associated with CB, uncinate, and fornix microstructure (Fig. 1), with greater adversity related to lower FA and/or higher MD indicative of impaired myelination and axonal development in white matter (5, 14–18). In contrast, we hypothesized that social disadvantage and psychosocial stress would not be associated with the CC or CST. Based upon prior work showing that postnatal exposure to maternal stress mediates the effect of social disadvantage on macrostructural brain development in childhood (25), we hypothesized that prenatal exposure to psychosocial stress would explain more variance in white matter microstructure than social disadvantage. However, we also acknowledge that other biological and environmental pathways (e.g., macro- and micronutrient deficiencies and direct and indirect neuroinflammatory effects of household, outdoor, and water pollutants) may link social disadvantage with infant brain development and that there may be some small proportion of remaining or unique variance in white matter attributable to social disadvantage after accounting for psychosocial stress.
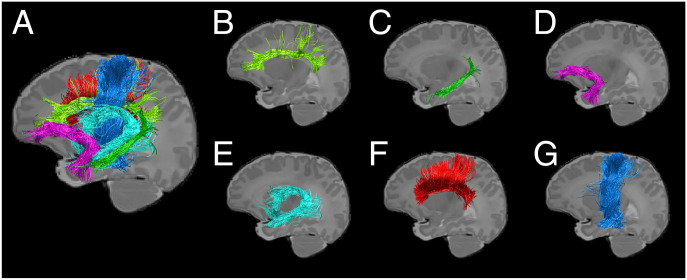
Fig. 1.
Demonstration of white-matter fibers of interest in a representative healthy, term-born neonate. Key fronto-limbic tracts (A) include the dorsal (B) and inferior (C) cingulum bundle, uncinate (D), and fornix (E). Control tracts include the corpus callosum (F) and corticospinal tract (G).
Given that severe socioeconomic hardship and maternal stress/depression tend to co-occur (13), the second objective of this study sought to understand the extent that severe socioeconomic hardship may exacerbate associations between psychosocial stress and aberrant white matter development. Formal moderation analysis was used to investigate whether psychosocial stress–white matter relationships were stronger among dyads facing severe socioeconomic hardships. Extremely low and lower-to-higher socioeconomic status (SES) was defined as INR below or at/above 200% of the national poverty threshold, respectively (26–31). Reasons for selecting INR at 200% were threefold. First, INR is an ecologically valid metric that determines family eligibility for social welfare programs, such as Children’s Health Insurance Program, Medicaid, and Supplemental Nutrition Assistance Program. Second, INR at 200% has been standardly used as a cut-point to define low-SES families in multiple large, longitudinal studies (26–31), making findings across studies more comparable, allowing for replication of moderation findings by future research, and making recommendations for intervention more directly applicable. Third, INR demonstrated the highest loading values on the latent social disadvantage factor, suggesting that the social disadvantage factor may primarily reflect INR. We hypothesized stronger psychosocial stress–white matter associations in dyads experiencing severe socioeconomic hardships. Supplementary aims included examining maternal medical risk (MMR) in pregnancy and prenatal drug exposure (cannabis and tobacco) as confounding factors. Sensitivity analysis was also undertaken to replicate key study findings in an age-restricted subsample of the cohort.
Results
Sample characteristics are shown in Table 1. First, prenatal exposure to social disadvantage and psychosocial stress were examined separately in relation to dMRI parameters, adjusted for infant postmenstrual age (PMA) at scan and sex (SI Appendix, Tables S1 and S2; see also SI Appendix, Fig. S1). Then, to delineate the conditional contribution of each latent factor when the other was included in the model, social disadvantage and psychosocial stress were fitted together in multivariable hierarchical regression models (results from step 2 of the regression models are summarized in Table 2). Results were multiple comparison corrected using Benjamini–Hochberg false discovery rate. Full results (SI Appendix, Tables S3–S6) show that infant PMA at scan explained 13–31% of the variance in MD, 18–32% of the variance in radial diffusivity (RD), 4–17% of the variance in axial diffusivity (AD), and 8–26% of the variance in FA across tracts.
Table 1.
Background characteristics of the sample (n = 289)
| N | Mean | SD | Range | |
|---|---|---|---|---|
| Infant characteristics | ||||
| Gestational age, weeks | 289 | 38.57 | 1.01 | 37–41 |
| Birthweight, grams | 289 | 3,254.59 | 489.29 | 2200–4627 |
| Sex assigned at birth, % (n) | 289 | … | … | … |
| Female | … | 45.0 (130) | … | … |
| Male | … | 55.0 (159) | … | … |
| Race, % (n)* | 289 | … | … | … |
| Black/African American | … | 62.3 (180) | … | … |
| White | … | 36.0 (104) | … | … |
| Asian | … | 2.0 (6) | … | … |
| Pacific Islander | … | 0.3 (1) | … | … |
| Other (not defined) | … | 0.7 (2) | … | … |
| Ethnicity, % (n) | 289 | … | … | … |
| Hispanic or Latina/o | … | 2.4 (7) | … | … |
| Not Hispanic or Latina/o | … | 96.9 (280) | … | … |
| Unspecified | … | 0.7 (2) | … | … |
| Postmenstrual age at MRI scan, weeks | 289 | 41.70 | 1.30 | 38–45 |
| Maternal background | ||||
| Age at delivery, years | 289 | 28.99 | 5.29 | 19–42 |
| Education, % (n) | 254 | … | … | … |
| Less than high school | … | 7.3 (21) | … | … |
| High school graduate | … | 47.8 (138) | … | … |
| College graduate | … | 13.5 (39) | … | … |
| Postgraduate degree | … | 19.4 (56) | … | … |
| Insurance status (public or underinsured), % (n) | 289 | 51.2 (148) | … | … |
| Race, % (n) | 289 | … | … | … |
| Black/African American | … | 61.6 (178) | … | … |
| White | … | 36.0 (104) | … | … |
| Asian | … | 1.7 (5) | … | … |
| Pacific Islander | … | 0.3 (1) | … | … |
| Other (not defined) | … | 0.3 (1) | … | … |
| Ethnicity, % (n) | 289 | … | … | … |
| Hispanic or Latina | … | 2.4 (7) | … | … |
| Not Hispanic or Latina | … | 97.2 (281) | … | … |
| Unspecified | … | 0.3 (1) | … | … |
| Tobacco use in pregnancy, % (n) | 289 | 13.5 (39) | … | … |
| Cannabis use in pregnancy and/or positive urine drug screen, % (n) | 289 | 26.6 (77) | … | … |
| Income-to-needs ratio† | 284 | 2.72 | 2.81 | 0.38–12.04 |
| Area Deprivation Index percentile | 283 | 68.77 | 24.94 | 1–100 |
| Healthy Eating Index | 230 | 58.33 | 10.06 | 32.96–80.67 |
| Edinburg Postnatal Depression Scale (EPDS)† | 289 | 4.61 | 3.94 | 0–21 |
| EPDS in the clinical range (score >13), % (n) | 289 | 10.0 (29) | … | … |
| Perceived Stress Scale (PSS)† | 289 | 13.11 | 6.46 | 1.00–32.00 |
| PSS in the clinical range (score >13), % (n) | 289 | 45.7 (132) | … | … |
| STRAIN, count | 269 | 7.27 | 5.73 | 0–27 |
| STRAIN, severity | 269 | 20.99 | 18.02 | 0–90 |
| Racial discrimination | 265 | 1.49 | 0.90 | 1–6 |
| Social disadvantage latent factor score‡ | 289 | 0.00 | 1.00 | −2.24–1.56 |
| Psychosocial stress latent factor score‡ | 289 | 0.00 | 1.00 | −1.79–3.19 |
*More than one race reported for four infants (Black/African American–White = 2, Black/African American–Chinese = 2).
†Mean value across trimesters 1, 2, and 3.
‡Standardized values.
Table 2.
Summary of hierarchical regression models linking infant characteristics and prenatal adversity factors with neonatal mean diffusivity (MD) fractional anisotropy (FA) (n = 289)
| MD | FA | |||||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | SE | q | Δ R2 | β (95% CI) | SE | q | Δ R2 | |
| R dorsal cingulum | R2 = 0.25* | … | R2 = 0.13* | … | ||||
| Sex | −0.02 (−0.12–0.08) | 0.05 | 0.87 | … | −0.11 (−0.22–−0.01) | 0.06 | 0.13 | … |
| PMA at scan | −0.50 (−0.61–−0.40) | 0.05 | <0.001 | … | 0.32 (0.21–0.43) | 0.06 | <0.001 | … |
| Social disadv. | −0.17 (−0.28–−0.06) | 0.06 | 0.008 | 0.03† | 0.18 (0.06–0.30) | 0.06 | 0.02 | 0.03‡ |
| Psych. stress | 0.02 (−0.09–0.12) | 0.06 | 0.94 | … | −0.07 (−0.19–0.05) | 0.06 | 0.57 | … |
| L dorsal cingulum | R2 = 0.22* | … | R2 = 0.13* | … | ||||
| Sex | −0.02 (−0.13–0.08) | 0.05 | 0.87 | … | −0.16 (−0.27–−0.05) | 0.06 | 0.03 | … |
| PMA at scan | −0.47 (−0.58–−0.37) | 0.05 | <0.001 | … | 0.30 (0.19–0.41) | 0.06 | <0.001 | … |
| Social disadv. | −0.10 (−0.22–0.01) | 0.06 | 0.07 | 0.02‡ | 0.14 (0.02–0.26) | 0.06 | 0.07 | 0.02 |
| Psych. stress | −0.06 (−0.17–0.05) | 0.06 | 0.94 | … | −0.03 (−0.14–0.09) | 0.06 | 0.83 | … |
| R inferior cingulum | R2 = 0.29* | … | R2 = 0.11* | … | ||||
| Sex | −0.10 (−0.20–0.01) | 0.05 | 0.16 | … | 0.06 (−0.05–0.17) | 0.06 | 38 | … |
| PMA at scan | −0.52 (−0.62–−0.41) | 0.05 | <0.001 | … | 0.34 (0.23–0.45) | 0.06 | <0.001 | … |
| Social disadv. | −0.25 (−0.36–−0.14) | 0.06 | <0.001 | 0.06* | 0.05 (−0.07–0.17) | 0.06 | 0.71 | <0.01 |
| Psych. stress | <0.01 (−0.10–0.11) | 0.06 | 0.94 | … | 0.02 (−0.10–0.14) | 0.06 | 0.83 | … |
| L inferior cingulum | R2 = 0.36* | … | R2 = 0.11* | … | ||||
| Sex | −0.11 (−0.20–−0.02) | 0.05 | 0.16 | … | −0.06 (−0.17–0.05) | 0.06 | 0.38 | … |
| PMA at scan | −0.59 (−0.68–−0.49) | 0.05 | <0.001 | … | 0.31 (0.19–0.42) | 0.06 | <0.001 | … |
| Social disadv. | −0.21 (−0.32–−0.11) | 0.05 | <0.001 | 0.06* | −0.01 (−0.13–0.12) | 0.06 | 0.97 | 0.01 |
| Psych. stress | −0.05 (−0.15–0.05) | 0.05 | 0.94 | … | 0.10 (−0.02–0.22) | 0.06 | 0.57 | … |
| R uncinate | R2 = 0.21* | … | R2 = 0.26* | … | ||||
| Sex | −0.06 (−0.16–0.05) | 0.05 | 0.69 | … | −0.11 (−0.21–−0.01) | 0.05 | 0.11 | … |
| PMA at scan | −0.46 (−0.57–−0.36) | 0.05 | <0.001 | … | 0.49 (0.39–0.59) | 0.05 | <0.001 | … |
| Social disadv. | −0.11 (−0.22–0.01) | 0.06 | 0.07 | 0.01 | <0.01 (−0.11–0.11) | 0.06 | 0.97 | <0.01 |
| Psych. stress | −0.01 (−0.13–0.10) | 0.06 | 0.94 | … | −0.01 (−0.12–0.10) | 0.06 | 0.84 | … |
| L uncinate | R2 = 0.16* | … | R2 = 0.19* | … | ||||
| Sex | −0.05 (−0.16–0.06) | 0.06 | 0.69 | … | <−0.01 (−0.11–0.10) | 0.05 | 0.94 | … |
| PMA at scan | −0.40 (−0.51–−0.29) | 0.06 | <0.001 | … | 0.43 (0.32–0.54) | 0.05 | <0.001 | … |
| Social disadv. | −0.17 (−0.29–−0.05) | 0.06 | 0.01 | 0.03† | 0.02 (−0.10–0.13) | 0.06 | 0.97 | 0.01 |
| Psych. stress | −0.01 (−0.12–0.11) | 0.06 | 0.94 | … | 0.08 (−0.03–0.20) | 0.06 | 0.57 | … |
| R fornix | R2 = 0.32* | … | R2 = 0.11* | … | ||||
| Sex | 0.01 (−0.09–0.11) | 0.05 | 0.87 | … | −0.08 (−0.20–0.03) | 0.06 | 0.22 | … |
| PMA at scan | −0.57 (−0.67–−0.47) | 0.05 | <0.001 | … | 0.31 (0.20–0.43) | 0.06 | <0.001 | … |
| Social disadv. | −0.14 (−0.25–−0.04) | 0.05 | 0.01 | 0.02‡ | 0.14 (0.02–0.26) | 0.06 | 0.07 | 0.02 |
| Psych. stress | 0.03 (−0.08–0.13) | 0.05 | 0.94 | … | −0.07 (−0.19–0.06) | 0.06 | 0.57 | … |
| L fornix | R2 = 0.31* | … | R2 = 0.10* | … | ||||
| Sex | 0.01 (−0.09–0.11) | 0.05 | 0.87 | … | −0.10 (−0.21–0.01) | 0.06 | 0.18 | … |
| PMA at scan | −0.56 (−0.66–−0.47) | 0.05 | <0.001 | … | 0.28 (0.16–0.39) | 0.06 | <0.001 | … |
| Social disadv. | −0.10 (−0.20–0.01) | 0.05 | 0.07 | 0.01 | 0.11 (−0.02–0.23) | 0.06 | 0.17 | 0.02 |
| Psych. stress | 0.01 (−0.09–0.12) | 0.05 | 0.94 | … | 0.04 (−0.08–0.16) | 0.06 | 0.83 | … |
q, significance value adjusted for multiple comparisons using Benjamini–Hochberg false discovery rate procedure (bold values indicate significant results (q < 0.05) after correction); Δ, change; R, right; L, left; PMA, postmenstrual age; disadv., Disadvantage; psych., Psychosocial.
*Model significance: P < 0.001.
†Model significance: P < 0.01.
‡Model significance: P < 0.05.
Social Disadvantage.
As shown in SI Appendix, Table S1, social disadvantage was related to lower MD in the bilateral dorsal and inferior CB, bilateral uncinate, and right fornix. Results persisted after multiple-comparison correction. In addition to MD findings, there were similar associations between social disadvantage and RD in the bilateral dorsal and inferior CB, left uncinate, and right fornix as well as AD in bilateral inferior CB and left uncinate. Associations between social disadvantage and higher FA in the bilateral dorsal CB and bilateral fornix did not survive multiple-comparison correction.
Psychosocial Stress.
As shown in SI Appendix, Table S2, psychosocial stress was associated with lower MD in the left inferior CB, with similar associations for reduced RD. This result persisted after multiple comparison correction. There were no associations for the uncinate or fornix.
Multivariable Analyses of Social Disadvantage and Psychosocial Stress.
When psychosocial stress and social disadvantage were fitted together in step 2 of the regression analyses (Table 2), social disadvantage continued to explain independent variance in MD for the right dorsal CB, bilateral inferior CB, left uncinate, and right fornix as well as higher FA in the right dorsal CB. Social disadvantage also continued to explain variance in RD for the bilateral dorsal and inferior CB and fornix as well as AD in the bilateral inferior CB and left uncinate (SI Appendix, Tables S4 and S5). Adding social disadvantage to step 2 of the multivariable model explained an additional 2–6% of the variance in dMRI parameters across white matter tracts (SI Appendix, Tables S3–S6). Importantly, the association between psychosocial stress and left inferior CB MD was attenuated after accounting for social disadvantage (Table 2). There were no unique associations between social disadvantage or psychosocial stress and FA in the uncinate or fornix after multiple-comparison correction (Table 2). These key study findings were unchanged in sensitivity analysis performed among an age-restricted subsample of infants scanned 41–43 wk PMA (SI Appendix, Table S7). Multivariable linear regression models were also consistent when INR was used as an independent variable in place of social disadvantage (SI Appendix, Table S8).
Negative Control Tracts.
Multivariable analyses were performed for the CC and CST as negative control tracts. As shown in SI Appendix, Table S9, there was no association between either social disadvantage or psychosocial stress and MD in the CC and right CST or FA in the CC and bilateral CST. However, greater social disadvantage was associated with lower MD in the left CST.
Psychosocial Stress in Extremely Low and Lower-to-Higher SES Groups.
To examine the extent that experiencing severe socioeconomic hardship may exacerbate associations between psychosocial stress and aberrant white matter development, formal moderation analysis was used to investigate whether psychosocial stress–white matter relationships were stronger among dyads facing severe socioeconomic hardships. This analysis was restricted to MD in the left inferior CB MD, because it was the only tract correlated with psychosocial stress prior to accounting for social disadvantage (SI Appendix, Table S2). The extremely low SES group (INR < 200%) had higher psychosocial stress scores and greater adversity across the other observed social disadvantage variables than the lower-to-higher SES group (INR ≥ 200%; see SI Appendix, Table S10), but there was no between-groups difference in MMR index scores.
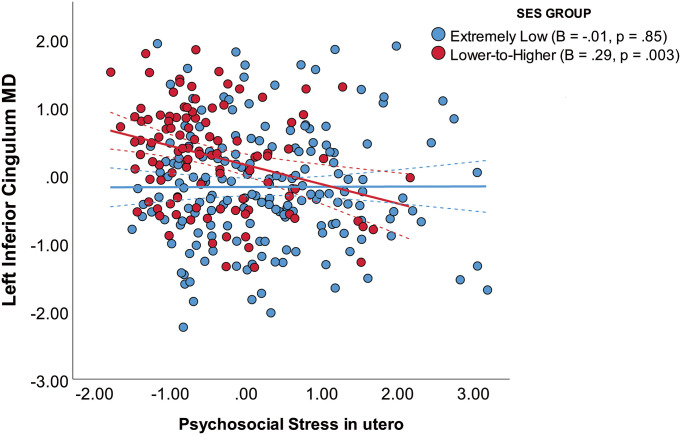
Moderation analysis (SI Appendix, Table S11) showed that there was an interaction between psychosocial stress and family SES group on left inferior CB MD (B = −0.31, P = 0.008), such that the association between psychosocial stress and left inferior CB MD was stronger in the lower-to-higher SES group than in the extremely low SES group (Fig. 2). Within the lower-to-higher SES group, association between psychosocial stress and MD in the inferior CB persisted after also accounting for individual differences in broader social disadvantage factor scores (SI Appendix, Table S12).
Fig. 2.
The interaction between psychosocial stress in utero and family socioeconomic status (SES) group on the left inferior cingulum bundle mean diffusivity (MD) at birth (n = 284). Here, MD values are shown as standardized residuals adjusted for infant PMA at scan. Solid lines represent the slope of each SES group, and dashed lines represent 95% CIs.
Confounding Factors.
As described in SI Appendix, mothers who used tobacco and cannabis during pregnancy had higher social disadvantage and psychosocial stress scores than mothers with no use (SI Appendix, Table S13). Prenatal exposure to cannabis was related to lower MD in the left inferior CB (P = 0.04) and higher FA in the left fornix (P = 0.02). Tobacco exposure was not significant. MMR was only correlated with higher MD in the left dorsal CB (P = 0.03). As reported in SI Appendix, Tables S14 and S15, cannabis exposure and MMR were not significant after accounting for infant PMA at scan and, therefore, did not alter the main conclusions of this study.
Discussion
In healthy, term-born neonates, prenatal exposure to social disadvantage was independently related to lower MD in the bilateral inferior CB, left uncinate, and right fornix and lower MD and higher FA in the right dorsal CB after accounting for covariate factors and maternal psychosocial stress. Similar results were also found for RD and AD. While maternal psychosocial stress was correlated with lower MD in the left inferior CB, this finding did not persist after accounting for social disadvantage. These findings were unchanged in an age-restricted subsample of infants scanned 41–43 wk PMA and when INR was tested as an independent variable in place of social disadvantage. However, moderation analysis showed that psychosocial stress was more strongly associated with lower MD in the left inferior CB among lower-to-higher SES dyads. Key study findings persisted after accounting for MMR and prenatal exposure to cannabis.
Consistent with Thompson et al. (5), social disadvantage in utero was associated with aberrant white matter connectivity at birth. Thompson et al. found alterations mainly in the uncinate, and we found effects in the CB, uncinate, and fornix. Our results suggest that, despite differences in timing of myelination, prenatal exposure to social disadvantage is associated with variability in white matter connectivity across fronto-limbic tracts of interest. Findings of lower MD or higher FA could reflect aberrant reductions in fiber branching or atypical pruning of white matter fibers, making the underlying dMRI tensors appear more directional (20, 23). Indeed, the CB and uncinate contain multiple branching fibers, and the fornix is a highly angular tract with projections into the prefrontal cortex. Lower MD or higher FA early in development may also be a marker of hypermaturation (32, 33). Preclinical studies suggest that deprivation and stress drive precocious oligodendrocyte differentiation (34) and premature myelination (35) in frontal and temporal brain regions, and human studies have shown that frontal and temporal brain regions contain projections from the CB, uncinate, and/or fornix (20, 22).The hypermaturation hypothesis is particularly relevant for the CB and uncinate, given that accelerated amygdala–frontal connectivity has been linked with the overactivation of the hypothalamic–pituitary–adrenal (HPA) axis and glucocorticoid expression in the context of early life adversity (33, 36). Clinically, higher neonatal and infant FA in the CB and uncinate has been shown to predict socioemotional problems and autism spectrum disorder (23, 24). Taking these findings together, social disadvantage in utero appears to be an important antecedent of aberrant microstructural development in key white matter tracts implicated in the development of socioemotional impairments. Consistent with this hypothesis, we also found that social disadvantage was not associated with the CC or the right CST, which were included in this study as negative control tracts. However, there was an association with left CST, suggesting that social disadvantage may be linked with altered microstructure in other early-developing tracts.
Multiple pathophysiological and environmental mechanisms likely play a role in linking social disadvantage with altered white matter development. Because maternal obesity, pre-eclampsia, and other health conditions are more common in the setting of social disadvantage (37) and have been linked to micronutrient deficiencies, vascular insufficiency, and/or oxidative stress likely to impair mitochondria-rich preoligodendrocytes in offspring (38, 39), we considered MMR in pregnancy. MMR was related to altered microstructure in the left CB, but this result did not persist after adjusting for infant PMA at scan, which captures expected age-related differences in neonatal white matter development (6, 7). As we focused on healthy, term-born neonates, mothers were also generally healthy. In addition, disadvantaged areas are more likely to have poorer quality housing, lack green spaces, and have greater lead toxicity and air pollution. Exposure to lead and air pollution in utero may disrupt highly sensitive oligodendrocyte differentiation, proliferation, and subsequent myelination (10–12). Social disadvantage may also be linked to infant brain development via heritable mechanisms such that disadvantage and trauma may be related to altered maternal brain structure or function, which is then passed to infants. Lead and air pollution, as well as intergenerational influences, should be examined in future work focusing on the mechanisms linking social disadvantage with fetal white matter development.
This study also examined prenatal exposure to psychosocial stress, which has been related to altered white matter connectivity at birth (14–18) and is one potential pathway by which social disadvantage may influence macrostructural brain development in childhood (25). The factor loadings for the psychosocial stress factor indicated that this factor may primarily reflect depression symptoms and perceived stress, and thus, maternal mood/affective problems and reactions to stress during pregnancy may be important targets for early intervention. As such, we hypothesized that psychosocial stress would explain more variance in neonatal white matter connectivity than social disadvantage. While psychosocial stress was correlated with MD in the left inferior CB, this finding was accounted for or subsumed by social disadvantage in multivariable analyses. Prior work focusing on maternal anxiety/depression has typically controlled for SES using narrow markers like income or education (14, 15, 17). We broadly assessed social disadvantage in an enriched sample, spanning demographics, family income, neighborhood disadvantage, and nutrition. Compared with narrow markers of SES, multifactorial indices of social disadvantage are thought to capture general inequality, reflect the cumulative effects of multiple risk exposure, and/or reduce measurement error (2). The presence of social disadvantage, a global and robust risk factor, may have a pervasive or more detectable association with white matter development. However, we also note that results of the multivariable linear regression models were similar when using INR in place of social disadvantage, such that INR was also more strongly associated with white matter than maternal psychosocial stress. This could reflect the fact that INR had the highest factor loadings on the social disadvantage factor as well as the extent to which the sample was enriched for low income and may have greater variability in INR than previous studies documenting effects of maternal depression and anxiety (14–18).
However, moderation analysis provided evidence of an interaction between psychosocial stress and family SES group, such that the association between psychosocial stress and the inferior CB was stronger in the lower-to-higher SES group than in the extremely low SES group. This suggests that maternal psychosocial stress may be more observable in the setting of less severe social disadvantage. Additionally, mothers in the lower-to-higher SES group are likely more demographically comparable to other samples in which associations between psychosocial stress and neonatal white-matter development have been found (14, 16) and where psychosocial stress may be arising for reasons other than severe social disadvantage. We note that, while we used a similar cut-point to define the lower-to-higher SES group as other large prospective studies (26–31), 200% of the poverty threshold is still relatively disadvantaged (e.g., income of $53,000 to support a four-person household) (40). Nonetheless, the specific association between psychosocial stress and the inferior CB may reflect the fact that inferior CB connects the amygdala with the hippocampus (20, 21). The amygdala and hippocampus are rich with glucocorticoid receptors that are highly sensitive to stress, with downstream effects on the HPA axis and production of cortisol that, in turn, alter neural plasticity (21, 41).
Study strengths included the prospective study design beginning in utero, oversampling for mothers with low income, broad assessment of social disadvantage and psychosocial stress, high quality dMRI data, and a large sample compared with existing studies of prenatal exposure to early life adversity with ≤100 term-born infants (15–18). Study limitations include the use of a self-report questionnaire to assess maternal depression. The Edinburgh Postnatal Depression Scale (EPDS) is commonly used in clinical and research settings, but mothers may have underreported symptoms. In our sample of mothers of healthy, term-born infants, 10% of mothers reported clinically significant depression symptoms, which is similar to population estimates (42). However, rates of perinatal depression are as high as 47% in mothers of infants born preterm (43). Study findings may, therefore, not generalize to mothers with clinical depression/anxiety or mothers of infants born preterm.
In this study of healthy, term-born neonates with dMRI data collected at birth, prenatal exposure to social disadvantage, but not psychosocial stress, was independently associated with white matter microstructure at birth. However, among lower-to-higher SES infants, prenatal exposure to psychosocial stress was more strongly associated with altered microstructure in the inferior CB. Taken together, findings suggest that prenatal exposure to early life adversity across multiple socioeconomic and psychosocial domains is an important antecedent of aberrant fronto-limbic white matter fiber development at birth. Interventions that reduce social disadvantage and increase access to welfare programs that address psychosocial stressors for expectant parents may be an important target to protect fetal brain development.
Materials and Methods
Sample.
This study draws data (44) from a longitudinal study of infants (born 2017–2020) and their mothers (recruited 09/2017–02/2020) participating in the Early Life Adversity and Biological Embedding (eLABE) study (19). Pregnant women were identified from the March of Dimes Prematurity Research Center at Washington University in St. Louis. eLABE study exclusion criteria spanned multiple gestations, infections known to cause congenital disease (e.g., syphilis), and alcohol or drug use other than tobacco and cannabis. The eLABE study recruited 395 pregnant women (declined participation n = 268) and their 399 singleton infants (four mothers had two singleton births during recruitment). Women were oversampled from an antenatal clinic serving low income women to enrich the sample for exposure to social disadvantage.
Procedure.
Mothers completed surveys in each trimester and at delivery to assess social background, mental health, and life experiences. Medical data were collected from surveys and chart review. In the first weeks of life, 385 nonsedated neonates underwent an MRI scan on a Siemens Prisma 3T scanner with a 64-channel head coil (SI Appendix, Fig. S2). After feeding, neonates were swaddled, positioned in a head-stabilizing vacuum fix wrap, fitted with noise-protection gear, and placed in the head coil on foam padding to decrease motion. MRI scans were not performed for 14 neonates due to the COVID-19 pandemic (March 2020). dMRI data were obtained for 365/385 neonates (no diffusion tensor imaging sequence collected n = 3, required frames not collected n = 4, sequence collected in one direction n = 8, artifact n = 5). Seventy-six neonates were excluded from analyses due to preterm birth (<37 wk gestation n = 53), low birthweight (<2,000 grams n = 1), neonatal intensive care unit admission >7 d (n = 8), or high-grade brain injury (n = 14). Thus, 289 healthy, term-born neonates were included in analyses (PMA at scan m = 42 wk, SD = 1.3, range 38–45) (Table 1). Study procedures were approved by the Washington University Institutional Review Board. Written informed consent was obtained from all mothers.
Measures.
Social disadvantage.
Maternal education level and health insurance status were collected in trimester 1. Mothers reported household income and persons in the home to calculate INR (40) in each trimester. Home addresses were collected at delivery to obtain Area Deprivation Index percentiles (45) to assess neighborhood SES, housing quality, and access to necessities using census block data. Prior work in the eLABE cohort has shown that, while 26% of mothers changed addresses during pregnancy, there was no significant change in block group (46). Mothers completed the Healthy Eating Index-2015 (47) in the third trimester or at delivery to assess nutrition. Observed social disadvantage variables were all correlated (range −0.66–0.74, all P < 0.001; see SI Appendix, Table S16).
Psychosocial stress.
In each trimester, mothers completed the EPDS (48) and the Cohen Perceived Stress Scale (49) to assess depression symptoms and perceived stress, respectively. Stressful/traumatic life events (count and severity) were assessed with the Stress and Adversity Inventory for Adults (STRAIN) (50), collected at the neonatal MRI scan (n = 183) or at the 1-y follow-up (n = 86). There was no difference in STRAIN count (d = 0.05, P = 0.71) or severity (d = 0.07, P = 0.58) between mothers who completed the STRAIN at the neonatal MRI scan or at follow-up. The Everyday Discrimination Survey (51) measured racial discrimination, collected at the neonatal MRI scan. Observed psychosocial stress variables were all correlated (range 0.21–0.93, all P < 0.01; see SI Appendix, Table S17).
Latent factors.
As described in Luby et al. (19), observed variables were analyzed for all eLABE mothers using structural equation analysis, resulting in two latent factors: social disadvantage and psychosocial stress (SI Appendix, Fig. S3). The two-factor model provided a superior fit of the data compared with an alternative three-factor model, the latter of which had poor fit statistics and low variable loadings ((19) see also SI Appendix). Thus, the two-factor model was selected for the current analyses. We also opted to use latent factors because the goal of this study was to account for multiple aspects of social disadvantage and psychosocial stress, rather than to examine interactions between observed variables within a latent factor. Social disadvantage and psychosocial stress were positively correlated (r = 0.40, P < 0.001). This modest correlation suggested the two factors, while related, are dissociable constructs. Social disadvantage was similar for subjects excluded or included in analyses (d = 0.03, P = 0.76), but there was a difference in psychosocial stress (d = 0.36, P = 0.001).
White matter microstructure.
Neonatal dMRI scans were acquired as two 5-min runs using MB4, TR/TE = 2,500/79.4 ms, (1.75 mm)3 voxels, with whole brain coverage (80 slices), 108 b values sampled on three shells of b = 500–2,500 s/mm2, and seven b = 0 images interspersed throughout each run with phase encoding direction reversal (anterior → posterior and posterior → anterior) for susceptibility- and eddy-current distortion correction. White matter tracts were defined using FA and FSL’s RGB V1 (primary vector) images. Referencing the FA and V1 images, seeds were placed at start points, waypoints, and endpoints of each tract using standard anatomical landmarks in subject native space (SI Appendix, Fig. S4) by two highly trained raters (interrater reliability coefficients: 0.80–0.98 for MD and 0.73–0.92 for FA). Depending on length, size, visibility, and shape of the tract, each tract was constructed with a standard set of seeds (dorsal CB [four], inferior CB [two], fornix [one], uncinate [three], CC [one], and CST [three]) and exclusion masks placed if necessary. Probabilistic tractography was then performed using FSL Probtrackx (version 5.0.9) to extract dMRI parameters for white matter tracts (Fig. 1). The diffusion tensor model was completed using FSL’s dtifit, and the tensors were fitted using FSL’s bedpost, which allows for the modeling of two crossing fibers. Curvature thresholds were determined according to the shape, length, and proximity of the tract to other white matter pathways, with curvature thresholds ranging from 0.2 to 0.94 across tracts. If more than one waypoint mask was required, we forced waypoint crossing in listed order. Probtrackx output files were then thresholded (inferior CB [2%], dorsal CB [4%], uncinate [8%], fornix [15%], CC [2%], and CST [1%]) to retain streamlines with highest probability values indicating greater certainty of white matter. After probabilistic tractography was completed and dMRI parameters obtained, stringent quality control checks were performed by identifying any dMRI value >2 SD above/below the mean of the distribution and visually inspecting each Probtrackx output file. Manual intervention (e.g., seed placement, altering curvature threshold) was undertaken if the tract output image was not found to be representative of the FA and tensors on the V1 image. If the tract output image was found to be representative of the FA and tensors on the V1 image, manual intervention was not deemed necessary. As such, none of the 289 healthy, term-born subjects with dMRI data failed probabilistic tractography (SI Appendix, Fig. S2).
Data Analysis.
Continuous variables were examined for distributions and outliers. Distributions were found to approximate normal. Four MD values (one each for the left uncinate, right uncinate, right inferior CB, and right fornix) were extreme outliers (>3 SD) and removed from analysis. Curve estimation was used to explore fit of associations between latent factors and dMRI parameters, with linear models providing the best overall fit. Tolerance and variance inflation factor values were inspected for all independent variables and found to be within the acceptable range, suggesting no violations of multicollinearity. Variables were standardized prior to analysis. Social disadvantage and psychosocial stress were examined separately in relation to dMRI parameters (MD, RD, AD, and FA) using regression models adjusted for infant PMA at scan and sex (male = 1, female = 2). Next, social disadvantage and psychosocial stress were fitted together in hierarchical, multivariable linear regression models. Standardized coefficients (with 95% CIs), SEs, and R2 values are reported. In step 1 of the regression, infant PMA at scan and sex were entered as covariates. Social disadvantage and psychosocial stress were entered in step 2, with R2 change statistics reported. Bivariate and multivariable analyses were multiple comparison corrected using the Benjamini–Hochberg false discovery rate (FDR) procedure (eight corrections [No. of tracts] for each dMRI parameter). The FDR rate was set at 5%. Sensitivity analyses were undertaken by performing multivariable linear regression models in an age-restricted sample of infants (n = 223) scanned from 41 to 43 wk PMA age. Supplementary multivariable linear regression models were also performed using INR in place of social disadvantage. To examine the extent that severe socioeconomic hardship may exacerbate associations between psychosocial stress and aberrant white matter development, formal moderation analysis was used to test interactions between psychosocial stress and family SES groups (INR at 200%) on white matter connectivity. Supplementary regression models were used to examine MMR during pregnancy and prenatal drug (cannabis and tobacco) exposure as confounding factors (SI Appendix).
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Mental Health (R01 MH113883, K01 MH122735, T32 MH100019, and F30 HD104313), March of Dimes Prematurity Research Center at Washington University, Washington University Intellectual and Developmental Disability Research Center (P50 HD103525), Children’s Discovery Institute, McDonnell Center for Systems Neuroscience, and a NARSAD Young Investigator Grant (No. 28521) from the Brain and Behavior Research Foundation. We thank the families involved in this study.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. N.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2204135119/-/DCSupplemental.
Data, Materials, and Software Availability
Data were collected with consent forms that allow data sharing. Deidentified data (prenatal adversity factors, birth diffusion parameters, and covariate variables) have been deposited in Open Science Framework "Prenatal Exposure to Maternal Social Disadvantage and Psychosocial Stress and Neonatal White Matter Connectivity at Birth" (https://osf.io/znwux/) (44). Please contact authors for additional details.
References
- 1.Kolb B., Gibb R., Brain plasticity and behaviour in the developing brain. J. Can. Acad. Child Adolesc. Psychiatry 20, 265–276 (2011). [PMC free article] [PubMed] [Google Scholar]
- 2.Rakesh D., Whittle S., Socioeconomic status and the developing brain — A systematic review of neuroimaging findings in youth. Neurosci. Biobehav. Rev. 130, 379–407 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Dufford A. J., et al. , Prospective associations, longitudinal patterns of childhood socioeconomic status, and white matter organization in adulthood. Hum. Brain Mapp. 41, 3580–3593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufford A. J., Kim P., Family income, cumulative risk exposure, and white matter structure in middle childhood. Front. Hum. Neurosci. 11, 547 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson D. K., et al. , Early life predictors of brain development at term-equivalent age in infants born across the gestational age spectrum. Neuroimage 185, 813–824 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Back S. A., et al. , Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 21, 1302–1312 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois J., et al. , The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 276, 48–71 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Bennett G. A., Palliser H. K., Walker D., Hirst J., Severity and timing: How prenatal stress exposure affects glial developmental, emotional behavioural and plasma neurosteroid responses in guinea pig offspring. Psychoneuroendocrinology 70, 47–57 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Wei L., et al. , Early life stress perturbs key cellular programs in the developing mouse hippocampus. Dev. Neurosci. 37, 476–488 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang P., et al. , Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatr. Dis. Treat. 11, 597–607 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Back S. A., Gan X., Li Y., Rosenberg P. A., Volpe J. J., Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 18, 6241–6253 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French H. M., Reid M., Mamontov P., Simmons R. A., Grinspan J. B., Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 87, 3076–3087 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez M., et al. , The social ecology of childhood and early life adversity. Pediatr. Res. 89, 353–367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lautarescu A., et al. , Maternal prenatal stress is associated with altered uncinate fasciculus microstructure in premature neonates. Biol. Psychiatry 87, 559–569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rifkin-Graboi A., et al. , Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J. Am. Acad. Child Adolesc. Psychiatry 54, 313–21.e2 (2015). [DOI] [PubMed] [Google Scholar]
- 16.D. C. Dean, 3rd, et al. , Association of prenatal maternal depression and anxiety symptoms with infant white matter microstructure. JAMA Pediatr. 172, 973–981 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posner J., et al. , Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry 6, e935–e935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham R. M., et al. , Maternal anxiety and depression during late pregnancy and newborn brain white matter development. AJNR Am. J. Neuroradiol. 41, 1908–1915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luby J. L., et al. , Modeling prenatal adversity/advantage: Effects on birth weight. medRxiv [Preprint] (2021), https://www.medrxiv.org/content/10.1101/2021.12.16.21267938v1. Accessed 17 December 2021.
- 20.Bubb E. J., Metzler-Baddeley C., Aggleton J. P., The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 92, 104–127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen B. S., Nasca C., Gray J. D., Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41, 3–23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Der Heide R. J., Skipper L. M., Klobusicky E., Olson I. R., Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 136, 1692–1707 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner R. G., et al. , Microstructure of the dorsal anterior cingulum bundle in very preterm neonates predicts the preterm behavioral phenotype at 5 years of age. Biol. Psychiatry 89, 433–442 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff J. J., et al. ; IBIS Network, Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169, 589–600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luby J., et al. , The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatr. 167, 1135–1142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendheim-Thoman Center for Research on Child Wellbeing, Disadvantaged Children who are Beating the Odds: Family, School, Neighborhood and City Contexts that Predict Academic Success among Socio-economically Disadvantaged Children (Princeton University, 2019). [Google Scholar]
- 27.Gonzalez M. R., et al. , Positive economic, psychosocial, and physiological ecologies predict brain structure and cognitive performance in 9-10-year-old children. Front. Hum. Neurosci. 14, 578822 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hair N. L., Hanson J. L., Wolfe B. L., Pollak S. D., Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 169, 822–829 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halle T., et al. , Disparities in Early Learning and Development: Lessons from the Early Childhood Longitudinal Study - Birth Cohort (ECLS-B) (Child Trends, 2009). [Google Scholar]
- 30.Hanson J. L., et al. , Family poverty affects the rate of human infant brain growth. PLoS One 8, e80954 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendzor D. E., Caughy M. O., Owen M. T., Family income trajectory during childhood is associated with adiposity in adolescence: A latent class growth analysis. BMC Public Health 12, 611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bath K. G., Manzano-Nieves G., Goodwill H., Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 82, 64–71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callaghan B. L., Sullivan R. M., Howell B., Tottenham N., The international society for developmental psychobiology Sackler symposium: Early adversity and the maturation of emotion circuits--a cross-species analysis. Dev. Psychobiol. 56, 1635–1650 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teissier A., et al. , Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol. Psychiatry 25, 1159–1174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., et al. , Alterations of gray matter volume and white matter integrity in maternal deprivation monkeys. Neuroscience 384, 14–20 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Tottenham N., Sheridan M. A., A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 3, 68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagahawatte N. T., Goldenberg R. L., Poverty, maternal health, and adverse pregnancy outcomes. Ann. N. Y. Acad. Sci. 1136, 80–85 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Cai Z., Zhang J., The effect of prepregnancy body mass index on maternal micronutrient status: A meta-analysis. Sci. Rep. 11, 18100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gumusoglu S. B., Chilukuri A. S. S., Santillan D. A., Santillan M. K., Stevens H. E., Neurodevelopmental outcomes of prenatal preeclampsia exposure. Trends Neurosci. 43, 253–268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Office of the Assistant Secretary for Planning and Evaluation, Poverty Guidelines. U.S. Federal Poverty Guidelines Used to Determine Financial Eligibility for Certain Programs (2022) https://aspe.hhs.gov/topics/poverty-economic-mobility/poverty-guidelines. Accessed 16 February 2022.
- 41.Fenoglio K. A., Chen Y., Baram T. Z., Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J. Neurosci. 26, 2434–2442 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavin N. I., et al. , Perinatal depression: A systematic review of prevalence and incidence. Obstet. Gynecol. 106, 1071–1083 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Winter L., et al. , Depression, posttraumatic stress and relationship distress in parents of very preterm infants. Arch. Women Ment. Health 21, 445–451 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Lean R. E., Data from “Prenatal Exposure to maternal social disadvantage and psychosocial stress and neonatal white matter connectivity at birth”. Open Science Framework. https://osf.io/znwux/. Deposited 26 September 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kind A. J. H., Buckingham W. R., Making neighborhood-disadvantage metrics accessible - The neighborhood atlas. N. Engl. J. Med. 378, 2456–2458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brady R. G., et al. , The effects of prenatal exposure to neighborhood crime on neonatal functional connectivity. Biol. Psychiatry 92, 139–148 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs-Smith S. M., et al. , Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 118, 1591–1602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox J. L., Holden J. M., Sagovsky R., Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 150, 782–786 (1987). [DOI] [PubMed] [Google Scholar]
- 49.Cohen S., Kamarck T., Mermelstein R., A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396 (1983). [PubMed] [Google Scholar]
- 50.Slavich G. M., Shields G. S., Assessing lifetime stress exposure using the stress and adversity inventory for adults (Adult STRAIN): An overview and initial validation. Psychosom. Med. 80, 17–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams D. R., Yu Yan, Jackson J. S., Anderson N. B., Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J. Health Psychol. 2, 335–351 (1997). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were collected with consent forms that allow data sharing. Deidentified data (prenatal adversity factors, birth diffusion parameters, and covariate variables) have been deposited in Open Science Framework "Prenatal Exposure to Maternal Social Disadvantage and Psychosocial Stress and Neonatal White Matter Connectivity at Birth" (https://osf.io/znwux/) (44). Please contact authors for additional details.