Significance
Older adults are frequently targets of financial fraud. Therefore, understanding how cognitive changes across adulthood impact social decisions with economic stakes is critical. We investigated, in adults aged 18 to 92, how memory for previous interactions influences social decisions and how those decisions differ from analogous, nonsocial choices. While their memory deficits led to poorer decisions in both domains, older adults had biases that made their social decisions especially maladaptive: they tended to remember that others were generous, they interacted with familiar others regardless of what they remembered about them, and they chose partners who appeared generous, even when they were not. Thus, older adults may make financially costly social decisions when those decisions depend on recalling specific past experiences.
Keywords: aging, episodic memory, social, decision-making, trust
Abstract
Older adults are frequent targets and victims of financial fraud. They may be especially susceptible to revictimization because of age-related changes in both episodic memory and social motivation. Here we examined these factors in a context where adaptive social decision-making requires intact associative memory for previous social interactions. Older adults made more maladaptive episodic memory-guided social decisions but not only because of poorer associative memory. Older adults were biased toward remembering people as being fair, while young adults were biased toward remembering people as being unfair. Holding memory constant, older adults engaged more with people that were familiar (regardless of the nature of the previous interaction), whereas young adults were prone to avoiding others that they remembered as being unfair. Finally, older adults were more influenced by facial appearances, choosing to interact with social partners that looked more generous, even though those perceptions were inconsistent with prior experience.
Older adults make many economic decisions, control substantial wealth, and are often targets of financial fraud. About 5% of cognitively unimpaired older adults are victims of financial fraud each year, a figure that is likely an underestimate (1, 2). Therefore, understanding how aging impacts economic decisions involving social partners is of critical importance. Many such decisions require people to retrieve details about specific previous episodes using episodic memory. For example, if you lose money to a scammer once, and that scammer contacts you again, you must remember the previous interaction to successfully avoid the scammer again. Aging might impact episodic memory-guided social decision-making in a few ways. Older adults display episodic memory deficits (3, 4) and impaired value-based decision-making (5–7). In addition, aging is associated with changes in social motivation (8–10), with older adults being relatively more prosocial and trusting (11–13). These factors might operate independently or interact with each other to yield financially costly decisions. The goal of the current study was to examine how age-related changes in episodic memory and social motivation impact episodic memory-guided social decision-making.
Most research on memory-guided decision-making has focused on how repeated experiences with actions and associated outcomes shape decisions (14). However, people often make decisions based on episodic memory for single previous experiences. A previous study of episodic memory-guided decision-making in young adults showed that associative memory—that is, memory for an association between a stimulus and its value—was necessary to support adaptive approach and avoidance decisions (15). In that study, participants viewed trial-unique images of houses (representing lotteries) along with their payouts. They later made decisions about whether to approach or avoid those stimuli. When participants correctly recalled the value of the stimulus, they made the financially advantageous choice to approach high-value stimuli and avoid low-value stimuli. When they only had recognition memory for an item, however, they were unable to discriminate between high- and low-value stimuli in their decisions. The same pattern was observed for social decisions (15, 16). Participants learned how much other people shared with an anonymous other out of $10 (in a dictator game). They later approached social partners that they remembered to have been more generous and avoided those who shared less. Thus, associative memory is necessary for adaptive decisions in both the social and nonsocial domains. However, social and nonsocial decisions differed in one sense: associative memory was better for unfair dictator game splits compared to low-value houses, which led to greater avoidance of unfair others. In other words, young adults were especially sensitive to unfairness, a finding consistent with other research showing that young adults have a negativity bias in the social domain (17, 18).
Aging might affect behavior in this paradigm in a few ways. First, older adults have well-documented deficits in associative memory (3, 4, 19). We therefore expect older adults to show a deficit in episodic memory-guided decision-making to the extent that their associative memory is worse than that of young adults. Second, since aging is associated with changes in social motivation (8, 10, 20), the social negativity bias observed in young adults may not extend to older adults. According to socioemotional selectivity theory (10, 21, 22), the limited time horizon faced by older adults leads them to prioritize the maintenance of social relationships over other goals, such as knowledge acquisition and financial preparedness. Young adults might therefore be more protective of their financial resources even if it means being less prosocial, whereas older adults might be more interested in cultivating and preserving social relationships than in increasing their financial resources. Thus, in line with the predictions of socioemotional selectivity theory, we expect that older adults will be less sensitive to fairness violations than young adults are and will instead make similar avoidance decisions in the social and nonsocial domains. They may even seek out interactions with familiar others, irrespective of the financial consequences of doing so.
Finally, if older adults are less able to use episodic memory to make social decisions, they may turn to a more automatic, heuristic strategy, such as evaluating a social partner based on their physical appearance. People form impressions of others based on their facial appearance, with some facial features being systematically associated with perceptions of trustworthiness and other traits (23–25). Appearance can then affect approach behavior (26) and memory (27, 28). It is unknown, however, whether appearance still influences social decisions when the decision-maker has had a previous interaction with that partner. We expected that older adults might rely on facial appearances more in their decisions, consistent with their inability to inhibit automatic processes and irrelevant information (29–31) and with their increased reliance on stereotypes in other contexts (32–34).
Here we recruited adults aged 18 to 92 to perform an episodic memory-guided decision task with social and nonsocial conditions (15). We hypothesized that older adults would make less adaptive decisions in both social and nonsocial contexts because of their worse associative memory. However, we also expected that older adults’ social decisions would be even more impaired than their nonsocial decisions due to their inclination to prioritize social relationships over monetary gains, as well as their increased reliance on facial appearances when making these decisions.
Results
Participant Characteristics.
Two hundred and ten participants from three age groups—young (ages 18 to 34; n = 76), middle-aged (ages 35 to 59; n = 70), and older adults (ages 60+; n = 64; Table 1)—were included in the final sample. Older adults were recruited primarily from the Clinical Core of Penn’s Alzheimer’s Disease Research Center (ADRC; n = 46). These participants are part of a longitudinal cohort that undergoes neuropsychological assessments and consensus conference designation to assess their cognitive status annually; all participants from this cohort were deemed cognitively normal. Additional participants were recruited through advertisements on campus (n = 111) or Facebook (n = 53). We found no differences in memory performance between the older adults from the ADRC cohort and those recruited separately (SI Appendix). We collected the Wechsler Test of Adult Reading (WTAR) from all participants to ensure that age groups were matched on intelligence [F(2,118) = 0.16; P = 0.854]. Age groups were also matched on gender composition (χ2 = 0.32; P = 0.854; n = 208).
Table 1.
Characteristics of participant groups
| Characteristic | Age group | ||
|---|---|---|---|
| Young | Middle-aged | Older | |
| Total N (in-laboratory n, remote n) | 76 (38 in-laboratory, 38 remote) | 70 (35 in-laboratory, 35 remote) | 64 (31 in-laboratory, 33 remote) |
| Mean age (SD, range) | 22.3 (3.58, 18–34) | 44.7 (7.08, 35–59) | 71.7 (7.23, 60–92) |
| Gender | 53 F, 22 M, 1 not reported | 46 F, 23 M, 1 not reported | 43 F, 21 M |
| Mean WTAR (SD, range) | 43.5 (4.47, 29–50)* | 42.1 (7.51, 17–50) | 42.8 (7.44, 19–50)† |
*n = 1 missing data.
†n = 4 missing data.
About half the participants completed the experiment in person, and about half completed it remotely while on a video call with an experimenter. When examining general item and associative memory performance, we found no differences between participants who did the task remotely and those who did it in the laboratory (SI Appendix), so we collapsed across testing modalities in our analyses.
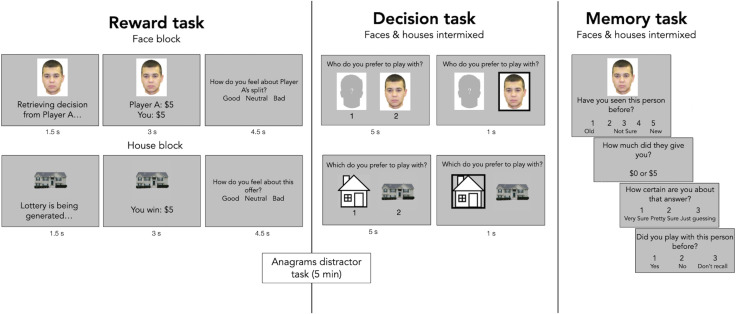
Participants first learned associations between stimuli and reward outcomes ($5 or $0). In one block of this reward phase, they saw faces of people who had previously come into the laboratory. These previous participants were asked how much of $10 they would share with an anonymous other person (i.e., they played a dictator game as proposer). We consider photos of those who split the $10 evenly as high-value stimuli (since they gave $5 to the participant) and those who kept all the money for themselves as low-value (since they gave $0). In another block of the reward phase (order counterbalanced), participants viewed photos of houses that were randomly paired with either $0 (low value) or $5 (high value) in a lottery. Following the reward phase, participants did a 5-min anagrams distractor task. Then they proceeded to the decision task, in which they decided whether to interact with each of the faces and houses again or instead with a face or house chosen at random. Finally, they did a memory task, in which item and associative memory, as well as confidence in associative memory, were assessed (Materials and Methods and Fig. 1).
Fig. 1.
Task layout. The reward task involved learning about 32 faces and 32 houses in blocks (counterbalanced order), with half of the faces and houses resulting in $5 rewards (high-value stimuli) and half resulting in $0 (low-value stimuli). Then, after a 5-min anagrams task (included to create a delay between study and test), participants saw 96 decision trials in the decision task, with face trials and house trials intermixed. All stimuli from the reward task were shown again, along with 32 novel stimuli (16 faces and 16 houses). In face trials, participants had 5 s to decide if they wanted to interact with the face again, knowing they would have to accept the offer proposed by the person pictured. In house trials, subjects decided whether they wanted to play the lottery associated with the house again. Then a bracket appeared for 1 s around the option that they chose. Finally, in the self-paced memory task, participants saw intermixed face and house trials, in which they judged whether they had seen the stimuli before on a scale from definitely old (1) to definitely new (5). If they selected 1, 2, or 3, they then saw three additional questions probing the value of the stimulus, the confidence in that value, and whether they remembered playing with that stimulus in the decision phase. All stimuli from the reward task were shown in the memory task, along with 32 novel stimuli. See Materials and Methods for full details.
In the reward phase, participants reported feeling better about high-value outcomes compared to low-value outcomes, and this difference was more pronounced in the social domain. These ratings did not, however, differ by age (SI Appendix).
Age Is Associated with Worse Associative Memory and a Response Bias in Associative Memory for Social Stimuli.
We took a signal detection approach to analyzing memory data, obtaining measures of d′ for item memory (i.e., discriminability between old and new stimuli) and for associative memory (i.e., among item hits, the ability to accurately categorize stimuli as high value or low value). As in previous research (15), we excluded responses of 3 (“not sure”) for the item memory question, counted responses of 1 and 2 as “old,” and counted responses of 4 and 5 as “new.” We also obtained measures of response bias for item and associative memory; higher response biases for item memory indicate a tendency to label items as “new,” and higher response biases for associative memory indicate a tendency to label items as low value (Materials and Methods).
Age was associated with both discriminability and response bias in item memory. In all age groups, for both faces and houses, item memory was above chance (SI Appendix). Age was negatively associated with item memory (d′) for both faces (r = −0.42; P < 0.001) and houses (r = −0.32, P < 0.001), such that older adults were worse at discriminating between old and new stimuli. Age was also negatively associated with response bias for both faces (r = −0.34; P < 0.001) and houses (r = −0.29; P < 0.001), with older adults being more likely to endorse having seen an item earlier.
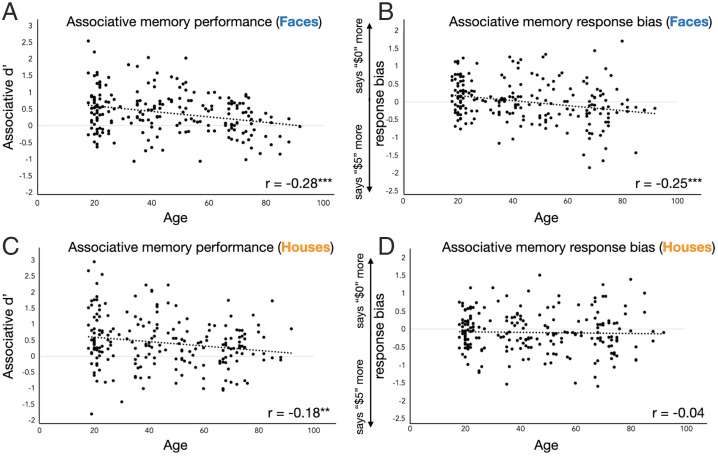
Age was also associated with both discriminability and response bias in associative memory. Associative memory was above chance in all age groups (SI Appendix). Age was negatively associated with associative memory performance, with older adults being less likely to correctly categorize previously seen items as being high ($5) or low ($0) value (faces, r = −0.28; P < 0.001; houses, r = −0.18; P = 0.008; Fig. 2 A and C). Finally, age was associated with associative memory response bias in the social domain (r = −0.25; P < 0.001) but not in the nonsocial domain (r = −0.04; P = 0.609), with these correlation coefficients being significantly different from each other (Fisher’s r-to-z, z = 2.19; P = 0.014; Fig. 2 B and D). When examining these biases in each age group, young adults showed a significant response bias toward saying that faces shared $0 (mean associative memory response bias = 0.15; SD = 0.49; t75 = 2.72; P = 0.008), and older adults displayed a significant response bias toward saying that faces shared $5 (M = −0.22; SD = 0.65; t63 = −2.76; P = 0.007). Middle-aged adults did not show a significant response bias (SI Appendix). Therefore, whereas young adults tended to report that a social partner acted unfairly, older adults were more likely to report that they split the money equitably.
Fig. 2.
Relationships between associative memory measures and age for (A and B) face stimuli and (C and D) house stimuli. Age was negatively associated with associative memory performance, such that older adults were less accurate in remembering whether stimuli were high value (worth $5) or low value (worth $0). Age was also negatively associated with response bias in the social domain, such that older adults were more likely to report that the people they had learned about shared $5, while young adults were more likely to report that people shared $0. Dotted lines are linear best fit lines. **P < 0.01, ***P < 0.001.
Adaptive Decision-Making, Which Declines with Age, Depends on Intact Associative Memory.
Next, we sought to replicate previous findings that adaptive decision-making based on single previous episodes depends on associative memory. This analysis was restricted to trials that were seen in the reward phase and correctly recognized. Trials were categorized into memory levels based on responses in the memory task. If associative memory was correct (participant said the stimulus was worth $0 when it was worth $0, and $5 when it was worth $5) and the subject was confident in that response (i.e., answered “pretty sure” or “very sure” regarding confidence), then this constituted “confident correct associative memory.” If associative memory was incorrect and the subject was confident in that answer, then this item was labeled as “confident incorrect associative memory.” If the participant said they were guessing about the associative memory question, regardless of if they got the answer right, that trial was labeled as “item memory only.” We ran a repeated-measures analysis of covariance (ANCOVA) with memory level (confident incorrect associative memory/item memory only/confident correct associative memory) and value ($0/$5) as within-subjects factors and choice (proportion of times that the old stimulus was chosen over the schematic image) as the dependent variable. Age was entered as a covariate.
We found that adaptive decision-making relied on associative memory. There was a significant main effect of value (F(1,163) = 20.39; P < 0.001; η2p = 0.111), such that people were more likely to select the stimulus when it was associated with $5 than when it was associated with $0 (t163 = −2.49; ptukey = 0.014). The main effect of memory level was not significant [F(2,326) = 0.11; P = 0.900; η2p = 0.001]. Critically, however, there was a significant value × memory level interaction [F(2,326) = 142.11; P < 0.001; η2p = 0.466; Fig. 3]. When participants recognized stimuli and correctly and confidently recalled their value, they were more likely to approach $5 stimuli than $0 stimuli (t163 = 25.42; ptukey < 0.001). In contrast, when they were confidently incorrect about the association, they were more likely to approach $0 stimuli than $5 stimuli (t163 = 19.35; ptukey < 0.001). Finally, when participants had item memory only, they did not show a significant difference between choices about $0 and $5 stimuli (t163 = 1.53; ptukey = 0.644). This finding corroborates previous research (15, 16, 35) showing that associative memory is necessary to make adaptive decisions about stimuli that were previously paired with values. Item memory alone led to an inability to differentiate between high-value and low-value stimuli in decision-making, and having a false memory for an association resulted in choosing low-value stimuli more than high-value stimuli. For full ANCOVA results, including interactions with age, see SI Appendix, Supplementary Text and SI Appendix, Fig. S1.
Fig. 3.
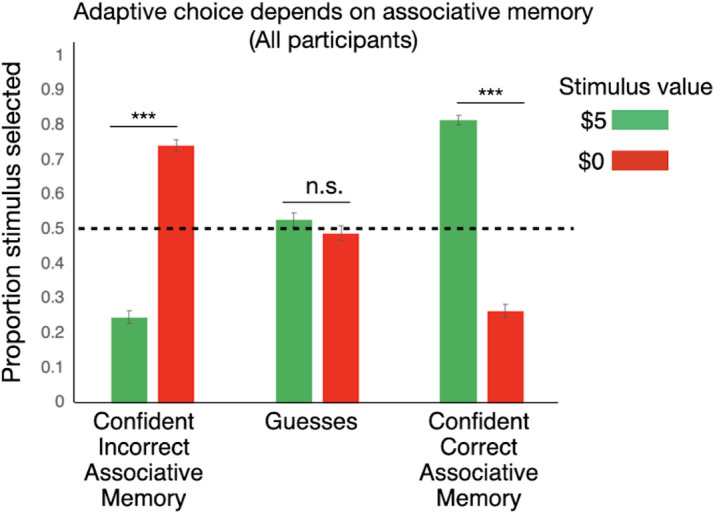
Adaptive decision-making depends on associative memory. When participants (n = 165 with full data in each bin) had intact item memory but were guessing about the value of the stimulus, there was no difference in their decision-making about stimuli that were actually worth $5 or $0. When they were confident and correct about the associated values, they made adaptive choices, significantly approaching high-value stimuli and avoiding low-value stimuli. They made maladaptive choices when they were confident and incorrect about the associated values (item hits only pictured, data collapsed across social and nonsocial trials, dotted line indicates chance levels of choosing stimulus over schematic image). Estimated marginal means are plotted, and error bars represent SE. ***p < 0.001; n.s. = p > 0.05.
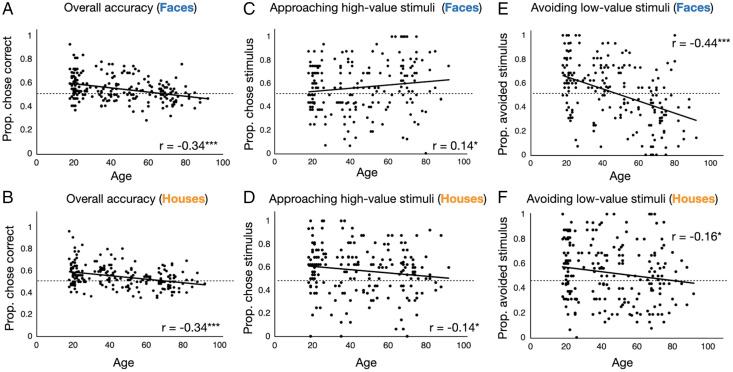
Given that aging was associated with worse associative memory and associative memory is needed for adaptive decision-making, it is perhaps unsurprising that aging was associated with less adaptive decision-making. We considered a decision to be adaptive if participants approached a high-value stimulus or avoided a low-value stimulus and maladaptive otherwise. We calculated the proportion of times that participants made the adaptive choice and found that age was negatively associated with that proportion in both the social (r = −0.34; P < 0.001) and nonsocial (r = −0.34; P < 0.001) domains (Fig. 4 A and B). When comparing the proportion of times that the correct option was chosen to chance levels (0.5), young adults performed above chance for both face trials (mean = 0.59; SD = 0.11; t75 = 7.06; P < 0.001) and house trials (mean = 0.59; SD = 0.11; t75 = 6.68; P < 0.001). Middle-aged adults also performed above chance (faces mean = 0.55; SD = 0.11; t69 = 3.81; P < 0.001; houses mean = 0.55; SD = 0.10; t69 = 4.78; P < 0.001). The performance of older adults, however, was indistinguishable from chance for both categories of stimuli (faces mean = 0.50; SD = 0.08; t63 = 0.37; P = 0.714; houses mean = 0.51; SD = 0.08; t63 = 0.88; P = 0.382).
Fig. 4.
Aging is associated with less adaptive episodic memory-based decision-making in both the social (A) and nonsocial (B) domains. A correct or adaptive choice is one in which participants approached a high-value ($5) stimulus or avoided a low-value ($0) stimulus. The proportion is out of all trials that featured previously seen stimuli (from the reward task). When separating adaptive decisions into two subtypes—approaching high-value stimuli (C and D) and avoiding low-value stimuli (E and F)—age was positively associated with successfully approaching high-value stimuli in the social domain (C) but negatively associated with adaptive approach behavior in the nonsocial domain (D). Age was negatively associated with adaptive avoidance behavior in both the social (E) and nonsocial domains (F), although the effect is stronger for social stimuli. The dashed line corresponds to chance level (0.5), and the solid lines are linear best-fit lines. ***P < 0.001, *P < 0.05.
Although older adults made less adaptive decisions in both domains, the nature of their errors differed between the social and nonsocial domains. Given that older adults were biased to remember others as fair (an associative memory response bias that was not present in the nonsocial domain), we examined decisions to approach high-value and avoid low-value stimuli separately, in both domains. On nonsocial trials, older adults approached high-value stimuli less (r = −0.14; P = 0.037; Fig. 4D) and avoided low-value stimuli less (r = −0.16; P = 0.019; Fig. 4F). In contrast, older adults were better than young adults at approaching generous others (r = 0.14; P = 0.042; Fig. 4C), but they were worse at avoiding unfair others (r = −0.44; P < 0.001; Fig. 4E). Thus, in the nonsocial domain, older adults showed similar impairments when deciding about high-value and low-value stimuli, but in the social domain, they specifically failed to avoid unfair others.
Unlike Young Adults, Older Adults Do Not Avoid Social Partners They Remember as Being Unfair.
These age-related changes in episodic memory-guided decision-making reflect older adults’ worse associative memory accuracy (across domains) and their associative memory response bias (in the social domain only). Next, we examined if maladaptive decision-making in older adults was driven solely by associative memory changes or if they made decisions that were maladaptive given the stimulus-value associations that they retrieved. At the same time, we investigated whether this pattern of decision-making differed if the stimuli were social vs. nonsocial. For this analysis, we excluded item memory only trials (i.e., associative memory guesses) and relabeled each trial according to whether the value of the stimulus on that trial was confidently remembered as being high value (“Remembered as $5”) or confidently remembered as being low value (“Remembered as $0”). Note that not all of these memories were accurate, but we were interested in whether subjects would act in accordance with what they believed to be the value of the stimulus. We ran a repeated-measures ANCOVA with choice as the dependent variable, remembered value (remembered as $5 vs. $0) and stimulus type (face vs. house) as within-subjects factors, and age as a covariate. Stimuli remembered as high value were chosen at a higher rate than stimuli remembered as low value [main effect of remembered value: F(1,174) = 245.80; P < 0.001; η2p = 0.586; t174 = 26.3; ptukey < 0.001], but there were no differences in behavior toward faces and houses across the whole sample (see SI Appendix for full ANCOVA results).
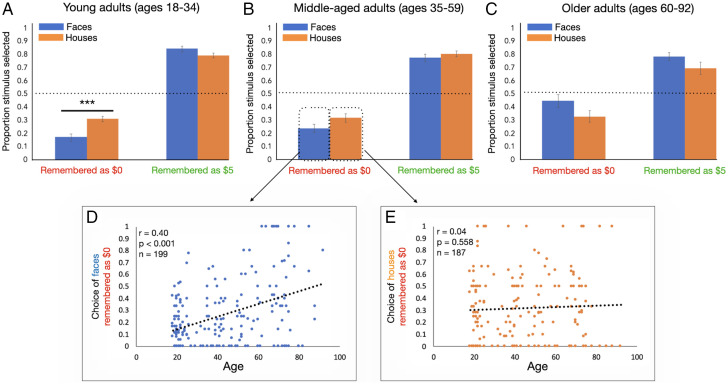
Participants did show differences in their behavior between social and nonsocial domains in a manner that was modulated by age, however (Fig. 5). First, there was a significant interaction between stimulus type (face vs. house) and age [F(1,174) = 8.69; P = 0.004; η2p = 0.048], such that older adults were more likely to approach familiar faces (regardless of retrieved value). Second, there was an interaction between stimulus type and remembered value [F(1,174) = 16.20; P < 0.001; η2p = 0.085] that was significantly modulated by age [F(1,174) = 10.17; P = 0.002; η2p = 0.055]. To interpret this three-way interaction, we ran post hoc Pearson correlations, relating age to choices about stimuli in each of the following bins: 1) faces remembered as having shared $5, 2) faces remembered as having shared $0, 3) houses remembered as worth $5, and 4) houses remembered as worth $0. There was a significant correlation between age and choice of faces remembered as being unfair (r = 0.40; P < 0.001; n = 199; Fig. 5D), but this did not extend to choices about low-value houses (r = 0.04; P = 0.558; n = 187; Fig. 5E and SI Appendix). The difference between these correlation coefficients was significant (Fisher’s r-to-z, z = 3.72; P < 0.001). Therefore, older adults were more likely than young adults to choose to interact with social others that they were confident had acted unfairly toward them.
Fig. 5.
(A) Young (n = 71), but not (B) middle-aged (n = 62) or (C) older (n = 43), adults are biased toward avoiding low-value social stimuli more than nonsocial stimuli. When examining only trials that were confidently endorsed as being worth $5 or $0 (regardless of associative memory accuracy), participants were more likely to approach stimuli remembered as high value and avoid stimuli remembered as low value. Estimated marginal means are plotted, and error bars represent SE. (D) The tendency to approach faces that were remembered as being unfair was associated with age. This was driven by young adults being especially prone to avoiding faces they remembered as being unfair, while older adults chose to interact with faces more, even if the retrieved value was low. (E) This association with age did not extend to nonsocial stimuli. ***P < 0.001.
To examine the trajectory of these age-related changes, we ran the same repeated-measures ANOVA separately in each age group (Fig. 5 A–C), removing the age covariate. Young adults were more likely to avoid unfair others compared to low-value house stimuli [remembered value × stimulus type interaction, F(1,70) = 21.37; P < 0.001; η2p = 0.234; t70 = −4.11; ptukey < 0.001], but there was no difference in their behavior toward faces and houses that they remembered as being high value (t70 = 1.98; ptukey = 0.206). There was a trend-level main effect of stimulus type [F(1,70) = 3.58; P = 0.063; η2p = 0.049], such that young adults tended to avoid faces more than houses overall (t70 = −1.89; ptukey = 0.063).
The middle-aged group, on the other hand, did not show a bias toward avoiding others remembered as unfair compared to low-value houses [remembered value × stimulus type interaction, F(1,61) = 0.97; P = 0.329; η2p = 0.016; $0 face vs. $0 house, t61 = −2.00; ptukey = 0.201; $5 face vs. $5 house, t61 = −0.97; ptukey = 0.766]. Therefore, young adults’ sensitivity toward social violations may not endure into middle age. Like the young adults, however, middle-aged adults tended to avoid faces more than houses in general [main effect of stimulus type, F(1,61) = 4.88; P = 0.031; η2p = 0.074; faces vs. houses, t61 = −2.21; ptukey = 0.031].
Finally, older adults approached low-value faces and houses similarly (t42 = 1.86; ptukey = 0.261) and high-value faces and houses similarly (t42 = 1.64; ptukey = 0.368; remembered value × stimulus type interaction, F1,42 = 0.13; P = 0.723; η2p = 0.003). However, older adults showed a main effect of stimulus type (F1,42 = 6.65; P = 0.013; η2p = 0.137), such that they approached faces more than houses (t42 = 2.58; ptukey = 0.013). Thus, older adults (but not young or middle-aged adults) approached social others more than nonsocial stimuli regardless of value, suggesting a general affinity for social interaction.
Older Adults Rely More on Facial Features Perceived as Trustworthy to Make Decisions.
So far, we have shown that older adults make maladaptive episodic memory-guided social decisions. This effect is driven not only by their worse associative memory and their prosocial associative memory response bias but also by their tendency to approach social partners even if they remember those partners as having been unfair. If older adults are not using episodic memory to guide their social choices, then how are they making these decisions? Next, we examined if the facial appearances of the social stimuli influenced participants’ choices. Face stimuli were rated by a separate sample (Materials and Methods) on perceived warmth, competence, trustworthiness, attractiveness, and dominance. Raters were also asked to guess how much of $10 the person pictured would share in a dictator game (perceived generosity, which was correlated with competence, warmth, and trustworthiness; SI Appendix).
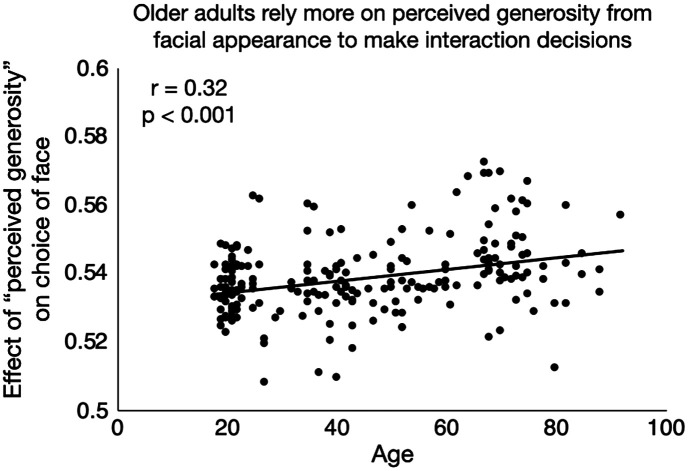
Older adults’ decisions relied more on these perceptions of others based on their facial features. The average rating for each of these questions was obtained for each of the face stimuli from the reward phase. These ratings were then entered into a series of mixed-effects logistic regressions with value ($5/$0), associative memory (correct/incorrect), and the value × associative memory interaction terms as covariates of no interest (Materials and Methods). Therefore, we controlled for normative influences on choice—value and the extent to which it could be recalled—to see if there was an effect of facial appearance above and beyond these influences. Perceived warmth, competence, trustworthiness, attractiveness, and generosity were all associated with an increased likelihood of interacting with the social partner in the decision phase (Table 2). Aging was associated with an increased reliance on all these features, however, such that older adults were more likely to select others who were rated as more trustworthy, warm, attractive, competent, and generous (Fig. 6). This association with age was not just a by-product of older adults’ worse associative memory, since the analysis controlled for memory effects, and there was no association between associative memory performance and the random-effects slopes on these facial attributes, even after controlling for age (SI Appendix). Thus, irrespective of their associative memory deficits, older adults chose to interact more with generous-looking partners.
Table 2.
Facial appearance attributes of stimuli predict interaction choices
| Face stimulus rating | Main effect on choice of stimulus (β) | 95% CI | Correlation between age and subject-specific random slope |
|---|---|---|---|
| Warmth | 0.384*** | 0.316–0.452 | r = 0.33*** |
| Trustworthiness | 0.498*** | 0.408–0.589 | r = 0.31*** |
| Competence | 0.384*** | 0.284–0.485 | r = 0.32*** |
| Dominance | −0.065 | −0.135–0.004 | r = 0.27*** |
| Attractiveness | 0.168*** | 0.115–0.221 | r = 0.26*** |
| Perceived generosity | 0.539*** | 0.437–0.641 | r = 0.32*** |
***P < 0.001.
Fig. 6.
Age is associated with a reliance on perceived generosity of the face stimulus (based on facial appearance alone) in decision-making. In a mixed-effects logistic regression predicting whether the participant chose to interact with a previously seen face in the decision phase, independent ratings of perceived generosity of that face predicted participants’ choices to interact with those faces. The effects of value (i.e., whether that face gave $5 or $0 in the reward phase) and associative memory (i.e., whether the value of the face was accurately remembered), as well as the interaction between value and associative memory, were controlled for in the regression. Older adults relied on perceived generosity more in their decisions: the random slope for the effect of perceived generosity on choice was estimated separately for each subject, and this slope was positively associated with age. In the scatterplot, the y axis shows the sum of the overall fixed effect and subject-specific random effect of perceived generosity on choice. Although only perceived generosity is depicted here, other facial appearance ratings (warmth, trustworthiness, competence, and attractiveness) were also predictive of choice, and their influence on choice was also associated with age (Table 2).
In an exploratory analysis (see SI Appendix for details), we investigated the effects of the age, gender (male or female), and race (Black, White, or Asian) of the face stimuli on decisions about whether to reengage with them. After controlling for normative influences on choice, participants were more likely to choose social partners that were younger (β = −0.020; P = 0.001), female (β = 0.448; P < 0.001), and Black (relative to White, β = 0.331; P < 0.001; no effect of Asian race relative to White, β = 0.061; P = 0.487). Although there were some relationships between these effects and participant age, they were weaker than the effects of the facial attribute ratings (all rs < 0.2) and would not hold when correcting for multiple comparisons (see SI Appendix for full results).
Post Hoc: Maladaptive Social Avoidance Decisions Are Driven Primarily by Prosocial Memory and Decision-Making Biases.
We found that the age-related decline in memory-guided social decision-making may be driven by a number of factors, including item and associative memory deficits, a bias toward remembering others as fair, an inclination toward approaching partners regardless of fairness, and an overreliance on perceptions of trustworthiness. In a post hoc multiple regression analysis, we tested which of these variables had the strongest influence on failures to avoid untrustworthy social partners. We found that prosocial motivations—both the propensity to recall that people were fair and the tendency to approach partners regardless of fairness—had the most significant impact on avoidance decisions (see SI Appendix for details).
Discussion
Here we examined age-related changes in social decision-making when those decisions rely on episodic memory for previous interactions. As expected, older adults had worse associative memory and displayed less adaptive decision-making in both social and nonsocial domains. However, in the social domain, older adults’ decision-making deficits were specific to failing to avoid people who were previously unfair. We found three age-related changes that each contributed to these maladaptive social decisions. First, older adults showed a bias toward remembering people as being fair, while young adults showed a bias toward remembering people as being unfair. Second, holding memory constant, older adults were more likely to engage with people they recognized—even if they remembered that the previous interaction was unfair—while young adults were especially avoidant of others they remembered as being unfair. Finally, older adults were more influenced by appearances, choosing to interact with others that are perceived as more generous, even though those perceptions did not accord with past experience.
Whether decisions were about social partners (represented by faces) or lotteries (represented by houses), memory for the association between an item and its value was necessary for adaptive approach/avoid decision-making, replicating previous research (15, 16, 35). Indeed, false associative memories—confident recollections that stimuli were worth the opposite of what they were actually worth—led to choices that were consistent with those memories but actually maladaptive. Aging was associated with worse associative memory, in line with the wealth of evidence for associative memory decline in older adults (3, 4, 36, 37). The ability to make adaptive episodic memory-based choices was thus impaired in older adults and was not even above chance, whether the stimuli were social or nonsocial.
When decoupling associative memory discriminability (d′) from response bias, we found that aging was associated with response bias in the social domain but not the nonsocial domain: older adults were more likely to remember social partners as being fair, while young adults were more likely to remember social partners as being unfair. Previous research with this paradigm found that young adults had better associative memory for unfair social others compared to low-value nonsocial stimuli (15). That study did not disentangle discriminability from response bias, however, so it is unclear whether a bias toward reporting that people were unfair was also present in the young adults in that study. These age-related differences in response bias can partially explain why older adults specifically failed to avoid unfair others; a bias toward remembering others as generous results in increased social approach behavior, even when it is inappropriate.
Even after accounting for differences in participants’ memories, however, age was positively associated with the tendency to approach social partners that were remembered as being unfair. Age group–specific follow-up analyses showed that older adults were more likely to approach social partners, regardless of their retrieved values, while young adults avoided unfair social partners more than low-value nonsocial stimuli. This finding is in line with the predictions of socioemotional selectivity theory (8, 10, 21, 22), which proposes that as people age, they focus more on preserving social relationships, rather than protecting financial resources. The older adults might have been choosing to interact with people who shared money inequitably because they cared more about engaging in a social interaction with a familiar person than about earning money for themselves. This finding also dovetails with previous research on age differences in trust (11). This literature has shown that older adults are more willing to trust others, but this age effect is strongest when older adults are faced with negative indicators of trust (i.e., unfair behavior) (11, 38). In line with this idea, we found that older adults were less likely to avoid others that they remembered as being unfair, but age was unrelated to the tendency to approach others that were remembered as being fair. Indeed, a post hoc multiple regression analysis suggested that prosocial biases in both memory and decision-making, which were more pronounced in older adults, explained poor social avoidance decisions even better than memory deficits did. Thus, interventions to improve social decision-making in older adults might target their disposition toward giving others the benefit of the doubt, both in their recollections and in their behavior.
Above and beyond the effects of associative memory, participants chose to interact more with people perceived as more trustworthy, and the extent to which they did so was modulated by age. Considering that the ratings of perceived generosity, trustworthiness, warmth, competence, and attractiveness were made by an independent group of subjects, this finding speaks to the consistency with which people judge trustworthiness based on facial features (24, 26). Older adults were more likely to use those spontaneous first impressions to make their choices. This is in line with research showing that older adults use schemas more (39–41) and mentalize less (42) when making social judgments, and they are less able to inhibit preexisting stereotypes and automatic processes (29–33). This result also corroborates previous research (11, 34) showing that older adults are more trusting of others both when trust cues are reliable (e.g., past behavior) and when they are unreliable (e.g., facial appearance). Importantly, this tendency to rely on first impressions was maladaptive from an economic standpoint since, consistent with past research (43), there was no relationship between how much a person was perceived as having shared and how much they actually shared.
Our study has a few notable strengths. First, we examined age-related changes continuously rather than using an “extreme group” design. Including middle-aged adults in our sample gave us a glimpse into the trajectory of age-related changes. For example, middle-aged adults did not show a significant negativity bias in either their memories or their decision-making about social others, suggesting that increased sensitivity to social violations in young adults may not even persist into middle age. Another strength of our study is that we obtained the sample size of an online study (200+) without sacrificing the rigor of an in-person study since even those participants who did the task remotely were monitored and instructed just as they would have been in the laboratory. Finally, the majority of our older adult participants were recruited from a longitudinal cohort at Penn’s ADRC, so we could ensure that they were cognitively unimpaired. We acknowledge that one limitation of our design is that the social stimuli were all pictures of young adults (ages 18 to 36). Restricting the stimulus age range was necessary since the images were of real people who had previously come into the laboratory, and they were mostly young. It will be important to replicate our findings with more age-diverse social partners. However, since we used a continuous age design and the majority of our participants were older than the people they were making decisions about, we think it is unlikely that our results are driven by the age composition of the stimulus set.
In sum, we found that older adults make less adaptive social decisions when they have to rely on associative episodic memories to make them. This decision-making deficit is driven both by memory decline—which affects both social and nonsocial choices—and by a tendency to approach familiar others even if they were previously unfair. While young adults showed a bias both toward reporting that people acted unfairly and toward avoiding interactions with those individuals, older adults showed a bias toward reporting that others were generous. Finally, older adults were even more likely than young adults to approach individuals who appeared more generous and trustworthy, despite the fact that these first impressions were not predictive of actual generosity. This research has important implications. Financial exploitation of older adults is highly prevalent, and in many cases, the perpetrator is either known to the victim or has victimized them before. Our findings suggest that revictimization in older adults may be driven by a combination of poor associative memory, a desire to interact with familiar others, and a reliance on inaccurate perceptions based on physical appearance. Future studies might uncover strategies that older adults can use to compensate for these effects. For example, since older adults are motivated by social concerns, it may be better for them to think of giving money to a scammer as a financial loss for one’s family rather than a financial loss for the self. The impact of older adults’ associative memory deficits and prosocial motivations on economic decision-making might be innocuous in some cases but devastating in others.
Materials and Methods
Participant Recruitment.
Two hundred and twenty-three participants (121 White non-Hispanic, 10 White Hispanic, 34 Asian non-Hispanic, 21 Black non-Hispanic, 1 Black Hispanic, 5 Other race non-Hispanic, 6 Other race Hispanic, 4 mixed race, and 21 not reported) completed the study, with 210 included in the final sample (SI Appendix, Exclusion Criteria). All participants provided informed consent, and all procedures were approved by the Institutional Review Board of the University of Pennsylvania. Efforts were made to recruit roughly equal numbers of participants in three age bins: young (18 to 34), middle-aged (35 to 59), and older adults (60+). Cognitively normal older adults were recruited primarily from the Clinical Core of Penn’s ADRC (n = 46 in final sample from this group). These participants are part of a longitudinal cohort in which they undergo neuropsychological assessments and consensus conference designation to assess their cognitive status annually. All other participants were recruited through advertisements on Penn’s campus (n = 111) or advertising on Facebook (n = 53). Participants recruited outside of the cohort reported that they were free of cognitive impairment, but we also collected the WTAR from all participants who were outside the cohort. Participants from the ADRC cohort already had WTAR scores available. While this test does not capture cognitive decline, it allowed us to test whether the age groups were matched on general intelligence.
Stimuli.
For information about how stimuli (photographs of previous participants and houses) were selected, see SI Appendix.
Procedure.
The procedure for this experiment was adapted from ref. 15. The session consisted of four tasks done in the following order: reward, distractor, decision, and memory tasks (presented in E-Prime 2.0; Psychology Software Tools). Participants were told at the outset that they would be learning about decisions made by participants who had previously come into the laboratory to play a dictator game. Then the experimenter explained the dictator game to participants and gave them the opportunity to play the role of proposer in the dictator game. They were told that the proposer was known as player A and that the people they would be learning about in the task would be referred to as player A in the reward task (described below). Participants were also given the opportunity to make a dictator game decision by answering the same multiple-choice question (potential splits in $1 increments) as the participants who were part of the stimulus database.
Reward Task.
The reward task consisted of a face block and a house block. The order in which these blocks were presented was counterbalanced across participants. On each trial of the face block of the reward task, participants saw a picture of a trial-unique face with the words “Retrieving decision from Player A” underneath. After 1.5 s, the text was replaced with that player A’s split (e.g., “Player A: $5, You: $5) for 3 s. Then participants viewed a screen asking them to rate how they felt about player A’s offer (1, good; 2, neutral; 3, bad). They had up to 4.5 s to respond. After a 1-s intertrial interval, the next player A was presented. There were 32 face trials all together, half of which offered a $5/$5 split and half of which offered a $10/$0 split, yielding 16 high-value and 16 low-value social stimuli. Each participant saw the same (randomly chosen) trial order, to minimize individual differences in accuracy due to order effects. In the house block of the reward task, participants viewed pictures of houses instead, with the text underneath the house initially saying “Lottery is being generated.” Participants were told that houses represented lotteries that were arbitrarily paired with rewards. This text was replaced with the participant’s outcome from that lottery (e.g., “You win: $5”). Half of the 32 house trials paid out $5, and half paid out $0, yielding 16 high-value and 16 low-value nonsocial stimuli. Just as in the face block, participants were asked to rate how they felt about the outcome from each house lottery.
Distractor Task.
After both blocks of the reward task, participants completed a 5-min anagrams task in which they were presented with a series of scrambled words and were given 30 s to unscramble each one. They were given a text entry box beneath each scrambled word to type in their responses, but they were not required to respond. Participants could not advance the trial themselves, and a new scrambled word was presented every 30 s.
Decision Task.
Next, participants completed a decision task, with house and face trials intermixed. Each participant saw the same (randomly chosen) trial order. Participants were instructed that on face trials, they would see two stimuli representing two different player As and they would have to decide which player A they would prefer to play with (i.e., who they would take a proposed split from). They were presented with two images, and they had 5 s to respond by pressing 1 for left or 2 for right. On the left side of the screen, they always saw a schematic drawing of a face, and on the right, they saw a trial-unique image of a player A from our stimulus database. Choosing the schematic drawing meant that a player A would be selected at random from the database, and their dictator game offer would be played out. Choosing the trial-unique image meant that the pictured player A’s dictator game split would be played out. Participants were encouraged to use the information they had learned in the reward task to make their decisions, and they were told that one of the face trial decisions would be randomly selected and the participant would receive the offer shared by the player A pictured (if they chose the trial-unique image) or they would receive the offer from a randomly drawn player A (if they chose the schematic). They were also told that the player As pictured would be contacted and would receive the portion of the $10 that they kept for themselves. All 32 faces from the reward task were presented, along with 16 novel player A images.
On house trials, participants were shown a schematic line drawing of a house on the left and a trial-unique image of a house on the right, and they had 5 s to choose between them by pressing 1 or 2. The 32 houses from the reward task were shown, along with 16 novel house images. Participants were told that one of the house trial decisions would be randomly selected and played out as well. Here choice of the schematic line drawing or choice of a novel house image resulted in a random payout of $0 or $5, but choice of a house image from the reward task would result in the same payout that the house was associated with earlier.
Participants thus completed 96 total decision trials, split into two blocks. They were never given feedback for their choices, but after the 5-s decision time was up, a black frame appeared for 1 s to indicate which choice the participant made. After a 1-s intertrial interval, the next trial appeared. At the end of the study, participants were paid according to their choices on one house trial and on one face trial.
Memory Task.
Finally, participants completed a memory task, in which they were presented with the 64 images that they saw in both the reward and decision tasks, as well as 32 never-before-seen images (16 houses and 16 faces). All participants saw the same (randomized) trial order. On each trial, they were asked to indicate (with a key press) on a scale from 1 to 5 whether the image had been shown before. The scale was anchored such that 1 corresponded to a high-confidence response that the stimulus was “old,” and 5 corresponded to a high-confidence response that the stimulus was “new,” with 3 corresponding to “not sure.” If the participant selected 1, 2, or 3, they were taken to another screen and asked what offer was given by the stimulus: $0 or $5. Then they were asked to rate their confidence in that answer (1, very sure; 2, pretty sure; 3, just guessing). Finally, they were asked to rate whether or not they chose to play with that stimulus in the decision task (1, yes; 2, no; 3, do not recall). If the participant selected 4 or 5 for the item memory question, the follow-up questions were omitted, and the next image was presented. The memory task was self-paced, with 1-s intertrial intervals between stimuli.
Although previous research showed no differences in memory performance whether memory was assessed before or after decision-making (15), we ensured in a separate online experiment that the effects of associative memory on decision-making did not differ whether interaction decisions were made before memory was assessed or after (SI Appendix).
WTAR.
After participants completed the task, they completed the WTAR (44), which involves pronouncing 50 irregularly spelled words. Each correct pronunciation is given a score of 1. This vocabulary test is considered a measure of intelligence, and performance is thought to be unaffected by cognitive decline. This measure was chosen because the participants from the ADRC cohort already had this measure collected when they joined the cohort.
For information about the procedure for remote data collection as well as exclusion criteria, see SI Appendix.
Analyses.
Participant characteristics.
To ensure that the three age groups did not differ from each other with respect to gender or intelligence, we ran a χ2 test to examine effects of age group on gender (male/female; two participants who reported a gender other than male or female were excluded from this analysis) and a one-way ANOVA to examine effects of age group on WTAR score. We also sought to ensure that memory performance did not differ between participants who performed the task remotely and those who performed it in the laboratory. We ran an independent-samples t test comparing overall item memory (d′) and overall associative memory (defined here as the proportion of hits that were correctly classified as having a $5 or $0 value) between participants from these two testing modality groups. Finally, we compared the cognitively normal older adults from the Penn ADRC cohort to those who were recruited through Facebook on these measures. Since we found no significant differences, we collapsed across testing modalities in our analyses.
Self-report.
We investigated effects of age, stimulus type, and value on participants’ self-reported ratings in the reward task by performing a repeated-measures ANOVA with stimulus type (face/house) and value ($5/$0) as within-subjects factors and age as a covariate.
Memory.
We took a signal detection approach to analyzing the memory data. First, we obtained a measure of item memory for each subject, separately for the social and nonsocial conditions. For the item memory analysis, we did not include responses in which participants indicated a “not sure” response (i.e., a 3). We calculated successful item memory as d′, in which the higher the d′, the better the subject’s discrimination between old and new stimuli. The d′ measure was calculated as z score (hit rate) − z score (false alarm rate). We implemented standard correction procedures to account for hit rates of 1 and false alarm rates of 0 by adjusting extreme values by 1/2N, where N is the number of old images for hit rates and the number of novel foils for false alarms. The d′ scores were submitted to one-sample t tests (comparing to 0), separately for each age group and stimulus type, to assess if item memory was above chance. Additionally, we estimated the response bias, as defined by −0.5 * [z score (hit rate) + z score (false alarm rate)]. We also compared these to zero using one-sample t tests, separately for each age group and stimulus type. Finally, we ran a Pearson correlation between age and 1) d′ for faces, 2) response bias for faces, 3) d′ for houses, and 4) response bias for houses.
In previous studies (15, 16) using a similar paradigm, associative memory was quantified as the percentage of times that the participant reported the correct stimulus value, out of the total number of item hits. However, we were interested not just in the overall associative memory accuracy but also in whether there were any memory biases that differed as a function of age. Therefore, we also took a signal detection approach to analyzing our associative memory data. We calculated d′ and response bias for associative memory for faces and houses, by considering an item as an associative memory hit if it was a $5 stimulus correctly identified as being worth $5 and as an associative memory false alarm if a $0 stimulus was identified as being worth $5 (note that this means that $0 stimuli correctly identified as being worth $0 are considered correct rejections rather than hits, but this labeling is arbitrary). Since the item hit rate was high (mean = 88%; SD = 11.5%) and did not significantly differ between $5 stimuli and $0 stimuli (t209 = 0.28; P = 0.782), we were confident that this method of analyzing the associative memory data would not be unduly biased by item memory performance. In addition to comparing the associative memory d′ and response bias measures to zero using one-sample t tests for each age group and stimulus type, we also performed Pearson correlations to investigate the relationship between age and associative memory d′ and response bias measures, separately for faces and houses.
Decision-making.
We were interested in the extent to which memory and stimulus value impacted decision-making, regardless of whether the stimuli were social or nonsocial. This analysis was restricted to trials that were 1) seen in the reward task phase of the experiment and 2) correctly recognized as old. Trials were categorized into different memory levels based on responses in the memory task, as follows. If associative memory was correct (participant said the stimulus was worth $0 when it was worth $0 or $5 when it was worth $5) and the subject expressed confidence in their associative memory response (i.e., answered “pretty sure” or “very sure” on the following question), then the item was labeled as indicative of confident correct associative memory. If associative memory for the item was incorrect and the subject expressed confidence in that answer, then this item was labeled as reflecting confident incorrect associative memory. Finally, if the participant indicated that they were guessing about their response for the associative memory question, regardless of if they got the answer right, that trial was labeled as reflecting item memory only. (Note that because this category collapses across correct and incorrect trials, it makes our analysis more conservative.) We performed a repeated-measures ANCOVA on choice data, with memory level (confident incorrect/item memory only/confident correct) and value ($0/$5) as within-subjects factors and age as a covariate. As a reminder, a stimulus was considered chosen if it was picked over the schematic image. If we found any effects of age, we planned to run post hoc Pearson correlations between age and choice in all six relevant trial bins, controlling for multiple comparisons using Bonferroni correction, to uncover the nature of the interaction.
We decided to exclude item misses from this analysis because since item memory was generally excellent, we would have had to exclude almost 50% (n = 98) of our sample in order to ensure that participants had at least one item in each memory level bin, including the “no item memory” bins. By excluding item misses, we could increase the sample size for this analysis to n = 165 (∼80% of the total sample).
Finally, we determined if age was associated with adaptive decision-making. We considered a decision to be adaptive if participants approached a high-value ($5) stimulus or avoided a low-value ($0) stimulus. We calculated the proportion of times out of all previously seen stimuli that participants made the adaptive choice, separately for the face and house trials, and we ran Pearson correlations between those proportions and age. In addition, we did these same analyses separately for high-value and low-value stimuli in both domains (i.e., we calculated the proportion of all high-value stimuli that were approached and the proportion of all low-value stimuli that were avoided), in order to examine whether age-related changes in decision-making were driven by maladaptive approach decisions, avoidance decisions, or both.
Social vs. nonsocial decision-making.
Next, we examined if memory-based decision-making differed if the stimuli were social or nonsocial. We were primarily interested in participants’ capacity to use their associative memory to make decisions, so we excluded the item memory only trials (i.e., associative memory guesses), and relabeled each trial according to whether the value of the stimulus on that trial was confidently remembered as being high value (“Remembered as $5”) or confidently remembered as being low value (“Remembered as $0”). We then ran a repeated-measures ANCOVA with choice as the dependent variable, remembered value (remembered as $5 vs. $0) and stimulus type (face vs. house) as within-subjects factors, and age as a covariate. If we found any effects of age, we planned to rerun this analysis separately for each age group. We also planned to conduct post hoc Pearson correlations between age and choice in each of the four relevant trial bins, Bonferroni-correcting for multiple comparisons.
Effects of face attributes.
Finally, we examined the effects of perceived 1) trustworthiness, 2) warmth, 3) dominance, 4) competence, 5) attractiveness, and 6) perceived dictator game generosity of the face stimuli on decision-making. To this end, for each of these qualities, we took the average rating (across the n = 20 independent raters; SI Appendix) and entered it as an independent variable in a mixed-effects logistic regression, with choice (1, chose stimulus; 0, chose schematic) as the dependent variable. Value (0, $0 stimulus; 1, $5 stimulus), associative memory (0, incorrect associative memory; 1, correct associative memory), and the value * associative memory interaction term were entered as covariates of no interest. Note that correct associative memory was defined differently in this analysis, not taking into account confidence ratings, in order to ensure that all trials were included and that each subject would have the same number of memory levels. We allowed intercepts and slopes (for the stimulus rating) to vary by subject. All participants across all age groups were included in these six mixed-effects regressions. If any of the models did not converge, we planned to drop random slopes from the model. In addition to examining the fixed effects of these face attributes, we were also interested in how these effects might vary with age. Therefore, we extracted the random-effects coefficients on the rating term for each subject and ran a Pearson correlation between these coefficients and age.
Supplementary Material
Acknowledgments
This work was funded by grant RF1AG058065 from the National Institute on Aging. We thank Nabil Khan, Ilyssa Delos Reyes, and Andy Garcia for help with data collection. We would also like to thank Oriel FeldmanHall and Russell Epstein for providing materials and Vishnu Murty for helpful discussion.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2208681119/-/DCSupplemental.
Data, Materials, and Software Availability
Anonymized behavioral data have been deposited in Open Science Framework (https://osf.io/39yqz/) (45).
References
- 1.Burnes D., et al. , Prevalence of financial fraud and scams among older adults in the United States: A systematic review and meta-analysis. Am. J. Public Health 107, e13–e21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson J. C., et al. , Financial exploitation of older adults: A population-based prevalence study. J. Gen. Intern. Med. 29, 1615–1623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyberg L., Lövdén M., Riklund K., Lindenberger U., Bäckman L., Memory aging and brain maintenance. Trends Cogn. Sci. 16, 292–305 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Buckner R. L., Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 44, 195–208 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Lighthall N. R., Neural mechanisms of decision-making in aging. Wiley Interdiscip. Rev. Cogn. Sci. 11, e1519 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Chung H.-K., Tymula A., Glimcher P., The reduction of ventrolateral prefrontal cortex gray matter volume correlates with loss of economic rationality in aging. J. Neurosci. 37, 12068–12077 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halfmann K., Hedgcock W., Kable J., Denburg N. L., Individual differences in the neural signature of subjective value among older adults. Soc. Cogn. Affect. Neurosci. 11, 1111–1120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mather M., Carstensen L. L., Aging and motivated cognition: The positivity effect in attention and memory. Trends Cogn. Sci. 9, 496–502 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Isaacowitz D. M., Freund A. M., Mayr U., Rothermund K., Tobler P. N., Age-related changes in the role of social motivation: Implications for healthy aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 76 (suppl. 2), S115–S124 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Carstensen L. L., Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychol. Aging 7, 331–338 (1992). [DOI] [PubMed] [Google Scholar]
- 11.Bailey P. E., Leon T., A systematic review and meta-analysis of age-related differences in trust. Psychol. Aging 34, 674–685 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Bailey P. E., Ruffman T., Rendell P. G., Age-related differences in social economic decision making: The ultimatum game. J. Gerontol. B Psychol. Sci. Soc. Sci. 68, 356–363 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Rosi A., Nola M., Lecce S., Cavallini E., Prosocial behavior in aging: Which factors can explain age-related differences in social-economic decision making? Int. Psychogeriatr. 31, 1747–1757 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Daw N. D., Doya K., The computational neurobiology of learning and reward. Curr. Opin. Neurobiol. 16, 199–204 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Murty V. P., FeldmanHall O., Hunter L. E., Phelps E. A., Davachi L., Episodic memories predict adaptive value-based decision-making. J. Exp. Psychol. Gen. 145, 548–558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FeldmanHall O., Montez D. F., Phelps E. A., Davachi L., Murty V. P., Hippocampus guides adaptive learning during dynamic social interactions. J. Neurosci. 41, 1340–1348 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell R., Buchner A., Source memory for faces is determined by their emotional evaluation. Emotion 11, 249–261 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Buchner A., Bell R., Mehl B., Musch J., No enhanced recognition memory, but better source memory for faces of cheaters. Evol. Hum. Behav. 30, 212–224 (2009). [Google Scholar]
- 19.Nyberg L., Functional brain imaging of episodic memory decline in ageing. J. Intern. Med. 281, 65–74 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Carstensen L. L., Mikels J. A., At the intersection of emotion and cognition: Aging and the positivity effect. Curr. Dir. Psychol. Sci. 14, 117–121 (2016). [Google Scholar]
- 21.Carstensen L. L., The influence of a sense of time on human development. Science 312, 1913–1915 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carstensen L. L., Socioemotional selectivity theory: The role of perceived endings in human motivation. Gerontologist 61, 1188–1196 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar M., Neta M., Linz H., Very first impressions. Emotion 6, 269–278 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Todorov A., Olivola C. Y., Dotsch R., Mende-Siedlecki P., Social attributions from faces: Determinants, consequences, accuracy, and functional significance. Annu. Rev. Psychol. 66, 519–545 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Todorov A., Said C. P., Engell A. D., Oosterhof N. N., Understanding evaluation of faces on social dimensions. Trends Cogn. Sci. 12, 455–460 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Zebrowitz L. A., Montepare J. M., Social psychological face perception: Why appearance matters. Soc. Personal. Psychol. Compass 2, 1497–1517 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassidy B. S., Zebrowitz L. A., Gutchess A. H., Appearance-based inferences bias source memory. Mem. Cognit. 40, 1214–1224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell R., Mieth L., Buchner A., Appearance-based first impressions and person memory. J. Exp. Psychol. Learn. Mem. Cogn. 41, 456–472 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Hasher L., Zacks R. T., Working memory, comprehension, and aging: A review and a new view. Psychol. Learn. Motiv. Adv. Res. Theory 22, 193–225 (1988). [Google Scholar]
- 30.Amer T., Campbell K. L., Hasher L., Cognitive control as a double-edged sword. Trends Cogn. Sci. 20, 905–915 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Peters E., Hess T. M., Västfjäll D., Auman C., Adult age differences in dual information processes: Implications for the role of affective and deliberative processes in older adults’ decision making. Perspect. Psychol. Sci. 2, 1–23 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Cassidy B. S., Hughes C., Lanie S. T., Krendl A. C., Effects of executive ability on bias and ingroup perceptions in aging. Psychol. Aging 35, 283–294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krendl A. C., Heatherton T. F., Kensinger E. A., Aging minds and twisting attitudes: An fMRI investigation of age differences in inhibiting prejudice. Psychol. Aging 24, 530–541 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A., Persistent reliance on facial appearance among older adults when judging someone’s trustworthiness. J. Gerontol. B Psychol. Sci. Soc. Sci. 73, 573–583 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Schaper M. L., Mieth L., Bell R., Adaptive memory: Source memory is positively associated with adaptive social decision making. Cognition 186, 7–14 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Salthouse T. A., When does age-related cognitive decline begin? Neurobiol. Aging 30, 507–514 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Old S. R., Naveh-Benjamin M., Differential effects of age on item and associative measures of memory: A meta-analysis. Psychol. Aging 23, 104–118 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Bailey P. E., Petridis K., McLennan S. N., Ruffman T., Rendell P. G., Age-related preservation of trust following minor transgressions. J. Gerontol. B Psychol. Sci. Soc. Sci. 74, 74–81 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Shi L. Z., Tang W. H., Liu X. P., Age-related schema reliance of judgments of learning in predicting source memory. Neuropsychol. Dev. Cogn. B. Aging Neuropsychol. Cogn. 19, 301–318 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Mather M., Johnson M. K., Affective review and schema reliance in memory in older and younger adults. Am. J. Psychol. 116, 169–189 (2003). [PubMed] [Google Scholar]
- 41.Hess T. M., Follett K. J., Adult age differences in the use of schematic and episodic information in making social judgments. Neuropsychol. Dev. Cogn. B. Aging Neuropsychol. Cogn. 1, 54–66 (2007). [Google Scholar]
- 42.Cassidy B. S., Hughes C., Krendl A. C., Age differences in neural activity related to mentalizing during person perception. Neuropsychol. Dev. Cogn. B. Aging Neuropsychol. Cogn. 28, 143–160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rule N. O., Krendl A. C., Ivcevic Z., Ambady N., Accuracy and consensus in judgments of trustworthiness from faces: Behavioral and neural correlates. J. Pers. Soc. Psychol. 104, 409–426 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D., Wechsler Test of Adult Reading: WTAR (The Psychological Corporation, 2001). [Google Scholar]
- 45.Lempert K.M., et al. , Aging is associated with maladaptive episodic memory-guided social decision-making. Open Science Framework. https://osf.io/39yqz/. Deposited 17 August 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized behavioral data have been deposited in Open Science Framework (https://osf.io/39yqz/) (45).