Significance
Primates from the Americas and Madagascar are predominantly arboreal but occasionally descend to the ground. This increased ground use was associated with multiple ecological drivers, including increased temperature and a decrease in canopy cover, as well as species-specific traits, including a dietary shift away from fruits and larger group size. As anthropogenic impacts to habitats and climate worsen, our results suggest that diurnal species already inhabiting hot, sparsely canopied sites, and exhibiting more generalized diets, are more likely to shift toward greater ground use.
Keywords: primate communities, primate evolution, evolutionary transitions, niche shift, climate change
Abstract
Among mammals, the order Primates is exceptional in having a high taxonomic richness in which the taxa are arboreal, semiterrestrial, or terrestrial. Although habitual terrestriality is pervasive among the apes and African and Asian monkeys (catarrhines), it is largely absent among monkeys of the Americas (platyrrhines), as well as galagos, lemurs, and lorises (strepsirrhines), which are mostly arboreal. Numerous ecological drivers and species-specific factors are suggested to set the conditions for an evolutionary shift from arboreality to terrestriality, and current environmental conditions may provide analogous scenarios to those transitional periods. Therefore, we investigated predominantly arboreal, diurnal primate genera from the Americas and Madagascar that lack fully terrestrial taxa, to determine whether ecological drivers (habitat canopy cover, predation risk, maximum temperature, precipitation, primate species richness, human population density, and distance to roads) or species-specific traits (body mass, group size, and degree of frugivory) associate with increased terrestriality. We collated 150,961 observation hours across 2,227 months from 47 species at 20 sites in Madagascar and 48 sites in the Americas. Multiple factors were associated with ground use in these otherwise arboreal species, including increased temperature, a decrease in canopy cover, a dietary shift away from frugivory, and larger group size. These factors mostly explain intraspecific differences in terrestriality. As humanity modifies habitats and causes climate change, our results suggest that species already inhabiting hot, sparsely canopied sites, and exhibiting more generalized diets, are more likely to shift toward greater ground use.
Eutherian mammal radiations are characterized by multiple evolutionary transitions between terrestrial, arboreal, fossorial, and aquatic lifestyles (1, 2). In primates, arboreality is hypothesized to be the ancestral condition (2–5). The evolutionary shift in some primate lineages to terrestrial niches is associated with various morphological/skeletal adaptations (6–10). Terrestriality is the prevalent strategy among some lineages of Catarrhini primates (i.e., African and Asian monkeys and great apes) (9, 11). Conversely, adaptations for predominantly terrestrial lifestyles are notably absent among living Platyrrhini of the Americas and Strepsirrhini of Africa (including Madagascar) and Asia (7, 12–14). However, some of these arboreal, diurnal primates periodically use the ground (15–21).
The evolutionary transition from arboreality to terrestriality is complex and carries debated costs and benefits (22, 23), of which three main areas are discussed. First, descending to the ground may come at the cost of greater predation risk (24, 25). Yet, it is unclear whether arboreal or terrestrial lifestyles are characterized by greater predation risks (22, 23, 26–28). Regardless, ground use by arboreal primates exposes them to novel predators and predation patterns. Recent natural and anthropogenically driven ecological changes, however, negatively impact native carnivore occupancy (29, 30), and may reduce terrestrial predation risk, thus facilitating ground use in primates (17, 20, 31–33). It should be noted, however, that native carnivores are often supplanted by nonnative carnivores, including dogs, which can have a negative impact on primate populations (29, 34, 35). Second, species occurring in naturally open canopy habitats have been shown to use the ground frequently (36). To such a degree, environmental changes and increasing anthropogenic encroachment on tropical forests may act as catalysts for species to adopt terrestrial habits as canopy cover becomes patchy and forest fragments provide fewer or lower quality resources. As a result, species may descend to the ground to cross open areas more frequently to fulfill their energetic requirements, access reproductive opportunities, or to disperse (17, 32, 37, 38). Therefore, plasticity in use of additional ecological niches (e.g., terrestrial stratum) may enhance resilience to disturbance and persistence in some fragmented landscapes (39–41). Third, extreme temperatures limit species’ biological functions (42, 43). As the understory and terrestrial environments are cooler than the upper canopy (43, 44), intense seasonal heat in previously dense tropical forest environments may drive arboreal species to seek thermoregulatory relief on the ground (45, 46). Arboreal primates during hot periods regularly descend to the ground to access terrestrial water sources for drinking or immersive cooling (18, 38, 47–51), and this behavior may become increasingly common given the cascading impacts of climate change (e.g., extreme heatwaves and droughts) (52, 53).
Primate community structure may also play an important role leading to terrestriality. Typically, sympatric species maintain separate niches to reduce ecological competition (54, 55). Therefore, in sites with high primate species richness (i.e., number of species) and greater potential for interspecific competition, species that can expand into terrestrial niches may experience reduced competition. As sympatric competitors, including other primate species, are potentially crowded into smaller ranges due to habitat losses, interspecific competition may increase until a new state is reached (56).
Species-specific factors have also been suggested to facilitate niche transition. Limited resource availability in the canopy may lead to shifts in foraging strategies (57), including increased terrestriality (11, 16, 38). For example, arboreal species reliant on seasonal resources may be more inclined to expand their dietary niche to include ground-based resources during periods of food scarcity (33). Furthermore, fully or semiterrestrial primates tend to be larger than strictly arboreal primates and tend to live in larger groups (22, 58, 59). Both characteristics are likely adaptations to high predation pressure and resource availability (28, 59–62) and may have facilitated the shift to terrestriality. Additionally, quadrupedal locomotion along the forest canopy, which mainly includes largely horizontal substrates, may have selected for hind- and forelimbs of similar length (7). This is in contrast to species using vertical clinging and leaping (VCL) locomotion from vertical substrates, which is associated with much longer hindlimbs (7). Species in the former category are predicted from a biomechanical perspective to have more effective cursorial quadrupedalism in a terrestrial environment (8, 63). Such species-specific factors may have facilitated the evolutionary transition of some primates to terrestrial lifestyles (9, 11, 64).
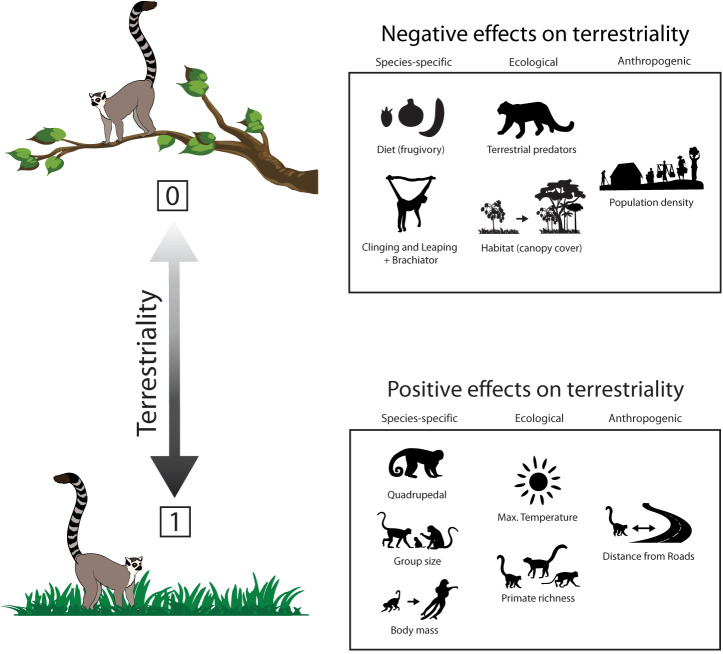
We focus on diurnal primates of the Americas and Madagascar to explore anthropogenic and ecological factors, and species-specific traits that are associated with greater use of the ground in two independent radiations. We did not include monkeys and apes from Africa and Asia as many of these species regularly exhibit semiterrestrial and terrestrial lifestyles (9, 11), and thus they experienced their niche transition presumably millions of years ago. Specifically, we are not interested in testing adaptations to terrestriality, but rather in the ecological, anatomical, and behavioral traits that make terrestriality a possible option for an arboreal primate. Regarding ecological and anthropogenic factors, we predict that terrestriality will be greater in species at sites: 1) where fewer native terrestrial predators pose a risk; 2) with more open, degraded, or fragmented forest areas, (i.e., less canopy cover); 3) with higher maximum temperatures favoring behavioral thermoregulation; 4) with high primate species richness; and 5) in greater distance from both roads and areas of higher human population densities (Fig. 1). Considering species-specific traits that may promote ground use, we predict that terrestriality will be greater in species: 1) that rely less on a diet of fruit as folivores tend to have gut adaptations more suitable for terrestrial resources; 2) with larger bodies; 3) that form larger groups; and 4) that exhibit anatomical adaptations for arboreal quadrupedalism (Fig. 1).
Fig. 1.
Hypothesized relationships between species-specific traits, and ecological and anthropogenic factors and ground use by monkeys in the Americas and lemurs in Madagascar, and not any specific transition in one species or another. For species-specific traits, taxa exhibiting quadrupedal locomotion (inferred from their intermembral index (IMI)), have a larger group size, and have greater body mass are hypothesized to use the ground more. Taxa with diets consisting of more fruit, and exhibiting vertical clinging and leaping (VCL) and brachiator locomotion (inferred from their IMI) are hypothesized to spend less time on the ground. Regarding ecological factors, taxa inhabiting sites with higher maximum temperatures and greater primate species richness are hypothesized to use the ground more. Taxa inhabiting sites with a greater number of terrestrial predators and greater continuous canopy coverage are hypothesized to spend less time on the ground. Regarding anthropogenic factors, taxa inhabiting sites that are greater distances from roads are hypothesized to use the ground more, whereas taxa inhabiting sites that are closer to denser human populations are hypothesized to spend less time on the ground.
Results
The 47 arboreal diurnal primate species we studied spent little time on the ground (2.5 ± 0.1% of the activity budget, monthly mean ± SE; n = 2,227 mo), and for over half of the species (61.7%) terrestrial behavior comprised less than 1% of their total monthly activity. Lemurs spent 4.8 ± 0.3% (monthly mean ± SE; n = 1,002 mo) of their time on the ground, whereas platyrrhine monkeys spent 2.4 ± 0.2% (monthly mean ± SE; n = 1,225 mo) of their time on the ground. Lemurs were on average more terrestrial than platyrrhine monkeys (Table 1 and SI Appendix, Table S2).
Table 1.
Summary results of the within-species model (variability within species) and the between-species model (variability between species) testing the influence of potential ecological drivers and species-specific factors on monthly terrestrial activity by arboreal primates from the Americas and Madagascar
| Model | Estimate | Error | CI | PD |
|---|---|---|---|---|
| Within-species model | ||||
| Intercept | −4.33 | 1.35 | −6.94 to −1.46 | 100% |
| Predation risk | 0.29 | 0.14 | 0.01 to 0.57 | 98% |
| Habitat (canopy cover) | −0.35 | 0.12 | −0.57 to −0.11 | 100% |
| Temperature maximum | 0.16 | 0.04 | 0.08 to 0.24 | 100% |
| Precipitation | 0.01 | 0.03 | −0.06 to 0.07 | 58% |
| Primate species richness | 0.28 | 0.15 | −0.01 to 0.56 | 97% |
| Habitat × Primate species richness | 0.17 | 0.08 | 0.01 to 0.33 | 98% |
| Diet (frugivory) | −0.17 | 0.04 | −0.25 to −0.10 | 100% |
| Group size | 0.10 | 0.05 | 0.00 to 0.19 | 98% |
| Posthabituation | −0.07 | 0.04 | −0.15 to 0.00 | 97% |
| Human population density | −0.04 | 0.15 | −0.33 to 0.25 | 60% |
| Distance to road | 0.23 | 0.12 | 0.00 to 0.46 | 97% |
| Between-species model | ||||
| Intercept | −3.84 | 2.39 | −8.74 to 0.79 | 95% |
| Region (Americas) | −1.29 | 2.74 | −6.73 to 4.25 | 70% |
| Predation risk | −0.02 | 0.46 | −0.94 to 0.84 | 52% |
| Habitat (canopy cover) | 0.26 | 0.38 | −0.48 to 1.00 | 76% |
| Temperature maximum | 0.17 | 0.35 | −0.55 to 0.82 | 69% |
| Precipitation | 0.45 | 0.40 | −0.34 to 1.22 | 87% |
| Primate species richness | −0.25 | 0.18 | −0.60 to 0.11 | 92% |
| Habitat × Primate species richness | −0.04 | 0.10 | −0.24 to 0.15 | 67% |
| Diet (frugivory) | −0.22 | 0.25 | −0.71 to 0.28 | 82% |
| Body mass | −0.43 | 0.34 | −1.08 to 0.25 | 90% |
| Group size | 0.41 | 0.27 | −0.10 to 0.94 | 94% |
| IMI (quadrupedal) | 0.24 | 1.30 | −2.43 to 2.70 | 58% |
| IMI (VCL) | 1.58 | 1.77 | −1.99 to 5.02 | 82% |
| Human population density | −0.12 | 0.37 | −0.84 to 0.61 | 62% |
| Distance to road | 0.33 | 0.41 | −0.48 to 1.12 | 79% |
Estimate, mean of the posterior distribution; Error, SD of the posterior distribution; CI, 95% credible intervals; PD, probability of direction indicating the probability of a coefficient being different from zero. Coefficients with PD > 90% are considered strong evidence of directional effects and are highlighted in bold. IMI intercept is a categorical variable and corresponds to brachiator, whereas VCL and quadrupedalism are the estimated differences from the intercept.
In the complete model, which accounts for both intra- and interspecific variability, the most important ecological pressure positively associated with terrestriality was maximum temperature, while habitat canopy cover was negatively associated with time spent on the ground (SI Appendix, Fig. S8 and Table S2). Considering anthropogenic factors, distance to roads was positively associated with ground use (SI Appendix, Fig. S8 and Table S2). For species-specific factors, a species’ degree of frugivory was negatively associated with terrestriality, whereas larger group size was positively associated with terrestriality (SI Appendix, Fig. S8 and Table S2). Furthermore, posthabituation time was negatively associated with ground use, meaning that species studied across a greater number of months were less likely to exhibit terrestriality (SI Appendix, Fig. S8 and Table S2).
For the within-species model, which evaluates the variability among conspecific populations (Table 1 and SI Appendix, Fig. S9), multiple ecological pressures influenced primate ground use. Similar to the complete model, maximum temperature and distance to roads were positively associated with terrestriality, while habitat canopy cover was negatively associated with terrestriality. Unlike the complete model, however, terrestriality decreased with increasing terrestrial predation risk, and increased with primate species richness. We also found a positive interaction between primate-rich habitats and habitat canopy cover, indicating an amplified effect of canopy cover on terrestriality in areas with higher primate species richness. Like the complete model, the three species-specific factors related to terrestriality were degree of frugivory (negative), group size (positive), and posthabituation (negative).
In the between-species model, which measures variability across species (Table 1 and SI Appendix, Fig. S10), none of the factors were strongly related to terrestriality. The only ecological factor that exhibited a clear association was primate species richness (negative). Species in habitats with denser canopy cover and with fewer sympatric primate species spent a greater proportion of time on the ground. Terrestriality was positively related with body mass and negatively with group size, indicating smaller species and larger groups, respectively, spending more time on the ground.
Discussion
We found more terrestrial activity in hotter environments with more mammalian predators, larger groups, and taxa with less frugivorous diets. However, the degree of terrestriality varies both within and between species, and when this variation is analyzed separately it reveals a more complex picture. Our within-species comparison shows that groups living in more open habitats, with more potential predators, and richer primate communities exhibit greater degrees of terrestriality. Species at more remote sites (i.e., greater distances from roads) also spent more time on the ground. By comparison, our between-species analysis reveals that species that descend more often to the ground tend to be smaller and live in larger groups. Contrary to previous single-species studies that showed an observer effect (15, 39, 65; but see ref. 33), shorter study duration (the number of posthabituation months) was strongly associated with ground use.
Ecological Correlates to Terrestriality.
Maximum temperature showed a positive relationship with the time spent on the ground in both the complete and intraspecific models, while our proxy for seasonality (i.e., monthly precipitation) was not influential within any of the models. Gradual and rapid temperature increases correlate with behavioral shifts (compare refs. 66 and 67). A possible explanation is that lemurs and platyrrhine monkeys increase their use of the ground as an adaptive thermoregulation strategy (68, 69). For example, we found that primate species like Eulemur fulvus and Eulemur rufifrons spent considerably more time on the ground in relatively hotter tropical deciduous forest habitats of Madagascar compared to their conspecifics inhabiting the cooler humid forest habitats, likely to access terrestrial water sources (50). This finding supports the idea that shifting between arboreality and terrestriality is an effective thermoregulatory response, with important implications considering current global warming trends (52, 70, 71).
Canopy cover has long been considered a factor in many evolutionary shifts (72). However, the degree to which this may result in a more terrestrial primate lifestyle is unclear (11). Denser canopy cover was associated with spending less time on the ground within species, but was not associated with ground use in the between-species model. The former is in line with our expectation that terrestrial activity tends to be higher in habitats with sparser canopies, such as those disturbed by anthropogenic activities (19, 38). Arboreal species in more open habitats (i.e., sparser canopies) may need to descend to the ground to forage and drink (19, 34, 38, 73, 74), although their ability to do so may be guided by species-specific characteristics acting as a predisposition (i.e., behavioral and anatomical exaptations) (75, 76).
Primate species richness had contrasting results, with a negative effect on terrestriality in the interspecific model and positive effect in the intraspecific model. As all primates within the communities examined are arboreal, greater numbers of species at a site may lead to higher competition for canopy resources, including both food and space. Under specific circumstances, descending to a rarely exploited niche (the forest floor) may be critical for coping with periods of limited resources (77). The positive interaction effect between canopy cover and primate species richness in the intraspecific model supports our hypothesis. Essentially, the negative effect of canopy cover on terrestriality was weaker as sympatric taxa richness increased. In other words, when canopy cover increases, the decrease in terrestriality is less pronounced in habitats with high primate diversity where we would expect higher competition. However, it is possible that at sites where a species may have recently become locally extirpated, this loss may result in competitive release, allowing one or more of the remaining species to partially, or fully, exploit newly available resources (78–80). Compared to many mammal taxa, primates tend to exhibit a high degree of behavioral flexibility (81, 82), and interindividual variation may be the mechanism underlying niche expansion (83).
Predation pressure is difficult to quantify and evaluate. The number of potential predator species provides a proxy with which to measure this risk (84, 85), and some site-/species-focused studies have noted that relaxation of predation pressure led to more ground-based activity (20, 32, 39). Interestingly, terrestrial predator species richness was associated with more terrestrial activity in our within-species model. Although we were unable to account for predator population abundance or the potential ecological and co-occurrence factors affecting these taxa (86), it appears that anthropogenic factors may play a role. Human population density and distance to roads may be considered as general proxies for various aspects of human encroachment, including feral dogs (Canis familiaris), which are known to prey upon wildlife (34, 35, 73). Of the two anthropogenic factors, conspecifics were more terrestrial at sites farther from roads.
Species-Specific Factors as Potential Facilitators of Terrestriality.
Frugivory was associated with decreased ground use in both the complete and intraspecific models, supporting previous assertions that diet is a driving force of terrestriality (38, 87). This link may be associated with folivores or species with a broad dietary spectrum using the ground more often to forage on different preferred foods (17, 88), or because they have gastrointestinal and dental adaptations allowing them to use terrestrial resources more efficiently (89). Despite the general reduced ground use by frugivores, periods of reduced fruit availability may lead facultative frugivores to search the ground for novel food resources to meet their seasonal nutritional needs (21, 90, 91). Many primates with broad dietary niches come to the ground to engage in geophagy and to access mineral licks (92, 93) and potentially fermented foods (94). However, given the supplementary nature of this feeding habit (95) that often involves short terrestrial travel, it has not been considered a key causative factor in any major shift in strata use. Primates may also descend to the ground to forage for arthropod prey (19, 21).
Group size had an effect in both the complete and within-species models. Large groups can facilitate terrestriality as they can potentially reduce predation risk. Folivores are in principle less constrained by group size compared to frugivores due to the less clumped spatiotemporal availability of preferred resources, although this is not always the case (96, 97). However, although it is conceivable that large groups foster terrestrial activity, it is also possible that groups that use the ground more often tend to form larger groups to reduce predation risk, leaving the causal relationship unclear. In both Brachyteles hypoxanthus in Caratinga (Brazil) and Hapalemur meridionalis in Mandena (Madagascar), it was the case that the largest group was considerably more terrestrial than smaller groups (17, 39).
Biomechanical (e.g., size-related and anatomical) challenges may impose various biological, ecological, and physiological constraints within both the arboreal and terrestrial strata (8). Such morphological factors could be species-specific consequences that evolve after, or in parallel with, the initial niche expansion into terrestrial activity. However, contrary to our hypothesis, we found a negative effect of body mass between species (i.e., smaller species showed increased terrestriality). Original hypotheses about the relationship between body size and terrestriality were proposed by Fleagle (7, 59) to explain the range of niche use in the entire Primate order, including the larger-bodied catarrhines. The primates included in this study, platyrrhines and lemuriformes, represent a more restricted range of body mass variation, and therefore it is possible that a different relationship between terrestriality and body mass is present for the entire order. We cannot evaluate the role that the relatively recent extinction of the larger and more terrestrial lemur species (98) may have had in releasing the competition for terrestrial resources with the extant smaller lemur species.
Although posthabituation months were used to control for a possible positive observer effect, our complete and within-species model showed that primates studied for shorter periods more strongly associated with ground use. Though this contrasts from some single-species studies (15, 39, 65), we believe our negative effect is more likely the result of the nonrandom distribution of study periods with respect to seasons, or the nonrandom distribution of species with respect to their average level of arboreality within our dataset.
Conclusions
We have shown that there are multiple factors that may lead arboreal primates to use the ground and that this transition is influenced by site-specific ecological pressures. Specifically, habitats with sparser canopies may be responsible for the evolutionary transition of nonhuman primates to terrestrial lifestyles (11, 19), whereas the more proximate causes of strata shift appear to be hotter environmental conditions (71) and dietary shifts away from frugivory. Considering species-specific traits, larger groups and smaller body mass facilitated ground use.
Although significant climate changes in both the Americas (99) and Madagascar (100) likely facilitated faunal turnover and speciation, it is not clear why terrestriality did not evolve there to the same extent as it is seen in catarrhines. Fossil records are sparse and the real extent of niche diversification that occurred in lemurs and platyrrhines over their evolutionary history is far from being understood (7). Examining primate behavioral and ecological flexibility alongside current environmental conditions, however, provides insight into evolutionary transitional periods that resulted in shifts to novel ecological niches. As human activity drives climate change, degrades primate habitats, and shifts plant phenological patterns, primate populations are facing unprecedented challenges that threaten their persistence (52, 70, 101–104). We expect that an increased use of the ground strata by species inhabiting hot, sparsely canopied sites and that exhibit a more generalized diet can buffer species against extinction. Productive future lines of research that will further clarify factors driving the evolution of terrestriality include comparing behavioral repertoires in terrestrial versus arboreal environments, evaluating potential ecological and life history drivers of annual variation in terrestrial behaviors, and if habitat structure explains variation in population-level terrestriality. All nonhuman primates, however, will be faced with challenges created by anthropogenic changes and for species less inclined to terrestrial activity, fast and effective conservation strategies will need to be implemented to ensure their survival.
Materials and Methods
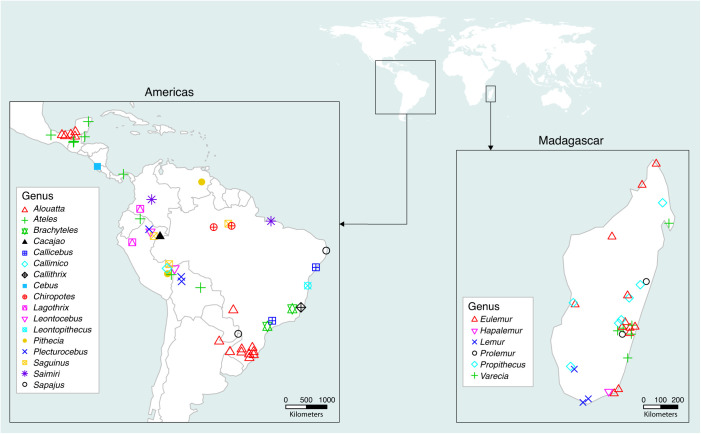
Coauthors contributed raw monthly behavioral ecology data from 47 primate taxa, specifically 15 lemur species representing two families (Lemuridae and Indriidae), and 32 platyrrhine species representing 4 families (Atelidae, Callitrichidae, Cebidae, and Pitheciidae) (Dataset S1). This collated dataset includes 150,961 observation hours across 2,227 months from species at 68 research sites, specifically 20 sites in Madagascar and 48 sites throughout the Americas (Fig. 2 and SI Appendix, Table S1). Our dataset includes 16 primate species (specifically 10 lemur and 6 platyrrhine monkey species) for which we have data from multiple sites.
Fig. 2.
Spatial distribution of primate genera included in our behavioral ecology dataset.
For each species, we provide monthly proportional data to account for different data-collection methods used in each study. Since nocturnal species are exposed to different ecological pressures compared to diurnal primates, we only focused on diurnal primates. Datasets included had a minimum of 12 h/mo to increase the chances that rare events, in our case terrestriality by arboreal species, would be recorded (105). We considered the monthly proportion of time spent terrestrially as our dependent variable.
Ecological Drivers.
We extracted site- and time-specific climate and habitat values in Google Earth Engine (earthengine.google.com) using the spatial coordinates and the year and month of the observations (106). We extracted monthly maximum temperatures and monthly total precipitation from the ERA5 Monthly Aggregates dataset (107). The latter is used as a conservative proxy for seasonality (108), incorporating the rainfall variation at research sites for the months included in our dataset. We obtained the relative canopy cover using a circular buffer around the coordinates of each study site from the Landsat Tree Cover Continuous Fields dataset (109) (SI Appendix, Fig. S1). Specifically, the buffer area was equal to twice the size of each study species' reported mean home range area.
We estimated the number of potential terrestrial mammalian predators per species per site from the number of carnivore species per location using International Union for the Conservation of Nature (IUCN) range maps (110). For each species per location, we only considered predators with a mean body mass greater than or equal to one-quarter of the mean body mass of the focal primate. This ratio was based on the minimum predator-prey ratio observed in terrestrial mammals (appendix S1 in ref. 111). The body mass threshold is very conservative and may lead to the inclusion of species that do not typically prey on adult primates; however, considering primates’ slow life histories and the additive risks to juveniles/infants, smaller predators can potentially trigger a fear reaction (112, 113). This approach is also limited by the nature of IUCN range maps and the consideration of predator–prey body mass ratios, which likely overestimates the presence of predators as large predators may have been extirpated by local hunting and habitat loss. However, this approach allowed us to estimate the spatial gradients of predator species richness at this scale of analysis for all sites and species, thereby avoiding potential author or publication reporting biases (compare with ref. 114). Although primates may also be preyed upon by birds of prey, snakes, and other primates, carnivores are considered their main terrestrial predators (115, 116).
Using IUCN range maps (110), we also estimated the number of sympatric primate species per site: that is, species richness (SI Appendix, Figs. S2 and S3). Given the potential increased effect of interspecific competition in sites with less canopy cover (potentially more fragmented), we examined the interaction between these two factors.
Finally, we considered two proxies of anthropogenic disturbance: human population density and distance to roads. The former accounts for the presence of humans, whereas the latter is a proxy of inverse of remoteness (i.e., inverse of accessibility to humans). We obtained the human population density data from the Socioeconomic Data and Applications Center (https://sedac.ciesin.columbia.edu/). We used the Gridded Population of the World dataset, v4 (117) for 2000, 2005, 2010, 2015, and 2020 at 30 arc-second resolution (∼1 km) (SI Appendix, Figs. S4 and S5). We matched the terrestriality data with the values of human population density using the closest layer in time. Road data for the countries of interest were extracted from the OpenStreetMap database (openstreetmap.org). From the vector files we only retained primary, secondary, and tertiary roads, motorways, trunks, all related “links,” and residential roads. Instead, we excluded all unclassified roads, paths, footways, and similar. We then rasterized the vector layer at 1-km resolution and calculated the distance from the nearest road for the entire study area (SI Appendix, Figs. S6 and S7). All raster data processing was conducted in R v3.6.3 (118) using the “raster” package (119).
Species-Specific Factors.
For each species’ specific site, coauthors contributed the monthly proportion of time spent feeding on fruit, the mean body mass, and the mean group size measured in the field. In the absence of mean body mass, we used data from the All the World’s Primates database (120). We inferred locomotion type via the Intermembral Index (IMI) (63), which is calculated as (length of humerus + length of radius)/(length of femur + length of tibia) × 100. Quadrupedal primates typically have an IMI between 67 and 104; of the arboreal quadrupeds, those falling below the lower threshold typically exhibit VCL, and those above the upper threshold are typically categorized as exhibiting brachiation, but also suspensory locomotion (7, 8, 63). Given potential for error when collecting field measurements, and the relative stability of the IMI within genera, we assigned each species to a category based on the IMI averaged at the genus level.
Statistical Analyses.
We tested our hypotheses by fitting a zero-inflated model with a β-family and logit link-function and using Bayesian inference. The use of a zero-inflation and β-family allowed accommodating for the highly skewed and zero-inflated distribution of terrestriality values bounded between 0 and 1. We added a group level to study site and one to species to control for multiple estimates in the same locations and multiple estimates per species, respectively. Considering climatic variation and its effect on resource phenology (108), we controlled for seasonality using monthly temperature and total precipitation at each site. We used study duration (i.e., the number of months posthabituation) to control for observer effect within the models. We controlled for phylogenetic effects by using a variance–covariance matrix derived from the phylogeny in Upham et al. (121). An additional observation level random effect was added to control for overdispersion. All fixed factors were scaled to a mean of 0 and SD of 1 to ensure comparability of the effect sizes, as well as improving numerical stability in their estimation. We used weakly informative priors using a normal distribution with an SD of 10 for the intercept, and an SD of 1.5 for all slope coefficients, thereby limiting the range to a plausible gradient of variation considering the logit link-function and scaled coefficients (122). All predictors were tested for multicollinearity prior to the modeling but none showed a correlation coefficient >0.7, so all variables were retained in the final model (123).
The complete model accounted for both intra- and interspecific variability in terrestriality; thus, we ran two additional models to disentangle the variability within- and between-species. To assess whether the detected effects could also explain the different degrees of terrestriality among conspecific populations (within-species model), we included only anthropogenic and ecological drivers, as well as site-specific species’ factors for which we had data (percent frugivory and group size). Prior to fitting this second model, we first subtracted the species’ mean from each observation value (species mean deviation) (124). Then, we fitted a model including both ecological drivers and species-specific traits to estimate the variability across species (between-species model), from which we subtracted the species mean deviation from each observation value. For both the within- and between-species model, we rescaled the variable to a mean of 0 and SD of 1 prior to model fitting and used the same weakly informative priors used for the complete model.
We ran 6,000 iterations over 10 Markov chain Monte Carlo chains for each model, with a “burn in” period of 2,000 iterations per chain leading to a total of 40,000 usable posteriors. We also checked models for chain convergence and parameter identifiability. We summarized the posterior distributions of coefficient estimates using 95% credible intervals. We considered credible intervals that did not overlap with zero as strong evidence of directionality. We also reported the probability of direction, a threshold-independent measure of evidence that varies from 50 to 100% and that indicates the probability of a coefficient being different from zero (125). We fitted the models in R v3.6.3 (118) using the ‘brms’ package (126), for model fitting, ‘bayestestR’ (125) for Bayesian summary statistics, and ‘ape’ (127) and ‘phytools’ (128) for handling the phylogenetic data.
Supplementary Material
Acknowledgments
T.M.E. was supported by funding from the American Society of Primatologists, Conservation International’s Primate Action Fund, IDEAWild, the Margot Marsh Biodiversity Fund, the Mohamed bin Zayed Species Conservation Fund (Project 11253008), Primate Conservation Inc., and the Primate Society of Great Britain/Knowsley Safari Park. D.B.A. was supported by the National Science Foundation Doctoral Dissertation Improvement Grants (NSF-DDIG) (BCS 1341174), the Animal Behavior Society, the Society for Integrative and Comparative Biology, the Tinker Foundation, and the Mansfield and Columbus campuses at The Ohio State University. N.A. was supported by Neotropical Primate Conservation through various grants. K.R.A. is supported as a fellow in the Canadian Institute for Advanced Research’s “Humans and the microbiome” program. J.C.B.-M. was supported by funding by the World Wildlife Fund-US (#6573), the Brazilian National Council for Scientific and Technological Development/Conselho Nacional de Desenvolvimento Cietifico e Tecnológico (CNPq) (PQ 1C #304475/2018-1), and Programa Nacional de Pós-Doutorado of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (Brazilian Higher Education Authority)/Comissao de Aperfeiçoamento de Pessoal de Nival Superior (CAPES) (Finance Code 001; PNPD Grant 2755/2010). S.A.B. received financial support from the Biological Dynamics of Forest Fragments Project, the Smithsonian Tropical Research Institute, Arizona State University, Fulbright/Institute of International Education, the Margot Marsh Biodiversity Foundation, Providing Educational Opportunities, Primate Conservation Inc., the Organization for Tropical Studies, and the American Society of Primatologists. T.S.C. was supported by a scholarship from Fundação de Amparo à Pesquisa (FUNAPE) and would like to thank the Mineração Rio do Norte (MRN) for their support. Ó.M.C. was supported by Programa Nacional de Pós-Doutorado of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (Brazilian Higher Education Authority)/CAPES (Finance Code 001; PNPD Grant 2755/2010). C.C.-K. received financial support from The NSF (NSF-BSC-1745371), Yale University MacMillan Center for International Studies, the National Geographic Society (EC-420R-18), The Explorers Club, Yale Institute for Biospheric Studies, Yale University Department of Anthropology, The International Primatological Society, and Primate Conservation Inc. Madagascar Ministry of the Environment and Madagascar National Parks permitted this research. I.C.C. received research support from the NSF (Dissertation Improvement Award Grant BNS-9101520), the National Geographic Society (Grant 4496-91), the Boise Fund of the University of Oxford, a Grant-in-Aid of Research from Sigma Xi–The Scientific Research Society, a Washington University Graduate Fellowship, the St. Louis Rainforest Alliance, and the St. Louis Zoo. L.M.F. was supported by Natural Sciences & Engineering Research Council of Canada, and the Canada Research Chairs Programme. A.M.F. was funded by the Wildlife Conservation Society, Conservation International, the Rufford Foundation, and the Primate Society of Great Britain. R.G.F. was supported by the Fundação Grupo Boticario (0973-2013-8) and CNPq. I.F. was supported by a scholarship from FUNAPE and would like to thank the MRN for their support. K.S.H. was supported by the Foundation for Wildlife Conservation, the Zoological Society of Milwaukee, Birds Without Borders, the University of Calgary, Athabasca University, and the Natural Sciences and Engineering Research Council of Canada. E.W.H. was supported by Deutsche Forschungsgemeinschaft, Deutscher Akademischer Austauschdienst (DAAD), Universitätsbund Göttingen. S.M.H. was supported by the Natural Sciences and Engineering Research Council of Canada, the Philanthropic Educational Organization, Conservation International, and Primate Conservation Inc. S.E.J. was supported by the Natural Sciences and Engineering Research Council of Canada, Conservation International, and Primate Conservation Inc. M.C.L. received funding from the National Science Foundation Graduate Research Fellowship (Award 1650042), University of California, Davis, Bucknell University, a Greenville Zoo Conservation grant, the Pittsburgh Zoo Conservation and Sustainability Fund, Primate Conservation Inc., an International Primatological Society Research Grant, and IDEAWild. F.R.d.M. was support by a scholarship from FUNAPE and would like to thank the MRN for their support. T.H.M. was funded primarily by The Aspinall Foundation through the “Saving Prolemur simus” project, with additional support from Beauval Nature and International Union for Conservation of Nature-“Save Our Species.” L.S.M. was supported by a scholarship from FUNAPE and would like to thank the MRN for their support. M.T.O. was supported in part by the NSF-DDIG (BCS 0851761), the J. William Fulbright Foundation, Sigma Xi, and the School of Human Evolution and Social Change at Arizona State University. B.P.-G. was supported by grants from the Comisión Nacional para el Conocimiento y Uso de la Bioversidad (HK009) and Consejo Nacional de Ciencia y Technología (CONACYT) (J51278), as well as graduate scholarships from CONACYT (2008–2010 and 2018–2021). G.P.-M. was supported by a doctorate and master’s degree scholarship from CONACYT (2007–2010) and by the Academic Division of Biological Sciences of the Universidad Juárez Autónoma de Tabasco. G.P.-M. also thanks the local people from Balancán for their guidance. B.E.R. was supported by funding provided by the Durrell Wildlife Conservation Trust, the Lion Tamarins of Brazil Fund, Margot Marsh Biodiversity Foundation, the Tulsa Zoo, Sigma Xi, and an NSF Research and Training grant to University of Maryland. G.R.-F. was supported by CONACYT Grants J51278, 157656, and CF263958 and by National Geographic Society Grant WW-R008-17. C.J.S. was supported by the Leakey Foundation, NSF-DDIG (BCS-0752683 to C. H. Janson), the National Geographic Society, and the Wenner-Gren Foundation. S.S. was supported by Neotropical Primate Conservation through various grants. P.G.A.d.S.L. received research support from the Fundação Grupo Boticario (0973-2013-8) and CNPq. A.C.S. was supported by Biotechnology and Biological Sciences Research Council Grant 98/S11498. S.E.S.A. was supported by CONACYT through student Grant 207883. Data were collected with the assistance of Augusto Canul, Eulogio Canul, Juan Canul, and Macedonio Canul. J.P.S.-A. was supported by DAAD, CAPES, and International Primatological Society Conservation grants, and currently supported by Fundação de Amparo a Ciência e Tecnologia (BFP-0149-2.05/19). V.K.S. was supported by the Coordination of Improvement of Higher Level Personnel (CAPES). K.J.E.S. was supported by Evangelisches Studienwerk Villigst, Universität Hamburg, and Kompetenzzentrum Nachhaltige Universität, Primate Conservation Inc. (PCI #1542), and the German Academic Exchange Service DAAD. M.P.T. was supported by the American Society of Mammalogists, the Consejo Nacional de Investigaciones Científicas y Técnicas, and IDEAWild. S.V.B. was supported by the National Autonomous University of Mexico (PAPIIT-Project IN200216).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2121105119/-/DCSupplemental.
Data, Materials, and Software Availability
All statistical codes and data used in the analyses have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.19344992.v1) (129). All other study data are included in the main text and supporting information.
References
- 1.Ji Q., et al. , The earliest known eutherian mammal. Nature 416, 816–822 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Szalay F. S., “Ancestral locomotor modes, placental mammals, and the origin of Euprimates: Lessons from history” in Primate Origins: Adaptations and Evolution, Ravosa M. J., Dagosto M., Eds. (Springer, 2007), pp. 457–487. [Google Scholar]
- 3.Sussman R. W., Primate origins and the evolution of angiosperms. Am. J. Primatol. 23, 209–223 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Kirk E. C., Lemelin P., Hamrick M. W., Boyer D. M., Bloch J. I., Intrinsic hand proportions of euarchontans and other mammals: Implications for the locomotor behavior of plesiadapiforms. J. Hum. Evol. 55, 278–299 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Boyer D. M., Toussaint S., Godinot M., Postcrania of the most primitive euprimate and implications for primate origins. J. Hum. Evol. 111, 202–215 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Gebo D. L., Sargis E. J., Terrestrial adaptations in the postcranial skeletons of guenons. Am. J. Phys. Anthropol. 93, 341–371 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Fleagle J. G., Primate Adaptation and Evolution (Academic Press, ed. 3, 2013). [Google Scholar]
- 8.Fleagle J. G., Lieberman D. E., “Major transformations in the evolution of primate locomotion” in Great Transformations in Vertebrate Evolution, Dial K. P., Shubin N. H., Brainerd E. L., Eds. (University of Chicago Press, 2015), pp. 257–278. [Google Scholar]
- 9.Hunt K. D., Why are there apes? Evidence for the co-evolution of ape and monkey ecomorphology. J. Anat. 228, 630–685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machnicki A. L., Spurlock L. B., Strier K. B., Reno P. L., Lovejoy C. O., First steps of bipedality in hominids: Evidence from the atelid and proconsulid pelvis. PeerJ 4, e1521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCrossin M. L., Benefit B. R., Gitau S. N., Palmer A. K., Blue K. T., “Fossil evidence for the origins of terrestriality among Old World higher primates” in Primate Locomotion: Recent Advances, Strasser J., Fleagle J., Rosenberger A., McHenry H., Eds. (Springer, 1998), pp. 353–396. [Google Scholar]
- 12.Fleagle J. G., Reed K. E., Comparing primate communities: A multivariate approach. J. Hum. Evol. 30, 489–510 (1996). [Google Scholar]
- 13.Heymann E. W., Giant fossil New World primates: Arboreal or terrestrial? J. Hum. Evol. 34, 99–101 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Kamilar J. M., Guidi L. M., The phylogenetic structure of primate communities: Variation within and across continents. J. Biogeogr. 37, 801–813 (2010). [Google Scholar]
- 15.Tabacow F. P., Mendes S. L., Strier K. B., Spread of a terrestrial tradition in an arboreal primate. Am. Anthropol. 111, 238–249 (2009). [Google Scholar]
- 16.Barnett A. A., et al. , Terrestrial foraging by Cacajao melanocephalus ouakary (primates) in Amazonian Brazil: Is choice of seed patch size and position related to predation risk? Folia Primatol. (Basel) 83, 126–139 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Eppley T. M., Donati G., Ganzhorn J. U., Determinants of terrestrial feeding in an arboreal primate: The case of the southern bamboo lemur (Hapalemur meridionalis). Am. J. Phys. Anthropol. 161, 328–342 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Serio-Silva J. C., Ramírez-Julián R., Eppley T. M., Chapman C. A., “Terrestrial locomotion and other adaptive behaviors in howler monkeys (Alouatta pigra) living in forest fragments” in Movement Ecology of Neotropical Forest Mammals, Reyna R., Chapman C. A., Eds. (Springer Nature, 2019), pp. 125–140. [Google Scholar]
- 19.Souza-Alves J. P., et al. , Terrestrial behavior in titi monkeys (Callicebus, Cheracebus, and Plecturocebus): Potential correlates, patterns, and differences between genera. Int. J. Primatol. 40, 553–572 (2019). [Google Scholar]
- 20.Monteza-Moreno C. M., Crofoot M. C., Grote M. N., Jansen P. A., Increased terrestriality in a Neotropical primate living on islands with reduced predation risk. J. Hum. Evol. 143, 102768 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Williamson R. E., et al. , Sharing spaces: Niche differentiation in diet and substrate use among wild capuchin monkeys. Anim. Behav. 179, 317–338 (2021). [Google Scholar]
- 22.Janson C. H., Goldsmith M. L., Predicting group size in primates: Foraging costs and predation risks. Behav. Ecol. 6, 326–336 (1995). [Google Scholar]
- 23.Shattuck M. R., Williams S. A., Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl. Acad. Sci. U.S.A. 107, 4635–4639 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheney D. L., Wrangham R. W., “Predation” in Primate Societies, Smuts B. B., Cheney D. L., Seyfarth R. M., Wrangham R. W., Struhsaker T. T., Eds. (University of Chicago Press, 1987), pp. 227–239. [Google Scholar]
- 25.Miller L. E., “An introduction to predator sensitive foraging” in Eat or Be Eaten: Predator Sensitive Foraging Among Primates, Miller L. E., Ed. (Cambridge University Press, 2002), pp. 1–17. [Google Scholar]
- 26.van Schaik C. P., Why are diurnal primates living in groups? Behaviour 87, 120–144 (1983). [Google Scholar]
- 27.Wrangham R. W., Gittleman J. L., Chapman C. A., Constraints on group size in primates and carnivores: Population density and day-range as assays of exploitation competition. Behav. Ecol. Sociobiol. 32, 199–209 (1993). [Google Scholar]
- 28.Isbell L. A., Predation on primates: Ecological patterns and evolutionary consequences. Evol. Anthropol. 3, 61–71 (1994). [Google Scholar]
- 29.Farris Z. J., et al. , Threats to a rainforest carnivore community: A multi-year assessment of occupancy and co-occurrence in Madagascar. Biol. Conserv. 210, 116–124 (2017). [Google Scholar]
- 30.Rich L. N., et al. , Assessing global patterns in mammalian carnivore occupancy and richness by integrating local camera trap surveys. Glob. Ecol. Biogeogr. 26, 918–929 (2017). [Google Scholar]
- 31.McGraw W. S., Bshary R., Association of terrestrial mangabeys (Cercocebus atys) with arboreal monkeys: Experimental evidence for the effects of reduced ground predator pressure on habitat use. Int. J. Primatol. 23, 311–325 (2002). [Google Scholar]
- 32.Campbell C. J., et al. , Terrestrial behavior of Ateles spp. Int. J. Primatol. 26, 1039–1051 (2005). [Google Scholar]
- 33.Souza-Alves J. P., et al. , For emergency only: Terrestrial feeding in Coimbra-Filho’s titis reflects seasonal arboreal resource availability. Primates 62, 199–206 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Pozo-Montuy G., Serio-Silva J. C., Chapman C. A., Bonilla-Sánchez Y. M., Resource use in a landscape matrix by an arboreal primate: Evidence of supplementation in black howlers (Alouatta pigra). Int. J. Primatol. 34, 714–731 (2013). [Google Scholar]
- 35.Farris Z. J., Chan S., Rafaliarison R., Valenta K., Occupancy modeling reveals interspecific variation in habitat use and negative effects of dogs on lemur populations. Int. J. Primatol. 40, 706–720 (2019). [Google Scholar]
- 36.Wright K. A., et al. , Positional behavior and substrate use in wild adult bearded capuchin monkeys (Sapajus libidinosus). Am. J. Primatol. 81, e23067 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Felton A. M., Engström L. M., Felton A., Knott C. D., Orangutan population density, forest structure and fruit availability in hand-logged and unlogged peat swamp forests in West Kalimantan, Indonesia. Biol. Conserv. 114, 91–101 (2003). [Google Scholar]
- 38.Barnett A. A., et al. , Terrestrial activity in pitheciins (Cacajao, Chiropotes, and Pithecia). Am. J. Primatol. 74, 1106–1127 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Mourthé I. M. C., et al. , Ground use by northern muriquis (Brachyteles hypoxanthus). Am. J. Primatol. 69, 706–712 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Wong B., Candolin U., Behavioral responses to changing environments. Behav. Ecol. 26, 665–673 (2015). [Google Scholar]
- 41.Bicca-Marques J. C., Chaves Ó. M., Hass G. P., Howler monkey tolerance to habitat shrinking: Lifetime warranty or death sentence? Am. J. Primatol. 82, e23089 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Cunningham S. J., Kruger A. C., Nxumalo M. P., Hockey P. A., Identifying biologically meaningful hot-weather events using threshold temperatures that affect life-history. PLoS One 8, e82492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bramer I., et al. , Advances in monitoring and modelling climate at ecologically relevant scales. Adv. Ecol. Res. 58, 101–161 (2018). [Google Scholar]
- 44.Samson D. R., Hunt K. D., A thermodynamic comparison of arboreal and terrestrial sleeping sites for dry-habitat chimpanzees (Pan troglodytes schweinfurthii) at the Toro-Semliki Wildlife Reserve, Uganda. Am. J. Primatol. 74, 811–818 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Kosheleff V. P., Anderson C. N., Temperature’s influence on the activity budget, terrestriality, and sun exposure of chimpanzees in the Budongo Forest, Uganda. Am. J. Phys. Anthropol. 139, 172–181 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Takemoto H., Acquisition of terrestrial life by human ancestors influenced by forest microclimate. Sci. Rep. 7, 5741 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bicca-Marques J. C., Calegaro-Marques C., Locomotion of black howlers in a habitat with discontinuous canopy. Folia Primatol. (Basel) 64, 55–61 (1995). [DOI] [PubMed] [Google Scholar]
- 48.Pozo-Montuy G., Serio-Silva J. C., Movement and resource use by a group of Alouatta pigra in a forest fragment in Balancán, México. Primates 48, 102–107 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Ferrari S. F., Hilário R. R., Use of water sources by buffy-headed marmosets (Callithrix flaviceps) at two sites in the Brazilian Atlantic Forest. Primates 53, 65–70 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Amoroso C. R., Kappeler P. M., Fichtel C., Nunn C. L., Water availability impacts habitat use by red-fronted lemurs (Eulemur rufifrons): An experimental and observational study. Int. J. Primatol. 41, 61–80 (2020). [Google Scholar]
- 51.Chaves Ó. M., et al. , Flower consumption, ambient temperature and rainfall modulate drinking behavior in a folivorous-frugivorous arboreal mammal. PLoS One 16, e0236974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvalho J. S., et al. , A global risk assessment of primates under climate and land use/cover scenarios. Glob. Change Biol. 25, 3163–3178 (2019). [DOI] [PubMed] [Google Scholar]
- 53.IPCC, “Climate change 2021: The physical science basis” in Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Masson-Delmotte V., et al., Eds. (Cambridge University Press, 2021). pp. 1–2391 [Google Scholar]
- 54.Ganzhorn J. U., “Body mass, competition and the structure of primate communities” in Primate Communities, Fleagle J. G., Janson C. H., Reed K. E., Eds. (Cambridge University Press, 1999), pp. 141–157. [Google Scholar]
- 55.Janson C. H., Chapman C. A., “Resources and primate community structure” in Primate Communities, Fleagle J. G., Janson C. H., Reed K. E., Eds. (Cambridge University Press, 1999), pp. 237–267. [Google Scholar]
- 56.Bregman T. P., et al. , Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology 96, 2692–2704 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Hemingway C. A., Bynum N., “The influence of seasonality on primate diet and ranging” in Seasonality in Primates: Studies of Living and Extinct Human and Non-Human Primates, Brockman D. K., van Schaik C. P., Eds. (Cambridge University Press, 2005), pp. 57–104. [Google Scholar]
- 58.Clutton-Brock T. H., Harvey P. H., Primate ecology and social organization. J. Zool. 183, 1–39 (1977). [Google Scholar]
- 59.Fleagle J. G., “Size and adaptation in primates” in Size and Scaling in Primate Biology, Jungers W. L., Ed. (Plenum Press, 1985), pp. 1–19. [Google Scholar]
- 60.Hamilton W. D., Geometry for the selfish herd. J. Theor. Biol. 31, 295–311 (1971). [DOI] [PubMed] [Google Scholar]
- 61.Cowlishaw G., Vulnerability to predation in baboon populations. Behaviour 131, 293–304 (1994). [Google Scholar]
- 62.Crofoot M. C., Why mob? Reassessing the costs and benefits of primate predator harassment. Folia Primatol. (Basel) 83, 252–273 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Napier J. R., Napier P. H., The Natural History of the Primates (MIT Press, 1985). [Google Scholar]
- 64.Benefit B. R., Victoriapithecus: The key to Old World monkey and catarrhine origins. Evol. Anthropol. 7, 155–174 (1999). [Google Scholar]
- 65.Nowak K., le Roux A., Richards S. A., Scheijen C. P., Hill R. A., Human observers impact habituated samango monkeys’ perceived landscape of fear. Behav. Ecol. 25, 1199–1204 (2014). [Google Scholar]
- 66.Maslin M. A., Shultz S., Trauth M. H., A synthesis of the theories and concepts of early human evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140064 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beever E. A., et al. , Behavioral flexibility as a mechanism for coping with climate change. Front. Ecol. Environ. 15, 299–308 (2017). [Google Scholar]
- 68.Boyles J. G., Seebacher F., Smit B., McKechnie A. E., Adaptive thermoregulation in endotherms may alter responses to climate change. Integr. Comp. Biol. 51, 676–690 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Terrien J., Perret M., Aujard F., Behavioral thermoregulation in mammals: A review. Front. Biosci. 16, 1428–1444 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Buchholz R., et al. , Behavioural research priorities for the study of animal response to climate change. Anim. Behav. 150, 127–137 (2019). [Google Scholar]
- 71.Oliveira B. F., Scheffers B. R., Vertical stratification influences global patterns of biodiversity. Ecography 42, 249 (2019). [Google Scholar]
- 72.Stockwell C. A., Hendry A. P., Kinnison M. T., Contemporary evolution meets conservation biology. Trends Ecol. Evol. 18, 94–101 (2003). [Google Scholar]
- 73.Galetti M., Sazima I., Impact of feral dogs in an urban Atlantic forest fragment in southeastern Brazil. Nat. Conserv. 4, 146–151 (2006). [Google Scholar]
- 74.Prates H. M., Bicca-Marques J. C., Age-sex analysis of activity budget, diet, and positional behavior in Alouatta caraya in an orchard forest. Int. J. Primatol. 29, 703–715 (2008). [Google Scholar]
- 75.Boyle S. A., Smith A. T., Can landscape and species characteristics predict primate presence in forest fragments in the Brazilian Amazon? Biol. Conserv. 143, 1134–1143 (2010). [Google Scholar]
- 76.Eppley T. M., Santini L., Tinsman J. C., Donati G., Do functional traits offset the effects of fragmentation? The case of large-bodied diurnal lemur species. Am. J. Primatol. 82, e23104 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Cowlishaw G., Dunbar R. I. M., Primate Conservation Biology (University of Chicago Press, 2000). [Google Scholar]
- 78.Peres C. A., Dolman P. M., Density compensation in neotropical primate communities: Evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 122, 175–189 (2000). [DOI] [PubMed] [Google Scholar]
- 79.Bolnick D. I., et al. , Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. Biol. Sci. 277, 1789–1797 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Segre H., DeMalach N., Henkin Z., Kadmon R., Quantifying competitive exclusion and competitive release in ecological communities: A conceptual framework and a case study. PLoS One 11, e0160798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLennan M. R., Spagnoletti N., Hockings K. J., The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. Int. J. Primatol. 38, 105–121 (2017). [Google Scholar]
- 82.Strier K. B., What does variation in primate behavior mean? Am. J. Phys. Anthropol. 162 (suppl. 63), 4–14 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Costa G. C., Mesquita D. O., Colli G. R., Vitt L. J., Niche expansion and the niche variation hypothesis: Does the degree of individual variation increase in depauperate assemblages? Am. Nat. 172, 868–877 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Vance-Chalcraft H. D., Soluk D. A., Ozburn N., Is prey predation risk influenced more by increasing predator density or predator species richness in stream enclosures? Oecologia 139, 117–122 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Griffin J. N., Byrnes J. E., Cardinale B. J., Effects of predator richness on prey suppression: A meta-analysis. Ecology 94, 2180–2187 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Davis C. L., et al. , Ecological correlates of the spatial co-occurrence of sympatric mammalian carnivores worldwide. Ecol. Lett. 21, 1401–1412 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Teaford M. F., Ungar P. S., Diet and the evolution of the earliest human ancestors. Proc. Natl. Acad. Sci. U.S.A. 97, 13506–13511 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mekonnen A., et al. , Dietary flexibility of Bale monkeys (Chlorocebus djamdjamensis) in southern Ethiopia: Effects of habitat degradation and life in fragments. BMC Ecol. 18, 4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lambert J. E., Primate digestion: Interactions among anatomy, physiology, and feeding ecology. Evol. Anthropol. 7, 8–20 (1998). [Google Scholar]
- 90.Ashbury A. M., et al. , Why do orangutans leave the trees? Terrestrial behavior among wild Bornean orangutans (Pongo pygmaeus wurmbii) at Tuanan, Central Kalimantan. Am. J. Primatol. 77, 1216–1229 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Lambert J. E., Rothman J. M., Fallback foods, optimal diets, and nutritional targets: Primate responses to varying food availability and quality. Annu. Rev. Anthropol. 44, 493–512 (2015). [Google Scholar]
- 92.Link A., Galvis N., Fleming E., Di Fiore A., Patterns of mineral lick visitation by spider monkeys and howler monkeys in Amazonia: Are licks perceived as risky areas? Am. J. Primatol. 73, 386–396 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Semel B. P., et al. , Assessing the function of geophagy in a Malagasy rain forest lemur. Biotropica 51, 769–780 (2019). [Google Scholar]
- 94.Amato K. R., et al. , Fermented food consumption in wild nonhuman primates and its ecological drivers. Am. J. Phys. Anthropol. 175, 513–530 (2021). [DOI] [PubMed] [Google Scholar]
- 95.Pebsworth P. A., Huffman M. A., Lambert J. E., Young S. L., Geophagy among nonhuman primates: A systematic review of current knowledge and suggestions for future directions. Am. J. Phys. Anthropol. 168 (suppl. 67), 164–194 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Steenbeek R., van Schaik C. P., Competition and group size in Thomas’s langurs (Presbytis thomasi): The folivore paradox revisited. Behav. Ecol. Sociobiol. 49, 100–110 (2001). [Google Scholar]
- 97.Snaith T. V., Chapman C. A., Primate group size and interpreting socioecological models: Do folivores really play by different rules? Evol. Anthropol. 16, 94–106 (2007). [Google Scholar]
- 98.Godfrey L. R., Jungers W. L., Schwartz G. T., “Ecology and extinction of Madagascar’s subfossil lemurs” in Lemurs: Ecology and Adaptation, Gould L., Sauther M. L., Eds. (Springer, 2006), pp. 41–64. [Google Scholar]
- 99.Silvestro D., et al. , Early arrival and climatically-linked geographic expansion of New World monkeys from tiny African ancestors. Syst. Biol. 68, 78–92 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Godfrey L. R., et al. , Mid-Cenozoic climate change, extinction, and faunal turnover in Madagascar, and their bearing on the evolution of lemurs. BMC Evol. Biol. 20, 97 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hockings K. J., et al. , Apes in the Anthropocene: Flexibility and survival. Trends Ecol. Evol. 30, 215–222 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Estrada A., et al. , Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 3, e1600946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galán-Acedo C., et al. , The conservation value of human-modified landscapes for the world’s primates. Nat. Commun. 10, 152 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chapman C. A., Peres C. A., Primate conservation: Lessons learned in the last 20 years can guide future efforts. Evol. Anthropol. 30, 345–361 (2021). [DOI] [PubMed] [Google Scholar]
- 105.Weatherhead P. J., How unusual are unusual events? Am. Nat. 128, 150–154 (1986). [Google Scholar]
- 106.Gorelick N., et al. , Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27 (2017). [Google Scholar]
- 107.Copernicus Climate Change Service, ERA5: Fifth Generation of the ECMWF Atmospheric Reanalyses of the Global Climate (Copernicus Climate Change Service Climate Data Store, (2017), https://cds.climate.copernicus.eu/cdsapp#!/home. Accessed 1 March 2021. [Google Scholar]
- 108.van Schaik C. P., Pfannes K. R., “Tropical climates and phenology: A primate perspective” in Seasonality in Primates: Studies of Living and Extinct Human and Non-Human Primates, Brockman D. K., van Schaik C. P., Eds. (Cambridge University Press, 2005), pp. 23–54. [Google Scholar]
- 109.Sexton J. O., et al. , Global, 30-m resolution continuous fields of tree cover: Landsat-based rescaling of MODIS vegetation continuous fields with lidar-based estimates of error. Int. J. Digit. Earth 6, 427–448 (2013). [Google Scholar]
- 110.International Union for Conservation of Nature, The IUCN Red List of Threatened Species (IUCN, Gland, Switzerland, 2017). [Google Scholar]
- 111.Tucker M. A., Rogers T. L., Examining predator-prey body size, trophic level and body mass across marine and terrestrial mammals. Proc. Biol. Sci. 281, 20142103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jones J. H., Primates and the evolution of long, slow life histories. Curr. Biol. 21, R708–R717 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barnett A. A., et al. , Honest error, precaution or alertness advertisement? Reactions to vertebrate pseudopredators in red‐nosed cuxiús (Chiropotes albinasus), a high‐canopy Neotropical primate. Ethology 124, 177–187 (2018). [Google Scholar]
- 114.Barsbai T., Lukas D., Pondorfer A., Local convergence of behavior across species. Science 371, 292–295 (2021). [DOI] [PubMed] [Google Scholar]
- 115.Hart D., “Predation on primates: A biogeographical analysis” in Primate Anti-Predator Strategies, Gursky S. L., Nekaris K. A. I., Eds. (Springer, 2007), pp. 27–59. [Google Scholar]
- 116.Fichtel C., “Predation” in The Evolution of Primate Societies, Mitani J. C., Call J., Kappeler P. M., Palombit R. A., Silk J., Eds. (Chicago University Press, 2012), pp. 169–194. [Google Scholar]
- 117.Center for International Earth Science Information Network, Gridded Population of the World, Version 4 (GPWv4): Population Density, Revision 11: NASA Socioeconomic Data and Applications Center (Socioeconomic Data and Applications Center, Columbia University, 2018). https://sedac.ciesin.columbia.edu/data/set/gpw-v4-population-density-rev11. Accessed 1 March 2021. [Google Scholar]
- 118.R Core Development Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020). [Google Scholar]
- 119.Hijmans R. J., et al. , Package ‘raster’. R package, 734 (2015). https://cran.r-project.org/web/packages/raster/index.html. Accessed 1 March 2021.
- 120.Rowe N., Myers M., All the World’s Primates (Primate Conservation, Inc., 2017), www.alltheworldsprimates.org. Accessed 13 January 2021. [Google Scholar]
- 121.Upham N. S., Esselstyn J. A., Jetz W., Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lemoine N. C., Moving beyond noninformative priors: Why and how to choose weakly informative priors in Bayesian analyses. Oikos 128, 912–928 (2019). [Google Scholar]
- 123.Dormann C. F., et al. , Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46 (2013). [Google Scholar]
- 124.Van de Pol M., Wright J., A simple method for distinguishing within-versus between-subject effects using mixed models. Anim. Behav. 77, 753–758 (2009). [Google Scholar]
- 125.Makowski D., Ben-Shachar M. S., Lüdecke D., bayestestR: Describing effects and their uncertainty, existence and significance within the Bayesian framework. J. Open Source Softw. 4, 1541 (2019). [Google Scholar]
- 126.Bürkner P., brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017). [Google Scholar]
- 127.Paradis E., et al. , Package ‘ape’. Analyses of phylogenetics and evolution, version 2 (2019). http://ape-package.ird.fr/. Accessed 1 March 2021.
- 128.Revell L. J., phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 129.Santini L., Hoeks S., Terrestriality 2022. Figshare. 10.6084/m9.figshare.19344992.v1. Deposited 28 August 2022. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All statistical codes and data used in the analyses have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.19344992.v1) (129). All other study data are included in the main text and supporting information.